Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/22/02. The contractual start date was in January 2010. The draft report began editorial review in January 2016 and was accepted for publication in March 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Debra Bick reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme, the NIHR Public Health Research programme, the NIHR Programme Grants for Applied Research programme and the Department of Health Policy Research Programme during the conduct of the study. Peter Brocklehurst reports personal fees from Oxford Analytica, grants from the National Institute for Health and Care Excellence, grants and personal fees from the Medical Research Council (MRC) and grants from the NIHR Health Services and Delivery Research programme, NIHR HTA programme and the Wellcome Trust outside the submitted work, and is chairperson of the NIHR HTA Maternal, Neonatal & Child Health Panel (MNCH). Pollyanna Hardy reports grants from the NIHR HTA programme outside the submitted work. Edmund Juszczak reports grants from the NIHR HTA programme and NIHR Efficacy and Mechanism Evaluation programme outside the submitted work, and is a member of the NIHR HTA programme Commissioning Board. Christine MacArthur reports grants from the NIHR outside the submitted work. Phillip Moore reports grants from the NIHR HTA programme during the conduct of the study. Oliver Rivero-Arias reports grants from MRC, NIHR HS&RD, NIHR HTA outside the submitted work and is a panel member of the NIHR Fellowship Awards and the UK National Screening Committee Fetal, Maternal and Child Health Group. Andrew Shennan reports grants from the MRC, the NIHR Research for Patient Benefit programme and the NIHR HTA programme outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Bick et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
As the most effective form of pain relief in labour, epidural analgesia is chosen by up to 30% of women in the UK each year,1 and this proportion has remained relatively stable over the last decade. 2 The uptake is greater in nulliparous women, with up to 40% of women having an epidural in large obstetric sites. 3 However, a systematic review of 23 randomised controlled trials (RCTs) that compared epidural analgesia with non-regional or no analgesia in labour found that epidural analgesia was associated with an increased risk of instrumental vaginal delivery (IVD) [risk ratio (RR) 1.42, 95% confidence interval (CI) 1.28 to 1.57]. 4
The trials that made the most contribution to the evidence base were conducted with epidural techniques that caused dense neuraxial blockade. Significant peripheral motor blockade, which can accompany conventional high-dose local anaesthetic epidural analgesia, inhibits mobility or the adoption of upright positions in labour. ‘Low-dose epidurals’, which use low-dose local anaesthetic in combination with opioids (usually fentanyl), were introduced in the early 1990s and are now in widespread use in the UK. This approach has been shown to result in a lower risk of IVD;5 however, the rate of IVD is still higher than that in women with no epidural. 6
Reducing the rate of IVD and increasing the spontaneous vaginal birth (SVB) rate would reduce short- and long-term morbidity for women by reducing the risk of perineal trauma and the effects of surgical repair. The incidence of perineal pain, dyspareunia and incontinence following IVD could also be reduced. 7–12 Although mobile epidurals preserve motor function (allowing greater mobility throughout labour) and can enable women to adopt upright positions, there is debate about whether or not an upright posture in the second stage of labour increases the SVB rate.
It is worth noting that the terms ‘ambulation’ and ‘mobilisation’ are often used interchangeably in the literature about epidural techniques that maintain motor function in the lower limbs. As the posture a woman adopts in labour is in part dependent on the motor power she retains, and this can be compromised by the peripheral motor blockade that accompanies effective epidural pain relief, it is clearly important to draw a distinction between mobilisation, the ability to move one’s legs, change position or move around the bed normally, and ambulation, which refers to the act of walking during labour. The ability to adopt upright postures in labour requires that women retain the capacity to mobilise, and some of these women will be able to ambulate.
A systematic review of the impact on mode of delivery of ambulation or upright positions in the first stage of labour (before full dilatation of the cervix) among women with epidurals found no significant difference between IVD (RR 1.16, 95% CI 0.93 to 1.44) and caesarean section (RR 0.91, 95% CI 0.70 to 1.19). 13 The second stage of labour may represent a period during which the adoption of an upright posture could exert the greatest influence and affect delivery mode by facilitating descent of the fetal head. A Cochrane review of position in the second stage of labour in women without epidurals found a reduction in IVD rate in the upright group (19 trials; RR 0.78, 95% CI 0.68 to 0.90). 14
Effectiveness of an upright position in the second stage of labour for women with epidurals
A Cochrane review of position in the second stage of labour among women with epidural analgesia was published in 2013,15 after the BUMPES trial started. This review included trials that compared upright positions with recumbent positions. The RR of SVB reported in the five included trials, including 879 women, was 1.02 (95% CI 0.81 to 1.28). There was clinical heterogeneity between the trials in relation to the eligibility criteria (some included multiparous women whereas others were restricted to nulliparous women) and in the nature of the interventions. In the upright group, for example, some women were actively encouraged to walk, and others were supported in a sitting position. In the recumbent group, some trials had allocated women to a sitting position and others to a lateral position. The authors concluded that there was no clear evidence about whether or not position in the second stage of labour made a difference to outcomes. 15
Effects on short- and longer-term maternal morbidity
An intervention that increases the rate of SVB by reducing the rate of IVD or caesarean section would also be expected to have an effect on short- and longer-term maternal morbidity. Faecal incontinence is clearly documented as being associated with forceps,11,16 including ongoing symptoms in women who only ever have one forceps delivery. 17 There may also be an increased risk of urinary incontinence, although this may be more closely associated with a longer second stage of labour;18,19 however, women who have a caesarean section have a lower risk of symptoms. 20,21 Other bowel problems such as haemorrhoids19,22,23 and constipation24 are more common after IVD, as are perineal pain and dyspareunia. 24,25 Caesarean section has many adverse sequelae, but, with the exception of faecal incontinence, most of these symptoms are less likely to occur in association with this delivery mode. It is therefore important to investigate positive impacts as well as any possible negative impacts of upright positions in the second stage of labour on maternal health outcomes.
There is increasing interest in obtaining maternity service users’ views of satisfaction with their experience of birth, as an indicator of the quality of their care and to inform organisational and policy changes. 26 Satisfaction is poorly defined and measured, although it is generally agreed that it is a multidimensional concept. 27,28 In a systematic review of factors influencing women’s satisfaction with birth, with a focus on the role of pain and pain relief, four factors (caregiver support, participation in decision-making, personal expectations and caregiver–patient relationship) were identified as important influences. 28 As position in the second stage of labour could influence a woman’s perceptions of the support she receives, her feelings of control and her expectations and experiences of labour and birth, satisfaction is an important consideration. The impact of negative consequences of the position adopted in the second stage of labour on these perspectives should also be identified.
Policy and practice at the time the trial commenced
Up to 30% of women in the UK use epidural analgesia for pain relief at some point in labour,1 with wide variation in the rate of epidural use between units. In a 1997 survey of UK units regarding epidural analgesia for labour, the epidural rate, including ‘low-dose’ epidurals, ranged from 0% to 85%, with an average rate of 24%. Of the 190 units that replied to the survey, 45 (24%) offered ‘low-dose epidurals’. 29 There is variation in the epidural technique employed to provide pain relief in labour and hospital policies governing maternal ambulation with an epidural in situ. A UK survey was conducted via the Obstetric Anaesthetists’ Association in 2008 to characterise national epidural practice and policy, with a response rate from lead clinicians of 80%. 30 It found that 95% of respondent units employed various epidural techniques consistent with the adoption of a range of upright positions, including ambulation, and that less than 50% of women actually did ambulate. Findings from the BUMPES trial are therefore widely generalisable to the majority of the nulliparous population that chooses epidural pain relief. With regard to reported hospital policies, 34% permitted maternal ambulation with low-dose epidural analgesia in situ. 30 Of those units that did not permit ambulation, 37% cited lack of evidence of a beneficial effect as a reason for this policy. This reluctance may reflect the current uncertainty in this field and that in general midwives have less experience of enabling women with epidurals to ambulate in second-stage labour rather than being in bed.
Rationale for a trial comparing upright with lying-down position
The National Institute for Health and Care Excellence (NICE) guidelines on intrapartum care published in 200731 (with no change in the update published in 2014) noted that there is ‘no effect of mobilisation following epidural analgesia on any maternal or neonatal outcomes’, and recommended that ‘women with regional analgesia should be encouraged to move and adopt whatever upright positions they find comfortable throughout labour’ (section 1.5.7, p. 22). This guidance is likely to lead to an increase in the use of upright positions, hence the need to compare upright positions with ‘lying-down’ positions rather than with usual care, given that usual care will increasingly include women assuming an upright position. Good-quality evidence is needed on whether or not upright positions in the second stage of labour in women with epidural analgesia have any beneficial effect on delivery mode and other important outcomes. It is crucial that the policies for the upright and comparison groups are clearly defined and monitored to ensure separation of the two approaches and to provide robust evidence about whether or not adopting an upright position does improve outcomes for women and their babies.
Chapter 2 Methods
Aim of the BUMPES trial
This was a multicentre RCT in which the primary objective was to evaluate whether or not, in nulliparous women who choose low-dose epidural analgesia, a policy of adopting an upright position throughout the second stage of labour is associated with an increase in the incidence of SVB, compared with a policy of adopting a lying-down position.
This objective was supported and supplemented by the following secondary objectives:
-
to evaluate whether or not there are differences between the two policies in important clinical outcomes for women and babies around the time of birth and 1 year post partum
-
to evaluate the cost-effectiveness of the two policies for position during second-stage labour from a NHS perspective
-
to measure women’s satisfaction with, and experience of, labour and delivery.
Trial design
The BUMPES study was a pragmatic, multicentre, individually randomised controlled trial that had a target recruitment of 3000 nulliparous women who had a low-dose epidural in situ. It was a two-arm parallel-group trial with one arm allocated to adopting an upright position during the second stage of labour and one arm allocated to adopting a lying-down position during the second stage of labour (Figure 1).
FIGURE 1.
Flow chart of participant recruitment.

Participant eligibility
The following inclusion criteria were applied throughout participant recruitment.
Inclusion criteria
Women admitted to a participating labour ward who fulfilled all of the following criteria were eligible to be randomised into the trial:
-
aged ≥ 16 years of age
-
≥ 37 weeks’ gestation
-
nulliparous (no previous delivery ≥ 24+0 weeks’ gestation)
-
singleton cephalic presentation
-
intended SVB
-
in the second stage of labour
-
with a low-dose epidural in situ during the first stage of labour, providing effective pain relief
-
able to understand printed documentation produced in English
-
able to give written answers in English.
Exclusion criteria
Women who did not fulfil all of the inclusion criteria were not included in the study.
Sample population
All women who met the inclusion criteria were considered potentially eligible to participate in the study.
Study setting
Trial recruitment was undertaken in the labour wards of participating NHS maternity hospitals.
Information for women and obtaining informed consent
Information about the trial was provided to all nulliparous women during the antenatal period, after their booking appointment. This process was individualised for each participating centre depending on their routine practice to maximise the number of women offered information well in advance of labour. For example, in some sites, women were provided with information about the trial at their routine anomaly scan appointment (18–22 weeks). All women had the opportunity to ask questions of their midwives or obstetricians at the hospital, or they could contact the trial office. When a woman in a participating centre had an effective epidural established during the first stage of labour, she could then be offered a participant information leaflet on the study. If, after reading this and having the opportunity to ask questions, she was willing to take part in the study, then informed consent was taken. The participant information made it clear that women were free to withdraw from the trial at any time for any reason without prejudice to their future care, and with no obligation to a give a reason for the withdrawal. Written informed consent was obtained by a health professional (e.g. midwife, obstetrician or anaesthetist) with delegated authority from the principal investigator at each site. Consent comprised a dated signature from the woman and a dated signature of the person who obtained informed consent. A copy of the signed informed consent document was given to the woman. In addition, one copy was retained in the woman’s medical notes, one was retained in the study site file and one was sent to the Trial Co-ordinating Centre.
Interventions
Women were allocated to a policy of either upright maternal position (intervention group) or lying-down maternal position (control group).
Intervention group
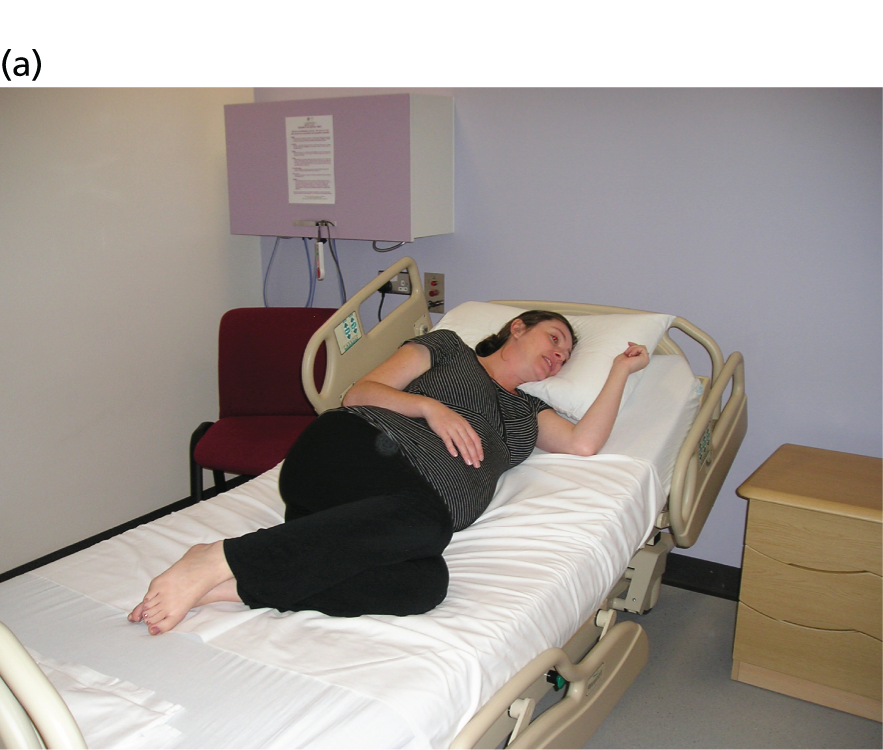
Women were allocated to a policy of upright maternal position that would maintain the pelvis in as vertical a plane as possible during the second stage of labour, with the intention of continuing this until the birth. Women allocated to the ‘upright’ group were encouraged by their midwife to adopt positions that allowed for as upright a posture as possible. This could include walking, standing, sitting out of bed, supported kneeling or completely upright in an obstetric bed (Figure 2) for as much of the second stage as possible.
FIGURE 2.
Possible positions for women randomised to the upright maternal position. (a) Seated; (b) supported kneeling; (c) seated with extended legs; and (d) completely upright.



Control group
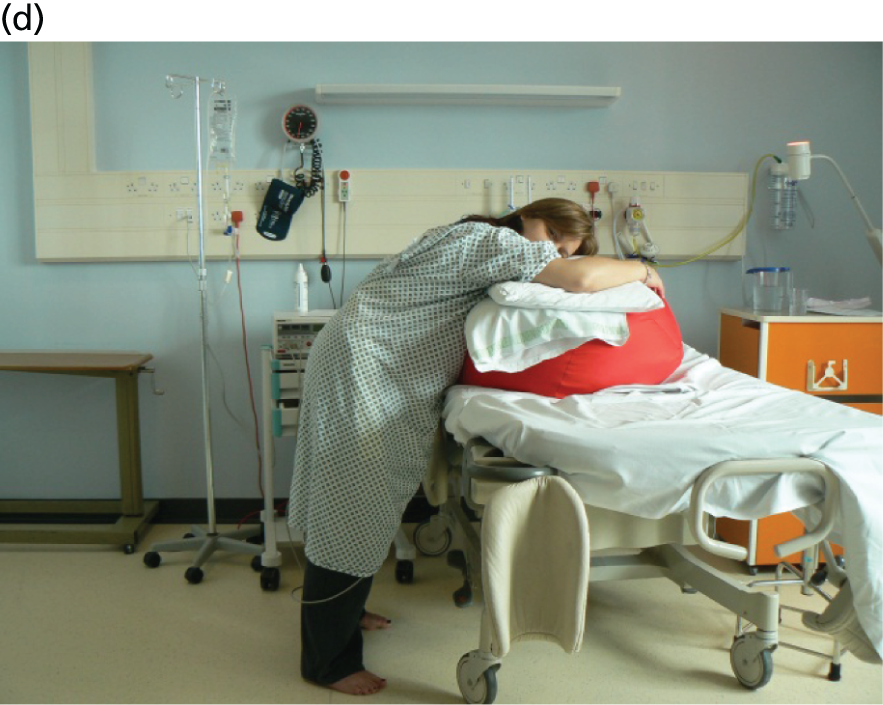
Women were allocated to a policy of a lying-down maternal position that would maintain the pelvis in as horizontal a plane as possible during the second stage of labour, with the intention of continuing this until the birth. Women allocated to the ‘lying-down’ group were encouraged to adopt a lying-down position that would mean lateral positions or lying down in bed for as much of the second stage of labour as possible. The bed could be tilted at up to a maximum of 30 degrees from horizontal (Figure 3).
FIGURE 3.
Possible positions for women randomised to the lying-down maternal position. (a) From in front; and (b) from behind. Note: a truly supine position (i.e. flat on the back) should not be used during labour because of the risk of aortocaval compression from the gravid uterus causing maternal hypotension.

Monitoring of adherence to allocation
In the second stage of labour, women with an effective epidural anaesthetic frequently have no desire to push. After confirmation of the second stage of labour, women were entered into the study. Midwives were encouraged to manage the second stage in two phases: a period of passive second-stage labour, allowing time for descent of the fetal head, followed by an active phase of expulsive pushing.
Training emphasised to the midwives the importance of supporting the woman in her allocated position, especially for the passive stage (which could last up to 2 hours). Positions were recorded on the trial worksheet at 15-minute intervals using a tick box, and midwives recorded ‘reason for change’ if the woman was moved out of her allocated position. As a pragmatic study, it was agreed that there would be expected reasons for changing position, for example fetal distress, fetal blood sampling or maternal discomfort, or to help improve pushing in the active second stage of labour. It was emphasised that midwives were required to record this information.
Randomisation
Participants were randomised to the allocated intervention (allocation ratio 1 : 1) using a web-based central service. To confirm eligibility, investigators were required to confirm the woman’s age and gestational age, that this was the woman’s first birth, that the fetus was a singleton with cephalic presentation and that an effective epidural was in situ, as well as obtaining signed consent. The randomisation software used random permuted blocks of sizes 2, 4, 6, 8 and 10, selected according to the proportions specified by Pascal’s triangle (1 : 4 : 6 : 8 : 10) to ensure that the staff recruiting women to the trial could not reliably predict the next allocation. Because of the large numbers of women recruited in each centre, no stratification by clinical characteristics was planned, although there was stratification by centre. The procedures for randomisation were fully documented, tested prior to the start of the trial, and monitored by the randomisation centre during the trial.
Outcome measures
Primary outcome measure
The primary outcome measure was the incidence of SVB.
Secondary outcomes
The following secondary outcomes were collected.
Mode of delivery
-
Instrumental delivery (forceps and ventouse)
-
and primary indication.
-
-
Caesarean section
-
and primary indication.
-
Outcomes from randomisation until delivery
-
Augmentation of labour.
-
Major interventions to maintain blood pressure (e.g. vasopressors).
-
Hypotension (systolic blood pressure of < 100 mmHg prior to delivery).
-
Application of fetal scalp electrode.
-
Fetal blood sampling.
-
Total doses of epidural local anaesthetic and opioids administered after randomisation.
-
Duration of active second stage of labour.
-
Total duration of second stage of labour.
-
Additional anaesthesia used for operative delivery.
Immediate post-delivery outcomes
-
Active management of the third stage of labour.
-
Episiotomy.
-
Pain during delivery.
-
Genital tract trauma (location and severity).
-
Manual removal of the placenta.
-
Primary post-partum haemorrhage requiring blood transfusion.
Postnatal period: woman
-
Duration of inpatient stay after delivery.
-
Satisfaction with experience of birth.
Postnatal period: infant
-
Cord artery pH of < 7.05 in second stage of labour [this is 2 standard deviations (SDs) below the mean] with a base deficit of ≥ 12 mmol/l (this is the threshold above which the risks of neurological damage increase).
-
Presence of meconium-stained liquor.
-
Apgar score of < 4 at 5 minutes.
-
Resuscitation at birth.
-
Skin-to-skin contact within the first hour of birth.
-
Initiation of breastfeeding within the first hour of birth.
-
Duration of inpatient stay.
-
Admission to neonatal unit and duration of stay.
One year after birth: woman
-
Urinary incontinence.
-
Faecal incontinence.
-
Other bowel problems.
-
Dyspareunia.
-
General physical and psychological health.
One year after birth: infant
-
Major morbidity, for example gross neurodevelopmental delay, including cerebral palsy (if a diagnosis has been made).
-
Hospital admissions.
Data collection schedule
Information at trial entry, including eligibility and maternal characteristics, was collected from hospital notes onto the specifically designed data collection booklet (DCB) (see Appendix 1). The position to which the woman was allocated was recorded on the DCB in two places – once in the eligibility section and again on the worksheet used to record the woman’s actual positions. As soon as possible after the woman was randomised, the attending midwife encouraged her into the allocated position and started recording on the DCB what position the woman was in ‘for the majority of the time in the last 15 minutes’, and if this position had changed from the allocated position and, if so, the reasons for this. Information on drugs taken after study entry and during labour was also recorded, as was other clinical information about the labour. The DCB also allowed for the collection of clinical outcome information on the delivery, as well as on neonatal outcomes and hospital stay.
If either the woman or the infant received a higher level of care (HLC), the relevant HLC form (see Appendices 2 and 3) was completed by the attending midwife.
As soon as possible after delivery, the woman was asked to complete a one-page questionnaire asking about her satisfaction with her birth experience, as well as asking her to provide an overview of what position she was in most of the time after study entry (see Appendix 4).
Women with surviving infants were followed up at 1 year with a self-administered questionnaire asking about their general health and well-being, with specific questions relating to any urinary and bowel problems. This questionnaire also requested information on the use of health services for themselves or their child (see Appendix 5). Prior to contact, mortality status and place of residence of both the woman and her infant were checked using NHS summary care records. Only women whose infants resided at the same address were contacted.
An overview of the time points at which trial data were collected is presented in Table 1.
| Data collection instrument | Time point | Person completing the data collection instrument | ||
|---|---|---|---|---|
| During labour | After delivery | 12 months | ||
| Woman and infant DCB | ✗ | ✗ | Completed by the attending midwife during labour and immediately after birth | |
| For all participating women and infants | ||||
| HLC form: woman | ✗ | Completed by the attending midwife during the woman’s admission and/or immediately after discharge from hospital; checked by the local principal investigator | ||
| Only completed for women receiving a HLC following delivery | ||||
| HLC form: infant | ✗ | Completed by the attending midwife during the infant’s admission and/or immediately after discharge from hospital; checked by the local principal investigator | ||
| Only completed for infants receiving a HLC following birth | ||||
| Maternal satisfaction form | ✗ | Completed by the woman as soon as possible after delivery | ||
| For all participating women | ||||
| Follow-up questionnaire | ✗ | Postal questionnaire completed by the woman | ||
| For all women whose babies were alive and both the woman and baby were resident at the same address | ||||
Sample size
The proposed sample size was 3000 women. At the time of writing the funding application, the assumed rate of the primary outcome of SVB in the control group was 55%. This was derived from data published from the Comparative Obstetric Mobile Epidural Trial (COMET) reflecting SVB rates in nulliparous women with a mobile epidural in the second stage of labour. 5 A total sample size of 3000 women (1500 in each arm) would have 90% power to detect a clinically significant (absolute) difference of 6% in the SVB rate between the two policies (with a 95% CI). The cost of implementing this technology is low; therefore, even modest differences in outcome are likely to be cost-effective. Detecting the smallest and most clinically relevant effect size possible was therefore desirable. A 6% absolute risk difference, which equates to a 10% RR reduction (approximately), was well within the uncertainty of the existing evidence (despite the existing trials’ heterogeneity) and was considered sufficient to change clinical practice.
The proportion of the upright group achieving a SVB was anticipated to be 61% under the null hypothesis. The test statistic used was the two-sided z-test with pooled variance. The significance level of the two-sided test was targeted at 5%. When considering longer-term outcomes, the proposed sample size of 3000 would be sufficient to detect a difference in the prevalence of faecal incontinence of 12% in the control group compared with 8% in the intervention group. The incidence of this outcome has been estimated as 14% among forceps deliveries and 10% among women with a SVB. 11
On collation of the pilot data for an interim analysis presented to the Data Monitoring Committee (DMC) in 2011, it was recognised that the combined primary outcome event rate was lower than anticipated. As of 6 December 2011, the overall SVB rate for BUMPES (combining upright and lying-down groups) was 33.8% [(95% CI 26.1% to 42.1%) based on 49/145 events]. With a reduction in the control group event rate (from an anticipated 55% to between 30% and 40%), keeping the sample size fixed at 3000 would mean that a RR of between 1.13 and 1.19 would be detectable, equivalent to an absolute risk reduction of 5–6%. Although there was not sufficient power to detect a RR as small as the planned 1.11, the absolute risk detectable is similar. The Trial Steering Committee (TSC) agreed that changes to the target sample size were therefore unnecessary.
Governance
Ethics arrangements
Favourable ethics approval for the study was granted by the National Research Ethics Service – Oxfordshire Research Ethics Committee (REC) B on 5 January 2010 (reference number 09/H0605/114). Approval was also sought from the Health and Social Care Information Centre (now known as NHS Digital) to establish the status of the mothers and their babies and details of the general practice at which they were registered. This was to ensure that 1-year follow-up questionnaires were not sent if either mother or baby may have died or if the family had changed address. NHS Digital approval was granted on 29 January 2013.
Approval was obtained from the research and development (R&D) departments for all participating hospitals. Table 2 provides details of the substantial amendments to the protocol approved by the REC. The R&D office of each participating hospital was notified of all amendments after REC approval was received. The REC were notified of all serious adverse events (SAEs) and progress reports were submitted annually.
| Amendment | Date | Description of main items in the request for approval (including version of protocol if revised) |
|---|---|---|
| Substantial amendment 1 | 9 June 2010 | Protocol version 2 (1 March 2010) |
| Key changes to the protocol were clarification on defining the term ‘nulliparous’, rewording the data collection section and updating the photographs of maternal positions. The PIL, consent form and antenatal leaflet were also updated | ||
| Substantial amendment 2 | 13 August 2010 | Submission of the maternal satisfaction questionnaire for approval |
| Non-substantial amendment 1 | 4 August 2010 | Administrative updates to the version numbers on the PIL and consent forms |
| Non-substantial amendment 2 | 23 September 2010 | Administrative update to the antenatal leaflet |
| Substantial amendment 3 | 7 March 2011 | Protocol version 3 (1 December 2010) |
| The majority of changes to the protocol were typographical or were made to increase clarity. The term ‘mobile epidural’ was replaced by ‘low-dose epidural’ throughout the document for consistency and to conform with clinical terminology. The secondary outcomes: ‘application of fetal scalp clip’ and ‘fetal blood sampling’ were added in order to assess concern over potential fetal distress | ||
| Recruitment posters were also designed to encourage midwives to recruit women to BUMPES | ||
| Substantial amendment 4 | 25 July 2011 | Protocol version 4 (20 July 2011) |
| Transfer of study sponsorship from the NPEU, University of Oxford to UCL. All study documents updated to reflect changes. The follow-up questionnaire entitled ‘You and Your First Child’s Health at One Year’ and a study poster were also submitted for approval | ||
| Substantial amendment 5 | 24 November 2011 | With study sponsorship and co-ordination being transferred from the NPEU to UCL, a letter was designed that notified women of the intention to also transfer their data (including name and address details) to UCL |
| Non-substantial amendment 3 | 14 February 2012 | Contact details updated on the study protocol, PIL, consent form, 1-year follow-up form, maternal satisfaction form and antenatal leaflet |
| Non-substantial amendment 4 | 13 March 2012 | Updated version number of PIL referenced in consent form |
| Substantial amendment 6 | 2 May 2012 | Protocol version 5 (2 March 2012) |
| Protocol changes made to reflect the change in contact details of the co-ordinating team, number of participating centres and minor typographical changes. For women with missing consent forms, a re-consent form and a covering letter for this form were also submitted for approval. The photos of the ‘lying-down’ positions were changed in the PIL and antenatal leaflet to reflect the positions more accurately. A life-size poster to be placed in the antenatal clinics and delivery suites was also submitted for approval | ||
| Substantial amendment 7 | 12 September 2012 | A follow-up reminder letter was designed to be sent to recruited women when no response was received following a 1-year follow-up questionnaire being sent out |
| Substantial amendment 8 | 7 January 2013 | Change of principal investigator at one of the participating centres. Minor amendment raised as substantial in error |
| Substantial amendment 9 | 16 April 2013 | The 1-year follow-up accompanying letter was designed to be sent with 1-year follow-up questionnaire. As the questionnaire sent out coincided with the infant’s first birthday, a gender-neutral birthday card was designed and submitted for approval. Minor changes were also made to the re-consent letter |
| Non-substantial amendment 5 | 16 May 2013 | Permission to use NIHR ‘OK to Ask’ promotion with the addition of the BUMPES logo and the words ‘Ask your midwife about’. This was used to promote BUMPES locally for International Clinical Trials Day (20 May 2012) |
| Substantial amendment 10 | 21 February 2014 | The 1-year follow-up accompanying letter content and layout were updated to include details of the online questionnaire. Approval was sought to remind participants to complete questionnaires via text message and/or e-mail. Details regarding a small incentive to complete the 1-year follow-up questionnaire in the form of a £5 shopping voucher were also included in the letter, e-mail and text message sent to women |
| Substantial amendment 11 | 14 July 2014 | Submission of the nested study protocol to assess the effectiveness on the return rate of the 1-year follow-up postal questionnaires. This was a promise of a monetary incentive (£10 voucher) made at the point of sending the initial follow-up questionnaire or on reminder letters only. One-year follow-up accompanying letters were also updated to include details on incentives |
| Substantial amendment 12 | 27 April 2015 | Protocol version 6 (27 April 2015) |
| Changes on how adherence to the allocated position would be analysed, minor clarification to the per diem cost calculation and clarification on how the study data would be analysed |
The trial was registered with the International Standard Randomised Controlled Trial Register under the reference number 35706297, and was adopted into the National Institute for Health Research (NIHR) portfolio under reference 8375.
Trial governance
Trial Steering Committee
The TSC included an independent chairperson, four other independent professional members (statistician, consultant anaesthetist, health economist, professor of midwifery) and one patient representative. Non-independent members included the chief investigator. Membership of the committee was approved by the NIHR Health Technology Assessment (HTA) programme. The TSC agreed a charter at its first meeting, based on that used by the Medical Research Council Clinical Trials Unit. The TSC met five times.
Data Monitoring Committee
The DMC was established for the trial and met as and when the DMC members requested. 32 The DMC comprised an independent chairperson and three independent members (a statistician, a professor of women’s health and a consultant in maternal and fetal medicine). Membership of the committee was approved by the NIHR HTA programme. During the period of recruitment to the trial, interim analyses were supplied, in strict confidence, to the DMC, together with any other analyses the DMC members requested. Meetings of the committee were arranged periodically, as considered appropriate by the chairperson. In the light of interim data, and other evidence from relevant studies (including updated overviews of the relevant RCTs), the DMC agreed to inform the TSC if, in its view, there was proof beyond reasonable doubt that the data indicated that any part of the protocol under investigation was either clearly indicated or clearly contraindicated, either for all women or for a particular subgroup of trial participants. A decision to inform the TSC would be based on statistical, clinical and ethical considerations.
The TSC and DMC members met jointly on two occasions: once at the beginning of the project before recruitment started, to review and comment on the protocol and data collection instruments, and to agree the TSC and DMC charters, and then again at the end of the project to agree the final analysis and provide feedback to the investigators about interpretation of the findings.
Clinical Investigators Group
The Clinical Investigators Group (CIG) comprised the chief investigator, co-applicants (including a lay member), clinical investigators from selected study sites, trial health economists and the trial statistician.
Appendix 6 lists the membership of the TSC, the DMC and the CIG.
Serious adverse event reporting
Serious adverse events were reported to the University College London (UCL) Trial Co-ordinating Office within 48 hours. The Trial Co-ordinating Office notified the chairperson of the DMC and the REC. All SAEs occurring during the trial observed by the investigator or reported by the participant, whether or not attributed to the trial, were reported on the DCB. SAEs considered to be related to the trial by the investigator were followed up until resolution or until the event was considered stable. The local investigator was asked to provide follow-up information when necessary. All related SAEs that could have resulted in a participant’s withdrawal from the trial, or which were present at the end of the trial, were followed up until a satisfactory resolution occurred.
The chief investigator submitted to the REC, once a year throughout the clinical trial, a safety report that included all SAEs.
