Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/136/120. The contractual start date was in June 2013. The draft report began editorial review in April 2016 and was accepted for publication in August 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Wendy Atkin reports grants from Cancer Research UK during the conduct of the study and from the National Institute for Health Research Health Technology Assessment programme for being a coprincipal investigator for study reference 02/02/01 [Halligan S, Dadswell E, Wooldrage K, Wardle J, von Wagner C, Lilford R, et al. Computed tomographic colonography compared with colonoscopy or barium enema for diagnosis of colorectal cancer in older symptomatic patients: two multicentre randomised trials with economic evaluation (the SIGGAR trials). Health Technol Assess 2015;19(54)], on which the current study is based. Steve Halligan also reports grants from the National Institute for Health Research Health Technology Assessment programme for being a coprincipal investigator for study reference 02/02/01, on which the current study is based.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Atkin et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Colorectal cancer diagnosis: the health service burden
Colorectal cancer (CRC), also known as bowel cancer, is a UK health priority. Over 40,000 men and women were newly diagnosed with this disease in the UK in 2012, and > 16,000 people died from it. 1 More than £1B in NHS expenditure per year was attributed to CRC management in a 2007 cost-of-illness evaluation, which included costs for screening and diagnosis through to those for treatment and palliative care. 2 Diagnostic costs were the single largest contributor to CRC NHS expenditure, accounting for approximately £291M (26%) of the total expenditure. 2 The overwhelming majority of CRC diagnostic costs (£270M; 92.9%) was attributed to investigations in those subsequently not found to have the disease. 2 The high costs associated with CRC diagnosis are likely to be a result of the very common nature of bowel cancer symptoms, as a result of which the majority of patients investigated will not have bowel cancer, and the high costs of the diagnostic tests used to investigate patients for suspected CRC. This is probably compounded by the lack of a cheaper, reliable, immediate test to triage patients.
In symptomatic patients, the recommended investigation for suspected CRC is endoscopic evaluation [by colonoscopy or flexible sigmoidoscopy (FS)], radiological imaging [by computerised tomography (CT) colonography or barium enema], or a combination of procedures, ‘where the aim is to achieve adequate visualisation of the entire colon and rectum’. 3 The choice of investigation depends on clinician and patient preference, local expertise and patient age/comorbidities. 3 In 2013/14, approximately 530,000 colonoscopies and 300,000 FS were performed in the NHS, compared with 60,000 CT colonography and 2000 barium enema evaluations. 4 In the UK, endoscopy services are currently overwhelmed by demand and under pressure to meet urgent referral targets. 4 As an indication of the increasing pressures on endoscopy services, it has been predicted that by 2020 an additional 750,000 endoscopies will be required per year to meet demand, which is a 44% increase on 2013/14. 4
Endoscopic whole-colon investigation: colonoscopy
In accordance with the National Institute for Health and Care Excellence (NICE) clinical guidelines, colonoscopy is the reference standard test for establishing a diagnosis of CRC. 3 The advantages of this procedure are that it can visualise the whole colorectum, has high sensitivity for CRC and also permits biopsy and the removal of lesions. 3 Colonoscopy is, however, an invasive procedure that is associated with a small risk of serious complications, including heavy bleeding (1 in 150), bowel perforation (1 in 1500) and, although rarely, death (approximately 1 in 10,000). 5 Colonoscopy can also be an uncomfortable experience for some patients,6 and the intravenous sedation and pain relief generally required for colonoscopy are also associated with cardiovascular and respiratory complications. 7 Moreover, patients who have been sedated are unable to return to work, operate machinery, drive a vehicle or make important decisions for 24 hours after the procedure. 8,9 Older patients or patients with comorbidities are at increased risk of complications and are also less likely to tolerate the purgative full bowel preparation that is required to cleanse the colon prior to colonoscopy. 10,11 The bowel preparation required for colonoscopy is often the part of this procedure that patients find most difficult,12 and failure to complete it results in loss of procedural accuracy. 10,13,14 There is also a small miss rate of colonoscopy for colorectal neoplasia, as demonstrated by tandem colonoscopy studies. 15
Radiological whole-colon investigations
The currently available radiological tests for imaging the large bowel include barium enema and CT colonography. These tests are recommended by NICE for use as alternatives to colonoscopy for the first-line investigation of older patients who are deemed to be at greater risk of complications – particularly those associated with sedation – for those who are unwilling to undergo colonoscopy or for whom a colonoscopy is deemed not possible. 16 In general, however, radiological imaging diagnostics are limited because further endoscopic investigation is required to collect biopsy specimens and/or remove suspicious lesions in the event that these are detected.
Barium enema
During this procedure, an enema containing barium suspension is passed through the bowel while the patient is positioned to facilitate the distribution of the enema throughout the colon. A series of radiographs are taken with the patient in a number of positions to ensure the adequate visualisation of the entire colorectal tract. Barium enema has the benefit over colonoscopy of improved safety17 and, although the bowel must also be prepared, sedation is not required. The diagnostic utility of barium enema is limited by its poor sensitivity. Current NICE guidelines recommend that barium enema is offered after an incomplete colonoscopy and in combination with FS for patients with major comorbidity as an alternative to colonoscopy. 3 Barium enema is being replaced by CT colonography when local facilities and expertise are available.
Computerised tomography colonography
Computerised tomography colonography is a relatively new technology for examining the entire colorectum. During this procedure, two- and three-dimensional images of the colorectal tract are produced. As with conventional colonoscopy, the patient must still undergo bowel preparation except when faecal tagging is used, during which a contrast reagent is orally administered; however, no sedation is required for the procedure. CT colonography is less invasive than colonoscopy and has an improved safety profile. 18,19 There is evidence that patient acceptability for CT colonography may be higher than that for colonoscopy, although this is not conclusive. 20–22 The sensitivity of CT colonography is comparable with that of colonoscopy and higher than that of barium enema. 18,23 CT colonography can detect extracolonic lesions/abnormalities, which can be useful in patients with vague abdominal symptoms. 19 However, many extracolonic abnormalities are incidental findings of benign origin that, nonetheless, prompt further diagnostic investigations, potentially exposing the patient to further risk and incurring additional costs. 18,24 The sensitivity of CT colonography for CRC in comparison with colonoscopy has been estimated at 96% in a systematic review. 25 In the Special Interest Group in Gastrointestinal and Abdominal Radiology (SIGGAR) trial, the detection rate of CRC and large polyps was considerably higher in the trial arm that received CT colonography than in the arm that received barium enema. 18
Alternative to whole-colon investigation: flexible sigmoidoscopy
Flexible sigmoidoscopy is an endoscopic procedure that is used to examine the distal colon and rectum. Isolated lesions in the proximal colon therefore go undetected with FS, unless a distal lesion is present that warrants a subsequent whole-colon investigation (WCI).
Flexible sigmoidoscopy can offer both clinical and resource use benefits over WCI. FS is a safer, quicker procedure than colonoscopy, and intravenous sedation or pain relief is not typically required, which makes this procedure potentially more appropriate for patients at a higher risk of sedation-related complications, and also means that the patient can generally return to normal activities immediately. 26,27 The lack of need for sedation removes the requirement for (1) recovery time in the endoscopy unit, (2) the patient to be accompanied home and (3) the patient to refrain from driving or operating machinery for 24 hours, as is necessary with sedation. Bowel preparation is more straightforward for FS than for WCI, as it can be achieved with an enema alone (either self-administered or administered by a health-care professional). 26 As with colonoscopy, biopsy tissue and small polyps can be removed during the procedure, although limited bowel preparation generally means that patients with larger lesions are referred for subsequent colonoscopy. Another advantage of FS is that it is a less complicated and less risky procedure than colonoscopy and can be carried out by an appropriately trained nurse specialist. 28
It has been suggested that there is scope within the NHS to reduce the diagnostic burden for patients and endoscopy services by implementing clinical protocols which incorporate the selective use of FS, in place of WCI, for the initial investigation of patients with symptoms suggestive of distal CRC. 29–33 For WCI to be avoided in favour of FS, diagnostic protocols using FS for first-line investigation must be able to demonstrate favourable risk–benefit profiles, in which the benefits of this less invasive procedure are balanced against the risk of a missed diagnosis of proximal cancer. 34–36 The use of such protocols is likely to be most relevant in clinical practice for which the clinical index of suspicion for proximal colon cancer is low, for example when patient and symptom profiles favour a diagnosis of distal CRC. 33,37
Prevalence of cancers in the proximal versus the distal colorectum
Up to 60% of CRCs diagnosed are in the distal colorectum. 38–40 The proportion of CRCs that are diagnosed at sites in the proximal colon increases with age in both men and women,40,41 although this effect is more pronounced in women. 41 More patients with proximal cancer present as an emergency (i.e. with intestinal obstruction)42,43 or with iron deficiency anaemia (IDA) and are referred directly to IDA clinics for gastroscopy and colonoscopy.
Colorectal cancer symptoms and signs
Identifying CRC as a cause of symptoms in a patient presenting to primary care is problematic. 44,45 The clinical features commonly associated with this disease, such as a change in bowel habit (CIBH), rectal bleeding, abdominal pain, weight loss and anaemia, are also common in the general population and are not specific to CRC. 46,47 Most often, these symptoms will be the result of other, more common conditions with a typically benign clinical course (e.g. irritable bowel syndrome and haemorrhoids) or, less frequently, more serious conditions such as inflammatory bowel disease (ulcerative colitis or Crohn’s disease). 48,49 Accordingly, the positive predictive values for many features suggestive of CRC in patients presenting to primary care are relatively low. 44,50 For rectal bleeding and abdominal pain in isolation, the positive predictive values reported by NICE are 5% and 2%, respectively. 46 Evidence suggests that positive predictive values for common symptoms and signs increase with age and can be higher when features are combined. 46,48–51 In patients referred by their general practitioner (GP) to hospital, positive predictive values for these features are increased; for example, the positive predictive value for rectal bleeding rises to 5–7% in the secondary care setting. 49
Symptoms suggestive of distal colorectal cancer
Cancers in the proximal and distal colorectum frequently present with different symptom profiles. 37,52,53 The majority of CRCs detected in patients with rectal bleeding alone, when the blood is bright red in colour, are located in parts of the colorectum distal to the splenic flexure. 37,53–57 The sensitivity of rectal bleeding for proximal lesions has been shown to be higher in elderly patients (those aged ≥ 80 years)58 or when bleeding is severe. 54 A CIBH with rectal bleeding is also associated with distal CRC,29 but it is less clear whether or not a CIBH without rectal bleeding can be used to distinguish distal from proximal CRC. 35
Symptoms and signs suggestive of proximal colon cancer
Cancers in the proximal colon are, in general, less likely to present with overt symptoms such as rectal bleeding. 16,52,58–60 IDA, with or without a palpable abdominal mass, is the most distinguishing clinical feature of proximal colon cancer. 16,31,33,52 Another symptom associated with a proximal colon cancer diagnosis is unexplained weight loss. 53 Weight loss, similar to IDA and abdominal mass, could be symptomatic of more advanced disease16 and might be related to the observation by some that proximal disease is often diagnosed at a more advanced stage. 61,62
The association between anaemia and proximal colon cancer
Anaemia is a condition in which a person has too few red blood cells, or the oxygen-carrying capacity of their red blood cells is diminished, to the extent that physiological needs are not met. 63 Iron deficiency, resulting for example from chronic blood loss, is the most common cause of anaemia and leads to IDA, in which iron stores are depleted and red blood cell production is accordingly compromised. 63,64 Decreased serum ferritin in the presence of low haemoglobin (Hb) and mean corpuscular volume (MCV) is most reliable for the diagnosis of IDA. 64 Blood loss from the gastrointestinal (GI) tract is the most common cause of IDA in postmenopausal women and in adult men, and in approximately 5–10% of cases IDA is caused by colonic neoplasia. 65 Proximal colon cancer in particular is likely to manifest clinically with the consequences of ‘silent’ GI blood loss such as IDA,37,56 which has been reported to be present in up to 75% of patients with proximal colon cancer. 56,60,66,67 Hb levels are generally lower in those with proximal colon cancer than in those with distal cancer,61,68 although there is evidence to suggest that Hb levels in a significant proportion of patients with proximal cancer are higher than locally and nationally defined diagnostic thresholds. 68 Thus, the diagnostic accuracy of anaemia for proximal colon cancer in clinical practice is likely to be influenced by laboratory thresholds68 and by which haematological parameters are used to determine anaemia status. 61 Similarly, the interpretation of clinical studies investigating the diagnostic value of anaemia for CRC by subsite is hampered by variations in the thresholds and haematological parameters used.
Tailoring initial investigations for suspected colorectal cancers based on clinical features
Whether or not the reported differences in the symptom profiles of proximal and distal CRCs can be used to adequately distinguish which patients are so unlikely to have proximal colon cancer that they are suitable for investigation by FS alone has been the subject of limited research in the UK health-care setting. A prospective cohort study by Thompson et al. 33 of 16,433 consecutive patients aimed to identify patient groups, based on presenting clinical features, who were most likely to benefit from WCI for investigation of CRC and which patients could safely be examined by FS because their risk of having a proximal cancer was so low. The patients included in this study had been referred by their GP to the colorectal clinic at St Mary’s Hospital, Portsmouth (and two peripheral hospitals), southern England, between 1986 and 2001. In this patient cohort, 815 (86.2%) out of 946 CRCs were located in the distal colorectum (and, therefore, possible to detect at FS). This percentage rose to 95.3% (750/787) in patients who did not have IDA or a palpable abdominal mass, which represented 96.3% (15,829/16,433) of all patients in this cohort. Although 4.7% (37/787) of CRCs diagnosed in patients without IDA and/or an abdominal mass were proximal, only 2.2% (17/787) would have been missed with FS as a first-line investigation because there was no additional indication for WCI, such as symptoms suggestive of obstruction or neoplasia detected by FS.
A retrospective cohort study published in 2010 similarly sought to identify features that were predictive of CRC anatomical site from the endoscopy and pathology records of 153 patients diagnosed with CRC between April 2005 and March 2006. 31 The findings of this study also supported the tailoring of initial investigations. In patients with CRC, distal lesions were associated with a CIBH and rectal bleeding. No patients with rectal bleeding alone had a proximal cancer31 and proximal disease was associated with anaemia (defined by low Hb level). A total of 88 (70%) out of 126 CRCs (the subset diagnosed in the outpatient setting) diagnosed in this cohort were in the distal colorectum. The initial diagnostic tool used to investigate the majority of patients with CRC diagnoses was colonoscopy; only 11% of patients were initially investigated by FS, compared with 50% initially investigated by colonoscopy. 31 In a response to this study, other authors carried out an audit of 835 colonoscopies performed at their London hospital. 30 In this audit, there were 177 CRC diagnoses between January 2008 and December 2009, and 45 (25%) of these were proximal colon cancers. 30 No patient who had presented with rectal bleeding or a CIBH alone was subsequently diagnosed with an isolated proximal cancer (i.e. without a synchronous distal cancer). 30
There have been other efforts to estimate proximal colon cancer miss rates for symptom-based tailoring of initial investigations. For the most part, these have been retrospective analyses of patients presenting through urgent 2-week wait referral pathways in England for the evaluation of symptoms suggestive of CRC. 29,32,34–36 In an analysis of presenting symptoms in 2-week wait patients with proximal cancer, only 3.4% (7/206) of patients with a CIBH and/or rectal bleeding would have had their proximal cancer missed if FS had been the only investigation. 29 A lower miss rate was calculated in a separate study of patients with distal symptoms referred to a rapid access colorectal clinic. Two (0.24%) proximal cancers were diagnosed in patients referred with distal symptoms in isolation after a cancer-free FS; however, the authors were not explicit as to whether or not there were any other indications for WCI in these patients. 32 Others have reported higher miss rates for proximal cancers and have suggested that FS is not an appropriate first-line investigation for patients with symptoms suggestive of CRC. 34,36 For example, Bhangu et al. 34 found that in a cohort of 1725 patients presenting at a 2-week wait clinic, 15.3% (13/85) of cancers in those presenting without IDA and/or an abdominal mass were proximal and would have been missed by FS. 34 However, the number of patients with proximal cancer who also had other criteria that would have warranted WCI after FS under current guidelines and standard of care was not reported and, notably, neither was the number of proximal cancers that were associated with synchronous distal lesions. 34
All of the studies described were either undertaken at single sites or in small numbers of patients, which limits the generalisability of their findings to the wider symptomatic population presenting to secondary care.
National clinical guidelines for symptom-based tailoring of diagnostic investigations for suspected colorectal cancer
There is a lack of consensus in national clinical guidelines regarding the requirement for WCI to investigate symptoms suggestive of CRC. Some UK organisations (detailed in the following paragraphs) have made explicit reference to the use of symptom-based tailoring of investigations and have recommended substitution of WCI with FS in certain scenarios, while others have not. This has probably had an impact on the implementation of symptom-driven FS protocols at a local level leading to variations in practice. 29,32,33,35,36
A cancer service recommendation was published by NICE in 2004,16 which stated that FS is an appropriate initial investigation for most patients with bowel symptoms, such as a CIBH and rectal bleeding, given that these symptoms are indicative of lesions in the distal colon and rectum. This publication also stated that WCI is necessary when the patient is deemed at risk of proximal disease because of risk factors/clinical features such as older age, an abdominal mass, IDA, abdominal pain and loss of appetite and weight. 16
In the 2011 NICE clinical guideline3 for the diagnosis and management of CRC in secondary care, no recommendations were made for the tailoring of diagnostic investigations based on symptoms as predictors of CRC subsite. In patients referred to secondary care with suspected CRC, these guidelines recommend colonoscopy, except for patients with major comorbidity, for whom FS followed by barium enema was recommended. 3 The Scottish Intercollegiate Guidelines Network69 published recommendations pertaining to the diagnosis of suspected CRC in 2011 (recommendation 126); these give guidance concerning the tailoring of investigations of symptoms and clinical features suggestive of large bowel pathology (including CRC), based on age and symptoms. They recommend that FS may be an appropriate investigation for patients with rectal bleeding alone who are aged < 50 years. However, when CRC is suspected, the recommendation is for visualisation of the whole large bowel. 69
Guidelines produced by the Association of Coloproctology of Great Britain and Ireland (ACPGBI) in 2007 are clearer in their recommendation for tailoring of CRC investigations based on symptoms. 59 These guidelines state that the majority of patients presenting with rectal bleeding and/or a CIBH and no other significant risk factors can be examined with FS. 59 The reasons for mandatory WCI listed in the ACPGBI guidelines are an abdominal mass, severe symptoms, a positive faecal occult blood test (FOBt) or strong family history. 59
In 2011, the British Society for Gastroenterology, the ACPGBI and the Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland made a joint multisociety position statement on the indications for diagnostic lower GI endoscopy, which stated that symptoms suggestive of CRC, including persistent rectal bleeding and/or a CIBH, blood in the stool and IDA, are indications for diagnostic colonoscopy. However, clinical indications for FS included those < 40 years of age with persistent and/or recurrent bleeding and/or a CIBH. 70
Study rationale
It is likely that the conflicting evidence about whether or not FS is adequate as an initial investigation for certain symptoms/symptom combinations suggestive of CRC, and the lack of consensus in clinical guidelines, has affected protocol implementation at the local level. 29,32,34,71 There have been calls for further clarity in this area, particularly in relation to diagnostic protocols for patients presenting with bowel symptoms alone without IDA and/or a palpable abdominal mass. 32,34 In 2011, we proposed the Symptoms of Colorectal Cancer Evaluation Research (SOCCER) study as an add-on study to the SIGGAR randomised controlled trials. 19 The SIGGAR trials had examined the diagnostic accuracy of CT colonography compared with colonoscopy or barium enema in patients with symptoms suggestive of CRC. 18,23 The SOCCER study was proposed in order to further contribute to the evidence base with respect to the predictive value of symptoms for CRC by subsite. In particular, it was designed to investigate whether or not the findings of the previous study by Thompson et al. 33 could be validated in a multicentre setting.
Aims and objectives
Primary objective
The primary objective of the SOCCER study was to investigate the link between patients’ symptoms at presentation and the risk of cancer in the proximal colon to determine whether or not there are particular symptoms or symptom combinations which indicate that a patient could be adequately cared for by a distal colorectum examination (FS) rather than a more extensive WCI. The primary outcome of the SOCCER study was the diagnostic yield of CRC (proximal/distal) within 3 years of presentation at clinic, by symptoms at presentation.
Secondary objectives
The secondary objectives of the SOCCER study were to:
-
measure the prevalence of proximal and distal CRC in referred patients presenting with symptoms suggestive of CRC
-
determine the number needed to be examined to diagnose one distal cancer, by symptoms at presentation
-
determine the number needed to be examined to diagnose one proximal cancer, by symptoms at presentation
-
determine the miss rate of CRC after FS
-
determine hypothetical proximal CRC miss rates if only patients with certain symptoms or combinations of symptoms are sent for WCI.
Study design and setting
This was a retrospective analysis of prospectively collected data from a cohort of patients who had been referred to 21 hospitals between 2004 and 2007, with symptoms or signs suggestive of CRC, and who had been assessed as potentially eligible for the SIGGAR randomised controlled trials. 18,19,23
Chapter 2 Methods
The SOCCER study was proposed as a follow-on study from the SIGGAR multicentre randomised controlled trials. 18,19,23 The SOCCER study is a retrospective analysis of a cohort of patients referred to secondary care who were assessed as potentially eligible for the SIGGAR trials, and includes patients regardless of whether or not they had been subsequently randomised. This approach was used to enhance the generalisability of the SOCCER study findings relating to symptoms at presentation, and subsequent cancer diagnosis, to the wider secondary care population. The clinical trial report for the SIGGAR trials, which contains information pertaining to trial design and full methodology, has been published elsewhere. 19 Methodology relevant to the SOCCER study cohort and analyses will be presented in this report. The reporting of this study is in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 72
Research governance and ethics arrangements
The SIGGAR trials were registered in the International Standard Randomised Controlled Trial Number registry under ISRCTN95152621. Imperial College London was the nominated sponsor for the SIGGAR and SOCCER studies. The research governance procedures in place at Imperial College London ensured that all appropriate regulations and guidelines were followed.
A study steering committee was convened to provide independent oversight of the SOCCER study and expert advice on aspects of the study. This committee also included a patient representative who provided input on study plans from the patient perspective.
Ethics approval and permission to use patient data without consent
Ethics approval for the SIGGAR randomised controlled trials was obtained from the Northern and Yorkshire Multi-Centre Research Ethics Committee on 15 January 2004 and, subsequently, from individual participating centres. Research ethics approval for the SOCCER study was granted as an extension to the SIGGAR randomised controlled trials by the North East (York) National Research Ethics Service. The SOCCER study was also granted Section 251 support under the National Health Service Act 200673 for the processing of patient identifiable information without consent [references ECC 5–04(E) 2011 and 14/CAG/1043]. To comply with the conditions of Section 251 support, the Cancer Screening and Prevention Research Group at Imperial College London (responsible for all aspects of trial and data management for this study) assessed its data handling procedures against Department of Health information governance standards. The Cancer Screening and Prevention Research Group holds an Information Governance toolkit to demonstrate compliance with these standards. 74
Recruitment
Selection of participating hospitals
Patients were recruited to the SIGGAR trials from hospital trusts in which a radiologist member of SIGGAR had expressed a prior interest in participating. Centres were expected to have an established and efficient fast-track referral system for patients with suspected CRC (usually an identified diagnostic clinic) to facilitate recruitment, and a named colorectal nurse specialist or researcher who would take responsibility for recruitment.
The final 21 NHS hospitals were selected via a ‘sham randomisation’ that identified centres likely to achieve a minimum monthly recruitment target (at least 18 patients). 23 These 21 hospital centres included teaching and general hospitals and were distributed across England (see Appendix 1).
SOCCER eligibility criteria
Patients who were considered potentially eligible for the SIGGAR trials were considered eligible for the SOCCER study, irrespective of whether or not they were randomised, unless they met the SOCCER study exclusion criteria.
SIGGAR trial eligibility assessment
Patients were assessed for eligibility for the SIGGAR trials between March 2004 and December 2007. Consecutive potentially eligible patients were identified by colorectal nurse specialists, research nurses or radiographers at these centres from CRC and gastroenterology outpatient clinics (including fast-track CRC clinics) and procedural lists (endoscopy and radiology). Patients who met the following SIGGAR trials inclusion criteria, and did not meet the exclusion criteria were considered potentially eligible for inclusion in the SOCCER study.
SIGGAR trials inclusion criteria
-
Had been referred to hospital for symptoms or signs suggestive of CRC.
-
Were aged ≥ 55 years.
-
Were clinically judged to need a WCI.
-
Were clinically judged as fit to undergo full bowel preparation.
SIGGAR trials exclusion criteria
-
Had a known genetic predisposition to cancer, for example familial adenomatous polyposis or hereditary non-polyposis CRC.
-
Had a known diagnosis of ulcerative colitis or Crohn’s disease.
-
Had undergone a WCI in the previous 6 months.
-
Had been referred for a WCI to follow up a previously diagnosed CRC.
SOCCER study exclusion criteria
Patients were randomised during the SIGGAR trials (CT colonography vs. colonoscopy or CT colonography vs. barium enema) only if they met eligibility criteria and had given informed consent, and if a consultant had consented to their participation. Some patients who were potentially eligible were, therefore, not randomised during the SIGGAR trials. These patients were included in the SOCCER study analysis unless they fulfilled the following exclusion criteria:
-
declined consent
-
gave consent and were randomised but subsequently dissented
-
were judged unable to give informed consent
-
had no symptoms recorded at presentation
-
were untraceable for follow-up CRC diagnoses through the Health and Social Care Information Centre (HSCIC)
-
had a duplicate study record.
Data collection
Patient data used in the SOCCER study were sourced from the SIGGAR trials and additional data were obtained from hospital records. All data were held in a de-identified format in a separate SOCCER study database.
Baseline characteristics
Patient baseline characteristics were collected when patients were originally assessed for eligibility for the SIGGAR trials, and data were collected for both randomised and non-randomised patients. This information had been recorded on the bespoke SIGGAR trials pro forma and included patient age, sex, date of referral, the urgency of the referral (‘2-week wait’, ‘urgent’, ‘soon’ or ‘routine’), the referral route, the diagnostic investigations requested, the outpatient clinic type (if applicable), other relevant diagnoses and whether or not the patient had initially been investigated by FS. For randomised patients, details of the main SIGGAR trial interventions (barium enema, CT colonography and colonoscopy) and outpatient appointments were also recorded on the trial pro forma.
Symptoms and clinical signs
Clinical features at presentation were recorded for potentially eligible patients at baseline during eligibility assessment for the SIGGAR trials. The SIGGAR trials pro forma contained tick boxes to record symptoms and clinical signs under ‘details/reason for referral’. Tick boxes were included for ‘rectal bleeding’, ‘abdominal pain’, ‘anaemia’, ‘weight loss’, ‘CIBH’ and ‘positive FOBt’. A free-text field to record additional symptoms was also included on the pro forma. Entries in the free-text field were manually coded by the trial team for use in the analysis. They were categorised into ‘abdominal mass’, ‘bloating/flatulence’, ‘tiredness/weakness’, ‘anal symptoms’, ‘nausea/vomiting’, ‘back pain’, ‘upper GI symptoms’, ‘rectal mass’, ‘family history’, ‘history of polyps’, ‘presence of cancer antibodies’, ‘elevated C-reactive protein’ and ‘liver problems’. A second free-text field to record the details of the CIBH was also included on the pro forma and was manually coded and categorised to ‘looser and/or more frequent’, ‘harder and/or less frequent’, ‘variable’ or ‘unspecified’.
Data pertaining to clinical features at presentation were also sourced from hospital records. Radiology, endoscopy and pathology records were requested for patients in the SOCCER study cohort and were interrogated for information concerning symptoms/clinical signs (specifically abdominal mass, rectal bleeding, abdominal pain, weight loss, a CIBH and rectal mass). Relevant data were extracted from text fields. For further details see Data extraction.
