Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 15/64/07. The protocol was agreed in June 2016. The assessment report began editorial review in December 2016 and was accepted for publication in May 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Fahd Quhill has received personal fees from Allergan for participation in medical education and training, and has participated in advisory boards given his clinical expertise in the role of steroids in diabetic macular oedema. He has also received support from Allergan to attend international conferences.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Squires et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Uveitis is a heterogeneous group of ocular disorders involving inflammation of the uveal tract of the eye, which consists of the iris, the ciliary body and the choroid,1–5 or surrounding tissues (e.g. sclera, retina and optic nerve). 6
Criteria for the classification of uveitis according to anatomical site of inflammation were formally developed by the International Uveitis Study Group in 1987. 7 These were later revised in 2004 following the Standardization of Uveitis Nomenclature (SUN) Workshop. 8 The SUN criteria included onset, duration and course of uveitis in the classification of the condition. There are currently no agreed guidelines for describing uveitis-related systemic conditions. 9 A summary of uveitis classification according to the SUN criteria8 is presented in Table 1.
| Type of uveitis | Primary site of inflammation |
|---|---|
| Anterior uveitis | Anterior chamber |
| Intermediate uveitis | Vitreous |
| Posterior uveitis | Retina and choroid |
| Panuveitis | Anterior chamber, vitreous, retina or choroid |
| Criteria | Description |
| Onset | |
| Sudden | (No detail provided) |
| Insidious | (No detail provided) |
| Duration | |
| Limited | < 3 months’ duration |
| Persistent | > 3 months’ duration |
| Course | |
| Acute | Episode characterised by sudden onset and limited duration |
| Recurrent | Repeated episodes with intermittent periods of inactivity not requiring treatment for > 3 months |
| Chronic | Persistent episodes with relapse in < 3 months of treatment discontinuation |
Anterior uveitis is inflammation of the anterior chamber (AC) involving the iris and the anterior aspect of the ciliary body; this is outside the scope of this assessment. Intermediate uveitis affects the posterior part of the ciliary body and the vitreous humour. Posterior uveitis affects the back of the eye, including the retina or the choroid. Intermediate and posterior uveitis may be referred to collectively as posterior segment-involving uveitis. Panuveitis is inflammation of the whole of the uveal tract (front and back of the eye), extending from the AC to the choroid or retina. 3 A diagram of the eye and the parts affected in anterior, intermediate and posterior uveitis is shown in Figure 1.
FIGURE 1.
Types of uveitis based on parts of the eye affected. Reproduced with permission from Phil Hibbert, Uveitis Information Group (Scotland). 12

Intermediate uveitis, posterior uveitis and panuveitis account for around 10% of uveitis cases in the UK10 but are more severe and more likely to cause vision loss. 11
Aetiology, pathology and prognosis
Uveitis may have an infectious or a non-infectious cause; this appraisal is restricted to non-infectious uveitis. Non-infectious uveitis may occur as an ocular manifestation of a systemic autoimmune condition such as Behçet’s disease, sarcoidosis, multiple sclerosis or Vogt–Koyanagi–Harada disease (VKH). 13,14 A study from the Netherlands including almost 400 patients with posterior uveitis, intermediate uveitis or panuveitis reported that around half of all cases were likely to be related to systemic disease. 15 In the remaining cases, no systemic association could be found; these cases are known as idiopathic uveitis, although it is presumed that the disease is still likely to be autoimmune in nature. 14 Specific forms of uveitis include birdshot chorioretinopathy (also referred to as birdshot uveitis).
One or both eyes may be affected in uveitis. Estimates of the proportion of bilateral cases from studies of uveitis patients in tertiary centres in the UK and Europe range from 41% to 67%. 11,16–18 Each of these centres included patients with both anterior and posterior segment-involving uveitis. Three of the authors (AD, IP and FQ) provided clinical advice throughout, hereafter referred to as clinical advisors to the Assessment Group (AG). They suggested that the proportion of bilateral cases is higher for posterior segment-involving uveitis patients only, with the proportion of bilateral cases in this group estimated to be 70–80%. Many patients have asymmetric disease, with some inflammation in both eyes but more severe disease in one eye (these patients may or may not be included in the above estimates for bilateral uveitis).
Symptoms of uveitis depend on the parts of the eye affected. The main symptoms of the forms of uveitis considered in this study include blurred vision and floaters in the eye. However, pain and redness in the eye, sensitivity to light, loss of peripheral vision and headaches may also be reported. 13 In general, clinical manifestations of uveitis of different aetiologies may be similar but treatment strategies are predominantly determined by the underlying pathophysiology3 and may often require a multidisciplinary approach.
The consequences of uveitis that may lead to loss of vision include early complications, such as cystoid macular oedema (swelling of the retina) and vitreous haze (VH) (inflammatory cell debris in the vitreous), and late complications, such as cataracts (cloudiness of the lens), glaucoma (optic nerve damage associated with increased pressure inside the eye) and irreversible damage to the retina. 14 Many patients with posterior segment-involving uveitis require cataract surgery at a relatively early age; however, as cataract surgery is relatively efficacious and safe, clinicians may be less concerned about cataract formation than other complications of uveitis (clinical advisors to the AG, personal communication).
Dick et al. 19 conducted a retrospective analysis of insurance claim data from 1998 to 2012 for patients with a diagnosis of non-infectious intermediate uveitis, posterior uveitis or panuveitis in the USA. In total, 1769 patients with uveitis were followed up for a mean period of 5.6 years. The reported 5-year risks for patients with non-infectious intermediate uveitis, posterior uveitis or panuveitis were as follows: glaucoma 20%, cataracts 35%, visual disturbance 29%, blindness or low vision 4.5%, retinal detachment 11% and retinal disorder 28%. The supplemental material included a Kaplan–Meier curve of time to blindness, which showed a 10-year risk of blindness or low vision of 6.6%.
Tomkins-Netzer et al. 18 conducted a cross-sectional study of all patients (n = 1076) who attended the uveitis clinic of a single consultant at Moorfields Eye Hospital in London. The mean follow-up duration was 7.97 years and vision loss [best corrected visual acuity (BCVA) ≤ 20/50] was reported in 19.2% of eyes. Macular scarring (4%), retinal detachment (1.33%) and chronic macular oedema (1.16%) were the most common causes of irreversible severe vision loss (BCVA ≤ 20/200). Twenty patients had bilateral severe vision loss and were registered as legally blind.
Another retrospective review of records of 315 patients with uveitis in the UK from January 1998 to December 2000 described visual impairment (BCVA ≤ 6/18 in at least one eye) in 220 out of 315 uveitis patients (70%) overall and in 149 of 192 patients with intermediate uveitis, posterior uveitis or panuveitis (78%) after a mean follow-up duration of 36.7 months. 11 Severe visual impairment (BCVA ≤ 6/60) occurred in 38% (n = 120/315) of patients. Permanent visual impairment was present in 17% (n = 54/315) of patients, with 15% (n = 46/315) of patients experiencing bilateral impairment. The World Health Organization (WHO)’s criteria for blindness (BCVA in better eye of < 3/60 or a visual field of ≤ 10°)20 were met in 36 out of 315 patients (11.4%). Cystoid macular oedema, cataract and the coexistence of both conditions were the predominant causes of visual loss in 26.8% (n = 59/220), 17.7% (n = 39/220) and 20% (n = 44/220) of uveitic patients respectively. Reported predictors of poor visual outcome were older age (p = 0.02 via logistic regression), bilateral inflammation (p = 0.0005 via t-test), panuveitis (p = 0.0005 via logistic regression) and increasing duration of reduced vision (p = 0.0005 via t-test). 11 Overall, around 10% of cases of blindness in the developed world are caused by uveitis. 18,21
Epidemiology and prevalence
Uveitis affects people of any age but generally presents in people of working age, aged 20–50 years. 3,14 The mean age at presentation for patients with all types of uveitis attending tertiary centres has been reported to range from 35 to 48 years across studies in the UK,11,18 the Netherlands15 and France. 16
There is extensive variation in the causes of uveitis worldwide, genetic factors and environmental features contributing significantly to its pathology. 14 Whereas infectious uveitis is frequently seen in developing countries, idiopathic non-infectious uveitis is more common in most of the developed world, including England. 3
Earlier epidemiological studies in Europe and the USA have estimated annual incidence rates of uveitis ranging from 14 to 22.5 per 100,000 people and prevalence rates of between 38 and 380 per 100,000 people. 11 Wide variations in epidemiological statistics have been explained by differences in the classification of uveitis, aetiological causes and demographic risk factors. 14 There are limited data on the prevalence of non-infectious posterior segment-involving uveitis in England. The Scottish Uveitis Network reported prevalence rates for patients with uveitis treated with immunosuppression (systemic corticosteroids, second-line immunosuppressants or a combined treatment of the two agents) collected prospectively over a 4-month period between August and November 2005; estimates ranged from 2 to 59 per 100,000 people. 22 A claims-based analysis conducted in the USA based on 2012 data from the OptumHealth Reporting and Insights claims database reported an overall prevalence of adult non-infectious uveitis (n = 4827 cases; 2086 men and 2741 women) of 121 cases per 100,000 people [95% confidence interval (CI) 117.5 to 124.3 cases per 100,000 people]. 23 The observed prevalence rates of non-infectious intermediate uveitis, posterior uveitis and panuveitis in adults were 1 case (95% CI 0.8 to 1.5 cases), 10 cases (95% CI 9.4 to 11.5 cases) and 12 cases (95% CI 10.6 to 12.7 cases) per 100,000 people respectively. 23 Earlier studies have generally provided no or limited data for patients with non-infectious uveitis24,25 or have had issues (e.g. missing data, use of administrative data, variations in referral patterns), making estimates less generalisable. 22,26 Between 3 and 16 out of 100,000 people are estimated to have non-infectious posterior segment-involving uveitis (see Chapter 5).
Impact of the health problem
Uveitis is the fifth leading cause of visual impairment in developed countries and accounts for 10% of cases of legal blindness. 23,27 Patients may experience sudden and temporary or progressive and permanent visual impairment. 11
With regard to anatomical classification of uveitis, patients with posterior segment-involving uveitis and panuveitis tend to suffer more severe visual impairment than those with anterior uveitis. 27 Compared with uveitis affecting only the posterior segment, patients with panuveitis (both posterior and anterior) tend to have a poorer prognosis. 11 Additionally, the underlying cause of uveitis may also significantly influence the prognosis of intraocular inflammation. 11 For example, patients with uveitis as a result of Behçet’s disease have poorer visual outcomes than patients with non-infectious uveitis without an associated systemic condition, even when intense treatment is initiated at early stages of the disease. 11 Complications of uveitis, namely cystoid macular oedema, cataract, glaucoma or a combination of any of these, significantly influence the visual morbidity.
A post hoc analysis of health-related quality of life (HRQoL) in patients with non-infectious intermediate or posterior uveitis participating in the HURON trial compared with that in a matched set of the general US population found that the uveitis group had lower mean scores on the following subscales of the National Eye Institute (NEI) 25-item Visual Function Questionnaire (VFQ-25):28 role emotional (p < 0.001), mental health (p < 0.001), role physical (p < 0.001), vitality (p < 0.001), general health (p = 0.01) and mental component summary (p < 0.001). 29 No statistically significant differences between the groups were found for the physical component summary, physical functioning, bodily pain and social functioning subscales of the VFQ-25 or for EuroQol-5 Dimensions (EQ-5D)30 scores.
Loss of visual function can lead to an inability to work and drive. It can also affect the ability to take part in leisure activities. In addition, the currently available treatments, including corticosteroids and immunosuppressants, are associated with substantial adverse events (AEs). The most common AEs associated with long-term use of corticosteroids include osteoporosis and fractures, gastric conditions, psychiatric conditions, skin conditions, hyperglycaemia, weight gain, ocular conditions (including cataract) and cerebrovascular disease. 31 The most common AEs associated with immunosuppressants include cataracts, ocular hypertension, headache, fever, nausea, diarrhoea, fatigue, paraesthesia, tremors and systemic infection. 32,33 These can lead to substantial reductions in HRQoL for the patient and may also have an impact on the patient’s family.
Significance for the NHS
Patients with uveitis often require referral to secondary care to confirm the diagnosis and for the provision of treatment. Patients require regular monitoring. There are substantial costs to the NHS and Personal Social Services (PSS) associated with treatment of the complications of uveitis and blindness, as well as treatment for the AEs associated with current practice. As the cause and presentation of uveitis varies between individuals, it is important for clinicians to have a range of treatment options available. In practice, a range of unlicensed immunosuppressants and corticosteroids are used to treat patients with uveitis. Clinical advisors to the AG suggest that dexamethasone (DEX) implants and adalimumab (ADA) are both used variably in current practice depending on funding availability. The number of patients who would be eligible for these treatments annually is uncertain, but Allergan and AbbVie, the manufacturers of DEX and ADA, respectively, estimate that it would be 589 and 175 patients respectively (see Chapter 5).
Measurement of disease
Outcome measures in uveitis may be grouped according to the different aspects that they measure: (1) disease activity or inflammation in the eye (e.g. VH, which is the degree of cloudiness in the vitreous humour, and acute cystoid macular oedema); (2) disease-associated tissue damage or complications (e.g. cataract, glaucoma, chronic cystoid macular oedema); (3) visual loss (e.g. visual acuity, visual field loss); and (4) patient-reported visual function (e.g. via the VFQ-25). 34
There are some issues worth highlighting about outcome measurements in patients with uveitis. Vision loss has a complex interaction with visual acuity [which is a measure of central vision according to a validated measure such as the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, Snellen chart or another similar tool35], visual field contrast sensitivity and colour vision. Visual acuity in patients with uveitis may reflect both the degree of intraocular inflammation and the extent of damage in the eye; whereas inflammation may vary over short time periods (days or weeks), damage may accrue slowly (months or years) and, with the important exception of cataract and acute cystoid macular oedema, is usually irreversible. It will be immediately evident that, whereas short-term effects on vision (related to inflammation) may be captured within a clinical trial, the commonly used time frames in studies are too short to capture important long-term consequences for vision of damage to the eye caused by inadequately controlled uveitis. This may lead to systematic underestimates of the effects of interventions for treating uveitis in clinical trials.
Markers of structural damage to the eye, such as macular oedema (swelling of the retina), cataract and glaucoma, are important outcomes because they are the mechanisms by which uveitis patients lose vision and they are objective measures. However, they may not be good markers of whether or not a treatment reduces inflammation because they indicate structural damage to the eye, which might not resolve when the inflammation is treated.
In clinical practice, a combination of several outcomes is used to assess the response of uveitic activity to treatment. Generally, outcomes related to uveitis are assessed by clinical examination (visual acuity, slit-lamp examination of AC cells, VH grading) and by imaging (e.g. optical coherence tomography).
The NEI system for VH grading and AC cell grading proposed by the SUN Working Group8 is the ‘current gold standard’ for assessing intraocular inflammation (i.e. AC cell grade and VH grade). 36 The SUN system was a formalisation and adoption of the Nussenblatt scale. 8,37 Grading requires the examination of a patient’s eye by an indirect ophthalmoscope followed by a comparison of the appearance with a series of photographs of varying grades of fundus VH. 37 Although the grading system is accepted by the US Food and Drug Administration and has been used in a number of recent studies of uveitis,36 it is a subjective grading of cloudiness in the vitreous humour caused by inflammatory cells and cell debris on a non-continuous scale (0, 0.5+, 1, 2, 3 and 4+). 7,8,34,37 Its poor discriminatory property for detecting changes in the lower VH grades and extensive inter-rater variations have been reported to be limitations of this system. 29,36,38
Inflammation in the AC is assessed on the basis of the number of cells per one field on standard slit-lamp examination or by high-speed optical coherence tomography. 8
Complications of structural changes in the eye as a result of uveitis are typically reported according to the type of complication. For example, the SUN Working Group suggests that macular oedema could be determined by clinical examination and additional tests, for example optical coherence tomography or fluorescein angiography. 8 A patient is considered to have an increased or elevated intraocular pressure (IOP) if the pressure rises above a specified limit or increases from a baseline value in a study in which patients are followed over time (i.e. longitudinal data). 8 Although no consensus has been reached on the threshold for considering elevated IOP, an increase of ≥ 10 mmHg is considered to be important. 8 However, the SUN Working Group recommends the reporting of IOPs above the following thresholds: 21 mmHg (above the accepted upper limit of normal), 24 mmHg (associated with a significant risk of glaucoma) and 30 mmHg (when treatment for raised IOP is often started). 8
Other outcomes reported in studies of patients with uveitis include generic utility measures such as the EQ-5D and vision-specific measures such as the VFQ-25. 39 These outcome measures capture broader considerations and hence may overcome some of the issues associated with the alternative outcome measures. The EQ-5D also allows treatments to be compared with treatments for other diseases and patient populations, although it may not be as sensitive as the VFQ-25. 40
Current service provision
Non-infectious intermediate uveitis, posterior uveitis and panuveitis are initially treated with corticosteroids. Corticosteroids may be administered systemically (oral or parenteral) or locally via periocular or intravitreal injections or intravitreal implants. Additionally, if the front of the eye is also affected, topical corticosteroids and dilating eye drops may be offered. Systemic corticosteroids carry significant morbidity (e.g. cataract, glaucoma, diabetes, osteoporosis, weight gain, raised blood pressure) and long-term use above 7.5 mg per day is not recommended. 41,42
In terms of second-line treatment, people with severe or chronic non-infectious uveitis whose disease has not adequately responded to corticosteroid treatment, for whom corticosteroids are not appropriate or whose uveitis recurs after tapering the corticosteroid dose may be given immunosuppressive drugs (such as methotrexate, mycophenolate mofetil, ciclosporin, tacrolimus and azathioprine). Immunosuppressive drugs can allow a reduction in the corticosteroid dose and associated complications. If the disease does not respond to these treatments or if they are not tolerated, especially in patients at high risk of losing their vision or those with systemic disease related to uveitis, biological tumour necrosis factor (TNF)-alpha inhibitors may be used. The majority of these treatments are not currently licensed.
There are currently no national guidelines on treating non-infectious uveitis; however, all three clinical advisors to the AG, who practise within different regions of the UK (Birmingham, Liverpool and Sheffield), were in agreement that the above description represents the general treatment pathway. This description is also consistent with three local treatment pathways, two referenced in the DEX submission from Allergan43 (North East Retinal Group4 and NHS Southern Derbyshire Clinical Commissioning Group5) and one obtained by personal communication from Alastair Denniston (West Midlands Regional Uveitis Service, August 2016). The general treatment pathway does not differ according to whether a patient has intermediate uveitis, posterior uveitis or panuveitis. However, specific treatment is individualised based on a broad range of factors. In particular, treatment depends on whether or not systemic disease is known to be present, whether or not any systemic disease is controlled (i.e. whether or not current inflammation is restricted to the eye) and whether the disease affects one or both eyes. Figure 2 shows the general treatment pathway developed based on the three local treatment pathways and input from the clinical advisors to the AG.
FIGURE 2.
General treatment pathway in patients with non-infectious uveitis.

For the purposes of this report, the following terminology is used:
-
Systemic disease. Known underlying systemic disease related to the uveitis.
-
Active systemic disease. Systemic disease that is currently requiring symptomatic treatment (in these patients, systemic treatment may be more appropriate to treat both the uveitis and the underlying disease).
-
No active systemic disease. Either no systemic disease related to uveitis or systemic disease that is currently controlled (in these patients, treatment local to the eye may be more appropriate).
-
Local treatment/local pathway. Treatments that are local to the eye (may be given to one or both eyes; little effect on systemic disease).
-
Systemic treatment/systemic pathway. Treatments that are given systemically (and by their nature treat both eyes and may also treat systemic disease).
-
Unilateral. Uveitis affecting one eye. This does not relate to treatment for one eye.
-
Bilateral. Uveitis affecting both eyes. This does not relate to treatment for both eyes. In the case of local treatment, it may be for one or both eyes and will be referred to as such.
-
Legal blindness. BCVA of ≤ 20/200 in the better-seeing eye and/or a visual field of ≤ 20°.
Description of the technologies under assessment
Adalimumab (Humira®; AbbVie Ltd, Maidenhead, UK) is a monoclonal antibody that inhibits the proinflammatory cytokine, TNF-alpha. ADA has a marketing authorisation from the European Medicines Agency (EMA) for the treatment of non-infectious intermediate uveitis, posterior uveitis and panuveitis in adult patients who have had an inadequate response to corticosteroids, in patients in need of corticosteroid sparing or in patients for whom corticosteroid treatment is inappropriate. 44 ADA is administered as a subcutaneous injection containing a 40-mg preparation of the active drug.
Dexamethasone intravitreal implant (Ozurdex®; Allergan Ltd, Marlow, UK) is a corticosteroid that suppresses inflammation by inhibiting the expression of proinflammatory mediators. The implant has a marketing authorisation from the EMA for treating adults with inflammation of the posterior segment of the eye presenting as non-infectious uveitis (i.e. intermediate uveitis, posterior uveitis and panuveitis). DEX intravitreal implant is a biodegradable ophthalmic implant that contains 0.7 mg of the active drug. Each implant is intravitreally administered using a single-use solid polymer drug delivery system or applicator. 45 The Summary of Product Characteristics (SmPC) for DEX notes that administration to both eyes concurrently is not recommended because of a lack of data. 45
Place of the interventions in the treatment pathway
Clinical advisors to the AG and three local treatment pathways from the North East Retinal Group4 and the NHS Southern Derbyshire Clinical Commissioning Group5 (as referenced in the DEX submission43) and the West Midlands Regional Uveitis Service (Alastair Denniston, personal communication) were consulted to determine the place of the interventions in the treatment pathway. A general view was that DEX and ADA would generally not be used for the same patients or at the same point in the pathway. Treatments local to the eye (including the DEX implant) are considered to be appropriate for unilateral uveitis or asymmetric bilateral uveitis (when disease is more severe in one eye) when systemic disease is not present or is well controlled. Systemic treatments (including ADA) are considered to be appropriate to treat patients with bilateral uveitis (i.e. affecting both eyes) and/or active systemic disease. According to clinical advice provided to the AG, systemic treatments would generally not be given to a patient with unilateral uveitis and no active systemic disease because of the adverse effects associated with them. Patients with bilateral uveitis but no active systemic disease could be treated using either a local or a systemic approach. Although the inclusion criteria for the clinical trials of these drugs46–48 were not limited by these factors, our clinical experts suggest that clinicians may have selected patients for the trials accordingly.
In addition, the licensing of ADA and DEX differ in that, to be eligible for ADA, patients must have had an inadequate response to corticosteroids or require steroid-sparing treatment or corticosteroid treatment must be inappropriate, whereas DEX implants can used as first-line treatment. Clinical advisors to the AG suggest that in practice it is likely that DEX would be used as second-line treatment following local or systemic treatment with corticosteroids, whereas ADA would be used as a third-line option for patients with insufficient control with, or intolerance to, systemic corticosteroids and immunosuppressants; however, for some patients this may be as a result of current funding availability rather than clinical need. Figure 2 shows the general treatment pathway, indicating the most likely place of DEX and ADA (based on the opinion of the clinical advisors to the AG).
Although for most patients there is a clear clinical rationale for providing DEX and ADA at different points in the treatment pathway, the licensing allows both treatments to be given at overlapping points in the pathway (i.e. for patients with an inadequate response to corticosteroids, in need of corticosteroid sparing or in whom corticosteroid treatment is inappropriate),44 although the DEX implant is also licensed in a less restricted group. 45 This overlap is reflected somewhat by their use in clinical trials (see Chapter 3). Table 2 presents the situations in which ADA and DEX may be used according to both licensing and clinical appropriateness. The most likely places in the pathway where these treatments would be used according to clinicians are shown in bold.
| Line of therapy (see Figure 2) | Unilateral (or temporary flare in one eye), no active systemic disease, local treatment appropriate | Bilateral, no active systemic disease, systemic or local treatment appropriate | Unilateral (or temporary flare in one eye), active systemic disease, systemic or local treatment appropriate | Bilateral, active systemic disease, systemic treatment appropriate |
|---|---|---|---|---|
| First line | DEX or ADA licensed if corticosteroid treatment is inappropriate | ADA licensed if corticosteroid treatment is inappropriate | ||
| Second line (after systemic corticosteroids) | DEX or ADAa | DEX or ADAa | DEX or ADAa | ADA |
| Third line (after systemic corticosteroids and immunosuppressants) | DEX or ADAa | DEX or ADA | DEX or ADA | ADA |
In addition to the issues described above, because uveitis covers a heterogeneous group of diseases, clinical advice suggests that maintaining a range of options is important depending on a patient’s requirements.
Identification of important subgroups
The following have been identified as important subgroups that might affect the treatment offered:
-
unilateral or bilateral uveitis
-
the presence or absence of underlying autoimmune or inflammatory disease
-
whether any underlying systemic disease is active or controlled
-
existing treatment with long-term systemic immunosuppressants
-
baseline visual acuity
-
patients for whom systemic or local corticosteroid treatments are not appropriate.
Current usage in the NHS
Dexamethasone implants and subcutaneous ADA injections are both used variably in current practice, which may partly depend on funding availability and/or clinician and patient preference.
Anticipated costs associated with the interventions
Table 3 shows the 6-monthly costs of DEX and ADA. One DEX implant is expected to last around 6 months, based on observational trial data18,50,51 and clinical advice. It should be noted that patients could receive more than one implant, either in succession or in the other eye, with staggered implementation; however, these options have not been assessed within a randomised controlled trial (RCT). ADA is administered every 2 weeks until treatment failure. In the VISUAL I trial of ADA in active patients, 50% of patients had failed on treatment by 6 months and 66% had failed by 1 year. 52 Clinical advisors to the AG suggest that some patients may remain on ADA treatment for many years.
| Drug | Licensed dose | Company | Cost (£) | Six-monthly cost (£) |
|---|---|---|---|---|
| ADA | 40 mg once every 2 weeks | AbbVie | 352.14 | 4578 |
| DEX | One 0.70-mg implant | Allergan | 870 | 870 |
Chapter 2 Definition of the decision problem
This study assessed the clinical effectiveness and cost-effectiveness of ADA (via subcutaneous injections) and a DEX intravitreal implant for treating inflammation of the posterior segment of the eye presenting as non-infectious uveitis. ADA is licensed for the treatment of non-infectious intermediate uveitis, posterior uveitis and panuveitis in adult patients who have had an inadequate response to corticosteroids or who are in need of corticosteroid-sparing therapy or for whom corticosteroid treatment is inappropriate, whereas DEX intravitreal implant is licensed for the treatment of adults with inflammation of the posterior segment of the eye presenting as non-infectious uveitis.
Decision problem
The decision problem was specified as in the following sections.
Population
-
Adults (aged ≥ 18 years) with non-infectious intermediate uveitis, posterior uveitis or panuveitis.
Interventions
-
Adalimumab (via subcutaneous injections).
-
Dexamethasone intravitreal implant.
Relevant comparators
Relevant comparators included:
-
periocular or intravitreal corticosteroid injections
-
intravitreal corticosteroid implants
-
systemic corticosteroids
-
systemic immunosuppressive therapies including azathioprine, methotrexate, cyclophosphamide, ciclosporin, chlorambucil, tacrolimus, mycophenolate mofetil and TNF-alpha inhibitors
-
intravitreal methotrexate
-
best supportive care (when all other treatment options have been tried)
-
placebo or a sham procedure.
Combinations of the above treatments were also considered as relevant comparators.
Outcomes
The following outcomes were considered relevant for this assessment:
-
visual acuity (the affected eye)
-
visual acuity (both eyes)
-
measured as the mean difference (MD) in BCVA according to a validated measure such as the ETDRS chart, Snellen chart or a similar tool
-
other measures of visual acuity would be considered if outcomes could be justified and validated in relation to accepted relevant standard measures
-
-
improvement in disease activity (e.g. VH grade, AC cell grade)
-
uveitis-related tissue damage or complications (e.g. cataract, macular oedema, retinal vascular occlusion)
-
reduction in systemic steroid use
-
mortality
-
adverse effects of treatment
-
HRQoL, including generic measures such as the EQ-5D and functional measures such as the VFQ-25
-
composite end points incorporating more than one of the above.
Overall aims and objectives of the study
The aims of the study were to:
-
evaluate the clinical effectiveness and safety of ADA subcutaneous injection and DEX intravitreal implant within their marketing authorisations for treating non-infectious intermediate uveitis, posterior uveitis or panuveitis in adults
-
estimate the incremental cost-effectiveness of ADA subcutaneous injection and DEX intravitreal implant within their marketing authorisations for treating non-infectious intermediate uveitis, posterior uveitis or panuveitis compared with each other and with current treatment
-
estimate the expected overall costs of ADA and DEX in England
-
identify key areas for primary research.
Chapter 3 Assessment of clinical effectiveness
A systematic review was undertaken to assess the clinical effectiveness and safety of ADA subcutaneous injection and DEX intravitreal implant within their marketing authorisations in adults with non-infectious intermediate uveitis, posterior uveitis or panuveitis. The review of the evidence of clinical effectiveness was carried out in accordance with the principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 53 First, the methods used in the systematic review of the clinical effectiveness evidence are presented. The results of the review are then reported followed by a summary of the results.
Methods for reviewing effectiveness
A registered protocol of this systematic review (CRD42016041799) is available on the PROSPERO website at www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016041799 (accessed 1 September 2016).
Identification of studies
The scope of the searches took into account the potential need to make simultaneous comparisons between all interventions, including, when appropriate, a network meta-analysis (NMA). The search strategy was designed to identify RCTs and systematic reviews of the relevant interventions, ADA and DEX intravitreal implant, as well as studies reporting on any comparators relevant to the scope, in patients with non-infectious intermediate uveitis, posterior uveitis and/or panuveitis. Given the broad range of possible comparators, the searches consisted only of terms for ‘uveitis’ combined with search filters for relevant study types and did not include terms for the interventions.
The search strategy consisted of medical subject headings (MeSH) or EMTREE Thesauri terms and free-text synonyms for ‘uveitis’. Searches were translated across databases and were not limited by language or publication date. Search strategies are presented in Appendix 1. Search filters designed to retrieve clinical trials, systematic reviews and economic evaluations were used in MEDLINE and other databases when appropriate.
Electronic database searches
The search approach involved the following:
-
searching of electronic databases and clinical trials registries
-
contact with experts in the field
-
examination of bibliographies of retrieved papers.
The following electronic databases and clinical trials registries were searched from inception for RCTs and systematic reviews:
-
MEDLINE (via Ovid) (1946 to 2016)
-
MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (1946 to 2016)
-
EMBASE (via Ovid) (1974 to 2016)
-
Cochrane Database of Systematic Reviews (CDSR) (via Wiley Online Library) (1996 to 2016)
-
Database of Abstracts of Reviews of Effects (DARE) (via Wiley Online Library) (1995–2015)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via Wiley Online Library) (1995 to 2016)
-
Health Technology Assessment (HTA) database (via Wiley Online Library) (1995 to 2016)
-
NHS Economic Evaluation Database (NHS EED) (via Wiley Online Library) (1995–2015)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost) (1982 to 2016)
-
Conference Proceedings Citation Index (CPCI) (Thomson Reuters) (1990 to June 2016)
-
the WHO’s International Clinical Trials Registry Platform [see http://apps.who.int/trialsearch/ (accessed 15 June 2016)].
Literature searching was undertaken in June 2016. Further searches were conducted in MEDLINE and CINAHL in October 2016.
Supplementary searches
References of relevant systematic reviews, primary studies and company submissions were checked to identify additional studies. Citation searching using Web of Science Citation Index (Thomson Reuters, 1899 to June 2016) was also undertaken. Searches were also conducted in Toxicology Literature Online (TOXLINE) in October 2016 to identify records reporting AEs for the technologies of interest.
Inclusion and exclusion criteria
The inclusion and exclusion criteria for selecting studies with relevant clinical effectiveness and safety data for ADA subcutaneous injection, DEX intravitreal implant or clinically relevant comparators in adults with non-infectious intermediate uveitis, posterior uveitis or panuveitis were consistent with the decision problem outlined in the National Institute for Health and Care Excellence (NICE) scope. 54
Population
The population of interest was adults with non-infectious intermediate uveitis, posterior uveitis or panuveitis. Eligible participants were considered for inclusion regardless of type of non-infectious posterior segment-involving uveitis (i.e. active or inactive uveitis; unilateral or bilateral uveitis; presence or absence of uveitis-related systemic disease or previous treatments for uveitis). Patients with infectious uveitis or uveitis as part of a masquerade syndrome were excluded from this review. In terms of patient age, studies were eligible if the enrolled patients were aged ≥ 18 years, or if separate data were provided for adults, or if at ≥ 80% of patients were adults. Studies conducted in paediatric populations were excluded.
Intervention
Interventions of interest were subcutaneous injection of ADA (40 mg) and DEX intravitreal implant (0.7 mg).
Comparators
Relevant comparators considered were as outlined in the NICE scope. 54 Studies reporting a comparison between subcutaneous injection of ADA and DEX intravitreal implant or between one of these interventions and any of the following comparator treatments were considered for inclusion:
-
periocular or intravitreal corticosteroid injections
-
intravitreal corticosteroid implants
-
systemic corticosteroids
-
systemic immunosuppressive therapies including azathioprine, methotrexate, cyclophosphamide, ciclosporin, chlorambucil, tacrolimus, mycophenolate mofetil and TNF-alpha inhibitors
-
intravitreal methotrexate
-
best supportive care (when all other treatment options have been tried)
-
placebo or a sham procedure.
In addition, studies reporting on any of the comparator treatments were considered for inclusion in a potential NMA.
Combinations of the above-mentioned interventions were also considered as relevant comparators.
Comparative studies in uveitis including interventions not specifically covered in the scope, or not considered to be clinically relevant comparators following consultation with clinical advisors to the AG, were excluded from the review. Excluded interventions included sirolimus, secukinumab, bevacizumab, acetazolamide, diclofenac, lisinopril, vitamin E, retinal antigens, echinacea and vitrectomy.
Outcomes
Outcomes of interest were:
-
visual acuity (the affected eye)
-
visual acuity (both eyes)
-
measured as the MD in BCVA according to a validated measure such as the ETDRS chart, Snellen chart or a similar tool
-
other measures of visual acuity would be considered if outcomes could be justified and validated in relation to accepted relevant standard measures
-
-
improvement in disease activity (e.g. VH grade, AC cell grade)
-
uveitis-related tissue damage or complications (e.g. cataract, macular oedema, retinal vascular occlusion)
-
reduction in systemic steroid use
-
mortality
-
adverse effects of treatment
-
HRQoL, including generic measures such as the EQ-5D and functional measures such as the VFQ-25
-
composite end points incorporating more than one of the above.
Study design
Data from RCTs were considered to be the most relevant for inclusion in the systematic review of the clinical effectiveness and safety of ADA subcutaneous injection and DEX intravitreal implant.