Data handling, checks, cleaning and processing
All data collection forms (i.e. DCBs, HLC forms, maternal satisfaction and 1-year follow-up forms) and consent forms, once completed and returned to the UCL Comprehensive Clinical Trials Unit (CCTU), were logged as received and date stamped. Data were double entered at the UCL CCTU using the study database, by independent data clerks. Validation routines checked for missing data and inconsistencies on an ongoing basis. This included screening for out-of-range data, with cross-checks for conflicting data within and between data collection forms using computerised logic-checking screens. Any validation errors on the DCBs and HLC forms were queried and documented. Queries were communicated to the appropriate centres by the trial manager. Errors on the maternal satisfaction questionnaire and the 1-year follow-up form were not queried with the woman.
Cost-effectiveness analysis
An economic evaluation was conducted as part of this trial and is reported in detail in Chapter 6.
Patient and public involvement
When the initial investigator group was being assembled to develop the trial, the National Childbirth Trust was approached to suggest a lay member who would be willing to join the group as a co-investigator. Mary Nolan agreed to join the group, and assumed equal membership of the co-investigator group at all planning meetings and trial conduct meetings, and in the drafting of the application, developing the detailed trial protocol and data collection forms, and report and paper writing. Mary took a lead in helping the team to develop participant information leaflets to be used in the antenatal period and at the time of labour, as well as helping to plan dissemination activities and drafting and developing the summary information for the public. During the course of the trial, Mary Nolan left the NCT to take up a position as Professor of Perinatal Education at the University of Worcester, but continued to represent the potential participant’s perspective in all aspects of the trial development, conduct and analysis.
Chapter 3 Analysis plan
The statistical analysis plan (SAP) was written and approved before unblinding the data for statistical analysis (see Appendix 7). The SAP provided details of the presentation and analysis of the results from the trial. The principles set out in the SAP were not intended to curtail exploratory analysis (e.g. to decide cut-off points for categorisation of continuous variables) or to prohibit accepted practices (e.g. data transformation prior to analysis), but they were intended to establish the rules that were followed, as closely as possible, when analysing and reporting the trial.
Any deviations from the SAP are described and justified in this report.
Patient groups for analysis
Losses to the trial post randomisation were defined as any of the following:
-
women for whom a valid consent form was not received
-
women for whom consent to use their data was withdrawn
-
women not in the second stage of labour when randomised and who did not reach the second stage before delivery
-
women not in labour or without an epidural in place at the time of randomisation.
The numbers (with percentages of the randomised population) of post-randomisation exclusions are reported by randomised treatment group, and the reasons summarised.
Women could specify whether or not data collected up to the point of withdrawal could be used. If the response was ‘no’, then they were counted as post-randomisation exclusions. If the response was ‘yes’, then they were reported as ‘missing’ for all subsequent outcomes.
For the primary analysis, participants were analysed in the groups into which they were randomly allocated, that is, comparing the outcomes of all women and infants for women allocated to a policy of an upright position with those of women allocated to a policy of lying down, regardless of position recorded at any time during the second stage of labour. Losses to the trial post randomisation are excluded from all analyses, with the exception of the safety-reporting population, which excluded women for whom a valid consent form was not received and women who withdrew and did not consent to use of their data.
The unit of analysis was the woman for all maternal outcomes and the infant for all infant outcomes.
Descriptive analyses
The flow of participants through each stage of the trial is summarised using a Consolidated Standards of Reporting Trials (CONSORT) diagram (see Figure 8). Specifically, for each intervention group we report the numbers of women randomly assigned and women for whom the incorrect allocation was recorded in the eligibility section of the DCB. The number of ineligible women randomised is reported, with reasons for ineligibility. The number of post-randomisation exclusions and women analysed for the primary outcome is also reported. We report numbers for the 1-year follow-up, women lost to follow-up and women who withdrew before 1 year. The total number of eligible women was not collected during the conduct of this study, as it was considered too great a burden for the participating centres and would not be sufficiently reliable.
Numbers (with percentages) for binary and categorical variables and means (and SDs) or medians (with lower and upper quartiles), or geometric means for continuous variables are presented; no tests of statistical significance were performed, nor CIs calculated, for differences between randomised groups on any baseline variable.
The number (with percentages) of losses to follow-up among women selected for the 1-year assessment is reported in the CONSORT flow chart by trial arm, and the reasons reported. Selected demographic and clinical characteristics, the primary outcome and selected short-term outcomes of women and their infants with 1-year data available were compared with those without 1-year data.
Missing data for primary and secondary outcomes, from baseline to the end of follow-up, are summarised for the two trial arms. Not all data were routinely collected by all hospitals; for example body mass index (BMI), cord artery pH and base deficit were sometimes omitted. The DCB allowed midwives to tick ‘data not recorded’. These data are summarised by trial arm and reported separately from data missing or unknown. Missing data for the primary outcome were negligible. If any data items were missing on the DCBs, every effort was made to extract these data from the hospital involved.
Primary effectiveness analyses
Outcomes are summarised by trial arm using counts and percentages for categorical variables, means and SDs for normally distributed continuous variables or medians and interquartile ranges for other continuous variables. In addition, geometric means are presented for durations of stages of labour, as these are inherently highly skewed data.
An adjusted analysis was performed on all comparative analyses adjusting for centre (the stratification factor at randomisation) as a random effect. Binary outcomes were analysed using log-binomial regression models and results presented as adjusted RRs with corresponding CIs. If the model did not converge, then log-Poisson regression models with robust variance estimation were used. 33 If the model was still unstable, then the centre was removed and unadjusted RRs presented. Continuous outcomes were analysed using linear regression models and results presented as adjusted differences in means with associated CIs. Unadjusted Hodges–Lehmann34 median differences (plus CIs) for skewed continuous variables are presented. The estimates are based on a difference between distributions. The Hodges–Lehmann median difference is calculated by forming all possible differences between the first treatment group and the second treatment group, and taking the median of those differences.
In addition, geometric mean ratios (GMRs) are presented for durations of the stages of labour as the distribution of these data is highly skewed. A geometric mean is a measure of central tendency that is based on the product of values (as opposed to an arithmetic mean that sums the values). A ratio of geometric means provides an indication of how large one geometric mean is relative to another.
Comparisons between randomised groups of all primary and secondary outcomes are reported in full for completeness and transparency, that is, there is no selective reporting of outcomes.
In order to take account of the number of comparisons, 95% CIs are presented for the primary outcome and 99% CIs for all other outcomes.
Description of adherence to allocation
As described in Chapter 2, Data collection schedule, a record was made every 15 minutes of the woman’s position ‘for the majority of the time since the last assessment’, and if this position had changed from the previous assessment the reasons for this change were recorded. Reasons for a change from a woman’s allocated position were recorded as free text.
Positions recorded on the DCB were categorised according to whether or not the women were ‘lying down’, ‘upright’ or in ‘other’ positions for each 15-minute interval. For each interval, the categorised position was compared with the position allocated for the woman, and if the allocated position was the same as the categorised position then that 15-minute interval was coded as ‘adherent’. All other positions were coded as ‘non-adherent’. Some manual coding was required for positions recorded as text. Positions recorded as lithotomy were categorised as ‘lying down’ as the pelvis was in a horizontal position.
A summary of adherence to allocated position is reported by trial arm for (1) the passive second stage (i.e. before pushing commenced); (2) the active second stage (i.e. pushing); and (3) the whole of the second stage. Summaries of adherence data are calculated as the proportion of 15-minute intervals a woman spends in the position to which she was allocated out of the total number of 15-minute intervals recorded in the passive, active or whole of the second stage of labour. Medians and interquartile ranges are presented owing to the skewed distribution of the data.
Hodges–Lehmann differences in medians with corresponding 95% CIs are presented by randomised group.
There are a variety of reasons why women change from their allocated position. Changing position to allow fetal blood sampling to be performed, to improve effective fetal heart rate monitoring, was considered ‘clinically unavoidable’. All reasons for change were reviewed and classified as clinically avoidable or unavoidable in accordance with these criteria. The analysis was performed for adherence by dealing with periods in which changes to a non-allocated position were considered necessary for ‘clinically unavoidable reasons’ as adherent.
Reasons for change from allocated position were coded by the trial statistician and an independent assessor, and are presented by trial arm using counts and percentages.
The self-completed maternal satisfaction questionnaire included a question asking the woman to record what position she was in for the majority of the time during the passive and active stages of labour with possible responses being ‘lying down’, ’upright’, ‘other’ and ‘can’t remember’. These data have been summarised by trial arm using counts and percentages along with 95% CIs for differences in percentages. A qualitative comparison has been made between these results and the results from the DCB data provided by the midwife, to ascertain the extent to which reporting bias may have occurred, if at all.
Additional effectiveness analyses
The primary analysis was adjusted further for the primary outcome (pre-specified in the SAP) to investigate the impact of the following known prognostic factors (in addition to centre): age as a continuous variable, ethnicity, diagnosis of delay and onset of labour (induced vs. spontaneous).
To examine whether or not the effect of policy of position during the second stage of labour was consistent across specific subgroups of women, the following prespecified subgroup analyses were undertaken:
-
gestational age (37+0 to 38+6 weeks; 39+0 to 40+6 weeks; and ≥ 41+0 weeks)
-
maternal age (≤ 24 years, 25–29 years, 30–34 years and ≥ 35 years)
-
augmentation with oxytocin (Syntocinon®; Novartis Pharmaceuticals UK Ltd, Frimley/Camberley, UK) in the first stage of labour (yes/no)
-
Index of Multiple Deprivation (IMD; population-based quintiles 1–5; derived using the postcode of the woman’s last known address based on the Indices of Multiple Deprivation 201035 and Ordnance Survey Code-Point Open36 February 2013).
For the trial primary outcome, results are presented as forest plots showing the RR plus 95% CI for each subgroup,37 by intervention group, with the p-value for the statistical test of interaction. 38 Centre was included as a stratifying factor in the list of subgroup analyses in the original protocol, as we were expecting to recruit to target using five centres only. Recruitment rates were poor and we expanded the number of recruiting centres to 41. A subgroup analysis on 41 centres was therefore not considered relevant.
A prespecified sensitivity analysis on the 1-year maternal outcomes was carried out on a restricted data set that excluded all women who were pregnant or had another child at the time of completing the follow-up questionnaire.
Statistical software
Stata/SE® for Windows version 13.1 (StataCorp LP, College Station, TX, USA) was used for all analyses.
Reliability
All outcome data, except for maternal satisfaction questionnaire data and 1-year questionnaire data, were recorded in the women’s hospital notes. Site-monitoring visits verified a random sample of data collected on the DCBs and HLC forms, by making comparisons with information recorded in hospital notes. Self-administered forms were not verified.
Data relating to the calculation of the process outcomes (i.e. maternal position at 15-minute intervals since study entry) were recorded by the midwife on the DCB only, and the DCB was itself the source documentation and can therefore not be verified directly with any other source. The maternal satisfaction questionnaire aimed to confirm these data with a question asking the women to record what position they were in for the majority of the time during the passive and active stages of labour.
The coding of position data and reasons for a change from allocated position recorded as text were validated by an independent clinician.
Protocol violations and deviations
A protocol violation is a failure to comply fully with the final study protocol as approved by the REC and research department, such as a serious non-compliance with the protocol resulting from error, fraud or misconduct, and results in the exclusion of a patient from the analysis for the study. There were no protocol violations.
A protocol deviation is a departure from the final study protocol as approved by the REC, with minor consequences on the integrity of the data. Protocol deviations are those that resulted in exclusion from the analysis reported in Chapter 5 (see Figure 8). There was only one other protocol deviation, and that was unrecognised at the time of randomisation; the woman had intrathecal analgesia.
Chapter 4 Trial conduct
There were two major challenges during the conduct of the trial: recruitment and monitoring of adherence to the intervention. These are explained below.
Recruitment
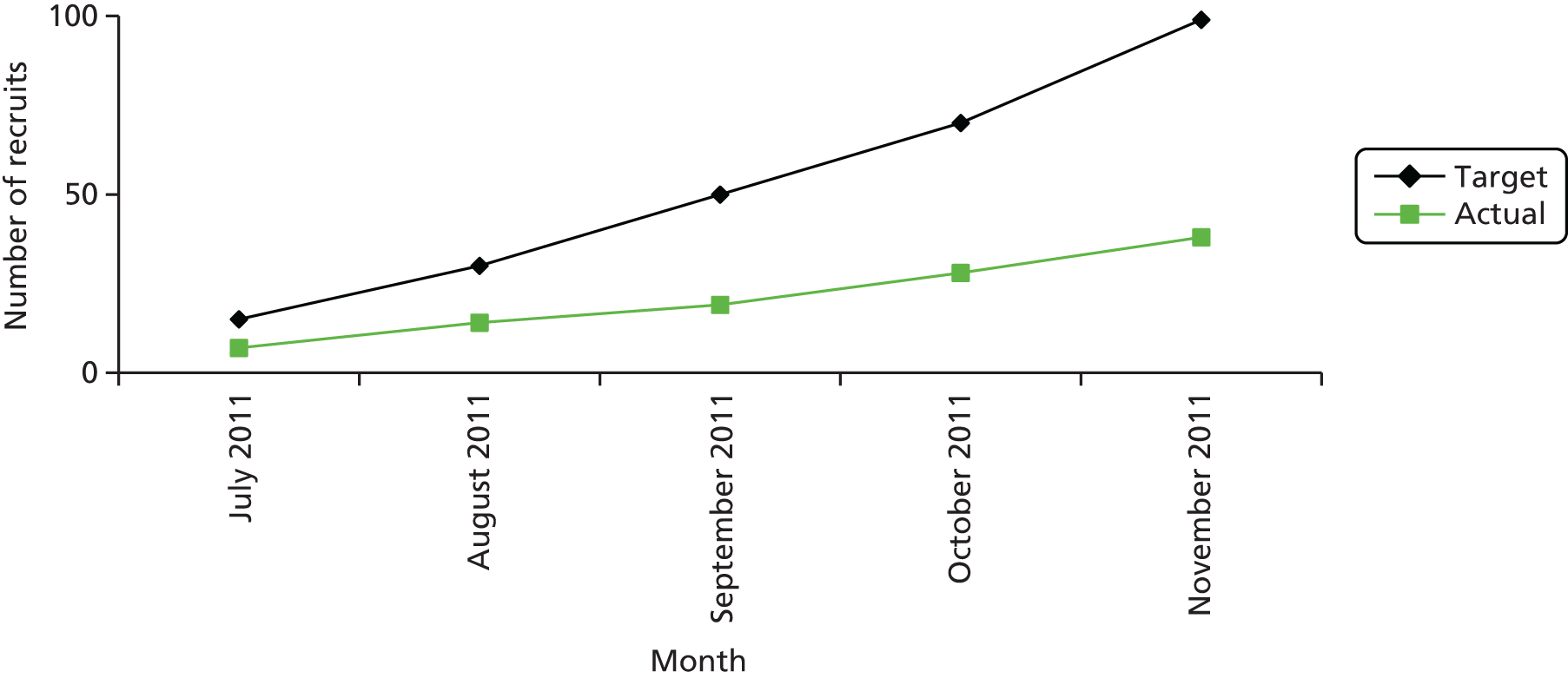
Trial recruitment was initially planned to be undertaken in the maternity units of four acute NHS trusts in England and one health board in Wales. The BUMPES study design originally outlined in the trial protocol described a single-centre internal pilot study to assess feasibility, develop teaching materials and field-test trial data collection processes. After 9 months of the pilot phase, it was noted that, although the trial infrastructure and data capture were satisfactory, accrual did not meet projected targets, despite accurate predictions of available participants. At the recommendation of the TSC, the trial was initiated at a second pilot site prior to ‘roll-out’, in order to establish if these limitations were site specific or reflected broader barriers to recruitment. It was noted that recruitment across the two pilot centres remained unsatisfactory, with an average of 49% of the overall recruitment target being met over the 6 months since the second pilot site opened to recruitment (Figures 4 and 5).
FIGURE 4.
Recruitment at first pilot centre.
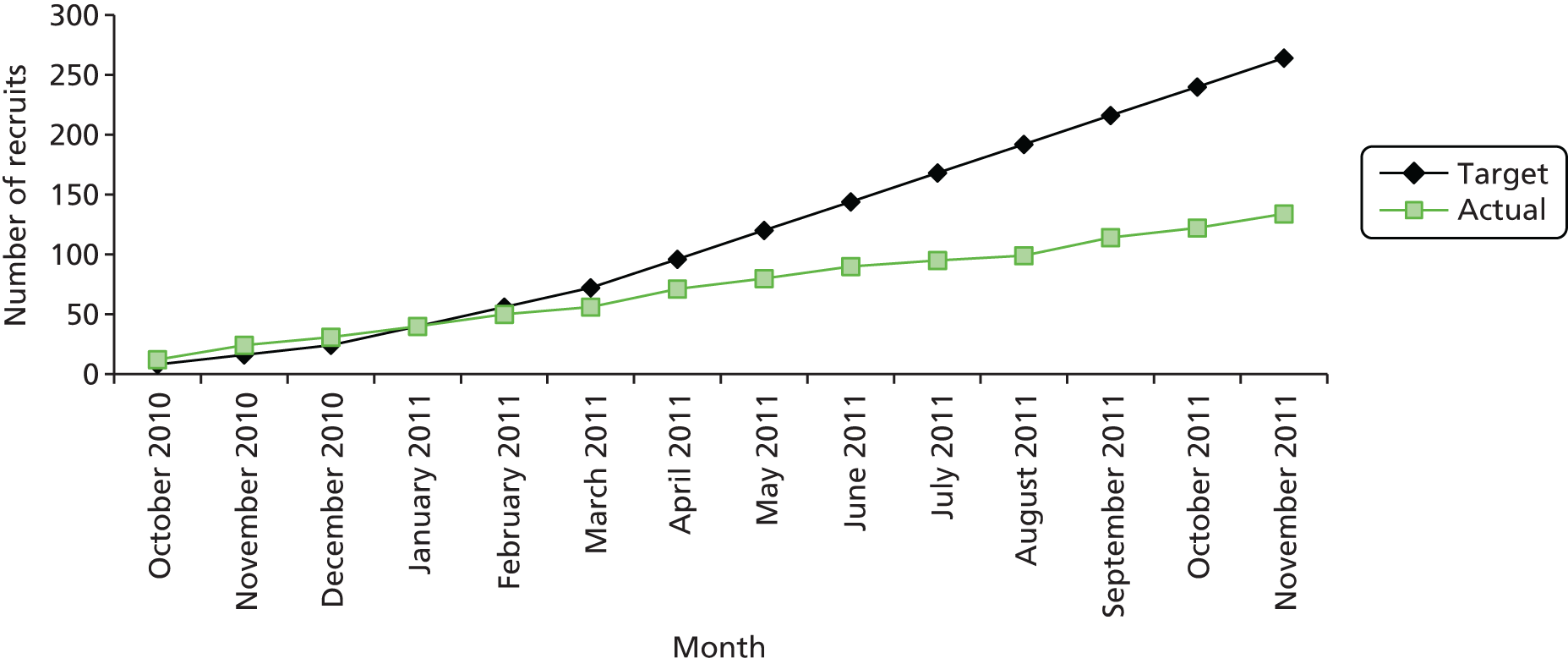
FIGURE 5.
Recruitment at second pilot centre.
Challenges to study recruitment
Equipoise
Engagement with clinical midwifery staff for feedback and exploration of obstacles to recruitment was repeatedly sought. Initially this process revealed a lack of equipoise regarding the trial intervention in some individuals. While unsupported by evidence, this was a powerful perceptual obstacle, which may in part have resulted from sources of conflicting information. As described earlier, the NICE Intrapartum Care Guidelines (2007)31 stated that:
Women with regional analgesia should be encouraged to move and adopt whatever upright position they find comfortable throughout labour.
NICE Clinical Guidelines, No. 190 (section 1.5.7, p. 22)31
These recommendations acknowledged that current evidence was inadequate and did not favour any specific positions. However, it is possible that misinterpretation of these guidelines could have affected equipoise and accounted for the perception that a particular position confers an advantage in birth outcomes. It may also explain the initial reluctance of midwives on the labour wards to identify with the study.
During the study, when women were approached to participate, they readily agreed. Thus there was nothing to suggest that those women who consented represented a distinct population that could reduce the generalisability of the trial findings. Furthermore, women in labour appeared to have genuine equipoise with respect to the intervention. Continued feedback to midwives and further training emphasised the importance of equipoise in order for the research to generate a definitive answer to the research question.
Clinical issues
Consent
Local R&D departments at participating units insisted that the clinical midwives attending women in labour required good clinical practice (GCP) training in order to take informed consent. The initiation visit allowed these issues to be explored and addressed locally either by arranging GCP training for midwives working on the labour wards or arranging for anaesthetists/research staff to be contacted to take consent. This inevitably led to delays in initiating recruitment and an ongoing barrier to recruitment, as most staff were not GCP trained, and many felt that this was unnecessary.
Competing studies
During the recruitment phase, several hospitals introduced other intrapartum studies, for which consent had been gained in the antenatal period or in early labour. The local staff felt that women could not be recruited to more than one study in the intrapartum period and midwives therefore did not approach these women.
As a consequence of these challenges, a decision was made that participation in the study should be expanded to more centres, in addition to the original five proposed.
Recruitment strategies
During the whole period of recruitment, a number of initiatives were launched to improve recruitment. These included:
-
Timing of informed consent. During the pilot phase, it was noted that gaining informed consent in the second stage of labour (from full dilatation of the cervix to birth of the baby) was delaying time to randomisation and therefore study entry. As the second stage of labour is a clinically demanding time on labour wards, this could potentially be a barrier to recruitment. Following approval (REC amendment 4, 25 July 2011), consent could be sought and obtained from potential study participants in the first stage of labour, once an effective epidural had been administered. Randomisation had to be delayed until the second stage of labour had been confirmed, but this process alleviated the burden of recruitment for the attending midwife.
-
To recruit at more sites. Following a proposal from the CIG and agreement from the TSC, there was approval to recruit a further 36 maternity units, which were opened to recruitment over a 24-month period (a total of 41 hospitals). Additional units that had a good track record of participation in health research in pregnancy were approached, along with hospitals that had already expressed an interest in participating. An initiation visit from the research midwife in the BUMPES team was arranged to fully explain the study to lead midwives, anaesthetists and local R&D departments, and also to evaluate their enthusiasm and the level of support that they would offer the study. Following R&D approvals, dates were arranged for the research midwife to attend the maternity units, and provide training to staff and support them during initial recruitment. This usually took 1 full week, covering day and night shifts, and involved small groups of midwives and anaesthetists. A training manual, posters, recruitment packs and randomisation flow charts, as well as 24-hour contact details, were in place for all centres prior to the start of recruitment. Further training was also provided to many units on request to support recruitment.
-
Change to funding model. Initially, BUMPES provided funding to appoint a ‘BUMPES midwife’ at each of the original five maternity units for 2 days per week. Their role was to support training, recruitment, data collection and the day-to-day running of the study. With the involvement of 36 more maternity units, the existing funding model was unsustainable within the trial budget, so this was changed to a ‘payment-per-recruit’ model (£85) for each of the maternity units. This proposal was approved by both the TSC (9 December 2011) and the NIHR HTA programme, and was in place from January 2012.
-
Development of local BUMPES champions and Comprehensive Local Research Network (CLRN) support (England). Given the change in the funding model, and the loss of specific BUMPES midwives, a revised model of local support was designed. This involved the introduction of BUMPES champions. Clinical midwives active on each labour ward shift were identified to promote the study, identify potential recruits, facilitate consent and support randomisation. This ensured that, as much as possible, someone was available who was knowledgeable about the trial and able to support recruiting midwives. This was supported in some units with extra CLRN funding and in others by the payment-per-recruit monies.
-
DCBs. Following feedback from the units, the DCBs were redesigned. Staff complained that DCBs were too long and the amount of information requested was too much, so that, on a busy labour ward shift, midwives were put off recruiting or completing the booklets. The DCBs were redesigned into parts 1 and 2. Part 1 was reduced to a single-page A3 worksheet and was the only section that the attending midwife during labour needed to complete. This requested information that could not be collected at a later date, for example visual analogue scale score (pain assessment), the date and time when the woman adopted the allocated position, times and positions every 15 minutes and reasons for change. CLRN and the National Institute for Social and Health Research [(NISCHR) Wales] research staff or staff employed using the BUMPES payment per recruit monies were able to complete part 2 at a later date with information from the maternal and neonatal notes.
-
Increasing midwifery ownership. A short article to raise awareness was published in the Royal College of Midwives Journal (2012). This was designed to encourage midwifery ownership of the study and the importance of the results, which could potentially have an impact on future midwifery practice and be beneficial to women. A Collaborators’ Study Day for recruiting units was arranged in November 2012 to improve networking, and for sharing ideas and identifying areas of good practice. This was attended by 34 midwives from 18 participating centres and feedback from the day was excellent.
-
Promoting BUMPES. Life-sized posters and other promotional items were designed and, when required, received REC approval. This helped to encourage promotion of the study to midwives, women and antenatal educators.
-
Recruitment updates and newsletters. Recruitment updates were sent to units monthly. Newsletters were published quarterly and included recruitment targets, the identity of new participating units and answers to frequently asked questions, to help improve awareness and address common errors and queries.
-
Incentives. Approved incentives for midwives such as fob watches, notebooks, Post-it® (3M, Cynthiana, KY, USA) notes, mugs, key rings, pens, tape measures, lanyards and lip gels were purchased and given during training sessions. This helped to identify and promote contact details of the study. Occasional gift vouchers were approved and given to support recruitment as well as seasonal gifts, such as Love Hearts (Swizzels Matlow, New Mills, Stockport, UK) for Valentine’s Day, Easter eggs, summer rock candy, Halloween-themed sweets and an advent calendar in December.
The combination of marketing the trial more actively and participation of the additional centres resulted in a substantial improvement in recruitment. The project management group continued to monitor recruitment closely throughout the trial. An example of the monitoring data reviewed is shown in Table 3 and Figure 6.
| Recruitment | Month, grand totals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| March 2012 | April 2012 | May 2012 | June 2012 | July 2012 | August 2012 | September 2012 | October 2012 | November 2012 | December 2012 | January 2013 | February 2013 | |
| Monthly targets | 25 | 44 | 63 | 75 | 90 | 111 | 114 | 141 | 164 | 176 | 188 | 192 |
| Monthly recruitment | 14 | 28 | 47 | 59 | 75 | 72 | 68 | 95 | 132 | 126 | 118 | 133 |
| Monthly target achieved (%) | 56 | 64 | 75 | 79 | 83 | 65 | 60 | 67 | 80 | 72 | 63 | 69 |
| Target cumulative total | 469 | 514 | 569 | 634 | 711 | 800 | 901 | 1014 | 1139 | 1276 | 1425 | 1586 |
| Actual cumulative total | 216 | 244 | 291 | 350 | 425 | 497 | 565 | 660 | 792 | 918 | 1036 | 1169 |
| Overall percentage of target | 46 | 47 | 51 | 55 | 60 | 62 | 63 | 65 | 70 | 72 | 73 | 74 |
FIGURE 6.
Study recruitment details from March 2012 to February 2013.
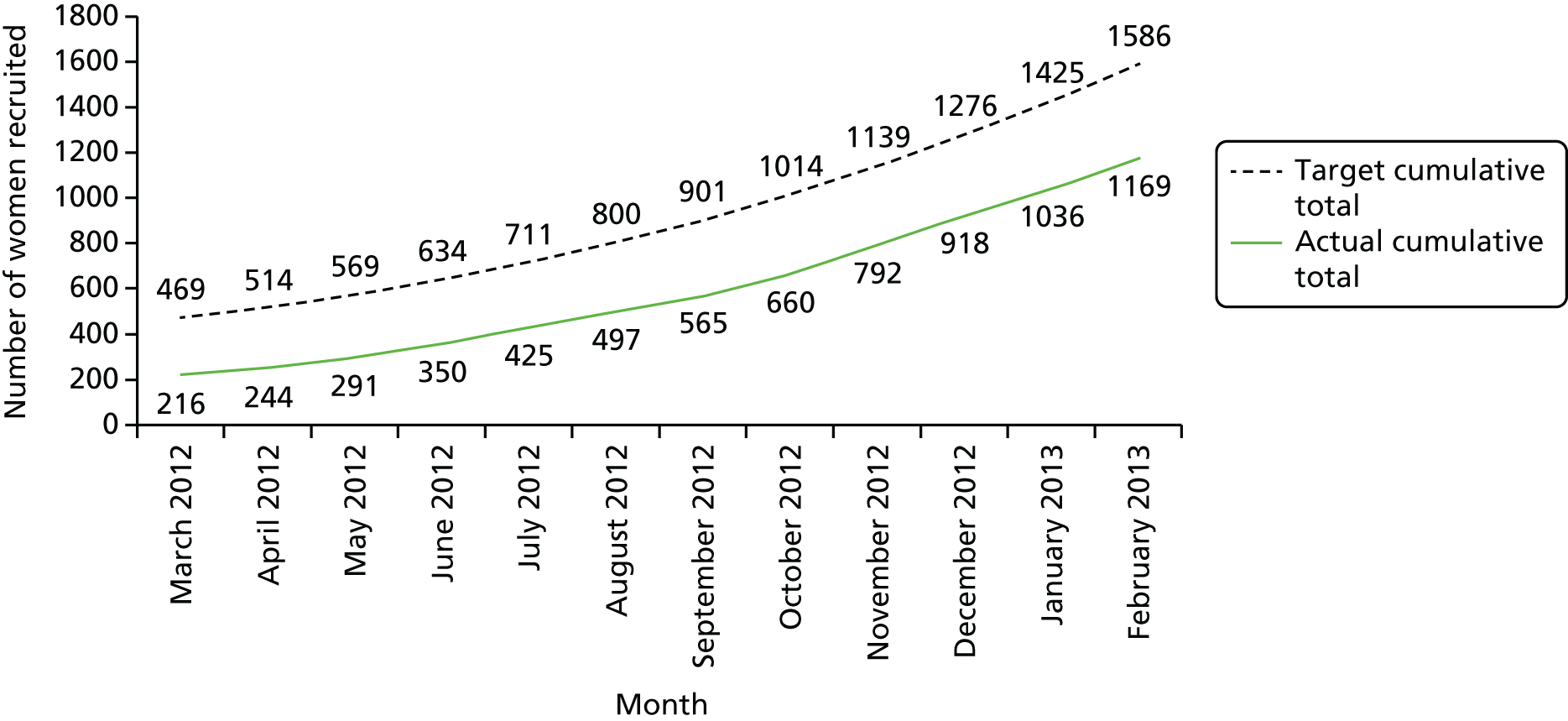
However, the delays inherent in establishing the participation of a greater number of centres resulted in a request to the NIHR HTA programme for a 12-month extension of the trial. This no-cost extension was granted in September 2013.
Chapter 5 Results
Between 4 October 2010 and 31 January 2014, 3236 women were randomised to the BUMPES trial from 41 participating centres (Figure 7).
FIGURE 7.
BUMPES final recruitment.
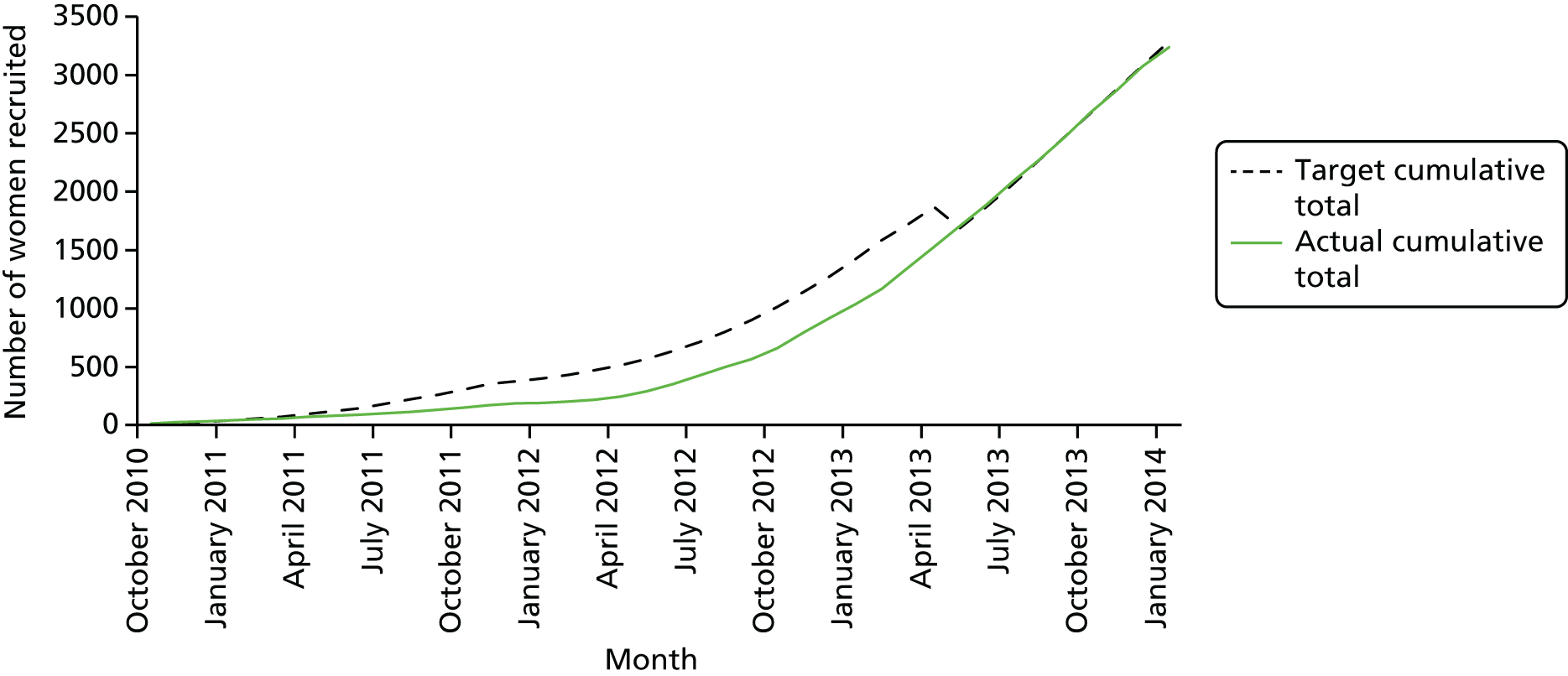
In accordance with the prespecified SAP, 143 women (4.4%) met the criteria to be excluded from the analysis of the primary outcome. The majority of these exclusions were because of missing or incomplete consent forms. For 32 women, exclusion was because they were randomised in error (19 were not in the second stage of labour at the time of randomisation and never reached the second stage of labour, having caesarean section prior to full dilatation of the cervix, and 12 were apparently randomised after delivery). These are detailed in the participant flow diagram (Figure 8). DCBs were available for all women recruited and analysed. Follow-up at 1 year was achieved for 61% of women (see Figure 8).