Anaemia
Anaemia and IDA are clinical signs that have been associated with proximal colon cancer in previous clinical studies33,56 and were therefore of key importance to the SOCCER study. Iron deficiency is the most common cause of anaemia and reflects more severe stages of the disease, when the body is no longer able to replenish iron stores. 64 Decreased MCV (microcytic anaemia) is often assumed to result from iron deficiency but is relatively non-specific for IDA;64 nonetheless, decreased MCV can be diagnostically useful in the investigation of GI causes of iron deficiency,65 for example when serum ferritin levels are not available. However, decreased serum ferritin levels are the most reliable sign for the diagnosis of IDA. 64
Owing to the significance to our study of anaemia status, we ideally would have had data on full blood counts for all patients in order to apply a uniform definition of anaemia and consistently classify the anaemia status of each patient based on their blood test results. Although a tick box for anaemia as a reason for referral had been included on the SIGGAR trials pro forma, the classification of anaemia was not necessarily consistent between hospitals. Therefore, we separated patients into those with blood test data and those without.
For patients for whom blood test data were available, we used laboratory data to confirm anaemia and excluded the tick box from our definition of anaemia. For these patients, anaemia status at presentation was determined from blood tests taken within 6 months before the date of referral (in the SIGGAR trials) and 3 months after. For patients with a diagnosis of CRC, any blood tests dated on or after the date of diagnosis were excluded. Blood test parameters [Hb level (g/dl), MCV (fl) and serum ferritin (µg/l)] were collected from hospital haematology databases (for further details see Data Extraction). When multiple results for a parameter were available for an individual patient, the lowest recorded value (within the relevant time period) was selected.
We considered four different definitions of anaemia: ‘broad anaemia’, ‘strict anaemia’, ‘broad IDA’ and ‘strict IDA’. Broad anaemia was defined solely by Hb level: < 13 g/dl in males and < 12 g/dl in females.Strict anaemia was defined as a Hb level of < 11 g/dl in males and < 10 g/dl in females, or a Hb level of ≥ 11 g/dl but < 13 g/dl in males or ≥ 10 g/dl but < 12 g/dl in females accompanied by microcytosis (MCV < 80 fl/cell) or low ferritin (< 20 µg/l). Broad IDA was defined as a Hb level of < 13 g/dl in males and < 12 g/dl in females accompanied by microcytosis (MCV < 80 fl/cell) or low ferritin (< 20 µg/l) and strict IDA was defined as a Hb level of < 13 g/dl in males and < 12 g/dl in females accompanied by low ferritin (< 20 µg/l).
For patients without blood test data, in the absence of any available full blood counts, we used the anaemia tick box on the SIGGAR trials pro forma to define the presence or absence of anaemia. In the analysis of the overall SOCCER study cohort, anaemia was defined as a Hb level of < 13 g/dl in men or < 12 g/dl in women for patients with blood test data and by using the anaemia tick box on the pro forma for patients without blood test data.
Flexible sigmoidoscopy
Details of FS procedures performed at the time of referral had been recorded on a separate pro forma during the SIGGAR trials and included room entry and exit times; procedure start and stop times; overall assessment of the examination by the endoscopist (‘very easy’, ‘quite easy’, ‘quite difficult’ or ‘very difficult’); assessment of bowel preparation quality by the endoscopist (‘excellent’, ‘good’, ‘adequate’ or ‘poor’); the segment of the colon reached and reasons (if any) the examination could not be completed; overall findings and details of polyps, cancers or biopsies and diverticula (with a severity rating of ‘none’, ‘mild’, ‘moderate’ or ‘severe’); and adverse events occurring during the procedure. Unfortunately, during scrutiny of these records, it was discovered that in many cases the information included had been taken from the electronic endoscopy record and that many items were missing.
Data extraction
Additional pathology, endoscopy, radiology and haematology data were collected from the relevant hospital databases for the SOCCER study patient cohort. When possible, data were bulk extracted; when this was not possible, data were extracted manually, either by staff at participating hospitals or by members of the study team who had been granted permission to do so.
A few databases at participating centres had reporting systems that permitted bulk extraction of the data according to specific criteria. When possible, data were extracted with the help of hospital staff who were familiar with the systems. For most hospital databases, the application interface was not designed for bulk data extraction, so acquiring and processing the data was complex and a number of problems were encountered; for example:
-
When the maintenance and support of the hospital databases had been outsourced to the database manufacturers, often only the manufacturers could help with extracting the data or by writing software enabling the study programmer to do so.
-
Some of the data were held on legacy systems; therefore, specialist support was required to extract data from these systems.
-
Information technology staff at the hospitals sometimes had to restore archived data temporarily so that they could be extracted.
-
Most hospitals had replaced databases over the intervening years and, therefore, some data were overlapping or were duplicated (e.g. records for the same patient were found on more than one system).
-
The data outputs from these databases were in a combination of structured and unstructured formats. Structured data could be cleaned easily and converted into a standardised format for uploading. In the case of unstructured data (usually large text fields), bespoke programs had to be written to extract, clean and convert the data into a suitable format.
Manually collected data
Data were collected manually in the following scenarios.
-
The hospital did not have the facilities or specialists to bulk extract the data for us.
-
The quoted cost for bulk extracting the data obtained from the suppliers of the system was excessive, making manual data collection more cost-effective.
-
It was possible to bulk extract the data only from a data warehouse/reporting system (not the main databases in which the raw data were held) and our findings showed that the data warehouse was not always up to date. In this scenario we collected the data manually from the applications that were linked to the main databases.
-
The hospital was unable to find specialists to help with bulk extraction within our required time frame, so we manually collected the data in order to meet our data collection deadlines.
-
Some hospitals were able to extract the type of test/examination and date but not provide a report. We used this information to identify the records of interest and narrowed down the task of manual data collection to the selected records.
-
The data were held on legacy systems and the hospital did not have a maintenance contract with the suppliers, with the result that there was no option but to extract the data manually.
Study researchers visited hospitals to manually collect data in a bespoke Microsoft Access® database (2010, Microsoft Corporation, Redmond, WA, USA) or spreadsheet which included patient study numbers. Patient identifiers from the SIGGAR trials were held at hospitals and were used to search for patients on hospital databases. De-identified data were returned to the study team, and the study programmer cleaned and uploaded it to a master SOCCER Oracle database (Oracle Database 11g Enterprise Edition, Oracle Corporation, Redwood Shores, CA, USA).
Data handling and quality assurance
The SOCCER database was created to store data in a standardised, structured format using a schema structure similar to the SIGGAR database. To facilitate statistical analysis, the data were classified into quantitative and qualitative variables, ensuring that data from different hospitals were classified in the same way as in the SIGGAR database, as there was wide variation in the raw data (e.g. field names were different, some data were coded or semicoded, whereas other data were in free-text fields, and data types varied).
The study programmer cleaned and uploaded the data from different hospitals into a standard database schema, and this involved several steps:
-
identifying the fields containing information required for the study, taking into account varying field names, data types and value representations
-
extracting information from free-text fields using programming techniques such as ‘regular expressions’ and ‘fuzzy matching’ and translating them into the codes used on the master database
-
translating values in the raw data into those used on the master database, if the information was already in a coded structured format (e.g. converting units for blood tests)
-
identifying and consolidating overlapping data and removing any redundancies (e.g. the same endoscopy or pathology reports extracted from two different systems)
-
identifying and correcting errors in the data (e.g. misspellings, different date formats or truncated data fields)
-
requesting missing data (e.g. missing patients, missing time periods, missing procedure types).
A graphical user interface that linked to the SOCCER database was designed, allowing the study researchers to efficiently read, interpret, check and manually code the endoscopy, pathology and symptoms data sets. Study researchers interrogated and linked the clinical reports and categorised the data in the same way as in the SIGGAR database. Reference data (sometimes referred to as look-up tables) were used to categorise and define permissible values for data fields on the database. This method restricted the values to be recorded in a data field, thereby preventing coding errors and also ensuring uniformity of data from different hospitals. The study researchers systematically reviewed a blinded random sample of records that had been coded by other study researchers to ensure accuracy and consistency.
Health and Social Care Information Centre colorectal cancer diagnoses
Colorectal cancer diagnoses within 3 years of referral were obtained from the HSCIC. A unique study number was allocated to all patients during the SIGGAR trials and the same study number was used for the SOCCER study cohort. This unique study number was used to collect cancer registrations from the HSCIC through their data linkage service. For patients who had not been randomised in the SIGGAR trials, participating hospitals provided the HSCIC with patient identifiers (name, date of birth, NHS number, etc.) to enable data linkage, as identifiers were not held by the central trial office for the non-randomised cohort. Hospital teams worked under instruction of the central trial team to prepare the data in the electronic format specified by the HSCIC. When local assistance was not available to collate the data required by the HSCIC, central trial team staff members were issued with letters of access by the hospitals concerned and visited sites personally to complete this task. For the cohort of patients who were randomised in the SIGGAR trials, the HSCIC already held the records and so no new information needed to be supplied to them. Following data linkage by the HSCIC, the central trial office received cancer registrations from the HSCIC for the full SOCCER study cohort in a de-identified format for analysis, which were linked only by study number.
Statistical methods
Sample size
Our original sample size assumed that we would have a total cohort of 8484 patients, in whom 421 distal cancers and 68 proximal cancers would be diagnosed. The analysis plan presented estimates for the precision for the estimated sensitivity under specific regimens, with the precision being conditional on the number of cancers diagnosed. We assumed that under a regimen offering WCI to patients with IDA and/or an abdominal mass we would detect 470 of the total 489 cancers, giving a sensitivity estimate of 96.1% with a 95% confidence interval (CI) of 94.0% to 97.6%. Although the final analysed cohort of 7380 patients was smaller than the proposed sample size, the number of cancers diagnosed was greater than expected, with a total of 429 distal cancers and 127 proximal cancers, thus providing a greater level of precision than originally estimated.
Primary outcome
The primary outcome was the diagnostic yield of distal or proximal cancer within 3 years of presentation at clinic, by symptom category at presentation. 18,19,23 CRC diagnoses were sourced from the HSCIC and from patient medical records. For cancers confirmed by a hospital pathology report but without corresponding verification by HSCIC, the local pathology report was taken as conclusive evidence of cancer. For the purposes of this study, CRCs included all cancers with International Classification of Diseases and Related Health Problems, Tenth Edition,75 site codes C18–C21 and with an International Classification of Diseases for Oncology, Third Edition,76 morphology code of 8000/3, 8010/3, 8070/3, 8123/3, 8140/2, 8140/3, 8144/3, 8210/3, 8261/2, 8261/3, 8263/2, 8263/3, 8480/3, 8481/3, 8490/3, 8510/3 or 8560/3. CRCs were classified as ‘distal’ if they were located in the anus, rectum, sigmoid colon or descending colon. Cancers located proximal to the descending colon were classed as ‘proximal’. Synchronous distal and proximal CRCs were included as separate cancers in the analysis.
Secondary outcomes
The secondary outcomes were the sensitivity of symptoms and symptom categories for distal and proximal cancer, the percentage of patients with cancer who had distal CRC by symptom and symptom category, the number needed to be examined to diagnose one distal or proximal cancer by symptom and symptom category at presentation, the miss rate for CRC at FS in the subgroup of patients with FS performed at baseline and the prevalence of proximal and distal CRC in the study cohort.
Analysis
Outcomes were first analysed separately in the cohort with blood test data and the cohort without blood test data. The findings in the two cohorts were then compared and outcomes analysed in the total combined cohort.
Sensitivity was calculated as the proportion of CRCs by cancer site (proximal/distal) that were identified by a particular symptom or symptom combination. Specificity was defined as the proportion of patients without CRC by cancer site who presented without a particular symptom/symptom combination.
Diagnostic yields were presented as percentages. The number needed to be examined was calculated as the inverse of the diagnostic yield. Binomial exact 95% CIs were calculated for key outcomes. The distributions of categorical variables (patient characteristics, referral details, symptoms, signs, indications and cancer outcomes) were compared between cohorts using Pearson’s chi-squared test or Fisher’s exact test, as appropriate, and all tests were two-tailed. Comparisons were made between: cohorts with and without blood test data; men and women; patients with distal cancer and patients with proximal cancer; and patients with and without FS performed at the time of referral. Data were analysed using Stata version 13.1 (StataCorp LP, College Station, TX, USA).
Chapter 3 Results
SOCCER patient cohort
In total, 8484 patients were assessed for eligibility for the SIGGAR trials, of whom 5448 were randomised to receive one of three interventions (colonoscopy, barium enema or CT colonography) used in the diagnosis of CRC and 3036 were not randomised (Figure 1; for reasons see Appendix 2). 18,19,23
FIGURE 1.
The SOCCER study profile.
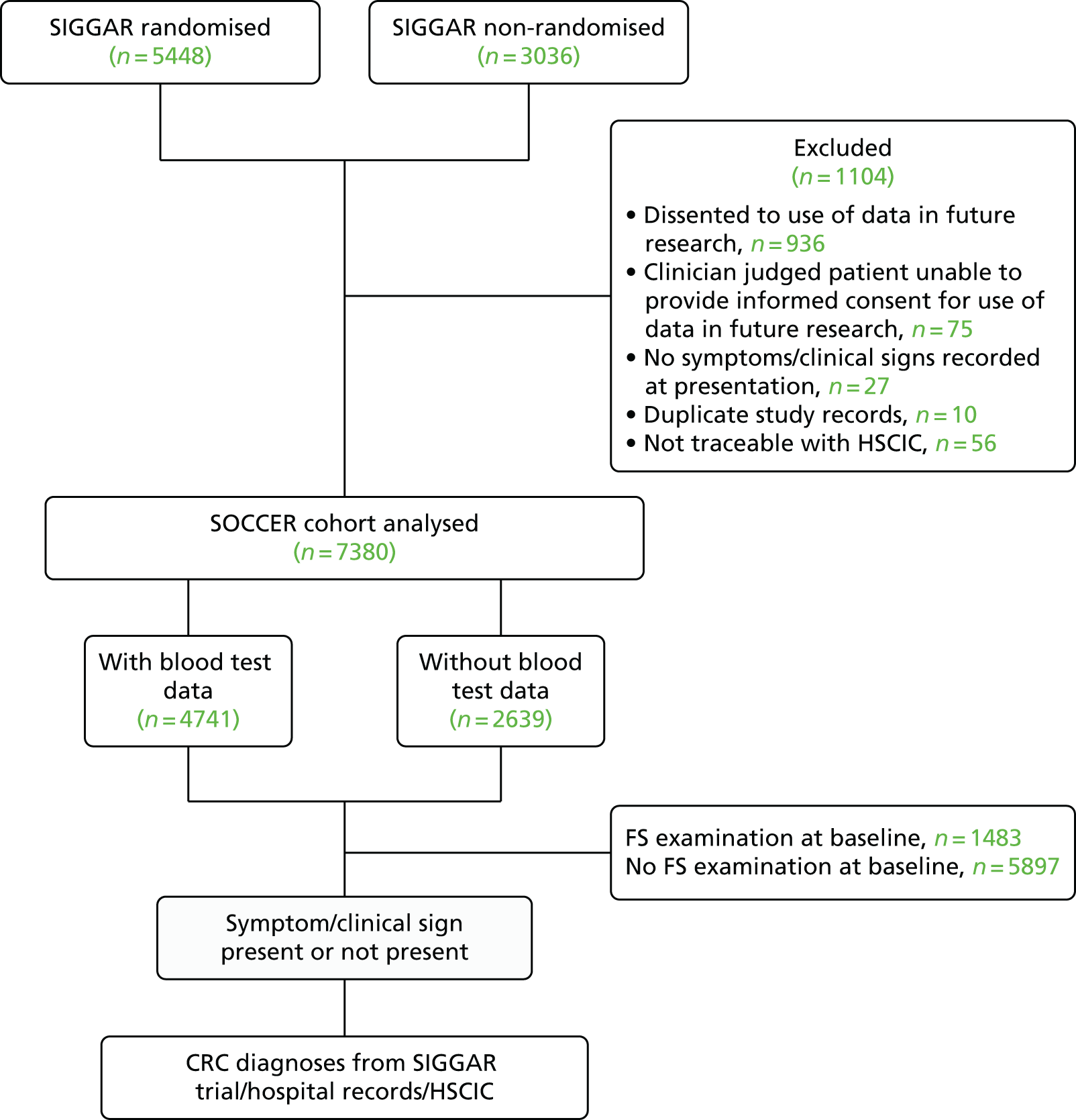
The SOCCER study used data from both randomised and non-randomised patients in the SIGGAR trials. Of 8484 patients, 1104 were excluded from the SOCCER study (see Figure 1). After exclusions, 7380 patients were included in the final cohort to be analysed.
Given the clinical significance attached to anaemia/IDA in CRC diagnosis, and the differences in national and locally defined laboratory parameters used to diagnose this condition, as part of our analyses we investigated the influence on study outcomes of varying the anaemia/IDA definition. These analyses were restricted to those patients for whom laboratory blood test data were available. Of the 7380 patients in the final SOCCER study cohort, blood test data were available for 4741 (64.2%) (see Figure 1).
Baseline characteristics of SOCCER patients overall and those with and without laboratory blood test data
Patient demographics and referral details
Overall, in the full cohort of 7380 patients, there were more women (59.0%) than men (Table 1). The majority of patients were referred via a colorectal outpatient clinic (84.5%). Just over half of all patients (n = 3976) were referred via the suspected cancer 2-week wait pathway, and a further 18% were considered ‘urgent’ referrals (n = 1315). Only 12% (n = 915) of patients in the full cohort were routine referrals.
| Characteristic | Total (N = 7380), n (%) | Cohort, n (%) | p-valuea | |
|---|---|---|---|---|
| With blood test data (N = 4741) | Without blood test data (N = 2639) | |||
| Sex | 0.83 | |||
| Men | 3027 (41.0) | 1949 (41.1) | 1078 (40.8) | |
| Women | 4353 (59.0) | 2792 (58.9) | 1561 (59.2) | |
| Age (years) | < 0.001 | |||
| 55–64 | 2410 (32.7) | 1418 (29.9) | 992 (37.6) | |
| 65–74 | 2739 (37.1) | 1800 (38.0) | 939 (35.6) | |
| 75–84 | 1898 (25.7) | 1288 (27.2) | 610 (23.1) | |
| ≥ 85 | 333 (4.5) | 235 (5.0) | 98 (3.7) | |
| Route of referral | < 0.001 | |||
| Colorectal surgical outpatient clinic | 6235 (84.5) | 3847 (81.1) | 2388 (90.5) | |
| Gastroenterology outpatient clinic | 638 (8.6) | 493 (10.4) | 145 (5.5) | |
| Other outpatient clinic | 50 (0.7) | 44 (0.9) | 6 (0.2) | |
| Straight to test | 396 (5.4) | 309 (6.5) | 87 (3.3) | |
| Hospital admission | 33 (0.4) | 26 (0.6) | 7 (0.3) | |
| Not recorded | 28 (0.4) | 22 (0.5) | 6 (0.2) | |
| Urgency of referral | < 0.001 | |||
| Two-week wait | 3976 (53.9) | 2638 (55.6) | 1338 (50.7) | |
| Urgent | 1315 (17.8) | 754 (15.9) | 561 (21.3) | |
| Soon | 660 (8.9) | 410 (8.7) | 250 (9.5) | |
| Routine | 915 (12.4) | 568 (12.0) | 347 (13.1) | |
| Not recorded | 514 (7.0) | 371 (7.8) | 143 (5.4) | |
Comparing the patients with and without blood test data available, those with blood test data were slightly older, less likely to be referred via a colorectal outpatient clinic and more likely to be referred via a gastroenterology outpatient clinic than patients without blood test data (all p < 0.001). Slightly more patients in the cohort with blood test data were referred via the 2-week-wait pathway than in the cohort without blood test data (p < 0.001).
Colorectal cancer symptoms and clinical signs at presentation
Overall, the most common symptom or clinical sign in the full cohort (n = 7380) was a CIBH: this symptom was reported in > 70% (n = 5382) of all patients (Table 2). Other common features included rectal bleeding (n = 2773), abdominal pain (n = 2126) and weight loss (n = 1148). Overall, the symptom/clinical sign profiles of the cohorts with and without blood test data were similar, although there were some differences. Notably, rectal bleeding and rectal mass were more common in the cohort without blood test results, but weight loss and tiredness/weakness were more common in the cohort with blood test results.
| Symptoms and signs/indications | Total (N = 7380), n (%) | Cohort, n (%) | p-valuea | |
|---|---|---|---|---|
| With blood test data (N = 4741) | Without blood test data (N = 2639) | |||
| Symptoms | ||||
| CIBH | 5382 (72.9) | 3472 (73.2) | 1910 (72.4) | 0.43 |
| Looser and/or more frequent | 2862 (38.8) | 1852 (39.1) | 1010 (38.3) | |
| Harder and/or less frequent | 865 (11.7) | 583 (12.3) | 282 (10.7) | |
| Variable | 648 (8.8) | 418 (8.8) | 230 (8.7) | |
| Unspecified | 1007 (13.6) | 619 (13.1) | 388 (14.7) | |
| Rectal bleeding | 2773 (37.6) | 1660 (35.0) | 1113 (42.2) | < 0.001 |
| Abdominal pain | 2126 (28.8) | 1367 (28.8) | 759 (28.8) | 0.95 |
| Weight loss | 1148 (15.6) | 881 (18.6) | 267 (10.1) | < 0.001 |
| Bloating/flatulence | 203 (2.8) | 131 (2.8) | 72 (2.7) | 0.93 |
| Tiredness/weakness | 152 (2.1) | 114 (2.4) | 38 (1.4) | 0.005 |
| Anal symptoms | 97 (1.3) | 56 (1.2) | 41 (1.5) | 0.18 |
| Nausea/vomiting | 44 (0.6) | 32 (0.7) | 12 (0.4) | 0.24 |
| Back pain | 13 (0.2) | 7 (0.1) | 6 (0.2) | 0.43 |
| Upper GI symptoms | 10 (0.1) | 10 (0.2) | 0 (0) | 0.018 |
| Signs/indications | ||||
| Abdominal mass | 216 (2.9) | 140 (3.0) | 76 (2.9) | 0.86 |
| Rectal mass | 165 (2.2) | 81 (1.7) | 84 (3.2) | < 0.001 |
| FOBt positive | 113 (1.5) | 76 (1.6) | 37 (1.4) | 0.50 |
| Family history | 117 (1.6) | 69 (1.5) | 48 (1.8) | 0.23 |
| History of polyps | 23 (0.3) | 14 (0.3) | 9 (0.3) | 0.74 |
| Other signsb | 16 (0.2) | 11 (0.2) | 5 (0.2) | 0.71 |
Colorectal cancer diagnoses by anatomical subsite
The prevalence of CRC in the whole cohort was 7.5% (Table 3). Overall, distal cancer was diagnosed in 5.8% of patients and proximal cancer was diagnosed in 1.7% of patients. Distal cancer was less common in the cohort with blood test data than in the cohort without (p < 0.001), whereas proximal cancer was more common in the cohort with blood test data (p = 0.007).
| CRCs diagnosed | Total (N = 7380), n (%) | Cohort, n (%) | p-valuea | |
|---|---|---|---|---|
| With blood test data (N = 4741) | Without blood test data (N = 2639) | |||
| Total patients with cancer | 551b (7.5) | 333c (7.0) | 218d (8.3) | 0.053 |
| Distal cancers | ||||
| Total patients with distal cancer | 429b (5.8) | 240c (5.1) | 189d (7.2) | < 0.001 |
| Anus | 10 (0.1) | 6 (0.1) | 4 (0.2) | |
| Rectum | 210 (2.8) | 103 (2.2) | 107 (4.1) | |
| Rectosigmoid | 57 (0.8) | 40 (0.8) | 17 (0.6) | |
| Sigmoid colon | 146 (2.0) | 87 (1.8) | 59 (2.2) | |
| Descending colon | 8 (0.1) | 6 (0.4) | 2 (0.1) | |
| Distal colorectum (no further specification) | 4 (0.1) | 1 (0.02) | 3 (0.1) | |
| Proximal cancers | ||||
| Total patients with proximal cancer | 127b (1.7) | 96c (2.0) | 31d (1.2) | 0.007 |
| Splenic flexure | 9 (0.1) | 9 (0.2) | 0 (0) | |
| Transverse colon | 18 (0.2) | 12 (0.3) | 6 (0.2) | |
| Hepatic flexure | 14 (0.2) | 13 (0.3) | 1 (0.04) | |
| Ascending colon | 36 (0.5) | 27 (0.6) | 9 (0.3) | |
| Caecum | 53 (0.7) | 38 (0.8) | 15 (0.6) | |
Anaemia and iron deficiency anaemia
Of the 4741 patients for whom blood test data at presentation were available, serum ferritin results were available for 1157 (approximately 24%) (Table 4). Among patients for whom ferritin data were available, low levels of serum ferritin (< 20 µg/l) were reported in approximately 31% (n = 353). MCVs were low (< 80 fl) in 9.0% (n = 176) of men and 8.2% (n = 229) of women. In total, 31.8% (n = 256) of men and 36.5% (n = 311) of women with low Hb levels (< 13 g/dl for men and < 12 g/dl for women) had either a low serum ferritin or a low MCV level. Only 1.1% (n = 13) of men and 2.7% (n = 53) of women with normal Hb also had low serum ferritin or MCV.
| Blood test result | Sex, n (%) | |||||
|---|---|---|---|---|---|---|
| Men (n = 1949) | Women (n = 2792) | |||||
| Hb < 11 g/dl (N = 359, 18.4%) | Hb 11–12.9 g/dl (N = 447, 22.9%) | Hb ≥ 13 g/dl (N = 1143, 58.7%) | Hb < 10 g/dl (N = 312, 11.2%) | Hb 10–11.9 g/dl (N = 541, 19.4%) | Hb ≥ 12 g/dl (N = 1939, 69.4%) | |
| Ferritin | ||||||
| Result collected | 190 (52.9) | 148 (33.1) | 155 (13.6) | 182 (58.3) | 201 (37.2) | 281 (14.5) |
| Result lowa | 97 (27.0) | 49 (11.0) | 6 (0.5) | 98 (31.4) | 74 (13.7) | 29 (1.5) |
| MCV lowb | 132 (36.8) | 36 (8.1) | 8 (0.7) | 119 (38.1) | 83 (15.3) | 27 (1.4) |
| Ferritin or MCV low | 184 (51.3) | 72 (16.1) | 13 (1.1) | 179 (57.4) | 132 (24.4) | 53 (2.7) |
| Neither ferritin nor MCV low | 175 (48.7) | 375 (83.9) | 1130 (98.9) | 133 (42.6) | 409 (75.6) | 1886 (97.3) |
Analyses in patients with blood test data
When applying the broadest definition of anaemia (based on low Hb level alone), > 40% (n = 806) of men and 30% (n = 853) of women were anaemic (Table 5). The proportions of men and women who were anaemic decreased as the definitions were tightened to improve specificity for anaemia resulting from iron deficiency, by either reducing the Hb threshold or introducing MCV and/or serum ferritin into the diagnostic criteria. The proportions of men and women with anaemia by the stricter definition were approximately half of those for the broad definition (35.0% vs. 18.5%). Only 12.0% of patients presented with probable IDA (broad definition IDA) and 6.7% presented with laboratory-confirmed IDA, for which iron deficiency was confirmed by low serum ferritin.
| Anaemia definition | Sex, n (%) | Total (N = 4741), n (%) | |
|---|---|---|---|
| Men (N = 1949) | Women (N = 2792) | ||
| Anaemia | |||
| Broad definition anaemia: Hb level of < 13 g/dl in men or < 12 g/dl in women | 806 (41.4) | 853 (30.6) | 1659 (35.0) |
| Strict definition anaemia: Hb level of < 11 g/dl in men or < 10 g/dl in women, or Hb level of ≥ 11 g/dl and < 13 g/dl in men or ≥ 10 g/dl and < 12 g/dl in women accompanied by microcytosis (MCV < 80 fl) or low ferritin (< 20 µg/l) | 431 (22.1) | 444 (15.9) | 875 (18.5) |
| IDA | |||
| Broad definition IDA: Hb level of < 13 g/dl in men or < 12 g/dl in women accompanied by microcytosis (MCV < 80 fl) or low ferritin (< 20 µg/l) | 256 (13.1) | 311 (11.1) | 567 (12.0) |
| Strict definition IDA: Hb level of < 13 g/dl in men or < 12 g/dl in women accompanied by low ferritin (< 20 µg/l) | 146 (7.5) | 172 (6.2) | 318 (6.7) |
Patient demographics and referral details in those with laboratory blood test data
To enable the consideration of anaemia and IDA clinical features, the analyses were restricted to those 4741 patients for whom blood test data were available, of whom 58.9% (n = 2792) were women (Table 6). Over one-third (38%; n = 1800) of patients were aged 65–74 years at presentation, with fewer than 5% (n = 235) aged ≥ 85 years. The proportions of men and women in each age group were similar, and the median age was 70 years (interquartile range 63–77 years) for both men and women. More than 80% (n = 3847) of patients were referred to colorectal surgical outpatient clinics and approximately 10% (n = 493) were referred to gastroenterology outpatient clinics. Over 50% of patients (n = 2638) were referred via the suspected cancer 2-week wait pathway and a further 16% were ‘urgent’ referrals (n = 754). Only 12% (n = 568) of patients in the cohort with blood test data were routine referrals.
