Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/144/51. The contractual start date was in September 2011. The draft report began editorial review in September 2016 and was accepted for publication in April 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Julia Boyd reports grants from the University of Edinburgh during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Walsh et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Summary of trial rationale, existing evidence, history of application (international collaboration)
Background
Red blood cell (RBC) transfusion is a widely practised intervention for critically ill patients. Approximately 85 million RBC units are transfused annually worldwide, and just under 2 million in the UK. In severe cases of anaemia or bleeding, reversal of dangerously low concentrations of haemoglobin can only be achieved by RBC transfusion. However, the practice has recognised risks, including transfusion-associated lung injury, transfusion-associated circulatory overload, bacterial contamination, infection transmission and allergic reactions, although all of these are rare. 1 Transfusion errors resulting in transfusion reactions are also an important cause of morbidity and mortality.
Meta-analysis of randomised controlled trials (RCTs) has demonstrated that ‘restrictive’ transfusion practice (using a lower haemoglobin concentration as a transfusion trigger, typically 70–80 g/l) appears to be safe and leads to a reduction in overall RBC usage. 2–4 The adoption of these restrictive haemoglobin transfusion triggers has resulted in a substantial reduction in RBC use, especially for surgery, and forms a key component of multimodal patient blood-management systems.
Donated RBCs can be stored for up to 35 days in the UK (and up to 42 days in some countries). Historically, the shelf life of RBCs was based on biochemical standards and RBC survival studies conducted in healthy volunteers. Despite the daily use of RBCs in hospitals worldwide, prior to the Age of BLood Evaluation (ABLE) trial (and other trials conducted over a similar time period) there was no high-quality clinical research to determine whether or not older stored RBCs deliver oxygen to tissues as effectively as fresher RBCs. Current standards for approval of RBC products are based on characteristics of the product, especially RBC survival in vivo at 24 hours, but not the ability of cells to transport oxygen to tissues. Prior to the ABLE trial, an accumulating body of laboratory and clinical research had raised the possibility that stored RBCs may be ineffective or even have harmful effects on patients. 5–8 None of this evidence was conclusive, but the signals seen in some uncontrolled observational clinical studies, together with the widespread use of RBC transfusions, meant that this research question was of vital importance to ensure that RBCs are used safely and effectively in the future. The implications of differences in effectiveness and safety of older versus fresher blood also have major practical and financial implications for blood services.
Transfusion laboratories actively manage RBC supplies to minimise wastage, because RBCs are a precious, limited and increasingly costly resource. As a result, RBCs stored for longer periods are frequently supplied to users, and older RBCs tend to be used first. RBCs are used for a wide range of patient groups as part of the management of a wide range of diseases and their complications. Importantly, indications for RBC transfusion range from life-threatening haemorrhage (e.g. trauma, gastrointestinal bleeding, postpartum haemorrhage) to the management of anaemia in otherwise stable patients (e.g. chronic inflammatory conditions, cancer and postoperative anaemia). Remarkably, prior to the ABLE trial, there was no high-quality evidence to reassure clinicians, blood services and patients that RBCs of all licensed storage ages are equally effective for any of these patient groups or clinical indications.
A systematic review undertaken in 2006 found only two small RCTs in adults relating to RBC transfusion. 8–10 Both trials were undertaken by members of the ABLE Canada or ABLE UK study groups. One was a study with physiological end points and the other was the feasibility study for the full ABLE trial. Neither provided conclusive information about the effectiveness and safety of stored blood in comparison with a fresher product (in total, only 89 patients were included).
Why were critically ill patients a suitable population in whom to study this question?
There was a strong rationale for undertaking research to assess the clinical effectiveness and safety of older versus fresher RBCs in critically ill patients. First, anaemia is very common and up to 95% of patients in intensive care units (ICUs) have haemoglobin values below the normal range after 2–3 days. 11,12 Second, RBC transfusion is one of the most common therapeutic interventions in the ICU;11 30–50% of all patients receive at least one RBC transfusion during their ICU stay, and a significant number receive RBC transfusions during the pre- and post-ICU hospital stay; in the UK, 10% of all RBCs are transfused to patients in general ICUs. 13 Third, there is strong evidence from a previous landmark trial [the Transfusion Requirements in Critical Care (TRICC) trial]14 that a restrictive haemoglobin transfusion trigger is safe for most critically ill patients. As a result, the use of RBCs is more consistent in the critically ill, which decreases the potential confounding effect of wide variation in RBC use among similar patient groups. Fourth, the need to improve the evidence base for RBC transfusion in critical care is widely supported by clinicians. A research priority-setting exercise undertaken by the UK Intensive Care Society had identified this research topic among active ICU clinicians as an area of urgent need and importance for further research. This led to a research topic proposal to the Health Technology Assessment (HTA) programme, submitted by the Intensive Care Society on behalf of the clinical community. These factors also indicated a willingness from the clinical community to participate in the ABLE trial in the UK. Fifth, the rationale for RBC transfusion is to restore oxygen-carrying capacity. Critically ill patients frequently have an oxygen supply–demand imbalance, so the biological plausibility of a difference in effectiveness between fresher and older stored RBCs was high. Sixth, mortality from critical illness is high, typically between 20% and 25% in UK ICUs, so this question was of particular relevance to this patient population. The incidence of morbidity that is potentially related to RBC transfusion (e.g. organ failures and infections) is also high, making these useful secondary outcomes. Seventh, critical illness is associated with high health-care costs over a prolonged period, and patients suffer significant long-term disability and reduced health-related quality of life (HRQoL). 15 This is therefore an appropriate population in which to assess cost-effectiveness of this intervention. Eighth, critically ill patients are cared for in dedicated ICUs with specialist staff experienced in the use of RBCs and undertaking complex research protocols, which meant that a trial was feasible and likely to be relatively efficient.
Changes to red blood cells during storage
Several reviews have summarised a large volume of literature characterising well-defined biochemical and cellular changes to RBCs during storage, collectively referred to as the storage lesion. 6,8,16 During storage, RBCs undergo a predictable change in structure, evolving from biconcave discs to spheroechinocytes. These changes are associated with a number of biochemical and biomechanical changes, including a depletion of adenosine triphosphate and 2,3-diphosphoglycerate (2,3-DPG), membrane phospholipid vesiculation and loss, protein oxidation, lipid peroxidation of cell membranes and loss of deformability. It is biologically highly plausible that these changes may have adverse clinical consequences by diminishing oxygen transport through capillary networks. Specifically, structural changes and the loss of cell deformability impair the ability of 8-µm RBCs to navigate the capillary networks, which typically have a smaller diameter. Even if RBCs are able to navigate capillaries, the depletion of 2,3-DPG in the stored RBCs may impair their ability to release oxygen to cells and tissues. Transfusing 2,3-DPG-depleted RBCs in primates, including humans, depletes systemic 2,3-DPG levels and shifts the oxygen dissociation curve leftwards, changes that require 24 hours to several days to reverse. Normal circulating RBCs also facilitate capillary transit by releasing nitric oxide, and this physiological effect is rapidly lost during storage.
The described changes occur at different rates over time, and can be modified but not eliminated with the use of storage solutions. Alterations are well established by 18–21 days, which is the typical age of RBCs currently transfused in the UK and other countries as standard practice. 17–19 At present, the standard used by blood providers to determine storage duration is that 75% of transfused RBCs should survive in the circulation 24 hours after transfusion. This is clearly not a measure of clinical or physiological effectiveness, but rather of physical survival. These measurements are also undertaken in relatively healthy individuals, whereas critically ill patients have an abnormal microcirculation that could further accentuate the clinical importance of the storage lesion.
Prolonged RBC storage changes the supernatant as well as the RBC. In the suspension medium, studies have noted the generation of cytokines and other bioactive substances, including histamine, complement, lipids and cytokines. 8,16 These bioactive substances may stimulate proinflammatory pathways and perhaps change flow patterns in the microcirculation. Other well-documented time-dependent changes include a progressive fall in pH, an increase in plasma potassium levels and release of free haemoglobin from lysed RBCs.
A further relevant factor relates to the use of leuco-reduced prior to storage in the blood bank. In the UK, since 1999, all RBC products must be leuco-reduced prior to storage. This was introduced to minimise the risk of variant Creutzfeldt–Jakob disease transmission, but a substantial body of evidence suggests that this may alter the nature and severity of the storage lesion. In general, evidence would suggest that the storage lesion, based on laboratory assays, is reduced by removing leucocytes from the RBC bag. A substantial proportion of the published observational literature relating to RBC storage and clinical outcomes used populations in which non-leuco-reduced RBCs were used. The generalisability of this literature to the current product was also questionable. Despite this, many countries, including Canada, where the ABLE trial was initiated, have introduced universal leuco-reduced.
In summary, prior to the ABLE trial there was substantial evidence that biochemical and structural changes during storage could decrease the ability of RBCs to transport and release oxygen through effects on oxygen uptake and release, and the process of capillary transit and storage-related accumulation of substances in the supernatant could have adverse clinical effects.
Animal evidence relating to red blood cell storage
Prior to the ABLE trial, animal models had been developed and used to test transfusion efficacy. Earlier studies demonstrated that old stored rat blood did not improve tissue oxygen consumption as compared with fresh RBCs. 20 By bleeding animals and replacing blood with RBC-free solutions (isovolaemic haemodilution), haemoglobin concentrations were so low that each RBC transfusion should result in increased uptake of oxygen by cells (a supply-dependent state). If RBCs worked properly in the supply-dependent state, there should be a noticeable decrease in serum lactate levels and an increase in oxygen consumption. Using this model, studies consistently noted that the transfusion of rat RBCs stored under standard conditions for 28 days when compared with fresh RBCs (stored for < 5 days) did not consistently improve measures of tissue oxygen consumption or hypoxia. However, it had subsequently been shown that these observations were in part explained by low post-transfusion survival of stored rat RBCs, but the experiments demonstrate the potentially limited efficacy of stored RBCs. 20,21 In the most comprehensive animal study, Raat et al. 22 compared the transfusion of 2- to 6-day-old, 2- to 3-week-old and 5- to 6-week-old human blood in a rat isovolaemic exchange model, and showed a decrease in microvascular partial pressure of oxygen in the gut with older RBCs compared with fresh and intermediate blood. However, these changes were not marked, and their clinical relevance to human disease was uncertain.
In summary, animal studies provided some evidence that older RBCs had lower efficacy to transport oxygen than fresh RBCs, but did not allow any conclusion regarding the clinical importance of these effects in humans to be made. The limitations of animal models to human disease supported the need for adequately powered effectiveness trials in a relevant human population, with clinically relevant outcome measures.
Clinical studies examining the importance of the red blood cell storage lesion
Cohort studies
Prior to the ABLE trial, a number of retrospective clinical studies had examined the association between prolonged storage times and adverse clinical outcomes. These had been reviewed shortly before the trial began. 16 These studies showed variable associations between storage age of RBCs and a wide range of adverse clinical outcomes, including mortality, pneumonia, serious infections, multiorgan failure and length of stay in many patient populations, including multiple-trauma victims, critically ill patients and patients undergoing cardiac surgical procedures. Associations between RBC storage age and adverse outcomes were not consistent across these studies, and the effect size observed among positive studies was also highly variable. The cohort study with the highest impact originated from a single-centre US cardiac study. Koch et al. 23 undertook a retrospective review of a cardiac surgery database and compared large cohorts that received either all RBCs stored for < 15 days (2872 patients) or all RBCs stored for ≥ 15 days (3130 patients). In adjusted analyses, the authors found strong associations between transfusion of older RBCs and excess in-hospital mortality, plus a range of other adverse patient outcomes (including renal failure and infection), which persisted 1 year after surgery. A major criticism of this study was that more patients in the cohort receiving older RBCs required massive transfusion (> 6 units of RBCs), which is known to be a strong predictor of adverse outcomes. The interpretation of cohort studies exploring the relationship between RBC transfusions and patient outcomes is notoriously difficult because of multiple forms of bias, including confounding by indication, variation in RBC exposure, residual confounding from unmeasured clinical factors (especially in complex populations such as the critically ill) and differences in the local RBC product used (especially leuco-reduced vs. non-leuco-reduced RBCs). These issues are particularly problematic when the effect of RBC storage duration is explored, because it is impossible to separate the known association between greater RBC exposure and adverse outcomes from the greater chance of receiving older RBCs when larger transfusions are required. 24 The confusing observational literature in this area was at best hypothesis-generating, and further justified the need for controlled trials with the current RBC product. The study by Koch et al. ,23 although inconclusive, substantially increased the importance of this question to clinicians, notably in cardiac surgery patients. The ABLE trial did not include patients undergoing uncomplicated cardiac surgery, but the importance of the question to this population was recognised in a trial undertaken concurrently with ABLE, RECESS (REd CEll Storage duration Study; NCT00991341).
Randomised controlled trials
Two RCTs in adults had been reported in the literature prior to commencing the ABLE trial. In a double-blind randomised trial, Walsh et al. 9 evaluated changes in gastric perfusion and oxygenation in 22 mechanically ventilated, critically ill patients who required a RBC transfusion in the absence of haemorrhage. The authors were not able to detect any adverse effects of older leuco-reduced RBCs (all units stored for > 20 days) on gastric intramucosal pH or arterial–gastric mucosal carbon dioxide gap when compared with fresh RBC transfusions (all units stored for < 5 days) or with baseline pre-transfusion values. These results contradicted a highly cited, uncontrolled observational before-and-after study conducted by Marik and Sibbald,25 who found an inverse relationship between the age of transfused red blood cells and gastric intramucosal pH (r = –0.71; p < 0.001), suggesting that older RBCs might reduce gastric perfusion and oxygenation. The second RCT was a pilot study undertaken in Canada (by the ABLE trial investigators), which aimed to establish the feasibility of undertaking a large RCT comparing standard storage age RBC transfusions with exclusive use of RBC transfusions stored for ≤ 7 days. 10 From the 57 patients included in the analysis, the median storage time was 4 days in the experimental group compared with 19 days in the group allocated to receive standard-aged blood [a difference of 15 days, interquartile range (IQR) of 12–16 days; p < 0.001]. Overall, 91% of patients allocated to receive fresh blood received RBCs with storage times of ≤ 7 days. There were important prognostic imbalances favouring the control group, including age and comorbidities, but no major differences in clinical outcomes or adverse events (AEs) were found. Key learning points from this study included a greater understanding of the RBC inventory processes required, the benefit of a run-in phase in each centre, the process of blinding RBC storage age from clinicians and the benefit of deferred consent at the time of the first transfusion decision.
Proposed mechanisms linking the red blood cell storage lesion with adverse clinical outcomes
Evidence is emerging that shows two general mechanisms could link prolonged RBC storage with adverse clinical outcomes, such as (1) an impaired ability of stored RBCs to transport or deliver oxygen effectively to tissues and (2) stimulation of the inflammatory cascade. A common end point of both mechanisms is organ failure, which is strongly associated with increased mortality during critical illness. RBC transfusion may trigger or worsen the systemic inflammatory response syndrome as one of the ‘multiple hits’ that typically occur during critical illness. Evidence of a direct pro-inflammatory effect from RBC transfusion could be explained by the many pro-inflammatory molecules detectable in RBC units. 8 Altered immune cell function post transfusion has been recognised for many years, and may predispose patients to further sepsis, especially nosocomial infections. Changes to RBC membrane composition, deformability and possibly nitric oxide release following prolonged storage may result in endothelial interactions that increase thrombosis, vasoconstriction and leucocyte adhesion, resulting in impaired flow, ischaemia and inflammation. Pre-storage leuco-reduction of RBC units may abrogate some of these storage effects, but it is unproven if they become clinically unimportant. A large before-and-after trial evaluating the impact of a universal pre-storage leuco-reduction programme found that rates of febrile episodes were reduced and a small (1%) mortality reduction occurred, but there was no change to infection rates. 26 Meta-analyses of trials of leuco-reduction have equivocal conclusions and do not uniformly indicate improved clinical outcomes with leuco-reduced blood. Therefore, although universal pre-storage leuco-reduction, which has been mandated since 1999 in the UK, has changed the standard RBC product, there is insufficient evidence to assume that this means that stored RBCs have identical effectiveness and safety compared with a fresher product.
Conclusions and summary of background in relation to existing research
Prior to the ABLE trial, the need for a trial was justified by a range of issues: (1) strong animal and laboratory evidence supporting the hypothesis that prolonged storage decreases RBC efficacy as an oxygen transporter and could result in deleterious clinical effects through inflammatory mechanisms; (2) observational studies in human populations reporting a number of associations between prolonged RBC storage and adverse clinical outcomes, including mortality and organ failure, but a clear recognition that this research design was inherently flawed by multiple forms of confounding and bias; and (3) the lack of completed or ongoing adequately powered trials addressing this question. In addition, the widespread use of RBCs in health care generally, and transfusion rates of 30–50% among critically ill patients, justified the need for a large, definitive trial to compare current RBC transfusion practice with exclusive use of fresher RBCs.
Context of the Age of BLood Evaluation trial in the UK
The importance of storage age of RBCs during critical illness was suggested as a topic to the National Institute for Health Research (NIHR) HTA programme and processed through the prioritisation and commissioning boards. Concurrently, the ABLE trial was funded and commenced recruitment in Canada, co-ordinated by the Ottawa Health Sciences Centre and run by the Canadian Critical Care Trials Group. The UK chief investigator (TSW) had been involved in the development and funding of the Canadian grant, but funding did not permit set-up of UK sites. The NIHR HTA programme commissioned a proposal for a UK arm of the ABLE trial through the UK Intensive Care Society, harmonised with the Canadian trial protocol, to ensure the proposed sample size was achieved. The UK proposal included a health economic evaluation, which was unique to the UK arm of the trial. This comprised a cost–utility analysis of fresh blood versus standard-aged blood and a methodological substudy to compare different versions of the EuroQol-5 Dimensions (EQ-5D) HRQoL measure in critical care patients. In addition, given the results of the primary analysis, a further substudy was subsequently added (following agreement with the funder) using the health economic data to evaluate factors associated with costs and quality-adjusted life-years (QALYs) in UK ABLE trial patients. Around the same time that the UK arm of the trial was funded, funding was obtained to extend recruitment to the Netherlands and France such that the ABLE trial became an international critical care trial supported by multiple funders and trial networks, but working to a single sample size and clinical trial protocol.
Chapter 2 Trial design and protocol
Objectives
Primary objective
The primary objective of the international ABLE trial was to answer the following research question.
In critically ill adult patients requiring RBC transfusion, does transfusing fresher RBCs stored for ≤ 7 days compared with standard-issue RBCs stored for up to 35 days decrease mortality, organ failures and new infections?
Secondary objectives
For the UK ABLE study, several additional objectives were defined.
-
To establish if, in critically ill adult patients, the use of RBCs stored for ≤ 7 days compared with standard-issue RBCs stored for up to 35 days improves HRQoL.
-
To establish the cost-effectiveness of transfusion using RBCs stored for ≤ 7 days compared with standard-issue RBCs stored for up to 35 days.
Study end points
Primary end point
Ninety-day all-cause mortality.
Secondary end points
-
Intensive care unit and hospital mortality; 28-day, and 6- and 12-month mortality; survival times.
-
Number of organ failures developing; multiple organ dysfunction score; time to development of organ failure; highest number of organ failures per patient.
-
New infections (including nosocomial pneumonia, deep-tissue infections and bacteraemia).
-
Duration of respiratory, haemodynamic and renal support.
-
Length of hospital and ICU stay.
-
Adverse event rates, including transfusion reactions.
-
Health-related quality of life at 6 and 12 months, measured using the EQ-5D.
Study design
The ABLE study was an international double-blind, multicentre, randomised clinical trial. A summary flow diagram describing the trial is shown in Figure 1.
FIGURE 1.
The design of the ABLE trial in the UK. NOK, next of kin.
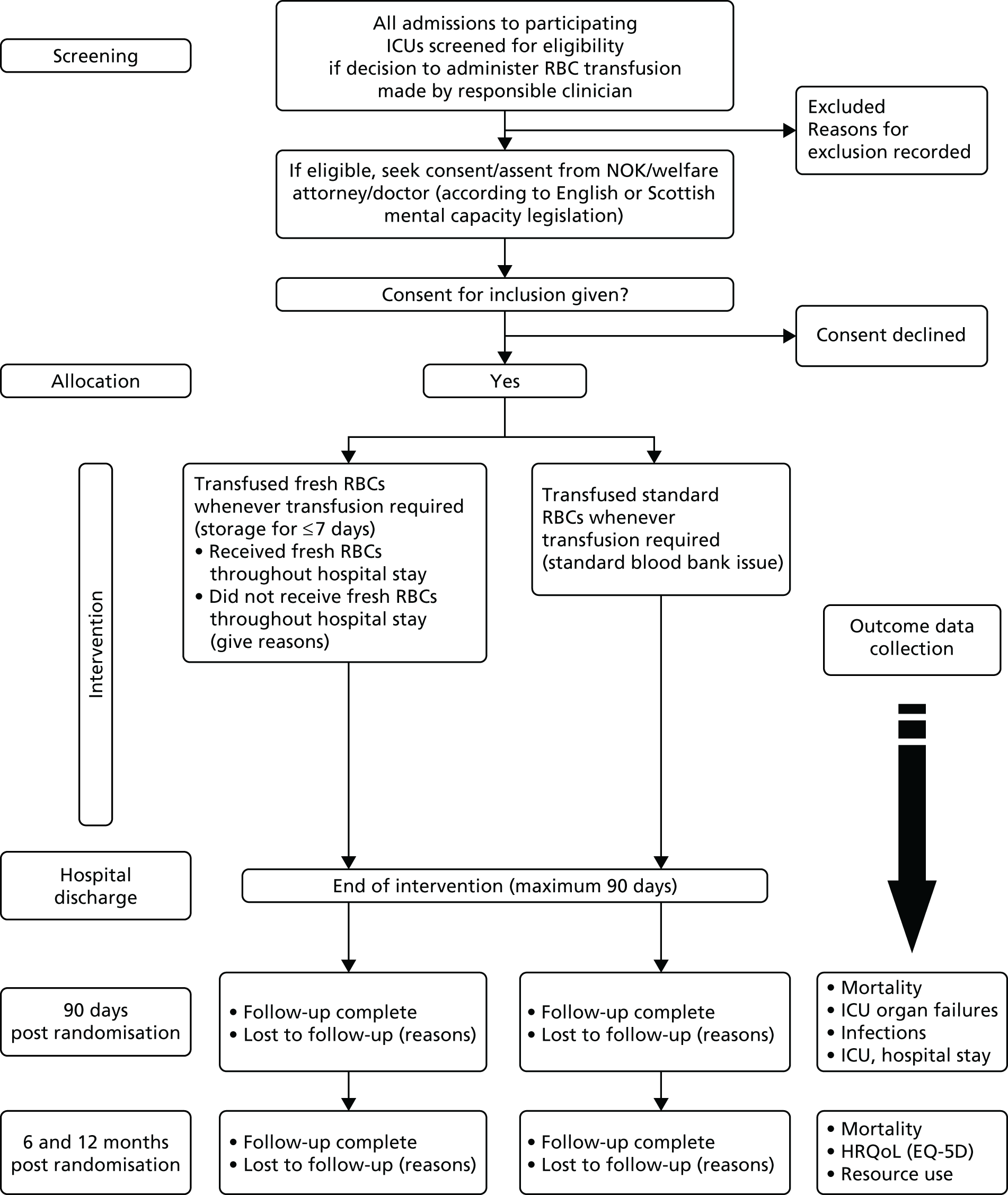
Patients were randomised to one of two groups, receiving either:
-
standard-issue RBCs (average storage age: 18–21 days)
-
red blood cells stored for ≤ 7 days (average storage age: 2–7 days).
Following randomisation, all decisions about when to transfuse were determined by caring clinicians unaware of group allocation, and there was no protocol for transfusion decision-making in the trial. The transfusion practice was, therefore, considered to represent current practice in terms of timing, triggers for transfusion and transfusion volumes. The only difference in treatment between the groups was, therefore, the storage age of RBCs used, with both groups receiving currently licensed RBC product. There were no additional blood samples or procedures beyond routine care, and all follow-up data were gathered from the patient record or from questionnaire-based follow-up.
Participant identification and selection
Study population
The ABLE trial population comprised a heterogeneous group of critically ill patients who received at least one RBC unit during the critical care phase of their illness. Only pre-storage leuco-reduced RBCs were used, because this is the standard-aged blood product in the UK and Canada, and most other health-care systems.
Inclusion criteria
All admissions to the ICU were potentially eligible for up to 7 days following admission and were tracked using screening logs for the following:
-
The patient had a request for a first RBC unit transfusion in the ICU.
-
The patient had an anticipated length of invasive and/or non-invasive mechanical ventilation (MV) of ≥ 48 hours once enrolled, as estimated by the attending physician.
Exclusion criteria
Exclusion criteria were classified into clinical criteria and transfusion laboratory criteria.
Clinical criteria
-
Patients who were aged < 16 years.
-
Patients who were previously enrolled in the ABLE trial.
-
Patients who had already been transfused with RBCs during the current hospitalisation.
-
Patients who had an obvious terminal illness documented in the medical record with a life expectancy of < 3 months.
-
Patients who had undergone routine cardiac surgical care (the proposed UK study would not take place in cardiac surgery ICUs).
-
Patients in whom a decision to withdraw/withhold critical care had been made (including patients with probable or proven brain death).
Transfusion laboratory criteria
-
No RBCs with a storage time of ≤ 7 days were available in the transfusion laboratory or could not be supplied for other reasons at the time of eligibility and potential randomisation.
-
Patients who required urgent transfusion of > 1 unit of uncross-matched RBCs.
-
Patients who had a known objection to blood transfusions.
-
Patients who planned to receive autologous-donated RBCs.
-
Patients who posed difficulties in securing blood products (i.e. those who had rare blood groups), and who were difficult to match.
Screening for eligibility
Patients eligible for the ABLE trial were identified by clinicians ordering RBC transfusion and via regular screening by research assistants/nurses. Screening logs tracking all patients admitted to the ICU who had not received a RBC transfusion were maintained by research staff to maximise recruitment potential. Reasons for non-enrolment were captured to understand barriers to enrolment and to implement strategies to improve recruitment rates.
Consent
Most patients were incapacitated at the time of eligibility (critical illness, MV or sedation), such that the Mental Capacity Act 200527 and Adults with Incapacity (Scotland) Act 200028 provided guidance. Because transfusion is usually a time-critical intervention, excessive delays in transfusion that were directly attributable to the trial could result in an enrolment bias by clinicians. In practice, the decision to enter the trial needed to be made within 1–2 hours in most cases (or sooner in cases with bleeding). Several approaches to obtaining consent were used, depending on the urgency of transfusion, mental capacity of the patient and availability of relatives. In addition, the different laws relating to research in incapacitated patients in Scotland versus the remainder of the UK influenced the processes.
-
Patients considered to have mental capacity were approached directly, and their informed consent requested.
