Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/201/02. The contractual start date was in January 2015. The draft report began editorial review in February 2017 and was accepted for publication in May 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Nefyn H Williams declares membership of the Health Technology Assessment (HTA) Primary Care, Community and Preventative Interventions Panel. Dyfrig A Hughes reports other grants from National Institute for Health Research (NIHR) during the conduct of the study and membership of the HTA Clinical Trials Board from 2010 to 2016, the HTA Funding Teleconference from 2015 to 2016 and the Pharmaceuticals Panel from 2008 to 2012. Eifiona Wood reports other grants from NIHR during the conduct of the study. Nadine E Foster declares membership of the HTA Primary Care, Community and Preventative Interventions Panel. David A Walsh reports grants from Pfizer Ltd and personal fees from Novartis Pharmaceuticals UK Ltd outside the submitted work. Kika Konstantinou reports other grants from NIHR during the conduct of the study. Jaro Karppinen reports personal fees from lectures (Pfizer Ltd, Merck & Co., Inc. and ORION Pharma GmbH), personal fees from a scientific advisory board (Axsome Therapeutics Inc.) and personal fees from stocks (ORION Pharma GmbH) outside the submitted work. Stephane Genevay reports grants from AbbVie Inc., Merck & Co., Inc., Pfizer Ltd, University Hospitals of Geneva, the Rheumasearch Foundation, Fondation de bienfaisance Eugenio Litta and Centre de Recherches outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Williams et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Sciatica is a symptom defined as unilateral, well-localised leg pain, with a sharp, shooting or burning quality, which approximates to the dermatomal distribution of the sciatic nerve down the posterior lateral aspect of the leg, and normally radiates to the foot or ankle. It is often associated with numbness or paraesthesia in the same distribution. 1 Sciatica is an important clinical problem for the NHS. Although prevalence rates vary widely between studies, in a trial that used a clinical assessment to establish the presence of sciatica, the point prevalence in the general population aged 30–64 years was 4.8%. 2 Some cohort studies have found that most cases resolve spontaneously, with 30% of patients having persistent troublesome symptoms at 1 year, with 20% of the total out of work. 3,4 However, another cohort found that 55% still had symptoms of sciatica 2 years later, and 53% after 4 years (which included 25% who had recovered after 2 years but had relapsed again by 4 years). 5 Current care pathways in the NHS typically involve the prescribing of analgesia by the patient’s general practitioner (GP) and, if troublesome symptoms persist, referral for physiotherapy either in community-based physiotherapy services, musculoskeletal interface services or secondary care spinal clinics. If pain persists, patients are referred for more invasive treatment, such as epidural corticosteroid injection and eventually disc surgery. 6 However, the evidence for most of these non-surgical treatments is poor;7 new treatment strategies are needed. At present between 5% and 15% of patients with sciatica undergo disc surgery. 3,4 In the NHS in England in 2013/14, 8330 lumbar discectomies were performed. 8 Based on a Dutch trial that indicated that the cost of sciatica to society represents 13% of all back pain-related costs, the annual impact on the UK economy is £268M in direct medical costs and £1.9B in indirect costs (inflated from 1998 figures). 9
Sciatica caused by lumbar nerve root pain usually arises from a prolapsed intervertebral disc,3 not only from compression of the nerve root,10 but also the release of proinflammatory factors from the damaged disc. 11,12 Internal disc rupture that does not result in prolapse can also induce disabling radicular pain,13 and the degree of disc displacement, nerve root enhancement and neural compression on magnetic resonance imaging (MRI) does not correlate with sciatic symptoms. 14 Corticosteroids have been used in an attempt to reduce the inflammation of the affected nerve root. Intramuscular corticosteroid injections have been tried, but two randomised controlled trials (RCTs) comparing them with placebo have found no evidence of efficacy. 15 Injection of corticosteroid into the epidural space should increase the amount of steroid reaching the affected nerve root, and it is a commonly used intervention in the NHS. However, systematic reviews of epidural steroid injections have reached conflicting views with regard to their efficacy compared with placebo, and their effectiveness compared with other treatments. 7,15–17 They also require to be administered by a specialist, usually as a hospital day case procedure, which increases their cost of administration. Other, less invasive, treatments to reduce inflammation in the affected nerve root are needed. The most important proinflammatory factor released from the prolapsed intervertebral disc is tumour necrosis factor alpha (TNF-α). 11,12 The monoclonal antibodies infliximab and adalimumab (Humira®; AbbVie Ltd, Maidenhead, UK) target TNF-α and are increasingly used to control inflammatory disease such as psoriasis, Crohn’s disease and rheumatoid arthritis. These so-called ‘biological agents’ bind specifically to TNF-α receptors on the cell surface and modulate biological responses that are induced or regulated by TNF-α, including the inflammatory process. 18 They may also have beneficial effects on the inflamed nerve root in sciatica,19 and have the additional advantage of being administered by intravenous (infliximab) or subcutaneous (adalimumab) injection in a hospital outpatient clinic, rather than by epidural injection as a hospital day case.
This research followed the recommendations of a Health Technology Assessment (HTA)-funded systematic review of management strategies for sciatica. 20 In this review the clinical effectiveness of different treatment strategies for sciatica were compared simultaneously using network meta-analysis. Network meta-analysis allows treatment strategies to be ranked in terms of clinical effectiveness with an estimate of the probability that each strategy is best, and provides estimates for all possible pairwise comparisons, based on both direct and indirect evidence. In terms of overall recovery or global effect, biological agents had the highest probability (0.5) of being best, with an odds ratio (OR) compared with inactive control of 16, but with very wide 95% credible intervals (CrIs) of 0.6 to 1002, reflecting the small number of included studies and lack of data that were available to inform these effect estimates. A CrI is a Bayesian confidence interval (CI). There were large but non-statistically significant effect estimates in favour of biological agents compared with the other treatment strategies, including traction (OR 13, 95% CrI 0.4 to 943), exercise therapy (OR 15, 95% CrI 0.4 to 1085) and passive physical therapies (OR 14, 95% CrI 0.5 to 975). In terms of pain intensity, biological agents had the second highest probability of being best (0.2), and were found to be statistically significantly better than the inactive control, but with wide CrIs, with a weighted mean difference (WMD) of –22 (95% CrI –36 to –8) compared with an opioid WMD of –31 (95% CrI –53 to –9) and a non-opioid analgesia WMD of –18 (95% CrI –33 to –2).
Following this HTA review we updated the literature search of biological agents for sciatica (from inception to February 2012). 21 We identified seven RCTs, one non-RCT and one historical cohort trial. We combined the results of six RCTs22–27 and one non-RCT28 comparing biological agents with placebo in meta-analyses. We found that biological agents resulted in better global effects in the short term (around 6 weeks’ follow-up) (OR 2.0, 95% CI 0.7 to 6.0), medium term (around 6 months’ follow-up) (OR 2.7, 95% CI 1.0 to 7.1) and long term (≥ 12 months’ follow-up) (OR 2.3, 95% CI 0.5 to 9.7); improved leg pain intensity in the short term (WMD –13.6, 95% CI –26.8 to –0.4) and medium term (WMD –7.0, 95% CI –15.4 to 1.5), but not the long term (WMD 0.2, 95% CI –20.3 to 20.8); and improved Oswestry Disability Index (ODI) score in the short term (WMD –5.2, 95% CI –14.1 to 3.7), medium term (WMD –8.2, 95% CI –14.4 to –2.0) and long term (WMD –5.0, 95% CI –11.8 to 1.8). It should be noted that there was heterogeneity in the leg pain intensity and ODI results, and improvements were no longer statistically significant when studies were restricted to RCTs. There was a reduction in the need for disc surgery, which was not statistically significant, limited evidence for improved employment outcomes and no difference in the number of adverse effects. There was limited evidence that a biological agent was superior to intravenous corticosteroids (one historical cohort trial),29 but not compared with epidural corticosteroid (two RCTs). 27,30 We concluded that there was some evidence of efficacy, but a paucity of evidence for clinical effectiveness for biological agents. Although there was insufficient evidence to change practice, there was sufficient evidence to suggest that a definitive RCT was warranted.
As part of the HTA review of management strategies for sciatica,20 a decision-analytic model was developed to estimate the relative cost-effectiveness of these different strategies. Three different treatment pathways were compared. The first pathway was primary care treatments alone (including the categories usual care, activity restriction, advice, non-opioid and opioid analgesia). The second pathway was stepped care starting with primary care treatments and, for those who did not improve, intermediate care treatment (exercise therapy, passive physical therapy, traction, manipulation, acupuncture and biological agents), epidural steroid injections then finally disc surgery. The third pathway was immediate referral to disc surgery following failed primary care management. The stepped care pathway was the most effective, with the most successful treatment strategy being non-opioid analgesia in primary care, followed by biological agents in intermediate care, followed by epidural corticosteroid injection and disc surgery. The place for biological agents in the therapeutic pathway is as a therapeutic option to be used by intermediate care services in patients for whom primary care treatment has failed, with the potential to reduce the need for more invasive treatments.
In summary, biological agents have the potential to reduce inflammation and nerve root pain in patients when primary care management has not relieved symptoms, but might they benefit the NHS? Apart from the economic model developed for the HTA review of management strategies for sciatica,31 there have been no economic evaluations of these agents. Although they might be beneficial for patients with sciatica, these agents are expensive costing £352 for 40 mg of adalimumab and £420 for 100 mg of infliximab. 32 Adalimumab is administered by subcutaneous injection, but infliximab confers the additional expense of intravenous injection. We intended to use adalimumab because of its ease of administration and, in order to provide a therapeutic effect lasting 1 month, two subcutaneous injections would be given 2 weeks apart. In order to initiate a rapid response, we used the typical starting dosage when treating psoriasis or Crohn’s disease of 80 mg followed by 40 mg. 18 Despite their cost, they may be cost-effective if shown to be sufficiently clinically effective and/or they reduce the need for more expensive treatments such as disc surgery, the average unit cost of which is between £3676 and £4971. 32 When the patent for adalimumab expires, it may result in the development of cheaper biosimilar drugs that can be used in its place. From searches of databases of current trials (inception to November 2013), we have not identified any large RCTs with a concurrent economic evaluation in a NHS setting.
Trial objectives
-
To evaluate the clinical effectiveness of subcutaneous injections of adalimumab plus physiotherapy compared with a placebo injection of 0.9% sodium chloride plus physiotherapy for patients with sciatica who have failed first-line primary care treatment. Potential participants were planned to be identified during primary care consultation, after referral to musculoskeletal service or following a practice database search.
The primary effectiveness outcome was sciatica-related health status using the ODI. 33 Secondary effectiveness outcomes included pain intensity, location, duration and anticipated trajectory; the risk of poor outcome; psychological measures, including fear-avoidance beliefs, self-efficacy, anxiety and depression; employment status; and adverse effects.
-
To evaluate the cost-effectiveness of subcutaneous injections of adalimumab plus physiotherapy compared with a placebo injection of 0.9% sodium chloride plus physiotherapy for patients with sciatica who have failed first-line primary care treatment from a health service and personal social care perspective. The primary economic outcome was the incremental cost per quality-adjusted life-year (QALY) gained. QALYs would be estimated by administering the EuroQol-5 Dimensions, 5-level version (EQ-5D-5L)34 at each follow-up visit.
Trial flow
The flow chart to show the different stages of the trial is presented in Figure 1. The flow chart to show the experience of the participant through the trial is in Figure 2.
FIGURE 1.
Consolidated Standards of Reporting Trials flow chart. MRI, magnetic resonance imaging; TB, tuberculosis.
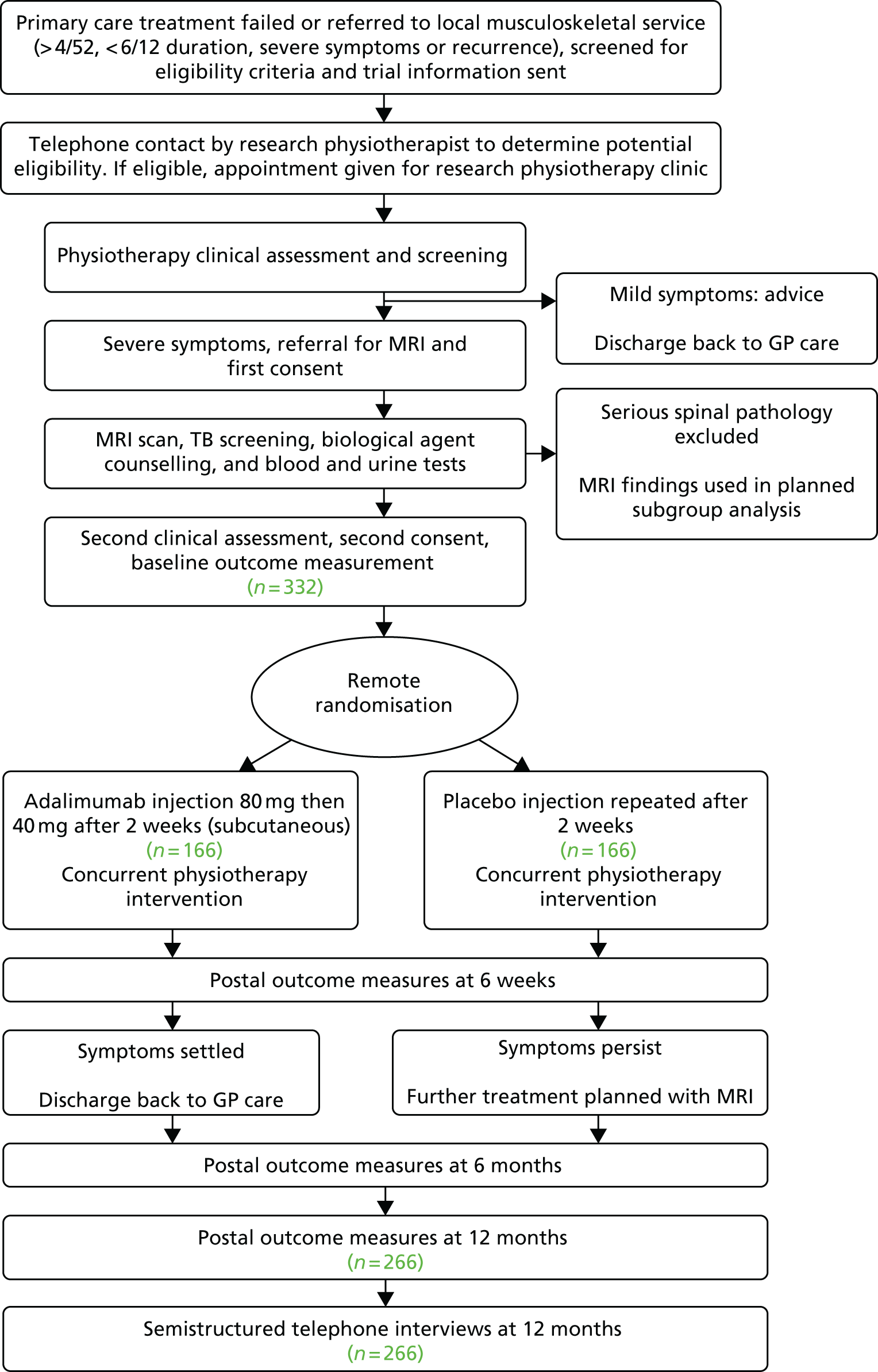
FIGURE 2.
Participant flow chart. CXR, chest radiography; MRI, magnetic resonance imaging; TB, tuberculosis.

Chapter 2 Methods
Design
Pragmatic, multicentre RCT with blinded participants, clinicians, outcome assessment and statistical analysis with concurrent economic evaluation and internal pilot.
Main centres
The RCT was planned to recruit from sites overseen by five collaborating centres in North Wales, London, Keele, Nottingham and Cardiff.
Selection and withdrawal of participants
Each collaborating centre oversaw one or more treatment sites that were delegated responsibility for delivering the interventions. Each treatment site had a number of patient identification centres, which consisted of general medical practices and local musculoskeletal services. The target population was adults with suspected sciatica for whom primary care treatment had failed. This was defined as troublesome symptoms (e.g. back and leg pain, pins and needles, numbness in leg, weakness), persisting for > 4 weeks and < 6 months. As the recruitment process took at least 4 weeks before participants were randomised, we did not have a lower time limit for duration of symptoms when identifying the target population and had an upper time limit of 20 weeks. These patients were identified in three ways:
-
by their GP
-
following a search of the general practice patient record database
-
after referral to local musculoskeletal services.
General practitioner referral
Patients identified during the primary care consultation with suspected sciatica were provided with information about the trial and invited, if interested, to return the reply slip to the research team in the pre-paid envelope. In North Wales and Keele, the primary care database displayed ‘pop-up’ screen messages to remind GPs about the study when potential patients were consulted.
Following a search of the general practice patient record database
Potential participants were identified by regular searches of the general practice patient record database by the practice management staff, directed by research officers from either the Health and Care Research Wales workforce or the local Clinical Research Network in England. The database was searched for diagnostic codes for sciatica. Participants were excluded if they had a known serious spinal pathology or a contraindication to adalimumab injection, such as serious infection [e.g. active or latent tuberculosis (TB)], transplanted organ, demyelinating disorders, malignancy, cardiac failure, low white blood cell count or pregnancy. Those identified as potentially eligible were invited to participate by a written invitation from their GP on the practice’s headed notepaper, and hand signed by a GP. Those who were interested returned the reply slip to the research team in the pre-paid envelope.