| Characteristic | Sex, n (%) | p-value | |
|---|---|---|---|
| Men (N = 1949) | Women (N = 2792) | ||
| Age (years) | 0.10 | ||
| 55–64 | 551 (28.3) | 867 (31.1) | |
| 65–74 | 772 (39.6) | 1028 (36.8) | |
| 75–84 | 536 (27.5) | 752 (26.9) | |
| ≥ 85 | 90 (4.6) | 145 (5.2) | |
| Route of referral | 0.50 | ||
| Colorectal surgical outpatient clinic | 1587 (81.4) | 2260 (80.9) | |
| Gastroenterology outpatient clinic | 199 (10.2) | 294 (10.5) | |
| Other outpatient clinic | 18 (0.9) | 26 (0.9) | |
| Straight to test | 128 (6.6) | 181 (6.5) | |
| Hospital admission | 6 (0.3) | 20 (0.7) | |
| Not recorded | 11 (0.6) | 11 (0.4) | |
| Urgency of referral | 0.48 | ||
| Two-week wait | 1076 (55.2) | 1562 (56.0) | |
| Urgent | 332 (17.0) | 422 (15.1) | |
| Soon | 165 (8.5) | 245 (8.8) | |
| Routine | 230 (11.8) | 338 (12.1) | |
| Not recorded | 146 (7.5) | 225 (8.1) | |
Colorectal cancer symptoms and clinical signs by sex and age
Clinical features that were more common in women than in men were CIBH (all subtypes with the exception of harder stools and/or less frequent defecation) (p < 0.001), abdominal pain (p < 0.001) and abdominal mass (p = 0.029) (Table 7). Rectal bleeding was more common in men than in women (p < 0.001). Similarly, more men than women presented with anaemia (p < 0.001) and IDA by the broad definition (p = 0.037).
| Symptoms and signs/indications | Sex, n (%) | p-value | |
|---|---|---|---|
| Men (N = 1949) | Women (N = 2792) | ||
| Symptoms | |||
| CIBH | 1352 (69.4) | 2120 (75.9) | < 0.001 |
| Looser and/or more frequent | 721 (37.0) | 1131 (40.5) | |
| Harder and/or less frequent | 259 (13.3) | 324 (11.6) | |
| Variable | 133 (6.8) | 285 (10.2) | |
| Unspecified | 239 (12.3) | 380 (13.6) | |
| Rectal bleeding | 744 (38.2) | 916 (32.8) | < 0.001 |
| Abdominal pain | 448 (23.0) | 919 (32.9) | < 0.001 |
| Weight loss | 386 (19.8) | 495 (17.7) | 0.071 |
| Bloating/flatulence | 51 (2.6) | 80 (2.9) | 0.61 |
| Tiredness/weakness | 51 (2.6) | 63 (2.3) | 0.43 |
| Anal symptoms | 23 (1.2) | 33 (1.2) | 0.99 |
| Nausea/vomiting | 8 (0.4) | 24 (0.9) | 0.072 |
| Back pain | 2 (0.1) | 5 (0.2) | 0.71 |
| Upper GI symptoms | 2 (0.1) | 8 (0.3) | 0.21 |
| Signs/indications | |||
| Anaemia | |||
| Anaemia (broad)a | 806 (41.4) | 853 (30.6) | < 0.001 |
| Anaemia (strict)b | 431 (22.1) | 444 (15.9) | < 0.001 |
| IDA (broad)c | 256 (13.1) | 311 (11.1) | 0.037 |
| IDA (strict)d | 146 (7.5) | 172 (6.2) | 0.072 |
| Abdominal mass | 45 (2.3) | 95 (3.4) | 0.029 |
| Rectal mass | 28 (1.4) | 53 (1.9) | 0.228 |
| FOBt positive | 31 (1.6) | 45 (1.6) | 0.95 |
| Family history | 21 (1.1) | 48 (1.7) | 0.070 |
| History of polyps | 10 (0.5) | 4 (0.1) | 0.028 |
| Other signse | 6 (0.3) | 5 (0.2) | 0.38 |
The proportions of men and women with rectal bleeding or abdominal pain decreased with increasing age (Table 8). Anaemia was substantially more common in older age groups in both men and women; 73% of men (n = 66) and more than half of women (n = 83) aged ≥ 85 years were anaemic (broad definition), compared with 24% of men (n = 131) and 19% of women (n = 161) aged 55–64 years. Similar trends with age for men and women were observed for all other definitions of anaemia and IDA.
| Symptoms and signs/indications | Sex, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 1949) | Women (n = 2792) | |||||||
| 55–64 years (N = 551) | 65–74 years (N = 772) | 75–84 years (N = 536) | ≥ 85 years (N = 90) | 55–64 years (N = 867) | 65–74 years (N = 1028) | 75–84 years (N = 752) | ≥ 85 years (N = 145) | |
| Symptoms | ||||||||
| CIBH | 382 (69.3) | 531 (68.8) | 365 (68.1) | 74 (82.2) | 679 (78.3) | 795 (77.3) | 546 (72.6) | 100 (69.0) |
| Looser and/or more frequent | 238 (43.2) | 261 (33.8) | 186 (34.7) | 36 (40.0) | 383 (44.2) | 418 (40.7) | 288 (38.3) | 42 (29.0) |
| Harder and/or less frequent | 49 (8.9) | 104 (13.5) | 83 (15.5) | 23 (25.6) | 79 (9.1) | 118 (11.5) | 109 (14.5) | 18 (12.4) |
| Variable | 26 (4.7) | 66 (8.6) | 37 (6.9) | 4 (4.4) | 92 (10.6) | 112 (10.9) | 65 (8.6) | 16 (11.0) |
| Unspecified | 69 (12.5) | 100 (12.9) | 59 (11.0) | 11 (12.2) | 125 (14.4) | 147 (14.3) | 84 (11.2) | 24 (16.6) |
| Rectal bleeding | 245 (44.5) | 290 (37.6) | 184 (34.3) | 25 (27.8) | 328 (37.8) | 338 (32.9) | 217 (28.9) | 33 (22.8) |
| Abdominal pain | 161 (29.2) | 176 (22.8) | 103 (19.2) | 8 (8.9) | 315 (36.3) | 357 (34.7) | 216 (28.7) | 31 (21.4) |
| Weight loss | 99 (18.0) | 132 (17.1) | 125 (23.3) | 30 (33.3) | 113 (13.0) | 170 (16.5) | 182 (24.2) | 30 (20.7) |
| Bloating/flatulence | 22 (4.0) | 24 (3.1) | 4 (0.8) | 1 (1.1) | 25 (2.9) | 32 (3.1) | 20 (2.7) | 3 (2.1) |
| Tiredness/weakness | 12 (2.2) | 21 (2.7) | 17 (3.2) | 1 (1.1) | 10 (1.2) | 29 (2.8) | 21 (2.8) | 2 (2.1) |
| Anal symptoms | 9 (1.6) | 10 (1.3) | 3 (0.6) | 1 (1.1) | 10 (1.2) | 15 (1.5) | 8 (1.1) | 0 (0) |
| Nausea/vomiting | 2 (0.4) | 2 (0.3) | 4 (0.8) | 0 (0) | 5 (0.6) | 9 (0.9) | 9 (1.2) | 1 (0.7) |
| Back pain | 1 (0.2) | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.1) | 2 (0.2) | 2 (0.3) | 0 (0) |
| Upper GI symptoms | 1 (0.2) | 1 (0.1) | 0 (0) | 0 (0) | 2 (0.2) | 4 (0.4) | 1 (0.1) | 1 (0.7) |
| Signs/indications | ||||||||
| Anaemia | ||||||||
| Anaemia (broad)a | 131 (23.8) | 314 (40.7) | 295 (55.0) | 66 (73.3) | 161 (18.6) | 280 (27.2) | 329 (43.7) | 83 (57.2) |
| Anaemia (strict)b | 74 (13.4) | 164 (21.2) | 156 (29.1) | 37 (41.1) | 86 (9.9) | 145 (14.1) | 167 (22.2) | 46 (31.7) |
| IDA (broad)c | 47 (8.5) | 108 (14.0) | 79 (14.7) | 22 (24.4) | 68 (7.8) | 105 (10.2) | 114 (15.2) | 24 (16.6) |
| IDA (strict)d | 27 (4.9) | 63 (8.2) | 49 (9.1) | 7 (7.8) | 39 (4.5) | 61 (5.9) | 58 (7.7) | 14 (9.7) |
| Abdominal mass | 12 (2.2) | 20 (2.6) | 10 (1.9) | 3 (3.3) | 18 (2.1) | 34 (3.3) | 27 (3.6) | 16 (11.0) |
| Rectal mass | 10 (1.8) | 8 (1.0) | 8 (1.5) | 2 (2.2) | 14 (1.6) | 14 (1.4) | 14 (1.9) | 11 (7.6) |
| FOBt positive | 3 (0.5) | 13 (1.7) | 13 (2.4) | 2 (2.2) | 13 (1.5) | 18 (1.7) | 13 (1.7) | 1 (0.7) |
| Family history | 8 (1.5) | 10 (1.3) | 3 (0.6) | 0 (0) | 23 (2.7) | 15 (1.5) | 7 (0.9) | 3 (2.1) |
| History of polyps | 3 (0.5) | 4 (0.5) | 3 (0.6) | 0 (0) | 1 (0.1) | 2 (0.2) | 1 (0.1) | 0 (0) |
| Other signse | 3 (0.5) | 0 (0) | 3 (0.6) | 0 (0) | 2 (0.2) | 2 (0.2) | 1 (0.1) | 0 (0) |
The proportions of patients presenting with a CIBH (all subtypes combined) were also influenced by age; however, the trends observed for men and women were not the same. A CIBH was more commonly reported in men aged ≥ 85 years (82.2%) than in men aged 55–64 years (69.3%) but was less common in older women (69% in those aged ≥ 85 years vs. 78.3% in those aged 55–64 years). The trend in men was largely a result of the increase in the proportion of men with harder stools and/or less frequent defecation with increasing age (8.9% in those aged 55–64 years vs. 25.6% in those aged ≥ 85 years), whereas fewer women reported a CIBH to looser and/or more frequent stools with increasing age (44.2% in those aged 55–64 years vs. 29.0% in those aged ≥ 85 years).
There was evidence of some association between age, gender and the presence of other less common clinical features, although in many cases the numbers available for analysis by age group were low. In women, but not in men, the presence of an abdominal mass as a reported clinical sign increased with increasing age. An abdominal mass was reported by 11.0% of women (n = 16) aged ≥ 85 years, compared with 2.1% of women (n = 18) aged 55–64 years. Similarly, there was evidence to suggest that a rectal mass was more commonly reported in older women (7.6% in women aged ≥ 85 years vs. 1.6% in those aged 55–64 years).
Colorectal cancer symptoms and clinical signs in isolation and in combination
Approximately 95% of patients (n = 4486) presented with between one and three features that are referral criteria in the NICE 2015 guidelines76 for suspected CRC; just over one-third (n = 1626) presented with a single NICE criteria symptom/sign (Table 9).
| Symptoms and signs/indications | Total, N | Number of NICE 2015 guideline symptoms or signs per patienta | ||||||
|---|---|---|---|---|---|---|---|---|
| 0, n (%) | 1, n (%) | 2, n (%) | 3, n (%) | 4, n (%) | 5, n (%) | 6, n (%) | ||
| Total | 4741 | 16 (0.3) | 1626 (34.3) | 1936 (40.8) | 923 (19.5) | 209 (4.4) | 30 (0.6) | 1 (0.0) |
| Symptoms | ||||||||
| CIBH | 3472 | 0 (0.0) | 903 (26.0) | 1522 (43.8) | 818 (23.6) | 198 (5.7) | 30 (0.9) | 1 (0.0) |
| Looser and/or more frequent | 1852 | 0 (0.0) | 521 (28.1) | 791 (42.7) | 417 (22.5) | 107 (5.8) | 15 (0.8) | 1 (0.1) |
| Harder and/or less frequent | 583 | 0 (0.0) | 96 (16.5) | 267 (45.8) | 172 (29.5) | 39 (6.7) | 9 (1.5) | 0 (0.0) |
| Variable | 418 | 0 (0.0) | 111 (26.6) | 197 (47.1) | 89 (21.3) | 20 (4.8) | 1 (0.2) | 0 (0.0) |
| Unspecified | 619 | 0 (0.0) | 175 (28.3) | 267 (43.1) | 140 (22.6) | 32 (5.2) | 5 (0.8) | 0 (0.0) |
| Rectal bleeding | 1660 | 0 (0.0) | 286 (17.2) | 733 (44.2) | 481 (29.0) | 137 (8.2) | 22 (1.3) | 1 (0.1) |
| Abdominal pain | 1367 | 0 (0.0) | 108 (7.9) | 629 (46.0) | 467 (34.2) | 135 (9.9) | 27 (2.0) | 1 (0.1) |
| Weight loss | 881 | 0 (0.0) | 11 (1.2) | 310 (35.2) | 379 (43.0) | 152 (17.3) | 28 (3.2) | 1 (0.1) |
| Bloating/flatulence | 131 | 3 (2.3) | 41 (31.3) | 57 (43.5) | 24 (18.3) | 6 (4.6) | 0 (0.0) | 0 (0.0) |
| Tiredness/weakness | 114 | 3 (2.6) | 16 (14.0) | 41 (36.0) | 32 (28.1) | 18 (15.8) | 4 (3.5) | 0 (0.0) |
| Anal symptoms | 56 | 1 (1.8) | 23 (41.1) | 17 (30.4) | 13 (23.2) | 2 (3.6) | 0 (0.0) | 0 (0.0) |
| Nausea/vomiting | 32 | 0 (0.0) | 7 (21.9) | 10 (31.3) | 12 (37.5) | 3 (9.4) | 0 (0.0) | 0 (0.0) |
| Back pain | 7 | 0 (0.0) | 1 (14.3) | 2 (28.6) | 3 (42.9) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Upper GI symptoms | 10 | 0 (0.0) | 3 (30.0) | 4 (40.0) | 1 (10.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) |
| Signs/indications | ||||||||
| Anaemia | ||||||||
| Anaemia (broad)b | 1659 | 0 (0.0) | 307 (18.5) | 606 (36.5) | 544 (32.8) | 173 (10.4) | 28 (1.7) | 1 (0.1) |
| Anaemia (strict)c | 875 | 0 (0.0) | 243 (27.8) | 306 (35.0) | 235 (26.9) | 78 (8.9) | 12 (1.4) | 1 (0.1) |
| IDA (broad)d | 567 | 0 (0.0) | 206 (36.3) | 181 (31.9) | 128 (22.6) | 46 (8.1) | 5 (0.9) | 1 (0.2) |
| IDA (strict)e | 318 | 0 (0.0) | 135 (42.5) | 91 (28.6) | 69 (21.7) | 21 (6.6) | 2 (0.6) | 0 (0.0) |
| Abdominal mass | 140 | 0 (0.0) | 2 (1.4) | 41 (29.3) | 53 (37.9) | 32 (22.9) | 11 (7.9) | 1 (0.7) |
| Rectal mass | 81 | 0 (0.0) | 9 (11.1) | 31 (38.3) | 27 (33.3) | 9 (11.1) | 5 (6.2) | 0 (0.0) |
| FOBt positive | 76 | 9 (11.8) | 32 (42.1) | 24 (31.6) | 10 (13.2) | 1 (1.3) | 0 (0.0) | 0 (0.0) |
| Family history | 69 | 1 (1.4) | 30 (43.5) | 27 (39.1) | 8 (11.6) | 3 (4.3) | 0 (0.0) | 0 (0.0) |
| History of polyps | 14 | 0 (0.0) | 6 (42.9) | 5 (35.7) | 3 (21.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other signsf | 11 | 2 (18.2) | 1 (9.1) | 4 (36.4) | 4 (36.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
The NICE qualifying features most commonly reported as single symptoms were a CIBH and rectal bleeding. For NICE criteria signs, between 36% and 43% of patients (depending on the definition used) presenting with IDA did so in the absence of any other NICE criteria. The proportions of patients presenting with anaemia/IDA in the absence of other NICE criteria symptoms/signs increased as the definition used for anaemia became stricter through inclusion of a requirement for iron deficiency.
The majority (67.4%) of the 3472 patients with a CIBH presented with this symptom in combination with one or two other NICE qualifying features. Similarly, the majority of patients (73.2%) with rectal bleeding presented with this symptom in combination with one or two other NICE qualifying features. Few patients with weight loss (1.2%) or abdominal pain (7.9%) presented without additional NICE qualifying features. Very few patients (n = 16) with features that were non-NICE qualifying presented without additional NICE criteria.
Patients with NICE qualifying features commonly presented with symptoms in combination with a CIBH, which probably reflected the high prevalence of this symptom in the cohort (73% overall prevalence) (Table 10). For instance, > 60% of patients (n = 1022) with rectal bleeding presented with a CIBH.
| Symptoms and signs/indications | Total, n | Occurrence of any additional NICE 2015 guideline symptoms or signsa | ||||||
|---|---|---|---|---|---|---|---|---|
| CIBH, n | Rectal bleeding, n | Abdominal pain, n | Weight loss, n | Anaemia (broad),b n | Abdominal mass, n | Rectal mass, n | ||
| Symptoms | ||||||||
| CIBH | 3472 | – | 1022 | 1032 | 698 | 989 | 92 | 45 |
| Rectal bleeding | 1660 | 1022 | – | 378 | 227 | 501 | 32 | 40 |
| Abdominal pain | 1367 | 1032 | 378 | – | 261 | 348 | 53 | 10 |
| Weight loss | 881 | 698 | 227 | 261 | – | 398 | 47 | 10 |
| Bloating/flatulence | 131 | 107 | 23 | 57 | 27 | 32 | 3 | 2 |
| Tiredness/weakness | 114 | 95 | 22 | 38 | 61 | 51 | 16 | 3 |
| Anal symptoms | 56 | 34 | 32 | 11 | 9 | 13 | 1 | 4 |
| Nausea/vomiting | 32 | 24 | 5 | 15 | 16 | 14 | 1 | 0 |
| Back pain | 7 | 6 | 3 | 2 | 4 | 3 | 0 | 1 |
| Upper GI symptoms | 10 | 9 | 2 | 4 | 3 | 4 | 1 | 0 |
| Signs/indications | ||||||||
| Anaemia | ||||||||
| Anaemia (broad)b | 1659 | 989 | 501 | 348 | 398 | – | 68 | 27 |
| Anaemia (strict)c | 875 | 430 | 231 | 153 | 202 | – | 34 | 13 |
| IDA (broad)d | 567 | 238 | 133 | 86 | 116 | – | 22 | 5 |
| IDA (strict)e | 318 | 119 | 69 | 44 | 56 | – | 7 | 5 |
| Abdominal mass | 140 | 92 | 32 | 53 | 47 | 68 | – | 1 |
| Rectal mass | 81 | 45 | 40 | 10 | 10 | 27 | 1 | – |
| FOBt positive | 76 | 35 | 11 | 18 | 16 | 34 | 0 | 0 |
| Family history | 69 | 50 | 29 | 20 | 7 | 12 | 1 | 1 |
| History of polyps | 14 | 7 | 8 | 4 | 0 | 6 | 0 | 0 |
| Other signsf | 11 | 8 | 2 | 3 | 5 | 3 | 0 | 0 |
Patients with anaemia/IDA commonly presented with these signs in combination with a CIBH, rectal bleeding and weight loss. Sixty per cent (n = 989) of patients with anaemia (broad definition) also had a CIBH, compared with 37% (n = 119) of patients with IDA (strict definition) (see Table 10).
Patient demographics and referral details in those with colorectal cancer diagnoses
Distal cancers were more likely to be diagnosed in men and proximal cancers were more likely to be diagnosed in women (p = 0.013) (Table 11). There were no major differences in subsite diagnoses by referral route (p = 0.21) or urgency of referral (p = 0.62).
| Characteristic | Cancer, n (%) | p-valuea | |
|---|---|---|---|
| Distal (N = 240) | Proximal (N = 96) | ||
| Sex | 0.013 | ||
| Men | 145 (60.4) | 44 (45.8) | |
| Women | 95 (39.6) | 52 (54.2) | |
| Age (years) | 0.44 | ||
| 55–64 | 54 (22.5) | 14 (14.6) | |
| 65–74 | 91 (37.9) | 39 (40.6) | |
| 75–84 | 80 (33.3) | 35 (36.5) | |
| ≥ 85 | 15 (6.3) | 8 (8.3) | |
| Route of referral | 0.21 | ||
| Colorectal surgical outpatient clinic | 198 (82.5) | 81 (84.4) | |
| Gastroenterology outpatient clinic | 22 (9.2) | 10 (10.4) | |
| Other outpatient clinic | 3 (1.2) | 2 (2.1) | |
| Straight to test | 17 (7.1) | 2 (2.1) | |
| Hospital admission | 0 (0) | 1 (1.0) | |
| Urgency of referral | 0.62 | ||
| Two-week wait | 169 (70.4) | 62 (64.6) | |
| Urgent | 29 (12.1) | 17 (17.7) | |
| Soon | 13 (5.4) | 7 (7.3) | |
| Routine | 12 (5.0) | 5 (5.2) | |
| Not recorded | 17 (7.1) | 5 (5.2) | |
Prevalence of distal colorectal and proximal colon cancers by age and sex
In stratified analyses, there were apparent trends in the prevalence of distal and proximal cancer by age and sex (Table 12). In women, the prevalence of distal cancer increased with age; 5.5% of women (n = 8) aged ≥ 85 years were diagnosed with distal cancer, compared with 2.2% of women (n = 19) aged 55–64 years. This trend in distal cancer prevalence by age was less apparent in men; however, distal cancer was more prevalent in men aged ≥ 65 years than in those aged 55–64 years. The prevalence of proximal cancer increased with increasing age in both men and women.
| CRC subsite | Sex, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 1949) | Women (n = 2792) | |||||||
| 55–64 years (N = 551) | 65–74 years (N = 772) | 75–84 years (N = 536) | ≥ 85 years (N = 90) | 55–64 years (N = 867) | 65–74 years (N = 1028) | 75–84 years (N = 752) | ≥ 85 years (N = 145) | |
| Distal | 35 (6.4) | 59 (7.6) | 44 (8.2) | 7 (7.8) | 19 (2.2) | 32 (3.1) | 36 (4.8) | 8 (5.5) |
| Proximal | 9 (1.6) | 15 (1.9) | 17 (3.2) | 3 (3.3) | 5 (0.6) | 24 (2.3) | 18 (2.4) | 5 (3.4) |
Symptomatic presentation of colorectal cancers by subsite
The symptom that was most commonly associated with distal cancer was a CIBH (Table 13), which was reported in 71.7% of distal CRC patients either as a single symptom or in combination with other clinical features. Looser and/or more frequent stools was the most commonly reported CIBH in those with distal cancer. Rectal bleeding was reported by 64.2% of those with distal cancer (n = 154).
| Characteristica | Cancer, n (%) | p-valueb | |
|---|---|---|---|
| Distal (N = 240) | Proximal (N = 96) | ||
| Symptoms | |||
| CIBH | 172 (71.7) | 52 (54.2) | 0.002 |
| Looser and/or more frequent | 97 (40.4) | 26 (27.1) | |
| Harder and/or less frequent | 28 (11.7) | 12 (12.5) | |
| Variable | 12 (5.0) | 5 (5.2) | |
| Unspecified | 35 (14.6) | 9 (9.4) | |
| Rectal bleeding | 154 (64.2) | 20 (20.8) | < 0.001 |
| Abdominal pain | 51 (21.3) | 33 (34.4) | 0.012 |
| Weight loss | 50 (20.8) | 24 (25.0) | 0.50 |
| Bloating/flatulence | 6 (2.5) | 3 (3.1) | 0.73 |
| Tiredness/weakness | 7 (2.9) | 4 (4.2) | 0.51 |
| Anal symptoms | 1 (0.4) | 0 (0) | 0.99 |
| Nausea/vomiting | 3 (1.2) | 0 (0) | 0.56 |
| Signs/indications | |||
| Anaemia | |||
| Anaemia (broad)c | 106 (44.2) | 77 (80.2) | < 0.001 |
| Anaemia (strict)d | 52 (21.7) | 60 (62.5) | < 0.001 |
| IDA (broad)e | 36 (15.0) | 48 (50.0) | < 0.001 |
| IDA (strict)f | 12 (5.0) | 20 (20.8) | < 0.001 |
| Abdominal mass | 10 (4.2) | 8 (8.3) | 0.17 |
| Rectal mass | 15 (6.3) | 0 (0) | 0.008 |
| FOBt positive | 6 (2.5) | 2 (2.1) | 0.99 |
| Family history | 3 (1.2) | 2 (2.1) | 0.62 |
| History of polyps | 0 (0) | 1 (1.0) | 0.28 |
Anaemia, by the broad and strict definitions, was the most frequently reported clinical sign for proximal cancer, reported in 80.2% and 62.5%, respectively, of proximal cancer patients. Anaemia was also reported frequently in patients with distal cancer (44.2% for the broad definition). The sensitivities of IDA for proximal cancer by the broad (50.0%) and strict (20.8%) definitions were lower than those of anaemia.
The NICE qualifying features that were more frequently reported by patients with distal cancer than by those with proximal cancer were a CIBH (p = 0.002), rectal bleeding (p < 0.001) and rectal mass (p = 0.008). By contrast, abdominal pain (p = 0.012) and anaemia/IDA (p < 0.001) were more frequently reported by patients with proximal cancer than by patients with distal cancer (see Table 13).