-
For patients who lacked capacity, the approaches differed between Scottish and English/Northern Irish sites in accordance with local legislation regarding the inclusion of incapacitated patients in medical research. This also arose because the ABLE trial was not considered to be a clinical trial of an investigational medicinal product. As such, the laws governing consent differed between UK countries.
In addition, patients who survived their illness and regained capacity were approached for consent to remain in the trial. The processes used, which reflected the differences in law between England/Northern Ireland and Scotland, are summarised in Figures 2 and 3.
FIGURE 2.
Consent flow chart used to determine the appropriate method for obtaining consent in Scotland. WA, welfare attorney.

FIGURE 3.
Consent flow chart used to determine the appropriate method for obtaining consent in England and Northern Ireland.

Randomisation
Group allocation
Group allocation was concealed from clinicians and researchers involved in care of the patients in the ICU. Randomisation was undertaken by research staff within the participating ICUs, in accordance with individual site arrangements via the ABLE trial web-based randomised system based in the Ottawa Health Research Centre, modified for UK centres. However, the group allocation was restricted to the hospital transfusion laboratory in order to maintain blinding of clinical and research teams in the ICU. Following randomisation, group allocation was concealed from research and clinical staff throughout the intervention period and follow-up, and was only known to transfusion laboratory staff.
Allocation was stratified by trauma versus other critically ill patients, and by study centre. The randomisation process comprised computer-generated random listing of the treatment allocations using a pre-established minimisation algorithm. Randomisation in the UK used the existing Canadian ABLE trial randomisation system modified to include UK centres. Only the study statistician and designate at the co-ordinating centre had knowledge of the randomisation codes.
Concealment following group allocation
All RBC units issued to patients had the expiry date (and date bled) concealed by application of an adhesive label by the transfusion laboratory technician/biomedical scientist prior to issue to patients. Accompanying documentation also had any expiry dates obscured. After the expiry date was obscured, the donations had a luggage label attached identifying the unit as part of the trial. The hospital transfusion laboratory completed full documentation to ensure that the expiry date of the product had been recorded and checked prior to issue. These procedures were all approved and carried out according to agreed standard operating procedures (SOPs) in each participating transfusion laboratory.
Protection against sources of bias
The following steps were undertaken to minimise the chance of bias: (1) concealment of randomisation; (2) masking of intervention; (3) maintaining a screening log at each centre to record the number of patients screened, number not randomised and the reason for exclusion, which enabled a comparison of the characteristics of ABLE trial patients with all eligible patients; (4) blinding of ICU staff from group allocation; (5) the Data and Safety Monitoring Committee (DSMC) and executive committee remained masked from group allocation throughout the trial, by using a designated statistician to prepare all randomisation schemes and interim analyses; and (6) adjustment for major co-interventions – potentially relevant major co-interventions, such as the use of all blood products, MV and vasoactive drugs that may influence oxygen transport, were recorded prospectively.
In the UK, an audit was also undertaken of a sample of all sequential ICU admissions to participating ICUs in which RBC use was collected to enable an assessment of how the enrolled cohort compared with all ICU patients receiving RBCs during hospitalisation.
Management and data collection during the intervention
Baseline data collection
Baseline data included age, sex, hospital and ICU admission dates, type of admission, most responsible ICU admission diagnosis, Acute Physiology and Chronic Health Evaluation II (APACHE II) score at ICU admission, transfusion history during the 4 weeks prior to admission and significant comorbidities. Co-interventions administered at randomisation were recorded, including MV, dialysis/renal replacement therapy and cardiovascular support.
Transfusion decisions during the intervention period
All RBC units were prepared in accordance with international standards, and, in the UK, represented usual practice. There was no control over transfusion decisions, which were at the discretion of the caring clinician.
Duration of the intervention
The intervention was intended to last from randomisation to hospital discharge, death or 90 days post randomisation, whichever occurred soonest.
Appropriate allocation of red blood cells to patients
The conduct of the study was reliant on the operation of the individual transfusion laboratories with respect to the administration of appropriately aged RBCs to patients in the two trial arms, whenever they were requested by clinicians during the intervention period.
Issue of red blood cells
All requests for RBC units were processed and blood issued according to standard local protocols. All participating ICUs utilised existing blood stock management, which usually involved issuing RBCs closest to expiry to minimise wastage. Blood banks that provided fresh RBCs to critically ill patients as a routine were not included in the trial. All patients enrolled in the study had their randomisation allocation ‘flagged’ in the local laboratory information management system.
Masking of expiry
To ‘blind’ the true expiry date of the RBC units from clinical staff, the following changes to the standard processing of RBC units were made once the unit was ready for issue to a named patient: (1) the transfusion laboratory technician/biomedical scientist covered the expiry date (and date bled) on the blood unit label with an ‘ABLE trial sticker’, and blacked out the expiry date on the hospital cross-match compatibility tag and the issue sheet (and any other accompanying documentation that had the true expiry date of the unit); (2) a separate label was attached to the RBC unit, advising that the patient was on the ABLE trial and that the expiry date had been shortened, using the wording ‘This unit of blood has been issued for transfusion to a patient on the ABLE (Age of Blood) Study. It must be transfused by 23:59 hrs on D/M/Y** or returned to hospital transfusion laboratory. The unit must be transfused within 4 hours of removal from blood fridge’; and (3) the unit of blood was then issued and collected following standard local procedures.
Checking/administration of red blood cell units in clinical area
Checking procedures were controlled locally according to SOPs consistent with national recommendations.
Monitoring of patients during transfusion followed local agreed policies. Each unit of blood was transfused within 4 hours of removal from the satellite blood fridge/temperature-controlled storage as per national recommendations. All documentation was completed according to local protocols, including signing and recording the donor component number on transfusion documentation. Traceability documentation was completed and followed as per local policy.
Recording co-interventions during the follow-up period
All blood components received during the follow-up period for up to 90 days post randomisation were recorded, including plasma, cryoprecipitate and platelets. Immunoglobulin, albumin and other relevant blood products, including recombinant factor VIIa and prothrombin complex, and receipt of starch solutions were also recorded.
Recording of clinically relevant complications during intensive care unit and hospital follow-up
Patient complications
The occurrence and date of onset of a range of clinically relevant complications were recorded for the period from randomisation to ICU discharge. These complications comprised:
-
acute lung injury and acute respiratory distress syndrome
-
pulmonary oedema
-
cardiovascular failure, cardiac ischaemia or infarction or cardiac arrest
-
deep-vein thrombosis or pulmonary embolism
-
acute transfusion reaction
-
severe sepsis or septic shock
-
multiple organ dysfunction syndrome (MODS)
-
infections (pneumonia, surgical site infection, bacteraemia, urinary tract infection or other infections)
-
other important outcomes (specified by the investigator).
For the remainder of the intervention period after ICU discharge, additional complications were reported as AEs at the discretion of the local principal investigator. All transfusion reactions occurring during the trial were reported as serious adverse blood reactions and events and serious hazards of transfusion in accordance with usual standard reporting practices in the NHS.
Recording of other important patient data during intensive care unit and hospital follow-up
Duration of organ support
The duration of ventilatory support, cardiovascular support and renal replacement therapy was recorded.
Change in organ dysfunction
Data to calculate the MODS score were recorded on a daily basis from day 1 to 7 post randomisation and then every 7 days until ICU discharge, death or 90 days, whichever was sooner. Haemoglobin concentration was also recorded at the same time points, when available.
Level of care
For the health economic analysis the total days of ICU (level 3), high dependency (level 2) and other ward-based care were recorded for the entire index hospital stay.
Follow-up
In the UK, the duration of follow-up extended to 12 months post randomisation, or until a participant died or withdrew from the trial. This duration was longer than for the other international sites, where follow-up for 6 months was undertaken. The longer UK follow-up was undertaken for the health economic analysis.
Follow-up during intensive care unit stay and subsequent hospital stay
Follow-up data during ICU stay and remaining hospitalisation were from the patient charts, records and laboratory databases. No study-specific tests, such as additional blood samples or investigations, were required. While in the ICU, daily recording of measures of organ failure and support measures was undertaken for the first 7 days and weekly thereafter, until ICU discharge, up to a maximum of 90 days. Data on hospital mortality and date of hospital discharge were collected from hospital statistics and/or hospital charts.
Follow-up following hospital discharge, including long-term follow-up
Ninety-day survival status
Ninety-day all-cause mortality was ascertained through hospital records for patients who died in hospital. Prior to any contact with surviving patients after discharge from hospital, the patient’s general practitioner (GP) was contacted to confirm their status.
Six-month follow-up
Patients were contacted according to a SOP at 6 months (± 4 weeks). After establishing/confirming survival status with the patient’s GP, the questionnaire-based follow-up included the following measures.
-
The EQ-5D29 HRQoL questionnaire. To enable a methodological substudy comparing the EQ-5D versions (see Chapter 6), patients at half of the UK sites received the EQ-5D, three-level version (EQ-5D-3L), and the remaining patients received the EQ-5D, five-level version (EQ-5D-5L). 30
-
A health economic questionnaire. Participants were asked about health and social care resource use retrospectively since hospital discharge. Resource-use items were: GP visits in the clinic and at home, and telephone consultations; district and practice nurse visits; physiotherapist, occupational therapist and speech and language therapist visits; dietitian, home care worker (e.g. ‘Meals on Wheels’), social worker, psychological therapist, counsellor and aids and adaptation worker visits; specialist nurse visits (substance misuse nurses, Macmillan Cancer Support nurses); day hospital visits; accident and emergency (A&E) visits; outpatient appointments; and readmissions to hospital, including surgical procedures and ICU spells.
-
For patients admitted to the ICU with traumatic brain injury, the Extended Glasgow Outcome Scale (GOSE) questionnaire was administered by telephone.
Initial contact was by post, with telephone contact being attempted in the event of non-response or data queries.
Twelve-month follow-up
Patients were contacted at 12 months (± 4 weeks). After establishing survival status, a questionnaire-based follow-up included the following measures.
-
The EQ-5D HRQoL questionnaire (EQ-5D-3L and EQ-5D-5L), as at the 6-month follow-up; patients received the same EQ-5D questionnaire version at both follow-up points.
-
A health economic questionnaire. Resource items were the same as for the 6-month questionnaire, covering the period from 6 to 12 months post discharge.
Initial contact was by post, with a second posting if questionnaires were not returned. Telephone contact was attempted in the event of non-response or data queries. At both the 6- and 12-month time points, a £5 gift voucher was sent with the follow-up questionnaires as a token of appreciation for involvement in the study.
Premature withdrawal of study participants
There were no predefined withdrawal criteria for patients following trial entry. We aimed to approach all patients for consent to remain in the trial if consent/assent/non-objection for randomisation was provided by a relative, welfare attorney, next of kin, personal consultee or independent clinician. For patients who chose not to remain in the trial or who withdrew during the intervention or follow-up period, no further contact was made. We asked these patients if they were happy for us to use data collected up to that point and data that could be acquired without contacting or involving them further. If they requested for all data to be removed, all data for that patient were destroyed. The same approach was used for situations in which a relative, welfare attorney, next of kin or personal consultee requested withdrawal in the absence of a mentally capacitated patient.
Statistical and data analysis
Sample size
The ABLE trial was designed to detect an absolute risk reduction in 90-day all-cause mortality of 5% from 25% to 20% (relative risk reduction of 20%). Justifications used in developing the trial in Canada were that (1) the Trial Steering Committee [(TSC) which includes experienced critical care and transfusion triallists and clinicians], international collaborators (which included the proposed chief investigator for the UK study) and representatives of blood providers agreed that a 5% mortality difference would justify a major change of the blood procurement and distribution system; (2) a subsequent survey of Canadian intensivists indicated that clinicians thought that a study that documented a 5% improvement in 90-day all-cause mortality with fresh blood compared with standard storage-age RBCs would justify a change in the blood supply; (3) a 5% difference was a biologically plausible treatment effect based on the results of the TRICC trial (in which different exposures to standard RBCs resulted in a trend to excess mortality in liberally transfused critically ill patients) and some more recent cohort studies (notably a trial in cardiac surgery patients);14,23 and (4) a baseline 90-day mortality of 25% was relevant to critical care patients who required transfusion, and was expected in Canada and the UK based on available data.
For 80% power at a 5% significance level, the ABLE trial required to recruit 1133 patients per arm (a total of 2266) to detect a 5% absolute risk reduction from baseline 90-day mortality of 25%. This total was increased to 2510 patients (1255 per arm) assuming a non-compliance rate of 5%, which was consistent with pre-trial pilot work. The UK sample size target was 500 at the time of funding.
Analyses
The analysis plan was developed by the international ABLE TSC, led by the Ottawa Health Research Centre.
Overall approach to analysis and baseline assessment
Baseline data of patients were assessed using frequency distributions and univariate descriptive statistics, including measures of central tendency and dispersion. As this was an effectiveness trial, all statistical analyses were based on an intention-to-treat approach. As a complementary approach, a treatment received as per-protocol analysis of the primary outcome measure was also undertaken.
Analysis of primary outcome
The influence of treatment groups (fresh vs. standard-issue blood) on the primary outcome of 90-day all-cause mortality was compared using chi-squared test procedures. Unadjusted but stratified relative risks with 95% confidence intervals (CIs) for the primary comparison were calculated. Secondary analyses using logistic regression models further elucidated the measure of effect while adjusting for possible confounding variables. Independent covariates such as centre, age, sex and comorbid illnesses, and severity of illness scores, were added to all logistic models. Pairs of variables were considered for inclusion into logistic models if there was sufficient statistical evidence and the interaction had clinical rationale.
Analysis of secondary outcomes
Treatment effects on other mortality rates such as ICU and hospital mortality as well as all-cause 6-month mortality were calculated using relative risks followed by logistic regression procedures using comparable models. We also compared Kaplan–Meier survival curves using a log-rank test followed by proportional hazards modelling for all mortality rates. We compared rates of organ failure and infections using a chi-squared test statistic. Finally, we compared processes of care, including length of hospital and ICU stay, the length of time requiring respiratory, haemodynamic and renal support by using a Wilcoxon rank-sum test statistic.
Co-interventions, compliance and losses to follow-up
A number of secondary analyses were conducted to better understand the influence of co-interventions, compliance and losses to follow-up on the robustness of the intention-to-treat analysis. These included an analysis of primary and secondary outcomes including only patients who completed the study as per protocol, inclusion of co-interventions in all multivariate procedures and an ‘as-treated’ analysis comparing all patients receiving all transfusions of ≤ 7 days storage age with those patients who received all transfusions of > 7 days storage age, regardless of allocation.
The conduct of the trial and the safety of participants were overseen by the DSMC, the members of which reviewed interim analyses after each consecutive group of 500 patients had been followed for 90 days. We adopted the O’Brien–Fleming group sequential stopping rules for the four interim analyses. All data management and statistical analyses were performed by the Methods Centre at the Ottawa Hospital Research Institute. Clinical co-ordination was conducted by the Research Centre of Sainte-Justine Hospital in Montreal.
Subgroup analyses
Using the approach outlined for primary and secondary analyses, similar steps for predefined subgroups of patients were undertaken, including age, exposure to RBCs using 1–3 RBC units compared with > 3 RBC units and an evaluation of severity of illness by comparing outcomes in patients with a low (< 20 points) versus higher (≥ 20 points) APACHE II score. Comparisons by admission status (medical, surgical, trauma) were also undertaken. These analyses were primarily hypothesis-generating or explanatory in nature.
UK analysis in the context of the international Age of BLood Evaluation trial
The protocol for the international ABLE trial was published. 17 The end of the main trial occurred when the predefined sample size was reached. This cohort was analysed, and the trial was published based on follow-up data for up to 90 days (the primary end point). 31 During recruitment, it was clear that the UK target sample of 500 patients would not be achieved by the time that the international trial completed recruitment. In order to acquire sufficient data to maximise the value of the economic evaluation, we sought agreement from the international ABLE TSC, the funder (the NIHR HTA programme) and ethics committees to continue recruitment in UK centres until the main database was locked for analysis. All patients enrolled in the UK part of the trial were followed up for 12 months to collect data for the health economic evaluation.
In this report, results from the prespecified analysis restricted to all patients enrolled in the UK cohort are presented, and compared with the previously reported data from the full international ABLE trial.
Adverse events
Adverse events were expected to occur frequently among enrolled patients as a result of ongoing critical illness. AEs that were expected in this population (i.e. events that are in keeping with the patient’s underlying medical condition) were not reported as AEs. Congenital abnormalities/birth defects were not relevant to this trial. Predefined clinically relevant complications recorded in the case report form, such as organ failures and infections, were not additionally reported as AEs, with the exception of acute transfusion reactions. AEs related to blood transfusions were reported, including all acute transfusion reactions occurring during the intervention period. This approach was used consistently across the international recruiting centres.
Serious adverse events (SAEs) were expected in many of the participants, consistent with critical illness. SAEs that were expected in this population (i.e. events that are in keeping with the patient’s underlying medical condition) and those that were collected as outcomes of the trial, including death and organ failure, were not reported as SAEs. Other SAEs were reported at the discretion of the research team at each site. The period of reporting of SAEs by each site research team was from randomisation to 90 days following randomisation for the index hospital admission or until hospital discharge, whichever was sooner.
Trial management and oversight
Trial Management Group
The ABLE UK trial was co-ordinated by a Trial Management Group (TMG), which comprised the UK chief investigator (Timothy S Walsh; Critical Care Lead), the transfusion medicine lead investigator (Simon Stanworth), the ABLE trial manager (Julia Boyd, Edinburgh Clinical Trials Unit), Douglas Watson (Scottish National Blood Transfusion Service), Fiona Goddard (NHS Blood Transfusion), a critical care co-ordinator (David Hope, Edinburgh), transfusion laboratory co-ordinators (Sue Hemmatapour and Helen Burrows; Oxford) and the health economic lead (Helen Campbell, Oxford; replaced by Stephen Morris, University College London, at a late stage in recruitment).
Trial Steering Committee
As the ABLE UK trial was part of the international trial, there was no separate TSC for the UK trial. Timothy S Walsh, Simon Stanworth and Helen Campbell were members of the international ABLE TSC.
Data and Safety Monitoring Committee
As the ABLE UK trial was part of the international trial, we did not establish a separate DSMC for the UK trial.
Membership of the international ABLE TSC and DSMC has been previously published. 31
Ethics approvals
The study was conducted in accordance with the principles of the International Conference on Harmonisation Tripartite Guideline for Good Clinical Practice. A favourable ethics opinion was obtained from the Scotland A Research Ethics Committee (REC; 11/AL/0111) and the Oxford C REC (11/SC/0417). Local research and development (R&D) approvals were obtained at all sites prior to commencing recruitment.
Amendments
Four amendments were made to the protocol during the study conduct (Table 1).
| Amendment and date | Summary of amendments |
|---|---|
| Amendment 1 | |
| September 2011 | UK ABLE trial protocol submitted (original ethics approval was for the Canadian/international protocol). Main differences between the Canadian and UK protocol reflected differences in the consent process in the UK and differences in health service organisation. There were additional outcomes specific to the UK, which included a HRQoL analysis and a health economic evaluation at 6 and 12 months. Various questionnaires were also submitted: the EQ-5D, GOSE and health economic questionnaires at 6 and 12 months. Consent forms were also revised to allow access to the participant’s medical records for the purposes of monitoring or inspections |
| Amendment 2 | |
| December 2011 | Protocol revision: changes limited to section on consent – there were differing processes of consent in Scotland and England/Northern Ireland reflecting the different legislation for adults with incapacity |
| Process of consent in Scotland: deferred consent removed from protocol. In cases in which the participant was incapacitated, written or witnessed oral consent from the relative/welfare guardian would need to be provided before the patient could be enrolled. The participant would always be approached for follow-on consent when they regained capacity. These changes resulted in revised consent forms and participant information sheets | |
| Process of consent in England/Northern Ireland: changes made to the terminology to ensure compliance with the Mental Capacity Act 2005.27 These changes resulted in revised consent forms and participant information sheets | |
| Two posters were also included in this amendment in order to raise study awareness: one for staff areas and one for relative waiting areas | |
| Amendment 3 | |
| March 2013 | Protocol revision: changes to study recruitment timelines and targets – UK recruitment target lowered to 400 participants, as the international study was recruiting ahead of schedule. It was agreed with the Canadian TMG to continue recruitment in the UK for a further 180 days beyond recruitment of the last participant in the international study. This enabled data from an estimated 100 additional participants to be included in the main trial analysis report for primary outcome and facilitated approximately 100 additional cases to be enrolled in the UK-only health economic evaluation. The number of participating UK sites was also increased and details of the TSC updated |
| Amendment 4 | |
| March 2014 | Protocol revision: relating to patients recruited into the study who did not have capacity at the time of enrolment. In these cases, patients were approached for consent at the earliest opportunity once they regained capacity. In some cases, patients were discharged before the research team had the opportunity to approach for consent. A process was added to the protocol to be followed in these cases. Research teams should attempt to contact the patient after discharge as soon as was reasonably practicable and provide them with information about the study and ascertain if they consented to remain in the trial. Consent forms were also updated |
Patient and public involvement in research
As the ABLE trial was funded in the UK to participate in the international trial, we did not involve patients or the public specifically in the UK project. Local patient representatives reviewed the study materials, specifically the information sheets for participants and relatives, and provided feedback on clarity and presentation.
Chapter 3 Trial management, governance and conduct
Approvals
The UK ABLE trial required a complex set of approvals to be in place to commence recruitment. These reflected the international nature of the trial, the large number of centres involved, and the need to set up both ICUs sand blood banks to enable randomisation and intervention management (Table 2). In total, over 25 separate contracts/agreements were required to be set up and agreed between the sponsor’s legal department and various organisations. This took considerable time and effort, and resulted in delays while agreements were finalised.
| Issues | Solution |
|---|---|
| International agreements | |
| Agreement to harmonise the Canadian and UK protocol and define data ownership, publication rules, authorship and the right to utilise data | Legal agreement between the UK sponsor (University of Edinburgh/NHS Lothian) and Canadian lead site |
| Data sharing agreement to enable UK use of the relevant data in the international data set | Separate data sharing agreement developed between Canada, the University of Edinburgh (sponsor) and University College London (for the health economic evaluation) |
| UK agreements/contracts | |
| Co-sponsorship agreement between the University of Edinburgh and NHS Lothian | Legal agreement between organisations |
| Subcontracts between sponsoring site (University of Edinburgh) and University of Oxford for health economic and blood bank co-ordinators | Legal agreements between institutions |
| Contracts with each of 20 participating sites | Managed in waves, prioritising larger higher-recruiting centres first |
| Contracts with other organisations receiving grant funding for the trial, including NHS Blood and Transplant, the Scottish National Blood Transfusion Service and the Intensive Care Foundation | Legal agreements with organisations |
| Ethics and R&D approvals | |
| Scottish A REC for Scottish sites [Adults with Incapacity (Scotland) Act 2000]28 | – |
| English (Oxford) C REC (Mental Capacity Act 2005, England);27 incorporating approval for the site in Northern Ireland | – |
One practical issue related to the definition of the UK protocol. The Canadian co-ordinating centre ran the international trial and acted as sponsor for the Canadian sites, and a Canadian version of the protocol was used. In the UK, with separate funding from the NIHR HTA programme to the University of Edinburgh, the UK co-sponsors (University of Edinburgh and NHS Lothian) required a separate document representing the UK protocol that was legally distinct from the Canadian protocol, despite the unification of protocols into a single international trial. A separate UK protocol therefore needed to be written and approved by the ethics committee.
Ethics considerations
The different legal frameworks for incapacitated patients in England and Scotland required separate ethics applications and approval processes, with trial materials using appropriate terminology for surrogate decision-makers. In many international centres, a true waiver of consent was granted because the two intervention groups were both part of standard care, and the decision to transfuse RBCs was determined by clinicians and not in accordance with the trial protocol. In addition, delays to transfusion were considered unacceptable, as these might have delayed treatment and reduced patient safety. UK law did not allow this approach, but resulted in different approaches in England/Northern Ireland from those used in Scotland (see Chapter 2). These differences made recruitment more difficult for some patients in Scotland, which adversely affected recruitment rates. Approval of consent by telephone was important in Scotland to decrease this impact. In all cases, the patient was approached wherever possible to obtain permission to continue in the trial if they survived their ICU admission and regained capacity. This took considerable research nurse resource and, in some cases, was difficult to achieve before patients were discharged home. The different approaches required to obtain consent or lack of objection to participate and remain in the trial illustrate the complex and time-consuming processes involved in trials recruiting critically ill, incapacitated patients with time-sensitive recruitment windows and interventions.
Site set-up
Site set-up required both the clinical (in the ICUs) and blood bank teams to be trained and SOPs established to execute the randomisation and group allocation. Blood bank set-up was challenging, but was facilitated by specialist blood bank co-ordinators employed for the purpose of set-up, monitoring and support throughout the trial. A detailed set of protocols and procedures was developed to enable potential participants to be rapidly screened to ascertain if allocation to ‘fresh’ RBCs was feasible based on blood group, cross-match and blood availability. In addition, procedures to ensure checking, blinding and modified blood issue procedures were established. There were frequently delays in this set-up as a result of the intense pressure many NHS blood banks worked under, and competing activities such as inspections and audits. A limited number of technician staff were generally available to undertake randomisation and group allocation procedures, which limited recruitment periods to weekdays in most centres. Despite dedicated funding for this activity, many blood banks did not have access to additional staff to support the trial beyond routine NHS work. This resulted in many potential participants being ‘missed’, and limited participation to mainly patients in whom first RBC transfusions were prescribed during weekday working hours. In addition, procedures to ‘tag’ participants on local blood bank systems to ensure that subsequent requests for RBCs maintained both group allocation and blinding were necessary, but did not delay blood issue. A modified blood-checking procedure at the bedside by clinical staff that ensured that national standards were adhered to but that maintained the blinding of RBC storage age was also needed in all centres.
Although the ABLE study was a trial based in the ICU, the major logistic challenges were in the blood banks. This was the first large, multicentre UK trial that required multiple NHS blood banks to allocate trial participants to receive different blood products under emergency conditions while maintaining blinding from clinical teams. The success of the trial reflected very considerable effort and support by blood bank staff and the blood bank co-ordinators working on the trial.
Timelines
A summary of the major trial set-up timelines is shown in Figure 4. We found a wide variation between sites for times to R&D approvals, and times to recruitment of the first patient from final approval (Figure 5). The reasons were multifactorial and varied between centres. Delays with final contracts between the sponsor and study site were prevalent, as a result of the slow responses from legal teams. These resulted in a ‘knock-on’ effect on final R&D approval; this was also delayed in some centres until clinical sites were ready to start screening, to minimise the impact of NIHR metrics that recorded time to first recruit. For clinical set-up, delays in blood bank training were a major source of delay in some centres. Frequent reasons were competing priorities, such as national inspections of the laboratory service (especially the Medicines and Healthcare products Regulatory Agency), unrelated to the ABLE trial, and staff shortages such that the organisational changes required to run the trial were delayed. The funding of dedicated blood bank co-ordinators in the trial was vital to minimise these delays.
