Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 13/74/01. The protocol was agreed in July 2016. The assessment report began editorial review in September 2016 and was accepted for publication in January 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Olga Ciccarelli received consultancy fees from Novartis, Biogen Idec Ltd General Electric and Genzyme. All payments were made to her employer, the UCL Institute of Neurology. She also received reimbursement from Novartis and the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) for attending a symposium and funds from the UK MS Society, Engineering and Physical Sciences Research Council (EPSRC), University College London Hospitals NHS Foundation Trust and UCL Hospitals Biomedical Research Centre for research. Aileen Clarke is an editor of the journal Health Technology Assessment. All payments are made to her employer, the Warwick Medical School. Carl Counsell received funding through Biogen Idec Ltd for a departmental multiple sclerosis (MS) nurse. He has also authored a paper that was critical of the UK risk-sharing scheme for disease-modifying therapies in MS (Sudlow CLM, Counsell CE. Problems with UK government’s risk sharing scheme for assessing drugs for multiple sclerosis. BMJ 2003;326:388–92). Jeremy Rodrigues holds a fellowship at the National Institute for Health and Care Excellence. This fellowship is unremunerated. Aileen Clarke and GJ Melendez–Torres are partly supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West Midlands at the University Hospitals Birmingham NHS Foundation Trust.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Melendez-Torres et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Introduction
Multiple sclerosis (MS) is a progressive, degenerative disease affecting the central nervous system (CNS). It is characterised by inflammation and demyelination of the neurons, mediated by an autoimmune response by T cells to white matter.
Although not yet fully understood, the aetiology of MS involves major genetic components,1 with two or more genes active in causing its development. 2,3 There is also a body of literature linking the development of MS with environmental factors or hypothesising the involvement of viral infections such as Epstein–Barr virus (EBV). 4–8
Within the UK, prevalence is around 203 per 100,000 person-years, whereas incidence was 9.6 per 100,000 person-years between 1990 and 2010, with a female-to-male ratio of 2.4. 9 Peak incidence is at around 40 and 45 years of age in men and women, respectively, with peaks in prevalence at 56 and 59 years for men and women respectively.
Types of multiple sclerosis
The disease can develop and progress in three major forms: (1) relapsing–remitting MS (RRMS), (2) primary progressive MS (PPMS) and (3) secondary progressive MS (SPMS), of which RRMS originates from a single demyelinating event known as clinically isolated syndrome (CIS). 10
Clinically isolated syndrome events are isolated events of neurological disturbance lasting for > 24 hours, which indicate the first clinical demyelination of the CNS,11 with clinical syndromes that are monofocal in nature (e.g. optic neuritis and transverse myelitis) or multifocal (e.g. optical neuritis, limb weakness from transverse myelitis and cerebellar signs). Patients presenting with a clinical history of one attack are given a diagnosis of CIS. In these cases, magnetic resonance imaging (MRI) helps to confirm whether a diagnosis of MS can be given instead at the onset of symptoms. A diagnosis of MS requires that disseminated in time and disseminated in space criteria are fulfilled, and these can be checked using the MRI scan performed at the onset of CIS. Patients with CIS who fulfil the disseminated in space criteria need evidence of disseminated in time to be diagnosed with MS; if disseminated in time criteria are not met at the baseline scan, it is necessary to either repeat the MRI scan to check whether there is a new lesion or wait for a second clinical attack. Notably, then, delays in the onset of a second ‘relapse’ for patients with CIS are equivalent to delays in MS progression.
In 80% of cases, RRMS is the form of MS at time of diagnosis. In RRMS, patients experience an exacerbation of symptoms followed by periods of remission. RRMS, as defined in research protocols, is characterised by episodes of relapses that last for > 24–48 hours. RRMS can be subtyped as rapidly evolving or highly active MS and, although these terms have not been precisely defined, they usually indicate two or more relapses within 1 year, with evidence of increasing lesion frequency on MRI scans. 12 This classification is mainly used in reference to newer therapies such as natalizumab (Tysabri®; Biogen Idec Ltd, Cambridge, MA, USA) and fingolimod (Gilenya®; Novartis, Basel, Switzerland). 13
Primary progressive multiple sclerosis has an older age at onset, with men having greater susceptibility,14 and is typically characterised by occasional plateaus in disease progression, with temporary minor improvements from onset. 15 Some PPMS patients experience relapses alongside disease progression.
Secondary progressive multiple sclerosis follows on from RRMS but the disease course is progressive, with or without temporary relapses, remissions and plateaus in symptoms. 15 The transition is gradual and often SPMS is diagnosed retrospectively. 15
The natural course of the disease is highly variable, with early stages of MS potentially developing into any of the subtypes. However, each subtype is associated with cumulative neurological dysfunction, which is often measured using the Expanded Disability Status Scale (EDSS). 16 Transition from RRMS to SPMS occurs in 60–70% of patients initially diagnosed with RRMS, approximately 10–30 years from disease onset. About 15% of RRMS patients may be diagnosed with ‘benign’ MS, thus avoiding the progression of disability and conversion to SPMS. 17
To date, there is no cure for MS. Currently approved drugs for MS act as immunomodulators or immunosuppressants, with the aim of reducing the pathological inflammatory reactions and reducing the frequency and severity of relapses and the rate of disease progression. Immunomodulation and immunosuppressing drugs used in MS are called disease-modifying therapies (DMTs).
Disease-modifying therapies
Beta-interferons
There are currently five licensed beta-interferon (IFN-β) drugs for MS: two IFN-β-1a drugs [Avonex® (Biogen Idec Ltd, Cambridge, MA, USA); Rebif® (Merck, Darmstadt, Germany)], pegylated (peg) IFN-β-1a (Plegridy®; Biogen Idec Ltd, Cambridge, MA, USA) and two IFN-β-1b drugs [Betaferon® (Bayer, Leverkusen, Germany) and Extavia® (Novartis, Basel, Switzerland)]. The two IFN-β-1b drugs are the same drug (both are manufactured on the same production line). These five drugs are recombinant forms of natural IFN-β, which is a 166 amino acid glycoprotein that can be produced by most body cells in response to viral infection or other biological inducers. 18 The two types of IFN-β-1a are structurally indistinguishable from natural IFN-β whereas the two types of IFN-β-1b are non-glycosylated forms that carry two structural changes compared with natural IFN-β (Met-1 deletion and Cys-17 to Ser mutation).
Depending on the formulation, the dose regimen is one intramuscular (IM) injection once a week (Avonex), one subcutaneous (SC) injection three times per week (Rebif) or one SC injection every other day (Betaferon, Extavia). Pegylated IFN-β-1a is a long-acting formulation of IFN-β-1a obtained by adding methoxy-polyethyleneglycol-O-2-methylpropionaldehyde to IFN-β-1a, which allows less frequent administration (one SC injection every 2 weeks).
The precise mechanism of action of IFN-β in MS is not fully understood. The immunological effects of IFN-β that are thought to have a potential action on MS are inhibition of T-cell co-stimulation/activation processes, modulation of anti-inflammatory and pro-inflammatory cytokines and decrease in aberrant T-cell migration. 19
The main indication for IFN-β is the treatment of RRMS. For some patients IFN-β is indicated in response to a single demyelinating event with an active inflammatory process when there is determined to be a high risk of development of clinically definite MS (CDMS). IFN-β-1b is also licensed for use in SPMS, as is IFN-β-1a (44 µg three times weekly by SC injection; Rebif) in cases in which SPMS remains with ongoing relapse activity. IFN-β drugs are not indicated for PPMS.
The most commonly reported adverse events (AEs) related to IFN-β are irritation at injection site reactions and flu-like symptoms. 20 Other AEs reported include pain, fatigue, headache and liver function abnormalities; a rare but important side effect is nephrotic syndrome. AEs may result in treatment discontinuation. Given the biological nature of recombinant IFN-β, patients are at risk of developing neutralising antibodies (NABs) against IFN-β. NABs are thought to increase relapse rates and the rate of disease progression.
Depending on the formulation, the current annual cost per patient of IFN-β treatment in the UK, assuming British National Formulary (BNF) list prices21 and considering a continuous treatment at the standard dose, is between £7264 and £10,572.
Glatiramer acetate
There are two licensed formulations of glatiramer acetate (GA) (Copaxone®; Teva Pharmaceutical Industries, Petah Tikva, Israel). GA consists of the acetate salts of synthetic polypeptides, containing four naturally occurring amino acids. The mechanisms by which GA exerts its effects in patients with MS are not fully understood but it is thought that it induces a broad immunomodulatory effect that modifies immune processes that are currently believed to be responsible for the pathogenesis of MS. 22
According to the Summary of Product Characteristics,22 GA is indicated for the treatment of RRMS, but not PPMS or SPMS. The dose regimen is 20 mg daily (formulation of 20 mg/ml) or 40 mg three times a week (formulation of 40 mg/ml) by SC injection. The most common AEs of GA are flushing, chest tightness, sweating, palpitations, headache and anxiety. 23 Injection site reactions are observed in up to half of patients.
The current annual cost of GA per patient in the UK, assuming BNF list prices21 and considering a continuous treatment at the standard dose, is £6681–6704.
Current use in the UK
Beta-interferon and GA are currently not recommended by the National Institute for Health and Care Excellence (NICE)24 as they were considered not to be cost-effective. However, IFN-β and GA have been available in the NHS through a risk-sharing scheme (RSS), with the exception of Extavia (a new brand of IFN-β-1b) and Plegridy (pegIFN-β-1a), which were released after the publication of technology appraisal (TA) 32. 24 Within the RSS, a registry has been set up to record long-term clinical outcomes of patients receiving IFN-β and GA. This review will consider the final data from this scheme alongside the clinical effectiveness evidence and their implications for the clinical effectiveness and cost-effectiveness of GA and IFN-β.
Description of the health problem
Multiple sclerosis is a neurodegenerative disorder characterised by inflammation and demyelination of neurons in the brain and spinal cord. It is a leading cause of non-traumatic disability in working-age adults and affects over 100,000 people in the UK. Although there is currently no cure for MS, a number of DMTs are available to help reduce the frequency of relapses and the rate of disease progression. IFN-β and GA are two such groups of drugs. However, at the time of TA32 in 2002,24 there was insufficient evidence of their clinical effectiveness and cost-effectiveness. A RSS was put in place, allowing patients to access the drugs and the NHS to adjust prices based on cost-effectiveness data, as well as monitor for long-term outcomes. This study aims to appraise the clinical effectiveness and cost-effectiveness of IFN-β and GA, integrating evidence from the literature with data on long-term outcomes collected from the RSS. The following sections summarise the pathogenesis, clinical course and epidemiology of MS and current service provision for MS.
Pathogenesis
Although the precise pathogenesis of MS is unclear, our current understanding is that it stems from autoreactive inflammatory responses targeting the myelin sheaths of CNS neurons. This inflammatory response begins in the periphery with activation of T helper cells that recognise CNS antigens. The subsequent inflammatory cascade leads and responds to disruption of the blood–brain barrier, allowing for increased transepithelial migration of activated immune cells, cytokines and chemokines into the CNS. Once in the CNS, the autoimmune response leads to demyelination and axonal degeneration.
More recently, MS has been recognised as consisting of both neurodegenerative and inflammatory processes. 25,26 Although neurodegeneration in MS is even less well understood than inflammation, it is thought to be mediated by degeneration of transected axons, defects in ion balance and loss of nutritional support to glial cells surrounding neurons. 27 Notably, investigations of autopsy specimens have shown that axonal loss can occur even in areas without acute inflammation, including in grey matter and normal-appearing white matter. 28 These neurodegenerative processes are thought to be responsible for progressive and permanent disability.
Aetiology
A large body of evidence suggests a multifactorial aetiology of MS, with some interaction of genetic and environmental triggers causing the peripheral immune system to become activated against CNS antigens. Although the precise interaction remains unknown, a number of risk factors for MS have been identified.
Genetic
Unsurprisingly, genetic polymorphisms linked to MS have been identified primarily in immune response proteins. The first and most significant genetic locus was identified in the 1970s on the human leucocyte antigen (HLA) complex. 29,30 HLAs encode part of the class II major histocompatibility complex (MHC) in humans, which presents processed foreign antigens to T cells for recognition. 30,31 Variations within the HLA region have been consistently associated with a risk of MS, with the HLA-DRB1*15:01 allele particularly implicated. 32–35 It is also thought that the HLA complex carries genetic determinants of MS clinical progression. 30
Although the HLA complex has the strongest and most long-standing linkage with MS, other genes are suspected of increasing disease susceptibility, determining age at onset and causing poorer prognoses for specific types of MS. 32 These genes have been identified based on evidence from genetic linkage studies, microarray studies and, more recently, genome-wide association studies (GWASs). 36 A seminal GWAS study performed by the International Multiple Sclerosis Consortium and the Wellcome Trust Case Control Consortium investigated 465,434 single-nucleotide polymorphisms in 9772 cases and 17,376 control subjects, implicating at least 59 non-HLA genes in MS inheritance. These genes include those involved in cytokine, immune stimulation and immunological signal transduction pathways. 32
Despite substantial data on the genetic risk for MS, the rate of concordance between monozygotic twins is modest at about 25%. 37 Additionally, a study reporting genome, epigenome and ribonucleic acid (RNA) sequences in MS-discordant monozygotic twins was able to find no substantial difference accounting for MS discordance. Such evidence points to the involvement of other causes in MS pathogenesis. 38
Viral
Among all environmental risk factors investigated in MS aetiology, EBV infection has shown the strongest consistent evidence of association. 39 EBV was first suggested as a potential causative agent of MS because of the similarity in epidemiological distribution across age, geography, ethnicity and socioeconomic status. 40 In total, 99.5% of patients with MS test seropositive for EBV antibodies, compared with 94.2% of the general population. 41 The current evidence for a role of EBV in MS is multifaceted: prospective studies note increased serum anti-EBV antibody titres before the onset of MS;42 a meta-analysis found that, for both adults and children testing negative for EBV, the odds ratio (OR) for developing MS was 0.18 [for adults, 95% confidence interval (CI) 0.13 to 0.26] compared with people who tested positive;43 and, at the molecular level, EBV can be isolated from B-cell infiltrates in meninges. 44 Although EBV is a demonstrated risk factor for MS, its role in causation remains unproven.
Other environmental risk factors
Populations living farther from the equator, both native and foreign born, have consistently shown increased MS risk. 45–50 In one meta-analysis, this correlation persisted even after adjusting for regional differences in genetic HLA-DRB1 alleles,50 although it was not replicated in a separate meta-analysis using incidence instead of prevalence. 51 One hypothesis is that this effect is mediated by sun exposure and vitamin D levels, with one supporting meta-analysis of 11 studies finding lower mean serum 25-hydroxyvitamin D levels in patients with MS. 45–49,52 Other possible explanations include confounding by socioeconomic factors or the ‘hygiene hypothesis’. Smoking is also implicated as a modest but consistent risk factor for MS, with smoking cessation suggested as an effective public health intervention that carries numerous other benefits. 39
Presentation
Clinical symptoms
Although the initial signs of MS are variable between patients, MS classically presents with focal neurological symptoms and signs of CNS dysfunction around the third decade of life. Relapses may present as painful loss of vision in one eye (optic neuritis), unilateral motor or sensory disturbance (corticobulbar/spinal tract involvement), double vision/vertigo/unsteadiness (brainstem or cerebellar syndrome), Lhermitte’s phenomenon (pain down the spine/body on flexing the neck, from a cervical cord lesion) or bilateral leg and bladder dysfunction (spinal cord syndrome). Fatigue is a common but non-specific symptom. As MS progresses in severity, it can also lead to cognitive decline as well as changes in mobility, bladder/bowel function and sexual function.
Imaging features
Magnetic resonance imaging modalities have an advantage over other imaging techniques, with their ability to dampen resonance signals from the cerebrospinal fluid (CSF) and intensify signals from sites of inflammation. 53 In sites of active inflammation, disruption of the blood–brain barrier allows lesions to be enhanced with the administration (and take-up) of contrast, whereas chronic lesions are generally non-enhancing. MRI formally joined the diagnostic criteria for MS in 200154 and has rapidly become a primary tool for characterising MS severity and progression. The characteristic MRI lesion is a cerebral or spinal plaque with high T2 signal, representing a region of demyelination with axon preservation. In the brain, plaques representing perivenular inflammation (and potential blood–brain barrier disruption) are known as Dawson’s fingers, and they are seen in the periventricular regions radiating perpendicularly away from ventricles. Outside the periventricular region, plaques are also commonly found in the corpus callosum, sub-/juxtacortical region, optic nerves and visual pathway. 55 Spinal cord lesions are nearly as common, although they more likely to be noticed clinically before MRI identification.
Pathology
Early acute-stage lesions are active plaques characterised by the breakdown of myelin, which may appear oedematous and inflamed histologically. Subacute-stage lesions appear paler in colour and have higher focal regions of macrophages. Chronic-stage lesions are inactive plaques with low levels of myelin breakdown, but are characterised by gliosis, leading to the production of scar tissue. 56–58 Within the chronic stages of the lesions, attempts at remyelination occur but the process may be hampered and unsuccessful because of the scar tissue formed by gliosis. 59,60
Diagnostic criteria
The diagnosis of MS is a clinical one, with supportive roles for neuroimaging and paraclinical findings. The fundamental requirement is for demonstrated CNS lesions disseminated in time and space. Initially, this demonstration was purely based on clinical findings and history; however, over time, laboratory results (such as CSF oligoclonal bands) and paraclinical evidence (such as neuroimaging) have been included as possible bases of diagnosis. 61
The McDonald criteria, newly revised in 2010,62 continue to form the standard diagnostic tool for investigating suspected MS in research settings and, to a more flexible degree, in clinical practice. 63 A MS attack, relapse or episode is defined by ‘patient-reported symptoms or objectively observed signs typical of an acute inflammatory demyelinating event in the CNS, current or historical, with duration of at least 24 hours, in the absence of fever or infection’. 62
The most ‘secure’ diagnoses are supported by two or more MS attacks, with objective clinical evidence of at least one lesion and ‘reasonable historical evidence’ of the second. Patients who have had two or more attacks with associated clinical signs of two or more separate lesions in the CNS are said to have CDMS. If objective clinical evidence for only one lesion is found, evidence for disseminated in space can come from T2 lesions on MRI if they occur in at least two of four locations characteristic for MS (juxtacortical, periventricular, infratentorial, spinal cord). Evidence for disseminated in time can be provided by new T2 or contrast-enhancing lesions on MRI appearing after disease onset or the simultaneous presence of contrast-enhancing (active) and non-enhancing (chronic) lesions on the scan performed at onset of CIS. Patients presenting with a clinical history of one attack and objective clinical evidence of one lesion, but without sufficient evidence of either disseminated in space or disseminated in time, are diagnosed with CIS.
Recent trends in the McDonald diagnostic criteria
The Poser et al. 64 criteria for MS diagnosis were published in 1983 and included two major categories of ‘definite’ or ‘probable’ MS, each with subgroups of ‘clinical’ or ‘laboratory supported’. Diagnosis was made based on the number of attacks and lesions with clinical evidence, paraclinical evidence and laboratory evidence. CIS or ‘possible MS’ was not included in the criteria, as such patients were not yet involved in research studies. The McDonald 2001 diagnostic criteria abolished the previous categories and instead focused on evidence for disseminated in time and disseminated in space. For the first time, it also explicitly allowed for MRI data to serve as evidence for disseminated in space and disseminated in time. Originally, demonstration of disseminated in space meant meeting the Barkhol–Tintoré criteria65 (or showing two MRI lesions and positive CSF) and demonstration of disseminated in time could be achieved only by enhancing lesions appearing 3 months after a clinical event. With a 2005 revision to the criteria,66 disseminated in time could also be demonstrated by the appearance of new T2 lesions 1 month after a ‘reference scan’ (which was required to be 3 months post clinical onset).
The McDonald 2010 revision62 further simplified previous diagnostic criteria. It allowed for lesions at two of four areas to provide evidence of disseminated in space, as opposed to the previous Barkhol–Tintoré criteria. 65 It also simplified the disseminated in time criteria by removing the requirement that the baseline MRI scan be carried out at least 30 days post clinical event and allowing for the presence of simultaneous enhancing and non-enhancing lesions on the scan at onset of CIS to serve for disseminated in time. After this revision, a diagnosis of MS could be confirmed based on just a single MRI scan (with enhancing and non-enhancing lesions disseminated in space). Because more patients meet the disseminated in space and disseminated in time criteria under the 2010 revision as opposed to the original guidelines or 2005 revision, more recently diagnosed patients are more likely to have a diagnosis of confirmed MS than a diagnosis of CIS.
Prognosis
Disability as part of prognosis
Quantification of disability in MS has been used extensively to standardise characterisations of functional disease progression. The three Kurtzke scales have commonly been used to describe MS progression. First, the Functional Systems Scale consists of measures of functionality in eight pre-chosen systems;16 second, the Disability Status Scale (DSS) is an 11-point scale measuring global disability;67 and, third, the EDSS is a modification of the DSS, measuring 20 points of disability. 68 The EDSS is currently used as the standard to measure disease progression in MS.
The EDSS quantifies disability in eight functional systems, specifically focusing on pyramidal, cerebellar, brain stem, sensory, bowel and bladder, visual and cerebral/mental function. 16 An EDSS score of 0.0 would indicate normal neurology with no impairment in any system; an EDSS score of 4 suggests full ambulation without aid despite relatively severe disability; a score of 6 suggests needing unilateral support (e.g. cane or crutch) to walk 100 m; and a score of 7 suggests wheelchair confinement, with an inability to walk > 5 m with support. 16
Prognoses for disease progression
Prognostic data are primarily taken from longitudinal cohort studies, many of which can include patients both on and off treatment. Patients who present with CIS have a 60–80% risk of developing CDMS within 10 years if they have MRI lesions at the time of presentation and a ≈20% risk if they do not (note that this prognosis will likely change with the revised McDonald 2010 diagnostic criteria for CIS) (reviewed in Marcus and Waubant69). RRMS is thought to last for around two decades before transition to SPMS. 70 Up to 15% of patients with RRMS may be retrospectively diagnosed with ‘benign’ MS. 17 There is significantly less consensus about the natural history of disability in the progressive phase of MS, with median times to EDSS 6 ranging from 15 to 32 years. 70 Very generally, progression to EDSS 4 is suspected to occur after one decade, EDSS 6 after 2 decades and EDSS 7 after three decades. 71,72 Median ages for EDSS 4, 6 and 7 were 42, 53 and 63 years, respectively, for a cohort study of 1844 patients in Lyon. 73
Risk factors for disease progression
Multiple sclerosis is notoriously heterogeneous and, even when all known risk factors are combined, they provide only moderate prognostic value. Generally, observational data have found male sex, older age at onset, progressive state at onset and higher number of MRI lesions to be predictive of a poor prognosis with faster disability progression. 74,75 A recent systematic review has identified several key factors related to relapse frequency and recovery. 75 Relapse activity appears to decrease with age and disease duration and cohort studies suggest that women experience relapses more frequently. Modifiable risk factors, including smoking, exposure to infectious disease and discontinuation of DMTs, are also associated with increased relapse frequency.
Relapse rates
There is some controversy over whether increased rates of relapse events represent an independent risk for disability progression in MS. Short-term studies suggest that relapses do not entirely regress; thus, EDSS scores, which are elevated during relapses, do not return to their previous baseline level. 76 Authors of these studies would conclude that a greater number of relapses would lead to earlier increases in EDSS scores. Longer cohort studies, however, have noted that the number of relapses is not associated with time to SPMS or EDSS 6. 71,77 A study examining placebo groups from two large Phase III trials also noted that half of the patients satisfying criteria for ‘confirmed progression’ (definitions ranging from a 1.0-point EDSS increase confirmed at 3 months to a 2.0-point EDSS increase confirmed at 6 months) were erroneously diagnosed, as their EDSS scores did not sustain progression, even through the end of the trial. 78 Thus, in short-term studies, EDSS scores measured months after relapse may still be reflecting changes in active, not progressive, disease. These longer timescales for recovery from relapse may need greater recognition.
Most recently, a longitudinal cohort study by Leray et al. 79 suggested that MS may be characterised by two distinct phases, with phase 1 lasting from diagnosis until irreversible EDSS 3 and phase 2 lasting from EDSS 3 until EDSS 6. Notably, disability progression in phase 1 did not influence disability progression in phase 2 and, similarly to previous studies, increased rates of relapse during the first 2 years of MS influenced only time in phase 1. Relapses after EDSS 3 were not associated with continued disability progression. Previously characterised risk factors of sex, age at onset and relapse history were not related to disability progression in phase 2. 79 These data are in line with previous studies suggesting that, although rates of relapse early in disease predict disease progression, relapses later in RRMS or during SPMS may not significantly predict or influence disability progression. 80,81
Prognoses for mortality
Patients with MS have an average lifespan that is 7–14 years shorter than that of matched control subjects. 82 A meta-analysis of standardised mortality rates (SMRs) found that, overall, patients had a SMR of 2.81 compared with control subjects, which suggests 181% more mortality per year than anticipated at any age. 83 This was especially increased for those at an EDSS score of > 7.5, who, in a separate study, were found to have a SMR of 4.0 compared with control subjects. 84 One review notes that, in most cohort studies of people with MS, MS is cited as the cause of death for between 50% and 75% of deaths. It also notes wide variation in the proportion of deaths ascribed to MS, resulting from variations in assessment, interpretation and coding practices. In particular, death from suicide is inconsistently reported as MS related, although there is a substantially increased risk of suicide among people with MS. 82
Epidemiology
Prevalence and incidence
An international survey including data from 92 countries estimated the median global prevalence of MS to be 33 per 100,000 or about 2.3 million people worldwide. 63 This prevalence has been increasing in the past few decades, primarily because of increased rates of survival and diagnosis, but a meta-regression analysis suggested that there is also likely a true increase in the incidence of MS. 51 This analysis also suggested that the increase is primarily in women, who already face double the burden of MS compared with men. 51,85–89
A recent systematic review reported estimates for MS prevalence in the UK ranging from 97.26 per 100,000 in England in 199890 to 230.60 per 100,000 in Northern Ireland in 2008. 85,91 Incidence estimates were less common and ranged from 4.4 to 12.2 per 100,000 person-years. 85 Analysis of the UK General Practice Research Database (GPRD) between 1990 and 20109 similarly showed an estimated prevalence of 258.5 per 100,000 women and 113.1 per 100,000 men, with an incidence of 11.52 per 100,000 women per year and 4.84 per 100,000 men per year. Incidence peaked in women at age 40 years and men at age 45 years. Although no systematic reviews of longitudinal incidence trends specifically look at the UK, analysis of the UK GPRD estimates that, although the overall prevalence of MS is increasing because of increased survival, incidence has decreased by 1.5% per year (although this may be because of a decrease in the number of false-positive diagnoses). 92 This analysis estimates that 126,669 people with MS were living in the UK in 2010, although this number may be inflated by about 20% because it includes inaccurate diagnoses. 92
Burden of disease
The effects of MS have major ramifications for patients and carers, as well as financial implications for patients and the state.
Disability
Multiple sclerosis has a wide range of effects, ranging from mobility problems to bladder/bowel dysfunction, sexual dysfunction, fatigue, visual disturbances, pain, depression and memory changes. 93 Interviews with 301 patients in Wales found that weakness, sensory changes and ataxia were the most commonly reported symptoms of MS,94 whereas a postal survey of 223 unrepresentative MS patients found that fatigue, bladder/bowel problems, balance problems and muscle weakness were the ‘worst’ symptoms. 93,95 In terms of functional impacts, mobility, the ability to use stairs and outdoor transport were cited as the activities most significantly impacted by disease, whereas activities such as dressing and feeding were more preserved. 96 Surveys of mobility in randomly sampled populations of patients with MS note that slightly less than half (41.4–53%) require walking aids or a wheelchair (EDSS 6+). 96–98
Quality of life
A survey based on the EuroQoL-5 Dimensions questionnaire (EQ-5D) suggested that 82.5% of 4516 patients had experienced difficulties in their daily activities and 76% had experienced pain and problems with mobility, with patients rating their mean health state as 5.97 out of 1099 (compared with a UK general population rating of 8.3100). Another study with 2708 participants living with MS established a mean utility of 0.49 (with perfect health equal to 1.00), with an inverse relationship between EDSS score and quality of life (QoL). 101 The study established that QoL was affected by the type of disease, recent relapse and length of time since diagnosis, with SPMS demonstrating the lowest QoL of the subtypes.
The lifetime prevalence of depression in patients with MS is approximately 50%, with an estimated annual prevalence of 20%. 102 Meta-analysis showed a SMR of 2.13 for suicide compared with the general population,83 although accuracy is difficult to assess because reporting of suicide as a cause of death continues to be heavily influenced by cultural biases. 82 Risk factors for suicide in patients with MS may include depression, social isolation, younger age, advanced disease subtype, low socioeconomic status and higher EDSS score. 103
Cost
A number of cost estimates for MS exist, most of them based on cost-of-illness analyses (which are contested),104 with significant variation in methodologies and costs accounted for. 93 Most recently, analyses estimated an average cost of between £30,460 and £39,500 per person-year. 105,106 Overall, indirect costs, including those from lost employment, are projected to be greater than the direct costs of care, and costs are greater for those in the later stages of disease. 93 The estimated cost of relapse ranges from £519107 to £2115,108 depending on the level of care required.
Cross-sectional surveys of disability in patients with MS demonstrate substantial changes in levels of employment. Surveys with an average age of respondents of 50 years have noted that most patients are not working,96,109 with most cases of early or partial retirement the result of MS. 98,109 In a study of 305 patients in England in the 1980s, 27% of patients reported a decreased standard of living because of employment changes and care costs and 36% of carers interviewed had also had their career impacted. 109 Lost employment is estimated to currently account for 34–40% of the total cost of MS. 105,106
Patient expectations and perceptions of disease
The literature describing qualitative experiences of patients is not as comprehensive as that surrounding pharmacological treatments and the pathology of MS. Collectively, however, what does exist unsurprisingly describes the experience of symptom onset and diagnosis as a negative one. 110–112 Patients inevitably experience distress and anxiety as they become aware of symptoms112 and this can continue or be amplified as they learn of their diagnosis; the diagnosis can, however, also be a source of relief because it provides an explanation for symptoms. 111 Receiving adequate information from health-care professionals at the time of diagnosis can have a positive effect on patients’ well-being and self-identification of relevant support services,111 whereas a lack of information or empathy can be linked to frustration, anxiety and fear. 112 The transition from RRMS to SPMS is also a challenging time for patients, as this requires adjusting to new ‘realities’ and preparing for forthcoming challenges in a declining trajectory. 113 A recent qualitative systematic review emphasises the importance of support from health-care providers and an accessible health-care system. 114 A comprehensive care plan including patient and carer support alongside therapeutics is described as key for successful management of MS. 115
Current service provision
At present there is no cure for MS, but treatment options exist based on the stage and subtype of disease. Currently approved drugs for MS act as immunomodulators or immunosuppressants, with the aim of reducing the pathological inflammatory reactions occurring in MS and thus the frequency and severity of relapses and the rate of disease progression. 116 Management of MS also includes non-pharmacological options such as lifestyle adjustments and rehabilitation, which are also included in the NICE guidelines for MS management. 117
Treatments to reduce the risk of relapses
Drugs aimed at reducing the risk of relapses are called DMTs. In addition to the DMTs introduced in Chapter 2, several newer drugs are licensed for use in the UK. Five newer drugs are recommended by NICE118–122 for the treatment of MS: alemtuzumab (Lemtrada®; Sanofi Genzyme, Cambridge, MA, USA), dimethyl fumarate (Tecfidera®; Biogen Idec Ltd, Cambridge, MA, USA), fingolimod, natalizumab and teriflunomide (Aubagio®; Sanofi Genzyme, Cambridge, MA, USA). A summary of these recommendations is provided in Table 1. DMTs are indicated in the treatment of classic RRMS, with the exception of natalizumab and fingolimod, which are recommended only in patients with highly active RRMS. Among DMTs, IFN-β-type drugs and GA are indicated for patients with CIS.
| Treatment | TA guidance | NICE recommendation |
|---|---|---|
| Alemtuzumab | TA312118 (May 2014) | Recommended as an option, within its marketing authorisation, for treating adults with active RRMSa |
| Dimethyl fumarateb | TA320119 (August 2014) | Recommended as an option for treating adults with active RRMS,a only if they do not have highly active or RES RRMSc |
| Fingolimodb | TA254120 (April 2012) | Recommended as an option for the treatment of highly active RRMS in adults, only if they have an unchanged or increased relapse rate or ongoing severe relapses compared with the previous year despite treatment with IFN-β |
| Natalizumab | TA127121 (August 2007) | Recommended as an option for the treatment only of RES RRMSc |
| Teriflunomideb | TA303122 (January 2014) | Recommended as an option for treating adults with active RRMSa only if they do not have highly active or RES RRMSb |
Immunosuppressive agents, such as azathioprine, cyclophosphamide, mitoxantrone and methotrexate, can also be used in the management of MS. These agents can provide potential benefit through downregulating pathogenic mediators of MS, but can also induce severe adverse effects on the immune system. Consequently, these drugs are indicated only in patients with aggressive forms of MS, including patients who experience very frequent and severe relapses. They are not currently included in any NICE guidelines, although they continue to be used for MS123 and a systematic review suggests their effectiveness in preventing relapse recurrence. 124
Treatment of acute relapses
Steroids are commonly used and recommended to treat acute relapses. Steroids are aimed at reducing the duration of relapses by shutting down the production of inflammatory cytokines and destroying activated lymphocytes that cause demyelination; these drugs are not, however, thought to induce long-term benefits with regard to the course of the disease. 125 NICE guidelines126 recommend the use of 0.5 g of oral methylprednisolone daily for 5 days in the first instance and to consider 1 g of intravenous methylprednisone daily for 3–5 days as an alternative if oral steroids are not tolerated or have failed or if hospital admission for severe relapse or monitoring is required. Patients should not be offered a supply of steroids to administer at home for prophylactic use for future relapses. Lastly, patient education should target the management of potential complications, such as mental health changes or irregularities in blood glucose levels.
Pharmacological treatment of symptoms
Current NICE guidelines offer advice to health-care professionals, patients and families on the management of MS symptoms. 117 Recommendations include amantadine use for fatigue (although it does not have marketing authorisation in this indication) and baclofen or gabapentin for spasticity, with combinations of baclofen and gabapentin possible if individual drugs cannot reach a dosage for adequate relief. 126 Other drugs such as tizanidine (Actavis UK Ltd, Devon, UK), dantrolene (Dantrium®; Norgine Ltd, Harefield, UK) or benzodiazepines should be considered as second- or third-line options. It should also be noted that fampridine (Fampyra®; Biogen Idec Ltd, Cambridge, MA, USA), recently approved in Europe to improve walking ability in people with MS, has not been recommended by NICE as a cost-effective treatment. A systematic review, however, concluded that the absolute and comparative efficacy and tolerability of anti-spasticity agents in MS was poorly documented and no recommendations could be made to guide prescription. 127
For treatment of psychological changes, rivastigmine, donepezil and memantine, which are classically used in Alzheimer’s disease, have been shown to improve cognitive impairment, but overall evidence for their efficacy in MS patients has proved inconclusive. 128 The treatment of depression includes consideration of both psychotherapy and antidepressant medication. Commonly used medications are selective serotonin reuptake inhibitors such as fluoxetine, paroxetine and sertraline. A recent systematic review showed that depression severity was improved in three pharmacological studies of depression treatment in MS. 129 NICE guidelines130 state that amitriptyline can be considered to treat emotional liability.
Managing disability
Non-pharmacological treatment options are directed towards a rehabilitative approach, with specialist assistance from a multidisciplinary team.
There is evidence that physical activity alone can improve fatigue and it has been linked to improvement in aerobic capacity, gait parameters and QoL. 131,132 Suggestions for an effective rehabilitation regime include progression of physical activity from basic to integrated functions,133 to utilise working muscles while avoiding muscle overload. Although randomised controlled trials (RCTs) have shown some evidence of improved mobility and QoL from exercise interventions, systematic reviews have not reached consensus on whether the studies – which are especially limited by small sample sizes and risk of bias from lack of blinding – provide enough evidence to make guided exercise prescriptions. 134–136 Urinary incontinence affects approximately 75% of patients and can substantially impact on QoL. 137 NICE guidelines on lower urinary tract dysfunction in neurological disease are available and should be used to inform treatment. 138
Care should also be taken in the management of the mental health of patients. Interventions should be aimed at regular monitoring of any depressive states and mental health services should be offered routinely to encourage participation. 139 Education for all health-care providers and patients in coping mechanisms may help improve QoL. 140
Chapter 2 Description of the technology under assessment
In accordance with the NICE scope,141 this multiple technology appraisal (MTA) focuses on IFN-β (including pegylated IFN-β-1a) and GA.
Beta-interferons
Interferons are proteins that bind to cell surface receptors, initiating a cascade of signalling pathways ending with the secretion of antiviral, antiproliferative and immunomodulatory gene products. 142 Natural IFN-β is a 166 amino acid glycoprotein that can be produced by most cells in response to viral infections or other biological inducers. 18 There are two types of recombinant IFN-β, known as IFN-β-1a and IFN-β-1b. IFN-β-1a is a glycosylated form that is structurally indistinguishable from natural IFN-β;18 IFN-β-1b is a non-glycosylated form that carries one amino acid substitution. 143 Several in vitro studies have concluded that the biological activity of some IFN-β-1a formulations is greater than that of IFN-β-1b,18,143,144 but the clinical implications of such differences are unknown. Furthermore, these studies have not compared all of the approved formulations of recombinant IFN-β.
The precise mechanism of action of IFN-β in MS is not fully understood, but some potential actions include inhibition of T-cell activation, modulation of inflammatory cytokines and reduction in aberrant T-cell migration into the CNS. 19
There are currently five licensed IFN-β drugs:
-
One formulation of IFN-β-1a (Avonex) is given at the recommended dosage of 30 µg [6 million international units (IU)] once a week, administered by IM injection.
-
The other formulation of IFN-β-1a (Rebif) is given at the recommended dosage of 22 µg (6 million IU) or 44 µg (12 million IU) three times per week, administered by SC injection.
-
IFN-β-1b (Betaferon and Extavia) is given at the recommended dosage of 250 µg every other day, administered by SC injection.
-
In pegIFN-β-1a (Plegridy), polyethylene glycol (PEG) is added to the N-terminus of IFN-β-1a, allowing for less frequent administration. Its recommended dosage is 125 µg every 2 weeks, administered by SC injection.
The current licensed indications for IFN-β are listed in Table 2. The main indication is for the treatment of patients with RRMS. Most IFN-β drugs (Avonex, Rebif and Betaferon/Extavia) also have indications in patients with a single demyelinating event with an active inflammatory process and at high risk of developing CDMS. IFN-β-1b is licensed for use in patients with SPMS. IFN-β-1a (Rebif) is licensed for SPMS with ongoing relapse activity. IFN-β drugs are not indicated for PPMS.
| Brand name | DMT | Recommended usage | Indications |
|---|---|---|---|
| Avonex | IFN-β-1a | Dose: 30 µg (6 million IU) | RRMS – in clinical trials, this was characterised by two or more acute exacerbations (relapses) in the previous 3 years without evidence of continuous progression between relapses; patients with a single demyelinating event with an active inflammatory process, if it is severe enough to warrant treatment with intravenous corticosteroids, if alternative diagnoses have been excluded and if they are determined to be at high risk of developing CDMS. Should be discontinued in patients who develop progressive MS |
| Administration: IM injection | |||
| Frequency: once a week | |||
| Rebif | IFN-β-1a | Dose: 22 µg (6 million IU) or 44 µg (12 million IU) | RRMS – in clinical trials, this was characterised by two or more relapses in the previous 2 years; patients with a single demyelinating event with an active inflammatory process, if alternative diagnoses have been excluded and if they are determined to be at high risk of developing CDMS. Efficacy has not been demonstrated in patients with SPMS without ongoing relapse activity |
| Administration: SC injection | |||
| Frequency: three times weekly | |||
| Betaferon/Extavia | IFN-β-1b | Dose: 250 µg (8 million IU) | Patients with a single demyelinating event with an active inflammatory process, if it is severe enough to warrant treatment with intravenous corticosteroids, if alternative diagnoses have been excluded and if they are determined to be at high risk of developing CDMS; patients with RRMS and two or more relapses within the last 2 years; patients with SPMS with active disease, evidenced by relapses |
| Administration: SC injection | |||
| Frequency: every other day | |||
| Plegridy | PegIFN-β-1a | Dose: 125 µg | Adult patients for the treatment of RRMS |
| Administration: SC injection | |||
| Frequency: every 2 weeks | |||
| Copaxone | GA | Dose: 20 mg or 40 mg | Treatment of relapsing forms of MS. It is not indicated in PPMS or SPMS. GA in the 20-mg formulation has been studied in both RRMS and CIS |
| Administration: SC injection | |||
| Frequency: daily (20 mg) or three times weekly (40 mg) |
The most commonly reported AEs of IFN-β are injection site reactions (mainly inflammation) and flu-like symptoms (including fever, chills and myalgias and headache), but these generally decline markedly after the first year of treatment. 20 Other AEs include hypersensitivity reactions, blood disorders (mainly leucopenia), menstrual disorders and mood and personality changes. AEs may be responsible for treatment discontinuation.
Because of its biological nature, recombinant IFN-β also carries a risk for patients of developing NABs,145 and this is thought to reduce the treatment efficacy. 146 The occurrence of NABs depends on patient-specific factors but also treatment-specific factors such as formulation, route of administration, dosage and frequency of administration. Given their different natures and routes of administration, the immunogenicity of IFN-β varies among the formulations of IFN-β. A systematic review of RCTs showed that the rate of patients developing NABs was 2.0–18.9% for Avonex, 16.5–35.4% for Rebif and 27.3–53.3% for Betaferon/Extavia. 147 Some guidelines recommend testing patients treated with IFN-β for the presence of NABs after 12 and 24 months of treatment. 145,148 In the UK, the monitoring of NABs is not performed in routine practice.
According to net prices listed in the BNF,21 the annual cost per patient of IFN-β in the UK is £8502 for Avonex, £7976/£10,572 for lower doses/higher doses of Rebif and £7264 for Betaferon/Extavia. The estimated cost in 2013–14 for IFN-β in England was £52,000,000, with 27.6% growth from 2012–13. 149
As of July 2016, no biosimilar version of IFN-β is available in the UK.
Glatiramer acetate
Glatiramer acetate is a synthetic molecule containing four naturally occurring amino acids: l-glutamic acid, l-alanine, l-tyrosine and l-lysine. It was initially created to mimic myelin basic protein, a suspected autoimmune antigen, and induce a mouse form of MS. Surprisingly, it prevented MS induction in mice, triggering clinical studies of GA as a treatment for MS. 142 It is now thought that GA induces a broad immunomodulatory effect, with actions including competition for the binding of antigen-presenting cells, antagonism at specific T-cell receptors and promotion of anti-inflammatory responses in dendritic cells, monocytes and B cells. 150
Two formulations of GA are currently used: 20 mg/ml and 40 mg/ml (Copaxone), equivalent to 18 mg or 36 mg of glatiramer base respectively. The dose regimen is 20 mg daily (formulation of 20mg/ml) or 40 mg three times a week (formulation of 40mg/ml) by SC injection (see Table 2). As of February 2016, no generic version of Copaxone is available in the UK.
Glatiramer acetate is indicated for the treatment of patients with RRMS. It is not indicated for PPMS or SPMS. The most common AEs of GA are flushing, chest tightness, sweating, palpitations and anxiety,23 with injection site reactions observed in up to half of patients.
The current annual cost per patient of GA in the UK is £6681–6704. 149 Generic prices are not yet available.
Care pathways for beta-interferon and glatiramer acetate
Beta-interferon and GA are considered first-line treatments for RRMS, except for patients with highly active RRMS, in which more advanced treatments (e.g. natalizumab) are considered most appropriate. Although some patients prefer dimethyl fumarate or teriflunomide because of their oral mode of administration, IFN-β and GA both have well-established long-term safety profiles that avoid some of the more severe side effects presented by other drugs, for example the rare but serious complications of progressive multifocal leukoencephalopathy associated with the reactivation of the John Cunningham virus with dimethyl fumarate treatment. Additionally, some patients may choose not to take IFN-β or GA, especially after CIS or if the course of MS appears to be benign. Patients receive specialist advice, including from neurologists and nurses specialising in MS care, when choosing which DMT to initiate. It is common for MS patients to see a neurologist about once a year for maintenance, and MRI scans are administered generally not more than once a year. Exacerbations may be managed by local GPs or by specialist neurology services depending on their severity and complexity.
Switching between first-line treatments mainly occurs because of side effects. Patients may escalate to a second-line treatment if MS is highly active, that is, characterised by multiple disabling relapses in a year or an unchanged relapse rate during first-line treatment.
On transition to SPMS – a diagnosis that is made retrospectively – patients are supposed to cease use of drugs that are not licensed for SPMS. However, there is anecdotal evidence that patients may continue on these drugs because of perceived benefits for relapse rate and the absence of any other treatment for SPMS.
The UK multiple sclerosis risk-sharing scheme
The last TA for IFN-β and GA for the treatment of MS (TA3224) did not find sufficient evidence of clinical effectiveness and cost-effectiveness to recommend treatment. The Department of Health151 set up a RSS to provide the then-licensed formulations of IFN-β-1a (Avonex and Rebif), interferon-β-1b (Rebif) and GA (Copaxone) to patients. Under this arrangement, the benefit of each drug would be regularly assessed using target outcomes agreed on with the manufacturers. The price for each drug would be scaled as necessary to reach a target level of cost-effectiveness, set at the start of the scheme as £36,000 per quality-adjusted life-year (QALY). As part of the RSS, patients meeting the criteria for treatment were enrolled in a cohort and monitored regularly for evidence of disability progression and treatment benefit.
Because all patients in the RSS received treatment, a comparator cohort including patients with measurement of disease progression without access to DMTs was needed. Several natural history cohorts meeting these criteria exist. The 6-year interim analyses used the British Columbia cohort, which was initiated in 1980, before DMTs were made routinely available in Canada. This cohort has prospectively recorded EDSS scores and covers about 80% of the relevant MS population in that area, providing a rich source of data about the natural history of MS. 152,153 Patients from the British Columbia cohort who would have met the criteria for prescribing IFN-β or GA were selected for comparison with those in the UK RSS. 153–155 Analysis of the 6-year data of the UK clinical cohort, comparing disease progression against the historical comparator cohort, suggested that, on the whole, the DMTs included in the RSS reduced disability progression and did so to the agreed level of cost-effectiveness. 154
Chapter 3 Definition of the decision problem
Decision problem and aim
The aim of this study was to appraise the clinical effectiveness and cost-effectiveness of IFN-β and GA within their marketing authorisations for treating MS, as an update to TA32. 24
In this assessment, IFN-β and GA were appraised using published data and taking account of additional data on long-term outcomes from the RSS.
As requested by NICE, IFN-β and GA were compared with best supportive care (BSC). NICE141 commented that:
Since Technology Appraisal 32 was published another interferon 1b (Extavia, Novartis), a pegylated interferon beta 1a (Plegridy, Biogen Idec [Ltd]) and a new formulation of glatiramer acetate (Copaxone, Teva pharmaceuticals) have been granted marketing authorisations. These technologies were not included in the risk sharing scheme because they were not appraised in Technology Appraisal 32. It has been determined by NICE that it is relevant to include these technologies in this appraisal so that guidance can be issued for all beta-interferons and formulations of glatiramer acetate currently licensed for MS in the UK. Further active treatments that have been licensed and recommended by NICE (including teriflunomide, fingolimod, natalizumab, alemtuzumab and dimethyl fumarate) will not be considered in this appraisal.
National Institute for Health and Care Excellence (2016). Multiple Technology Appraisal. Beta Interferon and Glatiramer Acetate for Treating Multiple Sclerosis (Review of TA32). Final Scope Updated Post Invitation. NICE has not checked the use of its content in publication to confirm that it accurately reflects the NICE publication from which it is taken
In addition, people with CIS were considered in this appraisal.
Objectives
Our first objective was to systematically review the evidence for the clinical effectiveness of IFN-β-1a, pegylated IFN-β-1a, IFN-β-1b and GA in people with relapsing MS (including people with RRMS and people with SPMS with active disease, evidenced by relapses) and CIS, that is, a single demyelinating event, who are considered at high risk of developing subsequent MS, compared with BSC without DMTs and with each other. The following outcomes were investigated:
-
relapse rate
-
transition to CDMS (in the case of CIS)
-
severity of relapse
-
disability (e.g. EDSS)
-
symptoms of MS such as fatigue, cognition and visual disturbance
-
freedom from disease activity
-
discontinuation as a result of NABs
-
mortality
-
adverse effects of treatment
-
health-related quality of life (HRQoL).
The second objective was to systematically review existing economic evaluations, including use of the existing RSS model; develop a de novo economic model for CIS; assess the cost-effectiveness of the treatments (IFN-β-1a, pegylated IFN-β-1a, IFN-β-1b and GA) for CIS and RRMS against the stated comparators, expressed in incremental costs per QALY, using a time horizon that was sufficiently long to reflect any differences in costs or outcomes between the technologies being compared and taking a NHS and Personal Social Services (PSS) perspective; and update model parameters and inputs to reflect available evidence from the literature, current costs, the NICE reference case,156 current practice and new data from the RSS.
Note
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 4 Methods for the assessment of clinical effectiveness
Protocol registration
We presented our protocol to a stakeholder information meeting on 29 February 2016 and subsequently registered it on PROSPERO as CRD42016043278.
Identification of studies
Initial scoping searches were undertaken in MEDLINE and The Cochrane Library in October 2015 to assess the volume and type of literature relating to the assessment question and to inform further development of the search strategy. Several relevant systematic reviews from the Cochrane Database of Systematic Reviews were identified. 157–161
The following search strategy was designed to capture RCTs of DMTs for patients with RRMS, SPMS or CIS. An iterative procedure was used to develop the planned searches, with reference to previous systematic reviews. 157–162 Clinical searches were restricted to RCT evidence. The included and excluded study lists from previous relevant Cochrane systematic reviews were checked. 159,160 The main database searches for MS were undertaken in January and February 2016 and were limited by date to the beginning of 2012 onwards [the year the searches were undertaken for the broad review and network meta-analysis (NMA) by Filippini et al. 160]. This review was chosen because of the breadth of its scope, search strategy and eligibility criteria. Other more recent reviews were considered to be more limited in terms of the types of MS covered and the types of studies included. 157,159 An additional targeted search for RCTs in CIS, not limited by date, was performed. A full record of the searches undertaken is provided in Appendix 1. The searches were developed for MEDLINE and adapted as appropriate for the other databases.
The search strategy included the following:
-
searching of electronic bibliographic databases, including trials in progress
-
scrutiny of references of included studies and relevant systematic reviews
-
contact with experts in the field
-
screening of websites for relevant publications.
We ran electronic searches on the following databases:
-
Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group Specialised Trials Register
-
MEDLINE (via Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
-
EMBASE (via Ovid)
-
The Cochrane Library (via Wiley Online Library), including the Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (NHS EED) and Health Technology Assessment (HTA) database
-
Science Citation Index and Conference Proceedings Citation Index – Science (Web of Science)
-
UK Clinical Research Network (UKCRN) Portfolio Database.
We also searched the trial registers at ClinicalTrials.gov and the World Health Organization (WHO)’s International Clinical Trials Registry Platform (ICTRP).
All bibliographic records identified through the electronic searches were collected in a managed reference database. The reference lists of included studies and relevant review articles were checked and the manufacturer websites were screened for relevant publications. The included studies and reference lists of manufacturer submissions were checked for relevant unpublished studies and any additional published studies. Other grey literature searches were undertaken using the online resources of the organisations shown in Table 3. More details of these website searches are provided in Appendix 1.
Inclusion criteria
We included studies that met the following criteria.
-
The study design was a RCT, a systematic review or a meta-analysis.
-
The population was people diagnosed with RRMS, SPMS or CIS.
-
The intervention was one of the following drugs, when used within its indication (see Table 2):
-
IFN-β-1a
-
pegylated IFN-β-1a
-
IFN-β–1b
-
GA.
-
We included drugs only when used within their marketing authorisation, that is, when the posology in the trial matched that in the indication, because of the extensive clinical use of these drugs and the corresponding safety and effectiveness profile of these established dosages. A wide variety of alternative dosages has been used across a variety of trials. It was judged that including dosages not matching the indication could present misleading estimates of effectiveness or safety and would introduce unnecessary heterogeneity.
-
The comparator was BSC without the use of DMTs or another of the interventions when used within its indication. In this review, BSC corresponded to arms of RCTs in which patients received either placebo added to standard care or no treatment.
-
The reported outcomes included at least one of the following:
-
relapse rate
-
progression to MS (for patients with CIS)
-
severity of relapse, defined as rate of steroid-treated relapses or rate of relapses graded as moderate or severe
-
disability, including as measured by the EDSS
-
MS symptoms, such as fatigue, cognition and visual disturbance
-
freedom from disease activity, defined as composite clinical and MRI outcomes
-
mortality
-
HRQoL
-
treatment-related AEs
-
discontinuation because of AEs
-
discontinuation because of loss of effectiveness attributed to NAB formation (we did not consider the rate of NAB formation alone because of its limited clinical relevance in practice).
-
-
The study was a full-text report in the English language.
Exclusion criteria
We excluded:
-
studies that compared an eligible intervention against an irrelevant comparator
-
studies that examined an eligible intervention used with a non-recommended dose regimen
-
studies reporting MRI outcomes alone
-
studies reporting early compared with late treatment only
-
studies that examined only MS subtypes other than those in the eligible population
-
studies that examined only patients with highly active or rapidly evolving MS, as BSC is not an appropriate comparator for these populations
-
studies reported as abstracts or conference proceedings or not reported in the English language.
Study selection process
We first examined relevant past systematic reviews (including those by Tramacere et al. ,159 Filippini et al. 160 and Clerico et al. 158) for studies meeting the inclusion criteria. We verified the inclusion of these studies by examining their full text.
For updated and new searches (including for studies addressing CIS), we collected all retrieved records in a specialised database and duplicate records were identified and removed. The reviewers piloted a screening form based on the predefined study inclusion and exclusion criteria. Subsequently, two reviewers (Xavier Armoiry and GJ Melendez-Torres) applied the inclusion/exclusion criteria and screened all identified bibliographic records at the title/abstract level (level I) and then at the full-text level (level II). Any disagreements over eligibility were resolved through consensus or by a third-party reviewer (Aileen Clarke). Reasons for the exclusion of full-text papers were documented. The study flow was documented using a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram. 163
Quality assessment strategy
Systematic reviews used to locate primary studies were appraised using the Assessing the Methodological Qualities of Systematic Reviews (AMSTAR) checklist. 164 All primary studies were appraised using the Cochrane risk of bias assessment tool. 165 Appraisal was undertaken by two reviewer (Jacoby Patterson and Jeremy Rodrigues). Uncertainty and/or any disagreements were cross-checked with a third reviewer and were resolved by discussion.
Data extraction strategy
For all included studies, the relevant data were extracted independently by two reviewers (Xavier Armoiry and GJ Melendez-Torres) using a data extraction form informed by the NHS Centre for Reviews and Dissemination. 166 Uncertainty and/or any disagreements were cross-checked with another reviewer and were resolved by discussion. The extracted data were entered into summary evidence tables (see Appendix 2 for a sample data extraction sheet). When multiple arms were presented, of which only some were relevant to our analysis, we extracted data only for those arms. The extracted information included:
-
study characteristics [i.e. first author name, country, design, study setting, sample size in each arm, funding source, duration of follow-up(s) and methodological features corresponding to the Cochrane risk of bias assessment tool165]
-
patient baseline characteristics [i.e. trial inclusion/exclusion criteria; number of participants enrolled and number of participants analysed; age, race and sex; disability (including as measured by the EDSS) at baseline; time from diagnosis of MS to study entry; and relapse rate at baseline]
-
treatment characteristics (i.e. type of drug; method of administration, dose and frequency; drug indication as stated; and definition of BSC as described by triallists)
-
outcome characteristics for each included outcome reported [i.e. definition of outcome measure; timing of measurement; scale of measurement; and effect size as presented, including mean difference, risk ratio, OR or hazard ratio (HR) or arm-level data necessary to calculate an effect size]. Measures of variability and statistical tests used were also extracted (standard deviation, 95% CI, standard error, p-value).
Data preparation
Many of the included studies did not present adequate data for key findings to enable inclusion prima facie in a meta-analysis model. We used a variety of published methods to derive the necessary data.
Across all studies, we used data for the point of greatest maturity (i.e. last available follow-up) for which effect sizes were estimable. In studies presenting estimates with confirmed relapses and with non-confirmed relapses, we selected estimates with confirmed relapses.
We used rate ratios (RRs) to examine relapse outcomes [e.g. the ratio of annualised relapse rates (ARRs) in two study arms]. We used summary statistics instead of attempting to approximate individual participant data for each arm, in part because of the use of stratification in estimating study findings. When necessary, we imputed standard errors by estimating the number of events in each arm (e.g. when relapse rates were analysed using an analysis of variance, or ANOVA, model with a Gaussian link, instead of the preferred Poisson distribution for count variables). When arm-level ARRs were presented without Poisson-based standard errors, we generally assumed that the ARR presented for study arms was a fair approximation and then re-estimated the standard errors for the RR using all available information on person-years of follow-up and number of relapses. RRs were then analysed using a log-normal distribution.
We used HRs to examine time-to-event outcomes (e.g. time to first relapse or time to confirmed disability progression). When HRs were not estimated from a Cox proportional hazards model, we used several methods in order of priority. First, we used methods published by Tierney et al. 167 to estimate the HR, in particular using the number of patients analysed, the number of total events and the p-value derived from a log-rank test. When these data were not available to us, we used the final predicted probabilities of survival in each study arm (generally estimated using Kaplan–Meier curves) and estimated the cumulative hazard using the equation –ln[S(t)], where S(t) is the probability of survival at time t. We then took the ratio of the cumulative hazards and used the log-rank p-value to approximate the standard errors for the HR, under the property that the p-value from the log-rank test for survival asymptotically approaches the p-value from a likelihood ratio test derived from a Cox proportional hazards model.
We used dichotomous outcomes to examine discontinuation as a result of AEs.
Narrative synthesis and meta-analysis
Narrative synthesis of studies and meta-analyses were organised hierarchically, first by MS subtype, then by intervention–comparator contrast and finally by each outcome for which data were available. Within each MS subtype, we examined included studies for similarity. When studies were sufficiently similar, we estimated both pairwise meta-analyses and NMAs. First, we pooled outcomes for each intervention–comparator contrast and by MS subtype using random-effects meta-analysis in Stata® 14 (StataCorp LP, College Station, TX, USA) and examined these pairwise meta-analyses for heterogeneity, measured as Cochran’s Q and I2.
Subsequently, we used the package -network-168 in Stata 14 to estimate NMAs. Because the package -network- operates in a frequentist paradigm, there was no need to carry out sensitivity analyses on prior distributions. When possible, we estimated meta-analyses using random effects; however, some sparse networks, in which there were few studies for each contrast between two treatments, required the use of a fixed-effects model. We used a common heterogeneity model, in which the between-studies variance is assumed to be equal across comparisons.
After estimating a consistency model (i.e. in which direct evidence for a contrast between two treatments is assumed to agree with indirect evidence for that contrast), we checked networks that were not star shaped in design for inconsistency using two methods. We estimated a design*treatment interaction model and examined both the design effects and the overall Wald test for evidence of inconsistency. We also used the side-splitting method to test for differences in the effectiveness estimates between direct and indirect evidence. When evidence of inconsistency existed, we considered the direction of that inconsistency.
Finally, we used a bootstrapping method to resample from our estimates of intervention effectiveness and develop probabilities of each treatment’s position relative to the other treatments. We then used the surface under the cumulative ranking curve (SUCRA) to produce a unified ranking of treatments.
Meta-analyses for clinically isolated syndrome
We estimated a NMA for time to CDMS in patients with CIS. This was the outcome most consistently reported across studies and matched most closely with the decision problem in the NICE scope. 141
Meta-analyses for relapsing–remitting multiple sclerosis and secondary progressive multiple sclerosis
Relapse outcomes and relapse severity
We elected to meta-analyse the RR of relapses as an overall measure of relapses in RRMS and SPMS. Although we narratively synthesised analyses for time to relapse and proportion free of relapses, both measures had significant issues. In particular, time to relapse data were inconsistently presented and at times impossible to impute whereas the proportion relapse free would have been especially dependent on the duration of follow-up and would not have captured the impact of drugs on multiple relapses per person.
We elected to meta-analyse two measures for relapse severity in RRMS: steroid-treated relapses and relapses described as moderate or severe. These were the most commonly reported measures.
Disability progression
We elected to meta-analyse time to disability progression as a measure of disability progression in RRMS and SPMS. We separated estimates for disability progression confirmed at 3 months and disability progression confirmed at 6 months, as we could not establish whether measures were commensurate. Although we narratively synthesised the proportions of patients with disability progression and the magnitude of EDSS change, we elected not to meta-analyse these data as they would have been especially dependent on the duration of follow-up. In particular, data for magnitude of EDSS change would have required extensive imputation.
Discontinuation as a result of adverse events
We estimated models for discontinuation as a result of AEs. To estimate these models, we examined three outcomes as reported: discontinuation of the study drug as a result of AEs, discontinuation of the study as a result of AEs and withdrawal from the study as a result of AEs. In the few studies that reported both discontinuation of the study drug as a result of AEs and discontinuation of the study as a result of AEs, we chose discontinuation of the study drug as a result of AEs as we believed that it would be better at capturing the relationship between study drugs and discontinuation. We also estimated one model with estimates of discontinuation closest to 24 months of follow-up, as available from included studies, as risk of discontinuation as a result of AEs is not an annualised measure, such as the ARR, or an ‘instantaneous’ measure, such as the HR, and we could not reliably estimate person-years of follow-up in each arm across all studies to convert study-level estimates to RRs.
Publication bias
If we had included > 10 studies for an intervention–comparator contrast, we would have used funnel plots to examine studies for the presence of publication bias in pairwise comparisons.
Industry submissions regarding the effectiveness of treatments
We examined manufacturer submissions and present summaries and an appraisal of their clinical effectiveness analyses in Chapter 6.
Chapter 5 Results of the assessment of clinical effectiveness
Search results
Included studies
The search identified 6420 potentially relevant records. We removed 6146 records that did not meet our inclusion criteria at title/abstract stage, leaving 274 records to be examined at the full-text stage. Of these studies we excluded 211, leaving 63 publications that met our inclusion criteria,170–232 corresponding to 35 primary studies. Of these primary studies, 32 were included in at least one meta-analysis. A flow diagram describing the process of identifying the relevant literature is provided in Figure 1.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart: clinical effectiveness reviews.

Excluded studies
The reasons for the exclusion of studies are presented both by type of reason for exclusion and for each record individually in Appendix 3.
Systematic reviews used to locate primary studies
Three Cochrane systematic reviews were identified as being of particular relevance to this study and contributed to the identification of original studies for inclusion. 158–160
Scope and aims
Overview
The study by Filippini et al. 160 aimed to review the clinical effectiveness of immunosuppressors and immunomodulators in all MS types and to rank them based on relapse rate, disability progression and acceptability. The study by Tramacere et al. 159 aimed to review and rank these agents in RRMS specifically. Clerico et al. 158 examined the role of IFN-β-1a, IFN-β-1b and GA in delaying the conversion of CIS into MS, although this analysis was undertaken before revised diagnostic criteria classified many CIS episodes as in fact being RRMS. 62
Diagnostic criteria used to identify studies
Tramacere et al. 159 used all four sets of diagnostic criteria54,62,64,66 to identify RCTs of treatment for RRMS in participants aged > 18 years.
Filipinni et al. 160 included RCTs only, investigating the treatment of adults aged > 18 years with MS diagnosed according to Poser et al. ,64 the original McDonald criteria54 or the 2005 modified McDonald criteria. 66 This review therefore included all types of MS. However, it did not incorporate the most recent revision of the McDonald criteria62 and so excluded CIS studies.
In contrast, Clerico et al. 158 used the Poser criteria64 to identify RCTs and pseudo-randomised double-blind trials of CIS, with reference to specific MRI findings. 169 No exclusion criterion based on participant age was specified.
Included interventions
Tramacere et al. 159 included all immunomodulators and immunosuppressors, even if unlicensed. This included the IFN-β and GA drugs specified in the NICE scope,141 as well as 11 other interventions. We noted that the review by Tramacere et al. 159 excluded the study by Calabrese et al. ,188 stating that it was non-randomised. To the best of our knowledge this study is a RCT and it has been included in our review.
The interventions studied by Filippini et al. 160 included IFN-β and GA formulations that were licensed at the time (i.e. not pegylated IFN), as well as seven other interventions. Clerico et al. 158 would have included licensed IFN-β and GA interventions (i.e. not pegylated IFN), but identified only three studies comparing IFN with placebo.
All three reviews included studies evaluating DMTs with a dose regimen currently not recommended or authorised [e.g. IFN-β-1a (Rebif) given once weekly instead of three times weekly]. The reviews did not account separately for the inclusion of studies with a DMT given under a non-recommended dose regimen in a sensitivity analysis.
Outcomes
Tramacere et al. 159 and Filippini et al. 160 examined risk of relapse over 12 months and 24 months as a dichotomous outcome, as well as the presence or absence of disability progression assessed using the EDSS. In the study by Filippini et al. ,160 which included progressive forms of MS as well as RRMS, risk of disability progression was reported as the first outcome.
Both reviews assessed AEs. Filippini et al. 160 also included the incidence of relapse over 36 months and assessments of the acceptability of treatment as measured by discontinuation as a result of AEs.
Clerico et al. 158 used the proportion converting to CDMS as the primary outcome, alongside the ARR and additional MRI outcomes.
Statistical methods
In the study by Tramacere et al. ,159 NMAs were performed for the primary outcomes. Random-effects models were used within a frequentist setting. In contrast, Filippini et al. 160 performed NMAs within a Bayesian framework. For both reviews, equal heterogeneity across comparisons was assumed and any correlations induced by multiarm studies were accounted for. Both used SUCRA to describe the ranking of treatments. 233
Review findings
Tramacere et al. 159 found that, in RRMS, the SUCRA for the chance of experiencing relapse over 12 months was 52% for GA, 36% for SC IFN-β-1a (Rebif), 33% for pegylated IFN-β-1a, 27% for IFN-β-1b and 25% for IM IFN-β-1a (Avonex). The RR for GA compared with placebo for this outcome was 0.80 (95% CI 0.68 to 0.93), whereas all other interventions of interest did not return significant results. The ranking of interventions of interest for the prevention of relapse over 24 months in RRMS was GA (most successful) followed by IFN-β-1b, SC IFN-β-1a (Rebif) and IM IFN-β-1a (Avonex).
The SUCRA plots for reducing the worsening of disability over 24 months in RRMS returned results of 58% for GA, 51% for IFN-β-1b, 36% for SC IFN-β-1a (Rebif) and 21% for IM IFN-β-1a (Avonex). The only interventions of interest with significant RRs compared with placebo were GA (0.77, 95% CI 0.64 to 0.92) and IFN-β-1b (0.79, 95% CI 0.65 to 0.97).
Thus, in the Tramacere et al. 159 review, GA performed the best of the interventions of interest. IM IFN-β-1a (Avonex) was consistently the least effective intervention. Other interventions included in the Cochrane review (but which were outside the scope of the current MTA) performed better, such as alemtuzumab (SUCRA 97%, RR vs. placebo 0.40, 95% CI 0.31 to 0.51).
Filippini et al. 160 returned similar rankings derived from SUCRA values for reducing the recurrence of relapses over 12 months. However, for reducing the recurrence of relapses at 24 months, the SUCRA values resulted in a different ranking: SC IFN-β-1a (Rebif), GA, IFN-β-1b and IM IFN-β-1a (Avonex). In terms of reducing disability progression over 24 months, GA was ranked best (SUCRA 67%) followed by IFN-β-1b (54%), SC IFN-β-1a (Rebif) (47%) and IM IFN-β-1a (Avonex) (18%).
In the study by Clerico et al. ,158 only direct treatment comparisons were performed, using conventional pairwise meta-analyses to compare IFN with placebo. No studies of GA were identified, but IFN was effective compared with placebo.
Review quality
All three Cochrane reviews scored 10 out of 11 on the AMSTAR checklist and were assessed as being of high methodological quality. Tramacere et al. 159 and Filippini et al. 160 inadequately reported grey literature searching and Clerico et al. 158 did not assess the risk of publication bias.
Study characteristics and methodological quality
Study and participant characteristics
We included 35 primary studies, represented by 63 articles170–232 published between 1987 and 2015, which involved 14,623 participants randomly assigned to IFN-β, GA or placebo added to standard care or BSC alone. The median follow-up was 24 months. Only four studies were conducted at single centres. 170,181,185,188 The median number of participating centres was 30.5 (range 1–200). The majority of the studies (57.1%) were international studies. Twenty-two (63%) were placebo-controlled studies,170–176,178–180,189,198–205,207–211,213,225,227,228,230–232 13 (34%) were head-to-head studies, with a comparison between one IFN-β and GA or between two IFNs,184–197,206,208,212–215,224,226,229,231 and two (6%) compared an IFN with no treatment (standard care). 76,78 Of the 22 placebo-controlled studies, three aimed to evaluate the effectiveness of DMTs that were excluded in the scope [laquinimod (Nerventra®; Teva Pharmaceutical Industries, Petah Tikva, Israel), daclizumab (Zinbryta®; Biogen Idec Ltd, Cambridge, MA, USA) and dimethyl fumarate] compared with placebo, with IFN-β or GA being added as a third descriptive arm. 198,199,216,230 Given the different posology and method of administration between these agents used in the three studies (two were oral drugs, one was an intravenous drug), the comparison of IFN-β or GA with placebo was not blinded.
The key characteristics of the included studies are provided in Table 4. A full list of publications is provided in Appendix 4.
| Study ID; MS type (diagnostic criteria); included in TA3224? | Study details | Characteristics of participants at baseline | Intervention | Participants |
|---|---|---|---|---|
| ADVANCE 2014;213 RRMS (2005 McDonald criteria66) |
|
|
Arm 1: pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy); arm 2: placebo | Randomised: arm 1, n = 512; arm 2, n = 500 |
| AVANTAGE 2014;182 RRMS/CIS (diagnostic criteria unclear) |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon) via Betaject; arm 2: IFN-β-1b 250 µg SC every other day (Betaferon) via Betaject light; arm 3: IFN-β-1a 44 µg SC three times weekly (Rebif) via Rebiject II | Included: arm 1, n = 73; arm 2, n = 79; arm 3, n = 68 |
| BECOME 2009;184 RRMS/CIS (likely 200154 or 200566 McDonald criteria) |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: GA 20 mg SC once daily (Copaxone) | Randomised: arm 1, n = 36; arm 2, n = 39 |
| BENEFIT 2006;171 CIS (Poser criteria,64 2001 McDonald criteria54) |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: injections of placebo | Randomised: arm 1, n = 305; arm 2, n = 182 |
| BEYOND 2009;190 RRMS (2005 McDonald criteria66) |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: GA 20 mg SC once daily (Copaxone) | Randomised: arm 1, n = 897; arm 2, n = 448 |
| Bornstein 1987;170 RRMS (Poser criteria64); included in TA32 |
|
|
Arm 1: GA 20 mg SC once daily (Copaxone); arm 2: placebo | Randomised: arm 1, n = 25; arm 2, n = 25 |
| BRAVO 2014;198 RRMS (2005 McDonald criteria66) |
|
|
Arm 1: IFN-β-1a 30 µg IM once weekly (Avonex); arm 2: oral placebo once daily with neurologist monitoring | Randomised: arm 1, n = 447; arm 2, n = 450 |
| Calabrese 2012;188 RRMS (2005 McDonald criteria66) |
|
|
Arm 1: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 2: IFN-β-1a 30 µg IM once weekly (Avonex); arm 3: GA 20 mg SC once daily (Copaxone) | Randomised: arm 1, n = 55; arm 2, n = 55; arm 3, n = 55 |
| CHAMPS 2000;172 CIS (Poser criteria64) |
|
|
Arm 1: IFN-β-1a 30 µg IM once weekly (Avonex); arm 2: placebo | Randomised: arm 1, n = 193; arm 2, n = 190 |
| CombiRx 2013;191 RRMS (2001 McDonald criteria,54 Poser criteria64) |
|
|
Arm 1: IFN-β-1a 30 µg IM once weekly (Avonex); arm 2: GA 20 mg SC once daily (Copaxone) | Randomised: arm 1, n = 250; arm 2, n = 259 |
| CONFIRM 2012;216 RRMS (2005 McDonald criteria66) |
|
|
Arm 1: GA 20 mg SC once daily (Copaxone); arm 2: two placebo capsules orally three times daily | Randomised: arm 1, n = 360; arm 2, n = 363 |
| Cop1 MSSG 1995;217 RRMS (Poser criteria64); included in TA32 |
|
|
Arm 1: GA 20 mg SC once daily (Copaxone); arm 2: placebo | Randomised: arm 1, n = 125; arm 2, n = 126 |
| ECGASG 2001;219 RRMS (Poser criteria64); included in TA32 (unpublished at the time) |
|
|
Arm 1: GA 20 mg SC once daily (Copaxone); arm 2: placebo SC injections | Randomised: arm 1, n = 119; arm 2, n = 120 |
| ESG 1998;222 SPMS (Poser criteria,64 Lublin and Reingold criteria15); included in TA32 |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: placebo SC injections | Randomised: arm 1, n = 360; arm 2, n = 358 |
| Etemadifar 2006;185 RRMS (Poser criteria64) |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: IFN-β-1a 30 µg IM once weekly (Avonex); arm 3: IFN-β-1a 44 µg SC three times weekly (Rebif) | Randomised: arm 1, n = 30; arm 2, n = 30; arm 3, n = 30 |
| EVIDENCE 2007;195 RRMS (Poser criteria64) |
|
|
Arm 1: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 2: IFN-β-1a 30 µg IM once weekly (Avonex) | Randomised: arm 1, n = 339; arm 2, n = 338 |
| GALA 2013;221 RRMS (2005 McDonald criteria66) |
|
|
Arm 1: GA 40 mg SC three times weekly (Copaxone); arm 2: SC placebo injections | Randomised: arm 1, n = 943; arm 2, n = 461 |
| GATE 2015;220 RRMS (2010 McDonald criteria62) |
|
|
Arm 1: GA 20 mg SC once daily (Copaxone); arm 2: placebo | Randomised: arm 1, n = 357; arm 2, n = 84 |
| IFNB MSSG 1995;209 RRMS (Poser criteria64); included in TA32 |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: SC placebo injections | Randomised: arm 1, n = 124; arm 2, n = 123 |
| IMPROVE 2012;207 RRMS (2005 McDonald criteria66) |
|
|
Arm 1: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 2: SC placebo injections | Randomised: arm 1, n = 120; arm 2, n = 60 |
| INCOMIN 2002;196 RRMS (Poser criteria64) |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: IFN-β-1a 30 µg IM once weekly (Avonex) | Randomised: arm 1, n = 92; arm 2, n = 96 |
| Kappos 2011;199 RRMS (2001 McDonald criteria54) |
|
|
Arm 1: IFN-β-1a 30 µg IM once weekly (Avonex); arm 2: placebo injection every other week | Randomised: arm 1, n = 55; arm 2, n = 54 |
| Knobler 1993;211 RRMS (Poser criteria64) |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: SC placebo injection | Randomised: arm 1, n = 6; arm 2, n = 7 |
| Mokhber 2014; RRMS (2005 McDonald criteria66) |
|
|
Arm 1: IFN-β-1a 30 µg IM once weekly (Avonex); arm 2: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 3: IFN-β-1b 250 µg SC every other day (Betaferon) | Randomised: arm 1, n = 23; arm 2, n = 23; arm 3, n = 23 |
| MSCRG 1996;200 RRMS (Poser criteria64); included in TA32 |
|
|
Arm 1: IFN-β-1a 30 µg IM once weekly (Avonex); arm 2: placebo | Randomised: arm 1, n = 158; arm 2, n = 143 |
| NASG 2004;223 SPMS (Poser criteria,64 Lublin and Reingold criteria15) |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: injectable placebo (note two types, one calibrated to body surface area) | Randomised: arm 1, n = 317; arm 2, n = 308 |
| Pakdaman 2007;173 CIS (Poser criteria64) |
|
|
Arm 1: IFN-β-1a 30 µg IM once weekly (Avonex); arm 2: injectable placebo | Randomised: arm 1, n = 104; arm 2, n = 98 |
| PreCISe 2009;174 CIS (2005 McDonald criteria,66 Poser criteria64) |
|
|
Arm 1: GA 20 mg SC once daily (Copaxone); arm 2: daily placebo injections | Randomised: arm 1, n = 243; arm 2, n = 238 |
| PRISMS 1998;189 RRMS (Poser criteria64); included in TA32 |
|
|
Arm 1: IFN-β-1a 22 µg SC three times weekly (Rebif); arm 2: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 3: placebo | Randomised: arm 1, n = 189; arm 2, n = 184; arm 3, n = 187 |
| REFLEX 2012;175 CIS (2005 McDonald criteria66) |
|
Arm 1: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 2: three times weekly SC injections of placebo | Randomised: arm 1, n = 146; arm 2, n = 146 | |
| REFORMS 2012;197 RRMS (2005 McDonald criteria,66 Poser criteria64) |
|
|
Arm 1: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 2: IFN-β-1b 250 µg SC every other day (Betaferon) | Randomised: arm 1, n = 65; arm 2, n = 64 |
| REGARD 2008;192 RRMS (2001 McDonald criteria54) |
|
|
Arm 1: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 2: GA 20 mg SC once daily (Copaxone) | Randomised: arm 1, n = 386; arm 2, n = 378 |
| REMAIN 2012;183 RRMS/SPMS (diagnostic criteria unclear) |
|
|
Arm 1: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 2: no treatment; presumably BSC | Randomised: arm 1, n = 15; arm 2, n = 15 |
| Schwartz 1997;181 RRMS (Poser criteria64) |
|
|
Arm 1: IFN-β-1b 250 µg SC every other day (Betaferon); arm 2: no placebo indicated; likely ongoing BSC | Randomised: arm 1, n = 34; arm 2, n = 45 |
| SPECTRIMS 2001;224 SPMS (Lublin and Reingold criteria15); included in TA32 |
|
|
Arm 1: IFN-β-1a 44 µg SC three times weekly (Rebif); arm 2: IFN-β-1a 22 µg SC three times weekly (Rebif); arm 3: placebo | Randomised: arm 1, n = 204; arm 2, n = 209; arm 3, n = 205 |
Risk of bias and methodological quality
The risk of bias graphs for all MS types and for each MS type across all included studies are presented in Figure 2. Table 5 also provides the assessment of risk of bias for each of the included studies.
FIGURE 2.
Risk of bias by MS type: (a) all MS types; (b) CIS; (c) RRMS; and (d) SPMS.
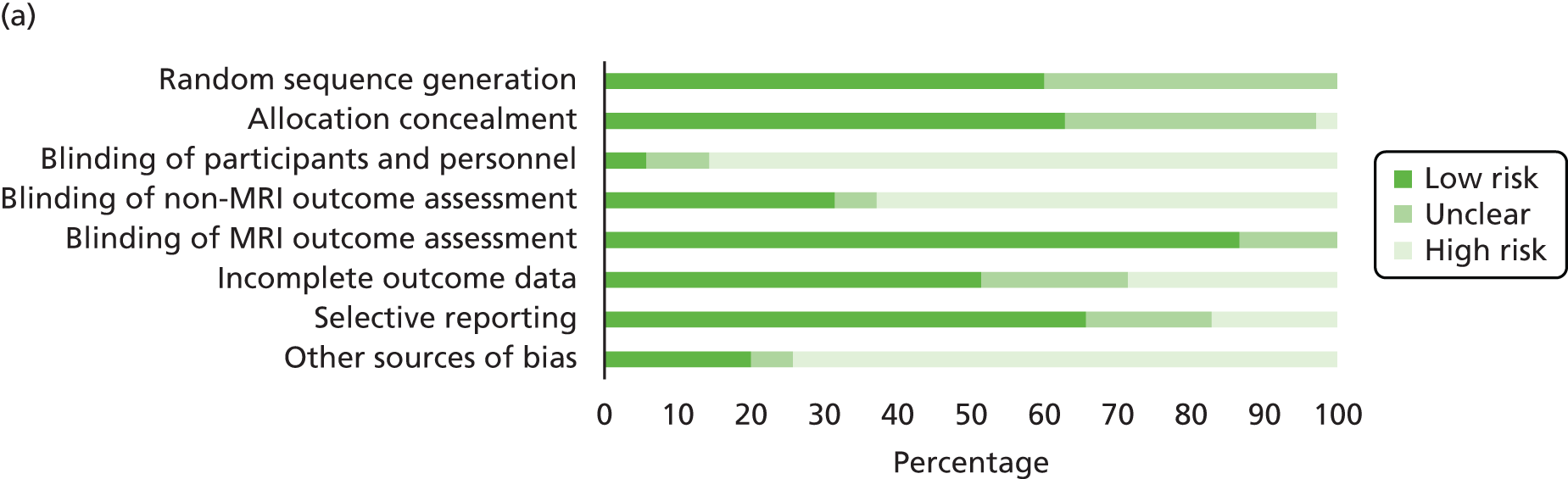
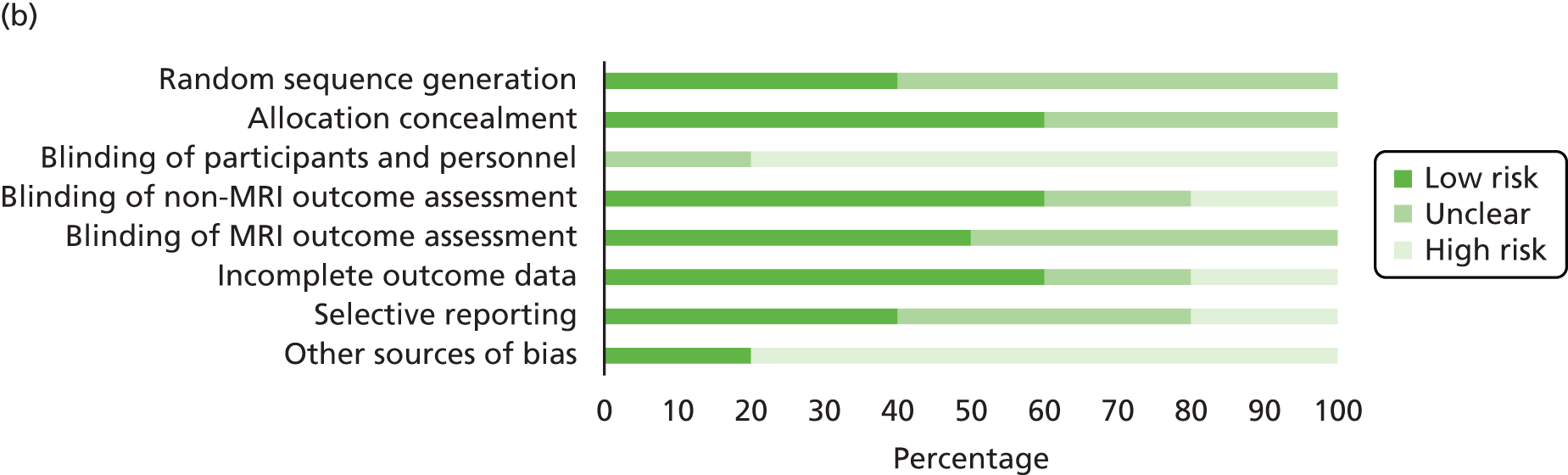

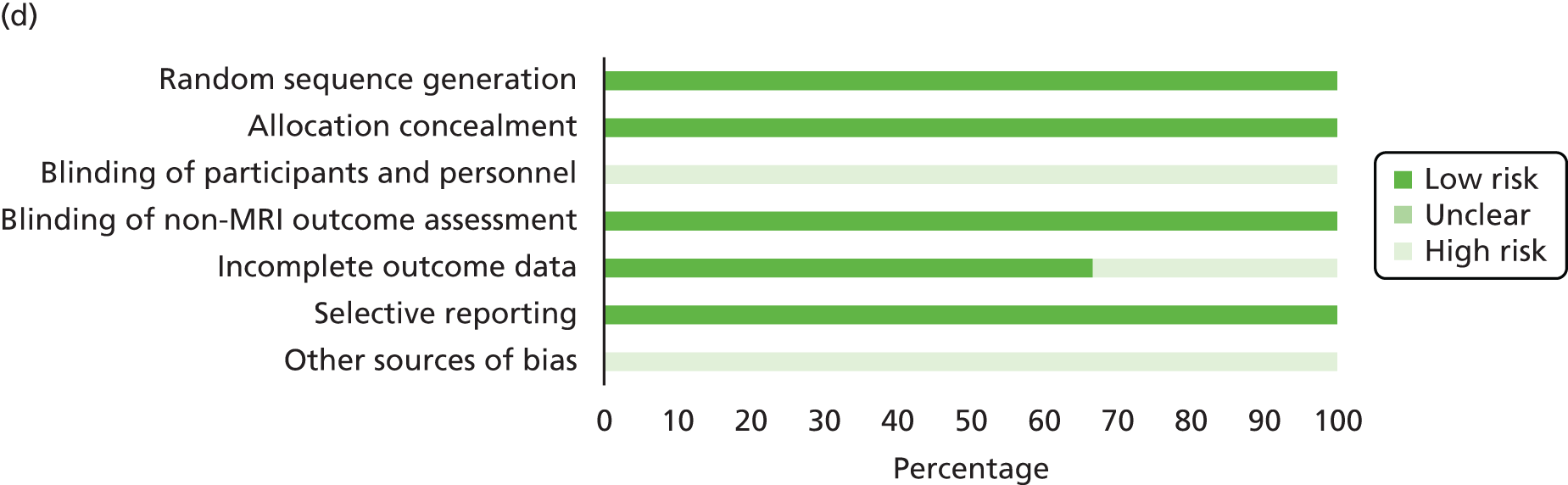
| MS type | Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment (except MRI) | Blinding of MRI outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias |
|---|---|---|---|---|---|---|---|---|---|
| CIS | BENEFIT 2006171 | Unclear | Low | High | Low | Low | Low | Unclear | High |
| CIS | CHAMPS 2000172 | Unclear | Unclear | High | Low | Low | Unclear | Low | High |
| CIS | Pakdaman 2007173 | Unclear | Unclear | Unclear | Unclear | NA | High | High | High |
| CIS | PreCISe 2009174 | Low | Low | High | High | Unclear | Low | Low | Low |
| CIS | REFLEX 2012175 | Low | Low | High | Low | Unclear | Low | Unclear | High |
| RRMS | ADVANCE 2014213 | Low | Low | High | High | Low | High | Low | High |
| RRMS | AVANTAGE 2014182 | Unclear | Unclear | High | High | NA | Unclear | Low | Low |
| RRMS | BECOME 2009184 | Unclear | Unclear | High | High | Low | Low | Low | High |
| RRMS | BEYOND 2009190 | Low | Low | High | High | NA | Unclear | Low | High |
| RRMS | Bornstein 1987170 | Unclear | High | High | High | NA | Low | Low | Low |
| RRMS | BRAVO 2014198 | Low | Low | High | Low | NA | Low | High | High |
| RRMS | Calabrese 2012188 | Low | Low | High | High | Low | Low | High | Low |
| RRMS | CombiRx 2013191 | Low | Low | Low | Low | NA | High | Low | Low |
| RRMS | CONFIRM 2012216 | Low | Low | High | High | Low | High | Low | High |
| RRMS | Cop1 MSSG 1995217 | Unclear | Low | High | High | NA | Low | Low | Low |
| RRMS | ECGASG 2001219 | Low | Low | High | High | Low | Low | Unclear | High |
| RRMS | Etemadifar 2006185 | Unclear | Unclear | High | High | NA | Low | Unclear | Unclear |
| RRMS | EVIDENCE 2007195 | Low | Low | High | High | NA | Low | Low | High |
| RRMS | GALA 2013221 | Unclear | Low | High | High | Low | Unclear | Low | High |
| RRMS | GATE 2015220 | Low | Low | High | High | Low | Low | Low | High |
| RRMS | IFNB MSSG 1995209 | Unclear | Unclear | High | High | Low | High | Low | High |
| RRMS | IMPROVE 2012207 | Unclear | Low | Unclear | Unclear | NA | Unclear | High | High |
| RRMS | INCOMIN 2002196 | Low | Low | High | High | Low | Low | Low | Low |
| RRMS | Kappos 2011199 | Low | Low | High | High | Low | Low | High | High |
| RRMS | Knobler 1993211 | Unclear | Unclear | High | High | NA | Unclear | Unclear | Unclear |
| RRMS | Mokhber 2014186 | Low | Unclear | High | High | NA | High | Low | High |
| RRMS | MSCRG 1996200 | Low | Unclear | Low | Low | Low | High | Unclear | High |
| RRMS | PRISMS 1998189 | Low | Low | Unclear | Low | NA | Low | Low | High |
| RRMS | REFORMS 2012197 | Low | Unclear | High | High | NA | Unclear | Low | High |
| RRMS | REGARD 2008192 | Low | Unclear | High | Low | NA | Low | Low | High |
| RRMS | REMAIN 2012183 | Unclear | Unclear | High | High | NA | High | Low | High |
| RRMS | Schwartz 1997181 | Unclear | Low | High | High | NA | High | High | Unclear |
| SPMS | ESG 1998222 | Low | Low | High | Low | NA | Low | Low | High |
| SPMS | NASG 2004223 | Low | Low | High | Low | NA | High | Low | High |
| SPMS | SPECTRIMS 2001224 | Low | Low | High | Low | NA | Low | Low | High |
Risk in randomisation or allocation methods
All studies that adequately detailed their method of randomisation (n = 21/35) used a method that was judged to be at low risk of bias. 174–175,186–192,195–200,213,216,219–220,222–224 Studies that reported methods of allocation concealment (the concealment of study allocation before the beginning of assigned treatment) were also judged to be at low risk of bias (n = 22/35),171,174–175,181,188–191,195–196,198–199,207,213,216–217,219–224 with the exception of one study that used open allocation. 170 All studies citing central allocation were judged as having a low risk of bias. 171,175,190,198,207,213,216–217,219–220,222
Risk in methods of blinding
In the studies examined, 86% (n = 30/35) were at high risk of bias from either complete or partial participant unblinding. 170–172,174–175,181–186,188,190,192,195–199,209,211,213,216–217,219–224 Fourteen studies, most of which were comparisons between different active drugs, specifically did not blind participants or practitioners;170,182–186,188,190,192,195–197,199,216 in another 15 studies, participants were initially blinded but were at high risk of unblinding from increased rates of side effects. 171–172,174–175,184,209,211,213,217,219–224 In particular, the lack of blinding in comparisons between different drugs meant that risk of bias was imbalanced across different comparisons for the same outcome. We designated all studies in which the rates of side effects (in particular, injection site reactions) in one study group were double those in another to be at high risk of bias from participant unblinding. In the two studies designated as being at low risk of bias for participant blinding, side effect rates were not increased by a factor of two in one group compared with the other (one study tested active vs. active treatments). 191,200
Blinding of outcome assessment was made similarly difficult by injection site reactions. Blinding of outcome assessment was designated as low risk only if injection site reaction rates were increased by less than a factor of two in the treatment group (two studies)172,200 or if participants were specifically instructed to cover their injection sites (eight studies). 171,175,189,192,198,222–224 In nine cases, outcome assessors were otherwise blinded but injection sites were not covered and these studies were designated to be at high risk of bias. 170,174,184,209,211,213,216–217,219 Additionally, studies in which participants were unblinded were designated as being at high risk of bias for outcome assessment if they did not report that participants were given specific instructions against sharing treatment information with assessors. 170,182–186,188,190,192,195–199,216 All studies that reported MRI outcomes and detailed methods for blinding of MRI assessment were found to be at low risk of bias (n = 13/15). 171–172,184,188,196,199–200,209,213,216,219–221
Risk in data analysis and reporting
In total, 29% (n = 10/35) of studies were found to be at high risk of bias from missing data, based on large numbers of missing data, a difference in rates of loss to follow-up between arms or lack of reporting of imputation methods. 173,181,183,186,191,200,209,213,216,223 In 17% (n = 6/35) of studies, outcomes were not reported as stated, and these were designated to be at high risk of bias from selective reporting. 173,181,188,198–199,207 Finally, all studies funded by drug manufacturers were designated as being at high risk of bias under the ‘other’ category,171–173,175,183–184,186,189–190,192,195,197–200,207,209,213,216,219–224 as this was not covered by other questions in the Cochrane risk of bias tool. 165
Summary: study characteristics and risk of bias
We located 35 primary studies from a variety of settings and covering all of the drugs listed in the NICE scope. 141 These studies were of variable quality, with particular issues posed by risk of unblinding of patients and outcome assessors because of injection site reactions, as well as imbalanced risk of bias from open-label comparisons. Many studies were sponsored by manufacturers and most studies were at high risk of bias because of missing data.
Clinical effectiveness: clinically isolated syndrome
Our analysis was informed by five trials. 171–175 It should be noted that triallists generally examined time to CDMS, defined using the Poser criteria64 and involving a second relapse or neurological deterioration, although some also presented analyses examining time to ‘McDonald MS’, in which MRI findings could be used with clinical findings to arrive at a diagnosis.
30 µg of interferon beta-1a intramuscularly once a week (Avonex) compared with placebo
Two trials evaluated 30 µg of IM IFN-β-1a once a week, both compared with placebo. 172,173
Time to diagnosis of multiple sclerosis
Both studies reported significant differences in favour of IFN-β-1a with regard to delaying time to confirmation of CDMS, diagnosed generally by a second relapse but in some cases by progressive neurological deterioration. The CHAMPS (Controlled High Risk Avonex Multiple Sclerosis Study) trial,172 which followed up 393 patients for up to 3 years, found a reduction in hazard of more than half (HR 0.49, 95% CI 0.33 to 0.73). The study by Pakdaman et al. ,173 which followed up 202 patients for up to 3 years, found a reduction in conversion to CDMS in the IFN-β-1a group (incidence 36.6% vs. 58.2%). We converted this to a HR of 0.54 (95% CI 0.36 to 0.81).
Separate publications also presented analyses stratified by risk levels, site of first lesion176 and type of first attack. 177 In analyses comparing patients with monofocal disease and patients with multifocal disease at first demyelinating event,177 patients with monofocal disease had a similar reduction in hazard to the whole trial population (HR 0.45, 95% CI 0.27 to 0.74), whereas patients with multifocal disease had a decreased reduction in hazard (HR 0.64, 95% CI 0.32 to 1.28).
Freedom from disease activity
The CHAMPS trial176 evaluated freedom from disease activity using several composite outcomes, each of which showed a reduction in hazard associated with IFN-β-1a. Patients receiving IFN-β-1a were less likely to have a composite outcome of CDMS or more than one new or enlarging T2 lesion, although this outcome may be closer to McDonald MS (adjusted HR 0.47, 95% CI 0.36 to 0.62); of CDMS or at least one new or enlarging T2 lesion (adjusted HR 0.55, 95% CI 0.42 to 0.71); or of CDMS, at least one new or enlarging T2 lesion or at least one gadolinium-enhancing lesion (adjusted HR 0.60, 95% CI 0.47 to 0.78).
Adverse events and mortality
Full results for AEs are available on request. Mortality was not reported in these studies.
44 µg of interferon beta-1a subcutaneously three times a week (Rebif) compared with placebo
One trial evaluated 44 µg of SC IFN-β-1a three times a week against placebo175 (this trial also included an arm testing 44 µg of SC IFN-β-1a once a week, which we do not consider further here as it is not covered by the recommended posology).
Time to diagnosis of multiple sclerosis
In the REFLEX (REbif FLEXible dosing in early MS) trial,175 340 patients in the relevant trial arms were followed for up to 2 years and a significant reduction in hazard for conversion to CDMS was found (HR 0.48, 95% CI 0.31 to 0.73). An additional analysis examined time to conversion to McDonald MS (i.e. using MRI criteria as well) and found a similar reduction in hazard (HR 0.49, 95% CI 0.38 to 0.64), corresponding to median days to diagnosis of 310 compared with 97 in the IFN-β-1a and placebo groups respectively.
Several subgroup analyses were undertaken on the study sample by risk level; key findings from Freedman et al. 178 are summarised here. In examining time to CDMS, patients with monofocal presentation (HR 0.58, 95% CI 0.40 to 0.84) and with multifocal presentation (HR 0.45, 95% CI 0.31 to 0.64) both experienced a decreased hazard of conversion to CDMS, but type of presentation did not appear to be a significant moderator. Similarly, an analysis that ‘rediagnosed’ patients as having McDonald MS or not based on the revised 2010 criteria62 found that patients who were McDonald 2010 MS negative had a significantly decreased hazard of conversion to McDonald 2005 MS (HR 0.49, p < 0.001), as did those who were McDonald 2010 MS positive at baseline (HR 0.54, p = 0.01).
Adverse events and mortality
Full results are available on request. Mortality was not significantly different between the groups, although no events occurred in the study drug arm and two deaths occurred in the placebo arm.
250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia) compared with placebo
One trial evaluated 250 µg of SC IFN-β-1b every other day against placebo. 171
Time to diagnosis of multiple sclerosis
In the BENEFIT (Betaferon®/Betaseron®/ in Newly Emerging Multiple Sclerosis for Initial Treatment) trial,171 468 patients were followed for up to 2 years. The study drug delayed time to CDMS (HR 0.50, 95% CI 0.36 to 0.70). This reduction in hazard corresponded to a difference in days to diagnosis of 618, compared with 255 at the 25th percentile. Triallists also considered time to McDonald MS; the effect of the study drug was similar in magnitude (HR 0.54, 95% CI 0.43 to 0.67).
Analyses stratified by risk levels, site of first lesion and type of first attack were also carried out in the BENEFIT trial. 179 In analyses comparing patients with monofocal and multifocal disease at first demyelinating event, patients with monofocal disease had a similar reduction in hazard to the whole trial population (HR 0.45, 95% CI 0.29 to 0.71), whereas patients with multifocal disease had a decreased reduction in hazard (HR 0.63, 95% CI 0.40 to 0.99).
Multiple sclerosis symptoms and health-related quality of life
Patients in the BENEFIT trial were assessed for cognitive performance using the Paced Auditory Serial Addition Test (PASAT). 180 At year 2, patients receiving the study drug had greater increases in score on this test than patients receiving placebo, including under conservative assumptions (2.0 vs. 0.6; p = 0.021). Additionally, patient-reported physical health and HRQoL data were collected in this trial. 171 Scores were not different between groups and were stable over the trial.
Adverse events and mortality
Full results are available on request. No deaths were reported in the BENEFIT trial. 171
20 mg of glatiramer acetate subcutaneously once daily (Copaxone) compared with placebo
One trial evaluated 20 mg of SC GA once daily against placebo. 174
Time to diagnosis of multiple sclerosis
The PreCISe (Presenting with Clinically Isolated Syndrome) trial174 followed up 481 patients for up to 3 years, although the trial was stopped early for benefit. Participants receiving 20 mg of SC GA once daily had a reduced hazard of conversion to CDMS (HR 0.55, 95% CI 0.4 to 0.77), although CDMS was defined here as the occurrence of a second exacerbation. The corresponding difference in days to diagnosis was 722, compared with 336 at the 25th percentile.
Adverse events and mortality
Full results are available on request. Mortality was not significantly different between groups, although the PreCISe trial174 reported only one death, in the study drug arm.
Meta-analyses: time to clinically definite multiple sclerosis
Pairwise meta-analyses
Direct evidence from comparisons is shown in Figure 3. All comparisons were against placebo. Only one comparison, 30 µg of IM IFN-β-1a once a week compared with placebo, included more than one study. The pooled effect size suggested that 30 µg of IM IFN-β-1a once a week reduces time to CDMS (HR 0.52, 95% CI 0.39 to 0.68), with low heterogeneity (I2 = 0%, p = 0.718).
FIGURE 3.
Pairwise meta-analyses: time to CDMS.

Network meta-analyses
The set of studies reporting HRs for time to CDMS formed a connected network (Figure 4). This network was star shaped, meaning that it contained no comparisons between active drugs. We estimated this model using random effects, as per the protocol.
FIGURE 4.
Network of studies: time to CDMS. ifn1a30, 30 µg of IM IFN-β-1a once a week; ifn1a44, 44 µg of SC IFN-β-1a three times weekly; ifn1b250, 250 µg of SC IFN-β-1b every other day; ga20, 20 mg of SC GA once daily.