FIGURE 8.
Participant flow diagram. a, If not contactable after 15 months since randomisation, then the questionnaire was not sent. Reproduced from The Epidural and Position Trial Collaborative Group. 39 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

Baseline characteristics were similar between the two arms of the trial (Table 4). Mean maternal age was 28.4 years (SD 5.6 years). The majority of women in both arms had a gestational age of between 37 and 41 completed weeks, although 7.5% of women were at ≥ 42 weeks. The vast majority of women participating in the trial were of white ethnic origin and mean BMI at booking was just over 25 kg/m2. Approximately 40% of women had their labour induced, which is higher than might be expected in the general maternity population. 40 However, as recruited women all had epidural analgesia, which is associated with longer and more painful labours, as is induction of labour, this proportion does not appear excessive. Similarly, 50% of women had augmentation with oxytocin during their labour, which is compatible with women requesting epidural analgesia because of a longer labour. 40
| Characteristic | Trial arm | |
|---|---|---|
| Upright (N = 1556) | Lying down (N = 1537) | |
| Centre, n (%) | ||
| Birmingham Women’s Hospital | 116 (7.5) | 118 (7.7) |
| St Thomas’ Hospital | 237 (15.2) | 241 (15.7) |
| Queen Alexandra Hospital, Portsmouth | 43 (2.8) | 42 (2.7) |
| University Hospital of Wales | 150 (9.6) | 146 (9.5) |
| Royal United Hospitals Bath | 99 (6.4) | 101 (6.6) |
| Bradford Royal Infirmary | 58 (3.7) | 55 (3.6) |
| Jessop Wing, Sheffield Teaching Hospital | 93 (6.0) | 94 (6.1) |
| Princess of Wales Hospital | 22 (1.4) | 19 (1.2) |
| Singleton Hospital, Swansea | 19 (1.2) | 18 (1.2) |
| Royal Gwent Hospital, Newport | 29 (1.9) | 25 (1.6) |
| Gloucestershire Royal Hospital | 26 (1.7) | 22 (1.4) |
| Nevill Hall Hospital | 9 (0.6) | 10 (0.7) |
| Frimley Park Hospital | 97 (6.2) | 96 (6.3) |
| Sunderland Royal Hospital | 21 (1.4) | 22 (1.4) |
| Pinderfields Hospital | 36 (2.3) | 36 (2.3) |
| Warrington Hospital | 29 (1.9) | 29 (1.9) |
| Tameside Hospital | 26 (1.7) | 24 (1.6) |
| Medway Maritime Hospital | 15 (1.0) | 10 (0.7) |
| South Tyneside District Hospital | 8 (0.5) | 7 (0.5) |
| Queen Mary’s Hospital, London | 64 (4.1) | 62 (4.0) |
| Queen Charlotte’s and Chelsea Hospital | 7 (0.5) | 11 (0.7) |
| Queen Elizabeth Hospital | 24 (1.5) | 21 (1.4) |
| Great Western Hospital | 27 (1.7) | 30 (2.0) |
| Royal Cornwall Hospital | 19 (1.2) | 20 (1.3) |
| Bedford Hospital | 26 (1.7) | 30 (2.0) |
| University College Hospital, London | 18 (1.2) | 13 (0.9) |
| Royal Sussex County Hospital | 16 (1.0) | 13 (0.9) |
| North Manchester General Hospital | 30 (1.9) | 28 (1.8) |
| New Cross Hospital, Wolverhampton | 22 (1.4) | 19 (1.2) |
| James Paget Hospital | 21 (1.4) | 23 (1.5) |
| St George’s Hospital | 32 (2.1) | 33 (2.2) |
| Princess Royal University Hospital | 6 (0.4) | 2 (0.1) |
| King’s College Hospital, London | 39 (2.5) | 41 (2.7) |
| St Mary’s Hospital | 3 (0.2) | 4 (0.3) |
| Dorset County Hospital | 10 (0.6) | 10 (0.7) |
| Kingston Hospital | 41 (2.6) | 46 (3.0) |
| Hillingdon Hospital | 7 (0.5) | 5 (0.3) |
| Arrowe Park Hospital | 7 (0.5) | 5 (0.3) |
| Lewisham Hospital | 2 (0.1) | 1 (0.1) |
| Prince Charles Hospital | 2 (0.1) | 5 (0.3) |
| Maternal age (years), n (%) | ||
| Mean (SD) | 28.4 (5.7) | 28.4 (5.6) |
| < 20 | 111 (7.1) | 99 (6.4) |
| 20–24 | 303 (19.5) | 292 (19.0) |
| 25–29 | 437 (28.1) | 463 (30.1) |
| 30–34 | 488 (31.4) | 482 (31.4) |
| 35–39 | 182 (11.7) | 161 (10.5) |
| ≥ 40 | 34 (2.2) | 40 (2.6) |
| Missing | 1 | 0 |
| Gestational age at entry (weeks) | ||
| Mean (SD) | 40.4 (1.2) | 40.4 (1.2) |
| 37+0 to 39+6, n (%) | 482 (31.0) | 500 (32.6) |
| 40+0 to 41+6, n (%) | 955 (61.5) | 921 (60.0) |
| ≥ 42+0, n (%) | 116 (7.5) | 115 (7.5) |
| Missing | 3 | 1 |
| IMD: quintile, n (%) | ||
| First (least deprived) | 205 (16.0) | 204 (16.0) |
| Second | 182 (14.2) | 201 (15.7) |
| Third | 246 (19.2) | 235 (18.4) |
| Fourth | 349 (27.2) | 345 (27.0) |
| Fifth (most deprived) | 299 (23.3) | 294 (23.0) |
| Wales – not derived | 224 | 217 |
| Postcode missing | 51 | 41 |
| Ethnic group, n (%) | ||
| White | 1305 (84.5) | 1275 (83.5) |
| Indian | 48 (3.1) | 57 (3.7) |
| Pakistani | 26 (1.7) | 30 (2.0) |
| Bangladeshi | 6 (0.4) | 3 (0.2) |
| Black African | 28 (1.8) | 30 (2.0) |
| Black Caribbean | 14 (0.9) | 11 (0.7) |
| Any other ethnic group | 117 (7.6) | 121 (7.9) |
| Not known/missing | 12 | 10 |
| BMI at booking visit (kg/m2) | ||
| Mean (SD) | 25.5 (5.4) | 25.2 (5.3) |
| Height and/or weight not known | 65 | 60 |
| Woman undergone FGM, n (%) | 6 (0.4) | 1 (0.1) |
| Missing | 6 | 7 |
| Onset of labour, n (%) | ||
| Spontaneous | 941 (60.6) | 904 (58.9) |
| Induced | 613 (39.5) | 632 (41.2) |
| Missing | 2 | 1 |
| Duration of first stage (minutes) | ||
| Median (IQR) | 510 (360–715) | 495 (350–705) |
| Geometric mean | 484.9 | 481.9 |
| Missing | 17 | 10 |
| Diagnosis of pre-eclampsia, n (%) | 52 (3.4) | 52 (3.4) |
| Missing | 5 | 3 |
| Continuous electronic fetal monitoring, n (%) | 1485 (95.5) | 1470 (95.8) |
| Missing | 1 | 2 |
| Diagnosis of delay requiring intervention, n (%) | 796 (51.2) | 770 (50.2) |
| Missing | 1 | 3 |
| Systemic opioids given prior to epidural, n (%) | 442 (28.4) | 435 (28.3) |
| Pethidine | 353 (79.9) | 330 (75.9) |
| Diamorphine | 77 (17.4) | 88 (20.2) |
| Remifentanil | 3 (0.7) | 4 (0.9) |
| Morphine | 0 (0.0) | 0 (0.0) |
| Meptazin | 12 (2.7) | 17 (3.9) |
| Missing | 1 | 1 |
| Epidural technique, n (%) | ||
| Epidural | 1492 (96.0) | 1481 (96.4) |
| Combined spinal epidural | 62 (4.0) | 55 (3.6) |
| Missing | 2 | 1 |
| Epidural maintained with PCEA/infusion, n (%) | 1224 (80.6) | 1196 (79.9) |
| Missing | 37 | 40 |
| Woman’s pain score for last contraction | ||
| Median (IQR) | 10 (0–30) | 10 (0–38) |
| Missing | 162 | 184 |
| Able to perform straight leg raise, n (%) | 1162 (78.7) | 1152 (80.2) |
| Missing | 79 | 101 |
| Position prior to study entry, n (%) | ||
| Lying down | 432 (29.0) | 546 (37.7) |
| Upright | 977 (65.6) | 832 (57.4) |
| Lithotomy | 5 (0.3) | 6 (0.4) |
| Semi-recumbent | 58 (3.9) | 53 (3.7) |
| Other | 17 (1.1) | 12 (0.8) |
| Missing | 67 | 88 |
| Time from VE diagnosing second stage to study entry (minutes) | ||
| Median (IQR) | 16 (9–30) | 16 (8–30) |
| Apparently randomised before diagnosis of second stagea | 70 | 79 |
| Time apparently > 180 minutesa | 6 | 7 |
| Missing | 7 | 2 |
| Time from study entry to start of recording positions (minutes) | ||
| Median (IQR) | 1 (–2 to 6) | 1 (–3 to 7) |
| Time from study entry to recording position > 15 minutes,b n (%) | 154 (10.1) | 150 (9.9) |
| Time apparently > 15 minutes before study entrya | 227 | 218 |
| Missing | 30 | 27 |
| Baby’s birth weight (grams)c | ||
| Mean (SD) | 3500 (450) | 3488 (442) |
| Missing | 1 | 1 |
Approximately 80% of women were able to perform a straight leg raise at the time of trial entry, suggesting that these women had reasonable mobility with their epidural analgesia.
There is an apparent disparity between the two groups in the position of the women at the time of trial entry. It appears that there was a higher proportion of women who were lying down in the group allocated to lying down than for women allocated to the upright position. The way these data were requested could have led to misclassification of this variable, in that midwives may have recorded the position of the women at the time of allocation, that is, after they had already assumed the allocated position. As all other characteristics of the women were similar at baseline, it appears very unlikely that this would represent the true position at the time of randomisation; rather, it would be a combination of this plus actual allocation.
The time from randomisation to trial entry, and all other durations recorded in the results section, are prone to errors because of time differences recorded in different parts of the labour ward. The time of randomisation is accurate, as this was recorded by the randomisation service. However, all other times will depend on the accuracy of the clocks in the different locations in the labour ward. For example, the clock in the central midwifery station may read a slightly different time from that in the labour room, and these may both be different from the clock in theatre. There were many (relatively minor) problems with derived duration variables in the data set (e.g. negative values), suggesting variation in actual time recorded between different settings.
There was a clear difference in the incidence of the primary outcome, SVB, between the groups, with 35.2% of women achieving a SVB in the upright group compared with 41.1% achieving a SVB in the lying-down group [adjusted RR 0.86, 95% CI 0.78 to 0.94 (Table 5)]. This represents a 5.9% absolute risk increase in the chance of a SVB in the lying-down group. The original and subsequently revised sample size estimation aimed to detect a 5–6% absolute risk reduction.
| Outcome | Trial arm | Adjusteda RR (95% CI) | |
|---|---|---|---|
| Upright (N = 1556), n (%) | Lying down (N = 1537), n (%) | ||
| SVB | 548 (35.2) | 632 (41.1) | 0.86 (0.78 to 0.94) |
| Missing | 1 | 0 | – |
As specified in the SAP, the primary outcome analysis was further adjusted for the characteristics age, ethnicity, the diagnosis of delay and the nature of the onset of labour (Table 6).
| Primary outcome | Adjusted RR (95% CI) |
|---|---|
| Full model:a adjusting for maternal age, ethnicity, diagnosis of delay and onset of labour | 0.86 (0.79 to 0.94) |
There was no evidence of a difference found for most of the secondary maternal outcomes after study entry and during labour, particularly with respect to epidural drug dosage, use of augmentation, fetal blood sampling or the use of fetal scalp electrodes (Table 7). There was a statistically significant difference at the 1% level in the duration of the active second stage of labour with a shorter duration of labour in the lying-down group (GMR 1.08 minutes, 99% CI 1.01 to 1.15 minutes).
| Outcome | Trial arm | Adjusteda effect measure (99% CI) | |
|---|---|---|---|
| Upright (N = 1556) | Lying down (N = 1537) | ||
| Epidural drugsb administered after study entry, n (%) | 832 (75.4) | 862 (76.7) | – |
| Missing | 453 | 413 | |
| Total-dose local anaestheticb (mg) | |||
| Bupivacaine | 814 | 849 | Median difference 0 (–2 to 0) |
| Mean (SD) | 26.4 (22.2) | 26.7 (21.2) | |
| Median (IQR) | 20 (10–31) | 20 (12–33) | |
| Lidocaine | 6 | 8 | Median difference 0 (–100 to 180) |
| Mean (SD) | 256.7 (88.0) | 205 (99.6) | |
| Median (IQR) | 200 (200–360) | 200 (180–250) | |
| Ropivicaine | 2 | 1 | Median difference 0 (–23 to 23) |
| Mean (SD) | 75 (31.8) | 75 | |
| Median (IQR) | 75 (53–98) | 75 (75–75) | |
| Total-dose opioidsb | |||
| Fentanyl (µg) | 809 | 840 | Median difference 0 (–4 to 0) |
| Mean (SD) | 49.4 (39.0) | 51.6 (41.6) | |
| Median (IQR) | 40 (20–60) | 40 (22–64) | |
| Diamorphine (mg) | 4 | 1 | Median difference 0 (0 to 0) |
| Mean (SD) | 3.0 (0.0) | 3.0 | |
| Median (IQR) | 3 (3–3) | 3 (3–3) | |
| Hypotension (systolic BP of < 100 mmHg), n (%) | 42 (2.7) | 49 (3.2) | RR 0.85 (0.50 to 1.44) |
| Missing | 3 | 4 | |
| Vasopressors to increase blood pressure, n (%) | 13 (0.8) | 12 (0.8) | RR 1.07 (0.39 to 2.99) |
| Missing | 3 | 2 | |
| Syntocinon for augmentation, n (%) | 172 (11.1) | 163 (10.6) | RR 1.04 (0.80 to 1.35) |
| Missing | 3 | 2 | |
| Fetal blood sampling performed, n (%) | 90 (5.8) | 72 (4.7) | RR 1.17 (0.82 to 1.68) |
| Missing | 4 | 3 | |
| Fetal scalp electrode applied, n (%) | 94 (6.1) | 85 (5.6) | RR 1.09 (0.76 to 1.57) |
| Missing | 6 | 11 | |
| Duration of active second stagec (minutes) | |||
| Geometric mean | 80.9 | 75.0 | GMR 1.08 (1.01 to 1.15) |
| Median (IQR) | 94 (56–133) | 88 (51–126) | Median difference 6 (1 to 11) |
| Missing | 14 | 12 | |
| Total duration of second staged (minutes) | |||
| Geometric mean | 130.5 | 125.1 | GMR 1.04 (0.98 to 1.10) |
| Median (IQR) | 149 (100–197) | 141 (95–188) | Median difference 7 (0 to 13) |
| Missing | 6 | 0 | |
Other secondary maternal outcomes, such as IVD and caesarean section, suggested an increased risk associated with the upright position, but again these differences were not statistically significant at the 1% level. For those women undergoing operative delivery (instrumental or caesarean section), the indications appeared to be similar between the two arms of the trial. Just over one-third were reported to be caused by fetal distress and nearly 60% caused by failure to progress.
With respect to genital tract trauma there was a suggestion that there may be an increase in the incidence of episiotomy in the upright group compared with the lying-down group, although this was not significant at the 1% level. There were no statistically significant differences in the risk of perineal trauma, although there appeared to be a higher incidence of obstetric anal sphincter injury in the upright group (6.7%) than in the lying-down group (5.3%), but again this difference was not statistically significant at the 1% level (Table 8).
| Outcome | Trial arm | Adjusteda effect measure (99% CI) | |
|---|---|---|---|
| Upright (N = 1556) | Lying down (N = 1537) | ||
| Mode of delivery, n (%) | |||
| IVDb | 849 (54.6) | 778 (50.6) | RR 1.08 (0.99 to 1.18) |
| Forceps | 578 (37.2) | 503 (32.7) | |
| Ventouse | 271 (17.4) | 275 (17.9) | |
| Caesarean sectionc | 158 (10.2) | 127 (8.3) | RR 1.23 (0.92 to 1.64) |
| Categoryd of caesarean section | |||
| 1 | 54 (34.2) | 33 (26.0) | |
| 2 | 95 (60.1) | 81 (63.8) | |
| 3 | 9 (5.7) | 13 (10.2) | |
| Missing | 1 | 0 | |
| Primary indication for assisted (non-spontaneous) delivery, n (%) | |||
| Instrumental | |||
| Fetal distress | 338 (39.9) | 304 (39.1) | |
| Failure to progress | 504 (59.4) | 468 (60.2) | |
| Other | 6 (0.7) | 5 (0.6) | |
| Missing | 1 | 1 | |
| Caesarean section | |||
| Fetal distress | 39 (24.7) | 32 (25.2) | |
| Failure to progress | 118 (74.7) | 94 (74.0) | |
| Other | 1 (0.6) | 1 (0.8) | |
| Anaesthesia required for instrumental/caesarean section delivery,e n (%) | 587 (58.5) | 515 (57.4) | RR 1.03 (0.95 to 1.12) |
| Technique usedf | |||
| Local infiltration | 65 (11.1) | 94 (18.3) | |
| Pudendal block | 16 (2.7) | 16 (3.1) | |
| High-dose epidural top-up | 439 (74.8) | 342 (66.4) | |
| Spinal anaesthesia | 68 (11.6) | 72 (14.0) | |
| General anaesthesia | 11 (1.9) | 6 (1.2) | |
| Missing | 4 | 7 | |
| Active management of third stage, n (%) | 1450 (98.0) | 1432 (98.2) | RR 1.00 (0.91 to 1.10) |
| Missing | 76 | 78 | |
| Genital tract trauma, n (%) | |||
| Episiotomy performed | 914 (58.8) | 838 (54.6) | RR 1.07 (0.99 to 1.16) |
| Missing | 1 | 1 | |
| Perineal tear evident, including perineal tear with episiotomy | 759 (48.9) | 785 (51.1) | RR 0.95 (0.87 to 1.04) |
| Missing | 4 | 1 | |
| Severity,g n (%) | |||
| 1 | 90 (11.9) | 96 (12.2) | |
| 2 | 563 (74.4) | 608 (77.5) | |
| 3a | 49 (6.5) | 53 (6.8) | |
| 3b | 33 (4.4) | 17 (2.2) | |
| 3c | 16 (2.1) | 7 (0.9) | |
| 4 | 6 (0.8) | 4 (0.5) | |
| Missing | 2 | 0 | |
| Obstetric anal sphincter injuryh | 104 (6.7) | 81 (5.3) | RR 1.27 (0.88 to 1.84) |
| Perineum sutured, n (%) | 1284 (82.6) | 1248 (81.4) | RR 1.02 (0.92 to 1.12) |
| Missing | 2 | 3 | |
| Anterior tear evident and sutured, n (%) | 102 (6.6) | 107 (7.0) | RR 0.95 (0.67 to 1.33) |
| Missing | 7 | 4 | |
| Manual removal of the placenta performed, n (%) | 99 (6.5) | 101 (6.7) | RR 0.97 (0.69 to 1.38) |
| Missing | 28 | 35 | |
| Post-partum haemorrhage requiring blood transfusion, n (%) | 63 (4.1) | 52 (3.4) | RR 1.20 (0.75 to 1.93) |
| Units transfused,i mean (SD) | 2.6 (1.5) | 2.2 (1.0) | MD –0.34 (–0.94 to 0.27) |
| Missing | 1 | 1 | |
| Woman’s pain score for birth | |||
| Median (IQR) | 15 (0–50) | 10 (0–50) | Median differencej 0 (0 to 0) |
| Missing | 345 | 347 | |
| Length of inpatient stay after delivery (hours) | |||
| Median (IQR) | 38.7 (24.9–59.7) | 37.5 (24.2–56.5) | Median differencej –1.2 (–3.2 to 0.7) |
| Missing | 48 | 34 | |
There was no evidence of a difference in maternal satisfaction in labour between the two arms of the trial, although there were interesting findings with respect to women’s views about the care they received in labour (Table 9). The majority of women ‘strongly agreed’ or ‘agreed’ with many of the statements, such as their satisfaction with their overall childbirth experience, that they were treated with respect by all the staff and that they were involved in decision-making during labour. However, in the case of factors such as whether or not their expectations for labour and birth were met, or if they felt in control, the proportions of women who ‘strongly agreed’ or ‘agreed’ were lower. Of particular interest in the context of BUMPES is that less than half of women ‘strongly agreed’ or ‘agreed’ that they were able to move as much as they wanted. Perhaps not surprisingly, the majority of women were satisfied with their pain relief during labour.
| Outcome | Trial arm | RRa,b (99% CI) | |
|---|---|---|---|
| Upright (N = 1556), n (%) | Lying down (N = 1537), n (%) | ||
| Number of questionnaires returned | 1208 (77.6) | 1165 (75.8) | – |
| Satisfied with overall childbirth experience | 0.97 (0.92 to 1.01) | ||
| Strongly agree | 553 (47.2) | 539 (47.1) | |
| Agree | 410 (35.0) | 434 (37.9) | |
| Neutral | 114 (9.7) | 100 (8.7) | |
| Disagree | 65 (5.6) | 40 (3.5) | |
| Strongly disagree | 30 (2.6) | 31 (2.7) | |
| Missing | 36 | 21 | |
| Treated with respect by all staff | 1.01 (0.99 to 1.02) | ||
| Strongly agree | 968 (82.0) | 937 (81.3) | |
| Agree | 178 (15.1) | 176 (15.3) | |
| Neutral | 19 (1.6) | 20 (1.7) | |
| Disagree | 7 (0.6) | 11 (1.0) | |
| Strongly disagree | 8 (0.7) | 8 (0.7) | |
| Missing | 28 | 13 | |
| Involved in making decisions | 0.99 (0.96 to 1.02) | ||
| Strongly agree | 824 (69.9) | 788 (68.5) | |
| Agree | 278 (23.6) | 299 (26.0) | |
| Neutral | 56 (4.8) | 45 (3.9) | |
| Disagree | 11 (0.9) | 10 (0.9) | |
| Strongly disagree | 10 (0.9) | 9 (0.8) | |
| Missing | 29 | 14 | |
| Expectations for labour and birth were met | 1.00 (0.93 to 1.08) | ||
| Strongly agree | 444 (38.0) | 437 (38.2) | |
| Agree | 359 (30.7) | 346 (30.2) | |
| Neutral | 209 (17.9) | 207 (18.1) | |
| Disagree | 118 (10.1) | 113 (9.9) | |
| Strongly disagree | 40 (3.4) | 41 (3.6) | |
| Missing | 38 | 21 | |
| Felt safe at all times | 1.01 (0.98 to 1.04) | ||
| Strongly agree | 793 (67.4) | 773 (67.2) | |
| Agree | 312 (26.5) | 299 (26.0) | |
| Neutral | 39 (3.3) | 51 (4.4) | |
| Disagree | 24 (2.0) | 16 (1.4) | |
| Strongly disagree | 9 (0.8) | 11 (1.0) | |
| Missing | 31 | 15 | |
| Good communication from staff | 1.01 (0.99 to 1.03) | ||
| Strongly agree | 913 (77.3) | 864 (75.3) | |
| Agree | 222 (18.8) | 230 (20.0) | |
| Neutral | 30 (2.5) | 33 (2.9) | |
| Disagree | 9 (0.8) | 10 (0.9) | |
| Strongly disagree | 7 (0.6) | 11 (1.0) | |
| Missing | 27 | 17 | |
| Felt in control | 1.01 (0.94 to 1.08) | ||
| Strongly agree | 428 (36.4) | 426 (37.2) | |
| Agree | 396 (33.6) | 368 (32.1) | |
| Neutral | 223 (19.0) | 232 (20.2) | |
| Disagree | 105 (8.9) | 93 (8.1) | |
| Strongly disagree | 25 (2.1) | 27 (2.4) | |
| Missing | 31 | 19 | |
| Able to move as much as wanted | 0.95 (0.85 to 1.06) | ||
| Strongly agree | 283 (24.5) | 310 (27.2) | |
| Agree | 285 (24.7) | 279 (24.5) | |
| Neutral | 239 (20.7) | 236 (20.7) | |
| Disagree | 253 (21.9) | 228 (20.0) | |
| Strongly disagree | 95 (8.2) | 86 (7.6) | |
| Missing | 53 | 26 | |
| Satisfied with position before pushing | 1.03 (0.99 to 1.07) | ||
| Strongly agree | 590 (50.3) | 566 (49.4) | |
| Agree | 460 (39.2) | 430 (37.5) | |
| Neutral | 83 (7.1) | 83 (7.2) | |
| Disagree | 29 (2.5) | 52 (4.5) | |
| Strongly disagree | 12 (1.0) | 15 (1.3) | |
| Missing | 34 | 19 | |
| Satisfied with position while pushing | 1.02 (0.98 to 1.06) | ||
| Strongly agree | 613 (52.2) | 570 (49.8) | |
| Agree | 425 (36.2) | 422 (36.9) | |
| Neutral | 94 (8.0) | 91 (8.0) | |
| Disagree | 29 (2.5) | 48 (4.2) | |
| Strongly disagree | 13 (1.1) | 14 (1.2) | |
| Missing | 34 | 20 | |
| Satisfied with labour pain relief | 1.00 (0.97 to 1.03) | ||
| Strongly agree | 791 (67.2) | 774 (67.4) | |
| Agree | 300 (25.5) | 288 (25.1) | |
| Neutral | 60 (5.1) | 51 (4.4) | |
| Disagree | 14 (1.2) | 23 (2.0) | |
| Strongly disagree | 12 (1.0) | 13 (1.1) | |
| Missing | 31 | 16 | |
Infant outcomes were extremely good overall, with very few babies having a low Apgar score at 5 minutes or evidence of metabolic acidosis (Table 10). There did appear to be more babies with metabolic acidosis in the lying-down group than in the upright group, but this difference was not statistically significant at the 1% level. Of interest, of the 23 babies with metabolic acidosis, 10 (six in the lying-down group and four in the upright group) were born in apparently normal condition with normal Apgar scores and with no evidence that they required resuscitation at the time of birth. Only eight of these babies received any HLC (six in the lying-down group and two in the upright group).
| Outcome | Trial arm | Adjusteda effect measure (99% CI) | |
|---|---|---|---|
| Upright (N = 1556) | Lying down (N = 1537) | ||
| Apgar score of < 4 at 5 minutes, n (%) | 2 (0.1) | 3 (0.2) | RR 0.66 (0.06 to 6.88) |
| Missing | 5 | 7 | |
| Metabolic acidosis,b n (%) | 6 (0.4) | 17 (1.2) | RR 0.35 (0.10 to 1.18) |
| pH and/or base deficit not donec | 531 (35.5) | 597 (40.4) | |
| Missing | 61 | 60 | |
| Meconium-stained liquor at delivery, n (%) | 347 (22.4) | 341 (22.2) | RR 1.01 (0.85 to 1.19) |
| Missing | 6 | 4 | |
| Resuscitation at birth, n (%) | 206 (13.3) | 180 (11.7) | RR 1.13 (0.89 to 1.45) |
| Missing | 1 | 2 | |
| Methodd | |||
| Facial oxygen | 122 (59.5) | 94 (52.2) | |
| Suction | 75 (36.6) | 74 (41.1) | |
| Bag and mask ventilation | 82 (40.0) | 82 (45.6) | |
| Intubation | 6 (2.9) | 8 (4.4) | |
| Complex resuscitation | 4 (2.0) | 1 (0.6) | |
| Missing | 1 | 0 | |
| Skin-to-skin contact in first hour after birth, n (%) | 1165 (77.1) | 1163 (78.4) | RR 0.98 (0.94 to 1.03)e |
| Missing | 45 | 53 | |
| Breastfeeding initiated in first hour after birth, n (%) | 780 (51.3) | 781 (52.1) | RR 0.98 (0.90 to 1.07) |
| Missing | 36 | 38 | |
| Length of inpatient hospital stay (hours) from birth | |||
| Median (IQR) | 38.7 (24.8–59.7) | 37.5 (24.2–56.9) | Median differencef –1.1 (–3.1 to 0.8) |
| Missing | 51 | 38 | |
| Admission to HLC,g n (%) | 108 (7.0) | 96 (6.3) | RR 1.11 (0.79 to 1.56) |
| Missing | 1 | 1 | |
| Length of stay in HLCh (days) | |||
| Total | 71 | 63 | |
| Median (IQR) | 2 (1–4) | 3 (1–6) | Median differencef 1 (0 to 2) |
| Missing | 4 | 5 | |
Overall, about 12% of babies required resuscitation at birth. This figure was slightly higher in the upright group than in the lying-down group, but this difference was not statistically significant at the 1% level. There was also a suggestion that babies in the upright group may have required more intensive resuscitation at birth, but again the numbers were very small and this difference was not statistically significant. There was no evidence of a difference in the proportions of babies with skin-to-skin contact in the first hour after birth, or in the proportions of babies who were breastfed in the first hour after birth. Median length of stay in hospital was approximately 38 hours for each group, and approximately 7% of babies were admitted for HLC (which included transitional care, special care or intensive care).
The data on adherence to the intervention are presented in Table 11 and Figure 9.
| Outcome | Trial arm | Median difference (95% CI) | |
|---|---|---|---|
| Upright (n = 1556) | Lying down (n = 1537) | ||
| During the passive second stage,a median (IQR) | 1.0 (1.0–1.0) | 1.0 (0.67–1.0) | 0 (0 to 0) |
| Missing: no passive time periods recorded | 320 | 314 | |
| Missing: time from study entry to start of recording positions at > 15 minutes | 227 | 217 | |
| Missing: pushing or birth dates/times not recorded | 13 | 10 | |
| Missing: position times not recorded | 50 | 36 | |
| During the active second stage,b median (IQR) | 0.88 (0.60–1.0) | 0.75 (0.38–1.0) | 0 (0 to 0) |
| Missing: no active time periods recorded | 11 | 19 | |
| Missing: time from study entry to start of recording positions at > 15 minutes | 227 | 217 | |
| Missing: pushing or birth dates/times not recorded | 13 | 10 | |
| Missing: position times not recorded | 50 | 36 | |
| During the whole second stage,c median (IQR) | 0.88 (0.67–1.0) | 0.78 (0.50–1.0) | 0 (0–0) |
| Missing: time from study entry to start of recording positions at > 15 minutes | 227 | 217 | |
| Missing: birth dates/times not recorded | 1 | 0 | |
| Missing: position times not recorded | 54 | 36 | |
| Reason for change from allocated position, n (%) | |||
| Passive stage | 201 | 343 | – |
| Clinical | 94 (50.0) | 78 (24.5) | |
| Non-clinical | 77 (41.1) | 218 (68.3) | |
| Clinical and non-clinical | 17 (9.0) | 23 (7.2) | |
| Missing | 13 | 24 | |
| Active stage | 699 | 981 | |
| Clinical | 416 (60.6) | 298 (31.1) | |
| Non-clinical | 136 (19.8) | 368 (38.5) | |
| Clinical and non-clinical | 135 (19.7) | 291 (30.4) | |
| Missing | 12 | 24 | |
| Whole of second stage | 788 | 1082 | |
| Clinical | 435 (56.6) | 306 (28.9) | |
| Non-clinical | 164 (21.3) | 419 (39.5) | |
| Clinical and non-clinical | 170 (22.1) | 335 (31.6) | |
| Missing | 19 | 22 | |
| Maternal reported adherence, n (%) | |||
| Passive stage | |||
| Mostly lying down | 226 (21.6) | 752 (72.3) | |
| Mostly upright | 794 (75.8) | 242 (23.3) | |
| Other | 24 (2.3) | 35 (3.4) | |
| Cannot remember | 3 (0.3) | 11 (1.1) | |
| Missing | 161 | 125 | |
| Form not completed | 348 | 372 | |
| Active stage | |||
| Mostly lying down | 202 (19.7) | 652 (63.7) | |
| Mostly upright | 745 (72.5) | 281 (27.4) | |
| Other | 78 (7.6) | 75 (7.3) | |
| Cannot remember | 3 (0.3) | 16 (1.6) | |
| Missing | 180 | 141 | |
| Form not completed | 348 | 372 | |
FIGURE 9.