In addition, the DEX company submission43 included efficacy and safety data from non-randomised retrospective studies of 0.7 mg of dexamethasone (DEX 700) for non-infectious posterior segment-involving uveitis, reported in English, which included at least 10 patients. These data are summarised here for information, as some non-RCTs assessed DEX repeat implants (in the same eye) or implants in both eyes, whereas the RCT of DEX assessed only one implant in one eye per patient. It was beyond the scope of this assessment to undertake further searches or check the study selection and data extraction process undertaken within the DEX company submission. Non-randomised studies of ADA are not included here as they were not provided in the company submission55 and it was beyond the scope of this assessment to undertake a de novo review of non-randomised studies of ADA.
The following publication types were excluded from the review: narrative reviews, systematic reviews, clinical guidelines, editorials, letters, opinion pieces, abstracts with insufficient detail to assess study quality or results and non-English-language articles. Studies of animal models and preclinical and biological studies were not included.
Study selection process
Study selection was undertaken using a two-stage process guided by prespecified inclusion and exclusion criteria, as presented in the previous section.
All retrieved records were exported into a reference management database (EndNote version X7; Thomson Reuters, CA, USA). After deduplication, records were assessed for relevance by initially examining titles/abstracts, followed by a detailed scrutiny of the related full-text versions of potentially includable studies. At each step, studies that did not satisfy the eligibility criteria were excluded. One reviewer (EP or KC) checked a set of records; this was followed by a 10% check of selected studies by a second reviewer (KC or EP). Disagreements were resolved by discussion and involvement of a third researcher (HS) if needed.
Data extraction
Data were extracted by one reviewer (EP or KC) using a standardised piloted data extraction form and checked by a second reviewer (KC or EP). Disagreements were resolved by discussion. Data relevant to the decision problem were extracted, with no blinding to authors or journal. In relation to the interventions of interest, namely ADA and DEX, data extraction was limited to patients randomised to treatment arms with doses consistent with their licensed indications. Extracted information for each study included the study name (when reported), first author with publication year, characteristics of the study population, interventions, comparators and outcomes. When multiple publications of the same study were identified, data were extracted and reported as a single study.
Quality assessment
The methodological quality of each included study was assessed using an adapted Cochrane risk-of-bias tool. 56 Quality assessment was undertaken by one reviewer (EP or KC) and checked by a second reviewer (KC or EP).
Data synthesis
It was initially anticipated that, to compare the interventions of interest with each other and with current standard care, pairwise meta-analyses and/or NMAs may be undertaken, depending on the availability of relevant RCTs with common comparators reporting consistent outcomes. However, conducting pairwise meta-analyses or NMAs was not possible for the reasons presented in Indirect comparison of treatments: rationale for not undertaking. Data from studies contributing to the review were therefore summarised and presented using tabular and narrative syntheses. Summary statistics, for example MDs between treatments for continuous outcomes and relative risks (RRs) for binary outcomes, were calculated if not provided in the study reports.
Results of the clinical effectiveness review
Quantity and quality of research available
Literature searches retrieved 10,585 records (10,582 from the database searches and three from searching reference lists). A total of 10,451 records were excluded at title and abstract stage. Of the 134 full-text articles obtained for detailed examination, 117 were excluded because they did not meet the eligibility criteria for the review. Details of the excluded full-text articles with reasons for exclusion are presented in Appendix 2. Seventeen potentially relevant articles (relating to 16 studies) were retained for potential inclusion in meta-analyses; 13 studies32,33,57–67 were related to comparators within the scope of the review and three studies (four articles46–48,68) evaluated ADA or DEX 700. It was not possible to include any of the 13 studies of comparators within a NMA (the reasons why this was not possible, and a summary of the 13 studies, are provided in Indirect comparison of treatments: rationale for not undertaking and Table 24). This section therefore focuses specifically on studies of DEX 700 and ADA. The selection of studies informing the clinical effectiveness review is summarised in Figure 3. An example data extraction form is provided in Appendix 3 and the criteria for the assessment of methodological quality are provided in Appendix 4.
FIGURE 3.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.

Assessment of effectiveness
Study characteristics
The characteristics of the two included studies of ADA and the one included study of DEX 700 in patients with non-infectious uveitis are summarised in Table 4.
| Study, company, study dates, setting | Population: sample size, mean age, % of females, type of uveitis | Population: diagnosis | Intervention and comparator | Previous treatments | Concomitant treatments | Outcomes |
|---|---|---|---|---|---|---|
| Idiopathic 37% (n = 81/217), sarcoidosis 8% (n = 18/217), Behçet’s disease 7% (n = 16/217), VKH 12% (n = 25/217), birdshot chorioretinopathy 20% (n = 44/217), multifocal choroiditis and panuveitis 5% (n = 11/217), other 10% (n = 22/217) |
|
All patients: high-dose oral corticosteroids |
|
|
||
| Idiopathic 31% (n = 69/226), sarcoidosis 14% (n = 32/226), Behçet’s disease 7% (n = 16/226), VKH 23% (n = 51/226), birdshot chorioretinopathy 13% (n = 30/226), multifocal choroiditis and panuveitis 3% (n = 7/226), other 9% (n = 21/226) |
|
All patients: high-dose oral corticosteroids |
|
|
||
| None specified (no patients had uncontrolled systemic conditions) |
|
|
|
|
All three included studies were international, multicentre RCTs conducted in regions including Europe, North America and Australia.
Two trials, VISUAL I46 (n = 223 patients) and VISUAL II47 (n = 229 patients), compared ADA administered subcutaneously as an 80-mg loading dose followed by 40 mg every other week with a corresponding placebo treatment in patients with active (VISUAL I)46 or inactive (VISUAL II)47 non-infectious intermediate uveitis, posterior uveitis or panuveitis. The treatment and follow-up period had a duration of up to 80 weeks (18 months) or until treatment failure. Main study data were available for 223 patients with active uveitis (67 study sites)46,52,55 and 229 patients with inactive uveitis (62 study sites). 47,55,72 The VISUAL I and VISUAL II trials also included a subpopulation of patients from Japan (n = 16 and n = 32 patients respectively). 55 Data for this subgroup were not included in related publications46,47 or the company submission. 55
One study, HURON48 (46 study sites; n = 229 patients), a 26-week Phase III trial, evaluated the effectiveness of two different dosages of DEX intravitreal implants, DEX 700 and DEX 350 (0.35 mg), compared with a sham procedure in patients with active, chronic non-infectious intermediate and posterior uveitis. Only data relating to the licensed DEX 700 arm are included in this review.
Patient characteristics
Patients included in the HURON trial48 (mean age 44.8 years) were slightly older than those included in the VISUAL I46 and VISUAL II47 trials (mean age 42.7 and 42.5 years respectively). The proportion of women varied from 57%46 to 63%. 48
Inclusion criteria for patients with active uveitis in the VISUAL I trial46 were based on the manifestation of one or more of the following: VH score of ≥ 2; AC cell grade of ≥ 2 and/or active inflammatory chorioretinal or retinal vascular lesions while on high-dose oral corticosteroids (10–60 mg/day) for ≥ 2 weeks. Inactive uveitis in patients included in the VISUAL II trial47 was characterised by a VH score of ≤ 0.5 and an AC cell grade of ≤ 0.5, with no active inflammatory chorioretinal or retinal vascular lesions (i.e. uveitis inactivity), while receiving 10–35 mg/day of oral prednisone or its equivalent to maintain an inactive state of inflammation ≥ 28 days before study entry. Patients were considered for inclusion if control of their disease was corticosteroid dependent, that is, they had had more than one uveitic flare in the past 18 months occurring within 1 month of tapering steroids. In the HURON trial,48 active intraocular inflammation was based on the presence of a VH score of ≥ 1.5+ and patients unresponsive to previous treatment with corticosteroids were excluded. 48
The mean duration of uveitis was shorter in the active treatment arms than in the comparator arms across all three studies (40.2 vs. 51.0 months for VISUAL I,46 59.5 vs. 62.9 months for VISUAL II46 and 50.5 vs. 61.2 months for HURON48). Intermediate uveitis was the most common site of inflammation in patients (81% of patients) in the HURON trial,48 whereas panuveitis was seen more frequently in patients in the VISUAL trials46,47 (approximately 46% panuveitis vs. 22% intermediate uveitis vs. 33% posterior uveitis). Uveitis-related systemic conditions reported for patients in the VISUAL trials46,47 included Behçet’s disease, sarcoidosis and VKH. More patients with active uveitis had no diagnosed systemic condition (73%) than those with inactive uveitis (56%) in the VISUAL trials. 46,47 Limited information about relevant coexisting systemic conditions was provided for the HURON trial in the journal article,48 company submission43 or clinical study report,71 only that no patients had uncontrolled systemic conditions. Over 90% of patients in the VISUAL trials46,47 presented with bilateral uveitis; outcomes in the left and right eyes were considered separately and were then averaged across eyes in the analysis of the study findings. Conversely, in the HURON trial,48 the proportion of patients with bilateral uveitis was not reported (the AG queried this and was informed by the company that these data were not collected). In patients with bilateral uveitis, the right eye was selected for treatment; only the study eye was analysed for relevant outcomes. Overall, 84% of patients received treatment in the worse-seeing eye.
Study treatment and follow-up
The active treatment in the HURON trial48 was a single DEX intravitreal implant. The study compared the licensed dose of 0.7 mg (DEX 700, n = 77 patients, reported here) and a dose of 0.35 mg (DEX 350, n = 76 patients, not reported here) with a sham procedure (n = 76 patients). One implant was received per patient; no repeat implants were given during the 26-week follow-up period and patients had an implant in only one eye.
The active treatment evaluated in the VISUAL trials46,47 was ADA. Patients randomised to the study arms (n = 11146 and 11547 patients) received a loading dose of 80 mg by subcutaneous injection and then a 40-mg dose, repeated every other week. 46 A corresponding placebo was administered to patients in the comparator arms (n = 11246 and 11447 patients). For patients with active uveitis,46 visits during the study were scheduled at baseline and weeks 1, 4, 6 and 8. Subsequently, further visits occurred every 4 weeks until the primary end point (treatment failure) was achieved or until completion of 80 weeks of treatment. The treatment and follow-up duration was therefore up to 80 weeks (18 months) or until treatment failure. The median duration of treatment and follow-up in the VISUAL I trial46 was 19 weeks for ADA and 13 weeks for placebo. In the VISUAL II trial,47 the median duration of treatment and follow-up was 35 weeks for ADA and 22 weeks for placebo. There was a longer duration of ADA treatment in both studies because patients in the placebo groups met the treatment failure end points earlier than patients in the ADA groups and were taken off treatment.
Previous treatments and concomitant treatments
All patients in the VISUAL trials46,47 had previously received high-dose oral corticosteroids (> 10 mg/day of prednisone or its equivalent) prior to study entry. Within the VISUAL I trial,46 all patients received standardised oral prednisone (60 mg/day; hereafter referred to as a steroid burst) from randomisation, which was gradually tapered to 0 mg by week 15 of the study. Furthermore, topical corticosteroids were permitted but were tapered and discontinued by week 9. In the VISUAL II trial,47 all patients were already receiving oral prednisone (10–35 mg/day); this was tapered to 0 mg by week 19 or earlier depending on the steroid dose at baseline. During the study, patients were eligible to receive at least one immunosuppressant including azathioprine, ciclosporin, mycophenolate mofetil or methotrexate, at the discretion of the study investigator(s).
Limited information on prior and concomitant treatments for uveitis was reported in the HURON trial,48 although one-quarter of patients in the relevant population (DEX 700 and sham groups) for this review had received or were using systemic immunosuppressants or anti-inflammatory treatment at baseline (n = 38/153, 25%). The company did, however, provide patient-level data, which showed that treatment received was generally similar across arms but that more patients received immunosuppressant rescue therapy in the sham arm (10.5%) than in the DEX 700 arm (1.3%).
In the HURON trial,48 patients were permitted to receive different treatments at the discretion of the investigator(s) if they were indicated. Permitted treatments before the study and at baseline as well as during the study included the following:71
-
perioperative prophylactic antibiotics (at the visit prior to implantation and 3 days postoperatively)
-
IOP-lowering treatments (if IOP was > 30 mmHg in the study eye)
-
topical corticosteroids or non-steroidal anti-inflammatory drugs (NSAIDs) in the study eye (if doses remained stable for ≥ 2 weeks before screening, were stable throughout the study visits and were anticipated to remain stable up to week 8)
-
intravitreal, topical or periocular corticosteroids in the non-study eye (if inflammation occurred in the non-study eye)
-
cycloplegics (indication not specified)
-
cataract surgery (if reduced visual acuity had a limiting impact on the patient, the cataract interfered with uveitis management and/or the cataract resulted in local inflammation or glaucoma; the decision to operate was at the discretion of the investigator and patient and a delay to surgery until after week 26 was encouraged)
-
systemic immunosuppressants, for example methotrexate or ciclosporin (if doses remained stable for ≥ 3 months before screening, were unchanged throughout study visits and were anticipated to remain stable up to week 8)
-
systemic corticosteroids, for example oral prednisone or equivalent (if doses remained stable and were ≤ 20 mg/day at ≥ 1 month before screening, were stable throughout study visits and were anticipated to remain stable up to week 8)
-
oral NSAIDs (indication not specified).
Within the HURON trial,48 new treatment or previous management requiring dose escalation with systemic corticosteroids or immunosuppressants or local (intravitreal, periocular and topical) corticosteroids was permitted only if any of these interventions was administered as rescue treatment. In general, rescue anti-inflammatory treatments were permissible if the VH score increased by ≥ 1 unit from week 3 to the start of week 8 and if VH = 1.5+ was recorded from week 8 to week 26. 48 Other rescue medications included anticoagulants and surgical procedures on the study eye. 48,71
Study outcomes
The primary study end point varied across the studies:
-
In the VISUAL I46 trial of ADA for active uveitis, the primary end point was time to treatment failure, a composite outcome including worsening of at least one of the following in one or more eyes (from best state achieved following the steroid burst, on or after week 6): AC cell grade, VH grade, BCVA or new active inflammatory retinal or chorioretinal vascular lesions.
-
In the VISUAL II47 trial of ADA for inactive uveitis, the primary end point was time to treatment failure, a composite outcome including worsening of at least one of the following in one or more eyes (from baseline, on or after week 2): AC cell grade, VH grade, BCVA or new active inflammatory retinal or chorioretinal vascular lesions.
-
In the HURON48 trial of DEX, the primary outcome was the proportion of patients with a VH score of zero at week 8 in the study eye (outcomes were also measured up to week 26).
Outcomes reported in the included studies and grading criteria for intraocular inflammation are presented in Tables 5 and 6 respectively.
| Outcome | Assessment method |
|---|---|
| VISUAL I46 | |
| Primary outcome (composite end point): time to treatment failure at or after 6 weeks – evidence of one or more of the following in one or more eyes | |
| AC cell grade: ≥ 0.5+ (at 6 weeks); two-step or greater increase in AC cell grade relative to best state achieved (after 6 weeks) | DIO, graded by SUN criteria |
| VH grade: ≥ 0.5+ (at 6 weeks); two-step or greater increase in VH grade relative to best state achieved (after 6 weeks) | DIO, graded by NEI/SUN criteria |
| New active, inflammatory chorioretinal or retinal lesions compared with baseline | DIO |
| Worsening of BCVA by ≥ 15 letters compared with best score previously observed | LogMAR units using ETDRS chart |
| VISUAL II47 | |
| Primary outcome (composite end point): time to treatment failure on or after 2 weeks – evidence of one or more of the following in one or more eyes | |
| New active, inflammatory chorioretinal or retinal lesions compared with baseline | DIO |
| AC cell grade: two-step or greater increase relative to baseline | DIO, graded by SUN criteria |
| VH grade: two-step or greater increase relative to baseline | DIO, graded by NEI/SUN criteria |
| Worsening of BCVA by ≥ 15 letters relative to baseline | LogMAR units using ETDRS chart |
| VISUAL I and VISUAL II46,47,73 | |
| Secondary outcomes: VISUAL I from best state achieved prior to week 6 to final or early termination visit; VISUAL II – from baseline to final or early termination (all measured for left and right eyes separately and then treatment effects averaged across eyes) | |
| Change in AC cell grade in each eye | DIO, graded by SUN criteria |
| Change in VH score in each eye | DIO, graded by NEI/SUN criteria |
| Change in BCVA in each eye | LogMAR units using ETDRS chart |
| Time to develop MO in at least one eye | Assessed in patients without MO at baseline |
| % change in CRT in each eye | Stratus OCT (Carl Zeiss Meditec, Inc., Dublin, CA, USA); Cirrus HD-OCT (Carl Zeiss Meditec, Inc., Dublin, CA, USA) or Spectralis (Heidelberg Engineering, Franklin, MA, USA) |
| Change in generic and vision-specific quality of life in each eye | EQ-5D score; VFQ-25 composite score, near vision subscore, distance vision subscore, ocular pain subscore |
| Disease quiescence | Absence of new active inflammatory lesions with AC cell and VH grade of ≤ 0.5+ |
| HURON43,48,71 | |
| Primary outcome (all in study eye only) | |
| VH score = 0 at week 8 | Scores consistent with published colour photographic scale |
| Secondary outcomes (all in study eye only) | |
| BCVA | AREDS-adapted ETDRS chart |
| Central macular thickness | OCT (at least six scans required at selected sites) |
| Early treatment failure | VH increase of ≥ 1 unit from baseline at week 3 |
| Late treatment failure | VH ≥ 1.5+ at week 8 or after week 8 |
| Use of escape medications | Medications administered to patients with early or late treatment failure |
| Patient-reported outcomes | VFQ-25 composite score and subscores |
| AC cell score | VH grade | ||||
|---|---|---|---|---|---|
| VISUAL I and II46,47 | VISUAL I and II46,47 | HURON48 | |||
| Grade | Criteria (number of cells)a | Grade | Criteria | Gradeb | Criteria |
| 0 | < 1 | 0 | No evident VH | 0 | No inflammation |
| 0.5+ | 1–5 | 0.5+ | Slight blurring of the optic disc margin because of the haze; normal striations and reflex of the nerve fibre layer cannot be visualised | +0.5 | Trace inflammation (slight blurring of the optic disc margins and/or loss of the nerve fibre layer reflex) |
| 1+ | 6–15 | 1+ | Permits a better definition of both the optic nerve head and the retinal vessels (compared with higher grades) | +1 | Mild blurring of retinal vessels and the optic nerve |
| 2+ | 16–25 | 2+ | Permits better visualisation of the retinal vessels (compared with higher grades) | +2 | Moderate blurring of the optic nerve head |
| 3+ | 26–50 | 3+ | Permits the observer to see the optic nerve head, but the borders are quite blurry | +3 | Marked blurring of the optic nerve head |
| 4+ | > 50 | 4+ | Optic nerve head is obscured | +4 | Optic nerve head not visible |
Secondary outcomes for the VISUAL I trial46 (see Table 5) were measured from the best state prior to week 6 (following the steroid burst), whereas secondary outcomes for the VISUAL II trial47 were measured from baseline. Secondary outcomes in the VISUAL trials were measured only up to treatment failure or the study end and, as treatment failure occurred in more patients in the placebo arms than in the ADA arms, the results may have been worse in the placebo arms at the point of outcome measurement. The last observation carried forward (LOCF) method was used for dealing with missing data.
Assessment of the methodological quality of the included studies
An overview of the methodological quality of the included studies is presented in Figure 4 and Table 7. Generally, all three studies performed well against all of the main quality items in the Cochrane risk-of-bias tool. 56 Suitable methods for random sequence generation were reported across all studies. In the VISUAL trials,46,47 the randomisation list was remotely generated by the statistics department of the manufacturer (AbbVie). Patients were subsequently allocated to study arms by means of a voice-response or web-response system. Similar methods were used in the HURON trial,48 with the manufacturer (Allergan) providing a centrally generated randomisation schedule, which was followed by an interactive allocation procedure for study participants that was remotely managed. In the VISUAL trials,46,47 randomisation to study arms was stratified according to prior immunosuppressant treatment; conversely, randomisation was stratified according to baseline VH in the HURON trial. 48 Blinding of participants and investigators was assessed as satisfactory across studies. In the VISUAL trials,46,47 unmasking of treatment allocation was permitted only in the event of a medical emergency. In the HURON trial,48 treatment investigators were responsible for the implantation procedure; however, outcome assessors were masked to the treatment received by patients. In terms of selective reporting, all studies reported prespecified primary outcomes. However, specific clinical outcomes (e.g. visual acuity or macular oedema) were assessed and reported differently across studies. This highlights the lack of standardisation in ophthalmic outcome reporting and makes it difficult to assess whether or not selective reporting occurred.
FIGURE 4.
Summary of the methodological quality of the included studies: review authors’ judgement about each quality item across the included studies.

| Study | Quality assessment itema | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| VISUAL I46 | Y | Y | Y | N | Y | Y | Y | Y | Y |
| VISUAL II47 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| HURON48 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
In terms of other biases, as noted in Study characteristics, secondary outcomes for the VISUAL I trial46 (see Table 5) were measured from the best state prior to week 6 (following the steroid burst); this use of a postrandomisation baseline may introduce bias. Conversely, secondary outcomes for the VISUAL II trial47 were measured from baseline. A further potential source of bias is that secondary outcomes in the VISUAL trials were measured only up to treatment failure or the study end and, as treatment failure occurred in more patients in the placebo arms than the ADA arms, the results may have been worse in the placebo arms at the point of outcome measurement. The LOCF method used for dealing with missing data may have introduced systematic bias as it assumes that data are missing at random, which is not the case here.
In terms of additional considerations for methodological and reporting quality, all studies reported prespecified inclusion and exclusion criteria. A priori sample size calculations for detecting between-group differences for the specified primary outcomes at a significance level of 5% indicated that 234 patients were needed to achieve a power of 90% in the VISUAL I trial46 (outcome time to treatment failure at or after 6 weeks); 220 patients were needed to achieve 80% power in the VISUAL II trial47 (outcome time to treatment failure at or after 2 weeks) and 73 patients were needed per study arm to achieve a power of 93% in the HURON trial48 (outcome proportion of patients with a VH score of 0). In the VISUAL I trial46 223 patients were randomised, slightly fewer than the 234 suggested by the power calculation; however, given that the study showed a significant between-group difference for the primary outcome (time to treatment failure), this was not an issue. Demographic and baseline characteristics between study arms were comparable for all studies with the exception of the duration of uveitis, which was slightly longer in the non-active comparator arms, as noted earlier. The impact of the non-study treatment options that were available throughout the duration of the studies is unclear, in particular in the HURON trial,48 in which patients with worsening of intraocular inflammation following the implantation procedure could receive rescue (escape) medication consisting of systemic corticosteroids or immunosuppressants or topical steroids. Indications for escape medication were early treatment failure (i.e. patients with a VH increase of ≥ 1 unit from baseline at week 3) or late treatment failure (i.e. patients with a VH grade of ≥ 1.5+ at week 8 or after week 8).
In the VISUAL I46 and II47 trials data for patients in the Japanese substudies were not included in the analyses. In the HURON trial,48 100% of the patients were included in the intention-to-treat (ITT) analyses, whereas in the VISUAL trials the analyses described as ‘ITT’ excluded 6 of 223 patients (3%)46 and 3 of 229 patients (1%)47 because of incomplete efficacy data and compliance issues at these sites.
Feasibility of meta-analysis
It was not considered appropriate to meta-analyse the findings of the VISUAL I46 and VISUAL II47 trials because the VISUAL I trial46 enrolled patients with active uveitis and the VISUAL II trial47 enrolled patients with inactive uveitis. Active uveitis refers to current inflammation in the eye whereas patients with inactive uveitis have limited inflammation, usually because of treatment with corticosteroids or immunosuppressants. In addition, the magnitude of the treatment effect is likely to be associated with the degree of disease activity and inflammation at baseline, with patients with little inflammation or vision loss at baseline less likely to show an improvement in outcomes. NMA was also not considered feasible or appropriate for the reasons discussed in Indirect comparison of treatments: rationale for not undertaking.
Effectiveness results from the included studies
Treatment failure
The primary outcome for the VISUAL trials46,47 of ADA was a composite treatment failure outcome, defined as worsening of at least one of the following in one or more eyes: AC cell grade, VH grade, BCVA or new active inflammatory retinal or chorioretinal vascular lesions (see Table 5). In the VISUAL I trial,46 outcomes were measured relative to the best state achieved following the initial steroid burst and treatment failure was assessed from week 6. In the VISUAL II trial,47 outcomes were measured relative to baseline and treatment failure was assessed from week 2.
In the VISUAL I trial46 (active uveitis), treatment failure was experienced by 54.5% of patients in the ADA arm and 78.5% of patients in the placebo arm (Table 8). The median time to treatment failure was 5.6 months for ADA and 3 months for placebo, giving a hazard ratio (HR) of 0.50 (95% CI 0.36 to 0.70; p < 0.001). Treatment failure related to each of the four individual criteria (worsening of AC cell grade, VH grade or BCVA or new lesions) was also significantly greater in the placebo arm than in the ADA arm (p = 0.04 to p < 0.001).
| Outcome | Study | |||||
|---|---|---|---|---|---|---|
| VISUAL I46,55 (active uveitis) | VISUAL II47,55 (inactive uveitis) | HURON48,71 (active uveitis) | ||||
| ADA | Placebo | ADA | Placebo | DEX 700 | Sham | |
| TF, n/N (%) | 60/110 (54.5)a | 84/107 (78.5)a | 45/115 (39)b | 61/111 (55)b | NRc | NRc |
| Comparison between groups | NR | NR | p < 0.001d | |||
| Time to TF in one or more eyes (months), median (IQR) | 5.6 (3.0 to not estimable) | 3.0 (1.5–5.6) | Not estimable (4.7 to not estimable) | 8.3 (3.0 to not estimable) | NR | NR |
| Comparison between groups, HR (95% CI) | 0.50 (0.36 to 0.70); p < 0.001 | 0.57 (0.39 to 0.84); p = 0.004 | NR | |||
| TF because of new lesions, n/N (%) | 17/110 (15.5) | 29/107 (27.1) | NR | NR | NR | NR |
| Comparison between groups, HR (95% CI) | 0.38 (0.21 to 0.69); p = 0.001 | 0.55 (0.26 to 1.15); p = 0.105 | NR | |||
| TF because of AC cell grade, n/N (%) | 24/110 (21.8) | 34/107 (31.8) | NR | NR | NR | NR |
| Comparison between groups, HR (95% CI) | 0.51 (0.30 to 0.86); p = 0.01 | 0.70 (0.42 to 1.18); p = 0.180 | NR | |||
| TF because of VH grade, n/N (%) | 16/110 (14.5) | 39/107 (36.4) | NR | NR | NR | NR |
| Comparison between groups, HR (95% CI) | 0.32 (0.18 to 0.58); p < 0.001 | 0.79 (0.34 to 1.81); p = 0.589 | NR | |||
| TF because of reduction in BCVA, n/N (%) | 23/110 (20.9) | 27/107 (25.2) | 10/115 (9) | 23/111 (21) | NR | NR |
| Comparison between groups, HR (95% CI) | 0.56 (0.32 to 0.98); p = 0.04 | 0.33 (0.16 to 0.70); p = 0.002 | NR | |||
In the VISUAL II trial47 (inactive uveitis), treatment failure was experienced by 39% of patients in the ADA arm and 55% of patients in the placebo arm (see Table 8). The median time to treatment failure was not estimable for the ADA arm (> 18 months), because fewer than half of the patients had experienced treatment failure, and was 8.3 months for the placebo arm (HR 0.57, 95% CI 0.39 to 0.84; p = 0.004). Treatment failure because of a reduction in BCVA was significantly greater in the placebo arm than in the ADA arm (p = 0.002), although failure related to the other three criteria (worsening of AC cell grade or VH grade or new lesions) was not statistically significant (p = 0.105 to p = 0.589).
Treatment failure in the HURON trial48 was defined as a VH grade increase of ≥ 1 unit at weeks 3–8 or a VH of ≥ +1.5 at weeks 8–26. No data were reported in the journal article,48 company submission43 or clinical study report,71 but a statistically significant difference between the DEX 700 arm and the sham arm (p < 0.001) was noted.
Best corrected visual acuity
The studies of ADA reported change in BCVA in units of logMAR (logarithm of the minimum angle of resolution) (Table 9). In the VISUAL I trial,46,52 change was measured from the best state reached prior to week 6 after the initial steroid burst rather than from baseline to the final value (week 80 or at the time of treatment failure). BCVA improved in both the ADA arm and the placebo arm following the initial steroid burst but worsened as time progressed, with greater worsening in the placebo arm. The change in BCVA (logMAR) from the best state prior to week 6 to final or early termination was 0.07 and 0.04 in the left and right eyes, respectively, in the ADA arm and 0.12 and 0.13 in the left and right eyes, respectively, in the placebo arm. The MD between groups in BCVA change, pooled across left and right eyes, was –0.07 (95% CI –0.11 to –0.02; p = 0.003).
| Outcome | Study | |||||||
|---|---|---|---|---|---|---|---|---|
| VISUAL I46,52 (active uveitis) | VISUAL II47,72 (inactive uveitis) | |||||||
| ADA | Placebo | ADA | Placebo | |||||
| Left eye (n = 101) | Right eye (n = 101) | Left eye (n = 103) | Right eye (n = 103) | Left eye (n = 115) | Right eye (n = 115) | Left eye (n = 110) | Right eye (n = 110) | |
| Mean VA, baseline (for VA change in VISUAL II47 only) | 0.22 (0.344) | 0.23 (0.277) | 0.24 (0.291) | 0.25 (0.307) | 0.14 (0.255) | 0.12 (0.222) | 0.16 (0.287) | 0.15 (0.274) |
| Mean VA, best value prior to week 6 following steroid burst (used as ‘baseline’ for changes in VISUAL I46) | 0.13 (0.290) | 0.14 (0.243) | 0.12 (0.262) | 0.14 (0.271) | NA | NA | NA | NA |
| Mean VA, final (week 80 or early termination) | 0.20 (0.370) | 0.18 (0.294) | 0.24 (0.319) | 0.27 (0.442) | 0.15 (0.338) | 0.11 (0.282) | 0.22 (0.388) | 0.16 (0.293) |
| Mean change in VA | ||||||||
| VISUAL I:46 from best state reached prior to 6 weeks to final or early termination | 0.07 (0.160) | 0.04 (0.143) | 0.12 (0.169) | 0.13 (0.320) | NA | NA | NA | NA |
| VISUAL II:47 from baseline to final or early termination | NA | NA | NA | NA | 0.01 (0.251) | –0.01 (0.165) | 0.06 (0.239) | 0.02 (0.198) |
| Difference in mean VA change between groups (pooled across left and right eyes), mean (95% CI) | –0.07 (–0.11 to –0.02); p = 0.003 | –0.04 (–0.08 to 0.01); p = 0.096 | ||||||
In the VISUAL II trial,47,72 change was measured from baseline to the final value (week 80 or at the time of treatment failure). BCVA stayed fairly constant from baseline to the final value in the ADA arm and worsened in the placebo arm (see Table 9). The change in BCVA (logMAR) from baseline to final or early termination was 0.01 and –0.01 in left and right eyes, respectively, in the ADA arm and 0.06 and 0.02 in left and right eyes, respectively, in the placebo arm. The MD between groups in BCVA change, pooled across left and right eyes, was reported as –0.04 (95% CI –0.08 to 0.01; p = 0.096).
In the HURON trial,43,48 BCVA was measured as the proportion of patients with a change of two or more or three or more ETDRS lines over the 26 weeks (Table 10). The proportion with an improvement of three or more lines was 43% in the DEX 700 arm and 7% in the sham arm at week 8 (p < 0.001) and 38% in the DEX 700 arm and 13% in the sham arm at week 26 (p < 0.001). Improvement of two or more lines followed a similar pattern (see Table 10). In the DEX 700 arm, there was a significant improvement in mean BCVA over weeks 3–26 (no values reported, p ≤ 0.002). 71
| Outcome | Treatment, n/N (%) | MD (95% CI) (%); p-value | RR (95% CI); p-value | |
|---|---|---|---|---|
| DEX 700 | Sham | |||
| Patients with BCVA improvement of three or more ETDRS lines (≥ 15 letters) | ||||
| Week 8 | 33/77 (43) | 5/76 (7) | 36.3 (24 to 49); p < 0.001 | 6.5 (2.7 to 15.8); p < 0.001 |
| Week 26 | 29/77 (38) | 10/76 (13) | 24.5 (11 to 38); p < 0.001 | 2.9 (1.5 to 5.5); p < 0.001 |
| Patients with BCVA improvement of two or more ETDRS lines (≥ 10 letters) | ||||
| Week 8 | 46/77 (60)a | 13/76 (17)a | 43 (29 to 56); p < 0.001 | 3.5 (2.1 to 5.9); p < 0.001 |
| Week 26 | 42/77 (55)a | 19/76 (25)a | 30 (15 to 44); p < 0.001 | 2.2 (1.4 to 3.4); p < 0.001 |
Patient-reported outcome measures
Data on patient-reported outcome measures (PROMs) derived from the publications and submissions related to the VISUAL and HURON studies are reported here. These data are presented in this report before additional clinical outcomes because of their importance for the cost-effectiveness modelling.
The main PROM reported in the journal articles for the VISUAL trials46,47 was the VFQ-25. Additional PROMS reported in the company’s submission55 and clinical study reports52,72 for the VISUAL trials included the EQ-5D, Hospital Anxiety and Depression Scale (HADS),74 Work Productivity and Activity Impairment (WPAI) questionnaire75 and Healthcare Resource Utilisation questionnaire. 76
The VFQ-25 is made up of 25 questions that cover 11 vision-specific quality-of-life subscales and one general health item. 28 Condition-specific subscales in the tool include those for general vision, ocular pain, distance activities, near activities, vision-specific dependency, vision-specific role difficulties, vision-specific social functioning, vision-specific mental health, driving, peripheral vision and colour vision. Responses to items in each subscale are coded and scored from 0 to 100. Summary scores for each subscale are derived from an average of scores for items within the subscale. A composite score is obtained by calculating the average of all of the scores from the 11 vision-specific subscales. The general health item score and blank items within the instrument are excluded when calculating the composite score. Higher scores indicate better visual functioning.