Local musculoskeletal services
Potential participants with suspected sciatica were also identified from referrals to local musculoskeletal services. Those identified were invited to participate by a written invitation from the local service on headed notepaper. Those who were interested returned the reply slip to the research team in the pre-paid envelope.
The centres in North Wales, Keele and Cardiff planned to identify and recruit participants in three ways: by their GP, following a search of the general practice patient record database or after referral to local musculoskeletal services. Nottingham and London planned to recruit and identify participants who had been referred to their local musculoskeletal services.
Telephone contact by the research physiotherapist
All those who had contacted the research team to state that they were interested in participating were sent a participant information sheet and were contacted by telephone by the research physiotherapist. The telephone call determined if they had unilateral leg pain and, if back pain was present, that leg pain intensity was worse than, or as bad as, the back pain. It also determined whether or not symptoms had persisted for > 20 weeks (to allow participants to be within the 6-month limit at randomisation). Finally, the telephone call allowed them to discuss any questions that they may have had about the study or their symptoms.
Research clinic
Those who satisfied the eligibility criteria were given an appointment slot in a research clinic run by research physiotherapists. At this research clinic all potential participants were assessed by the research physiotherapist for eligibility. Eligible participants who gave initial consent were registered and provided with a unique participant identification number. The following data were recorded on case report forms:
-
demographic details such as age, sex, height and weight
-
clinical findings such as pain location, pain duration, other presenting complaints, straight-leg raise test (left and right), femoral stretch test, muscle power, pinprick and light-touch sensation, quadriceps and Achilles tendon reflexes.
The research physiotherapist arranged for the participant to have the following blood tests taken by the phlebotomist to exclude haematological and biochemical abnormalities: full blood count, urea and electrolytes, estimated glomerular filtration rate, liver function test and glycosylated haemoglobin. The participant received TB screening in accordance with local practice and biological agents counselling. The research physiotherapist then arranged an appointment for MRI to exclude serious spinal pathology. All of these tests were completed within 2–3 weeks of the initial clinic visit and were recorded on the case report forms. The presence or absence of a disc prolapse on MRI was not to be used as an inclusion criterion, because the degree of disc displacement, nerve root enhancement or neural compression found on MRI does not correlate with sciatic symptoms. 14 MRI was reported by the local radiologist using a trial-specific standard operating procedure. The initial report stated whether or not the participant had serious spinal pathology that required a different treatment. A full MRI report was available only after completion of the study. Individual results were made available if a report was needed in an emergency, or if a spinal surgery referral was contemplated.
The research physiotherapist ensured that the participant received all the required tests. If there was any issue that required action, then the participant’s referring GP or musculoskeletal clinician was informed. When MRI had excluded serious spinal pathology, participants were contacted by the research physiotherapists either by telephone or post to attend the research clinic, where they received a second clinical assessment by the research physiotherapist, 2–3 weeks after their initial visit, to assess if they were still eligible. If they were still eligible, further consent was obtained for trial entry and randomisation when they were provided with a unique participant identification number. Table 1 shows the schedule of forms and procedures.
| Time point | Study period | Follow-up period | |||||
|---|---|---|---|---|---|---|---|
| Enrolment | Randomisation (within 2–3 weeks of registration) | Treatment visit 1 (within 3 days of randomisation) | Treatment visit 2 | 6 weeks | 6 months | 12 months | |
| Eligibility | ✗ | ✗ | |||||
| Informed consent | ✗ | ✗ | |||||
| Registration to trial | ✗ | ||||||
| FBC | ✗ | ||||||
| Urine pregnancy test | ✗ | ||||||
| U&E | ✗ | ||||||
| TB screening | ✗ | ||||||
| MRI | ✗ | ||||||
| MRI reportinga | ✗a | ✗a | |||||
| Eligibility confirmed | ✗ | ✗ | |||||
| Randomisation | ✗ | ||||||
| Subcutaneous injection of allocated treatment | ✗ | ✗ | |||||
| Physiotherapy treatment | ✗ | ✗ | |||||
| ODI | ✗ | ✗ | ✗ | ✗ | |||
| EQ-5D-5L | ✗ | ✗ | ✗ | ✗ | |||
| RMDQ | ✗ | ✗ | ✗ | ✗ | |||
| SBI | ✗ | ✗ | ✗ | ✗ | |||
| STarT Back Screening Tool | ✗ | ||||||
| PSEQ | ✗ | ✗ | ✗ | ✗ | |||
| HADS | ✗ | ✗ | ✗ | ✗ | |||
| TSK | ✗ | ✗ | ✗ | ✗ | |||
| RUQ | ✗ | ✗ | ✗ | ✗ | |||
| Pain outcome | ✗ | ✗ | ✗ | ✗ | |||
| Manikin pain diagram | ✗ | ✗ | ✗ | ✗ | |||
| Pain duration | ✗ | ||||||
| Pain trajectory | ✗ | ||||||
| Days of work | ✗ | ✗ | ✗ | ✗ | |||
| Global assessment of change | ✗ | ✗ | ✗ | ||||
| Adverse events | ✗ | ✗ | ✗ | ✗ | ✗ | ||
Inclusion criteria
-
Aged ≥ 18 years.
-
Clinical features of sciatica.
-
Leg pain worse or as bad as back pain, elicited by asking the participant.
-
Unilateral leg pain approximating a dermatomal distribution (contralateral buttock pain permitted if it did not succeed the inferior gluteal margin), obtained by asking the participant.
-
One of the following:
-
positive neural tension test, such as straight-leg raise test restricted to < 50° by leg pain; positive femoral stretch test; muscle weakness or loss of tendon reflex affecting one myotome
-
loss of sensation in a dermatomal distribution.
-
-
Persistent symptoms for at least 4 weeks and < 6 months despite first-line treatment in primary care, obtained by asking the participant.
-
Moderate to high severity (score of ≥ 30 points on the ODI). 33
Female partners of sexually active male participants had to use adequate contraception for at least 5 months after the last injection. Female participants were required to have had a negative urine pregnancy test within 2 weeks prior to randomisation, unless they were post menopause or had been sterilised. Sexually active male partners of female participants were also required to use adequate contraceptive methods. The researcher ensured that the risks, and consequences, of not using adequate contraception were fully understood by the participants and provided information and pathways as deemed necessary.
Exclusion criteria
-
Symptoms persisting for > 6 months (elicited by asking the participant).
-
A previous episode of sciatica in the last 6 months.
-
Unable to undergo MRI (e.g. magnetic metal implants, potential metallic intraocular foreign bodies, claustrophobia, extreme obesity), obtained from the medical records and by asking the participant.
-
Serious spinal pathology (including cauda equina syndrome, malignancy, recent fracture, infection or very large disc prolapse), which might require an urgent spinal surgery opinion, identified from participants’ previous medical history in their medical records or from MRI.
-
Incidental serious pathology identified by MRI (e.g. adrenal tumour).
-
Neurological deficit involving muscle weakness requiring an urgent spinal surgery assessment (e.g. foot drop).
-
Widespread pain throughout the body including the upper limb. 35 Pain was considered widespread when all of the following were present: pain in the left side of the body, pain in the right side of the body, pain above the waist and pain below the waist. Axial skeletal pain (cervical spine or anterior chest or thoracic spine or low back) had also to be present.
-
Prior use of biological agents targeting TNF-α within the previous 6 months obtained from the medical records and by asking the participant.
-
Previous lumbar spinal surgery, elicited from the medical records and by asking the participant.
-
Contraindications to adalimumab injection including serious infection such as active or latent TB, transplanted organ, demyelinating disorders, malignancy, cardiac failure, low white blood cell count, pregnancy (determined from the medical records, the results of investigations and by asking the participant).
-
Pregnant or breastfeeding (women must not breastfeed for at least 5 months after the last adalimumab injection).
-
Unable to communicate in English or Welsh.
-
Unable or unwilling to give informed consent.
Informed consent
At the initial physiotherapy clinic, the research physiotherapist determined preliminary eligibility, explained the nature of the trial and gave a repeat participant information sheet. The participant information sheet had been approved by the ethics committee and set out all of the key information, including the practicalities of the trial, the possible benefits and risks, and trial assessments. Participants were registered onto the trial and details were recorded on a database and a screening log at each of the trial treatment sites, and were assigned a unique participant identification number. Anonymised details labelled with the unique participant identification number were transferred to a separate database in the trials unit, which was used for recording all of the trial data. This ensured that the outcome measurement and statistical analysis would be performed blind to treatment allocation. All databases were password protected. The participant consent forms were stored in a locked filing cabinet in each treatment site. Clinical findings were recorded on case report forms.
Eligible participants who gave initial consent had blood tests to exclude haematological and biochemical abnormalities (full blood count, urea and electrolytes, estimated glomerular filtration rate, liver function test, glycated haemoglobin levels). They received TB screening, including plain chest radiography, biological agents counselling and MRI to exclude serious spinal pathology within 2–3 weeks of their initial visit. When MRI had excluded serious spinal pathology, and TB screening, a pregnancy test (in the case of eligible women) and biological agent counselling had been completed, participants attended a further appointment with the research physiotherapist. A second clinical assessment was performed and those who were still eligible were asked to complete a second informed consent form, approved by the ethics committee. In order to enter the RCT, the participant was randomised by the research physiotherapist using a remote web-based system. The treatment site sent a letter to each participant’s GP, informing them of their patient’s participation in the trial and requesting that the GP make a note of this in the patient’s record. In addition, GPs were asked to inform the trial team if they became aware that the participant had experienced an adverse event during the trial.
Three copies of the consent form were signed by the participant. The original was kept by the research team, one copy was kept by the participant and the third was filed in the participant’s hospital medical records. All participant information sheets, letters of invitation and consent forms were provided in Welsh and English in the two Welsh centres.
Magnetic resonance imaging
Participants who had given initial informed consent underwent MRI to exclude serious spinal pathology, but the presence or absence of a disc prolapse was not used as an inclusion criterion. The MRI scans were read and reported by a local radiologist, using a trial-specific standard operating procedure at each treatment site, who was independent of the trial team. Only results that showed serious pathology or suspected serious pathology were revealed to the research team, referring GP and the musculoskeletal clinician, who would then exclude the participant and refer for urgent assessment. Otherwise, the research team were informed that no serious spinal pathology was identified. The findings of MRI would be made available to the participant’s treating clinician only after completion of the study. Individual results were made available if a report was needed in an emergency, or if a spinal surgery or epidural injection referral was being contemplated, and were distributed to the clinical team, referring GP and the musculoskeletal clinician. The MRI findings were to be used in a planned a priori subgroup analysis. For clinical purposes, radiologists from each site provided a clinical report of the MRI. For the purpose of reporting standardised findings for research, two independent radiologists reported the MRI findings for all trial participants. The radiologists interpreted the report according to the MRI findings only.
Tuberculosis screening and biological agent counselling
The screening and counselling protocols used routinely by the rheumatology departments in each of the treatment sites were used and administered by an experienced rheumatology specialist nurse. All of the participating centres had access to either a specialist TB clinic or an infectious disease service, where any identified cases were referred and managed.
Second physiotherapy assessment
Participants attended a second appointment with the research physiotherapist 2–3 weeks after the initial appointment, after the MRI results had been reported and following TB screening and biological agent counselling. A second clinical assessment was performed and all the results of the tests performed were checked. If the participant remained eligible, a second consent form was completed. Participants completed a baseline questionnaire and were randomised using a remote web-based system. If they no longer fulfilled the criteria for trial entry, because their symptoms had improved at or below the 30-point threshold on the ODI, they were given advice about managing their remaining symptoms and discharged back to the care of their GP. Clinical findings were recorded on case report forms.
Registration
Once the first consent had been obtained, participants’ details were recorded on a database in the trial centre and each participant was assigned a unique participant identification number. Anonymised details labelled with the unique participant identification number were transferred to a separate database in the trials unit, which was used for recording all of the trial data. All databases were password protected. The participant consent forms were stored in a locked filing cabinet in each treatment site. Participants’ GPs were informed in writing about their participation in the trial.
Randomisation
After completion of the second consent form and once baseline outcome measures had been collected, participants were individually randomised. Randomisation to the Subcutaneous Injection of Adalimumab Trial compared with Control (SCIATiC) was achieved by secure web access to the remote randomisation system at the trials unit. This system was maintained and monitored independently of the trial statistician and any trial staff who needed to remain blind to the treatment allocation. In order to protect against subversion, while ensuring that the trial maintained good balance to the allocation ratio of 1 : 1 both within each stratification variable and across the trial, the randomisation was performed using a dynamic adaptive randomisation algorithm. 36 Participants were stratified by (1) treatment centre and (2) presence of neurological signs (motor weakness or sensory loss). The research physiotherapist who obtained informed consent requested the randomisation code from the web-based randomisation system, the result of which was e-mailed to the pharmacy and the rheumatology nurse, but not the research physiotherapist. The dispensing pharmacist logged and dispensed the appropriate injection in line with Medicines and Healthcare products Regulatory Agency (MHRA) guidelines. The injection was given on the day of randomisation; if this was not possible, a further appointment was arranged by the research physiotherapist so that the treatment could be given within 3 days from randomisation.
Withdrawal of participants
Withdrawal from the trial did not affect participants’ medical care, something that was emphasised in the participant information sheet. Failure to complete any one follow-up assessment did not constitute formal withdrawal from the trial, and, unless participants requested complete withdrawal of their data, data were used to impute values for the analysis. The imputation of missing values ensured that the data set was utilised to its full power. The full imputation details were prespecified as part of the statistical analysis plan.
Expected duration of trial
We planned to recruit participants over a 20-month period and to follow them up for 12 months.
Subcutaneous injections
All participants were randomised to receive two doses of subcutaneous injection, 2 weeks apart, into the posterior thigh. The intervention group received 80 mg of adalimumab followed by 40 mg,18 in order to achieve a therapeutic dose of adalimumab for a period of 4 weeks. The control group received an equivalent volume of 0.9% sodium chloride.
Injection process
The injections were prescribed by a consultant rheumatologist and administered by a rheumatology nurse experienced in the administration of these injections. The first injection was given on the same day as randomisation; if this was not possible, a further appointment was arranged by the research physiotherapist so that the treatment could be given within 3 days of randomisation. It was not possible to make the adalimumab and placebo syringes indistinguishable in appearance, nor was it possible to blind the pharmacy or the rheumatology nurse who administered the injections. Blinding of participants and the other clinicians was maintained using the following strategies. The rheumatologist wrote a prescription for ‘SCIATiC trial injection’ and was kept blind to treatment allocation. The research physiotherapist who obtained informed consent requested the randomisation code from a web-based randomisation system. The randomisation code was not sent to this physiotherapist, but was e-mailed to the pharmacy and the rheumatology nurse. The rheumatology nurse collected the injection from the pharmacy, which was transported in an undistinguishable box containing the adalimumab inside its original packaging or the 0.9% sodium chloride-containing ampoules. Communication between the participant and rheumatology nurse concerning the injection was kept to a minimum, and the rheumatology nurse administered the injections into the participant’s posterior thigh. The research physiotherapist was not present and did not communicate with the rheumatology nurse about the injection. In addition, in order to provide reassurance that other clinicians were not present, a log was kept of all people present in the room when each injection was administered. All research staff received full training on the blinding procedures. In order to assess whether or not blinding had been maintained, the participants were asked to complete a five-point Likert scale that asked if the participant considered the treatment to be:
-
definitely in the 0.9% sodium chloride injection group
-
more likely to be in the 0.9% sodium chloride injection group
-
equally likely to be in the 0.9% sodium chloride injection group or the adalimumab injection group
-
more likely to be in the adalimumab injection group
-
definitely in the adalimumab injection group.
Concurrent physiotherapy
Physiotherapy is usually considered normal practice for those participants who fail to improve with GP care alone. In this trial we aimed to investigate the clinical effectiveness of adalimumab in addition to physiotherapy. Current evidence on physiotherapy interventions for participants with sciatica indicates that specific exercise approaches (directional preference-based exercises or ‘McKenzie’ exercises based on certain spinal movements with or without manual therapy techniques) seem to relieve pain. 37 There was also evidence that exercise-based physiotherapy treatment added to GP care was beneficial. 38 Regimes including strengthening exercises of the lumbar and pelvic muscles also show some promise in terms of improvements in this group of participants. In this trial, both groups received a concurrent course of physiotherapy intervention that could be described as ‘best conservative care’. It was delivered in local physiotherapy departments by ‘treating’ physiotherapists and not by the ‘research’ physiotherapists who were carrying out the assessments of eligibility and randomisation. The physiotherapy intervention consisted of a package of directional preference (McKenzie), strengthening exercises or other exercises,37,38 and manipulative techniques that had been determined by consensus using a panel of extended scope physiotherapists. Treatments were intended to take into account and address participants’ individual needs, including clinical monitoring; appropriate advice and reassurance; assessment of psychosocial obstacles to recovery, such as excessive worrying or unhelpful beliefs about physical activity; and encouragement of appropriate, gradual return to full function, including work when applicable. The first session was expected to last approximately 45 minutes, with subsequent sessions lasting 30 minutes each. The therapy sessions were to be provided over a period of 12 weeks. The number of sessions provided would be determined by participant and therapist preference, and also response to treatment. We captured and described these aspects of physiotherapy treatment as part of the trial. The physiotherapy treatment started at the same time as the injection intervention in both arms of the trial. Participants were discouraged from receiving any other NHS-based co-intervention until this physiotherapy treatment had finished.