Symptomatic presentation of proximal colon cancer and distal colorectal cancer diagnoses by clinical features in isolation and in combination
A small proportion (9.3%; n = 16) of the 172 distal cancers diagnosed in patients with a CIBH were diagnosed in those with a CIBH alone (Table 14), in comparison to a high proportion (59.9%; n = 103) diagnosed among individuals who also reported rectal bleeding. Of the 52 proximal cancers diagnosed in patients with a CIBH, only four (7.7%) were diagnosed in those with a CIBH alone and 11 (21.2%) in those who also had rectal bleeding. However, when anaemia was also reported, 35 (67.3%) of these proximal cancers were captured.
| Symptoms and signs/indications | Total | Occurrence of any additional NICE 2015 guideline symptoms or signsa | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | CIBH | Rectal bleeding | Abdominal pain | Weight loss | Anaemia (broad)b | Abdominal mass | Rectal mass | ||||||||||||
| N | Distal, n | Proximal, n | Distal, n | Proximal, n | Distal, n | Proximal, n | Distal, n | Proximal, n | Distal, n | Proximal, n | Distal, n | Proximal, n | Distal, n | Proximal, n | Distal, n | Proximal, n | Distal, n | Proximal, n | |
| Symptoms | |||||||||||||||||||
| CIBH | 3472 | 172 | 52 | 16 | 4 | – | – | 103 | 11 | 40 | 22 | 37 | 16 | 67 | 35 | 8 | 5 | 7 | 0 |
| Rectal bleeding | 1660 | 154 | 20 | 19 | 2 | 103 | 11 | – | – | 26 | 5 | 23 | 5 | 59 | 14 | 2 | 3 | 13 | 0 |
| Abdominal pain | 1367 | 51 | 33 | 1 | 0 | 40 | 22 | 26 | 5 | – | – | 16 | 10 | 21 | 22 | 5 | 6 | 0 | 0 |
| Weight loss | 881 | 50 | 24 | 0 | 0 | 37 | 16 | 23 | 5 | 16 | 10 | – | – | 31 | 21 | 4 | 3 | 0 | 0 |
| Bloating/flatulence | 131 | 6 | 3 | 0 | 0 | 4 | 3 | 3 | 0 | 2 | 0 | 1 | 1 | 2 | 2 | 1 | 0 | 0 | 0 |
| Tiredness/weakness | 114 | 7 | 4 | 0 | 0 | 6 | 4 | 3 | 1 | 1 | 2 | 6 | 1 | 5 | 4 | 2 | 1 | 0 | 0 |
| Anal symptoms | 56 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea/vomiting | 32 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 |
| Back pain | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Upper GI symptoms | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Signs/indications | |||||||||||||||||||
| Anaemia | |||||||||||||||||||
| Anaemia (broad)b | 1659 | 106 | 77 | 8 | 22 | 67 | 35 | 59 | 14 | 21 | 22 | 31 | 21 | – | – | 5 | 7 | 3 | 0 |
| Anaemia (strict)c | 875 | 52 | 60 | 6 | 20 | 32 | 27 | 30 | 9 | 10 | 16 | 18 | 13 | – | – | 3 | 5 | 1 | 0 |
| IDA (broad)d | 567 | 36 | 48 | 6 | 18 | 20 | 21 | 21 | 5 | 7 | 13 | 11 | 10 | – | – | 1 | 5 | 1 | 0 |
| IDA (strict)e | 318 | 12 | 20 | 2 | 8 | 7 | 8 | 8 | 1 | 5 | 4 | 3 | 5 | – | – | 0 | 0 | 1 | 0 |
| Abdominal mass | 140 | 10 | 8 | 0 | 0 | 8 | 5 | 2 | 3 | 5 | 6 | 4 | 3 | 5 | 7 | – | – | 0 | 0 |
| Rectal mass | 81 | 15 | 0 | 1 | 0 | 7 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | – | – |
| FOBt positive | 76 | 6 | 2 | 0 | 0 | 3 | 0 | 1 | 0 | 2 | 1 | 2 | 0 | 5 | 2 | 0 | 0 | 0 | 0 |
| Family history | 69 | 3 | 2 | 0 | 0 | 3 | 2 | 3 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| History of polyps | 14 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Other signsf | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abdominal pain and weight loss as single clinical features were not predictive of either distal or proximal cancer (see Table 14). The majority of distal cancers in patients with abdominal pain (78.4%) and weight loss (74.0%) were diagnosed in those who also had a CIBH. Furthermore, the majority of proximal cancers in those with abdominal pain (n = 22) were in patients who also had a CIBH (66.7%) or anaemia (66.7%). Similarly, the majority of proximal cancers in those with weight loss occurred in patients who had this symptom in combination with a CIBH (66.7%) or anaemia (87.5%). No cancers were diagnosed in patients with weight loss alone.
Anaemia was notable out of all the NICE qualifying features for indicating a high ratio of proximal to distal cancers, which was in contrast to any other NICE guideline symptom (see Table 14). The ratios of proximal to distal cancer increased as the definitions for anaemia/IDA were tightened. The highest ratio was observed in patients with IDA by the strict definition (20 proximal cancers vs. 12 distal cancers). By contrast, anaemia by the broadest definition was not as specific for proximal cancer (77 proximal cancers vs. 106 distal cancers). Out of the 77 proximal cancers found in patients with anaemia (broad definition), 22 (28.6%) were found in patients with no other NICE qualifying features. Similarly, 8 out of 20 (40.0%) proximal cancers in patients with IDA were diagnosed in those who had this sign alone. Relative yields of distal cancers were higher in patients with anaemia/IDA (by any definition used) in combination with rectal bleeding. Using the broad definition of anaemia, 59 distal cancers were diagnosed in patients who also reported rectal bleeding, compared with 14 proximal cancers.
The NICE qualifying symptom associated with the highest proportionate number of patients diagnosed with distal cancer versus proximal cancer, regardless of other presenting clinical features, was rectal bleeding: when patients presented with this symptom, 89.5% of those diagnosed with cancer had distal cancer (Table 15).
| Symptoms and signs/indications | Total patients with CRC,a n | Patients with cancer, n | Percentage of patients with cancer who have distal cancer | |
|---|---|---|---|---|
| Distal | Proximal | |||
| Total | 333 | 240 | 96 | 72 |
| Symptoms | ||||
| CIBH | 222 | 172 | 52 | 77 |
| Looser and/or more frequent | 121 | 97 | 26 | 80 |
| Harder and/or less frequent | 40 | 28 | 12 | 70 |
| Variable | 17 | 12 | 5 | 71 |
| Unspecified | 44 | 35 | 9 | 80 |
| Rectal bleeding | 172 | 154 | 20 | 90 |
| Abdominal pain | 83 | 51 | 33 | 61 |
| Weight loss | 72 | 50 | 24 | 69 |
| Bloating/flatulence | 9 | 6 | 3 | 67 |
| Tiredness/weakness | 11 | 7 | 4 | 64 |
| Anal symptoms | 1 | 1 | 0 | 100 |
| Nausea/vomiting | 3 | 3 | 0 | 100 |
| Back pain | 0 | 0 | 0 | – |
| Upper GI symptoms | 0 | 0 | 0 | – |
| Signs/indications | ||||
| Anaemia | ||||
| Anaemia (broad)b | 181 | 106 | 77 | 59 |
| Anaemia (strict)c | 111 | 52 | 60 | 47 |
| IDA (broad)d | 83 | 36 | 48 | 43 |
| IDA (strict)e | 32 | 12 | 20 | 37 |
| Abdominal mass | 18 | 10 | 8 | 56 |
| Rectal mass | 15 | 15 | 0 | 100 |
| FOBt positive | 8 | 6 | 2 | 75 |
| Family history | 5 | 3 | 2 | 60 |
| History of polyps | 1 | 0 | 1 | 0 |
| Other signsf | 0 | 0 | 0 | – |
Rectal bleeding was examined in further detail as a symptom alone and in combination with other symptoms (Table 16). Among patients presenting with rectal bleeding alone, 89.5% (n = 17) of those diagnosed with cancer had a distal tumour. When rectal bleeding and a CIBH were reported together in the absence of anaemia, 95.8% (n = 69) of patients diagnosed with cancer had a distal cancer. This increased to 100.0% (n = 10) when the CIBH was reported as harder and/or less frequent defecation.
| Symptoms and signs/indications | Total patients with CRC,a n | Patients with cancer, n | Percentage of patients with cancer who have distal cancer | |
|---|---|---|---|---|
| Distal | Proximal | |||
| Rectal bleeding | 172 | 154 | 20 | 90 |
| Rectal bleeding with anaemiab | 72 | 59 | 14 | 82 |
| Rectal bleeding without anaemiab | 100 | 95 | 6 | 95 |
| + CIBH | 72 | 69 | 4 | 96 |
| Looser and/or more frequent | 43 | 40 | 4 | 93 |
| Harder and/or less frequent | 10 | 10 | 0 | 100 |
| Variable | 2 | 2 | 0 | 100 |
| Unspecified | 17 | 17 | 0 | 100 |
| + Abdominal pain | 16 | 15 | 2 | 94 |
| + Weight loss | 10 | 10 | 1 | 100 |
| + Abdominal mass | 1 | 0 | 1 | 0 |
| + Rectal mass | 10 | 10 | 0 | 100 |
| + FOBt positive | 0 | 0 | 0 | – |
| + Any non-guideline symptom | 7 | 7 | 0 | 100 |
| Rectal bleeding with neither anaemiab nor CIBH | 28 | 26 | 2 | 93 |
| + Abdominal pain | 2 | 2 | 0 | 100 |
| + Weight loss | 1 | 1 | 0 | 100 |
| + Abdominal mass | 0 | 0 | 0 | – |
| + Rectal mass | 5 | 5 | 0 | 100 |
| + FOBt positive | 0 | 0 | 0 | – |
| + Any non-guideline symptom | 2 | 2 | 0 | 100 |
| Rectal bleeding alone | 19 | 17 | 2 | 89 |
A more detailed examination of CIBH revealed generally lower proportions of distal cancer than when presenting symptoms included rectal bleeding (Table 17). When patients presented with a CIBH alone, 83.3% (n = 15) of those diagnosed with cancer had distal cancer. Of patients with cancer who presented with a CIBH and anaemia, between 57.9% and 71.7% had distal cancers, with proportionately more distal cancers in those reporting a change to looser and/or more frequent stools and anaemia (n = 38) than in those with a change to harder stools and/or less frequent defecation and anaemia (n = 11).
| Symptoms and signs/indications | Total patients with CRC,a n | Patients with distal cancer, n | Patients with proximal cancer, n | Percentage of patients with cancer who have distal cancer |
|---|---|---|---|---|
| CIBH | 222 | 172 | 52 | 77 |
| CIBH with anaemiab | 101 | 67 | 35 | 66 |
| Looser and/or more frequent | 53 | 38 | 16 | 72 |
| Harder and/or less frequent | 19 | 11 | 8 | 58 |
| Variable | 9 | 6 | 3 | 67 |
| Unspecified | 20 | 12 | 8 | 60 |
| CIBH without anaemiab | 121 | 105 | 17 | 87 |
| Looser and/or more frequent | 68 | 59 | 10 | 87 |
| Harder and/or less frequent | 21 | 17 | 4 | 81 |
| Variable | 8 | 6 | 2 | 75 |
| Unspecified | 24 | 23 | 1 | 96 |
| + Rectal bleeding | 72 | 69 | 4 | 96 |
| + Abdominal pain | 36 | 26 | 11 | 72 |
| + Weight loss | 20 | 18 | 3 | 90 |
| + Abdominal mass | 5 | 4 | 1 | 80 |
| + Rectal mass | 6 | 6 | 0 | 100 |
| + FOBt positive | 1 | 1 | 0 | 100 |
| + Any non-guideline symptom | 10 | 8 | 2 | 80 |
| CIBH with neither anaemiab nor rectal bleeding | 49 | 36 | 13 | 73 |
| + Abdominal pain | 22 | 13 | 9 | 59 |
| + Weight loss | 11 | 9 | 2 | 82 |
| + Abdominal mass | 4 | 4 | 0 | 100 |
| + Rectal mass | 1 | 1 | 0 | 100 |
| + FOBt positive | 1 | 1 | 0 | 100 |
| + Any non-guideline symptom | 5 | 3 | 2 | 60 |
| CIBH alone | 18 | 15 | 3 | 83 |
It has previously been reported that proportionately fewer proximal cancers are diagnosed in patients without anaemia and/or an abdominal mass. 31,33 Here, of the 187 patients diagnosed with cancer who presented with anaemia or an abdominal mass, 59.4% (n = 111) had distal cancer (Table 18). In patients diagnosed with cancer who had presented without anaemia or an abdominal mass, the proportions with distal cancer varied by symptom combination. Overall, 88.4% of patients (n = 129) without anaemia or an abdominal mass who had cancer had distal cancer; among these patients, a report of rectal bleeding increased this proportion to 96.0% (n = 95). By contrast, in patients without anaemia or abdominal mass who reported a CIBH but without rectal bleeding, only 71.1% (n = 32) of patients with cancer had distal cancer.
| Symptom/signs combinations | Total patients with CRC,a n | Patients with cancer, n | Percentage of patients with cancer who have distal cancer | |
|---|---|---|---|---|
| Distal | Proximal | |||
| Total | 333 | 240 | 96 | 72 |
| Anaemiab or abdominal mass | 187 | 111 | 78 | 59 |
| No anaemiab or abdominal mass | 146 | 129 | 18 | 88 |
| Rectal bleeding, no anaemia or abdominal mass | ||||
| Total | 99 | 95 | 5 | 96 |
| Rectal bleeding alone | 19 | 17 | 2 | 89 |
| Rectal bleeding and CIBH | 71 | 69 | 3 | 97 |
| Rectal bleeding and either weight loss or abdominal pain, and no CIBH | 2 | 2 | 0 | 100 |
| Rectal bleeding and only other symptoms or signs | 7 | 7 | 0 | 100 |
| CIBH, no anaemia, abdominal mass or rectal bleeding | ||||
| Total | 45 | 32 | 13 | 71 |
| CIBH alone | 18 | 15 | 3 | 83 |
| Looser and/or more frequent | 9 | 9 | 0 | 100 |
| Harder and/or less frequent | 5 | 3 | 2 | 60 |
| Variable | 3 | 3 | 0 | 100 |
| Unspecified | 1 | 0 | 1 | 0 |
| CIBH and weight loss or abdominal pain | 24 | 15 | 9 | 62 |
| CIBH and only other symptoms or signs | 3 | 2 | 1 | 67 |
| No anaemia, abdominal mass, rectal bleeding or CIBH | ||||
| Abdominal pain or weight loss | 1 | 1 | 0 | 100 |
| Only other symptoms or signs | 1 | 1 | 0 | 100 |
Diagnostic yields of clinical features for proximal and distal colorectal cancers
The overall diagnostic yield for distal cancer was more than twice that for proximal cancer (5.1% vs. 2.0%) and, as a corollary, fewer patients needed to be examined to diagnose one distal (20 patients, 95% CI 18 to 23 patients) than one proximal cancer (50 patients, 95% CI 41 to 61 patients) (Table 19). The diagnostic yields were higher for distal cancer than for proximal cancer for all NICE qualifying symptoms. The yields for proximal cancer were < 3% for all NICE qualifying symptoms. The highest diagnostic yield for distal cancer was observed in patients with rectal bleeding; out of the 1660 patients who presented with rectal bleeding, 154 (9.3%) were diagnosed with distal cancer, resulting in a low number who needed to be examined to diagnose one cancer (11, 95% CI 10 to 13). By contrast, only 1.2% patients (n = 20) with rectal bleeding were diagnosed with proximal cancer.
| Symptoms and signs/indications | Total (N = 4741), n (%) | Cancera | |||||
|---|---|---|---|---|---|---|---|
| Distal | Proximal | ||||||
| Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| Total | 4741 (100) | 240 | 5.1 | 20 (18 to 23) | 96 | 2.0 | 50 (41 to 61) |
| Symptoms | |||||||
| CIBH | 3472 (73.2) | 172 | 5.0 | 21 (18 to 24) | 52 | 1.5 | 67 (52 to 90) |
| Looser and/or more frequent | 1852 (39.1) | 97 | 5.2 | 20 (16 to 24) | 26 | 1.4 | 72 (49 to 109) |
| Harder and/or less frequent | 583 (12.3) | 28 | 4.8 | 21 (15 to 32) | 12 | 2.1 | 49 (29 to 94) |
| Variable | 418 (8.8) | 12 | 2.9 | 35 (21 to 68) | 5 | 1.2 | 84 (37 to 257) |
| Unspecified | 619 (13.1) | 35 | 5.7 | 18 (13 to 26) | 9 | 1.5 | 69 (37 to 150) |
| Rectal bleeding | 1660 (35.0) | 154 | 9.3 | 11 (10 to 13) | 20 | 1.2 | 83 (54 to 136) |
| Abdominal pain | 1367 (28.8) | 51 | 3.7 | 27 (21 to 36) | 33 | 2.4 | 42 (30 to 60) |
| Weight loss | 881 (18.6) | 50 | 5.7 | 18 (14 to 24) | 24 | 2.7 | 37 (25 to 58) |
| Bloating/flatulence | 131 (2.8) | 6 | 4.6 | 22 (11 to 59) | 3 | 2.3 | 44 (16 to 211) |
| Tiredness/weakness | 114 (2.4) | 7 | 6.1 | 17 (9 to 40) | 4 | 3.5 | 29 (12 to 104) |
| Anal symptoms | 56 (1.2) | 1 | 1.8 | 56 (11 to 2213) | 0 | 0 | – |
| Nausea/vomiting | 32 (0.7) | 3 | 9.4 | 11 (4 to 51) | 0 | 0 | – |
| Back pain | 7 (0.1) | 0 | 0 | – | 0 | 0 | – |
| Upper GI symptoms | 10 (0.2) | 0 | 0 | – | 0 | 0 | – |
| Signs/indications | |||||||
| Anaemia | |||||||
| Anaemia (broad)b | 1659 (35.0) | 106 | 6.4 | 16 (14 to 20) | 77 | 4.6 | 22 (18 to 28) |
| Anaemia (strict)c | 875 (18.5) | 52 | 5.9 | 17 (13 to 23) | 60 | 6.9 | 15 (12 to 19) |
| IDA (broad)d | 567 (12.0) | 36 | 6.3 | 16 (12 to 23) | 48 | 8.5 | 12 (10 to 16) |
| IDA (strict)e | 318 (6.7) | 12 | 3.8 | 27 (16 to 51) | 20 | 6.3 | 16 (11 to 26) |
| Abdominal mass | 140 (3.0) | 10 | 7.1 | 14 (8 to 29) | 8 | 5.7 | 18 (10 to 41) |
| Rectal mass | 81 (1.7) | 15 | 18.5 | 6 (4 to 10) | 0 | 0 | – |
| FOBt positive | 76 (1.6) | 6 | 7.9 | 13 (7 to 34) | 2 | 2.6 | 38 (11 to 313) |
| Family history | 69 (1.5) | 3 | 4.3 | 23 (9 to 111) | 2 | 2.9 | 35 (10 to 284) |
| History of polyps | 14 (0.3) | 0 | 0 | – | 1 | 7.1 | 14 (3 to 554) |
| Other signsf | 11 (0.2) | 0 | 0 | – | 0 | 0 | – |
Using NICE qualifying clinical signs, the highest diagnostic yield for proximal cancer was observed in patients with IDA (by the broad definition), of whom 8.5% were diagnosed with proximal cancer. The diagnostic yields in patients who presented with an abdominal mass were comparatively high for both proximal (5.7%) and distal cancer (7.1%). None of the 81 patients with rectal mass were diagnosed with proximal cancer, but this sign was highly predictive of distal cancer (diagnostic yield 18.5%).
In the 1660 patients with rectal bleeding for whom blood test data were available, diagnostic yields for distal cancer were as high, at 34.5% among those with a rectal mass and 11.9% for a CIBH to looser and/or more frequent stools (Table 20). By contrast, diagnostic yields for proximal cancer were approximately ≤ 1%, except in patients with anaemia (2.8%) or an abdominal mass (5.9%), and there was a large degree of uncertainty in the numbers needed to be examined to diagnose one proximal cancer. The diagnostic yield for distal cancer was notably higher in non-anaemic patients who had rectal bleeding in combination with rectal mass (34.5%); however, > 1 in 10 patients with rectal bleeding and anaemia were also diagnosed with distal cancer (diagnostic yield 11.8%).
| Symptoms and signs/indications | Total patients, n | Cancera | |||||
|---|---|---|---|---|---|---|---|
| Distal | Proximal | ||||||
| Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| Rectal bleeding | 1660 | 154 | 9.3 | 11 (10 to 13) | 20 | 1.2 | 83 (54 to 136) |
| Rectal bleeding with anaemiab | 501 | 59 | 11.8 | 9 (7 to 12) | 14 | 2.8 | 36 (22 to 66) |
| Rectal bleeding without anaemiab | 1159 | 95 | 8.2 | 13 (11 to 15) | 6 | 0.5 | 194 (90 to 526) |
| + CIBH | 736 | 69 | 9.4 | 11 (9 to 14) | 4 | 0.5 | 184 (73 to 675) |
| Looser and/or more frequent | 367 | 40 | 10.9 | 10 (7 to 13) | 4 | 1.1 | 92 (37 to 336) |
| Harder and/or less frequent | 144 | 10 | 6.9 | 15 (9 to 30) | 0 | 0 | – |
| Variable | 82 | 2 | 2.4 | 41 (12 to 337) | 0 | 0 | – |
| Unspecified | 143 | 17 | 11.9 | 9 (6 to 15) | 0 | 0 | – |
| + Abdominal pain | 289 | 15 | 5.2 | 20 (12 to 35) | 2 | 0.7 | 145 (41 to 1192) |
| + Weight loss | 132 | 10 | 7.6 | 14 (8 to 28) | 1 | 0.8 | 132 (25 to 5215) |
| + Abdominal mass | 17 | 0 | 0 | – | 1 | 5.9 | 17 (4 to 672) |
| + Rectal mass | 29 | 10 | 34.5 | 3 (2 to 6) | 0 | 0 | – |
| + FOBt positive | 6 | 0 | 0 | – | 0 | 0 | – |
| + Any non-guideline symptom | 85 | 7 | 8.2 | 13 (7 to 30) | 0 | 0 | – |
| Rectal bleeding with neither anaemiab nor CIBH | 423 | 26 | 6.1 | 17 (12 to 25) | 2 | 0.5 | 212 (59 to 1745) |
| + Abdominal pain | 100 | 2 | 2.0 | 50 (15 to 412) | 0 | 0 | – |
| + Weight loss | 37 | 1 | 2.7 | 37 (8 to 1462) | 0 | 0 | – |
| + Abdominal mass | 7 | 0 | 0 | – | 0 | 0 | – |
| + Rectal mass | 15 | 5 | 33.3 | 3 (2 to 9) | 0 | 0 | – |
| + FOBt positive | 3 | 0 | 0 | – | 0 | 0 | – |
| + Any non-guideline symptom | 31 | 2 | 6.5 | 16 (5 to 127) | 0 | 0 | – |
| Rectal bleeding alone | 260 | 17 | 6.5 | 16 (10 to 26) | 2 | 0.8 | 130 (37 to 1072) |
In patients with a CIBH, the highest diagnostic yields were once again observed for distal cancer (Table 21). Yields of up to 21.4% were observed for distal cancer in patients with a CIBH in combination with rectal mass, although only 15 out of 833 patients who presented with a CIBH alone were diagnosed with distal cancer (diagnostic yield 1.8%). Yields for proximal cancer were generally ≤ 4% in patients with a CIBH.
| Symptoms and signs/indications | Total patients, n | Cancera | |||||
|---|---|---|---|---|---|---|---|
| Distal | Proximal | ||||||
| Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| CIBH | 3472 | 172 | 5.0 | 21 (18 to 24) | 52 | 1.5 | 67 (52 to 90) |
| CIBH with anaemiab | 989 | 67 | 6.8 | 15 (12 to 19) | 35 | 3.5 | 29 (21 to 41) |
| Looser and/or more frequent | 509 | 38 | 7.5 | 14 (10 to 19) | 16 | 3.1 | 32 (20 to 56) |
| Harder and/or less frequent | 201 | 11 | 5.5 | 19 (11 to 37) | 8 | 4.0 | 26 (14 to 58) |
| Variable | 105 | 6 | 5.7 | 18 (9 to 48) | 3 | 2.9 | 35 (13 to 169) |
| Unspecified | 174 | 12 | 6.9 | 15 (9 to 28) | 8 | 4.6 | 22 (12 to 50) |
| CIBH without anaemiab | 2483 | 105 | 4.2 | 24 (20 to 29) | 17 | 0.7 | 147 (92 to 251) |
| Looser and/or more frequent | 1343 | 59 | 4.4 | 23 (18 to 30) | 10 | 0.7 | 135 (74 to 280) |
| Harder and/or less frequent | 382 | 17 | 4.5 | 23 (15 to 39) | 4 | 1.0 | 96 (38 to 350) |
| Variable | 313 | 6 | 1.9 | 53 (25 to 142) | 2 | 0.6 | 157 (44 to 1291) |
| Unspecified | 445 | 23 | 5.2 | 20 (14 to 31) | 1 | 0.2 | 445 (81 to 17,578) |
| + Rectal bleeding | 736 | 69 | 9.4 | 11 (9 to 14) | 4 | 0.5 | 184 (73 to 675) |
| + Abdominal pain | 784 | 26 | 3.3 | 31 (12 to 46) | 11 | 1.4 | 72 (41 to 143) |
| + Weight loss | 414 | 18 | 4.3 | 23 (15 to 39) | 3 | 0.7 | 138 (48 to 669) |
| + Abdominal mass | 52 | 4 | 7.7 | 13 (6 to 47) | 1 | 1.9 | 52 (10 to 2055) |
| + Rectal mass | 28 | 6 | 21.4 | 5 (3 to 13) | 0 | 0 | – |
| + FOBt positive | 24 | 1 | 4.2 | 24 (5 to 949) | 0 | 0 | – |
| + Any non-guideline symptom | 223 | 8 | 3.6 | 28 (15 to 65) | 2 | 0.9 | 112 (32 to 920) |
| CIBH with neither anaemiab nor rectal bleeding | 1747 | 36 | 2.1 | 49 (36 to 70) | 13 | 0.7 | 135 (79 to 253) |
| + Abdominal pain | 595 | 13 | 2.2 | 46 (27 to 86) | 9 | 1.5 | 67 (36 to 145) |
| + Weight loss | 319 | 9 | 2.8 | 36 (19 to 78) | 2 | 0.6 | 160 (45 to 1316) |
| + Abdominal mass | 42 | 4 | 9.5 | 11 (5 to 38) | 0 | 0 | – |
| + Rectal mass | 14 | 1 | 7.1 | 14 (3 to 554) | 0 | 0 | – |
| + FOBt positive | 21 | 1 | 4.8 | 21 (5 to 830) | 0 | 0 | – |
| + Any non-guideline symptom | 169 | 3 | 1.8 | 57 (20 to 273) | 2 | 1.2 | 85 (24 to 697) |
| CIBH alone | 833 | 15 | 1.8 | 56 (34 to 99) | 3 | 0.4 | 278 (96 to 1346) |
Of the NICE qualifying features, the presence of anaemia and/or an abdominal mass was associated with the highest diagnostic yield for proximal cancer, with 4.5% of these patients (n = 78) being diagnosed with proximal cancer (Table 22). In patients without anaemia or an abdominal mass, no other NICE qualifying features, either in isolation or in combination, exhibited diagnostic yields for proximal cancer of more than approximately 1%, with the exception of a CIBH to harder stools and/or less frequent defecation (diagnostic yield 2.2%). There was evidence to suggest that NICE qualifying features were more predictive of distal cancer than of proximal cancer in patients without anaemia or an abdominal mass (overall diagnostic yield of 4.3% vs. 0.6% for distal and proximal cancer, respectively). In particular, rectal bleeding, either alone or in combination with other clinical features, was associated with diagnostic yields of up to 17.5% (range 1.7–17.5%) for distal cancer but < 0.8% for proximal cancer.
| Symptom/sign combinations | Total patients, n (%) | Cancera | |||||
|---|---|---|---|---|---|---|---|
| Distal | Proximal | ||||||
| Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| Total | 4741 (100) | 240 | 5.1 | 20 (18 to 23) | 96 | 2.0 | 50 (41 to 61) |
| Anaemiab or abdominal mass | 1731 (36.5) | 111 | 6.4 | 16 (14 to 19) | 78 | 4.5 | 23 (18 to 28) |
| No anaemiab or abdominal mass | 3010 (63.5) | 129 | 4.3 | 24 (20 to 28) | 18 | 0.6 | 168 (106 to 282) |
| Rectal bleeding, no anaemia or abdominal mass | |||||||
| Total | 1142 (24.1) | 95 | 8.3 | 13 (10 to 15) | 5 | 0.4 | 229 (99 to 703) |
| Rectal bleeding alone | 260 (5.5) | 17 | 6.5 | 16 (10 to 26) | 2 | 0.8 | 130 (37 to 1072) |
| Rectal bleeding and CIBH | 726 (15.3) | 69 | 9.5 | 11 (9 to 14) | 3 | 0.4 | 242 (84 to 1173) |
| Rectal bleeding and either weight loss or abdominal pain, and no CIBH | 116 (2.4) | 2 | 1.7 | 58 (17 to 478) | 0 | 0 | – |
| Rectal bleeding and only other symptoms or signs | 40 (0.8) | 7 | 17.5 | 6 (4 to 14) | 0 | 0 | – |
| CIBH, no anaemia, abdominal mass or rectal bleeding | |||||||
| Total | 1705 (36.0) | 32 | 1.9 | 54 (38 to 78) | 13 | 0.8 | 132 (77 to 246) |
| CIBH alone | 833 (17.6) | 15 | 1.8 | 56 (34 to 99) | 3 | 0.4 | 278 (96 to 1346) |
| Looser and/or more frequent | 477 (10.1) | 9 | 1.9 | 53 (29 to 116) | 0 | 0 | – |
| Harder and/or less frequent | 90 (1.9) | 3 | 3.3 | 30 (11 to 145) | 2 | 2.2 | 45 (13 to 371) |
| Variable | 100 (2.1) | 3 | 3.0 | 34 (12 to 161) | 0 | 0 | – |
| Unspecified | 166 (3.5) | 0 | 0 | – | 1 | 0.6 | 166 (31 to 6558) |
| CIBH and weight loss or abdominal pain | 790 (16.7) | 15 | 1.9 | 53 (33 to 94) | 9 | 1.1 | 88 (47 to 192) |
| CIBH and only other symptoms or signs | 82 (1.7) | 2 | 2.4 | 41 (12 to 337) | 1 | 1.2 | 82 (16 to 3240) |
| No anaemia, abdominal mass, rectal bleeding or CIBH | |||||||
| Abdominal pain or weight loss | 138 (2.9) | 1 | 0.7 | 138 (26 to 5452) | 0 | 0 | – |
| Only other symptoms or signs | 25 (0.5) | 1 | 4.0 | 25 (5 to 988) | 0 | 0 | – |
We examined the proportions of patients diagnosed with proximal and distal cancers by different definitions of anaemia (anaemia broad, anaemia strict and IDA broad) (Table 23). Of the 95 patients with cancer who presented with IDA and/or an abdominal mass, 47.4% (n = 45) were diagnosed with distal cancer. Similar observations were made for patients with strict-definition anaemia and/or an abdominal mass (n = 59; 48.8% distal cancer). There was some evidence to suggest that a slightly greater proportion of cancers diagnosed in patients with broad definition anaemia and/or an abdominal mass were distal (n = 111, 59.4%). Similar proportions of cancers diagnosed were distal in patients with a symptom profile including rectal bleeding irrespective of the anaemia definition used (see Table 23).