In the VISUAL I trial,46 ADA produced a statistically significant and clinically meaningful improvement in the VFQ-25 composite score for patients with active uveitis relative to patients in the placebo arm (MD 4.20, 95% CI 1.02 to 7.38; p = 0.01), from the best state achieved following the initial steroid burst to time of treatment failure (early termination) or study end (80 weeks), as shown in Table 11. Of the three subscales predefined as secondary outcomes in the VISUAL trials, statistically significant and clinically meaningful differences favouring ADA over placebo were observed for changes in the near vision subscale (MD 5.12, 95% CI 0.34 to 9.90; p = 0.036) and the ocular pain subscale (MD 10.02, 95% CI 4.86 to 15.19; p < 0.001); differences in the distance vision subscale were not statistically significant (MD 1.86, 95% CI –2.03 to 5.75; p = 0.346).
| Outcome | Study | |||||
|---|---|---|---|---|---|---|
| VISUAL I46 (active uveitis) | VISUAL II47 (inactive uveitis) | |||||
| Treatment, mean (SD) | MD (95% CI), p-value | Treatment, mean (SD) | MD (95% CI), p-value | |||
| Placebo (n = 102) | ADA (n = 101) | Placebo (n = 109) | ADA (n = 115) | |||
| Composite score | –5.50 (11.97) | –1.30 (10.98) | 4.20 (1.02 to 7.38), p = 0.010 | 1.24 (10.7) | 3.36 (11.7) | 2.12 (–0.84 to 5.08), p = 0.16 |
| Distance vision subscale | –5.64 (14.65) | –3.77 (13.41) | 1.86 (–2.03 to 5.75), p = 0.346 | 0.76 (16.3) | 2.64 (17.2) | 1.88 (–2.53 to 6.29), p = 0.40 |
| Near vision subscale | –8.09 (17.75) | –2.97 (16.78) | 5.12 (0.34 to 9.90), p = 0.036 | 3.98 (17.4) | 3.88 (18.3) | –0.10 (–4.81 to 4.61), p = 0.97 |
| Ocular pain subscale | –12.62 (21.44) | –2.6 (15.34) | 10.02 (4.86 to 15.19), p < 0.001 | 2.87 (17.2) | 3.42 (21.3) | 0.56 (–4.56 to 5.68), p = 0.83 |
In the VISUAL II trial,47 differences between the ADA arm and the placebo arm were not statistically significant for change in VFQ-25 composite score (MD 2.12, 95% CI –0.84 to 5.08; p = 0.16) or for the distance vision, near vision or ocular pain subscales (p = 0.40 to p = 0.97; see Table 11).
In the VISUAL I trial, EQ-5D estimates were higher in ADA-treated patients than in the placebo group (Table 12). Reported EQ-5D predicted values, assessed from change from the best state achieved before week 6 to the final visit or early termination, demonstrated statistical significance, favouring ADA over placebo (MD 0.04, 95% CI 0.00 to 0.07; p = 0.044). 52,55 According to estimates based on the WPAI, compared with patients treated with placebo, those receiving ADA had less time off work (MD –10.61 days, p = 0.011). There were no significant differences between treatment groups for the remaining outcomes. 52,55
| Outcome | Study, mean (SD) | |||
|---|---|---|---|---|
| VISUAL I52,55 (active uveitis) | VISUAL II72 (inactive uveitis) | |||
| ADA (n = 101) | Placebo (n = 100) | ADA (n = 115) | Placebo (n = 108) | |
| EQ-5D score, baseline | 0.83 (0.15) | 0.82 (0.164) | 0.86 (0.160) | 0.85 (0.138) |
| EQ-5D score, best value prior to week 6 | 0.89 (0.128) | 0.88 (0.142) | NA | NA |
| EQ-5D score, final or early termination | 0.86 (0.153) | 0.80 (0.187) | 0.85 (0.165) | 0.84 (0.177) |
| Mean change in EQ-5D score | ||||
| VISUAL I:46 from best state prior to week 6 to final or early termination | –0.04 (0.129) | –0.07 (0.135) | NA | NA |
| VISUAL II:47 from baseline to final or early termination | NA | NA | –0.01 (0.134) | –0.01 (0.161) |
| MD (95% CI) | 0.04 (0.00 to 0.07); p = 0.044 | 0.00 (–0.03 to 0.04); p = 0.836 | ||
There was no significant improvement in patients’ EQ-5D scores in the VISUAL II trial (MD 0.00, 95% CI –0.03 to 0.04; p = 0.836). 72 No other significant differences were reported for the other outcomes.
Table 13 provides a summary of the VFQ-25 composite scores at baseline and weeks 8 and 26 in the HURON trial. 43,68,71 At baseline, the mean composite VFQ-25 score was 63.7 in the DEX 700 group and 71.3 in the sham group. 43
| Time point | Absolute values | Change from baseline | MD (95% CI); p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DEX 700 | Sham | DEX 700 | Sham | ||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | ||
| Baselinea | 73 | 63.7 (20.74) | 73 | 71.3 (19.0) | NR | NA | NR | NA | |
| Week 8 LOCFa | 73 | 75.1 (NR) | 74 | 74.2 (NR) | 73 | 9.6 (NR) | 74 | 4.2 (NR) | 5.4 (NR); p = 0.007 |
| Week 8 PP | 69 | 74.4 (17.3) | 70 | 74.5 (18.1) | 69 | 11.6 (14.7) | 69 | 3.4 (11.2) | 8.2 (NR); p < 0.001 |
| Week 16 LOCFa | 73 | 75.9 (NR) | 74 | 75.3 (NR) | NR | NR | NR | NR | NR |
| Week 16 PP | 69 | 75.3 (18.12) | 70 | 75.6 (19.1) | 69 | 10.5 (14.3) | 69 | 4.5 (12.7) | 6.0 (NR); p = NR |
| Week 26 LOCFa | 73 | 76.2 (NR) | 74 | 73.2 (NR) | 73 | 10.1 (NR) | 74 | 2.8 (NR) | 7.3 (NR); p = 0.001 |
| Week 26 or early exit (CSR71) | 72 | 74.6 (19.32) | 73 | 74.3 (20.4) | 72 | 10.3 (16.7) | 72 | 2.8 (13.9) | 7.5 (NR); p = NR |
By week 8 (based on analyses using raw scores for patients available at each time point), the change from baseline in composite VFQ-25 score in the DEX 700 group was 11.6 points and in the sham group was 3.4 points (p < 0.001). 71 Change at week 8 using LOCF analyses was 9.6 for the DEX 700 group and 4.2 for the sham group (p = 0.007). 68 Change at week 26 using LOCF analyses was 10.1 and 2.8 for patients in the DEX 700 and sham groups respectively (p = 0.001).
Statistically significant differences between the DEX 700 group and the sham group for changes in distance vision (p = 0.023), near vision (p = 0.031), peripheral vision (p = 0.045) and vision-specific social functioning (p = 0.019) were reported at the primary time point (week 8). 46,71
More patients in the DEX 700 group than the sham group had a ≥ 5-point improvement in VFQ-25 composite score (week 8: 54.8% vs. 27.0%, p < 0.001; week 26: 57.5% vs. 32.4%, p < 0.05; Table 14). More patients in the DEX 700 group than the sham group also had a ≥ 10-point improvement in VFQ-25 composite score (week 8: 45.2% vs. 14.9%, p < 0.001; week 26: p-value reported as significant but no value given; see Table 14). 43,71 Additionally, statistically significant differences between the groups were reported for the percentage of patients with ≥ 5-point and ≥ 10-point improvements in distance vision, general vision, peripheral vision, colour vision, ocular pain, vision-specific social functioning and vision-specific mental health (all p < 0.05).
| VFQ-25 scores | DEX 700, n/N (%) | Sham, n/N (%) | MD (95% CI) (%); p-value |
|---|---|---|---|
| Patients with a ≥ 5-point improvement | |||
| Week 8 | 40/73 (54.8) | 20/74 (27.0) | 27.8 (13 to 43); p < 0.001 |
| Week 16 | NR | NR | NR (NR); p = significant (NR) |
| Week 26 | 42/73 (57.5) | 24/74 (32.4) | 25.1 (10 to 41); p < 0.05 |
| Patients with a ≥ 10-point improvement | |||
| Week 8 | 33/73 (45.2) | 11/74 (14.9) | 30.3 (16 to 44); p < 0.001 |
| Week 16 | NR | NR | NR (NR); p = significant (NR) |
| Week 26 | NR | NR | NR (NR); p = significant (NR) |
EuroQol-5 Dimensions (US tariff), Short Form questionnaire-6 Dimensions (SF-6D)77 and Short Form questionnaire-36 items (SF-36)78 estimates were presented at baseline in the HURON trial but not beyond this and no other outcomes were reported. 43,71
Vitreous haze grade
Vitreous haze was measured by dilated indirect ophthalmoscopy in the VISUAL46,47 and HURON48 studies. In both cases grading was based on the original scale proposed by Nussenblatt et al. 37 and later adopted by SUN8 (with the minor modification of ‘trace’ being replaced by 0.5+ in the ordinal scale). An important difference between the VISUAL trials and the HURON trial, however, was that the HURON trial48 proposed an additional 1.5+ grade for cases that were deemed to lie between the 1+ and 2+ grades (see Table 6). Vitreous grade scores are presented in Study outcomes.
In the VISUAL trials,46,47 VH outcomes were considered as criteria contributing to the primary composite end points of treatment failure. In the VISUAL I trial,46 VH was assessed as the change from the best achieved score following a mandatory steroid burst until the final or early termination visit. In the VISUAL II trial,47 VH was assessed as the change from baseline to the final or early termination visit. Higher scores are correlated with increased severity of uveitis.
A statistically significant difference in change in VH score was reported for patients in the ADA group compared with patients in the placebo group in the VISUAL I trial46 (–0.27, 95% CI –0.43 to –0.11; p < 0.001; Table 15). Lower mean VH scores were also noted for the ADA group compared with the placebo group in the VISUAL II trial,47 but the difference between the groups was not statistically significant (–0.13, 95% CI –0.28 to 0.01; p = 0.070). In the VISUAL I trial,46 VH worsening was the least common cause of treatment failure in the ADA group (15% of events) and the most common reason for treatment failure in the placebo group (36% of events; HR 0.32, 95% CI 0.18 to 0.58; p < 0.001). Conversely, increases in VH grade in the VISUAL II trial47 were not significantly different between the treatment groups and did not affect the time to treatment failure (HR 0.79, 95% CI 0.34 to 1.81; p = 0.569).
| Outcome | Study | |||||||
|---|---|---|---|---|---|---|---|---|
| VISUAL I46 (active uveitis) | VISUAL II47 (inactive uveitis) | |||||||
| ADA | Placebo | ADA | Placebo | |||||
| Left eye (n = 101) | Right eye (n = 101) | Left eye (n = 103) | Right eye (n = 103) | Left eye (n = 115) | Right eye (n = 115) | Left eye (n = 110) | Right eye (n = 110) | |
| Mean (SD) VH score, final (week 80 or early termination) | 0.44 (0.736) | 0.47 (0.636) | 0.73 (0.795) | 0.78 (0.865) | 0.32 (0.594) | 0.32 (0.601) | 0.48 (0.728) | 0.42 (0.630) |
| Mean (SD) change in VH score | ||||||||
| VISUAL I:46 from best state reached prior to week 6 to final or early termination | 0.11 (0.559) | 0.13 (0.648) | 0.33 (0.666) | 0.45 (0.781) | NA | NA | NA | NA |
| VISUAL II:47 from baseline to final or early termination | NA | NA | NA | NA | 0.16 (0.601) | 0.18 (0.604) | 0.33 (0.733) | 0.27 (0.605) |
| Difference in mean change in VH score between groups (pooled across left and right eyes) (95% CI) | –0.27 (–0.43 to –0.11); p < 0.001 | –0.13 (–0.28 to 0.01); p = 0.070 | ||||||
Study entry eligibility criteria included, among others, a VH score of ≥ +1.5. 48 At baseline, more patients had a VH score of +1.5 to +2 (DEX 700: 84%, n = 65/77; sham: 87%, n = 66/76) than a score of +3 to +4 (DEX 700: 16%, n = 12/77; sham: 13%, n = 10/76). Patients were stratified using these two VH cut-off points. The primary efficacy outcome was the proportion of patients with a VH score of zero. The analysis was based on an ITT population and the primary time point was week 8 following implantation; outcomes were also measured up to week 26.
Compared with patients receiving a sham procedure, a statistically significantly higher proportion of patients in the DEX 700 arm achieved a VH score of zero at week 8 (MD 34.9%, 95% CI 22% to 48%; p < 0.001) and week 26 (MD 16.7%, 95% CI 4% to 30%; p = 0.014)48 (Table 16). Subgroup analyses by baseline VH score and previous systemic therapy are also shown in Table 16.
| Outcome | DEX 700 | Sham | MD (95% CI) (%); p-value | RR (95% CI); p-value |
|---|---|---|---|---|
| VH score = 0, % (n) | ||||
| Week 8: all patients | 46.8 (36/77) | 11.8 (9/76) | 34.9 (22 to 48); p < 0.001 | 4.0 (2.0 to 7.6); p < 0.001 |
| Week 26: all patients | 31.2 (24/77) | 14.5 (11/76) | 16.7 (4 to 30); p = 0.014 | 2.2 (1.1 to 4.1); p = 0.02 |
| Week 8a | ||||
| Baseline VH +1.5 or +2 | 48.4 (31/64) | 12.1 (8/66) | 36 (22 to 51); p < 0.001 | 4.0 (2.0 to 8.0); p < 0.001 |
| Baseline VH +3 or +4 | 41.7 (5/12) | 10.0 (1/10) | 32 (–2 to 65); p = 0.06 | 4.2 (0.6 to 30.1); p = 0.16 |
| Week 26a | ||||
| Previous systemic therapy | 28.6 (4/14) | 7.1 (1/14) | 21 (–6 to 49); p = 0.12 | 4.0 (0.5 to 31.5); p = 0.19 |
| No previous systemic therapy | 31.7 (20/63) | 16.1 (10/62) | 16 (1 to 30); p = 0.04 | 2.0 (1.0 to 3.9); p = 0.05 |
| Improvement of ≥ 1 in VH score, % (n) | ||||
| Week 8 | 94.8 (73/77) | 44.7 (34/76) | 50.1 (38 to 62); p < 0.001 | 2.1 (1.6 to 2.7); p < 0.001 |
| Week 26 | 81.8 (63/77) | 51.3 (39/76) | 30.5 (16 to 45); p < 0.001 | 1.6 (1.3 to 2.0); p < 0.001 |
| Improvement of ≥ 2 in VH score, % (n) | ||||
| Week 8 | 44.2 (34/77) | 13b (10/76) | Approximately 31; p < 0.001 | Approximately 3.4; p < 0.001 |
| Week 26 | 33.8 (26/77) | 14b (11/76) | Approximately 19; p = 0.001 | Approximately 2.3; p = 0.008 |
| Mean (SD) VH score | ||||
| Week 8 | 0.47 (NR) | 1.44 (NR) | –0.97; p < 0.001 | |
| Week 26 | 0.72 (NR) | 1.30 (NR) | –0.58; p < 0.001 | |
| Time to VH score = 0 | ||||
| Cumulative response rate | NR | NR | NR; p < 0.001 | |
Time to a VH score of zero was measured from day 0 (day of implantation) to the first event of VH of zero. Time points considered included weeks 3, 6 and 8 and any unplanned visit or early exit from study before week 8. A decrease in VH score to zero occurred earlier and was of a greater magnitude in effect in patients in the DEX 700 group than in those in the sham arm (p < 0.001; see Table 16). 48
The VH score for each study eye was assessed at each study visit. 48 Mean VH scores were significantly lower in the DEX 700 group than in the sham group at week 8 (–0.97; p < 0.001) and week 26 (–0.58; p < 0.001). The proportion of patients with an improvement in VH score of ≥ 1 unit was significantly greater in the DEX 700 group than in the sham group (p < 0.001 throughout the study), as was the proportion with an improvement of ≥ 2 units (DEX 700 vs. sham: week 3, p = 0.023; weeks 6–26, p ≤ 0.002).
Anterior chamber cell grade
In the VISUAL I trial,46,52,55 AC cell grade (see Table 6 for criteria) worsened to a greater extent in the placebo group than in the ADA group (MD –0.29; 95% CI –0.51 to –0.07; p = 0.011). In the VISUAL II trial,47,55,72 no significant difference in worsening of AC cell grade was noted between patients in the ADA group and patients in the placebo arm (MD –0.14; 95% CI –0.37 to 0.08; p = 0.218; Table 17).
| Outcome | Study | |||||||
|---|---|---|---|---|---|---|---|---|
| VISUAL I46,52,55 (active uveitis) | VISUAL II47,55,72 (inactive uveitis) | |||||||
| ADA | Placebo | ADA | Placebo | |||||
| Left eye (n = 101) | Right eye (n = 101) | Left eye (n = 102) | Right eye (n = 102) | Left eye (n = 115) | Right eye (n = 115) | Left eye (n = 110) | Right eye (n = 110) | |
| Mean (SD) change in AC cell grade | ||||||||
| VISUAL I: from best state reached prior to week 6 to final or early termination | 0.35 (NR) | 0.36 (NR) | 0.59 (NR) | 0.69 (NR) | NA | NA | NA | NA |
| VISUAL II: from baseline to final or early termination | NA | NA | NA | NA | 0.41 (NR) | 0.40 (NR) | 0.57 (NR) | 0.53 (NR) |
| MD (95% CI) in change in AC cell grade between groups (pooled across left and right eyes) | –0.29 (–0.51 to –0.07); p = 0.011 | –0.14 (–0.37 to 0.08); p = 0.218 | ||||||
In the HURON trial,48 the difference in the percentage of patients with ≥ 1 cell in the AC was statistically significant between the DEX 700 group and the sham group (14.5% vs. 38.7%; p = 0.002 between all three groups).
In the VISUAL trials,46,47 intraocular inflammation (assessed by VH grade and AC cell grade) was used to define disease quiescence and steroid-free quiescence as follows:73
-
disease quiescence:
-
no new active inflammatory lesions
-
AC cell grade of ≤ 0.5
-
VH grade of ≤ 0.5+
-
-
steroid-free quiescence (when not receiving steroid therapy):
-
no active inflammatory lesions
-
AC cell grade of zero
-
VH grade of zero.
-
In both studies, a statistically significant higher proportion (p-values not available) of patients in the ADA group were reported to have experienced disease quiescence and steroid-free quiescence at all assessment time points except for weeks 6 and 12 in the VISUAL I trial46 and week 16 in the VISUAL II trial. 47
Macular oedema
Measures of macular oedema were reported in terms of change in central macular thickness for patients with macular oedema at baseline and time to optical coherence tomography evidence of macular oedema for patients who developed the condition during the studies.
In the VISUAL trials,46,47 ADA did not significantly reduce the time to optical coherence tomography evidence of macular oedema in patients with active uveitis (HR 0.70, 95% CI 0.39 to 1.26; p = 0.231) or in patients with inactive uveitis (HR 0.75, 95% CI 0.34 to 1.69; p = 0.491). Additional post hoc analyses presented by the company for patients without a macular hole and/or retinal detachment in the VISUAL I trial showed that ADA resulted in statistically significant reductions in time to optical coherence tomography evidence of macular oedema in at least one eye at or after week 6 (HR 0.33, 95% CI 0.12 to 0.90; p = 0.023).
There was a significant difference between the groups in the percentage change in central macular thickness for patients with active uveitis (MD –11.4%, 95% CI –20.9% to –1.8%; p = 0.020)46 but not for those with inactive uveitis (MD –2.3%, 95% CI –8.5% to 3.8%; p = 0.451)47 (Table 18).
| Outcome | Study | |||||||
|---|---|---|---|---|---|---|---|---|
| VISUAL I46 (active uveitis)a | VISUAL II47 (inactive uveitis) | |||||||
| ADA (n = 101) | Placebo (n = 102) | ADA (n = 114) | Placebo (n = 107) | |||||
| Left eye | Right eye | Left eye | Right eye | Left eye | Right eye | Left eye | Right eye | |
| Percentage change in macular thickness, mean (SD) | 9.6 (29.8) | 8.2 (25.8) | 20.2 (52.0) | 22.0 (62.5) | 4.5 (29.8) | 5.4 (34.8) | 6.4 (20.7) | 7.7 (28.9) |
| MD (95% CI) (pooled across left and right eyes) | –11.4 (–20.9 to –1.8); p = 0.02 | –2.3 (–8.5 to 3.8); p = 0.451 | ||||||
| Outcome | ADA (n = 55) | Placebo (n = 45) | ADA (n = 90) | Placebo (n = 95) | ||||
| Median time to macular oedema in one or more eyes, months (IQR) | ||||||||
| Time frame: at or after week 6 | 11.1 (2.6–15.9) | 6.2 (1.4 to not estimable) | NA | NA | ||||
| Time frame: at or after week 2 | NA | NA | Not estimableb | Not estimableb | ||||
| MD (95% CI) | 0.70 (0.39 to 1.26); p = 0.23 | 0.75 (0.34 to 1.69); p = 0.49 | ||||||
| Outcome | HURON48 | |||||||
| DEX 700 (n = 39) | Sham (n = 43) | |||||||
| Decrease in macular thickness (µm), mean (SD) | ||||||||
| Week 8 | –99.4 (151.8) | –12.4 (123.7) | ||||||
| Week 26 | –50.2 (102.9) | –35.5 (134.9) | ||||||
| Week 8: MD (95% CI) (µm) | –87.0 (–147 to –27); p = 0.004 | |||||||
| Week 26: MD (95% CI) (µm) | –14.7 (–66 to 37); p = 0.58 | |||||||
Central macular thickness was assessed by optical coherence tomography at a number of study sites in the HURON trial. 48 The MD in decrease in central macular thickness between patients in the DEX 700 group and patients in the sham group was statistically significant at week 8 (MD –87.0 µm, 95% CI –147 to –27 µm; p = 0.004) but not at week 26 (MD –14.7 µm, 95% CI –66 to 37 µm; p = 0.58) (see Table 18).
The incidence of macular oedema is discussed further in Safety of the included interventions.
Effectiveness data from non-randomised studies of dexamethasone and adalimumab
Dexamethasone studies
A summary of effectiveness data from 11 non-randomised, non-comparative studies of the DEX 700 implant is shown in Appendix 5. 18,50,51,79–86 This is based on data from the company submission for DEX;43 the original study publications have not been examined because of time constraints. These data are included here as they provide some data on repeat implants, implants in both eyes and corticosteroid reduction, which were not assessed in the HURON trial. 48 Non-randomised study data for ADA are not included here as they were not provided in the company submission and it was beyond the scope of this assessment to undertake a de novo review of such data.
Following a single implant, two studies reported significant improvements in BCVA at 2–3 months, with a return to baseline values by 6 months,18,82 and significant improvements in VH up to 6 months,18,82 with a return to baseline by 12 months in the study with a longer follow-up. 18 Significant improvements in central retinal thickness were reported in one study up to 6 months after a single implant82 and in another study up to 3 months, with a return to baseline by 6 months. 83
Studies in which patients received between one and four implants reported improvements in BCVA at 12 months,50,83,86 with the improvements reported to be significant in one study. 50 Among studies with patients having a mix of single or multiple implants and macular oedema, significant improvements in central retinal thickness were reported up to 12 months in one study,50 with another study reporting significant improvements at 3 months but not at 6 months. 85
In terms of repeat implants, one study reported that after the second implant BCVA improved significantly by 1 month but then decreased, with a similar trend following up to six implants (not significant but small patient numbers). 18 Central retinal thickness and VH also showed a significant temporary improvement after the second implant, with similar (non-significant) improvements after the third and fourth implants. 18 Another study reported that the improvements in BCVA and central retinal thickness at 1 month were similar (not significantly different) following the first and second implants. 84
In a study of uveitis patients the median time from first to second implant was 10 months50 whereas in four studies of uveitic macular oedema the mean/median time from first to second implant was 4.7,81 5.0,51 7.183 and 1086 months. The mean time from second to third implant was 3.4 months in one study of uveitic macular oedema. 81
Implants in both eyes were assessed in one study, with 3 out of 11 patients (27%) receiving implants in both eyes having a response (reduced central retinal thickness and improved BCVA) in the second eye. 18
In terms of reduction in other therapies following a single implant, one study reported that 21 out of 27 patients (78%) reduced or stopped systemic or local treatment,18 whereas in another study 3 out of 12 patients (25%) reduced their corticosteroid dose79 and in another study systemic corticosteroids were reduced or discontinued in 14 out of 32 patients (44%) and discontinued in 8 out of 32 patients (25%) at 6 months. 82 Among studies using a mix of single or multiple implants, in one study systemic corticosteroids or immunosuppressants were reduced in 62% of patients and steroids were discontinued in 36% of patients at 12 months50 and in another study systemic corticosteroids were reduced or discontinued in 78% of patients and discontinued in 32% of patients at 12 months. 51
Adalimumab studies
Non-RCT data were presented in the company submission72 and these were based on a retrospective audit presented in the Clinical Commissioning Policy for anti-TNF treatment options for adult patients with severe refractory uveitis. 87 The study evaluated data for patients aged > 18 years with different clinical forms of uveitis receiving ADA (40 mg every other week) or infliximab (3–5 mg/kg every other week). The main findings of the audit were as follows:
-
Clinical remission of uveitis was observed in all patients (n = 41) on biologics [mean ± standard deviation (SD) follow-up of 1.36 ± 0.88 person-years].
-
In total, 48.78% of patients experienced visual acuity improvement (mean ± SD follow-up of 2.51 ± 2.01 person-years).
-
Fewer patients (17.07%) had worsening of visual acuity (mean ± SD follow-up of 4.38 ± 3.50 person-years).
-
Patients receiving biologics, in due course, required fewer or reduced concomitant treatments:
-
88.89% of patients showed a reduction in steroid dose to ≤ 10 mg (mean ± SD follow-up of 3.06 ± 2.32 person-years)
-
75.85% of patients showed a reduction in steroid dose to ≤ 5 mg (mean ± SD follow-up of 3.15 ± 1.76 person-years)
-
45.16% of patients discontinued steroid treatment (mean ± SD follow-up of 3.49 ± 1.59 person-years)
-
83.33% of patients showed a reduction in the number and/or frequency of immunomodulatory therapy (mean ± SD follow-up of 1.54 ± 0.99 person-years).
-
-
Patient-reported outcomes reported in the audit87 are summarised as follows:
-
A significant decrease in vision-related quality of life [Vision Core Measure (VCM) scale88] was directly associated with a decrease in visual acuity in the worse eye within 1 year of starting biologics (p = 0.0064).
-
Median vision-related VCM scores decreased with increasing follow-up time from the time of starting treatment with biologics.
-
Mean SF-36 physical component summary scores (< 47) were lower than estimates for the general population. However, the SF-36 mental component summary scores (> 47) were higher than estimates for the general population, with the exception of scores obtained at year 3 (duration of audit period not reported).
-
Safety of the included interventions
Safety information from Summaries of Product Characteristics
The SmPC for the DEX implant45 states that the most commonly reported AEs are those frequently observed with ophthalmic steroid treatment or intravitreal injections, including elevated IOP, cataract and conjunctival or vitreal haemorrhage. Less frequently reported but more serious adverse reactions include endophthalmitis (severe eye infection), necrotising retinitis (viral infection of the retina), retinal detachment and retinal tear. 45
The SmPC for ADA44 summarises AEs from studies of 9506 patients across a range of conditions. The SmPC states that the most commonly reported AEs are infections (such as nasopharyngitis, upper respiratory tract infection and sinusitis), injection site reactions (erythema, itching, haemorrhage, pain or swelling), headache and musculoskeletal pain. TNF antagonists such as ADA affect the immune system and their use may affect the body’s defence against infection and cancer. Fatal and life-threatening infections (including sepsis, opportunistic infections and tuberculosis), hepatitis B virus reactivation and various malignancies (including leukaemia, lymphoma and hepatosplenic T-cell lymphoma) have also been reported with use of ADA. Serious haematological, neurological and autoimmune reactions have also been reported, including rare reports of pancytopenia, aplastic anaemia, central and peripheral demyelinating events and lupus, lupus-related conditions and Stevens–Johnson syndrome. 44
Safety data from pivotal randomised controlled trials
Safety data from the RCTs are based on the published journal articles for the HURON trial48 and VISUAL I46 and II47 trials, the company submissions43,55 and the clinical study reports. 52,71,72 In the case of the HURON trial the safety data are based on all patients who were randomised to a group and received treatment [n = 76/77 (99%) for the DEX 700 group and n = 75/76 (99%) for the sham group]. Within the 26-week trial, the mean exposure to the intervention was 25.9 weeks in both groups. For the two RCTs of ADA compared with placebo, safety data are based on all randomised patients in both trials [n = 111 (100%, ADA) and 112 (100%, placebo) in VISUAL I and n = 115 (100%, ADA) and 114 (100%, placebo) in VISUAL II]. It should be noted that, in these trials, exposure to ADA was longer than exposure to placebo because treatment failure (and cessation of study treatment) occurred earlier in the placebo groups (median exposure: VISUAL I – 19 weeks ADA vs. 13 weeks placebo; VISUAL II – 35 weeks ADA vs. 22 weeks placebo). Therefore, one might expect more events in the ADA groups than in the placebo groups.
A summary of AEs is provided in Table 19. An AE of any type occurred in 80% of patients in the DEX 700 group compared with 68% in the sham group in the HURON trial48 and in 85–91% of patients in the ADA group compared with 79–84% of patients in the placebo group in the two VISUAL trials. 46,47 Serious AEs (SAEs) occurred in 9% of patients in the DEX 700 group compared with 6.7% of the sham group in the HURON trial48 and in 6–14% of patients in the ADA group compared with 5–8% of patients in the placebo group in the VISUAL trials. 46,70 There were no deaths in the HURON trial48 and one death in the ADA arm in each of the VISUAL trials;46,70 neither death was considered to be treatment related.
| AE summary | Study | |||||
|---|---|---|---|---|---|---|
| HURON48 (active uveitis) | VISUAL I46 (active uveitis) | VISUAL II47 (inactive uveitis) | ||||
| DEX 700 | Sham | ADA | Placebo | ADA | Placebo | |
| Time over which AEs were measured (weeks) | 26 (mean 25.9) | 26 (mean 25.9) | ≤ 80 (median 19) | ≤ 80 (median 13) | ≤ 80 (median 35) | ≤ 80 (median 22) |
| AEs (all), n/N (%) | 61/76 (80.3) | 51/75 (68.0) | 94/111 (84.7) | 88/112 (78.6) | 105/115 (91.3) | 96/114 (84.2) |
| AEs considered possibly treatment related, n/N (%) | 46/76 (60.5) | 21/75 (28.0) | ADA related: 45/111 (40.5) | ADA related: 35/112 (31.3) | ADA related: 64/115 (55.7) | ADA related: 52/114 (45.6) |
| Steroid related: 57/111 (51.4) | Steroid related: 53/112 (47.3) | Steroid related: 50/115 (43.5) | Steroid related: 48/114 (42.1) | |||
| SAEs, n/N (%) | 7/76 (9.2) | 6/75 (8.0) | 15/111 (13.5) | 5/112 (4.5) | 7/115 (6.1) | 9/114 (7.9) |
| SAEs considered possibly treatment related, n/N (%) | NR | NR | ADA related: 6/111 (5.4) | ADA related: 2/112 (1.8) | ADA related: 2/115 (1.7) | ADA related: 2/114 (1.8) |
| Steroid related: 2/111 (1.8) | Steroid related: 2/112 (1.8) | Steroid related: 0/115 (0) | Steroid related: 3/114 (2.6) | |||
| Discontinuation because of AEs, n/N (%) | 2/76 (2.6) | 0/75 (0) | 11/111 (9.9) | 4/112 (3.6) | 10/115 (8.7) | 7/114 (6.1) |
Systemic adverse events
Serious systemic AEs are shown in Table 20. Table 21 lists other systemic AEs that (1) occurred in ≥ 5% of patients in any treatment group in the HURON trial,48 (2) occurred in ≥ 5% of patients in the ADA groups in the VISUAL trials46,89 and (3) were noted as potentially important within uveitis treatments by clinical advisors to the AG. No reported systemic AEs (serious or non-serious) had a substantially higher rate in the DEX 700 arm than in the sham arm. The rate of serious infections was higher in the ADA group than the placebo group in the VISUAL I trial46 (4.5% vs. 1.8%) but not the VISUAL II trial47 (1.7% vs. 1.8%). Malignancies and chronic renal failure each occurred in a total of three patients across the ADA arms of both VISUAL trials but in no patients in the placebo arms. The majority of the listed systemic AEs had a somewhat higher rate in the ADA arms than the placebo arms.
