Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/57/43. The contractual start date was in September 2012. The draft report began editorial review in July 2016 and was accepted for publication in February 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Catherine Hewitt is a member of the Health Technology Assessment (HTA) Commissioning Board. David A Richards reports other National Institute for Health Research grants (NIHR) from the HTA programme (COBRA; CADENCE), a personal award (Senior Investigator) and a Programme Development Grant (ESSENCE) and the European Science Foundation during the conduct of the study. He is also a member of NIHR funding panels, including the Fellowships and Senior Investigator panels. Karen Spilsbury is a member of the NIHR Health Services and Delivery Commissioning Board. Simon Gilbody is a member of the HTA Evidence Synthesis Board and HTA Efficient Study Designs Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Bosanquet et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Depression in older adults
Depression accounts for the greatest burden of disease among all mental health conditions, and is expected to become the second highest among all general health problems by 2020. 1 It is currently estimated that in the UK around 10–20% of people aged ≥ 65 years have depression. 2 Projected demographic changes mean that population strategies to tackle depression will increasingly have to address the specific needs of older adults. 3 Depression often occurs alongside long-term physical health conditions4 and/or cognitive impairment and it is more prevalent among people who live alone in social isolation. All these factors tend to disproportionately affect the older adult population. Among older adults, a clinical diagnosis of a major depressive disorder is the strongest predictor for impaired quality of life. 5 Indeed, beyond personal suffering and family disruption, depression worsens the outcomes of many medical disorders and promotes disability. 6 In 2009, the National Institute for Health and Care Excellence (NICE) published guidelines that acknowledged the coexistence of physical health problems and depression. 7,8 Furthermore, it was recognised that the impairments in quality of life associated with depression are comparable to those of major physical illness. 5
Rationale for the CollAborative care for Screen-Positive EldeRs plus trial
Depression in older people is relatively common. 9 The effects on the individual include poor quality of life, increased morbidity and early mortality,10 and increased health and social care use. 11 Depression is often under-recognised and undertreated in primary care. 12,13 At present, the management of depression tends to be limited to the prescription of antidepressants, with poor adherance an associated problem. 12 In particular, older adults seem to be less likely than working-age adults to be offered psychological treatments. 14,15 So far, the evidence for psychological interventions relates to higher-intensity models of care that cannot feasibly be delivered at scale in primary care. Collaborative care is a framework model for organising and delivering psychosocial interventions at scale. 16 It represents a brief, patient-centred, psychosocial package of care delivered by a case manager who works to a defined protocol and co-ordinates the patient’s medication management with their general practitioner (GP). The case manager is supervised by a specialist who facilitates liaison across the primary care–secondary care interface. 17 In the USA, collaborative care has shown promising trial results among older people;16 however, the transferability of this model of service to the UK NHS cannot be assumed. Consequently, the CollAborative care for Screen-Positive EldeRs with major depression (CASPER) plus trial will substantially enhance the randomised evidence base in the care of older people with depression and inform future service provision.
Collaborative care: an organisational model of providing care
The vast majority of depression in older adults is managed entirely in primary care without recourse to specialist mental health services. 3,18 Although a range of individual treatments have been shown to be effective in the management of clinical depression in older adults, including antidepressants and psychosocial interventions,18 a repeated observation among those with depression has been the failure to integrate these effective elements of care into routine primary care services. 19 In addition, the implementation of any form of care will require a strategy that is low intensity and can be offered within primary care. 20
In recent years, an organisational model of care has been introduced called collaborative care. 21 Collaborative care borrows much from chronic disease management and ensures the delivery of effective forms of treatment (such as pharmacotherapy and/or brief psychological therapy) through augmenting the role of non-medical specialists in primary care. Collaborative care is a model whereby the non-medical specialists, or case managers, form a close collaboration with the person with depression and others involved in their care. The case manager acts as a conduit for the passage of information between all individuals involved and supports the participant to enable effective discussion of important problems. Case managers provide information and help participants to access appropriate services, such as social care and voluntary sector services.
The ubiquity of depression in primary care settings, along with the poor integration and co-ordination of care, has led to the development of, and increased use of, this model of care. In a 2012 Cochrane review22 of 79 randomised controlled trials (RCTs) (24,308 participants), clear and robust evidence of the effectiveness of collaborative care was shown. It improved depression outcomes in both the short and medium term. Moreover, there was evidence to suggest that collaborative care can be cost-effective by reducing health-care utilisation and improving overall quality of life. 23,24 However, the greater proportion of studies related to working-age adults. A relative lack of any evidence for older adults was identified, which led to calls for further research on collaborative care among that age group. One important exception was the evidence provided by the US Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) study of the effectiveness of collaborative care for older adults.
The IMPACT study was conducted by Unützer et al. 16 for those aged > 60 years with case-level clinical depression. The main finding was that, at 12 months, depression severity was at least 50% improved from baseline in almost half the participants in the intervention group, but only one in five of those receiving usual care. In 2007, a UK feasibility trial9 of collaborative care in older adults showed some positive results. In recent years, the evidence base has expanded, although not with direct reference to older adults. The CollAborative DEpression Trial (CADET)11 showed that collaborative care was effective at improving depression outcomes in a UK primary care population, and the Collaborative Interventions for Circulation and Depression: study protocol for a cluster randomized controlled trial of collaborative care for depression in people with diabetes and/or coronary heart disease (COINCIDE) trial10 showed a modest effect at reducing depression and improving self-management of chronic disease.
In addition to the provision of collaborative care, the studies also provide information and support to enable participants to undertake brief psychological therapies, in this case behavioural activation. Behavioural activation for the CASPER plus trial was adapted from the behavioural activation intervention delivered in CADET. 11 Manualised psychological interventions, such as behavioural activation, may benefit individuals experiencing depressive symptoms. It focuses on addressing the behavioural deficits common among those with depression by reintroducing positive reinforcement and reducing avoidance. Such interventions aim to manipulate the behavioural consequence of a trigger (environmental or cognitive) rather than directly interpret or restructure cognitions. 25 Behavioural activation is about helping patients to ‘act their way out’ of depression rather than wait until they are ready to ‘think their way out’. Helping people to identify and reintroduce valued activities that they have stopped doing, or to introduce ones they would like to take up, is an important component. The effectiveness of this psychological approach is now well demonstrated. 26,27 Behavioural activation can be readily delivered by a trained case manager either over the telephone or face to face (for those who experience difficulty using or accessing telephone-based therapy). 28
Limitations of previous trials
The major limitation of previous trials was an absence of a definitive UK trial of collaborative care in older adults with depression. The absence of UK trials of collaborative care was highlighted in 2009 guidance for depression issued by NICE, and the need for such trials was highlighted as a research priority. 7 We proposed to measure the clinical effectiveness and cost-effectiveness of using collaborative care on older adults with major depression in response to a lack of evidence of its benefit to the older population in UK primary care.
Chapter 2 Research objectives
The research objectives of this trial were to:
-
establish the clinical effectiveness of a collaborative care intervention for older people with screen-positive above-threshold (‘major depressive episode’) depression within a definitive RCT
-
examine the cost-effectiveness of a collaborative care intervention for older people with screen-positive above-threshold (‘major depressive episode’) depression across a range of health and social care costs within a definitive RCT.
The definitive RCT was preceded by a developmental phase to produce a manualised collaborative care intervention for older people and an internal pilot trial to optimise recruitment, randomisation and retention, and we report these preparatory objectives within the body of this report.
Chapter 3 Methods
For CASPER plus, those patients identified at the screening phase as having above-threshold, case-level depression will be eligible to enter the CASPER plus substudy.
Trial design
We conducted a pragmatic, multicentred, two-arm, parallel, open RCT. Participants with major depression were individually randomised (1 : 1) to receive either collaborative care in addition to usual GP care, or just usual GP care.
Approvals obtained
This study was approved by NHS Leeds East Research Ethics Committee (REC) on 28 September 2010 (REC reference number 10/H1306/61). Research management and governance approval was obtained for each trial centre thereafter (see Appendix 1). This trial was assigned the International Standard Randomised Controlled Trial Number of ISRCTN45842879.
Trial centres
Four centres in the north of England were selected as trial sites: (1) York centre (the core study centre) covering the city of York, Harrogate, Hull and the surrounding areas; (2) Leeds centre and the surrounding area; (3) Durham centre and the surrounding area; and (4) Newcastle upon Tyne centre, including Northumberland and North Tyneside. Each centre was responsible for co-ordinating the recruitment of participants into the study (trial and epidemiological cohort).
Duration of follow-up
All participants were followed up by questionnaire at 4, 12 and 18 months (see Chapter 4).
Participant eligibility
Inclusion criteria
People for whom both of the following criteria applied:
-
aged ≥ 65 years
-
identified by GP practice as being able to take part in collaborative care.
Exclusion criteria
Potential participants were excluded if identified by primary care clinicians as meeting one of the following criteria:
-
known alcohol dependency (as recorded on GP records)
-
known to be experiencing psychotic symptoms (as recorded on GP records)
-
any known comorbidity that would, in the GP’s opinion, make entry to the trial inadvisable (e.g. recent evidence of suicidal risk or self-harm, significant cognitive impairment)
-
other factors that would make an invitation to participate in the trial inappropriate (e.g. recent bereavement, terminal malignancy).
Sample size
To detect a minimum standard effect size of 0.35 (aligning with the US IMPACT study16 and our previous CASPER trial29,30) with 80% power and a two-sided 5% significance level, 260 patients (130 per arm) would be required. Although this is an individually randomised trial, there may be potential clustering at the level of each collaborative care case manager, and hence the sample size was inflated to account for this. Based upon an estimated intracluster correlation coefficient (ICC) of 0.02 and a case load size of 20, the design effect would be 1.38 {1 + [(20 – 1) × 0.02]} and 360 patients (180 in each arm) would be required. Allowing for 20% loss to follow-up, the final sample size needed was 450 patients (225 per arm).
Epidemiological cohort
During the first year of the CASPER plus trial, an epidemiological cohort was assembled. This consisted of people who had consented to participate in the trial but who were not depressed. Through our broad inclusion criteria we successfully recruited a total of 4668 patients aged ≥ 65 years into the CASPER cohort, from who we identified those with major depression who were eligible to participate in the CASPER plus trial. The reasons for this strategy were twofold: first, to recruit an adequate number of potential participants who would subsequently be identified as having depression, as we believed this would not always be recorded on GP records; and, second, to establish an epidemiological cohort of older adults who could be followed up and who would help inform the knowledge base around the health and well-being of older adults. This type of study design is termed a cohort multiple RCT. 31
Recruitment into the trial
Recruitment of all participants into the trial took place through primary care. GP practices agreed to participate after a member of the study team had introduced it to them with written information, followed by a face-to-face visit to explain the study and what participation would involve. Patients were identified by a computer search and then invited to participate in the CASPER study by their general practice, which posted an invitation pack to all eligible patients. The packs comprised an invitation letter (see Appendix 2) signed from the general practice, a consent form (see Appendix 3), a decline form (see Appendix 4), a participant information sheet (see Appendix 5), a background information sheet (see Appendix 6) and a prepaid return envelope addressed to the core study centre. No patient-identifiable data were available to the study teams until patients returned their consent form.
Consenting participants
During the consent stage, potential participants were asked to complete the Whooley questions,32 a two-item depression-screening/case-finding tool. These questions were asked at two different time points – on the background information sheet at invitation and in the baseline questionnaire – both times as self-reports. At the consent stage, participants were informed about the opportunity of participating in other related studies (e.g. qualitative studies) and were asked to indicate if they agreed to be approached in the future by ticking a box on the consent form. All participants who consented to take part in the CASPER study at this stage became part of the CASPER cohort. Participants did not become part of the CASPER plus trial until they had been subsequently assessed for suitability by completing a standardised diagnostic interview and randomisation.
Baseline assessment
On receipt of written consent from participants by the return of their consent form via post, baseline data were collected through a self-report questionnaire. All participants who returned completed consent forms to the core study centre were sent a baseline questionnaire (see Appendix 7). Participants were asked to respond to the Whooley questions32 for a second time and to provide self-report medication data. They were also asked to complete a range of health surveys, which consisted of the Patient Health Questionnaire-9 items (PHQ-9)33 – a measure of depression severity using a nine-item depression scale in reference to how a respondent has been feeling over the past 2 weeks; the Short Form questionnaire-12 items (SF-12)34 – a measure of health-related quality of life to obtain health-state utility by estimating the Short Form questionnaire-6 Dimensions (SF-6D); the EuroQol-5 Dimensions, 3 levels (EQ-5D-3L)35 – a standardised measure of health-state utility, designed primarily for self-completion by respondents; the Generalised Anxiety Disorder-7 item (GAD-7)36 scale – a severity measure of generalised anxiety used to gauge the past 2 weeks; the Patient Health Questionnaire-15 items (PHQ-15)37 – a measure of somatic complaints using a 15-item scale in reference to the last month; and the Connor–Davidson Resilience Scale-2 items (CD-RISC2)38 – used to measure an individual’s resilience and ability to bounce back.
Randomisation
Randomisation was carried out by the York Trials Unit Randomisation Service [www.yorkrand.com (accessed 23 June 2016)], accessed by a trained researcher from the study team. Participants were automatically randomised by a computer on a 1 : 1 basis by simple unstratified randomisation to either the intervention group or control group, following the completion of a diagnostic interview. All diagnostic interviews were conducted over the telephone by a trained researcher from the study team. The major depressive episode module of the Mini International Neuropsychiatric Interview (MINI) was used to ascertain the presence or absence of core depressive symptoms. 39 The MINI shows good agreement with other semistructured diagnostic interviews conducted to internationally recognised standards. 40–42 This allowed potential recruits to be identified as having major depressive disorder (five or more symptoms), subthreshold depression (two to four symptoms) or no depression (one symptom) (Table 1). 39,43,44 All participants diagnosed with major depressive disorder were randomised to either the intervention or the control arm.
| Key symptoms | Other symptoms |
|---|---|
| Depressed mood | Substantial changes in weight/appetite |
| Loss of interest | Change in sleep patterns |
| Change in energy levels | |
| Movement slowing down or speeding up | |
| Feeling guilty or worthless | |
| Unable to make decisions | |
| Thinking of death or suicide |
Once participants had been randomised, they were sent a letter informing them of the outcome of their diagnostic interview. If their MINI outcome was major depression, they were informed of their group allocation, either collaborative care or usual care. The participant’s GP was also sent a letter informing them that the named patient was eligible to take part in the CASPER plus trial owing to the major depression outcome of their diagnostic interview. It also specified which arm of the trial they had been randomised to.
Ineligible participants
All participants whose outcome was not major depression (either non-depressed or subthreshold) were sent a letter informing them that they were ineligible for the CASPER plus trial but that they would remain in the CASPER epidemiological cohort and continue to be followed up via questionnaires. Their GPs were also informed of this. This process of following up non-trial participants was discontinued once the original CASPER trial completed (see Chapter 4).
Trial interventions
Control group
Participants in the control group were allocated to receive usual GP care. They received no care additional to the usual primary care management of major depression offered by their GP in line with NICE depression guidance as implemented by their GP and local service provision. 7,8
Intervention group
Participants in the intervention group were allocated to receive a low-intensity programme of collaborative care using behavioural activation, designed specifically for those aged ≥ 65 years with major depression. Collaborative care was delivered by a case manager [a primary care mental health worker/Improving Access to Psychological Therapies (IAPT) worker] for an intended 8–10 weeks. This took place alongside participants’ usual GP care. The defining feature of collaborative care is a collaboration of expertise to help support the participant. A case manager works alongside the participant, sharing any relevant information with the GP and a mental health specialist (psychiatrist or psychologist). The case manager is a cohesive link between the participant and other professionals involved in their care. For example, a case manager who deemed a participant’s depressive symptoms to have deteriorated would pass this information on to the participant’s GP, who would optimise the management of the patient’s condition.
Collaborative care in the CASPER plus trial consisted of telephone support, symptom monitoring and active surveillance, facilitated by a computerised Patient Case-Management Information System (PC-MIS) [www.york.ac.uk/healthsciences/pc-mis (accessed 23 June 2016)] and low-intensity psychosocial management (behavioural activation). Participants randomised to the collaborative care intervention group were contacted by a case manager within 1 week of their randomisation to arrange their first collaborative care session. This was carried out face to face, usually at the participant’s home unless an alternative venue was preferred. After this initial meeting, subsequent sessions were carried out on a, more or less, weekly basis by telephone unless the participant had sensory impairments or preferred face-to-face visits. Case managers worked collaboratively with the participants, liaising with GPs and other health professionals involved in their care to discuss issues relating to participants’ mental and physical health, both during routine updates and when any concerns were identified. This included liaising with GPs as necessary to consider reviews of medication, which could relate to depression but also to comorbid physical health problems. It also included discussing with GPs referrals to other services, such as health services (e.g. pain clinics) or engagement with social services. Case managers worked with the participants to identify problems and agree goals for the intervention. They also worked with participants to identify, and subsequently provide, information about other services that may be useful, such as voluntary and statutory sector organisations and services.
Refinement of collaborative care/behavioural activation
The delivery of collaborative care and behavioural activation had been established in working-age adults for whom an appropriate training package and manual already existed. 28 However, these had not been tailored for use with older adults diagnosed with major depression. Before the study began, necessary changes were made to both the training package and manual (detailed in this section) to account for differences that may exist in the older adult population.
Training occurred over 2 days and involved a combination of brief lectures and role-play. Topics covered were the collaborative care approach as applied to older adults, medication management in older adults, behaviour theory and behavioural activation as adapted for older adults.
Adaptations to language and content
Adaptations were made to the information gathered at the initial assessment. Older adults are more likely to experience long-term health problems and a reduced level of functioning, with their psychological status often closely linked to their physical functioning. 45 Additional questions regarding health conditions and their impact were added to the standard assessment format. However, case managers were reminded to deliver a person-centred approach and not let preconceptions about the level of functioning of older adults influence their information gathering. Liaison with health professionals who were involved in treating the participant’s long-term health conditions was encouraged to promote a depth of understanding of these issues. Depression in older adults is associated with impaired social support;46 therefore, additional questions regarding social contacts and family were added. The risk assessment (see Appendix 8) was also adapted to enquire about past passive and past active suicide ideation as well as current plans and preparations, as past suicidality is a risk factor for current suicidal behaviour. 47
Information in the manual was tailored to meet the needs of older adults. Age-appropriate examples were used, such as bereavement and loss of role, to facilitate engagement and make it easier to relate to. The psychoeducation material given to participants was also modified to include information about depressive symptoms that occur specifically in older adulthood. As depression is associated with cognitive impairment in older people,48 a larger font and increased space for writing was introduced. In addition, when individuals displayed mild cognitive impairment, simpler language was used and the number of steps in each session, along with the homework, was reduced. Questions were also added to help the case manager assess the participant’s understanding of the treatment principles.
Functional equivalence and keeping well
Case managers were made aware of the importance of helping patients to identify functionally equivalent activities and a section was added to the Keeping Well Plan to prompt participants to identify functionally equivalent activities that may replace enjoyable or rewarding activities they were no longer able to undertake. Further details of the adaptations made can be found in Pasterfield et al. 49
Participant follow-up
All participants in the CASPER plus trial were followed up with questionnaires at 4 months (see Appendix 9), 12 months (see Appendix 10) and 18 months (see Appendix 11). All post-randomisation questionnaires were posted to participants from the York Trials Unit along with a pre-addressed prepaid envelope. Participants could complete the questionnaires manually and return them by post to York Trials Unit or they could complete the questionnaire online; an instruction sheet explaining how to log on to the CASPER study site and complete the process was included with each questionnaire. Reminder letters were sent by post at 2 weeks to any participants who had not returned their questionnaire. Telephone follow-up by one of the study team’s researchers was conducted for any participants who did not return the reminder questionnaire in order to complete the primary outcome measure (PHQ-9) at the very least.
Trial completion and exit
Participants were deemed to have exited the trial when they:
-
withdrew consent (wished to exit the trial with no further contact for follow-up or objective data)
-
had been in the trial for 18 months post randomisation
-
had reached the end of the trial
-
died
-
moved general practice to one not participating in the CASPER study
-
had another reason to exit according to clinical judgement from a health professional.
Withdrawals
Withdrawal could occur at any point during the study at the request of the participant. If a participant indicated that he or she wished to withdraw from the study, a researcher would speak to the participant to clarify to what extent they wished to withdraw: from the intervention, from the follow-up or from all aspects of the study. When withdrawal was only from the intervention, then follow-up data continued to be collected. Data were retained for all participants up to the date of withdrawal, unless they specifically requested for their details to be removed.
Objective data
Once the CASPER plus trial participants of a general practice had completed their follow-up, objective data were collected for each trial participant. Objective data consisted of details on each participant’s prescribed medication and the number of contacts they had with their general practice during their time in the trial. The only exception was for those participants who had withdrawn in full, thereby withdrawing consent to access their medical records. Objective data were collected from general practices via request from the core study centre. A spreadsheet template was e-mailed to the key contact of each general practice that included the identification codes of each trial participant for the practice with prewritten frozen headings: there were no identifiable data. The search dates for each participant were also listed, from the date they were randomised until either the date they completed the study 18 months later or the date that they had died, if that was the case. Data were still collected on participants who had withdrawn from treatment or follow-up, as they had provided us with consent to access their health records for the 18 months that they would have been in the study. The transfer of all objective data via e-mail was approved on the basis that no identifiable data were shared either with the general practice at the request stage or with the core study centre at the stage that objective data were returned.
Suicide protocol
A small but elevated risk of suicide and self-harm was inherent in the study population, all members of which had been identified as having major depression. All participants (both usual care and collaborative care) were subject to usual GP care and GPs were responsible for the day-to-day management of major depression. GPs were accountable for all treatment and management decisions including prescribing of medication, referral and assessment of risk. This arrangement was made clear to all clinicians and general practices that agreed to participate in the study. The pragmatic nature of the CASPER plus trial meant that we did not seek to influence this arrangement. However, we did follow good clinical practice by monitoring for suicide risk during all our encounters with participants. When a patient expressed a risk through thoughts of suicide or self-harm, we followed the study-specific procedure for suicide risk (see Appendix 8).
Patient and public involvement in research
The CASPER plus trial was informed by the involvement of users of mental health services and carers throughout the research period. An advisory group was established in the early stages of study. This consisted of a number of older adults, some of whom had mental health conditions, along with a carer representative. This group provided valuable insights into the relevance and readability of the study documentation. In the future, we plan to engage patient and public involvement in our dissemination strategies to guide on how best to share the findings.
Further studies
Following completion of the CASPER trial, the Self Help At Risk Depression (SHARD) substudy (not described in this report) was introduced to randomise participants identified with subthreshold depression to receive a self-help workbook or usual GP care. Results from the SHARD study will follow.
Clinical effectiveness
Primary outcome
The primary end point for the trial was patient-reported depression severity, as measured by the PHQ-933 at 4 months’ follow-up. Each item is scored from 0 to 3; thus, PHQ-9 scores can range from 0 to 27, with higher scores indicating more severe depression. Total scores from 0 (non-depressed) to 27 (severely depressed) were calculated based on the nine PHQ-9 items. These data were collected via self-report on the follow-up questionnaires. Any participants who did not return a completed questionnaire were sent a reminder, and those participants who did not respond were telephoned by one of the study team’s researchers to ask them to complete the PHQ-9 over the telephone. Missing items were replaced with the mean of the remaining items if one or two items were missing.
The PHQ-9 data were collected at baseline and randomisation (at the diagnostic interview), as well as at 4, 12 and 18 months’ follow-up. Scores at baseline and randomisation are reported in Chapter 5, Baseline characteristics. When analyses were adjusted for initial PHQ-9 score, the score at randomisation was used. The primary end point for the CASPER plus trial was at 4 months’ follow-up. At that point, treatment differences in the magnitude of a standard effect size of 0.35 were sought, which is of moderate size for psychological interventions and in line with collaborative care effects observed in other studies. Cohen50 classifies a standard effect size of 0.3 as a small to medium effect size, and this is in line with NICE guidelines for depression, which adopts a similar grading of clinical significance. Four months was selected as the primary end point, because it would occur soon after the end of the planned treatment but allow some additional time in the event that it was not possible to see participants on a weekly basis for practical reasons (e.g. holidays).
Secondary outcomes
The secondary outcome measures used were:
-
depression severity and symptomatology at 12 and 18 months (PHQ-9)
-
binary depression severity at 4, 12 and 18 months (PHQ-9), using scores of ≥ 10 to designate moderate depression caseness
-
quality of life at 4, 12 and 18 months (SF-12 and EQ-5D-3L)
-
psychological anxiety at 4, 12 and 18 months (GAD-7)
-
mental health medication at 4, 12 and 18 months
-
physical health problems at baseline, 4, 12 and 18 months (PHQ-15)
-
psychological resilience at baseline, 4, 12 and 18 months (CD-RISC2)
-
mortality at 4, 12 and 18 months.
Short Form questionnaire-12 items
The SF-1234 is a generic health status measure and a short form of the Short Form questionnaire-36 items health survey. It consists of 12 questions measuring eight domains (physical, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health) rated over the past month. Questions have three or five response categories, and responses are summarised into a physical component summary (PCS) score and mental component summary (MCS) score. The PCS and MCS scores range from 0 (the lowest level of health) to 100 (the highest level of health) and were designed to have a mean score of 50 in a representative sample of the US population. Therefore, scores > 50 represent above average health status, and vice versa. The SF-6D was estimated from responses to the SF-12 questionnaire and provided health-state utilities to inform cost–utility analysis.
EuroQol-5 Dimensions, 3 levels
The EQ-5D-3L35 is a standardised measure of current health status developed by the EuroQol Group for clinical and economic appraisal. The EQ-5D-3L consists of five questions each assessing a different quality-of-life dimension (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Each dimension is rated on three levels: no problems (score = 1), some problems (score = 2) and extreme problems (score = 3). A weighted summary index can be derived to give a score between 1 (perfect health) and 0 (death). For the purpose of the clinical effectiveness analysis, only scores of the individual dimensions were utilised. Health-state utilities (along with SF-6D) were estimated to potentially inform the cost–utility analysis; however, the SF-6D was ultimately found to be more sensitive to change in this cohort.
Generalised Anxiety Disorder-7 items scale
The GAD-736 is a brief measure of symptoms of anxiety based on diagnostic criteria described in Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition. 43 It consists of seven questions and is calculated by assigning scores of 0, 1, 2 and 3 to the response categories of ‘not at all’, ‘several days’, ‘more than half the days’ and ‘nearly every day,’ respectively. GAD-7 total score for the seven items ranges from 0 to 21. Scores of 5, 10 and 15 represent cut-off points for mild, moderate and severe anxiety, respectively.
Patient Health Questionnaire-15 items
The PHQ-1537 is a 15-item physical health problems questionnaire. Each health issue is rated as 0 (not bothered), 1 (bothered a little) or 2 (bothered a lot). Items are added to form a scale from 0 to 30, higher scores indicating worse symptom severity. Scores of 5, 10 and 15 have been used as cut-off points for low, medium and high symptom severity. Item 4 of the PHQ-15 (menstrual problems) was deemed not relevant for the older CASPER patient population and omitted from all questionnaires. Therefore, the total possible PHQ-15 score was 28.
Connor–Davidson Resilience Scale two-items
The CD-RISC238 is a two-item short form of the full Connor–Davidson Resilience Scale-25 items. It is a psychological resilience measure with specific items for bounce-back from adversity and adaptability to change. Agreement with the two items is scored from 0 to 4, resulting in a total score of 0 to 8, where a higher score indicates greater resilience.
Mental health medication
Medication data were captured by self-report on the follow-up questionnaires. Participants indicated prescribed medication by selecting from a list of 10 antidepressants, as well as listing any other medications they were prescribed.
Mortality data
A data linkage service was established with the NHS Digital to provide regular updates from the Office for National Statistics (ONS) mortality data on any trial participants who had died while in the study. Members of the research team recorded any identified deaths, date and cause of death on the study management database.
Other collected patient questionnaire data
Adverse events
The CASPER plus study was not a Clinical Trial of an Investigational Medicinal Product and was, therefore, not subject to any additional restrictions. Decisions regarding prescription of medications were made by the participant in conjunction with their GP: participation in the study had no bearing on this process. Any participants who asked a member of the CASPER plus study team for an opinion on medication issues were strongly encouraged to seek advice from their GP.
The study recorded details of all serious adverse events (SAEs). Any judged to have been related to the study were required to be reported to the REC under the terms of the standard operating procedures for RECs. 51 In the context of the older adult population of the CASPER plus study, many of the SAEs were expected: unscheduled hospitalisations, life-threatening conditions, incapacitating illnesses and deaths. These were not perceived as unexpected events; therefore, they would be reported as SAEs only if they appeared to be related to an aspect of taking part in the study (e.g. participation in treatment, completion of follow-up questionnaires, participation in qualitative substudies or telephone contact).
When a SAE was identified, the trial manager was informed by e-mail using a participant’s trial identification number, and not by any identifiable data. He or she then informed the chief investigator and two members of the Trial Management Group, who jointly decided if the event should be reported to the REC as a SAE. A SAE form was completed and a copy was filed securely at the core study centre. Any unexpected SAEs that were also judged to have been related should have been reported to the main REC within 15 days of the chief investigator becoming aware of the event. In the CASPER plus study, none of the SAEs were judged to have been related to the trial.
The occurrence of adverse events during the trial was monitored by an independent Data Monitoring Ethics Committee and the Trial Steering Committee. The Data Monitoring Ethics Committee/Trial Steering Committee would have seen immediately all SAEs thought to be treatment related.
Data collection schedule
An overview of the time points at which trial data were collected is presented in Table 2.
| Data collected | Time point | |||||
|---|---|---|---|---|---|---|
| Invitation | Baseline | Diagnostic interview/randomisation | 4 months’ follow-up | 12 months’ follow-up | 18 months’ follow-up | |
| Consent/decline | ✓ | |||||
| Demographics | ✓ | |||||
| Whooley questions | ✓ | ✓ | ||||
| Physical health problems | ✓ | |||||
| MINI major depressive module | ✓ | |||||
| PHQ-9 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| SF-12 | ✓ | ✓ | ✓ | ✓ | ||
| EQ-5D-3L | ✓ | ✓ | ✓ | ✓ | ||
| GAD-7 | ✓ | ✓ | ✓ | ✓ | ||
| PHQ-15 | ✓ | ✓ | ✓ | ✓ | ||
| CD-RISC2 | ✓ | ✓ | ✓ | ✓ | ||
| Mental health medication | ✓ | ✓ | ✓ | ✓ | ||
| Mortality | ✓ | ✓ | ✓ | |||
| SAEs | ✓ | ✓ | ✓ | |||
Statistical assumptions
Participants, care deliverers and the study team were not blinded to treatment allocation. However, allocations were concealed (group A and group B) for interim study reports, for example for the purpose of independent data monitoring reporting. The trial statistician who was responsible for the final statistical analysis was kept blind to group allocation until the primary analysis had been completed.
All analyses were conducted on intention-to-treat basis, using a two-sided statistical significance level of 0.05 unless otherwise stated. A full specification of the statistical analyses is documented in the CASPER plus statistical analysis plan (version 1.0). Any additional data assumptions for data, once received from the York Trials Unit data management team and the CASPER plus trial management team, for the purpose of this report, are documented separately.
Statistical analysis
Baseline characteristics
All participant baseline data (demographics from the background information form, outcome data from the baseline questionnaire, PHQ-9 and MINI responses from the diagnostic interview) were summarised descriptively by trial arm for all randomised participants and all participants included in the primary analysis.
The analysis population included all patients in their randomised groups with available outcome data (for the primary analysis: PHQ-9 score at 4, 12 or 18 months’ follow-up) as well as complete baseline covariates specified for the analysis.
Primary analysis
Unadjusted descriptives of depression severity (PHQ-9) at all follow-up time points were presented. A covariance pattern linear mixed-effects model was used to compare collaborative care with usual care on PHQ-9 scores at 4 months. Effects of interest and baseline covariates were specified as fixed effects, and the correlation of observations within patients over time was modelled by a covariance structure to describe the random effects. The mixed model provided increased statistical power by utilising all patients with outcomes for at least one follow-up time point.
The outcome modelled was PHQ-9 at 4, 12 and 18 months. The model included time, trial arm and time-by-treatment interaction as fixed effects, adjusting for PHQ-9 score at randomisation and physical/functional limitations (as measured by the baseline SF-12 PCS score). Different covariance structures for the repeated measurements available in the analysis software were explored, and the most appropriate pattern was used for the final model based on the model Akaike information criterion. The primary end point was the estimate of the effect of the intervention at 4 months, which is presented with 95% confidence intervals (CIs) and associated p-values.
Secondary analyses
The primary analysis model was repeated (1) including case managers as a random effect to account for clustering within case managers, (2) including additional covariates predictive of PHQ-9 scores at 4 months as identified by univariate regressions, (3) including additional covariates predictive of non-response at 4 months as identified by univariate regressions and (4) using multiply imputed data. Results from the secondary analyses were compared with those from the primary analysis in order to ascertain the robustness of any observed treatment differences.
Secondary outcomes
Patient Health Questionnaire 9-items depression severity estimates at 12 and 18 months were extracted from the primary analysis model and presented with 95% CIs and associated p-value. A logistic mixed-effects model was used to compare PHQ-9 depression caseness (scores of ≥ 10), using the same covariates as the primary analysis. Odds ratios and 95% CIs are presented for the effect of the intervention at 4, 12 and 18 months. Analyses of other secondary outcomes were conducted using linear or logistic mixed models, depending on the outcome measure, adjusting for PHQ-9 score at randomisation and baseline SF-12 PCS score as well as the outcome measure at baseline. Treatment effects at each time point were reported. EQ-5D-3L responses were reported descriptively as part of the statistical analysis and analysed fully as part of the economic analysis. Frequencies of adverse events were reported descriptively by treatment arm, including breakdown by type and estimated relatedness to the intervention. The number of deaths occurring in the 18-month trial period was summarised by trial arm and overall. A chi-squared test was used to compare proportions between trial arms if more than five participants died in each arm.
Economic analysis
Economic analysis took the form of a cost-effectiveness analysis and, in line with NICE guidance,52,53 adopted the perspective of the health and personal social services. The aim of the analysis was to estimate the value for money of providing collaborative care as compared with usual care. The time horizon for the analysis was 18 months from the date of randomisation; therefore, costs and quality-adjusted life-years (QALYs) were discounted at 3% for observations beyond 12 months. The analysis was conducted in Stata® version 13.1 (StataCorp LP, College Station, TX, USA).
Quality-adjusted life-years were estimated from responses to the SF-12 questionnaire to estimate SF-6D health state utilities. 54 This enables comparisons to be made across different health interventions and provides extra information for decision-makers. QALYs were estimated by measuring the area under the curve55 that joins the baseline and follow-up SF-6D utility scores, which was derived from population-based values.
