Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/104/19. The contractual start date was in November 2011. The draft report began editorial review in October 2016 and was accepted for publication in May 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Mike Thomas is a member of the Health Technology Assessment (HTA) Primary Care, Community and Preventive Interventions (PCCPI) Panel. In the last 3 years he has received speaker’s honoraria for speaking at sponsored meetings or satellite symposia at conferences from the following companies, marketing respiratory and allergy products: Aerocrine, GlaxoSmithKline (GSK) and Novartis International AG. He has received honoraria for attending advisory panels with Aerocrine, AstraZeneca, Boehringer Ingelheim, GSK and Novartis. He has received sponsorship to attend international scientific meetings from GSK and AstraZeneca and has received funding for research projects from GSK. He is a member of the British Thoracic Society (BTS)/Scottish Intercollegiate Guidelines Network (SIGN) Asthma Guideline Group and the National Institute for Health and Care Excellence (NICE) Asthma Guideline Group. Paul Little is Editor-in-Chief of the Programme Grants for Applied Research journal and is a member of the National Institute for Health Research (NIHR) Journals Library Board. Lucy Yardley Is a member of the Public Health Research journal Research Funding Board and a member of the HTA Efficient Study Designs Board and reports grants from the NIHR during the conduct of the study. James Raftery is a member of the NIHR Journals Library Editorial Group and the HTA and EME Editorial Board and was previously Director of the Wessex Institute and head of the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC). He is also a former member of the NIHR HSDR Research Led Board. Ian Pavord has received speaker’s honoraria for speaking at sponsored meetings in the last 5 years from AstraZeneca, Boehringer Ingelheim, Aerocrine, Almirall Ltd, Novartis and GSK. He has received honoraria for attending advisory panels with Almirall, AstraZeneca, Boehringer Ingelheim, GSK, MSD, Schering-Plough, Novartis, Dey Pharma, Napp Pharmaceuticals and RespiVert Ltd. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca and Napp. He is Chief Medical Advisor to Asthma UK, a member of the UK Department of Health Asthma Strategy Group, a member of the BTS/SIGN Asthma Guideline Group and joint Editor-in-Chief of Thorax. Neither Ian Pavord nor any member of his family has any shares in pharmaceutical companies. David Price reports other Board Membership (fees paid to Research in Real Life Ltd) from Aerocrine, Almirall, Amgen Inc., AstraZeneca, Boehringer Ingelheim, Chiesi Ltd, Meda, Mundipharma, Napp, Novartis and Teva Pharmaceutical Industries Ltd; consultancy (fees paid to Research in Real Life Ltd) from Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Meda, Mundipharma, Napp, Novartis, Pfizer Inc. and Teva; grants from the UK NHS, British Lung Foundation, Aerocrine, AKL Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly and Co., GSK, Meda, Merck & Co., Inc., Mundipharma, Napp, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva and Zentiva; lectures/speaking engagement fees (paid to Research in Real Life Ltd) from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla Ltd, GSK, Kyorin Pharmaceutical Co., Inc., Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda and Teva; manuscript preparation fees (paid to Research in Real Life Ltd) from Mundipharma and Teva; payment for travel/accommodation/meeting expenses (paid to Research in Real Life Ltd) from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis and Teva; funding for patient enrolment or completion of research (paid to Research in Real Life Ltd) from Almirall, Chiesi, Teva and Zentiva; and payment for the development of educational materials (paid to Research in Real Life Ltd) from GSK and Novartis, outside the submitted work. In addition, David Price has an AKL Ltd patent pending and owns shares in AKL Ltd, which produces phytopharmaceuticals. He owns 80% of Research in Real Life Ltd (which is subcontracted by Observational and Pragmatic Research Institute Pte Ltd), 75% of the social enterprise Optimum Patient Care Ltd and 75% of the Observational and Pragmatic Research Institute Pte Ltd. Ratko Djukanovic has received fees for lectures at symposia organised by Novartis and Teva and for consultation for these two companies as a member of advisory boards. He is a co-founder and current consultant, and has shares in, Synairgen, a University of Southampton spin-out company.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Thomas et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Asthma affects > 5 million people in the UK and costs the NHS in excess of £1B. Although pharmacotherapy is effective and can provide full control for some patients,1 surveys repeatedly show that outcomes remain suboptimal. A recent European survey showed that fewer than half of adults with asthma achieved good symptom control2 and that quality of life (QoL) is affected for most, with consequent costs to the community both directly from health service use and indirectly from lost productivity. Many patients have concerns about taking regular medication, particularly inhaled corticosteroids. Surveys of complementary and alternative medicines in asthma show high levels of use, with up to 79% of adults and 78% of children reporting trying different treatments, include breathing modification. 3 Breathing techniques are among the most commonly used complementary techniques, with up to 30% reporting having used them to control their symptoms. 4 The James Lind Alliance and the patient organisation Asthma UK have both identified breathing exercises for asthma as a priority area for research. 5 Asthma encompasses a variety of phenotypes and different therapeutic approaches may be effective in different patients. 6 Symptoms attributed to dysfunctional breathing have been reported to be more frequent in people with asthma than in the general population. 7,8 A number of controlled studies have investigated breathing modification techniques and have reported beneficial outcomes. Breathing control techniques investigated have included the Butekyo breathing method9–13 and yogic breathing. 14–16 Recent studies have shown clinically important effectiveness of physiotherapist-administered breathing exercises for people with asthma in the UK. 17–19 The evidence base for the effectiveness of breathing therapies for treating asthma has been assessed in several reviews. A recent systematic review of the effectiveness of physiotherapist-taught breathing retraining was carried out as part of a review of physiotherapy interventions in the treatment of respiratory diseases in adults. 20 This was a collaborative multidisciplinary review undertaken by the British Thoracic Society (BTS) and the Association of Chartered Physiotherapists in Respiratory Care (ACPRC), the respiratory clinical interest group of the Chartered Society of Physiotherapy (CSP). Its purpose was to critically appraise the evidence for respiratory physiotherapy techniques in respiratory diseases and it used an explicit evidence-based methodology. This consisted of an initial literature search, conducted by the Centre for Research and Dissemination (CRD), York, UK. Papers and abstracts identified were appraised and graded by two trained assessors using Scottish Intercollegiate Guidelines Network (SIGN) methodology, with recourse to a third assessor in the event of disagreements. The review found that ‘Breathing exercises, incorporating reducing respiratory rate and/or tidal volume and relaxation training, should be offered to patients to help control the symptoms of asthma and improve QoL (Grade A)’. In the latest iterations of both the BTS/SIGN UK national asthma guideline21 and the World Health Organization (WHO)-endorsed Global Initiative for Asthma (GINA) guideline,22 breathing exercises are endorsed as adjuvant treatment for people with inadequately controlled asthma despite standard pharmacological treatment. Previous research from members of this study group has provided evidence supporting this recommendation. A prior Cochrane review of breathing exercises for asthma was performed in 2004,23 before several large studies informing the BTS review had reported. This review stated that, because of the diversity of breathing exercises and outcomes used, it was impossible at that time to draw conclusions from the available evidence. The review stated that trends for improvements were noted in a number of outcomes and that large-scale studies were warranted to clarify the effectiveness of breathing exercises in the management of asthma. Subsequently, Slader et al. 3 reported a double-blind randomised controlled trial (RCT) of breathing techniques in asthma and concluded that breathing techniques may be useful in patients with mild asthma who use a reliever inhaler frequently. This Australian study investigated the effects of two different breathing retraining programmes taught by physiotherapists and delivered as videotaped instruction programmes that the participants completed at home, without face-to-face supervision. Both programmes were associated with improved health status and major reductions in bronchodilator use compared with baseline values.
These instructional interventions have subsequently been made available as internet downloads and have been used in Australia to improve asthma control in routine clinical practice. This study provided provisional evidence that breathing retraining programmes delivered in a self-guided audio-visual format are feasible and may potentially produce beneficial outcomes in asthma. A 2007 UK primary care-based RCT18 demonstrated that breathing retraining taught by a physiotherapist in face-to-face sessions significantly reduced respiratory symptoms and improved health-related QoL compared with usual care. The population studied consisted of community-treated asthmatics with mild and moderate disease. The contents of the breathing retraining programme in this study were very similar to those in our study, but only face-to-face instruction was investigated and no economic analysis was carried out. A Canadian RCT published in 200813 added further support for breathing retraining in asthma, also finding significant reductions in asthma symptoms. In this study, a breathing retraining intervention delivered by physiotherapists in a face-to-face setting was compared with the Butekyo breathing method (also taught in face-to-face sessions by a therapist). Large magnitude but similar improvements in health status and symptoms from baseline levels were seen in both treatment arms.
A further RCT published in 2009 investigated the effects of a physiotherapist-delivered breathing retraining intervention, with similar content to that included in the face-to-face arm of our trial. 17 This study controlled for the non-specific ‘placebo-like’ effect of professional contact and sympathetic attention by giving the control group the same amount of professional contact time (with an experienced respiratory nurse providing asthma education). Significant improvements from baseline were seen in patient-reported asthma outcomes for both treatment arms after 1 month, with trends favouring the breathing retraining group; at 6 months a large and significant difference between treatment arms was found in favour of breathing retraining. Significant improvements were seen between treatment arms in asthma-related QoL, anxiety and depression and Nijmegen questionnaire scores (measuring hyperventilation-related symptoms) and a trend was seen for an improvement in symptomatic asthma control. No effect on airway inflammation or physiology was found. No economic evaluation was carried out.
The addition of these subsequent trials to those in the Cochrane review23 as part of the BTS review20 led the authors to conclude that the evidence supporting breathing retraining for people with asthma was of 1++ strength. However, no recommendation on the most clinically effective or cost-effective way of providing breathing retraining was made. Most of the studies contributing to the evidence base involved face-to-face interventions and it is here that the evidence is strongest. Only two preliminary studies have investigated the use of instructional interventions delivered by videotape or DVD,9,14 with some evidence that this modality may also be effective. To our knowledge, no previous studies have compared a DVD breathing retraining intervention with a face-to-face breathing retraining intervention. In our study we aimed to assess the effectiveness of the intervention not only in comparison with usual care or a placebo but also in comparison with an intervention of known benefit. The logistic and economic implications of making this intervention available to all who could potentially benefit in the UK through a face-to-face physiotherapy programme are considerable. We felt that if comparable effectiveness could be shown for a self-guided breathing retraining programme, this is likely to provide a more efficient, convenient and cost-effective service to patients. The available evidence prior to this study suggested that a programme of breathing retraining consisting of three or more face-to-face sessions delivered by a specialist respiratory physiotherapist was effective in improving patient-reported end points, particularly health status (the outcome measure that most accurately captures patient experiences and QoL impairment) and psychological well-being, for people with asthma, and may be effective in reducing rescue bronchodilator medication usage. There were suggestions that similar beneficial effects may be achieved through the use of self-guided interventions instead of face-to-face instruction. However, the relative clinical effectiveness and cost-effectiveness of different approaches to breathing retraining have not been adequately assessed. If similar benefits could be demonstrated without face-to-face contact with a health-care professional, the health resource implications of providing breathing retraining would be improved and this intervention could realistically be made available to the many people with asthma who could potentially benefit from it. Therefore, we proposed to transfer the key components of the physiotherapist-delivered programme that we (and others) have shown to be effective into a self-guided format (delivered in this study through a DVD, but able to be delivered through internet-based technologies) and to compare the effects of this intervention with those of face-to-face sessions with a physiotherapist and with usual care. Our study included a full health economic evaluation, as previous research has focused on the clinical effectiveness, rather than the cost-effectiveness, of breathing retraining. We also included qualitative research to capture patient perspectives on the interventions and a full process evaluation.
Chapter 2 Methods
Trial design
The BREATHE (Breathing REtraining for Asthma – Trial of Home Exercises) trial was a pragmatic, observer-blinded, three-arm, parallel-group RCT comparing breathing retraining delivered using a DVD with face-to-face physiotherapy and with usual care (control) for adults with asthma and impaired health status.
Participants
We recruited 655 adult patients with a diagnosis of asthma and impaired asthma-related health status from 34 primary care sites (UK NHS general practices) in the Wessex region. Patients were recruited between November 2012 and January 2015, with follow-up ending in February 2016. Practices were recruited and supported by the UK Clinical Research Network (CRN), who also supported patient recruitment and follow-up.
Inclusion criteria
Inclusion criteria
-
Full practice registration for 12 months prior to enrolment.
-
Age 16–70 years.
-
Physician-diagnosed asthma in medical records.
-
One or more anti-asthma medication prescriptions in the previous year (determined from physician prescribing records).
-
Impaired asthma-related health status [Asthma Quality of Life Questionnaire (AQLQ)24 score of < 5.5].
-
Able to give informed consent.
Exclusion criteria
Exclusion criteria
-
Asthma judged at the baseline assessment to be dangerously unstable and in need of urgent medical review (if these participants were referred back to their usual primary care clinician for review).
-
Patients with an additional documented diagnosis of chronic obstructive pulmonary disease (COPD) with a forced expiratory volume in 1 second (FEV1) of < 60% predicted.
We aimed to allow broad entry criteria (with the inclusion of smokers and not insisting on physiological demonstration of reversible airflow obstruction) to allow the generalisability of the research findings to mild-to-moderate UK asthma populations treated in primary care NHS practice.
Interventions
Development of the self-guided intervention
The development process for the self-guided intervention is described in detail in Chapter 5.
Briefly, in phase 1 of the study, the development phase, we transferred an existing face-to-face programme of breathing retraining taught by physiotherapists to patients with dysfunctional breathing, and previously shown to be effective for people with poorly controlled asthma, to audio-visual media (trialled in DVD format in this study), and developed printed supportive materials and undertook qualitative piloting of these materials to optimise their acceptability and effectiveness.
Patient educational materials were developed by members of the team including physicians, physiotherapists, health psychologists, communications technology specialists and patient representatives. The DVD and accompanying booklet were created iteratively, with extensive qualitative patient input. The DVD content consisted of:
-
a detailed explanation and illustration of how to carry out the exercises
-
motivational components explaining the rationale for the exercises and addressing common doubts and concerns.
The materials were piloted with a panel of 29 members of the target population, purposively sampled for diversity in terms of age, sex, education and symptom profile. In audio-recorded face-to-face and telephone interviews we used open-ended questions to explore attitudes to the proposed treatment method in the context of health beliefs and then used ‘think-aloud’ methods12 to elicit spontaneous reactions to all of the proposed materials. We modified the scripts based on this feedback. Professional production of the DVD and booklet were undertaken and the materials were reviewed by members of our panel, who provided final feedback in face-to-face and telephone interviews.
Face-to-face physiotherapy
Participants randomised to the face-to-face physiotherapy arm were treated by a single, very experienced respiratory physiotherapist over three sessions, based on the face-to-face breathing retraining interventions studied in previous research and on the standard Papworth breathing retraining programme widely taught by physiotherapists in the UK and globally. 17–19 The content of the programme was very similar to that in the DVD arm of the study. The patient support booklet produced in the development phase to support the DVD-based breathing retraining, as described in the previous section, was also provided to patients in this arm of the study. The details and fidelity assessment of the physiotherapy intervention are described in more detail in Chapter 5.
Control group
Patients randomly assigned to the control arm (usual care) received no additional treatment or care. They underwent the same baseline and 12-month assessments and completed the 3- and 6-month postal questionnaires. Control participants were informed that they would subsequently be offered the DVD intervention if it was shown to be effective in the study, to encourage participation and retention.
Randomised controlled trial processes
We performed a parallel-group, three-arm RCT over a 12-month period to assess the effect of the DVD programme compared with usual care and face-to-face physiotherapist-led training with a similar content on the following parameters: asthma-related health status, parameters of symptomatic and physiological asthma control and asthma-related health resource use. Consenting participants were randomly assigned to (1) receipt of the DVD intervention plus supporting written material, (2) three sessions of face-to-face physiotherapy breathing instruction, consisting of an initial ‘small-group’ (up to five patients) session of approximately 45 minutes and two subsequent individual sessions of up to 45 minutes or (3) usual care, which included recruitment and follow-up assessments but no additional intervention or care.
Identification, recruitment and randomisation of participants
Potentially eligible patients in the participating practices were identified by searches of the practice computerised clinical record and prescribing systems. The searches were facilitated by the CRN, based on the inclusion criteria described earlier. Potentially eligible participants were sent information about the study, an AQLQ questionnaire to complete and return if interested in participating and a stamped return envelope. Contact telephone numbers were provided for patients who wished to discuss the study.
Those returning information and interested in participating in the study and who met the inclusion criteria were seen by one of the study research nurses [from the CRN Primary Care Research Network (PCRN) team] at their own general practice during a prearranged appointment. Patients who met the inclusion criteria at the research nurse review and who still wanted to participate provided written informed consent, had baseline measurements taken and were randomly allocated to one of the three study arms following a telephone call to the randomisation service of the Southampton Clinical Trials Unit (SCTU). Follow-up and intervention arrangements were made according to participant convenience and availability. All participants were informed that they would receive postal questionnaires to complete and return at 3 and 6 months, and would be invited for a further research nurse appointment at 12 months. Usual care for their asthma was otherwise allowed to continue.
DVD arm
Participants allocated to the DVD arm received a copy of the self-guided programme in DVD format and the printed support booklet. To cover the possibility of lack of access to a DVD player we were able to provide an inexpensive DVD player for participant use; however, none of the participants allocated to the DVD arm required this.
Physiotherapy arm
Those allocated to the face-to-face physiotherapy arm consented to be contacted by the physiotherapist delivering the intervention by telephone within the following week to arrange the first session. This took place at their general practice at a mutually convenient time. Subsequent sessions were arranged during the first session.
Usual-care arm
Participants in the control arm received no additional information or care during the study beyond their usual care.
Postal questionnaire
The AQLQ questionnaires were posted to all participants at 3 and 6 months after baseline along with a prepaid return envelope. A single reminder telephone call was made after 4 weeks.
Final visit
All participants were invited to a final study visit with a blinded research nurse (a different research nurse from the research nurse who carried out the baseline assessments) at their usual general practice 12 months after baseline. All baseline physiological and questionnaire measurements were repeated. Process evaluation questionnaires were completed and information on personal costs was collected. Participants who did not attend were sent a reminder and, if they were unwilling or unable to attend for a face-to-face visit but were willing to answer questions over the telephone, the AQLQ was completed to allow maximum collection of the primary efficacy outcome.
Health resource use
Health resource use information was extracted from medical records for all participants following the 12-month assessment. This included all respiratory-related medical encounters (primary and secondary care), investigations relating to asthma and respiratory-related prescribing.
Outcomes
Prespecified outcome measures were between-group and within-group changes from baseline to the end of the study (12 months). The statistical analysis plan (SAP) is provided below (see Statistical analysis plan).
The primary outcome was the between-group [intention-to-treat (ITT) population] change in asthma-specific health status [AQLQ (short version)] score, adjusted for potential confounders.
Secondary outcome measures were between-group (ITT population) change in Asthma Control Questionnaire (ACQ)25 score, lung function [FEV1, forced volume vital capacity (FVC), FEV1-to-FVC ratio, peak expiratory flow rate (PEFR)], fraction of exhaled nitric oxide (FeNO), health status [EuroQol-5 Dimensions (EQ-5D)26], anxiety and depression [Hospital Anxiety and Depression Scale (HADS)27], hyperventilation (Nijmegen) questionnaire28 score, oral corticosteroid courses for asthma exacerbations and bronchodilator use.
Sensitivity analyses included analyses of the primary and secondary outcomes in both the ITT and per-protocol (PP) populations. We also performed a prespecified sensitivity analysis including participants with missing baseline or outcome data for the primary efficacy parameter, the AQLQ, as described below and in the SAP. In addition, health economic and process evaluation analyses were carried out and are presented in Chapters 4 and 6 respectively.
Sample size
For equivalence of the DVD-delivered and face-to-face programmes
In a previous Health Technology Assessment (HTA) study,29 treatments were deemed to be equivalent if the 95% confidence interval (CI) for the mean difference in AQLQ score between treatment arms was wholly included between –0.3 and +0.3. The BREATHE sample size calculation for equivalence used the same equivalence boundary (i.e. between –0.3 and +0.3). However, we assumed that the standard deviation (SD) of the between-group difference in AQLQ score would be a conservative 25% smaller (i.e. SD 0.77) than that reported in a previous study (SD 1.03). 18 The justification for this was that the proposed equivalence analysis compared two breathing retraining interventions as opposed to a breathing intervention compared with usual care, as in the GLAD study. As this was an equivalence study, as opposed to a non-inferiority study, a two-tailed 5% significance level was used in the calculations.
Sample sizes of 210 in the DVD breathing retraining group and 105 in the face-to-face physiotherapy group were required to assess treatment equivalence with 90% power using an equivalence boundary for AQLQ score of 0.3. This assumed that the expected between-group difference in mean AQLQ score was zero; a two-tailed 5% significance level; a common SD for the AQLQ score of 0.77; and a lower/upper limit of –0.3/+0.3 for the 95% CI of the between-group difference in AQLQ score.
In the unlikely event that the between-group AQLQ score SD was higher than our estimated 0.77, assuming that all other parameters stayed the same, we would still have 80% power to declare equivalence between the DVD breathing retraining group and the face-to-face physiotherapy group as long as the between-group SD was no higher than 0.89.
For superiority of both the DVD-delivered and the face-to-face programme over usual care
For the superiority sample size calculations, there was no widely acceptable minimal clinically important difference (MCID) for between-group change in AQLQ score, although the MCID for within-person change in AQLQ score was reported to be 0.5 (SD 0.41). 24 Therefore, two approaches were used for the superiority sample size calculation: (1) using the published within-person MCID of 0.5 and (2) using the estimate from the previous study18 – a between-group mean (SD) difference in AQLQ score at 6 months of 0.38 (1.03). Although the original sample size calculations for the superiority study were carried out using a one-sided 5% significance level, subsequent open discussions between the trial team and the Data Monitoring and Ethics Committee (DMEC) and the Trial Management Group (TMG) resulted in a protocol change and the decision to use a two-sided 5% significance level. The following superiority study sample size calculations have been updated to reflect this change:
-
Using a MID of 0.5. A two-group t-test with a two-sided 5% significance level will have 90% power to detect a difference in mean AQLQ score of ≥ 0.5, assuming that the common SD is 0.41, when the sample sizes are 12 in the face-to-face breathing retraining group and 24 in the usual-care group. Similarly, a two-group t-test with a two-sided 5% significance level will have 90% power to detect a difference in mean AQLQ score of ≥ 0.5, assuming that the common SD is 0.41, when the sample size is 16 in both the DVD-delivered breathing retraining group and the usual-care group.
-
Using a MID of 0.38. A two-group t-test with a two-sided 5% significance level will have 90% power to detect a difference in mean AQLQ score of ≥ 0.38, assuming that the common SD is 1.03, when the sample sizes are 117 in the face-to-face breathing retraining group and 234 in the usual-care group. Similarly, a two group t-test with a two-sided 5% significance level will have 90% power to detect a difference in mean AQLQ score of ≥ 0.38, assuming that the common SD is 1.03, when the sample size is 156 in both the DVD-delivered breathing retraining group and the usual-care group.
Final sample size
Assuming a 10% dropout rate and a two-sided 5% significance level for each of the equivalence and superiority studies, we therefore aimed to recruit a total sample size of 650 patients (260 each in the DVD and usual-care arms and 130 in the face-to-face physiotherapy arm).
Changes to the original protocol
There were two significant amendments to the original protocol developed in 2012. There was concern from the DMEC that the attrition rate may be higher than the 10% initially anticipated. The protocol was subsequently amended to allow recruitment to be extended to ensure that the original target of 525 complete data sets was reached.
The second noteworthy amendment was to the hypothesis tests of the statistical analysis. The decision was made to change the trial’s superiority sample size calculations from a one-sided 5% analysis to a two-sided 5% analysis. The trial was adequately powered for either option but, after extensive discussions with the funder, sponsor, Trial Steering Committee (TSC), TMG and DMEC, it was agreed that the use of a two-sided sample size calculation would be optimum.
Statistical analysis plan
Statistical analysis plan objective
The objective of this SAP is to describe the quantitative statistical analyses to be carried out for the equivalence and superiority studies within BREATHE. This SAP is based on protocol version 7 (25 November 2015).
General principles
Categorical variables will be described with number and percentage in each category. Continuous variables will be described with mean and SD or median and interquartile range (IQR) depending on their distribution. The number of missing data will be provided for each variable.
Software
All analyses will be carried out using Stata® version 14 (StataCorp LP, College Station, TX, USA) or SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Data will be stored on a secure drive, with limited access to those who need it.
Study populations
All primary efficacy analyses are carried out on the ITT population. Safety analyses are carried out on the safety population.
Intention-to-treat population
All participants who were randomised and for whom at least one follow-up observation of the primary outcome (AQLQ score) is available. Participants will be analysed in the treatment arm to which they were randomised.
Per-protocol population
All participants of the ITT population excluding participants who were not compliant with their randomised study arm. Non-compliance is defined as those not having any primary outcome data and/or those not attending at least two physiotherapy appointments in the physiotherapy arm.
Effectiveness outcomes
Primary outcome
The primary outcome measure will be the 12-month post-intervention AQLQ score.
Secondary outcomes
Clinical
-
ACQ score.
-
Lung function (FEV1, FEV1-to-FVC ratio, PEFR).
-
FeNO.
-
Health status (EQ-5D).
-
Anxiety and depression scores (HADS questionnaire).
-
Hyperventilation (Nijmegen) questionnaire.
-
Oral corticosteroid courses.
-
Bronchodilator use.
-
Smoking status.
-
Process evaluations.
-
Patient-reported process evaluations (qualitative).
Engagement
-
Estimates of engagement with use of physiotherapy exercises in the physiotherapy arm.
-
Estimates of engagement with the breathing retraining.
Economic
-
Quality-adjusted life-years (QALYs).
-
Asthma-related health resource use.
-
Cost-effectiveness/utility.
Safety outcomes
-
Serious adverse events.
-
Non-serious adverse events.
-
Suspected unexpected serious adverse reactions.
Analysis
General principles
For the equivalence study, the adjusted repeated-measures mixed-model ITT analysis will be deemed the primary analysis with an adjusted repeated-measures mixed-model per-protocol analysis as a sensitivity analysis. For the superiority study, in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines, all comparative analysis will be conducted on an ITT repeated-measures mixed-model basis with a per-protocol repeated-measures mixed-model analysis performed as a sensitivity analysis. All analyses will be governed by this comprehensive SAP which will be agreed by the TSC and approved by the independent DMEC prior to any analyses being undertaken. There will be no formal interim analyses undertaken. Unless prespecified, a 5% two-sided significance level will be used to denote statistical significance throughout. As we specify a clear sequence of tests with a priori effect sizes to inform treatment selection, no adjustments will be made for multiple testing.
Data description
All variables will be described for each treatment group separately and for all participants together. This will be the number and percentage within each category for binary and categorical variables. Continuous variables will be described using the mean and SD (if normally distributed) or median (IQR) if skewed.
Missing data
A CONSORT flow diagram will provide the detail of the flow of trial participants, withdrawals and post-randomisation exclusions.
Missing baseline data
As this is collected at clinic by the research nurse it is anticipated that missing baseline data will be minimal (< 10%). Missing baseline data will be reported in the form of the number and percentage [n (%)] by variable and by randomised group. If a baseline variable has more than 10% missing data, we will use the missing indicator method recommended by White and Thompson. 30 This method is deemed a good approach irrespective of whether any missing baseline data does or does not predict the outcome. For missing baseline AQLQ scores, the method specified in Juniper et al. 24 was adopted. Values for each domain were calculated provided at least two-thirds of the items were scored; otherwise the domain value was set to missing. If any domain score was missing the overall AQLQ score was set to missing.
-
Missing primary outcome data. The repeated-measures mixed-model approach will account for missing data as long as at least one assessment of outcome is available. Compared to analysis of covariance (ANCOVA), a mixed-model approach is thought to give a more precise estimate of treatment effect at the final time point. Participants who do not provide any primary outcome follow-up data will therefore be excluded from the analysis. However, a prespecified sensitivity analysis will be carried out to include all randomised participants using the strategy reported by White et al. 31 This involves substituting the missing baseline AQLQ score by the specific trial arm mean and for missing 12-month AQLQ scores the method of last observation carried forward (LOCF) will be applied. If 12-month value is missing, the 6-month AQLQ score will be used to replace. If both 12-month and 6 month are missing, the 3-month AQLQ score will be used to replace the 12-month score. If all 3-month, 6-month and 12-month are missing, i.e. no follow-up information is available, then it will be assumed that the patient returned to their baseline AQLQ value.
-
Missing secondary outcome data. The number of baseline missing data per secondary outcome will be reported per randomised group and overall. Secondary outcomes analysed by repeated-measures mixed-effects models will assume missing at random. If this assumption is thought to be violated, we will consider alternative modelling strategies such as pattern mixture models.
Analysis of the primary outcome
Equivalence study
A 95% CI will be constructed for the mean difference in 12-month total AQLQ score between the DVD arm and the ‘face-to-face’ breathing retraining arm. Since the equivalence boundary is set at 0.3, equivalence will be declared if the 95% Cl is wholly included between –0.3 and +0.3. If equality is not evident, then a repeated-measures mixed-model will be used to examine whether the ‘face-to-face’ breathing retraining arm is superior to the DVD arm via examination of the difference (and 95% CI) of 12-month total AQLQ score (and each of the four domain scores) before and after adjustment for baseline AQLQ score and a set of prespecified variables. The prespecified variables are AQLQ score at baseline and the fixed effects of treatment arm, time, arm by time interaction plus the random effects of general practice and the patient-level covariates of age, sex, smoking status, BTS treatment step, baseline HADS score and baseline Nijmegen score. Correlations between baseline variables will be explored for the overall population on unblinded data and, if collinearity is evident, the most appropriate variables will be included in the final model. Models with different covariance assumptions were compared by using the Akaike information criterion (AIC); changes at 12 months between physiotherapy vs. usual care; DVD vs. usual care; DVD vs. physiotherapy were estimated from these models. Results from the adjusted ITT repeated-measures mixed model are deemed the primary analyses.
For participants that are lost to follow-up at some time during the 12-month follow-up their information will be included in the statistical analysis up to the point that they are lost to follow-up. The mixed-effects model assumes that data are missing at random and allows for unbalance or missing observations within subject.
Superiority study
Baseline comparability between the three arms of the trial will be evaluated by examination of summary statistics (the mean and SD or median and IQR for continuous variables, dependent on their distribution, and the number and percentage for categorical variables). In accordance with CONSORT guidelines, all comparative analysis will be conducted on an ITT basis with a per-protocol analysis performed as a sensitivity analysis.
For the primary outcome, a repeated-measures mixed model will be used to examine the 12-month total AQLQ score (and each of the four domain scores) across the three arms with adjustment for baseline AQLQ score and a set of prespecified variables. The same model procedure used in the equivalence study will be adapted here. Pairwise comparisons of AQLQ differences will be examined between the ‘usual-care’ arm and each of the DVD and ‘face-to-face’ breathing retraining arms via calculation of two sided 95% CIs. If the CI includes +0.3 then superiority of either the DVD or ‘face-to-face’ breathing retraining arms over usual care will be rejected.
Analysis of secondary outcomes
Equivalence study
A repeated-measures mixed model will be used to analyse the difference (and 95% CI) for the secondary outcome measures (ACQ, EQ-5D, HADS score) at 12 months before and after adjustment for baseline values and a set of prespecified variables. The list of prespecified variables is general practice (as a random effect), age, sex, smoking status, BTS treatment step, baseline HADS score and baseline Nijmegen score. For those secondary outcomes collected only at baseline and 12 months’ follow-up such as lung function, FeNO, Nijmegen score, ANCOVA will be used to analyse 12-month group differences before and after adjustment for baseline values and a set of aforementioned prespecified variables.
For those secondary outcomes that involve count data (i.e. oral corticosteroid courses, bronchodilator use, asthma-related health-care resource use), Poisson regression analysis (or negative binomial regression on failure of the assumptions of Poisson regression) with a log link function will be performed to give rate ratios (and their 95% CIs) in the DVD and ‘face-to-face’ breathing retraining arm both before and after adjustment for prespecified variables of general practice, age, sex, smoking status, BTS treatment step, baseline HADS score and baseline Nijmegen score. The exact number of covariates that will be included in the adjusted model will be dependent on the distribution of the secondary outcome and avoidance of loss of power.
Superiority study
A repeated-measures mixed model will be used to analyse the continuous secondary outcome measures [ACQ, EQ-5D and HADS score (both anxiety and depression scores)]. The ‘usual-care’ arm will be compared with each of the DVD and ‘face-to-face’ breathing retraining arms in turn.
For those secondary outcomes collected only at baseline and 12 months’ follow-up, such as lung function, FeNO and Nijmegen score, ANCOVA will be used to analyse 12-month group differences before and after adjustment for baseline values and a set of aforementioned prespecified variables.
For those secondary outcomes that involve count data (i.e. oral corticosteroid courses, bronchodilator use, asthma-related health-care resource use), Poisson regression (or negative binomial regression on failure of the assumption of Poisson regression) with a log link function will be performed to give rate ratios (and their 95% CIs) in the DVD and ‘face-to-face’ breathing retraining arm compared with ‘usual care’ both before and after adjustment for list of prespecified variables such as general practice, age, sex, smoking status, BTS treatment step, baseline HADS score and baseline Nijmegen score. The exact number of covariates that will be included in the adjusted model will be dependent on the distribution of the secondary outcome and avoidance of loss of power.
Engagement
The proportion (n) of participants who attended none, one, two and all three physiotherapy sessions in the physiotherapy arm will be tabulated.
Primary and secondary efficacy (equivalence study) analyses relating to the AQLQ (and each of the four domain scores) at 3 and 12 months will be repeated as a sensitivity analyses in two ways:
-
Engagement. Participants who did not engage with the breathing retraining at 3 months will be excluded. Engagement will be included as a binary variable defined by any response above ‘never started’ to any of the first three questions (number of weeks, days per week and times per day) in the ‘carrying out the breathing retraining’ block of treatment engagement questions at 3 months.
-
Amount of practice. Participants who did not engage with the breathing retraining at 3 months will be excluded and a new covariate – the amount of practice undertaken – will be added to the models. Amount of practice is a continuous variable which will be calculated by multiplying weights from each of the three questions shown in Table 1.
| Question | Response | Score | Example |
|---|---|---|---|
| Q1. For how many weeks did you carry out the breathing retraining? | Never started | 0 | If a participant carried out breathing retraining for 3–5 weeks, for 3–4 days at least twice a day, this would be a score of 32 (4 × 4 × 2) for the number of practice sessions completed |
| 1 week | 1 | ||
| 1–2 weeks | 2 | ||
| 3–5 weeks | 4 | ||
| 6–8 weeks | 7 | ||
| ≥ 9 weeks | 10 | ||
| Q2. How many times a week, on average, did you carry out the breathing retraining? | Never started | 0 | |
| 1–2 days | 2 | ||
| 3–4 days | 4 | ||
| 5–6 days | 6 | ||
| Most days | 7 | ||
| Q3. How many times a day, on average, did you carry out the breathing retraining? | Never started | 0 | |
| Once a day | 1 | ||
| At least twice a day | 2 |
Chapter 3 Results
Recruitment
We recruited patients from 34 primary care sites in Wessex through the PCRN (subsumed during the study into the CRN). We identified potential recruits to the study by searching general practice electronic medical records for patients with a coded diagnosis for asthma, undergoing current treatment and meeting inclusion criteria. Potential recruits were sent information about the study, the AQLQ (as impaired disease-specific health status was an inclusion criteria to enable an improvement to be demonstrated) and a prepaid return envelope.
A total of 15,203 invitation letters were mailed to potential participants and 1481 responses were received (a response rate of 9.7%). In total, 680 (45.9%) respondents were deemed ineligible, with lack of impairment according to AQLQ score being the most common reason for ineligibility; 655 participants (81.8% of the eligible respondents) were randomised, 261 (39.8% of randomised participants) to the DVD-delivered breathing retraining group, 262 (40.0%) to the usual-care group and 132 (20.2%) to the physiotherapist breathing retraining group (Table 2). The numbers of patients randomised from the different practices are shown in Appendix 1. The CONSORT diagram showing the flow of participants through the trial is provided in Figure 1.
FIGURE 1.
Consolidated Standards of Reporting Trials diagram.
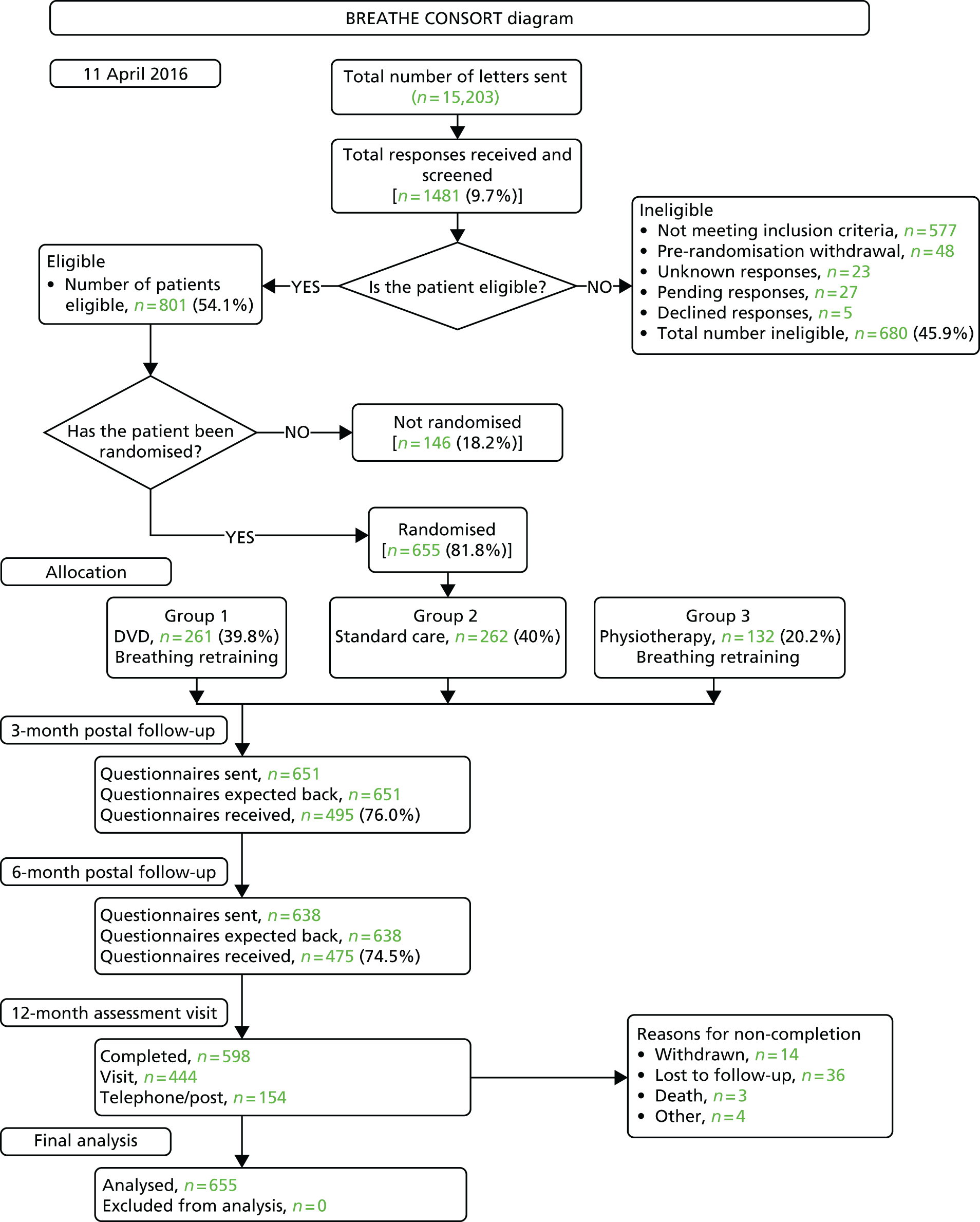
| Population | Treatment arm, n | Overall, n | ||
|---|---|---|---|---|
| DVD | Physiotherapy | Usual care | ||
| ITT | 261 | 132 | 262 | 655 |
| PP | 215 | 110 | 231 | 556 |
Because of errors in completion of the primary outcome (AQLQ) at baseline for 45 out of 655 participants (7%), it was not possible to assign an AQLQ score to these patients. However, one or more AQLQ returns was achieved for all randomised participants.