FIGURE 4.
Summary of the major logistic milestones during the set-up of the UK ABLE trial. CSP, Coordinated System for gaining NHS Permission.

FIGURE 5.
Summary of the time (days) for R&D approval and the time (days) from approval to first recruitment for the 20 ICUs that participated in the UK ABLE trial. FT, foundation trust.

Trial management
A TMG met every 4–6 weeks throughout recruitment by teleconference. These meetings were supplemented by regular investigator teleconferences with research staff (from both ICUs and blood banks), at which representatives from all sites were encouraged to share both positive and negative experiences of recruitment and trial conduct. These meetings were supplemented by regular newsletters and recruitment tables circulated by e-mail. Screening logs were returned and examined in real time. This was useful for a number of reasons: (1) it provided a rapid indication of when staffing or other problems were affecting recruitment; and (2) it provided valuable data on the reasons that potential patients were ‘missed’, which were collated and shared with all sites. This enabled a focus on solving common recruitment problems that occur across all trial sites. For example, it rapidly became apparent that the most common reason for missed recruitment was that the first transfusion took place out of hours when research staff and/or blood bank staff were unavailable. Several ICUs used local audit to change their practice and minimise night-time transfusions, consistent with current guidance.
These data enabled the reasons for non-enrolment of eligible patients to be clearly tracked during trial conduct (Table 3) and were used to implement improvements in real time.
| Reasons why eligible patients were not randomised in the ABLE UK trial | Number of patients |
|---|---|
| Insufficient time | 97 |
| Randomisation system N/A | 1 |
| Maximum number of locally active patients enrolled | 1 |
| RBCs needed urgently | 5 |
| Transfusion cancelled | 2 |
| Large-volume RBC transfusion | 9 |
| Transfused outside working hours | 957 |
| Transfused prior to screening completed | 13 |
| Transfused in operating theatre before randomisation | 27 |
| Other | 167 |
| Total | 1279 |
A continuously updated ‘top tips for recruitment’ checklist and sharing of solutions to problems was a focus of these meetings, which maintained momentum. The ‘top tips’ checklist was used as an audit tool to help each site explore whether or not they could optimise recruitment (Table 4).
| Tip | Tick if in use at your site |
|---|---|
| Study awareness | |
| Include a talk on ABLE in induction for all new staff, especially junior medical staff. We have provided a short presentation or a one-page summary of the trial that you can use | |
| Distribute ABLE lanyards with contact numbers to all new staff. These include the entry criteria and can have your contact details too | |
| Ensure ABLE posters are on display (in staff areas and relatives’ room) | |
| Mention ABLE in nursing and/or medical handovers, especially to highlight patients with low haemoglobin concentrations or needing a transfusion during the shift | |
| Add an ABLE sticker to daily charts, drug charts or other documentation for patients who are potentially eligible for ABLE | |
| Use electronic or white-board systems that are used to describe and track current patients, to highlight patients potentially eligible for ABLE | |
| Provide lists of potential ABLE patients to clinical staff prior to rounds | |
| Screening | |
| Try to screen twice per day (a.m. and p.m.) to catch new admissions | |
| Try to join some clinical rounds to highlight potentially eligible ABLE patients | |
| Encourage double-checking of blood gas haemoglobin values to minimise transfusion at night based on blood gas measurements alone | |
Review screening logs regularly to explore missed patients. Particular cases to highlight are:
|
|
| Feedback and incentives | |
| Provide positive feedback and small prizes to any staff highlighting potential ABLE patients. ABLE pens are a handy small token of appreciation; ask us if you require some more | |
| Provide certificates for nurses, doctors or others who flag a potential ABLE patient or help to recruit a patient. Suggest that these are included in continuing professional development or training files (e.g. as an e-portfolio research item) | |
| Reminder e-mails to clinical staff highlighting local recruitment, with thanks | |
| Regular reminders to staff by e-mail regarding the ABLE enrolment criteria, why the study is important, how it is going internationally and locally, how the unit is getting on in relation to recruited and missed patients . . . can you highlight why patients are being missed? | |
| Win–win strategies | |
| Use ABLE screening as local audit data. Transfusions overnight should be avoided as a quality issue in most hospitals. Feed back the number of overnight transfusions that were not urgent every month . . . try to target reductions in these linked to consideration for ABLE | |
| Feed back local transfusion data collected as ABLE screening to mortality and morbidity meetings, quality improvement meetings, etc. | |
| Engage trainees in performing local audits linked to ABLE screening | |
| If you have local transfusion protocols . . . link ABLE screening data to audit against local protocols to show compliance. Include audit against haemoglobin transfusion trigger, timing of transfusion (day/night, etc.) and any other local protocol items | |
| For research staff | |
| Reduce the number of patients being missed because they are transfused out of hours. Use strategies that detect these, and feed them back in real time to clinical teams. Use them as an educational opportunity for junior staff | |
| Try to undertake individual case review of each missed patient to define why the patient was missed and explore how this could be avoided . . . did it need education of a staff member about the trial? Try to do this when the event occurred . . . it will be more effective! | |
| Explore whether or not weekend recruitment may be feasible for clinical and blood bank teams. If it is, let us know . . . we may be able to negotiate additional payments for weekend recruits | |
| Can you identify patients who will be transfused in theatre prior to ICU admission and randomise them pre-theatre? It is possible to randomise patients pre-theatre if RBCs have been requested by the surgical or anaesthetic team. A flow chart and further information is included in the new protocol, which will be distributed soon. Consider whether or not this could be done at your site | |
| Ensure other teams are aware of the ABLE study, for example anaesthetists, A&E teams | |
| Do you have any competing trials in which there is a possibility of coenrolment? We have many coenrolment agreements in place now, but if there is a new study, please alert the trial office, which will investigate whether or not coenrolment is possible | |
| Is there any difficulty finding independent clinicians to sign the clinician agreement form? You need a balance between the number of clinicians on the delegation log and those consultants not on the log who can sign the clinician agreement form. Do you need to ‘fine tune’ or change this? | |
| For the blood bank | |
| Consider flagging potentially eligible patients in the blood bank on its IT system. Research staff can alert the blood bank when they identify a potentially eligible patient and individuals could be flagged electronically. If a request is during working hours, research staff could be contacted by the blood bank if a flag is on the patient. If out of hours, the requesting clinical staff could be asked to consider whether or not transfusion can be delayed until morning, reducing night-time non-urgent transfusions and potentially enabling enrolment the next morning | |
| If flagging is not possible on blood bank systems, ask the blood bank to call the research team during daytime hours, when non-urgent RBC requests are made for ICU patients | |
Follow-up
A predefined strategy for follow-up at 6 and 12 months post randomisation was used. First, the patient’s GP was contacted to ascertain survival status. Survivors were sent questionnaires by post, accompanied by a £5 gift token. At 12 months, non-response to postal questionnaire was followed by a second postal questionnaire. Failure to respond to postal follow-up was followed by up to three attempted contacts by telephone to complete the questionnaires. In addition, any queries were resolved by telephone contact, with up to three attempted contacts. As follow-up is known to be challenging in critical care survivors, we collected data to summarise the total time needed to achieve follow-up data. We also analysed the effectiveness of different approaches to follow-up in this population.
Audit data indicated that the average time required for each follow-up was 26 minutes at 6 months and 25 minutes at 12 months. Table 5 shows the success rate of the different follow-up strategies. Telephone contact was attempted at least three times, and this significantly improved follow-up response rates, especially at the 6-month time point.
| Process | Participants contacted for follow-up at | |
|---|---|---|
| 6 months | 12 months | |
| Total number of participants | 359 | 359 |
| Death prior to follow-up time point, n | 132 | 138 |
| Patient alive at this time point, n (%) | 227 (63) | 221 (62) |
| Site advised no follow-up was feasible, n | 2 | 2 |
| Lost to follow-up at this time point, n | 5 | 6 |
| Withdrawn from the trial at this time point, n | 18 | 19 |
| Participants sent postal questionnaire, n | 202 | 194 |
| Follow-up questionnaire returned after first postal questionnaire, survivors, n (%) | 94 (41) | 85 (39) |
| Follow-up questionnaire not returned after first postal questionnaire, survivors, n (%) | 108 (48) | 109 (49) |
| Reminder postal questionnaire sent | N/A | 107a |
| Follow-up questionnaire returned after second postal questionnaire, survivors, n (%) | N/A | 40 (18) |
| Follow-up questionnaire not returned after second postal questionnaire, survivors, n (%) | N/A | 67 (30) |
| Attempted completion via telephone: successful, survivors, n (%) | 45 (20) | 3 (1) |
| Attempted completion via telephone: unsuccessful, survivors, n (%) | 63b (28) | 66b (30) |
| Follow-up completed, survivors, n (%) | 139 (61) | 128 (58) |
Audit of blood transfusion in participating intensive care units
In order to understand how the patients recruited to the ABLE trial compared with all patients receiving blood transfusion in UK ICUs, we undertook an audit of 489 sequential ICU admissions to 15 out of 20 ABLE trial sites. Each site audited at least 30 sequential admissions, and used blood bank data to ascertain and record all RBC transfusions during the hospital stay, including the numbers of transfusions and whether or not the transfusion occurred pre, during or post ICU care.
Transfusion data were unavailable for six patients. The audit showed that 222 out of 483 (46%) of all patients admitted to the typical UK ICUs received a RBC transfusion during their hospitalisation. Transfused patients received a mean of 6.8 RBC units during hospitalisation. Data showed that 110 out of 483 (23%) patients received RBCs prior to ICU admission [49/483 (10%), exclusively pre ICU admission]. These patients were ineligible for the ABLE trial. A total of 135 out of 483 (28%) patients received RBCs during ICU stay [66/483 (14%), exclusively during ICU stay]. The mean RBC use for transfused patients during ICU care was 4.4 RBC units per patient. A total of 73 out of 483 (15%) patients received RBCs during the post-ICU discharge period [26/483 (5%), exclusively during the post-ICU period]. Many patients received RBCs at multiple time points during their hospitalisation.
These data, collected during the ABLE trial recruitment, indicated that many patients received RBCs but were either not eligible for the ABLE trial or were not included.
Concurrently, we examined the screening logs for the ABLE trial and found that of 3754 patients who received RBCs during their ICU care, only 1035 (28%) were eligible for the ABLE trial.
Together, these data clearly showed that, despite the pragmatic design of the trial, the ABLE trial population was only around 25% of ICU admissions, and this accounted for a minority of patients receiving RBCs during ICU care.
Chapter 4 Results of process of care and the clinical outcomes
The results of the international ABLE trial, which included the majority of the UK cohort of patients, have been published previously. 31 We present here a summary of the UK cohort, with comparison with effects observed in the main trial cohort. The UK cohort was underpowered for the primary and secondary trial outcomes, as expected from the outset of the project, but comparison with the main trial is useful to understand any possible differences between the UK cohort and the international trial population.
Patients
The international trial cohort was recruited between March 2009 and May 2014. The UK cohort was recruited between January 2012 and October 2014. The UK patients recruited after the international trial sample size was achieved (over a period of 6 months) were included in this summary of the UK cohort and the health economic evaluation. There were 293 patients recruited to the UK trial who were included in the main trial analysis, previously published. 31 In total, 359 patients were recruited in the UK (90% of the revised 400 patient sample size; see Figure 6 for timelines). In total, 354 patients were included in the UK cohort reported here for clinical outcomes (multiple imputation enabled the inclusion of 357 patients in the economic evaluation). These included 58 patients recruited after achievement of the international sample size. In four cases there were no primary outcome data, and in one case a patient withdrew consent.
FIGURE 6.
Recruitment accrual over time to the UK ABLE trial. The revised trial target of 400 patients was agreed with the HTA programme when the end date for the international trial was predicted.

In the international trial, 2510 patients underwent randomisation; 80 (3.2%) were withdrawn after randomisation because primary outcome data could not be obtained, leaving 2430 patients (1211 in the group allocated to receive fresh blood and 1219 in the group allocated to receive standard-aged blood) in the intention-to-treat analysis.
Baseline data were available for 2412 of the 2430 patients with primary outcome data. Of these 2430 patients, 94 (3.9%) did not receive any RBC transfusions. The overall rate of loss to follow-up was 3.2% at 90 days.
For the UK, the Consolidated Standards of Reporting Trials (CONSORT) flow diagram describing the trial is shown in Figure 7. In total, 5989 patients received a blood transfusion during the first 7 days of ICU stay. However, of these, 2081 did not meet the other inclusion criteria. Of the 3908 patients who met all inclusion criteria, 2270 were excluded in accordance with the protocol. By far the most common reason for exclusion, in 2021 patients, was the patient having had a transfusion earlier during their hospital stay. This included transfusions undertaken prior to ICU admission, for example to treat haemorrhage or perioperative blood loss. Of the 1638 patients who were eligible for randomisation, a high proportion (n = 1279, 78%) were excluded for a variety of practical reasons that meant that randomisation and group allocation were not feasible. The most common reason was transfusion at night (n = 957). In these situations, delay of transfusion to enable randomisation was not considered clinically appropriate or safe, but the randomisation procedure could not be organised either by clinical research staff or at the blood bank level. In total, 359 patients were randomised in the UK cohort, out of 1638 (22%) eligible patients according to the trial protocol. Five patients were not included in the primary analysis. In four cases, there were no primary outcome data; in one case, a patient withdrew consent. Of these 354 patients, 179 were randomised to the group allocated to receive fresh blood and 175 patients to the group allocated to receive standard-aged blood.
FIGURE 7.
The CONSORT flow diagram for the UK ABLE trial. a, No admission data from three sites. N/A, not applicable.

Baseline characteristics of the patients for the ABLE trial UK cohort, and for the overall international trial, are shown in Table 6. Patients had an age and sex profile typical of general ICU populations in the UK. The illness severity, based on APACHE II score and requirement for organ support, was high and the majority had significant levels of organ dysfunction based on the MODS score. Almost all patients were non-elective ICU admissions. The majority had a medical diagnosis, most likely to be consistent with the non-eligibility of patients receiving blood transfusion prior to ICU admission, which will include many surgical and trauma patients. The UK ABLE trial cohort was similar to the international cohort, although there were more elective ICU admissions in the UK ABLE trial cohort.
| Characteristic | Treatment group for | |||||
|---|---|---|---|---|---|---|
| The UK ABLE cohort | The international ABLE cohort | |||||
| Fresh blood (n = 179) | Standard-aged blood (n = 175) | Total (n = 354) | Fresh blood (n = 1206) | Standard-aged blood (n = 1206) | Total (n = 2412) | |
| Age (year) | 60.8 ± 15.6 | 62.3 ± 17.0 | 61.6 ± 16.3 | 61.3 ± 16.7 | 61.0 ± 16.7 | 61.2 ± 16.7 |
| Male sex | 101 (56.4) | 87 (49.7) | 188 (53.1) | 682 (56.6) | 643 (53.3) | 1325 (54.9) |
| Coexisting illness | 67 (37.4) | 71 (40.6) | 138 (39.0) | 512 (42.5) | 514 (42.6) | 1325 (54.5) |
| APACHE II score | 22.5 ± 7.3 | 21.9 ± 6.7 | 22.2 ± 7.0 | 21.9 ± 7.7 | 21.6 ± 7.6 | 21.8 ± 7.6 |
| Time from ICU admission to randomisation (days) | 3.3 ± 2.0 | 3.3 ± 1.8 | 3.3 ± 1.9 | 2.4 ± 2.0 | 2.4 ± 2.1 | 2.4 ± 2.1 |
| Time from hospitalisation to ICU admission (days) | 3.2 ± 6.4 | 3.3 ± 6.9 | 3.2 ± 6.6 | 2.6 ± 6.6 | 2.7 ± 6.5 | 2.6 ± 6.5 |
| Organ injury and support | ||||||
| MODS score | 5.5 ± 3.1 | 5.4 ± 3.0 | 5.5 ± 3.1 | 5.0 ± 3.1 | 4.7 ± 3.1 | 4.9 ± 3.1 |
| Invasive MV | 175 (97.8) | 164 (93.7) | 339 (95.8) | 1176 (97.5) | 1174 (97.3) | 2350 (97.4) |
| Renal replacement therapy | 40 (22.4) | 48 (27.4) | 88 (24.9) | 324 (26.9) | 354 (29.4) | 678 (28.1) |
| Vasoactive support | 109 (60.9) | 112 (64.0) | 221 (62.4) | 750 (62.2) | 765 (63.4) | 1515 (62.8) |
| Type of admission | ||||||
| Emergency | 166 (92.7) | 161 (92.0) | 327 (92.4) | 1164 (96.5) | 1169 (96.9) | 2333 (96.7) |
| Elective | 13 (7.3) | 14 (8.0) | 27 (7.6) | 42 (3.5) | 37 (3.1) | 79 (3.3) |
| Major admission category | ||||||
| Medical | 108 (60.3) | 112 (64.0) | 220 (62.1) | 845 (70.1) | 867 (71.9) | 1712 (71.0) |
| Surgical | 50 (27.9) | 41 (23.4) | 91 (25.7) | 175 (14.5) | 152 (12.6) | 327 (13.6) |
| Trauma | ||||||
| Brain injury | 15 (8.4) | 16 (9.1) | 31 (8.8) | 110 (9.1) | 106 (8.8) | 216 (9.0) |
| No brain injury | 6 (3.4) | 6 (3.4) | 12 (3.4) | 76 (6.3) | 80 (6.6) | 156 (6.5) |
Intervention
Table 7 shows the comparison of transfusion practice between the groups, between the UK ABLE trial and the international trial. In the international trial, a total of 5198 RBC units were given to patients in the group allocated to receive fresh blood and 5210 to patients in the group allocated to receive standard-aged blood. The pre-transfusion haemoglobin concentration was similar in the international cohort to the UK ABLE trial and the values indicated a restrictive transfusion practice consistent with current evidence. Patients received a similar number of RBC units in both groups. This was the case for the overall trial, and for the UK cohort. There was excellent separation of storage-age profile, which was similar in the UK to the international cohort. The difference between the distribution of storage age in each group was clinically relevant (see Figure 8; reproduced from full trial data). Protocol compliance was high. The rate of adherence to the transfusion protocol was 95.4% for all red blood cells transfused, with 100% of patients allocated to receive standard-aged blood receiving only standard-issue RBCs and 84.0% of patients allocated to receive fresh blood receiving only red blood cells stored for ≤ 7 days. In the group allocated to receive fresh blood, only 6.6% of the patients received > 1 RBC unit that had been stored for > 7 days, and only 4.6% received > 2 units that had been stored for > 7 days. Most patients in the group allocated to receive fresh blood [238 of 249 patients (95.6%)] who received only one RBC transfusion received exclusively RBC units that had been stored for < 8 days.
| Variable | Treatment group for | |||||
|---|---|---|---|---|---|---|
| The ABLE UK cohort | The international ABLE trial | |||||
| Fresh blood | Standard-aged blood | p-value | Fresh blood | Standard-aged blood | p-value | |
| Haemoglobin concentration in the ICU | ||||||
| Number of patients evaluated | 179 | 175 | – | 1207 | 1206 | – |
| Concentration (g/dl) before first transfusiona | 7.67 ± 0.86 | 7.77 ± 0.83 | 0.26 | 7.69 ± 1.28 | 7.64 ± 1.09 | 0.27 |
| Lowest concentration (g/dl) after randomisationa | 7.43 ± 0.72 | 7.56 ± 0.69 | 0.08 | 7.34 ± 1.46 | 7.31 ± 1.41 | 0.61 |
| RBC transfusions after randomisation | ||||||
| Patients who received at least one transfusion, n/N (%) | 175/179 (97.8) | 173/175 (98.9) | 0.68 | 1163/1207 (96.4) | 1173/1208 (97.1) | 0.31 |
| Time from randomisation to first transfusion (hours) | 8.55 ± 35.1 | 5.74 ± 22.4 | 0.37 | 10.3 ± 16.2 | 9.7 ± 16.2 | 0.43 |
| Number of RBC units per patient who received at least one transfusion | 4.0 ± 4.8 | 3.6 ± 3.7 | 0.40 | 4.3 ± 5.2 | 4.3 ± 5.5 | 0.98 |
| Duration of storage of all RBC units (days) | 5.2 ± 2.8 | 20.7 ± 7.3 | < 0.001 | 6.1 ± 4.9 | 22.0 ± 8.4 | < 0.001 |
| Adherence to transfusion protocol, n/N (%) | ||||||
| Patients | 152/175 (86.9) | 154/173 (89.0)b | – | 977/1163 (84.0) | 1206/1206 (100) | – |
| RBC units | 680/721 (94.3) | 605/637 (95.0)b | – | 4723/5198 (90.9) | 5210/5219 (100) | – |
FIGURE 8.
Distribution of RBC units in accordance with length of storage, as transfused to patients allocated to the fresh blood arm (green bars) and to the standard arm (black bars). Source: Lacroix et al. 31

Primary outcome
In the international trial, at 90 days after randomisation, 37.0% of patients in the group allocated to receive fresh blood and 35.3% of patients in the group allocated to receive standard-aged blood had died. The unadjusted and adjusted risk differences are shown in Table 8. There was no evidence of any clinically or statistically important difference between the groups. The proportion of patients who died was similar in the UK ABLE trial cohort.
| Outcome | Treatment group for the ABLE-UK cohort | Treatment group for the international ABLE trial cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Fresh blood | Standard-aged blood | Crude absolute risk difference (95% CI) | Adjusted absolute risk difference (95% CI) | Fresh blood | Standard-aged blood | Crude absolute risk difference (95% CI) | Adjusted absolute risk difference (95% CI) | |
| Patients, n/N (%) | Patients, n/N (%) | Percentage points | Percentage points | % | % | Percentage points | Percentage points | |
| Primary outcome: death by day 90 | 58/179 (32.4) | 61/175 (34.9) | –2.5 (–12.3 to 7.4) | –1.3 (–8.7 to 6.1) | 37.0 | 35.3 | 1.7 (–2.1 to 5.5) | 1.7 (–1.6 to 4.9) |
| Secondary outcomes | ||||||||
| Death in ICU | 42/179 (23.5) | 47/175 (26.9) | –3.4 (–12.4 to 5.6) | – | 27.0 | 24.2 | 2.5 (–1.0 to 5.9) | – |
| Death in hospital | 52/178 (29.2) | 59/175 (33.7) | –4.5 (–14.2 to 5.2) | – | 33.3 | 31.9 | 1.9 (–2.3 to 5.1) | – |
| Death by day 28 | 48/179 (26.8) | 53/175 (30.3) | –3.5 (–12.9 to 5.9) | – | 30.6 | 28.8 | 1.7 (–1.9 to 5.4) | – |
| Major illness | ||||||||
| MODS score | 24/179 (13.4) | 30/175 (17.1) | –3.7 (–11.2 to 3.8) | – | 13.4 | 13.0 | 0.4 (–2.3 to 3.1) | – |
| Acute respiratory distress syndrome | 10/179 (5.6) | 10/175 (5.7) | –0.1 (–4.9 to 4.7) | – | 5.7 | 6.6 | –0.9 (–2.8 to 1.0) | – |
| Cardiovascular failure | 6/179 (3.35) | 7/175 (4.0) | –0.65 (–4.6 to 3.3) | – | 5.1 | 4.2 | 0.8 (–0.8 to 2.5) | – |
| Cardiac ischaemia or infarction | 6/179 (3.35) | 6/175 (3.43) | –0.08 (–3.9 to 3.7) | – | 4.5 | 3.7 | 0.8 (–0.7 to 2.4) | – |
| Deep-vein thrombosis or pulmonary embolism | 0/179 (0) | 2/175 (1.14) | –1.1 (–2.7 to 0.4) | – | 3.6 | 3.6 | 0.0 (–1.5 to 1.5) | – |
| Nosocomial infection | 64/179 (35.75) | 52/175 (29.71) | 6.04 (–3.7 to 15.8) | – | 34.1 | 31.3 | 2.8 (–0.9 to 6.5) | – |
| Acute transfusion reaction | 2/179 (1.12) | 1/175 (0.57) | 0.55 (–1.4 to 2.5) | – | 0.3 | 0.5 | –0.2 (–0.7 to 0.3) | – |
Secondary analyses
In the international trial, the survival analysis of the time to death showed a hazard ratio in the group allocated to receive fresh blood, compared with the group allocated to receive standard-aged blood, of 1.1 (95% CI 0.9 to 1.2; p = 0.38). No significant difference in mortality was observed between the groups on the basis of follow-up duration, age, number of units transfused, APACHE II score or admission category. Differences between the groups in the UK ABLE trial cohort were similar to those in the international cohort (see Table 8).
In the international trial, no significant differences were observed with respect to major illnesses, duration of respiratory, haemodynamic or renal support, or length of stay in the ICU or hospital. There were also no differences in reported transfusion reactions. Data were comparable for the UK ABLE trial subgroup (see Table 8).