A base-case cost of collaborative care was estimated, based on the case manager training manual, which describes the treatment protocol (the manual is available from the authors on request). Over the full intended duration of the study (i.e. 18 months), participants’ health-care resource use was collected to estimate total cost of health care during treatment and the follow-up period. Various methods of collecting resource use data were initially considered (e.g. self-report questionnaires and medical record checks). Objective data were obtained from general practices giving information on participants’ (1) contacts with GPs (appointments, home visits or telephone consultations), (2) contacts with practice nurses (appointments or telephone consultations) and (3) prescriptions (although we were unable to analyse these data owing to methodological challenges). Given the sample age (≥ 65 years), additional ‘self-report questions’ were not added in order to limit overall questionnaire burden. National unit costs applied to the quantities of resources utilised. 56
For decision analysis, costs of the intervention, health-care use and changes in QALYs in the RCT will be combined to calculate the incremental cost-effectiveness ratio (ICER) using the following formula:
where C is the costs and E is the effects (as QALYs) in the intervention (I) or control (C) arm.
To estimate the joint distributions of cost and QALYs, non-parametric bootstrapping was conducted on the observed data. 57 This non-parametric bootstrap resampling technique allows us to assess uncertainty in the ICER. 58 First, results of the bootstrapped cost and QALYs are presented on the cost-effectiveness plane. The confidence ellipse indicates the incremental costs and QALYs on the 50%, 75% and 95% CIs, indicating the probability space in the cost-effectiveness plane within which we are confident that the true ICER is found.
To further evaluate the joint distributions of costs and benefits, a cost-effectiveness acceptability curve (CEAC) is generated. 59 The CEAC summarises information on uncertainty in cost-effectiveness estimate and illustrates how the probability that collaborative care will be cost-effective as the willingness-to-pay of decision-makers increases. According to NICE, the willingness-to-pay threshold for an additional QALY ranges between £20,000 and £30,000; the CEAC indicates the probability that collaborative care is within this range.
Participants’ take-up of collaborative care was recorded during sessions by case managers. This allowed deterministic sensitivity analysis of the potential variation in direct costs of intervention. Over the course of treatment, the case managers recorded information on the duration of the contact and how this took place for each contact with the participants. This information was used to adjust the expected cost of collaborative care when the patient, the case manager and supervisors agreed to deviation from the manualised intervention. The results were expressed on a CEAC and adjusted probabilities of falling within the NICE range of willingness to pay are presented.
Sensitivity analysis was performed to examine the implication to fidelity to intervention sessions and an ex post adjustment of the expected direct cost of collaborative care. The prescription of a programme of collaborative care is based on an assumption that all participants received the full course of treatment (i.e. 8–10 sessions) and this is an ex ante assumption underlying our base-case cost-effectiveness analysis.
Given that a service provider has intention to treat, the resources required to supply all of the intended sessions for collaborative care must be allocated and, therefore, the budget must include the total expected cost. However, after the allocation of a treatment package, individuals will have varying levels of fidelity to the programme and the expected direct cost of collaborative care may be adjusted when non-attendance of sessions is clearly documented.
All case managers were asked to log their activities with patients on PC-MIS (Patient Case Management Information System; www.york.ac.uk/healthsciences/pc-mis/; accessed 29 May 2016), which has been designed for IAPT. As collaborative care involves both assessment and treatment, demand may vary in relation to the specific levels of need of individuals. The number and duration of a participant’s contact with the case manager was contemporaneously logged on PC-MIS. It was noted whether or not these occurred face to face or by telephone.
Chapter 4 Protocol changes
The following changes were made to the original protocol, after it was initially approved by the REC on 28 September 2010 and the substantial amendment (number 6 of the CASPER trial) to run CASPER plus was approved on 20 April 2012 (see www.ncbi.nlm.nih.gov/pubmed/25409776; accessed 7 June 2016).
CollAborative care for Screen-Positive EldeRs plus trial
In the original CASPER protocol, the objective was to evaluate the clinical effectiveness and cost-effectiveness of a collaborative care intervention for older adults with subthreshold depression. In order to broaden the reach of CASPER, the CASPER plus trial and qualitative substudy were introduced to run concurrently, using the same recruitment procedure, interventions and measures to evaluate an adapted intervention for case-level depression. A separate CASPER plus protocol and amended study documents were developed and approved on 20 April 2012.
Recruitment methods
Direct referral
In order to maximise recruitment in an often difficult to reach group, an additional method of recruitment was introduced. In addition to the original strategy of sending an invitation pack by post to all patients (aged ≥ 65 years) who were identified by computer search as eligible for invitation by the general practice, this was supplemented with direct GP referral at patient consultation. GPs from participating practices were given a number of patient invitation packs (identical to those currently sent by post) that could be handed to patients aged ≥ 65 years who may be consulting about depression and who did not meet the exclusion criteria.
Targeted search
In order to optimise the search strategy, there was a move from an all-inclusive approach to a more targeted approach. The targeted search strategy was developed to include only patients who had a diagnosis of depression or those who were prescribed depression medication and had other conditions associated with an increased risk of depression (e.g. depression, low mood, antidepressant medication, ischaemic heart disease, diabetes, chronic obstructive pulmonary disease, arthritis or being a carer). This was done using the aforementioned Read codes (a coded thesaurus of clinical terms that have been used in the NHS since 1985), the choice of which was left to the discretion of the participating general practice.
Follow-up
Eighteen-month follow-up questionnaire
An 18-month follow-up questionnaire was introduced to obtain longer-term outcomes.
Cohort
In the original protocol, it was stated that all participants who returned screening questionnaires would be followed up at 4 and 12 months via post or online. This included participants both in the RCT and those in the epidemiological cohort. On completion of the CASPER trial of subthreshold depression, this policy was discontinued in order to maximise recruitment and retention to the CASPER plus trial. For the remainder of the trial, only CASPER plus trial participants were followed up. All potential cohort participants who had consented but did not meet the criteria for major depression at diagnostic interview were not followed up.
Inclusion/exclusion criteria
In the original protocol participants were excluded owing to alcohol dependency, any known comorbidity that would, in the GP’s opinion, make entry to the trial inadvisable (e.g. recent evidence of self-harm, known current thoughts of self-harm, significant cognitive impairment) and other factors that would make an invitation to participate in the trial inappropriate (e.g. recent bereavement, terminal malignancy) and/or because they were currently experiencing psychotic symptoms. During the trial there were several withdrawals from CASPER plus collaborative care condition as participants were already undergoing therapies and wished to continue with those therapies. Therefore, a screening question was added at the end of the diagnostic interview. This was not done at the invitation stage, to allow for people who had been referred to psychological services but had either not engaged with the service or who were still on a waiting list to participate in the study. People who answered ‘yes’ to this question did not proceed with the diagnostic interview and were excluded from the trial.
Recording of sessions
In the original protocol, there was no quality assurance procedure in place. In order to monitor and improve the quality of the collaborative care intervention delivered during the trial, a purposive sample of sessions was to be recorded. The sample was selected to reflect a range of backgrounds and experience of case managers. The allocation letter received by participants following randomisation was adapted from one that simply informed the participant that a case manager would be in touch shortly to a new letter that informed participants that we may wish to record some of their sessions with their case manager as a quality evaluation, stressing that the decision to agree to this was the participant’s alone and would not affect the treatment that he or she would receive. They also received an additional participant information sheet and consent form regarding the audio-recording.
Telephone delivery
In the original protocol all collaborative care participants were seen for their first session face to face. In the final stages of recruitment it was necessary to enable initial contacts to also be delivered by telephone to ensure that all participants could begin their collaborative care programme without delay. Some IAPT services deal exclusively with their patients via the telephone and so this mix of contacts reflects current practice in IAPT.
Chapter 5 Clinical results
Recruitment and flow of participants through the trial
Recruitment and follow-up
Participants were randomised into the CASPER plus trial between September 2012 and August 2014 from four UK sites and their surrounding areas in the north of England: York, Leeds, Durham and Newcastle upon Tyne. A total of 74 general practices screened their practice lists and identified patients who met the initial inclusion criteria. Exclusion criteria consisted of any known alcohol dependency and/or psychotic symptoms as recorded on GP records, any known adverse comorbidities or any other factors that GPs deemed made it inadvisable to invite patients, such as recent bereavement.
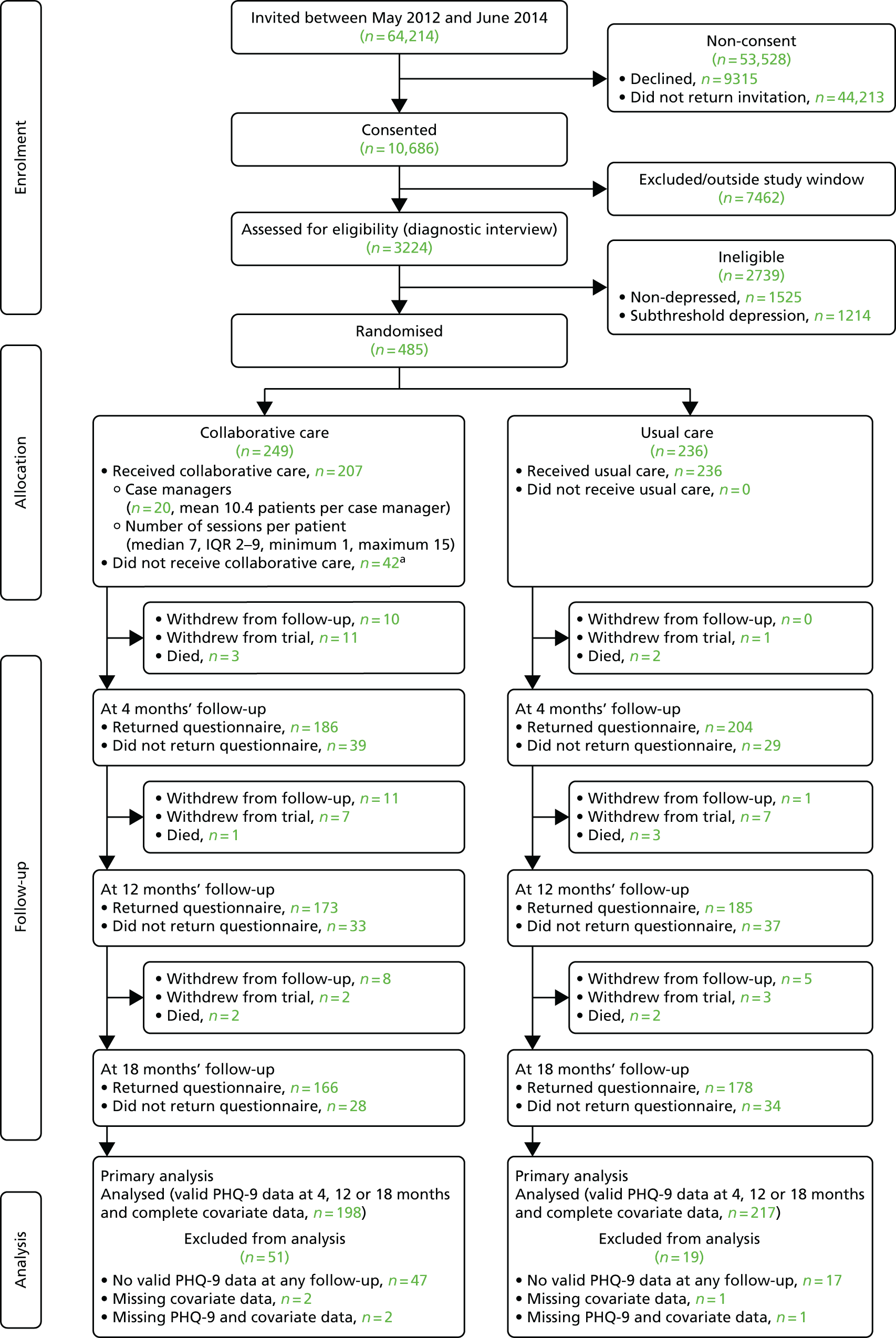
A total of 64,214 patients were identified by GP practices between 5 May 2012 and 10 June 2014 and invited by letter to take part in the CASPER study. Of 10,686 patients who consented, 3224 patients were assessed for eligibility by diagnostic interview. Based on the diagnostic interview, 485 (15%) patients were identified to have a major depressive episode and were randomised into the CASPER plus trial. Of the 485 participants randomised, 249 were allocated to collaborative care and 236 to usual care. The remaining patients were classified as having either below threshold depression (n = 1525) or subthreshold depression (n = 1214). They became part of the epidemiological cohort or were entered into the CASPER or CASPER SHARD trials if within the recruitment window for these trials. The randomised number of 485 participants exceeded that of the planned sample size of 450. The flow of participants is illustrated in Figure 1.
FIGURE 1.
Consolidated Standards of Reporting Trials diagram. a, Reasons for not receiving collaborative care: Carer – no time (n=1), causing marital unrest (n = 2), cognitive impairment (n = 1), did not wish to engage (n = 10), died (n = 2), invasive (n = 6), lost interest (n = 3), not low in mood (n = 1), physical disability (poor hearing) (n = 1), physical ill health (n = 8), receiving other counselling (n = 1), too busy (n = 2), too severely depressed (n = 1) and unable to contact (n = 3). IQR, interquartile range.
Trial withdrawals
Participants were able to withdraw from the study at any point. They were offered the options of withdrawing from the intervention only, from questionnaire follow-up (allowing continued collection of objective data) or from all aspects of the study. Data up to the date of withdrawal were retained for all participants, unless they specifically requested for their details to be removed. This happened on one occasion. The total number of trial withdrawals by trial arm is given in Table 3. Participants could withdraw from only collaborative care treatment but remain in the trial for follow-up purposes. A total of 83 participants (33%) in the collaborative care arm withdrew from treatment at some point, and the numbers of full or partial withdrawals were greater in this arm (n = 55) than in the usual-care group (n = 24).
| Type of withdrawal | Trial arm | |||
|---|---|---|---|---|
| Collaborative care (N = 55 withdrawn) | Usual care (N = 24 withdrawn) | |||
| n | % of 249 | n | % of 236 | |
| By 4 months’ follow-up | ||||
| Withdrawal from follow-up | 10 | 4.0 | 0 | 0.0 |
| Full withdrawal | 11 | 4.4 | 1 | 0.6 |
| Died | 3 | 1.2 | 2 | 1.1 |
| By 12 months’ follow-up | ||||
| Withdrawal from follow-up | 21 | 8.4 | 1 | 0.4 |
| Full withdrawal | 18 | 7.2 | 8 | 3.4 |
| Died | 4 | 1.6 | 5 | 2.1 |
| By 18 months’ follow-up | ||||
| Withdrawal from follow-up | 29 | 11.7 | 6 | 2.5 |
| Full withdrawal | 20 | 8.0 | 11 | 4.7 |
| Died | 6 | 2.4 | 7 | 3.0 |
When reasons for withdrawal were provided by the participant, these were documented in the study management database. Following completion of the trial, reasons were grouped into common categories, and these are listed in Tables 4–6 for the different types of follow-up.
| Reason for withdrawal | Trial arm | |||
|---|---|---|---|---|
| Collaborative care (N = 83 withdrawn) | Usual care (N = 0 withdrawn) | |||
| n | % | n | % | |
| Carer – no time | 4 | 4.8 | – | – |
| Causing marital unrest | 3 | 3.6 | – | – |
| Cognitive impairment | 1 | 1.2 | – | – |
| Did not wish to engage | 23 | 27.7 | – | – |
| Died | 2 | 2.4 | – | – |
| Does not need further support | 2 | 2.4 | – | – |
| Intervention not useful | 1 | 1.2 | – | – |
| Invasive | 7 | 8.4 | – | – |
| Lost interest | 3 | 3.6 | – | – |
| Not low in mood | 5 | 6.0 | – | – |
| Physical disability (poor hearing) | 1 | 1.2 | – | – |
| Physical ill health | 13 | 15.7 | – | – |
| Receiving other counselling | 3 | 3.6 | – | – |
| Too busy | 5 | 6.0 | – | – |
| Too severely depressed | 1 | 1.2 | – | – |
| Unable to contact | 7 | 8.4 | – | – |
| Unknown | 2 | 2.4 | – | – |
| Total | 83 | 100.0 | – | – |
| Reason for withdrawal | Trial arm | |||
|---|---|---|---|---|
| Collaborative care (N = 29 withdrawn) | Usual care (N = 6 withdrawn) | |||
| n | % | n | % | |
| Carer – no time | 1 | 3.5 | 0 | 0.0 |
| Did not wish to engage | 1 | 3.5 | 0 | 0.0 |
| Invasive | 3 | 10.3 | 0 | 0.0 |
| Lost interest | 14 | 48.3 | 1 | 16.7 |
| Moved out of area | 1 | 3.5 | 2 | 33.3 |
| Physical disability (poor sight) | 1 | 3.5 | 1 | 16.7 |
| Physical ill health | 4 | 13.8 | 0 | 0.0 |
| Suffered recent bereavement | 1 | 3.5 | 0 | 0.0 |
| Too busy | 2 | 6.9 | 1 | 16.7 |
| Too much effort | 0 | 0.0 | 1 | 16.7 |
| Too severely depressed | 1 | 3.5 | 0 | 0.0 |
| Total | 29 | 100.0 | 6 | 100.0 |
| Reason for withdrawal | Trial arm | |||
|---|---|---|---|---|
| Collaborative care (N = 20 withdrawn) | Usual care (N = 11 withdrawn) | |||
| n | % | n | % | |
| Carer – no time | 0 | 0.0 | 1 | 9.1 |
| Cognitive impairment | 1 | 5.0 | 1 | 9.1 |
| Did not wish to engage | 5 | 25.0 | 0 | 0.0 |
| Does not need further support | 1 | 5.0 | 0 | 0.0 |
| Invasive | 2 | 10.0 | 3 | 27.3 |
| Lost interest | 2 | 10.0 | 2 | 18.2 |
| Moved out of area | 2 | 10.0 | 2 | 18.2 |
| Physical ill health | 4 | 20.0 | 1 | 9.1 |
| Too much effort | 1 | 5.0 | 0 | 0.0 |
| Unable to contact | 1 | 5.0 | 1 | 9.1 |
| Unknown | 1 | 5.0 | 0 | 0.0 |
| Total | 20 | 100.0 | 11 | 100.0 |
The trial sample size calculation allowed for losses to follow-up of 20% at the primary end point at 4 months. The primary outcome (PHQ-9 depression severity) was available for 390 patients at that point, equating to an actual loss to follow-up of 19.6% (25.3% in the collaborative care arm and 13.6% in the usual-care arm).
The intervention: collaborative care
Collaborative care was offered to all patients in the intervention arm. A total of 21 case managers were trained to deliver the intervention, although only 12 delivered it in practice (a case load of 11.9 randomised patients per case manager). In practice, the intervention was delivered by 20 case managers (a case load of 10.4 patients who completed at least one session). Further details on the case load of each individual case manager are given as part of the practitioner analysis (see Chapter 3, Secondary analyses).
An overview of received treatments is provided in the Consolidated Standards of Reporting Trials diagram in Figure 1 and further details are presented in Tables 7 and 8. Of 249 randomised patients, 83% had at least one collaborative care session. Participants received on average six sessions over 8–9 weeks, of which, on average, one was delivered face to face and five were delivered over the telephone. The average session duration was 37 minutes. The most frequent reasons for not wanting to receive any collaborative care were not wishing to engage, physical ill health and invasiveness (Table 9).
| Collaborative care status | Patients randomised to collaborative care (N = 249) | |
|---|---|---|
| n | % | |
| Did not start treatment | 42 | 16.9 |
| Started treatment | 207 | 83.1 |
| Collaborative care details | Patients who received some collaborative care (N = 207) | |||||
|---|---|---|---|---|---|---|
| n | Mean | SD | Median | Minimum | Maximum | |
| Days from referral to first session | 207 | 34.4 | 25.5 | 27 | 7 | 220 |
| Number of sessions received | 207 | 6.0 | 3.48 | 7 | 1 | 15 |
| Face to face | 207 | 1.3 | 1.44 | 1 | 0 | 11 |
| Telephone | 207 | 4.8 | 3.37 | 5 | 0 | 11 |
| Average length of session (minutes) | 207 | 36.7 | 8.24 | 37 | 0 | 62 |
| Days from first to last session | 207 | 62.0 | 50.38 | 58 | 0 | 333 |
| Reason | Patients who received no collaborative care (N = 42) | |
|---|---|---|
| n | % | |
| Carer – no time | 1 | 2.4 |
| Causing marital unrest | 2 | 4.8 |
| Cognitive impairment | 1 | 2.4 |
| Did not wish to engage | 10 | 23.8 |
| Invasive | 6 | 14.3 |
| Lost interest | 3 | 7.1 |
| Not low in mood | 1 | 2.4 |
| Physical disability (poor hearing) | 1 | 2.4 |
| Physical ill health | 8 | 19.1 |
| Receiving other counselling | 1 | 2.4 |
| Too busy | 2 | 4.8 |
| Too severely depressed | 1 | 2.4 |
| Unable to contact | 3 | 7.1 |
| Died | 2 | 4.8 |
Baseline characteristics
Characteristics at consent, baseline and diagnostic interview (point of randomisation) for randomised participants and participants included in the primary analysis (‘as analysed’ population: patients with a valid PHQ-9 score at 4, 12 or 18 months’ follow-up) and valid covariate data (PHQ-9 score at randomisation and baseline SF-12 PCS score) are presented in Tables 10–12.
| Characteristic | As randomised | As analyseda | ||
|---|---|---|---|---|
| Collaborative care (N = 249) | Usual care (N = 236) | Collaborative care (N = 198) | Usual care (N = 217) | |
| Age at consent (years) | ||||
| n | 248 | 236 | 198 | 217 |
| Mean (SD) | 72.5 (6.57) | 71.8 (6.07) | 71.9 (6.03) | 71.6 (5.96) |
| Median (minimum, maximum) | 71 (64, 98) | 70 (65, 92) | 70 (64, 88) | 70 (65, 92) |
| Sex, n (%) | ||||
| Male | 98 (39.4) | 85 (36.0) | 81 (40.9) | 80 (36.9) |
| Female | 150 (60.2) | 151 (64.0) | 117 (59.1) | 137 (63.1) |
| Educated past 16 years of age, n (%) | 108 (43.4) | 101 (42.8) | 88 (44.4) | 95 (43.8) |
| Degree or equivalent professional qualification | 57 (22.9) | 68 (28.8) | 44 (22.2) | 62 (28.6) |
| Smoking (yes), n (%) | 30 (12.0) | 28 (11.9) | 25 (12.6) | 27 (12.4) |
| Three or more alcohol units/day, n (%) | 31 (12.4) | 26 (11.0) | 23 (11.6) | 23 (10.6) |
| Ethnicity, n (%) | ||||
| White | 241 (96.8) | 233 (98.7) | 193 (97.5) | 215 (99.1) |
| Asian or Asian British | 1 (0.4) | 0 (0) | 1 (0.5) | 0 (0) |
| Black or black British | 1 (0.4) | 0 (0) | 1 (0.5) | 0 (0) |
| Other | 3 (1.2) | 2 (0.8) | 3 (1.5) | 2 (0.9) |
| Health problems, n (%) | ||||
| Diabetes | 59 (23.7) | 47 (19.9) | 49 (24.7) | 42 (19.4) |
| Osteoporosis | 36 (14.5) | 25 (10.6) | 28 (14.1) | 22 (10.1) |
| High blood pressure | 120 (48.2) | 111 (47.0) | 96 (48.5) | 103 (47.5) |
| Rheumatoid arthritis | 50 (20.1) | 36 (15.3) | 38 (19.2) | 31 (14.3) |
| Osteoarthritis | 81 (32.5) | 75 (31.8) | 60 (30.3) | 71 (32.7) |
| Stroke | 21 (8.4) | 22 (9.3) | 18 (9.1) | 18 (8.3) |
| Cancer | 31 (12.4) | 21 (8.9) | 23 (11.3) | 20 (9.2) |
| Respiratory conditions | 71 (28.5) | 68 (28.8) | 52 (26.3) | 64 (29.5) |
| Eye condition | 84 (33.7) | 67 (28.4) | 64 (32.3) | 62 (28.6) |
| Heart disease | 55 (22.1) | 71 (30.1) | 42 (21.2) | 64 (29.5) |
| Other | 63 (25.3) | 50 (21.2) | 54 (27.3) | 47 (21.7) |
| Whooley: Over the past month have you been bothered by feeling down, depressed or hopeless?, n (%) | ||||
| Yes | 227 (91.2) | 202 (85.6) | 178 (89.9) | 186 (85.7) |
| No | 21 (8.4) | 34 (14.4) | 20 (10.1) | 31 (14.3) |
| Whooley: Over the past month have you been bothered by having little or no interest or pleasure in doing things?, n (%) | ||||
| Yes | 210 (84.3) | 186 (78.8) | 164 (82.8) | 171 (78.8) |
| No | 38 (15.3) | 50 (21.2) | 34 (17.2) | 46 (21.2) |
| Characteristic | As randomised | As analyseda | ||
|---|---|---|---|---|
| Collaborative care (N = 249) | Usual care (N = 236) | Collaborative care (N = 198) | Usual care (N = 217) | |
| PHQ-9 | ||||
| n | 248 | 236 | 198 | 217 |
| Mean score (SD) | 12.4 (5.43) | 12.1 (5.31) | 12.3 (5.43) | 12.0 (5.32) |
| Median score (minimum, maximum) | 12 (0, 27) | 12 (1, 27) | 12 (0, 27) | 12 (1, 27) |
| PHQ-9 grouping, n (%) | ||||
| No depression | 19 (7.6) | 15 (6.4) | 16 (8.1) | 15 (6.9) |
| Mild depression | 64 (25.7) | 64 (27.1) | 52 (26.3) | 59 (27.2) |
| Moderate depression | 79 (31.7) | 85 (36.0) | 61 (30.8) | 77 (35.5) |
| Moderately severe depression | 67 (26.9) | 51 (21.6) | 53 (26.8) | 47 (21.7) |
| Severe depression | 19 (7.6) | 21 (8.9) | 16 (8.1) | 19 (8.8) |
| PHQ-15 | ||||
| n | 246 | 234 | 196 | 215 |
| Mean score (SD) | 12.3 (4.51) | 11.9 (4.33) | 12.0 (4.46) | 11.9 (4.36) |
| Median score (minimum, maximum) | 12 (2, 26) | 11 (2, 24) | 12 (2, 24) | 11 (2, 24) |
| SF-12 (PCS) | ||||
| n | 245 | 234 | 198 | 217 |
| Mean score (SD) | 35.6 (13.08) | 36.8 (13.32) | 36.1 (13.16) | 36.6 (13.39) |
| Median score (minimum, maximum) | 34.5 (7.1, 66.3) | 35.8 (5.9, 69.6) | 34.9 (7.1, 66.3) | 38.8 (5.9, 69.6) |
| SF-12 (MCS) | ||||
| n | 245 | 234 | 198 | 217 |
| Mean score (SD) | 35.4 (9.51) | 35.7 (10.53) | 35.5 (9.66) | 36.0 (10.52) |
| Median score (minimum, maximum) | 35.8 (10.3, 60.2) | 36.2 (2.2, 62.9) | 35.9 (10.3, 60.2) | 36.4 (2.2, 62.9) |
| GAD-7 | ||||
| n | 247 | 234 | 197 | 215 |
| Mean score (SD) | 9.4 (5.03) | 9.3 (4.92) | 9.4 (5.12) | 9.2 (4.95) |
| Median score (minimum, maximum) | 9 (0, 21) | 9 (0, 21) | 9 (0, 21) | 9 (0, 21) |
| EQ-5D-3L, n (%) | ||||
| Mobility | ||||
| No problems | 71 (28.5) | 76 (32.2) | 61 (30.8) | 70 (32.3) |
| Some problems | 176 (70.7) | 157 (66.5) | 136 (68.7) | 144 (66.4) |
| Confined to bed | 0 (0.0) | 2 (0.8) | 0 (0.0) | 2 (0.9) |
| Self-care | ||||
| No problems | 163 (65.5) | 175 (74.2) | 134 (67.7) | 160 (73.7) |
| Some problems | 75 (30.1) | 55 (23.3) | 58 (29.3) | 52 (24.0) |
| Unable to wash/dress | 5 (2.0) | 4 (1.7) | 2 (1.0) | 3 (1.4) |
| Usual activities | ||||
| No problems | 64 (25.7) | 66 (28.0) | 52 (26.3) | 62 (28.6) |
| Some problems | 159 (63.9) | 151 (64.0) | 131 (66.2) | 138 (63.6) |
| Unable to perform | 24 (9.6) | 18 (7.6) | 14 (7.1) | 16 (7.4) |
| Pain/discomfort | ||||
| No pain | 34 (13.7) | 27 (11.4) | 30 (15.2) | 24 (11.1) |
| Moderate pain | 156 (62.7) | 152 (64.4) | 129 (65.2) | 140 (64.5) |
| Extreme pain | 57 (22.9) | 54 (22.9) | 38 (19.2) | 50 (23.0) |
| Anxiety/depression | ||||
| Not anxious/depressed | 26 (10.4) | 25 (10.6) | 21 (10.6) | 25 (11.5) |
| Moderately anxiety/depression | 176 (70.7) | 178 (75.4) | 141 (71.2) | 161 (74.2) |
| Extremely anxiety/depression | 44 (17.7) | 31 (13.1) | 34 (17.2) | 29 (13.4) |
| Prescribed antidepressants | 82 (32.9) | 79 (33.5) | 67 (33.8) | 77 (35.5) |
| Whooley: Over the past month have you been bothered by feeling down, depressed or hopeless?, n (%) | ||||
| Yes | 238 (95.6) | 219 (92.8) | 190 (96.0) | 201 (92.6) |
| No | 10 (4.0) | 17 (7.2) | 8 (4.0) | 16 (7.4) |
| Whooley: Over the past month have you been bothered by having little or no interest or pleasure in doing things?, n (%) | ||||
| Yes | 220 (88.4) | 210 (89.0) | 177 (89.4) | 195 (89.9) |
| No | 28 (11.2) | 26 (11.0) | 21 (10.6) | 22 (10.1) |
| Characteristic | As randomised | As analyseda | ||
|---|---|---|---|---|
| Collaborative care (N = 249) | Usual care (N = 236) | Collaborative care (N = 198) | Usual care (N = 217) | |
| PHQ-9 | ||||
| N | 248 | 236 | 198 | 217 |
| Mean score (SD) | 14.0 (5.37) | 14.0 (4.93) | 13.9 (5.42) | 13.9 (4.80) |
| Median score (minimum, maximum) | 14 (3, 27) | 14 (4, 27) | 14 (3, 26) | 14 (4, 27) |
| PHQ-9 grouping, n (%) | ||||
| No depression | 2 (0.8) | 4 (1.7) | 1 (0.5) | 4 (1.8) |
| Mild depression | 60 (24.1) | 46 (19.5) | 51 (25.8) | 41 (18.9) |
| Moderate depression | 77 (30.9) | 77 (32.6) | 57 (28.8) | 73 (33.6) |
| Moderately severe depression | 69 (27.7) | 76 (32.2) | 57 (28.8) | 73 (33.6) |
| Severe depression | 40 (16.1) | 33 (14.0) | 32 (16.2) | 26 (12.0) |
| From MINI: Were you ever depressed or down, most of the day, nearly every day for 2 weeks?, n (%) | ||||
| Yes | 213 (85.5) | 207 (87.7) | 170 (85.9) | 191 (88.0) |
| No | 35 (14.1) | 29 (12.3) | 28 (14.1) | 26 (12.0) |
| From MINI: For the past 2 weeks, were you depressed or down, most of the day, nearly every day?, n (%) | ||||
| Yes | 182 (73.1) | 172 (72.9) | 141 (71.2) | 157 (72.4) |
| No | 31 (12.4) | 35 (14.8) | 29 (14.6) | 34 (15.7) |
| From MINI: Were you ever much less interested in most things or much less able to enjoy things you used to enjoy most of the time for 2 weeks?, n (%) | ||||
| Yes | 234 (94.0) | 218 (92.4) | 190 (96.0) | 202 (93.1) |
| No | 14 (5.6) | 18 (7.6) | 8 (4.0) | 15 (16.9) |
| From MINI: In the past 2 weeks, were you much less interested in most things or much less able to enjoy the things you used to enjoy, most of the time?, n (%) | ||||
| Yes | 214 (85.9) | 205 (86.9) | 178 (89.9) | 190 (87.6) |
| No | 20 (8.0) | 13 (5.5) | 12 (6.1) | 12 (5.5) |
Primary outcome
Near-complete PHQ-9 responses were available for participants at diagnostic interview (one participant asked for all data to be destroyed at the point of withdrawal). At follow-up, 300 patients (62%) had valid PHQ-9 scores at all three follow-up times, 118 patients (24%) had a valid PHQ-9 score at 4 months or 12 months only, and for 67 patients (14%) no PHQ-9 scores were available at 18 months’ follow-up.