Baseline characteristics and demographics
Baseline demographic and clinical features of the participants in the study are shown in Table 3. The demographic and clinical profiles of the recruited population were typical of adult patients with mild-to-moderate asthma in the community and were similar between treatment arms. More women than men consented to participate in the trial and the median age of participants was 57 years, with approximately one-third of participants aged < 50 years. The median (IQR) age at diagnosis of asthma was 29 (11–45) years. In total, 8% of participants were current smokers and 33% were ex-smokers. Approximately one-third of participants had a family history of asthma. The most common self-reported asthma triggers were dust (77%), pollen (64%), smoke (64%), exercise (63%), stress (46%), cats (41%), dogs (24%) and food (19%).
| Characteristic | Treatment arm | Overall (N = 655) | ||
|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | ||
| Sex, n (%) | ||||
| Male | 97 (37.2) | 41 (31.1) | 98 (37.4) | 236 (36.0) |
| Female | 164 (62.8) | 91 (68.9) | 164 (62.6) | 419 (63.9) |
| Age (years), median (IQR) | 56 (45–65) | 55 (47–63) | 57 (47–65) | 57 (46–64) |
| Age group (years), n (%) | ||||
| ≤ 40 | 47 (18.0) | 21 (15.9) | 43 (16.4) | 111 (16.9) |
| 41–50 | 54 (20.7) | 28 (21.2) | 45 (17.2) | 127 (19.4) |
| 51–60 | 59 (22.6) | 35 (26.5) | 73 (27.9) | 167 (25.5) |
| > 60 | 101 (38.7) | 48 (36.4) | 101 (38.5) | 250 (38.2) |
| Weight (kg) | ||||
| Number included | 258 | 132 | 261 | 651 |
| Mean (SD) | 79.9 (17.6) | 80.6 (20.2) | 83.1 (18.1) | 81.3 (18.4) |
| Height (cm) | ||||
| Number included | 258 | 132 | 261 | 651 |
| Mean (SD) | 167.1 (9.0) | 165.7 (8.8) | 166.1 (9.1) | 166.4 (9.0) |
| Smoking status, n (%) | ||||
| Current smoker | 16 (6.1) | 13 (9.8) | 21 (8.0) | 50 (7.6) |
| Ex-smoker | 74 (28.4) | 43 (32.6) | 102 (38.9) | 219 (33.4) |
| Never | 169 (64.8) | 76 (57.6) | 139 (53.1) | 384 (58.6) |
| What they currently smoke, n (%) | ||||
| Cigarettes | 10 (3.8) | 8 (6.1) | 15 (5.7) | 33 (5.0) |
| Tobacco | 6 (2.3) | 5 (3.8) | 7 (2.7) | 18 (2.7) |
| Cigars | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Cigarettes/tobacco | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Tobacco/cigars | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| What they used to smoke, n (%) | ||||
| Cigarettes | 69 (26.4) | 40 (30.3) | 96 (36.6) | 205 (31.3) |
| Tobacco | 6 (2.3) | 4 (3.0) | 8 (3.1) | 18 (2.7) |
| Cigars | 3 (1.1) | 3 (2.3) | 1 (0.4) | 7 (1.1) |
| Cigarettes/tobacco | 2 (0.8) | 1 (0.8) | 2 (0.8) | 5 (0.8) |
| Tobacco/cigars | 1 (0.4) | 1 (0.8) | 0 (0.0) | 2 (0.3) |
| Cigarettes/cigars | 2 (0.8) | 2 (1.5) | 1 (0.4) | 5 (0.8) |
| Cigarettes/tobacco/cigars | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Average number of cigarettes per day for ever smokers | ||||
| Number included | 78 | 47 | 111 | 236 |
| Median (IQR) | 20 (10–21) | 15 (10–20) | 15 (6–20) | 15 (8–20) |
| Minimum, maximum | 1, 80 | 3, 60 | 1, 100 | 1, 100 |
| Pack-years of smoking among ever smokers | ||||
| Number included | 82 | 49 | 113 | 244 |
| Median (IQR) | 15 (5–30) | 13 (3–27) | 10 (3–23) | 12 (3–25) |
| Minimum, maximum | 0.05, 112.5 | 0.25, 159 | 0.05, 300 | 0.05, 300 |
| Age diagnosed with asthma (years) | ||||
| Number included | 259 | 132 | 259 | 650 |
| Median (IQR) | 27 (12–45) | 32 (14–45) | 28 (8–46) | 29 (11–45) |
| Family history of asthma, n (%) | ||||
| Mother | ||||
| Yes | 35 (13.4) | 28 (21.2) | 43 (16.4) | 106 (16.2) |
| No | 220 (84.3) | 99 (75.0) | 212 (80.9) | 531 (81.1) |
| Unknown | 2 (0.8) | 4 (3.0) | 7 (2.7) | 13 (2.0) |
| Father | ||||
| Yes | 31 (11.9) | 17 (12.9) | 26 (9.9) | 74 (11.3) |
| No | 218 (83.5) | 108 (81.8) | 219 (83.6) | 545 (83.2) |
| Unknown | 8 (3.1) | 6 (4.6) | 17 (6.5) | 31 (4.7) |
| Siblings | ||||
| Yes | 67 (25.7) | 35 (26.5) | 61 (23.3) | 163 (24.9) |
| No | 180 (69.0) | 84 (63.6) | 176 (67.2) | 440 (67.2) |
| n/a | 7 (2.7) | 13 (9.9) | 23 (8.8) | 43 (6.6) |
| Children | ||||
| Yes | 85 (32.6) | 38 (28.8) | 83 (31.7) | 206 (31.5) |
| No | 125 (47.9) | 66 (50.0) | 129 (49.2) | 320 (48.9) |
| n/a | 48 (18.4) | 27 (20.5) | 50 (19.1) | 125 (19.1) |
| Other family members | ||||
| Yes | 87 (33.3) | 50 (37.9) | 94 (35.9) | 231 (35.2) |
| No | 159 (60.9) | 76 (57.6) | 159 (60.7) | 394 (60.2) |
| Unknown | 5 (1.9) | 3 (2.3) | 4 (1.5) | 12 (1.8) |
| Asthma triggers, n (%) | ||||
| Cats | 119 (45.6) | 60 (45.5) | 109 (41.6) | 288 (43.9) |
| Dogs | 68 (26.1) | 37 (28.0) | 73 (27.9) | 178 (27.2) |
| Dust | 214 (81.9) | 108 (81.8) | 215 (82.1) | 537 (81.9) |
| Exercise | 185 (70.9) | 100 (75.8) | 191 (72.9) | 476 (72.7) |
| Pollen | 164 (62.8) | 89 (67.4) | 177 (67.6) | 430 (65.7) |
| Smoke | 186 (71.3) | 97 (73.5) | 174 (66.4) | 457 (69.8) |
| Stress | 133 (50.9) | 67 (50.8) | 133 (50.8) | 333 (50.8) |
| Food | 42 (16.1) | 33 (25.0) | 58 (22.1) | 133 (20.3) |
| Other | 211 (80.8) | 92 (69.7) | 198 (75.6) | 501 (76.5) |
| FeNO (p.p.b.) | ||||
| Number included | 238 | 126 | 242 | 606 |
| Median (IQR) | 21 (14–35) | 23 (15–23) | 23 (14–34) | 22 (14–34) |
| FEV1 (l) | ||||
| Number included | 246 | 130 | 253 | 629 |
| Mean (SD) | 2.6 (0.8) | 2.5 (0.7) | 2.6 (0.8) | 2.6 (0.8) |
| FVC (l) | ||||
| Number included | 246 | 130 | 253 | 629 |
| Mean (SD) | 3.5 (0.9) | 3.3 (0.9) | 3.4 (0.9) | 3.4 (0.9) |
| FEV1-to-FVC ratio | ||||
| Number included | 246 | 130 | 253 | 629 |
| Mean (SD) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) |
| FEV1% predicted | ||||
| Number included | 246 | 130 | 253 | 629 |
| Mean (SD) | 90.5 (18.8) | 88.8 (18.1) | 91.9 (21.6) | 90.7 (19.8) |
| PEFR (l/second) | ||||
| Number included | 244 | 129 | 249 | 622 |
| Mean (SD) | 425.5 (115.8) | 414.9 (110.0) | 423.4 (120.7) | 422.5 (116.5) |
| BTS treatment step,a n (%) | ||||
| 1 | 19 (7.3) | 8 (6.1) | 20 (7.6) | 47 (7.2) |
| 2 | 65 (24.9) | 41 (31.1) | 69 (26.3) | 175 (26.7) |
| 3 | 107 (41.0) | 52 (39.4) | 117 (44.7) | 276 (42.1) |
| 4 | 26 (10.0) | 16 (12.1) | 33 (12.6) | 75 (11.5) |
| 5 | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| Unknown/unspecified | 44 (16.9) | 15 (11.4) | 22 (8.4) | 81 (12.4) |
Participants had mildly impaired lung function, with a mean FEV1% predicted of 91% and a mean FEV1-to-FVC ratio of 0.8. The median (IQR) FeNO, a measure of eosinophilic airway inflammation, was 22 (14–34) parts per billion, which is at the top end of the normal range; over one-quarter of participants had a raised reading, indicative of persisting active inflammation despite inhaled steroid treatment. In terms of the BTS treatment step, our patient sample was typical of adult asthma patients treated in the community, with 7% at step 1 (needing short-acting beta-agonists only), 27% at step 2, 42% at step 3 and 12% at step 4 or 5. Self-reported atopic comorbidity was common (Table 4), with approximately two-thirds of participants reporting allergic problems including allergic rhinitis (69%) and eczema (31%), with most requiring pharmacological treatment. Over one-third of participants reported psychological problems (anxiety and depression), again with most requiring medication. Diagnosed COPD was present in only 2% of participants and had to be mild to meet entry criteria.
| Comorbidity | Treatment arm | Overall (N = 655) | ||
|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | ||
| Allergies | ||||
| Yes, n (%) | 179 (68.6) | 81 (61.4) | 177 (67.6) | 437 (66.7) |
| If yes, on current medication, n | 179 | 81 | 175 | 435 |
| Unknown, n | 4 | 2 | 3 | 9 |
| Hay fever/rhinitis | ||||
| Yes, n (%) | 183 (70.1) | 94 (71.2) | 174 (66.4) | 451 (68.9) |
| If yes, on current medication, n | 183 | 94 | 173 | 450 |
| Unknown, n | 3 | 0 | 8 | 12 |
| Eczema | ||||
| Yes, n (%) | 75 (28.7) | 46 (34.8) | 80 (30.5) | 201 (30.7) |
| If yes, on current medication, n | 75 | 46 | 78 | 199 |
| Unknown, n | 2 | 4 | 3 | 9 |
| Heart disease | ||||
| Yes, n (%) | 19 (7.3) | 13 (9.8) | 22 (8.4) | 54 (8.2) |
| If yes, on current medication, n | 19 | 13 | 22 | 54 |
| Unknown, n | 2 | 1 | 3 | 6 |
| Depression/anxiety | ||||
| Yes, n (%) | 92 (35.2) | 50 (37.9) | 91 (34.7) | 233 (35.6) |
| If yes, on current medication, n | 92 | 50 | 90 | 232 |
| Unknown, n | 1 | 0 | 1 | 2 |
| Documented diagnosis of COPD | ||||
| Yes, n (%) | 2 (0.8) | 2 (1.5) | 11 (4.2) | 15 (2.3) |
| No, n (%) | 259 (99.2) | 130 (98.5) | 251 (95.8) | 640 (97.7) |
Baseline questionnaire data (Table 5) showed moderate impairment in asthma-specific QoL, with a mean (SD) AQLQ score of 4.3 (0.9), and also impaired symptomatic asthma control, with a mean (SD) ACQ score of 1.5 (0.9). The baseline psychological assessment with the HADS questionnaire showed a median (IQR) anxiety score of 6 (4–9) and depression score of 3 (1–5). A score of ≤ 7 on this tool is considered ‘normal’ and so > 255 patients had scores suggestive of significant anxiety, in keeping with population-based survey data. Across the five domains of the EQ-5D generic QoL questionnaire, 42% of participants reported problems with pain or discomfort, 31% with activities, 27% with anxiety or depression, 23% with mobility and 5% with self-care.
| Questionnaire | Treatment arm | Overall (N = 655) | ||
|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | ||
| Mini AQLQ | ||||
| n | 244 | 120 | 246 | 610 |
| Mean (SD) score | 4.3 (0.9) | 4.2 (0.9) | 4.3 (0.9) | 4.3 (0.9) |
| Nijmegen questionnaire | ||||
| n | 259 | 132 | 262 | 653 |
| Mean (SD) score | 19.0 (8.8) | 19.0 (10.5) | 19.4 (9.4) | 19.2 (9.4) |
| HADS | ||||
| n | 257 | 131 | 261 | 649 |
| Anxiety, median (IQR) score | 7.0 (4–8) | 6.0 (4–9) | 6.0 (4–9) | 6.0 (4–9) |
| Depression, median (IQR) score | 3.0 (1–5) | 2.0 (1–5) | 3.0 (1–5) | 3.0 (1–5) |
| EQ-5D | ||||
| Mobility | ||||
| n | 259 | 132 | 262 | 653 |
| No problems, n (%) | 202 (78.0) | 97 (73.5) | 203 (77.5) | 502 (76.9) |
| Some problems, n (%) | 57 (22.0) | 35 (26.5) | 59 (22.5) | 151 (23.1) |
| Self-care | ||||
| n | 258 | 131 | 262 | 651 |
| No problems, n (%) | 251 (96.3) | 120 (91.6) | 246 (93.9) | 617 (94.8) |
| Some problems, n (%) | 7 (2.7) | 11 (8.4) | 16 (6.1) | 34 (5.2) |
| Usual activities | ||||
| n | 259 | 132 | 262 | 653 |
| No problems, n (%) | 186 (71.8) | 82 (62.1) | 183 (69.8) | 451 (69.1) |
| Some problems, n (%) | 73 (28.2) | 50 (37.9) | 79 (30.2) | 202 (30.9) |
| Pain/discomfort | ||||
| n | 259 | 131 | 261 | 651 |
| No problems, n (%) | 161 (62.2) | 79 (60.3) | 137 (52.5) | 377 (57.9) |
| Some problems, n (%) | 98 (37.8) | 52 (39.7) | 124 (47.5) | 274 (42.1) |
| Anxiety/depression | ||||
| n | 259 | 132 | 262 | 653 |
| No problems, n (%) | 187 (72.2) | 99 (75.0) | 192 (73.3) | 478 (73.0) |
| Some problems, n (%) | 72 (27.8) | 33 (25.0) | 70 (26.7) | 175 (27.0) |
| EQ-5D VAS | ||||
| n | 256 | 131 | 258 | 645 |
| Median (IQR) | 80 (69.3–88.8) | 75 (60–85) | 80 (68–89) | 80 (67.5–88) |
| Mean (SD) | 74.9 (16.8) | 71.7 (19.5) | 74.4 (16.9) | 74.1 (17.4) |
| ACQ | ||||
| n | 258 | 132 | 262 | 652 |
| Mean (SD) | 1.5 (0.9) | 1.6 (0.8) | 1.5 (0.9) | 1.5 (0.9) |
In summary, we successfully recruited a population of primary care asthma patients with mild-to-moderate asthma and with evidence of incomplete asthma control and QoL impairment, our target group. Their demographic profile was similar between randomisation arms and appears to be typical of the demographic and disease control profile of UK primary care adult asthma populations reported in recent surveys of asthma control.
Missing data, withdrawals and study retention
Missing baseline data (see Appendix 2)
Because of errors in completion of the primary outcome questionnaire (AQLQ) at baseline for 45 out of 655 participants (7%), it was not possible to assign an AQLQ score to these patients. There was a very low level of missing baseline data for the other questionnaires (< 2%).
Spirometry values (FEV1, FVC and FEV1-to-FVC ratio) were missing for 4% of participants, PEFR values for 5% and FeNO values for 7.5%, with similar proportions of missing data between randomisation arms.
Withdrawals
Only 21 of the 655 randomised patients withdrew from the study (3.2%), with similar withdrawal rates between the DVD (5.4%), physiotherapy (2.3%) and control (2.3%) arms. The reasons for withdrawals are provided in Appendix 3. The baseline characteristics of those who withdrew were similar to the baseline characteristics of the randomised population (see Appendix 4).
Primary outcome measure: disease-specific health status measured using the Asthma Quality of Life Questionnaire
The primary efficacy analysis was a comparison of between-group changes in AQLQ scores in the ITT population, with adjustments for prespecified covariates. Secondary analyses included an unadjusted comparison of between-group changes in AQLQ scores in the ITT population, adjusted and unadjusted comparisons of between-group changes in AQLQ scores in the PP population and analyses of the subdomains measured by the AQLQ instrument. Prespecified sensitivity analyses are also reported. We also assessed the time course of changes in AQLQ score from 3-month and 6-month AQLQ postal questionnaire data.
Table 6 presents the baseline and 12-month mean (SD) AQLQ scores and the unadjusted mean changes in AQLQ scores (with 95% CIs) in the DVD, physiotherapy and usual-care arms in the ITT and PP populations. Table 7 presents the primary efficacy analysis, the adjusted mean difference in 12-month AQLQ scores in the DVD, physiotherapy and usual-care treatment arms in the ITT and PP populations, with comparisons between the DVD arm and the control arm and between the physiotherapy arm and the control arm (superiority analysis) and between the DVD arm and the physiotherapy arm (equivalence analysis). The total AQLQ score (the primary efficacy measure) and the scores on the four subdomains of the AQLQ (which may be analysed and compared individually, measuring symptoms, activity, emotions and environment) are presented. The time course of AQLQ score changes in the ITT and PP populations is shown in Table 8.
| AQLQ domain | Time point, mean (SD) score | Unadjusted mean difference (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | ||||||||
| DVD | Physiotherapy | Usual care | DVD | Physiotherapy | Usual care | Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | |
| ITT population | |||||||||
| n | 244 | 120 | 246 | 231 | 121 | 246 | |||
| Total | 4.3 (0.9) | 4.2 (0.9) | 4.3 (0.9) | 5.4 (1.1) | 5.3 (1.1) | 5.1 (1.2) | 0.32 (0.08 to 0.56)** | 0.27 (0.08 to 0.47)** | –0.04 (–0.28 to 0.20) |
| Symptoms | 4.2 (1.0) | 4.0 (1.1) | 4.2 (1.1) | 5.2 (1.3) | 5.2 (1.1) | 4.9 (1.2) | 0.41 (0.13 to 0.70)** | 0.24 (0.01 to 0.47)* | –0.17 (–0.45 to 0.11) |
| Activities | 5.0 (1.4) | 4.8 (1.5) | 5.0 (1.3) | 5.9 (1.3) | 5.7 (1.4) | 5.7 (1.3) | 0.23 (–0.04 to 0.51) | 0.23 (0.002 to 0.44)* | –0.01 (–0.29 to 0.28) |
| Emotion | 4.0 (1.3) | 4.0 (1.4) | 3.9 (1.4) | 5.4 (1.5) | 5.5 (1.3) | 5.0 (1.6) | 0.42 (0.08 to 0.75)* | 0.36 (0.08 to 0.63)** | –0.06 (–0.39 to 0.28) |
| Environment | 4.0 (1.1) | 3.8 (1.2) | 3.9 (1.2) | 5.1 (1.5) | 5.0 (1.3) | 4.8 (1.5) | 0.28 (–0.02 to 0.57) | 0.31 (0.05 to 0.56)* | 0.03 (–0.27 to 0.33) |
| PP population | |||||||||
| n | 215 | 110 | 231 | 215 | 110 | 231 | |||
| Total | 4.3 (0.9) | 4.2 (0.9) | 4.3 (0.9) | 5.4 (1.2) | 5.3 (1.1) | 5.1 (1.2) | 0.32 (0.08 to 0.55)** | 0.28 (0.08 to 0.47)* | –0.04 (–0.28 to 0.20) |
| Symptoms | 4.2 (0.9) | 4.0 (1.1) | 4.2 (1.0) | 5.2 (1.3) | 5.1 (1.1) | 4.9 (1.2) | 0.39 (0.10 to 0.68)** | 0.24 (0.01 to 0.47)* | –0.16 (–0.44 to 0.13) |
| Activities | 5.0 (1.4) | 4.8 (1.4) | 5.0 (1.3) | 5.9 (1.3) | 5.6 (1.4) | 5.7 (1.3) | 0.22 (–0.06 to 0.50) | 0.22 (0.01 to 0.44) | 0.01 (–0.28 to 0.28) |
| Emotion | 4.0 (1.2) | 4.0 (1.4) | 4.0 (1.3) | 5.4 (1.5) | 5.5 (1.3) | 5.0 (1.5) | 0.43 (0.09 to 0.77)* | 0.37 (0.09 to 0.65)** | –0.06 (–0.40 to 0.28) |
| Environment | 3.9 (1.1) | 3.8 (1.2) | 3.9 (1.2) | 5.1 (1.5) | 4.9 (1.4) | 4.8 (1.5) | 0.26 (–0.04 to 0.56) | 0.31 (0.06 to 0.57)* | 0.05 (–0.24 to 0.35) |
| AQLQ domain | ITT population | PP population | ||||
|---|---|---|---|---|---|---|
| Adjusted mean differencea (95% CI) | Adjusted mean differencea (95% CI) | |||||
| Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | |
| Total | 0.24 (0.04 to 0.44)* | 0.28 (0.11 to 0.44)** | 0.04 (–0.16 to 0.24) | 0.22 (0.02 to 0.43)* | 0.26 (0.10 to 0.43)** | 0.04 (–0.17 to 0.25) |
| Symptoms | 0.27 (0.04 to 0.49)* | 0.24 (0.05 to 0.42)* | –0.03 (–0.26 to 0.20) | 0.25 (0.02 to 0.49)* | 0.21 (0.02 to 0.40)* | –0.04 (–0.27 to 0.19) |
| Activities | 0.08 (–0.14 to 0.31) | 0.21 (0.04 to 0.41)* | 0.13 (–0.10 to 0.36) | 0.08 (–0.15 to 0.30) | 0.21 (0.02 to 0.39)* | 0.13 (–0.10 to 0.36) |
| Emotion | 0.43 (0.16 to 0.71)** | 0.38 (0.16 to 0.60)** | –0.06 (–0.33 to 0.22) | 0.41 (0.14 to 0.68)** | 0.35 (0.13 to 0.58)** | –0.05 (–0.33 to 0.22) |
| Environment | 0.19 (–0.06 to 0.44) | 0.32 (0.11 to 0.53)** | 0.13 (–0.12 to 0.39) | 0.18 (–0.07 to 0.44) | 0.32 (0.11 to 0.54)** | 0.14 (–0.12 to 0.40) |
| AQLQ domain | Time point, mean (SD) score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | |||||||||
| DVD | Physiotherapy | Usual care | DVD | Physiotherapy | Usual care | DVD | Physiotherapy | Usual care | DVD | Physiotherapy | Usual care | |
| ITT population | ||||||||||||
| n | 244 | 120 | 246 | 171 | 105 | 226 | 163 | 101 | 217 | 231 | 121 | 241 |
| Total | 4.3 (0.9) | 4.2 (0.9) | 4.3 (0.9) | 5.1 (1.2) | 5.2 (1.0) | 4.9 (1.1) | 5.3 (1.3) | 5.3 (1.0) | 4.9 (1.2) | 5.4 (1.1) | 5.3 (1.1) | 5.1 (1.2) |
| Symptoms | 4.2 (1.0) | 4.0 (1.1) | 4.2 (1.1) | 5.1 (1.2) | 5.1 (1.1) | 4.7 (1.2) | 5.2 (1.2) | 5.3 (1.1) | 4.7 (1.3) | 5.2 (1.3) | 5.2 (1.1) | 4.9 (1.2) |
| Activities | 5.0 (1.4) | 4.8 (1.5) | 5.0 (1.3) | 5.7 (1.4) | 5.6 (1.3) | 5.5 (1.4) | 5.7 (1.4) | 5.7 (1.2) | 5.4 (1.4) | 5.9 (1.3) | 5.7 (1.4) | 5.7 (1.3) |
| Emotion | 4.0 (1.3) | 4.0 (1.4) | 3.9 (1.4) | 5.0 (1.4) | 5.1 (1.4) | 4.8 (1.6) | 5.2 (1.4) | 5.3 (1.4) | 4.8 (1.5) | 5.4 (1.5) | 5.5 (1.3) | 5.0 (1.6) |
| Environment | 4.0 (1.1) | 3.8 (1.2) | 3.9 (1.2) | 4.8 (1.4) | 4.8 (1.4) | 4.5 (1.3) | 4.9 (1.4) | 5.0 (1.2) | 4.5 (1.4) | 5.1 (1.5) | 5.0 (1.3) | 4.8 (1.5) |
| PP population | ||||||||||||
| n | 215 | 110 | 231 | 154 | 94 | 204 | 148 | 92 | 197 | 215 | 110 | 231 |
| Total | 4.3 (0.9) | 4.2 (0.9) | 4.3 (0.9) | 5.2 (1.1) | 5.2 (1.0) | 4.9 (1.1) | 5.3 (1.2) | 5.3 (1.0) | 4.9 (1.2) | 5.4 (1.2) | 5.3 (1.0) | 5.1 (1.2) |
| Symptoms | 4.2 (0.9) | 4.0 (1.1) | 4.2 (1.0) | 5.1 (1.2) | 5.1 (1.1) | 4.7 (1.2) | 5.1 (1.2) | 5.3 (1.1) | 4.7 (1.3) | 5.2 (1.3) | 5.1 (1.1) | 4.9 (1.2) |
| Activities | 5.0 (1.4) | 4.8 (1.4) | 5.0 (1.3) | 5.7 (1.3) | 5.6 (1.2) | 5.4 (1.3) | 5.7 (1.4) | 5.7 (1.2) | 5.4 (1.4) | 5.9 (1.3) | 5.6 (1.4) | 5.7 (1.3) |
| Emotion | 4.0 (1.2) | 4.0 (1.4) | 3.9 (1.3) | 5.0 (1.4) | 5.2 (1.4) | 4.7 (1.5) | 5.2 (1.4) | 5.4 (1.3) | 4.8 (1.5) | 5.4 (1.5) | 5.5 (1.3) | 5.0 (1.5) |
| Environment | 3.9 (1.1) | 3.8 (1.2) | 3.9 (1.1) | 4.8 (1.4) | 4.8 (1.4) | 4.5 (1.3) | 4.9 (1.4) | 5.0 (1.2) | 4.5 (1.4) | 5.1 (1.5) | 4.9 (1.4) | 4.8 (1.5) |
Within-group Asthma Quality of Life Questionnaire score changes
There was a large within-group change in mean AQLQ score of 1.1 from baseline to the 12-month assessment in both breathing retraining arms. There was also a smaller improvement of 0.8 in the control arm. The MCID in AQLQ score for an individual patient is 0.5 and a change of 1.0 equates to a large difference.
Between-group Asthma Quality of Life Questionnaire score changes
In the primary efficacy analysis, the between-group comparison of AQLQ scores in the ITT population adjusted for the prespecified covariates, we observed a statistically significant improvement in mean AQLQ score of 0.28 (95% CI 0.11 to 0.44; p < 0.001) in the DVD arm compared with the control arm and of 0.24 (95% CI 0.04 to 0.44; p < 0.05) in the physiotherapy arm compared with the control arm, confirming the superiority of the two active interventions over usual care. The adjusted mean difference between the DVD arm and the physiotherapy arm was 0.04 (95% CI –0.16 to 0.24), which was not significantly different; the 95% CI was within the prespecified equivalence margin, confirming the equivalence of the two active interventions. Across the AQLQ subdomains, the largest improvements in AQLQ scores for the active treatments compared with usual care were in the emotions subdomain (DVD vs. control: adjusted mean difference 0.38, 95% CI 0.16 to 0.60; p < 0.001; physiotherapy vs. control: adjusted mean difference 0.43, 95% CI 0.16 to 0.71; p < 0.001); significant improvements were also seen in the symptoms, activities and environment domains in the DVD arm compared with the usual-care arm and in the symptoms domain in the physiotherapy arm compared with the usual-care arm (with non-significant numerical improvements in the physiotherapy arm compared with the usual-care arm for activities and environment). There were no significant differences in subdomain scores between the DVD arm and the physiotherapy arm.
The overall messages were unchanged in the PP population analyses, with superiority of both active treatment arms over usual care and equivalence between the active arms, with the magnitude of the improvements very similar to the magnitude of the improvements in the ITT population. Similarly, in the unadjusted analyses in both the ITT and the PP populations, the overall messages were identical, with superiority of both interventions over usual care and equivalence of the active interventions and only minor differences in the magnitude of the differences between treatment arms.
Time course of Asthma Quality of Life Questionnaire score improvements
The improvements in AQLQ scores in both active arms compared with the control arm were observed at the first post-intervention assessment at 3 months and were maintained or increased over the 12-month study period. In the DVD arm, the improvement in mean total AQLQ score compared with baseline in the ITT population was 0.9 at 3 months, 1.0 at 6 months and 1.1 at 12 months; the equivalent values in the physiotherapy arm were 1.0, 1.1 and 1.1, respectively, and in the control arm were 0.6, 0.6 and 0.8 respectively. Similar patterns of change were seen in the PP population and for the subdomain scores in both the ITT and the PP populations.
Number needed to treat to achieve a clinically important improvement in the primary outcome measure, Asthma Quality of Life Questionnaire score
The number needed to treat (NNT) was calculated using the formula recommended by Guyatt et al. 32 (Juniper and Guyatt produced the AQLQ24 and ACQ25 tools). This analysis is based on an individual patient assessment of the proportions in each treatment arm showing a clinically significant improvement (≥ 0.5), the proportions showing unchanged scores (–0.49 to 0.49) and the proportions with a clinically significant deterioration (≤ –0.5) (see Appendix 5).
We found that, in the ITT population, 62% of participants in the DVD group reported a clinically significant improvement compared with 64% in the physiotherapy group and 56% in the control group (Table 9). The corresponding figures for deterioration were 5% in the DVD group, 4% in the physiotherapy group and 9% in the control group. The proportions in the PP population were slightly higher (see Table 9), with 74% of the DVD group, 76% of the physiotherapy group and 62% of the control group showing an improvement in QoL and 6% of the DVD group, 5% of the physiotherapy group and 10% of the control group showing a deterioration in QoL.
| Change in AQLQ score | Treatment arm, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| DVD | Physiotherapy | Usual care | ||
| ITT population | ||||
| n | 261 | 132 | 262 | 655 |
| Improved | 161 (61.7) | 85 (64.4) | 146 (55.7) | 392 (59.8) |
| Stayed the same | 47 (18.0) | 24 (18.2) | 71 (27.1) | 142 (21.7) |
| Deteriorated | 14 (5.4) | 5 (3.8) | 24 (9.2) | 43 (6.6) |
| Could not be calculated | 39 (14.9) | 18 (13.6) | 21 (8.0) | 78 (11.9) |
| Total | 261 (100.0) | 132 (100.0) | 262 (100.0) | 655 (100.0) |
| PP population | ||||
| n | 215 | 110 | 231 | 556 |
| Improved | 159 (74.0) | 83 (75.5) | 142 (61.5) | 384 (69.1) |
| Stayed the same | 43 (20.0) | 22 (20.0) | 67 (29.0) | 132 (23.7) |
| Deteriorated | 13 (6.0) | 5 (4.5) | 22 (9.5) | 40 (7.2) |
| Total | 215 (100.0) | 110 (100.0) | 231 (100.0) | 556 (100.0) |
In between-group comparisons, these figures equated to a NNT for one patient to have a clinically relevant improvement in QoL in the ITT population of eight for the DVD arm compared with the usual-care arm (Table 10), seven for the physiotherapy arm compared with the usual-care arm (Table 11) and 41 for the physiotherapy arm compared with the DVD arm (Table 12). In the PP population the corresponding NNTs were eight, seven and 56 (Tables 13–15).
| Usual care | DVD | ||
|---|---|---|---|
| Improved (0.725) | Stayed the same (0.212) | Deteriorated (0.063) | |
| Improved (0.606) | 0.44 | 0.13 | 0.04 |
| Stayed the same (0.295) | 0.21 | 0.06 | 0.02 |
| Deteriorated (0.010) | 0.07 | 0.02 | 0.01 |
| NNT for DVD vs. usual carea | 8.2 | ||
| Usual care | Physiotherapy | ||
|---|---|---|---|
| Improved (0.746) | Stayed the same (0.211) | Deteriorated (0.044) | |
| Improved (0.606) | 0.45 | 0.13 | 0.03 |
| Stayed the same (0.295) | 0.22 | 0.06 | 0.01 |
| Deteriorated (0.010) | 0.07 | 0.02 | 0.00 |
| NNT for physiotherapy vs. usual carea | 6.8 | ||
| Physiotherapy | DVD | ||
|---|---|---|---|
| Improved (0.725) | Stayed the same (0.212) | Deteriorated (0.063) | |
| Improved (0.746) | 0.54 | 0.16 | 0.05 |
| Stayed the same (0.211) | 0.15 | 0.04 | 0.01 |
| Deteriorated (0.044) | 0.03 | 0.01 | 0.00 |
| NNT for physiotherapy vs. DVDa | 41.0 | ||
| Usual care | DVD | ||
|---|---|---|---|
| Improved (0.725) | Stayed the same (0.212) | Deteriorated (0.063) | |
| Improved (0.614) | 0.45 | 0.12 | 0.04 |
| Stayed the same (0.290) | 0.21 | 0.06 | 0.02 |
| Deteriorated (0.010) | 0.07 | 0.02 | 0.01 |
| NNT for DVD vs. usual carea | 7.92 | ||
| Usual care | Physiotherapy | ||
|---|---|---|---|
| Improved (0.755) | Stayed the same (0.2) | Deteriorated (0.045) | |
| Improved (0.614) | 0.46 | 0.12 | 0.03 |
| Stayed the same (0.290) | 0.22 | 0.06 | 0.01 |
| Deteriorated (0.010) | 0.07 | 0.02 | 0.00 |
| NNT for physiotherapy vs. usual carea | 6.86 | ||
| Physiotherapy | DVD | ||
|---|---|---|---|
| Improved (0.725) | Stayed the same (0.212) | Deteriorated (0.063) | |
| Improved (0.755) | 0.56 | 0.15 | 0.05 |
| Stayed the same (0.2) | 0.15 | 0.04 | 0.01 |
| Deteriorated (0.045) | 0.03 | 0.01 | 0.00 |
| NNT for physiotherapy vs. DVDa | 55.52 | ||
Prespecified sensitivity analysis on the primary outcome
As per the prespecified SAP, we carried out a sensitivity analysis on the primary outcome data, the change in AQLQ scores between baseline and 12 months adjusted for the prespecified covariates. In this analysis we included all randomised patients regardless of whether baseline or follow-up AQLQ data were present and we used the following rules (based on recommendations from Juniper) to assign values to missing data:
-
For missing baseline AQLQ scores, the following rules were adopted. Values for each AQLQ subdomain (symptoms, emotions, activities, environment) were calculated provided at least two-thirds of the items were scored, otherwise the domain value was set to missing. If any domain score was missing, the overall AQLQ score was set to missing and step 2 was followed.
-
Following step 1, any remaining missing baseline AQLQ scores were replaced by their cohort mean (as specified in the SAP).
-
For missing 12-month AQLQ scores, the method of LOCF was applied. If the 12-month score was missing, the 6-month score was used. If both the 12-month and 6-month scores were missing, the 3-month score was used to replace the 12-month score. If the 3-month, 6-month and 12-month scores were missing, that is, no follow-up information was available, then it was assumed that the patient returned to his or her baseline AQLQ score. Hence, the baseline and 12-month scores were the same.
Table 16 shows the baseline and 12-month unadjusted scores and the unadjusted mean differences in the three treatment arms in the ITT and PP populations, and Table 17 shows the adjusted mean difference in 12-month AQLQ scores in the three treatment arms in the ITT and PP populations. This sensitivity analysis provides a similar message to the primary analyses: we see that there are significant improvements in both of the active arms (DVD and physiotherapy) above usual care in both the ITT and PP populations, although predictably of slightly lower magnitude (as we assume ‘no change’ in cases with missing data) than in the main analysis. We continue to show equivalence between the two active arms.
| AQLQ domain | Time point, mean (SD) score | Unadjusted mean difference (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | ||||||||
| DVD | Physiotherapy | Usual care | DVD | Physiotherapy | Usual care | Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | |
| ITT population | |||||||||
| n | 261 | 132 | 262 | 261 | 132 | 262 | |||
| Total | 4.3 (0.9) | 4.2 (0.9) | 4.3 (0.9) | 5.3 (1.1) | 5.3 (1.1) | 5.1 (1.2) | 0.26 (0.05 to 0.48)* | 0.17 (–0.01 to 0.35) | –0.09 (–0.31 to 0.12) |
| Symptoms | 4.2 (1.0) | 4.1 (1.1) | 4.2 (1.0) | 5.1 (1.3) | 5.1 (1.2) | 4.9 (1.2) | 0.37 (0.10 to 0.63)** | 0.17 (–0.04 to 0.38) | –0.19 (–0.45 to 0.06) |
| Activities | 5.0 (1.3) | 4.8 (1.4) | 5.0 (1.3) | 5.8 (1.4) | 5.6 (1.4) | 5.6 (1.4) | 0.16 (–0.09 to 0.42) | 0.11 (–0.09 to 0.32)* | –0.05 (–0.30 to 0.20) |
| Emotion | 4.0 (1.3) | 4.1 (1.3) | 4.0 (1.3) | 5.2 (1.6) | 5.4 (1.4) | 5.0 (1.6) | 0.31 (0.001 to 0.62)* | 0.25 (–0.01 to 0.50) | –0.06 (–0.37 to 0.24) |
| Environment | 4.0 (1.1) | 3.8 (1.2) | 3.9 (1.1) | 5.0 (1.5) | 5.0 (1.4) | 4.8 (1.5) | 0.21 (–0.05 to 0.48) | 0.13 (–0.10 to 0.37) | –0.08 (–0.36 to 0.20) |
| PP population | |||||||||
| n | 261 | 123 | 262 | 261 | 123 | 262 | |||
| Total | 4.3 (0.9) | 4.2 (0.9) | 4.3 (0.9) | 5.3 (1.2) | 5.3 (1.1) | 5.1 (1.2) | 0.30 (0.08 to 0.52)* | 0.17 (–0.01 to 0.35) | –0.13 (–0.35 to 0.09) |
| Symptoms | 4.2 (1.0) | 4.0 (1.1) | 4.2 (1.0) | 5.1 (1.3) | 5.1 (1.2) | 4.9 (1.2) | 0.40 (0.12 to 0.67)* | 0.17 (–0.04 to 0.38) | –0.22 (–0.49 to 0.04) |
| Activities | 5.0 (1.3) | 4.8 (1.4) | 5.0 (1.3) | 5.8 (1.4) | 5.6 (1.4) | 5.6 (1.4) | 0.19 (–0.08 to 0.45) | 0.11 (–0.09 to 0.32) | –0.08 (–0.33 to 0.18) |
| Emotion | 4.0 (1.3) | 4.0 (1.3) | 4.0 (1.3) | 5.2 (1.6) | 5.4 (1.4) | 5.0 (1.6) | 0.36 (0.04 to 0.68)* | 0.25 (–0.01 to 0.50) | –0.11 (–0.43 to 0.20) |
| Environment | 4.0 (1.1) | 3.8 (1.2) | 3.9 (1.1) | 5.0 (1.5) | 5.0 (1.4) | 4.8 (1.5) | 0.27 (–0.005 to 0.53) | 0.13 (–0.10 to 0.37) | –0.13 (–0.42 to 0.15) |
| AQLQ domain | ITT population | PP population | ||||
|---|---|---|---|---|---|---|
| Adjusted mean differenceb (95% CI) | Adjusted mean differenceb (95% CI) | |||||
| Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | |
| Total | 0.21 (0.02 to 0.40)* | 0.17 (0.02 to 0.33)* | –0.04 (–0.23 to 0.15) | 0.25 (0.06 to 0.44)* | 0.17 (0.01 to 0.33)* | –0.08 (–0.27 to 0.12) |
| Symptoms | 0.26 (0.04 to 0.47)* | 0.16 (–0.02 to 0.34) | –0.09 (–0.31 to 0.12) | 0.29 (0.07 to 0.51)* | 0.16 (–0.02 to 0.34) | –0.13 (–0.35 to 0.09) |
| Activities | 0.06 (–0.14 to 0.27) | 0.13 (–0.05 to 0.30) | 0.06 (–0.15 to 0.27) | 0.10 (–0.11 to 0.31) | 0.12 (–0.05 to 0.30) | 0.02 (–0.19 to 0.24) |
| Emotion | 0.32 (0.06 to 0.58)* | 0.23 (0.02 to 0.45)* | –0.09 (–0.34 to 0.17) | 0.36 (0.10 to 0.62)* | 0.23 (0.02 to 0.44)* | –0.13 (–0.39 to 0.14) |
| Environment | 0.16 (–0.07 to 0.40) | 0.18 (–0.02 to 0.38) | 0.01 (–0.23 to 0.25) | –0.18 (–0.46 to 0.9) | 0.22 (–0.03 to 0.46) | –0.04 (–0.29 to 0.21) |
Secondary outcome measures
Physiological measures
Physiological outcome measures studied related-to-lung function (FEV1, FEV1% predicted, FVC, FEV1/FVC ratio, PEFR) and airway inflammation (FeNO). Measures were taken at baseline and 12 months. The primary analysis (Table 18) assessed change in physiological parameters between baseline and 12 months in the ITT population, adjusted for the prespecified covariates; secondary analyses assessed these parameters in the unadjusted ITT population (Table 19) and the adjusted and unadjusted PP populations (see Tables 14 and 15). In the primary analysis we observed no significant between-group changes in our measures of airway physiology or inflammation, indicating that the interventions did not change obstruction or inflammation in the airways and so were not disease modifying. The within-group changes from baseline to 12 months were small and non-significant.
| Parameters | ITT population | PP population | ||||
|---|---|---|---|---|---|---|
| Adjusted mean differencea (95% CI) | Adjusted mean differencea (95% CI) | |||||
| Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | |
| FEV1 (l) | –0.04 (–0.11 to 0.04) | –0.001 (–0.07 to 0.07) | 0.03 (–0.05 to 0.12) | 0.02 (–0.06 to 0.11) | –0.01 (–0.08 to 0.07) | –0.03 (–0.12 to 0.06) |
| FVC (l) | –0.04 (–0.16 to 0.08) | –0.03 (–0.14 to 0.07) | 0.01 (–0.12 to 0.13) | 0.03 (–0.09 to 0.16) | 0.02 (–0.09 to 0.13) | –0.01 (–0.14 to 0.12) |
| FEV1-to-FVC ratio | –0.01 (–0.02 to 0.01) | 0.004 (–0.01 to 0.02) | 0.01 (–0.01 to 0.03) | 0.01 (–0.02 to 0.02) | –0.003 (–0.02 to 0.01) | –0.004 (–0.03 to 0.02) |
| FEV1% predicted | 0.44 (–3.23 to 4.12) | 0.53 (–2.75 to 3.81) | 0.09 (–3.81 to 3.99) | –1.49 (–5.33 to 2.36) | –0.98 (–4.35 to 2.39) | 0.51 (–3.55 to 4.57) |
| PEFR (l/second) | –4.79 (–22.35 to 12.77) | –1.99 (–17.83 to 13.85) | 2.80 (–15.94 to 21.54) | 3.19 (–15.41 to 21.80) | 2.91 (–13.66 to 19.48) | –0.29 (–20.10 to 19.53) |
| FeNOb (p.p.b.) | 1.05 (0.95 to 1.23) | 1.13 (0.98 to 1.29) | 1.07 (0.91 to 1.25) | 1.05 (0.89 to 1.23) | 1.14 (0.98 to 1.31) | 1.08 (0.92 to 1.28) |
| Parameters | Time point | Unadjusted mean difference (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | ||||||||
| DVD | Physiotherapy | Usual care | DVD | Physiotherapy | Usual care | Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | |
| ITT population | |||||||||
| n | 261 | 132 | 262 | 143 | 92 | 189 | |||
| FEV1 (l), mean (SD) | 2.6 (0.8) | 2.5 (0.7) | 2.6 (0.8) | 2.6 (0.8) | 2.4 (0.7) | 2.5 (0.7) | 0.01 (–0.06 to 0.09) | –0.02 (–0.08 to 0.04) | –0.03 (–0.09 to 0.02) |
| FVC (l), mean (SD) | 3.5 (0.9) | 3.3 (0.9) | 3.4 (0.9) | 3.6 (1.0) | 3.2 (0.8) | 3.4 (0.9) | 0.01 (–0.11 to 0.13) | –0.01 (–0.09 to 0.09) | –0.01 (–0.10 to 0.09) |
| FEV1/FVC ratio, mean (SD) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.7 (0.1) | 0.8 (0.1) | 0.003 (–0.02 to 0.02) | –0.02 (–0.02 to 0.02) | –0.005 (–0.02 to 0.01) |
| FEV1% predicted, mean (SD) | 90.5 (18.8) | 88.8 (18.1) | 91.9 (21.6) | 90.5 (19.2) | 89.5 (19.5) | 91.9 (17.4) | –2.05 (–5.78 to 1.68) | –2.02 (–4.9 to 0.89) | 0.03 (–2.89 to 2.96) |
| PEFR (l/second), mean (SD) | 425.5 (115.7) | 414.9 (110.0) | 423.4 (120.7) | 422.7 (122.3) | 400.1 (114.7) | 415.1 (117.2) | 4.49 (–11.15 to 20.13) | 2.32 (–11.36 to 15.99) | –2.17 (–15.54 to 11.19) |
| FeNO (p.p.b.), median (IQR) | 21 (14–35) | 23 (15–33) | 23 (14–34) | 20 (13–33) | 21 (13–32) | 20 (13–31) | Unadjusted median difference, p-value: | ||
| Z = –1.09, r = –0.07;a p = 0.28 | Z = –2.412, r = –0.14;a p = 0.02 | Z = –0.941, r = –0.06;a p = 0.35 | |||||||
| PP population | |||||||||
| n | 215 | 110 | 231 | 134 | 83 | 180 | |||
| FEV1 (l), mean (SD) | 2.6 (0.8) | 2.4 (0.7) | 2.6 (0.8) | 2.6 (0.8) | 2.4 (0.7) | 2.5 (0.7) | –0.003 (–0.08 to 0.08) | –0.03 (–0.001 to 0.07) | –0.02 (–0.08 to 0.03) |
| FVC (l), mean (SD) | 3.5 (0.9) | 3.2 (0.8) | 3.4 (0.9) | 3.5 (1.0) | 3.3 (0.8) | 3.4 (0.9) | –0.004 (–0.13 to 0.13) | –0.02 (–0.13 to 0.09) | –0.01 (–0.1 to 0.09) |
| FEV1/FVC ratio, mean (SD) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.7 (0.1) | 0.8 (0.1) | –0.0004 (–0.02 to 0.02) | –0.001 (–0.02 to 0.02) | –0.001 (–0.02 to 0.02) |
| FEV1% predicted, mean (SD) | 90.3 (19.3) | 87.6 (17.9) | 92.2 (21.8) | 90.5 (16.6) | 89.8 (18.9) | 91.6 (17.3) | –3.29 (–7.16 to 0.57) | –2.39 (–5.45 to 0.66) | 0.90 (–1.99 to 3.79) |
| PEFR (l/second), mean (SD) | 422.5 (118.8) | 410.5 (108.1) | 421.9 (120.4) | 419.6 (121.4) | 401.3 (110.2) | 413.3 (118.0) | 3.0 (–13.56 to 19.57) | 3.3 (–11.05 to 17.71) | 0.33 (–13.67 to 14.33) |
| FeNO (p.p.b.), median (IQR) | 21 (15–35) | 22 (15–34) | 22.5 (14–34.5) | 19 (13–33) | 21 (13–32) | 20 (13–31) | Unadjusted median difference, p-value: | ||
| Z = –0.85, r = –0.05;a p = 0.40 | Z = –2.12, r = –0.13;a p = 0.03 | Z = –0.91, r = –0.06;a p = 0.36 | |||||||
We observed no significant changes in the within-group mean scores (geometric mean for FeNO readings) between baseline and 12 months for any of these parameters. In the unadjusted analyses there was a small but statistically significant difference in the change in median FeNO score from baseline to 12 months in the ITT population in the DVD group (from 21 to 20) compared with the control group (from 23 to 20; p = 0.03), but this difference was not seen in the primary (i.e. adjusted for covariates) analysis. None of the lung function parameters changed significantly from baseline in any of the treatment arms in the ITT and PP populations.
