Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/01/16. The contractual start date was in August 2013. The draft report began editorial review in August 2016 and was accepted for publication in July 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Peter Langhorne received funding from the National Institute for Health Research; National Health Medical Research Council Australia; Chest, Heart and Stroke Scotland; the Stroke Association, UK; and Chest Heart and Stroke Association of Northern Ireland to complete this trial. Peter Langhorne is a member of Health Technology Assessment (HTA) Clinical Trials Board. Olivia Wu is a member of HTA Evidence Synthesis Board and Systematic Review Programme Advisory Group. Helen Rodgers reports grants from Newcastle University during the tenure of this grant. Julie Bernhardt reports grants from National Health and Medical Research Council Australia; NIHR; Singapore Health, Singapore; Chest, Heart and Stroke Scotland, UK; Chest Heart and Stroke Association of Northern Ireland; Stroke Association, UK, during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Langhorne et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Modern stroke unit care
The management of stroke patients has progressed greatly in the last two decades1,2 and several interventions have provided good evidence of benefit for acute stroke patients. 1–8 These include:
-
stroke unit care3 (a complex package of specialist multidisciplinary stroke care involving nurses, therapists and doctors)
-
aspirin for ischaemic stroke4
-
intravenous thrombolysis with recombinant tissue plasminogen activator (rtPA) for ischaemic stroke5
-
mechanical thrombectomy for major ischaemic stroke6
-
emergency decompressive surgery for malignant middle cerebral artery syndrome. 7
Among these interventions, the stroke unit effect has potentially the greatest population impact as it combines both moderate effectiveness and broad applicability. 1,2 However, as it is a complex intervention it is difficult to be certain about the key components of stroke unit care. 8 Descriptive studies have reported that early mobilisation (EM) (starting out of bed, sitting, standing and walking early after stroke) is widely thought to be an important contributor to the stroke unit effect. 8–10 The other potentially important components include (1) co-ordinated multidisciplinary care, (2) skilled and specialised staff, (3) training and education of staff and (4) protocols of care covering common problems. 8,9 This trial focuses on the mobilisation component of the stroke unit rehabilitation intervention.
Rehabilitation
The term rehabilitation covers a broad philosophy and range of interventions aiming to help an individual recovering from disabling illness to minimise the impact of that illness on their level of dependence on external support. 11 The modern classification of diseases in the International Classification of Functioning, Disability and Health framework considers rehabilitation to comprise an interaction between the impact of the disease, the characteristics of the individual and the nature of their environment. 11 Rehabilitation professionals aim to act on different levels of the illness to minimise the impact on the individual. 11
In the context of acute stroke, early rehabilitation usually covers the key impairments experienced by patients in the acute stage of the illness. 11 These include swallowing impairment, language and speech impairment, motor impairment, reduced mobility, reduced balance and reduced ability to carry out self-care activities. An early focus on mobilisation is one that is likely to be relevant to a substantial majority of acute stroke patients.
Early mobilisation
Early mobilisation comprises the commencement of sitting, standing and walking training out of bed early after stroke. Early descriptions of stroke units frequently refer to EM and it is thought to make an important contribution to the effectiveness of stroke unit care. 9,10 However, there are disagreements about the role of EM. 10
Arguments around mobilisation
The biological rationale for EM is based on three principal lines of argument: (1) there is good evidence that bed rest has a harmful impact on cardiovascular, respiratory, muscular, skeletal and immune systems across many conditions11,12 and is likely to slow recovery; (2) some of the most common and serious complications after stroke are those related to immobility13–16 (we know that the routine day of most acute stroke patients is largely inactive;17,18 therefore, introducing frequent training out of bed may reduce the risk of complications of immobility); and (3) current concepts of biological recovery after brain injury suggest a narrow window of opportunity for brain plasticity and repair. 19 If the brain indeed remodels itself based on experience20 then early task-specific training may well have an important contribution to improving recovery. 21,22
However, we must acknowledge that there are also concerns about potential harm of EM,10,23 particularly in the first 24 hours after stroke onset. These concerns include haemodynamic considerations, such as fears that raising the patient’s head early after stroke will impair cerebral blood flow and cerebral perfusion23 or, in the case of intracerebral haemorrhage, increase the risk of inducing further bleeding. 24 As a result of these theoretical concerns, some clinicians have advocated initial bed rest for stroke patients. 23
Given these uncertainties about the practice of EM in acute stroke patients we sought to carry out A Very Early Rehabilitation Trial (AVERT) in acute stroke patients that focused on very early (commencing within 24 hours of stroke onset), frequent out-of-bed mobilisations in the first 14 days.
AVERT programme
The AVERT programme of work that was run by Professor Julie Bernhardt of the University of Melbourne and began with Phase I observational studies. These studies demonstrated that most acute stroke patients were inactive for most of the time17,18 but that this pattern of inactivity varied between hospitals. 17 She also demonstrated that there was considerable variation of opinion and clinical uncertainty among health-care professionals about the value of very early mobilisation (VEM). 23 These studies led to the AVERT Phase II safety and feasibility randomised controlled trial (RCT)25,26 and the closely related Very Early Rehabilitation or Intensive Telemetry After Stroke (VERITAS) trial27 carried out in Glasgow by Professor Peter Langhorne. These trials indicated that VEM was feasible and in the case of AVERT Phase II could be carried out within 24 hours of stroke onset. This approach was observed to be safe,25–27 showed signals for improvements in recovery25–28 as well as indicating that EM was probably cost-effective. 29
Justification for the current study
The preparatory work carried out in AVERT Phases I and II led to the planning and conduct of the definitive AVERT Phase III trial. 30 This was planned as a pragmatic, international, multicentre Phase III RCT with the power to evaluate the efficacy and safety of VEM after stroke. This report outlines the AVERT Phase III international trial with some specific emphasis on the UK contribution. Much of this work has already been published by the AVERT group. 30–36 We will also refer to two related studies that were nested within the AVERT programme. These studies contribute to the understanding of AVERT, but were not specifically included in the original Health Technology Assessment (HTA) programme trial application. These comprise (1) a qualitative process evaluation37 and (2) a study of the generalisability of the AVERT results. 38
Chapter 2 Methods
Aims and objectives
The primary aim of this trial was to investigate the effectiveness of a protocol to implement VEM after stroke; an earlier start with frequent out-of-bed activity compared with usual care (UC), which is traditionally started later (> 24 hours).
The objectives of AVERT were designed addressed four main questions:
-
Does VEM reduce death and disability at 3 months post stroke?
-
Does VEM reduce the number and severity of complications at 3 months post stroke?
-
Does VEM improve quality of life (QoL) at 12 months post stroke?
-
Is VEM cost-effective? [Note: this aspect of the trial programme was not funded by the current National Institute for Health Research (NIHR) HTA programme grant.]
Our clinical hypotheses were as follows:
-
VEM would improve functional outcome at 3 months.
-
VEM would reduce immobility related complications.
-
VEM would accelerate walking recovery with no increase in neurological complications.
-
VEM would result in improved QoL at 12 months.
-
VEM would be cost-effective.
We aimed to carry out a large multicentre pragmatic trial recruiting a broad range of acute stroke patients including those aged > 80 years, those with intracerebral haemorrhage, those who had received rtPA and those admitted to stroke units in a range of different hospital types (small and large, urban and regional).
Trial design
We carried out a pragmatic, prospective, parallel-group, multicentre, international Phase III RCT with blinded assessment of outcomes and an intention-to-treat analysis. Full details of the trial rationale and statistical analysis plan30 were published in advance.
Study settings
The trial was carried out in the acute stroke unit of 56 hospitals in five countries: UK (England, Scotland, Northern Ireland and Wales), Australia, New Zealand, Singapore and Malaysia. Stroke units were housed in a range of hospital settings including local and regional hospitals (see list in Appendix 2).
Participants
We aimed to include all eligible patients aged ≥ 18 years with a confirmed first or recurrent stroke (infarct or intracerebral haemorrhage) who were admitted to a stroke unit within 24 hours of onset. Exclusion criteria are listed below and included significant premorbid disability, acute deterioration, admission to the intensive care unit, competing care needs or physiological instability. Recruitment and informed consent could take place in the emergency room or in the acute stroke unit.
Eligibility
All patients (aged ≥ 18 years) admitted with stroke diagnosis (first or recurrent stroke, infarct or haemorrhage) were screened for suitability for inclusion into the trial. If a patient was found to be ineligible for inclusion into the trial, the reason was recorded on the stroke patient-screening log. A diagnosis of transient ischaemic attack (TIA) would not have been considered eligible and the patient would not have been recruited into the trial. However, if a patient was recruited into AVERT who, clinically, appeared to have stroke symptoms and was considered eligible but later assessment confirmed a TIA or other diagnosis, the patient remained in the trial and continued to be followed up until completion.
Inclusion criteria
-
Informed consent obtained from the patient or a responsible third party.
-
Patients aged ≥ 18 years with a clinical diagnosis of first or recurrent stroke, infarct or haemorrhage.
-
Patients admitted to hospital within 24 hours of the onset of stroke.
-
Patient for admission to the acute stroke unit.
-
Patients who receive thrombolysis could be recruited if the attending physician permits and if mobilisation within 24 hours of stroke was permitted.
-
Consciousness: at a minimum, the patient must at least be able to react to verbal commands.
-
Patients could participate in AVERT if they were already recruited to non-intervention trials (e.g. imaging) if dual recruitment was permitted by the ethics committee.
Exclusion criteria
-
Too disabled before stroke [prestroke modified Rankin scale (mRS) score of 3, 4 or 5].
-
Patient diagnosed with TIA.
-
Deterioration in patient’s condition in the first hour of admission resulting in direct admission to intensive care unit, a documented clinical decision for palliative treatment (e.g. those with devastating stroke) or immediate surgery.
-
Concurrent diagnosis of rapidly deteriorating disease (e.g. terminal cancer).
-
A suspected or confirmed lower limb fracture at the time of stroke preventing the implementation of the mobilisation protocol.
-
Patients could not be concurrently recruited to drug or other intervention trials.
-
Unstable coronary or other medical condition that were judged by the investigator to impose a hazard to the patient by involvement in the trial.
-
Unstable physiological variables:
-
systolic blood pressure of < 110 mmHg or > 220 mmHg
-
oxygen saturation of < 92% with supplementation
-
resting heart rate of < 40 or > 110 beats per minute (b.p.m.)
-
temperature of > 38.5°C.
-
Randomisation and masking
Ethics review boards approved the study at all sites. Informed consent was obtained from all patients or their nominated representative. Eligible participants were invited to participate in a trial that was testing ‘different types of rehabilitation’ but were not given specific information about the two approaches. 30
After informed consent was obtained, a medical history and physical examination was performed. The following stroke assessments were carried out:
-
premorbid mRS39
-
baseline mRS39
-
National Institutes of Health Stroke Scale (NIHSS) score40
-
Oxfordshire Community Stroke Project41 classification. A paper case report form (CRF) was completed by the AVERT team member (see Appendix 3).
Baseline NIHSS, OSCP (Oxfordshire Community Stroke Project) classification, premorbid mRS and the date of the stroke were all entered into the AVERT Online electronic data capture system prior to randomisation. AVERT Online randomly allocated the treatment group with the result immediately notified to the investigator. Participants were randomised (in a 1 : 1 ratio) through a secure remote, web-based, computer-generated block randomisation procedure with an average block size of six. Permuted blocks of various lengths were used to ensure allocation concealment.
Randomisation was stratified by:
-
study site
-
stroke severity using the NIHSS score,40 for which mild is a NIHSS score of 1–7, moderate is a NIHSS score of 8–16 and severe is a NIHSS score of > 16. 42
Participants were allocated to receive either usual stroke unit care alone, or usual stroke unit care in addition to the experimental intervention, VEM. VEM patients were provided the first mobilisation as soon as they were recruited and additional mobilisations according to the protocol. The intervention period lasted 14 days or until the patient was discharged from stroke unit care, whichever was sooner.
Patients were not aware of their treatment group and outcome assessors plus the investigators involved in the conduct of the trial and data management were blinded to the group assignment. To try and reduce the risk of contamination of the UC intervention, staff providing the VEM protocol were trained to conceal the mobilisation protocol and group allocation. The patient’s participation would be terminated if consent had been withdrawn or if the patient’s safety had been considered to be at risk.
Procedures
Following randomisation, the trial staff obtained the following patient data within 24 hours:
The AVERT intervention protocol
The intervention protocol was not published or distributed except to trials intervention staff. AVERT staff from within the stroke unit team (i.e. site investigators, physiotherapists, nurses) were trained by clinical trial managers to deliver the AVERT intervention protocol at site initiation and investigator meetings, with refresher and new staff training provided on an ongoing basis. 35 This complex intervention required staff to work together to achieve the VEM and UC mobility targets. Trial staff agreed not to distribute or disseminate the protocol and to keep the protocol in a secure location.
To aid protocol description, the key intervention definitions are summarised in Box 1.
Dose: a session of mobilisation given to AVERT patients.
Very early: the earliest possible time after a consented patient had suffered a stroke to their first mobilisation intervention (≤ 24 hours).
Mobilisation: the patient was assisted and encouraged in functional tasks, including activities such as sitting over the edge of the bed, standing up, sitting out of bed and walking. Upper limb movement would have been integrated into functional activities as appropriate. Mobilisations were performed by the AVERT nurse and/or the AVERT physiotherapist. Support staff such as therapy assistants and students could also be trained to provide mobilisations.
Counting mobilisations: when a patient performed a mobilisation (e.g. walked to the toilet with help or was sat out of bed) and rested for ≥ 5 minutes, then their next mobilising activity (e.g. walking back from the toilet or getting back into bed) would have constituted another mobilisation.
TTFM: this is the time from stroke onset to the time the patient is first mobilised out of bed (assisted or independent). This does not include the initial assessment by the AVERT physiotherapist.
Physiotherapist’s record of mobilisation sessions: the date, time, minutes and content of each session were recorded via AVERT Online. If the online forms were unavailable, paper forms could be used to temporarily collect the information until such time as it could be entered online.
Nurse’s record of mobilisation sessions: the date and time it started and the type of each mobilisation would have been recorded on AVERT Online or, if the website was not available, data were temporarily recorded on the paper nurse recording form until such time as they could be entered online.
Excessive fatigue: if the patient reported a score of > 13 on the Borg Perceived Exertion Scale and/or AVERT staff assess that the patient is excessively fatigued (e.g. the patient’s functional performance worsened significantly during the intervention).
Contamination: when the witnessing of a different intervention makes others change their UC practice consciously or unconsciously.
The intervention protocol was followed for all randomised patients. Information about the group to which the patient had been randomised was known only by the AVERT physiotherapist and nursing staff. The protocol for the interventions was not intended to replace any clinical decision-making of the individual therapists and nurses involved in the treatment delivery. However, the expectation was that they would adhere to the protocol whenever possible.
The protocol for VEM interventions and UC was continued until day 14 of the patient’s stay in the stroke unit or until discharge from the stroke unit (whichever was sooner). If the patient was palliated then VEM was discontinued and follow-up with trial assessments continued until death or 12-month follow-up.
Very early mobilisation interventions
The components of usual stroke unit care, including normal physiotherapy and nursing procedures, were provided at the discretion of the individual sites. In addition to UC, the VEM intervention included four important features.
-
It had to begin within 24 hours of stroke onset.
-
The focus had to be on sitting, standing and walking activities (i.e. out of bed).
-
VEM delivered in at least three out-of-bed sessions per day in addition to UC.
-
Nursing and physiotherapy mobilisations were titrated each day, according to patient’s functional level.
The content of nursing and physiotherapy mobilisations were detailed, task-specific activities targeting the recovery of standing and walking. It was tailored to accommodate four levels of functional ability and was adjusted daily in line with participant recovery. The usual risk assessments and lifting policies were applied to all mobilisations. Even following the protocol, clinician judgement was still required when the patient’s suitability to get out of bed was assessed.
Principles of very early mobilisation
The principles of the VEM intervention were developed in consultation with the early rehabilitation team in the acute stroke unit in Trondheim, Norway,9 and used in AVERT Phase II. 25 Trained physiotherapy and nursing staff helped patients to continue task-specific out-of-bed activity that was focused on recovery of active sitting, standing and walking. The frequency and intensity (amount) was guided by the intervention protocol. This was titrated according to functional activity baseline and monitored daily and adjusted with recovery. For example, low-functioning dependent patients (level 1) had a target of active sitting with assistance with each session lasting between 10 and 30 minutes. Higher-functioning patients (level 4) would have a target of standing and walking with each session lasting 10 minutes and with no restricted maximum. The frequencies of sessions were varied according to the patient’s functional level. Passive sitting was not classified as a mobilisation activity and sitting for > 50 minutes at a time was discouraged. The intervention continued for 14 days or until discharge. Physiotherapists and nurses had separate intervention targets but worked together to deliver the intervention dose. Mobilisation activities were all recorded online.
The key principles were as follows.
-
The target dose (the number of interventions, the type of intervention and the amount of time spent with each VEM patient) was additional to usual stroke unit nursing and therapy and was titrated according to the patient’s level of functional ability.
-
Patients were recruited as soon as possible after stroke onset, until 24 hours (day 0). The first VEM commenced as soon as possible after recruitment and could be provided when the patient arrived on the ward, or earlier if they were in the emergency department. The VEM target TTFM was within 24 hours from stroke. When patients were routinely mobilised within 24 hours at any site, the target VEM time was 5 hours less than UC.
-
Patients should not rest in bed for long periods of the day unless they were medically unstable.
-
If medically stable (not specifically restricted to bed), patients were helped to perform functional (out of bed) activities for the prescribed VEM dose according to the patient’s functional level.
-
Patients who were stable enough to sit out of bed or sit up over the side of the bed with help were assisted to do so for the prescribed VEM dose according to the patient’s functional level. If, on the first 3 days, they required the moderate or maximum assistance of others to move themselves from chair to bed, they could not be left to sit out of bed for longer than 50 minutes each time.
-
When sat out of bed, patients would have been comfortably seated in a supportive chair or wheelchair with the hemiplegic upper limb supported.
-
The VEM safety assessment was strictly adhered to for the first mobilisation out of bed. This involved measurement of vital signs and was critical to the safety of the mobilisation.
-
For patients randomised to the VEM group, the AVERT nurse and physiotherapist worked together to achieve a daily target for the frequency of sessions and minutes of physiotherapy mobilisation.
The target number of sessions and minutes of physiotherapy to be delivered each day was dictated by the level of functional ability at the start of each day
Level of functional ability
The AVERT staff assessed the patient’s functional ability using the MSAS as soon as possible after recruitment and then at the start of each day. The patient was assessed as one of the four functional levels (Table 1). The daily assessment of level and the daily mobilisation targets were communicated to the study team. The level assigned to the patient was not changed throughout the day. If the patient’s level of performance fluctuated, clinicians adjusted VEM interventions according to patient status. Usual risk assessments and lifting policies were applied to all mobilisations.
| Level | Definition | Patient description |
|---|---|---|
| 1 | Equivalent to sitting from supine, MSAS | Low arousal (responded to voice but required physical prompting) |
| Score = 1–4 | Fully dependent. Unable to sit on the edge of the bed without the assistance of 1 or 2 people | |
| 2 | Equivalent to sitting from supine, MSAS | Followed commands (verbal or non-verbal/gestures) |
| Score = 5–6 | Moderate to high dependence. Able to sit on the edge of the bed but would requires assistance and/or supervision. Able to stand with assistance | |
| 3 | Equivalent to gait MSAS | Follows commands |
| Score = 2–4 | Moderate dependence. Able to walk with moderate to maximum assistance | |
| 4 | Equivalent to gait MSAS | Low/no dependence |
| Score = 5–6 | Able to walk with minimal/no assistance |
First very early mobilisation safety assessment
The first VEM mobilisation out of bed was strictly governed by a safety assessment. If the safety assessment failed then the first mobilisation did not commence until the patient achieved the safety criteria.
For the first VEM sit out-of-bed mobilisation post stroke, the following procedure was followed.
Before first mobilisation
Step 1
The following physiological variables were required:
-
systolic blood pressure of 110–220 mmHg
-
oxygen saturation of ≤ 92% supplementation (see note on page 9)
-
resting heart rate of 40–110 b.p.m.
-
temperature of < 38.5 °C.
Note:
-
Oxygen saturation was measured without supplemental oxygen.
-
Oxygen was stopped for 1 minute. If SpO2 was < 92%, oxygen supplementation was resumed and maintained throughout the mobilisation.
-
Blood pressure was measured in the unaffected arm. Oxygen saturation was measured on the affected arm.
-
If blood pressure, heart rate and oxygen saturation were within acceptable limits then the patient proceeded to the next step.
Performing the first mobilisation
Step 2
-
The back of the bed was raised to > 70° of hip flexion. The measures of blood pressure, heart rate and oxygen saturation were repeated.
-
If blood pressure, heart rate and oxygen saturation were within acceptable limits then the patient proceeded to the next step.
-
If blood pressure drop was > 30 mmHg then the patient remained in bed, but was reassessed at a later time.
Step 3
-
The patient was assisted to sit over the edge of the bed (feet on the floor if able). This may have required the assistance of one or two people and the patient may have required the assistance of one person to maintain sitting balance.
-
The measures of blood pressure, heart rate and oxygen saturation were repeated.
-
If blood pressure, heart rate and oxygen saturation were within acceptable limits then the intervention would have proceeded to the next step.
-
If blood pressure drop was > 30 mmHg then the patient remained in bed and would have been reassessed at a later time.
Step 4
-
Sitting was for 5 minutes and measures of blood pressure, heart rate and oxygen saturation were repeated.
-
If blood pressure, heart rate and oxygen saturation were within acceptable limits, the intervention proceeded to the next step.
-
If blood pressure drop was > 30 mmHg then the patient remained in bed, and reassessed at a later time.
Step 5
-
An appropriate level of assistance was used (hoist or manual assistance dependent on routine assessment findings) and the patient was transferred to a comfortable chair with adjustable back to allow an angle of 90°–100° hip flexion.
-
Measures of blood pressure, heart rate and oxygen saturation were repeated.