Clinical management of persistent symptoms
The protocol was designed such that, once the participants had completed their course of physiotherapy, and symptoms had settled or were improving, then no further intervention would be organised. They were to be discharged to the care of their GP and followed up by the research team. If troublesome symptoms persisted, then further treatment could be planned, as appropriate, by referral to musculoskeletal interface clinics or secondary care specialists according to local arrangement in each of the centres. The plans for further treatment were at the discretion of the treating clinicians and could include epidural corticosteroid injections or referral for disc surgery. The full result of MRI was to be made available if a spinal surgery referral was contemplated. All additional treatments were recorded in detail in a case report form.
Internal pilot trial
The pilot built on previous research in this participant group undertaken by team members,20,21,30,39 which had already provided information on trial administration, the characteristics of sciatica participants and the effects of biological treatments from previous studies. The internal pilot was designed to rehearse the procedures and logistics to be undertaken in the main trial. It was designed to assess the feasibility of the arrangements for delivering the interventions, recruitment rate and initial retention rate. The internal pilot would be based on the first 50 participants recruited into the trial. We planned to start recruitment in two centres (North Wales and London) and then to roll out recruitment in the other three centres over the following 3 months. We expected the recruitment rate to build up over the first 3 months to the target rate of four participants per collaborating centre per month. We anticipated that this would take 7 months. The indicative stopping criteria at the end of this internal pilot were recruitment, which failed to reach 80% of the planned recruitment rate target, dropouts up until the 6-week postal questionnaire assessment exceeding 20% or more than one centre failing to commence recruiting. Any procedural changes identified in the pilot would be implemented across all trial sites subject to ethics approval of the appropriate major amendment. Data from participants in the internal pilot would be automatically rolled into the main trial data unless the Trial Management Group (TMG) believed that data were incompatible with the remaining data. No interim analysis at the primary end point (1 year) was proposed and, therefore, this internal pilot would not affect the overall power of the trial. Wittes and Brittain’s40 method would be used for sample size recalculation if required.
Primary outcome
The primary clinical outcome was back pain-specific disability measured using the ODI33 at 12 months. The primary economic outcome was the incremental cost per QALY gained, estimated by administering the EQ-5D-5L34 at each follow-up visit.
Outcome measures
Condition-specific outcomes
-
Back pain-specific disability using the ODI. 33
The ODI is an outcome assessment tool that is used to measure a participant’s impairment and quality of life (i.e. how badly the pain has affected their life). The participant questionnaire contains items concerning intensity of pain, lifting, ability to care for oneself, ability to walk, ability to sit, sexual function, ability to stand, social life, sleep quality and ability to travel. Each topic category is followed by six statements describing different potential scenarios in the participant’s life relating to the topic. The participant then checks the statement that most closely resembles their situation. Each question is scored on a scale of 0–5, with the first statement being zero and indicating the least amount of disability and the last statement scoring 5 and indicating the most severe disability. The index is converted to a percentage score from 0 to 100. Zero is equated with no disability and 100 with maximum disability. It was used at the first clinical assessment to assess eligibility and also at the second clinical assessment to confirm eligibility. If recruited onto the trial, this score was used as the baseline measurement. It would also be measured at follow-up after 6 weeks, and at 6 and 12 months.
-
Leg pain-related functional disability using the leg pain version of the Roland–Morris Disability Questionnaire (RMDQ). 41,42
The RMDQ is a measure of disability in which greater levels of disability are reflected by higher numbers on a 24-point scale. The RMDQ is a self-administered outcome measure. Participants are asked to read the list of 24 sentences and place a tick against appropriate questions based on how they feel each sentence describes them on that day. If the sentence does not describe their symptoms that day, participants are asked to leave the space next to the sentence blank. The RMDQ is scored by adding up the number of items checked by the participant. The score can therefore vary from 0 to 24 points. If a participant indicates in any way that an item is not applicable to them, the item is scored ‘no’ (i.e. the denominator remains 24). It was measured at baseline and at 6 weeks’ and 6 and 12 months’ follow-up.
-
Leg pain interference using the Sciatica Bothersomeness Index. 43
This is an index based on participants reporting symptoms that reflected the trouble the participant is going through with his/her sciatica symptoms. The index included self-reported ratings of symptom intensity of leg pain; numbness or tingling in the leg, foot or groin; weakness in the leg/foot; or back or leg pain while sitting. Each symptom item is rated on a scale from 0 to 6, with 0 being not bothersome, 3 somewhat bothersome and 6 extremely bothersome. It was measured at baseline and at 6 weeks’ and 6 and 12 months’ follow-up.
-
Pain location using a pain manikin. 44
This is a picture of a human figure (manikin) on which pain is indicated by the participant and can be used to measure musculoskeletal pain. It was used at baseline, 6 weeks’, and 6 and 12 months’ follow-up.
Generic outcomes
-
Health utility using the EQ-5D-5L. 34
This is a participant-completed index of health-related quality of life, which gives a weight to different health states. It consists of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each has five levels of severity (no problems/some/moderate problems/extreme problems and unable to). It was used at baseline and at 6 weeks’ and 6 and 12 months’ follow-up. It allowed the calculation of QALYs, using the area under the curve method, which would be used as part of the economic analysis.
-
Global assessment of change since baseline.
The global assessment of change is a measure of changes in levels of pain over a set time period. It was measured at 6 weeks’ and at 6 and 12 months’ follow-up.
Psychological outcomes
-
Anxiety and depression using the Hospital Anxiety and Depression Scale. 45
This is a participant-completed outcome measure of anxiety and depression. It is designed to measure anxiety and depression in participants with physical health problems. It has seven items related to common symptoms of anxiety and seven items for depression. Participants are asked whether they experience the symptom definitely, sometimes, not much or not at all. The Hospital Anxiety and Depression Scale was designed for use in the hospital setting but has been used successfully with the general population. It was used at baseline and at 6 weeks’ and 6 and 12 months’ follow-up.
Use of health-care and social-care services
Employment
-
Questions on employment status, work absence, sick certification and self-certification.
These were used at baseline and at 6 weeks’ and 6 and 12 months’ follow-up.
Process measures (potential predictors and mediators of outcome)
Risk of persistent disabling pain was assessed using the following tools:
-
STarT Back Screening Tool. 48
This screening tool assesses patients’ risk of persistent disabling pain. Patients’ risk subgroup (low, medium or high risk) has been shown by team members to be predictive of outcomes, including patients with back pain and with suspected sciatica. This was measured at baseline only.
-
Pain trajectory based on a single question. 49
This question is used to classify low back pain duration and asks ‘How long is it since you had a whole month without any back pain?’. There are seven discrete response categories: < 3 months, 3–6 months, 7–12 months, 1–2 years, 3–5 years, 6–10 years and > 10 years. This shows that recalled duration of pain is a predictor of outcome in patients with low back pain, independent of baseline severity and psychological status.
-
Pain Self-Efficacy Questionnaire (PSEQ). 50
The PSEQ is a 10-item questionnaire, developed to assess the confidence people with ongoing pain have in performing activities while in pain. The PSEQ is applicable to all persisting pain presentation. It covers a range of functions, including household chores, socialising and work, as well as coping with pain without medication. It was used at baseline and at 6 weeks’ and 6 and 12 months’ follow-up.
-
Fear of movement using the Tampa Scale of Kinesiophobia. 51
The Tampa Scale of Kinesiophobia is a 17-item checklist that is used to measure the fear of movement (re)injury related to chronic back pain. The scale is based on the model of fear avoidance, fear of work-related activities and fear of movement/reinjury. It was used at baseline and at 6 weeks’ and 6 and 12 months’ follow-up.
Follow-up
The questionnaires followed best practice in their design, to maximise response rate. The baseline questionnaire was administered by the research physiotherapists and completed by the participant. We planned to send follow-up postal questionnaires at 6 weeks, and at 6 and 12 months. Non-responders were to be sent an additional copy of the questionnaire. Persistent non-responders were to be contacted by telephone in order to collect a minimum data set. We planned to contact all participants by telephone 2 weeks after the 12-month questionnaire was sent. This would allow us to collect a minimum data set from non-responders, and to conduct a a brief semistructured interview with all participants asking about their overall experience of the trial and subsequent follow-up treatment. Blinding to treatment allocation would be maintained during these telephone interviews. Once again, in order to assess if blinding had been maintained, participants would be asked to complete a five-point Likert scale about which treatment group they believed that they were in.
Assessment of safety
As part of site initiation, training included an overview of possible side effects/potential adverse reactions associated with adalimumab.
Recording adverse events and adverse reactions
All trial staff and clinicians in contact with trial participants were responsible for noting adverse events reported by participants and making them known to appropriate medical staff. Trial participants were encouraged from the outset to contact the research team at the time that an event occurred. Participants were given a leaflet or card containing a contact address and telephone number. All adverse events, including non-serious adverse events, were recorded in the participant’s medical records and on their case report form. All adverse events were reported up to 1 month after the conclusion of the physiotherapy intervention. Adverse events included:
-
an exacerbation of a pre-existing illness
-
an increase in frequency or intensity of a pre-existing episodic condition
-
a condition (even though it may have been present prior to the start of the trial) detected after trial drug administration
-
continuous persistent disease or symptoms present at baseline that worsened following administration of the trial treatment.
The following were not included as adverse events:
-
medical or surgical procedures in which the condition that led to the procedure was the adverse event
-
pre-existing disease or conditions present before treatment that did not worsen
-
overdose of medication without signs or symptoms.
Recording serious adverse events and serious adverse reactions
The definition of a serious adverse event was any medical event that:
-
resulted in death
-
was life-threatening (refers to an event during which the participant was at risk of death at the time of the event; it does not refer to an event that might have caused death had it been more severe in nature)
-
required hospitalisation, or prolongation of existing hospitalisation
-
resulted in persistent/significant disability or incapacity
-
was a congenital abnormality or birth defect.
Serious adverse events also included were other important medical events that, based on appropriate medical judgement, may have jeopardised the participant and may have required medical or surgical intervention.
Any serious adverse events and serious adverse reactions were recorded in the ‘Investigator Site File’ and the ‘Trial Master File’.
Statistics
Sample size
From the WMD in our previous meta-analysis,21 we found a relative improvement of 8 points in the ODI at 6 months’ follow-up in the group receiving biological agents compared with placebo, with a standard deviation of 16 points, giving an effect size of 0.5. In order to detect a more conservative effect size of 0.4 with 90% power, with a significance level of 5% for a two-tailed t-test, a sample size of 133 in each treatment group was needed. We aimed for a 90% return rate of the final questionnaires but, for a more conservative retention rate of 80%, 332 participants needed to be recruited. If, as is likely, there was any correlation between the baseline and outcome measure, the size of effect detectable would be smaller (or the power to detect a 0.4 effect enhanced).
Recruitment rate
Calculations of recruitment rates for SCIATiC were based on data available from an observational study, led by co-applicants at Keele, which recruited adult patients seeking treatment in primary care for low back-related leg pain including sciatica [Assessment and Treatment of Leg pain Associated with the Spine (ATLAS) trial cohort]. 39 The ATLAS study recruited 609 patients from 17 general medical practices (approximate total adult population of 90,200) over 24 months. Analysis of the recruitment data shows that 219 (36%) participants in this cohort had sciatica with pain in one leg only (with > 80% diagnostic confidence,) with a RMDQ score of > 7 points (equivalent to an ODI score of ≥ 30 points). On average, per month, 86 potential participants were identified by GPs and referred to the ATLAS study, 54 attended the physiotherapy-led research clinic and 25 gave consent and were eligible for the study, nine of whom had a clinical diagnosis of sciatica (spinal nerve root pain) satisfying the conditions described in Inclusion criteria and Exclusion criteria in terms of disability score and diagnostic confidence. Based on these figures and taking into account that in the ATLAS study cohort approximately nine participants per month were recruited, and making the assumption that half this number would consent to be randomised in a RCT, our target rate of recruitment was four participants per collaborating centre per month, with centres covering similar sized populations. For SCIATiC, North Wales, Keele and Cardiff aimed to recruit from GP practices with a combined registered population of at least 100,000 per centre.
Data analysis
All data were anonymised and coded so that data collection and statistical analysis were performed blind to treatment allocation. The code would be broken only after the primary analysis had been completed. The analysis would be performed on a ‘treatment as allocated’ principle to ensure protection against unintended bias. The data would be fully imputed using a multiple imputation by chain equations approach52 in line with a predefined statistical analysis plan to minimise data loss as a result of missing values or time points. Participants who needed to be referred for disc surgery would be labelled as ‘treatment failures’ and their last test results prior to surgery would be carried forward in the analysis. Sensitivity analyses (best case/worst case) would be performed to assess the influence of different imputation assumptions. All trial reporting was Consolidated Standards of Reporting Trials53 compliant.
Primary analysis
The main outcome variable was the ODI measured at 12 months. A linear mixed-model approach for repeated measures would be used to assess the effects of time and group, while time × group effects would further describe and explain the overall finding (the interaction term would assess whether or not the effect of the intervention was the same at each time point). The use of a linear mixed model for analysis should take care of missing data; however, if imputation was required, then the multiple imputation by chain equations approach described would have been used. This model would be fully defined in the statistical analysis plan prior to all analyses. This statistical analysis plan would be approved by all lead investigators and site principal investigators (PIs), and available for comment by the independent committees prior to sign-off.
Secondary analysis
Secondary continuous outcome variables would be assessed in a similar way to the primary outcome variable, with the exception of time to referral for surgery, which would be measured from trial entry (this is the date of second consent) and analysed using Kaplan–Meier survival analyses and the log-rank test. Dichotomous variables would be explored using logistic regression. These analyses would be repeated using prespecified participant subgroups (including the presence of neurological deficit on entry to the trial and MRI findings). Subgroups would be defined within the statistical analysis plan prior to the analyses beginning.
Economic analysis
The health economic analysis would adopt the perspective of the NHS and Personal Social Services, with the inclusion of indirect costs (e.g. time off work) as a secondary analysis. Costs included those of treatment, tests, procedures and investigations, contact with primary and secondary care services and personal social services. Resource use would be obtained from participants’ self-reporting of resource use, captured by questionnaire administration. 46,47 Unit cost data would be obtained from standard sources54 and other resources such as the British National Formulary. 32 The primary economic outcomes would be the incremental cost per QALY gained, estimated by administering the EQ-5D-5L at each follow-up point. The number of QALYs gained by each participant would be calculated as the area under the curve, using the trapezoidal rule, applying the UK tariffs and corrected for baseline utility score. When appropriate, missing resource use or health outcome data would be imputed. 55 Non-parametric bootstrapped 95% CIs would be estimated (10,000 replicates). Stratified cost-effectiveness analyses would be conducted on important, prespecified participant subgroups. Total costs would be combined with QALYs to calculate the incremental cost–utility ratio of the package of adalimumab plus physiotherapy compared with a 0.9% sodium chloride injection plus physiotherapy. Estimates of incremental cost–utility ratios would be compared with the £20,000–30,000 per QALY threshold of cost-effectiveness, and a range of one-way sensitivity analyses would be conducted to assess the robustness of the analysis. Multivariate sensitivity analyses would be applied when interaction effects were suspected. The joint uncertainty in costs and benefits would be considered through the application of bootstrapping and the estimation of cost-effectiveness acceptability curves. 56
Trial management
Trial Management Group
Individuals responsible for the day-to-day running of the trial were included in a TMG, which included the chief investigator, lead investigators, PIs, trial manager, statistician, health economist, site co-ordinators, research staff, data manager and collaborating clinicians, as necessary. The TMG’s role was to monitor all aspects of the trial’s set-up, conduct and progress. The group ensured that the protocol was adhered to, and would take appropriate action to safeguard participants and ensure the overall quality of the trial. The TMG reported to the Data Monitoring and Ethics Committee (DMEC) and Trial Steering Committee (TSC), and met every 1–2 months.
Trial Steering Committee
A TSC was set up to oversee the running of the trial on behalf of the sponsor and funder, and had the overall responsibility for the continuation or termination of the trial. The TSC had an independent chairperson and a majority of independent members and included a patient representative. The role of the TSC was to ensure that the trial was conducted in accordance with the principles of ‘good clinical practice’ and the relevant regulations, and it provided advice on all aspects of the trial. The trial protocol and any subsequent amendments were agreed by the TSC. The TSC reported to the TMG, the sponsor and the funder and it met every 6 months.
Data Monitoring and Ethics Committee
A DMEC monitored the progress of the trial and reviewed all adverse events. The DMEC would have reviewed results from the internal pilot trial and advised the TSC as needed. It met every 6 months.
Reporting
The TMG reported to the DMEC and TSC. The DMEC reported to the TSC and the TSC reported to the TMG, the sponsor and the funder. Safety reports were submitted every 6 months to the Research Ethics Committee (REC), the sponsor and the funder. Development update safety reports were submitted to the MHRA.
Direct access to source data and documents
Source data were the hospital-written and NHS electronic medical records. Access to these data was through the participant’s clinicians, physiotherapist and research nurse. Trial-related monitoring, audits, REC reviews and regulatory compliance inspections were permitted, allowing access to data and documents when required.
Quality assurance
This trial was conducted in line with the trial protocol and followed the principles of good clinical practice outlined by the International Committee of Harmonisation Good Clinical Practice E6 (R1) Current Step 4 Version57 and complied with the European Union directive 2001/20/EC. 58
A monitoring plan was developed based on a trial risk assessment, which provided details of day-to-day quality control, audits, etc., and was delegated to members of the trial team to ensure that collected data adhered to the requirements of the protocol; only authorised persons completed case report forms; the potential for missing data was minimised; data were valid through validation checks (e.g. range and consistency checks); and recruitment rates, withdrawals and losses to follow-up were reviewed overall and by hospital site.