Box and whisker plots of adherence (proportion of time spent in allocated position). (a) Passive stage; (b) active stage; and (c) whole of second stage. Reproduced from The Epidural and Position Trial Collaborative Group. 39 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.


The prespecified subgroup analyses are presented as forest plots in Figure 10 and Table 12. There is no evidence of heterogeneity between any of the prespecified subgroups for the primary outcome of SVB.
FIGURE 10.
Subgroup analyses for SVB (forest plot). a, All models adjusted for centre as a random effect; and b, diagnosis of delay prior to study entry requiring oxytocin. Reproduced from The Epidural and Position Trial Collaborative Group. 39 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
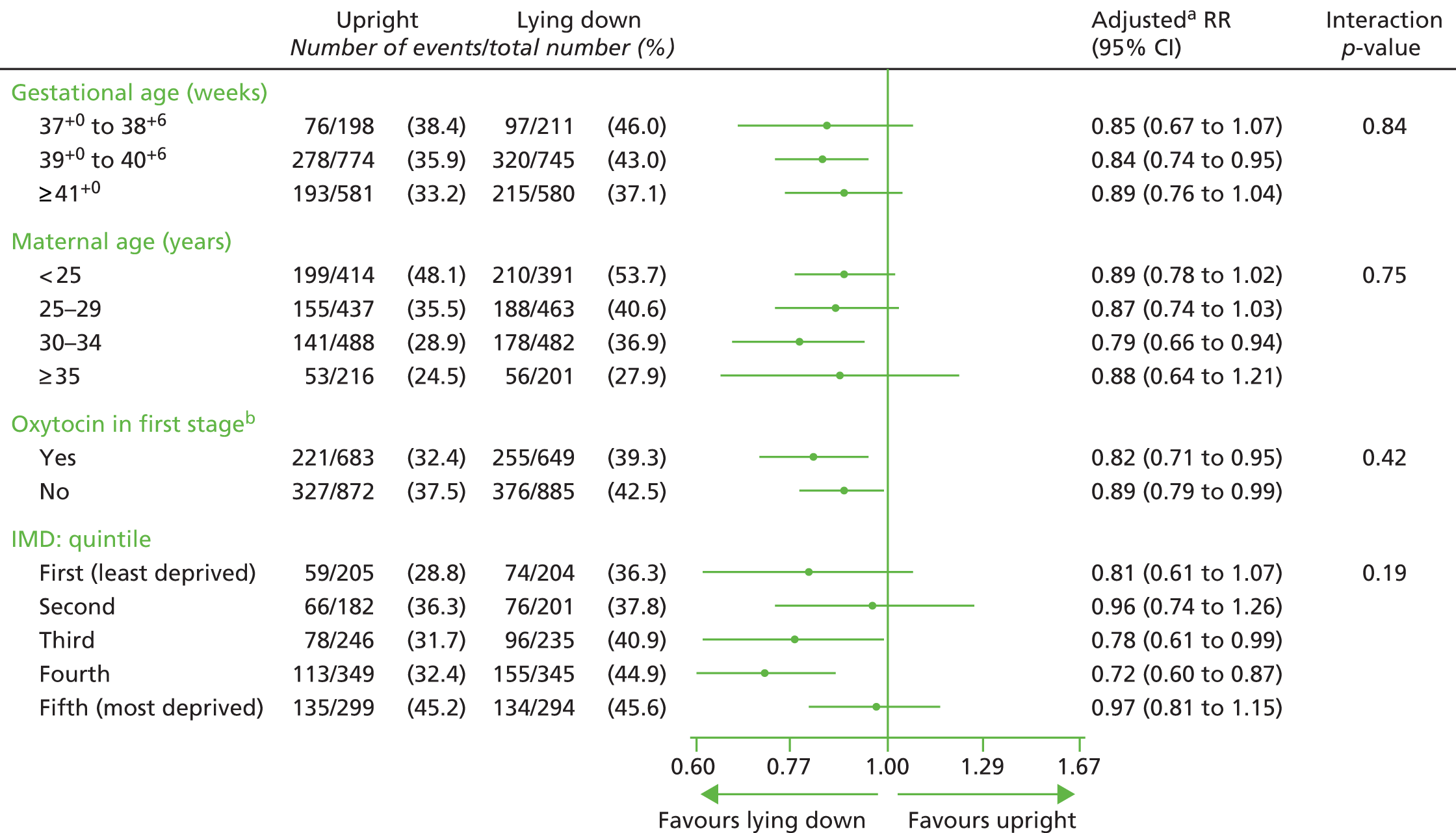
| Factor | Trial arm | Adjusteda RR (95% CI) | Interaction p-value | |
|---|---|---|---|---|
| Upright (N = 1556), n (%) | Lying down (N = 1537), n (%) | |||
| Gestational age (weeks) | ||||
| 37+0 to 38+6 | 76/198 (38.4) | 97/211 (46.0) | 0.85 (0.67 to 1.07) | 0.839 |
| 39+0 to 40+6 | 278/774 (35.9) | 320/745 (43.0) | 0.84 (0.74 to 0.95) | |
| ≥ 41+0 | 193/581 (33.2) | 215/580 (37.1) | 0.89 (0.76 to 1.04) | |
| Maternal age (years) | ||||
| < 25 | 199/414 (48.1) | 210/391 (53.7) | 0.89 (0.78 to 1.02) | 0.747 |
| 25–29 | 155/437 (35.5) | 188/463 (40.6) | 0.87 (0.74 to 1.03) | |
| 30–34 | 141/488 (28.9) | 178/482 (36.9) | 0.79 (0.66 to 0.94) | |
| ≥ 35 | 53/216 (24.5) | 56/201 (27.9) | 0.88 (0.64 to 1.21) | |
| Oxytocin in the first stageb | ||||
| Yes | 221/683 (32.4) | 255/649 (39.3) | 0.82 (0.71 to 0.95) | 0.417 |
| No | 327/872 (37.5) | 376/885 (42.5) | 0.89 (0.79 to 0.99) | |
| IMD: quintile | ||||
| First (least deprived) | 59/205 (28.8) | 74/204 (36.3) | 0.81 (0.61 to 1.07) | 0.187 |
| Second | 66/182 (36.3) | 76/201 (37.8) | 0.96 (0.74 to 1.26) | |
| Third | 78/246 (31.7) | 96/235 (40.9) | 0.78 (0.61 to 0.99) | |
| Fourth | 113/349 (32.4) | 155/345 (44.9) | 0.72 (0.60 to 0.87) | |
| Fifth (most deprived) | 135/299 (45.2) | 134/294 (45.6) | 0.97 (0.81 to 1.15) | |
Responses to postal questionnaires at 1 year after the birth were received from 61% of women. The characteristics of women who did and did not respond to the questionnaire are detailed in Table 13. Responders were more likely to be slightly older and live in less deprived areas, and to be white. They were also less likely to have had a SVB, and more likely to have had an instrumental delivery. There were no differences between responders and non-responders in the risk of caesarean section or the onset of labour (spontaneous or induced), or in the incidence of neonatal resuscitation or for their babies to be admitted to a HLC.
| Characteristic | 1-year follow-up | p-value | |
|---|---|---|---|
| Received (N = 1892) | Not received (N = 1201) | ||
| Maternal age (years) | |||
| Mean (SD) | 29.7 (5.2) | 26.5 (5.7) | p < 0.001a |
| Missing | 0 | 1 | |
| Gestational age at entry (weeks) | |||
| Mean (SD) | 40.4 (1.2) | 40.3 (1.2) | p = 0.048a |
| Missing | 1 | 3 | |
| IMD: quintile, n (%) | p < 0.001b | ||
| First (least deprived) | 279 (17.6) | 130 (13.3) | |
| Second | 259 (16.4) | 124 (12.7) | |
| Third | 318 (20.1) | 163 (16.7) | |
| Fourth | 431 (27.2) | 263 (26.9) | |
| Fifth (most deprived) | 295 (18.7) | 298 (30.5) | |
| Wales: not derived | 265 | 176 | |
| Postcode missing | 45 | 47 | |
| Ethnic group, n (%) | p < 0.001b | ||
| White | 1624 (86.5) | 956 (80.1) | |
| Indian | 58 (3.1) | 47 (3.9) | |
| Pakistani | 22 (1.2) | 34 (2.9) | |
| Bangladeshi | 3 (0.2) | 6 (0.5) | |
| Black African | 30 (1.6) | 28 (2.4) | |
| Black Caribbean | 11 (0.6) | 14 (1.2) | |
| Any other ethnic group | 129 (6.9) | 109 (9.1) | |
| Not known/missing | 15 | 7 | |
| BMI at booking visit (kg/m2) | |||
| Mean (SD) | 25.2 (5.2) | 25.6 (5.6) | p = 0.030a |
| Height and/or weight not known, n | 70 | 55 | |
| Onset of labour, n (%) | |||
| Spontaneous | 1121 (59.3) | 724 (60.3) | p = 0.573b |
| Induced | 769 (40.7) | 476 (39.7) | |
| Missing | 2 | 1 | |
| Diagnosis of delay requiring intervention, n (%) | 985 (52.1) | 581 (48.4) | p = 0.043b |
| Missing | 3 | 1 | |
| SVB, n (%) | 677 (35.8) | 503 (41.9) | p = 0.001b |
| Missing | 0 | 1 | |
| IVD,c n (%) | 1040 (55.0) | 587 (48.9) | p = 0.001b |
| Missing | 0 | 1 | |
| Caesarean section,d n (%) | 175 (9.3) | 110 (9.2) | p = 0.94b |
| Missing | 0 | 1 | |
| Episiotomy performed, n (%) | 1120 (59.2) | 632 (52.7) | p < 0.001b |
| Missing | 1 | 1 | |
| Obstetric anal sphincter injury,e n (%) | 116 (6.1) | 69 (5.8) | p = 0.675b |
| Missing | 2 | 5 | |
| Perineum sutured, n (%) | 1585 (83.9) | 947 (79.1) | p = 0.001b |
| Missing | 2 | 3 | |
| Resuscitation at birth, n (%) | 241 (12.8) | 145 (12.1) | p = 0.584b |
| Missing | 2 | 1 | |
| Breastfeeding initiated in the first hour after birth, n (%) | 994 (53.8) | 567 (48.4) | p = 0.004b |
| Missing | 45 | 29 | |
| Infant admission to HLC,f n (%) | 121 (6.4) | 83 (6.9) | p = 0.572b |
| Missing | 1 | 1 | |
Table 14 list the secondary maternal outcomes up to 1 year after birth in women who responded to the questionnaires. There was no evidence of any differences between the groups in relation to the incidence or severity of urinary incontinence, faecal incontinence, constipation, haemorrhoids or dyspareunia. Similarly, there was no evidence of a difference in the incidence of diagnosed cerebral palsy or severe neurodevelopmental delay in any of the infants at 1 year (see Table 15).
| Outcome | Trial arm | Adjusteda effect measure (99% CI) | |
|---|---|---|---|
| Upright (N = 950) | Lying down (N = 942) | ||
| Urinary incontinence, n (%) | |||
| Leaking in the first 3 months | 462 (48.8) | 461 (49.2) | RR 0.99 (0.88 to 1.12) |
| Missing | 4 | 4 | |
| ICIQ-UI scoreb over the past 4 weeks | |||
| Median (IQR) | 0 (0–4) | 0 (0–4) | Median differencef 0 (0 to 0) |
| Missing, n | 39 | 34 | |
| Faecal incontinence, n (%) | |||
| No bowel control and/or soiling | |||
| In the first 3 months | 108 (11.5) | 132 (14.2) | RR 0.81 (0.59 to 1.11) |
| Missing | 7 | 9 | |
| In the past 4 weeks | 32 (3.4) | 27 (2.9) | RR 1.18 (0.61 to 2.28) |
| Missing | 10 | 8 | |
| No bowel control and/or soiling and/or feel need to go, n (%) | |||
| In the first 3 months | 215 (22.8) | 251 (26.9) | RR 0.85 (0.69 to 1.05) |
| Missing | 8 | 8 | |
| In the past 4 weeks | 113 (12.1) | 102 (10.9) | RR 1.10 (0.79 to 1.53) |
| Missing | 12 | 10 | |
| No bowel control at times,c n (%) | |||
| Never | 829 (87.9) | 806 (86.1) | |
| In the first 3 months | 83 (8.8) | 103 (11.0) | |
| In the past 4 weeks | 13 (1.4) | 19 (2.0) | |
| At any other time | 29 (3.1) | 20 (2.1) | |
| Missing | 7 | 5 | |
| Soiling from back passage on underwear,c n (%) | |||
| Never | 836 (88.6) | 838 (89.5) | |
| In the first 3 months | 70 (7.4) | 75 (8.0) | |
| In the past 4 weeks | 24 (2.5) | 14 (1.5) | |
| At any other time | 24 (2.5) | 22 (2.4) | |
| Missing | 6 | 6 | |
| Feel need to go and have to go immediately,c n (%) | |||
| Never | 640 (67.9) | 616 (65.8) | RR 0.85 (0.67 to 1.08) |
| In the first 3 months | 173 (18.4) | 202 (21.6) | |
| In the past 4 weeks | 98 (10.4) | 90 (9.6) | RR 1.08 (0.76 to 1.55) |
| At any other time | 77 (8.2) | 82 (8.8) | |
| Missing | 8 | 6 | |
| Constipation,c n (%) | |||
| Never | 367 (38.9) | 406 (43.2) | RR 1.12 (0.96 to 1.29) |
| In the first 3 months | 395 (41.8) | 353 (37.6) | |
| In the past 4 weeks | 94 (10.0) | 107 (11.4) | RR 0.87 (0.62 to 1.23) |
| At any other time | 140 (14.8) | 154 (16.4) | |
| Missing | 6 | 2 | |
| Haemorrhoids,c n (%) | |||
| Never | 495 (52.4) | 518 (55.1) | RR 1.03 (0.87 to 1.23) |
| In the first 3 months | 308 (32.6) | 297 (31.6) | |
| In the past 4 weeks | 108 (11.4) | 116 (12.3) | RR 0.93 (0.67 to 1.28) |
| At any other time | 108 (11.4) | 115 (12.2) | |
| Missing | 6 | 2 | |
| Dyspareunia,c,d n (%) | |||
| Never | 366 (40.7) | 363 (40.6) | RR 0.95 (0.82 to 1.10) |
| In the first 3 months | 364 (40.5) | 381 (42.6) | |
| In the past 4 weeks | 80 (8.9) | 79 (8.8) | RR 1.01 (0.68 to 1.49) |
| At any other time | 160 (17.8) | 151 (16.9) | |
| Missing | 5 | 2 | |
| Not applicable (not had sexual intercourse since the birth) | 46 | 45 | |
| Outcome | Trial arm | Adjusteda RR (99% CI) | |
|---|---|---|---|
| Upright (N = 950), n (%) | Lying down (N = 942), n (%) | ||
| Major morbidityb | 1 (0.11) | 4 (0.42) | 0.25 (0.01 to 4.40) |
The prespecified sensitivity analysis, which excluded women who had another birth or were pregnant at the time of the 1-year follow-up, demonstrates no change in the conclusions of the study (see Table 16).
| Outcome | Trial arm | Adjusteda effect measure (99% CI) | |
|---|---|---|---|
| Upright (N = 950) | Lying down (N = 942) | ||
| Women who have had another baby, n (%) | 6 (0.6) | 4 (0.4) | |
| Missing | 6 | 14 | |
| Women pregnant at time of completing questionnaire, n (%) | 61 (6.5) | 72 (7.8) | |
| Missing | 9 | 20 | |
| Denominator excluding women who were pregnant/had another baby | 883 | 866 | |
| Urinary incontinence, n (%) | |||
| Leaking in the first 3 months | 432 (49.2) | 426 (49.4) | RR 0.99 (0.88 to 1.13) |
| Missing | 4 | 4 | |
| ICIQ-UI scoreb over the past 4 weeks | |||
| Median (IQR) | 0 (0–4) | 0 (0–4) | Median difference 0 (0 to 0) |
| Missing | 38 | 30 | |
| Faecal incontinence | |||
| No bowel control and/or soiling | |||
| In the first 3 months | 101 (11.5) | 122 (14.2) | RR 0.81 (0.59 to 1.12) |
| Missing | 7 | 9 | |
| In the past 4 weeks | 28 (3.2) | 27 (3.2) | RR 1.02 (0.51 to 2.02) |
| Missing | 10 | 8 | |
| No bowel control and/or soiling and/or feel the need to go | |||
| In the first 3 months | 203 (23.2) | 235 (27.4) | RR 0.85 (0.68 to 1.05) |
| Missing | 8 | 7 | |
| In the past 4 weeks | 106 (12.2) | 93 (10.9) | RR 1.12 (0.79 to 1.58) |
| Missing | 12 | 9 | |
| Feel need to go and have to go immediatelyc | |||
| In the first 3 months | 161 (18.4) | 191 (22.2) | RR 0.83 (0.65 to 1.06) |
| In the past 4 weeks | 92 (10.5) | 81 (9.4) | RR 1.12 (0.77 to 1.62) |
| Missing | 8 | 5 | |
| Constipation,c n (%) | |||
| In the first 3 months | 368 (42.0) | 328 (38.0) | RR 1.11 (0.95 to 1.29) |
| In the past 4 weeks | 82 (9.4) | 90 (10.4) | RR 0.90 (0.62 to 1.30) |
| Missing | 6 | 2 | |
| Haemorrhoids,c n (%) | |||
| In the first 3 months | 291 (33.2) | 278 (32.2) | RR 1.03 (0.86 to 1.23) |
| In the past 4 weeks | 100 (11.4) | 102 (11.8) | RR 0.97 (0.69 to 1.36) |
| Missing | 6 | 2 | |
| Dyspareunia,c,d n (%) | |||
| In the first 3 months | 339 (40.7) | 351 (42.9) | RR 0.95 (0.82 to 1.10) |
| In the past 4 weeks | 75 (9.0) | 78 (9.5) | RR 0.95 (0.64 to 1.41) |
| Missing | 5 | 2 | |
| Not applicable (not had sexual intercourse since the birth) | 45 | 45 | |
There were a number of adverse events reported during the course of the trial. The majority of these did not appear to be related to the intervention (Table 17).
| Adverse event | Trial arm | |
|---|---|---|
| Upright (N = 1556) | Lying down (N = 1537) | |
| Woman, n | ||
| Dizziness | 29 | 21 |
| Post-partum haemorrhage | 1 | 1 |
| Seizure | 0 | 2 |
| Other | 3 | 3 |
| Baby, n | ||
| Stillbirth | 1 | 0 |
| Birth trauma | 1 | 0 |
| Other | 2 | 0 |
Chapter 6 Economic evaluation
In this chapter we present the results of the economic evaluation conducted alongside the BUMPES trial. A within-trial cost–consequences analysis with a time horizon of a 1-year follow-up and a NHS perspective was conducted. The aim of this analysis was to inform about health-care utilisation and costs for the treatment pathways followed by mothers and their babies from trial entry up to 1 year after birth. In addition to the consequences of the different positions during the late stages of labour in women with an epidural presented in previous chapters, we also report maternal health-related quality of life (HRQoL) at 1 year after birth. Details of each component of the economic analysis are provided in this chapter.
Methods
A health economics analysis plan was developed to guide the health economics team during the development of the economic evaluation (see Appendix 8). Overall, the team followed this analysis plan during the conduct of the economic evaluation, but there was one deviation from the plan that affected the final presentation of results of the economic analysis. The original analysis plan stated quality-adjusted life-years (QALYs) as the primary health outcome measure and used a cost–utility analysis to present the results of the economic evaluation. However, quality-of-life data at randomisation were not collected owing to the intrinsic difficulties in collecting this information during labour. In addition, it was not planned to collect data during the early postnatal period as part of the trial. As a result, the calculation of a QALY profile for the trial duration could not be derived. The next alternative could have been to conduct a cost-effectiveness analysis using the primary outcome in the trial or another clinical end point to present the results of the economic analysis. However, given that this was, to our knowledge, the first economic evaluation of position during labour, the results of such an analysis would have been difficult to interpret by decision-makers in the absence of a willingness to pay for health gains for the selected outcome. Therefore, we decided to conduct a cost–consequences evaluation as the primary analysis for the economic evaluation. In a cost–consequences analysis, the different components of costs and benefits of the interventions under evaluation are presented in a disaggregated manner without an attempt to estimate a summary measure [e.g. incremental cost-effectiveness ratio (ICER)]. 41 As a secondary outcome of the economic analysis we conducted a cost-effectiveness analysis using the number of additional cases of SVB as the outcome measure. The latter analysis was included as a benchmark for future economic evaluations studies in obstetrics.
Health outcome measures
Health outcomes for women and their babies before discharge and at the 1-year follow-up were evaluated as potential consequences to include in the economic evaluation. Chapter 5 reported a number of secondary maternal, neonatal and longer-term outcomes in addition to the primary outcome in the trial. Most of these outcomes led to health-care service utilisation, which was accounted for in our analysis, and only a selection of maternal and infant outcomes were therefore included in the cost–consequences analysis. For women, we selected the incidence of SVB, maternal satisfaction with labour as reported immediately after the birth and urinary and faecal incontinence at 1 year’s follow-up. An Apgar score of < 4 at 5 minutes and major morbidity at 1 year’s follow-up were the consequences selected for infants. When reporting the results of difference in estimates between upright and lying-down positions for any of these outcomes, we refer to the appropriate tables in Chapter 5 rather than replicating the tables here.
In addition to the above consequences, maternal HRQoL information using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L) and the Short Form questionnaire-12 items (SF-12) instruments was collected at the 1-year follow-up. The EQ-5D-3L is an increasingly widely used multiattribute generic instrument for measuring HRQoL in cost–utility analyses. 42 It has two components: a descriptive system covering five dimensions (mobility, self-care, usual activity, pain/discomfort and anxiety/depression), each of which has three levels (no problem, some problems and extreme problems), and a ‘feeling thermometer’ using a visual analogue scale. The 243 health states of the EQ-5D-3L can be converted into preference-based utility values using a value set obtained from a British general population sample. 43 The SF-12 is a generic HRQoL instrument derived from its longer counterpart, the Short Form questionnaire-36 items (SF-36),44 designed to measure general health functioning. The SF-12 items measure physical or emotional limitations, physical functioning, pain, general health, vitality, social functioning and mental health problems. The number of levels in each SF-12 question varies depending on the version used, and in this study the SF-12 version 2 was administered. Health states from the SF-12 instrument can be converted into preference-based utilities using the Short Form questionnaire-6 Dimensions (SF-6D) algorithm, which was used in this study. 45
NHS health-care resource use
Detailed information about secondary care usage was collected and included resources consumed during the late stages of labour to hospital discharge, and during the first 12 months after birth as reported at the 1-year follow-up. Chapter 5 reported some data about health-care usage that we also present in this chapter with additional information. Data from trial entry up to postnatal hospital discharge were collected from hospital records and included in the DCB (see Appendix 1). A postal questionnaire was used to collect secondary care information at the 1-year follow-up, and this was sent by the trial management team, which also dealt with reminders and appropriate double-data entry and data cleaning. Information was collected for women and their babies. The different items of resource use collected for each category of secondary care health service are summarised in Table 18.
| Resource use item | Unit cost (£) | Source | Notes |
|---|---|---|---|
| Maternal | |||
| Birth related | |||
| Augmentation (oxytocin) | 1 | BNF 201546 | Oxytocin, injection, price for 10 units/ml, 1-ml ampoule |
| Fetal blood sampling | 28 | John Radcliffe Hospital Women’s Centre (Oxford) | Obtained from hospital finance department |
| Fetal scalp electrode | 5 | Schroeder et al.47 | |
| Hypotension medication | 10 | BNF 201546 | Injection, phenylephrine hydrochloride 10 mg/ml (1%), 1-ml ampoule = £9.91 |
| Mode of birth | |||
| Vaginal delivery | 1462 | NHS Reference Costs 2013–14 48 | Normal delivery with a complication score of 0 (HRG data) |
| Assisted delivery | 1860 | NHS Reference Costs 2013–14 48 | Assisted delivery with a complication score of 0 (HRG data) |
| Caesarean section delivery | 3674 | NHS Reference Costs 2013–14 48 | Emergency caesarean section |
| Episiotomy | 27 | Schroeder et al.47 | |
| Perineal tear | |||
| First- and second-degree tears | 23 | Schroeder et al.47 | |
| Third- and fourth-degree tears | 64 | Schroeder et al.47 | |
| Manual removal of the placenta | 74 | Schroeder et al.47 | |
| Post-partum haemorrhage | 154 | Eddama et al.49 | |
| Blood transfusion | 157 | Schroeder et al.47 | Per blood pack |
| HLC admissions | |||
| Level of care (per day) | |||
| Level 0 | 643 | NHS Reference Costs 2013–14 48 | |
| Level 1 | 890 | NHS Reference Costs 2013–14 48 | |
| Level 2 | 1266 | NHS Reference Costs 2013–14 48 | |
| Level 3 | 1449 | NHS Reference Costs 2013–14 48 | |
| Investigations | |||
| MRI | 139 | NHS Reference Costs 2013–14 48 | |
| CT | 80 | NHS Reference Costs 2013–14 48 | |
| Radiography | 48 | NHS Reference Costs 2013–14 48 | |
| Transfer to another hospital | 435 | Schroeder et al.47 | |
| Outpatient visits | |||
| Perineal care clinic | 13 | NHS Reference Costs 2013–14 48 | |
| Gynaecological | 13 | NHS Reference Costs 2013–14 48 | |
| Surgical | 11 | NHS Reference Costs 2013–14 48 | |
| Other | 127 | – | Average cost of outpatient visits |
| Hospital visits | |||
| Hospital inpatient (per day) | 757 | NHS Reference Costs 2013–14 48 | Average cost of regular day or night admissions |
| Postnatal ward stay (per day) | 103 | Schroeder et al.47 | |
| Infant | |||
| Birth related | |||
| Cord blood sampling | 0.05 | Schroeder et al.47 | |
| HLC admissions | |||
| Level of care (per day) | |||
| Special care | 41 | NHS Reference Costs 2013–14 48 | |
| High dependency | 839 | NHS Reference Costs 2013–14 48 | |
| Intensive care | 1118 | NHS Reference Costs 2013–14 48 | |
| Investigations | |||
| Radiography | 85 | NHS Reference Costs 2013–14 48 | |
| CT scans | 37 | NHS Reference Costs 2013–14 48 | |
| MRI | 48 | NHS Reference Costs 2013–14 48 | |
| Transfer to another hospital | 1259 | NHS Reference Costs 2013–14 48 | Neonatal critical care, transportation |
| Neonatal death | 696 | Schroeder et al.47 | |
| Outpatient visits | |||
| Orthopaedic | 148 | NHS Reference Costs 2013–14 48 | |
| Paediatric | 289 | NHS Reference Costs 2013–14 48 | |
| Hearing | 138 | NHS Reference Costs 2013–14 48 | |
| Eye | 115 | NHS Reference Costs 2013–14 48 | |
| Dermatology | 144 | NHS Reference Costs 2013–14 48 | |
| Other | 167 | – | Average cost of outpatient |
| Hospital visits | |||
| Hospital inpatient (per day) | 886 | NHS Reference Costs 2013–14 48 | Paediatric high-dependency ward |
No intervention-specific costs were assigned to either upright or lying-down position as neither was associated with the use of any additional resources . Given that all randomised women already had epidural analgesia and that any remaining medication after the birth was considered to be waste, epidural-specific costs were excluded from the cost analysis. In addition, any top-up epidural drugs costs in both arms were excluded from the cost analysis as there was no evidence of a difference between groups (see Table 7).
At the study design stage, there was a general concern about including primary care and community care visits as part of the data collection because these tend to be frequent and poorly recalled by new mothers compared with secondary care visits. 50 It was agreed that hospital care constituted the main cost driver for this population and the target source data to collect in the study. Therefore, primary care and community care visit data were not collected. However, urinary and faecal incontinence are important outcomes following birth and may be related to the mode of delivery, and can have long-lasting effects on HRQoL and additional visits to primary care. 17,21 Therefore, it was decided that, if necessary, primary care visits related to these adverse events would be estimated using recent data from the literature if significant differences between treatment arms were observed. Nevertheless, this was not the case (see Table 14), and such visits were not incorporated as part of the categories of resource use in the cost analysis and are presented as part of the health outcomes in the cost–consequences analysis. We also assumed that any costs for specific surgeries were reflected in the length of stay and the unit cost attached to the admission. Therefore, we did not conduct a micro-costing approach for the maternal and infant surgeries performed in different time periods.
Unit cost data collection
Sources and associated estimates of unit costs for the different categories of resource use are presented in Table 18. Unit costs were mainly extracted from national sources, including the Personal Social Services Research Unit51 and the NHS Reference Costs 2013–14,48 and from a recent published cost-effectiveness analysis of alternative planned places of birth in woman at low risk of complications. 47 The unit cost of undertaking fetal blood sampling for the assessment of metabolic acidosis was not available in any of the sources consulted and was provided by the finance department of a large obstetric unit in Oxford. All costs were expressed in 2013/14 pounds sterling inflated to this base using the Hospital and Community Health Service Inflation Index52 where appropriate.
Statistical analysis
Volumes of categories of resource use were multiplied by the corresponding unit cost to estimate the cost per woman or baby in a particular category. This was then averaged across each trial arm to obtain a mean cost per woman or baby of a particular category. We estimated health-care resource use deriving mean estimates and SDs either across all women and their babies in an arm of the trial or only for those consuming the resource category. The latter analysis using standard descriptive statistics was expected to provide information about potential outliers influencing the estimation of mean resource use and costs for a particular category of resource use in each arm of the trial. Categories of resource use and associated costs are presented separately for women and their babies, but the estimation of summary costs for a particular resource use category (e.g. total costs at 12-month follow-up) was calculated adding information from the pair. Health-care resource use between treatment arms was compared using RRs for binomial variables and mean differences for continuous covariates. Costs were compared using mean differences between treatment arms. Recent evidence suggests that both parametric and non-parametric methods accurately estimate the true standard errors (SEs) of means, even when data are highly skewed, and moderate to large (n > 50) sample sizes for continuous variables. 53 Hence, mean resource use and cost differences and associated uncertainty for particular categories of resource use and costs between the two positions during the late stage of labour were estimated using parametric methods. In line with the statistical analysis of the primary outcome, differences between treatment arms were adjusted using a random intercept binomial (for RRs) or linear (for mean differences) model using hospital centre as a random effect. In order to be consistent with the original SAP, a 95% significance level was defined to determine significant differences in health-care resource use for the primary outcome (SVB) whereas a 99% significance level was used for the other categories of resource use. A 95% significance level was used to compare main categories of costs between treatment arms.
Mean differences and associated uncertainty in EQ-5D-3L and SF-6D utilities between the treatment arms were assessed using parametric methods and adjusted using a random intercept linear model for hospital centre.
The number of missing data for secondary care resource use up to hospital discharge was very low in each treatment arm (< 1%), and we present the health-care resource use and costs during this period using a complete-case analysis. The proportion of missing data on both resource use and HRQoL was higher at the 1-year follow-up. In this case, resource use was presented using a complete-case analysis, but in the case of maternal quality-of-life scores and costs we implemented a multiple imputation framework with chained equation. 54 Current guidance on handling missing data in cost-effectiveness analysis was followed to inform such analysis. 55 Only missing utility scores (for both EQ-5D-3L index and SF-6D utilities) and individual cost items were imputed and the distribution of responses for both instruments was reported for data available. We constructed an imputation model that included covariates with complete data on trial entry characteristics (maternal age, gestational age at entry, BMI and baby’s birth weight), HRQoL variables (EQ-5D-3L and SF-6D scores), and all individual categories of cost variables. We used prediction mean-matching, estimated 50 different imputations, and the imputation model was implemented separately by trial allocation. Mean estimates and estimates of SE were combined between imputed data sets using Rubin’s rule56 and adjusted using a random intercept linear model for hospital centre.
Mean differences in the total costs at 12 months’ follow-up and the number of additional cases of SVB between treatment arms were combined into the ICER to determine cost-effectiveness as a secondary outcome in the economic evaluation. The lying-down position was used as the comparator in the ICER calculation. Uncertainty around the ICER was evaluated parametrically using 95% CIs. 57 The five parameters needed (difference in costs, SE of difference in costs, difference in effects, SE of difference in effects and correlations of differences in costs and effects) to estimate parametrically the uncertainty around the ICER were obtained from the multiple imputation analysis.
The statistical analysis was conducted in Stata/SE for Windows version 13.1.