Data for other secondary outcomes are shown in Table 9. There were no differences between the groups in the international trial, and data were similar for the UK ABLE trial cohort.
| Outcome | Treatment group for the ABLE UK cohort | Treatment group for the international ABLE trial cohort | ||||
|---|---|---|---|---|---|---|
| Fresh blood | Standard-aged blood | Mean difference (95% CI) | Fresh blood | Standard-aged blood | Mean difference (95% CI) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| MODS score | ||||||
| Highest score | 7.1 ± 3.4 | 6.9 ± 3.1 | 0.2 (–0.5 to 0.9) | 6.4 ± 3.2 | 6.2 ± 3.2 | 0.2 (–0.1 to 0.4) |
| Delta score | 1.6 ± 2.0 | 1.4 ± 1.8 | 0.1 (–0.3 to 0.5) | 1.4 ± 1.8 | 1.4 ± 1.9 | –0.1 (–0.2 to 0.1) |
| Duration of supportive care (days) | ||||||
| MV | 13.8 ± 11.4 | 14.1 ± 11.2 | –0.3 (–2.6 to 2.1) | 15.0 ± 18.0 | 14.7 ± 14.9 | 0.3 (–1.1 to 1.6) |
| Cardiac or vasoactive drugs | 6.9 ± 7.6 | 6.6 ± 8.3 | 0.3 (–1.4 to 1.9) | 7.1 ± 10.2 | 7.5 ± 11.2 | –0.4 (–1.2 to 0.5) |
| Renal replacement therapy | 4.4 ± 10.1 | 4.3 ± 9.8 | 0.1 (–2.0 to 2.1) | 2.5 ± 10.1 | 2.3 ± 8.3 | 0.2 (–0.6 to 0.9) |
| Length of stay (days) | ||||||
| In ICU | 13.8 ± 9.6 | 14.3 ± 10.5 | –0.5 (–2.6 to 1.6) | 15.3 ± 15.4 | 15.3 ± 14.8 | 0.1 (–1.2 to 1.3) |
| In hospital | 32.7 ± 28.0 | 32.0 ± 31.5 | 0.7 (–5.5 to 7.0) | 34.4 ± 39.5 | 33.9 ± 38.8 | 0.5 (–2.6 to 3.7) |
The per-protocol analysis of the primary outcome, which included only patients who received a transfusion, showed no difference between the groups in the international trial, and data were comparable for the UK ABLE trial cohort. In the sensitivity analysis of the primary outcome, in which outcomes of the patients in the fresh-blood group who received only RBCs that had been stored for ≤ 7 days, were compared with the outcomes in patients in the standard-aged blood group, who received RBCs that had been stored for > 7 days, also showed no differences between the groups. Data are shown in Tables 10 and 11.
| Outcome | Treatment group for the ABLE UK cohort | Treatment group for the international ABLE trial cohort | ||||
|---|---|---|---|---|---|---|
| Fresh blood | Standard-aged blood | Crude absolute risk difference (95% CI) | Fresh blood | Standard-aged blood | Crude absolute risk difference (95% CI) | |
| Patients, n/N (%) | Patients, n/N (%) | Percentage points | % | % | Percentage points | |
| Primary outcome: death by day 90 | 57/175 (32.6) | 60/173 (34.7) | –2.1 (–12.0 to 7.8) | 36.7 | 34.2 | 2.5 (–1.4 to 6.4) |
| Outcome | Treatment group for the ABLE UK cohort | Treatment group for the international ABLE trial cohort | ||||
|---|---|---|---|---|---|---|
| Fresh blood | Standard-aged blood | Crude absolute risk difference (95% CI) | Fresh blood | Standard-aged blood | Crude absolute risk difference (95% CI) | |
| Patients, n/N (%) | Patients, n/N (%) | Percentage points | % | % | Percentage points | |
| Primary outcome: death by day 90 | 50/152 (32.9) | 54/154 (35.1) | –2.2 (–12.8 to 8.4) | 36.9 | 34.1 | 2.8 (–1.4 to 6.9) |
Chapter 5 Cost–utility analysis of fresh blood versus standard-aged blood in the Age of BLood Evaluation study
Aim
In this study we investigated the cost-effectiveness of fresh blood versus standard-aged blood in critical care patients. The sample comprised patients in the UK ABLE trial.
Methods
Overview of economic evaluation
We undertook a cost–utility analysis to compare the costs and outcomes of fresh blood versus standard-aged blood for the 359 patients in the UK arm of the ABLE trial. Two patients had no study measurements and so were not included in the analysis, giving a sample size of 357 for the cost–utility analysis. This is a slightly larger number than the sample size for the main UK analysis described in the previous chapter, made possible by the use of multiple imputation in the economic analysis to deal with missing data. Fresh blood was defined as blood donated within the past 6.1 (± SD 4.9) days and standard-aged blood within 22.0 (± SD 8.4) days. The outcome measure was QALYs, which combine length of life and quality of life, based on the National Institute for Health and Care Excellence (NICE)’s recommendations. 32 Cost-effectiveness was expressed as incremental net monetary benefits (INMBs). 32 The analysis took a UK NHS and personal social services perspective. 32 Costs were calculated in 2015 UK pounds and inflated where necessary. 33 The time horizon was 1 year, reflecting the follow-up period in the trial. Extrapolation beyond the end of the trial was not undertaken because the within-trial analysis found no evidence of significant differences in costs or benefits between groups; 1 year was long enough to reflect all important differences in costs or outcomes between treatments with fresh blood and standard-aged blood. Given the time horizon, discounting was not applied to costs or outcomes.
Resource use and costs
For every patient we calculated the cost of the index hospitalisation (from hospital admission to hospital discharge) and of follow-up to 12 months post randomisation based on resource use data collected in the trial. We included the costs of blood transfusions, fresh-frozen plasma and platelets, length of stay in hospital regular inpatient wards and in the ICU, use of MV, vasopressors, inotropes, continuous renal replacement therapy (CRRT), haemodialysis and peritoneal dialysis in the ICU. Post-discharge costs included GP visits in the clinic and at home, and telephone consultations; district and practice nurse visits; physiotherapist, occupational therapist and speech and language therapist visits; dietitian, home care worker (e.g. Meals on Wheels), social worker, psychological therapist, counsellor and aids and adaptation worker visits; specialist nurse visits (substance misuse nurses, Macmillan Cancer Support nurses); day hospital visits; A&E visits; outpatient appointments; and readmissions to hospital, including surgical procedures and ICU spells. The questionnaire used to collect the data is included in Appendix 5.
Unit costs were obtained from published sources and inflated where appropriate. 33–38 The unit costs per unit of fresh blood and standard-aged blood were assumed to be the same, as there is no differential pricing by age of blood, but the sensitivity of the findings to this assumption was investigated in the sensitivity analysis. We included blood transfusion laboratory issue costs. 34 Administration costs were assumed to be included in the ICU and inpatient costs per stay, although in any case, since there were no between-group differences in the number of blood products received, these costs would be the same in both groups and would not affect the incremental costs. Consultant, district, practice and specialist nurse, and specialist therapist visits were assumed to last 1 hour; GP clinic visits were assumed to last 11.7 minutes. Unit costs of hospital inpatient and ICU stays were daily costs applied to length of stay data collected in the trial.
Utilities and quality-adjusted life-years
Generic health status was described at 6 and 12 months post randomisation, using the EQ-5D descriptive system. 29,39 More frequent measurement was not considered to be feasible, given the challenges of complete follow-up in this group. The EQ-5D contains five dimensions: mobility, self-care, usual activities, pain and discomfort, anxiety and depression). The UK study centres were randomised to using either the EQ-5D-3L or the EQ-5D-5L at both follow-up time points (to enable the methodological substudy described in Chapter 6). Fifty per cent of centres were randomised to each version and the same version was used at both follow-up points. For each dimension, there are three levels in the EQ-5D-3L (no problems, some problems and unable to/extreme problems) and five levels in the EQ-5D-5L version (no problems, slight problems, moderate problems, severe problems and unable to/extreme problems). As patients recruited to the trial were initially critically ill, completion of the EQ-5D at randomisation was not possible. Therefore, baseline utility was assumed to be zero for all patients. This assumption has been made in previous studies in which baseline utility measurement was not possible. 40 EQ-5D health states were converted into utility values using formulae that attach weights to each level in each dimension based on valuations by general population samples. Different formulae and value sets were used to calculate utility values for the EQ-5D-3L and EQ-5D-5L, although both value sets were based on the UK general population. 30,41 Utility values of one represent full health, values of zero are equivalent to death and negative values represent states worse than death. Patients who died were assigned a utility value of zero at all subsequent time periods. A utility profile was constructed for every patient, assuming a straight-line relation between their utility values at each measurement point. QALYs for every patient from baseline to 12 months were calculated as the area under the utility profile. The implications of our assumptions were as follows: for patients who survived up to 6 months, QALYs up to 6 months were calculated using the EQ-5D scores at 6 months, assuming a EQ-5D score of zero units at baseline, and a linear interpolation between baseline and 6 months; for patients who died between baseline and 6 months, we assumed that they accrued zero QALYs; for patients who survived up to 12 months, we used a linear interpolation between EQ-5D scores at 6 and 12 months; and for patients who died between 6 and 12 months, and for whom a EQ-5D score at 6 months was available, a linear interpolation was applied between the 6-month EQ-5D score and the date of death, at which point a zero EQ-5D score was applied thereafter. We assigned all the EQ-5D data that were collected to either the 6- or 12-month measurement points, irrespective of the precise time when these were actually measured.
Dealing with missing data
The extent of missing data across all of the individual variables in the analysis ranged from 0% to 25.6%. Multiple imputation was used to impute missing data for index hospitalisation costs, costs of follow-up up to 12 months, and utility values at 6 and 12 months. Age, sex, study centre, treatment allocation, comorbidities at hospital admission [separate indicators for pre-existing significant comorbid illnesses comprising severe lung disease with symptoms at rest, previous myocardial infarction, severe heart disease with symptoms at rest, disabling stroke, chronic renal failure (dialysis dependent), type 1 or type 2 diabetes mellitus with evidence of end-organ damage, metastatic cancer, immunosuppressive therapy, chronic institutionalisation for > 6 months, deep-vein thrombosis], APACHE II score on ICU admission and MODS score on ICU admission were included in the imputation as additional explanatory variables. We used an iterative Markov chain Monte Carlo procedure based on multivariate normal regression, generating 20 imputed data sets.
Statistical methods
Mean costs, outcomes and net monetary benefits (NMBs) were compared between UK patients randomly assigned to receive fresh blood or standard-aged blood, irrespective of which treatment was administered. We calculated differences in mean costs, QALYs and INMBs between groups. NMBs for patients allocated to receive fresh (F) and standard-aged blood (S) were calculated as the mean QALYs per patient (Q) multiplied by the maximum willingness to pay for a QALY (R) minus the mean cost per patient (C):
The NMBs are likely to be negative, reflecting a relatively high cost incurred by each treatment group in the first 12 months compared with the likelihood of limited QALYs being accrued in each group over the same period. The treatment option with the highest INMB (either most positive or least negative) is preferred on cost-effectiveness grounds. The INMB was calculated as the difference in mean QALYs per patient with fresh blood versus standard-aged blood multiplied by the maximum willingness to pay for a QALY minus the difference in mean cost per patient:
We used the cost-effectiveness threshold range recommended by NICE (£20,000 to £30,000)32 as the lower and upper limits of the maximum willingness to pay for a QALY (R). If the INMB is positive (negative) then fresh blood (standard-aged blood) was preferred on cost-effectiveness grounds. QALYs gained and incremental costs were adjusted for age, sex, study centre, comorbidities, APACHE II score and MODS score using regression analysis. For each of the 20 imputed data sets, we ran 1000 bootstrap replications using non-parametric bootstrapping, resampling observations with replacement. Results were combined using equations described by Briggs et al. 42 to calculate standard errors around mean values accounting for uncertainty in imputed values, skewness of cost and utility data and sampling variation. Standard errors were used to calculate 95% CIs around point estimates.
Sensitivity analyses
A cost-effectiveness acceptability curve43 showing the probability that fresh blood was cost-effective compared with standard-aged blood at a range of values for the maximum willingness to pay for a QALY was generated based on the proportion of the bootstrap replications across all 20 imputed data sets with positive INMBs. 44 The probability that fresh blood was cost-effective at a maximum willingness to pay for a QALY of £20,000 and £30,000 was based on the proportion of bootstrap replications with positive INMBs at these values. This shows the probability that fresh blood would be cost-effective compared with standard-aged blood based on the estimated costs and outcomes in the two treatment groups and the uncertainty in these estimates. Note the probability that standard-aged blood is cost-effective is one minus this probability figure. We undertook further univariate sensitivity analyses: (1) increasing the cost of fresh blood by 25%; (2) increasing the cost of standard-aged blood by 25%; (3) changing the daily cost of non-invasive and invasive MV by ± 25%; (4) changing the daily cost of vasopressors and inotropes by ± 25%; (5) changing the daily cost of CRRT, haemodialysis and peritoneal dialysis by ± 25%; (6) changing the daily cost of ICU admission by ± 25%; and (7) changing the daily cost of an inpatient stay by ± 25%.
Results
In our sample, the mean number of units of RBCs transfused was 4.1 RBC units [standard deviation (SD) 4.9 RBC units] in the group allocated to receive fresh blood (n = 181) and 3.7 RBC units (SD 3.7 RBC units) in the group allocated to receive standard-aged blood (n = 176; Table 12). The mean number of units of fresh-frozen plasma transfused was 1.1 RBC units (SD 5.1 RBC units) in the group allocated to receive fresh blood (n = 181) and 0.6 RBC units (SD 2.1 RBC units) in the group allocated to receive standard-aged blood (n = 176). The mean number of units of platelets transfused was 0.6 RBC units (SD 2.3 RBC units) in the group allocated to receive fresh blood (n = 181) and 0.3 RBC units (SD 1.1 RBC units) in the group allocated to receive standard-aged blood (n = 176).
| Item of resource use | Trial group | Unit cost (£) | Missing (%) | |
|---|---|---|---|---|
| Fresh blood | Standard-aged blood | |||
| Blood units (all patients),a mean (SD) | ||||
| Number of units of RBCs | 4.1 (4.9), 181 | 3.7 (3.7), 176 | 121.85 | 0.6 |
| Number of units of fresh-frozen plasma | 1.1 (5.1), 181 | 0.6 (2.1), 176 | 28.46 | 0.6 |
| Number of units of platelets | 0.6 (2.3), 181 | 0.3 (1.1), 176 | 303.51 | 0.6 |
| Inpatient and ICU costs (all patients), mean (SD) | ||||
| Number of days on non-invasive MV before invasive MV | 0.4 (2.6), 181 | 0.5 (2.5), 176 | 874.88 | 0.6 |
| Number of days on invasive MV | 12.3 (9.4), 181 | 12.5 (10.8), 176 | 874.88 | 0.6 |
| Number of days on non-invasive MV after invasive MV | 1.1 (6.9), 181 | 1.0 (3.7), 176 | 874.88 | 0.6 |
| Number of days on vasopressors | 5.5 (6.8), 181 | 5.1 (6.4), 176 | 113.35 | 0.6 |
| Number of days on inotropes | 1.3 (3.5), 181 | 1.5 (3.9), 176 | 113.35 | 0.6 |
| Number of days on CRRT | 3.2 (6.4), 181 | 3.2 (6.2), 176 | 152.49 | 0.6 |
| Number of days on haemodialysis | 0.9 (7.2), 181 | 1.1 (6.2), 176 | 152.49 | 0.6 |
| Number of days on peritoneal dialysis | 0.1 (2.0), 181 | 0.01 (0.2), 176 | 152.49 | 0.6 |
| Number of days in ICU | 14.8 (9.5), 179 | 15.3 (10.5), 175 | 640.65 | 1.4 |
| Number of days in hospital | 36.9 (28.8), 178 | 36.3 (33.2), 174 | 214.00b | 1.9 |
| Primary/community care costs post discharge (survivors only), mean (SD) | ||||
| Number of GP visits in clinic | 6.3 (6.8), 76 | 3.9 (4.2), 83 | 65.00 | – |
| Number of GP visits at home | 1.0 (1.8), 76 | 1.8 (4.5), 83 | 114.00 | |
| Number of GP telephone consultations | 1.7 (2.2), 76 | 1.6 (2.3), 83 | 27.00 | |
| Number of district nurse visits (community nurse) | 11.6 (32.5), 76 | 12.5 (29.6), 83 | 67.00 | |
| Number of practice nurse visits | 2.8 (5.3), 76 | 3.4 (8.8), 83 | 56.00 | |
| Number of NHS physiotherapist visits (hospital) | 3.0 (5.8), 76 | 2.9 (6.7), 83 | 38.00 | |
| Number of occupational therapist (community) visits | 1.4 (5.5), 76 | 1.7 (4.9), 83 | 44.00 | |
| Number of speech and language therapist (hospital) visits | 0.9 (4.9), 76 | 0.7 (4.6), 83 | 38.00 | |
| Number of dietitian visits | 1.3 (2.5), 76 | 0.6 (1.7), 83 | 38.00 | |
| Number of home care worker visits | 13.5 (57.1), 76 | 19.4 (107.9), 83 | 24.00 | |
| Number of social worker visits | 0.4 (1.4), 76 | 0.4 (1.2), 83 | 57.00 | |
| Number of psychological therapist (psychologist, psychiatrist, psychology counsellor) visits | 1.2 (5.3), 76 | 0.6 (2.0), 83 | 139.00 | |
| Number of counsellor visits | 0.7 (3.2), 76 | 0.1 (0.7), 83 | 44.00 | |
| Number of day hospital visits | 1.6 (4.8), 76 | 1.9 (12.2), 83 | 113.00 | |
| Number of aids and adaptations worker visits | 0.5 (1.2), 76 | 0.7 (1.7), 83 | 57.00 | |
| Number of substance misuse nurse visits | 0.03 (0.3), 76 | 0.1 (0.5), 83 | 56.00 | |
| Number of Macmillan Cancer Support nurse visits | 0.2 (1.1), 76 | 0.0 (0.0), 83 | 25.00 | |
| Number of other types of visit | 1.0 (4.4), 76 | 11.0 (80.2), 83 | Various | |
| Secondary care costs post discharge (survivors only) | ||||
| Number of A&E visits, mean (SD) | 0.7 (1.3), 76 | 0.9 (2.8), 83 | 140.59 | – |
| Number of outpatient clinic visits, mean (SD) | 4.6 (4.6), 76 | 6.0 (8.2), 83 | 113.00 | |
| Number (%) of patients readmitted to hospitalc | ||||
| 0 times | 44 (58), 76 | 55 (66), 83 | – | – |
| 1 time | 16 (21), 76 | 13 (16), 83 | – | |
| 2 times | 8 (11), 76 | 4 (5), 83 | – | |
| 3 times | 6 (8), 76 | 5 (6), 83 | – | |
| 4 times | 2 (3), 76 | 2 (2), 83 | – | |
| 5 times | 0 (0), 76 | 4 (5), 83 | – | |
| Number of days in hospital if readmitted, mean (SD) | 8.0 (15.5), 60d | 7.9 (16.4), 66c | 530–641e | |
The mean length of stay in hospital was 36.9 days (SD 28.8 days) in the group allocated to receive fresh blood and 36.3 days (SD 33.2 days) in the group allocated to receive standard-aged blood. Mean length of stay in the ICU was 14.8 days (SD 9.5 days) in the group allocated to receive fresh blood (n = 179) and 15.3 days (SD 10.5 days) in the group allocated to receive standard-aged blood (n = 175). While in the ICU, the mean length of use of invasive MV was 12.3 days (SD 9.4 days) in the group allocated to receive fresh blood (n = 181) and 12.5 days (SD 10.8 days) in the group allocated to receive standard-aged blood (n = 176). The mean number of days on vasopressors and inotropes was 5.5 days (SD 6.8 days) and 1.3 days (SD 3.5 days), respectively, in the group allocated to receive fresh blood and 5.1 days (SD 6.4 days) and 1.5 days (SD 3.9 days), respectively, in the group allocated to receive standard-aged blood. Mean number of days of CRRT was 3.2 days (SD 6.4 days) in the group allocated to receive fresh-blood (n = 181) and 3.2 days (SD 6.4 days) in the group allocated to receive standard-aged blood (n = 176).
Post discharge, surviving patients in both groups used primary care and community care services. The mean number of GP visits in the clinic up to 12 months was 6.3 visits (SD 6.8 visits) in the group allocated to receive fresh blood (n = 76) and 3.9 visits (SD 4.2 visits) in the group allocated to receive standard-aged blood (n = 83). GP visits at home or GP telephone consultations were less frequent. The mean number of district nurse visits was 11.6 visits (SD 32.5 visits) in the group allocated to receive fresh blood group and 12.5 visits (SD 29.6 visits) in the group allocated to receive standard-aged blood. The mean number of home care worker visits was 13.5 visits (SD 57.1 visits) in the group allocated to receive fresh blood and 19.4 visits (SD 107.9 visits) in the group allocated to receive standard-aged blood. Less frequent in both groups were visits to a practice or specialist nurse, physiotherapist, psychologist, occupational therapist, speech and language therapist, dietitian, counsellor or social worker. Post discharge, patients in both groups used secondary care services. The mean number of A&E visits was 0.7 visits (SD 1.3 visits) in the group allocated to receive fresh blood group and 0.9 visits (SD 2.8 visits) in the group allocated to receive standard-aged blood. The mean number of outpatient clinic visits was 4.6 visits (SD 4.6 visits) in the group allocated to receive fresh blood and 6.0 visits (SD 8.2 visits) in the group allocated to receive standard-aged blood. In the group allocated to receive fresh blood, 42% of patients were readmitted to hospital at least once for a mean period of 8.0 days; in the group allocated to receive standard-aged blood, these figures were 34% and 7.9 days.
Accounting for missing data, the mean total cost per patient was £32,346 (95% CI £29,306 to £35,385) in patients receiving fresh blood (n = 181) and £33,353 (95% CI £29,729 to £36,978) in patients receiving standard-aged blood (n = 176; Table 13). In both groups, approximately 85% of the total costs were incurred during the index hospital admission and 15% during follow-up.
| Variable | Trial group, mean (95% CI) | |
|---|---|---|
| Fresh blood | Standard-aged blood | |
| Cost (£) of index admission | 28,158 (25,646 to 30,669) | 28,666 (25,589 to 31,743) |
| Cost (£) of follow-up | 4188 (2599 to 5778) | 4687 (2961 to 6413) |
| Total cost (£) | 32,346 (29,306 to 35,385) | 33,353 (29,729 to 36,978) |
| Utility values | ||
| Baseline | 0 (0 to 0) | 0 (0 to 0) |
| 6 months | 0.273 (0.205 to 0.340) | 0.284 (0.222 to 0.345) |
| 12 months | 0.284 (0.217 to 0.351) | 0.283 (0.221 to 0.346) |
| QALYs | 0.207 (0.158 to 0.256) | 0.213 (0.170 to 0.257) |
| NMB (£) | ||
| 20,000 | –28,201 (–31,283 to –25,119) | –29,102 (–32,596 to –25,609) |
| 30,000 | –26,128 (–29,345 to –22,911) | –26,978 (–30,489 to –23,465) |
Mean utility values were similar for the two groups and over time (see Table 13). Mean total QALYs per patient were 0.207 (95% CI 0.158 to 0.256) in the group allocated to receive fresh blood and 0.213 in the group allocated to receive standard-aged blood (95% CI 0.170 to 0.257).
As expected, at a maximum willingness to pay for a QALY of £20,000, the NMBs for the group allocated to receive fresh blood and the group allocated to receive standard-aged blood were negative: –£28,201 (95% CI –£31,323 to –£25,119) and –£29,102 (95% CI –£32,596 to –£25,609), respectively (see Table 13). Furthermore, at a maximum willingness to pay for a QALY of £30,000, the NMBs for the group allocated to receive fresh blood and the group allocated to receive standard-aged blood were negative: –£26,128 (95% CI –£29,345 to –£22,911) and –£26,978 (95% CI –£30,489 to –£23,465), respectively (see Table 13).
Mean costs and QALYs and NMBs were similar for complete cases (Table 14), with 30–50% missing data on total costs and QALYs.
| Variable | Trial group | |||
|---|---|---|---|---|
| Fresh blood | Standard-aged blood | |||
| Mean (95% CI) | Missing, n (%) | Mean (95% CI) | Missing, n (%) | |
| Cost (£) of index admission | 26,178 (23,244 to 29,111) | 178 (1.7) | 24,468 (21,471 to 27,466) | 174 (3.9) |
| Cost (£) of follow-up | 3857 (2093 to 5621) | 102 (43.7) | 4395 (2543 to 6246) | 116 (35.9) |
| Total cost (£) | 30,034 (26,377 to 33,692) | 102 (43.7) | 28,863 (25,312 to 32,414) | 116 (35.9) |
| Utility values | ||||
| Baseline | 0 (0 to 0) | 181 (0) | 0 (0 to 0) | 176 (2.8) |
| 6 months | 0.27 (0.19 to 0.34) | 122 (32.6) | 0.26 (0.20 to 0.33) | 131 (27.6) |
| 12 months | 0.28 (0.21 to 0.36) | 126 (30.4) | 0.26 (0.20 to 0.33) | 132 (27.6) |
| QALYs | 0.20 (0.15 to 0.26) | 111 (38.7) | 0.20 (0.15 to 0.25) | 117 (35.4) |
| NMB (£) | ||||
| 20,000 | –25,972 (–29,630 to –22,313) | 98 (45.9) | –24,898 (–28,238 to –21,557) | 108 (38.6) |
| 30,000 | –23,940 (–27,717 to –20,163) | 98 (45.9) | –22,915 (–26,250 to –19,579) | 108 (38.6) |
There were no significant differences in costs between the two groups (mean incremental cost for the group allocated to receive fresh blood vs. the group allocated to receive standard-aged blood: –£231, 95% CI –£4876 to £4415) or in outcomes (mean QALYs gained –0.010, 95% CI –0.078 to 0.057) (Table 15). The INMBs for the group allocated to receive fresh blood versus the group allocated to receive standard-aged blood were not significantly different from zero at a maximum willingness to pay for a QALY of £20,000 (mean £25, 95% CI –£4587 to £4637) and £30,000 (mean –£77, 95% CI –£4821 to £4666).
| Variable | Incremental cost (£), mean (95% CI) | QALYs gained, mean (95% CI) | INMB (£), mean (95% CI) | |
|---|---|---|---|---|
| 20,000 | 30,000 | |||
| Base casea | –231 (–4876 to 4415) | –0.010 (–0.078 to 0.057) | 25 (–4587 to 4637) | –77 (–4821 to 4666) |
| No adjustmentb | –1007 (–5736 to 3722) | –0.0053 (–0.071 to 0.061) | 902 (–3769 to 5572) | 849 (–3931 to 5628) |
| Complete-case analysisc | 1778 (–3480 to 7036) | 0.013 (–0.065 to 0.090) | –1526 (–6648 to 3596) | –1399 (–6626 to 3827) |
At a maximum willingness to pay for a QALY of £20,000 and £30,000, the probability that fresh blood is cost-effective was 0.52 and 0.49, respectively (Figure 9); as noted, the probability that standard-aged blood is cost-effective is one minus this figure, indicating that the costs and outcomes for the two treatment groups were similar.
FIGURE 9.
Cost-effectiveness acceptability curve showing the probability that fresh blood is cost-effective vs. standard-aged blood at different values of the maximum willingness to pay for a QALY.

Incremental costs, QALYs gained and INMBs for the group allocated to receive fresh blood versus the group allocated to receive standard-aged blood remained not significantly different from zero when rerunning the analysis without adjusting for potential confounding factors, and using complete cases (see Table 15).