Data handling
The sources of data for the trial were as follows: recruitment details; baseline outcome measures captured electronically onto password-protected and encrypted computers by the research physiotherapists or research nurses; postal questionnaires at 6 weeks’ and at 6 and 12 months’ follow-up entered into the MACRO system (version 4; InferMed, London, UK); and telephone minimum data collection from non-responders captured on computers by researchers. Additional health service use data obtained from primary and secondary care records, with participants’ consent, would be recorded electronically on the computers. Each centre would input data into the MACRO data management program, which is a web-based system allowing controlled access to data by all centres and allows a full audit trail.
Trial sponsor
Bangor University (reference number 12/201/02; contact Dr Huw Roberts).
Ethics approval
Wales REC-3 granted approval on 27 May 2015 (15/WA/105). Clinical trial authorisation was approved from the MHRA on 15 April 2015 (21996/0002/001-0001).
Chapter 3 Results
Trial progress
Funding for the trial was approved by the HTA programme on 11 August 2014; the intention at that time was for the trial to open in January 2015 and close in June 2018. It had been planned that all the trial documentation for the regulatory approval for the trial would be completed between July 2014 and December 2014 so that regulatory approval could be obtained by January 2015.
Five collaborating sites planned to participate in the trial – North Wales, Cardiff, London, Keele and Nottingham – with training at the five sites taking place between February 2015 and April 2015. The initial plan was that the trial would be set up and recruiting participants at North Wales and London by April 2015, with the other sites opening to recruitment in June to October 2015. The trial documentation for the regulatory approval was not in place until December 2014. Regulatory approval was obtained from the MHRA on 15 April 2015 and from the REC on 27 May 2015. During this time the three English sites submitted requests for excess treatment costs (ETCs) to the NHS England for Clinical Commissioning Groups, ETCs for the two Welsh sites were agreed, and contracts were sent to both lead and collaborating sites from the sponsor, Bangor University. There were delays and unforeseen complexities in obtaining the ETCs for the English sites. Contracts also proved an issue and caused major delays because of difficulties with the delegation of the roles and responsibilities and what was required within the different contracts between university and university, and university and NHS sites. One of the sites withdrew from the trial in February 2016 because of concerns about the dosage of the biologic used and its patient population, which was higher than the standard dose used for patients with rheumatoid arthritis but similar to dosage in other conditions. This withdrawal led to a risk review for the other sites, which felt that, as no new data were available, the risk of infection for participants was acceptable, and they all agreed to continue participating in the trial.
The trial opened to recruitment on 8 December 2015 at North Wales and Nottingham, with Keele opening to recruitment on 11 August 2016. At the time of trial closure, contractual discussions were still ongoing between Bangor University, Cardiff University and Cardiff and Vale University Health Board.
During this time all sites dealt with a number of challenges. In North Wales, a research physiotherapist was seconded to the trial in September 2015 but, because of a shortage of physiotherapists in the department, was required by the health board to return to their previous employment and then left the post in February 2016 for personal reasons. As a result of physiotherapy staffing shortages within Betsi Cadwaladr University Health Board (BCUHB), the site was unable to employ a replacement research physiotherapist for the required research physiotherapist time. Participants were identified and recruited via the musculoskeletal clinic and physiotherapy clinics. The PI at the site contacted fellow consultants throughout the health board to ensure that all potential participants were identified. Only three participants were recruited at this site between March and July 2016.
Nottingham had intended to recruit its participants for the trial from the Sherwood Forest Hospitals NHS Foundation Trust Back Pain Unit diagnostics clinics only, based on pre-study clinic data. During the first 3 months after the start of recruitment at that site, no eligible participants were identified. The site PI extended screening to include orthopaedic clinics in addition to back pain unit clinics, but without any significant increase in recruitment. The PI at this site investigated whether or not there had been a change in referral pathways for people with sciatica in the region that might have affected referrals into these clinics. The PI confirmed, in February 2016, that this had been the case. GPs had been given direct access to MRI scans for sciatica, and were requesting and reviewing the results of MRI before requesting opinion or treatment from the back pain service. As well as introducing delay to referrals, the local GPs were tending to refer those with sciatica and a congruent disc prolapse on MRI to an alternative spinal surgical unit (e.g. Nottingham University Hospitals NHS Trust). A potential participant referred to the PI had already received MRI, which made them ineligible. Trial progress was an agenda item at the TMG. Owing to changes in pathways at Sherwood Forest Hospitals NHS Foundation Trust Back Pain Unit, it was agreed by the TMG that the protocol should be amended to include participants who had already undergone MRI. This was approved by the REC on 15 April 2016 and by the MHRA on 27 May 2016. During this time, Sherwood Forest Hospitals NHS Foundation Trust had also been in discussions with local primary care colleagues to arrange identification of potentially eligible participants from local GP practices through database searches or opportunistic referral. This was agreed and implemented in June 2016. Sherwood Forest Hospitals NHS Foundation Trust recruited five participants to the trial between February and September 2016.
During this period, Keele had obtained agreements for its ETCs and contract negotiations were finalised between Bangor University and Keele University, and between Bangor University, Keele University and Royal Wolverhampton NHS Trust, and also between Bangor University, Keele University and Staffordshire and Stoke-on-Trent Partnership Trust. The Royal Wolverhampton NHS Trust was opened to recruitment on 11 August 2016; one potential participant was identified prior to the trial closure on 20 September 2016. Randomisation of this participant was not permitted as a result of trial closure on 26 September 2016.
The TSC met on 11 January 2016. The problems with recruitment were discussed at the meeting, and the members were very sympathetic to the trial team’s frustration of the poor recruitment to the study. The TSC recommended the following steps if the funders allowed the trial to continue:
-
optimise recruitment for the two sites that were open
-
work harder to reduce bottlenecks (e.g. more MRI slots in North Wales)
-
increase the number of general practices searching for potential participants
-
approach the musculoskeletal triage clinics to join the study as sites
-
inform Keele and Cardiff at the next management group meeting that if they were not about to start recruiting then they would no longer be part of the trial
-
contact other possible sites that would be able to recruit to the study within the next 6 months.
The chief investigator contacted four sites one each in north, south and mid-Wales, and also the Royal Free Hospital in London.
Although the additional North Wales site was eager to participate and there was sufficient staff, there was a lack of clinical space to accommodate the trial within the rheumatology department. The rheumatology consultant submitted a case to the hospital managers with plans to increase the space available to carry out clinical trials. Unfortunately, this request was turned down because of competing demands on clinical space in the hospital, so it was not possible for the rheumatology department to be involved with SCIATiC and other clinical trials.
The hospital site in south Wales had expressed interest in participating in the trial in April 2016, and the site was arranging to accommodate the trial when we had to notify them that the trial was terminating early.
The hospital site in mid-Wales had also expressed interest in participating and had notified us on 17 August 2016 that its physiotherapy team had agreed to accommodate the trial. Unfortunately, we informed the site on 18 August 2016 about the discussions that we had with the funders and the expected termination of the trial.
The Royal Free Hospital had been discussing increasing their portfolio of clinical trials within the physiotherapy department and in April 2016 had expressed interest in participating. Unfortunately, it later informed us that it was doubtful if it would receive funding for the intervention arm of the trial. The hospital also felt that it would have difficulty recruiting participants with a symptom duration of < 6 months. In addition, its rheumatology department did not have spare capacity as it was already busy with existing research activities.
Owing to concerns with the slow recruitment, the chief investigator and Bangor trial team met with the funders on 28 January 2016, who informed the trial team that they should:
-
finalise existing contracts immediately
-
open sites that had not yet opened within the month
-
start recruiting from all sites
-
complete recruitment to the internal pilot of 50 participants by June 2016.
Another meeting with the funders was held on 16 August 2016. At the meeting the funders requested that the project team should submit closedown proposals as soon as possible and, ideally, by no later than 31 August 2016. Two different scenarios were proposed:
-
an immediate closedown of recruitment with submission of the project report by the end of December 2016
-
closure of recruitment in 6 months, until which time the study team should seek to establish the most effective recruitment routes for any future study, with submission of the project report by the end of June 2017.
From 16 August to 26 September 2016 only one participant was randomised to the trial by the Sherwood Forest Hospitals NHS Foundation Trust site. This information was relayed back to the funder, and on 26 September 2016 the chief investigator confirmed that the funders had asked for the trial to be closed immediately and the project report to be completed by the end of December 2016.
Trial timetable
The trial timetable is outline in Table 2.
| Event | Date of completion | |
|---|---|---|
| Expected | Actual | |
| Finalised protocol and trial documentation | July–December 2014 | January 2015 |
| Ethics and NHS R&D permission/MHRA approvals | September 2014–February 2015 | MHRA – April 2015 |
| REC – May 2015 | ||
| BCUHB R&D approval – July 2015 | ||
| SFHT R&D approval – November 2015 | ||
| RWT R&D approval – August 2016 | ||
| Contracts signed and completed | January–March 2015 | BCUHB – April 2015 |
| SFHT – October 2015 | ||
| Bart’s Health NHS Trust – September 2015 | ||
| RWT – July 2016 | ||
| Staff training and site initiation | March–May 2015 and November–December 2015 | June 2015, July 2015, September 2015 and November 2015 |
| Set up of centres to recruitment | February–March 2015 | BCUHB – December 2015 |
| SFHT – December 2015 | ||
| RWT – August 2016 | ||
| Identification of potential participants | February 2015–August 2016 | December 2015 |
| Telephone screening | March 2015–September 2016 | December 2015 |
| Physiotherapy clinical assessment | April 2015–October 2016 | December 2015 |
Trial recruitment
Recruitment data for the trial are presented in Figure 3 and reasons for withdrawal or exclusion in Table 3. Sherwood Forest Hospitals NHS Foundation Trust and BCUHB recruited from December 2015 to September 2016. The Royal Wolverhampton NHS Trust recruited from August 2016 to September 2016. During this time, eight participants were randomised. No adverse events or adverse reactions were recorded for any of the participants.
FIGURE 3.
Participant flow diagram for Sherwood Forest Hospitals NHS Foundation Trust, BCUHB and the Royal Wolverhampton NHS Trust.
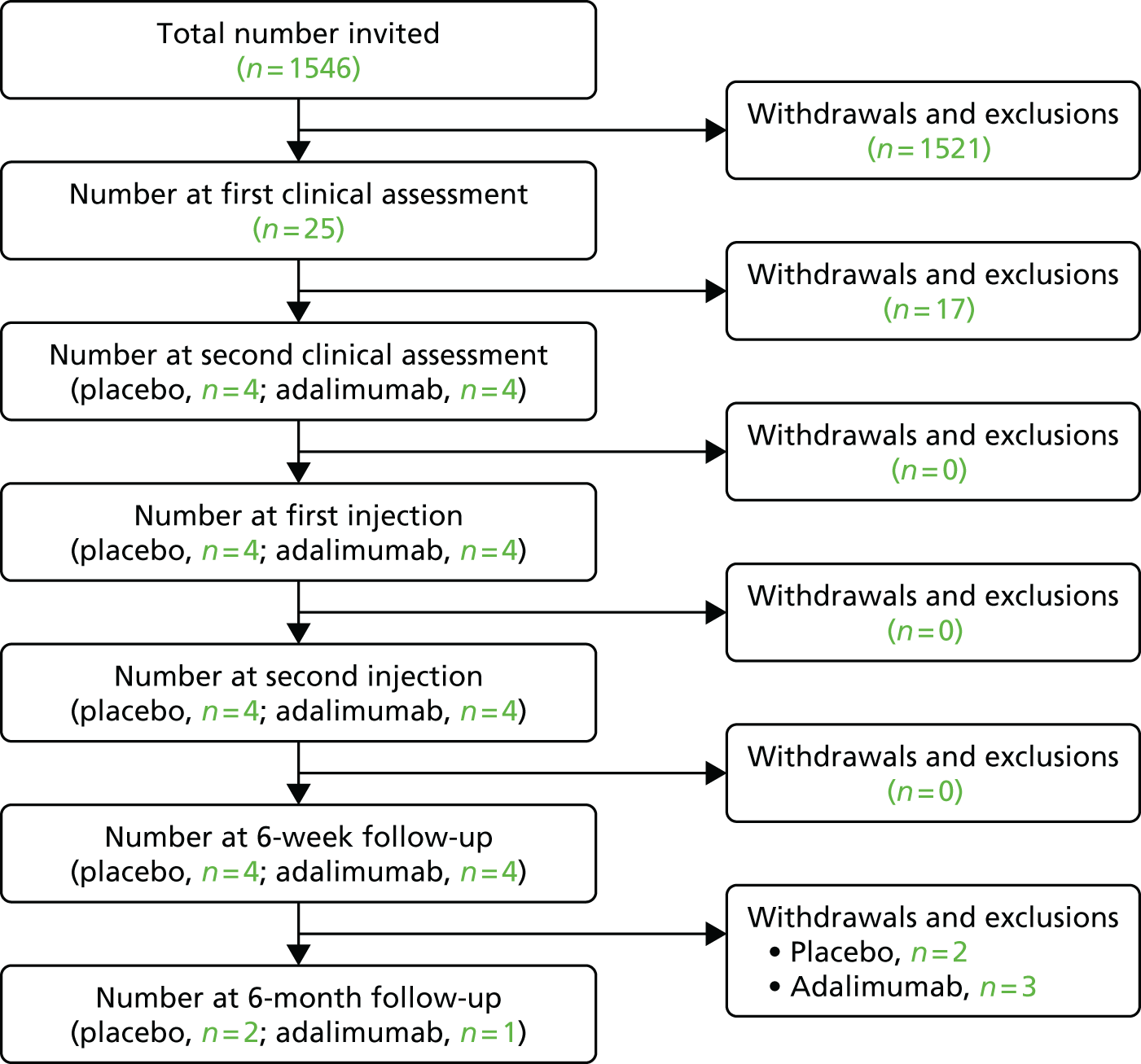
| Reason for withdrawal and exclusion | n |
|---|---|
| From invitation to first clinical assessment | 1520 |
| Did not confirm interest | 963 |
| Symptoms persisting for > 6 months | 173 |
| Previous episode of sciatica in the last 6 months | 2 |
| Contraindications to MRI | 6 |
| Serious spinal pathology | 4 |
| Incidental serious pathology identified by MRI | 1 |
| Widespread pain throughout body | 25 |
| Previous use of biological agents targeting TNF-α | 1 |
| Previous lumbar spinal surgery | 16 |
| Contraindications to adalimumab | 1 |
| Pregnant or breastfeeding | 1 |
| Unable to communicate in English or Welsh | 3 |
| Mental health problems | 3 |
| No sciatica | 210 |
| Previous surgery | 11 |
| No leg pain | 20 |
| Complicated symptoms | 18 |
| Pain in both legs | 7 |
| Expressed interest but delay in telephone screening attributable to site staffing issues means no longer meet criteria for inclusion (e.g. no longer in pain or have recently breached the > 22-week exclusion window since replying) | 6 |
| No response or no longer interested | 23 |
| Symptoms resolved/improved | 10 |
| Current leg pain worse than or as bad as back pain | 3 |
| Trial closed early to recruitment | 14 |
| From first to second clinical assessment | 17 |
| Over time limit for second clinical assessment | 1 |
| Study closure | 5 |
| Mild symptoms – discharged to GP care | 7 |
| TB screening failed | 1 |
| Participant revealed long-term history of widespread pain at screening – particularly in shoulders | 1 |
| No positive neurological test | 1 |
| Patient did not attend appointment and could not be contacted | 1 |
| From 6-week to 6-month follow-up | 4 |
| Study closure | 4 |
Contracting delays
Site contracts were a major issue. Initial contract templates were drafted in November 2014 but could not proceed further until the contract and finances were agreed with the funder, and the funder did not provide these until February 2015. Draft subcontracts were sent to the relevant parties from the Bangor University contracts department on 31 March 2015.
Betsi Cadwaladr University Health Board: Peter Maddison Rheumatology Centre, Llandudno Hospital
The subcontract was signed on 8 April 2015; the time taken was 9 days.
Nottingham University/Sherwood Forest Hospitals NHS Foundation Trust: Rheumatology Department, King’s Mill Hospital
The subcontract with Sherwood Forest Hospitals NHS Foundation Trust was signed on 20 October 2015; the time taken was 197 days. The collaborative agreement with Nottingham University was signed on 7 July 2016; the time taken was 378 days.
Initial agreement was sent to Sherwood Forest Hospitals NHS Foundation Trust and Nottingham University on 31 March 2015. A draft academic agreement was sent to Nottingham University by Bangor University on 25 June 2015, along with a reminder regarding the NHS contract, and a response was received from the PI on 16 July 2015. Nottingham queried their costings on 3 August 2015, and costings were confirmed by Bangor University on 4 May 2016; the collaborative agreement was signed on 7 July 2016. Bangor University received an update from the Sherwood Forest Hospitals NHS Foundation Trust site on 15 September 2015 stating that they were awaiting an internal response, and the NHS trust contract was signed on 20 October 2015.
Queen Mary University of London/Bart’s Health NHS Trust: The Royal London Hospital
The collaborative agreement with Queen Mary University of London was signed on 24 July 2015; the time taken 115 days. The subcontract with Bart’s Health NHS Trust was signed on 14 August 2015; the time taken was 131 days.
An initial agreement was sent to Bart’s Health NHS Trust and Queen Mary University of London on 31 March 2015. Bangor University received a response from the site on 21 July 2015 stating that the site agreed with the terms of the contract. On 24 July 2015, the site contacted Bangor University to say that, as the template had been approved by the funder, they could move to signature. The collaborative agreement was signed on 24 July 2015 and the subcontract with the NHS trust was signed on 14 August 2015.