| Symptom/sign combinations | Anaemia (broad)a | Anaemia (strict)b | IDA (broad)c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total,d n | Distal, n | Proximal, n | % with distal cancer | Total,d n | Distal, n | Proximal, n | % with distal cancer | Total,d n | Distal, n | Proximal, n | % with distal cancer | |
| Total | 333 | 240 | 96 | 72 | 333 | 240 | 96 | 72 | 333 | 240 | 96 | 72 |
| Anaemia or abdominal mass | 187 | 111 | 78 | 59 | 121 | 59 | 63 | 49 | 95 | 45 | 51 | 47 |
| No anaemia or abdominal mass | 146 | 129 | 18 | 88 | 212 | 181 | 33 | 85 | 238 | 195 | 45 | 82 |
| Rectal bleeding, no anaemia or abdominal mass | ||||||||||||
| Total | 99 | 95 | 5 | 96 | 132 | 123 | 10 | 93 | 145 | 132 | 14 | 91 |
| Rectal bleeding alone | 19 | 17 | 2 | 89 | 28 | 25 | 3 | 89 | 31 | 27 | 4 | 87 |
| Rectal bleeding and CIBH | 71 | 69 | 3 | 97 | 90 | 85 | 6 | 94 | 98 | 91 | 8 | 93 |
| Rectal bleeding and either weight loss or abdominal pain, and no CIBH | 2 | 2 | 0 | 100 | 6 | 5 | 1 | 83 | 8 | 6 | 2 | 75 |
| Rectal bleeding and only other symptoms or signs | 7 | 7 | 0 | 100 | 8 | 8 | 0 | 100 | 8 | 8 | 0 | 100 |
| CIBH, no anaemia, abdominal mass or rectal bleeding | ||||||||||||
| Total | 45 | 32 | 13 | 71 | 66 | 50 | 17 | 76 | 74 | 54 | 21 | 73 |
| CIBH alone | 18 | 15 | 3 | 83 | 28 | 25 | 3 | 89 | 32 | 28 | 4 | 87 |
| Looser and/or more frequent | 9 | 9 | 0 | 100 | 17 | 17 | 0 | 100 | 18 | 18 | 0 | 100 |
| Harder and/or less frequent | 5 | 3 | 2 | 60 | 5 | 3 | 2 | 60 | 5 | 3 | 2 | 60 |
| Variable | 3 | 3 | 0 | 100 | 5 | 5 | 0 | 100 | 6 | 6 | 0 | 100 |
| Unspecified | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 1 | 2 | 33 |
| CIBH and weight loss or abdominal pain | 24 | 15 | 9 | 62 | 34 | 22 | 13 | 65 | 38 | 23 | 16 | 61 |
| CIBH and only other symptoms or signs | 3 | 2 | 1 | 67 | 4 | 3 | 1 | 75 | 4 | 3 | 1 | 75 |
| No anaemia, abdominal mass, rectal bleeding or CIBH | ||||||||||||
| Abdominal pain or weight loss | 1 | 1 | 0 | 100 | 9 | 5 | 4 | 56 | 12 | 6 | 6 | 50 |
| Only other symptoms or signs | 1 | 1 | 0 | 100 | 5 | 3 | 2 | 60 | 7 | 3 | 4 | 43 |
Comparing the diagnostic yields and numbers needed to be examined in patients with anaemia or an abdominal mass, with anaemia status defined by the strict definition (Table 24) and IDA by the broad definition (Table 25), diagnostic yields for proximal cancer were similar (6.4% and 7.4%, respectively); both of these estimates are higher than the 4.5% yield when the broad definition of anaemia is used (see Table 22). The diagnostic yields for proximal cancer in patients without anaemia or an abdominal mass by any definition were similar (range 0.6–1.1%). The number needed to be examined to diagnose one proximal cancer in patients without IDA or an abdominal mass was 91 (95% CI 68 to 124; see Table 25), compared with 114 (95% CI 82 to 166; see Table 24) for the strict definition of anaemia and 168 (95% CI 106 to 282; see Table 22) for the broad definition of anaemia.
| Symptom/sign combinations | Total patients, n (%) | Cancera | |||||
|---|---|---|---|---|---|---|---|
| Distal | Proximal | ||||||
| Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| Total | 4741 (100) | 240 | 5.1 | 20 (18 to 23) | 96 | 2.0 | 50 (41 to 61) |
| Anaemiab or abdominal mass | 981 (20.7) | 59 | 6.0 | 17 (14 to 22) | 63 | 6.4 | 16 (13 to 21) |
| No anaemiab or abdominal mass | 3760 (79.3) | 181 | 4.8 | 21 (19 to 25) | 33 | 0.9 | 114 (82 to 166) |
| Rectal bleeding, no anaemia or abdominal mass | |||||||
| Total | 1405 (29.6) | 123 | 8.8 | 12 (10 to 14) | 10 | 0.7 | 141 (77 to 293) |
| Rectal bleeding alone | 337 (7.1) | 25 | 7.4 | 14 (10 to 21) | 3 | 0.9 | 113 (39 to 544) |
| Rectal bleeding and CIBH | 887 (18.7) | 85 | 9.6 | 11 (9 to 13) | 6 | 0.7 | 148 (69 to 403) |
| Rectal bleeding and either weight loss or abdominal pain, and no CIBH | 134 (2.8) | 5 | 3.7 | 27 (12 to 82) | 1 | 0.7 | 134 (25 to 5294) |
| Rectal bleeding and only other symptoms or signs | 47 (1.0) | 8 | 17.0 | 6 (4 to 14) | 0 | 0 | – |
| CIBH, no anaemia, abdominal mass or rectal bleeding | |||||||
| Total | 2083 (43.9) | 50 | 2.4 | 42 (32 to 56) | 17 | 0.8 | 123 (77 to 211) |
| CIBH alone | 994 (21.0) | 25 | 2.5 | 40 (28 to 62) | 3 | 0.3 | 332 (114 to 1606) |
| Looser and/or more frequent | 571 (12.0) | 17 | 3.0 | 34 (22 to 58) | 0 | 0 | – |
| Harder and/or less frequent | 115 (2.4) | 3 | 2.3 | 39 (14 to 185) | 2 | 1.7 | 58 (17 to 474) |
| Variable | 120 (2.5) | 5 | 4.2 | 24 (11 to 74) | 0 | 0 | – |
| Unspecified | 188 (4.0) | 0 | 0 | – | 1 | 0.5 | 188 (35 to 7427) |
| CIBH and weight loss or abdominal pain | 988 (20.8) | 22 | 2.2 | 45 (30 to 72) | 13 | 1.3 | 76 (45 to 143) |
| CIBH and only other symptoms or signs | 101 (2.1) | 3 | 3.0 | 34 (12 to 163) | 1 | 1.0 | 101 (19 to 3990) |
| No anaemia, abdominal mass, rectal bleeding or CIBH | |||||||
| Abdominal pain or weight loss | 182 (3.8) | 5 | 2.7 | 37 (16 to 112) | 4 | 2.2 | 46 (19 to 167) |
| Only other symptoms or signs | 90 (1.9) | 3 | 3.3 | 30 (11 to 145) | 2 | 2.2 | 45 (13 to 371) |
| Symptom/sign combinations | Total patients, n (%) | Cancera | |||||
|---|---|---|---|---|---|---|---|
| Distal | Proximal | ||||||
| Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| Total | 4741 (100) | 240 | 5.1 | 20 (18 to 23) | 96 | 2.0 | 50 (41 to 61) |
| IDAb or abdominal mass | 685 (14.4) | 45 | 6.6 | 16 (12 to 21) | 51 | 7.4 | 14 (11 to 18) |
| No IDAb or abdominal mass | 4056 (85.6) | 195 | 4.8 | 21 (19 to 24) | 45 | 1.1 | 91 (68 to 124) |
| Rectal bleeding, no anaemia or abdominal mass | |||||||
| Total | 1501 (31.7) | 132 | 8.8 | 12 (10 to 14) | 14 | 0.9 | 108 (65 to 196) |
| Rectal bleeding alone | 369 (7.8) | 27 | 7.3 | 14 (10 to 21) | 4 | 1.1 | 93 (37 to 338) |
| Rectal bleeding and CIBH | 938 (19.8) | 91 | 9.7 | 11 (9 to 13) | 8 | 0.9 | 118 (60 to 272) |
| Rectal bleeding and either weight loss or abdominal pain, and no CIBH | 144 (3.0) | 6 | 4.2 | 24 (12 to 65) | 2 | 1.4 | 72 (21 to 593) |
| Rectal bleeding and only other symptoms or signs | 50 (1.1) | 8 | 16.0 | 7 (4 to 14) | 0 | 0 | – |
| CIBH, no anaemia, abdominal mass or rectal bleeding | |||||||
| Total | 2215 (46.7) | 54 | 2.4 | 42 (32 to 55) | 21 | 0.9 | 106 (70 to 171) |
| CIBH alone | 1058 (22.3) | 28 | 2.6 | 38 (27 to 57) | 4 | 0.4 | 265 (104 to 970) |
| Looser and/or more frequent | 608 (12.8) | 18 | 3.0 | 34 (22 to 57) | 0 | 0 | – |
| Harder and/or less frequent | 119 (2.5) | 3 | 2.5 | 40 (14 to 192) | 2 | 1.7 | 60 (17 to 490) |
| Variable | 131 (2.8) | 6 | 4.6 | 22 (11 to 59) | 0 | 0 | – |
| Unspecified | 200 (4.2) | 1 | 0.5 | 200 (37 to 7901) | 2 | 1.0 | 100 (29 to 825) |
| CIBH and weight loss or abdominal pain | 1051 (22.2) | 23 | 2.2 | 46 (31 to 72) | 16 | 1.5 | 66 (41 to 115) |
| CIBH and only other symptoms or signs | 106 (2.2) | 3 | 2.8 | 36 (13 to 171) | 1 | 0.9 | 106 (20 to 4188) |
| No anaemia, abdominal mass, rectal bleeding or CIBH | |||||||
| Abdominal pain or weight loss | 210 (4.4) | 6 | 2.9 | 35 (17 to 95) | 6 | 2.9 | 35 (17 to 95) |
| Only other symptoms or signs | 130 (2.7) | 3 | 2.3 | 44 (16 to 210) | 4 | 3.1 | 33 (14 to 119) |
We also examined the diagnostic yields and numbers needed to be examined for distal and proximal cancers in patients with anaemia (all definitions) separate from an abdominal mass (Table 26). For distal cancers, the highest diagnostic yields were observed in patients with anaemia with/without additional symptoms (range 3.8–6.4%). Anaemia by the broadest definition was most predictive of distal cancer in this group of patients, whereas IDA strict definition was least predictive. The diagnostic yield for proximal cancer for all patients with anaemia, by any definition and regardless of whether or not they had additional symptoms, ranged from 4.6% to 8.5%. For patients with anaemia, IDA by the broadest definition and IDA by the strictest definition were the most and least predictive of proximal cancer, respectively.
| Anaemia definition and other signs/symptoms | Total, n (%) | Cancer | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distal or proximal | Distala | Proximala | |||||||||||
| Number of patients | % of cancers | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | % of distal cancers | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | % of proximal cancers | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| Total | 4741 (100) | 333 | 100 | 7.0 | 15 (13 to 16) | 240 | 100 | 5.1 | 20 (18 to 23) | 96 | 100 | 2.0 | 50 (41 to 61) |
| Anaemia and/or other symptoms | |||||||||||||
| Anaemia (broad)b | 1659 (35.0) | 181 | 54 | 10.9 | 10 (8 to 11) | 106 | 44 | 6.4 | 16 (14 to 20) | 77 | 80 | 4.6 | 22 (18 to 28) |
| Anaemia (strict)c | 875 (18.5) | 111 | 33 | 12.7 | 8 (7 to 10) | 52 | 22 | 5.9 | 17 (13 to 23) | 60 | 63 | 6.9 | 15 (12 to 19) |
| IDA (broad)d | 567 (12.0) | 83 | 25 | 14.6 | 7 (6 to 9) | 36 | 15 | 6.3 | 16 (12 to 23) | 48 | 50 | 8.5 | 12 (10 to 16) |
| IDA (strict)e | 318 (6.7) | 32 | 10 | 10.1 | 10 (8 to 15) | 12 | 5 | 3.8 | 27 (16 to 51) | 20 | 21 | 6.3 | 16 (11 to 26) |
| Anaemia and no other NICE symptoms | |||||||||||||
| Anaemia (broad)b | 307 (6.5) | 30 | 9 | 9.8 | 11 (8 to 15) | 8 | 3 | 2.6 | 39 (20 to 89) | 22 | 23 | 7.2 | 14 (10 to 23) |
| Anaemia (strict)c | 243 (5.1) | 26 | 8 | 10.7 | 10 (7 to 15) | 6 | 3 | 2.5 | 41 (19 to 110) | 20 | 21 | 8.2 | 13 (9 to 20) |
| IDA (broad)d | 206 (4.3) | 24 | 7 | 11.7 | 9 (6 to 14) | 6 | 3 | 2.9 | 35 (17 to 93) | 18 | 19 | 8.7 | 12 (8 to 20) |
| IDA (strict)e | 135 (2.8) | 10 | 3 | 7.4 | 14 (8 to 28) | 2 | 1 | 1.5 | 68 (20 to 556) | 8 | 8 | 5.9 | 17 (9 to 39) |
| Anaemia and no other reported symptoms | |||||||||||||
| Anaemia (broad)b | 282 (5.9) | 26 | 8 | 9.2 | 11 (8 to 17) | 6 | 3 | 2.1 | 47 (22 to 128) | 20 | 21 | 7.1 | 15 (10 to 23) |
| Anaemia (strict)c | 230 (4.9) | 23 | 7 | 10.0 | 10 (7 to 16) | 5 | 2 | 2.2 | 46 (21 to 141) | 18 | 19 | 7.8 | 13 (9 to 22) |
| IDA (broad)d | 193 (4.1) | 21 | 6 | 10.9 | 10 (7 to 15) | 5 | 2 | 2.6 | 39 (17 to 119) | 16 | 17 | 8.3 | 13 (8 to 21) |
| IDA (strict)e | 125 (2.6) | 8 | 2 | 6.4 | 16 (9 to 36) | 2 | 1 | 1.6 | 63 (18 to 515) | 6 | 6 | 4.8 | 21 (10 to 57) |
Analyses in patients without blood test data
To compare the results in the patients with blood test data with the rest of the SOCCER study, proximal and distal cancer diagnoses by clinical feature were examined in the 2639 patients in whom no blood test data were available (Table 27). In this subset, anaemia was simply defined by a question (yes/no) on the SIGGAR referral form and was not confirmed by laboratory data. The proportion of cancer diagnoses that were distal was very similar in the cohort of patients in whom non-laboratory-confirmed anaemia or an abdominal mass was the reason for referral (see Table 27) and in the cohort with laboratory-confirmed anaemia status or an abdominal mass (58.1% vs. 59.4%) (see Table 18). Similarly, in patients without anaemia or an abdominal mass, there was little difference in the proportion of cancer diagnoses that were distal in patients with and without blood test data (88.4% vs. 93.7%, respectively).
| Symptom/signs combinations | Total patients with CRC,a n | Patients with cancer, n | Percentage of patients with cancer who have distal cancer | |
|---|---|---|---|---|
| Distal | Proximal | |||
| Total | 218 | 189 | 31 | 87 |
| Anaemiab or abdominal mass | 43 | 25 | 18 | 58 |
| No anaemiab or abdominal mass | 175 | 164 | 13 | 94 |
| Rectal bleeding, no anaemia or abdominal mass | ||||
| Total | 135 | 126 | 9 | 93 |
| Rectal bleeding alone | 28 | 26 | 2 | 93 |
| Rectal bleeding and CIBH | 94 | 87 | 7 | 93 |
| Rectal bleeding and either weight loss or abdominal pain, no CIBH | 6 | 6 | 0 | 100 |
| Rectal bleeding and only other symptoms or signs | 7 | 7 | 0 | 100 |
| CIBH, no anaemia, abdominal mass or rectal bleeding | ||||
| Total | 38 | 36 | 4 | 95 |
| CIBH alone | 17 | 16 | 2 | 94 |
| Looser and/or more frequent | 6 | 6 | 0 | 100 |
| Harder and/or less frequent | 2 | 2 | 0 | 100 |
| Variable | 6 | 5 | 1 | 83 |
| Unspecified | 3 | 3 | 1 | 100 |
| CIBH and weight loss or abdominal pain | 20 | 19 | 1 | 95 |
| CIBH and only other symptoms or signs | 1 | 1 | 1 | 100 |
| No anaemia, abdominal mass, rectal bleeding or CIBH | ||||
| Abdominal pain or weight loss | 1 | 1 | 0 | 100 |
| Only other symptoms or signs | 1 | 1 | 0 | 100 |
Among the 2639 patients referred without blood test data, more were diagnosed with distal cancer (n = 189, 7.2%) than with proximal cancer (n = 31, 1.2%) (Table 28). The diagnostic yields for distal and proximal cancer in patients presenting with anaemia or an abdominal mass in the cohort without blood test data were high (8.6% and 6.2%, respectively) and were similar to the yields in patients with blood test data (see Table 22) (6.4% and 4.5%, respectively). The diagnostic yields for proximal cancer in the patient cohorts with and without blood test data were also very similar for patients who presented without anaemia or an abdominal mass (both 0.6%). By contrast, there was some evidence to suggest that the diagnostic yield for distal cancer in patients presenting without anaemia or an abdominal mass was slightly higher in the cohort without blood test data than in the cohort with blood test data (7.0% vs. 4.3%).
| Symptom/signs combinations | Total patients, n (%) | Cancera | |||||
|---|---|---|---|---|---|---|---|
| Distal | Proximal | ||||||
| Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| Total | 2639 (100) | 189 | 7.2 | 14 (13 to 17) | 31 | 1.2 | 86 (61 to 126) |
| Anaemiab or abdominal mass | 290 (11.0) | 25 | 8.6 | 12 (9 to 18) | 18 | 6.2 | 17 (11 to 27) |
| No anaemiab or abdominal mass | 2349 (89.0) | 164 | 7.0 | 15 (13 to 17) | 13 | 0.6 | 181 (106 to 339) |
| Rectal bleeding, no anaemia or abdominal mass | |||||||
| Total | 1054 (39.9) | 126 | 12.0 | 9 (8 to 10) | 9 | 0.9 | 118 (62 to 256) |
| Rectal bleeding alone | 312 (11.8) | 26 | 8.3 | 12 (9 to 19) | 2 | 0.6 | 156 (44 to 1287) |
| Rectal bleeding and CIBH | 619 (23.5) | 87 | 14.1 | 8 (6 to 9) | 7 | 1.1 | 89 (44 to 220) |
| Rectal bleeding and either weight loss or abdominal pain, and no CIBH | 86 (3.3) | 6 | 7.0 | 15 (7 to 39) | 0 | 0 | – |
| Rectal bleeding and only other symptoms or signs | 37 (1.4) | 7 | 18.9 | 6 (3 to 13) | 0 | 0 | – |
| CIBH, no anaemia, abdominal mass or rectal bleeding | |||||||
| Total | 1161 (44.0) | 36 | 3.1 | 33 (24 to 46) | 4 | 0.3 | 291 (114 to 1065) |
| CIBH alone | 631 (23.9) | 16 | 2.5 | 40 (25 to 69) | 2 | 0.3 | 316 (88 to 2604) |
| Looser and/or more frequent | 359 (13.6) | 6 | 1.7 | 60 (28 to 163) | 0 | 0 | – |
| Harder and/or less frequent | 84 (3.2) | 2 | 2.4 | 42 (12 to 346) | 0 | 0 | – |
| Variable | 66 (2.5) | 5 | 7.6 | 14 (6 to 40) | 1 | 1.5 | 66 (13 to 2608) |
| Unspecified | 122 (4.6) | 3 | 2.5 | 41 (15 to 197) | 1 | 0.8 | 122 (23 to 4820) |
| CIBH and weight loss or abdominal pain | 463 (17.5) | 19 | 4.1 | 25 (16 to 41) | 1 | 0.2 | 463 (84 to 18,289) |
| CIBH and only other symptoms or signs | 67 (2.5) | 1 | 1.5 | 67 (13 to 2647) | 1 | 1.5 | 67 (13 to 2647) |
| No anaemia, abdominal mass, rectal bleeding or CIBH | |||||||
| Abdominal pain or weight loss | 103 (3.9) | 1 | 1.0 | 103 (19 to 4069) | 0 | 0 | – |
| Only other symptoms or signs | 31 (1.2) | 1 | 3.2 | 31 (6 to 1225) | 0 | 0 | – |
Analyses in the full SOCCER patient cohort
In the full SOCCER cohort, 77.9% (n = 429) of patients diagnosed with cancer were diagnosed with distal cancer (Table 29). Among those diagnosed with cancer, 59.1% (n = 136) of all patients who presented with anaemia or an abdominal mass had distal cancer, which was similar to the percentage in the cohort with blood test data (59.4%) (see Table 18). Of the 321 cancer patients in the full cohort who did not present with anaemia or an abdominal mass, 293 had distal cancer (91.3%), which was similar to the proportion with distal cancer observed in the cohort of patients with blood test data (88.4%). Furthermore, the proportions of patients diagnosed with distal cancer among those who presented without anaemia or an abdominal mass and with rectal bleeding were similar in the full cohort and the reduced cohort (94.4% and 96.0%, respectively). Similar to the findings in the cohort with blood test data, there was more variation in the proportions of patients without anaemia or an abdominal mass with distal cancer who presented with symptom profiles including a CIBH (range 71.4–100.0%).
| Symptom/signs combinations | Total patients with CRC,a n | Patients with cancer, n | Percentage of patients with cancer who have distal cancer | |
|---|---|---|---|---|
| Distal | Proximal | |||
| Total | 551 | 429 | 127 | 78 |
| Anaemiab or abdominal mass | 230 | 136 | 96 | 59 |
| Anaemia,b no abdominal mass | 191 | 117 | 76 | 61 |
| Abdominal mass, no anaemiab | 21 | 11 | 10 | 52 |
| Anaemiab and abdominal mass | 18 | 8 | 10 | 44 |
| No anaemiab or abdominal mass | ||||
| Total | 321 | 293 | 31 | 91 |
| Rectal bleeding, no anaemia or abdominal mass | ||||
| Total | 234 | 221 | 14 | 94 |
| Rectal bleeding alone | 47 | 43 | 4 | 91 |
| Rectal bleeding and CIBH | 165 | 156 | 10 | 95 |
| Rectal bleeding and either weight loss or abdominal pain, no CIBH | 8 | 8 | 0 | 100 |
| Rectal bleeding and only other symptoms or signs | 14 | 14 | 0 | 100 |
| CIBH, no anaemia, abdominal mass or rectal bleeding | ||||
| Total | 83 | 68 | 17 | 82 |
| CIBH alone | 35 | 31 | 5 | 89 |
| Looser and/or more frequent | 15 | 15 | 0 | 100 |
| Harder and/or less frequent | 7 | 5 | 2 | 71 |
| Variable | 9 | 8 | 1 | 89 |
| Unspecified | 4 | 3 | 2 | 75 |
| CIBH and weight loss or abdominal pain | 44 | 34 | 10 | 77 |
| CIBH and only other symptoms or signs | 4 | 3 | 2 | 75 |
| No anaemia, abdominal mass, rectal bleeding or CIBH | ||||
| Abdominal pain or weight loss | 2 | 2 | 0 | 100 |
| Only other symptoms or signs | 2 | 2 | 0 | 100 |
In the full cohort, 27.4% (n = 2021) of patients were referred with anaemia or an abdominal mass (Table 30); the diagnostic yields for proximal and distal cancer were similar (4.8% and 6.7%, respectively) and were similar to the yields for proximal and distal cancer obtained in the patient cohort with blood test data (4.5% and 6.4%, respectively) (see Table 22).
| Symptom/signs combinations | Total patients, n (%) | Cancera | |||||
|---|---|---|---|---|---|---|---|
| Distal | Proximal | ||||||
| Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| Total | 7380 (100) | 429 | 5.8 | 18 (16 to 19) | 127 | 1.7 | 59 (49 to 70) |
| Anaemiab or abdominal mass | 2021 (27.4) | 136 | 6.7 | 15 (13 to 18) | 96 | 4.8 | 22 (18 to 26) |
| Anaemia,b no abdominal mass | 1805 (24.5) | 117 | 6.5 | 16 (13 to 19) | 76 | 4.2 | 24 (20 to 31) |
| Abdominal mass, no anaemiab | 133 (1.8) | 11 | 8.3 | 13 (7 to 24) | 10 | 7.5 | 14 (8 to 28) |
| Anaemiab and abdominal mass | 83 (1.1) | 8 | 9.6 | 11 (6 to 24) | 10 | 12.0 | 9 (5 to 17) |
| No anaemiab or abdominal mass | 5359 (72.6) | 293 | 5.5 | 19 (17 to 21) | 31 | 0.6 | 173 (122 to 255) |
| Rectal bleeding, no anaemia or abdominal mass | |||||||
| Total | 2196 (29.8) | 221 | 10.1 | 10 (9 to 12) | 14 | 0.6 | 157 (94 to 287) |
| Rectal bleeding alone | 572 (7.8) | 43 | 7.5 | 14 (11 to 19) | 4 | 0.7 | 143 (57 to 524) |
| Rectal bleeding and CIBH | 1345 (18.2) | 156 | 11.6 | 9 (8 to 11) | 10 | 0.7 | 135 (74 to 281) |
| Rectal bleeding and either weight loss or abdominal pain, and no CIBH | 202 (2.7) | 8 | 4.0 | 26 (14 to 58) | 0 | 0 | – |
| Rectal bleeding and only other symptoms or signs | 77 (1.0) | 14 | 18.2 | 6 (4 to 10) | 0 | 0 | – |
| CIBH, no anaemia, abdominal mass or rectal bleeding | |||||||
| Total | 2866 (38.8) | 68 | 2.4 | 43 (34 to 55) | 17 | 0.6 | 169 (106 to 290) |
| CIBH alone | 1464 (19.8) | 31 | 2.1 | 48 (34 to 70) | 5 | 0.3 | 293 (126 to 902) |
| Looser and/or more frequent | 836 (11.3) | 15 | 1.8 | 56 (34 to 100) | 0 | 0 | – |
| Harder and/or less frequent | 174 (2.4) | 5 | 2.9 | 35 (16 to 107) | 2 | 1.1 | 87 (25 to 717) |
| Variable | 166 (2.2) | 8 | 4.8 | 21 (11 to 48) | 1 | 0.6 | 166 (31 to 6558) |
| Unspecified | 288 (3.9) | 3 | 1.0 | 96 (34 to 465) | 2 | 0.7 | 144 (41 to 1188) |
| CIBH and weight loss or abdominal pain | 1253 (17.0) | 34 | 2.7 | 37 (27 to 54) | 10 | 0.8 | 126 (69 to 261) |
| CIBH and only other symptoms or signs | 149 (2.0) | 3 | 2.0 | 50 (18 to 240) | 2 | 1.3 | 75 (21 to 614) |
| No anaemia, abdominal mass, rectal bleeding or CIBH | |||||||
| Abdominal pain or weight loss | 241 (3.3) | 2 | 0.8 | 121 (34 to 994) | 0 | 0 | – |
| Only other symptoms or signs | 56 (0.8) | 2 | 3.6 | 28 (9 to 230) | 0 | 0 | – |
The diagnostic yield for proximal cancer was lower in the 72.6% (n = 5359) of patients referred without anaemia or an abdominal mass in the full cohort and was similar to that obtained in the cohort with blood test data (both 0.6%). The diagnostic yields for distal cancer for the 29.8% (n = 2196) of patients referred with a symptom profile including rectal bleeding were high (diagnostic yield range 4.0–18.2%) in the full cohort and were similar to the yields obtained in the subgroup with blood test data (diagnostic yield range 1.7–17.5%). This was also reflected in the low numbers needed to examine for distal cancer. Only 10 patients (95% CI 9 to 12 patients) with rectal bleeding would need to be examined to diagnose one distal cancer in the full cohort; by contrast, 157 patients (95% CI 94 to 287 patients) with rectal bleeding would need to be examined to diagnose one proximal cancer. Notably, none of the 2.7% (n = 202) of patients referred with rectal bleeding and weight loss/abdominal pain in the absence of a CIBH had proximal cancer (see Table 30).