Rankings from the NMA suggested that 44 µg of SC IFN-β-1a three times weekly was ranked best, followed by 250 µg of SC IFN-β-1b every other day, 30 µg of IM IFN-β-1a once a week and 20 mg of SC GA once daily (Table 6). Placebo was ranked last.
| Drug | SUCRA | 44 µg of SC IFN-β-1a three times weekly | 250 µg of SC IFN-β-1b every other day | 30 µg of IM IFN-β-1a weekly | 20 mg of SC GA daily | Placebo |
|---|---|---|---|---|---|---|
| 44 µg of SC IFN-β-1a three times weekly | 0.70 | 0.96 (0.56 to 1.65) | 0.93 (0.56 to 1.55) | 0.87 (0.51 to 1.50) | 0.48 (0.31 to 0.74) | |
| 250 µg of SC IFN-β-1b every other day | 0.68 | 0.97 (0.63 to 1.50) | 0.91 (0.57 to 1.45) | 0.50 (0.36 to 0.70) | ||
| 30 µg of IM IFN-β-1a weekly | 0.62 | 0.94 (0.61 to 1.45) | 0.52 (0.39 to 0.68) | |||
| 20 mg of SC GA daily | 0.5 | 0.55 (0.40 to 0.76) | ||||
| Placebo | 0 |
Findings for comparisons between active drugs and placebo were identical, as expected, to those in the pairwise meta-analyses. Findings for indirect comparisons between drugs did not suggest the superiority of any one drug over another.
Because the network was star shaped, we could not test for inconsistency.
Sensitivity analysis
We also re-estimated the network with effect sizes for time to conversion to McDonald MS for those studies reporting it. Effectiveness estimates were robust to this change.
Meta-analyses: not possible for adverse events in clinically isolated syndrome
Of the four studies171,172,174,175 reporting discontinuations as a result of AEs, two reported discontinuations over 36 months172,174 and two reported discontinuations over 24 months. 171,175 As a result, we did not estimate a NMA for discontinuations in CIS. Estimates can be found in Table 7.
| Study | Comparison | Follow-up (months) | Treatment arm events | Treatment group (n) | Treatment events proportion (%) | Placebo arm events | Placebo group (n) | Placebo events proportion (%) |
|---|---|---|---|---|---|---|---|---|
| PreCISe 2009174 | GA 20 mg daily vs. placebo | 36 | 14 | 243 | 5.8 | 4 | 238 | 1.7 |
| REFLEX 2012175 | IFN-β-1a 44 µg SC three times weekly vs. placebo | 24 | 5 | 171 | 2.9 | 6 | 171 | 3.5 |
| CHAMPS 2000172 | IFN-β-1a 30 µg IM weekly vs. placebo | 36 | 1 | 193 | 0.5 | 7 | 190 | 3.7 |
| BENEFIT 2006171 | IFN-β-1b 250 µg SC every other day vs. placebo | 24 | 24 | 292 | 8.2 | 1 | 176 | 0.6 |
Summary: clinically isolated syndrome
Comparisons for included drugs all relied on one or two trials, but each comparison suggested that IFN or GA delayed time to CDMS over a 2- to 3-year follow-up. This finding appeared to be robust to the diagnostic criteria used to establish a definitive MS diagnosis. The NMA did not suggest the superiority of one drug over another. The rate of AEs tended to be higher in trial arms receiving the active drugs, although, when mortality was reported, it was not significantly higher in patients receiving the study drug. Findings on additional outcomes (MS symptoms, HRQoL) were infrequently reported.
Clinical effectiveness: relapsing–remitting multiple sclerosis
Our analysis was informed by 27 trials. 170,181–221,226,229–231 Of these 27 trials, one evaluated HRQoL measures alone181 and one evaluated AEs alone. 182 In addition, two trials reported on mixed populations. 183,184 The REMAIN (REbif compared with no treatment in the therapy of relapsing Multiple sclerosis After mItoxaNtrone) trial,183 which followed up 30 participants over 96 weeks, included a mixed RRMS (n = 13) and SPMS (n = 17) population. Because of the size of this open-label trial, because data were not stratified by type of MS and because treatment switching was allowed, we decided to include this trial in narrative synthesis but not in meta-analyses. In contrast, the BECOME (Betaseron vs. Copaxone in Multiple Sclerosis with Triple-Dose Gadolinium and 3-Tesla MRI Endpoints) trial,184 which followed up 75 participants over 2 years, included 14 patients diagnosed with CIS before the revision of the McDonald criteria. Because we judged it likely that many of the 14 patients originally diagnosed as having CIS would have been classed as having RRMS under the most recent criteria, we analysed this trial alongside other RRMS-only trials. Thus, 24 relevant trials reported key clinical outcomes.
Several characteristics of the ‘epidemiology’ of the trial network bear discussing first: the design of included multiarm trials, two-arm trials comparing active drugs against each other and trials with mixed populations.
Of the 24 trials reporting clinical outcomes, four trials had three relevant treatment arms:
-
Both Etemadifar et al. 185 and Mokhber et al. 186,187 evaluated 44 µg of SC IFN-β-1a three times a week against 30 µg of IM IFN-β-1a once a week against 250 µg of SC IFN-β-1b every other day.
-
Calabrese et al. 188 evaluated 44 µg of SC IFN-β-1a three times a week against 30 µg of IM IFN-β-1a once a week against 20 mg of SC GA once daily.
-
The PRISMS (Prevention of Relapses and Disability by Interferon Beta-1a Subcutaneously in Multiple Sclerosis) trial189 compared 44 µg of SC IFN-β-1a three times a week against 22 µg of SC IFN-β-1a three times a week against placebo.
An additional seven two-arm trials compared active drugs against each other:
-
Two trials184,190 compared 250 µg of SC IFN-β-1b every other day against 20 mg of SC GA once daily.
-
The CombiRx (Combination Therapy in Patients with Relapsing–Remitting Multiple Sclerosis) trial. 191 compared 30 µg of IM IFN-β-1a once a week against 20 mg of SC GA once daily.
-
The REGARD (REbif vs. Glatiramer Acetate in Relapsing MS Disease) trial192 compared 44 µg of SC TFN-β-1a three times a week against 20 mg of SC GA once daily.
-
The EVIDENCE (Evidence of Interferon Dose–Response: European North American Comparative Efficacy) trial193–195 compared 44 µg of SC IFN-β-1a three times a week against 30 µg of IM IFN-β-1a once a week.
-
The INCOMIN (Independent Comparison of Interferon) trial196 compared 250 µg of SC IFN-β-1b every other day against 30 µg of IM IFN-β-1a once a week.
-
The REFORMS (Rebif New Formulation versus Betaseron Tolerability Study) trial197 compared 44 µg of SC IFN-β-1a three times a week against 250 µg of SC IFN-β-1b every other day.
30 µg of interferon beta-1a intramuscularly once a week (Avonex) compared with placebo
Our analysis was informed by three trials comparing 30 µg of IM IFN-β-1a once a week against placebo. 198–200 The BRAVO (Benefit–Risk Assessment of AVonex and LaquinimOd) trial198 compared oral laquinimod against 30 µg of IM IFN-β-1a once a week and oral placebo, whereas Kappos et al. 199 compared intravenous ocrelizumab against 30 µg of IM IFN-β-1a once a week and intravenous placebo. The Multiple Sclerosis Collaborative Research Group (MSCRG) trial200 compared 30 µg of IM IFN-β-1a once a week against an IM placebo.
An additional six trials compared 30 µg of IM IFN-β-1a once a week against other drugs: three multiarm trials185–188 and three two-arm trials. 191,193–196
Relapse outcomes
Findings for relapse outcomes relied on three trials with different follow-up times,198–200 including two of the largest trials in this review. 198,200 All three studies suggested a beneficial effect of 30 µg of IM IFN-β-1a once a week in terms of reducing the rate of relapses. The BRAVO trial,198 which followed 887 patients in the relevant trial arms for 24 months, found that patients receiving 30 µg of IM IFN-β-1a once a week had a 26% reduction in the ARR (RR 0.74, 95% CI 0.60 to 0.92). In the study by Kappos et al. ,199 108 patients were followed up over 24 weeks and, although the ARR was lower in patients receiving 30 µg of IM IFN-β-1a once a week (ARR 0.36, 95% CI 0.22 to 0.60) than in patients receiving placebo (ARR 0.64, 95% CI 0.43 to 0.94), this difference was only marginally significant (p = 0.07). Finally, in the MSCRG trial,200 301 patients were followed up for up to 3 years, although the study was stopped early for efficacy and thus patients had variable times to follow-up. In analyses including all patients, the ARR for patients receiving the study drug was significantly less than the ARR for patients receiving placebo (0.67 vs. 0.82; p = 0.04).
Only the MSCRG trial200 reported time to first relapse. This was not presented with a HR estimate, but a log-rank test suggested that 30 µg of IM IFN-β-1a once a week did not significantly delay time to first exacerbation compared with placebo (IFN-β-1a vs. placebo: median 47.3 vs. 36.1 weeks; p = 0.34).
Finally, all three studies reported the proportion of patients who were relapse free, although the findings were somewhat heterogeneous and comparability is limited by the differential follow-up. The BRAVO trial198 found that 69% of patients receiving 30 µg of IM IFN-β-1a once a week were relapse free compared with 61% of patients receiving placebo (p = 0.023). This difference was narrower in the study by Kappos et al. 199 (30 µg of IM IFN-β-1a once a week 78% vs. placebo 76%), with a RR for experiencing any relapses of 0.92 (95% CI 0.46 to 1.84). The MSCRG trial200 reported proportions only for those patients who completed the intended 104 weeks of study, excluding those who were enrolled but who did not complete the 104 weeks before the study was stopped. For the 85 patients included who received 30 µg IM of IFN-β-1a once a week, 38% were free of relapses, compared with 26% of the 87 patients receiving placebo. A significance test was not presented.
Relapse severity
We could not locate any relevant comparisons between 30 µg of IM IFN-β-1a once a week and placebo for outcomes relating to moderate or severe relapses or steroid-treated relapses.
Disability progression
Only the BRAVO trial198 estimated time to disability progression confirmed at 3 months. Patients receiving 30 µg of IM IFN-β-1a once a week and placebo were delayed, but not significantly so, in time to progression (HR 0.74, 95% CI 0.51 to 1.09). The results for disability progression confirmed at 6 months were similar (HR 0.73, 95% CI 0.47 to 1.14). The MSCRG trial200 also reported time to progression confirmed at 6 months. Based on a Kaplan–Meier analysis, the predicted probability of progression at 2 years was 21.9% in patients receiving 30 µg of IM IFN-β-1a once a week compared with 34.9% in patients receiving placebo (log-rank p = 0.02), indicating a slowing of time to progression. 200,201 In a separate publication, the reduction in hazard was reported as 43.0% (i.e. HR 0.570; p = 0.03). 202
Empirical proportions of patients with progression confirmed at 3 months were also reported in the BRAVO trial198 (30 µg of IM IFN-β-1a once a week 11% vs. placebo 13%). The proportions with progression at 6 months were similarly low (30 µg of IM IFN-β-1a once a week 8% vs. placebo 10%). In the MSCRG trial, empirical proportions of patients with progression confirmed at 6 months were reported for the full sample in a separate publication from the main study report. 202 Patients receiving 30 µg of IM IFN-β-1a once a week had a lower probability of progression than patients receiving placebo (15% vs. 25%), although follow-up was variable. Significance tests were not presented for these proportions per se (i.e. not as part of survival analysis, discussed in the previous paragraph) in any of the three trials.
The magnitude of change from baseline in EDSS score was presented only for the MSCRG trial. 200 Among patients completing 104 weeks on the study, those receiving 30 µg of IM IFN-β-1a once a week had a smaller increase in EDSS score than those receiving placebo (0.25 vs. 0.74; p = 0.02). This finding was similar in patients examined to week 130 (0.02 vs. 0.61; p = 0.02), with the lower of the scores at week 104 or week 130 taken as a measure of ‘sustained’ change. In the BRAVO trial,198 patients receiving 30 µg of IM IFN-β-1a once a week had a smaller decrease in the Multiple Sclerosis Functional Composite score at 24 months, but this difference was not significant (z-scores –0.045 vs. –0.14; p = 0.21).
Freedom from disease activity
We could not locate any relevant comparisons between 30 µg of IM IFN-β-1a once a week and placebo for the combined clinical–MRI measures of freedom from disease activity.
Multiple sclerosis symptoms and health-related quality of life
The MSCRG trial203 reported performance on both the Comprehensive and Brief Neuropsychological Batteries by examining change from baseline to 2 years and estimated models with both no covariates and with baseline performance as a covariate. Although exact effect sizes were not provided, the study found that, in patients completing 104 weeks on the study, compared with placebo, 30 µg of IM IFN-β-1a once a week improved information processing and memory (p = 0.036 unadjusted, p = 0.011 adjusted for baseline performance) and visuospatial abilities and executive functions (p = 0.005 unadjusted, p = 0.085 adjusted), but not verbal abilities and attention span (p = 0.603 unadjusted, p = 0.917 adjusted). Findings were similar for the Brief Neuropsychological Battery (p = 0.020 for both unadjusted and adjusted), although 30 µg of IM IFN-β-1a once a week did not significantly delay time to onset of deterioration confirmed at 6 months (log-rank p = 0.094). Analyses of PASAT scores indicated that, although the difference in magnitude of change did not rise to significance (p = 0.119 unadjusted, p = 0.090 adjusted), patients receiving 30 µg of IM IFN-β-1a once a week did delay time to sustained deterioration (log-rank p = 0.023).
Additionally, patients receiving 30 µg of IM IFN-β-1a once a week had a decreased hazard of sustained worsening in the timed 25-foot walk (HR 0.401; p = 0.04). However, this decreased hazard was not evidenced in the nine-hole peg test with the dominant hand (HR 0.514; p = 0.07) or the non-dominant hand (HR 0.494; p = 0.10) or the box and block test with the dominant hand (HR 0.581; p = 0.45) or the non-dominant hand (HR 0.835; p = 0.75). 202 A variety of combinations of these end points was also tested. In a separate publication, use of an instrument to examine functional independence showed that change over 104 weeks in cognitive aspects of functional independence was not significant. 204 This was the case when considered as a difference in both means (p = 0.08) and time to sustained worsening (log-rank p = 0.188), with similar findings for difference in means for motor aspects of functional independence (p = 0.10, log-rank p = 0.368). Total changes in functional independence were significant at 104 weeks (p = 0.03).
Finally, the MSCRG trial reported on the effects of 30 µg of IM IFN-β-1a once a week on the Sickness Impact Profile as a measure of QoL. 205 In the study population as a whole, there were no differences between placebo and the study drug on the overall measure, nor on its physical or psychosocial components. However, when considering patients with low HRQoL at baseline (defined as a score of ≥ 10 on the measure), patients receiving the study drug had a greater improvement on physical aspects of the measure than those receiving placebo (–3.78 vs. 3.57; p < 0.05).
Adverse events and mortality
We stratified the comparison of AEs by type of placebo, as local AEs (e.g. injection site reactions) would not apply in studies with oral or intravenous placebos. Full results are available on request.
Mortality was not different between groups for either type of placebo. However, only one death occurred in the MSCRG trial200 (in the study drug arm), no deaths occurred in the study by Kappos et al. 199 and only one death occurred in the BRAVO trial198 (in the study drug arm).
Summary of the narrative synthesis: 30 µg of interferon beta-1a intramuscularly once a week (Avonex) compared with placebo
Findings from three trials198–200 suggested that, relative to placebo, 30 µg of IM IFN-β-1a once a week reduces relapse rates, although findings were less clear for other relapse-related outcomes. Findings from two trials198,200 suggested that 30 µg of IM IFN-β-1a once a week also has a beneficial effect in terms of delaying disability progression, although only the MSRCG trial200 presented significant results. Findings from the MSCRG trial200–204 with regard to MS symptoms were inconsistent across tests. We were unable to find any relevant comparisons for relapse severity, defined as moderate/severe or steroid-treated relapses, or the combined clinical–MRI measures of freedom from disease activity. Mortality was rare and not significantly different between groups.
30 µg of interferon beta-1a intramuscularly once a week (Avonex) compared with 44 µg of interferon beta-1a subcutaneously three times a week (Rebif)
Four trials compared 30 µg of IM IFN-β-1a once a week against 44 µg of SC IFN-β-1a three times a week. 185–188,193–195
Relapse outcomes
Findings for relapse outcomes relied on three trials, of which the EVIDENCE trial193–195 was the largest by far. Calabrese et al. 188 analysed 141 patients randomised to either 30 µg of IM IFN-β-1a once a week (n = 47), 44 µg of SC IFN-β-1a three times a week (n = 46) or 20 mg of SC GA once daily (n = 48) over 2 years, with complete follow-up for analysed patients. Relapses were apparently analysed using a normal distribution, although formal significance tests were not presented. At 2 years, patients receiving 30 µg of IM IFN-β-1a once a week had an average ARR of 0.5 [standard deviation (SD) 0.6], whereas patients receiving 44 µg of SC IFN-β-1a three times a week had an average ARR of 0.4 (SD 0.6). We estimated a RR of 1.25 (95% CI 0.81 to 1.92). Etemadifar et al. 185 analysed 90 patients randomised 1 : 1 : 1 to either 30 µg of IM IFN-β-1a once a week, 44 µg of SC IFN-β-1a three times a week or 250 µg of SC IFN-β-1b every other day. Because relapses were analysed using a repeated measures ANOVA method with normal distributions, we re-estimated RRs based on the number of relapses in each arm. Based on a total of 57 relapses in patients receiving 30 µg of IM IFN-β-1a once a week and 66 relapses in patients receiving 44 µg of SC IFN-β-1a three times a week, we estimated a RR of 0.86 (95% 0.61 to 1.23). Finally, the EVIDENCE trial194,195 randomised 677 patients and followed them up for an intended period of at least 48 weeks, with a median follow-up of 64 weeks. Patients receiving 30 µg of IM IFN-β-1a once a week had a higher ARR (0.65) than patients receiving 44 µg of SC IFN-β-1a three times a week (0.54); this difference was statistically significant (RR 1.20; p = 0.033).
Only the EVIDENCE trial194,195 presented data for time to first relapse. The 40th percentile of patients receiving 30 µg of IM IFN-β-1a once a week had their first relapse at 6.7 months whereas the 40th percentile of patients receiving 44 µg of SC IFN-β-1a three times a week had their first relapse at 13.5 months. Relative to patients receiving 30 µg of IM IFN-β-1a once a week, patients receiving 44 µg of SC IFN-β-1a three times a week had a decreased hazard of first relapse (HR 0.70, 95% CI 0.56 to 0.88).
Both studies presenting data on the proportions of patients free of relapse were in agreement on the direction of effect. In the study by Etemadifar et al. ,185 patients receiving 30 µg of IM IFN-β-1a once a week were less likely to be free of relapses than patients receiving 44 µg of SC IFN-β-1a three times a week (20.0% vs. 56.7%), but a pairwise significance test was not presented. In the EVIDENCE trial,194,195 patients receiving 30 µg of IM IFN-β-1a once a week were less likely to be relapse free (48%) than patients receiving 44 µg of SC IFN-β-1a three times a week (56%), that is, the OR for being relapse free at the end of the study favoured patients receiving 44 µg of SC IFN-β-1a three times a week (OR 1.5, 95% CI 1.1 to 2.0).
Relapse severity
Only the EVIDENCE trial194,195 reported outcomes related to relapse severity, in this case ARRs for steroid-treated relapses. Patients receiving 30 µg of IM IFN-β-1a once a week had an ARR of 0.28 for steroid-treated relapses, whereas patients receiving 44 µg of SC IFN-β-1a three times a week had an ARR of 0.19 for steroid-treated relapses. Thus, the RR for steroid-treated relapses is 1.47 (p = 0.009).
Disability progression
The EVIDENCE trial193 was the only trial that reported time to disability progression and the proportions of patients progressing. Drawing from interim data on all patients at 48 weeks of follow-up, patients receiving 30 µg of IM IFN-β-1a once a week appeared to progress faster than patients receiving 44 µg of SC IFN-β-1a three times a week. However, this finding was not significant for either progression confirmed at 3 months (44 µg SC vs. 30 µg IM: HR 0.87, 95% CI 0.58 to 1.31) or progression confirmed at 6 months (HR 0.70, 95% CI 0.39 to 1.25). At the end of the study, there was no statistically significant difference in the proportion of patients with disability progression confirmed at 3 months between those receiving 30 µg of IM IFN-β-1a once a week and those receiving 44 µg of SC IFN-β-1a three times a week (17% vs. 16%; p = 0.710).
In the study by Calabrese et al. ,188 the magnitude of change in EDSS score did not appear to be numerically different between 30 µg of IM IFN-β-1a once a week [0.2 (SD 0.4)] and 44 µg of SC IFN-β-1a three times a week [0.2 (SD 0.5)], but formal significance testing was not reported. However, in the study by Etemadifar et al. ,185 patients receiving 30 µg of IM IFN-β-1a once a week had a reduction in EDSS score of 0.1 (95% CI –0.2 to 0.5), a numerically smaller decrease than that seen for patients receiving 44 µg of SC IFN-β-1a three times a week (0.3, 95% CI 0.03 to 0.5). Again, formal significance testing was not reported. Finally, Mokhber et al. 186,187 found no difference in EDSS score between baseline and 12 months’ follow-up for 30 µg of IM IFN-β-1a once a week (0.0, n = 20; p = 0.548), whereas a test for change was significant for 44 µg of SC IFN-β-1a SC three times a week (–1.0, n = 21; p = 0.001). Pairwise testing was not performed but an overall test was not significant.
Freedom from disease activity
We could not locate any relevant comparisons between 30 µg of IM IFN-β-1a once a week and 44 µg of SC IFN-β-1a three times a week for the combined clinical–MRI measures of freedom from disease activity.
Multiple sclerosis symptoms and health-related quality of life
Mokhber et al. 186 presented tests of cognitive function, although without pairwise comparisons. Except for the symbol digit modalities test, for all tests presented (selective reminding test, spatial recall test, symbol digit modalities test, PASAT and word list generation), comparisons across all three treatment groups were not statistically significant. Post hoc tests found evidence that patients receiving 30 µg of IM IFN-β-1a once a week did not improve as much as patients receiving 44 µg of SC IFN-β-1a three times a week on the word list generation and PASAT-easy tests.
Additionally, Mokhber et al. 187 disaggregated the Multiple Sclerosis Quality of Life-54 scale into its subcomponents, including mental health (five components) and physical health (eight components). There were few significant within-group differences in this small trial and pairwise significance tests, as well as estimates of change from baseline, were not presented in a standard format, permitting only discussion of direction and significance of differences. Patients receiving 30 µg of IM IFN-β-1a once a week significantly worsened in terms of energy and fatigue compared with patients receiving 44 µg of SC IFN-β-1a three times a week, who improved. However, patients receiving 30 µg of IM IFN-β-1a once a week significantly improved in terms of physical role limitations compared with patients receiving 44 µg of SC IFN-β-1a three times a week, who also improved. Patients receiving 30 µg of IM IFN-β-1a once a week also significantly improved in terms of both emotional role limitations and cognitive function compared with patients receiving 44 µg of SC IFN-β-1a three times a week. Differences were not significant for physical function, health perceptions, pain, sexual function, social function, health distress, overall QoL or emotional well-being.
Adverse events and mortality
The EVIDENCE trial206 was the only trial that reported AEs. No studies reported mortality. Full results are available on request.
Summary of the narrative synthesis: 30 µg of interferon beta-1a intramuscularly once a week (Avonex) compared with 44 µg of interferon beta-1a subcutaneously three times a week (Rebif)
Findings from three trials, of which one was considerably larger than the others, suggested that 30 µg of IM IFN-β-1a once a week was less effective than 44 µg of SC IFN-β-1a three times a week in terms of reducing and delaying relapses. Findings from the EVIDENCE trial194,195 suggested that 30 µg of IM IFN-β-1a once a week was also less effective than 44 µg of SC IFN-β-1a three times a week at reducing steroid-treated relapses. Across disability progression outcomes, the findings did not show a clear pattern, and the largest trial193 did not find a significant difference in terms of disability progression outcomes. Findings on MS symptoms and HRQoL were poorly reported and inconsistent and relied on one small trial. We were unable to locate any comparisons for combined clinical–MRI measures of freedom from disease activity and the included studies did not report mortality.
30 µg of interferon beta-1a intramuscularly once a week (Avonex) compared with 250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia)
Three trials compared 30 µg of IM IFN-β-1a once a week against 250 µg of SC IFN-β-1b every other day. 185–187,196
Relapse outcomes
Findings for relapse outcomes relied on two trials, both with 24 months of follow-up. In the study by Etemadifar et al. ,185 patients receiving 30 µg of IM IFN-β-1a once a week had fewer relapses over 2 years of follow-up than patients receiving 250 µg of SC IFN-β-1b every other day (57 vs. 65; n = 30 in both groups). We estimated this as a RR of 0.88 (95% CI 0.61 to 1.25). However, in the INCOMIN trial,196 which followed up 188 patients over 24 months, patients receiving 30 µg of IM IFN-β-1a once a week had a higher ARR (0.7) than patients receiving 250 µg of SC IFN-β-1b SC every other day (0.5). Because the authors presented the effect size estimate as a standardised mean difference, we re-estimated the RR as 1.4 (95% CI 1.07 to 1.83).
Both trials suggested that the proportion of patients who were relapse free was comparatively higher in the group receiving 250 µg of SC IFN-β-1b every other day. The proportions of patients experiencing relapses were significantly different between the relevant arms in the study by Etemadifar et al. ,185 with patients receiving 30 µg of IM IFN-β-1a once a week less likely to be free of relapse (20% vs. 43.3%, p = 0.049). In the INCOMIN trial,196 patients receiving 30 µg of IM IFN-β-1a once a week were also less likely to be free of relapse than patients receiving 250 µg of SC IFN-β-1b every other day (36% vs. 51%; risk ratio 0.76, 95% CI 0.59 to 0.99).
Relapse severity
Only the INCOMIN trial196 presented findings for relapse severity, specifically ARRs for steroid-treated relapses. Although patients receiving 30 µg of IM IFN-β-1a once a week were more likely to have steroid-treated relapses than those receiving 250 µg of SC IFN-β-1b every other day (0.5 vs. 0.38), this difference was not significant (estimated RR 1.32, 95% CI 0.96 to 1.80).
Disability progression
Only the INCOMIN trial196 presented differences in time to disability progression confirmed at 6 months and proportions with disability progression. More patients receiving 30 µg of IM IFN-β-1a once a week progressed than patients receiving 250 µg of SC IFN-β-1b every other day (30% vs. 13%), with patients in the 250 µg of SC IFN-β-1b every other day group having a reduction in risk of progression of 56% (p = 0.005). In combination with a log-rank test reported as p < 0.01, this gives an estimated HR of 2.24 (95% CI 1.21 to 4.13).
Findings from all three trials suggested that 30 µg of IM IFN-β-1a once a week did not have as beneficial an effect on the magnitude of change in EDSS score as 250 µg of SC IFN-β-1b every other day. In the study by Etemadifar et al. ,185 patients receiving 30 µg of IM IFN-β-1a once a week had a reduction in EDSS score of 0.1 (95% CI –0.2 to 0.5), a numerically smaller decrease than that seen in patients receiving 250 µg of SC IFN-β-1b every other day (0.7, 95% CI 0.5 to 0.9). Again, formal pairwise significance testing was not reported. Moreover, in a comparatively small trial, Mokhber et al. 186,187 found no evidence of a significant difference in EDSS score between baseline and 12 months’ follow-up for 30 µg of IM IFN-β-1a once a week (0.0, n = 20; p = 0.548), whereas a test for change was significant for 250 µg of SC IFN-β-1b every other day (–0.6, n = 19; p = 0.028). Pairwise testing was not performed but an overall test was not significant. Finally, using ANCOVA-adjusted estimates, the INCOMIN trial196 found that patients receiving 30 µg of IM IFN-β-1a once a week had a higher EDSS score at the end of the trial than patients receiving 250 µg of SC IFN-β-1b every other day (2.5 vs. 2.1; p = 0.004).
Multiple sclerosis symptoms and health-related quality of life
Mokhber et al. 186 presented tests of cognitive function, although without pairwise comparisons. It should be reiterated that this trial was a small trial, with 39 patients analysed in total for the relevant comparisons. Except for the symbol digit modalities test, for all tests presented (selective reminding test, spatial recall test, symbol digit modalities test, PASAT and word list generation), comparisons across all three treatment groups were not statistically significant. Post hoc tests found evidence that patients receiving 30 µg of IM IFN-β-1a once a week did not improve as much as patients receiving 250 µg of SC IFN-β-1b every other day on the symbol digit modalities and PASAT-easy tests.
Additionally, Mokhber et al. 187 disaggregated the Multiple Sclerosis Quality of Life-54 scale into its subcomponents, including mental health (five components) and physical health (eight components). There were few significant within-group differences in this small trial and pairwise significance tests, as well as estimates of change from baseline, were not presented in a standard format, permitting only discussion of direction and significance of differences. Patients receiving 30 µg of IM IFN-β-1a once a week significantly improved in terms of health perceptions and pain compared with patients receiving 250 µg of SC IFN-β-1b every other day, who declined on both measures. However, patients receiving 250 µg of SC IFN-β-1b every other day improved more on overall QoL, overall mental health aspects of QoL and emotional well-being than patients receiving 30 µg of IM IFN-β-1a once a week. Differences were not significant for overall physical health aspects of QoL, physical function, energy/fatigue, physical role limitations, sexual function, social function, health distress, emotional role limitations or cognitive function.
Adverse events and mortality
Only the INCOMIN trial196 reported AEs. No studies reported mortality. Full results are available on request.
Summary of the narrative synthesis: 30 µg of interferon beta-1a intramuscularly once a week (Avonex) compared with 250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia)
Although the included trials were in conflict with regard to the relative effect of the drugs on relapse rates, the INCOMIN trial196 suggested that 30 µg of IM IFN-β-1a once a week was less effective than 250 µg of SC IFN-β-1b every other day in reducing relapse rates and both studies found that the proportion of patients free of relapses was lower in the group receiving 30 µg of IM IFN-β-1a once a week. The INCOMIN trial196 did not find a difference between groups with regard to relapse severity, measured as steroid-treated relapses, but both studies agreed that 30 µg of IM IFN-β-1a once a week was less effective than 250 µg of SC IFN-β-1b every other day for disability progression. Findings on MS symptoms and HRQoL relied on one small trial with inconsistent effects and poor reporting. No studies reported mortality.
30 µg of interferon beta-1a intramuscularly once a week (Avonex) compared with 20 mg of glatiramer acetate subcutaneously once daily (Copaxone)
Two trials compared 30 µg of IM IFN-β-1a once a week against 20 mg of SC GA once daily. 188,191
Relapse outcomes
Findings for relapse outcomes relied on both trials, with substantial follow-up; one trial, the CombiRx trial,191 was considerably larger than the other. In the trial by Calabrese et al. ,188 patients receiving 30 µg of IM IFN-β-1a once a week (n = 47) did not appear to have a numerically different ARR [0.5 (SD 0.6) vs. 0.5 (SD 0.4)] after 2 years’ follow-up than patients receiving 20 mg of SC GA once daily (n = 48). A formal significance test was not reported, but we re-estimated the RR as 1.00 (95% CI 0.67 to 1.50). However, in the larger CombiRx trial,191 with 36 months’ follow-up, patients receiving 30 µg of IM IFN-β-1a once a week (n = 250) had a higher ARR than patients receiving 20 mg of SC GA once daily (0.16 vs. 0.11). This difference was tested using a Cox proportional hazards model with correction for repeated events, which found statistically significant evidence of a shorter time between relapses for 30 µg of IM IFN-β-1a once a week than for 20 mg of SC GA once daily (HR 1.43, 95% CI 1.04 to 1.95). This finding was robust to a sensitivity analysis including non-protocol-defined relapses.
However, the CombiRx trial191 did not find a significant difference between groups in time to first relapse (p = 0.19). Additional information was not reported. The CombiRx trial191 also did not find a significant difference between groups in the proportion with protocol-defined relapses at 36 months (74.0% vs. 79.5%; p = 0.14).
Relapse severity
We were unable to locate any relevant comparisons between 30 µg of IM IFN-β-1a once a week and 20 mg of SC GA once daily on outcomes relating to moderate or severe relapses or steroid-treated relapses.
Disability progression
The CombiRx trial191 reported the proportions of patients with EDSS progression at 6 months. Fewer patients receiving 30 µg of IM IFN-β-1a once a week progressed than patients receiving 20 mg of SC GA once daily (21.6% vs. 24.8%), but this difference was reported as not statistically significant.
In the trial by Calabrese et al. ,188 patients receiving 30 µg of IM IFN-β-1a once a week had a numerically lower increase in EDSS score at 2 years [0.2 (SD 0.4)] than patients receiving 20 mg of SC GA once daily [0.3 (SD 0.5)], but formal significance testing was not reported.
Freedom from disease activity
Only the CombiRx trial191 reported freedom from disease activity outcomes for this comparison. In this trial, the proportion with freedom from disease activity (defined as the absence of exacerbation, EDSS progression or combined unique lesion activity, that is, no new enhanced lesions, unenhanced T2 lesions or enlarged unenhanced T2 lesions) was not different between patients receiving 30 µg of IM IFN-β-1a once a week and patients receiving 20 mg of SC GA once daily (21.2% vs. 19.4%; p = 0.62). This finding was robust to the inclusion of non-protocol-defined exacerbations (17.1% vs. 16.1%; p = 0.762).
Multiple sclerosis symptoms and health-related quality of life
In the CombiRx trial,191 change from baseline to 36 months was measured for the Multiple Sclerosis Functional Composite and several of its components, but none of the differences between groups was statistically significant. Overall, the Multiple Sclerosis Functional Composite score improved slightly in both the 30 µg of IM IFN-β-1a once a week group [mean 0.1 (SD 0.5)] and the 20 mg of SC GA once-daily group [mean 0.2 (SD 0.5)]. Time in seconds to complete the timed 25-foot walk increased slightly in both groups [0.2 (SD 1.1) vs. 0.2 (SD 1.7)] but time in seconds to complete the nine-hole peg test decreased slightly [–0.4 (SD 3.8) vs. –0.1 (SD 4.1)]. In addition, both groups improved in terms of the number of questions answered correctly on the PASAT [3.5 (SD 8.1) vs. 4.3 (SD 7.4)].
Adverse events and mortality
Only the CombiRx trial191 reported AEs or mortality. Full results are available on request. One death occurred in each of the relevant arms of the CombiRx trial and thus differences were not significant.
Summary of the narrative synthesis: 30 µg of interferon beta-1a intramuscularly once a week (Avonex) compared with 20 mg of glatiramer acetate subcutaneously once daily (Copaxone)
Findings from the two studies were mixed with regard to relapse outcomes, but the results of the larger of the two trials suggested that 30 µg of IM IFN-β-1a once a week was less effective than 20 mg of SC GA once daily at reducing relapses. Findings for disability progression, combined clinical–MRI measures of freedom from disease activity and MS symptoms did not suggest a difference between the two drugs. We were unable to locate any evidence for relapse severity, defined as moderate or severe relapses or steroid-treated relapses. Mortality was rare and not different between treatments in the CombiRx trial. 191
44 µg and 22 µg of interferon beta-1a subcutaneously three times a week (Rebif) compared with placebo
Our analysis was informed by three trials comparing 44 µg of SC IFN-β-1a three times a week against no treatment. 183,189,207 The REMAIN trial183 used BSC alone as a comparator, whereas the other two trials used placebo. As noted earlier, the REMAIN trial is of limited interest but is included here for completeness. The PRISMS trial189 also compared 22 µg of SC IFN-β-1a three times a week against no treatment.
An additional six trials compared 44 µg of SC IFN-β-1a three times a week against other drugs: three multiarm trials185–188 and three two-arm trials. 192–195,197 Comparisons in the EVIDENCE trial193–195 were discussed earlier.
Relapse outcomes
Both key studies reported relapse outcomes. The PRISMS trial,189 which tested both doses of SC IFN-β-1a three times a week, followed up 560 patients (n = 184 in the 44-µg arm, n = 189 in the 22-µg arm, n = 187 in the placebo arm) over 2 years. Relative to placebo, both the 44-µg dose (RR 0.67, 95% CI 0.56 to 0.79) and the 22-µg dose (RR 0.73, 95% CI 0.61 to 0.86) reduced the rate of relapses. The IMPROVE (Investigating MRI Parameters with Rebif imprOVEd formulation) trial,207 a comparatively short trial that followed up 180 patients over 16 weeks (n = 120 in the 44-µg arm, n = 60 in the placebo arm), also showed a substantial decrease in the rate of relapses in those receiving the study drug (RR 0.43, 95% CI 0.23 to 0.82). Time to first relapse outcomes were cursorily presented in the PRISMS trial. 189 Both the 44-µg dose and the 22-µg dose delayed time to first relapse, by 5 months and 3 months respectively, although a significance test was not presented. However, (confidential information has been removed).
Finally, the PRISMS trial189 reported the proportions of patients who were free of relapse. Higher proportions of patients were relapse free in both treatment groups than in the placebo group at 2 years of follow-up: in the placebo arm 16% of patients were free of relapses, patients receiving 44 µg of SC IFN-β-1a three times a week had a 32% chance of being free of relapses (OR 2.57, 95% CI 1.56 to 4.25) and patients receiving 22 µg of SC IFN-β-1a three times a week had a 27% chance of being free of relapses (OR 2.01, 95% CI 1.21 to 3.35).
The REMAIN trial,183 which followed up 30 patients with either RRMS or SPMS for 96 weeks, did not find a significant difference between arms in time to first relapse or the proportion of patients relapse free.
Relapse severity
The PRISMS trial189 presented data for both moderate or severe relapses and steroid-treated relapses. Patients receiving placebo had, on average, more moderate or severe relapses over the course of the study (0.99) than patients receiving 44 µg of SC IFN-β-1a three times a week (0.62) or patients receiving 22 µg of SC IFN-β-1a three times a week (0.71). We re-estimated the corresponding RR for 44 µg of SC IFN-β-1a three times a week as 0.64 (95% CI 0.53 to 0.74) and for 22 µg of SC IFN-β-1a three times a week as 0.72 (95% CI 0.61 to 0.84). Correspondingly, patients receiving 44 µg of SC IFN-β-1a three times a week were more likely to be free of any moderate or severe relapses (OR 2.32, 95% CI 1.47 to 3.37). Findings were similar for the 22 µg of SC IFN-β-1a three times a week compared with placebo (OR 2.13, 95% CI 1.41 to 3.21).
The pattern of findings in the PRISMS trial189 for steroid treatment was similar. Patients receiving placebo had, on average, more courses of steroids for MS relapses over the course of the study (1.39) than patients receiving 44 µg (0.75) or 22 µg (0.97) of SC IFN-β-1a three times a week. We re-estimated the corresponding RR for 44 µg of SC IFN-β-1a three times a week compared with placebo as 0.54 (95% CI 0.46 to 0.63) and for 22 µg of SC IFN-β-1a three times a week compared with placebo as 0.70 (95% CI 0.61 to 0.80). Correspondingly, patients receiving 44 µg of SC IFN-β-1a three times a week were more likely to be free of any steroid-treated relapses (OR 1.99, 95% CI 1.32 to 3.02), as were patients receiving 22 µg of SC IFN-β-1a three times a week (OR 1.71, 95% CI 1.14 to 2.57).
Disability progression
In the PRISMS trial,189 time to disability progression confirmed at 3 months was slowed by both doses of SC IFN-β-1a three times a week compared with placebo. The 25th percentile of the distribution of time to progression was 21.3 months for patients receiving 44 µg of SC IFN-β-1a three times a week and 18.5 months for patients receiving 22 µg of SC IFN-β-1a three times a week, compared with 11.9 months for patients receiving placebo. Corresponding HRs showed evidence of a statistically significant delay in progression (44 µg: HR 0.62, 95% CI 0.43 to 0.91; 22 µg: HR 0.68, 95% CI 0.48 to 0.98).
Both the PRISMS trial189 and the IMPROVE trial207 reported the magnitude of change in EDSS score. In the PRISMS trial,189 compared with placebo, both 44 µg and 22 µg of SC IFN-β-1a three times a week resulted in a smaller increase in EDSS score, with a difference of 0.25 EDSS points (both p < 0.05). The IMPROVE trial207 did not report a standard significance test, although the median change in EDSS score in both the 44-µg arm and the placebo arm was 0.
In the REMAIN trial,183 the magnitude of change in EDSS score, time to progression and proportions of patients with progression were not significantly different between arms.
Freedom from disease activity
We were unable to locate any relevant comparisons between 44 µg or 22 µg of SC IFN-β-1a three times a week and placebo for the combined clinical–MRI measures of freedom from disease activity.
Multiple sclerosis symptoms and health-related quality of life
The PRISMS trial reported the effects of 44 µg and 22 µg of SC IFN-β-1a three times a week on various MS symptoms. 189,208 As noted in the original trial report,189 patients receiving the 44-µg dose were less likely to have a sustained worsening in ambulation than those receiving placebo (7% vs. 13%; p < 0.05); however, the corresponding value for those receiving the 22-µg dose (12%) was not significantly different from that for placebo. Subsequently, Gold et al. 231 reported that, although patients in all three groups increased their scores from baseline on the Center for Epidemiologic Studies Depression Rating Scale, these changes were not significantly different between the groups (44 µg: 0.2, 22 µg: 1.8, placebo: 0.9; p = 0.60). Similarly, the risk of exceeding the cut-off score for depression on this scale was not significantly different between either the 44-µg arm (RR 0.7, 95% CI 0.3 to 1.6) or the 22-µg arm (RR 0.8, 95% CI 0.3 to 1.8) and the placebo arm and the proportions of patients exceeding the cut-off on the Beck Hopelessness Scale were not significantly different between the placebo arm (6.9%) and either the 44-µg arm (6.9%; p = 1.0) or the 22-µg arm (10.5%; p = 0.55). Finally, data were not presented numerically, but it was reported that there was no difference between the groups in scores on the General Health Questionnaire or its subscales.
Adverse events and mortality
All studies presented AEs. Full results are available on request. None of the studies reported deaths related to the study drugs.
Summary of the narrative synthesis: 44 µg and 22 µg of interferon beta-1a subcutaneously three times a week (Rebif) compared with placebo
Findings from two trials suggested a beneficial effect of 44 µg of SC IFN-β-1a three times a week compared with placebo for relapse outcomes. Additionally, findings from the PRISMS trial189 suggested a beneficial effect of 44 µg of SC IFN-β-1a three times a week on relapse severity (both moderate/severe relapses and steroid-treated relapses) and on delaying disability progression. Findings from the PRISMS trial189,208 also suggested a beneficial effect of 44 µg of SC IFN-β-1a three times a week on ambulation, but not mental health. Findings for the 22-µg dose in the PRISMS trial189,208 were similar except for ambulation. Mortality was not reported.
44 µg of interferon beta-1a subcutaneously three times a week (Rebif) compared with 250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia)
Three trials compared 44 µg of SC IFN-β-1a three times a week against 250 µg of SC IFN-β-1b every other day. 185–187,197 An additional trial182 compared these drugs with regard to AEs.
Relapse outcomes
Assessment of relapse outcomes for this comparison relied on two small studies with very different follow-up times. In the study by Etemadifar et al. ,185 over 2 years of follow-up, patients receiving 44 µg of SC IFN-β-1a three times a week had 66 relapses whereas patients receiving 250 µg of SC IFN-β-1b every other day had 65 relapses (n = 30 in both groups). We estimated this as a RR of 1.02 (95% CI 0.72 to 1.43). In the REFORMS trial,197 patients receiving 44 µg of SC IFN-β-1a three times a week had an ARR of 0.15 whereas patients receiving 250 µg of SC IFN-β-1b every other day had an ARR of 0.11. This difference was statistically significant (p < 0.001), although this was a relatively small trial (n = 129), patients were followed up for only 12 weeks and patient relapses were self-reported rather than assessed by a neurologist.
In the study by Etemadifar et al. ,185 the proportion of patients without relapses at 2 years was numerically higher in the group receiving 44 µg of SC IFN-β-1a three times a week than in the group receiving 250 µg of SC IFN-β-1b every other day (56.7% vs. 43.3%), but no pairwise significance testing was performed.
Relapse severity
We were unable to find any comparisons between 44 µg of SC IFN-β-1a three times a week and 250 µg of SC IFN-β-1b every other day for outcomes relating to moderate or severe relapses or steroid-treated relapses.
Disability progression
Analysis of disability progression in both trials was by magnitude of EDSS score change, although both trials reported inadequate details of the analysis. In the study by Etemadifar et al. ,185 patients receiving 44 µg of SC IFN-β-1a three times a week had a decrease in EDSS score of 0.3 (95% CI 0.03 to 0.5), whereas patients receiving 250 µg of SC IFN-β-1b every other day had a decrease in EDSS score of 0.7 (95% CI 0.5 to 0.9). A pairwise significance test was not performed. Patients in the study by Mokhber et al. 186,187 in both treatment groups also showed a decrease in EDSS score, but in the opposite direction (44 µg: –1.0, p = 0.001; 250 µg: –0.6, p = 0.028). Again, a pairwise significance test was not performed.
Freedom from disease activity
We were unable to find any comparisons between 44 µg of SC IFN-β-1a three times a week and 250 µg of SC IFN-β-1b every other day for combined clinical–MRI measures of freedom from disease activity.
Multiple sclerosis symptoms and health-related quality of life
As noted previously, analyses in the study by Mokhber et al. 186 for cognitive function found no significant differences between the groups, except for the symbol digit modalities test. Post hoc analyses indicated that patients receiving 44 µg of SC IFN-β-1a three times a week improved more than those receiving 250 µg of SC IFN-β-1b every other day on the symbol digit modalities test and the PASAT-easy.
Across the QoL domains tested in the study by Mokhber et al. ,187 44 µg of SC IFN-β-1a three times a week was not significantly different from 250 µg of SC IFN-β-1b every other day except for overall mental health aspects of HRQoL, with patients receiving 250 µg of SC IFN-β-1b every other day improving significantly more.
Adverse events and mortality
Adverse events were reported only in the AVANTAGE trial182 and the REFORMS trial. 197 Only the AVANTAGE trial182 reported mortality, with no events occurring in either study arm. Full results are available on request.
Summary of the narrative synthesis: 44 µg of interferon beta-1a subcutaneously three times a week (Rebif) compared with 250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia)
Findings were derived from three small trials and should thus be treated with caution. The two trials reporting relapse outcomes disagreed on the comparative effectiveness of these two drugs, although there was some evidence from the REFORMS trial197 that patients receiving 44 µg of SC IFN-β-1a three times a week had a higher ARR. Findings for disability progression, MS symptoms and HRQoL were inconsistent and poorly reported. We were unable to find comparisons for relapse severity or combined clinical–MRI measures of freedom from disease activity. No deaths were reported.
44 µg of interferon beta-1a subcutaneously three times a week (Rebif) compared with 20 mg of glatiramer acetate subcutaneously once daily (Copaxone)
Two trials compared 44 µg of SC IFN-β-1a three times a week against 20 mg of SC GA once daily. 188,192
Relapse outcomes
In the study by Calabrese et al. ,188 patients receiving 44 µg of SC IFN-β-1a three times a week had a numerically lower ARR than patients receiving 20 mg of SC GA once daily after 2 years of follow-up [0.4 (SD 0.6) vs. 0.5 (SD 0.4)], but formal significance testing was not reported and relapses were analysed using a normal distribution. We re-estimated this as a RR of 0.80 (95% CI 0.52 to 1.23). In the larger REGARD trial,192 764 patients were followed up for 96 weeks. The ARR was not significantly different between patients receiving 44 µg of SC IFN-β-1a three times a week and patients receiving 20 mg of SC GA once daily (0.30 vs. 0.29; p = 0.828).
The REGARD trial192 did not find a significant difference in time to first relapse between patients receiving 44 µg of SC IFN-β-1a three times a week and those receiving 20 mg of SC GA once daily (HR 0.94, 95% CI 0.74 to 1.21); there was also no significant difference between the groups in the proportion of patients who were free of relapses at 96 weeks (62% vs. 62%; p = 0.96).
Relapse severity
In the REGARD trial,192 the ARR for steroid-treated relapses was not significantly different between patients receiving 44 µg of SC IFN-β-1a three times a week and those receiving 20 mg of SC GA once daily (0.19 vs. 0.17; p = 0.386).
Disability progression
The REGARD trial192 reported the proportions of patients with disability progression confirmed at 6 months. The proportions were not significantly different between patients receiving 44 µg of SC IFN-β-1a three times a week (11.7%) and those receiving 20 mg of SC GA once daily (8.7%) (p = 0.117).
In the study by Calabrese et al. ,188 patients receiving 44 µg of SC IFN-β-1a three times a week had a numerically lower increase in EDSS score at 2 years [0.2 (SD 0.5)] than patients receiving 20 mg of SC GA once daily [0.3 (SD 0.5)], but formal significance testing was not reported.
Freedom from disease activity
We were unable to locate any comparisons between 44 µg of SC IFN-β-1a three times a week and 20 mg of SC GA once daily for combined clinical–MRI measures of freedom from disease activity.
Multiple sclerosis symptoms and health-related quality of life
We were unable to locate any comparisons between 44 µg of SC IFN-β-1a three times a week and 20 mg of SC GA once daily for MS symptoms or HRQoL.
Adverse events and mortality
Adverse events and mortality were reported in the REGARD trial. 192 Only one death occurred, in the IFN arm, and thus mortality was not significantly different between groups. Full results are available on request.
Summary of the narrative synthesis: 44 µg of interferon beta-1a subcutaneously three times a week (Rebif) compared with 20 mg of glatiramer acetate subcutaneously once daily (Copaxone)
Findings from two trials did not suggest a difference between the two drugs for relapse outcomes, relapse severity or disability progression. We could not locate comparisons relating to combined clinical–MRI measures of freedom from disease activity or MS symptoms or HRQoL. There was no difference in mortality between the groups.
250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia) compared with placebo
We included two trials comparing 250 µg of SC IFN-β-1b every other day with placebo. 209–211 Schwartz et al. 181 examined QoL outcomes only and used BSC as the comparator instead of placebo.
An additional six trials compared 250 µg of SC IFN-β-1b every other day against other drugs: two multiarm trials185–187 and four two-arm trials. 184,190,196,197 Comparisons from the trials by Etemadifar et al. 185 and Mokhber et al. 186,187 and the INCOMIN trial196 and REFORMS trial197 have been discussed in previous sections.
Relapse outcomes
Both studies reporting ARRs suggested a beneficial effect of 250 µg of SC IFN-β-1b every other day, although only the trial by the IFNB Multiple Sclerosis Study Group (IFNB MSSG)209,210 may have been powered to detect a difference between the groups. In the IFNB MSSG trial,209,210 247 patients in the relevant arms were followed up for variable amounts of time, with the initial 2-year study phase continuing into a blinded extension; thus, some patients were followed for up to 5.5 years, with a median follow-up of 46.0 months in the placebo arm and 48.0 months in the relevant study drug arm. At the end of the study, patients receiving 250 µg of SC IFN-β-1b every other day had a lower ARR than patients receiving placebo (0.78, 95% CI 0.70 to 0.88 vs. 1.12, 95% CI 1.02 to 1.23; p = 0.0006). In a comparatively small trial, Knobler et al. 211 followed up 30 patients over 3 years, including a 6-month dose-finding period at the start of the study. The 24 patients receiving 250 µg of SC IFN-β-1b every other day had an ARR of 0.7 whereas the six patients receiving placebo had an ARR of 0.9. This difference was not significant (p = 0.33).
Both studies also reported information on time to first relapse. Knobler et al. 211 reported that the median time to first relapse was delayed, but not significantly so, in patients receiving 250 µg of SC IFN-β-1b every other day compared with patients receiving placebo (14 months vs. 2 months; log-rank p = 0.07). The comparatively larger IFNB MSSG trial209 reported a similar finding at the 3-year follow-up, albeit of a smaller magnitude and rising to statistical significance. The median time to first exacerbation was delayed in patients receiving 250 µg of SC IFN-β-1b every other day compared with placebo (264 days vs. 147 days; log-rank p = 0.028).
The proportions of patients who were free of relapse were also available only at the 3-year follow-up for the IFNB MSSG trial. 209 The proportions free of relapse were not significantly different between the groups (250 µg of SC IFN-β-1b every other day 21.8% vs. placebo 13.8%; p = 0.097). Three-year results from the study by Knobler et al. 211 showed a similar trend (42% vs. 17%), with these findings also not significant (p = 0.37).
Relapse severity
Relapse severity was reported based on both 2-year and final data from the IFNB MSSG trial,209,210 but only the 2-year data were usable. At 2 years of follow-up, patients receiving 250 µg of SC IFN-β-1b every other day had a lower ARR for moderate or severe relapses than those receiving placebo (0.23 vs. 0.45; p = 0.002). Similar findings based on final data were reported, but only a p-value (p = 0.012) for a relationship in the same direction was provided. Knobler et al. 211 did not find a significant relationship for ‘attack severity’, although the findings were reported only as a non-significant p-value (p = 0.67) and relapse severity was not defined.
Disability progression
The IFNB MSSG trial210 reported that 250 µg of SC IFN-β-1b every other day delayed disability progression confirmed at 3 months, but not significantly so, with a median time to progression of 4.79 years, compared with 4.18 years in the placebo group (log-rank p = 0.096). The proportions of patients with confirmed progression showed a similar trend (35% vs. 46%). We re-estimated this as a HR of 0.71 (95% CI 0.48 to 1.06). Knobler et al. 211 examined change in EDSS score from baseline between the groups but noted only that the difference between the groups was not statistically significant (p = 0.42).
Freedom from disease activity
We were unable to locate any relevant comparisons between 250 µg of SC IFN-β-1b every other day and placebo for combined clinical–MRI measures of freedom from disease activity.
Multiple sclerosis symptoms and health-related quality of life
In the study by Schwartz et al. ,181 34 patients receiving 250 µg of SC IFN-β-1b every other day were compared with 45 patients receiving BSC. Over the course of a year, there was no difference between the groups in quality-adjusted time without symptoms and toxicity, measured in months (10.6 vs. 10.4; p = 0.50).
Adverse events and mortality
Adverse events were reported in the IFNB MSSG trial210 and the study by Knobler et al. 211 None of the studies reported mortality. Full results are available on request.
Summary of the narrative synthesis: 250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia) compared with placebo
Findings from two studies suggested a beneficial effect of 250 µg of SC IFN-β-1b every other day on relapse outcomes compared with placebo (although not on the proportions of patients who were relapse free). Findings from the IFNB MSSG trial209,210 suggested a reduction in the rate of moderate or severe relapses in the group receiving 250 µg of SC IFN-β-1b every other day, but findings from the study by Knobler et al. 211 were uninterpretable. Neither study found evidence of a delay in time to disability progression. One small study comparing 250 µg of SC IFN-β-1b every other day against BSC did not find differences in HRQoL over a year. We were unable to find any comparisons for combined clinical–MRI measures of freedom from disease activity. None of the studies reported mortality.
250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia) compared with 20 mg of glatiramer acetate subcutaneously once daily (Copaxone)
Two trials compared 250 µg of SC IFN-β-1b every other day against 20 mg of SC GA once daily. 184,190
Relapse outcomes
Both the BECOME trial184 and the larger BEYOND (Betaferon Efficacy Yielding Outcomes of a New Dose) trial190 reported ARRs. In the BECOME trial,184 75 patients were followed up for up to 2 years. Patients receiving 250 µg of SC IFN-β-1b every other day did not have a significantly different ARR from patients receiving 20 mg of SC GA once daily (0.37 vs. 0.33; p = 0.68). Findings from the BEYOND trial,190 in which 1345 patients from the relevant trial arms were followed up for at least 2 and up to 3.5 years, suggested a similar trend (ARR 0.36 vs. 0.34; one-tailed p = 0.79). This was expressed using a Cox proportional hazards model with modification for repeated events (HR 1.06, 95% CI 0.89 to 1.26).
Time to first relapse was also not significantly different between arms in either study. In the BECOME trial,184 of patients who had relapses, the median time to first relapse for those receiving 250 µg of SC IFN-β-1b every other day (123 days) was not very different from the median time to first relapse for those receiving 20 mg of SC GA once daily (121 days), with a non-significant log-rank test on the whole sample (p = 0.12). In the BEYOND trial,190 there was no substantial difference in days to first relapse for patients at the 25th percentile (250 µg of SC IFN-β-1b every other day 283 days vs. 20 mg of SC GA once daily 271 days; one-sided log-rank p = 0.75). This was supported by the proportions of patients who were relapse free at 2 years, estimated from a Kaplan–Meier model, which were very similar (59% vs. 58%).
Finally, only the BECOME trial184 reported the empirical proportions of patients relapsing. Fewer patients receiving 250 µg of SC IFN-β-1b every other day were relapse free than patients receiving 20 mg of SC GA once daily, but this difference was not significant (53% vs. 72%; p = 0.10).
Relapse severity
Only the BEYOND trial190 reported ARRs for severity of relapse. ARRs for major relapse were not significantly different between patients receiving 250 µg of SC IFN-β-1b every other day and those receiving 20 mg of SC GA once daily (0.19 vs. 0.18; one-sided p = 0.36). Time to first major relapse was also not significantly different between the arms, with both arms having proportions with a relapse at 2 years of 27%, as predicted by a Kaplan–Meier model (log-rank p = 0.56).
Both studies reported the empirical proportions of patients receiving steroid treatment for MS. In the BECOME trial,184 more patients receiving 250 µg of SC IFN-β-1b every other day (44%) required steroid treatment for relapses than patients receiving 20 mg of SC GA once daily (23%), but this difference was only of marginal significance (p = 0.09). In contrast, the proportions of patients requiring steroid treatment for relapses were not significantly different in the BEYOND trial190 (34% vs. 32%; p = 0.43).
Disability progression
The BEYOND trial190 reported time to disability progression confirmed at 3 months. Because median time to progression was not reached, the time to progression at the 10th percentile was reported. The 10th percentile of patients receiving 250 µg of SC IFN-β-1b every other day progressed after 274 days, whereas patients receiving 20 mg of SC GA once daily progressed after 268 days (log-rank p = 0.35). Alternative estimates were provided based on Kaplan–Meier models, in which the probability of progression at the end of 2 years was 21% in those receiving 250 µg of SC IFN-β-1b every other day and 20% in those receiving 20 mg of SC GA once daily (log-rank p = 0.68). We estimated a HR of 1.06 (95% CI 0.81 to 1.37) from these statistics.
In a separate publication to the main trial report, the BECOME trial212 reported time to disability progression confirmed at 6 months. The empirical proportions of patients progressing in each arm were dissimilar (250 µg of SC IFN-β-1b every other day 12.1% vs. 20 mg of SC GA once daily 17.6%), but the log-rank test was non-significant (p = 0.51). Based on these statistics, we estimated a HR of 0.66 (95% CI 0.19 to 2.28). The BECOME trial212 also reported progression based on the Multiple Sclerosis Functional Composite, in which an increase of 0.2 SDs confirmed at 6 months constitutes evidence of progression. The same trend was apparent (5.7% vs. 10.3%, log-rank p = 0.39).
Freedom from disease activity
We were unable to locate any relevant comparisons between 250 µg of SC IFN-β-1b every other day and 20 mg of SC GA once daily for combined clinical–MRI measures of freedom from disease activity.
Multiple sclerosis symptoms and health-related quality of life
We were unable to locate any relevant comparisons between 250 µg of SC IFN-β-1b every other day and 20 mg of SC GA once daily for MS symptoms or HRQoL. However, the BECOME trial212 did present results for the Multiple Sclerosis Functional Composite, discussed earlier in Disability progression.
Adverse events and mortality
Both studies reported AEs, but only the BEYOND trial190 reported mortality. The difference between the groups was not significant for mortality, although only one death occurred, in the GA arm. Full results are available on request.
Summary of the narrative synthesis: 250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia) compared with 20 mg of glatiramer acetate subcutaneously once daily (Copaxone)
Findings from two trials – one small and one large – did not suggest a difference between the two drugs in terms of relapse outcomes, relapse severity or disability progression. We were unable to locate any comparisons for combined clinical–MRI measures of freedom from disease activity. The differences between the groups was not significant for mortality.
125 µg of pegylated interferon beta-1a subcutaneously every 2 weeks (Plegridy) compared with placebo
We included one trial comparing 125 µg of SC pegylated IFN-β-1a every 2 weeks against placebo. 213 We were unable to locate any trials including comparisons between 125 µg of SC pegylated IFN-β-1a every 2 weeks and other drugs. In its placebo-controlled phase, the ADVANCE trial213 compared 125 µg of SC pegylated IFN-β-1a every 2 weeks and every 4 weeks with placebo for 48 weeks. In total, 1012 patients in the relevant arms were analysed.
Relapse outcomes
Participants receiving 125 µg of SC pegylated IFN-β-1a every 2 weeks showed a decrease in ARR (RR 0.644, 95% CI 0.500 to 0.831). 213 Time to first relapse was also delayed in patients receiving the active drug (HR 0.61, 95% CI 0.47 to 0.80).
Relapse severity
Publications arising from this study did not report relapse severity.
Disability progression
Participants receiving 125 µg of SC pegylated IFN-β-1a every 2 weeks experienced a delay in time to disability progression confirmed at 3 months (HR 0.62, 95% CI 0.40 to 0.97). 213 As reported in the Summary of Product Characteristics filed by the European Medicines Agency,234 the time to disability progression confirmed at 6 months was longer in patients receiving the study drug than in patients receiving placebo (HR 0.46, 95% CI 0.26 to 0.81).
Freedom from disease activity
In the ADVANCE trial, measures of freedom from disease activity included mixed clinical and MRI, clinical-only and MRI-only definitions and were reported in a separate publication214 to the main study report. As stated in the methods, we report here the mixed clinical and MRI definition, which included both the absence of relapses and the absence of onset of disability progression confirmed at 3 months, as well as no gadolinium-enhancing lesions and no new or newly enlarging T2 hyperintense lesions. Between baseline and week 48 of the trial, 33.9% of patients (n = 466 in this analysis) receiving the study drug had no evidence of disease activity, whereas 15.1% of patients (n = 484 in this analysis) receiving placebo had no evidence of disease activity (OR 2.89, 95% CI 2.11 to 3.95). This finding was robust to sensitivity analysis of data missingness.
Multiple sclerosis symptoms and health-related quality of life
In the ADVANCE trial,215 patients receiving 125 µg of SC pegylated IFN-β-1a every 2 weeks did not significantly worsen over 48 weeks on the Multiple Sclerosis Impact Scale (MSIS-29) physical subscale (mean change 0.08, 95% CI –1.10 to 1.27), whereas placebo patients did (mean change 1.24, 95% CI 0.05 to 2.44). Both groups improved on the MSIS-29 psychological subscale, with no statistically significant difference between the groups (pegylated IFN-β-1a: –2.06, 95% CI –3.58 to –0.53; placebo: –2.17, 95% CI –3.63 to –0.70). Participants also completed the Short Form questionnaire-12 items (both the physical component summary and the mental component summary), EQ-5D and EQ-5D visual analogue scale. None of the differences between groups or within groups was statistically significant (the authors did not present specific data), but patients receiving 125 µg of SC pegylated IFN-β-1a every 2 weeks did show a significant improvement on the visual analogue scale (mean change 2.06, 95% CI 0.58 to 3.54).
Adverse events and mortality
The ADVANCE trial213 reported AEs and mortality. Full results are available on request. Differences between groups for mortality were not significant, with one event occurring in the study drug arm and two events occurring in the placebo arm.
Summary of the narrative synthesis: 125 µg of pegylated interferon beta-1a subcutaneously every 2 weeks (Plegridy) compared with placebo
Findings from one study included in this comparison suggested a beneficial effect of 125 µg of SC pegylated IFN-β-1a every 2 weeks compared with placebo for relapse outcomes, disability progression and freedom from disease activity. For HRQoL measures, 125 µg of SC pegylated IFN-β-1a every 2 weeks was not different from placebo. Relapse severity outcomes were not reported. Groups were not significantly different with respect to mortality.
20 mg of glatiramer acetate subcutaneously once daily and 40 mg of glatiramer acetate subcutaneously three times a week (Copaxone) compared with placebo
We included five trials comparing 20 mg of SC GA once daily against placebo. 170,216–220 One trial221 tested 40 mg of SC GA three times a week against placebo.
Additionally, one multiarm trial188 and four two-arm trials184,190–192 compared 20 mg of SC GA once daily against other drugs. These comparisons have been discussed in the previous sections.
Relapse outcomes
All five trials comparing 20 mg of SC GA once daily against placebo reported relapse rates, as did the trial comparing 40 mg of SC GA three times a week against placebo. The study by Bornstein et al. 170 followed up 48 patients over 2 years. There were 16 relapses over 2 years in the 25 patients receiving 20 mg of SC GA once daily and 62 relapses in the 23 patients receiving placebo, which gives an estimated RR of 0.25 (95% CI 0.14 to 0.43). In another early trial by the Copolymer 1 Multiple Sclerosis Study Group (Cop1 MSSG),217,218 251 patients were followed up over at least 2 years, with an extension of up to 11 months. At 2 years, the ARR in patients receiving 20 mg of SC GA once daily was 0.59, whereas the ARR in patients receiving placebo was 0.84. 217 This difference was statistically significant (p = 0.007). Subsequent studies found similar reductions in ARR in the treatment arms. In a trial by the European/Canadian Glatiramer Acetate Study Group (ECGASG),219 which followed up 239 patients over 9 months, the ARR in the study drug group was 0.81 whereas the ARR in the placebo group was 1.21 (RR 0.67; p = 0.012). The CONFIRM (Comparator and an Oral Fumarate in Relapsing–Remitting Multiple Sclerosis) trial216 followed up 713 patients in the relevant study arms for 2 years and also found a significant difference in ARRs between the groups (20 mg of SC GA once daily 0.29 vs. placebo 0.40; RR 0.71, 95% CI 0.55 to 0.93). However, in a trial following up 357 patients receiving branded GA and 84 patients receiving placebo for 9 months,220 the ARRs were not substantially different between groups (20 mg of SC GA once daily 0.40, 95% CI 0.26 to 0.62 vs. placebo 0.38, 95% CI 0.22 to 0.66), although a standard significance test was not presented. The GALA (Glatiramer Acetate Low-Frequency Administration) trial221 compared 40 mg of SC GA three times a week against placebo (40 mg of SC GA three times a week, n = 943 vs. placebo, n = 461) over 12 months. Patients receiving the study drug had a significantly lower ARR than patients receiving placebo (40 mg of SC GA three times a week 0.33, 95% CI 0.28 to 0.39 vs. placebo 0.51, 95% CI 0.42 to 0.61), with an associated significant RR (0.66, 95% CI 0.54 to 0.80).
Two studies reported time to relapse. Including the extension phase, patients receiving 20 mg of SC GA once daily in the Cop1 MSSG trial218 had a delayed time to first relapse compared with patients receiving placebo, but this difference was not significant (median days to first relapse 287 vs. 198; p = 0.057). However, in the larger CONFIRM trial,216 patients receiving 20 mg of SC GA once daily did have a significant delay in time to relapse (HR 0.71, 95% CI 0.55 to 0.92). In the GALA trial,221 patients receiving 40 mg of SC GA three times a week also had a longer median time to first relapse (393 days vs. 377 days), with a HR of 0.61 (95% CI 0.49 to 0.74).
Finally, greater empirical proportions of patients receiving 20 mg of SC GA once daily tended to be free of relapse than patients receiving placebo, but this trend was not completely consistent. In the study by Bornstein et al. ,170 56% of patients receiving the study drug were relapse free at 2 years compared with 26% of patients receiving placebo (adjusted OR 4.6; p = 0.036). Similarly, the Cop1 MSSG trial218 found that, over the whole trial, patients receiving the study drug were more likely to be free of relapses than those receiving placebo (33.6% vs. 24.6%; p = 0.002). In the ECGASG trial219 this trend did not rise to significance (55.5% vs. 49.2%; OR 1.47, 95% CI 0.84 to 2.56) and in the GATE (Glatiramer Acetate Clinical Trial to Assess Equivalence with Copaxone) trial220 the proportions who were relapse free were not substantially different between the groups (73.9% vs. 73.8%), although a significance test was not provided. In the GALA trial,221 patients receiving 40 mg of SC GA three times a week were more likely to be free of relapses than patients receiving placebo (77.0% vs. 65.5%; OR 1.93, 95% CI 1.49 to 2.49).
Relapse severity
In the ECGASG trial,219 patients receiving 20 mg of SC GA once daily had fewer steroid-treated relapses than those receiving placebo (54 vs. 84). We estimated this as a RR for steroid-treated relapses of 0.65 (95% CI 0.46 to 0.91). The proportion of patients with steroid-treated relapses was correspondingly lower in the study drug arm (33.6% vs. 39.2%), but this was not tested for significance. In the GALA trial,221 patients receiving 40 mg of SC GA three times weekly had a lower ARR (0.30, 95% CI 0.25 to 0.36) for ‘severe’ relapses, defined as steroid-treated or hospitalised relapses, than patients receiving placebo (ARR 0.47, 95% CI 0.38 to 0.57). This translated into a RR of 0.64 (95% CI 0.53 to 0.79).
Disability progression
Three studies170,216,218 presented data on time to disability progression confirmed at 3 months, whereas only the CONFIRM trial216 presented data on time to progression confirmed at 6 months. Studies suggested a beneficial, but generally not significant, impact of 20 mg of SC GA once daily on confirmed disability progression. In the study by Bornstein et al. ,170 the median time to progression confirmed at 3 months was not reached for patients receiving 20 mg of SC GA once daily, but was 18 months for patients receiving placebo. This difference was significant (log-rank p = 0.05). Together with the proportions of patients with progression of 20% in the study drug arm and 48% in the placebo arm, we estimated the HR of progression as 0.37 (95% CI 0.14 to 1.00). In the Cop1 MSSG trial,218 the probability of non-progression was 76.8% in the 20 mg of SC GA once-daily arm and 70.6% in the placebo arm. Using the value from a related significance test (p = 0.199), we estimated the HR as 0.76 (95% CI 0.50 to 1.16). Finally, the CONFIRM trial216 did not find that 20 mg of SC GA once daily slowed time to progression confirmed at 3 months (HR 0.93, 95% CI 0.63 to 1.37). This finding was similar when disability progression was confirmed at 6 months (HR 0.87, 95% CI 0.55 to 1.38).
Only two studies presented data on the proportions of patients with confirmed disability progression in comparisons between 20 mg of SC GA once daily and placebo. As noted above, in the study by Bornstein et al. ,170 20% of patients receiving 20 mg of SC GA once daily progressed over 2 years, whereas 48% of patients receiving placebo progressed. In univariate analyses, this finding was not significant (p = 0.064), but multivariate analyses found a significant effect on the probability of progression (p = 0.033). In the Cop1 MSSG trial,218 the proportion with progression confirmed at 3 months was 23.2% in patients receiving 20 mg of SC GA once daily and 29.4% in patients receiving placebo over the whole trial. In the GALA trial,221 which compared 40 mg of SC GA three times weekly against placebo, 95.5% of patients receiving the study drug were free of confirmed progression compared with 96.3% of patients receiving placebo, but a formal significance test was not presented.
Finally, the magnitude of change in EDSS score was reported by most studies, with changes small across studies. In the study by Bornstein et al. ,170 the findings were presented as the proportions improving or worsening by magnitude of improvement/worsening. We estimated that patients receiving 20 mg of SC GA once daily improved by 0.12 EDSS points and patients receiving placebo worsened by 0.74 EDSS points, with a significant difference between groups (p < 0.05). In the Cop1 MSSG trial,218 patients receiving 20 mg of SC GA once daily did not show a significant improvement in EDSS score (–0.11, 95% CI –0.31 to 0.10) whereas patients receiving placebo showed a significant worsening (0.34, 95% CI 0.13 to 0.54). This difference was statistically significant (p = 0.006). In the ECGASC trial,219 mean EDSS change from baseline was not significantly different between groups (20 mg of SC GA once daily 0.02 vs. placebo 0.05), but a p-value or CIs were not presented. In the GATE trial,220 neither patients receiving the study drug (–0.08, 95% CI –0.19 to 0.03) nor patients receiving placebo (–0.02, 95% CI –0.17 to 0.14) showed a significant improvement in EDSS score. Change in the GALA trial221 was also negligible [40 mg of SC GA three times weekly 0.0 (SD 0.6) vs. placebo 0.1 (SD 0.6)].
Freedom from disease activity
The GATE trial220 was the only study that reported combined clinical–MRI findings for freedom from disease activity. The proportion free from disease activity was slightly greater in the arm receiving 20 mg of SC GA once daily than in the placebo arm (9.2% vs. 7.1%), with similar findings once proportions were adjusted for stratification variables (8.5% vs. 6.6%). A formal significance test was not presented.
Multiple sclerosis symptoms and health-related quality of life
The CONFIRM trial230 presented data for HRQoL disaggregated by subscale of the Short Form questionnaire-36 items (SF-36). Compared with placebo, which showed a negative trend, change from baseline in the 20 mg of SC GA once-daily group was positive for the whole scale and the two groups were significantly different on the physical component summary (p = 0.0259). However, the groups were not significantly different on the mental component summary. The group receiving 20 mg of SC GA once daily significantly improved (p < 0.05) compared with the group receiving placebo with regard to physical functioning (0.3 vs. –2.2), bodily pain (2.3 vs. –1.3) and general health (1.9 vs. –0.6), but not physical (0.3 vs. –2.2) or emotional (1.4 vs. –3.3) aspects of role limitation, vitality (1.1 vs. 0.4), social functioning (–0.6 vs. –0.1) or mental health (0.3 vs. 0.6). Changes in EQ-5D scores were not presented, but scores were stated to be stable in all groups over the course of the study. Compared with patients receiving placebo, patients receiving 20 mg of SC GA once daily were not more likely to have been stable or improved in either the physical component (OR 1.24, 95% CI 0.83 to 1.85) or the mental component (1.22, 95% CI 0.82 to 1.83) of the SF-36.
In the Cop1 MSSG trial,217 at 2 years the mean ambulation index scores were similar between patients receiving 20 mg of SC GA once daily (0.27) and patients receiving placebo (0.28).
Adverse events and mortality
We stratified comparisons by type of placebo. All studies reported AEs but only the GALA,221 GATE220 and CONFIRM216 trials reported deaths. Only one death occurred in trials with matched placebos, in the placebo arm of the GALA trial;221 in the CONFIRM trial,216 one death occurred in each arm. Full results are available on request.
Summary of the narrative synthesis: 20 mg of glatiramer acetate subcutaneously once daily and 40 mg of glatiramer acetate subcutaneously three times a week (Copaxone) compared with placebo
Taken together, findings from the five trials testing 20 mg of SC GA once daily and the one trial testing 40 mg of SC GA three times a week suggested a beneficial effect on relapse outcomes. Both trials (20 mg of GA;219 40 mg of GA221) reporting relapse severity outcomes also found that the study drug decreased the rate of steroid-treated relapses. Findings for disability progression were less convincing and studies generally did not present significant results. Only one study presented combined clinical–MRI measures of freedom from disease activity and this study did not show a large difference between groups, although significance testing was not undertaken. One study showed some effects of 20 mg of SC GA once daily on HRQoL measures. Groups were not significantly different with regard to mortality.
Meta-analyses: relapse rate
Pairwise meta-analyses
Direct evidence from comparisons against placebo is shown in Figure 5. All drugs had a statistically significant beneficial effect on relapse rate compared with placebo. Findings for 125 µg of SC pegylated IFN-β-1a every 2 weeks, 40 mg of SC GA three times weekly and 22 µg of SC IFN-β-1a three times weekly all relied on one study. Comparisons that relied on multiple studies were diverse in terms of heterogeneity: heterogeneity ranged from an I2 of 0% (250 µg of SC IFN-β-1b every other day, 30 µg of IM IFN-β-1a once a week) to an I2 of 43% (44 µg of SC IFN-β-1a three times weekly) and 73% (20 mg of SC GA once daily). However, there were too few studies in each comparison to enable exploration of heterogeneity.
FIGURE 5.
Pairwise meta-analyses: ARRs for active drug vs. placebo trials in RRMS.
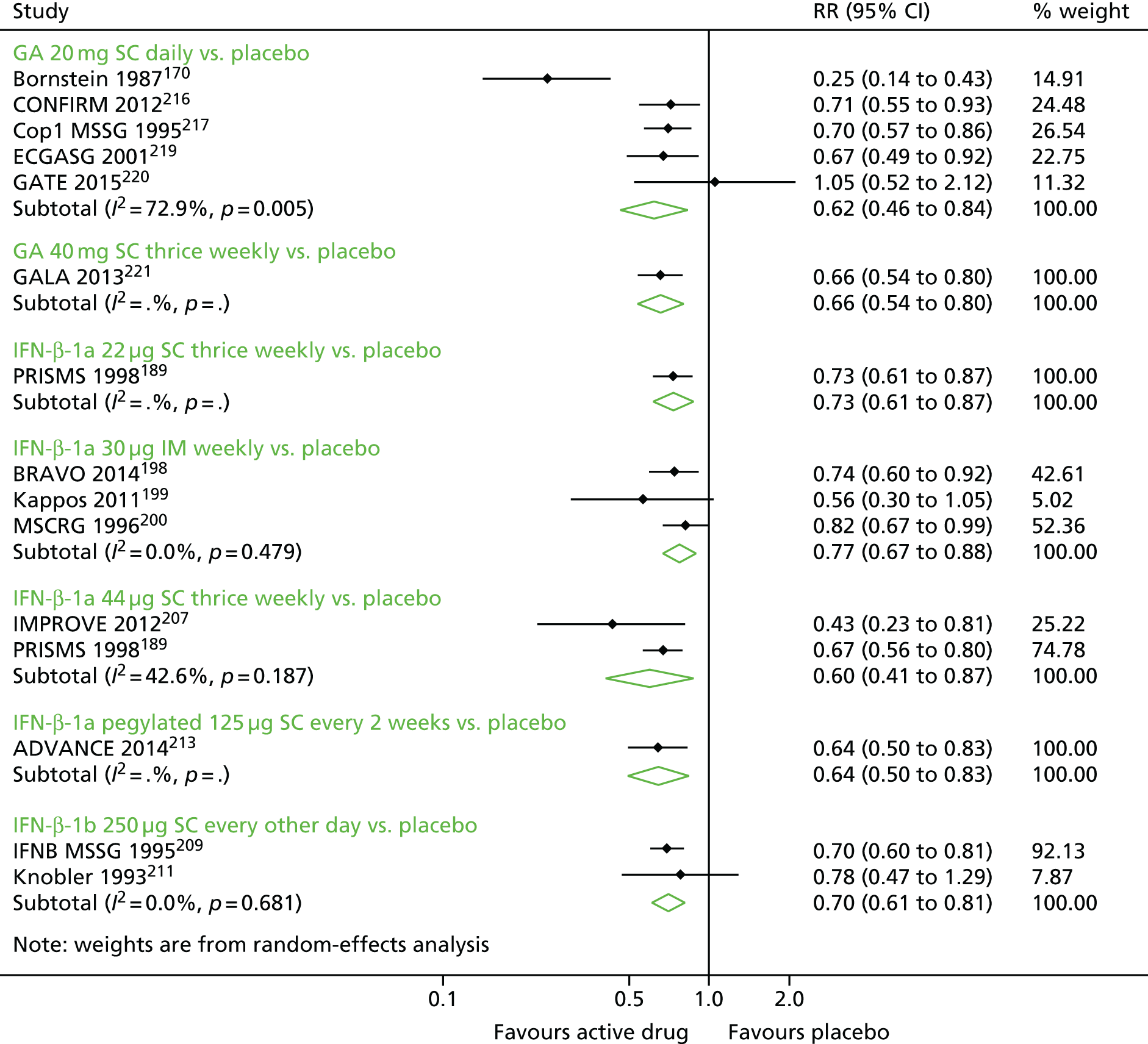
Direct evidence from comparisons between active drugs is shown in Figure 6. None of the pooled comparisons showed evidence of a statistically significant effect favouring one drug over another. Although several analyses had high I2 values each comparison had too few studies to permit exploration of heterogeneity.
FIGURE 6.
Pairwise meta-analyses: ARRs for active drug vs. active drug trials in RRMS.
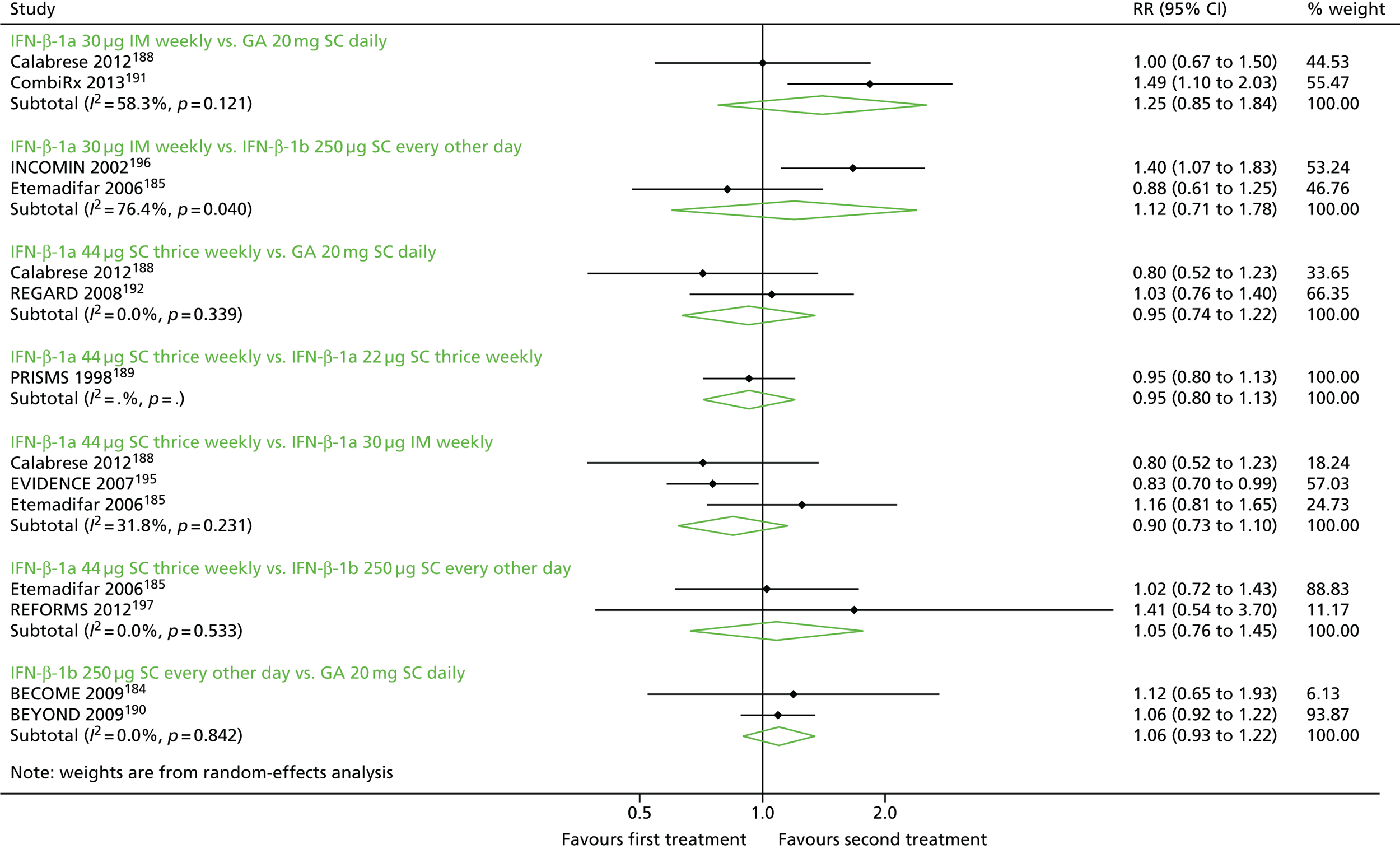
Network meta-analyses
The set of studies reporting ratios of relapse rates formed a connected network (Figure 7). In the network, all drugs were compared against placebo, but 40 mg of SC GA three times weekly and 125 µg of SC pegylated IFN-β-1a every 2 weeks were not compared against other active drugs in the network; 22 µg of SC IFN-β-1a three times weekly was connected to the network because of its inclusion in the PRISMS trial,189 which also tested the 44-µg dose.
FIGURE 7.
Network of studies: ARRs in RRMS. ga20, 20 mg of SC GA once daily; ga40, 40 mg of SC GA once daily; ifn1a22, 22 µg of SC IFN-β-1a three times weekly; ifn1a30, 30 µg of IM IFN-β-1a once a week; ifn1a44, 44 µg of SC IFN-β-1a three times weekly; ifn1b250, 250 µg of SC IFN-β-1b every other day; peg, 125 µg of SC pegylated IFN-β-1a every 2 weeks.

Random-effects NMA generated estimates for each drug compared with placebo and with every other drug (Table 8). Ranking of the drugs suggested that the drug with the highest cumulative probability SUCRA of being the best was 20 mg of SC GA once daily, followed by 125 µg of SC pegylated IFN-β-1a every 2 weeks and 40 mg of SC GA three times weekly, with 30 µg of IM IFN-β-1a once a week ranked second to last and placebo ranked last.
| Drug | SUCRA | GA 20 mg SC once daily | Pegylated IFN-β-1a 125 µg SC every 2 weeks | GA 40 mg SC three times weekly | IFN-β-1a 44 µg SC three times weekly | IFN-β-1b 250 µg SC every other day | IFN-β-1a 22 µg SC three times weekly | IFN-β-1a 30 µg IM weekly | Placebo |
|---|---|---|---|---|---|---|---|---|---|
| GA 20 mg SC once daily | 0.77 | 1.01 (0.77 to 1.33) | 1.00 (0.80 to 1.24) | 0.97 (0.85 to 1.10) | 0.95 (0.86 to 1.05) | 0.91 (0.76 to 1.08) | 0.82 (0.73 to 0.92) | 0.65 (0.59 to 0.72) | |
| Pegylated IFN-β-1a 125 µg SC every 2 weeks | 0.73 | 0.98 (0.71 to 1.35) | 0.95 (0.72 to 1.26) | 0.94 (0.71 to 1.23) | 0.89 (0.66 to 1.21) | 0.81 (0.62 to 1.06) | 0.64 (0.50 to 0.83) | ||
| GA 40 mg SC three times weekly | 0.70 | 0.97 (0.77 to 1.22) | 0.96 (0.77 to 1.19) | 0.91 (0.71 to 1.17) | 0.82 (0.66 to 1.03) | 0.66 (0.54 to 0.80) | |||
| IFN-β-1a 44 µg SC three times weekly | 0.64 | 0.99 (0.86 to 1.13) | 0.94 (0.80 to 1.10) | 0.85 (0.76 to 0.95) | 0.68 (0.60 to 0.76) | ||||
| IFN-β-1b 250 µg SC every other day | 0.56 | 0.95 (0.79 to 1.14) | 0.86 (0.76 to 0.97) | 0.69 (0.62 to 0.76) | |||||
| IFN-β-1a 22 µg SC three times weekly | 0.43 | 0.91 (0.76 to 1.08) | 0.72 (0.61 to 0.85) | ||||||
| IFN-β-1a 30 µg IM weekly | 0.18 | 0.80 (0.72 to 0.88) | |||||||
| Placebo | 0 | ||||||||
| Wald test for inconsistency (χ2, df, p-value) | 11.71, 11, 0.38 |
Findings derived from the NMA for comparisons between each drug and placebo substantially mirrored those of the pairwise comparisons and reflected statistically significant reductions in relapse rates in patients receiving active drugs. Pairwise comparisons between drugs mostly revealed little evidence of superiority of one drug over another, although 20 mg of SC GA once daily (RR 0.82, 95% CI 0.73 to 0.92), 44 µg of SC IFN-β-1a three times weekly (RR 0.85, 95% CI 0.76 to 0.95) and 250 µg of SC IFN-β-1b every other day (RR 0.86, 95% CI 0.76 to 0.97) all produced significant reductions in the relapse rate compared with 30 µg of IM IFN-β-1a once a week. These findings from the pairwise comparisons in the NMA, which all included direct (i.e. head-to-head) evidence, were similar in magnitude of effect to findings from the pairwise meta-analyses, but may have benefited from a ‘stabilised’ heterogeneity parameter because of the assumption of equal between-studies variance.
Tests of inconsistency in the network did not suggest disagreement between direct and indirect evidence. A Wald test for overall inconsistency derived from a design*treatment interaction model was not statistically significant (p = 0.38) and comparisons between the direct and the indirect evidence derived from the side-splitting model did not show any statistically significant differences.
Sensitivity analyses
Several characteristics of the trials included in this network suggested that additional analyses would confirm the robustness of our findings. All of these analyses were post hoc. First, we excluded the REFORMS trial197 from the analysis, as it was the only study in which relapses were self-reported by subjects instead of documented by an examining neurologist. Effect estimates remained essentially unchanged for all pairwise comparisons.
Second, we compared findings from studies with ‘true’, blinded placebos with findings from studies that did not use blinded placebos, that is, studies that did not deliver placebos using the same route of administration as for the study drugs. Specifically, the BRAVO,198 CONFIRM216 and Kappos et al. 199 trials did not administer placebo by the same route as the relevant IFN or GA arm in each trial. We found that the effects of these drugs compared with placebo were robust to inclusion of a covariate in the model for trials without a blinded placebo.
Third, we noticed that the study by Bornstein et al. 170 was an outlier in the comparison between 20 mg of SC GA once daily and placebo. When we excluded this trial from the pairwise meta-analysis, the pooled RR for relapses still suggested a reduction in ARR compared with placebo (RR 0.71, 95% CI 0.62 to 0.82), with an I2 of 0%. Re-estimation of the NMA yielded a change in the SUCRA-based rankings, with 20 mg of SC GA once daily now ranked third, but point estimates and CIs were not substantially different in the new model (Table 9).
| Drug | SUCRA | Pegylated IFN-β-1a 125 µg SC every 2 weeks | GA 40 mg SC three times weekly | GA 20 mg SC once daily | IFN-β-1a 44 µg SC three times weekly | IFN-β-1b 250 µg SC every other day | IFN-β-1a 22 µg SC three times weekly | IFN-β-1a 30 µg IM weekly | Placebo |
|---|---|---|---|---|---|---|---|---|---|
| Pegylated IFN-β-1a 125 µg SC every 2 weeks | 0.76 | 0.98 (0.71 to 1.35) | 0.95 (0.73 to 1.25) | 0.94 (0.71 to 1.24) | 0.92 (0.70 to 1.21) | 0.89 (0.66 to 1.20) | 0.80 (0.61 to 1.05) | 0.64 (0.50 to 0.83) | |
| GA 40 mg SC three times weekly | 0.73 | 0.97 (0.78 to 1.21) | 0.96 (0.77 to 1.20) | 0.94 (0.75 to 1.17) | 0.91 (0.70 to 1.17) | 0.82 (0.65 to 1.02) | 0.66 (0.54 to 0.80) | ||
| GA 20 mg SC once daily | 0.69 | 0.99 (0.87 to 1.12) | 0.98 (0.86 to 1.12) | 0.93 (0.78 to 1.12) | 0.84 (0.74 to 0.95) | 0.68 (0.61 to 0.75) | |||
| IFN-β-1a 44 µg SC three times weekly | 0.65 | 0.98 (0.86 to 1.12) | 0.94 (0.80 to 1.11) | 0.85 (0.76 to 0.95) | 0.68 (0.61 to 0.76) | ||||
| IFN-β-1b 250 µg SC every other day | 0.55 | 0.96 (0.80 to 1.15) | 0.87 (0.77 to 0.98) | 0.70 (0.63 to 0.77) | |||||
| IFN-β-1a 22 µg SC three times weekly | 0.45 | 0.90 (0.76 to 1.07) | 0.72 (0.62 to 0.85) | ||||||
| IFN-β-1a 30 µg IM weekly | 0.17 | 0.80 (0.73 to 0.89) | |||||||
| Placebo | 0.00 | ||||||||
| Wald test for inconsistency (χ2, df, p-value) | 12.59, 11, 0.32 |
Meta-analyses: relapse severity – moderate or severe relapses
Pairwise meta-analyses
Direct evidence from pairwise comparisons is shown in Figure 8. Each comparison was informed by one study. All drugs compared with placebo had a statistically significant beneficial effect in terms of reducing the rate of moderate or severe relapses. In comparisons based on active drugs, there was no evidence that one dose of SC IFN-β-1a three times weekly was statistically better than the other (44 µg vs. 22 µg) or that 250 µg of SC IFN-β-1b every other day was different from 20 mg of SC GA once daily. The active drugs 40 mg of SC GA three times weekly, 30 µg of IM IFN-β-1a once a week and 125 µg of SC pegylated IFN-β-1a every 2 weeks were not represented in this analysis.
FIGURE 8.
Pairwise estimates: ARRs for moderate or severe relapses in RRMS.

Network meta-analyses
The set of studies reporting ratios of relapse rates for moderate and severe relapses formed a connected network (Figure 9). In the network, direct evidence for 20 mg of SC GA once daily was only available for the comparison with 250 µg of SC IFN-β-1b every other day.
FIGURE 9.
Network of studies: ARRs for moderate or severe relapses in RRMS. ga20, 20 mg of SC GA once daily; ifn1a22, 22 µg of SC IFN-β-1a three times weekly; ifn1a44, 44 µg of SC IFN-β-1a three times weekly; ifn1b250, 250 µg of SC IFN-β-1b every other day.
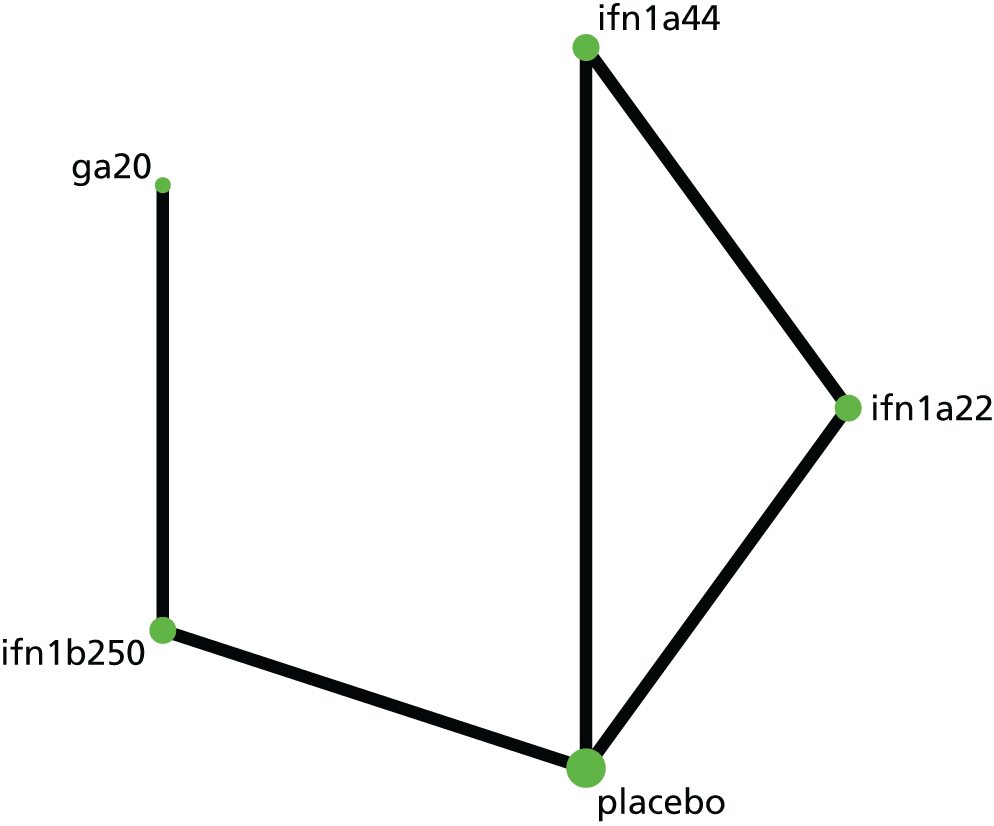
Because of the shape of the network, in which there was no opportunity for inconsistency and in which no direct comparison was informed by more than one trial, the model was estimated using fixed effects instead of random effects, as in the protocol. Ranking of drugs suggested that 20 mg of SC GA once daily was best, followed by 250 µg of SC IFN-β-1b every other day and 44 µg and 22 µg of SC IFN-β-1a three times weekly, with placebo ranked last (Table 10).
| Drug | SUCRA | GA 20 mg SC once daily | IFN-β-1b 250 µg SC every other day | IFN-β-1a 44 µg SC three times weekly | IFN-β-1a 22 µg SC three times weekly | Placebo |
|---|---|---|---|---|---|---|
| GA 20 mg SC once daily | 0.85 | 0.95 (0.70 to 1.27) | 0.77 (0.48 to 1.24) | 0.68 (0.42 to 1.08) | 0.48 (0.31 to 0.76) | |
| IFN-β-1b 250 µg SC every other day | 0.80 | 0.82 (0.56 to 1.19) | 0.71 (0.49 to 1.03) | 0.51 (0.37 to 0.71) | ||
| IFN-β-1a 44 µg SC three times weekly | 0.57 | 0.87 (0.74 to 1.03) | 0.63 (0.53 to 0.74) | |||
| IFN-β-1a 22 µg SC three times weekly | 0.28 | 0.72 (0.61 to 0.84) | ||||
| Placebo | 0.00 |
Findings derived from the NMA for comparisons between each drug and placebo were similar to findings from comparisons between each drug and placebo from the direct evidence, as would be expected. In an indirect comparison, 20 mg of SC GA once daily reduced the rate of moderate and severe relapses compared with placebo (RR 0.48, 95% CI 0.31 to 0.76). Pairwise comparisons between active drugs did not yield evidence of the superiority of any one drug over another.
Because there was no possibility of inconsistency in the network, we did not test for it.
Meta-analyses: relapse severity – steroid-treated relapses
Pairwise meta-analysis
Direct evidence from comparisons against placebo is shown in Figure 10. Each comparison was informed by one study. All drugs that were compared with placebo had a significant effect in terms of reducing the rate of steroid-treated relapses. In head-to-head comparisons between active drugs, 44 µg of SC IFN-β-1a three times weekly produced a greater reduction in steroid-treated relapses than the 22-µg dose of the same drug (RR 0.77, 95% CI 0.67 to 0.89) and 30 µg of IM IFN-β-1a once a week (RR 0.68, 95% CI 0.51 to 0.91). Pairwise comparisons between 30 µg of IM IFN-β-1a once a week and 250 µg of SC IFN-β-1b every other day and between 44 µg of SC IFN-β-1a three times weekly and 20 mg of SC GA once daily did not show statistical evidence of superiority. The active drug 125 µg of SC pegylated IFN-β-1a every 2 weeks was not included in this analysis.
FIGURE 10.
Pairwise estimates: ARRs for steroid-treated relapses in RRMS.
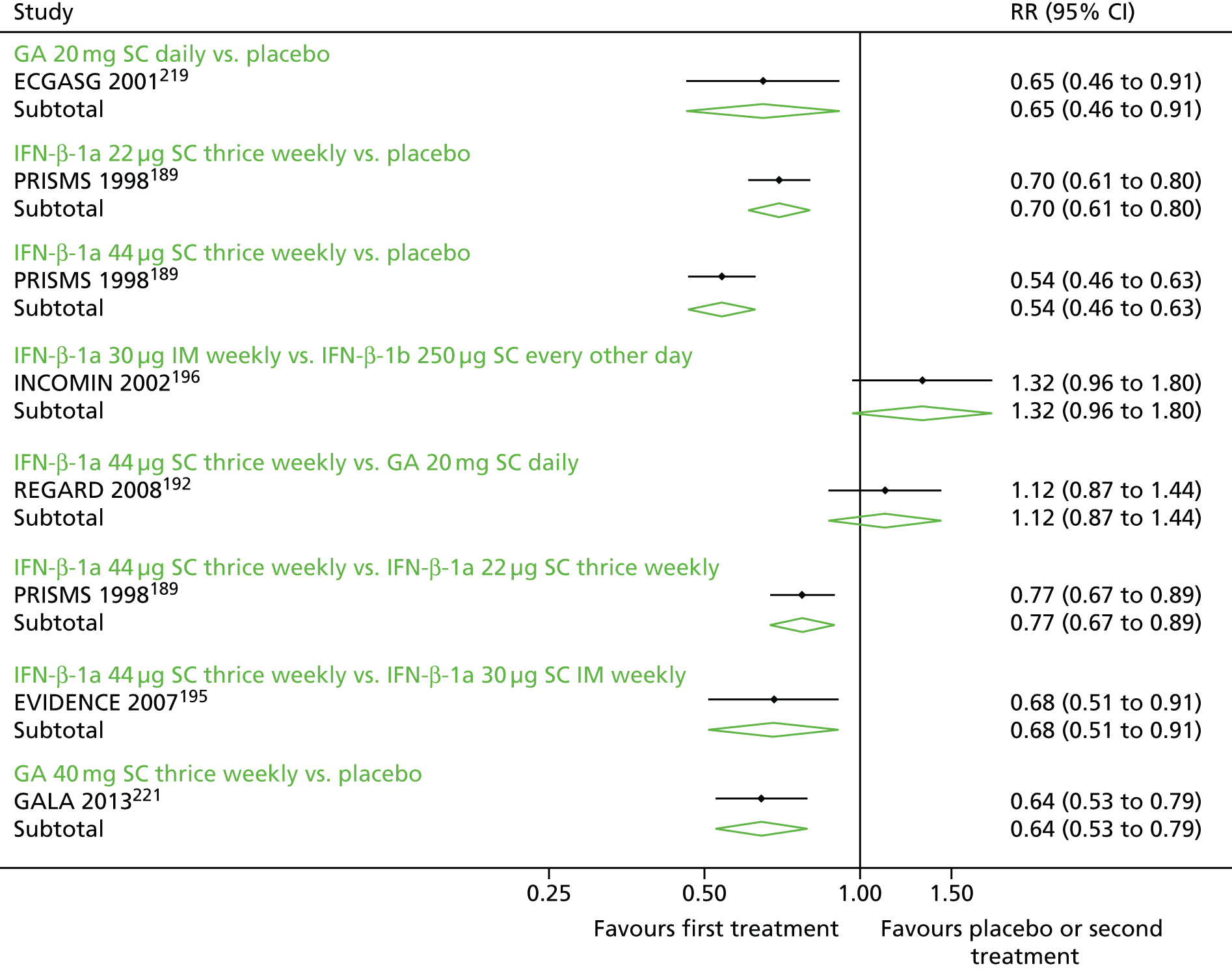
Network meta-analyses
The set of studies reporting ratios of steroid-treated relapse rates formed a connected network (Figure 11). In the network, each comparison was informed by one study, but there were closed loops between studies, suggesting the possibility of inconsistency. Because in this parameterisation of the model inconsistency is regarded as a source of heterogeneity, even though there is no potential for heterogeneity in any of the comparisons informed by direct evidence, we estimated the model as both a fixed-effects and a random-effects model.
FIGURE 11.
Network of studies: ARR for steroid-treated relapses in RRMS. ga20, 20 mg of SC GA once daily; ga40, 40 mg of SC GA three times weekly; ifn1a22, 22 µg of SC IFN-β-1a three times weekly; ifn1a30, 30 µg of IM IFN-β-1a once a week; ifn1a44, 44 µg of SC IFN-β-1a three times weekly; ifn1b250, 250 µg of SC IFN-β-1b every other day.

Numerical estimates of intervention effectiveness were not meaningfully different between the random-effects model and the fixed-effects model (Table 11). However, the random-effects model did not support a significant reduction in the rate of steroid-treated relapses with 250 µg of SC IFN-β-1b every other day (fixed-effects RR 0.62, 95% CI 0.40 to 0.98; random-effects RR 0.64, 95% CI 0.36 to 1.14). The random-effects model also did not support the superiority of any one drug over another, except for 44 µg of SC IFN-β-1a three times weekly over 30 µg of IM IFN-β-1a once a week (RR 0.68, 95% CI 0.48 to 0.97). However, in the fixed-effects model, 44 µg of SC IFN-β-1a three times weekly was superior to both 30 µg of IM IFN-β-1a once a week (RR 0.68, 95% CI 0.51 to 0.91) and 22 µg of SC IFN-β-1a three times weekly (RR 0.79, 95% CI 0.68 to 0.91), both of which comparisons were informed by direct evidence; 20 mg of SC GA once daily was also superior to both 30 µg of IM IFN-β-1a once a week (RR 0.67, 95% CI 0.47 to 0.95) and 22 µg of SC IFN-β-1a three times weekly (RR 0.77, 95% CI 0.61 to 0.98), although neither comparison was informed by direct evidence.
| Drug | SUCRA | GA 20 mg SC once daily | IFN-β-1a 44 µg SC three times weekly | IFN-β-1b 250 µg SC every other day | GA 40 mg SC three times weekly | IFN-β-1a 22 µg SC three times weekly | IFN-β-1a 30 µg IM weekly | Placebo |
|---|---|---|---|---|---|---|---|---|
| Fixed-effects model | ||||||||
| GA 20 mg SC once daily | 0.85 | 0.98 (0.80 to 1.21) | 0.88 (0.55 to 1.41) | 0.85 (0.63 to 1.15) | 0.77 (0.61 to 0.98) | 0.67 (0.47 to 0.95) | 0.55 (0.44 to 0.68) | |
| IFN-β-1a 44 µg SC three times weekly | 0.83 | 0.89 (0.58 to 1.37) | 0.87 (0.68 to 1.11) | 0.79 (0.68 to 0.91) | 0.68 (0.51 to 0.91) | 0.56 (0.48 to 0.64) | ||
| IFN-β-1b 250 µg SC every other day | 0.64 | 0.97 (0.59 to 1.58) | 0.88 (0.56 to 1.38) | 0.76 (0.56 to 1.04) | 0.62 (0.40 to 0.98) | |||
| GA 40 mg SC three times weekly | 0.56 | 0.91 (0.71 to 1.16) | 0.79 (0.54 to 1.15) | 0.64 (0.53 to 0.79) | ||||
| IFN β–1a 22 µg SC three times weekly | 0.40 | 0.86 (0.63 to 1.19) | 0.71 (0.62 to 0.81) | |||||
| IFN-β-1a 30 µg IM weekly | 0.20 | 0.82 (0.59 to 1.13) | ||||||
| Placebo | 0.02 | |||||||
| Wald test for inconsistency (χ2, df, p-value) | 1.65, 1, 0.20 | |||||||
| Random-effects model | ||||||||
| GA 20 mg once daily | 0.82 | 0.98 (0.75 to 1.29) | 0.88 (0.49 to 1.58) | 0.87 (0.57 to 1.34) | 0.78 (0.56 to 1.10) | 0.67 (0.43 to 1.05) | 0.56 (0.41 to 0.77) | |
| IFN-β-1a 44 µg SC three times weekly | 0.81 | 0.89 (0.53 to 1.50) | 0.89 (0.60 to 1.31) | 0.80 (0.62 to 1.03) | 0.68 (0.48 to 0.97) | 0.57 (0.44 to 0.74) | ||
| IFN-β-1b 250 µg SC every other day | 0.64 | 0.99 (0.52 to 1.90) | 0.89 (0.50 to 1.58) | 0.76 (0.52 to 1.11) | 0.64 (0.36 to 1.14) | |||
| GA 40 mg three times weekly | 0.59 | 0.90 (0.61 to 1.32) | 0.67 (0.43 to 1.05) | 0.64 (0.48 to 0.86) | ||||
| IFN-β-1a 22 µg SC three times weekly | 0.44 | 0.85 (0.55 to 1.32) | 0.72 (0.56 to 0.92) | |||||
| IFN-β-1a 30 µg IM weekly | 0.23 | 0.84 (0.54 to 1.30) | ||||||
| Placebo | 0.06 | |||||||
| Wald test for inconsistency (χ2, df, p-value) | 1.63, 1, 0.20 | |||||||
Because the overall Wald test for inconsistency did not provide evidence of a difference between direct and indirect evidence (p = 0.20), the fixed-effects model may be preferable.
Meta-analyses: time to disability progression confirmed at 3 months
Pairwise meta-analyses
Direct evidence from comparisons is shown in Figure 12. Only one comparison, 20 mg of SC GA once daily compared with placebo, included more than one study and 40 mg of SC GA three times weekly was not represented in this analysis.
FIGURE 12.
Pairwise meta-analyses: time to disability progression confirmed at 3 months in RRMS.

Comparison of drugs against placebo showed a mixed pattern of results. The active drugs 20 mg of SC GA once daily (HR 0.79, 95% CI 0.60 to 1.05), 30 µg of IM IFN-β-1a once a week (HR 0.74, 95% CI 0.51 to 1.08) and 250 µg of SC IFN-β-1b every other day (HR 0.71, 95% CI 0.48 to 1.06) did not show evidence of delaying disability progression. However, both 44 µg of SC IFN-β-1a three times weekly (HR 0.62, 95% CI 0.43 to 0.90) and 22 µg of SC IFN-β-1a three times weekly (HR 0.68, 95% CI 0.48 to 0.97) and 125 µg of SC pegylated IFN-β-1a every 2 weeks (HR 0.62, 95% CI 0.40 to 0.97) did show evidence of delaying disability progression. None of the three direct comparisons between active drugs suggested a benefit of one over another.
Network meta-analyses
The set of studies reporting HRs for time to disability progression confirmed at 3 months formed a connected network (Figure 13). In the network, all active drugs were compared against placebo and three comparisons between active drugs were present as well.
FIGURE 13.
Network of studies: time to disability progression confirmed at 3 months in RRMS. ga20, 20 mg of SC GA once daily; ifn1a22, 22 µg of SC IFN-β-1a three times weekly; ifn1a30, 30 µg of IM IFN-β-1a once a week; ifn1a44, 44 µg of SC IFN-β-1a three times weekly; ifn1b250, 250 µg of SC IFN-β-1b every other day; peg, 125 µg of SC pegylated IFN-β-1a every 2 weeks.
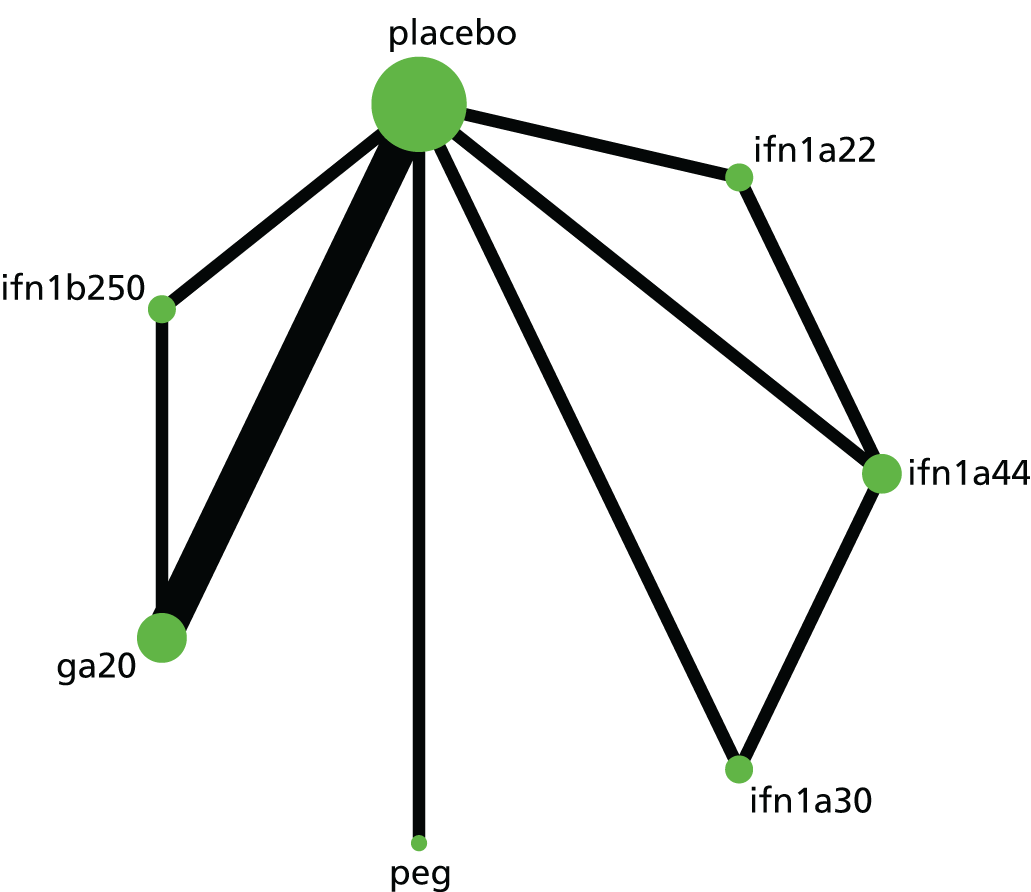
The NMA, which was estimated with random effects as per the protocol, generated HR estimates for each drug compared with placebo and with every other drug (Table 12). Ranking of the drugs suggested that the drug with the highest cumulative probability of being the best was 44 µg of SC IFN-β-1a three times weekly, followed by 125 µg of SC pegylated IFN-β-1a every 2 weeks and 22 µg of SC IFN-β-1a three times weekly, with 250 µg of SC IFN-β-1b every other day ranked second to last and placebo ranked last.
| Drug | SUCRA | IFN-β-1a 44 µg SC three times weekly | Pegylated IFN-β-1a 125 µg SC every 2 weeks | IFN-β-1a 22 µg SC three times weekly | IFN-β-1a 30 µg IM weekly | GA 20 mg SC once daily | IFN-β-1b 250 µg SC every other day | Placebo |
|---|---|---|---|---|---|---|---|---|
| IFN-β-1a 44 µg SC three times weekly | 0.77 | 1.01 (0.59 to 1.74) | 0.92 (0.65 to 1.30) | 0.86 (0.62 to 1.19) | 0.82 (0.56 to 1.22) | 0.81 (0.53 to 1.22) | 0.63 (0.46 to 0.86) | |
| Pegylated IFN-β-1a 125 µg SC every 2 weeks | 0.75 | 0.91 (0.52 to 1.59) | 0.85 (0.49 to 1.46) | 0.81 (0.49 to 1.34) | 0.80 (0.47 to 1.34) | 0.62 (0.40 to 0.97) | ||
| IFN-β-1a 22 µg SC three times weekly | 0.62 | 0.94 (0.62 to 1.42) | 0.90 (0.59 to 1.36) | 0.88 (0.57 to 1.36) | 0.68 (0.49 to 0.96) | |||
| IFN-β-1a 30 µg IM weekly | 0.50 | 0.96 (0.65 to 1.42) | 0.94 (0.62 to 1.43) | 0.73 (0.53 to 1.00)* | ||||
| GA 20 mg SC once daily | 0.44 | 0.98 (0.78 to 1.24) | 0.76 (0.60 to 0.97) | |||||
| IFN-β-1b 250 µg SC every other day | 0.39 | 0.78 (0.59 to 1.02) | ||||||
| Placebo | 0.02 | |||||||
| Wald test for inconsistency (χ2, df, p-value) | 0.35, 2, 0.84 |
Comparisons of active drugs with placebo were similar between the NMA and the pairwise meta-analyses. Notably, additional information from indirect comparisons yielded a more precise estimate of effectiveness for both 30 µg of IM IFN-β-1a once a week compared with placebo (HR 0.73, 95% CI 0.53 to 1.00; p = 0.0499) and 20 mg of SC GA once daily compared with placebo (HR 0.76, 95% CI 0.60 to 0.97). Comparisons between active drugs in the NMA did not indicate that any one drug was statistically better than any other, as all pairwise comparisons were not statistically significant.
Tests of inconsistency in the network did not suggest any disagreement between direct and indirect evidence. An overall Wald test derived from a design*treatment interaction model returned a non-significant result (p = 0.84) and comparisons between the direct evidence and the indirect evidence derived from the side-splitting model did not show any statistically significant differences.
Meta-analyses: time to disability progression confirmed at 6 months
Pairwise meta-analyses
Direct evidence from comparisons is shown in Figure 14. All comparisons were based on a single study, except for 30 µg of IM IFN-β-1a once a week compared with placebo. The active drug 40 mg of SC GA three times weekly was not represented in this analysis.
FIGURE 14.
Pairwise meta-analyses: time to disability progression confirmed at 6 months in RRMS.

Three drugs were compared against placebo: 20 mg of SC GA once daily did not delay confirmed disability progression compared with placebo, but 30 µg of IM IFN-β-1a once weekly (HR 0.66, 95% CI 0.47 to 0.92) and 125 µg of SC pegylated IFN-β-1a every 2 weeks (HR 0.46, 95% CI 0.26 to 0.81) did. Of the three comparisons between active drugs, only 30 µg of IM IFN-β-1a once a week yielded a significant difference, when compared with 250 µg of SC IFN-β-1b every other day.
Network meta-analyses
The set of studies reporting HRs for time to disability progression confirmed at 6 months formed a connected network (Figure 15). In the network, 250 µg of SC IFN-β-1b every other day and 44 µg of SC IFN-β-1a three times weekly are not compared with placebo, but only with other active drugs.
FIGURE 15.
Network of studies: time to disability progression confirmed at 6 months in RRMS. ga20, 20 mg of SC GA once daily; ifn1a30, 30 µg of IM IFN-β-1a once a week; ifn1a44, 44 µg of SC IFN-β-1a three times weekly; ifn1b250, 250 µg of SC IFN-β-1b every other day; peg, 125 µg of SC pegylated IFN-β-1a every 2 weeks.

The NMA, which was estimated with random effects as per the protocol, generated HR estimates for each drug compared with placebo and with every other drug (Table 13). Ranking of the drugs suggested that the drug with the highest cumulative probability of being the best was 250 µg of SC IFN-β-1b every other day, followed by 125 µg of SC pegylated IFN-β-1a every 2 weeks, 44 µg of SC IFN-β-1a three times weekly and 30 µg of IM IFN-β-1a once a week; 20 mg of SC GA once daily was ranked second to last and placebo was ranked last.
| Drug | SUCRA | IFN-β-1b 250 µg SC every other day | Pegylated IFN-β-1a 125 µg SC every 2 weeks | IFN-β-1a 44 µg SC three times weekly | IFN-β-1a 30 µg IM weekly | GA 20 mg SC once daily | Placebo |
|---|---|---|---|---|---|---|---|
| IFN-β-1b 250 µg SC every other day | 0.90 | 0.74 (0.32 to 1.71) | 0.71 (0.32 to 1.60) | 0.50 (0.29 to 0.87) | 0.42 (0.21 to 0.83) | 0.34 (0.18 to 0.63) | |
| Pegylated IFN-β-1a 125 µg SC every 2 weeks | 0.71 | 0.97 (0.40 to 2.33) | 0.68 (0.35 to 1.31) | 0.56 (0.28 to 1.15) | 0.46 (0.26 to 0.81) | ||
| IFN-β-1a 44 µg SC three times weekly | 0.70 | 0.70 (0.39 to 1.25) | 0.58 (0.27 to 1.27) | 0.47 (0.24 to 0.93) | |||
| IFN-β-1a 30 µg IM weekly | 0.40 | 0.83 (0.49 to 1.41) | 0.68 (0.49 to 0.94) | ||||
| GA 20 mg SC once daily | 0.25 | 0.82 (0.53 to 1.26) | |||||
| Placebo | 0.05 | ||||||
| Wald test for inconsistency (χ2, df, p-value) | 0.77, 1, 0.38 |
When compared with placebo in the NMA, 20 mg of SC GA once daily had a similar estimate of effectiveness (HR 0.82, 95% CI 0.53 to 1.26) as in the direct evidence, as did 30 µg of IM IFN-β-1a once a week (HR 0.68, 95% CI 0.49 to 0.94) and 125 µg of SC pegylated IFN-β-1a every 2 weeks (HR 0.46, 95% CI 0.26 to 0.81). Both 44 µg of SC IFN-β-1a three times weekly (HR 0.47, 95% CI 0.24 to 0.93) and 250 µg of SC IFN-β-1b every other day (HR 0.34, 95% CI 0.18 to 0.63) showed evidence of delaying disability progression compared with placebo. However, both of these estimates are based solely on indirect evidence and findings from the INCOMIN trial,196 which informed the comparison between 250 µg of SC IFN-β-1b every other day and 30 µg of IM IFN-β-1a once a week and relied on a HR estimated from summary statistics.
Comparisons between active drugs in from the NMA suggested that 250 µg of SC IFN-β-1b every other day is superior to both 30 µg of IM IFN-β-1a once a week (HR 0.50, 95% CI 0.29 to 0.87) and 20 mg of SC GA once daily (HR 0.41, 95% CI 0.21 to 0.83). In particular, the result of the comparison between 250 µg of SC IFN-β-1b every other day and 20 mg of SC GA once daily was greater in magnitude than the direct evidence suggested. No other comparisons between active drugs yielded statistically significant evidence of superiority of one drug over any other.
Tests of inconsistency in the network did not suggest that direct and indirect evidence disagreed to a statistically significant level; however, the network was sparse and only one comparison included more than one study. An overall Wald test for inconsistency returned a statistically non-significant result (p = 0.38).
Meta-analyses: adverse events
Summary of the adverse events meta-analyses
Full results for pairwise meta-analyses of AEs are available on request. Although the diversity and heterogeneity of AEs precluded detailed examination of each, several trends were apparent across pairwise comparisons:
-
comparing 30 µg of IFN-β-1a (Avonex) with equivalent placebo, 30 µg of IFN-β-1a was associated with more chills, flu-like symptoms, NABs and myalgia
-
comparing 30 µg of IFN-β-1a (Avonex) with 44 µg of SC IFN-β-1a (Rebif), 44 µg of IFN-β-1a was associated with more injection site reactions, liver disorders, NABs and white blood cell abnormalities, whereas 30 µg of IFN-β-1a was associated with more fatigue
-
comparing 30 µg of IFN-β-1a (Avonex) with IFN-β-1b (Betaferon/Extavia), IFN-β-1b was associated with more injection site reactions and NABs
-
comparing 30 µg of IFN-β-1a (Avonex) with GA (Copaxone), there were no significant differences in AEs
-
comparing 44 µg of IFN-β-1a (Rebif) with placebo, 44 µg of IFN-β-1a was associated with more injection site reactions, flu-like symptoms, liver disorders, granulocytopenia, leucopenia, lymphopenia and NABs
-
comparing 44 µg of IFN-β-1a (Rebif) with IFN-β-1b (Betaferon/Extavia), 44 µg of IFN-β-1a was associated with more alanine aminotransferase disorders and IFN-β-1b was associated with more injection site pain
-
comparing 44 µg of IFN-β-1a (Rebif) with GA (Copaxone), 44 µg of IFN-β-1a was associated with more liver enzyme disorders, NABs, headache, flu-like symptoms and myalgia and GA was associated with more injection site reactions, immediate post-injection reactions and binding antibodies
-
comparing IFN-β-1b (Betaferon/Extavia) with placebo, IFN-β-1b was associated with more injection site inflammation and NABs
-
comparing IFN-β-1b (Betaferon/Extavia) with GA (Copaxone), IFN-β-1b was associated with more flu-like symptoms, insomnia and disordered liver enzymes and GA was associated with more injection site reactions, itching, pain, inflammation and induration and immediate post-injection reactions
-
comparing GA (Copaxone) with equivalent placebo, GA was associated with more injection site induration, itching, injection site mass, erythema, pain, inflammation and reactions and more immediate post-injection systemic reactions
-
comparing pegIFN-β-1a (Plegridy) with placebo, pegylated IFN-β-1a was associated with more injection site erythema, pain, itching, chills and/or fever, headache, flu-like symptoms, myalgia, pyrexia, any AE possibly related to the drug, patients who discontinued the study because of AEs and severe AEs.
Discontinuation because of adverse events: modal follow-up
Pairwise meta-analyses
Pairwise meta-analyses for discontinuation because of AEs, combined across studies at the modal follow-up, are presented in Figure 16. The modal follow-up was approximately 24 months and thus we included studies with an intended follow-up period around this point. We included 12 estimates in these meta-analyses. There was no visual evidence of a systematic difference between the groups based on the strict definition of the outcome. In every pairwise meta-analysis, CIs were wide, as would be expected. Three pooled estimates relied on multiple studies: 20 mg of SC GA once daily compared with placebo, 30 µg of IM IFN-β-1a once a week compared with placebo and 250 µg of SC IFN-β-1b every other day compared with 20 mg of SC GA once daily. There was no evidence in this analysis for 40 mg of SC GA three times weekly or 125 µg of SC pegylated IFN-β-1a every 2 weeks.
FIGURE 16.
Pairwise meta-analyses: discontinuation because of AEs at 24 months in RRMS. RR, risk ratio.
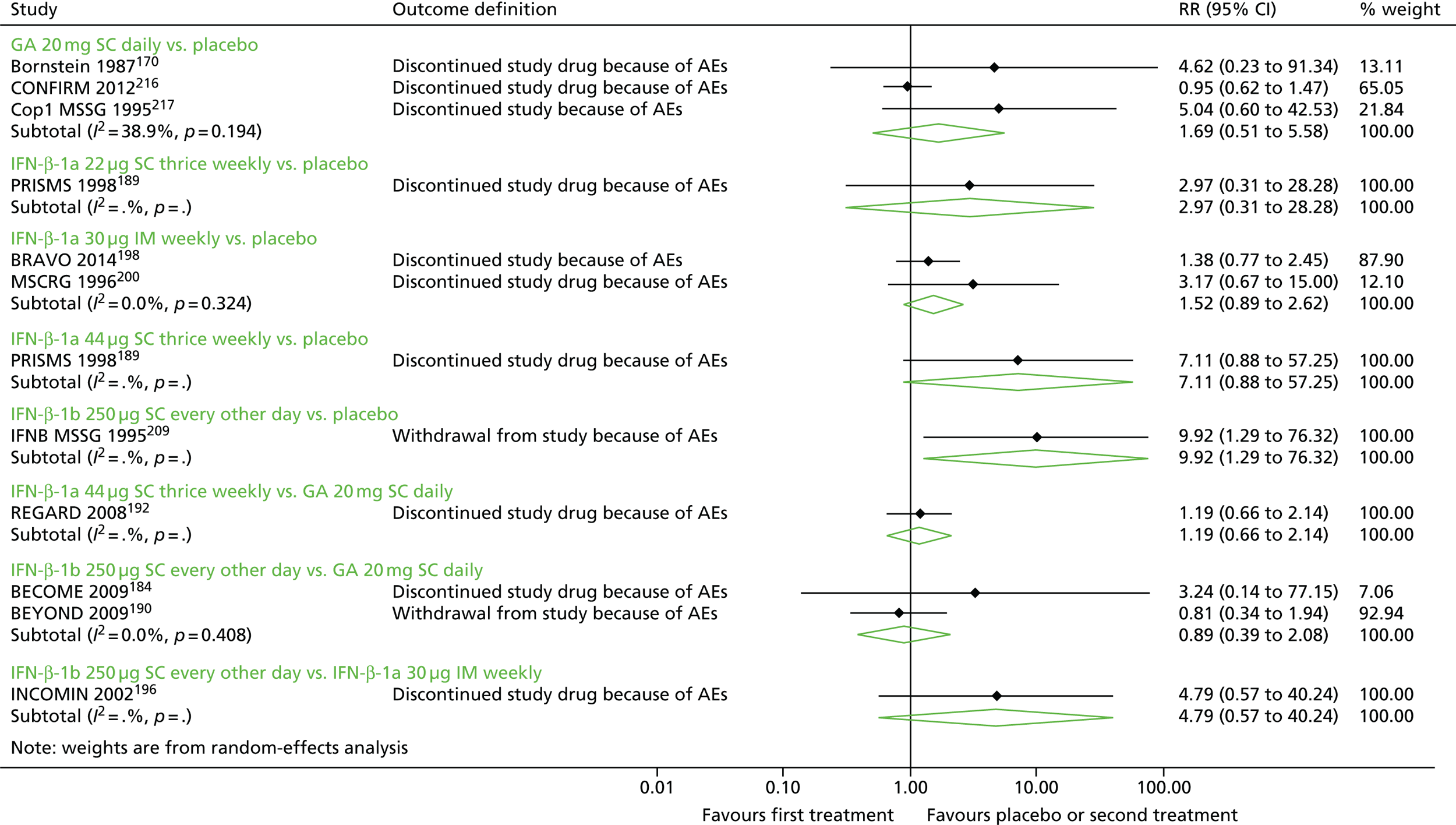
Direction of effect in pooled estimates and single studies generally suggested that discontinuation because of AEs was more likely in study arms testing active drugs than in study arms testing placebo, but these findings were generally not statistically significant. The one exception was the IFNB MSSG trial,210 from which we used 24-month data. In this study, which compared 250 µg of SC IFN-β-1b every other day with placebo, patients receiving the study drug were more likely to withdraw from the study because of an AE (risk ratio 9.92, 95% CI 1.29 to 76.32).
Network meta-analyses
The set of studies included in the NMA formed a connected network (Figure 17). All drugs were compared with placebo; 40 mg of SC GA three times weekly and 125 µg of SC pegylated IFN-β-1a every 2 weeks were not included in this analysis.
FIGURE 17.
Network of studies: discontinuation because of AEs at 24 months in RRMS. ga20, 20 mg of SC GA once daily; ifn1a22, 22 µg of SC IFN-β-1a three times weekly; ifn1a30, 30 µg of IM IFN-β-1a once a week; ifn1a44, 44 µg of SC IFN-β-1a three times weekly; ifn1b250, 250 µg of SC IFN-β-1b every other day.