Keele University/Royal Wolverhampton NHS Trust: Cannock Chase Hospital and New Cross Hospital
The collaborative agreement with Keele University was signed on 2 June 2016; the time taken was 427 days. The subcontract with the Royal Wolverhampton NHS Trust was signed on 7 July 2016; the time taken was 459 days.
The initial agreement was sent to Keele University on 31 March 2015, with service-level agreements to be sent to the NHS sites by Keele University. Initial feedback was received from Keele University in May 2015 with a response to these from Bangor University in June 2015. At a meeting on 22 July 2015 in Keele, contracts were discussed with the trial manager and the research team at Keele University, considering either (1) a tripartite contract between Bangor University, Keele University and the two sites at Royal Wolverhampton Hospitals NHS Trust (Cannock Hospital and New Cross Hospital) or (2) a contract from the sponsor directly to the NHS sites. During further discussions between the contract departments of Bangor and Keele universities, it was agreed that the following subcontracts would be used: Bangor University and Keele University, and Bangor University and Royal Wolverhampton Hospitals NHS Trust. Another contract was also required between Bangor University and Staffordshire and Stoke-on-Trent Partnership Trust, as patients in the trial would be treated by physiotherapists in Royal Wolverhampton Hospitals or from Staffordshire and Stoke-on-Trent Partnership Trust. The collaborative agreement was signed on 2 June 2016. Further discussions concerning the delegated duties and wording of the contract to clarify the role of the NHS sites meant that the subcontract was not agreed and signed until 7 July 2016.
Cardiff University/Cardiff and Vale University Health Board
The subcontract was still being discussed at the termination of the trial in September 2016, 18 months after the initial draft had been sent. There were several unresolved issues. Cardiff University decided that it wanted a tripartite subcontract between Bangor University, Cardiff University and Cardiff and Vale University Health Board. However, after agreeing to this tripartite agreement, it decided not to sign, as it wanted to use the Brunswick model agreement. 59 Initially MRI was to be undertaken by Cardiff University, but because this was allocated to the university, rather than the health board, only 80% of the cost would be reimbursed, and who should pay for this underspend was left unresolved. This delay in signing the contract also meant that the research physiotherapist seconded to the post was not able to sign her secondment contract and had to return to her original post. A teleconference took place on 21 January 2016 to resolve the outstanding issues. As a result, an updated subcontract was sent with an updated work schedule, but remained unsigned.
The funder also requested oversight of all the subcontracts before they were signed. Delays with the subcontracts led to delays with recruitment and retention of staff at the trial sites. It also meant that a great deal of trial management time that could have been spent on finding solutions to the slow recruitment was instead expended on contracting issues.
Withdrawal of site
Eight months after initiation, Bart’s London, one of the larger sites, revisited the risk assessment of the trial and felt the participant population in its area meant that the 80-mg initial dose would confer a risk of infection that was higher than acceptable. Bart’s London therefore decided to withdraw its participation in the trial. This withdrawal led to a risk review for the other sites, all of which felt that, as there were no new available data, the risk of infection for their participants was acceptable and agreed to continue participating in the trial.
Recruiting research physiotherapists at sites
It took longer than anticipated to obtain research and development (R&D) approval and set up the site, and this led to delays in recruiting research physiotherapists. Just after the BCUHB site had opened to recruitment, the research physiotherapist, who had been seconded to the post from the NHS physiotherapy department, was required to return to her clinical duties because of staff shortages. This had a negative effect on the trial, as it halved the physiotherapist time available for screening and recruiting participants to the study at that site. The chief investigator complained to the department that this removal was jeopardising the trial, and it was agreed that it would only be for 6 weeks until a locum could be employed. Unfortunately, the same research physiotherapist went on long-term sick leave and was advised by the health board’s occupational health department not to return to the post of research physiotherapist. This led to delays in recruiting participants into the trial and resulted in the loss of some potential participants who had expressed an interest, as there was no physiotherapist to screen and recruit them.
This resulted in a change of protocol whereby a medically qualified member of staff could be used to screen potential participants if a research physiotherapist was unavailable. In the interim, Health and Care Research Wales nurses assisted with the screening of potential participants and another physiotherapist was assigned to assist with the recruitment of participants to work on the trial for one session per week for 5 months. A rheumatology registrar was trained in the trial procedures, but was only available to recruit potential participants for 1 month after regulatory approvals were complete.
Slow recruitment at open trial sites
Recruitment was slower than anticipated in both BCUHB and Sherwood Forest Hospitals NHS Foundation Trust, which were opened to recruitment on 8 December 2015. Royal Wolverhampton NHS Trust opened on 11 August 2016; the trial was closed on 26 September 2016. No participants could be recruited by Royal Wolverhampton NHS Trust in this time.
In BCUHB, 16 GP practices identified eligible patients presenting at the practice by database searches or opportunistic referral. An application was made to increase the funding for NHS support costs to cover the costs of 30 practices, but this was not in place before the trial closed. Musculoskeletal clinics and physiotherapy departments also searched for eligible patients presenting to their clinics.
At Sherwood Forest Hospitals NHS Foundation Trust, patients referred to the musculoskeletal service at the rheumatology clinics were screened for eligibility. It was noted by the PI at this site that the number of sciatica patients referred to the clinics had fallen as a result of a change in the referral pathway commissioned by the local commissioning group. At this site it was then decided to invite GP practices to identify eligible patients presenting at the practice, by database search or opportunistic referral. Database searches commenced at 12 practices in June 2016. A total of 756 potential participants were identified, 11 were invited to first clinical assessment screening and five participants provided consent to participation before the trial was terminated.
At Royal Wolverhampton NHS Trust, R&D approval was agreed and contracts signed on 7 July 2016, just before the trial was terminated. One patient was screened but was not invited to provide consent to participation prior to trial closure.
Excess treatment costs
Applications for ETCs were agreed by the Welsh Government by the two Welsh sites, but agreement for the three English sites was more problematic. On 4 February 2014, the trial chief investigator asked all participating sites to submit an application for ETCs. This was submitted by Sherwood Forest Hospitals NHS Foundation Trust in June 2014, and the ETCs were approved on 11 March 2015. Discussion had also taken place in Wolverhampton and an application submitted, but ETCs had been declined because of insufficient funds. This site subsequently explored other potential sources of funding (e.g. from primary and secondary care, or the pharmaceutical company Abbott UK, if they could provide a discounted or free drug). On 1 June 2015, the chief investigator requested details of how to obtain a subvention from the National Institute for Health Research. The chief investigator then contacted the Department of Health and requested assistance with the matter and was told that the subvention budget is not used to fund specific study sites and that, as sites for the trial are already signed up for ETCs, they would expect the trust to cover the costs. Keele led negotiations with both the trust and the Clinical Commissioning Groups in the West Midlands (Wolverhampton). Owing to anti-TNF-α falling outside payments by results tariff, both parties argued that they were not funded to support the ETCs associated with this trial. Following negotiation it was agreed that the local Clinical Commissioning Groups and the trust (charitable funds) would fund an equal split of the ETCs. ETCs were approved for Royal Wolverhampton NHS Trust on 19 August 2015 and provisional ETCs agreed for Bart’s Health NHS Trust; the trust was told that it would be finalised once R&D approval was given.
Outcome measure results
The original intention was to use a linear mixed-model approach to assess the effects of time, group and time × group. This was not possible as data were only available for eight randomised participants on trial closure. The data for the eight participants are presented. The demographic information is presented in Appendix 1. Results from all the measures that were collected at different time points are reported in Appendix 2, with the pain manikin drawings presented separately in Appendix 3. Resource use results are presented in Appendix 4 and concomitant medications in Appendix 5. Finally, the physiotherapy information is presented in Appendix 6 and concomitant medications in Appendix 7. It is not possible to make any conclusions from these data as no analysis was performed.
Patient and public involvement
The patient and public involvement representative contributed to trial design by commenting on the trial protocol and patient-facing documents, as well as participating in the TSC meetings. Unfortunately, we lost contact with the patient and public involvement representative when trial recruitment was closed.
Comments and feedback from the trial management team and research teams
The trial management team and all sites were asked to reflect on the trial about what worked, and what did not. The following was noted.
Comments from the trial management team
-
We would recommend for future studies that a site feasibility questionnaire be designed and sent out at an early stage in the trial to all potential sites to inform them on all aspects of the trial design, to ascertain any potential problems with recruitment and to highlight any logistical challenges that the potential sites may face.
-
Documentation was a problem as the TMG had to approve all of the documents used. Members of the group would respond separately, not at all or after the documents had been finalised. In order to provide sufficient clarity and accuracy in the trial documents, we would recommend that documents should only be sent to the lead investigator and PIs, and discussed at individual site team meetings. We would recommend that document meetings, to approve trial documents, should be held on a regular basis and realistic time frames given.
-
There were long delays negotiating subcontracts, as much of the contracting discussions focused on whether the academic partners or the clinical sites were responsible for delivering the randomised treatment and physiotherapy to the participants. The contracts needed to be between the NHS sites and the sponsor, with the local universities supporting the process rather than being the contracting party. There needs to be full discussion between the sponsor’s contracting department and all academic partners and clinical sites to obtain an early agreement about what the contracts need to include, and how the contracting process should be arranged, so that the academic partners and clinical sites have a clear understanding of their delegated roles and tasks.
-
Misattribution of costs was another difficulty for this trial. Sites were asked to provide their own costings on what they required, but some sites had requested funding for their university when it should have been attributed to the NHS site. We would recommend that each site discuss with its finance and R&D department where the funding should be attributed and what costings are required. This needs to be at the application for funding stage.
-
Excess treatment costs remained an issue, with no clarity about who was responsible for funding these in the English sites. Commissioners were investing in a study that might not produce savings in the long term, and any savings would be realised within secondary care sites and remain hidden from the commissioners.
-
Recruitment was difficult, especially as sciatica participants at this stage in their illness would be managed in primary care, and would not have necessarily been referred to secondary care. These were not insurmountable issues, but early and regular communication between primary and secondary care staff would have been beneficial. Changing patient pathways within the NHS between trial design and completion resulted in challenges at Sherwood Forest Hospitals NHS Foundation Trust. Risk management plans for recruitment were agreed in advance, and implementation should have been timely.
-
Difficulties with recruiting patients within 6 months of symptom onset might prove challenging for future research in this area. If the proposed mechanism of action of treatment, and lack of availability of other effective treatments, permits, a longer symptom duration eligibility criterion would be likely to increase recruitment. Several participants who expressed interest in participation had their symptoms for > 6 months. It would have been useful to collect data on exactly how far over the threshold these potential participants were.
-
Our experience raises concerns about secondment of service physiotherapists into research roles, in which changes in service demands might result in suspension or termination of their secondment. Availability of dedicated research physiotherapists at sites, or recruitment of research physiotherapists for the specific study, might have reduced staffing issues. Physiotherapists could have been employed directly by the trial rather than seconded, as because of departmental shortages, seconded staff had to resume their previous roles during the agreed seconded period of the trial; this caused major problems and hindered the recruitment of participants in North Wales.
Comments from the Betsi Cadwaladr University Health Board site in the Peter Maddison Rheumatology Centre, Llandudno
-
Identifying participants from searches of the general practice record database was not very successful; the uptake from this was very low.
-
There were too many components and individuals involved, which affected the success of the trial.
-
The trial would have worked better if carried out entirely in primary care.
-
Training a research officer with rheumatology experience, or giving a physiotherapist training in biologic treatments, may have been better than involving a physiotherapist and secondary care rheumatology nurses.
Comments from the Betsi Cadwaladr University Health Board Physiotherapy Department
Two main areas of difficulty were identified as barriers to the success of SCIATiC from the research physiotherapist perspective.
-
Recruitment.
-
The search criteria for the general practice record database were not able to be specific enough and were poor in identifying exclusion criteria. At telephone triage, many patients had symptoms for many months, in some cases years, meaning that they were ineligible for the trial. A large proportion of the research physiotherapist’s time was wasted telephoning people who were not eligible.
-
Primary care was the main source of patients, but GPs were not universally on board with identifying patients. Not all of the GPs in the participating practices were aware of the trial, so suitable patients may have been missed.
-
-
Logistics.
-
In the final 6 months from April until September 2016, the time available for the research physiotherapist to contact patients was limited to 2 hours on one afternoon per week. For 5 weeks in December 2015 and January 2016 the research physiotherapist had her hours cut by 50% as she was required to cover the outpatient clinics in the Llandudno physiotherapy department.
-
The number of patients who were available when telephoned in the afternoons was very low.
-
The availability of staff such as research nurses and senior clinicians to support the research physiotherapist was limited as a result of other commitments. It was very difficult to co-ordinate the biological agent counselling, blood tests etc. Because of the restricted staff availability, it was difficult to make timely appointments for eligible participants. It was also difficult to co-ordinate the large number of tests (MRI, T-spot for TB and chest radiography) on 1 day.
-
Owing to the large geographical surface area of BCUHB, some patients had to travel (in pain) for up to 2 hours to attend appointments.
-
Suggestions for future trials:
-
The involvement of the research physiotherapist during the initial planning stage could have helped recruitment and communication. The funding for the post was delayed and then the appointment of the research physiotherapist was later than hoped.
-
First-contact physiotherapists were not identified as a source of recruitment. Designing the trial to work with these physiotherapists would have helped identify suitable patients in a timely way.
-
Rather than identifying participants from retrospective database searches, clinicians in GP practices could have identified eligible patients during face-to-face consultations and would have been able to check for eligibility before they were invited to participate, resulting in a better conversion rate for trial participation.
-
A laminated checklist for each consulting or treatment room containing the inclusion and exclusion criteria may have helped remind staff about the trial.
-
Rather than having a single research physiotherapist with limited days and location, it would have been better having physiotherapists in a couple of locations, trained to identify suitable patients on the outpatient waiting list and able to telephone triage to assess eligibility; this would have improved recruitment.
Comments from the Keele team
Trial set-up
-
Two key challenges were agreeing the ETCs at one of our two clinical sites, and the merging of two clinical rheumatology services at the time of trial set-up. Both of these challenges delayed the start of the trial significantly.
-
Completion of contractual agreements. This trial was a clinical trial of an investigational medicinal product, and Keele clinical trials unit had recent experience of a MHRA inspection in autumn 2015; thus, the Keele team was particularly keen to ensure that all the required contracts and sponsorship arrangements for this trial were clearly in place and appropriate agreements reached about delegation of responsibilities.
-
Many of the clinical staff did not feel fully prepared to commence participant recruitment and treatment following the November 2015 training session. Further training was developed and delivered to the research physiotherapists and usual care physiotherapists, supplemented by local working instructions and/or training packs that the Keele team developed. Further discussions were also undertaken with the rheumatological nursing staff involved in the trial in the NHS sites to clarify the study processes and procedures.
Recruitment
-
The clinical sites supported by Keele did not have a chance to test the success, or otherwise, of identification and recruitment processes fully because of the short time between open and close to recruitment at their sites (from 11 August to 23 September 2016). However, during this time, the number of potentially eligible patients identified through the GP system searches was smaller than expected.
-
There was a reasonable response rate (14 out of 43) to invitations to the trial, and nearly half of these (6 out of 14) were from GPs handing trial information packs to patients in the consultation. It is therefore possible that patients may be more likely to respond if the GP has given them the pack and potentially discussed the trial with them.
Comments from the Sherwood Forest Hospitals NHS Foundation Trust: King’s Mill Hospital Rheumatology Department and Back Pain Unit
Recruitment
-
Initial recruitment was through back pain clinics within Sherwood Forest Hospitals NHS Foundation Trust.
-
It would have been beneficial to have had GP recruitment from the start.
-
GPs would have screened for patients on a monthly rolling search.
-
Good working relationship with the people from the clinical research network who were identifying participating GPs.
-
We could have potentially had five more patients on the study who had given their first consent and had been screened, but were unable to be randomised as the study was closed to recruitment.
-
The clinical research network facilitated recruitment through primary care, which became increasingly productive. The average recruitment figures did reflect the higher recruitment rate at the time the study was closed.
Comments from Cardiff
-
Very good contact and support form Bangor Trials Unit. Always helpful on the telephone.
-
Good support from the PI, as above.
-
Flexibility with training packages.
-
A major stumbling block was sorting out contracts, which was the main hurdle that, unfortunately, was not overcome in a timely fashion.
Chapter 4 Discussion
Summary of main findings
We have demonstrated that a RCT of adalimumab for persistent sciatica of 3–6 months’ duration is acceptable to some participants. The trial methods were feasible in terms of randomisation method and outcome measurement. However, recruitment rates were lower than expected and several other factors contributed to trial termination prior to pilot study completion. We were therefore unable to demonstrate that this trial was feasible. There was a long delay agreeing and exchanging subcontracts with participating centres and sites. The contracting discussions with one site were never concluded and another centre dropped out of the study before recruitment started. There were delays negotiating ETCs for the English centres and sites. We did attempt to recruit additional sites in Wales, but negotiations were not concluded at the time of trial closure. Delays in the contracting process also caused delays in recruiting research staff, in particular the research physiotherapists. In the two sites that did open in time to recruit participants, recruitment was slow. In one site, the sciatica management pathway changed around the time that it opened to recruitment. This site initially only relied on referrals to its secondary care musculoskeletal service, but later involved the primary care research network, which was starting to identify participants just before trial closure. In the other site there were operational issues identifying the research physiotherapist resource. This site relied on retrospective GP record review to identify potential participants and although large numbers of invitations were sent to potential participants, there was a low rate of uptake with only a small proportion seen for an initial assessment, and entered into the trial. We were in the process of increasing the number of GP practices within the North Wales area to assist with increasing recruitment; this was not concluded prior to the trial closure. Recruitment was improving just before the trial was shut down with five potential participants ready to be recruited within the following month.