The diagnostic yields for distal cancer in patients referred with a symptom profile including a CIBH were generally lower than those for rectal bleeding in the full cohort (see Table 30). Among the 2866 patients (38.8% of the full cohort) referred with a CIBH without rectal bleeding, distal cancer was diagnosed in 2.4% (n = 68). This yield was very similar to that observed in the cohort with blood test data (1.9%). Diagnostic yields for distal cancer were similar for all CIBH subtypes (range 1.0–4.8%). CIBH was less specific for proximal cancer than for distal cancer, reflected in the high numbers needed to examine (≥ 75), and nearly all of the diagnostic yields were lower than 1% (range 0–1.3%). This was similar to the observations in the cohort with blood test data. In the small number of patients (n = 297, 4.0%) in the full cohort referred without anaemia, an abdominal mass, rectal bleeding or a CIBH, no proximal cancers were diagnosed, which was also the case in the cohort with blood test data.
Patient profiles of proximal cancers diagnosed in those presenting with rectal bleeding in the absence of anaemia or an abdominal mass
A total of 14 (45.2%) out of the 31 proximal cancers in patients without anaemia or an abdominal mass were diagnosed in those with rectal bleeding (Table 31). Five of these patients had pathology or a clinical finding in the distal colorectum that would probably have warranted a follow-on WCI after an initial FS examination, of whom four were female and four were aged ≥ 69 years. Three of these five patients had cancer located in the caecum and the majority (n = 3) presented with a CIBH to looser stools and/or more frequent defecation in addition to rectal bleeding.
| Patient | Sex | Age (years) | Cancer site | Cancer size (mm) | Symptoms/signs/indications | Distal findings |
|---|---|---|---|---|---|---|
| Distal findings that would necessitate referral for WCI | ||||||
| 1 | Male | 80 | CM | Unknown | RB, CIBH (looser/increase), AP | FS finding: suspected cancer in RM |
| 2 | Female | 59 | AC | 45 | RB, CIBH (looser/increase) | 20-mm tubulovillous adenoma in SC |
| 3 | Female | 69 | CM | 50 | RB | Transported blood observed distally; multiple sessile polyps in RM (< 4 mm) |
| 4 | Female | 77 | CM | Unknown | RB | FS finding: ≥ 3 lesions |
| 5 | Female | 82 | HF | 35 | RB, CIBH (looser/increase), AP, WL | Synchronous 48-mm distal cancer in SC |
| No reason for WCI | ||||||
| 6 | Male | 63 | SF | 35 | RB | 8-mm tubulovillous adenoma in RM |
| 7 | Male | 69 | TC | 35 | RB, CIBH (looser/increase) | Multiple diverticula |
| 8 | Male | 71 | TC | 25 | RB, CIBH (looser/increase) | Three hyperplastic polyps in SC (all ≤ 7 mm), multiple diverticula |
| 9 | Male | 78 | CM | 40 | RB | FS finding: complete and normal |
| 10 | Male | 79 | AC | 30 | RB, CIBH (looser/increase) | 5-mm tubular adenoma in SC, proctitis in RM |
| 11 | Male | 81 | CM | 30 | RB, CIBH (looser/increase) | No abnormality reported |
| 12 | Female | 65 | CM | 70 | RB, CIBH (looser/increase) | Multiple diverticula |
| 13 | Female | 83 | CM | 70 | RB, CIBH (looser/increase) | No abnormality reported |
| No information on findings known | ||||||
| 14 | Female | 68 | CM | Unknown | RB, CIBH (looser/increase) | No information |
The majority (57.1%, n = 8) of the 14 patients with proximal cancer who presented with rectal bleeding without anaemia or an abdominal mass did not have pathology or a clinical finding in the distal colorectum that would have warranted follow-up by WCI. Three-quarters (n = 6) of these patients were male and all were aged ≥ 63 years. Four of the proximal tumours in this group of patients were located in the caecum and three-quarters (n = 6) of these patients had a CIBH to looser stools and/or more frequent defecation in addition to rectal bleeding; only two patients in this group presented with rectal bleeding alone.
Proximal and distal cancer diagnostic yields in men and women aged < 70 years or ≥ 70 years by symptoms/clinical signs
We looked at the influence of age and sex on the diagnostic yields for distal and proximal cancer by symptoms that met criteria for FS (rectal bleeding or a CIBH to looser stools and/or more frequent defecation only in the absence of anaemia/an abdominal mass) compared with symptoms that did not (Table 32). Symptoms that met the criteria for FS were almost twice as predictive of distal cancer in men aged ≥ 70 years as in men aged < 70 years (15.8% vs. 8.1%); similarly, the distal cancer diagnostic yield in women aged ≥ 70 years with these symptoms was approximately three times that for women aged < 70 years (8.3% vs. 3.1%). Distal cancer diagnostic yields in men and women with symptoms that did not meet the criteria for FS were generally lower. Numbers needed to be examined to diagnose one distal cancer were quite low for men irrespective of age group or whether or not their presenting symptoms met FS criteria (range 7–18). In women, the numbers needed to be examined to diagnose one distal cancer were generally higher than those for men and were higher in those aged < 70 years (32 and 46 for those meeting FS and not meeting FS criteria, respectively) than in those aged ≥ 70 years (12 and 26 for those meeting and not meeting FS criteria, respectively).
| Sex, age group and whether or not the patient fits the criteria for FSa | Total patients, n (%) | Cancer | |||||
|---|---|---|---|---|---|---|---|
| Distalb | Proximalb | ||||||
| Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | Number of patients | Diagnostic yield (%) | Number needed to be examined to diagnose one cancer (95% CI) | ||
| Total | 7380 (100) | 429 | 5.8 | 18 (16 to 19) | 127 | 1.7 | 59 (49 to 70) |
| Men | |||||||
| Aged < 70 years | |||||||
| Symptoms meet criteria for FS | 770 (10.4) | 62 | 8.1 | 13 (10 to 17) | 2 | 0.3 | 385 (107 to 3178) |
| Symptoms do not meet criteria for FS | 735 (10.0) | 43 | 5.9 | 18 (13 to 24) | 14 | 1.9 | 53 (32 to 96) |
| Aged ≥ 70 years | |||||||
| Symptoms meet criteria for FS | 519 (7.0) | 82 | 15.8 | 7 (6 to 8) | 5 | 1.0 | 104 (45 to 319) |
| Symptoms do not meet criteria for FS | 1003 (13.6) | 69 | 6.9 | 15 (12 to 19) | 36 | 3.6 | 28 (21 to 40) |
| Women | |||||||
| Aged < 70 years | |||||||
| Symptoms meet criteria for FS | 1024 (13.9) | 32 | 3.1 | 32 (23 to 47) | 4 | 0.4 | 256 (101 to 939) |
| Symptoms do not meet criteria for FS | 1193 (16.2) | 26 | 2.2 | 46 (32 to 71) | 17 | 1.4 | 71 (45 to 121) |
| Aged ≥ 70 years | |||||||
| Symptoms meet criteria for FS | 719 (9.7) | 60 | 8.3 | 12 (10 to 16) | 3 | 0.4 | 240 (83 to 1162) |
| Symptoms do not meet criteria for FS | 1417 (19.2) | 55 | 3.9 | 26 (20 to 35) | 46 | 3.2 | 31 (24 to 42) |
Proximal cancer diagnostic yields were higher in men whose symptoms did not meet FS criteria than in those whose did, irrespective of age (1.9% vs. 0.3% for those aged < 70 years and 3.6% vs. 1.0% for those aged ≥ 70 years); this pattern was also observed in women. Importantly, in those presenting with symptoms that met criteria for FS, diagnostic yields for proximal cancer were ≤ 1% (range 0.3–1.0%), irrespective of age. The highest diagnostic yield for proximal cancer in patients with symptoms meeting FS criteria was in men aged ≥ 70 years (1%). The number needed to be examined in order to diagnose one proximal cancer in patients with symptoms meeting FS criteria was > 100 (range 104–385), irrespective of age group and sex.
Flexible sigmoidoscopy examinations
A subset of patients received a FS examination at baseline, which presented an opportunity to assess the CRC miss rate in this cohort (Table 33). Out of the total 7380 patients in the cohort, 20.1% (n = 1483) were examined by FS at the time of referral. Men were as likely as women to be examined by FS (p = 0.67) and there were no differences in the age profiles between those examined by FS and those not examined by FS (p = 0.056). By contrast, the route and urgency of referral differed, with proportionately more patients who underwent FS being referred to a colorectal surgical outpatient clinic than those not examined by FS (91.2% vs. 82.8%). Approximately twice as many patients who did not receive a FS examination were referred to a gastroenterology outpatient clinic as patients who did receive FS (9.5% vs. 5.1%).
| Characteristic | Total (N = 7380), n (%) | Cohort, n (%) | p-valuea | |
|---|---|---|---|---|
| With FS performed (N = 1483) | Without FS performed (N = 5897) | |||
| Sex | 0.67 | |||
| Men | 3027 (41.0) | 601 (40.5) | 2426 (41.1) | |
| Women | 4353 (59.0) | 882 (59.5) | 3471 (58.9) | |
| Age (years) | 0.056 | |||
| 55–64 | 2410 (32.7) | 472 (31.8) | 1938 (32.9) | |
| 65–74 | 2739 (37.1) | 594 (40.1) | 2145 (36.4) | |
| 75–84 | 1898 (25.7) | 357 (24.1) | 1541 (26.1) | |
| ≥ 85 | 333 (4.5) | 60 (4.0) | 273 (4.6) | |
| Route of referral | < 0.001 | |||
| Colorectal surgical outpatient clinic | 6235 (84.5) | 1353 (91.2) | 4882 (82.8) | |
| Gastroenterology outpatient clinic | 638 (8.6) | 76 (5.1) | 562 (9.5) | |
| Other outpatient clinic | 50 (0.7) | 6 (0.4) | 44 (0.7) | |
| Straight to test | 396 (5.4) | 39 (2.6) | 357 (6.1) | |
| Hospital admission | 33 (0.4) | 6 (0.4) | 27 (0.5) | |
| Not recorded | 28 (0.4) | 3 (0.2) | 25 (0.4) | |
| Urgency of referral | < 0.001 | |||
| Two-week wait | 3976 (53.9) | 748 (50.4) | 3228 (54.7) | |
| Urgent | 1315 (17.8) | 444 (29.9) | 871 (14.8) | |
| Soon | 660 (8.9) | 74 (5.0) | 586 (9.9) | |
| Routine | 915 (12.4) | 179 (12.1) | 736 (12.5) | |
| Not recorded | 514 (7.0) | 38 (2.6) | 476 (8.1) | |
Some major differences were observed in the symptomatic presentation of patients who had received a FS examination compared with patients who had not (Table 34). Of the common symptoms, patients who had received a FS examination were more likely to present with a CIBH (p < 0.001), rectal bleeding (p = 0.001) and abdominal pain (p = 0.002). There was evidence to suggest that some of the less common symptoms (and those not explicitly included in the NICE 2015 suspected cancer referral guidelines76), specifically bloating/flatulence and tiredness/weakness, were slightly more common in the cohort who had not been examined by FS (p < 0.001 for both).
| Symptoms and signs/indications | Total (N = 7380), n (%) | Cohort with FS performed (N = 1483), n (%) | Cohort without FS performed (N = 5897), n (%) | p-valuea |
|---|---|---|---|---|
| Symptoms | ||||
| CIBH | 5382 (72.9) | 1133 (76.4) | 4249 (72.1) | < 0.001 |
| Looser and/or more frequent | 2862 (38.8) | 575 (38.8) | 2287 (38.8) | |
| Harder and/or less frequent | 865 (11.7) | 139 (9.4) | 726 (12.3) | |
| Variable | 648 (8.8) | 132 (8.9) | 516 (8.8) | |
| Unspecified | 1007 (13.6) | 287 (19.4) | 720 (12.2) | |
| Rectal bleeding | 2773 (37.6) | 612 (41.3) | 2161 (36.6) | 0.001 |
| Abdominal pain | 2126 (28.8) | 476 (32.1) | 1650 (28.0) | 0.002 |
| Weight loss | 1148 (15.6) | 221 (14.9) | 927 (15.7) | 0.44 |
| Bloating/flatulence | 203 (2.8) | 21 (1.4) | 182 (3.1) | < 0.001 |
| Tiredness/weakness | 152 (2.1) | 14 (0.9) | 138 (2.3) | < 0.001 |
| Anal symptoms | 97 (1.3) | 17 (1.2) | 80 (1.4) | 0.53 |
| Nausea/vomiting | 44 (0.6) | 7 (0.5) | 37 (0.6) | 0.49 |
| Back pain | 13 (0.2) | 1 (0.1) | 12 (0.2) | 0.49 |
| Upper GI symptoms | 10 (0.1) | 1 (0.1) | 9 (0.2) | 0.70 |
| Signs/indications | ||||
| Anaemia | ||||
| Blood test data collected | 4741 (62.2) | 916 (61.8) | 3825 (64.9) | 0.026 |
| Anaemiab | 1888 (25.6) | 318 (21.4) | 1570 (26.6) | < 0.001 |
| Abdominal mass | 216 (2.9) | 63 (4.3) | 153 (2.6) | < 0.001 |
| Rectal mass | 165 (2.2) | 47 (3.2) | 118 (2.0) | 0.007 |
| FOBt positive | 113 (1.5) | 18 (1.2) | 95 (1.6) | 0.27 |
| Family history | 117 (1.6) | 15 (1.0) | 102 (1.7) | 0.048 |
| History of polyps | 23 (0.3) | 1 (0.1) | 22 (0.4) | 0.067 |
| Other signsc | 16 (0.2) | 2 (0.1) | 14 (0.2) | 0.75 |
Fewer patients who were examined by FS, compared with those patients not examined by FS, had blood test data available at presentation (p = 0.026) and fewer were reported to be anaemic (p < 0.001) (see Table 34). By contrast, more patients who had received a FS examination had an abdominal (p < 0.001) or a rectal (p = 0.007) mass.
The proportion of patients diagnosed with cancer was higher among those who had been examined by FS than among those who had not (9.6% vs. 6.9%, respectively; p < 0.001) (Table 35). Of the 1483 patients who were investigated with FS, 142 were diagnosed with CRC (see Table 35). Of these 142 patients, 112 (78.9%) were diagnosed with distal cancer. Patients examined by FS were also more likely to be diagnosed with distal cancer (p = 0.001) than those who did not have a FS and there was some evidence to suggest that this difference was attributable to a higher rate of rectal cancer in the cohort examined by FS (4.5% vs. 2.4%). There was no difference in the rates of proximal cancer between the two subgroups (p = 0.22).
| CRCs diagnosed | Total (N = 7380), n (%) | Cohort with FS performed (N = 1483), n (%) | Cohort without FS performed (N = 5897), n (%) | p-valuea |
|---|---|---|---|---|
| Total patients with cancer | 551b (7.5) | 142c (9.6) | 409d (6.9) | < 0.001 |
| Distal cancers | ||||
| Total patients with distal cancer | 429b (5.8) | 112c (7.6) | 317d (5.4) | 0.001 |
| Anus | 10 (0.1) | 3 (0.2) | 7 (0.1) | |
| Rectum | 210 (2.8) | 66 (4.5) | 144 (2.4) | |
| Rectosigmoid | 57 (0.8) | 11 (0.7) | 46 (0.8) | |
| Sigmoid colon | 146 (2.0) | 31 (2.1) | 115 (2.0) | |
| Descending colon | 8 (0.1) | 2 (0.1) | 6 (0.1) | |
| Distal colorectum (no further specification) | 4 (0.1) | 3 (0.2) | 1 (0.0) | |
| Proximal cancers | ||||
| Total patients with proximal cancer | 127b (1.7) | 31c (2.1) | 96d (1.6) | 0.22 |
| Splenic flexure | 9 (0.1) | 1 (0.1) | 8 (0.1) | |
| Transverse colon | 18 (0.2) | 6 (0.4) | 12 (0.2) | |
| Hepatic flexure | 14 (0.2) | 3 (0.2) | 11 (0.2) | |
| Ascending colon | 36 (0.5) | 9 (0.6) | 27 (0.5) | |
| Caecum | 53 (0.7) | 12 (0.8) | 41 (0.7) | |
Rates of distal cancer were approximately twice as high in men as in women, whereas rates of proximal cancer were similar between the sexes (Table 36). The yield of distal cancer increased with age; among men and women aged ≥ 85 years, the proportion diagnosed with distal cancer (21.4% and 8.7%, respectively) was more than twice that among those aged 55–64 years (9.0% and 4.1%, respectively). The rate of proximal cancer was much lower than that of distal cancer but rates increased with increasing age in both men and women.
| CRC subsite | Men (N = 601) | Women (N = 882) | ||||||
|---|---|---|---|---|---|---|---|---|
| 55–64 years (N = 201), n (%) | 65–74 years (N = 242), n (%) | 75–84 years (N = 144), n (%) | ≥ 85 years (N = 14), n (%) | 55–64 years (N = 271), n (%) | 65–74 years (N = 352), n (%) | 75–84 years (N = 213), n (%) | ≥ 85 years (N = 46), n (%) | |
| Distal | 18 (9.0) | 31 (12.8) | 17 (11.8) | 3 (21.4) | 11 (4.1) | 18 (5.1) | 10 (4.7) | 4 (8.7) |
| Proximal | 1 (0.5) | 4 (1.7) | 8 (5.6) | 0 (0) | 2 (0.7) | 6 (1.7) | 8 (3.8) | 2 (4.3) |
Flexible sigmoidoscopy miss rates for distal cancers
Of the 112 patients diagnosed with distal cancer who had been examined by FS, most (90.2%) had cancer diagnosed by FS (Table 37). Of the 11 distal cancers (9.8%) not diagnosed at FS, eight might have been found if current practice was applied, as patients in whom FS was incomplete or in whom a lesion was detected would have gone on to receive a repeat examination and/or a WCI. Only in the three patients with a ‘complete and normal’ FS examination would cancer have been missed by FS (miss rate 2.7%).
| Findings at FS | Total patients, n (%) | Patients diagnosed with distal cancer,a n (%) | Patients diagnosed with proximal cancer,a n (%) |
|---|---|---|---|
| Total | 1483 (100) | 112b (7.6) | 31b (2.1) |
| Cancer suspected | 108 (7.3) | 101 (93.5) | 1 (0.9) |
| ≥ 10-mm lesion detected | 54 (3.6) | 2b (3.7) | 3b (5.6) |
| ≥ 3 lesions detected | 10 (0.7) | 0 (0) | 1 (10.0) |
| Incomplete owing to pain | 158 (10.7) | 2 (1.3) | 1 (0.6) |
| Incomplete owing to faeces | 157 (10.6) | 3 (1.9) | 2 (1.3) |
| Incomplete owing to technical issuesc | 50 (3.4) | 1 (2.0) | 1 (2.0) |
| Incomplete for unknown reason | 8 (0.5) | 0 (0) | 0 (0) |
| Complete and normal | 938 (63.3) | 3 (0.3) | 22 (2.3) |
There were 31 patients who were initially examined by FS and subsequently diagnosed with proximal cancer. Two-thirds of these (n = 22) were diagnosed after a ‘complete and normal’ FS examination and would not have gone on to have a WCI after this outcome, unless other diagnostic indicators were present.
Flexible sigmoidoscopy miss rates for proximal and distal colorectal cancer by clinical features
Approximately one in four patients (n = 359) who underwent FS at baseline presented with anaemia and/or an abdominal mass, of whom slightly more were diagnosed with proximal than with distal cancer (n = 25 vs. 23; Table 38). Of the 23 patients diagnosed with distal cancer, 18 (78.3%) were diagnosed in those in whom cancer had been suspected at FS and four (17.4%) were diagnosed in patients who had an incomplete examination. Only one patient with distal cancer was diagnosed after a ‘complete and normal’ FS examination (potential miss rate 4%). Notably, 19 out of 25 patients (76.0%) with proximal cancer had a ‘normal and complete’ FS examination.
| Findings at FS | Total, n | Anaemiaa and/or abdominal mass | Rectal bleeding, no anaemiaa or abdominal mass | CIBH to looser and/or more frequent, no anaemia,a abdominal mass or rectal bleeding | CIBH to other than looser and/or more frequent, no anaemia,a abdominal mass or rectal bleeding | Abdominal pain or weight loss, no anaemia,a abdominal mass, rectal bleeding or CIBH | Only other symptoms or signs/indications | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n (%) | Distal,b n | Proximal,b n | Patients, n (%) | Distal,b n | Proximal,b n | Patients, n (%) | Distal,b n | Proximal,b n | Patients, n (%) | Distal,b n | Proximal,b n | Patients, n (%) | Distal,b n | Proximal,b n | Patients, n (%) | Distal,b n | Proximal,b n | ||
| Total | 1483 | 359 (24.2) | 23 | 25 | 493 (33.2) | 73 | 3 | 321 (21.6) | 6 | 0 | 257 (17.3) | 9c | 3c | 44 (3.0) | 0 | 0 | 9 (0.6) | 1 | 0 |
| Cancer suspected | 108 | 22 (20.4) | 18 | 0 | 71 (65.7) | 69 | 1 | 7 (6.5) | 6 | 0 | 7 (6.5) | 7 | 0 | 0 (0.0) | 0 | 0 | 1 (0.9) | 1 | 0 |
| ≥ 10-mm lesion detected | 54 | 10 (18.5) | 0 | 2 | 33 (61.1) | 1 | 0 | 5 (9.3) | 0 | 0 | 6 (11.1) | 1c | 1c | 0 (0.0) | 0 | 0 | 0 (0.0) | 0 | 0 |
| ≥ 3 lesions detected | 10 | 0 (0.0) | 0 | 0 | 5 (50.0) | 0 | 1 | 3 (30.0) | 0 | 0 | 1 (10.0) | 0 | 0 | 1 (10.0) | 0 | 0 | 0 (0.0) | 0 | 0 |
| Incomplete owing to pain | 158 | 43 (27.2) | 2 | 1 | 43 (27.2) | 0 | 0 | 39 (24.7) | 0 | 0 | 29 (18.4) | 0 | 0 | 4 (2.5) | 0 | 0 | 0 (0.0) | 0 | 0 |
| Incomplete owing to faeces | 157 | 54 (34.4) | 1 | 2 | 46 (29.3) | 1 | 0 | 27 (17.2) | 0 | 0 | 24 (15.3) | 1 | 0 | 5 (3.2) | 0 | 0 | 1 (0.6) | 0 | 0 |
| Incomplete owing to technical issuesd | 50 | 13 (26.0) | 1 | 1 | 18 (36.0) | 0 | 0 | 10 (20.0) | 0 | 0 | 7 (14.0) | 0 | 0 | 2 (4.0) | 0 | 0 | 0 (0.0) | 0 | 0 |
| Incomplete for unknown reason | 8 | 3 (37.5) | 0 | 0 | 2 (25.0) | 0 | 0 | 1 (12.5) | 0 | 0 | 2 (25.0) | 0 | 0 | 0 (0.0) | 0 | 0 | 0 (0.0) | 0 | 0 |
| Complete and normal | 938 | 214 (22.8) | 1 | 19 | 275 (29.3) | 2 | 1 | 229 (24.4) | 0 | 0 | 181 (19.3) | 0 | 2 | 32 (3.4) | 0 | 0 | 7 (0.7) | 0 | 0 |
Half of the patients diagnosed with cancer (53.5%, 76/142) who were examined by FS presented with the symptom profile of rectal bleeding without anaemia or an abdominal mass (see Table 38). In this group, 73 (96.0%) had distal cancer and the large majority of these cancers (n = 69) were diagnosed at FS. Only three patients with this symptom combination were diagnosed with proximal cancer after a FS examination, and two of these patients had findings at FS that might have warranted a WCI.
In total, 39.0% (n = 578) of patients examined by FS presented with a CIBH (of any subtype) without rectal bleeding, anaemia or an abdominal mass, 18 of whom were diagnosed with cancer. The majority (83.3%, n = 15) had distal cancer diagnosed. Only three patients overall were diagnosed with proximal cancer in this group, all of whom had a CIBH to subtypes other than looser and/or more frequent stools. One patient with a CIBH was diagnosed with proximal and distal cancer after FS examination, although this patient also had a large lesion detected at FS, which might have warranted a WCI; the remaining two proximal cancers were diagnosed after complete and normal FS examinations. Fewer patients who presented with a CIBH without other common NICE referral criteria symptoms/clinical signs (anaemia, an abdominal mass or rectal bleeding) were diagnosed with cancer (see Table 38).
Approximately 3% (n = 44) of patients examined by FS presented with abdominal pain/weight loss (without anaemia, an abdominal mass, rectal bleeding or a CIBH) (see Table 38). No patients with this symptom profile were diagnosed with cancer.