The results were not sensitive to changes in the cost of fresh blood and standard-aged blood, nor to changes of admission and follow-up costs; INMBs did not vary much from baseline values and in every case the INMB was not significantly different from zero (Table 16).
| Scenario | INMB (£), mean (95% CI) | |
|---|---|---|
| 20,000 | 30,000 | |
| Base case | 25 (–4587 to 4637) | –77 (–4821 to 4666) |
| Unit cost of fresh blood increased by 25% | –142 (–4919 to 4636) | –257 (–5171 to 4658) |
| Unit cost of standard-aged blood increased by 25% | 269 (–4375 to 4914) | 208 (–4579 to 4994) |
| Daily cost of non-invasive and invasive MV increased by 25% | 272 (–4692 to 5236) | 175 (–4909 to 5260) |
| Daily cost of non-invasive and invasive MV decreased by 25% | –126 (–4441 to 4190) | –216 (–4677 to 4244) |
| Daily cost of vasopressor and inotropes increased by 25% | 244 (–4313 to 4800) | 155 (–4532 to 4841) |
| Daily cost of vasopressor and inotropes decreased by 25% | 91 (–4411 to 4593) | 1.7 (–4634 to 4637) |
| Daily cost of CRRT, haemodialysis and peritoneal dialysis increased by 25% | 212 (–4394 to 4818) | 134 (–4597 to 4866) |
| Daily cost of CRRT, haemodialysis and peritoneal dialysis decreased by 25% | 26 (–4513 to 4565) | –55 (–4743 to 4632) |
| Daily cost of ICU admission increased by 25% | 142 (–5082 to 5367) | 50 (–5293 to 5394) |
| Daily cost of ICU admission decreased by 25% | 186 (–4498 to 4870) | 84 (–4732 to 4900) |
| Daily cost of inpatient stay increased by 25% | 204 (–4927 to 5336) | 95 (–5154 to 5344) |
| Daily cost of inpatient stay decreased by 25% | 190 (–4403 to 4782) | 82 (–4615 to 4778) |
Summary
Our economic analysis to evaluate the cost-effectiveness of using fresh blood units versus standard-aged blood units for transfusions in critically ill patients in the UK showed that there are no differences in terms of costs and outcomes. The univariate sensitivity analysis showed that the results were not sensitive to assumptions made, and the probabilistic sensitivity analysis indicated that the probability that the use of fresh blood was cost-effective was close to the probability that standard-aged blood was cost-effective. The findings mean that there is no reason to prefer fresh blood to standard-aged blood on the basis of differences in quality or length of life, or on cost grounds.
Chapter 6 A nested study comparing the EuroQol-5 Dimensions utility scores in critical care survivors
Background and aims
Health-related quality of life is an important outcome in critical care survivors. The EQ-5D is a generic measure of HRQoL that allows the calculation of QALYs for undertaking cost–utility analyses. This is a recommended measure of HRQoL in critical care45 and it has been used in critical care trials. 46–48 Until recently, the EQ-5D-3L has been commonly used, in which each dimension in the descriptive system is assessed using three levels of severity. In order to reduce ceiling effects (the proportion of respondents reporting the best possible health) and to increase sensitivity to changes and differences in health status, the EQ-5D-5L was developed. 49 Several studies have compared the EQ-5D-3L and the EQ-5D-5L in different countries in both general population samples and specific patient groups, but none of these has examined the two measures in critical care patients. 49–59 The aim of the present study was to compare EQ-5D-3L scores with EQ-5D-5L scores in critical care survivors in the UK. The sample for the present study comprised 359 patients treated at any of the 20 participating UK centres.
Methods
Measures
The EQ-5D utility scores were measured among survivors at 6 and 12 months post randomisation. Patients at 10 centres were assigned to receive the EQ-5D-3L (165 randomised patients) and those at the other 10 centres received the EQ-5D-5L (194 randomised patients). The EQ-5D descriptive system contains five dimensions (mobility, self-care, usual activities, pain and discomfort, and anxiety and depression). For each dimension, there are three levels in the EQ-5D-3L (no problems, some problems and unable to/extreme problems) and five levels in the EQ-5D-5L (no problems, slight problems, moderate problems, severe problems and unable to/extreme problems). Each EQ-5D-3L health state was converted into a single summary index (utility score), applying a formula that attaches weights to each of the levels in each dimension based on valuations by general population samples. Given the perspective of our analysis, we used a value set for the UK population to calculate utility values at each time point for every participant. 60 Each EQ-5D-5L health state was similarly converted into a utility score using a recently published value set for England. 30 For both measures, utility scores of 1 represent full health, scores of zero are equivalent to death, negative scores represent states worse than death. With the value set we have applied, the EQ-5D-3L has values that range from 1 for no problems on any dimension to –0.594 for the worst health state (level-3 problems on every dimension); the EQ-5D-5L has values ranging from 1 to –0.281 (level-5 problems on every dimension).
Statistical analysis
The mean and median scores of both measures at 6 and 12 months were calculated. We tested for unadjusted differences in means between the two measures using two-sample t-tests. The distribution of both measures was plotted, and visual inspection of the distributions suggested that the use of classical parametric tests to compare the two sets of utility scores may not be appropriate. Therefore, we also used the two-sample Wilcoxon rank-sum (Mann–Whitney) test and the two-sample median test to test differences in distributions and medians.
Different patients completed the two measures, and to reduce the probability that differences in utility scores were attributable to differences in patient characteristics, we ran adjusted analyses controlling for treatment allocation (standard-aged blood, fresh blood), age (six categories), sex, comorbidities at hospital admission [separate indicators for pre-existing significant comorbid illnesses comprised severe lung disease with symptoms at rest, previous myocardial infarction, severe heart disease with symptoms at rest, disabling stroke, chronic renal failure (dialysis dependent), type 1 or 2 diabetes mellitus with evidence of end-organ damage, metastatic cancer, immunosuppressive therapy, chronic institutionalisation for > 6 months, deep-vein thrombosis], APACHE II score on ICU admission and MODS score on ICU admission. We tested for differences in characteristics between patients receiving each EQ-5D utility measure at each time point; for continuous variables we used two-sample t-tests, and for categorical variables we used chi-squared tests. We regressed utility scores at 6 months and at 12 months (two separate regressions) against an indicator for whether or not the patient had completed the EQ-5D-5L (1 = yes; 0 = otherwise) plus the covariates. We used both ordinary least squares (OLS) regression (whereby the objective is to estimate the conditional mean of the dependent variable) and median regression (to estimate the conditional median).
We tested for floor and ceiling effects based on the proportion of patients reporting the best and worst health states on the two versions [state 11111 (best) and state 33333 (worst EQ-5D-3L state) or 55555 (worst EQ-5D-5L state)]. We tested for differences in the proportions of patients reporting the best and worst health states on the two versions at each time point using chi-squared tests.
To determine the extent of variation in utility scores according to respondent characteristics, we analysed utility scores separately by age (higher or lower than the median age of 64 years), sex, the 10 individual comorbidities, APACHE II score on ICU admission and MODS score on ICU admission. We regressed utility score against each characteristic, also controlling for treatment allocation. For each of the 14 characteristics, we ran eight (2 × 2 × 2) regressions, running separate models for each combination of EQ-5D version, time (6 months and 12 months) and regression model (OLS and median regression); we ran 112 models in total.
We also explored whether or not the impact of treatment allocation (fresh blood or standard-aged blood) on HRQoL was different using the two EQ-5D measures at both time points. We calculated mean and median scores of the two measures at 6 and 12 months for each treatment group, and tested for unadjusted differences between treatment groups with each measure at each time point. We tested for adjusted differences using OLS and median regression, regressing utility scores at 6 months and at 12 months with both EQ-5D measures (four separate regressions) against the covariates (including treatment allocation).
Robust standard errors are reported throughout and p-values of < 0.05 were considered statistically significant. All analyses were undertaken using Stata® version 13 (StataCorp LP, College Station, TX, USA).
Results
In total, 227 of the 359 patients were alive at 6 months, and, of these, 121 returned an EQ-5D questionnaire (53%). A total of 221 patients were alive at 12 months, and 119 returned a questionnaire (54%). The distribution of both sets of utility scores at each time point was wide and discontinuous (Figures 10–13). The mean utility scores at 6 months for the EQ-5D-3L and EQ-5D-5L were 0.51 (SD 0.37) and 0.58 (SD 0.31), respectively (Table 17). The median utility scores were 0.59 (IQR 0.19–0.73) and 0.65 (IQR 0.34–0.82), respectively. At 12 months, the mean values were 0.50 (SD 0.33) and 0.60 (SD 0.33), respectively, and the median values were 0.62 (IQR 0.19–0.69) and 0.70 (IQR 0.42–0.84), respectively. The means, distributions and medians were not significantly different at 6 months (all p-values were ≥ 0.22), and neither was the mean difference at 12 months (p = 0.11), but the differences in distributions and medians at 12 months were significant (both p-values = 0.03).
FIGURE 10.
Distribution of EQ-5D-3L utility scores at 6 months.
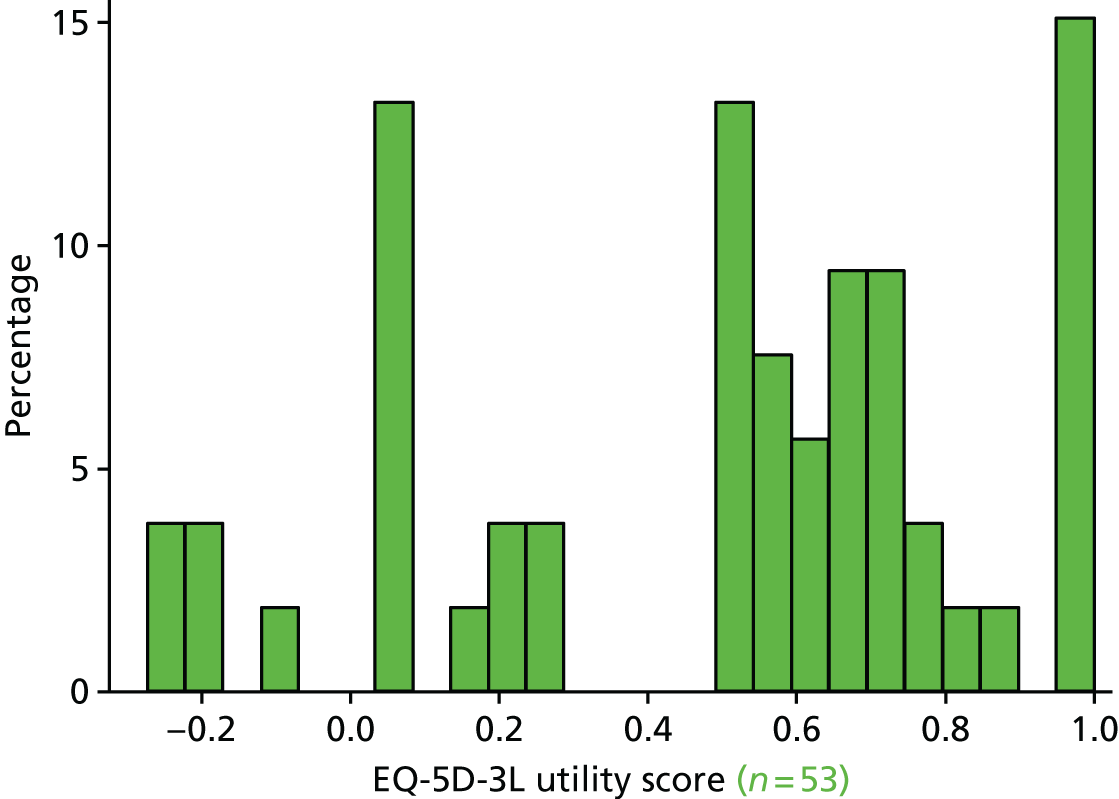
FIGURE 11.
Distribution of EQ-5D-5L utility scores at 6 months.
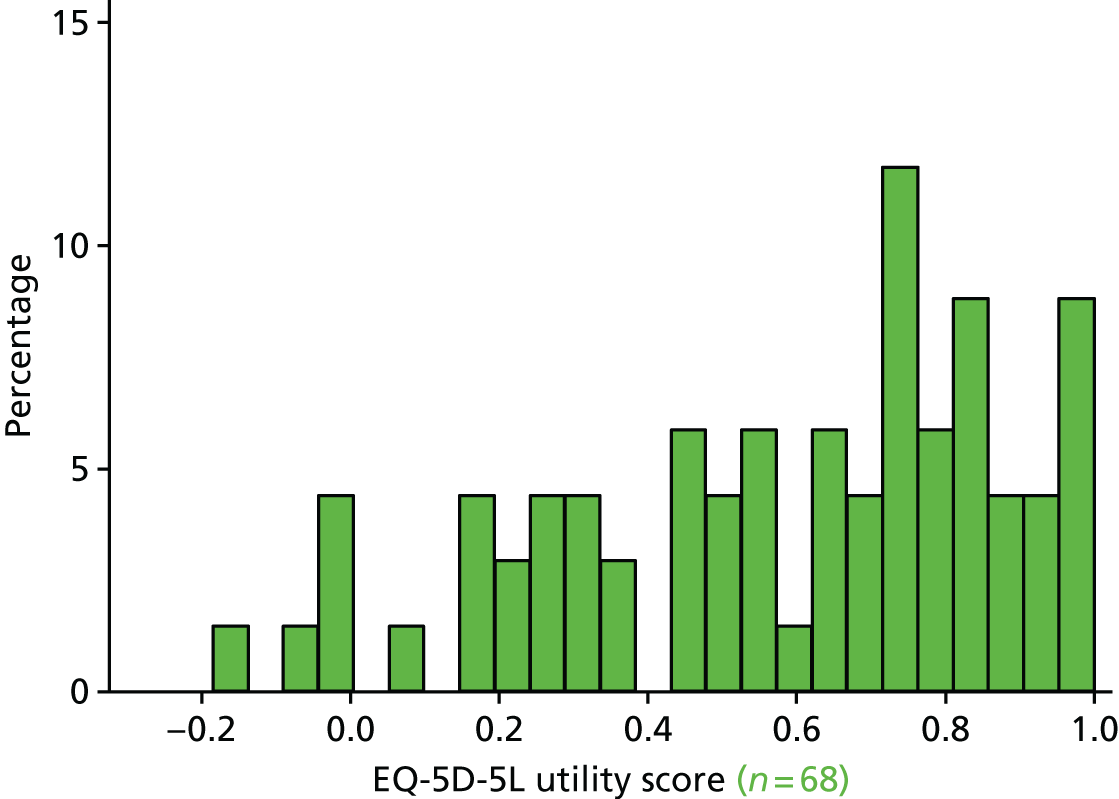
FIGURE 12.
Distribution of EQ-5D-3L utility scores at 12 months.

FIGURE 13.
Distribution of EQ-5D-5L utility scores at 12 months.
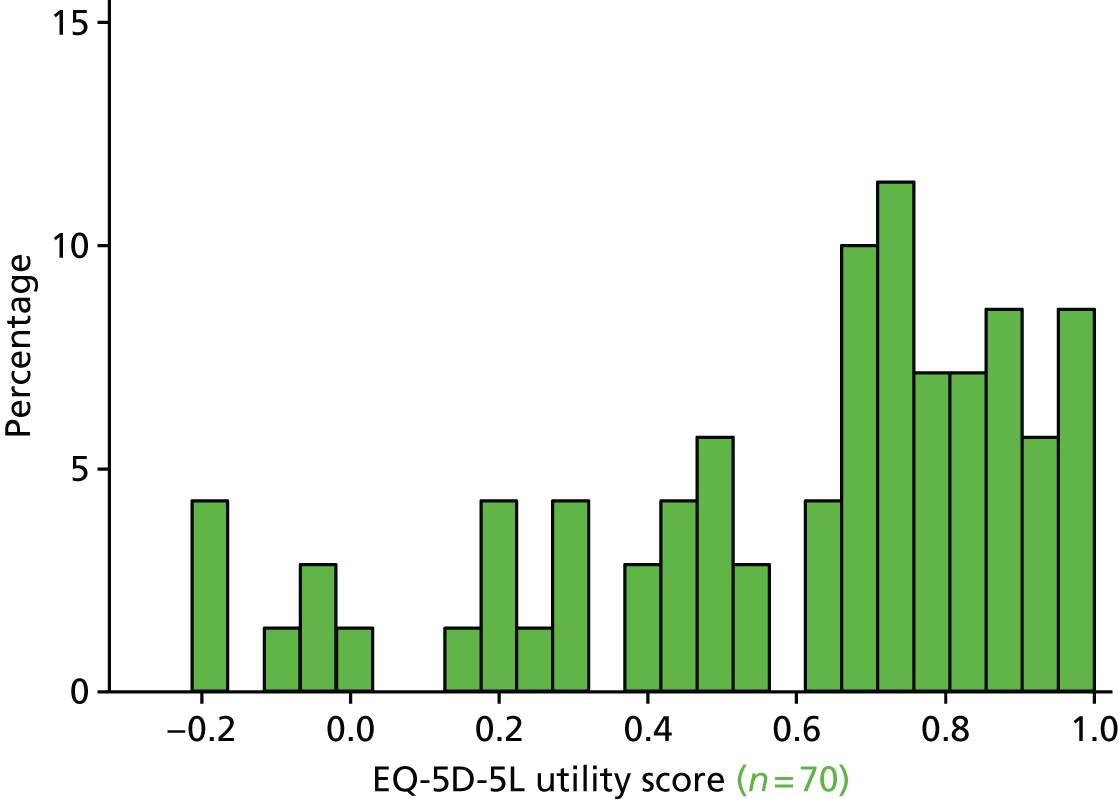
| Variable | Time point | |
|---|---|---|
| 6 months | 12 months | |
| EQ-5D-3L | ||
| Number of observations | 53 | 49 |
| Mean (SD) | 0.51 (0.37) | 0.50 (0.33) |
| Median (IQR) | 0.59 (0.19–0.73) | 0.62 (0.19–0.69) |
| EQ-5D-5L | ||
| Number of observations | 68 | 70 |
| Mean (SD) | 0.58 (0.31) | 0.60 (0.33) |
| Median (IQR) | 0.65 (0.34–0.82) | 0.70 (0.42–0.84) |
| Tests of differences (unadjusted), p-value | ||
| Difference in meansa | 0.22 | 0.11 |
| Difference in distributionsb | 0.25 | 0.03 |
| Difference in mediansc | 0.22 | 0.03 |
There were no differences in observed characteristics between patients receiving the two utility measures at each time point, with the exception that a higher proportion of respondents in the EQ-5D-3L group at 12 months had been allocated to receive fresh blood than that of the EQ-5D-5L group (p = 0.04; Table 18).
| Variable | Time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| EQ-5D-3L (n = 53) | EQ-5D-5L (n = 68) | Tests of differences (p-value) | EQ-5D-3L (n = 49) | EQ-5D-5L (n = 70) | Tests of differences (p-value) | |
| Allocated to receive fresh blood (%) | 53 | 43 | 0.27 | 59 | 40 | 0.04 |
| Age (years), mean (SD) | 59 (15) | 61 (15) | 0.60 | 59 (17) | 59 (16) | 0.92 |
| Male (%) | 58 | 54 | 0.65 | 55 | 57 | 0.83 |
| Severe lung disease with symptoms at rest (%) | 8 | 7 | 0.97 | 6 | 7 | 0.83 |
| Previous myocardial infarction (%) | 9 | 15 | 0.38 | 8 | 14 | 0.31 |
| Severe heart disease with symptoms at rest (%) | 6 | 7 | 0.72 | 6 | 7 | 0.83 |
| Disabling stroke (%) | 0 | 1 | 0.38 | 0 | 0 | |
| Chronic renal failure [dialysis dependent (%)] | 0 | 4 | 0.12 | 0 | 4 | 0.14 |
| Type 1 or 2 diabetes mellitus with evidence of end-organ damage (%) | 9 | 15 | 0.38 | 8 | 14 | 0.31 |
| Metastatic cancer (%) | 2 | 4 | 0.44 | 0 | 6 | 0.09 |
| Immunosuppressive therapy (%) | 4 | 9 | 0.27 | 4 | 11 | 0.16 |
| Chronic institutionalisation for > 6 months (%) | 2 | 1 | 0.86 | 2 | 3 | 0.78 |
| Deep-vein thrombosis (%) | 6 | 1 | 0.20 | 4 | 3 | 0.72 |
| APACHE II score (points), mean (SD) | 26 (8) | 23 (7) | 0.14 | 25 (8) | 23 (7) | 0.19 |
| MODS score (points), mean (SD) | 5 (3) | 5 (3) | 0.51 | 5 (3) | 5 (3) | 0.73 |
After adjusting for patient characteristics, there were no significant differences between the conditional mean and median EQ-5D-3L and EQ-5D-5L utility scores at 6 months (p ≥ 0.17; Table 19), but there were significant differences at 12 months (p < 0.05); mean EQ-5D-5L scores were 0.15 units higher with the EQ-5D-5L than with the EQ-5D-3L, and median EQ-5D-5L scores were 0.20 units higher.
| Variable | Time point | |
|---|---|---|
| 6 months | 12 months | |
| OLS | ||
| Marginal effect (95% CI; p-value) | 0.10 (–0.04 to 0.23; 0.17) | 0.15 (0.02 to 0.28; 0.02) |
| R2 | 0.16 | 0.12 |
| Number of observations | 121 | 119 |
| Median regression | ||
| Marginal effect (95% CI; p-value) | 0.10 (–0.06 to 0.26; 0.22) | 0.20 (0.09 to 0.30; < 0.01) |
| Pseudo-R2 | 0.11 | 0.10 |
| Number of observations | 121 | 119 |
No patients reported being in the worst EQ-5D-3L state or the worst EQ-5D-5L state at 6 or 12 months. Eight patients (15%) reported being in the best health state on the EQ-5D-3L at 6 months compared with six patients (9%) on the EQ-5D-5L. Seven patients (14%) reported being in the best health state on the EQ-5D-3L at 12 months compared with six patients (9%) on the EQ-5D-5L. These figures were not significantly different (p ≥ 0.29).
For the 112 regression models used to determine the extent of variation in utility score according to patient characteristics, in only three models was the coefficient on the patient characteristic significantly different from zero. In all three cases, the significant effect was found using the EQ-5D-5L in patients at the 12-month follow-up using median regression (sample size: n = 70 patients). These analyses showed that immunosuppressive therapy, chronic institutionalisation for > 6 months and deep-vein thrombosis had a significant negative impact on median EQ-5D scores; the marginal effects were –0.23 (95% CI –0.47 to –0.01; p-value = 0.05; number of patients with condition, 8), –0.68 (95% CI –1.22 to –0.13; p-value = 0.02; number of patients with condition, 2) and –0.87 (95% CI –1.42 to –0.31; p-value = 0.01; number of patients with condition, 2), respectively.
At 6 months, there were no unadjusted differences in utility scores (means, distributions and medians) between trial treatment groups using either the EQ-5D-3L or the EQ-5D-5L (p ≥ 0.13; Table 20). At 12 months, there were no unadjusted differences in utility scores between treatment groups using the EQ-5D-3L (p ≥ 0.40), but EQ-5D-5L distributions were significantly different between treatment groups (p-value of –0.03). When we controlled for patient characteristics, there were no significant differences in utility scores between treatment groups for either EQ-5D measure at either time point (p ≥ 0.20; Table 21).
| Time point | Trial group | Tests of differences (unadjusted) | |
|---|---|---|---|
| Fresh blood | Standard-aged blood | ||
| 6 months | |||
| EQ-5D-3L | |||
| Number of observations | 28 | 25 | |
| Mean (SD) | 0.45 (0.43) | 0.57 (0.28) | p = 0.24a |
| Median (IQR) | 0.55 (0.08–0.78) | 0.59 (0.52–0.71) | p = 0.55b |
| p = 0.87c | |||
| EQ-5D-5L | |||
| Number of observations | 29 | 39 | |
| Mean (SD) | 0.63 (0.31) | 0.54 (0.30) | p = 0.26a |
| Median (IQR) | 0.74 (0.50–0.82) | 0.54 (0.28–0.83) | p = 0.19b |
| p = 0.14c | |||
| 12 months | |||
| EQ-5D-3L | |||
| Number of observations | 29 | 20 | |
| Mean (SD) | 0.47 (0.35) | 0.55 (0.31) | p = 0.46a |
| Median (IQR) | 0.59 (0.08–0.69) | 0.64 (0.26–0.69) | p = 0.40b |
| p = 0.59c | |||
| EQ-5D-5L | |||
| Number of observations | 28 | 42 | |
| Mean (SD) | 0.70 (0.29) | 0.54 (0.33) | p = 0.05a |
| Median (IQR) | 0.76 (0.60–0.87) | 0.66 (0.30–0.80) | p = 0.03b |
| p = 0.06c | |||
| Time point | Score | |
|---|---|---|
| EQ-5D-3L | EQ-5D-5L | |
| 6 months | ||
| OLS | ||
| Marginal effect (95% CI; p-value) | –0.10 (–0.33 to 0.12; 0.37) | 0.06 (–0.10 to 0.23; 0.47) |
| R2 | 0.44 | 0.24 |
| Number of observations | 53 | 68 |
| Median regression | ||
| Marginal effect (95% CI; p-value) | –0.11 (–0.45 to 0.24; 0.53) | 0.07 (–0.23 to 0.37; 0.64) |
| Pseudo-R2 | 0.35 | 0.22 |
| Number of observations | 53 | 68 |
| 12 months | ||
| OLS | ||
| Marginal effect (95% CI; p-value) | 0.03 (–0.17 to 0.23; 0.76) | 0.13 (–0.07 to 0.33; 0.20) |
| R2 | 0.45 | 0.15 |
| Number of observations | 49 | 70 |
| Median regression | ||
| Marginal effect (95% CI; p-value) | 0.05 (–0.43 to 0.53; 0.84) | 0.12 (–0.12 to 0.37; 0.31) |
| Pseudo-R2 | 0.35 | 0.09 |
| Number of observations | 49 | 70 |
Summary
In conclusion, this study compared the performance of the EQ-5D-3L and the EQ-5D-5L in measuring the HRQoL in critical care survivors in the UK. There was some evidence of difference between the two measures, and that the EQ-5D-5L discriminated between subgroups of patients with major comorbidities whereas the EQ-5D-3L did not, but further research is needed to confirm these findings.
Chapter 7 An analysis of the UK Age of BLood Evaluation trial data set to explore factors associated with costs and quality-adjusted life-years in critically ill adults
Background and aims
Intensive care units provide potentially life-saving interventions to critically ill patients, but incur considerable costs when treating patients. 61 Length of stay is a driver of ICU costs, and factors affecting this include institutional factors (such as organisational structure and resources), medical factors (especially cause of admission and severity of illness) and psychosocial factors (such as communication between clinical staff, and with the patient’s family). 62 On discharge from the ICU, survivors often have long-term health problems that affect their quality of life and survival, and health service utilisation and costs; factors associated with increased resource use include increasing age, comorbidities, organ dysfunction score and previous resource use. 15,61 The aim of the present study was to investigate the factors associated with hospital costs and total costs and QALYs up to 12 months after ICU admission in critically ill adults in the UK.
Methods
The sample for the present study comprised 359 patients treated at any of the 20 participating UK centres. Two patients had no study measurements and so were not included in the analysis, giving a sample of 357 patients.