Score distribution
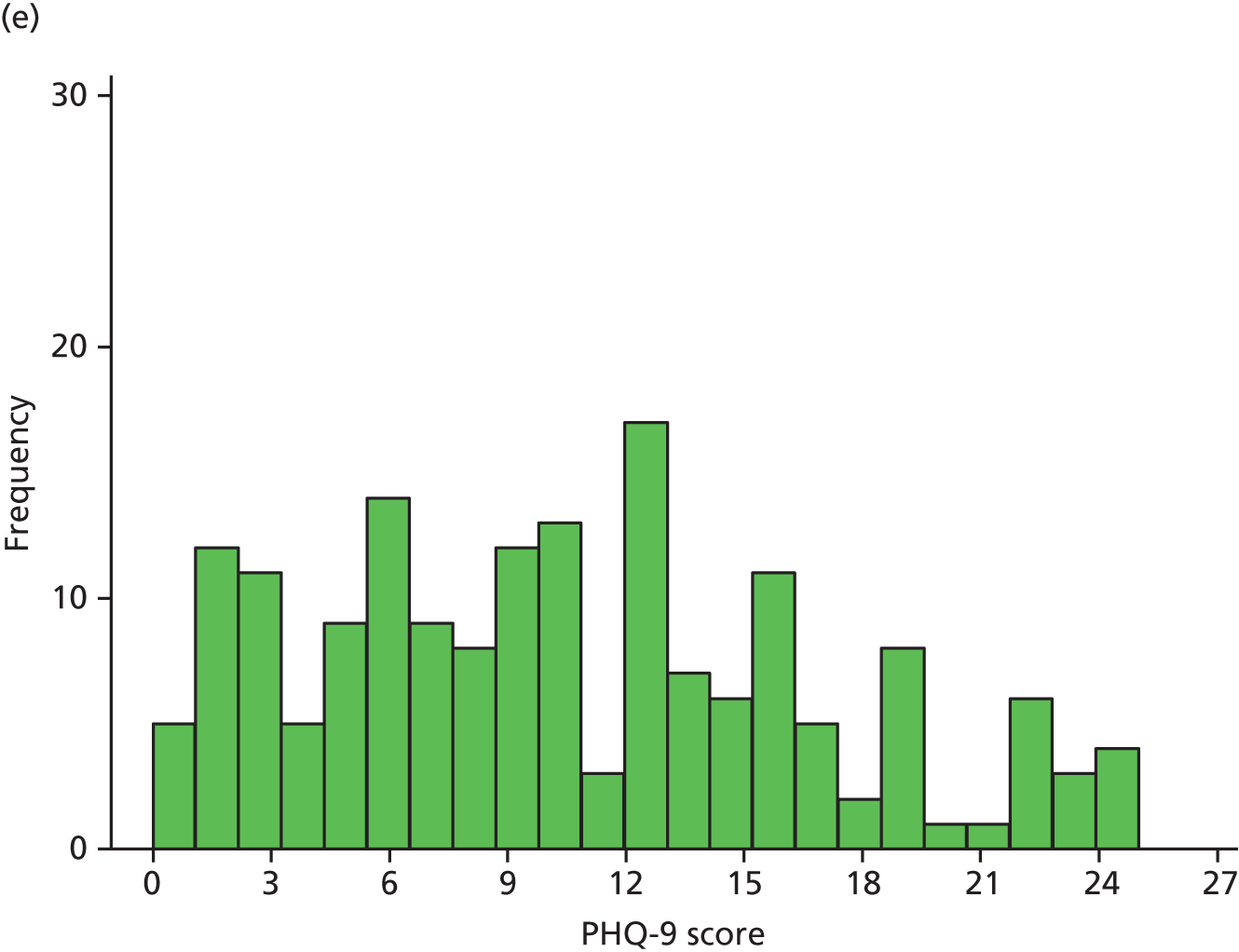
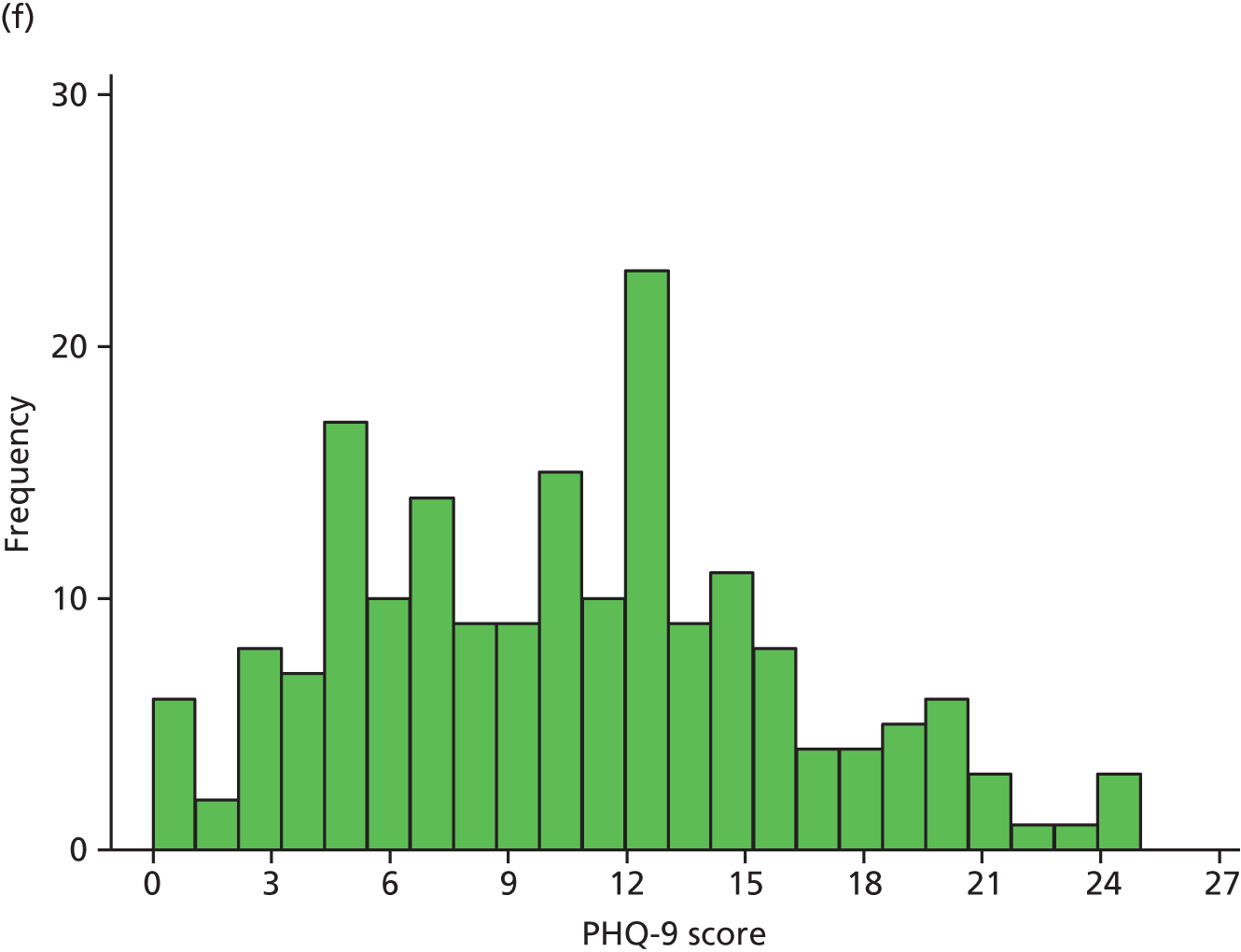
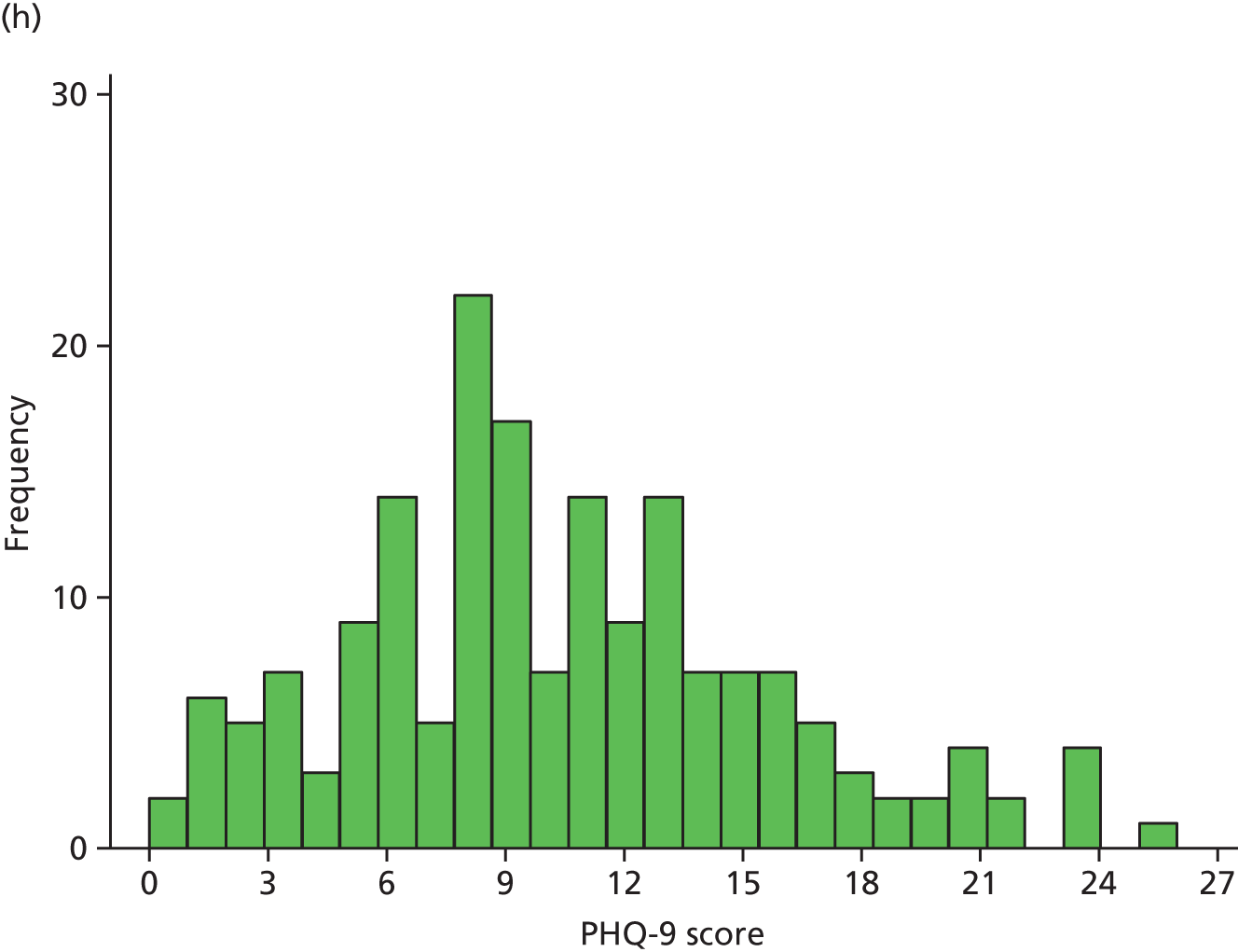
Figure 2 illustrates the distribution of PHQ-9 scores for each trial arm over time. At randomisation, scores were distributed approximately normal with a slight right skew, which became more pronounced over the follow-up period, as patients in both arms improved.
FIGURE 2.
Distribution of PHQ-9 scores by trial arm. (a) Randomisation, collaborative care; (b) randomisation, usual care; (c) 4 months’ follow-up, collaborative care; (d) 4 months’ follow-up, usual care; (e) 12 months’ follow-up, collaborative care; (f) 12 months’ follow-up, usual care; (g) 18 months’ follow-up, collaborative care; and (h) 18 months’ follow-up, usual care.





Unadjusted summary statistics
Summary statistics of the raw PHQ-9 scores are given in Table 13 and are illustrated in Figure 3. Average depression severity, as measured by the PHQ-9, was around 14 score points at randomisation. Scores in both treatment arms improved between randomisation and 4 months’ follow-up, but to a greater extent in the collaborative care group (to a score of around 9) than in the usual-care group (to a score of around 11). By 12 and 18 months’ follow-up, average depression scores continued to improve slightly in the usual-care group, whereas scores in the collaborative care group increased again to similar levels.
| Time | Trial arm | Total (N = 485) | |
|---|---|---|---|
| Collaborative care (N = 249) | Usual care (N = 236) | ||
| Randomisation, n (%) | 248 (99.6) | 236 (100) | 484 (99.8) |
| Mean (SD) | 14.0 (5.37) | 14.0 (4.93) | 14.0 (5.15) |
| Median (minimum, maximum) | 14 (3, 27) | 14 (4, 27) | 14 (3, 27) |
| 4 months, n (%) | 186 (75) | 204 (86) | 390 (80) |
| Mean (SD) | 8.9 (5.53) | 10.9 (5.89) | 9.9 (5.79) |
| Median (minimum, maximum) | 8 (0, 24) | 11 (0, 26) | 9 (0, 26) |
| 12 months, n (%) | 172 (69) | 185 (78) | 357 (74) |
| Mean (SD) | 10.4 (6.25) | 10.6 (5.52) | 10.5 (5.87) |
| Median (minimum, maximum) | 10 (0, 25) | 10 (0, 25) | 10 (0, 25) |
| 18 months, n (%) | 165 (66) | 178 (75) | 343 (71) |
| Mean (SD) | 10.4 (6.09) | 10.3 (5.50) | 10.4 (5.79) |
| Median (minimum, maximum) | 10 (0, 25) | 9 (0, 26) | 10 (0, 26) |
FIGURE 3.
Unadjusted mean PHQ-9 scores (with 95% CIs).
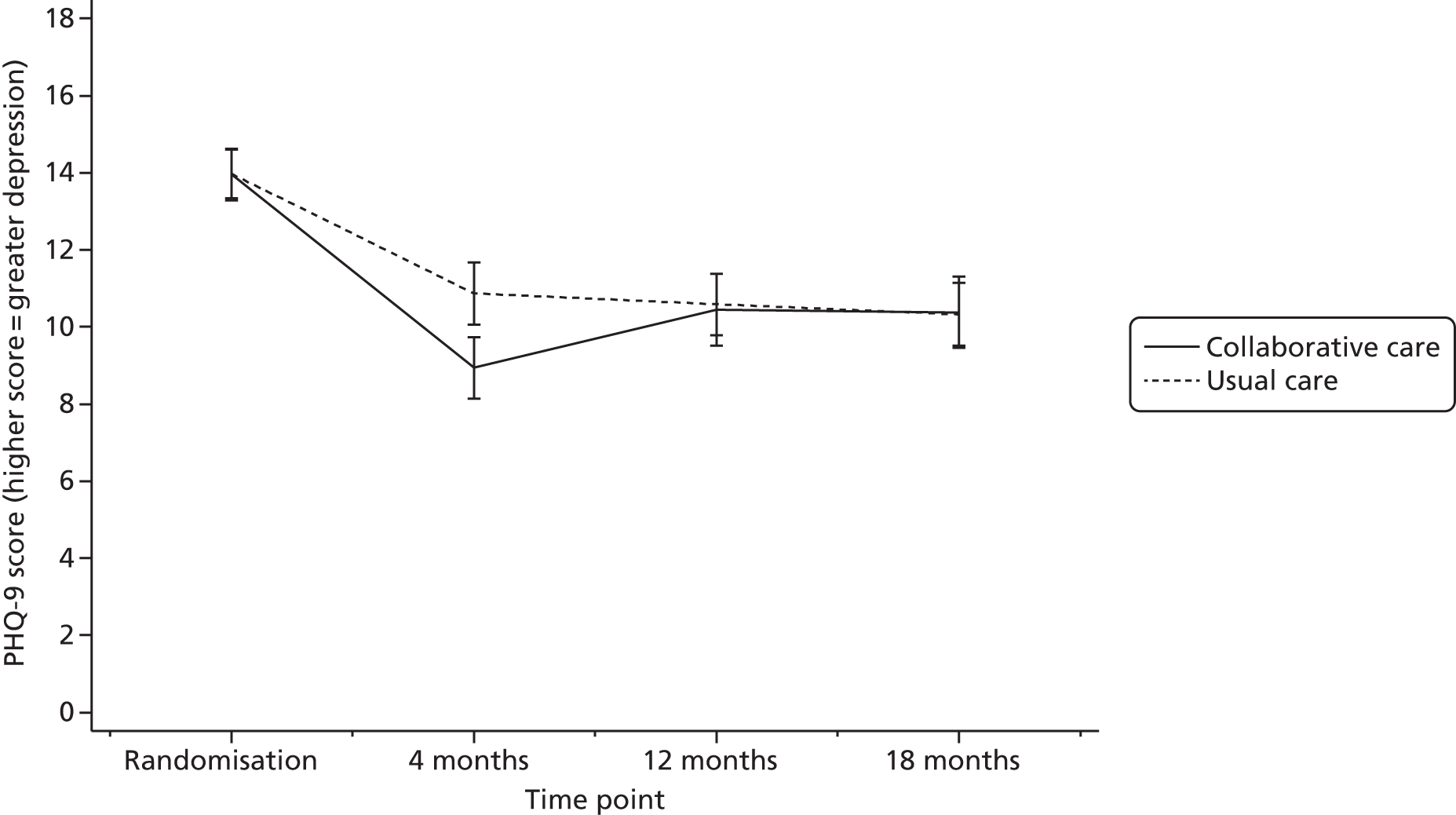
Primary analysis
The primary outcome was analysed by a covariance pattern linear mixed model using PHQ-9 score at 4 and 12 months as the outcome. The model included as fixed effects: time, trial arm and time-by-treatment interaction, adjusting for PHQ-9 depression at randomisation and physical/functional limitations as measured by the baseline SF-12 PCS score. Patients were included in the analysis if they had a valid PHQ-9 score at 4, 12 or 18 months’ follow-up and complete covariate data. Patients were analysed as part of the group to which they had been randomised (intention to treat).
The correlation of observations within patients over time was modelled by a covariance structure to describe the random effects. Different types of available covariance structures were investigated for this model (unstructured, independent, exchangeable, autoregressive and exponential). The exchangeable covariance structure (estimating one covariance parameter to model the relatedness between any two time points) displayed the lowest and therefore best-fitting log likelihood values, and was not significantly worse fitting than the full-parameter unstructured model when compared using the chi-squared test. Therefore, the exchangeable covariance pattern was selected.
Diagnostics of model fit showed an acceptable distribution of standard residuals with a small number of outliers at the higher end of the distribution. There was uniform variance between predicted and actual residuals, and no transformation of PHQ-9 scores was carried out for the analysis.
Adjusted PHQ-9 score means and group differences for the primary analysis model as specified above are presented in Table 14. The analysis revealed significant differences between trial arms at each 4 months’ follow-up in favour of collaborative care, but not at 12 or 18 months’ follow-up: 1.92 score points (95% CI 0.85 to 2.99 score points; p < 0.001) for the primary end point at 4 months; 0.19 score points (95% CI –0.92 to 1.29 score points; p = 0.741) at 12 months and 0.002 score points (95% CI –1.12 to 1.12 score points; p = 0.997) at 18 months. Using the overall residual standard deviation (SD, 5.72), the score difference at 4 months equates to a standard effect size of 0.34 (the trial was powered for a standard effect size of 0.35).
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 monthsa | 198 | 8.98 | 8.20 to 9.75 | 217 | 10.90 | 10.16 to 11.64 | 1.92 | 0.85 to 2.99 | < 0.001 |
| 12 months | 198 | 10.44 | 9.65 to 11.24 | 217 | 10.63 | 9.87 to 11.40 | 0.19 | –0.92 to 1.29 | 0.741 |
| 18 months | 198 | 10.53 | 9.72 to 11.34 | 217 | 10.53 | 9.76 to 11.31 | 0.002 | –1.12 to 1.12 | 0.997 |
Secondary outcomes analyses
Adjusting for clustering by case manager
It was expected in the planning and sample size calculation for this trial that collaborative care case managers would work with an average case load of 20 patients, and the clustering of outcomes within case managers was expected to be described by an ICC of 0.02. In total, there were 20 case managers (four in York, four in Leeds, 10 in Durham and two in Newcastle upon Tyne) for a total of 246 participants in the collaborative care arm, that is, an average case load of 12.3 randomised patients per case manager. Case loads varied considerably between 1 and 46 patients. Three patients withdrew before they were assigned a case manager.
The average ICC for clustering within case managers was found to negligible: ICC ≤ 0.0001 (95% CI 0 to 0.0757) for PHQ-9 scores at 4 months.
In order to quantify the impact of the grouping by case managers with respect to the primary outcome, case manager identifiers were included as a random effect in the primary linear mixed-analysis model, nested within treatment arm. Participants in the usual-care arm were coded as their own case managers for the purpose of analysis, and the covariance structure was estimated separately for each treatment arm in order to account for the differences in variability for the random effect.
Adjusted PHQ-9 score means and group differences for this analysis are given in Table 15. Group differences remained significant in favour of collaborative care at 4 months’ follow-up (a difference of 1.92 PHQ-9 score points), and outcomes did not significantly differ between groups at 12 or 18 months. Thus, accounting for the clustering by case manager did not affect the size of the treatment effect compared with the primary analysis.
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 198 | 8.98 | 8.20 to 9.76 | 217 | 10.90 | 10.16 to 11.63 | 1.92 | 0.85 to 2.99 | < 0.001 |
| 12 months | 198 | 10.45 | 9.65 to 11.25 | 217 | 10.63 | 9.87 to 11.39 | 0.18 | –0.92 to 1.29 | 0.744 |
| 18 months | 198 | 10.54 | 9.73 to 11.35 | 217 | 10.53 | 9.76 to 11.30 | –0.01 | –1.13 to 1.11 | 0.991 |
Adjusting for covariates predictive of Patient Health Questionnaire 9-items at 4 months
The primary analysis was adjusted for PHQ-9 depression at randomisation and baseline physical limitations (SF-12 PCS score). In order to identify any other relevant covariates of depression severity at follow-up, a number of selected demographics and baseline measures were used as predictors of PHQ-9 depression at 4 months in individual regressions followed by a combined regression to avoid issues of multicollinearity, using a non-conservative significance level of p < 0.10 at each stage. All analyses adjusted for PHQ-9 scores at randomisation.
Considered predictors were age, sex, an indicator of whether or not any selected antidepressants had been prescribed at baseline, a history of depression [as measured by two questions of the MINI at randomisation: (1) whether or not patients had ever been consistently depressed for a minimum of 2 weeks and (2) whether or not patients had ever experienced a lack of interest or enjoyment for a minimum of 2 weeks], baseline anxiety (as measured by the GAD-7) and baseline physical functioning (as measured by the PHQ-15).
Results of the individual regressions and summary regression are given in Table 16. Positive coefficients indicate increased depression at 4 months for higher values of the predictor variable (or for the condition specified in the table for categorical variables). Initial identified predictors following individual regressions were prescribed medication, a history of depression (both indicators) as well as baseline GAD-7 and PHQ-15 scores. Higher levels of anxiety, physical health problems, a greater likelihood of being described antidepressants and having a history of depression were associated with higher PHQ-9 depression severity at 4 months. Of these predictors, all but prescribed antidepressants remained significant in a summary regression and were included in the primary analysis model. Age and sex were not significant predictors of PHQ-9 scores.
| Characteristic | Coefficient | Standard error | p-valuea |
|---|---|---|---|
| Individual regressions | |||
| Age | –0.04 | 0.046 | 0.363 |
| Sex (being female) | 0.70 | 0.567 | 0.217 |
| Prescribed antidepressants (any) | 1.18 | 0.578 | 0.043 |
| Ever having been depressed or down for 2 weeks | 2.90 | 0.804 | < 0.001 |
| Ever having lost interest or enjoyment for 2 weeks | 2.78 | 1.189 | 0.020 |
| Baseline GAD-7 score | 0.33 | 0.056 | < 0.001 |
| Baseline PHQ-15 score | 0.21 | 0.066 | 0.002 |
| Summary regression | |||
| Prescribed antidepressants (any) | 0.63 | 0.558 | 0.259 |
| Ever having been depressed or down for 2 weeks | 2.65 | 0.788 | 0.001 |
| Ever having lost interest or enjoyment for 2 weeks | 2.20 | 1.174 | 0.062 |
| Baseline GAD-7 score | 0.26 | 0.058 | < 0.001 |
| Baseline PHQ-15 score | 0.14 | 0.065 | 0.034 |
Adjusted PHQ-9 score means and group differences for the primary analysis model [additionally adjusting for history of depression (two questions), GAD-7 and PHQ-15 at baseline] are given in Table 17. Group differences remained significant in favour of collaborative care at 4 months’ follow-up (a difference of 1.95 PHQ-9 score points), whereas differences at 12 and 18 months remained not statistically significant. Thus, accounting for additional predictors of the primary outcome did not affect treatment differences. An overall comparison of treatment effects for PHQ-9 depression severity from different analyses is presented in Table 18.
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 196 | 7.68 | 6.41 to 8.94 | 214 | 9.62 | 8.41 to 10.84 | 1.95 | 0.92 to 2.98 | < 0.001 |
| 12 months | 196 | 9.09 | 7.81 to 10.37 | 214 | 9.35 | 8.12 to 10.58 | 0.26 | –0.81 to 1.32 | 0.633 |
| 18 months | 196 | 9.21 | 7.93 to 10.49 | 214 | 9.25 | 8.02 to 10.47 | 0.04 | –1.04 to 1.11 | 0.945 |
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| Unadjusted means | |||||||||
| 4 months | 186 | 8.94 | 8.14 to 9.74 | 204 | 10.87 | 10.06 to 11.68 | 1.93 | – | – |
| 12 months | 172 | 10.44 | 9.50 to 11.38 | 185 | 10.59 | 9.79 to 11.39 | 0.15 | – | – |
| 18 months | 165 | 10.38 | 9.44 to 11.32 | 178 | 10.33 | 9.51 to 11.14 | –0.05 | – | – |
| Primary analysisa | |||||||||
| 4 monthsb | 198 | 8.98 | 8.20 to 9.75 | 217 | 10.90 | 10.16 to 11.64 | 1.92 | 0.85 to 2.99 | < 0.001 |
| 12 months | 198 | 10.44 | 9.65 to 11.24 | 217 | 10.63 | 9.87 to 11.40 | 0.19 | –0.92 to 1.29 | 0.741 |
| 18 months | 198 | 10.53 | 9.72 to 11.34 | 217 | 10.53 | 9.76 to 11.31 | 0.00 | –1.12 to 1.12 | 0.997 |
| Analysis adjusted for clustering by case managerc | |||||||||
| 4 months | 198 | 8.98 | 8.20 to 9.76 | 217 | 10.90 | 10.16 to 11.63 | 1.92 | 0.85 to 2.99 | < 0.001 |
| 12 months | 198 | 10.45 | 9.65 to 11.25 | 217 | 10.63 | 9.87 to 11.39 | 0.18 | –0.92 to 1.29 | 0.744 |
| 18 months | 198 | 10.54 | 9.73 to 11.35 | 217 | 10.53 | 9.76 to 11.30 | –0.01 | –1.13 to 1.11 | 0.991 |
| Analysis adjusted for additional covariates predictive of PHQ-9 score at 4 monthsd | |||||||||
| 4 months | 196 | 7.68 | 6.41 to 8.94 | 214 | 9.62 | 8.41 to 10.84 | 1.95 | 0.92 to 2.98 | < 0.001 |
| 12 months | 196 | 9.09 | 7.81 to 10.37 | 214 | 9.35 | 8.12 to 10.58 | 0.26 | –0.81 to 1.32 | 0.633 |
| 18 months | 196 | 9.21 | 7.93 to 10.49 | 214 | 9.25 | 8.02 to 10.47 | 0.04 | –1.04 to 1.11 | 0.945 |
| Analysis adjusted for covariates predictive of non-response at 4 monthse | |||||||||
| 4 months | 196 | 8.94 | 7.77 to 10.11 | 215 | 10.91 | 9.81 to 12.00 | 1.97 | 0.93 to 3.00 | < 0.001 |
| 12 months | 196 | 10.35 | 9.16 to 11.53 | 215 | 10.60 | 9.49 to 11.72 | 0.26 | –0.81 to 1.33 | 0.637 |
| 18 months | 196 | 10.47 | 9.28 to 11.66 | 215 | 10.54 | 9.42 to 11.65 | 0.07 | –1.01 to 1.15 | 0.903 |
| Analysis using multiply imputed dataf | |||||||||
| 4 months | 249 | 9.01 | 8.21 to 9.81 | 236 | 10.94 | 10.20 to 11.68 | 1.93 | 0.89 to 2.96 | < 0.001 |
| 12 months | 249 | 10.51 | 9.70 to 11.33 | 236 | 10.74 | 9.96 to 11.52 | 0.23 | –0.86 to 1.32 | 0.679 |
| 18 months | 249 | 10.66 | 9.82 to 11.51 | 236 | 10.57 | 9.78 to 11.37 | –0.09 | –1.18 to 1.00 | 0.869 |
Adjusting for missingness
No valid PHQ-9 response at the primary end point of 4 months’ follow-up was available for 25.3% (n = 63) of patients in the collaborative care arm and 13.6% (n = 32) of patients in the usual-care arm. In order to investigate the impact of missing data on the treatment effect, any baseline predictors of non-response at 4 months’ follow-up (no valid PHQ-9 score) were identified by individual and a summary logistic regression using p < 0.10 and included as covariates in the primary analysis model.
Considered predictors were age, sex, an indicator of whether or not any selected antidepressants had been prescribed at baseline, a history of depression (as measured by two questions of the MINI at randomisation), depression at randomisation (PHQ-9 score), baseline mental well-being (SF-12 MCS score), baseline anxiety (GAD-7 score) and baseline physical functioning (PHQ-15 score, SF-12 PCS score).
The results of the individual and summary regressions are presented in Table 19. Odds ratios > 1 indicate a greater likelihood of non-response at 4 months for higher values of the predictor variable (or for the condition specified in the table for categorical variables). The initial identified predictors were age, GAD-7 score, SF-12 MCS score, PHQ-15 score, prescribed antidepressants and ever having lost interest or enjoyment for ≥ 2 weeks. PHQ-9 response at 4 months was more likely to be missing for older participants, participants with greater anxiety, reduced mental functioning or more physical problems, those not on antidepressants and those who reported ever having lost interest or enjoyment for more than 2 weeks. Of these predictors, age, the SF-12 MCS score, PHQ-15 score, antidepressant use and loss of interest remained significant in a summary regression and were included in the primary analysis model.
| Characteristic | Odds ratio | Standard error | p-valuea |
|---|---|---|---|
| Individual regressions | |||
| Age | 1.04 | 0.018 | 0.005 |
| Sex (being female) | 1.09 | 0.260 | 0.715 |
| Baseline GAD-7 score | 1.04 | 0.024 | 0.074 |
| Baseline SF-12 MCS score | 0.98 | 0.011 | 0.059 |
| Baseline SF-12 PCS score | 0.99 | 0.009 | 0.207 |
| Baseline PHQ-15 score | 1.07 | 0.037 | 0.011 |
| Randomisation PHQ-9 score | 1.03 | 0.023 | 0.164 |
| Prescribed antidepressants (any) | 0.63 | 0.164 | 0.078 |
| Ever having been depressed or down for 2 weeks | 1.05 | 0.361 | 0.884 |
| Ever having lost interest or enjoyment for 2 weeks | 0.50 | 0.201 | 0.085 |
| Summary regression | |||
| Age | 1.05 | 0.019 | 0.007 |
| Baseline GAD-7 score | 1.01 | 0.030 | 0.738 |
| Baseline SF-12 MCS score | 0.98 | 0.014 | 0.085 |
| Baseline PHQ-15 score | 1.06 | 0.030 | 0.058 |
| Prescribed antidepressants (any) | 0.62 | 0.171 | 0.084 |
| Ever having lost interest or enjoyment for 2 weeks | 0.44 | 0.186 | 0.051 |
Adjusted PHQ-9 score means and group differences for the primary analysis model are given in Table 20. Group differences remained significant in favour of collaborative care at 4 months’ follow-up (a difference of 1.97 PHQ-9 score points), and remained not statistically significant at 12 and 18 months’ follow-up. Thus, accounting for predictors of non-response did not affect the treatment effect. An overall comparison of treatment effects for PHQ-9 depression severity from different analyses is presented in Table 18.
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 196 | 8.94 | 7.77 to 10.11 | 215 | 10.91 | 9.81 to 12.00 | 1.97 | 0.93 to 3.00 | < 0.001 |
| 12 months | 196 | 10.35 | 9.16 to 11.53 | 215 | 10.60 | 9.49 to 11.72 | 0.26 | –0.81 to 1.33 | 0.637 |
| 18 months | 196 | 10.47 | 9.28 to 11.66 | 215 | 10.54 | 9.42 to 11.65 | 0.07 | –1.01 to 1.15 | 0.903 |
In addition, the primary analysis was repeated using complete data derived from multiple imputation by chained equations. Data were imputed from all additional predictors identified in the previous two analyses (age, baseline SF-12, GAD-7 and PHQ-15 scores, antidepressant use and depression history) as well as treatment allocation and available PHQ-9 scores at any time points. Adjusted PHQ-9 score means and group differences for the primary analysis model are given in Table 21 (results based on 20 imputations). Group differences remained significant in favour of collaborative care at 4 months’ follow-up (a difference of 1.93 PHQ-9 score points), and remained not statistically significant at 12 and 18 months’ follow-up. Thus, using complete data did not affect the treatment effect. An overall comparison of treatment effects for PHQ-9 depression severity from different analyses is presented in Table 18.
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 249 | 9.01 | 8.21 to 9.81 | 236 | 10.94 | 10.20 to 11.68 | 1.93 | 0.89 to 2.96 | < 0.001 |
| 12 months | 249 | 10.51 | 9.70 to 11.33 | 236 | 10.74 | 9.96 to 11.52 | 0.23 | –0.86 to 1.32 | 0.679 |
| 18 months | 249 | 10.66 | 9.82 to 11.51 | 236 | 10.57 | 9.78 to 11.37 | –0.09 | –1.18 to 1.00 | 0.869 |
Summary of Patient Health Questionnaire-9 items analysis models
Table 18 provides an overview of group means and treatment effect estimates from the primary analysis and secondary analyses of depression severity at 4, 12 and 18 months as measured by PHQ-9 scores. Unadjusted means are presented for reference. Adjusted average estimates of group differences at the primary end point at 4 months ranged from 1.92 to 1.97 PHQ-9 score points in favour of collaborative care.
Binary Patient Health Questionnaire 9-items outcome
Using the cut-off point of ≥ 10 PHQ-9 score points, Table 22 presents the number and percentage of moderately to severely depressed participants at randomisation and follow-up by treatment arm. The figures are illustrated in Figure 4. Approximately 77% of randomised CASPER plus participants were depressed at randomisation. At 4 months’ follow-up, this percentage improved to 40% in the collaborative care arm and 55% in the usual-care arm. This difference was not maintained at 12 or 18 months’ follow-up.
| Time point | Trial arm | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Total | % | n | Total | % | n | Total | % | |
| Randomisation | 186 | 248 | 75.0 | 186 | 236 | 78.8 | 372 | 484 | 76.9 |
| 4 months | 75 | 186 | 40.3 | 113 | 204 | 55.4 | 188 | 390 | 48.2 |
| 12 months | 87 | 172 | 50.6 | 103 | 185 | 55.7 | 190 | 357 | 53.2 |
| 18 months | 88 | 165 | 53.3 | 88 | 178 | 49.4 | 176 | 343 | 51.3 |
FIGURE 4.
Unadjusted per cent of patients (with 95% CIs) with moderate to severe depression.

Data were analysed by logistic mixed-effects modelling, including moderate to severe PHQ-9 depression (yes or no) at 4, 12 and 18 months as the outcome, predicted by trial arm, time (4, 12 or 18 months), group by time interaction, depression severity at randomisation (PHQ-9 score) and baseline physical functioning (SF-12 PCS score). Resulting treatment effect estimates are presented in Table 23.
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Odds ratio | 95% CI | n | Odds ratio | 95% CI | Odds ratio | 95% CI | p-value | |
| 4 months | 198 | 0.95 | 0.58 to 1.33 | 217 | 2.08 | 1.27 to 2.89 | 2.18 | 1.36 to 3.51 | 0.001 |
| 12 months | 198 | 1.65 | 0.79 to 2.52 | 217 | 2.31 | 1.14 to 3.49 | 1.40 | 0.72 to 2.72 | 0.319 |
| 18 months | 198 | 2.29 | 0.76 to 3.81 | 217 | 1.66 | 0.62 to 2.69 | 0.72 | 0.31 to 1.71 | 0.461 |
The greater reduction in moderately to severely depressed cases seen in the collaborative care arm compared with the usual-care arm was statistically significant at 4 months’ follow-up (odds ratio 2.18, 95% CI 1.36 to 3.51; p < 0.001), but was not statistically significant at 12 or 18 months.
Secondary outcomes
Continuous and binary secondary outcomes were analysed by longitudinal linear and logistic mixed models, adjusting for the baseline assessment of the outcome, PHQ-9 score at randomisation and SF-12 PCS score. Estimates of the effect of the intervention were derived and are presented for each follow-up time point. In addition, adverse events are reported descriptively and the number of deaths are compared by chi-squared test.