Patient and cancer profiles of distal cancers missed at initial flexible sigmoidoscopy examination
Of the 11 patients subsequently diagnosed with distal cancer whose diagnosis was missed at FS, six (54.5%) were men and seven (63.6%) were aged ≥ 73 years (Table 39). The majority of these patients (n = 8) had cancer in the sigmoid colon. One male patient was subsequently diagnosed with a large tumour (90 mm) in the sigmoid colon after a FS examination that was incomplete owing to the presence of faeces. Distal cancers in two female patients that were missed at FS were also subsequently missed at WCI (CT colonography).
| Patient | Sex | Age (years) | Cancer site | Cancer size (mm) | Findings at FS |
|---|---|---|---|---|---|
| 1 | Male | 56 | Distal | Unknown | Incomplete owing to faeces |
| 2 | Male | 63 | SC | 90 | Incomplete owing to faeces |
| 3 | Male | 63 | SC and DC | 50 (annular); unknown | Incomplete owing to faeces |
| 4 | Male | 73 | SC | 52 | Incomplete owing to pain |
| 5 | Male | 78 | SC | 70 | Complete and normal |
| 6 | Male | 78 | DC and TC | Unknown | 10-mm polyp in SC |
| 7 | Female | 59 | SC | Unknown | ≥ 10-mm polypoidal swelling in RM and diagnosed proctitis |
| 8 | Female | 73 | SC | Unknown | Incomplete as could not advance scope, diverticular disease |
| 9 | Female | 74 | RM | Unknown | Complete and normal |
| 10 | Female | 74 | SC | Unknown | Complete and normal. Cancer also missed by CT colonography |
| 11 | Female | 80 | SC | 50 | Incomplete owing to pain. Cancer also missed by CT colonography |
Chapter 4 Discussion
It is widely recognised that the frequency of particular signs or symptoms differs between patients with proximal and distal CRC. Patients with proximal CRC more frequently present with anaemia, whereas patients with distal CRC are more likely to present with rectal bleeding or a CIBH. 33,35,37,54,58,62,66,67,77–82
There have been a number of attempts to use clinical features at presentation to inform decisions about the most appropriate diagnostic investigation. An early attempt was that of Majumdar et al. ,37 who created an algorithm to predict distal location of CRC based on the presence of anaemia, rectal bleeding, constipation, anorexia, vomiting, nausea, fatigue and abdominal pain. 37
More recent strategies have focused on IDA, abdominal mass, a CIBH and rectal bleeding. A number of authors have found that IDA and abdominal mass are strongly associated with proximal cancer and that patients without either of these two signs are unlikely to have proximal cancer. 29,32,33,83 Others have noted that rectal bleeding and/or a CIBH are predominantly distal symptoms and proximal cancer is only rarely diagnosed in patients with these symptoms, particularly in the absence of other signs or symptoms. 29–31 However, the suitability of these symptom combinations to identify patients with low risk of proximal cancer, in whom WCI could be avoided, is not fully agreed,34,36 and the current NICE guideline recommends WCI for all patients referred to secondary care with symptoms and signs indicative of CRC. 3
The study by Thompson et al. ,33 which is the largest to date and which our study aimed to validate, collected data prospectively on 16,433 patients newly referred to a colorectal clinic in Portsmouth between 1986 and 2001 with clinical features suggestive of CRC. In that study the diagnostic yield for proximal cancer in patients without IDA or an abdominal mass was 0.2% (37/15,829), with the proportion of cancers located proximally in this symptom group being 4.7%. Similarly, in patients without IDA or an abdominal mass who presented with rectal bleeding or a CIBH alone, the diagnostic yield for proximal cancer was only 0.2% (21/11,867) and only 3.1% (21/671) of cancers in this symptom group were proximally located.
In the study by Thompson et al. ,33 the diagnostic yield for proximal cancer was slightly higher in those with a CIBH to less frequent stools than in those who experienced a CIBH to more frequent stools (0.7% vs. 0.2%, respectively). Furthermore, the proportion of cancers that were proximal in those with a CIBH to less frequent stools was substantially higher (25.0%, 1/4) than in in those with a CIBH to more frequent stools (3.1%, 2/64). 33 FS was the initial investigation in 98.9% of patients, and the diagnostic yield of WCI after FS was low (2.3%). These findings led the authors to recommend FS for investigation of patients without IDA or an abdominal mass, together with careful treat-watch-and-wait diagnostic strategies and WCI for patients with persistent or recurrent symptoms.
We studied an independent cohort of 7380 patients with clinical features suggestive of CRC who were referred to 21 hospitals in England between 2004 and 2007. We confirmed that for a proportion of patients with specific symptoms alone, an examination of the distal colorectum only, rather than a WCI, may be a safe option for the diagnosis or exclusion of CRC. In our study, we focused initially on the proportion of diagnosed cancers that were located distally as a measure of the likely sensitivity of a distal examination for detection of cancer in patients with a particular symptom profile. Using this method, we showed that a high proportion (41.4%) of cancers diagnosed in patients with anaemia (Hb < 13 g/dl in men or < 12 g/dl in women) or an abdominal mass were located in the proximal colon and that WCI is therefore necessary for these patients. The proportion of diagnosed cancers that were proximal was even higher in patients with both anaemia and an abdominal mass (55.3%). By contrast, we identified two symptomatic patient groups (without anaemia or an abdominal mass) for whom FS would be an acceptable examination. These were:
-
patients with rectal bleeding either as a single symptom or in combination with other symptoms, including a CIBH
-
patients with a CIBH to looser and/or more frequent stools as a single symptom.
Among patients with rectal bleeding and no anaemia or abdominal mass, 94.0% of cancers were located distally. Having additional symptoms such as a CIBH, abdominal pain or weight loss did not increase the proportion of proximal cancers, suggesting that the presence of rectal bleeding is a strong indicator of a distal location (see Table 29). In this group, only 14 proximal cancers were diagnosed in 2196 patients (diagnostic yield 0.6%), suggesting that 157 WCIs would have been required to detect a single proximal cancer.
Patients with a CIBH without rectal bleeding (and no anaemia or abdominal mass) were a more heterogeneous group, in whom, overall, 20.0% of cancers were located proximally. However, of the 15 cancers diagnosed in those with a CIBH to looser and/or more frequent stools as a single symptom, none was a proximal cancer.
Patients without anaemia or an abdominal mass but with rectal bleeding constituted 29.8% of the cohort (2196/7380), while those who presented with a CIBH as a single symptom to looser and/or more frequent stools constituted 11.3% of the cohort (836/7380). Therefore, for approximately 40% of patients in our cohort, investigation by FS alone might have been sufficient.
Our findings are supported by a number of studies that have looked at either the proportion of cancers that are proximal according to clinical features at presentation or the proportion of proximal cancers diagnosed in prospectively collected series of patients with rectal bleeding or a CIBH referred under the 2-week wait pathway. Kent et al. 31 audited all CRCs diagnosed over a 2-year period and found that, out of 45 patients with proximal cancers, 41 presented with anaemia or an abdominal mass, four presented with a CIBH (to unspecified frequency) and abdominal pain and none presented with a CIBH and/or rectal bleeding as sole symptoms. In a similar audit of cancers diagnosed over a 2-year period, Ingham Clark et al. 30 found that, out of 38 patients with proximal cancers, none presented with rectal bleeding and/or a CIBH alone. Ingham Clark et al. 30 also audited all colonoscopies performed in 2010 to investigate rectal bleeding and/or a CIBH. Of 21 CRCs diagnosed, only two were located proximal to the splenic flexure, and in both cases the symptoms were accompanied by abdominal pain. In an audit by Royle et al. 32 of 1690 patients referred to a rapid access FS with ‘red-flag’ symptoms, but no anaemia or abdominal mass, only two proximal cancers (0.24%) were diagnosed following a cancer-free FS. Similarly, Couch et al. 35 reported that in 968 patients referred from primary care for colonoscopy for the investigation of ‘red-flag’ symptoms, none of the 17 cancers located proximal to the splenic flexure were diagnosed in patients with rectal bleeding. In the series reported by Bhangu et al. ,34 of 85 patients referred to a 2-week wait clinic with either rectal bleeding, a CIBH or abdominal pain, 13 (15.3%) were diagnosed with proximal cancer. 34 This unacceptably high figure may have been the result of inclusion of abdominal pain, which our present study suggests could be a symptom of proximal cancer. These studies support the theoretical use of FS for patients with rectal bleeding and/or a CIBH alone, without anaemia or an abdominal mass, but are more equivocal about the most appropriate investigation for patients with a CIBH without rectal bleeding. However, most studies do not draw a distinction between a CIBH to looser and/or more frequent stools, which seems to be a distal symptom, and a CIBH to harder and/or less frequent stools, which can also be a symptom of proximal cancer. These previous studies also confirm that if a CIBH is accompanied by abdominal pain, then a WCI is needed.
Sensitivity and specificity of anaemia and iron deficiency anaemia for proximal cancer
We have confirmed the findings of others that anaemia with and without evidence of IDA (and/or abdominal mass) confers a high yield of cancer in patients referred to secondary care. 33,34,84,85 Although anaemia is seen in both proximal and distal disease, it is the most frequent clinical feature in proximal cancers, symptoms of which tend to be vague. Anaemia has therefore been used to define a population of patients in whom a WCI is warranted and, conversely, its absence has been used to define patients in whom FS might be safe. 33
However, there is no consensus on the precise definition of anaemia that should be used. To address this, we investigated the influence of varying definitions of anaemia and IDA on outcomes; we investigated different diagnostic thresholds with four definitions of anaemia/IDA in analyses. The definitions used were ‘broad anaemia’, ‘strict anaemia’, ‘broad IDA’ and ‘strict IDA’, which incorporated Hb, MCV and/or ferritin values (see Table 5). Of the 96 proximal cancers diagnosed in patients with blood test data in the present study, 77 (80.2%) were found in the 1659 patients with anaemia defined by the broadest definition, 60 (62.5%) were found in 875 patients with anaemia by the strict definition, 48 (50.0%) were found in 567 patients with IDA by the broad definition and 20 (20.8%) were found in 172 patients with IDA according to the most strict definition. Thus, widening the definition of anaemia increased the sensitivity of this clinical sign for proximal cancer. Broad definition anaemia is therefore likely to be the most diagnostically useful for excluding proximal cancer. The downside of using the broadest definition of anaemia is decreased specificity. Widening the definition from the strictest to the broadest definition of anaemia decreased diagnostic yields from 8.5% with IDA (broad definition) to 4.6% (for the broad definition of anaemia). However, even with the broadest definition, anaemia was the feature with the highest yield for proximal cancer of any symptom or sign. As anaemia is also found in patients with distal cancer, the overall yield of CRC (proximal or distal) in those with anaemia was 10.9%, with a number needed to be examined to detect a CRC at any site of only 10. Other studies investigated the associations between anaemia/IDA and CRC site using varying definitions of anaemia/IDA, based on local laboratory thresholds or national/international guidelines;29,32,33,37 however, in some instances, the definitions used are not clear. 30,31,35 We have demonstrated the impact of using different definitions for anaemia/IDA on diagnostic study outcomes. In the Thompson et al. 33 series, patients were considered to have IDA on the basis of low Hb (reference range 13–18 g/dl men and 12–16 g/dl women) and low MCV (reference range 80–95 fl). This is most comparable with the broad IDA definition used in our present study.
Sensitivity of flexible sigmoidoscopy in diagnosis of distal cancer
Although we have shown that certain groups of patients are more likely to have distal cancer, there are various reasons why cancers may not be detected at FS. First, we have defined a distal cancer as one that occurs distal to the splenic flexure. However, one problem with FS is that there are no landmarks to determine the anatomical site reached by the instrument. The length of scope inserted is also not a good marker because of the propensity for looping in the sigmoid colon. However, using magnetic endoscopic imaging, which permits visualisation of the anatomical location of the endoscope tip, Painter et al. 86 found that the splenic flexure was not reached in 60.7% of 117 FS procedures and the sigmoid colon/descending colon junction was not reached in 24.8% of procedures. Similar findings were also reported by others. 87,88
In our cohort, 11 out of 112 (9.8%) distal cancers were not diagnosed at FS, although only three of these cancers were diagnosed following a FS that was reported to be complete and normal. In two patients, a large (≥ 10 mm) distal lesion that would have warranted WCI was detected, and in six patients FS was incomplete owing to pain or the presence of faeces.
In other series of patients undergoing FS as their initial investigation, few have reported the miss rate of distal cancer. In the Thompson et al. 33 series, 786 out of 813 distal cancers (96.7%) were detected at the initial FS and a further 22 were diagnosed at the subsequent WCI, which in 19 cases was carried out because FS was incomplete, in two cases was carried out because of suspicious symptoms and in one case was carried out because of a family history of bowel cancer. A further five cancers were subsequently diagnosed during 3 years of follow-up among patients who did not have further WCI, giving a miss rate of 0.6%. In a more recent series of 1690 patients referred to a rapid-access, straight-to-test FS clinic for symptomatic urgent symptoms (excluding a right-sided mass or IDA), a distal cancer was diagnosed in 82 patients at FS, and no patient with a cancer-free FS who had a subsequent WCI was found to have cancer within 3 years. 32 In a series of 591 patients referred to a rapid-access FS clinic in 2006, Lim et al. 89 reported that 34 distal cancers were diagnosed, of which 32 (94.1%) were diagnosed at FS. These findings conflict with a study of the odds of an interval distal CRC after FS compared with colonoscopy among 15,484 older patients in the USA. 90 The study used the Surveillance, Epidemiology and End-Results-Medicare linked database, and an interval CRC was defined as one diagnosed between 6 and 36 months after lower endoscopy. The authors found that 8.8% of CRCs diagnosed after FS were interval, compared with 2.5% after colonoscopy (odds ratio 3.52). The authors speculated that the increased rate might be related to differences in bowel preparation quality, sedation use or depth of insertion. However, it was also noted that an incomplete colonoscopy not reaching the splenic flexure is billed to Medicare as FS, which could be a potential bias. Procedures performed in an office setting are more likely to be incomplete and older adults are less likely to be offered a follow-up colonoscopy. 91–93
Thus, despite FS not reaching the splenic flexure in around 60% of patients (according to literature from the 1980s and 1990s),87–89 few distal cancers are missed when the examination is declared to be normal and complete. Only 8 of the 551 cancers diagnosed in our series (1.5%) were located in the descending colon (see Table 3), the distal segment least likely to be examined at FS. Moreover, only two of these descending colon cancers had the symptom profile that would fit suitability for FS.
Whole-colon investigations performed after flexible sigmoidoscopy
A proportion of patients who have FS at their initial investigation will have a subsequent WCI. In some series this proportion is as high as 70%. 32,89 In the Thompson et al. 33 series, 34.8% of patients (5665/16,256) had WCI after FS. In studies in lower-risk patients, or when criteria for WCI referral after FS were more tightly controlled, referral rates were slightly lower, ranging from 16% to 31%. 71,94,95 It is not possible to determine the proportion of patients referred for WCI after FS in the SOCCER cohort, as patients were eligible for the SIGGAR trials on the basis that they required a WCI. Very few studies report reasons for referral for WCI after FS, but clearly this is an important question, as it profoundly affects the cost-effectiveness of offering FS. In the few studies that do provide details, the reasons provided include the presence of distal pathology (neoplastic and non-neoplastic),71,89,94–97 suboptimal bowel preparation and/or incomplete FS,89,94,95,97 symptoms not adequately explained at FS32,57,97 and the presence of symptoms/signs (anaemia, abdominal pain, weight loss and faecal occult blood) that are suggestive of proximal pathology. 89,95,96
In the Thompson et al. 33 series, the yield of proximal cancer in patients receiving WCI after FS was particularly low in patients with distal symptoms when FS yielded no clear indication for further investigation. Similarly, in the SOCCER series only three proximal cancers were diagnosed in this scenario (see Table 38). Very few studies have looked at the yield of benign proximal pathology (neoplastic or non-neoplastic) from WCI after FS in patients with distal symptoms;32,71 however, this can be estimated from studies in patients with distal symptoms having WCI in which isolated proximal pathology that would not be detected by FS is reported. 98,99 In the SOCCER cohort, 1057 patients had a colonoscopy as part of the SIGGAR trials and 36 were diagnosed with colitis; however, only three had isolated colitis in the proximal colon. Just one of these patients had a CIBH to looser and/or more frequent stools as their only symptom; the remaining two presented with anaemia or weight loss (data not presented). We can find no other studies that report isolated benign proximal findings in patients with rectal bleeding and/or a CIBH but no proximal symptom or signs. One study of 1766 patients undergoing colonoscopy for rectal bleeding found non-malignant proximal pathology (including diverticular disease, polyps and colitis) in 53.0% of patients overall and in 9.5% of patients in whom no significant distal pathology had been found. 98 In this series, patients with abdominal pain and some right-sided symptoms were excluded but those with anaemia were not. In the only study to examine the additional yield of colonoscopy over FS in patients with diarrhoea, the diagnostic yield for isolated inflammatory bowel disease and microscopic colitis in the proximal colon was 1.6%. 99 This study was in a young cohort (aged ≤ 50 years) of 615 patients, 62.1% of whom presented with chronic diarrhoea. Although patients with IDA or rectal bleeding were excluded, some presented with abdominal pain.
Another potential reason for performing WCI after FS is to complete an examination that had to be terminated because of pain or poor bowel preparation. FS is usually performed without intravenous sedation or pain relief and a proportion of patients may not tolerate the procedure. In our series, pain led to termination of the procedure in 10.7% of patients (see Table 37). Some endoscopy units now offer a 50 : 50 mix of nitrous oxide and air (Entonox®, BOC Healthcare, Manchester, UK) to help to manage procedural pain. An advantage of Entonox is that patients recover rapidly and driving ability is not impaired. 100 To achieve clearance of the sigmoid colon and rectum, it is usual to use a single phosphate enema, either self-administered by the patient at home33,89 or administered in the endoscopy unit around 1 hour prior to the procedure. 32,33,89,101 However, the rate of incomplete FS owing to poor bowel preparation in symptomatic series varies from 4% to 9%. 32,71,101,102 In our series it was 10.6% (157/1483; see Table 37). For patients found to have suboptimal bowel preparation at FS, some units now administer a second enema via the endoscope and repeat the procedure after the patient has opened their bowels. There are no published studies on whether the use of Entonox for patients experiencing discomfort, or the administration of a second enema when the first one has not achieved adequate distal cleansing, has increased completion rates for FS.
Strengths and limitations
This study has a number of strengths. It was a multicentre study of > 7000 patients for whom detailed data concerning clinical features at presentation and diagnoses were collected. Most previous studies examining the associations between symptoms and risks of distal and proximal cancer in the UK health-care setting have been single-site studies and/or involved small numbers of patients. 29–34,36 In addition, we have applied four definitions of anaemia in the data analyses, which has provided deeper insight into the diagnostic implications of anaemia in CRC. However, this study also has some limitations. The impact of selection bias in the SOCCER study was minimised through consecutive patient enrolment, the multicentre setting and the inclusion of patients who had originally been excluded from the SIGGAR trials; however, some residual impact is likely to remain and the degree to which this has had an impact on the findings has not been quantified. For example, this cohort is not fully representative of all patients being assessed for symptoms suggestive of CRC in secondary care as only patients aged > 55 years were included.
The SIGGAR trials were not initially designed to answer the question of whether or not FS is suitable for a certain subset of patients as defined by presenting symptom. For this reason, the trial pro forma did not include tick boxes for a number of clinical features. Instead, many symptoms had to be extracted from free-text fields, which may have introduced information bias. Furthermore, the eligibility criteria for SIGGAR meant that patients were recruited if they were deemed to need a WCI. The exclusion of participants who were not deemed to need a WCI may have inflated the diagnostic yields for proximal cancer in our study.
In England, up to one-quarter of all CRCs present as an emergency103 and it has been demonstrated that proximal cancers are more likely than distal cancer to present as an emergency. 42,43 Clearly, the patients with proximal cancer included in the study presented here are not representative of all patients with proximal cancer as most were recruited via outpatient clinics. However, the findings of the SOCCER study do apply to non-emergency patients (i.e. the large majority) when there is scope to improve outcomes for those with CRC by ensuring that they receive effective and efficient diagnostic investigation(s).
The generalisability of our findings may be affected by changes in the population presenting to secondary care with suspected CRC that have occurred since the trial ended in 2007 or that may occur in the future. New NICE referral guidelines were introduced in 2015,46 which are likely to alter the population of patients referred for potential CRC by GPs. New recommendations include the use of FOBt in primary care. Furthermore, the introduction of FS screening (bowel scope) and accompanying accreditation of bowel scope screening endoscopists may influence the quality of FSs performed within the NHS. Other potential factors that may influence the generalisability of our findings include the introduction of screening using FOBt in 2008 and an increasingly ageing population.
We have made some assumptions for conclusions relating to the suitability of FS as an initial investigation. We have assumed that the diagnostic accuracy of FS for distal cancer is 100% and that colonoscopy detects 100% of distal and proximal cancers, which does not reflect clinical practice. We were unable to assess the quality of FS procedures because it appears that information on the quality of bowel preparation and the completeness of examination is not routinely recorded.
Conclusions
We corroborated previous findings and demonstrated that the proportion of CRC diagnoses that are proximally located in patients without anaemia or abdominal mass is low in those with:
-
rectal bleeding as a single symptom or in combination with other symptoms
-
a CIBH to looser stools and/or more frequent defecation as a single symptom.
Flexible sigmoidoscopy alone should be a safe examination in these two groups of patients. Rapid-access clinics offering FS should be a safe and efficient mechanism to diagnose or exclude CRC in the many patients with these symptoms.
A high proportion of CRCs diagnosed in patients with anaemia, abdominal mass or other symptoms or signs, such as abdominal pain, weight loss or a CIBH to less frequent stools, were located in the proximal colon, and a WCI is necessary for these patients.
Implications for practice
The risk of CRC in primary care by presenting clinical features was the subject of a review and update by the NICE guidelines in 2015. 76 To improve the sensitivity of the referral pathway for suspected CRC, the recommended referral threshold was set at a positive predictive value of 3%, where previously thresholds had been disparate but generally > 5%. 46 It was acknowledged that, owing to the common and non-specific nature of CRC symptoms, this would correspondingly lower the specificity of referral but the 3% positive predictive value threshold was considered the level at which potential benefits generally outweighed potential risks. It is inevitable, however, that the lowered referral threshold for suspected CRC will result in many more patients having further diagnostic investigations but, ultimately, not being found to have this disease. 104 This study adds to the body of evidence that supports the selective use of FS as an initial investigation to confirm or exclude CRC diagnosis in clinical practice. It is expected that reducing the number of WCIs performed in the NHS could reduce both the patient and health service diagnostic burdens for this disease, which will inevitably become more pressing if specificity of referral pathways is reduced. At present, despite the accumulating evidence supporting symptom-based tailoring of initial investigations for CRC, and the fact that FS is being employed in clinical practice, there are no recommendations for this practice in the current NICE guideline. 3
Recommendations for research
This study was unable to provide answers to some questions, which we believe may be priorities for future research. Avenues for future research might include:
-
A cost-effectiveness analysis of symptom-based tailoring of diagnostic investigations for CRC. Such an analysis should incorporate both neoplastic and non-neoplastic disease outcomes.
-
Investigating the age/sex/symptom profile of all consecutive patients referred to hospital with the lower threshold (positive predictive value 3%) given in the NICE 2015 suspected cancer referral guidelines;76 doing so would answer the question of the potential role of FS in terms of reducing the requirement for a WCI.
-
Assessing the proportion of FS examinations for which pain or poor bowel preparation are reported, how many of these result in an incomplete examination, and the local measures (e.g. use of Entonox, administering a second enema and repeating the examination after a short delay) used to increase completion rates for FS examinations. In addition, assessing any improvement in diagnostic yields, both for cancer and significant polyps, achieved as a result of these additional measures.
Acknowledgements
Contributions of authors
Wendy Atkin (Professor in GI Epidemiology) planned, designed and executed the study, and provided guidance on statistical analysis. She drafted the monograph and acts as guarantor for this monograph.
Kate Wooldrage (Medical Statistician) devised statistical analysis plan, analysed data and drafted the monograph.
Urvi Shah (Senior Data Analyst) devised data analysis plan, co-ordinated and managed data collection and data cleaning.
Kate Skinner (Medical Writer) drafted the monograph.
Jeremy Brown (Epidemiologist) provided guidance on data interpretation and edited the manuscript.
Willie Hamilton (Professor in Primary Care Diagnostics) provided clinical advice on anaemia, patient referrals, and data analysis/interpretation.
Ines Kralj-Hans (Clinical Trial Manager) advised on clinical trial methodology, data collection, and analysis/interpretation.
Michael R Thompson (Consultant Colorectal Surgeon) provided clinical advice on patient referrals for colon examinations, and data interpretation.
Karen G Flashman (Research Co-ordinator) provided guidance on data collection, interpretation and analysis.
Steve Halligan (Professor in GI Radiology and Head of the University College London Centre for Medical Imaging) provided clinical advice on patient referrals for colon examinations and data interpretation.
Siwan Thomas-Gibson (Consultant Gastroenterologist) provided clinical advice on patient referrals for colon examinations and data interpretation.
Margaret Vance (Nurse Consultant) provided clinical advice on patient referrals for colon examinations and data interpretation.
Amanda J Cross (Senior Lecturer in Cancer Epidemiology) provided guidance on statistical analysis and data interpretation and drafted the monograph.
All authors assisted with data interpretation and revision of the monograph for intellectual content, and reviewed and approved the final version.
Study sites, study oversight and participating investigators
SOCCER trial steering committee
Greg Rubin: chairperson, independent (Professor of General Practice and Primary Care, Durham University).
Omar Faiz: non-independent (Consultant Colorectal Surgeon, Imperial College London).
Pawan Randev: independent (GP/Primary Care Lead, London Cancer Alliance).
John de Caestecker: independent (Consultant Gastroenterologist, Leicester General Hospital).
Richard Logan: independent (Professor of Epidemiology/Consultant Gastroenterologist, University of Nottingham).
Helen Watson: patient representative, independent (arranged through Bowel Cancer UK).
SIGGAR trial principal investigators
Darren Beech (Royal Cornwall Hospital, Truro), Anthony Higginson (Queen Alexandra Hospital, Portsmouth), Clive Kay (Bradford Teaching Hospitals NHS Foundation Trust), Craig Jobling (Nottingham City Hospital/Queen’s Medical Centre), Dominic Blunt (Charing Cross Hospital, London), Andrew Slater (Oxford Radcliffe Hospital, Oxford), Sathi Sukumar (University Hospital of South Manchester, Manchester), Nick Hughes (Frimley Park Hospital, Frimley), Philip Woolfall (University Hospital of North Tees, Stockton-on-Tees), Ian Crighton (Royal Lancaster Infirmary/Furness General Hospital), David Cade (Leighton Hospital, Crewe), Dion Morton (Queen Elizabeth Hospital Birmingham) and Paul Ziprin (St Mary’s Hospital, London).
SOCCER data collection
Andrew Taylor (Royal United Hospitals Bath NHS Foundation Trust), Sophie Stephenson (Bradford Teaching Hospitals NHS Foundation Trust), Vijay Dabhi (University Hospitals Birmingham NHS Foundation Trust), Michael Mulcahy (EndoSoft®), Paul Nacmanson (Imperial College Healthcare NHS Trust), Edwin Turner (Imperial College Healthcare NHS Trust), Andrew Cooper (Mid Cheshire Hospitals NHS Foundation Trust), John Madine (Royal Cornwall Hospitals NHS Trust), Alexander Dengler (Royal Cornwall Hospitals NHS Trust), Eddy McClements (University Hospitals of Morecambe Bay NHS Foundation Trust), Ann Worley (Nottingham University Hospitals NHS Trust), Martin Hand (Nottingham University Hospitals NHS Trust), Kevin Downes (North Tees and Hartlepool Hospitals NHS Foundation Trust), Deborah Wilson (North Tees and Hartlepool Hospitals NHS Foundation Trust), Gareth Frederickson (Pennine Acute Hospitals NHS Trust), Gary Walton (Pennine Acute Hospitals NHS Trust), Niki Solesbury (Oxford University Hospitals NHS Foundation Trust), Kevin Paddon (Oxford University Hospitals NHS Foundation Trust), Helen Hemsworth (Oxford University Hospitals NHS Foundation Trust), Maria Higgins (Oxford University Hospitals NHS Foundation Trust), Jocelyn Elmes (Plymouth Hospitals NHS Trust), Alan Reid (Portsmouth Hospitals NHS Trust), Dipak Bagga (London North West Healthcare NHS Trust), Jean Manning (London North West Healthcare NHS Trust) and Heather Slim (University Hospital of South Manchester NHS Foundation Trust).
SOCCER study staff
Kevin Pack: data collection, acquisition, cleaning and coding.
Iain Stenson: data collection and cleaning and information governance.
Laura Turner: information governance, ethics approvals and project management.
Paula Kirby: project management.
Jeremy Brown: literature review and editorial assistance.
Fiona Lucas: literature review and editorial assistance.
Data sharing statement
Data sharing requests should be directed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0: Cancer Incidence Estimated, Mortality and Prevalence Worldwide: IARC CancerBase No. 11. Lyon: International Agency for Research on Cancer; 2013.
- Bowel Cancer Services Cost and Benefits: Summary Report to the Department of Health. York: York Health Economics Consortium; 2007.
- Colorectal Cancer: The Diagnosis and Management of Colorectal Cancer. London: NICE; 2011.
- Brown H, Wyatt S, Croft S, Gale N, Turner A, Mulla A. Scoping the Future: An Evaluation of Endoscopy Capacity Across the NHS in England 2015. https://www.cancerresearchuk.org/sites/default/files/scoping_the_future_-_final.pdf (accessed January 2016).
- Department of Health . Bowel Cancer Screening: The Facts 2012. www.gov.uk/government/uploads/system/uploads/attachment_data/file/423840/bowel-cancer-the-facts.pdf (accessed September 2016).