| AE | Study, n/N (%) | |||||
|---|---|---|---|---|---|---|
| HURON48 (active uveitis) | VISUAL I46 (active uveitis) | VISUAL II47 (inactive uveitis) | ||||
| DEX 700 | Sham | ADA | Placebo | ADA | Placebo | |
| Deaths | 0/76 (0) | 0/75 (0) | 1/111 (0.9) (not treatment related) | 0/112 (0) | 1/115 (0.9) (not treatment related) | 0/114 (0) |
| Hospitalisation | NR | NR | NR | NR | NR | NR |
| Infections (serious) | NR | NR | 5/111 (4.5) | 2/112 (1.8) | 2/115 (1.7) | 2/114 (1.8) |
| Tumours/malignancy | NR | NR | 2/111 (1.8) | 0/112 (0) | 1/115 (0.9) | 0/114 (0) |
| Anaphylactic reaction | NR | NR | 1/111 (0.9) | 0/112 (0) | NR | NR |
| Demyelinating disease | NR | NR | 1/111 (0.9) | 0/112 (0) | 0/115 (0) | 0/114 (0) |
| Renal failure, chronic | NR | NR | 1/111 (0.9) | 0/112 (0) | 2/115 (1.7) | 0/114 (0) |
| Accidental overdose | NR | NR | 1/111 (0.9) | 0/112 (0) | NR | NR |
| Ligament/tendon rupture | NR | NR | 1/111 (0.9) | 0/112 (0) | NR | NR |
| Fracture | NR | NR | 0/111 (0) | 1/112 (0.9) | 1/115 (0.9) | 1/114 (0.9) |
| Hepatitis, acute | NR | NR | 0/111 (0) | 1/112 (0.9) | NR | NR |
| Abortion, induced | NR | NR | 0/111 (0) | 1/112 (0.9) | NR | NR |
| Neutropenia | NR | NR | NR | NR | 1/115 (0.9) | 0/114 (0) |
| Dysphagia (difficulty swallowing) | NR | NR | NR | NR | 1/115 (0.9) | 0/114 (0) |
| Dysarthria (unclear speech) | NR | NR | NR | NR | 1/115 (0.9) | 0/114 (0) |
| Status migrainosus | NR | NR | NR | NR | 1/115 (0.9) | 0/114 (0) |
| Epistaxis (nosebleed) | NR | NR | NR | NR | 1/115 (0.9) | 0/114 (0) |
| Pleurisy | NR | NR | NR | NR | 1/115 (0.9) | 0/114 (0) |
| Cardiac tamponade | NR | NR | NR | NR | 1/115 (0.9) | 0/114 (0) |
| Aortic dissection | NR | NR | NR | NR | 1/115 (0.9) | 0/114 (0) |
| Deep-vein thrombosis | NR | NR | NR | NR | 0/115 (0) | 2/114 (1.8) |
| Hypertensive crisis | NR | NR | NR | NR | 0/115 (0) | 1/114 (0.9) |
| Arthritis | NR | NR | NR | NR | 0/115 (0) | 1/114 (0.9) |
| Cerebrovascular accident | 1/76 (1.3) | 0/75 (0) | NR | NR | NR | NR |
| Pelvic inflammatory disease | 1/76 (1.3) | 0/75 (0) | NR | NR | NR | NR |
| Cerebellar infarction | 1/76 (1.3) | 0/75 (0) | NR | NR | NR | NR |
| Pyelonephritis | 0/76 (0) | 1/75 (1.3) | NR | NR | NR | NR |
| Ankylosing spondylitis | 0/76 (0) | 1/75 (1.3) | NR | NR | NR | NR |
| AE | Study, n/N (%) | |||||
|---|---|---|---|---|---|---|
| HURON48 (active uveitis) | VISUAL I46 (active uveitis) | VISUAL II47 (inactive uveitis) | ||||
| DEX 700 | Sham | ADA | Placebo | ADA | Placebo | |
| Systemic AEs (≥ 5% in any group for the HURON trial or ≥ 5% in the treatment group in the VISUAL trials) | ||||||
| Nasopharyngitis | NR | NR | 21/111 (18.9) | 8/112 (7.1) | 18/115 (15.7) | 19/114 (16.7) |
| Headache | 5/76 (6.6) | 5/75 (6.7) | 12/111 (10.8) | 15/112 (13.4) | 17/115 (14.8) | 17/114 (14.9) |
| Fatigue | 0/76 (0) | 2/75 (2.7) | 12/111 (10.8) | 7/112 (6.3) | 14/115 (12.2) | 9/114 (7.9) |
| Arthralgia (joint pain) | 0/76 (0) | 2/75 (2.7) | 10/111 (9.0) | 11/112 (9.8) | 27/115 (23.5) | 12/114 (10.5) |
| Back pain | NR | NR | 9/111 (8.1) | 2/112 (1.8) | 9/115 (7.8) | 7/114 (6.1) |
| Injection site reactions | NR | NR | 7/111 (6.3) | 7/112 (6.3) | 23/115 (20.0) | 15/114 (13.2) |
| Urinary tract infection | NR | NR | 7/111 (6.3) | 0/112 (0) | 13/115 (11.3) | 10/114 (8.8) |
| Cough | NR | NR | 7/111 (6.3) | 4/112 (3.6) | 11/115 (9.6) | 6/114 (5.3) |
| Bronchitis | NR | NR | 7/111 (6.3) | 4/112 (3.6) | NR | NR |
| Hyperhidrosis (increased sweating) | NR | NR | 7/111 (6.3) | 3/112 (2.7) | NR | NR |
| Muscle spasms | NR | NR | 7/111 (6.3) | 4/112 (3.6) | NR | NR |
| Nausea | 0/76 (0) | 4/75 (5.3) | 6/111 (5.4) | 7/112 (6.3) | 2/115 (1.7) | 3/114 (2.6) |
| Paraesthesia (‘pins + needles’) | NR | NR | 6/111 (5.4) | 0/112 (0) | NR | NR |
| Insomnia | NR | NR | 5/111 (4.5) | 8/112 (7.1) | 8/115 (7.0) | 3/114 (2.6) |
| Myalgia (muscle pain) | NR | NR | 5/111 (4.5) | 2/112 (1.8) | 6/115 (5.2) | 2/114 (1.8) |
| Hypertension | 2/76 (2.6) | 3/75 (4.0) | 4/111 (3.6) | 1/112 (0.9) | 7/115 (6.1) | 5/114 (4.4) |
| Liver changes: elevated alanine aminotransferase | NR | NR | 1/111 (0.9) | 2/112 (1.8) | 8/115 (7.0) | 1/114 (0.9) |
| Liver changes: elevated aspartate aminotransferase levels | NR | NR | 1/111 (0.9) | 1/112 (0.9) | 6/115 (5.2) | 1/114 (0.9) |
| Pain in extremity | NR | NR | NR | NR | 10/115 (8.7) | 3/114 (2.6) |
| Upper respiratory tract infection | NR | NR | NR | NR | 10/115 (8.7) | 3/114 (2.6) |
| Injection site pain | NR | NR | NR | NR | 8/115 (7.0) | 9/114 (7.9) |
| Sinusitis | NR | NR | NR | NR | 8/115 (7.0) | 4/114 (3.5) |
| Additional systemic AEs (noted as potentially important by clinical advisors) | ||||||
| Anxiety | NR | NR | 5/111 (4.5) | 0/112 (0) | 5/115 (4.3) | 2/114 (1.8) |
| Renal: elevated creatinine | NR | NR | 4/111 (3.6) | 2/112 (1.8) | 2/115 (1.7) | 3/114 (2.6) |
| Weight gain | NR | NR | 3/111 (2.7) | 2/112 (1.8) | 2/115 (1.7) | 0/114 (0) |
| Anaemia | NR | NR | 3/111 (2.7) | 0/112 (0) | 0/115 (0) | 2/114 (1.8) |
| Muscle weakness (myasthenia) | NR | NR | 3/111 (2.7) | 0/112 (0) | NR | NR |
| Cushing syndrome | NR | NR | 2/111 (1.8) | 1/112 (0.9) | 1/115 (0.9) | 0/114 (0) |
| Depression | NR | NR | 1/111 (0.9) | 1/112 (0.9) | 2/115 (1.7) | 3/114 (2.6) |
| Diabetes | NR | NR | 1/111 (0.9) | 2/112 (1.8) | 2/115 (1.7) | 0/114 (0) |
| Osteoporosis | NR | NR | 1/111 (0.9) | 1/112 (0.9) | 0/115 (0) | 2/114 (1.8) |
Immunogenicity
In the VISUAL I trial,46 anti-ADA antibodies were detected in 3 out of 110 patients (2.7%) in the ADA group. These three patients had treatment failure at 16, 44 and 48 weeks (compared with a median time to treatment failure of 24 weeks among the remaining 107 patients). In the VISUAL II trial,47 anti-ADA antibodies were detected in 6 out of 115 patients (5.2%) in the ADA group. Five of these six patients had treatment failure at weeks 13, 16, 16, 24 and 31 (not estimable for the remaining patient).
Ocular adverse events
Ocular AEs are shown in Table 22. In terms of serious ocular AEs, endophthalmitis (severe eye infection) and severe uveitis worsening occurred in one patient each in the DEX 700 group and in no patients in the sham group in the HURON trial. 48 Conjunctival haemorrhage occurred in 30% of patients in the DEX 700 group and 21% of patients in the sham group in the HURON trial,48 whereas rates for this AE were low in the VISUAL trials. 46,47
| AE | Study, n/N (%) | |||||
|---|---|---|---|---|---|---|
| HURON48 (active uveitis) | VISUAL I46 (active uveitis) | VISUAL II47 (inactive uveitis) | ||||
| DEX 700 | Sham | ADA | Placebo | ADA | Placebo | |
| Serious ocular AEs in the study eyea (all reported in trials) | ||||||
| Retinal detachment | 2/76 (2.6) | 2/75 (2.7) | 1/111 (0.9) | 1/112 (0.9) | 0/115 (0) | 1/114 (0.9) |
| Endophthalmitis (severe eye infection) | 1/76 (1.3) | 0/75 (0) | NR | NR | NR | NR |
| Uveitis worsening (as SAE) | 1/76 (1.3) | 0/75 (0) | NR | NR | NR | NR |
| Cataract (as SAE) | 0/76 (0) | 1/75 (1.3) | NR | NR | NR | NR |
| Choroidal neovascularisation | NR | NR | 1/111 (0.9) | 0/112 (0) | 0/115 (0) | 1/114 (0.9) |
| Transient blindness | NR | NR | NR | NR | 1/115 (0.9) | 0/114 (0) |
| Subretinal fluid | NR | NR | NR | NR | 0/115 (0) | 1/114 (0.9) |
| Ocular AEs in the study eyea (≥ 5% in any group for the HURON trial and ≥ 5% in the treatment group for the VISUAL trials) | ||||||
| Raised IOP | 19/76 (25.0) | 5/75 (6.7) | 3/111 (2.7) | 2/112 (1.8) | 3/115 (2.6) | 2/114 (1.8) |
| ≥ 25 mmHg | Week 3: 5/70 (7.1) | Week 3: 1/70 (1.4) | NR | NR | NR | NR |
| Week 8: 3/73 (4.1) | Week 8: 0/71 (0) | |||||
| Week 26: 0/74 (0) | Week 26: 3/72 (4.2) | |||||
| ≥ 35 mmHg | Week 3: 1/70 (1.4) | Week 3: 0/70 (0) | NR | NR | NR | NR |
| Week 8: 2/73 (2.7) | Week 8: 0/71 (0) | |||||
| Week 26: 0/74 (0) | Week 26: 0/72 (0) | |||||
| Conjunctival haemorrhage | 23/76 (30.3) | 16/75 (21.3) | 0/111 (0) | 1/112 (0.9) | 3/115 (2.6) | 2/114 (1.8) |
| Vitreous haemorrhage | NR | NR | Eye haemorrhage: 1/111 (0.9) | Eye haemorrhage: 0/112 (0) | 1/115 (0.9) | 0/114 (0) |
| Retinal haemorrhage: 1/111 (0.9) | Retinal haemorrhage: 2/112 (1.8) | |||||
| Ocular discomfort | 10/76 (13.2) | 6/75 (8.0) | NR | NR | NR | NR |
| Eye pain | 9/76 (11.8) | 10/75 (13.3) | 9/111 (8.1) | 2/112 (1.8) | 9/115 (7.8) | 6/114 (5.3) |
| Cataract | ||||||
| All patients | 9/76 (11.8) | 4/75 (5.3) | 4/111 (3.6) | 2/112 (1.8) | 2/115 (1.7) | 6/114 (5.3) |
| Phakic eyes at baseline | 9/62 (14.5) | 4/55 (7.3) | NR | NR | NR | NR |
| Phakic eyes with no cataract at baseline | 9/42 (21.4) | 4/28 (14.3) | NR | NR | NR | NR |
| Iridocyclitis | 7/76 (9.2) | 4/75 (5.3) | 1/111 (0.9) | 0/112 (0) | 3/115 (2.6) | 2/114 (1.8) |
| Ocular hypertension | 6/76 (7.9) | 0/75 (0) | 3/111 (2.7) | 1/112 (0.9) | 0/115 (0) | 2/114 (1.8) |
| Myodesopsia (floaters or vitreal cells) | 6/76 (7.9) | 5/75 (6.7) | NR | NR | NR | NR |
| Uveitis/uveitis worsening | 6/76 (7.9) | 7/75 (9.3) | 11/111 (9.9) | 8/112 (7.1) | 6/115 (5.2) | 9/114 (7.9) |
| Conjunctival hyperaemia (red eye) | 5/76 (6.6) | 7/75 (9.3) | NR | NR | NR | NR |
| Vision blurred | 5/76 (6.6) | 3/75 (4.0) | 8/111 (7.2) | 2/112 (1.8) | NR | NR |
| Macular oedema | 3/76 (3.9) | 6/75 (8.0) | NR | NR | 7/115 (6.1) | 7/114 (6.1) |
| Eye pruritus (itching) | 3/76 (3.9) | 5/75 (6.7) | NR | NR | NR | NR |
| Visual acuity reduced | 1/76 (1.3) | 4/75 (5.3%) | NR | NR | 6/115 (5.2) | 10/114 (8.8) |
| Eye swelling | 1/76 (1.3) | 4/75 (5.3) | NR | NR | NR | NR |
| Conjunctivitis | 0/76 (0) | 4/75 (5.3) | NR | NR | NR | NR |
| Additional ocular AEs in the study eyea (noted as potentially important by clinical advisors) | ||||||
| Cataract surgery | ||||||
| All patients | 1/76 (1.3) | 2/75 (2.7) | NR | NR | 1/115 (0.9) | 2/114 (1.8) |
| Phakic eyes at baseline | 1/62 (1.6) | 2/55 (3.6) | NR | NR | NR | NR |
| Phakic eyes with no cataract at baseline | 1/42 (2.4) | 2/28 (7.1) | NR | NR | NR | NR |
| IOP-lowering medications | Up to 16/71 (23) at any single time point | NR, presumed 0% | NR | NR | NR | NR |
| IOP-lowering surgery | ||||||
| Incisional surgery, laser trabeculoplasty, cryotherapy | 0/76 (0) | 0/75 (0) | NR | NR | NR | NR |
| Laser iridotomy | 2/76 (2.6) | 0/75 (0) | NR | NR | NR | NR |
| Glaucoma | 0/76 (0) | 2/75 (2.7) | 1/111 (0.9) | 0/112 (0) | NR | NR |
| Low IOP (hypotony) | 1/76 (1.3) | 0/75 (0) | NR | NR | NR | NR |
Raised IOP occurred in 25% of the DEX 700 group compared with 7% of the sham group in the HURON trial,48 whereas there was little difference between the ADA group and the placebo group in the VISUAL trials. 46,47 In the DEX 700 group, IOP of ≥ 25 mmHg peaked at week 3 (7.1% vs. 1.4% in the sham group), whereas IOP of ≥ 35 mmHg peaked at week 12 (4.1% vs. 0% in the sham group). By week 26, no patients in the DEX 700 group had an IOP of ≥ 25 mmHg whereas 4.2% of patients in the sham group had an IOP of ≥ 25 mmHg.
Glaucoma rates showed little difference between the DEX 700 group (0%) and the sham group (2.7%) in the HURON trial48 or between the ADA group (0.9%) and the placebo group (0%) in the VISUAL I trial. 46 In the HURON trial48 no patients required incisional surgery for glaucoma, whereas two patients (2.6%) in the DEX 700 group required laser iridotomy in the study eye for iris bombe and raised IOP. At any single time point across the 26 weeks, up to 23% of patients in the DEX 700 group required IOP-lowering medication (the percentage requiring this at any point in the study is not reported).
In the HURON trial,48 9 out of 62 eyes (15%) in the DEX 700 group and 4 out of 55 eyes (7%) in the sham group had cataracts in eyes that were phakic (had a natural lens) at baseline. Among phakic eyes with no cataract at baseline, 9 out of 42 eyes (21%) in the DEX 700 group and 4 out of 28 eyes (14%) in the sham group had cataracts. For the ADA group, no data were reported on whether eyes were phakic or had cataract at baseline; among all patients there were more cataracts in the ADA group than in the placebo group in the VISUAL I trial46 (3.6% vs. 1.8%) but more in the placebo group than in the ADA group in the VISUAL II trial47 (1.7% vs. 5.3%). Cataract surgery among phakic eyes occurred in 1 out of 62 patients (1.6%) in the DEX 700 group and 2 out of 55 patients (3.6%) in the sham group; in the VISUAL II trial47 cataract surgery occurred in one patient in the ADA group and two patients in the placebo group.
Safety data from non-randomised studies of dexamethasone
A summary of safety data from 11 non-randomised, non-comparative studies of the DEX implant is shown in Appendix 6. 18,50,51,79–86 This is based on data presented within the company submission for DEX. 43 The proportion of patients with an increased IOP is typically higher in real-world studies than in a RCT, which may reflect the inclusion of patients with a prior need for IOP-lowering medications, who were excluded from the HURON trial. 43 Implant migration to the AC has been reported in a few patients and occurred in eyes that were aphakic (no lens) or pseudophakic (artificial lens). 43 A few cases of endophthalmitis or retinal detachment were reported after administration of DEX 700. 43 Non-randomised studies of ADA are not included here as they were not included in the company submission and it was beyond the scope of this assessment to undertake a de novo review of these data.
Ongoing studies
Ongoing studies relevant to the decision problem are shown in Table 23. These were identified through a search of the ClinicalTrials.gov database (using terms for uveitis plus ADA or DEX) and from the DEX company submission. 43
| Study name/company | Type and estimated number of participants | Population | Interventions | Key outcomes | Follow-up | Start and end dates | Reference |
|---|---|---|---|---|---|---|---|
| DEX 700 | |||||||
| PeriOcular and INTravitreal Corticosteroids for Uveitic Macular Edema Trial (POINT); Johns Hopkins Bloomberg School of Public Health (JHSPH) Center for Clinical Trials/NEI | RCT, n = 267 |
|
|
|
8 and 24 weeks | March 2015–July 2018 | ClinicalTrials.gov (NCT02374060) |
| Macular Edema Ranibizumab v. Intravitreal Anti-inflammatory Therapy Trial (MERIT); JHSPH Center for Clinical Trials/NEI | RCT, n = 240 |
|
|
|
8 weeks and 6 months | November 2016–March 2019 | ClinicalTrials.gov (NCT02623426) |
| A Long-Term Safety Study of Ozurdex in Clinical Practice; Allergan | Cohort, n = 875 |
|
|
|
2 years | March 2012–March 2016 (CSR available September 2016a) | ClinicalTrials.gov (NCT01539577) |
| ADA | |||||||
| Adalimumab in Uveitis Refractory to Conventional Therapy (ADUR); Heidelberg University/Abbott | RCT, n = 25 |
|
|
|
Up to 24 weeks | August 2006–March 2013 | ClinicalTrials.gov (NCT00348153); Mackensen 201290 (abstract) |
| Randomized Trial Comparing Efficacy of Adalimumab, Anakinra and Tocilizumab in Non-infectious Refractory Uveitis (RUBI); Assistance Publique – Hôpitaux de Paris | RCT, n = 120 |
|
|
|
16 weeks | October 2016–May 2019 | ClinicalTrials.gov (NCT02929251) |
| Intravitreal Adalimumab Versus Subcutaneous Adalimumab in Non-infectious Uveitis (IVAS) | RCT, n = 32 |
|
|
|
26 weeks | February 2016–June 2019 | ClinicalTrials.gov (NCT02706704) |
| A Study of the Long-term Safety and Efficacy of Adalimumab in Subjects with Intermediate-, Posterior-, or Panuveitis (VISUAL III); AbbVie (previously Abbott) | Non-RCT, n = 400 |
|
|
|
Up to 330 weeks (6.3 years) | November 2010–March 2018 | ClinicalTrials.gov (NCT01148225); Suhler 201691 (abstract) |
Ongoing studies of dexamethasone
Two ongoing RCTs of DEX 700 were identified, both in patients with macular oedema as a result of uveitis. Both compared DEX 700 against other local treatments. The POINT trial [PeriOcular and INTravitreal Corticosteroids for Uveitic Macular Edema Trial (NCT02374060), due to complete in 2018] compares DEX 700 with intravitreal triamcinolone or periocular triamcinolone, whereas the MERIT trial [Macular Edema Ranibizumab v. Intravitreal Anti-inflammatory Therapy Trial (NCT02623426), due to complete in 2019] compares DEX 700 with intravitreal methotrexate or intravitreal ranibizumab. In addition, a long-term safety cohort study of DEX 700 (NCT01539577) in 875 patients with posterior segment-involving uveitis or central or branch retinal vein occlusion was due to complete in March 2016, but no published results were identified.
Ongoing studies of adalimumab
Three ongoing RCTs of ADA were identified. One small RCT [Adalimumab in Uveitis Refractory to Conventional Therapy (ADUR) (NCT00348153)]90 compared ADA plus corticosteroids and immunosuppressants with corticosteroids in combination with immunosuppressants and was due to be completed in March 2013. This study is potentially of interest because of its active comparator arm. However, no published results were identified other than an abstract reporting intermediate results for 20 of 25 patients;90 this was not included in the clinical effectiveness review because of the limited results presented. The two other RCTs of ADA are due to complete in 2019. The RUBI trial [Randomized Trial Comparing Efficacy of Adalimumab, Anakinra and Tocilizumab in Non-infectious Refractory Uveitis (NCT02929251)] aims to compare ADA against two further biological therapies: anakinra (an interleukin-1 receptor antagonist) and tocilizumab (an antibody against the interleukin-6 receptor). The IVAS trial [Intravitreal Adalimumab Versus Subcutaneous Adalimumab in Non-infectious Uveitis (NCT02706704)] aims to compare subcutaneous ADA against intravitreal ADA.
In addition, a non-randomised extension study of ADA [A Study of the Long-term Safety and Efficacy of Adalimumab in Subjects with Intermediate-, Posterior-, or Panuveitis (VISUAL III) (M11-327, NCT01148225)] enrolled patients from the VISUAL I and VISUAL II studies (ADA or placebo arms) who either completed these trials or experienced treatment failure. Patients who discontinued the VISUAL I or II study because of treatment failure were defined as having active disease on entry to the VISUAL III study, whereas patients who completed the VISUAL I or II study were defined as having inactive disease. Patients received open-label ADA (40 mg every other week) and were followed up for 78 weeks (active uveitis patients) or 54 weeks (inactive uveitis patients). The study is due to be completed in 2018. Preliminary data are available from a conference abstract. 91 This states that, of 243 patients with active uveitis after 78 weeks, 96.3% had no new inflammatory lesions relative to week 8, 91.0% had an AC cell grade of ≤ 0.5+ and 87.8% had a VH grade of ≤ 0.5+. Of 128 patients with inactive uveitis after 54 weeks, 98.5% had no new inflammatory lesions relative to baseline, 98.5% had an AC cell grade of ≤ 0.5+ and 92.6% had a VH grade of ≤ 0.5+. The mean systemic corticosteroid daily dose decreased from 12.7 to 3.68 prednisone equivalents by year 1 for patients with active uveitis and remained stable from 1.48 to 1.21 prednisone equivalents for inactive patients. AE rates were stated to be comparable to those in the VISUAL I and VISUAL II trials but no data were presented in terms of numbers of patients with events. No data were presented for visual acuity or the VFQ-25.
Indirect comparison of treatments: rationale for not undertaking
The decision problem states that relevant comparators include periocular or intravitreal corticosteroid injections, intravitreal corticosteroid implants, systemic corticosteroids, systemic immunosuppressants, TNF-alpha inhibitors and intravitreal methotrexate (see Chapter 2). The trials of DEX 700 and ADA compared these interventions only with placebo/sham procedure. In the absence of direct evidence comparing ADA and DEX 700 and the absence of direct evidence comparing either of these treatments with a comparator reflective of current UK practice, an indirect comparison using a NMA was considered. A NMA allows a simultaneous comparison between interventions based on a synthesis of any direct and indirect evidence about treatment effects across RCTs that share at least one treatment in common with at least one other study.
Consideration of indirect comparisons for all studies of clinically relevant comparators
Randomised controlled trials that included any of the treatments in the comparator decision set for posterior segment-involving uveitis were sought. In addition to the trials of DEX 700 (HURON48) and ADA (VISUAL I46 and II47), 13 additional trials of relevant comparators were identified. 32,33,57–67
Unfortunately, it was considered infeasible and inappropriate to conduct a NMA for the reasons outlined in Table 24. However, a brief summary of all identified trials of relevant comparators is provided in this section for information: study characteristics are provided in Table 25 and a summary of reported outcomes is provided in Table 26. The reasons for not including the additional identified trials in a NMA were:
-
No link to the network containing ADA and DEX 700, that is, no common comparator. This applied to studies of fluocinolone implant,57,58 periocular steroids,60 methotrexate32,67 and mycophenolate mofetil. 32 The use of elicitation of experts’ beliefs to inform the parameters required to link disconnected networks was considered in depth but was not implemented for two reasons. It was deemed to be infeasible in the time frame and, moreover, would be of questionable benefit given the concerns related to the comparability of the two main trials (see Consideration of indirect comparisons for trials of adalimumab and dexamethasone) and hence the validity of the resulting connected network.
-
Heterogeneity in patient populations in terms of active/inactive uveitis. It was not considered appropriate to pool studies of patients with active and inactive uveitis. Active uveitis refers to current inflammation in the eye, whereas patients with inactive uveitis have limited inflammation, usually because they have received treatment with corticosteroids or immunosuppressants. The treatment effect is likely to be related to the degree of activity/inflammation at baseline. The trial of etanercept,61 one trial of ADA (VISUAL II)47 and one trial of voclosporin65,66 could not be analysed with the HURON48 and VISUAL I46 studies for this reason. In terms of trials in patients with inactive uveitis, the trials of etanercept61 and voclosporin65,66 had no comparable outcome data to enable a NMA to be conducted with the VISUAL II trial. 47
-
Heterogeneity in patient populations for other reasons. The trial of intravitreal triamcinolone59 was carried out in patients who all had uveitic macular oedema, whereas in most trials only a subset of patients had uveitic macular oedema. The treatment effect is likely to be associated with the proportion of patients with uveitic macular oedema at baseline because this condition causes vision loss. Therefore, treating uveitic macular oedema is likely to lead to greater gains in vision than treating patients with uveitis but no uveitic macular oedema. The trial of azathioprine62 was carried out in patients who all had Behçet’s disease, whereas most trials were carried out in a mixed population with only a small percentage of patients having Behçet’s disease and other systemic diseases; again, these are clinically very different populations. In addition, as noted in Consideration of indirect comparisons for trials of adalimumab and dexamethasone, there are many differences in the populations and the prior and concomitant treatments used between the DEX 700 (HURON7) and the ADA (VISUAL I46) studies for active uveitis.
-
Lack of comparable outcomes. Within the trials that had a common comparator to DEX 700 or ADA (i.e. a placebo arm),59,61–63,65,66 none reported outcomes that were consistent with those in the DEX 700 and ADA trials (see Table 26). Change in VFQ-25 score was reported for both the HURON trial48 and the VISUAL I trial46 but a NMA was not considered appropriate for the reasons listed in Indirect comparison of treatments: rationale for not undertaking.
| Study | Intervention | Comparator | Reasons for non-inclusion in NMA |
|---|---|---|---|
| HURON48,68 | DEX 700 implant (local steroid) | Placebo (sham) |
|
| VISUAL I46 | ADA (anti-TNF) | Placebo |
|
| VISUAL II47 | ADA (anti-TNF) | Placebo |
|
| Multicenter Uveitis Steroid Treatment Trial Research Group57 | Fluocinolone implant (local steroid) | Steroids and immunosuppressants |
|
| Pavesio 201058 | Fluocinolone implant (local steroid) | Steroids and immunosuppressants |
|
| Shin 201559 | Triamcinolone intravitreal injection (local steroid) | Placebo (sham) |
|
| Ferrante 200060 | Triamcinolone periocular injection (local steroid) | Methylprednisolone periocular injection |
|
| Foster 200361 | Etanercept (anti-TNF) | Placebo |
|
| Yazici 199062 | Azathioprine (immunosuppressant) | Placebo |
|
| Murphy 200533 | Ciclosporin (immunosuppressant) | Tacrolimus |
|
| de Vries 199063 | Ciclosporin (immunosuppressant) | Placebo |
|
| Nussenblatt 199164 | Ciclosporin (immunosuppressant) | Prednisolone |
|
| Bodaghi 2012 (active)65,66 | Voclosporin (immunosuppressant) | Placebo |
|
| Bodaghi 2012 (maintenance)65,66 | Voclosporin (immunosuppressant) | Placebo |
|
| Mackensen 201367 | Methotrexate (immunosuppressant) | Interferon-beta |
|
| Rathinam 200432 | Methotrexate (immunosuppressant) | Mycophenolate mofetil |
|
| Study | Intervention | Comparator | Patients randomised, n | Age (years); mean (range) | Location of uveitis | Duration of uveitis (months) | Bilateral uveitis (%) | % with MO | Systemic conditions | Current inflammation (active, non-active) | Inclusion criteria: VA and inflammation | % prior HD steroids/immunosuppressants | Concomitant treatment | Eyes treated | Eyes analysed | Duration: treatment and follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HURON48,68 | DEX 700 | Placebo (sham) | 153 (DEX 700 + sham) | ≥ 18; 45 (18–82) | Int/post | DEX 700 51, sham 61 | NR | NR | No uncontrolled systemic condition | Active | VH ≥ 1.5, BCVA 10–75 letters | 26% steroids or immunosuppressants | 26% stable dose steroids or immunosuppressants; rescue: local steroids, systemic medications (new or increased) | One (right if bilateral) | Study eye only | Single implant, follow-up 6 months (26 weeks) |
| VISUAL I46 | ADA (40 mg every 2 weeks) | Placebo | 223 | ≥ 18; 43 (18–81) | Int/post/pan | ADA 40, placebo 51 | 91 | Left 36, right 37 | None 73%, sarcoid 8%, Behçet’s disease 7%, VKH 12% | Active | At least one of VH ≥ 2, AC cell grade ≥ 2, inflammatory lesions | 100% HD steroids | All: prednisone 60 mg/day, tapered by week 15; some: immunosuppressants, maximum 1 | NA (systemic) | Left and right separately | Up to 80 weeks (1.5 years); ADA 19 weeks (median), placebo 13 weeks (median) |
| VISUAL II47 | ADA (40 mg every 2 weeks) | Placebo | 229 | ≥ 18; 43 (NR) | Int/post/pan | 61 | 96 | NR | None 56%, sarcoid 16%, Behçet’s disease 6%, other 8% | Inactive (≥ 28 days) | VH ≤ 0.5, AC cell grade ≤ 0.5, no inflammatory lesions, steroid dependent | 100% HD steroids, some immunosuppressants | All: prednisone 10–35 mg/day, tapered by week 19; some: immunosuppressants, maximum 1 | NA (systemic) | Left and right separately | Up to 80 weeks (1.5 years); ADA 35 weeks (median), placebo 22 weeks (median) |
| Multicenter Uveitis Steroid Treatment Trial Research Group57 | Fluocinolone implant (0.59 mg) | Systemic steroids and immunosuppressants | 255 | ≥ 13; 46 (NR) | Int/post/pan | Fluocinolone 47, control 43 | 88 | 41 | None 73%, systemic 27%; none requiring systemic therapy | Active (or recently active) | No VH criteria (some had VH = 0), BCVA = hand motions or better | Some steroids, some immunosuppressants (% NR) | Fluocinolone arm: steroids and immunosuppressants discontinued; control arm: steroids (tapered), immunosuppressants (86%) | Both if bilateral | All uveitic eyes | Repeat if recurs, follow-up 2 years |
| Pavesio 201058 | Fluocinolone implant (0.59 mg) | Systemic steroids and immunosuppressants | 140 | ≥ 6; 42 (12–75) | Int/post/pan | NR | NR | NR | None requiring systemic therapy | Inactive (‘clinically quiet’) | VH ≤ 2, AC cells ≤ 10, visual acuity ≥ 1.4 logMAR (6/150) | 100% HD steroids, some immunosuppressants | Fluocinolone arm: steroids and immunosuppressants discontinued; control arm: HD steroids ± immunosuppressants; rescue: steroids | One (worse if bilateral) | Study eye only | Single implant, follow-up 2 years |
| Shin 201559 | Triamcinolone intravitreal injection | Placebo (sham) | 50 | ≥ 20; 52 (NR) | NR | NR | NR | 100 | None 48%, systemic 52% (sarcoid, Behçet’s disease, VKH) | NR | UMO, BCVA 25–80 ETDRS letters | 100% HD steroids, some immunosuppressants | All: systemic steroids or immunosuppressants and topical steroids | One (worse if bilateral) | Study eye only | Repeat if MO recurs, follow-up 6 months |
| Ferrante 200060 | Triamcinolone periocular injection | Methylprednisolone periocular injection | 36 | NR; NR (NR) | Int/post | NR | NR | NR | NR | Active (vitritis or UMO) | UMO or vitritis | NR | NR | NR (assume one) | NR | Repeat at 6 weeks if needed, follow up 3 months |
| Foster 200361 | Etanercept (25 mg SC twice a week) | Placebo | 20 | ≥ 18; 47 (NR) | NR | NR (6 months MTX) | NR | NR | None 60%, SLE 15%, HLA-B27 15%, arthritis 10% | Inactive, VH ≥ 1.5, BCVA 10–75 letters | NR | 100% MTX (immunosuppressant) | All: MTX (tapered); steroid eye drops if needed | NA (systemic) | Both eyes, all patients | 6 months (24 weeks) |
| Yazici 199062 | Azathioprine (2.5 mg/kg daily) | Placebo | 48 | Any age, 32 (NR) | NR | Azathioprine 103, placebo 83 | 71 | NR | Behçet’s disease 100% | NR | NR | No steroids or immunosuppressants (past month) | Rescue: systemic steroids if required | NA (systemic) | Unclear | 2 years |
| Murphy 200533 | Ciclosporin (2.5–5.0 mg/kg/day) | Tacrolimus (0.03–0.08 mg/kg daily) | 37 | NR; median 43 (NR) | Int/post/pan | 12–24 | 76 | NR | None 70%, Behçet’s disease 11%, sarcoidosis 8% | NR | NR | 100% HD steroids (or as required) | Some: oral steroids only | NA (systemic) | Per patient | 3 months |
| de Vries 199063 | Ciclosporin (10 mg/kg/day) | Placebo | 27 | ≥ 18; 45 (22–75) | Int/post/pan | Ciclosporin 67, placebo 78 | NR | NR | None 74%, Behçet’s disease 15%, sarcoidosis 11% | Active | BCVA ≤ 0.5 in best eye (or Behçet’s or trauma) | 100% HD steroids | All: oral steroids (tapered) | NA (systemic) | Unclear | Up to 1 year |
| Nussenblatt 199164 | Ciclosporin (10 mg/kg/day orally) | Prednisolone (42–64 mg/day orally) | 56 | ≥ 10; 38 (10–61) | Int/post | NR | 100 | 55 | None 82%, sarcoidosis 13%, VKH 5% | Active | VA 20/40 or worse, both eyes; inflammation (VH, VA decrease, retinal lesions) | No steroids or immunosuppressants (past month) | No systemic treatments; topical medications permitted | NA (systemic) | Per patient | 3 months |
| Bodaghi 2012 (active)65,66 | Voclosporin (0.2, 0.4, 0.6 mg/kg b.i.d.) | Placebo | 218 | ≥ 13; median 42 (NR) | Int/post/pan | 52 | NR | NR | NR | Active | VH ≥ 2 | 100% HD steroids (or contraindicated refused) | Some: oral steroids | NA (systemic) | Study eye or either | 6 months (24 weeks) |
| Bodaghi 2012 (maintenance)65,66 | Voclosporin (0.2, 0.4, 0.6 mg/kg b.i.d.) | Placebo | 232 | ≥ 13; median 43 (NR) | Int/post/pan | 52 | NR | NR | NR | Inactive | NR | 100% HD steroids | Some: oral steroids | NA (systemic) | Study eye or either | 6 months (26 weeks) |
| Mackensen 201367 | MTX (20 mg SC weekly) | Interferon-beta (44 µg SC three times weekly) | 19 | ≥ 18; median 42 (NR) | Intermediate | ≥ 1 year | NR | 100 | None 74%, multiple sclerosis 26% | Active | Uveitic macular oedema (≥ 250 um); visual acuity ≤ 20/30 (0.2 logMAR) | 100% HD steroids and acetazolamide | NR | NA (systemic) | Study eye (worse) | 3 months |
| Rathinam 200432 | MTX (25 mg orally weekly) | Mycophenolate mofetil (1 g twice/day) | 80 | ≥ 16; 39 (NR) | Int/post/pan | NR | 81 | 41 | None 35.5%, VKH 54%, Behçet’s disease 8%, sarcoidosis 2.5% | Active | At least one of: VH ≥ 1, AC cell grade ≥ 1, Vitreous cells ≥ 1, Active lesions | 100% HD steroids | All: oral steroids (tapered); some: topical steroid | NA (systemic) | All uveitic eyes | 6 months |
| Study | Intervention | Comparator | VA | Inflammatory activity | Complications | Composite outcomes | HRQoL | AEs | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Final value | Change | % improved three or more lines | % improved two or more lines | VH: final | % VH = 0 | % VH improved ≥ 1 | % VH improved ≥ 2 | AC cell grade: change | Cataract: incidence | Cataract: % surgery | MO incidence | Time to MO | Macular thickness: change | % eyes MO improved | Steroid reduction | % reduced steroids | % rescue steroids | Time to treatment failure (active uveitis) | Uveitis recurrence | Composite (positive) | Generic HRQoL | VFQ-25 composite: final | VFQ-25 composite: change | Systemic AEs | Ocular AEs | |||
| HURON48,68 | DEX 700 | Placebo (sham) | Y (ETDRS) (6 months) | Y (2, 6 months) | Y (2, 6 months) | Y (final, no SD) | Y | Y | Y | Y | Y | Y | Y | Y (intravitreal/systemic) | Y (2, 4, 6 months) | Y (no SD/SE) (2, 6 months) | Y | Y | ||||||||||
| VISUAL I46 | ADA | Placebo | Y (logMAR) | Y (logMAR) | Y (final and change) | Y | Y | Y | Y | Y | Y | Y (worse AC cells, VH, VA, lesions)a | Y (EQ-5D, HADS, WPAI) | Y | Y | Y | Y | |||||||||||
| VISUAL II47 | AD | Placebo | Y (logMAR) | Y (logMAR) | Y (change) | Y | Y | Y | Y | Y | Y (AC, VH, VA, lesions) | Y | Y | Y | ||||||||||||||
| Multicenter Uveitis Steroid Treatment Trial Research Group57 | Fluocinolone implant | Systemic steroids and immunosuppressants | Y (ETDRS) (2, 6, 24 months) | Y (ETDRS) (6, 12, 24 months) | Y (24 months) | Y | HR only | Y | Y | Y | Y (EQ-5D, SF-36) | Y (6, 12, 24 months) | Y (6, 12, 24 months) | Y | Y | |||||||||||||
| Pavesio 201058 | Fluocinolone implant | Systemic steroids and immunosuppressants | Y (24 months) | Y | Y | Y (improved) | Y (AC, VH, VA) | Y | Y | |||||||||||||||||||
| Shin 201559 | Triamcinolone intravitreal injection | Placebo (sham) | (No data just p = NS) | Y | Y | No data, p-value | Y (% reduced) | Y | ||||||||||||||||||||
| Ferrante 200060 | Triamcinolone periocular injection | Methylprednisolone periocular injection | Y | Y (intravitreal) | Y | |||||||||||||||||||||||
| Foster 200361 | Etanercept | Placebo | Y | Y (uveitis flare-ups) | Y | |||||||||||||||||||||||
| Yazici 199062 | Azathioprine | Placebo | Unclear data | Y (intravenous) | Y | |||||||||||||||||||||||
| Murphy 200533 | Ciclosporin | Tacrolimus | Y | Y (previous responders) | Y (VA two or more lines or ophthalmoscopy = 0) | Y | ||||||||||||||||||||||
| de Vries 199063 | Ciclosporin | Placebo | Y (Landolt C, p-value only) | Y (% stopped) | No data, p-values | Y | ||||||||||||||||||||||
| Nussenblatt 199164 | Ciclosporin | Prednisolone | Y | Y | Y | Y (resolved) | Y (VA three or more lines or VH improvement ≥ 2) | Y | ||||||||||||||||||||
| Bodaghi 2012 (active)65,66 | Voclosporin | Placebo | Unclear data | |||||||||||||||||||||||||
| Bodaghi 2012 (maintenance)65,66 | Voclosporin | Placebo | Y (recurrence) | |||||||||||||||||||||||||
| Mackensen 201367 | MTX | Interferon-beta | Y (Snellen, logMAR) | Y (ETDRS, logMAR) | Y | Y (final) | Y | Y | Y (improved/resolved) | SF-36, no data | Y | Y | Y | |||||||||||||||
| Rathinam 200432 | MTX | Mycophenolate mofetil | Y (logMAR) | Y | Y (resolved) | Y (% with minimal inflammation while on low or no steroid treatment) | Y | Y | ||||||||||||||||||||
Consideration of indirect comparisons for trials of adalimumab and dexamethasone
The outcomes reported for the ADA and DEX 700 trials varied from trial to trial (see Study characteristics) and so the potential networks of evidence were considered separately for each outcome of interest. Outcomes considered for the NMA were VFQ-25, visual acuity, VH and AEs. This was driven by the potential to undertake a NMA for these outcomes.