Antidepressants
Patients indicated on questionnaires whether or not they were currently prescribed any of a list of 10 antidepressants (see Table 24 for details and the frequencies of prescriptions by trial arm). Citalopram was the most commonly prescribed antidepressant.
| Antidepressant | Trial arm | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | |||||||||||
| Month | Month | Month | ||||||||||
| 0 | 4 | 12 | 18 | 0 | 4 | 12 | 18 | 0 | 4 | 12 | 18 | |
| Dosulepin | 3 | 3 | 3 | 2 | 8 | 4 | 4 | 3 | 11 | 7 | 7 | 5 |
| Sertraline | 17 | 18 | 17 | 16 | 13 | 14 | 17 | 16 | 30 | 32 | 34 | 32 |
| Venlafaxine | 10 | 7 | 9 | 8 | 6 | 6 | 4 | 5 | 16 | 13 | 13 | 13 |
| Lofepramine | 1 | 1 | 0 | 1 | 4 | 3 | 2 | 1 | 5 | 4 | 2 | 2 |
| Fluoxetine | 14 | 8 | 7 | 7 | 15 | 13 | 9 | 6 | 29 | 21 | 16 | 13 |
| Duloxetine | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 3 | 2 | 4 | 3 |
| Citalopram | 27 | 19 | 17 | 19 | 24 | 18 | 18 | 19 | 51 | 37 | 35 | 38 |
| Paroxetine | 3 | 3 | 4 | 3 | 2 | 3 | 2 | 1 | 5 | 6 | 6 | 4 |
| Trazodone | 6 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | 9 | 7 | 6 | 8 |
| Mirtazapine | 9 | 9 | 5 | 7 | 11 | 8 | 11 | 10 | 20 | 17 | 16 | 17 |
A binary variable was created to indicate whether or not patients had been prescribed any of the listed antidepressants. Table 25 presents the number and percentage of patients on antidepressants at baseline and follow-up by treatment arm. The figures are illustrated in Figure 5. Approximately 33% of patients were prescribed antidepressants at baseline. Of the participants remaining in the trial at follow-up, antidepressants were prescribed to a greater percentage at 4, 12 and 18 months than at baseline, around 36% on average. Differences between treatment arms were small.
| Time point | Trial arm | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Total | % | n | Total | % | n | Total | % | |
| Baseline | 82 | 248 | 33.1 | 79 | 236 | 33.5 | 161 | 484 | 33.3 |
| 4 months | 70 | 186 | 37.6 | 68 | 204 | 33.3 | 138 | 390 | 35.4 |
| 12 months | 61 | 173 | 35.3 | 68 | 185 | 36.8 | 129 | 358 | 36.0 |
| 18 months | 61 | 166 | 36.8 | 61 | 178 | 34.3 | 122 | 344 | 35.5 |
FIGURE 5.
Unadjusted per cent of patients (with 95% CIs) who were prescribed antidepressants.
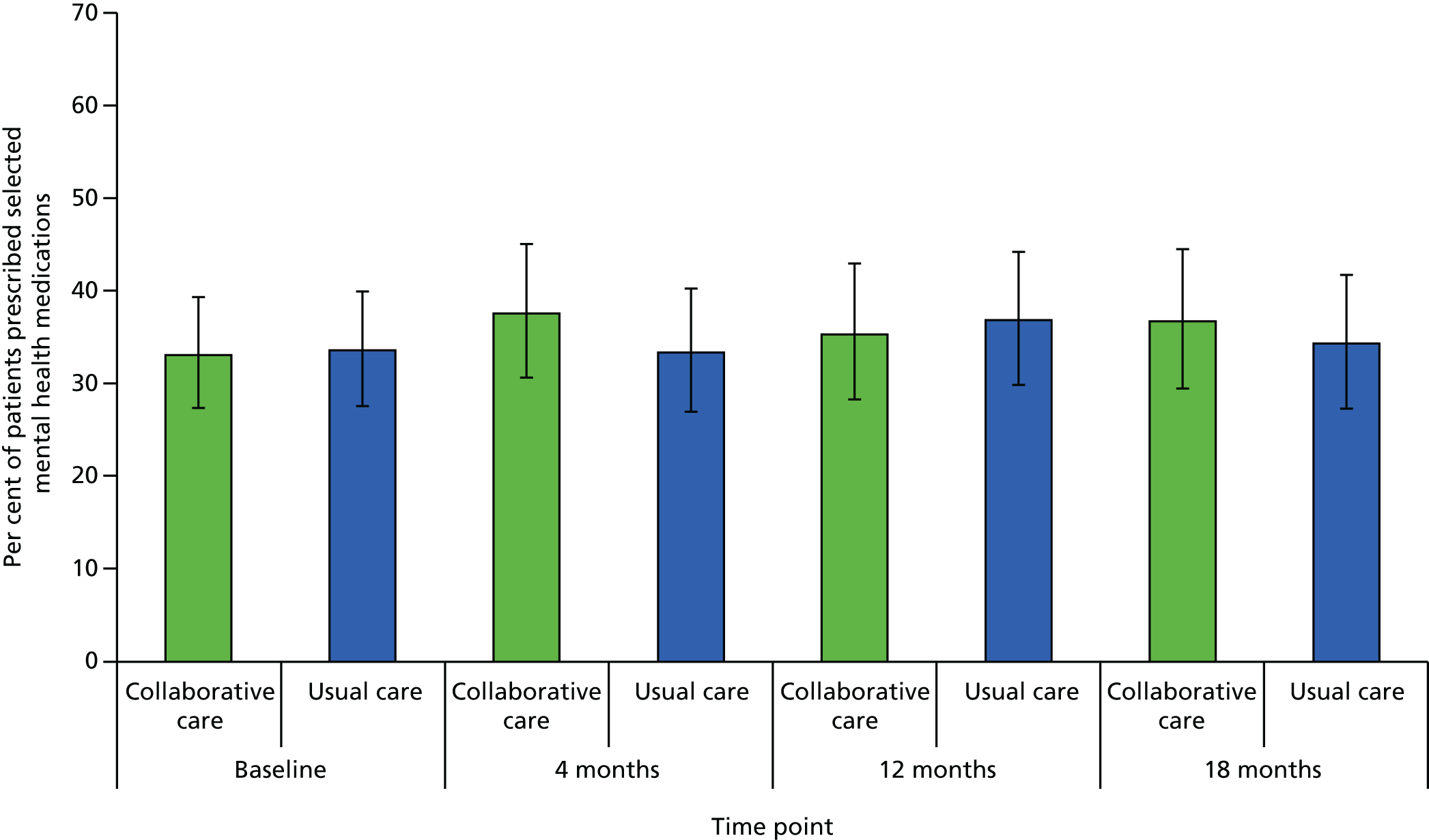
Data were analysed by a logistic mixed-effects model, including prescribed medication (yes or no) at 4, 12 and 18 months as the outcome, and were predicted by trial arm, time (4, 12 and 18 months), group by time interaction and prescribed antidepressants at baseline. Treatment effect estimates are presented in Table 26.
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Odds ratio | 95% CI | n | Odds ratio | 95% CI | Odds ratio | 95% CI | p-value | |
| 4 months | 198 | 6.86 | 1.22 to 12.50 | 217 | 2.70 | 0.88 to 4.53 | 0.39 | 0.17 to 0.89 | 0.025 |
| 12 months | 198 | 2.77 | 0.40 to 5.15 | 217 | 4.03 | 0.62 to 7.45 | 1.45 | 0.55 to 3.88 | 0.454 |
| 18 months | 198 | 2.28 | 0.15 to 4.40 | 217 | 2.07 | 0.17 to 3.97 | 0.91 | 0.30 to 2.79 | 0.868 |
The adjusted relative odds of being prescribed antidepressants were higher in the collaborative care arm than the usual-care arm at 4 months (odds ratio 0.39, 95% CI 0.17 to 0.89; p = 0.025); however, differences between treatment arms were not statistically significant at 12 or 18 months’ follow-up.
Generalised Anxiety Disorder-7 item scale psychological anxiety
The GAD-7 is a brief measure of symptoms of anxiety with a score range of 0–21, with higher scores indicating more severe anxiety. Unadjusted means for psychological anxiety based on the GAD-7 are presented in Table 27 and Figure 6, and the results of the formal statistical analysis by mixed modelling are given in Table 28.
| Time | Trial arm | Total (N = 485) | |
|---|---|---|---|
|
Collaborative care (N = 249) |
Usual care (N = 236) |
||
| Baseline n | 247 | 234 | 481 |
| Mean (SD) | 9.4 (5.03) | 9.3 (4.92) | 9.4 (4.97) |
| Median (minimum, maximum) | 9 (0, 21) | 9 (0, 21) | 9 (0, 21) |
| 4 months n | 181 | 191 | 372 |
| Mean (SD) | 6.7 (5.07) | 8.3 (5.25) | 7.5 |
| Median (minimum, maximum) | 6 (0, 20) | 7 (0, 21) | 6 (0, 21) |
| 12 months n | 166 | 176 | 342 |
| Mean (SD) | 7.4 (5.71) | 8.4 (5.36) | 7.9 (5.55) |
| Median (minimum, maximum) | 6 (0, 21) | 8 (0, 21) | 7 (0, 21) |
| 18 months n | 161 | 171 | 322 |
| Mean (SD) | 7.5 (5.22) | 7.9 (4.94) | 7.7 (5.07) |
| Median (minimum, maximum) | 7 (0, 21) | 8 (0, 20) | 7 (0, 21) |
FIGURE 6.
Unadjusted mean GAD-7 scores (with 95% CIs).
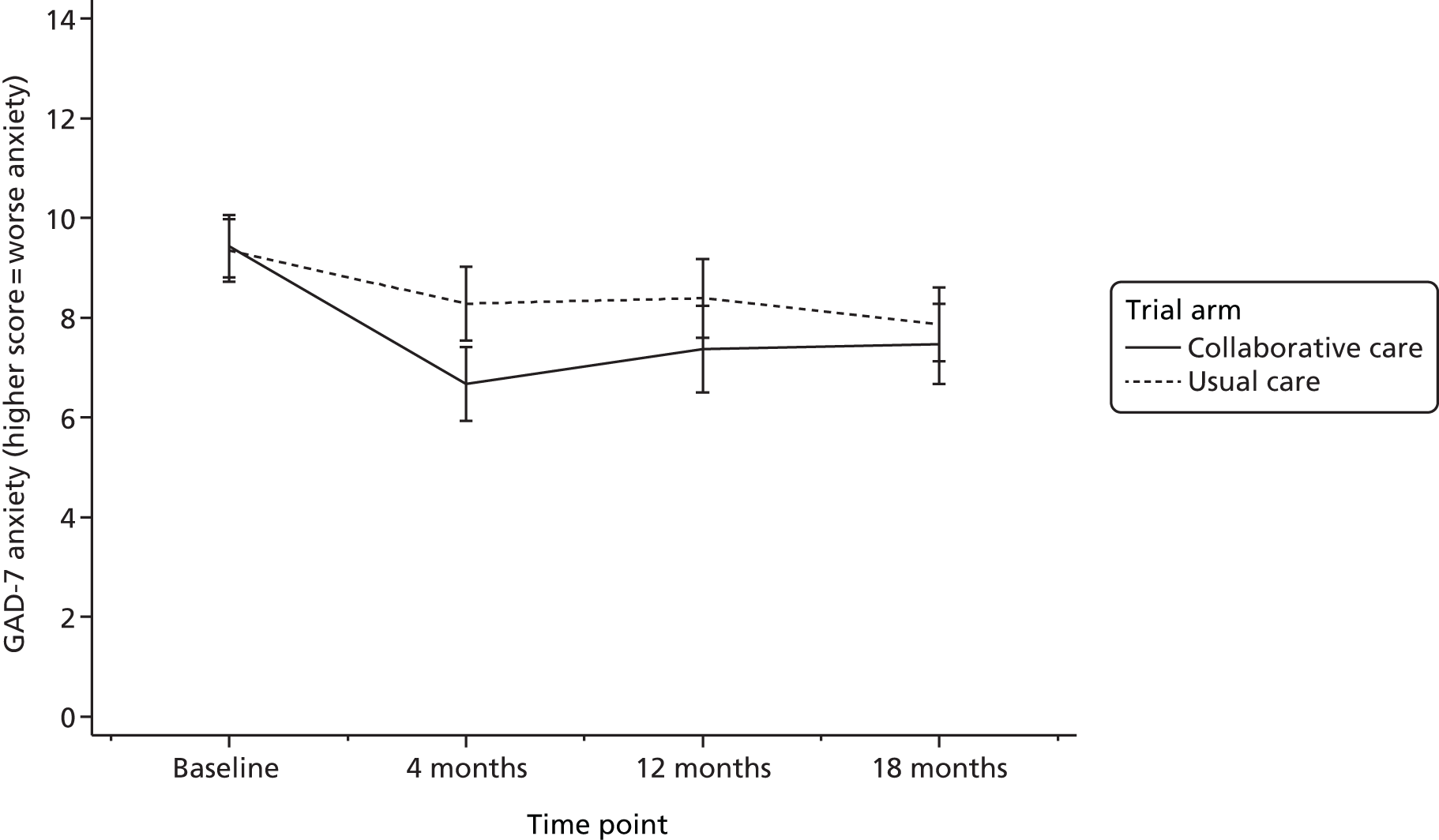
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 195 | 6.60 | 5.94 to 7.25 | 210 | 8.27 | 7.64 to 8.91 | 1.68 | 0.77 to 2.59 | < 0.001 |
| 12 months | 195 | 7.33 | 6.65 to 8.01 | 210 | 8.42 | 7.76 to 9.07 | 1.09 | 0.14 to 2.03 | 0.024 |
| 18 months | 195 | 7.59 | 6.91 to 8.27 | 210 | 7.91 | 7.25 to 8.57 | 0.32 | –0.63 to 1.27 | 0.511 |
The figures indicate that anxiety was on average at around nine score points for all participants at baseline. Both trial arms improved anxiety levels at 4 months’ follow-up, significantly more so in the collaborative care arm (mean score difference 1.68, 95% CI 0.77 to 2.59; p < 0.001). Group differences decreased in magnitude but remained statistically significant at 12 months’ follow-up (mean score difference 1.09, 95% CI 0.14 to 2.03; p = 0.024), but not at 18 months.
Short Form questionnaire-12 items physical component summary score
The SF-12 PCS score ranges from 0 to 100, with higher scores indicating better functioning. Unadjusted means for physical functioning are presented in Table 29 and Figure 7, and the results of the formal statistical analysis by mixed modelling are given in Table 30.
| Time | Trial arm | Total (N = 485) | |
|---|---|---|---|
| Collaborative care (N = 249) | Usual care (N = 236) | ||
| Baseline n | 245 | 234 | 479 |
| Mean (SD) | 35.6 (13.08) | 36.8 (13.32) | 36.2 (13.20) |
| Median (minimum, maximum) | 34.5 (7.1, 66.3) | 35.8 (5.9, 69.6) | 35.3 (5.9, 69.6) |
| 4 months n | 178 | 188 | 366 |
| Mean (SD) | 35.2 (13.53) | 35.8 (12.14) | 35.5 (12.82) |
| Median (minimum, maximum) | 33.5 (7.3, 64.0) | 34.0 (12.9, 65.5) | 33.8 (7.3, 65.5) |
| 12 months n | 166 | 171 | 337 |
| Mean (SD) | 34.3 (13.17) | 34.3 (12.02) | 34.3 (12.58) |
| Median (minimum, maximum) | 33.8 (7.7, 69.6) | 33.0 (9.4, 61.0) | 33.7 (7.7, 69.6) |
| 18 months n | 158 | 167 | 325 |
| Mean (SD) | 34.0 (13.51) | 35.1 (12.11) | 34.6 (12.80) |
| Median (minimum, maximum) | 30.7 (8.7, 70.9) | 33.7 (11.8, 63.0) | 33.3 (8.7, 70.9) |
FIGURE 7.
Unadjusted mean SF-12 PCS scores (with 95% CIs).
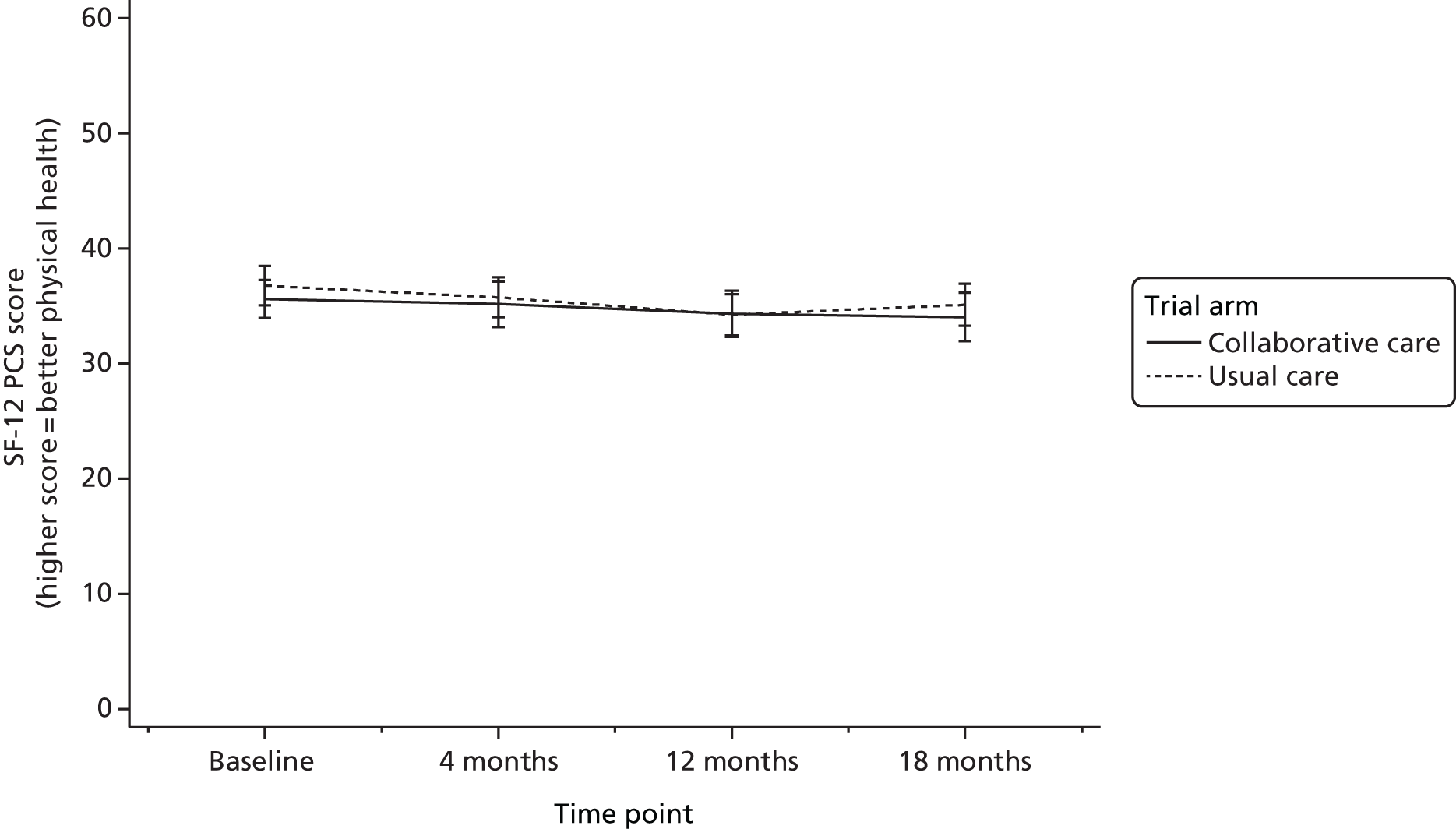
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 196 | 35.5 | 34.4 to 36.7 | 211 | 35.1 | 34.0 to 36.2 | –0.44 | –2.00 to 1.23 | 0.583 |
| 12 months | 196 | 34.7 | 33.6 to 35.9 | 211 | 34.5 | 33.4 to 35.6 | –0.24 | –1.86 to 1.37 | 0.769 |
| 18 months | 196 | 34.1 | 33.0 to 35.3 | 211 | 34.7 | 33.5 to 35.8 | 0.55 | –1.09 to 2.18 | 0.514 |
The figures indicate that physical functioning was below average adult physical health status (scores of < 50) for participants throughout the trial period, as would be expected in an elderly population. Patients maintained similar functioning levels between baseline and 18 months, and group differences in physical functioning were not statistically significant based on the mixed-effects analysis at 4 months (mean score difference –0.44, 95% CI –2.00 to 1.23; p = 0.583) or any other follow-up.
Short Form questionnaire-12 items mental component summary score
The SF-12 MCS scores range from 0 to 100, with higher scores indicating better functioning. Unadjusted means for psychological functioning are presented in Table 31 and Figure 8 and the results of the formal statistical analysis by mixed modelling are given in Table 32.
| Time | Trial arm | Total (N = 485) | |
|---|---|---|---|
| Collaborative care (N = 249) | Usual care (N = 236) | ||
| Baseline (n) | 245 | 234 | 479 |
| Mean (SD) | 35.4 (9.51) | 35.7 (20.53) | 35.5 (10.01) |
| Median (minimum, maximum) | 35.8 (10.3, 60.2) | 36.2 (2.2, 63.9) | 35.9 (2.2, 62.9) |
| 4 months (n) | 178 | 188 | 366 |
| Mean (SD) | 41.6 (11.22) | 38.6 (10.86) | 40.1 (11.12) |
| Median (minimum, maximum) | 41.6 (13.4, 65.5) | 37.8 (7.8, 71.3) | 39.7 (7.8, 71.3) |
| 12 months (n) | 166 | 171 | 337 |
| Mean (SD) | 40.4 (12.12) | 38.9 (10.82) | 39.7 (11.49) |
| Median (minimum, maximum) | 40.4 (9.6, 67.4) | 38.5 (16.0, 68.6) | 39.5 (9.6, 68.6) |
| 18 months (n) | 158 | 167 | 325 |
| Mean (SD) | 40.1 (11.34) | 38.9 (10.84) | 39.5 (11.09) |
| Median (minimum, maximum) | 41.3 (14.5, 62.9) | 38.4 (1.7, 63.0) | 39.0 (1.7, 63.0) |
FIGURE 8.
Unadjusted mean SF-12 MCS scores (with 95% CIs).
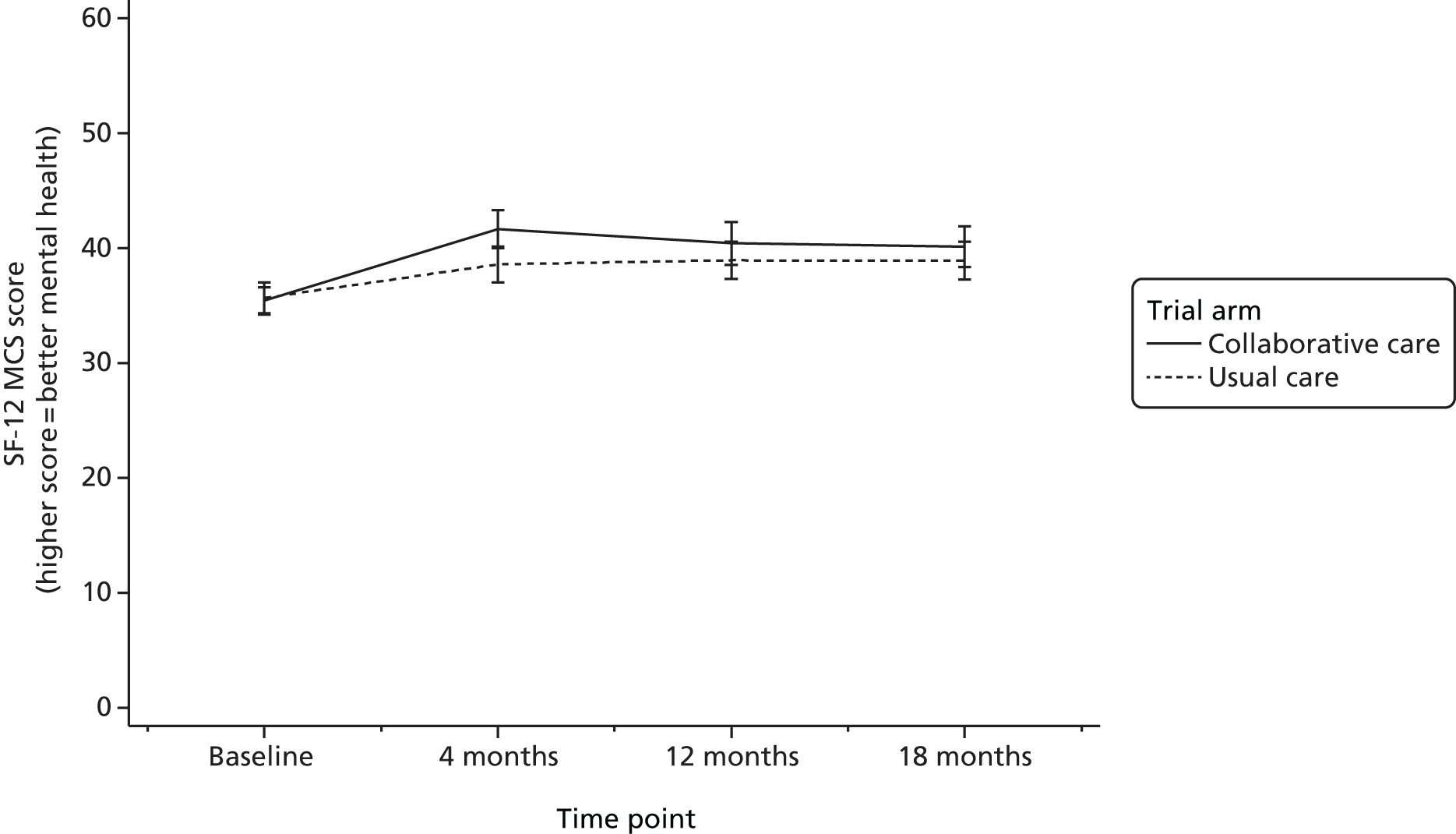
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| N | Mean | 95% CI | N | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 196 | 41.7 | 40.2 to 43.1 | 211 | 38.7 | 37.3 to 40.1 | –3.02 | –5.04 to –0.99 | 0.004 |
| 12 months | 196 | 40.5 | 39.0 to 42.0 | 211 | 38.9 | 37.4 to 40.3 | –1.63 | –3.73 to 0.46 | 0.125 |
| 18 months | 196 | 40.0 | 38.5 to 41.5 | 211 | 38.8 | 37.3 to 40.3 | –1.18 | –3.30 to 0.93 | 0.273 |
The figures indicate that participants’ average mental functioning was below the general average for adults (scores of < 50) throughout the trial period, which may be expected in a population with major depressive episodes. At 4 months’ follow-up, mental functioning had improved in patients in both arms, but to a greater extent in the collaborative care arm, and this difference between arms was statistically significant (mean score difference –3.02, 95% CI –5.04 to –0.99; p = 0.004). Participants in both trial arms maintained a score of around 40 at 12 and 18 months’ follow-up, and group differences were not statistically significant at these time points.
EuroQol-5 Dimensions, 3 levels
Quality of life using the EQ-5D-3L is measured on five dimensions – mobility, self-care, usual activities, pain/discomfort and anxiety/depression – and participants are given three response options to indicate their level of problems for each dimension. The weighted summary index derived from these dimensions is summarised and formally analysed as part of the CASPER plus health economic evaluation. For the purpose of exploring differences in quality of life between treatment arms, the frequencies of responses for each category in each dimension are presented descriptively in Table 33 and illustrated in Figures 9–13.
| Severitya | Trial arm | |||||
|---|---|---|---|---|---|---|
| Collaborative care (N = 249) | Usual care (N = 236) | |||||
| Total n | n | % | Total n | n | % | |
| EQ-5D-3L mobility | ||||||
| Baseline | ||||||
| Level 1 | 247 | 71 | 29 | 235 | 76 | 32 |
| Level 2 | 247 | 176 | 71 | 235 | 157 | 67 |
| Level 3 | 247 | 0 | 0 | 235 | 2 | 1 |
| 4 months | ||||||
| Level 1 | 181 | 56 | 31 | 193 | 36 | 36 |
| Level 2 | 181 | 124 | 69 | 193 | 69 | 64 |
| Level 3 | 181 | 1 | 1 | 193 | 1 | 1 |
| 12 months | ||||||
| Level 1 | 168 | 48 | 29 | 175 | 49 | 28 |
| Level 2 | 168 | 119 | 71 | 175 | 124 | 71 |
| Level 3 | 168 | 1 | 1 | 175 | 2 | 1 |
| 18 months | ||||||
| Level 1 | 161 | 49 | 30 | 171 | 51 | 30 |
| Level 2 | 161 | 111 | 69 | 171 | 120 | 70 |
| Level 3 | 161 | 1 | 1 | 171 | 0 | 0 |
| EQ-5D-3L self-care | ||||||
| Baseline | ||||||
| Level 1 | 243 | 163 | 67 | 234 | 175 | 75 |
| Level 2 | 243 | 75 | 31 | 234 | 55 | 24 |
| Level 3 | 243 | 5 | 2 | 234 | 4 | 2 |
| 4 months | ||||||
| Level 1 | 179 | 127 | 71 | 192 | 145 | 76 |
| Level 2 | 179 | 50 | 28 | 192 | 45 | 23 |
| Level 3 | 179 | 2 | 1 | 192 | 2 | 1 |
| 12 months | ||||||
| Level 1 | 168 | 115 | 68 | 175 | 119 | 68 |
| Level 2 | 168 | 48 | 29 | 175 | 55 | 31 |
| Level 3 | 168 | 5 | 3 | 175 | 1 | 1 |
| 18 months | ||||||
| Level 1 | 161 | 110 | 68 | 171 | 121 | 71 |
| Level 2 | 161 | 49 | 30 | 171 | 48 | 28 |
| Level 3 | 161 | 2 | 1 | 171 | 2 | 1 |
| EQ-5D-3L usual activities | ||||||
| Baseline | ||||||
| Level 1 | 338 | 136 | 40 | 356 | 124 | 35 |
| Level 2 | 338 | 189 | 56 | 356 | 209 | 59 |
| Level 3 | 338 | 13 | 4 | 356 | 23 | 6 |
| 4 months | ||||||
| Level 1 | 253 | 123 | 49 | 314 | 108 | 34 |
| Level 2 | 253 | 116 | 46 | 314 | 187 | 60 |
| Level 3 | 253 | 14 | 6 | 314 | 19 | 6 |
| 12 months | ||||||
| Level 1 | 231 | 112 | 48 | 277 | 89 | 48 |
| Level 2 | 231 | 108 | 47 | 277 | 165 | 47 |
| Level 3 | 231 | 11 | 5 | 277 | 23 | 5 |
| 18 months | ||||||
| Level 1 | 247 | 64 | 26 | 235 | 66 | 28 |
| Level 2 | 247 | 159 | 64 | 235 | 151 | 64 |
| Level 3 | 247 | 24 | 10 | 235 | 18 | 8 |
| EQ-5D-3L pain/discomfort | ||||||
| Baseline | ||||||
| Level 1 | 247 | 34 | 14 | 233 | 27 | 12 |
| Level 2 | 247 | 156 | 63 | 233 | 152 | 65 |
| Level 3 | 247 | 57 | 23 | 233 | 54 | 23 |
| 4 months | ||||||
| Level 1 | 180 | 26 | 14 | 192 | 26 | 14 |
| Level 2 | 180 | 113 | 63 | 192 | 121 | 63 |
| Level 3 | 180 | 41 | 23 | 192 | 45 | 23 |
| 12 months | ||||||
| Level 1 | 168 | 25 | 15 | 176 | 20 | 11 |
| Level 2 | 168 | 102 | 61 | 176 | 114 | 65 |
| Level 3 | 168 | 41 | 24 | 176 | 42 | 24 |
| 18 months | ||||||
| Level 1 | 161 | 23 | 14 | 171 | 23 | 13 |
| Level 2 | 161 | 95 | 59 | 171 | 115 | 67 |
| Level 3 | 161 | 43 | 27 | 171 | 33 | 19 |
| EQ-5D-3L anxiety/depression | ||||||
| Baseline | ||||||
| Level 1 | 246 | 26 | 11 | 234 | 25 | 11 |
| Level 2 | 246 | 176 | 72 | 234 | 178 | 76 |
| Level 3 | 246 | 44 | 18 | 234 | 31 | 13 |
| 4 months | ||||||
| Level 1 | 178 | 49 | 28 | 192 | 42 | 22 |
| Level 2 | 178 | 116 | 65 | 192 | 130 | 68 |
| Level 3 | 178 | 13 | 7 | 192 | 20 | 10 |
| 12 months | ||||||
| Level 1 | 168 | 46 | 27 | 177 | 38 | 21 |
| Level 2 | 168 | 104 | 62 | 177 | 123 | 69 |
| Level 3 | 168 | 18 | 11 | 177 | 16 | 9 |
| 18 months | ||||||
| Level 1 | 161 | 36 | 22 | 172 | 34 | 3 |
| Level 2 | 161 | 104 | 65 | 172 | 123 | 72 |
| Level 3 | 161 | 21 | 13 | 172 | 15 | 1 |
FIGURE 9.
The EQ-5D-3L mobility dimension: per cent of patients in each severity category.

FIGURE 10.
The EQ-5D-3L self-care dimension: per cent of patients in each severity category.
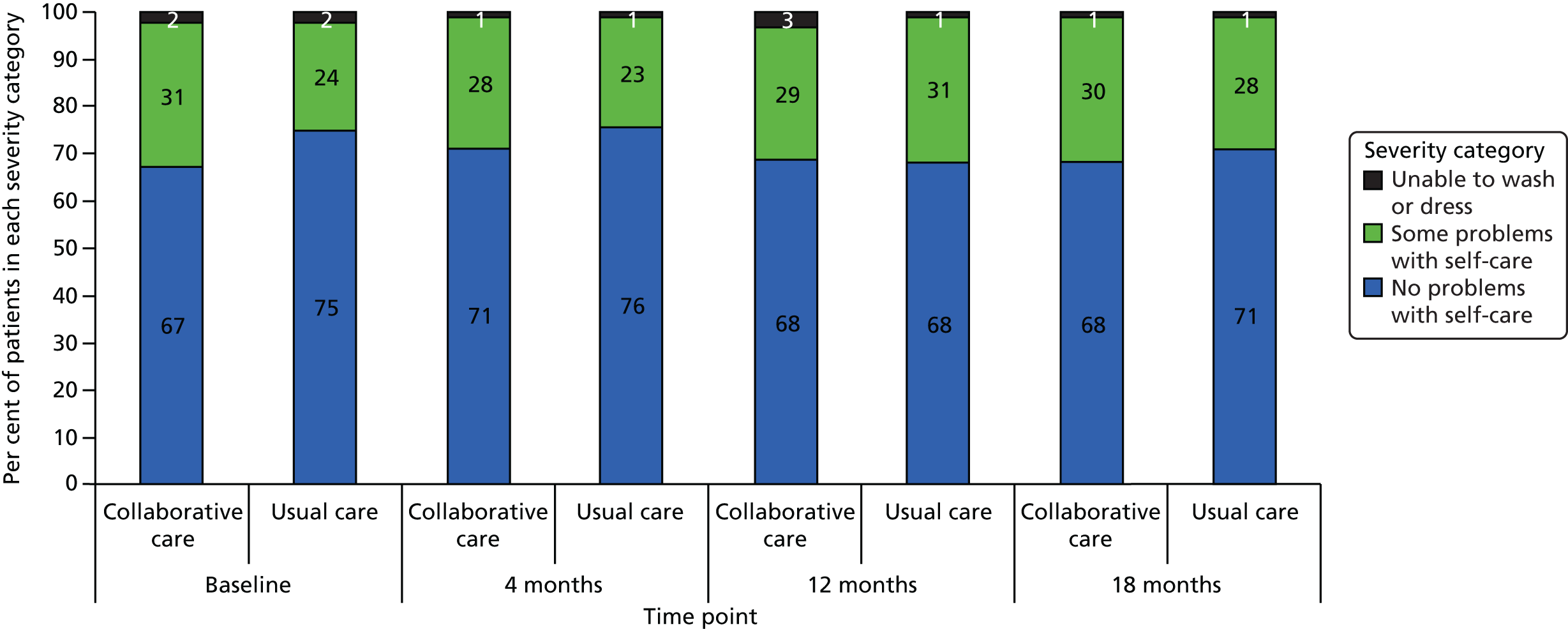
FIGURE 11.