-
If measures were within acceptable limits then the patient maintained sitting and was monitored for comfort.
The first out-of-bed mobilisation was interrupted and the patient returned to bed if:
-
in the clinician’s judgement, the patient was not tolerating the mobilisation (i.e. became less responsive, developed a headache, became nauseated or vomited or became pale or clammy)
-
systolic blood pressure was < 100 mmHg or > 230 mmHg
-
systolic blood pressure decrease was > 30 mmHg
-
heart rate was > 120 b.p.m.
-
oxygen saturation was < 90%.
Maximum sitting time for sitting out of bed was 50 minutes each time, for the first 3 days.
What if a very early mobilisation patient could not have achieved the first mobilisation?
Factors that would have affected a patient’s ability to mobilise may have included (but were not limited to) (1) vital signs not within the normal listed limits, (2) an adverse event (AE) that would have led to a mobilisation restriction for a period of time (e.g. acute myocardial infarction, lower limb fracture, pneumonia, carotid endarterectomy) or (3) a deterioration which led to palliation.
In the case of a temporary interruption to mobilisation due to an event similar to those listed above, mobilisation was recommenced as soon as possible. The patient’s physiological variables were reviewed every few hours (step 1). Clinical judgement was used and the first mobilisation was attempted when the patient physiological variables were within limits. When a mobilisation was planned, but not able to be performed, staff submitted a therapist or nurse recording form with the time and reason not mobilised. Whenever possible, VEM resumed at the earliest opportunity.
Usual care group
Participants who were randomised to receive UC received the usual post-stroke unit care. Prior to trial commencement, baseline UC was reported by trial staff at each site. Typical UC is described in Table 2. Mobilisation activity was not prescribed but all mobilisations were recorded. UC patient mobilisations at each site were monitored for change during the trial.
| Level | Patient description | Nursing activities | Physiotherapy activities |
|---|---|---|---|
| 1 | Low arousal | Approximately one mobilisation every 1 or 2 days | Approximately one treatment every 1 or 2 days |
| Fully dependent | |||
| 2 or 3 | Moderate – high dependence | Approximately two mobilisations per day | Approximately one treatment per day, including a mobilisation |
| 4 | Low dependence | Approximately four mobilisations per day | Approximately may/may not have received treatment, would have been encouraged to have mobilised independently |
| Note: this aspect varied greatly | Note: this aspect varied greatly |
Recording of mobilisation sessions
Mobilisation data for both UC and VEM patients were recorded by the AVERT physiotherapist, ward physiotherapist and occupational therapist and/or the AVERT nurse(s) using therapist and nurse recording forms, respectively, on AVERT Online. For convenience, paper therapist and nurse recording forms (Figures 1 and 2) were sometimes used to initially record mobilisations and then the data were transferred to AVERT Online. VEM interventions were not recorded in routine medical records.
FIGURE 1.
Example of the paper therapist recording form.

FIGURE 2.
Example of the paper nurse recording form.
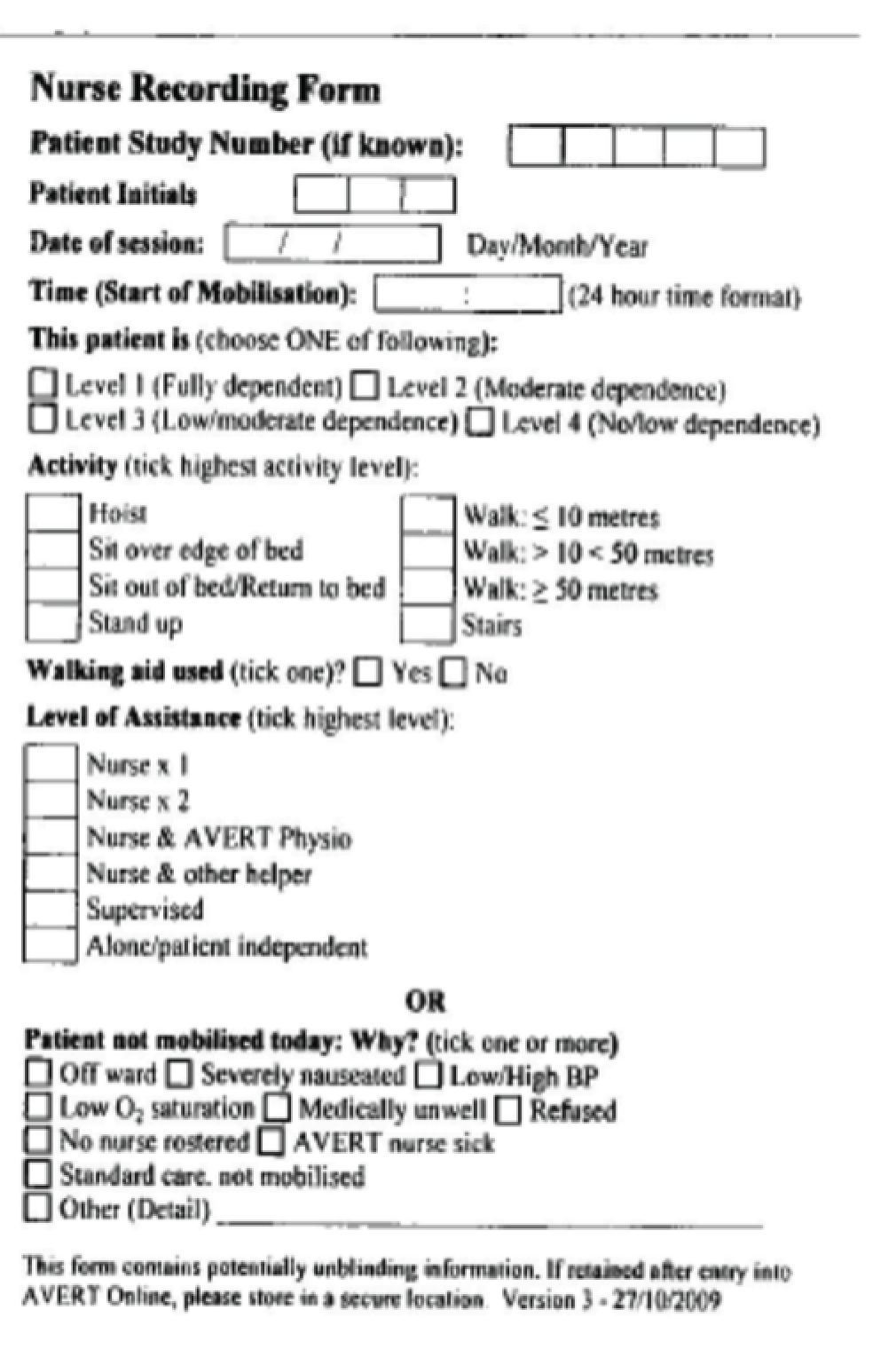
The AVERT nurse(s) recorded all mobilisations that they were responsible for initiating that were conducted alone or with an AVERT physiotherapist, ward physiotherapist, occupational therapist or other assistant. Mobilisations in which the AVERT nurse helped either the AVERT physiotherapist or ward physiotherapist/occupational therapist for study patients (either group) were recorded by the therapist that initiated the mobilisation. The nurse involvement was acknowledged in the recording of the mobilisation. This prevented double reporting of a same mobilisation.
Equipment
Existing equipment (e.g. beds, standing hoists, standing frames, tilt tables, chairs, lap trays, gait aids, arm supports, safety belts, etc.) from each hospital were utilised as per usual ward policy and availability. Ward lifting policies were applied to all mobilisations for AVERT patients. The vulnerable hemiplegic shoulder was cared for with the use of lap trays when the patient was seated and the provision of slings used for transfer and walking activities.
Adherence to protocols
The online recording system allowed the intervention staff to document all mobilisations, including attempted mobilisations and reasons for when the patient was not mobilised according to protocol. Intervention staff received feedback from an external monitor about their compliance with the trial protocol. These were provided in quarterly compliance summaries and were reviewed regularly by the Data Safety and Monitoring Committee.
Contamination was a potential problem for this trial. This was because all patients in this study were situated on a single ward. This made it difficult to keep other staff on the ward from seeing the intervention staff work with patients who had been randomised to VEM. If contamination had occurred, the results of the trial would have been diluted because intervention and UC would have become more alike.
Contamination was considered to have occurred if VEM was provided to UC patients or became UC for a large number of patients. Measures to reduce the potential of the intervention practices to be adopted by staff other than the AVERT staff included security of the intervention protocol and procedures to stop ward staff observing VEM sessions. The Data Safety and Monitoring Committee monitored contamination throughout the trial.
Data collection
Source data relating to each patient were maintained in the patient’s medical record.
Source data relating to the intervention therapy given to the patient were not recorded in the patient’s medical record. The therapy information was recorded in the web-based therapy/nurse forms (see Figures 1 and 2) on AVERT Online; data were also recorded in the individual patient’s paper CRF, which would have been supported by information documented in the patient’s medical record or clinical notes.
Blinding
We recognise that blinding is vital for the integrity of any RCT. As the AVERT physiotherapists and nurses were delivering the interventions, they were not blinded to the interventions but protocols were in place to conceal allocation group to all other ward staff.
The following measures were followed to maintain blinding for the AVERT study:
-
A patient or their family were never told of the group to which they had been randomly allocated, even if they asked.
-
The AVERT physiotherapists never wrote VEM interventions in the medical record and AVERT nurses recorded only standard information and did not refer to frequency of intervention provided.
-
Anyone who did not need to know the patients group were never told, even if they asked.
-
The AVERT staff ensured that other staff and AVERT patients did not become aware of the details of the VEM. VEM activities were conducted behind curtains with patients screened from other ward staff, and mobilisations performed off the ward were provided whenever this was possible.
-
The blinded assessors assigned to the trial site were not on the ward when the trial took place and did not witness treatments that patients had received.
-
The blinded assessor, who conducted assessments, was never told the group to which patients were allocated. The assessor had been trained in what they could and could not ask participants, therapists, nurses and other staff whom they encountered.
-
The blinded assessor informed AVERT ward staff if it was necessary to visit the ward for any reason, which minimised the risk of an intervention session being witnessed. Every effort was made by AVERT staff to ensure that a session was not witnessed by the blinded assessor.
Participant assessments
Outcome assessments were done in person or by telephone by a trained assessor who was not working in the study stroke unit and was blinded to treatment allocation.
Three-month assessment
At 3 months post stroke, the assessor located the patient and conducted the assessment, which included the following. 30
-
mRS:39 a commonly used scale for measuring the degree of disability or dependence in the daily activities of people who have suffered a stroke or other causes of neurological disability.
-
Irritability, anxiety and depression assessment (IDA):44,45 following stroke.
-
Barthel Index:46 an ordinal scale used to measure performance in activities of daily living.
-
assessment of quality of life (AQoL). 47
-
Rivermead Motor Assessment Scale48 (RMAS): assesses the motor performance of patients with stroke and was developed for both clinical and research use.
-
50-metre walk:30 assessed if the patient had not achieved walking during the 14-day intervention period.
-
Montreal Cognitive Assessment (MoCA):49 a cognitive screening test designed to assist health professionals in detecting mild cognitive impairment.
-
Cost of care: the cost CRF collects resource use on acute hospital length of stay, discharge location, ambulance services, rehabilitation, stroke-related rehospitalisations, change of accommodation, aids, home modifications, community services, return to work, informal care hours and country-specific services (e.g. UK outpatient therapy; Asian maid services in the home).
-
AEs, important medical events (IMEs) and serious adverse events (SAEs) (see below).
Adverse events
An AE is defined as any untoward medical occurrence in any participant involved in the study and that does not have a causal relationship to the study intervention. This included any worsening of a pre-existing event. AEs were recorded in the patient’s medical record and reporting commenced from the time of informed consent. Events were recorded in the patient’s CRF and included the date of onset, description, severity and duration and whether or not it was thought to be related to the study intervention.
All AEs were collected from the time of the patient’s consent until the end of the intervention period and were followed until the event was resolved or had been stabilised.
Important medical events
The IMEs were prespecified events that are important outcomes measures for this study. These events included:30
-
falls (with no soft tissue injury, with soft tissue injury, with bone fracture)
-
stroke progression (defined as a worsening stroke, in the clinician’s view, in the same vascular territory as the initial event occurring during the first 14 days)
-
recurrent stroke (defined as a new stroke event beyond 14 days (in the clinician’s view)
-
pulmonary embolism
-
deep-vein thrombosis
-
myocardial infarction
-
angina
-
urinary tract infection
-
pressure sores
-
pneumonia
-
depression (clinically diagnosed).
Serious adverse events
A SAE was an AE or IME that met any one of the following criteria:
-
resulted in death
-
was life-threatening
-
required inpatient hospitalisation
-
prolonged hospitalisation (if an event occurs while the patient is in hospital, which in itself prolongs the patient stay)
-
resulted in persistent or significant disability.
Up to 3-month follow-up all IMEs, serious or not serious, were reported. After 14 days, we recorded new AEs that were classified as serious but were not IMEs. From 3–12 months, SAEs were collected.
Twelve-month assessment
At 12 months, the final assessment was conducted by the blinded assessor. The assessor made contact with the patient/relative/carer and organised the meeting.
At this visit, all 3-month assessments were repeated, except for the MoCA.
Outcomes
Primary outcome
The primary outcome was survival without major disability (mRS score of 0–2) at 3 months after stroke. A favourable outcome was defined as mRS score of 0 (no symptoms), 1 (impairment but no disability) or 2 (independent but with minor disability). A poor outcome was defined as mRS score of 3 (disability but able to walk), mRS score of 4 (disabled and unable to walk), mRS score of 5 (bed-bound and in need of full nursing care) or mRS score of 6 (death).
Secondary outcomes
Secondary outcomes included an assumption-free ordinal shift39,40 of the mRS across the full range of the scale. This measures a change in the mRS across the whole range of the scale rather than just across one threshold. We also obtained time taken to achieve unassisted walking for 50 m and the proportion of patients achieving walking by 3 months. Death and the number of non-fatal SAEs were recorded up to 3 months.
Serious adverse events were recorded according to standard definitions and included IMEs relevant to acute stroke patient recovery (see above). Serious adverse events and deaths were independently adjudicated by an outcome committee who were blinded to treatment allocation. This included a review of the source data if necessary. The classification of complications of interest were neurological (stroke progression and recurrent stroke) and complications of immobility (deep-vein thrombosis, pulmonary embolism, pneumonia, urinary tract infection and pressure sores). At 12 months, we recorded health-related quality of life (HRQoL) and items of patient costs.
Subgroup analyses
In view of the complex nature of the VEM intervention a number of exploratory analyses were prespecified. 30 In particular, subgroup analysis by age, stroke severity, stroke subtype (infarct or haemorrhage), treatment with thrombolysis (rtPA) and TTFM. We also prespecified an exploration of the dose of the intervention in terms of (1) TTFM, (2) frequency of mobilisation and (3) total amount of time undergoing the intervention.
Withdrawal from treatment and data collection
We anticipated that some participants would wish to withdraw from treatment. In that circumstance, the reason and date of withdrawal were documented and they were invited to allow further collection of follow-up data. Clearly, if the participant refused further follow-up then all treatment and data collection ceased at that point. We considered analyses based on last result carried forward in the event of significant loss of information.
Data retention
All study documents were confidential. Each site was issued with an investigator site file in which to store study documents. All of the study-related documents were stored in a locked area and accessible only to study staff.
At the completion of the study, all site study data and materials have been archived, at site, and have been stored in a secure area for a period of ≥ 7 years if required by hospital procedures.
Power calculation and sample size
The trial was powered to detect an absolute risk reduction of a poor outcome (mRS score of 3–6) of at least 7.1%. This threshold was based on (1) a consensus among clinicians and researchers that an absolute risk reduction of this size would be clinically meaningful and (2) observational data indicating that a hospital routinely practising EM compared with a similar Australian data set had a 9.1% better outcome on the similar variable of death or institutional care (31.8% vs. 40.9%). If EM accounted for 78% of this benefit9 then the absolute difference would be 7.1%.
We estimated that a sample of 2104 patients would be required to provide 80% power to detect a significant intervention effect (two-sided p = 0.05) with adjustments for 5% drop-in and 10% drop-out. Statistical analysis was prespecified and published in advance. 30 Stata® (StataCorp LP, College Station, TX, USA)/IC (version 13) was used for all analysis. For the primary analysis we used an intention-to-treat approach with the assumption that data were missing at random. We also explored the sensitivity of our results to plausible departures from this assumption. This used both a selection model (to model the mechanism of missing data) and a pattern mixture model (modelling the differences between observed and missing data).
Statistical methods
We used standard methods for handling of missing data. 30,50 The primary efficacy analysis was carried out on an intention to treat basis with an assumption that data were missing at random. 30 We explored the sensitivity of our conclusions to plausible departures from this assumption and used both a selection model and pattern mixture model of the differences between observed and missing data. The results were plotted out over a range of assumptions.
We did the primary efficacy analysis used the binary logistic regression model, with treatment group as an independent variable and mRS outcome at 3 months as the dependent variable. This was dichotomised into scores of 0–2 as favourable outcome and scores of 3–6 as poor outcome. Baseline stroke severity (NIHSS) and age were included as treatment covariates for adjustment purposes.
The primary outcome analysis included subgroup analysis based on age (< 65 years, 65–80 years and > 80 years), stroke severity (mild NIHSS 1–7, moderate 8–16 and severe > 16), stroke type (ischaemic vs. haemorrhagic), treatment with tissue plasminogen activator, TTFM (< 12 hours, 12–24 hours and > 24 hours) and geographical region (Australia/New Zealand vs. UK, Australia/New Zealand vs. Asia), with adjustment for stroke severity and age.
We also estimated the treatment effect on the mRS using an ordinal analysis at 3 months with the assumption-free Wilcoxon Mann–Whitney U-test generalised odds ratio (OR) approach. 51,52 This provided a measure of effect size with confidence interval (CIs), which was stratified by age and stroke severity. Time (days) taken to achieve unassisted walking of 50 m was analysed using the Cox regression model with treatment group as the independent variable, the time to unassisted walking (censored at 3 months) as the dependent variable, and age and baseline NIHSS as covariates. The estimated effect size is presented as a hazard ratio (HR) with corresponding 95% CI. The analysis of walking status (yes or no) was analysed with a binary logistic model using treatment group as the independent variable and walking status as the dependent variable.
We used a binary logistic regression model to analyse mortality outcomes. Treatment group was the independent variable and death at 3 months was the dependent variable. Age and stroke severity were treatment covariates. We used negative binomial regression to compare the expected counts of serious complications between groups at 3 months. We report the estimated effect sizes and corresponding 95% CI as incidence rate ratios (IRRs) adjusted for age and stroke severity.
We wished to determine whether or not practice had shifted during the course of this trial. We did this by testing the association between treatment effect and trial duration by including an appropriate interaction term into the logistic regression model used in the primary analysis. We also did an exploratory analysis in which we examined the effect of time since the start of the trial on differences in dose characteristics between the two groups. We used regression models with an interaction term for treatment by time since the start of the trial; a median regression model was used for TTFM and median session frequency and a binomial regression model was used for median daily minutes per session and total treatment time over the intervention period (total minutes).
End-point analyses
Primary end point: the primary outcome was planned as a ‘between-group’ comparison of mRS at 3 months, analysed across the whole distribution of scores subject to the validity of shift analysis model assumptions. If the assumptions for shift analysis were not met, 3-month mRS was to be dichotomised into good outcome (mRS score of 0–2) and poor outcome (mRS score of 3–6), and the groups were compared using a binary logistic model. Although the trial was under way, new ordinal approaches to analysis were developed, tested and gained acceptance in acute stroke trial (see the statistical analysis plan). The management committee determined that an assumption-free ordinal approach to analysis should be included as a secondary outcome (statistical analysis plan). Therefore, the analysis plan was changed such that the 3-month mRS results were dichotomised into good outcome (mRS score of 0–2) and poor outcome (mRS score of 3–6), and the groups compared using a binary logistic model. 30 The primary analysis was adjusted with baseline NIHSS and premorbid mRS as covariates. Unadjusted results were also to be shown. The intervention effect was represented in terms of ORs. Other potential prognostic variables such as age, stroke type and side of stroke were included in subgroup efficacy analyses.
Secondary patient end points: regression models for count data were used to compare SAEs between groups at 3 months. Risk ratios, adjusted as per primary analysis, including age and NIHSS as covariates, were reported.
The odds of achieving unassisted walking at 3 months was analysed using binary logistic regression analyses [adjusted odds ratios (aORs) and 95% CIs]. Cox regression analysis was used to analyse the time (days) to achieve unassisted walking. This was presented as adjusted hazard ratios (aHR) with 95% CIs and was censored at 3 months.
Mortality outcomes at 3 months were analysed using binary logistic regression with death as the dependent variable (aOR with 95% CI). The dose effect on counts of SAE was analysed using binomial regression (adjusted incident rate ratio with 95% CI). Different subtypes of SAE (immobility related, neurological) were analysed separately.
Health-related QoL analysis was planned as a multivariable median regression model with a treatment group as independent variable and the AQoL score as the dependent variable. To estimate the effect of intervention group on AQoL scores at 12 months, treatment covariates for adjustment purposes would include baseline NIHSS, age and sex.
Data sharing and archiving
All deidentified trial data have been archived in secure facilities for a minimum period of 7 years. The options of data sharing arrangements were not available at the trial commencement and were not included in participant consent processes.
Economic evaluation at 12 months
The economic evaluation was not included in the NIHR HTA programme funding. However, the wider AVERT programme did include a health economic analysis;34 therefore, we summarised the resource use data collected for an economic evaluation. We prospectively collected resource use data within the trial using standard data collection tools. The primary economic evaluation planned is a cost-effectiveness analysis comparing resource use during the 12 months of follow-up. The health outcomes of the VEM intervention were measured against a UC comparator. It was also intended to have included a cost–utility analysis.
For the cost-effectiveness analysis, the primary outcome is a mRS score of 0–2 at 12 months. It was intended that the cost–utility analysis used HRQoL expressed as quality-adjusted life-years gained over a 12-month period. This was measured using the mRS and the assessment of QoL.