The NMA, which was estimated with random effects, generated estimates for each drug compared with placebo and with every other drug (Table 14). Because CIs were wide in pairwise, direct meta-analyses, CIs were wide in the NMA, and estimates compared with placebo were often numerically different. The NMA did not offer statistical evidence that any one drug was more likely to result in discontinuation because of AEs than any other drug. Based on SUCRAs, 250 µg of SC IFN-β-1b every other day was ranked highest for discontinuation because of AEs, followed by 44 µg of SC IFN-β-1a three times weekly. Placebo was ranked last.
| Drug | SUCRA | IFN-β-1b 250 µg SC every other day | IFN-β-1a 44 µg SC three times weekly | GA 20 mg SC once daily | IFN-β-1a 22 µg SC three times weekly | IFN-β-1a 30 µg IM weekly | Placebo |
|---|---|---|---|---|---|---|---|
| IFN-β-1b 250 µg SC every other day | 0.79 | 1.15 (0.20 to 6.56) | 1.70 (0.50 to 5.81) | 2.37 (0.22 to 25.84) | 2.74 (0.56 to 13.38) | 4.41 (1.07 to 18.29) | |
| IFN-β-1a 44 µg SC three times weekly | 0.76 | 1.48 (0.39 to 5.57) | 2.07 (0.32 to 13.44) | 2.39 (0.38 to 15.22) | 3.85 (0.81 to 18.29) | ||
| GA 20 mg SC once daily | 0.57 | 1.40 (0.17 to 11.76) | 1.61 (0.38 to 6.91) | 2.60 (0.88 to 7.64) | |||
| IFN-β-1a 22 µg SC three times weekly | 0.41 | 1.15 (0.10 to 13.09) | 1.86 (0.21 to 16.83) | ||||
| IFN-β-1a 30 µg IM weekly | 0.35 | 1.61 (0.52 to 5.02) | |||||
| Placebo | 0.12 | ||||||
| Wald test for inconsistency (χ2, df, p-value) | 2.38, 3, 0.50 |
In comparison with the direct evidence from the IFNB MSSG trial,210 the risk ratio for discontinuation because of AEs for 250 µg of SC IFN-β-1b every other day compared with placebo was lower but remained statistically significant (risk ratio 4.41, 95% CI 1.07 to 18.29). The risk ratio for 44 µg of SC IFN-β-1a three times weekly compared with placebo was lower in the NMA (risk ratio 3.85, 95% CI 0.81 to 18.29) than the pairwise estimate derived from the PRISMS trial189 (risk ratio 7.11, 95% CI 0.88 to 57.25), as was the risk ratio for 22 µg of SC IFN-β-1a three times weekly compared with placebo (NMA: risk ratio 1.86, 95% CI 0.21 to 16.83; PRISMS trial:189 risk ratio 2.97, 95% CI 0.31 to 28.28). However, the risk ratio for 20 mg of SC GA once daily compared with placebo was higher in the NMA (risk ratio 2.60, 95% CI 0.88 to 7.64) than in the pairwise meta-analysis (risk ratio 1.69, 0.51 to 5.58).
An overall test for inconsistency across the network did not suggest the presence of inconsistency (p = 0.50). However, a side-splitting test did find that direct and indirect evidence were in conflict for the comparison between 20 mg of SC GA once daily and placebo, with a suggestion that the risk of discontinuation because of AEs in the indirect evidence was higher than that presented in the direct evidence (p = 0.037). Thus, there is some evidence of inconsistency in this network.
Discontinuation because of adverse events: all follow-up times
Pairwise meta-analyses
Pairwise meta-analyses for discontinuation because of AEs across all time points are shown in Figure 18. There was no visual evidence of a systematic difference between the groups based on the strict definition of the outcome. In every pairwise meta-analysis, CIs were wide, as would be expected. Five pooled estimates relied on multiple studies: 20 mg of SC GA once daily compared with placebo, 30 µg of IM IFN-β-1a once a week compared with placebo and 250 µg of SC IFN-β-1b every other day compared with each placebo, 20 mg of SC GA once daily and 44 µg of SC IFN-β-1a three times weekly.
FIGURE 18.
Pairwise meta-analyses: discontinuation because of AEs at all time points in RRMS. RR, risk ratio.
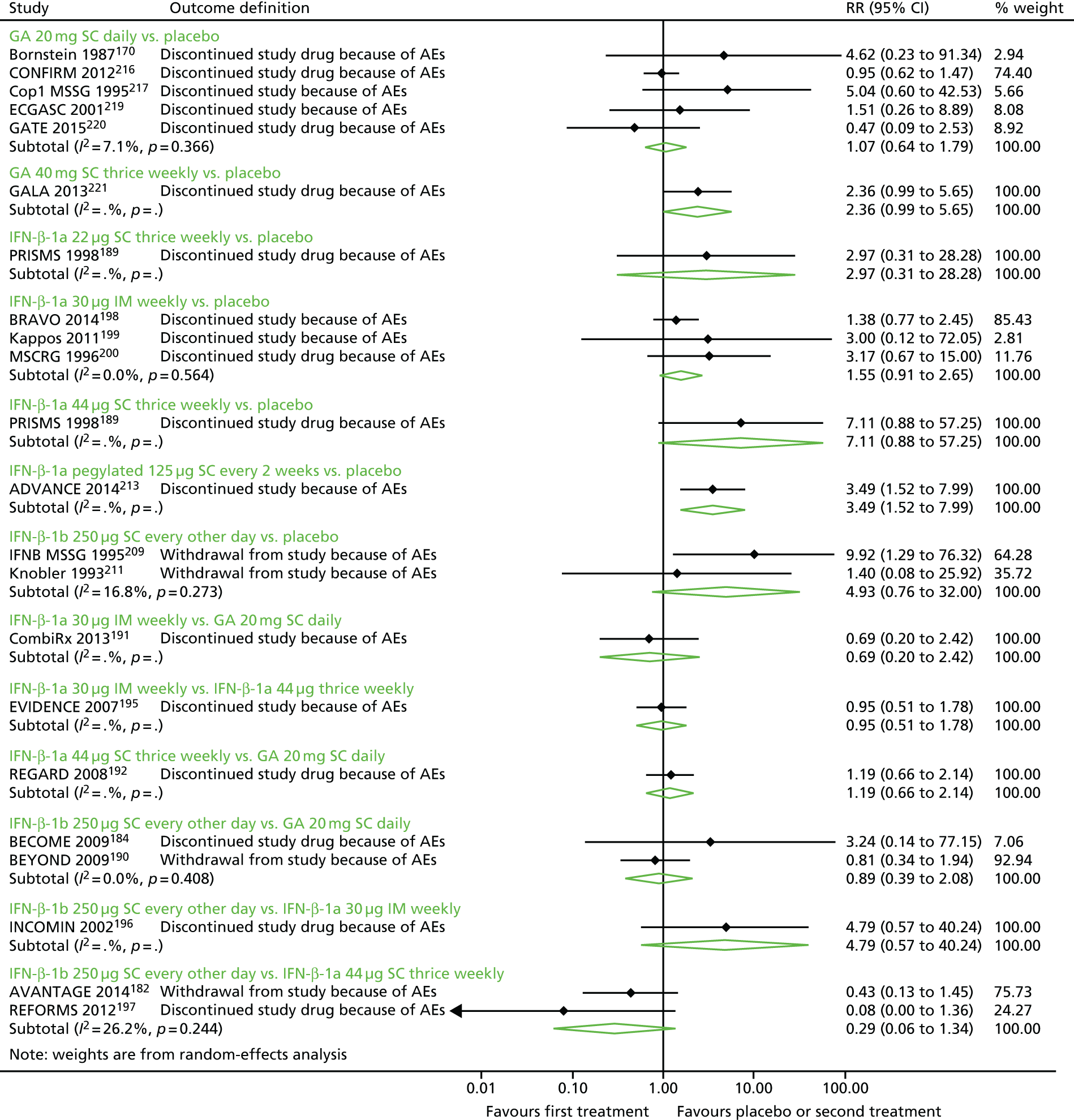
Despite visual evidence suggesting that discontinuation because of AEs was more likely in study arms testing active drugs than in study arms testing placebo, almost all individual study estimates and pooled estimates did not suggest that discontinuation was more likely in trial arms corresponding to one drug over another to a statistically significant level. The one exception was 125 µg of SC pegylated IFN-β-1a every 2 weeks compared with placebo, in which patients receiving the study drug were more likely to discontinue the study because of AEs (risk ratio 3.49, 95% CI 1.52 to 7.99). Estimates for 40 mg of SC GA three times weekly compared with placebo were marginally non-significant (risk ratio 2.36, 95% CI 0.99 to 5.65). Again, both estimates were based on one study. Of note is that comparisons between 20 mg of SC GA once daily and placebo, which included five studies, did not suggest a substantial relationship between the study drug and discontinuation (risk ratio 1.07, 95% CI 0.64 to 1.79), but this was driven (at least in part) by the null finding from the CONFIRM trial216 (risk ratio 0.95, 95% CI 0.62 to 1.47).
Network meta-analyses
The studies included in this analysis formed a connected network (Figure 19). All drugs were compared with placebo and all drugs were included in this analysis.
FIGURE 19.
Network of studies: discontinuation because of AEs at all time points in RRMS. ga20, 20 mg of SC GA once daily; ga40, 40 mg of SC GA three times weekly; ifn1a22, 22 µg of SC IFN-β-1a three times weekly; ifn1a30, 30 µg of IM IFN-β-1a once a week; ifn1a44, 44 µg of SC IFN-β-1a three times weekly; ifn1b250, 250 µg of SC IFN-β-1b every other day; peg, 125 µg of SC pegylated IFN-β-1a every 2 weeks.
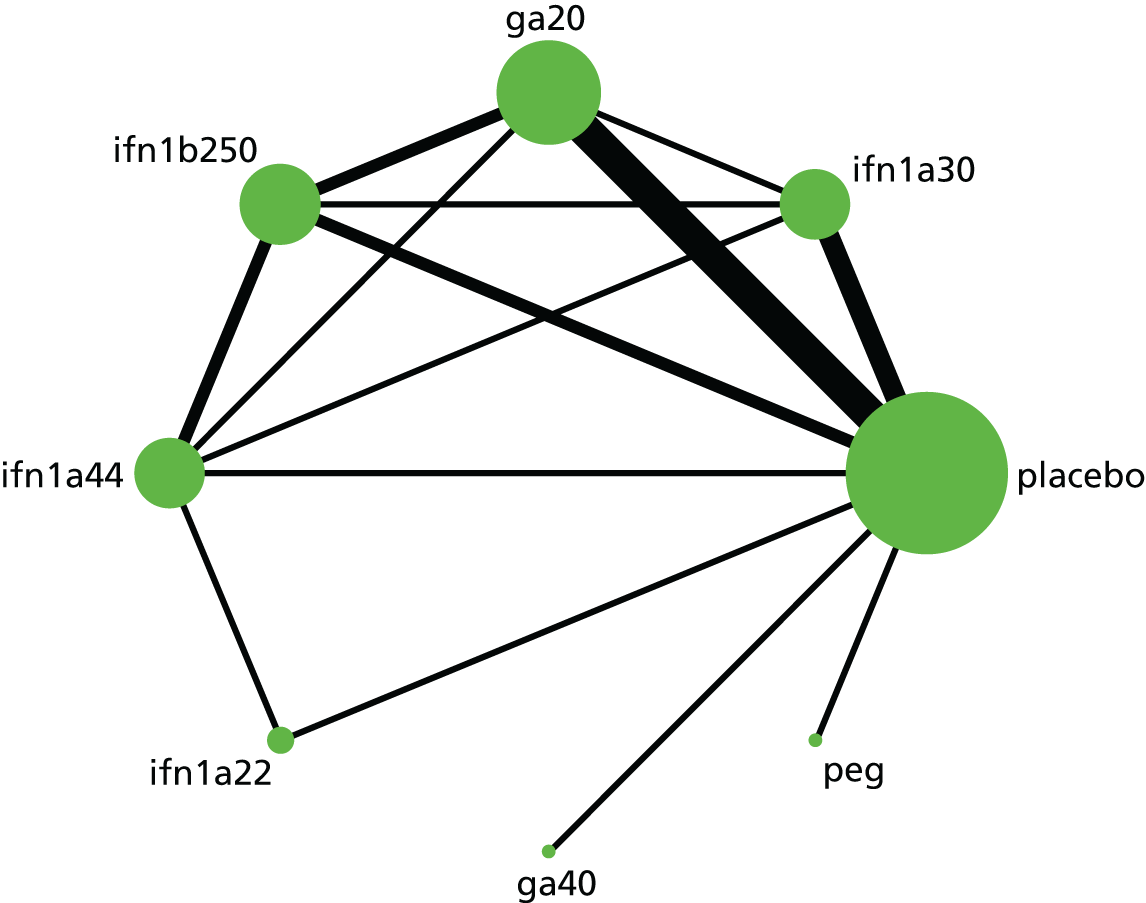
The NMA, which was estimated with random effects as per the protocol, generated estimates for each drug compared with placebo and with every other drug (Table 15). The NMA did not offer statistical evidence that any one drug was more likely to result in discontinuation because of AEs than any other drug. Based on SUCRAs, 125 µg of SC pegylated IFN-β-1a every 2 weeks was ranked highest for risk of discontinuation because of AEs (i.e. greatest risk of discontinuation), followed by 44 µg of SC IFN-β-1a three times weekly. Placebo was ranked last.
| Drug | SUCRA | Pegylated IFN-β-1a 125 µg SC every 2 weeks | IFN-β-1a 44 µg SC three times weekly | GA 40 mg SC three times weekly | IFN-β-1b 250 µg SC every other day | IFN-β-1a 30 µg IM weekly | GA 20 mg SC once daily | IFN-β-1a 22 µg SC three times weekly | Placebo |
|---|---|---|---|---|---|---|---|---|---|
| Pegylated IFN-β-1a 125 µg SC every 2 weeks | 0.82 | 1.40 (0.31 to 6.45) | 1.48 (0.29 to 7.43) | 1.99 (0.43 to 9.15) | 2.15 (0.57 to 8.04) | 2.24 (0.59 to 8.44) | 2.82 (0.35 to 23.04) | 3.49 (1.13 to 10.76) | |
| IFN-β-1a 44 µg SC three times weekly | 0.73 | 1.05 (0.22 to 4.95) | 1.42 (0.61 to 3.30) | 1.53 (0.65 to 3.59) | 1.60 (0.76 to 3.36) | 2.01 (0.45 to 9.01) | 2.49 (0.89 to 6.95) | ||
| GA 40 mg SC three times weekly | 0.66 | 1.35 (0.29 to 6.35) | 1.45 (0.38 to 5.60) | 1.52 (0.39 to 5.89) | 1.91 (0.23 to 15.88) | 2.36 (0.74 to 7.53) | |||
| IFN-β-1b 250 µg SC every other day | 0.50 | 1.08 (0.42 to 2.79) | 1.12 (0.51 to 2.49) | 1.42 (0.26 to 7.71) | 1.75 (0.63 to 4.89) | ||||
| IFN-β-1a 30 µg IM weekly | 0.45 | 1.04 (0.51 to 2.13) | 1.32 (0.24 to 7.17) | 1.62 (0.82 to 3.23) | |||||
| GA 20 mg SC once daily | 0.40 | 1.26 (0.24 to 6.50) | 1.56 (0.77 to 3.14) | ||||||
| IFN-β-1a 22 µg SC three times weekly | 0.33 | 1.24 (0.21 to 7.26) | |||||||
| Placebo | 0.12 | ||||||||
| Wald test for inconsistency (χ2, df, p-value) | 11.04, 6, 0.09 |
Because CIs were frequently wide in pairwise, direct meta-analyses, CIs were wide in the NMAs, and estimates compared with placebo were often numerically different. Compared with direct estimates from the PRISMS trial,189 evidence from the NMA suggested a numerically lower risk of discontinuation because of AEs for 44 µg of SC IFN-β-1a three times weekly compared with placebo (NMA: risk ratio 2.49, 95% CI 0.89 to 6.95; PRISMS trial:189 risk ratio 7.11, 95% CI 0.88 to 57.25), that is, the magnitude of the risk of discontinuation compared with placebo was smaller in the NMA than in the one trial informing the direct comparison. The same applied for 22 µg of SC IFN-β-1a three times weekly (NMA: risk ratio 1.24, 95% CI 0.21 to 7.26; PRISMS trial:189 risk ratio 2.97, 95% CI 0.31 to 28.28). Similarly, estimates for discontinuation because of AEs for 250 µg of SC IFN-β-1b every other day compared with placebo were lower in the NMA than in the pairwise meta-analysis (NMA: risk ratio 1.75, 95% CI 0.63 to 4.89; pairwise meta-analysis: risk ratio 4.93, 95% CI 0.76 to 32.00). Estimates of discontinuation because of AEs were higher in the NMA for 20 mg of SC GA once daily compared with placebo (NMA: risk ratio 1.56, 95% CI 0.77 to 3.14; pairwise meta-analysis: risk ratio 1.07, 95% CI 0.64 to 1.79).
An overall Wald test for inconsistency in the network did not reach significance, but suggested some conflict between direct and indirect evidence (p = 0.09). Examination of the specific design effects from the design*treatment interaction model suggested that direct estimates of discontinuation because of AEs for 250 µg of SC IFN-β-1b every other day compared with placebo could be driving this result (design effect p = 0.075). However, a side-splitting test did not suggest an obvious source of conflict between direct and indirect evidence. Thus, although there is no statistically significant evidence of inconsistency in this network, the findings should be viewed with caution.
Comparison of network meta-analyses: modal follow-up compared with all time points
Neither NMA found evidence that one drug was superior to any other.
However, estimates for discontinuation because of AEs for active drugs compared with placebo tended to be lower in the network including all time points, possibly because the majority of studies included in this analysis that were set aside in the modal follow-up analysis included shorter follow-up periods (generally of ≤ 1 year). Estimates were essentially unchanged for 30 µg of IM IFN-β-1a once a week compared with placebo (modal follow-up: risk ratio 1.61, 95% CI 0.52 to 5.02; all time points: risk ratio 1.62, 95% CI 0.82 to 3.23).
Supplementary analyses: pooled effectiveness of disease-modifying therapies used in the risk-sharing scheme
In preparation for the cost-effectiveness analyses, we undertook a post hoc, supplementary, pairwise meta-analysis of all trials comparing DMTs in the RSS with placebo (i.e. excluding trials of pegylated IFN and 40 mg of GA, as well as head-to-head-only trials). We used a random-effects model and estimated outcomes for ARR and time to disability progression confirmed at 3 months. Based on 12 relevant trials examining the ARR, on-scheme DMTs reduced the relapse rate (RR 0.65, 95% CI 0.56 to 0.76). Across six relevant trials examining time to disability progression confirmed at 3 months, on-scheme DMTs delayed disability progression (HR 0.70, 95% CI 0.55 to 0.87).
Summary: relapsing–remitting multiple sclerosis
The studies suggested, and meta-analyses confirmed, that IFNs and GA reduce the relapse rate, reduce the rate of severe relapses (both measured using neurological rating scales and steroid treatment) and generally delay disability progression in RRMS. However, the findings were clearer for disability progression confirmed at 3 months than disability progression confirmed at 6 months. There was little evidence that any one drug was superior to any other, except for disability progression confirmed at 6 months, but networks were especially sparse. Findings for disability progression confirmed at 3 months did not match the findings for disability progression confirmed at 6 months. Freedom from disease activity, MS symptoms and HRQoL were infrequently reported and evidence for MS symptoms and HRQoL also suffered from poor reporting. Findings for discontinuations because of AEs, which were intended to be indicative, did not suggest that one drug was more likely to result in discontinuation than any other or, with few exceptions, than placebo. However, findings for discontinuations relied on networks with some limited evidence of inconsistency.
In Table 16 we summarise the main clinical effectiveness and safety outcome results from the NMAs for each DMT compared with placebo.
| Drug | ARR | Time to sustained disability progression confirmed at 3 months | Time to sustained disability progression confirmed at 6 months | Discontinuation because of AEs at all time points | ||||
|---|---|---|---|---|---|---|---|---|
| SUCRA | RR (95% CI) | SUCRA | HR (95% CI) | SUCRA | HR (95% CI) | SUCRA | Risk ratio (95% CI) | |
| IFN-β-1a 22 µg SC three times weekly | 0.43 | 0.72 (0.61 to 0.85) | 0.62 | 0.68 (0.49 to 0.96) | – | – | 0.33 | 1.24 (0.21 to 7.26) |
| IFN-β-1a 44 µg SC three times weekly | 0.64 | 0.68 (0.60 to 0.76) | 0.77 | 0.63 (0.46 to 0.86) | 0.70 | 0.47 (0.24 to 0.93) | 0.73 | 2.49 (0.89 to 6.95) |
| IFN-β-1a 30 µg IM weekly | 0.18 | 0.80 (0.72 to 0.88) | 0.50 | 0.73 (0.53 to 1.00) | 0.40 | 0.68 (0.49 to 0.94) | 0.45 | 1.62 (0.82 to 3.23) |
| IFN-β-1b 250 µg SC every other day | 0.56 | 0.69 (0.62 to 0.76) | 0.39 | 0.78 (0.59 to 1.02) | 0.90 | 0.34 (0.18 to 0.63) | 0.5 | 1.75 (0.63 to 4.89) |
| Pegylated IFN-β-1a 125 µg SC every 2 weeks | 0.73 | 0.64 (0.50 to 0.83) | 0.75 | 0.62 (0.40 to 0.97) | 0.71 | 0.46 (0.26 to 0.81) | 0.82 | 3.49 (1.13 to 10.76) |
| GA 20 mg SC once daily | 0.77 | 0.65 (0.59 to 0.72) | 0.44 | 0.76 (0.60 to 0.97) | 0.25 | 0.82 (0.53 to 1.26) | 0.4 | 1.56 (0.77 to 3.14) |
| GA 40 mg SC three times weekly | 0.70 | 0.66 (0.54 to 0.80) | – | – | – | – | 0.66 | 2.36 (0.74 to 7.53) |
Clinical effectiveness: secondary progressive multiple sclerosis
This analysis was informed by three trials. 222–224 It should be noted that, although all studies included both relapsing and non-relapsing patients, only the SPECTRIMS (Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS) trial224 presented subgroup analyses by history of previous relapses in SPMS.
44 µg and 22 µg of interferon beta-1a subcutaneously three times a week (Rebif) compared with placebo
One trial evaluated both the 44-µg dose and the 22-µg dose of SC IFN-β-1a three times a week compared with placebo. 224
Relapse outcomes
In the SPECTRIMS trial,224 618 patients were followed up for 3 years. RRs based on ARRs were numerically identical for both active arms compared with placebo (44 µg: RR 0.69, 95% CI 0.56 to 0.85; 22 µg: RR 0.69, 95% CI 0.56 to 0.84).
Subgroup analyses stratifying by whether patients had a history of relapse showed a pattern of significant results for those previously relapsing and non-significant results for those not previously relapsing. 224 For those previously relapsing, ARRs for the 44-µg dose (0.67; p < 0.001) and the 22-µg dose (0.57; p < 0.001) were significantly different from the ARR in the placebo arm (1.08). For those not previously relapsing, ARRs for both dosages (44 µg: 0.43, p > 0.05; 22 µg: 0.36, not significant) were not significantly different from the ARR in the placebo arm (0.39).
Both active arms also showed a similar delay in time to first relapse, although only the 44-µg dose had a significant effect compared with placebo (HR 0.77, 95% CI 0.61 to 0.98), corresponding to a median time to first relapse of 494 days in the 44-µg dose group compared with 281 days in the placebo group. 224 Although the results for the 22-µg dose group compared with the placebo group were similar (476 days compared with 281 days), this did not translate into a significant effect (HR 0.87, 95% CI 0.69 to 1.10). The difference between the two active arms was not calculated in this trial, although the HR for the 44-µg dose compared with the 22-µg dose can be approximated as 0.77/0.87 = 0.89, which is not statistically different from unity.
Relapse severity
Both active arms in the SPECTRIMS trial224 showed a similar reduction in the annualised rate of moderate or severe relapses (44 µg: RR 0.68, 95% CI 0.44 to 0.81; 22 µg: RR 0.66, 95% CI 0.51 to 0.86). 224 The findings were similar for annualised rates of steroid courses used to treat relapses (44 µg: RR 0.66, 95% CI 0.49 to 0.89; 22 µg: RR 0.59, 95% CI 0.44 to 0.81).
Disability progression
In the SPECTRIMS trial,224 disability progression was confirmed at 3 months. Neither active drug was associated with a significant decrease in hazard for time to confirmed disability progression in the main analysis (44 µg: HR 0.83, 95% CI 0.65 to 1.07; 22 µg: 0.88, p = 0.305), nor were the active arms substantially different. However, an analysis controlling for disease characteristics found a significant difference in the 44-µg arm (HR 0.78, 95% CI 0.60 to 1.00) compared with the control arm.
Subgroup analyses combined the two active drug dosages into one arm and stratified models by whether patients had a history of relapse. 224 The HR for time to confirmed disability progression suggested a positive, although non-significant, effect in previously relapsing patients (0.74; p = 0.055), whereas the HR approached unity in non-relapsing patients (1.01; p = 0.934). However, among previously relapsing patients, the proportion of patients with confirmed disability progression was significantly different between those receiving 44/22 µg of the active drug and those receiving placebo (OR 0.52, 95% CI 0.29 to 0.93), whereas among those not previously relapsing, the proportion of patients with confirmed disability progression was not significantly different between those receiving 44/22 µg of the active drug and those receiving placebo (OR 1.07, 95% CI 0.64 to 1.78).
Freedom from disease activity
We were unable to locate any relevant comparisons between 44 µg or 22 µg of SC IFN-β-1a three times a week and placebo for combined clinical–MRI measures of freedom from disease activity.
Multiple sclerosis symptoms and health-related quality of life
We were unable to locate any relevant comparisons between 44 µg or 22 µg of SC IFN-β-1a three times a week and placebo for MS symptoms and HRQoL.
Adverse events and mortality
The SPECTRIMS trial224 reported AEs and mortality. Full results are available on request. Mortality was not significantly different between the groups: one patient died in the placebo arm whereas two patients died in the 44-µg arm and one patient died in the 22-µg arm.
250 µg of interferon beta-1b subcutaneously every other day (Betaferon/Extavia) compared with placebo
Two trials evaluated 250 µg of SC IFN-β-1b every other day. 222,223,225 The North American Study Group on Interferon beta-1b in Secondary Progressive MS (NASG) trial223 included a dosage of IFN-β-1b that is not recommended and thus this arm was not included in the analysis.
Relapse outcomes
In the European Study Group on Interferon β-1b in Secondary Progressive MS (ESG) trial,222,225 718 patients were followed for up to 2 years. Patients receiving the study drug had a significantly lower ARR than those in the placebo arm (0.42 vs. 0.57; p = 0.003). We approximated this as a RR of 0.74 (95% CI 0.65 to 0.83). Similarly, for the 623 patients enrolled in the relevant study arms in the NASG trial223 and followed for up to 3 years before early study termination, patients receiving the study drug had a significantly lower ARR than placebo patients (0.16 vs. 0.28; p = 0.009). We approximated this as a RR of 0.57 (95% CI 0.43 to 0.75).
Both studies also demonstrated a statistically significant delay in time to first relapse in the active drug arm. In interim data from the ESG trial,222 the median time to first relapse was 644 days in the study drug arm compared with 403 days in the placebo arm (log-rank p = 0.003). In the NASG trial,223 end-of-study data demonstrated a time to relapse at the 30th percentile of 1051 days in the study drug arm compared with 487 days in the placebo arm (log-rank p = 0.01). However, in the ESG trial222 the proportions relapsing were not significantly different between the groups (57.5% in the study drug arm vs. 62.0% in placebo, p = 0.083), whereas in the NASG trial there was a significant difference between the groups (29% vs. 38%; p = 0.018).
Relapse severity
Both studies showed a significant difference between the study drug arm and the placebo arm in the proportion of patients experiencing moderate or severe relapses (ESG trial interim data:222 43.6% vs. 53.1%, p = 0.0083; NASG trial:223 21% vs. 30%, p = 0.012). In the NASG trial,223 the annualised rate of moderate or severe relapses was significantly lower in the study drug arm than in the placebo arm (0.10 vs. 0.19; p = 0.022). However, it should be noted that outcome tables for the NASG trial presented two markedly different estimates of relapse severity. Under the second set of estimates, neither the proportion of patients with moderate or severe relapses (3% vs. 6%; p = 0.056) or the annualised rate of moderate or severe relapses (0.01 vs. 0.02; p = 0.052) was significantly different between the arms. Contact with the study investigators did not yield clarification.
In both studies, the percentage of patients treated with steroids also decreased significantly in the study drug arm compared with the placebo arm (ESG trial interim data:222 53.6% vs. 67.9%, p < 0.0001; NASG trial:223 37% vs. 46%, p = 0.023).
Disability progression
In the ESG trial,225 progression was measured using a variety of criteria, including progression of at least 1.0 EDSS points confirmed at 3 months and at 6 months and progression of 2.0 EDSS points confirmed at 3 months. Each of these measures was estimated both excluding data collected during relapses (the default) and including relapse data, but proportions were similar in all cases between measures including and measures excluding data collected during relapses and thus only the default measures are discussed here. The proportion of patients progressing at least 1.0 EDSS point confirmed at 3 months was significantly lower in the study drug arm than in the placebo arm (45.3% vs. 53.9%; p = 0.031). Combined with estimated probabilities from a life table model (estimated non-progression at 33 months 53% vs. 44%) and a log-rank p-value of 0.003, this yielded an approximate HR of 0.75 (95% CI 0.61 to 0.92). The proportions with confirmed progression of at least 1.0 EDSS point at 6 months (40.8% vs. 48.6%; p = 0.049) and with confirmed progression of at least 2.0 EDSS points at 3 months (16.4% vs. 22.6%; p = 0.032) showed similar trends. However, in the NASG trial,223 disability progression was confirmed at 6 months and did not show a significant difference in terms of time to progression (study drug 32% vs. placebo 34%; log-rank p = 0.61).
Similarly, although patients in the ESG trial225 showed a significant difference between arms in the average EDSS progression score (0.47 vs. 0.69 points; p = 0.003), patients in the NASG trial223 did not (0.53 vs. 0.62 points; p = 0.634).
Freedom from disease activity
We were unable to locate any relevant comparisons between 250 µg of SC IFN-β-1b every other day and placebo for combined clinical–MRI measures of freedom from disease activity.
Multiple sclerosis symptoms and health-related quality of life
In the NASG trial,223 change from baseline was not significantly different between patients in the study drug arm and patients in the placebo arm for fatigue (Environmental Status Scale score change 1.7 vs. 1.2; p = 0.125), cognition (composite neuropsychological score change –0.28 vs. –0.32; p = 0.42) or depression (Beck Depression Inventory score change –0.5 vs. –1.0; p = 0.652; percentage newly treated with antidepressants 29% vs. 29%; p = 0.987). Changes in overall Multiple Sclerosis Quality of Life Inventory scores were also not significantly different (p = 0.502).
Adverse events and mortality
Both studies reported AEs and mortality. Full results are available on request. Studies were not significantly different with regard to mortality: there were seven deaths in the combined 250 µg of SC IFN-β-1b every other day arms of the two trials and two deaths in the combined placebo arms.
Meta-analyses: relapse rate
Pairwise meta-analyses
Direct evidence from comparisons is shown in Figure 20. The SPECTRIMS trial224 compared 44 µg of SC IFN-β-1a three times weekly, 22 µg of SC IFN-β-1a three times weekly and placebo, whereas the other two included trials compared 250 µg of SC IFN-β-1b every other day with placebo. The pooled effect of 250 µg of SC IFN-β-1b every other day compared with placebo suggested that the drug reduces the rate of relapse (RR 0.71, 95% CI 0.63 to 0.79).
FIGURE 20.
Pairwise meta-analyses: ARRs in SPMS.
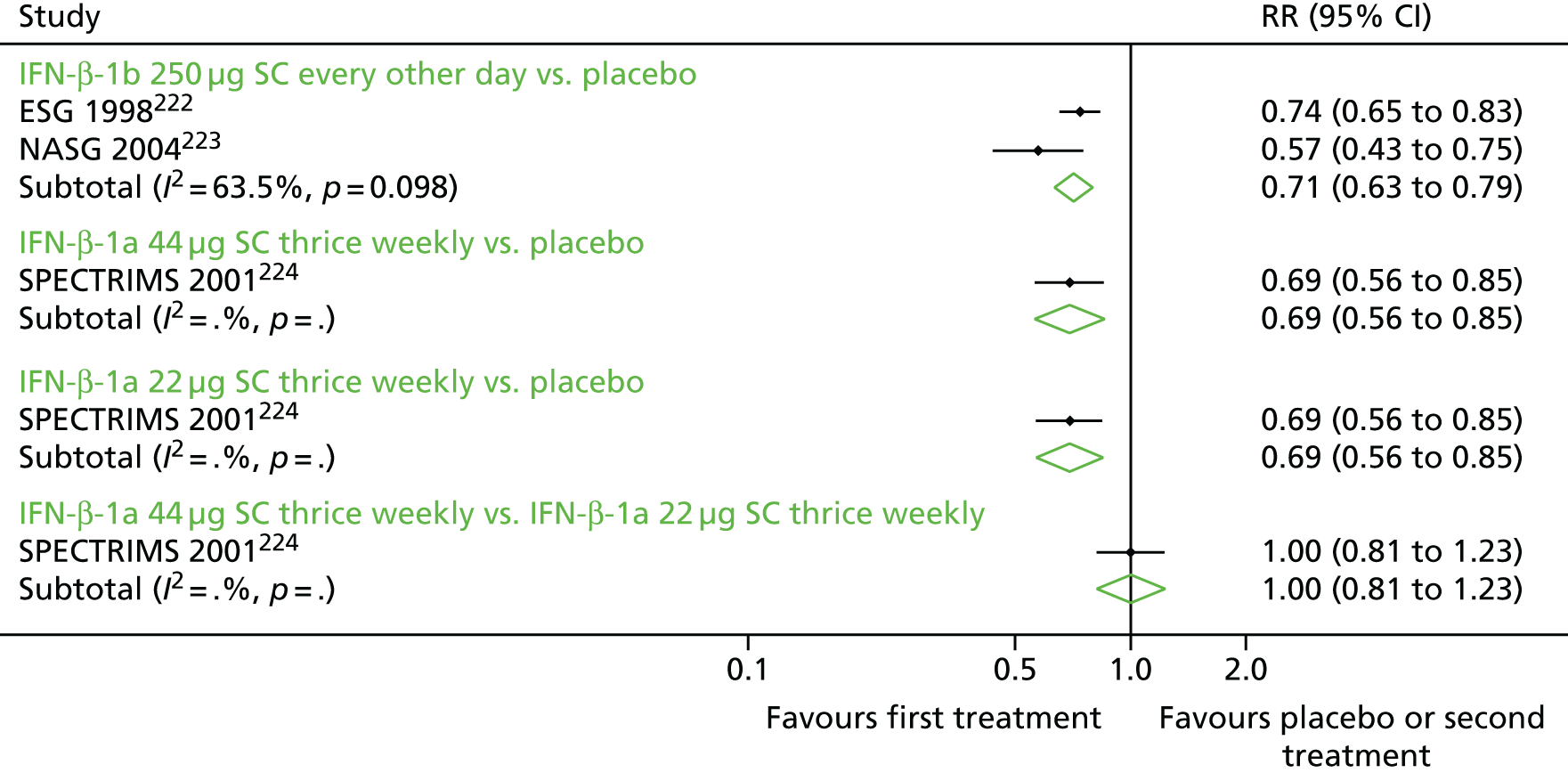
Network meta-analyses
Ranking of the drugs in the resultant network suggested that 250 µg of SC IFN-β-1b every other day was superior to the equally ranked 44 µg of SC IFN-β-1a three times weekly and 22 µg of SC IFN-β-1a three times weekly (Table 17). Placebo was ranked last. Findings for comparisons between active drugs and placebo were, as would be expected, essentially the same as in the direct evidence. Comparisons between 250 µg of SC IFN-β-1b every other day and both 44 µg of SC IFN-β-1a three times weekly and 22 µg of SC IFN-β-1a three times weekly did not suggest a statistically significant difference between the drugs in terms of effectiveness (44 µg: HR 0.97, 95% CI 0.63 to 1.50; 22 µg: HR 0.97, 95% CI 0.63 to 1.49). Because there was no possibility of inconsistency in the network, we did not test for it.
| Drug | SUCRA | IFN-β-1b 250 µg SC every other day | IFN-β-1a 44 µg SC three times weekly | IFN-β-1a 22 µg SC three times weekly | Placebo |
|---|---|---|---|---|---|
| IFN-β-1b 250 µg SC every other day | 0.71 | 0.97 (0.63 to 1.50) | 0.97 (0.63 to 1.49) | 0.67 (0.52 to 0.86) | |
| IFN-β-1a 44 µg SC three times weekly | 0.64 | 1.00 (0.71 to 1.42) | 0.69 (0.49 to 0.98) | ||
| IFN-β-1a 22 µg SC three times weekly | 0.64 | 0.69 (0.49 to 0.98) | |||
| Placebo | 0.01 |
Meta-analyses: relapse severity
We did not undertake meta-analyses for relapse severity in SPMS because of the quality and scarcity of the data.
Meta-analyses: time to disability progression confirmed at 3 months
Pairwise meta-analyses
Direct evidence from comparisons is shown in Figure 21. Comparisons included two trials. 222,224,225 The findings are the same as for the individual trials.
FIGURE 21.
Pairwise comparisons: time to disability progression confirmed at 3 months in SPMS.

Network meta-analyses
Because of the shape of the network, in which there was no opportunity for inconsistency and in which no direct comparison was informed by more than one trial, the model was estimated using fixed effects instead of random effects, as in the protocol. Ranking of drugs in the resultant network suggested that 250 µg of SC IFN-β-1b every other day was superior to 44 µg of SC IFN-β-1a three times weekly and 22 µg of SC IFN-β-1a three times weekly (Table 18). Placebo was ranked last. Findings for comparisons between active drugs and placebo were, as would be expected, essentially the same as in the direct evidence. Comparisons between 250 µg of SC IFN-β-1b every other day and both 44 µg of SC IFN-β-1a three times weekly and 22 µg of SC IFN-β-1a three times weekly did not suggest a statistically significant difference between the drugs in terms of effectiveness (44 µg: HR 0.91, 95% CI 0.65 to 1.25; 22 µg: HR 0.85, 95% CI 0.62 to 1.18). Because there was no possibility for inconsistency in the network, we did not test for it.
| Drug | SUCRA | IFN-β-1b 250 µg SC every other day | IFN-β-1a 44 µg SC three times weekly | IFN-β-1a 22 µg SC three times weekly | Placebo |
|---|---|---|---|---|---|
| IFN-β-1b 250 µg SC every other day | 0.85 | 0.91 (0.65 to 1.25) | 0.85 (0.62 to 1.18) | 0.75 (0.61 to 0.92) | |
| IFN-β-1a 44 µg SC three times weekly | 0.64 | 0.94 (0.74 to 1.21) | 0.83 (0.65 to 1.06) | ||
| IFN-β-1a 22 µg SC three times weekly | 0.44 | 0.88 (0.69 to 1.12) | |||
| Placebo | 0.07 |
Meta-analyses: time to disability progression confirmed at 6 months
Only one trial223 reported an effect size for time to disability progression confirmed at 6 months. In the comparison between 250 µg of SC IFN-β-1b every other day and placebo, there was no statistically significant effect of the study drug on time to disability progression. We imputed this HR as 0.93 (95% CI 0.71 to 1.22).
Meta-analyses: adverse events
Summary of adverse events meta-analyses
Full results for pairwise meta-analyses of AEs are available on request. Although the diversity and heterogeneity of AEs precluded detailed examination of each, several trends were apparent across pairwise comparisons. SC IFN-β-1a three times weekly was associated with more application site disorders, more cases of necrosis, higher levels of alanine aminotransferase and aspartate aminotransferase, more cases of leucopenia and lymphopenia, higher levels of NABs and a higher number of patients who discontinued study treatment because of AEs than placebo. Comparing 250 µg of SC IFN-β-1b every other day with placebo, IFN-β-1b was associated with more injection site inflammation, more cases of necrosis, pain, injection site reactions, chest pain, chills only, chills and fever, fever only, flu syndrome, hypertonia, leucopenia, lymphadenopathy, lymphopenia, higher levels of NABs, rash and a higher number of patients who discontinued study treatment because of AEs.
Meta-analyses: discontinuation because of adverse events
Pairwise meta-analyses
All three studies presented data for discontinuation of the study drug because of AEs and all studies included follow-up of 36 months. Pairwise estimates are provided in Figure 22. Compared with placebo, all drugs were associated with a significant increase in the risk of discontinuation because of AEs.
FIGURE 22.
Pairwise meta-analyses: discontinuation because of AEs in SPMS. RR, risk ratio.

Network meta-analyses
Studies formed a star-shaped network. Examination of SUCRAs in the resultant network suggested that 44 µg of SC IFN-β-1a three times weekly was ranked highest for discontinuation of the study drug because of AEs (i.e. associated with the greatest risk), followed by 22 µg of SC IFN-β-1a three times weekly and 250 µg of SC IFN-β-1b every other day (Table 19). Placebo was ranked last.
| Drug | SUCRA | IFN-β-1a 44 µg SC three times weekly | IFN-β-1a 22 µg SC three times weekly | IFN-β-1b 250 µg SC every other day | Placebo |
|---|---|---|---|---|---|
| IFN-β-1a 44 µg SC three times weekly | 0.81 | 1.23 (0.64 to 2.37) | 1.32 (0.46 to 3.83) | 3.62 (1.37 to 9.56) | |
| IFN-β-1a 22 µg SC three times weekly | 0.60 | 1.08 (0.37 to 3.18) | 2.94 (1.09 to 7.95) | ||
| IFN-β-1b 250 µg SC every other day | 0.58 | 2.73 (1.78 to 4.19) | |||
| Placebo | 0.01 |
As would be expected, estimates from comparisons with placebo were unchanged in the NMA compared with the pairwise meta-analysis. There was no evidence from the NMA that any one drug was more likely to result in discontinuations because of AEs than any other drug.
Because there was no opportunity for inconsistency in the network, we did not test for it.
Summary: secondary progressive multiple sclerosis
Findings for SPMS patients with a recent history of relapses were not reported consistently by studies; thus, the findings should be regarded with caution. Taken together, the three studies suggested that the included drugs reduced the relapse rate and relapse severity relative to placebo, although we were unable to clarify issues related to relapse severity data from one trial. Findings for disability progression were mixed. We were unable to locate any relevant comparisons for combined clinical–MRI measures of freedom from disease activity. One study reported MS symptom data and did not find evidence of differences between the study drug and placebo. There were no significant differences between the study drugs and placebo in terms of mortality. Each drug was associated with an increased risk of discontinuation because of AEs.
Network meta-analyses of ARR and time to disability progression confirmed at 3 months did not suggest superiority of one drug over another, nor did NMAs of discontinuation because of AEs suggest that one drug was more likely to result in discontinuation than any other. We did not undertake meta-analyses for relapse severity because of unresolved questions about one of the three included studies and because only one study reported time to disability progression confirmed at 6 months.
Overall summary of clinical effectiveness findings
In CIS, each included drug showed evidence of delaying time to CDMS. The NMA did not show evidence of the superiority of one drug over another, although the network was sparse and only one drug was represented by more than one trial. In RRMS, drugs showed good evidence of reducing the relapse rate, including the rate of moderate or severe relapses and, in most cases, the rate of steroid-treated relapses. There was little evidence of the superiority of one drug over another in reducing the relapse rate. Some drugs, but not all, delayed time to disability progression confirmed at 3 months, although there was no evidence of the superiority of one drug over any other. The NMA for time to disability progression confirmed at 6 months indicated that most drugs showed improvement over placebo in delaying time to progression, but this analysis was sparse and several comparisons against placebo relied solely on indirect evidence. Finally, in SPMS, all drugs reduced the relapse rate, although the network was sparse and relied on three studies. Time to confirmed disability progression at 3 months was measured in only two studies, which showed variable effects across treatments. Analyses of discontinuation because of AEs in RRMS and SPMS were indicative, but again did not point to one drug being more likely than any other to result in discontinuation because of an AE.
We were unable to undertake meta-analyses for additional outcomes – MS symptoms, HRQoL and freedom from disease activity – because of heterogeneity and sparsity of data and poor reporting for these outcomes. Additionally, no studies reported discontinuation because of loss of effect attributed to NABs.
The conclusions are tempered by several considerations. Analyses did not show a clear ‘winner’ across outcomes and, again, comparisons between drugs as part of NMA models were in the main inconclusive. Although the main model for ARR was the best-populated model, analyses for relapse severity were sparse. Analyses for time to disability progression confirmed at 6 months were especially sparse. In particular, several comparisons of drugs with placebo as part of this last model relied exclusively on indirect evidence. Moreover, analyses for time to progression confirmed at 3 and 6 months did not show a consistent pattern, except that all drugs were beneficial in delaying disability progression. This is particularly concerning, as progression confirmed at 6 months is considered to be a ‘stronger’ outcome than progression confirmed at 3 months. NMA models also had an imbalanced risk of bias across the networks of studies. For example, most active drug compared with active drug trials were open-label trials. Finally, trials relied on short follow-up times of mostly < 2 years in duration.
We used the drug-specific estimates for ARR, disability progression sustained at 3 months and disability progression sustained at 6 months derived from our NMAs in the economic modelling presented in Chapter 12. Our NMAs informed key clinical parameters in sensitivity analyses for our base-case model.
Chapter 6 Manufacturers’ submissions: clinical effectiveness
Three submissions were received from:
-
Merck for 44 µg and 22 µg of IM IFN-β-1a three times weekly (Rebif)
-
Teva Pharmaceutical Industries for 20 mg of SC GA daily or 40 mg of SC GA three times weekly (Copaxone)
-
Biogen Idec Ltd for 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) and 30 µg of IM IFN-β-1a weekly (Avonex).
44 µg and 22 µg of interferon beta-1a intramuscularly three times weekly (Rebif): summary of the Merck submission
The clinical effectiveness section of the submission presented an overview of the relevant trials sponsored by the manufacturer, including the following clinical effectiveness data.
Clinical effectiveness of Rebif in relapsing–remitting multiple sclerosis
The manufacturer’s submission stated that, in patients with RRMS, Rebif demonstrated short-term and long-term efficacy in reducing relapses and delaying disease progression compared with BSC. The submission included findings from the PRISMS trial,189 including its long-term and observational extensions, to support this claim. The manufacturer’s submission also presented findings from head-to-head trials, including the EVIDENCE,195 IMPROVE207 and REGARD192 trials.
Clinical effectiveness of Rebif in clinically isolated syndrome
The manufacturer’s submission stated that, in patients with CIS, Rebif demonstrated a reduction in the number of patients who progressed to a diagnosis of MS over the short and long term compared with BSC. The submission included findings from the REFLEX trial,175 including its long-term and observational extension, to support this claim.
Clinical effectiveness of Rebif in secondary progressive multiple sclerosis
The manufacturer’s submission stated that, in trials including subsets of patients with SPMS with relapses, Rebif has some, but not a significant, effect in terms of reducing the time to disability progression and a significant effect in terms of reducing the relapse rate. The submission included findings from the SPECTRIMS trial224 to support this claim.
Risk sharing scheme findings on the clinical effectiveness of Rebif
The year 10 RSS analysis and data for Rebif were included in the manufacturer’s submission. The submission stated that the HRs estimated from the RSS for disability progression for Rebif compared with BSC (confidential information has been removed) were within the 10% range for the target HR needed to result in clinical effectiveness. The manufacturer’s submission also noted that the RSS yielded an estimate of effectiveness for Rebif that was similar to estimates from the PRISMS trial.
Our assessment of the Merck submission
Our AMSTAR assessment of the manufacturer’s submission is provided in Table 20.
| AMSTAR checklist | Manufacturer’s submission |
|---|---|
| 1. Was an ‘a priori’ design provided? | Yes – the systematic review protocol was described in the submission appendix |
| 2. Was there duplicate study selection and data extraction? | Yes – all abstracts were reviewed by two experienced systematic reviewers according to the eligibility criteria; any differences in opinion regarding eligibility were resolved through discussion with a third reviewer. The same process was applied to the subsequent review of full papers |
| 3. Was a comprehensive literature search performed? | Yes – searches were performed in the following electronic databases: MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid SP); EMBASE (via Ovid SP); CENTRAL; and PubMed (for e-publications ahead of print). Abstracts from the following key international conferences were searched: Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Annual Meeting (2015); European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) Annual Congress (2015); ACTRIMS and ECTRIMS joint meeting (2014); American Academy of Neurology (AAN) Annual Meeting (2015); and American Neurological Association (ANA) Annual Meeting (2014 and 2015). Searches were run on 5 October 2015 |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? | No inclusion of grey literature |
| 5. Was a list of studies (included and excluded) provided? | Included studies were listed; excluded studies were not listed in the main submission but those excluded from the NMA were listed in the NMA document |
| 6. Were the characteristics of the included studies provided? | Interventions, doses, regimens, numbers of participants and the data arising from the review that was used to inform the NMA are shown in the appendix. Comparison tables of patient baseline characteristics and for the outcomes of ARR and sustained disability progression in the identified RCTs are available on request |
| 7. Was the scientific quality of the included studies assessed and documented? | Quality appraisal tables are available on request; not supplied because of the number of pages |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | Not stated that quality of studies used in formulating conclusions; no mention of sensitivity analyses by study quality |
| 9. Were the methods used to combine the findings of studies appropriate? | Methods appear appropriate |
| 10. Was the likelihood of publication bias assessed? | Not stated |
| 11. Was the conflict of interest included? | Manufacturer’s submission |
Review of the network meta-analyses methods
Model type
Network meta-analyses models were estimated in the Bayesian framework. Both fixed-effects and random-effects models were assessed according to the relative treatment-specific effect. The fit of the fixed-effects and random-effects models was compared using the deviance information criterion (DIC). A lower DIC is indicative of better fit. The best-fitting model was identified for each analysis. When the fit was similar between the fixed-effect model and the random-effects model, the random-effects model was adopted as a conservative approach. Moreover, the NMA included a comparison of the posterior distribution of between-study SDs with the prior distributions to assess whether it was updated by the available evidence (i.e. the additional information had had an effect). Consistency was assessed using node-splitting analyses.
(Confidential information has been removed.)
Prior distributions and estimation
The models were fitted using the OpenBUGS software package version 3.2.2 (Free Software Foundation, Inc., Boston, MA, USA). Models used 100,000 burn-in simulations with 150,000 simulations used. Flat priors were used in all cases for the treatment-specific, study-specific and between-study variance terms.
Interventions
The NMA included all trials testing licensed drugs with dosages at or below the recommended dose. Interventions and comparators of interest were immunosuppressives or immunomodulators: alemtuzumab (Lemtrada), dimethyl fumarate (Tecfidera), fingolimod (Gilenya), GA (Copaxone), IFN-β-1a (Avonex), IFN-β-1b (Betaferon/Extavia), pegylated IFN-β-1a, natalizumab (Tysabri) and teriflunomide (Aubagio).
Outcomes and data preparation
The NMA included analyses for ARR and disability progression. Models for disability progression included progression confirmed at 6 months, with additional data from confirmation at 3 months when 6-month data were not available, and the converse, that is, disability progression confirmed at 3 months, with additional data from confirmation at 6 months when 3-month data were not available. One potential issue with this method is that analyses are not strictly interpretable and rely on an assumption that progression estimates from 3 months and 6 months are exchangeable, but this is unclear and may be questionable.
Authors used an optimisation algorithm to estimate person-years and number of relapses to be used with an exact Poisson likelihood. Authors also used summary HRs in estimating disability progression models.
One strength of the reporting in this NMA was the transparency about included effect sizes for each model.
Participants
The NMA included all patients with a diagnosis of RRMS or progressive relapsing MS (PRMS). The NMA included an informal assessment of the similarity of baseline characteristics across trials. The authors did not undertake meta-regression or subgroup analyses.
Included trials
Unlike the assessment group’s NMA, the NMA in the manufacturer’s submission included trials with comparators outside the NICE scope. 141 However, even though the NMA in the manufacturer’s submission did not set explicit restrictions on the duration of follow-up, several trials appeared to be missing, including the BRAVO trial,198 IMPROVE trial,207 trial by Knobler et al. ,211 trial by Kappos et al. 199 and the GATE trial. 220 Although some of these trials may have been published after the last search, it is not clear why they were excluded.
Findings from the network meta-analyses presented in the manufacturer’s submission
Annualised relapse rate findings
A lower ARR is indicative of a better response. Although the submitted NMA covered a variety of doses and drugs, we summarise here only those results relating to licensed doses of the drugs under consideration.
(Confidential information has been removed.)
Sustained disability progression findings
(Confidential information has been removed.)
Results compared with the results of the assessment group’s network meta-analyses
For ARR, the results for 22 µg of IFN-β-1a three times weekly and 44 µg of IFN-β-1a three times weekly compared with placebo were similar in the manufacturer’s NMA and in the assessment group’s NMA.
(Confidential information has been removed.) This was also the case in the assessment group’s NMA.
The ‘blending’ method used in the manufacturer’s NMA for analyses of sustained disability progression at 3 months and 6 months means that its analyses are not strictly commensurate with the assessment group’s NMAs. Over both analyses, the assessment group’s NMAs suggested a significant effect of 22 µg of IFN-β-1a three times weekly and 44 µg of IFN-β-1a three times weekly. (Confidential information has been removed.)
Summary of the Merck submission
The quality of the submitted systematic review and NMA was reasonable and appropriate and the findings matched in magnitude and direction, although not always in significance, the corresponding findings from the assessment group’s NMAs. The assessment group did note challenges with the interpretation of the combined disability progression models and observed that several ostensibly relevant trials were not included in the NMA. Additionally, the submission included trials of patients with PRMS, which was outside the NICE scope141 for this submission. NMAs were not presented for CIS or SPMS.
20 mg of glatiramer acetate subcutaneously daily or 40 mg of glatiramer acetate subcutaneously three times weekly (Copaxone): summary of the Teva Pharmaceutical Industries submission
Clinical effectiveness of Copaxone in relapsing–remitting multiple sclerosis and clinically isolated syndrome
The manufacturer’s submission stated that GA in both doses (20 mg SC daily and 40 mg SC three times weekly) reduces the ARR and disability progression. It cited the trial by Bornstein et al. ,170 the Cop1 MSSG trial,217 the ECGASG trial,219 the trial by Calabrese et al. ,188 the CONFIRM trial216 and the GALA trial221 in support of this claim. It further noted that GA in its 20-mg SC daily dose delays progression to CDMS, citing the PreCISe trial174 and its extension. 235
Risk sharing scheme findings on the clinical effectiveness of Copaxone
The manufacturer’s submission stated that, based on the year 10 RSS analysis, 20 mg of SC GA once daily reduced EDSS disability progression at 10 years (confidential information has been removed), with no evidence of a treatment waning effect at 10 years compared with the updated 6-year analysis. Based on the year 6 data, the manufacturer’s submission stated that, compared with the total IFN-β cohort together, the Copaxone cohort (confidential information has been removed).
Our assessment of the Teva Pharmaceutical Industries submission
Our assessment of the systematic review contained in the Teva submission is provided in Table 21.
| AMSTAR checklist | Manufacturer’s submission |
|---|---|
| 1. Was an ‘a priori’ design provided? | Yes – protocol in the submission appendix |
| 2. Was there duplicate study selection and data extraction? | Not stated |
| 3. Was a comprehensive literature search performed? | Yes – searches were performed in PubMed, EMBASE and The Cochrane Library |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? | No mention of grey literature |
| 5. Was a list of studies (included and excluded) provided? | Included studies were listed in the appendix; a list of excluded studies was not provided |
| 6. Were the characteristics of the included studies provided? | Yes – in the appendix |
| 7. Was the scientific quality of the included studies assessed and documented? | Yes – in the appendix |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | An analysis of heterogeneity in the included studies was carried out and a number of potential sources of heterogeneity were identified. The main sources of heterogeneity and their impacts were investigated further through sensitivity analyses. The following sensitivity analyses were conducted: exclusion of studies with < 2 years’ follow-up; exclusion of studies with < 50 patients per treatment arm; and a separate analysis of 3-month and 6-month confirmed disability progression. However, it does not appear that sensitivity analyses were carried out using overall quality scores. The results of RCTs were shown separately from the results of non-randomised studies |
| 9. Were the methods used to combine the findings of studies appropriate? | Results were tabulated but not combined in forest plots |
| 10. Was the likelihood of publication bias assessed? | Not stated |
| 11. Was the conflict of interest included? | Manufacturer’s submission |
Review of the network meta-analyses methods
Model type
Models were estimated in the Bayesian framework. Both fixed-effects and random-effects models were estimated and then compared on fit. The authors also estimated pairwise meta-analyses and heterogeneity statistics.
Prior distributions and estimation
The authors used non-informative prior distributions. The authors used WinBUGS version 1.4.3 software (MRC Biostatistics Unit, Cambridge, UK) in all NMAs. In each model, two parallel chains were run, with a 50,000 iteration burn-in period. A total of 20,000 iterations against a thinning fact of 10 were sampled from each of the two chains. Convergence was assessed using Brooks–Gelman–Rubin diagnostics.
Interventions
All licensed drugs were included. Dosages were not specified, which poses significant ambiguity about whether all dosages in the literature were considered or only those that correspond to the marketing authorisation. It appears that both dosages of GA were pooled into one node in the analysis, but this was not clear.
Outcomes and data preparation
For disability progression, the authors estimated the number of events and the person-years of follow-up in each study and analysed data using a binomial likelihood with a complementary log-log link. Analyses used a model in which disability progression confirmed at 6 months was preferred, with 3-month data used when 6-month data were not available. Analyses of ARR used an arm-level data approach with a Poisson likelihood.
Although the authors presented relevant arm-level data for trials including GA in the text of the submission, it was not clear what the NMA inputs were. No forest plots for individual study estimates were presented.
Participants
Only participants with RRMS were included in the NMA.
Included trials
Unlike the assessment group’s NMA, the NMA in the manufacturer’s submission included trials with comparators outside the NICE scope. 141 However, the authors also excluded studies with a follow-up of < 6 months. Within these restrictions, it appears that the authors captured all relevant trials, although the trial by Knobler et al. 211 was not included.
Findings from the network meta-analyses presented in the manufacturer’s submission
Annualised relapse rate findings
(Confidential information has been removed.)
Sustained disability progression findings
(Confidential information has been removed.)
Results compared with the results of the assessment group’s network meta-analyses
(Confidential information has been removed.) HRs for disability progression at 3 months and 6 months were blended and pooled across Copaxone doses in the manufacturer’s submission, but were analysed separately in the assessment group’s NMA; thus, the findings are not strictly commensurate. (Confidential information has been removed) in the assessment group’s NMA the HR for disease progression for GA compared with placebo was significantly better at 3 months (HR 0.76, 95% CI 0.60 to 0.97) only and not at 6 months (HR 0.82, 95% CI 0.53 to 1.26). Point estimates for disability progression were similar.
Summary of the Teva Pharmaceutical Industries submission
The quality of the submitted systematic review and NMA was reasonable and appropriate and the findings matched in magnitude and direction, although not always in significance, the corresponding findings from the assessment group’s NMAs. The assessment group did note challenges with the interpretation of the combined disability progression models, but found that the inclusion of trials was reasonable and clear. However, there was a considerable lack of transparency about the inputs that were used for each NMA model and no forest plots were presented. Additionally, it was not clear how dosages were used in the included models. NMAs were not presented for CIS.
30 µg of interferon beta-1a intramuscularly weekly (Avonex) and 125 µg of pegylated interferon beta-1a subcutaneously every 2 weeks (Plegridy): summary of the Biogen Idec Ltd submission
Clinical effectiveness of Avonex in relapsing–remitting multiple sclerosis and clinically isolated syndrome
The manufacturer’s submission stated that 30 µg of IM IFN-β-1a weekly is effective in reducing the relapse rate and disability progression compared with placebo, citing the MSCRG trial200 and its observational extension236 as evidence. The submission further stated that 30 µg of IM IFN-β-1a weekly is effective in delaying CDMS in patients with CIS, citing the CHAMPS trial172 and its open-label extension237 in support of this.
Risk sharing scheme findings on the clinical effectiveness of Avonex
The clinical effectiveness of Avonex in the RSS showed that, in the year 10 analysis, (confidential information has been removed).
Clinical effectiveness of Plegridy in relapsing–remitting multiple sclerosis
The manufacturer’s submission stated that 125 µg of SC pegylated IFN-β-1a every 2 weeks is effective in reducing the relapse rate and disability progression compared with placebo, citing the ADVANCE trial213 as well as its extension238 in support of this. Plegridy was not included in the RSS.
Our assessment of the Biogen Idec Ltd submission
Our assessment of the systematic review contained in the Biogen Idec Ltd submission is provided in Table 22.
| AMSTAR checklist | Manufacturer’s submission |
|---|---|
| 1. Was an ‘a priori’ design provided? | Yes – Table 37 |
| 2. Was there duplicate study selection and data extraction? | Yes – the literature searches for this review were conducted as part of a wider programme of research on treatments for MS. Search strategies included terms designed to identify studies of all European Union (EU)-approved treatments or treatments expected to be approved in the near future in CIS, RRMS or SPMS patients. Identified studies were independently assessed by a reviewer to ascertain whether they met the predefined inclusion and exclusion criteria [based on population, interventions, comparators and outcomes (PICOS)], with any uncertainties resolved by discussion with a second reviewer. Data were extracted from eligible publications into a predefined table by a reviewer. All studies meeting the inclusion criteria described in Table 37 were initially included in the systematic review. These studies were then screened by two reviewers against the PICOS criteria of the NICE MTA of IFN-β and GA (NICE scope141) for treating MS to identify relevant studies for inclusion in meta-analyses and narrative syntheses |
| 3. Was a comprehensive literature search performed? | Yes – searches were conducted in October 2014 and updated on 9 November 2015 in MEDLINE (including MEDLINE In-Process & Other Non-Indexed Citations and MEDLINE Daily Update), EMBASE, CENTRAL and Science Citation Index, with no restrictions on date. Using Boolean operators, the searches combined terms [including medical subject headings (MeSH) as appropriate] for the condition, the treatments and the outcomes of interest. A rapid appraisal was also conducted to identify relevant systematic reviews, TAs, guidelines and guidance in the following databases: Cochrane Database of Systematic Reviews, DARE, HTA database, NICE, National Institute for Health Research (NIHR), Canadian Agency for Drugs and Technologies in Health (CADTH) and PROSPERO. In addition, searches were conducted in the following clinical trial registers to identify data from ongoing or unpublished clinical trials: ClinicalTrials.gov, Current Controlled Trials, WHO ICTRP, PharmNet.Bund and EU Clinical Trials Register (EUCTR). The full search strategies were provided in appendix E. Hand searching of reference lists from included studies and relevant systematic reviews was also conducted |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? | Unpublished trials were sought |
| 5. Was a list of studies (included and excluded) provided? | A summary of the 16 studies included in the multiple treatment comparison was provided in appendix G (Table 55). Details of studies included in the systematic review but excluded from the multiple treatment comparison were provided in appendix F (Table 54), along with the rationale for their exclusion. Excluded studies were listed in appendix 3 |
| 6. Were the characteristics of the included studies provided? | Yes – appendix G |
| 7. Was the scientific quality of the included studies assessed and documented? | Yes – Table 57 and appendix G |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | Not stated |
| 9. Were the methods used to combine the findings of studies appropriate? | Yes – sensitivity analyses took into account heterogeneity |
| 10. Was the likelihood of publication bias assessed? | As stated in the submission, ‘Publication bias would have been assessed using funnel plots (e.g. SE (log [RR]) vs. RR) where at least ten studies were included in an analysis; however, there were no head-to-head comparisons that included enough studies to produce a funnel plot’ (p. 205) |
| 11. Was the conflict of interest included? | Manufacturer’s submission |
Review of the network meta-analyses methods
Model type
Random-effects and fixed-effects models were both estimated and compared on the DIC, with random-effects models preferred throughout. Further iterations were captured if convergence was in question.
Prior distributions and estimation
Network meta-analyses were estimated in the Bayesian framework using gemtc in the R environment (The R Foundation for Statistical Computing, Vienna, Austria). After 50,000 burn-in iterations, a further 50,000 iterations were captured. Convergence was assessed using the Brooks–Gelman–Rubin diagnostic. Prior distributions were non-informative.
Interventions
All studies testing comparisons of the drugs in the NICE scope141 and at the dosages contained in the marketing authorisations were included. Thus, dosages were clearly specified.
Outcomes and data preparation
Analyses included ARR for studies with follow-up of at least 12 months; HRs for disability progression confirmed at 3 months and, separately, at 6 months, with follow-up data at 12 or 24 months; and ORs for either any AE or serious AE. Data were analysed as log RRs, log HRs or log ORs with corresponding standard errors. The authors did not provide a justification for models that were intended to be estimated at either 12 or 24 months’ follow-up or why they chose to stratify estimates in this way. There was a lack of clarity regarding study inputs and no forest plots for individual study estimates were presented.
Participants
Although the searches included patients with RRMS, CIS and SPMS, it appears that only RRMS trials were meta-analysed.
Included trials
Studies excluded from the NMA and reasons for exclusion were, overall, clearly documented. However, the Biogen Idec Ltd NMA excluded several studies on what would appear to be the basis of short-term follow-up. This was not made explicit.
Findings from the network meta-analyses presented in the manufacturer’s submission
The NMA found that 30 µg of IM IFN-β-1a weekly significantly reduced the ARR relative to placebo, but not relative to other treatments. In fact, in the manufacturer’s NMA, 20 mg of SC GA once daily was more effective at reducing the ARR than 30 µg of IM IFN-β-1a weekly. Findings for disability progression confirmed at 3 or 6 months were not significant relative to other treatments or placebo.
The NMA found that, for ARR, no significant treatment effects were observed in the comparisons between 125 µg of SC pegylated IFN-β-1a every 2 weeks and other treatments or between 125 µg of SC pegylated IFN-β-1a every 2 weeks and placebo, although the last finding was marginally non-significant (RR 0.64, 95% CI 0.41 to 1.04). For disability progression sustained at 3 or 6 months, no statistically significant differences were observed between 125 µg of SC pegylated IFN-β-1a every 2 weeks and other treatments or placebo.
Analyses for AEs were conducted only for 30 µg of IFN-β-1a weekly. No differences were found relative to placebo or other treatments.
The authors carried out a wide variety of sensitivity analyses, which were summarised in appendix H.
Results compared with the results of the assessment group’s network meta-analyses
Biogen Idec Ltd’s NMA on the whole did not identify a statistically significant benefit of 125 µg of pegylated IFN-β-1a every 2 weeks or 30 µg of IFN-β-1a weekly with regard to the key outcomes – ARR and disability progression confirmed at 3 months and 6 months. However, both drugs demonstrated statistically significant effectiveness for these three outcomes in the assessment group’s NMA. Point estimates were generally similar between the NMA for ARR and the NMA for time to disability progression confirmed at 3 months. This discrepancy may result from the choice of prior distribution for between-trial variance in the base case of the manufacturer’s NMA, as well as the apparent exclusion of studies with short-term follow-up. Notably, the assessment group considered several more drugs in the analysis of disability progression confirmed at 6 months than it would appear were included in the manufacturer’s NMA for this outcome.
Summary of the Biogen Idec Ltd submission
The quality of the submitted systematic review was both reasonable and appropriate. Although a strength of the models was the explicit approach to dosages of comparators included, inputs in the NMA models were opaque and no study-level forest plots were presented with specific estimates. Moreover, the initial decision to stratify estimates by 12 or 24 months was not clearly explained and apparent exclusions based on follow-up were not explicitly declared.
Chapter 7 Methods for the assessment of cost-effectiveness studies
Identification of studies: clinically isolated syndrome
Introduction
The purpose of this systematic review was to identify existing cost-effectiveness model designs in CIS and to identify parameter values (e.g. health state utilities and costs) suitable for use in a decision-analytic model. We did not identify a suitable systematic review in CIS in the overview of systematic reviews (see Appendix 5) and scoping searches did not find many existing models. Therefore, our searches were broad and not limited by date.
Search strategy
The following electronic databases were searched: MEDLINE (via Ovid); MEDLINE In-Process & Other Non-Indexed Citations and MEDLINE Daily Update (via Ovid); EMBASE (via Ovid); The Cochrane Library (via Wiley Online Library), including the NHS EED and HTA database; Science Citation Index (Web of Knowledge); Research Papers in Economics (RePEc) and the Cost-effectiveness Analysis (CEA) Registry. The database searches were designed to be broad in nature, with search terms for CIS combined with terms for economic/HRQoL generic measures (based on recognised search filters239–242) when appropriate. A full record of the searches carried out is provided in Appendix 6. The searches were not limited by publication date. All bibliographic records identified through the electronic searches were collected in a managed reference database. The reference lists of included studies were also checked. Grey literature searches were undertaken concurrently for both clinical effectiveness and cost-effectiveness evidence using the online resources of various regulatory bodies, health service research agencies, professional societies and patient organisations. For a record of these searches, see Appendix 1.
We undertook several additional searches. We checked the reference lists of primary studies identified through database searches for studies on the natural history of people with CIS and CIS patient registries. We also undertook targeted database searches to identify any additional CIS patient registries including data from before 1995 (see Appendix 7). We searched studies citing included studies to identify more recent literature.
Inclusion and exclusion criteria
Studies meeting the following criteria were included in the review:
-
population – adults (aged ≥ 18 years) diagnosed with CIS, defined as people who had experienced a single demyelinating event in one or several areas of the CNS within the previous 2 months
-
interventions – DMTs (IFN-β-1a, IFN-β-1b and GA) licensed for the treatment of CIS
-
comparators – BSC without DMTs or another DMT (IFN-β-1a, IFN-β-1b or GA) licensed for the treatment of CIS
-
outcomes – cost per QALY gained, cost per life-year gained and cost per case of MS delayed
-
study design – economic analysis consisting of a decision-analytic model
-
language – English and Spanish.
All publication types were included.
Other studies that contained information on parameter values (e.g. health state utilities, costs, natural history outcomes) suitable for use in a decision-analytic model were identified at this stage and set aside for later review.
Studies in people diagnosed with RRMS, SPMS or PPMS were excluded.
Study selection
Studies were first reviewed on title and abstract by two reviewers working independently (Hendramoorthy Maheswaran and Peter Auguste). Subsequently, full-text studies were accessed and checked against the criteria for inclusion. As mentioned in the previous section, studies that presented information on costs and outcomes related to the natural history of, or DMT for people with, CIS were also examined at this stage and set aside for later review.
Data extraction
Data extraction was conducted by two reviewers (Hendramoorthy Maheswaran and Peter Auguste). Information extracted by one reviewer was cross-checked by the other. Any disagreements were resolved by discussion or by recourse to a third-party reviewer (Jason Madan). We extracted study details (title, author and year of study), background characteristics (population, intervention, comparator and outcomes), methods (study perspective, time horizon, discount rate, measure of effectiveness, assumptions and analytical methods), results (study parameters, base-case and sensitivity analyses), discussion (study findings, limitations of the models and generalisability) and ‘other’ details (source of funding and conflicts of interests). The data extraction sheet is presented in Appendix 6.
Quality assessment
The studies were appraised using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS)243 and Philips et al. 244 frameworks for best practice in economic evaluation and decision-analytic modelling respectively. The CHEERS assessment tool243 consists of six dimensions: title and abstract, introduction, methods, results, discussion and other. A series of questions is used to check whether these dimensions/attributes have been satisfactorily reported (see Appendix 6). The Philips et al. 244 tool consists of two main dimensions: structure of the model and information used to parameterise the model. A series of questions is used to check whether these have been satisfactorily conducted (see Appendix 6).
The quality assessment was undertaken by two reviewers (Hendramoorthy Maheswaran and Peter Auguste). Study quality assessed by Hendramoorthy Maheswaran was cross-checked by Peter Auguste and vice versa. Any disagreements were resolved by discussion or by recourse to a third-party reviewer (Jason Madan).
Data synthesis
Findings from the included studies were synthesised narratively with the goal of summarising current modelling methods.
Identification of studies: relapsing–remitting multiple sclerosis
Introduction
The purpose of this systematic review was to identify existing cost-effectiveness model designs in RRMS and to identify parameter values (e.g. health state utilities, costs) suitable for use in a decision-analytic model. We identified several related systematic reviews of cost-effectiveness evaluations in RRMS in the overview of systematic reviews. 245–253 Therefore, we performed searches for primary cost-effectiveness studies from the earliest search date found in these selected reviews (i.e. 2012) to April 2016. We performed separate searches for relevant HRQoL studies, with no date limits applied. We used well-established methods that are used for undertaking systematic reviews of clinical studies. 166
Search strategy
The following electronic databases were searched separately for cost-effectiveness studies and HRQoL studies: MEDLINE (via Ovid); MEDLINE In-Process & Other Non-Indexed Citation and MEDLINE Daily Update (via Ovid); EMBASE (via Ovid); The Cochrane Library (via Wiley Online Library), including the NHS EED and HTA database; Science Citation Index (Web of Knowledge); RePEc; and the CEA Registry. The database searches were kept broad, with search terms for MS combined with terms for economics/HRQoL generic measures (based on recognised search filters239–242) when appropriate. A full record of the searches carried out is provided in Appendix 7. The searches for primary cost-effectiveness studies were limited by publication date from January 2012 to April 2016. HRQoL searches were not limited by publication date. All bibliographic records identified through the electronic searches were collected in a managed reference database. The reference lists of included studies were also checked. Grey literature searches were undertaken using the online resources of various regulatory bodies, health service research agencies, professional societies and patient organisations.
The following additional searches were undertaken. We checked the reference lists of primary studies identified through the searches described in the paragraph above for studies on the natural history of people with RRMS and RRMS patient registries. We also undertook targeted database searches to identify any additional RRMS patient registries that included data from before 1995 (see Appendix 7). Citation searches on the included studies were undertaken to identify more recent literature.
Inclusion and exclusion criteria
Studies meeting the following criteria were included in the review:
-
population – adults (≥ 18 years) diagnosed with RRMS
-
interventions – DMTs (IFN-β-1a, pegylated IFN-β-1a, IFN-β-1b or GA) licensed for the treatment of RRMS
-
comparators – BSC without DMTs or another DMT (IFN-β-1a, IFN-β-1b or GA) licensed for the treatment of RRMS
-
outcomes – cost per QALY gained, cost per life-year gained and cost per case of MS delayed
-
study design – economic analysis consisting of a decision-analytic model.
Other studies that contained information on parameter values (e.g. health state utilities, costs, natural history outcomes) suitable for use in a decision-analytic model were identified at this stage and set aside for later review.
Studies were excluded if they included people diagnosed with CIS. Additionally, studies were excluded if they were reported in the form of an abstract or conference proceeding or were not published in the English language.
Study selection
Studies were first reviewed on title and abstract by two reviewers working independently (HM and PA). Subsequently, full-text studies were accessed and checked against the criteria for inclusion. As mentioned in the previous section, studies that presented information on costs and outcomes related to the natural history of, or DMT for people with, RRMS were also examined at this stage and set aside for later review.
Data extraction
Data extraction was conducted by two reviewers (Hendramoorthy Maheswaran and Peter Auguste). Information extracted by one reviewer was cross-checked by the other. Any disagreements were resolved by discussion or by recourse to a third-party reviewer (Jason Madan). We extracted study details (title, author and year of study), background characteristics (population, intervention, comparator and outcomes), methods (study perspective, time horizon, discount rate, measure of effectiveness, assumptions and analytical methods), results (study parameters, base-case and sensitivity analyses), discussion (study findings, limitations of the models and generalisability) and ‘other’ details (source of funding and conflicts of interests). The data extraction sheet is presented in Appendix 7.
Quality assessment
The studies were appraised against the CHEERS243 and Philips et al. 244 frameworks for best practice in economic evaluation and decision-analytic modelling respectively. The CHEERS assessment tool consists of six dimensions: title and abstract, introduction, methods, results, discussion and other. A series of questions is used to check whether these have been satisfactorily reported (see Appendix 7). The Philips et al. 244 tool consists of two main dimensions: structure of the model and information used to parameterise the model. A series of questions is used to check whether these have been satisfactorily reported (see Appendix 7).
The quality assessment was undertaken by two reviewers (Hendramoorthy Maheswaran and Peter Auguste). Study quality assessed by one reviewer was cross-checked by the other. Any disagreements were resolved by discussion or by recourse to a third-party reviewer (Jason Madan).
Data synthesis
Information extracted from the included studies was summarised in a table. The findings from these studies have been compared narratively to show the current modelling methods used and our recommendations for future modelling of RRMS are discussed.
Chapter 8 Results of the systematic review of the cost-effectiveness literature
Results of the searches for clinically isolated syndrome studies
The electronic database searches identified 614 records (Figure 23). After removing duplicates, 452 records were screened for inclusion. On the basis of title and abstract, 435 records were excluded and the remaining 17 records were included for full-text screening. A further eight articles were excluded at the full-text stage, with the reasons for exclusion provided in Appendix 6, leaving nine studies254–262 that included a decision-analytic model, which were used to estimate the cost-effectiveness of DMTs for treating people with CIS.
FIGURE 23.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart: economic studies relating to CIS.
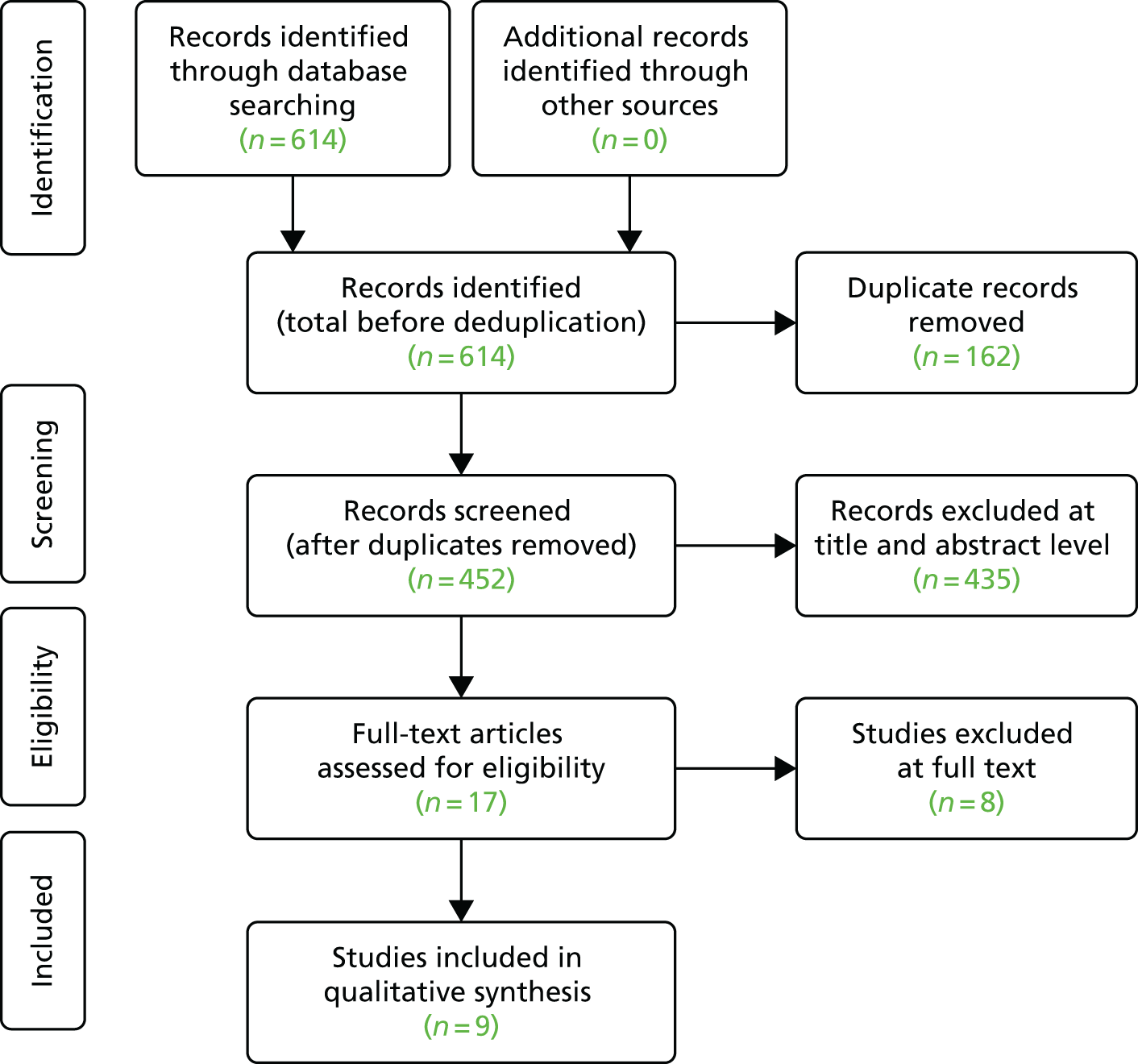
Description of the included studies
Summary of economic studies comparing disease-modifying therapies for people with clinically isolated syndrome
Fredrikson et al.254
Fredrikson et al. 254 used a Markov model structure to assess the cost-effectiveness of 44 µg SC IFN-β-1a three times weekly compared with no treatment for people who had experienced a single demyelinating event in one or several areas of the CNS within the previous 2 months. The model simulated the pathway for people with CIS who received DMTs compared with no treatment and cost-effectiveness was estimated over the model’s time horizon. The model started with a hypothetical cohort with a mean age of 31 years, which reflected the participants in the REFLEX trial,175 and continued with participants occupying/progressing to one of the following health states: CIS and on treatment, CIS no treatment or RRMS defined by the McDonald 2005 criteria. 66 Fredrikson et al. 254 made a number of simplifying assumptions: once people converted to RRMS they could progress in single-step increments; the treatment effect was assumed to continue over the model time horizon, based on clinical judgement; a maximum duration of 25 years for treatment was applied; the probability of discontinuation of DMTs was derived based on the 3-year rate from the REFLEXION (REbif FLEXible dosing in early MS extensION) trial263 (this probability was applied from year 3 for the remainder of the model duration); 95% of people with CIS would convert to MS using the McDonald criteria; and people with MS who progressed to EDSS 7 or who converted to SPMS would discontinue treatment.
Information required to populate the model was obtained from the REFLEX175 and REFLEXION263 trials as well as resource use and costs from published sources. Information was required for utility values associated with CIS and MS (by EDSS state), the conversion rate from CIS to CDMS according to McDonald MRI criteria, the annual average dropout rate over 25 years and the market share of DMTs prescribed for MS. Resource use and costs included those related to informal care, services, investments (house and car modifications, walking aides, wheelchairs), symptom management medication, tests (MRI scans of the brain and spinal cord in the first year of diagnosis and a brain MRI scan every year), ambulatory care, inpatient care, loss of productivity because of early retirement and short-term absence. The analysis was conducted from a societal perspective and the results were presented in terms of costs per progression-free life-years and costs per QALY gained over a 40-year time horizon. All costs were reported in 2012 Swedish kronors and converted to euros using a historical average exchange rate from 2005. All costs and outcomes were discounted at 3% per annum. Along with the cost-effectiveness analysis, Fredrikson et al. 254 conducted univariate and probabilistic sensitivity analyses.
The results showed that there was an incremental gain of 1.63 progression-free life-years for people who received a DMT compared with no treatment. Additionally, there was a 0.53 incremental QALY gain for people who received treatment. From a societal perspective, the base-case results showed a cost saving of approximately SEK 270,260.
Kobelt et al.255
Kobelt et al. 255 used a Markov structure to assess the cost-effectiveness of 250 µg of SC IFN-β-1b every other day compared with no treatment for people with CIS. The model simulated disease progression for a hypothetical cohort of people being treated for CIS and cost-effectiveness was estimated over a 20-year time horizon. The model started with a cohort of people who received either 250 µg of SC IFN-β-1b every other day or no treatment and continued with them remaining in the CIS health state or progressing to mild, moderate or severe MS disability. An illustrative Markov structure was not presented as this was an abstract.
The authors did not elaborate on the sources of information used to populate the model. All costs were reported in 2006 euros. The primary outcome measure of effectiveness was QALYs gained over the 20-year time horizon; however, the authors did not elaborate on the descriptive tools used to value these health states. All costs and benefits were discounted at 3% per annum. The analysis was conducted from a societal perspective and the results were presented in terms of incremental cost-effectiveness ratios (ICERs) expressed as the cost per QALY gained. Kobelt et al. 255 conducted sensitivity analyses by changing key model input parameters to determine the impact on the deterministic results. Additionally, a probabilistic sensitivity analysis was undertaken.
The base-case results showed that IFN-β-1b dominated the no-treatment arm. In sensitivity analyses the base-case results were robust to changes in model input parameters. The results from the probabilistic sensitivity analysis showed that IFN-β-1b was the preferred option, with a > 50% probability of being cost-effective compared with no treatment at a willingness-to-pay threshold of €50,000 per QALY.
Lazzaro et al.256
Lazzaro et al. 256 developed an epidemiological/survival model to estimate the cost-effectiveness of 250 µg of SC IFN-β-1b every other day for people with a monofocal or multifocal CIS diagnosis compared with postponing disease-modifying disease treatment until subsequent conversion to CDMS.
Information required to populate the model was obtained from published sources. Information on the incidence of CIS, the utility value of CIS, the conversion rate from CIS to CDMS according to McDonald MRI criteria and the annual average dropout rate over 25 years was obtained. All resource use and costs (for disease-modifying drugs and other drugs, outpatient diagnostic procedures, consultations and laboratory tests, hospitalisation, physical therapy, walking aids, transport, working days lost by patients and their caregivers and informal care) were obtained from published sources and were presented in 2006 euros. The results were presented as ICERs, expressed as cost per QALY gained over the 25-year time horizon. The measurement and valuation of preference-based outcomes were not reported. The base-case analysis was undertaken from the Italian National Health Service (INHS) perspective and all costs and benefits were discounted at 3% per annum. To have a workable model, a number of simplifying assumptions were made. The authors undertook a number of one-way (annual consumption of and average annual compliance rate for 250 µg of SC IFN-β-1b every other day; replacement of IFN-β-1b with 44 µg of SC IFN-β-1a three days a week; CDMS-related patient utility values) and multiway (annual conversion rates to CDMS during year 1 and 2) sensitivity analyses and also conducted a probabilistic sensitivity analysis.
From the INHS perspective, the base-case results showed that the mean incremental cost for people who received early treatment compared with delayed treatment was approximately €894. The mean incremental gain for people who received early treatment compared with delayed treatment was 0.35, which equated to an ICER of approximately €2575 per QALY. From the societal viewpoint, early treatment dominated delayed treatment, meaning that early treatment was cheaper than delayed treatment and more effective. The results from the one-way and multiway sensitivity analyses showed that the base-case results were sensitive to change in the DMT and the lower-limit 95% CI CDMS conversion rates during years 1 and 2 of the epidemiological model. The results from the probabilistic sensitivity analysis showed that, at a willingness-to-pay for an incremental QALY of €5500, early treatment was likely to be cost-effective, with a probability of 100%.
Iskedjian et al.257
Iskedjian et al. 257 used two Markov model structures to assess the cost-effectiveness of 30 µg of IM IFN-β-1a once weekly compared with current treatment (four intravenous injections of 1 g of methylprednisolone for 3 days followed by 14 days of 1 mg of oral steroids twice daily) for people who had experienced a single, clinically diagnosed, demyelinating event. The model simulated the pathway for people with CIS who received DMTs compared with symptom management and cost-effectiveness was estimated over a 12-year time horizon. The first model started with a hypothetical cohort of people receiving one of the two treatments and captured the costs and outcomes associated with progression to CDMS and the second model estimated the long-term costs and outcomes of progression through various EDSS states [mild (EDSS score of ≤ 3.5), moderate (EDSS score of 4–5.5) and severe (EDSS score of ≥ 6)]. Iskedjian et al. 257 made a number of simplifying assumptions, for example that people who progressed to CDMS received no treatment benefit but accrued costs associated with their EDSS health states and people in both arms of the model received 30 µg of IM IFN-β-1a once weekly once diagnosed with CDMS. Relapse rates were fixed to one every 2 years, relapses were assumed to last for 2 months and people did not discontinue treatment (i.e. 100% compliance was assumed).
Information on transition probabilities, resource use and costs was obtained from the literature. The analysis was conducted from the Canadian Ministry of Health and societal perspectives and the results were presented in terms of costs per mono-symptomatic life-years (MLYs) gained and QALYs gained over a 12-year time horizon. Utility values were derived based on the Health Utilities Index (HUI) questionnaire, which was administered to Canadian MS patients. A separate analysis was undertaken that used utility values derived from the EQ-5D questionnaire. All costs were reported in 2001 Canadian dollars and all costs and outcomes were discounted by 5% per annum. Along with the cost-effectiveness analysis, Iskedjian et al. 257 conducted univariate (20- and 30-year time horizons, using utility values based on the EQ-5D questionnaire and varying the discount rate) and probabilistic sensitivity analyses.
Results from the Canadian Ministry of Health perspective showed that, over the 12-year time horizon, mean costs were CA$173,000 and CA$108,000 for the Avonex arm and current treatment arm respectively. Expected mean MLYs gained were 4.69 and 3.48 for the Avonex and current treatment arms, respectively, which equated to an ICER of CA$53,110 per MLY gained. Results from the societal perspective showed that, over the 12-year time horizon, mean costs were CA$317,000 and CA$262,000 for the Avonex and current treatment arms respectively. Expected mean MLYs gained were 4.69 and 3.48 for the Avonex and current treatment arms, respectively, which equated to an ICER of approximately CA$44,800 per MLY gained. The ICERs per QALY gained were approximately CA$227,600 and CA$189,300 from the Canadian Ministry of Health and societal perspectives respectively. Using utilities derived from the EQ-5D, the ICERs per QALY gained were approximately CA$116,100 and CA$91,200 from the Canadian Ministry of Health and societal perspectives respectively. The sensitivity analysis demonstrated that, in the progression to CDMS model, the results were sensitive to the time horizon and the rate of progression to CDMS. Using a 6-year time horizon resulted in an incremental cost per MLY gained of CA$85,100 and CA$79,300 for the Canadian Ministry of Health and societal perspectives respectively. Increasing the probability of progressing to CDMS reduced the incremental cost per MLY gained to CA$44,700 and CA$35,600 for the Canadian Ministry of Health and societal perspectives respectively. Decreasing the probability of progression to CDMS resulted in an increase in the incremental cost per MLY gained to CA$67,800 and CA$60,200 for the Canadian Ministry of Health and societal perspectives respectively.
Arbizu et al.258
The study by Arbizu et al. 258 was presented in abstract form. Arbizu et al. 258 undertook a cost–utility analysis comparing the costs and consequences of providing supportive care with those of treatment with IFN-β-1b in Spanish patients with incident CIS. Costs were estimated from a societal perspective and adjusted to 2008 euros. A 3% discount rate was applied to future costs and health benefits. A Markov model was used and EDSS scores defined initial health states. In the analyses it was assumed that those who progressed to RRMS would start IFN-β-1b and would remain on treatment until the EDSS score worsened to 6.5. The BENEFIT trial171 findings were used to model EDSS progression over time and transitions from CIS to MS. Costs and utility scores were predominantly obtained from published sources.
Their main findings suggested that, when the model was run over a 50-year time horizon, the ICER for IFN-β-1b compared with no treatment was €20,500 per QALY gained. The findings were sensitive to the time horizon, the cost of IFN-β-1b and the risk of disease progression on treatment.
Caloyeras et al.260
The study by Caloyeras et al. 260 was presented in abstract form. Caloyeras et al. 260 undertook a cost–utility analysis comparing the costs and consequences of providing supportive care with those of treatment with IFN-β-1b in Australian patients with incident CIS. The authors used findings from the BENEFIT trial171 to determine initial EDSS scores for those with CIS, the subsequent risk of progression in EDSS score and the risk of progressing to RRMS. Costs were estimated from a societal perspective and adjusted to 2007 Australian dollars. A discount rate of 5% was applied to future costs and health benefits, in accordance with Australian policy guidelines. A Markov model was used and EDSS scores defined initial health states for CIS and RRMS. The costs and utilities attached to treatment health states for CIS and RRMS were identical and dependent on the EDSS score. It was assumed that DMTs were discontinued when disability worsened to an EDSS score of 6.5. Published sources were used to estimate costs and utility weights for health states.
When the model was run over a 25-year time horizon the ICER for IFN-β-1b compared with supportive care was AU$20,000 (US$14,000) per QALY gained.
Caloyeras et al.259
The study by Caloyeras et al. 259 was presented in abstract form, with the poster presentation retrieved for appraisal. Caloyeras et al. 259 undertook a cost–utility analysis comparing the costs and consequences of providing supportive care with those of treatment with IFN-β-1b in Australian patients with incident CIS. The authors used findings from the BENEFIT trial171 to determine initial EDSS scores for those with CIS, the subsequent risk of progression in EDSS scores and the risk of progressing to RRMS. Costs were estimated from a societal perspective and adjusted to 2007 Australian dollars. A discount rate of 5% was applied to future costs and health benefits, in accordance with Australian policy guidelines. A Markov model was used and EDSS scores defined initial health states for CIS and RRMS. The costs and utilities attached to treatment health states for CIS and MS were the same and dependent on EDSS score. It was assumed that DMTs were not discontinued unless disability worsened to an EDSS score of 6.5. Patients were limited to one AE per annum.
The main findings suggested that, when the model was run over a 25-year time horizon, the ICER for IFN-β-1b compared with no treatment was AU$68,000 per QALY gained.
It is of note that these findings are presented by the same group as the study by Caloyeras et al. 260 A different cost per QALY was derived, even though it appears that the same setting/perspective, time horizon, model structure and underlying data from the BENEFIT trial171 were used.
Caloyeras et al.261
Caloyeras et al. 261 used a Markov model structure to assess the cost-effectiveness of 250 µg of IFN-β-1b once daily compared with BSC for people with their first clinical event suggestive of MS. The model simulated the pathway for people with CIS who received DMTs compared with BSC and cost-effectiveness was estimated over the model’s time horizon. The model started with a hypothetical cohort of people aged 30 years who were diagnosed with CIS and who had an EDSS score of 0–5.5 and continued with people occupying/progressing to one of the following seven health states: EDSS 0.0, EDSS 1.0–1.5, EDSS 2.0–2.5, EDSS 3.0–3.5, EDSS 6.0–7.5 non-relapse, EDSS 8.0–9.5 non-relapse and EDSS 10 (MS-related death). Caloyeras et al. 261 made a number of assumptions: progression in EDSS levels was modelled independently of progression to MS; two types of relapses were modelled – relapse resulting in progression from CIS to MS and relapse after progression to MS; all-cause mortality was estimated using life tables; MS-specific mortality occurred only when the EDSS score was 10 and people who discontinued treatment did not restart DMTs.
Clinical information (e.g. HRs for DMTs compared with placebo) required to populate the model was obtained from the BENEFIT trial. 171 Information on utilities associated with EDSS levels was obtained from published sources. Resource use and costs included those for hospital inpatient care, ambulatory care, tests, drugs (DMTs and other drugs), services, adaptations/investments and informal care. Costs associated with relapses were estimated from a cross-sectional web-based survey. The analysis was conducted from a Swedish societal perspective and the results were presented in terms of costs per QALY gained over a 50-year time horizon. All costs were reported in 2009 Swedish kronor and all costs and outcomes were discounted at 3% per annum. Along with the cost-effectiveness analysis, Caloyeras et al. 261 undertook one-way sensitivity analysis (acquisition costs, EDSS threshold for discontinuation, time horizon of the model, EDSS progression probability and discount rates) and probabilistic sensitivity analysis (drug acquisition costs, direct and indirect costs, utilities, EDSS progression probabilities, treatment discontinuation rate, relapse rate) using a uniform distribution and varying model parameters by ±2.5%.
The base-case results showed that treatment with IFN-β-1b dominated BSC arm (commencing treatment when people progressed to RRMS). People who started on early treatment accumulated slightly higher direct medical costs per patient but lower direct non-medical costs. The sensitivity analyses demonstrated that the base-case results were robust to changes made to model parameters. However, the model findings were sensitive to changes made to the time horizon of the analysis. Undertaking the analysis over a shorter 5-year time horizon found that early treatment was not cost-effective (ICER SEK 1.32M).
Zarco et al.262
Zarco et al. 262 used a decision tree structure to assess the cost-effectiveness of IFN-β-1a or IFN-β-1b compared with BSC for people diagnosed with CIS. The model started with a hypothetical cohort of people with CIS and continued with a proportion of people having a relapse or not having a relapse at a 1-year time horizon. At the 2-year time horizon, the model considered the proportion of people progressing to CDMS and those remaining in a CIS health state. The report was unclear on the assumptions made in the model.
Information on progression from CIS to CDMS in an untreated population was obtained from the BENEFIT trial. 171 Information on the treatment efficacy of DMTs was obtained from clinical trials. Resource use and costs were estimated from a hospital-level microcosting study and treatment costs were estimated from national health insurance data. The analysis was conducted from the Colombian societal perspective and the results were presented in terms of costs per QALY and costs per disability-adjusted life-year over a 2-year time horizon. All costs were reported in 2011 US dollars and all costs and outcomes were discounted in the second year by 3%. The authors undertook univariate and probabilistic sensitivity analyses.
In terms of costs per QALY, the base-case results showed that interferons were not cost-effective compared with BSC for treating people with CIS.
Characteristics of the included studies
The characteristics of the studies included in this review are presented in Table 23. All of the studies included an economic model to estimate the cost-effectiveness of DMTs for treating people with CIS. The economic evaluations were conducted in Sweden,254,255,261 Australia,259,260 Italy,256 Colombia,262 Spain258 and Canada. 257
| Author, year and country | Attributes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Intervention and comparator | Perspective | Model type and cycle length | Health states | Time horizon | Evidence synthesis | Outcomes | Source of preference data | Discount rate | Sensitivity analysis | |
| Fredrikson 2013;254 Sweden | People who experienced a single demyelinating event in one or several areas of the CNS within the previous 2 months | 44 µg of SC IFN-β-1a three-times weekly compared with no treatment | Societal perspective | Cohort Markov model with 1-year cycle length | CIS and on treatment, CIS no treatment or RRMS defined by McDonald 2005 criteria66 | 40 years | Not based on a systematic review | Progression-free life-years gained, QALYs gained | Not reported (authors suggested that utility values associated with each EDSS level were obtained from a study in MS patients) | 3% per annum for costs and outcomes | RRMS defined by the Poser criteria |
| Kobelt 2007;255 Sweden | People with a clinically isolated event | 250 µg of SC IFN-β-1b every other day compared with no treatment | Societal perspective | Cohort Markov model with 1-year cycle length | Progression from CIS to mild, moderate and severe MS | 20 years | Not reported | QALYs gained | Not reported | 3% per annum for costs and outcomes | Changes to time horizon, treatment duration and proportion of people treated at conversion |
| Lazzaro 2009;256 Italy | People with a monofocal or multifocal CIS diagnosis (McDonald MRI criteria) | 250 µg of SC IFN-β-1b every other day compared with no treatment | INHS and societal perspective | Epidemiological/survival model | Not reported | 25 years | Not reported | QALYs gained | Not reported | 3% per annum for costs and outcomes | Annual consumption of and average annual compliance rate to IFN-β-1b; replacement of IFN-β-1b with 44 µg of SC IFN-β-1a three days a week; CDMS-related patient utility values; and probabilistic sensitivity analysis |
| Iskedjian 2005;257 Canada | People who experienced a single, clinically diagnosed, demyelinating event | 30 µg of IM IFN-β-1a once weekly compared with four intravenous injections of 1 g of methylprednisolone for 3 days followed by 14 days of 1 mg of oral steroids twice daily | Ministry of Health and societal perspective | Two cohort Markov models, each with a 1-year cycle length | The first model captured costs and outcomes associated with progression to CDMS and the second model estimated the long-term costs and outcomes of progression through various EDSS states [mild (EDSS score of ≤ 3.5), moderate (EDSS score of 4–5.5) and severe (EDSS score of ≥ 6)] | 12 years | Not reported | MLYs gained, QALYs gained | Utility values were derived based on the HUI questionnaire and EQ-5D questionnaire | 5% per annum for costs and outcomes | 20- and 30-year time horizons; utility values based on the EQ-5D questionnaire; varying discount rates |
| Arbizu 2009;258 Spain | People with CIS | 250 µg of SC IFN-β-1b every other day compared with no treatment | Not reported | Not reported | Markov model | 50 years | Not reported | QALYs gained | Not reported | 3% per annum for costs and benefits | Sensitivity analysis was undertaken but the extent of the analysis was unclear |
| Caloyeras 2008;259 Australia | Adults with CIS | 250 µg of SC IFN-β-1b every other day compared with BSC | Societal perspective | Markov model | CIS health states and RRMS health states defined by the same EDSS strata (0; 1–1.5; 2–2.5; 3–5.5; 6) | 25 years | Based on results from a RCT | QALYs gained | EQ-5D data from the BENEFIT trial171 and published literature | 5% per annum for costs and benefits | Unclear but looks like one-way sensitivity analysis only |
| Caloyeras 2009;260 Australia | Adults with CIS | 250 µg of SC IFN-β-1b every other day compared with BSC | Australian perspective but unclear if health provider or societal perspective | Markov model | Health states defined by EDSS levels | 25 years | Based on results from a RCT | QALYs gained | Obtained from published studies | 5% per annum for costs and benefits | Unclear but looks like one-way sensitivity analysis only |
| Caloyeras 2012;261 Sweden | Patients with a first clinical event suggestive of MS (CIS) | 250 µg of SC IFN-β-1b every other day compared with BSC | Societal perspective | Markov model | First clinical event suggestive of MS (EDSS 0–5.5), RRMS (EDSS 0–5.5), non-relapsing forms of MS (EDSS 6–9.5) and EDSS 10 (death) and death from all causes | 50 years | Based on results from a RCT | QALYs gained | EQ-5D data from the BENEFIT trial171 and published literature | 3% per annum for costs and benefits | Univariate and probabilistic sensitivity analyses |
| Zarco 2014;262 Columbia | People meeting standard indications for initiation of treatment with IFN-β-1a and with a diagnosis of CIS/MS | IFN-β-1a and IFN-β-1b | Societal perspective | Decision tree | Conversion to MS | 2 years | Unclear | Disability-adjusted life-years and QALYs | Obtained from published tables | 3% for costs and outcomes in the second year | Relapse management, conversion probabilities and indirect costs; probabilistic sensitivity analysis |
Studies mainly compared DMT with no treatment. 254–256,258–261 Treatment included SC IFN-β-1a three times weekly254 and SC IFN-β-1b. 255,256,258–261 One study262 compared IFN-β-1a with IFN-β-1b and one study257 compared DMTs (30 µg of IM IFN-β-1a once weekly) with current treatment (four intravenous injections of 1 g of methylprednisolone for 3 days followed by 14 days of 1 mg of oral steroids twice daily).
Seven studies254,255,257,258–261 used a cohort Markov model structure and one study262 used a decision tree structure and the remaining study256 used an epidemiological/survival model and affixed costs and benefits accrued over time for occupying health states. One study258 used a decision tree structure and, in the remaining study,262 it was unclear what model structure was used. Model cycle lengths ranged from 6 months261 to 1 year and time horizons ranged from 2 years257 up to 50 years. 261 Most studies254,255,257,259–261 included longer-term progression through to RRMS and estimated cost-effectiveness.
Five studies254,255,259,261,262 analysed cost-effectiveness from the societal perspective, whereas two studies256,257 analysed cost-effectiveness from both the health service perspective and the societal perspective. Two studies258,260 were unclear on the perspective of the analysis. Six studies254–256,258,261,262 used a discount rate of 3% per annum for costs and outcomes, whereas three studies257,259,260 applied an annual 5% discount rate for costs and outcomes. Six studies255,256,258–261 presented the results in terms of cost per QALY alone, one study used progression-free survival in addition to cost per QALY,254 one study used MLYs gained in addition to cost per QALY257 and one study used disability-adjusted life-years and QALYs. 262
Definition of clinically isolated syndrome
The definitions used to characterise people with CIS were consistent. The majority of the studies defined their hypothetical cohort as adults who had experienced a single demyelinating event suggestive of MS. Two studies254,256 elaborated on this definition and suggested that their cohorts consisted of adults who had experienced a single demyelinating event in one or several areas of the CNS. To our knowledge, no studies included in this systematic review defined their population based on the McDonald 2010 criteria. 62
Characteristics of clinically isolated syndrome models
Four studies254,255,257,261 modelled the longer-term impact of treating CIS with DMTs, incorporating progression to RRMS. No studies modelled conversion from RRMS to SPMS. All studies except that conducted by Iskedjian et al. 257 considered progression until death in the analysis; there was no justification for omitting this health state in the analysis by Iskedjian et al. 257 Disease progression in the RRMS health states was stratified by severity (mild, moderate and severe)255,257 or by predicting changes in EDSS scores. 254,256,258–261 In the majority of the studies the risk of death was obtained from country-specific lifetime tables for the general population. In one study,254 mortality rates were adjusted to reflect the increased risk of mortality associated with MS. Here, background mortality was multiplied by EDSS-specific adjustment factors to reflect MS-specific mortality. All other studies accounted for death by assuming that people died on progression to EDSS 10. Adjusting the background mortality and including progression to EDSS 10 leads to double counting of people who may die from MS-related causes.
Treatment effect of disease-modifying therapies in the clinically isolated syndrome health state
Three studies254,256,261 clearly stated that treatment discontinuation was considered in the analysis. One study257 assumed that people did not discontinue treatment. The remaining studies255,258–260 were unclear on whether treatment discontinuation was included in the analysis. Treatment discontinuation was assumed to be a result of AEs from drug utilisation and/or progression to an EDSS score of ≥ 6. Discontinuation rates ranged from 6% every 2 years254 to 17.7% annually. 256 It appeared that the study by Fredrikson et al. 254 assumed a constant hazard over time for discontinuation of treatment in the first 2 years and, in subsequent years, used information from a follow-on trial. In the analysis undertaken by Caloyeras et al. ,261 a Weibull parametric model was fitted to Swedish registry data to derive time-dependent transition probabilities for people discontinuing treatment. Here, discontinuation of treatment was assumed to be the same for both early and delayed treatment (waiting until people developed MS).
Quality assessment of the modelling methods in clinically isolated syndrome studies
In this section we present a summary of the reporting quality of the studies included in the current review against the Philips et al. 244 checklist, which is presented in Appendix 6.
Model structures
Models presented in full publications were generally of good quality. The studies clearly stated their decision problem, the perspective of the analysis and the objectives of the analysis, all of which were consistent with the decision problem and disease progression. However, analyses were often limited in scope. Most studies compared one DMT with BSC and thus did not include and analyse all treatment options available for people with CIS. All studies clearly stated the time horizon of their analysis, but studies with shorter time horizons may not have been able to capture all of the costs and consequences of treating or not treating CIS with DMTs.
Information required for models
In general, methods used in the published studies to identify relevant information to populate the models were satisfactory. 254,256,257,261,262 As expected, less information was available from published abstracts. 255,258–260 All studies provided references for their model inputs, but authors were not clear on how the evidence was synthesised (e.g. search strategy, quality assessment). In all studies, information was required on the effect of DMTs on disease progression, resource use and costs, outcomes and mortality. The effect of DMTs on delaying progression from CIS to RRMS was modelled using HRs. The relative reduction in progression that was associated with DMTs was then applied to the predicted baseline cohort of people with CIS. All studies except that by Zarco et al. 262 derived a HR directly from a trial. In contrast, the study by Zarco et al. 262 obtained this HR by combining the treatment effects from a number of studies. However, the authors of this study did not elaborate on the quality assessment of these RCTs or on how information on treatment effects was meta-analysed. The effect of DMTs can be applied to a baseline cohort of people to show the treatment effect on conversion to RRMS. Baseline information can be obtained from CIS registries, a natural history cohort or a placebo arm of a clinical trial. In all studies, information on disease progression in a baseline cohort was obtained from RCTs. Most studies undertook analyses based on a long time horizon, which is in line with the NICE reference case. 156 However, only two studies254,261 elaborated on the techniques used to extrapolate treatment effects beyond the time horizon of the RCTs. These studies provided information on the parametric models chosen and justified their choice of survival model.
Most studies254,256,257,261,262 justified and referenced the costs used in their analyses. Costs required for the models were mainly obtained from published sources and these were inflated to current prices using the appropriate indices. In some studies256,261 the authors provided detailed information on resource use. All authors stated the perspective of the analyses and the resource use and costs reflected the viewpoint/perspective of the analyses. All authors discounted costs and benefits using the appropriate rates.
In the studies that reported the model results in terms of QALYs, the authors provided the references used to obtain the utility weights. However, the majority of the studies did not elaborate on the descriptive tools/measures used to value these health states in these populations or did not elaborate on the quality assessment or choices made between sources. Additionally, authors did not elaborate on whether or not the sources of utility information used were relevant to their population of interest. To our knowledge, utility weights were obtained primarily from studies undertaken in a RRMS population.
Uncertainty
All studies addressed parameter uncertainty in the analyses, but none attempted to address all types (methodological, structural, parameter and generalisability) of uncertainty. All studies made changes to key model input parameters to explore the impact on the results. Two studies255,257 ran their analyses over shorter time horizons to explore the impact on the ICER estimates. However, it was unclear whether these studies also assumed that the duration of the treatment effect had been reduced.
Summary of the clinically isolated syndrome cost-effectiveness evidence
The evidence base offers insight into the decision-analytic models used to estimate the cost-effectiveness of DMTs for reducing conversion of CIS to MS. We identified nine studies, which included six full-text articles254–257,261,262 and three abstracts. 258–260
In general, the modelling methodology appeared to draw on current approaches to evaluating cost-effectiveness of DMTs in RRMS. The authors used EDSS levels to define health states for CIS, with DMTs impacting on progression from CIS to RRMS. Once individuals progressed to RRMS, their disease progression was modelled using increasing EDSS scores and progression to SPMS. This seems a reasonable approach as EDSS levels were commonly used to describe populations recruited in clinical trials evaluating DMTs in CIS. In addition, it enables cost and utility data for RRMS patients to be utilised in the CIS model. For example, utility weights for EDSS levels among CIS patients could be assumed to be equivalent to utility weights for comparable EDSS levels among RRMS patients.
The shorter time horizons that some studies used to evaluate costs and consequences were of concern. As CIS patients progress to RRMS, and DMTs reduce this progression, it would seem important to incorporate the long-term costs and consequences of RRMS (either treatment with DMTs or BSC) in a cost-effectiveness analysis of treatment strategies for patients with CIS.
We appraised studies against the CHEERS243 and Philips et al. 244 checklists for best practice for reporting economic evaluation and economic modelling studies. Based on our appraisal, the majority of the full-text articles scored well in terms of defining the decision problem, outlining the study perspective, listing the intervention and comparators, presenting an illustrative model structure and providing a clear outline of the assumptions. Abstracts were limited in the amount of information that could be provided. From our review, we have raised some limitations/concerns that mainly relate to the information required to populate the economic models. First, it was unclear how authors made choices between data sources, especially utility values. It was unclear whether utility values had been obtained by undertaking a systematic review. The majority of the studies reporting their results in terms of QALYs provided references for these utility values. However, authors did not provide details on the descriptive tools/measures used to measure HRQoL and also insufficient information was provided on who (CIS/MS patient or public) valued these health states. Second, the study undertaken by Zarco et al. 262 estimated the treatment effect on conversion to MS from a number of trials. However, little information was provided on how a point estimate for the treatment effect was derived. Third, only two studies254,261 provided sufficient information on extrapolating the treatment effect beyond the trial time horizon. Finally, it was unclear whether studies accounted for the uncertainty around extrapolating beyond the trail time horizon.
In Chapter 12, we have used information from this review to develop a de novo model structure, which we used to estimate the cost-effectiveness of DMTs for treating people with CIS.
Results of the searches for relapsing–remitting multiple sclerosis studies
The electronic database searches identified 2451 records (Figure 24). After removing duplicates, 1393 records were screened for inclusion. On the basis of title and abstract, 1168 records were excluded and the remaining 225 records were included for full-text screening. A further 213 articles were excluded at the full-text stage (see Appendix 7 for a list of excluded studies with reasons), leaving 10 studies154,264–272 that included a decision-analytic model used to estimate the cost-effectiveness of DMTs for treating people with RRMS.
FIGURE 24.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart: economic studies relating to RRMS.