The EQ-5D-3L usual activities dimension: per cent of patients in each severity category.
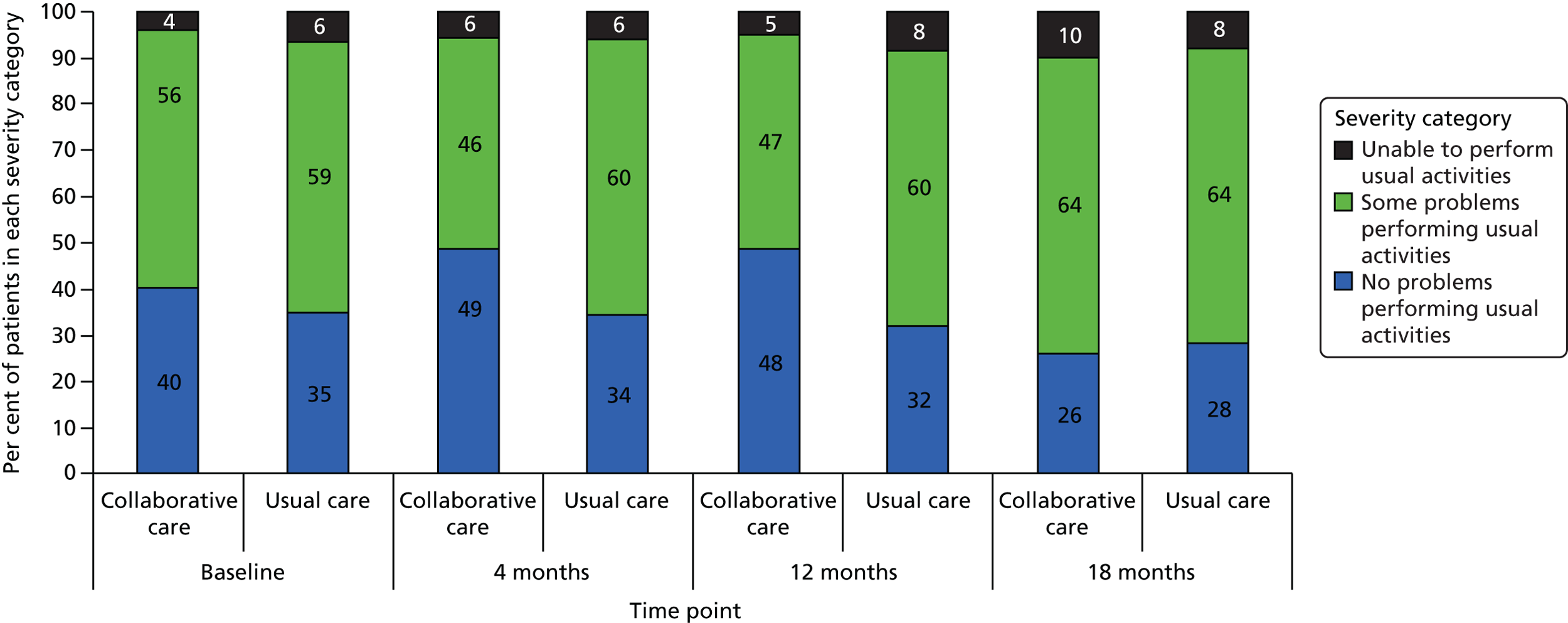
FIGURE 12.
The EQ-5D-3L pain/discomfort dimension: per cent of patients in each severity category.

FIGURE 13.
The EQ-5D-3L anxiety/depression dimension: per cent of patients in each severity category.
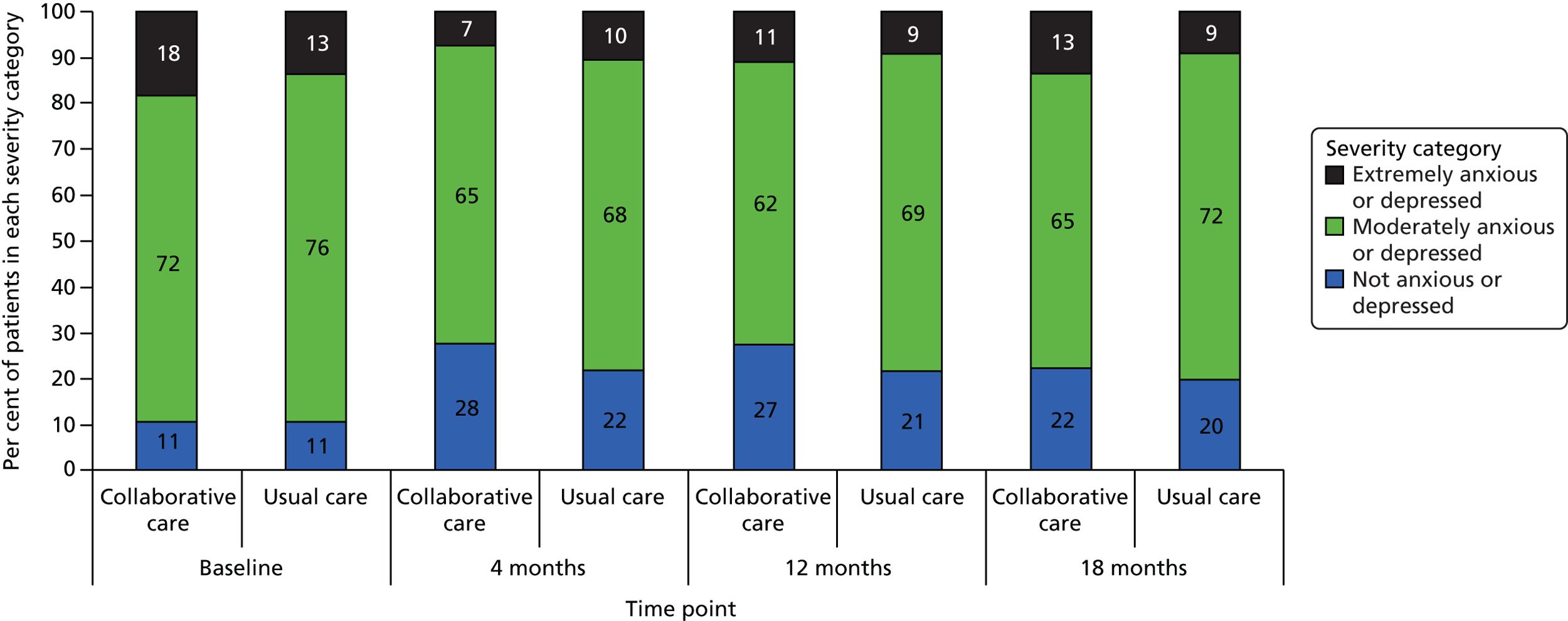
The majority of CASPER plus participants indicated no problems or some problems in each of the EQ-5D-3L areas, with few patients having severe difficulties. The most frequent use of the severe category was in the pain/discomfort dimension (around one-quarter of participants). The greatest trial arm differences were seen for usual activities, with the number of patients who had no problems performing usual activities increasing from 40% to 49% at 4 months’ follow-up in the collaborative care arm, whereas rates remained stable in the usual-care arm. This difference was maintained at 12 months but not at 18 months. Relatively greater improvements in favour of the intervention arm were also seen for anxiety and depression at 4 and 12 months, the number of people not anxious or depressed being higher in the collaborative care arm, although group differences were of moderate magnitude (around 6%). There were no substantial group differences in the mobility, self-care or pain/discomfort dimensions.
Patient Health Questionnaire-15 items physical health problems
The PHQ-15 is a measure of physical health problems. In this study it had a score range of 0–28 (usual maximum is 30), as a question regarding menstrual problems was removed for the elderly CASPER plus patient population.
Unadjusted means for physical health problems are presented in Table 34 and Figure 14, and the results of the formal statistical analysis by mixed modelling are given in Table 35. Physical health problems significantly decreased in the collaborative care arm at 4 months’ follow-up; in contrast, in the usual-care group, symptoms remained constant throughout follow-up (mean score difference 1.67, 95% CI 0.98 to 2.36; p < 0.001). This difference became smaller but remained statistically significant at 12 months (mean score difference 1.19, 95% CI 0.47 to 1.90; p = 0.001), whereas follow-up scores returned to near baseline levels for both groups at 18 months.
| Time | Trial arm | Total (N = 485) | |
|---|---|---|---|
| Collaborative care (N = 249) | Usual care (N = 236) | ||
| Baseline n | 146 | 234 | 480 |
| Mean (SD) | 12.3 (4.51) | 11.9 (4.33) | 12.1 (4.42) |
| Median (minimum, maximum) | 12 (2, 26) | 11 (2, 24) | 12 (2, 26) |
| 4 months n | 178 | 187 | 365 |
| Mean (SD) | 9.9 (4.63) | 11.5 (4.60) | 10.7 (4.68) |
| Median (minimum, maximum) | 10 (2, 22) | 11 (1, 22) | 10 (1, 22) |
| 12 months n | 165 | 178 | 343 |
| Mean (SD) | 10.5 (4.65) | 11.7 (4.59) | 11.1 (4.64) |
| Median (minimum, maximum) | 10 (3, 23) | 11.5 (1, 23) | 11 (1, 23) |
| 18 months n | 161 | 168 | 329 |
| Mean (SD) | 11.2 (5.22) | 11.4 (4.73) | 11.3 (4.97) |
| Median (minimum, maximum) | 11 (2, 23) | 11 (1, 22) | 11 (1, 23) |
FIGURE 14.
Unadjusted mean PHQ-15 scores (with 95% CIs).

| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 195 | 10.02 | 9.52 to 10.52 | 209 | 11.69 | 11.21 to 12.17 | 1.67 | 0.98 to 2.36 | < 0.001 |
| 12 months | 195 | 10.42 | 9.91 to 10.94 | 209 | 11.61 | 11.12 to 12.10 | 1.19 | 0.47 to 1.90 | 0.001 |
| 18 months | 195 | 10.04 | 10.53 to 11.56 | 209 | 11.34 | 10.84 to 11.84 | 0.30 | –0.43 to 1.02 | 0.423 |
Connor–Davidson Resilience Scale-2 items resilience
The two-item CD-RISC2 resilience measure has a score range of 0 to 8, with a higher score indicating greater psychological resilience. Unadjusted means for psychological resilience are presented in Table 36 and Figure 15, and the results of the formal statistical analysis by mixed modelling are given in Table 37. Average resilience at baseline was around 5 score points and remained consistent over the 18 months’ follow-up for patients in the usual-care group. Among patients in the collaborative care group, average resilience marginally improved but dropped back to baseline levels at 18 months. The group difference was statistically significant at 12 months’ follow-up (mean score difference –0.35, 95% CI –0.68 to –0.03; p = 0.034).
| Time | Trial arm | Total (N = 485) | |
|---|---|---|---|
| Collaborative care (N = 249) | Usual care (N = 236) | ||
| Baseline n | 247 | 235 | 482 |
| Mean (SD) | 4.9 (1.81) | 4.9 (1.74) | 4.9 (1.78) |
| Median (minimum, maximum) | 5 (0, 8) | 5 (0, 8) | 5 (0, 8) |
| 4 months n | 176 | 191 | 367 |
| Mean (SD) | 5.2 (1.78) | 5.0 (1.91) | 5.1 (1.85) |
| Median (minimum, maximum) | 5 (0, 8) | 5 (0, 8) | 5 (0, 8) |
| 12 months n | 168 | 177 | 345 |
| Mean (SD) | 5.1 (1.84) | 4.9 (1.82) | 5.0 (1.83) |
| Median (minimum, maximum) | 5 (0, 8) | 5 (0, 8) | 5 (0, 8) |
| 18 months n | 161 | 171 | 332 |
| Mean (SD) | 5.0 (2.03) | 4.9 (1.88) | 5.0 (1.95) |
| Median (minimum, maximum) | 5 (0, 8) | 5 (0, 8) | 5 (0, 8) |
FIGURE 15.
Unadjusted mean CD-RISC2 scores (with 95% CIs).
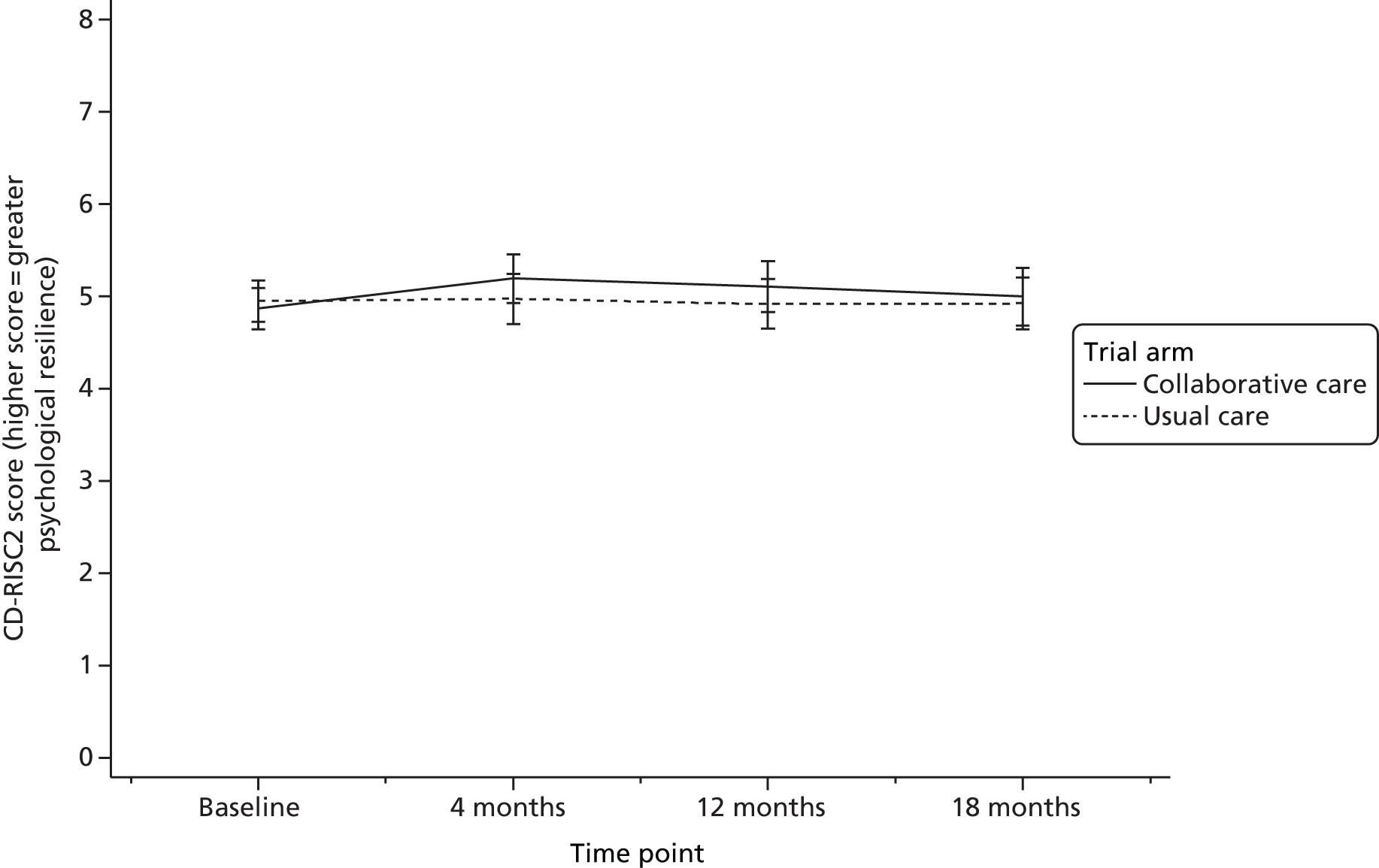
| Estimate at | Trial arm | Group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | ||||||||
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 196 | 5.19 | 4.96 to 5.42 | 211 | 4.91 | 4.69 to 5.13 | –0.28 | –0.59 to 0.04 | 0.089 |
| 12 months | 196 | 5.21 | 4.97 to 5.44 | 211 | 4.86 | 4.63 to 5.08 | –0.35 | –0.68 to –0.03 | 0.034 |
| 18 months | 196 | 5.01 | 4.77 to 5.25 | 211 | 4.85 | 4.62 to 5.08 | –0.16 | –0.49 to 0.17 | 0.352 |
Adverse events
A total of 81 SAEs including deaths were identified in CASPER plus participants over the 18-month follow-up period: 47 events occurred in 41 patients in the collaborative care arm and 34 events occurred in 33 patients in the usual-care arm (Table 38). The maximum number of SAEs per person was three, and the average number of SAEs experienced per CASPER plus participant was 0.19 in the collaborative care arm and 0.14 in the usual-care arm.
| Adverse event statistic | Trial arm | Total | |
|---|---|---|---|
| Collaborative care | Usual care | ||
| Total number of adverse events | 47 | 34 | 81 |
| Number of patients with any adverse event | 41 | 33 | 74 |
| Per cent of patients with any adverse event | 16.5 | 14.0 | 15.3 |
| Average number of events per patient | |||
| Mean | 0.19 | 0.14 | 0.17 |
| Median | 0 | 0 | 0 |
| Minimum, maximum | 0, 3 | 0, 2 | 0, 3 |
The majority of SAEs (98%) were assessed as being unrelated to the intervention, and the remaining SAEs were unlikely to be related. A breakdown of these figures by trial arm, as well as by the type and nature of the events, is presented in Table 39. The majority of events were unscheduled hospitalisations, with cardiovascular and miscellaneous events being the most likely reason for admissions. Causes of death are further detailed in the Mortality section of this report.
| SAE characteristic | Trial arm | Total (N = 81 events) | ||||
|---|---|---|---|---|---|---|
| Collaborative care (N = 47 events) | Usual care (N = 34 events) | |||||
| n | % | n | % | n | % | |
| Relatedness to the intervention | ||||||
| Unrelated | 46 | 98.9 | 33 | 97.1 | 79 | 97.5 |
| Unlikely to be related | 1 | 2.1 | 1 | 2.9 | 2 | 2.5 |
| Possibly related | – | – | – | – | – | – |
| Probably related | – | – | – | – | – | – |
| Definitely related | – | – | – | – | – | – |
| Type | ||||||
| Unscheduled hospitalisation | 24 | 51.1 | 19 | 55.9 | 43 | 53.1 |
| Other medically important condition | 17 | 36.2 | 8 | 23.5 | 25 | 30.9 |
| Death | 6 | 12.8 | 7 | 20.6 | 13 | 16.1 |
| Nature of adverse event | ||||||
| Cancer | 3 | 6.4 | 3 | 8.8 | 6 | 7.4 |
| Cardiovascular | 15 | 31.9 | 10 | 29.4 | 25 | 30.9 |
| Infection | 5 | 10.6 | 5 | 14.7 | 10 | 12.4 |
| Acute infection | 4 | 8.5 | 1 | 2.9 | 5 | 6.2 |
| Injury from falls | 5 | 10.6 | 4 | 11.8 | 9 | 11.1 |
| Miscellaneous | 14 | 29.8 | 11 | 32.4 | 25 | 30.9 |
| Unknown | 1 | 2.1 | 0 | 0.0 | 1 | 1.2 |
Mortality
A total of 13 participants died during the 18-month follow-up period, six patients in the collaborative care arm (2.4% of randomised patients) and seven patients in the usual-care arm (3.0% of randomised patients). Causes of death are summarised in Table 40. A chi-squared test revealed that the difference in mortality rates between treatment arms was statistically significant (χ21 = 0.14; p = 0.705).
| Trial arm | Cause of death |
|---|---|
| Collaborative care | 11436 – bilateral pneumonia |
| Collaborative care | 12507 – pneumonia |
| Collaborative care | 15355 – ischaemic heart disease and duodenal adenoma |
| Collaborative care | 16870 – congestive cardiac failure |
| Collaborative care | 17898 – chronic obstructive pulmonary disease and breast cancer |
| Collaborative care | 18977 – congestive cardiac failure |
| Usual care | 13133 – cardiac failure |
| Usual care | 15608 – myocardial infarction and bronchial pneumonia |
| Usual care | 18051 – cardiac failure |
| Usual care | 18497 – double pneumonia and kidney failure |
| Usual care | 18913 – lung cancer |
| Usual care | 21395 – ischaemic colitis |
| Usual care | 21800 – small cell carcinoma of the lung |
All deaths were further recorded as SAEs, and potential relatedness to the trial treatment was assessed as part of the adverse event processing. In total, 92% (12 events) of deaths were categorised as being unrelated to treatment, and 8% (one event) as unlikely to be related to treatment.
Summary of clinical effectiveness analysis
A total of 485 elderly patients in the north of England with a major depressive episode were randomised into the CASPER plus trial: 249 participants were allocated to collaborative care and 236 participants were allocated to usual care. Of those in the collaborative care arm, 83% received at least one treatment session and, on average, participants received a total of six sessions (one face to face and five over the telephone). A total of 83 participants (33%) withdrew from collaborative care treatment before or during treatment, with the most common reasons being not wishing to engage and physical ill health.
Participants were followed up by postal questionnaire at 4 months (80%), 12 months (74%) and 18 months (71%). Trial dropout was greater in the collaborative care arm (22% withdrew) than in the usual-care arm (10%). The primary trial outcome was PHQ-9 depression severity, analysed by a covariance pattern mixed model, adjusting for PHQ-9 depression at randomisation and baseline SF-12 physical functioning. As data from all time points were included in the model, 415 participants (86%) participants were included in the primary analysis. Model estimates at the primary end point of 4 months revealed a statistically significant effect in favour of collaborative care (mean difference 1.92 score points, 95% CI 0.85 to 2.99 score points; p < 0.001). However, this difference was not maintained during the long-term follow-up at 12 months (p = 0.741) or 18 months (p = 0.997).
Secondary analyses demonstrated robustness of these results when adjusting for clustering by case managers (20 case managers, ICC < 0.001), additional predictors of depression severit or predictors of non-response and when using multiply imputed data. All mean group differences at 4 months ranged between 1.92 and 1.97 score points. Results were mirrored by the greater reduction of moderately to severely depressed cases (PHQ-9 score of ≥ 10) for collaborative care patients at 4 months’ follow-up (p = 0.001), which was not maintained long term.
Of the secondary outcomes, collaborative care was associated with decreased anxiety (GAD-7 score) at 4 and 12 months (p < 0.001 and p = 0.024, respectively), better mental health functioning (SF-12 MCS score) at 4 months (p = 0.004) and greater psychological resilience at 12 months (p = 0.034). Self-reported prescription of selected antidepressants increased among collaborative care patients at 4 months (p = 0.025). Although there were no trial arm differences in physical functioning (SF-12 PCS score), patients in the collaborative care arm had fewer physical health problems (PHQ-15 score) at 4 and 12 months’ follow-up than patients in the usual-care arm (p < 0.001 and p = 0.001, respectively). Group differences were not statistically significant for any of the outcomes at 18 months’ follow-up.
A comparable number of SAEs occurred in each trial arm (collaborative care, 47 events; usual care, 31 events). Six participants in the collaborative care arm died during the trial, compared with seven in the usual-care arm (χ21 = 0.14; p = 0.705).
Chapter 6 Health economics
The health economic component of the CASPER plus trial was an incremental cost-effectiveness analysis exploring the value for money of the intervention over and above usual care. An analysis of uncertainty is also included to demonstrate the robustness of the results. First, the resource use and costs are estimated, including the costs of providing collaborative care and associated training of health-care professionals, and also the wider costs to the NHS. Second, health outcomes are quantified using QALYs using the SF-6D algorithm.
Resource use and costs
Collaborative care: required resources and associated costs
Case managers were psychological well-being practitioners (PWPs) employed at NHS band 5. Case managers each received training to provide collaborative care as part of the CASPER plus trial. In total, three training events were held covering four regions of the study (York, Leeds, Durham and Newcastle upon Tyne), each consisting of 2 consecutive days of training. The number of attendees per training event varied and efforts were made to provide training in a manner that ensured that the overall costs of travel and accommodation were minimal.
During the training, PWPs were orientated to the case managers’ manual, which outlined the overall principles of collaborative care and a ‘session-by-session overview’ of what case managers aimed to achieve with patients. The training courses for case managers were predominantly provided by two trainers; subsequently, these trainers also supervised case managers during implantation of the collaborative care programme implementation.
The manual stipulated that the programme of treatment should consist of ‘8–10 mainly telephone contacts with occasional face-to-face contacts over a period of 12 weeks’. In terms of the expectation for each session, it further stated that ‘contacts last 45 minutes for session one and 20–30 minutes for each subsequent contact’. The first session was generally held face to face and took place at participants’ homes, GP surgeries or other community venues.
Case managers received weekly supervision from a designated supervisor. The schedule of supervision followed a standardised agenda whereby for each patient there was a weekly discussion and case managers would prepare feedback to discuss each case with their supervisor. Supervisors were responsible for providing support to case managers on the process of collaborative care and medication management and on specific psychological interventions. On average, each patient contact was discussed between the case manager and supervisor for approximately 5 minutes.
Case managers provided participant-specific feedback to GPs. In the first instance, case managers worked with and advised participants’ GPs on their care. During treatment, case managers would provide a letter to update the GP on participants’ progress and, when appropriate, whether or not GPs might consider further treatment. At the end of the programme, case managers also sent a participant-specific summary report to the GP. Supervisors were available to advise case managers on next steps and consultation with GPs. Three letters were prepared and sent over the 12 weeks, requiring approximately 30 minutes of administration per letter. Case managers would also speak to GPs directly if they had any concerns about a participant’s medication or overall well-being.
Case managers were also charged with a duty of care to engage outside agencies (such as social services or in response to safeguarding issues) in situations in which they became aware of safety or risk (including abuse). However, the client group had a generally low level of clinical need in this respect, and this additional responsibility was not generally required.
To estimate the personnel costs required to provide collaborative care (as intended within the manual), estimates of NHS unit costs were derived from national reference costs56 (Table 41).
| Item | Unit cost (£) | Referencea |
|---|---|---|
| PWP (band 5) | ||
| Per hourb | 39 | Nurse (mental health) |
| Patient-related workb | 52 | Nurse (mental health) |
| Face-to-face contactb | 74 | Nurse (mental health) |
| PWP (band 6) | ||
| Supervisionb | 49 | Nurse team leader |
| GP | ||
| Appointment | 45 | ‘Per patient contact lasting 11.7 minutes’ |
| Home visit | 114 | ‘Per out of surgery visit lasting 23.4 minutes’ |
| Telephone consultation | 27 | ‘Per telephone consultation lasting 7.1 minutes’ |
| Practice nurse | ||
| Appointment | 13.43 | ‘£52 per hour of face-to-face contact, duration of contact 15.5 minutes’ |
| Telephone consultation | 6.15 | ‘£52 per hour of face-to-face contact, assumed similar time as GP: 7.1 minutes’ |
Table 42 summarises the resources required over the 12-week programme of collaborative care and indicates our estimate of the direct cost for base-case analysis. The direct cost of collaborative care (based on the prior estimation within the manual) was calculated to be £494.73. This cost is adopted for the base-case cost as, ex ante, there is insufficient information to anticipate actual levels of required care; however, deviation that did occur will be explored within our sensitivity analyses.
| Item | Frequency | Duration | Total quantity | Cost (£) | Description |
|---|---|---|---|---|---|
| Training case managers | |||||
| Case managers attending | 16 case managers | 13 hours | 208 | 8112a | 2 days, 6.5 hours each |
| Supervision of course | Two trainers, three sessions | 13 hours | 96 hours | 4704b | 2 days, 6.5 hours each |
| Manual | One manual/case manager | – | 16 | 80 | Printing |
| Travel and accommodation | For two trainers × two sessions | 1 night | 4 nights | 600 | Sessions in Durham and Leeds |
| Subtotal (total cost of training) | 13,496 | Cost to train all case mangers | |||
| Subtotal (total cost of training per participant) | 39.23 | 249 allocated to the programme | |||
| Collaborative care | |||||
| Session 1 | One per patient | 45 minutes | 45 minutes | 55.50 | Assumed by home visitc |
| Sessions 2–10 | Median of nine sessions per patient | 30 minutes | 4.5 hours | 234 | Assumed by telephoned |
| Supervisions | One per week (12) | 5 minutes | 1 hour | 88 | 1 hour over 12 weeksa,b |
| GP communication | Three letters | 30 minutes | 1.5 hours | 78 | Patient-related work4 |
| Engaging outside agencies | 0 | 0 | 0 | 0 | Not required in CASPER |
| Subtotal (total cost of intervention per participant) | 455.50 | ||||
| Total cost (training + intervention) | 494.73 | Cost for base-case analysis | |||
Consequences for health care by trial arm
Patient contacts over the duration of the trial are presented in Table 43, which compares the summary statistics for those who accessed collaborative care with the summary statistics for those who accessed usual care. Initial observation suggests that collaborative care in depression results in a small marginal increase in contacts with most services (except GP home visits). However, the mean contact rate with any service is dependent on access to the service and the subsequent level of utilisation.
| Categoriesof health-care resources | Trial arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | |||||||||
| Mean | SD | Minimum | Maximum | n | Mean | SD | Minimum | Maximum | n | |
| GP | ||||||||||
| Appointment | 10.12 | 7.74 | 0 | 41 | 226 | 9.63 | 7.36 | 0 | 45 | 221 |
| Home visit | 0.76 | 2.48 | 0 | 21 | 226 | 0.80 | 2.52 | 0 | 26 | 221 |
| Telephone consultation | 2.42 | 3.75 | 0 | 27 | 226 | 2.20 | 3.00 | 0 | 15 | 221 |
| Practice nurse | ||||||||||
| Appointment | 5.40 | 6.60 | 0 | 54 | 226 | 5.10 | 6.11 | 0 | 40 | 221 |
| Nurse | ||||||||||
| Telephone consultation | 0.37 | 2.06 | 0 | 24 | 226 | 0.33 | 0.89 | 0 | 7 | 221 |
To test whether or not differences in service use may be attributed to collaborative care, statistical tests must accommodate highly skewed distributions with significant numbers of zero service users and, therefore, specific analytical procedures are required. 60 Applying zero-inflated negative binomial regression61 allows inference on the effect of collaborative care on two factors: access (using the logistic model) and overall change in the contact rate (using the full model). For full regression outputs see Appendix 12.
Having any access to services is indicated by outputs of the logistic models. Across all five resource use categories we may conclude that participants are generally unlikely to access any services. Examining logistic regression outputs related to nurse appointment (see Appendix 12, Table 55) suggest that collaborative care may increase the likelihood of access (log odd = 14.1944; p = 0.01). However, small sample numbers available from this trial mean that inferences regarding the effect of collaborative care should be made with caution.
The full model specification accounts for access and subsequent use to test any overall change in the contact rate. Over resource use categories, there is generally no significant difference between groups. However, inference of the effect of collaborative care on nurse telephone consultations suggests an overall increase in the contact rate of 2.25 (95% CI 0.9285 to 5.4403; p = 0.073). Again, given the sample size, inferences on the effect of collaborative care should be made with caution.
Cost–consequences and total costs
Unit costs (as presented in Table 41) were multiplied by resource utilisation to derive patient-level costs of health care (Table 44). Health-care costs of treatment therefore extend beyond the cost of the collaborative care programme (£494.73), increasing wider costs by a mean of £682.27. Overall, the mean total cost in the collaborative care group was £1171.45 (95% CI £1166.99 to £1175.92, n = 226), compared with £654.14 (95% CI £650.78 to £657.52, n = 221) in the usual-care group.
| Categories of cost | Trial arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | |||||||||
| Mean | SD | Minimum | Maximum | n | Mean | SD | Minimum | Maximum | n | |
| Collaborative care | 489.18 | 0.00 | 489.18 | 489.18 | 249 | 0.00 | 0.00 | 0.00 | 0.00 | 236 |
| GP | ||||||||||
| Appointment | 455.18 | 348.12 | 0.00 | 1845.00 | 226 | 433.51 | 331.03 | 0.00 | 2025.00 | 221 |
| Home visit | 86.76 | 282.44 | 0.00 | 2394.00 | 226 | 90.79 | 286.95 | 0.00 | 2964.00 | 221 |
| Telephone consultation | 65.47 | 101.30 | 0.00 | 729.00 | 226 | 59.38 | 81.07 | 0.00 | 405.00 | 221 |
| Practice nurse | ||||||||||
| Appointment | 72.58 | 88.62 | 0.00 | 725.40 | 226 | 68.44 | 82.02 | 0.00 | 537.33 | 221 |
| Nurse | ||||||||||
| Telephone consultation | 2.29 | 12.68 | 0.00 | 147.68 | 226 | 2.03 | 5.49 | 0.00 | 43.07 | 221 |
| Total cost | 1171.45 | 523.60 | 489.18 | 4273.94 | 226 | 654.15 | 506.38 | 0.00 | 3548.87 | 221 |
Health benefits
Health-state utility by time point
Utility scores for each participant were estimated from the responses to the SF-6D at baseline and at 4, 12 and 18 months. Table 45 presents a summary of the unadjusted utility scores by time point and trial arm across all available respondents at each time point.
| Trial arm | Mean | SD | Median | Minimum | Maximum | n |
|---|---|---|---|---|---|---|
| Collaborative care (utility) | ||||||
| Baseline | 0.551 | 0.105 | 0.543 | 0.345 | 0.863 | 243 |
| 4 months | 0.580 | 0.144 | 0.580 | 0.000 | 0.937 | 180 |
| 12 months | 0.565 | 0.153 | 0.569 | 0.000 | 0.922 | 171 |
| 18 months | 0.540 | 0.154 | 0.553 | 0.000 | 0.895 | 175 |
| Usual care (utility) | ||||||
| Baseline | 0.559 | 0.100 | 0.565 | 0.345 | 0.863 | 233 |
| 4 months | 0.566 | 0.139 | 0.566 | 0.000 | 0.922 | 195 |
| 12 months | 0.550 | 0.143 | 0.580 | 0.000 | 0.859 | 179 |
| 18 months | 0.535 | 0.159 | 0.545 | 0.000 | 0.895 | 165 |
However, as we can observe, the available sample number by group and across time points declines as the study progresses. For the purpose of illustration, health-state utilities were estimated using a linear-mixed model and estimate group marginal effect for the mean for each time point; Figure 16 plots the outputs and illustrates trends in estimated utilities by trial arm over the trial period.