These findings suggest that the improvements in QoL seen in our primary outcome measure (AQLQ score) were not mediated by changes in the pathophysiology of asthma; this will be considered in Chapter 7.
Patient-reported outcome measures (questionnaires)
We included a number of validated questionnaires as secondary outcome measures: the ACQ (measuring asthma symptoms), the Nijmegen questionnaire (measuring symptoms related to hyperventilation and dysfunctional breathing) and the HADS, with separate domains measuring anxiety and depression. Our main analysis was a comparison of the mean score changes between baseline and 12 months in the ITT population between the study arms, with adjustments for prespecified covariates (Table 20). In secondary analyses we compared mean score changes between baseline and 12 months in the PP population between study arms, with adjustments for prespecified covariates (see Table 16) and unadjusted changes in questionnaire scores in the ITT population and the PP population (Table 21).
| Questionnaire | ITT population | PP population | ||||
|---|---|---|---|---|---|---|
| Adjusted mean differencea (95% CI) | Adjusted mean differencea (95% CI) | |||||
| Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | |
| ACQ | –0.06 (–0.23 to 0.13) | –0.09 (–0.25 to 0.06) | –0.04 (–0.23 to 0.15) | –0.04 (–0.22 to 0.15) | –0.05 (–0.21 to 0.11) | –0.01 (–0.20 to 0.19) |
| HADS | ||||||
| Anxiety | –0.04 (–0.73 to 0.64) | –0.22 (–0.81 to 0.38) | –0.18 (–0.89 to 0.54) | 0.03 (–0.71 to 0.75) | –0.16 (–0.79 to 0.47) | –0.17 (–0.94 to 0.58) |
| Depression | –0.55 (–1.14 to 0.04) | –0.56 (–1.07 to –0.05)* | –0.01 (–0.63 to 0.60) | –0.58 (–1.19 to 0.04) | –0.56 (–1.10 to –0.03)* | 0.02 (–0.62 to 0.66) |
| Nijmegen questionnaire | 1.28 (–0.55 to 3.12) | 0.90 (–0.71 to 2.51) | –0.38 (–2.30 to 1.55) | 1.41 (–0.50 to 3.32) | 0.99 (–0.67 to 2.65) | –0.42 (–2.42 to 1.58) |
| Questionnaire | Time point | Unadjusted mean difference (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | ||||||||
| DVD | Physiotherapy | Usual care | DVD | Physiotherapy | Usual care | Physiotherapy vs. usual care | DVD vs. usual care | DVD vs. physiotherapy | |
| ITT population | |||||||||
| n | 260 | 132 | 262 | 159 | 96 | 194 | |||
| ACQ, mean (SD) | 1.5 (0.9) | 1.6 (0.8) | 1.5 (0.9) | 1.2 (0.9) | 1.3 (0.8) | 1.2 (0.8) | –0.15 (–0.3 to 0.04) | –0.09 (–0.27 to 0.08) | 0.06 (–0.13 to 0.26) |
| Nijmegen questionnaire, mean (SD) | 19.0 (8.8) | 18.9 (10.5) | 19.4 (9.4) | 16.9 (9.4) | 17.1 (9.8) | 18.3 (9.3) | 0.87 (–0.7 to 2.5) | 0.8 (–0.5 to 2.2) | –0.03 (–1.6 to 1.5) |
| HADS | |||||||||
| Anxiety | |||||||||
| Median (IQR) | 7 (4–8) | 6 (4–9) | 6 (4–9) | 5 (3–8) | 6 (3–8) | 6 (3–9) | Unadjusted median difference, p-value: | ||
| Minimum, maximum | 0, 19 | 0, 20 | 0, 20 | 0, 16 | 0, 18 | 0, 18.7 | Z = –0.04, r = –0.0021; p = 0.96 | Z = –0.66, r = –0.041; p = 0.5 | Z = –0.50, r = –0.031; p = 0.62 |
| Depression | |||||||||
| Median (IQR) | 3 (1–5) | 2 (1–5) | 3 (1–5) | 2 (1–5) | 3 (1–4) | 3 (1–5) | Unadjusted median difference, p-value: | ||
| Minimum, maximum | 0, 17 | 0, 17 | 0, 14 | 0, 13 | 0, 17 | 0, 16 | Z = –1.92, r = –0.11;a p = 0.6 | Z = –2.94,* r = –0.161;a p = 0.03 | Z = –0.65, r = –0.041;a p = 0.52 |
| PP population | |||||||||
| n | 215 | 110 | 231 | 147 | 91 | 193 | |||
| ACQ, mean (SD) | 1.5 (0.8) | 1.6 (0.8) | 1.5 (0.9) | 1.2 (0.9) | 1.3 (0.8) | 1.3 (0.8) | 0.13 (–0.07 to 0.34) | 0.05 (–0.13 to 0.24) | –0.08 (–0.28 to 0.12) |
| Nijmegen questionnaire, mean (SD) | 18.6 (8.7) | 18.7 (10.5) | 19.6 (9.2) | 16.8 (9.5) | 17.1 (9.5) | 18.7 (9.2) | 0.96 (–0.74 to 2.66) | 0.81 (–0.61 to 2.22) | –0.15 (–1.77 to 1.47) |
| HADS | |||||||||
| Anxiety | |||||||||
| Median (IQR) | 6 (4–8) | 6 (4–9) | 6 (4–9) | 5 (3–9) | 6 (3–8) | 6 (4–9) | Unadjusted median difference, p-value: | ||
| Minimum, maximum | 0, 19 | 0, 18 | 0, 18 | 0, 16 | 0, 18 | 0, 19 | Z = –0.12, r = –0.01;a p = 0.91 | Z = –0.37, r = –0.02;a p = 0.7 | Z = –0.28, r = –0.02;a p = 0.78 |
| Depression | |||||||||
| Median (IQR) | 3 (1–5) | 2 (1–5) | 3 (1–4) | 2 (1–5) | 3 (1–5) | 3 (1–6) | Unadjusted median difference, p-value: | ||
| Minimum, maximum | 0, 17 | 0, 17 | 0, 14 | 0, 13 | 0, 17 | 0, 16 | Z = –2.07, r = –0.001;a p = 0.04 | Z = –2.7, r = –0.15;a p = 0.01 | Z = –0.23, r = –0.02;a p = 0.82 |
We found no significant changes in asthma symptom control, anxiety scores or Nijmegen questionnaire scores in the active intervention groups compared with the control group in either the ITT or the PP population. We did observe a small magnitude but statistically significant improvement in depression scores in the DVD treatment arm compared with the control arm, with a similar magnitude but statistically non-significant difference in the physiotherapy arm compared with the control arm. However, the baseline depression scores were low and few participants met ‘caseness’ criteria for depression, with depression scores being lower than anxiety scores in the study population. This suggests that depression was not a significant factor in these patients and that the low magnitude changes in depression scores are unlikely to explain the improvements in QoL observed in the active treatment arms. We did observe within-group changes from baseline of 0.2–0.3 units in the mean ACQ score (with a change of 0.5 signifying a clinically important change for an individual patient), indicating that there were modest improvements in the asthma symptoms experienced in all treatment arms. Similarly, although there were improvements in anxiety and Nijmegen questionnaire scores between baseline and 12 months in each group, there was no significant between-group difference in these parameters, indicating that the active interventions did not significantly improve anxiety or hyperventilation-related symptoms and that the mechanism responsible for the improved disease-specific QoL scores in the active treatment groups was not related to improvements in these factors.
Asthma exacerbations
Asthma exacerbations (attacks) were assessed [using the European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force on asthma outcomes recommendations for defining an exacerbation33] according to prescribed short courses of oral corticosteroids for worsening of asthma by the usual physician, with data obtained from the manual and electronic reviews of the patient medical record at 12 months. In keeping with the mild asthma population recruited, only 12% of the ITT population (13% of the PP population) had one or more asthma attack over the 12-month period (Table 22). The percentage of patients in the ITT population randomisation treatment arms (DVD, physiotherapy, control) having one or more asthma attack was 9%, 11% and 15%, respectively, and in the PP population was 10%, 12% and 16% respectively (Table 23). There was no statistically significant difference in the crude exacerbation rate between the DVD arm and the physiotherapy arm (p = 0.6 in the ITT population, p = 0.8 in the PP population; Table 24) or between the physiotherapy arm and the control arm (p = 0.4 for both populations; Table 25). The DVD group narrowly failed to reach a statistically significant reduction in exacerbations compared with the control group, with a p-value of 0.06 in the ITT population and 0.12 in the PP population) (Table 26).
| Oral corticosteroid courses | ITT population, n (%) | PP population, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | Total (N = 655) | DVD (N = 215) | Physiotherapy (N = 110) | Usual care (N = 231) | Total (N = 556) | |
| None | 237 (90.8) | 117 (88.6) | 223 (85.1) | 577 (88.1) | 193 (89.8) | 97 (88.2) | 195 (84.4) | 485 (87.2) |
| 1 | 17 (6.5) | 10 (7.6) | 26 (9.9) | 53 (8.1) | 16 (7.4) | 9 (8.2) | 26 (11.3) | 51 (9.2) |
| 2 | 2 (0.8) | 4 (3.0) | 10 (3.8) | 16 (2.4) | 2 (0.9) | 3 (2.7) | 8 (3.5) | 13 (2.3) |
| 3 | 2 (0.8) | 0 (0.0) | 1 (0.4) | 3 (0.5) | 2 (0.9) | 0 (0.0) | 1 (0.4) | 3 (0.5) |
| ≥ 4 | 3 (1.1) | 1 (0.8) | 2 (0.8) | 6 (0.9) | 2 (0.9) | 1 (0.9) | 1 (0.4) | 4 (0.7) |
| Total | 261 (100.0) | 132 (100.0) | 262 (100.0) | 655 (100.0) | 215 (100.0) | 110 (100.0) | 231 (100.0) | 556 (100.0) |
| Oral corticosteroid courses | ITT population, n (%) | PP population, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | Total (N = 655) | DVD (N = 215) | Physiotherapy (N = 110) | Usual care (N = 231) | Total (N = 556) | |
| None | 237 (90.8) | 117 (88.6) | 223 (85.1) | 577 (88.1) | 193 (89.8) | 97 (88.2) | 195 (84.4) | 485 (87.2) |
| ≥ 1 | 24 (9.2) | 15 (11.4) | 39 (14.9) | 78 (11.9) | 22 (10.2) | 13 (11.8) | 36 (15.6) | 71 (12.8) |
| Total | 261 (100.0) | 132 (100.0) | 262 (100.0) | 655 (100.0) | 215 (100.0) | 110 (100.0) | 231 (100.0) | 556 (100.0) |
| Oral corticosteroid courses | ITT population, n | PP population, n | ||||
|---|---|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | p-value | DVD (N = 215) | Physiotherapy (N = 110) | p-value | |
| None | 237 | 117 | 0.62 | 193 | 97 | 0.81 |
| ≥ 1 | 24 | 15 | 22 | 13 | ||
| Total | 261 | 132 | 215 | 110 | ||
| Oral corticosteroid courses | ITT population, n | PP population, n | ||||
|---|---|---|---|---|---|---|
| Physiotherapy (N = 132) | Usual care (N = 262) | p-value | Physiotherapy (N = 110) | Usual care (N = 231) | p-value | |
| None | 117 | 223 | 0.42 | 97 | 195 | 0.45 |
| ≥ 1 | 15 | 39 | 13 | 36 | ||
| Total | 132 | 262 | 110 | 231 | ||
| Oral corticosteroid courses | ITT population, n | PP population, n | ||||
|---|---|---|---|---|---|---|
| DVD (N = 261) | Usual care (N = 262) | p-value | DVD (N = 215) | Usual care (N = 231) | p-value | |
| None | 237 | 223 | 0.06 | 193 | 195 | 0.12 |
| ≥ 1 | 24 | 39 | 22 | 36 | ||
| Total | 261 | 132 | 215 | 231 | ||
A negative binomial regression model was constructed to assess the between-group differences in asthma exacerbation frequency in the ITT (Tables 27–29 and Figure 2) and PP (Figure 3 and see Appendix 6) populations, adjusting for differences in exacerbation frequency at baseline and for covariates (age, sex, BTS treatment step and smoking status). In the adjusted analysis for the ITT population, the risk of exacerbations was lower in the DVD arm than in the usual-care arm [incidence rate ratio (IRR) 0.68), but the 95% CI crossed the line of unity (95% CI 0.34 to 1.38) and so this finding was not statistically significant and could have occurred through chance. The analysis of the physiotherapy arm compared with the usual-care arm produced a similar result, with a (lower magnitude) non-significant reduction in the risk of an asthma attack in the physiotherapy arm (IRR 0.85, 95% CI 0.36 to 1.98). There was also no significant difference in the risk of an exacerbation in the DVD group compared with the physiotherapy group, but the DVD group was favoured (IRR 0.81, 95% CI 0.33 to 1.99). The unadjusted negative binomial regression model produced very similar results, as did the adjusted and unadjusted models in the PP population. Our study was not powered to show an effect on exacerbations and, as the annual exacerbation rate is modest in patients with mild and moderate asthma treated in the community, a larger sample would be needed to show a statistically significant reduction in exacerbations. This will be considered further in Chapter 7.
| Oral corticosteroid courses | Treatment arm, n | Total, n | ||
|---|---|---|---|---|
| DVD | Physiotherapy | Usual care | ||
| 0 | 237 | 117 | 223 | 577 |
| 1 | 17 | 10 | 26 | 53 |
| 2 | 2 | 4 | 10 | 16 |
| 3 | 2 | 0 | 1 | 3 |
| 4 | 2 | 1 | 1 | 4 |
| 6 | 1 | 0 | 0 | 1 |
| 10 | 0 | 0 | 1 | 1 |
| Total | 261 | 132 | 262 | 655 |
| Comparison | Unadjusted IRR | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 0.69 | 0.34 to 1.41 | 0.31 |
| DVD vs. usual care | 0.65 | 0.37 to 1.16 | 0.15 |
| DVD vs. physiotherapy | 0.94 | 0.45 to 1.96 | 0.87 |
| Comparison | Adjusted IRRa | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 0.85 | 0.36 to 1.98 | 0.89 |
| DVD vs. usual care | 0.68 | 0.34 to 1.38 | 0.42 |
| DVD vs. physiotherapy | 0.81 | 0.33 to 1.99 | 0.84 |
FIGURE 2.
Frequency of one or more exacerbations in the 12 months post randomisation by treatment arm in the ITT population. OC, oral corticosteroid.
FIGURE 3.
Frequency of one or more exacerbations in the 12 months post randomisation by treatment arm in the PP population. OC, oral corticosteroid.
Bronchodilator use
In the 12 months following the baseline assessment, 177 of 261 (67.8%) participants randomised to the DVD arm required one or more prescriptions for rescue bronchodilators compared with 104 of 132 (78.8%) in the physiotherapy arm and 206 of 262 (78.6%) in the control arm. The number of inhalers prescribed over the 12 months post randomisation by treatment arm in the ITT population is shown in Table 30 and the proportions of participants having each number of inhalers are shown graphically in Figure 4. A negative binomial regression model was constructed for the ITT population to estimate the difference in rescue bronchodilator use between arms. In the unadjusted analysis (Table 31) there was a non-significant trend for lower bronchodilator use in the DVD arm compared with the usual-care arm (IRR 0.83, 95% CI 0.68 to 1.03; p = 0.09) and for the DVD arm compared with the physiotherapy arm (IRR 0.81, 95% CI 0.63 to 1.04; p = 0.10), with little difference between physiotherapy arm and the usual-care arm (IRR 1.03, 95% CI 0.81 to 1.33; p = 0.80). In the regression model adjusted for covariates (age, sex, BTS treatment step, baseline smoking status, baseline HADS scores and baseline Nijmegen questionnaire score; Table 32), a similar pattern was seen, with the adjusted IRR being 0.91 (95% CI 0.72 to 1.15; p = 0.61) for the DVD arm compared with the control arm, 0.88 (95% CI 0.66 to 1.15; p = 0.50) for the DVD arm compared with the physiotherapy arm and 1.03 (95% CI 0.79 to 1.37; p = 0.93) for the physiotherapy arm compared with the usual-care arm. The same analyses were performed on the PP population, which also found no significant differences in rescue bronchodilator use between randomisation treatment arms in the adjusted or the unadjusted analysis (Figure 5 and see Appendix 5).
| Number of rescue inhalers | Treatment arm, n | Total, n | ||
|---|---|---|---|---|
| DVD | Physiotherapy | Usual care | ||
| 0 | 84 | 28 | 56 | 168 |
| 1 | 36 | 26 | 61 | 123 |
| 2 | 39 | 16 | 32 | 87 |
| 3 | 20 | 18 | 26 | 64 |
| 4 | 23 | 14 | 24 | 61 |
| 5 | 17 | 4 | 12 | 33 |
| 6 | 12 | 6 | 11 | 29 |
| 7 | 6 | 4 | 5 | 15 |
| 8 | 6 | 3 | 11 | 20 |
| 9 | 5 | 2 | 3 | 10 |
| 10 | 5 | 2 | 6 | 13 |
| 11 | 2 | 1 | 2 | 5 |
| 12 | 1 | 3 | 5 | 9 |
| 13 | 1 | 2 | 0 | 3 |
| 14 | 2 | 2 | 0 | 4 |
| 16 | 1 | 0 | 1 | 2 |
| 17 | 0 | 0 | 1 | 1 |
| 18 | 0 | 0 | 3 | 3 |
| 22 | 0 | 0 | 2 | 2 |
| 26 | 0 | 0 | 1 | 1 |
| 28 | 1 | 0 | 0 | 1 |
| 40 | 0 | 1 | 0 | 1 |
| Total | 261 | 132 | 262 | 655 |
FIGURE 4.
Distribution of rescue bronchodilator prescriptions in the 12 months post randomisation by treatment arm in the ITT population.

| Comparison | Unadjusted IRR | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 1.03 | 0.81 to 1.33 | 0.80 |
| DVD vs. usual care | 0.83 | 0.68 to 1.03 | 0.09 |
| DVD vs. physiotherapy | 0.81 | 0.63 to 1.04 | 0.10 |
| Comparison | Adjusted IRRa | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 1.03 | 0.79 to 1.37 | 0.93 |
| DVD vs. usual care | 0.91 | 0.72 to 1.15 | 0.61 |
| DVD vs. physiotherapy | 0.88 | 0.66 to 1.15 | 0.50 |
FIGURE 5.
Distribution of rescue bronchodilator prescriptions in the 12 months post randomisation by treatment arm in the PP population.

Respiratory-related general practitioner consultations
In the 12 months following randomisation, the number of times that each patient saw a general practitioner (GP) for a respiratory-related reason varied between none and 21 (Table 33). In total, 71.2% of the DVD group, 75.1% of the physiotherapy group and 76.7% of the control group had one or more GP consultation. Unadjusted and adjusted negative binomial regression models were constructed to estimate the difference between treatment arms in GP consultation rates. In both adjusted and unadjusted analyses of the ITT population (Tables 34 and 35), there were non-significant trends for lower consultation rates in the active arms than in the control arm, with minimal differences between the active arms (DS vs. control: unadjusted IRR 0.87, 95% CI 0.72 to 1.05; adjusted IRR 0.93, 95% CI 0.74 to 1.15; physiotherapy vs. control: unadjusted IRR 0.90, 95% CI 0.72 to 1.12; adjusted IRR 0.94, 95% CI 0.72 to 1.24; DVD vs. physiotherapy: unadjusted IRR 0.97, 95% CI 0.77 to 1.22; adjusted IRR 0.98, 95% CI 0.75 to 1.29). Similar results were found for the PP population (Tables 36 and 37 and see Appendix 7).
| Number of GP consultations | Treatment arm, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| DVD | Physiotherapy | Usual care | ||
| 0 | 75 (44.4) | 33 (19.5) | 61 (36.1) | 169 (100.0) |
| 1 | 103 (39.6) | 55 (21.2) | 102 (39.2) | 260 (100.0) |
| 2 | 42 (39.6) | 24 (22.6) | 40 (37.7) | 106 (100.0) |
| 3 | 19 (38.8) | 8 (16.3) | 22 (44.9) | 49 (100.0) |
| 4 | 8 (32.0) | 3 (12.0) | 14 (56.0) | 25 (100.0) |
| 5 | 4 (17.4) | 6 (26.1) | 13 (56.5) | 23 (100.0) |
| 6 | 4 (40.0) | 1 (10.0) | 5 (50.0) | 10 (100.0) |
| 7 | 2 (50.0) | 0 (0.0) | 2 (50.0) | 4 (100.0) |
| 8 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| 9 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| 11 | 1 (33.3) | 1 (33.3) | 1 (33.3) | 3 (100.0) |
| 12 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| 15 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| 16 | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| 21 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Total | 261 (39.8) | 132 (20.2) | 262 (40.0) | 655 (100.0) |
| Comparison | Unadjusted IRR | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 0.90 | 0.72 to 1.12 | 0.35 |
| DVD vs. usual care | 0.87 | 0.72 to 1.05 | 0.14 |
| DVD vs. physiotherapy | 0.97 | 0.77 to 1.22 | 0.78 |
| Comparison | Adjusted IRRa | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 0.94 | 0.72 to 1.24 | 0.87 |
| DVD vs. usual care | 0.93 | 0.74 to 1.15 | 0.69 |
| DVD vs. physiotherapy | 0.98 | 0.75 to 1.29 | 0.95 |
| Comparison | Unadjusted IRR | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 0.90 | 0.71 to 1.14 | 0.37 |
| DVD vs. usual care | 0.85 | 0.71 to 1.04 | 0.11 |
| DVD vs. physiotherapy | 0.95 | 0.75 to 1.21 | 0.68 |
| Comparison | Adjusted IRRa | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 0.93 | 0.71 to 1.22 | 0.84 |
| DVD vs. usual care | 0.88 | 0.69 to 1.10 | 0.35 |
| DVD vs. physiotherapy | 0.96 | 0.72 to 1.24 | 0.84 |
It is not possible to determine whether the interventions reduced the need for GP consultations, as the study was underpowered to test this hypothesis, but there is a suggestion that there may have been some reduction in consultation rates (by approximately one-tenth in this sample).
Overall health resource use
The analysis of health resource use is considered in detail in Chapter 4.
Overall health resource use was assessed through direct medical costs. The mean NHS cost per patient by arm was aggregated from costs of prescriptions, consultations and hospital admissions. Although there were few hospital admissions (usual-care arm, n = 8; physiotherapy arm, n = 0; DVD arm, n = 4), they were by far the most costly item. The main cost items for each group were asthma-related medications and GP consultations. The mean cost per patient was highest in the control group (£356), with a similar cost in the physiotherapy group (£355) and the lowest cost in the DVD group (£296).
Patient engagement with the intervention in the active treatment arms
Patient engagement was assessed as part of our prespecified analysis plan; the data are presented in Tables 38 and 39. Engagement was good in the physiotherapy arm, with 95% of participants attending at least one of the three scheduled sessions and 93% attending all three. Patient experience of the different components of the intervention was generally favourable and the majority of patients spent a considerable amount of time practising the various techniques taught, with over half spending half a day or more practising each technique. The main factor hindering the practising of techniques was practical problems, for example finding time.
| Measure | Physiotherapy arm (N = 132) |
|---|---|
| Number of sessions attended, n (%) | |
| n | 132 |
| None | 6 (4.5) |
| At least one | 126 (95.5) |
| At least two | 123 (93.2) |
| At least three | 123 (93.2) |
| Treatment experience scorea | |
| n | 104 |
| Stomach breathing, median (IQR); minimum, maximum | 8 (6–9); 3, 10 |
| Nose breathing, median (IQR); minimum, maximum | 8 (6–9); 3, 10 |
| Slow breathing, median (IQR); minimum, maximum | 7 (5–8); 1, 10 |
| Controlled breath holding, median (IQR); minimum, maximum | 5 (4–7); 1, 10 |
| Relaxation training, median (IQR); minimum, maximum | 8 (6–9); 1, 10 |
| Total time spent on each breathing technique,b n (%)c | |
| Stomach breathing | |
| Weeks – did not use | 1 (1.0) |
| 1–5 weeks | 20 (19.0) |
| ≥ 6 weeks | 79 (75.2) |
| Hours – did not use | 1 (1.0) |
| Up to 1 hour | 44 (41.9) |
| Up to half a day or more | 54 (51.4) |
| Nose breathing | |
| Weeks – did not use | 1 (1.0) |
| 1–5 weeks | 20 (19.0) |
| ≥ 6 weeks | 80 (76.2) |
| Hours – did not use | 3 (2.9) |
| Up to 1 hour | 33 (31.4) |
| Up to half a day or more | 65 (61.9) |
| Slow breathing | |
| Weeks – did not use | 2 (1.9) |
| 1–5 weeks | 32 (30.5) |
| ≥ 6 weeks | 67 (63.8) |
| Minutes – did not use | 3 (2.9) |
| Up to 10 minutes | 54 (51.4) |
| > 10 minutes | 44 (41.9) |
| Controlled breath holding | |
| Weeks – did not use | 10 (9.5) |
| 1–5 weeks | 38 (36.2) |
| ≥ 6 weeks | 53 (50.5) |
| Minutes – did not use | 12 (11.4) |
| Up to 5 minutes | 64 (61.0) |
| > 5 minutes | 25 (23.8) |
| Relaxation training | |
| Weeks – did not use | 13 (12.4) |
| 1–5 weeks | 38 (36.2) |
| ≥ 6 weeks | 50 (47.6) |
| Minutes – did not use | 15 (14.3) |
| Up to 10 minutes | 69 (65.7) |
| > 10 minutes | 17 (16.2) |
| Treatment engagement score | |
| n | 104 |
| Problems due to symptoms, median (IQR); minimum, maximum | 0 (0–0); 0, 12 |
| Problems due to uncertainty about the therapy, median (IQR); minimum, maximum | 0 (0–0); 0, 12 |
| Problems due to doubts about the therapy, median (IQR); minimum, maximum | 0 (0–0); 0, 12 |
| Practical problems, median (IQR); minimum, maximum | 4.5 (1–9); 0, 20 |
| Problems due to lack of support, median (IQR); minimum, maximum | 0 (0–0); 0, 12 |
| Measure | DVD arm (N = 244) |
|---|---|
| Treatment experience scorea | |
| n | 160 |
| Stomach breathing, median (IQR); minimum, maximum | 6 (5–8); 1, 10 |
| Nose breathing, median (IQR); minimum, maximum | 6 (5–8); 1, 10 |
| Slow breathing, median (IQR); minimum, maximum | 6 (5–8); 1, 10 |
| Controlled breath holding, median (IQR); minimum, maximum | 5 (4–7); 1, 10 |
| Relaxation training, median (IQR); minimum, maximum | 7 (6–8); 1, 10 |
| Total time spent on each breathing technique,b n (%)c | |
| Stomach breathing | |
| Weeks – did not use | 10 (4.1) |
| 1–5 weeks | 59 (24.2) |
| ≥ 6 weeks | 90 (36.9) |
| Hours – did not use | 12 (4.9) |
| Up to 1 hour | 104 (42.6) |
| Up to half a day or more | 41 (16.8) |
| Nose breathing | |
| Weeks – did not use | 12 (4.9) |
| 1–5 weeks | 53 (21.7) |
| ≥ 6 weeks | 94 (38.5) |
| Hours – did not use | 13 (5.3) |
| Up to 1 hour | 75 (30.7) |
| Up to half a day or more | 69 (28.3) |
| Slow breathing | |
| Weeks – did not use | 11 (4.5) |
| 1–5 weeks | 64 (26.2) |
| ≥ 6 weeks | 85 (34.8) |
| Minutes – did not use | 11 (4.5) |
| Up to 10 minutes | 100 (41.0) |
| > 10 minutes | 48 (19.7) |
| Controlled breath holding | |
| Weeks – did not use | 19 (7.8) |
| 1–5 weeks | 74 (30.3) |
| ≥ 6 weeks | 67 (27.5) |
| Minutes – did not use | 20 (8.2) |
| Up to 5 minutes | 103 (42.2) |
| > 5 minutes | 36 (14.8) |
| Relaxation training | |
| Weeks – did not use | 25 (10.2) |
| 1–5 weeks | 59 (24.2) |
| ≥ 6 weeks | 76 (31.1) |
| Minutes – did not use | 24 (9.8) |
| Up to 10 minutes | 91 (37.3) |
| > 10 minutes | 45 (18.4) |
| Treatment engagement score | |
| n | 158 |
| Problems due to symptoms | 0 (0–2); 0, 12 |
| Problems due to uncertainty about the therapy | 0.5 (0–3); 0, 12 |
| Problems due to doubts about the therapy | 1.0 (0–4); 0, 10 |
| Practical problems | 6.5 (3–11); 0, 20 |
| Problems due to lack of support | 0 (0–3); 0, 12 |
Similarly, engagement was good in the DVD arm, although the overall engagement scores for the different components of the intervention were generally slightly lower than those in the physiotherapy arm and the time spent practising the various techniques was lower. Practical problems were again the main hindering factor, although some participants reported uncertainty or doubts over the instructions provided.
These findings are discussed further in Chapter 6.
As a prespecified analysis, we compared changes in the primary outcome (AQLQ) between the physiotherapy arm and the DVD arm, including the ‘amount of practice’ as a covariate and excluding participants who did not engage with the breathing retraining at 3 months in the physiotherapy arm, with data presented for the 3-month evaluation and the 12-month evaluation (see Appendix 8). This was carried out to assess whether engagement with the exercises was different between the active arms and whether this could be reflected in differences in primary outcome scores, as the physiotherapists suspected that some patients failed to engage and so had poor outcomes from the face-to-face training programmes, whereas those who did engage generally did well.
Compared with the primary efficacy analysis, this analysis including engagement and time spent practising increased the magnitude of the AQLQ changes in the physiotherapy arm at 3 months, but did not result in significant differences between the DVD arm and the physiotherapy arm, which continued to reach the equivalence margin at the 12-month evaluation, and did not change the message emerging from the study. These findings are discussed further in Chapter 6.
Adverse events
Adverse events were collected for all randomised patients by patients and study centre principal investigators (PIs) on adverse event report forms and by manual and electronic review of medical records at 12 months. Adverse events were categorised by PIs according to the classification scheme provided in Appendix 9.
Serious adverse events
The serious adverse event rate was low, with no study-related serious adverse events and an overall rate of 5%. The highest serious adverse event rate was in the control group (7.6%), with lower rates in the DVD group (4.2%) and the physiotherapy group (3%) (Table 40). A full list of all serious adverse events reported is provided in Appendix 10.
| Event | Treatment arm, n (%) | Total (N = 655), n (%) | ||
|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | ||
| SUSARs | None | |||
| Expected serious adverse reactions | None | |||
| Other serious adverse events | 11 (4.2) | 4 (3.0) | 20 (7.6) | 35 (5.3) |
Other adverse events
In total, 744 adverse events were reported from 272 patients (41.5% of the randomised population), with quantification by category and randomisation group provided in Table 41. Of the adverse events, 47% occurred in the control arm, 36% occurred in the DVD arm and 17% occurred in the physiotherapy arm (note that there was a 2 : 1 : 2 randomisation schedule between the DVD, physiotherapy and control arms and so the adverse event rate was very similar between the DVD arm and the physiotherapy arm). A summary of the adverse events reported is provided in Table 42. A full list of each event reported as well as severity gradings is provided in Appendix 11.
| Categorya | Treatment arm, n (%) | Total (N = 655), n (%) | ||
|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | ||
| Abdominal/GIT | 19 (35.2) | 5 (9.3) | 30 (55.6) | 54 (100.0) |
| Acute exacerbation of asthma | 32 (45.1) | 13 (18.3) | 26 (36.6) | 71 (100.0) |
| Chest pain | 24 (41.4) | 7 (12.1) | 27 (46.6) | 58 (100.0) |
| Increased asthma symptoms | 29 (29.9) | 28 (28.9) | 40 (41.2) | 97 (100.0) |
| Malignancy | 3 (75.0) | 1 (25.0) | 0 (0.0) | 4 (100.0) |
| Musculoskeletal | 22 (35.5) | 15 (24.2) | 25 (40.3) | 62 (100.0) |
| Neurological | 8 (33.3) | 3 (12.5) | 13 (54.2) | 24 (100.0) |
| Psychological/psychiatric | 12 (28.6) | 2 (4.8) | 28 (66.7) | 42 (100.0) |
| Respiratory tract infection/cough | 79 (35.1) | 31 (13.8) | 115 (51.1) | 225 (100.0) |
| Rhinitis/rhinosinusitis | 20 (43.5) | 6 (13.0) | 20 (43.5) | 46 (100.0) |
| Miscellaneous | 23 (37.7) | 12 (19.7) | 26 (42.6) | 61 (100.0) |
| Total | 271 (36.4) | 123 (16.5) | 350 (47.0) | 744 (100.0) |
| Characteristic | Treatment arm | Total (N = 655) | ||
|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | ||
| Patients experiencing at least one AE,a n (%) | 101 (38.7) | 55 (41.7) | 132 (50.4) | 288 (44.0) |
| PI assessment | ||||
| Number of AEs | 271 | 123 | 350 | 744 |
| Number pending | 0 | 0 | 0 | 0 |
| Total number | 271 | 123 | 350 | 744 |
| Severity of AE, n (%)b | ||||
| 1 – mild | 129 (47.6) | 66 (53.7) | 155 (44.3) | 350 (47.0) |
| 2 – moderate | 112 (41.3) | 44 (35.8) | 141 (40.3) | 297 (39.9) |
| 3 – severe | 25 (9.2) | 13 (10.6) | 50 (14.3) | 88 (11.8) |
| 4 – life threatening | 4 (1.5) | 0 (0.0) | 2 (0.6) | 6 (0.8) |
| 5 – death related to AE | 1 (0.4) | 0 (0.0) | 2 (0.6) | 3 (0.4) |
| Pending | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 271 (100.0) | 123 (100.0) | 350 (100.0) | 744 (100.0) |
Overall, the adverse event profile was as expected in the recruited population, with fewer adverse events in the active arms than in the control arm. There was no indication that any of the adverse events were related to either the DVD programme or the physiotherapy programme, which appeared to be well tolerated.
Chapter 4 Economic evaluation
Introduction
The health economic analysis evaluated the superiority of each intervention compared with usual care and made a non-inferiority comparison between the DVD arm and the face-to face physiotherapy arm. The ITT population was employed, with imputation of missing values. The time frame was that of the trial. Adjustment was made for baseline values and for repeated measurement. The perspective was that of the NHS, but with exploration of non-NHS costs.
Data collected
Resource use data were collected using three forms. The medication review form was used to record data on all asthma-related NHS consultations and prescriptions over the period of the trial, collected from patient records held by each general practice. It was also used to record outpatient visits, accident and emergency (A&E) visits, out-of-hours consultations and hospital admissions related to asthma. A concomitant medications form was used to record data on six conditions that might be linked to differences in outcomes. A third form was used to record data from patients relating to over-the-counter medications, non-NHS consultations and any other privately accessed items related to asthma or its palliation.
The results presented here relate only to the medication review data. The data on concomitant medications proved unusable for several reasons, principally because of lack of detail provided on relevant medications and dates. The data on patient-reported costs also proved of little use as very few items were recorded. This we interpret as indicating that few such costs were incurred; hence, this justified taking a NHS perspective as opposed to a societal perspective.
Intervention costs
Intervention costs (shown in Appendix 12) were £83.45 per patient in the face-to-face physiotherapy breathing retraining group and £2.85 in the DVD breathing retraining group. The physiotherapy group cost was based on the duration of the planned three consultations with the practice nurse, adjusted for attendance. The cost of the DVD intervention was that of printing the discs and documentation. The development cost of these materials was treated as a research cost and was not included here.
Resource use and costs
The unit costs for each of the resource groups (GP consultations, outpatient visits, A&E attendances, out-of-hours consultations, inpatient admissions) and sources are shown in Appendix 12.
EuroQol-5 Dimensions and Asthma Quality of Life Questionnaire
Asthma Quality of Life Questionnaire data collection was discussed earlier (see Chapter 3). EQ-5D data were collected at the initial visit at baseline and at 3 and 6 months (both by post) and 12 months (assessment visit).
Analyses
Cost–utility analyses were based on all patients randomised. Utility scores were derived based on the UK tariff. 34 QALYs were calculated based on the area under the curve approach. The time frame of the analysis was that of the trial (12 months).
Bootstraps based on 1000 samples with replacements were used to estimate the cost per AQLQ score improvement and cost per QALY gained, with CIs presented to illustrate the uncertainty. Scatterplots are presented to illustrate the uncertainty in the cost–utility estimates.
Results
The medical review form was used to extract patient resource use data in the 12 months following randomisation. Three patients died and two patients left their practice. We assumed that no additional costs were incurred for these patients after the date of their last visit.