Data collection tools to capture resource use were piloted in the AVERT pilot study29 and then further adapted to accommodate local service provision in different countries. An exploratory analysis of the resource use data was planned to consider the relationship between patterns of service use in health outcomes within the trial. These health outcomes included QoL. A further objective explored economic impacts of stroke on patients, families, the broader community and the health sector.
The methods for assessing safety, effectiveness and QoL have already been published in the statistical analysis plan. 30 The economic analysis plan complements the statistical analysis plan and was finalised prior to the 12-month data collection period being completed. 34
The economic analysis plan describes key study variables for the economic evaluation, outlines the primary cost-effectiveness analysis and describes proposed exploratory analysis. The development of the economic analysis plan was guided using recommended standards. 53 The economic analyses are under way; however, a UK-specific economic evaluation will not be undertaken as not supported with NIHR HTA programme funding.
Exploratory analyses
To further investigate the interaction between dose characteristics and patients and a favourable outcome we used (1) binary regression analysis and (2) a classification and regression tree (CART) analysis (Salford predictive modeller software suite version 7, Salford Systems, San Diego, CA, USA).
The CART is a binary partitioning statistical method that starts with the total sample. It then uses a stepwise approach to split the sample in to subsamples that are homogeneous in a defined outcome. 54 The input variable that achieves the most effective split is dichotomised by automated analysis at an optimal threshold, maximising the homogeneity within, and separation between, resulting subgroups. A 10-fold internal cross-validation is used to maximise model performance that is assessed as the area under the receiver operating characteristic (ROC) curve. The internal cross-validation divides the data randomly into 10 groups with nine used to build the model (training data set) and one used to validate the model (testing data set). CART also numerically ranks each input to build the tree by relative importance. In our analysis, we included all prespecified subgroup variables (patient age, NIHSS, stroke type, treatment with rtPA), group allocation and the three dose characteristics (TTFM, frequency and daily amount). This analysis explored the relative importance of each variable in association with achieving a favourable outcome (mRS score of 0–2). A further analysis (CART II) investigated multidimensional relationships between dose characteristics alone and favourable outcome. 55
Both approaches to exploratory analysis examined the three main characteristics of treatment dose:
-
TTFM out of bed (hours)
-
frequency – median number of out-of-bed sessions per patient per day
-
daily amount – median minutes of out-of-bed activity per patient per day.
We also recorded total amount (total minutes of out-of-bed activity over the whole intervention period) to account for varying lengths of stay in hospital.
Nurses recorded the type of activity and the time of the day each activity began. This did not include total time in minutes as this was not routine practice. Physiotherapists recorded the type of activity, the time that the activity began and the total out-of-bed activity (minutes), as this was incorporated in normal practice. Therefore, physiotherapy data alone contributed to the variables of daily amount (minutes) and total amount (minutes) of out-of-bed activity. Both nursing and physiotherapy data contributed to TTFM and frequency of mobilisations. For the definition of frequency of mobilisation, episodes of sitting, standing or walking activity had to be separated from another episode of activity by more than 5 minutes of rest (e.g. in a chair).
In an attempt to avoid excessive collinearity between daily amount and total amount we tested two different models that were adjusted for age and baseline stroke severity for all the analysis:
-
Model 1 – TTFM, frequency (median daily number of out-of-bed sessions) and amount (median daily out-of-bed session time in 5-minute increments),
-
Model 2 – TTFM, frequency (median daily number of out-of-bed sessions) and amount (total minutes out-of-bed activity over the whole intervention period in 5-minute increments).
The primary exploratory analysis was carried out using binary logistic regression models with favourable outcome (mRS score of 0–2) at 3 months as the dependent variable.
Meta-analysis of comparable trials
We wished to set the AVERT results in the context of other similar RCTs. We updated the searches of the existing systematic review,56 searching MEDLINE, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Cochrane Stroke Group trials register, several international ongoing trials registers, reference lists of articles and also performed citation searching up to 2015. Foreign language translations were sought. Two review authors assessed trial eligibility, quality and performed data extraction. We included any trial that compared EM after stroke (within 48 hours) with a more delayed mobilisation. The primary outcome was death or poor outcome (dependency or institutionalisation) at follow-up with the use of a mRS score of 3–5 as the preferred definition of poor outcome. We used a fixed-effects model to estimate ORs and 95% CIs, with the use of a random-effects model in the event of substantial heterogeneity (I2 > 50%).
Patient and public involvement
Stroke survivors in Australia contributed to the original trial development. In particular, Ms Brooke Parsons (a stroke survivor) was involved in the development of the proposal and the monitoring and progress of the trial through her role on the Trial Steering Committee. Each individual study site had varying degrees of patient and public involvement.
Role of the funding source
The various funders of the AVERT international trial had no role in study design, data collection, analysis and interpretation or in the writing of the report. The author team had full access to all data in the study.
Chapter 3 Qualitative process evaluation
Introduction
As stated in the introduction, we also refer to two related studies that were not specifically included in the original HTA programme trial application but were nested within the AVERT programme and contribute to its understanding. These are a qualitative process evaluation,37 which is summarised here, and a study of the generalisability of AVERT,38 which appears in the results (see Chapter 4).
It is particularly challenging to implement multidisciplinary stroke rehabilitation interventions when the intervention is both complex and multifaceted. This part of the trial programme aimed to better understand how the implementation of the VEM intervention was experienced by the staff involved. It has been reported that efforts to implement evidence-based recommendations in acute stroke units have had mixed success. In particular, changing clinician behaviour is particularly challenging when incorporated within pragmatic trials. 57 The qualitative process evaluation summarised here aimed to help us better understand the implementation of the VEM intervention protocol from the perspective of the health-care professionals who were responsible for its delivery. We believed that understanding the knowledge and perspectives of these staff would be important to both develop effective guidance in the future and implement the VEM intervention if it was found to be effective.
The main component of this chapter was published in Luker et al. 37 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text in this chapter includes minor additions and formatting changes to the original text.
Methods
We used the standard qualitative methodological methodologies58,59 involving AVERT trial collaborators in Scotland, Australia and New Zealand. Ethics approvals were obtained for all three countries. The Scottish component of recruitment was carried out as part of a Stroke Association-funded Doctor of Philosophy (PhD) by Ms Louise Craig, who was also our first AVERT manager in the UK. The raw Scottish data relevant to the main AVERT trial were reanalysed by colleagues in Australia using the same approach for all included study sites.
Study sample
We used purposive sampling at participating AVERT sites. This was overseen by the trial manager in Australia and New Zealand and by Louise Craig in Scotland. Of the 72 staff who expressed interest, six did not eventually consent to take part and one moved abroad. We obtained informed consent from 33 physiotherapists, 18 nurses, one physiotherapy assistant and one speech pathologist. These staff members are based in four stroke units in Scotland, 14 in Australia and one in New Zealand.
The qualitative data were collected and analysed before the primary outcome of the trial was available. We conducted semistructured interviews facilitated by interview guides that permitted additional questions or probes if interesting information arose. 37 The main focus of the interview was on the implementation of the VEM protocol. The Scottish interviews were conducted between 2010 and 2011 and the Australian and New Zealand interviews took place in 2014. They could be by telephone or face to face. Telephone interviews commonly ran for 30 minutes, while face-to-face interviews averaged 59 minutes. Interviews were audio-recorded, transcribed, cross-checked with participants and deidentified prior to analysis.
Analysis
We used a thematic analysis to explore the experience and perspectives of staff involved in the trial. This approach is said to be especially relevant to multimethods health research58,59 and uses low-inference interpretation. We inductively coded the data and set about identifying themes. Each stage incorporated independent consideration by two or more researchers with subsequent discussion and consensus forming. We coded the transcripts to small sections of meaning and then through an iterative process we grouped the codes into logical and meaningful clusters in a hierarchical tree structure. This resulted in categories, descriptive themes and subthemes (Table 3). Emergent themes, each with subthemes, were grouped into three categories: staff experience of implementing the trial intervention, barriers to implementation of the trial intervention and strategies to overcome barriers to intervention (see Table 3). Stroke unit staff described the challenges of taking part in the trial and how their unit set about implementing the VEM protocol. The recent publication37 describes the findings in detail but for the purposes of this report we have summarised the main themes as follows.
-
Staff experience of implementing the trial intervention.
-
Extra work but rewarding: the extra work was felt to be justified by the hope that the trial might benefit stroke patient outcomes.
-
Team practice changes: several staff reported a positive impact on teamwork at their site. In particular, closer working of nurses and physiotherapists and some changes in their professional roles.
-
– Changes to usual practice: over the duration of the trial some staff perceived a change in UC. This was not a universal perception but was noted by a substantial minority.
-
-
-
Barriers to intervention implementation.
The main reported barriers related to the general implementation of a trial and also those specific to the VEM intervention, in particular, the frequency of the intervention.
-
Team challenges: implementation difficulties were notable at sites that appeared to lack established interdisciplinary team working practices.
-
Staffing challenges: a common theme was that inadequate staffing levels made it difficult to consistently implement the VEM protocol particularly in the face of competing demands. It was recognised that experienced and trained staff were essential for successful implementation of the protocol.
-
Organisational or workplace barriers.
-
– The acute model and culture: there was a common view that the rapid pace and focus on early discharge of acute hospitals rendered rehabilitation a low priority.
-
– Barriers to acute stroke unit access: a particular problem was that significant delays were experienced while patients waited for a bed in the acute stroke unit.
-
– Competing priorities: competing organisational priorities such as discharge pressure, accreditation work and transfer policies were commonly reported as being a challenge.
-
-
Physical environmental barriers: environmental barriers such as lack of equipment and chairs were reported to be barriers.
-
Staff attitudes and beliefs.
-
– Resistance to change of practice: resistance to changing practice was identified at some sites particularly among staff who were not experienced in clinical trials.
-
– Beliefs about roles and capabilities: mobilisation delays at some sites were due to nurses awaiting physiotherapists to begin mobilisation.
-
– Beliefs about consequences: many staff assumed a positive treatment effect from VEM, which appeared to influence their perceptions of the treatment and anecdotal outcomes.
-
-
Patient barriers.
-
– Acuity, instability and complexity: acute health problems including unstable medical conditions and complex problems were viewed as a common barrier.
-
– Severity of stroke: early delivery of the VEM protocol was viewed as challenging in patients with drowsiness or reduced cognition. They would require more staff to assist mobilisation. The challenges with milder strokes were mainly due to the high frequency mandated by the VEM protocol.
-
– Fatigue: staff reported that fatigue was a common problem reported by patients sometimes preventing mobilisation.
-
– Family anxiety: on some occasions families raised concerns that the VEM intervention was preventing necessary rest.
-
-
-
Overcoming implementation barriers.
Many interviewees described strategies for implementing the VEM intervention in the face of the observed barriers. These are summarised below.
-
Teamwork is central to success: units that felt they had been successful in providing the VEM protocol frequently reported shared interdisciplinary roles with nurses, physiotherapists and others working closely together through flexible work practices and mutual trust.
-
– Communication and co-ordination: a component of good interdisciplinary working was effective communication and co-ordination between different members of staff.
-
-
Getting staff on board: an almost universal theme was the importance of spending time and effort to get staff engaged with the new VEM practice.
-
– Staff education and training: this was felt to be a cornerstone of getting staff involved.
-
– Leadership for change: implementing the VEM protocol was seen as a whole team responsibility but required leadership.
-
-
Working differently: a positive attitude to implementing the VEM protocol was seen in sites determined to work around organisational barriers and foster a culture appropriate to the trial. This included a willingness to relinquish some control of traditional roles and practices.
-
– Staffing model changes: some units reported using different staffing models involving nursing or allied health assistants.
-
– Managing patient fatigue: some units reported innovative approaches to timetabling therapy to accommodate patient fatigue.
-
-
| Themes | Interviewsa | Subthemes | |
|---|---|---|---|
| Category 1: Staff experience of implementing the trial intervention | |||
| 1 | Extra work but rewarding | 27 | |
| 2 | Team practice changes | 24 | |
| Changes to UC | |||
| Category 2: Barriers to intervention implementation | |||
| 3 | Team challenges | 19 | |
| 4 | Staffing challenges | 37 | |
| 5 | Organisational or workplace barriers | 28 | |
| The acute model and culture | |||
| Barriers to Acute Stroke Unit access | |||
| Competing priorities | |||
| Physical environment barriers | |||
| 6 | Staff attitudes and beliefs | 32 | |
| Not ‘on board’ | |||
| Beliefs about roles and capabilities | |||
| Beliefs about consequences | |||
| 7 | Patients’ barriers | 35 | |
| Acuity, instability and complexity | |||
| Severity of stroke | |||
| Fatigue | |||
| Family anxiety | |||
| Category 3: Overcoming implementation barriers | |||
| 8 | Teamwork central to success | 43 | |
| Communication and coordination | |||
| 9 | Getting staff ‘on board’ | 35 | |
| Staff education and training | |||
| Leadership for change | |||
| 10 | Working differently | 29 | |
| ‘This is what we do here’ | |||
| Shifting control | |||
| Staffing model changes | |||
| Dealing with fatigue | |||
Discussion
The interview with staff identified some common themes. 37 First, implementing the VEM protocol within AVERT was acknowledged as being a challenging task. However, despite the challenges encountered there was substantial enthusiasm about participation in the trial. This enthusiasm was largely driven by an interest in the research question and the potential benefit to future stroke patients. A strong feature of these interviews was the importance of highly effective interdisciplinary teamwork. This was widely acknowledged as being an important factor in implementation of the VEM protocol. A second key feature was the importance of effective leadership to champion and encourage the trial within their units.
A strength of the qualitative study was that information was collected and analysed prior to the main AVERT trial results becoming available. Participants were generally optimistic that the trial would be positive and this seemed to be a factor in sustaining interest over a number of years. Another study strength was that experiences were collected across three countries, and, although there were some minor intercountry differences,37 the similarities were more striking than the differences.
Chapter 4 Statistical trial results
The main component of this chapter was published by the AVERT Trial Collaboration Group,31 (an Open Access article) distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text in this chapter includes minor additions and formatting changes to the original text.
Screening and exploring threats to generalisability in the AVERT
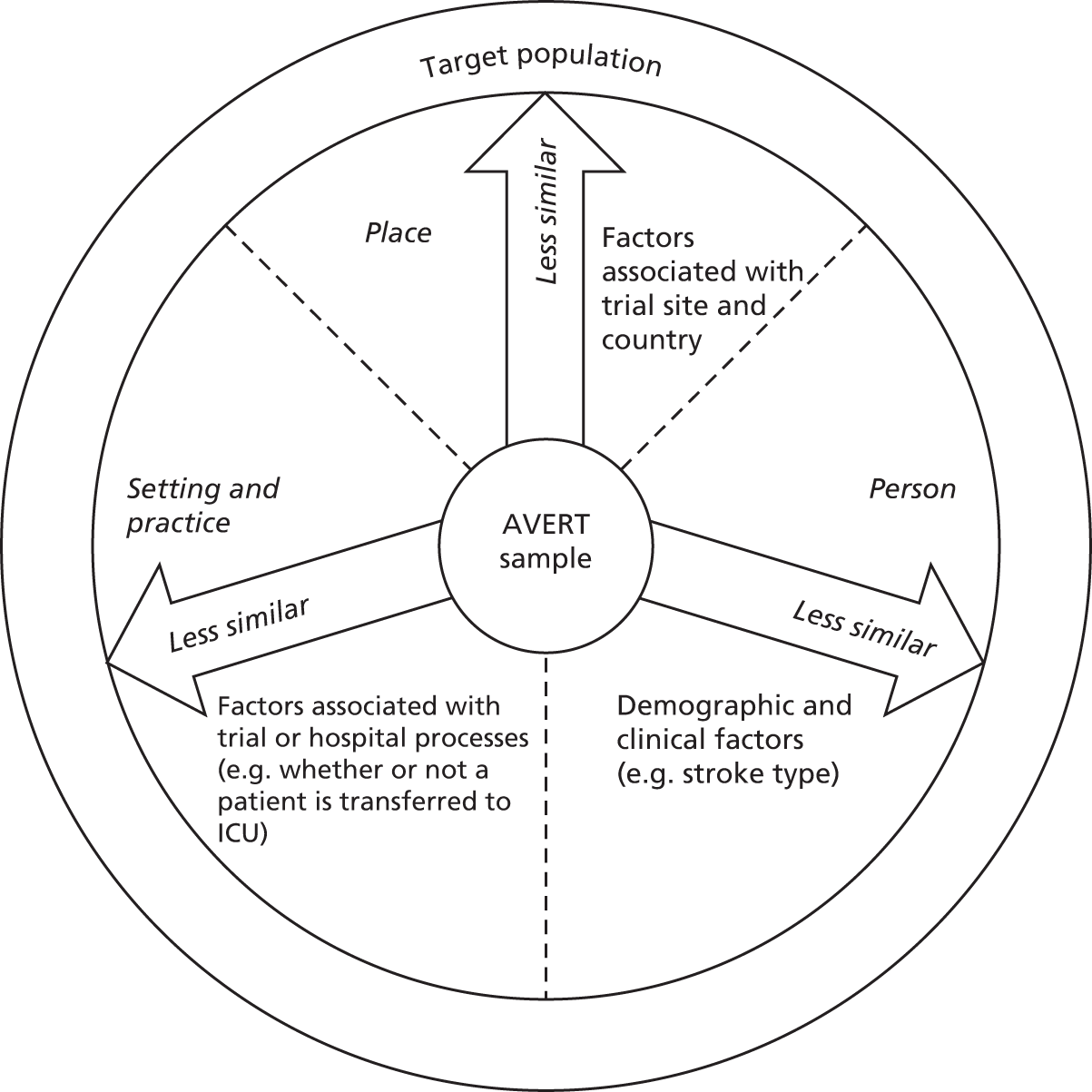
In parallel with the main trial of AVERT, we also carried out a study to explore potential threats to generalisability of the main results of AVERT. 38 We wished to consider the impact of person, place, setting and practice as a framework for considering generalisability. Therefore, we used a proximal similarity model (Figure 3) to carry out this analysis of the first 20,000 patients screened for inclusion in AVERT, which involved 44 hospitals in five countries. Of the first 20,000 patients screened for inclusion, 1158 were recruited and randomised in AVERT.
FIGURE 3.
Proximal similarity framework applied to the AVERT trial: a model for conceptualising the dimensions along which the sample of patients may be similar to the target population. Each dimension (person, place, setting and practice) is affected by specific factors that may threaten external validity. ICU, intensive care unit. Reproduced with permission from Bernhardt et al. 38 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
We compared recruited patients with the target population and also explored the factors (demographic, clinical process and site factors) that were associated with participant recruitment using a proximal similarity model (see Figure 3) that incorporated inclusion and exclusion criteria (Figure 4).
FIGURE 4.
Relationship between trial inclusion/exclusion criteria and screening log categories in AVERT. a, other reasons included (but are not limited to) patients not admitted to a stroke unit, lower limb fractures and no treating therapist available. ICU, intensive care unit. Reproduced with permission from Bernhardt et al. 38 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.

The characteristics of participants included in the trial were broadly similar in terms of demographic and stroke characteristics with the exception that recruited participants had a greater proportion of men (Table 4). Late arrival to hospital (after 24 hours) was the most commonly reported reason for non-recruitment. Overall, older and female participants were less likely to be recruited to the trial. The reasons for exclusion of women rather than men applied to a range of reasons including refusal. Among severe stroke patients, the odds of exclusion because of early deterioration was particularly common (OR 10.4, 95% CI 9.3 to 11.7, p < 0.001).
| Features | AVERT, non-recruited | AVERT, recruited | Difference (p-value) – recruited : non-recruited |
|---|---|---|---|
| n (%) | 18,842 (94) | 1158 (6) | |
| Age (years), median (IQR) | 75 (64–82) | 73 (63–80) | < 0.001 |
| Range | 15–102 | 18–100 | |
| Female age (years), median (IQR) | 78 (61–80) | 76 (66–82) | |
| Male age (years), median (IQR) | 71 (68–85) | 71 (61–79) | |
| Female, % (95% CI) | 47 (47 to 48) | 37 (34 to 40) | < 0.001 |
| NIHSS, n (%) | < 0.001 | ||
| Mild (1–7) | 10,012 (53) | 619 (53) | |
| Moderate (8–16) | 4934 (26) | 358 (31) | |
| Severe (> 16) | 3896 (21) | 181 (16) | |
| Stroke type, n (%) | 0.504 | ||
| Ischaemic | 16,328 (87) | 1012 (87) | |
| ICH | 2514 (13) | 146 (13) |
We found that using a screening log that captured a broad range of reasons for non-recruitment added to the collection of demographic data. 38 Similarly, the use of a model to explicitly explore generalisability was informative. However, a large screening log can only collect a limited amount of demographic and clinical information and it is quite possible that other factors may have influenced our recruitment. The proximal similarity model which explores person, place, practice and setting did provide some important information about the generalisability of this trial. Overall, the external validity appeared reasonably good.