FIGURE 16.
Plot of mean (95% CI) of SF-6D indexes over the trial period, by trial arm.
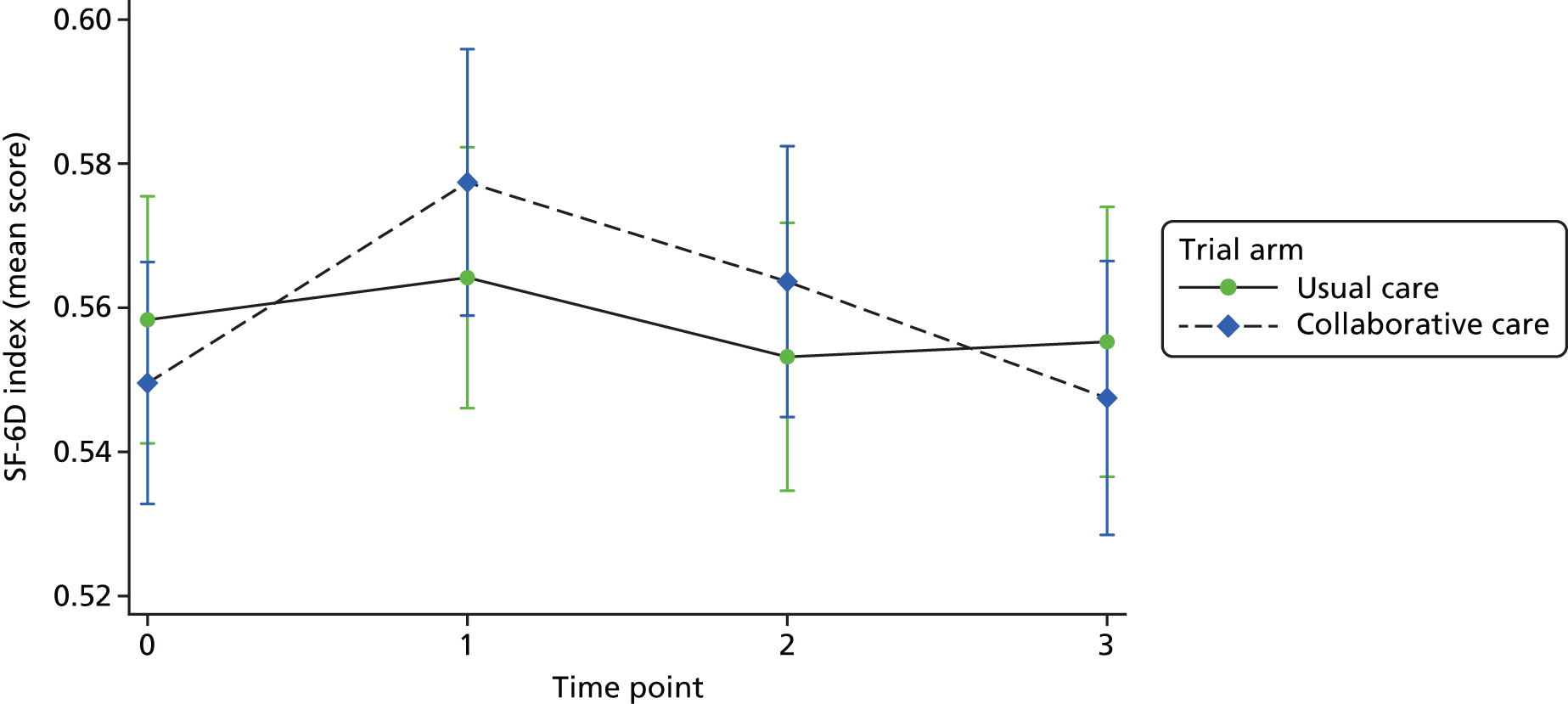
Observing differences in baseline utility scores by trial arms suggests that control for baseline utility to estimate cost-effectiveness is important.
Quality-adjusted life-years
The QALYs were estimated by summing the time-weighted averages of the utility scores between the four time points up to 18 months, for all individuals with information available for complete-case cost-effectiveness analysis. Table 46 compares the undiscounted QALYs, as well as QALYs discounted at 3% beyond 12 months, by trial arm.
| Trial arm | Mean | SD | Median | Minimum | Maximum | n |
|---|---|---|---|---|---|---|
| Without a 3% discount beyond 12 months | ||||||
| Collaborative care | 0.900 | 0.241 | 0.889 | 0.036 | 1.573 | 175 |
| Usual care | 0.889 | 0.224 | 0.914 | 0.044 | 1.412 | 187 |
| With a 3% discount beyond 12 months | ||||||
| Collaborative care | 0.893 | 0.238 | 0.881 | 0.036 | 1.573 | 175 |
| Usual care | 0.882 | 0.222 | 0.907 | 0.044 | 1.395 | 187 |
The incremental QALY gained of collaborative care compared with usual care would be 0.011, and this result did not change when QALYs were discounted beyond 12 months. To adjust for baseline utility, we apply ordinary least squares to explain QALYs and controlled for trial arm, age and baseline utility. Table 47 presents the outputs of the ordinary least squares regression.
| Variables | Coefficient | Standard error | t | p > t | 95% CI |
|---|---|---|---|---|---|
| Baseline utility | 1.275 | 0.091 | 14.070 | 0.000 | 1.097 to 1.453 |
| Collaborative care | 0.019 | 0.019 | 0.960 | 0.338 | –0.020 to 0.057 |
| Age | –0.026 | 0.016 | –1.570 | 0.118 | –0.058 to 0.007 |
| Constant | 0.094 | 0.071 | 1.320 | 0.188 | –0.046 to 0.233 |
Adjusting for baseline utility scores, the collaborative care baseline is associated with an incremental QALY gain of 0.019 (95% CI –0.020 to 0.057; p = 0.338). Independent of treatment, baseline utility is significantly predictive of overall QALYs. Given the potential implications of group differences in baseline utility and age, the adjusted incremental QALY gain for collaborative care using the complete-case sample (n = 362) informs all subsequent estimates of cost-effectiveness.
Cost-effectiveness and uncertainty
Collaborative care for depression resulted in a small but non-significant mean increase in QALYs over the 18-month period, with a higher associated health-care cost. Based on the generic health gains, the mean cost per incremental QALY was £26,016. Examining this ICER, collaborative care for depression falls within the explicit willingness-to-pay range (£20,000–30,000 per QALY)53 and may represent value for money to the NHS. However, a risk-averse decision-maker may wish to consider the uncertainty in the ICER. Non-parametric bootstrap of the difference in cost and QALYs generates 10,000 replications.
Figure 17 presents results of the bootstrap, depicting the uncertainty surrounding the mean difference in cost and QALYs on the cost-effectiveness plane. The results of the bootstrap indicate the average incremental cost of collaborative care over usual care to be £479.58 (bootstrapped 95% CI £380.55 to £578.61). This demonstrates that a large proportion of the replications fall within the north-east quadrant (82.36%), suggesting that the most likely scenario is that collaborative care in depression increases costs and also creates QALY gains. Figure 18 illustrates the uncertainty surrounding the ICER and provides 50%, 75% and 95% confidence ellipses. Inference on the 50% confidence ellipse suggests that, based on the current sample size, we cannot absolutely exclude the possibility that collaborative care may reduce health status.
FIGURE 17.
Cost-effectiveness plane (controlling for baseline utility). Bootstrap with 10,000 replications.

FIGURE 18.
Confidence ellipse (controlling for baseline utility).
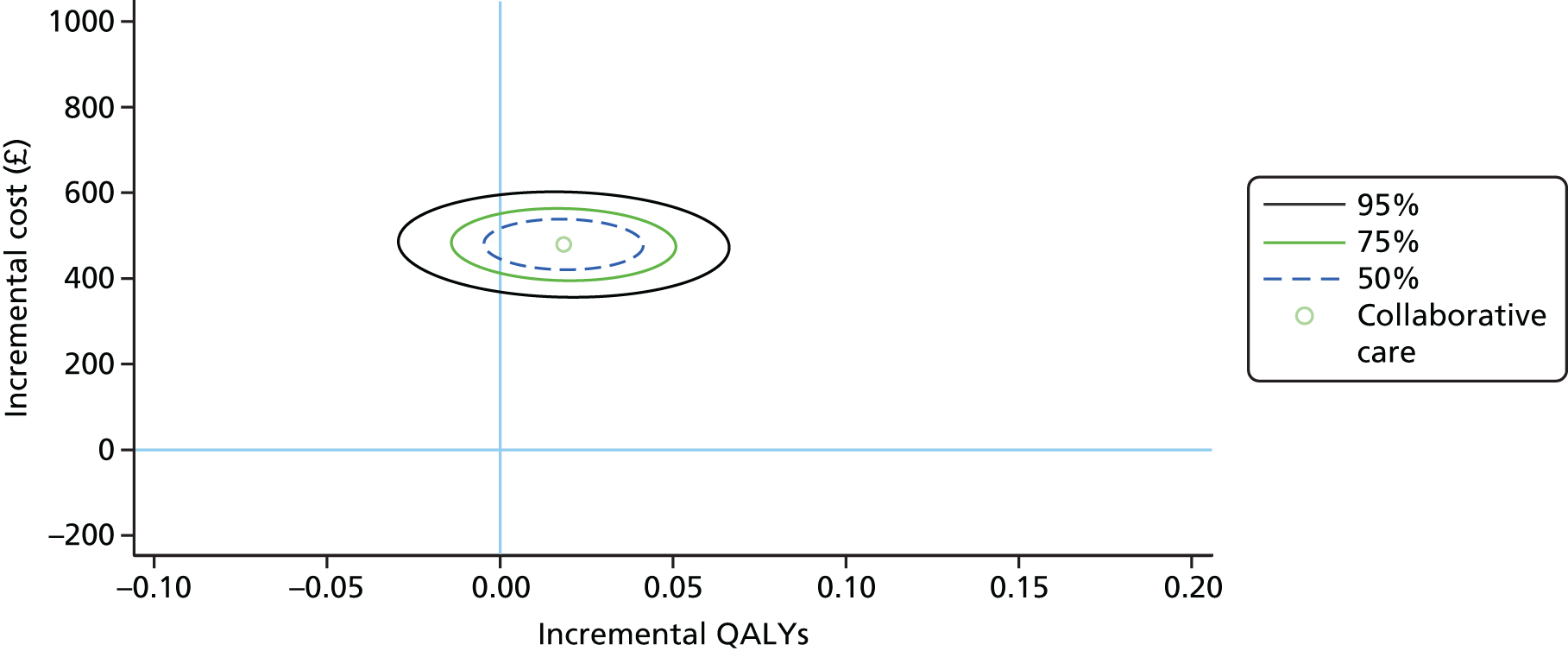
Figure 19 presents the CEAC illustrating the relationship between willingness to pay and the probability that collaborative care would be cost-effective. With reference to the NICE’s cost-effectiveness threshold, the likelihood that collaborative care would be cost-effective at £20,000 per QALY is 38.84% and at £30,000 per QALY is 54.94%.
FIGURE 19.
Cost-effectiveness acceptability curve (controlling for baseline utility) for the cost per QALY analysis.
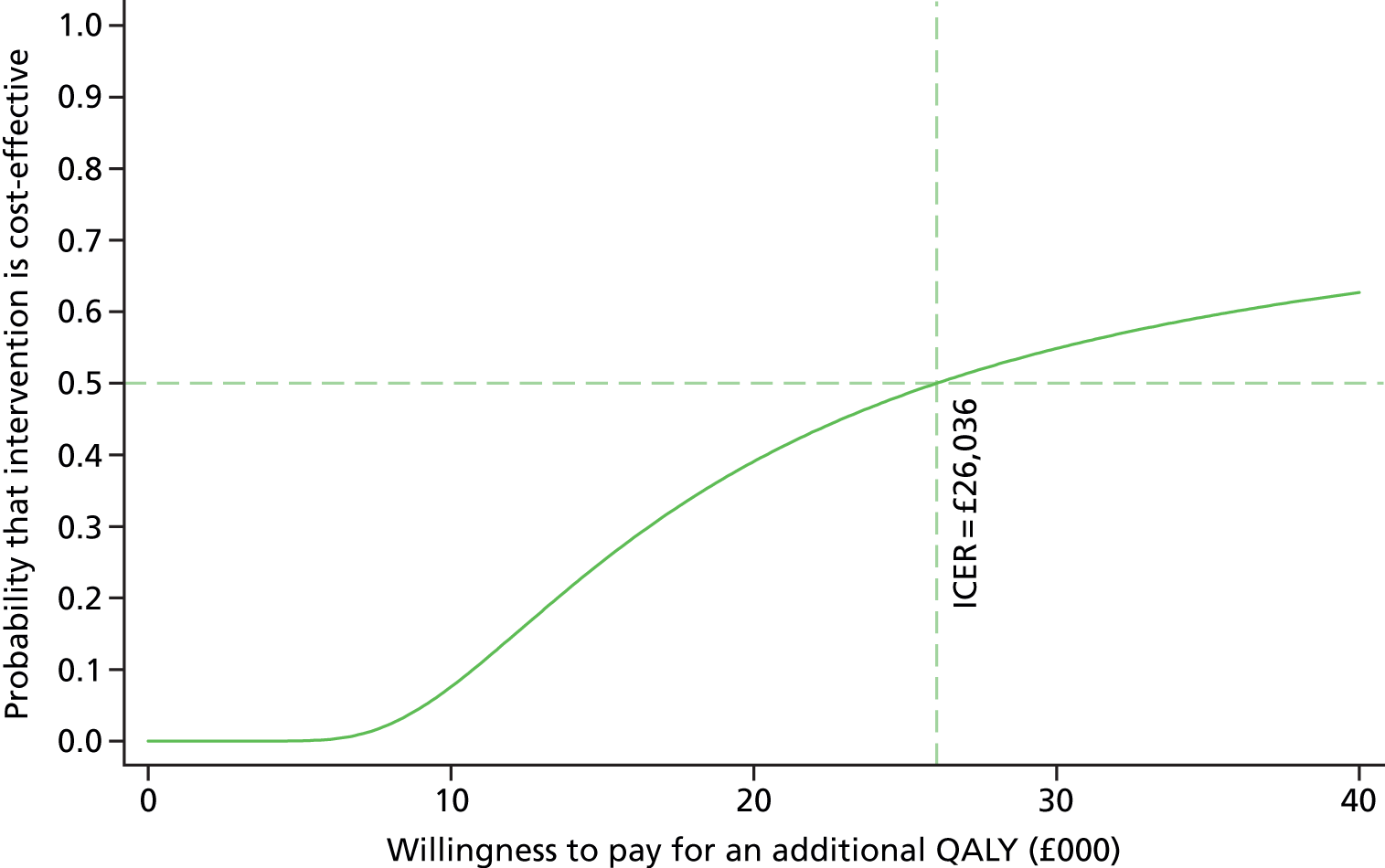
Sensitivity analysis
Sensitivity analysis: fidelity to intervention sessions and ex post adjustment of the expected direct cost of collaborative care
This analysis seeks to examine documented fidelity of participants to treatment (as observed from data collected using PC-MIS) and to consider how this may adjust our expectation of the cost of implementing collaborative care. Figure 20 summarises distribution in the number of contacts. The total number of registered sessions shows significant variation, and a bimodal distribution of participant sessions appears evident. This raises the question of whether or not there exists a selection process in early sessions (by consumer, provider or both) that divides patients into two groups. For example, 128 of participants (51%) received five or fewer sessions in the early stage of care and the remaining 49% were most likely to receive 10 sessions.
FIGURE 20.
Number of sessions of collaborative care. CC, collaborative care.

To examine if health status explains the number of received sessions, Table 48 presents baseline scores to PHQ-9, GAD-7 and SF-6D contingent on whether or not participants received more or fewer than five sessions. With respect to all three measures, this suggests that the group that received six or more sessions, on average, had poorer health status at baseline. However, reference to 95% CIs would suggest that the between-group difference is not significant.
| Collaborative care | Baseline scores, mean (95% CI) | ||
|---|---|---|---|
| PHQ-9 | GAD-7 | SF-6D | |
| Number of sessions | |||
| ≤ 5 | 12.01 (10.26 to 13.76) | 9.2 (7.77 to 10.64) | 0.553 (0.482 to 0.624) |
| ≥ 6 | 12.75 (10.96 to 14.55) | 9.67 (8.19 to 11.14) | 0.548 (0.475 to 0.621) |
The next question is, therefore, whether or not the number of sessions is influential on the treatment effect. Figure 21 illustrates the mean (95% CI) of SF-6D indexes over the trial period comparing usual care with treatment. The trial arms are subdivided into participants who received five or fewer sessions of collaborative care and those who received more than six sessions of care. This provides a clear illustration that a dose–response relation is likely to exist between the number of sessions received and generic health status. Fidelity to, and engagement with, the treatment programme appears to be an important feature in threshold depression.
FIGURE 21.
Mean (95% CI) of SF-6D indexes over the trial period comparing the usual-care group with trial arms that received either five or fewer sessions of collaborative care or more than six sessions of collaborative care. CC, collaborative care.

Table 49 calculates the adjusted direct costs of collaborative care using data from PC-MIS based on session from 174 trial participants. This suggests that, on average, collaborative care received by participants cost £198.25 (95% CI £196.16 to £200.35). Given the available information on health gains, it is difficult to determine how this should be interpreted compared with the expected ex ante cost of £494.73. One interpretation is that, in practice, collaborative care cost £296.48 less than expected.
| Session | Type of contact (%) | Mean duration (minutes) | Mean cost (£) | Poisson exact (95% CI) (£) | ||
|---|---|---|---|---|---|---|
| Face to face | Telephone | |||||
| 1 | 90.34 | 9.66 | – | 60 | 64.02 | 62.83 to 65.22 |
| 2 | 5.71 | 94.29 | 1.27 | 31 | 21.41 | 20.73 to 22.11 |
| 3 | 6.33 | 92.41 | – | 30 | 19.11 | 18.47 to 19.77 |
| 4 | 6.34 | 93.66 | – | 30 | 17.95 | 17.32 to 18.59 |
| 5 | 7.58 | 92.42 | 0.83 | 29 | 16.68 | 16.08 to 17.30 |
| 6 | 5.79 | 93.39 | – | 29 | 14.99 | 14.42 to 15.58 |
| 7 | 6.25 | 93.75 | – | 29 | 14.09 | 13.53 to 14.66 |
| 8 | 7.14 | 92.86 | 1.32 | 28 | 11.87 | 11.37 to 12.40 |
| 9 | 7.89 | 90.79 | – | 26 | 8.30 | 7.88 to 8.74 |
| 10 | 7.14 | 92.86 | – | 27 | 6.18 | 5.81 to 6.56 |
| 11 | 7.14 | 92.86 | – | 26 | 2.73 | 2.49 to 2.99 |
| 12 | 14.29 | 85.71 | – | 26 | 0.43 | 0.33 to 0.53 |
| 13 | 50 | 50 | – | 25 | 0.21 | 0.15 to 0.29 |
| 14 | – | 100 | – | 30 | 0.15 | 0.10 to 0.22 |
| 15 | – | 100 | – | 30 | 0.15 | 0.10 to 0.22 |
| Total cost | 198.25 | 196.16 to 200.35 | ||||
To examine the value underlying the observed rate of fidelity to treatment, Figure 22 presents an adjusted CEAC (with ICER) to examine whether, with an intention-to-treat perspective, collaborative care for threshold depression represents value for money or not.
FIGURE 22.
Cost-effectiveness acceptability curve (controlling for baseline utility) using ex post estimate of the direct costs of collaborative care.
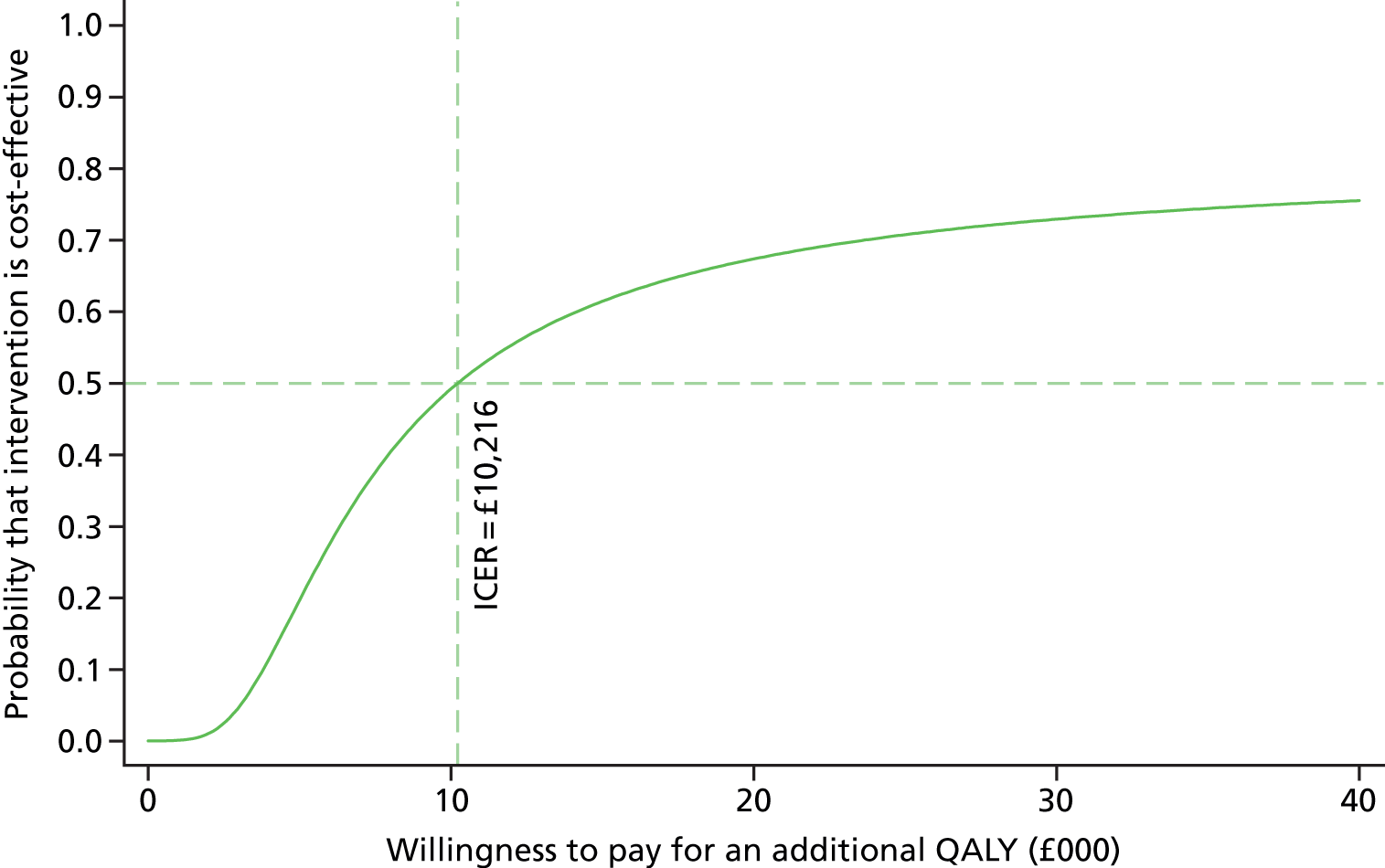
Firstly, given the ratio of average treatment effect to the adjusted cost of collaborative care, an ICER of £10,216 per incremental QALY can be estimated. Costs and QALYs can also be examined by subgroup (i.e. contingent on whether or not participants received more than five sessions of collaborative care). Table 50 performs a seemingly unrelated regression to inform the cost-effectiveness analysis related to these subgroups.
| Coefficients | Total costs (£) (95% CI) | QALY (95% CI) |
|---|---|---|
| Collaborative care: five or fewer sessions | £12 (–£120 to £143) | –0.0004 (–0.0517 to 0.0509) |
| Collaborative care: six or more session | £307 (£193 to £421.93)**** | 0.0311 (–0.01375 to 0.0760)* |
| Age | –£33 (–£117 to £51) | –0.02533 (–0.0582 to 0.0075)* |
| Baseline utility | – | 1.2638 (1.0840 to 1.4436)**** |
| Constant | £560 (£302 to £819)**** | 0.105 (–0.0365 to 0.2466)* |
The results indicate that receiving five or fewer sessions of collaborative care is associated with an average cost of £12 (95% CI –£120 to £143) and results in an average QALY gain of –0.0004 (95% CI –0.0517 to 0.0509); therefore, this strategy is dominated by usual care.
We can also determine that the overall cost of receiving six or more sessions of collaborative care is associated with an average cost of £307 (95% CI £193 to £421.93; p < 0.001) and an average QALY gain of 0.0311 (95% CI –0.01375 to 0.0760; p = 0.174). Although the statistical significance of the difference in QALY gain is low, despite the reduction in sample size, it remains higher than the average treatment effect presented in Table 47. Overall, this suggests that, for individuals who receive six or more sessions of collaborative care, the ICER will be £9876 per QALY.
Figure 23 presents confidence ellipses on the cost-effectiveness plane for each subgroup and clearly illustrates that collaborative care requires a strict minimum number of sessions (i.e. six). Examining the CEAC, we can observe that (for session numbers greater than six) the probability that collaborative care is cost-effective is significantly higher over the explicit reimbursement range (£20,000–30,000 per QALY). These findings suggest that collaborative care may be cost-effective with improved fidelity and that further research to better understand reasons why certain participants do not adhere to the treatment programme (e.g. patient preferences or supply-side competing priorities) is required.
FIGURE 23.
(a) Confidence ellipses (comparing collaborative care with more or fewer than six sessions: session number ≤ 5 vs. ≥ 6) and (b) CEAC (for collaborative care with six or more sessions: session number ≥ 6).
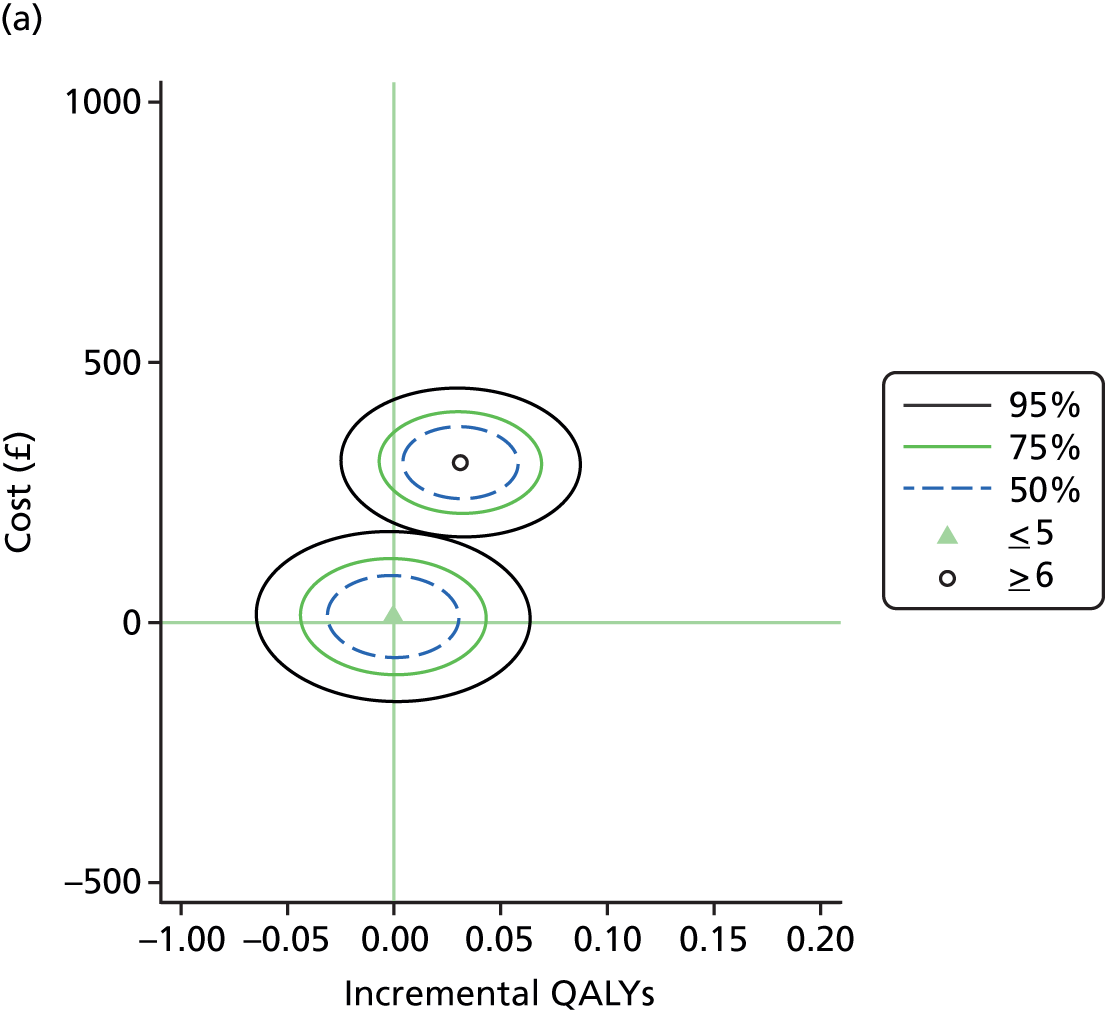
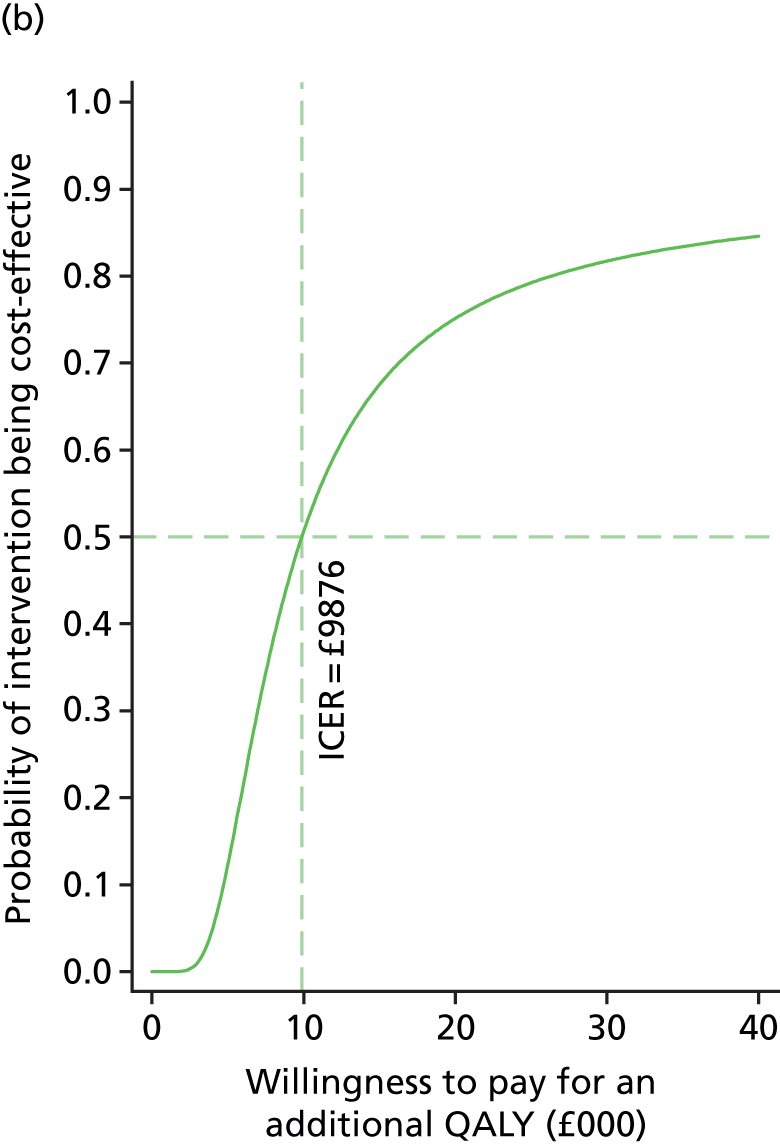
Chapter 7 Qualitative findings
Background
Gunn et al. 62 reported that GPs perceive patient engagement to be of fundamental importance in dealing with depression. Older people may be reluctant to define their distress as a mental health problem, with implications for treatment acceptance. 13 Simpson et al. 63 reported on the experiences of depressed participants receiving collaborative care in the UK, finding that case managers were able to reduce the sense of stigma of being diagnosed with a mental health problem and resolve misconceptions around medication prescribed by the GPs.
Aims
The nested qualitative process evaluation explored the views and experiences of the CASPER plus intervention within the collaborative care framework for the management of depression in older people from the perspectives of participants, case managers and GPs. It considered:
-
Older people’s experiences of receiving treatment for depression within a collaborative care framework and the acceptability of the collaborative care intervention. We sought participants’ views on depression and their experiences of receiving the intervention from case managers.
-
Case managers’ experiences of delivering an intervention for depression within a collaborative care framework.
-
GPs’ perspectives of the management of depression and views on the CASPER plus intervention.
The process evaluation explored patient and professional views to determine whether or not service-level integration of care is effective and how it is experienced by participants. It explored whether or not the model of collaborative care intervention fitted within routine practice and was viewed as sustainable. The findings from the CASPER plus RCT (see Chapter 5) revealed a statistically significant difference in the primary outcome of depression severity (PHQ-9) between trial arms at 4 months’ follow-up in favour of collaborative care, but not at 12 or 18 months’ follow-up. Of the secondary outcomes, collaborative care was associated with decreased anxiety (GAD-7 score) at 4 and 12 months, improved mental health functioning (SF-12 MCS score) at 4 months and greater psychological resilience at 12 months. None of the outcomes had a statistically significant difference at 18 months’ follow-up.
The qualitative data, presented here, provide insight into:
-
recognising and identifying depression in older people
-
components of the intervention within the collaborative care framework valued by participants
-
how the collaborative care framework fits into current practice.
Methods
Ethics approvals
Ethics approval for the RCT and this qualitative study was gained by Leeds East Research Ethics Committee, Yorkshire & Humber (reference number 10/H1306/61).
Design
We conducted semistructured interviews with trial participants, case managers and GPs to gather in-depth information on their views and experiences of receiving and delivering the intervention and how they perceived the acceptability, engagement and implementation of patient and collaborative care, respectively. Interviews were conducted with trial participants at the end of the intervention period and with case managers delivering the intervention and patient GPs during the intervention.