The mean NHS cost per patient by treatment arm was aggregated from the costs of asthma-related prescriptions, consultations and hospital admissions. The unadjusted mean cost was highest in the usual-care arm (£356; Table 43), with a similar cost in the physiotherapy arm (£335) and the lowest cost in the DVD arm (£296). The main cost items for each group were asthma-related medications and GP consultations, with lower costs reflecting low levels of use of other services; although there were few hospital admissions (usual-care arm, n = 8; physiotherapy arm, n = 0; DVD arm, n = 4), they were by far the most costly item. The numbers of patients using each service are also shown in Table 43.
| Resource use | Category | Mean (SD) for those using service | Mean (SD) for all |
|---|---|---|---|
| Usual care (N = 262) | |||
| Costs (£)a | Total costs | 379 (672) (n = 246) | 356 (657) |
| Medication | 183 (217) (n = 242) | 169 (214) | |
| GP consultation | 100 (84) (n = 201) | 77 (85) | |
| Outpatient attendance | 347 (321) (n = 21) | 28 (130) | |
| Hospital admission | 2581 (0) (n = 8) | 79 (445) | |
| Number of cases | Medication usage | 9.71 (10.14) (n = 242) | 8.97 (10.08) |
| GP consultation | 2.22 (1.87) (n = 201) | 1.71 (1.89) | |
| Outpatient attendance | 2.57 (2.38) (n = 21) | 0.21 (0.96) | |
| Hospital admission | 1 (0) (n = 8) | 0.03 (0.17) | |
| Physiotherapy (N = 132) | |||
| Costs (£)a | Total costs | 335 (254) (n = 132) | 335 (254) |
| Medication | 173 (199) (n = 120) | 157 (196) | |
| GP consultation | 92 (93) (n = 99) | 69 (90) | |
| Outpatient attendance | 203 (88) (n = 14) | 21 (68) | |
| Hospital admission | 0 (0) (n = 0) | 0 (0) | |
| Intervention | 83 (0) (n = 132) | 83 (0) | |
| Number of cases | Medication usage | 9.27 (7.49) (n = 120) | 8.42 (7.63) |
| GP consultation | 2.04 (2.07) (n = 99) | 1.53 (2) | |
| Outpatient attendance | 1.5 (0.65) (n = 14) | 0.16 (0.51) | |
| Hospital admission | 0 (0) (n = 0) | 0 (0) | |
| Intervention | 1 (0) (n = 132) | 1 (0) | |
| DVD (N = 261) | |||
| Costs (£)a | Total costs | 296 (715) (n = 261) | 296 (715) |
| Medication | 167 (177) (n = 222) | 142 (174) | |
| GP consultation | 94 (99) (n = 186) | 67 (94) | |
| Outpatient attendance | 221 (122) (n = 25) | 21 (75) | |
| Hospital admission | 3872 (2581) (n = 4) | 59 (551) | |
| Intervention | 3 (0) (n = 261) | 3 (0) | |
| Number of cases | Medication usage | 9.04 (8.03) (n = 222) | 7.69 (8.08) |
| GP consultation | 2.08 (2.2) (n = 186) | 1.48 (2.08) | |
| Outpatient attendance | 1.64 (0.91) (n = 25) | 0.16 (0.56) | |
| Hospital admission | 1.5 (1) (n = 4) | 0.02 (0.21) | |
| Intervention | 1 (0) (n = 261) | 1 (0) | |
The mean costs in each arm changed only slightly when bootstrap methods were used: £377 in the usual-care arm, £333 in the physiotherapy arm and £293 in the DVD arm (Table 44). The differences were not statistically significant at the 5% level.
| Treatment arm | Cost (£), mean (95% CI) | Incremental cost (£), mean (95% CI) |
|---|---|---|
| Usual care | 377 (310 to 459) | – |
| Physiotherapy | 333 (299 to 369) | –41 (–134 to 33) |
| DVD | 293 (228 to 374) | –83 (–187 to 12) |
Given that the intervention costs were higher in the physiotherapy arm (£83.5) and the DVD arm (£2.85) than in the usual-care arm, the inclusion of NHS costs offset these higher costs, leading to lower overall mean costs in both intervention arms than in the usual-care arm.
EuroQol-5 Dimensions data were collected at baseline (n = 653/655), 3 months (n = 519/655), 6 months (n = 472/655) and 12 months (n = 437/655) (see Table 45). For the whole sample, completion fell from an estimated 99% at baseline to 67% at 12 months. Some participants missed some but not all time points, but data for at least two time point were available for 87% of participants. Linear interpolation was used for those with two or three time points, with imputation for those with fewer time points.
| Time | EQ-5D score, mean (SD) | Number completing | % completion |
|---|---|---|---|
| Usual care (n = 262) | |||
| Baseline | 0.805 (0.236) | 261 | 100 |
| 3 months | 0.773 (0.269) | 222 | 85 |
| 6 months | 0.751 (0.301) | 212 | 81 |
| 12 months | 0.797 (0.246) | 191 | 73 |
| Physiotherapy (n = 132) | |||
| Baseline | 0.794 (0.269) | 130 | 98 |
| 3 months | 0.789 (0.275) | 105 | 80 |
| 6 months | 0.774 (0.272) | 98 | 74 |
| 12 months | 0.747 (0.318) | 93 | 70 |
| DVD (n = 261) | |||
| Baseline | 0.82 (0.235) | 258 | 99 |
| 3 months | 0.775 (0.275) | 167 | 64 |
| 6 months | 0.794 (0.269) | 162 | 62 |
| 12 months | 0.826 (0.221) | 153 | 59 |
The mean EQ-5D QoL scores varied between 0.751 and 0.826, with small differences at baseline and over time.
The mean EQ-5D QoL scores based on complete cases are shown in Table 46.
| Treatment arm | EQ-5D score, mean (95% CI) | Number of patients with complete data at all points | Percentage used in the calculation |
|---|---|---|---|
| Usual care (n = 262) | 0.801 (0.765 to 0.836) | 154 | 58.8 |
| Physiotherapy (n = 132) | 0.764 (0.696 to 0.831) | 70 | 53.0 |
| DVD (n = 261) | 0.817 (0.776 to 0.858) | 113 | 43.3 |
Cost-effectiveness analysis
The primary analysis of AQLQ score differences was based on the population with baseline measurement of AQLQ scores (n = 244, 120 and 246 in the DVD, physiotherapy and usual-care arms respectively; see Chapter 3). The cost-effectiveness analyses were based on the same population (n = 610 in total).
Bootstrapped mean costs per person in this population were £380 (95% CI £310 to £459), £296 (95% CI £228 to £374) and £334 (95% CI £299 to £269) for the DVD, physiotherapy and usual-care arms respectively. This gave a difference in costs of –£83 (95% CI –£187 to £12) for the DVD arm and –£45 (95% CI –£134 to £33) for the physiotherapy arm compared with the usual-care arm (Table 47).
| Treatment arm | Cost (£), mean (95% CI) | Difference in costs (£), mean (95% CI) | Difference in AQLQ score, mean (95% CI) | Incremental cost (£) per AQLQ score improvement (95% CI) |
|---|---|---|---|---|
| Usual care (n = 246) | 380 (310 to 459) | |||
| DVD (n = 244) | 296 (228 to 374) | |||
| Physiotherapy (n = 120) | 334 (299 to 269) | |||
| Physiotherapy vs. usual care | –45 (–134 to 33) | 0.23 (0.06 to 0.40) | –400 (–1545 to 106) | |
| DVD vs. usual care | –83 (–187 to 12) | 0.26 (0.11 to 0.41) | –340 (–986 to 52) |
The estimated differences in AQLQ scores, based on bootstrap methods with adjustments for prespecified covariates, were similar to those in the primary analyses, with differences of 0.23 (95% CI 0.06 to 0.40) for the physiotherapy arm compared with the usual-care arm and 0.26 (95% CI 0.11 to 0.41) for the DVD arm compared with the usual-care arm (see Table 47).
The incremental cost per AQLQ score improvement was –£400 (95% CI –£1545 to £106) for the physiotherapy arm compared with the usual-care arm and –£340 (95% CI –£986 to £52) for the DVD arm compared with the usual-care arm (see Table 47). Both interventions dominated usual care. Given the non-inferiority of the DVD intervention compared with the physiotherapy intervention, cost-minimisation analysis favoured the DVD intervention over the physiotherapy intervention. This was the base case for the cost-effectiveness analysis.
Cost–utility analysis
Incremental differences between the arms in terms of costs and QALYs and the incremental cost-effectiveness ratios (ICERs) are shown in Table 48. There were small baseline adjusted QALY differences between the arms, with the face-to-face physiotherapy arm having 0.007 more QALYs than the usual-care arm and the DVD arm having 0.02 more QALYs than the usual-care arm. The DVD arm showed a gain of 0.014 QALYs compared with the face-to-face physiotherapy arm. The gains were not statistically significant.
| Treatment arm | Cost (£), mean (95% CI) | Incremental cost (£), mean (95% CI) | QALYs, mean (95% CI) | Incremental QALYs, mean (95% CI) | ICER (£/QALY) (95% CI) |
|---|---|---|---|---|---|
| Usual care | 377 (310 to 459) | 0.767 (0.738 to 0.79) | |||
| Physiotherapy | 333 (299 to 369) | –41 (–134 to 33) | 0.771 (0.735 to 0.807) | 0.005 (–0.039 to 0.05) | –671 (–14,269 to 13,814) |
| DVD | 293 (228 to 374) | –83 (–187 to 12) | 0.788 (0.764 to 0.811) | 0.022 (–0.013 to 0.058) | –2754 (–17,739 to 12,017) |
| DVD vs. physiotherapy | 40 (–43 to 116) | 0.017 (–0.025 to 0.06) | –941 (–12,260 to 11,620) | ||
| Treatment arm | Cost (£), mean (95% CI) | Incremental cost (£) (95% CI) | QALYs adjusted for baseline QoL, mean (95% CI) | Incremental adjusted QALYs (95% CI) | ICER (£/adjusted QALY) (95% CI) |
| Usual care | 377 (310 to 459) | 0.767 (0.741 to 0.788) | |||
| Physiotherapy | 333 (299 to 369) | –41 (–134 to 33) | 0.773 (0.74 to 0.805) | 0.007 (–0.033 to 0.047) | –877 (–15,555 to 18,573) |
| DVD | 293 (228 to 374) | –83 (–187 to 12) | 0.787 (0.765 to 0.807) | 0.02 (–0.011 to 0.053) | –3057 (–18,877 to 10,864) |
| DVD vs. physiotherapy | 40 (–43 to 116) | 0.014 (–0.023 to 0.052) | –1145 (–14,982 to 9843) |
In the adjusted analysis (bottom part of Table 48), the ICERs had negative values for both the physiotherapy arm and the DVD arm compared with the usual-care arm. The physiotherapy arm cost £877 less per QALY than the usual-care arm and the DVD arm cost £3057 less per QALY than the usual-care arm. Both interventions dominated usual care. The CIs for these ICERs were wide and not statistically significant. Similar results were found in the unadjusted analysis.
The probability of dominance was investigated for each of the comparisons. This can be interpreted as the proportion of the simulated results (points on a scatterplot) that fall into the lower right-hand (south-east) quadrant. Scatterplots are shown of the joint distribution of the incremental mean cost and mean QALYs for the DVD arm compared with the usual-care arm (Figure 6) and for the physiotherapy arm compared with the usual-care arm (Figure 7).
FIGURE 6.
Scatterplot of the joint distribution of the incremental mean cost from a NHS perspective and mean QALYs adjusted for baseline QoL over 12 months based on the imputed data: 95% CI ellipse DVD arm compared with the usual-care arm.

FIGURE 7.
Scatterplot of the joint distribution of incremental mean cost from NHS perspective and mean QALYs adjusted baseline QoL over 12 months based on the imputed data: 95% CI ellipse physiotherapy arm compared with the usual-care arm.

The probability of usual care being dominated by the DVD intervention (lower costs and higher QALYs) was 82%. The probability of usual care being dominated compared with the face-to-face physiotherapy intervention (lower costs and higher QALYs) was 51%.
Discussion and conclusions
This well-conducted randomised trial provides evidence of the likely cost-effectiveness of both face-to-face physiotherapy and breathing retraining delivered using a DVD and booklet. Limitations include the time frame used, the reliance of the cost-effectiveness estimates on a single trial and the restriction of the costing perspective to the NHS. Although longer-term follow-up would be desirable, it seems likely that patients who have been able to improve their QoL using breathing retraining would continue to apply the techniques that they have learned. If so, the benefits recorded here may have been understated. Although cost-effectiveness should ideally be based on the totality of evidence, such as a meta-analytical estimate of effect size, we note similar results for the one similar trial of breathing exercises in asthma. 17 Our adoption of a NHS, as opposed to a societal, perspective was based on the returns from a questionnaire, which indicated that few costs fell outside the NHS.
The cost/AQLQ analysis favoured both the DVD intervention and the face-to-face physiotherapy intervention compared with usual care, with the DVD intervention achieving equivalent outcomes to the face-to-face physiotherapy intervention at a lower cost. The cost/QALY analysis showed similar results for the DVD and face-to-face physiotherapy comparisons with usual care, albeit based on small non-significant differences in outcomes. Both interventions dominated usual care. The DVD intervention had similar outcomes to the face-to-face physiotherapy intervention but at lower costs. The probabilities of the interventions being dominant were 85% for the DVD intervention compared with usual care and 51% for the face-to-face physiotherapy intervention compared with usual care. Thus, both the cost-effectiveness analysis and the cost–utility analysis provided congruent results. Both interventions achieved outcome gains at lower total costs than usual care. The lower cost in the DVD group meant that the DVD intervention was preferable to face-to-face physiotherapy. The low cost of the DVD (£2.85) meant that, if effective, it was highly likely to be cost-effective. Further, if made available on the internet, its cost would fall to close to zero.
In conclusion:
-
the QALY differences between the treatment arms were in the same direction as the differences in the primary outcome, but were smaller
-
the increased cost of each intervention was offset by reductions in total costs so that both interventions dominated usual care
-
the DVD/booklet training was preferable to face-to-face physiotherapy, as the outcomes were within the equivalence margin and the DVD intervention had a lower cost.
Chapter 5 Intervention development
The BREATHE trial was a three-armed trial, with two active arms and one control arm. The active arms consisted of physiotherapy breathing retraining delivered face-to-face and physiotherapy breathing retraining delivered using a DVD, with an accompanying booklet (Breathe Freely) provided to both active arms. This chapter describes the process of developing these three intervention components prior to carrying out the pilot RCT.
Face-to-face physiotherapy
Although breathing retraining for asthma is provided by some physiotherapists within the NHS and privately, in clinical practice there may be considerable variation in the content and frequency of face-to-face sessions. For this research we needed to standardise the intervention as much as possible to ensure that each participant received similar treatment and potentially to allow for comparability across different therapists. Our initial plan was to recruit two to three part-time physiotherapists to provide the face-to-face intervention during the life of the BREATHE trial.
The first step was to decide on the frequency of sessions. In clinical practice, physiotherapists have the option to tailor their treatments to the needs of individual patients, which results in large variations in treatment session numbers from patient to patient. However, previous related research trials by the chief investigator17,19 had demonstrated that three face-to-face sessions were sufficient to have a positive effect on the primary outcome, and discussions with local physiotherapists also suggested that three was the average number of sessions provided within the NHS. It was therefore decided that all participants should be offered three sessions with the physiotherapist, with intervention adherence being predefined as attending any two out of these three sessions.
The content for each session was derived from discussions with physiotherapists, from AB’s own experience and from discussion with other members of the research team. From these discussions, AB produced two documents: a complete description of the protocol for face-to-face physiotherapy [Face-To-Face Physiotherapy Protocol (Anne Burton, University of Southampton, 2012)] and a reduced version of the protocol to be given to intervention physiotherapists during training workshops [Training Manual for Physiotherapists (Anne Burton, University of Southampton, 2012)].
Training workshops were held by AB at various locations, at which the physiotherapists received information about the trial and the training manual. Details of the training provided at these workshops can be found in the manual. Although five physiotherapists attended these workshops, ultimately, only one physiotherapist (Ruth DeVos) provided all of the intervention sessions throughout the life of the trial. Fidelity of intervention delivery was determined in two ways: first, by the physiotherapist completing specifically designed checklists (see the Face-To-Face Physiotherapy Protocol for details) and, second, through the use of direct observation, whereby AB went out to the general practices during both the pilot trial and the main trial to observe the physiotherapist delivering a session of the intervention for approximately 5% of the participants (6 out of the 132 recruited to this arm). There was 100% adherence to the intervention protocol by the physiotherapist (physiotherapist adherence to the protocol had been predefined as conforming to 90% of the checklist).
Booklet development (Breathing Freely)
Previous experience from within the research team had indicated that accompanying materials (such as a written booklet) containing behavioural content to motivate and reassure patients were very useful to support user self-management. 35 Our booklet, Breathing Freely: Your Guide to Breathing Retraining for Asthma, was developed by a team of health psychologists, physiotherapists, physicians and patient representatives (Emily Arden-Close, Sarah Kirby, Emma Teasdale, Anne Bruton, Mike Thomas, Mark Stafford-Watson, Denise Gibson, Lucy Yardley, University of Southampton, 2013).
A draft booklet was first created by three of the authors (EAC, LY), based on advice from physiotherapists (AB, DG) and a GP with expertise in asthma (MT) and a similar booklet that LY had created that had successfully supported the exercise-based self-management of symptoms in patients with dizziness. 35 The first part of the booklet (pages 1–8) was designed to build motivation (in terms of social cognitive theory and positive outcome expectancies) and confidence (self-efficacy) to undertake breathing retraining. To convince users that breathing retraining would be effective, the cognitive–behavioural rationale for breathing retraining was first explained in terms of the vicious cycle of breathing problems, anxiety and hyperventilation and how this could be reversed by breathing retraining. Positive expectancies for treatment benefit were promoted by explaining how breathing retraining can improve not only breathing but also psychological well-being, and can allow users to do more without becoming breathless. The second part of the booklet (pages 10–15) contained detailed instructions (with illustrations) on how to progressively master breathing retraining, with reassurance and advice addressing common difficulties and barriers. The final part of the booklet (pages 16–21) encouraged users to use their new breathing skills to engage in more daily activities that they might have previously found difficult because of asthma (e.g. exercise, stressful situations). Implementation of breathing retraining was encouraged by providing success stories from other patients and charts to plan when to carry out the breathing exercises and physical activity and log progress.
Using our ‘person-based approach’ to developing accessible, persuasive and helpful intervention materials,36 feedback on the draft version was sought through the use of semistructured think-aloud interviews lasting approximately 1 hour with 29 people with asthma. The interviews included two components: open-ended questions exploring attitudes to breathing retraining exercises in the context of health beliefs and lifestyle and think-aloud methods to elicit spontaneous reactions to the booklet. Interview questions covered participants’ experiences of managing their asthma. This included questions about breathing difficulties, previous experience of breathing techniques for asthma management and the perceived relevance of breathing training exercises to asthma control. Participants then read the draft booklet and provided feedback about each page. Further open-ended questions explored impressions of the booklet, attitudes to breathing exercises, perceived attitudes of relevant others (family, health-care professionals), barriers to carrying out breathing exercises at home and possible ways of overcoming these barriers.
After each interview the feedback on each page was tabulated into recommended changes and positive aspects of the booklet. After the first few interviews had been conducted, the recommended changes were collated and a MoSCoW (must, should, could, would) analysis was conducted (breaking changes down into things we must, could, should and were not going to do), based on the nature of the comments and how many people mentioned each comment. The booklet was then revised before further interviews were conducted. This cycle continued until no further major changes were suggested. Further details of this process and the changes made in response to feedback can be found in two publications. 36,37
DVD development
Our aim was to produce an intervention that replicated the experience of face-to-face physiotherapy breathing retraining in an accessible self-guided format. We acquired the media in a high-quality video file format to ‘future-proof’ our content as much as possible and facilitate distribution using different technologies. The available technologies for distribution at the time of the original funding application were videotape, DVD and the internet. Although we knew that distributing video via the internet was possible, we chose the DVD as the distribution format for this project because, at the time, DVD players were becoming more popular and fewer people in the UK had access to the internet. In 2009 (when the funding application was written) the broadband network and hardware required to view internet-distributed video was not established or as widespread as it is now. Since then, distributing video via the internet has become ubiquitous. Reformatting our original video files for distribution via the internet will be relatively straightforward.
To enable people to identify with the patients seen on screen on the DVD, it was decided to involve genuine individuals with asthma (rather than actors) to take on the roles of patients. A local specialist respiratory physiotherapist (Denise Gibson) was asked to play the role of the breathing retraining physiotherapist. Dr Mark Porter (who has considerable experience of fronting medical programmes on both radio and television) kindly agreed to be the ‘presenter’ for the DVD.
The DVD production team consisted of AB, MT and DG, the psychology team developing the booklet, patient representatives, a commercial company (Zemedia) and staff at Solent University Studios, Southampton. AB produced a draft script for the DVD, based on a combination of the written Face-To-Face Protocol, the developing Breathing Freely booklet and discussions with physiotherapists. This was then shared with our psychology team members, patient representatives and others in the research team. Input from TMG patient representatives was of great importance in these revisions, and these representatives were involved in and commented on all revision steps, providing verbal and written comment on patient perspectives of the intervention. In addition, the iterative person-based think-aloud process for the development of digital behavioural interventions that was used in the development process for this intervention (described in detail in Chapter 6) used a representative sample of 29 adults with asthma purposively sampled for diversity of age, sex, education and symptom profile. These patients looked at draft versions of the intervention and provided feedback and comment using a formal qualitative methodology, and this was used to modify the intervention based on their reactions and comments. Several revisions to the script were subsequently made in response to comments and feedback from within the team. A professional media production team (Zemedia) had been identified by AB in 2009 during the initial funding application process and its main representative (Tim O’Riordan) had been regularly attending project meetings from 2011. Zemedia were employed to translate our script into a filmable story, to film the scenes and to create and produce the final DVD. Script refinement and preparation for filming continued over the first year of the research programme, during which period there were several team meetings to agree on patient roles and locations and to design ‘story boards’ for individual scenes. Filming took place over 3 days in May and June 2012, in Solent University Studios and on location in patients’ homes, with Steve Bowles of Zemedia and AB directing. Clips from the filming were shared within the research team for comment in terms of what to include/exclude, what voiceovers were appropriate and general formatting of the DVD. It was not felt appropriate to seek patient feedback on the developing DVD itself at that stage as it would not have been possible to refilm scenes because of time and financial constraints. Tim O’Riordan was responsible for the editing and post-production work that resulted in the final version of the DVD used in the trial. Feedback on the DVD as used by participants was sought as part of the research trial and is described elsewhere in this report.
Patient and public involvement (PPI) was central to this project, with two PPI representatives on the TMG attending TMG meetings and commenting on all stages of the process, and with the patient charity Asthma UK being a partner in the process. With the partnership of the charity and the active involvement of patients in all stages of the trial, in particular in the development and optimisation of the DVD intervention and booklet, we feel that there was complete PPI involvement overall. We did not experience any difficulty in obtaining PPI or in acting on it and would recommend that further trials in this clinical area integrate PPI into their processes as much as possible and involve patient charities if possible.
Chapter 6 Process evaluation
In line with Medical Research Council (MRC) guidance for developing and evaluating complex interventions38 and the MRC process evaluation framework,39 qualitative and quantitative process evaluations were nested within the trial to allow for a more detailed exploration of aspects that may be relevant to trial outcomes that might inform policy and practice. The qualitative process evaluation was conducted after the BREATHE trial 3-month follow-up with a sample of participants who had taken part in the intervention arms of the trial, to capture their perspectives on the interventions and understand what aspects were perceived as strengths and weaknesses.
The aim of the quantitative process evaluation was to explore participants’ reported expectations of the interventions, their experiences and their level of engagement with the interventions. Measures for the quantitative process evaluation were taken immediately after randomisation and at each of the 3-month, 6-month and 12-month follow-up time points. Like the qualitative process evaluation, only those who had taken part in the intervention arms of the trial were included.
The methodology and findings of the qualitative and quantitative process evaluations are each reported in more detail in the following sections.
Qualitative process evaluation
The qualitative process evaluation aimed to explore the experiences of participants in the intervention arms (DVD and face-to-face physiotherapy) of the BREATHE trial, to understand what patients perceive as the strengths and weaknesses of the different modes of delivery of breathing retraining.
Methodology
Semistructured telephone interviews were used to explore participants’ experiences of breathing retraining for asthma delivered by DVD or face-to-face physiotherapy. Participants were eligible for inclusion if they had taken part in one of the two intervention arms of the trial. Initially, consecutive sampling of participants in the pilot for the main trial was used. Purposive sampling was used towards the end to ensure adequate representation of male participants. Recruitment continued until the data reached saturation (i.e. no new themes were being raised). The final sample consisted of 11 women and five men between the ages of 23 and 70 years [mean (SD) age 55.2 (12.9) years]. There were eight participants (two men) in the DVD group and eight participants (three men) in the face-to-face physiotherapy group.
The transcripts were analysed by EAC using inductive thematic analysis. Transcripts were read carefully several times to ensure familiarity with the data. A coding manual was developed on the first few transcripts to ensure transparent and systematic coding of data and this was frequently revised. Themes were continually compared with newly coded transcripts to ensure that they applied to the data. The coding manual was then checked with LY and a sample of texts second coded, to ensure good inter-rater reliability. Themes were checked for differences as a function of group (intervention arm).
Results
Five main themes emerged: reasons for taking part, experience of breathing retraining, impact of breathing retraining, benefits of breathing retraining and problems with breathing retraining. These are discussed in the following sections.
Reasons for taking part
Reasons for taking part included being asked to, to enhance progress in research, to feel better/reduce symptoms and to reduce the use of medication. Some participants took part because a health-care professional asked them to:
I had my um annual asthma check-up and they just asked me if I would do it kind of there and then so I just said that I would.
P9, female, DVD
Some participants had always supported research and felt that it was their duty to give something back to help enhance progress with regard to research and knowledge:
because I would support research that is going to improve things for human beings.
P16, male, face-to-face physiotherapy
Many participants felt that it would help improve their health and symptoms:
I was just hoping it would . . . help my breathing when I went up hills . . . because that’s what I was particularly concerned with.
P2, female, DVD
Related to this, many participants wanted to reduce their use of medication. Although they took it as required, they wanted to be less dependent on it:
I liked the idea of a natural solution to the asthma rather than having to take medication.
P4, female, face-to-face physiotherapy
Experience of breathing retraining
The participants in the face-to-face group had a positive impression of the physiotherapist, who tailored the treatment to their needs, and found the sessions motivational.
All of the participants in the face-to-face group had a positive impression of the physiotherapist as friendly, very helpful, supportive and patient:
the lady that did them was really, really nice. She wasn’t condescending in any way, she was really patient, she was very quick to praise when you did it right.
P14, female, face-to-face physiotherapy
Participants mentioned that the physiotherapist tailored the treatment to their needs, rather than offering a ‘one size fits all’ approach. When they were experiencing difficulties, she reviewed the techniques and helped to break down goals into more manageable ones. For example, one participant described this experience of improving nose breathing:
When I spoke to [the physiotherapist] about it um, originally because I was having problems with it, she said just try and set myself little goals. So what I sort of do is when sort of leave the house I sort of set myself a goal to breathe through my nose to a certain point and then I will do it again, you know, I will get to this point and then I will try and breathe through my nose again.
P5, female, face-to-face physiotherapy
The physiotherapist also tailored support as participants needed, which facilitated mastery of the techniques:
. . . when she said, ‘Now, I need you to do these exercises at home’, she saw the look on my face and she said, ‘Would you like me to write the instructions down?’ And I said, ‘Yes, please’. And so that, because I’d already done the actual training bit in my session the instructions put it back into my mind what I had to do and I found it really, really informative.
P14, female, face-to-face physiotherapy
The face-to-face physiotherapy group also found seeing the physiotherapist motivational. Knowing that they would attend appointments prompted them to prioritise practising breathing techniques:
I liked having the person there. It is not so much that she told me off when I hadn’t done the exercises but it is like an extra conscience.
P5, female, face-to-face physiotherapy
The materials used by participants in the DVD group (booklet and DVD) were also considered useful. Participants liked the booklet as it reminded them to carry out their exercises, was helpful to refer to when practising and enabled them to log results. Participants also found that the DVD helped when practising the exercises. However, although some preferred the DVD because it showed them how to perform the exercises, some preferred the booklet because it could be carried anywhere:
. . . with the DVD it was actually showing you.
P11, male, DVD
I liked the booklet better . . . because I could just pick it up and, you know, look at it and um do some of the exercises when I wanted to.
P2, female, DVD
Generally, participants felt that the booklet and the DVD complemented each other:
I found by reading the booklet and then watching the DVD the two matched and I could see what was meant.
P13, female, DVD
Impact of breathing retraining
The three main ways that participants felt that their lives were impacted by breathing retraining were in undergoing regular practice, having an increased awareness of breathing and developing new habits. Many participants reported initially practising breathing techniques regularly (more than three times a day), in line with recommendations, and felt that this had facilitated development of new habits. Also, many mentioned increased awareness of their breathing. Talking to the physiotherapist or watching the DVD and practising the exercises made them aware that they had been breathing incorrectly:
I’m a habitual mouth breather and to realise that I’d been breathing wrong all my life was a bit of an eye opener.
P10, female, face-to-face physiotherapy
I do try to make myself aware of breathing through my nose all the time.
P11, male, DVD
Many participants mentioned being able to perform stomach breathing and nose breathing automatically. They had internalised this new way of breathing so it became a habit:
I can at rest actually do the stomach breathing pretty much naturally now.
P3, female, DVD
I still try and do it [nose breathing].
P12, male, face-to-face physiotherapy
Benefits of breathing retraining
Participants also mentioned many health benefits that they associated with breathing retraining, including increased control over breathing, reduced need for medication, feeling more relaxed and improved health and QoL. Almost all participants mentioned increased control over breathing. They reported being able to use the techniques to breathe through asthma attacks:
I had two asthma attacks last year . . . and um actually being able to do this breathing helped a lot and I didn’t have to go to hospital.
P14, female, face-to-face physiotherapy
Related to this, breathing retraining was often associated with a reduced need for medication. Many participants were able to use breathing techniques rather than reaching for a reliever inhaler when they felt symptoms coming on:
I don’t have to keep getting my inhaler and taking my inhaler um, I can literally just um do some of these breathing and I feel much better.
P5, female, face-to-face physiotherapy
Participants also mentioned that the breathing techniques helped them to relax:
. . . when things have got a bit busy I have sort of been very conscious to do it and I’ve found it very helpful and very calming.
P15, male, face-to-face physiotherapy
Other benefits attributed to breathing retraining under the umbrella of improved health and QoL included being less wheezy, sleeping better and having more energy:
I sleep so much better.
P14, female, face-to-face physiotherapy
I also used to get very wheezy first thing in the morning and that doesn’t seem to be happening now.
P3, female, DVD
Problems with breathing retraining
Participants also mentioned problems with breathing retraining. These included difficulties finding time to practise and difficulties mastering techniques. Many participants said that it was difficult to find time to practise the breathing techniques before they were able to fit them fitted into their daily routine:
Initially quite hard to get started. It was finding the time I think um and putting aside a regular time so that I didn’t skip things.
P13, female, DVD
Barriers included busy schedules and difficulties trying to fit the exercises in during the daytime while at work, meaning that high levels of motivation were required to carry them out:
I could do as many BREATHE’s in the evening when I’m sat at home . . . but finding the time throughout the day when I’m at work, that was a bit more challenging.
P5, female, face-to-face physiotherapy
Many participants also mentioned difficulties with mastering the techniques. Many participants found breath holding most difficult:
. . . the most difficult I found holding my breath.
P4, female, face-to-face physiotherapy
Ease of mastering the techniques varied. Participants usually found it easier to carry out the techniques if they had previous experience of them. Apart from one participant in the face-to-face group who experienced severe problems with breath holding, an inability to carry out the techniques appeared more common in the DVD group:
I have to say um, no matter how I tried, and on the DVD it said it would come eventually, I cannot breathe through my diaphragm.
P7, female, DVD
Some participants found it difficult to apply the techniques in particular situations:
I can’t quite master the stomach breathing when I am moving around, but no doubt that will come.
P3, female, DVD
Discussion
Reasons for taking part in the BREATHE trial included being asked to, to enhance progress in research, to feel better/reduce symptoms and to reduce medication use. Participants in the face-to-face group had a positive impression of the physiotherapist, liked receiving treatment tailored to their needs and found meetings motivational. All participants liked the materials. The impact of breathing retraining included regular practice of the exercises leading to increased awareness of breathing and the development of new habits. The perceived benefits of breathing retraining included increased control over breathing, a reduced need for medication, feeling more relaxed and improved health and QoL. However, problems included difficulties in finding time to practise and with mastering techniques.
Strengths and limitations of the study
This is the first full analysis of patients’ experiences of breathing retraining for asthma self-management. However, it has several limitations. Although participants were randomised to their treatment arm of the trial, they self-selected into this nested qualitative process evaluation. Despite attempts to reduce socially desirable responding, participants may have felt pressure to report positive outcomes. In addition, the interviewer may have brought bias from being involved in creating the intervention, although participants were unaware of this.
Interpretation of the findings in relation to previously published work
There was very little intentional non-engagement in breathing retraining. Participants wanted to improve their asthma control and reduce their reliance on medication. Previous research has also shown that people with asthma adopt non-pharmacological methods of management to reduce reliance on medication. 37,40 Most participants reported practising intensively (more than three times a day) for the initial 3–4 weeks, as recommended by the physiotherapist and in the booklet. Following this, participants reported increased awareness of their breathing and that breathing techniques had become habitual, in line with research showing that habit formation is an effective health behaviour change strategy. 41
Participants in the face-to-face group were very positive about the physiotherapist. Many said that the meetings motivated them to carry on with their exercises, with participants in the DVD group stating that they would have preferred to see the physiotherapist. Similarly, in a trial of vestibular rehabilitation for dizziness delivered with or without telephone support, all participants reported preferring telephone support. 42 A reported inability to master certain techniques appeared to be more common in the DVD group. Although many face-to-face physiotherapy participants mentioned finding diaphragm breathing, breath holding and slow breathing difficult initially, several in the DVD group reported being unable to master diaphragm breathing. Those who saw the physiotherapist said that she had tailored the treatment to their needs and they felt able to improve with this support and encouragement. However, the BREATHE trial showed that face-to-face physiotherapy was only marginally more effective than the DVD. Similarly, in a trial of vestibular rehabilitation, telephone support did not lead to greater improvements than a booklet. 35 Although personal contact may enhance confidence in carrying out techniques, this does not necessarily lead to greater benefits. However, a minority of individuals may need face-to-face instruction to master novel techniques. The perceived benefits of breathing retraining included better asthma control, meaning that participants were able to breathe their way through asthma attacks and, in some cases, possibly even avoid hospital admissions; reduced use of reliever medication, meaning that participants felt more in control of their asthma and less worried if an inhaler was not available; feeling more relaxed; and sleeping better. Participants also reported having more energy and feeling better. These benefits are similar to those found in previous breathing retraining trials. 3,17 Many participants reported difficulties with finding time to perform the breathing exercises until they were able to make them part of their routine. One participant reported dropping out of the trial because of difficulty finding time to practise. People who saw the booklet during the development phase predicted that it would be difficult for those working full-time and/or with young children to find time to practise the exercises. 37 However, in contrast to previous findings,37 all participants saw the relevance of breathing retraining, possibly because the trial was open only to those whose QoL was affected by asthma.
Implications for health care and recommendations for research
Breathing retraining was well received by participants in both groups, with many reporting improved well-being, and is likely to be acceptable and valued as an adjuvant treatment in general practice, whether delivered face-to-face or by DVD. Given the greatly increased availability of DVD delivery, plus the limited increased benefit provided by face-to-face physiotherapy, making the breathing training DVD (and booklet) widely available may lead to improved asthma control in the general population. This could be tested in a large-scale RCT. To increase the confidence of individuals carrying out breathing retraining delivered by DVD, it might be helpful to inform them that research has demonstrated equivalent benefit of the DVD delivery method to face-to-face physiotherapy. Also, individuals offered breathing retraining delivered by DVD could be provided with a telephone number to contact in case they experienced difficulties in mastering the techniques, to enhance the benefits of breathing retraining. Overall, though, breathing training is likely to be well received as a method of asthma management.
Quantitative process evaluation
The objective of the quantitative process evaluation was to quantify patient-reported expectations of treatment, experiences of treatment and engagement. This process evaluation included five main sets of analyses:
-
Sensitivity analyses – of engagement with and amount of practice of the BREATHE exercises reported at 3 months’ follow-up. The rationale for these analyses was to assess treatment efficacy in participants who met ‘threshold engagement’ levels; that is, efficacy is expected to be greater in those who have looked at the DVD/booklet and carried out breathing retraining at least once, and to investigate whether participants who practised the exercises more regularly derived more benefit.
-
Between-group differences – to explore whether there was a difference between the two intervention groups on measures of expectancy, experience, treatment engagement and practical barriers.
-
Predictors of the amount of practice and continuous engagement – to assess whether expectancy, experience and practical barriers are associated with the amount of practice (at 3 months) and continuous engagement (at 6 and 12 months).
-
Analyses were carried out to model the theory of planned behaviour and explore whether intentions, the amount of practice, engagement and continued engagement can be predicted by the model.
-
The factor structure of the Problematic Experiences of Therapy Scale (PETS)43 was checked using exploratory factor analysis as it has not previously been used in an asthma population.
Methodology
Immediately after randomisation, participants in the two intervention groups (DVD or face-to-face physiotherapy) were shown the booklet and asked to complete a questionnaire assessing expectancy (their beliefs about asthma and their first impressions of the intervention to which they had been allocated). At the 3-month follow-up, participants in the two intervention groups were asked to complete brief questionnaires relating to their experiences of treatment, engagement with treatment and perceived barriers to carrying out the treatment. Questions to assess whether or not participants continued using the two treatments were included at the 6- and 12-month follow-ups.
Measures
Expectancy (baseline)
-
Perceived causes. A single item from the Brief Illness Perceptions Questionnaire (IPQ) was used. 44 People with asthma commonly attribute the cause of their asthma to external factors such as being hereditary or being caused by a respiratory virus, pollution or allergies. 44,45 According to the common sense model of illness, such beliefs can have an impact on a variety of illness outcomes. 46 Research has shown that external causal attributions are associated with adherence to asthma medication; however, it is unclear whether these beliefs are associated with higher or lower levels of adherence. 47,48 The impact of causal attributions on illness outcome has not yet been explored in relation to other types of asthma treatment such as breathing retraining. The Brief IPQ item asks people to write their own top three (in rank order) perceived causes of their illness; therefore, we did not need to use or create a list. However, as the most common items selected by people with asthma are hereditary, a respiratory virus, pollution and allergies, we expected these to be the main responses to this question.
-
Perceived chronicity. A single item was used to measure whether or not participants believed that asthma was a chronic condition. 49 Many people with asthma perceive their illness to be an acute episodic illness rather than a chronic one. The belief that they have asthma only if they are having symptoms (the ‘no symptoms, no asthma’ belief) is associated with low adherence to inhaled corticosteroids and beliefs that inhaled corticosteroids are important to use when symptomatic but not asymptomatic, and with lower participation in self-management tasks such as peak flow monitoring or routine doctor visits. 49
-
Expectancy. Expectancy (improvements that a person believes will be personally achieved during treatment)50 is an important non-specific factor that can impact on the adherence to and outcome of treatment. 51 As well as being an important non-specific factor, outcome expectancy (along with self-efficacy) is also a central tenet of Bandura’s social cognitive theory. 52 It was expected that the face-to-face treatment group would reach greater levels of expectancy than the home-based treatment group. Expectancy was measured using the three expectancy items from the Credibility/Expectancy Questionnaire,50 which were standardised and summed for analysis.
-
Self-efficacy. Self-efficacy is a well-documented predictor of treatment outcome and, together with expectancy, has been associated with subjective recovery and adherence to physiotherapy53 and outcomes such as AQLQ score in people with asthma. 54,55 The items selected were based on Lorig’s three-item Exercise Regularly Scale,56 which was created to assess self-efficacy to perform self-management behaviours in people with chronic disease, correctly, every day and without making their symptoms worse. The three self-efficacy items are rated on a scale from 0 to10. Responses were summed and averaged for analysis.
-
Perceived need for support (baseline). A single-item question was included to assess how important the participants (in both active treatment groups) felt it would be to receive physiotherapist support (i.e. whether the physiotherapist sessions were anticipated by participants to be useful and adding value beyond the booklet itself, regardless of whether or not they would be receiving this support).
-
Theory of planned behaviour. The theory of planned behaviour model hypothesises that attitudes, subjective norms and perceived behavioural control will predict intentions and that intentions and perceived behavioural control will predict behaviour (engagement and amount of practice). Consistent with guidance on constructing a theory of planned behaviour questionnaire,57 semistructured qualitative interviews were conducted to elicit salient beliefs to create appropriate items relating to the components of the model. 37 Items in each subscale were summed to create a composite score for analysis. The factor structure of the constructs was checked using exploratory factor analysis, with principal axis factoring and direct oblimin rotation, and internal reliability was checked using Cronbach’s alpha. The structure and internal reliability of the constructs were suitable for further analysis, with the exception of control-related beliefs, which were of borderline acceptability (α = 0.69). Therefore, the two items in the control-related beliefs construct were used separately as single items in further analyses. Redundancy (indicated by multicollinearity) were also noted within the attitudes, subjective norms and intentions constructs. Although the constructs were summed for these analyses, future work could reduce participant burden by using a single item for each of these constructs.
-
Attitudes – three items were created to assess the belief that carrying out the intervention would be good/bad, beneficial/harmful or useful/useless. Items were reverse coded so that higher scores reflected a more positive attitude. The internal reliability of the construct was excellent (α = 0.94).
-
Subjective norms – three items were created to assess the perception that important others believed that carrying out the intervention would be good/bad, beneficial/harmful or useful/useless. Items were reverse coded so that higher scores reflected a more positive view (α = 0.98).
-
Perceived behavioural control – two items were created to assess the perception that it would be possible to carry out the intervention and that participants were confident that they could carry out the intervention (α = 0.89).
-
Intentions – intentions were assessed by three items measuring whether participants would try to, intended to and wanted to carry out the intervention (α = 0.96). Because of a ceiling effect (n = 168, 44% of participants endorsing the maximum scores), the data were recoded into a binary variable (high = 21/low < 21).
-
Beliefs (attitude related) – four items were created to assess whether the intervention would be relevant, reduce the use of a reliever inhaler, help the patient to feel more in control of asthma and help the patient to feel more relaxed (α = 0.87).
-
Beliefs (control related) – two items were created to assess whether the intervention was perceived to be time-consuming and difficult to fit into the daily routine. These were used in the analysis as single items.