Outcome measures
Utilities and quality-adjusted life-years
Generic health status was described at 6 and 12 months post randomisation using the EQ-5D descriptive system. 29,39 This contains five dimensions (mobility, self-care, usual activities, pain and discomfort, and anxiety and depression). UK study centres were randomised to use of either the EQ-5D-3L or the EQ-5D-5L at both follow-up time points. For each dimension, there are three levels in the EQ-5D-3L (no problems, some problems and unable to/extreme problems) and five levels in the EQ-5D-5L (no problems, slight problems, moderate problems, severe problems and unable to/extreme problems). As patients recruited to the trial were initially critically ill, completion of the EQ-5D at randomisation was not possible. Therefore, baseline utility was assumed to be zero for all patients. This assumption has been made in previous studies where baseline utility measurement was not possible. 40 EQ-5D health states were converted into utility values using formulae that attach weights to each level in each dimension based on valuations by general population samples. Different formulae and value sets were used to calculate utility values for the EQ-5D-3L and the EQ-5D-5L, although both value sets were based on the UK general population. 30,41 Utility values of 1 represent full health, values of zero are equivalent to death and negative values represent states worse than death. Patients who died were assigned a utility value of zero at all subsequent time periods. A utility profile was constructed for every patient, assuming a straight-line relation between their utility values at each measurement point. QALYs for every patient from baseline to 12 months were calculated as the area under the utility profile. The implications of our assumptions were as follows. For patients who survived up to 6 months, QALYs up to 6 months were calculated using the EQ-5D scores at 6 months, assuming an EQ-5D score of zero units at baseline, and a linear interpolation between baseline and 6 months. For patients who died between baseline and 6 months, we assumed that they accrued zero QALYs. For patients who survived up to 12 months, we used a linear interpolation between EQ-5D scores at 6 and 12 months. For patients who died between 6 and 12 months and for whom an EQ-5D score at 6 months was available, a linear interpolation was applied between the 6-month EQ-5D score and the date of death, at which point, a EQ-5D score of zero units was applied thereafter. The maximum QALYs achievable in the study were 0.75. We assigned all the EQ-5D data that were collected to either the 6- or 12-month measurement points, irrespective of the precise time when these were actually measured.
Resource use and costs
The analysis took a UK NHS and personal social services perspective. 32 Costs were calculated in 2015 UK pounds and inflated where necessary. 33 For every patient, we calculated the cost of the index hospitalisation (from hospital admission to hospital discharge) and of follow-up to 12 months based on resource use data collected in the trial. We included the costs of blood transfusions; fresh-frozen plasma and platelets; length of stay in hospital regular inpatient wards and in the ICU; and use of MV, vasopressors, inotropes, CRRT, haemodialysis and peritoneal dialysis in the ICU. Post-discharge costs included GP visits in the clinic and at home, and telephone consultations; district and practice nurse visits; physiotherapist, occupational therapist and speech and language therapist visits; dietitian, home care worker (e.g. Meals on Wheels), social worker, psychological therapist, counsellor, and aids and adaptation worker visits; specialist nurse visits (substance misuse nurses, Macmillan Cancer Support nurses); day hospital visits; A&E visits; outpatient appointments; and readmissions to hospital, including surgical procedures and ICU spells. Unit costs were obtained from published sources and inflated where appropriate (see Chapter 5 for further details about costing methodology and the unit costs used). The unit costs per unit of fresh blood and standard-aged blood were assumed to be the same. Unit costs of hospital inpatient and ICU stays were daily costs applied to length of stay data collected in the trial. Given the time horizon, discounting was not applied to costs (or outcomes).
Statistical analysis
Outcome measures and samples
We ran seven sets of regression models, with outcomes and samples described in Table 22. We ran models for utilities measured at 6 and 12 months on survivors only, as including zero values for those who died would not be informative (132 patients died before 6 months, and six patients died between 6 and 12 months). Similarly, we examined costs of follow-up to 12 months for survivors only.
| Model | Outcome measure | Sample |
|---|---|---|
| 1 | EQ-5D score at 6 months | Survivors only |
| 2 | EQ-5D score at 12 months | Survivors only |
| 3 | QALYs up to 12 months | Survivors only |
| 4 | QALYs up to 12 months | All patients (survivors plus those who died) |
| 5 | Costs of index admission | All patients (survivors plus those who died) |
| 6 | Costs of follow-up | Survivors only |
| 7 | Total costs (index admission plus follow-up) | All patients (survivors plus those who died) |
Covariates
We included the following variables as covariates in the regression models: age (linear term), sex, comorbidities at hospital admission [separate indicators for pre-existing significant comorbid illnesses comprising severe lung disease with symptoms at rest, previous myocardial infarction, severe heart disease with symptoms at rest, disabling stroke, chronic renal failure (dialysis dependent), type 1 or 2 diabetes mellitus with evidence of end-organ damage, metastatic cancer, immunosuppressive therapy, chronic institutionalisation for > 6 months, deep-vein thrombosis], APACHE II score on ICU admission and lowest MODS score during ICU stay, treatment allocation (fresh blood or standard-aged blood) and study centre. We did not include separate controls for whether or not patients completed the EQ-5D-3L or the EQ-5D-5L measure, as this was determined by study centre. Covariates measured after baseline assessment were not included in the analyses, because these may have been influenced by the costs incurred and utilities accrued. In models that included all patients (models 4, 5 and 7), we also included covariates for whether or not the patient died in the ICU or during follow-up. Previous studies have shown that important drivers of ICU and post-discharge costs are cause of admission and severity of illness, age and pre-existing comorbidities. 15,62,63 To reflect this we included age, pre-existing significant comorbid illnesses and illness severity measured by APACHE II score and MODS score in our analyses.
Regression models
For utility scores and QALYs, we used both OLS regression (whereby the objective is to estimate the conditional mean of the dependent variable) and median regression (to estimate the conditional median). For costs, to account for skewness of the cost data, we used a generalised linear model with gamma family and log-link. 64 We also considered using log-normal, Gaussian, inverse Gaussian and negative binomial distributions, but the gamma model gave the best fit in terms of residual plots and the Akaike information criterion.
Dealing with missing data
The extent of missing data for some of the outcome measures included in the analysis was large (the range across the seven measures was 1–56%). Multiple imputation was used to impute missing data separately for each outcome. For analyses using survivors only (models 1, 2, 3 and 6), age, sex, study centre, treatment allocation, comorbidities at hospital admission, APACHE II score on ICU admission and worst MODS score during ICU stay were included in the imputation as additional explanatory variables (these were variables in the trial data set with no missing values). For analyses using all patients (models 4, 5 and 7), we additionally included whether or not the patient died in the ICU or during follow-up. For utility scores and QALYs among survivors only (models 1, 2 and 4), we imputed utility scores at 6 and 12 months for survivors only and calculated QALYs up to 12 months from the imputed values. We repeated this approach to impute QALYs up to 12 months for all patients, but did not limit the sample to survivors only (model 3). We imputed each of the cost measures directly, and for follow-up costs to 12 months (model 6), we ran the imputation procedure on survivors only, whereas for index hospitalisation costs and total costs up to 12 months (models 5 and 7), we included all patients. We used an iterative Markov chain Monte Carlo procedure based on multivariate normal regression, generating 20 imputed data sets. We ran the regression model described above on each of the imputed data sets and computed aggregate coefficients and standard errors using combination rules. 65
Variable selection
Each of the outcome measures was regressed against the covariates. The predictors of each outcome were selected using forward and backward stepwise selection techniques, and only statistically significant variables were retained after each iteration. We also ran univariate models for every outcome measure, including each covariate individually. Study centre was included in every model. Robust standard errors are reported throughout and p-values of < 0.05 were considered statistically significant. All analyses were undertaken using Stata version 13.
Results
In total, 219 of the 357 patients were alive at 12 months, and, of these, 121 returned an EQ-5D questionnaire at 6 months (55% of survivors) and 119 at 12 months (54%). Data on QALYs up to 12 months were available for 96 patients (44%). Including those patients who died, QALY data up to 12 months were available for 228 patients (64% of all patients). The distribution of utility scores at each time point and QALYs up to 12 months was wide and discontinuous (Figures 14–17). The high frequency of zero values in QALYS up to 12 months across all patients reflects the number of patients who died before 6 months (132 patients). The mean utility scores at 6 and 12 months were 0.54 (SD 0.33) and 0.56 (SD 0.33), respectively (Table 23). The medians were 0.62 (IQR 0.28–0.80) and 0.66 (IQR 0.31–0.80), respectively. Mean and median QALYs up to 12 months across all patients were 0.18 (SD 0.26) and 0 (IQR 0–0.40), again reflecting the number of patients who died before 6 months. Values for survivors only were 0.43 (SD 0.23) and 0.46 (IQR 0.27–0.59), respectively.
FIGURE 14.
Distribution of utility scores at 6 months (survivors only).
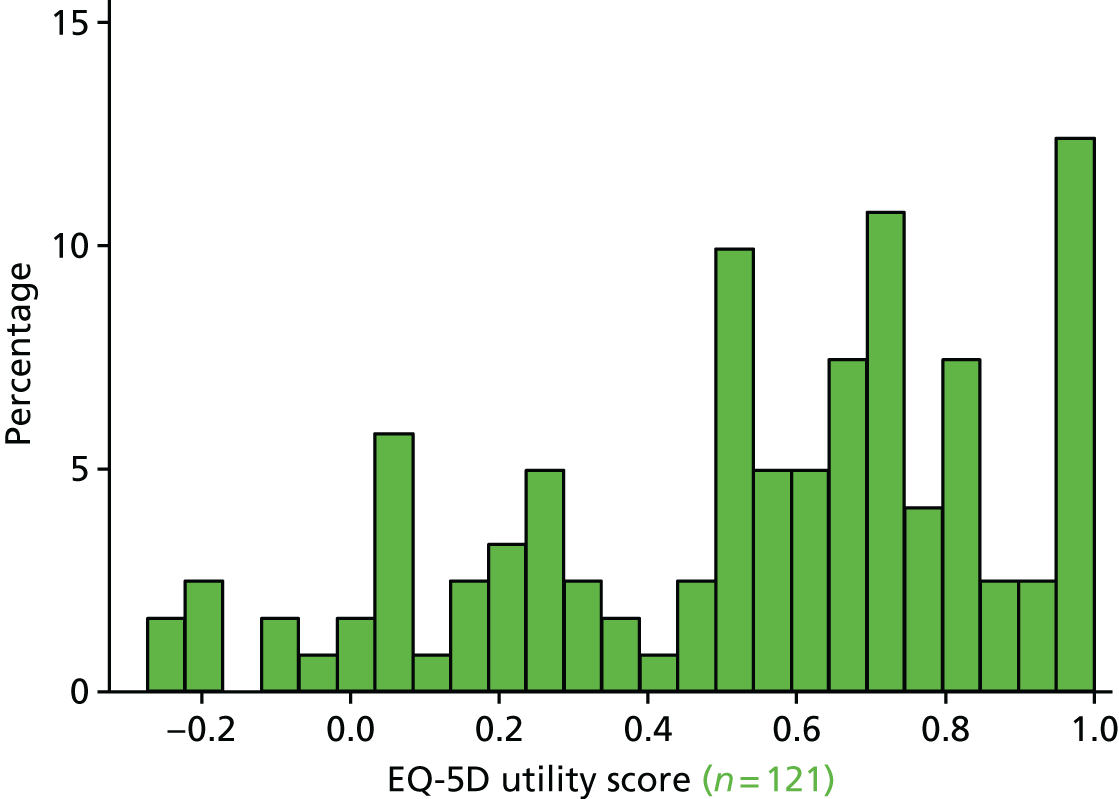
FIGURE 15.
Distribution of utility scores at 12 months (survivors only).
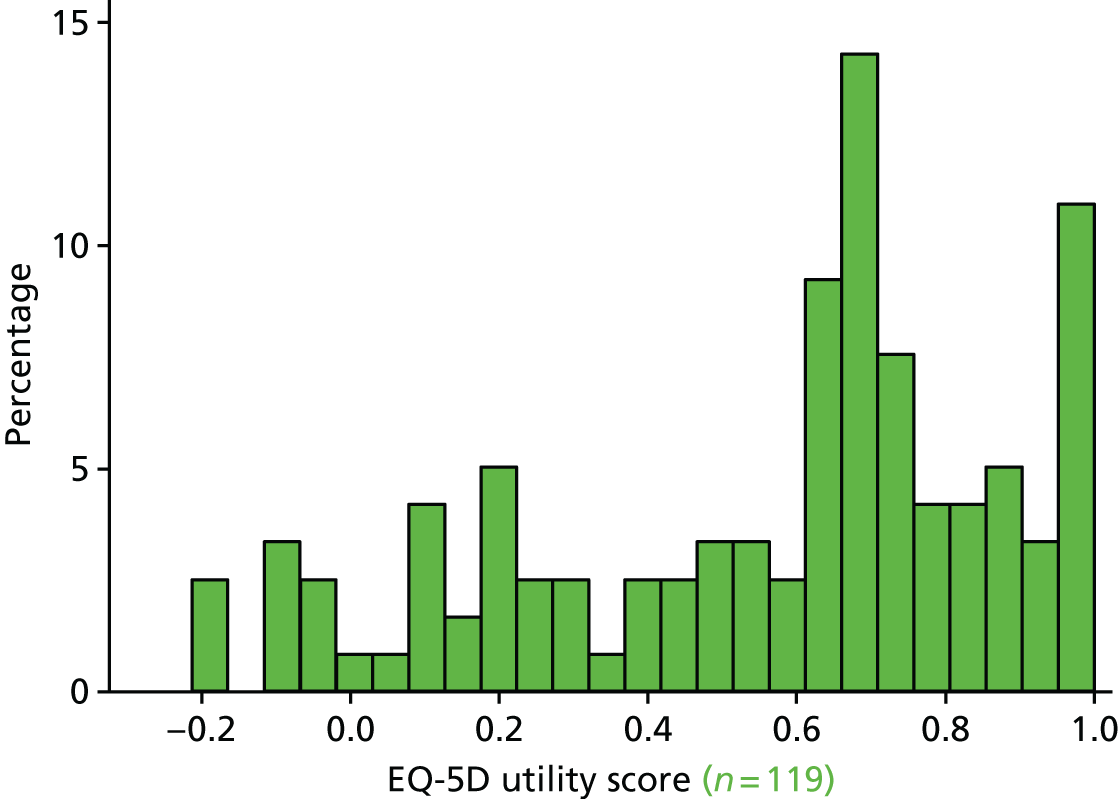
FIGURE 16.
Distribution of QALYs up to 12 months (all patients).

FIGURE 17.
Distribution of QALYs up to 12 months (survivors only).

| Variable | Value | Observations | Missing (%) |
|---|---|---|---|
| Dependent variables | |||
| Utility scores at 6 months (survivors only) | |||
| Mean (SD) | 0.54 (0.33) | 121 | 45 |
| Median (IQR) | 0.62 (0.28–0.80) | 121 | 45 |
| Utility scores at 12 months (survivors only) | |||
| Mean (SD) | 0.56 (0.33) | 119 | 46 |
| Median (IQR) | 0.66 (0.31–0.80) | 119 | 46 |
| QALYs up to 12 months (all patients) | |||
| Mean (SD) | 0.18 (0.26) | 228 | 36 |
| Median (IQR) | 0 (0–0.40) | 228 | 36 |
| QALYs up to 12 months (survivors only) | |||
| Mean (SD) | 0.43 (0.23) | 96 | 56 |
| Median (IQR) | 0.46 (0.27–0.59) | 96 | 56 |
| Index hospitalisation costs (all patients) | |||
| Mean (SD) | 30,803 (20,501) | 352 | 1 |
| Median (IQR) | 26,771 (15,974–39,744) | 352 | 1 |
| Follow-up costs to 12 months (survivors only) | |||
| Mean (SD) | 8324 (11,908) | 108 | 51 |
| Median (IQR) | 3819 (1549–10,617) | 108 | 51 |
| Total costs up to 12 months (all patients) | |||
| Mean (SD) | 31,867 (21,130) | 218 | 39 |
| Median (IQR) | 26,972 (16,073–41,590) | 218 | 39 |
| Independent variables (all patients) | |||
| Age (years), mean (SD) | 61.5 (16.3) | 357 | 0 |
| Male (%) | 53.2 | 357 | 0 |
| Severe lung disease with symptoms at rest (%) | 7.6 | 357 | 0 |
| Previous myocardial infarction (%) | 10.9 | 357 | 0 |
| Severe heart disease with symptoms at rest (%) | 8.1 | 357 | 0 |
| Disabling stroke (%) | 1.1 | 357 | 0 |
| Chronic renal failure (dialysis dependent) (%) | 4.2 | 357 | 0 |
| Type 1 or 2 diabetes mellitus with evidence of end-organ damage (%) | 11.2 | 357 | 0 |
| Metastatic cancer (%) | 2.0 | 357 | 0 |
| Immunosuppressive therapy (%) | 7.6 | 357 | 0 |
| Chronic institutionalisation for > 6 months (%) | 1.1 | 357 | 0 |
| Deep-vein thrombosis (%) | 2.8 | 357 | 0 |
| APACHE II score (points), mean (SD) | 24.6 (7.3) | 357 | 0 |
| Worst MODS score (points) during ICU stay, mean (SD) | 7.0 (3.3) | 357 | 0 |
| 0–4 (%) | 23.8 | 85 | – |
| 5–8 (%) | 50.0 | 178 | – |
| 9–12 (%) | 19.9 | 71 | – |
| ≥ 13 (%) | 6.4 | 23 | – |
| Died in ICU (%) | 25.2 | 357 | 0 |
| Died in ICU or during 12-month follow-up (%) | 38.9 | 357 | 0 |
Index hospitalisation costs were available for 352 patients (99% all patients), and the mean and median values were £30,803 (SD £20,501) and £26,771 (IQR £15,974–39,744), respectively. Follow-up costs to 12 months were available for 108 survivors (49% of survivors), with mean and median values of £8324 (SD £11,908) and £3819 (IQR £1549–10,617), respectively. Total costs up to 12 months across all patients had mean and median values of £31,867 (SD £21,130) and £26,972 (IQR £16,073–41,590), respectively. The distribution of all three cost variables was positively skewed (Figures 18–20).
FIGURE 18.
Distribution of index hospitalisation costs (all patients).
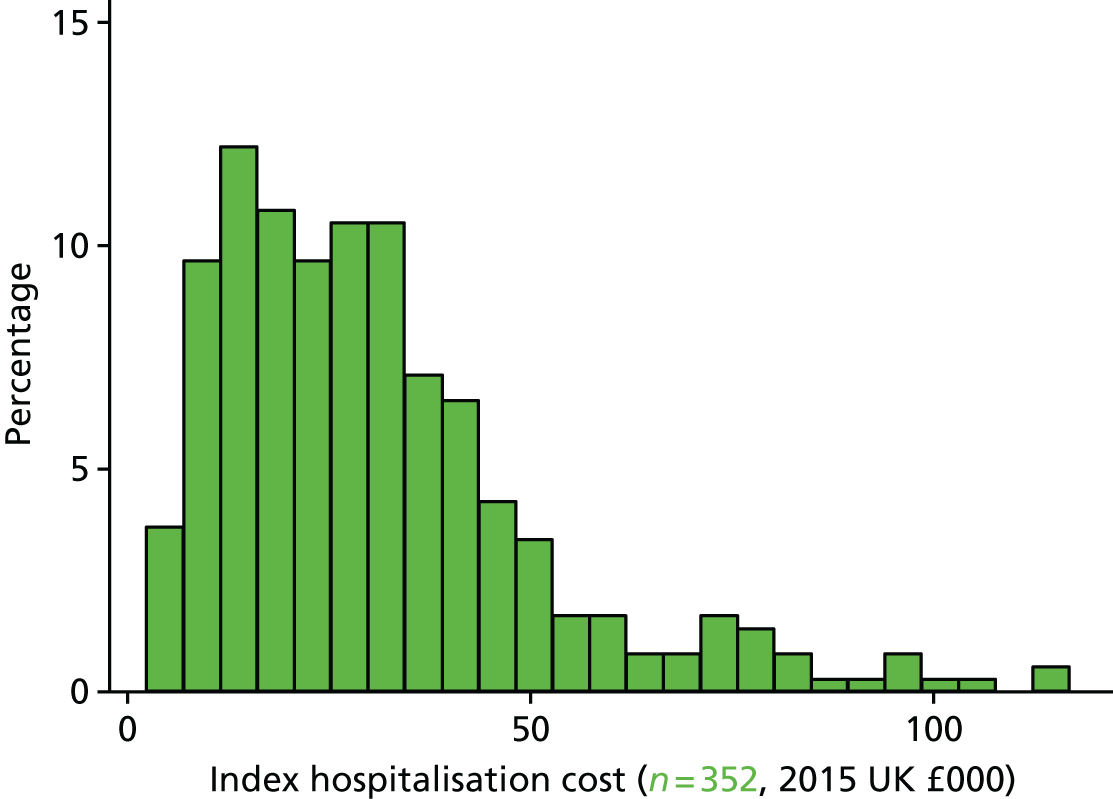
FIGURE 19.
Distribution of follow-up costs up to 12 months (survivors only).
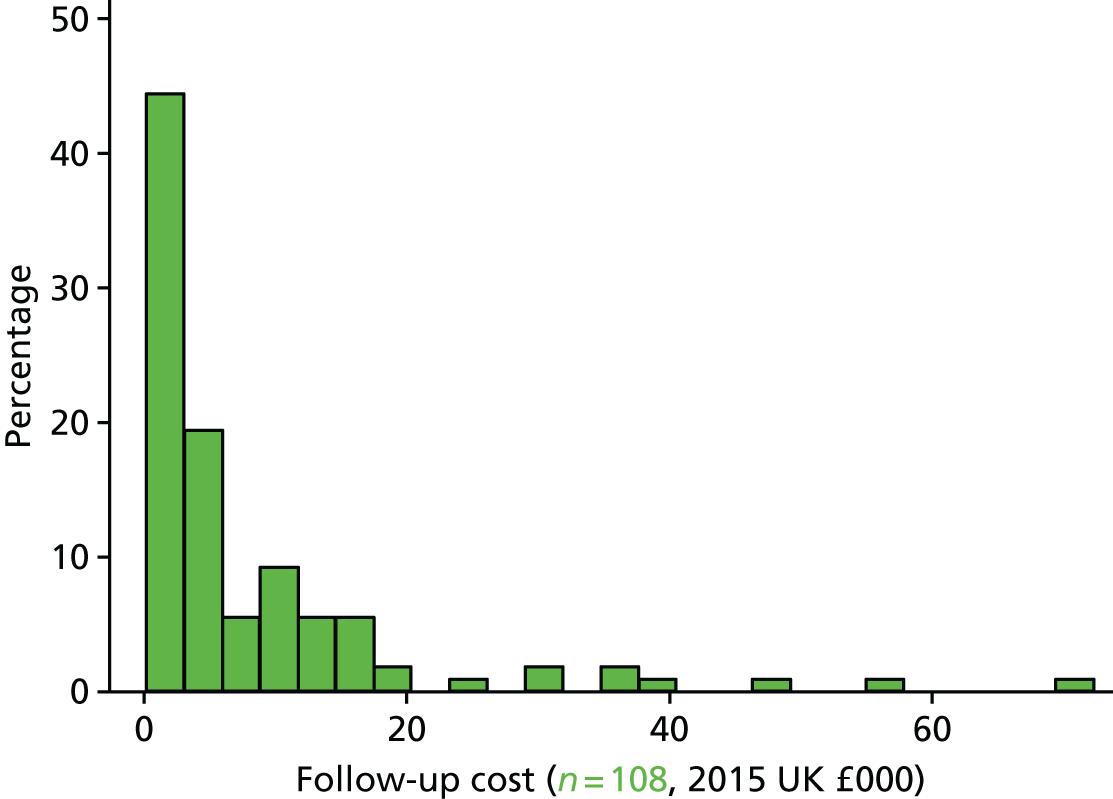
FIGURE 20.
Distribution of total costs up to 12 months (all patients).
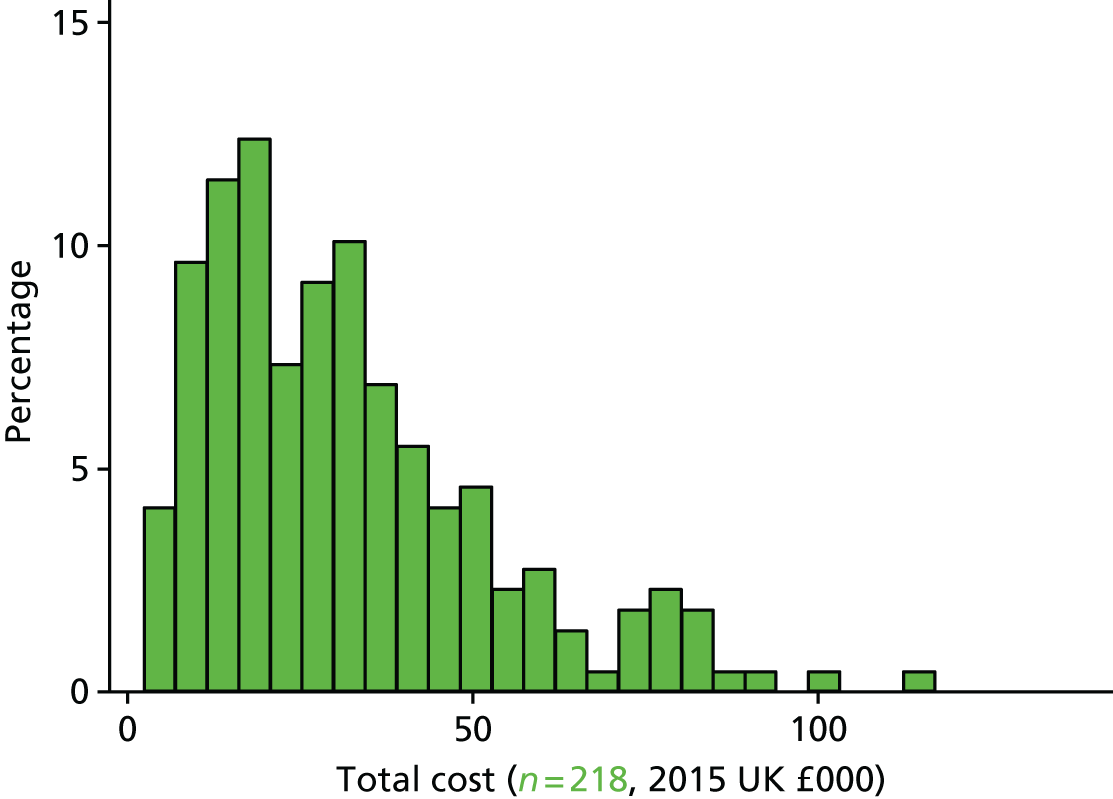
The mean age of patients was 62 years and 53% were male (see Table 23). The prevalence of pre-existing significant comorbid illnesses ranged from 1% (disabling stroke, chronic institutionalisation for > 6 months) to 11% (type 1 or 2 diabetes mellitus with evidence of end-organ damage). The mean APACHE II score at ICU admission was 25 points (SD 7 points) and the mean worst MODS score during ICU stay was 7 points (SD 3 points), with the modal category 5–8 points (50% of patients). Twenty-five per cent of patients died during the ICU stay, and 39% died by the end of the 12-month follow-up.
None of the covariates was a statistically significant predictor of utility scores at 6 or 12 months or of QALYs up to 12 months, either for all patients or survivors only (models 1–4; results not shown).