Sampling
Our aim was to interview a purposive sample of GPs and trial participants, including some participants who declined to take part or who withdrew from the intervention, alongside all the case managers who delivered the intervention. Our approach was to sample participants and GPs from recruiting practices in both urban and rural areas in the north of England and to gather data from areas of differing deprivation indices to achieve a spread in sex, age and socioeconomic status (SES). We aimed to interview all 12 case managers and supervisors and up to 15–20 GPs (or until category saturation was achieved) along with 7–10 participants who did not engage in the intervention and 15 participants who completed the intervention (or until category saturation achieved).
Initially, as numbers were small and recruitment to the trial was slow, we invited all participants who had completed the intervention to take part in a semistructured interview of up to 1 hour. All case managers were invited to be interviewed once they had delivered a course of treatment to at least three participants, and GPs from practices with at least five participants from the collaborative care arm of the trial were invited to be interviewed. Once we had recruited approximately half of our participants this way, we then used a purposive sampling strategy with the aim of gaining a more varied sample of patient and GP participants.
At the start of the CASPER plus qualitative study, following the order of GP practice recruitment, all participants invited to take part were from the central site of York, which included urban and rural practices in the surrounding areas from Harrogate to Hull. Given that most of these areas are of relatively low to moderate deprivation, we used an active selection process to ensure some participants from areas of higher deprivation were invited to be interviewed, such as inner-city Hull.
Participants were sent an invitation pack by post, which comprised a letter, an information leaflet and a consent form with a pre-paid envelope to return to the research team. GPs and case managers were sent an invitation letter, information leaflet and a consent form by e-mail. Before interviews commenced, written informed consent was obtained from all participants (see Appendices 13–15).
Data collection
Interviews were carried out by Karen Overend, Katherine Bosanquet and Sarah Nutbrown in a place convenient to the participant and lasted between 20 and 60 minutes. The majority of GPs chose to be interviewed at their practice, although 5 out of 12 asked to be interviewed by telephone. Ten out of the 12 participants requested to be interviewed in their home, with the remaining two choosing to be interviewed by telephone. Nearly all case managers were interviewed in the researcher’s office, with three opting for a telephone interview. Interviews were conducted between May 2013 and November 2014. All interviews were digitally recorded (with participants’ signed consent), transcribed verbatim and anonymised before data analysis.
A topic guide was developed for each of the three groups (see Appendices 16–18). The topic guides were designed with reference to the literature, approved by the research team and developed iteratively as data collection commenced.
Consent
In accordance with ethics guidelines, informed consent was gained by the researcher from each study participant before the interview commenced. An information sheet was sent to the participant in advance, as part of the invitation pack. Before starting the interview, the researcher (interviewer) ensured that this was signed by the participant (interviewee), repeated the main points of the information sheet and aim of the study and gave the participant an opportunity to ask any questions. The researcher assured the participant of the anonymity and confidentiality of their personal information. GPs and case managers were also given the opportunity to ask questions about the study and were assured anonymity and confidentiality. Consent was obtained from GPs and case managers using the same process as for trial participants.
Data analysis
The interview transcriptions formed the data, through the use of thematic analysis and principles of constant comparison. 64 This was developed iteratively and the topic guides were modified as analysis progressed. The main qualitative researcher on the project (KO) worked closely with the data to identify descriptive coding; this was informed by regular discussion with qualitative supervisor (CC-G) and co-researcher (KB). Analysis was undertaken by individual researchers Karen Overend, Carolyn Chew-Graham and Katherine Bosanquet. Data analysis involved a process of organising the data, descriptive coding, interpretive coding, writing and theorising. Deviant cases were actively sought throughout the analysis and emerging ideas and themes modified in response. Following analysis by individual researchers, themes were agreed during discussion with the full research team.
Findings
In total, 12 GPs, 13 participants (12 who had completed the intervention and one who had withdrawn before commencing therapy) and eight case managers were interviewed (see Appendix 19). The main themes identified in the data were ‘revealing hidden depression’, ‘reducing the blind spots’, ‘an opportunity to talk’ and ‘moving on’ from depression. Our findings are reported in a recent qualitative paper. 65
Data are presented to support analysis and are labelled by identifier and number.
Revealing hidden depression
For most of the older people we interviewed, being invited to participate in the CASPER plus study seemed to raise their awareness of low mood:
It crept up on me really, how I felt. I think it had been coming on for a long time and I didn’t realise how bad I’d got until I filled that form in and I just ticked the boxes and posted it.
Participant (PT)6
Several GPs described how taking part in the CASPER plus trial helped to raise awareness of depression in their older population. One GP said:
I think it’s probably alerted us to one or two of the . . . more needy participants who perhaps were not coming to us for help . . . people have been brought into the system that . . . had sort of dropped out from seeing the GP.
GP3
Some case managers described how some participants admitted they had not spoken to others, including their GPs, about how low they felt:
One gentleman that I saw, he said the most useful thing had been the diagnostics, as risk was identified, and so we wrote to the GP about that. And it was . . . the risk was still there when I saw him for the first time so I put that in a letter as well and he said that had kind of opened the door. He would have never gone and spoken to his GP about it.
Case manager (CM)2
. . . they [the patient] wouldn’t do anything and they wouldn’t commit suicide but they feel ashamed I guess of having some thoughts [that they’d be better off dead] . . . and those are the sorts of things they don’t always like us to share with the GP because it’s back to that stigma, isn’t it?
CM1
Although some patient participants did not use labels such as ‘depression’ or ‘low mood’, those who did suggested that other older people may fail to recognise or admit their feelings because of the perceived stigma of doing so:
. . . people don’t talk about it do they, they think it’s a weakness don’t they? But it is something that you can’t help when you are in it, you know as I say you don’t realise you are going in it and as much as you try you know sometimes you can’t get out it, it gets deeper you know.
PT6
A few patient participants commented on the ‘invisibility of depression’:
. . . you know if I broke an arm I’d get a sling wouldn’t I, you know it’s fairly obvious, but I suppose with any mental illness you can’t see it, you don’t know.
Withdrawn participant (PTW)1
Several GPs reported an awareness of the stigma associated with depression, especially in this age group, that may impact on whether or not the patient would raise it within a consultation:
It’s sort of an age group where they’re not as open about depression as maybe younger people are, there’s a bit of a stigma attached to it still.
GP8
A few GPs described how they normalised depression in older people; one admitted possibly colluding with the patient in ignoring cues within the primary care:
You’re sort of aware there are people who have depressive episodes that aren’t possibly addressed, they may themselves not really recognise it, and they just think it’s part of, you know, getting older.
GP3
You’d like to think that primary care is fairly aware of it [depression] anyway. But maybe the temptation is to let sleeping dogs lie, I don’t know. So you know, if you diagnose someone with depression you’ve got to do something about it haven’t you?
GP6
Some GPs described a tension between a desire to consider the ‘whole’ patient and, owing to limited time and treatment options, a tendency to prescribe antidepressants to older people:
We often go down a medication route because, well it does help them, and it’s very difficult to get other services. And the psychiatry for the elderly tends to be more focused on dementia.
GP8
Several GPs recognised that depression in older people often materialises alongside complex physical conditions or social problems, including loneliness. Some of these GPs disclosed a reluctance to identify the condition, partly because of the absence of a psychological treatment pathway for depression in the over-sixty-fives and a tendency to prioritise physical symptoms over emotional health:
I suppose in a busy clinic we probably don’t have time to sort of delve into depression along with the sort of 12 and a half minutes of consulting on chronic diseases that’s squeezed into 10 minutes, so depression would take another 5 or 6, so . . . we’ll probably skip over that unless they bring it to us.
GP12
Being invited to participate in the CASPER plus trial provided an opportunity for some people to talk about depression, enabling them to recognise and seek help for low mood.
Reducing the ‘blind spots’
Several case managers and three GPs described how two practitioners working with a patient helped to reduce the ‘blind spots’, as each professional offered a different perspective:
So you’ve got the benefit of somebody who’s looking at a person, never having met them before who can see certain things, versus somebody who has known somebody for some time and can see certain things but, those two people, will have, probably have, blind spots . . . because one person doesn’t know that person very well and the other has maybe, over the years, has just sort of formed a fixed idea about somebody. Collaborative working, not only will it progress the patient forward but it will also . . . reduce blind spots, I think, in their care.
GP1
One GP saw the case manager as helping to ‘patch up’ the gaps in the patient’s support network:
I think a lot of the difficulty . . . is their support networks have become a bit more fragmented . . . especially those that are bereaved, or have families spread around the country or spread around the world . . . so I can see that maybe we can patch that fragmentation up a little bit . . . it’s not the same as having your relatives but having some kind of support, I can see that as a benefit.
GP3
The case managers viewed their role as a facilitator, or ‘go-between’, who is able to convey information to the GP that the patient may be reluctant to disclose directly:
Sometimes, if people can’t talk to their GP or don’t understand that maybe they had a problem like depression, and don’t know how to approach a GP because of stigma and things like that then I’ve been that facilitator, I’ve helped them with that process.
CM1
For example, one case manager reported advocating on behalf of a patient who was having problems with pain:
. . . she was using cannabis to manage the pain and she felt there was nothing else the doctors could do, so I spoke to her GP and they said she could get a referral to the pain clinic . . . She [the patient] had given up all hope, but she was happy for me to pester them a little bit.
CM3
The GPs and case managers offered different perspectives on participants’ health needs, which was seen to reduce ‘blind spots’ in depression care.
An opportunity to talk
Being offered an opportunity to talk outside the GP consulting room was valued by the majority of participants:
The most startling thing about the experience was all my life I’ve never had anybody to talk to, there’re things I wouldn’t even discuss with my wife and to have an outsider person that didn’t really know me who was impartial . . . that helped me a great deal, just by having someone to discuss things with.
PT5
. . . having someone to talk to . . . about things in my life that I would talk to say the family about or friends unless they were extremely close friends, it gave me someone objective to talk to you know, that was removed from my situation.
PT2
Some participants suggested that GPs were not always receptive to discussing problems with mood:
You know and the GPs, well they don’t, they don’t seem to be interested I don’t think. Oh, it’s depression, take a pill, go away.
PT12
I just have a bit of a problem with doctors because I just don’t think they do the job that they maybe should be doing, it’s a 2-minute interview or whatever, they don’t really know your records, they don’t know the history, they don’t tie things up.
PTW1
In contrast, most participants described the case manager as providing empathic support, being able to offer more time than the GP and knowing how to direct participants to voluntary organisations:
. . . she did everything she possibly could . . . I mean she went the extra mile. She spoke to the people at Parkinson’s – Parkinson’s UK – to see if there was a network somewhere, an advice centre, and things I didn’t know she found out for me.
PT7
Patient participants spoke about the benefit of having someone to talk to in confidence, outside the primary care consultation, who was said to listen without judging, allowing them to talk openly about feelings and personal issues:
I thought it was very good. And I think the fact that people were bothered, to see how the older people felt . . . I think that was good. You didn’t feel like you just got a script thrown at you and you were waiting for God sort of thing . . . it was the fact that someone was interested in how you felt.
PT1
Giving participants an opportunity to talk outside the clinical setting of the primary care consultation room appears to be valued by most of the older people we interviewed and by their GPs.
‘Moving on’ from depression
Some participants reported how the case manager encouraged them to increase activity and social contact, which the participants felt had improved both their physical health and mood. For example:
The telephone conversations for me were helpful. She got me to think about doing things. I’m doing a computer course now and there’s a chance I might be able to help them at [voluntary organisation].
PT9
It has helped me thinking about things I can do . . . I go in the pool, only in the baby pool but it’s good for my legs and my shoulder . . . and you know it makes you feel better once you’ve done it, not just my legs, but in yourself, you know . . .
PT6
A few participants valued the practical aspects and the techniques learned from the case manager:
I’ve kept a diary all my working life and by going – a daily diary that is – and by going through it we could highlight various things that tip the balance if you like of the scales of happiness and depression and it was highlighted [depression] and between us we figured out a way of coming through it basically.
PT5
When we moved onto the technical part of it where they are asking specific questions and giving specific ideas, I find these very useful and in fact I’ve continued to do those. The ones I am talking about are where you identify things to do . . . and make a list.
PT4
Case management with behavioural activation provides older people with tools to help manage their depressive symptoms and to understand that behaviour and mood are closely linked. Behavioural activation promotes participation in social and physical activity, which may enable older people to ‘move on’ from depression and to experience improved well-being.
Discussion
To our knowledge this is the first qualitative study to explore the perspectives of older people, case managers and GPs, all of whom were participants in a trial of collaborative care for older people. Our findings support previous studies that suggest that depression in older people may be hidden and that invitation to participate in a trial can serve to uncover depression in participants and to raise awareness in GPs. The findings also illustrate that interaction with the case manager provides older participants with an opportunity to talk outside the primary care consultation, to deal with their low mood and to move forward.
The findings support the literature, which suggests that participation in a trial and active case management can help to reduce stigma and may improve the care for mental health problems, such as depression,10,66 and that being invited to participate in a trial acted as a catalyst for older people to reflect on their feelings and depression, which may not have been identified outside the trial setting.
Both GPs and participants may normalise depression and view it as an expected consequence of having one or more chronic health conditions. 12,67 GPs may be reluctant to address signs and symptoms of the condition, partly because of the lack of treatment options for older depressed adults and the limited consultation time in which to address the problem. Our results add to the evidence that there is insufficient capacity within existing primary care for psychosocial support of older people with depression68 and that older people may value a separate space to discuss their problems.
Strengths and weaknesses
This study explored multiple perspectives on the views and experiences of those receiving and delivering a psychosocial intervention for depression within a collaborative care framework. Although we aimed to interview people across a wide demographic range, we found it difficult to recruit GPs and participants from areas of low SES. We believe this may be a reflection of the demographics of trial participation, as people with higher levels of deprivation are less likely to respond to invitation. This means that the group of CASPER plus trial participants we recruited from was disproportionately less deprived than the general population. Similarly, GPs in areas of lower SES were less likely to respond to an invitation to be interviewed. In addition, ethnicity was poorly recorded at GP practices so we were unable to sample on this basis.
Conclusions
Depression is commonly hidden and coexists with physical conditions that are prioritised by both participants and GPs. Being invited to participate in a trial about depression seems to facilitate acceptance of symptoms and may reduce stigma and allow older people to disclose their feelings, name the problem and access care. Older people value an opportunity to talk outside the GP consultation. The findings from this nested qualitative study suggest that a psychosocial intervention delivered by a case manager can provide a valuable resource, which fills a gap in the care of older people with depression. Behavioural activation encourages increased activity and social contact, which may improve physical health symptoms as well as mood. Furthermore, it can enable older people to ‘move on’ from depression, providing them with the tools to manage their symptoms.
Chapter 8 Discussion
The CASPER plus trial is, to our knowledge, the first large-scale evaluation of the clinical effectiveness and cost-effectiveness of collaborative care in older adults with case-level depression in the UK. The area of research was one that was prioritised by the National Institute for Health Research (NIHR) Health Technology Assessment programme and was identified as a research priority in NICE guidelines on the management of depression. 7 We designed a collaborative care intervention suitable for older people with clinical depression that could feasibly be delivered via expansion of psychological care by the IAPT programme. In the CASPER plus trial outcomes were measured across a broad range of domains including psychological well-being, quality of life, resilience and health-state utility. Important aspects of health service resource use were also recorded. The CASPER plus trial included concurrent qualitative and economic evaluations.
The main findings of the CASPER plus study in relation to (1) trial-based estimates of the clinical effectiveness of collaborative care, (2) trial-based estimates of cost-effectiveness and (3) qualitative examination of acceptability and use of collaborative care will now be discussed in turn.
Trial-based estimate of the clinical effectiveness of collaborative care for subthreshold depression
A group of older adults with Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition Major Depressive Disorder were recruited to the CASPER plus study. The mean age of the population was 72 years. There was a high prevalence of coexisting long-term health problems, such as diabetes, arthritis, ischaemic heart disease or chronic respiratory illness.
When offered collaborative care, the majority of participants (83%) engaged with this telephone-based intervention and the mean number of sessions was six.
At 4 months’ follow-up there was improvement over time in both groups in terms of depression severity as measured by a commonly used measure of depression severity (the PHQ-9), but a greater level of improvement was recorded in the collaborative care group. There was a statistically significant benefit for collaborative care in terms of the primary outcome of depression severity at 4 months. The magnitude of difference in favour of collaborative care at 4 months was 1.92 PHQ-9 score points (95% CI 0.85 to 2.99 score points; p < 0.001). This benefit for collaborative care was not sustained at 12 or 18 months. The score difference at 4 months equates to a standard effect size of 0.34 and is in the range of the effect size that the trial was powered to detect. This finding was robust to a range of sensitivity analyses.
An effect in reducing the prevalence of case-level depression at 4 months was also observed. At 4 months’ follow-up, 40% of participants in the collaborative care arm were found to be moderately to severely depressed, compared with 55% in the usual-care group (odds ratio 2.18, 95% CI 1.36 to 3.51). By 12 and 18 months there was no effect for collaborative care.
When a number of secondary outcomes were analysed there was also a benefit for collaborative care. There was a significant and sustained 4- and 12-month improvement in anxiety (as measured by the GAD-7) and somatic complaints (as measured by the PHQ-15). Of note was the fact that common somatic complaints among older people (such as pain, constipation and disrupted sleep patterns) were found to be specifically improved in the collaborative care group compared with the usual-care group.
The population of older adults had important limitations of function consistent with the high levels of physical comorbidity, and this was reflected in low scores on the SF-12 PCS. Physical functioning was below average adult physical health status (scores of < 50) for participants throughout the trial period, as would be expected in an older population; however, collaborative care had little impact on physical function. Improvements and between-group differences were observed for the MCS of the SF-12 in favour of collaborative care, and in line with changes on other psychological function scales. Improvements were also noted for resilience, as measured by the CD-RISC2 measure at 12 months.
In summary, statistically significant improvements in depression severity were observed in favour of collaborative care in both the short term (4 months) and the medium term (12 months) for secondary outcomes of anxiety and somatisation.
Summary of trial-based estimates of the cost-effectiveness of collaborative care
There was a concurrent cost-effectiveness analysis within the CASPER plus trial, and we were able to derive utility-based estimates of quality of life alongside resource use derived from scrutiny of routinely collected administrative data (GP databases and IAPT databases). Collaborative care was a relatively brief intervention delivered by a low-intensity IAPT therapist. When all costs associated with a fully completed episode of collaborative care were accounted for, the cost to the NHS was £495 per patient. Only around half of the collaborative care participants completed six or more of the eight planned sessions and, when the costs of collaborative care as may be delivered within a typical IAPT service were accounted for, the cost was £198 per patient. There was a non-significant improvement in health-state utilities associated with collaborative care compared with usual care (adjusted QALY gains = 0.019; p = 0.338). Resource use was not substantially offset in the collaborative care group, with the total costs reduced by around £51 in the collaborative care group. In the base-case analysis, the incremental cost-effectiveness of collaborative care achieved gains at a cost of £26,010 per QALY. The probability that the incremental cost-effectiveness of collaborative care was less than £20,000 per QALY was 39% and the probability that it fell below the willingness-to-pay threshold of £30,000 per QALY was 55%. When participants who engaged with six or more sessions were included in the analysis, the cost per QALY estimate fell to £9876 per QALY.
Summary of main findings from qualitative examination of acceptability and uptake of collaborative care
The qualitative evaluation explored the perspectives of older people with depression being offered and receiving treatment for depression within a collaborative care framework. It obtained multiple perspectives on the understanding of depression and depression management in older people by investigating both patient and professional views, which provided bottom-up evidence on the acceptability and practicality of the intervention. This type of collaborative care represents an innovative treatment in the NHS, as it involves the delivery of a psychological intervention by a novel mode of delivery (over the telephone).
The qualitative evaluation showed that the intervention was acceptable to a large proportion of participants but that some did not engage with it. Some participants had misgivings about the potential benefits of behaviourally based programmes. Some participants disliked certain aspects of behavioural activation, such as the need to reflect and self-monitor. Others found the activity diaries and ‘homework’ difficult, requiring too much time and effort. However, case managers learned to adapt treatment and tailor collaborative care to the individual, and this process improved as case managers gained experience.
The qualitative evaluation provided evidence that participants appreciated their personal relationship with the case manager, who was able to facilitate communication with their GP as well as provide them with the opportunity to talk, outside the clinical setting of the primary care consultation room.
Discussion of main findings
The observed standard effect of 0.34 for the primary outcome represents a moderate effect size according to criteria used to classify the magnitude of effect for psychological interventions. 50 The effect size is consistent with findings from systematic reviews of collaborative care, as summarised in a recent Cochrane review,22 and is also of the same order of magnitude as that seen in UK trials of collaborative care for working-age adults, such as those observed in the recently published CADET69 and also in the recently completed CASPER trial for older people with lower-severity depression. 29,30 The CASPER plus trial also showed benefits across a range of secondary outcomes, and it was notable that there were improvements in anxiety symptoms, somatoform symptoms and quality of life (mental domain as measured by the SF-12). These benefits were seen in the short term (4 months) and were also sustained at 12 months for secondary outcomes (but not for the outcome of depression severity). At 18 months’ follow-up there were no discernible differences between groups.
The proportion of participants with case-level depression at 4 months was reduced among those who received collaborative care. We note that other studies have found longer-term benefits of collaborative care,70 including studies of collaborative care for older populations,16 but this finding was not replicated in the CASPER plus trial. When we looked at the prescription of antidepressants in this population, we noted that only a minority of participants were in receipt of any kind of antidepressant. The provision of collaborative care had an impact on the prescription of antidepressants in the short term, with a doubling of antidepressant prescriptions at 4 months’ follow-up, but this was not sustained at 12 months. It was in the short term that the greatest benefits were apparent for collaborative care, and this is in line with research which shows a strong relationship between antidepressant prescription rates and the magnitude of benefit from collaborative care. 21 Collaborative care is a complex intervention with multiple components and it is, as yet, unclear how the different components of treatment relate to outcome both in the short and longer term.
We noted from the rates of uptake of the intervention that the majority of participants (83%) engaged well and completed a large number of planned sessions (median six out of eight planned sessions). The qualitative evaluation of collaborative care pointed to aspects of the intervention that participants found helpful. The initial appointment was face to face in order to establish a relationship between the case manager and participant before continuing the sessions as telephone appointments. What was notable was that participants were generally happy to receive collaborative care over the telephone, but that the initial face-to-face meeting was felt to be important. There was some uncertainty whether or not a telephone intervention would be acceptable to older people with depression. It was encouraging to find, from the qualitative study and comments made to case managers, that this was seen by most people to be an acceptable method of delivery. This is important for those who plan services or for therapists who may have misgivings about the telephone-based mode of delivery of a psychosocial intervention. These results are in line with our earlier study of the use of collaborative care for older people with subthreshold depression.
The evidence-supported psychological intervention at the centre of collaborative care in the CASPER plus trial was behavioural activation. 71 The psychological intervention was adapted for use in an older age group at the developmental pilot phase of the CASPER and CASPER plus studies. 49 A reduction in social isolation is an important aspect of the intervention and much of the collaborative care for some participants was focused around this. Although face-to-face contact with the case manager may have provided initial social contact, it would only be in the short term. The case managers sought to reduce social isolation in the long term by ascertaining a participant’s needs and preferences regarding social contact. Putting them in touch with organisations, groups and individuals who could help them to increase their social network and opportunities for interaction afforded them long-term benefits.
Case managers worked in a patient-centred way with each participant. There was also a significant use of ‘functional equivalence’. If the participant had identified an activity that they had been forced to stop doing in the past, the way they had managed this could be used to illustrate the principle of functional equivalence.
We also found that a small but significant minority of participants did not engage with a psychologically based intervention. Nevertheless, it is notable that the uptake of collaborative care in the context of the CASPER plus trial was broadly in line with (or higher than) a range of primary care-based low-intensity interventions, such as those offered by IAPT services. 72 The results of the CASPER plus trial, therefore, add to an emerging evidence base that behavioural activation is effective for older adults. 73
The results of the economic evaluation provide robust evidence relating to cost-effectiveness of collaborative care for older people with depression. The CASPER plus trial provides estimates of the overall costs of the intervention, which will be useful for those who may plan services. Within a range of scenarios, collaborative care was found to provide QALY gains within a range of willingness-to-pay thresholds. There are relatively few cost-effectiveness analyses of collaborative care from the perspective of the UK health-care system. The randomised economic research worldwide generally shows that collaborative care is cost-effective. 23 The results of the CASPER plus trial add to emerging evidence of cost-effectiveness of collaborative care in the UK. The economic results of the CASPER trial are broadly in line with the only other UK cost-effectiveness analysis of collaborative care (reporting results of cost per QALY of £14,248 in working-age adults69) and also replicate findings from large-scale US studies of collaborative care in older people. 74
The most recent NICE guidance7 in relation to the management of depression was unable to recommend collaborative care in this population, and the CASPER plus trial represents a significant advance in the development of randomised knowledge in this area. This research knowledge will be helpful to those who formulate guidelines in the management of depression, including the next iteration of NICE guidelines in the care of depression and the care of psychological problems in the context of long-term physical ill health. 8
Limitations
The results of the CASPER plus trial need to be considered in the light of limitations that emerged during the study. First, regarding trial design, blinding was not feasible, which means there was potential for contamination at the GP level as well as at an individual level. In addition, many participants would be living geographically close to one another in the same catchment area and in a population of that age it is reasonable to assume that some participants would know each other and share their trial experiences. In either case, we expect that contamination would result in additional benefits to control arm participants, thereby reducing any group differences during follow-up and rendering our result a conservative estimate of the treatment effect. In addition, relating to study design, participants were recruited by means of postal screening of general practice lists, which included patients without a diagnosis of depression; therefore, participants who were identified with depression may not have necessarily presented in usual GP care. Therefore, the results of the CASPER plus trial may not automatically apply to older people who screen-positive for depression in the context of primary care attendance or physical health checks for older people.
Retention and differential attrition between the trial arms was a further limitation. Although follow-up rates were high overall (80% at 4 months), and exceeded the expected trial retention on which the trial was powered, there was a higher rate of attrition in the collaborative care arm compared with the usual-care arm (25% in the collaborative care arm and 14% in the usual-care arm). This was in part accounted for by a number of participants who disengaged from the collaborative care intervention and fully withdrew from the trial at the same time. It remains possible, however, that the patients who withdrew from the trial and did not provide outcome data may have presented a very different outcome profile to those who continued, which may have biased the treatment effect. Based on the very similar baseline characteristics between randomised patients and those available for the primary analysis, as well as our exploration of the impact of missingness, such bias appears less likely. In addition, results of statistical tests relating to the trial’s secondary outcomes should be interpreted as exploratory, as no adjustments for multiple testing were made for these analyses.
Another limitation relates to the trial recruitment method whereby participants were invited by their general practices. This resulted in a large number of patients aged ≥ 65 years being invited from each practice, although with relatively low consent rates (mean average 17%), which reduces generalisability. This will have produced a selective sample; however, given that everyone who was invited had equal opportunity to take part and participation was based on patient choice, it was a pragmatic method that would produce similar results if the intervention was rolled out in practice.
Finally, we did not formally assess cognitive impairment. Instead, we asked GPs to screen out any participants with known marked cognitive impairment. For randomised participants, if cognitive impairment was suspected, we informed the GP of this, but we also sought to engage the participant in the intervention for those who were allocated to collaborative care. We do not know the level of cognitive impairment in the current study and the extent to which its presence moderates treatment outcomes.
There were also some important limitations to note on performing the cost-effectiveness analysis. First, although data were collected on secondary care and social care use at each follow-up time point, the data were collected via self-report questionnaires, which were not deemed to be accurate enough data sets to conduct the cost-effective analysis. Therefore, only objective data, obtained from GP administrative systems, informed the cost analysis. Second, it was not possible to provide a reading of participant resource use at baseline, as the study design had approvals to collect health resource use data only from the randomisation date to the study completion date. The baseline was, therefore, outside the period in which participants had consented to provide information.
Chapter 9 Conclusions
There is currently little provision of psychosocial care for older adults with depression. Depression is relatively common among older people and is often associated with long-term health conditions. The CASPER plus trial represents the largest UK trial-based evaluation of a psychosocial intervention for this group. It was found to be effective across a range of depression, psychological and quality-of-life outcomes in the short term. Collaborative care resulted in accelerated improvements in clinical depression at 4 months’ follow-up. The effects were less apparent but still present at 12 months’ follow-up. The longer-term benefits at 18 months had disappeared when there was no discernible difference between those who received collaborative care and those who received usual care. The intervention was delivered over the telephone by low-intensity psychological therapists, such as those who work in NHS IAPT services. Qualitative research showed this to be an acceptable and valued treatment by the majority of people who were offered collaborative care. A concurrent economic evaluation found that the intervention resulted in gains in QALYs at a cost threshold that is acceptable to the UK health system.
Implications for health care
Collaborative care was acceptable for many of the older adults with depression and could readily be delivered over the telephone, following a first face-to-face meeting. However, although there is, at the policy level, a clearly identified aim to increase uptake of IAPT services in older adults,75 this has not as yet translated to changes in practice. For example, the most recent annual report on the use of IAPT services76 indicates that, of over 1,250,000 referrals to IAPT in April 2014 to March 2015, only 79,000 were adults aged ≥ 65 years (6.4%). The most recent ONS data (2016)77 indicate that 17.7% of the UK population is aged ≥ 65 years. As a result, it may be worth exploring other methods of delivering the intervention, such as through nurses who conduct comorbidity checks or healthy-living workers. The evaluation of the feasibility and acceptability of delivery by these other professional groups should be a research priority. This may include nurses but should also include any other professional or paraprofessional group that may allow the treatment to be delivered at scale. Certainly, health-care providers will need to ensure that IAPT services have sufficient capacity to enable the provision of collaborative care for older people with depression.
Collaborative care proved clinically effective at improving depression scores and reducing the incidence of case-level depression for older people with depression. The small to moderate effect size of 0.34 may represent limited change at the individual level but it has substantial impact at the population level. 50 Moreover, the robust cost-effectiveness estimates on using collaborative care to treat depression were cost-effective under conventional willingness-to-pay thresholds. This study has shown that collaborative care represents a feasible and effective means of treating depression in primary care. Depression is a relatively common condition, affecting about 5% of older adults. The CASPER plus trial evidence could be used by policy-makers and primary care to improve services and reduce the disease burden of our ageing population.
A final implication for health care relates to the higher drop-out rate from the collaborative care arm and what this would mean for take-up of the intervention in the real world. Some participants found the intervention intrusive and felt that talking and thinking about their symptoms made them feel uncomfortable. This may signal a potential problem if collaborative care were offered in NHS services. As with all psychological services, this type of intervention will not necessarily suit everyone and care should be taken to ascertain the likelihood of this being the case prior to any referral to such a service. Coupled with this is the finding that the greatest level of benefit in relation to costs was found for those who engaged with the intervention for more than five sessions.
Recommendations for research
Analysis of the CASPER plus trial results highlighted a number of future research priorities listed below in order of perceived importance.
-
First, a large proportion of the CASPER plus trial had at least one long-term physical health condition, and, although there were some improvements in function and quality of life among participants, there remains little evidence on the clinical effectiveness and cost-effectiveness of collaborative care at treating comorbidities. Evidence from a US trial78 that tested collaborative care for the treatment of comorbid depression and diabetes mellitus found that it helped improve depression care and outcomes but did not result in improved glycaemic control. Future trials of collaborative care are therefore required to investigate the clinical effectiveness and cost-effectiveness of collaborative care at improving physical and mental health outcomes on older adults with multimorbidities. Given the complexities associated with managing multiple conditions and the increasing number of older adults in our population as it ages, future research in this area is critical. There may also be value in examining the effect of collaborative care in the presence of cognitive impairment.
-
Second, many patients in the collaborative care arm discontinued treatment or dropped out of the trial. Further qualitative and quantitative work should explore reasons for this. This should also include maximising the acceptability and effectiveness of collaborative care for this population and identifying the most appropriate target population for the intervention.
-
Third, translating the research findings into clinical practice will be challenging and would benefit from further research. This relates both to enabling capacity to deliver the intervention to patients and to be able to target it at those most likely to complete the process and make use of the resource. Future research should also evaluate the feasibility and effectiveness of collaborative care when the case manager is not someone with specific training in mental health. This may include nurses working in primary care but should also include other professionals.
-
Fourth, collaborative care is a complex intervention and there is, as yet, little information on how different components relate to outcomes both in the short and longer term. Further work is needed to establish the relationship between treatment components and outcomes.
-
Finally, this was a brief intervention and benefit was truncated beyond 12 months. Future research should be conducted to establish how minimal interventions may be offered to ensure that the early gains from treatment are sustained. Trials of 12-month top-up sessions for collaborative care (delivered by telephone) are needed. This would allow the longer term impact of collaborative care and its impact on relapse rates to be investigated. Depression is a recurrent disorder and it would be useful to judge longer term impact on relapse and the prevention of future case-level depression.
Acknowledgements
We would especially like to thank all the participants who took part in this trial. Thanks also to the GPs and other professionals from all participating general practices who enabled patients to be recruited and data to be collected, ensuring the success of the trial. We are grateful to York Trials Unit for managing and storing the data securely and to the PC-MIS team for providing support in using their PC-MIS. We also wish to thank the Trial Steering Committee and Data Monitoring and Ethics Committee members for overseeing the study from inception to completion.
The CASPER trial also benefited from working with the Yorkshire and Humber NHS IAPT and the North East NHS IAPT services. In addition, it was supported by North and East Yorkshire and Northern Lincolnshire Primary Care Research Network; Yorkshire and Humber Clinical Research Network (CRN; previously known as Comprehensive Local Research Network); North East and North Cumbria CRN; Tees, Esk and Wear Valleys NHS Foundation Trust; and Northumberland, Tyne and Wear NHS Foundation Trust.
CollAborative care for Screen-Positive EldeRs with major depression trial team (past and present)
Chief investigator
Simon Gilbody.
Trial managers
Katharine Bosanquet and Helen Lewis.
Principal investigators
David Ekers, Simon Gilbody, John Holmes and Esther Cohen-Tovee.
Trial co-ordinators
Katharine Bosanquet, Emily Clare, Lesley Haley, Jahnese Hamilton, Shaista Meer and Gemma Traviss-Turner.
Statisticians
Rhian Gabe, Catherine Hewitt and Ada Keding.
Health economists
Steve Parrott and Dominic Trépel.
Qualitative researchers
Carolyn Chew-Graham and Karen Overend.
Trial administrators
Pauline Holloway, Sarah Mercer, Alice North and Denise Womersley.
Data management team
Matthew Bailey, Ben Cross, Tanya Pawson, Kevin Sherratt, Claire Stewart and Val Wadsworth.
Research team
Katie Atherton, Della Bailey, Jules Beresford-Dent, Jacqueline Birtwistle, Daniel Brett, Simon Carver, Catherine Donegan, Deborah Foster, Samantha Gascoyne, Rebecca Hargate, Gillian Johnson, Rachel Mann, Sarah Nutbrown, Karen Overend, Jodi Pervin, Norah Phipps, Katherine Richardson, Helen Riding and Rebecca Woodhouse.