Two networks of evidence were considered. A diagram of network 1 is provided in Figure 5. Network 1 consists of two trials (HURON48 and VISUAL I46) and allows pairwise comparisons to be made between ADA and DEX 700 and placebo/sham procedure (the common comparator of the two trials). The trials share common assessment time points of 8, 16 and 26/27 weeks (26 weeks for the HURON trial48 and 27 weeks for the VISUAL I trial46). Given that the HURON trial48 was a 26-week trial, comparison beyond this time point is not possible based on the observed data.
FIGURE 5.
Network 1 for the VFQ-25 outcome: indirect comparison of ADA, DEX and placebo/sham.
A diagram of network 2 is provided in Figure 6. Network 2 is an extension of network 1 including the Multicenter Uveitis Steroid Treatment (MUST) trial of fluocinolone corticosteroid implant compared with systemic corticosteroids and immunosuppressants,57 under the assumption that the efficacy of the fluocinolone implant is the same as that of DEX 700. This allows an indirect comparison with treatment with systemic corticosteroids and immunosuppressants, which may be considered more reflective of current UK practice than treatment with placebo/sham procedure. An indirect comparison using this network is possible only at 26 weeks (the first follow-up time point in the MUST trial).
FIGURE 6.
Network 2 for the VFQ-25 outcome: indirect comparison of ADA, DEX, placebo/sham and immunosuppressants.
The AG began with a question about the best way to compare the treatment options within a network, with the prior belief that such an analysis could be undertaken. However, after substantial deliberation between all members of the AG and discussion with the clinical advisors, it was reluctantly decided that a NMA was inappropriate and may provide misleading results. The main issues were as follows.
-
Baseline systemic therapy: in the HURON trial48 only 26% of patients were receiving systemic therapy at baseline, whereas in the VISUAL I trial46 all patients were receiving systemic high-dose corticosteroids. Therefore, patients in these studies may have been on different ‘lines’ of treatment. In addition, in the VISUAL I trial46 91% of patients had bilateral uveitis, whereas the corresponding proportion was not reported in the HURON trial;48 this may be a further difference between the patient populations in these studies.
-
Rescue therapy: a greater proportion of patients in the sham arm than in the DEX 700 arm in the HURON trial48 received rescue therapy (38.2% vs. 22.1%). In the VISUAL I trial46 there was no reported difference in concomitant therapy between the two arms. It may be misleading to attribute an indirect effect of ADA compared with DEX 700 to these interventions alone.
-
Comparability of the baseline treatments in the HURON48 and VISUAL I46 studies: the VISUAL I trial46 included an initial steroid burst; this was not included in the HURON trial. 48 Thus, the baseline interventions were different and it would be meaningful to combine the treatment effects across studies only if the initial steroid burst did not affect the treatment effect. However, clinical advice suggests that the treatment effect will depend on the initial steroid burst. Patients experience an initial improvement from the steroid burst and there is less scope during this period for patients to demonstrate further improvement (i.e. the effect of ADA is not additive to the effect of the steroids). In the analyses undertaken by AbbVie this issue was addressed by considering the change from the peak within the first 6 weeks to the final/termination visit for each individual. This approach was not considered appropriate for estimating the treatment effect because patients are comparable only at baseline and treatment effects should be estimated relative to baseline.
-
Validity of the comparable efficacy assumption for dexamethasone and fluocinolone (network 2 only): although DEX 700 and fluocinolone are both corticosteroid intravitreal implants, they cannot be considered clinically equivalent because the fluocinolone implant has a higher potency (median duration of effect 30 months)92 than the DEX 700 implant (median duration of effect 6 months). 43 There are no head-to-head trials comparing DEX 700 and fluocinolone implants.
-
Issues with the reported data: patients in the VISUAL I trial46 were followed up to the time of treatment failure only and missing data beyond this point were imputed using LOCF. No other methods for dealing with missing data were considered and it is possible that the use of LOCF may provide a biased estimate of the treatment effect as it assumes that the data are missing at random, which is not true in this case. Although LOCF was also used in the HURON trial48 the issue is less problematic in this case because most patients were followed up for 26 weeks and treatment could not be discontinued (because the implants are not removed). Estimates of the treatment effect for secondary outcomes (including VFQ-25 score, EQ-5D score, visual acuity and VH) may be biased because data were collected only until treatment failure. Evidence about key outcome measures could be synthesised using either absolute values at each time point or change from baseline. The use of absolute values was ruled out because of differences in response at baseline between the sham arm and the treatment arm in the HURON trial48 for the VFQ-25. The sham arm had a higher mean VFQ-25 score at baseline, whereas clinical advice suggests that the lower mean VFQ-25 score associated with the treatment arm is likely to be more representative of the population. It was not possible to account appropriately for baseline differences.
-
Treatment with adalimumab and dexamethasone is generally appropriate for different patient groups: as discussed in Description of the technologies under assessment, there is only a small patient group in which it would be appropriate to compare DEX 700 and ADA, with the most likely group being patients with bilateral uveitis with a temporary flare-up. Consequently, an analysis that assumes that clinicians would be prepared to treat any patient in the population with any of the treatments is inappropriate.
Summary of clinical effectiveness and safety (randomised controlled trials)
Three RCTs were included in the review of clinical effectiveness; a summary of the results is provided in Table 27. Two RCTs compared ADA with placebo, for up to 80 weeks or until treatment failure, in patients with intermediate uveitis, posterior uveitis or panuveitis on high-dose oral corticosteroids: VISUAL I46 (active uveitis) and VISUAL II47 (inactive uveitis). Oral corticosteroids were tapered from baseline and patients could receive up to one systemic immunosuppressant. One RCT (HURON48) compared DEX 700 (a single 0.7-mg implant) with a sham procedure over 26 weeks’ follow-up in patients with intermediate or posterior uveitis. At baseline, 25% of participants were on systemic therapies, which could be continued at a stable dose. 48 Thirteen additional studies of clinically relevant comparator treatments (vs. placebo or one another) were identified. However, because of clinical heterogeneity, differences in outcomes and a lack of common comparators, it was not feasible to undertake a NMA. Therefore, the summary of clinical efficacy evidence presented here is restricted to the VISUAL I,46 VISUAL II47 and HURON48 studies.
| Outcome | Difference between groups: treatment effect (95% CI), p-value | |||
|---|---|---|---|---|
| ADA: VISUAL I (active uveitis) | ADA: VISUAL II (inactive uveitis) | DEX 700: HURON – 8 weeks | DEX 700: HURON – 26 weeks | |
| Time to treatment failure (worsening of AC, VH or BCVA or new lesions) | HR 0.50 (0.36 to 0.70), p < 0.001 | HR 0.57 (0.39 to 0.84), p = 0.004 | NR | NR |
| BCVA (change) (logMAR) | MD –0.07 (–0.11 to –0.02), p = 0.003 | –0.04 (–0.08 to 0.01), p = 0.096 | NR | MD NR, p = 0.002 |
| BCVA improvement of three of more lines (15 letters) | NR | NR | MD 36.3% (24 to 49), p < 0.001; RR 6.5 (2.7 to 15.8), p < 0.001 | MD 24.5 (11 to 38), p < 0.001; RR 2.9 (1.5 to 5.5), p = 0.001 |
| BCVA improvement of two or more lines (10 letters) | NR | NR | MD 43 (29 to 56), p < 0.001; RR 3.5 (2.1 to 5.9), p < 0.001 | MD 30 (15 to 44), p < 0.001; RR 2.2 (1.4 to 3.4), p < 0.001 |
| VH grade (change) | MD –0.27 (–0.43 to –0.11), p < 0.001 | MD –0.13 (–0.28 to 0.01), p = 0.070 | NR | NR |
| VH grade (final) | NR | NR | MD –0.97 (NR), p < 0.001 | MD –0.58 (NR), p < 0.001 |
| % with VH = 0 | NR | NR | MD 34.9 (22 to 48), p < 0.001; RR 4.0 (2.0 to 7.6), p < 0.001 | MD 16.7 (4 to 30), p = 0.014; RR 2.2 (1.1 to 4.1), p = 0.02 |
| % with VH improvement of ≥ 2 | NR | NR | MD NR, p < 0.001 | MD NR, p = 0.001 |
| AC cell grade (change) | MD –0.29 (–0.51 to –0.07), p = 0.011 | MD –0.14 (–0.37 to 0.08), p = 0.218 | NR | NR |
| Macular oedema (change in macular thickness) (µm) | NR | NR | MD –87.0 (–147 to –27), p = 0.004 | MD –14.7 (–66 to 37), p = 0.58 |
| Macular oedema (change in macular thickness) (% change) | MD –11.4 (–20.9 to –1.8), p = 0.020) | MD –2.3 (–8.5 to 3.8), p = 0.451 | NR | NR |
| VFQ-25 composite score (change) | MD 4.20 (1.02 to 7.38), p = 0.010 | MD 2.12 (–0.84 to 5.08), p = 0.160 | MD NR, p = 0.007 | MD NR, p = 0.001 |
| % with ≥ 5-point improvement in VFQ-25 score | NR | NR | MD NR, p < 0.001 | MD NR, p < 0.05 |
| EQ-5D score (change) | MD 0.04 (0.00 to 0.07), p = 0.044 | MD 0.00 (–0.03 to 0.04), p = 0.836 | NR | NR |
| % requiring rescue medication | NR | NR | NR | MD 16, p = 0.030 |
Treatment failure in the VISUAL trials of ADA was defined as worsening of any of the following in either eye: AC cell grade, VH grade, BCVA or new inflammatory lesions. In the VISUAL I trial46 (active uveitis), the median time to treatment failure was 5.6 months for ADA compared with 3 months for placebo (HR 0.50, 95% CI 0.36 to 0.70; p < 0.001). Treatment failure was experienced by 54.5% of participants on ADA compared with 78.5% of participants on placebo. In the VISUAL II trial47 (inactive uveitis), the median time to treatment failure was not estimable for ADA and was 8.3 months for placebo (HR 0.57, 95% CI 0.39 to 0.84; p = 0.004). Treatment failure was experienced by 39% of participants on ADA compared with 55% of participants on placebo. In the VISUAL I trial46,52 there were significant benefits of ADA compared with placebo for changes in the following (averaged across both eyes): visual acuity (p = 0.003), inflammation [VH (p < 0.001) and AC cell grade (p = 0.011)], macular oedema [change in central retinal thickness (p = 0.020)], VFQ-25 composite score (p = 0.010) and EQ-5D score (p = 0.044). In the VISUAL II trial,47,72 differences were not significant for ADA compared with placebo for changes in any of the following (averaged across both eyes): visual acuity (p = 0.096), inflammation [VH (p < 0.070) and AC cell grade (p = 0.218)], macular oedema [change in central retinal thickness (p = 0.451)], VFQ-25 composite score (p = 0.160) or EQ-5D score (p = 0.836).
In the HURON trial48 there were significant benefits of DEX 700 compared with the sham procedure for the following (measured in the study eye only): percentage of patients with a VH score of zero at 8 weeks (p < 0.001) and 26 weeks (p = 0.014), percentage of patients with a VH improvement of ≥ 2 units at 8 weeks (p < 0.001) and 26 weeks (p = 0.001), percentage of patients with a BCVA improvement of three or more lines over weeks 3–26 (p < 0.001), mean BCVA improvement over weeks 3–26 (p ≤ 0.002), central retinal thickness at 8 weeks (p ≤ 0.004) although not 26 weeks (p ≥ 0.227), change in VFQ-25 composite score (per patient as opposed to study eye) at 8 weeks (p = 0.007) and 26 weeks (p = 0.001) and percentage of patients with a ≥ 5-point improvement in VFQ-25 score at 8 weeks (p < 0.001) and 26 weeks (p < 0.05). Rescue medication (corticosteroid injections in the study eye or new/increased use of systemic corticosteroids or immunosuppressants) were required in 22% of participants in the DEX 700 arm and 38% of participants in the sham arm (p = 0.030).
As ADA affects the immune system, the potential risks of treatment with ADA include infections and malignancy. 44 The rate of serious infections was higher in the ADA group than in the placebo group in the VISUAL I trial46 (4.5% vs. 1.8%) but not the VISUAL II trial47 (1.7% vs. 1.8%). Malignancies and chronic renal failure each occurred in a total of three patients across both trials in the ADA group, with no cases in the placebo group. Systemic AEs that had a higher rate of occurrence in the ADA group than in the placebo group in at least one of the VISUAL trials46,47 included infections, injection site reactions, fatigue, arthralgia, myalgia, paraesthesia, hypertension and elevations of liver enzymes. Anti-ADA antibodies in patients receiving ADA occurred in 2.7% in the VISUAL I trial46 and 5% in the VISUAL II trial. 47 There was little difference between ADA and placebo in the rates of ocular AEs.
In terms of safety, the risks of DEX 700 include those associated with intraocular steroids, that is, increased IOP, cataract and glaucoma, as well as infection and bleeding. 45 In the HURON trial,48 raised IOP occurred in 25% of participants in the DEX 700 group compared with 7% of participants in the sham group, whereas IOP of ≥ 25 mmHg occurred in 7.1% of participants in the DEX 700 group compared with 1.4% of participants in the sham group. Glaucoma rates were lower in the DEX 700 group (0%) than in the sham group (2.7%); no patients required incisional surgery for glaucoma, whereas 2.6% of participants in the DEX 700 group required a laser iridotomy and, at any single time point, up to 23% of participants in the DEX 700 group required IOP-lowering medication (not reported for the sham group). Cataracts in eyes that were phakic (had a natural lens) at baseline occurred in 15% of participants in the DEX 700 group compared with 7% of participants in the sham group and cataract surgery was carried out in 1.6% and 3.6% of the DEX 700 and sham groups respectively. Endophthalmitis (severe eye infection) and severe uveitis worsening occurred in one patient each in the DEX 700 group; there were no cases in the sham group. Conjunctival haemorrhage occurred in 30% of participants in the DEX 700 group compared with 21% of participants in the sham group. No systemic AEs had a substantially higher rate in the DEX 700 group than in the sham group.
Chapter 4 Assessment of cost-effectiveness
This chapter first presents a systematic review of existing cost-effectiveness evidence for treatments given to mainly adult patients with non-infectious uveitis. This is followed by a description of the de novo model developed by the AG to assess the cost-effectiveness of DEX in patients with active uveitis, ADA in patients with active uveitis and ADA in patients with inactive uveitis, all compared with current practice, and the results of the model and discussion of the analysis. Finally, a summary of the key model results is presented.
Systematic review of existing cost-effectiveness evidence
Methods
A comprehensive search was undertaken to systematically identify economic evaluations and quality-of-life studies in patients with active non-infectious intermediate uveitis, posterior uveitis and/or panuveitis.
The following electronic databases and clinical trials registries were searched from inception:
-
MEDLINE (via Ovid) (1946–2016)
-
MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (1946–2016)
-
EMBASE (via Ovid) (1980–2016)
-
The Cochrane Library (via Wiley Online Library)
-
HTA database (1995–2016)
-
NHS EED (1995–2015)
-
-
CINAHL (via EBSCOhost) (1982–2016)
-
Web of Science Citation Index (Thomson Reuters) (1899–2016)
-
CPCI (Thomson Reuters) (1990–2016)
The search strategy consisted of MeSH or EMTREE Thesauri terms and free-text synonyms for ‘uveitis’. Searches were translated across databases and were limited by neither language nor publication date. The search strategies are presented in Appendix 1. Search filters designed to identify economic evaluations and quality-of-life studies were used on MEDLINE and other databases when appropriate. Reference and citation searching of included papers was undertaken.
The inclusion criterion was economic evaluations of treatments given to mainly adult patients for non-infectious uveitis. This took a deliberately broad perspective and was not limited to treatment with ADA or DEX. Studies that reported only costs were excluded, although these were marked as being potentially useful for informing the model parameters. Study selection was undertaken by one reviewer (IB) and checked by a second reviewer (HS). Critical appraisal of the included studies was undertaken using a combination of key components of the BMJ checklist for economic evaluations93 together with the Eddy checklist for mathematical models94 (see Table 28).
Results
The electronic literature searches identified 1177 potentially relevant economic analyses of treatment for non-infectious uveitis. Of these, only seven studies appeared to relate to the economic evaluation of non-infectious uveitis and full texts of these papers were obtained. 95–101 Two of these studies met the inclusion criteria,100,101 one of which was published only as a conference abstract. 100 The numbers of studies screened and included within the review are shown in Figure 7.
FIGURE 7.
Summary of selection and exclusion of economic evaluation studies.

Justification for excluding studies at the full paper screening stage
The review by the Health Technology Inquiry Service95 was excluded following full paper screening as it did not identify any cost-effectiveness studies. The study reported by Ang et al. 96 was excluded because it related to an analysis of interventions for tuberculous uveitis rather than non-infectious uveitis and compared diagnostic testing strategies rather than treatments for diagnosed disease. The studies by Ramanan et al. 97 and Ramanan et al. 98 were excluded because they were limited to children and they did not include an economic analysis. The study reported by Nguyen et al. 99 was excluded because it was not an economic evaluation.
Included economic evaluations
The key characteristics of the two studies100,101 identified for inclusion within the review are shown in Table 28 and are discussed briefly below. Neither of these studies included ADA or DEX as interventions or comparators. One of the economic analyses was based on a semi-Markov model100 whereas the other101 extrapolated cost and HRQoL data collected during the MUST trial. 57,102 The two economic evaluations compared a different set of treatments.
| Characteristics | Study | |
|---|---|---|
| Padula et al.100 | Sugar et al.101 | |
| Country and year of publication | USA, 2011 | USA, 2014 |
| Type of economic analysis | Cost–utility analysis | Cost–utility analysis |
| Health economic perspective | Societal | Payer’s perspective for costs and the patient’s perspective for outcomes |
| Health economic comparisons (listed interventions) | Infliximab, systemic steroids, methotrexate | Fluocinolone acetonide intraocular implant, oral corticosteroid with immunosuppressive agents as needed |
| Population | Patients with sarcoid posterior uveitis | Patients aged ≥ 13 years with non-infectious intermediate uveitis, posterior uveitis or panuveitis in one or both eyes (active within ≤ 60 days) for which systemic corticosteroids were indicated (excluding those requiring systemic therapy for non-ocular indications) |
| Time horizon | Lifetime | 3 years |
| Health economic outcomes | Incremental cost per QALY gained | Incremental cost per QALY gained |
| Modelling approach | Semi-Markov model | Extrapolation of trial data |
Padula et al.:100 a cost-effectiveness analysis of off-label biologics to treat sarcoid posterior uveitis versus standard of care: comparing infliximab to methotrexate and systemic steroids
The study by Padula et al. 100 was reported only as a conference abstract. The authors presented the methods and results of a cost-effectiveness analysis of infliximab compared with methotrexate and systemic steroids over a lifetime horizon. The economic evaluation used a semi-Markov approach to estimate health outcomes and costs. Patients entered the model following the onset of sarcoid posterior uveitis. No further information was provided about the population reflected in the model. Cost-effectiveness was evaluated in terms of the incremental cost per quality-adjusted life-year (QALY) gained from a societal perspective.
Probabilities, health utilities and costs used in the model were reported to be taken from the literature, although parameter values were not reported in the abstract. It was not specified whether or not a systematic review was conducted. Costs and health outcomes were discounted at a rate of 3% per annum. Costs were expressed in 2010 US dollars. The authors conducted univariate sensitivity analyses, threshold analyses and probabilistic sensitivity analysis (PSA) using 10,000 simulations.
The incremental cost-effectiveness ratio (ICER) for methotrexate compared with systemic steroids was estimated to be US$10,053 per QALY gained. Methotrexate dominated infliximab in the base case. However, if a patient’s health utility after successful recovery was < 0.750 (base-case value of 0.84), then infliximab produced a greater net benefit than methotrexate, assuming a willingness-to-pay (WTP) threshold of US$50,000 per QALY gained. The PSA suggested that the probability of methotrexate dominating infliximab was 0.60.
It is not possible to assess the validity of the model as only limited information is provided within the conference abstract. The AG notes that this analysis does not include either of the interventions being assessed within this appraisal (DEX and ADA) and the model does not appear to differentiate between unilateral and bilateral uveitis, which may be associated with different cost-effectiveness results. There is insufficient information provided within the abstract for this analysis to be useful in the current appraisal.
Sugar et al.:101 cost-effectiveness of fluocinolone acetonide implant versus systemic therapy for noninfectious intermediate, posterior, and panuveitis
Sugar et al. 101 presented a cost-effectiveness analysis of fluocinolone acetonide intraocular implant compared with oral corticosteroids and immunosuppressive agents. Costs and health benefits were estimated from data collected during the MUST trial. 57 The economic analysis used a time horizon of 3 years and costs and benefits were discounted at a rate of 3% per annum. Costs were expressed in US dollars (year unclear). The authors used a payer’s perspective for costs and the patient’s perspective for outcomes. The authors estimated the cost to a payer to maximise health benefits by using the more effective, but more expensive, treatment.
The within-trial data (differences in costs and utilities), reported at 2 years’ follow-up, were extrapolated by a further year for a 3-year time horizon. The difference in the mean total costs of the treatments was determined with a linear regression with a saturated means model. The history of the disease was modelled through a sequence of utility values measured during the trial at different points in time. No health states were used. Uncertainty was assessed using bootstrapping and was represented using cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs).
For bilateral uveitis, the fluocinolone acetonide implant for both eyes was estimated to generate 0.057 additional QALYs at an additional cost of US$16,900; the ICER was reported to be US$297,800 per QALY gained. The probabilities of the fluocinolone acetonide implant being cost-effective compared with systemic therapy at WTP thresholds of US$50,000 and US$100,000 per QALY gained were 0.003 and 0.04 respectively. For unilateral uveitis, the implant resulted in 0.130 additional QALYs at an additional cost of US$5300; the ICER was reported to be US$41,200 per QALY. The probabilities of the implant being cost-effective compared with systemic therapy at WTP thresholds of US$50,000 and US$100,000 per QALY gained were 0.53 and 0.74 respectively.
The study highlights the importance of considering unilateral and bilateral uveitis separately within future economic evaluations in terms of (1) the cost difference between types of treatments, (2) quality-of-life impacts and (3) the greater risk to vision of an operative procedure on both eyes compared with one eye. However, this study does not consider the cost-effectiveness of the implant in one eye for patients with bilateral uveitis as all patients with bilateral uveitis within the MUST trial were given an implant in both eyes. 57 The model has several additional key limitations:
-
All relevant comparators were not included in the model. Systemic steroids and immunosuppressants were assumed to be the gold standard as they were the only included comparators of the fluocinolone acetonide implant, with no discussion about whether or not this was appropriate.
-
AEs were not taken into consideration.
-
It is not clear how the 2 years of data from the MUST trial were extrapolated to the 3-year time horizon.
-
It is not clear whether or not the implant would have benefits after this 3-year period.
-
No model validation was reported.
-
The analysis of uncertainty was not well described.
Company submissions
Neither AbbVie55 (ADA) nor Allergan43 (DEX) submitted a health economic model. Within its submission, AbbVie provided no discussion of cost-effectiveness and presented a budget impact estimate based on the acquisition costs of ADA only. 55
Within its submission, Allergan argued that DEX has been recommended by NICE for the treatment of macular oedema secondary to retinal vein occlusion103 and that the costs per patient associated with DEX are comparable for posterior segment uveitis and patients with macular oedema secondary to retinal vein occlusion, whereas the incremental gains in visual acuity are greater in posterior segment uveitis based on the trial data from the individual trials. This argument fails to consider the incremental (rather than absolute) cost of DEX treatment compared with current treatment. Allergan also submitted a budget impact model, which takes into account the costs of treatment and monitoring but not the costs of treating events associated with uveitis or AEs associated with treatment.
Summary of the review of existing cost-effectiveness studies
No existing studies have assessed the cost-effectiveness of either DEX or ADA within this patient population. Only one published health economic model of non-infectious uveitis exists. This study was subject to several limitations, including poor reporting of some of the methods, validation and uncertainty analysis, not taking into account AEs and the use of a 3-year time horizon, which may not fully capture all impacts of the treatments.
Independent economic assessment
Methods
This section provides details of the Markov model developed by the AG, which was used to evaluate the cost-effectiveness of ADA and DEX within their licensed indications for non-infectious posterior segment-involving uveitis compared with current practice, from a NHS and PSS perspective. A cohort of patients with a mean age of 44.8 years was followed over a lifetime. All costs and QALYs were discounted at a rate of 3.5% per year. ADA and DEX were not compared against each other. This is because of their different use in clinical practice (see Chapter 1, Description of the technologies under assessment) and because, for the limited indications for which the clinician has a choice regarding which treatment to use, there is a lack of evidence, as detailed in Chapter 3 (see Indirect comparison of treatments: rationale for not undertaking). Table 29 describes the key features of the model for both ADA and DEX.
| Characteristic | Intervention | |
|---|---|---|
| ADA | DEX | |
| Population | People with non-infectious intermediate uveitis, posterior uveitis or panuveitis with (1) active disease (VISUAL I46) and (2) inactive disease (VISUAL II47) | People with non-infectious intermediate uveitis, posterior uveitis or panuveitis with active disease (HURON48) |
| Intervention | ADA until treatment failure + LCP(VI), ADA until treatment failure + LCP(VII) | One DEX implant + LCP(H) |
| Comparator | LCP(VI), LCP(VII) | LCP(H) |
| Outcome used from trial | EQ-5D | VFQ-25 |
| Time horizon | Lifetime | Lifetime |
| Discounting | 3.5% per year for costs and QALYs | 3.5% per year for costs and QALYs |
| Treatment discontinuation | Parametric survival curve of time to treatment failure fitted to VISUAL I and II trial data | Patients are given only one DEX implant |
| Method for estimating QALYs during the trial period | Use directly measured EQ-5D scores at each time point until treatment failure, when patients revert to baseline utility, adjusted for age | Use VFQ-25 data captured at each time point in the trial mapped onto EQ-5D scores |
| Method for estimating QALYs following the trial period | Patients who have not failed treatment retain the averaged utility from months 12–18 of the trial (because of small patient numbers), adjusted for age. Patients who fail treatment revert to baseline utility, adjusted for age | Assumes that utility remains the same for 4 weeks following the trial and then returns to baseline by week 30, adjusted for age |
| AEs (except blindness) | Cataract, raised IOP, glaucoma, serious infections, hypertension, fractures, diabetes. Impact on HRQoL associated with these AEs was assumed to be captured within the VFQ-25/EQ-5D scores | |
| Permanent blindness (comparator) | No blindness prior to treatment failure. Constant rate of blindness after treatment failure based on Dick et al.19 | Constant rate of blindness based on Dick et al.19 |
| Permanent blindness (intervention) | No blindness prior to treatment failure. Constant rate of blindness after treatment failure based on Dick et al.19 | RR for blindness of 0.5 for 30 weeks following implantation |
| Treatment following remission | For all patients, treatment will continue until treatment failure | For all patients, treatment will continue until treatment failure |
Because of the substantial uncertainties associated with the above assumptions because of the limited evidence base, most of the assumptions were altered within exploratory analyses to test their impact on the model results.
Model description
Patient population
The model population consists of people with non-infectious intermediate uveitis, posterior uveitis or panuveitis. Patients receiving DEX were assumed to have active disease, whereas the model assessed the cost-effectiveness of ADA separately for patients with active and inactive disease. An analysis was undertaken to explore the cost-effectiveness of DEX use in one eye in patients with unilateral disease as a separate subgroup; the trial did not provide data separately for this group and hence this analysis is considered to be exploratory. Because of a lack of evidence, it was not possible to explore additional subgroups. A cohort of uveitis patients was assumed to enter the model with a mean age of 44.8 years, based on the mean age within the HURON trial,48 and was followed over a lifetime. The model population was limited to adults aged ≥ 18 years because the marketing authorisations for the technologies being considered relate only to this group.
Interventions
The two technologies considered were ADA (40 mg every 2 weeks until treatment failure) and the DEX implant (0.7 mg, once only in the base case).
Within the clinical trials of ADA (VISUAL I46 and II47), patients were already receiving high-dose corticosteroids at randomisation, with a corticosteroid burst given to all patients at the start of the VISUAL I trial; corticosteroids were tapered to zero by week 15 (VISUAL I) or week 19 (VISUAL II). Clinical advisors to the AG suggested that this is also likely to reflect clinical practice, although the SmPC suggests that ADA may be given alongside corticosteroids or alone. 44 Given the evidence available, for patients with active disease, the model considers the cost-effectiveness of ADA plus an initial oral corticosteroid burst, rather than ADA alone.
The DEX implant can be administered in the affected eye to unilateral patients, in one eye for patients with bilateral disease or in both eyes at staggered intervals for patients with bilateral disease. Patients could also receive more than one consecutive implant. Clinical advisors to the AG suggested that the DEX implant would most probably be used when disease affects only one eye (or is more severe in one eye in the case of asymmetric disease), or to treat a temporary flare-up in one or both eyes, when systemic disease is not present or is well controlled. The base-case model assumed that patients would receive one DEX implant in one affected eye, as in the HURON trial. 48 There are no RCTs that assess the use of more than one consecutive implant or the use of implants in two affected eyes. However, there are several non-randomised trials, with 12–24 months of follow-up, that allow the use of repeat implants. 18,50,51 These studies consistently report that, after around 6 months, patients’ outcomes return to those at baseline and that up to three repeat implants are each likely to have a similar treatment effect. Given the limited evidence around repeat implants, this was explored within sensitivity analysis. In one of the studies that assessed implants in both eyes, 3 out of 11 patients (27%) receiving implants had a response (reduced central retinal thickness and improved BCVA) in the second eye. 18 Clinical advisors to the AG suggested that it is more likely that systemic treatment would be used if both eyes required treatment; however, the direction of the ICER for treatment in both eyes compared with one eye is considered in Chapter 6.
Comparators
The two technologies were compared independently with current practice, which includes a range of immunosuppressants (such as methotrexate, mycophenolate mofetil, ciclosporin and azathioprine) and corticosteroids. Given the concerns regarding the robustness of undertaking a NMA (see Chapter 3, Indirect comparison of treatments: rationale for not undertaking), within the base-case analysis, current practice was assumed to be equivalent to practice in the control arm (sham or placebo) of the clinical trials of the interventions. In the VISUAL trials of ADA,46,47 patients received initial corticosteroids, which were tapered by 15 weeks (VISUAL I46) and 19 weeks (VISUAL II47), and 32% in the VISUAL I trial46 and 48% in the VISUAL II47 study were receiving one immunosuppressant at baseline (across arms), which they were able to maintain according to the study protocol. Given that a greater proportion of patients in practice are likely to receive systemic corticosteroids, these comparators are denoted throughout as limited current practice based on the VISUAL I trial [LCP(VI)] or the VISUAL II trial [LCP(VII)]. In the HURON trial of DEX,48 patients were allowed to receive rescue therapy with corticosteroids or immunosuppressants and 25% were using systemic immunosuppressants or anti-inflammatory treatment at baseline, which they were able to maintain according to the study protocol. This comparator is denoted throughout as limited current practice based on the HURON trial [LCP(H)]. Apart from rescue therapy with immunosuppressants within the HURON trial, the proportions receiving immunosuppressants and corticosteroids were similar across the arms of the HURON48 and VISUAL I46 and II47 trials. In current practice, a greater proportion of patients would receive systemic immunosuppressants or anti-inflammatory treatment than in the control arms of the pivotal studies; consequently, the base-case analysis is likely to underestimate both the effectiveness and the AE profile of current practice, as well as the costs associated with treatment. Within exploratory analyses, the AG assessed the impact on the results of increasing the value of these parameters within the model. However, it should also be noted that in clinical practice a greater proportion of patients being treated with ADA and DEX are also likely to receive concomitant treatment.