Screening and recruitment
A summary of trial recruitment in the UK and other recruiting regions, during the period of the NIHR HTA programme grant (2012–16), is shown in Table 5. Recruitment from UK sites stood at 319 participants at the start of 2013 (average UK recruitment rate of seven participants per month). This compared with 19 participants per month in all other sites in Australia, New Zealand and Asia. The expansion that was possible through the NIHR HTA programme grant allowed us to recruit 291 UK participants in 2013–14 (average recruitment per month of 14 participants compared with 16 participants per month in all other sites).
| Country/centre | January to December 2011 | January to December 2012 | January to December 2013 | January to October 2014 | Final total |
|---|---|---|---|---|---|
| Northern Ireland total | 20 | 12 | 2 | 2 | 59 |
| Antrim | 8 | 7 | 1 | 2 | 18 |
| Belfast City | 5 | – | – | – | 15 |
| Craigavon | 0 | – | – | – | 0 |
| Daisy Hill | 1 | – | – | – | 1 |
| Ulster | 6 | 5 | 1 | – | 25 |
| Wales total | 5 | 1 | 5 | 12 | |
| Neville Hall | 5 | 1 | 5 | 12 | |
| Scotland total | 13 | 26 | 25 | 30 | 171 |
| Aberdeen | 5 | 9 | 8 | 11 | 33 |
| Crosshouse | – | – | – | – | 3 |
| Edinburgh | – | 2 | 4 | – | 6 |
| Forth Valley | 2 | 12 | 13 | 20 | 65 |
| Monklands | 3 | 1 | 26 | ||
| Western | – | – | – | – | 10 |
| Wishaw | 3 | 2 | – | – | 28 |
| England total | 26 | 107 | 116 | 111 | 368 |
| Blackpool | – | – | – | 17 | 17 |
| Calderdale | – | 2 | 5 | 7 | |
| Harrogate | – | 4 | 4 | 7 | 15 |
| Hexham | 2 | 3 | – | – | 5 |
| Imperial College | 9 | 7 | 8 | 5 | 29 |
| London St George | – | – | 2 | 5 | 7 |
| North Devon | 1 | 4 | 1 | 6 | |
| North Tyneside | 1 | 8 | 7 | 1 | 17 |
| QEQMH | – | 4 | 9 | 8 | 21 |
| Royal Bournemouth | – | 5 | 18 | 9 | 32 |
| Royal Devon | 2 | 7 | 7 | 8 | 24 |
| Royal Victoria | 5 | 6 | 13 | 11 | 35 |
| South Tyneside | 2 | 2 | 3 | 1 | 8 |
| St Mary’s IoW | – | 10 | 1 | 2 | 13 |
| Wansbeck | 3 | 8 | 4 | 3 | 18 |
| Yeovil | 1 | 23 | 24 | 13 | 61 |
| York | – | 15 | 11 | 28 | 54 |
| UK total (number/month) | 64 (5.3) | 146 (12.2) | 149 (12.4) | 142 (15.8) | 610 |
| Australia/New Zealand/Asia total (number/month) | 237 (19.8) | 164 (13.7) | 179 (14.9) | 149 (15.3) | 1494 |
| Total (number/month) | 301 (25.1) | 309 (25.3) | 322 (26.8) | 271 (30.1) | 2104 |
The trial profile is provided in Figure 5. A total of 25,237 patients were admitted within 24 hours of stroke onset, of whom 23,133 were ineligible. The most common reasons were no recruiting staff available at the time of admission, medical instability, or premorbid disability. A smaller number were enrolled in other clinical trials or refused trial entry. Between 18 July 2006 and 16 October 2014, we randomly allocated 2104 patients to receive either VEM (n = 1054) or UC (n = 1050).
FIGURE 5.
AVERT profile. a, More than one reason possible per patient. Reproduced with permission from the AVERT Trial Collaboration Group (2015). 31 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Eligibility violations
A total of 34 patients were found to have a non-stroke diagnosis (n = 13 in VEM, and n = 21 in UC). A total of 26 were never mobilised (n = 12 in VEM, and n = 14 in UC) (see Figure 5). These patients remained in the trial and, if they agreed, were followed through until completion.
Participant baseline characteristics
Table 6 outlines the participant baseline characteristics that were similar between study groups. The median time to randomisation was 18 hours after stroke and was the same in both groups. The majority of patients (80%) were experiencing their first stroke and a large minority (45%) were classified as having moderate or severe stroke (NIHSS of > 7). Approximately one-quarter of patients were aged > 80 years (26%) and 24% received rtPA.
| Features | VEM (n = 1054) | UC (n = 1050) |
|---|---|---|
| Recruitment region, n (%) | ||
| Australia and New Zealand | 617 (59) | 626 (60) |
| Asia | 126 (12) | 125 (12) |
| UK | 311 (29) | 299 (28) |
| Age (years), mean (IQR) | 72.3 (62.3–80.3) | 72.7 (63.4–80.4) |
| < 65, n (%) | 331 (31%) | 298 (28%) |
| 65–80, n (%) | 448 (43%) | 481 (46%) |
| > 80, n (%) | 275 (26%) | 271 (26%) |
| Sex, n (%) | ||
| Female | 411 (39) | 407 (39) |
| Male | 643 (61) | 643 (61) |
| Risk factors, n (%) | ||
| Hypertension | 707 (67) | 717 (68) |
| Ischaemic heart disease | 235 (22) | 251 (24) |
| Hypercholesterolaemia | 421 (40) | 423 (40) |
| Diabetes mellitus | 239 (23) | 228 (21) |
| Smoking, n (%) | ||
| Never smoked | 454 (43) | 491 (47) |
| Smokera | 227 (22) | 204 (19) |
| Ex-smokera | 352 (33) | 341 (33) |
| Unknown | 21 (2) | 14 (1) |
| Atrial fibrillation | 229 (22) | 237 (23) |
| Premorbid history, n (%) | ||
| Premorbid mRS | ||
| 0 | 799 (76) | 786 (75) |
| 1 | 145 (14) | 158 (15) |
| 2 | 110 (10) | 106 (10) |
| Living arrangement at time of admission, n (%) | ||
| Home alone | 257 (25) | 275 (26) |
| Home with someone | 781 (74) | 761 (73) |
| Supported accommodation | 16 (1) | 14 (1) |
| Independent walking, n (%) | ||
| Without aid | 908 (86) | 925 (88) |
| With aid | 146 (14) | 125 (12) |
| Time to randomisation (hours), mean (IQR) | 18.2 (12.1–21.8) | 18.2 (12.5–21.8) |
| Stroke history, n (%) | ||
| First stroke | 878 (83) | 843 (80) |
| NIHSS score, mean (IQR) | 7 (4–12) | 7 (4–12) |
| Mild (1–7) | 592 (56) | 578 (55) |
| Moderate (8–16) | 315 (30) | 328 (31) |
| Severe (> 16) | 147 (14) | 144 (14) |
| Stroke type (Oxfordshire Stroke Classification), n (%) | ||
| Total anterior circulation infarct | 224 (21) | 232 (22) |
| Partial anterior circulation infarct | 340 (32) | 328 (31) |
| Posterior circulation infarct | 93 (9) | 106 (10) |
| Lacunar infarct | 255 (24) | 268 (26) |
| Intracerebral haemorrhage | 142 (14) | 116 (11) |
| rtPA treatment, n (%) | ||
| Yes | 247 (23) | 260 (25) |
| Baseline walking (MSAS walking score), n (%) | ||
| Independent | 439 (42) | 416 (40) |
| Supervised or assisted | 522 (49) | 538 (51) |
| Unable to walk | 91 (9) | 96 (9) |
| Unknown | 2 (< 1) | 0 (0) |
Participant withdrawals
A total of 2083 (99%) of patients were included in the 3-month follow-up assessment (see Figure 5). The main reasons for withdrawal were refusal (15 participants) and unknown (six participants). At the 12-month follow-up, 2052 (97%) completed an assessment, which showed that 24 patients were missing and 28 refused follow-up.
For UK participants (n = 610), 3-month follow-up assessments were complete for ≥ 98% of participants (nine withdrew). The 12-month follow-up assessments were complete for > 96% of UK participants (15 withdrew, 10 could not be contacted).
Treatment compliance
Table 7 summarises the three crucial elements of the VEM protocol. Patients allocated to VEM began mobilisation within 24 hours of stroke.
| Features | VEM (n = 1054) | UC (n = 1050) | p-value | Median shift (95% CI) |
|---|---|---|---|---|
| TTFM (hours) | 18.5 (12.8–22.3; n = 1042a) | 22.4 (16.5–29.3; n = 1036a) | < 0.0001 | 4.8 (4.1 to 5.7) |
| Frequency per personb | 6.5 (4.0–9.5) | 3 (2.0–4.5) | < 0.0001 | 3 (3 to 3.5) |
| Daily amount per person (minutes)c | 31 (16.5–50.5) | 10 (0–18) | < 0.0001 | 21.0 (20 to 22.5) |
| Total amount per person (minutes)d | 201.5 (108–340) | 70 (32–130) | < 0.0001 | 117 (107 to 128) |
Patients in the VEM group successfully commenced mobilisation early after randomisation (median 18.5 hours after stroke). In the UC group, the median time to mobilisation was almost 5 hours later, but still within 24 hours of stroke onset. The categorisation of TTFM in the VEM and UC groups is outlined in Table 7. It is notable that 965 (92%) VEM patients had mobilised within 24 hours compared with 623 (59%) patients in the UC group. It was noted that the median TTFM in the UC group actually reduced during the study period. The rate of reduction was 28 minutes per year (95% CI 11.3 to 44.6 minutes; p = 0.001). There was no significant change in the VEM group over the same time period. As a result, there was a significant interaction between time since commencing the trial and TTFM (p = 0.017). In contrast, during the study period there was no significant change in the daily frequency or daily amount of out-of-bed intervention or in the total intervention time.
Content of physical therapy and nursing
Further exploration of the intervention differences between the VEM and UC groups are shown in Figure 6. TTFM was substantially reduced in the VEM group in all subgroups of patients except those recruited in Asia. The frequency of out-of-bed activity was increased in all VEM subgroups, especially younger patients with milder strokes. A similar pattern was seen in the amount of out-of-bed activity regardless of whether it was measured per day or over the whole intervention period.
FIGURE 6.
Intervention characteristics by group (VEM vs. UC) for each subgroup. (a) TTFM (hours); (b) frequency, median daily sessions of out-of-bed activity; (c) daily amount, median minutes per day in out-of-bed activity; and (d) total amount, minutes, over the intervention period. The forest plot shows the effect size and 95% CI for earlier TTFM, more frequent out-of-bed sessions, higher daily amount of out-of-bed activity and higher total dose over the intervention period in the VEM group. Titration of dose to the stroke severity is evident. Stroke severity (NIHSS): mild = 1–7, moderate = 8–16, and severe > 16. Reproduced with permission from the AVERT Trial Collaboration Group (2015). 31 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
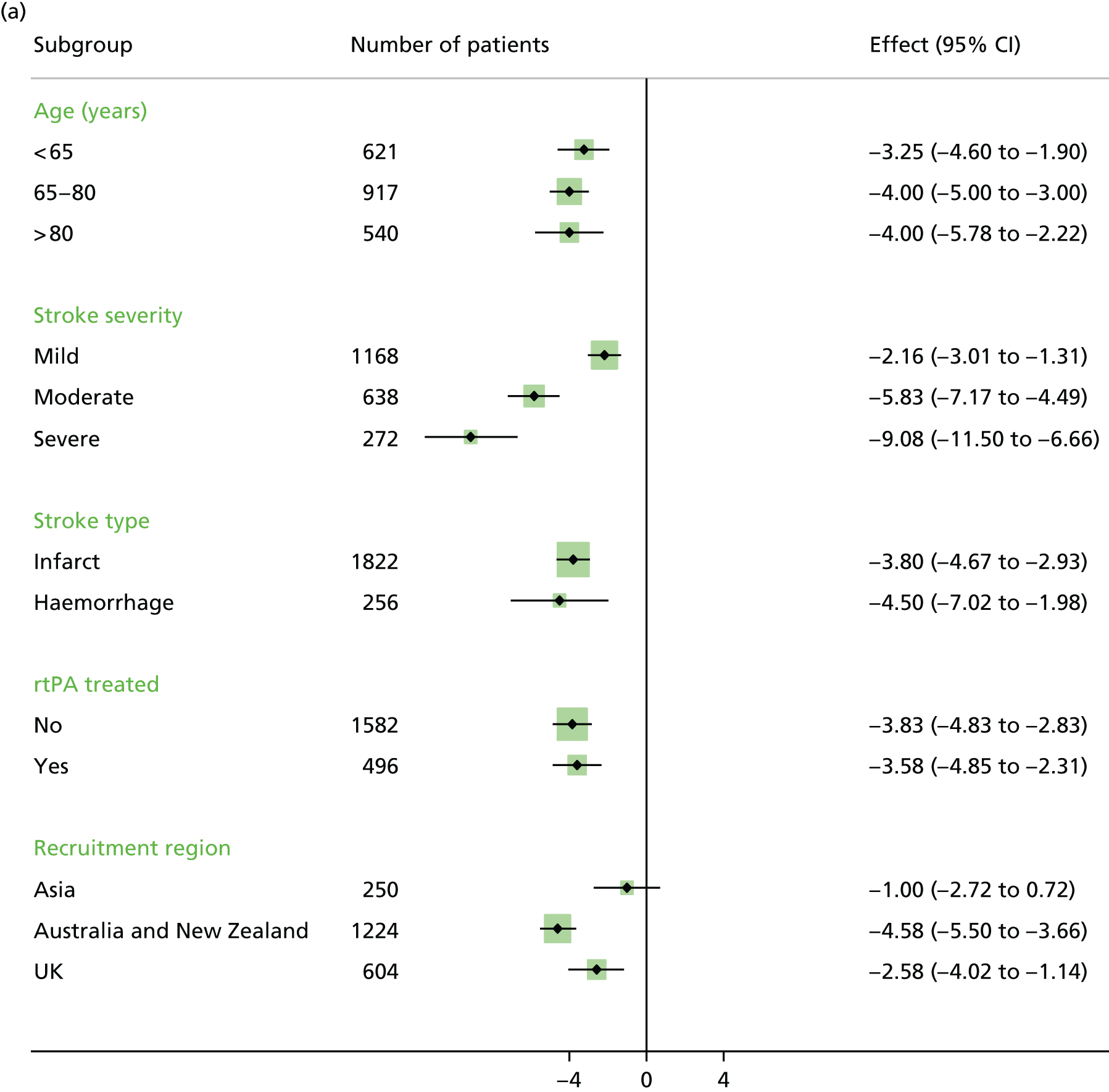



Regional differences
Figure 6 also shows the successful delivery of VEM compared with UC regimes in the UK sites. In general, the differences were slightly less marked in the UK than other regions but, overall, UK sites delivered a 2.6-hour reduction in TTFM, with three more out-of-bed activity sessions per day. This equated to 15 minutes more out-of-bed activity per day or 90 minutes during the intervention period.
Participant assessments
Out of the 2104 participants randomly assigned to either VEM (n = 1054) or UC (n = 1050), 2083 (99%) were available for 3-month mRS follow-up assessments (see Figure 5), with smaller numbers available for other outcome measures. At the 12-month follow-up, 2052 (97%) completed a mRS assessment, which showed that 24 patients were missing and 28 refused follow-up.
Primary end point: 3 months
At 3-month follow-up, fewer patients in the VEM group had a favourable outcome than the UC group (Table 8). This resulted in a significant difference between the groups on the prespecified analysis, which adjusted for baseline age and NIHSS (see Table 8): 480 (46%) in the VEM group had a favourable outcome compared with 525 (50%) in the UC group (aOR 0.73, 95% CI 0.59 to 0.90; p = 0.004). Sensitivity analysis produced similar results (Figure 7) and the treatment effect showed no interaction with time since the commencement of the trial. Unadjusted analysis of the primary outcome showed a reduction in favourable outcome that did not achieve statistical significance (p = 0.068).
| Features | VEM (n = 1038a) | UC (n = 1045a) | Adjusted analysis | Unadjusted analysis | ||
|---|---|---|---|---|---|---|
| OR, generalised OR or HRb (95% CI) | p-value | OR, generalised OR or HRb (95% CI) | p-value | |||
| Primary | ||||||
| Favourable outcomec | 480 (46) | 525 (50) | 0.73 (0.59 to 0.90) | 0.004 | 0.85 (0.72 to 1.0) | 0.068 |
| Secondary | ||||||
| mRS category | – | – | 0.94 (0.85 to 1.03) | 0.193 | 0.94 (0.85 to 1.03) | 0.202 |
| 0 | 90 (9) | 96 (9) | – | – | – | – |
| 1 | 200 (19) | 204 (19) | – | – | – | – |
| 2 | 190 (18) | 225 (22) | – | – | – | – |
| 3 | 238 (23) | 218 (21) | – | – | – | – |
| 4 | 140 (14) | 127 (12) | – | – | – | – |
| 5 | 92 (9) | 103 (10) | – | – | – | – |
| 6 | 88 (8) | 72 (7) | – | – | – | – |
| Walking 50 m unassistedd | 6 (5–7; n = 1051) | 7 (6–8; n = 1049) | 1.04 (0.94 to 1.15) | 0.459 | 1.05 (0.95 to 1.16) | 0.331 |
FIGURE 7.
Sensitivity analysis for the primary outcome, favourable outcome (mRS score of 0–2) at 3 months. The intention-to-treat analysis assumes that data are missing at random. The sensitivity of the results to plausible departures from this assumption were explored. Assumptions about the missing data were expressed via a parameter delta [exp(delta)], which measures the degree of departure from missing at random assumption (range 0–1). The upper 95% CI is below 1 (OR 0.71, 95% CI 0.57 to 0.87; p = 0.001) for all the values of delta indicating that the results remain significant on the sensitivity analysis. PMM, pattern mixture model. Reproduced with permission from the AVERT Trial Collaboration Group (2015). 31 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
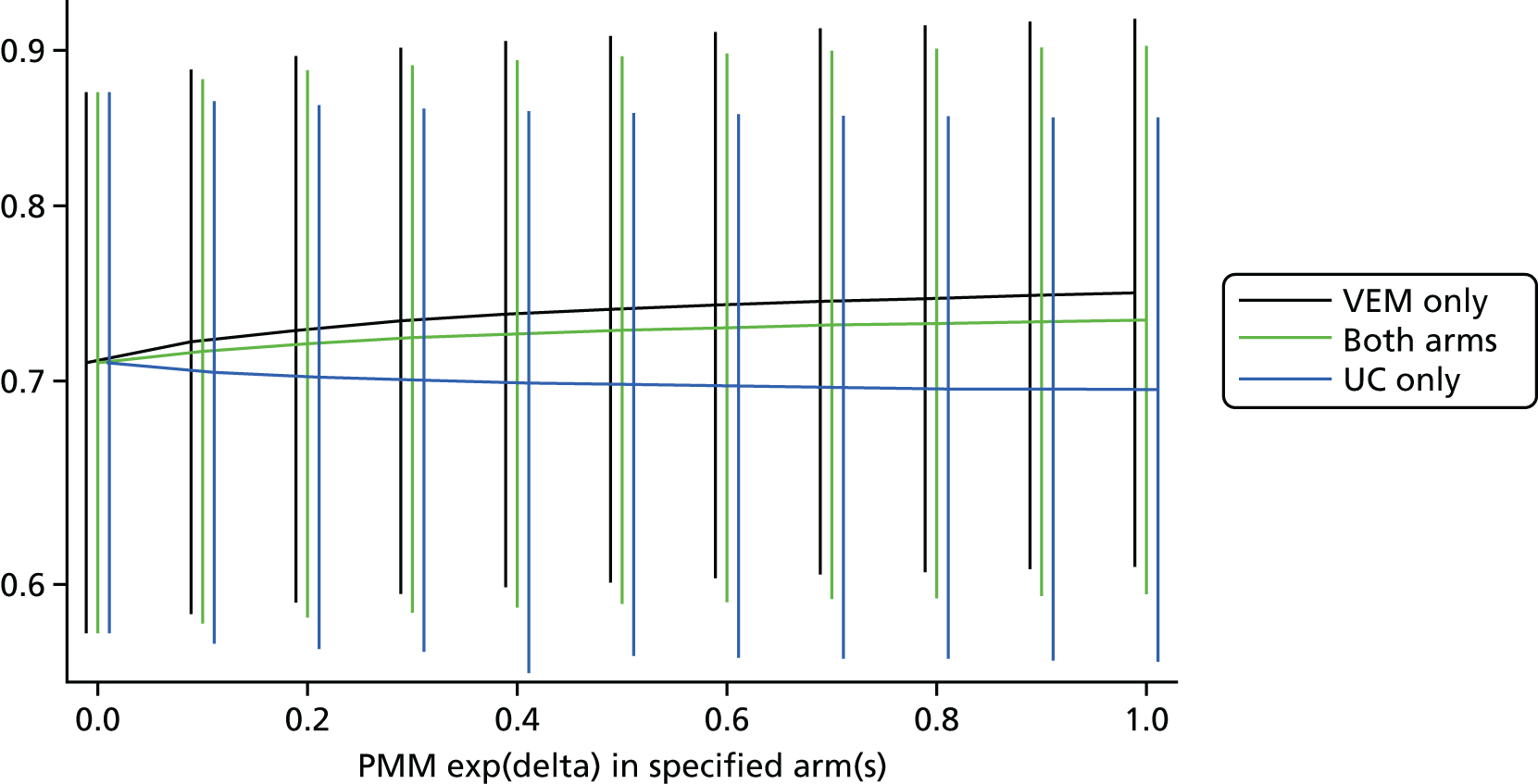
Secondary patient end point: 3 months
The secondary analysis included the assumption-free ordinal analysis. This did not show a significant difference between groups across the whole mRS (Figure 8). OR (95% CI) for an improved outcome was 0.94 (0.85 to 1.03; p = 0.202) for an unadjusted analysis and 0.94 (0.85 to 1.03; p = 0.193) for an adjusted analysis (see Table 8).
FIGURE 8.
Patients achieving each mRS score at 3 months. Reproduced with permission from the AVERT Trial Collaboration Group (2015). 31 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Walking ability
By 7 days after stroke, 50% of patients were able to walk unassisted and 784 (75%) were walking by 3 months in the VEM group. In the UC group, at 3 months, 796 (76%) were walking (aOR 0.83, 95% CI 0.64 to 1.07; p = 0.143). There was no significant difference in the groups in the time to walking unassisted (Figure 9 and see Table 8).
FIGURE 9.