-
Treatment experience (3 months’ follow-up)
-
Enjoyment. Intrinsic motivation is an important factor in adherence to rehabilitation and exercise. 58,59 Enjoyment is a factor that is considered to underlie intrinsic motivation. 60,61 Given the different aspects of the trial intervention, it was expected that participants would enjoy the therapist appointments, relaxation and controlled breathing, but dislike the controlled breath holding. It was unclear whether they would enjoy or dislike carrying out additional physical exercise/activity. The single item measuring enjoyment used by Reeve,60 with responses measured on a scale from 0 to 10, ranging from not at all enjoyable to extremely enjoyable, was selected for this study. To allow for the expectation that some might find the controlled breath holding aversive (a stronger response than not enjoyable), the scale was adapted from extremely enjoyable to not at all enjoyable to extremely enjoyable to extremely unpleasant. This question wording presents less bias, allowing a non-judgemental basis for participants to provide more honest answers. The question was repeated six times in relation to the different aspects of the intervention (stomach breathing, nose breathing, slow breathing, controlled breath holding, relaxation training and, in the physiotherapy group only, appointments with the physiotherapist).
-
Perceived need for support. A single-item question was included to assess change since baseline in how important the participants (in both the DVD group and the physiotherapy group) perceived the physiotherapist support to be (i.e. whether the physiotherapist sessions were perceived by participants to be useful and to add value beyond the booklet itself, regardless of whether they received this support or not).
-
Perceptions of the physiotherapist (physiotherapy group only). The Treatment Appraisal Questionnaire62 contains five single items (measured on a scale from 1 to 7) relating to ‘perceptions of the therapist’. The items assess the degree of trust in the therapist, confidence in the therapist’s qualifications and competence and the extent to which participants feel comfortable talking to the therapist about their health and believe that the therapist wants to help them. These factors may all be relevant to participants undergoing face-to-face treatment. Because of a ceiling effect (n = 74, 77% of participants endorsing the maximum score), the data were recoded into a binary variable (high = 35/low < 35).
-
Problematic Experiences of Therapy Scale. Participants’ perceptions of any adherence problems were measured using the PETS. 43 The PETS was developed to measure patient perceptions of barriers to adherence to home-based rehabilitation. The four validated PETS subscales were included (problems due to symptoms – three items; problems due to uncertainty about the therapy – three items; problems due to doubts – three items; and practical problems – five items), along with a new theoretically derived subscale to measure problems due to lack of support (three items).
-
Treatment engagement. A range of items was included to measure participants’ level of engagement with breathing retraining in terms of how much they carried out breathing retraining and which techniques they used. The following items were included:
-
Carrying out the breathing retraining:
-
Number of weeks that the breathing retraining was carried out for.
-
Days per week on average that the breathing retraining was carried out for.
-
Times per day on average that the breathing retraining was carried out for.
-
Engagement – engagement is a composite binary variable in which ‘engaged’ was defined as giving any response above ‘never started’ to any of the first three questions above (number of weeks, days per week and times per day). Participants were defined as non-engaged if they did not start breathing retraining at all.
-
Reason for stopping regular breathing retraining (because they no longer have symptoms of asthma or for other reasons).
-
Continuation with occasional breathing retraining (despite not carrying out regular breathing retraining) – specified in relation to as many as applied out of stomach breathing, nose breathing, slow breathing, controlled breath holding or relaxation training.
-
-
Total time spent on each type of technique:
-
stomach breathing (number of weeks/hours per day)
-
nose breathing (number of weeks/hours per day)
-
slow breathing (number of weeks/minutes per day)
-
controlled breath holding (number of weeks/minutes per day)
-
relaxation training (number of weeks/minutes per day).
-
-
Amount of practice – amount of practice is a continuous variable that was calculated by multiplying the responses to the first three questions within the ‘Carrying out the breathing retraining’ section. These questions related to the number of weeks, number of days per week and number of times per day, on average, that the breathing retraining was carried out. Participants who did not engage with the breathing retraining were excluded from the analyses.
-
Continued treatment engagement (6 and 12 months’ follow-up)
-
Five items were included to measure whether participants had continued to carry out breathing retraining at 6 and 12 months. The five items consisted of one question for each technique (stomach breathing, nose breathing, slow breathing, controlled breath holding and relaxation training) to find out how often participants had carried out the exercises [measured on a five-point scale ranging from never to regularly (most days)].
Results
Sensitivity analyses for engagement with and amount of practice of breathing exercises reported at 3 months’ follow-up
These are reported with the main trial results (see Chapter 3, Patient engagement with the intervention in the active treatment arms.)
Analyses of between-group differences to explore whether there is a difference between the two intervention groups on measures of expectancy, experience, treatment engagement and practical barriers
T-tests and chi-squared tests were used to explore whether there were any differences between the treatment groups on measures of expectancy, experience, treatment engagement and practical barriers. Content analysis was performed on the three open-ended text items that measured perceived causes. A total of 937 responses were given, the majority of which could be grouped into eight main themes: allergies (245 responses), health issues (142 responses, such as viral infections, radiotherapy, obesity, pregnancy/menopause, glandular fever and childhood conditions), smoking (114 responses), pollution/environmental causes (104 responses), hereditary causes (79 responses), stress/psychological causes (65 responses), weather (65 responses) and exercise (59 responses). The three categories with the highest frequencies (allergy, health issues and smoking) were binary coded into present/absent in the data and used in between-group analyses. Participants could attribute up to three causes. Using this recoded data, a total of 192 participants (49%) believed that their asthma was caused by allergies, 120 (31%) by health issues and 110 (28%) by smoking.
With regard to perceived chronicity, 44% of participants thought of asthma as a chronic disease, with the remainder reporting some degree of the ‘no symptoms, no asthma’, acute episodic disease belief (16% believed that they had asthma most of the time, 20% some of the time and 21% only when they were having symptoms). The data were analysed in relation to each of these four categories separately.
There were no significant differences between the two treatment arms with regard to beliefs about the causes or chronicity of asthma. However, having been given the booklet to look through and told what group they had been allocated to, the face-to-face physiotherapy group had more positive perceptions of breathing retraining than the DVD group. (Table 49). Being aware that they would be receiving physiotherapist support led to significantly more positive attitudes and attitude-related beliefs, a greater sense of perceived behavioural control and higher intentions to carry out breathing retraining in the physiotherapy group. Those anticipating physiotherapist support were significantly less likely to believe that carrying out breathing retraining would be too time-consuming or difficult to fit into their daily routine. Those who were aware that they had been randomised to the physiotherapist group felt a significantly greater need for the physiotherapist support than those who were aware that they had been randomised to the DVD group.
| Beliefs about asthma and first impressions of treatment | Treatment arm, baseline | Group differencea | p-value | |
|---|---|---|---|---|
| DVD | Physiotherapy | |||
| Beliefs about asthma, n (%) | ||||
| Perceived causes | ||||
| Cause 1 – allergy | 125 (48) | 67 (51) | 0.29 | 0.592 |
| Cause 2 – health issues | 81 (31) | 39 (30) | 0.09 | 0.762 |
| Cause 3 – smoking | 69 (26) | 41 (31) | 0.93 | 0.335 |
| Perceived chronicity | 2.03 | 0.566 | ||
| All the time | 112 (45) | 54 (42) | ||
| Most of the time | 41 (16) | 18 (14) | ||
| Some of the time | 45 (18) | 31 (24) | ||
| Only when symptoms present | 52 (21) | 27 (21) | ||
| First impressions of treatment, mean (SD)b | ||||
| Expectancy (n = 243, n = 124) | –0.11 (2.74) | 0.24 (2.66) | –1.20 (–0.95 to 0.23) | 0.233 |
| Self-efficacy (n = 252, n = 129)c | 7.70 (1.62) | 8.01 (1.48) | –1.84 (–0.62 to 0.05) | 0.067 |
| Perceived need for physiotherapist support – baseline (n = 250, n = 127)c | 2.15 (1.26) | 3.43 (0.87) | –11.48 (–1.49 to –1.06) | 0. 001 |
| Theory of planned behaviour constructs | ||||
| Attitudes (n = 239, n = 119)c | 16.45 (3.75) | 17.74 (2.82) | –3.64 (–1.96 to –0.66) | 0.001 |
| Subjective norms (n = 235, n = 117)c | 16.82 (3.89) | 17.62 (3.72) | –1.85 (–1.62 to –0.04) | 0.059 |
| Perceived behavioural control (n = 251, n = 130)c | 11.05 (2.64) | 12.07 (2.37) | –3.84 (–1.56 to –0.47) | 0.001 |
| Intentions, n (%) (n = 249, n = 129) | 99 (40) | 69 (54) | 6.49 | 0.011 |
| Beliefs (attitude related) (n = 245, n = 125)c | 21.99 (4.23) | 23.07 (4.37) | –2.31 (–1.98 to –0.19) | 0.020 |
| Beliefs (control related) | ||||
| Time-consuming (n = 252, n = 127)c | 4.10 (1.80) | 3.65 (1.73) | –2.31 (0.03 to 0.83) | 0.024 |
| Difficult to fit into routine (n = 252, n = 126)c | 3.33 (1.74) | 2.86 (1.73) | –2.47 (0.08 to 0.86) | 0.013 |
The majority of participants in the face-to-face physiotherapy group found the appointments with the physiotherapist to be extremely enjoyable (93% rated the appointments as ≥ 8 out of 10) and 77% had the best possible perceptions of their physiotherapist. Those in the face-to-face physiotherapy group continued to perceive a significantly greater need for physiotherapist support than those in the DVD group and they also reported a significantly more positive treatment experience in relation to enjoyment of stomach breathing, nose breathing and relaxation training than those in the DVD group. They also experienced significantly fewer problems as a result of symptoms, uncertainty, doubt and lack of support. There were no differences between the face-to-face physiotherapy group and the DVD group in terms of the level of enjoyment of controlled breath holding or practical problems (Table 50).
| Treatment experiencea | Treatment arm, 3-month follow-up | Group differenceb | p-value | |
|---|---|---|---|---|
| DVD | Physiotherapy | |||
| Enjoyment of treatment, mean (SD) | ||||
| Stomach breathing (n = 159, n = 104) | 6.13 (1.99) | 7.42 (1.67) | –5.71 (–1.75 to –0.85) | < 0.001 |
| Nose breathing (n = 160, n = 104) | 6.06 (2.18) | 7.52 (1.78) | –5.96 (–1.95 to –0.98) | < 0.001 |
| Slow breathing (n = 159, n = 103) | 6.22 (2.01) | 6.69 (2.12) | –1.81 (–0.98 to 0.04) | 0.072 |
| Controlled breath holding (n = 158, n = 103) | 5.54 (2.29) | 5.43 (2.18) | 0.41 (–0.44 to 0.68) | 0.681 |
| Relaxation training (n = 155, n = 102) | 6.97 (1.80) | 7.62 (2.38) | –2.32 (–1.19 to –0.10) | 0.022 |
| Appointments with physiotherapist (physiotherapy group only) (n = 103), median (IQR); minimum, maximum | 9 (9–10); 1, 10 | |||
| Perceived need for physiotherapist support (3 months) (n = 158, n = 101), mean (SD) | 1.85 (1.38) | 3.64 (0.72) | –13.73 (–2.05 to –1.54) | < 0.001 |
| Perceptions of physiotherapist (physiotherapy group only) (n = 96), n (%) | 74 (77) | |||
| PETS, n (%) | ||||
| Problems due to symptoms (n = 155, n = 103) | 49 (31.6) | 21 (20.4) | 3.94 | 0.047 |
| Problems due to uncertainty (n = 156, n = 101) | 75 (48.1) | 14 (13.9) | 31.71 | < 0.001 |
| Problems due to doubts (n = 166, n = 105) | 90 (54.2) | 24 (22.9) | 25.95 | < 0.001 |
| Practical problems (n = 166, n = 105) | 141 (84.9) | 84 (80.0) | 1.11 | 0.291 |
| Problems due to lack of support (n = 166, n = 105) | 74 (44.6) | 17 (16.2) | 23.24 | < 0.001 |
Overall, engagement with breathing retraining was extremely high, with 98.1% of responders reporting that they had attempted at least one of the breathing retraining techniques (Table 51). Only five participants (1.9%), who were all allocated to the DVD group, reported not attempting any breathing retraining at all. Participants who had received face-to-face physiotherapy were significantly more likely than those in the DVD group to have carried out breathing retraining more times per day, more days per week and for more weeks, and to have completed more practice sessions. This particularly applied to the stomach breathing and nose breathing, but also to the slow breathing and controlled breath holds to a lesser, but still significant, extent. Among those who stopped regular practice (n = 97), those in the face-to-face physiotherapy group were significantly more likely to continue with occasional practice of nose breathing.
| Engagement and number of practice sessionsa | Treatment arm, 3-month follow-up | Group differenceb | p-value | |
|---|---|---|---|---|
| DVD | Physiotherapy | |||
| Carrying out the breathing retraining | ||||
| Engagement with breathing retraining (overall) (n = 261, n = 132), n (%) | 256 (98.1) | 132 (100) | 2.56 | 0.110 |
| Number of weeksc,d (n = 165, n = 103), mean (SD) | 3.68 (1.38) | 4.32 (0.87) | –4.67 (–0.92 to –0.38) | 0.001 |
| Days per weekc,e (n = 165, n = 103), mean (SD) | 2.52 (1.20) | 3.08 (1.02) | 4.07 (–0.83 to –0.28) | 0.001 |
| At least twice per day (n = 164, n = 105), n (%) | 46 (28.0) | 58 (55.2) | 19.96 | < 0.001 |
| Amount of practice sessions completed (overall)c (n = 164, n = 102), mean (SD) | 48.56 (44.71) | 75.01 (46.38) | –26.45 (–37.68 to –14.97) | 0.001 |
| Stopped regular practice as no asthma symptoms (n = 162, n = 105), n (%) | 14 (8.6) | 5 (4.8) | 1.45 | 0.228 |
| Stopped regular practice for other reasons (n = 160, n = 101), n (%) | 61 (38.1) | 27 (26.7) | 3.60 | 0.058 |
| Stopped regular practice but continued with occasional practice, n (%) | ||||
| Overall (n = 66, n = 31) | 61 (92.4) | 30 (96.8) | 0.69 | 0.407 |
| Stomach breathing (n = 61, n = 30) | 44 (72.1) | 26 (86.7) | 2.39 | 0.122 |
| Nose breathing (n = 61, n = 30) | 38 (62.3) | 26 (86.7) | 5.72 | 0.017 |
| Slow breathing (n = 61, n = 30) | 42 (68.9) | 19 (63.3) | 0.28 | 0.599 |
| Controlled breath holding (n = 61, n = 30) | 13 (21.3) | 6 (20.0) | 0.02 | 0.885 |
| Relaxation training (n = 61, n = 30) | 27 (44.3) | 10 (33.3) | 1.00 | 0.318 |
| Total time spent on each breathing technique, mean (SD) | ||||
| Stomach breathing | ||||
| Number of weeksd (n = 159, n = 100) | 3.53 (1.56) | 4.35 (1.05) | –5.03 (–1.16 to –0.49) | 0.001 |
| Hours per dayf (n = 157, n = 99) | 1.39 (0.99) | 2.11 (1.20) | –5.00 (–1.01 to –0.42) | 0.001 |
| Nose breathing | ||||
| Number of weeksd (n = 159, n = 101) | 3.52 (1.66) | 4.33 (1.03) | –4.81 (–1.14 to –0.47) | 0.001 |
| Hours per dayf (n = 157, n = 101) | 1.81 (1.27) | 2.45 (1.34) | –3.81 (–0.97 to –0.34) | 0.001 |
| Slow breathing | ||||
| Number of weeksd (n = 160, n = 101) | 3.44 (1.53) | 3.89 (1.27) | –2.55 (–0.80 to –0.11) | 0.012 |
| Minutes per dayg (n = 159, n = 101) | 2.10 (1.35) | 2.45 (1.28) | –2.05 (–0.68 to –0.02) | 0.039 |
| Controlled breath holding | ||||
| Number of weeksd (n = 160, n = 101) | 2.88 (1.76) | 3.32 (1.57) | –2.08 (–0.79 to –0.07) | 0.020 |
| Minutes per dayh (n = 159, n = 101) | 1.74 (1.09) | 1.83 (1.11) | –0.69 (–0.37 to 0.19) | 0.497 |
| Relaxation training | ||||
| Number of weeksd (n = 160, n = 101) | 3.01 (1.86) | 3.29 (1.66) | –1.27 (–0.70 to 0.16) | 0.217 |
| Minutes per dayi (n = 160, n = 101) | 1.86 (1.24) | 1.64 (1.20) | 1.41 (–0.10 to 0.50) | 0.159 |
The positive effect of physiotherapist support on stomach breathing and nose breathing also had lasting effects, with participants in the physiotherapy group continuing to carry out the stomach breathing and nose breathing significantly more often than those in the DVD group at both 6 months’ and 12 months’ follow-up (Table 52).
| Exercisea | Treatment arm, mean (SD) | Group differenceb | p-value | |
|---|---|---|---|---|
| DVD | Physiotherapy | |||
| 6-month follow-up | ||||
| Number included | 156 | 96 | ||
| Stomach breathing | 2.18 (1.24) | 2.94 (1.02) | –5.04 (–1.05 to –0.46) | < 0.001 |
| Nose breathing | 2.43 (1.31) | 2.83 (1.19) | –2.46 (–0.73 to –0.08) | 0.014 |
| Slow breathing | 2.22 (1.20) | 2.48 (1.11) | –1.73 (–0.56 to 0.36) | 0.085 |
| Controlled breath holding | 1.92 (1.26) | 1.81 (1.16) | 0.70 (–0.20 to 0.42) | 0.512 |
| Relaxation training | 1.83 (1.24) | 2.01 (1.30) | –1.12 (–0.51 to 0.14) | 0.264 |
| 12-month follow-up | ||||
| Number included | 153 | 96 | ||
| Stomach breathing | 2.18 (1.18) | 2.72 (1.12) | –3.59 (–0.84 to –0.25) | < 0.001 |
| Nose breathing | 2.31 (1.25) | 2.66 (1.26) | –2.09 (–0.67 to –0.02) | 0.037 |
| Slow breathing | 2.20 (1.17) | 2.38 (1.07) | –1.21 (–0.47 to 0.11) | 0.226 |
| Controlled breath holding | 1.84 (1.20) | 1.66 (1.03) | 1.22 (–0.11 to 0.47) | 0.225 |
| Relaxation training | 1.75 (1.22) | 1.93 (1.19) | –1.16 (–0.49 to 0.13) | 0.249 |
Correlates and predictors of the amount of practice and continued engagement
Analyses were carried out to assess whether expectancy, experience and practical barriers were associated with amount of practice (at 3 months) and continued engagement (at 6 months and 12 months). As engagement was 98% in the DVD group and 100% in the face-to-face physiotherapy group, there was no variation in the data to be able to carry out the planned analyses in relation to non-engagement.
For the continuous outcomes (amount of practice at 3 months and continued engagement at 6 months and 12 months), point-biserial (for binary expectancy variables) and bivariate (for continuous expectancy variables, treatment experience and practical barriers) correlations were used to identify significant variables to be entered into multiple linear regressions for each outcome. All of the correlations are presented in Appendix 13 and significant correlations are presented in Table 53.
| Amount of practice at 3 months | Continued engagement at | ||||
|---|---|---|---|---|---|
| 6 months | 12 months | ||||
| DVD | Physiotherapy | DVD | Physiotherapy | DVD | Physiotherapy |
|
|
|
|
|
|
Among the correlations, a moderate-sized effect size was found for the relationship between self-efficacy and amount of practice at 3 months and continued engagement at both 6 and 12 months, but only in the DVD group. Believing that asthma was caused by allergy was associated with more practice at 3 months in the face-to-face physiotherapy group. Believing that asthma was caused by health issues was associated with less continued engagement at 12 months in the DVD group. Believing that asthma was caused by smoking was associated with both more practice at 3 months in the DVD group and greater continued engagement at 12 months in the face-to-face physiotherapy group.
In the DVD group, higher expectancy (agreeing strongly that asthma symptoms will be improved by breathing retraining), greater self-efficacy for breathing retraining and greater perceived behavioural control regarding breathing retraining were associated with more practice at 3 months and greater continued engagement at 6 months. However, the only one of these factors that predicted greater continued engagement at 12 months was self-efficacy for breathing retraining. In addition, stronger intentions to carry out breathing retraining were associated with greater continued engagement at 6 and 12 months in the DVD group. Although the perceived need for support was high in the face-to-face physiotherapy group at baseline, it did not relate to the amount of practice or continued engagement.
In the DVD group, greater enjoyment of treatment was associated with more practice at 3 months and greater continued engagement at 6 and 12 months for all aspects of breathing retraining (stomach breathing, nose breathing, slow breathing, controlled breath holds and relaxation). However, the findings were less consistent for the face-to-face physiotherapy group. Greater enjoyment of treatment was associated with more practice at 3 months for stomach and nose breathing, greater continued engagement at 6 months for stomach breathing, nose breathing, controlled breath holding and relaxation and greater continued engagement at 12 months for stomach breathing, slow breathing, controlled breath holding and relaxation. Experience of appointments with the physiotherapist was not related to practice or continued engagement.
With regard to practical barriers, in the DVD group problems due to uncertainty were associated with less practice at 3 months and less continued engagement at 6 months. Problems due to doubts, practical problems and lack of support were associated with less practice at 3 months in both the DVD group and the face-to-face physiotherapy group. Problems due to doubts and practical problems were associated with less continued engagement at 6 and 12 months in the DVD group only. Problems due to lack of support were associated with less continued engagement at 6 months in the DVD group only. Problems due to symptoms were associated with less continued engagement at 6 months in the DVD group only.
The significant correlates shown in Table 53 were input into the multiple regression detailed in Appendix 14 to predict the amount of practice at 3 months. More practice at 3 months was predicted by fewer practical problems and fewer problems due to lack of support (R2 = 0.33, (F17,66 = 1.92; p < 0.05). This model explained 33% of the variance in practice at 3 months. This means that individuals who experienced fewer practical problems in carrying out breathing retraining and fewer problems due to lack of support practised more.
The significant correlates shown in Table 53 were input into the multiple regression detailed in Appendix 15 to predict continued engagement at 6 months. More engagement at 6 months was predicted by fewer problems due to symptoms, fewer problems due to doubts and finding relaxation training enjoyable. This model explained 24% of the variance in continued engagement at 6 months (F14,174 = 3.97; p < 0.001). According to this model, individuals who experienced fewer problems due to symptoms and doubts and who found relaxation training more enjoyable were more likely to continue to engage with breathing training at 6 months.
The significant correlates shown in Table 53 were input into the multiple regression in Appendix 16 to predict variables associated with continued engagement at 12 months. The overall model was significant, explaining 17% of the variance in continued engagement at 12 months (F12,173 = 3.05; p < 0.001). However, no individual predictors of continued engagement at 12 months were identified.
Analyses to model the theory of planned behaviour and explore whether intentions, amount of practice, engagement and continued engagement can be predicted by the model
The theory of planned behaviour hypothesises that attitudes, subjective norms and perceived behavioural control will predict intentions and that intentions and perceived behavioural control will predict behaviour (amount of practice and continued engagement at 6 months and 12 months). Correlational analyses indicated that there is a strong relationship among most of the variables (see Appendix 17).
A logistic regression was carried out to assess the role of attitudes, subjective norms and perceived behavioural control in the prediction of intentions (see Appendix 18). Overall, the model was a good fit to the data (χ2 (6) = 154.23; p < 0.001). Perceived behavioural control was found to be a significant predictor of intentions (odds ratio 2.21, 95% CI 1.79 to 2.72), but attitudes and subjective norms were not.
A hierarchical linear regression assessed the role of intentions and perceived behavioural control in predicting continued engagement at 6 months (see Appendix 19). The final model, in which perceived behavioural control was the only significant predictor of continued engagement, explained 3% of the variance in continued engagement at 6 months: (F2,242 = 7.31; p < 0.001).
A multiple regression was carried out to assess the role of intentions and perceived behavioural control in predicting continued engagement at 12 months (see Appendix 20). Intention was entered in the first step and was a significant predictor (95% CI 0.40 to 2.71). Although neither factor was significant at the second step, the model was a significant predictor, explaining 3% of the variance (F2,238 = 3.87; p < 0.05).
Finally, a multiple regression was carried out to assess the role of intentions and perceived behavioural control in predicting the amount of practice at 3 months (see Appendix 21). No significant predictors were identified. The model explained 1% of the variance in the amount of practice at 3 months (F2,257 = 2.99; p = 0.05).
Checking the factor structure of the Problematic Experiences of Therapy Scale
The factor structure of the PETS was checked using exploratory factor analysis [principal axis factoring, with oblique (direct oblimin) rotation to allow for correlations between factors] as (1) it has not previously been used in an asthma population and (2) the ‘problems due to lack of support’ subscale questions were new and had not previously been validated. The model was forced into the hypothesised five-factor solution and items 3 and 5 were excluded from the analysis because of multicollinearity. The fit of the final model was good. The determinant (0.00002) indicated no further multicollinearity and the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy indicated that the data were suitable for factor analysis (KMO = 0.86). Bartlett’s test of sphericity was significant (χ2 (105) = 2283.81; p < 0.001), indicating that there was a sufficient relationship between the variables. Communalities were all > 0.5, indicating sufficient common variance between the variables. The pattern matrix (see Appendix 22) showed that all items loaded clearly onto their intended factors (all loadings were > 0.63, with no cross-loading). The biggest amount of variance in the data set was explained by the new ‘problems due to lack of support’ subscale, which accounted for 44.49% of the total variance, substantially more than any of the other subscales (which ranged between 6.64% and 15.25%).
All subscales showed good internal consistency. The inter-item correlation matrices were all > 0.3 and the corrected item–total correlations were also well above 0.3. The overall Cronbach’s alpha values were all good and ranged from 0.76 to 0.90 (see Appendix 22). A higher Cronbach’s alpha value (increasing from 0.85 to 0.90) could have been achieved if item 15 were deleted from the ‘problems due to lack of support’ subscale. However, it was decided to retain the item as it had a good theoretical fit and seemed suitable according to all other aspects of the exploratory factor and Cronbach’s alpha analyses. Appendix 23 shows the component correlation matrix between the five factors. It is interesting to note that there are both positive and negative relationships between the factors.
Discussion
After being informed of group allocation, the face-to-face physiotherapy group had more positive perceptions of breathing retraining than the DVD group, including greater intentions to carry out the exercises and being less likely to believe that carrying out breathing retraining would be too time-consuming or difficult to fit into their daily routine. This suggests that physiotherapy delivered by a physiotherapist may be more positively received than a DVD, and health-care professionals need to think of ways to enhance the appeal of breathing retraining delivered by DVD in practice. Those who were aware that they had been randomised to the physiotherapist group also felt a significantly greater need for physiotherapist support than those who were aware that they had been randomised to the DVD group. This suggests that the anticipation of support can raise expectations about how much that support is needed. This should be taken into account when considering patient expectation management in clinical practice. This is particularly important as the face-to-face physiotherapy group continued to perceive a need for physiotherapist support.
The face-to-face physiotherapy group found the appointments with the physiotherapist to be extremely enjoyable and reported greater enjoyment of the stomach breathing, nose breathing and relaxation exercises than those in the DVD group. They also experienced significantly fewer problems due to symptoms, uncertainty, doubt and lack of support. This shows that the participants in the face-to-face physiotherapy group felt supported by the physiotherapist and suggests that seeing a physiotherapist is the ideal treatment for maximising enjoyment of breathing retraining. However, as the trial was carried out by a single physiotherapist, it is not clear whether these findings were the result of individual factors related to the physiotherapist or simply the act of seeing a physiotherapist. Further research is needed to explore experiences of breathing retraining carried out by more than one physiotherapist. Overall, levels of engagement with breathing retraining were extremely high, suggesting that access to a DVD plus the Breathing Freely booklet is sufficient for enabling engagement.
Those in the face-to-face physiotherapy group also spent significantly more time practising breathing retraining and were more likely to continue to carry out the stomach breathing and nose breathing exercises at 6 and 12 months than those in the DVD group. This may be because their initial greater levels of practice led to stomach and nose breathing becoming new habits, in line with research that habit formation is an effective behaviour change strategy. 41
A moderate effect size was found for the relationship between self-efficacy and amount of practice at 3 months and continued engagement at both 6 and 12 months, but only in the DVD group. This suggests that if people with asthma are being expected to undertake breathing retraining with just the support of a DVD, it is important that they are confident that they can carry out breathing retraining correctly every day and without making their symptoms worse. However, although the perceived need for support was high in the face-to-face physiotherapy group at baseline, it did not relate to the amount of practice or continued engagement.
Believing asthma to be caused by health issues was associated with less continued engagement at 12 months in the DVD group. This is in line with the medical model: if people believe that asthma is not under their control, they may be less likely to engage with interventions to reduce symptoms.
In the DVD group, greater enjoyment of treatment was associated with more practice at 3 months and greater continued engagement at 6 and 12 months, for all aspects of breathing retraining. This suggests that if people with asthma are being expected to undertake breathing retraining delivered by DVD, it may be helpful to maximise enjoyment by presenting it in an attractive way. However, these findings were less consistent for those in the face-to-face physiotherapy group, maybe because seeing the physiotherapist provided them with greater extrinsic motivation to practise initially.
In the DVD group, more problems due to uncertainty, doubts, practical problems and lack of support were associated with less practice at 3 months and less continued engagement. This suggests that, if people with asthma are not confident about breathing retraining and do not feel supported in carrying it out, they may experience problems in engaging with it. Future research could examine whether or not this issue might be addressed by providing some (e.g. telephone or online) access to physiotherapist support.
Conclusions
Implications for health care and recommendations for research
Almost all participants engaged with breathing retraining, suggesting that it is likely to be valued as a method of asthma management. Participants in the face-to-face physiotherapy group enjoyed the techniques more, reported practising more at 3 months and demonstrated greater continued engagement at 6 and 12 months, but this was not associated with better outcomes. In the DVD group, those who enjoyed the techniques more and reported greater confidence in their ability to carry them out also practised more at 3 months and demonstrated greater continued engagement with the techniques at 6 and 12 months.
Chapter 7 Discussion
Study findings
Overview
We report the results of a pragmatic, three-arm, parallel-group RCT comparing the effectiveness of a breathing retraining programme for people with suboptimal asthma control delivered by a self-guided programme (as a DVD with a supporting booklet, both developed for the study) with the effectiveness of a three-session face-to-face breathing retraining programme delivered by a respiratory physiotherapist and a control consisting of usual care. To our knowledge this is by far the largest and most rigorous study on breathing retraining exercise to be reported in the world literature. This was designed as a ‘pragmatic’ study, that is, one that had broad entry criteria to allow the participation of most people with asthma in the community and that included study measurements and procedures that interfered as little as possible with usual clinical care. Only two study-related visits were made (at baseline and 12 months), with two further postal questionnaires at 3 and 6 months, and normal care was allowed to proceed over the 12 months of the study. We supplemented visit measurements and questionnaire data with routinely collected clinical data from the GP medical record. Our primary outcome was asthma-related QoL, with measurement of a range of other patient-reported outcomes, objective physiological outcomes and health resource use outcomes. A formal health economic evaluation was built into the programme and both qualitative and quantitative process evaluations were carried out. The study was set in primary care, recruiting from research general practices in Wessex through the CRN, and we successfully achieved recruitment targets, with a high level of retention in the study. We received good feedback from the practices hosting the study, from patients and from staff; it was an enjoyable and happy study to be involved in for all concerned.
Key outcomes
The study was powered to show the superiority of both active arms over usual care in terms of asthma-related QoL and to show equivalence between the active arms, with equivalence margins being those used in a previous HTA asthma study, which resulted in a New England Journal of Medicine publication. 63 Health-related QoL is the outcome measure that best captures the overall effect of asthma on a patient and we used a very well-validated and widely used instrument to measure this key patient-centred factor in our study as the primary outcome. We were indeed able to show superiority of both active treatment arms over usual care and to show equivalence between the two active treatment arms. The improvements in AQLQ scores were observed by the first (3-month) post-intervention assessment and were maintained or increased over the course of the study. We were also able to show lower asthma-related health-care costs in both active treatment arms than in the usual-care arm, which represents a ‘dominant’ health economic assessment, that is, better outcomes were achieved for lower costs to the NHS. The costs were lowest in the DVD arm, with an 82% chance that usual care was dominated by the provision of the DVD programme.
There was no significant observed change in lung function or in airway inflammation either within or between randomisation treatment arms over the 12 months of the study. There were consistent trends towards modest improvements in asthma symptom scores and other patient-reported outcome measures, asthma attacks, respiratory-related GP consultations and rescue bronchodilator use in the breathing retraining arms compared with the usual-care arm, but none of these reached statistical significance thresholds, other than a low-magnitude improvement in the (already low) depression scores in the DVD treatment arm compared with the usual-care arm. The consistent trends towards improvement with breathing retraining but lack of statistical significance observed for most patient-reported and health resource use measures may indicate that these are chance findings or, more likely, a lack of power of the study to show differences between groups for these outcomes. The reduction in number of asthma attacks is particularly interesting and has not previously been reported in association with breathing retraining, but this study is much larger than previous studies that have been carried out and so is better able to detect a signal. Asthma attacks are relatively uncommon in patients with mild-to-moderate asthma and so a larger study would be needed to provide a large enough sample to determine whether breathing exercises can indeed reduce asthma exacerbations. However, asthma attacks (as defined by the prescribing of oral corticosteroids for worsening asthma, as recommended by the ERS/ATS Task Force on asthma outcomes)33 were numerically reduced in this study by approximately one-third in those undergoing breathing retraining. As there was no significant effect on lung function or airway inflammation, it seems that, if a reduction in exacerbations is confirmed in further studies, the mechanism is likely to reflect consulting behaviour and tolerance of symptoms, rather than pathophysiological changes in objective asthma severity. Presented with a patient with asthma attending for worsening asthma symptoms, GPs have limited therapeutic options, and prescription of a course of oral steroids is the most likely action. Some of these episodes may spontaneously resolve without steroids and it is plausible that breathing exercises allowed better symptom control or tolerance on the part of the patient, and so they deferred presentation to a GP, which could have resulted in the prescription of oral steroids. Adverse events were rare, were similar between treatment arms and were of the order of magnitude expected in a 12-month study of this size. There was no suggestion that breathing retraining delivered by either modality caused problems for patients or had any associated adverse events.
The process evaluations confirmed that breathing retraining was acceptable to participants in the study, most of whom felt it to be relevant and useful. Some of the participants in the DVD arm felt that they would liked to have had access to a professional to clarify certain things and to help them through the programme, although the equivalence in outcomes indicates that this was not something that was needed for the breathing retraining to have an effect in this group of patients. We found a NNT of 40 for one patient to benefit from face-to-face physiotherapy compared with the self-guided programme, indicating that for the great majority the self-guided intervention can provide effective breathing retraining. It remains possible that different groups, for example those with more severe asthma or those with more severely impaired QoL, may benefit more from seeing a physiotherapist. Future studies are needed to identify the minority who would benefit from seeing a professional rather than undertaking a self-guided programme.
Clinical relevance and magnitude of effect
We achieved a statistically significant improvement in both active arms compared with usual care, but, as this was a large study, it is important to consider the clinical relevance of the improvements that we observed as well as the statistical significance. The MCID for a patient to notice a difference in QoL is 0.5 for the AQLQ instrument,24 with a change of 1.0 equating to a large improvement. In both the DVD arm and the physiotherapy arm we observed a mean improvement of 1.1 from baseline, so on average patients felt considerably better with regard to how their asthma was affecting their life. Approximately two-thirds of all patients in both breathing retraining arms achieved the MCID of 0.5 over the study period, with three-quarters reaching the MCID for improvement in the PP population, which may better reflect those who actually received the breathing retraining. In terms of how large or small these improvements are in relation to improvements seen with pharmacological interventions commonly used as add-on treatments to inhaled corticosteroids for people with asthma, we may compare them with those seen in another HTA programme-funded UK primary care pragmatic asthma study reported by Price et al. 63 in the New England Journal of Medicine, which compared the effectiveness of add-on long-acting beta-agonists (LABAs) with the effectiveness of leukotriene receptor antagonists (LTRAs) in a directly comparable population to that in our study, UK primary care-treated adult asthma patients found by screening to have suboptimally controlled asthma. This study lacked a control arm but, compared with baseline values, the mean improvements in AQLQ score in the ITT population were 1.0 in the LABA group and 0.8 in the LTRA group, so slightly lower than those that we observed with breathing retraining.
In our study, we did observe an improvement in AQLQ score in the usual-care arm of a lesser but large magnitude, with a mean improvement of 0.8 in the ITT population at 12 months and with 56% reaching the MCID threshold. Improvements in QoL are generally seen in the control and placebo arms in asthma RCTs. This improvement is likely to relate partly to the well-described beneficial effects of trial involvement and partly to a ‘regression to the mean’ effect consequent to our recruitment criterion of impaired QoL. However, we did find significant improvements over the usual-care arm in both active intervention arms, with the mean improvements over usual care being 0.28 (95% CI 0.11 to 0.44) in the DVD arm and 0.24 (95% CI 0.04 to 0.44) in the physiotherapy arms. Although these levels are below the 0.5 threshold for the MCID, it should be noted that 0.5 relates to the MCID for the individual patient and not for the mean between-group differences. Indeed, it has recently been reported by Bateman et al. 64 in a networked meta-analysis of the magnitude of AQLQ changes associated with pharmacological interventions in asthma that this threshold is never reached in RCTs. This study reports the mean improvement in AQLQ score over the control for patients not fully controlled with inhaled corticosteroids as 0.35 (95% CI 0.27 to 0.43) for the addition of a LABA, 0.20 (95% CI 0.13 to 0.27) for the addition of a LTRA, 0.01 (95% CI –0.20 to 0.22) for the addition of theophylline, 0.30 (95% CI 0,20 to 0.40) for the addition of omalizumab and 0.06 (95% CI –0.18 to 0.30) for the addition of a short-acting beta-2 agonist. The improvements over the control that we observed for breathing retraining are therefore of comparable magnitude to those seen with commonly used pharmacological strategies in RCTs, slightly less than those associated with LABAs and slightly greater than those associated with LTRAs.
As recommended by Guyatt et al. 32 in the interpretation of AQLQ data in clinical trials, and using the formula that they recommend, we included a NNT for one patient to benefit above usual-care analysis. This provided NNTs of eight for the DVD group and seven for the physiotherapy group, which we could consider low compared with many commonly used interventions in clinical practice.
These findings suggest that breathing retraining has an important and clinically relevant role for patients who are uncontrolled on standard asthma therapy and may be considered as well as, or instead of, an increase in medication. Further studies incorporating ‘responder analyses’ are needed to clarify whether there are specific characteristics of individual patients to indicate whether a pharmacological or a non-pharmacological adjuvant treatment (or both) will be most effective. The heterogeneity of asthma is increasingly recognised6 and the concepts of stratified and ‘personalised, precision’ medicine are increasingly being applied. With the demonstration in our study of the effectiveness of breathing retraining delivered through a simple and cost-effective self-guided intervention, we feel that this could now become a standard treatment option for people with asthma and that breathing dysfunction could be viewed as a ‘treatable trait’ when personalising treatment for individual patients. 65
Mechanism of effect
The mechanism of effect of breathing retraining on improving QoL in this study cannot be definitively ascertained, but it does not appear to reflect changes in the pathophysiology of the condition, as reflected by lung function or airway inflammation. Likewise, improvements in anxiety or depression between treatment arms were either not significant or of low magnitude and so cannot fully explain the patient-experienced benefits. It is interesting that asthma symptom scores showed only a trend towards a modest improvement over the study period in those undergoing breathing retraining, which was of lower magnitude (in terms of the MCID) than the change in QoL. This implies that reduced symptoms did not fully explain why patients felt so much better and unaffected by their asthma and may indicate a greater tolerance, understanding and acceptance of the symptoms; these were still present but were less distressing and were accepted and coped with better. It is plausible that the possession of a simple non-pharmacological strategy for dealing with symptoms when they occur provided patients with more confidence and improved self-management skills. Indeed, the qualitative interviews carried out as part of the process evaluation would tend to support this hypothesis.
The AQLQ instrument has four subdomains that can be analysed separately: symptoms, activity, environment and emotions. Other than the activity and environment subdomains in the physiotherapy arm, all of these domains showed statistically significant improvements in both breathing retraining arms compared with the control arm, with the largest magnitude improvements being in the emotions domain. This implies that breathing retraining has a wide-ranging impact on the way that asthma affects a patient, which includes less impact of symptoms and environmental triggers, the ability to undertake higher levels of activity and, in particular, less emotional impact. The mechanism of effect is therefore likely to be complex and multifaceted, with effects on the perception of symptoms, behaviours and emotions but not on the underlying pathophysiology of the illness.
Patient perspectives and process evaluation
The process evaluation, with both quantitative (questionnaire-based) and qualitative components, showed that patients engaged well with both of the breathing retraining programmes and felt that both the DVD and the booklet were useful. Some participants in the DVD arm felt that they would have liked to see a health professional as part of the retraining process, although the lack of difference in outcomes between the active treatment arms indicates that this was probably not necessary for the majority of patients. As we recruited patients with milder disease from the broad asthma population, who had not actually presented with problems and asked for professional help, it is possible that there remains a subgroup with more severe problems who would gain greater benefits from seeing a physiotherapist in person. However, in the broad asthma population, a simple self-guided programme (a DVD with our theory-based behaviour change booklet) would seem to be capable of providing an inexpensive and convenient intervention for many people with asthma that does not necessitate a commitment of resources to allow far greater access to respiratory physiotherapists in the community.
Adverse events
There have been no suggestions of treatment-related adverse effects from breathing retraining exercises in the literature to date, but in this large study we carried out a careful analysis of any potential downsides for patients undergoing breathing retraining programmes. We collected information on all respiratory adverse events and adverse events that could plausibly be related to the breathing programmes, including musculoskeletal and psychological/psychiatric adverse events, and on all significant adverse events. Overall, the adverse event profile was as expected in the recruited population and adverse events were not felt by the PIs to be study related, with fewer adverse events in the active arms than in the control arm and DVD and physiotherapy programmes appearing to be well tolerated.