Description of the included studies
Summary of economic studies comparing disease-modifying therapies for people with relapsing–remitting multiple sclerosis
Sanchez-de la Rosa et al.264
Sanchez-de la Rosa et al. 264 used a Markov model structure to assess the cost-effectiveness of IM IFN-β-1a, SC IFN-β-1a, SC IFN-β-1b and SC GA compared with symptomatic treatment for people in Spain diagnosed with RRMS. The model simulated the pathway for people with RRMS who received DMTs compared with symptomatic treatment and cost-effectiveness was estimated over the model’s time horizon. The model started with a hypothetical cohort of adults diagnosed with RRMS and continued with people occupying/progressing to one of the following health states: EDSS 0.0–2.5, relapse EDSS 0.0–2.5, EDSS 3.0–5.5, relapse EDSS 3.0–5.5, EDSS 6.0–7.5, EDSS 8.0–9.5 and dead). The authors made a number of simplifying assumptions: people could die from natural causes in all health states except for EDSS 8.0–9.5, all people in the model received symptomatic treatment for MS, people who discontinued treatment were assumed to receive symptom management alone, treatment reduced the amount of sick leave and people were always working regardless of EDSS level.
The model required information on the starting distribution by EDSS level, probability of progression, incidence of NABs, resource use and costs and utility values by EDSS level. Information on utilities associated with RRMS was obtained from an observational study that was undertaken in Spain, which included a sample of people with MS who responded to the EQ-5D questionnaire. Resource use and costs, stratified by EDSS level, were obtained from published sources. Costs included pharmacological, MS management and loss of productivity costs. The analysis was conducted from the Spanish societal perspective and the results were presented in terms of costs per life-year gained and costs per QALY gained over a 10-year time horizon. All costs were reported in 2010 euros and all costs and outcomes were discounted at 3% per annum. The authors undertook one-way sensitivity analysis (applied 0% and 5% discount rates; varied the time horizon to 2, 4, 6 and 8 years; and changed the incidence of NABs and loss of productivity costs).
The base-case results in terms of costs per QALY showed that IM IFN-β-1a was dominant compared with SC IFN-β-1b. However, treatment with IM IFN-β-1a was not cost-effective compared with SC GA at a willingness-to-pay threshold of €30,000 per QALY. The sensitivity analyses demonstrated that the base-case results were robust and stable to changes made to model parameters.
Nikfar et al.265
Nikfar et al. 265 estimated the cost-effectiveness of using symptom management in combination with IM IFN-β-1a, SC IFN-β-1a or SC IFN-β-1b compared with symptom management alone for the treatment of RRMS. The authors developed a Markov structure to demonstrate the clinical pathway (RRMS defined by EDSS levels and transitioning to SPMS) that people would undergo for the treatment of RRMS. The model started with a hypothetical cohort of adults (aged 30 years) who received one of four treatment strategies. One of the simplifying assumptions was that people started in EDSS 1–3.5. People could transition from RRMS to SPMS from the third cycle (approximately 5 years after diagnosis of RRMS, with an assumption that this took place between EDSS 4–6 and EDSS 6–9.5). In the case of withdrawal from IFN-β treatment in cycles 4–15, patients were allocated to the transition probabilities for relapse and disease progression used in the symptom management arm. Information required to populate the model (probabilities of clinical events and probabilities of switching to other IFN-β treatment or symptomatic treatments and relapse rates) was obtained from published sources through a literature review. Information on utility values, resource use and costs was obtained from a cross-sectional study undertaken by the authors. Briefly, 200 MS patients were recruited randomly from three referral hospitals in two cities, three private offices of MS specialists and members of the MS Iranian Society. The authors elicited utility values directly from participants using a visual analogue scale and the EQ-5D and HUI-3 validated questionnaires. Information on resource use and costs was obtained using a retrospective approach, in which information was collected at a single time point and covered the 1-year period before inclusion in the study. All prices were extracted from official tariffs and were reported in 2012 US dollars. The analysis was conducted from the Iranian societal perspective and the base-case results were expressed as ICERs based on the outcome of cost per QALY gained. All costs and outcomes were discounted at 7.2% and 3% per annum respectively. The base-case results showed that, when using the World Health Organization’s recommendation for willingness-to-pay thresholds (for developing countries, an ICER of less than three times the national gross domestic product is considered cost-effective), all interventions except IM IFN-β-1a were cost-effective compared with symptom management alone. However, using utility values based on the EQ-5D, IM IFN-β-1a was shown to be cost-effective. The sensitivity analyses showed that these results were robust except when changes were made to the use of copied biopharmaceuticals and biosimilars, when these interventions were shown to be dominant.
Agashivala and Kim266
Agashivala and Kim266 undertook a cost-effectiveness analysis using a decision tree. The authors simulated the costs and benefits of using fingolimod in both the first and second years as compared to using IFN-β in the first year and then using fingolimod in the second year, as in the extension to the TRANSFORMS (TRial Assessing injectable interferoN vS. FTY720 Oral in RRMS) trial. 273 They did not provide a description or diagrammatic representation of their model. The authors estimated the costs of providing treatments over the model time horizon and compared these with the observed rates of relapse from the TRANSFORMS trial and thereby estimated the additional costs per relapse avoided. Their definition of relapse, which was based on the definition used in the TRANSFORMS trial, was new, worsening or recurrent neurological symptoms occurring 30 days from the onset of a preceding relapse and lasting for at least 24 hours, without fever or infection. Relapses were confirmed if they were accompanied by an increase of at least half a point on the EDSS, 1 point on two different functional systems of the EDSS or 2 points on one of the functional systems (bowel, bladder or cerebral functional systems were excluded). Resource use data were extracted from the literature and unit costs were obtained from the 2010 Physicians’ Fee and Coding Guide. 274 Costs were estimated from a US private payer perspective (health insurance) and included drug acquisition costs and the costs of monitoring and relapses. The analysis was undertaken over a time horizon of 2 years. Costs were adjusted to 2011 US dollars and future costs and outcomes were not discounted. The authors undertook one-way sensitivity analysis by varying input parameters by ± 10%.
The main finding was that it is more cost-effective to start fingolimod than to start IFN-β and then switch to fingolimod after 1 year of treatment. The estimated cost per relapse avoided was lower when fingolimod was started as first-line treatment than when it was started in the second year, with a cost per relapse avoided of US$20,499 more in the delayed fingolimod group than in the early fingolimod group. The findings are limited by the scope of the analysis undertaken. The analysis did not take into account (or did not describe) potential differences between the two treatments in terms of long-term health and cost impacts, the impact on disability/QoL or the consequences of adverse reactions to treatment. In addition, the parameter for risk of relapse was derived from a single clinical trial with inclusion and exclusion criteria that may limit generalisability to the general population.
Palace et al.154
Palace et al. 154 developed a Markov model to simulate the long-term experiences of people with RRMS. To model the natural history of RRMS, information from a baseline cohort was obtained from the British Columbia Multiple Sclerosis (BCMS) database. The clinical course of RRMS was modelled using health states that captured the long-term disability progression. Health states in RRMS were defined by EDSS levels 0–10. People who progressed to an EDSS score of ≥ 6 were assumed to have converted to SPMS. In all health states people were subjected to the risk of all-cause mortality or MS-related mortality. The treatment effect of DMTs (IFN-β or GA) on disability progression and relapse rates was obtained from the RSS year 6 analysis. Transitions for both the treated and the untreated cohorts occurred annually. In each model cycle, people incurred costs and accrued benefits based on the health state that they occupied. Costs incurred were related to drug acquisition costs, costs of management by EDSS level and the cost of relapse. Benefits accrued were measured in terms of HRQoL and this information was obtained from published sources.
Palace et al. 154 projected the cost-effectiveness of DMTs included in the RSS over a 20-year time horizon. The analysis was conducted from the UK NHS and PSS perspective and the results were presented in terms of ICERs, expressed as costs per QALY gained. All costs were reported in 2014 UK pounds and all costs and benefits were discounted at 3.5% per annum. The authors undertook a sensitivity analysis to determine whether the base-case results were sensitive to the choice of the natural history cohort.
Pan et al.267
Pan et al. 267 used a Markov model and estimated the cost-effectiveness of 250 µg of IFN-β-1b compared with no treatment for people with RRMS. The model simulated the pathway for two cohorts (intervention vs. no treatment) and cost-effectiveness was estimated over a 70-year time horizon. The model started with a hypothetical cohort of people who were aged ≥ 18 years and who had been diagnosed with CDMS or laboratory-supported definite MS for > 1 year and who were ambulatory with an EDSS score of ≥ 5.5, with at least two acute relapses during the previous 2 years. In the Markov model structure, the authors considered seven health states: EDSS 0.0–1.5, EDSS 2.0–2.5, EDSS 3–3.5, EDSS 4–5.5, EDSS 6–7.5, EDSS 8–9.5 and dead. In the model, people remained or progressed to more severe RRMS health states over 6-monthly cycles. To have a workable model structure, the following assumptions were made: people who received mixed treatments during the post-trial period were assumed to have the same treatment efficacy as those who received IFN-β-1b during the trial period, a utility decrement of 0.0235 was applied to people who relapsed and this was assumed to last for 6 months, the model assumed no backward/regressive transitions, that is, MS was seen as a progressive disease, the effectiveness of treatment was assumed to last for the duration of treatment, people who discontinued treatment were assumed to progress at the same rate as people in a natural history cohort, the model assumed that people with RRMS (EDSS score of < 6.0) received treatment and people who discontinued treatment were assumed not to reinitiate treatment.
Data required to populate the model were obtained from published sources. Clinical information on the risk of EDSS progression and relapse rates was based on a meta-analysis undertaken by the authors. Information on utility values was obtained from a published source and these values were based on the EQ-5D. Utility values were allocated according to EDSS health state. Utility decrements were applied to people who relapsed, independent of EDSS state. No disutilities for carers were included in the analysis. Resource use and costs stratified by EDSS level were obtained from published sources. Costs included drug treatment costs, health state costs stratified by EDSS state, informal care costs and indirect (loss of productivity) costs. The authors applied a 10% discount to drug prices for IFN-β-1b and mixed DMTs. The analysis was conducted from a US societal perspective and the results were presented in terms of ICERs, expressed as costs per QALY gained. All costs were reported in 2011 US dollars and all costs and outcomes were discounted at 3% per annum. Pan et al. 267 undertook one-way sensitivity analyses on key model input parameters (changing the time horizon, exclusion of productivity losses as a result of premature death and changing the discount rate and starting EDSS distribution) but did not undertake probabilistic sensitivity analysis.
The base-case results in terms of life-years gained showed that the discounted mean incremental cost of IFN-β-1b was approximately US$86,200, with a reduction in life-years lost of 2.8 years, which equated to an ICER of approximately US$31,000 per life-years gained compared with no treatment. The results in terms of QALYs gained showed that the discounted mean incremental gain was approximately US$86,200, with a 1.9-year increase in QALYs, which equated to an ICER of approximately US$46,400 per QALY gained. Changes made to the treatment discontinuation rate together with discounting on DMT drug costs resulted in moderate changes to the ICER. However, changes made to the time horizon (from 70 years to 20 years) resulted in the ICER (approximately US$163,600) becoming less cost-effective. Additionally, changing the starting distribution to 50% in EDSS 0.0–1.5 and 50% in EDSS 2.0–2.5 resulted in a more cost-effective ICER of approximately US$19,600.
Darbà et al.268
Darbà et al. 268 undertook a cost-effectiveness analysis and compared the costs and consequences of treating RRMS with GA, IM IFN-β-1b and combination therapy with GA and IM IFN-β-1b. The analysis used a Spanish payer perspective, future costs and outcomes were discounted and costs were adjusted to 2013 euros. The authors built a Markov model with five health states relating to outcomes observed in the CombiRx trial191 and estimated the incremental costs per relapses avoided. The model was run over 10 years with a 1-year cycle length. Transition probabilities were derived from the CombiRx trial,191 whereas health-care resource use was obtained from other published sources. The authors assumed that the risk of exacerbation/relapses decreased over time (for the years after the end of the trial). One-way and probabilistic sensitivity analyses were carried out.
The main finding was that treatment with GA monotherapy dominated (was less costly and resulted in fewer relapses) the other treatment options. The authors did not take into account the costs associated with AEs and it is unclear exactly what the health state ‘information lost’ represents (likely to be dropouts from the main trial). These two issues may impact on the findings. The findings have limited generalisability as no other DMTs were considered and disability and QoL were not included in the model.
Imani and Golestani269
Imani and Golestani269 undertook a cost-effectiveness analysis to estimate the incremental costs and benefits of four DMTs in comparison to BSC in Iran. They used a Markov model structure and estimated costs and consequences over a lifetime horizon and from the Iranian societal perspective. Costs were estimated in 2011 US dollars and discount rates used reflected Iranian policy. Direct health provider costs included the costs of treatment, monthly costs associated with EDSS states and the costs of relapses. It was unclear whether other medical costs were included, for example the costs of adverse drug events. Indirect costs included the costs of loss of productivity from absenteeism. In the model, nearly 75% of those modelled started with some degree of disability (EDSS score of > 2.5). Fewer health states, defined by EDSS score, were used to model disability progression and to assign costs/utilities to and no diagrammatic representation of the model was provided.
The authors found that, of the DMTs, treatment with IFN-β-1a was the most cost-effective option. However, the ICER for IFN-β-1a in comparison to BSC was US$607,397 per QALY gained at the societal level. One-way sensitivity analysis found that the ICER was higher when the analysis was undertaken over a shorter time horizon. The findings have limited generalisability because of the analysis setting, as resource use reflected care and costs in Iran.
Dembek et al.270
Dembek et al. 270 undertook a cost-effectiveness analysis to estimate the incremental costs and benefits of injectable DMTs in comparison with BSC in Spain. They compared three different regimens of IFN and GA. They used a Markov model structure and estimated costs and consequences over a 30-year time horizon and from the Spanish societal perspective. Costs were estimated in 2010 euros. Direct health provider costs included the costs of treatment, monitoring, AEs and relapses. Indirect costs included the costs of loss of productivity from absenteeism and early retirement. Other non-medical costs were also included (e.g. costs of walking aids, informal care and transportation). In the model, the authors assumed that most MS patients start DMTs early, at the point of minimal or no disability, and stop once the EDSS score progresses to 6.0. In addition, they used fewer health states by EDSS score to model disability progression and to assign costs/utilities to and assumed that there was no additional mortality risk from MS.
The authors found that, of the DMTs, treatment with IM IFN-β-1a was more cost-effective than treatment with SC IFN-β-1a, IFN-β-1b or GA. The probabilistic sensitivity analysis showed that IM IFN-β-1a was the most cost-effective treatment in 79–97% of simulations. However, the ICER for IM IFN-β-1a in comparison with BSC was €168,629 per QALY gained at the societal level. One-way sensitivity analysis found that the findings were sensitive to DMT costs, cycle utilities and disutility weights assigned to relapse events. The authors discussed their findings in relation to previous economic analyses but did not discuss the policy implications of the high ICER for DMT in comparison with BSC. The study is also limited by the fact that the findings were not presented from the health payer perspective as well.
Chevalier et al.271
Chevalier et al. 271 undertook a cost-effectiveness analysis to estimate the incremental costs and benefits of other DMTs in comparison to delayed-release dimethyl fumarate. They compared dimethyl fumarate with three different dosing regimens of IFN and three other DMTs. They used the same model structure as in a previous NICE HTA of DMTs in MS119 and estimated the cost-effectiveness from the French societal and payer perspectives. The model was run over 30 years with a 1-year cycle length and followed French guidelines for discounting. Costs were estimated in 2013 euros, although the costs of drugs were in 2015 prices. Direct health provider costs included the costs of drugs, monitoring, AEs and management associated with EDSS health states and relapses. Indirect costs included the costs of loss of productivity as a result of absenteeism and early retirement.
The authors found that GA, 30 µg of IFN-β-1a, 250 µg of IFN-β-1b, fingolimod and teriflunomide were dominated (i.e. had higher costs and fewer QALYs) by 44 µg of IFN-β-1a and dimethyl fumarate at both the societal and the health payer level. The ICER for 44 µg of IFN-β-1a in comparison to dimethyl fumarate was €29,047 per QALY and €13,110 per QALY from the health payer and societal perspectives respectively. The probabilistic sensitivity analysis found that, at a willingness-to-pay threshold of €30,000, the probability that dimethyl fumarate was the most cost-effective option was 0.65. The one-way sensitivity analysis suggested that, under the majority of scenarios investigated, dimethyl fumarate continued to dominate other DMTs, except 44 µg of IFN-β-1a. The ICER was most influenced by the dimethyl fumarate disability progression rate, the dimethyl fumarate acquisition cost, the EDSS state cost and the dimethyl fumarate relapse rate. The main finding was that dimethyl fumarate is the optimal choice of DMT.
Lee et al.272
Lee et al. 272 undertook a cost-effectiveness analysis to estimate the incremental costs and benefits of fingolimod in comparison to IM IFN-β-1a. They estimated the cost-effectiveness from the US societal perspective. The model was run over 10 years, with a 1-year cycle length, and followed US guidelines for discounting, with costs adjusted to 2011 US dollars. The model simulated costs and outcomes for hypothetical MS patients aged 37 years with minimal or no disability (EDSS score of < 2.5). Health states in the model reflected the current EDSS score and whether patients were on treatment. The authors assumed that relapses lasted for only 1 month and graded the severity of relapses and assumed that treatment was stopped once the EDSS score was > 5.5. The direct health provider costs included the costs of drugs, monitoring and management associated with EDSS health states and relapses. Indirect costs included the costs of loss of productivity as a result of absenteeism, but it was unclear whether these costs also included the costs of early retirement. QoL weights were derived from US-based studies.
The authors found that, in comparison to 30 µg of IM IFN-β-1a once weekly, the ICER for fingolimod was US$73,975 per QALY gained at the societal level. The ICER was higher from the health payer perspective (US$81,794 per QALY). The probabilistic sensitivity analysis found that fingolimod was not cost-effective at a willingness-to-pay threshold of US$50,000 per QALY, but would be cost-effective if the cost of the drug were to drop.
Characteristics of the included studies
The characteristics of the studies included in this review are presented in Table 24. All of the studies included an economic model to estimate the cost-effectiveness of DMTs for treating people with RRMS. The economic evaluations were mainly conducted in the USA266,267,272 and Spain,264,268,270 with two studies carried out in Iran265,269 and the remaining studies carried out in the UK154 and France. 271 Studies mainly compared IM IFN-β-1a once weekly, SC IFN-β-1a three times weekly, SC IFN-β-1b or SC GA with symptom management. 264,265,269,270 Two studies compared IM IFN-β-1a once weekly with GA,154,268 one study compared SC IFN-β-1b with symptom management,267 two studies included IM IFN-β-1a once weekly in their intervention compared with fingolimod266,272 and the remaining study included comparisons between IFN-β-1a, IFN-β-1b or GA and dimethyl fumarate. 271
| Author, year and country | Attributes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Intervention and comparator | Perspective | Model type and cycle length | Health states | Time horizon | Evidence synthesis | Outcomes | Source of preference data | Discount rate | Sensitivity analysis | |
| Sanchez-de la Rosa 2012;264 Spain | People with RRMS in Spain | IM IFN-β-1a (Avonex), 44 µg SC IFN-β-1a (Rebif), SC IFN-β-1b (Betaferon/Extavia) and SC GA (Copaxone) compared with symptomatic treatment | Spanish societal perspective | Markov model with 1-month cycle length | Relapse EDSS 0.0–2.5, relapse EDSS 3.0–5.5, EDSS 0.0–2.5, EDSS 3.0–5.5, EDSS 6.0–7.5, EDSS 8.0–9.5 and death | 10 years | Clinical information on disease progression and relapses obtained from a published study | Relapse rate estimation, disease progression estimation for EDSS 0.0–2.5 to EDSS 3.0–5.5 and disease progression estimation for EDSS 3.0–5.5 to EDSS 6.0–7.5 | Utility values obtained from observational study undertaken in Spain, based on participants with MS who completed an EQ-5D questionnaire | 3% per annum for both health outcomes and costs; 7.5% for drug costs | Discount rate was set to 0% and 5%; the incidence of NABs was set to IM IFN-β-1a 2.0%, SC IFN-β-1a 12.5%, SC IFN-β-1b 27.8% and GA 0%; time horizon was set to 2, 4, 6 and 8 years |
| Nikfar 2013;265 Iran | People with RRMS | Symptom management in combination with IM IFN-β-1a (Avonex), 44 µg SC IFN-β-1a (Rebif) or SC IFN-β-1b (Betaferon) compared with symptom management alone | Iranian societal perspective | Markov model with biennial cycle lengths | RRMS (EDSS 1–3.5, EDSS 4–6, EDSS 6.5–9.5), SPMS (EDSS 6.5–9.5), withdrawal, switching, death | 30 years | Treatment effects were obtained from RCTs and long-term follow-up studies | Number of people remaining in the RRMS state, number of people remaining relapse free, QALYs gained, total costs and productivity losses | Directly elicited from people with MS using a visual analogue scale and EQ-5D and HUI-3 instruments | 7.2% per annum for costs and 3% for outcomes | Authors assessed the impact of using copied biosimilars and biosimilars in the analysis and using different sources of utility estimates and the sensitivity of discounting costs and outcomes |
| Agashivala 2012;266 USA | People with RRMS who had experienced at least one documented relapse in the last 2 years | 2 years of fingolimod therapy (0.5 or 1.25 mg/day orally) compared with IFN-β-1a 30 µg weekly for 1 year followed by 1 year of fingolimod therapy (0.5 or 1.25 mg/day orally) | US commercial health plan (private insurance perspective) | Decision tree | No clear description or diagram with the modelling approach reported | 2 years | Clinical evidence from the TRANSFORMS trial273 | Relapses avoided | Not applicable | Not reported | Univariate sensitivity analyses undertaken |
| Palace 2015;154 UK | People aged ≥ 18 years with RRMS, two clinically significant relapses in the previous 2 years and an EDSS level of ≤ 5.5 and, for SPMS, ambulant with relapses as the main driver of advancing disability | 30 µg of IM IFN-β-1a (Avonex), 44 or 22 µg of SC IFN-β-1a (Rebif), 250 µg of SC IFN-β-1b (Betaferon) and SC GA (Copaxone) compared with a natural history cohort with symptom management | NHS and PSS perspective | Markov model with annual cycle lengths | EDSS 0–9 | 20 years | Clinical information from the RSS | Loss of utility (primary outcome), EDSS progression (secondary outcome) | HRQoL information was collected from the EQ-5D questionnaire | 3.5% per annum for both health outcomes and costs | Scenario analyses around discontinuation of DMTs, loss to follow-up, inclusion of SPMS at baseline, using information up to 4 years from the RSS and changing the natural history cohort |
| Pan 2012;267 USA | People aged ≥ 18 years with CDMS or laboratory-supported definite MS for > 1 year, ambulatory with an EDSS level of ≥ 5.5 and at least two acute relapses during the previous 2 years | 250 µg of SC IFN-β-1b every other day compared with no treatment | Societal perspective | Markov model with 6-month cycle length | EDSS 0.0–1.5, EDSS 2.0–2.5, EDSS 3–3.5, EDSS 4–5.5, EDSS 6–7.5, EDSS 8–9.5 and death | 70 years | Authors stated that the risk of EDSS progression and relapse rates were obtained from published sources | Life-years gained and QALYs gained | Utility values were obtained from a published source and these were based on information collected on the EQ-5D questionnaire | 3% per annum for costs and outcomes | One-way sensitivity analyses: changing the time horizon, exclusion of productivity losses because of premature death, changing the discount rate and changing the starting EDSS distribution |
| Darbà 2014;268 Spain | Spanish patients aged 18–60 years with established RRMS, EDSS score 0–5.5 and at least two exacerbations | 20 mg of GA SC every day (Copaxone) combined with 30 µg of IM IFN-β-1a weekly (Avonex) compared with either 20 mg of GA SC every day (Copaxone) or with 30 µg of IM IFN-β-1a weekly (Avonex) | Spanish NHS perspective | Markov model with annual cycle lengths | No relapses, suspected exacerbations, non-protocol-defined exacerbations, protocol-defined exacerbations and information lost | 10 years | Clinical evidence from the CombiRx trial191 | Relapses avoided | Not applicable | 3% per annum for both health outcomes and costs; 7.5% for drug costs | Authors carried out one-way sensitivity analysis and probabilistic sensitivity analysis |
| Imani 2012;269 Iran | MS patients in Iran | DMTs for MS [IFN-β-1a (Avonex), IFN-β-1b (Betaferon/ Extavia), IFN-β-1a (Rebif) and IFN-β-1a (CinnoVex®; CinnaGen, Shahrak Gharb, Tehran, Iran)] compared with symptom management/ supportive care | Iranian Ministry of Health perspective, but costing perspective was societal (including lost worker productivity) | Markov model with annual cycle lengths | Four RRMS states determined by EDSS score (0–2.5, 3–5.5, 6–7.5, 8–9.5), two relapsed states determined by EDSS score (0–2.5, 3–5.5), death | Until death | Unclear | Time spent in EDSS 0.0–5.5, time spent relapse free, life-years gained and QALYs gained | Published literature | 3% per annum for both health outcomes and costs | Unclear on the type of sensitivity analysis (e.g. one way) undertaken |
| Dembek 2014;270 Spain | MS patients aged 30 years and with no or minimal disability (57% with EDSS score of 1–1.5 and 43% with EDSS score of 2–2.5) | 30 µg of IM IFN-β-1a once weekly, 44 µg of SC IFN-β-1a three times weekly, 250 µg of SC IFN-β-1b every other day and 20 mg of SC GA daily | Societal perspective | Markov model with annual cycle lengths | Four RRMS states determined by EDSS score (0–2.5, 3–5.5, 6–7.5, 8–9.5), two relapsed states determined by EDSS score (0–2.5, 3–5.5), death | 30 years | Unclear | QALYs | Published literature | 3% per annum for health outcomes and costs | Univariate sensitivity analysis and probabilistic sensitivity analysis carried out |
| Chevalier 2016;271 France | People with RRMS | 44 µg of SC IFN-β-1a, 30 µg of IM IFN-β-1a, 250 µg of SC IFN-β-1b, GA, teriflunomide and fingolimod compared with delayed-release dimethyl fumarate | Health payee and societal perspectives | Markov model with annual cycle lengths | RRMS and SPMS health states | 30 years | Information on the risk of AEs obtained from a systematic review undertaken by the authors | QALYs | EQ-5D responses from a study undertaken among MS patients in France; utility scores derived using French tariff set | 4% per annum for first 30 years, then 2% thereafter | Probabilistic sensitivity analysis |
| Lee 2012;272 USA | People with RRMS with a mean age of 37 years | 0.5 mg of fingolimod orally once a day compared with 30 µg of IM IFN-β-1a once weekly | US societal perspective | Markov model with annual cycle lengths | RRMS non-treatment states determined by EDSS score (0–2.5, 3–5.5, 6–7.5, 8–9.5), two treatment states determined by EDSS level (0–2.5, 3–5.5), temporary relapse health state, death | 10 years | Unclear | QALYs | Unclear | 3% per annum for both costs and outcomes | One-way and probabilistic sensitivity analyses |
All studies except that by Agashivala and Kim266 used a Markov cohort model structure to determine the cost-effectiveness of DMTs for RRMS. Agashivala and Kim266 used a decision tree structure. For those studies using a Markov model structure, model cycle lengths were 1 month,264 6 months,267 annual154,268–272 or biennial265 and time horizons ranged from 2 years266 up to death. 269 Five studies264,265,267,270,272 analysed cost-effectiveness from a societal perspective alone, two studies154,268 analysed cost-effectiveness from a NHS perspective, two studies269,271 analysed cost-effectiveness from both a NHS and a societal perspective and one study266 analysed cost-effectiveness from a third-party provider perspective. Six studies264,267–270,272 used a discount rate of 3% per annum for costs and outcomes, one study271 applied an annual 4% discount rate for costs and outcomes, one study154 applied a 3.5% annual discount rate, one study265 used a discount rate of 7.2% for costs and 3% for outcomes and the final study266 did not explicitly state the discounting approach. Additionally, two studies264,268 included a discount rate of 7.5% for the cost of drugs. The results were mainly presented in terms of relapses avoided, life-years gained and QALYs gained.
Definition of relapsing–remitting multiple sclerosis
The definitions used to characterise people with RRMS were consistent across all studies. However, to our knowledge no studies elaborated on the definitions used to define MS from the clinical studies that were used to obtain treatment effects of DMTs.
Characteristics of relapsing–remitting multiple sclerosis
All studies considered disease progression based on the use of EDSS levels to capture disability progression in people with RRMS. All models also captured the relapsing nature of MS. Nine studies264–272 grouped EDSS health states (e.g. EDSS 1–3.5265) but authors did not provide justification for how these groupings were derived. In contrast, Palace et al. 154 modelled each EDSS level to show disease progression. One study265 clearly presented definitions for each health state included in the model. Three studies154,265,272 included the conversion of RRMS to SPMS. Only one study154 allowed people to transition to less severe health states. In studies that considered relapses in their models,154,265,272 authors assumed that relapses occurred up to an EDSS level of 5.5. At this level, authors assumed that people discontinued treatment and followed the same pathway as people who were at the same EDSS level but untreated.
In general the risk of death was obtained from country-specific lifetime tables for the general population. Two studies264,272 assumed that people were at risk of MS-related death at EDSS 8–9.5. However, it was unclear whether Sanchez-de la Rosa et al. 264 varied the risk of death by age. Nikfar et al. 265 used another method to account for death. These authors assumed that MS increased the risk of death by threefold across age- and sex-adjusted mortality rates. Pan et al. 267 modelled mortality based on extrapolating survival data from an observational study. These authors fitted a Weibull parametric model to the placebo (no treatment) group to project survival over a lifetime, then mortality for IFN-β-1b was modified by applying the HR derived from a comparison between the treatment group and the placebo group. Evidence on other parametric model fits was not presented by the authors.
Treatment effect of disease-modifying therapy in relapsing–remitting multiple sclerosis
The effect of treatment on disability progression and the frequency of relapses was considered in all studies by applying a HR/relative risk to a baseline cohort of people with RRMS. All studies drew on evidence from RCTs. However, only one study264 was clear on the meta-analytical methods used to estimate the treatment effect from clinical trials. These authors used log-linear regression to estimate the treatment effect of DMTs on disease progression and relapse frequency.
It was unclear whether studies modelled the direct impact of DMTs in the conversion to SPMS. All studies considered an indirect impact of DMTs on mortality by showing that DMTs delay disease progression.
It was not clear whether any studies accounted for the waning effect of DMTs. One study264 considered the effect of NABs on the efficacy of DMTs.
Discontinuation of treatment in relapsing–remitting multiple sclerosis
Discontinuation rates were considered in all studies except for that by Agashivala and Kim. 266 Treatment discontinuation was assumed to occur as a result of AEs from drug utilisation and/or progression to an EDSS score of ≥ 6 or a perceived lack of efficacy. 265 To our knowledge, no studies fitted a parametric model to long-term data to derive time-dependent transition probabilities for people discontinuing treatment. Studies used short-term information on discontinuation rates from trials and assumed a constant hazard over time for the duration of the model.
Quality assessment
We present a summary of the reporting quality of the studies included in the current review assessed against the CHEERS243 and Philips et al. 244 criteria, which cover model structure, information required for the model and uncertainty. Details of the quality assessment for each study are presented in Appendix 7.
Model structures
Structures of the models included in this review were generally of satisfactory quality. In accordance with best practice for developing model structures, studies clearly stated the decision problem and the viewpoint/perspective of the analysis and the objectives of the model, all of which were consistent with the decision problem. Additionally, illustrative structures captured the relapsing nature of MS and followed the pathway for people treated for RRMS. Although good reporting quality was noted in most studies, some structural issues were noted. These related to the time horizon, the model structure, half-cycle corrections and the generalisability of the results. In four studies154,264,266,272 the time horizon was possibly too short to capture all of the costs and benefits of treatment with DMTs. Agashivala and Kim266 used a decision tree structure and affixed probability estimates for progression at discrete/fixed time points. As a result, this does not reflect the true nature of RRMS. A Markov model would have been more appropriate because of the chronic nature of the disease and the long-term horizons for progressing to more severe EDSS levels. Additionally, the health states included in the model structure were not clearly described. One study264 used a 1-month cycle length in the model, but this does not reflect the routine follow-up for people with RRMS; an annual cycle length would have been more appropriate. On the other hand, Nikfar et al. 265 used a model cycle over 2 years, although it was unclear whether a half-cycle correction was used.
In general, all studies154,264–272 stated the location of the analyses but not the setting, which prevents assessment of the generalisability of the results.
Information required for models
The methods used to identify relevant information to populate the models were satisfactory in most studies. 264–266,268,270–272 All studies provided references for their model inputs but quality appraisal and selection of relevant inputs were rarely made transparent. In all studies information was required on the treatment effect of DMTs on progression and relapse rates, resource use and costs, outcomes and mortality. 154,264–272
The effects of treatment with DMTs on disease progression compared with no treatment were modelled using HRs. The relative reduction in disability progression associated with DMTs was applied to the predicted baseline cohort of people with RRMS. In some analyses, studies obtained this HR directly from a trial or through reviewing the clinical effectiveness literature. However, studies that used the latter approach did not elaborate on the quality assessment of these RCTs or provide sufficient detail on how the HR had been derived. Information on a baseline cohort of people could be obtained from MS registries, a natural history cohort or the placebo arm of a trial. In all studies, information on disease progression in a baseline cohort was obtained from RCTs. All models considered the treatment effect on reduction in relapses. The treatment effect on the average number of relapses experienced by EDSS level was obtained from published sources. Most studies undertook analyses based on a long time horizon, which is in line with the NICE reference case. 156 However, authors did not elaborate on the techniques used to extrapolate the treatment effects beyond the time horizon of the RCTs. Studies using a shorter time horizon, for example that by Lee et al. ,272 did not assume treatment benefit beyond the length of the follow-up study.
Information on resource use and costs was obtained from published sources and these were well documented in some studies. Resource use by EDSS level was well documented in the study undertaken by Nikfar et al. 265
Uncertainty
All studies included one-way sensitivity analysis, undertaken by changing key model inputs to determine the robustness of the base-case results. Changes were made to discount rates, the time horizon, the initial EDSS distribution of people in the starting cohort, the perspective of the analysis, the discontinuation rate and utility values. To our knowledge, authors did not use information from a natural history cohort of people to model disease progression as part of their sensitivity analyses or allow for waning treatment effect over time.
Summary of the relapsing–remitting multiple sclerosis cost-effectiveness evidence
We identified 10 studies154,264–272 that used an economic model to estimate the cost-effectiveness of DMTs for treating people with RRMS. The evidence offers insight into the modelling methodology, including the illustrative structures to depict MS progression, key model inputs and assumptions made to assess cost-effectiveness. These methods appeared to be feasible across all studies.
We appraised studies against the CHEERS243 and Philips et al. 244 checklists for best practice for reporting economic evaluation and economic modelling studies respectively. Studies performed well against these checklists in terms of reporting sufficient information on the decision problem, outlining the study perspective, listing the intervention and comparators, presenting an illustrative model structure and providing a clear outline of the assumptions. Our review highlights some limitations of the studies; these are related to the structure of the models and the information required to populate the models. In terms of the model structure, the time horizon was short in some studies and the choice of model structure did not accurately reflect or capture the disability progression associated with MS. Limitations associated with model information relate to the lack of detail provided on the quality assessment of clinical effectiveness studies, the methods used to meta-analyse information from clinical studies and extrapolating the treatment effect beyond the trial time horizons. Additionally, we noted some limitations in the methods used to model mortality.
In Chapter 11 we draw on the information from this review in terms of model design and model inputs to estimate the cost-effectiveness of DMTs for treating people with RRMS.
Chapter 9 Risk-sharing scheme submission
Overview of the risk-sharing scheme model
In the RSS model, an economic analysis was conducted to assess the cost-effectiveness of the combined treatment effect of 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif), 20 mg of SC GA once daily (Copaxone), 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) and 30 µg of IM IFN-β-1a once weekly (Avonex) compared with BSC for people with RRMS. 154
In the analysis, a Markov model was used to depict the natural history of people with RRMS, including progression to SPMS. Information required on the natural history of people with RRMS was based on the BCMS cohort. Two sets of transition probabilities were reported: transitions based on the age at onset of RRMS below (subgroup 1) and above (subgroup 2) the median age. In both the natural history cohort and the RSS cohort, disability progression was characterised using the EDSS, which ranges from 0 to 10 (death). In addition to progressing to more severe EDSS states, people were allowed to regress to less severe EDSS states, which reflected the natural course of the disease. In the model, only those in EDSS level 7–9 could progress to EDSS level 10 (death). Additionally, it was assumed that the SMR increased by twofold, regardless of the age at onset or severity of MS.
In the treatment arm (RSS model), the model assumed a discontinuation rate of 5% per annum for patients with an EDSS score of 0–6 and that all patients reaching an EDSS score of ≥ 7 would discontinue treatment. It was assumed that people who discontinued treatment would remain off treatment for the remainder of their life.
The analysis was undertaken from the UK NHS and PSS perspective in a secondary care setting. Health outcomes were measured in QALYs and the analysis was undertaken over a 50-year time horizon. Information on utilities by EDSS state was obtained by pooling utility estimates from the 2002 and 2005 MS Trust surveys, based on information collected on the EQ-5D, which were subsequently converted to an EQ-5D index score. Information on resource use and unit costs was obtained from Tappenden et al. 275 and subsequently inflated to current prices. The results were presented as ICERs, expressed as cost per QALY gained. Both costs and benefits were discounted at 3.5% per annum.
The base-case results showed that, for people in subgroup 1, the mean cost per person in the treatment arm was approximately £357,100, with a mean of 7.987 QALYs gained per person. For BSC, the mean cost per person was approximately £328,800, with a mean of 6.947 QALYs gained per person. Consequently, the ICER was approximately £27,200 per QALY. In subgroup 2, the mean cost per person in the treatment arm was approximately £379,300, with 8.022 QALYs gained per person, whereas the mean cost per person in the BSC arm was approximately £355,500, with 7.028 QALYs gained per person. This gave an ICER of approximately £23,900 per QALY. Overall, the mean incremental cost of DMTs compared with BSC was approximately £25,600, with a corresponding 1.013 QALYs gained and an ICER of approximately £25,300 per QALY.
A number of sensitivity analyses were undertaken:
-
excluding from the analyses those who switched to a non-scheme DMT
-
using imputation techniques to account for missing values in the multilevel model examining patient trajectories in EDSS scores, with observations nested within people
-
changing the assumption made in the Markov model about the treatment effect of DMTs on backward transitions
-
supplementing transition probabilities derived from the BCMS with imputed values.
Sensitivity analysis 1 showed a marginal increase in the treatment effect for the ‘base run’. For sensitivity analysis 2, slight differences were seen between treatment effects. No probabilistic sensitivity analyses were undertaken. Table 25 gives a summary of the RSS model.
| Parameter | RSS model |
|---|---|
| Natural history cohort | BCMS cohort |
| Population | People initially diagnosed with RRMS and those who progress to SPMS |
| Intervention | DMTs available in the RSS: 30 µg of IM IFN-β-1a once a week (Avonex), 44 or 22 µg of SC IFN-β-1a three times per week (Rebif), 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia), 20 mg of SC GA daily (Copaxone) |
| Comparator | BSC |
| Type of model | Markov model |
| HR | Targeted outcomes were agreed for each of the four DMTs included in the RSS, expressed as HRs of disability progression for treated compared with no treatment |
| Resource use and costs | DMT costs, health state/EDSS costs and cost of relapses |
| HRQoL | Utility values were pooled from the 2002 and 2005 MS Trust surveys |
| Discontinuation of treatment | Assumed that 5% of people would discontinue treatment every year, as seen in the RSS |
| Relapse | Weighted average of the frequency of relapses for people with RRMS and SPMS, irrespective of EDSS level |
| AEs | Utility decrement of 0.02 associated with AEs from DMTs. It was assumed that this decrement would apply only to the first year of treatment |
| Mortality | MS-related death for people in EDSS 7–9. For all states, a SMR was estimated and multiplied by two to take account of MS-related and non-MS-related mortality |
| Time horizon | 50 years |
| Base-case analysis results | Using the ‘base run’ model, an ICER of approximately £25,300 per QALY was derived. Using the ‘time-varying model’, an ICER of approximately £33,700 per QALY was derived |
| Sensitivity analysis (and probabilistic sensitivity analysis) results | No probabilistic sensitivity analysis was undertaken |
Evidence used to parameterise the risk-sharing scheme multiple sclerosis model
The model was populated with clinical information from the RSS and secondary sources. Information required to parameterise the model included evidence on the natural history of people with RRMS, the aggregate treatment effect of DMTs, AEs, resource use and costs, mortality and HRQoL.
Natural history of relapsing–remitting multiple sclerosis
The natural history of RRMS and SPMS was estimated using the BCMS database. Details of the BCMS cohort have been published elsewhere. 153 In brief, the BCMS database is a population-based database established in the 1980s that captures about 80% of people with MS in British Columbia, Canada. 154 EDSS scores were recorded by a MS specialist after a face-to-face consultation with patients and this usually occurred at the annual visit to the MS clinic. In the database, people who progressed to SPMS were not censored. However, all patients were censored in 1996 as a result of the introduction of DMTs in British Columbia. This database is considered to be large (by 2004 the BCMS database included > 5900 participants), with prospectively collected information (e.g. EDSS scores, relapses, AEs) and a long-term follow-up (> 25,000 cumulative years), and the database covers a relatively recent time period. 154
Expanded Disability Status Scale progression in the British Columbia Multiple Sclerosis cohort
The method of Jackson et al. 276 was used to depict the natural history of MS, based on the observation of people with RRMS in the BCMS cohort. Transition matrices were derived for people whose age at onset of MS was below and above the median age (Tables 26 and 27 respectively). Disability progression was characterised using the EDSS. In addition to progressing to more severe EDSS states, people were allowed to improve to less severe EDSS states, which reflects the natural course of the disease. From the transition matrix, only people in EDSS 7–9 could progress to EDSS 10 (MS-related death).
| From EDSS state | To EDSS state | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 0 | 0.6870 | 0.0612 | 0.0169 | 0.0062 | 0.0018 | 0.0005 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0 |
| 1 | 0.2110 | 0.6787 | 0.1265 | 0.0522 | 0.0225 | 0.0056 | 0.0014 | 0.0002 | 0.0000 | 0.0000 | 0 |
| 2 | 0.0720 | 0.1664 | 0.5955 | 0.1165 | 0.0662 | 0.0291 | 0.0045 | 0.0005 | 0.0000 | 0.0000 | 0 |
| 3 | 0.0224 | 0.0646 | 0.1729 | 0.5439 | 0.1210 | 0.0594 | 0.0252 | 0.0026 | 0.0003 | 0.0000 | 0 |
| 4 | 0.0043 | 0.0170 | 0.0454 | 0.0945 | 0.4874 | 0.0915 | 0.0321 | 0.0073 | 0.0006 | 0.0000 | 0 |
| 5 | 0.0014 | 0.0047 | 0.0184 | 0.0573 | 0.1009 | 0.4727 | 0.0424 | 0.0042 | 0.0005 | 0.0000 | 0 |
| 6 | 0.0018 | 0.0067 | 0.0219 | 0.1148 | 0.1664 | 0.2810 | 0.7283 | 0.1220 | 0.0187 | 0.0014 | 0 |
| 7 | 0.0001 | 0.0005 | 0.0018 | 0.0107 | 0.0262 | 0.0396 | 0.1151 | 0.6814 | 0.0570 | 0.0045 | 0 |
| 8 | 0.0000 | 0.0001 | 0.0005 | 0.0037 | 0.0069 | 0.0191 | 0.0457 | 0.1628 | 0.8544 | 0.1301 | 0 |
| 9 | 0.0000 | 0.0000 | 0.0000 | 0.0004 | 0.0007 | 0.0014 | 0.0052 | 0.0189 | 0.0608 | 0.6252 | 0 |
| 10 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0077 | 0.2387 | 1 |
| From EDSS state | To EDSS state | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 0 | 0.6954 | 0.0583 | 0.0159 | 0.0059 | 0.0017 | 0.0005 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0 |
| 1 | 0.2029 | 0.6950 | 0.1213 | 0.0496 | 0.0221 | 0.0053 | 0.0013 | 0.0001 | 0.0000 | 0.0000 | 0 |
| 2 | 0.0725 | 0.1578 | 0.6079 | 0.1201 | 0.0666 | 0.0294 | 0.0044 | 0.0005 | 0.0000 | 0.0000 | 0 |
| 3 | 0.0217 | 0.0609 | 0.1680 | 0.5442 | 0.1152 | 0.0587 | 0.0250 | 0.0025 | 0.0003 | 0.0000 | 0 |
| 4 | 0.0042 | 0.0164 | 0.0446 | 0.0911 | 0.4894 | 0.0874 | 0.0307 | 0.0073 | 0.0005 | 0.0000 | 0 |
| 5 | 0.0014 | 0.0046 | 0.0185 | 0.0584 | 0.1039 | 0.4869 | 0.0408 | 0.0038 | 0.0005 | 0.0000 | 0 |
| 6 | 0.0018 | 0.0064 | 0.0216 | 0.1165 | 0.1681 | 0.2731 | 0.7407 | 0.1168 | 0.0187 | 0.0013 | 0 |
| 7 | 0.0001 | 0.0005 | 0.0017 | 0.0103 | 0.0258 | 0.0388 | 0.1089 | 0.6926 | 0.0553 | 0.0043 | 0 |
| 8 | 0.0000 | 0.0001 | 0.0005 | 0.0036 | 0.0067 | 0.0188 | 0.0438 | 0.1606 | 0.8964 | 0.1326 | 0 |
| 9 | 0.0000 | 0.0000 | 0.0000 | 0.0003 | 0.0006 | 0.0010 | 0.0042 | 0.0156 | 0.0205 | 0.6230 | 0 |
| 10 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0077 | 0.2387 | 1 |
Types of multiple sclerosis
The model included people who commenced in a RRMS health state and who progressed to SPMS. People with CIS, PPMS or benign disease were not included in the RSS as treatment options included in the scheme were not licensed for these types of MS. 275
Interventions
The RSS model analysed the combined treatment effects of IFN-β and GA compared with BSC for people with RRMS. Table 28 shows the drugs and dose regimes included in the RSS with their licensed indications in the UK. The year 10 analyses included people whose EDSS scores were recorded after they had switched to non-scheme DMTs. The assessment group was not clear on the non-scheme DMTs included in the RSS. Sensitivity analysis was conducted around the treatment effect, which involved censoring people whose EDSS scores were recorded after switching treatment. Censoring these people resulted in an increase in the combined treatment effect (HR 0.7666).
| Trade name | Drug | Dose regime | Route of administration | Licensed indications |
|---|---|---|---|---|
| Avonex | IFN-β-1a | 30 µg once a week | IM | RRMS |
| Rebif | IFN-β-1a | 44 µg three times per week (22 µg three times per week for patients unable to tolerate the higher dose) | SC | RRMS, SPMS |
| Betaferon | IFN-β-1b | 250 µg every other day | SC | RRMS, SPMS |
| Copaxone | GA | 20 mg once daily | SC | RRMS |
Population
The population included in the RSS model was similar to the population in the BCMS cohort. In the RSS, the population was stratified by age at onset of RRMS and by EDSS score. The initial distribution of people in each EDSS state is presented in Table 29.
| EDSS level | Age at onset below median, n | Age at onset above median, n | Total |
|---|---|---|---|
| 0 | 61 | 74 | 135 |
| 1 | 295 | 394 | 689 |
| 2 | 411 | 677 | 1088 |
| 3 | 401 | 569 | 970 |
| 4 | 273 | 379 | 652 |
| 5 | 162 | 279 | 441 |
| 6 | 76 | 166 | 242 |
| 7 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 |
| Total | 1679 | 2538 | 4217 |
Mortality rate
Two types of mortality were included in the model, MS-related death (EDSS 10) and death from other causes. General population mortality was obtained from the Office for National Statistics (ONS)277 and a weighted average was taken to represent the distribution of men and women in the economic model. People with RRMS and SPMS were assumed to have a higher mortality rate than those in the general population. It was assumed that the SMR increased twofold, regardless of the age at onset or severity of MS and EDSS level. The assessment group noted that the same transition probabilities from EDSS 7–9 to MS-related death were used for both natural history subgroups and also for both active therapy subgroups. The assessment group was concerned that MS-related mortality may have been overestimated, as individuals in the model also died as a result of progression to EDSS 10 (death).
Resource use and costs
All resource use and costs included in the analysis were those directly related to the NHS and PSS perspective and were reported in UK pounds in 2015/16 prices. The RSS model included the following costs:
-
DMT costs
-
health state/EDSS costs
-
cost of relapse.
Disease-modifying therapy costs
Table 28 shows the DMTs included in the RSS model. Drug prices were agreed as part of the RSS. A weighted average cost of these treatments was taken, with a mean cost of £7300 per year derived for people who received treatment. However, it was not clear how this weighted average cost was derived.
Health state/Expanded Disability Status Scale costs
Information on resource use and costs associated with treating MS from a UK perspective was obtained from a cross-sectional observational study undertaken by Kobelt et al. 108 This study obtained resource use information to derive the costs of MS from a societal perspective (direct and indirect costs), but also provided disaggregated information relating to the direct costs of MS (detection, treatment, rehabilitation and long-term care). The direct costs included the costs of inpatient care, ambulatory care, social care, drug treatment, investments made to the home and informal care (care provided in the absence of family). The study reported that direct costs (including informal care) accounted for 54% of the total costs, with the remaining 46% representing indirect costs. However, excluding informal care from the analysis, direct costs accounted for 38% of the total costs per patient per year. The costs were estimated for each individual patient in the study and an average cost per patient was reported with respect to the different levels of disability (mild, moderate and severe). All costs were reported in UK pounds at 1999/2000 prices.
The previous report by the School of Health and Related Research (ScHARR), University of Sheffield,275 suggested that 244 out of the 622 records of resource use and costs for treating MS in the UK were excluded because respondents had PPMS or benign MS or information on EDSS state was missing. Mean direct costs by EDSS state and mean cost of a relapse reported in the economic model submitted by the Department of Health were based on information supplied to the ScHARR team in confidence and the assessment group did not have access to this information. Costs in the ScHARR submission were subsequently inflated to current prices (2015/16) using the appropriate indices from the Hospital and Community Health Services (HCHS) pay and price index 2015/16278 and the assessment group believes that these have been appropriately derived. Table 30 shows the costs included in the model.
| EDSS state | Unit costs (£), 1999/2000 prices | Unit costs (£), 2015/16 prices |
|---|---|---|
| 0 | 756 | 1164 |
| 1 | 756 | 1164 |
| 2 | 756 | 1164 |
| 3 | 1394 | 2147 |
| 4 | 1444 | 2225 |
| 5 | 5090 | 7840 |
| 6 | 5678 | 8746 |
| 7 | 17,327 | 26,688 |
| 8 | 26,903 | 41,439 |
| 9 | 34,201 | 52,679 |
| 10 | 0 | 0 |
Despite these mean costs being correctly derived, the RSS model assumed that resource use and patient management have not changed since 1990/2000. The assessment group believes that a systematic review could have been conducted to obtain more recent information on resource use.
The assessment group is unable to provide comment on:
-
the resource use information valued to derive the mean unit costs per EDSS state
-
the number of people reporting on resource use in each health state
-
the percentage of people receiving each drug treatment
-
the distribution of resource use and the techniques used to account for the skewness of costs, if this existed
-
the techniques used to account for missing data, if they existed
-
‘mapping’ from mild, moderate and severe disability onto the EDSS.
Cost of relapse
The cost of a relapse included in the RSS model was obtained from Tappenden et al. 275 and subsequently inflated to current prices using the HCHS pay and price index 2015/16. 278 The cost represents an average cost regardless of the severity of the relapse. The cost of a relapse was the same in the treatment and no treatment arms of the model. As with health state costings, the assessment group noted that the original cost year was 1999/2000 and assumptions were made that resource use and management have not changed since the base year. Despite this assumption, the assessment group considers the cost of relapse (£4263) to have been derived correctly. However, the assessment group is unclear on the components/resources costed to derive this cost. Additionally, the assessment group believes that a review of the literature could have been undertaken to obtain more recent information.
The costs included in the model were related to drug treatment, health state/EDSS and relapse costs. The assessment group was not clear whether the costs of treating AEs, administering the drugs or monitoring treatments were included in the analysis. For example, IFN-β-1a (Avonex) is administered intramuscularly and would incur additional directs costs (e.g. training patients or carers to administer injections).
Health state utility values
The primary outcome measure used in the model was a ‘deviation score of the average observed loss of utility’ (Department of Health, 10 January 2017, personal communication). Health outcomes were measured in QALYs, with utility weights assigned to the health states in the model. The utilities used in the RSS model were derived by first pooling values from two MS Trust surveys (2002 and 2005) and then subtracting carers’ disutility. Utilities obtained from Boggild et al. ,279 as used in Tappenden et al. ,275 were derived based on information from a two-stage survey of 1554 respondents from the MS Trust database. To our understanding, these three sets (data from the two MS Trust surveys and Boggild et al. 279) formed the three-pooled data set. Utility estimates, by EDSS, were derived based on information collected on the EQ-5D questionnaire, which was subsequently converted to an EQ-5D index score. Alternative utility values were derived based on pooled data sets from the ScHARR model275 and also from the UK MS Trust surveys. Table 31 shows the utility values used in the RSS model.
| EDSS state | Boggild et al.279 data set | Three-pooled data set | Two-pooled data set | Carers’ disutility |
|---|---|---|---|---|
| 0 | 0.7850 | 0.8722 | 0.9248 | –0.002 |
| 1 | 0.7480 | 0.7590 | 0.7614 | –0.002 |
| 2 | 0.6900 | 0.6811 | 0.6741 | –0.002 |
| 3 | 0.5827 | 0.5731 | 0.5643 | –0.002 |
| 4 | 0.5827 | 0.5731 | 0.5643 | –0.045 |
| 5 | 0.5790 | 0.5040 | 0.4906 | –0.142 |
| 6 | 0.4740 | 0.4576 | 0.4453 | –0.167 |
| 7 | 0.3650 | 0.2825 | 0.2686 | –0.063 |
| 8 | 0.2640 | 0.0380 | 0.0076 | –0.095 |
| 9 | –0.1770 | –0.2246 | –0.2304 | –0.095 |
| 10 | 0 | 0 | 0 | 0 |
Carers’ disutility
An analysis was undertaken that included carers’ disutility by EDSS state. Table 31 shows the disutility values used in the model. Initially, the assessment group was unclear on the source of these disutilities. However, on clarification the Department of Health suggested that these values were obtained from a study by Acaster et al. 280 The assessment group examined the literature to identify other potential sources of disutilities associated with providing care for people with MS.
Treatment effect
The effect of treatment with DMTs was modelled for the relative reduction in the annual frequency of relapses and the relative risk of disease progression between EDSS states. In the RSS model, both treatment effects were estimated based on observed relapses and progressions in EDSS scores in people in the RSS. Although not clear, it appeared that similar methods used to derive transition matrices for the BCMS cohort were used to derive transition matrices for the RSS model. From the comparison between both cohorts, a mean HR of 0.7913 for disability progression was derived, based on the RSS year 10 analyses. The model assumed that the treatment effect reduced the instantaneous rate of forward transitions by this HR, independent of EDSS level, and that there was no effect on backward transitions. The report suggested that the HRs for backward transitions were similar to that for forward transitions; however, these ratios were not reported. Additionally, in the model (base run) it was assumed that the HR remained the same over the entire duration (50 years) of the model time horizon.
Relapse frequency
In the RSS model, a weighted average of the frequency of relapse for people with RRMS and SPMS, at the same EDSS level, was derived based on information obtained from the 2002 survey by the MS Trust (Table 32). However, because of the paucity of information reported on the aggregate treatment effect of DMTs on reducing relapse frequencies, we are unable to provide further commentary on this estimate.
| EDSS | Relapse frequency | Mean frequency | ||||
|---|---|---|---|---|---|---|
| RRMS | SPMS | % RRMS | % SPMS | Untreated | Treated | |
| 0 | 0.8895 | 0.0000 | 1.000 | 0.000 | 0.8895 | 0.6405 |
| 1 | 0.7885 | 0.0000 | 0.861 | 0.139 | 0.6790 | 0.4888 |
| 2 | 0.6478 | 0.6049 | 0.861 | 0.139 | 0.6418 | 0.4621 |
| 3 | 0.6155 | 0.5154 | 0.806 | 0.194 | 0.5961 | 0.4292 |
| 4 | 0.5532 | 0.4867 | 0.545 | 0.455 | 0.5230 | 0.3765 |
| 5 | 0.5249 | 0.4226 | 0.343 | 0.657 | 0.4577 | 0.3295 |
| 6 | 0.5146 | 0.3595 | 0.270 | 0.730 | 0.4014 | 0.2890 |
| 7 | 0.4482 | 0.3025 | 0.053 | 0.947 | 0.3103 | 0.2234 |
| 8 | 0.3665 | 0.2510 | 0.000 | 1.000 | 0.2510 | 0.1807 |
| 9 | 0.2964 | 0.2172 | 0.000 | 1.000 | 0.2172 | 0.1564 |
| 10 | 0.0000 | 0.0000 | 0.000 | 0.000 | 0 | 0 |
Treatment discontinuation
In the treatment arm of the economic model it was assumed that 5% of people discontinue treatment every year as a result of AEs and that treatment would be discontinued among individuals progressing to an EDSS score of ≥ 7. However, the reasons for this were unclear, for example people may discontinue treatment because the therapy is no longer working. 275
The assessment group noted that no sensitivity analysis or probabilistic sensitivity analysis was undertaken around these key assumptions about discontinuation. The justification for these assumptions was based on the proportion of people discontinuing treatment as seen in the RSS. However, published evidence suggests that the proportion of people discontinuing treatment in clinical trials of the DMTs included in the RSS may range from 0%197 to 10%. 216 Additionally, it appears that people who discontinued treatment continued to accrue treatment benefits without additional costs. When people progressed to EDSS 7–9, the model used ‘on treatment’ transition probabilities. The assessment group would expect that people who discontinued treatment would progress to more severe health states in a similar way to people in the natural history cohort.
Analysis (cycle length, time horizon and perspective)
For the base-case analysis, a Markov model was developed and programmed to assess the cost-effectiveness of the combined treatment effect of DMTs in the RSS compared with no treatment (BSC) for people with RRMS. The model cycled yearly, with a starting age of 30 years, and estimated the mean costs and effects associated with treatment compared with no treatment over a 50-year time horizon. The analysis was conducted from the NHS and PSS perspective and the results were reported in terms of ICERs, expressed as cost per QALY gained. Both costs and benefits were discounted at 3.5% per annum.
Time-varying model
The RSS submission also included a sensitivity analysis using a ‘time-varying model’ to take account of a perceived lack of fit of the RSS in taking account of the trajectories of patients with higher EDSS levels at baseline. The model had two sets of transition probabilities, one for years 0–2 and one for all subsequent years.
Summary of the critical appraisal of the risk-sharing model
In general, the assessment group considered the RSS model to be appropriate to estimate the cost-effectiveness of DMTs compared with BSC. In most cases the model draws on the best available evidence on progression through RRMS and SPMS by EDSS level, resource use and costs and utility values. We have considered and provided a critique of the RSS model against the NICE reference case156 and of the economic model inputs and we have checked the model used to estimate cost-effectiveness. However, some uncertainties remain, which are presented below. Additionally, in Chapter 11, we describe alternative analyses that address our concerns:
-
The model applied a constant rate of 5% for people discontinuing treatment. However, there is little evidence to support this assumption.
-
The difference between combined DMTs and BSC in reducing the frequency of relapses was 0.72, but it was unclear how this value was derived. The report suggested that a weighted average of the frequency of relapses for people with RRMS and SPMS, irrespective of EDSS level, was used and that this was derived from information obtained from the 2002 survey undertaken by the MS Trust.
-
The assessment group noted that there was an assumption of increased risk of mortality for people with MS compared with the general population. This was in addition to transition probabilities to EDSS 10 (MS-related death). Using this assumption would lead to double counting of MS-related deaths in the model.
-
The model considers the prices of drugs agreed between the companies and the Department of Health. However, it was unclear to the assessment group how these prices were derived.
-
In the analysis, the model included carers’ disutilities. The assessment group agrees that people may experience a loss in utility when caring for people with MS. However, this analysis used a NHS and PSS perspective.
-
A probabilistic sensitivity analysis to incorporate uncertainty in the estimates for model parameters was not undertaken.
Chapter 10 Manufacturers’ submissions: economic evidence
Biogen Idec Ltd
Background
This section focuses on the economic evidence submitted by Biogen Idec Ltd. This section is set out as follows: first, we present an overview/summary and then we provide a critique of the economic model submitted, which describes in detail the evidence (e.g. natural history information, effectiveness of interventions included in the analysis, resource use and costs, mortality and HRQoL) used to parameterise the model. In the Biogen Idec Ltd model, an economic analysis was conducted to assess the cost-effectiveness of 30 µg of IM IFN-β-1a once weekly (Avonex), 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif), 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia), 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) and 20 mg of SC GA daily or 40 mg of SC GA three times weekly (Copaxone) compared with BSC for people with RRMS.
In the analysis, a Markov model was used to depict the natural history of people with RRMS through the SPMS. Information required on the natural history of people with RRMS was based on extrapolating the ADVANCE placebo arm data213 with the BCMS cohort data.
In the intervention arms it was assumed that treatment with DMTs was not discontinued after reaching a particular EDSS level, which the authors suggested is in accordance with the current Association of British Neurologists guidelines. 281 It was assumed that people would discontinue treatment only having progressed to the SPMS health state.
The analysis was undertaken from the payer perspective. The outcome measure used in the analysis was QALYs gained, over a 50-year time horizon. Treatment effects were assumed to delay the progression of the disease and reduce the frequency of relapses. Utilities for RRMS by EDSS level were based on information from the ADVANCE trial213 and Orme et al. ,101 which was derived from utility values from the UK MS Trust survey. Utility values for SPMS by EDSS level were based on information from the UK MS Trust survey, as cited in the manufacturer’s submission. Carers’ disutilities were based on information obtained from the manufacturer’s submission to NICE for TA127. 282 Disutility values for AEs associated with each DMT were included in the economic analysis.
Information on resource use and unit costs was obtained from various sources. The results were presented as ICERs and expressed as cost per life-year gained and cost per QALY gained. Both costs and benefits were discounted at 3.5% per annum. The authors carried out a number of sensitivity analyses (considering the societal perspective, patient baseline characteristics, transition probabilities, treatment efficacy, relapse rates, discontinuation rates, utility values, mortality multipliers, patients’ out-of-pocket costs, carers’ costs, loss of productivity for people with MS and AEs) and probabilistic sensitivity analysis to determine the robustness of the base-case results.
The base-case results showed that treatment with 125 µg of SC pegylated IFN-β-1a every 2 weeks resulted in the highest mean life-years gained (20.658) and mean QALYs gained (9.642) compared with all other interventions included in the analysis. Compared with BSC, 125 µg of SC pegylated IFN-β-1a every 2 weeks had a mean incremental cost of approximately £25,200, with corresponding incremental QALYs of 0.810, which equated to an ICER of approximately £31,000 per QALY.
The results from the sensitivity analyses showed that the base-case results were robust to univariate changes made to key input parameters, except for changes to the HR for confirmed disability progression, which had the greatest impact. The probabilistic sensitivity analysis suggested that, at a £30,000 per QALY willingness-to-pay threshold, 125 µg of SC pegylated IFN-β-1a every 2 weeks had a < 0.4 probability of being cost-effective compared with BSC.
Types of multiple sclerosis
The model included people who commenced in a RRMS health state and progressed to SPMS. People with CIS, PPMS or benign disease were not included in the analysis.
Model structure
The illustrative Markov model structure submitted by the manufacturer was based on the original ScHARR model,275 with developments to include other interventions. The manufacturer used a cohort-based Markov model to depict the natural history of people with RRMS. The model simulated disability progression, progression from RRMS to SPMS and the relapsing nature of the disease. People with RRMS were able to occupy one of the EDSS health states, which ranged from 0 to 10 in increments of 0.5. The model allowed for people to progress, regress or stay in the same EDSS health state or progress from RRMS to SPMS. When people progressed to SPMS they either remained in the same EDSS state or progressed to more severe EDSS states.
In the model, people incurred costs and accrued benefits depending on the EDSS state for RRMS and SPMS. Benefits were measured using QALYs, whereby each model cycle a utility is assigned to people occupying a specific health state.
The assessment group was uncertain whether a review of the economic literature was undertaken to inform the model design and/or its inputs. Based on our review there appears to be some inconsistency in the model structures used to estimate the cost-effectiveness of DMTs for people with RRMS. These discrepancies may be a result of the complex nature of MS. In Biogen Idec Ltd’s model, people could progress from health states of an EDSS score of ≥ 1 to SPMS. However, in some models identified in the review people could progress only from health states of an EDSS score of ≥ 6 to SPMS.
Interventions
The interventions considered in the economic analyses were 30 µg of IM IFN-β-1a once weekly (Avonex), 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif), 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia), 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) and 20 mg of SC GA daily or 40 mg of SC GA three times weekly (Copaxone). These comparisons are all in line with the NICE scope. 141 The interventions were compared against BSC for people with RRMS. The manufacturer suggested that BSC would not currently be offered as a starting point to RRMS patients.
Population
The population included in the economic analysis was similar to the population included in the ADVANCE trial213 (i.e. 71% female, RRMS with a starting age of 36 years). The initial distribution of people in each EDSS state is presented in Table 33.
| EDSS state | Distribution (%) |
|---|---|
| 0 | 6 |
| 1 | 26 |
| 1.5–2 | 28 |
| 2.5–3 | 24 |
| 3.5–4 | 12 |
| 4.5–5 | 4 |
| 5.5–6 | 0 |
| 6.5–7 | 0 |
| 7.5–8 | 0 |
| 8.5–9.5 | 0 |
| 10 | 0 |
Transitions
To simulate how people transitioned between the health states in the model, information was required on transitions between RRMS health states, progression from RRMS to SPMS and transitions between SPMS health states for both the comparator arm and the intervention arm. In the comparator arm (natural history receiving BSC), in the base case the transition probabilities were derived from information from the ADVANCE trial,213 supplemented with information from the BCMS data set. 153 Table 34 shows the annual transition probabilities between RRMS health states used in the natural history arm. In sensitivity analysis, the manufacturer derived other transition probabilities, using information from the ADVANCE trial213 extrapolated with information from the BCMS data set153 or the London Ontario data set. 80 The transition probabilities for RRMS to SPMS were based on information from the London Ontario data set. 80 The manufacturer suggested that these values were not available in the BCMS cohort but did not elaborate on how they were derived. Table 35 shows the transition probabilities for RRMS to SPMS by EDSS level. Transition probabilities for people progressing within SPMS health states were estimated from the BCMS cohort. 153 These annual probabilities were derived using a multistate model. Table 36 shows the transitions between SPMS states.
| From EDSS state | To EDSS state | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 1.5–2 | 2.5–3 | 3.5–4 | 4.5–5 | 5.5–6 | 6.5–7 | 7.5–8 | 8.5–9.5 | 10 | |
| 0 | 0.850 | 0.050 | 0.100 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0 |
| 1 | 0.024 | 0.830 | 0.114 | 0.024 | 0.000 | 0.000 | 0.006 | 0.001 | 0.001 | 0.000 | 0 |
| 1.5–2 | 0.014 | 0.152 | 0.670 | 0.104 | 0.048 | 0.000 | 0.010 | 0.001 | 0.001 | 0.000 | 0 |
| 2.5–3 | 0.000 | 0.008 | 0.125 | 0.693 | 0.084 | 0.017 | 0.064 | 0.005 | 0.004 | 0.000 | 0 |
| 3.5–4 | 0.000 | 0.022 | 0.000 | 0.216 | 0.519 | 0.086 | 0.141 | 0.009 | 0.007 | 0.000 | 0 |
| 4.5–5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.041 | 0.532 | 0.375 | 0.028 | 0.023 | 0.000 | 0 |
| 5.5–6 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.894 | 0.049 | 0.056 | 0.001 | 0 |
| 6.5–7 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.807 | 0.189 | 0.004 | 0 |
| 7.5–8 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.995 | 0.006 | 0 |
| 8.5–9.5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 | 0 |
| 10 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1 |
| EDSS state | Probability of transition to SPMS (one EDSS state higher) |
|---|---|
| 1 | 0.003 |
| 1.5–2 | 0.032 |
| 2.5–3 | 0.117 |
| 3.5–4 | 0.210 |
| 4.5–5 | 0.299 |
| 5.5–6 | 0.237 |
| 6.5–7 | 0.254 |
| 7.5–8 | 0.153 |
| 8.5–9.5 | 1.000 |
| From EDSS state | To EDSS state | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 1.5–2 | 2.5–3 | 3.5–4 | 4.5–5 | 5.5–6 | 6.5–7 | 7.5–8 | 8.5–9.5 | 10 | |
| 0 | 0.695 | 0.203 | 0.073 | 0.022 | 0.004 | 0.001 | 0.002 | 0.000 | 0.000 | 0.000 | 0 |
| 1 | 0.058 | 0.695 | 0.158 | 0.061 | 0.016 | 0.005 | 0.006 | 0.000 | 0.000 | 0.000 | 0 |
| 1.5–2 | 0.016 | 0.121 | 0.608 | 0.168 | 0.045 | 0.018 | 0.022 | 0.002 | 0.001 | 0.000 | 0 |
| 2.5–3 | 0.006 | 0.050 | 0.120 | 0.544 | 0.091 | 0.058 | 0.116 | 0.010 | 0.004 | 0.000 | 0 |
| 3.5–4 | 0.002 | 0.022 | 0.067 | 0.115 | 0.489 | 0.104 | 0.168 | 0.026 | 0.007 | 0.001 | 0 |
| 4.5–5 | 0.001 | 0.005 | 0.029 | 0.059 | 0.087 | 0.487 | 0.273 | 0.039 | 0.019 | 0.001 | 0 |
| 5.5–6 | 0.000 | 0.001 | 0.004 | 0.025 | 0.031 | 0.041 | 0.741 | 0.109 | 0.044 | 0.004 | 0 |
| 6.5–7 | 0.000 | 0.000 | 0.001 | 0.002 | 0.007 | 0.004 | 0.117 | 0.693 | 0.161 | 0.016 | 0 |
| 7.5–8 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.019 | 0.056 | 0.903 | 0.021 | 0 |
| 8.5–9.5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.006 | 0.174 | 0.818 | 0 |
| 10 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1 |
Treatment effects of 30 µg of interferon beta-1a intramuscularly once weekly (Avonex)
For disability progression the manufacturer derived a HR based on a Cox proportional hazard model as a measure of relative risk. In the RSS model, the treatment effect of 30 µg of IM IFN-β-1a once weekly (Avonex) was shown to be (confidential information has been removed). The year 10 implied HR of (confidential information has been removed) for 30 µg of IM IFN-β-1a once weekly was used in the manufacturer’s model. Assuming no waning, the transition matrices are presented in Tables 37 and 38 for age at onset of < 28 years and age at onset of > 28 years respectively. The implied HR was applied to the model to show the relative effect of treatment on disability progression.
| From EDSS state | To EDSS state | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 1.5–2 | 2.5–3 | 3.5–4 | 4.5–5 | 5.5–6 | 6.5–7 | 7.5–8 | 8.5–9.5 | 10 | |
| 0 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 1 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 1.5–2 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 2.5–3 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 3.5–4 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 4.5–5 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 5.5–6 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 6.5–7 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 7.5–8 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 8.5–9.5 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 10 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| From EDSS state | To EDSS state | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 1.5–2 | 2.5–3 | 3.5–4 | 4.5–5 | 5.5–6 | 6.5–7 | 7.5–8 | 8.5–9.5 | 10 | |
| 0 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 1 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 1.5–2 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 2.5–3 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 3.5–4 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 4.5–5 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 5.5–6 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 6.5–7 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 7.5–8 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 8.5–9.5 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| 10 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
Resource use and costs
All costs included in the analysis were those directly related to the NHS and PSS perspective. Costs were reported in UK pounds in 2015/16 prices. The model included the following costs:
-
drug acquisition costs
-
administration costs
-
monitoring costs
-
health state/EDSS costs
-
cost of relapse
-
treatment-related AE costs.
Drug acquisition costs
Treatment costs for the DMTs are presented in Table 39. Annual costs were presented for the list and net price for each DMT available at the time of the RSS. From the Microsoft Excel® model submitted (2013; Microsoft Corporation, Redmond, WA, USA), the annual cost of each drug was derived from the dosage (per week and per year) and the price per packet. The assessment group considered these acquisition costs to be correctly derived.
| Treatment | Administration | Doses per year | Annual acquisition cost (£, 2014/15 prices) | |||
|---|---|---|---|---|---|---|
| List price | Net price | |||||
| Year 1 | Subsequent years | Year 1 | Subsequent years | |||
| IFN-β-1a IM (Avonex) | 30 µg once weekly | 52.18 | 8502 | 8502 | (Confidential information has been removed) | (Confidential information has been removed) |
| PegIFN-β-1a SC (Plegridy) | 125 µg every 2 weeks | 26.1 | 8502 | 8502 | 8502 | 8502 |
| IFN-β-1a SC (Rebif) | 22 µg three times weekly | 156.18 | 7914 | 7976 | (Confidential information has been removed) | (Confidential information has been removed) |
| IFN-β-1a SC (Rebif) | 44 µg three times weekly | 156.18 | 10,311 | 10,572 | (Confidential information has been removed) | (Confidential information has been removed) |
| IFN-β-1b SC (Betaferon/Extavia) | 250 µg every other day | 182.63 | 7239 | 7239 | (Confidential information has been removed) | (Confidential information has been removed) |
| IFN-β-1b SC (Extavia) | 250 µg every other day | 182.63 | 7239.11 | 7239.11 | 7239.11 | 7239.11 |
| GA SC (Copaxone) | 20 mg once daily | 365.25 | 6681 | 6681 | (Confidential information has been removed) | (Confidential information has been removed) |
| GA SC (Copaxone) | 40 mg once daily | 156.18 | 6681 | 6681 | 6681 | 6681 |
When no net prices for DMTs were available [SC pegIFN-β-1a (Plegridy), SC IFN-β-1b (Extavia) and GA (Copaxone)], the list prices of these drugs were used in the analysis. The assessment group noted that the annual drug acquisition cost for 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) was reported as £7239 but the model used £7239.11 in the analysis.
Administration costs
Annual administration costs encompassed the costs associated with teaching people to self-administer the drugs. The administration costs are presented in Table 40. The assessment group considered the resource use and costs to be appropriate.
| Treatment | Annual administration cost for year 1 (£, 2014/15 prices) | Resource use | Annual administration cost for subsequent years (£, 2014/15 prices) | Resource use |
|---|---|---|---|---|
| IFN-β-1a 30 µg IM once weekly (Avonex) | 177.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| IFN-β-1a 44 or 22 µg SC three times weekly (Rebif) | 177.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 177.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 177.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| GA 20 mg SC once daily or 40 mg SC three times weekly (Copaxone) | 177.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
Monitoring costs
Annual monitoring costs for each treatment were presented in appendix K of the main report. The manufacturer clearly outlined the resource use used to derive monitoring costs. Monitoring costs were presented for year 1 and for subsequent years. The monitoring costs for all interventions are presented in Table 41. These annual monitoring costs appeared to have been derived and used in the model correctly.
| Treatment | Monitoring cost for (£, 2014/15 prices) | |
|---|---|---|
| Year 1 | Subsequent years | |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 190.73 | 10.78 |
| IFN-β-1a 44 or 22 µg SC three times weekly (Rebif) | 203.25 | 10.78 |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 190.73 | 10.78 |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 191.92 | 10.78 |
| GA 20 mg SC once daily or 40 mg SC three times weekly (Copaxone) | 175.75 | 10.78 |
Health state/Expanded Disability Status Scale costs
Health state costs (payer perspective) by EDSS level and MS type (RRMS/SPMS) are presented in Table 42. These costs were related to MS management (expected/unexpected visits to health-care providers). The manufacturer also identified and presented cost estimates from the study by Karampampa et al. 283 and the burden of illness (BOI) study. Costs obtained from Karampampa et al. 283 were inflated using the HCHS index and these seemed to have been correctly derived. These cost estimates were used in sensitivity analyses. Costs were presented from the payer, government and societal perspectives in the sensitivity analyses. It appears that the cost estimates by EDSS state varied between studies. There appears to be a gradual increase in the management cost estimates derived in the submission and the BOI study from EDSS 0 to 6, followed by larger increases beyond EDSS 6. However, in the Karampampa et al. 283 study, management costs seem to increase gradually from EDSS 0 to 10.
| EDSS state | RRMS (£, 2014/15 prices) | SPMS (£, 2014/15 prices) | ||||
|---|---|---|---|---|---|---|
| Biogen Idec Ltd | Karampampa et al.283 | BOI study | Biogen Idec Ltd | Karampampa et al.283 | BOI study | |
| 0 | 937 | 1179 | 4301 | 1263 | 1470 | 4301 |
| 1 | 974 | 1399 | 4783 | 1301 | 1745 | 4783 |
| 1.5–2 | 714 | 1674 | 8666 | 1040 | 2088 | 8666 |
| 2.5–3 | 3906 | 2006 | 7720 | 4232 | 2502 | 7720 |
| 3.5–4 | 1892 | 2393 | 7159 | 2218 | 2985 | 7159 |
| 4.5–5 | 3210 | 2837 | 9147 | 3537 | 3538 | 9147 |
| 5.5–6 | 4285 | 3337 | 12,830 | 4611 | 4161 | 12,830 |
| 6.5–7 | 11,279 | 3892 | 17,971 | 11,605 | 4854 | 17,971 |
| 7.5–8 | 27,472 | 4503 | 29,915 | 27,798 | 5616 | 29,915 |
| 8.5–9.5 | 21,982 | 5170 | 37,656 | 22,309 | 6449 | 37,656 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 |
Cost of relapse
In the main report of the manufacturer’s submission, the cost of a relapse (£2697) was obtained from the ScHARR model275 and subsequently inflated to current prices (£4265) using the HCHS pay and price index 2014/15. 278 Using a cost from a dated source assumes that the management of and resource use for treating relapses have not changed post 2001. The assessment group considered this to be a strong assumption.
In critiquing the economic model submitted (and as stated in the appendices), the assessment group noted that the cost of relapse used was obtained from the study by Hawton and Green107 and then subsequently inflated to current prices using the HCHS pay and price index 2014/15. 278 This cost represents an average cost regardless of the severity of the relapse. Costs were derived for relapses not requiring hospitalisation (£568) and for relapses requiring hospitalisation (£3651). The assessment group noted that these costs were the same in all arms (intervention and comparator arms) of the model. These costs appear to have been correctly derived. However, the manufacturer did not elaborate on the resource use estimates used to derive the unit cost of a relapse. Resource use information in the Hawton and Green study107 was obtained from information collected in the UK South West Impact of Multiple Sclerosis (SWIMS) project. 284 The SWIMS project is a prospective, longitudinal cohort study of people with MS in Devon and Cornwall, with follow up every 6 months. In this study information was collected on type of MS, disease severity measured by the EDSS, number of relapses in the previous 6 months, length of relapse, whether relapses led to hospital admittance and treatment received for relapses. Additional information was collected on health or social care use in the previous 6 months and the frequency of contact with a health-care professional. Resource use was valued using the Personal Social Services Research Unit (PSSRU),278 NHS reference costs285 and the BNF. 21 All costs derived were reported in UK pounds in 2012 prices. The assessment group considered this study to be methodologically robust. However, these costs represented people with various types of MS (RRMS, PPMS, SPMS, benign, combination or not known) who experienced relapses over a 6-month period. Resource use and costs were not reported by type of MS in the Hawton and Green study. 107 The assessment group considers that the costs used in the model were underestimates of the cost of a relapse.
Cost of adverse events
The model included the costs of AEs resulting from the use of DMTs. Estimates of resource use were presented in appendix K of the manufacturer’s submission. Health-care resource use for each AE was validated by a Delphi panel conducted by the manufacturer in December 2013. The manufacturer provided the percentages of people who developed these AEs by DMT. Table 43 shows the annual cost of treatment for AEs by DMT used in the model. These annual costs for the treatment of AEs appear to have been correctly derived.
| Treatment | Unit cost (£, 2014/15 prices) |
|---|---|
| IFN-β-1a 30 µg IM once weekly (Avonex) | 154.97 |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 76.95 |
| IFN-β-1a 22 µg SC three times weekly (Rebif) | 127.33 |
| IFN-β-1a 44 µg SC three times weekly (Rebif) | 140.89 |
| IFN-β-1b 250 µg SC every other day (Betaferon) | 104.12 |
| IFN-β-1b 250 µg SC every other day (Extavia) | 104.12 |
| GA 20 mg SC once daily (Copaxone) | 74.78 |
| GA 40 mg SC three times weekly (Copaxone) | 74.78 |
Health state utility values
Utilities were derived by EDSS level and MS type (RRMS and SPMS). In the base case these were derived by combining information from the placebo arm of the ADVANCE trial213 (EDSS 0–5) with information from the UK MS Trust survey (EDSS score of ≥ 6). Utility values for EDSS 6 were derived by adding the utility value for EDSS 5 (taken from the ADVANCE trial213) to the difference in utility values between EDSS 6 and EDSS 5 from the UK MS Trust survey. The same method was used to derive utility values for EDSS scores from ≥ 7 to 9. Utility values used in the model are presented in Table 44. The manufacturer also included disutilities associated with relapses experienced in a RRMS health state (–0.071) and in a SPMS health state (–0.045). These disutilities were applied across all EDSS levels by MS type (RRMS and SPMS). Disutilities were obtained from the study by Orme et al. 101 An analysis was also undertaken that included carers’ disutility by EDSS state. Table 44 shows the disutility values used in the model. Because of a lack of information, the burden associated with caring for people with RRMS or SPMS was assumed to be the same.
| EDSS state | Utility value | Carers’ disutility | ||
|---|---|---|---|---|
| RRMS | SPMS | RRMS | SPMS | |
| 0 | 0.879 | 0.834 | 0.000 | 0.000 |
| 1 | 0.866 | 0.821 | –0.001 | –0.001 |
| 1.5–2 | 0.771 | 0.726 | –0.003 | –0.003 |
| 2.5–3 | 0.662 | 0.617 | –0.009 | –0.009 |
| 3.5–4 | 0.573 | 0.528 | –0.009 | –0.009 |
| 4.5–5 | 0.549 | 0.504 | –0.020 | –0.020 |
| 5.5–6 | 0.491 | 0.446 | –0.027 | –0.027 |
| 6.5–7 | 0.328 | 0.283 | –0.053 | –0.053 |
| 7.5–8 | –0.018 | –0.063 | –0.107 | –0.107 |
| 8.5–9.5 | –0.164 | –0.209 | –0.140 | –0.140 |
| Relapse disutility in the RRMS states | –0.071 | |||
| Relapse disutility in the SPMS states | –0.045 | |||
Adverse event disutilities
The disutilities associated with AEs resulting from treatment with the DMTs are presented in Table 45.
| Treatment | Annual disutility |
|---|---|
| IFN-β-1a 30 µg IM once weekly (Avonex) | –0.024 |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | –0.016 |
| IFN-β-1a 44 µg SC three times weekly (Rebif) | –0.019 |
| IFN-β-1b 250 µg SC every other day (Betaferon) | –0.018 |
| IFN-β-1b 250 µg SC every other day (Extavia) | –0.018 |
| GA 20 mg SC once daily (Copaxone) | –0.007 |
| GA 40 mg SC three times weekly (Copaxone) | –0.007 |
Mortality rate
Mortality was assumed to be equivalent between RRMS and SPMS and dependent on EDSS state. All patients were modelled to be at risk of mortality from MS and other causes. This was modelled by first estimating SMRs using data from the ONS,277 as cited in the Biogen Idec Ltd submission, and applying a mortality multiplier to reflect both causes of death. Additionally, individuals in EDSS 7–9 could die from MS-specific mortality from transition to EDSS 10 (death).
Relapse frequency
The ARRs were obtained from the ADVANCE trial213 up to EDSS 5.5 and were supplemented with rates derived from the study by Patzold et al. ,286 as cited in the manufacturer’s submission. Table 46 shows the relapse rates by EDSS level used in the base case (ADVANCE trial placebo arm213) and other relapse rates used in scenario analyses.
| EDSS state | Study | |||||
|---|---|---|---|---|---|---|
| ADVANCE trial213 placebo arm | Patzold et al.286 and UK MS Trust survey (TA254,120 TA320119 methods) |
Patzold et al.286 and UK MS Trust survey (TA303,122 TA312118 methods) |
||||
| RRMS | SPMS | RRMS | SPMS | RRMS | SPMS | |
| 0 | 0.260 | 0.000 | 0.709 | 0.000 | 0.725 | 0.000 |
| 1 | 0.237 | 0.000 | 0.729 | 0.000 | 0.743 | 0.000 |
| 1.5–2 | 0.460 | 0.315 | 0.676 | 0.465 | 0.690 | 0.447 |
| 2.5–3 | 0.495 | 0.602 | 0.720 | 0.875 | 0.723 | 0.788 |
| 3.5–4 | 0.670 | 0.515 | 0.705 | 0.545 | 0.707 | 0.567 |
| 4.5–5 | 0.181 | 0.160 | 0.591 | 0.524 | 0.599 | 0.517 |
| 5.5–6 | 0.150 | 0.139 | 0.490 | 0.453 | 0.508 | 0.445 |
| 6.5–7 | 0.156 | 0.104 | 0.508 | 0.340 | 0.504 | 0.312 |
| 7.5–8 | 0.156 | 0.104 | 0.508 | 0.340 | 0.504 | 0.312 |
| 8.5–9.5 | 0.156 | 0.104 | 0.508 | 0.340 | 0.504 | 0.312 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 |
Relapse rates per person per year for EDSS levels > 5.5 were derived based on the relative increase in ARR reported in the study by Patzold et al. 286 Patzold et al. 286 reported ARRs based on the year of diagnosis of RRMS. ARRs by year were converted to ARRs by EDSS level by taking the mean number of relapses per year for each health state from the UK MS Trust survey and multiplying by the relative relapse rates per person reported by Patzold et al. 286
Treatment discontinuation
In the model, people who progressed to a SPMS health state discontinued treatment. However, treatment was assumed not to be discontinued after reaching a particular EDSS level. This is in accordance with current Association of British Neurologists guidelines. 281 Annual discontinuation rates used in the model are presented in Table 47.
| Treatment | Annual withdrawal (%) |
|---|---|
| IFN-β-1a 30 µg IM once weekly (Avonex) | 7.9 |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 10.4 |
| IFN-β-1a 22 µg SC three times weekly (Rebif) | 6.0 |
| IFN-β-1a 44 µg SC three times weekly (Rebif) | 12.3 |
| IFN-β-1b 250 µg SC every other day (Betaferon) | 5.7 |
| IFN-β-1b 250 µg SC every other day (Extavia) | 5.7 |
| GA 20 mg SC once daily (Copaxone) | 7.2 |
| GA 40 mg SC three times weekly (Copaxone) | 7.2 |
Analysis (cycle length, time horizon and perspective)
The analysis was undertaken from a NHS and PSS perspective. The outcome measure used in the analysis was QALYs gained, over a 50-year time horizon with an annual cycle length. The starting age of the population was 36 years. The results were presented as ICERs, expressed as cost per QALY gained. Both costs and benefits were discounted at 3.5% per annum.
Assumptions
To have a workable model, the manufacturer made the following assumptions:
-
The probability of transitioning to a health state in the next cycle depends only on the health state in the present cycle.
-
Transition from RRMS to SPMS is accompanied by an increase in EDSS state of 1.0.
-
The population at baseline in the ADVANCE trial213 is representative of the RRMS population in clinical practice.
-
Each year, the EDSS score can remain the same, increase or decrease.
-
In the base case, treatments affect EDSS progression but not EDSS regression.
-
Treatment effects on relapse and EDSS progression are independent.
-
In the base case, treatments have the same effect on progression in each EDSS state.
-
In the base case, treatment efficacy is constant over time.
-
Treatments do not directly impact on transitions to SPMS but impact on patients’ EDSS state, which influences the transition to SPMS.
-
Treatment discontinuation is constant for all years.
-
The mortality rate for age > 100 years is same as that for age 100 years.
-
Annualised AE risks are applied every year – this may overestimate the incidence of AEs as patients who have AEs may discontinue treatment in the initial years on treatment.
-
RRMS patients in all EDSS states may receive treatments depending on the maximum EDSS limit.
-
SPMS patients receive BSC only.
-
Patient access schemes, when publicly available, are considered in the base case.
Summary of the Biogen Idec Ltd submission results
The base-case results showed that treatment with 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) resulted in the highest mean life-years gained (20.658) and mean QALYs (9.642) compared with all other interventions included in the analysis. Compared with BSC, 125 µg of SC pegylated IFN-β-1a every 2 weeks had a mean incremental cost of approximately £25,200 with corresponding incremental QALYs of 0.810, which equated to an ICER of approximately £31,000 per QALY.
The results from the sensitivity analyses showed that the base-case results were robust to univariate changes made to key input parameters, except for changes to the HR for confirmed disability progression, which had the greatest impact. The probabilistic sensitivity analysis suggested that, at a £30,000 per QALY willingness-to-pay threshold, 125 µg of SC pegylated IFN-β-1a every 2 weeks had a < 0.4 probability of being cost-effective compared with BSC.
Teva UK Limited
Background
This section focuses on the economic evidence on GA (Copaxone) submitted by Teva UK Ltd. This section is set out as for the previous manufacturer’s submission: first, we present an overview/summary and then we provide a critique of the economic model submitted by Teva. This section describes in detail the evidence (e.g. natural history information, effectiveness of the interventions included in the analysis, resource use and costs, mortality and HRQoL) used to parameterise the model.
The economic submission to NICE included:
-
a description of the de novo economic model from Teva, which assessed the cost-effectiveness of DMTs for the treatment of RRMS; this included details on the intervention and comparators, study population, resource use and costs, the modelling methodology and assumptions made
-
appendices with details of the evidence used to inform the model and a description of a NMA carried out to generate alternative estimates of efficacy that were used in sensitivity analysis.
Overview
In the Teva model, an economic analysis was conducted to assess the cost-effectiveness of the DMTs 30 µg of IM IFN-β-1a once weekly (Avonex), 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif), 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia), 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) and 20 mg of SC GA daily or 40 mg of SC GA three times weekly (Copaxone), as well as fingolimod, natalizumab and dimethyl fumarate, for use in second-line therapy, compared with BSC for people with RRMS.
In the analysis, a Markov model was used to depict the natural history of people with RRMS through progression to SPMS. The model included 21 health states, defined by EDSS score and disease stage (RRMS or SPMS). Only integer EDSS values were allowed, with fractional values rounded down. Disease progression rates during RRMS on BSC were based on the BCMS database, as in the RSS model. 153 Transition rates to SPMS were estimated using HRs observed in the London Ontario data set,80 following assumptions made in the ScHARR model. 275 The Teva model assumed that progression to SPMS increases EDSS scores by 1. Progression between EDSS scores for SPMS was calculated using the same transition probabilities as for RRMS. Treatment was assumed to continue until patients progressed to SPMS or reached an EDSS score of ≥ 7 and was not reinitiated.
The analysis was undertaken from the payer perspective, although sensitivity analyses were included from a societal perspective. The outcome measure used in the analysis was QALYs gained, over a 50-year time horizon. Treatment effects were assumed to delay the progression of the disease and reduce the frequency of relapses. The assumed HR (applied to all forward transitions) for GA compared with BSC was (confidential information has been removed) in the base case, based on the subset of patients in the RSS who received this DMT. Utilities for RRMS by EDSS level were based on pooling data from the MS Trust survey and Orme et al. ,101 following the RSS. 153 Utilities for SPMS by EDSS level were assumed to be the same as for RRMS. Carers’ disutilities were based on information obtained from the manufacturer’s submission to NICE for TA127. 282 Disutility values for AEs associated with each DMT were taken from a range of sources, including the NICE appraisal of alemtuzumab118 and the study by Maruszczak et al. 287
Information on resource use and unit costs was obtained from various sources (BNF,21 PSSRU,278 NHS reference costs285). The results were presented as ICERs and expressed as cost per life-year gained and cost per QALY gained. Both costs and benefits were discounted at 3.5% per annum. The authors undertook a number of sensitivity analyses (considering the societal perspective, patient baseline characteristics, transition probabilities, treatment efficacy, relapse rates, discontinuation rates, utility values, mortality multipliers, patients’ out-of-pocket costs, carers’ costs, loss of productivity for people with MS and AEs) and probabilistic sensitivity analysis to determine the robustness of the base-case results. The base-case results showed that treatment with GA resulted in a mean gain per patient of (confidential information has been removed) life-years or (confidential information has been removed) QALYs, at a net discounted cost of (confidential information has been removed), giving an ICER of (confidential information has been removed) per QALY. The probability of the cost-effectiveness of GA relative to BSC was (confidential information has been removed) at £20,000 per QALY and (confidential information has been removed) at £30,000 per QALY. Results from deterministic sensitivity analyses showed that the base-case results were robust to univariate changes made to key input parameters, except for changes to the HR for confirmed disability progression, which had the greatest impact, and EDSS score-related costs, which influenced whether GA was cost-effective relative to BSC.
Evidence used to parameterise the Teva model
Natural history of relapsing–remitting multiple sclerosis
Two key sources informed the analysis of natural history of RRMS: the London Ontario data set80 for transition to SPMS and SPMS transitions and the BCMS data set153 for EDSS progression. Tables 48 and 49 show the natural history transition matrices from the BCMS data set for people with an age at onset below the median age and an age at onset above the median age respectively.
| From EDSS state | To EDSS state | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 0 | 0.68701 | 0.21104 | 0.07196 | 0.02236 | 0.00434 | 0.00136 | 0.00176 | 0.00012 | 0.00003 | 0.00000 | 0.00000 |
| 1 | 0.06122 | 0.67867 | 0.16643 | 0.06463 | 0.01698 | 0.00474 | 0.00667 | 0.00052 | 0.00014 | 0.00001 | 0.00000 |
| 2 | 0.01692 | 0.12654 | 0.59552 | 0.17292 | 0.04538 | 0.01842 | 0.02190 | 0.00182 | 0.00054 | 0.00005 | 0.00000 |
| 3 | 0.00620 | 0.05215 | 0.11649 | 0.54385 | 0.09451 | 0.05729 | 0.11479 | 0.01070 | 0.00366 | 0.00035 | 0.00000 |
| 4 | 0.00176 | 0.02251 | 0.06617 | 0.12104 | 0.48739 | 0.10090 | 0.16645 | 0.02622 | 0.00689 | 0.00067 | 0.00000 |
| 5 | 0.00055 | 0.00562 | 0.02915 | 0.05935 | 0.09154 | 0.47268 | 0.28098 | 0.03961 | 0.01909 | 0.00143 | 0.00000 |
| 6 | 0.00012 | 0.00141 | 0.00447 | 0.02516 | 0.03209 | 0.04241 | 0.72834 | 0.11509 | 0.04566 | 0.00525 | 0.00000 |
| 7 | 0.00001 | 0.00016 | 0.00052 | 0.00260 | 0.00730 | 0.00419 | 0.12198 | 0.68147 | 0.16283 | 0.01895 | 0.00000 |
| 8 | 0.00000 | 0.00001 | 0.00004 | 0.00030 | 0.00057 | 0.00053 | 0.01885 | 0.05747 | 0.86099 | 0.06124 | 0.00000 |
| 9 | 0.00000 | 0.00000 | 0.00000 | 0.00002 | 0.00004 | 0.00004 | 0.00178 | 0.00596 | 0.17091 | 0.82124 | 0.00000 |
| 10 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 1.00000 |
| From EDSS state | To EDSS state | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 0 | 0.69537 | 0.20294 | 0.07251 | 0.02170 | 0.00422 | 0.00137 | 0.00175 | 0.00011 | 0.00003 | 0.00000 | 0.00000 |
| 1 | 0.05826 | 0.69503 | 0.15781 | 0.06087 | 0.01638 | 0.00458 | 0.00643 | 0.00048 | 0.00013 | 0.00001 | 0.00000 |
| 2 | 0.01586 | 0.12135 | 0.60786 | 0.16796 | 0.04458 | 0.01849 | 0.02160 | 0.00174 | 0.00052 | 0.00004 | 0.00000 |
| 3 | 0.00594 | 0.04961 | 0.12008 | 0.54421 | 0.09107 | 0.05844 | 0.11651 | 0.01029 | 0.00355 | 0.00030 | 0.00000 |
| 4 | 0.00165 | 0.02214 | 0.06660 | 0.11518 | 0.48936 | 0.10387 | 0.16812 | 0.02580 | 0.00671 | 0.00056 | 0.00000 |
| 5 | 0.00052 | 0.00533 | 0.02942 | 0.05866 | 0.08738 | 0.48692 | 0.27312 | 0.03880 | 0.01883 | 0.00102 | 0.00000 |
| 6 | 0.00012 | 0.00133 | 0.00444 | 0.02497 | 0.03069 | 0.04080 | 0.74072 | 0.10894 | 0.04377 | 0.00423 | 0.00000 |
| 7 | 0.00001 | 0.00015 | 0.00052 | 0.00247 | 0.00727 | 0.00385 | 0.11683 | 0.69268 | 0.16063 | 0.01559 | 0.00000 |
| 8 | 0.00000 | 0.00001 | 0.00004 | 0.00029 | 0.00055 | 0.00050 | 0.01880 | 0.05573 | 0.90340 | 0.02067 | 0.00000 |
| 9 | 0.00000 | 0.00000 | 0.00000 | 0.00002 | 0.00004 | 0.00003 | 0.00176 | 0.00568 | 0.17414 | 0.81832 | 0.00000 |
| 10 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 1.00000 |
Types of multiple sclerosis
The model included people who commenced in a RRMS health state and progressed to SPMS. People with CIS, PPMS or benign disease were not included in the analysis.
Interventions
The interventions considered in the economic analyses are presented in Table 50. The interventions included 30 µg of IM IFN-β-1a once weekly (Avonex), 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif), 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia), 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) and 20 mg of SC GA daily or 40 mg of SC GA three times weekly (Copaxone), as well as fingolimod (Gilenya), natalizumab (Tysabri) and dimethyl fumarate (Tecfidera) as second-line therapies. It was assumed that the split between these second-line therapies would be 50%, 30% and 20%, respectively, based on expert opinion. The interventions were compared with BSC for people with RRMS.
| Trade name | Drug | Dose regime | Route of administration | Licensed indication |
|---|---|---|---|---|
| Avonex | IFN-β-1a | 30 µg once a week | IM | RRMS |
| Rebif | IFN-β-1a | 22 or 44 µg three times per week | SC | RRMS |
| Betaferon/Extavia | IFN-β-1b | 250 µg every other day | SC | RRMS |
| Plegridy | PegIFN-β-1a | 125 µg every 2 weeks | SC | RRMS |
| Copaxone | GA | 20 mg once daily/40 mg three times weekly | Oral | RRMS |
| Gilenya | Fingolimod | 0.5 mg once daily | Oral | RRMS |
| Tysabri | Natalizumab | 300 mg once every 4 weeks | IVI | RRMS |
| Tecfidera | Dimethyl fumarate | 240 mg twice daily | Oral | RRMS |
Model structure
The illustrative Markov model structure submitted by the manufacturer was based on the original ScHARR model275 with developments to include other interventions. The manufacturer used a cohort-based Markov model to depict the natural history of people with RRMS. The model simulated disability progression, progression from RRMS to SPMS and the relapsing nature of the disease. People with RRMS were able to occupy one of the EDSS health states, which ranged from 0 to 10. The model allowed for people to progress, regress or stay in the same EDSS health state or progress from EDSS to SPMS. When people progressed to SPMS, they could progress, regress or remain in the same EDSS state.
In the model, people incurred costs and accrued benefits depending on the EDSS state for RRMS and SPMS. Benefits were measured using QALYs, with a utility being assigned to people occupying a specific health state each model cycle.
Population
The population included in the economic analysis was similar to the population in the RSS data set (confidential information has been removed). The initial distribution of people in each EDSS state is presented in Table 51.
| EDSS state | Distribution (%) |
|---|---|
| 0 | 3 |
| 1 | 16 |
| 2 | 26 |
| 3 | 23 |
| 4 | 16 |
| 5 | 10 |
| 6 | 6 |
| 7 | 0 |
| 8 | 0 |
| 9 | 0 |
| 10 | 0 |
Resource use and costs
Costs included in the analysis were those directly related to the NHS and PSS perspective. Costs were reported in UK pounds in 2015/16 prices. The model included the following costs:
-
drug acquisition costs
-
administration costs
-
monitoring costs
-
health state/EDSS costs
-
cost of relapse
-
treatment-related AE costs.
Drug acquisition costs
Treatment costs for GA along with the other DMTs are presented in Table 52. Annual costs were presented for the list and net price for each DMT that was available at the time of the RSS. From the Microsoft Excel (2013) model submitted, the annual cost of each drug was based on the dosage (per week and year) and the price per packet.
| Treatment | Annual acquisition cost (£, 2014/15 prices) | |
|---|---|---|
| List price | Net price | |
| GA 20 mg once daily or 40 mg SC three times weekly (Copaxone) | 6704.29 | (Confidential information has been removed) |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 8531.20 | (Confidential information has been removed) |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 7264.82 | (Confidential information has been removed) |
| IFN-β-1a 44 µg SC three times weekly (Rebif) | 10,608.43 | (Confidential information has been removed) |
| IFN-β-1a 22 µg SC three times weekly (Rebif) | 8003.67 | (Confidential information has been removed) |
| Fingolimod (Gilenya) | 19,175.63 | 19,175.63 |
| Natalizumab (Tysabri) | 14,740.45 | 14,740.45 |
| Dimethyl fumarate (Tecfidera) | 17,910.29 | (Confidential information has been removed) |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 8531.20 | 8531.20 |
When no net prices for DMTs were available because of treatments not being included in the RSS [fingolimod, natalizumab and 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy)], the list price of these drugs were used in the analysis.
Administration costs
Annual administration costs encompassed the costs associated with teaching people to self-administer the drugs. The administration costs are presented in Table 53.
| Treatment | Annual administration cost for year 1 (UK£, 2014/15 prices) | Resource use | Annual administration cost for subsequent years (UK£, 2014/15 prices) | Resource use |
|---|---|---|---|---|
| GA 20 mg SC once daily/40 mg SC three times weekly (Copaxone) | 174.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 174.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 174.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| IFN-β-1a 44 µg SC three times weekly (Rebif) | 174.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| IFN-β-1a 22 µg SC three times weekly (Rebif) | 174.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 174.00 | 3 hours of nurse’s time to teach self-administration | 0.00 | None |
| Fingolimod (Gilenya) | 144.99 | Continuous electrocardiography and blood pressure monitoring for 6 hours following the first dose | 0.00 | None |
| Natalizumab (Tysabri) | 5199.02 | Thirteen infusions per year with 1 g of methylprednisolone per infusion | 5199.02 | Thirteen infusions per year with 1 g of methylprednisolone per infusion |
| Dimethyl fumarate (Tecfidera) | 0 | None | 0 | None |
Monitoring costs
Annual monitoring costs for each treatment were presented in appendix 6 of the main report. The manufacturer clearly outlined the resource use used to derive monitoring costs. Monitoring costs were presented for year 1 and for subsequent years. The annual monitoring costs for all interventions are presented in Table 54. These annual monitoring costs appeared to be derived and used in the model correctly. The monitoring costs for second-line therapies were not presented in appendix 6 of the submission.
| Treatment | Monitoring costs for (£, 2014/15 prices) | |
|---|---|---|
| Year 1 | Subsequent years | |
| GA 20 mg SC once daily/40 mg SC three times weekly (Copaxone) | 414.00 | 414.00 |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 521.08 | 512.54 |
| IFN-β-1a 22 µg SC three times weekly (Rebif) | 521.08 | 512.54 |
| IFN-β-1a 44 µg SC three times weekly (Rebif) | 521.08 | 512.54 |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 521.08 | 512.54 |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 521.08 | 512.54 |
Health state/Expanded Disability Status Scale costs
Health state costs (payer perspective) by EDSS level and MS type (RRMS/SPMS) are presented in Table 55. These costs were related to MS management (expected/unexpected visits to health-care providers). The costs were taken from the ScHARR model275 and inflated to 2015 prices. Sensitivity analyses were carried out using health state costs sourced from Tyas et al. 288 and Karampampa et al. 283 The former set of costs involved lower costs for high EDSS scores leading to an increase in the ICER for GA to £29,000 per QALY. The latter involved higher costs for high EDSS scores, which resulted in GA dominating BSC.
| EDSS state | Cost (£) |
|---|---|
| 0 | 1195 |
| 1 | 1195 |
| 2 | 1195 |
| 3 | 2204 |
| 4 | 2284 |
| 5 | 8049 |
| 6 | 8978 |
| 7 | 27,398 |
| 8 | 42,541 |
| 9 | 54,080 |
Cost of relapse
The cost of a mild relapse was estimated as £870 and the cost of a severe relapse requiring hospitalisation was estimated as £5580. The submission stated that these costs were sourced from the manufacturer’s submission for NICE TA312118 (alemtuzumab for treating RRMS), which took these costs from a budget impact analysis in the Republic of Ireland. 289 This raises questions about the robustness of the estimate and its relevance to a UK setting. The assessment group for TA312 conducted its own sensitivity analysis in which the cost of a severe relapse was assumed to be lower (£3039). A justification for this was not presented in the report, but it implies that the assessment group at the time thought that the higher figure might be an overestimate.
Cost of adverse events
The model included the costs of AEs resulting from the use of DMTs. Estimates of resource use were presented in appendix 6 of the manufacturer’s submission. Unit costs of resources used to manage AEs were sourced from the PSSRU,278 national reference costs285 and the manufacturer’s submission for TA312,118 although insufficient detail is presented for the accuracy of the costs assumed for AEs to be fully verified. Table 56 shows the annual cost of treatment for AEs by DMT used in the model.
| Treatment | Unit cost (£, 2014/15 prices) | |
|---|---|---|
| Year 1 | Year 2 | |
| GA (Copaxone) | 44.61 | 44.61 |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 32.81 | 32.81 |
| IFN-β-1a 22 µg SC three times weekly (Rebif) | 20.59 | 20.59 |
| IFN-β-1a 44 µg SC three times weekly (Rebif) | 26.90 | 26.90 |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 13.64 | 22.66 |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 30.75 | 30.75 |
Health state utility values
Utilities were derived by EDSS level and were assumed to be independent of MS type (RRMS and SPMS). In the base case, these were derived from the same sources as in the RSS model. 153 Utility values used in the model are presented in Table 57.
| EDSS state | Utility | Carers’ disutility |
|---|---|---|
| 0 | 0.925 | 0.002 |
| 1 | 0.761 | 0.002 |
| 2 | 0.674 | 0.002 |
| 3 | 0.564 | 0.002 |
| 4 | 0.564 | 0.045 |
| 5 | 0.491 | 0.142 |
| 6 | 0.445 | 0.167 |
| 7 | 0.269 | 0.063 |
| 8 | 0.008 | 0.095 |
| 9 | –0.230 | 0.095 |
Carers’ disutility
An analysis was undertaken that included carers’ disutility by EDSS state. Table 57 shows the disutility values used in the model.
Mortality rate
Expanded Disability Status Scale-dependent mortality multipliers were used to estimate mortality from UK general population rates (sourced from ONS data for 2012–14290). These multipliers (which were themselves adapted from Pokorski et al. 291) were taken from the manufacturer’s submission to NICE for teriflunomide. 292 This raises concerns around the robustness of the assumed mortality rates and raises questions around whether a more up-to-date source could have been identified.
Adverse event disutilities
The assumed annual disutilities resulting from AEs are provided in Table 58. These were calculated from AE rates derived from clinical trials of the treatments included in the submission. Disutilities for AEs were obtained from the study by Maruszczak et al. ,287 from the manufacturers’ submissions to NICE for alemtuzumab,118 teriflunomide122 and dimethyl fumarate119 and from the Summary of Product Characteristics for 44 µg of SC IFN-β-1a three times weekly (Rebif). 293
| Treatment | Annual disutility | |
|---|---|---|
| Year 1 | Subsequent years | |
| GA (Copaxone) | –0.0043 | –0.0043 |
| IFN-β-1a 30 µg IM once weekly (Avonex) | –0.0009 | –0.0009 |
| IFN-β-1a 22 µg SC three times weekly (Rebif) | –0.0027 | –0.0027 |
| IFN-β-1a 44 µg SC three times weekly (Rebif) | –0.0034 | –0.0034 |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | –0.0043 | –0.0037 |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | –0.0028 | –0.0028 |
Relapse
The disutility per relapse was assumed to be 0.058 if the relapse was severe and 0.009 otherwise. The lower utility value was based on the study by Orme et al. 101 The manufacturer was unable to identify a UK source for estimating the disutility associated with severe relapse. An estimate for a US population was identified, but the manufacturer argued that this was an overestimate for an equivalent UK population. This utility value was therefore degraded by the ratio of UK to US disutilities for non-severe relapse (0.071/0.091), which resulted in a reduction in the severe disutility from 0.302 to 0.236. This was combined with an assumed duration of 90 days to provide the estimate of 0.058.
Treatment discontinuation
In the Teva model, people who progressed to a SPMS health state discontinued treatment. Accordingly, treatment was assumed to discontinue at EDSS 7, in agreement with Association of British Neurologists guidelines. 281
Analysis (cycle length, time horizon and perspective)
The analysis was undertaken from a NHS and PSS perspective. The outcome measure used in the analysis was QALYs gained, over a 50-year time horizon with an annual cycle length. The starting age of the population was 30 years. The results were presented as ICERs and were expressed as cost per QALY gained. Both costs and benefits were discounted at 3.5% per annum.
Summary of the model assumptions
In summary, the following assumptions were made in the Teva model:
-
The probability of transitioning to a health state in the subsequent cycle depends only on the health state in the present cycle.
-
Transition from RRMS to SPMS is accompanied by an increase in EDSS state of 1.
-
Each year, the EDSS score can remain the same, increase or decrease.
-
In the base case, treatments affect EDSS progression but not EDSS regression.
-
Treatment effects on relapse and EDSS progression are independent.
-
In the base case, treatments have the same effect on progression in each EDSS state.
-
In the base case, treatment efficacy is constant over time.
-
Treatments do not directly impact on transitions to SPMS but impact on patients’ EDSS state, which influences transition to SPMS.
-
Treatment discontinuation is constant for all years.
-
The annualised AE risks are applied every year – this may overestimate the incidence of AEs as patients who undergo AEs may discontinue treatment in the initial years of treatment.
-
Patients who discontinue treatment move on to one of three second-line treatments: Gilenya (50%), Tysabri (30%) and Tecfidera (20%).
-
SPMS patients receive BSC only.
-
The list price of GA was considered in the base case.
Summary of the results
The base-case results showed that treatment with GA (Copaxone) resulted in a mean gain per patient of (confidential information has been removed) life-years or (confidential information has been removed), at a net discounted cost of (confidential information has been removed), giving an ICER of (confidential information has been removed) per QALY. The probability of GA (Copaxone) being cost-effective relative to BSC was (confidential information has been removed) at £20,000 per QALY and (confidential information has been removed) at £30,000 per QALY.
Merck Biopharma
Background
This section of the report focuses on the economic evidence submitted to NICE by Merck on 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif). In this section we provide a summary of the economic analysis presented by Merck and then critically appraise the analysis and findings. Merck’s economic model and cost-effectiveness analysis considered 44 µg/22 µg of SC IFN-β-1a three times weekly compared with BSC for the treatment of RRMS, SPMS and CIS. Details were provided of the intervention and comparators, the study population, resource use and costs, the modelling methodology and assumptions made.
Merck initially conducted a systematic review of the cost-effectiveness literature relating to MS and identified four studies that met its inclusion criteria; two of these studies examined DMTs in CIS. 256,261 In addition, it reviewed cost-effectiveness analyses undertaken as part of HTAs for NICE (four publications118–120,122) and the Canadian Agency for Drugs and Technologies in Health (CADTH) (one publication294). It concluded that the majority of studies used a comparable approach to that used in the ScHARR analysis,275 undertaken for TA32. 24 In addition, it highlighted that it adopted a commonly used approach to modelling mortality for MS patients, although it did not specify which studies from its review used this approach.
Merck relapsing–remitting multiple sclerosis model
For the RRMS analysis, a Markov model was used to depict the natural history of people with RRMS. The analysis was undertaken from the UK NHS and PSS perspective. The outcome measure used in the analysis was QALYs. The model was run over a 50-year time horizon with 1-year cycles and a half-cycle correction was applied. A 3.5% discount rate was applied to all future costs and health outcomes.
The model used EDSS scores, increasing in increments of 1, to model disability progression with and without DMTs. The model did not have separate health states for SPMS and assumed that all patients discontinued DMTs on reaching EDSS 7. The BCMS natural history model153 was used to model disease progression in people with RRMS. For those not on treatment, disability could improve (backward transition in EDSS states). The model included information from both doses of the drug; thus, Merck estimated outcomes for patients given the different doses, based on numbers given the different doses in the RSS cohort, and then pooled the outcomes. Of note, Merck used dose-specific parameters to populate its models (e.g. costs, treatment effects).
In its analysis, the initial distribution of EDSS scores was based on what was observed in the RSS data set for those treated with 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif). Treatment effects were assumed to delay the progression of the disease and reduce the frequency of relapses. HRs from the 10-year RSS data provided by the Department of Health were used to model the impact of DMTs on disability progression (worsening EDSS scores). The ‘waning effect’ of DMTs on disability progression hazards was also incorporated. For relapse rates, Merck used findings from the PRISMS study. 189 In its base-case analysis, Merck modelled mortality in the same way as in the ScHARR model275 by applying a SMR of 2.0 to life table mortality estimates, with an additional MS-specific mortality risk applied to those whose EDSS score reached 6.
Health outcomes were measured in QALYs. Merck assigned utility weights to the EDSS health states and included utility decrements for carers, relapses and adverse drug reactions. Utility estimates were derived by pooling data from the UK MS Trust postal survey, as cited in the submission, and the Heron data set. 101 The pooling of these data was undertaken by IMS Health on behalf of the UK MS Trust. Merck assumed that the duration of the utility decrement from a relapse was 46 days and that approximately 5% per annum would experience a utility decrement from an AE. Health-care resource use and cost estimates used in the model were derived from the Department of Health/ScHARR estimates275 and adjusted accordingly. The costs were assigned to EDSS health states and to relapses. The costs of DMTs were based on the annual per-patient NHS acquisition costs.
Merck undertook a number of sensitivity analyses to investigate the impact of discounting, shorter time horizons, alternative approaches to deriving mortality rates and HRs, alternative sources of utilities and costs and alternative assumptions regarding AEs and discontinuation rates. In addition, it undertook probabilistic sensitivity analysis to determine the robustness of the base-case results.
In its base case analysis, Merck estimated that the treatment of RRMS with 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) would result in an additional (confidential information has been removed) QALYs gained at an additional cost of (confidential information has been removed) over a 50-year time horizon. The ICER was estimated to be (confidential information has been removed) per QALY gained. The ICER estimated in the probabilistic sensitivity analysis was (confidential information has been removed) per QALY gained. In sensitivity analyses, Merck found that the base-case results were robust to univariate changes made to key input parameters. The majority of the sensitivity analyses resulted in lower ICERs. The ICERs were higher when different approaches were used to estimate EDSS health state costs (provided in appendix 17 of the submission).
Merck secondary progressive multiple sclerosis model
Merck also undertook an economic analysis of 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) for patients with SPMS. It used the same model structure and modelling techniques as before and populated the model with patient characteristics and treatment effects for treatment with 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) in SPMS patients. As highlighted before, the model did not include separate health states for SPMS and assumed that all patients discontinued DMTs on reaching EDSS 7. For the characteristics of the population modelled Merck used observational data from the SPECTRIMS study224 (64% female, mean age 43 years and EDSS score 5 or 6 at baseline). Additional assumptions made included the presence of a constant relapse rate independent of EDSS level.
In the base-case deterministic analysis, Merck estimated that treatment of SPMS with 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) would result in an additional (confidential information has been removed) QALYs gained at an additional cost of (confidential information has been removed) over a 50-year time horizon. The ICER was estimated to be (confidential information has been removed) per QALY gained. The ICER estimated from the probabilistic sensitivity analysis was (confidential information has been removed) per QALY gained. In sensitivity analyses, Merck found that the base-case results were robust to univariate changes made to key input parameters. The majority of the sensitivity analyses resulted in comparable ICER estimates (provided in appendix 17 of the submission).
Merck clinically isolated syndrome model
Merck also undertook an economic analysis of treatment with 44 µg/22 µg of SC IFN-β-1a (Rebif) in patients with CIS. It estimated the ICERs for starting DMTs in CIS patients, to providing BSC for CIS patients with DMTs when patients progress to RRMS. Merck used the same model structure and modelling techniques as before and populated the model with patient characteristics and treatment effects for treatment with 44 µg/22 µg of SC IFN-β-1a (Rebif) in CIS patients. The characteristics of the population modelled were based on participants in the REFLEX trial. 175 The relative risks for conversion from CIS to RRMS for the first and second year on DMTs and the relative risk of relapse were extracted from the REFLEX trial. 175 In addition, Merck assumed that there was no treatment effect of DMTs on risk of progression to RRMS after 2 years. For delayed therapy we considered that the rates of conversion and relapse were also based on the placebo arm of the REFLEX trial, although this is not clear from the submission. Merck also assumed that for CIS patients EDSS scores remained constant until conversion to RRMS, at which point the EDSS scores were based on EDSS scores while in the CIS state.
In the base-case deterministic analysis, Merck estimated that early treatment of CIS with 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) would result in an additional (confidential information has been removed) QALYs gained at an additional cost of (confidential information has been removed) over a 50-year time horizon. The ICER was estimated to be (confidential information has been removed) per QALY gained. The ICER estimated from the probabilistic sensitivity analysis was (confidential information has been removed) per QALY gained. In sensitivity analyses, Merck found that the base-case results were robust to univariate changes made to key input parameters. The majority of the sensitivity analyses resulted in comparable ICER estimates (provided in appendix 17 of the submission).
Evaluation of Merck’s submission
Types of multiple sclerosis
Merck undertook an economic analysis of 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) for the treatment of RRMS, SPMS and CIS. The base-case analysis examined costs and health outcomes for MS patients aged < 30 years.
Model structure
The illustrative Markov model structure submitted by the manufacturer was based on the original ScHARR model. 275 The manufacturer used a cohort-based Markov model to depict the natural history of people with RRMS. The model simulated disability progression, progression from RRMS to SPMS and the relapsing nature of the disease. People with RRMS/SPMS were able to occupy one of the EDSS health states, which ranged from 0 to 9, in increments of 1.0. The model allowed for people to progress, regress or stay in the same EDSS health state or progress from RRMS to SPMS. For those on DMTs no backward transition in EDSS states was permitted.
Merck used the same model structure for the economic analysis of DMTs for the treatment of SPMS and parameterised the model with patient characteristics and treatment effects for treatment with 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) in SPMS patients. The CIS model had an additional five on-treatment and five off-treatment health states defined by EDSS score (0–4, in increments of 1). In addition, in the CIS model it was assumed that EDSS scores remained constant until conversion to RRMS, at which point EDSS scores were based on EDSS scores while in the CIS state.
Interventions
The interventions considered in the economic analyses are presented in Table 59. For RRMS and SPMS Merck compared 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) with BSC and for CIS it compared 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) with BSC, with DMTs started on progression to RRMS. For all those started on DMTs, treatment was discontinued once the EDSS score was ≥ 7. In addition, it was assumed that 5% per annum discontinued treatment as a result of AEs. For the DMT treatment strategy, the model aggregated the observed RSS data across both doses of the drug.
| Trade name | Drug | Dose | Route of administration | Type of MS |
|---|---|---|---|---|
| Rebif | IFN-β-1a | 44 or 22 µg | SC | RRMS |
| Rebif | IFN-β-1a | 44 or 22 µg | SC | SPMS |
| Rebif | IFN-β-1a | 44 or 22 µg | SC | CIS |
Population
In the RRMS model, the population included in the economic analysis was similar to the population who started IFN-β-1a (Rebif) in the RSS cohort. 153 In the base-case RRMS analysis, Merck examined the costs and health outcomes for MS patients aged < 30 years and MS patients aged ≥ 30 years. In the SPMS model, the population included in the economic analysis was similar to the population included in the SPECTRIMS study224 and, in the CIS model, the population included in the economic analysis was similar to the population included in the REFLEX study. 175 The initial distribution of people in each EDSS state is presented in Table 60. Of note, the distribution of initial EDSS scores for the RRMS population was taken from the Microsoft Excel (2013) file and is not the same as that presented in the manufacturer’s final written summary.
| MS type/treatment | Population | EDSS score (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| RRMS: 44 µg < 30 years | (Confidential information has been removed) female, mean age at onset 30 years | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| RRMS: 22 µg < 30 years | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | |
| SPMS (all) | 64.0% female, mean age at onset 43 years | 0 | 0 | 0 | 0 | 0 | 50 | 50 |
| CIS | 67.0% female, mean age at onset 31 years | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
Mortality rate
In the base-case analysis, the manufacturer modelled mortality in the same way as in the ScHARR model275 by applying a SMR of 2.0 to life table mortality estimates, with an additional MS-specific mortality risk applied to those whose EDSS score reached 6. In sensitivity analyses Merck used an alternative approach to modelling mortality. Briefly, this approach resulted in lower mortality rates assigned to early EDSS health states and higher mortality rates assigned to those with more advanced disability. Although this approach may be valid, the data used to derive these values were published > 20 years ago, when BSC was likely to have been less optimal than current provision, especially for those with more advanced disability.
Treatment effects of disease-modifying therapy
Merck followed the same approach used in the Department of Health RSS model analysis to model the impact of DMTs on disability progression. The BCMS natural history model153 was used to model disease progression in people with RRMS, allowing for improvements in disability (backward transition in EDSS states).
In the RRMS model, the DMT strategy utilised the 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif)-specific HRs supplied by the Department of Health from the year 10 RSS data. These HRs were applied to the natural history model to model the on-treatment impact. Of note, Merck individually modelled the treatment impact for the two different dosages of the drug and pooled the final costs and health outcomes to estimate the ICERs. It also assumed that there would be no improvement in disability (backward transition in EDSS states) for those on DMTs. In the models it assumed that 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) would be discontinued when disability progressed to an EDSS level of ≥ 7. In addition, it assumed that 5% of patients stopped treatment for other reasons (i.e. dropouts) every year. It also incorporated the ‘waning effect’ of DMTs on disability progression hazards. For relapse rates it used the findings from the PRISMS study. 189
In the CIS model, progression to RRMS in the delayed treatment strategy (DMTs once progressed to RRMS) and the rates of conversion and relapse were based on the outcomes of the placebo arm of the REFLEX trial. 175 In the CIS DMT treatment strategy the relative risks for conversion from CIS to RRMS for the first and second year on DMTs and the relative risk of relapse were extracted from the REFLEX trial. 175 The manufacturer assumed that there was no treatment effect of DMTs on risk of progression to RRMS after 2 years. It also assumed that for CIS patients EDSS scores remained constant until conversion to RRMS, at which point the EDSS scores were based on the EDSS scores while in the CIS state.
Resource use and costs
All costs included in the analysis were those directly related to the NHS and PSS perspective. Costs were reported in 2015 UK pounds, with future costs discounted at a rate of 3.5% per annum. The model included the following costs:
-
drug acquisition costs
-
health state/EDSS costs
-
cost of relapse
-
AE costs.
Drug acquisition costs
In the model, the drug acquisition costs represent the annual per-patient NHS acquisition costs (confidential information has been removed). The drug acquisition costs for the two dosages of SC IFN-β-1a three times weekly (Rebif), 44 µg and 22 µg, were (confidential information has been removed) and (confidential information has been removed) respectively. In the model Merck utilised the observed numbers of patients on the two different dosages in the RSS cohort and assigned costs accordingly. Hence, the true modelled cost of the drugs will be a RSS sample weighted average. The costs of administering the drugs and monitoring response to treatment were not included.
Health state/Expanded Disability Status Scale costs
Resource use/costs were assigned to each EDSS health state. In the base-case analysis Merck utilised the same costs as in the ScHARR analysis,275 with adjustment to 2015 UK pounds. This is the same approach used in the Department of Health RSS model analysis. In sensitivity analyses Merck used costs reported by Tyas et al. 288 and Karampampa et al. ,283 again with adjustment to 2015 UK pounds.
Cost of relapse
In the base-case analysis the manufacturer utilised the same cost of relapse as in the ScHARR analysis,275 with adjustment to 2015 UK pounds. This is the same approach used in the Department of Health RSS model analysis.
Adverse event costs
In the base-case analysis the manufacturer did not include costs incurred as a result of AEs, in accordance with the Department of Health RSS model analysis. In a sensitivity analysis it incorporated costs incurred as a result of AEs, using data on AEs reported in the PRISMS study. 189
Health state utility values
Health outcomes were measured in QALYs and future health outcomes were discounted at a rate of 3.5% per annum. Utility weights were assigned to the EDSS health states, including utility decrements for carers, relapses and adverse drug reactions. Utility estimates were derived by pooling data from the UK MS Trust postal survey and the Heron data set. 101 The data were pooled using sample size-weighted averages, with pooling undertaken by IMS Health on behalf of the MS Trust. Merck assumed that the duration of the utility decrement from a relapse was 46 days and that approximately 5% per annum would experience a utility decrement from an AE.
Table 61 shows the utility weights used in the base-case analysis. Of note, the pooled values do not take into account differences between the two samples in terms of age, sex and other variables that may be independently associated with HRQoL. The pooled utility values were used in the Department of Health RSS model analysis, including the impact on carers. Merck stated that, as the pooled values were not provided with standard errors for the probabilistic sensitivity analysis, it used the standard errors reported in one of the two data sets that were pooled. 101 For this the company extracted the standard errors from the multivariable regression analysis and therefore these represent the standard errors for the adjusted coefficients.
| EDSS state | Utility value, mean (standard error) | |
|---|---|---|
| Patient health states | Carer decrements | |
| 0 | 0.925 (0.045) | –0.002 (0.053) |
| 1 | 0.761 (0.048) | –0.002 (0.053) |
| 2 | 0.674 (0.048) | –0.045 (0.057) |
| 3 | 0.564 (0.052) | –0.045 (0.057) |
| 4 | 0.564 (0.048) | –0.142 (0.062) |
| 5 | 0.491 (0.047) | –0.16 (0.055) |
| 6 | 0.445 (0.047) | –0.173 (0.054) |
| 7 | 0.269 (0.049) | –0.03 (0.038) |
| 8 | 0.008 (0.050) | –0.095 (0.075) |
| 9 | –0.23 (0.074) | 0 |
| Relapse | –0.22 (0.089) for 46 (10) days | |
| AE | –0.321 (0.051) in 5.1% (8.6%) of patients | |
In a sensitivity analysis Merck estimated the ICERs using utility values derived from an unpublished study by Boggild et al. and using utility values derived from pooling all three data sets (unpublished data from the UK MS Trust postal survey, the Heron data set101 and unpublished data from Boggild et al. ). The utility values assigned to health states in the sensitivity analysis were lower (poorer HRQoL).
Analysis (cycle length, time horizon and perspective)
The analysis was undertaken from the NHS and PSS perspective. The outcome measure used in the analysis was QALYs gained, over a 50-year time horizon and with an annual cycle length. The results were presented as ICERs and expressed as cost per QALY gained. Both costs and benefits were discounted at 3.5% per annum.
Assumptions
Merck made a range of assumptions in the model analysis. For the RRMS model it assumed that:
-
The year 10 RSS data set reflects the future MS population characteristics, the initial EDSS level on starting DMTs, the dosage of 44 µg/22 µg of SC IFN-β-1 three times weekly (Rebif) and the treatment impact on disability progression.
-
Age at MS diagnosis was 30 years.
-
The natural history progression of MS, resource use, HRQoL, waning effect of DMTs and mortality rates were the same as those used by the UK Department of Health in its RSS model analysis.
-
Uncertainty around the HRs characterising the treatment impact of DMTs was assumed to have an upper limit of 1.0 in the probabilistic sensitivity analysis.
-
DMTs were discontinued once the EDSS score was ≥ 7.
-
Five per cent of patients discontinued DMTs for other reasons (dropouts).
The model included additional assumptions relating to SPMS:
-
A starting EDSS level of 5 and 6 (50% each) was assumed.
-
An untreated relapse rate of 1.08 per patient-year was assumed.
-
HRs for progression and relative risks for relapse were assumed to be as for the SPECTRIMS trial224 relapsing population.
Finally, the model included several assumptions relating to CIS:
-
Patients’ baseline EDSS scores were assumed to be as in the REFLEX trial. 175
-
Conversion from CIS was assumed to be as in the REFLEX trial175 for delayed treatment, with relative risks for years 1 and 2 calculated from the REFLEX trial. 175
-
No treatment effect was applied beyond year 2, although patients with CIS were assumed to remain on treatment for up to 5 years.
-
Patients were assumed to remain in the starting EDSS state during and on conversion to MS diagnosed by the McDonald criteria. 62
Summary of results
In the base-case analysis, Merck estimated that treatment of RRMS with 44 µg/22 µg of SC IFN-β-1a three times weekly (Rebif) would result in an additional (confidential information has been removed) QALYs gained at an additional cost of (confidential information has been removed) over a 50-year time horizon. The ICER was estimated to be (confidential information has been removed) QALY gained. The ICER estimated from the probabilistic sensitivity analysis was (confidential information has been removed) per QALY gained.
Summary and critique of the manufacturers’ submissions
Overview of the manufacturers’ submissions
This section provides an overview of the economic evidence submitted by the three companies: (1) Biogen Idec, (2) Teva UK Ltd and (3) Merck Biopharma. We provide a summary of the manufacturers’ submissions and an assessment of how they compare with the NICE reference case156 and how they differ from each other and from the Department of Health RSS model analysis.
The economic evaluations are summarised in Table 62. Biogen Idec Ltd undertook an economic analysis to assess the cost-effectiveness of its DMTs, 30 µg of IM IFN-β-1a once weekly (Avonex) and 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy), and other DMTs on the market, including 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif), 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) and 20 mg of SC GA daily or 40 mg of SC GA three times weekly (Copaxone). Teva undertook a comparable economic analysis of its DMT, 20 mg of SC GA daily or 40 mg of SC GA three times weekly (Copaxone), and others on the market, including 30 µg of IM IFN-β-1a once weekly (Avonex), 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy), 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif), 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia), fingolimod (Gilenya), natalizumab (Tysabri) and dimethyl fumarate (Tecfidera), whereas Merck undertook an economic analysis of only its DMT, 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif).
| Parameter | Company | ||
|---|---|---|---|
| Biogen Idec Ltd: IFN-β-1a 30 µg IM once weekly (Avonex), pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | Merck: IFN-β-1a 44 or 22 µg SC three times weekly (Rebif) | Teva: GA 20 mg SC once daily or 40 mg SC three times weekly (Copaxone) | |
| Natural history cohort | Natural history cohort based on extrapolating data from the ADVANCE trial placebo arm213 with information from the BCMS data set153 | Natural history cohort based on the BCMS natural history model | Natural history cohort based on the BCMS natural history data set for RRMS states153 and the London Ontario natural history cohort for transitions from RRMS to SPMS and SPMS transitions80 |
| Population | Adults (≥ 18 years) with RRMS | RRMS: adults, mean age 30 years, (confidential information has been removed) female (based on RSS data153); SPMS: adults, mean age 43 years, 64% female (based on the SPECTRIMS trial224); CIS: adults, mean age 31 years, 67% female (based on the REFLEX trial175) | Adults (≥ 18 years) with RRMS |
| Interventions | IFN-β-1a 30 µg IM once weekly (Avonex), pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy), IFN-β-1a 44 µg SC three times weekly (Rebif), IFN-β-1b 250 µg SC every other day (Betaferon), IFN-β-1b 250 µg SC every other day (Extavia), GA 20 mg SC three times weakly (Copaxone), GA 40 mg SC three times weekly (Copaxone) | IFN-β-1a 44 or 22 µg SC three times weekly (Rebif) | GA 20 mg SC once daily (Copaxone), IFN-β-1a 30 µg IM once weekly (Avonex), pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy), IFN-β-1a 22 µg SC three times weekly (Rebif), IFN-β-1a 44 µg SC three times weekly (Rebif), IFN-β-1b 250 µg SC every other day (Betaferon), fingolimod 0.5 mg once daily (Gilenya), natalizumab 300 mg once every 4 weeks (Tysabri), dimethyl fumarate 240 mg twice daily (Tecfidera) |
| Comparator | BSC | CIS: BSC for CIS and DMTs for RRMS; RRMS: BSC; SPMS: BSC | BSC |
| Type of model and health states | Cohort-based Markov model with 21 health states (10 for RRMS, 10 for SPMS and one for the dead state) characterised by EDSS levels, which ranged from 0–10 in increments of 0.5 | CIS: cohort-based Markov model with an additional five on-treatment and five off-treatment health states for CIS defined by EDSS score (0–4, in increments of 1) (otherwise includes the same health states as for the RRMS model); RRMS and SPMS: cohort-based Markov model with 21 health states: 10 EDSS not-on-treatment states, 10 EDSS on-treatment states and absorbing death state (EDSS health states 0–9, in increments of 1.0) | Cohort-based Markov model with 21 health states (10 for RRMS, 10 for SPMS and one for the dead state) characterised by EDSS levels, which ranged from 0 to10 in increments of 1 |
| HRs | HRs based on confirmed disability progression. The year 10 implied HR of (confidential information has been removed) for 30 µg of IM IFN-β-1a was used in the manufacturer’s model | CIS: conversion rate for CIS to RRMS based on the REFLEX trial;175 RRMS: HRs for sustained disability progression supplied to Merck by the Department of Health based on analysis of year 10 RSS data: progression – (confidential information has been removed), relapse – HR (44 µg) 0.67, HR (22 µg) 0.71; SPMS: relapse rate for SPMS not on treatment based on the placebo arm of the SPECTRIMS trial,224 HR for SPMS on treatment with 44-µg dosage derived from the SPECTRIMS trial:224 progression – HR (44 µg) (confidential information has been removed), relapse – HR (44 µg) 0.62, HR (22 µg) 0.53 | HRs for GA of (confidential information has been removed) for disability progression and (confidential information has been removed) derived from Teva’s NMA. Additional sensitivity analysis of Teva’s NMA assuming (confidential information has been removed) HR for progression compared with BSC was used in scenario analyses in the model |
| Resource use and costs | Drug acquisition costs, monitoring costs, administration costs, relapse costs (including a percentage requiring hospitalisation as a proxy for severity), health state costs, treatment-related AE costs | RRMS: based on Department of Health/ScHARR275 resource use and costs, adjusted to 2015 UK pounds, with costs including drug acquisition costs, monitoring costs, administration costs, relapse costs, health state costs and treatment-related AE costs; SPMS and CIS: based on the RRMS model approach | Drug acquisition costs, monitoring costs, administration costs, relapse costs (including a percentage requiring hospitalisation as a proxy for severity), health state costs and treatment-related AE costs |
| HRQoL | Utility values by EDSS level were based on information from the ADVANCE trial213 and Orme et al.,101 which was derived from utility values from the UK MS Trust survey. Carers’ disutilities were derived based on information obtained from the manufacturer’s submission to NICE for TA127121 | Utility values by EDSS score were derived by pooling data from a UK MS Trust postal survey and the Heron data set.101 Data were pooled using sample size-weighted averages, with pooling undertaken by IMS Health on behalf of the MS Trust | Utility values by EDSS level were based on information from Orme et al.,101 which was derived from utility values from the UK MS Trust survey. A sensitivity analysis was performed using smoothed data from three RSS data sets. Carers’ disutilities were derived based on information obtained from the manufacturer’s submission to NICE for TA127121 |
| Discontinuation of treatment | Only people who progressed to SPMS discontinued DMTs | Treatment was stopped when the EDSS score was ≥ 7. In addition, 5% discontinued treatment irrespective of EDSS level (derived from the observed dropout rate from the 8-year RSS data) | Withdrawal rate of 5% per year as per the RSS model. Treatment was also discontinued for those with an EDSS score of ≥ 7 |
| Relapse | Relative risks of a relapse per person in the RRMS health states were estimated from the ADVANCE trial213 for EDSS levels up to 5.5. ARRs for EDSS levels > 5.5 were based on the relative increases in ARR reported in the study by Patzold et al.286 | RRMS: relapse rates were assigned to each EDSS health state. These estimates were based on the MS Trust survey in 2001. For those receiving treatment it was assumed that patients would benefit from a risk reduction for relapse. The relative risk reduction of relapse was taken from data presented in the 2002 ScHARR model.275 The original source of these estimates is the PRISM study.189 RRMS: IFN-β-1a 44 µg: RR 0.67 (95% CI 0.67 to 1.00), IFN-β-1a 22 µg: RR 0.71 (95% CI 0.60 to 0.84); SPMS: IFN-β-1a 44 µg: RR 0.62 (95% CI 0.52 to 0.74), IFN-β-1a 22 µg: RR 0.53 (95% CI 0.44 to 0.64) | Relative risks of relapse were estimated from Teva’s NMA. A distinction was made between moderate and severe relapse. The ARR was applied to the proportion of relapses that were severe. For GA this was 0.796.219 For other DMTs this ranged from 0.495 (pegylated IFN-β-1a) to 1.282 (Tecfidera) |
| AEs | Annualised risks for AEs were considered for all treatments. AEs for people in the BSC arm were not considered. Annualised risks for each treatment were qualitatively analysed. AEs reported in the ADVANCE trial (> 5% for any DMT or > 3% for all treatments) were included in the economic analysis | 5.1% experienced AEs every year on DMTs. AEs were associated with a utility decrement of 0.02 | The nature and rate of AEs were derived from pooled clinical trial data. The assumed probability of an AE on GA was 0.481 (first and second year). For other DMTs probabilities ranged from 0.32 (Tecfidera) to 0.752 (pegylated IFN-β-1a). The disutility of an AE was 0.004 QALYs for GA and ranged from 0.000 QALYs (Gilenya, Tecfidera) to 0.004 QALYs (Copaxone, pegylated IFN-β-1a) |
| Mortality | Mortality was assumed to be equivalent between RRMS and SPMS and dependent on the EDSS level | Utilised the Department of Health/RSS approach to mortality in the base-case analysis. This involved applying a SMR of 2.0 to life table estimates and a MS-specific mortality rate for those with a EDSS score of ≥ 6 | An EDSS-dependent mortality multiplier was used to estimate mortality from UK general population rates (sourced from ONS data for 2012–14290). These multipliers (which were adapted from Pokorski et al.291) were taken from the manufacturer’s submission on teriflunomide to NICE122 |
| Time horizon | 50 years | 50 years | 50 years |
| Base-case analysis results | Pegylated IFN-β-1a 125 µg SC every 2 weeks had an ICER of approximately £31,000 per QALY compared with BSC | CIS: ICER: (confidential information has been removed) gained; RRMS: ICER: (confidential information has been removed) gained; SPMS: ICER: (confidential information has been removed) gained | GA had an ICER of (confidential information has been removed) per QALY compared with BSC (confidential information has been removed) when excluding support for nursing/infrastructure cost) in the Department of Health-agreed analysis. In the de novo model (confidential information has been removed) per QALY for GA compared with BSC. GA was (confidential information has been removed) |
| Sensitivity analysis (and probabilistic sensitivity analysis) results | All base-case results except for the HR for confirmed disability progression were robust to sensitivity analysis. At the willingness-to-pay threshold for a QALY, 125 µg of SC pegylated IFN-β-1a every 2 weeks had a < 0.4 probability of being cost-effective compared with BSC | CIS: ICER: (confidential information has been removed) gained; RRMS: ICER: (confidential information has been removed) gained; SPMS: ICER: (confidential information has been removed) gained | (Confidential information has been removed) of cost-effectiveness at £20,000 compared with BSC. The cost-effective results were most sensitive to the choice of data informing the HR for progression |
In the primary analysis all three companies undertook an economic analysis of DMTs compared with BSC for people with RRMS. The three companies clearly stated their decision problem, which was consistent with NICE’s scope141 for the appraisal.
Types of multiple sclerosis
Biogen Idec Ltd and Teva undertook a cost-effectiveness analysis of DMTs for those with RRMS only. Merck also evaluated the cost-effectiveness of its DMT in patients presenting with SPMS and CIS.
Analysis (cycle length, time horizon and perspective)
All three manufacturers followed the same approach with regard to the model analysis, perspectives, outcome measures and time horizon for analysis. They all undertook a cost–utility analysis from the NHS and PSS perspective, in accordance with the NICE reference case. 156 The outcome measure used in the analysis was QALYs gained, over a 50-year time horizon with an annual cycle length, with a starting age for the population modelled of ≥ 30 years. The time horizon used should be sufficiently long to reflect differences in costs and outcomes. The results were presented as ICERs, expressed as cost per QALY gained. Both costs and benefits were discounted at 3.5% per annum.
Model structure
All three submissions utilised a Markov cohort model, based on the original ScHARR model,275 to undertake their cost-effectiveness analysis. Broadly, all of the submissions used EDSS scores to define RRMS and SPMS health states, with 10 mutually exclusive EDSS-defined health states. In all of the models, people with RRMS could progress, regress or stay in the same EDSS health state or progress from RRMS to SPMS. People could not move from SPMS to RRMS and, once progressed to SPMS, individuals’ EDSS scores could not improve.
There were some differences between the manufacturers’ submissions with regard to when DMTs were stopped in the model analysis. In the Biogen Idec Ltd model it was assumed that DMTs were discontinued once patients progressed to SPMS. In the Teva model DMTs were discontinued once the EDSS score was ≥ 7 or when patients had progressed to SPMS. In the Merck model DMTs were discontinued once the EDSS score was ≥ 7, irrespective of whether patients had progressed to SPMS. In all three submissions, DMTs were stopped if patients experienced adverse drug reactions. When DMTs are stopped is likely to impact on the modelled lifetime costs and therefore the ICER estimates.
Interventions evaluated
All three manufacturers compared treatment with DMTs with BSC in patients with RRMS. For SPMS Merck compared 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif) with BSC and for CIS it compared 44 or 22 µg of SC IFN-β-1a three times weekly (Rebif) with BSC followed by DMTs on progression to RRMS.
Population modelled
There were differences between the three manufacturer submissions in how they determined the population to be modelled. Teva and Merck used the population characteristics (age; sex distribution; starting EDSS scores) observed in the RSS cohort data,153 whereas Biogen Idec Ltd used the baseline characteristics observed in the ADVANCE trial. 213 A major difference between the two approaches is the mean age at onset of RRMS used. In the Biogen Idec Ltd model this was 36 years whereas in the Teva and Merck models this was 30 years. Also, in the Biogen Idec Ltd model approximately 32% of the cohort modelled started with an EDSS score of ≤ 1, whereas in the Teva and Merck models between 19% and 23% of the cohort modelled started with an EDSS score of ≤ 1. The age of the population is likely to impact on modelled lifetime costs and lifetime QALYs. For example, modelling cost-effectiveness of DMTs in an older population will likely result in lower total lifetime costs and lower total lifetime QALYs, but how this impacts on the ICER estimate may be complex. In addition, the initial distribution of EDSS scores in the population modelled will also have an impact on lifetime costs and QALYs, especially as higher EDSS health states are associated with higher costs and poorer utility weights than lower EDSS health states. Again, how this impacts on the ICER is complex. The assessment group considers that the age, sex and EDSS scores among those in the RSS data set better reflect the UK RRMS population than participants recruited into a clinical trial.
Transition probabilities: disease progression, relapse and mortality
The manufacturers’ submissions used different approaches to model disease progression for those on BSC. Biogen Idec Ltd derived transition probabilities using disability progression observed in the placebo arm of the ADVANCE trial,213 supplemented with information from the BCMS data set. 153 Teva used the London Ontario data80 to derive the majority of its transition probabilities to model progression, whereas Merck used the BCMS data set. 153 The data sources used to model disease progression for the BSC strategy are likely to impact on the ICER. Although it may be difficult to argue which of the London Ontario or BCMS data sets provides the optimal representation of disease progression in MS patients not receiving DMTs, it would seem unorthodox to use patients recruited into the placebo arm of a clinical trial to represent this.
For relapse rates (ARRs) there were some differences in the data used by each manufacturer. All three manufacturers applied EDSS health state-specific relapse rates. Biogen Idec Ltd estimated relapse rates using data obtained from the ADVANCE trial213 up to an EDSS score of 5.5 and supplemented this with rates derived from the study by Patzold et al. 286 Teva and Merck both followed the Department of Health RSS model approach and used the same relapse rates as in the previous ScHARR model. 275 The relapse rates (for BSC) used by Biogen Idec Ltd tended to be lower, translating into fewer episodes and lower modelled lifetime costs and higher lifetime QALYs. How this impacts on the ICER estimate will also depend on the relapse rates assigned to the DMT strategy.
All three manufacturers used comparable approaches to modelling mortality. As with the RSS model, background all-cause mortality was derived from age- and sex-specific mortality rates. In addition, an MS-specific mortality rate was included through a mortality multiplier assigned to each EDSS health state.
Transition probabilities: treatment effect
All three manufacturers followed comparable approaches to modelling the treatment effect of DMTs; however, there were some differences in the data sources used. Treatment effects included the impact of DMTs on disease progression and on relapses. A HR was applied to the natural history progression matrices to determine disease progression for those on DMTs. Biogen Idec Ltd and Teva stated that they undertook a NMA to estimate the HRs for disability progression. Of note, implied HRs for 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) are not available from the year 10 RSS data set. However, Merck stated that it used the implied HR for disability progression from the 10-year RSS data provided by the Department of Health. Of note, the implied HRs from the RSS data sets tended to be higher than those obtained from the NMA. A higher HR for disability progression will result in higher ICER estimates.
For relapse rates on DMTs, Biogen Idec Ltd and Teva undertook a NMA whereas Merck extracted the value from the previous ScHARR model. 275 As previously mentioned Biogen Idec Ltd used a different data source from the other two manufacturers for relapse rates for BSC, with the relapse rates that it used for BSC being lower. The relapse rates on DMTs obtained from NMA s tended to be lower than those obtained from the 10-year RSS data sets. Untangling the impact on the final ICER is complex, especially in the case of Biogen Idec Ltd’s model. However, a greater effect of DMTs on reducing relapse rates will lead to smaller ICER estimates.
There were minor differences in how treatment discontinuation was modelled in the three manufacturer submissions. Biogen Idec Ltd reported that it used the discontinuation rates observed in clinical trials of the DMTs. Teva and Merck followed the Department of Health RSS model and assumed that 5% of patients would discontinue treatment per annum. The discontinuation rates used by Biogen Idec Ltd were generally higher than 5% per annum for the DMTs that it evaluated. A higher discontinuation rate will lead to lower lifetime costs but also fewer QALYs for treatment with DMTs. This may potentially impact on ICER estimates.
There were significant differences in how the treatment waning effect was modelled in the three manufacturer submissions. Biogen Idec Ltd assumed that there would be no treatment waning effect in its base-case analysis and that the efficacy of DMTs would be maintained. Teva and Merck followed the approach taken in the RSS model and assumed that, after 10 years on DMTs, efficacy would be lower. Not including a waning effect will not impact on lifetime costs on DMTs but will increase QALYs on DMTs and will likely result in lower ICER estimates.
Although the NICE reference case156 highlights that systematic reviews should be undertaken to obtain evidence on outcomes, the RSS cohort long-term outcome data may be a more valid data source.
Resource use and costs
In all three manufacturer submissions, costs included in the analysis were those directly related to the NHS and PSS perspective, with costs inflated to 2015 UK pounds. There were some differences in the costs included by the three manufacturers. All three manufacturers included:
-
drug acquisition costs
-
administration costs
-
monitoring costs
-
health state/EDSS costs
-
cost of relapse
-
treatment-related AE costs.
There were some differences in how the cost of providing DMTs (acquisition, administration and monitoring) was estimated and/or described. Biogen Idec Ltd and Teva provided a detailed breakdown of the costs included by drug acquisition, administration and monitoring. Merck provided a single total cost for treatment with DMTs. It is unclear whether this estimate included the cost of administering and/or monitoring of treatment with DMTs. Additionally, the estimate used in the model analysis was classified as commercial-in-confidence material and may not represent the list price for the drug. The total cost involved in providing DMTs to patients will be an important driver of cost-effectiveness. It does not appear that any of the three manufacturers included infrastructure costs (e.g. nursing infrastructure) in the drug treatment costs.
Teva and Merck used resource use data reported in the ScHARR analysis275 to determine the costs to assign to EDSS-defined health states. The costs assigned by Teva and Merck, adjusted to 2015 UK pounds, were approximately the same. Biogen Idec Ltd reported that it used cost data reported in the UK MS Trust survey and assigned different costs depending on both EDSS state and whether patients had RRMS or SPMS. The costs assigned to the EDSS states in Biogen Idec Ltd’s submission tended to be lower than those used by Teva and Merck. This is likely to result in lower lifetime costs but will affect both the DMT strategy and the BSC strategy.
For the cost of relapse, the three manufacturers followed the same approach. A proportion of those experiencing relapses would experience mild relapses (not requiring hospitalisation) whereas the others would experience severe relapses (requiring hospitalisation). The costs of each type of relapse differed and so an average cost of relapse was estimated (based on the proportions). The sources of the data differed, with Biogen Idec Ltd using data from the study by Hawton and Green107 and Teva and Merck inflating the costs reported in the ScHARR model. 275 The cost estimates used in Biogen Idec Ltd’s model were lower than those used in the Teva and Merck models.
Merck did not include the cost of treatment-related AEs in the primary analysis but included this cost in sensitivity analysis. Biogen Idec Ltd and Teva included the cost of AEs. Biogen Idec Ltd undertook its own study with specialists (a Delphi panel) to estimate resource use for AEs and consequently the unit costs. Teva derived the unit costs for AEs using a combination of information from the PSSRU,278 national reference costs285 and the manufacturer’s submission for TA312. 118
Health state utility values
There were some differences between the manufacturers’ submissions with regard to the sources of health state utility weights and how these were assigned to the health states. In the submissions by Teva and Merck, health state utilities for EDSS health states were derived by pooling data from the MS Trust and the Heron data sets. 101 Both assumed that the current EDSS score determined the utility scores for both the RRMS and the SPMS health states. This was the approach used in the RSS model. Biogen Idec Ltd derived utility weights differently in its model analysis. It used a combination of utility data from the ADVANCE trial213 and the UK MS Trust survey and its approach to pooling the data was driven by data availability and not by standard methodological approaches to pooling data. In addition, Biogen Idec Ltd assigned different utility weights to the EDSS health states according to whether or not a patient had RRMS or SPMS. As the EDSS state provides an assessment of disability, it may not be appropriate to apply a lower utility weight to the same EDSS state if patients had SPMS.
All three manufacturers used different approaches to quantify the disutility from relapses. Teva and Merck assigned a disutility weight for a relapse and assumed that the disutility from a relapse would last for between 46 and 90 days, with Teva further stratifying relapse disutility by the severity of the relapse (mild vs. severe). Although not clear, it appears that Biogen Idec Ltd assumed that the disutility from a relapse would persist and assigned an additional disutility to all EDSS health states (by subtracting the relapse disutility from the EDSS utility) for those who had a relapse.
The above two issues highlight major differences in the utility weights assigned to the EDSS health states between Biogen Idec Ltd and Teva/Merck. The way that this impacts on the ICER estimates is multifactorial and complex. There is a potential that this may lead to more favourable ICERs (greater QALY gain from DMTs) in the Biogen Idec Ltd model as one of the benefits of DMTs is to reduce relapses and delay progression to SPMS.
There were also some minor differences in the manufacturers’ submissions in the data sources used to quantify carers’ disutility. Teva and Merck followed the approach used in the RSS model, using data reported by Acaster et al. ,280 whereas Biogen Idec Ltd used data from the study by Orme et al. 101 Overall, this translated to Biogen Idec Ltd assigning predominantly lower disutility weights to the lower EDSS health states and higher disutility weights to the two highest EDSS health states.
There were some minor differences in how disutilities from adverse drug reactions were modelled. All three manufacturers assigned an average disutility, as in the RSS model. The average disutility was based on the proportion experiencing AEs and the disutility weights attached to adverse drug reactions. Overall, the values were not too dissimilar and are unlikely to impact on ICER estimates.
Summary
The assessment group reviewed the three manufacturer submissions from Biogen Idec Ltd, Teva and Merck. Overall, the methodological approaches used by the three companies were in accordance with the NICE reference case156 (Table 63). However, there were significant differences in the modelling approach and data sources used by the three manufacturers and this is likely to explain the differences in the estimated ICERs. Importantly, there were significant differences between the approaches used by the manufacturers and the approach used in the Department of Health RSS model analysis. Biogen Idec Ltd’s submission differed the most from the Department of Health RSS model analysis and Merck’s submission differed the least.
| Element of HTA | Company | Reference case156 | ||
|---|---|---|---|---|
| Biogen Idec Ltd | Teva | Merck | ||
| Defining the decision problem | ✓ | ✓ | ✓ | The scope developed by NICE141 |
| Comparator(s) | ✓ | ✓ | ✓ | As listed in the scope developed by NICE141 |
| Perspective on outcomes | ✓ | ✓ | ✓ | All direct health effects, whether for patients or, when relevant, carers |
| Perspective on costs | ✓ | ✓ | ✓ | NHS and PSS |
| Type of economic evaluation | ✓ | ✓ | ✓ | Cost–utility analysis with fully incremental analysis |
| Time horizon | ✓ | ✓ | ✓ | Long enough to reflect all important differences in costs or outcomes between the technologies being compared |
| Synthesis of evidence on health effects | ✓ | ✓ | ✓ | Based on a systematic review |
| Measuring and valuing health effects | ✓ | ✓ | ✓ | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults |
| Source of data for measurement of HRQoL | ✓ | ✓ | ✓ | Reported directly by patients and/or carers |
| Source of preference data for valuation of changes in HRQoL | ✓ | ✓ | ✓ | Representative sample of the UK population |
| Equity considerations | ✓ | ✓ | ✓ | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit |
| Evidence on resource use and costs | ✓ | ✓ | ✓ | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS |
| Discounting | ✓ | ✓ | ✓ | The same annual rate for both costs and health effects (currently 3.5%) |
Impact on the results of the assumptions made by the manufacturers
To understand the consequences of the assumptions made by the manufacturers, we calculated the results using the manufacturer-submitted treatment effects and list prices but otherwise mainly used the RSS model assumptions.
In these analyses we retained the majority of the assumptions made in the RSS model but made the following changes:
-
we excluded carers’ disutilities
-
we used the HRs for disability progression submitted by each manufacturer
-
we used the list prices for the DMTs.
Using the RSS base run and time-varying models, we estimated the cost-effectiveness of the DMTs [30 µg of IM IFN-β-1a weekly (Avonex), 44 µg of SC IFN-β-1a three times weekly (Rebif) and 20 mg of SC GA daily (Copaxone)] included in the RSS model and manufacturer submissions compared with BSC for people with RRMS. We present the results in terms of total mean costs and total mean QALYs and ICERs based on the cost per QALY gained. We report the results based on pairwise comparisons (each DMT compared with BSC) and on an incremental analysis. In the incremental analysis the strategies are ranked in ascending order according to mean cost. We eliminated strategies that were dominated, that is, strategies that were more expensive and less effective. If there was a linear combination of two other strategies that were more costly and less effective (extended dominance), these were eliminated. For the remaining strategies we derived an incremental cost per QALY gained.
Results in terms of quality-adjusted life-years gained
Over a 50-year time horizon, the results from the base-run model showed that the BSC arm had expected mean costs of approximately £344,900 and mean QALYs of 8.451. Mean costs for 30 µg of IM IFN-β-1a weekly (Avonex) were approximately (confidential information has been removed) and corresponding mean QALYs were (confidential information has been removed). Mean costs for 44 µg of SC IFN-β-1a three times weekly (Rebif) and 20 mg of SC GA daily (Copaxone) were approximately (confidential information has been removed), with corresponding mean QALYs of (confidential information has been removed) respectively. Results from the incremental analysis (Table 64) showed that (confidential information has been removed). Excluding strategies that were dominated resulted in the comparison between BSC and (confidential information has been removed). Our pairwise analysis (Table 65) showed that ICERs for each drug compared with BSC were different between the manufacturers’ analyses and our estimates from the RSS model.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 344,900 | – | 8.451 | – | – |
| GA 20 mg SC once daily (Copaxone) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| IFN-β-1a 30 µg IM once weekly (Avonex) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| IFN-β-1a 44 µg SC three times weekly (Rebif) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| DMT and manufacturer | Manufacturer’s incremental costs (£) | Incremental costs based on RSS model (£) | Manufacturer’s incremental QALYs | Incremental QALYs based on RSS model | Manufacturer’s ICER (£) | ICER (£) based on RSS model |
|---|---|---|---|---|---|---|
| IFN-β-1a 30 µg IM once weekly (Avonex) (Biogen Idec Ltd) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| IFN-β-1a 44 µg SC three times weekly (Rebif) (Merck) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| GA 20 mg SC once daily (Copaxone) (Teva) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
Discussion and conclusion
In this analysis we compared DMTs with BSC and reported the incremental costs and QALYs for each manufacturer and those derived using the RSS model. Of note, we had concerns about the total QALYs estimated in the manufacturers’ submissions. The RSS model and our own cost-effectiveness model analysis estimated mean QALYs of 8.5 for BSC in the base-case analysis, whereas Teva’s model estimated approximately (confidential information has been removed) QALYs and Merck’s model estimated approximately (confidential information has been removed) QALYs. When we adapted the RSS model to use disability progression from the Teva and Merck submissions, the mean QALYs approximated to 8.5. We looked at a range of parameters that might affect estimated mean QALYs: natural history cohort, utility values, mortality rates and starting EDSS distributions. Teva used the London Ontario data set80 to model disease progression and this may explain why its estimate was different. We could not explain the difference between the findings from the RSS model and the findings in Merck’s submission. All other aforementioned parameters were comparable between the models.
Chapter 11 Health economic assessment: relapsing–remitting multiple sclerosis
Objectives and methods
Objective
In Chapter 9, the assessment group outlined some limitations of the RSS model. We undertook several sensitivity analyses to address these concerns, using alternative information sources and assumptions. The additional analyses undertaken by the assessment group are presented in this chapter.
To assess the impact of DMTs used to treat people diagnosed with RRMS, we developed a decision-analytic modelling framework that used longitudinal data from natural history cohorts to provide information on the progression of RRMS. The objective of the model was to estimate the cost-effectiveness of DMTs within their marketing authorisation for treating people diagnosed with RRMS. In the model, health outcomes were measured in QALYs and we present the results in terms of incremental cost per QALY gained. In the UK, an ICER of < £20,000–30,000 per QALY is considered cost-effective by decision-makers. 156
Developing the model structure
To estimate the cost-effectiveness of DMTs for treating people with RRMS, we used, and developed the model structure for the RSS scheme submitted by the Department of Health. Details of the RSS model are outlined elsewhere in this report (see Chapter 9). Briefly, the RSS model is a cohort-based Markov model. The model cycled yearly, with a starting age of 30 years, and estimated the mean costs and effects associated with treatment compared with no treatment (BSC) over a 50-year time horizon. The analysis was conducted from the NHS and PSS perspective and the results were reported in terms of ICERs, expressed as the cost per QALY gained. Both costs and benefits were discounted at 3.5% per annum. Health states for people with RRMS or SPMS were characterised by EDSS levels ranging from 0 to 10. In the model, transition matrices are applied to show how people move through the model. People are able to progress to more severe EDSS levels or regress to less severe EDSS levels or there is a probability of dying from MS-related or other causes.
Changes made in our analyses to the model assumptions and characteristics from the risk-sharing scheme model
The assessment group assessed the impact of the following changes to the RSS model:
-
use of discontinuation rates obtained from our clinical effectiveness review
-
use of alternative estimates of treatment effectiveness (ARRs and HRs for disability progression) derived from our clinical effectiveness review
-
changes to mortality assumptions
-
use of list prices for DMTs
-
exclusion of carers’ disutilities
-
impact of varying key model input parameters
-
implementation of probabilistic sensitivity analysis.
Discontinuation rates
In the treatment arm of the RSS model it was assumed that every year 5% of people discontinued treatment as a result of AEs. However, it was unclear whether this assumption was based on empirical evidence. We undertook further analyses to derive a combined discontinuation rate based on all of the drugs used in the RSS model and discontinuation rates based on each individual drug used in the RSS model. These proportions were derived from the RRMS studies included in our clinical review. Studies reported the instantaneous rates of people who discontinued treatment as a result of DMTs. We converted these rates to annual probabilities using the equation [probability = 1 – exp(–rt)], where r is rate and t is time.
Table 66 shows the annual discontinuation rates for each DMT, as well as the annual discontinuation rate for all DMTs combined. Our combined annual probability of 2.29% is lower than the discontinuation rate assumed in the RSS model. Using this value in the model would lead to more people remaining on treatment. Discontinuation rates reported by each manufacturer tended to be higher than those derived from our clinical review.
| Treatment | Reported in RSS model | Derived from assessment group clinical review | Reported by each manufacturer | Derived from assessment group clinical review |
|---|---|---|---|---|
| IFN-β-1a 30 µg IM once a week (Avonex) | 0.0500 | 0.0229 | 0.0790 | 0.0150 |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 0.0500 | 0.0229 | 0.1040 | 0.0150a |
| IFN-β-1a 44/22 µg SC three times per week (Rebif) | 0.0500 | 0.0229 | 0.0500 | 0.0263 |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 0.0500 | 0.0229 | Not submitted | 0.0219 |
| GA 20 mg SC once daily or 40 mg SC three times a week (Copaxone) | 0.0500 | 0.0229 | 0.0500 | 0.0263 |
Treatment effectiveness: annualised relapse rates
In the RSS model the ARR for those treated with DMTs compared with those not treated was 0.72. We undertook further analyses to derive the ARR based on the studies identified in our clinical effectiveness review to see how this compared with the value reported in the RSS model and with the values reported in the manufacturers’ submissions. From our meta-analysis we derived a combined ARR of 0.6494 (95% CI 0.5572 to 0.7567). Our ARR is lower than the ARR presented in the RSS model. The combined treatment effect from our NMA of the published studies suggests that there is a discrepancy in the assessment of the effectiveness of DMTs depending on the data source used. RCT evidence appears to show that DMTs are more effective than is suggested by the RSS model (Table 67). In addition, we compared the ARRs for each individual DMT derived from our NMA with the ARRs reported by each manufacturer. These ARRs appear to be very similar.
| Treatment | Reported by RSS (95% CI) | Derived from assessment group clinical review (95% CI) | Reported by each manufacturer (95% CI) | Derived from assessment group clinical review (95% CI) |
|---|---|---|---|---|
| IFN-β-1a 30 µg IM once a week (Avonex) | 0.7200 (0.6118 to 0.8309) | 0.6494 (0.5572 to 0.7567) | (Confidential information has been removed) | 0.80 (0.72 to 0.88) |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 0.7200 (0.6118 to 0.8309) | 0.6494 (0.5572 to 0.7567) | 0.6420 (0.4070 to 1.0380) | 0.64 (0.50 to 0.83) |
| IFN-β-1a 44/22 µg SC three times per week (Rebif) | 0.7200 (0.6118 to 0.8309) | 0.6494 (0.5572 to 0.7567) | 0.670 (0.57 to 0.79) | 0.68 (0.61 to 0.76) |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 0.7200 (0.6118 to 0.8309) | 0.6494 (0.5572 to 0.7567) | Not submitted | 0.69 (0.62 to 0.76) |
| GA 40 mg SC three times a week (Copaxone) | 0.7200 (0.6118 to 0.8309) | 0.6494 (0.5572 to 0.7567) | (Confidential information has been removed) | 0.66 (0.54 to 0.80) |
| GA 20 mg SC once daily (Copaxone) | 0.7200 (0.6118 to 0.8309) | 0.6494 (0.5572 to 0.7567) | (Confidential information has been removed) | 0.66 (0.59 to 0.72) |
Treatment effectiveness: time to disability progression
We used both pooled and DMT-specific estimates of disability progression relative to BSC from our NMAs and compared them with other relevant inputs.
First, we estimated a combined treatment effect of DMTs by pooling relevant active versus placebo trials for on-scheme DMTs. The results showed a reduced hazard of sustained confirmed disability progression for people treated with DMT compared with BSC (HR 0.6955, 95% CI 0.5530 to 0.8747). In contrast, the RSS model reported a HR of 0.7913 (95% CI 0.7705 to 0.8122).
Second, we compared the estimates for disease progression reported by each manufacturer with the estimates derived from our analysis. Again, our results demonstrate a discrepancy between the effect sizes generated by the different sources of data (the RSS model, the pooled RCT evidence, the effects reported by the manufacturers and the DMT-specific effects estimated in our NMAs). Table 68 shows the treatment effects on disability progression from the assessment group and manufacturer estimates. Assessment group values are for disability progression confirmed at 3 months. We additionally considered disability progression confirmed at 6 months (Table 69).
| Treatment | Reported by the RSS model, HRa (95% CI) | Derived from the assessment group clinical review, HR (95% CI) | Reported by each manufacturer, HR (95% CI) | Derived from the assessment group clinical review, HR (95% CI) |
|---|---|---|---|---|
| IFN-β-1a 30 µg IM once a week (Avonex) | 0.7913 (0.7705 to 0.8122) | 0.6955 (0.5530 to 0.8747) | (Confidential information has been removed) | 0.7300 (0.5300 to 1.0000) |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 0.620 (0.2090 to 1.8150) | 0.6200 (0.4000 to 0.9700) | ||
| IFN-β-1a 44/22 µg SC three times per week (Rebif) | (Confidential information has been removed) | 0.6300 (0.4600 to 0.8600) | ||
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | Not submitted | 0.7800 (0.5900 to 1.0200) | ||
| GA 40 mg SC three times a week (Copaxone) | (Confidential information has been removed) | Not derived | ||
| GA 20 mg SC once daily (Copaxone) | 0.7600 (0.6000 to 0.9700) |
| Treatment | Derived from the assessment group clinical review, HR (95% CI) |
|---|---|
| IFN-β-1a 30 µg IM once a week (Avonex) | 0.68 (0.49 to 0.94) |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 0.46 (0.26 to 0.81) |
| IFN-β-1a 44/22 µg SC three times per week (Rebif) | 0.47 (0.24 to 0.93) |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 0.34 (0.18 to 0.63) |
| GA 40 mg SC three times a week (Copaxone) | Not reported |
| GA 20 mg SC once daily (Copaxone) | 0.82 (0.53 to 1.26) |
Mortality
The assessment group previously highlighted concerns regarding overestimation of MS-related mortality. In the RSS model we noted that individuals were subject to MS-related mortality (modelled as twice the standardised mortality rate from other causes) in addition to mortality from transition to EDSS 10 (MS-related death). We highlighted that this would theoretically lead to double counting of MS-related deaths in the model and that the results would therefore show a reduction in life-years and QALYs gained. Hence, we changed the risk of MS-related death to be the same as that for the general population, as the risk of MS-related death is already captured in the transition matrices. An alternative approach that we did not explore in these analyses would have been to consider using mortality multipliers for lower EDSS levels to capture the increased risk of mortality for those with MS compared with the general population.
Resource use and costs
The costs of DMTs were obtained from the BNF. 21 The annual cost of £8502 for treatment with IFN-β-1a (Avonex) was derived based on the recommended dosage of 30 µg once a week. The annual cost of £10,572 for treatment with IFN-β-1a (Rebif) was derived based on a dosage of 44 µg three times per week. We derived annual costs of £7264 and £6681 (£6704) for treatment with IFN-β-1b 250 µg every other day (Betaferon/Extavia) and GA (Copaxone) 40 mg SC three times weekly (20 mg SC once daily) respectively. Table 70 presents the costs for each DMT. Of note, we did not specifically take into account that those on 44 µg of IFN-β-1a (Rebif) three times per week may subsequently have their dosage reduced to 22 µg three times per week.
| Treatment | Cost (£, 2015 prices) | Source |
|---|---|---|
| IFN-β-1a 30 µg IM once a week (Avonex) | 8502 | BNF21 |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 8502 | |
| IFN-β-1a 44 µg SC three times per week (Rebif) | 10,572 | |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) | 7264 | |
| GA 20 mg SC once daily | 6704 | |
| GA 40 mg three times a week with at least 48 hours between doses | 6681 |
Utility values including carers’ disutilities
The assessment group considered the utility values used in the RSS analyses to be appropriate. However, we identified through literature searching other sources of utility estimates. In sensitivity analyses we explored the impact of using these other sources of utility values.
Disutilities associated with caring for people with MS were included in the RSS analyses. However, it appears that carers included in the analysis represent informal/unpaid carers. The NICE reference case suggests that the perspective should be all direct health effects, whether for patients or other people. Hence, the assessment group has excluded carers’ disutilities from the main analysis. We present analyses including carer disutilities in Appendix 8.
Base case cost-effectiveness analysis
The Markov model was developed and programmed to choose the base-case model inputs to assess the cost-effectiveness of DMTs for the management of people with RRMS. The model estimated the mean costs and health benefits associated with each DMT and assumed that the starting age of the population was 30 years. We considered the RSS model base case with changes made to avoid double counting of mortality and the removal of carer disutilities to be our base case. The analysis was undertaken from a NHS and PSS perspective in a specialist MS care setting and outcomes were reported as ICERs, expressed in terms of cost per QALY gained. All costs and outcomes were discounted at 3.5% per annum.
Sensitivity analyses
The following multiway sensitivity analyses were undertaken:
-
SA1 – pooled estimates of effectiveness for on-scheme DMTs from the assessment group review. In this analysis we used inputs from our review of the evidence pooled across all on-scheme DMTs. We used the aggregated HR for disability progression confirmed at 3 months, the aggregated ARR and the aggregated discontinuation rate.
-
SA2 – estimates of effectiveness of individual drugs from the assessment group review.
-
Individual drugs from the assessment group review, progression confirmed at 3 months: we used the HR for disability progression confirmed at 3 months derived from our clinical effectiveness review and the RR for ARR derived from our clinical effectiveness review, as well as relevant discontinuation rates and list prices.
-
Individual drugs from the assessment group review, progression confirmed at 6 months: we used the HR for disability progression confirmed at 6 months derived from our clinical effectiveness review and the RR for ARR derived from our clinical effectiveness review, as well as relevant discontinuation rates and list prices.
-
-
SA3 – HRs from the manufacturers’ submissions. We used the HRs (confirmed disease progression) reported by each manufacturer with the ARRs reported by each manufacturer, as well as relevant discontinuation rates and list prices.
-
SA4 – time horizon changed. In this analysis we used estimates of effectiveness of individual drugs from the assessment group review, progression confirmed at 3 months and relapse rates from the clinical effectiveness review and relevant discontinuation rates and list prices, using a time horizon of 20 years or 30 years.
-
SA5 – parameter uncertainty analysis for the base case and SA1 models. We varied the HR for disability progression, the RR for ARRs, the costs of the DMTs and the annual discontinuation rate by ±10% for the base case and SA1 models.
Probabilistic sensitivity analysis
We undertook probabilistic sensitivity analyses on the base case and SA1 models to determine the uncertainty of the key model input parameters.
In the probabilistic sensitivity analysis we varied the HR for disability progression, the RR for ARR, utility values for each EDSS state, the disutility associated with relapses, management costs by EDSS state and the cost of relapses and assigned distributions, which reflected the amount and pattern of variation.
The cost-effectiveness results were calculated by simultaneously selecting random values from each distribution. The process was repeated 1000 times in a Monte Carlo simulation of the model to give an indication of how variation in the model parameters leads to variation in the ICERs for a given treatment combination (e.g. DMT compared with BSC).
In Table 71 we present the point estimates and the appropriate distribution for the input parameters. This type of analysis allows all parameter uncertainties to be incorporated into the analysis. Sampling parameter values from probability distributions rather than from a simple range defined by the upper and lower bounds places greater weight on the likely combinations of parameter values and the simulation results quantify the impact of uncertainties on the model in terms of the confidence that can be placed in the analysis results.
| Variable | Base-case value | 95% CIs | Distribution | Source |
|---|---|---|---|---|
| Baseline distribution of people in the RSS model, n | ||||
| EDSS 0 | 135 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 1 | 689 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 2 | 1088 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 3 | 970 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 4 | 652 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 5 | 441 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 6 | 242 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 7 | 0 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 8 | 0 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 9 | 0 | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 10 | 0 | – | Fixed | Base-case values obtained from the RSS model |
| RRMS: relapse frequency (% of RRMS patients) | ||||
| EDSS 0 | 0.8895 (100) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 1 | 0.7885 (86.1) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 2 | 0.6478 (86.1) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 3 | 0.6155 (80.6) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 4 | 0.5532 (54.5) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 5 | 0.5249 (34.3) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 6 | 0.5146 (27.0) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 7 | 0.4482 (5.3) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 8 | 0.3665 (0.0) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 9 | 0.2964 (0.0) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 10 | 0.0000 (0.0) | – | Fixed | Base-case values obtained from the RSS model |
| SPMS: relapse frequency (% of SPMS patients) | ||||
| EDSS 0 | 0.0000 (0.0) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 1 | 0.0000 (13.9) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 2 | 0.6049 (13.9) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 3 | 0.5154 (19.4) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 4 | 0.4867 (45.5) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 5 | 0.4226 (65.7) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 6 | 0.3595 (73.0) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 7 | 0.3025 (94.7) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 8 | 0.2510 (100) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 9 | 0.2172 (100) | – | Fixed | Base-case values obtained from the RSS model |
| EDSS 10 | 0.0000 (100) | – | Fixed | Base-case values obtained from the RSS model |
| HR | ||||
| Disability progression in the RSS model | 0.7913 | 0.7708 to 0.8123 | Log-normal | |
| Disability progression in the assessment group model | 0.6955 | 0.5530 to 0.8747 | Log-normal | Derived from the assessment group analysis |
| RR | ||||
| ARR in the RSS model | 0.7200 | 0.6118 to 0.8309 | Log-normal | Base-case value obtained from the RSS model and CIs estimated by the Department of Health |
| ARR in the assessment group model | 0.6494 | 0.5572 to 0.7567 | Log-normal | Derived from the assessment group analysis |
| Management costs by EDSS state (£) | ||||
| EDSS 0 | 1195 | Assumed to be log-normally distributed with a standard error of 10% of the mean value | Log-normal | Base-case values obtained from the RSS model |
| EDSS 1 | 1195 | Log-normal | ||
| EDSS 2 | 1195 | Log-normal | ||
| EDSS 3 | 2203 | Log-normal | ||
| EDSS 4 | 2283 | Log-normal | ||
| EDSS 5 | 8045 | Log-normal | ||
| EDSS 6 | 8974 | Log-normal | ||
| EDSS 7 | 27,385 | Log-normal | ||
| EDSS 8 | 42,521 | Log-normal | ||
| EDSS 9 | 54,055 | Log-normal | ||
| EDSS 10 | 0 | Fixed | ||
| Management of relapse | ||||
| Cost of relapse (£) | 4263 | Assumed to be log-normally distributed with a standard error of 10% of the mean value | Log-normal | Base-case values obtained from the RSS model |
| Utility values | ||||
| EDSS 0 | 0.9248 | 0.8650 to 0.9581 | Log-normal | Base-case values obtained from the RSS model and the ScHARR model275 |
| EDSS 1 | 0.7614 | 0.7079 to 0.8051 | Log-normal | |
| EDSS 2 | 0.6741 | 0.6165 to 0.7230 | Log-normal | |
| EDSS 3 | 0.5643 | 0.5143 to 0.6092 | Log-normal | |
| EDSS 4 | 0.5643 | 0.4965 to 0.6230 | Log-normal | |
| EDSS 5 | 0.4906 | 0.4333 to 0.5421 | Log-normal | |
| EDSS 6 | 0.4453 | 0.3722 to 0.5099 | Log-normal | |
| EDSS 7 | 0.2686 | 0.2190 to 0.3150 | Log-normal | |
| EDSS 8 | 0.0076 | –0.0705 to 0.0800 | Log-normal | |
| EDSS 9 | –0.2304 | –0.3086 to –0.1569 | Log-normal | |
| Dead | 0 | – | Fixed | By definition |
| Disutility of a relapse | –0.0277 | ±10% | Log-normal | Assumption |
| Other | ||||
| Mortality (age-specific death rates) | Life tables | – | Fixed | ONS,290 as cited in the Biogen Idec Ltd submission |
| Discount rate per annum (costs and QALYs) | 3.5% | – | Fixed | |
Sensitivity analyses 1–4 are summarised in Table 72 with respect to key model parameters.
| Parameter | Base-case analysis | Sensitivity analysis | ||||
|---|---|---|---|---|---|---|
| SA1: Pooled estimates of effectiveness from on-scheme DMTs from AG review | SA2a: Estimates of effectiveness of individual drugs from the AG review, progression confirmed at 3 months | SA2b: Estimates of effectiveness of individual drugs from the AG review, progression confirmed at 6 months | SA3: HRs from the manufacturers’ submissions | SA4: Time horizon changed | ||
| Cost of DMTs (£) | 8000 | 8000 | IFN-β-1a 30 µg IM once a week (Avonex) 8502; pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 8502; IFN-β-1a 44 µg SC three times per week (Rebif) 10,572; IFN-β-1b 250 µg every other day (Betaferon/Extavia) 7264; GA 20 mg SC once daily (Copaxone) 6704 | IFN-β-1a 30 µg IM once a week (Avonex) 8502; pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 8502; IFN-β-1a 44 µg SC three times per week (Rebif) 10,572; IFN-β-1b 250 µg every other day (Betaferon/Extavia) 7264; GA 20 mg SC once daily (Copaxone) 6704 | IFN-β-1a 30 µg IM once a week (Avonex) 8502; pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 8502; IFN-β-1a 44 µg SC three times per week (Rebif) 10,572; IFN-β-1b 250 µg every other day (Betaferon/Extavia) 7264; GA 20 mg SC once daily (Copaxone) 6704 | IFN-β-1a 30 µg IM once a week (Avonex) 8502; pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 8502; IFN-β-1a 44 µg SC three times per week (Rebif) 10,572; IFN-β-1b 250 µg every other day (Betaferon/Extavia) 7264; GA 20 mg SC once daily (Copaxone) 6704 |
| Pooled on-scheme DMTs on disability progression, HR (95% CI) | 0.7913a | 0.6955 (0.5530 to 0.8747) | Not applicable | Not applicable | Not applicable | Not applicable |
| Individual drug time to disability progression, HR (95% CI) | Not applicable | Not applicable | IFN-β-1a 30 µg IM once a week (Avonex) 0.73 (0.53 to 1.00); pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.62 (0.40 to 0.97); IFN-β-1a 44 µg SC three times per week (Rebif) 0.63 (0.46 to 0.86); IFN-β-1b 250 µg every other day (Betaferon/Extavia) 0.78 (0.59 to 1.0); GA 20 mg SC once daily (Copaxone) 0.76 (0.60 to 0.97) | IFN-β-1a 30 µg IM once a week (Avonex) 0.68 (0.49 to 0.94); pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.46 (0.26 to 0.81); IFN-β-1a 44 µg SC three times per week (Rebif) 0.47 (0.24 to 0.93); IFN-β-1b 250 µg every other day (Betaferon/Extavia) 0.34 (0.18 to 0.63); GA 20 mg SC once daily (Copaxone) 0.82 (0.53 to 1.26) | IFN-β-1a 30 µg IM once a week (Avonex) (confidential information has been removed); pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.620 (0.21 to 1.82); IFN-β-1a 44 µg SC three times per week (Rebif) (confidential information has been removed); IFN-β-1b 250 µg every other day (Betaferon/Extavia) NS; GA 20 mg SC once daily (Copaxone) (confidential information has been removed) | IFN-β-1a 30 µg IM once a week (Avonex) 0.73 (0.53 to 1.00); pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.62 (0.40 to 0.97); IFN-β-1a 44 µg SC three times per week (Rebif) 0.63 (0.46 to 0.86); IFN-β-1b 250 µg every other day (Betaferon/Extavia) 0.78 (0.59 to 1.0); GA 20 mg SC once daily (Copaxone) 0.76 (0.60 to 0.97) |
| Aggregated ARR (95% CI) | 0.72 | 0.6494 (0.5572 to 0.7567) | Not applicable | Not applicable | Not applicable | Not applicable |
| Individual drug ARRs (95% CI) | Not applicable | Not applicable | IFN-β-1a 30 µg IM once a week (Avonex) 0.80 (0.72 to 0.88); pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.64 (0.50 to 0.83); IFN-β-1a 44 µg three times per week (Rebif) 0.68 (0.61 to 0.76); IFN-β-1b 250 µg every other day (Betaferon/Extavia) 0.69 (0.62 to 0.76); GA 20 mg SC once daily (Copaxone) 0.66 (0.59 to 0.72) | IFN-β-1a 30 µg IM once a week (Avonex) 0.80 (0.72 to 0.88); pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.64 (0.50 to 0.83); IFN-β-1a 44 µg three times per week (Rebif) 0.68 (0.61 to 0.76); IFN-β-1b 250 µg every other day (Betaferon/Extavia) 0.69 (0.62 to 0.76); GA 20 mg SC once daily (Copaxone) 0.66 (0.59 to 0.72) | IFN-β-1a 30 µg IM once a week (Avonex) 0.7870 (0.5990 to 0.9790); pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.6420 (0.4070 to 1.0380); IFN-β-1a 44 µg three times per week (Rebif) 0.670 (0.670 to 1.000); IFN-β-1b 250 µg every other day (Betaferon/Extavia) NS; GA 20 mg SC once daily (Copaxone) (confidential information has been removed) | IFN-β-1a 30 µg IM once a week (Avonex) 0.80 (0.72 to 0.88); pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.64 (0.50 to 0.83); IFN-β-1a 44 µg three times per week (Rebif) 0.68 (0.61 to 0.76); IFN-β-1b 250 µg every other day (Betaferon/Extavia) 0.69 (0.62 to 0.76); GA 20 mg SC once daily (Copaxone) 0.66 (0.59 to 0.72) |
| Annual discontinuation of treatment rate | 0.05 | 0.0229 | IFN-β-1a 30 µg IM once a week (Avonex) 0.0150; pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.0150; IFN-β-1a 44 µg SC three times per week (Rebif) 0.0263; IFN-β-1b 250 µg every other day (Betaferon/Extavia) 0.0219; GA 20 mg SC once daily (Copaxone) 0.0263 | IFN-β-1a 30 µg IM once a week (Avonex) 0.0150; pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.0150; IFN-β-1a 44 µg SC three times per week (Rebif) 0.0263; IFN-β-1b 250 µg every other day (Betaferon/Extavia) 0.0219; GA 20 mg SC once daily (Copaxone) 0.0263 | IFN-β-1a 30 µg IM once a week (Avonex) 0.0790; pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.1040; IFN-β-1a 44 µg SC three times per week (Rebif) 0.0500; IFN-β-1b 250 µg every other day (Betaferon/Extavia) NS; GA 20 mg SC once daily (Copaxone) 0.0500 | IFN-β-1a 30 µg IM once a week (Avonex) 0.0150; pegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) 0.0150; IFN-β-1a 44 µg SC three times per week (Rebif) 0.0263; IFN-β-1b 250 µg every other day (Betaferon/Extavia) 0.0219; GA 20 mg SC once daily (Copaxone) 0.0263 |
| Time horizon | 50 years | 50 years | 50 years | 50 years | 50 years | 20 years, then at 30 years |
Results of the cost-effectiveness analysis
In this section we present the results relating to the base-run model. Further results relating to the time-varying model are provided in Appendix 8.
Base-case and sensitivity analyses
Base case
In Table 73 we present the findings from our base-case analysis, taking into account the concerns described above. The results showed that over a 50-year time horizon the DMT strategy was more costly and more effective than BSC. The DMT strategy was approximately £31,900 more costly per person than the BSC strategy and produced 0.943 more QALYs, with an ICER of approximately £33,800 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 8.664 | – | – |
| DMTs | 394,000 | 31,900 | 9.607 | 0.943 | 33,800 |
SA1: Pooled estimates of effectiveness for on-scheme disease-modifying therapies from the assessment group review
We used two key estimates of treatment effectiveness from our clinical effectiveness review: the aggregated HR for disability progression confirmed at 3 months and the aggregated ARR.
In Table 74 the results are presented in terms of cost per QALY. The results show that the DMT strategy was more costly and more effective than BSC alone. The DMT strategy was approximately £23,300 more costly than BSC and produced 1.822 more QALYs, which equated to an ICER of approximately £12,800 per QALY. This indicates that for every additional QALY from DMTs there is an incremental cost of £12,800.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 8.664 | – | – |
| DMTs | 385,400 | 23,300 | 10.486 | 1.822 | 12,800 |
SA2a: Estimates of effectiveness of individual drugs from the assessment group review, progression confirmed at 3 months (preferred analysis)
In this model we used the HRs (DMT vs. placebo) for disability progression confirmed at 3 months (see Table 68) and ARR (see Table 67) derived from our clinical effectiveness review and applied them to the individual DMTs.
The results from this sensitivity analysis (Table 75) show that BSC was the least expensive strategy and 30 µg of IM IFN-β-1a once weekly (Avonex) was the most expensive strategy. In terms of QALYs, BSC is expected to result in the fewest QALYs (8.664) and 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) is expected to yield the most QALYs (11.223). Treatment with 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) dominated all other DMT strategies, being less costly and more effective. Compared with BSC, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) was approximately £17,800 more costly and more effective by an expected mean gain of 2.559 QALYs, with an ICER of £7000 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 8.664 | – | – |
| PegIFN-β-1a 125 µg every 2 weeks (Plegridy) | 379,900 | 17,800 | 11.223 | 2.559 | 7000 |
| GA 20 mg SC once daily (Copaxone) | 381,400 | 1500 | 10.012 | –1.211 | Dominated |
| IFN-β-1b 250 µg every other day (Betaferon/Extavia) | 393,400 | 13,500 | 9.934 | –1.289 | Dominated |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 404,800 | 24,900 | 10.867 | –0.356 | Dominated |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 406,400 | 26,500 | 10.348 | –0.875 | Dominated |
SA2b: Estimates of effectiveness of individual drugs from the assessment group review, progression confirmed at 6 months
In this sensitivity analysis we used HRs for disability progression confirmed at 6 months derived from our clinical effectiveness review. The findings showed that 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) was the least costly and most effective treatment strategy, dominating other treatment strategies included in this analysis (Table 76). We did not include 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) in this analysis as its value for progression confirmed at 6 months was (1) extreme, (2) derived from indirect evidence and (3) driven by one open-label trial using an imputed HR.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 347,000 | – | 12.583 | – | – |
| BSC | 362,100 | 15,100 | 8.664 | –3.919 | Dominated |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 377,600 | 30,600 | 12.041 | –0.542 | Dominated |
| GA 20 mg SC once daily (Copaxone) | 391,800 | 44,800 | 9.650 | –2.933 | Dominated |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 397,200 | 50,200 | 10.717 | –1.866 | Dominated |
SA3: Hazard ratios from manufacturer submissions
When we used the estimates for treatment effectiveness (ARR and disability progression) reported by each manufacturer, BSC was the least expensive strategy and 44 µg of SC IFN-β-1a three times a week (Rebif) was the most expensive (Table 77). In terms of QALYs, BSC is expected to result in the fewest QALYs (8.664) and 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) is expected to yield the most QALYs (9.931). The results showed that 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) dominated all other DMT strategies. Compared with BSC, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) had an ICER of £3300 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 8.664 | – | – |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 366,300 | 4200 | 9.931 | 1.267 | 3300 |
| GA 40 mg SC three times weekly (Copaxone) | 374,600 | 8300 | 9.821 | –0.11 | Dominated |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 387,600 | 21,300 | 9.563 | –0.368 | Dominated |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 412,900 | 46,600 | 9.719 | –0.212 | Dominated |
SA4: Time horizon changed from 50 years to 20 years and 30 years
Tables 78 and 79 show the results of the sensitivity analyses based on a 20-year and 30-year time horizon, respectively. The GA treatment strategy was extendedly dominated by 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) in both analyses. Additionally, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) dominated both 30 µg of IM IFN-β-1a once weekly (Avonex) and 44 µg of SC pegIFN-β-1a three times a week (Rebif). Excluding all dominated strategies, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) compared with BSC had ICERs of approximately £21,200 and £10,600 per QALY for the 20-year and 30-year time horizons respectively.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 196,900 | – | 6.644 | – | – |
| GA 20 mg SC once daily (Copaxone) | 220,900 | 24,000 | 7.436 | 0.792 | Extendedly dominated |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 225,800 | 28,900 | 8.007 | 1.363 | 21,200 |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 242,900 | 17,100 | 7.570 | –0.437 | Dominated |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 245,200 | 19,400 | 7.882 | –0.125 | Dominated |
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 279,400 | – | 7.774 | – | – |
| GA 20 mg SC once daily (Copaxone) | 299,400 | 20,000 | 8.874 | 1.1 | Extendedly dominated |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 300,400 | 21000 | 9.756 | 1.982 | 10,600 |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 322,900 | 22500 | 9.532 | –0.224 | Dominated |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 323,300 | 22,900 | 9.103 | –0.653 | Dominated |
SA5: Parameter uncertainty analysis
Figure 25 shows a graphical representation (also known as a tornado diagram) of the impact on the base case of varying key model input parameters. In this analysis we varied the HR for disability progression, the RR for ARRs, the cost of DMTs and the annual discontinuation rate by ±10%. Additionally, we assessed the impact on the base-case results of varying the model time horizon by ±10%. The results show that changes to the HR for disability progression have the greatest impact on the cost-effectiveness results. A decrease in the treatment effect (increase in the HR) by 10% resulted in an ICER of approximately £74,500 per QALY. An increase in the treatment effect (decrease in the HR) by 10% resulted in an ICER of approximately £15,300 per QALY. The model remained robust to changes to the treatment discontinuation rate and the model time horizon.
FIGURE 25.
Tornado diagram for DMTs compared with BSC: base case.