Index hospitalisation costs were predicted by worst MODS score during ICU stay and whether or not the patient died in the ICU (Table 24). Controlling for whether or not the patient died, hospitalisation costs increased with worsening MODS score; patients with a MODS score of 5–8 points incurred around £10,000 higher mean costs than those with values of 0–4 points, and those patients with scores of ≥ 9 points incurred mean costs that were £16,000–19,000 higher than those with scores of 0–4 points (note that the values for the 9- to 12-point and ≥ 13-point categories were not significantly different from one another). Patients who died incurred, on average, £18,000 lower costs than those who did not die, depending on their MODS score. Survivors with prior chronic institutionalisation incurred costs during the 12 months’ follow-up that were, on average, around £24,000 higher than those without chronic institutionalisation. In the model of total costs up to 12 months, the following covariates were significant: costs were higher with worsening MODS score for those who died in the ICU and those with prior chronic institutionalisation. Controlling for whether or not the patient died, total costs increased with worsening MODS score by, on average, around £8000, £19,000 and £21,000 per patient for those with scores of 5–8, 9–12 and ≥ 13 points, respectively, compared with those with a score of 0–4 points. On average, those who died incurred £30,000 lower costs than those who did not, and those with prior chronic institutionalisation incurred total costs that were, on average, around £24,000 higher than those without chronic institutionalisation. None of the other covariates was individually statistically significant as a predictor of any of the cost measures, nor were they jointly significant when added to the models just described.
| Sample | Index hospitalisation costs | Follow-up costs to 12 months | Total costs up to 12 months |
|---|---|---|---|
| All patients | Survivors only | All patients | |
| Worst MODS score (points) during ICU stay: 5–8 points | 0.32 (< 0.01; £9989) | – | 0.26 (0.03; £8345) |
| Worst MODS score (points) during ICU stay: 9–12 points | 0.61 (< 0.01; £18,672) | – | 0.54 (< 0.01; £18,527) |
| Worst MODS score (points) during ICU stay: ≥ 13 points | 0.53 (< 0.01; £16,311) | – | 0.51 (< 0.01; £21,207) |
| Died in ICU | –0.58 (< 0.01; –£17,850) | – | –0.68 (< 0.01; –£29,771) |
| Chronic institutionalisation for > 6 months | – | 1.29 (< 0.01; £24,137) | 0.86 (< 0.01; £36,889) |
| Number of observations | 357 | 219 | 357 |
Summary
We evaluated the factors associated with hospital costs and total costs and QALYs up to 12 months after ICU admission in critically ill adults in the UK. None of the variables in our data was a significant predictor of utility scores at 6 or 12 months, or of QALYs up to 12 months. The lack of an effect may reflect the limitations of our data set in terms of available measures, sample size and data missingness. Consistent with previous studies, we found that ICU costs were an increasing function of illness severity, measured in terms of MODS score and mortality. 62 Contrary to previous studies, costs from hospital discharge up to 12 months were largely unaffected by acute illness factors, age or comorbidities; however, there was some evidence that chronic institutionalisation, included as an indicator of pre-existing morbidity, was associated with 12-month costs. 15,63
Chapter 8 Discussion and implications for future practice
Study conduct
The ABLE UK study was specifically funded to collaborate with the Canadian ABLE trial [funded by the Canadian Institutes of Health Research (CIHR)]. Specifically, the aim was to ensure the recruitment of the intended sample size of 2510 patients, and to include a UK-specific health economic evaluation. At the time of funding, Canadian recruitment was slower than anticipated and there was a risk that the trial would not achieve the target sample, which would have significantly reduced its external validity, generalisability and impact. Funding by the HTA programme, together with recruitment in France and the Netherlands, meant that recruitment was completed to target.
There were several relevant learning issues from this model of funding. First, despite the HTA programme ‘fast-tracking’ the application following the commissioned call, the process of review and final funding, followed by contract finalisation, meant that the ABLE UK trial came ‘on-line’ almost 2 years after recruitment to the international trial began. The further time required to set up recruitment in the UK further delayed contribution to the main trial. Second, several practical issues resulted from the international arrangements: collaboration and data sharing agreements were needed between the UK and Canada; separate sponsorship arrangements for the UK were required; and the trial protocol required adaptation to the UK, mainly as a result of different legal frameworks for the inclusion of incapacitated patients. Third, the legal and contractual burden for the trial was very high as a result of the multiple stakeholders, which created a significant workload for the sponsor and delays in trial set-up, despite attempts to expedite delays. Of relevance, there was no funding allocated to the very high legal/contracting burden for the sponsor (the University of Edinburgh). The consequence of these issues was that, despite intense effort by the trial management team, the set-up time to start recruitment meant that approximately 40% of the international trial sample size had been achieved before UK recruitment began. The recruitment rates in the UK were close to those predicted, and comparable to non-UK centres, but the international sample size was achieved before the UK target of 500 (revised to 400 during the trial) was achieved. A consequence was a risk that insufficient longer-term outcome data would be available for the health economic evaluation. Through collaboration between the international trial team, UK funder, sponsor and ethics committees, we were able to gain approval to extend recruitment in the UK until the database for the international cohort was locked for analysis. This approach allowed us to maximise the use of funds in the UK and obtain a larger data set for economic evaluation. These processes could have been improved and shortened if funding across international collaborators could be considered in a more parallel approach, or the review process for commissioned research could be shortened further once an international trial was under way. In addition, pre-agreed arrangements for sponsorship, shared protocols and data governance between the NIHR and equivalent international funders might have reduced delays and workload.
The UK ABLE trial was the first, to our knowledge, to require considerable research activity from blood bank staff across multiple NHS sites. Blood banks were required to manage group allocation and blinding, which involved implementation of study-specific SOPs and blood inventory management. We encountered several challenges that resulted in significant delays and limited recruitment. First, some blood banks were managed by the blood transfusion service, some by the NHS, and some were contracted partly/wholly to private providers; this resulted in contractual and governance challenges that delayed agreements. Second, almost all blood banks worked under intense pressure with staff shortages and competing activities that took priority over research, notably inspections. As a result, and despite the provision of set-up funding and generous per-patient remuneration, many blood banks were unable to complete training and site set-up procedures in a timely manner, because they were unable to free up dedicated time for these activities. This occurred despite strong support from clinical and technical staff within centres, and enthusiasm to participate in the trial. The nature of the work also required relatively senior staff to implement set-up and training, and the group allocation procedures. Employing blood bank co-ordinators on the project management team, who visited and supported all blood banks, was crucial in minimising these delays and ensuring governance issues were addressed in a consistent manner. Our experience strongly supports the use of similar dedicated individuals in any future trials involving extensive blood bank procedures across multiple sites. We would also recommend identifying methods to protect blood bank staff time early in the trial to ensure set-up times are minimised, as this was the most frequent cause of delays. Finding ways to enable blood bank procedures to occur outside normal working hours would also have significantly increased recruitment, as this was the reason for non-inclusion of otherwise eligible patients in 75% of cases.
The UK ABLE trial required recruitment of incapacitated patients in a time-sensitive manner, as decisions were required within a few hours in order to avoid delays in blood transfusions that had been prescribed for clinical reasons. This resulted in a high level of complexity in the consent procedures, which resulted in significant resource use and limited recruitment. First, different approaches were required in England/Northern Ireland versus Scotland, as a result of different mental capacity legislation. Specifically, in Scotland, patients could not be included unless consent was obtained from a relative/welfare guardian prior to randomisation. This meant that patients for whom a relative/welfare guardian was unavailable during the short recruitment window could not be included in the trial. The provision for telephone consent was essential to decrease the impact of this limitation. The experience of research staff, based on informal feedback, was that this was acceptable to most relatives. In England/Northern Ireland, the ability to ask a professional consultee was available, which reduced the pressure on research staff and made enrolment potentially easier. Experience during the trial suggested that the English legislation improved efficiency and was easier to implement. In all cases (Scotland and England/Northern Ireland), the intention was to discuss ongoing continuation in the trial with survivors once they regained capacity. This was often many days/weeks after randomisation and after discharge from the ICU, which required significant time commitment from research staff and was sometimes unsuccessful if patients had been discharged home. There was only one withdrawal of consent for a patient who regained capacity, suggesting that these processes were acceptable. After seeking clarification from the ethics committee, we amended the protocol (amendment 4) to enable patients to be contacted after hospital discharge with information and for consent to remain in the trial. This appeared to be acceptable to patients; however, when no response was obtained, it meant that the patient remained in the trial based on the agreement provided in hospital by the professional or personal consultee (England/Northern Ireland) or relative/welfare guardian (Scotland). The complexity of these processes illustrates the ethics and governance challenges of critical care research, even when the intervention and control group practices were all within current routine care, as was the case in the trial. Our experience strongly supports the ability to use telephone consent when appropriate (especially in Scotland), and the use of professional consultees when relatives were not available (in England/Northern Ireland). Early clarification of procedures to be used when follow-on consent is not obtained should also be considered in future similar research, including contact post hospital discharge. Our experience also highlights the high resource costs of obtaining consent in critical care trials, which should be recognised in funding applications.
We found the use of regular teleconferences with site representatives useful in maintaining enthusiasm, recruitment rates and, especially, for identifying and sharing common problems and finding solutions. The development of a ‘top tips’ resource and a summary of common blocks to recruitment and solutions that worked well and less well was effective, especially as a ‘living’ document that was regularly updated and circulated. The use of recruitment competitions and prizes for key milestones was also popular and maintained the engagement of research nurses/co-ordinators who were key to ongoing patient identification. Our experience supports the use of multiple strategies to support sites and maintain buy-in, which we believe especially important in critical care trials, which are challenging at multiple levels.
Our experience confirms the challenges found in other critical care trials, which included long-term follow-up for economic evaluation by obtaining questionnaire-based follow-up for up to 12 months. We achieved 61% and 58% completed follow-up at 6 and 12 months, respectively, for questionnaires for surviving patients, which equated to 75% and 74% complete follow-up when death was included as an outcome, respectively. This compared favourably with other recent UK-based critical care trials in which follow-up rates of 30–40% were typical. After a single postal questionnaire, completion rates were only 48% and 49% at 6 and 12 months, respectively. We included a gift token with these questionnaires, which may have improved responses, but this is difficult to assess. The use of a second questionnaire request (at 12 months only) followed by attempted telephone contact improved response rates by 18–20%. Given the increasing interest in longer-term HRQoL outcomes following critical illness, the recognition that these are significantly reduced in many patients, and of their impact on QALYs and cost-effectiveness analysis, obtaining high follow-up rates are important. Our data suggest that including a multifaceted strategy that includes both postal and telephone-based methods, and costing the resources to undertake this, is important in future research. Our embedded audit suggesting an average of 30 minutes per patient follow-up is useful for future grant-costing in this population.
Clinical results
The results of the international ABLE trial were published in March 2015, before the UK ABLE trial follow-up was completed. 31 No significant effects (beneficial or harmful) were found from the use of fresh versus standard-aged RBCs on mortality at 90 days, or any of the predefined secondary outcomes. These included the duration of respiratory, cardiovascular and renal support, length of stay or any predefined AEs, especially transfusion reactions. There were also no differences in outcomes within any of the predefined subgroups, including medical, surgical and trauma admissions. Importantly, the RBC transfusion triggers used were similar to those recommended in current guidelines11,66,67 and a large separation in RBC age was achieved between the groups (mean 6.1 vs. 22.0 days). The standard-aged RBC group was similar to the RBC storage age profile that is used in the NHS. A high rate of compliance with group allocation was achieved, and the findings of sensitivity analyses that included only patients who received RBC transfusions and those that included patients who received only RBCs with storage age according to group allocation were almost identical. The study population had a very high illness severity based on their illness severity score, the degree of organ failure at baseline and during the intervention, and the high mortality rate of 33% at 90 days. This observed control group mortality rate was higher than the 25% on which the sample size was calculated for the international trial. However, the aim was to include patients with a mortality rate that was at least 25%, such that the trial achieved recruitment of the target patient group. The mean length of ICU and hospital stay of 14 days and 32 days, respectively, highlights the extremely high resource use associated with these patients. The 95% CIs around the effect observed in the international trial ranged from 2.1% lower mortality with fresher RBCs to 5.5% lower mortality with standard-aged RBCs. These data provided compelling evidence that fresher RBCs conveyed no clinical benefit to the study population included in the trial, especially as the non-significant direction of effect favoured the standard-aged blood group.
In this report, we analysed the UK cohort according to the predefined analysis plan, and also compared findings with the international cohort. This analysis was underpowered for the clinical outcomes and was undertaken in order to assess if any major differences were present in the UK population in terms of baseline characteristics, transfusion practice, study conduct or clinical outcomes. Our data show that the critically ill patients included in the UK ABLE trial were very similar to those included in the international trial. The only possible exception was a greater representation of surgical patients and those undergoing elective procedures, but these differences were small and unlikely to be clinically relevant. The clinical outcomes were very similar for both the primary and all secondary outcomes. In addition, the process measures, especially the transfusion practice based on the mean pre-transfusion haemoglobin concentration, were very similar.
In summary, the ABLE trial found no clinically or statistically important benefit from transfusing exclusively RBCs stored for ≤ 7 days compared with the current standard practices used in blood banks. There were no important differences between the UK cohort and the international cohort in patient characteristics or outcomes.
Strengths and weaknesses of the clinical trial
The strengths of the international ABLE trial included the sufficiently large sample size to detect a clinically important difference in mortality, and a range of relevant secondary outcome measures. The CIs around the estimations of effect make an important beneficial effect from using fresh RBCs compared with the current standard very unlikely in the population studied. This population included a heterogeneous spectrum of critically ill patients whose baseline characteristics were typical of the sickest patients admitted to ICUs in the UK and other countries. The international participation increased the external generalisability of the trial results. The design minimised the chance of bias through allocation concealment, blinding and low loss to follow-up for the outcomes at 90 days. There was a high rate of protocol compliance, which achieved excellent separation of RBC storage age. The pre-planned sensitivity analyses also all showed similar effects to the main analysis.
The trial has potential weaknesses. The recruited population was heterogeneous, which means that some subgroups in whom different effects may occur could have been under-represented. The enrolment criteria meant that previously transfused patients and those in whom transfusion could not be delayed were not included. This probably explains the relatively low representation of massively transfused patients, trauma patients and patients with primary bleeding diagnoses, such as gastrointestinal bleeding. The embedded audit undertaken during the UK trial showed that 46% of all ICU admissions received RBCs during their hospitalisation, mostly before and during ICU care. Twenty-three per cent of patients received RBCs before ICU admission, and were excluded from the trial for this reason. We also found that only 28% of all transfused patients were potentially eligible for the trial, and only 22% of these were enrolled. By far the most common reason for non-inclusion was transfusion at night or outside usual working hours. These data raise the possibility that the patients not enrolled based on inclusion criteria and/or logistic reasons were systematically different from the trial cohort. As these patients may have been over-represented by diagnoses or complications that mandated more rapid or larger RBC transfusions, this might limit the generalisability to all critically ill patients. The transfusion practice was close to current evidence-based guidelines, which suggest that transfusion triggers close to 70 g/l for most patients, especially in the absence of cardiovascular disease. However, there was clearly some variation between clinicians and centres in the use of RBCs. Although this was inevitable in a pragmatic trial in which this aspect of care was not controlled, it could potentially create some ‘noise’ in the effects observed. Any variation in transfusion decision-making was balanced between the groups, so the impact of this potential confounder was unlikely to be important.
Importantly, the ABLE trial did not test the hypothesis that fresh RBCs were superior to ‘old’ RBCs with a storage duration close to the current maximum storage times of 35–42 days. It is possible that the additional effects of storage during this period could alter the effectiveness of the blood product. The conclusions of the ABLE trial are therefore limited to the comparison of the current typical storage age with exclusively fresher RBCs.
Economic evaluation
We undertook a pre-planned series of analyses that were specific to the UK ABLE trial, and also of potentially more generic value to critical care economic evaluations. These comprised a cost–utility analysis of the value of fresh RBCs compared with standard-aged RBCs, a nested study comparing the EQ-5D-3L and EQ-5D-5L utility scores in critical care survivors and an exploration of factors associated with health-care resource use and QALYs following critical illness. All analyses used the data collected from the UK ABLE trial data set.
Cost–utility analysis
Our economic analysis to evaluate the cost-effectiveness of using fresh blood units versus standard-aged blood for transfusions in critically ill patients in the UK showed that there were no differences in terms of costs and outcomes. The univariate sensitivity analysis showed that the results were not sensitive to the assumptions made, and the probabilistic sensitivity analysis indicated that the probability that use of fresh blood was cost-effective was close to the probability that standard-aged blood was cost-effective. The findings mean that there is no reason to prefer fresh blood to standard-aged blood on the basis of differences in quality or length of life, or on cost grounds.
The main strength of the analysis is that it is based on a large multicentre randomised trial with detailed information on resource use, utility values and mortality up to 12 months. However, there are several limitations. First, we assumed that the cost of fresh and standard-aged blood units were the same, but this might not be true. On the one hand, standard-aged blood might incur extra costs as a result of the longer storage time; on the other hand, organising blood supplies and blood banks to provide exclusively fresh blood might incur greater costs, plus there might be a higher likelihood of wasting unused blood. Our sensitivity analysis showed that when changing the costs of blood, the conclusions do not change. Second, during the 12-month follow-up we made assumptions regarding the duration of contacts during follow-up, as this information was not collected in the trial. These costs represent a small proportion of the total costs, and the mean number of contacts per patient was similar in the two groups, so these assumptions are unlikely to make any difference to the overall findings. Third, during follow-up the reason for the hospital appointments and some hospital readmissions were not always stated; we therefore used a diagnosis-specific unit cost where possible, and an average cost where diagnostic information was not available. Fourth, for hospital readmissions, if the patient was admitted to the ICU then data were not collected on the number of days spent in the ICU, only whether or not the patient was admitted to the ICU. We assumed that each ICU admission lasted 1 day. This would almost certainly underestimate the costs of readmissions, but was unlikely to affect the relative cost-effectiveness of the two groups, as the number of ICU admissions was small. Fifth, QALY data were collected using either the EQ-5D-3L or the EQ-5D-5L in different patients; each UK study centre was randomly allocated to one version. As shown in Chapter 6, the use of different measures did result in a difference in utility scores by treatment allocation. Sixth, because of the nature of the patients who are treated in the ICU at randomisation (incapacitated emergency admissions), no baseline data were collected for the QALYs, so we assumed a baseline value of zero, which is consistent with previous studies. Further research would be beneficial to investigate the impact and validity of this approach, and if other methodological approaches should be adopted. Seventh, utility data were collected at two time points only, 6 and 12 months. Data from more frequent measurement of utilities would have been useful, but was not considered to be feasible given the challenges of complete follow-up in this group. Additionally, more frequent measurement is unlikely to have affected the results of the economic analysis, given that there were no between-group differences in any of the primary or secondary outcome measures, and nor were there differences in utilities at 6 and 12 months. Eighth, we did not have complete data for every participant in the trial and used multiple imputation. However, conclusions regarding the cost-effectiveness of fresh blood versus standard-aged blood were the same for analyses using multiple imputation and complete cases. Ninth, a wider perspective (e.g. societal) could have been taken, including costs to patients, families and productivity. Given that the trial found no differences in QALYs between the two groups, it is highly unlikely that this would affect the incremental costs between the two groups and therefore the overall conclusions of the analysis.
Comparison of the two EuroQol-5 Dimensions versions for assessing health-related quality of life in critical care survivors
We evaluated two versions of the EQ-5D at different time points and found some evidence of disagreement between the two measures, even after controlling for differences in patient characteristics. Although there was some evidence that EQ-5D-5L scores were higher than EQ-5D-3L scores, these differences did not affect utility scores associated with fresh versus standard-aged blood, which were not different for either measure at both time points when controlling for differences in patient characteristics. This result is unsurprising, given the results of the ABLE trial, which found no significant differences in the primary and secondary outcomes between treatment groups.
There have been several studies comparing the EQ-5D-5L and EQ-5D-3L in different populations, generally showing that the EQ-5D-5L performs better than the EQ-5D-3L as a result of reduction in ceiling effects49–51,53–55,57–59 and ability to discriminate between patient characteristics. 52–54,56,57 Some studies have shown a high level of agreement between the two measures. 50,51,54,57,59 There have been no studies comparing the two instruments in critical care survivors.
There were no differences in ceiling effects between the two utility measures, and there was some evidence that the EQ-5D-5L discriminated between subgroups of patients with major comorbidities, while the EQ-5D-3L did not. The lack of a difference in ceiling effects may be a result of the relatively low proportion of patients in our sample reporting best possible quality of life compared with respondents in other studies. For example, in our sample, 9–15% of patients reported being in the best possible health state, compared with 47–56% in a recent study using an English general population sample. 49 This difference is likely to reflect the substantial long-term health burden on patients associated with critical care survivorship. 15
There are two major limitations to this analysis. The first is that the two EQ-5D measures were not collected in the same patients, so between-measure differences in utility may reflect differences in patient characteristics. We controlled for a range of patient characteristics, including pre-existing significant comorbid illnesses, but we cannot rule out the possibility that differences in unobserved patient characteristics may explain the observed difference in utility scores at 12 months. The second major limitation is the sample size; our study originally included 359 patients, but mortality, questionnaire non-response and analyses by subgroups meant that sample sizes in the study were ultimately small, potentially reducing the likelihood of finding statistically significant differences between groups. A further limitation is that the patient characteristics, especially the comorbidities, were measured at hospital admission, 6–12 months prior to EQ-5D measurement. This might partly explain the non-significant impact of many of the patient characteristics on utility scores in our analysis. On the other hand, the comorbidities were collected in the trial because they are likely to have a significant impact on health and persist over time. Nonetheless, analyses with repeated characteristic measurement at the same time as utility measurement would be useful, and would also permit an evaluation of the two utility measures as health changes over time. Finally, we did not achieve a full response rate to the EQ-5D questionnaires among survivors; response rates were 53–54% at the two time points and the non-responders may not be a random sample of trial participants; on the plus side, the response rate was higher than in another recent study examining differences in utility scores between critical care patients (36%). 68
Further research would be beneficial to repeat the analysis with a larger cohort of critical care survivors, using a study design in which patients completed both EQ-5D questionnaires, along with repeated assessment of health and other characteristics. This would increase confidence that any observed differences between the two utility measures did not result from differences in patient characteristics, and would permit more sophisticated analyses.
Factors associated with health-care costs and quality-adjusted life-years
We evaluated the factors associated with hospital costs and total costs and QALYs up to 12 months after ICU admission in critically ill adults in the UK. None of the variables in our data was a significant predictor of utility scores at 6 or 12 months or of QALYs up to 12 months. The lack of an effect may reflect the limitations of our data set in terms of available measures, sample size and data missingness. Consistent with previous studies,62 we found that ICU costs were an increasing function of illness severity, measured in terms of MODS score and mortality. Contrary to previous studies, costs from hospital discharge up to 12 months were largely unaffected by acute illness factors, age or comorbidities, although there was some evidence that chronic institutionalisation, included as an indicator of pre-existing morbidity was associated with 12-month costs. 15,63
There are several limitations to this analysis. First, our study did not include a control group, for example hospital patients not admitted to the ICU or a before-and-after comparison of ICU patients, which have been used in previous studies. 15,63 Second, there were limited covariates in our data set, in terms of pre-existing morbidities and other variables that have been shown to predict long-term outcomes and resource use in previous studies, such as prior hospitalisations. Third, the sample size was limited; our study originally included 357 patients, but mortality and questionnaire non-response meant that sample sizes in the study were ultimately small, potentially reducing the likelihood of finding statistically significant differences between groups. We did not achieve a full response rate to the follow-up questionnaires among survivors; response rates were 45–55% and the non-responders may not be a random sample of trial participants.
In conclusion, we found that ICU costs were an increasing function of illness severity, measured in terms of MODS score and mortality. Further research would be beneficial to investigate the factors associated with longer-term costs and outcomes.
Results in context of other research
Several systematic reviews have been published during the conduct of the ABLE trial. A meta-analysis by Wang et al. 69 in 2012 of 18 observational studies and three RCTs, predominantly in cardiac surgery and trauma patients, concluded that older blood was associated with an increased risk of death. A larger meta-analysis by Lelubre and Vincent in 201370 included 55 studies, of which only eight were RCTs. This showed no superiority of fresher RBCs over a range of morbidity outcomes (infection, bleeding, duration of MV, multiorgan dysfunction, length of stay) and mortality. Authors commented on significant confounding factors in interpreting these analyses, namely retrospective designs, heterogeneous populations, small studies and the exposed volume of RBCs. Sicker patients with comorbidities tend to receive more RBC units, such that inferring causality of adverse effects to RBC storage duration is challenging, as evidenced by a meta-analysis, which showed positive results for studies that did not adjust for this compared with those that did.
A Cochrane review of RCTs assessed the effects of age of stored RBCs in people requiring transfusion. 71 Only RCTs were included, with participants of any age (from neonates to adults) requiring transfusion for anaemia of any aetiology. The primary outcome was mortality within 7 days and 30 days after transfusion, with longer-term mortality as a secondary outcome. In total, 16 RCTs were eligible for inclusion in the review with a total of 1864 participants of all ages; five RCTs on neonates, one on paediatric patients and 10 on adults. All included studies followed similar design and analysis strategies; there were two randomised groups of participants, one receiving ‘fresher’ blood than the other, implying dichotomous outcomes, which were examined based on risk ratios when comparing the two groups. There were five trials dealing with transfusion in low-birthweight neonates, with the rest of the trials dealing with anaemia (including malarial anaemia in a paediatric population), cardiac surgery and treatment for critical illness, trauma and the ICU. There were only 2 out of the 16 studies that assigned > 100 patients, with the two larger trials assigning 377 and 910 patients. Five feasibility trials were also included as precursors to larger trials, including the two trials that underpinned the ABLE trial. 9,10 There were differences recorded between the included studies in the additive solutions used to preserve RBCs and whether or not RBCs were leuco-reduced and/or irradiated, and four studies did not report on this information. There were differences between the protocol definitions and the actual age of stored blood used, and many studies reported significant protocol violations, often apparently because of daily fluctuations of stored blood availability. The lack of agreed definitions of the ‘age of blood’ and, as a consequence, the variability of the recorded cut-off points between the included studies were major impediments in exploring the relationship between the length of time of stored blood and the risk of transfusion in this and other reviews. The multiple transfusions required by many patients and the inconsistent reporting of data details regarding the actual age of transfused blood served as compounding factors for the methodological challenges encountered. The studies in the review reported in total three groupings for comparison of the RBC product: ‘fresher’, ‘standard practice’ and ‘actively older’ blood. Eight trials compared transfusion of ‘fresher’ with ‘actively older’ blood, with the remaining eight comparing ‘fresher’ with ‘standard practice’ blood. The definitions of ‘fresh’, ‘standard’ and ‘older’ were not consistent among studies. In many trials, the ranges overlapped such that the distinction between trial groups was small and of questionable relevance. No conclusions could be drawn from the available evidence from RCTs that preceded the ABLE trial.