Health resource use and asthma-related costs
The asthma-related costs in the study were lower in the active arms than in the usual-care arm, implying that the interventions have the potential to both improve outcomes and reduce costs, with lowest costs in the DVD arm. The main cost items for each group were asthma-related medications and GP consultations. There was no single factor driving the lower costs in the active arms, but we saw trends towards lower GP consultation rates, lower rescue bronchodilator use and lower exacerbation risk. None of these reductions was individually statistically significant when comparing the active arms with the control arm, although the sample size was inadequate to provide sufficient statistical power to test these outcomes. The mean number of respiratory-related GP consultations was 1.8 in the control arm compared with 1.5 in both the physiotherapy arm and the DVD arm and the percentage of participants having one or more asthma attacks requiring oral steroids was 15% in the control arm, 11% in the physiotherapy arm and 9% in the DVD arm. There was an 82% probability of usual care being dominated by the breathing retraining programme delivered by a DVD (lower costs and better outcomes) and the economic evaluation concluded that in this patient sample the self-guided programme was more cost-effective than the provision of face-to-face physiotherapy, by having outcomes within the equivalence margin and costing less.
Strengths and weaknesses of the study
This study has many strengths and a few weaknesses. This is by far the largest study of breathing retraining in asthma to have been reported and we know of no other studies of comparable size currently under way or planned. To our knowledge, this is the first study of breathing retraining in asthma that has compared a face-to-face programme with a self-guided programme and the first to have a rigorous, prospective health economic evaluation and process evaluations embedded within it.
In terms of study design, this study followed two previous smaller studies from the study team17,19 that together represent an evaluation strategy in keeping with the recommendations of the MRC framework for the evaluation of complex interventions,38 with this project being a Phase IV study. The study design was a ‘pragmatic’ RCT, with a focus on confirming the clinical effectiveness and cost-effectiveness of breathing retraining in a broad and representative primary care population. We therefore had wide entry criteria and study procedures that were designed to be as easy to comply with as possible and to interfere with normal care (other than the provision of the interventions) as little as possible. This allowed primary care sites to host the study without too much disruption to usual care and, we believe, encouraged patient participation. We were able to recruit and retain 655 primary care patients into a 12-month study that required informed consent, study visits and questionnaire completion. The study was designed and supervised by a multidisciplinary team, with strong PPI input at all stages provided by our very active PPI representatives and by the patient charity Asthma UK, which was a full partner in the project from its inception. The multidisciplinary team included physiotherapists, primary and secondary care clinicians, scientists, primary and secondary care respiratory nurses, health psychologists, behaviour change experts specialising in developing and evaluating self-guided behaviour change interventions, statisticians and health economists. The involvement of researchers with a general practice background facilitated a study design that was feasible and acceptable in a busy ‘routine care’ setting and acceptable to patients with mild-to-moderate asthma. The use of an internal pilot allowed hindrances to recruitment and study procedures to be identified and corrected early in the study, with subsequent smooth running of the main trial. Regular steering group meetings were well attended and active, and the team was fortunate to have the support of very interested and active TSC and DMEC members. There was a regular exchange of information with the funder, the National Institute for Health Research (NIHR) HTA programme, as well as the provision of support. Other NIHR structures also provided excellent support to the study. The SCTU undertook trial management most effectively, with several changes in key personnel not impairing the continuity and effectiveness of the team. The friendly, accessible and proactive involvement of the SCTU team was a key strength in delivering a successful study. Invaluable support was provided by the NIHR CRN team involved in the study, with strong support from the research nurse team with regard to the recruitment of practices and patients. There was good partnership with the NHS organisations (clinical commissioning groups and trusts) involved in the study, who also provided support. The general practices hosting the study were invariably supportive and appreciated the pragmatic design of the study and the involvement of practice asthma nurses in the study. Very good feedback was received from the recruiting sites and the GPs, practice nurses and practice managers involved, who were positive about the study and enjoyed their involvement in it. A key strength underlying these effective partnerships was good and open communication throughout the study, allowing ‘buy-in’ and effective team working. As a result of these good relationships, coupled with a sound study design, we were able to recruit beyond the original recruitment targets and to achieve high retention rates.
Another strength of the study is that we used a variety of clinically relevant outcome measures, including a panel of validated patient-reported outcome measures in questionnaire format, physiological lung function measures (that included quality-assured spirometry measured by trained and accredited respiratory nurses) and airway inflammation measures. In addition, patients provided consent to access their routine primary care medical records, which allowed us to collect consultation, prescribing and other health resource use data. This combination of outcome measures allowed us to assess the effects of the intervention on QoL, the key patient-focused outcome measure that reflects patients’ experience of their disease and the amount of disturbance that it causes in their lives, and relate this to other physiological and psychological measures. This multidimensional assessment allows some insight into the mechanisms of effect of this complex intervention and further helps to dispel the myths propagated by some proponents of breathing retraining (such as some of those advocating the Butekyo method) that breathing exercises can ‘cure’ asthma and replace the need for anti-inflammatory and bronchodilator medication. 66–68 In keeping with our previous studies, we found no significant effect of the interventions on parameters measuring the pathophysiology of asthma, such as lung function and airway inflammation, despite a significant lessening of the impact of asthma on people’s lives.
A key strength of the study was the rigorous development process used for transferring the physiotherapy-based programme to a self-guided intervention, with printed support material. The development process that we have described is based on a well-established methodology using an iterative process and extensive and effective patient input. Members of the intervention development team were very experienced, having previously developed a number of effective self-guided interventions in other clinical areas, and applied a tried and tested procedure to develop and refine the materials. The involvement of experienced qualitative researchers allowed patient perspectives to be efficiently and accurately gathered and assessed and to be effectively incorporated into the intervention. A professional media production firm was used to produce the high-quality and patient-friendly self-guided intervention. The self-guided intervention proved to be accessible and well received by patients of all educational levels. The same team designed and performed the process evaluation (including both the qualitative evaluation and the quantitative evaluation), which has contributed to the assessment of the intervention and will inform subsequent implementation work. We were also fortunate to identify experienced local physiotherapists with expertise in breathing retraining who were able to assist with the DVD development and provide the face-to-face physiotherapy intervention.
Similarly, a strength of the study was the involvement of health economists at all stages, from protocol design to writing of the report. The health economic evaluation was seamlessly integrated into the study design and execution. High-quality health economic information is imperative in the current medicopolitical environment if a new intervention is going to be accepted and utilised in clinical care and promoted by commissioners. We believe that the powerful and persuasive health economic arguments, combined with the simplicity and ease of provision of the self-guided intervention, will allow rapid uptake and implementation throughout the NHS, to the benefit of patients.
A further strength of the study was the full involvement of the statistical team at all stages of the project, with input from statisticians on the TSC and the DMEC throughout the study. The choice of primary outcome, primary and secondary statistical analyses and regression models was made with the help of consensus processes after long and constructive debate. The use of the full ITT population in the primary effectiveness analysis, with a number of prespecified sensitivity analyses on the PP population, using different ways of handling missing data and using different regression models, allows confidence to be had in the key messages of the study. The statistically significant superiority of usual care and the equivalence of the self-guided and physiotherapy-based programmes (using prespecified equivalence margins) were maintained in all sensitivity analyses, adding to the confidence that this was a correct finding.
In terms of the limitations of the study, the CONSORT diagram shows that only 10% of the total primary care asthma population invited by post to participate in the study responded to the invitation. It is possible that these respondents represent an atypical sample of patients and that the response to the intervention could be different in the total population. The demographic and asthma severity profile of respondents were, however, typical of those of UK asthma populations. We also feel that our recruitment rate was much higher than that achieved by most asthma studies and it is well documented that the evidence base for asthma guidelines comes from studies with tight inclusion and exclusion criteria that result in < 2% of all community-treated patients being eligible. 69 One cannot force patients to enter trials and we feel that changes in the design of the study and the processes used would not improve on the recruitment rate that we achieved. However, there is now the need for evaluated implementation studies to confirm that the benefits seen in our ‘real-world’ but trial-consenting population can be translated to the wider adult asthma population, as the intervention is made available. Such assessments will need to use routinely collected data or minimally intrusive outcome measures if they are to capture outcomes in the wider population.
Approximately 40% of our responders had unimpaired asthma-related QoL and so were ineligible for the study. This is in keeping with previous population-based studies on QoL in asthma, with the majority being found to have impaired health. We feel that those with unimpaired QoL are probably already coping well with their illness and so are not in need of further help from an intervention such as breathing retraining; however, this applies only to a minority of adult asthma sufferers in the UK and so there is potentially a wide use for this intervention in routine practice.
Our study sample size was powered on the primary outcome measure, the AQLQ. For a number of the secondary outcome measures, including asthma attacks, GP consultations and rescue medication prescriptions, we observed numerical, but non-significant or marginally significant, improvements in outcome in the breathing retraining arms compared with the usual-care arm. We cannot say whether these improvements were chance findings or whether lack of statistical power prevented a significant result being seen. This is particularly true of asthma attacks as these occur only in about 1 in 10 patients with mild-to-moderate asthma treated in the community over a single year. Larger studies would be needed to investigate this fully. As all of these factors (consultations, asthma attacks and rescue medication prescriptions) can be assessed through the analysis of routinely collected data, this could be investigated in implementation studies using analysis of anonymised routine data.
Participants in our study were aware of which study arm they had been allocated to. Although this is a limitation, it is clearly not possible to ‘blind’ a participant in such a study to group allocation. The study was, however, ‘observer blinded’, with the research nurses collecting data and the statistical team blinded as to study arm allocation until the statistical report was finalised. The control arm in this study consisted of usual care and so those in the active arms had a greater provision of care. Although it is possible that participants randomised to the usual-care arm were disappointed not to be receiving an active intervention, they were assured that at the completion of the study they would be offered the intervention if it was found to be effective. It is noteworthy that there was a large improvement in QoL in the control arm during the study, probably reflecting the beneficial effects of trial involvement and a ‘regression to the mean’ effect. Those in the DVD arm received audio-visual and printed support materials but there was no provision of additional care, whereas those in the physiotherapy arm underwent three face-to-face visits with a respiratory physiotherapist and also received the printed support materials. Additional professional care can improve health outcomes in a non-specific placebo-like way. In previous studies earlier in the MRC complex intervention framework process, we included additional professional contact in the control arm17,19 and observed similar improvements in the breathing retraining arm compared with the control arm. As this was a Phase IV pragmatic study, we felt that usual care was the appropriate comparator as it provided a better estimate for the health economic evaluation and provides better information for pragmatic implementation. In this situation the new intervention is provided in addition to current usual care. In our study we did see large improvements in the usual-care arm, although less than in the active arms, reflecting the well-described phenomenon whereby patients benefit simply from taking part in research trials.
Our study included only adults. The intervention materials were designed for adults and would not be transferable to younger age groups without modification. There is anecdotal and very limited study evidence that breathing retraining is feasible and effective in adolescents and in younger children. 70 Asthma has a high prevalence in younger people and there is considerable public and parental interest in non-drug interventions for asthma in children. There is therefore still a need for studies to clarify the clinical effectiveness and cost-effectiveness of breathing retraining interventions in younger people. As we excluded children and adolescents, the groups in whom asthma is most prevalent, the average age of our trial population was higher than that in population-based asthma demographic data. It is possible that there was some under-representation of younger adults, who are generally harder to recruit to time-consuming clinical trials, but the demography in our trial was similar to that in other pragmatic community-based adult asthma clinical trials. For example, in the study by Price et al. ,63 the mean age of subjects was 46 years, 56% were aged ≥ 46 years and the overall demographic profile was similar to ours.
We were able to provide the intervention only to English-language speakers in this study. There is therefore a need to transfer the intervention into other languages and to frame it in culturally appropriate forms for ethnic minorities. Consideration of adaptations for people with limited literacy skills or learning disabilities is also needed.
Our study provided part of the self-guided intervention in the form of a DVD, although potentially this could be provided in other formats, including for the internet and smartphone-based platforms. The DVD format was chosen, first, to control access to the programme in the trial and, second, as this format was very commonly used at the time of the study. We had the ability to provide inexpensive DVD players to anyone randomised to this arm of the study who did not already have access to a DVD player, but no one needed this. However, with advances in digital technology and the widespread use of streaming and downloads over the internet, and the wide ownership and use of smartphones, DVDs are now used less frequently. We cannot guarantee that the intervention would provide identical outcomes if provided as an internet-based platform and so this needs to be investigated in further studies. We are currently transferring the content of the DVD to an internet-streamed breathing retraining programme and piloting its use.
Comparison with the results of other studies
The literature on breathing retraining for asthma provided through health professional-delivered face-to-face programmes is reasonably extensive and convincing, such that the UK BTS/SIGN asthma guideline71 and the WHO GINA guideline72 both recommend breathing retraining as an option for patients uncontrolled on standard therapy, on the basis of a meta-analysis of the improvement in asthma in RCTs. Our finding that physiotherapy was superior to usual care was therefore in keeping with this evidence base. Our study extends this evidence as the pragmatic design allows greater external validity and confidence in generalising the findings than those of previous studies, which have often used selected populations and atypical clinical settings as opposed to UK general practice. The lack of improvement in lung function and airway inflammation is in accordance with the majority of the current literature, although there are some small studies (some with methodical problems) that have reported improvements in pathophysiological parameters.
Very few previous studies have investigated audio-visual programmes for breathing retraining that do not involve contact with a health professional and none have compared face-to-face breathing retraining with such programmes. The equivalence that we have demonstrated between face-to-face physiotherapy and the DVD programme is therefore a novel finding and of considerable significance in terms of implementing the intervention. Despite the evidence of effectiveness for breathing retraining and the guideline recommendations, the vast majority of patients who could benefit do not currently have access to such training and many who do have to pay a private practitioner (often unregulated) for it.
Other novel aspects of our study include its size, duration and range of outcome measures and the rigorous health economic evaluation and process evaluations. These evaluations suggest that the widespread provision of the self-guided breathing retraining programme is likely to be well accepted and to reduce NHS costs for asthma patients.
Implications for services and future research
We report a Phase IV study of breathing retraining for adults with asthma who are uncontrolled on their current treatment, which has shown improved outcomes and reduced costs of a breathing retraining programme delivered by DVD. On the basis of this evidence, we feel that this intervention is potentially of benefit to the majority of adults with asthma in the community and can be delivered to them as a low-cost, and logistically viable, self-guided programme that has the potential to reduce NHS costs as well as benefit patients. We therefore feel that implementation studies are now needed, to optimise delivery and to assess the effects of providing the intervention in a wide and community-based programme. This has implications for service delivery.
Specific research issues that should be addressed include assessing the effectiveness of the programme delivered though internet-based and smartphone-based platforms. We are currently in the process of carrying out the technical work necessary for this. Comparisons of the acceptability and effectiveness of different delivery methods are needed. We would hope to make these comparisons and are in discussion with our charity partner, Asthma UK, on how to structure and deliver the intervention most effectively.
Given the effectiveness of the intervention in an adult population, we feel that there is a need for research to be carried out in a paediatric population, among whom there is great interest in such interventions. It is not justifiable to automatically extrapolate the results of asthma trials in older age groups to children, although this has frequently been carried out, often inappropriately. In addition, the framing of the intervention and the language and directions provided will need to be adapted for younger people. As mentioned earlier, there is also a need to frame and translate the intervention for specific groups, such as ethnic minorities, those with health literacy problems and those with learning disabilities.
Chapter 8 Conclusions
The majority of adults with asthma have impaired QoL, despite the wide availability of effective pharmacotherapy. Breathing retraining exercises have a good evidence base as adjuvant treatment to improve QoL for people with asthma when taught by a physiotherapist in a face-to-face programme. However, although recommended in national and international guidelines, such programmes are under-used because suitably trained specialist therapists are not available to most people who could benefit. We created a self-guided intervention (DVD plus supporting booklet), involving a multidisciplinary professional team, with extensive patient input, and using a qualitative, iterative methodology. Our aim was to transfer the content of a physiotherapy breathing retraining programme to an attractive and accessible format suitable for patients to use at home, at a time convenient to them. We performed a 12-month, three-armed, parallel-group, observer-blinded RCT involving consenting adults with asthma treated in the community in a primary care setting to compare the effects of the new self-guided intervention with the effects of a three-session face-to-face physiotherapy breathing retraining programme plus the booklet and usual care. Asthma-related QoL was the primary outcome. The study was powered to show the superiority of both breathing retraining programmes over usual care and the equivalence of the self-guided and face-to-face physiotherapy programmes and succeeded in doing so. The improvement in QoL was similar to that reported in a meta-analysis of the effects on QoL of add-on pharmacological interventions for uncontrolled patients. There was no significant change in lung function or airway inflammation associated with breathing retraining by either route, implying that the interventions did not alter the underlying biological pathophysiology of asthma. Consistent (but statistically non-significant) trends in improvement in other patient-reported outcome measures (including symptom scores and anxiety and depression scores) and in asthma attacks, GP consultations and rescue medication use were observed with the active interventions compared with usual care. Both active programmes were well received, acted on and accepted by patients and there was no evidence of adverse effects. Asthma-related health-care costs were lower in both of the active arms than in the usual-care arm, with the self-guided intervention having the lowest costs and a > 80% probability of being the ‘dominant’ health economic strategy, that is, the strategy with better outcomes at lower costs.
Physiotherapy breathing retraining exercises are therefore acceptable, clinically effective and cost-effective for adults with asthma and may be delivered by a simple self-guided intervention (our DVD plus our theory-based behaviour change booklet). There is now a need for research on effectively implementing this intervention within usual care and to investigate the effects of similar interventions adapted for other patient groups not studied during this project.
Acknowledgements
The authors would like to acknowledge the key role played by the following: all patients volunteering for the study; those providing PPI input, in particular Mark Stafford-Watson; all practices hosting the study; Asthma UK; the CRN research nurses; Darren Taylor, Lisa McDermott and Mark Stafford-Watson, who were the patients on the DVD; Denise Gibson, the physiotherapist advisor for intervention development; Dr Mark Porter, the presenter on the DVD; Nick Das, Mike Radford and Sarah Benge of the SCTU; NIHR Research Design Service South Central; Ruth DeVos, the face-to-face intervention physiotherapist; Solent NHS trust, which provided non-research costs for the face-to-face physiotherapy; and Tim O’Riordan from Zemedia.
Funding
The authors acknowledge funding from the UK NIHR HTA Board and the NIHR CRN and support from the NIHR CRN.
Sponsor
University of Southampton.
Data Monitoring and Ethics Committee members
Professor Robert McKinley, Dr Rachel Garrod and Dr Ly-Mee Yu.
Trial Steering Committee members
Professor Sally Singh, Dr Christopher Cates, Professor Christopher Griffiths and Mr John Price.
BREATHE trial general practices
-
Adelaide Surgery.
-
Alma Medical Practice.
-
Barton-on-Sea Surgery.
-
Bosmere Medical Practice.
-
Brook Lane Surgery.
-
Clanfield Surgery.
-
Cowplain Family Practice.
-
Derby Road Surgery.
-
Forest End Surgery.
-
Forton Medical Centre.
-
Friarsgate Surgery.
-
Gosport Medical Centre.
-
Heyward Road Surgery.
-
Highcliffe Medical Centre.
-
Highlands Practice.
-
Homewell Practice.
-
Kirklands Surgery.
-
Lordshill Health Centre.
-
New Forest Medical Centre.
-
Nightingale Surgery.
-
North Baddesley Surgery.
-
Old Fire Station Surgery.
-
Osborne Practice.
-
Park and St Francis Surgery.
-
Portsdown Group Practice.
-
Ramillies Surgery.
-
Regents Park Surgery.
-
Ringwood Medical Centre.
-
Stoke Road Medical Centre.
-
Sunnyside Medical Centre.
-
Three Swans Surgery.
-
Waterbrook Medical Practice.
-
Waterside Medical Practice.
-
Woolston Lodge Surgery.
Contributions of authors
Mike Thomas (Professor of Primary Care Research) was the chief investigator of the BREATHE trial. He was responsible for the study conception, provided overall leadership and was the primary care lead.
Anne Bruton (Professor of Respiratory Rehabilitation, NIHR Senior Research Fellow) was a co-applicant and lead physiotherapist for the BREATHE trial. She was responsible for the study conception, provided overall leadership and was the physiotherapy lead.
Paul Little (Professor of Primary Care Research) was a co-applicant and TMG member and was involved in the design of the study.
Stephen Holgate (MRC Clinical Professor of Immunopharmacology and Honorary Consultant Physician) was a co-applicant and TMG member and was involved in the design of the study.
Amanda Lee (Chair in Medical Statistics) was a statistician on the study.
Lucy Yardley (Professor of Health Psychology) was the study lead for the digital intervention development and behaviour change and process evaluation.
Steve George (Emeritus Fellow) was a co-applicant and TMG member and was involved in the design of the study.
James Raftery (Professor of Health Technology Assessment and previously Chairperson of NETSCC and Director of the Wessex Institute) was a TMG member and was the health economic lead for the study.
Jennifer Versnel (Patient Representative and previously Executive Director of Research and Policy at Asthma UK) was a TMG member, was responsible for the study conception and was the PPI co-lead.
David Price (Chair of Primary Care Respiratory Medicine) was a co-applicant and TMG member and was involved in the design of the study.
Ian Pavord (Professor of Respiratory Medicine) was a co-applicant and TMG member and was involved in the design of the study.
Ratko Djukanovic (Professor of Medicine, Director of the Southampton NIHR Respiratory Biomedical Research Unit, Director of the NIHR Southampton Centre for Biomedical Research) was a co-applicant and TMG member and was involved in the design of the study.
Michael Moore (Professor of Primary Health Care Research) was a co-applicant and TMG member and was involved in the design of the study.
Sarah Kirby (Associate Professor) was involved in the process evaluation and qualitative components of the study.
Guiqing Yao (Associate Professor in Health Economics) was involved in the health economic component of the study.
Shihua Zhu (Senior Research Fellow in Health Economics) was involved in the health economic component of the study.
Emily Arden-Close (Lecturer in Health Psychology) was involved in the digital intervention development and the qualitative components of the study.
Manimekalai Thiruvothiyur (Research Assistant) was a statistician on the study.
Frances Webley (BREATHE Trial Manager, SCTU) was responsible for the delivery and co-ordination of the RCT.
Mark Stafford-Watson (Patient Representative) was a TMG member and was the PPI co-lead.
Elizabeth Dixon (BREATHE Trial Manager, SCTU) was responsible for the delivery and co-ordination of the RCT.
Lynda Taylor (BREATHE Trial Co-ordinator, SCTU) was responsible for the delivery and co-ordination of the RCT.
Contribution of other
Denise Gibson (Consultant Respiratory Physiotherapist) was responsible for the physiotherapy intervention design and development.
Publications
Arden-Close E, Teasdale E, Tonkin-Crine S, Pitre N, Stafford-Watson M, Gibson D, et al. Patients’ perceptions of the potential of breathing training for asthma: a qualitative study. Prim Care Respir J 2013;22:449–53. http://dx.doi.org/10.4104/pcrj.2013.00092
Bruton A, Kirby S, Arden-Close E, Taylor L, Webley F, George S, et al. The BREATHE study: Breathing REtraining for Asthma – Trial of Home Exercises. A protocol summary of a randomised controlled trial. Prim Care Respir J 2013;22:PS1–7. http://dx.doi.org/10.4104/pcrj.2013.00047
Data sharing statement
Data included in this report can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. GOAL Investigators Group . Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004;170:836-44. https://doi.org/10.1164/rccm.200401-033OC.
- Demoly P, Gueron B, Annunziata K, Adamek L, Walters RD. Update on asthma control in five European countries: results of a 2008 survey. Eur Respir Rev 2010;19:150-7. https://doi.org/10.1183/09059180.00002110.
- Slader CA, Reddel HK, Spencer LM, Belousova EG, Armour CL, Bosnic-Anticevich SZ, et al. Double blind randomised controlled trial of two different breathing techniques in the management of asthma. Thorax 2006;61:651-6. http://dx.doi.org/10.1136/thx.2005.054767.
- Ernst E. Breathing techniques – adjunctive treatment modalities for asthma? A systematic review. Eur Respir J 2000;15:969-72. https://doi.org/10.1183/09031936.00.15596900.
- James Lind Alliance . Asthma Top 10 n.d. www.jla.nihr.ac.uk/priority-setting-partnerships/asthma/top-10-priorities/ (accessed 1 August 2017).
- Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008;372:1107-19. https://doi.org/10.1016/S0140-6736(08)61452-X.
- Thomas M, McKinley RK, Freeman E, Foy C. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: cross sectional survey. BMJ 2001;322:1098-100. https://doi.org/10.1136/bmj.322.7294.1098.
- Thomas M, McKinley RK, Freeman E, Foy C, Price D. The prevalence of dysfunctional breathing in adults in the community with and without asthma. Prim Care Respir J 2005;14:78-82. https://doi.org/10.1016/j.pcrj.2004.10.007.
- Opat AJ, Cohen MM, Bailey MJ, Abramson MJ. A clinical trial of the Buteyko breathing technique in asthma as taught by a video. J Asthma 2000;37:557-64. https://doi.org/10.3109/02770900009090810.
- Bowler SD, Green A, Mitchell CA. Buteyko breathing techniques in asthma: a blinded randomised controlled trial. Med J Aust 1998;169:575-8.
- Berlowitz D, Denehy L, Johns DP, Bish RM, Walters EH. The Buteyko asthma breathing technique. Med J Aust 1995;162.
- McHugh P, Aitcheson F, Duncan B, Houghton F. Buteyko breathing technique for asthma: an effective intervention. N Z Med J 2003;116.
- Cowie RL, Conley DP, Underwood MF, Reader PG. A randomised controlled trial of the Buteyko technique as an adjunct to conventional management of asthma. Respir Med 2008;102:726-32. https://doi.org/10.1016/j.rmed.2007.12.012.
- Cooper S, Oborne J, Newton S, Harrison V, Thompson Coon J, Lewis S, et al. Effect of two breathing exercises (Buteyko and pranayama) in asthma: a randomised controlled trial. Thorax 2003;58:674-9. https://doi.org/10.1136/thorax.58.8.674.
- Singh V, Wisniewski A, Britton J, Tattersfield A. Effect of yoga breathing exercises (pranayama) on airway reactivity in subjects with asthma. Lancet 1990;335:1381-3. https://doi.org/10.1016/0140-6736(90)91254-8.
- Manocha R, Marks GB, Kenchington P, Peters D, Salome CM. Sahaja yoga in the management of moderate to severe asthma: a randomised controlled trial. Thorax 2002;57:110-15. https://doi.org/10.1136/thorax.57.2.110.
- Thomas M, McKinley RK, Mellor S, Watkin G, Holloway E, Scullion J, et al. Breathing exercises for asthma: a randomised controlled trial. Thorax 2009;64:55-61. https://doi.org/10.1136/thx.2008.100867.
- Holloway EA, West RJ. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax 2007;62:1039-42. https://doi.org/10.1136/thx.2006.076430.
- Thomas M, McKinley RK, Freeman E, Foy C, Prodger P, Price D. Breathing retraining for dysfunctional breathing in asthma: a randomised controlled trial. Thorax 2003;58:110-15. https://doi.org/10.1136/thorax.58.2.110.
- Bott J, Blumenthal S, Buxton M, Ellum S, Falconer C, Garrod R, et al. Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax 2009;64:i1-5. http://dx.doi.org/10.1136/thx.2008.110726.
- British Thoracic Society/Scottish Intercollegiate Guidelines Network . British Guideline on the Management of Asthma 2016. www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/ (accessed 16 August 2017).
- Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention 2016. www.ginasthma.org (accessed 6 March 2017).
- Holloway E, Ram FS. Breathing exercises for asthma. Cochrane Database Syst Rev 2004;1. https://doi.org/10.1002/14651858.CD001277.pub2.
- Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest 1999;115:1265-70. https://doi.org/10.1378/chest.115.5.1265.
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902-7. https://doi.org/10.1034/j.1399-3003.1999.14d29.x.
- EuroQOL Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res 1985;29:199-206. https://doi.org/10.1016/0022-3999(85)90042-X.
- Price D, Musgrave S, Wilson E, Sims E, Shepstone L, Blyth A, et al. A pragmatic single-blind randomised controlled trial and economic evaluation of the use of leukotriene receptor antagonists in primary care at steps 2 and 3 of the national asthma guidelines (ELEVATE study). Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15210.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. https://doi.org/10.1136/bmj.d40.
- Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ 1998;316:690-3. https://doi.org/10.1136/bmj.316.7132.690.
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180:59-9. http://dx.doi.org/10.1164/rccm.200801-060ST.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Yardley L, Barker F, Muller I, Turner D, Kirby S, Mullee M, et al. Clinical and cost effectiveness of booklet based vestibular rehabilitation for chronic dizziness in primary care: single blind, parallel group, pragmatic, randomised controlled trial. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2237.
- Yardley L, Ainsworth B, Arden-Close E, Muller I. The person-based approach to enhancing the acceptability and feasibility of interventions. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0033-z.
- Arden-Close E, Teasdale E, Tonkin-Crine S, Pitre N, Stafford-Watson M, Gibson D, et al. Patients’ perceptions of the potential of breathing training for asthma: a qualitative study. Prim Care Respir J 2013;22:449-53. https://doi.org/10.4104/pcrj.2013.00092.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h1258.
- Brien SB, Bishop FL, Riggs K, Stevenson D, Freire V, Lewith G. Integrated medicine in the management of chronic illness: a qualitative study. Br J Gen Pract 2011;61:e89-96. https://doi.org/10.3399/bjgp11X556254.
- Lally P, Chipperfield A, Wardle J. Healthy habits: efficacy of simple advice on weight control based on a habit-formation model. Int J Obes 2008;32:700-7. https://doi.org/10.1038/sj.ijo.0803771.
- Muller I, Kirby S, Yardley L. Understanding patient experiences of self-managing chronic dizziness: a qualitative study of booklet-based vestibular rehabilitation, with or without remote support. BMJ Open 2015;5. http://dx.doi.org/10.1136/bmjopen-2015-007680.
- Kirby S, Donovan-Hall M, Yardley L. Measuring barriers to adherence: validation of the problematic experiences of therapy scale. Disabil Rehabil 2014;36:1924-9. https://doi.org/10.3109/09638288.2013.876106.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7. https://doi.org/10.1016/j.jpsychores.2005.10.020.
- Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised Illness Perception Questionnaire (IPQ-R). Psychol Health 2002;17:1-16. http://dx.doi.org/10.1080/08870440290001494.
- Leventhal H, Meyer D, Nerenz D, Rachman S. Contributions to Medical Psychology. New York, NY: Pergamon Press; 1980.
- Jessop DC, Rutter DR. Adherence to asthma medication: the role of illness representations. Psychol Health 2003;18:595-612. http://dx.doi.org/10.1080/0887044031000097009.
- Byer B, Myers LB. Psychological correlates of adherence to medication in asthma. Psychol Health Med 2000;5:389-93. https://doi.org/10.1080/713690213.
- Halm EA, Mora P, Leventhal H. No symptoms, no asthma: the acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest 2006;129:573-80. https://doi.org/10.1378/chest.129.3.573.
- Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry 2000;31:73-86. http://dx.doi.org/10.1016/S0005-7916(00)00012-4.
- Paterson C, Dieppe P. Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. BMJ 2005;330:1202-5. https://doi.org/10.1136/bmj.330.7501.1202.
- Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: WH Freeman; 1997.
- Tijou I, Yardley L, Sedikides C, Bizo L. Understanding adherence to physiotherapy: findings from an experimental simulation and an observational clinical study. Psychol Health 2010;25:231-47. http://dx.doi.org/10.1080/08870440802372431.
- Mancuso CA, Rincon M, McCulloch CE, Charlson ME. Self-efficacy, depressive symptoms, and patients’ expectations predict outcomes in asthma. Med Care 2001;39:1326-38. https://doi.org/10.1097/00005650-200112000-00008.
- Lavoie KL, Bouchard A, Joseph M, Campbell TS, Favreau H, Bacon SL. Association of asthma self-efficacy to asthma control and quality of life. Ann Behav Med 2008;36:100-6. https://doi.org/10.1007/s12160-008-9053-8.
- Lorig K. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks, CA: Sage Publications; 1996.
- Fishbein M, Ajzen I. Predicting and Changing Behavior. New York, NY: Psychology Press; 2010.
- Maclean N, Pound P. A critical review of the concept of patient motivation in the literature on physical rehabilitation. Soc Sci Med 2000;50:495-506.
- Ryan RM, Frederick CM, Lepes D, Rubio N, Sheldon KM. Intrinsic motivation and exercise adherence. Int J Sport Psychol 1997;28:335-54.
- Reeve J. The interest–enjoyment distinction in intrinsic motivation. Motiv Emot 1989;13:83-103. https://doi.org/10.1007/BF00992956.
- Mcauley E, Duncan T, Tammen VV. Psychometric properties of the intrinsic motivation inventory in a competitive sport setting – a confirmatory factor-analysis. Res Q Exerc Sport 1989;60:48-5. https://doi.org/10.1080/02701367.1989.10607413.
- Bishop FL, Yardley L, Lewith GT. Treatment appraisals and beliefs predict adherence to complementary therapies: a prospective study using a dynamic extended self-regulation model. Br J Health Psychol 2008;13:701-18. http://dx.doi.org/10.1348/135910707x249570.
- Price D, Musgrave SD, Shepstone L, Hillyer EV, Sims EJ, Gilbert RF, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med 2011;364:1695-707. https://doi.org/10.1056/NEJMoa1010846.
- Bateman ED, Esser D, Chirila C, Fernandez M, Fowler A, Moroni-Zentgraf P, et al. Magnitude of effect of asthma treatments on Asthma Quality of Life Questionnaire and Asthma Control Questionnaire scores: systematic review and network meta-analysis. J Allergy Clin Immunol 2015;136:914-22. http://dx.doi.org/10.1016/j.jaci.2015.03.023.
- Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016;47:410-19. https://doi.org/10.1183/13993003.01359-2015.
- BBC News . The ‘Breathless’ Way to Cure Asthma 1998. http://news.bbc.co.uk/1/hi/health/153320.stm (accessed 16 August 2017).
- YouTube . The Buteyko Method: A Natural Cure for Asthma n.d. www.youtube.com/watch?v=TOpJefLbBJE (accessed 16 August 2017).
- Smith C. Independent 2006. www.independent.co.uk/life-style/health-and-families/health-news/buteyko-breathing-technique-a-cure-for-asthma-411996.html (accessed 16 August 2017).
- Price D, Bjermer L, Popov TA, Chisholm A. Integrating evidence for managing asthma in patients who smoke. Allergy Asthma Immunol Res 2014;6:114-20. https://doi.org/10.4168/aair.2014.6.2.114.
- Macedo TM, Freitas DA, Chaves GS, Holloway EA, Mendonca KM. Breathing exercises for children with asthma. Cochrane Database Syst Rev 2016;4. https://doi.org/10.1002/14651858.CD011017.pub2.
- British Thoracic Society . BTS/SIGN/British/Guideline/on/the/Management/of/Asthma 2016. www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/ (accessed 24 October 2016).
- World Health Organization . WHO ‘GINA’ Asthma Guideline n.d. http://ginasthma.org/ (accessed 24 October 2016).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Department of Health . NHS Reference Costs 2014 to 2015 n.d. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 16 September 2016).