Time taken to regain walking unassisted 50 m by 3 months. a, Number of patients who had not achieved walking. Reproduced with permission from the AVERT Trial Collaboration Group (2015). 31 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Case fatality
At the 3-month follow-up, the overall case fatality was 8% (Table 9); 88 (8%) patients died in the VEM group and 72 (7%) in the UC group. The main causes of SAEs are outlined in Table 9. These accounted for two-thirds of all deaths and included stroke progression (n = 31 in the VEM group and n = 19 in the UC group), pneumonia (n = 19 in the VEM group and n = 15 in the UC group) and recurrent stroke (n = 11 in the VEM group and n = 7 in the UC group).
| Features | VEM (n = 1054) | UC (n = 1050) | OR or IRRa (95% CI) | p-value |
|---|---|---|---|---|
| Death | 88/1048 (8)b | 72 (7) | 1.34 (0.93 to 1.93) | 0.113 |
| Non-fatal SAEs | 0.88 (0.72 to 1.07) | 0.194 | ||
| 0 | 853 (81) | 842 (80) | – | – |
| 1 | 157 (15) | 146 (14) | – | – |
| 2 | 32 (3) | 41 (4) | – | – |
| 3 | 10 (1) | 16 (2) | – | – |
| 4 | 2 (< 1) | 4 (< 1) | – | – |
| 5 | 0 | 1 (< 1) | – | – |
| Immobility SAEsc | 0.92 (0.62 to 1.35) | 0.665 | ||
| 0 | 1000 (95) | 997 (95) | – | – |
| 1 | 50 (5) | 46 (4) | – | – |
| 2 | 4 (< 1) | 5 (1) | – | – |
| 3 | 0 | 2 (< 1) | – | – |
| 4 | 0 | 0 | – | – |
| 5 | 0 | 0 | – | – |
| Neurological SAEsc | 1.26 (0.95 to 1.66) | 0.108 | ||
| 0 | 947 (90) | 967 (92) | – | – |
| 1 | 104 (10) | 78 (7) | – | – |
| 2 | 3 (< 1) | 4 (< 1) | – | – |
| 3 | 0 | 1 (< 1) | – | – |
| 4 | 0 | 0 | – | – |
Length of hospital stay
For patients in the VEM group, the median length of hospital stay including acute care and rehabilitation was 16 days [interquartile range (IQR) 5–44 days]. Patients in the UC group had a hospital stay of a median of 18 days (6–43 days). The equivalent figure for acute care alone was 7 days (IQR 4–13 days) for both patients receiving VEM or UC. The rehabilitation length of stay was 28 days (15–49 days) for the VEM group and 30 days (16–51 days) for the UC group. The number of patients transferring from acute care to patient rehabilitation was 492 (46%) in the VEM group and 523 (49%) in the UC group.
Adverse events
Serious adverse events: most patients did not have a SAE in the first 3 months of follow-up (see Table 9). There was no significant difference in the proportion of patients who had non-fatal SAEs (see Table 9). We also examined SAEs by prespecified category of complication (immobility vs. neurological). Relatively few patients in either group had a fatal or non-fatal serious complication related to immobility (see Table 9). The final number was 8% in each group. Serious neurological complications were recorded in < 10% of patients in either group and there were no significant differences between the groups (see Table 9). The most common neurological complication was stroke progression and was recorded in 72 (7%) participants in the VEM group and 56 (5%) participants in the UC group.
Staff safety: one staff injury was reported in the VEM group.
Secondary patient end point: 12 months
A total of 2052 (97.5%) participants completed the 12-month follow-up (Figure 10). In the VEM group, 139 participants had died, 19 refused and 16 were lost, compared with 118, nine and eight participants, respectively, in the UC group. For the UK participants (n = 610), assessments were complete for ≥ 96% (15 withdrew, 10 could not be contacted). Therefore, 52 (2.5%) patients did not complete mRS at 12-month follow-up (see Figure 7). The aOR (adjusted for age and baseline NIHSS) for a favourable outcome (mRS score of 0–2) in the VEM group was 0.84 (95% CI 0.68 to 1.03; p = 0.089).
FIGURE 10.
Trial profile at 12-month follow-up.
Health-related QoL: the AQoL could not be collected for 87 out of 2104 (4.1%) participants. In the VEM group, 139 had died, 36 could not be completed (refused, incomplete, not collected by assessor) and 16 could not be contacted. In the UC group, 118 had died, 27 could not be completed and eight could not be contacted. A death outcome was scored as zero and included in the analysis. A score of < 0 was classified as ‘worse than death’ and 0.9–1.0 was ‘excellent’. Treatment covariates for adjustment are baseline NIHSS, age and sex. The per cent of proxy completions (when the patients is alive but the AQoL was completed by a family member, friend or carer) was 11.9% in the VEM group and 12.6% in the UC group. The median AQoL IQR for the VEM group was 0.47 (95% CI 0.07 to 0.81) and in the UC group was 0.49 (95% CI 0.08 to 0.81; p = 0.865). The adjusted median regression result was –0.0036 (95% CI –0.045 to 0.038; p = 0.865).
Walking ability: at 12 months, 24% (434/1836) of alive patients were not walking 50 m. The aOR between groups for walking at 12 months was 0.85 (95% CI 0.65 to 1.10; p = 0.222). The between-group comparison of proportion of the number of days before patients recovered walking ability was not statistically significantly different between groups (HR 1.02, 95% CI 0.94 to 1.13; p = 0.553).
Subgroup analyses
The prespecified subgroup analysis of the primary outcome (favourable outcome of mRS score of 0–2 at 3 months) is outlined in Figure 11. The pattern of results tended to favour the UC intervention across all the main subgroups. The point estimate suggested that the poorest outcomes in the VEM group were in patients with severe stroke and patients with intracerebral haemorrhage. However, within each individual subgroup analysis, no statistically significant interactions were recorded (p > 0.05), but the trial is underpowered to detect subgroup interactions.
FIGURE 11.
Prespecified subgroup analyses: primary outcome at 3 months. None of the individual subgroup analyses had significant treatment-by-subgroup interactions (all p > 0.05). Stroke severity (NIHSS): mild = 1–7, moderate = 8–16 and severe > 16. Reproduced with permission from the AVERT Trial Collaboration Group (2015). 31 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

The subgroup analysis of the secondary outcome (death at 3 months) is outlined in Figure 12. The pattern of results tended to favour the UC intervention across all the main subgroups. The point estimate suggesting that the poorest outcomes in the VEM group were in patients with intracerebral haemorrhage did not achieve a statistically significant level of interactions (p > 0.05, see Figure 11).
FIGURE 12.
Death at 3 months by group shown for each subgroup. None of the individual subgroup analyses had significant treatment-by-subgroup interactions (all p > 0.05). Stroke severity (NIHSS): mild = 1–7, moderate = 8–16 and severe > 16. Reproduced with permission from the AVERT Trial Collaboration Group (2015). 31 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Moderator analysis
Functional outcome: further prespecified analysis explored the relationship between treatment received and patient outcomes; this analysis included all patients in a single cohort analysis and explored relationships within the group using binary logistic regression models and CART analysis. Baseline characteristics of the combined participant groups are shown in Table 10. It was notable that the group were representative of the stroke population: 25% were ≥ 80 years of age and 43% had a moderate or severe stroke (NIHSS score of > 7) while 12% were diagnosed with intracerebral haemorrhage.
| Features | Patients (N = 2104) |
|---|---|
| Recruitment region, n (%) | |
| Australia/New Zealand | 1243 (59) |
| Asia | 251 (12) |
| UK | 610 (29) |
| Patient characteristics | |
| Age (years), median (IQR) | 73 (63–80) |
| Female, n (%) | 818 (40) |
| Risk factors, n (%) | |
| Hypertension | 1424 (68) |
| Ischaemic heart disease | 487 (23) |
| Hypercholesterolaemia | 929 (40) |
| Diabetes mellitus | 467 (22) |
| Atrial fibrillation | 466 (22) |
| Smoking, n (%) | |
| Never smoked | 945 (45) |
| Smoker | 431 (20) |
| Ex-smoker | 693 (33) |
| Living arrangement at time of admission | |
| Home alone | 532 (25) |
| Home with someone else, n (%) | 1542 (73) |
| Time (hours) to randomisation, median (IQR) | 18 (12–22) |
| First stroke, n (%) | 1721 (82) |
| NIHSS score at baseline | |
| Median (IQR) | 7 (4–12) |
| Mild (NIHSS 1–7), n (%) | 1170 (56) |
| Moderate (NIHSS 8–16), n (%) | 643 (31) |
| Severe (NIHSS score of > 16), n (%) | 291 (14) |
| Stroke type (Oxfordshire Stroke Classification), n (%) | |
| Total anterior circulation infarct | 456 (22) |
| Partial anterior circulation infarct | 668 (32) |
| Posterior circulation infarct | 199 (9) |
| Lacunar infarct | 523 (25) |
| Intracerebral haemorrhage | 258 (12) |
| Treated with rtPA | 507 (24) |
| Baseline walking (based on MSAS), n (%) | |
| Independent | 855 (41) |
| Supervised or assisted | 1060 (50) |
The intervention characteristics for all patients are summarised in Table 11. The median TTFM was short [20.2 hours (IQR 14.7–23.8)] and 1588 (75%) of all participants began out-of-bed activities within 24 hours of stroke onset.
| Characteristics | All included patients (N = 2104) |
|---|---|
| TTFM (hours), median (IQR) | 20.2 (14.7–23.8)a (n = 2078) |
| Frequency per personb [median daily sessions of out-of-bed activity (IQR)] | 5 (3–8) |
| Daily amount per personc [median minutes per day spent in out-of-bed activity (IQR)] | 17.5 (6–35) |
| Total amount per personc [minutes over the intervention periodd (median and IQR)] | 120 (50–235) |
In the logistic regression analysis (Table 12) a longer TTFM was associated with reduced odds of favourable outcome (OR 0.99, 95% CI 0.98 to 1.00; p = 0.036). In the first model, the effect of timed first mobilisation was adjusted for the median daily number of sessions (frequency) and the median daily number of minutes (daily amount) as well as age and baseline severity (NIHSS). This should be interpreted as follows: for two patients with a similar age and baseline stroke severity who receive a similar frequency and daily amount of out-of-bed activity, the patient who starts mobilisation earlier has an increased odds of a favourable outcome.
| Features | Favourable outcome (mRS score of 0–2) | Walking 50 m unassisted | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p-value | Binary OR (95% CI) | p-value | Cox HR (95% CI) | p-value |
| Model 1 | ||||||
| TTFM (per extra hour of time) | 0.99 (0.98 to 1.0) | 0.036 | 1.0 (0.99 to 1.0) | 0.40 | 0.99 (0.98 to 0.99) | < 0.001 |
| Frequency, median daily sessionsa (per one extra session of mobilisation) | 1.13 (1.09 to 1.18) | < 0.001 | 1.66 (1.53 to 1.80) | < 0.001 | 1.10 (1.09 to 1.13) | < 0.001 |
| Daily amount, median (per extra 5 minutes of mobilisation activity) | 0.94 (0.91 to 0.97) | < 0.001 | 0.85 (0.81 to 0.89) | < 0.001 | 0.96 (0.94 to 0.97) | < 0.001 |
| Model 2 | ||||||
| TTFM (per extra hour of time) | 0.99 (0.98 to 1.0) | 0.025 | 1.0 (0.99 to 1.0) | 0.48 | 0.99 (0.98 to 0.99) | < 0.001 |
| Frequency, median daily sessionsa (per one extra session of mobilisation) | 1.14 (1.10 to 1.18) | < 0.001 | 1.63 (1.51 to 1.76) | < 0.001 | 1.11 (1.10 to 1.13) | < 0.001 |
| Total amountb (per extra 5 minutes of mobilisation activity over intervention period) | 0.99 (0.98 to 0.99) | < 0.001 | 0.98 (0.98 to 0.99) | < 0.001 | 0.99 (0.99 to 0.99) | < 0.001 |
We found a similar pattern of association with each of the dose characteristics for both favourable outcome (mRS score of 0–2) and walking by 3 months (see Table 12). All three intervention variables (timed to first mobilisation, frequency, daily amount) were significantly associated with outcome in model 1. When keeping other variables constant, every extra 5 minutes of out-of-bed activity per day was associated with reduced odds of favourable outcome. In contrast, increasing the frequency of sessions was associated with an improved odds of a favourable outcome by 13% (95% CI 9% to 18%; p < 00.1) and also and improved odds of walking 50 m unassisted (66%, 95% CI 53% to 80%; p < 0.001). The pattern was similar when the alternative model (model 2) was used.
Adverse outcomes: when exploring associations between intervention characteristics and death within 3 months, the only characteristic that reduced the odds of death was increasing session frequency (Table 13). Non-fatal AEs showed less consistent associations with dose characteristics. It should be noted that relatively few mobility and neurological SAEs were reported.
| Features | Deaths | Non-fatal SAEs | Fatal or non-fatal neurological SAEs | Fatal or non-fatal immobility SAEs | ||||
|---|---|---|---|---|---|---|---|---|
| Binary OR (95% CI) | p-value | IRR (95% CI) | p-value | IRR (95% CI) | p-value | IRR (95%) | p-value | |
| Model 1 | ||||||||
| TTFM (per extra hour of time) | 0.99 (0.98 to 1.00) | 0.07 | 1.0 (0.99 to 1.00) | 0.71 | 1.0 (0.99 to 1.00) | 0.45 | 1.00 (0.99 to 1.00) | 0.59 |
| Frequency, median daily sessionsa (per one extra session of mobilisation) | 0.78 (0.70 to 0.88) | < 0.01 | 0.99 (0.95 to 1.03) | 0.55 | 0.89 (0.84 to 0.95) | < 0.01 | 0.94 (0.87 to 1.01) | 0.11 |
| Daily amount, medianb (per extra 5 minutes of mobilisation activity) | 0.96 (0.89 to 1.04) | 0.30 | 0.96 (0.93 to 0.99) | 0.01 | 1.03 (0.99 to 1.08) | 0.17 | 0.94 (0.89 to 1.00) | 0.06 |
| Model 2 | ||||||||
| TTFM (per extra hour of time) | 0.99 (0.98 to 1.00) | 0.07 | 0.99 (0.99 to 1.00) | 0.81 | 1.00 (0.99 to 1.00) | 0.35 | 1.00 (0.99 to 1.00) | 0.59 |
| Frequency, median daily sessionsa (per one extra session of mobilisation) | 0.79 (0.71 to 0.88) | < 0.01 | 0.96 (0.93 to 0.99) | 0.02 | 0.93 (0.88 to 0.98) | < 0.01 | 0.91 (0.85 to 0.97) | < 0.01 |
| Total amountb (per extra 5 minutes over intervention period of mobilisation activity) | 0.99 (0.98 to 1.00) | 0.06 | 1.00 (1.00 to 1.00) | 0.49 | 1.00 (0.99 to 1.00) | 0.32 | 1.0 (0.99 to 1.00) | 0.41 |
Classification and regression tree analysis
The CART analysis exploring the relationships with a good functional outcome (mRS score of 0–2) is outlined in Figure 13. This includes timed first mobilisation, intervention frequency, daily amount, patient age, baseline NIHSS, stroke subtype, treatment with thrombolysis and randomisation group. We observed good to excellent performance with a training data set (ROC 0.78) and ROC of 0.77 in a testing data set. The relative contribution of each variable is shown on the figure. In this analysis, treatment group was not an important discriminator of patient outcome. As expected, younger patients and those with low baseline NIHSS scores had a higher probability of a favourable outcome. The association with intervention characteristics was evident further down the analysis tree. For example, at terminal node 4 (see Figure 13) the greater probability of favourable outcome was associated with more frequent short mobilisation sessions (no more than 13.5 minutes). Mobilisation frequency also split the tree for terminal nodes 5 and 6, suggesting that more frequent sessions to achieve a higher dose was associated with an improved odds of a good outcome. In further CART analysis (Figures 14–16), TTFM intervention frequency and amount plus group are all influential splitters in these models.
FIGURE 13.
Classification and Regression Tree (CART1) advanced analysis investigating associations between dose and patient characteristics and the odds of a favourable outcome (mRS score of 0–2). Each box shows the number (N) with the headline characteristic plus the number of them (%, highlighted in bold) with a favourable outcome (mRS score of 0–2). Terms used include median daily number of out-of-bed sessions per day (frequency), median daily out-of-bed activity session time (amount), age (years) and stroke severity (NIHSS). Frequency is derived from nursing and physiotherapist data and amount (minutes) is derived from physiotherapist data only. Adapted with permission from Bernhardt et al. (2016). 33 Copyright © 2016 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0 (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

FIGURE 14.
CART2. CART advanced analysis investigating associations between dose characteristics and odds of a favourable outcome (mRS score of 0–2). Each box shows the number (N) with the headline characteristic plus the number of them (%, highlighted in bold) with a favourable outcome (mRS score of 0–2). Terms used are TTFM, median daily number of out-of-bed sessions per day (frequency) and median daily out-of-bed activity session time (amount). Frequency is derived from nursing and therapist data and amount (minutes) is derived from physiotherapist data only. Note: the model performed well with ‘training’ and ‘testing’ and ROC results were 0.78 and 0.69, respectively. The relative importance of each characteristic was frequency (100%), daily amount (32%) and TTFM (28%). CART2 shows that frequency of out-of-bed activity is an important splitter in the model, with higher frequency and lower amounts of out-of-bed activity generally associated with better outcome. Adapted with permission from Bernhardt et al. (2016). 33 Copyright © 2016 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0 (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

FIGURE 15.
CART3. Further exploration of terminal node one from CART1 (NIHSS score of ≤ 7.5, aged ≤ 76.3 years). This CART investigates associations between dose characteristics, patient characteristics and treatment with favourable outcome (mRS score of 0–2). Each box shows the number (N) with the headline characteristic plus the number of them (%, highlighted in bold) with a favourable outcome (mRS score of 0–2). Variables include TTFM, median daily number of out-of-bed sessions per day (frequency) and median daily out-of-bed activity time (amount), age, baseline NIHSS, stroke type and rtPA treatment. Note: the ‘training’ and ‘testing’ ROC results were 0.68 and 0.60, respectively. Relative importance of each characteristic: frequency (100%), NIHSS score (99%), daily amount (84%), age (83%), infarct/haemorrhage (10%), TTFM (4%) and VEM group (3%). CART3 indicates that higher frequency and lower amounts are important splitters in the model. Adapted with permission from Bernhardt et al. (2016). 33 Copyright © 2016 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0 (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
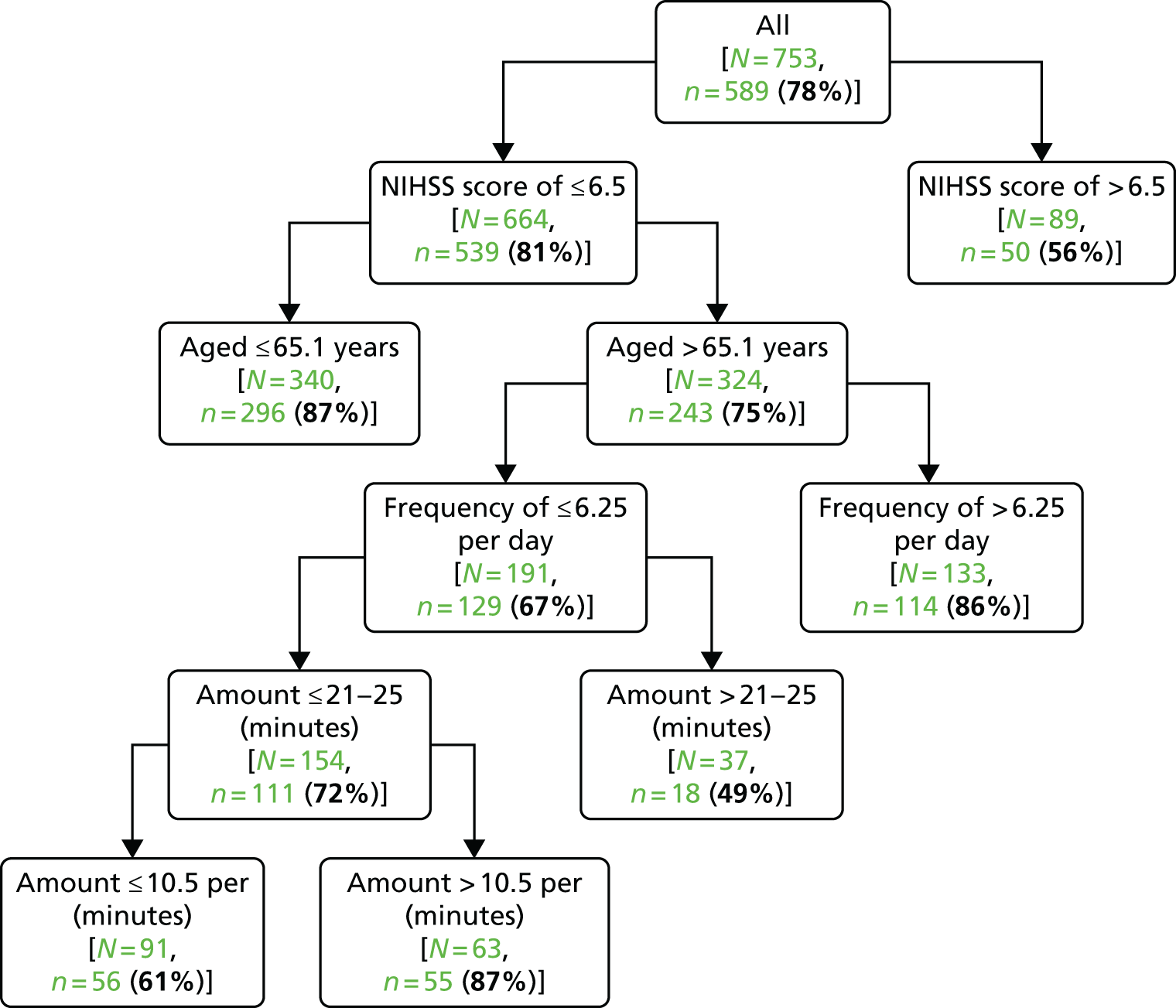
FIGURE 16.