In Figure 26 we show the impact on the model estimated in SA1 of varying key model input parameters. In SA1, model input parameters were based on pooled estimates of treatment effectiveness for on-scheme DMTs. To determine the robustness of these results we varied the HR for disability progression, the RR for ARRs, the cost of DMTs, the annual discontinuation rate and the model time horizon. The results show that the model was sensitive to changes in the cost of DMTs. An increase of 10% in the cost of DMTs led to an increase in the ICER of 41%. A decrease of 10% in the cost of DMTs led to a decrease in the ICER of approximately 42%. The results remained robust to changes made to the ARR, the model time horizon and the annual discontinuation rate.
FIGURE 26.
Tornado diagram for DMTs compared with BSC: SA1.

Probabilistic sensitivity analysis conducted on the base case
Table 80 presents the results of the probabilistic sensitivity analysis conducted on the base case, that is, when the RSS data were used to estimate the HR for disability progression and the RR for ARRs. These results show that the DMT strategy was more costly and more effective than BSC, with an ICER of approximately £34,000 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 363,600 | – | 8.64 | – | – |
| DMTs | 395,200 | 31,600 | 9.57 | 0.93 | 34,000 |
Figure 27 shows the cost-effectiveness plane for the results from the 1000 simulations from the probabilistic sensitivity analysis conducted on the base case and Figure 28 shows the proportion of these simulations at various willingness-to-pay thresholds in the form of a cost-effectiveness acceptability curve. The cost-effectiveness plane shows that all of the simulations are in the north-east quadrant, where DMTs are more effective and more costly than BSC. We believe that the HR for disability progression is likely to be one of the key drivers of the economic model. The results from the cost-effectiveness acceptability curve show that, at a willingness-to-pay threshold of £30,000 per QALY, DMTs have a probability of being cost-effective of 0.23 compared with BSC.
FIGURE 27.
Cost-effectiveness plane: probabilistic sensitivity analysis conducted on the base case.
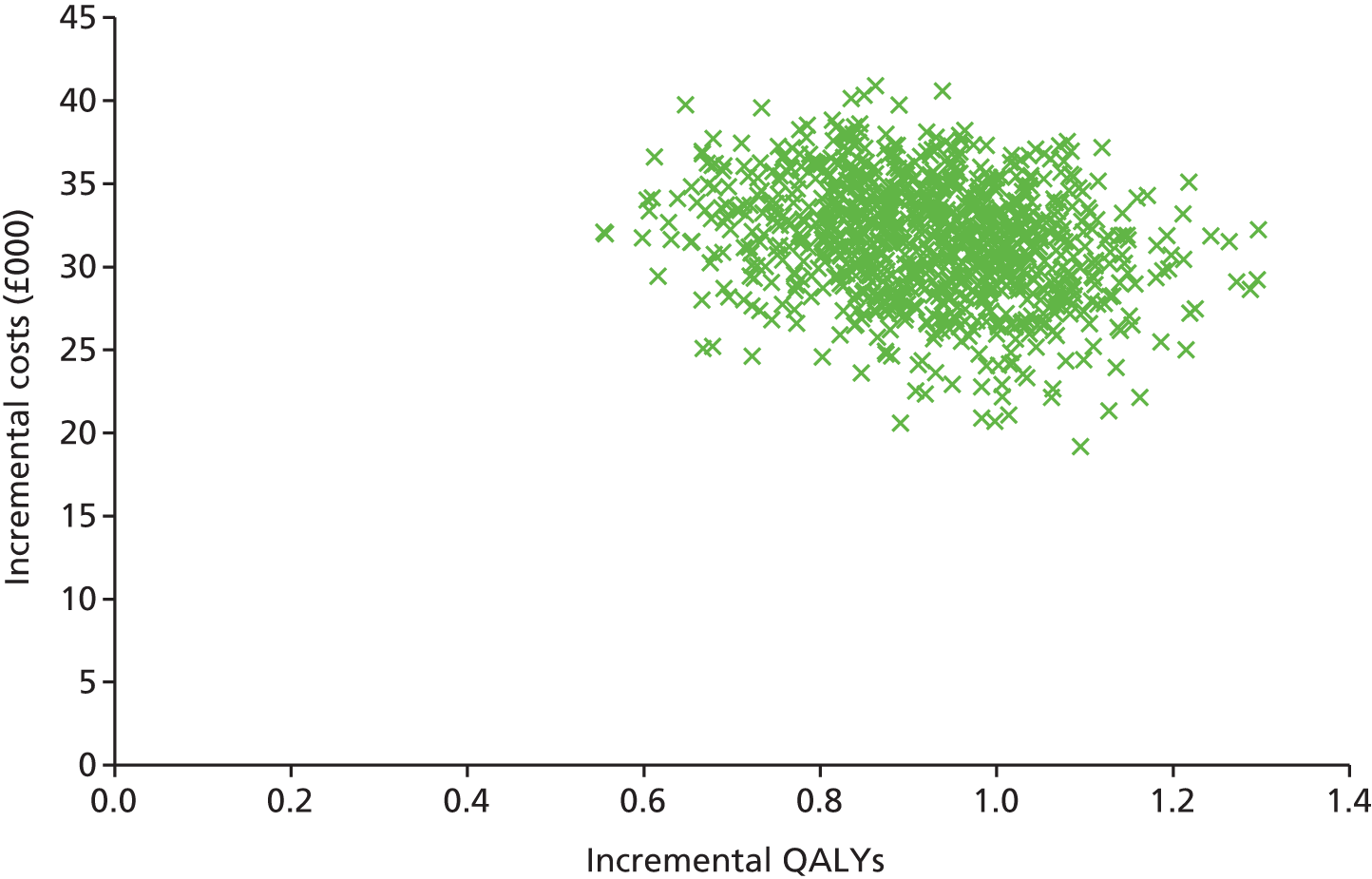
FIGURE 28.
Cost-effectiveness acceptability curve: probabilistic sensitivity analysis conducted on the base case. WTP, willingness to pay.
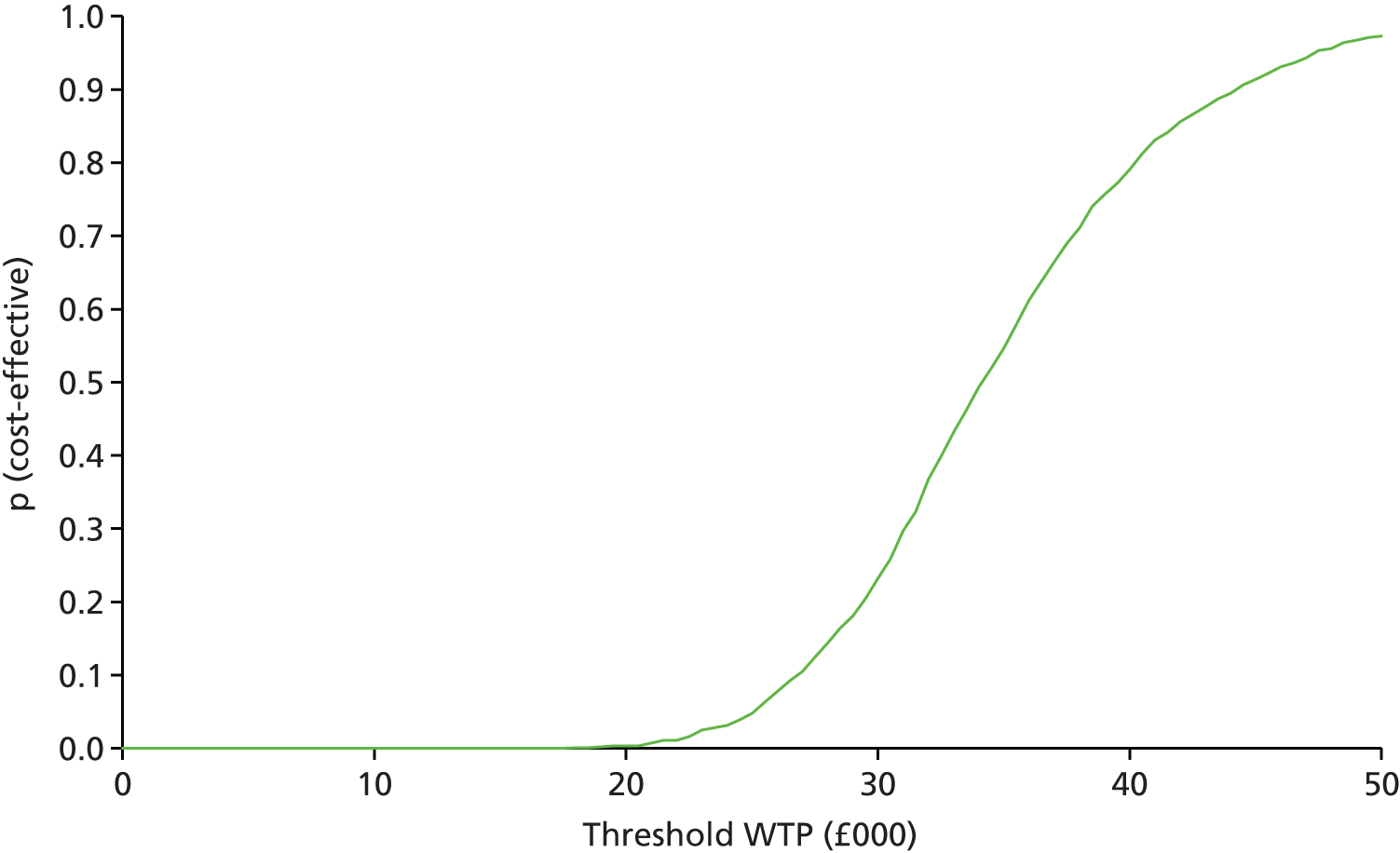
Probabilistic sensitivity analysis conducted on SA1
Table 81 presents the results of the probabilistic sensitivity analysis when the findings from the assessment group review were used to estimate the pooled HR for disability progression and the pooled RR for ARRs. The ICER for DMTs compared with BSC was approximately £10,100 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 363,500 | – | 8.635 | – | – |
| DMTs | 383,100 | 19,600 | 10.573 | 1.938 | 10,100 |
The results of the simulations are presented on a cost-effectiveness plane in Figure 29. Figure 30 provides the cost-effectiveness acceptability curve. The results from the 1000 simulations show that a substantial number of points are in the north-east quadrant. Importantly, a significant number of simulations are in the south-east quadrant, where DMTs could be considered more effective and less costly than BSC. The cost-effectiveness acceptability curve shows that, at a willingness-to-pay threshold of £30,000 per QALY, DMTs have a probability of being cost-effective of 0.84 compared with BSC.
FIGURE 29.
Cost-effectiveness plane: probabilistic sensitivity analysis conducted on SA1.
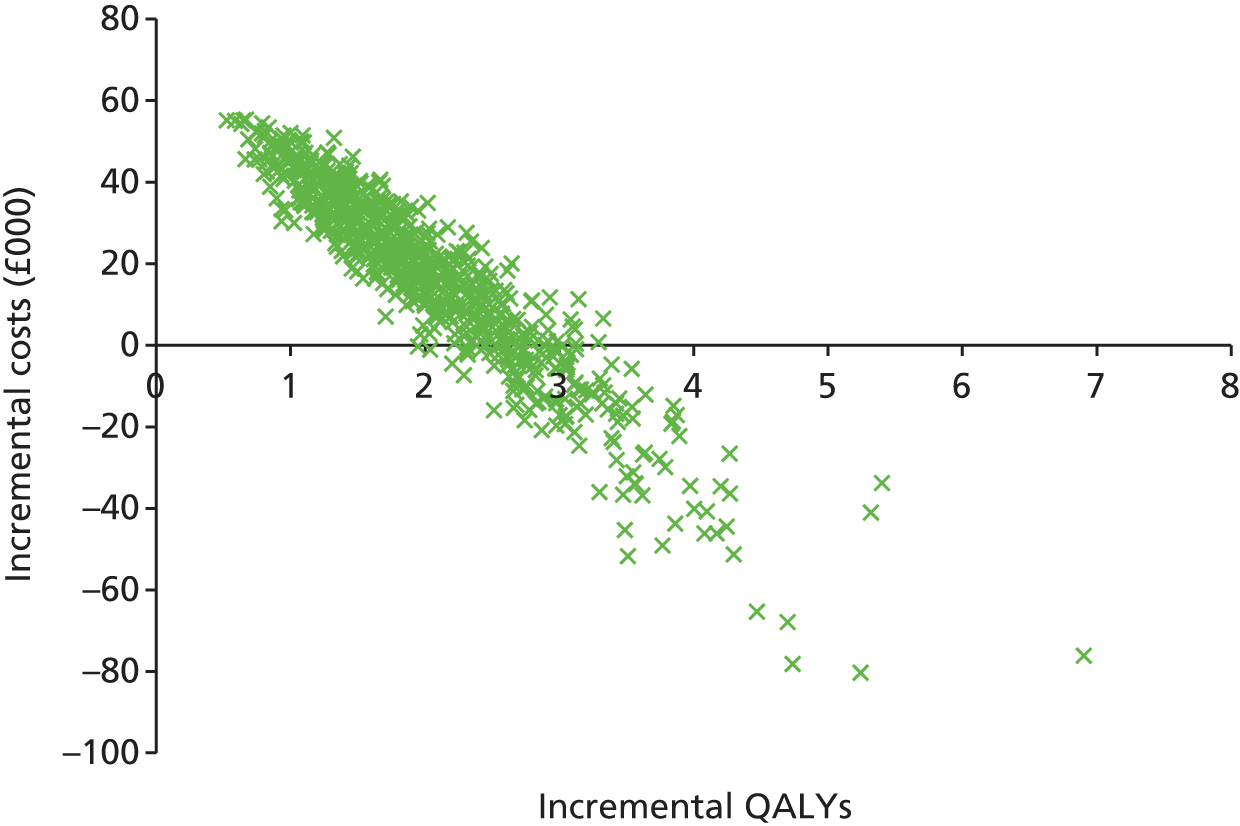
FIGURE 30.
Cost-effectiveness acceptability curve: probabilistic sensitivity analysis conducted on SA1. WTP, willingness to pay.

Through visual inspection of the cost-effectiveness plane, it appears that the incremental cost of providing DMTs is correlated with the incremental effects of receiving treatment. We undertook further model simulations (not presented here) in which we kept the HR for disability progression constant and varied other parameters. This resulted in the majority of the plots concentrated in the north-east quadrant, with no correlation seen. This finding, in addition to the findings presented in Figures 29 and 30, highlights the fact that the HR for disability progression is likely to be one of the key drivers in the economic model. The more effective DMTs are at slowing disease progression, the more likely they are to be cost-effective.
Discussion of the economic assessment of disease modifying treatments for relapsing–remitting multiple sclerosis
Summary of the results
In this chapter we describe a variety of sensitivity analyses that were carried out to address our concerns with the RSS model. In the base case we drew on the RSS model and made a number of changes relating to mortality and carers’ disutilities. Additionally, we undertook probabilistic sensitivity analyses to incorporate uncertainty around key model input parameters. The deterministic results showed that DMT was more costly and more effective than BSC, with an ICER of approximately £33,800 per QALY. The probabilistic sensitivity analysis results, using the RSS data to estimate the parameters for treatment effectiveness, showed that DMT had a probability of 0.23 of being cost-effective compared with BSC at a willingness-to-pay threshold of £30,000 per QALY. Even at higher willingness-to-pay thresholds (e.g. £50,000 per QALY), the probability of DMTs being cost-effective did not reach 1.
We undertook a number of further sensitivity analyses in which we used HRs for disability progression and RRs for ARRs derived from our NMAs. The deterministic results showed that DMT had an ICER of approximately £12,800 per QALY compared with BSC. The probabilistic results, using the assessment group data, showed that, compared with BSC, DMT had a probability of 0.84 of being cost-effective at a willingness-to-pay threshold of £30,000 per QALY.
Strengths and limitations
There were several strengths to our analyses. First, we assessed the RSS model in detail and undertook a number of sensitivity analyses, including probabilistic sensitivity analyses, to explore our concerns with the model. Second, we drew on rigorous evidence to carry out a comprehensive set of sensitivity analyses and used probabilistic sensitivity analyses to explore uncertainty. We were able to use clinical inputs from our own rigorous systematic review of the clinical effectiveness evidence, including our NMAs, for key treatment effectiveness parameters. This enabled us to compare the implications of different estimates of treatment effectiveness, including the RSS model estimates, the pooled on-scheme DMT effect sizes from our clinical effectiveness review, effect sizes for individual DMTs from the NMAs contained in our clinical effectiveness review and effectiveness estimates supplied in the manufacturers’ submissions.
However, there were also limitations to our analyses. When CIs for input parameters were not provided for probabilistic sensitivity analyses, we had to apply commonly used approaches to model uncertainty. The effect of these strategies may be to incorrectly estimate the uncertainty around input parameters and thus overestimate or underestimate the probability of DMTs being cost-effective at given willingness-to-pay thresholds. We were unable to include uncertainty around parameters for the natural history cohort used as a comparator in the RSS model.
Moreover, any cost-effectiveness analyses undertaken using the estimates from our clinical effectiveness review propagate the major weaknesses identified with that evidence, including the sparse networks of evidence, the generally short-term follow-up times used and the differential risk of bias across comparisons. In particular, some estimates of intervention effectiveness relied on few studies; our assessment of 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy), relied on one trial with 1 year of follow-up connected to evidence networks through the placebo arm only.
Finally, we chose as our base case the RSS model, which draws on observational evidence from a non-contemporaneous, historical cohort. However, we believe that the long-term follow-up, relevance to the NHS and to current clinical practice and the rigorous methods used to collect and report data made it the best choice as the base case. In contrast, the evidence derived from the clinical effectiveness review had serious limitations, discussed in the conclusion to Chapter 5. These limitations led us to believe, on balance, that the RSS model was a better choice for the base case.
Conclusions of the cost-effectiveness analysis
Based on the model and its inputs, the results of the base case, which draws on the evidence from the RSS, suggest that, compared with BSC, DMT has a probability of 0.23 of being cost-effective at a willingness-to-pay threshold of £30,000 per QALY gained. The results from our pooled analysis of RCTs suggest a probability of 0.84 of DMT being cost-effective compared with BSC at a willingness-to-pay threshold of £30,000 per QALY gained. The impact of DMTs on disability progression was found to be a key driver of cost-effectiveness. Previously we have highlighted the differences between estimates of effectiveness of DMTs derived from the RSS data and those derived from the NMA of clinical trials. The cost-effectiveness analysis in this section highlights how these differences in clinical effectiveness translate into apparent differences in conclusions related to cost-effectiveness. However, any analyses undertaken on data from our review of clinical effectiveness propagate the weaknesses in that evidence, including the short-term follow-up times used and the sparse number of data for each comparison.
Chapter 12 Health economic assessment: clinically isolated syndrome
Health economics methods
Objective
Our objective was to undertake a cost-effectiveness analysis to estimate the incremental cost per QALY gained from providing DMTs to patients with CIS. We developed a decision-analytic modelling framework, which used longitudinal data from natural history cohorts and RCTs to provide information on progression from CIS to RRMS. The modelling framework was informed by literature searches on model-based economic evaluations of interventions used to treat people with CIS and longitudinal studies that tracked the progression/conversion of CIS to RRMS. The objective of the model was to estimate the cost-effectiveness of DMTs within their marketing authorisation for people with CIS. In the model, the results are presented in terms of cost per QALY gained.
Developing the model structure
To assess the cost-effectiveness of DMTs for treating CIS, we developed a de novo economic model using TreeAge Pro 2013 software (TreeAge Software, Inc., Williamstown, MA, USA).
The model represents, as far as possible, the clinical pathways that people would take while receiving treatment for CIS. Figure 31 shows an illustrative model structure. The model was structured in two stages: (1) treatment of people with CIS and further progression to RRMS and (2) disease progression while in the RRMS health state. In the model we compared six strategies:
-
BSC for people with CIS and RRMS
-
BSC for people with CIS and DMTs for people converting to RRMS
-
treatment with 30 µg of IM IFN-β-1a once weekly (Avonex) for people with CIS, continuing on DMTs after converting to RRMS
-
treatment with 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) for people with CIS, continuing on DMTs after converting to RRMS
-
treatment with 44 µg of SC IFN-β-1a three times weekly (Rebif) for people with CIS, continuing on DMTs after converting to RRMS
-
treatment with 20 mg of SC GA once daily (Copaxone) for people with CIS, continuing on DMTs after converting to RRMS.
FIGURE 31.
Illustrative model structure.

Overview of strategies
An overview of how these strategies relate to the decision-analytic model is provided in Figure 32.
FIGURE 32.
Pathway for the strategies being compared.