In summary, these reviews highlight the lack of high-quality evidence prior to the completion and publication of the ABLE trial, and the uncertainty regarding the clinical importance of RBC storage age for clinical outcomes.
An important concurrent RCT to the ABLE trial was RECESS,72 which was a multicentre RCT conducted between 2010 and 2014. Participants were aged ≥ 12 years and undergoing complex cardiac surgery, and likely to require RBC transfusion. Patients were randomly assigned to receive leucocyte-reduced RBCs stored for ≤ 10 days (shorter-term storage group) or for ≥ 21 days (longer-term storage group) for all intraoperative and postoperative transfusions. The primary outcome was the change in MODS score (range of 0–24 points, with higher scores indicating more severe organ dysfunction) from the pre-operative score to the highest composite score through day 7 or the time of death or discharge. The median storage time of RBCs provided to the 1098 participants who received RBC transfusion was 7 days in the shorter-term storage group and 28 days in the longer-term storage group. The mean change in MODS score was an increase of 8.5 points and 8.7 points, respectively (95% CI for the difference: −0.6 to 0.3 points; p = 0.44). The 7-day mortality was 2.8% in the shorter-term storage group and 2.0% in the longer-term storage group (p = 0.43); 28-day mortality was 4.4% and 5.3%, respectively (p = 0.57). AEs did not differ significantly between groups, except that hyperbilirubinaemia was more common in the longer-term storage group. This trial found no evidence of a clinically or statistically important difference in development of organ dysfunction or any of the other secondary outcome measures, associated with the storage age of RBCs. Importantly, RECESS provides similar findings to the ABLE trial in groups at a much lower risk of death, and compared a fresher RBC storage age group with a group allocated to receive RBCs that were, on average, 7 days older than the standard age of RBCs used in the ABLE trial control group.
Another relevant trial, INFORM (INforming Fresh versus Old Red cell Management; ISRCTN08118744),73 was published since the main publication describing the ABLE trial. This was a hospital-wide study randomising any patient requiring a RBC transfusion to receive the freshest versus the oldest available RBCs in the blood bank during their hospital stay in a 1 : 2 ratio. 18 The trial was undertaken in six hospitals and included 20,858 patients with type A or O blood. Of these patients, 6936 were assigned to the short-term storage group and 13,922 to the long-term storage group. The primary outcome was in-hospital mortality, which was estimated by means of a logistic regression model after adjustment for study centre and patient blood type. The mean storage duration was 13.0 days in the short-term storage group and 23.6 days in the long-term storage group (achieving the pre-planned mean separation of 10 days). There were 634 deaths (9.1%) in the short-term storage group and 1213 (8.7%) in the long-term storage group (odds ratio 1.05, 95% CI 0.95 to 1.16; p = 0.34). The results were consistent in three prespecified high-risk subgroups (patients undergoing cardiovascular surgery, those admitted to intensive care and those with cancer) indicating consistent lack of differences in three contrasting groups at different risk of short- and long-term mortality. Importantly, the effects seen in critically ill patients were similar to those found in the ABLE trial, in whom a similar ‘usual-care’ comparator storage age was compared with an overall ‘fresher’ storage age. The risk of death was lower in the INFORM trial ICU population (older storage age, 12.8%; shorter storage age, 13.3%), but these results provide evidence that the ABLE trial findings are likely to be applicable to a large number of ICU patients, despite the narrower inclusion criteria used in the trial.
The TRANSFUSE trial74 (a multi-centre randomised double-blinded Phase III trial of the effect of standard issue red blood cell blood units on mortality compared to freshest available red blood cell units; ClinicalTrials.gov NCT01638416) is a large trial in critically ill patients (n = 5000), in which patients requiring RBC transfusion are randomised to receive either the freshest RBC units available in the blood bank at the time of request or standard-issue RBCs (similar in design to the INFORM trial). 73 This trial is ongoing and the results are not yet available at the time of writing.
Implications for practice
The ABLE trial provides evidence that there is no clinical or economic rationale for transfusing exclusively fresher RBCs to patients at high risk of death in the ICU, when RBC transfusion decisions are at the discretion of the clinician. Blood banks should not change their current practice of providing standard-aged RBCs to this patient group, assuming the blood product is typically aged, on average, 20–22 days, and is leuco-reduced. Taken together with RECESS72 and INFORM73 trial findings, the current evidence base does not support the routine or widespread use of fresher RBCs more than those provided as usual practice (with a mean storage age of 20–22 days).
The ABLE trial does not provide evidence that RBCs with an older storage age are as safe as standard-aged RBCs, especially those with a storage age close to the end of current storage times of 35–42 days. However, most blood banks and blood services actively manage blood stocks such that the proportion of RBCs transfused at the end of storage lifespan is relatively small.
Acknowledgements
Contributions of authors
Timothy S Walsh (Professor of Critical Care, University of Edinburgh; specialty: critical care and trial design/management) secured funding, contributed to the study design, led the UK trial and contributed to analysis and interpretation.
Simon Stanworth (Consultant Haematologist; specialty: transfusion medicine and trial design/management) secured funding, contributed to the study design, was haematology lead, contributed to trial management and contributed to analysis and interpretation.
Julia Boyd (Trial Manager, Edinburgh Clinical Trials Unit, University of Edinburgh; specialty: trial management) was trial manager and contributed to analysis and interpretation.
David Hope (Critical Care Research Co-ordinator, NHS Lothian; specialty: trial set-up and management) was research co-ordinator and contributed to trial management.
Sue Hemmatapour (Blood Bank Trial Co-ordinator, University of Oxford; specialty: transfusion scientist) was blood bank research co-ordinator and contributed to trial management.
Helen Burrows (Blood Bank Trial Co-ordinator, University of Oxford; specialty: transfusion scientist) was blood bank research co-ordinator and contributed to trial management.
Helen Campbell (Health Economist, University of Oxford; specialty: health economics) secured funding, designed the health economic evaluation and contributed to trial design and management.
Elena Pizzo (Senior Research Associate in Health Economics, University College London; specialty: health economics) contributed to the analysis and interpretation of the health economic evaluation.
Nicholas Swart (Research Associate in Health Economics, University College London; specialty: health economics) contributed to the analysis and interpretation of the health economic evaluation.
Stephen Morris (Professor of Health Economics, University College London; specialty: health economics) led and contributed to the analysis and interpretation of the health economic evaluation.
Publications
The international ABLE trial protocol was published in 2011:
Lacroix J, Hébert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, et al. The Age of Blood Evaluation (ABLE) randomised controlled trial: study design. Transfus Med Rev 2011;25:197–205.
The results of the international ABLE trial were published in 2015:
Lacroix J, Hébert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015;372:1410–18.
Data sharing statement
The international ABLE trial data are governed by data sharing agreements between the co-investigators. This states that any application to view or utilise the data should be made through the international TSC via the chairperson of the group and ABLE trial chief investigator, Jacques Lacroix.
For the UK health economic evaluation and longer-term follow-up for health-care costs and HRQoL, access to data can be facilitated through the UK chief investigator, Professor Timothy S Walsh, or the UK health economic lead investigator, Professor Stephen Morris.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, et al. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev 2006;20:273-82. https://doi.org/10.1016/j.tmrv.2006.05.002.
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015;350. https://doi.org/10.1136/bmj.h1354.
- Carson JL, Carless PA, Hébert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA 2013;309:83-4. https://doi.org/10.1001/jama.2012.50429.
- Carson JL, Carless PA, Hébert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD002042.pub3.
- D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus 2010;8:82-8.
- Vamvakas EC. Meta-analysis of clinical studies of the purported deleterious effects of ‘old’ (versus ‘fresh’) red blood cells: are we at equipoise?. Transfusion 2010;50:600-10. https://doi.org/10.1111/j.1537-2995.2009.02465.x.
- Wilkinson KL, Brunskill SJ, Dorée C, Hopewell S, Stanworth S, Murphy MF, et al. The clinical effects of red blood cell transfusions: an overview of the randomized controlled trials evidence base. Transfus Med Rev 2011;25:145-55.e2. https://doi.org/10.1016/j.tmrv.2010.11.006.
- Tinmouth A, Fergusson D, Yee IC, Hébert PC. ABLE Investigators . Clinical consequences of red cell storage in the critically ill. Transfusion 2006;46:2014-27. https://doi.org/10.1111/j.1537-2995.2006.01026.x.
- Walsh TS, McArdle F, McLellan SA, Maciver C, Maginnis M, Prescott RJ, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients?. Crit Care Med 2004;32:364-71. https://doi.org/10.1097/01.CCM.0000108878.23703.E0.
- Hébert PC, Chin-Yee I, Fergusson D, Blajchman M, Martineau R, Clinch J, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg 2005;100:1433-8. https://doi.org/10.1213/01.ANE.0000148690.48803.27.
- Napolitano LM, Kurek S, Luchette FA, Anderson GL, Bard MR, Bromberg W, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. J Trauma 2009;67:1439-42. https://doi.org/10.1097/TA.0b013e3181ba7074.
- Walsh TS, Wyncoll DL, Stanworth SJ. Managing anaemia in critically ill adults. BMJ 2010;341. https://doi.org/10.1136/bmj.c4408.
- Walsh TS, Garrioch M, Maciver C, Lee RJ, MacKirdy F, McClelland DB, et al. Red cell requirements for intensive care units adhering to evidence-based transfusion guidelines. Transfusion 2004;44:1405-11. https://doi.org/10.1111/j.1537-2995.2004.04085.x.
- Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999;340:409-17. https://doi.org/10.1056/NEJM199902113400601.
- Lone NI, Gillies MA, Haddow C, Dobbie R, Rowan KM, Wild SH, et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med 2016;194:198-20. https://doi.org/10.1164/rccm.201511-2234OC.
- Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang 2009;96:93-103. https://doi.org/10.1111/j.1423-0410.2008.01117.x.
- Lacroix J, Hébert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, et al. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev 2011;25:197-205. https://doi.org/10.1016/j.tmrv.2011.03.001.
- Heddle NM, Cook RJ, Arnold DM, Crowther MA, Warkentin TE, Webert KE, et al. The effect of blood storage duration on in-hospital mortality: a randomized controlled pilot feasibility trial. Transfusion 2012;52:1203-12. https://doi.org/10.1111/j.1537-2995.2011.03521.x.
- Walsh TS, Boyd JA, Watson D, Hope D, Lewis S, Krishan A, et al. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med 2013;41:2354-63. https://doi.org/10.1097/CCM.0b013e318291cce4.
- d’Almeida MS, Gray D, Martin C, Ellis CG, Chin-Yee IH. Effect of prophylactic transfusion of stored RBCs on oxygen reserve in response to acute isovolemic hemorrhage in a rodent model. Transfusion 2001;41:950-6. https://doi.org/10.1046/j.1537-2995.2001.41070950.x.
- d’Almeida MS, Jagger J, Duggan M, White M, Ellis C, Chin-Yee IH. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfus Med 2000;10:291-303. https://doi.org/10.1046/j.1365-3148.2000.00267.x.
- Raat NJ, Verhoeven AJ, Mik EG, Gouwerok CW, Verhaar R, Goedhart PT, et al. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model. Crit Care Med 2005;33:39-45. https://doi.org/10.1097/01.CCM.0000150655.75519.02.
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008;358:1229-39. https://doi.org/10.1056/NEJMoa070403.
- Middelburg RA, van de Watering LM, van der Bom JG. Blood transfusions: good or bad? Confounding by indication, an underestimated problem in clinical transfusion research. Transfusion 2010;50:1181-3. https://doi.org/10.1111/j.1537-2995.2010.02675.x.
- Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA 1993;269:3024-9. https://doi.org/10.1001/jama.1993.03500230106037.
- Hébert PC, Fergusson D, Blajchman MA, Wells GA, Kmetic A, Coyle D, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA 2003;289:1941-9. https://doi.org/10.1001/jama.289.15.1941.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Adults with Incapacity (Scotland) Act 2000. Edinburgh: Scottish Government; 2000.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Devlin N, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England 2016. eprints.whiterose.ac.uk/97964/ (accessed 26 July 2016).
- Lacroix J, Hébert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015;372:1410-18. https://doi.org/10.1056/NEJMoa1500704.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2015.
- Campbell HE, Stokes EA, Bargo DN, Curry N, Lecky FE, Edwards A, et al. Quantifying the healthcare costs of treating severely bleeding major trauma patients: a national study for England. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-0987-5.
- NHS Reference Costs 2015 to 2016. London: Department of Health; 2016.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2013.
- Blood and Transplant Price List 2014–15. London: NHS; 2015.
- Sepsis: Recognition, Diagnosis and Early Management. London: NICE; 2016.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Improve Trial Investigators . Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial. Eur Heart J 2015;36:2061-9. https://doi.org/10.1093/eurheartj/ehv125.
- Szende A, Oppe M, Devlin N. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. Rotterdam: Springer; 2007.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing. . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. https://doi.org/10.1002/hec.766.
- Briggs AH. Handling uncertainty in cost-effectiveness models. PharmacoEconomics 2000;17:479-500. http://dx.doi.org/10.2165/00019053-200017050-00006.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- Angus DC, Carlet J. 2002 Brussels Roundtable Participants. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med 2003;29:368-77. https://doi.org/10.1007/s00134-002-1624-8.
- Harrison DA, Prabhu G, Grieve R, Harvey SE, Sadique MZ, Gomes M, et al. Risk Adjustment In Neurocritical care (RAIN) – prospective validation of risk prediction models for adult patients with acute traumatic brain injury to use to evaluate the optimum location and comparative costs of neurocritical care: a cohort study. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17230.
- Harvey SE, Parrott F, Harrison DA, Sadique MZ, Grieve RD, Canter RR, et al. A multicentre, randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of early nutritional support via the parenteral versus the enteral route in critically ill patients (CALORIES). Health Technol Assess 2016;20. http://dx.doi.org/10.3310/hta20280.
- Hernández RA, Jenkinson D, Vale L, Cuthbertson BH. Economic evaluation of nurse-led intensive care follow-up programmes compared with standard care: the PRaCTICaL trial. Eur J Health Econ 2014;15:243-52. https://doi.org/10.1007/s10198-013-0470-7.
- Feng Y, Devlin N, Herdman M. Assessing the health of the general population in England: how do the three- and five-level versions of EQ-5D compare?. Health Qual Life Outcomes 2015;13. https://doi.org/10.1186/s12955-015-0356-8.
- Ferreira LN, Ferreira PL, Ribeiro FP, Pereira LN. Comparing the performance of the EQ-5D-3L and the EQ-5D-5L in young Portuguese adults. Health Qual Life Outcomes 2016;14. https://doi.org/10.1186/s12955-016-0491-x.
- Agborsangaya CB, Lahtinen M, Cooke T, Johnson JA. Comparing the EQ-5D 3L and 5L: measurement properties and association with chronic conditions and multimorbidity in the general population. Health Qual Life Outcomes 2014;12. https://doi.org/10.1186/1477-7525-12-74.
- Greene ME, Rader KA, Garellick G, Malchau H, Freiberg AA, Rolfson O. The EQ-5D-5L Improves on the EQ-5D-3L for Health-related Quality-of-life Assessment in Patients Undergoing Total Hip Arthroplasty. Clin Orthop Relat Res 2015;473:3383-90. https://doi.org/10.1007/s11999-014-4091-y.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- Jia YX, Cui FQ, Li L, Zhang DL, Zhang GM, Wang FZ, et al. Comparison between the EQ-5D-5L and the EQ-5D-3L in patients with hepatitis B. Qual Life Res 2014;23:2355-63. https://doi.org/10.1007/s11136-014-0670-3.
- Kim SH, Kim HJ, Lee SI, Jo MW. Comparing the psychometric properties of the EQ-5D-3L and EQ-5D-5L in cancer patients in Korea. Qual Life Res 2012;21:1065-73. https://doi.org/10.1007/s11136-011-0018-1.
- Pan CW, Sun HP, Wang X, Ma Q, Xu Y, Luo N, et al. The EQ-5D-5L index score is more discriminative than the EQ-5D-3L index score in diabetes patients. Qual Life Res 2015;24:1767-74. https://doi.org/10.1007/s11136-014-0902-6.
- Pattanaphesaj J, Thavorncharoensap M. Measurement properties of the EQ-5D-5L compared to EQ-5D-3L in the Thai diabetes patients. Health Qual Life Outcomes 2015;13. https://doi.org/10.1186/s12955-014-0203-3.
- Scalone L, Ciampichini R, Fagiuoli S, Gardini I, Fusco F, Gaeta L, et al. Comparing the performance of the standard EQ-5D 3L with the new version EQ-5D 5L in patients with chronic hepatic diseases. Qual Life Res 2013;22:1707-16. https://doi.org/10.1007/s11136-012-0318-0.
- Yfantopoulos JN, Chantzaras AE. Validation and comparison of the psychometric properties of the EQ-5D-3L and EQ-5D-5L instruments in Greece. Eur J Health Econ 2017;18:519-31. http://dx.doi.org/10.1007/s10198-016-0807-0.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Lone NI, Seretny M, Wild SH, Rowan KM, Murray GD, Walsh TS. Surviving intensive care: a systematic review of healthcare resource use after hospital discharge. Crit Care Med 2013;41:1832-43. https://doi.org/10.1097/CCM.0b013e31828a409c.
- Gruenberg DA, Shelton W, Rose SL, Rutter AE, Socaris S, McGee G. Factors influencing length of stay in the intensive care unit. Am J Crit Care 2006;15:502-9.
- Hill AD, Fowler RA, Pinto R, Herridge MS, Cuthbertson BH, Scales DC. Long-term outcomes and healthcare utilization following critical illness – a population-based study. Critical Care 2016;20. https://doi.org/10.1186/s13054-016-1248-y.
- Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197-204. https://doi.org/10.1258/1355819042250249.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987.
- Retter A, Wyncoll D, Pearse R, Carson D, McKechnie S, Stanworth S, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol 2013;160:445-64. https://doi.org/10.1111/bjh.12143.
- Klein AA, Arnold P, Bingham RM, Brohi K, Clark R, Collis R, et al. AAGBI guidelines: the use of blood components and their alternatives 2016. Anaesthesia 2016;71:829-42. https://doi.org/10.1111/anae.13489.
- Vainiola T, Pettilä V, Roine RP, Räsänen P, Rissanen AM, Sintonen H. Comparison of two utility instruments, the EQ-5D and the 15D, in the critical care setting. Intensive Care Med 2010;36:2090-3. https://doi.org/10.1007/s00134-010-1979-1.
- Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion 2012;52:1184-95. https://doi.org/10.1111/j.1537-2995.2011.03466.x.
- Lelubre C, Vincent JL. Relationship between red cell storage duration and outcomes in adults receiving red cell transfusions: a systematic review. Crit Care 2013;17. https://doi.org/10.1186/cc12600.
- Brunskill SJ, Wilkinson KL, Doree C, Trivella M, Stanworth S. Transfusion of fresher versus older red blood cells for all conditions. Cochrane Database Syst Rev 2015;5. https://doi.org/10.1002/14651858.CD010801.pub2.
- Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med 2015;372:1419-29. https://doi.org/10.1056/NEJMoa1414219.
- Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med 2016;375:1937-45. https://doi.org/10.1056/NEJMoa1609014.
- Kaukonen KM, Bailey M, Ady B, Aubron C, French C, Gantner D, et al. A randomised controlled trial of standard transfusion versus fresher red blood cell use in intensive care (TRANSFUSE): protocol and statistical analysis plan. Crit Care Resusc 2014;16:255-61.
Appendix 1 Grant co-applicants and list of Age of BLood Evaluation UK investigators
Grant co-applicants
Professor Timothy S Walsh (University of Edinburgh; Chief Investigator), Professor Danny McAuley (Queens University, Belfast; Co-director of Research UK Intensive Care Society), Professor Gavin Perkins (University of Warwick; Co-director of Research UK Intensive Care Society), Dr Simon Stanworth (NHS Blood and Transplant; University of Oxford), Professor Gordon Murray (University of Edinburgh), Mr Douglas Watson (Scottish National Blood Transfusion Service), Professor Duncan Young (University of Oxford), Dr Helen Campbell (University of Oxford), Dr Duncan Wyncholl (St Thomas’ Hospital, London), Professor Rupert Pearse (Queens Mary’s University, London), Dr Jacques Lacroix (ABLE trial Chief Investigator; University of Montreal, QC, Canada), Dr Stephen Wright (Freeman Hospital, Newcastle), Dr Alan Tinmouth (Ottawa Hospital, ON, Canada) and Dr Dean Fergusson (Ottawa Hospital Research Institute, ON, Canada).
The Age of BLood Evaluation UK investigators
UK Trial Management Group
Timothy S Walsh, Simon Stanworth, Helen Campbell, David Hope, Julia Boyd, Douglas Watson, Helen Burrows, Sue Hemmatapour, Fiona Goddard and Claire Dyer.
UK centres
Royal Infirmary of Edinburgh
Timothy S Walsh, Lynn Manson, Carol McFarlane and David Hope.
Ninewells Hospital, Dundee
Stephen Cole, Sam Rawlinson, Graeme Paterson, Louise Cabrelli and Jackie Duff.
Heartlands Hospital, Birmingham
Gavin Perkins, Matthew Lumley, James Taylor, Teresa Melody, Keith Cooper and Peter Sutton.
Bristol Royal Infirmary
Jeremy Bewley, Tom Latham, Adele Wardle, Louise Flintoff, Denise Webster and Lisa Grimmer.
Leicester Royal Infirmary
Jonathan Thompson, Hafiz Quereshi, Gregg Byrne, Natalie Rich and Sarah Bowrey.
King’s College London
Ritesh Maharaj, Aleksander Mijovic, Matthew Free, Kenneth Amenyah, Daniel Hadfield, Sarah Casboult, Clare Mellis, Clair Harris, Georgina Parsons and Fiona Wade-Smith.
Royal London Hospital
Rupert Pearse, Shubha Allard, Colin Barber, Paul Grist, Kirsty Everingham, Edyta Niebrzegowska and Eleanor McAlees.
St Thomas’ Hospital, London
Duncan Wyncoll, Susan Robinson, Tim Maggs, Katie Lei, Kathryn Chan, John Smith and Barnaby Sanderson.
Freeman Hospital and the Royal Victoria Infirmary, Newcastle
Stephen Wright, Jonathan Wallis, Yvonne Scott, Verity Calder, Carmen Scott and Kayla Harris.
John Radcliffe Hospital and the Churchill Hospital, Oxford
Stuart McKechnie, Simon Stanworth, Julie Staves and Paula Hutton.
Southampton General Hospital
Ravi Gill, Rashid Kazmi, Dawn Smith, Kerry Dowling, Clare Bolger, Karen Salmon and Jessica Piper.
Royal Victoria Hospital, Belfast
James McNamee, Robert Cuthbert, Matt Gillespie, Lia McNamee, Griania White and Leonna Bannon.
Western General Hospital, Edinburgh
Charles Wallis, Huw Roddie, Jonathon Falconer, Heidi Dawson and Gosha Wojcik.
Leeds General Infirmary and St James’s University Hospital
Mark Bellamy, Marina Karakantza, Richard Haggas, Terrence Haines, Diane Howarth, Stuart Elliot and Zoe Beardow.
Royal Berkshire Hospital, Reading
Andrew Walden, Rebecca Sampson, Nicola Mundy, Nicola Jacks and Abby Brown.
Norfolk and Norwich University Hospital
Simon Fletcher, Gillian Turner, Deborah Asher, Melissa Rosbergen and Georgina Glister.
Northampton General Hospital
Jonathan Wilkinson, Sajjan Mittal, Karen Spreckley, Jennifer Spimpolo and Andrea Kempa.
Intensive care unit and blood bank investigators
The intensive care unit principal investigator is listed first and then the blood bank principal investigator.
Royal Infirmary of Edinburgh
Timothy S Walsh and Lynn Manson.
Ninewells Hospital, Dundee
Stephen Cole and Sam Rawlinson.
Heartlands Hospital, Birmingham
Gavin Perkins and Matthew Lumley.
Bristol Royal Infirmary
Jeremy Bewley and Tom Latham.
Leicester Royal Infirmary
Jonathan Thompson and Hafiz Quereshi.
King’s College London
Ritesh Maharaj and Aleksander Mijovic.
Royal London Hospital
Rupert Pearse and Shubha Allard.
St Thomas’ Hospital, London
Duncan Wyncoll and Susan Robinson.
Freeman Hospital and the Royal Victoria Infirmary, Newcastle
Stephen Wright and Jonathan Wallis.
John Radcliffe Hospital and the Churchill Hospital, Oxford
Stuart McKechnie and Simon Stanworth.
Southampton General Hospital
Ravi Gill and Rashid Kazmi.
Royal Victoria Hospital, Belfast
James McNamee and Robert Cuthbert.
Western General Hospital, Edinburgh
Charles Wallis and Huw Roddie.
Leeds General Infirmary and St James’s University Hospital
Mark Bellamy and Marina Karakantza.
Royal Berkshire Hospital, Reading
Andrew Walden and Rebecca Sampson.
Norfolk and Norwich University Hospital
Simon Fletcher and Gillian Turner.
Northampton General Hospital
Jonathan Wilkinson and Sajjan Mittal.
Appendix 2 Participant information sheet
Appendix 3 Participant consent form
Appendix 4 EuroQol-5 Dimensions, three-level version questionnaire
This sample questionnaire has been reproduced with permission from the EuroQol Group. 29
UK (English) © 1990 EuroQol Group EQ-5D™ is a trade mark of the EuroQol group
Appendix 5 EuroQol-5 Dimensions, five-level version questionnaire
This sample questionnaire has been reproduced with permission from the EuroQol Group. 29
UK (English) © 2009 EuroQol Group EQ-5D™ is a trade mark of the EuroQol group
Appendix 6 Health economic questionnaires used in the Age of BLood Evaluation trial
List of abbreviations
- 2,3-DPG
- 2,3-diphosphoglycerate
- A&E
- accident and emergency
- ABLE
- Age of BLood Evaluation
- AE
- adverse event
- APACHE II
- Acute Physiology and Chronic Health Evaluation II
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRRT
- continuous renal replacement therapy
- DSMC
- Data and Safety Monitoring Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GOSE
- Extended Glasgow Outcome Scale
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICU
- intensive care unit
- INFORM
- INforming Fresh versus Old Red cell Management
- INMB
- incremental net monetary benefit
- IQR
- interquartile range
- MODS
- multiple organ dysfunction syndrome
- MV
- mechanical ventilation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OLS
- ordinary least squares
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RBC
- red blood cell
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RECESS
- REd CEll Storage duration Study
- SAE
- serious adverse event
- SD
- standard deviation
- SOP
- standard operating procedure
- TMG
- Trial Management Group
- TRICC
- Transfusion Requirements in Critical Care
- TSC
- Trial Steering Committee