CART4. Further explanation of terminal node eight from CART1 (NIHSS score of > 7.5). This CART investigates associations between dose characteristics, patient characteristics and treatment with favourable outcome (mRS score of 0–2). Each box shows the number (N) with the headline characteristic plus the number of them (%, highlighted in bold) with a favourable outcome (mRS score of 0–2). Variables include TTFM, median daily number of out-of-bed sessions per day (frequency) and median daily out-of-bed activity time (amount), age, baseline NIHSS, stroke type and rtPA treatment Note: the ‘training’ and ‘testing’ ROC results were 0.81 and 0.71, respectively. Relative importance of each characteristic: NIHSS score (100%), frequency (95%), age (45%), TTFM (27%), VEM group (21%), daily amount (6%) and infarct/haemorrhage (2%). CART4 indicates that higher session frequency is associated with higher proportion of patients with a favourable outcome. Adapted with permission from Bernhardt et al. (2016). 33 Copyright © 2016 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0 (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Finally, we repeated the regression analysis for the UC group alone because of the potential risk of any unmeasured confounding by treatment group (additional unmeasured differences between treatment groups). This repeat analysis (Table 14) indicated that the same factors were important within the UC group alone.
| Features | Favourable outcome OR (95% CI) | p-value |
|---|---|---|
| Model 1 | ||
| TTFM (per extra hour of time) | 0.98 (0.97 to 0.99) | 0.002 |
| Frequency, median daily sessions (per one extra session of out-of-bed activity) | 1.12 (1.04 to 1.21) | 0.004 |
| Daily amount, median (per extra 5 minutes of out-of-bed activity) | 1.00 (0.93 to 1.07) | 0.942 |
| Model 2 | ||
| TTFM (per extra hour of time) | 0.98 (0.97 to 0.99) | 0.002 |
| Frequency, median daily sessions (per one extra session of out-of-bed activity) | 1.15 (1.06 to 1.23) | 0.0001 |
| Total amount (per extra 5 minute minutes of out-of-bed activity over intervention period) | 0.98 (0.98 to 0.99) | 0.001 |
Specific UK perspectives
Figure 6 also showed the delivery of VEM in different regions. This shows the successful delivery of VEM versus UC regimes in the UK sites. In general, the differences between VEM and UC were slightly less marked in the UK than other regions but, overall, UK sites delivered a 2.6-hour reduction in TTFM, with three more out-of-bed activity sessions per day. This equated to 15 minutes more out-of-bed activity per day or 90 minutes during the intervention period.
The subgroup analyses of the primary outcome (alive and independent at 3 months) and secondary outcome (death at 3 months) are outlined in Figures 10 and 11. The UK results were indistinguishable from those of Asia and Australia and New Zealand, with none of the regional subgroups achieving a statistically significant level of interaction (p > 0.05).
Economic analysis
An economic analysis was not funded in the context of this NIHR grant. The economic analysis plan34 had anticipated further analysis would be important in the event of the VEM intervention having a positive effect. As this was not the case, these analyses are not yet completed.
Meta-analysis of early mobilisation trials
In our systematic review of similar RCTs, we identified eight eligible trials25,27,31,60–64 that currently have data available. Of these eight trials (2618 participants), AVERT provided the most information (2104 participants).
The median (range) delay to starting mobilisation after stroke was 18.5 (13.1–43) hours in the EM group and 33.3 (22.5–71.5) hours in the delayed group. The median difference within trials was 12.7 (4–45.6) hours. Other differences in intervention varied between trials. In at least four trials, the EM group also received more time in therapy or mobilisation activity.
Complete 3-month outcome data were available for 2542 (97%) participants (Figure 17). Compared with delayed mobilisation, EM showed non-significant increases in the odds of death or dependency (OR 1.10, 95% CI 0.94 to 1.29), death (OR 1.27, 95% CI 0.95 to 1.70) and a decreased odds of experiencing any complication (OR 0.89, 95% CI 0.73 to 1.08).
FIGURE 17.
Meta-analysis of EM trials: early vs. delayed mobilisation in acute stroke patients. df, degrees of freedom; M–H, Mantel–Haenszel.

Repeating the analysis using a random-effects model did not alter these conclusions. There was substantial heterogeneity of intervention but the average TTFM was not significantly related to the odds of death or dependency or death alone (test for subgroup differences was p = 0.35 and p = 0.19, respectively).
Figure 17 shows the number (events) with death or poor outcome (mRS score of 3–6) at 3 months after stroke of the total number of patients (total) allocated to the EM (experimental) or delayed (control) mobilisation group. Results are presented as the OR (95% CI) of the early versus delayed mobilisation group.
Chapter 5 Discussion
This project met its initial objectives of effectively delivering our VEM protocol with a resulting change in practice. 30 We observed earlier, more frequent and higher dose (amount of) out-of-bed mobility in terms of sitting, standing and walking activity. However, the unexpected feature was that the VEM intervention reduced the odds of a favourable outcome at 3 months after stroke compared with lower-dose UC, which started, on average, 5 hours later. It should be recognised that the outcome of this trial was observed against a background of a very high level of recovery overall. Despite having more than one-quarter of participants aged > 80 years and almost half recording a moderate or severe stroke, almost 50% had a favourable outcome in terms of independence at 3 months. Across both groups, the case fatality rate averaged only 8%. A further point to note is that HRQoL did not differ significantly between groups at the 12-month follow-up.
The prespecified subgroup analysis raised the possibility that patients with more severe stroke and those with intracerebral haemorrhage may do less well with VEM, but there was no statistically significant interaction across these subgroups. Exploration of case fatality within subgroups also suggested the possibility that intracerebral haemorrhage patients may be at higher risk of harm, but these analyses had wide CIs and were not statistically significant. Although the trial was not powered to detect differences between subgroups, these apparent differences may raise potentially important questions and warrant further investigation. In particular, there have been concerns10,23,24,32,64 about the safety of VEM in frailer individuals (older patients and those with intracerebral haemorrhage). It is notable that patients receiving tissue plasminogen activator had outcomes that were similar to those who did not receive this treatment, hence there is no evidence that EM is particularly harmful in the context of thrombolysis.
The results of this trial are intriguing particularly because the results of smaller trials suggested that early, frequent and higher-dose VEM would result in a favourable outcome. 25–28 A favourable outcome for the VEM group was also observed in a similar pilot trial in the UK27 and in an individual-patient meta-analysis of two small EM trials. 28 However, a non-significant increase in unfavourable outcome was reported in a more recent small Norwegian trial65 comparing VEM (< 24 hours) versus later mobilisation (> 24 hours). It is not yet clear if the results of the current AVERT are simply providing greater precision around these smaller estimates or if there is some qualitative difference in the nature of the intervention.
We were surprised to observe the very low rates of AEs overall and, in particular, the low rates of immobility-related complications. We had anticipated that VEM would result in fewer immobility-related complications but there were no statistically significant differences between groups. One explanation could be that UC now includes a sufficiently early onset of mobilisation, which may have reduced the risk of immobility-related complications compared with historical comparisons. The modern high-quality stroke unit care in the hospitals taking part in AVERT included 75% of patients undergoing out-of-bed mobilisation within 24 hours and only 7% of patients remaining in bed for > 48 hours. It is striking that UC in the present trial (median TTFM of 22 hours) was substantially lower than in previous studies (> 30 hours). Unfortunately, we do not have access to directly comparable information from other acute stroke trials.
The AVERT is, to our knowledge, the largest acute stroke rehabilitation trial to date with a complex intervention provided by existing clinical staff. Our aim had been to undertake a trial that met the exacting quality standards of a drug or device trial but that was sufficiently inclusive to be relevant to routine practice. We achieved our aim of high intervention fidelity and complete primary end-point follow-up in > 99% of participants. We also succeeded in careful characterisation of the intervention and UC and successfully adjudicated a large number of safety outcomes. We aimed to enhance the external validity of the trial by establishing it within routine hospital care across five countries. For these reasons, we believe the results of the trial are robust and provide important new evidence. The detailed description of the dose characteristics allowed us to proceed with an exploration of the interaction between intervention and outcome.
Exploratory analysis
The prespecified exploratory analysis found a consistent association between the odds of recovery with independence (mRS score of 0–2) at 3 months and some intervention characteristics. These were irrespective of treatment group and were independently seen in the control group as well as in the combined group analysis. The odds of a favourable outcome increased with mobilisation frequency: by 13% with each additional session per day of out-of-bed activity. In contrast, an increasing amount of time spent in out-of-bed activity was associated with a reduced odds of a favourable outcome when keeping constant intervention frequency and TTFM. The same pattern of potential beneficial effect with increasing the frequency (but not the amount) of out-of-bed activity was seen consistently across most of the clinical and safety analyses.
The purpose of these prespecified analyses was to unpack the primary results of the VEM intervention, which was essentially complex in nature. 66 The VEM intervention was defined in terms of TTFM but also included more frequent and higher-dose out-of-bed activity. The dose–response analysis suggests that increasing the frequency of mobilisation may help reduce disability and immobility while, in contrast, increasing the total time of out-of-bed activity in the early phase after stroke was associated with poorer outcomes. In summary, the exploratory analyses indicate that short, frequent sessions may be preferable for many stroke patients early after stroke.
The potential impact of TTFM was less clear probably because of a relatively compact distribution of this variable; therefore, the optimal time to commence out-of-bed activity is still uncertain. Animal studies have suggested that very high-dose training in the early post-stroke phase may increase brain lesion volume,67 but were not associated with the behavioural outcomes that are analogous to disability measures. However, conflicting results have also been reported in which moderate exercise reduced lesion volume and protected ischaemic tissue against secondary damage. 68–70
The conventional multivariable analysis was supplemented with the CART analysis. This was to provide an independent exploration using methodology based on different assumptions. Even when we included patient characteristics that strongly predict outcome after stroke, such as age and stroke severity, the intervention characteristics had an important role in defining patient groups. In particular, in patients with more severe stroke (NIHSS score of > 13), more frequent mobilisation sessions were associated with a more favourable outcome.
Particular strengths of this exploratory study are that the dose–response analysis was prespecified in the expectation that we would need to explore the intervention in greater detail. One potential criticism of the exploratory analysis is the possibility that the intervention protocol could have influenced our findings because the intervention dose was titrated to stroke severity and patients with less severe stroke would get a higher intervention dose. However, to exclude this possibility we repeated the analysis within the UC group alone and found the same relationship between TTFM, mobilisation frequency and the amount of time in mobilisation. In particular, when you maintain mobilisation time and the median minutes of out-of-bed activity is constant, more frequent sessions in the control group were associated with an improved odds of a good outcome of 1.12 (95% CI 1.04 to 1.21; p = 0.004). Table 12 outlines the association between TTFM and a marginally improved outcome and the lack of association between total amount of out-of-bed activity and outcome. Although the intervention protocol may have provided some confounding of the observed associations, it cannot explain all the findings observed. The key observations from the exploratory analysis are as follows:
-
Mobility interventions embedded within routine care and delivered in the acute phase can influence a patient’s long-term outcomes. It is very important that triallists carefully define and measure these aspects of care.
-
The generally accepted philosophy that more practice is always better requires reconsideration particularly early after stroke.
-
The frequency of mobility may be more important than other aspects of delivery. This requires further investigation.
Strengths and limitations
We found that using a screening log that captured a broad range of reasons for non-recruitment added to the collection of demographic data. 38 Similarly, the use of a model to explicitly explore generalisability was informative. However, a large screening log can collect only a limited amount of demographic and clinical information and it is quite possible that other factors influenced our recruitment.
The AVERT is largest acute stroke rehabilitation trial with a complex intervention provided by existing clinical staff. We achieved our aim of undertaking a trial that met the exacting quality standards of a drug or device trial but that was relevant to routine practice. It was established within routine hospital care across five countries. We achieved complete primary end-point follow-up in > 99% of participants and successfully adjudicated a large number of safety outcomes. For these reasons, we believe that the results of the trial are robust and provide important new evidence.
We believe that we achieved our aim of high intervention fidelity (the extent to which staff adheres to treatment protocols). This is a challenging part of trials of a complex intervention. 66 Within AVERT, sites were monitored on their delivery of VEM and UC and successful delivered differences in the intervention. 36 We provided trial protocols to treating staff who were trained in protocol intervention. Site initiation sessions were used to discuss and resolve local barriers and we provided reminders, decision tools and ongoing support for queries. The use of site champions and arrangements such as coleadership from a nurse and a physiotherapist were seen to be positive factors. It seems advisable that trial protocols for complex interventions include an implementation plan with the approach that would be used to achieve, measure and monitor acceptable fidelity standards. We also succeeded in careful characterisation of the VEM intervention and UC. Establishing fidelity provides confidence that the intervention was properly tested and that the outcome results can be correctly attributed to the intervention.
Despite these efforts, it is notable that UC TTFM had changed over time (but not the other aspects of mobilisation, frequency and total time of out-of-bed activities). Although UC was not standardised, careful trial monitoring allowed tracking of UC over time and provided reassurance that time to start mobilisation differences between groups were maintained. Mobilisation dose did not change over time. Although the reasons for earlier UC TTFM over time are unclear, it remains possible that many staff assumed that commencing earlier was safe and/or effective, and this unconsciously influenced their delivery of UC over time. External influences include more recent recommendations in clinical practice guidelines to mobilise early and intensively.
The AVERT has several limitations which are largely due to the large study size. In a large international trial it is difficult to collect more than a small amount of information about potential modifying or confounding factors such as physiological variations. It was also difficult to collect detailed information about staff–patient interactions. As AVERT was a pragmatic trial, we were not prescriptive about UC and it is interesting to note that TTFM appeared to change substantially during the period of the trial. This occurred despite independent monitoring, reporting and feedback to the study sites about the nature of their UC and VEM. As a result of the changes in standard mobilisation practice, by the end of the trial approximately two-thirds of patients receiving UC had started out-of-bed activity within 24 hours of stroke onset. It is uncertain if this change is a consequence of contamination from the trial protocol or the result of changing attitudes to EM over time, as was reflected in some recent clinical guidelines.
Comparison with other trials
Our systematic review was dominated by AVERT but confirmed that EM (within 48 hours) was not associated with improved outcomes compared with delayed mobilisation. However, it should be noted that in the majority of trials included in the review, mobilisation commenced within 48 hours of stroke onset rather than 24 hours. Furthermore, it is important to recognise that despite public education efforts to improve identification of stroke and seek early medical attention, patients are often delayed in reaching hospital. Generally, discussion of the timing of commencement of mobilisation is relative to time of admission which may be significantly later than time of stroke onset. All AVERT data are relative to time of stroke onset. In view of the complexity of the intervention and the uncertainty around the effect estimates, more detailed analyses are warranted.
Implications for practice
Delivery of AVERT required commitment to delivering a VEM intervention that needed strong interdisciplinary collaboration between nurses and physiotherapists and some modification of current care models. The qualitative analysis contributed some unique insights into what factors may be important to successful teams aiming to deliver a complex multidisciplinary intervention.
The results of AVERT should influence clinical practice. Most clinical practice guidelines had recommended EM10 but there was little specific advice provided. We would conclude that our high-dose frequent mobilisation protocol within 24 hours of stroke onset was less effective than UC and should not be routinely applied. However, because the UC protocol is also complex in nature, and increasingly featured a shift to early onset mobilisation, then it is over-simplistic to simply advise UC. When mobilisations are attempted early after stroke, short, frequent mobilisations are associated with better outcomes. Further exploration of this data set is essential and, as outlined in our published statistical analysis plan, we propose further dose–response analyses to explore the effect of dose rehabilitation on clinical and safety outcomes.
Implications for research
The AVERT results challenge several previous assumptions and raise several important research questions that can be listed as follows:
-
What should mobilisation entail: are there aspects that can safely be implemented?
-
Who should we target for early intervention? In particular, are there patient groups for whom EM is safe or unsafe?
-
Are important physiological and molecular changes induced by early physical activity in ischaemic tissue? In particular, does early active mobilisation induce early neurological changes that are detrimental to recovery?
-
How do we best describe the characteristics of EM? We still lack a clear and widely accepted descriptive framework for EM activities.
The AVERT group are planning a new trial to unpack the combined influences of mobilisation frequency and dose (duration).
A more detailed meta-analysis of existing trials is also needed to explore the limitations of the AVERT results in the context of other similar trials.
Acknowledgements
Contributions of authors
Professor Peter Langhorne was the chief investigator for the UK, was the lead grant holder and compiled, drafted and revised the manuscript.
Professor Olivia Wu, Professor Helen Rodgers, and Professor Ann Ashburn were grant holders and revised the manuscript.
Professor Julie Bernhardt was principal investigator for the international trial, was a grant holder and revised the manuscript.
Other contributions
We particularly want to thank the participants in this trial, and their families, who have supported this study. We are very grateful to all the AVERT collaboration investigators for their hard work and dedication and the many individuals not specifically mentioned in the report.
We specifically want to thank Sheila Lennon and Michael Power for their role in developing the Northern Ireland phase of the trial. We also received exceptional support from Jan Chamberlain (Florey Institute) and the office staff at the University of Glasgow (Beverly Armstrong, Louise Craig, Fiona Graham, Lynn Legg, Rosemary Morrison, Heather Moorhead, Lorraine O’Donohue, Susan Rogers and Myra Smith).
Patient and public involvement
Stroke survivors formed part of the original trial development team in Australia. The AVERT protocol was developed in consultation with service users and a stroke survivor (Ms Brooke Parsons) who took part in monthly management committee meetings. Ms Parsons sat on both the Management and Trial Steering Committee for the AVERT project and was a regular attendee at meetings. Ms Parsons also attended the AVERT triallists’ meeting held in Glasgow on 16 April 2015 to precede the presentation of the 3-month outcome results at the inaugural European Stroke Organisation conference in Glasgow on 17–19 April 2015.
Information newsletters with trial updates and stroke survivor stories were provided to all interested trial participants twice a year. These were posted, e-mailed or delivered to people involved in the trial by the blinded assessors. In addition, there was a website that could be accessed at any time to obtain more up-to-date information about news of the trial (www.florey.edu.au/very-early-rehabilitation-trial-avert).
Data sharing statement
A considerable number of analyses are currently under way for this trial data set. As these analyses are completed we anticipate that data will become available to the scientific community with as few restrictions as feasible. All deidentified trial data in Chapter 2 have been archived in secure facilities for a minimum period of 7 years. The options of data sharing arrangements were not available at the trial commencement and were not included in participant consent processes.
AVERT collaboration group
Management Committee
Julie Bernhardt (chairperson), Leonid Churilov, Janice Collier, Helen Dewey, Geoffrey Donnan, Fiona Ellery, Peter Langhorne, Richard Lindley, Marjory Moodie, Brooke Parsons (consumer representative) and Amanda Thrift.
Trial Steering Committee
Geoffrey Donnan (cochairperson), Helen Dewey (cochairperson), Julie Bernhardt, Peter Langhorne, Richard Lindley, Amanda Thrift, Marjory Moodie, Brooke Parsons (consumer representative) and main investigators (MIs) from all participating hospitals.
International advisors
Bent Indredavik, Anne Loege and Torunn Askim.
Data Monitoring Committee
Phillip Bath (University of Nottingham, Nottingham, UK; chairperson), Christopher Bladin (Box Hill Hospital, Melbourne, VA, Australia), Christopher Reid (Monash University, Melbourne, VA, Australia), Stephen Read (Royal Brisbane and Women’s Hospital, Melbourne, VA, Australia) and Cathy Said (Austin Health, Melbourne, VA, Australia).
Outcomes Committee
Sandy Middleton (Australian Catholic University, Sydney, NSW, Australia; chairperson), Judith Frayne (Alfred Hospital, Melbourne, VA, Australia) and Velandai Srikanth (Monash Health, Melbourne, VA, Australia).
Country leaders and grant holders
Australia: Julie Bernhardt (National Health and Medical Research Council: Helen Dewey, Julie Bernhardt, Geoffrey Donnan, Amanda Thrift, Robert Carter and Richard Lindley), (National Health and Medical Research Council: Julie Bernhardt, Geoff Donnan, Richard Lindley, Amanda Thrift, Peter Langhorne, Marjory Moodie, Helen Dewey and Leonid Churilov).
UK: Peter Langhorne (Chest Heart and Stroke Scotland: Peter Langhorne, Olivia Wu, Julie Bernhardt, Matthew Walters Claire Ritchie and Lorraine Smith), (The Stroke Association: Peter Langhorne, Olivia Wu, Anne Ashburn, Helen Rodgers and Julie Bernhardt), (HTA: Peter Langhorne, Anne Ashburn, Julie Bernhardt, Helen Rogers and Olivia Wu).
Northern Ireland: Sheila Lennon (Northern Ireland Chest Heart and Stroke: Sheila Lennon, Michael Power and Julie Bernhardt).
Singapore: Shahul Hameed (Singhealth: Shahul Hameed, Ratnagopal Pavanni, Peter Lim, Julie Bernhardt and Dawn Tan).
Statistics and data management
Leonid Churilov, Tim Brewer, Janice Collier, Nick Haritos, Edwin Leong, Cecilia Li, Caesar NayWin, Marcus Nicol, Liudmyla Olenka and Li Chun Quang.
Health economics
Marjory Moodie, Robert Carter, Silvia Hope, Lauren Sheppard, Kiusiang Tay-Teo and Olivia Wu.
Cognition
Toby Cumming and Thomas Linden.
Trial Coordinating Centres
The Florey Institute of Neuroscience and Mental Health, Melbourne, VA, Australia. Karen Borschmann, Jan Chamberlain, Janice Collier, Toby Cumming, Fiona Ellery, Teresa Occhiodoro, Helen Palfreeman, Tara Purvis, Bernadette Sirgo, Nick Tiliacos, John Van Holsteyn and Henry Zhao.
University of Glasgow, Glasgow, UK. Beverly Armstrong, Louise Craig, Fiona Graham, Lynn Legg, Rosemary Morrison, Heather Moorhead, Lorraine O’Donohue, Susan Rogers and Myra Smith.
University of Central Lancashire, Preston, UK. Denise Forshaw and Jane Fitzgerald.
List of AVERT collaborators, countries and recruitment at each participating hospital
Figures in parentheses are the number of patients recruited by the centre. MIs are listed first for each site. Some investigators worked across multiple sites, but they are listed for one site only.