Outcomes
The model was used to estimate the incremental cost per QALY gained for each intervention compared with current practice.
The VISUAL trials and the HURON trial48 each reported VFQ-25 HRQoL data at baseline and at each follow-up visit. The VISUAL trials46,47 also reported EQ-5D data at baseline and at each follow-up visit. The model used the EQ-5D data directly for modelling the effectiveness of ADA. The HURON trial48 reported EQ-5D data at baseline but not at subsequent time points. It was therefore not possible to use the EQ-5D data directly; however, Allergan shared patient-level data from the HURON trial with the AG, which allowed an analysis of the relationship between the VFQ-25 and the EQ-5D using the baseline data (see Model structure). It was necessary to convert VFQ-25 data to EQ-5D utilities to estimate QALYs associated with treatment with DEX and LCP(H). 104
The use of the outcomes from the HURON trial48 representing vision and inflammation (visual acuity, VH) was considered by the AG as an alternative to the use of the VFQ-25 for estimating QALYs; however, the VFQ-25 outcome was preferred because of the difficulties associated with using vision as an outcome in uveitis and capturing all impacts of the interventions (see Chapter 1, Description of the health problem). Clinical advisors to the AG suggested that clinicians measure ocular outcomes based on multiple factors, including visual acuity, VH and macular oedema. The VFQ-25 captures multiple components of vision, as well as broader considerations such as general health and the vision-related impact on the ability to drive and undertake normal activities. It is also essential to capture the AEs associated with the treatments and it is difficult to determine the utility decrements associated with the multiple interacting AEs associated with these treatments. The AG considered that the VFQ-25 should largely capture the impact of AEs, as well as treatment effects, on HRQoL.
The presence of unilateral or bilateral uveitis is important in terms of estimating outcomes for several reasons. The BCVA in the better-seeing eye is more representative of quality of life than the BCVA in the worst-seeing eye. 105 In addition, a patient with bilateral disease is expected to have a lower quality of life on average than a patient with unilateral disease. Thus, a person with bilateral disease has more scope to benefit from treatment. However, in patients with bilateral disease receiving local treatment, the choice of study eye is important in determining the extent to which quality of life can increase.
In the VISUAL I46 and VISUAL II47 trials, 91% and 96% of patients had bilateral disease respectively. Clinical advice received by the AG suggests that this is representative of patients who would be given ADA in practice because it is a systemic treatment. Within the HURON trial48 it was not recorded whether patients had unilateral or bilateral disease. Based on the patient-level data provided by Allergan, the proportion of patients with VH that was greater than zero in the non-study eye was 51%; clinical advisors to the AG stated that this suggests that ≥ 51% of patients had bilateral disease. Within the HURON trial,48 when patients had bilateral uveitis, the right eye was chosen for treatment. This resulted in the better-seeing eye being treated in 10.7% and 17.1% of cases for DEX 700 and the sham procedure respectively.
Given that the presence of unilateral or bilateral uveitis was not reported in the HURON trial,48 it was not possible for the AG to undertake robust subgroup analysis around this factor. The base-case model is therefore dependent on the assumption that the patients included within the HURON trial and the way in which DEX was used within the trial would be representative of current practice. It was not possible to draw robust conclusions about the subgroups separately in terms of cost-effectiveness; however, an exploratory subgroup analysis was undertaken (see Model evaluation methods). As described within Chapter 1 (see Description of the health problem), it is expected that around 70–80% of this patient population would have bilateral disease. However, it may be that, because DEX is a local treatment, patients with unilateral disease would be more likely to be selected for DEX treatment, both within the trial and in practice. Given that patients with bilateral disease have a greater capacity to benefit from treatment because of the BCVA of the better-seeing eye being the best predictor of quality of life, and that treatment of one eye would cost the same whether given to a person with unilateral or bilateral disease, if the trial had a lower proportion of bilateral cases than in practice, the effectiveness of DEX may be underestimated. Conversely, if the trial had a higher proportion of bilateral cases than in practice, the effectiveness of DEX may be overestimated.
Time horizon
The time horizon of the model was the lifetime of patients (up to age 100 years) and a starting age of 44.8 years was used, representing the average age of patients with non-infectious posterior segment-involving uveitis within the HURON trial. 48 A cycle length of 2 weeks was chosen as this is the time between administration of ADA doses and assessment of patients for disease progression. This is also a sufficiently short cycle length to capture all relevant clinical events associated with DEX and current practice.
Discounting
All costs and QALYs were discounted at a rate of 3.5% per year.
Model structure
The structure of the AG model is presented in Figure 8. The model includes five health states: (1) treatment: no permanent blindness, (2) treatment failure: no permanent blindness, (3) permanent blindness, (4) remission (no treatment) and (5) death. For DEX, treatment was one implant, which was assumed to be effective for 6 months, at which time patients move to the treatment failure health state if they have remained in the treatment state until this time. Patients in the LCP(H) group begin in the treatment failure health state. Patients may discontinue ADA because of treatment failure, defined by the VISUAL trial criteria,46,47 at which time they will move to the treatment failure health state if they have remained in the treatment state until this time. Patients in the LCP(VI) and LCP(VII) groups also begin in the treatment health state and move to the treatment failure health state once they have met criteria for treatment failure. Within the treatment health state, HRQoL (defined using the VFQ-25 or EQ-5D) can be improved as a result of the treatment effect or as a result of a reduction in AEs. The HRQoL estimates should capture any impacts of the interventions on visual loss while treatment is provided. However, treatment may also reduce the risk of experiencing permanent damage to the eye, resulting in a decreased risk of permanent legal blindness. Once a patient experiences legal blindness in the model, they can either remain in this health state or progress to death.
FIGURE 8.
State transition diagram of the decision model.

Patients may also enter remission, whereby they do not receive further treatment but they continue to receive the benefits of the previous treatment. Within the base case, the proportion of patients experiencing remission was assumed to be zero; the impact of increasing this proportion was considered within the exploratory analyses.
Estimation of model parameters
Treatment discontinuation
In the base-case analysis, the DEX implant was assumed to be administered only once to one eye and to have an efficacy of 30 weeks, based on data from the HURON trial. 48
Patients could discontinue ADA as a consequence of any of the four criteria for treatment failure used within the VISUAL trials,46,47 including (1) the development of new inflammatory lesions, (2) worsening of AC cell grade, (3) worsening of VH grade or (4) worsening of visual acuity. Treatment discontinuation was modelled using parametric curves fitted to Kaplan–Meier curves for time to treatment failure from the trials. The Kaplan–Meier curves for time to treatment failure included in the VISUAL I52 and II72 clinical study reports were digitised and individual patient data were reconstructed using the methods described by Guyot et al. 106 A number of parametric curves were fitted to the data using the flexsurv R package (R version 3.3.2, flexsurv version 1.0.0; The R Foundation for Statistical Computing, Vienna, Austria). Tables 30 and 31 present the Akaike information criterion (AIC) and Bayesian information criterion (BIC) scores for statistical goodness of fit.
| Criterion | Arm | Log-normal | Gamma | Weibull | Gompertz | Exponential | Log-logistic |
|---|---|---|---|---|---|---|---|
| AIC | ADA | 374.7 | 388.5 | 384.7 | 370.3 | 403.4 | 377.5 |
| Placebo | 435.9 | 465.7 | 456.5 | 438.4 | 486.7 | 438.9 | |
| BIC | ADA | 377.4 | 391.2 | 387.4 | 373.0 | 407.1 | 380.2 |
| Placebo | 438.5 | 468.4 | 459.2 | 441.1 | 490.3 | 441.5 |
| Criterion | Arm | Log-normal | Gamma | Weibull | Gompertz | Exponential | Log-logistic |
|---|---|---|---|---|---|---|---|
| AIC | ADA | 370.6 | 378.9 | 377.3 | 364.9 | 382.2 | 373.1 |
| Placebo | 403.5 | 408.4 | 406.1 | 403.7 | 428.5 | 403.2 | |
| BIC | ADA | 373.3 | 381.6 | 380.0 | 367.7 | 385.9 | 375.8 |
| Placebo | 406.2 | 411.2 | 408.8 | 406.4 | 432.3 | 406.0 |
It should be noted that these are relative measures of goodness of fit and it is possible that other models not tested here could provide a better fit to the data. Figures 9–12 show the Kaplan–Meier data and the fitted parametric distributions for the VISUAL I46 and II47 trials for the ADA and comparator groups.
FIGURE 9.
Observed and fitted curves for time to treatment discontinuation in the ADA arm in the VISUAL I trial.
FIGURE 10.
Observed and fitted curves for time to treatment discontinuation in the placebo arm in the VISUAL I trial.

FIGURE 11.
Observed and fitted curves for time to treatment discontinuation in the ADA arm in the VISUAL II trial.

FIGURE 12.
Observed and fitted curves for time to treatment discontinuation in the placebo arm in the VISUAL II trial.
The statistical analysis suggested that, of those tested, the parametric distributions with the best fit to the data were the Gompertz and the log-normal distributions for both the ADA group and the placebo group in the VISUAL I46 and II47 trials. Clinical advisors to the AG suggested that it is clinically plausible that some patients would remain on ADA for years; hence, the plateauing of these curves seems potentially reasonable. However, the Gompertz curve seems clinically implausible as observational studies of ADA in similar patient populations have suggested that patients are likely to continue to fail treatment in the longer term. 107,108 The log-normal distribution appears to be the most plausible for the placebo arm so that patients do fail on treatment relatively quickly. The log-normal distribution also appears clinically reasonable for the ADA group. It should be noted that, although based on these predictions alone some patients would continue to receive treatment for an implausibly long period of time, within the model patients may die of other causes, which negates the need for a cure model to be employed.
It was assumed that after patients fail and discontinue treatment with ADA, or 6 months after the DEX implant is injected, patients receive limited current practice, which includes a range of immunosuppressants (such as methotrexate, mycophenolate mofetil, ciclosporin and azathioprine) and corticosteroids for a proportion of patients. It was also assumed that ADA and DEX are effective only while they are being given. Therefore, patients who are no longer being treated with ADA, and patients who received the DEX implant > 6 months ago, accrue no additional health gains.
Permanent legal blindness
The VISUAL46,47 and HURON48 trials did not report any occurrence of blindness, which is likely to be because of the short duration of these trials. However, it may be that the use of ADA or DEX could prevent damage to the eye, which may in turn prevent future blindness. Conversely, it is possible that the AEs associated with treatment (such as raised IOP) could lead to an increased risk of blindness via glaucoma. There is no evidence about any longer-term (positive or negative) impacts of the interventions on vision loss beyond the treatment duration. As such, it was not possible to include a complex model of long-term outcomes associated with the interventions. However, as these interventions ultimately aim to reduce permanent damage to the eye, a state for becoming permanently legally blind was included as this has the largest impact on quality of life and costs.
We defined blindness as a BCVA of ≤ 20/200 in the better-seeing eye, according to the UK definition of legal blindness. 109 The AG considered two approaches for modelling permanent blindness based on the evidence from the key RCTs. The first was to extrapolate the decrease in BCVA over time using the mean change and distribution from the trials and estimate the proportion of patients who would go below the legal blindness threshold in each group. The AG considered that this approach had three weaknesses: (1) the follow-up period of the clinical trials was not long enough to capture the total impact on visual acuity because damage to the eye does not always immediately impact on visual acuity; (2) there are different trajectories according to the cause of the damage to the eye, which could not be appropriately captured by a single parametric distribution; and (3) for patients with unilateral disease, additional assumptions about the probability of blindness in both eyes would need to be made.
The second approach considered by the AG was to use outcomes from the trials such as glaucoma and macular oedema as surrogate outcomes for blindness in the future. In principle, this would allow a more accurate estimate of blindness over time to be made, and could exclude outcomes such as cataract, for which blindness is reversible through surgery. However, the AG did not identify any evidence that could provide a link between these shorter-term outcomes and blindness. The only evidence of blindness caused by uveitis identified by the AG was cross-sectional and did not specify time to blindness. 18,27 This means that populating the model would have required elicitation or an assumed distribution for how long it would take patients to become blind and for this to be extended beyond the period of the cross-sectional study data. In addition, the key long-term outcome to include in the model is blindness in both eyes, given that the BCVA in the better eye is the best predictor of quality of life and blindness in both eyes would incur the greatest costs. The cross-sectional studies do not provide sufficient information to estimate the probability of blindness in both eyes; hence, numerous assumptions would be required. The identified studies also include patients with anterior uveitis. The AG requested the patient-level data from the authors of one of the cross-sectional studies18 to be able to predict blindness over time from the outcomes reported within the clinical trials; however, these data were not provided. Given the number of assumptions that would be required to undertake this analysis in the absence of patient-level data, and the low proportions of patients reported to have glaucoma (< 3% in any arm) and new cases of macular oedema (< 8% in any arm) in the clinical trials,46–48 the AG decided that adopting such a complex analysis within the model may produce potentially misleading results.
Therefore, a simpler approach was taken and the assumptions were tested within exploratory analyses. For the base-case analysis, clinical experts to the AG helped to identify sources that could be used to estimate a constant blindness rate associated with (limited) current practice. All studies identified were cross-sectional rather than longitudinal. The best source of evidence was considered to be a study by Dick et al. ,19 a retrospective analysis of insurance claims data, because all patients (n = 1769) had posterior segment, non-infectious uveitis. A constant rate of blindness and uncertainty around this parameter was estimated by the AG based on the proportion of patients going blind within the study by Dick et al. 19 and the mean follow-up time. By 10 years, this study predicted that 6.6% of patients would go legally blind, in the absence of death from other causes. The proportions of patients who had unilateral and bilateral disease are not reported within the study. Two alternative similar sources were also identified as being potentially relevant, studies by Tomkins-Netzer et al. 18 and Durrani et al. 11 The constant blindness rate derived from the study by Tomkins-Netzer et al. 18 was deemed to be an underestimate by one of the clinicians consulted by the AG (Alastair Denniston, personal communication) and the study included a wider population than the target population of the current appraisal (including patients with infectious and anterior uveitis). The rate derived from the study by Durrani et al. 11 was substantially higher than the rate derived from Dick et al. 19 but this study also included a wider population. As the authors warned, ‘being a tertiary referral centre, more patients are likely to suffer from severe, often bilateral uveitis’ and they acknowledged that the results of their study ‘could not be applied to the general population because of the tertiary nature of the patient population’. 11 The AG explored the impact of using blindness rates based on these other two sources in exploratory analyses (see Exploratory sensitivity analyses and Results).
As discussed earlier, there is no evidence on the treatment effect of ADA or DEX on legal blindness. To model the impact of treatment with ADA on the rate of blindness, given the strict criteria for treatment failure within the VISUAL trials,46,47 it was assumed that patients could not go blind before treatment failure. This was assumed for both the intervention and the comparator. The rate of blindness following treatment failure was then approximated so that the rate of blindness at each cycle in the placebo group was equivalent to the estimate from Dick et al. 19 It was not considered clinically reasonable that a DEX implant would prevent all cases of blindness during treatment, but it was deemed equally unreasonable to assume that it would prevent no cases of blindness. In light of the absence of evidence around this parameter, the AG sampled from a uniform distribution between 0 and 1 within the PSA and used the mean of this distribution (0.5) for the deterministic analysis. Therefore, the AG assumed that half of the cases of blindness in this group would be avoided for the period in which the treatment effect was applied (30 weeks in the base case). It was assumed that patients in the comparator group would have the same blindness rate as in the general population.
The AG heard from clinicians that around 20–30% of patients with uveitis remain unilateral and that patients treated with DEX are more likely to be unilateral. The AG assumed that patients who remained unilateral would not go blind and therefore the rate of blindness in the DEX target population would be lower than that in the general population and this is turn would be lower than that in the ADA target population. For the base case, the AG assumed that, in the general population, 25% of patients would remain unilateral whereas in the DEX target population 30% of patients would remain unilateral. For ADA, the proportions of patients with unilateral uveitis as reported in the VISUAL I46 (9.2%) and VISUAL II47 (4.4%) trials were used for active and inactive patients respectively. The blindness rate for bilateral patients was determined by dividing the blindness rate estimated from Dick et al. 19 by the proportion of bilateral patients in the general population. The incidence of blindness in each analysis was adjusted by multiplying the rate of blindness for bilateral patients by the proportion of bilateral patients in each population.
Adverse events
One of the key drivers for new treatment options is the substantial AE profile of existing treatments, which reduce HRQoL and incur treatment costs. In addition, treatment with ADA and DEX is associated with AEs. Given that the main outcome measures being used from the clinical trials were the VFQ-25 and EQ-5D, it was assumed that these will capture the quality-of-life impacts associated with AEs during the period in which the treatments are provided. The incidence of AEs from the trials was therefore used only to calculate the additional costs associated with their management. As such, AEs included within the model are limited to those for which the cost of treatment is substantial. Based on advice from the clinical experts to the AG, the AEs associated with substantial costs of treatment are cataract, raised IOP, glaucoma, serious infections, hypertension, fractures and diabetes.
There are no longer-term safety data for ADA and DEX for uveitis beyond the data reported within the key clinical trials. 46–48 However, Burmester et al. 110 reported the results of a study on the long-term safety of ADA in 23,458 patients with other indications compared with the general population in terms of the incidence of disease. The study found that overall malignancy and lymphoma rates were not increased as a result of ADA use. Although the incidence of non-melanoma skin cancer was greater for patients receiving ADA for some of the indications included, for all indications death rates were equivalent to or lower than those expected in the general population. Given the findings of this study, the model does not include any longer-term cumulative impacts of ADA on patient outcomes. For DEX, the model assumed that patients will receive only one implant. The application of more than one implant is considered later in the discussion section.
There is no clinical rationale for the AEs associated with corticosteroid use to differ between study arms because usage is similar between the arms within the trials. Therefore, although diabetes and osteoporosis are associated with substantial costs, there are no real differences in incidence between the arms of the trials. Within the exploratory analysis assessing the impact of greater corticosteroid use in the comparator groups, the proportion of patients with these AEs was increased according to their incidence in the MUST trial. 57
The probabilities of AEs per cycle used in the model (Table 32) were calculated based on their incidence in the trials and the mean follow-up time of each trial.
| AE | DEX 700 | LCP(H) | Active uveitis | Inactive uveitis | SSI | ||
|---|---|---|---|---|---|---|---|
| ADA | LCP(VI) | ADA | LCP(VII) | ||||
| Raised IOP | 0.019 | 0.005 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 |
| Cataract | 0.016 | 0.011 | 0.002 | 0.002 | 0.001 | 0.003 | 0.008 |
| Glaucoma | 0.000 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 |
| Hypertension | 0.002 | 0.003 | 0.002 | 0.001 | 0.003 | 0.003 | 0.002 |
| Serious infections | 0.000 | 0.000 | 0.003 | 0.003 | 0.001 | 0.001 | 0.000 |
| Fracture | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 |
| Diabetes | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
Quality of life
The AG considered the published studies for mapping the VFQ-25 to the EQ-5D included in the database of mapping studies by Dakin. 111 However, none of the published mapping studies was based on a population with uveitis and, considering that the AG had access to the VFQ-25 and EQ-5D patient-level data at baseline from the HURON trial,48 the AG decided to fit a new mapping model. The AG used the approach that produced the best fit according to Browne et al. 112 (ordinary least squares) and noticed that the mapping resulted in similar coefficient values to those presented by Payakachat et al. ,113 who used an alternative modelling method (censored least absolute deviation). The mapping was used for all of the analyses involving DEX, within the exploratory analyses comparing the interventions with current practice, as provided in the MUST trial,57 and within a sensitivity analysis for ADA.
The patient-level data from the HURON trial48 were used to test for a correlation between the VFQ-25 and the EQ-5D at baseline. The scatterplot is presented in Figure 13. A linear regression model was fitted to the data to predict EQ-5D utilities from VFQ-25 scores. One regression model was fitted to all three arms of the HURON trial (sham, DEX 350 implant and DEX 700 implant) to maximise the sample size for the regression analysis. The underlying assumption was that the relationship between VFQ-25 scores and EQ-5D utilities would be independent of treatment. The fitted regression used in the economic model was:
FIGURE 13.
The relationship between the VFQ-25 and the EQ-5D based on patient-level data from the HURON trial. 48

It is recognised that a linear model is not bounded and is likely to have poor performance for utility values at the extremes. However, given that the mapping was only used for means, no extreme values were used. Alternative non-linear models (e.g. quadratic regression) were also tested but did not significantly improve the fit to the data. The variance–covariance matrix of the slope and the intercept of the regression model is presented in Table 33. To represent the uncertainty in the regression model, the matrix was used to sample the two coefficients of the regression model in the PSA.
| Intercept | VFQ-25 | |
| Intercept | 1.75E-03 | |
| VFQ-25 | –2.42E-05 | 3.63E-07 |
The baseline utilities, that is, the utilities for patients at week 0, were estimated based on the patient-level data from each trial: the HURON trial48 for DEX and its comparator [LCP(H)], the VISUAL I trial46 for ADA and its comparator in active patients [LCP(VI)] and the VISUAL II trial for ADA and its comparator in inactive patients [LCP(VII)]. In the HURON trial,48 the baseline utility and visual acuity were substantially different between the sham arm and the DEX arm (visual acuity was 71.3 for the sham arm and 63.7 for the DEX 700 arm). Clinical advisors to the AG were asked to consider whether or not the baseline difference in both utility and visual acuity were reasonably due to random variation. All three experts agreed that a difference in visual acuity of ≥ 10 letters is considered to be clinically significant and a difference below this level could be owing to random variation and therefore it is plausible that the difference between the arms at baseline in the HURON trial was due to random variation. The baseline utilities were not varied to represent any population subgroups because these data were not available from the trials. The impact of changing the baseline utility has been assessed within the univariate sensitivity analysis; however, this analysis assumes that the relative treatment effect remains the same. This is unlikely to be the case for subgroups with differing baseline utilities such as patients with unilateral or bilateral uveitis. However, there is no evidence from the trials around outcomes for these subgroups that would enable a robust subgroup analysis.
The VFQ-25 data from each follow-up point within the HURON trial48 (weeks 0, 8, 16 and 26) and the EQ-5D data from each follow-up point of the VISUAL trials46,47 (weeks 0, 1, 4, 6, 8, 12, 16, 20, 24, 27 and 32 and then every 4 weeks until week 80) were used to estimate the change in utility for each treatment group over the time period of the trials. These were adjusted according to the average baseline utilities but maintaining the change from baseline in each arm.
When comparing DEX with its comparator, the AG assumed that the utility of patients who received DEX would drop to that of patients in the comparator arm after the duration of the treatment effect. Within the base-case analysis, the treatment effect was assumed to last for 30 weeks (4 weeks longer than the trial period). Within the sensitivity analyses the utility was assumed to decrease to the baseline utility over varying time periods. When comparing ADA with its comparator, for patients who fail and hence discontinue treatment it was assumed that utility returns to the baseline utility score, adjusted for any reduction in utility associated with age. For patients who receive ADA beyond the duration of the trial (80 weeks), it was assumed that their utility remains constant after the last follow-up point until treatment discontinuation. This utility is based on the mean of the last 6 months of data. Figures 14–16 present the predicted mean utility values over time, excluding any adjustments for blindness, for DEX compared with LCP(H) for active patients, ADA compared with LCP(VI) for active patients and ADA compared with LCP(VII) for inactive patients respectively.
FIGURE 14.
Mean utilities for DEX compared with LCP(H) for active patients over time.

FIGURE 15.
Mean utilities for ADA compared with LCP(VI) for active patients over time.
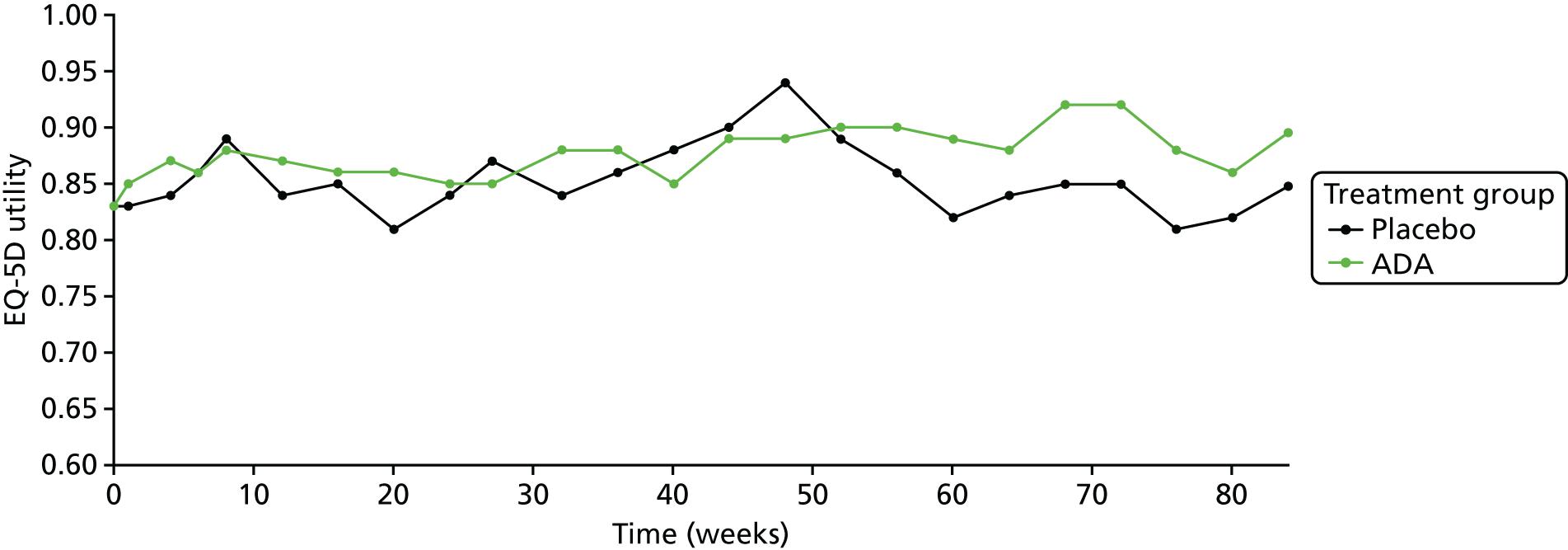
FIGURE 16.
Mean utilities for ADA compared with LCP(VII) for inactive patients over time.

Age adjustments to utility were based on the regression equation reported by Ara and Brazier. 114 Age-related utility was calculated using the following formula:
where A = 0.0212126, B = –0.0002587, C = –0.0000332 and D = 0.9508566.
The ratio between the utility for the general population at the starting age and that of the mean cohort age at each cycle was applied within the model.
Given that the main outcome measures being used from the clinical trials are the VFQ-25 and EQ-5D, it was assumed that these will capture the quality-of-life impacts associated with AEs during the period in which the treatments are provided.
Two UK-based studies report utilities associated with blindness,109,115 which the AG thought to be the best sources of evidence. Both studies have been used within previous NICE appraisals. 116–120 Czoski-Murray et al. 109 used contact lenses to simulate blindness associated with macular degeneration, whereas Brown115 estimated utilities according to valuations by patients with a range of conditions associated with blindness. The AG used the time trade-off values reported in these studies. Each study provided utilities for different levels of blindness and the AG calculated a weighted average based on the number of patients within the studies falling into each category. This assumed that patients with uveitis would have a similar distribution for the severity of blindness. The study by Czoski-Murray et al. 109 was used in the base-case analysis as it was based on public valuations of utility; however, it does not provide utilities for the worst states of blindness and may therefore overestimate the overall utility associated with blindness. This resulted in a utility associated with blindness of 0.38. Uncertainty around this parameter was modelled using the variance–covariance matrix provided within the study. The utility estimated from the study by Brown115 (0.57) was employed within sensitivity analysis.
Resource use and costs
The costs of ADA, DEX, immunosuppressants and corticosteroids were based on the latest drug tariff. 49 Drug acquisition costs included within the model are presented in Table 34.
| Drug | Dose | Brand name | 6-monthly cost (£) |
|---|---|---|---|
| ADA | 40 mg every 2 weeks | Humira | 4578 |
| DEX | One 0.7-mg implant | Ozurdex | 870 |
| Mycophenolate mofetil | 1 g twice daily | NA | 136 |
| Methotrexate | 15 mg weekly | NA | 16 |
| Ciclosporin | 2 mg/kg twice daily | NA | 985 |
| Azathioprine | 1 mg/kg daily | NA | 27 |
| Systemic prednisolone | 7.5 mg daily | NA | 12 |
The cost of treatment with immunosuppressants was calculated separately for each comparison [DEX vs. LCP(H), ADA vs. LCP(VI) and ADA vs. LCP(VII)] as a weighted average of mycophenolate mofetil, methotrexate, ciclosporin and azathioprine, based on their usage in the relevant trials (HURON,48 VISUAL I46 and VISUAL II47).
The two arms of each clinical trial were similar in terms of the use of corticosteroids and other medications, when reported. There was, however, an imbalance in the use of rescue therapy within the HURON trial. 48 The clinical study report states that the proportions of patients who received rescue therapy, which involved systemic and local corticosteroid use and immunosuppressants, in the DEX arm and the sham arm were 22.1% and 38.2% respectively. 71 Based on the patient-level data from the HURON trial,48 the largest imbalance is in the provision of immunosuppressants as rescue therapy; these therapies are also more costly than corticosteroids. Of those patients who were not already taking immunosuppressants at baseline, only one patient from the DEX arm (1.3%) received an immunosuppressant whereas eight patients in the sham arm (10.5%) received an immunosuppressant. Of these, three patients received an immunosuppressant for 1–2 months and the remaining five patients did not stop immunosuppressant use within the trial period. This suggests that DEX may reduce the need for immunosuppressants. The model includes the costs of the additional immunosuppressants provided to the proportion of patients receiving this rescue therapy. The use of corticosteroid rescue therapy within the HURON trial48 was more similar between the DEX group and the sham group (20.7% for DEX vs. 27.7% for sham) and such therapy is generally provided for only 2–4 weeks based on the patient-level data. Given that corticosteroids are inexpensive, this would result in a minimal cost difference between the groups and hence these costs have not been incorporated within the model. Within the base case, all other treatment costs were assumed to be the same between the DEX group and the LCP(H) group and between the ADA group and the LCP(VI)/LCP(VII) groups. An exploratory analysis was undertaken to explore the impact of an increase in the costs and utilities of the comparators.
The DEX implant was assumed to be administered within one outpatient appointment at a cost of £113.42, based on NHS Reference Costs 2014–15121 (minor vitreous retinal procedures, ≥ 19 years). ADA was assumed to be self-administered; the base-case model assumed that 10% of patients will need help from a district nurse to administer the injections, at a cost of £44, based on Unit Costs of Health and Social Care 2015122 (district nurse cost per hour). All other treatments would be administered by the patient and therefore there would be no extra costs of administration for corticosteroids or immunosuppressants.
The model assumed that all patients would receive monitoring every 6 weeks, irrespective of treatment. Monitoring consists of outpatient visits for visual function monitoring to assess the efficacy of the treatments and to monitor the risk of AEs. The AG assumed that monitoring for AEs was conducted alongside regular visual function monitoring follow-ups. It was also assumed that patients receiving immunosuppressants would receive six additional blood monitoring visits annually. Both regular monitoring and blood monitoring appointments were assumed to cost £96.11, based on NHS Reference Costs 2014–15121 (outpatient attendance visit, ophthalmology, face-to-face visit).
The management of cataract and glaucoma was based on surgery costs taken from NHS Reference Costs 2014–15. 121 Raised IOP was assumed to be treated with two doses of bimatoprost (Lumigan®, Allergan) on average (most patients will need just one dose but others will need many). Serious infection was assumed to be treated with hospitalisation and the cost was based on an average of NHS Reference Costs 2014–15 for the infections reported within the VISUAL trials. 46,47 Treatment for hypertension was based on the cost of antihypertensive treatment taken from the study by Breeze et al. 123
A focused search was undertaken in October 2016 to identify cost and utility studies of blindness (see Appendix 1 for the MEDLINE search strategy). Free-text terms for blindness and sight or vision loss (in the titles field) were combined with either an economic filter (balance of sensitivity and sensitivity) or a sensitive quality-of-life studies filter. The search was carried out in MEDLINE and MEDLINE-in-Process & Other Non-Indexed Citations (Ovid). The search for cost studies was limited from 2006 until 2016. Based on this review, the AG considered that the most recent good-quality evidence associated with the costs of blindness was presented within a health technology assessment of treatment for age-related macular degeneration. 124 The costs of each component included within the calculation of the total annual cost of blindness to the NHS and PSS have been updated with the most recent data and uplifted to 2015 prices using the Hospital & Community Health Services index,122 as shown in Table 35.
| Component | % of patients receiving service | Cost (£) | Source |
|---|---|---|---|
| Blind registrationa | 95 | 146 | Meads and Hyde125 |
| Low-vision aidsa | 33 | 191 | Meads and Hyde125 |
| Low-vision rehabilitationa | 11 | 329 | Meads and Hyde125 |
| Depression | 39 | 2378c | McCrone et al.126 |
| Hip replacement | 5 | 4086c | NHS Reference Costs 2014–15 121 |
| Community care | 6 | 281c | Curtis and Burns122 (social care for older people) |
| Residential care | 30 | 21,732b,c | Curtis and Burns122 (private residential care) |
| Annual total | 7659 |
Fracture and diabetes have been shown to be the largest cost items associated with the long-term use of corticosteroids. 31 The cost of fracture was based on evidence from a HTA monograph by Davis et al. 127 and includes the costs of hospitalisations, accident and emergency visits, referrals, prescriptions and general practitioner contacts. The cost of diabetes was based on the annual hospitalisation cost from the UK Prospective Diabetes Study, which is the largest study of the costs of diabetes and its complications in the UK,128 and the treatment costs from the study by Breeze et al. 123 Table 36 summarises the resource use and costs associated with the AEs included in the model.
| AE | Resource use | Cost (£) | Frequency | Source |
|---|---|---|---|---|
| Cataract | Cataract surgery | 852.40 | One-off cost | NHS Reference Costs 2014–15 121 |
| Raised IOP | Treatment with two doses of bimatoprost | 23.42 | One-off cost | British National Formulary 49 |
| Glaucoma | Glaucoma surgery | 581.25 | One-off cost | NHS Reference Costs 2014–15 121 |
| Serious infection | Hospitalisation | 5940.50 | One-off cost | NHS Reference Costs 2014–15 121 |
| Hypertension | Antihypertensive prescription | 7.04 | One-off cost | Breeze et al.123 |
| Permanent blindness | See Table 35 | 237 | Transition | See Table 35 |
| 7659 | Annual | |||
| Fracture | Hospitalisations, accident and emergency visits, referrals, prescriptions and general practitioner contacts | 2116.17–6022.62 depending on age and sex | One-off fracture cost | Davis et al.127 |
| Diabetes | Diabetes treatment and hospitalisation for complications of diabetes | 1521.46 | Annual | Alva et al.128 and Breeze et al.123 |
An important reason for developing new technologies is that existing treatments for non-infectious uveitis are associated with substantial AEs. In particular, long-term high-dose systemic corticosteroid use is associated with significant morbidity, including glaucoma, raised blood pressure, diabetes and osteoporosis. 41,42 Ideally, corticosteroid-sparing benefits would be taken into account in the comparison with current treatment. However, the VISUAL trials46,47 did not allow corticosteroid use in either arm following the initial corticosteroid burst and taper and in the HURON trial48 there was a minimal difference in corticosteroid usage between the arms of the trial. If corticosteroid usage is higher in clinical practice than in the trials, the effectiveness of the comparator may also increase, as well as the AE rate. Corticosteroid-sparing treatment was considered only within the exploratory analyses, in which the comparator was based on the MUST trial. 57
Based on advice received from the clinical advisors to the AG, an additional state was added to the model to reflect the possibility of patients achieving remission after a stable period, for example after 2 years on ADA. This would mean that patients would discontinue treatment on achieving remission but continue to experience the benefits of ADA until they were predicted to fail treatment from the extrapolated survival curves. Given that there is no evidence around this, within the base case we assumed that no patients would be taken off treatment because of remission; however, alternative assumptions around continued benefit following discontinuation because of remission were considered within the exploratory analyses.