Appendix 1 Numbers of patients randomised by study practice and treatment arm
| Practice | Treatment arm, n | Overall, n | ||
|---|---|---|---|---|
| DVD | Physiotherapy | Usual care | ||
| Adelaide Surgery | 1 | 1 | 1 | 3 |
| Alma Medical Practice | 3 | 2 | 4 | 9 |
| Barton-on-Sea Surgery | 19 | 9 | 19 | 47 |
| Bosmere Medical Practice | 14 | 7 | 15 | 36 |
| Brook Lane Surgery | 7 | 4 | 7 | 18 |
| Clanfield Surgery | 4 | 2 | 4 | 10 |
| Cowplain Family Practice | 5 | 3 | 5 | 13 |
| Derby Road Surgery | 6 | 2 | 5 | 13 |
| Forest End Surgery | 6 | 3 | 6 | 15 |
| Forton Medical Centre | 12 | 6 | 13 | 31 |
| Friarsgate Surgery | 18 | 9 | 19 | 46 |
| Gosport Medical Centre | 8 | 4 | 8 | 20 |
| Heyward Road Surgery | 2 | 1 | 3 | 6 |
| Highcliffe Medical Centre | 6 | 3 | 5 | 14 |
| Highlands Practice | 8 | 4 | 7 | 19 |
| Homewell Practice | 13 | 7 | 14 | 34 |
| Kirklands Surgery | 7 | 4 | 7 | 18 |
| Lordshill Health Centre | 8 | 4 | 6 | 18 |
| New Forest Medical Centre | 4 | 3 | 4 | 11 |
| Nightingale Surgery | 9 | 5 | 9 | 23 |
| North Baddesley Surgery | 4 | 1 | 3 | 8 |
| Old Fire Station Surgery | 5 | 3 | 6 | 14 |
| Osborne Practice | 9 | 4 | 8 | 21 |
| Park and St Francis Surgery | 11 | 5 | 10 | 26 |
| Portsdown Group Practice | 18 | 9 | 19 | 46 |
| Ramillies Surgery | 4 | 2 | 5 | 11 |
| Regents Park Surgery | 3 | 1 | 2 | 6 |
| Ringwood Medical Centre | 10 | 6 | 10 | 26 |
| Stoke Road Medical Centre | 5 | 3 | 6 | 14 |
| Sunnyside Medical Centre | 4 | 1 | 4 | 9 |
| Three Swans Surgery | 7 | 3 | 6 | 16 |
| Waterbrook Medical Practice | 9 | 4 | 8 | 21 |
| Waterside Medical Practice | 10 | 6 | 11 | 27 |
| Woolston Lodge Surgery | 2 | 1 | 3 | 6 |
| Total | 261 | 132 | 262 | 655 |
Appendix 2 Missing baseline primary and secondary outcomes by treatment arm
| Outcome | Treatment arm, n (%) | Overall (N = 655), n (%) | ||
|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | ||
| AQLQ | 17 (6.5) | 12 (9.1) | 16 (6.1) | 45 (6.9) |
| Nijmegen questionnaire | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| HADS | 4 (1.5) | 1 (0.8) | 1 (0.4) | 6 (0.9) |
| EQ-5D | ||||
| Mobility | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| Self-care | 3 (1.1) | 1 (0.8) | 0 (0.0) | 4 (0.6) |
| Usual activities | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| Pain/discomfort | 2 (0.8) | 1 (0.8) | 1 (0.4) | 4 (0.6) |
| Anxiety/depression | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| EQ-5D VAS | 5 (1.9) | 1 (0.8) | 4 (1.5) | 10 (1.5) |
| ACQ | 3 (1.1) | 0 (0.0) | 0 (0.0) | 3 (0.5) |
| FEV1 | 15 (5.7) | 2 (1.5) | 9 (3.4) | 26 (4.0) |
| FeNO | 23 (8.8) | 6 (4.5) | 20 (7.6) | 49 (7.5) |
| FVC | 15 (5.7) | 2 (1.5) | 9 (3.4) | 26 (4.0) |
| FEV1-to-FVC ratio | 15 (5.7) | 2 (1.5) | 9 (3.4) | 26 (4.0) |
| FEV1% predicted | 15 (5.7) | 2 (1.5) | 9 (3.4) | 26 (4.0) |
| PEFR | 17 (6.5) | 3 (2.3) | 13 (5.0) | 33 (5.0) |
| Smoking status | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| Weight | 3 (1.1) | 0 (0.0) | 1 (0.4) | 4 (0.6) |
| Height | 2 (0.8) | 0 (0.0) | 1 (0.4) | 3 (0.5) |
Appendix 3 Patient withdrawals by treatment arm
| Reason for withdrawal | Treatment arm, n (%) | Total (N = 655) n (%) | ||
|---|---|---|---|---|
| DVD (N = 261) | Physiotherapy (N = 132) | Usual care (N = 262) | ||
| Withdrew from the study | 13 (5.0) | 3 (2.3) | 5 (1.9) | 21 (3.2) |
| Primary reason for withdrawal | ||||
| Away for several months | 0 (0.0) | 1 (33.3) | 0 (0.0) | 1 (4.8) |
| No longer wants to take part | 1 (7.7) | 0 (0.0) | 2 (40.0) | 3 (14.3) |
| Did not attend 12-month follow-up | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Family reasons | 1 (7.7) | 1 (33.3) | 1 (20.0) | 3 (14.3) |
| Husband unwell/family illness | 2 (15.4) | 0 (0.0) | 0 (0.0) | 2 (9.5) |
| Left practice | 2 (15.4) | 0 (0.0) | 0 (0.0) | 2 (9.5) |
| Did not receive DVD | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Requested to be deleted | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Notified nurse that since starting the trial asthma worsened | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Study has not helped asthma | 0 (0.0) | 1 (33.3) | 0 (0.0) | 1 (4.8) |
| Too busy – life commitments | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Death | 1 (7.7) | 0 (0.0) | 2 (40.0) | 3 (14.3) |
| No reason given | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
Appendix 4 Baseline characteristics of the patient withdrawals and non-withdrawals
| Characteristic | Withdrawals (N = 21) | Non-withdrawals (N = 634) |
|---|---|---|
| Sex, n (%) | ||
| Male | 5 (23.8) | 231 (36.4) |
| Female | 16 (76.2) | 403 (63.6) |
| Age (years) | ||
| Number included | 21 | 634 |
| Median (IQR) | 59 (51.5–64.5) | 56 (46–64) |
| Minimum, maximum | 23, 70 | 16, 70 |
| Weight (kg) | ||
| Number included | 21 | 630 |
| Median (IQR) | 77 (68–90) | 81.42 (18–46) |
| Minimum, maximum | 54, 118 | |
| Height (cm) | ||
| Number included | 21 | 630 |
| Median (IQR) | 161.5 (157–170) | 166.56 (9.06a) |
| Minimum, maximum | 152, 175 | |
| Smoking status, n (%) | ||
| Current smoker | 0 (0.0) | 50 (7.9) |
| Previous smoker | 6 (28.6) | 212 (33.4) |
| Never smoker | 15 (71.4) | 370 (58.4) |
| What they currently smoke, n (%) | ||
| Cigarettes | 0 (0.0) | 33 (5.2) |
| Tobacco | 0 (0.0) | 18 (2.8) |
| Cigars | 0 (0.0) | 1 (0.2) |
| What they used to smoke, n (%) | ||
| Cigarettes | 5 (23.8) | 191 (30.1) |
| Tobacco | 0 (0.0) | 10 (1.6) |
| Cigars | 1 (4.8) | 1 (0.2) |
| Cigarettes/tobacco | 0 (0.0) | 4 (0.6) |
| Cigarettes/cigars | 0 (0.0) | 4 (0.6) |
| Tobacco/cigars | 0 (0.0) | 1 (0.2) |
| Cigarettes/tobacco/cigars | 0 (0.0) | 1 (0.2) |
| Average cigarettes/day for ever smokers, n | ||
| Median (IQR) | 10 (6.5–20) | 15 (8–20) |
| Minimum, maximum | 5, 20 | 1, 100 |
| Pack-years of smoking | ||
| Number included | 6 | 238 |
| Median (IQR) | 23.25 (6.5–57.25) | 11.85 (3–25) |
| Minimum, maximum | 2, 100 | 0.05, 300 |
| Age diagnosed with asthma (years) | ||
| Number included | 21 | 629 |
| Median (IQR) | 37 (14–52) | 28 (11–45) |
| Minimum, maximum | 3, 60 | 1, 68 |
| Family history of asthma, n (%) | ||
| Mother | ||
| Yes | 3 (14.3) | 103 (16.2) |
| No | 18 (85.7) | 513 (80.9) |
| Unknown | 0 (0.0) | 13 (2.1) |
| Father | ||
| Yes | 2 (9.5) | 72 (11.4) |
| No | 17 (81.0) | 528 (83.3) |
| Unknown | 2 (9.5) | 29 (4.6) |
| Siblings | ||
| Yes | 6 (28.6) | 157 (24.8) |
| No | 14 (66.7) | 426 (67.2) |
| n/a | 0 (0.0) | 43 (6.8) |
| Children | ||
| Yes | 4 (19.0) | 202 (31.9) |
| No | 15 (71.4) | 305 (48.1) |
| n/a | 2 (9.5) | 123 (19.4) |
| Other family members | ||
| Yes | 5 (23.8) | 226 (35.6) |
| No | 15 (71.4) | 379 (59.8) |
| Unknown | 1 (4.8) | 11 (1.7) |
| Asthma triggers, n (%) | ||
| Cats | 13 (61.9) | 275 (43.4) |
| Dogs | 8 (38.1) | 170 (26.8) |
| Dust | 16 (76.2) | 521 (82.2) |
| Exercise | 14 (66.7) | 462 (72.9) |
| Pollen | 13 (61.9) | 417 (65.8) |
| Smoke | 13 (61.9) | 444 (70.0) |
| Stress | 10 (47.6) | 323 (50.9) |
| Food | 5 (23.8) | 128 (20.2) |
| Others | 17 (81.0) | 484 (76.3) |
| FeNO (p.p.b.) | ||
| Number included | 18 | 588 |
| Median (IQR) | 21.5 (15.75–32.75) | 22 (14–34) |
| Minimum, maximum | 5, 202 | 0, 159 |
| FEV1 (l) | ||
| Number included | 20 | 609 |
| Median (IQR) | 2.3 (1.9–2.9) | 2.6 (0.8a) |
| Minimum, maximum | 1.5, 4.1 | |
| FVC (l) | ||
| Number included | 20 | 609 |
| Median (IQR) | 3.0 (2.3–3.5) | 3.4 (0.9a) |
| Minimum, maximum | 2.1, 5.0 | |
| FEV1-to-FVC ratio | ||
| Number included | 20 | 609 |
| Median (IQR) | 0.8 (0.8–0.9) | 0.8 (0.1a) |
| Minimum, maximum | 0.6, 0.9 | |
| FEV1% predicted | ||
| Number included | 20 | 609 |
| Median (IQR) | 90.5 (77.8–103.8) | 90.7 (20.0a) |
| Minimum, maximum | 69, 112 | |
| PEFR | ||
| Number included | 20 | 602 |
| Median (IQR) | 406.0 (355–529) | 422.2 (117.1a) |
| Minimum, maximum | 292, 623 | |
Appendix 5 Supplementary information and sensitivity analyses on rescue inhaler use
| Number of rescue inhalers | Treatment arm, n | Total, n | ||
|---|---|---|---|---|
| DVD | Physiotherapy | Usual care | ||
| 0 | 63 | 22 | 44 | 129 |
| 1 | 30 | 22 | 57 | 109 |
| 2 | 31 | 13 | 28 | 72 |
| 3 | 18 | 16 | 23 | 57 |
| 4 | 21 | 12 | 23 | 56 |
| 5 | 16 | 3 | 10 | 29 |
| 6 | 10 | 6 | 9 | 25 |
| 7 | 5 | 2 | 5 | 12 |
| 8 | 6 | 3 | 11 | 20 |
| 9 | 4 | 2 | 3 | 9 |
| 10 | 5 | 1 | 5 | 11 |
| 11 | 1 | 0 | 1 | 2 |
| 12 | 1 | 3 | 5 | 9 |
| 13 | 1 | 2 | 0 | 3 |
| 14 | 1 | 2 | 0 | 3 |
| 16 | 1 | 0 | 1 | 2 |
| 17 | 0 | 0 | 1 | 1 |
| 18 | 0 | 0 | 2 | 2 |
| 22 | 0 | 0 | 2 | 2 |
| 26 | 0 | 0 | 1 | 1 |
| 28 | 1 | 0 | 0 | 1 |
| 40 | 0 | 1 | 0 | 1 |
| Total | 215 | 110 | 231 | 556 |
| Comparison | Unadjusted IRR | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 1.04 | 0.80 to 1.35 | 0.76 |
| DVD vs. usual care | 0.86 | 0.69 to 1.07 | 0.18 |
| DVD vs. physiotherapy | 0.83 | 0.63 to 1.08 | 0.17 |
| Comparison | Adjusted IRRa | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 1.04 | 0.78 to 1.40 | 0.94 |
| DVD vs. usual care | 0.93 | 0.73 to 1.19 | 0.78 |
| DVD vs. physiotherapy | 0.89 | 0.66 to 1.21 | 0.66 |
Appendix 6 Supplementary information and sensitivity analyses on asthma exacerbation frequency
| Oral corticosteroid courses | Treatment arm, n | Total, n | ||
|---|---|---|---|---|
| DVD | Physiotherapy | Usual care | ||
| 0 | 193 | 97 | 195 | 485 |
| 1 | 16 | 9 | 26 | 51 |
| 2 | 2 | 3 | 8 | 13 |
| 3 | 2 | 0 | 1 | 3 |
| 4 | 2 | 1 | 0 | 3 |
| 10 | 0 | 0 | 1 | 1 |
| Total | 215 | 110 | 231 | 556 |
| Comparison | Unadjusted IRR | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 0.73 | 0.35 to 1.50 | 0.38 |
| DVD vs. usual care | 0.66 | 0.37 to 1.20 | 0.18 |
| DVD vs. physiotherapy | 0.92 | 0.43 to 1.95 | 0.82 |
| Comparison | Adjusted IRRa | 95% CI | p-value |
|---|---|---|---|
| Physiotherapy vs. usual care | 0.79 | 0.33 to 1.90 | 0.81 |
| DVD vs. usual care | 0.69 | 0.33 to 1.42 | 0.45 |
| DVD vs. physiotherapy | 0.87 | 0.34 to 2.19 | 0.93 |
Appendix 7 Numbers of participants in the per-protocol population having none or one or more respiratory-related general practitioner consultations in the 12 months post randomisation
| Number of GP consultations | Treatment arm, n | Total, n | ||
|---|---|---|---|---|
| DVD | Physiotherapy | Usual care | ||
| None | 52 | 25 | 45 | 122 |
| ≥ 1 | 163 | 85 | 186 | 434 |
| Total | 215 | 110 | 231 | 556 |
Appendix 8 Supplementary information and sensitivity analyses on the primary outcome (Asthma Quality of Life Questionnaire)
| AQLQ subdomain | Treatment arm, adjusted mean differencea (95% CI) | Adjusted mean differenceb (95% CI) | ||
|---|---|---|---|---|
| Physiotherapyc vs. usual care | DVD vs. usual care | DVD vs. physiotherapyc | DVD vs. physiotherapyc | |
| Total | 0.22 (0.003 to 0.43)* | 0.28 (0.12 to 0.45)* | 0.07 (–0.15 to 0.28) | 0.03 (–0.20 to 0.27) |
| Symptoms | 0.25 (0.01 to 0.49)* | 0.24 (0.05 to 0.43)* | –0.01 (–0.25 to 0.24) | –0.01 (–0.28 to 0.25) |
| Activities | 0.07 (–0.17 to 0.30) | 0.23 (0.05 to 0.41)* | 0.16 (–0.07 to 0.40) | 0.13 (–0.13 to 0.38) |
| Emotion | 0.37 (0.08 to 0.65)* | 0.38 (0.16 to 0.60)** | 0.01 (–0.28 to 0.30) | 0.02 (–0.29 to 0.33) |
| Environment | 0.21 (–0.06 to 0.48) | 0.33 (0.11 to 0.54)* | 0.12 (–0.16 to 0.39) | 0.09 (–0.20 to 0.39) |
| AQLQ subdomain | Treatment arm, adjusted mean differencea (95% CI) | Adjusted mean differenceb (95% CI) | ||
|---|---|---|---|---|
| Physiotherapyc vs. usual care | DVD vs. usual care | DVD vs. physiotherapyc | DVD vs. physiotherapyc | |
| Total | 0.41 (0.20 to 0.63)** | 0.29 (0.11 to 0.47)** | –0.12 (–0.35 to 0.10) | –0.10 (–0.34 to 0.13) |
| Symptoms | 0.51 (0.26 to 0.76)** | 0.38 (0.18 to 0.59)** | –0.13 (–0.38 to 0.13) | –0.09 (–0.36 to 0.17) |
| Activities | 0.24 (–0.001 to 0.48)* | 0.29 (0.09 to 0.48)* | 0.05 (–0.20 to 0.30) | 0.03 (–0.22 to 0.29) |
| Emotion | 0.42 (0.13 to 0.71)* | 0.23 (–0.01 to 0.47) | –0.19 (–0.49 to 0.11) | –0.13 (–0.44 to 0.18) |
| Environment | 0.42 (0.15 to 0.70)** | 0.26 (0.03 to 0.49)* | –0.17 (–0.45 to 0.12) | –0.16 (–0.46 to 0.13) |
Appendix 9 Adverse event categories
-
Abdominal/gastrointestinal: vaginal discharge, testicular pain, rectal bleeding, abdominal pain – complains of adhesions, altered bowel habit – complains of adhesions, diarrhoea, menorrhagia, bacterial vaginosis, amenorrhoea, gastric band erosion, laparoscopic vaginal hysterectomy, gastritis, vaginal discharge, oral thrush, haemorrhoids, vaginal thrush, suspected urinary tract infection, vulvovaginal candidiasis, epigastric pain plus dysfunctional breathing, pain from pre-existing umbilical hernia, caecal volvulus, small bowel obstruction, colitis, vomiting, vomiting (flu), non-specific abdominal pain, gastric bypass surgery, irritable bowel syndrome, abdominal cramps and diarrhoea, epigastric pain episode linked to ongoing acid reflux, abdominal cramps – gastroenteritis, lower abdominal pain, abdominal pain, breathlessness related to abdominal pain, increased nausea, abdominal pain (right iliac fossa), lower abdominal pain with loose stools, per vaginal bleed post large loop excision of the transformation zone of the cervix, small bowel obstruction, ileus, umbilical hernia repair.
-
Acute exacerbation of asthma: acute asthma exacerbation, asthma exacerbation, exacerbation of asthma/COPD, asthma flair, green sputum worsening (acute exacerbation of asthma), productive cough worsening (acute exacerbation of asthma), mild exacerbation of asthma, chesty cough (acute asthma exacerbation), cough/wheeze (infected exacerbation of asthma), asthma attack, chest infection/asthma exacerbation, infective exacerbation of asthma.
-
Chest pain and cardiovascular: chest pain, chest pains, chest pains with palpitations, chest wall pain, costochondritis, chest pain? – hiatus hernia, rib pain, chest pain – acute coronary syndrome, myocardial infarction, chest pain secondary to indigestion, exertional chest pain (while hovering), chest pain (musculoskeletal), dull pain around right lateral chest wall, pleuritic left-sided chest pain.
-
Increased asthma symptoms: cough, wheeze tachycardia, worsening of asthma, wheeze, chest tightness, wheeze plus shortness of breath, shortness of breath, mild wheeze, shortness of breath on exertion, cough, dyspnoea, asthma triggered by pollen, ongoing asthma symptoms, wheezy cough, acute wheezy bronchitis, respiratory symptoms limiting exercise activities, poor asthma control, nocturnal cough/wheeze, feels unable to fully inhale.
-
Malignancy: malignant lymphoma, malignant neoplasm breast – review, prostate cancer, follicular papillary carcinoma.
-
Musculoskeletal: Baker’s cyst, cervicalgia, golfer’s elbow, degenerative change in lumbosacral region, left leg pain, lacerated left middle finger, back pain, aching muscles, shoulder pain, left arm pain, leg cramps, back pain following fall, arthralgia of hands/shoulders, knee pain, shoulder pain, tennis elbow, lateral epicondylitis, ankle pain, ankle and hip pain, sprain (left) lateral collateral knee ligament, hip pain, acute lumbar back pain, sprain (left) lateral collateral ligament, neck pain, joint aches (virus), joint ache, right knee replacement surgery, left-sided jaw pain, neck pain, lower back pain, fracture of lower vertebra, right shoulder rotator cuff tear, gout, fractured right rib, prolapsed intervertebral lumbar disc (worsening of), right shoulder rotator cuff sprain, right bicep tendon rupture, frozen shoulder (right), worsening of frozen shoulder (right), rotator cuff syndrome, left-sided muscle tightness in neck, septic arthritis.
-
Neurological: tremor, right-hand numbness, headache – different from migraine, neuralgic pain, neuropathic pain, headache, right-sided body numbness, transient ischaemic attack, chronic inflammatory demyelinating polyneuropathy, fatigue, dizziness, double vision, nausea, cerebral venous thrombosis, left-sided numbness, tinnitus, giant cell arteritis, post-FeNO test breathlessness, light-headedness and clammy, migraine.
-
Psychological/psychiatric: shortness of breath (panic attack), anxiety, depression, insomnia, low mood, panic attacks, recurrence of depressive episode, low mood, overbreathing, stress.
-
Respiratory tract infection/cough: acute laryngitis, bibasilar collapse, bronchitis, chest infection, chest infection/shortness of breath on exertion, chest rattle, chesty cough, cold, cold virus – hoarseness and cough, cold virus – sore throat and cough, congestion with cold, cough and shortness of breath, coryzal, cough (flu), cough (post viral), cough (upper respiratory tract infection), cough? – viral laryngitis, cough with green phlegm, wheeze, shortness of breath, crackles and phlegm, cough/cold, dry cough, ear infection, fever (flu), flu-like virus, green sputum, green sputum (upper respiratory tract infection), haemoptysis, influenza A, laryngitis, lower respiratory tract infection, mild cough, night cough, pharyngitis, pleural effusion, pneumonia, post viral cough and wheeze, productive cough, productive cough (lower respiratory tract infection), Pseudomonas – chest infection, Pseudomonas aeruginosa infection, respiratory heart infection, respiratory tract infection, shortness of breath (pneumonia), sore throat (virus), stridor, upper respiratory chest infection, viral cold and cough, viral illness causing wheeze, fever, chest tightness, viral infection, viral upper respiratory tract infection, worsening cough with wheeze and shortness of breath.
-
Rhinitis/rhinosinusitis: (sinusitis) blocked nose, (sinusitis) earache, (sinusitis) sore eyes, acute sinusitis, allergic rhinitis, blocked ear – left sided, catarrh, chronic rhinitis, congestion, earache/congested sinuses, Eustachian tube dysfunction, inflamed left ear canal, nasal congestion, otitis media, post-nasal drip, runny nose, sinusitis, worsening hay fever, worsening of otitis externa.
-
Miscellaneous: acute tonsillitis, anaemia, balanitis, benign paroxysmal positional vertigo, blepharitis, calf pain, chalazion (eyelid cyst), conjunctivitis, contact dermatitis, contraception, cough? gastric reflux? post-nasal drip, dysfunctional breathing, Eustachian tube dysfunction (bilateral), excision of thyroglossal cyst, eye symptoms, facial pain, fever, flu symptoms, heterozygous factor V Leiden mutation, hoarseness, itch, left otalgia, neck swelling, occasional feeling below sternum of ‘racing’ – fluttering, feels winded, open wound on scalp, oral thrush, otitis externa, pleural effusion, post-spirometry chest discomfort, problems with crumbling teeth, pyrexia (virus), scolding injury, seborrheic dermatitis, sensation of mucus in throat, snoring symptoms, swollen tongue, tight/sore throat, tonsillitis, urinary tract infection, urticarial rash, virus affecting ears, throat and stomach, watery eyes.
Appendix 10 List of all serious adverse events reported
| Arm | Patient ID | Date of onset of SAE | Main symptom | Seriousa | Causalityb |
|---|---|---|---|---|---|
| DVD | 11011 | 14 January 2013 | Gastric band erosion | 3 | 5 |
| 11023 | 30 December 2012 | Chest pain | 3 | 5 | |
| 12114 | 03 April 2014 | Acute exacerbation of asthma | 3 | 3 | |
| 15207 | 26 November 2013 | Chest pain (musculoskeletal) | 3 | 5 | |
| 17121 | 08 August 2013 | Non-specific abdominal pain | 3 | 5 | |
| 20244 | 07 February 2014 | Asthma exacerbation | 3 | 3 | |
| 21269 | 09 March 2014 | Transient ischaemic attack | 3 | 3 | |
| 31447 | 03 March 2015 | Abdominal pain | 3 | 5 | |
| 32446 | 05 June 2015 | Chest infection/coronary artery disease | 3 | 3 | |
| 39613 | 20 July 2015 | Acute exacerbation of asthma | 3 | 3 | |
| 40596 | 05 June 2015 | Death – cardiac arrest | 1 | 5 | |
| Physiotherapy | 10014 | 17 April 2013 | Malignant lymphoma | 2 | 5 |
| 12067 | 28 October 2013 | Chest pain with palpitations | 3 | 5 | |
| 38576 | 17 November 2014 | Septic arthritis | 3 | 5 | |
| 38582 | 16 March 2015 | Asthma exacerbation | 3 | 3 | |
| Usual care | 12057 | 24 May 2014 | Hospitalised – chest infection | 3 | 3 |
| 12091 | 28 December 2013 | Chest pain | 3 | 5 | |
| 12127 | 31 October 2013 | Gastritis | 3 | 5 | |
| 12195 | 26 May 2014 | Chest pain secondary to hiatus hernia | 3 | 5 | |
| 01 September 2014 | Abdominal pain | ||||
| 14095 | 26 April 2014 | Death – pulmonary oedema | 1 | 5 | |
| 15203 | 16 June 2014 | Caecal volvolus | 3 | 5 | |
| 31 July 2014 | Colitis | ||||
| 17173 | 23 July 2014 | Pneumonia | 3 | 3 | |
| 18184 | 02 March 2014 | Stridor | 3 | 5 | |
| 20238 | 11 April 2014 | Death – pleural effusion | 1 | 5 | |
| 25294 | 27 July 2014 | Urinary tract infection | 3 | 3 | |
| 30395 | 26 December 2014 | Influenza A | 3 | 5 | |
| 31434 | 24 March 2015 | Pneumonia | 3 | 4 | |
| 31501 | 04 July 2014 | Cerebral venous thrombosis | 3 | 3 | |
| 32449 | 10 September 2014 | Acute coronary syndrome | 3 | 5 | |
| 34477 | 24 December 2014 | Chest pain – non cardiac | 3 | 5 | |
| 27 June 2014 | |||||
| 22 June 2014 | |||||
| 37547 | 15 July 2015 | Per vaginal bleed post large loop excision of the transformation zone of the cervix | 3 | 5 | |
| 38570 | 22 November 2014 | Pulmonary embolism | 2 | 5 | |
| 02 December 2014 | Lower respiratory tract infection | ||||
| 38572 | 14 February 2015 | Small bowel obstruction | 3 | 5 | |
| 38587 | 10 September 2015 | Tonsillitis | 3 | 5 | |
| 39575 | 09 January 2015 | Infective exacerbated asthma | 3 | 3 |
Appendix 11 List of all adverse events (maximum grade) reported by treatment arm
| Category of main symptom | Nature of adverse event | Patient ID | Date of onset | Severity of adverse eventa | Seriousb | Causalityc |
|---|---|---|---|---|---|---|
| Abdominal/gastrointestinal | Testicular pain | 10004 | 11 October 2013 | 2 | 5 | |
| Gastric band erosion | 11011 | 14 January 2013 | 3 | 3 | 5 | |
| Haemorrhoids | 13051 | 06 May 2014 | 3 | 5 | ||
| Suspected urinary tract infection | 13053 | 11 June 2014 | 2 | 5 | ||
| Pain from pre-existing umbilical hernia | 15201 | UNK May 2013 | 2 | 5 | ||
| Non-specific abdominal pain | 17121 | 08 August 2013 | 2 | 3 | 5 | |
| Irritable bowel syndrome | 24307 | 15 October 2014 | 1 | 5 | ||
| Abdominal cramps and diarrhoea | 25303 | 18 December 2014 | 1 | 4 | ||
| Abdominal cramps – gastroenteritis | 27466 | 30 May 2015 | 2 | 5 | ||
| Abdominal pain | 31447 | 23 March 2015 | 3 | 3 | 5 | |
| Breathlessness related to abdominal pain | 32409 | 17 April 2015 | 2 | 5 | ||
| Acute exacerbation of asthma | Acute exacerbation of asthma | 10003 | 11 February 2013 | 2 | 3 | |
| Asthma exacerbation | 10031 | 11 July 2013 | 2 | 3 | ||
| Exacerbation of COPD | 12064 | 03 March 2014 | 2 | 3 | ||
| Acute asthma exacerbation | 12114 | 03 April 2014 | 3 | 3 | ||
| Asthma flair | 15157 | 29 August 2014 | 1 | 3 | ||
| Asthma exacerbation | 19221 | UNK February 2014 | 1 | 3 | ||
| Asthma exacerbation | 20244 | 07 February 2014 | 2 | 3 | 3 | |
| Exacerbation of asthma | 31433 | 09 January 2015 | 1 | 3 | ||
| Acute exacerbation of asthma | 31453 | 21 April 2015 | 2 | 3 | ||
| Acute respiratory distress syndrome | 32446 | 05 June 2015 | 3 | 3 | 3 | |
| Cough/wheeze (infected exacerbation of asthma) | 33511 | 15 December 2014 | 2 | 3 | ||
| Acute exacerbation of asthma | 33521 | 27 December 2014 | 1 | 3 | ||
| Acute exacerbation of asthma | 33522 | UNK February 2015 | 2 | 3 | ||
| Asthma exacerbation | 34476 | 15 May 2015 | 3 | |||
| Acute exacerbation of asthma | 34516 | 22 June 2015 | 2 | 3 | ||
| Asthma attack | 35545 | 15 August 2015 | 2 | 3 | ||
| COPD exacerbation | 36529 | 22 March 2015 | 3 | 3 | ||
| Exacerbation of asthma | 37538 | 15 December 2014 | 2 | 3 | ||
| Asthma exacerbation | 38562 | 17 March 2015 | 2 | 3 | ||
| Asthma exacerbation | 39613 | 20 July 2015 | 3 | 3 | 3 | |
| Chest pain and cardiovascular | Atrial fibrillation | 10020 | 23 March 2013 | 2 | 5 | |
| Chest pain | 11023 | 30 December 2012 | 2 | 3 | 5 | |
| Hypertension | 13053 | 30 October 2013 | 3 | 5 | ||
| Chest pain (musculoskeletal) | 15207 | 26 November 2013 | 2 | 3 | 5 | |
| Chest pain | 17108 | 25 January 2014 | 2 | 4 | ||
| chest pain | 24307 | 26 May 2014 | 2 | 4 | ||
| Palpitations | 31481 | 17 March 2015 | 1 | 4 | ||
| Chest pain related to abdominal pain | 32409 | 17 April 2015 | 2 | 5 | ||
| Cardiac disorder | 32446 | 26 May 2015 | 4 | 3 | 5 | |
| Chest soreness | 39613 | 01 November 2015 | 1 | 3 | 3 | |
| Cardiac arrest | 40596 | 05 June 2015 | 5 | 5 | ||
| Palpitations | 40609 | 26 February 2015 | 1 | 5 | ||
| Left lateral chest wall pain | 40614 | 02 December 2015 | 1 | 5 | ||
| Increased asthma symptoms | Worsening of asthma | 12114 | 24 February 2014 | 1 | 3 | |
| Worsening of asthma | 13053 | 06 August 2013 | 2 | 3 | ||
| Cough | 15157 | 09 June 2014 | 1 | 3 | ||
| Wheeze | 16136 | UNK April 2014 | 1 | 3 | ||
| Cough | 16160 | 31 March 2014 | 1 | 3 | ||
| Shortness of breath | 18172 | 18 October 2013 | 1 | 3 | ||
| Cough | 18172 | 28 May 2014 | 1 | 3 | ||
| Cough | 19221 | UNK February 2014 | 1 | 3 | ||
| Cough | 21269 | 06 March 2014 | 1 | 3 | ||
| Cough | 23300 | 27 December 2014 | 1 | 3 | ||
| Cough | 25298 | 01 November 2014 | 1 | 3 | ||
| Shortness of breath from hay fever | 25308 | 09 June 2014 | 1 | 3 | ||
| Wheezy cough | 25332 | 01 April 2014 | 1 | 3 | ||
| Cough | 26336 | 24 June 2014 | 1 | 3 | ||
| Tight chest | 30391 | 13 October 2014 | 1 | 3 | ||
| Increasing shortness of breath | 31401 | 15 May 2014 | 2 | 3 | ||
| Poor asthma control | 31455 | UNK November 2014 | 1 | 3 | ||
| Worsening of asthma | 32451 | UNK February 2015 | 2 | 3 | ||
| Shortness of breath | 34438 | 15 September 2014 | 1 | 3 | ||
| Cough | 34476 | 24 December 2014 | 1 | 3 | ||
| Cough/wheeze | 34516 | 15 May 2015 | 1 | 3 | ||
| Worsening of asthma (cough and wheeze) | 37539 | 05 December 2014 | 3 | 3 | ||
| Chest tightness | 37549 | 20 February 2015 | 2 | 3 | ||
| Feels unable to fully inhale | 38562 | 11 October 2015 | 2 | 3 | ||
| Malignancy | Malignant neoplasm breast – review | 13051 | 01 April 2014 | 3 | 5 | |
| Prostate cancer | 24289 | 05 January 2015 | 3 | 5 | ||
| Follicular papillary carcinoma | 30426 | 21 May 2014 | 4 | 3 | 5 | |
| Musculoskeletal | Baker’s cyst | 10003 | 30 September 2013 | 1 | 5 | |
| Golfer’s elbow | 10004 | 11 February 2013 | 2 | 4 | ||
| Lacerated left middle finger | 10020 | 23 September 2013 | 1 | 5 | ||
| Aching muscles | 10033 | 06 August 2013 | 1 | 5 | ||
| Left arm pain | 12064 | 17 September 2013 | 2 | 4 | ||
| Leg cramps | 12085 | 06 May 2014 | 1 | 5 | ||
| Back pain following fall | 12089 | 20 June 2014 | 2 | 5 | ||
| Lateral epicondylitis | 13051 | 06 May 2014 | 3 | 5 | ||
| Right shoulder rotator cuff strain | 31433 | 14 October 2014 | 2 | 5 | ||
| Neck pain | 33522 | UNK November 2014 | 2 | 4 | ||
| Spinal stenosis | 40596 | 13 March 2015 | 2 | 5 | ||
| Right frozen shoulder | 40598 | 01 May 2015 | 1 | 5 | ||
| Back pain | 42618 | 28 October 2015 | 2 | 5 | ||
| Lower back pain | 42629 | 19 August 2015 | 1 | 5 | ||
| Hip pain | 43651 | 02 July 2015 | 1 | 5 | ||
| Neurological | Right-hand numbness | 12049 | 31 October 2013 | 1 | 4 | |
| Headache – different from migraine | 12089 | 07 November 2013 | 2 | 4 | ||
| Right-sided body numbness | 21269 | 09 March 2014 | 4 | 4 | ||
| Transient ischaemic attack | 21269 | 09 March 2014 | 3 | 3 | 5 | |
| Fatigue | 31420 | 27 November 2014 | 1 | 4 | ||
| Dizziness | 33495 | 22 September 2014 | 2 | 4 | ||
| Tinnitus | 36535 | UNK April 2015 | 2 | 5 | ||
| Psychological/psychiatric | Anxiety with depression | 13045 | 30 December 2013 | 2 | 3 | |
| Insomnia | 13051 | 04 October 2013 | 3 | 3 | ||
| Depression | 16136 | UNK September 2013 | 2 | 3 | ||
| Depression | 20248 | 01 September 2014 | 1 | 3 | ||
| Depression | 22275 | UNK January 2014 | 1 | 3 | ||
| Anxiety | 32404 | UNK March 2015 | 1 | 3 | ||
| Anxiety | 35540 | 28 December 2014 | 1 | 3 | ||
| Depression | 35545 | UNK May 2015 | 2 | 3 | ||
| Low mood | 38580 | UNK January 2015 | 1 | 3 | ||
| Memory loss | 40596 | 22 December 2014 | 2 | 3 | ||
| Anxiety attack | 43637 | 21 October 2015 | 1 | 3 | ||
| Respiratory tract infection/cough | Chest infection | 12064 | 21 May 2014 | 2 | 5 | |
| Chesty cough | 12114 | 11 October 2013 | 2 | 5 | ||
| Upper respiratory tract infection | 13061 | 26 September 2013 | 1 | 4 | ||
| Chest infection | 15155 | 31 December 2013 | 1 | 4 | ||
| Chest infection | 15157 | 07 May 2014 | 1 | 5 | ||
| Productive cough | 16136 | UNK April 2014 | 1 | 5 | ||
| Green sputum | 16160 | 03 April 2014 | 1 | 5 | ||
| Chesty cough | 17108 | 08 April 2014 | 2 | 4 | ||
| Laryngitis | 17144 | 16 December 2013 | 1 | 5 | ||
| Worsening of cough | 18172 | 13 June 2014 | 1 | 5 | ||
| Bronchitis | 19221 | UNK November 2013 | 1 | 5 | ||
| Chest infection | 20249 | 12 April 2014 | 1 | 5 | ||
| Cold | 21269 | 04 November 2014 | 1 | 5 | ||
| Cold | 23315 | 01 January 2015 | 1 | 5 | ||
| Upper respiratory tract infection | 23316 | 13 March 2014 | 1 | 5 | ||
| Chest infection | 23359 | 23 January 2015 | 1 | 5 | ||
| Chest infection | 24289 | 15 July 2014 | 1 | 5 | ||
| Viral cough | 25332 | 14 December 2014 | 1 | 5 | ||
| Lower respiratory tract infection | 25376 | 25 December 2014 | 1 | 5 | ||
| Viral infection | 26323 | 18 January 2015 | 1 | 5 | ||
| Chest infection | 26374 | 02 December 2014 | 1 | 5 | ||
| Upper respiratory tract infection | 27337 | 05 July 2014 | 1 | 5 | ||
| Cold | 27367 | 05 October 2014 | 1 | 5 | ||
| Upper respiratory tract infection | 27466 | 05 January 2015 | 2 | 5 | ||
| Sore throat | 29463 | 08 December 2014 | 1 | 5 | ||
| Chesty cough | 30391 | 24 March 2015 | 1 | 5 | ||
| Productive cough | 31401 | 07 November 2014 | 2 | 4 | ||
| Upper respiratory tract infection | 31420 | 29 December 2014 | 2 | 4 | ||
| Chest infection | 31447 | 09 June 2015 | 2 | 4 | ||
| Upper respiratory tract infection | 31481 | 01 February 2015 | 1 | 4 | ||
| Chest infection | 32407 | UNK December 2014 | 2 | 5 | ||
| Chest infection | 32446 | 05 June 2015 | 3 | 3 | 5 | |
| Lower respiratory tract infection | 32451 | 11 March 2015 | 2 | 5 | ||
| Chesty cough | 33511 | 26 January 2015 | 2 | 4 | ||
| Productive cough (lower respiratory tract infection) | 33520 | 10 December 2014 | 1 | 4 | ||
| Chest infection | 33521 | 12 December 2014 | 1 | 4 | ||
| Cold | 33522 | UNK February 2015 | 1 | 4 | ||
| Upper respiratory tract infection | 34456 | 22 May 2015 | 2 | 5 | ||
| Cough and hoarse voice | 34476 | 16 January 2015 | 2 | 5 | ||
| Worsening cough with wheeze and shortness of breath | 34516 | 02 October 2014 | 2 | 4 | ||
| Cold | 35531 | 01 December 2014 | 1 | 5 | ||
| Upper respiratory tract infection | 35540 | 01 January 2015 | 2 | 4 | ||
| Sore throat | 36554 | 30 July 2015 | 1 | 5 | ||
| Post viral cough and wheeze | 37549 | 06 January 2015 | 2 | 5 | ||
| Viral cold and cough | 38560 | 28 December 2014 | 1 | 5 | ||
| Chest infection/shortness of breath on exertion | 38561 | 02 January 2015 | 2 | 4 | ||
| Chest infection | 38579 | 09 April 2015 | 2 | 5 | ||
| Cold virus – hoarseness and cough | 38580 | 08 October 2015 | 1 | 5 | ||
| Chest infection | 38592 | 19 May 2015 | 2 | 4 | ||
| Cough | 38602 | 11 December 2014 | 2 | 5 | ||
| Cough and brown phlegm | 39613 | 08 December 2014 | 2 | 5 | ||
| Upper respiratory tract infection | 41648 | 20 December 2015 | 1 | 5 | ||
| Lower respiratory tract infection | 42618 | 20 April 2015 | 1 | 5 | ||
| Acute bronchitis | 43638 | 18 November 2015 | 1 | 5 | ||
| Cough | 43646 | 23 March 2015 | 1 | 5 | ||
| Chest infection | 43651 | 03 January 2016 | 1 | 5 | ||
| Cough | 43652 | 05 January 2016 | 2 | 5 | ||
| Rhinitis/rhinosinusitis | Chronic rhinitis | 10031 | 07 August 2013 | 2 | 5 | |
| Worsening hay fever | 12114 | 23 May 2014 | 1 | 5 | ||
| Otitis media | 13051 | 17 January 2014 | 3 | 5 | ||
| Nasal congestion | 15155 | 17 August 2013 | 1 | 4 | ||
| (Sinusitis) sore eyes | 16196 | 26 December 2013 | 1 | 5 | ||
| Inflamed left ear canal | 23316 | 18 February 2014 | 1 | 5 | ||
| Sinusitis | 25308 | 25 January 2015 | 1 | 5 | ||
| Congestion | 29463 | 08 December 2014 | 1 | 5 | ||
| Worsening of otitis externa | 31401 | 15 May 2014 | 2 | 5 | ||
| Eustachian tube dysfunction | 31447 | 10 December 2014 | 1 | 4 | ||
| Earache | 34476 | 26 March 2015 | 1 | 5 | ||
| Acute sinusitis | 35531 | 01 December 2014 | 2 | 4 | ||
| Blocked ear | 36535 | UNK April 2015 | 2 | 5 | ||
| Post-nasal drip | 38568 | 08 May 2015 | 2 | 4 | ||
| Eustachian tube dysfunction | 43638 | 08 October 2015 | 1 | 5 | ||
| Miscellaneous | Watery eyes | 10004 | 08 April 2013 | 2 | 5 | |
| Scolding injury | 10020 | 02 October 2013 | 1 | 5 | ||
| Eye symptoms | 10033 | 31 October 2013 | 1 | 5 | ||
| Occasional feeling below sternum of ‘racing’ – fluttering, feels winded | 12085 | 06 May 2014 | 1 | 5 | ||
| Urticarial rash | 13045 | 13 January 2014 | 2 | 5 | ||
| Urinary tract infection | 13051 | 30 May 2014 | 2 | 5 | ||
| Snoring symptoms | 13052 | 02 January 2014 | 2 | 5 | ||
| Facial pain | 20244 | 07 March 2014 | 1 | 4 | ||
| Benign paroxysmal positional vertigo | 29463 | 25 June 2014 | 1 | 4 | ||
| Sensation of mucus in throat | 31435 | UNK September 2014 | 2 | 4 | ||
| Facial pain | 34476 | 26 March 2015 | 1 | 5 | ||
| Post-spirometry chest discomfort | 37549 | 02 September 2014 | 1 | 5 | ||
| Temporomandibular joint disorder | 38597 | UNK February 2015 | 3 | 5 | ||
| Blocked ear right | 38603 | UNK April 2015 | 1 | 5 | ||
| Lower back pain (secondary to car accident in 2006) | 38622 | 10 July 2015 | 1 | 5 | ||
| Claudication left calf | 40596 | 26 January 2015 | 1 | 5 | ||
| Fever | 42629 | 30 August 2015 | 1 | 5 | ||
| Vertigo | 43631 | 21 June 2015 | 1 | 5 |
| Category of main symptom | Nature of adverse event | Patient ID | Date of onset | Severity of adverse eventa | Seriousb | Causalityc |
|---|---|---|---|---|---|---|
| Abdominal/gastrointestinal | Vaginal discharge | 10001 | 22 January 2013 | 1 | 5 | |
| Abdominal pain | 10032 | 08 March 2013 | 2 | 5 | ||
| Haemorrhoids | 13060 | 22 January 2014 | 2 | 5 | ||
| Gastric bypass surgery | 20267 | 29 March 2014 | 2 | 3 | 5 | |
| Increased nausea | 33507 | UNK October 2014 | 2 | 4 | ||
| Acute exacerbation of asthma | Asthma exacerbation | 10001 | 24 September 2013 | 2 | 3 | |
| Productive cough worsening (acute exacerbation of asthma) | 18183 | 05 February 2014 | 1 | 3 | ||
| Acute exacerbation of asthma | 25379 | 22 November 2014 | 2 | 3 | ||
| Exacerbation of asthma | 25379 | 13 February 2015 | 2 | 3 | ||
| Exacerbation of asthma | 31417 | 15 July 2014 | 2 | 3 | ||
| Asthma exacerbation | 31432 | 12 October 2014 | 2 | 3 | ||
| COPD exacerbation | 37537 | 03 August 2015 | 3 | 3 | ||