Results
Clinical consequences
In Chapter 5 we reported that there were significantly fewer SVBs in the upright group (see Table 5) and that we found no evidence of differences between the two intervention groups in maternal satisfaction (see Table 9), maternal urinary and faecal incontinence (see Table 14), frequency of Apgar score of < 4 at 5 minutes (see Table 10) or major morbidity (see Table 15).
NHS health-care resource use
Tables 19 and 20 present the maternal and infant health-care resource use from trial entry to postnatal hospital discharge for the birth-related, HLC admissions and hospital length of stay categories of resource use. In line with reporting of the primary outcome in Chapter 5, the only statistically significant difference observed between trial arms was the higher number of SVBs in the lying-down arm. The number of infants in whom cord blood was sampled was slightly higher in the upright group than in the lying-down group, yielding a statistically significant RR (99% CI) of 1.012 (1.010 to 1.013). No other significant differences were detected.
| Health-care resource use | Trial arm | RR/MD (99% CI)a | |
|---|---|---|---|
| Upright (N = 1556) | Lying down (N = 1537) | ||
| Delivery-related, n (%) | |||
| Augmentation (oxytocin) | 172 (11.08) | 163 (10.62) | 1.04 (0.80 to 1.35) |
| Missing | 3 | 2 | |
| Fetal blood sampling | 90 (5.80) | 72 (4.69) | 1.17 (0.82 to 1.68) |
| Missing | 4 | 3 | |
| Fetal scalp electrode | 94 (6.06) | 85 (5.57) | 1.09 (0.76 to 1.57) |
| Missing | 6 | 11 | |
| Hypotension medication | 13 (0.84) | 12 (0.78) | 1.07 (0.39 to 2.99) |
| Missing | 3 | 2 | |
| Mode of birth | |||
| SVB | 548 (35.24) | 632 (41.12) | 0.86 (0.78 to 0.94)* |
| IVD | 849 (54.60) | 778 (50.62) | 1.08 (0.98 to 1.18) |
| Caesarean section | 158 (10.16) | 127 (8.26) | 1.23 (0.92 to 1.64) |
| Missing | 1 | 0 | |
| Episiotomy | 914 (58.78) | 838 (54.56) | 1.08 (0.99 to 1.16) |
| Missing | 1 | 1 | |
| Perineal tear | 760 (48.97) | 785 (51.11) | 0.95 (0.87 to 1.04) |
| First- or second-degree tear | 654 (42.19) | 704 (45.83) | |
| Third- or fourth-degree tear | 104 (6.71) | 81 (5.27) | |
| Missing | 6 | 1 | |
| Manual removal of the placenta | 99 (6.48) | 101 (6.72) | 0.97 (0.69 to 1.38) |
| Missing | 28 | 35 | |
| Post-partum haemorrhage with blood transfusion | 63 (4.05) | 52 (3.39) | 1.20 (0.75 to 1.92) |
| Missing | 1 | 1 | |
| HLC admissions | |||
| Level of care (days), mean (SD) | |||
| Level 0 | 0.035 (0.339) | 0.025 (0.320) | 0.010 (–0.020 to 0.040) |
| Level 1 | 0.041 (0.222) | 0.029 (0.201) | 0.011 (–0.007 to 0.031) |
| Level 2 | 0.021 (0.202) | 0.023 (0.191) | –0.002 (–0.020 to 0.016) |
| Level 3 | 0.005 (0.076) | 0.001 (0.036) | 0.003 (–0.002 to 0.009) |
| Missing | 3 | 2 | |
| Surgery performed | 7 (0.45) | 4 (0.26) | |
| Missing | 3 | 2 | |
| Transfer to another hospital | 2 (0.13) | 0 (0.00) | |
| Missing | 3 | 2 | |
| Investigations, mean (SD) | |||
| Radiography | 0.0032 (0.0671) | 0.0039 (0.0721) | –0.0007 (–0.007 to 0.006) |
| CT | 0.0013 (0.0359) | 0.0007 (0.0255) | 0.0006 (–0.002 to 0.005) |
| MRI | 0 (0) | 0.0007 (0.0255) | –0.0007 (–0.002 to 0.001) |
| Missing | 3 | 2 | |
| Hospital length of stay, mean (SD) | |||
| Length of stay (days) | 2.73 (2.28) | 2.74 (2.94) | –0.003 (–0.242 to 0.243) |
| Missing | 5 | 5 | |
| Health-care resource use | Trial arm | RR/MD (99% CI)a | |
|---|---|---|---|
| Upright (N = 1556) | Lying down (N = 1537) | ||
| Birth-related, n (%) | |||
| Cord blood sampling | 1025 (66.21) | 940 (61.56) | 1.012 (1.010 to 1.013)* |
| Missing | 8 | 10 | |
| Resuscitation | 206 (13.24) | 180 (11.71) | 1.32 (0.89 to 1.44) |
| Facial oxygen | 122 (7.85) | 94 (6.12) | |
| Suction | 75 (4.83) | 74 (4.82) | |
| Bag and mask ventilation | 82 (5.28) | 82 (5.34) | |
| Intubation | 6 (0.39) | 8 (0.52) | |
| Complex resuscitation | 4 (0.26) | 1 (0.07) | |
| Missing | 2 | 2 | |
| HLC admissions | |||
| Level of care (in days), mean (SD) | |||
| Special care | 0.10 (0.65) | 0.14 (1.16) | –0.042 (–0.127 to 0.046) |
| Missing | 13 | 7 | |
| High dependency | 0.02 (0.17) | 0.03 (0.33) | –0.010 (–0.035 to 0.015) |
| Missing | 9 | 4 | |
| Intensive care | 0.04 (0.83) | 0.03 (0.32) | 0.017 (–0.041 to 0.076) |
| Missing | 9 | 3 | |
| Surgery performed, n (%) | 2 (0.13) | 3 (0.20) | |
| Missing | 9 | 4 | |
| Transfer to another hospital, n (%) | 2 (0.13) | 4 (0.26) | |
| Missing | 9 | 3 | |
| Neonatal death, n (%) | 0 | 0 | |
| Missing | 33 | 29 | |
| Investigations, mean (SD) | |||
| Radiography | 0.02 (0.21) | 0.04 (0.49) | –0.015 (–0.050 to 0.197) |
| Missing | 11 | 7 | |
| CT | 0 (0) | 0.002 (0.447) | –0.002 (–0.005 to 0.001) |
| Missing | 10 | 6 | |
| MRI | 0.0020 (0.0440) | 0.0007 (0.0256) | 0.0013 (–0.002 to 0.005) |
| Missing | 10 | 6 | |
| Hospital length of stay, mean (SD) | |||
| Length of stay (days) | 3 (1.93) | 3.07 (3.15) | –0.067 (–0.308 to 0.174) |
| Missing | 6 | 5 | |
A breakdown of health-care resources consumed at 12-month follow-up for women and their babies for outpatient and hospital visits is presented in Table 21 using a complete-case analysis. No significant differences for any of the categories between treatment groups were observed.
| Resource use category | Trial arm | MD (99% CI)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Upright (N = 1556) | Lying down (N = 1537) | ||||||||||
| n | Missing | Min. | Max. | Mean (SD) | n | Missing | Min. | Max. | Mean (SD) | ||
| Maternal | |||||||||||
| Outpatient visits | |||||||||||
| Perineal care clinic | 941 | 615 | 0 | 10 | 0.140 (0.781) | 936 | 601 | 0 | 10 | 0.109 (0.711) | 0.030 (–0.059 to 0.118) |
| Gynaecological | 925 | 631 | 0 | 8 | 0.144 (0.590) | 931 | 606 | 0 | 10 | 0.179 (0.815) | –0.035 (–0.120 to 0.050) |
| Surgical | 944 | 612 | 0 | 6 | 0.035 (0.322) | 936 | 601 | 0 | 5 | 0.049 (0.346) | –0.014 (–0.054 to 0.025) |
| Other | 927 | 629 | 0 | 50 | 0.514 (2.371) | 920 | 617 | 0 | 35 | 0.414 (1.814) | 0.099 (–0.154 to 0.715) |
| Hospital visits | |||||||||||
| Hospital inpatient (days) | 935 | 621 | 0 | 28 | 0.198 (1.328) | 927 | 610 | 0 | 24 | 0.201 (1.257) | –0.003 (–0.120 to 0.115) |
| Number of operations | 950 | 606 | 0 | 2 | 0.036 (0.202) | 942 | 595 | 0 | 2 | 0.050 (0.232) | –0.014 (–0.040 to 0.012) |
| Infant | |||||||||||
| Outpatient visits | |||||||||||
| Orthopaedic | 943 | 613 | 0 | 20 | 0.060 (0.760) | 935 | 602 | 0 | 10 | 0.056 (0.466) | 0.005 (–0.070 to 0.080) |
| Paediatric | 923 | 633 | 0 | 8 | 0.250 (0.745) | 912 | 625 | 0 | 20 | 0.279 (1.077) | –0.027 (–0.138 to 0.084) |
| Hearing | 942 | 614 | 0 | 4 | 0.037 (0.272) | 937 | 600 | 0 | 10 | 0.043 (0.411) | –0.006 (–0.047 to 0.036) |
| Eye | 935 | 621 | 0 | 3 | 0.042 (0.248) | 932 | 605 | 0 | 3 | 0.062 (0.305) | –0.021 (–0.054 to 0.013) |
| Dermatology | 944 | 612 | 0 | 6 | 0.039 (0.328) | 938 | 599 | 0 | 5 | 0.036 (0.282) | 0.003 (–0.033 to 0.039) |
| Other | 930 | 626 | 0 | 10 | 0.206 (0.855) | 924 | 613 | 0 | 22 | 0.297 (1.378) | –0.090 (–0.225 to 0.048) |
| Hospital visits | |||||||||||
| Hospital inpatient (days) | 928 | 628 | 0 | 21 | 0.374 (1.441) | 919 | 618 | 0 | 45 | 0.491 (2.245) | –0.123 (–0.344 to 0.103) |
| Number of operations | 950 | 606 | 0 | 2 | 0.015 (0.129) | 942 | 595 | 0 | 1 | 0.023 (0.151) | –0.009 (–0.025 to 0.008) |
Similar information to that in Tables 19–20 is presented in Tables 22 and 23, but only for women and their babies consuming the health-care resource use. The results from such analysis confirmed the presence of a few individuals (as indicated by the range) consuming more of some resource use categories than the remaining participants (e.g. one infant admitted for intensive care for 31 days in the upright position or women having more than 30 visits as an outpatient in both groups). However, the impact of these outliers was minimal when averaging across all participants, given the low number of participants consuming any of these resources.
| Health-care resource use | Trial arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Upright | Lying down | |||||||||
| n | Mean (SD) | Min. | Max. | Width of IQR | n | Mean (SD) | Min. | Max. | Width of IQR | |
| Mothers admitted to HLC | ||||||||||
| Level of care (days) | ||||||||||
| Level 0 | 28 | 1.928 (1.676) | 1 | 8 | 1 | 17 | 2.235 (2.137) | 1 | 8 | 2 |
| Level 1 | 56 | 1.125 (0.384) | 1 | 3 | 0 | 38 | 1.184 (0.512) | 1 | 3 | 0 |
| Level 2 | 23 | 1.391 (0.941) | 1 | 4 | 0 | 26 | 1.346 (0.629) | 1 | 3 | 1 |
| Level 3 | 6 | 1.667 (0.408) | 1 | 2 | 0 | 2 | 1 (0) | 1 | 1 | 0 |
| Surgery performed | 7 | – | – | – | – | 4 | – | – | – | – |
| Transfer to another hospital | 2 | – | – | – | – | 0 | – | – | – | – |
| Investigations | ||||||||||
| Radiography | 4 | 1.2 (0.5) | 1 | 2 | 1 | 5 | 1.2 (0.447) | 1 | 2 | 1 |
| CT | 2 | 1 (0) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| MRI | – | – | – | – | – | 1 | 1 | 1 | 1 | 0 |
| Infants admitted to HLC | ||||||||||
| Level of care (days) | ||||||||||
| Special care | 58 | 2.66 (2.15) | 1 | 11 | 3 | 53 | 4.08 (4.78) | 1 | 30 | 13 |
| High dependency | 15 | 1.53 (0.92) | 1 | 3 | 2 | 14 | 2.71 (2.27) | 1 | 9 | 8 |
| Intensive care | 17 | 3.88 (7.16) | 1 | 31 | 2 | 14 | 2.79 (1.89) | 1 | 7 | 6 |
| Surgery performed | 2 | – | – | – | – | 3 | – | – | – | – |
| Transfer to another hospital | 4 | – | – | – | – | 2 | – | – | – | – |
| Neonatal death | 0 | – | – | – | – | 0 | – | – | – | – |
| Investigations | ||||||||||
| Radiography | 26 | 1.35 (0.85) | 1 | 5 | 0 | 21 | 2.76 (3.27) | 1 | 13 | 9 |
| CT | – | – | – | – | – | 3 | 1 (0) | 1 | 1 | 0 |
| MRI | 3 | 1 (0) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| Health-care resource use | Trial arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Upright | Lying down | |||||||||
| n | Min. | Max. | Width of IQR | Mean (SD) | n | Min. | Max. | Width of IQR | Mean (SD) | |
| Maternal | ||||||||||
| Outpatient visits | ||||||||||
| Perineal care clinic | 55 | 1 | 10 | 2 | 2.4 (2.257) | 38 | 1 | 10 | 2 | 2.684 (2.384) |
| Gynaecological | 75 | 1 | 8 | 1 | 1.773 (1.192) | 80 | 1 | 10 | 1 | 2.088 (1.943) |
| Surgical | 16 | 1 | 6 | 2 | 2.063 (1.436) | 26 | 1 | 5 | 1 | 1.770 (1.142) |
| Other | 135 | 1 | 50 | 3 | 3.526 (5.303) | 113 | 1 | 35 | 2 | 3.372 (4.117) |
| Hospital visits | ||||||||||
| Hospital inpatient (days) | 49 | 1 | 28 | 2 | 3.776 (4.534) | 48 | 1 | 24 | 4 | 3.875 (4.072) |
| Number of operations | 31 | 1 | 2 | 0 | 1.097 (0.301) | 44 | 1 | 2 | 0 | 1.068 (0.255) |
| Infant | ||||||||||
| Outpatient visits | ||||||||||
| Orthopaedic | 21 | 1 | 20 | 1 | 2.714 (4.429) | 24 | 1 | 10 | 24 | 2.167 (2.014) |
| Paediatric | 130 | 1 | 8 | 1 | 1.777 (1.109) | 118 | 1 | 20 | 1 | 2.153 (2.229) |
| Hearing | 23 | 1 | 4 | 0 | 1.522 (0.898) | 23 | 1 | 10 | 14 | 1.739 (2.027) |
| Eye | 30 | 1 | 3 | 1 | 1.3 (0.535) | 43 | 1 | 3 | 1 | 1.349 (0.529) |
| Dermatology | 19 | 1 | 6 | 1 | 1.947 (1.311) | 21 | 1 | 5 | 1 | 1.619 (1.024) |
| Other | 84 | 1 | 10 | 2 | 2.286 (1.834) | 87 | 1 | 22 | 3 | 3.149 (3.360) |
| Hospital visits | ||||||||||
| Hospital inpatient (days) | 102 | 1 | 21 | 4 | 3.402 (2.943) | 113 | 1 | 45 | 4 | 3.991 (5.218) |
| Number of operations | 13 | 1 | 2 | 0 | 1.077 (0.277) | 22 | 1 | 1 | 0 | 1 (0) |
NHS costs
Patterns of missing data for summary cost categories using a complete-case analysis are presented in Figures 11 and 12. It was evident from the plots that in each trial arm the number of missing data from trial entry to hospital discharge was low following a primarily monotonic missing data pattern. A larger number of missing data out of the overall data available was observed at the 12-month follow-up. The charts showed the potential cost information available (indicated by the grey area) to inform the multiple imputation model. The frequency of missing information in each trial arm and for all categories of health-care resource use are reported in Tables 19–21.
FIGURE 11.
Pattern of missing data for main categories of costs for the upright group. Blue shading represents missing data for one or more individuals (horizontal axis) for a particular cost variable (vertical axis); green shading represents observed data.
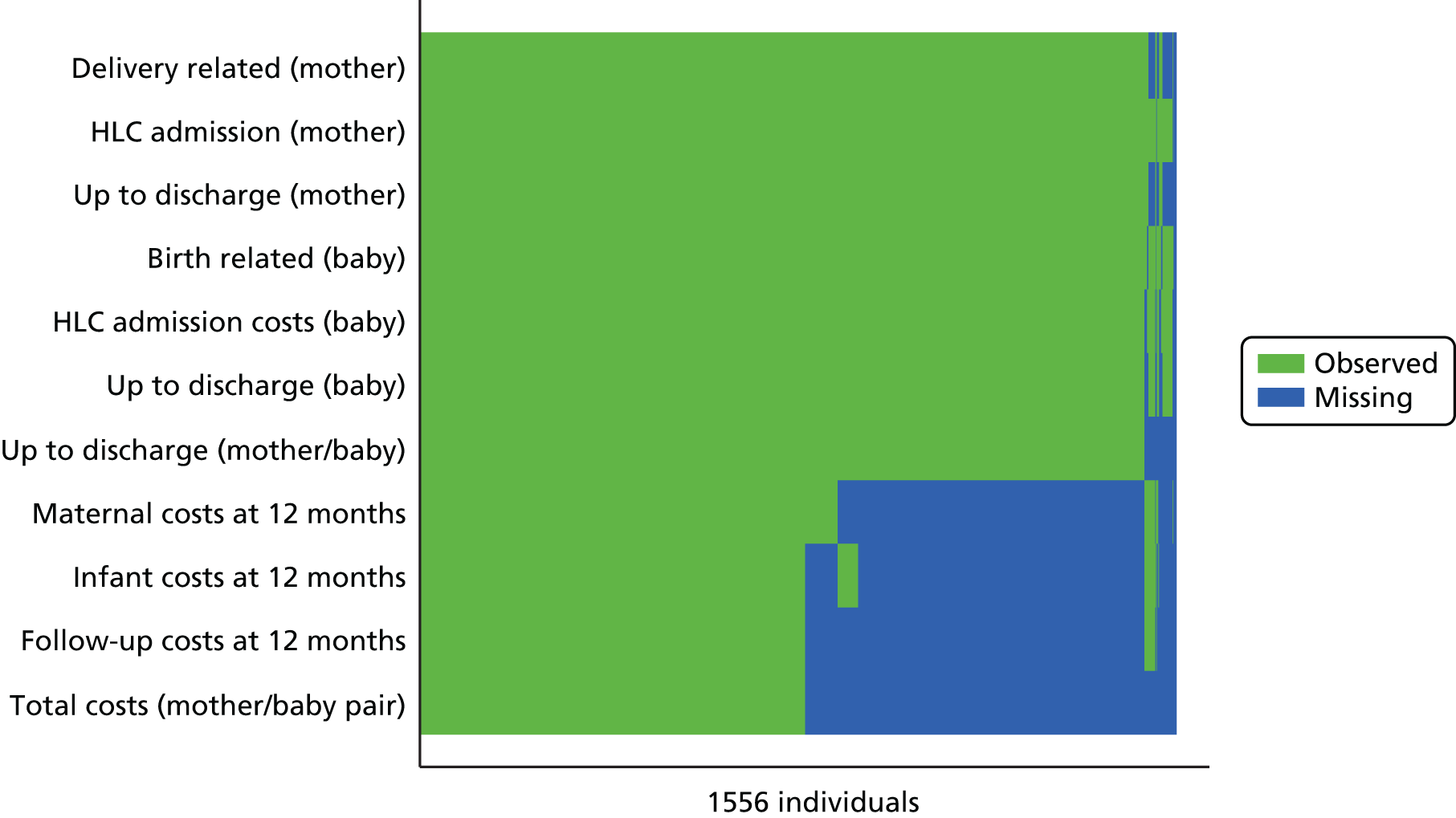
FIGURE 12.
Pattern of missing data for main categories of costs for the lying-down group. Blue shading represents missing data for one or more individuals (horizontal axis) for a particular cost variable (vertical axis); green shading represents observed data.
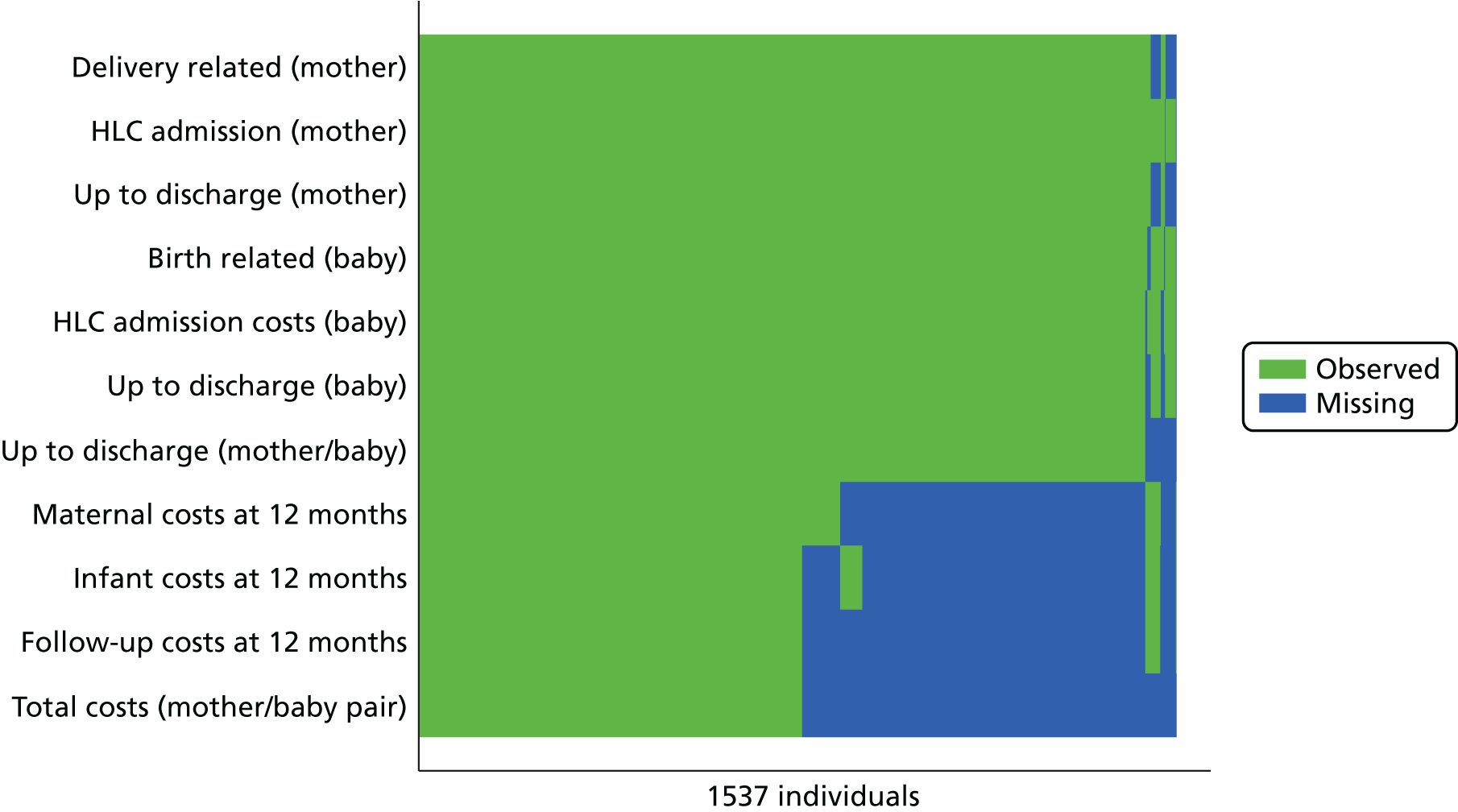
Table 24 reports the results of the cost analysis using multiple imputation over the study period for mothers and their babies. A statistically significant mean cost difference (95% CI) of £59 (£6 to £111) between trial arms favouring the lying-down policy was detected in the delivery-related cost category. This translated into a statistically significant mean difference (95% CI) of £78 (£13 to £143) from trial entry to hospital discharge per delivery favouring the lying-down strategy. A significant mean cost difference (95% CI) of £0.002 (£0.001 to £0.004) favouring the lying-down position was observed for the birth-related cost category. This difference disappeared when adding the cost of HLC admissions to the total cost for infants from trial entry to hospital discharge. A summary of the overall costs from trial entry to 12-month follow-up for women and their infants using multiple imputation is also shown at the end of Table 24. The mean (SE) total cost per woman/infant pair at 12-month follow-up (adding all categories of costs) was estimated to be £3207 (£73) and £3252 (£81) in the upright and lying-down positions, respectively, a non-significant mean cost difference (95% CI) of –£42 (–£254 to £169) favouring the upright position.
| Cost category | Trial arm | MD (95% CI)a | |
|---|---|---|---|
| Upright (N = 1556) | Lying down (N = 1537) | ||
| Mean (SE) | Mean (SE) | ||
| Maternal | |||
| From trial entry discharge | |||
| Total delivery related | 2283 (19) | 2225 (19) | 59 (6 to 111)* |
| Total cost HLC admissions | 92 (12) | 73 (11) | 20 (–11 to 51) |
| Total cost per delivery | 2375 (24) | 2298 (23) | 78 (13 to 143)* |
| Follow-up costs | |||
| Total outpatient visits | 104 (11) | 94 (9) | 10 (–17 to 37) |
| Hospital admissions | 159 (35) | 159 (30) | 0.09 (–96 to 97) |
| Total cost per delivery | 263 (37) | 252 (33) | 13 (–84 to 109) |
| Infant | |||
| From trial entry to discharge | |||
| Total birth related | 0.033 (0.001) | 0.031 (0.001) | 0.002 (0.001 to 0.004)* |
| Total cost HLC admissions | 108 (28) | 114 (22) | –6 (–76 to 64) |
| Total cost per baby | 108 (28) | 114 (22) | –6 (76 to 63) |
| Follow-up costs | |||
| Total outpatient visits | 129 (9) | 153 (13) | –23 (–54 to 8) |
| Hospital admissions | 333 (0) | 434 (56) | –102 (–238 to 34) |
| Total cost per baby | 462 (42) | 587 (68) | –123 (–273 to 34) |
| Total 12-month follow-up costs | 725 (58) | 839 (71) | –113 (–293 to 66) |
| Total costs per woman/baby pair | 3207 (73) | 3252 (81) | –42 (–254) to 169) |
Maternal health-related quality of life at 12-month follow-up
Summary EQ-5D-3L and SF-6D scores at 12-month follow-up for each trial arm using a multiple imputation analysis is reported in Table 25. Quality-of-life scores were very similar (almost identical) between trial arms for both instruments and therefore no significant mean differences were detected.
| HRQoL instrument | n | Mean | SE | n | Mean | SE | MD (95% CI)a |
|---|---|---|---|---|---|---|---|
| EQ-5D-3L score | 1556 | 0.919 | 0.005 | 1537 | 0.922 | 0.004 | –0.003 (–0.016 to 0.011) |
| SF-6D score | 1556 | 0.802 | 0.004 | 1537 | 0.805 | 0.004 | –0.004 (–0.015 to 0.006) |
The distribution of responses of available data for both instruments is reported in detail in Tables 26 and 27, respectively. It is clear that, using both instruments, the majority of women participating in BUMPES reported a good quality of life at 1 year post delivery.
| EQ-5D-3L dimension | Trial arm | |
|---|---|---|
| Upright (N = 1556), n (%) | Lying down (N = 1537), n (%) | |
| Mobility | ||
| No problems | 809 (94.5) | 811 (95.6) |
| Some problems | 46 (5.4) | 37 (4.4) |
| Confined to bed | 1 (0.1) | 0 (0.0) |
| Missing | 700 | 689 |
| Self-care | ||
| No problems | 850 (99.1) | 845 (99.7) |
| Some problems | 6 (0.7) | 3 (0.4) |
| Unable to wash or dress | 1 (0.1) | 0 (0.0) |
| Missing | 699 | 689 |
| Usual activities | ||
| No problems | 798 (93.3) | 787 (92.7) |
| Some problems | 56 (6.6) | 61 (7.2) |
| Unable to perform activities | 1 (0.1) | 1 (0.1) |
| Missing | 701 | 688 |
| Pain/discomfort | ||
| No pain | 687 (80.4) | 669 (79.1) |
| Moderate pain | 161 (18.9) | 171 (20.2) |
| Extreme pain | 6 (0.7) | 6 (0.7) |
| Missing | 702 | 691 |
| Anxiety/depression | ||
| Not anxious or depressed | 702 (82.0) | 708 (83.5) |
| Moderately anxious or depressed | 140 (16.4) | 133 (15.7) |
| Extremely anxious or depressed | 14 (1.6) | 7 (0.8) |
| Missing | 700 | 689 |
| SF-12 dimension | Trial arm | |
|---|---|---|
| Upright (N = 1556), n (%) | Lying down (N = 1537), n (%) | |
| General health | ||
| Excellent | 172 (18.2) | 168 (17.9) |
| Very good | 465 (49.2) | 473 (50.5) |
| Good | 261 (27.6) | 259 (27.6) |
| Fair | 38 (4.0) | 36 (3.8) |
| Poor | 10 (1.1) | 1 (0.1) |
| Missing | 610 | 600 |
| Moderate activities | ||
| Yes, limited a lot | 24 (2.5) | 21 (2.2) |
| Yes, limited a little | 76 (8.0) | 79 (8.4) |
| No, not limited at all | 848 (89.5) | 837 (89.3) |
| Missing | 608 | 600 |
| Climbing several flights of stairs | ||
| Yes, limited a lot | 28 (3.0) | 24 (2.6) |
| Yes, limited a little | 119 (12.7) | 120 (13.0) |
| No, not limited at all | 792 (84.4) | 782 (84.5) |
| Missing | 617 | 611 |
| Accomplished less than would like (physical health) | ||
| All of the time | 10 (1.1) | 4 (0.4) |
| Most of the time | 28 (3.0) | 25 (2.7) |
| Some of the time | 64 (6.8) | 69 (7.4) |
| A little of the time | 152 (16.1) | 146 (15.6) |
| None of the time | 690 (73.1) | 693 (74.0) |
| Missing | 612 | 600 |
| Limited in the kind of work/activities (physical health) | ||
| All of the time | 8 (0.9) | 5 (0.5) |
| Most of the time | 20 (2.1) | 12 (1.3) |
| Some of the time | 44 (4.7) | 52 (5.6) |
| A little of the time | 130 (13.8) | 109 (11.7) |
| None of the time | 741 (78.6) | 754 (80.9) |
| Missing | 613 | 605 |
| Accomplished less than would like (emotional health) | ||
| All of the time | 8 (0.9) | 2 (0.2) |
| Most of the time | 26 (2.8) | 21 (2.2) |
| Some of the time | 88 (9.3) | 79 (8.4) |
| A little of the time | 184 (19.5) | 179 (19.1) |
| None of the time | 638 (67.6) | 658 (70.1) |
| Missing | 612 | 598 |
| Less careful than usual (emotional health) | ||
| All of the time | 6 (0.6) | 2 (0.2) |
| Most of the time | 17 (1.8) | 15 (1.6) |
| Some of the time | 70 (7.4) | 56 (6.0) |
| A little of the time | 153 (16.3) | 152 (16.3) |
| None of the time | 695 (73.9) | 709 (75.9) |
| Missing | 615 | 603 |
| Pain interfering with normal work | ||
| Not at all | 719 (76.2) | 706 (75.1) |
| A little bit | 165 (17.5) | 186 (19.8) |
| Moderately | 35 (3.7) | 26 (2.8) |
| Quite a bit | 21 (2.2) | 18 (1.9) |
| Extremely | 4 (0.4) | 4 (0.4) |
| Missing | 612 | 597 |
| Felt calm and peaceful | ||
| All of the time | 81 (8.6) | 87 (9.3) |
| Most of the time | 510 (54.0) | 490 (52.1) |
| Some of the time | 240 (25.4) | 244 (26.0) |
| A little of the time | 86 (9.1) | 103 (11.0) |
| None of the time | 27 (2.9) | 16 (1.7) |
| Missing | 612 | 597 |
| Have a lot of energy | ||
| All of the time | 47 (5.0) | 46 (4.9) |
| Most of the time | 432 (45.7) | 440 (46.9) |
| Some of the time | 310 (32.8) | 302 (32.2) |
| A little of the time | 109 (11.5) | 115 (12.3) |
| None of the time | 47 (5.0) | 35 (3.7) |
| Missing | 611 | 599 |
| Felt downhearted and low | ||
| All of the time | 10 (1.1) | 9 (1.0) |
| Most of the time | 35 (3.7) | 36 (3.8) |
| Some of the time | 174 (18.5) | 180 (19.2) |
| A little of the time | 346 (36.8) | 341 (36.4) |
| None of the time | 376 (40.0) | 372 (39.7) |
| Missing | 615 | 599 |
| Physical/emotional health interfered with social activities | ||
| All of the time | 7 (0.7) | 5 (0.5) |
| Most of the time | 26 (2.8) | 21 (2.2) |
| Some of the time | 105 (11.1) | 102 (10.9) |
| A little of the time | 173 (18.3) | 186 (19.9) |
| None of the time | 636 (67.2) | 623 (66.5) |
| Missing | 609 | 600 |
Cost-effectiveness analysis as a secondary outcome in the economic evaluation
A summary of cost-effectiveness results comparing upright with lying-down positions during the second stage of labour is shown in Table 28. The ICER (95% CI) was estimated to be £722 (–£2968 to £6358) per additional case of SVB.
| Position during labour | Total cost (2013/14 UK £) | Incremental costs | Proportion of SVBs | Incremental effect (additional cases of SVB) | ICER |
|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (95% CI) | |
| Lying down | 3252 (81) | – | 0.411 (0.012) | – | – |
| Upright | 3207 (73) | –42 (108) | 0.352 (0.012) | –0.06 (0.02) | 722 (–2968 to 6358) |
Discussion
This chapter has described in detail the methods and results of the economic evaluation conducted as part of the BUMPES trial. We have implemented a robust methodology based on more recent guidance to present these results. 58
Our results suggested that women randomised to the lying-down position consumed significantly fewer resources than those randomised to an upright policy during the original hospital stay. Such results were driven by more SVBs in the lying-down treatment arm. Infants incurred similar costs in both arms of the trial during this time. At the 12-month follow-up, no significant overall cost differences were observed between upright and lying-down positions for mothers or their babies. The significantly higher costs incurred from trial entry to discharge by women in the upright group were offset by the slightly, but non-significantly, higher costs incurred during follow-up by the babies of women in the lying-down arm. A possible explanation is that SVB was associated with higher follow-up costs than instrumental delivery or caesarean section. However, additional analysis assessing follow-up costs by mode of delivery between the trial groups suggested that this was not the case (data available from the corresponding author). No evidence of differences was found for the other secondary maternal outcomes, neonatal clinical outcomes or maternal HRQoL. Therefore, the results of the cost–consequences analysis provided robust evidence clearly in favour of lying down, at no risk to women or their babies, and at no extra cost to the NHS.