Best supportive care arm for clinically isolated syndrome and relapsing–remitting multiple sclerosis
In this strategy, people receive BSC as treatment for CIS. People who are alive can remain in this health state or progress to RRMS. People who progress to the RRMS health state are assumed to follow the pathway for people in the natural history cohort of the RSS model.
Best supportive care for clinically isolated syndrome and disease-modifying therapies for people with relapsing–remitting multiple sclerosis
In this strategy, people receive BSC as treatment for CIS. People who are alive can remain in this health state or progress to RRMS. People who progress to the RRMS health state are assumed to follow the pathway for people in the DMT arm of the RSS model.
Disease-modifying therapy for clinically isolated syndrome and relapsing–remitting multiple sclerosis
People in this strategy receive a DMT for CIS. People can continue receiving treatment or discontinue treatment. People who continue treatment can remain in this health state or progress to the RRMS health state. People who convert to RRMS are assumed to follow the pathway for people in the DMT arm of the RSS model. People who discontinue CIS treatment can remain in this health state while receiving BSC or can convert to RRMS. We assumed that people who convert to RRMS follow the pathway for people in the DMT arm of the RSS model. The pathway for people in the DMT arm of the RSS model reflects the pooled estimates for all DMTs in the RSS model (e.g. drug acquisition costs) and consequently takes into account that, although patients with CIS may discontinue the modelled DMT, when they progress to RRMS they may be started on an alternative DMT. The pathways for all DMTs for CIS being compared in the model are the same.
Model assumptions
A number of assumptions were required to undertake these analyses:
-
Starting population: people aged 30 years and with CIS, that is, who had experienced a clinically diagnosed, single demyelinating event in one or several areas of the CNS within the last 2 months and with no evidence of RRMS on a MRI scan.
-
People who have converted to RRMS have no residual treatment benefit based on previous treatment in the CIS health state.
-
People who have converted to RRMS are assumed to follow the same pathway as people in the RSS model.
-
Patients with CIS who discontinue a DMT (e.g. because of AEs) will be started on an alternative DMT once they progress to RRMS. The risk of patients with RRMS discontinuing a DMT is not dependent on whether they had discontinued a DMT while they had CIS.
Data required for the model
The model was populated with information identified from the clinical effectiveness and cost-effectiveness review and supplemented with information from secondary sources. Information required to parameterise the model included transition probabilities, resource use and costs and utilities. These are discussed in turn in the following sections.
Transition probabilities and proportions
Information was required on the risk of disease progression from CIS to RRMS, for an untreated cohort and for a treated cohort of people with CIS. For the untreated cohort, progression rates could be derived from a natural history cohort, a patient registry or from CIS patients registered on the placebo arm of a trial. In the base case for the BSC arm, we identified one study295 based on a literature review that provided useful information on time to progression to RRMS for people diagnosed with CIS with no asymptomatic lesions on MRI. We reconstructed the Kaplan–Meier survival curve of time from first attack to conversion to RRMS based on baseline MRI (no asymptomatic lesion) and then fitted various parametric models to these data. According to the Akaike information criterion and Bayesian information criterion, we found that the Weibull and log-logistic models provided the best fits to the Kerbrat et al. 295 data. Figure 33 shows the reconstructed Kaplan–Meier curve with the Weibull parametric model. From this, annual transition probabilities generated by the Weibull model were used for the BSC arm. To derive the transition probabilities for conversion to RRMS for the treatment arms, we applied the HRs derived from our clinical review. Table 82 shows the estimates used to derive transition probabilities for conversion to RRMS in the model.
FIGURE 33.
Reconstructed Kaplan–Meier survival curve and Weibull model for time to conversion to RRMS on BSC by annual cycles. Source: Kerbrat et al. 295
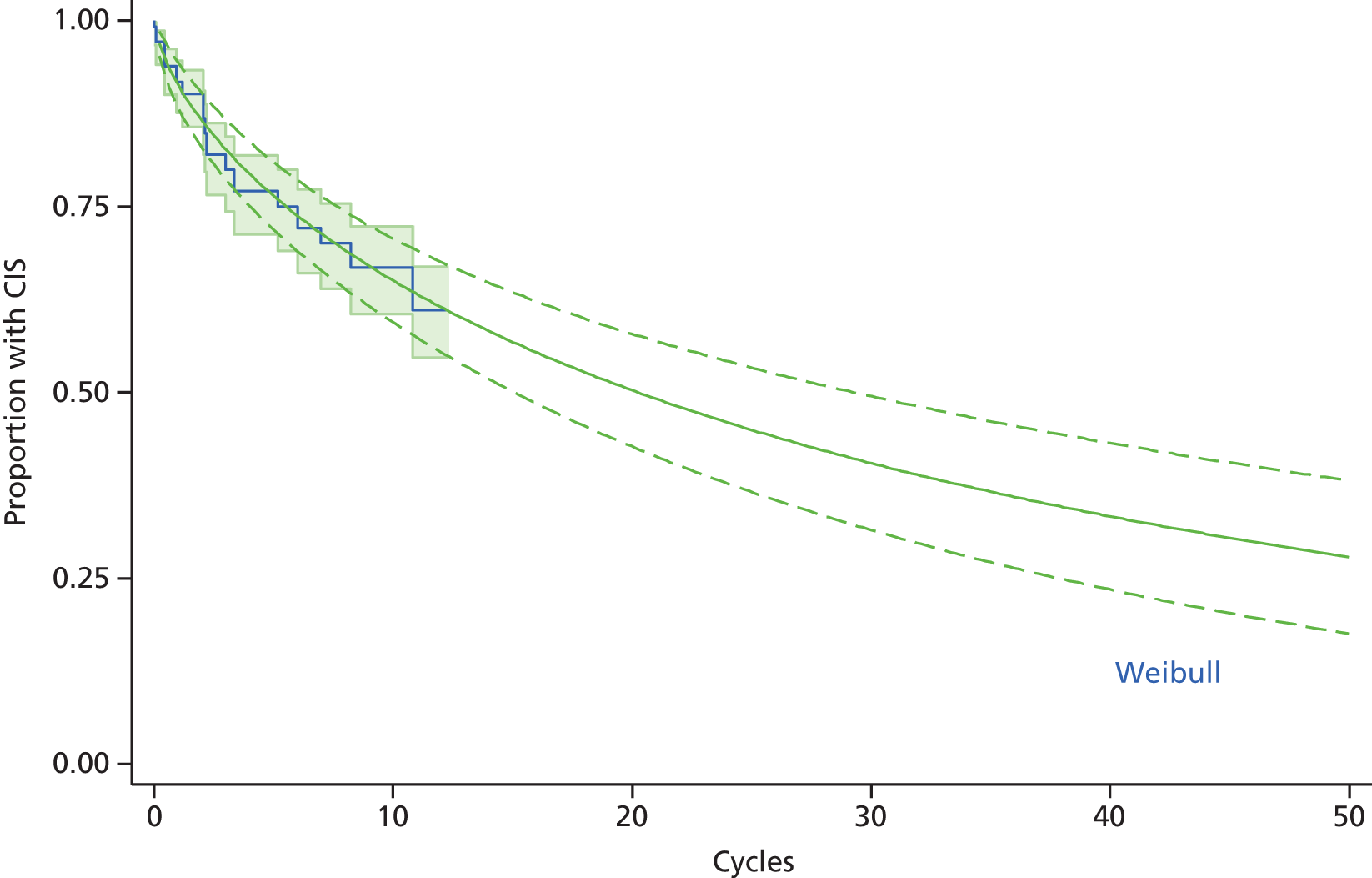
| Treatment | Base-case value | HR (95% CI) |
|---|---|---|
| BSC | Weibull (λ = 0.0906; γ = 0.6768) | – |
| IFN-β-1a 30 µg IM once a week (Avonex) | 0.516 (0.389 to 0.684) | |
| IFN-β-1a 44 µg SC three times per week (Rebif) | 0.480 (0.314 to 0.738) | |
| IFN-β-1b 250 µg every other day (Betaferon/Extavia) | 0.500 (0.36 to 0.699) | |
| GA 20 mg SC once daily (Copaxone) | 0.549 (0.397 to 0.762) |
Proportion of people discontinuing disease-modifying therapy
We included the annual proportion of people who discontinued DMTs as a result of AEs in the model. These proportions were derived from the CIS and RRMS studies included in our clinical review. Studies reported the instantaneous rate of people who discontinued treatment as a result of AEs. We converted this to an annual probability using the equation [probability = 1 – exp(–rt)], where r is rate and t is time. When discontinuation rates were not available from CIS studies, we used studies that followed up people with RRMS and assumed that the discontinuation rates would be applicable to those with CIS. Table 83 shows the proportions obtained from the studies and the annual probability of discontinuation for each DMT used in the base-case analysis.
| Parameter | Type of MS | Cumulative hazard (%) | Length of trial (years) | Annual probability | Reference |
|---|---|---|---|---|---|
| IFN-β-1a 30 µg IM once a week (Avonex) | RRMS | 4.4 | 2 | 0.0222 | Derived from Jacobs et al.172 |
| IFN-β-1a 44 µg SC three times per week (Rebif) | RRMS | 6.0 | 1.85 | 0.0330 | Derived from Mikol et al.192 |
| IFN-β-1b 250 µg every other day (Betaferon/Extavia) | CIS | 8.2 | 2 | 0.0419 | Derived from Kappos et al.171 |
| GA 20 mg SC once daily (Copaxone) | CIS | 5.8 | 3 | 0.0197 | Derived from Comi et al.174 |
Resource use and costs
The resource use and costs utilised were those directly incurred by the NHS and PSS. Resource use and costs were required for DMTs, drug administration and monitoring and CIS with no treatment. Unit costs are presented in Table 84 and resource use estimates used to derive costs are provided in Appendix 9.
| Parameter | Base-case value (£, 2015 prices) | Source |
|---|---|---|
| DMTs | ||
| IFN-β-1a 30 µg IM once a week (Avonex) | 8502 | BNF21 |
| IFN-β-1a 44 µg SC three times per week (Rebif) | 10,572 | BNF21 |
| IFN-β-1b 250 µg every other day (Betaferon/Extavia) | 7264 | BNF21 |
| GA 20 mg SC once daily (Copaxone) | 6704 | BNF21 |
| Monitoring costs | ||
| IFN-β-1a 30 µg IM once a week (Avonex) | 553.20 | Estimates of resource use obtained from clinical expert (see Appendix 9) and unit costs obtained from the BNF,21 NHS reference costs 2014/15285 and Curtis and Burns278 |
| IFN-β-1a 44 µg SC three times per week (Rebif) | 560.33 | |
| IFN-β-1b 250 µg every other day (Betaferon/Extavia) | 553.20 | |
| GA 20 mg SC once daily (Copaxone) | 553.20 | |
| Cost of subsequent monitoring | 323.77 | |
| Other costs | ||
| Drug administration | 225.00 | Assumption with regard to resource use information and unit costs from Curtis and Burns278 |
| Health state costs | ||
| CIS no treatment | 350.49 | Assumption with regard to resource use information and unit costs from Curtis and Burns278 and NHS reference costs 2014/15285 |
The costs of DMT were obtained from the BNF. 21 The annual cost of £8502 for treatment with IFN-β-1a (Avonex) was based on a dosage of 30 µg once a week. The annual cost of £10,572 for treatment with IFN-β-1a (Rebif) was based on a dosage of 44 µg three times per week. We derived annual costs of £7264 and £6704 for treatment with 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) and 20 mg of SC GA daily (Copaxone) respectively.
The costs of monitoring were derived based on clinical expert opinion for resource use, which was valued using NHS reference costs285 and information from Curtis and Burns. 278 Monitoring costs were derived for initiating treatment and for subsequent monitoring. We derived a cost of £553.20 for monitoring those who received treatment with 30 µg of IM IFN-β-1a once a week (Avonex), 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) and 20 mg of SC GA daily (Copaxone) during the first year of commencing treatment. We assumed that patients required visits to a neurologist and a MS nurse and received a series of blood tests and a MRI scan. For those who commenced treatment with 44 µg of SC IFN-β-1a three times per week (Rebif), we derived a cost of £560.33. This included the costs of the same resources used in the monitoring of other DMTs plus the cost of a thyroid function test. For subsequent monitoring, we derived a cost of £323.77 for all DMTs. For this we assumed that patients required visits to a neurologist and a MS nurse and received an annual MRI scan.
We calculated an annual cost of drug administration of £225. For this we assumed that a specialist nurse (community), employed at band 6 on the NHS Agenda for Change scale (£75 per hour of patient-related work), would spend 3 hours of contact time teaching patients how to self-administer DMTs.
Utility values
Health outcomes were measured in QALYs. In the model, we assigned the same utility values to all the CIS health states. For this we have derived a weighted utility value based on two pooled utility values by EDSS health states (MS Trust survey 2002 and 2005) and weighted by the proportion of individuals at each EDSS health state observed on entry to the RSS cohort. The disutility associated with AEs from DMTs was based on the estimates from Tappenden et al. 275 This was the approach used in the cost-effectiveness analysis of DMTs in RRMS. Table 85 shows the utility values used in the model.
| Parameter | Base-case value | Source |
|---|---|---|
| Health state utility value | ||
| CIS | 0.6218 | Assumption |
| Disutility associated with AEs | ||
| IFN-β-1a 30 µg IM once a week (Avonex) | –0.02 | Tappenden et al.275 |
| IFN-β-1a 44 µg SC three times per week (Rebif) | –0.02 | Tappenden et al.275 |
| IFN-β-1b 250 µg every other day (Betaferon/Extavia) | –0.02 | Tappenden et al.275 |
| GA 20 mg SC once daily (Copaxone) | –0.02 | Tappenden et al.275 |
Cost-effectiveness analysis
A Markov model was constructed and programmed to choose the base-case model inputs to assess the cost-effectiveness of various DMTs for the management of people with CIS. The model estimated the mean costs and health benefits associated with each DMT and assumed that the starting age of the population was 30 years. The analysis was undertaken from a NHS and PSS perspective and outcomes were reported as ICERs, expressed in terms of cost per QALY gained. All costs and outcomes were discounted at 3.5% per annum.
Sensitivity analyses
Deterministic sensitivity analyses were undertaken for the base-case results for the cost per QALY outcome measures:
-
SA1 – changing the time horizon to 20 years and 30 years
-
SA2 – assuming that 5% of people with CIS would discontinue treatment with DMTs.
In addition, we assessed the impact of varying key model input parameters on our base-case results.
Results of the cost-effectiveness analysis
Base-case cost-effectiveness analysis
In the base-case analysis, providing BSC for people with CIS and continuing BSC on conversion to RRMS was the least costly strategy, with a mean cost of approximately £136,800, and the least effective strategy, with a mean of 12.78 QALYs gained (Table 86). The strategy in which people with CIS receive treatment with 20 mg of SC GA daily (Copaxone) and then receive a DMT when they convert to RRMS dominated the 30 µg of IM IFN-β-1a once weekly (Avonex) and 44 µg of SC IFN-β-1a three times weekly (Rebif) treatment strategies. Excluding all dominated and extendedly dominated strategies, the optimal strategy was treatment with 20 mg of SC GA daily (Copaxone) for CIS followed by a DMT for those with progression to RRMS. In comparison to BSC, providing 20 mg of SC GA once daily (Copaxone) for patients with CIS and then a DMT on progression to RRMS was associated with an ICER of £16,500 per QALY gained.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC for CIS and RRMS | 136,800 | – | 12.78 | – | – |
| BSC for CIS and DMTs for RRMS | 176,400 | 39,600 | 13.16 | 0.38 | Extendedly dominated |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) for CIS and DMTs for RRMS | 216,800 | 80,000 | 16.85 | 3.69 | Extendedly dominated |
| GA 20 mg SC once daily (Copaxone) for CIS and DMTs for RRMS | 235,200 | 98,400 | 18.73 | 5.95 | 16,500 |
| IFN-β-1a 30 µg IM once a week (Avonex) for CIS and DMTs for RRMS | 252,100 | 16,900 | 18.57 | –0.16 | Dominated |
| IFN-β-1a 44 µg SC three times per week (Rebif) for CIS and DMTs for RRMS | 260,300 | 25,100 | 17.61 | –1.12 | Dominated |
SA1: Changing the time horizon to 20 years and 30 years
Tables 87 and 88 show the findings when the model was run over time horizons of 20 years and 30 years respectively. Over these shorter time horizons, treatment of CIS with 20 mg of SC GA daily (Copaxone) and then a DMT for those who progress to RRMS remained cost-effective, and the 30 µg of IM IFN-β-1a weekly (Avonex) and 44 µg of SC IFN-β-1a (Rebif) treatment strategies continued to be dominated by the 20 mg of SC GA daily (Copaxone) strategy.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC for CIS and RRMS | 155,100 | – | 10.33 | – | – |
| BSC for CIS and DMTs for RRMS | 166,400 | 21,600 | 10.73 | 0.40 | Extendedly dominated |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) for CIS and DMTs for RRMS | 181,600 | 33,600 | 11.99 | 1.66 | Extendedly dominated |
| GA 20 mg SC once daily (Copaxone) for CIS and DMTs for RRMS | 190,400 | 42,700 | 12.46 | 2.13 | 20,000 |
| IFN-β-1a 30 µg IM once a week (Avonex) for CIS and DMTs for RRMS | 204,100 | 13,400 | 12.39 | –0.07 | Dominated |
| IFN-β-1a 44 µg SC three times per week (Rebif) for CIS and DMTs for RRMS | 215,000 | 24,000 | 12.15 | –0.31 | Dominated |
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC for CIS and RRMS | 173,100 | – | 12.02 | – | – |
| BSC for CIS and DMTs for RRMS | 197,100 | 24,000 | 12.46 | 0.44 | Extendedly dominated |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) for CIS and DMTs for RRMS | 220,600 | 47,500 | 14.89 | 2.87 | Extendedly dominated |
| GA 20 mg SC once daily (Copaxone) for CIS and DMTs for RRMS | 234,700 | 61,600 | 15.88 | 3.86 | 16,000 |
| IFN-β-1a 30 µg IM once a week (Avonex) for CIS and DMTs for RRMS | 249,800 | 15,100 | 15.78 | –0.10 | Dominated |
| IFN-β-1a 44 µg SC three times per week (Rebif) for CIS and DMTs for RRMS | 259,300 | 24,600 | 15.28 | –0.60 | Dominated |
SA2: Assuming that 5% of people with clinically isolated syndrome discontinue treatment with disease-modifying therapies
Table 89 shows the findings when we assumed that approximately 5% of those treated with DMTs for CIS discontinue treatment every year. In this scenario, the 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) treatment strategy was cost-effective, with an ICER of £20,900 per QALY. Treatment with 20 mg of SC GA daily (Copaxone) followed by a DMT for those progressing to RRMS remained cost-effective. The 30 µg of IM IFN-β-1a weekly (Avonex) treatment strategy continued to be dominated and the 44 µg of SC IFN-β-1a three times weekly (Rebif) treatment strategy was associated with an extremely high ICER.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC for CIS and RRMS | 136,800 | – | 12.78 | – | – |
| BSC for CIS and DMTs for RRMS | 176,400 | 39,600 | 13.16 | 0.38 | Extendedly dominated |
| GA 20 mg SC once daily (Copaxone) for CIS and DMTs for RRMS | 209,800 | 73,000 | 16.22 | 3.44 | Extendedly dominated |
| IFN-β-1b 250 µg SC every other day (Betaferon/Extavia) for CIS and DMTs for RRMS | 211,500 | 74,700 | 16.36 | 3.58 | 20,900 |
| IFN-β-1a 30 µg IM once a week (Avonex) for CIS and DMTs for RRMS | 224,700 | 13,200 | 16.31 | –0.05 | Dominated |
| IFN-β-1a 44 µg SC three times per week (Rebif) for CIS and DMTs for RRMS | 242,300 | 30,800 | 16.41 | 0.05 | 616,000 |
In Figure 34 we present graphically the impact of varying key model input parameters on the cost-effectiveness results. To determine the robustness of the results, we varied the utility value for the CIS health state and the probability of treatment discontinuation, as well as the mode of drug administration, the disutility associated with AEs and the annual cost of BSC. The results show that the model was most sensitive to a ±10% change in the utility of the CIS health state. A 10% increase in the health state utility of CIS would give a value of 0.6898. However, this would still give an ICER for 20 mg of SC GA daily (Copaxone) compared with BSC of £18,600, well within the normal expected levels of willingness to pay.
FIGURE 34.
Tornado diagram for 20 mg of SC GA daily compared with BSC.
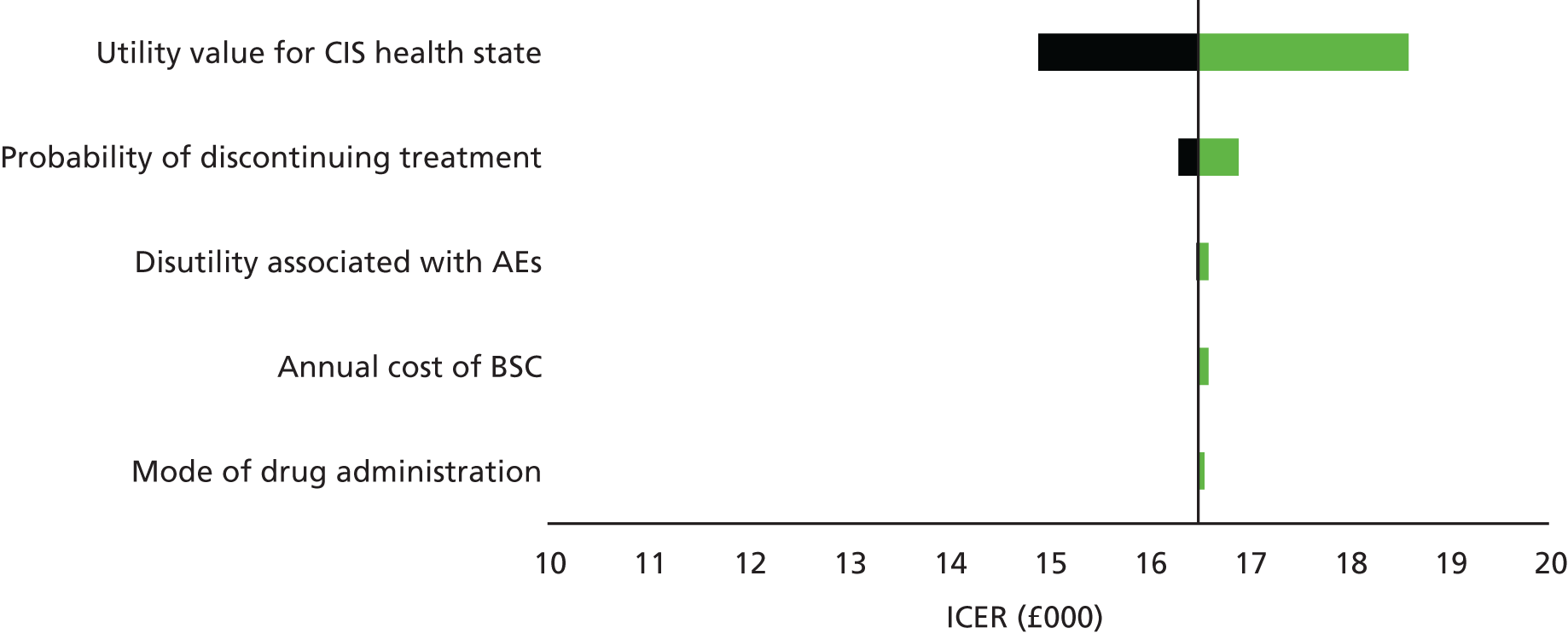
Discussion of the economic assessment of disease-modifying therapies for clinically isolated syndrome
Summary of the results
Having estimated the treatment effect of each DMT on conversion to RRMS, we then assessed the cost-effectiveness of DMTs in people who were diagnosed with CIS in the absence of evidence for RRMS on a MRI scan. We developed a decision-analytic model, taking the NHS and PSS perspective, and presented outcomes in terms of cost per QALY gained. We considered six strategies in our analysis, which included treatment with BSC in addition to the DMTs available for people with CIS. The base-case deterministic results showed that treating people with 20 mg of SC GA daily (Copaxone) followed by a DMT on conversion to RRMS dominated the 30 µg of IM IFN-β-1a once a week (Avonex) and 44 µg of SC IFN-β-1a three times per week (Rebif) treatment strategies. We found that the 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) treatment strategy was extendedly dominated and, although it was cost-effective in comparison to BSC, the ICER was higher than that for the 20 mg of SC GA once daily (Copaxone) treatment strategy. Excluding all dominated strategies, the ICER for 20 mg of SC GA once daily (Copaxone) for patients with CIS and then a DMT on progression to RRMS was approximately £16,500 per QALY gained.
The sensitivity analysis showed that treatment of CIS with 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) followed by a DMT on progression to RRMS would also be a cost-effective option if discontinuation rates for all of the drug treatments were comparable. The sensitivity analysis did not suggest that the 30 µg of IM IFN-β-1a once a week (Avonex) treatment strategy or the 44 µg of SC IFN-β-1a three times per week (Rebif) treatment strategy was a cost-effective option in the UK. The results further showed that the model is likely to be sensitive to the utility associated with the CIS health state and to the treatment discontinuation rate while in the CIS state.
Strengths and limitations
Our analysis had several strengths. We built a de novo model for CIS and we were able to incorporate evidence from our systematic review of clinical effectiveness. We also incorporated long-term costs and consequences of progressing to, and receiving DMTs for, RRMS. We also used evidence from the RSS observational cohort to model the effect of conversion to RRMS.
However, our analysis was limited in several important ways. We did not undertake probabilistic sensitivity analysis. Moreover, because of the paucity of HRQoL information in people with CIS, we assumed CIS to be comparable to early-phase RRMS. However, we investigated the effect of varying this input parameter by ±10% on the cost-effectiveness results and found that the ICERs were still well within the expected levels of willingness to pay. Finally, our findings from the clinical effectiveness review relied on a population diagnosed with CIS before the revised 2010 McDonald criteria54 reclassified many who would have previously been classified as having CIS as in fact having RRMS.
Conclusions
Our cost-effectiveness findings suggest that, in people with CIS, it would be cost-effective to start DMTs. We found that, of the evaluated DMTs, 20 mg of SC GA daily (Copaxone) was the optimal choice. Greater understanding around discontinuation rates of DMTs in CIS patients would be valuable, as these may impact on whether or not 250 µg of SC IFN-β-1b every other day (Betaferon/Extavia) is also a cost-effective option. The results are presented in the light of some limitations/uncertainty, mainly around the utility values for the CIS health state and disutilities associated with AEs. Our analyses drew on utility values obtained from people with RRMS and, because of the complexity of the modelling approach and lack of data, we were unable to quantify this uncertainty by undertaking probabilistic sensitivity analysis. Until more reliable information on utility values becomes available, these results should be interpreted with caution.
Chapter 13 Discussion
Summary
Clinical effectiveness
We systematically reviewed and synthesised the evidence relating to the effectiveness of IFNs and GA within their marketing authorisations for CIS, RRMS and SPMS. We exhaustively searched databases to update previous high-quality reviews for each of these MS types and used standard systematic review methodology to select, appraise and extract data from relevant studies. Our search identified 35 primary studies: five in CIS, 27 in RRMS, of which 24 reported clinical effectiveness outcomes of interest, and three in SPMS. We synthesised the findings from these trials narratively and, when appropriate, using pairwise meta-analyses and NMAs. Across MS types, studies were variable in quality. Most studies were sponsored by the manufacturers of the DMTs. We also judged that many studies were at high risk of unblinding of participants and personnel because of injection site reactions, with potential implications for the blinding of outcome assessors. Many trials, especially of head-to-head comparisons, were open-label trials.
The clinical effectiveness evidence suggested that IFNs and GA were effective for key outcomes and across MS types and there was little evidence from the NMAs that any one drug was superior to any other for different clinical outcomes. In CIS, each drug included showed evidence of delaying time to CDMS. In RRMS, drugs showed good evidence of reducing the relapse rate, including the rate of moderate or severe relapses and, in most cases, the rate of steroid-treated relapses. Most drugs delayed disability progression confirmed at 3 months, although the findings were less consistent for disability progression confirmed at 6 months. Finally, in SPMS, all drugs reduced the relapse rate, although the network was sparse and relied on three studies. Time to confirmed disability progression at 3 months was measured in only two studies, which showed variable effects across treatments. We undertook analyses of discontinuation following AEs in RRMS and SPMS. These analyses, which were intended to be indicative, did not offer evidence that one drug was more likely than any other to result in discontinuation following an AE.
We synthesised the findings for additional outcomes in the scope141 (MS symptoms, HRQoL and freedom from disease activity) narratively but were unable to undertake meta-analyses because of heterogeneity, sparsity of data and poor reporting for these outcomes. The findings suggested a generally beneficial effect of DMTs on freedom from disease activity, but findings for MS symptoms and HRQoL were poorly reported and inconsistent. Additionally, no studies reported discontinuation because of loss of effect attributed to NABs.
Cost-effectiveness
As part of our assessment of cost-effectiveness, we undertook four related work packages. First, we systematically reviewed, appraised and synthesised the recent cost-effectiveness evidence on DMTs for people with CIS and MS. Second, we critically appraised the year 10 RSS economic model, including checking the model and reviewing inputs to and assumptions made in the model. Third, we assessed the cost-effectiveness of DMTs for the treatment of RRMS. Fourth, we assessed the cost-effectiveness of DMTs for the treatment of CIS. We assessed cost-effectiveness using a modified RSS model, with clinical effectiveness inputs in the base case derived from the year 10 RSS analyses. We conducted several additional analyses: (1) using pooled estimates of the effectiveness of on-scheme DMTs from our systematic review of clinical effectiveness, (2) using estimates of the effectiveness of each DMT from our systematic review of clinical effectiveness and (3) using estimates of the effectiveness of each DMT from manufacturer submissions.
We identified 10 studies in RRMS and nine studies in CIS that reported evidence on a decision model used to estimate the cost-effectiveness of DMTs. In general, most studies used appropriate model structures to capture/simulate disease progression. According to best practice for reporting cost-effectiveness analyses, all studies performed satisfactorily in terms of outlining the decision problem, stating the perspective of the analysis, adhering to the scope of the model and outlining the structural assumptions. However, there were some limitations of these studies. First, we consider the time horizon to be short in some studies and these analyses may not have captured the full costs and benefits of DMTs. Second, the choice of model structure in several studies did not accurately reflect disability progression associated with MS. Third, the authors did not provide sufficient details on the meta-analytical methods used to estimate the treatment effects of DMTs or sufficient details on how treatment effects had been extrapolated beyond the trial time horizons.
We considered the RSS model to be appropriate for estimating the cost-effectiveness of DMTs compared with BSC. The model drew on the best available evidence on disease progression, resource use and costs and utility values. However, our appraisal highlighted concerns with the RSS model relating to mortality, carers’ disutilities, discontinuation rates and how the ARR was estimated.
In our base-case assessment of the cost-effectiveness of DMTs for RRMS, our results suggested that it is not cost-effective to treat people who have RRMS with DMTs. Using as our base case the RSS model with modifications made to the assumptions relating to mortality and carers’ disutilities, we found that DMTs were more costly and more effective than BSC, with an ICER of approximately £33,800 per QALY gained. We also used pooled estimates derived from our clinical effectiveness review for all on-scheme DMTs, which showed that, although DMTs were more costly than BSC, they also produced more QALYs, with an ICER of approximately £12,800 per QALY gained. When we compared each DMT, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) appeared to be the most cost-effective treatment, but clinical effectiveness estimates for this drug were based on one trial with 1 year of follow-up. Results from the probabilistic sensitivity analysis conducted on the RSS data showed that, at a willingness-to-pay threshold of £30,000 per QALY gained, DMTs had a probability of being cost-effective of 0.23.
We also assessed the cost-effectiveness of DMTs for CIS. Our base-case analysis suggested that treatment with 20 mg of SC GA daily (Copaxone) followed by a DMT on progression to RRMS was cost-effective relative to BSC, with an ICER of £16,500 per QALY gained, and dominated all other strategies in the base case.
Strengths and limitations
Study searches, inclusion and exclusion criteria and study selection
We used a rigorous and exhaustive search strategy to locate primary studies, including updating high-quality systematic reviews. Additionally, we used auditable and transparent methods to select and synthesise studies. When appropriate, we undertook post hoc sensitivity analyses in our clinical effectiveness study to check the robustness of our findings.
A limitation of our work, inherent to all systematic reviews, is publication bias. Methods for detecting publication bias in NMAs are still in development and we did not have enough studies for any one comparison to test for small-study bias. This may be especially relevant as many of the early trials of IFNs and GA for MS were small trials.
Another important limitation was the selective and inconsistent reporting of outcomes. For example, one of the reasons that we did not undertake a meta-analysis of time to first relapse estimates was the inconsistent and often poor reporting, especially across multiple reports of the same study, which prevented imputation of HRs. This was especially a problem for the findings relating to MS symptoms and QoL in individual trials, with the findings often reported as significance thresholds (e.g. p < 0.05 or p > 0.05), without reporting of the magnitude of the effects.
Finally, we elected to include only studies and arms of studies examining interventions within their marketing authorisations, that is, we did not include study arms examining additional, non-licensed doses of the study drugs. Although this means that our analysis perhaps more closely represents clinical practice today, it does mean that additional information on the effectiveness of these drugs was not included in the analysis. Moreover, because our scope was limited to IFNs and GA, we could not include information on additional newer drugs. This was a limitation in that additional trials would have strengthened the resultant study networks analysed (see the following section).
Synthesis methods and statistical analyses of clinical effectiveness
For most outcomes we were able to complement narrative syntheses with pairwise and NMAs, but this was not always possible (e.g. magnitude of EDSS change in RRMS or relapse severity in SPMS).
Our analyses also had several statistical advantages. In examining the effect of IFNs and GA on disability progression, we used time-to-event outcomes and HRs instead of calculating risk ratios or ORs at different follow-up points. Thus, trial findings were reported at their fullest ‘maturity’167 and all relevant data were included. Although HRs are not immune to selection bias, they may be less likely than relative risks to depend on the time points chosen in the analysis.
Related to our decision to use HRs, we were able to use the full complement of methods to estimate effect sizes from available study-level data. This meant that more studies were included in our analyses than would otherwise have been the case. However, this may also be a limitation in that indirect methods (e.g. integrating underneath the survivor function to estimate the cumulative hazard) are not preferable to direct estimates of intervention effects.
Our decision to estimate NMAs with effects for relapse rate, relapse severity and time to confirmed disability progression across time points was justified in that RRs for relapses account for person-years and thus, under an assumption of a constant rate, should not depend on time to follow-up. Similarly, HRs represent ‘instantaneous’ risk and thus, under a proportional hazards assumption, should not depend on time to follow-up. However, this decision is not without its drawbacks. On the one hand, we were unable to verify empirically whether HRs and RRs were time varying because of few comparisons on every node of the study networks. On the other hand, we judged that stratifying analyses by time to follow-up would have resulted in excessively sparse networks that would have been difficult to interpret collectively. Thus, our decision to pool study estimates across follow-up times for analyses of clinical outcomes was both a strength and a potential limitation. Notably, we did stratify analyses by time to follow-up in NMAs of discontinuations as a result of AEs because we judged that the only feasible estimator in these analyses was the risk ratio.
Finally, one issue inherent to the clinical effectiveness evidence was that different sources of bias were spread differentially throughout the networks. Most notably, trials involving an active drug compared with an active drug in RRMS were frequently open label in design and thus participants were aware of the drugs that they were receiving. This might have posed a greater risk to the unblinding of outcome assessors than in ostensibly double-blinded trials.
Synthesis methods and statistical analyses of cost-effectiveness
One strength of our cost-effectiveness analyses was the considerable effort made to identify the best available evidence on model input parameters and model structure. In addition, several of our analyses were based on estimates derived from our systematic review and NMAs of clinical effectiveness, which were themselves based on rigorous searches and analysis. We also appraised the RSS model and were then able to modify the assumptions that we found concerning. Our extensive sensitivity analyses, both deterministic and probabilistic, allowed us to explore a variety of data sources. Finally, we were able to develop a de novo model structure for a hypothetical cohort of people with CIS.
However, one limitation of the analyses undertaken using data from the NMAs is that they at times relied on sparse networks with an uneven risk of bias throughout the networks. For example, analyses relating to 125 µg of SC pegIFN-β-1a (Plegridy) relied on one trial that was not connected to any other trials except by a placebo comparator. Thus, any issues with the estimates derived from our review of clinical effectiveness would have been propagated through the analysis of cost-effectiveness.
Another limitation was the difficulty of estimating uncertainty for key parameters in the RSS model. In conducting our probabilistic sensitivity analysis based on our modified RSS model, we used uncertainty estimates for the ARRs derived from the clinical effectiveness review rather than from the RSS itself.
Additionally, our findings were restricted to IFNs and GA. It is possible that other RRMS or CIS treatments may be more cost-effective.
Choice of the base case for the economic analysis
As noted above, we used a modified version of the RSS model as our base case. Although cost-effectiveness estimates derived from the RSS model and from the review of clinical effectiveness evidence have comparative strengths and weaknesses, we decided on balance that estimates from the RSS model provided the best estimates of cost-effectiveness. Although the RSS model relied on a historical (i.e. non-contemporaneous) comparator, and thus non-randomised evidence likely to be prone to selection bias, we believe that the long-term follow-up, relevance to the NHS and to current clinical practice and rigorous methods used in collecting and reporting data made it the best choice for the base case. In contrast, although the estimates from our review of clinical effectiveness were derived from randomised evidence, the predominantly short-term nature of the included trials, the high risk of other biases (including as a result of manufacturer sponsorship and from open-label active drug vs. active drug trials), the imbalance of these risks of bias across the networks of evidence and the sparseness of the evidence for some DMTs raised doubts about its value for the base case. Although both sources of evidence were at high risk of bias, we believe that the RSS model best represented a relevant base case for MS treatment in the NHS.
Views of patients and carers
The submission from the RSS supports the use of DMTs for MS, including the use of IFNs and GA, based on the results of the RSS, clinical trial data and research on perspectives gathered by the MS Society. These perspectives included several patient case studies reporting that DMTs had significantly reduced or prevented relapses and symptoms, enabling patients to lead more independent active lifestyles. The treatment had improved their mental health by reducing their fear of future relapses and increasing feelings of confidence and control. The MS Society noted that DMTs promote patient choice by allowing individuals to weigh up lower-risk moderate-efficacy treatments compared with higher-risk and higher-efficacy treatments. The range of treatment options allows for the differential way that MS can affect individuals and their differential responses to DMTs.
Previous research
Our findings updated a number of previous reviews, although the comparability of the findings is limited. Compared with the review by Clerico et al. ,158 the key review that we used for analyses of CIS, we included only trials reporting the use of IFNs and GA within their marketing authorisations. We also included several trials published after the publication of their review. 173–175 We were also able to use NMAs for time to CDMS to examine the relative effectiveness of drugs. Our findings substantially update their review and provide additional evidence of the effectiveness of IFNs and GA for CIS.
Compared with the review by Tramacere et al. ,159 which broadly examined immunomodulators and immunosuppressants for RRMS, we included only trials examining IFNs and GA against each other and against a no-treatment comparator and only doses and formulations within the marketing authorisations. Because Tramacere et al. 159 included studies across different dosages, drug classes and indications and because they used risk ratios as the sole outcome estimator, our analyses and theirs are largely incommensurate. However, our analyses of treatment discontinuation as a result of AEs agreed with their analyses in that neither review suggested that any one drug had a significant effect on discontinuation as a result of AEs relative to placebo.
Implications for practice
We did not include formulations outside the recommended usage in the UK. In addition, our study was specifically designed to exclude the clinical effectiveness and cost-effectiveness of newer MS treatments such as newer monoclonal antibodies (alemtuzumab, daclizumab). This review should be considered in conjunction with newer NICE and other guidance on the clinical effectiveness and cost-effectiveness of these agents.
Our findings agree with the Association of British Neurologists classification of IFNs and GA as drugs of ‘moderate efficacy’. 281 Our analysis does suggest that these drugs are effective in controlling the relapse rate and disability progression.
Protocol variations
We originally presented our protocol at a stakeholder information meeting and subsequently registered this protocol on the PROSPERO database (registration number CRD42016043278). Our methods differed slightly from those detailed in the protocol in the following ways.
In the clinical effectiveness systematic review we did not use data from the RSS as prior distributions in a Bayesian meta-analysis. This was because of the mismatch between the time to follow-up in the trials and the time to follow-up in the year 10 RSS data and the different analytical methods used between the trials and the RSS analyses. Subsequently, we did not use Bayesian methodology in our NMA models. We also decided to exclude trials that examined only IFN or GA doses outside the marketing authorisation. Finally, we did not search the database Current Controlled Trials as this would have duplicated searches already carried out.
Although these were not strictly variations from our protocol, we subsequently refined our definitions of several outcomes. We operationalised relapse severity as the RRs of relapses graded as moderate or severe or as the RRs of relapses requiring steroid treatment. We also took advice from our clinical consultants and examined combined clinical–MRI outcomes for freedom from disease activity.
Recommendations for future research
One key flaw in the assembled clinical effectiveness evidence was the lack of long-term follow-up. The RSS was designed to collect longer-term observational data in this area; however, a large-scale, longitudinal RCT comparing active first-line agents would contribute meaningfully towards resolving uncertainty about the relative benefits of different IFN or GA formulations. We note that the submission from the MS Society identified a similar research priority. It may be that using blinded adjudicator panels for relapses and disease progression could attenuate the risk of bias accruing in an open-label trial. Because of this lack of long-term follow-up, DMT trials are generally not informative on whether drugs delay progression to SPMS.
There is also a need to reach consensus on the different stages of MS, the distinctiveness of which are open to question. Related to this, there is a need to understand how changing imaging technologies and changes in clinical practice (e.g. changes in the classification of CIS under new diagnostic criteria) impact on diagnosis and management. From an epidemiological perspective, a priority for research should be to understand how and under what circumstances MS progresses through different types (e.g. from CIS to RRMS and then to SPMS). We note that the submission from the MS Society identified a similar research priority. Related to this, there is a need to develop outcomes that meaningfully reflect MS symptoms, such as disability progression. Many have enumerated the issues with the EDSS, and it is possible that time to progression sustained at 3 months does not reliably capture disability progression, given the variable time for recovery from relapses.
The scope of this assessment was limited to IFNs and GA and thus we did not include more recently approved DMTs, such as oral agents or monoclonal antibodies, that are increasingly used as first-line treatment for patients with RRMS. Therefore, future research comparing the clinical effectiveness and cost-effectiveness of IFNs and GA with those of these new agents could be considered to better reflect the therapeutic options available to patients within the NHS.
Another priority for research is to focus on patients who are not on the lower end of the EDSS. This may be of value for populations with MS as survival rates improve and advances are made in terms of support and aids for those with disabilities.
Additionally, valuation of health benefits continues to be a vexing area for MS. This was an issue identified in the original guidance resulting from TA32. 24 One possible way to address this issue is through systematic review and metasynthesis of qualitative studies relating to the lived experience of MS, with particular attention given to the dominant clinical features, for example relapse and disability progression. This could provide a basis for an understanding of the relevant health states and benefits that more closely matches the preferences and experiences of people living with the target condition.
Finally, above and beyond the population-average evidence that DMTs reduce the relapse rate, there is a need to understand who responds best to DMTs, especially who does not respond to IFNs or GA early on, to enable more targeted therapeutic decisions to be made. Although several trials included in our clinical effectiveness review used subgroup analyses based, for example, on presenting lesions or demographic characteristics, a more fine-grained understanding could help patients and clinicians make better-informed decisions.
Acknowledgements
The authors gratefully acknowledge the assistance of Ms Karoline Munro and Dr Martin Connock. The authors also acknowledge the work and assistance of Charles Dobson and the RSS Steering Group.
Contributions of authors
GJ Melendez-Torres co-ordinated the project, led the review of clinical effectiveness and led the drafting of the report.
Peter Auguste led the review of cost-effectiveness, the critique of the RSS submission and the economic modelling and contributed to drafting of the report.
Xavier Armoiry co-led the review of clinical effectiveness and contributed to the drafting of the report.
Hendramoorthy Maheswaran contributed to the economic evaluation work and the drafting of the report.
Rachel Court contributed to the reviews of clinical effectiveness and cost-effectiveness through search and information specialist support and to the drafting of the report.
Jason Madan contributed to the economic evaluation work and the drafting of the report.
Alan Kan contributed to the review of clinical effectiveness and to the drafting of the report.
Stephanie Lin contributed to the review of clinical effectiveness and to the drafting of the report.
Carl Counsell contributed as a clinical expert and to the drafting of the report.
Jacoby Patterson contributed to the review of clinical effectiveness and to the drafting of the report.
Jeremy Rodrigues contributed to the review of clinical effectiveness and to the drafting of the report.
Olga Ciccarelli contributed as a clinical expert and to the drafting of the report.
Hannah Fraser contributed to the drafting of the report.
Aileen Clarke supervised the project and contributed to the drafting of the report.
Data sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Ebers GC, Bulman DE, Sadovnick AD, Paty DW, Warren S, Hader W, et al. A population-based study of multiple sclerosis in twins. N Engl J Med 1986;315:1638-42. https://doi.org/10.1056/NEJM198612253152603.
- Mumford CJ, Wood NW, Kellar-Wood H, Thorpe JW, Miller DH, Compston DA. The British Isles survey of multiple sclerosis in twins. Neurology 1994;44:11-5. https://doi.org/10.1212/WNL.44.1.11.
- Sadovnick AD, Armstrong H, Rice GP, Bulman D, Hashimoto L, Paty DW, et al. A population-based study of multiple sclerosis in twins: update. Ann Neurol 1993;33:281-5. https://doi.org/10.1002/ana.410330309.
- Granieri E, Casetta I, Tola MR, Ferrante P. Multiple sclerosis: infectious hypothesis. Neurol Sci 2001;22:179-85. https://doi.org/10.1007/s100720170021.
- Lassmann H, Niedobitek G, Aloisi F, Middeldorp JM. NeuroproMiSe EBV Working Group . Epstein–Barr virus in the multiple sclerosis brain: a controversial issue – report on a focused workshop held in the Centre for Brain Research of the Medical University of Vienna, Austria. Brain 2011;134:2772-86. https://doi.org/10.1093/brain/awr197.
- Owens GP, Bennett JL. Trigger, pathogen, or bystander: the complex nexus linking Epstein–Barr virus and multiple sclerosis. Mult Scler 2012;18:1204-8. https://doi.org/10.1177/1352458512448109.
- Pohl D. Epstein–Barr virus and multiple sclerosis. J Neurol Sci 2009;286:62-4. https://doi.org/10.1016/j.jns.2009.03.028.
- Radić M, Martinović Kaliterna D, Radić J. Infectious disease as aetiological factor in the pathogenesis of systemic sclerosis. Neth J Med 2010;68:348-53.
- Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O’Riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry 2014;85:76-84. https://doi.org/10.1136/jnnp-2013-305450.
- Confavreux C, Vukusic S. The natural history of multiple sclerosis. Rev Prat 2006;56:1313-20. https://doi.org/10.1016/b978-0-443-07271-0.50006-9.
- National Multiple Sclerosis Society . Clinically Isolated Syndrome (CIS) n.d. www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/diagnosing-ms/cis/index.aspx (accessed 23 October 2015).
- MS-UK . Choices: Types of MS 2014. www.ms-uk.org/files/choices_types.pdf (accessed 4 November 2015).
- Multiple Sclerosis Trust . Types of MS: Rapidly Evolving Severe Relapsing Remitting MS 2014. www.mstrust.org.uk/a-z/types-ms (accessed 4 November 2015).
- Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol 2007;6:903-12. https://doi.org/10.1016/S1474-4422(07)70243-0.
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907-11. https://doi.org/10.1212/WNL.46.4.907.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-52. https://doi.org/10.1212/WNL.33.11.1444.
- Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc 2014;89:225-40. http://dx.doi.org/10.1016/j.mayocp.2013.11.002.
- Runkel L, Meier W, Pepinsky RB, Karpusas M, Whitty A, Kimball K, et al. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta). Pharm Res 1998;15:641-9. https://doi.org/10.1023/A:1011974512425.
- Zhang J, Hutton G, Zang Y. A comparison of the mechanisms of action of interferon beta and glatiramer acetate in the treatment of multiple sclerosis. Clin Ther 2002;24:1998-2021. https://doi.org/10.1016/S0149-2918(02)80094-7.
- Plosker GL. Interferon-β-1b: a review of its use in multiple sclerosis. CNS Drugs 2011;25:67-88. http://dx.doi.org/10.2165/11206430-000000000-00000.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Teva Pharmaceuticals Ltd . Copaxone 20 mg Ml, Solution for Injection, Pre-Filled Syringe. Summary of Product Characteristics n.d. www.medicines.org.uk/emc/medicine/17516#PHARMACODYNAMIC_PROPS (accessed 21 April 2017).
- La Mantia L, Munari LM, Lovati R. Glatiramer acetate for multiple sclerosis. Cochrane Database Syst Rev 2010;5. http://dx.doi.org/10.1002/14651858.CD004678.pub2.
- National Institute for Health and Care Excellence . Beta Interferon and Glatiramer Acetate for the Treatment of Multiple Sclerosis 2002. www.nice.org.uk/Guidance/TA32 (accessed 15 May 2016).
- Trapp BD, Nave K-A. Multiple sclerosis: an immune or neurodegenerative disorder?. Annu Rev Neurosci 2008;31:247-69. https://doi.org/10.1146/annurev.neuro.30.051606.094313.
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278-85. http://dx.doi.org/10.1056/NEJM199801293380502.
- Minagar A, Granger DN, Granger JP. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. San Rafael, CA: Morgan & Claypool Life Sciences; 2014.
- Bjartmar C, Kinkel RP, Kidd G, Rudick RA, Trapp BD. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology 2001;57:1248-52. https://doi.org/10.1212/WNL.57.7.1248.
- Gourraud PA, Harbo HF, Hauser SL, Baranzini SE. The genetics of multiple sclerosis: an up-to-date review. Immunol Rev 2012;248:87-103. http://dx.doi.org/10.1111/j.1600-065X.2012.01134.x.
- Jersild C, Fog T, Hansen GS, Thomsen M, Svejgaard A, Dupont B. Histocompatibility determinants in multiple sclerosis, with special reference to clinical course. Lancet 1973;2:1221-5. https://doi.org/10.1016/S0140-6736(73)90970-7.
- Naito S, Namerow N, Mickey MR, Terasaki PI. Multiple sclerosis: association with HL-A3. Tissue Antigens 1972;2:1-4. https://doi.org/10.1111/j.1399-0039.1972.tb00111.x.
- Sawcer S, Hellenthal G, Pirinen M, Spencer CC, . Wellcome Trust Case Control Consortium . Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011;476:214-19. https://doi.org/10.1038/nature10251.
- Lincoln MR, Montpetit A, Cader MZ, Saarela J, Dyment DA, Tiislar M, et al. A predominant role for the HLA class II region in the association of the MHC region with multiple sclerosis. Nat Genet 2005;37:1108-12. https://doi.org/10.1038/ng1647.
- Oksenberg JR, Barcellos LF, Cree BA, Baranzini SE, Bugawan TL, Khan O, et al. Mapping multiple sclerosis susceptibility to the HLA-DR locus in African Americans. Am J Hum Genet 2004;74:160-7. https://doi.org/10.1086/380997.
- Schmidt H, Williamson D, Ashley-Koch A. HLA-DR15 haplotype and multiple sclerosis: a HuGE review. Am J Epidemiol 2007;165:1097-109. https://doi.org/10.1093/aje/kwk118.
- Muñoz-Culla M, Irizar H, Otaegui D. The genetics of multiple sclerosis: review of current and emerging candidates. Appl Clin Genet 2013;6:63-7. http://dx.doi.org/10.2147/TACG.S29107.
- Willer CJ, Dyment DA, Risch NJ, Sadovnick AD, Ebers GC. Canadian Collaborative Study Group . Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci USA 2003;100:12877-82. http://dx.doi.org/10.1073/pnas.1932604100.
- Baranzini SE, Mudge J, van Velkinburgh JC, Khankhanian P, Khrebtukova I, Miller NA, et al. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature 2010;464:1351-6. http://dx.doi.org/10.1038/nature08990.
- Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol 2015;14:263-73. http://dx.doi.org/10.1016/S1474-4422(14)70267-4.
- Warner HB, Carp RI. Multiple sclerosis and Epstein–Barr virus. Lancet 1981;2. https://doi.org/10.1016/S0140-6736(81)91527-0.
- Goodin DS. The causal cascade to multiple sclerosis: a model for MS pathogenesis. PLOS ONE 2009;4. http://dx.doi.org/10.1371/journal.pone.0004565.
- Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernán MA, Olek MJ, et al. Epstein–Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA 2001;286:3083-8. https://doi.org/10.1001/jama.286.24.3083.
- Pakpoor J, Disanto G, Gerber JE, Dobson R, Meier UC, Giovannoni G, et al. The risk of developing multiple sclerosis in individuals seronegative for Epstein–Barr virus: a meta-analysis. Mult Scler 2013;19:162-6. http://dx.doi.org/10.1177/1352458512449682.
- Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P, et al. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J Exp Med 2007;204:2899-912. https://doi.org/10.1084/jem.20071030.
- Carlyle IP. Multiple sclerosis: a geographical hypothesis. Med Hypotheses 1997;49:477-86. https://doi.org/10.1016/S0306-9877(97)90065-7.
- Esparza ML, Sasaki S, Kesteloot H. Nutrition, latitude, and multiple sclerosis mortality: an ecologic study. Am J Epidemiol 1995;142:733-7.
- Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 2007;69:381-8. https://doi.org/10.1212/01.wnl.0000268266.50850.48.
- Resch J. Geographic distribution of multiple sclerosis and comparison with geophysical values. Soz Praventivmed 1995;40:161-71. https://doi.org/10.1007/BF01318637.
- Rosen LN, Livingstone IR, Rosenthal NE. Multiple sclerosis and latitude: a new perspective on an old association. Med Hypotheses 1991;36:376-8. https://doi.org/10.1016/0306-9877(91)90014-P.
- Simpson S, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatr 2011;82:1132-41. http://dx.doi.org/10.1136/jnnp.2011.240432.
- Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010;9:520-32. http://dx.doi.org/10.1016/S1474-4422(10)70064-8.
- Duan S, Lv Z, Fan X, Wang L, Han F, Wang H, et al. Vitamin D status and the risk of multiple sclerosis: a systematic review and meta-analysis. Neurosci Lett 2014;570:108-13. http://dx.doi.org/10.1016/j.neulet.2014.04.021.
- Wattjes MP, Barkhof F. High field MRI in the diagnosis of multiple sclerosis: high field-high yield?. Neuroradiology 2009;51:279-92. http://dx.doi.org/10.1007/s00234-009-0512-0.
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7. https://doi.org/10.1002/ana.1032.
- Ge Y. Multiple sclerosis: the role of MR imaging. AJNR Am J Neuroradiol 2006;27:1165-76.
- Okuda DT, Mowry EM, Beheshtian A, Waubant E, Baranzini SE, Goodin DS, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 2009;72:800-5. https://doi.org/10.1212/01.wnl.0000335764.14513.1a.
- Nusbaum AO, Lu D, Tang CY, Atlas SW. Quantitative diffusion measurements in focal multiple sclerosis lesions: correlations with appearance on TI-weighted MR images. AJR Am J Roentgenol 2000;175:821-5. https://doi.org/10.2214/ajr.175.3.1750821.
- Honce JM. Gray matter pathology in MS: neuroimaging and clinical correlations. Mult Scler Int 2013;2013. http://dx.doi.org/10.1155/2013/627870.
- Lisanti CJ, Asbach P, Bradley WG. The ependymal ‘dot–dash’ sign: an MR imaging finding of early multiple sclerosis. AJNR Am J Neuroradiol 2005;26:2033-6.
- Janardhan V, Suri S, Bakshi R. Multiple sclerosis: hyperintense lesions in the brain on nonenhanced T1-weighted MR images evidenced as areas of T1 shortening. Radiology 2007;244:823-31. https://doi.org/10.1148/radiol.2443051171.
- Poser CM, Brinar VV. Diagnostic criteria for multiple sclerosis: an historical review. Clin Neurol Neurosurg 2004;106:147-58. http://dx.doi.org/10.1016/j.clineuro.2004.02.004.
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292-30. http://dx.doi.org/10.1002/ana.22366.
- MS International Federation . Atlas of MS 2013: Mapping Multiple Sclerosis Around the World 2013. www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf (accessed 7 June 2016).
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227-31. http://dx.doi.org/10.1002/ana.410130302.
- Tintoré M, Rovira A, Martinez MJ, Rio J, Diaz-Villoslada P, Brieva L, et al. Isolated demyelinating syndromes: comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. AJNR Am J Neuroradiol 2000;21:702-6.
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald criteria’. Ann Neurol 2005;58:840-6. http://dx.doi.org/10.1002/ana.20703.
- Kurtzke JF. On the origin of EDSS. Mult Scler Relat Disord 2015;4:95-103. http://dx.doi.org/10.1016/j.msard.2015.02.003.
- Hobart J, Freeman J, Thompson A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain 2000;123:1027-40. https://doi.org/10.1093/brain/123.5.1027.
- Marcus JF, Waubant EL. Updates on clinically isolated syndrome and diagnostic criteria for multiple sclerosis. Neurohospitalist 2013;3:65-80. http://dx.doi.org/10.1177/1941874412457183.
- Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology 2010;74:2004-15. http://dx.doi.org/10.1212/WNL.0b013e3181e3973f.
- Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;343:1430-8. http://dx.doi.org/10.1056/NEJM200011163432001.
- Kremenchutzky M, Rice GPA, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain 2006;129:584-94. https://doi.org/10.1093/brain/awh721.
- Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain 2006;129:595-60. https://doi.org/10.1093/brain/awh714.
- Confavreux C, Vukusic S. The clinical course of multiple sclerosis. Handb Clin Neurol 2014;122:343-69. http://dx.doi.org/10.1016/B978-0-444-52001-2.00014-5.
- Kalincik T. Multiple sclerosis relapses: epidemiology, outcomes and management. A systematic review. Neuroepidemiology 2015;44:199-214. http://dx.doi.org/10.1159/000382130.
- Hirst C, Ingram G, Pearson O, Pickersgill T, Scolding N, Robertson N. Contribution of relapses to disability in multiple sclerosis. J Neurol 2008;255:280-7. http://dx.doi.org/10.1007/s00415-008-0743-8.
- Confavreux C. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 2003;126:770-82. https://doi.org/10.1093/brain/awg081.
- Liu C, Blumhardt LD. Disability outcome measures in therapeutic trials of relapsing–remitting multiple sclerosis: effects of heterogeneity of disease course in placebo cohorts. J Neurol Neurosurg Psychiatr 2000;68:450-7. https://doi.org/10.1136/jnnp.68.4.450.
- Leray E, Yaouanq J, Le Page E, Coustans M, Laplaud D, Oger J, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010;133:1900-13. https://doi.org/10.1093/brain/awq076.
- Scalfari A, Neuhaus A, Degenhardt A, Rice GP, Muraro PA, Daumer M, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain 2010;133:1914-29. https://doi.org/10.1093/brain/awq118.
- Tremlett H, Yousefi M, Devonshire V, Rieckmann P, Zhao Y. UBC Neurologists . Impact of multiple sclerosis relapses on progression diminishes with time. Neurology 2009;73:1616-23. http://dx.doi.org/10.1212/WNL.0b013e3181c1e44f.
- Scalfari A, Knappertz V, Cutter G, Goodin DS, Ashton R, Ebers GC. Mortality in patients with multiple sclerosis. Neurology 2013;81:184-92. http://dx.doi.org/10.1212/WNL.0b013e31829a3388.
- Manouchehrinia A, Tanasescu R, Tench CR, Constantinescu CS. Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios. J Neurol Neurosurg Psychiatr 2016;87:324-31. http://dx.doi.org/10.1136/jnnp-2015-310361.
- Sadovnick AD, Ebers GC, Wilson RW, Paty DW. Life expectancy in patients attending multiple sclerosis clinics. Neurology 1992;42:991-4. https://doi.org/10.1212/WNL.42.5.991.
- Kingwell E, Marriott JJ, Jetté N, Pringsheim T, Makhani N, Morrow SA, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol 2013;13. http://dx.doi.org/10.1186/1471-2377-13-128.
- Jobin C, Larochelle C, Parpal H, Coyle PK, Duquette P. Gender issues in multiple sclerosis: an update. Womens Health 2010;6:797-820. http://dx.doi.org/10.2217/whe.10.69.
- Greer JM, McCombe PA. Role of gender in multiple sclerosis: clinical effects and potential molecular mechanisms. J Neuroimmunol 2011;234:7-18. http://dx.doi.org/10.1016/j.jneuroim.2011.03.003.
- Eikelenboom MJ, Killestein J, Kragt JJ, Uitdehaag BM, Polman CH. Gender differences in multiple sclerosis: cytokines and vitamin D. J Neurol Sci 2009;286:40-2. http://dx.doi.org/10.1016/j.jns.2009.06.025.
- Sadovnick AD. European Charcot Foundation Lecture: the natural history of multiple sclerosis and gender. J Neurol Sci 2009;286:1-5. http://dx.doi.org/10.1016/j.jns.2009.09.005.
- Ford HL, Gerry E, Airey CM, Vail A, Johnson MH, Williams DR. The prevalence of multiple sclerosis in the Leeds Health Authority. J Neurol Neurosurg Psychiatr 1998;64:605-10. https://doi.org/10.1136/jnnp.64.5.605.
- Gray OM, McDonnell GV, Hawkins SA. Factors in the rising prevalence of multiple sclerosis in the north-east of Ireland. Mult Scler 2008;14:880-6. http://dx.doi.org/10.1177/1352458508090663.
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4-14. http://dx.doi.org/10.1111/j.1365-2125.2009.03537.x.
- Richards R, Sampson F, Beard S, Tappenden P. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess 2002;6. https://doi.org/10.3310/hta6100.
- Swingler RJ, Compston DA. The morbidity of multiple sclerosis. QJ Med 1992;83:325-37.
- Symptom Management Survey. London: MS Society; 1998.
- Einarsson U, Gottberg K, Fredrikson S, von Koch L, Holmqvist LW. Activities of daily living and social activities in people with multiple sclerosis in Stockholm County. Clin Rehabil 2006;20:543-51. https://doi.org/10.1191/0269215506cr953oa.
- Rodriguez M, Siva A, Ward J, Stolp-Smith K, O’Brien P, Kurland L. Impairment, disability, and handicap in multiple sclerosis: a population-based study in Olmsted County, Minnesota. Neurology 1994;44:28-33. https://doi.org/10.1212/WNL.44.1.28.
- Wynia K, van Wijlen AT, Middel B, Reijneveld SA, Meilof JF. Change in disability profile and quality of life in multiple sclerosis patients: a five-year longitudinal study using the Multiple Sclerosis Impact Profile (MSIP). Mult Scler 2012;18:654-61. https://doi.org/10.1177/1352458511423935.
- Jones KH, Ford DV, Jones PA, John A, Middleton RM, Lockhart-Jones H, et al. How people with multiple sclerosis rate their quality of life: an EQ-5D survey via the UK MS register. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0065640.
- Szende A, Bas J, Cabases J. Self-Reported Population Health: An International Perspective Based on EQ-5D. Dordrecht: Springer Netherlands; 2014.
- Orme M, Kerrigan J, Tyas D, Russell N, Nixon R. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health 2007;10:54-60. https://doi.org/10.1111/j.1524-4733.2006.00144.x.
- Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry 2005;76:469-75. https://doi.org/10.1136/jnnp.2004.054635.
- Pompili M, Forte A, Palermo M, Stefani H, Lamis DA, Serafini G, et al. Suicide risk in multiple sclerosis: a systematic review of current literature. J Psychosom Res 2012;73:411-17. http://dx.doi.org/10.1016/j.jpsychores.2012.09.011.
- Byford S, Torgerson DJ, Raftery J. Economic note: cost of illness studies. BMJ 2000;320. https://doi.org/10.1136/bmj.320.7245.1335.
- Kobelt G, Berg J, Lindgren P, Kerrigan J, Russell N, Nixon R. Costs and quality of life of multiple sclerosis in the United Kingdom. Eur J Health Econ 2006;7:96-104. http://dx.doi.org/10.1007/s10198-006-0380-z.
- McCrone P, Heslin M, Knapp M, Bull P, Thompson A. Multiple sclerosis in the UK: service use, costs, quality of life and disability. Pharmacoeconomics 2008;26:847-60. https://doi.org/10.2165/00019053-200826100-00005.
- Hawton AJ, Green C. Multiple sclerosis: relapses, resource use, and costs. Eur J Health Econ 2016;17:875-84. http://dx.doi.org/10.1007/s10198-015-0728-3.
- Kobelt G, Lindgren P, Parkin D, Francis DA, Johnson M, Bates D, et al. Costs and Quality of Life in Multiple Sclerosis. A Cross-Sectional Observational Study in the UK. Stockholm: Stockholm School of Economics; 2000.
- Hakim EA, Bakheit AM, Bryant TN, Roberts MW, McIntosh-Michaelis SA, Spackman AJ, et al. The social impact of multiple sclerosis – a study of 305 patients and their relatives. Disabil Rehabil 2000;22:288-93. https://doi.org/10.1080/096382800296755.
- Edwards RG, Barlow JH, Turner AP. Experiences of diagnosis and treatment among people with multiple sclerosis. J Eval Clin Pract 2008;14:460-4. http://dx.doi.org/10.1111/j.1365-2753.2007.00902.x.
- Johnson J. On receiving the diagnosis of multiple sclerosis: managing the transition. Mult Scler 2003;9:82-8. https://doi.org/10.1191/1352458503ms856oa.
- Malcomson KS, Lowe-Strong AS, Dunwoody L. What can we learn from the personal insights of individuals living and coping with multiple sclerosis?. Disabil Rehabil 2008;30:662-74. https://doi.org/10.1080/09638280701400730.
- Davies F, Edwards A, Brain K, Edwards M, Jones R, Wallbank R, et al. ‘You are just left to get on with it’: qualitative study of patient and carer experiences of the transition to secondary progressive multiple sclerosis. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-007674.
- Methley AM, Chew-Graham C, Campbell S, Cheraghi-Sohi S. Experiences of UK health-care services for people with multiple sclerosis: a systematic narrative review. Health Expect 2015;18:1844-55. http://dx.doi.org/10.1111/hex.12228.
- Embrey N. Multiple sclerosis: managing a complex neurological disease. Nurs Stand 2014;29:49-58. http://dx.doi.org/10.7748/ns.29.11.49.e9190.
- Zajicek J, Freeman J, Porter B. Multiple Sclerosis Care: A Practical Manual. Oxford: Oxford University Press; 2007.
- Multiple Sclerosis: Management of Multiple Sclerosis in Primary and Secondary Care. London: NICE; 2014.
- National Institute for Health and Care Excellence . Alemtuzumab for Treating Relapsing –Remitting Multiple Sclerosis 2014. www.nice.org.uk/guidance/ta312 (accessed 1 June 2016).
- Dimethyl Fumarate for Treating Relapsing–Remitting Multiple Sclerosis. London: NICE; 2014.
- Fingolimod for the Treatment of Highly Active Relapsing–Remitting Multiple Sclerosis. London: NICE; 2012.
- Natalizumab for the Treatment of Adults with Highly Active Relapsing–Remitting Multiple Sclerosis. London: NICE; 2007.
- Teriflunomide for Treating Relapsing–Remitting Multiple Sclerosis. London: NICE; 2014.
- Hommes OR, Weiner HL. Results of an international questionnaire on immunosuppressive treatment of multiple sclerosis. Mult Scler 2002;8:139-41. https://doi.org/10.1191/1352458502ms791oa.
- Casetta I, Iuliano G, Filippini G. Azathioprine for multiple sclerosis. Cochrane Database Syst Rev 2007;4. http://dx.doi.org/10.1002/14651858.CD003982.pub2.
- Frohman EM, Shah A, Eggenberger E, Metz L, Zivadinov R, Stüve O. Corticosteroids for multiple sclerosis: I. Application for treating exacerbations. Neurotherapeutics 2007;4:618-26. https://doi.org/10.1016/j.nurt.2007.07.008.
- Perry M, Swain S, Kemmis-Betty S, Cooper P. Guideline Development Group of the National Institute for Health and Care Excellence . Multiple sclerosis: summary of NICE guidance. BMJ 2014;349. https://doi.org/10.1136/bmj.g5701.
- Shakespeare DT, Boggild M, Young C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev 2003;4. https://doi.org/10.1002/14651858.cd001332.
- He D, Zhang Y, Dong S, Wang D, Gao X, Zhou H. Pharmacological treatment for memory disorder in multiple sclerosis. Cochrane Database Syst Rev 2013;12. http://dx.doi.org/10.1002/14651858.CD008876.pub3.
- Fiest KM, Walker JR, Bernstein CN, Graff LA, Zarychanski R, Abou-Setta AM, et al. Systematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosis. Mult Scler Relat Disord 2016;5:12-26. http://dx.doi.org/10.1016/j.msard.2015.10.004.
- National Institute for Health and Care Excellence . Multiple Sclerosis in Adults: Management 2014. www.nice.org.uk/guidance/cg186 (accessed 1 June 2016).
- Gallien P, Nicolas B, Robineau S, Pétrilli S, Houedakor J, Durufle A. Physical training and multiple sclerosis. Ann Readapt Med Phys 2007;50:373-6.
- Gutierrez GM, Chow JW, Tillman MD, McCoy SC, Castellano V, White LJ. Resistance training improves gait kinematics in persons with multiple sclerosis. Arch Phys Med Rehabil 2005;86:1824-9. https://doi.org/10.1016/j.apmr.2005.04.008.
- Petajan JH, White AT. Recommendations for physical activity in patients with multiple sclerosis. Sports Med 1999;27:179-91. https://doi.org/10.2165/00007256-199927030-00004.
- Wiles CM, Newcombe RG, Fuller KJ, Shaw S, Furnival-Doran J, Pickersgill TP, et al. Controlled randomised crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J Neurol Neurosurg Psychiatr 2001;70:174-9. https://doi.org/10.1136/jnnp.70.2.174.
- Asano M, Dawes DJ, Arafah A, Moriello C, Mayo NE. What does a structured review of the effectiveness of exercise interventions for persons with multiple sclerosis tell us about the challenges of designing trials?. Mult Scler 2009;15:412-21. https://doi.org/10.1177/1352458508101877.
- Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: exercise, education, and medication. Mult Scler Int 2014;2014. https://doi.org/10.1155/2014/798285.
- Rashid WC, Jackson A, McFadden K, Meriman E, Vernon K. Symptomatic Management of Multiple Sclerosis in Primary Care 2013. https://community.mssociety.org.uk/sites/default/files/resources/resource_files/Symptomatic%20management%20of%20MS%20in%20primary%20care%20-%20MGP%20guideline.pdf (accessed 28 October 2015).
- National institute for Health and Care Excellence . Urinary Incontinence in Neurological Disease: Assessment and Management 2012. www.nice.org.uk/guidance/cg148 (accessed 15 October 2015).
- Flood S, Foley FW, Zemon V, Picone M, Bongardino M, Quinn H. Predictors of changes in suicidality in multiple sclerosis over time. Disabil Rehabil 2014;36:844-7. http://dx.doi.org/10.3109/09638288.2013.822570.
- Mikula P, Nagyova I, Krokavcova M, Vitkova M, Rosenberger J, Szilasiova J, et al. Coping and its importance for quality of life in patients with multiple sclerosis. Disabil Rehabil 2014;36:732-6. http://dx.doi.org/10.3109/09638288.2013.808274.
- National Institute for Health and Care Excellence . Multiple Technology Appraisal. Beta Interferon and Glatiramer Acetate for Treating Multiple Sclerosis (Review of TA32). Final Scope Updated Post Invitation 2016.
- Dhib-Jalbut S. Mechanisms of action of interferons and glatiramer acetate in multiple sclerosis. Neurology 2002;58:3-9. https://doi.org/10.1212/WNL.58.8_suppl_4.S3.
- Scagnolari C, Selvaggi C, Di Biase E, Fraulo M, Dangond F, Antonelli G. In vitro assessment of the biologic activity of interferon beta formulations used for the treatment of relapsing multiple sclerosis. J Immunoassay Immunochem 2014;35:288-99. https://doi.org/10.1080/15321819.2013.848815.
- Antonetti F, Finocchiaro O, Mascia M, Terlizzese MG, Jaber A. A comparison of the biologic activity of two recombinant IFN-beta preparations used in the treatment of relapsing–remitting multiple sclerosis. J Interferon Cytokine Res 2002;22:1181-4. https://doi.org/10.1089/10799900260475696.
- Sørensen PS, Deisenhammer F, Duda P, Hohlfeld R, Myhr KM, Palace J, et al. Guidelines on use of anti-IFN-beta antibody measurements in multiple sclerosis: report of an EFNS Task Force on IFN-beta antibodies in multiple sclerosis. Eur J Neurol 2005;12:817-27. https://doi.org/10.1111/j.1468-1331.2005.01386.x.
- Sørensen PS. Neutralizing antibodies against interferon-beta. Ther Adv Neurol Disord 2008;1:125-41. http://dx.doi.org/10.1177/1756285608095144.
- Govindappa K, Sathish J, Park K, Kirkham J, Pirmohamed M. Development of interferon beta-neutralising antibodies in multiple sclerosis – a systematic review and meta-analysis. Eur J Clin Pharmacol 2015;71:1287-98. http://dx.doi.org/10.1007/s00228-015-1921-0.
- Bertolotto A, Capobianco M, Amato MP, Capello E, Capra R, Centonze D, et al. Guidelines on the clinical use for the detection of neutralizing antibodies (NABs) to IFN beta in multiple sclerosis therapy: report from the Italian Multiple Sclerosis Study group. Neurol Sci 2014;35:307-16. https://doi.org/10.1007/s10072-013-1616-1.
- Health and Social Care Information Centre . Hospital Prescribing: England, 2013–14 2014. http://digital.nhs.uk/catalogue/PUB15883 (accessed 20 October 2015).
- Aharoni R. The mechanism of action of glatiramer acetate in multiple sclerosis and beyond. Autoimmun Rev 2013;12:543-53. http://dx.doi.org/10.1016/j.autrev.2012.09.005.
- Department of Health . Cost Effective Provision of Disease Modifying Therapies for People With Multiple Sclerosis 2002. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/Healthservicecirculars/DH_4004332 (accessed 4 October 2015).
- Kingwell E, van der Kop M, Zhao Y, Shirani A, Zhu F, Oger J, et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia, Canada. J Neurol Neurosurg Psychiatry 2012;83:61-6. https://doi.org/10.1136/jnnp-2011-300616.
- Palace J, Bregenzer T, Tremlett H, Oger J, Zhu F, Zhu F, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004073.
- Palace J, Duddy M, Bregenzer T, Lawton M, Zhu F, Boggild M, et al. Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6 years: a clinical cohort study with natural history comparator. Lancet Neurol 2015;14:497-505. https://doi.org/10.1016/S1474-4422(15)00018-6.
- Palace J, Bregenzer T, Tremlett H, Duddy M, Boggild M, Zhu F, et al. Modelling Natural History for the UK Multiple Sclerosis Risk-Sharing Scheme n.d.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- La Mantia L, Di Pietrantonj C, Rovaris M, Rigon G, Frau S, Berardo F, et al. Interferons-beta versus glatiramer acetate for relapsing–remitting multiple sclerosis. Cochrane Database Syst Rev 2014;7. https://doi.org/10.1002/14651858.cd009333.pub2.
- Clerico M, Faggiano F, Palace J, Rice G, Tintore M, Durelli L. Recombinant interferon beta or glatiramer acetate for delaying conversion of the first demyelinating event to multiple sclerosis. Cochrane Database Syst Rev 2008;2. https://doi.org/10.1002/14651858.cd005278.pub3.
- Tramacere I, Del Giovane C, Salanti G, D’Amico R, Filippini G. Immunomodulators and immunosuppressants for relapsing–remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev 2015;9. http://dx.doi.org/10.1002/14651858.CD011381.pub2.
- Filippini G, Del Giovane C, Vacchi L, D’Amico R, Di Pietrantonj C, Beecher D, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev 2013;6. http://dx.doi.org/10.1002/14651858.CD008933.pub2.
- La Mantia L, Vacchi L, Di Pietrantonj C, Ebers G, Rovaris M, Fredrikson S, et al. Interferon beta for secondary progressive multiple sclerosis. Cochrane Database Syst Rev 2012;1. http://dx.doi.org/10.1002/14651858.CD005181.pub3.
- Northern and Yorkshire Regional Drug & Therapeutics Centre . Assessment of Interferon-Beta and Glatiramer for the Treatment of Multiple Sclerosis 2000. www.nice.org.uk/guidance/TA32/documents/original-hta-report-april-20002 (accessed 10 October 2015).
- Moher D, Liberati A, Tetzlaff J, Altman DG. the PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013-20. http://dx.doi.org/10.1016/j.jclinepi.2008.10.009.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8. https://doi.org/10.1186/1745-6215-8-16.
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98-110. http://dx.doi.org/10.1002/jrsm.1044.
- Frohman EM, Goodin DS, Calabresi PA, Corboy JR, Coyle PK, Filippi M, et al. The utility of MRI in suspected MS: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2003;61:602-11. https://doi.org/10.1212/01.WNL.0000082654.99838.EF.
- Bornstein MB, Miller A, Slagle S, Weitzman M, Crystal H, Drexler E, et al. A pilot trial of Cop 1 in exacerbating–remitting multiple sclerosis. N Engl J Med 1987;317:408-14. https://doi.org/10.1056/NEJM198708133170703.
- Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006;67:1242-9. https://doi.org/10.1212/01.wnl.0000237641.33768.8d.
- Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000;343:898-904. https://doi.org/10.1056/NEJM200009283431301.
- Pakdaman H, Sahraian MA, Fallah A, Pakdaman R, Ghareghozli K, Ghafarpour M, et al. Effect of early interferon beta-1a therapy on conversion to multiple sclerosis in Iranian patients with a first demyelinating event. Acta Neurol Scand 2007;115:429-31. https://doi.org/10.1111/j.1600-0404.2007.00813.x.
- Comi G, Martinelli V, Rodegher M, Moiola L, Bajenaru O, Carra A, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 2009;374:1503-11. https://doi.org/10.1016/S0140-6736(09)61259-9.
- Comi G, De Stefano N, Freedman MS, Barkhof F, Polman CH, Uitdehaag BMJ, et al. Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol 2012;11:33-41. https://doi.org/10.1016/S1474-4422(11)70262-9.
- Beck RW, Chandler DL, Cole SR, Simon JH, Jacobs LD, Kinkel RP, et al. Interferon beta-1a for early multiple sclerosis: CHAMPS trial subgroup analyses. Ann Neurol 2002;51:481-90. https://doi.org/10.1002/ana.10148.
- O’Connor P, Kinkel RP, Kremenchutzky M. Efficacy of intramuscular interferon beta-1a in patients with clinically isolated syndrome: analysis of subgroups based on new risk criteria. Mult Scler 2009;15:728-34. http://dx.doi.org/10.1177/1352458509103173.
- Freedman MS, De Stefano N, Barkhof F, Polman CH, Comi G, Uitdehaag BM, et al. Patient subgroup analyses of the treatment effect of subcutaneous interferon beta-1a on development of multiple sclerosis in the randomized controlled REFLEX study. J Neurol 2014;261:490-9. https://doi.org/10.1007/s00415-013-7222-6.
- Polman C, Kappos L, Freedman MS, Edan G, Hartung HP, Miller DH, et al. Subgroups of the BENEFIT study: risk of developing MS and treatment effect of interferon beta-1b. J Neurol 2008;255:480-7. http://dx.doi.org/10.1007/s00415-007-0733-2.
- Penner IK, Stemper B, Calabrese P, Freedman MS, Polman CH, Edan G, et al. Effects of interferon beta-1b on cognitive performance in patients with a first event suggestive of multiple sclerosis. Mult Scler 2012;18:1466-71. https://doi.org/10.1177/1352458512442438.
- Schwartz CE, Coulthard-Morris L, Cole B, Vollmer T. The quality-of-life effects of interferon beta-1b in multiple sclerosis. An extended Q-TWiST analysis. Arch Neurol 1997;54:1475-80. https://doi.org/10.1001/archneur.1997.00550240029009.
- Bayer HealthCare . Clinical Study Synopsis: The AVANTAGE Study – A Randomized, Multicenter, Phase IV, Open-Label Prospective Study Comparing Injection Site Reaction and Injection Site Pain in Patients With Relapsing Remitting Multiple Sclerosis (RRMS) or After a First Demyelinating Event Suggestive of MS Newly Started on Interferon Beta-1b (Betaferon®) or Interferon Beta-1a (Rebif®) 2013. http://trialfinder.pharma.bayer.com/omr/online/91489_Study_Synopsis_CTP.pdf (accessed 1 May 2016).
- Rieckmann P, Heidenreich F, Sailer M, Zettl UK, Zessack N, Hartung HP, et al. Treatment de-escalation after mitoxantrone therapy: results of a phase IV, multicentre, open-label, randomized study of subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis. Ther Adv Neurol Disord 2012;5:3-12. https://doi.org/10.1177/1756285611428503.
- Cadavid D, Wolansky LJ, Skurnick J, Lincoln J, Cheriyan J, Szczepanowski K, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009;72:1976-83. http://dx.doi.org/10.1212/01.wnl.0000345970.73354.17.
- Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of Betaferon, Avonex, and Rebif in treatment of relapsing–remitting multiple sclerosis. Acta Neurol Scand 2006;113:283-7. https://doi.org/10.1111/j.1600-0404.2006.00585.x.
- Mokhber N, Azarpazhooh A, Orouji E, Rao SM, Khorram B, Sahraian MA, et al. Cognitive dysfunction in patients with multiple sclerosis treated with different types of interferon beta: a randomized clinical trial. J Neurol Sci 2014;342:16-20. https://doi.org/10.1016/j.jns.2014.01.038.
- Mokhber N, Azarpazhooh A, Orouji E, Khorram B, Modares Gharavi M, Kakhi S, et al. Therapeutic effect of Avonex, Rebif and Betaferon on quality of life in multiple sclerosis. Psychiatry Clin Neurosci 2015;69:649-57. http://dx.doi.org/10.1111/pcn.12308.
- Calabrese M, Bernardi V, Atzori M, Mattisi I, Favaretto A, Rinaldi F, et al. Effect of disease-modifying drugs on cortical lesions and atrophy in relapsing–remitting multiple sclerosis. Mult Scler 2012;18:418-24. http://dx.doi.org/10.1177/1352458510394702.
- PRISMS Study Group . Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998;352:1498-504. https://doi.org/10.1016/S0140-6736(98)03334-0.
- O’Connor P, Filippi M, Arnason B, Comi G, Cook S, Goodin D, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing–remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol 2009;8:889-97. https://doi.org/10.1016/S1474-4422(09)70226-1.
- Lublin FD, Cofield SS, Cutter GR, Conwit R, Narayana PA, Nelson F, et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol 2013;73:327-40. http://dx.doi.org/10.1002/ana.23863.
- Mikol DD, Barkhof F, Chang P, Coyle PK, Jeffery DR, Schwid SR, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs. Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol 2008;7:903-14. https://doi.org/10.1016/S1474-4422(08)70200-X.
- Panitch H, Goodin DS, Francis G, Chang P, Coyle PK, O’Connor P, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE Trial. Neurology 2002;59:1496-506. https://doi.org/10.1212/01.WNL.0000034080.43681.DA.
- Panitch H, Goodin DS, Francis G, Chang P, Coyle PK, O’Connor P, et al. Benefits of high-dose, high-frequency interferon beta-1a in relapsing–remitting multiple sclerosis are sustained to 16 months: final comparative results of the EVIDENCE trial. J Neurol Sci 2005;239:67-74. https://doi.org/10.1016/j.jns.2005.08.003.
- Schwid SR, Panitch HS. Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clin Ther 2007;29:2031-48. https://doi.org/10.1016/j.clinthera.2007.09.025.
- Durelli L, Verdun E, Barbero P, Bergui M, Versino E, Ghezzi A, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 2002;359:1453-60. https://doi.org/10.1016/S0140-6736(02)08430-1.
- Singer B, Bandari D, Cascione M, LaGanke C, Huddlestone J, Bennett R, et al. Comparative injection-site pain and tolerability of subcutaneous serum-free formulation of interferonbeta-1a versus subcutaneous interferonbeta-1b: results of the randomized, multicenter, Phase IIIb REFORMS study. BMC Neurol 2012;12. https://doi.org/10.1186/1471-2377-12-154.
- Vollmer TL, Sorensen PS, Selmaj K, Zipp F, Havrdova E, Cohen JA, et al. A randomized placebo-controlled Phase III trial of oral laquinimod for multiple sclerosis. J Neurol 2014;261:773-83. http://dx.doi.org/10.1007/s00415-014-7264-4.
- Kappos L, Li D, Calabresi PA, O’Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing–remitting multiple sclerosis: a Phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779-87. https://doi.org/10.1016/S0140-6736(11)61649-8.
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285-94. https://doi.org/10.1002/ana.410390304.
- Rudick RA, Goodkin DE, Jacobs LD, Cookfair DL, Herndon RM, Richert JR, et al. Impact of interferon beta-1a on neurologic disability in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology 1997;49:358-63. https://doi.org/10.1212/WNL.49.2.358.
- Goodkin DE, Priore RL, Wende KE, Campion M, Bourdette DN, Herndon RM, et al. Comparing the ability of various compositive outcomes to discriminate treatment effects in MS clinical trials. The Multiple Sclerosis Collaborative Research Group (MSCRG). Mult Scler 1998;4:480-6. https://doi.org/10.1177/135245859800400604.
- Fischer JS, Priore RL, Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, et al. Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Group. Ann Neurol 2000;48:885-92. https://doi.org/10.1002/1531-8249(200012)48:6<885::AID-ANA9>3.0.CO;2-1.
- Granger CV, Wende K, Brownscheidle CM. Use of the FIM instrument in a trial of intramuscular interferon beta-1a for disease progression in relapsing–remitting multiple sclerosis. Am J Phys Med Rehabil 2003;82:427-36. https://doi.org/10.1097/01.PHM.0000069189.86581.08.
- Miller DM, Weinstock-Guttman B, Bourdette D, You X, Foulds P, Rudick RA. Change in quality of life in patients with relapsing–remitting multiple sclerosis over 2 years in relation to other clinical parameters: results from a trial of intramuscular interferon {beta}-1a. Mult Scler 2011;17:734-42. https://doi.org/10.1177/1352458510397221.
- Sandberg-Wollheim M, Bever C, Carter J, Färkkilä M, Hurwitz B, Lapierre Y, et al. Comparative tolerance of IFN beta-1a regimens in patients with relapsing multiple sclerosis. The EVIDENCE study. J Neurol 2005;252:8-13. http://dx.doi.org/10.1007/s00415-005-0589-2.
- De Stefano N, Sormani MP, Stubinski B, Blevins G, Drulovic JS, Issard D, et al. Efficacy and safety of subcutaneous interferon β-1a in relapsing–remitting multiple sclerosis: further outcomes from the IMPROVE study. J Neurol Sci 2012;312:97-101. https://doi.org/10.1016/j.jns.2011.08.013.
- Patten SB, Metz LM. Interferon beta-1 a and depression in relapsing–remitting multiple sclerosis: an analysis of depression data from the PRISMS clinical trial. Mult Scler 2001;7:243-8.
- IFNB Multiple Sclerosis Study Group . Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 1993;43:655-61. https://doi.org/10.1212/WNL.43.4.655.
- University of British Columbia MS/MRI Analysis Group . Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995;45:1277-85. https://doi.org/10.1212/WNL.45.7.1277.
- Knobler RL, Greenstein JI, Johnson KP, Lublin FD, Panitch HS, Conway K, et al. Systemic recombinant human interferon-beta treatment of relapsing–remitting multiple sclerosis: pilot study analysis and six-year follow-up. J Interferon Res 1993;13:333-40. https://doi.org/10.1089/jir.1993.13.333.
- Cadavid D, Kim S, Peng B, Skurnick J, Younes M, Hill J, et al. Clinical consequences of MRI activity in treated multiple sclerosis. Mult Scler 2011;17:1113-21. http://dx.doi.org/10.1177/1352458511405375.
- Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ, Boyko A, Pelletier J, et al. Pegylated interferon beta-1a for relapsing–remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol 2014;13:657-65. https://doi.org/10.1016/S1474-4422(14)70068-7.
- Arnold DL, Calabresi PA, Kieseier BC, Sheikh SI, Deykin A, Zhu Y, et al. Effect of peginterferon beta-1a on MRI measures and achieving no evidence of disease activity: results from a randomized controlled trial in relapsing–remitting multiple sclerosis. BMC Neurol 2014;14. https://doi.org/10.1186/s12883-014-0240-x.
- Newsome SD, Guo S, Altincatal A, Proskorovsky I, Kinter E, Phillips G, et al. Impact of peginterferon beta-1a and disease factors on quality of life in multiple sclerosis. Mult Scler Relat Disord 2015;4:350-7. http://dx.doi.org/10.1016/j.msard.2015.06.004.
- Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97. https://doi.org/10.1056/NEJMoa1206328.
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45:1268-76. https://doi.org/10.1212/WNL.45.7.1268.
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology 1998;50:701-8. https://doi.org/10.1212/WNL.50.3.701.
- Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging-measured disease activity and burden in patients with relapsing multiple sclerosis. Ann Neurol 2001;49:290-7. https://doi.org/10.1002/ana.64.
- Cohen J, Belova A, Selmaj K, Wolf C, Sormani MP, Oberyé J, et al. Equivalence of generic glatiramer acetate in multiple sclerosis: a randomized clinical trial. JAMA Neurol 2015;72:1433-41. http://dx.doi.org/10.1001/jamaneurol.2015.2154.
- Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R. GALA Study Group . Three times weekly glatiramer acetate in relapsing–remitting multiple sclerosis. Ann Neurol 2013;73:705-13. http://dx.doi.org/10.1002/ana.23938.
- Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis . European Study Group on interferon beta-1b in secondary progressive MS. Lancet 1998;352:1491-7. https://doi.org/10.1016/S0140-6736(98)10039-9.
- Panitch H, Miller A, Paty D, Weinshenker B. North American Study Group on Interferon beta-1b in Secondary Progressive MS . Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology 2004;63:1788-95. https://doi.org/10.1212/01.WNL.0000146958.77317.3E.
- SPECTRIMS Study Group . Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology 2001;56:1496-504. https://doi.org/10.1212/WNL.56.11.1496.
- Kappos L, Polman C, Pozzilli C, Thompson A, Beckmann K, Dahlke F. European Study Group in Interferon beta-1b in Secondary-Progressive MS . Final analysis of the European multicenter trial on IFNbeta-1b in secondary-progressive MS. Neurology 2001;57:1969-75. https://doi.org/10.1212/WNL.57.11.1969.
- Filippi M, Rocca MA, Camesasca F, Cook S, O’Connor P, Arnason BG, et al. Interferon β-1b and glatiramer acetate effects on permanent black hole evolution. Neurology 2011;76:1222-8. http://dx.doi.org/10.1212/WNL.0b013e3182143577.
- CHAMPS Study Group . Interferon beta-1a for optic neuritis patients at high risk for multiple sclerosis. Am J Ophthalmol 2001;132:463-71. https://doi.org/10.1016/S0002-9394(01)01209-0.
- O’Connor P. CHAMPS . The effects of intramuscular interferon beta-1a in patients at high risk for development of multiple sclerosis: a post hoc analysis of data from CHAMPS. Clin Ther 2003;25:2865-74. https://doi.org/10.1016/S0149-2918(03)80339-9.
- Lindsey JW, Scott TF, Lynch SG, Cofield SS, Nelson F, Conwit R, et al. The CombiRx trial of combined therapy with interferon and glatiramer acetate in relapsing remitting MS: design and baseline characteristics. Mult Scler Relat Disord 2012;1:81-6. https://doi.org/10.1016/j.msard.2012.01.006.
- Kita M, Fox RJ, Phillips JT, Hutchinson M, Havrdova E, Sarda SP, et al. Effects of BG-12 (dimethyl fumarate) on health-related quality of life in patients with relapsing–remitting multiple sclerosis: findings from the CONFIRM study. Mult Scler 2014;20:253-7. https://doi.org/10.1177/1352458513507818.
- Gold R, Rieckmann P, Chang P, Abdalla J. PRISMS Study Group . The long-term safety and tolerability of high-dose interferon beta-1a in relapsing–remitting multiple sclerosis: 4-year data from the PRISMS study. Eur J Neurol 2005;12:649-56. https://doi.org/10.1111/j.1468-1331.2005.01083.x.
- Anon. Common Drug Review: CDEC Final Recommendation: Interferon Beta-1a (Rebif – EMD Serono Canada Inc.) Indication: Clinically Isolated Syndrome 2013. www.cadth.ca/media/cdr/complete/cdr_complete_Rebif_Aug-19-13.pdf (accessed 1 June 2016).
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. http://dx.doi.org/10.1016/j.jclinepi.2010.03.016.
- European Medicines Agency . Plegridy 125 Micrograms Solution for Injection in Pre-Filled Pen. Summary of Product Characteristics n.d. www.medicines.org.uk/emc/medicine/29370 (accessed 21 April 2017).
- Comi G, Martinelli V, Rodegher M, Moiola L, Leocani L, Bajenaru O, et al. Effects of early treatment with glatiramer acetate in patients with clinically isolated syndrome. Mult Scler 2013;19:1074-83. https://doi.org/10.1177/1352458512469695.
- Kinkel RP, Kollman C, O’Connor P, Murray TJ, Simon J, Arnold D, et al. IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology 2006;66:678-84. https://doi.org/10.1212/01.wnl.0000200778.65597.ae.
- Kinkel RP, Dontchev M, Kollman C, Skaramagas TT, O’Connor PW, Simon JH. Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance Investigators . Association between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: a 10-year follow-up of the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. Arch Neurol 2012;69:183-90. https://doi.org/10.1001/archneurol.2011.1426.
- Kieseier BC, Arnold DL, Balcer LJ, Boyko AA, Pelletier J, Liu S, et al. Peginterferon beta-1a in multiple sclerosis: 2-year results from ADVANCE. Mult Scler 2015;21:1025-35. https://doi.org/10.1177/1352458514557986.
- Centre for Reviews and Dissemination . Search Strategies: NHS EED n.d. www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 3 January 2016).
- Glanville J, Kaunelis D, Mensinkai S. How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. Int J Technol Assess Health Care 2009;25:522-9. http://dx.doi.org/10.1017/S0266462309990523.
- Royle P, Waugh N. Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7340.
- Paisley S, Booth A, Mensinkai S. Etext on Health Technology Assessment (HTA) Information Resources: Chapter 12: Health-Related Quality of Life Studies 2005. www.nlm.nih.gov/archive/20060905/nichsr/ehta/chapter12.html (accessed 5 January 2016).
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care 2013;29:117-22. https://doi.org/10.1017/S0266462313000160.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Allen F, Montgomery S, Maruszczak M, Kusel J, Adlard N. Convergence yet continued complexity: a systematic review and critique of health economic models of relapsing–remitting multiple sclerosis in the United Kingdom. Value Health 2015;18:925-38. https://doi.org/10.1016/j.jval.2015.05.006.
- Castrop F, Haslinger B, Hemmer B, Buck D. Review of the pharmacoeconomics of early treatment of multiple sclerosis using interferon beta. Neuropsychiatr Dis Treat 2013;9:1339-49. http://dx.doi.org/10.2147/NDT.S33949.
- Guo S, Pelligra C, Saint-Laurent Thibault C, Hernandez L, Kansal A. Cost-effectiveness analyses in multiple sclerosis: a review of modelling approaches. Pharmacoeconomics 2014;32:559-72. https://doi.org/10.1007/s40273-014-0150-1.
- Hawton A, Shearer J, Goodwin E, Green C. Squinting through layers of fog: assessing the cost effectiveness of treatments for multiple sclerosis. Appl Health Econ Health Policy 2013;11:331-41. http://dx.doi.org/10.1007/s40258-013-0034-0.
- Owens GM, Olvey EL, Skrepnek GH, Pill MW. Perspectives for managed care organizations on the burden of multiple sclerosis and the cost–benefits of disease-modifying therapies. J Manag Care Pharm 2013;19:41-53. https://doi.org/10.18553/jmcp.2013.19.s1.S41.
- Thompson JP, Abdolahi A, Noyes K. Modelling the cost effectiveness of disease-modifying treatments for multiple sclerosis: issues to consider. Pharmacoeconomics 2013;31:455-69. http://dx.doi.org/10.1007/s40273-013-0063-4.
- Yamamoto D, Campbell JD. Cost-effectiveness of multiple sclerosis disease-modifying therapies: a systematic review of the literature. Autoimmune Dis 2012;2012. https://doi.org/10.1155/2012/784364.
- Zalesak M, Greenbaum JS, Cohen JT, Kokkotos F, Lustig A, Neumann PJ, et al. The value of specialty pharmaceuticals – a systematic review. Am J Manag Care 2014;20:461-72.
- Kuspinar A, Mayo NE. A review of the psychometric properties of generic utility measures in multiple sclerosis. Pharmacoeconomics 2014;32:759-73. https://doi.org/10.1007/s40273-014-0167-5.
- Fredrikson S, McLeod E, Henry N, Pitcher A, Lowin J, Cuche M, et al. A cost-effectiveness analysis of subcutaneous interferon beta-1a 44mcg 3-times a week vs no treatment for patients with clinically isolated syndrome in Sweden. J Med Econ 2013;16:756-62. http://dx.doi.org/10.3111/13696998.2013.792824.
- Kobelt G, Lindgren P, Miltenburger C, Hillert J. Economic evaluation of interferon-angstrom-1b in the treatment of patients with a clinically isolated syndrome (CIS). Value Health 2007;10:A385-6. https://doi.org/10.1016/S1098-3015(10)65357-0.
- Lazzaro C, Bianchi C, Peracino L, Zacchetti P, Uccelli A. Economic evaluation of treating clinically isolated syndrome and subsequent multiple sclerosis with interferon beta-1b. Neurol Sci 2009;30:21-3. https://doi.org/10.1007/s10072-009-0015-0.
- Iskedjian M, Walker JH, Gray T, Vicente C, Einarson TR, Gehshan A. Economic evaluation of Avonex (interferon beta-1a) in patients following a single demyelinating event. Mult Scler 2005;11:542-51. https://doi.org/10.1191/1352458505ms1211oa.
- Arbizu T, Pinol C, Casado V. Cost–utility of interferon beta-1b in the treatment of patients with a clinically isolated syndrome suggestive of multiple sclerosis in Spain. Value Health 2009;12. https://doi.org/10.1016/S1098-3015(10)74820-8.
- Caloyeras JP, Wang C, Bauer L, Lee WC, Lanius V, Gondek K. Cost–utility of interferon beta-1b in the treatment of patients with a clinically isolated syndrome suggestive of multiple sclerosis. Value Health 2008;11. https://doi.org/10.1016/S1098-3015(10)70448-4.
- Caloyeras JP, Harrow B, Wang C, Beckmann K, Knappertz V, Pohl C, et al. Cost–utility of interferon beta-1B in the treatment of patients with a clinically isolated syndrome suggestive of multiple sclerosis: model utilizing five year benefit data. Value Health 2009;12. https://doi.org/10.1016/S1098-3015(10)73129-6.
- Caloyeras JP, Zhang B, Wang C, Eriksson M, Fredrikson S, Beckmann K, et al. Cost-effectiveness analysis of interferon beta-1b for the treatment of patients with a first clinical event suggestive of multiple sclerosis. Clin Ther 2012;34:1132-44. https://doi.org/10.1016/j.clinthera.2012.03.004.
- Zarco LA, Millán SP, Londoño D, Parada L, Taborda A, Borda MG. The cost-effectiveness of interferon beta treatment in patients with a clinically isolated syndrome in Colombia. Biomedica 2014;34:110-17. http://dx.doi.org/10.1590/S0120-41572014000100014.
- Comi G, De Stefano N, Freedman MS, Barkhof F, Uitdehaag BMJ, de Vos M, et al. Subcutaneous interferon beta-1a in the treatment of clinically isolated syndromes: 3-year and 5-year results of the Phase III dosing frequency-blind multicentre REFLEXION study. J Neurol Neurosurg Psychiatry 2017;88:285-94. https://doi.org/10.1136/jnnp-2016-314843.
- Sanchez-de la Rosa R, Sabater E, Casado MA, Arroyo R. Cost-effectiveness analysis of disease modifying drugs (interferons and glatiramer acetate) as first line treatments in remitting–relapsing multiple sclerosis patients. J Med Econ 2012;15:424-33. https://doi.org/10.3111/13696998.2012.654868.
- Nikfar S, Kebriaeezadeh A, Dinarvand R, Abdollahi M, Sahraian MA, Henry D, et al. Cost-effectiveness of different interferon beta products for relapsing–remitting and secondary progressive multiple sclerosis: decision analysis based on long-term clinical data and switchable treatments. Daru 2013;21. https://doi.org/10.1186/2008-2231-21-50.
- Agashivala N, Kim E. Cost-effectiveness of early initiation of fingolimod versus delayed initiation after 1 year of intramuscular interferon beta-1a in patients with multiple sclerosis. Clin Ther 2012;34:1583-90. http://dx.doi.org/10.1016/j.clinthera.2012.06.012.
- Pan F, Goh JW, Cutter G, Su W, Pleimes D, Wang C. Long-term cost-effectiveness model of interferon beta-1b in the early treatment of multiple sclerosis in the United States. Clin Ther 2012;34:1966-76. http://dx.doi.org/10.1016/j.clinthera.2012.07.010.
- Darbà J, Kaskens L, Sánchez-de la Rosa R. Cost-effectiveness of glatiramer acetate and interferon beta-1a for relapsing–remitting multiple sclerosis, based on the CombiRx study. J Med Econ 2014;17:215-22. http://dx.doi.org/10.3111/13696998.2014.890936.
- Imani A, Golestani M. Cost–utility analysis of disease-modifying drugs in relapsing–remitting multiple sclerosis in Iran. Iran J Neurol 2012;11:87-90.
- Dembek C, White LA, Quach J, Szkurhan A, Rashid N, Blasco MR. Cost-effectiveness of injectable disease-modifying therapies for the treatment of relapsing forms of multiple sclerosis in Spain. Eur J Health Econ 2014;15:353-62. http://dx.doi.org/10.1007/s10198-013-0478-z.
- Chevalier J, Chamoux C, Hammès F, Chicoye A. Cost-effectiveness of treatments for relapsing remitting multiple sclerosis: a French societal perspective. PLOS ONE 2016;11. http://dx.doi.org/10.1371/journal.pone.0150703.
- Lee S, Baxter DC, Limone B, Roberts MS, Coleman CI. Cost-effectiveness of fingolimod versus interferon beta-1a for relapsing remitting multiple sclerosis in the United States. J Med Econ 2012;15:1088-96. http://dx.doi.org/10.3111/13696998.2012.693553.
- Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X, et al. Comparison of fingolimod with interferon beta-1a in relapsing–remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 2011;10:520-9. https://doi.org/10.1016/S1474-4422(11)70099-0.
- Physician’s Fee and Coding Guide. Duluth, GA: MAG Mutual HealthCare Solutions, Inc.; 2010.
- Tappenden P, Chilcot J, O’Hagan T, McCabe C, Cooper N, Abrams K, et al. Cost Effectiveness of Beta Interferons and Glatiramer Acetate in the Management of Multiple Sclerosis: Final Report to the National Institute for Clinical Excellence. London; 2001.
- Jackson CH, Sharples LD, Thompson SG, Duffy SW, Couto E. Multistate Markov models for disease progression with classification error. JR Stat Soc Ser D Stat 2003;52:193-209. https://doi.org/10.1111/1467-9884.00351.
- Mortality and Survival. London: ONS; 2010.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Boggild M, Palace J, Barton P, Ben-Shlomo Y, Bregenzer T, Dobson C, et al. Multiple sclerosis risk sharing scheme: two year results of clinical cohort study with historical comparator. BMJ 2009;339. https://doi.org/10.1136/bmj.b4677.
- Acaster S, Perard R, Chauhan D, Lloyd AJ. A forgotten aspect of the NICE reference case: an observational study of the health related quality of life impact on caregivers of people with multiple sclerosis. BMC Health Serv Res 2013;13. http://dx.doi.org/10.1186/1472-6963-13-346.
- Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol 2015;15:273-9. https://doi.org/10.1136/practneurol-2015-001139.
- Natalizumab (Tysabri®) for the Treatment of Adults with Highly Active Relapsing Remitting Multiple Sclerosis: Biogen Idec Single Technology Appraisal (STA) Submission to the National Institute for Health and Clinical Excellence. London: NICE; 2007.
- Karampampa K, Gustavsson A, Miltenburger C, Eckert B. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Mult Scler 2012;18:7-15. http://dx.doi.org/10.1177/1352458512441566.
- Zajicek JP, Ingram WM, Vickery J, Creanor S, Wright DE, Hobart JC. Patient-orientated longitudinal study of multiple sclerosis in south west England (the South West Impact of Multiple Sclerosis Project, SWIMS) 1: protocol and baseline characteristics of cohort. BMC Neurol 2010;10. https://doi.org/10.1186/1471-2377-10-88.
- NHS Reference Costs 2014 to 2015. London: Department of Health; 2015.
- Patzold U, Pocklington PR. Course of multiple sclerosis. First results of a prospective study carried out of 102 MS patients from 1976–1980. Acta Neurol Scand 1982;65:248-66. https://doi.org/10.1111/j.1600-0404.1982.tb03084.x.
- Maruszczak MJ, Montgomery SM, Griffiths MJ, Bergvall N, Adlard N. Cost–utility of fingolimod compared with dimethyl fumarate in highly active relapsing–remitting multiple sclerosis (RRMS) in England. J Med Econ 2015;18:874-85. http://dx.doi.org/10.3111/13696998.2015.1056794.
- Tyas D, Kerrigan J, Russell N, Nixon R. The distribution of the cost of multiple sclerosis in the UK: how do costs vary by illness severity?. Value Health 2007;10:386-9. https://doi.org/10.1111/j.1524-4733.2007.00192.x.
- Dee A, Hutchinson M, De La Harpe D. A budget impact analysis of natalizumab use in Ireland. Ir J Med Sci 2012;181:199-204. http://dx.doi.org/10.1007/s11845-011-0773-6.
- Office for National Statistics . National Life Tables, United Kingdom: 2012–2014 2015. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2015-09-23 (accessed June 2017).
- Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med 1997;29:101-6.
- Single Technology Appraisal (STA): Teriflunomide for the Treatment of Relapsing Remitting Multiple Sclerosis in Adults: Manufacturer/Sponsor Submission of Evidence. London: NICE; 2013.
- electronic Medicines Compendium (eMC) . Beta Interferon Summary of Product Characteristics (Avonex®, Betaferon®, Extavia®, Plegridy®, Rebif®) n.d. www.medicines.org.uk/ (accessed June 2017).
- Common Drug Review Pharmacoeconomic Review Report: Teriflunomide (Aubagio). Ottawa: CADTH; 2014.
- Kerbrat A, Hamonic S, Leray E, Tron I, Edan G, Yaouanq J. West Neuroscience Network of Excellence (WENNE) . Ten-year prognosis in multiple sclerosis: a better outcome in relapsing–remitting patients but not in primary progressive patients. Eur J Neurol 2015;22:507-e35. http://dx.doi.org/10.1111/ene.12600.
- Tolley K, Hutchinson M, You X, Wang P, Sperling B, Taneja A, et al. A network meta-analysis of efficacy and evaluation of safety of subcutaneous pegylated interferon beta-1a versus other injectable therapies for the treatment of relapsing–remitting multiple sclerosis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0127960.
Appendix 1 Searches undertaken for the systematic reviews of clinical effectiveness
Multiple sclerosis searches
Review articles checked for both included studies and studies excluded with reasons
MEDLINE (via Ovid)
Database: Ovid MEDLINE® 1946 to January week 2 2016.
Searched on 27 January 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | exp Multiple Sclerosis/ | 46,764 |
| 2 | multiple sclerosis.tw. | 49,799 |
| 3 | 1 or 2 | 57,188 |
| 4 | randomized controlled trial.pt. | 403,450 |
| 5 | controlled clinical trial.pt. | 89,937 |
| 6 | clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ | 35,683 |
| 7 | (random* or ‘controlled trial*’ or ‘clinical trial*’ or rct).tw. | 873,696 |
| 8 | 4 or 5 or 6 or 7 | 1,065,585 |
| 9 | Animals/ | 5,743,229 |
| 10 | Humans/ | 15,593,111 |
| 11 | 9 not 10 | 4,140,900 |
| 12 | 8 not 11 | 964,542 |
| 13 | 3 and 12 | 4921 |
| 14 | (metaanalys* or ‘meta analys*’ or ‘meta-analys*’).tw. | 69,140 |
| 15 | ‘systematic* review*’.mp. | 61,461 |
| 16 | meta analysis.pt. | 60,117 |
| 17 | 14 or 15 or 16 | 122,687 |
| 18 | 3 and 17 | 635 |
| 19 | limit 3 to systematic reviews | 1136 |
| 20 | 18 or 19 | 1233 |
| 21 | 13 or 20 | 5694 |
| 22 | limit 21 to yr=‘2012 -Current’ | 1545 |
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Database: Ovid MEDLINE® In-Process & Other Non-Indexed Citations 26 January 2016.
Searched on 27 January 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | multiple sclerosis.tw. | 4892 |
| 2 | (random* or ‘controlled trial*’ or ‘clinical trial*’ or rct).tw. | 108,317 |
| 3 | 1 and 2 | 610 |
| 4 | (metaanalys* or ‘meta analys*’ or ‘meta-analys*’).tw. | 14,094 |
| 5 | ‘systematic* review*’.tw. | 15,189 |
| 6 | 4 or 5 | 23,570 |
| 7 | 1 and 6 | 118 |
| 8 | 3 or 7 | 684 |
| 9 | limit 8 to yr=‘2012 -Current’ | 563 |
EMBASE (via Ovid)
Database: Ovid EMBASE 1974 to 2016 week 04.
Searched on 27 January 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | *multiple sclerosis/ | 64,389 |
| 2 | multiple sclerosis.tw. | 80,240 |
| 3 | 1 or 2 | 87,466 |
| 4 | randomized controlled trial/ | 392,971 |
| 5 | (random* or ‘controlled trial*’ or ‘clinical trial*’ or rct).tw. | 1,306,964 |
| 6 | 4 or 5 | 1,388,801 |
| 7 | 3 and 6 | 8813 |
| 8 | meta analysis/ | 103,317 |
| 9 | (metaanalys* or ‘meta analys*’ or ‘meta-analys*’).tw. | 110,582 |
| 10 | ‘systematic review’/ | 100,520 |
| 11 | ‘systematic* review*’.tw. | 96,391 |
| 12 | 8 or 9 or 10 or 11 | 222,654 |
| 13 | 3 and 12 | 1280 |
| 14 | 7 or 13 | 9616 |
| 15 | limit 14 to yr = ‘2012 -Current’ | 4527 |
| 16 | limit 15 to (conference abstract or conference paper or conference proceeding) | 2363 |
| 17 | 15 not 16 | 2164 |
The Cochrane Library (via Wiley Online Library)
Searched on 27 January 2016.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Multiple Sclerosis] explode all trees | 1916 |
| #2 | multiple sclerosis:ti,ab,kw (Word variations have been searched) | 4921 |
| #3 | #1 or #2 | 4925 |
| #4 | #1 or #2 Publication Year from 2012 to 2016 | 1861 |
Distribution of results from The Cochrane Library search:
-
Cochrane reviews, n = 44:
-
reviews, n = 39
-
protocols, n = 5
-
-
other reviews (DARE), n = 60
-
trials (CENTRAL), n = 1702
-
methods studies, n = 0
-
technology assessments (HTA database), n = 28
-
economic evaluations, n = 27
-
Cochrane groups, n = 0.
Science Citation Index (Web of Knowledge)
Searched on 27 January 2016.
| ID | Hits | Search |
|---|---|---|
| #1 | 85,913 | TS = ‘multiple sclerosis’ Indexes = SCI-EXPANDED Timespan = All years |
| #2 | 1,388,789 | TS = (random* or (clinical NEAR/1 trial*) or (controlled NEAR/1 trial*) or rct) Indexes = SCI-EXPANDED Timespan = All years |
| #3 | 80,440 | TS = (systematic* NEAR/1 review*) Indexes = SCI-EXPANDED Timespan = All years |
| #4 | 166,410 | TS = (metaanalys* or meta-analys* or (meta NEAR/1 analys*)) Indexes = SCI-EXPANDED Timespan = All years |
| #5 | 216,848 | #4 OR #3 Indexes = SCI-EXPANDED Timespan = All years |
| #6 | 8425 | #2 AND #1 Indexes = SCI-EXPANDED Timespan = All years |
| #7 | 1326 | #5 AND #1 Indexes = SCI-EXPANDED Timespan = All years |
| #8 | 9263 | #7 OR #6 Indexes = SCI-EXPANDED Timespan = All years |
| #9 | 3485 | #8 Indexes = SCI-EXPANDED Timespan = 2012–2016 |
| #10 | 237 | (#9) AND DOCUMENT TYPES: (Meeting Abstract OR Proceedings Paper) Indexes = SCI-EXPANDED Timespan = All years |
| #11 | 3248 | #9 not #10 Indexes = SCI-EXPANDED Timespan = All years |
UK Clinical Research Network (UKCRN) Portfolio Database
Searched on 27 January 2016.
Total hits: 265.
Search
Keyword: multiple sclerosis
AND
Status: closed
AND
Study Design: Interventional
Total hits: 41.
Cochrane MS Group Specialised Register
Searched 26 February 2016
Keywords
(interferon\*) OR (interferon beta) OR (beta-1 interferon) OR (beta 1 interferon) OR (interferon beta-1\*) OR (rebif) OR (avonex) OR (Betaseron) OR (beta-seron) OR (betaferon) OR (beta-IFN-1\*) OR (interferon beta-1\*) OR (Interferon-beta\*) OR (interferon beta\*) OR (recombinant interferon beta-1\*) OR (beta-1a interferon) OR (beta 1a interferon) OR (interferon beta-1a) OR (beta 1b interferon) OR (interferon beta1b) OR (IFNb-1b) OR (IFNbeta-1b) OR (interferon beta-1b) OR (copolymer-1) OR (cop-1) OR (copaxone) OR (glatiramer acetate) OR (cpx) OR (cop1) OR (copolymer) OR (glatiramer) OR (polyethylene glycol-interferon-beta-1a) OR (PEG IFN-beta-1a) OR (Pegylated interferon beta-1a) OR (Ocrelizumab)
AND
(relapsing remitting) OR (relapsing–remitting) OR (remitting-relapsing) OR (remitting relapsing) OR (secondary progressive)
Clinically isolated syndrome searches
Review articles checked for included studies and studies excluded with reasons
-
Cochrane reviews: Clerico et al. 158
MEDLINE (Ovid)
Database: Ovid MEDLINE® 1946 to January week 4 2016.
Searched on 9 February 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | Demyelinating Diseases/ | 10,446 |
| 2 | Myelitis, Transverse/ | 1153 |
| 3 | exp Optic Neuritis/ | 6737 |
| 4 | Encephalomyelitis, Acute Disseminated/ | 1689 |
| 5 | Demyelinating Autoimmune Diseases, CNS/ | 316 |
| 6 | demyelinating disease*.tw. | 4725 |
| 7 | transverse myelitis.tw. | 1356 |
| 8 | neuromyelitis optica.tw. | 1735 |
| 9 | optic neuritis.tw. | 3792 |
| 10 | acute disseminated encephalomyelitis.tw. | 1098 |
| 11 | devic.tw. | 107 |
| 12 | ADEM.tw. | 574 |
| 13 | demyelinating disorder.tw. | 335 |
| 14 | clinically isolated syndrome.tw. | 644 |
| 15 | first demyelinating event.tw. | 68 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 24,564 |
| 17 | randomised controlled trial.pt. | 404,260 |
| 18 | (random* or ‘controlled trial*’ or ‘clinical trial*’ or rct).tw. | 875,933 |
| 19 | 17 or 18 | 975,513 |
| 20 | (metaanalys* or ‘meta analys*’ or ‘meta-analys*’).tw. | 69,583 |
| 21 | ‘systematic* review*’.mp. | 61,879 |
| 22 | meta analysis.pt. | 60,490 |
| 23 | 20 or 21 or 22 | 123,386 |
| 24 | 16 and 19 | 661 |
| 25 | 16 and 23 | 74 |
| 26 | 24 or 25 | 713 |
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Database: Ovid MEDLINE® In-Process & Other Non-Indexed Citations 8 February 2016.
Searched on 9 February 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | demyelinating disease*.tw. | 405 |
| 2 | transverse myelitis.tw. | 148 |
| 3 | neuromyelitis optica.tw. | 317 |
| 4 | optic neuritis.tw. | 356 |
| 5 | acute disseminated encephalomyelitis.tw. | 128 |