Strengths and weaknesses
The trial methods, in terms of randomisation method and outcome measurement, worked smoothly but for only eight participants, so it is not possible to claim that the methods were feasible. An internal pilot study was planned, but unfortunately we were unable to recruit sufficient numbers.
We had modelled the numbers of eligible participants for our recruitment projections on the ATLAS cohort study. 39 However, we made unrealistic assumptions about the numbers of identified participants who would be willing to participate in a clinical trial of an investigational medicinal product. Although we identified large numbers of potential participants, only small numbers returned reply slips indicating a willingness to participate. It was not known why eligible participants did not wish to participate. Presumably, some found the trial procedures too burdensome, such as the complex two-stage recruitment process, whereas others did not want to participate in a RCT, especially in a clinical trial of an investigational medicinal product involving a medication with known potential side effects. The ATLAS study cohort was led by the team at Keele University and recruited 610 participants in 23 months. 60 The two clinical sites supported by Keele signed their contracts just prior to trial closure. Although potential participants had started to be identified, there was insufficient time to recruit them. Because of this, it was not possible to compare rates of recruitment into the RCT with those found in the ATLAS cohort study, nor the more recent HTA-funded Sciatica Outcomes in Primary Care trial using similar methods of identifying participants. One site in east London dropped out of the trial just before recruitment began because the PI had concerns about patient safety. The PIs in the other sites reviewed the safety risks to potential participants and felt that these risks were justified and that measures were in place to mitigate, detect and address any adverse effects. Although there were only short delays in obtaining research permissions and finalising the trial contract with the funder, there were very long delays negotiating subcontracts with three of the sites. The difficulty of achieving clarity of delegation of functions, and clarity about what needed to be in the different contracts (university to university vs. university to NHS trusts) are key learning experiences from this trial. Negotiations concerning ETCs in England were protracted and complex, which added to the delay negotiating contracts and setting up sites.
Comparison with previous literature
The previous systematic review of biological agents for sciatica found a small number of RCTs and other studies with small numbers of participants recruited. 21 Many of these studies also had poor rates of recruitment, especially in the UK NHS. 24
Implications for future research
A number of factors contributed to the lack of recruitment to the trial. There were delays in contracting, the process for identifying and recruiting participants was inefficient, there were delays in site set-up and a lack of investigator engagement (possibly because of a lack of equipoise). After the London centre withdrew, we asked the other PIs whether or not the research question was still in equipoise. They all agreed that the risk of infection from the dose of drug administered in this trial was acceptable, and that they were still in equipoise.
In order to reduce delays in the contracting process, there needs to be an early agreement about what the contracts need to include, and how the contracting process should be arranged, so that the academic partners and clinical sites have a clear understanding of their delegated roles and tasks. Early discussions about site requirements, perhaps using a site feasibility questionnaire, early dialogue with sites’ R&D departments and the early appointment of research staff in each site would facilitate trial set-up.
We are unable to make any recommendations for future practice in this area because of a lack of trial results. Without any results from the internal pilot study, it is difficult to make recommendations for future research in this area. It may be that there is insufficient equipoise around the question of adalimumab for sciatica among patients and some clinicians. However, this would need to be addressed in further qualitative research. The two-stage recruitment process was complicated and not feasible. We had modelled the number of potentially eligible patients on results from the ATLAS cohort study. However, ATLAS did not use the same two-stage process, nor did it rely on retrospective searches of GP records, but rather relied on the identification and invitation of eligible patients who were currently consulting their GP for sciatica. The same recruitment process for ATLAS, which relies on GPs entering relevant diagnostic Read codes into their computer systems, triggering a ‘pop-up’ reminder about sending eligible patients to dedicated clinics for further assessment and eligibility checking, has worked in the Sciatica Outcomes in Primary Care (SCOPiC) trial. 61 Similar recruitment methods were going to be used in the two sites supported by the Keele co-applicants. Unfortunately, because of delays in finalising contracts and setting up sites there was insufficient time to recruit any participants and to test these recruitment methods before trial closure. So, it is still not possible to say whether or not the use of the recruitment method used in the SCOPiC trial would be feasible for a similar RCT in the NHS testing an investigational medicinal product.
A trial of biological therapy in patients with sciatica still needs to be done, but would require a clearer contracting process, qualitative research to ensure that patients would be willing to participate and simpler recruitment methods.
Acknowledgements
Particular thanks go to all the SCIATiC study participants who gave their time to take part in the study.
The authors would also like to thank:
Betsi Cadwaladr University Health Board: Fiona Ford, Annabel Meayers and Anna White for screening patients and recruiting participants, the BCUHB Physiotherapy Department staff who provided support for the study and its staff who were involved.
Betsi Cadwaladr University Health Board rheumatology consultants: Yasmeen Ahmad and Sarang Chitale for supporting the study and providing access for researchers to the rheumatology clinic.
Betsi Cadwaladr University Health Board rheumatology ward staff: Anne Breslin and Catherine Owen for biologic counselling, recruiting participants and delivering the intervention.
Betsi Cadwaladr University Health Board radiology consultants: Mradul Gupta and Bethan Wyn Owen, and BCUHB radiology department staff who provided support for the study and the staff who were involved.
Betsi Cadwaladr University Health Board GP surgeries: Beech House, Denbigh; Clarence Medical Centre, Rhyl; Pen Y Bont Surgery, St Asaph; Canolfan Iechyd Amlwch, Amlwch; Plas Menai Surgery, Llanfairfechan; Canolfan Iechyd Y Felinheli, Y Felinheli; Berllan Surgery, Denbigh; St Mark’s Dee View Surgery, Deeside; Plas Meddyg Surgery, Ruthin; Madryn House Surgery, Rhyl; Bronyffynnon Surgery, Denbigh; Llanberis Surgery, Llanberis; Bradley’s Practice, Buckley; Penrhyn Bay Surgery, Llandudno; Roseneath Medical Practice, Buckley; and Longford House Surgery, Holyhead, for its contribution in identifying participants.
Health and Care Research Wales: Jayne Jones, Lucie Hobson, Hayley Tapping, Sarah Evans, Melissa Van der Bijl, Karen Jones and Arwel Jones for their contribution in patient recruitment and data collection.
North Wales Organisation for Randomised Trials in Health: particularly Lexi Bastable for constructing and maintaining the MACRO online databases, and Roumen Kountchev for construction of the remote online randomisation system. Debbie Skelhorn for quality assurance and Michelle Williams for clerical support. In addition, Rhiannon Whittaker for her contribution to the study protocol.
Sherwood Forest Hospitals NHS Foundation Trust: Debbie Bottomley, Deborah Wilson and Stephen Bliss for identifying screening participants, recruiting participants and delivering the intervention.
Sherwood Forest Hospitals NHS Foundation Trust Physiotherapy Department: to the staff who provided support for the study and its staff who were involved in the trial.
Sherwood Forest Hospitals NHS Foundation Trust rheumatology consultant: David Walsh for supporting the study and providing access for researchers to the rheumatology clinic.
Sherwood Forest Hospitals NHS Foundation Trust GP surgeries: for their contribution in identifying participants.
Royal Wolverhampton NHS Trust: Julie Edwards and Joanne Logan for screening participants.
Royal Wolverhampton NHS Trust rheumatology consultants: Hem Sapkota and Tom Sheeran for supporting the study and providing access for researchers to the rheumatology clinic.
Royal Wolverhampton NHS Trust Radiology Department staff: who provided support for the study and the staff who were involved.
Staffordshire and Stoke-on-Trent Partnership NHS Trust: Tina Hadley Barrows and Treena Larkin for screening patients and recruiting participants, and the Staffordshire and Stoke-on-Trent Partnership NHS Trust Physiotherapy Department staff who provided support for the study and its staff who were involved.
National Institute for Health Research Clinical Research Network: for its contribution in identifying participants and participant recruitment.
Keele University: Liz Hartshorne and Stephanie Butler were involved in the conduct of the study, including maintenance of trial documentation. George Tony, who provided radiological support for the study.
Independent members of the steering committee: Martin Underwood (chairperson) (Martin Underwood is an editor for the HTA journal and a member of the National Institute for Health Research Journals Library Board), Kim Burton, John O’Dowd, Elaine Buchanan and Yvonne Sylvestre. The patient representative was Jackie McCarthy.
Independent members of the DMEC: Paul Little (chairperson) [Paul Little is Programme Director of Programme Grants for Applied Research (PGfAR), Editor-in-Chief for the Programme Grants for Applied Research journal and a member of the National Institute for Health Research Journals Library Board], Karen Walker-Bone, Keith Bush and Gareth Ambler.
Contributions of authors
Nefyn H Williams (Clinical Senior Lecturer in General Practice) was the chief investigator and grant holder, was responsible for study design, conduct and analysis, led the writing of Chapters 1, 2 and 4, contributed to all other chapters, led the discussions of the implications of study findings and had overall responsibility for the study and final report.
Alison Jenkins (Trial Manager) was responsible for overseeing day-to-day conduct, contributed to all chapters of the report and contributed to the discussion of the implications of study findings.
Nia Goulden (Trial Statistician) conducted the statistical analysis for the trial, led the writing of Chapter 3 and gave feedback on other chapters of the report.
Zoe Hoare (Principal Trial Statistician) gave input to study design, was responsible for the statistical analysis design, provided methodological oversight and support for the trial statistician, and contributed to the discussion of the implications of study findings.
Dyfrig A Hughes (Professor of Health Economics) was a co-investigator, contributed to the study design, was responsible for the economic evaluation, and commented on all chapters of the final report and to the discussion of the implications of study findings.
Eifiona Wood (Senior Research Fellow in Pharmacoeconomics) was the trial health economist, commented on all chapters of the final report and contributed to the discussion of the implications of study findings.
Nadine E Foster (National Institute for Health Research Professor of Musculoskeletal Health in Primary Care) was a co-investigator, contributed to methodology and study design, commented on all chapters of the final report and contributed to discussion of the implications of study findings.
David A Walsh (Professor of Rheumatology) was a co-investigator, was responsible for study design, provided methodological oversight throughout the study, commented on all chapters of the final report and contributed to the discussion of the implications of study findings.
Dawn Carnes (Senior Lecturer in Musculoskeletal Health) was a co-investigator, and contributed to the methodology and study design.
Valerie Sparkes (Reader in Arthritis Research, Director of Impact and Innovation) was a co-investigator, contributed to the methodology and study design, and provided physiotherapy expertise.
Elaine M Hay (Professor of Community Rheumatology) contributed to the methodology and study design.
John Isaacs (Professor of Clinical Rheumatology) gave input to the study design.
Kika Konstantinou (Spinal Physiotherapy Specialist) was a co-investigator, contributed to methodology and study design, provided physiotherapy expertise, commented on all chapters of the final report and contributed to the discussion of the implications of study findings.
Dylan Morrissey (Consultant Physiotherapist, Clinical Reader) was a co-investigator, contributed to the methodology and study design, and provided physiotherapy expertise.
Jaro Karppinen (Professor of Physical and Rehabilitation Medicine) contributed to the methodology and study design.
Stephane Genevay (Head of the Multidisciplinary Back Pain Clinic) contributed to the methodology and study design.
Clare Wilkinson (Deputy Head of Research, School of Healthcare Sciences) was a co-investigator, provided feedback on study protocol and contributed to the discussion of the implications of study findings.
Data sharing statement
All available data are included as appendices or can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain?. JAMA 1992;268:760-5. https://doi.org/10.1001/jama.1992.03490060092030.
- Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine 2008;33:2464-72. https://doi.org/10.1097/BRS.0b013e318183a4a2.
- Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine 1993;18:1433-8. https://doi.org/10.1097/00007632-199309010-00006.
- Bush K, Cowan N, Katz DE, Gishen P. The natural history of sciatica associated with disc pathology. A prospective study with clinical and independent radiologic follow-up. Spine 1992;17:1205-12. https://doi.org/10.1097/00007632-199210000-00013.
- Tubach F, Beauté J, Leclerc A. Natural history and prognostic indicators of sciatica. J Clin Epidemiol 2004;57:174-9. https://doi.org/10.1016/S0895-4356(03)00257-9.
- Koes BW, van Tulder MW, Peul WC. Diagnosis and treatment of sciatica. BMJ 2007;334:1313-17. https://doi.org/10.1136/bmj.39223.428495.BE.
- Luijsterburg PA, Verhagen AP, Ostelo RW, van Os TA, Peul WC, Koes BW. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: a systematic review. Eur Spine J 2007;16:881-99. https://doi.org/10.1007/s00586-007-0367-1.
- Hospital Episode Statistics, Admitted Patient Care England, 2013–14: Procedures and Interventions. Leeds: Health and Social Care Information Centre; 2015.
- van Tulder MW, Koes BW, Bouter LM. A cost-of-illness study of back pain in the Netherlands. Pain 1995;62:233-40. https://doi.org/10.1016/0304-3959(94)00272-G.
- Mixer W, Barr J. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med 1934;211:210-15. https://doi.org/10.1056/NEJM193408022110506.
- Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disk herniation-associated radiculopathy. Semin Arthritis Rheum 1998;28:60-71. https://doi.org/10.1016/S0049-0172(98)80029-2.
- Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine 1984;9:7-15. https://doi.org/10.1097/00007632-198401000-00004.
- Ohnmeiss DD, Vanharanta H, Ekholm J. Relation between pain location and disc pathology: a study of pain drawings and CT/discography. Clin J Pain 1999;15:210-17. https://doi.org/10.1097/00002508-199909000-00008.
- Karppinen J, Malmivaara A, Tervonen O, Pääkkö E, Kurunlahti M, Syrjälä P, et al. Severity of symptoms and signs in relation to magnetic resonance imaging findings among sciatic patients. Spine 2001;26:E149-54. https://doi.org/10.1097/00007632-200104010-00015.
- Vroomen PC, de Krom MC, Slofstra PD, Knottnerus JA. Conservative treatment of sciatica: a systematic review. J Spinal Disord 2000;13:463-9. https://doi.org/10.1097/00002517-200012000-00001.
- Boswell MV, Hansen HC, Trescot AM, Hirsch JA. Epidural steroids in the management of chronic spinal pain and radiculopathy. Pain Physician 2003;6:319-34.
- Abdi S, Datta S, Trescot AM, Schultz DM, Adlaka R, Atluri SL, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician 2007;10:185-212.
- European Medicines Agency . 04/04/2012/Humira/–/EMEAHC00481-II0082./Product/Information n.d. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000481/human_med_000822.jsp&mid=WC0b01ac058001d124&murl=menus/medicines/medicines.jsp (accessed 7 August 2014).
- Cooper RG, Freemont AJ. TNF-alpha blockade for herniated intervertebral disc-induced sciatica: a way forward at last?. Rheumatology 2004;43:119-21. https://doi.org/10.1093/rheumatology/keh013.
- Lewis R, Williams N, Matar HE, Din N, Fitzsimmons D, Phillips C, et al. The clinical effectiveness and cost-effectiveness of management strategies for sciatica: systematic review and economic model. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15390.
- Williams N, Lewis R, Din NU, Matar HE, Fitzsimmons D, Phillips CJ, et al. A systematic review and meta-analysis of biological treatments targeting tumour necrosis factor alpha for sciatica. Eur Spine J 2013;22:1921-35. https://doi.org/10.1007/s00586-013-2739-z.
- Cohen S, Bogduk N, Dragovich A, Buckenmaier CC, Griffith S, Kurihara C, et al. Randomized, double-blind, placebo controlled, dose-response and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology 2009;110:1116-26. https://doi.org/10.1097/ALN.0b013e3181a05aa0.
- Genevay S, Viatte S, Finckh A, Zufferay P, Balagué F, Gabay C. Adalimumab in severe and acute sciatica: a multicenter, randomized, double-blind, placebo-controlled trial. Arthrit Rheum 2010;62:2339-46. https://doi.org/10.1002/art.27499.
- Okoro T, Tafazl S, Longworth S, Sell PJ. Tumour necrosis factor α-blocking agent (etanercept); a triple blind randomized controlled trial of its use in treatment of sciatica. J Spinal Disord Tech 2010;23:74-7. https://doi.org/10.1097/BSD.0b013e31819afdc4.
- Karppinen J, Korhonen T, Hammond A, Bowman C, Malmivaara A, Veeger N, et al. The efficacy of infliximab in sciatica induced by disc herniations located at L3/4 or L4/5: a small-scale randomized controlled trial. Open Spine J 2009;1:9-13. https://doi.org/10.2174/1876532700901010009.
- Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Bowman C, et al. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine 2006;31:2759-66. https://doi.org/10.1097/01.brs.0000245873.23876.1e.
- Cohen SP, White RL, Kurihara C, Larkin TM, Chang A, Griffith SR, et al. Epidural steroids, etanercept, or saline in subacute sciatica: a multicenter, randomized trial. Ann Intern Med 2012;156:551-9. https://doi.org/10.7326/0003-4819-156-8-201204170-00002.
- Korhonen T, Karppinen J, Malmivaara A, Autio R, Niinimäki J, Paimela L, et al. Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up. Spine 2004;29:2115-19. https://doi.org/10.1097/01.brs.0000141179.58778.6c.
- Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis 2004;63:1120-3. https://doi.org/10.1136/ard.2003.016451.
- Becker C, Heidersdorf S, Drewlo S, de Rodriguez SZ, Krämer J, Willburger RE. Efficacy of epidural perineural injections with autologous conditioned serum for lumbar radicular compression: an investigator-initiated, prospective, double-blind, reference-controlled study. Spine 2007;32:1803-8. https://doi.org/10.1097/BRS.0b013e3181076514.
- Fitzsimmons D, Phillips CJ, Bennett H, Jones M, Williams N, Lewis R, et al. Cost-effectiveness of different strategies to manage patients with sciatica. Pain 2014;155:1318-27. https://doi.org/10.1016/j.pain.2014.04.008.
- British National Formulary. London: BMJ Group and the Royal Pharmaceutical Society of Great Britain; 2012.
- Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271-3.
- EuroQol Group . EuroQol: a new facility for the measurement of health related quality of life. Health Policy 1999;16:199-208.
- A General Practice Approach to Management of Widespread Musculoskeletal Pain and Fibromyalgia. Chesterfield: Arthritis Research UK; 2003.
- Russell D, Hoare ZS, Whitaker R, Whitaker CJ, Russell IT. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:922-34. https://doi.org/10.1002/sim.4175.
- Bakhtiary A, Safavi-Farokhi Z, Rezasoltani A. Lumbar stabilizing exercises improve activities of daily living in patients with lumbar disc herniation. BMR 2005;18:55-60. https://doi.org/10.3233/BMR-2005-183-401.
- Albert H, Manniche C. The efficacy of systematic active conservative treatment for patients with severe sciatica: a single-blinded randomized clinical controlled trial. Spine 2012;37:531-42. https://doi.org/10.1097/BRS.0b013e31821ace7f.
- Konstantinou K, Beardmore R, Dunn K, Lewis M, Hider S, Sanders T, et al. Clinical course, characteristics and prognostic indicators in patients presenting with back and leg pain in primary care. The ATLAS study protocol. BMC Musculoskel Disord 2012;13. https://doi.org/10.1186/1471-2474-13-4.
- Wittes J, Brittain E. The role of internal pilot studies in increasing the efficiency of clinical trials. Stat Med 1990;9:65-71. https://doi.org/10.1002/sim.4780090113.
- Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 1983;8:141-4. https://doi.org/10.1097/00007632-198303000-00004.
- Kim M, Guilfoyle MR, Seeley HM, Laing RJ. A modified Roland–Morris disability scale for the assessment of sciatica. Acta Neurochir 2010;152:1549-53. https://doi.org/10.1007/s00701-010-0679-5.
- Grøvle L, Haugen AJ, Keller A, Natvig B, Brox JI, Grotle M. The bothersomeness of sciatica: patients’ self-report of paresthesia, weakness and leg pain. Eur Spine J 2010;19:263-9. https://doi.org/10.1007/s00586-009-1042-5.
- Lacey RJ, Lewis M, Jordan K, Jinks C, Sim J. Interrater reliability of scoring of pain drawings in a self-report health survey. Spine 2005;30:E455-8. https://doi.org/10.1097/01.brs.0000174274.38485.ee.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Ridyard C, Hughes DA. Methods for the collection of resource use data within clinical trials: a systematic review of studies funded by the UK Health Technology Assessment Program. Value Health 2010;13:867-72. https://doi.org/10.1111/j.1524-4733.2010.00788.x.
- Thorn JC, Coast J, Cohen D, Hollingworth W, Knapp M, Noble SM, et al. Resource-use measurement based on patient recall: issues and challenges for economic evaluation. Appl Health Econ Health Policy 2013;11:155-61. https://doi.org/10.1007/s40258-013-0022-4.
- Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum 2008;59:632-41. https://doi.org/10.1002/art.23563.
- Dunn KM, Croft PR. The importance of symptom duration in determining prognosis. Pain 2006;121:126-32.
- Nicholas MK. The Pain Self-Efficacy Questionnaire: taking pain into account. Eur J Pain 2007;11:153-63. https://doi.org/10.1016/j.ejpain.2005.12.008.
- Vlaeyen J, Kole-Snijders AMJ, Boren RGB, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioural performance. Pain 1995;62:363-72. https://doi.org/10.1016/0304-3959(94)00279-N.
- van Buuren S. Flexible Imputation of Missing Data. Boca Raton, FL: Chapman & Hall, CRC Press; 2012.
- Schulz K, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;152. https://doi.org/10.1136/bmj.c332.
- National Schedule of Reference Costs 2013–14. London: Department of Health; 2014.
- Noble SM, Hollingworth W, Tilling K. Missing data in trial-based cost-effectiveness analysis: the current state of play. Health Econ 2012;21:187-200. https://doi.org/10.1002/hec.1693.
- Glick H, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human use. Guideline for Good Clinical Practice E6 (R1) 1996.
- European Commission . Clinical Trials – Directive 2001 20 EC n.d. https://ec.europa.eu/health/human-use/clinical-trials/directive_en (accessed 25 September 2017).
- Association of Research Managers and Administrators . Brunswick Agreements n.d. www.arma.ac.uk/resources/brunswick-agreements (accessed 12 December 2016).
- Konstantinou K, Dunn KM, Ogollah R, Vogel S, Hay EM. ATLAS study research team . Characteristics of patients with low back and leg pain seeking treatment in primary care: baseline results from the ATLAS cohort study. BMC Musculoskelet Disord 2015;16. https://doi.org/10.1186/s12891-015-0787-8.
- ISRCTN Registry . Stratified Care for Patients With Sciatica in Primary Care 2017. www.isrctn.com/ISRCTN75449581 (accessed 2 August 2017).
Appendix 1 Demographic information for each of the eight randomised participants
| Characteristic | Treatment group, participant ID | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Adalimumab | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Site | Llandudno General Hospital | Llandudno General Hospital | King’s Mill Hospital | King’s Mill Hospital | Llandudno General Hospital | King’s Mill Hospital | King’s Mill Hospital | King’s Mill Hospital |
| Age (years) | 46 | 72 | 64 | 59 | 20 | 62 | 68 | 41 |
| Ethnicity | Welsh | British | English | English | Welsh | English | English | English |
| Sex | Male | Male | Female | Male | Female | Female | Male | Female |
| Height (cm) | 183 | 180 | 159 | 175 | 167 | 170 | 186 | 164 |
| Weight (kg) | 90 | 83 | 96 | 78 | 88 | 134 | 93 | 76 |
| Employment status | FT | PT | Retired | FT | PT | FT | Retired | FT |
| Absent from work as a result of sciatica? | Yes | No | N/A | Yes | Yes | Yes | N/A | Yes |
| Sickness certificate? | Yes | No | N/A | Yes | Yes | Yes | N/A | Yes |
Appendix 2 Scores from all of the outcome measures for each of the eight randomised participants
| Outcome measure | Treatment group, participant ID | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Adalimumab | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| ODI | ||||||||
| First clinical assessment | 66 | 36 | 78 | 66 | 76 | 54 | 36 | 60 |
| Second clinical assessment | 80 | 48 | 76 | 46 | 76 | 54 | 36 | 64 |
| 6-week follow-up | 18 | 38 | 72 | 34 | 76 | 64 | 20 | 58 |
| 6-month follow-up | 6 | Not completed | 74 | Not completed | Not completed | 64 | Not Completed | Not completed |
| Global Assessment | ||||||||
| Back at 6-week follow-up | Much better | No change | Much worse | Much better | Worse | Better | No change | Worse |
| Leg at 6-week follow-up | Better | No change | Much worse | Much better | Worse | No change | No change | No change |
| Back at 6-month follow-up | Much better | Not completed | Better | Not completed | Not completed | Better | Not completed | Not completed |
| Leg at 6-month follow-up | Better | Not completed | No change | Not completed | Not completed | No change | Not completed | Not completed |
| Sciatica Bothersomeness Index | ||||||||
| Pain in leg at first treatment | 5 | 4 | 6 | 6 | 5 | 4 | 4 | 6 |
| Numbness or tingling in leg, foot or groin at first treatment | 5 | 5 | 3 | 6 | 4 | 6 | 4 | 5 |
| Weakness in foot or leg at first treatment | 5 | 4 | 0 | 0 | 6 | 6 | 4 | 5 |
| Back or leg pain while sitting at first treatment | 4 | 6 | 6 | 6 | 5 | 4 | 4 | 5 |
| Pain in leg at 6-week follow-up | 2 | 4 | 6 | 3 | 5 | 4 | 6 | 5 |
| Numbness or tingling in leg, foot or groin at 6-week follow-up | 4 | 5 | 6 | 3 | 3 | 6 | 6 | 5 |
| Weakness in foot or leg at 6-week follow-up | 5 | 3 | 6 | 4 | 5 | 6 | 6 | 5 |
| Back or leg pain while sitting at 6-week follow-up | 2 | 5 | 5 | 2 | 6 | 4 | 4 | 5 |
| Pain in leg at 6-month follow-up | 0 | Not completed | 5 | Not completed | Not completed | 4 | Not completed | Not completed |
| Numbness or tingling in leg, foot or groin at 6-month follow-up | 4 | Not completed | 3 | Not completed | Not completed | 6 | Not completed | Not completed |
| Weakness in foot or leg at 6-month follow-up | 4 | Not completed | 0 | Not completed | Not completed | 6 | Not completed | Not completed |
| Back or leg pain while sitting at 6-month follow-up | 1 | Not completed | 6 | Not completed | Not completed | 4 | Not completed | Not completed |
| EQ-5D-5L | ||||||||
| State at first treatment | 33333 | 32231 | 43452 | 33342 | 33543 | 45433 | 32332 | 32442 |
| Score at first treatment | 0.63 | 0.76 | 0.15 | 0.46 | 0.31 | 0.38 | 0.67 | 0.37 |
| Scale at first treatment | 70 | 55 | 40 | 65 | 40 | 25 | 55 | 30 |
| State at 6-week follow-up | 21131 | 22331 | 43453 | 11231 | 43542 | 43434 | 21232 | 32332 |
| Score at 6-week follow-up | 0.88 | 0.76 | 0.12 | 0.88 | 0.19 | 0.19 | 0.75 | 0.67 |
| Scale at 6-week follow-up | 80 | 65 | 30 | 80 | 40 | 40 | 50 | 35 |
| State at 6-month follow-up | 21221 | Not completed | 43452 | Not completed | Not completed | 43434 | Not completed | Not completed |
| Score at 6-month follow-up | 0.84 | Not completed | 0.15 | Not completed | Not completed | 0.19 | Not completed | Not completed |
| Scale at 6-month follow-up | 85 | Not completed | 50 | Not completed | Not completed | 40 | Not completed | Not completed |
| Hospital Anxiety and Depression Scale | ||||||||
| Anxiety score at first treatment | 12 | 3 | 6 | 4 | 15 | 17 | 10 | 10 |
| Depression score at first treatment | 7 | 4 | 10 | 13 | 9 | 14 | 7 | 10 |
| Total score at first treatment | 19 | 7 | 16 | 17 | 24 | 31 | 17 | 20 |
| Anxiety score at 6-week follow-up | 2 | 3 | 12 | 9 | 11 | 13 | 5 | 10 |
| Depression score at 6-week follow-up | 3 | 3 | 16 | 6 | 13 | 12 | 5 | 10 |
| Total score at 6-week follow-up | 5 | 6 | 28 | 15 | 24 | 25 | 10 | 20 |
| Anxiety score at 6-month follow-up | 2 | Not completed | 6 | Not completed | Not completed | 13 | Not completed | Not completed |
| Depression score at 6-month follow-up | 1 | Not completed | 10 | Not completed | Not completed | 12 | Not completed | Not completed |
| Total score at 6-month follow-up | 3 | Not completed | 16 | Not completed | Not completed | 25 | Not completed | Not completed |
| Keele STarT Back Screening Tool | ||||||||
| First treatment | 6 | 5 | 7 | 3 | 8 | 8 | 6 | 5 |
| Subscore at first treatment | 3 | 1 | 4 | 2 | 4 | 4 | 3 | 4 |
| Tampa Scale of Kinesiophobia | ||||||||
| First treatment | 45 | Not completed | 40 | 56 | 42 | 40 | 44 | 41 |
| 6-week follow-up | 34 | 32 | 41 | 47 | 48 | 42 | 40 | 41 |
| 6-month follow-up | 38 | Not completed | 40 | Not completed | Not completed | 43 | Not completed | Not completed |
| Group Likert scale | ||||||||
| 6-week follow-up | Equally likely to be in the 0.9% sodium chloride injection group or the adalimumab injection group | Equally likely to be in the 0.9% sodium chloride injection group or the adalimumab injection group | More likely to be in the 0.9% sodium chloride injection group | Equally likely to be in the 0.9% sodium chloride injection group or the adalimumab injection group | More likely to be in the adalimumab injection group | Equally likely to be in the 0.9% sodium chloride injection group or the adalimumab injection group | Definitely in the 0.9% sodium chloride injection group | More likely to be in the adalimumab injection group |
| 6-month follow-up | More likely to be in the 0.9% sodium chloride injection group | Not completed | More likely to be in the 0.9% sodium chloride injection group | Not completed | Not completed | Equally likely to be in the 0.9% sodium chloride injection group or the adalimumab injection group | Not completed | Not completed |
| PSEQ | ||||||||
| First treatment | 26 | 48 | 8 | 17 | 9 | 14 | 30 | 9 |
| 6-week follow-up | 41 | 34 | 3 | 46 | 7 | 21 | 37 | 9 |
| 6-month follow-up | 50 | Not completed | 10 | Not completed | Not completed | 21 | Not completed | Not completed |
| RMDQ | ||||||||
| Back at first treatment | 21 | Not completed | 19 | 0 | 21 | 23 | 1 | 0 |
| Leg at first treatment | 21 | Not completed | 18 | 19 | 21 | 23 | 19 | 24 |
| Back at 6-week follow-up | 3 | Not completed | 20 | 7 | 21 | 21 | 0 | 0 |
| Leg at 6-week follow-up | Not completed | Not completed | 16 | 10 | 18 | 22 | 9 | 23 |
| Back at 6-month follow-up | 2 | Not completed | 19 | Not completed | Not completed | 21 | Not completed | Not completed |
| Leg at 6-month follow-up | 3 | Not completed | 18 | Not completed | Not completed | 21 | Not completed | Not completed |
Appendix 3 Completed pain manikins for all eight participants randomised
Appendix 4 Results from the Resource Use Questionnaire for all eight randomised participants
| Time point of assessment | Treatment group, participant ID | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Adalimumab | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| First clinical assessment | ||||||||
| Bought medicines from pharmacy or other retailer? | Yes | No | No | No | Yes | No | No | Yes |
| Cost to nearest pound (reasons related to sciatica) | 20 | N/A | N/A | N/A | 36 | N/A | N/A | 100 |
| Cost to nearest pound (other reasons) | 0 | N/A | N/A | N/A | 0 | N/A | N/A | 0 |
| Did you travel by private car for any of your visits? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Miles (reasons related to sciatica) | 25 | 0 | 22 | 3 | N/A | 28 | 3 | 10 |
| Miles (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Did you travel by bus, train or taxi for any of your visits to GP surgeries or hospital visits? | No | No | No | No | No | No | No | No |
| Cost (reasons related to sciatica) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Cost (other reasons) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Number of GP surgery visits (reasons related to sciatica) | 0 | 2 | 2 | 3 | 12 | 3 | 3 | 7 |
| Number of GP surgery visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of GP out-of-hours surgery visits (reasons related to sciatica) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of GP out-of-hours surgery visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of GP home visits (reasons related to sciatica) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of GP home visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of A&E visits (reasons related to sciatica) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Number of A&E visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of outpatient clinic visits (reasons related to sciatica) | 0 | 0 | 3 | 0 | 9 | 3 | 0 | 0 |
| Number of outpatient clinic visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of outpatient day-case visits (reasons related to sciatica) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of outpatient day-case visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of inpatient visits | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of visits to physiotherapist | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cost (£) | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Number of acupuncture sessions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cost (£) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Number of visits to osteopath/chiropractor | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Cost (£) | N/A | N/A | N/A | 37 | N/A | N/A | N/A | N/A |
| Number of other services outside hospital | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cost (£) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Your number of days off (to nearest half day) (reasons related to sciatica) | 27 | 0 | 0 | 0 | 80 | 84 | 0 | 0 |
| Lost earnings (to nearest pound) (reasons related to sciatica) | 0 | N/A | N/A | N/A | 600 | 0 | N/A | N/A |
| Your number of days off (to nearest half day) (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lost earnings (to nearest pound) (other reasons) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Family member/friend number of days off (to nearest half day) (reasons related to sciatica) | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 0 |
| Family member/friend lost earnings (to nearest pound) (reasons related to sciatica) | N/A | N/A | N/A | N/A | 700 | N/A | N/A | N/A |
| Family member/friend number of days off (to nearest half day) (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Family member/friend lost earnings (to nearest pound) (other reasons)s | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 6-week follow-up | ||||||||
| Bought medicines from pharmacy or other retailer? | No | Not completed | Yes | No | Yes | No | No | Yes |
| Cost to nearest pound (reasons related to sciatica) | N/A | Not completed | 50 | N/A | 84 | N/A | N/A | 100 |
| Cost to nearest pound (other reasons) | N/A | Not completed | 0 | N/A | 0 | N/A | N/A | 0 |
| Did you travel by private car for any of your visits? | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Miles (reasons related to sciatica) | 2 | 40 | 60 | N/A | N/A | 20 | 4 | 20 |
| Miles (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Did you travel by bus, train or taxi for any of your visits to GP surgeries or hospital visits? | No | No | No | No | No | No | No | No |
| Cost (reasons related to sciatica) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Cost (other reasons) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Number of GP surgery visits (reasons related to sciatica) | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 7 |
| Number of GP surgery visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of GP out-of-hours surgery visits (reasons related to sciatica) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of GP out-of-hours surgery visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of GP home visits (reasons related to sciatica) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of GP home visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of A&E visits (reasons related to sciatica) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Number of A&E visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of outpatient clinic visits (reasons related to sciatica) | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 |