Future studies can use the results of our cost-effectiveness analysis using the cost per additional case of SVB as an outcome measure as the benchmark for future comparisons in the area. However, we recommend that such comparisons are used with caution owing to well-known problems of comparability to other disease areas and ‘double-counting’ when estimating the ICER. 59,60
To our knowledge, this is the first study reporting an economic evaluation of upright versus lying-down positions during the second stage of labour in nulliparous women with an epidural. The most comprehensive systematic review of economic evaluations of potential interventions during intrapartum care has been recently updated in a NICE clinical guideline. 31 The review aimed to identify all published cost-effectiveness evaluations in the areas within the remit of the guideline that included the second stage of labour, but no published economic evaluations were identified. Therefore, the results reported here should be considered the most up-to-date evidence about the cost-effectiveness of alternative position strategies during the second stage of labour in women with an epidural.
We were not able to collect HRQoL data at trial entry (or late pregnancy) or in the early postnatal period, precluding the estimation of QALYs. Therefore, one of the limitations of the current analysis is the limited comparability of our study with evaluations in other health areas using a cost-per-QALY approach, as recommended by NICE. 58 The use of QALYs has the potential to be a feasible and responsive outcome measure to evaluate the effectiveness of delivery position interventions during labour. A recent study has assessed the impact of mode of delivery on maternal HRQoL postnatally, suggesting that caesarean section is associated with larger quality-of-life decrements, followed by instrumental delivery and then SVB. 61 Collecting quality-of-life information during late pregnancy and in the early postnatal period to use in cost-effectiveness analysis is controversial, because there is limited evidence about the validity of recommended instruments such as the EQ-5D-3L in this context. 62,63 However, it would be possible to estimate QALYs in the BUMPES study using a modelling exercise that synthesises available quality-of-life information during late pregnancy and in the early postpartum period from a literature review with the collected EQ-5D-3L data at 12-month follow-up. Such analysis is of interest to the research team and will be explored in future research. However, we feel that our cost–consequences analysis provides important evidence that can be considered by decision-makers in obstetrics when making recommendations about position during the late stages of labour in women with an epidural.
Chapter 7 Discussion and conclusion
BUMPES is the largest RCT yet undertaken of different positions in labour and their effects on the mode of birth. There is clear evidence of a benefit of adopting a lying-down position in the second stage of labour for nulliparous women with epidural analgesia, and there are no apparent disadvantages in relation to either short- or long-term outcomes for either mother or baby. Thus, a clinical improvement (increase in SVBs) could be achieved by encouraging nulliparous women with epidural analgesia to adopt a lying-down position during the second stage of labour. The intervention has no resource implications and has no additional risk to women or their babies, and thus could be implemented rapidly without additional cost.
Like all pragmatic trials, the study has limitations. The reporting of adherence is complex for this trial. Various options were considered for recording adherence, but, given the size of the trial, many of these, such as video recordings of the second stage of labour, were both impractical and intrusive. As midwives recorded observations of the women and the fetus in utero every 15 minutes during labour, it was felt that asking the midwife to record position of these epochs would facilitate good data collection. However, it was also recognised that there were a number of clinical reasons why position could not be maintained as allocated, for example to facilitate the taking of a fetal blood sample or because the fetal heart could not be easily monitored using an external monitor. Representing these as non-adherent in the analysis would have been unhelpful.
With an intervention such as this, masking is impossible, so the results may be influenced by the women’s and the midwives’ perceptions of the different positions in their ability to achieve a SVB. Given that existing NICE guidance suggests that women with an epidural should be encouraged to adopt whatever upright position they find comfortable, we might expect the trial results to suggest an improvement in SVB with an upright position if midwives’ and women’s behaviour was altered in these positions because of a firm belief that these were preferable. The finding that the lying-down position increases the chances of achieving a SVB suggests that this potential bias was either absent or minimal in its impact, or that the benefit of the lying-down position may be even greater in leading to a SVB.
The original commissioning brief for this trial asked for a measure of maternal satisfaction with the allocated position during labour. This proved particularly challenging, as there are no validated quantitative measures of maternal satisfaction specific to the labour and birth episode, and unpicking which aspects of care could improve an individual woman’s satisfaction with her allocated position is difficult. The measure that was used was therefore created for this trial and so has limited external validity, even though it has internal validity.
The incidence of SVB in the trial overall was lower than anticipated when the study was being designed. The estimate of the risk of SVB was calculated using the COMET, which was published in 2001. 5 In the COMET, there were almost no caesarean sections undertaken during the second stage of labour, reflecting the change in clinical practice in obstetrics between the late 1990s and now.
We can only speculate about the mechanism by which a lying-down position increases the chance of a SVB. We have no direct measurements of the density of the epidural block in the two positions nor the level of the block. It is possible that women in the upright position acquired a more dense block around the birth canal because of the effect of gravity on the epidural drugs, which could have made expulsive efforts more difficult; however, the similarity of drug doses used in each group would suggest that this is unlikely. In women in the upright group, who may have been sitting, the pelvic outlet may have been restricted by the position on the coccyx or by lower genital tract oedema and venous obstruction causing swelling of the soft tissues obstructing the pelvic outlet. In addition, it is possible that lying down, by easing pressure of the fetal head on the pelvis, results in improved uterine blood flow and therefore improved uterine activity. This would suggest a difference in the risk of operative delivery associated with failure to progress; however, the distribution of indications for operative delivery appeared to be the same in both groups. In addition, there was little difference in the use of oxytocin because of delay in labour progress after trial entry.
The mechanism by which the lying-down position improves the risk of SVB remains unknown; however, the results of this trial are clear. Among women with an epidural during their first birth, adopting a lying-down position in the second stage of labour increases the chance of achieving a SVB. The results cannot be directly extrapolated to multiparous women with an epidural, or to women who do not have an epidural, in whom the situation may be very different.
Longer-term outcomes
The response rate to the 1-year follow-up was 61%. Therefore, there is a possibility that the follow-up results are less than robust because of non-response bias. This poor response, despite many attempts to improve the rate (described in Chapter 8), is a phenomenon increasingly being seen with contemporary clinical trials in this area. We provided a variety of options for women to complete their questionnaire: on paper, via the internet or by telephone. The majority of women still prefer to use paper, but, despite these various offers, the response rate remained poor. Responders and non-responders exhibited clear differences in a variety of demographic and clinical factors that were anticipated. Women who responded were more likely to be older, more likely to be white, less likely to live in a deprived area and less likely to have achieved a SVB. There were, however, no apparent differences between the two randomised groups in their response rates or characteristics, suggesting that there were minimal biases in the comparison between the two groups.
The lack of an impact of the risk of SVB on longer-term outcomes, such as faecal incontinence, is of interest. The observation that IVD is associated with increased risks of faecal incontinence is robust; however, in the BUMPES trial the differences between the randomised groups of women in their risk of SVB and instrumental delivery were relatively small, so, although there are associations between different modes of birth and long-term outcomes, these are likely to be diluted in a trial in which these differences in actual mode of birth are relatively modest (only a 6% absolute difference in the risk of SVB). This is likely to explain the lack of an observed difference in long-term outcomes.
Adherence
Adherence is clearly important when considering an intervention of this nature. The observation that there was greater adherence in the upright group than in the lying-down group suggests that there may be even greater benefit from adopting a lying-down position if adherence could be improved. However, the ability to comply with the allocated intervention may be a function of the intervention itself. For example, women in the lying-down group may be more difficult to monitor when using an external cardiotocograph monitor because of the ability to position the monitor accurately to pick up the fetal heart rate. If this is the case, then non-adherence for clinical reasons is to be expected. It would therefore be difficult to improve adherence in these women if that meant that they could not be effectively and safely monitored. Further work needs to be undertaken on the association between adherence and various characteristics of the nature of the non-adherence, and the risk of the various outcomes, before much more can be said about whether or not improved adherence may improve the benefit of the lying-down position.
Challenges with equipoise
Although the trial was initially expected to recruit from five UK centres, the expansion to 41 centres suggests that the results have good generalisability, in that a large number of centres participated throughout the country. However, the reason for the expansion in the number of centres was because of the concerns expressed by midwives in terms of recruiting to the trial. It was clear that many midwives did not have equipoise about position in the second stage of labour for women with an epidural, and in all of the participating centres it was a relatively small number of midwives who recruited the women in those centres. At no stage in the trial was there any suggestion that women were expressing a preference not to participate in the trial if they were approached. This effect of midwives having particular views about the benefits of position may limit the generalisability if midwives are not prepared to accept the evidence produced by the BUMPES trial. When there are strong views, for example that an upright position is preferable to a lying-down position, if the results of BUMPES fail to be implemented, then the population benefit of a lying-down position in these women will be relatively small. By producing results that contradict current NICE guidance and what is widely accepted, that is, that an upright position improves the chances of achieving a SVB, these results challenge an orthodoxy that may make their implementation more challenging. The results of the trial, however, are clear. If women giving birth to their first babies and their carers are keen to increase the chances of achieving a SVB, then adopting a lying-down position in the second stage of labour, for as much of that second stage of labour as can be achieved, results in a modest but real increase in their chances of doing so. There is no evidence to suggest that this improvement carries a risk either to the women themselves, in relation to perineal trauma, or to their babies, in relation to newborn compromise. Similarly, there is no evidence of any long-term risks to the health and well-being of the women or their babies. Given that there was no evidence of increasing resource consumption with the intervention, the authors believe that it could be straightforward to implement and therefore realise benefits for a substantial number of women having their first babies throughout the UK and other countries.
Monitoring adherence
Another observation from the conduct of this trial is whether or not a trial management group can and should monitor adherence. In general, adherence is often monitored by the DMC, which is the only group that sees any data by allocated intervention. For drug trials, in which monitoring of adherence may be relatively straightforward, this may not be a problem. But for interventions that are less well circumscribed, such as that in BUMPES, it is important that people with a good knowledge of the trial and its interventions are able to monitor adherence to ensure that there is reasonable separation between the arms of the trial. This can be achieved by the DMC; however, given the complexity of monitoring adherence to this intervention, particularly with respect to taking account of clinically acceptable reasons for periods of non-compliance, such as fetal blood sampling, the nature of DMCs (in that they tend to meet annually) means that the DMC is removed from the conduct of the trial, which may be a disadvantage. In a pragmatic trial, there is a clear reluctance to impose strict adherence rules, but it is helpful and useful to identify centres or particular individuals with very poor adherence so that behaviour can be modified. In BUMPES, with 41 centres, it was possible to monitor adherence by centre and identify at least one outlier centre that had particularly poor adherence to one of the allocated arms, which was at odds with all the other participating centres. This difference did not come to light until the trial investigators had received agreement from the DMC that the trial management group should be able to monitor adherence by allocated intervention on an ongoing basis. We believe that there is an important lesson to be learnt here about the nature of monitoring adherence, and agreements should be in place early in the trial on who should undertake this, what parameters should be monitored and how this should be overseen carefully to ensure that the degree of monitoring is reasonable, and that monitoring adherence cannot be used by the trial management group to make any inferences about outcomes.
Chapter 8 Nested study
This chapter describes a nested study within the BUMPES trial, which has been published. 64
Rationale for nested study
The return rate of 1-year follow-up questionnaires for BUMPES was lower than expected and the use of incentives to evaluate the impact on questionnaire return rate was discussed by the TSC. It was noted by the TSC that there was a lack of evidence relating to the value of incentives to increase collection of follow-up data in trials. As the evidence base for incentives included a variety of populations, it could be that postnatal women respond differently. A proposal for a nested study within a trial (SWAT) was therefore developed.
Background
Maximising follow-up rates for postal questionnaires for RCTs is an important aspect of a well-designed and well-conducted study. Loss to follow-up can lead to bias and compromise the internal and external validity of the results.
Use of incentives to promote questionnaire return in clinical trials has been researched. Existing systematic reviews suggest that they are effective,65,66 but not all studies have sufficient funds to use them. Promising an incentive once data are returned can reduce the cost burden of this approach. Brueton et al. 66 found that an offer of a monetary incentive was comparable with the addition of a monetary incentive with the questionnaire (pooled RR 1.04, 95% CI 0.91 to 1.19). However, it may be possible to provide further cost-savings if the offer was restricted to the reminder letters only.
We evaluated the effect of promising a monetary incentive at first mail-out versus a promise on reminder letters only, with the incentive being posted out on receipt of a completed follow-up questionnaire.
Objective
To assess the effectiveness on the return rate of the 1-year follow-up postal questionnaires for BUMPES of the promise of a monetary incentive made at the point of sending the questionnaire for the first time compared with a promise made on reminder letters only.
Trial design
Parallel-group RCT nested within BUMPES.
Study setting
All women randomised into the BUMPES study who consented to be contacted at 12 months and who had not yet been sent their 1-year follow-up questionnaire were included.
Participant eligibility
Inclusion criteria
-
Recruited to BUMPES.
-
Consented at recruitment to receive follow-up questionnaire.
-
One-year questionnaire not sent.
Exclusion criteria
-
Women who had a stillbirth.
-
Women whose infant had died.
-
Address details unknown.
-
Woman and infant not living at the same address.
Interventions
Women were randomly allocated to the following two groups.
-
Incentive cover letter: this contained details of the promise of a monetary incentive when the questionnaire was first sent. A £10 gift voucher redeemable at high-street shops was sent to the woman on return of a completed questionnaire. The cover letter included a sentence explaining that the voucher was to thank participants for their time and effort. All reminder letters included a sentence about the incentive.
-
Incentive reminder letters: the standard cover letter did not mention any incentive. All subsequent reminder letters sent if the questionnaire was not returned detailed the promise of an incentive. A £10 gift voucher redeemable at high-street shops was sent to the woman on return of a completed questionnaire.
For both groups, women were contacted electronically and via text messaging if the contact details had been collected. The content of the e-mails and texts sent reflected the group to which the woman was randomised. All women were provided with an option of completing the questionnaire online.
Outcome measure
Primary outcome measure
The primary outcome measure was questionnaire return, defined as receipt of a completed or partially completed questionnaire at the BUMPES office.
Secondary outcome measures
The following secondary outcomes were analysed:
-
the number of questionnaires returned without chasing by the study team
-
the total cost of the vouchers sent out by nested study arm.
Data collection
Recording of questionnaire receipt, date received and voucher sent was made using internal trial administration systems. Postal versus online receipt was also recorded.
Sample size
The sample size was predetermined by the numbers of questionnaires remaining to be sent at the point of start of the nested study.
BUMPES started recruiting in October 2010 and finished in January 2014. A total of 3236 women were randomised. It was estimated that approximately 1150 women remained to be followed up at the start date of this study (beginning August 2014). Assuming that approximately 15% of these women would be excluded from receiving the questionnaire due to stillbirth, infant death, or address details unknown or different from the infant, 980 women would be eligible to be randomised in the nested study (approximately 490 per group).
In order to assess the detectable effect size possible with the given sample size, we estimated the control group risk based on current literature. Khadjesari et al. 67 investigated the use of an offer of an incentive [a £10 Amazon (Amazon.com, Inc., Bellevue, WA, USA) gift voucher] versus no offer of an incentive on follow-up rates in an online trial. They found an increase of 9% (95% CI 5% to 12%) when using the offer of an incentive. Kenyon et al. 68 investigated the use of a monetary incentive included in reminder letters versus no incentive and found an improvement in the response rate between the two groups of 11.7% (95% CI 4.7% to 18.6%).
The follow-up questionnaire return rate for BUMPES up to June 2014 was 59%. Assuming that this could increase by at least 5% with use of the offer of an incentive either with an incentive cover letter or with an incentive reminder letter only, a sample size of 980 would be sufficient to demonstrate an increase in questionnaire return rate of 8% from 64% in the incentive reminder letter group to 72% in the incentive cover letter group at a two-sided 5% significance level with 80% power. Figure 13 illustrates the proportion detectable in the incentive cover letter group for control group risk varying between 60% and 70%. The detectable difference lies between 8% and 8.5% for varying control group estimates.
FIGURE 13.
Proportion in incentive cover letter group by proportion in control group. Significance = 0.05; power = 0.80; sample size per group = 490.
Randomisation
Allocation was by computerised random number generation stratified by BUMPES allocation and by centre. Randomisation to incentive cover letter or incentive reminder letter occurred at each woman’s next follow-up point during the conduct of the BUMPES study, with a block size of four. Each BUMPES participant was randomised to incentive cover letter or incentive reminder letter once only.
Blinding
Trial staff were aware of allocation as a result of the nature of the interventions and the practicalities involved in sending the letters and the vouchers.
Statistical analysis
For all analyses, participants were analysed in the groups into which they were randomly allocated, that is, comparing outcomes for women allocated to the incentive cover letter with outcomes for women allocated to the incentive reminder letter, regardless of allocation received.
All analyses were based on all women randomised for whom data were available.
The flow of participants through the trial was summarised using a CONSORT flow diagram (see Figure 14). Specifically, the number of women recruited to the BUMPES main trial and subsequently recruited to the BUMPES SWAT is reported along with reasons for not being included in the SWAT.
Participants in the two randomised groups are described separately with respect to baseline demographics and clinical characteristics, including the primary outcome for the main BUMPES study, and recorded on the BUMPES DCB.
Numbers (with percentages) for binary and categorical variables and means (and SDs) or medians (with lower and upper quartiles) for continuous variables are reported.
The return rate and chase rate before the introduction of the randomised interventions (i.e. before the SWAT started) and at the end of the study (with both SWAT trial arms combined) are presented using numbers and percentages.
The return rate and chase rate by method of completion (online vs. postal) are described by trial arm using numbers and percentages.
An adjusted analysis was performed on the two return rate outcomes adjusting for centre (the stratification factor at randomisation) as a random effect. The analysis was carried out using log-binomial regression models and results are presented as adjusted RRs with 95% CIs.
To examine whether or not the effect of when vouchers were sent was consistent across specific subgroups of women, a subgroup analysis by IMD quintile was prespecified. Results are presented as RR plus 95% CI for each subgroup, by intervention group, with the p-value for the statistical test of interaction.
Stata/SE for Windows (version 13.1) was used for all analyses.
Results
Randomisation to the incentive nested study started on 31 July 2014 and continued until all questionnaires and reminders had been sent (last letter sent 6 March 2015). The total number of women in the SWAT was 1026. Eight women were excluded from the analysis as it was discovered after they had been randomised to the SWAT that they had changed address (Figure 14).
FIGURE 14.
Nested study participant flow diagram. a, If not contactable after 15 months since randomisation, questionnaire was not sent.
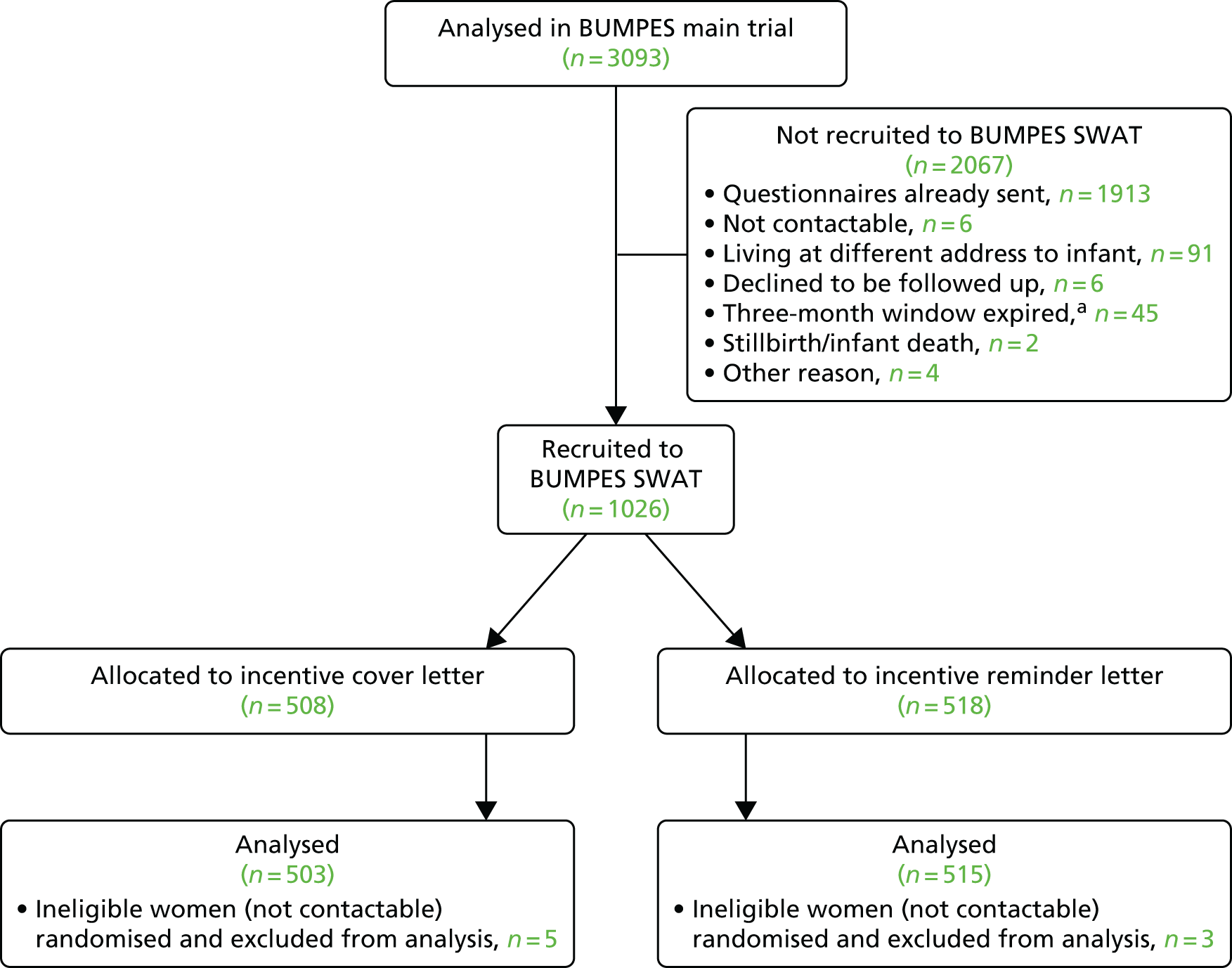
Balance between the SWAT trial arms in baseline characteristics and centre of recruitment to BUMPES was good. There were only small imbalances in onset of labour (spontaneous or induced), diagnosis of pre-eclampsia and SVB (the BUMPES primary outcome) (Table 29).
| Characteristic | Letter | |
|---|---|---|
| Cover (N = 503) | Reminder (N = 515) | |
| Centre, n (%) | ||
| Birmingham Women’s Hospital | 13 (2.6) | 16 (3.1) |
| St Thomas’ Hospital | 61 (12.1) | 60 (11.7) |
| Queen Alexandra Hospital, Portsmouth | 9 (1.8) | 8 (1.6) |
| University Hospital of Wales | 36 (7.2) | 36 (7.0) |
| Royal United Hospitals Bath | 27 (5.4) | 31 (6.0) |
| Bradford Royal Infirmary | 10 (2.0) | 12 (2.3) |
| Jessop Wing, Sheffield Teaching Hospital | 20 (4.0) | 20 (3.9) |
| Princess of Wales Hospital | 3 (0.6) | 2 (0.4) |
| Singleton Hospital, Swansea | 3 (0.6) | 1 (0.2) |
| Royal Gwent Hospital, Newport | 17 (3.4) | 14 (2.7) |
| Gloucestershire Royal Hospital | 9 (1.8) | 9 (1.8) |
| Nevill Hall Hospital | 4 (0.8) | 4 (0.8) |
| Frimley Park Hospital | 25 (5.0) | 25 (4.9) |
| Sunderland Royal Hospital | 4 (0.8) | 6 (1.2) |
| Pinderfields Hospital | 15 (3.0) | 14 (2.7) |
| Warrington Hospital | 8 (1.6) | 9 (1.8) |
| Tameside Hospital | 4 (0.8) | 5 (1.0) |
| Medway Maritime Hospital | 2 (0.4) | 1 (0.2) |
| South Tyneside District Hospital | 1 (0.2) | 4 (0.8) |
| Queen Mary’s Hospital, London | 13 (2.6) | 13 (2.5) |
| Queen Charlotte’s and Chelsea Hospital | 3 (0.6) | 4 (0.8) |
| Queen Elizabeth Hospital | 6 (1.2) | 7 (1.4) |
| Great Western Hospital | 12 (2.4) | 10 (1.9) |
| Royal Cornwall Hospital | 12 (2.4) | 12 (2.3) |
| Bedford Hospital | 9 (1.8) | 9 (1.8) |
| University College Hospital, London | 12 (2.4) | 12 (2.3) |
| Royal Sussex County Hospital | 7 (1.4) | 9 (1.8) |
| North Manchester General Hospital | 14 (2.8) | 16 (3.1) |
| New Cross Hospital, Wolverhampton | 8 (1.6) | 10 (1.9) |
| James Paget Hospital | 11 (2.2) | 10 (1.9) |
| St George’s Hospital | 24 (4.8) | 23 (4.5) |
| Princess Royal University Hospital | 2 (0.4) | 1 (0.2) |
| King’s College Hospital, London | 36 (7.2) | 37 (7.2) |
| St Mary’s Hospital | 1 (0.2) | 2 (0.4) |
| Dorset County Hospital | 8 (1.6) | 8 (1.6) |
| Kingston Hospital | 38 (7.6) | 38 (7.4) |
| Hillingdon Hospital | 6 (1.2) | 6 (1.2) |
| Arrowe Park Hospital | 6 (1.2) | 6 (1.2) |
| Lewisham Hospital | 1 (0.2) | 2 (0.4) |
| Prince Charles Hospital | 3 (0.6) | 3 (0.6) |
| Maternal age (years) | ||
| Mean (SD) | 28.9 (5.6) | 29.3 (5.5) |
| < 20, n (%) | 24 (4.8) | 24 (4.7) |
| 20–24, n (%) | 93 (18.5) | 79 (15.3) |
| 25–29, n (%) | 133 (26.4) | 148 (28.7) |
| 30–34, n (%) | 177 (35.2) | 180 (35.0) |
| 35–39, n (%) | 66 (13.1) | 71 (13.8) |
| ≥ 40, n (%) | 10 (2.0) | 13 (2.5) |
| Missing | 0 | 0 |
| Gestational age at entry (weeks) | ||
| Mean (SD) | 40.4 (1.2) | 40.3 (1.2) |
| 37+0 to 39+6, n (%) | 150 (29.9) | 167 (32.5) |
| 40+0 to 41+6, n (%) | 320 (63.8) | 315 (61.3) |
| ≥ 42+0, n (%) | 32 (6.4) | 32 (6.2) |
| Missing | 1 | 1 |
| IMD: quintile, n (%) | ||
| First (least deprived) | 64 (15.0) | 72 (16.3) |
| Second | 72 (16.9) | 63 (14.2) |
| Third | 83 (19.5) | 91 (20.5) |
| Fourth | 112 (26.3) | 129 (29.1) |
| Fifth (most deprived) | 95 (22.3) | 88 (19.9) |
| Wales – not derived | 66 | 59 |
| Postcode missing | 11 | 13 |
| Ethnic group, n (%) | ||
| White | 415 (83.7) | 434 (85.1) |
| Indian | 20 (4.0) | 14 (2.8) |
| Pakistani | 9 (1.8) | 7 (1.4) |
| Bangladeshi | 2 (0.4) | 1 (0.2) |
| Black African | 14 (2.8) | 10 (2.0) |
| Black Caribbean | 7 (0.7) | 2 (0.4) |
| Any other ethnic group | 29 (5.9) | 42 (8.2) |
| Not known/missing | 7 | 5 |
| BMI at booking visit (kg/m2) | ||
| Mean (SD) | 25.2 (5.2) | 25.2 (5.3) |
| Height and/or weight not known | 17 | 11 |
| Woman undergone FGM, n (%) | 2 (0.4) | 0 (0.0) |
| Missing | 1 | 1 |
| Onset of labour, n (%) | ||
| Spontaneous | 309 (61.6) | 293 (56.9) |
| Induced | 193 (38.5) | 222 (43.1) |
| Missing | 1 | 0 |
| Duration of first stage (minutes) | ||
| Median (IQR) | 490 (345–675) | 505 (360–720) |
| Geometric mean | 473.8 | 492.2 |
| Missing | 4 | 6 |
| Diagnosis of pre-eclampsia, n (%) | 12 (2.4) | 22 (4.3) |
| Missing | 2 | 1 |
| Continuous electronic fetal monitoring, n (%) | 484 (96.4) | 492 (95.7) |
| Missing | 1 | 1 |
| Diagnosis of delay requiring intervention, n (%) | 266 (53.1) | 272 (52.8) |
| Missing | 2 | 0 |
| Systemic opioids given prior to epidural, n (%) | 142 (28.3) | 137 (26.6) |
| Pethidine | 103 (72.5) | 97 (70.8) |
| Diamorphine | 38 (26.8) | 38 (27.7) |
| Remifentanil | 0 (0.0) | 1 (0.7) |
| Morphine | 0 (0.0) | 0 (0.0) |
| Meptid | 3 (2.1) | 3 (2.2) |
| Missing | 1 | 0 |
| Epidural technique, n (%) | ||
| Epidural | 485 (96.6) | 496 (96.5) |
| Combined spinal epidural | 17 (3.4) | 18 (3.5) |
| Missing | 1 | 1 |
| Epidural maintained with PCEA/infusion, n (%) | 359 (73.1) | 369 (73.1) |
| Missing | 12 | 10 |
| Woman’s pain score for last contraction | ||
| Median (IQR) | 10 (0–32) | 10 (0–30) |
| Missing | 59 | 55 |
| Able to perform straight leg raise, n (%) | 381 (80.4) | 408 (82.8) |
| Missing | 29 | 22 |
| Time from VE diagnosing second stage to study entry (minutes) | ||
| Median (IQR) | 13 (7–26) | 15 (8–26) |
| Apparently randomised before diagnosis of second stagea | 33 | 19 |
| Time apparently > 180 minutesb | 4 | 2 |
| Missing | 1 | 1 |
| Time from study entry to start of recording positions (minutes) | ||
| Median (IQR) | 0 (–4 to 5) | 1 (–3 to 5) |
| Time from study entry to recording position of > 15 minutes,a n (%) | 38 (7.7) | 44 (8.7) |
| Time apparently > 15 minutes before study entryb | 79 | 68 |
| Missing | 9 | 7 |
| SVB, n (%) | 197 (39.2) | 181 (35.2) |
| Missing | 0 | 0 |
The percentage of questionnaires returned before the SWAT started was considerably lower than the overall percentage returned from participants included in the SWAT (55.6% vs. 73.0%, respectively). This trend is also seen in the percentage returned without any reminder letters being sent [35.3% vs. 46.8%, respectively (Table 30)].
| Return rate, n (%) | SWAT | |
|---|---|---|
| Pre (N = 2067) | Post (N = 1018) | |
| Questionnaire returned | 1149 (55.6) | 743 (73.0) |
| Missing | 0 | 0 |
| Questionnaire returned without chasing by study team | 729 (35.3) | 476 (46.8) |
| Missing | 0 | 0 |
Return rates by postal and online completion between the two SWAT arms are presented in Table 31. A total of 152 questionnaires (20.5% of all questionnaires returned) were completed online, with slightly more being returned online in the reminder letter group than in the cover letter group (18.0% vs. 23.0%).
| Return rate, n (%) | Letter | |
|---|---|---|
| Cover (N = 503) | Reminder (N = 515) | |
| Questionnaire returned | 373 (74.2) | 370 (71.8) |
| Postal | 306 (82.0) | 285 (77.0) |
| Online | 67 (18.0) | 85 (23.0) |
| Questionnaire returned without chasing by study team | 259 (51.5) | 217 (42.1) |
| Postal | 207 (79.9) | 161 (72.2) |
| Online | 52 (20.1) | 56 (25.8) |
Figure 15 and Table 32 present the percentages of questionnaires returned, according to how many times a reminder letter was sent, and broken down by postal versus online completion. A higher percentage of questionnaires were returned without chasing after receipt of a cover letter promising an incentive than in the group receiving a standard cover letter (51.5% vs. 42.1%, respectively). However, if a reminder was sent, fewer women returned the questionnaire in the group receiving the promise of an incentive in the cover letter than those receiving a promise in the reminder letter (11.5% vs. 14.6%, respectively, for the first reminder, and 8.9% vs. 11.5%, respectively, for the second reminder).