Trial Management Group
Joy Adamson, Katie Atherton, Della Bailey, Jacqueline Birtwistle, Katharine Bosanquet, Emily Clare, Esther Cohen-Tovee, Jaime Delgadillo, David Ekers, Deborah Foster, Samantha Gascoyne, Simon Gilbody, Lesley Haley, Jahnese Hamilton, Catherine Hewitt, John Holmes, Ada Keding, Peter Knapp, Helen Lewis, Rachel Mann, Dean McMillan, Shaista Meer, Sarah Nutbrown, Karen Overend, Steve Parrott, Jodi Pervin, Gemma Traviss-Turner, Dominic Trépel and Rebecca Woodhouse.
Trial Steering Committee
Mr Mike Beckett, Director of York Mind, York (now Director of Development at the Retreat, York).
Dr David Geddes, Medical Director of NHS North Yorkshire and York; GP at Clifton Medical Practice, York (now National Head of Primary Care Commissioning, NHS Commissioning Board, Leeds).
Dr Alison Layton (chairperson), Co-director of North & East Yorkshire & North Lincolnshire Comprehensive Local Research Network; Harrogate and District NHS Foundation Trust lead for Research and Development, Harrogate District Hospital (now Clinical Director of CRN Yorkshire and Humber).
Dr Waquas Waheed, Academic Consultant Psychiatrist, Lancashire Care NHS Foundation Trust, Preston (now National Primary Care Research and Development Centre, University of Manchester, Manchester).
Plus the members of the CASPER plus Trial Management Group.
Data Monitoring and Ethics Committee
Dr David Kessler (chairperson) NIHR Clinical Lecturer, Primary Health Care, University of Bristol, Bristol.
Dr Judy Leibowitz, Primary Care Mental Health Development Co-ordinator, Camden Primary Care Trust, London (now Head of IAPT, Camden and Islington NHS Foundation Trust).
Professor Stephen Walters, Medical Statistics and Clinical Trials, Health Services Research, School of Health and Related Research (ScHARR), University of Sheffield.
Patient and public involvement in research
The CASPER trial owes great thanks to the users of mental health services and carers who were part of the advisory group, which was established at the end of the pilot phase; their insights and understanding helped improve the relevance and readability of the study documentation.
Contributions of authors
Katharine Bosanquet (Research Fellow, Health Sciences) acted as CASPER study manager since the end of recruitment phase, previously co-ordinated recruitment and the running of the core site, York, managed the collection of objective data from GP practices across all sites and drafted the report.
Joy Adamson (Senior Research Fellow, Health Sciences) contributed to the development of the grant application and trial protocol.
Katie Atherton (Clinical Studies Officer, Health Sciences) was as case manager and contributed to the day-to-day running of the trial.
Della Bailey (Research Fellow, Health Sciences) developed the manual and intervention, was a case manager who also trained and supervised case managers and contributed to writing the report.
Catherine Baxter (Clinical Studies Officer, Health Sciences) was a case manager and contributed to the day-to-day running of the trial.
Jules Beresford-Dent (Clinical Studies Officer, Health Sciences) contributed to the day-to-day running of the trial.
Jacqueline Birtwistle (Research Assistant, Health Sciences) contributed to the day-to-day running of the trial.
Carolyn Chew-Graham (Professor of General Practice Research) contributed to the development of the grant application and trial protocol and supervised the qualitative research and analysis.
Emily Clare (Clinical Studies Officer, CRN) co-ordinated recruitment and the running of the trial at the Newcastle upon Tyne site.
Jaime Delgadillo (Researcher and Cognitive Behavioural Psychotherapist, Leeds Community Healthcare NHS Trust) supervised case managers and gave clinical input and advice during the trial.
David Ekers (Senior Clinical Lecturer, Health Sciences) contributed to the development of the grant application and trial protocol, provided expertise and training in behavioural activation, gave clinical input and advice during the trial and was a local principal investigator.
Deborah Foster (Research Fellow, Health Sciences) developed the manual and intervention and was a case manager who also trained and supervised case managers.
Rhian Gabe (Senior Statistician, Health Sciences) provided statistical support during the study.
Samantha Gascoyne (Trial Support Officer, Health Sciences) contributed to the day-to-day running of the trial.
Lesley Haley (Clinical Studies Officer, CRN) co-ordinated recruitment and the running of the trial at the Durham site.
Jahnese Hamilton (Clinical Studies Officer, CRN) co-ordinated recruitment and the running of the trial at the Newcastle upon Tyne site.
Rebecca Hargate (Clinical Studies Officer, Health Sciences) contributed to the day-to-day running of the trial.
Catherine Hewitt (Senior Statistician, Health Sciences) contributed to the development of the grant application and trial protocol, provided statistical support throughout the study, supervised the conduct of the statistical analysis and undertook the second checking of the final analysis for the report.
John Holmes (Senior Clinical Lecturer, Health Sciences) contributed to the development of the grant application and trial protocol, provided expertise in design and evaluation of psychosocial interventions for older adults with comorbidity, gave clinical input and advice during the trial and was a local principal investigator.
Ada Keding (Statistician, Health Sciences) wrote the statistical analysis plan and performed the statistical analysis and contributed to writing the report.
Helen Lewis (Research Fellow, Health Sciences) acted as CASPER study manager until the end of recruitment phase.
Dean McMillan (Senior Clinical Lecturer, Health Sciences and Hull York Medical Schools) contributed to the development of the grant application and trial protocol. Gave clinical input and advice during the trial alongside a supervisory role of supervisors.
Shaista Meer (Research Officer, Health Sciences) co-ordinated recruitment and the running of the trial at the Leeds site.
Natasha Mitchell (Senior Research Fellow) contributed to the development of the grant application and trial protocol.
Sarah Nutbrown (Research Fellow) contributed to the day-to-day running of the trial and developed site-specific procedures.
Karen Overend (Trial Support Officer, Health Sciences) wrote the trial protocol, conducted the qualitative research and analysis, contributed to the day-to-day running of the trial and contributed to writing the report.
Steve Parrott (Reader Health Economics) contributed to the development of the grant application and trial protocol and supervised the conduct of the economic analysis.
Jodi Pervin (Trial Support Officer, Health Sciences) acted as a case manager and contributed to the day-to-day running of the trial.
David A Richards (Professor, Mental Health Services Research) contributed to the development of the grant application and trial protocol and provided content expertise in the design of low-intensity collaborative care.
Karen Spilsbury (Professor Nursing) contributed to the development of the grant application and trial protocol.
David Torgerson (Professor, Trial Methodology) provided advice on efficient and effective trial conduct and contributed to the development of the grant application and trial protocol.
Gemma Traviss-Turner (Senior Research Fellow, Health Sciences) co-ordinated recruitment and the running of the trial at the Leeds site, collected Leeds site objective data and contributed to writing the report.
Dominic Trépel (Health Economist, Health Sciences) conducted all the cost-effectiveness analysis and contributed to writing the report.
Rebecca Woodhouse (Trial Support Officer, Health Sciences) contributed to the day-to-day running of the trial and contributed to writing the report.
Simon Gilbody (Professor, Psychological Medicine and Health Services Research) contributed to the development of the grant application and trial protocol, gave clinical input and advice during the trial, was the Chief Investigator who oversaw the study and contributed to writing the report.
All authors approved and/or commented on the final manuscript.
Publications
Papers
Lewis H, Hems D, Bosanquet K, Overend K. Is enough being done to treat depression in the elderly? Aging Health 2013;9:243–5.
Pasterfield M, Bailey D, Hems DJ, McMillan D, Richards D, Gilbody SM. Adapting manualized Behavioural Activation treatment for older adults with depression. The Cognitive Behaviour Therapist 2014;7:e5.
Overend K, Lewis H, Bailey D, Bosanquet K, Chew-Graham C, Ekers D, et al. CASPER plus (CollAborative care in Screen-Positive EldeRs with major depressive disorder): study protocol for a randomised controlled trial. Trials 2014;15:451.
Bosanquet K, Mitchell N, Gabe R, Lewis H, McMillan D, Ekers D, et al. Diagnostic accuracy of the Whooley depression tool in older adults in UK primary care. J Affect Disord 2015;182:39–43.
Overend K, Bosanquet K, Bailey D, Foster D, Gascoyne S, Lewis H, et al. Revealing hidden depression in older people: a qualitative study within a randomised controlled trial. BMC Fam Pract 2015;16:142.
Bosanquet K, Bailey D, Gilbody SM, Harden M, Manea LE, Nutbrown SE, McMillan D. Diagnostic accuracy of the Whooley questions for the identification of depression: a diagnostic meta-analysis. BMJ Open 2015;5:e008913.
Donoghue HM, Traviss-Turner GD, House AO, Lewis H, Gilbody S. Life adversity in depressed and non-depressed older adults: a cross-sectional comparison of the brief LTE-Q questionnaire and life events and difficulties interview as part of the CASPER study. J Affect Disord 2016;193:31–8.
Lewis H, Keding A, Bosanquet K, Bailey D, Gilbody SM, Torgerson D. An randomized controlled trial of Post-it® notes did not increase postal response rates in older depressed participants. J Eval Clin Pract 2016;23:102–7.
Presentations
Overend K. Collaborative Care for Depression: What is the Magic Ingredient? Systematic Review and Qualitative Meta-Synthesis of Provider and Patient Perspectives. Presented at the Primary Care Mental Health Research Conference, Amsterdam, the Netherlands, 12 May 2016.
Bosanquet K. Geographic Variation in Consent Rates during CASPER plus Randomized Controlled Trial. Presented at the Primary Care Mental Health Research Conference, Durham, UK, 26 March 2015.
Overend K. The CASPER Plus Qualitative Study: Collaborative Care for Older People with Depression. Presented at the Primary Care Mental Health Research Conference, Durham, UK, 26 March 2015.
Chew-Graham C. Case Management for Older People with Depression – A Qualitative Study: ‘Reducing the Blind Spots’. Presented at the Society for Academic Primary Care Annual Scientific Meeting Conference, Edinburgh, UK, 20 June 2014.
Overend K, Bosanquet, K. Reducing ‘Blind Spots’ to Achieve Patient-Centred Care: Early Insights from a Qualitative Study. Presented at the Society for Academic Primary Care North Conference, Kendal, UK, 21–22 November 2013.
Posters
Traviss G, Holmes J, Lewis H, Mitchell N, McMillan D, Hems D, et al. CASPER – Collaborative cAre for Screen Positive EldeRs. Presented at British Psychological Society Annual Conference, Harrogate, UK, 2013.
Bosanquet K, Mitchell N, Lewis H, Bailey D, Gabe R, McMillan D, Gilbody S. Diagnostic Accuracy of Whooley Depression Tool in Older Adults Based in Primary Care in the UK. Presented at the UK Primary Care Mental Health Research Conference, Manchester, UK, 2013. Won best academic poster award.
Overend K, Lewis H, Bosanquet K, Mann R, Hems D, Bailey D, Chew-Graham C. The CASPER Plus Nested Qualitative Research Study. Presented at the UK Primary Care Mental Health Research Conference, Manchester, UK, 2013.
Overend K, Lewis H, Atherton K, Bosanquet K, Hall R, Hems D, et al. The CASPER Plus Trial. Presented at the UK Primary Care Mental Health Research Conference, Manchester, UK, 2013.
Overend K, Bosanquet K, Lewis H, Nutbrown S, Bailey D, Hems D, et al. Reducing ‘Blind Spots’ to Achieve Patient-Centred Care: Preliminary Results from a Qualitative Study. Presented at Primary Care Mental Health Research Conference, Exeter, UK, 2014.
Lewis H, Hems D, Bailey D, McMillan D, Overend K, Woodhouse R, et al. Self-Help for Those at Risk of Depression: The SHARD Trial, A Study Protocol. Presented at Primary Care Mental Health Research Conference, Exeter, UK, 2014.
Chew-Graham C. Case Management for Older People with Depression – A Qualitative Study: Offering a New Perspective to Patients. Presented at World Association of Social Psychiatry Conference, London, UK, 2014.
Bosanquet K. Objective Data: Measuring Cost-Effectiveness of Healthcare Interventions. Presented at NIHR CRN Mental Health National Scientific Meeting Conference, York, UK, 2015.
Overend K. Case Management for Older People with Depression: A Qualitative Study. Presented at NIHR CRN Mental Health National Scientific Meeting Conference, York, UK, 2015.
Overend K. The CASPER Plus Qualitative Study: Collaborative Care for Older People with Depression. Presented at Primary Care Mental Health Research Conference, Durham, UK, 2015.
Bosanquet K. Objective Data: Measuring Cost-Effectiveness of Healthcare Interventions. Presented at Primary Care Mental Health Research Conference, Durham, UK, 2015.
Bailey D. Collaborative Care is Valued by Older Adults with Depression: The CASPER Trial. Presented at Primary Care Mental Health Research Conference, Durham, UK, 2015.
Keding A, Lewis H, Bosanquet K, Gilbody S, Torgerson D. Post-It Notes to Improve Questionnaire Response Rates in RCTs Findings from a Randomised Sub-Study. Presented at the International Clinical Trials Methodology Conference, Glasgow, UK, 2015.
Traviss-Turner GD. Measuring Life Events in Health Research. Presented at Primary Care Mental Health Research Conference, Amsterdam, the Netherlands, 2016.
Overend K, Groom M, Kirby N, Teahan A, Delgadillo J. Using Outcome Feedback in Psychological Therapy: Qualitative Findings from a Feasibility Study. Presented at Primary Care Mental Health Research Conference, Amsterdam, the Netherlands, 2016.
Radio broadcast interviews
-
British Broadcasting Corporation (BBC1). Inside Out North East & Cumbria [interview with Gilbody S, Foster DJ and Bailey D]. London, 15 October 2012.
-
British Broadcasting Corporation (BBC Radio York). BBC Radio York [interview with Gilbody S]. York, 15 October 2012.
-
British Broadcasting Corporation (BBC1). Look North [interview with a GP whose practice participated in the CASPER study]. London, 15 October 2012.
Data sharing statement
Reasonable requests for patient-level data should be made to the corresponding author and will be considered by the CASPER plus trial management group. Consent for data sharing was not obtained but the presented data are anonymised and risk of identification is low.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Murray C, Lopez A. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Disease, Injuries and Risk Factors in 1990. Boston, MA: Harvard School of Public Health on behalf of the World Bank; 1996.
- Davies SC. Annual Report of the Chief Medical Officer 2013: Public Mental Health Priorities – Investing in the Evidence. London: Department of Health; 2014.
- Chew-Graham C, Baldwin R, Burns A. Treating depression in later life. BMJ 2004;329:181-2. http://dx.doi.org/10.1136/bmj.329.7459.181.
- Rapp SR, Parisi SA, Walsh DA. Psychological dysfunction and physical health among elderly medical inpatients. J Consult Clin Psychol 1988;56:851-5. https://doi.org/10.1037/0022-006X.56.6.851.
- Chachamovich E, Fleck M, Laidlaw K, Power M. Impact of major depression and subsyndromal symptoms on quality of life and attitudes toward aging in an international sample of older adults. Gerontologist 2008;48:593-602. https://doi.org/10.1093/geront/48.5.593.
- Walker Z, Leek CA, D’ath PJ, Katona CLE. Psychiatric morbidity in elderly attenders at an accident and emergency department. Int J Geriatr Psychiatry 1995;10:951-7. https://doi.org/10.1002/gps.930101107.
- Depression in Adults: The Treatment and Management of Depression in Adults (Update – NICE Clinical Guideline 90). Manchester: NICE; 2009.
- Depression in Adults with a Chronic Physical Health Problem. London: NICE; 2009.
- Chew-Graham CA, Lovell K, Roberts C, Baldwin R, Morley M, Burns A, et al. A randomised controlled trial to test the feasibility of a collaborative care model for the management of depression in older people. Br J Gen Pract 2007;57:364-70.
- Coventry P, Lovell K, Dickens C, Bower P, Chew-Graham C, McElvenny D, et al. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h638.
- Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4913.
- Chew-Graham C, Kovandžić M, Gask L, Burroughs H, Clarke P, Sanderson H, et al. Why may older people with depression not present to primary care? Messages from secondary analysis of qualitative data. Health Soc Care Community 2012;20:52-60. http://dx.doi.org/10.1111/j.1365-2524.2011.01015.x.
- Burroughs H, Lovell K, Morley M, Baldwin R, Burns A, Chew-Graham C. ‘Justifiable depression’: how primary care professionals and patients view late-life depression? A qualitative study. Fam Pract 2006;23:369-77. https://doi.org/10.1093/fampra/cmi115.
- Rodda J, Walker Z, Carter J. Depression in older adults. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5219.
- Prina AM, Marioni RE, Hammond GC, Jones PB, Brayne C, Dening T. Improving access to psychological therapies and older people: findings from the Eastern Region. Behav Res Ther 2014;56:75-81. http://dx.doi.org/10.1016/j.brat.2014.03.008.
- Unützer J, Katon W, Callahan CM, Williams JW, Hunkeler E, Harpole L, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288:2836-45. https://doi.org/10.1001/jama.288.22.2836.
- Gunn J, Diggens J, Hegarty K, Blashki G. A systematic review of complex system interventions designed to increase recovery from depression in primary care. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-88.
- Baldwin RC, Anderson D, Black S, Evans S, Jones R, Wilson K, et al. Royal College of Psychiatrists . Guideline for the management of late-life depression in primary care. Int J Geriatr Psychiatry 2003;18:829-38. http://dx.doi.org/10.1002/gps.940.
- Iliffe S, Haines A, Gallivan S, Booroff A, Goldenberg E, Morgan P. Assessment of elderly people in general practice. 1. Social circumstances and mental state. Br J Gen Pract 1991;41:9-12.
- Lovell K, Richards D. Multiple access points and levels of entry (MAPLE): ensuring choice, accessibility and equity for CBT services. Behav Cogn Psychother 2000;28:379-91.
- Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care. Making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry 2006;189:484-93. https://doi.org/10.1192/bjp.bp.106.023655.
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10. http://dx.doi.org/10.1002/14651858.CD006525.pub2.
- Gilbody S, Bower P, Whitty P. Costs and consequences of enhanced primary care for depression: systematic review of randomised economic evaluations. Br J Psychiatry 2006;189:297-308. https://doi.org/10.1192/bjp.bp.105.016006.
- Hudson JL, Bower P, Archer J, Coventry PA. Does collaborative care improve social functioning in adults with depression? The application of the WHO ICF framework and meta-analysis of outcomes. J Affect Disord 2016;189:379-91. http://dx.doi.org/10.1016/j.jad.2015.09.034.
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, et al. A component analysis of cognitive-behavioural treatment for depression. J Consult Clin Psychol 1996;64:295-304. https://doi.org/10.1037/0022-006X.64.2.295.
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol Med 2008;38:611-23. https://doi.org/10.1017/S0033291707001614.
- Ekers D, Webster L, Van Straten A, Cuijpers P, Richards D, Gilbody S. Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0100100.
- Richards DA, Lovell K, Gilbody S, Gask L, Torgerson D, Barkham M, et al. Collaborative care for depression in UK primary care: a randomized controlled trial. Psychol Med 2008;38:279-87. https://doi.org/10.1017/S0033291707001365.
- Lewis H, Adamson J, Atherton K, Bailey D, Birtwistle J, Bosanquet K, et al. CollAborative care and active surveillance for Screen-Positive EldeRs with subthreshold depression (CASPER): a multicentred randomised controlled trial of clinical effectiveness and cost-effectiveness. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21080.
- Gilbody S, Lewis H, Adamson J, Atherton K, Bailey D, Birtwistle J. Effect of collaborative care vs usual care on depressive symptoms in older adults with subthreshold depression: the CASPER randomized clinical trial. JAMA 2017;317:728-37. https://doi.org/10.1001/jama.2017.0130.
- Relton C, Torgerson D, O’Cathain A, Nicholl J. Rethinking pragmatic randomised controlled trials: introducing the ‘cohort multiple randomised controlled trial’ design. BMJ 2010;340. https://doi.org/10.1136/bmj.c1066.
- Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med 1997;12:439-45. https://doi.org/10.1046/j.1525-1497.1997.00076.x.
- Kronke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002;32:1-7. https://doi.org/10.3928/0048-5713-20020901-06.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. https://doi.org/10.1001/archinte.166.10.1092.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258-66. https://doi.org/10.1097/00006842-200203000-00008.
- Vaishnavi S, Connor K, Davidson JR. An abbreviated version of the Connor-Davidson Resilience Scale (CD-RISC), the CD-RISC2: psychometric properties and applications in psychopharmacological trials. Psychiatry Res 2007;152:293-7. https://doi.org/10.1016/j.psychres.2007.01.006.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33.
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Harnett Sheehan K, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry 1997;12:224-31. https://doi.org/10.1016/S0924-9338(97)83296-8.
- Sheehan DV, Lecrubier Y, Harnett Sheehan K, Janavs J, Weiller E, Keskiner A, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry 1997;12:232-41. https://doi.org/10.1016/S0924-9338(97)83297-X.
- Amorim P, Lecrubier Y, Weiller E, Hergueta T, Sheehan D. DSM-IH-R psychotic disorders: procedural validity of the Mini International Neuropsychiatric Interview (MINI). Concordance and causes for discordance with the CIDI. Eur Psychiatry 1998;13:26-34. https://doi.org/10.1016/S0924-9338(97)86748-X.
- Gruenberg A, Goldstein R, Pincus H, Licinio J, Wong M-L. . Biology of Depression: From Novel Insights to Therapeutic Strategies. Weinheim: Wiley-VCH; 2005.
- Adams KB, Moon H. Subthreshold depression: characteristics and risk factors among vulnerable elders. Aging Ment Health 2009;13:682-92. http://dx.doi.org/10.1080/13607860902774501.
- Zeiss AM, Lewinsohn PM, Rohde P, Kato P, Mann T. Handbook of Diversity Issues in Health Psychology. New York, NY: Plenum Press; 1996.
- Blazer DG. Depression in late life: review and commentary. Focus 2009;7:118-36. https://doi.org/10.1176/foc.7.1.foc118.
- Joiner TE, Conwell Y, Fitzpatrick KK, Witte TK, Schmidt NB, Berlim MT, et al. Four studies on how past and current suicidality relate even when ‘everything but the kitchen sink’ is covaried. J Abnorm Psychol 2005;114:291-303. https://doi.org/10.1037/0021-843X.114.2.291.
- Vinkers DJ, Gussekloo J, Stek ML, Westendorp RGJ, van der Mast RC. Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ 2004;329:881-5. https://doi.org/10.1136/bmj.38216.604664.DE.
- Pasterfield M, Bailey D, Hems D, McMillan D, Richards D, Gilbody S. Adapting manualized behavioural activation treatment for older adults with depression. The Cognitive Behaviour Therapist 2014;7. https://doi.org/10.1017/S1754470X14000038.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Lawrence Erlbaum Associates; 1988.
- National Research Ethics Service . Standard Operating Procedures for Research Ethics Committees in the United Kingdom 2009.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. http://dx.doi.org/10.1002/hec.901.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- O’Brien BJ, Briggs A. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68. https://doi.org/10.1191/0962280202sm304ra.
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2015.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Manning WG, Mullahy J. Estimating log models: to transform or not to transform?. J Health Econ 2001;20:461-94. https://doi.org/10.1016/S0167-6296(01)00086-8.
- Vuong Q. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica 1989;57:307-33. https://doi.org/10.2307/1912557.
- Gunn JM, Palmer VJ, Dowrick CF, Herrman HE, Griffiths FE, Kokanovic R, et al. Embedding effective depression care: using theory for primary care organisational and systems change. Implement Sci 2010;5. http://dx.doi.org/10.1186/1748-5908-5-62.
- Simpson AE, Richards D, Gask L, Hennessy S, Escott D. Patients’ experiences of receiving collaborative care for the treatment of depression in the UK: a qualitative investigation. Ment Health Fam Med 2008;5:95-104.
- Glaser BG. The constant comparative method of qualitative analysis. Social Problems 1965;12:436-45. https://doi.org/10.2307/798843.
- Overend K, Bosanquet K, Bailey D, Foster D, Gascoyne S, Lewis H, et al. Revealing hidden depression in older people: a qualitative study within a randomised controlled trial. BMC Fam Pract 2015;16. http://dx.doi.org/10.1186/s12875-015-0362-2.
- Knowels SE, Chew-Graham C, Coupe N, Adeyemi I, Keyworth C, Thampy H, et al. Better together? a naturalistic qualitative study of inter-professional working in collaborative care for co-morbid depression and physical health problems. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-110.
- Coventry PA, Hays R, Dickens C, Bundy C, Garrett C, Cherrington A, et al. Talking about depression: a qualitative study of barriers to managing depression in people with long term conditions in primary care. BMC Fam Pract 2011;12. http://dx.doi.org/10.1186/1471-2296-12-10.
- Lewis HJ, Hems DJ, Bosanquet KN, Overend KJ. Is enough being done to treat depression in the elderly?. Aging Health 2013;9:243-5. https://doi.org/10.2217/ahe.13.9.
- Green C, Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, et al. Cost-effectiveness of collaborative care for depression in UK primary care: economic evaluation of a randomised controlled trial (CADET). PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0104225.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314-21. https://doi.org/10.1001/archinte.166.21.2314.
- Kanter JW, Manos RC, Bowe WM, Baruch DE, Busch AM, Rusch LC. What is behavioural activation? A review of the empirical literature. Clin Psychol Rev 2010;30:608-20. https://doi.org/10.1016/j.cpr.2010.04.001.
- IAPT Three-Year Report: The First Million Patients. London: DH; 2012.
- Samad Z, Brealey S, Gilbody S. The effectiveness of behavioural therapy for the treatment of depression in older adults: a meta-analysis. Int J Geriatr Psychiatry 2011;26:1211-20. http://dx.doi.org/10.1002/gps.2680.
- Katon WJ, Schoenbaum M, Fan MY, Callahan CM, Williams J, Hunkeler E, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry 2005;62:1313-20. https://doi.org/10.1001/archpsyc.62.12.1313.
- How to Make IAPT More Accessible to Older People: A Compendium. London: DH; 2013.
- Psychological Therapies: Annual Report on the Use of IAPT Services England, 2014/15. London: NHS Digital; 2015.
- Humby P. Overview of the UK Population: February 2016. London: ONS; 2016.
- Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042-9. https://doi.org/10.1001/archpsyc.61.10.1042.
Appendix 1 Regulatory approvals
| Trust | Research and development approval granted |
|---|---|
| NHS East Riding of Yorkshire | 18 November 2010 |
| NHS Hull | 6 January 2011 |
| NHS North Yorkshire and York | 18 November 2010 |
| NHS Leeds | 29 September 2011 |
| NHS County Durham | 21 October 2011 |
| NHS Darlington | 21 October 2011 |
| NHS Middlesbrough | 21 October 2011 |
| NHS Stockton-on-Tees | 21 October 2011 |
| NHS Hartlepool | 21 October 2011 |
| NHS Redcar and Cleveland | 21 October 2011 |
| Northumberland Tyne and Wear NHS Foundation Trust | 15 February 2013 |
| NHS North of Tyne | 5 March 2013 |
Appendix 2 CollAborative care for Screen-Positive EldeRs plus participant invite letter
Appendix 3 CollAborative care for Screen-Positive EldeRs plus participant consent form
Appendix 4 CollAborative care for Screen-Positive EldeRs plus decline form
Appendix 5 CollAborative care for Screen-Positive EldeRs plus participant information sheet
Appendix 6 CollAborative care for Screen-Positive EldeRs plus background information sheet
Appendix 7 CollAborative care for Screen-Positive EldeRs plus baseline questionnaire
Appendix 8 Exploring risk in research interviews assessment form
Appendix 9 CollAborative care for Screen-Positive EldeRs plus 4-month follow-up questionnaire
Appendix 10 CollAborative care for Screen-Positive EldeRs plus 12-month follow-up questionnaire
Appendix 11 CollAborative care for Screen-Positive EldeRs plus 18-month follow-up questionnaire
Appendix 12 Zero-inflated negative binomial regression
| GP appointments | IRR | Standard error | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Full model | |||||
| Collaborative care | 0.9726 | 0.0733 | –0.3700 | 0.713 | 0.8391 to 1.1274 |
| Constant | 10.3623 | 0.5436 | 44.5700 | 0.000 | 9.3498 to 11.4845 |
| Logistic model | |||||
| Collaborative care | 0.5384 | 0.9378 | 0.5700 | 0.566 | –1.2996 to 2.3764 |
| Constant | –3.9940 | 0.7827 | –5.1000 | 0.000 | –5.5281 to –2.4599 |
| GP home visits | IRR | Standard error | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Collaborative care | 1.2358 | 0.5291 | 0.4900 | 0.621 | 0.5340 to 2.8599 |
| Constant | 0.6066 | 0.1356 | –2.2400 | 0.025 | 0.3914 to 0.9401 |
| Logistic model | |||||
| Collaborative care | 17.3135 | 15682.5500 | 0.0000 | 0.999 | –30720 to 30755 |
| Constant | –19.0563 | 15682.5500 | 0.0000 | 0.999 | –30756 to 30718 |
| GP telephone consultation | IRR | Standard error | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Collaborative care | 1.3146 | 0.2366 | 1.5200 | 0.129 | 0.9238 to 1.8707 |
| Constant | 2.1911 | 0.3857 | 4.4600 | 0.000 | 1.5517 to 3.0939 |
| Logistic model | |||||
| Collaborative care | 1.4852 | 2.2885 | 0.6500 | 0.516 | –3.0003 to 5.9706 |
| Constant | –2.8972 | 2.7483 | –1.0500 | 0.292 | –8.2838 to 2.4894 |
| Nurse appointment | IRR | Standard error | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Collaborative care | 0.9935 | 0.1244 | –0.0500 | 0.959 | 0.7774 to 1.2698 |
| Constant | 5.3825 | 0.4368 | 20.7400 | 0.000 | 4.5911 to 6.3104 |
| Logistic model | |||||
| Collaborative care | 14.1944 | 2213.3500 | 0.0100 | 0.995 | –4324 to 4352 |
| Constant | –18.0387 | 2213.3490 | –0.0100 | 0.993 | –4356 to 4320 |
| Nurse telephone consultation | IRR | Standard error | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Collaborative care | 2.2476 | 1.0137 | 1.8000 | 0.073 | 0.9285 to 5.4403 |
| Constant | 0.3607 | 0.0757 | –4.8600 | 0.000 | 0.2390 to 0.5441 |
| Logistic model | |||||
| Collaborative care | 16.5231 | 3127.0730 | 0.0100 | 0.996 | –6112 to 6145 |
| Constant | –16.7359 | 3127.0740 | –0.0100 | 0.996 | –6146 to 6112 |
Appendix 13 CollAborative care for Screen-Positive EldeRs plus participant interview consent form
Appendix 14 CollAborative care for Screen-Positive EldeRs plus case manager/supervisor interview consent form
Appendix 15 CollAborative care for Screen-Positive EldeRs plus general practitioner interview consent form
Appendix 16 Qualitative case manager topic guide
Appendix 17 Qualitative case manager topic guide
Appendix 18 Qualitative general practitioner topic guide
Appendix 19 Qualitative demographics tables
| Identification number | Sex | Age range (years) | Index of Multiple Deprivation number (decile) | Face to face or telephone? | Urban/rural general practice |
|---|---|---|---|---|---|
| PT1 | Female | 75–80 | 1 | Face to face | Urban |
| PT2 | Male | 75–80 | 9 | Face to face | Urban |
| PT3 | Male | 65–70 | 5 | Face to face | Rural |
| PT4 | Male | 81–85 | 8 | Face to face | Rural |
| PT5 | Male | 65–70 | 2 | Face to face | Urban |
| PT6 | Female | 65–70 | 10 | Face to face | Rural |
| PT7 | Female | 65–70 | 10 | Face to face | Rural |
| PT8 | Female | 65–70 | 10 | Face to face | Urban |
| PT9 | Male | 65–70 | 2 | Face to face | Urban |
| PT10 | Female | 65–70 | 8 | Telephone | Urban |
| PT11 | Female | 75–80 | 9 | Face to face | Urban |
| PT12 | Female | 65–70 | 9 | Telephone | Urban |
| PT1(withdrawn) | Male | 65–70 | 6 | Face to face | Rural |
All eight case managers who agreed to be interviewed were female and aged between 27 and 50 years. All case managers had been trained as NHS PWPs as part of the IAPT initiative. They each had several years experience of delivering low-intensity psychological interventions. In addition, two of the case managers were involved in training case managers for the CASPER plus trial and in their supervision.
| Identification number | Sex | Years of experiencea | Interview type |
|---|---|---|---|
| CASE MANAGER1 | Female | 8 | Face to face |
| CASE MANAGER2 | Female | 9 | Face to face |
| CASE MANAGER3 | Female | 4 | Face to face |
| CASE MANAGER4 | Female | 4 | Face to face |
| CASE MANAGER5 | Female | 4 | Telephone |
| CASE MANAGER6 | Female | 3 | Telephone |
| CASE MANAGER7 | Female | 3 | Telephone |
| CASE MANAGER8 | Female | 5 | Face to face |
| Identification number | Sex | Practice size | Index of Multiple Deprivation numbera | Urban/rural general practice |
|---|---|---|---|---|
| GP1 | Male | 14,886 | 5 | Urban |
| GP2 | Male | 10,150 | 6 | Urban |
| GP3 | Male | 19,879 | 10 | Rural |
| GP4 | Female | 18,083 | 8 | Rural |
| GP5 | Male | 24,353 | 5 | Urban |
| GP6 | Male | 15,915 | 4 | Urban |
| GP7 | Male | 6961 | 6 | Urban |
| GP8 | Female | 13,000 | 3 | Urban |
| GP9 | Female | 18,083 | 8 | Rural |
| GP10 | Female | 11,893 | 6 | Rural |
| GP11 | Male | 7183 | 10 | Rural |
| GP12 | Male | 15,432 | 5 | Rural |
Appendix 20 CollAborative care for Screen-Positive EldeRs plus protocol version 2.1 (original)
Appendix 21 CollAborative care for Screen-Positive EldeRs plus protocol version 2.6 (final version)
List of abbreviations
- CADET
- CollAborative DEpression Trial
- CASPER
- CollAborative care for Screen-Positive EldeRs with major depression
- CD-RISC2
- Connor–Davidson Resilience Scale-2 items
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CM
- case manager
- CRN
- Clinical Research Network
- EQ-5D-3L
- EuroQol-5 Dimensions, 3 levels
- GAD-7
- Generalised Anxiety Disorder-7 items
- GP
- general practitioner
- IAPT
- Improving Access to Psychological Therapies
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IMPACT
- Improving Mood-Promoting Access to Collaborative Treatment
- MCS
- mental component summary
- MINI
- Mini International Neuropsychiatric Interview
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- ONS
- Office for National Statistics
- PC-MIS
- Patient Case-Management Information System
- PCS
- physical component summary
- PHQ-9
- Patient Health Questionnaire-9 items
- PHQ-15
- Patient Health Questionnaire-15 items
- PT
- participant
- PTW
- withdrawn participant
- PWP
- psychological well-being practitioner
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SES
- socioeconomic status
- SF-12
- Short Form questionnaire-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SHARD
- Self Help At Risk Depression