Mortality
Mortality rates within the model were assumed to reflect those of the general population, based on the most recent Office for National Statistics life tables for England. 129
The model assumed that AEs have no impact on mortality, although it is recognised that in practice diabetes, osteoporosis and blindness would have some impact on mortality.
Model evaluation methods
The cost-effectiveness results for DEX and ADA compared with limited current practice are presented based on both the probabilistic and the deterministic versions of the model. In total, 5000 probabilistic samples were run to estimate the expected costs and QALYs. Uncertainty surrounding incremental costs, outcomes and cost-effectiveness was represented using CEACs and cost-effectiveness planes. It should, however, be noted that the uncertainty analysis is likely to underestimate the true uncertainty surrounding the cost-effectiveness of each option because of the numerous structural uncertainties associated with the model that are not captured within the PSA. A range of exploratory scenario analyses were undertaken to explore the sensitivity of the model results to key structural assumptions. A univariate sensitivity analysis was also undertaken to explore the impact of alternative plausible parameters on the model results. All model results are presented for the entire patient population of interest as evidence did not allow a subgroup analysis to be undertaken; the potential direction of the results for key subgroups such as patients with unilateral and bilateral uveitis are considered in the discussion section.
Probabilistic sensitivity analysis
To assess the uncertainty around the parameters used in the model, the AG defined probability distributions for most parameters using the available evidence and undertook PSA. Gamma distributions were generally used for costs and beta distributions for utility values and probabilities. The RR of blindness for DEX was based on a uniform distribution because of a lack of evidence. Table 37 summarises the input parameters and their base-case mean values and distributions used in the PSA. In addition to the parameters listed in Table 37, beta distributions were defined for utility scores at each time point in each arm, as well as the prevalence of concomitant therapy and the incidence of AEs and rescue therapy. Multivariate normal distributions were used for the parameters of the survival curves used to determine time to treatment failure. A Dirichlet distribution was used for the weight distribution of the cohort, which determined the mean dose cost of azathioprine and ciclosporin.
| Parameter | Mean | Distribution | Source |
|---|---|---|---|
| Age (years) | 44.8 | Fixed | |
| Discount rate (costs and utilities) (%) | 3.5 | Fixed | NICE104 |
| Sex, male (%) | 36.7 | Fixed | HURON trial48 |
| Cycle length (weeks) | 2 | Fixed | |
| Utilities | |||
| Baseline VFQ-25 score for DEX and LCP(H) arms | 66.63 | Beta | HURON trial (data on file) |
| Baseline EQ-5D score for patients with active uveitis | 0.83 | Beta | AbbVie52 |
| Baseline EQ-5D score for patients with inactive uveitis | 0.85 | Beta | AbbVie72 |
| Blindness utility | 0.38 | Multivariate normal (using variance–covariance matrix) | Czoski-Murray et al.109 |
| Regression model for relationship between VFQ-25 and EQ-5D scores | |||
| Intercept | 0.445 | Multivariate normal (using variance–covariance matrix in Table 33) | Based on patient-level data from the HURON trial (data on file) |
| Slope | 0.005 | ||
| Proportion of bilateral patients (%) | |||
| General population | 75 | Beta | Assumption |
| DEX population | 70 | Beta | Assumption |
| Active uveitis population | 90.8 | Beta | AbbVie52 |
| Inactive uveitis population | 95.6 | Beta | AbbVie72 |
| Blindness | |||
| Probability of blindness (annual) | 0.0068 | Beta | Dick et al.19 |
| RR of blindness for DEX during 6-month period following implantation | 0.5 | Uniform | Assumption |
| RR of blindness for ADA while on treatment | 0 | Fixed | Assumption |
| Remission | |||
| Rate of remission when treatment is stopped but the treatment effect continues | 0 | Fixed | Assumption |
| Drug costs (£) | |||
| DEX 700 mg | 870 | Fixed | BNF49 |
| ADA 40 mg | 352.14 | Fixed | BNF49 |
| Prednisolone | 1.24 | Fixed | BNF49 |
| Mycophenolate mophetil | 9.31 | Fixed | BNF49 |
| Methotrexate | 2.40 | Fixed | BNF49 |
| Ciclosporin | 48.50 | Fixed | BNF49 |
| Azathioprine | 3.24 | Fixed | BNF49 |
| Bimatoprost | 11.71 | Fixed | BNF49 |
| Adcal D3® (NextPharma, Göttingen, Germany) | 7.49 | Fixed | BNF49 |
| Omeprazole | 1.17 | Fixed | BNF49 |
| Administration and monitoring | |||
| Monitoring visit frequency (weeks) | 6 | Jabs et al.130 | |
| Monitoring visit cost (£) | 96.11 | Gamma | NHS Reference Costs 2014–15121 (outpatient attendance, ophthalmology, consultant-led) |
| DEX implant administration cost (£) | 113.42 | Gamma | NHS Reference Costs 2014–15121 (minor vitreous retinal procedures) |
| % of self-injectors needing district nurse for ADA | 10 | Beta | NICE131 |
| ADA administration cost (£) (patients who need help from a nurse) | 44 | Gamma | Curtis and Burns122 and NHS Reference Costs 2014–15121 (district nurse) |
| AE costs (£) | |||
| Cataract surgery | 852.40 | Gamma | NHS Reference Costs 2014–15121 (phacoemulsification cataract extraction and lens implant, with CC score 4+) |
| Raised IOP | 23.42 | Gamma | BNF49 |
| Glaucoma procedure | 581.25 | Gamma | NHS Reference Costs 2014–15121 (weighted average of glaucoma procedures) |
| Serious infection | 5940.50 | Gamma | NHS Reference costs 2014–15121 [average of infection hospitalisations (based on the proportions of each infection in the VISUAL trials46,47)] |
| Hypertension | 7.04 | Gamma | Breeze et al.123 |
| Blindness (transition) | 237 | Gamma | See Table 35 |
| Blindness (annual) | 7659 | Gamma | See Table 35 |
| Fracture | 2116.17–6022.62 | Gamma | Davis et al.127 |
| Diabetes | 1521.46 | Gamma | Alva et al.128 and Breeze et al.123 |
Exploratory sensitivity analyses
A number of exploratory analyses were undertaken to explore the uncertainties within the model. Although there was a lack of evidence to fully inform these exploratory analyses, the aim was to provide an indication of the impact of alternative assumptions on the results.
-
A greater proportion of patients are treated with immunosuppressants and corticosteroids in the comparator groups. In clinical practice it would be expected that a higher proportion of patients would receive systemic therapy. This would result in greater efficacy associated with the comparator, with a higher AE rate and higher costs.
As discussed in Chapter 3 (see Indirect comparison of treatments: rationale for not undertaking), it was not possible to undertake a NMA to compare DEX or ADA with an alternative comparator, which might be more representative of current practice. However, the comparator arm of the MUST trial57 (identified within the systematic review) was made up of patients who received systemic corticosteroids, supplemented in 86% of cases with immunosuppressants, and was thought by the clinical experts to the AG to be reasonably representative of clinical practice. Hence, this study was used to inform an exploratory analysis. This exploratory analysis was not undertaken for patients with inactive uveitis because the MUST trial included only patients with active uveitis. For active patients, data from the comparator arm of the MUST trial were used relating to (1) an estimate of the total proportion of patients receiving (i) corticosteroids and (ii) immunosuppressants, to estimate costs; (2) an estimate of the HRQoL of patients; and (3) the rates of any AEs associated with substantial resource use. With respect to the total proportion of patients receiving corticosteroids and immunosuppressants, it was unclear from the MUST trial publication exactly which immunosuppressants were used57 and hence the composition was assumed to be the same as that for the VISUAL I trial. 46 It should be noted that using the data from the MUST trial without performing any formal mixed treatment comparison assumes that the trial population was comparable with the populations within the VISUAL and HURON trials and does not include any measure of uncertainty around the comparison.
Within the base-case analysis, HRQoL was assumed to return to baseline levels following treatment failure with ADA or after 6 months following DEX implantation. Given that the comparator arm patients were able to receive immunosuppressants and corticosteroids, it was assumed within this exploratory analysis that patients treated with ADA or DEX were also able to receive immunosuppressants and corticosteroids, as in the comparator arm of the MUST trial following the end of treatment with the intervention. Therefore, the overall effectiveness of DEX and ADA was expected to increase as well as the effectiveness of the comparator.
The analysis assumed that treatment with prednisolone includes concomitant therapy with Adcal D3 (£47.58) and 20 mg of omeprazole once daily (£15.25).
-
Incidence and HRQoL impact of blindness. As there is limited evidence around the rate of legal blindness for this patient group, and there is no evidence around the impact of treatment on this rate, the AG performed exploratory analyses around these parameters. This was done by varying the rate of legal blindness in patients with uveitis who are treated with (limited) current practice (from 0 to 0.0374) based on alternative sources11,18 (see Permanent legal blindness) and varying the RR of legal blindness cases avoided as a result of treatments (from 0 to 1).
These analyses were also undertaken using (1) alternative utilities from Brown115 and (2) a higher cost of blindness based on the upper bound of the 95% CI for this parameter.
-
Patients who go into remission after ADA treatment. A proportion of patients who continue treatment with ADA may achieve remission. The base-case analysis assumed that these patients would continue to receive ADA until treatment failure; however, the clinical advisors to the AG suggested that, after around 2 years of stable disease, patients may no longer require treatment but because they are in remission they may maintain the same level of HRQoL as that while on treatment. This sensitivity analysis therefore assessed the impact of assuming that, after 2 years on treatment, a varying proportion of patients (0–1) would no longer receive ADA but their HRQoL would decrease only with age, until the treatment failure curve predicts failure or they die from other causes.
-
Using the VFQ-25 data from the VISUAL trials of ADA46,47 to map to EQ-5D utility data. This sensitivity analysis assessed the impact of using the regression analysis of the HURON trial data48 to map the VFQ-25 data from the VISUAL trials46,47 to EQ-5D utilities.
-
Extrapolation of time to treatment discontinuation for ADA. The impact of using alternative plausible parametric distributions (Weibull, Gompertz) for time to treatment discontinuation was explored.
-
Varying the time period over which the utility decreases to that of baseline after treatment. The treatment effect beyond 6 months for DEX and beyond treatment discontinuation for ADA is unknown. Within the base case, patients receiving DEX were assumed to take 4 weeks to return to baseline utility beyond the trial follow-up of 6 months. HRQoL for patients receiving ADA was assumed to return to baseline levels immediately on treatment discontinuation. Within this exploratory analysis the treatment effect beyond 6 months for DEX was varied from 0 to 8 weeks and the treatment effect beyond discontinuation for ADA was increased to 4 weeks.
Univariate sensitivity analyses
Each parameter within the base case was varied to assess its impact on the model results, as shown in Table 38.
| Parameters | Mean | Lower value | Upper value | Source |
|---|---|---|---|---|
| Utilities | ||||
| Baseline | 0.79 | 0.77 | 0.80 | HURON trial individual patient data (data on file) |
| 0.83 | 0.81 | 0.85 | AbbVie52 | |
| 0.85 | 0.83 | 0.87 | AbbVie72 | |
| Blindness | 0.35 | 0.28 | 0.42 | Czoski-Murray et al.109 |
| Administration and monitoring | ||||
| Monitoring visit frequency (weeks) | 6 | 4 | 8 | Jabs et al.130 |
| Monitoring visit cost (£) | 96.11 | 77.27 | 114.95 | NHS Reference Costs 2014–15121 (outpatient attendance, ophthalmology, consultant-led) |
| DEX implant administration cost (£) | 113.42 | 91.15 | 135.65 | NHS Reference Costs 2014–15121 (minor vitreous retinal procedures) |
| % of self-injectors needing district nurse for ADA | 10 | 0 | 20 | NICE131 |
| ADA administration cost (£) (patients who need help from a nurse) | 44 | 29.96 | 44.56 | Curtis and Burns122 |
| AE costs (£) | ||||
| Raised IOP | 23.42 | 11.71 | 46.84 | BNF49 |
| Cataract surgery | 852.40 | 658.33 | 1019.47 | NHS Reference Costs 2014–15121 (phacoemulsification cataract extraction and lens implant, with CC score 4+) |
| Glaucoma procedure | 581.25 | 467.32 | 695.17 | NHS Reference Costs 2014–15121 (weighted average of glaucoma procedures) |
| Hypertension | 7.04 | 5.66 | 8.42 | Breeze et al.123 |
| Serious infections | 5940.50 | 4776 | 7105 | NHS Reference Costs 2014–15121 [average of infection hospitalisations (based on the proportions of each infection in the VISUAL trials46,47)] |
| Blindness (transition) | 236.95 | 191 | 283 | See Table 35 |
| Blindness (annual) | 7658.71 | 6158 | 9160 | See Table 35 |
Results
Dexamethasone
Base case
The base-case results are presented in Table 39. Based on the probabilistic version of the model, a single DEX implant combined with limited current practice as provided in the HURON trial48 [DEX 700 + LCP(H)] was estimated to produce 0.029 incremental QALYs compared with LCP(H) alone at an additional cost of £573, resulting in an ICER of £19,509 per QALY gained. Figure 17 presents the CEAC. Assuming WTP thresholds of £20,000 and £30,000 per QALY gained, the probability that a single DEX implant produces more net benefit than LCP(H) is estimated to be 0.47 and 0.72 respectively. The deterministic results were similar to those generated using the probabilistic model (Table 40), with an estimated ICER of £20,058 per QALY gained for DEX 700 + LCP(H) compared with LCP(H). A breakdown of the results of the deterministic analysis is provided in Appendix 7.
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) | Probability of cost-effectiveness at WTP threshold of | |
|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | ||||||
| LCP(H)a | 14.599 | 39,992 | 0.53 | 0.28 | |||
| DEX 700 + LCP(H)a | 14.629 | 40,565 | 0.029 | 573 | 19,509 | 0.47 | 0.72 |
FIGURE 17.
Cost-effectiveness acceptability curve for DEX 700 + LCP(H) vs. LCP(H).
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|
| LCP(H)a | 14.613 | 39,655 | |||
| DEX 700 + LCP(H)a | 14.641 | 40,235 | 0.029 | 580 | 20,058 |
The small differences in both costs and QALYs between the two groups mean that the ICER is very sensitive to alternative model parameters and assumptions, as shown within subsequent sensitivity analyses.
Figure 18 shows the cost-effectiveness scatterplot for DEX 700 + LCP(H) compared with LCP(H). The scatterplot shows that there is a negative correlation between incremental costs and incremental QALYs. The AG believes that this is because blindness has a strong impact both on QALYs gained and on costs, and the impact of DEX on blindness is very uncertain. A low RR of blindness would lead to increased QALY gains and important cost savings.
FIGURE 18.
Cost-effectiveness plane scatterplot of DEX 700 + LCP(H) vs. LCP(H).

Exploratory analyses
This exploratory analysis suggests that injecting a DEX implant before applying a treatment considered to be current practice (a mix of systemic steroids and immunosuppressants, based on the comparator within the MUST trial57) is expected to produce 0.011 additional QALYs at an incremental cost of £216 compared with current practice, resulting in an ICER of £19,899 per QALY gained, as shown in Table 41.
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) | Probability of cost-effectiveness at WTP threshold of | |
|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | ||||||
| CP(M)a | 15.152 | 63,465 | 0.54 | 0.45 | |||
| DEX 700 + LCP(H)b before CP(M)a | 15.163 | 63,681 | 0.011 | 216 | 19,899 | 0.47 | 0.55 |
Within this exploratory analysis, the total QALYs associated with DEX 700 increase compared with the base case because of the assumption that patients would be able to receive more immunosuppressants and corticosteroids (equivalent to the comparator group) after 6 months following the DEX implant. It should be noted that the ICER estimated for DEX 700 compared with current practice as provided in the MUST trial57 [CP(M)] is only slightly higher than that estimated for DEX 700 compared with LCP(H). The difference would be higher if different rates of blindness had been applied for CP(M) and LCP(H). It is reasonable to assume that CP(M) would lead to a lower incidence of blindness than LCP(H) because of the more intensive treatment, but the AG assumed the same rate of blindness for both given the absence of evidence to estimate rates for both.
The AG analysed the combined impact of different blindness rates based on different sources in the literature and assuming different RRs for blindness on DEX. As shown in Table 42, the impact of the RR of blindness on the ICER for DEX 700 + LCP(H) compared with LCP(H) alone is very important and there is no evidence describing the impact that DEX will have on the rate of blindness. The higher the rate of blindness, the greater the impact of the RR on the model results. Assuming a rate of blindness from Durrani et al. 11 and a RR of 1 (i.e. a DEX implant has no effect on blindness), the ICER for DEX 700 + LCP(H) compared with LCP(H) alone is £56,329 per QALY gained, whereas DEX 700 dominates if the RR is ≤ 0.25 based on the same rate of blindness.
| Annual rate of blindness (source) | RR of blindness on DEX | ||||
|---|---|---|---|---|---|
| 0 (no blindness) | 0.25 | 0.50a | 0.75 | 1 (no effect) | |
| 0 (assumption) | 48,937 | 48,937 | 48,937 | 48,937 | 48,937 |
| 0.0038 (Tomkins-Netzer et al.18) | 17,100 | 21,816 | 28,089 | 36,844 | 49,915 |
| 0.0066a (Dick et al.19) | 8688 | 13,314 | 20,058a | 30,805 | 50,627 |
| 0.0374 (Durrani et al.11) | Dominates | Dominates | 557 | 10,900 | 56,329 |
The AG also explored the impact of assuming a different source for the utility for patients following the onset of blindness. The base case used an estimate based on the study by Czoski-Murray et al. ;109 the exploratory analysis was undertaken using an estimate reported by Brown115 The results of these exploratory analyses are presented in Table 43, showing that the ICERs for DEX 700 + LCP(H) compared with LCP(H) were higher than those based on the study by Czoski-Murray et al. 109 (in cases in which the rate of blindness is higher than zero and DEX 700 has an impact on the rate of blindness). This is because the utility for blindness based on the study by Czoski-Murray et al. 109 (0.38) was lower than that based on the study by Brown115 (0.57).
| Annual rate of blindness (source) | RR of blindness on DEX | ||||
|---|---|---|---|---|---|
| 0 (no blindness) | 0.25 | 0.50a | 0.75 | 1 (no effect) | |
| 0 (assumption) | 48,937 | 48,937 | 48,937 | 48,937 | 48,937 |
| 0.0038 (Tomkins-Netzer et al.18) | 22,015 | 26,972 | 32,988 | 40,440 | 49,915 |
| 0.0066a (Dick et al.19) | 12,108 | 17,782 | 25,257a | 35,550 | 50,627 |
| 0.0374 (Durrani et al.11) | Dominates | Dominates | 853 | 15,198 | 56,329 |
To explore the impact of the cost of blindness, the AG undertook an analysis using the upper bounds of the 95% CIs for the annual cost of blindness and the cost of the transition to blindness. Table 44 presents the result of these exploratory analyses, which lead to lower ICERs for DEX 700 + LCP(H) compared with LCP(H) than in the analyses using the mean costs of blindness (in cases in which the rate of blindness is higher than zero and DEX 700 has an impact on the rate of blindness).
| Annual rate of blindness (source) | RR of blindness on DEX | ||||
|---|---|---|---|---|---|
| 0 (no blindness) | 0.25 | 0.50a | 0.75 | 1 (no effect) | |
| 0 (assumption) | 48,937 | 48,937 | 48,937 | 48,937 | 48,937 |
| 0.0038 (Tomkins-Netzer et al.18) | 15,195 | 20,185 | 26,822 | 36,085 | 49,915 |
| 0.0066a (Dick et al.19) | 6283 | 11,174 | 18,305a | 29,668 | 50,627 |
| 0.0374 (Durrani et al.11) | Dominates | Dominates | Dominates | 8534 | 56,329 |
For the above analyses, when the annual rate of blindness is set to 0, the results could be used to give an indication of the cost-effectiveness of DEX for patients with unilateral disease (as patients with unilateral disease are unlikely to become legally blind, unless their disease progresses to become bilateral). The ICER when the annual rate of blindness is set to 0 is £48,937. It is important to note that the treatment effect may also be different (expected to be reduced) for unilateral patients compared with a pooled group of unilateral and bilateral patients; however, there is no evidence available to model this.
In the base case it was assumed that the health-related gain from DEX as measured at the end of the HURON trial48 (week 26) is maintained for 4 weeks (up to week 30) and then falls to that of the comparator arm. Table 45 shows the impact of varying the treatment effect duration on the cost-effectiveness estimates. The ICER for DEX 700 + LCP(H) compared with LCP(H) varies from £24,715 per QALY gained assuming 26 weeks of treatment effect to £12,154 per QALY gained assuming 42 weeks of treatment effect.
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|
| LCP(H) | 14.613 | 39,655 | |||
| DEX 700: 26 weeks | 14.637 | 40,256 | 0.024 | 600 | 24,715 |
| DEX 700: 30 weeksa | 14.641 | 40,235 | 0.029 | 580 | 20,058 |
| DEX 700: 34 weeks | 14.646 | 40,214 | 0.033 | 559 | 16,692 |
| DEX 700: 42 weeks | 14.655 | 40,173 | 0.043 | 518 | 12,154 |
Univariate sensitivity analyses
The AG explored the impact of different parameters on the results of the model, as shown in Table 46.
| Parameter | Base case, lower value, upper value | ICER based on lower value (£) | ICER based on upper value (£) |
|---|---|---|---|
| Utilities | |||
| Baseline | 0.79, 0.77, 0.80 | 20,346 | 19,783 |
| Blindness | 0.38, 0.31, 0.57 | 18,551 | 25,257 |
| Administration and monitoring | |||
| Monitoring visit frequency (weeks) | 6, 4, 8 | 20,545 | 19,814 |
| Monitoring visit cost (£) | 44, 35.80, 53.03 | 19,854 | 20,282 |
| DEX implant administration cost (£) | 113.42, 91.15, 135.65 | 19,326 | 20,863 |
| AE costs (£) | |||
| Raised IOP | 23.42, 19.06, 28.23 | 20,024 | 20,095 |
| Cataract surgery | 852.40, 658.33, 1019.47 | 19,534 | 20,635 |
| Glaucoma procedure | 581.25, 467.32, 695.17 | 20,173 | 19,931 |
| Hypertension | 7.04, 5.66, 8.42 | 20,058 | 20,057 |
| Blindness (transition) | 237, 191, 283 | 20,061 | 20,054 |
| Blindness (annual) | 7659, 6158, 9160 | 21,807 | 18,308 |
The model results were generally robust to changes in the values of these parameters. The model was most sensitive to assumptions around the comparator, assumptions around permanent blindness and the duration of the treatment effect.
Adalimumab: active uveitis patients
Base case
The base-case results are presented in Table 47. In the base case, ADA + LCP(VI), as provided in the VISUAL I trial,46 was estimated to produce 0.194 incremental QALYs compared with LCP(VI) alone in patients with active uveitis at an additional cost of £18,321, resulting in an ICER of £94,523 per QALY gained. The ICER generated using the deterministic version of the model (£95,506) (Table 48) was similar to that in the probabilistic model. A breakdown of the results of the deterministic analysis is provided in Appendix 7. Figure 19 shows the CEAC for ADA + LCP(VI) compared with LCP(VI) in patients with active uveitis. It should be noted that within the VISUAL I trial46 both treatment groups included an initial systemic steroid burst, which was tapered by week 15, and around 30% of patients in both arms received systemic immunosuppressants.
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) | Probability of cost-effectiveness at WTP threshold of | |
|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | ||||||
| LCP(VI)a | 14.897 | 47,776 | 1.00 | 1.00 | |||
| ADA + LCP(VI)a | 15.091 | 66,098 | 0.194 | 18,321 | 94,523 | 0.00 | 0.00 |
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|
| LCP(VI)a | 14.919 | 47,186 | |||
| ADA + LCP(VI)a | 15.110 | 65,401 | 0.191 | 18,215 | 95,506 |
FIGURE 19.
Cost-effectiveness acceptability curve for ADA + LCP(VI) vs. LCP(VI) in patients with active uveitis.
Figure 20 shows the cost-effectiveness plane for ADA + LCP(VI) compared with LCP(VI). The scatterplot shows that there is a positive correlation between the incremental costs and the incremental QALYs. The AG believes that this is because longer ADA treatments lead to more QALYs but also incur important additional costs.
FIGURE 20.
Cost-effectiveness plane scatterplot of ADA + LCP(VI) vs. LCP(VI) in patients with active uveitis.
Exploratory analyses
The AG undertook an exploratory analysis (Table 49) whereby patients who fail ADA are assumed to receive a treatment that the AG considered representative of current practice, a mix of systemic steroids and immunosuppressants based on the MUST trial57 [CP(M)]. The analysis shows that ADA + LCP(VI) before CP(M) is expected to produce 0.0159 additional QALYs at an incremental cost of £17,183 compared with CP(M), resulting in an ICER of £109,044 per QALY gained.
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) | Probability of cost-effectiveness at WTP threshold of | |
|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | ||||||
| CP(M)a | 15.655 | 66,171 | 1.00 | 1.00 | |||
| ADA + LCP(VI)b before CP(M)a | 15.813 | 83,355 | 0.158 | 17,183 | 109,044 | 0.00 | 0.00 |
Within this exploratory analysis, the total QALYs associated with the ADA group increase compared with the base case because of the assumption that more patients would be able to receive immunosuppressants and corticosteroids (equivalent to the proportion in the comparator group) after ADA treatment failure. It should be noted that the ICER estimated for ADA compared with CP(M) is only slightly higher than that for ADA compared with LCP(VI). The difference would be higher if different rates of blindness had been applied for CP(M) and LCP(VI). It is reasonable to assume that CP(M) would lead to a lower incidence of blindness than LCP(VI) because of the more intensive treatment, but the AG assumed the same rate of blindness for both given the absence of evidence to estimate rates for both.
The AG analysed the combined impact of different blindness rates based on different sources in the literature and assuming different RRs for patients before treatment failure. As shown in Table 50, the impact of the RR of blindness on the ICER for ADA + LCP(VI) compared with LCP(VI) in patients with active uveitis is highly influential. The higher the rate of blindness, the greater the impact of the RR. Assuming the highest rate of blindness from the literature (based on the study by Durrani et al. 11) resulted in an ICER for ADA + LCP(VI) compared with LCP(VI) of £202,592 per QALY gained for a RR of 1 (i.e. ADA has no effect on blindness) and £33,003 per QALY gained for a RR of 0 (i.e. no patient goes blind before treatment failure).
| Annual rate of blindness (source) | RR of blindness before treatment failure | ||||
|---|---|---|---|---|---|
| 0 (no blindness)a | 0.25 | 0.50 | 0.75 | 1 (no effect) | |
| 0 (assumption) | 192,808 | 192,808 | 192,808 | 192,808 | 192,808 |
| 0.0038 (Tomkins-Netzer et al.18) | 121,908 | 134,773 | 150,325 | 169,503 | 193,740 |
| 0.0066a (Dick et al.19) | 95,506a | 110,263 | 129,611 | 156,077 | 194,471 |
| 0.0374 (Durrani et al.11) | 33,003 | 44,570 | 63,587 | 100,494 | 202,592 |
The AG also explored the impact of assuming a different source for the utility for patients following the onset of blindness. In the base case the utility estimate was based on the study by Czoski-Murray et al. ;109 the exploratory analysis was undertaken using the utility estimate reported by Brown115 The results of this exploratory analysis are shown in Table 51. The ICERs for ADA + LCP(VI) compared with LCP(VI) were higher using the utility estimate from Brown115 than when using the utility estimate from Czoski-Murray et al. 109 This is because the utility estimate for blindness is lower in the study by Czoski-Murray et al. 109 (0.38) than in the study by Brown115 (0.57).
| Annual rate of blindness (source) | RR of blindness before treatment failure | ||||
|---|---|---|---|---|---|
| 0 (no blindness)a | 0.25 | 0.50 | 0.75 | 1 (no effect) | |
| 0 (assumption) | 192,808 | 192,808 | 192,808 | 192,808 | 192,808 |
| 0.0038 (Tomkins-Netzer et al.18) | 142,399 | 152,827 | 164,646 | 178,154 | 193,740 |
| 0.0066a (Dick et al.19) | 119,012a | 132,539 | 148,886 | 169,031 | 194,471 |
| 0.0374 (Durrani et al.11) | 48,876 | 63,923 | 86,679 | 124,952 | 202,592 |
To explore the impact of the cost of blindness, the AG undertook an analysis using the upper bounds of the 95% CIs for the annual cost of blindness and the cost of the transition to blindness. Table 52 presents the results of this exploratory analysis, which show that the ICERs for ADA + LCP(VI) compared with LCP(VI) are lower than in the analyses using the mean costs of blindness, except when a blindness rate of 0 or a RR before treatment failure of 1 is assumed.
| Annual rate of blindness (source) | RR of blindness before treatment failure | ||||
|---|---|---|---|---|---|
| 0 (no blindness)a | 0.25 | 0.50 | 0.75 | 1 (no effect) | |
| 0 (assumption) | 192,808 | 192,808 | 192,808 | 192,808 | 192,808 |
| 0.0038 (Tomkins-Netzer et al.18) | 120,637 | 133,725 | 149,546 | 169,056 | 193,712 |
| 0.0066a (Dick et al.19) | 93,765a | 108,775 | 128,453 | 155,372 | 194,422 |
| 0.0374 (Durrani et al.11) | 30,187 | 41,936 | 61,245 | 98,713 | 202,352 |
In the base case, the AG assumed that patients would stay on ADA until treatment failure. However, based on clinical advice received by the AG, an additional analysis was undertaken assuming that, after 2 years of successful treatment, a proportion of patients discontinue treatment as they are in remission and retain the benefits of treatment. Table 53 presents the results for different annual discontinuation rates for patients who have completed 2 years of successful treatment. As expected, only the cost of treatment with ADA varies with the different rates of treatment discontinuation after remission: the cost of ADA treatment decreases as the rate of discontinuation increases and therefore the ICER for ADA + LCP(VI) compared with LCP(VI) also decreases. If all patients who have not failed treatment (according to the discontinuation criteria defined in the VISUAL trials46,47) by 2 years could discontinue ADA and retain the benefits accrued from treatment, the ICER for ADA + LCP(VI) compared with LCP(VI) would be £35,299 per QALY gained.
| Annual rate of treatment discontinuation | Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|---|
| 0.00a | LCP(VI)b | 14.919 | 47,186 | |||
| ADA + LCP(VI)b | 15.110 | 65,401 | 0.191 | 18,215 | 95,506 | |
| 0.10 | LCP(VI)b | 14.919 | 47,186 | |||
| ADA + LCP(VI)b | 15.110 | 60,034 | 0.191 | 12,848 | 67,363 | |
| 0.25 | LCP(VI)b | 14.919 | 47,186 | |||
| ADA + LCP(VI)b | 15.110 | 57,239 | 0.191 | 10,052 | 52,707 | |
| 1.00 | LCP(VI)b | 14.919 | 47,186 | |||
| ADA + LCP(VI)b | 15.110 | 53,918 | 0.191 | 6732 | 35,299 |
It should be noted that the clinical advisors to the AG suggested that the treatment failure criteria for the VISUAL trials46,47 are more strict than would be used in clinical practice and hence it is possible that a greater proportion of patients would still be receiving ADA treatment at 2 years. However, there is no evidence around the extent of the benefit of ADA in these patients.
The AG undertook an exploratory analysis using EQ-5D scores mapped from VFQ-25 scores captured in the VISUAL I trial46 instead of directly measured EQ-5D scores. This analysis resulted in a higher incremental QALY gain and therefore a slightly lower ICER for ADA + LCP(VI) compared with LCP(VI) than in the base case (Table 54).
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|
| LCP(VI)a | 14.350 | 47,186 | |||
| ADA + LCP(VI)a | 14.546 | 65,401 | 0.196 | 18,215 | 92,884 |
To assess the impact of uncertainty around the extrapolation of time to treatment failure, the AG undertook an exploratory analysis using alternative parametric curves (Table 55). The ICER for ADA + LCP(VI) compared with LCP(VI) was considerably higher when using a Gompertz distribution (£101,429 per QALY) and a Weibull distribution (£103,369 per QALY) than when using a log-normal distribution, as in the base case (£95,506 per QALY).
| Parametric curve | Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Log-normala | LCP(VI)b | 14.919 | 47,186 | |||
| ADA + LCP(VI)b | 15.110 | 65,401 | 0.191 | 18,215 | 95,506 | |
| Gompertz | LCP(VI)b | 14.947 | 47,186 | |||
| ADA + LCP(VI)b | 15.569 | 110,215 | 0.621 | 63,029 | 101,429 | |
| Weibull | LCP(VI)b | 14.917 | 47,186 | |||
| ADA + LCP(VI)b | 15.031 | 58,938 | 0.114 | 11,751 | 103,369 |
Univariate sensitivity analyses
The AG explored the impact of different parameters on the results of the model, as shown in Table 56.
| Parameters | Base case, lower value, upper value | ICER based on lower value (£) | ICER based on upper value (£) |
|---|---|---|---|
| Utilities | |||
| Baseline | 0.83, 0.81, 0.85 | 97,804 | 93,419 |
| Blindness | 0.38, 0.31, 0.57 | 88,602 | 119,012 |
| Administration and monitoring | |||
| Monitoring visit frequency (weeks) | 6, 4, 8 | 95,983 | 95,267 |
| Monitoring visit cost (£) | 96.11, 77.27, 114.95 | 95,290 | 95,744 |
| ADA administration cost (£) (help from a nurse) | 44, 35.80, 53.03 | 95,272 | 95,763 |
| % of self-injectors needing district nurse for ADA | 10, 0, 20 | 94,253 | 96,758 |
| AE costs (£) | |||
| Cataract surgery | 852.40, 658.33, 1019.47 | 95,465 | 95,551 |
| Glaucoma procedure | 581.25, 467.32, 695.17 | 95,487 | 95,527 |
| Serious infections | 5940, 4776, 7105 | 95,272 | 95,763 |
| Hypertension | 7.04, 5.66, 8.42 | 95,505 | 95,506 |
| Blindness (transition) | 237, 191, 283 | 95,510 | 95,502 |
| Blindness (annual) | 7659, 6158, 9160 | 97,243 | 93,769 |
Of those parameters tested within the univariate sensitivity analysis, the parameters relating to baseline utility and the utility of blindness had the greatest impact on the ICER for ADA + LCP(VI) compared with LCP(VI). However, the model was most sensitive to assumptions around the comparator, assumptions around permanent blindness and the proportion of patients who would discontinue treatment on achieving remission and retain the benefits of treatment.