| Number of outpatient clinic visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of outpatient day-case visits (reasons related to sciatica) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of outpatient day-case visits (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of inpatient visits | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of visits to physiotherapist | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cost (£) | N/A | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| Number of acupuncture sessions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cost (£) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Number of visits to osteopath/chiropractor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cost (£) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Number of other services outside hospital | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cost (£) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Your number of days off (to nearest half day) (reasons related to sciatica) | 0 | 0 | 0 | 0 | 120 | 42 | 0 | 18 |
| Lost earnings (to nearest pound) (reasons related to sciatica) | 0 | 0 | 0 | 0 | 950 | 0 | 0 | 0 |
| Your number of days off (to nearest half day) (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lost earnings (to nearest pound) (other reasons) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Family member/friend number of days off (to nearest half day) (reasons related to sciatica) | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Family member/friend lost earnings (to nearest pound) (reasons related to sciatica) | N/A | N/A | N/A | N/A | 240 | N/A | N/A | N/A |
| Family member/friend number of days off (to nearest half day) (other reasons) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Family member/friend lost earnings (to nearest pound) (other reasons) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 6-month follow-up visits | ||||||||
| Bought medicines from pharmacy or other retailer? | Yes | Not completed | No | Not completed | Not completed | No | Not completed | Not completed |
| Cost to nearest pound (reasons related to sciatica) | 2 | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
| Cost to nearest pound (other reasons) | N/A | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Did you travel by private car for any of your visits? | No | Not completed | Yes | Not completed | Not completed | Yes | Not completed | Not completed |
| Miles (reasons related to sciatica) | N/A | Not completed | 20 | Not completed | Not completed | 20 | Not completed | Not completed |
| Miles (other reasons) | N/A | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Did you travel by bus, train or taxi for any of your visits to GP surgeries or hospital visits? | No | Not completed | No | Not completed | Not completed | No | Not completed | Not completed |
| Cost (reasons related to sciatica) | N/A | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
| Cost (other reasons) | N/A | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
| Number of GP surgery visits (reasons related to sciatica) | 0 | Not completed | 2 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of GP surgery visits (other reasons) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of GP out-of-hours surgery visits (reasons related to sciatica) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of GP out-of-hours surgery visits (other reasons) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of GP home visits (reasons related to sciatica) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of GP home visits (other reasons) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of A&E visits (reasons related to sciatica) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of A&E visits (other reasons) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of outpatient clinic visits (reasons related to sciatica) | 0 | Not completed | 2 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of outpatient clinic visits (other reasons) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of outpatient day-case visits (reasons related to sciatica) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of outpatient day-case visits (other reasons) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of inpatient visits | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Number of visits to physiotherapist | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Cost (£) | N/A | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
| Number of acupuncture sessions | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Cost (£) | N/A | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
| Number of visits to osteopath/chiropractor | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Cost (£) | N/A | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
| Number of other services outside hospital | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Cost (£) | N/A | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
| Your number of days off (to nearest half day) (reasons related to sciatica) | 0 | Not completed | 0 | Not completed | Not completed | 43 | Not completed | Not completed |
| Lost earnings (to nearest pound) (reasons related to sciatica) | N/A | Not completed | N/A | Not completed | Not completed | 0 | Not completed | Not completed |
| Your number of days off (to nearest half day) (other reasons) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Lost earnings (to nearest pound) (other reasons) | N/A | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
| Family member/friend number of days off (to nearest half day) (reasons related to sciatica) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Family member/friend lost earnings (to nearest pound) (reasons related to sciatica) | N/A | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
| Family member/friend number of days off (to nearest half day) (other reasons) | 0 | Not completed | 0 | Not completed | Not completed | 0 | Not completed | Not completed |
| Family member/friend lost earnings (to nearest pound) (other reasons) | N/A | Not completed | N/A | Not completed | Not completed | N/A | Not completed | Not completed |
Appendix 5 Concomitant medications for all eight randomised participants
| Medication | Treatment group, participant ID | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Adalimumab | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Concomitant medications first clinical assessment | ||||||||
| Number of concomitant medications | 0 | 5 | 8 | 4 | 9 | 5 | 10 | 0 |
| Medication name |
|
|
|
|
|
|
||
| Concomitant medications first treatment | ||||||||
| Number of concomitant medications | 0 | 5 | 0 | 0 | 11 | 5 | 0 | 0 |
| Medication name |
|
|
|
|||||
| Concomitant medications second treatment | ||||||||
| Number of concomitant medications | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Medication name |
|
|||||||
Appendix 6 Physiotherapy treatment received for all eight randomised participants
| Physiotherapy treatment | Treatment group, participant ID | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Adalimumab | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Number of physiotherapy courses | 1 | Not completed | 4 | 6 | 1 | 6 | 6 | 4 |
| Advice, education and reassurance? | 0 | Not completed | 4 | 5 | 5 | 4 | 5 | 5 |
| Medication usage discussion/review? | 0 | Not completed | 1 | 0 | 2 | 1 | 2 | 0 |
| Specific exercise: stability | 0 | Not completed | 2 | 2 | 0 | 3 | 3 | 0 |
| Specific exercise: McKenzie | 1 | Not completed | 2 | 4 | 0 | 2 | 3 | 0 |
| Specific exercise: neural glides | 1 | Not completed | 0 | 4 | 0 | 0 | 3 | 1 |
| Specific exercise: other | 1 | Not completed | 2 | 1 | 1 | 4 | 3 | 2 |
| Details | Gluteus maximus maximum stretch | Not completed | Mobilisation exercises, reduced exercise | Strength and relaxation | Posture | Mobilisation exercises, balance | Iliotibial band, piriformis stretch | General mobility and flare up |
| Joint mobilisations/manipulations | 0 | Not completed | 0 | 0 | 0 | 0 | 0 | 0 |
| Soft tissue techniques | 0 | Not completed | 0 | 0 | 0 | 0 | 0 | 0 |
| Other treatment | 0 | Not completed | 2 | 4 | 5 | 0 | 4 | 3 |
| Details | N/A | Not completed | Relaxation CD, paired walking | Posture limitations and postural structure | Acupuncture | N/A | Advice on overactivity, swimming, use of heat | Relaxation and visualisation |
| Action plan for relapse discussed | Not completed | Not completed | 2 | Not completed | Not completed | 4 | 1 | Not completed |
| Outcome | Not completed | Not completed | Interface | GP | Spinal orthopaedics | GP | GP | Spinal orthopaedics |
| Comments | Patient seen for initial assessment. Given exercises, however, patient cancelled follow-up appointments | Not completed | Not completed | Not completed | Patient continued to have five further treatments of acupuncture | Not completed | Not completed | Not completed |
Appendix 7 Concomitant medications reported during physiotherapy for all eight randomised participants
| Medication | Treatment group, participant ID | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Adalimumab | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Number of concomitant medications | 1 | 8 | 7 | 5 | 10 | |||
| Medication name |
|
|
|
|
|
|||
Appendix 8 Milestones
| Project milestone | Completion date | Delay (months) | |
|---|---|---|---|
| Proposed | Actual | ||
| Year 1 | |||
| Finalise protocol and trial documentation | December 2014 | December 2014 | 0 |
| Set up TMG | September 2014 | October 2014 | 1 |
| Ethics approval | December 2014 | May 2015 | 5 |
| R&D approval | December 2014 | July 2015–August 2016 | 7–20 |
| Design patient packs and CRFs | December 2014 | April 2015 | 4 |
| Design, validation and set-up of study database | January 2015 | August 2015 (testing) | 7 |
| Randomisation set-up | February 2015 | April 2015 | 2 |
| DMEC and TSC meetings | November 2014 | DMEC: June 2015 TSC: November 2014 |
DMEC: 7 TSC: 0 |
| Physiotherapists recruited and in post | Month 1: January 2015 | BCUHB: September 2015 | 8 |
| SFHT: already in post | 0 | ||
| Keele: already in post | 0 | ||
| Cardiff: March 2016 | 14 | ||
| London: December 2015 | 11 | ||
| Set-up of centres | Month 1: January 2015 | BCUHB: site opened December 2015 | 11 |
| SFHT: site opened December 2015 | 11 | ||
| Keele: site opened July 2016 | 18 | ||
| Cardiff | Not obtained before trial closed by funder | ||
| London | Withdrew February 2016 | ||
| BCUHB: further training June, September, November 2015 | 5 | ||
| Keele: further training November 2015 | 10 | ||
| SFHT: further training July 2015 | 6 | ||
| Cardiff: further training January, March 2016 | 12 | ||
| London: further training June 2015 | 5 | ||
| Identification of potential participants | Month 1- February 2015 | BCUHB: December 2015 | 10 |
| SFHT: January 2016 | 11 | ||
| Keele: August 2016 | 18 | ||
| Cardiff | Not obtained before trial closed by funder | ||
| London | Not applicable | ||
| Telephone screening | Month 3: March 2015 | BCUHB: December 2015 | 9 |
| SFHT: February 2016 | 11 | ||
| Keele: July 2016 | 17 | ||
| Cardiff | Not obtained before trial closed by funder | ||
| London | Not applicable | ||
| Recruitment of participants – pilot study | Month 4: April 2015 | BCUHB: March 2016 | 11 |
| SFHT: February 2016 | 10 | ||
| Keele | Not obtained before trial closed by funder | ||
| Cardiff | Not obtained before trial closed by funder | ||
| London | Not applicable | ||
| Baseline and randomisation – pilot study | Month 5: May 2015 | BCUHB: March 2016 | 10 |
| SFHT: February 2016 | 9 | ||
| Keele | Not obtained before trial closed by funder | ||
| Cardiff | Not obtained before trial closed by funder | ||
| London | Not applicable | ||
| Physiotherapy clinical assessment | Month 4: April 2015 | BCUHB: March 2016 | 11 |
| SFHT: March 2016 | 11 | ||
| Keele | Not obtained before trial closed by funder | ||
| Cardiff | Not obtained before trial closed by funder | ||
| London | Not applicable | ||
| Post out 6-week follow-up – pilot study | Month 7: July 2015 | BCUHB: April 2016 | 9 |
| SFHT: April 2016 | 9 | ||
| Keele | Not obtained before trial closed by funder | ||
| Cardiff | Not obtained before trial closed by funder | ||
| London | Not applicable | ||
| Year 2 | |||
| Post out 6-month follow-up | Month 10: October 2015 | BCUHB: September 2016 | 11 |
| SFHT: August 2016 | 10 | ||
| Keele | Not obtained before trial closed by funder | ||
| Cardiff | Not obtained before trial closed by funder | ||
| London | Not applicable | ||
| Complete 6-month follow-up | Month 11: November 2015 | BCUHB: September 2016 | 10 |
| SFHT: September 2016 | 10 | ||
| Keele | Not obtained before trial closed by funder | ||
| Cardiff | Not obtained before trial closed by funder | ||
| London | Not applicable | ||
| Post out 12-month follow-up | Month 16: April 2016 | Due February 2017 | Not obtained before trial closed by funder |
| Complete 12-month follow-up | Month 17: May 2016 | Due March 2017 | Not obtained before trial closed by funder |
| Pilot study analysis review and report | Month 11: November 2015 | Eight participants recruited – November 2016 | Early termination |
| Data cleaning and preparation for analysis | Month 10: October 2015 | Eight participants recruited – November 2016 | Early termination |
| Statistical and economic analysis | Month 20: August 2016 | Eight participants recruited – November 2016 | Early termination |
| Statistical and economic write-up | Months 4 and 37: April 2015 and January 2018 | Eight participants recruited – November 2016 | Early termination |
| Data monitoring, quality assurance and cleaning | Months 6–39: July 2015–March 2018 | Trial monitored throughout set-up and during trial as per normal procedures | 0 |
| Site closure, preparation for archiving | Months 27 and 29: March 2017 and March 2018 | Owing to early termination, site closure and archiving December 2016 | Early termination |
| Year 3 | |||
| Write up of final report | Month 37: January 2018 | Eight participants recruited – November 2016 | Early termination |
| Dissemination | Month 40: April 2018 | Owing to early termination, dissemination will be January 2017 | Early termination |
Appendix 9 Progress summary
| Description | Timeline | Future action required | Current status |
|---|---|---|---|
| Finalisation of all documentation | Agreed by TSC and TMG: 26 January 2015 | None | Complete |
| Approval by Bangor University Ethics Committee | 9 March 2015 | None | Complete |
| MHRA approval | 15 April 2015 | None | Granted/complete |
| REC approval | 27 May 2015 | None | Granted/complete |
| Trial closed as a result of poor recruitment | 23 September 2016 | Report written, sites notified and regulatory bodies notified | Ongoing |
Five sites scheduled to participate in SCIATiC
Betsi Cadwaladr University Health Board: Peter Maddison Rheumatology Centre Llandudno Hospital
| Description | Timeline | Future action required | Current status |
|---|---|---|---|
| R&D approval | 9 July 2015 | None | Complete |
| Contracts signed | 8 April 2015 | None | Complete |
| Research physiotherapist | 15 September 2015 | None | In place/complete |
| Site initiation | 1 June 2015; further training provided on 28 September 2015 and 23 November 2015 | None | Complete |
| Site opened to recruitment | 8 December 2015 | None | Complete |
| Participant screening | Started 8 December 2015 | None | Complete |
| Trial participants | January 2016 | Scheduled for randomisation on 18 January 2016 – participants did not attend. First participant randomised 1 March 2016, three participants recruited | Complete |
Sherwood Forest Hospitals NHS Foundation Trust: Rheumatology Department, King’s Mill Hospital
| Description | Timeline | Future action required | Current status |
|---|---|---|---|
| R&D approval | 20 November 2015 | None | Granted/complete |
| Contracts signed | 20 October 2015 | None | Complete |
| Site initiation | 28 July 2015 | None | Complete |
| Site opened to recruitment | 8 December 2015 | None | Complete |
| Participant screening | Screening of participants in clinic started January 2016 | None | Ongoing |
| Trial participants | March 2016 | Five participants recruited | Ongoing |
Bart’s Health NHS Trust: The Royal London Hospital
| Description | Timeline | Future action required | Current status |
|---|---|---|---|
| R&D approval | Ongoing at time of site withdrawal | None | Not applicable as site withdrew |
| Contracts signed | 23 September 2015 | None | Complete |
| Research physiotherapist | Ongoing at time of site withdrawal | None | Not applicable as site withdrew |
| Initiation performed | 23 June 2015 | None | Complete |
Royal Wolverhampton NHS Trust: Cannock Chase Hospital and New Cross Hospital
| Description | Timeline | Future action required | Current status |
|---|---|---|---|
| R&D outstanding | 10 August 2016 | None | Complete |
| Contracts outstanding | 7 July 2016 | None | Complete |
| Initiation | 26 November 2015 | None | Complete |
| Site opened to recruitment | August 2016 | None | Complete |
| Participant screening | September 2016 | One participant screened; no participants randomised before trial closure | Complete |
Cardiff and Vale University Health Board
| Description | Timeline | Future action required | Current status |
|---|---|---|---|
| R&D approval | Ongoing at time of early termination of trial | Final queries to be answered by site | Outstanding |
| Contracts | Ongoing at time of early termination of trial | In discussion with Bangor University and Cardiff and Vale University Health Board | Outstanding |
| Initiation | Ongoing at time of early termination of trial | Due | Outstanding |
Appendix 10 Participant information sheets in English and Welsh
Appendix 11 Consent forms in English and Welsh
Appendix 12 Case report forms including telephone screening and baseline questionnaire
The following questionnaires were also used:
-
STarT Back Tool47
-
SBI42
-
HADS44
-
Graddfa Pryder ac Iselder Ysbyty (HADS)44
-
Pain Manikin43
-
PSEQ49
-
fear of movement using the TSK. 50
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Williams et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. UK (English) © 2009 EuroQol Group EQ-5D™ is a trade mark of the EuroQol GroupWales (Weish) © 2013 EuroQol Group EQ-5D™ is a trade mark of the EuroQol Group
List of abbreviations
- ATLAS
- Assessment and Treatment of Leg pain Associated with the Spine
- BCUHB
- Betsi Cadwaladr University Health Board
- CI
- confidence interval
- CrI
- credible interval
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, 5-level version
- ETC
- excess treatment cost
- GP
- general practitioner
- HTA
- Health Technology Assessment
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MRI
- magnetic resonance imaging
- ODI
- Oswestry Disability Index
- OR
- odds ratio
- PI
- principal investigator
- PSEQ
- Pain Self-Efficacy Questionnaire
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RMDQ
- Roland–Morris Disability Questionnaire
- SCIATiC
- Subcutaneous Injection of Adalimumab Trial compared with Control
- SCOPiC
- Sciatica Outcomes in Primary Care
- TB
- tuberculosis
- TMG
- Trial Management Group
- TNF-α
- tumour necrosis factor alpha
- TSC
- Trial Steering Committee
- WMD
- weighted mean difference