Australia
Austin Hospital (253): E Hibbert, R Melling, S Petrolo, T Purvis, H Williamson (MIs), P Adams, L Augoustakis, S Batcheler, S Berney, V Cobani, B Cohen, H Dewey, S Gangi, N Giofre, C Gordon, L Hegarty, M Hindson, F Horvath, S Kalinowski, A Kleine, S Kramer, J Lawrence, S Lindquist, N Logan, A Macdonell, J Matlioski, N McDonough, S McLennan, M McNamee, L Miller, C Nall, E Nelson, K Ng, Z Nicholas, C Nunn, K Owen, E Plant, L Proud, D Quah, K Rodway, S Sertori, V Sheldon, L Sherry, S Speare, K Stansfeld, N Studden, Z Teoh, L Twist, G Velupillai, L Walker, K Wall, A Warwick, R Wharrie, J Wilson, H Worboys and D Young. Royal Perth Hospital (149): J Ancliffe (MI), M Bryant, B Doran, M Field, P Fogliani, A Haber, G Hankey, D Hendrie, V Jackaman, A Jacobsen, S Jose, R Lim, R Louis, S Nanthakumar, S Pain, A Power, B Rappeport, J Reynolds, L Smith, S Tombe, A Wesseldine and T West. The Royal Melbourne Hospital (95): K Clarke, H Maccanti, L Marr, S Plumb, J Quiney, L Werner (MIs), E Abeykoon, W Apirutvorrachod, L Attard, S Behanan, D Brown, K Buchanan, D Butler, M Camac, S Davis, D Diocera, N Gan, C Gendre, J Germaine, P Hand, L Maurenbrecher, J McCulloch, S Mcritchie, M Ong, R Pachett, L Pesavento, H Power, R Reilly, M Sawers, G Silva, C Stevens, L Taylor, T Timms, M Ugalde, A Vardy, J Wallace, S Walsh, E Whatley and E Winter. Frankston Hospital (90): M Baxter, M Davis, L Sundararajan (MIs), E Butler, K Caspers, E Coulter, S Shaw, F Kent, H Lack, F Leavold, J Lord, J Martin-Francisco, R Mohanraj, R Nelson, T O’Neill, R Otto, J Parker, V Rees and B Stevens. Westmead Hospital (84): R Chen (MI), RI Lindley, J Bindra, R Dongre, N Downey, M Ferris, L Gibson, R Gonzalez, M Kinniburgh, M Lazaridou, D McCormack, R Singh, A Stepney and Y Tria. Geelong Hospital (74): K Bainbridge, B Killey, R Sheedy (MIs), O Aitchison, L Bray, K Clatworthy, S Coghill, M Collins, L Cornwall, J Dow, P Gates, S Gillett, N Johnson, S Joseph, K Kopelke, R Lam, R Levy, N Lloyd, S Logan, G McPherson, M Newth, C Parsons, K Powles, M Rebis, T Samakowidic, L Sanders, S Savickas, J Shrimpton, H Smith, L Smith, J Spehr, J Summers, G Taylor, M Thackeray and B Wilkinson. The Alfred (42) K Richardson (MI), J Frayne, E Barber, L Bode, A Brakey, K Chand, P Christin, G Crook, D Delrosario-Kelly, R Descallar, A Deutsch, S Easo, M Farquhar, P Fergus, J Ford, E Hamson, M Hlaing, E Hope, J Lacivita, J Laurenson, K Lock, N Ly, K McKay, C Mill, K Moloney, L Price, Tristan Terry, A Tyers, S Willems and R Woolstencroft. Flinders Medical Centre (40): N Crawshaw, J Luker, C Wood, S Choat (MIs), C Archer, D Benham, M Billinger, M Bronca, S Curchin, C Dickie, M Dixon, D Douglass, M Enomoto, K Ernst, L Fries, S George, E Green, L Hamilton, Z Harris, T Heard, G Hunt, N Jamieson, M Mackenzie, H McKearney, B Oermann, C O’Reilly, T Pearson, N Reid, L Rodda, D Scutcheon, C Simons, R Smith, L Tait, J Troake and D Usher. Western Hospital (37): L Mackey, T Wijeratne (MIs), C Abela, S Ashoka, C Chen, T Cheng, V Chong, S Cooke, A Fok, L Galang, C Grant, S Karageorge, K Kat, L Keo, B Lee, A Luscombe, J Mackay, M Minett, J Mizen, P Nim, N Nunlist, V Patel, M Pathirage, A Paton, M Pombuena, N Rathnayake, L Rhodes, M Sequeira, S Smart, S Somaratne, N Sta Maria, L Talbot and R Tecle. Epworth Richmond (23): M Shannon, R Gerraty (MIs), S Allen, R Boyle, N Fatchen, N Hendley, A Hyde, M Inal, P Kalubowilage, M Laverde, K Lawless, A McFadyen, K Peters, C Pugh, C Qin, J Robertson, S Smee, R Tomlinson, V Wang, F Williams, D Woolley and R Yawieriin. St George Hospital (23): N Austin, S Pomfret, M Tinsley (MIs), L Allport, C Ang, L Armitage, E Blundell, A Courtney, M Dela Costa, T Devi Thapa, P Diwakar, M Dulleh, J Francis, P Cic, G Gellie, C Gill, D James, S Lee, T Mai, K Majcher, C Mawson, G Newton, N Qiu, E Ragonton, L Roberts, H Saitamis, L Stanwell, L Ting, P Xu and L Yin. Albury Hospital (23): V Crosby (MI), K Broadhead, J Church, R Collins, K Everitt, M Fisher, K Hochmuth, N Jones, A Lieschke, E McCarthy, C McGlone, D Morey, D Neilson, S Spry and M Vile. Nambour General Hospital (20): R Grimley, D Rowley (MIs), I Rosbergen, E Ahern, L Anderson, J Boreham, R Devin, R Doolan, M Dyke, L Griffiths, K Guest, D Hecita, N Kendal, J Koltermann, M Lacy, S Lebeter, D Lloyd, M Matthews, C McAuley, A Pollock, M Pyke, T Rogers, S Street, G Styles, A Tampiyappa, J Trinder, T Verral, K Walker and C White. Sir Charles Gairdner Hospital (15): T Beckwith, L Cormack (MIs), J Arriagada, C Babenschneider, D Blacker, S Bennett, S Connor, J Cowmeadow, N Daniel, G Edmonds, M Faulkner, M Garcia-Vega, K Kruger, B Martial, P McGinley, H Mountford, V Riley, N Smith, F Stepan, S Tilley and S Whisson. Warrnambool Base Hospital (15): P Groot (MI), J Bailey, K Ballinger, C Bell, B Camilleri, C Charnley, D Crabbe, S Crossland, N Edirimanna, C Fitzgerald, C Gibbins, J Gibbs, K Hirst, A Kennedy, E Klose, K McDowall, S Miller, R Morgan, A Noonan, M North, M Oliver, K Richards, T Russell, N Scott, A Shlanski and A Traynor. West Gippsland Hospital (12): S Smith (MI), R Adams, C Banks, K Burke, S Hewat, B McKenna, M McKimmie, L Polmear, M Traumanis and S Whiteman. St Vincent’s Hospital (12): V Bramah, R Errey, M Halpin, V Molan, D Wheelwright, N Wilson, W Zhang (MIs), M Bakshi, S Bracher, M Bryant, W Byrnes, T Denton, N DeVries, P Fay, P Galbraith, T Gallaher, O Haidar, K Holgate, K Hozack, N Jackson, S Kipps, S Lerner, R Marcus, R Merheb, C Naismith, G Nolan, R Odelli, K Page, P Sangvatanakul, T Simpson, P van Vliet, K Walch, S Walker and T Yasue. Wyong Public Hospital (11): G Auld (MI), R Baker, K Cousins, M Fairbrother, K Hutchinson, M Maclean, E Maher, D Mills, S Ohlback, J Sturm, M Tooth and J Watkins. The Wesley Hospital (9): J Cramb (MI), P Atkinson, J Conrad, D Fichera, S Follent, C Gilbert, M Herzig, S Kohler, S McCracken, L Nunan, S Roberts, J Shelley, S Varendorff and A Wills. Calvary Mater Newcastle Hospital (8): A Moore, A Robertson (MIs), J Britton, A Burgess, T Coates, J Croft, E Greening, J Holland, P O’Brien and R Strong. Wodonga Hospital (8): L Tighe (MI), S Bilston, J Black, K De Rivera, I Dwyer, S Gissane, K Heckenberg, S Jackson, A Maclagan, L O’Hare, H Patel, J Pearce, C Scanlan, K Seymour, M Symington, A Tyers, A Waite and K Wiesner. Belmont Hospital (5): O Katalinic, M Spear (MIs), P Brown, E Difuntorum, S Gilbert, J Henderson, D James, H Janssen, E Lane, S Lowndes, D Smith, S Thompson, D Weaver, S Weston and S Wright. Wollongong Hospital (4): S Cox, C Tse (MIs), J Adrian, M Doughty, J Kok, R McGrath, T Morris, A Pickup, E Ray, R Richardson, M Sims, C Thompson, K Trinh, N Walton and F Whittaker. Gosford Hospital (2): P Andersen (MI), J Burrows, M Dawson, D Griffiths, G Harris, P Kavalieros, B O’Brien, K Roberts, J Watkins and C Whyatt.
New Zealand
Auckland City Hospital (189): A McRae, G Wavish (MIs), F Anos, J Armstrong, E Au, A Barber, C Bates, M Bertulfo, A Boggs, F Burgess, K Cassels-Brown, M Chiu, S Dass, N Duff, J Farrell, W Foster, D Fuertez, C Gadhvi, S George, A Green, L Harvey-Fitzgerald, L Hau, L Hayward, D Holman, K Huggins, M Jacobs, A John, H Kaur, T Lagerstedt, J Lee, R Llenes, L Lyons, S Magandi, M Martin, S Mathew, T Mathew, D McKellar, E Moss, KL Nand, K Nicol, F Peterson, A Prasad, K Quick, E Revell, S Roy, J Ryan, N Samadi, B Scrivener, J Slow, S Tharakan, J Torrens, E van Bysterveldt, C Villaluz and S Yang.
Malaysia
UKM Medical Centre (123): Katijjahbe MA (MI), Ai Sing G, Azlina A, Azmi MI, Efri MH, Fadilah AZ, Fathuddeen H, Haryani H, H Hussien, Izuani M, ZC Man, Man Ying C, Mashitoh KM, Noor Azah A, Norlinah MI, Norliza I, Ravinder KB, Rohaizah R, Rosnita K, Rozita A, Safwan J, R Sahathevan, Shahrul I, Sharifah SM, Tan HJ, Wan Nafisah WY, YL Yee, Zaharah MA and Zunaidah AS.
Singapore
Singapore General Hospital (128): D Tan, MT Ahmad, S Hameed (MIs), MFB Bakari, J Britto, JJ Chen, S Choo, M Faizal, FK Fong, S Hong, J Ja’afar, Z Ke, G Koh, CK Lee, YF Lee, P Lim, GM Lim, SH Ninhadi, G Ong, T Pei Pei, V Penero, N Rahim, P Ratnagopal, K Saleh, HC Seow, E Sim, CK Tan, PY Tay, I Teo, S Thilarajah, PHJ Wong, WP Wong and S Yeap.
UK
Forth Valley Royal Hospital (65): M Macleod (MI), A Anderson, K Armstrong, K Baird, D Balfour, M Boyd, J Cameron, C Carswell, C Clanachan, L Cuthill, I Devoy, S Forsyth, J Gavin, M Hughes, E Marr, S McAuley, E McCagherty, K McCallum, N McDonald, C McGhee, TA McIntyre, L Noonan, A Smart and R Walshe. Yeovil District Hospital (61): D Neal (MI), J Allison, G Ball, S Board, H Brunt, C Buckley, C Carroll, D Hayward, T Hutchinson, E Jones, E Keeling, E Marsh, N Mead, H Smith, C Vickers, B Williams-Yesson and D Wood. York Hospital (54): J Coyle, M Keeling (MIs), L Ackroyd, C Brown, K Donnan, N Dyer, H Green, G Kilbride, C Nicholson and M Porteous. Royal Victoria Infirmary (35): S Louw (MI), A Annamalai, A Barkat, S Crawford, M Fawcett, D Harvey, V Hogg, A Hughes, J Kemp, J Morrison, K Storey and T Thompson. Aberdeen Royal Infirmary (33): J Furnace, MJ Macleod (MIs), J Bell, K Bennett, M Bruce, R Clarke, H Cowie, H Gow, J Irvine, A Joyson, S MacDonald, A Macvicar, N Murphy and J Robertson. Royal Bournemouth Hospital (32): C Gordon, J Kwan, L Redpath, K Saunders (MIs), J Bell, R Burrow, C Clarke, C Dickson, G Hann, M Heath, S Heath, A Hewett, R Humphrey, B Longland, A Orpen, C Ovington, J Page, E Rogers and K Toombs. Imperial College Healthcare, St Mary’s Hospital (29): R Howes, A Lacey, P Meakin (MIs), D Ames, S Banerjee, E Beranova, S Berry, MJ Burke, V Cassama, K Collins, J Crow, A Dunne, C Gomez, A Hawkins, K Hellier, SA Howard, A Kar, E Lambert, H Lee, C Mandri, J Moye, E Murtagh, J Pushpa-Rajah, J Richardson, T Sachs, J Stilwell, V Tilley, P Wilding and N Wilson. Wishaw General Hospital (28): E Feely, S Kirk (MIs), P Cassidy, A Chalmers, C Duguid, N Hughes, J Hutton, K Lapsley, J Lee, A Murray, L Weir and M Whitelaw. Monklands Hospital (26): M Barber, D Esson (MIs), H Armit, C Devlin, R Duncan, C Forman, K Frame, L Hogg, J Lee, P McLeod, R McWhinney, J Porter, M Purves and L Snowball. Ulster Hospital (25): B Wroath (MI), L Fearson, M Gibson, S Gillespie, N Ignatius, T Kane, J Kwant, M Matthews, C McCallion, C McConville, M McDowell, C McNally, L Moore, P Murphy, A Nesbitt, J Newell, M Power, E Reid and K Robinson. Royal Devon and Exeter Hospital (23): C Charnley, M James (MIs), S Bacon, N Booth, A Bowring, L Boxall, J Burt, J Cageao, N Green, K Gupwell, S Keenan, H Kingwell, M Kryszkowska, J Mortimore, B Peace and C Roughan. Queen Elizabeth The Queen Mother Hospital (21): G Gunathilagan, J Sampson, G Thomas (MIs), T Allen, G Dane, K Harris, S Hart, SA Jones, M Reader, J Sampson and G Thomas. Antrim Area Hospital (18): P Browne, C McGoldrick, D Mullan (MIs), P Adair, J Armstrong, E Beggs, I Bell, C Edwards, L Gilligan, C Kelly, M Kennedy, J Kurian, L Leal, A McAtamney, E McKay, E Rogan, M Smyth, E Wiseman and J Vahidassr. Wansbeck General Hospital (18): C Price, V Riddell (MIs), E Bendix, K Craig, R Davison, A Harrison and A Smith. Blackpool Hospital (17): V Green (MI), K Ashton, W Barkhuizen, A Daniel, C Dickinson, H Durdu, D Eastwood, H Goddard, R Hodkin, J Howard, C Jeffs, S Joyce, C Kelly, G Kerr, J Lanes, B Magnall, M McMahon, M Moody, S Patton, R Taylor and A Watson. North Tyneside General Hospital (17): K Mitchelson, L Mokoena (MIs), L Aird, R Lakey, J Murdy, K Nelson and G Storey. Belfast City Hospital (15): R McGeown, S Tauro (MIs), R Brady, D Holland, M Kinnaird, L Maltman, D Martin, K McCord, S McKenna, C Morgan, C Shannon, A Steele and I Wiggam. Harrogate District Hospital (15): S Appleby, S Brotheridge, M Prescott (MIs), P Bagot, D Baston, C Bennett, J Featherstone, C Hare, A McCluskey, S Wade and R Worton. St Mary’s Hospital, Isle of Wight (13): E Hakim, J Herman, T Norman (MIs), L Beale, E Buckley, K Byrne, M Gasior, B Robles, C Smallwood, S Stevens, M Thomas and V Williams. Nevill Hall Hospital (12): K Buck (MI), S Armstrong, V Brice, A Edwards, S Gething, A Griffiths, T Hills, D Howells, S Langdon, S Moseley, G Powell, G Reynolds, B Richard, E Scott, R White and J Zebedee. Western Infirmary (10): M Walters (MI), J Alexander, L Brand, E Colquhoun, A Hill, D Macartney, H MacDonald, B Manak, H Morgan and C Ritchie. South Tyneside District Hospital (8): H Hunter (MI), T Blair, M Duffy, J Graham, J Scott, T Vu and P Yorston. Calderdale Royal Hospital (7): A Nair (MI), I Shakir, C Button, M Friend, J Greig, B Hairsine, S Wade and S Williamson. St George’s Hospital (7): G Cloud (MI), T Adedoyin, N Dayal, S Gawned, R Ghatala, N Jeyaraj, L Kerin, L Montague, C Orefo, J O’Reilly, J Styles, S Trippier, C Watchurst and F Watson. North Devon District Hospital (6): J Hunt, R Latif (MIs), C Barrett, J Cox, F Hammonds, K Quick, K Robinson, A Skinner and C Vernon. Royal Infirmary of Edinburgh (6): S Burgess, T Elder-Gracie (MIs), C Browne, W Cameron, V Coleman, C Fulgencio, L Gibson, P Halliday, D Heaney, L Main, K McGavin, G Mead, F Proudfoot, A Redpath, C Rodger and S Scott. Hexham General Hospital (5): K. Robinson. Crosshouse Hospital (3): K Mason (MI), L Baxter, A Bryce, M Halkett, J Halliday, A McAllister, M McGuiness, M Munro, A Robb, A Thompson, B Tougher, J Weadon and J Young. Daisy Hill Hospital (1): C Douglas (MI), M McParland, S Boyle, B Byrne, L Comiskey, J Gilpin, S Gilpin, A Harris, S Harshaw, J Haughey, F McArdle, L McConnell, E McEneaney, M Millar, M Murphy and J Tilley.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Langhorne P, Sandercock P, Prasad K. Evidence-based practice for stroke. Lancet Neurol 2009;8:308-9. https://doi.org/10.1016/S1474-4422(09)70060-2.
- Gilligan AK, Thrift AG, Sturm JW, Dewey HM, Macdonell RA, Donnan GA. Stroke units, tissue plasminogen activator, aspirin and neuroprotection: which stroke intervention could provide the greatest community benefit?. Cerebrovasc Dis 2005;20:239-44. https://doi.org/10.1159/000087705.
- Stroke Unit Trialists Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2007;4.
- Sandercock P, Counsell C, Gubitz G, Tseng MC. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD000029.pub2.
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929-35. https://doi.org/10.1016/S0140-6736(14)60584-5.
- Balami JS, Sutherland BA, Edmunds LD, Grunwald IQ, Neuhaus AA, Hadley G, et al. A systematic review and meta-analysis of randomized controlled trials of endovascular thrombectomy compared with best medical treatment for acute ischemic stroke. Int J Stroke 2015;10:1168-78. https://doi.org/10.1111/ijs.12618.
- Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 2007;6:215-22. https://doi.org/10.1016/S1474-4422(07)70036-4.
- Langhorne P, de Villiers L, Pandian JD. Applicability of stroke-unit care to low-income and middle-income countries. Lancet Neurol 2012;11:341-8. https://doi.org/10.1016/S1474-4422(12)70024-8.
- Indredavik B, Bakke F, Slordahl SA, Rokseth R, Hâheim LL. Treatment in a combined acute and rehabilitation stroke unit: which aspects are most important?. Stroke 1999;30:917-23. https://doi.org/10.1161/01.STR.30.5.917.
- Bernhardt J, English C, Johnson L, Cumming TB. Early mobilization after stroke: early adoption but limited evidence. Stroke 2015;46:1141-6. https://doi.org/10.1161/STROKEAHA.114.007434.
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377:1693-702. https://doi.org/10.1016/S0140-6736(11)60325-5.
- Allen C, Glasziou P, Del Mar C. Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet 1999;354:1229-33. https://doi.org/10.1016/S0140-6736(98)10063-6.
- Mutin-Carnino M, Carnino A, Roffino S, Chopard A. Effect of muscle unloading, reloading and exercise on inflammation during a head-down bed rest. Int J Sports Med 2014;35:28-34. https://doi.org/10.1055/s-0033-1343407.
- Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke: a multicenter study. Stroke 2000;31:1223-9. https://doi.org/10.1161/01.STR.31.6.1223.
- Bamford J, Dennis M, Sandercock P, Burn J, Warlow C. The frequency, causes and timing of death within 30 days of a first stroke: the Oxfordshire Community Stroke Project. J Neurol Neurosurg Psychiatry 1990;53:824-9. https://doi.org/10.1136/jnnp.53.10.824.
- Govan L, Langhorne P, Weir CJ. Stroke Unit Trialists Collaboration . Does the prevention of complications explain the survival benefit of organized inpatient (stroke unit) care? Further analysis of a systematic review. Stroke 2007;38:2536-40. https://doi.org/10.1161/STROKEAHA.106.478842.
- Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke 2004;35:1005-9. https://doi.org/10.1161/01.STR.0000120727.40792.40.
- West T, Bernhardt J. Physical activity in hospitalised stroke patients. Stroke Res Treat 2012;2012. https://doi.org/10.1155/2012/813765.
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 2009;10:861-72. https://doi.org/10.1038/nrn2735.
- Johansson BB. Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke 2000;31:223-30. https://doi.org/10.1161/01.STR.31.1.223.
- Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models?. Neurorehabil Neural Repair 2012;26:923-31. https://doi.org/10.1177/1545968312440745.
- Pekna M, Pekny M, Nilsson M. Modulation of neural plasticity as a basis for stroke rehabilitation. Stroke 2012;43:2819-28. https://doi.org/10.1161/STROKEAHA.112.654228.
- Skarin M, Bernhardt J, Sjöholm A, Nilsson M, Linden T. ‘Better wear out sheets than shoes’: a survey of 202 stroke professionals’ early mobilisation practices and concerns. Int J Stroke 2011;6:10-5. https://doi.org/10.1111/j.1747-4949.2010.00534.x.
- Olavarría VV, Arima H, Anderson CS, Brunser AM, Muñoz-Venturelli P, Heritier S, et al. Head position and cerebral blood flow velocity in acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2014;37:401-8. https://doi.org/10.1159/000362533.
- Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke 2008;39:390-6. https://doi.org/10.1161/STROKEAHA.107.492363.
- Cumming TB, Thrift AG, Collier JM, Churilov L, Dewey HM, Donnan GA, et al. Very early mobilisation after stroke fast tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke 2011;42:153-8. https://doi.org/10.1161/STROKEAHA.110.594598.
- Langhorne P, Stott DJ, Knight A, Barer D, Bernhardt J, Watkins C. Very Early Rehabilitation or Intensive Telemetry after Stroke (VERITAS): a pilot randomised controlled trial. Cerebrovasc Dis 2010;29:352-60. https://doi.org/10.1159/000278931.
- Craig LE, Bernhardt J, Langhorne P, Wu O. Early mobilization after stroke: an example of an individual patient data meta-analysis of a complex intervention. Stroke 2010;41:2632-6. https://doi.org/10.1161/STROKEAHA.110.588244.
- Tay-Teo K, Moodie M, Bernhardt J, Thrift AG, Collier J, Donnan G, et al. Economic evaluation alongside a phase II, multi-centre, randomised controlled trial of very early rehabilitation after stroke (AVERT). Cerebrovasc Dis 2008;26:475-81. https://doi.org/10.1159/000155984.
- Bernhardt J, Churilov L, Dewey H, Lindley R, Moodie M, Collier J, et al. for the AVERT Collaborators . Statistical Analysis Plan (SAP) for A Very Early Rehabilitation Trial (AVERT): An international trial to determine the efficacy and safety of commencing out of bed standing and walking training (very early mobilisation) within 24 h of stroke onset vs usual stroke unit care. Int J Stroke 2015;10:23-4. https://doi.org/10.1111/ijs.12423.
- The AVERT Trial Collaboration Group . Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015;386:46-55. https://doi.org/10.1016/S0140-6736(15)60690-0.
- Bernhardt J. on behalf of the AVERT Investigators . Could upright posture be harmful in the early stages of stroke? – Author’s Reply. Lancet 2015;386:1734-5. https://doi.org/10.1016/S0140-6736(15)00692-3.
- Bernhardt J, Churilov L, Ellery F, Collier J, Chamberlain J, Langhorne P, et al. on behalf of the AVERT Collaboration Group . Pre-specified dose response analysis for A Very Early Rehabilitation Trial (AVERT). Neurology 2016;86:2138-45. https://doi.org/10.1212/WNL.0000000000002459.
- Sheppard L, Dewey H, Bernhardt J, Collier JM, Ellery F, Churilov L, et al. on behalf of the AVERT Trial Collaboration Group . Economic Evaluation Plan (EEP) for A Very Early Rehabilitation Trial (AVERT): An international trial to compare the costs and cost-effectiveness of commencing out of bed standing and walking training (very early mobilization) within 24 hours of stroke onset with usual stroke unit care. Int J Stroke 2016;11:492-4. https://doi.org/10.1177/1747493016632254.
- Bernhardt J, Lindley RI, Lalor E, Ellery F, Chamberlain J, Van Holsteyn J, et al. on behalf of the AVERT Collaboration Group . AVERT2 (a very early rehabilitation trial, a very effective reproductive trigger): retrospective observational analysis of the number of babies born to trial staff. BMJ 2015;351. https://doi.org/10.1136/bmj.h6432.
- van Wijk R, Cumming T, Churilov L, Donnan G, Bernhardt J. An early mobilization protocol successfully delivers more and earlier therapy to acute stroke patients: further results from phase II of AVERT. Neurorehabil Neural Repair 2012;26:20-6. https://doi.org/10.1177/1545968311407779.
- Luker JA, Craig L, Bennett L, Ellery F, Langhorne P, Wu O, et al. Implementing a complex rehabilitation intervention in a stroke trial: a qualitative process evaluation of AVERT. BMC Med Res Methodol 2016;16. https://doi.org/10.1186/s12874-016-0156-9.
- Bernhardt J, Raffelt A, Churilov L, Lindley RI, Speare S, J. A, et al. on behalf of the AVERT Trialists’ Collaboration . Exploring threats to generalisability in a large international rehabilitation trial (AVERT). BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008378.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. https://doi.org/10.1161/01.STR.19.5.604.
- Brott T, Adams H, Olinger C, Marler J, Barsan W, Biller J, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989;20:864-70. https://doi.org/10.1161/01.STR.20.7.864.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. https://doi.org/10.1016/0140-6736(91)93206-O.
- Briggs DE, Felberg RA, Malkoff MD, Bratina P, Grotta JC. Should mild or moderate stroke patients be admitted to an intensive care unit?. Stroke 2001;32:871-6. https://doi.org/10.1161/01.STR.32.4.871.
- Simondson J, Goldie P, Brock K, Nosworthy J. The Mobility Scale for Acute Stroke patients: intra-rater and inter-rater reliability. Clin Rehab 1996;10:295-300. https://doi.org/10.1177/026921559601000406.
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 1970;2:92-8.
- Snaith RP, Constantopoulos AA, Jardine MY, McGuffin P. A clinical scale for the self-assessment of irritability. Br J Psychiatry 1978;132:164-71. https://doi.org/10.1192/bjp.132.2.164.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5.
- Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res 1999;8:209-24. https://doi.org/10.1023/A:1008815005736.
- Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. Int Disabil Stud 1990;12:6-9. https://doi.org/10.3109/03790799009166594.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. https://doi.org/10.1136/bmj.d40.
- Howard G, Waller JL, Voeks JH, Howard VJ, Jauch EC, Lees KR, et al. A simple, assumption-free, and clinically interpretable approach for analysis of modified Rankin outcomes. Stroke 2012;43:664-9. https://doi.org/10.1161/STROKEAHA.111.632935.
- Churilov L, Arnup S, Johns H, Leung T, Roberts S, Campbell BC, et al. An improved method for simple, assumption-free ordinal analysis of the modified Rankin Scale using generalized odds ratios. Int J Stroke 2014;9:999-1005. https://doi.org/10.1111/ijs.12364.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- James G, Witten D, Hastie T, Tibshirani R, James G, Witten D, et al. An Introduction to Statistical Learning with Applications in R. New York, NY: Springer Science and Business Media; 2013.
- Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disabil Rehabil 2006;28:823-30. https://doi.org/10.1080/09638280500534861.
- Bernhardt J, Thuy MN, Collier JM, Legg LA. Very early versus delayed mobilisation after stroke. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD006187.pub2.
- Brady MC, Stott DJ, Norrie J, Chalmers C, St George B, Sweeney PM, et al. Developing and evaluating the implementation of a complex intervention: using mixed methods to inform the design of a randomised controlled trial of an oral healthcare intervention after stroke. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-168.
- Neergaard MA, Olesen F, Andersen RS, Sondergaard J. Qualitative description – the poor cousin of health research?. BMC Med Res Methodol 2009;9. https://doi.org/10.1186/1471-2288-9-52.
- Nayer S, Stanley M. Qualitative Research Methodologies for Occupational Science and Therapy. London: Routledge; 2015.
- Chippala P, Sharma R. Effect of very early mobilisation on functional status in patients with acute stroke: a single-blind randomized controlled trial. Clin Rehabil 2015:1-7.
- Poletto SR, Rebello LC, Valenca MJM, Rossato D, Almeida AG, Brondani R, et al. Early mobilization in ischemic stroke: a pilot randomized trial of safety and feasibility in a public hospital in Brazil. Cerebrovasc Dis Extra 2015;5:31-40. https://doi.org/10.1159/000381417.
- Herisson F, Godard S, Volteau C, Le Blanc E, Guillon B, Gaudron M. SEVEL study group . Early sitting in ischemic stroke patients (SEVEL): a randomized controlled trial. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0149466.
- Chippala P, Sharma R. Effect of Very early mobilisation on disability and adverse events in the first 3 months post stroke: a single-blind, randomisd controlled trial. Int J Health Sci Res 2015;5:166-74.
- Bernhardt J, Indredavik B, Langhorne P. When should rehabilitation begin after stroke?. Int J Stroke 2013;8:5-7. https://doi.org/10.1111/ijs.12020.
- Sundseth A, Thommessen B, Rønning OM. Outcome after mobilization within 24 hours of acute stroke: a randomized controlled trial. Stroke 2012;43:2389-94. https://doi.org/10.1161/STROKEAHA.111.646687.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:979-83.
- Austin MW, Ploughman M, Glynn L, Corbett D. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neurosci Res 2014;8:8-15. https://doi.org/10.1016/j.neures.2014.06.007.
- Egan KJ, Janssen H, Sena ES, Longley L, Speare S, Howells DW, et al. Exercise reduces infarct volume and facilitates neurobehavioral recovery: results from a systematic review and meta-analysis of exercise in experimental models of focal ischemia. Neurorehabil Neural Repair 2014;28:800-12. https://doi.org/10.1177/1545968314521694.
- Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res 1998;783:286-92. https://doi.org/10.1016/S0006-8993(97)01356-5.
- Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, et al. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-centre RCT. Neurology 2009;73:195-201. https://doi.org/10.1212/WNL.0b013e3181ab2b27.
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e1-72. https://doi.org/10.1161/STR.0000000000000098.
- Herbert D, Lindsay MP, McIntyre A, Kirton A, Rumney PG, Bragg S, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke 2016;11:459-84. https://doi.org/10.1177/1747493016643553.
- Intercollegiate Stroke Working Party/Fifth Edition 2016/Royal College of Physicians . Stroke Guidelines n.d. www.rcpLondon.ac.uk/guidelines-policy/stroke-guidelines (accessed 28 August 2017).
- Stroke Foundation . 2017 Draft Guidelines 2017. https://strokefoundation.org.au/What-we-do/Treatment-programs/Clinical-guidelines (accessed 28 August 2017).
Appendix 1 Project outputs
Publications
The main aspects of this project have already been published.
Bernhardt J, Lindley RI, Lalor E, Ellery F, Chamberlain J, Van Holsteyn J, et al. on behalf of the AVERT Collaboration Group. AVERT2 (a very early rehabilitation trial, a very effective reproductive trigger): retrospective observational analysis of the number of babies born to trial staff. BMJ 2015;351:h6432.
The AVERT Trial Collaboration Group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015;386:46–55.
Bernhardt J, Raffelt A, Churilov L, Lindley RI, Speare S, Ancliffe J, et al. on behalf of the AVERT Trialists’ Collaboration. Exploring threats to generalisability in a large international rehabilitation trial (AVERT). BMJ Open 2015;5:e008378.
Bernhardt J, Churilov L, Ellery F, Collier J, Chamberlain J, Langhorne P, et al. on behalf of the AVERT Collaboration Group. Pre-specified dose response analysis for A Very Early Rehabilitation Trial (AVERT). Neurology 2016;86(Suppl. 23):2138–45.
Bernhardt J, Churilov L, Dewey H, Lindley R, Moodie M, Colier J, et al. for the AVERT Collaborators. Statistical analysis plan (SAP) for A Very Early Rehabilitation Trial (AVERT): an international trial to determine the efficacy and safety of commencing out of bedstanding and walking training (very early mobilisation) within 24 h of stroke onset vs. usual stroke unit care. Int J Stroke 2015;10:23–4.
Luker JA, Craig L, Bennett L, Ellery F, Langhorne P, Wu O, Bernhardt J. Implementing a complex rehabilitation intervention in a stroke trial: a qualitative process evaluation of AVERT. BMC Med Res Methodol 2016;16:52.
Bernhardt J, Dewey H, Collier J, Thrift A, Lindley R, Moodie M, Donnan G. A Very Early Rehabilitation Trial (AVERT). Int J Stroke 2006;1(Suppl. 3):169–71.
Bernhardt J, Churilov L, Dewey H, Lindley R, Moodie M, Collier J, et al. for the AVERT Collaborators. Statistical Analysis Plan (SAP) for A Very Early Rehabilitation Trial (AVERT): an international trial to determine the efficacy and safety of commencing out of bed standing and walking training (very early mobilisation) within 24 h of stroke onset vs usual stroke unit care. Int J Stroke 2015;10:23–4.
The AVERT Trial Collaboration group, Bernhardt J, Langhorne P, Lindley RI, Thrift AG, Ellery F, et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015;386:46–55.
Bernhardt J, AVERT investigators. Could upright posture be harmful in the early stages of stroke? - Author’s reply. Lancet 2015;386:1734–5.
Bernhardt J, Lindley RI, Lalor E, Ellery F, Chamberlain J, Van Holsteyn J, et al. on behalf of the AVERT Collaboration Group. AVERT2 (a very early rehabilitation trial, a very effective reproductive trigger): retrospective observational analysis of the number of babies born to trial staff. BMJ 2015;351:h6432.
Bernhardt J, Churilov L, Ellery F, Collier J, Chamberlain J, Langhorne P, et al. on behalf of the AVERT Collaboration Group. Pre-specified dose response analysis for A Very Early Rehabilitation Trial (AVERT). Neurology 2016;86(Suppl. 23):2138–45.
Sheppard L, Dewey H, Bernhardt J, Collier JM, Ellery F, Churilov L, et al. on behalf of the AVERT Trial Collaboration Group. Economic Evaluation Plan (EEP) for A Very Early Rehabilitation Trial (AVERT): an international trial to compare the costs and cost-effectiveness of commencing out of bed standing and walking training (very early mobilization) within 24 hours of stroke onset with usual stroke unit care. Int J Stroke 2016;11(Suppl. 4):492–4.
Conference presentations
There have been a large number of conference presentations (> 30) by various members of the AVERT team. These included European Stroke Organisation Conference 2015 and 2016, International Stroke Conference 2015, 2016, Stroke Society of Australia 2014–16, and the UK Stroke Forum 2014–15.
Presentations to investigators, professional associations and UK stroke network
A large number of local and regional presentations were carried out and have been reported in the investigators newsletters. This has included presentations at the UK Stroke Forum (2011, 2012, 2013, 2014), local meetings (March 2013, April 2013, European Stroke congress 2013). In addition, we hosted two large UK contributor meetings in Glasgow in October 2013 and April 2015.
Consumers
We have disseminated the 3-month results for distribution from each hospital to the patients recruited. After the 12-month results are published, patients will be allowed to find out what group they were in (from the local hospital) and we will publish a final patient report. Alongside this, we plan to disseminate results to consumers via consumer organisations [e.g. the Stroke Association (UK) and the National Stroke Foundation (Australia)].
Media/social media
We have promoted and publicised the trial on social media [Twitter (www.twitter.com; Twitter, Inc., San Francisco, CA, USA), Facebook (www.facebook.com; Facebook, Inc., Menlo Park, CA, USA) and blogs].
Clinical guidelines
Two stroke clinical practice guidelines (USA,71 Canada72) have recently changed. Updates of the UK73 and Australian74 guidelines are underway.
Clinical trials websites
The NIHR HTA and AVERT websites are up to date, with grant publications online. www.nets.nihr.ac.uk/projects/hta/120116 (accessed 28 August 2017).
www.gla.ac.uk/researchinstitutes/icams/staff/peterlonghorne/#/grants,researchinterests (accessed 28 August 2017).
www.florey.edu.au/very-early-rehabilitation-trial-avert (accessed 28 August 2017).
Appendix 2 Participating sites
| Hospital site | Numbers recruited | Principal investigator |
|---|---|---|
| Australia | 1043 | |
| Austin Hospital | 253 | H Williamson |
| Royal Perth Hospital | 149 | J Ancliffe |
| Royal Melbourne Hospital | 95 | L Werner |
| Frankston Hospital | 90 | L Sundararajan |
| Westmead Hospital | 84 | R Chen |
| Geelong Hospital | 74 | R Sheedy |
| Alfred Hospital | 42 | K Richardson |
| Flinders Medical Centre | 40 | S Choat |
| Western Hospital | 37 | T Wijeratne |
| Albury Hospital | 23 | V Crosby |
| Epworth Hospital, Richmond | 23 | S Gerraty |
| St George Hospital | 23 | M Tinsley |
| Nambour Hospital | 20 | D Rowley |
| Warrnambool Hospital | 15 | P Groot |
| Sir Charles Gairdner Hospital | 15 | L Cormack |
| St Vincent’s Hospital | 12 | W Zhang |
| West Glippsland Hospital | 12 | S Smith |
| Wyong Public Hospital | 11 | G Auld |
| The Wesley Hospital | 9 | J Cramb |
| Calvary Mater Newcastle | 8 | A Robertson |
| Wodonga Hospital | 8 | L Tighe |
| Belmont Hospital | 5 | M Spear |
| Wollongong Hospital | 4 | C Tse |
| Gosford Hospital | 2 | P Andersen |
| New Zealand | 189 | |
| Auckland Hospital | 189 | G Wavish |
| Singapore and Malaysia | 251 | |
| Singapore General Hospital | 128 | S Hameed |
| UKM Malaysia | 123 | MA Katijjahbe |
| UK | 610 | |
| Forth Valley Royal Hospital | 65 | M Macleod |
| Yeovil District Hospital | 61 | D Neal |
| York Hospital | 54 | M Keeling |
| Royal Victoria Infirmary | 35 | S Louw |
| Aberdeen Royal Infirmary | 33 | MJ Macleod |
| Royal Bournemouth | 32 | K Saunders |
| Imperial College Hospital (St Marys) | 29 | P Meakin |
| Wishaw General Hospital | 28 | S Kirk |
| Monklands Hospital | 26 | M Barbour |
| Ulster Hospital | 25 | B Wroath |
| Royal Devon & Exeter Hospital | 23 | C Charnley, et al. |
| Queen Elizabeth The Queen Mother Hospital | 21 | J Sampson, et al. |
| Antrim Area Hospital | 18 | D Mullan, et al. |
| Wansbeck General Hospital | 18 | C Price, et al. |
| Blackpool Hospital | 17 | V Green |
| North Tyneside General Hospital | 17 | L Mokoena, et al. |
| Belfast City Hospital | 15 | S Tauro, et al. |
| Harrogate District Hospital | 15 | S Brotheridge, et al. |
| St Mary’s Hospital Isle of Wight | 13 | T Norman, et al. |
| Nevill Hall Hospital | 12 | K Buck |
| Western Infirmary | 10 | M Walters |
| South Tyneside District Hospital | 8 | H Hunter |
| Calderdale Royal Hospital | 7 | A Nair |
| London St George Hospital | 7 | G Cloud |
| North Devon District Hospital | 6 | R Latif, et al. |
| Royal Infirmary Edinburgh | 6 | T Elder-Gracie, et al. |
| Hexham General Hospital | 5 | K Robinson |
| Crosshouse Hospital | 3 | K Mason |
| Daisy Hill | 1 | C Douglas |
Appendix 3 Protocol version 3
Glossary
- AVERT intervention protocol
- A protocol for use by clinical staff to guide the delivery of the very early mobilisation and usual care interventions.
- AVERT Online
- A password-protected, trial-specific web-based management system.
- Contamination
- When the witnessing of a different intervention makes others change their usual care practice (consciously or unconsciously).
- Counting mobilisations
- If a patient performs a mobilisation (e.g. walks to toilet with help or is sat out of bed) and then rests for 5 minutes, then their next mobilising activity (e.g. walking back from the toilet or getting back into bed) constitutes another mobilisation.
- Dose
- A session of mobilisation given to AVERT patients.
- Early mobilisation
- Starting out of bed, sitting, standing and walking early after stroke with no defined time from stroke onset.
- Excessive fatigue
- When the patient reports a score of > 13 on the Borg Perceived Exertion Scale and/or AVERT staff assess that the patient is excessively fatigued (e.g. the patient’s functional performance worsens significantly during the intervention).
- Mobilisation
- The patient is assisted and encouraged in functional tasks, including activities such as sitting over the edge of the bed, standing up, sitting out of bed and walking. Upper limb movement was intended to be integrated into functional activities as appropriate. Mobilisations were performed by the AVERT nurse and/or the AVERT physiotherapist. Support staff, such as therapy assistants and students, could also be trained to provide mobilisations.
- Nurse’s record of mobilisation sessions
- The time each session started and the type of each session was recorded on AVERT Online or, if the website was not available, data were temporarily recorded on the paper nurses recording form until such time it could be entered online.
- Physiotherapist’s record of mobilisation session
- The date and time each session started, the minutes and content of each session were recorded via AVERT Online. If the online forms were unavailable, paper therapist recording forms could be used to temporarily collect the information until such time it could be entered online.
- Time to first mobilisation
- The time from stroke onset to the time of the patient’s first mobilisation out of bed (assisted or independent). This did not include the initial assessment by the AVERT physiotherapist.
- Transient ischaemic attack
- Stroke-like symptoms that resolve completely within 24 hours.
- Very early
- The earliest possible time after a consented patient had suffered a stroke to their first mobilisation intervention (≤ 24 hours).
- Very early mobilisation
- The earliest possible time after a consented patient had a stroke to their first out-of-bed mobilisation.
List of abbreviations
- AE
- adverse event
- aHR
- adjusted hazard ratio
- aOR
- adjusted odds ratio
- AQoL
- assessment of quality of life
- AVERT
- A Very Early Rehabilitation Trial
- b.p.m.
- beats per minute
- CART
- classification and regression tree
- CI
- confidence interval
- CRF
- case report form
- EM
- early mobilisation
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IDA
- irritability, anxiety and depression assessment
- IME
- important medical event
- IQR
- interquartile range
- IRR
- incidence rate ratio
- MI
- main investigator
- MoCA
- Montreal Cognitive Assessment
- mRS
- modified Rankin scale
- MSAS
- Mobility Scale for Acute Stroke
- NIHR
- National Institute for Health Research
- NIHSS
- National Institutes of Health Stroke Scale
- OR
- odds ratio
- QoL
- quality of life
- RCT
- randomised controlled trial
- RMAS
- Rivermead Motor Assessment Scale
- ROC
- receiver operating characteristic
- rtPA
- recombinant tissue plasminogen activator
- SAE
- serious adverse event
- TIA
- transient ischaemic attack
- TTFM
- time to first mobilisation
- UC
- usual care
- VEM
- very early mobilisation
- VERITAS
- Very Early Rehabilitation on Intensive Telemetry After Stroke