FIGURE 15.
Percentages of questionnaires returned according to how many times a reminder letter was sent, broken down by postal vs. online completion.
| Rate, n (%) | Letter | |
|---|---|---|
| Cover (N = 503) | Reminder (N = 515) | |
| Questionnaire returned without chasing by study team | 259 (51.5) | 217 (42.1) |
| Postal | 207 (79.9) | 161 (74.2) |
| Online | 52 (20.1) | 56 (25.8) |
| Questionnaire returned after first reminder | 58 (11.5) | 75 (14.6) |
| Postal | 48 (82.8) | 55 (73.3) |
| Online | 10 (17.2) | 20 (26.7) |
| Questionnaire returned after second reminder | 56 (11.1) | 78 (15.2) |
| Postal | 51 (91.1) | 69 (88.5) |
| Online | 5 (8.9) | 9 (11.5) |
| Did not return questionnaire | 130 (25.8) | 145 (28.2) |
For the primary outcome, the percentage of questionnaires returned overall for those receiving the incentive cover letter was slightly higher than those receiving the incentive reminder letter (74.2% vs. 71.8%, respectively), but this was not statistically significant at the 5% level (adjusted RR 1.03, 95% CI 0.96 to 1.11) suggesting no evidence of an effect resulting from when the incentive is offered (Table 33). However, women who received a cover letter promising an incentive were more likely to return their questionnaire without a reminder letter being required than those who received a standard cover letter (adjusted RR 1.22, 95% CI 1.07 to 1.39). The mean difference in the total cost of the vouchers was £4.56 (95% CI £4.02 to £5.11), with the cost being higher in the group receiving the standard cover letter.
| Outcome | Letter | Adjusted effect measure (95% CI) | |
|---|---|---|---|
| Cover (N = 503) | Reminder (N = 515) | ||
| Primary outcome | |||
| Questionnaire returned, n (%) | 373 (74.2) | 370 (71.8) | RRa 1.03 (0.96 to 1.11) |
| Missing | 0 | 0 | |
| Secondary outcomes | |||
| Questionnaire returned without chasing by study team, n (%) | 259 (51.5) | 217 (42.1) | RRa 1.22 (1.07 to 1.39) |
| Missing | 0 | 0 | |
| Total cost of vouchers (£) | 3790 | 1530 | |
| Cost of vouchers,b mean (SD) | 7.5 (0.2) | 3.0 (0.2) | MD 4.56 (4.02 to 5.11) |
| Missing | 0 | 0 | |
The pre-specified subgroup analysis is presented as a forest plot in Figure 16. There is no evidence of heterogeneity for IMD subgroups for the primary outcome of overall response rate (p = 0.43).
FIGURE 16.
Subgroup analysis for questionnaires returned.

Discussion
In this SWAT, there is no evidence to suggest that the offer of a monetary incentive at first mail-out, compared with only when a reminder letter is sent, makes a substantial difference to the overall return rate of a 1-year follow-up postal questionnaire. Although slightly more questionnaires were returned in the group receiving the offer at first mail-out (an absolute difference of 3.4%), the corresponding RR of 1.03 (95% CI 0.96 to 1.11) was not statistically significant at the 5% level.
The return rate for women included in the SWAT compared with that before the SWAT was introduced showed a marked improvement (absolute difference 17%). Although this is not a randomised comparison, it is consistent with that found by Kenyon et al. 68 in a randomised study investigating the inclusion of a high-street voucher versus no voucher sent with a reminder letter to parents of 7-year-old children, which showed an increase in the return rate of 11.7% (95% CI 4.7% to 18.6%). This study is included in a systematic review that showed that the addition of monetary incentives was more effective than no incentive at increasing response rates to postal questionnaires (RR 1.18, 95% CI 1.09 to 1.28). 66
This SWAT used a £10 high-street gift voucher as a monetary incentive. The mean cost of vouchers per participant was greater in the group receiving the offer at first mail-out (£7.50 vs. £3.00). Coupled with the lack of evidence of a difference in the overall return rate, this would indicate that sending the offer of an incentive with a reminder letter only is a cost-effective approach to improving return rates. However, there is evidence to suggest that the return rate without requiring reminders is higher in the group for whom the incentive is offered in the first mail-out (absolute difference 9.4%). The cost of administering the additional reminder letters was not calculated, but is a serious consideration that would need to be offset against the expected cost of the vouchers and could depend on the resources available as well as the sample size of the study.
There are ethical issues to consider with the approach of only sending an offer of an incentive to those participants who do not return their questionnaire promptly. Consideration should be given to the chance that participants in a study may communicate with each other, and share their experiences regarding whether or not they received an incentive.
This is the first known SWAT to investigate the use of incentives for improving questionnaire return rates in a population of first-time mothers with infants around 1 year old. This study suggests that offering incentives when a reminder is required could be cost-effective depending on the sample size of the study and hence the resources required to administer the reminder letters.
Acknowledgements
Contributions of authors
Professor Debra Bick (Professor of Evidence Based Midwifery Practice, King’s College London): trial design and oversight, advice on measures of maternal satisfaction and interpretation of the results.
Dr Annette Briley (Consultant Midwife, Guy’s and St Thomas’ NHS Foundation Trust): trial oversight and interpretation of the results.
Professor Peter Brocklehurst (Professor of Women’s Health, UCL): trial design, oversight, analysis and interpretation of the findings. Responsible for the first draft and co-ordination of the production of this report and is its guarantor.
Ms Pollyanna Hardy (Senior Trials Statistician, National Perinatal Epidemiology Unit, University of Oxford): trial design, oversight of production of SAP, analysis and interpretation of the results.
Professor Edmund Juszczak (Associate Professor–Director, National Perinatal Epidemiology Unit, University of Oxford): trial design and oversight, statistical advice and interpretation of the results.
Ms Lynn Lynch (Midwifery Lecturer, Cardiff University): co-ordination of local staff at all of the participating centres, central day-to-day management of the centres and interpretation of the results.
Professor Christine MacArthur (Professor of Maternal and Child Epidemiology, University of Birmingham): trial design, oversight, advice on measures of post-partum well-being and morbidity, and analysis and interpretation of the findings.
Dr Phillip Moore (Consultant Anaesthetist, University Hospital Birmingham NHS Trust): trial design, oversight, advice on anaesthetic issues, analysis and interpretation of the findings.
Professor Mary Nolan (Professor of Perinatal Education, University of Worcester): trial design, oversight, advice on participant information material, analysis and interpretation of the findings.
Professor Oliver Rivero-Arias (Associate Professor/Senior Health Economist, National Perinatal Epidemiology Unit, University of Oxford): oversight of production of health economics analysis plan, analysis and interpretation of the results.
Dr Julia Sanders (Consultant Midwife/Reader in Midwifery, Cardiff University): trial design, oversight, analysis and interpretation of the findings.
Professor Andrew Shennan (Professor of Obstetrics, King’s College London): trial design, oversight, analysis and interpretation of the findings.
Dr Matt Wilson (Consultant in Obstetric Anaesthesia/Senior Lecturer in Anaesthesia, Sheffield Teaching Hospital/University of Sheffield): trial design, oversight, advice on anaesthetic issues, analysis and interpretation of the findings.
Co-investigators group
-
Professor Debra Bick, Professor of Evidence Based Midwifery Practice, King’s College London.
-
Dr Annette Briley, Consultant Midwife, Guy’s and St Thomas’ NHS Foundation Trust (replaced Geraldine O’Sullivan in 2012).
-
Professor Peter Brocklehurst, Professor of Women’s Health at UCL.
-
Oya Eddama, Health Economist, National Perinatal Epidemiology Unit, University of Oxford.
-
Professor Janesh Gupta, Professor/Honorary Consultant in Obstetrics and Gynaecology, Birmingham University/Birmingham Women’s Foundation NHS Trust.
-
Pollyanna Hardy, Senior Trials Statistician, National Perinatal Epidemiology Unit, University of Oxford.
-
Professor Edmund Juszczak, Associate Professor–Director, National Perinatal Epidemiology Unit, University of Oxford.
-
Lynn Lynch, Senior Research Midwife, Cardiff University.
-
Professor Christine MacArthur, Professor of Maternal and Child Epidemiology, University of Birmingham.
-
Professor Rona McCandlish, Epidemiologist: Maternal Health, National Perinatal Epidemiology Unit, University of Oxford (until 2012).
-
Dr Phillip Moore, Consultant Anaesthetist, University Hospital Birmingham NHS Trust.
-
Professor Mary Nolan, Professor of Perinatal Education, University of Worcester.
-
Dr Felicity Plaat, Lead Clinician and Consultant Anaesthetist, Queen Charlotte’s and the Hammersmith Hospital/Senior Lecturer, Imperial College London.
-
Dr Dean Regier, Senior Health Economist, National Perinatal Epidemiology Unit, University of Oxford (until 2012).
-
Dr Julia Sanders, Consultant Midwife/Reader in Midwifery, Cardiff University.
-
Professor Andrew Shennan, Professor of Obstetrics, King’s College London.
-
Dr Geraldine O’Sullivan, Lead Clinician in Obstetric Anaesthesia, Guy’s and St Thomas’ NHS Foundation Trust (deceased 2012).
-
Dr Matt Wilson, Consultant in Obstetric Anaesthesia/Senior Lecturer in Anaesthesia, Sheffield Teaching Hospital/University of Sheffield.
Trial Steering Committee
Independent members:
-
Dr Paul Howell, Consultant Anaesthetist, St Bartholomew’s Hospital
-
Professor Dame Tina Lavender, Professor in Midwifery, University of Manchester
-
Professor Alan Montgomery (Vice-Chairperson), Professor of Medical Statistics and Clinical Trials, Nottingham Clinical Trials Unit
-
Professor Stephen Palmer, Professor of Health Economics, University of York
-
Ms Justine Pepperell (Consumer Representative)
-
Professor Steve Robson (Chairperson), Professor of Fetal Medicine, Medical School, University of Newcastle.
Data Monitoring Committee
Independent members:
-
Professor Christine Kettle, Professor of Women’s Health, Academic Unit of Obstetrics and Gynaecology, University Hospital of North Staffordshire and Staffordshire University
-
Mr Stephen Walkinshaw (Vice-Chairperson), Consultant in Maternal and Fetal Medicine, Liverpool Women’s NHS Foundation Trust
-
Dr Steve Yentis (Chairperson), Consultant Anaesthetist, Chelsea and Westminster Hospital
-
Dr Pat Yudkin, Emeritus Reader in Medical Statistics, University of Oxford.
Clinical Trials Unit Staff
Comprehensive Clinical Trials Unit, University College London
-
Julie Bakobaki, Clinical Project Manager (until April 2014).
-
Laura Custins, Trial Manager (until November 2012).
-
Suzanne Drake, Data Entry Assistant (until June 2015).
-
Amber Gibney, Data Manager (until October 2013).
-
Steve Hibbert, IT Manager (until September 2015).
-
Elizabeth Howden, Trial Manager (until December 2013).
-
Alycia Kopec, Data Manager (until 2012).
-
Tola Lawal, Data Manger (until January 2015).
-
Sawretse Leslie, Trial Manager (until September 2015).
-
Lynn Lynch, Senior Research Midwife (until June 2014).
-
Kate Maclagan, Clinical Project Manager (until September 2015).
-
Garrie Powers, IT Developer (until September 2015).
-
Guy Schroter, Clinical Project Manager (until April 2015).
National Perinatal Epidemiology Unit, Oxford
-
Ursula Bowler, Senior Trials Manager (until June 2011).
-
Sonali De Silva-Mitter, Trial Manager (until June 2011).
-
Oya Eddama, Health Economist (until September 2015).
-
Ann Kennedy, Assistant Trials Manager (until June 2011).
-
Andy King, Head of Trials Programming (until June 2011).
-
Andy Kirk, Webmaster and Design Coordinator (until June 2011).
-
Claire Nelis, Statistician (until June 2011).
-
Rachel Roberts, Trial Manager (until June 2011).
-
Suzanne Williams, Data Coordinator (until June 2011).
Independent midwife assessor for clinical coding, London
Sarah Bryan
Recruiting centres
Centre: Arrowe Park Hospital, Wirral. Principal investigator: Suresh Singaravelu. Recruiting staff: Carly Nulty, Carolyn Bragg, Gerri Griffiths, Helen Burghall, Jane Murphy, Julie Dale, K Mccoy, Lynne Lacy, Rachel Roberts, Sandra Hutcheon, Tanya Wynne, Tracy Green and Vickie Heller.
Centre: Bedford Hospital. Principal investigator: Yaqub Latoo. Recruiting staff: Anita Males, Anni Price, Babs Harris, C Dyer, Carla Ball, Carol Handrahan, Donna James, Elizabeth Carlyle, Emma Clarke, Hayley Smith, Jen Welsh, Jenny Cowland, K Emery, Katie Summers, L Church, Liz Dodd, Lucy Wills, Marion Moore, Melissa Coles, Paula Griffiths, Rachel Pressley, Rebecca Adcock, Ruth Croot, Ruth Steward, S Harris, Sarah Gates, Sarah Johnson-Clarke and Sue Hill.
Centre: Birmingham Women’s Hospital. Principal investigator: Phil Moore. Recruiting staff: A Connolly, Alexandra Bellamy, Anna Zhao, Anya Chruscinska, B Oniono Kuafor, Becky Cullen, Bethany Lunn, Bobby Sharma, C Bishop, C Graves, Charlotte Bowman, Charlotte Davies, Charlotte Hinks, Chloe O’Hara, Claire Bissell, Debbie Baker, Deborah Robinson, Elisha Randell, Elizabeth Ewers, Emily Byrne, Emma Wright, Erica Henry, Fiona Musgrave, G George, Gemma Barnfield, Gemma Wadsworth, Hannah Wood, Harriet Fisher, J Rose, Jane McNair, Jennifer Prescot, Jenny Pledger, Jess Shaw, Jessica Gregory, Joelle Rowland, Juillete Webster, Julia Cheshire, Justine Craig, K Horton, Karen Davies, Karen Elkin, Katie Freitas, Kerry Hudson, Kimberley McMahon, Kiranjit Sehmi, Kirsten Emson, Kirsty Elwell, Laura Mulryan, Lauren Hill, Lauren Webley, Lisa Salmon, Lucy O’Grady, Madeleine Parry, Margarita Bariou, Maureen Joseph, Megan Corbett, Michaela Dzioba, Michaelene Cole, Michelle Bennett, Michelle Neal, Nichol Ross, Nicola Mcenery, Nikki Robbins, Novia Samuels, Orphelia Atkins, Pam Simpson, Paula Trinham, Rachel Singer, Rebecca Cullen, Rebecca Gallimore, Rebecca Leon, Rebecca Mckenzie, Rhea Bond, Ruth Cavey-Wilcox, Samuel Todd, Sarah Blythe, Sarah Ketley, Sasha Hamilton, Sethenia Beckford, Sian Wilkie, Stella Bibb, Stephanie Henry, Teresa Vann, Tracey Bond, V Preece and Victoria McDonagh.
Centre: Bradford Royal Infirmary. Principal investigator: Diane Farrar. Recruiting staff: Alexandra Fozard, Alice Tunney, Alison Chapman, Amanda Wilson-Thompson, Aongola Ngenda, Carrie Owens, Christina Scott, Clare Cummings, Fozia Arshad, Geraldine Atkinson, Gillian Butterfield, Heather Darlow, Helen M Sharp, Jennie Robertshaw, Joanne Mortimer, Joanne Watson, Josephine Hartley, Judith Lowther, Katarzyna Denkiewicz, L Jarockyj, Laura Mckenna, Lisa Thompson, Lydia Brookes, Maryanne Naylor, Nicola Davies, Rachael Jones, Rachel Hemers, Rachel Wild, Rebecca Skelton, S Marriott, S Nicholson, Sania Iqbal, Stacey Ryles, Susie Weekes, Talitha Grandison, Tracey Germaine-Rylance and Victoria Jones.
Centre: Brighton Royal Sussex County Hospital. Principal investigator: Vanessa Fludder. Recruiting staff: Claire Miles, Emma May, Florence Crawley, Hannah Tomms, Igone Sesma, Jayne Denyer, Kate McCambridge, Melanie Wight, Nichola Tuck, Omotoyin Awonuga, Paula Alonso-Gonzalez, Rachael Chatterton, Rachel Cox, Rosie Darling and Victoria Wright.
Centre: Dorset County Hospital, Dorchester. Principal investigator: Christine Grother. Recruiting staff: Allison Hamilton, Bev Robertson, Carly Smith, Christine Grother, Hilary Fletcher, Jane Linger, Jo Hartley, Julie Bonifacio, Julie Younger, Karen Myers, Kathryn Dyer, Louise Shreiber, Nichola Coliandris, Sarah Haigh and Tina Parker.
Centre: Frimley Park Hospital. Principal investigator: Karen Plews. Recruiting staff: Abbey Ford, Alexandra Mayrs, Alison Shilton, Amanda Dowling, Anna Holland, Anna Kemsley, C Green, Cara Gambon, Catherine Bressington, Charlie Thompson, Christina Longman, Cindy Port, Claire Smith-White, Danielle Perkins, Deidre Hussey, Di Wyeth, Fiona Allison, Florence Chauyara, Frances Warner, Gifty Dadzie, Hannah Brown, Helen Hawkins, Irene Tan, J Willard, Jackie White, Jaimie Sutherland, Jannine Bailey, Jayne Moss, Jenny Evans, Jessica Main, Jo Green, Joanna Broomham, K Wren, Karen Plews, Karen Spencer, Karen Wrigley, Kasia Russel, Katie Harrald, Kirsty Fisher, Laura Kirk, Lauren Barnett, Lauren Bartlett, Leah Pueschel, Lisa Rudall, Liz Treen, Louise Wylie, Melanie Taylor, Michelle Chuter, Michelle Hardy, Michelle Mcloughlin, Michelle Nicholls, Naomi Davies, Nicola Wimshurst, Pat Harris, Paula Ball, Penny Schnabel, R Warner, Rachel Bright, Rachel Rouen, Rebecca Beddows, Ruth Beesley, Sabine Everett, Sarah Heath, Saras Bishop, Sham Shelbourn, Sharon Phipps, Shian Fethney, Sophie Adams, Sophie Hutton, Susan Meyjes, Tabitha Stuthridge, Tania Gaffney, Theresa Thomas, Tina Longman, Tracy Hopkins, Vicky Donovan, Vivienne Novis, Wendy Bascal and Zoe Farr.
Centre: Gloucestershire Royal Hospital. Principal investigator: Louis Khor. Recruiting staff: Angela Smith, Angela Stevens, Bryony Bell, C Pearson, Carly Avis, Caroline Broadhurst, Cassy King, Chantel Coleman, Charlotte Barwick, Cody Allen, Ellen Reeves, Fiona Liddle, Hayley Marvill, Jane Barradale-Smith, Jane Soule, Jenna Surman, Jennifer Pratley, Kerrie Lotsu, Lisa Frattolillo, Louise Broadley, Lucy Broad, Michelle Dimery, Michelle Partington, Nikki Dobson, Nina Kellow, Rachael Harris, Rachel Midwinter-Marland, Ridwana Pandor, Sarah Pilcher, Sian Harrington and Tracey Miller.
Centre: Great Western Hospital, Swindon. Principal investigator: Tracey Sargent. Recruiting staff: Amy Pitcher, Angela Bunce, Anita Long, Ashley Heal, Danielle Heywood, Debra Hunt, Denise Selby, Elaine Price, Jade Gordon, Jane McGregor, Jennie Hone, Joanna Coulson, Joanne Lewis, Julia Sewell, Kate Welsh, Kathryn Owen, Kelly Greenslade, Kimberley Tubb, Lisa Nicholson, Nicola George, Rachel Ravati, Rebekah Tollafield, Ruth Davies, Sara Brown, Sophie Stewart, Susan Bint, Tamara Byrne, Tanya Miles, Victoria Norman, Viv Cutler and Zhilla Majadabadi.
Centre: Hillingdon Hospital. Principal investigator: Jane Terry. Recruiting staff: Denise Ahmed, Emma Speirs, Fiona White, IR Howarth, Kirsty Griffith, Kirsty Stark, Licricia Ngahan-Tchaptchet, M Lawlor, Manjit Matharu and Sarah Bell-Ryan.
Centre: James Paget University Hospital, Great Yarmouth. Principal investigator: Mumtaz Rashid. Recruiting staff: Andrea Bedford, Angela Oram, Ann Pye, Caroline Fox, Elsie Gibbs, Emily Boyce, Emily Cole, Faye Hewitt, Helen Cullimore, Jane Ward, Jennifer Thompson, Joan Timewell, Kerry Burwood, Laura Jarrett, Lauren Goodfellow, Lesley Yates, Nora Hassan, Pauline Studley, Sinead Osborne, Sophie Neville, Tracey Porter and Victoria Reeve.
Centre: King’s College Hospital, London. Principal investigator: Cathy Walton. Recruiting staff: A Tully, Agnes Kimbowa, Alice-Amanda Hinton, Amelia Evans, Anna Mazzarelli, Bridget Rance, Bryanna Chenoweth, C Beckmann, Christine Murphy, Clare Patterson, Clemmie Hooper, Dorcas Appah, Dorisilla Adolwa, Emily Stockton, Erica White, Erika Glenny, Esther Annan, Halina Szajna, Iqra Khan, Katherine Clark, Katrice Currie, Kelly Macfadyen, Laura Santos, Mary Bollard, Mary Obud, Michelle Lynch, Modupe Adebayo, Olivia Snowball, Omatalani R Sangare, Rachael Waldron, Rachel Barlow, Rebecca Macleod, Rebecca Manners, Ruth Graham, Ruth Landis, Sadie Holland, Sarah Skivens, Sarah-Ann Evans, Sophie Halton-Nathan, Sophie Steward, Stacey Robinson, Sue Byrne and Susie Urquhart.
Centre: Kingston Hospital. Principal investigator: Arezou Rezvani. Recruiting staff: Alexandra Frost, Alice Cox, Amanda Carey, Amisha Chauhan, Anne-Marie Greaves, Annie Stott, B Hellmich, Bronwen Kenward, C Caton, Charlotte Rose, Chloe Du Parcq, Elka Dimitrova, Fiona Smith, Hui Tam, Jana Durtova, Jennifer Slee, Joanna Pitcher, Laura Grainger, Laura Sowell, Leanne Bateman, Lizzanne Roman, Louise Jones, Lucy Bellinger, Lyndsey Smith, Morwenna Trevan, N Haysum, Nicky Ni, Nicole James-Lowe, Nikki West, Perrine Dhaisne, Rachel Bell, Rachel Rolfe, Rebekah Hoadley, Rosemary Mukasa, Ruth Sentenga, Sam Frewin, Sarah Lowe, Scarlett Beland-Tyce, Sophie Wismer, Suesan Beirouty, Susan Leahy, Susan O’Callaghan, Toni Brown, V Gunawardena and Zandra Rubia-Mendoza.
Centre: Lewisham Hospital, London. Principal investigator: Frances Jones. Recruiting staff: Ellen Madamombe, Jade Johnson, Kay Holford, Mabinty Leigh Sian Turner and Suzannah Sheerin.
Centre: Medway Maritime Hospital, Gillingham. Principal investigator: Dorothea Smith. Recruiting staff: Andrea Curling, Belinda Newman, Deborah Simmons, Debra Nwosa, Dorothy Smith, Helen Jones, Jane Simmons, K Ashwell, Kerry Sturgess, Lovelace Oti, Ludmila Wilson, Lynn Deller, Michelle Hayes, N Jones, Patricia Chaplin, Sarah-Jane Cross, Sharon Small, Tatjana Molotkova, Valerie Andrew and Zoe Wood.
Centre: Nevill Hall Hospital, Abergavenny. Principal investigator: Louise Taylor. Recruiting staff: Amanda Lisle, Andrea Priddle, B Markey, Cath Barwise, Deb Oliver, Kath Barwise, Kerry Owen, Kim McKie, Leanne Ball, Mandy Jones, Pippa Nicholas, Sue Jordan and Wendy Howells-Smith.
Centre: New Cross Hospital, Wolverhampton. Principal investigator: Gowri Simon. Recruiting staff: Becci Leathley, Claire Morgans, Deborah Brettle, Ellen Poniatowska, Emma Clews, Hazel Peden, Hazel Remmet-Booth, Jane Hussellbee, Joanne Ridley, Julia Icke, Karen Evans, Laura Brooks, Louise Wood, Marni Fassett, Pip Grocott, Siva Basra and Tracy Willetts.
Centre: North Manchester General Hospital. Principal investigator: Viv Dickinson. Recruiting staff: Andrea Kerti, C Sinclair, Catherine Holt, Catherine Hughes, Colette Robinson, Collette Riley, Dawn Littler, E Foley, Ellie Cardnell, Emma Baxter, Emma Groom, Emma Park, G Charles, Georgina Cartridge, Helena Spencer, Jean Davis, Jenna Haycocks, Jo Jakubowski, Joanna Ward, Julie Ainsworth, Julie Whitby, Karen Robbins, Kate O’Hagan, Kerri Delaney, Laura Ashton, Laurene Mannix, Lileath Fisher, Louise Blinkhorn, Lyndsay Yates, Lynsay Ingham, Margaret Kerins, Michelle Salt, Pam Whittle, Rachel Tully, Sacha Jackson, Stella Oakes, Tina Affleck, Valerie Julie Walker and Zoe Davies.
Centre: Pinderfields Hospital, Wakefield. Principal investigator: Nicolas Akerman. Recruiting staff: Anna Warburton, Caroline Paterson, Chiew Poskitt, Rachel Stock, Muhammad Faisal Ehsan, Elise McShee, Jacqueline Edge, James Bedford, Jill Greenwood, Julie Wormstone, Karen Simeson, Laura King, Marie Knox, Michelle Mowbray, Penny Barker, Rosalind Morley, Stacey Dunn, Sue Winrow, Tracy Langcake, Ujala Ahmad and Veronica Walker.
Centre: Prince Charles Hospital, Merthyr Tydfil. Principal investigator: Liz Edwards. Recruiting staff: Bev Jones, Catherine Bush, Ceri Hill-Jones, Diane Lewis, Jodie Hodges and Theresa Jones.
Centre: Princess of Wales Hospital, Bridgend. Principal investigator: Sarah Fox. Recruiting staff: Angela Davies, Annette Jones, Christie-Ann Lang, Donna Hall, Elinor Taylor, Elizabeth James, Emily Grainger, Gemma Griffiths, Hannah Lambert, Joy James, Julie Roberts, Kate Richards, Kathryn Greaves, Lauren Yaw, Lynne Grieves, Mari Davies, Megan Cave, Michelle Bassett, Rachel Morgan, Sarah Jones, Sian Middleton and Tracey Bowman.
Centre: Queen Alexandra Hospital, Portsmouth. Principal investigator: Aneeta Sinha. Recruiting staff: Amy Pollard, Andrea Grey, Ann Going, B Edge, Beryl Pullen, Carol Richardson, Carole Longley, Ella Edwards, Ellie Jenkins, Emma Connelly, Emma Kellagher, Fiona Moore, Genevieve O’Docherty, Gill Allen, Isla Campbell, Isobel Murtagh, Jemma Cave, Jill Hall, Jo Jordan, Jo Warwick, Jodie Carolan, Karen Darr, Karen Munks, Karen Wellspring, Katherine O’Mara, Katrina Walker, Kim Leonard, Laura Davis, Linda Lishman, Lucy Galloway, Lulu Russell Smith, Lynda Baker, Lynne Groves, Mandy Whittle, Mary Taylor, Mary-Ann Sheehan, Melanie Say, Mo Turnbull, Naomi Simpson, Penny Bone, Penny Cox, Sally Griffiths, Sarah Burr, Sharon Buttriss, Suzanne LeBrocq, Tracey Hall, Tracey Lasisi, Vanessa Garlish, Wendy Bessant, Wendy Marsh and Zoe Garner.
Centre: Queen Charlotte’s and Chelsea Hospital, London. Principal investigator: Felicity Platt. Recruiting staff: Alice Gautreau, Grace Kember, Igbeka Hayes, Karen McArtney-Roberts, Lisa Rickwood, Lucy Simpson and Suzanne Ridley.
Centre: Queen Elizabeth Hospital, Kings Lynn. Principal investigator: Anoop Surendran. Recruiting staff: Beverley Golding, Caroline Tucker, Catherine Bent, Debby Ramsdale, Donna Allen, E Cervi, Emma Chapman, Helen Parker, Jacinta Baptista, Jean Keen, Jodie Cully, Jodie Jupe, Lisa Gormley, Liz Tyler, Michaela Bouskova, Naomi Seaman, Rachel McCabe, Rosie Hucklesby, S Tennant, S Wingfield, Sarah Russell, Tracey Stafford, Tracy Cooke and Yvonne Fulcher.
Centre: Royal Cornwall Hospital, Truro. Principal investigator: Nila Cota. Recruiting staff: Amy Dunstan, Charlie Fulcher, Dariel Rowe, Eddi Theedham, Jane Parke, Jane Stubbs, Jenny Heron, Jo Bennett, Josie Dodgson, Julie Wallis, Karen Needham, Katherine Holland, Kerry Youngman, Kim Hewlett, Kimberly Fanson, Laura Quinn, Lisa Marchetti, Lizzie Cowan, Lorraine Kennedy-Snaith, M Hobson, M Underwood, NPM Middleton, Samantha Broughton, Sarah Grigg, Suzanne Bryant, Tracey Rowe, Victoria Bassett and Wendy Preen.
Centre: Royal Gwent Hospital, Newport. Principal investigator: Louise Taylor. Recruiting staff: Amie Cook, Beth Comben, Carmen Rubio-Batanas, Chloe Rowsell, Donna Crocombe, Donna Fleming, Elleanor Griffiths, Fay Smith-Warren, Fiona Carter, Helen Bishop, Jane Morgan, Janet Lawson, Jessica Waters, Karen Halford, Lesley-Ann Bushell, Margot Jones, Michelle Haggart, Naomi Martin, Nicola Smith, R Green, Rose Thomas, Sophie Savigar-Jones, Tara Welch and Tracey Griffiths.
Centre: Royal Hallamshire Hospital, Sheffield. Principal investigator: Vicky Wilson. Recruiting staff: Alison Morison, Alison Norris, Amanda Muller, Amy King, Anne Hemingway, Benash Nazmeen, Caroline Dabinett, Carollynn Jones, Carolyn Metcalfe, Cheryl Popovich, Claire Craine, Claire Sayan, Clara Mwatati, Clare Hennessey, Clare Lord, Clare Newton, Dalia Peretz, Deborah Cresswell, Faith Tye, Gill Hunt, Hannah Tebbs, Heather Croft, Helen Frow, Holly Hickman, Jessica Brookes, Jill Parton, Jo Varley, Joy Herdman, Judy Chang, Julia Thackray, Julie Hawksworth, Justine Todd, Kate Fish, Kathleen Farrand-Green, Kay Crowch, Laura Asher, Laura Chadwick, Leanne Rutkowski, Louise Roberts, Lucy Bon, Medeline Mudehwe, Melinda Pagden, P Pokorna, R Nye, Rebecca Bustani, Rebecca Weston, Rio Cooper, Rosie Barker, Sally Dawn, Samantha Young, Sara Calow, Sarah Bell, Sarah Senbeto, Sarah Swift, Steven Mackie, Sylwia Szarwark, Tracy Hobson, Victoria Lee, Victoria Wilkins, Wendy Few, Wendy Murphy and Zoe Riley.
Centre: Royal Sussex County Hospital, Brighton. Principal investigator: Vanessa Fludder. Recruiting staff: Alanna Dunkerton, Emma Peck, Helen Williams, Kate Clark and Rosheen Baker.