| 6 | devic.tw. | 6 |
| 7 | ADEM.tw. | 83 |
| 8 | demyelinating disorder.tw. | 55 |
| 9 | clinically isolated syndrome.tw. | 115 |
| 10 | first demyelinating event.tw. | 6 |
| 11 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 | 1249 |
| 12 | (random* or ‘controlled trial*’ or ‘clinical trial*’ or rct).tw. | 108,853 |
| 13 | (metaanalys* or ‘meta analys*’ or ‘meta-analys*’).tw. | 14,202 |
| 14 | ‘systematic* review*’.tw. | 15,358 |
| 15 | 13 or 14 | 23,763 |
| 16 | 11 and 12 | 63 |
| 17 | 11 and 15 | 17 |
| 18 | 16 or 17 | 73 |
EMBASE (via Ovid)
Database: Ovid EMBASE 1974 to 2016 week 06.
Searched on 9 February 2016.
| ID | Search | Hit |
|---|---|---|
| 1 | demyelinating disease/ | 12,216 |
| 2 | myelitis/ | 6771 |
| 3 | optic neuritis/ | 6979 |
| 4 | acute disseminated encephalomyelitis/ | 1378 |
| 5 | myelooptic neuropathy/ | 4897 |
| 6 | demyelinating disease*.tw. | 7443 |
| 7 | transverse myelitis.tw. | 2462 |
| 8 | neuromyelitis optica.tw. | 4162 |
| 9 | optic neuritis.tw. | 6551 |
| 10 | acute disseminated encephalomyelitis.tw. | 1762 |
| 11 | devic.tw. | 229 |
| 12 | ADEM.tw. | 1211 |
| 13 | demyelinating disorder.tw. | 624 |
| 14 | clinically isolated syndrome.tw. | 1758 |
| 15 | first demyelinating event.tw. | 159 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 34,739 |
| 17 | randomized controlled trial/ | 394,252 |
| 18 | (random* or ‘controlled trial*’ or ‘clinical trial*’ or rct).tw. | 1,311,256 |
| 19 | 17 or 18 | 1,393,301 |
| 20 | meta analysis/ | 103,826 |
| 21 | (metaanalys* or ‘meta analys*’ or ‘meta-analys*’).tw. | 111,288 |
| 22 | ‘systematic review’/ | 101,172 |
| 23 | ‘systematic* review*’.tw. | 97,114 |
| 24 | 20 or 21 or 22 or 23 | 223,913 |
| 25 | 16 and 19 | 1706 |
| 26 | 16 and 24 | 322 |
| 27 | 25 or 26 | 1914 |
| 28 | limit 27 to (conference abstract or conference paper or conference proceeding or ‘conference review’) | 493 |
| 29 | 27 not 28 | 1421 |
| 30 | limit 29 to human | 1340 |
| 31 | limit 29 to animals | 59 |
| 32 | 31 not 30 | 59 |
| 33 | 29 not 32 | 1362 |
The Cochrane Library (via Wiley Online Library)
Searched on 9 February 2016.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Multiple Sclerosis] explode all trees | 2125 |
| #2 | multiple sclerosis:ti,ab,kw (Word variations have been searched) | 5081 |
| #3 | #1 or #2 | 5081 |
| #4 | first or early or ‘clinically isolated’:ti,ab,kw (Word variations have been searched) | 166,444 |
| #5 | #3 and #4 | 1037 |
| #6 | MeSH descriptor: [Demyelinating Diseases] this term only | 71 |
| #7 | MeSH descriptor: [Myelitis, Transverse] this term only | 6 |
| #8 | MeSH descriptor: [Optic Neuritis] explode all trees | 95 |
| #9 | MeSH descriptor: [Encephalomyelitis, Acute Disseminated] this term only | 3 |
| #10 | MeSH descriptor: [Demyelinating Autoimmune Diseases, CNS] this term only | 2 |
| #11 | demyelinating next disease*:ti,ab,kw (Word variations have been searched) | 186 |
| #12 | transverse myelitis:ti,ab,kw (Word variations have been searched) | 14 |
| #13 | neuromyelitis optica:ti,ab,kw (Word variations have been searched) | 20 |
| #14 | optic neuritis:ti,ab,kw (Word variations have been searched) | 220 |
| #15 | acute disseminated encephalomyelitis:ti,ab,kw (Word variations have been searched) | 13 |
| #16 | devic:ti,ab,kw (Word variations have been searched) | 2 |
| #17 | ADEM:ti,ab,kw (Word variations have been searched) | 4 |
| #18 | demyelinating disorder:ti,ab,kw (Word variations have been searched) | 49 |
| #19 | clinically isolated syndrome:ti,ab,kw (Word variations have been searched) | 114 |
| #20 | first demyelinating event:ti,ab,kw (Word variations have been searched) | 72 |
| #21 | single demyelinating event:ti,ab,kw (Word variations have been searched) | 9 |
| #22 | #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 | 1436 |
Distribution of results from The Cochrane Library search:
-
Cochrane reviews, n = 41
-
other reviews, n = 8
-
trials, n = 1369
-
methods studies, n = 4
-
technology assessments, n = 6
-
economic evaluations, n = 8
-
Cochrane Groups, n = 0.
Science Citation Index (Web of Knowledge)
Searched on 10 February 2016.
| ID | Hits | Search |
|---|---|---|
| #1 | 6786 | TS = (demyelinating NEAR/2 (disease* OR disorder*)) Indexes = SCI-EXPANDED Timespan = All years |
| #2 | 1699 | TS = (transverse NEAR/1 myelitis) Indexes = SCI-EXPANDED Timespan = All years |
| #3 | 4584 | TS = ‘optic neuritis’ Indexes = SCI-EXPANDED Timespan = All years |
| #4 | 3531 | TS = ‘neuromyelitis optica’ Indexes = SCI-EXPANDED Timespan = All years |
| #5 | 1596 | TS = (‘acute disseminated’ NEAR/1 encephalomyelitis) Indexes = SCI-EXPANDED Timespan = All years |
| #6 | 462 | TS = ‘devic’ Indexes = SCI-EXPANDED Timespan = All years |
| #7 | 687 | TS = ‘ADEM’ Indexes = SCI-EXPANDED Timespan = All years |
| #8 | 1195 | TS = ‘clinically isolated syndrome’ Indexes = SCI-EXPANDED Timespan = All years |
| #9 | 96 | TS = ‘first demyelinating event’ Indexes = SCI-EXPANDED Timespan = All years |
| #10 | 16,869 | #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes = SCI-EXPANDED Timespan = All years |
| #11 | 1,393,569 | TS = (random* or (clinical NEAR/1 trial*) or (controlled NEAR/1 trial*) or rct) Indexes = SCI-EXPANDED Timespan = All years |
| #12 | 80,440 | TS = (systematic* NEAR/1 review*) Indexes = SCI-EXPANDED Timespan = All years |
| #13 | 167,718 | TS = (metaanalys* or meta-analys* or (meta NEAR/1 analys*)) Indexes = SCI-EXPANDED Timespan = All years |
| #14 | 216,848 | #13 OR #12 Indexes = SCI-EXPANDED Timespan = All years |
| #15 | 1039 | #11 AND #10 Indexes = SCI-EXPANDED Timespan = All years |
| #16 | 122 | #14 AND #10 Indexes = SCI-EXPANDED Timespan = All years |
| #17 | 1123 | #16 OR #15 Indexes = SCI-EXPANDED Timespan = All years |
| #18 | 93 | (#17) AND DOCUMENT TYPES: (Meeting Abstract OR Proceedings Paper) Indexes = SCI-EXPANDED Timespan = All years |
| #19 | 1030 | #17 NOT #18 Indexes = SCI-EXPANDED Timespan = All years |
Cochrane MS Group Specialised Register
Searched on 26 February 2016.
Total hits: 188.
Keywords for CIS
(interferon\*) OR (interferon beta) OR (beta-1 interferon) OR (beta 1 interferon) OR (interferon beta-1\*) OR (rebif) OR (avonex) OR (Betaseron) OR (beta-seron) OR (betaferon) OR (beta-IFN-1\*) OR (interferon beta-1\*) OR (Interferon-beta\*) OR (interferon beta\*) OR (recombinant interferon beta-1\*) OR (beta-1a interferon) OR (beta 1a interferon) OR (interferon beta-1a) OR (beta 1b interferon) OR (interferon beta1b ) OR (IFNb-1b) OR (IFNbeta-1b) OR (interferon beta-1b) OR (copolymer-1) OR (cop-1) OR (copaxone) OR (glatiramer acetate) OR (cpx) OR (cop1) OR (copolymer) OR (glatiramer) OR (polyethylene glycol-interferon-beta-1a) OR (PEG IFN-beta-1a) OR (Pegylated interferon beta-1a) OR (Ocrelizumab)
AND
clinically isolated syndrome* OR first demyelinating event* OR first demyelinating episode OR first demyelinating attack OR First event OR first episode OR first clinical episode OR single clinical episodes OR first demyelinating event/* OR clinically isolated syndrome*
Additional searches for both multiple sclerosis and clinically isolated syndrome
ClinicalTrials.gov
Searched on 3 May 2016.
Hits: 182.
Advanced search
Interventional Studies | multiple sclerosis OR clinically isolated syndrome OR CNS demyelinating OR transverse myelitis OR neuromyelitis optica | interferon OR glatiramer OR betaferon OR betaseron OR avonex OR plegridy OR rebif OR extavia OR copaxone | Phase 2, 3, 4
World Health Organization (WHO)’s International Clinical Trials Registry Platform (ICTRP)
Searched on 14 July 2016.
Hits: 588 records for 175 trials.
(Relapsing Remitting Multiple Sclerosis OR RRMS OR clinically isolated syndrome OR CNS demyelinating OR transverse myelitis OR neuromyelitis optica) in the Condition
AND
(interferon OR glatiramer OR betaferon OR betaseron OR avonex OR plegridy OR rebif OR extavia OR copaxone) in the Intervention
Websites
| Name (trade name) | URL | Date searched |
|---|---|---|
| Companies and sponsors | ||
| Bayer (Betaferon) | www.bayer.co.uk/http://pharma.bayer.com/ | 26 April 2016 |
| Biogen Idec Ltd (Avonex and Plegridy) | www.biogen-international.com/https://www.biogen.uk.com/ | 28 April 2016 |
| Merck Serono (Rebif) | http://biopharma.merckgroup.com/en/index.html | |
| Novartis (Extavia) | www.novartis.com https://www.novartis.co.uk/ | 28 April 2016 |
| Teva Pharmaceuticals (Copaxone) | www.tevapharm.com/research_development/http://www.tevauk.com/ | 1 May 2016 |
| Patient/carer groups | ||
| Brain and Spine Foundation | www.brainandspine.org.uk | 1 May 2016 |
| Multiple Sclerosis National Therapy Centres | www.msntc.org.uk | 1 May 2016 |
| MS UK | www.ms-uk.org | 1 May 2016 |
| Multiple Sclerosis Society | www.mssociety.org.uk | 1 May 2016 |
| Multiple Sclerosis Trust | www.mstrust.org.uk | 1 May 2016 |
| Neurological Alliance | www.neural.org.uk | 1 May 2016 |
| The Brain Charity (formerly known as Neurosupport) | www.thebraincharity.org.uk | 1 May 2016 |
| Sue Ryder | www.sueryder.org | 1 May 2016 |
| Professional groups | ||
| Association of British Neurologists | www.theabn.org | 1 May 2016 |
| British Neuropathological Society | www.bns.org.uk | 1 May 2016 |
| Institute of Neurology | www.ucl.ac.uk/ion | 1 May 2016 |
| www.ucl.ac.uk/ion/departments/neuroinflammation | 5 May 2016 | |
| http://discovery.ucl.ac.uk | 10 May 2016 | |
| Primary Care Neurology Society | www.p-cns.org.uk | 1 May 2016 |
| Therapists in MS | www.mstrust.org.uk/health-professionals/professional-networks/therapists-ms-tims/research | 1 May 2016 |
| UK Multiple Sclerosis Specialist Nurse Association | www.ukmssna.org.uk | 1 May 2016 |
| Relevant research groups | ||
| Brain Research Trust | www.brt.org.uk/research | 1 May 2016 |
| British Neurological Research Trust | www.ukscf.org; www.ukscf.org/about-us/bnrt.html | 1 May 2016 |
| Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group | www.cochranelibrary.com; http://msrdcns.cochrane.org/our-reviews | 1 May 2016 |
| National Institute for Health Research | www.nihr.ac.uk/research/; www.nihr.ac.uk/industry/; www.nihr.ac.uk/policy-and-standards/ | 1 May 2016 |
Appendix 2 Sample data extraction sheet for clinical effectiveness reviews
| Study acronym/ID: | ||
| Name of reviewer: | ||
| Number of publications extracted: | ||
| Study details | ||
|---|---|---|
| Study ID (EndNote): | ||
| First author surname: | ||
| Year of publication: | ||
| Country: | ||
| Study setting: | ||
| Number of centres: | ||
| Study period: | ||
| Follow-up period: | ||
| Funding: | ||
| Subtypes of MS included: | ||
| Definition of CIS used: | ||
| Aim of the study: | ||
| Participants: | ||
| Inclusion criteria: | ||
| Exclusion criteria: | ||
| Total number of participants: | ||
| Sample attrition/dropout: | ||
| Number of participants analysed: | ||
| Characteristics of participants | ||
| Mean age: | ||
| Mean sex: | ||
| Race: | ||
| EDSS score at baseline: | ||
| Relapse rate at baseline: | ||
| Time from diagnosis of MS: | ||
| Other clinical features of MS: | ||
| Intervention (repeat if necessary for multiple intervention arms) | ||
| Type of drug: | ||
| Method of administration: | ||
| Dose: | ||
| Frequency: | ||
| Drug indication as stated: | ||
| Best supportive care as described | ||
| Outcomes | ||
| Primary outcomes: | ||
| Secondary outcomes: | ||
| Method of assessing outcomes: | ||
| If freedom from disease activity is an outcome, how was it defined? | ||
| Timing of assessment: | ||
| Adverse event: | ||
| Health-related quality of life: yes/no; which measures used? | ||
| Number of participants | Intervention | Comparator, if present |
| Screened | ||
| Excluded | ||
| Randomised/included | ||
| Missing participants (people who were lost to follow-up during the trial) | ||
| Withdrawals (all who did not complete, including those lost to follow-up) | ||
| Patient baseline characteristics | ||
| Age (years) | ||
| Sex | ||
| Race | ||
| EDSS score at baseline | ||
| Relapse rate at baseline | ||
| Time from diagnosis of MS | ||
| Outcome data: relapses, disability | ||
| Relapse rate | ||
| Severity of relapse | ||
| Disability, including as measured by the EDSS | ||
| Freedom from disease activity | ||
| Outcome data: MS symptoms (add rows as necessary) | ||
| Fatigue | ||
| Visual disturbance | ||
| Cognition | ||
| Outcome data: additional outcomes | ||
| Mortality | ||
| Health-related quality of life | ||
| Progression to MS (CIS only) | ||
| Discontinuation due to neutralising antibody formation | ||
| Adverse events (add rows as necessary for adverse events reported in RCTs) | ||
| Risk of bias assessment | ||
| Random sequence generation | High risk Unclear risk Low risk | |
| Description in trial | ||
| Allocation concealment | High risk Unclear risk Low risk | |
| Description in trial | ||
| Blinding of participants and personnel | High risk Unclear risk Low risk | |
| Description in trial | ||
| Blinding of outcome assessment | High risk Unclear risk Low risk | |
| Description in trial | ||
| Incomplete outcome data | High risk Unclear risk Low risk | |
| Description in trial | ||
| Selective reporting | High risk Unclear risk Low risk | |
| Description in trial | ||
| Other sources of bias | High risk Unclear risk Low risk | |
| Description in trial | ||
| Authors’ conclusion | ||
| Reviewer’s conclusion | ||
Appendix 3 Documentation of excluded studies
| Reasons | Number |
|---|---|
| Conference abstract | 10 |
| DMT used with a non-recommended dose regimen | 15 |
| Irrelevant comparator/intervention | 58 |
| Irrelevant comparator/intervention/outcome | 1 |
| Irrelevant comparator/intervention/population | 1 |
| Irrelevant comparator/intervention/study type | 4 |
| Irrelevant comparator/population | 5 |
| Irrelevant comparator/population/study type | 1 |
| Irrelevant intervention | 7 |
| Irrelevant intervention/population | 2 |
| Irrelevant intervention/study type | 8 |
| Irrelevant outcome | 13 |
| Irrelevant outcome/study type | 2 |
| Irrelevant outcome/study type/population | 1 |
| Irrelevant population | 11 |
| Irrelevant population/outcome | 1 |
| Irrelevant population/study type | 7 |
| Irrelevant study type | 24 |
| Non-English-language study | 1 |
| No results are provided, refers to results from a conference abstract | 1 |
| Not a primary research study | 3 |
| Protocol only with no results | 15 |
| Study evaluating a treatment-switch strategy | 1 |
| Systematic review that did not enable location of further primary studies | 18 |
| Use of an unlicensed drug formulation | 1 |
| Total | 211 |
| Reference | Reason for exclusion |
|---|---|
| Aggarwal S, Kumar S, Topaloglu H. Comparison of network meta-analysis and traditional meta-analysis for prevention of relapses in multiple sclerosis. Value Health 2015;18:A660. http://dx.doi.org/10.1016/j.jval.2015.09.2394 | Conference abstract |
| Agius M, Meng X, Chin P, Grinspan A, Hashmonay R. Fingolimod therapy in early multiple sclerosis: an efficacy analysis of the TRANSFORMS and FREEDOMS studies by time since first symptom. CNS Neurosci Ther 2014;20:446–51 | Irrelevant comparator/intervention |
| Åivo J, Lindsrom BM, Soilu-Hanninen M. A randomised, double-blind, placebo-controlled trial with vitamin D3 in MS: subgroup analysis of patients with baseline disease activity despite interferon treatment. Mult Scler Int 2012;2012:802796 | Irrelevant comparator/intervention |
| Andersen O, Elovaara I, Färkkilä M, Hansen HJ, Mellgren SI, Myhr KM, et al. Multicentre, randomised, double blind, placebo controlled, phase III study of weekly, low dose, subcutaneous interferon beta-1a in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2004;75:706–10 | DMT used with a non-recommended dose regimen |
| Andersen O, Elovaara I, Färkkilä M, Hansen HJ, Mellgren SI, Myhr KM, et al. Multicentre, randomised, double blind, placebo controlled, phase III study of weekly, low dose, subcutaneous interferon beta-1a in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2004;75:706–10 | DMT used with a non-recommended dose regimen |
| Anderson G, Meyer D, Herrman CE, Sheppard C, Murray R, Fox EJ, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol 2010;257:1917–23 | Use of an unlicensed drug formulation |
| Anonymous. Visual function 5 years after optic neuritis: experience of the Optic Neuritis Treatment Trial. The Optic Neuritis Study Group. Arch Ophthalmol 1997;115:1545–52 | Irrelevant comparator/intervention |
| Anonymous. Early administration of interferon-beta-1a in multiple sclerosis. Eur J Pediatr 2001;160:135–6 | Irrelevant study type |
| Anonymous. Baseline MRI characteristics of patients at high risk for multiple sclerosis: results from the CHAMPS trial. Controlled High-risk subjects Avonex Multiple sclerosis Prevention Study. Mult Scler 2002;8:330–8 | Irrelevant outcome |
| Anonymous. Developing Neuroprotection and Repair Strategies in MS: Phase IIa Randomized, Controlled Trial of Minocycline in Acute Optic Neuritis (ON). ClinicalTrials.gov. National Institutes of Health; 2010. URL: https://clinicaltrials.gov/ct2/show/NCT01073813 (accessed 1 June 2016) | Irrelevant intervention |
| A Phase II Study Comparing Low- and High-Dose Alemtuzumab and High-Dose Rebif® in Patients With Early, Active Relapsing–Remitting Multiple Sclerosis. ClinicalTrials.gov, National Institutes of Health; 2002. URL: https://clinicaltrials.gov/ct2/show/NCT00050778 (accessed 15 June 2017) | Protocol only with no results |
| A Randomized, International, Multi Centre Study to Assess the Efficacy and Safety of Intravenous PEG-Liposomal Prednisolone Sodium Phosphate (Nanocort®) vs Intravenous Methylprednisolone (Solu-Medrol®) Treatment in Patients with Acute Exacerbation of Relapsing–Remitting Multiple Sclerosis or in Patients with Clinically Isolated Syndrome. ClinicalTrials.gov, National Institutes of Health; 2009. URL: https://clinicaltrials.gov/ct2/show/NCT01039103 (accessed 15 June 2017) | Protocol only with no results |
| Arnold DL, Narayanan S, Antel S. Neuroprotection with glatiramer acetate: evidence from the PreCISe trial. J Neurol 2013;260:1901–6. http://dx.doi.org/10.1007/s00415-013-6903-5 | Irrelevant outcome |
| Ashtari F, Savoj MR. Effects of low dose methotrexate on relapsing–remitting multiple sclerosis in comparison to Interferon β-1α: a randomized controlled trial. J Res Med Sci 2011;16:457–62 | Irrelevant intervention |
| Balak DM, Hengstman GJ, Cakmak A, Thio HB. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler 2012;18:1705–17 | Systematic review that did not enable location of further primary studies |
| Balcer LJ, Galetta SL, Calabresi PA, Confavreux C, Giovannoni G, Havrdova E, et al. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurol 2007;68:1299–304 | Irrelevant comparator/intervention |
| Bandari D, Wynn D, Miller T, Singer B, Wray S, Bennett R, et al. Rebif(®) Quality of Life (RebiQoL): a randomized, multicenter, Phase IIIb study evaluating quality-of-life measures in patients receiving the serum-free formulation of subcutaneous interferon beta-1a for the treatment of relapsing forms of multiple sclerosis. Mult Scler Relat Disord 2013;2:45–56 | Irrelevant comparator/intervention |
| Barkhof F, Polman CH, Radue EW, Kappos L, Freedman MS, Edan G, et al. Magnetic resonance imaging effects of interferon beta-1b in the BENEFIT study: integrated 2-year results. Arch Neurol 2007;64:1292–8 | Irrelevant outcome |
| Barkhof F, Rocca M, Francis G, Van Waesberghe JH, Uitdehaag BM, Hommes OR, et al. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon beta1a. Ann Neurol 2003;53:718–24 | DMT used with a non-recommended dose regimen |
| Beck RW. The optic neuritis treatment trial: three-year follow-up results. Arch Ophthalmol 1995;113:136–7 | Irrelevant comparator/intervention/study type |
| Beck RW, Trobe JD. The Optic Neuritis Treatment Trial. Putting the results in perspective. The Optic Neuritis Study Group. J Neuroophthalmol 1995;15:131–5 | Irrelevant comparator/intervention |
| Berkovich, R, Amezcua L, Subhani D, Cen S. Pilot study of monthly pulse adrenocorticotropic hormone (ACTH) or methylprednisolone as an add-on therapy to beta-interferons for long-term treatment of multiple sclerosis. Neurol 2013;80:e205-6 | Irrelevant comparator/intervention |
| Bermel RA, Weinstock-Guttman B, Bourdette D, Foulds P, You X, Rudick RA. Intramuscular interferon beta-1a therapy in patients with relapsing–remitting multiple sclerosis: a 15-year follow-up study. Mult Scler 2010;16:588–96 | Irrelevant comparator/intervention |
| Bornstein MB, Miller A, Slagle S, Weitzman M, Drexler E, Keilson M, et al. A placebo-controlled, double-blind, randomised, two-centre, pilot trial of Cop 1 in chronic progressive multiple sclerosis. Neurol 1991;41:533–9 | Irrelevant population |
| Brex PA, Molyneux PD, Smiddy P, Barkhof F, Filippi M, Yousry TA, et al. The effect of IFNbeta-1b on the evolution of enhancing lesions in secondary progressive MS. Neurology 2001;57:2185–90 | Irrelevant outcome |
| Brunetti L, Wagner ML, Maroney M, Ryan M. Teriflunomide for the treatment of relapsing multiple sclerosis: a review of clinical data. Ann Pharmacother 2013;47:1153–60. http://dx.doi.org/10.1177/1060028013500647 | Irrelevant intervention/study type |
| Calkwood J, Cree B, Crayton H, Kantor D, Steingo B, Barbato L, et al. Impact of a switch to fingolimod versus staying on glatiramer acetate or beta interferons on patient- and physician-reported outcomes in relapsing multiple sclerosis: post hoc analyses of the EPOC trial. BMC Neurol 2014;14:220. http://dx.doi.org/10.1186/s12883-014-0220-1 | Irrelevant comparator/intervention |
| Canadian Agency for Drugs and Technologies in Health. Clinical Review Report. Teriflunomide (Aubagio – Genzyme Canada) Indication: Relapsing–Remitting Multiple Sclerosis. 2014. URL: www.cadth.ca/sites/default/files/cdr/clinical/SR0350_Aubagio_CL_Report_e.pdf (accessed 1 June 2016) | Irrelevant intervention/study type |
| Chan CK, Lam DS. Optic neuritis treatment trial:10-year follow-up results. Am J Ophthalmol 2004;138:695 | Irrelevant study type |
| Chinea Martinez AR, Correale J, Coyle PK, Meng X, Tenenbaum N. Efficacy and safety of fingolimod in Hispanic patients with multiple sclerosis: pooled clinical trial analyses. Adv Ther 2014;31:1072–81 | Irrelevant comparator/intervention |
| Clerico M, Contessa G, Durelli L. Interferon-beta1a for the treatment of multiple sclerosis. Expert Opin Biol Ther 2007;7:535–42. http://dx.doi.org/10.1517/14712598.7.4.535 | Irrelevant study type |
| Clerico M, Schiavetti I, Mercanti SF, Piazza F, Gned D, Morra VB, et al. Treatment of relapsing–remitting multiple sclerosis after 24 doses of natalizumab: evidence from an Italian spontaneous, prospective, and observational study (the TY-STOP study). JAMA Neurol 2014;71:954–60 | Irrelevant study type |
| ClinicalTrials.gov. An Efficacy and Safety Comparison Study of Two Marketed Drugs in Patients With Relapsing–Remitting MS (ABOVE). URL: https://clinicaltrials.gov/ct2/show/NCT00206648 (accessed 1 June 2016) | Study evaluating a treatment-switch strategy |
| Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–15. http://dx.doi.org/10.1056/NEJMoa0907839 | Irrelevant comparator/intervention |
| Cohen JA, Barkhof F, Comi G, Izquierdo G, Khatri B, Montalban X, et al. Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol 2013;260:2023–32. http://dx.doi.org/10.1007/s00415-013-6932-0 | Irrelevant comparator/intervention |
| Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing–remitting multiple sclerosis: a randomised controlled Phase 3 trial. Lancet 2012;380:1819–28 | Irrelevant comparator/intervention |
| Cohen JA, Coles AJ, Arnold DL, Confavreu C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing–remitting multiple sclerosis: a randomised controlled Phase 3 trial. Lancet 2012;380:1819–28 | Irrelevant comparator/intervention |
| Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR, Kooijmans MF, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 2002;59:679–87 | DMT used with a non-recommended dose regimen |
| Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR, Kooijmans MF, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 2002;59:679–87 | DMT used with a non-recommended dose regimen |
| Cohen JA, Rovaris M, Goodman AD, Ladkani D, Wynn D, Filippi M, 9006 Study Group. Randomized, double-blind, dose-comparison study of glatiramer acetate in relapsing–remitting MS. Neurology 2007;68:939–44 | Irrelevant comparator/intervention/population |
| Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008;359:1786–801. http://dx.doi.org/10.1056/NEJMoa0802670 | Irrelevant comparator/intervention |
| Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008;359:1786–801. http://dx.doi.org/10.1056/NEJMoa0802670 | Irrelevant comparator/intervention |
| Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab versus interferon β-1a in early relapsing–remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol 2011;10:338–48 | Irrelevant comparator/population |
| Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab more effective than interferon β-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology 2012;78:1069–78. http://dx.doi.org/10.1212/WNL.0b013e31824e8ee7 | Irrelevant comparator/intervention |
| Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled Phase 3 trial. Lancet 2012;380:1829–39 | Irrelevant comparator/intervention |
| Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled Phase 3 trial. Lancet 2012;380:1829–39 | Irrelevant comparator/intervention |
| Comi G, Barkhof F, Durelli L, Edan G, Fernandez O, Filippi M, et al. Early treatment of multiple sclerosis with Rebif (recombinant human interferon beta): design of the study. Mult Scler 1995;1:S24–7 | Protocol only with no results |
| Comi G, Cohen JA, Arnold DL, Wynn D, Filippi M, FORTE Study Group. Phase III dose-comparison study of glatiramer acetate for multiple sclerosis. Ann Neurol 2011;69:75–82. http://dx.doi.org/10.1002/ana.22316 | Irrelevant comparator/intervention |
| Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernández O, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 2001;357:1576–82 | DMT used with a non-recommended dose regimen |
| Comi G, Martinelli V, Rodegher M, Moiola L, Leocani L, Bajenaru O, et al. Effects of early treatment with glatiramer acetate in patients with clinically isolated syndrome. Mult Scler 2013;19:1074–83 | Irrelevant population/study type |
| Comi G, O’Connor P, Montalban X, Antel J, Radue EW, Karlsson G, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler 2010;16:197–207. http://dx.doi.org/10.1177/1352458509357065 | Irrelevant comparator/intervention |
| Cooper K, Bryant J, Harris P, Loveman E, Jones J, Welch K. Alemtuzumab for the Treatment of Relapsing-Remitting Multiple Sclerosis: a Single Technology Appraisal. 2013. URL: www.nets.nihr.ac.uk/projects/hta/128301 (accessed 21 April 2017) | Irrelevant comparator/intervention |
| Dalfampridine After Optic Neuritis to Improve Visual Function in Multiple Sclerosis. ClinicalTrials.gov, National Institutes of Health; 2011. URL: http://clinicaltrials.gov/ct2/show/NCT01337986 (accessed June 2017) | Protocol only with no results |
| Daniels GH, Vladic A, Brinar V, Zavalishin I, Valente W, Oyuela P, et al. Alemtuzumab-related thyroid dysfunction in a Phase 2 trial of patients with relapsing–remitting multiple sclerosis. J Clin Endocrinol Metab 2014;99:80–9 | Irrelevant comparator/intervention |
| De Stefano N, Comi G, Kappos L, Freedman MS, Polman CH, Uitdehaag BM, et al. Efficacy of subcutaneous interferon beta-1a on MRI outcomes in a randomised controlled trial of patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry 2014;85:647–53 | Irrelevant outcome |
| De Stefano N, Curtin F, Stubinski B, Blevins G, Drulovic J, Issard D, et al. Rapid benefits of a new formulation of subcutaneous interferon beta-1a in relapsing–remitting multiple sclerosis. Mult Scler 2010;16:888–92. http://dx.doi.org/10.1177/1352458510362442 | Irrelevant outcome |
| Deisenhammer F, Hegen H. Alemtuzumab more effective than interferon β-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology 2012;79:1071–2. http://dx.doi.org/10.1212/01.wnl.0000419501.12719.38 | Irrelevant study type |
| Del Santo F, Maratea D, Fadda V, Trippoli S, Messori A. Treatments for relapsing–remitting multiple sclerosis: summarising current information by network meta-analysis. Eur J Clin Pharmacol 2012;68:441–8 | Systematic review that did not enable location of further primary studies |
| Double-Blind Extension of the Study 27025 (REFLEX) to Obtain Long-Term Follow-up Data in Patients with Clinically Definite MS and Patients with a First Demyelinating Event at High Risk of Converting to MS, Treated With Rebif® New Formulation (REFLEXION). ClinicalTrials.gov, National Institutes of Health; 2009. URL: https://clinicaltrials.gov/ct2/show/NCT00813709 (accessed 15 June 2017) | Protocol only with no results |
| Edan G, Kappos L, Montalbán X, Polman CH, Freedman MS, Hartung HP, et al. Long-term impact of interferon beta-1b in patients with CIS: 8-year follow-up of BENEFIT. J Neurol Neurosurg Psychiatr 2014;85:1183–9. http://dx.doi.org/10.1136/jnnp-2013-306222 | Irrelevant comparator/population/study type |
| Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of interferon beta products and azathioprine in the treatment of relapsing–remitting multiple sclerosis. J Neurol 2007;254:1723–8. http://dx.doi.org/10.1007/s00415-007-0637-1 | Irrelevant comparator/intervention |
| Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of interferon beta products and azathioprine in the treatment of relapsing–remitting multiple sclerosis. J Neurol 2007;254:1723–8. http://dx.doi.org/10.1007/s00415-007-0637-1 | Irrelevant comparator/population |
| Evidence of interferon beta-1a dose response in relapsing–remitting MS: the OWIMS Study. The Once Weekly Interferon for MS Study Group. Neurol 1999;53:679–86 | DMT used with a non-recommended dose regimen |
| Filippi M, Rovaris M, Inglese M, Barkhof F, Stefano N, Smith S, et al. Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet 2004;364:1489–96 | DMT used with a non-recommended dose regimen |
| Fox E, Arnold D, Brinar V, Cohen J, Coles A, Confavreux C. Relapse outcomes with alemtuzumab vs. Rebif(®) in treatment-naive relapsing–remitting multiple sclerosis (CARE-MS I): secondary and tertiary endpoints. Neurol 2010;78 | Irrelevant comparator/intervention |
| Fox RJ, Cree BA, De Sèze J, Gold R, Hartung HP, Jeffery D, et al. MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurol 2014;82:1491–8. [Erratum in Neurology 201;84:862.] | Irrelevant comparator/intervention |
| Fox RJ, Cree BA, De Sèze J, Gold R, Hartung HP, Jeffery D, et al. MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurol 2014;82:1491–8 | Irrelevant population |
| Fox E, Edwards K, Burch G, Wynn DR, LaGanke C, Crayton H, et al. Outcomes of switching directly to oral fingolimod from injectable therapies: results of the randomized, open-label, multicenter, Evaluate Patient OutComes (EPOC) study in relapsing multiple sclerosis. Mult Scler Relat Disord 2014;3:607–19 | Irrelevant comparator/intervention |
| Freedman MS. Evidence for the efficacy of interferon beta-1b in delaying the onset of clinically definite multiple sclerosis in individuals with clinically isolated syndrome. Ther Adv Neurol Disord 2014;7:279–88 | Irrelevant intervention/population |
| Freedman MS, Truffinet P, Comi G, Kappos L, Miller AE, Olsson TP, et al. A randomized trial of teriflunomide added to glatiramer acetate in relapsing multiple sclerosis. Mult Scler J Exp Transl Clin 2015;1:1–10 | Irrelevant intervention |
| Freedman MS, Wolinsky JS, Wamil B, Confavreux C, Comi G, Kappos L, et al. Teriflunomide added to interferon-β in relapsing multiple sclerosis: a randomized Phase II trial. Neurology 2012;78:1877–85 | Irrelevant intervention |
| Freedman MS, Wolinsky JS, Wamil B, Confavreux C, Comi G, Kappos L, et al. Teriflunomide added to interferon-β in relapsing multiple sclerosis: a randomized Phase II trial. Neurology 2012;78:1877–85. http://dx.doi.org/10.1212/WNL.0b013e318258f7d4 | Irrelevant comparator/intervention |
| Frohman EM, Havrdova E, Lublin F, Barkhof F, Achiron A, Sharief MK, et al. Most patients with multiple sclerosis or a clinically isolated demyelinating syndrome should be treated at the time of diagnosis. Arch Neurol 2006;63:614–19 | Irrelevant study type |
| Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg-Sorensen P. Safety and efficacy of oral cladribine in patients with relapsing–remitting multiple sclerosis: results from the 96 week Phase IIIB extension trial to the clarity study. Neurology 2013;80(7 Suppl.):P07.119 | Irrelevant intervention |
| Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler 2012;18:932–46 | Systematic review that did not enable location of further primary studies |
| Gobbi C, Meier DS, Cotton F, Sintzel M, Leppert D, Guttmann CR, Zecca C. Interferon beta 1b following natalizumab discontinuation: one year, randomized, prospective, pilot trial. BMC Neurol 2013;13:101. http://dx.doi.org/10.1186/1471-2377-13-101 | Irrelevant comparator/intervention/study type |
| Goodin DS, Ebers GC, Cutter G, Cook SD, O’Donnell T, Reder AT, et al. Cause of death in MS: long-term follow-up of a randomised cohort, 21 years after the start of the pivotal IFNbeta-1b study. BMJ Open 2012;2(6) | Irrelevant intervention/study type |
| Goodin DS, Reder AT, Ebers GC, Cutter G, Kremenchutzky M, Oger J, et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNbeta-1b trial. Neurology 2012;78:1315–22 | Irrelevant comparator/intervention/study type |
| Gotkine M. Neuromyelitis optica and the Optic Neuritis Treatment Trial. Arch Neurol 2008;65:1545–6. http://dx.doi.org/10.1001/archneur.65.11.1545-c | Irrelevant study type |
| Govindappa K, Sathish J, Park K, Kirkham J, Pirmohamed M. Development of interferon beta-neutralising antibodies in multiple sclerosis – a systematic review and meta-analysis. Eur J Clin Pharmacol 2015;71:1287–98 | Irrelevant outcome/study type |
| Hadden RD, Sharrack B, Bensa S, Soudain SE, Hughes RA. Randomized trial of interferon beta-1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 1999;53:57–61 | Irrelevant population |
| Hadjigeorgiou GM, Doxani C, Miligkos M, Ziakas P, Bakalos G, Papadimitriou D, et al. A network meta-analysis of randomized controlled trials for comparing the effectiveness and safety profile of treatments with marketing authorization for relapsing multiple sclerosis. J Clin Pharm Ther 2013;38:433–9 | Systematic review that did not enable location of further primary studies |
| Hartung HP, Freedman MS, Polman CH, Edan G, Kappos L, Miller DH, et al. Interferon beta-1b-neutralizing antibodies 5 years after clinically isolated syndrome. Neurology 2011;77:835–43. [Erratum in Neurology 2011;77(13):1317.] | Irrelevant study type |
| Hartung H, Vollmer T, Arnold D, Cohen J, Coles A, Confavreux C. Alemtuzumab reduces MS disease activity in active relapsing–remitting multiple sclerosis patients who had disease activity on prior therapy. Neurology 2013;80(7 Suppl.):P07.093 | Conference abstract |
| Havrdova E, Giovannoni G, Stefoski D, Umans K, Greenberg S, Mehta L. Proportion of disease-activity free patients with relapsing–remitting multiple sclerosis following 1 year of treatment with daclizumab high-yield process in the select study. Neurology 2013;80(7 Suppl.):P07.105 | Conference abstract |
| Havrdova E, Zivadinov R, Krasensky J, Dwyer MG, Novakova I, Dolezal O, et al. Randomized study of interferon beta-1a, low-dose azathioprine, and low-dose corticosteroids in multiple sclerosis. Mult Scler 2009;15:965–76. http://dx.doi.org/10.1177/1352458509105229 | Irrelevant comparator/intervention |
| Hersh CM, Cohen JA. Alemtuzumab for the treatment of relapsing–remitting multiple sclerosis. Immunotherapy 2014;6:249–59. http://dx.doi.org/10.2217/imt.14.7 | Irrelevant study type |
| Hutchinson M, Fox RJ, Havrdova E, Kurukulasuriya NC, Sarda SP, Agarwal S, et al. Efficacy and safety of BG-12 (dimethyl fumarate) and other disease-modifying therapies for the treatment of relapsing–remitting multiple sclerosis: a systematic review and mixed treatment comparison. Curr Med Res Opin 2014;30:613–27 | Systematic review that did not enable location of further primary studies |
| Hutchinson M, Fox RJ, Miller DH, Phillips JT, Kita M, Havrdova E. Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing–remitting multiple sclerosis: subgroup analyses of the CONFIRM study. J Neurol 2013;260:2286–96 | Systematic review that did not enable location of further primary studies |
| Jacobs LD, Beck RW, Simon JH. Interferon beta-1a prevented the development of clinically definite multiple sclerosis after a first demyelinating event. Evid Based Med 2001;6:78 | Irrelevant study type |
| Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. A Phase III trial of intramuscular recombinant interferon beta as treatment for exacerbating–remitting multiple sclerosis: design and conduct of study and baseline characteristics of patients. Multiple Sclerosis Collaborative Research Group (MSCRG). Mult Scler 1995;1:118–35 | Protocol only with no results |
| Jacque F, Gaboury I, Christie S, Grand’Maison F. Combination therapy of interferon beta-1b and tacrolimus: a pilot safety study. Mult Scler Int 2012;2012:935921 | Irrelevant comparator/intervention |
| Johnson KP, Brooks BR, Ford CC, Goodman AD, Lisak RP, Myers LW, et al. Glatiramer acetate (Copaxone): comparison of continuous versus delayed therapy in a six-year organized multiple sclerosis trial. Mult Scler 2003;9:585–91 | Irrelevant population |
| Kalincik T, Horakova D, Dolezal O, Krasensky J, Vaneckova M, Seidl Z, et al. Interferon, azathioprine and corticosteroids in multiple sclerosis: 6-year follow-up of the ASA cohort. Clin Neurol Neurosurg 2012;114:940–6 | Irrelevant comparator/intervention |
| Kamm CP, El-Koussy M, Humpert S, Findling O, Burren Y, Schwegler G, et al. Atorvastatin added to interferon beta for relapsing multiple sclerosis: 12-month treatment extension of the randomized multicenter SWABIMS trial. PLOS ONE 2014;9:e86663 | Irrelevant comparator/intervention |
| Kamm CP, El-Koussy M, Humpert S, Findling O, von Bredow F, Burren Y, et al. Atorvastatin added to interferon beta for relapsing multiple sclerosis: a randomized controlled trial. J Neurol 2012;259:2401–13 | Irrelevant comparator/intervention |
| Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006;355:1124–40 | Irrelevant comparator/intervention |
| Kappos L, Edan G, Freedman M, Montalban X, Miller D, Polman C. Benefit 11: long-term follow-up study of patients with clinically isolated syndrome treated with interferon beta-1b. J Neurol Sci 2013;333:e383 | Conference abstract |
| Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 2007;370:389–97 | Irrelevant intervention/study type |
| Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 2009;8:987–97 | Irrelevant intervention/study type |
| Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, et al. Efficacy and safety of oral fumarate in patients with relapsing–remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled Phase IIb study. Lancet 2008;372:1463–72 | Irrelevant comparator/intervention |
| Kappos L, Traboulsee A, Constantinescu C, Erälinna JP, Forrestal F, Jongen P, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing–remitting MS. Neurology 2006;67:944–53 | Irrelevant population/study type |
| Kappos L, Wiendl H, Selmaj K, Arnold DL, Havrdova E, Boyko A, et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2015;373:1418–28. http://dx.doi.org/10.1056/NEJMoa1501481 | Irrelevant comparator/intervention |
| Katz B. The Tübingen Study on Optic Neuritis Treatment – a prospective, randomized and controlled trial. Surv Ophthalmol 1994;39:262–3 | Irrelevant comparator/intervention |
| Keltner JL, Johnson CA, Cello KE, Dontchev M, Gal RL, Beck RW, et al. Visual field profile of optic neuritis: a final follow-up report from the optic neuritis treatment trial from baseline through 15 years. Arch Ophthalmol 2010;128:330–7 | Irrelevant comparator/intervention/outcome |
| Keltner JL, Johnson CA, Spurr JO, Beck RW. Visual field profile of optic neuritis. One-year follow-up in the Optic Neuritis Treatment Trial. Arch Ophthalmol 1994;112:946–53 | Irrelevant comparator/intervention/study type |
| Kieseier BC, Arnold DL, Balcer LJ, Boyko AA, Pelletier J, Liu S, et al. Peginterferon beta-1a in multiple sclerosis: 2-year results from ADVANCE. Mult Scler 2015;21:1025–35. http://dx.doi.org/10.1177/1352458514557986 | Irrelevant comparator/intervention |
| Kinkel RP, Dontchev M, Kollman C, Skaramagas TT, O’Connor PW, Simon JH, et al. Association between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: a 10-year follow-up of the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. Arch Neurol 2012;69:183–90 | Irrelevant comparator/intervention |
| Kinkel RP, Kollman C, O’Connor P, Murray TJ, Simon J, Arnold D, et al. IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology 2006;66:678–84 | Irrelevant comparator/intervention |
| Kinkel RP, Simon JH, O’Connor P, Hyde R, Pace A. Early MRI activity predicts treatment nonresponse with intramuscular interferon beta-1a in clinically isolated syndrome. Mult Scler Relat Disord 2014;3:712–19 | Irrelevant population |
| Koch-Henriksen N, Sørensen PS. The Danish National Project of interferon-beta treatment in relapsing–remitting multiple sclerosis. The Danish Multiple Sclerosis Group. Mult Scler 2000;6:172–5 | DMT used with a non-recommended dose regimen |
| Koch-Henriksen N, Sørensen PS, Christensen T, Frederiksen J, Ravnborg M, Jensen K, et al. A randomized study of two interferon-beta treatments in relapsing–remitting multiple sclerosis. Neurology 2006;66:1056–60 | DMT used with a non-recommended dose regimen |
| Kott E, Kessler A, Biran S. Optic neuritis in multiple sclerosis patients treated with Copaxone. J Neurol 1997;244:S23–4 | Conference abstract |
| La Mantia L, Di Pietrantonj C, Rovaris M, Rigon G, Frau S, Berardo F, et al. Interferons-beta versus glatiramer acetate for relapsing–remitting multiple sclerosis. Cochrane Database Syst Rev 2014;7:CD009333 | Systematic review that did not enable location of further primary studies |
| La Mantia L, Di Pietrantonj C, Rovaris M, Rigon G, Frau S, Berardo F, et al. Comparative efficacy of interferon beta versus glatiramer acetate for relapsing–remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 2015;86:1016–20 | Systematic review that did not enable location of further primary studies |
| La Mantia L, Vacchi L, Di Pietrantonj C, Ebers G, Rovaris M, Fredrikson S, et al. Interferon beta for secondary progressive multiple sclerosis. Cochrane Database Syst Rev 2012;1:CD005181 | Systematic review that did not enable location of further primary studies |
| La Mantia L, Vacchi L, Rovaris M, Di Pietrantonj C, Ebers G, Fredrikson S, et al. Interferon beta for secondary progressive multiple sclerosis: a systematic review. J Neurol Neurosurg Psychiatry 2013;84:420–6 | Systematic review that did not enable location of further primary studies |
| Lacy M, Hauser M, Pliskin N, Assuras S, Valentine MO, Reder A. The effects of long-term interferon-beta-1b treatment on cognitive functioning in multiple sclerosis: a 16-year longitudinal study. Mult Scler 2013;19:1765–72 | Irrelevant comparator/intervention |
| Lam S, Wang S, Gottesman M. Interferon-beta1b for the treatment of multiple sclerosis. Expert Opin Drug Metab Toxicol 2008;4:1111–17. http://dx.doi.org/10.1517/17425255.4.8.1111 | Irrelevant study type |
| Leary SM, Miller DH, Stevenson VL, Brex PA, Chard DT, Thompson AJ. Interferon beta-1a in primary progressive MS: an exploratory, randomized, controlled trial. Neurology 2003;60:44–51 | Irrelevant population |
| Lessell S. Corticosteroid treatment of acute optic neuritis. N Engl J Med 1992;326:634–5. http://dx.doi.org/10.1056/NEJM199202273260909 | Irrelevant study type |
| Likhar N, Mothe RK, Esam H, Kinra G, Shah C, Dang A. Epidemiology and current treatment of neuromyelitis optica: a systematic review. Value Health 2015;18:A750–1. http://dx.doi.org/10.1016/j.jval.2015.09.2904 | Conference abstract |
| Liu Y, Duan Y, He Y, Wang J, Xia M, Yu C, et al. Altered topological organization of white matter structural networks in patients with neuromyelitis optica. PLOS ONE 2012;7:e48846. http://dx.doi.org/10.1371/journal.pone.0048846 | Irrelevant study type |
| Mahdi-Rogers M, van Doorn PA, Hughes RAC. Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev 2013;6:CD003280. http://dx.doi.org/10.1002/14651858.CD003280.pub4 | Irrelevant population/study type |
| Manova MG, Kostadinova II, Akabaliev VC. A clinical study of multiple sclerosis patients treated with betaferon. Folia Med 2008;50:24–9 | Irrelevant intervention/population |
| Manova MG, Kostadinova II. Adverse drug reactions after 24-month treatment with two-dosage regimens of betaferon in patients with multiple sclerosis. Folia Med 2009;51:31–6 | Irrelevant population |
| Martínez Férez IM, Flores Moreno S, Rodríguez López R. Efficacy and Safety of the Immunoregulatory Drugs Interferon Beta and Glatiramer in the Treatment of Relapsing Remitting Multiple Sclerosis. 2013. URL: www.juntadeandalucia.es/salud/servicios/contenidos/nuevaaetsa/up/AETSA_4_2013_InterferonGlatiramero_EM.pdf (accessed 1 June 2016) | Non-English-language study |
| Massacesi L, Tramacere I, Amoroso S, Battaglia MA, Benedetti MD, Filippini G, et al. (2014). Azathioprine versus beta interferons for relapsing–remitting multiple sclerosis: a multicentre randomized non-inferiority trial. PLOS ONE 2014;9:e113371 | Irrelevant comparator/intervention |
| Mazdeh M, Mobaien AR. Efficacy of doxycycline as add-on to interferon beta-1a in treatment of multiple sclerosis. Iran J Neurol 2012;11:70–3 | Irrelevant comparator/intervention |
| Meca-Lallana JE, Hernández-Clares R, Carreón-Guarnizo E. Spasticity in multiple sclerosis and role of glatiramer acetate treatment. Brain Behav 2015;5:e00367. http://dx.doi.org/10.1002/brb3.367 | Irrelevant study type |
| Melo A, Rodrigues B, Bar-Or A. Beta interferons in clinically isolated syndromes: a meta-analysis. Arq Neuropsiquiatr 2008;66:8–10 | Systematic review that did not enable location of further primary studies |
| Meng X, Chin PS, Hashmonay R, Zahur Islam M, Cutter G. Effect of switching from intramuscular interferon beta-1a to oral fingolimod on time to relapse in patients with relapsing–remitting multiple sclerosis enrolled in a 1-year extension of TRANSFORMS. Contemp Clin Trials 2015;41:69–74 | Irrelevant comparator/intervention |
| Menon V, Saxena R, Misra R, Phuljhele S. Management of optic neuritis. Indian J Ophthalmol 2011;59:117–22. http://dx.doi.org/10.4103/0301-4738.77020 | Irrelevant study type |
| Messori A, Fadda V, Maratea D, Trippoli S. Indirect meta-analytical comparison of azathioprine and of beta interferon effectiveness in all forms of multiple sclerosis pooled together. J Neurol Sci 2014;347:408–10 | Irrelevant study type |
| Miller D, Rudick RA, Hutchinson M. Patient-centered outcomes: translating clinical efficacy into benefits on health-related quality of life. Neurology 2010;74(Suppl. 3):24–35. http://dx.doi.org/10.1212/WNL.0b013e3181dbb884 | No results are provided, refers to results from a conference abstract |
| Miller DH, Fox RJ, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 CONFIRM study. Neurology 2015;84:1145–52. http://dx.doi.org/10.1212/WNL.0000000000001360 | Irrelevant outcome |
| Minagara A, Murray TJ, PROOF Study Investigators. Efficacy and tolerability of intramuscular interferon beta-1a compared with subcutaneous interferon beta-1a in relapsing MS: results from PROOF. Curr Med Res Opin 2008;24:1049–55. http://dx.doi.org/10.1185/030079908X280545 | Irrelevant population/study type |
| Minocycline in Clinically Isolated Syndromes (CIS). ClinicalTrials.gov, National Institutes of Health; 2010. URL: https://clinicaltrials.gov/ct2/show/NCT00666887 (accessed 15 June 2017) | Protocol only with no results |
| Montalban X, Sastre-Garriga J, Tintore M, Brieva L, Aymerich FX, Rio J, et al. A single-centre, randomised, double-blind, placebo-controlled study of interferon beta-1b on primary progressive and transitional multiple sclerosis. Mult Scler 2009;15:1195–205 | Irrelevant population |
| Motamed MR, Najimi N, Fereshtehnejad SM. The effect of interferon-beta1a on relapses and progression of disability in patients with clinically isolated syndromes (CIS) suggestive of multiple sclerosis. Clin Neurol Neurosurg 2007;109:344–9 | DMT used with a non-recommended dose regimen |
| Nafissi S, Azimi A, Amini-Harandi A, Salami S, Shahkarami MA, Heshmat R. Comparing efficacy and side effects of a weekly intramuscular biogeneric/biosimilar interferon beta-1a with Avonex in relapsing remitting multiple sclerosis: a double blind randomized clinical trial. Clin Neurol Neurosurg 2012;114:986–9 | Irrelevant comparator/intervention |
| Nagtegaal GJ, Pohl C, Wattjes MP, Hulst HE, Freedman MS, Hartung HP, et al. Interferon beta-1b reduces black holes in a randomised trial of clinically isolated syndrome. Mult Scler 2014;20:234–42 | Irrelevant outcome |
| National Horizon Scanning Centre. Glatiramer Acetate (Copaxone) for a Single Demyelinating Event with an Active Inflammatory Process. Horizon Scanning Technology Briefing. Birmingham: National Horizon Scanning Centre; 2008 | Not a primary research study |
| National Horizon Scanning Centre. Laquinimod for Multiple Sclerosis: Relapsing–Remitting – First or Second Line. Horizon Scanning Review. Birmingham: National Horizon Scanning Centre; 2011 | Not a primary research study |
| National Horizon Scanning Centre. Teriflunomide for Relapsing Multiple Sclerosis (MS) – First Line. Horizon Scanning Review. Birmingham: National Horizon Scanning Centre; 2011 | Not a primary research study |
| Neuroprotection with Riluzole Patients with Early Multiple Sclerosis. ClinicalTrials.gov, National Institutes of Health; 2006. URL: https://clinicaltrials.gov/ct2/show/NCT00501943 (accessed 15 June 2017) | Protocol only with no results |
| Nicholas R, Straube S, Schmidli H, Pfeiffer S, Friede T. Time-patterns of annualized relapse rates in randomized placebo-controlled clinical trials in relapsing multiple sclerosis: a systematic review and meta-analysis. Mult Scler 2012;18:1290–6 | Irrelevant intervention/study type |
| NIHR Horizon Scanning Centre. Ocrelizumab for Relapsing–Remitting Multiple Sclerosis. 2014. URL: www.hsric.nihr.ac.uk/topics/ocrelizumab-for-relapsing–remitting-multiple-sclerosis/ (accessed 1 June 2016) | Irrelevant study type |
| Norman G, Rice S, O’Connor J, Lewis-Light K, Craig D, McDaid C. Dimethyl Fumarate for the Treatment of Relapsing Remitting Multiple Sclerosis. CRD and CHE Technology Assessment Group Report. 2013. URL: www.nets.nihr.ac.uk/projects/hta/128101 (accessed 1 June 2016) | Irrelevant study type |
| Optic Neuritis Study Group. The 5-year risk of MS after optic neuritis. Experience of the optic neuritis treatment trial. Neurology 1997;49:1404–13 | Irrelevant comparator/intervention |
| Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol 2008;65:727–32 | Irrelevant study type |
| Optic Neuritis Study Group. Visual function 15 years after optic neuritis: a final follow-up report from the Optic Neuritis Treatment Trial. Ophthalmology 2008;115:1079–82.e1075 | Irrelevant comparator/intervention |
| Optic Neuritis Treatment Trial (ONTT). ClinicalTrials.gov, National Institutes of Health; 2006. URL: https://clinicaltrials.gov/ct2/show/NCT00000146 (accessed 15 June 2017) | Protocol only with no results |
| Oral Cladribine in Early Multiple Sclerosis (MS). ClinicalTrials.gov, National Institutes of Health; 2010. URL: https://clinicaltrials.gov/ct2/show/NCT00725985 (accessed 15 June 2017) | Protocol only with no results |
| Pakdaman H, Fallah A, Sahraian MA, Pakdaman R, Meysamie A. Treatment of early onset multiple sclerosis with suboptimal dose of interferon beta-1a. Neuropediatrics 2006;37:257–60. http://dx.doi.org/10.1055/s-2006-924723 | DMT used with a non-recommended dose regimen |
| Panitch HS. Interferons in multiple sclerosis. A review of the evidence. Drugs 1992;44:946–62 | Irrelevant study type |
| Paolillo A, Pozzilli C, Giugni E, Tomassini V, Gasperini C, Fiorelli M, et al. A 6-year clinical and MRI follow-up study of patients with relapsing–remitting multiple sclerosis treated with interferon-beta. Eur J Neurol 2002;9:645–55 | Irrelevant population |
| Patten SB, Metz LM. Hopelessness ratings in relapsing–remitting and secondary progressive multiple sclerosis. Int J Psychiatry Med 2002;32:155–65 | Irrelevant outcome |
| Perry M, Swain S, Kemmis-Betty S, Cooper P, Guideline Development Group of the National Institute for Health and Care Excellence. Multiple sclerosis: summary of NICE guidance. BMJ 2014;349:g5701. http://dx.doi.org/10.1136/bmj.g5701 | Irrelevant study type |
| Phase III Study with Teriflunomide Versus Placebo in Patients with First Clinical Symptom of Multiple Sclerosis. ClinicalTrials.gov, National Institutes of Health; 2008. URL: https://clinicaltrials.gov/ct2/show/NCT00622700 (accessed 15 June 2017) | Protocol only with no results |
| Phase IV, Rater-blinded, Randomized Study, Comparing the Effects of 250 mg of Betaseron with 20 mg of Copaxone in Patients with the Relapsing–Remitting or Clinically Isolated Forms of Multiple Sclerosis using 3 Tesla MRI with Triple-Dose Gadolinium. ClinicalTrials.gov, National Institutes of Health; 2003. URL: https://clinicaltrials.gov/ct2/show/NCT00176592 (accessed 15 June 2017) | Protocol only with no results |
| Pöllmann W, Erasmus LP, Feneberg W, Straube A. The effect of glatiramer acetate treatment on pre-existing headaches in patients with MS. Neurology 2006;66:275–7 | Irrelevant population/study type |
| Putzki N, Bell SH, Reynolds JN, Kinkel RP, Dontchev M, Tanner JP, et al. CHAMPIONS extension: 10-year outcomes in interferon beta-1a-treated patients at high risk for developing multiple sclerosis after a clinically isolated syndrome. J Neurol Sci 2009;285:S119–20 | Conference abstract |
| Qizilbash N, Mendez I, Sanchez-de la Rosa R. Benefit–risk analysis of glatiramer acetate for relapsing–remitting and clinically isolated syndrome multiple sclerosis. Clin Ther 2012;34:159–76.e155 | Systematic review that did not enable location of further primary studies |
| REbif FLEXible Dosing in Early Multiple Sclerosis (MS). ClinicalTrials.gov, National Institutes of Health; 2010. URL: https://clinicaltrials.gov/ct2/show/NCT00404352 (accessed 15 June 2017) | Protocol only with no results |
| Remington GM, Treadaway K, Frohman T, Salter A, Stuve O, Racke MK, et al. A one-year prospective, randomized, placebo-controlled, quadruple-blinded, Phase II safety pilot trial of combination therapy with interferon beta-1a and mycophenolate mofetil in early relapsing–remitting multiple sclerosis (TIME MS). Ther Adv Neurol Disord 2010;3:3–13 | Irrelevant comparator/population |
| Roskell NS, Zimovetz EA, Rycroft CE, Eckert BJ, Tyas DA. Annualized relapse rate of first-line treatments for multiple sclerosis: a meta-analysis, including indirect comparisons versus fingolimod. Curr Med Res Opin 2012;28:767–80 | Systematic review that did not enable location of further primary studies |
| Rovaris M, Comi G, Rocca MA, Valsasina P, Ladkani D, Pieri E, et al. Long-term follow-up of patients treated with glatiramer acetate: a multicentre, multinational extension of the European/Canadian double-blind, placebo-controlled, MRI-monitored trial. Mult Scler 2007;13:502–8 | Irrelevant population/study type |
| Rovaris M, Comi G, Rocca MA, Wolinsky JS, Filippi M. Short-term brain volume change in relapsing–remitting multiple sclerosis: effect of glatiramer acetate and implications. Brain 2001;124:1803–12 | Irrelevant outcome |
| Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006;354:911–23 | Irrelevant comparator/intervention |
| Rudick R, Miller DM, Weinstock-Guttman B, Bourdette DN, Foulds P, You X. The relationship between baseline clinical measures and quality of life in patients with relapsing multiple sclerosis: analyses from the Phase 3 trial of intramuscular interferon beta-1a. Mult Scler 2008;14:S293 | Conference abstract |
| Saida T, Kikuchi S, Itoyama Y, Hao Q, Kurosawa T, Nagato K, et al. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Mult Scler 2012;18:1269–77. http://dx.doi.org/10.1177/1352458511435984 | Irrelevant comparator/intervention |
| Saida T, Tashiro K, Itoyama Y, Sato T, Ohashi Y, Zhao Z, Interferon Beta-1b Multiple Sclerosis Study Group of Japan. Interferon beta-1b is effective in Japanese RRMS patients: a randomized, multicenter study. Neurology 2005;64:621–30 | DMT used with a non-recommended dose regimen |
| Seddighzadeh A, Hung S, Selmaj K, Cui Y, Liu S, Sperling B, et al. Single-use autoinjector for peginterferon-beta1a treatment of relapsing–remitting multiple sclerosis: safety, tolerability and patient evaluation data from the Phase IIIb ATTAIN study. Expert Opin Drug Deliv 2014;11:1713–20 | Irrelevant intervention/study type |
| Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol 2010;17:1019–32. http://dx.doi.org/10.1111/j.1468-1331.2010.03066.x | Irrelevant intervention/study type |
| Siddiqui MA, Wellington K. Intramuscular interferon-beta-1a: in patients at high risk of developing clinically definite multiple sclerosis. CNS Drugs 2005;19:55–61 | Irrelevant study type |
| Simon, JH, Jacobs LD, Campion M, Wende K, Simonian N, Cookfair DL, et al. Magnetic resonance studies of intramuscular interferon beta-1a for relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group. Ann Neurol 1998;43:79–87 | Irrelevant outcome |
| Simvastatin Treatment of Patients with Acute Optic Neuritis. ClinicalTrials.gov, National Institutes of Health; 2006. URL: https://clinicaltrials.gov/ct2/show/NCT00261326 (accessed 15 June 2017) | Protocol only with no results |
| Soilu-Hanninen M, Aivo J, Lindstrom BM, Elovaara I, Sumelahti ML, Farkkila M, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon beta-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2012;83:565–71 | Irrelevant comparator/intervention |
| Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al. Safety and efficacy of ofatumumab in relapsing–remitting multiple sclerosis: a Phase 2 study. Neurology 2014;82:573–81. http://dx.doi.org/10.1212/WNL.0000000000000125 | Irrelevant comparator/intervention |
| Sormani MP, Bruzzi P, Beckmann K, Wagner K, Miller DH, Kappos L, et al. MRI metrics as surrogate endpoints for EDSS progression in SPMS patients treated with IFN beta-1b. Neurology 2003;60:1462–6 | Irrelevant outcome |
| Stępień A, Chalimoniuk M, Lubina-Dabrowska N, Chrapusta SJ, Galbo H, Langfort J. Effects of interferon β-1a and interferon β-1b monotherapies on selected serum cytokines and nitrite levels in patients with relapsing–remitting multiple sclerosis: a 3-year longitudinal study. Neuroimmunomodulation 2013;20:213–22 | Irrelevant population/outcomes |
| Study to Compare Double-Dose Betaferon to the Approved Dose, for Patients with Early Secondary Progressive Multiple Sclerosis (SPMS). ClinicalTrials.gov, National Institutes of Health; 2008. URL: https://clinicaltrials.gov/ct2/show/NCT00313976 (accessed 15 June 2017) | Protocol only with no results |
| Sühs KW, Hein K, Pehlke JR, Käsmann-Kellner B, Diem R. Retinal nerve fibre layer thinning in patients with clinically isolated optic neuritis and early treatment with interferon-beta. PLOS ONE 2012;7:e51645. http://dx.doi.org/10.1371/journal.pone.0051645 | Irrelevant study type |
| Tolley K, Hutchinson M, You X, Wang P, Sperling B, Taneja A, et al. A Network meta-analysis of efficacy and evaluation of safety of subcutaneous pegylated interferon beta-1a versus other injectable therapies for the treatment of relapsing–remitting multiple sclerosis. PLOS ONE 2015;10:e0127960 | Systematic review that did not enable location of further primary studies |
| Tsivgoulis G, Katsanos AH, Grigoriadis N, Hadjigeorgiou GM, Heliopoulos I, Kilidireas C. The effect of disease modifying therapies on brain atrophy in patients with relapsing–remitting multiple sclerosis: a systematic review and meta-analysis. PLOS ONE 2015;10:e0116511 | Irrelevant outcome/study type |
| Tsivgoulis G, Katsanos AH, Grigoriadis N, Hadjigeorgiou GM, Heliopoulos I, Papathanasopoulos P, et al. The effect of disease modifying therapies on disease progression in patients with relapsing–remitting multiple sclerosis: a systematic review and meta-analysis. PLOS ONE 2015;10:e0144538 | Systematic review that did not enable location of further primary studies |
| Tsivgoulis G, Katsanos AH, Grigoriadis N, Hadjigeorgiou GM, Heliopoulos I, Papathanasopoulos P, et al. The effect of disease-modifying therapies on brain atrophy in patients with clinically isolated syndrome: a systematic review and meta-analysis. Ther Adv Neuro Disord 2015;8:193–202 | Irrelevant outcome/study type/population |
| Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled Phase 3 trial. Mult Scler 2014;20:705–16 | Irrelevant comparator/intervention |
| Vollmer T, Jeffery D, Goodin D, Kappos L, Lublin F, Radue EW. Long-term safety of fingolimod in patients with relapsing–remitting multiple sclerosis: results from Phase 3 FREEDOMS II extension study. Neurology 2013;80(7 Suppl.):P01.165 | Irrelevant comparator/intervention |
| Vollmer T, Panitch H, Bar-Or A, Dunn J, Freedman MS, Gazda SK, et al. Glatiramer acetate after induction therapy with mitoxantrone in relapsing multiple sclerosis. Mult Scler 2008;14:663–70 | Irrelevant comparator/population |
| Vollmer TL, Sorensen PS, Selmaj K, Zipp F, Havrdova E, Cohen JA, et al. A randomized placebo-controlled Phase III trial of oral laquinimod for multiple sclerosis. J Neurol 2014;261:773–83. http://dx.doi.org/10.1007/s00415-014-7264-4 | Conference abstract |
| Voskuhl RR, Wang H, Wu TC, Sicotte NL, Nakamura K, Kurth F, et al. Estriol combined with glatiramer acetate for women with relapsing–remitting multiple sclerosis: a randomised, placebo-controlled, Phase 2 trial. Lancet Neurol 2016;15:35–46 | Irrelevant comparator/intervention |
| Waubant E, Maghzi AH, Revirajan N, Spain R, Julian L, Mowry EM, et al. A randomized controlled Phase II trial of riluzole in early multiple sclerosis. Ann Clin Transl Neurol 2014;1:340–7 | Irrelevant comparator/intervention |
| Waubant E, Pelletier D, Mass M, Cohen JA, Kita M, Cross A, et al. Randomized controlled trial of atorvastatin in clinically isolated syndrome. The STAyCIS study. Neurology 2012;78:1171–8 | Irrelevant comparator/intervention |
| Weinshenker BG. Review: in relapsing–remitting multiple sclerosis, disease-modifying agents reduce annual relapse rates. Ann Intern Med 2014;160:JC5 | Conference abstract |
| Weinstock-Guttman B, Galetta SL, Giovannoni G, Havrdova E, Hutchinson M, Kappos L, et al. Additional efficacy endpoints from pivotal natalizumab trials in relapsing–remitting MS. J Neurol 2012;259:898–905. http://dx.doi.org/10.1007/s00415-011-6275-7 | Irrelevant comparator/intervention |
| Wolinsky JS, Borresen TE, Dietrich DW, Wynn D, Sidi Y, Steinerman JR, et al. GLACIER: an open-label, randomized, multicenter study to assess the safety and tolerability of glatiramer acetate 40 mg three-times weekly versus 20 mg daily in patients with relapsing–remitting multiple sclerosis. Mult Scler Relat Disord 2015;4:370–6 | Irrelevant study type |
| Wolinsky JS, Narayana PA, O’Connor P, Coyle PK, Ford C, Johnson K, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol 2007;61:14–24 | Irrelevant population |
| Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a Phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol 2010;9:381–90 | Irrelevant intervention |
| Zagmutt FJ, Carroll CA. Meta-analysis of adverse events in recent randomized clinical trials for dimethyl fumarate, glatiramer acetate and teriflunomide for the treatment of relapsing forms of multiple sclerosis. Int J Neurosci 2015;125:798–807 | Systematic review that did not enable location of further primary studies |
| Ziemssen T, Hoffman J, Apfel R, Kern S. Effects of glatiramer acetate on fatigue and days of absence from work in first-time treated relapsing–remitting multiple sclerosis. Health Qual Life Outcomes 2008;6:67. http://dx.doi.org/10.1186/1477-7525-6-67 | Irrelevant population/study type |
| Zintzaras E, Doxani C, Mprotsis T, Schmid CH, Hadjigeorgiou GM. Network analysis of randomized controlled trials in multiple sclerosis. Clin Ther 2012;34:857–69.e859 | Systematic review that did not enable location of further primary studies |
| Zivadinov R, Dwyer MG, Ramasamy DP, Davis MD, Steinerman JR, Khan O. The effect of three times a week glatiramer acetate on cerebral T1 hypointense lesions in relapsing–remitting multiple sclerosis. J Neuroimaging 2015;25:989–95 | Irrelevant outcome |
Appendix 4 Studies included in the clinical effectiveness review with relevant publications
| Study ID | Title | Full article(s): main | Full article(s): other |
|---|---|---|---|
| ADVANCE 2014 | A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study to Evaluate the Efficacy and Safety of PEGylated Interferon Beta-1a (BIIB017) in Subjects With Relapsing Multiple Sclerosis | Calabresi et al.213 | Arnold et al.214 (MRI), Newsome et al.215 (HRQoL) |
| AVANTAGE 2014 | Safety Study in Relapsing–remitting Multiple Sclerosis (RRMS) Patients Receiving Betaferon or Rebif | No formal publication; results on company website182 and ClinicalTrials.gov | |
| BECOME 2009 | Phase IV, Rater-blinded, Randomized Study, Comparing 250 mg of Betaseron With 20 mg of Copaxone in Patients With the Relapsing–remitting (RR) or CIS Forms of ms Using 3 Tesla(3 T) Magnetic Resonance Imaging (MRI) With Triple-dose Gadolinium | Cadavid et al.184 | Cadavid et al.212 |
| BENEFIT 2006 | The BEtaferon in Newly Emerging Multiple Sclerosis for Initial Treatment (BENEFIT) trial | Kappos et al.171 | Polman et al.179 (subgroup analysis), Penner et al.180 (cognitive performance in CIS) |
| BEYOND 2009 | International, Randomized, Multicenter, Phase IIIb Study in Patients With Relapsing-Remitting Multiple Sclerosis Comparing Over a Treatment Period of at Least 104 Weeks: 1. Double-Blinded Safety, Tolerability, and Efficacy of Betaseron/Betaferon 250 µg (8 MIU) and Betaseron/-Betaferon 500 µg (16 MIU), Both Given Subcutaneously Every Other Day, and 2. Rater-Blinded Safety, Tolerability, and Efficacy of Betaseron/-Betaferon s.c. Every Other Day With Copaxone 20 mg s.c. Once Daily | O’Connor et al.190 | Filippi et al.226 (post hoc analysis of MRI scans) |
| Bornstein 1987 | A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis | Bornstein et al.170 | |
| BRAVO 2014 | A Multinational, Multicenter, Randomized, Parallel-group Study Performed in Subjects With RRMS to Assess the Efficacy, Safety and Tolerability of Laquinimod Over Placebo in a Double-blind Design and a Reference Arm of Interferon β–1a (Avonex®) in a Rater-blinded Design | Vollmer et al.198 | |
| Calabrese 2012 | Effect of disease-modifying drugs on cortical lesions and atrophy in relapsing–remitting multiple sclerosis | Calabrese et al.188 | |
| CHAMPS 2000 | Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis | Jacobs et al.172 | Beck et al.176 (subgroup analysis, CHAMPS Study Group227 (subgroup of acute optic neuritis), O’Connor228 (subgroup analysis), O’Connor et al.177 (subgroup analysis) |
| CombiRx 2013 | A Multi-Center, Double-Blind, Randomized Study Comparing the Combined Use of Interferon Beta-1a and Glatiramer Acetate to Either Agent Alone in Patients With Relapsing-Remitting Multiple Sclerosis (CombiRx) | Lublin et al.191 | Lindsey et al.229 (protocol) |
| CONFIRM 2012 | A Randomized, Multicenter, Placebo-Controlled and Active Reference (Glatiramer Acetate) Comparison Study to Evaluate the Efficacy and Safety of BG00012 in Subjects With Relapsing-Remitting Multiple Sclerosis | Fox et al.216 | Kita et al.230 (HRQoL) |
| Cop1 MSSG 1995 | Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial | Johnson et al.217 (initial findings) | Johnson et al.218 (final results) |
| ECGASG 2001 | European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis | Comi et al.219 | |
| ESG 1998 | Placebo-controlled multicentre randomised trial of interferon-1b in treatment of secondary progressive multiple sclerosis | European Study Group on Interferon Beta-1b in Secondary Progressive MS222 | Kappos et al.225 (final results) |
| Etemadifar 2006 | Comparison of Betaferon, Avonex, and Rebif in treatment of relapsing–remitting multiple sclerosis | Etemadifar et al.185 | |
| EVIDENCE 2007 | Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis | Schwid and Panitch195 | Panitch et al.193 (comparative results), Panitch et al.194 (final comparative results), Sandberg-Wollheim et al.206 (AEs) |
| GALA 2013 | Three times weekly glatiramer acetate in relapsing–remitting multiple sclerosis | Khan et al.221 | |
| GATE 2015 | Multi-centre, Randomized, Double-blind, Placebo-controlled, Parallel-group, 9 Month, Equivalence Trial Comparing the Efficacy and Safety and Tolerability of GTR (Synthon BV) to Copaxone® (Teva) in Subjects With Relapsing Remitting Multiple Sclerosis Followed by an Open-label 15 Month GTR Treatment Part Evaluating the Long-term GTR Treatment Effects | Cohen et al.220 | |
| IFNB MSSG 1995 | Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial | IFNB Multiple Sclerosis Study Group209 | IFNB Multiple Sclerosis Study Group210 (additional data and further details) |
| IMPROVE 2012 | A Two-arm, Randomized, Double-blind, Control Group-compared, Multicenter, Phase IIIb Study With Monthly MRI and Biomarker Assessments to Evaluate the Efficacy, Safety, and Tolerability of Rebif® New Formulation (IFN Beta-1a) in Subjects With Relapsing Remitting Multiple Sclerosis | De Stefano et al.207 | |
| INCOMIN 2002 | Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN) | Durelli et al.196 | |
| Kappos 2011 | Phase II, Multicenter, Randomized, Parallel-Group, Partially Blinded, Placebo and Avonex Controlled Dose Finding Study to Evaluate the Efficacy As Measured by Brain MRI Lesions, and Safety of 2 Dose Regimens of Ocrelizumab in Patients With RRMS | Kappos et al.199 | |
| Knobler 1993 | Systemic recombinant human interferon-β treatment of relapsing-remitting multiple sclerosis: pilot study analysis and six-year follow-up | Knobler et al.211 | |
| Mokhber 2014 | Cognitive dysfunction in patients with multiple sclerosis treated with different types of interferon beta: a randomized clinical trial | Mokhber et al.186 | Mokhber et al.187 (HRQoL) |
| MSCRG 1996 | Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis | Jacobs et al.200 | Fischer et al.,203 Goodkin et al.,202 Granger et al.,204 Miller et al.,205 Rudick et al.201 |
| NASG 2004 | Interferon beta-1b in secondary progressive MS | Panitch et al.223 | |
| Pakdaman 2007 | Effect of early interferon beta-1a therapy on conversion to multiple sclerosis in Iranian patients with a first demyelinating event | Pakdaman et al.173 | |
| PreCISe 2009 | A Multinational, Multicenter, Randomized, Double-Blind, Placebo Controlled, Parallel Group Study to Evaluate the Effect of Early Glatiramer Acetate Treatment in Delaying the Conversion to Clinically Definite Multiple Sclerosis (CDMS) of Subjects Presenting With Clinically Isolated Syndrome (CIS) | Comi et al.174 | |
| PRISMS 1998 | Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis | PRISMS Study Group189 | Patten and Metz208 (depression), Gold et al.231 (4-year safety and tolerability) |
| REFLEX 2012 | A Phase III, Randomized, Double-blind, Placebo-controlled, Multicenter Clinical Trial of Rebif New Formulation (44 Microgram [Mcg] Three Times Weekly [Tiw] and 44 Mcg Once Weekly [ow]) in Subjects at High Risk of Converting to Multiple Sclerosis (REFLEX) | Comi et al.175 | Freedman et al.178 (subgroup analysis), CADTH232 |
| REFORMS 2012 | A Randomized, Multicenter, Two Arm, Open Label, Twelve Week Phase IIIb Study to Evaluate the Tolerability of Rebif (New Formulation) (IFN Beta-1a) and Betaseron (IFN Beta-1b) in IFN-naive Subjects With Relapsing Remitting Multiple Sclerosis (RRMS) Followed by a Single Arm, Eighty-two Week Minimum, Rebif (New Formulation) Only Safety Extension | Singer et al.197 | |
| REGARD 2008 | Phase IV, Multicenter, Open Label, Randomized Study of Rebif® 44 mcg Administered Three Times Per Week by Subcutaneous Injection Compared With Copaxone® 20 mg Administered Daily by Subcutaneous Injection in the Treatment of Relapsing Remitting Multiple Sclerosis | Mikol et al.192 | |
| REMAIN 2012 | Phase IV, Multicenter, Open Label, Randomized Study of Rebif® 44mcg Administered Three Times Per Week by Subcutaneous Injection Compared With no Treatment in the Therapy of Relapsing Multiple Sclerosis After Mitoxantrone | Rieckmann et al.183 | |
| Schwartz 1997 | The quality-of-life effects of interferon beta-1b in multiple sclerosis | Schwartz et al.181 | |
| SPECTRIMS 2001 | Randomized controlled trial of interferon beta-1a in secondary progressive MS | SPECTRIMS Study Group224 |
Appendix 5 Overview of systematic reviews in relapsing–remitting multiple sclerosis, secondary progressive multiple sclerosis and clinically isolated syndrome: methods and results
Objective
To provide an overview of systematic reviews, published in the last 5 years, of studies that assessed the cost-effectiveness of treating RRMS, SPMS and/or CIS.
Search strategy
The following electronic databases were searched from January 2011 to January 2016: MEDLINE (via Ovid); MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) and MEDLINE Daily Update (via Ovid); EMBASE (via Ovid); The Cochrane Library (via Wiley Online Library), including the NHS EED and HTA database; Science Citation Index (Web of Knowledge); RePEc; and the CEA Registry. The database searches were kept broad with search terms for MS and CIS combined with economic/HRQoL terms and systematic review terms (based on recognised search filters239–242 when appropriate). Searches for MS and CIS were performed separately but the results were deduplicated and then combined for assessment. A full record of the searches is provided at the end of this appendix. The searches were limited to reviews published in or after 2011. All bibliographic records identified through the electronic searches were collected in a managed reference database. The reference lists of included studies were also checked. Grey literature searches were undertaken using the online resources of various regulatory bodies, health service research agencies, professional societies and patient organisations.
Study selection and inclusion criteria
The selection of studies was undertaken by Peter Auguste and checked by Hendramoorthy Maheswaran using the following defined criteria. Systematic reviews of economic evaluations that involved the use of economic models in RRMS/SPMS/CIS were included. Systematic reviews of HRQoL studies in RRMS/SPMS/CIS were also selected at this stage for later review.
Quality appraisal
The studies were appraised against the AMSTAR framework for best practice in undertaking systematic reviews. 164 The AMSTAR assessment tool consists of a series of criteria/questions (e.g. a priori design, study selection and data extraction, comprehensive literature search or methods used to combine the findings) to assess key quality indicators in systematic reviews. Appraisal of the methodological quality of the studies was undertaken by two reviewers (Hendramoorthy Maheswaran and Peter Auguste). Study quality assessed by one reviewer was cross-checked by the other reviewer. Any disagreements were resolved by discussion or by recourse to a third-party reviewer (Jason Madan).
Results
The electronic database searches identified 1566 records (Figure 35). After removing duplicates, 1023 records were screened for inclusion. On the basis of title and abstract, 966 records were excluded and the remaining 57 records were included for full-text screening. A further 48 articles were excluded at the full-text stage, leaving nine systematic reviews,245–253 of which eight were economic evaluation studies245–252 and one was a systematic review of studies that used a generic tool to measure HRQoL in people with multiple sclerosis. 253
FIGURE 35.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart: systematic reviews of economic evaluations.
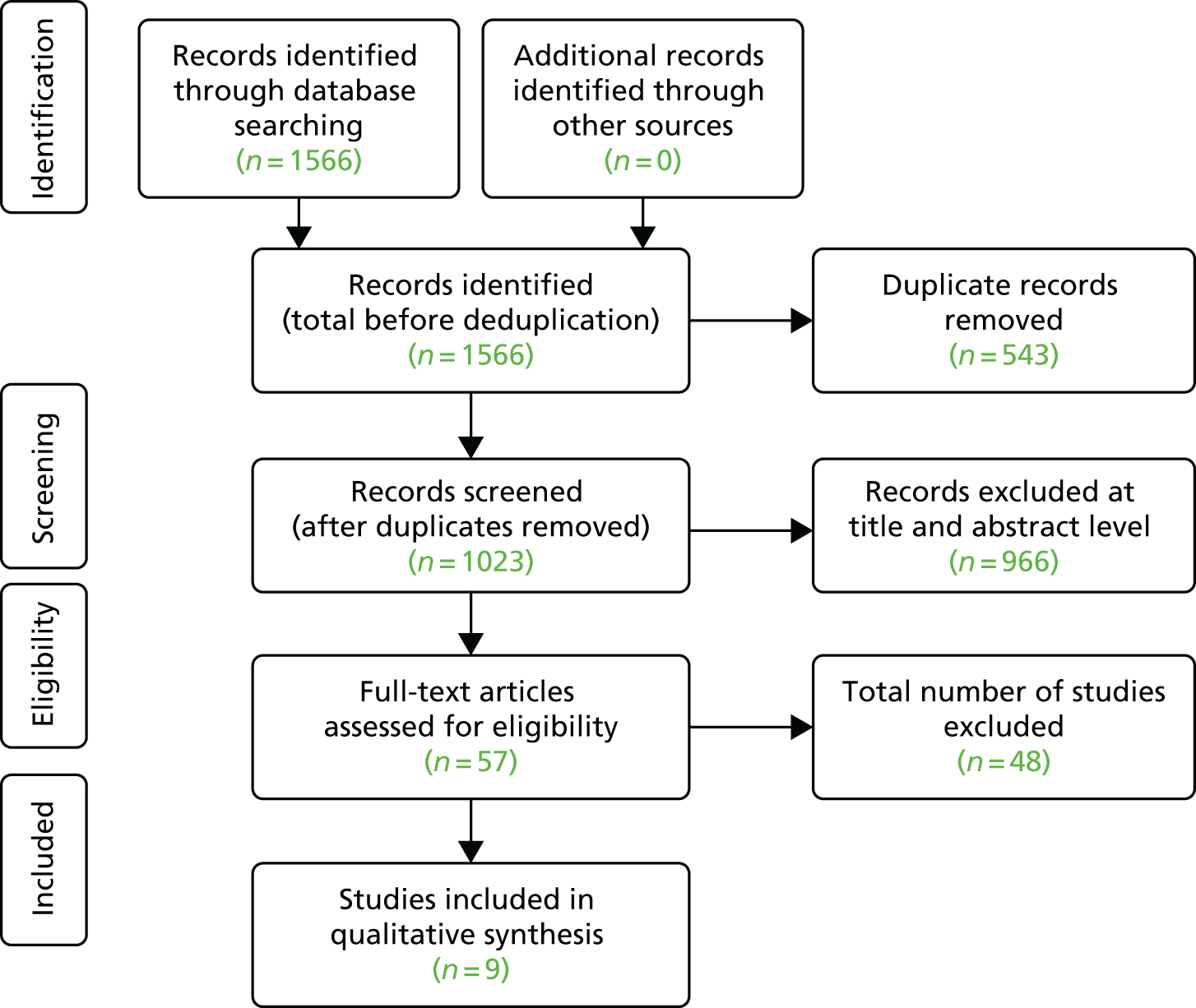
Summary
We identified nine245–253 systematic reviews published since January 2011, which included eight reviews of economic evaluation studies245–252 and one review that looked at generic tools used to measure HRQoL in people with MS. 253
We appraised these studies against the AMSTAR methodological assessment tool. Details on how each review performed are provided in Table 104. Based on our appraisal, systematic reviews generally performed satisfactorily in terms of stating an a prori design of the review, stating the characteristics of all included studies and stating the status of the publication. However, these reviews were also subject to some limitations. First, most studies were unclear on whether study selection and data extraction were carried out in duplicate or did not carry out study selection and data extraction in duplicate. Second, although some authors245–251,253 provided a list of included studies, not all of these authors246,248–250,253 provided a list of excluded studies. Third, it was unclear or not stated whether some authors assessed and/or documented the scientific quality of the included studies.
| Criteria | Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Allen 2015245 | Castrop 2013246 | Guo 2014247 | Hawton 2013248 | Owens 2013249 | Thompson 2013250 | Yamamoto 2012251 | Zalesak 2014252 | Kuspinar 2014253 | |
| Was an ‘a priori’ design provided? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was there duplicate study selection and data extraction? | N | U | Y | U | N | N | U | U | N |
| Was a comprehensive literature search performed? | Y; sensitive subject search used in multiple sources including NICE website, but UK terms added to database searches using .mp., which may be a concern because it reduced numbers of studies considerably | N; only MEDLINE (PubMed) using only two broad MeSH terms – one for MS and one for ‘costs and cost analysis’ (assume exploded?); no free-text or other searching and no specific terms for CIS | Y; MEDLINE (PubMed) using only specific ‘cost–benefit analysis’ MeSH term with MS in all fields and generic and brand names for DMTs; references of included studies after title/abstract sift were checked | Y; multiple sources searched; references of retrieved studies and existing review articles were checked and citation searches were undertaken | N; non-systematic search, only MEDLINE (PubMed); unclear whether MeSH heading ‘Health Care Economics and Organizations’ was exploded, but some free-text terms used; no other searching for results section undertaken | Y?; MEDLINE (Ovid and PubMed) using only two exploded broad MeSH terms – one for MS and one for ‘costs and cost analysis’; references of published (included?) studies checked | Y?; MEDLINE (PubMed) using only specific ‘cost–benefit analysis’ with ‘the general search term’ MS (assume free text and MeSH?); generic and brand names for DMTs incorporated – unclear how, but numbers in flow chart imply combined with AND; CEA Registry and NHS EED also searched | U | Y; multiple databases searched using search strategy appropriate to the specific measures of interest, but no general HRQoL terms used |
| Was the status of publication (i.e. grey literature) used as an inclusion criterion? | N | N | Y | Y | Y | Y | Y | Y | Y |
| Was a list of studies (included and excluded) provided? | Included – Y (n = 18, relating to 12 models); excluded – Y (n = 8) | Included – Y (n = 4); excluded – N | Included – Y (n = 12); excluded – Y (n = 13) | Included – Y (n = 38); excluded – Y (n = 20) | Included – Y (n = 53 on costs, cost-effectiveness, productivity decline or absenteeism); excluded – N | Included – Y (n = 35); excluded – N | Included – Y (n = 22); excluded – Y (n = 28) | N | Included – Y (n = 15); excluded – N |
| Were the characteristics of the included studies provided? | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Was the scientific quality of the included studies assessed and documented? | Y | Y | U | N | N | N | Y | N | Y |
| Was the scientific quality of the included studies used appropriately in formulating conclusions? | Y | Y | U | NA | NA | NA | Y | NA | Y |
| Were the methods used to combine the findings of studies appropriate? | NA | NA | NA | NA | NA | NA | NA | NA | Y |
| Was the likelihood of publication bias assessed? | NA | NA | NA | NA | NA | NA | NA | NA | Y |
| Was the conflict of interest stated? | Y | Y | Y | N | Y | Y | Y | Y | Y |
| Additional criteria used by the assessment group | |||||||||
| Search date | 3 March 2014 | 14 December 2012 | 1 April 2013 | December 2011 | 15 September 2011 | 26 April 2012 | September 2012 | Unclear | 8 October 2013 |
| Scope | RRMS, DMTs, UK, cost-effectiveness models | CIS, IFN-β, comparative, cost and cost-effectiveness | MS, DMTs, cost-effectiveness models | MS, cost-effectiveness | MS, DMTs, cost and cost-effectiveness | MS, DMTs, cost-effectiveness models | MS, DMTs, cost-effectiveness | MS, breast cancer and rheumatoid arthritis, specialty medicines, market research and cost-effectiveness | MS, specific generic utility measures (HUI, EQ-5D, SF-6D, Quality of Well-Being Scale) |
Based on the quality assessment of these reviews, we considered six studies245,247–251 to be methodologically robust and likely to capture economic analyses pre 2012. Hence, we undertook a search of primary studies (for RRMS) with the search date limited to 2012 and later.
Full record of searches
Relapsing–remitting multiple sclerosis searches
MEDLINE (via Ovid)
Database: Ovid MEDLINE® 1946 to January week 2 2016.
Searched on 26 January 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | exp Multiple Sclerosis/ | 46,764 |
| 2 | multiple sclerosis.tw. | 49,799 |
| 3 | 1 or 2 | 57,188 |
| 4 | exp Economics/ | 517,314 |
| 5 | exp ‘Costs and Cost Analysis’/ | 193,082 |
| 6 | Health Status/ | 63,909 |
| 7 | exp ‘Quality of Life’/ | 131,614 |
| 8 | exp Quality-Adjusted Life Years/ | 7896 |
| 9 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 475,628 |
| 10 | (health state* or health status).tw. | 41,055 |
| 11 | (qaly* or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or HUI).tw. | 140,813 |
| 12 | (markov or time trade off or TTO or standard gamble or hrql or hrqol or disabilit* or disutilit*).tw. | 133,533 |
| 13 | (quality adj2 life).tw. | 154,937 |
| 14 | (decision adj2 model).tw. | 4073 |
| 15 | (visual analog* scale* or discrete choice experiment* or health* year* equivalen* or (willing* adj2 pay)).tw. | 33,173 |
| 16 | (‘resource use’ or resource utili?ation).tw. | 9570 |
| 17 | (well-being or wellbeing).tw. | 46,483 |
| 18 | 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 | 1,328,233 |
| 19 | 3 and 18 | 9165 |
| 20 | (metaanalys* or meta analys* or meta-analys*).tw. | 69,140 |
| 21 | (systematic* and review*).mp. | 94,951 |
| 22 | meta analysis.pt. | 60,117 |
| 23 | (literature and review*).mp. | 315,101 |
| 24 | (review* adj10 (model* or cost* or economic* or ‘quality of life’ or HRQoL or HRQL or utilit*)).tw. | 37,856 |
| 25 | 20 or 21 or 22 or 23 or 24 | 452,492 |
| 26 | 19 and 25 | 551 |
| 27 | limit 19 to systematic reviews | 409 |
| 28 | 26 or 27 | 698 |
| 29 | limit 28 to yr=‘2011 -Current’ | 305 |
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Database: Ovid MEDLINE® In-Process & Other Non-Indexed Citations 25 January 2016.
Searched on 26 January 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | multiple sclerosis.tw. | 4878 |
| 2 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 69,030 |
| 3 | (health state* or health status).tw. | 4219 |
| 4 | (qaly* or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or HUI).tw. | 19,706 |
| 5 | (markov or time trade off or TTO or standard gamble or hrql or hrqol or disabilit* or disutilit*).tw. | 16,928 |
| 6 | (quality adj2 life).tw. | 22,185 |
| 7 | (decision adj2 model).tw. | 500 |
| 8 | (visual analog* scale* or discrete choice experiment* or health* year* equivalen* or (willing* adj2 pay)).tw. | 5276 |
| 9 | (‘resource use’ or resource utili?ation).tw. | 1372 |
| 10 | (well-being or wellbeing).tw. | 6440 |
| 11 | 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 | 126,738 |
| 12 | 1 and 11 | 1295 |
| 13 | (metaanalys* or meta analys* or meta-analys*).tw. | 14,035 |
| 14 | (systematic* and review*).tw. | 18,717 |
| 15 | (literature and review*).tw. | 40,052 |
| 16 | (review* adj10 (model* or cost* or economic* or ‘quality of life’ or HRQoL or HRQL or utilit*)).tw. | 6244 |
| 17 | 13 or 14 or 15 or 16 | 62,995 |
| 18 | 12 and 17 | 93 |
| 19 | limit 12 to systematic reviews | 63 |
| 20 | 18 or 19 | 105 |
| 21 | limit 20 to yr=‘2011 -Current’ | 91 |
EMBASE (via Ovid)
Database: Ovid EMBASE 1974 to 2016 week 04.
Searched on 26 January 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | multiple sclerosis/ | 93,609 |
| 2 | multiple sclerosis.tw. | 80,240 |
| 3 | 1 or 2 | 101,212 |
| 4 | exp health economics/ | 677,659 |
| 5 | exp health status/ | 164,988 |
| 6 | exp ‘quality of life’/ | 325,811 |
| 7 | exp quality adjusted life year/ | 15,391 |
| 8 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 713,057 |
| 9 | (health state* or health status).tw. | 57,400 |
| 10 | (qaly* or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF6D or SF-6D or HUI).tw. | 223,035 |
| 11 | (markov or time trade off or TTO or standard gamble or hrql or hrqol or disabilit* or disutilit*).tw. | 208,655 |
| 12 | (quality adj2 life).tw. | 270,996 |
| 13 | (decision adj2 model).tw. | 6739 |
| 14 | (visual analog* scale* or discrete choice experiment* or health* year* equivalen*).tw. | 49,099 |
| 15 | (‘resource use’ or resource utili?ation).tw. | 17,555 |
| 16 | (well-being or wellbeing or (willing* adj2 pay)).tw. | 74,545 |
| 17 | 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 | 1,972,705 |
| 18 | 3 and 17 | 20,936 |
| 19 | meta analysis/ | 103,317 |
| 20 | (metaanalys* or meta analys* or meta-analys*).tw. | 110,582 |
| 21 | ‘systematic review’/ | 100,520 |
| 22 | (systematic* adj3 review*).tw. | 103,537 |
| 23 | (literature adj3 review*).tw. | 245,646 |
| 24 | (review* adj10 (model* or cost* or economic* or ‘quality of life’ or HRQoL or HRQL or utilit*)).tw. | 56,320 |
| 25 | 19 or 20 or 21 or 22 or 23 or 24 | 486,435 |
| 26 | 18 and 25 | 994 |
| 27 | limit 18 to ‘systematic review’ | 312 |
| 28 | 26 or 27 | 994 |
| 29 | limit 28 to yr=‘2011 -Current’ | 566 |
Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library)
Searched on 13 January 2016.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Multiple Sclerosis] explode all trees | 1916 |
| #2 | multiple sclerosis:ti,ab,kw | 4938 |
| #3 | #1 or #2 | 4942 |
| #4 | MeSH descriptor: [Economics] explode all trees | 25,789 |
| #5 | MeSH descriptor: [Costs and Cost Analysis] explode all trees | 23,940 |
| #6 | MeSH descriptor: [Health Status] explode all trees | 5540 |
| #7 | MeSH descriptor: [Quality of Life] explode all trees | 15,431 |
| #8 | MeSH descriptor: [Quality-Adjusted Life Years] explode all trees | 3942 |
| #9 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*):ti,ab,kw | 51,646 |
| #10 | (health next (state* or status)):ti,ab,kw | 7475 |
| #11 | (qaly* or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or HUI):ti,ab,kw | 12,645 |
| #12 | (markov or ‘time trade off’ or TTO or ‘standard gamble’ or hrql or hrqol or disabilit* or disutilit*):ti,ab,kw | 18,569 |
| #13 | (quality near/2 life):ti,ab,kw | 42,732 |
| #14 | (decision near/2 model):ti,ab,kw | 393 |
| #15 | ((visual next analog* next scale*) or (‘discrete choice’ next experiment*) or (health* next year* next equivalen*) or (willing* near/2 pay)):ti,ab,kw | 19,706 |
| #16 | (‘resource use’ or resource next utili?ation):ti,ab,kw | 1571 |
| #17 | (well-being or wellbeing):ti,ab,kw | 5981 |
| #18 | #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 | 125,705 |
| #19 | #3 and #18 Publication Year from 2011 to 2016 | 1048 |
Total all databases: 1048.
Other reviews (DARE): 11.
Health Technology Assessment (HTA) database (Centre for Reviews and Dissemination)
Searched on 13 January 2016.
Total: 38.
Search strategy
Any field: multiple sclerosis
AND
Publication year 2011 to 2016
AND
HTA selected
NHS Economic Evaluation Database (NHS EED) (The Cochrane Library)
Searched on 13 January 2016.
Note: since March 2015, NHS EED is no longer updated.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Multiple Sclerosis] explode all trees | 1916 |
| #2 | multiple sclerosis:ti,ab,kw | 4938 |
| #3 | #1 or #2 | 4942 |
| #4 | (metaanalys* or (meta next analys*) or meta-analys*):ti,ab,kw | 26655 |
| #5 | review* or literature or systematic*:ti,ab,kw | 112066 |
| #6 | #4 or #5 | 114328 |
| #7 | #3 and #6 Publication Year from 2011 to 2016 | 282 |
All databases: 282.
Economic evaluations (NHS EED): 31.
Science Citation Index (Web of Knowledge)
Searched on 26 January 2016.
| ID | Hits | Search |
|---|---|---|
| #1 | 29,661 | TS = ‘multiple sclerosis’ Indexes = SCI-EXPANDED Timespan = 2011–2016 |
| #2 | 573,437 | TS = (‘quality of life’ or QoL or hrql or hrqol or (‘quality adjusted life’ NEAR/1 year*) or QALY* or cost* or economic* or pharmacoeconomic* or pharmaco-economic* or euro-qol or utilit* or disutilit* or euroqol or ‘euro qol’ or EQ5D or EQ-5D or SF-36 or SF36 or SF-6D or SF6D or HUI or (time NEAR/1 trade*) or TTO or ‘standard gamble’ or markov or (decision NEAR/2 model*) or (visual NEAR/1 analog*) or ‘discrete choice’ or ((health* NEAR/1 year*) NEAR/1 equivalen*) or (health NEAR/1 stat*) or ‘willingness to pay’ or ‘resource use’ or (resource NEAR/1 utili?ation) or wellbeing or well-being) Indexes = SCI-EXPANDED Timespan = 2011–2016 |
| #3 | 102,963 | TS = (metaanalys* or (meta NEAR/1 analys*)) Indexes = SCI-EXPANDED Timespan = 2011–2016 |
| #4 | 60,945 | TS = (systematic* AND review*) Indexes = SCI-EXPANDED Timespan = 2011–2016 |
| #5 | 99,993 | TS = (literature AND review*) Indexes = SCI-EXPANDED Timespan = 2011–2016 |
| #6 | 24,398 | TS = (review* NEAR/10 (model* or cost* or economic* or ‘quality of life’ or HRQoL or HRQL or utilit*)) Indexes = SCI-EXPANDED Timespan = 2011–2016 |
| #7 | 232,254 | #6 OR #5 OR #4 OR #3 Indexes = SCI-EXPANDED Timespan = 2011–2016 |
| #8 | 394 | #7 AND #2 AND #1 Indexes = SCI-EXPANDED Timespan = 2011–2016 |
Research Papers in Economics (RePEc)
Searched on 13 January 2016.
-
EconPapers.
-
Free text: ‘multiple sclerosis’.
-
125.
-
Sorted by item date.
-
Total number published from 2011 to 2016: 36.
Cost-effectiveness Analysis (CEA) Registry
Searched on 13 January 2016.
Contained details of articles up to 2013 at time of search.
-
Basic search.
-
Articles.
-
Full search contents: multiple sclerosis.
-
Total number published from 2011 to 2016: 14.
School of Health and Related Research (ScHARR) Health Utilities Database (HUD)
Searched on 13 January 2016.
Total: nine.
Search strategy
multiple sclerosis in any field
AND
2011 to 2016 in Year Published
Clinically isolated syndrome searches
MEDLINE (via Ovid)
Database: Ovid MEDLINE® 1946 to January week 4 2016.
Searched on 10 February 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | Demyelinating Diseases/ | 10,446 |
| 2 | Myelitis, Transverse/ | 1153 |
| 3 | exp Optic Neuritis/ | 6737 |
| 4 | Encephalomyelitis, Acute Disseminated/ | 1689 |
| 5 | Demyelinating Autoimmune Diseases, CNS/ | 316 |
| 6 | demyelinating disease*.tw. | 4725 |
| 7 | transverse myelitis.tw. | 1356 |
| 8 | neuromyelitis optica.tw. | 1735 |
| 9 | optic neuritis.tw. | 3792 |
| 10 | acute disseminated encephalomyelitis.tw. | 1098 |
| 11 | devic.tw. | 107 |
| 12 | ADEM.tw. | 574 |
| 13 | demyelinating disorder.tw. | 335 |
| 14 | clinically isolated syndrome.tw. | 644 |
| 15 | first demyelinating event.tw. | 68 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 24,564 |
| 17 | exp Economics/ | 517,857 |
| 18 | exp ‘Costs and Cost Analysis’/ | 193,384 |
| 19 | Health Status/ | 64,061 |
| 20 | exp ‘Quality of Life’/ | 131,967 |
| 21 | exp Quality-Adjusted Life Years/ | 7948 |
| 22 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 476,878 |
| 23 | (health state* or health status).tw. | 41,167 |
| 24 | (qaly* or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or HUI).tw. | 141,292 |
| 25 | (markov or time trade off or TTO or standard gamble or hrql or hrqol or disabilit* or disutilit*).tw. | 133,897 |
| 26 | (quality adj2 life).tw. | 155,431 |
| 27 | (decision adj2 model).tw. | 4092 |
| 28 | (visual analog* scale* or discrete choice experiment* or health* year* equivalen* or (willing* adj2 pay)).tw. | 33,282 |
| 29 | (‘resource use’ or resource utili?ation).tw. | 9601 |
| 30 | (well-being or wellbeing).tw. | 46,641 |
| 31 | 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 | 1,331,084 |
| 32 | (metaanalys* or meta analys* or meta-analys*).tw. | 69,583 |
| 33 | (systematic* and review*).mp. | 95,472 |
| 34 | meta analysis.pt. | 60,490 |
| 35 | (literature and review*).mp. | 315,829 |
| 36 | (review* adj10 (model* or cost* or economic* or ‘quality of life’ or HRQoL or HRQL or utilit*)).tw. | 37,973 |
| 37 | 32 or 33 or 34 or 35 or 36 | 453,843 |
| 38 | 16 and 31 | 1437 |
| 39 | 37 and 38 | 82 |
| 40 | limit 38 to systematic reviews | 51 |
| 41 | 39 or 40 | 107 |
| 42 | limit 41 to yr=‘2011 -Current’ | 51 |
Total not including reviews already screened as part of the search for cost systematic reviews in RRMS: 11
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Database: Ovid MEDLINE® In-Process & Other Non-Indexed Citations 10 February 2016.
Searched on 11 February 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | demyelinating disease*.tw. | 406 |
| 2 | transverse myelitis.tw. | 148 |
| 3 | neuromyelitis optica.tw. | 322 |
| 4 | optic neuritis.tw. | 360 |
| 5 | acute disseminated encephalomyelitis.tw. | 128 |
| 6 | devic.tw. | 6 |
| 7 | ADEM.tw. | 84 |
| 8 | demyelinating disorder.tw. | 56 |
| 9 | clinically isolated syndrome.tw. | 118 |
| 10 | first demyelinating event.tw. | 6 |
| 11 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 | 1259 |
| 12 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 69,098 |
| 13 | (health state* or health status).tw. | 4217 |
| 14 | (qaly* or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or HUI).tw. | 19,723 |
| 15 | (markov or time trade off or TTO or standard gamble or hrql or hrqol or disabilit* or disutilit*).tw. | 16,916 |
| 16 | (quality adj2 life).tw. | 22,287 |
| 17 | (decision adj2 model).tw. | 492 |
| 18 | (visual analog* scale* or discrete choice experiment* or health* year* equivalen* or (willing* adj2 pay)).tw. | 5321 |
| 19 | (‘resource use’ or resource utili?ation).tw. | 1372 |
| 20 | (well-being or wellbeing).tw. | 6423 |
| 21 | 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 | 126,925 |
| 22 | (metaanalys* or meta analys* or meta-analys*).tw. | 13,978 |
| 23 | (systematic* and review*).tw. | 18,746 |
| 24 | (literature and review*).tw. | 40,310 |
| 25 | (review* adj10 (model* or cost* or economic* or ‘quality of life’ or HRQoL or HRQL or utilit*)).tw. | 6282 |
| 26 | 22 or 23 or 24 or 25 | 63,191 |
| 27 | 11 and 21 | 186 |
| 28 | limit 27 to systematic reviews | 7 |
| 29 | 26 and 27 | 12 |
| 30 | 28 or 29 | 14 |
| 31 | limit 30 to yr = ‘2011 -Current’ | 11 |
Total not including reviews already screened as part of the search for cost systematic reviews in RRMS: 5
EMBASE (via Ovid)
Database: Ovid EMBASE 1974 to 2016 week 06.
Searched on 11 February 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | demyelinating disease/ | 12,216 |
| 2 | myelitis/ | 6771 |
| 3 | optic neuritis/ | 6979 |
| 4 | acute disseminated encephalomyelitis/ | 1378 |
| 5 | myelooptic neuropathy/ | 4897 |
| 6 | demyelinating disease*.tw. | 7443 |
| 7 | transverse myelitis.tw. | 2462 |
| 8 | neuromyelitis optica.tw. | 4162 |
| 9 | optic neuritis.tw. | 6551 |
| 10 | acute disseminated encephalomyelitis.tw. | 1762 |
| 11 | devic.tw. | 229 |
| 12 | ADEM.tw. | 1211 |
| 13 | demyelinating disorder.tw. | 624 |
| 14 | clinically isolated syndrome.tw. | 1758 |
| 15 | first demyelinating event.tw. | 159 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 34,739 |
| 17 | exp health economics/ | 679,154 |
| 18 | exp health status/ | 165,534 |
| 19 | exp ‘quality of life’/ | 327,227 |
| 20 | exp quality adjusted life year/ | 15,498 |
| 21 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 715,448 |
| 22 | (health state* or health status).tw. | 57,542 |
| 23 | (qaly* or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF6D or SF-6D or HUI).tw. | 223,904 |
| 24 | (markov or time trade off or TTO or standard gamble or hrql or hrqol or disabilit* or disutilit*).tw. | 209,301 |
| 25 | (quality adj2 life).tw. | 272,302 |
| 26 | (decision adj2 model).tw. | 6788 |
| 27 | (visual analog* scale* or discrete choice experiment* or health* year* equivalen*).tw. | 49,341 |
| 28 | (‘resource use’ or resource utili?ation).tw. | 17,623 |
| 29 | (well-being or wellbeing or (willing* adj2 pay)).tw. | 74,888 |
| 30 | 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 | 1,979,047 |
| 31 | meta analysis/ | 103,826 |
| 32 | (metaanalys* or meta analys* or meta-analys*).tw. | 111,288 |
| 33 | ‘systematic review’/ | 101,172 |
| 34 | (systematic* adj3 review*).tw. | 104,294 |
| 35 | (literature adj3 review*).tw. | 246,476 |
| 36 | (review* adj10 (model* or cost* or economic* or ‘quality of life’ or HRQoL or HRQL or utilit*)).tw. | 56,523 |
| 37 | 31 or 32 or 33 or 34 or 35 or 36 | 488,476 |
| 38 | 16 and 30 | 3989 |
| 39 | 37 and 38 | 212 |
| 40 | limit 38 to ‘systematic review’ | 64 |
| 41 | 39 or 40 | 212 |
| 42 | limit 41 to yr=‘2011 -Current’ | 113 |
Total not including reviews already screened as part of the search for cost systematic reviews in RRMS: 47
Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library)
Searched on 13 January 2016.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Demyelinating Diseases] this term only | 71 |
| #2 | MeSH descriptor: [Myelitis, Transverse] this term only | 6 |
| #3 | MeSH descriptor: [Optic Neuritis] explode all trees | 95 |
| #4 | MeSH descriptor: [Encephalomyelitis, Acute Disseminated] this term only | 3 |
| #5 | MeSH descriptor: [Demyelinating Autoimmune Diseases, CNS] this term only | 2 |
| #6 | demyelinating next disease*:ti,ab,kw (Word variations have been searched) | 186 |
| #7 | transverse myelitis:ti,ab,kw (Word variations have been searched) | 14 |
| #8 | neuromyelitis optica:ti,ab,kw (Word variations have been searched) | 20 |
| #9 | optic neuritis:ti,ab,kw (Word variations have been searched) | 220 |
| #10 | acute disseminated encephalomyelitis:ti,ab,kw (Word variations have been searched) | 13 |
| #11 | devic:ti,ab,kw (Word variations have been searched) | 2 |
| #12 | ADEM:ti,ab,kw (Word variations have been searched) | 4 |
| #13 | demyelinating disorder:ti,ab,kw (Word variations have been searched) | 49 |
| #14 | clinically isolated syndrome:ti,ab,kw (Word variations have been searched) | 114 |
| #15 | first demyelinating event:ti,ab,kw (Word variations have been searched) | 72 |
| #16 | single demyelinating event:ti,ab,kw (Word variations have been searched) | 9 |
| #17 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 | 561 |
| #18 | MeSH descriptor: [Economics] explode all trees | 26,697 |
| #19 | MeSH descriptor: [Costs and Cost Analysis] explode all trees | 24,728 |
| #20 | MeSH descriptor: [Health Status] explode all trees | 6149 |
| #21 | MeSH descriptor: [Quality of Life] explode all trees | 17,692 |
| #22 | MeSH descriptor: [Quality-Adjusted Life Years] explode all trees | 4063 |
| #23 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*):ti,ab,kw | 53,199 |
| #24 | (health next (state* or status)):ti,ab,kw | 7906 |
| #25 | (qaly* or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or HUI):ti,ab,kw | 13,317 |
| #26 | (markov or ‘time trade off’ or TTO or ‘standard gamble’ or hrql or hrqol or disabilit* or disutilit*):ti,ab,kw | 19,514 |
| #27 | (quality near/2 life):ti,ab,kw | 44,945 |
| #28 | (decision near/2 model):ti,ab,kw | 418 |
| #29 | ((visual next analog* next scale*) or (‘discrete choice’ next experiment*) or (health* next year* next equivalen*) or (willing* near/2 pay)):ti,ab,kw | 20,672 |
| #30 | (‘resource use’ or resource next utili?ation):ti,ab,kw | 1657 |
| #31 | (well-being or wellbeing):ti,ab,kw | 6305 |
| #32 | #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 | 130,941 |
| #33 | #17 and #32 Publication Year from 2011 to 2016 | 97 |
Total all databases: 97.
Other reviews (DARE): none.
NHS Economic Evaluation Database (NHS EED) and Health Technology Assessment (HTA) database (The Cochrane Library)
Searched on 11 February 2016.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Demyelinating Diseases] this term only | 71 |
| #2 | MeSH descriptor: [Myelitis, Transverse] this term only | 6 |
| #3 | MeSH descriptor: [Optic Neuritis] explode all trees | 95 |
| #4 | MeSH descriptor: [Encephalomyelitis, Acute Disseminated] this term only | 3 |
| #5 | MeSH descriptor: [Demyelinating Autoimmune Diseases, CNS] this term only | 2 |
| #6 | demyelinating next disease*:ti,ab,kw (Word variations have been searched) | 186 |
| #7 | transverse myelitis:ti,ab,kw (Word variations have been searched) | 14 |
| #8 | neuromyelitis optica:ti,ab,kw (Word variations have been searched) | 20 |
| #9 | optic neuritis:ti,ab,kw (Word variations have been searched) | 220 |
| #10 | acute disseminated encephalomyelitis:ti,ab,kw (Word variations have been searched) | 13 |
| #11 | devic:ti,ab,kw (Word variations have been searched) | 2 |
| #12 | ADEM:ti,ab,kw (Word variations have been searched) | 4 |
| #13 | demyelinating disorder:ti,ab,kw (Word variations have been searched) | 49 |
| #14 | clinically isolated syndrome:ti,ab,kw (Word variations have been searched) | 114 |
| #15 | first demyelinating event:ti,ab,kw (Word variations have been searched) | 72 |
| #16 | single demyelinating event:ti,ab,kw (Word variations have been searched) | 9 |
| #17 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 Publication Year from 2011 to 2016 | 241 |
Total all databases: 241.
Technology assessments (HTA database): one.
Economic evaluations (NHS EED): two.
Science Citation Index (Web of Knowledge)
Searched on 24 February 2016.
| ID | Hits | Search |
|---|---|---|
| #1 | 6814 | TS = (demyelinating NEAR/2 (disease* OR disorder*)) Indexes = SCI-EXPANDED Timespan = All years |
| #2 | 1703 | TS = (transverse NEAR/1 myelitis) Indexes = SCI-EXPANDED Timespan = All years |
| #3 | 4593 | TS = ‘optic neuritis’ Indexes = SCI-EXPANDED Timespan = All years |
| #4 | 3547 | TS = ‘neuromyelitis optica’ Indexes = SCI-EXPANDED Timespan = All years |
| #5 | 1605 | TS = (‘acute disseminated’ NEAR/1 encephalomyelitis) Indexes = SCI-EXPANDED Timespan = All years |
| #6 | 464 | TS = ‘devic’ Indexes = SCI-EXPANDED Timespan = All years |
| #7 | 690 | TS = ‘ADEM’ Indexes = SCI-EXPANDED Timespan = All years |
| #8 | 1202 | TS = ‘clinically isolated syndrome’ Indexes = SCI-EXPANDED Timespan = All years |
| #9 | 96 | TS = ‘first demyelinating event’ Indexes = SCI-EXPANDED Timespan = All years |
| #10 | 16,921 | #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes = SCI-EXPANDED Timespan = All years |
| #11 | 1,495,884 | TS = (‘quality of life’ or QoL or hrql or hrqol or (‘quality adjusted life’ NEAR/1 year*) or QALY* or cost* or economic* or pharmacoeconomic* or pharmaco-economic* or euro-qol or utilit* or disutilit* or euroqol or ‘euro qol’ or EQ5D or EQ-5D or SF-36 or SF36 or SF-6D or SF6D or HUI or (time NEAR/1 trade*) or TTO or ‘standard gamble’ or markov or (decision NEAR/2 model*) or (visual NEAR/1 analog*) or ‘discrete choice’ or ((health* NEAR/1 year*) NEAR/1 equivalen*) or (health NEAR/1 stat*) or ‘willingness to pay’ or ‘resource use’ or (resource NEAR/1 utili?ation) or wellbeing or well-being) Indexes = SCI-EXPANDED Timespan = All years |
| #12 | 168,986 | TS = (metaanalys* or (meta NEAR/1 analys*)) Indexes = SCI-EXPANDED Timespan = All years |
| #13 | 104,464 | TS = (systematic* AND review*) Indexes = SCI-EXPANDED Timespan = All years |
| #14 | 253,207 | TS = (literature AND review*) Indexes = SCI-EXPANDED Timespan = All years |
| #15 | 62,256 | TS = (review* NEAR/10 (model* or cost* or economic* or ‘quality of life’ or HRQoL or HRQL or utilit*)) Indexes = SCI-EXPANDED Timespan = All years |
| #16 | 497,345 | #15 OR #14 OR #13 OR #12 Indexes = SCI-EXPANDED Timespan = All years |
| #17 | 59 | #16 AND #11 AND #10 Indexes = SCI-EXPANDED Timespan = All years |
| #18 | 41 | #17 Indexes = SCI-EXPANDED Timespan = 2011–2016 |
Total not including reviews already screened as part of the search for cost systematic reviews in RRMS: four.
Research Papers in Economics (RePEc)
Searched on 24 February 2016.
-
EconPapers first search.
-
Free text: demyelinating OR myelitis OR ‘neuromyelitis optica’ OR ‘optic neuritis’ OR ‘acute disseminated encephalomyelitis’ OR ‘clinically isolated syndrome’.
-
Two.
-
Sorted by item date.
-
Total number published from 2011 to 2016: one.
-
-
EconPapers second search.
-
Keywords and title: devic OR ADEM.
-
none.
-
-
Total: one.
-
Total not including reviews already screened as part of the search for cost systematic reviews in RRMS: one.
Cost-effectiveness Analysis (CEA) Registry
Searched on 24 February 2016.
-
Contained details of articles up to 2013 at time of search.
-
Basic Search.
-
Articles.
-
Full Search Contents: demyelinating: three.
-
Full Search Contents: myelitis: one.
-
Full Search Contents: neuromyelitis optica: none.
-
Full Search Contents: optic neuritis: none.
-
Full Search Contents: encephalomyelitis: none.
-
Full Search Contents: clinically isolated syndrome: two.
-
Total: six.
-
Total number published from 2011 to 2016: one.
-
Total not including reviews already screened as part of the search for cost systematic reviews in RRMS: none.
School of Health and Related Research (ScHARR) Health Utilities Database (HUD)
Searched on 24 February 2016.
-
Demyelinating in any field: none.
-
Myelitis in any field: none.
-
Neuromyelitis optica in any field: none.
-
Optic neuritis in any field: none.
-
Acute disseminated encephalomyelitis in any field: none.
-
Clinically isolated syndrome in any field: none.
-
Total: none.
Grey literature
Searches of websites were undertaken concurrently for both clinical effectiveness and cost-effectiveness studies. For a record of these searches, see Appendix 1.
Appendix 6 Cost-effectiveness review of clinically isolated syndrome studies
Full record of searches
Main searches
MEDLINE (via Ovid)
Database: Ovid MEDLINE® 1946 to March week 4 2016.
Searched on 6 April 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | Demyelinating Diseases/ | 10,532 |
| 2 | Myelitis, Transverse/ | 1165 |
| 3 | exp Optic Neuritis/ | 6821 |
| 4 | Encephalomyelitis, Acute Disseminated/ | 1696 |
| 5 | Demyelinating Autoimmune Diseases, CNS/ | 323 |
| 6 | demyelinating disease*.tw. | 4779 |
| 7 | transverse myelitis.tw. | 1371 |
| 8 | neuromyelitis optica.tw. | 1786 |
| 9 | optic neuritis.tw. | 3828 |
| 10 | acute disseminated encephalomyelitis.tw. | 1109 |
| 11 | devic.tw. | 107 |
| 12 | ADEM.tw. | 583 |
| 13 | demyelinating disorder.tw. | 339 |
| 14 | clinically isolated syndrome.tw. | 660 |
| 15 | first demyelinating event.tw. | 69 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 24,812 |
| 17 | exp Economics/ | 522,024 |
| 18 | exp ‘Costs and Cost Analysis’/ | 195,358 |
| 19 | exp Quality-Adjusted Life Years/ | 8146 |
| 20 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 484,557 |
| 21 | (decision adj2 model).tw. | 4186 |
| 22 | (‘resource use’ or resource utili?ation).tw. | 9821 |
| 23 | (qaly* or (generic adj2 (instrument* or measure*)) or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or Health Utilities Index or HUI or 15D or Assessment of Quality of Life or AQOL or Quality of Well-Being or QWB).tw. | 27,152 |
| 24 | 17 or 18 or 19 or 20 or 21 or 22 or 23 | 885,600 |
| 25 | 16 and 24 | 195 |
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Database: Ovid MEDLINE® In-Process & Other Non-Indexed Citations 5 April 2016.
Searched on 6 April 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | demyelinating disease*.tw. | 415 |
| 2 | transverse myelitis.tw. | 150 |
| 3 | neuromyelitis optica.tw. | 329 |
| 4 | optic neuritis.tw. | 380 |
| 5 | acute disseminated encephalomyelitis.tw. | 136 |
| 6 | devic.tw. | 6 |
| 7 | ADEM.tw. | 85 |
| 8 | demyelinating disorder.tw. | 58 |
| 9 | clinically isolated syndrome.tw. | 122 |
| 10 | first demyelinating event.tw. | 6 |
| 11 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 | 1298 |
| 12 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 71,278 |
| 13 | (decision adj2 model).tw. | 511 |
| 14 | (‘resource use’ or resource utili?ation).tw. | 1444 |
| 15 | (qaly* or (generic adj2 (instrument* or measure*)) or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or Health Utilities Index or HUI or 15D or Assessment of Quality of Life or AQOL or Quality of Well-Being or QWB).tw. | 3504 |
| 16 | quality-adjusted life year*.tw. | 949 |
| 17 | 12 or 13 or 14 or 15 or 16 | 74,654 |
| 18 | 11 and 17 | 23 |
EMBASE (via Ovid)
Database: Ovid EMBASE 1974 to 2016 week 14.
Searched on 6 April 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | demyelinating disease/ | 12,351 |
| 2 | myelitis/ | 6889 |
| 3 | optic neuritis/ | 7109 |
| 4 | acute disseminated encephalomyelitis/ | 1437 |
| 5 | myelooptic neuropathy/ | 4987 |
| 6 | demyelinating disease*.tw. | 7511 |
| 7 | transverse myelitis.tw. | 2498 |
| 8 | neuromyelitis optica.tw. | 4242 |
| 9 | optic neuritis.tw. | 6631 |
| 10 | acute disseminated encephalomyelitis.tw. | 1792 |
| 11 | devic.tw. | 231 |
| 12 | ADEM.tw. | 1224 |
| 13 | demyelinating disorder.tw. | 633 |
| 14 | clinically isolated syndrome.tw. | 1789 |
| 15 | first demyelinating event.tw. | 159 |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 35,248 |
| 17 | multiple sclerosis/ | 94,999 |
| 18 | multiple sclerosis.tw. | 81,514 |
| 19 | 17 or 18 | 102,763 |
| 20 | exp *health economics/ | 212,668 |
| 21 | exp quality adjusted life year/ | 15,786 |
| 22 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).ti. | 164,671 |
| 23 | (decision adj2 model).tw. | 6901 |
| 24 | (‘resource use’ or resource utili?ation).tw. | 17,938 |
| 25 | (qaly* or (generic adj2 (instrument* or measure*)) or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or Health Utilities Index or HUI or 15D or Assessment of Quality of Life or AQOL or Quality of Well-Being or QWB).tw. | 50,631 |
| 26 | 20 or 21 or 22 or 23 or 24 or 25 | 371,080 |
| 27 | 16 and 26 | 173 |
NHS Economic Evaluation Database (NHS EED) and Health Technology Assessment (HTA) database (The Cochrane Library)
Searched on 6 April 2016.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Demyelinating Diseases] this term only | 71 |
| #2 | MeSH descriptor: [Myelitis, Transverse] this term only | 6 |
| #3 | MeSH descriptor: [Optic Neuritis] explode all trees | 95 |
| #4 | MeSH descriptor: [Encephalomyelitis, Acute Disseminated] this term only | 3 |
| #5 | MeSH descriptor: [Demyelinating Autoimmune Diseases, CNS] this term only | 2 |