| Chest infection/asthma exacerbation | 38576 | 28 September 2015 | 3 | 3 | ||
| Asthma exacerbation | 38582 | 16 March 2015 | 3 | 3 | 3 | |
| Asthma exacerbation | 40600 | 24 March 2015 | 1 | 3 | ||
| Chest pain and cardiovascular | Deep-vein thrombosis | 10014 | 18 September 2013 | 3 | 5 | |
| Chest pains with palpitations | 12067 | 28 October 2013 | 2 | 3 | 5 | |
| Pleuritic left-sided chest pain | 19220 | 04 July 2014 | 1 | 4 | ||
| Chest pain | 33507 | 20 October 2014 | 2 | 4 | ||
| Costochondritis | 34460 | 15 July 2014 | 2 | 5 | ||
| Costal margin chest pain | 43650 | 15 June 2015 | 1 | 5 | ||
| Increased asthma symptoms | Cough | 10032 | 21 November 2013 | 1 | 3 | |
| Wheeze plus shortness of breath | 17110 | 15 March 2014 | 3 | 3 | ||
| Worsening of asthma | 17123 | 11 December 2013 | 2 | 3 | ||
| Cough | 17128 | 10 August 2014 | 1 | 3 | ||
| Wheeze | 18175 | 17 September 2013 | 1 | 3 | ||
| One wheeze | 18183 | 05 February 2014 | 1 | 3 | ||
| Wheeze | 19220 | 06 January 2014 | 1 | 3 | ||
| Cough, dyspnoea and wheeze | 23360 | 18 June 2014 | 1 | 3 | ||
| Cough | 23362 | 27 October 2015 | 1 | 3 | ||
| Cough | 24311 | 26 October 2014 | 1 | 3 | ||
| Worsening of asthma | 25291 | 13 April 2014 | 1 | 3 | ||
| Increase in asthma symptoms | 25305 | 01 October 2014 | 1 | 3 | ||
| Cough and wheeze | 25379 | 13 March 2015 | 2 | 3 | ||
| Cough | 26341 | 04 May 2014 | 1 | 3 | ||
| Cough | 27338 | 23 February 2015 | 1 | 3 | ||
| Respiratory symptoms limiting exercise activities | 31424 | 16 May 2015 | 1 | 3 | ||
| Cough | 31486 | 03 September 2014 | 2 | 3 | ||
| Cough | 32492 | 13 January 2015 | 2 | 3 | ||
| Increased cough, wheeze and shortness of breath | 37550 | 13 April 2015 | 2 | 3 | ||
| Cough | 38564 | 05 September 2015 | 1 | 3 | ||
| Malignancy | Malignant lymphoma | 10014 | 17 April 2013 | 3 | 5 | |
| Musculoskeletal | Shoulder pain | 12040 | 01 July 2013 | 2 | 5 | |
| Ankle and hip pain | 13060 | 09 September 2013 | 3 | 4 | ||
| Back pain | 13060 | 03 February 2014 | 3 | 5 | ||
| Joint aches (virus) | 18183 | 12 September 2014 | 1 | 5 | ||
| Left-sided jaw pain | 25325 | 12 September 2014 | 1 | 5 | ||
| Fractured right rib | 31424 | UNK January 2015 | 2 | 5 | ||
| Septic arthritis | 38576 | 18 November 2014 | 3 | 3 | 5 | |
| Sciatica | 43635 | 19 March 2015 | 1 | 5 | ||
| Knee pain | 43639 | 01 July 2015 | 1 | 5 | ||
| Shoulder pain | 43643 | 28 July 2015 | 1 | 5 | ||
| Neurological | Neuropathic pain | 13060 | 27 June 2013 | 2 | 5 | |
| Chronic inflammatory demyelinating polyneuropathy | 21346 | 19 November 2014 | 2 | 5 | ||
| Tinnitus | 36543 | UNK November 2014 | 1 | 5 | ||
| Psychological/psychiatric | Anxiety | 26321 | UNK May 2014 | 1 | 3 | |
| Stress | 33519 | UNK October 2014 | 2 | 4 | ||
| Respiratory tract infection/cough | Sore throat | 10032 | 01 September 2013 | 1 | 5 | |
| Congestion with cold, cough and shortness of breath | 17110 | 10 February 2014 | 2 | 5 | ||
| Sore throat (virus) | 18183 | 12 September 2014 | 1 | 5 | ||
| Bronchitis | 18219 | UNK January 2014 | 1 | 5 | ||
| Green sputum | 19220 | 06 January 2014 | 1 | 5 | ||
| Cough/cold | 20250 | 24 December 2013 | 1 | 5 | ||
| Chest infection | 21268 | 22 March 2014 | 1 | 4 | ||
| Viral cough | 23284 | 29 December 2014 | 1 | 5 | ||
| Chest infection | 23312 | 24 November 2014 | 1 | 5 | ||
| Viral illness causing wheeze, cough, fever and chest tightness | 23343 | 28 December 2014 | 1 | 5 | ||
| Upper respiratory chest infection | 23360 | 09 December 2014 | 1 | 5 | ||
| Cold | 23362 | 26 January 2015 | 1 | 5 | ||
| Viral illness | 25291 | 15 December 2014 | 1 | 5 | ||
| Cough and cold | 25325 | 09 September 2014 | 1 | 5 | ||
| Chest infection | 25330 | 17 July 2014 | 1 | 5 | ||
| Chest infection | 25379 | 24 February 2015 | 2 | 5 | ||
| Cold | 26321 | 28 January 2015 | 1 | 5 | ||
| Cold | 26381 | 20 January 2015 | 1 | 5 | ||
| Chest infection | 27338 | 11 December 2014 | 1 | 5 | ||
| Upper respiratory tract infection | 28383 | 20 February 2015 | 1 | 5 | ||
| Upper respiratory tract infection | 30399 | 27 December 2014 | 1 | 5 | ||
| Lower respiratory tract infection | 31432 | 12 October 2014 | 2 | 4 | ||
| Cold virus (sore throat and cough) | 37537 | 24 July 2015 | 2 | 5 | ||
| Sore throat | 37550 | 13 April 2015 | 1 | 5 | ||
| Chest infection | 38593 | 25 September 2015 | 2 | 4 | ||
| Cough | 39574 | 15 December 2014 | 2 | 5 | ||
| Rhinitis/rhinosinusitis | Chronic rhinitis | 18183 | 12 September 2014 | 1 | 5 | |
| Earache/congested sinuses | 25325 | 20 October 2014 | 1 | 5 | ||
| Nasal congestion | 28383 | 2 | 5 | |||
| Left ear infection | 31419 | UNK June 2014 | 2 | 4 | ||
| Runny nose | 32492 | 13 January 2015 | 2 | 5 | ||
| Miscellaneous | Urinary tract infection | 10014 | 29 May 2013 | 2 | 5 | |
| Itch | 12040 | 19 February 2014 | 1 | 4 | ||
| Otitis externa | 13047 | 21 October 2013 | 1 | 5 | ||
| Calf pain | 13060 | 18 July 2013 | 3 | 5 | ||
| Blepharitis | 13072 | 13 March 2014 | 1 | 5 | ||
| Pyrexia (virus) | 18183 | 12 September 2014 | 1 | 5 | ||
| Virus affecting ears, throat and stomach | 28387 | 13 March 2015 | 1 | 5 | ||
| Oral thrush | 31474 | 03 February 2015 | 1 | 5 | ||
| Acute tonsillitis | 34514 | 20 June 2015 | 2 | 5 | ||
| Hoarseness | 38564 | 14 November 2014 | 1 | 5 |
| Category of main symptom | Nature of adverse event | Patient ID | Date of onset | Severity of adverse eventa | Seriousb | Causalityc |
|---|---|---|---|---|---|---|
| Abdominal/gastrointestinal | Rectal bleeding | 10005 | 01 July 2013 | 2 | 5 | |
| Altered bowel habit – complains of adhesions | 10013 | 14 December 2013 | 2 | 5 | ||
| Bacterial vaginosis | 10021 | 19 June 2013 | 2 | 5 | ||
| Laparoscopic vaginal hysterectomy | 12068 | 17 March 2014 | 3 | 3 | 5 | |
| Gastritis | 12127 | 31 October 2013 | 2 | 3 | 5 | |
| Abdominal pain | 12195 | 01 September 2014 | 3 | 3 | 5 | |
| Epigastric pain plus dysfunctional breathing | 13073 | 23 September 2013 | 2 | 5 | ||
| Colitis | 15203 | 31 July 2014 | 3 | 3 | 5 | |
| Vomiting (flu) | 16187 | UNK December 2013 | 1 | 5 | ||
| Epigastric pain episode linked to ongoing acid reflux | 27349 | 07 October 2014 | 1 | 5 | ||
| Lower abdominal pain | 31434 | 13 June 2014 | 2 | 4 | ||
| Gastritis | 32416 | UNK September 2014 | 2 | 5 | ||
| Abdominal pain | 34459 | 12 May 2015 | 1 | 5 | ||
| Lower abdominal pain with loose stools | 34500 | 01 December 2014 | 2 | 4 | ||
| Per vaginal bleed post large loop excision of the transformation zone of the cervix | 37547 | 15 July 2015 | 2 | 3 | 5 | |
| Umbilical hernia repair | 38572 | 17 February 2015 | 3 | 3 | 5 | |
| Acute exacerbation of asthma | Asthma exacerbation | 10030 | 03 January 2014 | 3 | 3 | |
| Exacerbation of asthma/COPD | 12057 | 08 January 2014 | 2 | 3 | ||
| Asthma exacerbation | 12068 | 25 August 2013 | 2 | 3 | ||
| Exacerbation of asthma | 12137 | 20 March 2014 | 2 | 3 | ||
| Asthma exacerbation | 17125 | 20 January 2014 | 2 | 3 | ||
| COPD | 17173 | 10 September 2013 | 2 | 3 | ||
| Asthma exacerbation | 18182 | UNK May 2014 | 2 | 3 | ||
| Exacerbation of asthma | 23283 | 17 March 2014 | 1 | 3 | ||
| Asthma exacerbation | 23342 | 01 August 2014 | 1 | 3 | ||
| One exacerbation of asthma | 26350 | 08 April 2014 | 1 | 3 | ||
| Exacerbation of asthma | 31425 | UNK November 2014 | 1 | 3 | ||
| One exacerbation of asthma | 31429 | 12 February 2015 | 1 | 3 | ||
| Acute exacerbation of asthma | 31434 | 13 November 2014 | 2 | 3 | ||
| Asthma exacerbation | 31436 | 24 May 2014 | 1 | 3 | ||
| Asthma exacerbation | 31503 | 30 October 2014 | 2 | 3 | ||
| Acute exacerbation of asthma | 34499 | 23 February 2015 | 2 | 3 | ||
| Acute exacerbation of asthma | 34500 | 21 January 2015 | 2 | 3 | ||
| Asthma exacerbation | 34515 | 06 May 2015 | 2 | 3 | ||
| Asthma exacerbation | 36555 | 09 February 2015 | 3 | 3 | ||
| Exacerbation of asthma | 38572 | 19 October 2014 | 3 | 3 | ||
| Infective exacerbation of asthma | 39575 | 09 January 2015 | 3 | 3 | 3 | |
| Exacerbation of asthma | 42616 | 13 July 2015 | 1 | 3 | ||
| Exacerbation of asthma | 43641 | 11 September 2015 | 1 | 3 | ||
| Chest pain and cardiovascular | Palpitations | 12043 | 15 August 2013 | 2 | 3 | |
| Chest pain | 12091 | 28 December 2013 | 2 | 3 | 5 | |
| Chest pain? – hiatus hernia | 12195 | 26 May 2014 | 2 | 3 | 5 | |
| High blood pressure | 13066 | 30 May 2014 | 3 | 4 | ||
| Chest pain | 13073 | 04 October 2013 | 3 | 5 | ||
| Collapsed and died – post mortem report found pulmonary oedema with a background of dilated cardiomyopathy and thyroid disease | 14095 | 26 April 2014 | 1 | 3 | 5 | |
| Chest pain | 18184 | 16 October 2013 | 1 | 5 | ||
| Rib pain | 20260 | 24 February 2014 | 1 | 5 | ||
| Chest pain | 28390 | UNK November 2014 | 2 | 5 | ||
| Palpitations | 31434 | 24 March 2015 | 3 | 3 | 4 | |
| Chest pain – acute coronary syndrome | 32449 | 10 September 2014 | 3 | 3 | 5 | |
| Exertional chest pain (while hovering) | 33510 | 02 September 2014 | 2 | 4 | ||
| Chest pain | 34477 | 24 December 2014 | 2 | 3 | 5 | |
| Pulmonary embolism | 38570 | 22 November 2014 | 3 | 3 | 5 | |
| Atrial fibrillation | 38572 | 02 August 2015 | 3 | 5 | ||
| Palpitations | 40608 | 09 November 2015 | 1 | 5 | ||
| Increased asthma symptoms | Wheeze tachycardia | 11017 | 02 May 2013 | 3 | 3 | 3 |
| Worsening of asthma | 12137 | 17 January 2014 | 2 | 3 | ||
| Wheeze | 16147 | UNK December 2013 | 2 | 3 | ||
| Cough | 17125 | 01 December 2013 | 2 | 3 | ||
| Cough | 17173 | 04 April 2014 | 1 | 3 | ||
| Cough | 18170 | 05 May 2014 | 1 | 3 | ||
| Shortness of breath | 18184 | 28 February 2014 | 2 | 3 | ||
| Cough | 18211 | 02 December 2013 | 1 | 3 | ||
| Wheeze | 19228 | 06 October 2014 | 1 | 3 | ||
| Cough | 21282 | 21 December 2014 | 1 | 3 | ||
| Cough | 23342 | 25 April 2014 | 1 | 3 | ||
| Cough and wheeze | 24357 | 16 March 2015 | 1 | 3 | ||
| Shortness of breath | 25293 | 02 June 2014 | 1 | 3 | ||
| Ongoing asthma symptoms | 25294 | 14 April 2014 | 1 | 3 | ||
| Slight breathlessness | 25324 | 17 September 2014 | 1 | 3 | ||
| Cough | 25412 | 31 August 2014 | 1 | 3 | ||
| Cough and wheeze | 27333 | 24 September 2014 | 1 | 3 | ||
| Asthma symptoms | 30398 | UNK September 2014 | 1 | 3 | ||
| Tight chest | 31422 | 16 May 2014 | 2 | 3 | ||
| Shortness of breath | 31470 | 26 April 2015 | 2 | 3 | ||
| Poor asthma control | 31503 | 18 February 2015 | 2 | 3 | ||
| Slight wheeze | 32400 | 08 January 2015 | 2 | 3 | ||
| Cough | 32403 | UNK November 2014 | 2 | 3 | ||
| Increasing shortness of breath | 33504 | 12 July 2014 | 2 | 3 | ||
| Cough | 34440 | 11 November 2014 | 1 | 3 | ||
| Breathlessness | 34459 | UNK December 2014 | 2 | 3 | ||
| Cough and wheeze | 34515 | 29 June 2015 | 2 | 3 | ||
| Asthma worsening | 37527 | UNK January 2015 | 1 | 3 | ||
| Cough and wheeze | 37547 | 18 May 2015 | 2 | 3 | ||
| Wheeze and shortness of breath | 38567 | 14 December 2014 | 2 | 3 | ||
| Shortness of breath on exertion | 38604 | 28 February 2015 | 2 | 3 | ||
| Cough | 42615 | 29 December 2014 | 1 | 5 | ||
| Musculoskeletal | Degenerative change in lumbosacral region | 10005 | 21 May 2013 | 2 | 5 | |
| Back pain | 10024 | 30 September 2013 | 2 | 5 | ||
| Arthralgia of hands/shoulders | 12100 | 24 March 2014 | 2 | 5 | ||
| Shoulder pain | 13046 | 18 March 2014 | 3 | 5 | ||
| Cervicalgia | 17099 | 09 June 2014 | 2 | 5 | ||
| Neck pain | 17173 | 20 May 2014 | 1 | 5 | ||
| Joint ache | 19228 | 06 October 2014 | 1 | 5 | ||
| Right knee replacement surgery | 23280 | 01 December 2014 | 2 | 3 | 5 | |
| Neck pain | 26317 | 13 July 2014 | 1 | 5 | ||
| Low back pain | 31406 | UNK July 2014 | 2 | 4 | ||
| Right shoulder rotator cuff tear | 31421 | 16 November 2014 | 3 | 5 | ||
| Gout | 31422 | 27 March 2015 | 2 | 4 | ||
| Prolapsed intervertebral lumbar disc (worsening of) | 31425 | 31 October 2014 | 2 | 4 | ||
| Right shoulder rotator cuff sprain | 31429 | 02 September 2014 | 2 | 4 | ||
| Worsening of frozen shoulder (right) | 31437 | UNK April 2015 | 2 | 4 | ||
| Rotator cuff syndrome | 31445 | UNK January 2014 | 1 | 4 | ||
| Back pain | 33513 | 23 February 2015 | 1 | 4 | ||
| Cervicalgia | 34500 | 13 May 2015 | 2 | 4 | ||
| Left-sided muscle tightness in neck | 35541 | 17 May 2015 | 1 | 4 | ||
| Cervicalgia | 35544 | UNK January 2015 | 3 | 5 | ||
| Tennis elbow | 43630 | 17 November 2015 | 1 | 5 | ||
| Rotator cuff syndrome | 43641 | 06 May 2015 | 2 | 5 | ||
| Neurological | Tremor | 10021 | 12 November 2013 | 1 | 5 | |
| Neuralgic pain | 13046 | 15 November 2013 | 2 | 5 | ||
| Headache | 19228 | 06 October 2014 | 1 | 5 | ||
| Dizziness | 31425 | UNK July 2014 | 2 | 4 | ||
| Nausea | 31434 | 24 March 2015 | 2 | 3 | 4 | |
| Cerebral venous thrombosis | 31501 | 04 July 2014 | 3 | 3 | 5 | |
| Headache | 33504 | 12 July 2014 | 2 | 4 | ||
| Light-headed symptoms | 33513 | 03 June 2015 | 1 | 4 | ||
| Giant cell arteritis | 38570 | 24 October 2014 | 3 | 3 | 5 | |
| Post FeNO test breathlessness, light-headedness and clammy | 38572 | 30 September 2015 | 2 | 5 | ||
| Migraine | 43649 | 05 March 2015 | 1 | 5 | ||
| Psychological/psychiatric | Mood swings | 10021 | 08 May 2013 | 2 | 3 | |
| Anxiety (worsening) | 12043 | 24 September 2013 | 3 | 3 | ||
| Lethargy | 13046 | 08 October 2013 | 2 | 5 | ||
| Anxiety with depression | 13073 | 13 June 2014 | 3 | 3 | ||
| Shortness of breath (panic attack) | 16151 | 15 April 2014 | 1 | 3 | ||
| Anxiety | 17099 | 06 August 2013 | 2 | 3 | ||
| Anxiety | 17125 | 27 June 2014 | 2 | 3 | ||
| Depression | 21282 | UNK November 2014 | 1 | 3 | ||
| Depression | 23344 | UNK October 2014 | 1 | 3 | ||
| Depression | 25378 | 15 October 2014 | 1 | 3 | ||
| Low mood | 31406 | UNK December 2014 | 2 | 3 | ||
| Depression | 31429 | 02 April 2015 | 2 | 3 | ||
| Recurrence of depressive episode | 31437 | UNK October 2014 | 2 | 3 | ||
| Low mood | 31484 | 28 January 2015 | 2 | 3 | ||
| Anxiety | 32416 | UNK September 2014 | 2 | 3 | ||
| Anxiety attack | 33504 | 30 March 2015 | 2 | 3 | 3 | |
| Respiratory tract infection/cough | Acute lower respiratory tract infection | 12057 | 24 May 2014 | 2 | 3 | 4 |
| Lower respiratory tract infection | 12062 | 11 February 2014 | 2 | 5 | ||
| Chest infection | 12068 | 26 March 2014 | 2 | 5 | ||
| Upper respiratory tract infection | 12075 | 24 December 2013 | 2 | 5 | ||
| Chesty cough | 12082 | 10 January 2014 | 1 | 5 | ||
| Cough ongoing | 12137 | 31 March 2014 | 2 | 5 | ||
| Sore throat | 16139 | 16 August 2014 | 1 | 5 | ||
| Productive cough | 16147 | UNK December 2013 | 2 | 5 | ||
| Shortness of breath (flu) | 16187 | UNK December 2013 | 1 | 5 | ||
| Pneumonia | 17173 | 23 July 2014 | 3 | 3 | 5 | |
| Cough (post viral) | 18182 | UNK March 2014 | 1 | 5 | ||
| Stridor | 18184 | 02 March 2014 | 2 | 3 | 5 | |
| Dry cough | 18209 | 15 August 2014 | 1 | 5 | ||
| Productive cough | 18216 | 19 March 2014 | 1 | 5 | ||
| Cough with green phlegm | 19240 | UNK December 2013 | 1 | 5 | ||
| Chest infection | 20246 | 11 November 2013 | 1 | 5 | ||
| Chest infection | 20260 | 10 November 2013 | 1 | 5 | ||
| Chesty cough | 20265 | 22 August 2014 | 1 | 5 | ||
| Chest infection | 20277 | UNK May 2014 | 1 | 5 | ||
| Cold | 21270 | 24 November 2014 | 1 | 5 | ||
| Lower respiratory tract infection | 21282 | 04 October 2014 | 1 | 5 | ||
| Bronchitis | 22276 | UNK December 2013 | 1 | 4 | ||
| One episode of coughing | 23280 | 15 October 2014 | 1 | 5 | ||
| Viral infection (green sputum, cough) | 23283 | 08 December 2014 | 1 | 5 | ||
| Chest infection | 23287 | 01 December 2014 | 1 | 5 | ||
| Flu-like virus | 23288 | 31 December 2014 | 1 | 5 | ||
| Chest infection | 23342 | 04 April 2014 | 1 | 5 | ||
| Chest infection | 24309 | 18 December 2014 | 1 | 5 | ||
| Chest infection | 24357 | 24 January 2015 | 1 | 5 | ||
| Respiratory heart infection | 25294 | 01 April 2014 | 1 | 5 | ||
| Sore throat | 25324 | 29 December 2014 | 1 | 5 | ||
| Pharyngitis | 25329 | 01 May 2014 | 1 | 5 | ||
| Cold | 25331 | 15 December 2014 | 1 | 5 | ||
| Cold | 25412 | 11 April 2015 | 1 | 5 | ||
| Cough? viral laryngitis | 26317 | 18 August 2014 | 1 | 5 | ||
| Cough with green sputum | 26335 | 25 November 2014 | 1 | 5 | ||
| Upper respiratory tract infection | 26339 | 18 October 2014 | 1 | 5 | ||
| Upper respiratory tract infection | 26350 | 30 January 2015 | 1 | 5 | ||
| Cough and cold | 26371 | 17 February 2015 | 1 | 5 | ||
| Chesty cough | 27349 | 28 May 2014 | 1 | 5 | ||
| Upper respiratory tract infection | 28388 | 26 February 2015 | 1 | 5 | ||
| Chest infection | 29413 | 01 April 2015 | 1 | 5 | ||
| Chest infection | 29462 | 15 April 2015 | 1 | 5 | ||
| Influenza A | 30395 | 26 December 2014 | 3 | 3 | 5 | |
| Lower respiratory tract infection | 30398 | 20 December 2014 | 1 | 5 | ||
| Sore throat | 31422 | 02 March 2015 | 1 | 4 | ||
| Haemoptysis | 31434 | 24 March 2015 | 2 | 3 | 4 | |
| Productive cough | 31436 | 24 May 2014 | 2 | 4 | ||
| Chest infection | 31482 | 30 December 2014 | 2 | 4 | ||
| Chesty cough | 31503 | 03 February 2015 | 2 | 4 | ||
| Sore throat | 32400 | 08 January 2015 | 2 | 5 | ||
| Night cough | 32488 | 01 March 2015 | 2 | 4 | ||
| Chesty cough | 33504 | UNK November 2014 | 1 | 4 | ||
| Cold virus | 34439 | 15 October 2014 | 1 | 5 | ||
| Chest infection | 34458 | UNK April 2015 | 1 | 5 | ||
| Chest infection | 34496 | 25 February 2015 | 2 | 4 | ||
| Chest infection | 34499 | 23 November 2014 | 2 | 4 | ||
| Coryzal | 35544 | 25 June 2015 | 3 | 5 | ||
| Pharyngitis | 36533 | 25 March 2015 | 1 | 5 | ||
| Chest rattle | 36552 | 10 August 2015 | 1 | 4 | ||
| Cough, wheeze, shortness of breath, crackles and phlegm | 36555 | 27 October 2014 | 3 | 4 | ||
| Upper respiratory tract infection | 37547 | 25 February 2015 | 2 | 5 | ||
| Pseudomonas aeruginosa infection | 38563 | 17 May 2015 | 3 | 4 | ||
| Acute laryngitis | 38567 | 22 June 2015 | 2 | 5 | ||
| Lower respiratory tract infection | 38570 | 02 December 2014 | 3 | 3 | 5 | |
| Sore throat – fungal mouth infection | 38581 | 27 January 2015 | 2 | 5 | ||
| Cold virus | 38583 | 11 October 2015 | 1 | 5 | ||
| Chest infection | 38590 | 24 January 2015 | 1 | 5 | ||
| Bronchiectasis – infective exacerbation | 38591 | 07 September 2015 | 1 | 5 | ||
| Cold virus | 38636 | 28 November 2015 | 1 | 5 | ||
| Cough | 39611 | 18 April 2015 | 1 | 5 | ||
| Cough | 42620 | 29 April 2015 | 1 | 5 | ||
| Rhinitis/rhinosinusitis | Acute sinusitis | 10036 | 30 January 2014 | 2 | 5 | |
| Acute sinusitis | 12137 | 27 May 2014 | 1 | 5 | ||
| Congestion | 18209 | 15 August 2014 | 1 | 5 | ||
| Nasal congestion | 19228 | 06 October 2014 | 1 | 5 | ||
| Acute sinusitis | 20260 | 15 September 2014 | 1 | 5 | ||
| Allergic rhinitis | 20265 | 05 May 2014 | 1 | 5 | ||
| Sinusitis | 23342 | 16 January 2015 | 1 | 5 | ||
| Sinusitis | 25293 | 06 August 2014 | 1 | 5 | ||
| Allergic rhinitis | 27349 | 12 June 2014 | 1 | 5 | ||
| Eustachian tube dysfunction | 31429 | 11 March 2015 | 1 | 4 | ||
| Acute sinusitis | 33510 | UNK December 2014 | 2 | 4 | ||
| Sinus pain | 34440 | UNK March 2015 | 1 | 5 | ||
| Sinusitis | 37547 | 01 March 2015 | 2 | 5 | ||
| Sinusitis | 38563 | 17 August 15 | 2 | 4 | ||
| Rhinitis | 38606 | 05 July 2015 | 2 | 5 | ||
| Acute sinusitis | 43641 | 09 December 2015 | 1 | 5 | ||
| Miscellaneous | Anaemia | 10021 | 18 February 2013 | 2 | 5 | |
| Open wound on scalp | 10030 | 28 June 2013 | 1 | 5 | ||
| Problems with crumbling teeth | 12042 | 21 August 2013 | 2 | 3 | 5 | |
| Balanitis | 12043 | 21 March 2014 | 1 | 5 | ||
| Contact dermatitis | 12068 | 12 November 2013 | 1 | 5 | ||
| Excision of thyroglossal cyst | 13046 | 01 October 2013 | 2 | 5 | ||
| Dysfunctional breathing | 13073 | 03 September 2013 | 2 | 5 | ||
| Pyrexia | 18184 | 28 February 2014 | 2 | 5 | ||
| Pleural effusion | 20238 | 11 April 2014 | 1 | 3 | 5 | |
| Flu symptoms | 23319 | UNK February 2014 | 1 | 5 | ||
| Urinary tract infection | 25294 | 27 July 2014 | 2 | 3 | 5 | |
| Cough? gastric reflux? post-nasal drip | 25375 | UNK August 2014 | 1 | 5 | ||
| Neck swelling | 26350 | 06 January 2015 | 1 | 5 | ||
| Oral thrush | 29462 | 24 September 2014 | 1 | 5 | ||
| Fever | 31434 | 24 March 2015 | 2 | 3 | 4 | |
| Seborrheic dermatitis | 31437 | UNK October 2014 | 2 | 5 | ||
| Oral thrush | 31450 | 18 December 2014 | 1 | 4 | ||
| Oral thrush | 31503 | 11 March 2015 | 2 | 4 | ||
| Tonsillitis | 38587 | 10 September 2015 | 3 | 3 | 5 |
Appendix 12 Unit costs of the interventions and primary care and other service use
| Service | Unit cost (£) | Source |
|---|---|---|
| GP consultation | 45.00 | PSSRU73 |
| Out-of-hours clinic | 135.00 | PSSRU73 |
| Walk-in clinic | 135.00 | PSSRU73 |
| Outpatient | 135.00 | PSSRU73 |
| A&E visit | 177.00 | PSSRU73 |
| Inpatient admission | 2581.00 | NHS Reference Costs 2014 to 2015 74 |
| Intervention: face-to-face physiotherapy | 83.45 | Trial estimate |
| Intervention: DVD | 2.85 | Trial estimate |
Appendix 13 Correlation coefficients (Pearson’s r) between expectancy, experience and practical barriers associated with amount of practice (at 3 months) and continued engagement (at 6 and 12 months)
| Expectancy, experience and practical barriers | Amount of practice at 3 months | Continued engagement at | ||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| DVD | Physiotherapy | DVD | Physiotherapy | DVD | Physiotherapy | |
| Expectancy | ||||||
| Beliefs about asthma | ||||||
| Perceived causes | ||||||
| Cause 1 – allergy | 0.14 | 0.20* | –0.00 | –0.02 | 0.03 | 0.01 |
| Cause 2 – health issues | –0.07 | –0.05 | –0.09 | –0.03 | –0.21** | 0.08 |
| Cause 3 – smoking | 0.20* | 0.05 | 0.05 | 0.17 | 0.10 | 0.27** |
| Perceived chronicity | ||||||
| All the time | 0.06 | 0.11 | 0.11 | 0.01 | 0.03 | 0.04 |
| Most of the time | –0.25** | –0.02 | –0.11 | 0.06 | –0.11 | 0.01 |
| Some of the time | 0.12 | –0.07 | 0.00 | –0.06 | 0.07 | –0.13 |
| Only when symptoms are present | 0.02 | –0.05 | –0.02 | –0.00 | –0.00 | 0.09 |
| First impressions of treatment | ||||||
| Expectancy | 0.21** | 0.02 | 0.23** | 0.14 | 0.16 | –0.05 |
| Self-efficacy | 0.33*** | –0.04 | 0.38*** | 0.20 | 0.35*** | –0.05 |
| Perceived need for support (baseline) | –0.01 | –0.03 | –0.03 | 0.06 | –0.10 | –0.06 |
| TPB: perceived behavioural control | 0.16* | –0.07 | 0.27*** | 0.06 | 0.15 | 0.03 |
| TPB: intentions | 0.14 | –0.01 | 0.17* | 0.13 | 0.19* | 0.09 |
| Treatment experience | ||||||
| Enjoyment of treatment | ||||||
| Stomach breathing | 0.39*** | 0.30** | 0.37*** | 0.29** | 0.34*** | 0.24* |
| Nose breathing | 0.39*** | 0.30** | 0.36*** | 0.23* | 0.39*** | 0.19 |
| Slow breathing | 0.36*** | 0.11 | 0.32*** | 0.18 | 0.32*** | 0.24* |
| Controlled breath holding | 0.26*** | 0.05 | 0.24** | 0.25* | 0.24** | 0.27* |
| Relaxation training | 0.39*** | 0.10 | 0.32*** | 0.35*** | 0.25** | 0.36*** |
| Appointments with physiotherapist (physiotherapy group only) | 0.19 | 0.01 | 0.04 | |||
| Perceived need for support (3 months) | –0.16* | 0.26* | –0.11 | 0.10 | –0.14 | 0.32** |
| Perceptions of physiotherapist (physiotherapy group only) | 0.21* | 0.09 | 0.09 | |||
| Practical barriers | ||||||
| PETS | ||||||
| Problems due to symptoms | –0.10 | –0.09 | –0.18* | 0.15 | –0.03 | 0.07 |
| Problems due to uncertainty | –0.32*** | –0.15 | –0.29*** | 0.10 | –0.07 | 0.07 |
| Problems due to doubts | –0.46*** | –0.20* | –0.45*** | –0.15 | –0.30*** | –0.05 |
| Practical problems | –0.38*** | –0.30** | –0.33*** | –0.15 | –0.32*** | –0.12 |
| Problems due to lack of support | –0.25*** | –0.25** | –0.24** | –0.16 | –0.18 | –0.01 |
Appendix 14 Multiple regression results for expectancy, experience and practical barrier variables associated with amount of practice (at 3 months)
| B | SE (B) | β | 95% CI | |
|---|---|---|---|---|
| Perceived causes | ||||
| Cause 1 – allergy | 20.15 | 10.65 | 0.22 | –1.11 to 41.41 |
| Cause 3 – smoking | 8.11 | 11.08 | 0.08 | –14.01 to 30.24 |
| Perceived chronicity | ||||
| Most of the time | 6.52 | 13.57 | 0.05 | –20.57 to 33.61 |
| First impressions of treatment | ||||
| Expectancy | –1.60 | 2.11 | –0.09 | –5.80 to 2.61 |
| Self-efficacy | 0.13 | 4.54 | 0.00 | –8.94 to 9.21 |
| TPB: perceived behavioural control | –2.32 | 2.29 | –0.11 | –6.89 to 2.24 |
| Enjoyment of treatment | ||||
| Stomach breathing | 1.91 | 4.91 | 0.07 | –7.89 to 11.71 |
| Nose breathing | 6.63 | 3.95 | 0.25 | –1.25 to 14.51 |
| Slow breathing | 1.61 | 3.68 | 0.07 | –5.74 to 8.96 |
| Controlled breath holding | –3.82 | 3.58 | –0.16 | –10.97 to 3.33 |
| Relaxation training | –2.25 | 2.46 | –0.12 | –7.15 to 2.65 |
| Perceived need for support (3 months) | 5.29 | 9.10 | 0.07 | –12.89 to 23.46 |
| Perceptions of physiotherapist (physiotherapy group only) | 9.02 | 14.52 | 0.08 | –19.97 to 38.00 |
| PETS | ||||
| Problems due to uncertainty | –11.37 | 15.69 | –0.08 | –42.69 to 19.96 |
| Problems due to doubts | –2.52 | 14.40 | –0.02 | –31.27 to 26.23 |
| Practical problems | –28.44 | 14.05 | –0.24* | –56.49 to –0.39 |
| Problems due to lack of support | –29.86 | 14.95 | –0.23* | –59.72 to –0.00 |
Appendix 15 Multiple regression results for expectancy, experience and practical barrier variables associated with continued engagement (at 6 months)
| B | SE (B) | β | 95% CI | |
|---|---|---|---|---|
| First impressions of treatment | ||||
| Expectancy | 0.10 | 0.13 | 0.06 | –0.15 to 0.35 |
| Self-efficacy | 0.17 | 0.30 | 0.05 | –0.43 to 0.76 |
| TPB: perceived behavioural control | –0.11 | 0.17 | –0.06 | –0.45 to 0.23 |
| TPB: intentions | 0.73 | 0.78 | 0.08 | –0.81 to 2.28 |
| Enjoyment of treatment | ||||
| Stomach breathing | 0.32 | 0.24 | 0.13 | –0.16 to 0.79 |
| Nose breathing | 0.31 | 0.23 | 0.13 | –0.15 to 0.76 |
| Slow breathing | –0.19 | 0.28 | –0.08 | –0.73 to 0.36 |
| Controlled breath holding | –0.00 | 0.22 | –0.00 | –0.44 to 0.43 |
| Relaxation training | 0.41 | 0.19 | 0.18* | 0.04 to 0.79 |
| PETS | ||||
| Problems due to symptoms | 1.75 | 0.88 | 0.16* | 0.00 to 3.49 |
| Problems due to uncertainty | 0.34 | 0.90 | 0.03 | –1.43 to 2.12 |
| Problems due to doubts | –2.20 | 0.86 | –0.22** | –3.89 to –0.50 |
| Practical problems | –1.28 | 0.93 | –0.10 | –3.11 to 0.54 |
| Problems due to lack of support | –0.88 | 0.82 | –0.08 | –2.51 to 0.74 |
Appendix 16 Multiple regression results for expectancy, experience and practical barrier variables associated with continued engagement (at 12 months)
| B | SE (B) | β | 95% CI | |
|---|---|---|---|---|
| Perceived causes | ||||
| Cause 2 – health issues | –0.65 | 0.66 | –0.07 | –1.95 to 0.65 |
| Cause 3 – smoking | 1.19 | 0.70 | 0.12 | –0.19 to 2.57 |
| First impressions of treatment | ||||
| Self-efficacy | 0.17 | 0.27 | 0.05 | –0.36 to 0.70 |
| TPB: intentions | –0.09 | 0.68 | –0.01 | –1.44 to 1.26 |
| Enjoyment of treatment | ||||
| Stomach breathing | 0.16 | 0.26 | 0.07 | –0.35 to 0.67 |
| Nose breathing | 0.36 | 0.22 | 0.17 | –0.08 to 0.80 |
| Slow breathing | –0.02 | 0.27 | –0.01 | –0.55 to 0.51 |
| Controlled breath holding | 0.01 | 0.21 | 0.01 | –0.40 to 0.42 |
| Relaxation training | 0.29 | 0.19 | 0.14 | –0.08 to 0.67 |
| Perceived need for support (3 months) | –0.17 | 0.23 | –0.06 | –0.62 to 0.28 |
| PETS | ||||
| Problems due to doubts | –0.28 | 0.74 | –0.03 | –1.74 to 1.19 |
| Practical problems | –1.27 | 0.87 | –0.11 | –2.99 to 0.45 |
Appendix 17 Correlation coefficients (Pearson’s r) between the components of the theory of planned behaviour
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Attitude (α = 94) | |||||||||
| 2. Subjective norms (α = 0.98) | 0.71*** | ||||||||
| 3. Perceived behavioural control (α = 0.89) | 0.26*** | 0.20*** | |||||||
| 4. Intentions (α = 0.96) | 0.24*** | 0.25*** | 0.58*** | ||||||
| 5. Beliefs (attitude related) (α = 0.87) | 0.44*** | 0.29*** | 0.55*** | 0.36*** | |||||
| 6. Beliefs (control related –1) – time-consuming | 0.21*** | 0.17*** | 0.23*** | 0.16** | 0.05 | ||||
| 7. Beliefs (control related –2) – fit daily routine | 0.26*** | 0.25*** | 0.35*** | 0.27*** | 0.15** | 0.53*** | |||
| 8. Amount of practice | 0.13* | 0.12 | 0.14* | 0.12 | 0.15** | 0.27*** | 0.21*** | ||
| 9. Continued engagement (6 months) | 0.13* | 0.05 | 0.24*** | 0.17** | 0.24*** | 0.32*** | 0.32*** | 0.51*** | |
| 10. Continued engagement (12 months) | 0.05 | 0.03 | 0.15* | 0.17** | 0.18** | 0.22*** | 0.19** | 0.55*** | 0.76*** |
Appendix 18 Hierarchical logistic regression results for the role of attitude, subjective norms and perceived behavioural control in the prediction of intentions
| B | SE (B) | Wald | Odds ratio [exp(B)] | 95% CI | |
|---|---|---|---|---|---|
| Attitude | –0.03 | 0.06 | 0.26 | 0.97 | 0.86 to 1.10 |
| Subjective norms | 0.09 | 0.05 | 3.20 | 1.10 | 0.99 to 1.22 |
| Perceived behavioural control | 0.79 | 0.11 | 54.95*** | 2.21 | 1.79 to 2.72 |
| Beliefs (attitude related) | 0.04 | 0.05 | 0.93 | 1.04 | 0.96 to 1.14 |
| Beliefs (control related –1) – time-consuming | –0.05 | 0.09 | 0.30 | 0.95 | 0.79 to 1.14 |
| Beliefs (control related –2) – fit daily routine | 0.01 | 0.10 | 0.01 | 1.01 | 0.83 to 1.23 |
Appendix 19 Hierarchical linear regression results for the role of intention and perceived behavioural control in the prediction of continued engagement (at 6 months)
| B | SE (B) | β | 95% CI | |
|---|---|---|---|---|
| Step 1 | ||||
| Intention | 1.72 | 0.63 | 0.17** | 0.48 to 2.95 |
| Step 2 | ||||
| Intention | 0.53 | 0.77 | 0.05 | –0.99 to 2.04 |
| Perceived behavioural control | 0.40 | 0.15 | 0.20** | 0.10 to 0.70 |
Appendix 20 Hierarchical multiple regression results for the role of intention and perceived behavioural control in the prediction of continued engagement (at 12 months)
| B | SE (B) | β | 95% CI | |
|---|---|---|---|---|
| Step 1 | ||||
| Intention | 1.55 | 0.59 | 0.17** | 0.40 to 2.71 |
| Step 2 | ||||
| Intention | 1.20 | 0.72 | 0.13 | –0.22 to 2.61 |
| Perceived behavioural control | 0.12 | 0.14 | 0.07 | –0.16 to 0.40 |
Appendix 21 Hierarchical multiple regression results for the role of intention and perceived behavioural control in the prediction of amount of practice (at 3 months)
| B | SE (B) | β | 95% CI | |
|---|---|---|---|---|
| Step 1 | ||||
| Intention | 10.71 | 5.80 | 0.11 | –0.72 to 22.14 |
| Step 2 | ||||
| Intention | 4.78 | 6.87 | 0.05 | –8.75 to 18.32 |
| Perceived behavioural control | 2.18 | 1.36 | 0.12 | –0.50 to 4.86 |
Appendix 22 Principal axis factoring (pattern matrix) and internal consistency of the Problematic Experiences of Therapy Scale items
| Items | I | II | III | IV | V |
|---|---|---|---|---|---|
| I. Problems due to symptoms (α = 0.76) | |||||
| 1. I had to skip the breathing retraining because it made my symptoms worse | 0.827 | ||||
| 2. I was prevented from carrying out the breathing retraining by severe symptoms | 0.919 | ||||
| 3. I could not carry out the breathing retraining because it caused more symptoms | |||||
| II. Problems due to uncertainty (α = 0.89) | |||||
| 4. I could not carry out the breathing retraining because I was unsure how to do it properly | –0.880 | ||||
| 5. I was unable to carry out the breathing retraining because it was difficult to know what to do | |||||
| 6. I did not carry out the breathing retraining because I was worried that I was doing it wrong | –0.755 | ||||
| III. Problems due to doubts (α = 0.90) | |||||
| 7. I skipped the breathing retraining because I was not sure if it was helping | –0.881 | ||||
| 8. I skipped the breathing retraining because it did not seem relevant to my symptoms and problems | –0.880 | ||||
| 9. I did not carry out the breathing retraining because I was not convinced it was right for me | –0.915 | ||||
| IV. Practical problems (α = 0.89) | |||||
| 10. Lack of time prevented me from carrying out the breathing retraining | 0.944 | ||||
| 11. It was not possible to find suitable opportunities to carry out the breathing retraining | 0.891 | ||||
| 12. I was too busy to carry out the breathing retraining | 0.910 | ||||
| 13. I was too tired to carry out the breathing retraining | 0.631 | ||||
| 14. I found it difficult to remember to carry out the breathing retraining | 0.646 | ||||
| V. Problems due to lack of support (α = 0.85) | |||||
| 15. I did not carry out the breathing retraining because I did not receive enough support from my family and friends | 0.792 | ||||
| 16. Lack of support from a health professional prevented me from carrying out the breathing retraining | 0.822 | ||||
| 17. I did not carry out the breathing retraining because I did not have anyone to support me | 0.931 | ||||
| Item variance accounted for by factor (%) | 6.64 | 4.77 | 15.25 | 15.25 | 44.49 |
Appendix 23 Component Pearson’s correlation matrices of the Problematic Experiences of Therapy Scale subscales
| Subscale | I | II | III | IV | V |
|---|---|---|---|---|---|
| I. Problems due to symptoms | |||||
| II. Problems due to uncertainty | –0.337 | ||||
| III. Problems due to doubts | –0.326 | 0.489 | |||
| IV. Practical problems | 0.166 | –0.299 | –0.292 | ||
| V. Problems due to lack of support | 0.371 | –0.501 | –0.448 | 0.491 |
List of abbreviations
- A&E
- accident and emergency
- ACQ
- Asthma Control Questionnaire
- ANCOVA
- analysis of covariance
- AQLQ
- Asthma Quality of Life Questionnaire
- BREATHE
- Breathing REtraining for Asthma – Trial of Home Exercises
- BTS
- British Thoracic Society
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CRN
- Clinical Research Network
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- EuroQol-5 Dimensions
- FeNO
- fraction of exhaled nitric oxide
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced volume vital capacity
- GINA
- Global Initiative for Asthma
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IPQ
- Illness Perceptions Questionnaire
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ITT
- intention to treat
- KMO
- Kaiser–Meyer–Olkin
- LABA
- long-acting beta-agonist
- LOCF
- last observation carried forward
- LTRA
- leukotriene receptor antagonist
- MCID
- minimal clinically important difference
- MRC
- Medical Research Council
- NIHR
- National Institute for Health Research
- NNT
- number needed to treat
- PCRN
- Primary Care Research Network
- PEFR
- peak expiratory flow rate
- PETS
- Problematic Experiences of Therapy Scale
- PI
- principal investigator
- PP
- per protocol
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAP
- statistical analysis plan
- SCTU
- Southampton Clinical Trials Unit
- SD
- standard deviation
- SIGN
- Scottish Intercollegiate Guidelines Network
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- WHO
- World Health Organization