Centre: Royal United Hospital, Bath. Principal investigator: Tracey Sargent. Recruiting staff: Angela Fitzpatrick-Nash, Anne Moffatt, Anne White, Annie Collingwood, Ashley Heal, Bridget Dack, Camilla Hawkett, Charley Reschwamm, Christie Harrison, Cindy Stamp, Donna Williams, Ellie Grant, Elly Doyle, Emily Craig, Emma Tanner, Gemma Day, Hannah Cross, Hannah Jewell, Hannah Reid, Helen-Marie Crooks, Hilary Paice, Jane Norris, Jemma Freegard, Jennifer Reid, Jenny Pullen, Jo Waldron, Jo Woodburn, Julia Grant, Karen Doran, Kate Boulton, Katherine Jackson, Kathryn Vosper, Kathy L Holford, Katie Gooding, Kerry Perkins, Kim Miles, Laura Friend, Leah Harrold, Linda Davis, Liz Norton, Martina Grey, Mirella Popescu, Naomi Bonett-Healy, Nora Seager-Wilkendorf, Rachel Brierley, Rachel Coleman, Rebecca Lamb, Rebecca Murdoch, Rebecca Pendry, Rebecca Walsh, Rhian Motean, Rose Jenkins-Hunt, Ruth Branson, Sara Burnard, Sara Driver, Sarah Marks, Sasha Cairns, Sharon Seager, Susan Collins, Tamara Carr-Gomm, Tina Coffey, Tracy Boakes, Wendy Duberry and Wendy Giles-Smart.
Centre: Singleton Hospital, Swansea. Principal investigator: Sarah Fox. Recruiting staff: Amanda Bates, Cath Harris, Danielle Clifton, Ellie Brown, Felicity Curtis, Julie Ellerton, Julie Thomas, Kate Phillips, Kim Hillier, Linda Richards, Lisa Rees, Lucie Warren, Nicky Court, Outi Morris, Rachel Williams, Rebecca James, Rebecca Lewis, Sarah Fox, Sharon Cooling, Sharon Evan, Sian Phillips and Vicki Lennon.
Centre: South Tyneside Hospital, South Shields. Principal investigator: Mrs Shamma Al-Inizi. Recruiting staff: A MacKay, Allison Nicholson, C Greaves, Delia Brennan, Emma Hindes, Helen M Parker, Judith Black, Linda McNamee, Louise Nicholson, Nicola Tindall, Shelley Bowie and Stacey McFarley.
Centre: St George’s Hospital, London. Principal investigator: Asma Khalil. Recruiting staff: Amy Ridout, Angel Segura, Astell Aikines-Aryeetey, Bridget Okereke, Carmen Martin Martinez, Christiana Appeah, Claire Davies, Cristina Perez, Danielle Holbrook, Dede Efueye, Elaine Sheehan, Eleonor Cowlard, Emma Corrigan, Emma Freeman, Emma McCheyne, Erin Hutchings, H Gardner, Iona Hughes, Iryna Santoskkaya-Marsh, Isabel Aylward, Isabelle Cornet, Jessica Welham, Joyce Adu-Amankwah, Judith Mugerwa, Julia Plana Soria, Leila Zahedi, Lorena Santana Cardenosa, N Karali, Natalie James, Ngozi Asika, Ojevwe Owereh, Ophelie Granger, Paula Lavandeira, Paulette Joy Palmer, Raquel Vives Font, Rosie Sands, Roxanne Vidal, Sarah Esegbona-Adeigbe, Silvia Campo, Steve-Samuel King-Inneh, Sylvia Zoldak and Zinab Jalloh-Conteh.
Centre: St Mary’s Hospital, London. Principal investigator: Felicity Platt. Recruiting staff: Birima Darego-Wokoma, Leticia Alvarez, Margarita Lopez-Liesa, Sarah Pilgrem, Suzanne Ridley and Teresa Ribera.
Centre: St Thomas’ Hospital, London. Principal investigator: Annette Briley. Recruiting staff: A Perlepe, A Veitch, Abby Stewart, Abosede Kako-Are, Agatha Okafar, Ailsa Gill, Alhan Javan, Alice Du Preez, Alison Armstrong, Amanda Stephens, Amy Davies, Amy Smith, Ana Elices, Ana Llamas, Anita Meagher, Ann Marie McHugh, Anna Dios, Anna Gaudion, Anna Kenny, Anne Cobell, Annie Bell, Beth King, Birima Darego-Wokoma, Blanca Rodrigo-Ibanez, Bree Cant, Camella Main, Dede Efueye, E Blasse, Edith Onyeneri, Elena Martinez-Zuddin, Eleonora Bruni, Elizabeth Connelly, Elsa Moro, Emilie Grantham, Emily Jelen, Emma Copley, Emma Grey, Emma Grey, Emma Paten, Emmeline Mudford, Erika Duncan, Faye Safari, Florence Awichi, Fran Lawrence, Funso Adegoke, Gail Roberts, Gemma Baillie, Gillian Donaldson, Gloria Brempong, Grace Obwona-Lanana, Hannah Delmar-Addy, Hannah Emerson, Hannah Levy, Hannah Rogers, Hannah Veazey, Harriett Ivey, Hayley Osborne, Henrietta Simire, Holly Hickman, Ida Bradley, Iro Perlepe, Isabelle Arrabel, Jacqueline Mhako, Jane Love, Janet Cooper, Jennifer Tubby, Jess Cavaya, Jess Floyd, Jessica Quaroni, Jessima Cavaya, Jo Hoffmann, Jo McCarthy, Jo Parker, Jordan Anderson, Juliet Falola, Juliette Falolu, Karine Tweddle, Katia Ciccarella, Kaz Herlihy, Kylie Gould, Laura Bridle, Lauren Chandler, Lianne Phipps, Lianne Prior, Lola Shomefun, Louise Higgs, Lydia Gerrie, Madelena Wilders, Maeve O’Connell, Maggie Lee, Mara Bruno, Marcia Trusty, Maria Pipi, Marina Daniele, Marisa Alvarez, Marta Fernandez Diez, Monika Franklin, Moronike Agboola, N Carlin, Namgyal Gonkatsang, Olivet Macfarlane, Olivia Wheeler-Robinson, P Blair, Pauline Jackson, Rachel Grazette, Rosalind Pouteaux, Sarah Driver, Sarah Evison, Sarah Fowlie, Sarah Kensington, Sarah Tanner, Selina Tettey, Sharon Mumford, Sonia Pereina, Sophie Robinson, Stacy Brown, Stefania Andrian, Stella Nanseera, Sue Turner, Sumaira Bashir, Vaishni Moorji, Vic Offredi, Vivienne Gosden, Yemisi Fadoungbo, Yvonne Mcgrath, Zahra Famili, Zainab Jalloh, Zeenath Uddin and Zekiye Degmenlibey.
Centre: Sunderland Royal Hospital. Principal investigator: Kim Hinshaw. Recruiting staff: Amanda Bargh, Carol Forrester, Christine Evans, Claire Liddel, Deb Holmes, Deborah Bonney, Denise Mace, Donna Rodgers, Donna Rogers, Eileen Walton, Hannah White, Janet Rooks, Julie Harris, Julie Taylorson, Karen Hutchinson, Karen L Armstrong, Kate Reedman, Kathryn Evans, Katrina Dowell, Leeann Adey, Linda Adamson, Lisa Wilson, Lyndsey Summerbell, Natalie Graham, Nicola Easton, Pam Cheek, Sheila Ford, Sonia Thompson, Sophie Robson, Stephanie Hepple, Suzanne Stelling and Victoria Young.
Centre: Tameside Hospital, Ashton-under-Lyne. Principal investigator: Gillian Singleton. Recruiting staff: Ann Gibson, Donna Saleh, Felicia Taylor, Gabby Greenwood, Gemma Lumley, Gillian Singleton, Helen Clase, Jackie Tomlinson, Jan Moriarty, Janet Danzi, Karen Rothera, Kate Firth, Kerry Jackson, Lisa Fisher, Louise Nelson, Paula Frazer, Rachel Drain, S Bungaree, Sharon Aldous, Sophie Hook, Teresa Quinn and Tracey Leicester.
Centre: University College Hospital, London. Principal investigator: Belinda Green. Recruiting staff: Abisola Bashua, Amy Tiltman, Ann Esquerdo, Anna White, C Amaning, Christine Haron, Constance Mvududu, Donata Hoesch, Edna Farah Dahir, Eleri Bates, Ellie Sanderson, Emily Nygaard, Fenya Jonas, Hayley Gilroy, Heidi Buhlmann, Ivan Bettinsoli, J Cole, J Evans, Jennifer Lang, Jenny Keys, Jes Surtees, Lucia Fitzsimons, Margarita Akyla, Meghan Jackson, Nellie Sarmiento, Teresa Okemadu and Zahra Khan.
Centre: University Hospital of Wales, Cardiff. Principal investigator: Rachel Collis. Recruiting staff: A Morgan, A Aimee Jones, Aime Symes, Alex Andrews, Alice Fairman, Alice Snell, Alyson Gardiner, Ami Wolstenholme, Amy Garrett, Amy Vaithilingam, Amy Welsh, Angela Amey-jones, Angela Jones, Anika Brodd, Anna Jones, Annie Kitchen, C Swallow, Cara Moore, Cara Moruzzi, Carla Blackshaw, Catherine Downing, Cheryl Cox, Debbie Grey, Debbie Hunt, Debbie Jones, Deborah Powell, Ed Cross, Elaine Patterson, Elin Phillips, Emily James, Emily Shaw, Emma Bull, Felicity Callan, G McElroy, Gloria Lane, Hannah Hills, Hannah Thomas, Helen Lock, Hollie Power, Jane Reid, Jayne Frank, Jenna Parsons, Jenna Terry, Jenny Rickson, Jess Holmes, Joanne Bowen, Jodie Clark, Jude Casey, Julia Morgan, Juliet Grimes, Karen Jennings, Karina Downing, Kate Lynch, Kate Murphy, Kate Siddal, Katherine Fischer, Katherine Williams, Kathryn Smith, Katie O’Bradovic, Katie Stubbs, Kelly Bennett, Kerri Hamblin, Kirsty Jones, Laura Mundy, Laura Rose, Laura Terry, Lauren Quirke, Lieska Hoes, Lindsey Hilldrup, Louise Houghton, Luisa Canale, Lynette Rowlands, Miranda Millett, Misha Harry, Natalie Rees, Nerys Kirtley, Nia Cleal, Nicola Savoury, Nicola Schilling, Patricia Chan, Polly Ferguson, Rachel Bain, Rachel Harry, Rebecca Boselli, Ros Howells, Ruth Leonard, Sally Alqaddo, Sally Meek, Samantha Crouch, Sara Davies, Sarah Heap, Sarah James, Sarah Lucas, Sarah Madley, Sarah Morris, Sarah Spencer, Sherrie Bird, Shirley Goodwin, Sian Jones, Sofia Odugleh, Tamsin Edwards, Tracey Lawrence, Trudy Thomas-Jones, Wendy Hoggan and Zoe Millichap.
Centre: Warrington Hospital. Principal investigator: Rita Arya. Recruiting staff: Alison Quine, Ann Pathmakumar, Ann-Marie Brooke, Ann-Marie Hatton, Cate Fitzpatrick, Cath Kidd, Danielle Stotton, Deborah Fletcher, Debra Clements, Donna Abbott, Eileen Fielding, Einir O’Neill, Elaine Armitage, Hannah Stevens, Hayley Axon, Heather Mee, Helen Ling, Helen Poulton, Jackie Richards, Jayne Wright, Katherine Conquest, Kerry Jones, Lesley Hampson, Linda Hennon, Mags Odell, Marie Wheatley, Mary Cubbon, Mary Hornby, Simone Peters, Susan Evans, Tamsin Hawkins, V Hodson and Vicky Littlewood.
Centre: West Middlesex University Hospital. Principal investigator: Louise Page. Recruiting staff: A Akodu, Adebola Aroboto, Adelaide Adubuffour, Alicia Thomas, Alyson Brown, Amanda Bray, Anna Piasecka, B Collard, B Snee, C Gordon-Jack, Deborah Reid, Eleanor Fraser, F Addow, Fiona Ghalustians, Grace Volo, Hannah Thomas, Ilaria Torre, J Fernandez, Jennifer Ryan, Jessica Howard, Joy Ataderie, Julia Harris, Juliet Joseph, Karen Beck, Karen Lundie, Kelly Mack, Kirsty Dolling, Lisa Takab, M Farrell, Maddie Saunders, Marie Garvey, Marie O’Connell, Marie Oliver, Mercy Batchelor, Neveen Jivan, Nikki Jaques, P Laurence, Pia Tomeldon, Po Ying Li, R Shadna, Renske van Gaans, Revai Chingwa, Risi Akodu, S Harrison, Sally Dauncey, Sally Seaman, Sarah Dixon, Silviya Giffin, Sophie Pike, Tsakani Tshavane, V Henry and W Fambe.
Publications
Bird H. Labour Position Study Launches. The Royal College of Midwives, 14 August 2012. URL: www.rcm.org.uk/news-views-and-analysis/news/labour-position-study-launches (accessed 2 October 2017).
Hardy P, Bell J, Brocklehurst P. Evaluation of the effects of an offer of a monetary incentive on the rate of questionnaire return during follow-up of a clinical trials: a randomised study within a trial. BMC Med Res Methodol 2016;16:82.
The Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ 2017;359:j4471.
Data sharing statement
Data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Khor LJ, Jeskins G, Cooper GM, Paterson-Brown S. National obstetric anaesthetic practice in the UK 1997/1998. Anaesthesia 2000;55:1168-72. https://doi.org/10.1046/j.1365-2044.2000.01720.x.
- Redshaw M, Henderson J. Safely Delivered: A National Survey of Women’s Experience of Maternity Care 2014. www.npeu.ox.ac.uk/reports/807-safely-delivered (accessed 9 September 2016).
- Redshaw M, Rowe R, Hockley C, Brocklehurst P. Recorded Delivery: A National Survey of Women’s Experience of Maternity Care. Oxford: National Perinatal Epidemiology Unit; 2006.
- Anim-Somuah M, Smyth RMD, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev 2011;12. https://doi.org/10.1002/14651858.CD000331.pub3.
- Comparative Obstetric Mobile Epidural Trial (COMET) Study Group UK . Effect of low-dose mobile versus traditional epidural techniques on mode of delivery: a randomised controlled trial. Lancet 2001;358:19-23. https://doi.org/10.1016/S0140-6736(00)05251-X.
- Liu EH, Sia AT. Rates of caesarean section and instrumental vaginal delivery in nulliparous women after low concentration epidural infusions or opioid analgesia: systematic review. BMJ 2004;328. https://doi.org/10.1136/bmj.38097.590810.7C.
- de Leeuw JW, Struijk PC, Vierhout ME, Wallenburg HC. Risk factors for third degree perineal ruptures during delivery. BJOG 2001;108:383-7. https://doi.org/10.1016/S0306-5456(00)00090-5.
- Dupuis O, Madelenat P, Rudigoz RC. Fecal and urinary incontinence after delivery: risk factors and prevention. Gynecol Obstet Fertil 2004;32:540-8. https://doi.org/10.1016/j.gyobfe.2004.02.020.
- Eason E, Labrecque M, Marcoux S, Mondor M. Anal incontinence after childbirth. CMAJ 2002;166:326-30.
- Fitzpatrick M, Behan M, O’Connell PR, O’Herlihy C. Randomised clinical trial to assess anal sphincter function following forceps or vacuum assisted vaginal delivery. BJOG 2003;110:424-9. https://doi.org/10.1046/j.1471-0528.2003.02173.x.
- MacArthur C, Glazener CM, Wilson PD, Herbison GP, Gee H, Lang GD, et al. Obstetric practice and faecal incontinence three months after delivery. BJOG 2001;108:678-83. https://doi.org/10.1111/j.1471-0528.2001.00183.x.
- MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG 2000;107:1460-70. https://doi.org/10.1111/j.1471-0528.2000.tb11669.x.
- Roberts CL, Algert CS, Olive E. Impact of first-stage ambulation on mode of delivery among women with epidural analgesia. Aust N Z J Obstet Gynaecol 2004;44:489-94. https://doi.org/10.1111/j.1479-828X.2004.00294.x.
- Gupta JK, Hofmeyr GJ, Shehmar M. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev 2012;5. https://doi.org/10.1002/14651858.CD002006.pub3.
- Kemp E, Kingswood CJ, Kibuka M, Thornton JG. Position in the second stage of labour for women with epidural anaesthesia. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD008070.pub2.
- Pretlove SJ, Thompson PJ, Toozs-Hobson PM, Radley S, Khan KS. Does the mode of delivery predispose women to anal incontinence in the first year postpartum? A comparative systematic review. BJOG 2008;115:421-34. https://doi.org/10.1111/j.1471-0528.2007.01553.x.
- Macarthur C, Glazener C, Lancashire R, Herbison P, Wilson D, Grant A. Faecal incontinence and mode of first and subsequent delivery: a six-year longitudinal study. BJOG 2005;112:1075-82. https://doi.org/10.1111/j.1471-0528.2005.00721.x.
- Macarthur C, Wilson D, Herbison P, Lancashire RJ, Hagen S, Toozs-Hobson P, et al. Faecal incontinence persisting after childbirth: a 12 year longitudinal study. BJOG 2013;120:169-79. https://doi.org/10.1111/1471-0528.12039.
- Brown S, Lumley J. Maternal health after childbirth: results of an Australian population based survey. Br J Obstet Gynaecol 1998;105:156-61. https://doi.org/10.1111/j.1471-0528.1998.tb10045.x.
- MacArthur C, Glazener CM, Wilson PD, Lancashire RJ, Herbison GP, Grant AM. Persistent urinary incontinence and delivery mode history: a six-year longitudinal study. BJOG 2006;113:218-24. https://doi.org/10.1111/j.1471-0528.2005.00818.x.
- Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med 2003;348:900-7. https://doi.org/10.1056/NEJMoa021788.
- MacArthur C, Lewis M, Knox EG. Health after childbirth. Br J Obstet Gynaecol 1991;98:1193-5. https://doi.org/10.1111/j.1471-0528.1991.tb15386.x.
- Ansara D, Cohen MM, Gallop R, Kung R, Schei B. Predictors of women’s physical health problems after childbirth. J Psychosom Obstet Gynaecol 2005;26:115-25. https://doi.org/10.1080/01443610400023064.
- Glazener CM, Abdalla M, Stroud P, Naji S, Templeton A, Russell IT. Postnatal maternal morbidity: extent, causes, prevention and treatment. Br J Obstet Gynaecol 1995;102:282-7. https://doi.org/10.1111/j.1471-0528.1995.tb09132.x.
- Barrett G, Pendry E, Peacock J, Victor C, Thakar R, Manyonda I. Women’s sexual health after childbirth. BJOG 2000;107:186-95. https://doi.org/10.1111/j.1471-0528.2000.tb11689.x.
- Towards Better Births: A Review of Maternity Services in England. London: Commission for Healthcare Audit and Inspection; 2008.
- Christiaens W, Bracke P. Assessment of social psychological determinants of satisfaction with childbirth in a cross-national perspective. BMC Pregnancy Childbirth 2007;7. https://doi.org/10.1186/1471-2393-7-26.
- Hodnett ED. Pain and women’s satisfaction with the experience of childbirth: a systematic review. Am J Obstet Gynecol 2002;186:160-72. https://doi.org/10.1016/S0002-9378(02)70189-0.
- Burnstein R, Buckland R, Pickett JA. A survey of epidural analgesia for labour in the United Kingdom. Anaesthesia 1999;54:634-40. https://doi.org/10.1046/j.1365-2044.1999.00894.x.
- Prabhu A, Plaat F. Use of ‘mobile epidurals’ in the UK. Int J Obstet Anesth 2009;18:28-33.
- Intrapartum Care for Healthy Women and Babies. London: RCOG Press; 2014.
- DAMOCLES Study Group . A proposed charter for clinical trial data monitoring committees: helping them do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)70939-9.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702-6. https://doi.org/10.1093/aje/kwh090.
- Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Statist 1963;34:598-611. https://doi.org/10.1214/aoms/1177704172.
- Department for Communities and Local Government . The English Indices of Deprivation 2010 2011. www.gov.uk/government/uploads/system/uploads/attachment_data/file/6871/1871208.pdf (accessed 11 July 2017).
- Ordnance Survey . Code-Point Open 2013. www.ordnancesurvey.co.uk/business-and-government/products/code-point-open.html (accessed 11 July 2017).
- Matthews JN, Altman DG. Interaction 3: how to examine heterogeneity. BMJ 1996;313. https://doi.org/10.1136/bmj.313.7061.862.
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7382.219.
- The Epidural and Position Trial Collaborative Group . Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ 2017;359. https://doi.org/10.1136/bmj.j4471.
- NHS Digital . Hospital Episode Statistics. NHS Maternity Statistics – England, 2013–14 2015. www.hscic.gov.uk/catalogue/PUB16725/nhs-mate-eng-2013-14-summ-repo-rep.pdf (accessed 9 September 2016).
- Mauskopf JA, Paul JE, Grant DM, Stergachis A. The role of cost-consequence analysis in healthcare decision-making. PharmacoEconomics 1998;13:277-88. https://doi.org/10.2165/00019053-199813030-00002.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy 1997;2:14-8.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Schroeder E, Petrou S, Patel N, Hollowell J, Puddicombe D, Redshaw M, et al. Cost effectiveness of alternative planned places of birth in woman at low risk of complications: evidence from the Birthplace in England national prospective cohort study. BMJ 2012;344. https://doi.org/10.1136/bmj.e2292.
- Department of Health . NHS Reference Costs 2013–14 2015. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed 9 September 2016).
- Eddama O, Petrou S, Schroeder L, Bollapragada S, Mackenzie F, Norrie J, et al. The cost-effectiveness of outpatient (at home) cervical ripening with isosorbide mononitrate prior to induction of labour. BJOG 2009;116:1196-203. https://doi.org/10.1111/j.1471-0528.2009.02236.x.
- Petrou S, Murray L, Cooper P, Davidson LL. The accuracy of self-reported healthcare resource utilization in health economic studies. Int J Technol Assess Health Care 2002;18:705-10. https://doi.org/10.1017/S026646230200051X.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit; University of Kent; 2014.
- Department of Health . NHS Finance Manual 2015. www.info.doh.gov.uk/doh/finman.nsf/Newsletters?OpenView (accessed 11 July 2017).
- Nixon RM, Wonderling D, Grieve RD. Non-parametric methods for cost-effectiveness analysis: the central limit theorem and the bootstrap compared. Health Econ 2010;19:316-33. https://doi.org/10.1002/hec.1477.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Little RJ, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: Wiley Online Library; 2002.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401. https://doi.org/10.1146/annurev.publhealth.23.100901.140534.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2010.
- Mullins CD. Double counting and the reporting of cost per event avoided. Clin Ther 2006;28:602-3. https://doi.org/10.1016/j.clinthera.2006.04.002.
- Prick BW, Bijlenga D, Jansen AJ, Boers KE, Scherjon SA, Koopmans CM, et al. Determinants of health-related quality of life in the postpartum period after obstetric complications. Eur J Obstet Gynecol Reprod Biol 2015;185:88-95. https://doi.org/10.1016/j.ejogrb.2014.11.038.
- Mogos MF, August EM, Salinas-Miranda AA, Sultan DH, Salihu HM. A systematic review of quality of life measures in pregnant and postpartum mothers. Appl Res Qual Life 2013;8:219-50. https://doi.org/10.1007/s11482-012-9188-4.
- Morrell CJ, Cantrell A, Evans K, Carrick-Sen DM. A review of instruments to measure health-related quality of life and well-being among pregnant women. J Repro Infant Psych 2013;31:512-30. https://doi.org/10.1080/02646838.2013.835795.
- Hardy P, Bell J, Brocklehurst P. Evaluation of the effects of an offer of a monetary incentive on the rate of questionnaire return during follow-up of a clinical trial: a randomised study within a trial. BMC Med Res Methodol 2016;16. https://doi.org/10.1186/s12874-016-0180-9.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Methods to increase response rates to postal questionnaires. Cochrane Database Methodol Rev 2003;4. https://doi.org/10.1002/14651858.MR000008.pub2.
- Brueton VC, Tierney J, Stenning S, Harding S, Meredith S, Nazareth I, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2013;12. https://doi.org/10.1002/14651858.MR000032.pub2.
- Khadjesari Z, Murray E, Kalaitzaki E, White IR, McCambridge J, Thompson SG, et al. Impact and costs of incentives to reduce attrition in online trials: two randomized controlled trials. J Med Internet Res 2011;13. https://doi.org/10.2196/jmir.1523.
- Kenyon S, Pike K, Jones D, Taylor D, Salt A, Marlow N, et al. The effect of a monetary incentive on return of a postal health and development questionnaire: a randomised trial [ISRCTN53994660]. BMC Health Serv Res 2005;5. https://doi.org/10.1186/1472-6963-5-55.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:698-702. https://doi.org/10.1136/bmj.c332.
- Kahan BC, Morris TP. Analysis of multicentre trials with continuous outcomes: when and how should we account for centre effects?. Stat Med 2013;32:1136-49. https://doi.org/10.1002/sim.5667.
- Metz CE. Basic principles of ROC analysis. Semin Nucl Med 1978;8:283-98. https://doi.org/10.1016/S0001-2998(78)80014-2.
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561-77.
- Fawcett T. An introduction to ROC analysis. Pattern Recognit Lett 2006;27:861-74. https://doi.org/10.1016/j.patrec.2005.10.010.
- Symon A. A review of mothers’ prenatal and postnatal quality of life. Health Qual Life Outcomes 2003;1. https://doi.org/10.1186/1477-7525-1-38.
- Ungar, WJ . Challenges in health state valuation in paediatric economic evaluation: are QALYs contraindicated?. PharmacoEconomics 2011;29:641-52. https://doi.org/10.2165/11591570-000000000-00000.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. https://doi.org/10.1097/00005650-199206000-00002.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Petrou S, Hockley C. An investigation into the empirical validity of the EQ-5D and SF-6D based on hypothetical preferences in a general population. Health Econ 2005;14:1169-89. https://doi.org/10.1002/hec.1006.
- Billingham LJ, Abrams KR. Simultaneous analysis of quality of life and survival data. Stat Methods Med Res 2002;11:25-48. https://doi.org/10.1191/0962280202sm269ra.
- Simons CL, Rivero-Arias O, Yu LM, Simon J. Multiple imputation to deal with missing EQ-5D-3L data: should we impute individual domains or the actual index?. Qual Life Res 2015;24:805-15. https://doi.org/10.1007/s11136-014-0837-y.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- Glick H, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. https://doi.org/10.1177/0272989X9001000308.
Appendix 1 BUMPES: woman and infant data collection booklet (version 9.0)
Appendix 2 BUMPES: higher level of care data collection form – woman (version 2.0)
Appendix 3 BUMPES: higher level of care data collection form – infant (version 2.0)
Appendix 4 BUMPES: ‘your labour and birth experience’ form (version 6.0)
Appendix 5 BUMPES: 1-year follow-up (version 3.0)
Pages of the 1-year follow-up questionnaire that contained copyrighted material have been removed.
Appendix 6 Membership of the Trial Steering Committee, Data Monitoring Committee and Clinical Investigator Group
Clinical Investigator Group
-
Professor Debra Bick, Professor of Evidence Based Midwifery Practice, King’s College London.
-
Dr Annette Briley, Consultant Midwife, Guy’s and St Thomas’ NHS Foundation Trust (replaced Geraldine O’Sullivan in 2012).
-
Professor Peter Brocklehurst, Professor of Women’s Health at UCL.
-
Oya Eddama, Health Economist, National Perinatal Epidemiology Unit, University of Oxford.
-
Professor Janesh Gupta, Professor/Honorary Consultant in Obstetrics and Gynaecology, Birmingham University/Birmingham Women’s Foundation NHS Trust.
-
Pollyanna Hardy, Senior Trials Statistician, National Perinatal Epidemiology Unit, University of Oxford.
-
Professor Edmund Juszczak, Associate Professor–Director, National Perinatal Epidemiology Unit, University of Oxford.
-
Lynn Lynch, Senior Research Midwife, Cardiff University.
-
Professor Christine MacArthur, Professor of Maternal and Child Epidemiology, University of Birmingham.
-
Professor Rona McCandlish, Epidemiologist: Maternal Health, National Perinatal Epidemiology Unit, University of Oxford (until 2012).
-
Dr Phillip Moore, Consultant Anaesthetist, University Hospital Birmingham NHS Trust.
-
Professor Mary Nolan, Professor of Perinatal Education, University of Worcester.
-
Dr Felicity Plaat, Lead Clinician and Consultant Anaesthetist, Queen Charlotte’s and the Hammersmith Hospital/Senior Lecturer, Imperial College London.
-
Dr Dean Regier, Senior Health Economist, National Perinatal Epidemiology Unit, University of Oxford (until 2012).
-
Dr Julia Sanders, Consultant Midwife/Reader in Midwifery, Cardiff University.
-
Professor Andrew Shennan, Professor of Obstetrics, King’s College London.
-
Dr Geraldine O’Sullivan, Lead Clinician in Obstetric Anaesthesia, Guy’s and St Thomas’ NHS Foundation Trust (deceased 2012).
-
Dr Matt Wilson, Consultant in Obstetric Anaesthesia/Senior Lecturer in Anaesthesia, Sheffield Teaching Hospital/University of Sheffield.
Trial Steering Committee
Independent members:
-
Dr Paul Howell, Consultant Anaesthetist, St Bartholomew’s Hospital
-
Professor Dame Tina Lavender, Professor in Midwifery, University of Manchester
-
Professor Alan Montgomery (Vice-Chairperson), Professor of Medical Statistics and Clinical Trials, Nottingham Clinical Trials Unit
-
Professor Stephen Palmer, Professor of Health Economics, University of York
-
Ms Justine Pepperell (Consumer Representative)
-
Professor Steve Robson (Chairperson), Professor of Fetal Medicine, Medical School, University of Newcastle.
Data Monitoring Committee
Independent members:
-
Professor Christine Kettle, Professor of Women’s Health, Academic Unit of Obstetrics and Gynaecology, University Hospital of North Staffordshire and Staffordshire University
-
Mr Stephen Walkinshaw (Vice-Chairperson), Consultant in Maternal and Fetal Medicine, Liverpool Women’s NHS Foundation Trust
-
Dr Steve Yentis (Chairperson), Consultant Anaesthetist, Chelsea and Westminster Hospital
-
Dr Pat Yudkin, Emeritus Reader in Medical Statistics, University of Oxford.
Appendix 7 BUMPES: statistical analysis plan
Appendix 8 BUMPES: health economics analysis plan
Appendix 9 List of hospitals contributing to the BUMPES study
| Centre number | Centre name |
|---|---|
| 384 | Arrowe Park Hospital |
| 373 | Bedford Hospital |
| 203 | Birmingham Women’s Hospital |
| 152 | Bradford Royal Infirmary |
| 379 | Dorset County Hospital Dorchester |
| 356 | Frimley Park Hospital |
| 353 | Gloucestershire Royal Hospital |
| 365 | Great Western Hospital |
| 381 | Hillingdon Hospital |
| 369 | James Paget University Hospital |
| 112 | Jessop Wing, Sheffield |
| 380 | King’s College London |
| 382 | Kingston Hospital |
| 377 | Lewisham Hospital |
| 363 | Medway Maritime Hospital |
| 358 | Neville Hall Hospital |
| 368 | New Cross Hospital |
| 370 | North Manchester General Hospital |
| 354 | Pinderfields Hospital |
| 378 | Prince Charles Hospital |
| 351 | Princess of Wales Hospital |
| 367 | Princess Royal Hospital |
| 279 | Queen Alexandra Hospital, Portsmouth |
| 140 | Queen Charlotte’s and Chelsea Hospital |
| 364 | Queen Elizabeth Hospital |
| 361 | Queen Mary’s Hospital |
| 374 | Royal Cornwall Hospital |
| 383 | Royal Glamorgan Hospital |
| 352 | Royal Gwent Hospital, Newport |
| 366 | Royal Sussex County Hospital |
| 350 | Royal United Hospital, Bath |
| 362 | Singleton Hospital, Swansea |
| 360 | South Tyneside District Hospital |
| 376 | St George’s Hospital |
| 375 | St Mary’s Hospital |
| 261 | St Thomas’ Hospital |
| 227 | Sunderland Royal Hospital |
| 359 | Tameside Hospital |
| 355 | University College Hospital, London |
| 173 | University Hospital of Wales |
| 357 | Warrington Hospital |
Appendix 10 BUMPES: serious adverse event form (version 2.0)
Appendix 11 BUMPES: incentive trial protocol (version 1.0, 8 July 2009)
Appendix 12 BUMPES: data collection worksheet (version 5.0)
Appendix 13 BUMPES: withdrawal form (version 4.0)
List of abbreviations
- BMI
- body mass index
- BP
- blood pressure
- CI
- confidence interval
- CIG
- Clinical Investigators Group
- CLRN
- Comprehensive Local Research Network
- CONSORT
- Consolidated Standards of Reporting Trials
- DCB
- data collection booklet
- DMC
- Data Monitoring Committee
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GCP
- good clinical practice
- GMR
- geometric mean ratio
- HLC
- higher level of care
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IVD
- instrumental vaginal delivery
- MD
- mean difference
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- risk ratio
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SVB
- spontaneous vaginal birth
- SWAT
- study within a trial
- TSC
- Trial Steering Committee
- UCL
- University College London