| #6 | demyelinating next disease*:ti,ab,kw (Word variations have been searched) | 187 |
| #7 | transverse myelitis:ti,ab,kw (Word variations have been searched) | 14 |
| #8 | neuromyelitis optica:ti,ab,kw (Word variations have been searched) | 20 |
| #9 | optic neuritis:ti,ab,kw (Word variations have been searched) | 222 |
| #10 | acute disseminated encephalomyelitis:ti,ab,kw (Word variations have been searched) | 13 |
| #11 | devic:ti,ab,kw (Word variations have been searched) | 3 |
| #12 | ADEM:ti,ab,kw (Word variations have been searched) | 4 |
| #13 | demyelinating disorder:ti,ab,kw (Word variations have been searched) | 49 |
| #14 | clinically isolated syndrome:ti,ab,kw (Word variations have been searched) | 116 |
| #15 | first demyelinating event:ti,ab,kw (Word variations have been searched) | 72 |
| #16 | single demyelinating event:ti,ab,kw (Word variations have been searched) | 9 |
| #17 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 | 566 |
Total all databases: 566.
Technology assessments (HTA database): two.
Economic evaluations (NHS EED): three.
Science Citation Index and Conference Proceedings Citation Index – Science (Web of Knowledge)
Searched on 6 April 2016.
| ID | Hits | Search |
|---|---|---|
| #1 | 6912 | TS = (demyelinating NEAR/2 (disease* OR disorder*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #2 | 1732 | TS = (transverse NEAR/1 myelitis) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #3 | 4703 | TS = ‘optic neuritis’ Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #4 | 3616 | TS = ‘neuromyelitis optica’ Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #5 | 1620 | TS = (‘acute disseminated’ NEAR/1 encephalomyelitis) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #6 | 474 | TS = ‘devic’ Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #7 | 711 | TS = ‘ADEM’ Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #8 | 1225 | TS = ‘clinically isolated syndrome’ Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #9 | 96 | TS = ‘first demyelinating event’ Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #10 | 17,216 | #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #11 | 1,280,769 | TS = (cost* or economic* or pharmacoeconomic* or pharmaco-economic*) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #12 | 80,174 | TS = ((‘quality adjusted life’ NEAR/1 year*) or QALY* or (generic NEAR/2 (instrument* or measure*)) or euro-qol or euroqol or ‘euro qol’ or EQ5D or EQ-5D or SF-36 or SF36 or SF-6D or SF6D or ‘health utilities index’ or HUI or 15D or ‘assessment of quality of life’ or AQOL or ‘Quality of Well-Being’ or QWB or (decision NEAR/2 model*) or ‘resource use’ or (resource NEAR/1 utili?ation)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #13 | 1,335,874 | #11 or #12 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| #14 | 210 | #13 AND #10 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
Research Papers in Economics (RePEc)
Searched on 6 April 2016.
-
EconPapers first search.
-
Free text: demyelinating OR myelitis OR ‘neuromyelitis optica’ OR ‘optic neuritis’ OR ‘acute disseminated encephalomyelitis’ OR ‘clinically isolated syndrome’.
-
Two.
-
-
EconPapers second search.
-
Keywords and Title: devic OR ADEM.
-
None.
-
-
Total: two.
Cost-effectiveness Analysis (CEA) Registry
Searched on 6 April 2016.
Contained details of articles up to 2014 at time of search
-
Basic search.
-
Articles.
-
Full search contents: demyelinating: three.
-
Full search contents: myelitis: one.
-
Full search contents: neuromyelitis optica: none.
-
Full search contents: optic neuritis: none.
-
Full search contents: encephalomyelitis: none.
-
Full search contents: clinically isolated syndrome: two.
-
Total: six.
School of Health and Related Research (ScHARR) Health Utilities Database (HUD)
Searched on 6 April 2016.
-
Demyelinating in any field: none.
-
Myelitis in any field: none.
-
Neuromyelitis optica in any field: none.
-
Optic neuritis in any field: none.
-
Acute disseminated encephalomyelitis in any field: none.
-
Clinically isolated syndrome in any field: none.
-
Total: none.
Additional searches
CIS (or RRMS post 2011) registers or cohort natural history.
MEDLINE (via Ovid)
Database: Ovid MEDLINE® 1946 to June week 1 2016.
Searched on 16 June 2016.
| ID | Search |
|---|---|
| 1 | Demyelinating Diseases/ |
| 2 | Myelitis, Transverse/ |
| 3 | exp Optic Neuritis/ |
| 4 | Encephalomyelitis, Acute Disseminated/ |
| 5 | Demyelinating Autoimmune Diseases, CNS/ |
| 6 | demyelinating disease*.tw. |
| 7 | transverse myelitis.tw. |
| 8 | neuromyelitis optica.tw. |
| 9 | optic neuritis.tw. |
| 10 | acute disseminated encephalomyelitis.tw. |
| 11 | devic.tw. |
| 12 | ADEM.tw. |
| 13 | demyelinating disorder.tw. |
| 14 | clinically isolated syndrome.tw. |
| 15 | first demyelinating event.tw. |
| 16 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 |
| 17 | exp Registries/ |
| 18 | (registry or registries).tw. |
| 19 | (register or registers).tw. |
| 20 | 17 or 18 or 19 |
| 21 | exp Cohort Studies/ |
| 22 | (cohort adj (study or studies)).tw. |
| 23 | cohort analy$.tw. |
| 24 | (follow up adj (study or studies)).tw. |
| 25 | 21 or 22 or 23 or 24 |
| 26 | natural history.tw. |
| 27 | natural course.tw. |
| 28 | untreated.tw. |
| 29 | ((‘no’ or ‘not’) adj2 (treat* or therap*)).tw. |
| 30 | (natural adj2 (progression or development)).tw. |
| 31 | 26 or 27 or 28 or 29 or 30 |
| 32 | 16 and 20 |
| 33 | 16 and 25 and 31 |
| 34 | 32 or 33 |
| 35 | Multiple Sclerosis, Relapsing-Remitting/ |
| 36 | relapsing remitting multiple sclerosis.tw. |
| 37 | 35 or 36 |
| 38 | limit 37 to yr=‘2011 -Current’ |
| 39 | 20 and 38 |
| 40 | 25 and 31 and 38 |
| 41 | 39 or 40 |
| 42 | 34 or 41 |
| Reference | Reason for exclusion |
|---|---|
| Casado V, Gubieras L, Romero-Pinel L, Matas E, Bau L, Lopez M, et al. Cost of the diagnosis of multiple sclerosis. J Neurol 2009;256:S126 | Not a full economic evaluation |
| Fredrikson S, Prayoonwiwat N, Wicklein EM, Scherer P, Langdon D. Psychosocial aspects of clinically isolated syndrome (CIS) in Asia: baseline data from the CogniCIS study Asian cohort. J Neurol Sci 2009;285:S95 | Not an economic analysis |
| Fredrikson S, Wicklein EM, Prayoonwiwat N, Beckmann K, Scherer P, Langdon D. Cognitive performance and health-related quality of life in clinically isolated syndrome (CIS) suggestive of multiple sclerosis: 2-year data from CogniCIS, a multinational, longitudinal study. Eur J Neurol 2010;17:57 | Not an economic analysis |
| Kinkel RP, Laforet G, You X. Disease-related determinants of quality of life 10 years after clinically isolated syndrome. Int J MS Care 2015;17:26–34 | Not an economic analysis |
| Prayoonwiwat N, Nidhinandana S, Chankrachang S, Asawavichienjinda T, Tantirittisak T, Fredrikson S, et al. Psychosocial aspects of clinically isolated syndrome (CIS) in Asia: baseline data from the CogniCIS study Asian cohort. Mult Scler 2010;16:266–7 | Not an economic analysis |
| Sanchez-Solino O, Grau C, Parra JC, Arroyo E. Quality of life in patients with high-risk clinically isolated syndrome treated with Avonex: interim results of the AREMIN study. J Neurol 2010;257:S190 | Not an economic analysis |
| Stourac P, Horakova D, Tyblova M, Klimova E, Szilasiova J, Fenclova I, et al. Interim analysis of AMETYST: a Phase 4 observational study of the impact of intramuscular interferon b-1a on quality of life, disability, and cognition in patients with clinically isolated syndrome/clinically definite multiple sclerosis. Mult Scler 2012;1:486 | No model included |
| Vermersch P, de Seze J, Delisse B, Lemaire S, Stojkovic T. Quality of life in multiple sclerosis: influence of interferon-beta1 a (Avonex) treatment. Mult Scler 2002;8:377–81 | Not an economic analysis |
Blank data extraction form for cost-effectiveness studies: clinically isolated syndrome
| Date: | |
| Study ID: | |
| Name of first reviewer: | |
| Name of second reviewer: | |
| Study details | |
|---|---|
| Study title | |
| First author | |
| Co-authors | |
| Source of publication: Journal yy;vol.(issue):pp | |
| Language | |
| Publication type | |
| Inclusion criteria/study eligibility/PICOS | |
| Population | |
| Intervention(s) | |
| Comparator(s) | |
| Outcome(s) | |
| Study design | |
| Methods | |
| Setting and location | |
| Study perspective | |
| Comparators | |
| Time horizon | |
| Discount rate | |
| Outcomes | |
| Measurement of effectiveness | |
| Measurement and valuation of preference-based outcomes | |
| Resource use and costs | |
| Currency, price date and conversion | |
| Model type | |
| Assumptions | |
| Analytical methods | |
| Results | |
| Study parameters | |
| Incremental costs and outcomes | |
| Characterising uncertainty | |
| Study findings | |
| Limitations | |
| Generalisability | |
| Source of funding | |
| Conflicts of interest | |
| Comments | |
| Authors’ conclusion | |
| Reviewer’s conclusion | |
Quality assessment of economic evaluations in clinically isolated syndrome
| Assessment | Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fredrikson 2013254 | Iskedjian 2005257 | Lazzaro 2009256 | Kobelt 2007255 | Arbizu 2009258 | Caloyeras 2009260 | Caloyeras 2008259 | Caloyeras 2012261 | Zarco 2014262 | |
| Title | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Abstract | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Introduction | |||||||||
| Background and objectives | Y | Y | Y | N | Y | Y | Y | Y | Y |
| Methods | |||||||||
| Target population and subgroups | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Setting and location | N | N | N | N | Y | Y | Y | Y | Y |
| Study perspective | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Comparators | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Time horizon | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Discount rate | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Choice of health outcomes | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Measurement of effectiveness | Y | Y | Y | Y | U | U | Y | Y | Y |
| Measurement and valuation of preference-based outcomes | N | N | N | N | U | U | Y | Y | NA |
| Estimating resources and costs | Y | Y | Y | N | Y | Y | Y | Y | Y |
| Currency, price date and conversion | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Choice of model | Y | Y | Y | Y | U | U | U | Y | Y |
| Assumptions | Y | Y | Y | N | U | U | Y | Y | U |
| Analytical methods | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Study parameters (results) | Y | Y | Y | Y | U | U | Y | N | Y |
| Incremental costs and outcomes | Y | Y | Y | Y | U | U | Y | Y | Y |
| Characterising uncertainty | Y | Y | Y | Y | U | U | U | N | Y |
| Study findings (discussion) | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Limitations | Y | Y | Y | N | U | U | U | Y | Y |
| Generalisability | Y | Y | Y | U | U | U | U | Y | Y |
| Other | |||||||||
| Source of funding (other) | Y | Y | Y | N | U | U | U | Y | N |
| Conflicts of interest | Y | Y | Y | N | U | U | U | Y | N |
| Criteria | Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fredrikson 2013254 | Iskedjian 2005257 | Lazzaro 2009256 | Kobelt 2007255 | Arbizu 2009258 | Caloyeras 2008259 | Caloyeras 2009260 | Caloyeras 2012261 | Zarco 2014262 | |
| Structure | |||||||||
| Is there a clear statement of the decision problem? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Is the objective of the model specified and consistent with the stated decision problem? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Is the primary decision-maker specified? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Is the perspective of the model stated clearly? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Are the model inputs consistent with the stated perspective? | Y | Y | Y | U | U | Y | Y | Y | Y |
| Has the scope of the model been stated and justified? | Y | Y | Y | U | U | Y | U | Y | Y |
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Y | Y | Y | Y | U | U | U | Y | Y |
| Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | Y | Y | U | U | U | U | Y | N |
| Are the sources of the data used to develop the structure of the model specified? | Y | Y | Y | U | U | Y | U | Y | Y |
| Are the causal relationships described by the model structure justified appropriately? | Y | Y | Y | U | U | U | U | Y | U |
| Are the structural assumptions transparent and justified? | Y | Y | Y | U | U | U | U | Y | U |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Y | Y | Y | U | U | U | U | Y | U |
| Is there a clear definition of the options under evaluation? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Have all feasible and practical options been evaluated? | N | N | N | N | Y | Y | Y | N | N |
| Is there justification for the exclusion of feasible options? | N | N | N | N | U | NA | U | N | N |
| Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Is the time horizon of the model sufficient to reflect all important differences between the options? | Y | N | Y | Y | Y | Y | Y | Y | N |
| Are the time horizon of the model and the duration of treatment described and justified? | Y | Y | Y | U | Y | Y | Y | Y | Y |
| Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Y | Y | Y | U | Y | Y | Y | Y | N |
| Is the cycle length defined and justified in terms of the natural history of disease? | Y | Y | Y | N | Y | Y | Y | Y | NA |
| Data | |||||||||
| Are the data identification methods transparent and appropriate given the objectives of the model? | Y | Y | Y | U | U | U | U | Y | U |
| Where choices have been made between data sources are these justified appropriately? | N | N | N | U | U | U | U | Y | U |
| Has particular attention been paid to identifying data for the important parameters of the model? | U | Y | Y | U | U | U | U | U | U |
| Has the quality of the data been assessed appropriately? | U | N | N | U | U | U | U | U | U |
| Where expert opinion has been used are the methods described and justified? | Y | Y | Y | U | U | U | U | N | U |
| Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Y | Y | Y | U | U | U | U | Y | U |
| Is the choice of baseline data described and justified? | Y | Y | Y | U | U | U | U | Y | Y |
| Are transition probabilities calculated appropriately? | Y | Y | Y | U | U | U | U | Y | U |
| Has a half-cycle correction been applied to both costs and outcomes? | N | N | N | U | U | U | U | N | NA |
| If not, has the omission been justified? | N | N | N | U | U | U | U | N | NA |
| If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | N | N | N | U | U | U | U | Y | U |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | Y | Y | Y | U | Y | Y | U | Y | NA |
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | N | N | N | U | U | U | U | Y | NA |
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | Y | Y | Y | U | U | U | U | Y | NA |
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | Y | N | N | U | U | U | U | U | Y |
| Are the costs incorporated into the model justified? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Has the source for all costs been described? | Y | Y | Y | N | U | U | U | Y | Y |
| Have discount rates been described and justified given the target decision-maker? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Are the utilities incorporated into the model appropriate? | U | Y | Y | Y | Y | Y | Y | Y | Y |
| Is the source of utility weights referenced? | Y | Y | Y | N | U | Y | U | Y | Y |
| Are the methods of derivation for the utility weights justified? | Y | Y | N | N | U | U | U | Y | U |
| Have all data incorporated into the model been described and referenced in sufficient detail? | Y | Y | N | N | U | U | U | Y | N |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate?) | Y | Y | Y | N | U | U | U | Y | U |
| Is the process of data incorporation transparent? | U | U | Y | N | U | U | U | U | N |
| If data have been incorporated as distributions, has the choice of distributions for each parameter been described and justified? | N | N | N | N | U | U | U | N | Y |
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | N | N | N | N | U | U | U | N | U |
| Have the four principal types of uncertainty been addressed? | N | N | N | N | U | U | U | N | N |
| If not, has the omission of particular forms of uncertainty been justified? | N | N | N | N | U | U | U | U | N |
| Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | Y | Y | Y | U | U | U | U | N | N |
| Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | N | N | N | N | U | U | U | N | N |
| Has heterogeneity been dealt with by running the model separately for different subgroups? | NA | NA | NA | U | U | U | U | N | N |
| Are the methods of assessment of parameter uncertainty appropriate? | Y | Y | Y | Y | U | U | U | Y | Y |
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | Y | Y | Y | U | U | U | U | NA | Y |
| Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | U | U | U | U | U | U | U | U | N |
| Are any counterintuitive results from the model explained and justified? | NA | Y | NA | U | U | U | U | U | NA |
| If the model has been calibrated against independent data, have any differences been explained and justified? | NA | NA | NA | U | U | U | U | NA | U |
| Have the results been compared with those of previous models and any differences in results explained? | Y | Y | Y | U | U | U | U | N | Y |
Appendix 7 Cost-effectiveness review of relapsing–remitting multiple sclerosis studies
Full record of searches
Main searches: 2012–16
MEDLINE (via Ovid)
Database: Ovid MEDLINE® 1946 to March week 4 2016.
Searched on 5 April 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | exp Multiple Sclerosis/ | 47,422 |
| 2 | multiple sclerosis.tw. | 50,604 |
| 3 | 1 or 2 | 58,051 |
| 4 | exp Economics/ | 522,024 |
| 5 | exp ‘Costs and Cost Analysis’/ | 195,358 |
| 6 | exp Quality-Adjusted Life Years/ | 8146 |
| 7 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 484,557 |
| 8 | (decision adj2 model).tw. | 4186 |
| 9 | (‘resource use’ or resource utili?ation).tw. | 9821 |
| 10 | (qaly* or (generic adj2 (instrument* or measure*)) or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or Health Utilities Index or HUI or 15D or Assessment of Quality of Life or AQOL or Quality of Well-Being or QWB).tw. | 27,152 |
| 11 | 4 or 5 or 6 or 7 or 8 or 9 or 10 | 885,600 |
| 12 | 3 and 11 | 1860 |
| 13 | limit 12 to yr=‘2012 -Current’ | 507 |
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Database: Ovid MEDLINE® In-Process & Other Non-Indexed Citations 4 April 2016.
Searched on 5 April 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | multiple sclerosis.tw. | 4995 |
| 2 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).tw. | 71,051 |
| 3 | (decision adj2 model).tw. | 511 |
| 4 | (‘resource use’ or resource utili?ation).tw. | 1438 |
| 5 | (qaly* or (generic adj2 (instrument* or measure*)) or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or Health Utilities Index or HUI or 15D or Assessment of Quality of Life or AQOL or Quality of Well-Being or QWB).tw. | 3483 |
| 6 | quality-adjusted life year*.tw. | 945 |
| 7 | 2 or 3 or 4 or 5 or 6 | 74,406 |
| 8 | 1 and 7 | 239 |
| 9 | limit 8 to yr=‘2012 -Current’ | 198 |
EMBASE (via Ovid)
Database: Ovid EMBASE 1974 to 2016 week 14.
Searched on 5 April 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | multiple sclerosis/ | 94,999 |
| 2 | multiple sclerosis.tw. | 81,514 |
| 3 | 1 or 2 | 102,763 |
| 4 | exp *health economics/ | 212,668 |
| 5 | exp quality adjusted life year/ | 15,786 |
| 6 | (pharmacoeconomic* or pharmaco-economic* or economic* or cost*).ti. | 164,671 |
| 7 | (decision adj2 model).tw. | 6901 |
| 8 | (‘resource use’ or resource utili?ation).tw. | 17,938 |
| 9 | (qaly* or (generic adj2 (instrument* or measure*)) or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or Health Utilities Index or HUI or 15D or Assessment of Quality of Life or AQOL or Quality of Well-Being or QWB).tw. | 50,631 |
| 10 | 4 or 5 or 6 or 7 or 8 or 9 | 371,080 |
| 11 | 3 and 10 | 2024 |
| 12 | limit 11 to yr=‘2012 -Current’ | 988 |
| 13 | limit 12 to (conference abstract or conference paper or conference proceeding or ‘conference review’) | 550 |
| 14 | 12 not 13 | 438 |
NHS Economic Evaluation Database (NHS EED) and Health Technology Assessment (HTA) database (The Cochrane Library)
Searched on 5 April 2016.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Multiple Sclerosis] explode all trees | 2127 |
| #2 | multiple sclerosis:ti,ab,kw | 5131 |
| #3 | #1 or #2 Publication Year from 2012 to 2016 | 2064 |
Total all databases: 2064.
Technology assessments (HTA database): 30.
Economic evaluations (NHS EED): 27.
Science Citation Index (Web of Knowledge)
Searched on 5 April 2016.
| ID | Hits | Search |
|---|---|---|
| #1 | 87,043 | TS = ‘multiple sclerosis’ Indexes = SCI-EXPANDED Timespan = All years |
| #2 | 53,184 | TI = (cost* or economic* or pharmacoeconomic* or pharmaco-economic*) Indexes = SCI-EXPANDED Timespan = 2012–2016 |
| #3 | 24,433 | TS = ((‘quality adjusted life’ NEAR/1 year*) or QALY* or (generic NEAR/2 (instrument* or measure*)) or euro-qol or euroqol or ‘euro qol’ or EQ5D or EQ-5D or SF-36 or SF36 or SF-6D or SF6D or ‘health utilities index’ or HUI or 15D or ‘assessment of quality of life’ or AQOL or ‘Quality of Well-Being’ or QWB or (decision NEAR/2 model*) or ‘resource use’ or (resource NEAR/1 utili?ation)) Indexes = SCI-EXPANDED Timespan = 2012–2016 |
| #4 | 73,283 | #3 OR #2 Indexes = SCI-EXPANDED Timespan = 2012–2016 |
| #5 | 472 | #4 AND #1 Indexes = SCI-EXPANDED Timespan = 2012–2016 |
| #6 | 157 | (#5) AND DOCUMENT TYPES: (Meeting Abstract OR Meeting Summary OR Proceedings Paper) Indexes = SCI-EXPANDED Timespan = 2012–2016 |
| #7 | 315 | #5 not #6 Indexes = SCI-EXPANDED Timespan = 2012–2016 |
Research Papers in Economics (RePEc)
Searched on 5 April 2016.
-
EconPapers.
-
Free text: ‘multiple sclerosis’.
-
128.
-
Sorted by item date.
-
Total number published from 2012 to 2016: 32.
Cost-effectiveness Analysis (CEA) Registry
Searched on 5 April 2016.
Contained details of articles up to 2014 at time of search
-
Basic search.
-
Articles.
-
Full search contents: multiple sclerosis.
-
Total number published from 2012 to 2016: 17.
School of Health and Related Research (ScHARR) Health Utilities Database (HUD)
Searched on 5 April 2016.
Total: seven.
Search strategy
multiple sclerosis in any field
AND
2012 to 2016 in Year Published
Main searches: health-related quality of life studies with generic measures up to 2011
MEDLINE (via Ovid)
Database: Ovid MEDLINE® 1946 to March week 4 2016.
Searched on 6 April 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | exp Multiple Sclerosis/ | 47,422 |
| 2 | multiple sclerosis.tw. | 50,604 |
| 3 | 1 or 2 | 58,051 |
| 4 | (qaly* or (generic adj2 (instrument* or measure*)) or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or Health Utilities Index or HUI or 15D or Assessment of Quality of Life or AQOL or Quality of Well-Being or QWB).tw. | 27,152 |
| 5 | 3 and 4 | 355 |
| 6 | limit 5 to yr=‘1902 - 2011’ | 248 |
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Database: Ovid MEDLINE® In-Process & Other Non-Indexed Citations 5 April 2016.
Searched on 6 April 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | multiple sclerosis.tw. | 5010 |
| 2 | (qaly* or (generic adj2 (instrument* or measure*)) or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or Health Utilities Index or HUI or 15D or Assessment of Quality of Life or AQOL or Quality of Well-Being or QWB).tw. | 3504 |
| 3 | 1 and 2 | 46 |
| 4 | limit 3 to yr=‘1860 - 2011’ | 7 |
EMBASE (via Ovid)
Database: Ovid EMBASE 1974 to 2016 week 14.
Searched on 6 April 2016.
| ID | Search | Hits |
|---|---|---|
| 1 | multiple sclerosis/ | 94,999 |
| 2 | multiple sclerosis.tw. | 81,514 |
| 3 | 1 or 2 | 102,763 |
| 4 | (qaly* or (generic adj2 (instrument* or measure*)) or EQ5D or EQ-5D or euroqol or euro-qol or SF-36 or SF36 or SF-6D or SF-6D or SF6D or Health Utilities Index or HUI or 15D or Assessment of Quality of Life or AQOL or Quality of Well-Being or QWB).tw. | 50,631 |
| 5 | 3 and 4 | 885 |
| 6 | limit 5 to yr = ‘1902 - 2011’ | 427 |
| 7 | limit 6 to (conference abstract or conference paper or conference proceeding or ‘conference review’) | 158 |
| 8 | 6 not 7 | 269 |
Science Citation Index (Web of Knowledge)
Searched on 6 April 2016.
| ID | Hits | Search |
|---|---|---|
| #1 | 61,623 | TS = ‘multiple sclerosis’ Indexes = SCI-EXPANDED Timespan = 1900–2011 |
| #2 | 20,713 | TS = (QALY* or (generic NEAR/2 (instrument* or measure*)) or euro-qol or euroqol or ‘euro qol’ or EQ5D or EQ-5D or SF-36 or SF36 or SF-6D or SF6D or ‘health utilities index’ or HUI or 15D or ‘assessment of quality of life’ or AQOL or ‘Quality of Well-Being’ or QWB) Indexes = SCI-EXPANDED Timespan = 1900–2011 |
| #3 | 351 | #2 AND #1 Indexes = SCI-EXPANDED Timespan = 1900–2011 |
| #4 | 19 | (#3) AND DOCUMENT TYPES: (Meeting Abstract OR Meeting Summary OR Proceedings Paper) Indexes = SCI-EXPANDED Timespan = 1900–2011 |
| #5 | 332 | #3 not #4 Indexes = SCI-EXPANDED Timespan = 1900–2011 |
Cost-effectiveness Analysis (CEA) Registry
Searched on 6 April 2016.
Contained details of articles up to 2014 at time of search.
-
Basic search.
-
Articles.
-
Full search contents: multiple sclerosis.
-
Total number published from 1997 to 2011: 22.
School of Health and Related Research (ScHARR) Health Utilities Database (HUD)
Searched on 6 April 2016.
Total: two.
Search strategy
multiple sclerosis in any field
AND
2000 to 2011 in Year Published
Additional searches
Targeted database search to identify any additional MS patient registries that included data from before 1995.
MEDLINE (via Ovid)
Database: Ovid MEDLINE® 1946 to May week 4 2016.
Searched on 31 May 2016.
| 1 | exp Multiple Sclerosis/ | 48,148 |
| 2 | multiple sclerosis.tw. | 51,476 |
| 3 | 1 or 2 | 58,975 |
| 4 | exp Registries/ | 67,800 |
| 5 | (registry or registries).tw. | 70,207 |
| 6 | (register or registers).tw. | 45,934 |
| 7 | 4 or 5 or 6 | 140,237 |
| 8 | 3 and 7 | 755 |
| 9 | limit 8 to yr=‘1902 - 2005’ | 178 |
Excluded studies (cost-effectiveness studies and health-related quality of life studies)
| Reference | Reason for exclusion |
|---|---|
| Abolfazli R, Hosseini A, Gholami K, Javadi MR, Torkamandi H, Emami S. Quality of life assessment in patients with multiple sclerosis receiving interferon beta-1a: a comparative longitudinal study of Avonex and its biosimilar CinnoVex. ISRN Neurology 2012;2012:786526 | Intervention not of interest |
| Acaster S, Perard R, Chauhan D, Lloyd AJ. A forgotten aspect of the NICE reference case: an observational study of the health related quality of life impact on caregivers of people with multiple sclerosis. BMC Health Serv Res 2013;13:346. http://dx.doi.org/10.1186/1472-6963-13-346 | Not relevant |
| Ayuso GI. [Multiple sclerosis: socioeconomic effects and impact on quality of life.] Med Clin (Barc) 2014;143(Suppl. 3):7–12 | Not relevant |
| Baumstarck K, Butzkueven H, Fernandez O, Flachenecker P, Stecchi S, Idiman E, et al. Responsiveness of the Multiple Sclerosis International Quality of Life questionnaire to disability change: a longitudinal study. Health Qual Life Outcomes 2013;11:127 | Generic preference-based measure not used |
| Baumstarck K, Pelletier J, Aghababian V, Reuter F, Klemina I, Berbis J, et al. Is the concept of quality of life relevant for multiple sclerosis patients with cognitive impairment? Preliminary results of a cross-sectional study. PLOS ONE 2012;7:e30627 | Generic preference-based measure not used |
| Baumstarck K, Pelletier J, Boucekine M, Auquier P, MusiQoL study group. Predictors of quality of life in patients with relapsing–remitting multiple sclerosis: a 2-year longitudinal study. Rev Neurol 2015;171:173–80. http://dx.doi.org/10.1016/j.neurol.2014.09.005 | Generic preference-based measure not used |
| Baumstarck K, Pelletier J, Butzkueven H, Fernández O, Flachenecker P, Idiman E, et al. Health-related quality of life as an independent predictor of long-term disability for patients with relapsing–remitting multiple sclerosis. Eur J Neurol 2013;20:907–14, e78–9. http://dx.doi.org/10.1111/ene.12087 | Generic preference-based measure not used |
| Beckerman H, Kempen JC, Knol DL, Polman CH, Lankhorst GJ, de Groot V. The first 10 years with multiple sclerosis: the longitudinal course of daily functioning. J Rehabil Med 2013;45:68–75. http://dx.doi.org/10.2340/16501977-1079 | Generic preference-based measure not used |
| Bergvall N, Tambour M, Henriksson F, Fredrikson S. Cost-minimization analysis of fingolimod compared with natalizumab for the treatment of relapsing–remitting multiple sclerosis in Sweden. J Med Econ 2013;16:349–57. http://dx.doi.org/10.3111/13696998.2012.755537 | Intervention not of interest |
| Boeru G, Milanov I, De Robertis F, Kozubski W, Lang M, Rojas-Farreras S, et al. ExtaviJect® 30G device for subcutaneous self-injection of interferon beta-1b for multiple sclerosis: a prospective European study. Med Devices (Auckl) 2013;6:175–84 | Not relevant |
| Boucekine M, Loundou A, Baumstarck K, Minaya-Flores P, Pelletier J, Ghattas B, Auquier P. Using the random forest method to detect a response shift in the quality of life of multiple sclerosis patients: a cohort study. BMC Med Res Methodol 2013;13:20. http://dx.doi.org/10.1186/1471-2288-13-20 | Generic preference-based measure not used |
| Brandes DW, Raimundo K, Agashivala N, Kim E. Implications of real-world adherence on cost-effectiveness analysis in multiple sclerosis. J Med Econ 2013;16:547–51 http://dx.doi.org/10.3111/13696998.2013.774281 | Not relevant |
| Brown MG. Cost of disease-modifying therapies for multiple sclerosis. Neurology 2015;84:e181–5 http://dx.doi.org/10.1212/WNL.0000000000001676 | Not a full economic analysis |
| Buchanan RJ, Johnson O, Zuniga MA, Carrillo-Zuniga G, Chakravorty BJ. Health-related quality of life among Latinos with multiple sclerosis. J Soc Work Disabil Rehabil 2012;11:240–57 http://dx.doi.org/10.1080/1536710X.2012.730846 | Generic preference-based measure not used |
| Buhse M, Della Ratta C, Galiczewski J, Eckardt P. Caregivers of older persons with multiple sclerosis: determinants of health-related quality of life. J Neurosci Nurs 2015;47:E2–12. http://dx.doi.org/10.1097/JNN.0000000000000117 | Not relevant |
| Calkwood J, Cree B, Crayton H, Kantor D, Steingo B, Barbato L, et al. Impact of a switch to fingolimod versus staying on glatiramer acetate or beta interferons on patient- and physician-reported outcomes in relapsing multiple sclerosis: analyses of the EPOC trial. BMC Neurol 2014;14:220 | Intervention not of interest |
| Caloyeras JP, Zhang B, Wang C, Eriksson M, Fredrikson S, Beckmann K, et al. Cost-effectiveness analysis of interferon beta-1b for the treatment of patients with a first clinical event suggestive of multiple sclerosis. Clin Ther 2012;34:1132–44. http://dx.doi.org/10.1016/j.clinthera.2012.03.004 | Not relevant for RRMS review |
| Campbell JD, Ghushchyan V, Brett McQueen R, Cahoon-Metzger S, Livingston T, Vollmer T, et al. Burden of multiple sclerosis on direct, indirect costs and quality of life: national US estimates. Mult Scler Relat Disord 2014;3:227–36 | Results not reported by EDSS level |
| Campbell JD, McQueen RB, Miravalle A, Corboy JR, Vollmer TL, Nair K. Comparative effectiveness of early natalizumab treatment in JC virus-negative relapsing–remitting multiple sclerosis. Am J Manag Care 2013;19:278–85 | Intervention not of interest |
| Carlson JJ, Hansen RN, Dmochowski RR, Globe DR, Colayco DC, Sullivan SD. Estimating the cost-effectiveness of onabotulinumtoxinA for neurogenic detrusor overactivity in the United States. Clin Ther 2013;35:414–24. http://dx.doi.org/10.1016/j.clinthera.2013.02.020 | Intervention not of interest |
| Chruzander C, Ytterberg C, Gottberg K, Einarsson U, Widen Holmqvist L, Johansson S. A 10-year follow-up of a population-based study of people with multiple sclerosis in Stockholm, Sweden: changes in health-related quality of life and the value of different factors in predicting health-related quality of life. J Neurol Sci 2014;339:57–63 | Results not reported by EDSS level |
| Cioncoloni D, Innocenti I, Bartalini S, Santarnecchi E, Rossi S, Rossi A, Ulivelli M. Individual factors enhance poor health-related quality of life outcome in multiple sclerosis patients. Significance of predictive determinants. J Neurol Sci 2014;345:213–19. http://dx.doi.org/10.1016/j.jns.2014.07.050 | Generic preference-based measure not used |
| Coleman CI, Sidovar MF, Roberts MS, Kohn C. Impact of mobility impairment on indirect costs and health-related quality of life in multiple sclerosis. PLOS ONE 2013;8:e54756. http://dx.doi.org/10.1371/journal.pone.0054756 | Generic measure not used; indirect costs estimated |
| Cooper K, Bryant J, Harris P, Loveman E, Jones J, Welch K. Alemtuzumab for the Treatment of Relapsing–Remitting Multiple Sclerosis: a Single Technology Appraisal. Southampton: SHTAC; 2013 | Intervention not of interest |
| Crespo C, Izquierdo G, García-Ruiz A, Granell M, Brosa M. Cost minimisation analysis of fingolimod vs natalizumab as a second line of treatment for relapsing–remitting multiple sclerosis. Neurologia 2014;29:210–17. http://dx.doi.org/10.1016/j.nrl.2013.04.003 | Interventions not of interest |
| de la Rosa RS, García-Bujalance L, Meca-Lallana J. Cost analysis of glatiramer acetate versus interferon-β for relapsing–remitting multiple sclerosis in patients with spasticity: the Escala study. Health Econ Rev 2015;5:1–9 | No decision-analytic model |
| Devy R, Lehert P, Varlan E, Genty M, Edan G. A short and validated multiple sclerosis-specific health-related quality of life measurement for routine medical practice. Eur J Neurol 2013;20:935–41 | Generic preference-based measure not used |
| Di Filippo M, Proietti S, Gaetani L, Gubbiotti M, Di Gregorio M, Eusebi P, et al. Lower urinary tract symptoms and urodynamic dysfunction in clinically isolated syndromes suggestive of multiple sclerosis. Eur J Neurol 2014;21:648–53 | Generic preference-based measure not used |
| Ertekin O, Ozakbas S, Idiman E. Caregiver burden, quality of life and walking ability in different disability levels of multiple sclerosis. Neurorehabilitation 2014;34:313–21 | Generic preference-based measure not used |
| Fernández-Muñoz JJ, Morón-Verdasco A, Cigarán-Méndez M, Muñoz-Hellín E, Pérez-de-Heredia-Torres M, Fernández-de-las-Peñas C. Disability, quality of life, personality, cognitive and psychological variables associated with fatigue in patients with multiple sclerosis. Acta Neurol Scand 2015;132:118–24. http://dx.doi.org/10.1111/ane.12370 | Generic preference-based measure not used |
| Fiest KM, Fisk JD, Patten SB, Tremlett H, Wolfson C, Warren S, et al. Comorbidity is associated with pain-related activity limitations in multiple sclerosis. Mult Scler Relat Disord 2015;4:470–6. http://dx.doi.org/10.1016/j.msard.2015.07.014 | Not relevant |
| Finès P, Garner R, Bancej C, Bernier J, Manuel DG. Development and implementation of microsimulation models of neurological conditions. Health Rep 2016;27:3–9 | Not relevant |
| Flensner G, Landtblom AM, Soderhamn O, Ek AC. Work capacity and health-related quality of life among individuals with multiple sclerosis reduced by fatigue: a cross-sectional study. BMC Public Health 2013;13:224 | Generic preference-based measure not used |
| Fogarty E, Walsh C, Adams R, McGuigan C, Barry M, Tubridy N. Relating health-related quality of life to disability progression in multiple sclerosis, using the 5-level EQ-5D. Mult Scler 2013;19:1190–6. http://dx.doi.org/10.1177/1352458512474860 | No decision-analytic model |
| Fredrikson S, McLeod E, Henry N, Pitcher A, Lowin J, Cuche M, et al. A cost-effectiveness analysis of subcutaneous interferon beta-1a 44mcg 3-times a week vs no treatment for patients with clinically isolated syndrome in Sweden. J Med Econ 2013;16:756–62. http://dx.doi.org/10.3111/13696998.2013.792824 | Not relevant for RRMS review |
| Garattini L, Ghislandi F, Da Costa MR. Cost-effectiveness modeling in multiple sclerosis: playing around with non-healthcare costs? Pharmacoeconomics 2015;33:1241–4. http://dx.doi.org/10.1007/s40273-015-0322-7 | Not relevant |
| Gavelova M, Nagyova I, Rosenberger J, Krokavcova M, Gdovinova Z, Groothoff JW, et al. Importance of an individual’s evaluation of functional status for health-related quality of life in patients with multiple sclerosis. Disabil Health J 2015;8:372–9 | Generic preference-based measure not used |
| Ghajarzadeh M, Azizi S, Moghadasi AN, Sahraian MA, Azimi A, Mohammadifar M, et al. Validity and reliability of the Persian version of the PERception de la Scle’rose En Plaques et de ses Pousse’es Questionnaire evaluating multiple sclerosis-related quality of life. Int J Prev Med 2016;7:25 | Generic preference-based measure not used |
| Giordano A, Ferrari G, Radice D, Randi G, Bisanti L, Solari A, POSMOS study. Health-related quality of life and depressive symptoms in significant others of people with multiple sclerosis: a community study. Eur J Neurol 2012;19:847–54. http://dx.doi.org/10.1111/j.1468-1331.2011.03638.x | Not relevant |
| Goodwin E, Green C. A quality-adjusted life-year measure for multiple sclerosis: developing a patient-reported health state classification system for a multiple sclerosis-specific preference-based measure. Value Health 2015;18:1016–24 | Generic preference-based measure not used |
| Goodwin E, Green C, Spencer A. Estimating a preference-based index for an eight-dimensional health state classification system for multiple sclerosis. Value Health 2015;18:1025–36 | Generic preference-based measure not used |
| Grytten N, Aarseth JH, Espeset K, Berg Johnsen G, Wehus R, Lund C, et al. Health-related quality of life and disease-modifying treatment behaviour in relapsing–remitting multiple sclerosis – a multicentre cohort study. Acta Neurol Scand Suppl 2012;195:51–7. http://dx.doi.org/10.1111/ane.12033 | Generic preference-based measure not used |
| Guia de Practica Clinica Sobre la Atencion a las Personas con Esclerosis Multiple. [Clinical Practice Guideline of Care for People with Multiple Sclerosis.] Barcelona: Catalan Agency for Health Information, Assessment and Quality (CAHIAQ); 2012 | Non-English language |
| Hadianfard H, Ashjazadeh N, Feridoni S, Farjam E. The role of psychological resilience, severity of disease and treatment adherence in the prediction of health-related quality of life in patients with multiple sclerosis. Neurol Asia 2015;20:263–8 | Generic preference-based measure not used |
| Hawton A, Green C, Telford CJ, Wright DE, Zajicek JP. The use of multiple sclerosis condition-specific measures to inform health policy decision-making: mapping from the MSWS-12 to the EQ-5D. Mult Scler 2012;18:853–61 | Excluded from systematic review, but retained for information on inputs |
| Hawton A, Green C, Telford C, Zajicek J, Wright D. Using the Multiple Sclerosis Impact Scale to estimate health state utility values: mapping from the MSIS-29, version 2, to the EQ-5D and the SF-6D. Value Health 2012;15:1084–91 | Excluded from systematic review, but retained for information on inputs |
| Heisen M, Treur MJ, van der Hel WS, Frequin ST, Groot MT, Verheggen BG. Fingolimod reduces direct medical costs compared to natalizumab in patients with relapsing–remitting multiple sclerosis in the Netherlands. J Med Econ 2012;15:1149–58 | Interventions not of interest; not full economic analysis |
| Jones KH, Ford DV, Jones PA, John A, Middleton RM, Lockhart-Jones H, et al. How people with multiple sclerosis rate their quality of life: an EQ-5D survey via the UK MS register. PLOS ONE 2013;8:e65640. http://dx.doi.org/10.1371/journal.pone.0065640 | Excluded from systematic review, but retained for information on inputs |
| Kappos L, Gold R, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Quality of life outcomes with BG-12 (dimethyl fumarate) in patients with relapsing–remitting multiple sclerosis: the DEFINE study. Mult Scler 2014;20:243–52. http://dx.doi.org/10.1177/1352458513507817 | Not relevant |
| Karampampa K, Gustavsson A, Miltenburger C, Eckert B. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Mult Scler 2012;18(2 Suppl.):7–15 | Excluded from systematic review, but retained for information on inputs |
| Karampampa K, Gustavsson A, Miltenburger C, Kindundu CM, Selchen DH. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis: the costs and utilities of MS patients in Canada. J Popul Ther Clin Pharmacol 2012;19:e11–25 | Excluded from systematic review, but retained for information on inputs |
| Karampampa K, Gustavsson A, Miltenburger C, Mora S, Arbizu T. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from Spain. Mult Scler 2012;18(2 Suppl.):35–9 | Excluded from systematic review, but retained for information on inputs |
| Karampampa K, Gustavsson A, Miltenburger C, Neidhardt K, Lang M. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from Germany. Mult Scler 2012;18(2 Suppl.):23–7 | Excluded from systematic review, but retained for information on inputs |
| Karampampa K, Gustavsson A, Miltenburger C, Teruzzi C, Fattore G. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from Italy. Mult Scler 2012;18(2 Suppl.):29–34 | Excluded from systematic review, but retained for information on inputs |
| Karampampa K, Gustavsson A, Miltenburger C, Tyas D. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from the United Kingdom. Mult Scler 2012;18(2 Suppl.):41–5 | Excluded from systematic review, but retained for information on inputs |
| Karampampa K, Gustavsson A, van Munster ET, Hupperts RM, Sanders EA, Mostert J, et al. Treatment experience, burden, and unmet needs (TRIBUNE) in Multiple Sclerosis study: the costs and utilities of MS patients in the Netherlands. J Med Econ 2013;16:939–50. http://dx.doi.org/10.3111/13696998.2013.807267 | Excluded from systematic review, but retained for information on inputs |
| Kerling A, Keweloh K, Tegtbur U, Kück M, Grams L, Horstmann H, Windhagen A. Physical capacity and quality of life in patients with multiple sclerosis. Neurorehabilitation 2014;35:97–104. http://dx.doi.org/10.3233/NRE-141099 | Generic preference-based measure not used |
| Khan F, Amatya B, Kesselring J. Longitudinal 7-year follow-up of chronic pain in persons with multiple sclerosis in the community. J Neurol 2013;260:2005–15. http://dx.doi.org/10.1007/s00415-013-6925-z | Generic preference-based measure not used |
| Kinkel RP, Laforet G, You X. Disease-related determinants of quality of life 10 years after clinically isolated syndrome. Int J MS Care 2015;17:26–34 | Generic preference-based measure not used |
| Kita M, Fox RJ, Gold R, Giovannoni G, Phillips JT, Sarda SP, et al. Effects of delayed-release dimethyl fumarate (DMF) on health-related quality of life in patients with relapsing–remitting multiple sclerosis: an integrated analysis of the phase 3 DEFINE and CONFIRM studies. Clin Ther 2014;36:1958–71 | Intervention not of interest |
| Klevan G, Jacobsen CO, Aarseth JH, Myhr KM, Nyland H, Glad S, et al. Health related quality of life in patients recently diagnosed with multiple sclerosis. Acta Neurol Scand 2014;129:21–6. http://dx.doi.org/10.1111/ane.12142 | Generic preference-based measure not used |
| Kohlmann T, Wang C, Lipinski J, Hadker N, Caffrey E, Epstein M, et al. The impact of a patient support program for multiple sclerosis on patient satisfaction and subjective health status. J Neurosci Nurs 2013;45:E3–14. http://dx.doi.org/10.1097/JNN.0b013e31828a4161 | Not relevant |
| Kohn CG, Sidovar MF, Kaur K, Zhu Y, Coleman CI. Estimating a minimal clinically important difference for the EuroQol 5-Dimension health status index in persons with multiple sclerosis. Health Qual Life Outcomes 2014;12:66 | Not relevant |
| Kuspinar A, Mayo NE. Do generic utility measures capture what is important to the quality of life of people with multiple sclerosis? Health Qual Life Outcomes 2013;11:71. http://dx.doi.org/10.1186/1477-7525-11-71 | Excluded from systematic review, but retained for information on inputs |
| Labuz-Roszak B, Kubicka-Baczyk K, Pierzchala K, Horyniecki M, Machowska-Majchrzak A, Augustynska-Mutryn D, et al. [Quality of life in multiple sclerosis – association with clinical features, fatigue and depressive syndrome.] Psychiatr Pol 2013;47:433–42 | Excluded from systematic review, but retained for information on inputs |
| Learmonth YC, Hubbard EA, McAuley E, Motl RW. Psychometric properties of quality of life and health-related quality of life assessments in people with multiple sclerosis. Qual Life Res 2014;23:2015–23. http://dx.doi.org/10.1007/s11136-014-0639-2 | Generic preference-based measure not used |
| Limone BL, Sidovar MF, Coleman CI. Estimation of the effect of dalfampridine-ER on health utility by mapping the MSWS-12 to the EQ-5D in multiple sclerosis patients. Health Qual Life Outcomes 2013;11:105. http://dx.doi.org/10.1186/1477-7525-11-105 | Intervention not of interest |
| Lukoschek C, Sterr A, Claros-Salinas D, Gütler R, Dettmers C. Fatigue in multiple sclerosis compared to stroke. Front Neurol 2015;6:116. http://dx.doi.org/10.3389/fneur.2015.00116 | Not relevant |
| Magistrale G, Pisani V, Argento O, Incerti CC, Bozzali M, Cadavid D, et al. Validation of the World Health Organization Disability Assessment Schedule II (WHODAS-II) in patients with multiple sclerosis. Mult Scler 2015;21:448–56 | Not relevant |
| Marrie RA, Horwitz R, Cutter G, Tyry T. Cumulative impact of comorbidity on quality of life in MS. Acta Neurol Scand 2012;125:180–6. http://dx.doi.org/10.1111/j.1600-0404.2011.01526.x | Generic measure not used |
| Maruszczak MJ, Montgomery SM, Griffiths MJ, Bergvall N, Adlard N. Cost–utility of fingolimod compared with dimethyl fumarate in highly active relapsing–remitting multiple sclerosis (RRMS) in England. J Med Econ 2015;18:874–85 | Interventions not in scope |
| Mäurer M, Comi G, Freedman MS, Kappos L, Olsson TP, Wolinsky JS, et al. Multiple sclerosis relapses are associated with increased fatigue and reduced health-related quality of life – a post hoc analysis of the TEMSO and TOWER studies. Mult Scler Relat Disord 2016;7:33–40 | Interventions not in scope |
| Mauskopf J, Fay M, Iyer R, Sarda S, Livingston T. Cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing forms of multiple sclerosis in the United States. J Med Econ 2016;19:432–42. http://dx.doi.org/10.3111/13696998.2015.1135805 | Interventions not in scope |
| Mikula P, Nagyova I, Krokavcova M, Vitkova M, Rosenberger J, Szilasiova J, et al. Social participation and health-related quality of life in people with multiple sclerosis. Disabil Health J 2015;8:29–34. http://dx.doi.org/10.1016/j.dhjo.2014.07.002 | Generic preference-based measure not used |
| Mitosek-Szewczyk K, Kułakowska A, Bartosik-Psujek H, Hożejowski R, Drozdowski W, Stelmasiak Z. Quality of life in Polish patients with multiple sclerosis. Adv Med Sci 2014;59:34–8. http://dx.doi.org/10.1016/j.advms.2013.07.002 | Not relevant |
| Motl RW, McAuley E. Physical activity and health-related quality of life over time in adults with multiple sclerosis. Rehabil Psychol 2014;59:415–21. http://dx.doi.org/10.1037/a0037739 | Generic preference-based measure not used |
| National Institute for Health and Care Excellence. Teriflunomide for Treating Relapsing–Remitting Multiple Sclerosis. Technology appraisal guidance TA303. London: NICE; 2014. URL: www.nice.org.uk/guidance/ta303 (accessed 15 June 2017) | Intervention not of interest |
| Newsome SD, Guo S, Altincatal A, Proskorovsky I, Kinter E, Phillips G, et al. Impact of peginterferon beta-1a and disease factors on quality of life in multiple sclerosis. Mult Scler Relat Disord 2015;4:350–7. http://dx.doi.org/10.1016/j.msard.2015.06.004 | Generic preference-based measure not used |
| Norman G, Rice S, O’Connor J, Lewis-Light K, Craig D, McDaid C. Dimethyl Fumarate for Treating Relapsing-Remitting Multiple Sclerosis: a Single Technology Appraisal. CRD and CHE Technology Assessment Group; 2013. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32013000873/frame.html (accessed 15 June 2017) | Intervention not of interest |
| O’Day K, Meyer K, Stafkey-Mailey D, Watson C. Cost-effectiveness of Natalizumab vs Fingolimod for the Treatment of Relapsing–Remitting Multiple Sclerosis: Analyses in Sweden. 2014. URL: http://onlinelibrary.wiley.com/o/cochrane/cleed/articles/NHSEED-22014043467/frame.html (accessed 25 April 2017) | Abstract |
| O’Day K, Meyer K, Stafkey-Mailey D, Watson C. Cost-effectiveness of natalizumab vs fingolimod for the treatment of relapsing-remitting multiple sclerosis: analyses in Sweden. J Med Econ 2015;18:295–302. http://dx.doi.org/10.3111/13696998.2014.991786 | Interventions not in scope |
| Oleen-Burkey M, Castelli-Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse: the patient’s perspective. Patient 2012;5:57–69 | Excluded from systematic review, but retained for information on inputs |
| Palace J, Bregenzer T, Tremlett H, Oger J, Zhu F, Boggild M, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open 2014;4:e004073. [Erratum published in BMJ Open 2014;4:e004073corr1.] | Not an economic analysis |
| Péntek M, Gulácsi L, Rózsa C, Simó M, Iljicsov A, Komoly S, Brodszky V. Health status and costs of ambulatory patients with multiple sclerosis in Hungary. Ideggyogy Sz 2012;65:316–24 | Excluded from systematic review, but retained for information on inputs |
| Pierzchala K, Adamczyk-Sowa M, Dobrakowski P, Kubicka-Baczyk K, Niedziela N, Sowa P. Demographic characteristics of MS patients in Poland’s upper Silesia region. Int J Neurosci 2015;125:344–51. http://dx.doi.org/10.3109/00207454.2014.937002 | Not relevant |
| Raikou M, Kalogeropoulou M, Rombopoulos G. A cost-effectiveness analysis of fingolimod versus dimethyl fumarate as a second-line disease modifying treatment in patients with highly active relapsing–remitting multiple sclerosis. Value Health 2015;18:A758 | Interventions not in scope |
| Reese JP, Wienemann G, John A, Linnemann A, Balzer-Geldsetzer M, Mueller UO, et al. Preference-based health status in a German outpatient cohort with multiple sclerosis. Health Qual Life Outcomes 2013;11:162 | Excluded from systematic review, but retained for information on inputs |
| Ruutiainen J, Viita AM, Hahl J, Sundell J, Nissinen H. Burden of illness in multiple sclerosis (DEFENSE) study: the costs and quality-of-life of Finnish patients with multiple sclerosis. J Med Econ 2016;19:21–33. http://dx.doi.org/10.3111/13696998.2015.1086362 | Excluded from systematic review, but retained for information on inputs |
| Sabanov AV, Luneva AV, Matveev NV. [Pharmacoeconomic analysis of the efficacy of natalizumab in relapsing–remitting multiple sclerosis.] Zh Nevrol Psikhiatr Im SS Korsakova 2014;114:65–9 | Query full economic analysis |
| Salehpoor G, Rezaei S, Hosseininezhad M. Quality of life in multiple sclerosis (MS) and role of fatigue, depression, anxiety, and stress: a bicenter study from north of Iran. Iran J Nurs Midwifery Res 2014;19:593–9 | Not relevant |
| Sanchez-de la Rosa R, Sabater E, Casado MA. Cost analysis of glatiramer acetate vs. fingolimod for the treatment of patients with relapsing–remitting multiple sclerosis in Spain. Health Econ Rev 2013;3:13 | Review |
| Sidovar MF, Limone BL, Coleman CI. Mapping of Multiple Sclerosis Walking Scale (MSWS-12) to five-dimension EuroQol (EQ-5D) health outcomes: an independent validation in a randomized control cohort. Patient Relat Outcome Meas 2016;7:13–18 | Excluded from systematic review, but retained for information on inputs |
| Sidovar MF, Limone BL, Lee S, Coleman CI. Mapping the 12-item multiple sclerosis walking scale to the EuroQol 5-dimension index measure in North American multiple sclerosis patients. BMJ Open 2013;3(5) | Excluded from systematic review, but retained for information on inputs |
| Svensson M, Fajutrao L. Costs of formal and informal home care and quality of life for patients with multiple sclerosis in Sweden. Mult Scler Int 2014;2014:529878. http://dx.doi.org/10.1155/2014/529878 | Results not stratified by EDSS level but by severity level |
| Takemoto ML, Lopes da Silva N, Ribeiro-Pereira AC, Schilithz AO, Suzuki C. Differences in utility scores obtained through Brazilian and UK value sets: a cross-sectional study. Health Qual Life Outcomes 2015;13:119 | Results not stratified by EDSS level but by fatigue level |
| Thomas S, Thomas PW, Kersten P, Jones R, Green C, Nock A, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatr 2013;84:1092–9. http://dx.doi.org/10.1136/jnnp-2012-303816 | No decision-analytic model |
| Tosh J, Dixon S, Carter A, Daley A, Petty J, Roalfe A, et al. Cost effectiveness of a pragmatic exercise intervention (EXIMS) for people with multiple sclerosis: economic evaluation of a randomised controlled trial. Mult Scler 2014;20:1123–30 | Intervention not of interest |
| Versteegh MM, Leunis A, Luime JJ, Boggild M, Uyl-de Groot CA, Stolk EA. Mapping QLQ-C30, HAQ, and MSIS-29 on EQ-5D. Med Decis Making 2012;32:554–68. http://dx.doi.org/10.1177/0272989X11427761 | No utility values available for people with an EDSS level of > 7 |
| Versteegh MM, Leunis A, Uyl-de Groot CA, Stolk EA. Condition-specific preference-based measures: benefit or burden? Value Health 2012;15:504–13. http://dx.doi.org/10.1016/j.jval.2011.12.003 | Not relevant |
| Yamout B, Issa Z, Herlopian A, El Bejjani M, Khalifa A, Ghadieh AS, Habib RH. Predictors of quality of life among multiple sclerosis patients: a comprehensive analysis. Eur J Neurol 2013;20:756–64. http://dx.doi.org/10.1111/ene.12046 | Not relevant |
| Zarco LA, Millán SP, Londoño D, Parada L, Taborda A, Borda MG. [The cost-effectiveness of interferon beta treatment in patients with a clinically isolated syndrome in Colombia.] Biomedica 2014;34:110–17. http://dx.doi.org/10.1590/S0120-41572014000100014 | Not relevant for RRMS review |
| Zhang X, Hay JW, Niu X. Cost effectiveness of fingolimod, teriflunomide, dimethyl fumarate and intramuscular interferon-beta1a in relapsing–remitting multiple sclerosis. CNS Drugs 2015;29:71–81 | Interventions not in scope |
| Reference | Reason for exclusion |
|---|---|
| Acquadro C, Lafortune L, Mear I. Quality of life in multiple sclerosis: translation in French Canadian of the MSQoL-54. Health Qual Life Outcomes 2003;1:70. http://dx.doi.org/10.1186/1477-7525-1-70 | No generic preference-based measure used |
| Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler 2001;7:340–4 | No generic preference-based measure used |
| Anonymous. Burden of illness of multiple sclerosis: part II: quality of life. The Canadian Burden of Illness Study Group. Can J Neurol Sci 1998;25:31–8 | No generic preference-based measure used |
| Argyriou AA, Karanasios P, Ifanti AA, Iconomou G, Assimakopoulos K, Makridou A, et al. Quality of life and emotional burden of primary caregivers: a case–control study of multiple sclerosis patients in Greece. Qual Life Res 2011;20:1663–8. http://dx.doi.org/10.1007/s11136-011-9899-2 | Not relevant |
| Arnoldus JH, Killestein J, Pfennings LE, Jelles B, Uitdehaag BM, Polman CH. Quality of life during the first 6 months of interferon-beta treatment in patients with MS. Mult Scler 2000;6:338–42 | No generic preference-based measure used |
| Aymerich M, Guillamon I, Jovell AJ. Health-related quality of life assessment in people with multiple sclerosis and their family caregivers. A multicenter study in Catalonia (Southern Europe). Patient Prefer Adherence 2009;3:311–21 | No generic preference-based measure used |
| Aymerich M, Guillamon I, Perkal H, Nos C, Porcel J, Berra S, et al. Spanish adaptation of the disease-specific questionnaire MSQOL-54 in multiple sclerosis patients. Neurologia 2006;21:181–7 | No generic preference-based measure used |
| Baker JG, Granger CV, Ottenbacher KJ. Validity of a brief outpatient functional assessment measure. Am J Phys Med Rehabil 1996;75:356–63 | No generic preference-based measure used |
| Baumstarck-Barrau K, Pelletier J, Simeoni MC, Auquier P, MusiQol Study G. [French validation of the Multiple Sclerosis International Quality of Life Questionnaire.] Rev Neurol (Paris) 2011;167:511–21 | No generic preference-based measure used |
| Baumstarck-Barrau K, Simeoni MC, Reuter F, Klemina I, Aghababian V, Pelletier J, Auquier P. Cognitive function and quality of life in multiple sclerosis patients: a cross-sectional study. BMC Neurol 2011;11:17. http://dx.doi.org/10.1186/1471-2377-11-17 | No generic preference-based measure used |
| Bermel RA, Weinstock-Guttman B, Bourdette D, Foulds P, You X, Rudick RA. Intramuscular interferon beta-1a therapy in patients with relapsing–remitting multiple sclerosis: a 15-year follow-up study. Mult Scler J 2010;16:588–96 | Not relevant |
| Brunet DG, Hopman WM, Singer MA, Edgar CM, MacKenzie TA. Measurement of health-related quality of life in multiple sclerosis patients. Can J Neurol Sci 1996;23:99–103 | No generic preference-based measure used |
| Casado V, Romero L, Gubieras L, Alonso L, Moral E, Martinez-Yelamos S, et al. An approach to estimating the intangible costs of multiple sclerosis according to disability in Catalonia, Spain. Mult Scler 2007;13:800–4 | Not relevant |
| Casetta I, Riise T, Wamme Nortvedt M, Economou NT, De Gennaro R, Fazio P, et al. Gender differences in health-related quality of life in multiple sclerosis. Mult Scler 2009;15:1339–46. http://dx.doi.org/10.1177/1352458509107016 | Not relevant |
| Delgado-Mendilívar JM, Cadenas-Díaz JC, Fernández-Torrico JM, Navarro-Mascarell G, Izquierdo G. [A study of the quality of life in cases of multiple sclerosis.] Rev Neurol 2005;41:257–62 | No generic preference-based measure used |
| Di Fabio RP, Choi T, Soderberg J, Hansen CR. Health-related quality of life for patients with progressive multiple sclerosis: influence of rehabilitation. Phys Ther 1997;77:1704–16 | No generic preference-based measure used |
| Drulovic J, Pekmezovic T, Matejic B, Mesaros S, Manigoda M, Dujmovic I, et al. Quality of life in patients with multiple sclerosis in Serbia. Acta Neurol Scand 2007;115:147–52 | No generic preference-based measure used |
| Drulovic J, Riise T, Nortvedt M, Pekmezovic T, Manigoda M. Self-rated physical health predicts change in disability in multiple sclerosis. Mult Scler 2008;14:999–1002 http://dx.doi.org/10.1177/1352458508088917 | No generic preference-based measure used |
| Earnshaw SR, Graham J, Oleen-Burkey M, Castelli-Haley J, Johnson K. Cost effectiveness of glatiramer acetate and natalizumab in relapsing–remitting multiple sclerosis. Appl Health Econ Health Policy 2009;7:91–108 | Economic analysis pre 2012 |
| Fernandez O, Baumstarck-Barrau K, Simeoni MC, Auquier P, MusiQoL Study Group. Patient characteristics and determinants of quality of life in an international population with multiple sclerosis: assessment using the MusiQoL and SF-36 questionnaires. Mult Scler 2011;17:1238–49 | No generic preference-based measure used |
| Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Mult Scler 1999;5:251–9 | No generic preference-based measure used |
| Fisk JD, Brown MG, Sketris IS, Metz LM, Murray TJ, Stadnyk KJ. A comparison of health utility measures for the evaluation of multiple sclerosis treatments. J Neurol Neurosurg Psychiatr 2005;76:58–63 | HRQoL results not presented by EDSS level |
| Forbes A, While A, Mathes L. Informal carer activities, carer burden and health status in multiple sclerosis. Clin Rehabil 2007;21:563–75 | Carers’ disutilities |
| Forbes A, While A, Mathes L, Griffiths P. Health problems and health-related quality of life in people with multiple sclerosis. Clin Rehabil 2006;20:67–78 | No generic preference-based measure used |
| Forbes RB, Lees A, Waugh N, Swingler RJ. Population based cost utility study of interferon beta-1b in secondary progressive multiple sclerosis. BMJ 1999;319:1529–33 | Population not of interest |
| Freeman JA, Hobart JC, Langdon DW, Thompson AJ. Clinical appropriateness: a key factor in outcome measure selection: the 36 item short form health survey in multiple sclerosis. J Neurol Neurosurg Psychiatr 2000;68:150–6 | No generic preference-based measure used |
| Freeman JA, Hobart JC, Thompson AJ. Does adding MS-specific items to a generic measure (the SF-36) improve measurement? Neurology 2001;57:68–74 | Results not presented by EDSS level |
| Freeman JA, Langdon DW, Hobart JC, Thompson AJ. Health-related quality of life in people with multiple sclerosis undergoing inpatient rehabilitation. J Neurol Rehabil 1996;10:185–94 | No generic preference-based measure used |
| Gani R, Giovannoni G, Bates D, Kemball B, Hughes S, Kerrigan J. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing–remitting multiple sclerosis in the UK. Pharmacoeconomics 2008;26:617–27 | Economic analysis pre 2012 |
| Gottberg K, Einarsson U, Ytterberg C, de Pedro Cuesta J, Fredrikson S, von Koch L, et al. Health-related quality of life in a population-based sample of people with multiple sclerosis in Stockholm County. MultScler 2006;12:605–12 | No generic preference-based measure used |
| Guarnaccia JB, Aslan M, O’Connor TZ, Hope M, Kazis L, Kashner CM, Booss J. Quality of life for veterans with multiple sclerosis on disease-modifying agents: relationship to disability. J Rehabil Res Dev 2006;43:35–44 | Not relevant |
| Haupts M, Elias G, Hardt C, Langenbahn H, Obert H, Pöhlau D, et al. [Quality of life in patients with remitting–relapsing multiple sclerosis in Germany.] Nervenarzt 2003;74:144–50. http://dx.doi.org/10.1007/s00115-002-1446-5 | Non-English language |
| Heiskanen S, Meriläinen P, Pietilä AM. Health-related quality of life – testing the reliability of the MSQOL-54 instrument among MS patients. Scand J Caring Sci 2007;21:199–206 | Generic measure not used |
| Hermann BP, Vickrey B, Hays RD, Cramer J, Devinsky O, Meador K, et al. A comparison of health-related quality of life in patients with epilepsy, diabetes and multiple sclerosis. Epilepsy Res 1996;25:113–18 | Not relevant |
| Hincapie-Zapata ME, Suarez-Escudero JC, Pineda-Tamayo R, Anaya JM. [Quality of life in multiple sclerosis and other chronic autoimmune and non-autoimmune diseases.] Rev Neurol 2009;48:225–30 | Mixed population |
| Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29) – a new patient-based outcome measure. Brain 2001;124:962–73 | No generic preference-based measure used |
| Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology 2003;60:31–6 | No generic preference-based measure used |
| Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome. Health Technol Assess 2004;8(9) | No generic preference-based measure used |
| Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. How responsive is the Multiple Sclerosis Impact Scale (MSIS-29)? A comparison with some other self report scales. J Neurol Neurosurg Psychiatr 2005;76:1539–43 | No generic preference-based measure used |
| Hopman WM, Coo H, Pavlov A, Day AG, Edgar CM, McBride EV, Brunet DG. Multiple sclerosis: change in health-related quality of life over two years. Can J Neurol Sci 2009;36:554–61 | No generic preference-based measure used |
| Jankovic SM, Kostic M, Radosavljevic M, Tesic D, Stefanovic-Stoimenov N, Stevanovic I, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing–remitting multiple sclerosis: a Markov model based on data a Balkan country in socioeconomic transition. Vojnosanit Pregl 2009;66:556–62 | Economic analysis pre 2012 |
| Jones CA, Pohar SL, Warren S, Turpin KV, Warren KG. The burden of multiple sclerosis: a community health survey. Health Qual Life Outcomes 2008;6:1. http://dx.doi.org/10.1186/1477-7525-6-1 | Results not presented by EDSS level |
| Kendrick M, Johnson KI. Long-term treatment of multiple sclerosis with interferon-beta may be cost effective. Pharmacoeconomics 2000;18:45–53 | Economic analysis pre 2012 |
| Kikuchi H, Kikuchi S, Ohbu S, Suzuki N, Maezaw M. [A survey on constitutive elements of quality of life in patients with multiple sclerosis.] Brain Nerve 2007;59:617–22 | No generic preference-based measure used |
| Kobelt G. Costs and quality of life for patients with multiple sclerosis in Belgium. Eur J Health Econ 2006;7(Suppl. 2):S24–33 | Excluded from systematic review, but retained for information on inputs |
| Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology 2006;66:1696–702 | Not relevant |
| Kobelt G, Berg J, Lindgren P, Anten B, Ekman M, Jongen PJ, et al. Costs and quality of life in multiple sclerosis in the Netherlands. Eur J Health Econ 2006;7(Suppl. 2):S55–64. [Erratum published in Eur J Health Econ 2007;8:359.] | < 30% of population with RRMS |
| Kobelt G, Berg J, Lindgren P, Battaglia M, Lucioni C, Uccelli A. Costs and quality of life of multiple sclerosis in Italy. Eur J Health Econ 2006;7(Suppl. 2):45–54. http://dx.doi.org/10.1007/s10198-006-0385-7 | 50% of the population had PPMS. Results not stratified by type of MS |
| Kobelt G, Berg J, Lindgren P, Gerfin A, Lutz J. Costs and quality of life of multiple sclerosis in Switzerland. Eur J Health Econ 2006;7(Suppl. 2):S86–95 | Excluded from systematic review, but retained for information on inputs |
| Kobelt G, Jönsson L, Fredrikson S. Cost–utility of interferon beta1b in the treatment of patients with active relapsing–remitting or secondary progressive multiple sclerosis. Eur J Health Econ 2003;4:50–9. http://dx.doi.org/10.1007/s10198-002-0163-0 | Economic analysis pre 2012 |
| Kobelt G, Jönsson L, Henriksson F, Fredrikson S, Jönsson B. Cost–utility analysis of interferon beta-1b in secondary progressive multiple sclerosis. Int J Technol Assess Health Care 2000;16:768–80 | Population not of interest |
| Kobelt G, Lindgren P, Smala A, Bitsch A, Haupts M, Kolmel HW, et al. Costs and quality of life in multiple sclerosis. An observational study in Germany. Eur J Health Econ 2001;2:60–8 | HRQoL results grouped by EDSS levels |
| Kobelt G, Texier-Richard B, Lindgren P. The long-term cost of multiple sclerosis in France and potential changes with disease-modifying interventions. Mult Scler 2009;15:741–51. http://dx.doi.org/10.1177/1352458509102771 | Economic analysis pre 2012 |
| Laosanguanek N, Wiroteurairuang T, Siritho S, Prayoonwiwat N. Reliability of the Thai version of SF-36 questionnaire for an evaluation of quality of life in multiple sclerosis patients in multiple sclerosis clinic at Siriraj Hospital. J Med Assoc Thai 2011;94(Suppl. 1):84–8 | No generic preference-based measure used |
| Malkova NA, Riabukhina OV, Babenko LA, Ionova TI, Kishtovich AV. [Health-related quality of life in patients with multiple sclerosis.] Zh Nevrol Psikhiatr Im SS Korsakova 2005;105:31–7 | Full text not available in English language |
| McCrone P, Heslin M, Knapp M, Bull P, Thompson A. Multiple sclerosis in the UK: service use, costs, quality of life and disability. Pharmacoeconomics 2008;26:847–60 | MS type not of interest |
| Michalski D, Liebig S, Thomae E, Singer S, Hinz A, Bergh FT. Anxiety, depression and impaired health-related quality of life are therapeutic challenges in patients with multiple sclerosis. Ment Illn 2010;2:e5 | Not relevant |
| Miller A, Dishon S. Health-related quality of life in multiple sclerosis: psychometric analysis of inventories. Mult Scler 2005;11:450–8 | No generic preference-based measure used |
| Miller A, Dishon S. Health-related quality of life in multiple sclerosis: the impact of disability, gender and employment status. Qual Life Res 2006;15:259–71. http://dx.doi.org/10.1007/s11136-005-0891-6 | No generic preference-based measure used |
| Mo F, Choi BC, Li FC, Merrick J. Using Health Utility Index (HUI) for measuring the impact on health-related quality of life (HRQL) among individuals with chronic diseases. Sci World J 2004;4:746–57 | Mixed population |
| Moore F, Wolfson C, Alexandrov L, Lapierre Y. Do general and multiple sclerosis-specific quality of life instruments differ? Can J Neurol Sci 2004;31:64–71 | HRQoL results grouped by EDSS levels |
| Morales Rde R, Morales Nde M, Rocha FC, Fenelon SB, Pinto Rde M, Silva CH. [Health-related quality of life in multiple sclerosis.] Arq Neuropsiquiatr 2007;65:454–60 | No generic preference-based measure used |
| Murrell RC, Kenealy PM, Beaumont JG, Lintern TC. Assessing quality of life in persons with severe neurological disability associated with multiple sclerosis: the psychometric evaluation of two quality of life measures. Br J Health Psychol 1999;4:349–62 | No generic preference-based measure used |
| Myers JA, McPherson KM, Taylor WJ, Weatherall M, McNaughton HK. Duration of condition is unrelated to health-state valuation on the EuroQoL. Clin Rehabil 2003;17:209–15 | Not relevant |
| Nicholl CR, Lincoln NB, Francis VM, Stephan TF. Assessing quality of life in people with multiple sclerosis. Disabil Rehabil 2001;23:597–603 | Results not presented by EDSS levels |
| Nicholl L, Hobart JC, Cramp AF, Lowe-Strong AS. Measuring quality of life in multiple sclerosis: not as simple as it sounds. Mult Scler 2005;11:708–12 | No generic preference-based measure used |
| Nortvedt MW, Riise T, Myhr KM, Nyland HI. Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology 1999;53:1098–103 | Intervention is not of interest |
| Nortvedt MW, Riise T, Myhr KM, Nyland HI. Performance of the SF-36, SF-12, and RAND-36 summary scales in a multiple sclerosis population. Med Care 2000;38:1022–8 | No generic preference-based measure used |
| Nortvedt MW, Riise T, Myhr KM, Nyland HI. Quality of life as a predictor for change in disability in MS. Neurology 2000;55:51–4 | No generic preference-based measure used |
| Nortvedt MW, Riise T, Myhr KM, Nyland HI, Hanestad BR. Type I interferons and the quality of life of multiple sclerosis patients. Results from a clinical trial on interferon alfa-2a. Mult Scler 1999;5:317–22 | Intervention is not of interest |
| Noyes K, Bajorska A, Chappel A, Schwid SR, Mehta LR, Weinstock-Guttman B, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based study. Neurology 2011;77:355–63. http://dx.doi.org/10.1212/WNL.0b013e3182270402 | Economic analysis pre 2012 |
| Nuijten MJ, Hutton J. Cost-effectiveness analysis of interferon beta in multiple sclerosis: a Markov process analysis. Value Health 2002;5:44–54 | Economic analysis pre 2012 |
| Orme M, Kerrigan J, Tyas D, Russell N, Nixon R. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health 2007;10:54–60 | Excluded from systematic review, but retained for information on inputs |
| Ozakbas S, Akdede BB, Kösehasanogullari G, Aksan O, Idiman E. Difference between generic and multiple sclerosis-specific quality of life instruments regarding the assessment of treatment efficacy. J Neurol Sci 2007;256:30–4 | Not relevant |
| Pakpour AH, Yekaninejad MS, Mohammadi NK, Molsted S, Zarei F, Patti F, Harrison A. Health-related quality of life in Iranian patients with multiple sclerosis: a cross-cultural study. Neurol Neurochir Pol 2009;43:517–26 | No generic preference-based measure used |
| Parkin D, Jacoby A, McNamee P, Miller P, Thomas S, Bates D. Treatment of multiple sclerosis with interferon beta: an appraisal of cost-effectiveness and quality of life. J Neurol Neurosurg Psychiatr 2000;68:144–9 | Economic analysis pre 2012 |
| Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D. A cost–utility analysis of interferon beta for multiple sclerosis. Health Technol Assess 1998;2(4) | Economic analysis pre 2012 |
| Parkin D, Rice N, Jacoby A, Doughty J. Use of a visual analogue scale in a daily patient diary: modelling cross-sectional time-series data on health-related quality of life. Soc Sci Med 2004;59:351–60. http://dx.doi.org/10.1016/j.socscimed.2003.10.015 | Not relevant |
| Patti F, Amato MP, Battaglia MA, Pitaro M, Russo P, Solaro C, Trojano M. Caregiver quality of life in multiple sclerosis: a multicentre Italian study. Mult Scler 2007;13:412–19 | Not relevant |
| Patti F, Cacopardo M, Palermo F, Ciancio MR, Lopes R, Restivo D, Reggio A. Health-related quality of life and depression in an Italian sample of multiple sclerosis patients. J Neurol Sci 2003;211:55–62 | Caregiver quality of life |
| Patti F, Russo P, Pappalardo A, Macchia F, Civalleri L, Paolillo A, FAMS study group. Predictors of quality of life among patients with multiple sclerosis: an Italian cross-sectional study. J Neurol Sci 2007;252:121–9 | Not relevant |
| Pfennings L, Cohen L, Adèr H, Polman C, Lankhorst G, Smits R, van der Ploeg H. Exploring differences between subgroups of multiple sclerosis patients in health-related quality of life. J Neurol 1999;246:587–91 | Not relevant |
| Pfennings LE, Van der Ploeg HM, Cohen L, Bramsen I, Polman CH, Lankhorst GJ, Vleugels L. A health-related quality of life questionnaire for multiple sclerosis patients. Acta Neurol Scand 1999;100:148–55 | No generic preference-based measure used |
| Phillips CJ. The cost of multiple sclerosis and the cost effectiveness of disease-modifying agents in its treatment. CNS Drugs 2004;18:561–74 | Economic analysis pre 2012 |
| Phillips CJ, Gilmour L, Gale R, Palmer M. A cost utility model of interferon beta-1b in the treatment of relapsing–remitting multiple sclerosis. J Med Econ 2001;4:35–50 | Economic analysis pre 2012 |
| Phillips JT, Giovannoni G, Lublin FD, O’Connor PW, Polman CH, Willoughby E, et al. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler 2011;17:970–9 | Not relevant |
| Pittock SJ, Mayr WT, McClelland RL, Jorgensen NW, Weigand SD, Noseworthy JH, Rodriguez M. Quality of life is favorable for most patients with multiple sclerosis: a population-based cohort study. Arch Neurol 2004;61:679–86. http://dx.doi.org/10.1001/archneur.61.5.679 | No relevant information |
| Popova EV, Riabukhina OV, Vorob’eva OV, Malkova NA, Boiko AN. Changes in quality of life in patients with remitted multiple sclerosis during the specific treatment with disease-modifying drugs: a comparative study of populations of Moscow and Novosibirsk. Zh Nevrol Psikhiatr Im SS Korsakova 2010;110:67–70 | No relevant information |
| Pozzilli C, Palmisano L, Mainero C, Tomassini V, Marinelli F, Ristori G, et al. Relationship between emotional distress in caregivers and health status in persons with multiple sclerosis. Mult Scler 2004;10:442–6 | Carers’ disutilities |
| Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Patient and community preferences for treatments and health states in multiple sclerosis. Mult Scler 2003;9:311–19 | Results not reported for all EDSS levels |
| Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health 2004;7:554–68. http://dx.doi.org/10.1111/j.1524-4733.2004.75007.x | Economic analysis pre 2012 |
| Putzki N, Fischer J, Gottwald K, Reifschneider G, Ries S, Siever A, et al. Quality of life in 1000 patients with early relapsing–remitting multiple sclerosis. Eur J Neurol 2009;16:713–20 | Excluded from systematic review, but retained for information on inputs |
| Riazi A, Hobart JC, Lamping DL, Fitzpatrick R, Freeman JA, Jenkinson C, et al. Using the SF-36 measure to compare the health impact of multiple sclerosis and Parkinson’s disease with normal population health profiles. J Neurol Neurosurg Psychiatry 2003;74:710–14 | No generic preference-based measure used |
| Rivera-Navarro J, Benito-León J, Oreja-Guevara C, Pardo J, Dib WB, Orts E, et al. Burden and health-related quality of life of Spanish caregivers of persons with multiple sclerosis. Mult Scler 2009;15:1347–55. http://dx.doi.org/10.1177/1352458509345917 | Carers’ disutilities |
| Robinson D Jr, Zhao N, Gathany T, Kim LL, Cella D, Revicki D. Health perceptions and clinical characteristics of relapsing–remitting multiple sclerosis patients: baseline data from an international clinical trial. Curr Med Res Opin 2009;25:1121–30 | No generic preference-based measure used |
| Rothwell PM, McDowell Z, Wong CK, Dorman PJ. Doctors and patients don’t agree: cross sectional study of patients’ and doctors’ perceptions and assessments of disability in multiple sclerosis. BMJ 1997;314:1580–3 | Not relevant |
| Rubio-Terres C, Aristegui Ruiz I, Medina Redondo F, Izquierdo Ayuso G. [Cost–utility analysis of multiple sclerosis treatment with glatiramer acetate or interferon beta in Spain.] Farm Hosp 2003;27:159–65 | Economic analysis pre 2012 |
| Rubio-Terres C, Dominguez-Gil Hurle A. [Cost–utility analysis of relapsing–remitting multiple sclerosis treatment with azathioprine or interferon beta in Spain.] Rev Neurol 2005;40:705–10 | Economic analysis pre 2012 |
| Rudick RA, Miller DM. Health-related quality of life in multiple sclerosis – current evidence, measurement and effects of disease severity and treatment. CNS Drugs 2008;22:827–39 | No generic preference-based measure used |
| Rudick RA, Miller D, Hass S, Hutchinson M, Calabresi PA, Confavreux C, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol 2007;62:335–46. http://dx.doi.org/10.1002/ana.21163 | Intervention is not of interest |
| Sehanovic A, Dostovic Z, Smajlovic D, Avdibegovic E. Quality of life in patients suffering from Parkinson’s disease and multiple sclerosis. Med Arh 2011;65:291–4 | No generic preference-based measure used |
| Senol V, Sipahioglu MH, Ozturk A, Argün M, Utaş C. Important determinants of quality of life in a peritoneal dialysis population in Turkey. Ren Fail 2010;32:1196–201. http://dx.doi.org/10.3109/0886022X.2010.517349 | Not relevant |
| Shawaryn MA, Schiaffino KM, LaRocca NG, Johnston MV. Determinants of health-related quality of life in multiple sclerosis: the role of illness intrusiveness. Mult Scler 2002;8:310–18 | Utility values not reported |
| Solari A, Radice D. Health status of people with multiple sclerosis: a community mail survey. Neurol Sci 2001;22:307–15 | No generic preference-based measure used |
| Szilasiova J, Krokavcova M, Gdovinova Z, Rosenberger J, Van Dijk JP. Quality of life in patients with multiple sclerosis in Eastern Slovakia. Disabil Rehabil 2011;33:1587–93. http://dx.doi.org/10.3109/09638288.2010.540292 | No generic preference-based measure used |
| Tatarinova M, Fokin IV, Boiko AN. [Quality of life in multiple sclerosis and pharmaco-economic studies.] Zh Nevrol Psikhiatr Im SS Korsakova 2002;(Suppl.):76–80 | Full text not available in English Language |
| Thompson JP, Noyes K, Dorsey ER, Schwid SR, Holloway RG. Quantitative risk–benefit analysis of natalizumab. Neurology 2008;71:357–64. http://dx.doi.org/10.1212/01.wnl.0000319648.65173.7a | Economic analysis pre 2012, but provides useful information on utility values by EDSS level |
| Turpin KV, Carroll LJ, Cassidy JD, Hader WJ. Deterioration in the health-related quality of life of persons with multiple sclerosis: the possible warning signs. Mult Scler 2007;13:1038–45 | Not relevant |
| Vermersch P, de Seze J, Delisse B, Lemaire S, Stojkovic T. Quality of life in multiple sclerosis: influence of interferon-beta1 a (Avonex) treatment. Mult Scler 2002;8:377–81 | No generic preference-based measure used |
| Vickrey BG, Hays RD, Genovese BJ, Myers LW, Ellison GW. Comparison of a generic to disease-targeted health-related quality-of-life measures for multiple sclerosis. J Clin Epidemiol 1997;50:557–69 | No generic preference-based measure used |
| Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res 1995;4:187–206 | No generic preference-based measure used |
Blank data extraction form for cost-effectiveness studies: relapsing–remitting multiple sclerosis
| Date: | |
| Study ID: | |
| Name of first reviewer: | |
| Name of second reviewer: | |
| Study details | |
|---|---|
| Study title | |
| First author | |
| Co-authors | |
| Source of publication: Journal yy;vol.(issue):pp | |
| Language | |
| Publication type | |
| Inclusion criteria/study eligibility/PICOS | |
| Population | |
| Intervention(s) | |
| Comparator(s) | |
| Outcome(s) | |
| Study design | |
| Methods | |
| Setting and location | |
| Study perspective | |
| Comparators | |
| Time horizon | |
| Discount rate | |
| Outcomes | |
| Measurement of effectiveness | |
| Measurement and valuation of preference-based outcomes | |
| Resource use and costs | |
| Currency, price date and conversion | |
| Model type | |
| Assumptions | |
| Analytical methods | |
| Results | |
| Study parameters | |
| Incremental costs and outcomes | |
| Characterising uncertainty | |
| Discussion | |
| Study findings | |
| Limitations | |
| Generalisability | |
| Other | |
| Source of funding | |
| Conflicts of interest | |
| Comments | |
| Authors’ conclusion | |
| Reviewer’s conclusion | |
Quality assessment of economic evaluations in relapsing–remitting multiple sclerosis
| Assessment | Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sanchez-de la Rosa 2012264 | Nikfar 2013265 | Agashivala 2012266 | Palace 2015154 | Pan 2012267 | Darbà 2014268 | Imani 2012269 | Dembek 2014270 | Chevalier 2016271 | Lee 2012272 | |
| Title | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Abstract | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Introduction | ||||||||||
| Background and objectives | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Methods | ||||||||||
| Target population and subgroups | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Setting and location | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Study perspective | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Comparators | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Time horizon | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Discount rate | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Choice of health outcomes | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Measurement of effectiveness | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Measurement and valuation of preference-based outcomes | Y | Y | NA | Y | Y | NA | N | Y | Y | Y |
| Estimating resources and costs | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Currency, price date and conversion | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Choice of model | Y | Y | N | Y | Y | Y | N | Y | Y | Y |
| Assumptions | Y | Y | Y | Y | Y | N | N | Y | Y | Y |
| Analytical methods | Y | Y | N | Y | Y | U | N | Y | Y | Y |
| Results | ||||||||||
| Study parameters | Y | Y | N | Y | Y | N | Y | N | N | Y |
| Incremental costs and outcomes | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Characterising uncertainty | Y | Y | N | Y | Y | N | N | N | Y | N |
| Discussion | ||||||||||
| Study findings | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Limitations | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Generalisability | Y | Y | N | Y | Y | N | Y | Y | N | Y |
| Other | ||||||||||
| Source of funding | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Conflicts of interest | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Criteria | Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sanchez-de la Rosa 2012264 | Nikfar 2013265 | Agashivala 2012266 | Palace 2015154 | Pan 2012267 | Darbà 2014268 | Imani 2012269 | Dembek 2014270 | Chevalier 2016271 | Lee 2012272 | |
| Structure | ||||||||||
| Is there a clear statement of the decision problem? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Is the objective of the model specified and consistent with the stated decision problem? | Y | Y | U | Y | Y | Y | Y | Y | Y | Y |
| Is the primary decision-maker specified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Is the perspective of the model stated clearly? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Are the model inputs consistent with the stated perspective? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Has the scope of the model been stated and justified? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | Y | U | Y | Y | U | Y | Y | Y | Y |
| Are the sources of the data used to develop the structure of the model specified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Are the causal relationships described by the model structure justified appropriately? | Y | Y | U | Y | Y | Y | Y | Y | Y | Y |
| Are the structural assumptions transparent and justified? | Y | Y | U | Y | Y | N | Y | Y | Y | Y |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Y | Y | U | Y | Y | U | Y | Y | Y | Y |
| Is there a clear definition of the options under evaluation? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Have all feasible and practical options been evaluated? | Y | N | N | N | N | N | Y | Y | Y | N |
| Is there justification for the exclusion of feasible options? | NA | N | U | N | N | N | NA | NA | NA | N |
| Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | Y | Y | U | Y | Y | N | Y | Y | Y | Y |
| Is the time horizon of the model sufficient to reflect all important differences between the options? | N | Y | N | N | Y | Y | Y | Y | Y | N |
| Are the time horizon of the model and the duration of treatment described and justified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Y | Y | U | Y | Y | N | Y | Y | Y | Y |
| Is the cycle length defined and justified in terms of the natural history of disease? | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Data | ||||||||||
| Are the data identification methods transparent and appropriate given the objectives of the model? | Y | Y | Y | Y | N | Y | N | Y | Y | Y |
| Where choices have been made between data sources are these justified appropriately? | U | N | U | N | N | Y | N | Y | Y | Y |
| Has particular attention been paid to identifying data for the important parameters of the model? | U | U | U | U | N | N | N | U | Y | U |
| Has the quality of the data been assessed appropriately? | N | N | N | N | N | N | N | N | N | N |
| Where expert opinion has been used are the methods described and justified? | NA | NA | N | NA | NA | NA | NA | NA | Y | N |
| Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Y | Y | U | Y | N | Y | U | N | Y | Y |
| Is the choice of baseline data described and justified? | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Are transition probabilities calculated appropriately? | U | U | U | U | U | U | U | U | U | U |
| Has a half-cycle correction been applied to both costs and outcomes? | NA | N | N | N | N | N | N | N | N | N |
| If not, has the omission been justified? | NA | N | N | N | N | N | N | N | N | N |
| If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | U | U | Y | Y | U | Y | U | Y | Y | Y |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | NA | U | NA | N | N | U | N | Y | U | N |
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | NA | N | Y | NA | N | N | U | N | U | N |
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | NA | N | NA | U | U | U | U | Y | Y | U |
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | NA | N | NA | N | N | N | U | N | Y | U |
| Are the costs incorporated into the model justified? | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Has the source for all costs been described? | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Have discount rates been described and justified given the target decision-maker? | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Are the utilities incorporated into the model appropriate? | Y | Y | NA | Y | Y | NA | N | Y | Y | Y |
| Is the source of utility weights referenced? | Y | Y | NA | Y | Y | NA | Y | Y | Y | Y |
| Are the methods of derivation for the utility weights justified? | Y | Y | NA | Y | Y | NA | N | Y | Y | Y |
| Have all data incorporated into the model been described and referenced in sufficient detail? | N | N | Y | N | N | Y | N | N | Y | Y |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate?) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Is the process of data incorporation transparent? | N | N | Y | N | N | N | N | N | N | Y |
| If data have been incorporated as distributions, has the choice of distributions for each parameter been described and justified? | N | NA | NA | N | N | N | U | N | N | N |
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | NA | NA | NA | NA | NA | U | U | U | Y | N |
| Have the four principal types of uncertainty been addressed? | N | N | N | N | N | N | U | Y | Y | N |
| If not, has the omission of particular forms of uncertainty been justified? | N | N | N | N | N | N | U | N | NA | N |
| Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | N | N | N | N | N | N | N | N | N | N |
| Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | N | N | N | N | N | N | N | N | N | N |
| Has heterogeneity been dealt with by running the model separately for different subgroups? | N | N | N | N | N | N | N | Y | N | N |
| Are the methods of assessment of parameter uncertainty appropriate? | Y | Y | U | Y | Y | U | N | U | Y | Y |
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | Y | Y | U | Y | Y | N | N | N | NA | N |
| Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | N | N | N | N | N | N | N | N | N | N |
| Are any counterintuitive results from the model explained and justified? | NA | NA | NA | NA | NA | NA | NA | Y | NA | Y |
| If the model has been calibrated against independent data, have any differences been explained and justified? | N | N | NA | NA | NA | N | N | N | NA | N |
| Have the results been compared with those of previous models and any differences in results explained? | Y | Y | N | Y | Y | N | Y | Y | N | Y |
Results of additional searches: potentially relevant studies from the patient registry and cohort search
Brønnum-Hansen H, Koch-Henriksen N, Hyllested K. Survival of patients with multiple sclerosis in Denmark: a nationwide, long-term epidemiologic survey. Neurology 1994;44:1901–7.
Brønnum-Hansen H, Koch-Henriksen NJ, Hyllested K. [Survival in disseminated sclerosis in Denmark. A nation-wide study of the period 1948-1986.] Ugeskr Laeg 1995;157:7131–5.
Confavreux C. Establishment and use of multiple sclerosis registers – EDMUS. Ann Neurol 1994;36:S136–9.
Flachenecker P, Zettl UK, Götze U, Haas J, Schimrigk S, Elias W, et al. [MS registry in Germany – design and first results of the pilot phase.] Nervenarzt 2005;76:967–75. http://dx.doi.org/10.1007/s00115-005-1907-8
Ford HL, Gerry E, Johnson M, Williams R. A prospective study of the incidence, prevalence and mortality of multiple sclerosis in Leeds. J Neurol 2002;249:260–5.
Koch-Henriksen N. The Danish Multiple Sclerosis Registry: a 50-year follow-up. Mult Scler 1999;5:293–6.
Trojano M. Can databasing optimise patient care? J Neurol 2004;251(Suppl. 5):v79–82. http://dx.doi.org/10.1007/s00415-004-1513-x
Appendix 8 Additional analyses undertaken by the assessment group
Time-varying model
In Table 142 the results are presented in terms of cost per QALY gained for the time-varying model. Analyses used information from the NMA. These results show that the DMT treatment strategy was more costly and more effective than BSC alone. The DMT treatment strategy was approximately £33,600 more costly than BSC and produced 1.461 more QALYs, which equated to an ICER of approximately £23,000 per QALY. This indicates that for every additional QALY from DMTs there is an incremental cost of £23,000.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 8.664 | – | – |
| DMTs | 395,700 | 33,600 | 10.125 | 1.461 | 23,000 |
SA2a: Estimates of effectiveness of individual drugs from the assessment group review, progression confirmed at 3 months and individual drug annualised relapse rates
Results based on the time-varying model by individual drug showed that BSC was the least costly and least effective strategy (Table 143). Of the DMTs considered, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) was the most cost-effective strategy by ICER. Both 30 µg of IM IFN-β-1a once weekly (Avonex) and 44 µg of SC IFN-β-1a three times a week (Rebif) were dominated by 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy).
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 8.664 | – | – |
| GA 20 mg (Copaxone) | 388,400 | 26,300 | 9.770 | 1.105 | Extendedly dominated |
| IFN-β-1b 250 µg every other day (Betaferon/Extavia) | 390,500 | 28,400 | 10.139 | 1.475 | Extendedly dominated |
| PegIFN-β-1a 125 µg (Plegridy) | 395,500 | 33,400 | 10.642 | 1.978 | 16,900 |
| IFN-β-1a 30 µg IM (Avonex) | 415,900 | 20,400 | 9.994 | –0.648 | Dominated |
| IFN-β-1a 44 µg (Rebif) | 416,100 | 20,600 | 10.420 | –0.222 | Dominated |
SA2b: Estimates of effectiveness of individual drugs from the assessment group review, progression confirmed at 6 months and individual drug annualised relapse rates
The results based on the time-varying model are reported in Table 144. Treatment with 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) dominated all other DMT treatment strategies. Compared with BSC, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) was more expensive and more effective and had an ICER of approximately £3200 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 8.664 | – | – |
| PegIFN-β-1a 125 µg every 2 weeks (Plegridy) | 371,500 | 9400 | 11.608 | 2.944 | 3200 |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 395,700 | 24,200 | 11.290 | –0.318 | Dominated |
| GA 20 mg SC once daily (Copaxone) | 396,500 | 25,000 | 9.485 | –2.123 | Dominated |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 409,200 | 37,700 | 10.267 | –1.341 | Dominated |
Incorporating carers’ disutilities
The following analyses relate to the base-run model.
Cost-effectiveness analysis results: base case and sensitivity analyses
Base case
In Table 145 we present the findings from our base-case analysis with the inclusion of carers’ disutilities. The DMT treatment strategy was more costly and more effective than BSC. The DMT treatment strategy was approximately £31,900 more costly per person than the BSC strategy and produced 1.046 more QALYs, with an ICER of approximately £30,500 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 7.148 | – | – |
| DMTs | 394,000 | 31,900 | 8.194 | 1.046 | 30,500 |
SA1: Pooled estimates of effectiveness for on-scheme disease-modifying therapies from the assessment group review
We used two key estimates of treatment effectiveness from our clinical effectiveness review: the aggregated HR for disability progression confirmed at 3 months and the aggregated ARR.
In Table 146 the results show that the DMT treatment strategy was more costly and more effective than BSC alone. The DMT treatment strategy was approximately £23,300 more costly than BSC and produced 2.031 more QALYs, which equated to an ICER of approximately £11,500 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 7.148 | – | – |
| DMTs | 385,400 | 23,300 | 9.179 | 2.031 | 11,500 |
SA2a: Estimates of effectiveness of individual drugs from the assessment group review, progression confirmed at 3 months (preferred analysis)
The results for this analysis were robust to the inclusion of carers’ disutilities (Table 147). Treatment with 125 µg of pegIFN-β-1a every 2 weeks (Plegridy) remained dominant compared with all other DMT treatment strategies. Compared with BSC, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) was approximately £17,800 more costly and produced 2.868 more QALYs, with an ICER of £6200 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 7.148 | – | – |
| PegIFN-β-1a 125 µg every 2 weeks (Plegridy) | 379,900 | 17,800 | 10.016 | 2.868 | 6200 |
| GA 20 mg SC once daily (Copaxone) | 381,000 | 1100 | 8.646 | –1.552 | Dominated |
| IFN-β-1b 250 µg every other day (Betaferon/Extavia) | 393,400 | 13,500 | 8.556 | –1.46 | Dominated |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 404,800 | 24,900 | 9.614 | –0.402 | Dominated |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 406,100 | 26,200 | 9.027 | –0.989 | Dominated |
SA2b: Estimates of effectiveness of individual drugs from the assessment group review, progression confirmed at 6 months
Similarly, the results for this analysis were robust to the inclusion of carers’ disutilities. Treatment with 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) remained dominant compared with all other strategies included in the analysis (Table 148).
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 347,000 | – | 11.584 | – | – |
| BSC | 362,100 | 15,100 | 7.148 | –4.436 | Dominated |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 377,600 | 30,600 | 10.966 | –0.618 | Dominated |
| GA 20 mg SC once daily (Copaxone) | 391,900 | 44,900 | 8.236 | –3.348 | Dominated |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 396,900 | 49,900 | 9.446 | –2.138 | Dominated |
SA3: Hazard ratios from manufacturer submissions
When we used the estimates for treatment effectiveness (ARR and disability progression) reported by each manufacturer and included carers’ disutilities, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) dominated all other DMT treatment strategies (Table 149). Compared with BSC, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) resulted in an ICER of £3000 per QALY.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 362,100 | – | 7.148 | – | – |
| PegIFN-β-1a 125 µg SC every 2 weeks (Plegridy) | 366,300 | 4200 | 8.566 | 1.418 | 3000 |
| GA 20 mg SC once daily (Copaxone) | 374,600 | 8300 | 8.432 | –0.134 | Dominated |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 387,600 | 21,300 | 8.149 | –0.417 | Dominated |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 412,900 | 46,600 | 8.318 | –0.248 | Dominated |
SA4: Time horizon changed from 50 years to 20 years and 30 years
Tables 150 and 151 show the results based on a 20-year and 30-year time horizon respectively. With the inclusion of carers’ disutilities, in both analyses the GA treatment strategy continued to be extendedly dominated by 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy). Additionally, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) dominated both 30 µg of IM IFN-β-1a once weekly (Avonex) and 44 µg of SC IFN-β-1a three times a week (Rebif). Excluding all dominated strategies, 125 µg of SC pegIFN-β-1a every 2 weeks (Plegridy) compared with BSC had an ICER of approximately £18,200 and £9300 per QALY for the 20-year and 30-year time horizons respectively.
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 196,900 | – | 5.710 | – | – |
| GA 20 mg SC once daily (Copaxone) | 220,500 | 23,600 | 6.628 | 0.918 | Extendedly dominated |
| PegIFN-β-1a 125 µg every 2 weeks (Plegridy) | 225,800 | 28,900 | 7.301 | 1.591 | 18,200 |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 242,600 | 16,800 | 6.789 | –0.512 | Dominated |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 245,200 | 19,400 | 7.156 | –0.145 | Dominated |
| Strategy | Cost (£) | QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | ||
| BSC | 279,400 | – | 6.540 | – | – |
| GA 20 mg SC once daily (Copaxone) | 298,900 | 19,500 | 7.790 | 1.25 | Extendedly dominated |
| PegIFN-β-1a 125 µg every 2 weeks (Plegridy) | 300,400 | 21,000 | 8.809 | 2.269 | 9300 |
| IFN-β-1a 44 µg SC three times a week (Rebif) | 322,900 | 22,500 | 8.551 | –0.258 | Dominated |
| IFN-β-1a 30 µg IM once weekly (Avonex) | 323,000 | 22,600 | 8.057 | –0.752 | Dominated |
Appendix 9 Details of resource use used to derive cost inputs
This appendix describes the cost calculations used in the CIS model.
| Resource use | Quantity | Description | Unit costs (£, 2015 prices) | Source |
|---|---|---|---|---|
| MRI | 1 | RD01A | 137.23 | NHS Reference Costs 2014 to 2015 285 |
| Neurologist visit | 1 | Outpatient attendance, Neurology 400 | 175.76 | Assumption and consultation with clinical expert (Professor Olga Ciccarelli, University College London, June 2016, personal communication); NHS Reference Costs 2014/15285 |
| MS nurse visit | 2 | 15 minutes | 18.75 | Assumption and consultation with clinical expert (Professor Olga Ciccarelli, personal communicationa); Curtis and Burns278 |
| Estimated cost for monitoring people with CIS receiving BSC | £350.49 | |||
| Resource use | Quantity | Description | Unit costs (£, 2015 prices) | Source |
|---|---|---|---|---|
| Full blood count | 5 | DAPS05 – haematology | 3.01 | Assumptions and consultation with clinical expert on the number of full blood count, liver functions and renal function tests required (Professor Olga Ciccarelli, University College London, June 2016, personal communication); NHS Reference Costs 2014 to 2015285 |
| Liver function tests | 5 | DAPS04 – clinical biochemistry | 1.19 | |
| Thyroid function test | 1 | DAPS09 – other | 7.13 | |
| Renal function tests | 5 | DAPS04 – clinical biochemistry | 1.19 | |
| MRI | 1 | RD01A | 137.23 | NHS Reference Costs 2014 to 2015 285 |
| Neurologist visit | 2 | Outpatient attendance, Neurology 400 | 175.76 | Assumption and consultation with clinical expert (Professor Olga Ciccarelli, personal communication); NHS Reference Costs 2014 to 2015285 |
| MS nurse visit | 2 | 15 minutes | 18.75 | Assumption and consultation with clinical expert (Professor Olga Ciccarelli, personal communicationa); Curtis and Burns278 |
| Estimated initial cost of monitoring people in the first year of receiving DMTs (Avonex/Plegridy, Betaferon and Copaxone) | £553.20 | |||
| Estimated initial cost of monitoring people in the first year of receiving Rebif (includes thyroid function test) | £560.33 | |||
| Resource use | Quantity | Description | Unit costs (£, 2015 prices) | Source |
|---|---|---|---|---|
| Full blood count | 2 | DAPS05 – haematology | 3.01 | Assumptions and consultation with clinical expert on the number of full blood count, liver functions and renal function tests required (Professor Olga Ciccarelli, University College London, June 2016, personal communication); NHS Reference Costs 2014 to 2015285 |
| Liver function tests | 2 | DAPS04 – clinical biochemistry | 1.19 | |
| Renal function tests | 2 | DAPS04 – clinical biochemistry | 1.19 | |
| MRI | 1 | RD01A | 137.23 | NHS Reference Costs 2014 to 2015 285 |
| Neurologist visit | 1 | Outpatient attendance, Neurology 400 | 175.76 | Assumption and consultation with clinical expert (Professor Olga Ciccarelli, personal communication) |
| Subsequent annual cost of monitoring people receiving DMTs | £323.77 | |||
Appendix 10 Results by age at onset of relapsing–remitting multiple sclerosis
Using the base-run RSS model, we derived mean costs and mean QALYs for the BSC and DMT arms for various ages of onset of RRMS.
| Age (years) | BSC | DMTs | ||
|---|---|---|---|---|
| Mean cost (£) | Mean QALYs | Mean cost (£) | Mean QALYs | |
| 30 | 362,128 | 8.664 | 393,966 | 9.607 |
| 31 | 360,392 | 8.643 | 392,218 | 9.583 |
| 32 | 358,487 | 8.620 | 390,300 | 9.557 |
| 33 | 356,426 | 8.596 | 388,226 | 9.528 |
| 34 | 354,182 | 8.569 | 385,967 | 9.497 |
| 35 | 351,763 | 8.540 | 383,532 | 9.464 |
| 36 | 349,145 | 8.508 | 380,898 | 9.428 |
| 37 | 346,303 | 8.474 | 378,039 | 9.388 |
| 38 | 343,252 | 8.437 | 374,970 | 9.345 |
| 39 | 339,985 | 8.397 | 371,685 | 9.299 |
| 40 | 336,479 | 8.354 | 368,160 | 9.250 |
| 41 | 332,764 | 8.309 | 364,429 | 9.197 |
| 42 | 328,825 | 8.261 | 360,475 | 9.141 |
| 43 | 324,639 | 8.208 | 356,273 | 9.081 |
| 44 | 320,230 | 8.153 | 351,850 | 9.017 |
| 45 | 315,615 | 8.095 | 347,226 | 8.950 |
| 46 | 310,782 | 8.034 | 342,385 | 8.879 |
| 47 | 305,740 | 7.969 | 337,339 | 8.804 |
| 48 | 300,491 | 7.901 | 332,087 | 8.725 |
| 49 | 295,059 | 7.829 | 326,658 | 8.642 |
| 50 | 289,449 | 7.754 | 321,055 | 8.555 |
| 51 | 283,682 | 7.677 | 315,301 | 8.465 |
| 52 | 277,718 | 7.595 | 309,353 | 8.371 |
| 53 | 271,632 | 7.511 | 303,291 | 8.273 |
| 54 | 265,398 | 7.423 | 297,085 | 8.171 |
| 55 | 259,060 | 7.333 | 290,784 | 8.067 |
| 56 | 252,565 | 7.239 | 284,327 | 7.957 |
| 57 | 245,948 | 7.141 | 277,753 | 7.844 |
| 58 | 239,201 | 7.040 | 271,050 | 7.726 |
| 59 | 232,326 | 6.934 | 264,220 | 7.604 |
| 60 | 225,352 | 6.825 | 257,293 | 7.477 |
| 61 | 218,270 | 6.712 | 250,254 | 7.346 |
| 62 | 211,077 | 6.595 | 243,098 | 7.210 |
| 63 | 203,763 | 6.472 | 235,810 | 7.068 |
| 64 | 196,405 | 6.345 | 228,471 | 6.922 |
| 65 | 189,004 | 6.216 | 221,080 | 6.772 |
| 66 | 181,530 | 6.081 | 213,596 | 6.616 |
| 67 | 174,037 | 5.942 | 206,079 | 6.457 |
| 68 | 166,497 | 5.798 | 198,486 | 6.292 |
| 69 | 158,995 | 5.652 | 190,914 | 6.124 |
| 70 | 151,501 | 5.501 | 183,319 | 5.951 |
| 71 | 144,046 | 5.347 | 175,732 | 5.775 |
| 72 | 136,611 | 5.187 | 168,119 | 5.593 |
| 73 | 129,248 | 5.024 | 160,536 | 5.407 |
| 74 | 121,999 | 4.858 | 153,024 | 5.219 |
| 75 | 114,851 | 4.688 | 145,559 | 5.027 |
| 76 | 107,837 | 4.515 | 138,172 | 4.833 |
| 77 | 101,019 | 4.342 | 130,933 | 4.637 |
| 78 | 94,362 | 4.165 | 123,791 | 4.440 |
| 79 | 87,944 | 3.989 | 116,838 | 4.243 |
| 80 | 81,775 | 3.814 | 110,087 | 4.048 |
Glossary
- Annualised relapse rate
- This indicates the number of relapses a patient would expect to have on average every year. Differences in the annualised relapse rate are measured as a rate ratio, which suggests the percentage difference in rate between two groups. A rate ratio of 0.75 in group 1 compared with group 2 means that group 1 has 25% fewer relapses than group 2. In contrast, a rate ratio of 1.25 suggests that group 1 has 25% more relapses than group 2. In multiple sclerosis, an improvement of one drug over another would be represented by a rate ratio of < 1.
- Surface under the cumulative ranking curve
- In network meta-analyses, it is possible to rank interventions on the size of their effect. This is carried out using the surface under the cumulative ranking curve. A higher surface under the cumulative ranking curve means a larger magnitude of effect. For clinical effectiveness outcomes, such as relapse rate and time to disability progression, interventions are ranked based on how much the intervention reduces relapse or slows down disability progression. For discontinuation as a result of adverse events, interventions are ranked on how much they increase the risk of discontinuation.
- Time to disability progression
- This indicates how quickly a patient would expect to have disability progression compared with another patient. This is measured as a hazard ratio. A hazard ratio of < 1 in group 1 compared with group 2 means that group 1 will take longer to have disability progression. Conversely, a hazard ratio of > 1 in group 1 compared with group 2 means that group 1 will have faster disability progression on average. For example, a hazard ratio of 0.75 in group 1 compared with group 2 means that, at a point in the future, people without progression in group 1 will have a 25% less chance of having disability progression than people without progression in group 2. In multiple sclerosis, an improvement of one drug over another would be represented by a hazard ratio of < 1.
- Time to disability progression confirmed at 3 (or 6) months
- To reduce the effect of ‘blips’ in disability progression on estimates of effectiveness, many trials require that an initial sign of disability progression be confirmed at a repeat visit 3 (or 6) months later. Thus, time to disability progression confirmed at 3 months is simply the time to disability progression when that disability progression has been subsequently confirmed 3 months after the visit when progression was first detected. Similarly, time to disability progression confirmed at 6 months is the time to progression when that progression has been subsequently confirmed 6 months after the visit when it was first detected.
List of abbreviations
- AE
- adverse event
- AMSTAR
- Assessing the Methodological Qualities of Systematic Reviews
- ANOVA
- analysis of variance
- ARR
- annualised relapse rate
- BCMS
- British Columbia Multiple Sclerosis
- BECOME
- Betaseron vs. Copaxone in Multiple Sclerosis with Triple-Dose Gadolinium and 3-Tesla MRI Endpoints
- BENEFIT
- Betaferon®/Betaseron® in Newly Emerging Multiple Sclerosis for Initial Treatment
- BEYOND
- Betaferon Efficacy Yielding Outcomes of a New Dose
- BNF
- British National Formulary
- BOI
- burden of illness
- BRAVO
- Benefit–Risk Assessment of AVonex and LaquinimOd
- BSC
- best supportive care
- CDMS
- clinically definite multiple sclerosis
- CEA Registry
- Cost-effectiveness Analysis Registry
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHAMPS
- Controlled High Risk Avonex Multiple Sclerosis Study
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CIS
- clinically isolated syndrome
- CNS
- central nervous system
- CombiRx
- Combination Therapy in Patients with Relapsing–Remitting Multiple Sclerosis
- CONFIRM
- Comparator and an Oral Fumarate in Relapsing–Remitting Multiple Sclerosis
- Cop1 MSSG
- Copolymer 1 Multiple Sclerosis Study Group
- CSF
- cerebrospinal fluid
- DARE
- Database of Abstracts of Reviews of Effects
- DIC
- deviance information criterion
- DMT
- disease-modifying therapy
- DSS
- Disability Status Scale
- EBV
- Epstein–Barr virus
- ECGASG
- European/Canadian Glatiramer Acetate Study Group
- EDSS
- Expanded Disability Status Scale
- EQ-5D
- EuroQol-5 Dimensions
- ESG
- European Study Group on Interferon β-1b in Secondary Progressive MS
- EVIDENCE
- Evidence of Interferon Dose–Response: European North American Comparative Efficacy
- GA
- glatiramer acetate
- GALA
- Glatiramer Acetate Low-Frequency Administration
- GATE
- Glatiramer Acetate Clinical Trial to Assess Equivalence with Copaxone
- GPRD
- General Practice Research Database
- GWAS
- genome-wide association study
- HCHS
- Hospital and Community Health Service
- HLA
- human leucocyte antigen
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- HUI
- Health Utilities Index
- ICER
- incremental cost-effectiveness ratio
- ICTRP
- International Clinical Trials Registry Platform
- IFN-β
- beta-interferon
- IFNB MSSG
- IFNB Multiple Sclerosis Study Group
- IM
- intramuscular
- IMPROVE
- Investigating MRI Parameters with Rebif imprOVEd formulation
- INCOMIN
- Independent Comparison of Interferon
- INHS
- Italian National Health Service
- IU
- international unit
- MeSH
- medical subject heading
- MLY
- mono-symptomatic life-year
- MRI
- magnetic resonance imaging
- MS
- multiple sclerosis
- MSCRG
- Multiple Sclerosis Collaborative Research Group
- MSIS-29
- Multiple Sclerosis Impact Scale
- MTA
- multiple technology appraisal
- NAB
- neutralising antibody
- NASG
- North American Study Group on Interferon beta-1b in Secondary Progressive MS
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- ONS
- Office for National Statistics
- OR
- odds ratio
- PASAT
- Paced Auditory Serial Addition Test
- peg
- pegylated
- PPMS
- primary progressive multiple sclerosis
- PreCISe
- Presenting with Clinically Isolated Syndrome
- PRISMS
- Prevention of Relapses and Disability by Interferon Beta-1a Subcutaneously in Multiple Sclerosis
- PRMS
- progressive relapsing multiple sclerosis
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REFLEX
- REbif FLEXible dosing in early MS
- REFLEXION
- REbif FLEXible dosing in early MS extensION
- REFORMS
- Rebif New Formulation versus Betaseron Tolerability Study
- REGARD
- REbif vs. Glatiramer Acetate in Relapsing MS Disease
- REMAIN
- REbif compared with no treatment in the therapy of relapsing Multiple sclerosis After mItoxaNtrone
- RePEc
- Research Papers in Economics
- RR
- rate ratio
- RRMS
- relapsing–remitting multiple sclerosis
- RSS
- risk-sharing scheme
- SC
- subcutaneous
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SMR
- standardised mortality rate
- SPECTRIMS
- Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS
- SPMS
- secondary progressive multiple sclerosis
- SUCRA
- surface under the cumulative ranking curve
- SWIMS
- South West Impact of Multiple Sclerosis
- TA
- technology appraisal
- TRANSFORMS
- TRial Assessing injectable interferoN vS. FTY720 Oral in RRMS
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.