- von Wagner C, Ghanouni A, Halligan S, Smith S, Dadswell E, Lilford RJ, et al. Patient acceptability and psychologic consequences of CT colonography compared with those of colonoscopy: results from a multicenter randomized controlled trial of symptomatic patients. Radiology 2012;263:723-31. http://dx.doi.org/10.1148/radiol.12111523.
- Amornyotin S. Sedation-related complications in gastrointestinal endoscopy. World J Gastrointest Endosc 2013;5:527-33. http://dx.doi.org/10.4253/wjge.v5.i11.527.
- Bowel Cancer UK. My Guide to Flexible Sigmoidoscopy and Colonoscopy. London: Bowel Cancer UK; 2013.
- St Mark’s Hospital and Academic Institute . Colonoscopy n.d. www.stmarkshospital.nhs.uk/services-a-z/wolfson-unit-for-endoscopy/colonoscopy/ (accessed December 2015).
- Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, et al. A consensus document on bowel preparation before colonoscopy: prepared by a Task Force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc 2006;63:894-909. https://doi.org/10.1016/j.gie.2006.03.918.
- Day LW, Kwon A, Inadomi JM, Walter LC, Somsouk M. Adverse events in older patients undergoing colonoscopy: a systematic review and meta-analysis. Gastrointest Endosc 2011;74:885-96. http://dx.doi.org/10.1016/j.gie.2011.06.023.
- Senore C, Ederle A, Fantin A, Andreoni B, Bisanti L, Grazzini G, et al. Acceptability and side-effects of colonoscopy and sigmoidoscopy in a screening setting. J Med Screen 2011;18:128-34. http://dx.doi.org/10.1258/jms.2011.010135.
- Sidhu S, Geraghty J, Karpha I, Wark L, Logan C, Sarkar S. Outcomes following an initial unsuccessful colonoscopy: a 5-year complete audit of teaching hospital colonoscopy practice. Gut 2011;60. http://dx.doi.org/10.1136/gut.2011.239301.423.
- Saltzman JR, Cash BD, Pasha SF, Early DS, Muthusamy VR, . ASGE Standards of Practice Committee . Bowel preparation before colonoscopy. Gastrointest Endosc 2015;81:781-94. https://doi.org/10.1016/j.gie.2014.09.048.
- Dominitz JA, Spiegel B. Editorial: On the quality of quality metrics: rethinking what defines a good colonoscopy. Am J Gastroenterol 2016;111:730-2. http://dx.doi.org/10.1038/ajg.2016.103.
- Guidance on Cancer Services. Improving Outcomes in Colorectal Cancers. Manual Update. London: NICE; 2004.
- Blakeborough A, Sheridan MB, Chapman AH. Complications of barium enema examinations: a survey of UK Consultant Radiologists 1992 to 1994. Clin Radiol 1997;52:142-8. https://doi.org/10.1016/S0009-9260(97)80108-0.
- Atkin W, Dadswell E, Wooldrage K, Kralj-Hans I, von Wagner C, Edwards R, et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet 2013;381:1194-202. http://dx.doi.org/10.1016/S0140-6736(12)62186-2.
- Halligan S, Dadswell E, Wooldrage K, Wardle J, von Wagner C, Lilford R, et al. Computed tomographic colonography compared with colonoscopy or barium enema for diagnosis of colorectal cancer in older symptomatic patients: two multicentre randomised trials with economic evaluation (the SIGGAR trials). Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19540.
- Svensson MH, Svensson E, Lasson A, Hellstrom M. Patient acceptance of CT colonography and conventional colonoscopy: prospective comparative study in patients with or suspected of having colorectal disease. Radiology 2002;222:337-45. https://doi.org/10.1148/radiol.2222010669.
- Taylor SA, Halligan S, Saunders BP, Bassett P, Vance M, Bartram CI. Acceptance by patients of multidetector CT colonography compared with barium enema examinations, flexible sigmoidoscopy, and colonoscopy. AJR Am J Roentgenol 2003;181:913-21. http://dx.doi.org/10.2214/ajr.181.4.1810913.
- van Gelder RE, Birnie E, Florie J, Schutter MP, Bartelsman JF, Snel P, et al. CT colonography and colonoscopy: assessment of patient preference in a 5-week follow-up study. Radiology 2004;233:328-37. http://dx.doi.org/10.1148/radiol.2331031208.
- Halligan S, Wooldrage K, Dadswell E, Kralj-Hans I, von Wagner C, Edwards R, et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. Lancet 2013;381:1185-93. https://doi.org/10.1016/S0140-6736(12)62124-2.
- Berland LL, Silverman SG, Gore RM, Mayo-Smith WW, Megibow AJ, Yee J, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 2010;7:754-73. http://dx.doi.org/10.1016/j.jacr.2010.06.013.
- Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection – systematic review and meta-analysis. Radiology 2011;259:393-405. http://dx.doi.org/10.1148/radiol.11101887.
- Atkin WS, Hart A, Edwards R, Cook CF, Wardle J, McIntyre P, et al. Single blind, randomised trial of efficacy and acceptability of oral picolax versus self administered phosphate enema in bowel preparation for flexible sigmoidoscopy screening. BMJ 2000;320:1504-8. https://doi.org/10.1136/bmj.320.7248.1504.
- Welchman S, Cochrane S, Minto G, Lewis S. Systematic review: the use of nitrous oxide gas for lower gastrointestinal endoscopy. Aliment Pharmacol Ther 2010;32:324-33. http://dx.doi.org/10.1111/j.1365-2036.2010.04359.x.
- Moshakis V, Ruban R, Wood G. Role of the nurse endoscopist in colorectal practice. Br J Surg 1996;83. https://doi.org/10.1002/bjs.1800831023.
- Badiani S, Desai A, Chapman MA. Is whole colonic imaging necessary for symptoms of change in bowel habit and/or rectal bleeding?. Colorectal Dis 2012;14:1197-200. http://dx.doi.org/10.1111/j.1463-1318.2011.02918.x.
- Ingham Clark CL, Zinkhan S, Ramar S, Shah S, Suri D. The use of symptoms to predict colorectal cancer site. Can we reduce the pressure on our endoscopy services?. Colorectal Dis 2010;12:834-5. http://dx.doi.org/10.1111/j.1463-1318.2010.02299.x.
- Kent AJ, Woolf D, McCue J, Greenfield SM. The use of symptoms to predict colorectal cancer site. Can we reduce the pressure on our endoscopy services?. Colorectal Dis 2010;12:114-18. http://dx.doi.org/10.1111/j.1463-1318.2009.01770.x.
- Royle TJ, Ferguson HJ, Mak TW, Simpson JA, Thumbe V, Bhalerao S. Same-day assessment and management of urgent (2-week wait) colorectal referrals: an analysis of the outcome of 1606 patients attending an endoscopy unit-based colorectal clinic. Colorectal Dis 2014;16:O176-81. http://dx.doi.org/10.1111/codi.12508.
- Thompson MR, Flashman KG, Wooldrage K, Rogers PA, Senapati A, O’Leary DP, et al. Flexible sigmoidoscopy and whole colonic imaging in the diagnosis of cancer in patients with colorectal symptoms. Br J Surg 2008;95:1140-6. http://dx.doi.org/10.1002/bjs.6234.
- Bhangu A, Khan M, Roberts L, Reynolds A, Desai A, Mathew G. Detection and survival of colorectal cancer from a 2 week wait service. Surgeon 2011;9:78-82. http://dx.doi.org/10.1016/j.surge.2010.07.012.
- Couch DG, Murphy JH, Boyle KM, Hemingway DM. Straight to flexible sigmoidoscopy: rationalization of 2-week wait referrals in suspected colorectal cancer. Colorectal Dis 2015;17:980-3. http://dx.doi.org/10.1111/codi.12988.
- Macdonald S, Radhakrishnan S, Seward E. PTH-028 Is flexible sigmoidoscopy ever enough? An audit of the rates of proximal disease during colonoscopy. Gut 2013;62. https://doi.org/10.1136/gutjnl-2013-304907.516.
- Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol 1999;94:3039-45. https://doi.org/10.1111/j.1572-0241.1999.01454.x.
- Strul H, Kariv R, Leshno M, Halak A, Jakubowicz M, Santo M, et al. The prevalence rate and anatomic location of colorectal adenoma and cancer detected by colonoscopy in average-risk individuals aged 40–80 years. Am J Gastroenterol 2006;101:255-62. http://dx.doi.org/10.1111/j.1572-0241.2006.00430.x.
- Matanoski G, Tao X, Almon L, Adade AA, Davies-Cole JO. Demographics and tumor characteristics of colorectal cancers in the United States, 1998–2001. Cancer 2006;107:1112-20. https://doi.org/10.1002/cncr.22008.
- Nawa T, Kato J, Kawamoto H, Okada H, Yamamoto H, Kohno H, et al. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol 2008;23:418-23. https://doi.org/10.1111/j.1440-1746.2007.04923.x.
- Snaebjornsson P, Jonasson L, Jonsson T, Möller PH, Theodors A, Jonasson JG. Colon cancer in Iceland – a nationwide comparative study on various pathology parameters with respect to right and left tumor location and patients age. Int J Cancer 2010;127:2645-53. http://dx.doi.org/10.1002/ijc.25258.
- Askari A, Malietzis G, Nachiappan S, Antoniou A, Jenkins J, Kennedy R, et al. Defining characteristics of patients with colorectal cancer requiring emergency surgery. Int J Colorectal Dis 2015;30:1329-36. http://dx.doi.org/10.1007/s00384-015-2313-8.
- Powell AG, Wallace R, McKee RF, Anderson JH, Going JJ, Edwards J, et al. The relationship between tumour site, clinicopathological characteristics and cancer-specific survival in patients undergoing surgery for colorectal cancer. Colorectal Dis 2012;14:1493-9. http://dx.doi.org/10.1111/j.1463-1318.2012.03048.x.
- Fletcher RH. The diagnosis of colorectal cancer in patients with symptoms: finding a needle in a haystack. BMC Med 2009;7. http://dx.doi.org/10.1186/1741-7015-7-18.
- Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng K, Marshall T. The risk of colorectal cancer with symptoms at different ages and between the sexes: a case-control study. BMC Med 2009;7. http://dx.doi.org/10.1186/1741-7015-7-17.
- Suspected Cancer: Recognition and Referral. London: NICE; 2015.
- Fijten GH, Blijham GH, Knottnerus JA. Occurrence and clinical significance of overt blood loss per rectum in the general population and in medical practice. Br J Gen Pract 1994;44:320-5.
- Thompson MR, Heath I, Ellis BG, Swarbrick ET, Wood LF, Atkin WS. Identifying and managing patients at low risk of bowel cancer in general practice. BMJ 2003;327:263-5. http://dx.doi.org/10.1136/bmj.327.7409.263.
- Hamilton W, Sharp D. Diagnosis of colorectal cancer in primary care: the evidence base for guidelines. Fam Pract 2004;21:99-106. https://doi.org/10.1093/fampra/cmh121.
- Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer 2005;93:399-405. http://dx.doi.org/10.1038/sj.bjc.6602714.
- Jellema P, van der Windt DA, Bruinvels DJ, Mallen CD, van Weyenberg SJ, Mulder CJ, et al. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1269.
- Colcock BP. Early diagnosis in carcinoma of the right colon. Dis Colon Rectum 1964;7:482-5. https://doi.org/10.1007/BF02616946.
- Oh SW, Kim YH, Choi YS, Chang DK, Son HJ, Rhee PL, et al. The comparison of the risk factors and clinical manifestations of proximal and distal colorectal cancer. Dis Colon Rectum 2008;51:56-61. https://doi.org/10.1007/s10350-007-9083-5.
- Church JM. Analysis of the colonoscopic findings in patients with rectal bleeding according to the pattern of their presenting symptoms. Dis Colon Rectum 1991;34:391-5. https://doi.org/10.1007/BF02053689.
- Eckardt VF, Schmitt T, Kanzler G, Eckardt AJ, Bernhard G. Does scant hematochezia necessitate the performance of total colonoscopy?. Endoscopy 2002;34:599-603. http://dx.doi.org/10.1055/s-2002-33249.
- Hreinsson JP, Jonasson JG, Bjornsson ES. Bleeding-related symptoms in colorectal cancer: a 4-year nationwide population-based study. Aliment Pharmacol Ther 2014;39:77-84. http://dx.doi.org/10.1111/apt.12519.
- Fine KD, Nelson AC, Ellington RT, Mossburg A. Comparison of the color of fecal blood with the anatomical location of gastrointestinal bleeding lesions: potential misdiagnosis using only flexible sigmoidoscopy for bright red blood per rectum. Am J Gastroenterol 1999;94:3202-10. https://doi.org/10.1111/j.1572-0241.1999.01519.x.
- Bat L, Pines A, Shemesh E, Levo Y, Zeeli D, Scapa E, et al. Colonoscopy in patients aged 80 years or older and its contribution to the evaluation of rectal bleeding. Postgrad Med J 1992;68:355-8. https://doi.org/10.1136/pgmj.68.799.355.
- Guidelines for the Management of Colorectal Cancer. London: ACPGBI; 2007.
- Kanellos D, Kitsios G, Kanellos I, Demetriades H, Pramateftakis MG, Angelopoulos S, et al. Anaemia as a symptom of right colon cancer. Tech Coloproctol 2004;8:62-4. https://doi.org/10.1007/s10151-004-0114-0.
- Sadahiro S, Suzuki T, Tokunaga N, Mukai M, Tajima T, Makuuchi H, et al. Anemia in patients with colorectal cancer. J Gastroenterol 1998;33:488-94. https://doi.org/10.1007/s005350050120.
- Alley PG, McNee RK. Age and sex differences in right colon cancer. Dis Colon Rectum 1986;29:227-9. https://doi.org/10.1007/BF02553021.
- The World Health Organization . Health Topics: Anaemia n.d. www.who.int/topics/anaemia/en/ (accessed April 2016).
- National Institute for Health and Care Excellence . Clinical Knowledge Summary. Anaemia – Iron Deficiency 2013. https://cks.nice.org.uk/anaemia-iron-deficiency (accessed April 2016).
- Goddard AF, James MW, McIntyre AS, Scott BB. British Society of Gastroenterology . Guidelines for the management of iron deficiency anaemia. Gut 2011;60:1309-16. http://dx.doi.org/10.1136/gut.2010.228874.
- Acher PL, Al-Mishlab T, Rahman M, Bates T. Iron-deficiency anaemia and delay in the diagnosis of colorectal cancer. Colorectal Dis 2003;5:145-8. https://doi.org/10.1046/j.1463-1318.2003.00415.x.
- Edna TH, Karlsen V, Jullumstrø E, Lydersen S. Prevalence of anaemia at diagnosis of colorectal cancer: assessment of associated risk factors. Hepatogastroenterology 2012;59:713-16. http://dx.doi.org/10.5754/hge11479.
- Masson S, Chinn DJ, Tabaqchali MA, Waddup G, Dwarakanath AD. Is anaemia relevant in the referral and diagnosis of colorectal cancer?. Colorectal Dis 2007;9:736-9. http://dx.doi.org/10.1111/j.1463-1318.2006.01200.x.
- Diagnosis and Management of Colorectal Cancer. Edinburgh: SIGN; 2011.
- The Association of Coloproctology of Great Britain and Ireland . Guidance on the Indications for Diagnostic Upper GI Endoscopy, Flexible Sigmoidoscopy and Colonoscopy 2011. www.bsg.org.uk/images/stories/docs/clinical/guidance/indications_diagnostic_endoscopy_13.pdf (accessed April 2016).
- Papagrigoriadis S, Arunkumar I, Koreli A, Corbett WA. Evaluation of flexible sigmoidoscopy as an investigation for ‘left sided’ colorectal symptoms. Postgrad Med J 2004;80:104-6. https://doi.org/10.1136/pmj.2003.008540.
- Elm EV, Altman D, Egger M, Pocock S, Gotzsche P, Vanderbroucke J. STROBE initiative . Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007;335:806-8. https://doi.org/10.1136/bmj.39335.541782.AD.
- National Health Service Act 2006. London: The Stationery Office; 2006.
- NHS Digital . Information Governance Toolkit Assessment Report 8HL46-FOM-CSPRG 2015. www.igt.hscic.gov.uk/AssessmentReportCriteria.aspx?tk=422975761626667&lnv=3&cb=8a4c9484-5085-4a2d-aa5e-3f983e41ebed&sViewOrgId=47550&sDesc=8HL46-FOM-CSPRG (accessed August 2016).
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems n.d. www.who.int/classifications/icd/en/ (accessed April 2016).
- International Classification of Diseases for Oncology. Geneva: WHO; 2013.
- Alexiusdottir KK, Möller PH, Snaebjornsson P, Jonasson L, Olafsdottir EJ, Björnsson ES, et al. Association of symptoms of colon cancer patients with tumor location and TNM tumor stage. Scand J Gastroenterol 2012;47:795-801. http://dx.doi.org/10.3109/00365521.2012.672589.
- Al-Saeed EF, Tunio MA, Al-Obaid O, Abdulla M, Al-Anazi A, AlShenaifi JY, et al. Correlation of pretreatment hemoglobin and platelet counts with clinicopathological features in colorectal cancer in Saudi population. Saudi J Gastroenterol 2014;20:134-8. https://doi.org/10.4103/1319-3767.129479.
- Ayyub MI, Al-Radi AO, Khazeindar AM, Nagi AH, Maniyar IA. Clinicopathological trends in colorectal cancer in a tertiary care hospital. Saudi Med J 2002;23:160-3.
- Beale AL, Penney MD, Allison MC. The prevalence of iron deficiency among patients presenting with colorectal cancer. Colorectal Dis 2005;7:398-402. http://dx.doi.org/10.1111/j.1463-1318.2005.00789.x.
- Elzouki AN, Habel S, Alsoaeiti S, Abosedra A, Khan F. Epidemiology and clinical findings of colorectal carcinoma in two tertiary care hospitals in Benghazi, Libya. Avicenna J Med 2014;4:94-8. http://dx.doi.org/10.4103/2231-0770.140659.
- Ho CH, Yu YB, Wu PH. The prevalence of iron deficiency anemia and its clinical implications in patients with colorectal carcinoma. J Chin Med Assoc 2008;71:119-22. http://dx.doi.org/10.1016/S1726-4901(08)70002-9.
- Thompson MR, Flashman K, O’Leary DP, Senapati A. Diagnosis of bowel cancer; most patients don’t require whole colonic imaging (WCI); are NICE guidelines misleading?. Gut 2015;64:A540-1. http://dx.doi.org/10.1136/gutjnl-2015-309861.1183.
- Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng KK, Marshall T. The importance of anaemia in diagnosing colorectal cancer: a case–control study using electronic primary care records. Br J Cancer 2008;98:323-7. http://dx.doi.org/10.1038/sj.bjc.6604165.
- Panagiotopoulou IG, Fitzrol D, Parker RA, Kuzhively J, Luscombe N, Wells AD, et al. The yield of colorectal cancer among fast track patients with normocytic and microcytic anaemia. Ann R Coll Surg Engl 2014;96:289-93. http://dx.doi.org/10.1308/003588414X13814021680076.
- Painter J, Saunders DB, Bell GD, Williams CB, Pitt R, Bladen J. Depth of insertion at flexible sigmoidoscopy: implications for colorectal cancer screening and instrument design. Endoscopy 1999;31:227-31. http://dx.doi.org/10.1055/s-1999-13673.
- Lehman GA, Buchner DM, Lappas JC. Anatomical extent of fiberoptic sigmoidoscopy. Gastroenterology 1983;84:803-8.
- Ott DJ, Wu WC, Gelfand DW. Extent of colonic visualization with the fiberoptic sigmoidoscope. J Clin Gastroenterol 1982;4:337-41. https://doi.org/10.1097/00004836-198208000-00009.
- Lim CS, McGeever L, Grey JH, Krishna A, Jabbar AA, Hendry WS. How important is it to investigate the whole of the colon after initial assessment at a rapid access colorectal clinic?. Int J Colorectal Dis 2009;24:1341-5. http://dx.doi.org/10.1007/s00384-009-0741-z.
- Wang YR, Cangemi JR, Loftus EV, Picco MF. Increased odds of interval left-sided colorectal cancer after flexible sigmoidoscopy compared with colonoscopy in older patients in the United States: a population-based analysis of the SEER-Medicare linked database, 2001–2005. Mayo Clin Proc 2013;88:471-8. http://dx.doi.org/10.1016/j.mayocp.2013.02.010.
- Bair D, Pham J, Seaton MB, Arya N, Pryce M, Seaton TL. The quality of screening colonoscopies in an office-based endoscopy clinic. Can J Gastroenterol 2009;23:41-7. https://doi.org/10.1155/2009/831029.
- Rizek R, Paszat LF, Stukel TA, Saskin R, Li C, Rabeneck L. Rates of complete colonic evaluation after incomplete colonoscopy and their associated factors: a population-based study. Med Care 2009;47:48-52. http://dx.doi.org/10.1097/MLR.0b013e31817d92bc.
- Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology 2007;132:2297-303. http://dx.doi.org/10.1053/j.gastro.2007.03.032.
- Badger SA, Gilliland R, Neilly PJ. The effectiveness of flexible sigmoidoscopy as the primary method for investigating colorectal symptoms in low-risk patients. Surg Endosc 2005;19:1349-52. https://doi.org/10.1007/s00464-004-2215-2.
- Vellacott KD, Roe AM, Mortensen NJ. An evaluation of a direct access flexible fibreoptic sigmoidoscopy service. Ann R Coll Surg Engl 1987;69:149-52.
- Niv Y, Asaf V. Open-access, flexible, fiberoptic sigmoidoscopy in a regional primary-care clinic. J Clin Gastroenterol 1992;15:218-21. https://doi.org/10.1097/00004836-199210000-00008.
- Toomey P, Asimakopoulos G, Zbar A, Kmiot W. ‘One-stop’ rectal bleeding clinics without routine flexible sigmoidoscopy are unsafe. Ann R Coll Surg Engl 1998;80:131-3.
- Mulcahy HE, Patel RS, Postic G, Eloubeidi MA, Vaughan JA, Wallace M, et al. Yield of colonoscopy in patients with nonacute rectal bleeding: a multicenter database study of 1766 patients. Am J Gastroenterol 2002;97:328-33. http://dx.doi.org/10.1111/j.1572-0241.2002.05465.x.
- Shale MJ, Walters JR, Westaby D. Adequacy of flexible sigmoidoscopy with biopsy for diarrhea in patients under age 50 without features of proximal disease. Gastrointest Endosc 2011;73:757-64. http://dx.doi.org/10.1016/j.gie.2010.11.037.
- Martin JP, Sexton BF, Saunders BP, Atkin WS. Inhaled patient-administered nitrous oxide/oxygen mixture does not impair driving ability when used as analgesia during screening flexible sigmoidoscopy. Gastrointest Endosc 2000;51:701-3. https://doi.org/10.1067/mge.2000.106113.
- Fincher RK, Osgard EM, Jackson JL, Strong JS, Wong RK. A comparison of bowel preparations for flexible sigmoidoscopy: oral magnesium citrate combined with oral bisacodyl, one hypertonic phosphate enema, or two hypertonic phosphate enemas. Am J Gastroenterol 1999;94:2122-7. https://doi.org/10.1016/s0002-9270(99)00364-0.
- McCallum RW, Meyer CT, Marignani P, Cane E, Contino C. Flexible sigmoidoscopy: diagnostic yield in 1015 patients. Am J Gastroenterol 1984;79:433-7.
- Public Health England . National Cancer Intelligence Network. Routes to Diagnosis 2006–2013 Workbook n.d. www.ncin.org.uk/publications/routes_to_diagnosis (accessed March 2016).
- Redaniel MT, Ridd M, Martin RM, Coxon F, Jeffreys M, Wade J. Rapid diagnostic pathways for suspected colorectal cancer: views of primary and secondary care clinicians on challenges and their potential solutions. BMJ Open 2015;5. http://dx.doi.org/10.1136/bmjopen-2015-008577.
Appendix 1 Participating hospitals
| Site | NHS trust | Total number of patients |
|---|---|---|
| Royal United Hospital, Bath | Royal United Hospitals Bath NHS Foundation Trust | 446 |
| Bradford Royal Infirmary | Bradford Teaching Hospitals NHS Foundation Trust | 729 |
| Queen Elizabeth Hospital, Birmingham | University Hospitals Birmingham NHS Foundation Trust | 414 |
| Charing Cross Hospital and Hammersmith Hospital | Imperial College Healthcare NHS Trust | 271 |
| Leighton Hospital, Crewe | Mid Cheshire Hospitals NHS Foundation Trust | 433 |
| Royal Cornwall Hospital, Truro | Royal Cornwall Hospitals NHS Trust | 521 |
| Frimley Park Hospital, Camberley | Frimley Health NHS Foundation Trust | 52 |
| Royal Lancaster Infirmary/Furness General Hospital | University Hospitals of Morecambe Bay NHS Foundation Trust | 518 |
| Queen’s Medical Centre, Nottingham/Nottingham City Hospital | Nottingham University Hospitals NHS Trust | 561 |
| University Hospital of North Tees, Stockton-on-Tees | North Tees and Hartlepool Hospitals NHS Foundation Trust | 64 |
| The Royal Oldham Hospital | Pennine Acute Hospitals NHS Trust | 412 |
| John Radcliffe Hospital, Oxford | Oxford University Hospitals NHS Foundation Trust | 150 |
| St Mary’s Hospital, Paddington | Imperial College Healthcare NHS Trust | 225 |
| Derriford Hospital, Plymouth | Plymouth Hospitals NHS Trust | 242 |
| Queen Alexandra Hospital, Portsmouth | Portsmouth Hospitals NHS Trust | 810 |
| St Mark’s Hospital, Harrow | London North West Healthcare NHS Trust | 1399 |
| Withington Community Hospital/Wythenshawe Hospital, Manchester | University Hospital of South Manchester NHS Foundation Trust | 133 |
| Total | 7380 |
Appendix 2 SIGGAR trial eligibility
| Reason | n (%) |
|---|---|
| Clinician-declined consent | 2176 (71.7) |
| CRC already diagnosed | 56 (1.8) |
| Other cancer already diagnosed | 69 (2.3) |
| Clinician requested specific procedure | |
| Colonoscopy | 731 (24.1) |
| CT colonography | 303 (10.0) |
| FS | 230 (7.6) |
| Oesophagogastroduodenoscopy | 218 (7.2) |
| Barium enema | 19 (0.6) |
| Ultrasound | 16 (0.5) |
| Magnetic resonance imaging | 5 (0.2) |
| Requested procedure unknown | 39 (1.3) |
| Clinical situation too urgent or waiting list too long | 52 (1.7) |
| Patient unfit for whole colon examination | 215 (7.1) |
| Patient unable to give informed consent | 75 (2.5) |
| No reason given | 148 (4.9) |
| Patient-declined consent | 834 (27.5) |
| Patient requested specific procedure | |
| Colonoscopy | 15 (0.5) |
| CT colonography | 3 (0.1) |
| Barium enema | 2 (0.07) |
| Requested procedure unknown | 128 (4.2) |
| Patient wanted to avoid specific procedure | |
| CT colonography because claustrophobic | 13 (0.4) |
| CT colonography for other reasons | 2 (0.07) |
| Colonoscopy | 1 (0.03) |
| Barium enema | 1 (0.03) |
| Patient had difficulty comprehending | 84 (2.8) |
| Patient died before consent obtained | 2 (0.07) |
| No reason given | 583 (19.2) |
| Reason for exclusion unknown | 26 (0.9) |
| Total excluded patients | 3036 |
List of abbreviations
- ACPGBI
- Association of Coloproctology of Great Britain and Ireland
- CI
- confidence interval
- CIBH
- change in bowel habit
- CRC
- colorectal cancer
- CT
- computerised tomography
- FOBt
- faecal occult blood test
- FS
- flexible sigmoidoscopy
- GI
- gastrointestinal
- GP
- general practitioner
- Hb
- haemoglobin
- HSCIC
- Health and Social Care Information Centre
- IDA
- iron deficiency anaemia
- MCV
- mean corpuscular volume
- NICE
- National Institute for Health and Care Excellence
- SIGGAR
- Special Interest Group in Gastrointestinal and Abdominal Radiology
- SOCCER
- Symptoms of Colorectal Cancer Evaluation Research
- WCI
- whole-colon investigation