Adalimumab: inactive uveitis patients
Base case
In the base case, ADA plus limited current practice as provided in the VISUAL II trial47 [LCP(VII)] was estimated to produce 0.118 incremental QALYs compared with LCP(VII) alone at an extra cost of £37,432, resulting in an ICER of £317,547 per QALY gained in patients with inactive uveitis, as shown in Table 57. The deterministic analysis produced a slightly higher ICER (£321,405), as shown in Table 58. A breakdown of the results of the deterministic analysis is provided in Appendix 7. Figure 21 shows the CEAC for ADA + LCP(VII) compared with LCP(VII) in patients with inactive uveitis and Figure 22 shows the cost-effectiveness plane scatterplot of ADA + LCP(VII) compared with LCP(VII). The scatterplot shows a positive correlation between incremental costs and incremental QALYs, as was the case for patients with active uveitis. However, in patients with inactive uveitis, the comparator was more effective than the intervention arm. It should be noted that around 47% of patients in both arms received systemic immunosuppressants.
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) | Probability of cost-effectiveness at WTP threshold of | |
|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | ||||||
| LCP(VII)a | 15.221 | 48,642 | 1.00 | 1.00 | |||
| ADA + LCP(VII)a | 15.339 | 86,074 | 0.118 | 37,432 | 317,547 | 0.00 | 0.00 |
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|
| LCP(VII)a | 15.244 | 48,111 | |||
| ADA + LCP(VII)a | 15.361 | 85,462 | 0.116 | 37,351 | 321,405 |
FIGURE 21.
Cost-effectiveness acceptability curve for ADA + LCP(VII) compared with LCP(VII) in patients with inactive uveitis.

FIGURE 22.
Cost-effectiveness plane scatterplot of ADA + LCP(VII) compared with LCP(VII) in patients with inactive uveitis.
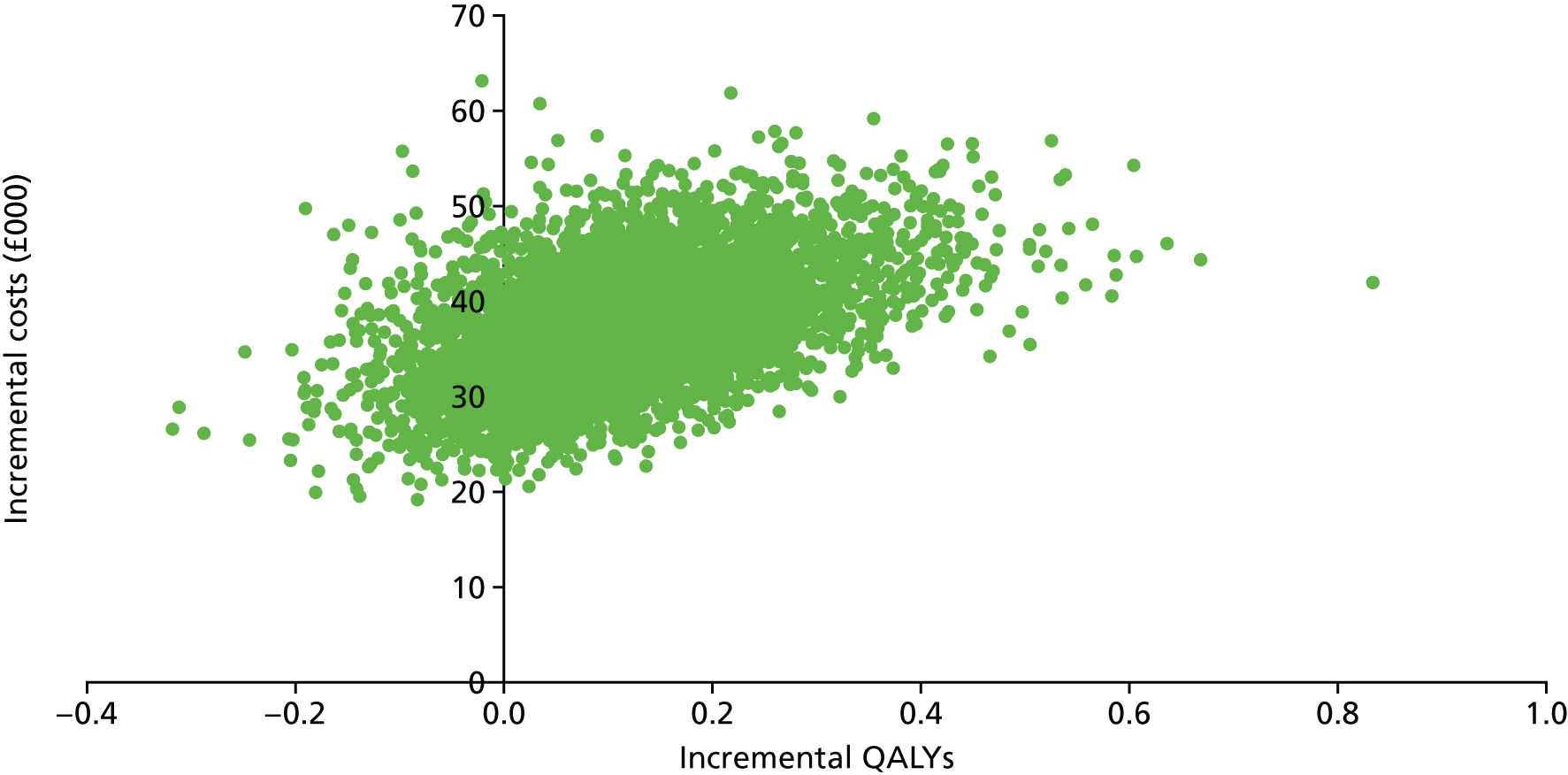
Exploratory analyses
The AG analysed the combined impact of different blindness rates based on different sources in the literature and assuming different RRs for patients before treatment failure. As shown in Table 59, the impact of the RR of blindness on the ICER for ADA + LCP(VII) compared with LCP(VII) in patients with inactive uveitis is very important. The higher the rate of blindness, the greater the impact of the RR. Assuming the highest rate of blindness from the literature (based on the study by Durrani et al. 11) resulted in an ICER for ADA + LCP(VII) compared with LCP(VII) of £7,411,362 per QALY gained for a RR of 1 (i.e. assuming that ADA has no effect on blindness) and an ICER of £85,544 per QALY for a RR of 0 (i.e. assuming that no patient goes blind before treatment failure).
| Annual rate of blindness (source) | RR of blindness before treatment failure | ||||
|---|---|---|---|---|---|
| 0 (no blindness)a | 0.25 | 0.50 | 0.75 | 1 (no effect) | |
| 0 (assumption) | 4,814,459 | 4,814,459 | 4,814,459 | 4,814,459 | 4,814,459 |
| 0.0038 (Tomkins-Netzer et al.18) | 527,056 | 679,863 | 956,162 | 1,606,857 | 4,988,973 |
| 0.0066a (Dick et al.19) | 321,405a | 420,805 | 607,928 | 1,089,865 | 5,133,625 |
| 0.0374 (Durrani et al.11) | 85,544 | 112,594 | 167,837 | 331,006 | 7,411,362 |
The AG also explored the impact of assuming a different source for the utility for patients following the onset of blindness. In the base case the utility estimate was based on the study by Czoski-Murray et al. ;109 the exploratory analysis was undertaken using the utility estimate reported by Brown. 115 The results of this exploratory analysis are shown in Table 60. The ICERs for ADA + LCP(VII) compared with LCP(VII) were higher using the utility estimate from Brown115 than when using the utility estimate from Czoski-Murray et al. 109 This is because the utility estimate for blindness is lower in the study by Czoski-Murray et al. 109 (0.38) than in the study by Brown115 (0.57).
| Annual rate of blindness (source) | RR of blindness before treatment failure | ||||
|---|---|---|---|---|---|
| 0 (no blindness)a | 0.25 | 0.50 | 0.75 | 1 (no effect) | |
| 0 (assumption) | 4,814,459 | 4,814,459 | 4,814,459 | 4,814,459 | 4,814,459 |
| 0.0038 (Tomkins-Netzer et al.18) | 821,798 | 1,040,149 | 1,414,808 | 2,206,843 | 4,988,973 |
| 0.0066a (Dick et al.19) | 514,958a | 665,947 | 940,350 | 1,593,079 | 5,133,625 |
| 0.0374 (Durrani et al.11) | 141,538 | 185,892 | 275,797 | 536,245 | 7,411,362 |
To explore the impact of the cost of blindness, the AG undertook an analysis using the upper bounds of the 95% CIs for the annual cost of blindness and the cost of the transition to blindness. Table 61 presents the results of this exploratory analysis, which show that the ICERs for ADA + LCP(VI) compared with LCP(VI) are lower than in the analyses using the mean costs of blindness, except when a blindness rate of 0 or a RR before treatment failure of 1 is assumed.
| Annual rate of blindness (source) | RR of blindness before treatment failure | ||||
|---|---|---|---|---|---|
| 0 (no blindness)a | 0.25 | 0.50 | 0.75 | 1 (no effect) | |
| 0 (assumption) | 4,814,459 | 4,814,459 | 4,814,459 | 4,814,459 | 4,814,459 |
| 0.0038 (Tomkins-Netzer et al.18) | 523,933 | 676,848 | 953,341 | 1,604,491 | 4,988,973 |
| 0.0066a (Dick et al.19) | 318,140a | 417,608 | 604,860 | 1,087,124 | 5,133,625 |
| 0.0374 (Durrani et al.11) | 82,177 | 109,245 | 164,519 | 327,767 | 7,411,362 |
In the base case, the AG assumed that patients would stay on ADA until treatment failure. However, based on clinical advice received by the AG, a further analysis was undertaken assuming that, after 2 years of successful treatment, a proportion of patients discontinue treatment as they are in remission and retain the benefits of treatment. Table 62 presents the results for different annual discontinuation rates for patients after 2 years of successful treatment. As expected, only the cost of treatment with ADA varies with the different rates of treatment discontinuation after remission: the cost of ADA treatment decreases as the rate of discontinuation increases and therefore the ICER for ADA + LCP(VII) compared with LCP(VII) also decreases. It is worth noting that, if all patients could discontinue ADA treatment after 2 years and still retain the benefits of treatment, the ICER for ADA + LCP(VII) compared with LCP(VII) would be £84,132 per QALY gained.
| Rate of remission | Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|---|
| 0.00a | LCP(VII)b | 15.244 | 48,111 | |||
| ADA + LCP(VII)b | 15.361 | 85,462 | 0.116 | 37,351 | 321,405 | |
| 0.10 | LCP(VII)b | 15.244 | 48,111 | |||
| ADA + LCP(VII)b | 15.361 | 71,241 | 0.116 | 23,130 | 199,031 | |
| 0.25 | LCP(VII)b | 15.244 | 48,111 | |||
| ADA + LCP(VII)b | 15.361 | 64,710 | 0.116 | 16,599 | 142,832 | |
| 1.00 | LCP(VII)b | 15.244 | 48,111 | |||
| ADA + LCP(VII)b | 15.361 | 57,888 | 0.116 | 9777 | 84,132 |
The AG undertook an exploratory analysis using EQ-5D scores mapped from VFQ-25 scores captured in the VISUAL II trial47 instead of directly measured EQ-5D scores. This analysis resulted in lower QALY gains and therefore a higher ICER for ADA + LCP(VII) compared with LCP(VII) than in the base case (Table 63).
| Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|
| LCP(VII)a | 15.100 | 48,111 | |||
| ADA + LCP(VII)a | 15.207 | 85,462 | 0.107 | 37,351 | 348,094 |
To assess the impact of uncertainty around the extrapolation of the time to treatment failure, the AG undertook an exploratory analysis using alternative parametric curves. The ICER for ADA + LCP(VII) compared with LCP(VII) was lower when using a Gompertz distribution (£297,746 per QALY) or a Weibull distribution (£235,916 per QALY) than when a log-normal distribution was used, as in the base case (£321,405 per QALY) (Table 64).
| Rate of remission | Treatment group | Total QALYs | Total costs (£) | Incremental QALYs | Incremental costs (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Log-normala | LCP(VII)b | 15.244 | 48,111 | |||
| ADA + LCP(VII)b | 15.361 | 85,462 | 0.116 | 37,351 | 321,405 | |
| Gompertz | LCP(VII)b | 15.305 | 48,101 | |||
| ADA + LCP(VII)b | 15.628 | 144,266 | 0.323 | 96,166 | 297,746 | |
| Weibull | LCP(VII)b | 15.225 | 48,114 | |||
| ADA + LCP(VII)b | 15.325 | 71,577 | 0.099 | 23,463 | 235,916 |
Univariate sensitivity analyses
The AG explored the impact of different parameters on the results of the model, as shown in Table 65.
| Parameters | Base case, lower value, upper value | ICER based on lower value (£) | ICER based on upper value (£) |
|---|---|---|---|
| Utilities | |||
| Baseline | 0.85, 0.83, 0.87 | 334,704 | 309,733 |
| Blindness | 0.38, 0.31, 0.57 | 279,904 | 514,958 |
| Administration and monitoring | |||
| Monitoring visit frequency (weeks) | 6, 4, 8 | 322,313 | 320,952 |
| Monitoring visit cost (£) | 96.11, 77.27, 114.95 | 320,956 | 321,900 |
| ADA administration cost (£) (help from a nurse) | 44, 35.80, 53.03 | 320,628 | 322,262 |
| % of self-injectors needing district nurse for ADA | 10, 0, 20 | 317,234 | 325,577 |
| AE costs (£) | |||
| Cataract surgery | 852.40, 658.33, 1019.47 | 321,741 | 321,035 |
| Glaucoma procedure | 581.25, 467.32, 695.17 | 321,405 | 321,405 |
| Serious infections | 5940, 4776, 7105 | 321,620 | 321,169 |
| Hypertension | 7.04, 5.66, 8.42 | 321,405 | 321,406 |
| Blindness (transition) | 237, 191, 283 | 321,409 | 321,402 |
| Blindness (annual) | 7659, 6158, 9160 | 324,667 | 318,144 |
As for patients with active disease, of those parameters tested within the univariate sensitivity analysis, the parameters relating to the baseline utility and the utility of blindness had the greatest impact on the ICER for ADA + LCP(VII) compared with LCP(VII). However, the ICER for ADA + LCP(VII) compared with LCP(VII) in patients with inactive uveitis did not fall below £84,000 per QALY gained for any of the analyses considered.
Discussion
Model results and key uncertainties
The base-case analysis undertaken by the AG estimated the ICER for one DEX implant in one eye for a combination of patients with unilateral and bilateral uveitis compared with limited current practice, as per the HURON trial,48 to be £19,509 per QALY gained. The ICER for ADA (systemic, therefore treatment for both eyes) for patients with mainly bilateral uveitis compared with limited current practice, as per the VISUAL trials,46,47 was estimated to be £94,523 and £317,547 per QALY gained in patients with active and inactive uveitis respectively.
The results of the model are highly uncertain because of the limited availability of evidence. There are three major issues with the existing evidence: (1) there is no evidence comparing DEX or ADA with current practice; (2) long-term outcomes, in particular the incidence of permanent blindness, are uncertain; and (3) there is no evidence around the proportion of patients who would experience remission and be taken off ADA (or an alternative) treatment or around long-term outcomes for these patients. These are structural uncertainties within the model and the complexity of these issues in combination with the lack of data meant that it was not possible to appropriately quantify the uncertainty associated with them within the PSA, as would be ideal. Instead, the potential impacts of these factors on the model results have been dealt with using exploratory analysis.
These analyses suggest that the rate of blindness in the comparator group and the RR of blindness for DEX and ADA substantially affect the ICER. The cost per QALY gained compared with the comparator within the HURON trial48 ranged from dominating to £56,329 for DEX. Under all assumptions tested for these parameters, the ICER associated with ADA compared with (limited) current practice remained above £30,000 and £82,000 for patients with active and inactive uveitis respectively. The choice of comparator did not have a substantial impact on the ICER, although it should be noted that the rate of blindness was assumed to be the same for all comparators independent of the proportion of patients receiving systemic treatment, which may have slightly overestimated the QALYs associated with the placebo and sham groups and hence the ICERs for these comparisons may be slightly overestimated. The exploratory analyses also suggest that the proportion of patients who would be taken off ADA treatment following remission and who would maintain the same quality of life is a key driver of the model results. Under the assumption that all patients who remain on ADA at 2 years achieve remission and are taken off treatment while retaining quality of life, the ICER for ADA compared with (limited) current practice decreases to £35,299 and £84,132 per QALY for patients with active and inactive uveitis respectively.
Use of adalimumab and dexamethasone in clinical practice
The clinical advisors to the AG suggested that there are several differences between the way in which the treatments are provided within the RCTs and the way in which they would be provided in practice. The clinical experts suggested that the proportion of patients who remain on ADA treatment is likely to be underestimated within the clinical trial because of the strict criteria for treatment failure. If more people were to remain on treatment, the additional group of patients on treatment would incur the same costs as those who remain on treatment in the VISUAL trial, whereas the effectiveness of ADA is likely to be reduced in these patients who were considered to have failed treatment in the trial and hence the ICER would increase for these patients.
The model predicts that ADA would have a substantially higher ICER for inactive patients than active patients. The VISUAL II trial47 captures the benefits of ADA over placebo for preventing the recurrence of uveitis symptoms in patients who were inactive while on high-dose steroids, once the steroids have been tapered and discontinued. However, our clinical advisors suggested that, for the ‘inactive’ group of patients, ADA is more likely to be used in patients who have to discontinue existing immunosuppressants because they are ineffective or not tolerated. However, there are no clinical data for this group of patients.
The model assumes that only one DEX implant would be provided to patients. There is no RCT evidence to assess the comparative effectiveness or safety of more than one DEX implant; however, there are several non-randomised trials with 12–24 months’ follow-up that have allowed repeat implants. 18,50,51 These studies consistently report that, after around 6 months, patients’ outcomes return to those at baseline and that up to three repeat implants are each likely to have a similar treatment effect. Each additional implant is associated with a higher incidence of AEs such as raised IOP and cataract. 18,50,51 The univariate sensitivity analyses suggested that the model is not sensitive to the costs of treating raised IOP or cataract and, hence, given that the cost of each implant is the same, the cost-effectiveness of up to three consecutive implants is expected to be similar to the cost-effectiveness of one implant. The ICER would be expected to decrease if there was also a cumulative impact on the reduction in blindness or if patients were to achieve remission after consecutive implants. The clinical experts to the AG suggested that the maximum number of implants likely to be provided to one eye per patient is four because of the increasing rate of raised IOP for each implant. Clinicians suggested that the increasing rate of cataract would not affect their decision regarding additional implants because the condition is reversible with surgery. If DEX implants were repeated at < 6 months following the previous implant, the DEX implants would be less cost-effective than currently predicted.
The clinical advisors suggested that patients would not usually receive an implant in both eyes because they are more likely to have a systemic treatment if both eyes require treatment; however, this may occur in some cases. There was insufficient evidence to assess the cost-effectiveness of using DEX implants in both eyes; however, because the costs would essentially be doubled (with the exception of some monitoring costs) and the increment in HRQoL is likely to be lower for the second eye, it is expected to be less cost-effective than treatment in one eye for a patient with bilateral disease.
The clinical experts to the AG suggested that ADA and DEX are likely to be provided alongside other treatment options in practice. In the clinical trials, around one-third of patients did receive other treatments in both arms. However, it is unclear whether or not the relative effectiveness of ADA and DEX predicted within the trials would be the same if the use of alternative treatments in both the intervention group and the comparator group was increased. If the relative effectiveness and costs remained the same, then the ICER would not change from the base-case predicted ICER. However, because of the lack of evidence for a comparator that represents current practice, it is unclear how both ADA and DEX may have an impact on the use of other treatments. The model incorporates the impact of DEX on use of rescue therapy, but this is based on the analysis using a sham comparator. If treatment with DEX or ADA led to a reduction in the use of immunosuppressants and/or corticosteroids without this having an impact on efficacy in these treatment groups, then DEX and ADA would be more cost-effective than currently predicted.
Potentially important subgroups
The model includes a heterogeneous population and it may be that the interventions are more cost-effectiveness in some groups than others. However, there was insufficient evidence to undertake any formal subgroup analyses. This discussion considers the key subgroups for which the interventions may be more cost-effective. Almost all patients receiving ADA will have bilateral uveitis; however, DEX may be given to patients with unilateral and bilateral uveitis. DEX is likely to be more cost-effective when given in one eye to patients with bilateral uveitis because BCVA in the better-seeing eye is the best predictor of quality of life and hence bilateral uveitis patients are generally able to benefit more from treatment than unilateral uveitis patients, for the same cost of treatment. When the annual rate of blindness is set to 0, the results can be used to give an indication around the cost-effectiveness of DEX for patients with unilateral disease (as patients with unilateral disease are unlikely to become legally blind, unless their disease progresses to become bilateral). The ICER in this case was £48,937. It is important to note that the treatment effect may also be different (expected to be reduced) for unilateral patients compared with a pooled group of unilateral and bilateral patients; however, there was no evidence available to model this.
Patients also have the potential to benefit more from treatment with ADA or DEX if they have more severe uveitis and hence the treatments are likely to be more cost-effective as the baseline disease worsens. In addition, patients with macular oedema are more likely to go blind and therefore the interventions of interest, in particular ADA, because of the longer duration of treatment, are more likely to prevent cases of blindness and hence are likely to be more cost-effective in this group.
Model perspective
The base-case analysis took a NHS and PSS perspective. However, sight problems and sight damage caused by uveitis can affect every aspect of daily life. The quality-of-life measures used within the health economic model aimed to largely capture these effects. However, if a societal perspective was taken, the cost-effectiveness of the interventions would be reduced. A societal perspective would capture the additional cost savings associated with increased leisure time and workplace productivity resulting from the benefits of the interventions. Given that non-infectious uveitis affects a working-age population, these cost savings would not be negligible. Therefore, there are likely to be additional non-NHS and -PSS cost savings of the interventions that are not captured within our analyses; however, analysis of these additional cost savings are beyond the scope of this NICE appraisal.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Many uveitis treatments used in clinical practice are not licensed for uveitis and injections of triamcinolone are contraindicated in the eye [Kenalog® formulation (Bristol-Myers Squibb Pharmaceuticals Ltd, Princeton, NJ, USA)] or are not available in the UK [Trivaris® (Allergan, Irvine, CA, USA)/Triesence® (Alcon Canada Inc., Mississauga, ON, Canada) formulation]. DEX implants and ADA are both used variably in current practice, depending on funding availability. Posterior segment-involving uveitis covers a broad spectrum of patients. DEX implants and ADA would generally be used in different populations in clinical practice (DEX for local disease or local flare-up and in unilateral cases; ADA for severe refractory disease, often bilateral and/or related to a systemic condition). There are few trial data relating to patients who have very severe uveitis or who are unresponsive to or contraindicated for immunosuppressants.
The prevalence of non-infectious posterior segment-involving uveitis is estimated to be between 3 and 10 in 100,000 people in the European Union, based on a population of 506,500,000, including people from the UK. 132 The mid-2015 estimate for the adult population of England is 43,108,471. 133 This results in an estimated prevalence of non-infectious posterior segment-involving uveitis in adults in England of between 1293 and 4311. Within its submission to NICE,55 Allergan, however, estimated a higher prevalence of non-infectious posterior segment-involving uveitis in adults of 16.14 per 100,000, based on a US study, which would result in a higher estimate of 6958 adults affected by non-infectious posterior segment-involving uveitis in England. In its submission,43 AbbVie predicted that 5389 adults would be affected by non-infectious posterior segment-involving uveitis in England. The proportion of patients who would receive DEX and ADA within this patient group is highly uncertain. Within its submission to NICE,55 Allergan predicted that 589 patients would be eligible for DEX annually (8.0% of the predicted number of patients with non-infectious posterior segment-involving uveitis), whereas, within its submission,43 AbbVie predicted that 175 patients would be eligible for ADA annually (3.2% of the predicted number of patients with non-infectious posterior segment-involving uveitis).
The provision of ADA and DEX does not usually engender significant additional management costs compared with current practice. Therefore, the burden on the NHS is generally related to the additional drug acquisition costs and differences in the treatment of AEs. Allergan and AbbVie submitted estimates of the annual additional cost to the NHS of DEX and ADA respectively. DEX was predicted by Allergan to cost an additional £74,672–1,057,706 in the first year, depending on whether the comparator was an immunosuppressant or a corticosteroid, rising to an additional £599,537–5,514,704 in year 5. These estimates were based on the costs of DEX (assuming that patients with unilateral and bilateral disease would have 1.64 implants and 3.28 implants on average annually respectively) minus the costs of immunosuppressants or corticosteroids, which assumes that DEX would eradicate the need for these comparators, and hence they are likely to be underestimates. They also include a reduction in monitoring costs for patients receiving DEX, but do not include the costs of the treatment of AEs. The increase in costs over time occurs because of the assumption that patients will continue to receive DEX implants, which is unlikely to be the case, and hence the first year cost is likely to be the most reasonable estimate.
Adalimumab was predicted by AbbVie to cost £1.55M in the first year, rising to £4.77M in year 5. This was based on the costs of ADA minus the costs of prednisolone (assuming that ADA would be given as third-line treatment and would eradicate the need for corticosteroids). It does not include the costs of the treatment of AEs. The increase in costs over time occurs because of an expected increase by the company in the use of biologics in this patient population. The calculation did not account for patients failing on treatment and hence the increase in costs over the 5 years is likely to have been overpredicted.
Given the substantial uncertainties around prevalence, the number of patients who would receive ADA and DEX, the impact of the interventions on existing immunosuppressant and corticosteroid use, the impact of the interventions on blindness, the number of DEX implants that patients would receive (bilateral and consecutive) in practice and whether or not patients would stop receiving ADA following remission, the AG has not undertaken an analysis of its own as the extent to which it could improve on the companies’ estimates is unclear.
Chapter 6 Discussion
Statement of principal findings
One RCT of ADA in patients with active uveitis (VISUAL I;46 n = 223, follow-up of up to 80 weeks) showed significant benefits over placebo for time to treatment failure as well as visual acuity, inflammation (VH and AC cell grade), macular oedema (change in central retinal thickness) and VFQ-25 score. Another RCT of ADA in patients with inactive uveitis controlled with corticosteroids (VISUAL II;47 n = 229, follow-up of up to 80 weeks) showed significant benefits over placebo for time to treatment failure but not for the other outcomes. There were some concerns regarding the use of LOCF to account for missing data after patients experienced treatment failure in the ADA studies as these data were not missing at random as more patients experienced treatment failure in the placebo arms than in the ADA arms. The ICERs for ADA (systemic, therefore treatment of both eyes) for patients with mainly bilateral uveitis compared with limited current practice, as in the VISUAL trials,46,47 were estimated to be £94,523 and £317,547 per QALY gained in patients with active and inactive uveitis respectively.
A 26-week study of DEX 700 compared with a sham procedure (HURON;48 n = 153 for relevant groups) showed significant improvements in the DEX 700 group for measures of visual acuity, inflammation (VH and AC cell grade), macular oedema (central retinal thickness) and VFQ-25 score. The base-case analysis undertaken by the AG estimated that the ICER for one DEX implant in one eye for a combination of patients with unilateral and bilateral uveitis compared with limited current practice, as in the HURON trial,48 was £19,509 per QALY gained.
Exploratory analyses suggest that two of the factors that have the largest impact on the ICERs, both of which are highly uncertain, are (1) the rate of blindness in the comparator group and (2) the RR of blindness for ADA and DEX. The ICER for DEX compared with (limited) current practice varied from dominating to £56,329 per QALY gained under different assumptions for these parameters. Setting the rate of legal blindness to zero was used to explore the potential cost-effectiveness of DEX for patients with unilateral uveitis; the ICER in this case was £50,627 per QALY gained. Under all assumptions tested for these parameters, the ICER associated with ADA compared with (limited) current practice remained above £30,000 and £82,000 per QALY gained for patients with active and inactive uveitis respectively. The proportion of patients taken off ADA treatment following remission and maintaining the same quality of life had the largest impact on the ICER for ADA, with ICERs of £35,299 and £84,132 per QALY gained for patients with active and inactive uveitis, respectively, when it was assumed that all patients go into remission after 2 years on ADA.
Strengths and limitations of the assessment
We have attempted to compare the two interventions being assessed with current practice; however, there is no RCT evidence comparing any two treatments within the scope of the assessment. ADA was compared with placebo in both studies of ADA identified (patients in both arms also received initial systemic corticosteroids, which were then tapered, and some also received an immunosuppressant) and DEX was compared with a sham procedure in the one study of DEX identified (25% also continued with a stable dose of systemic corticosteroids or immunosuppressants and 22% received rescue therapy, either a local steroid injection or new/increased systemic therapy, in both the DEX 700 arm and the sham arm). The placebo/sham arms could be considered to represent standard practice to some extent because other therapies were permitted in both the active treatment and the placebo arms in all three studies. However, the main comparison was with placebo/sham procedure as opposed to active management with other therapies.
It was not possible to conduct meta-analyses or NMAs because of clinical heterogeneity, lack of common comparators (disconnected network) and differences in the reported outcomes.
The health economic model is the first model that has attempted to assess the cost-effectiveness of ADA or DEX for the treatment of non-infectious uveitis. However, the results are highly uncertain because of the limited availability of evidence and the differences between the trial evidence and clinical practice (as discussed in Chapter 4, Independent economic assessment).
The model includes a heterogeneous population and it may be that the interventions are more cost-effective in some groups than others. However, there was no evidence from the trials to allow subgroup analyses to be undertaken. Patients have the potential to benefit more from treatment with ADA or DEX if they have more severe uveitis and hence the treatments are likely to be more cost-effective as the baseline disease worsens. In addition, patients with macular oedema are more likely to go blind and hence the interventions of interest, in particular ADA, because of the longer duration of treatment, are more likely to prevent cases of blindness and hence are likely to be more cost-effective in this group. The exploratory analysis in which the rate of blindness was varied to represent patients with unilateral uveitis suggests that the ICER for DEX compared with (limited) current practice increases substantially for this patient group; however, the treatment effect for this subgroup is assumed to remain unchanged.
The analysis presented here takes a NHS and PSS perspective. However, non-infectious uveitis affects a working-age population and can reduce workplace productivity. In addition, the disease can affect leisure time. Therefore, there are likely to be additional non-NHS and -PSS costs and benefits of the interventions not captured within our analyses.
Uncertainties
The key uncertainties associated with this evaluation are:
-
the comparative effectiveness and cost-effectiveness of DEX and ADA and their effectiveness and cost-effectiveness compared with those of systemic immunosuppressants and corticosteroids, as would be used in practice
-
how short-term improvements in visual acuity and inflammation relate to long-term effects on vision loss and blindness
-
how ADA and DEX would be used in practice, particularly with regard to taking patients off treatment following remission and the number of DEX implants that would be provided
-
the impact of the expected differences between clinical practice and the trial evidence on estimated outcomes
-
the effectiveness and cost-effectiveness of ADA and DEX in subgroups of patients, including patients with unilateral and bilateral uveitis, those with more and less severe uveitis, patients who are unresponsive to or who are contraindicated for immunosuppressants, patients with macular oedema and patients with underlying autoimmune or inflammatory diseases
-
the long-term impacts of corticosteroids.
Other relevant factors
The number of patients who would be eligible for these treatments is low. DEX implants and ADA are currently generally used in very different patient populations in clinical practice.
Chapter 7 Conclusions
Two RCTs of systemic ADA and one RCT of a unilateral, single DEX implant showed significant benefits over placebo or a sham procedure for outcomes including visual acuity, inflammation (VH and AC cell grade), macular oedema (central retinal thickness), VFQ-25 score and time to treatment failure. One DEX implant in a mixed group of unilateral and bilateral patients had an estimated ICER of £19,509 per QALY gained compared with (limited) current practice. The ICER associated with ADA compared with (limited) current practice did not fall below £30,000 per QALY gained in any of the analyses carried out.
There is substantial uncertainty around the evidence, in particular with regard to the comparative effectiveness and cost-effectiveness of DEX and ADA and their effectiveness and cost-effectiveness compared with those of systemic immunosuppressants and corticosteroids, as would be used in practice, and how short-term improvements in visual acuity and inflammation relate to long-term effects on vision loss and blindness. In addition, the way in which ADA and DEX would be used in practice and the impact of the expected differences between clinical practice and the trial evidence on estimated outcomes are uncertain. Finally, there are important subgroups for which the interventions may be more or less effective and cost-effective; however, there was insufficient evidence to make robust conclusions around this.
Implications for service provision
The provision of ADA and DEX does not usually engender significant additional management costs. Therefore, the burden on the NHS is generally related to the drug acquisition costs and the treatment of AEs.
Suggested research priorities
-
Development of guidance on which outcomes should be used and how they should be reported in trials of non-infectious uveitis, outlining primary clinical outcomes as well as considering a standardised way of reporting reduction in corticosteroid use.
-
Primary research comparing:
-
DEX with immunosuppressants over the long term in patients with localised (uveitis affecting one eye or a flare in one eye) active uveitis
-
ADA with immunosuppressants and other anti-TNFs over the long term in patients with systemic bilateral active uveitis
-
ADA, DEX, immunosuppressants and other anti-TNFs over the long term in patients with systemic unilateral uveitis (or a flare in one eye).
-
-
Researchers should consult a statistician and follow the Consolidated Standards of Reporting Trials (CONSORT) statement134 when designing and undertaking RCTs. 135
-
Research on how short-term improvements in visual acuity or inflammation relate to long-term effects on moderate-to-severe vision loss and blindness.
-
An assessment of the impact of treatments within important subgroups, including patients with unilateral and bilateral uveitis, those with severe uveitis, patients who are unresponsive to or who are contraindicated for immunosuppressants, patients with macular oedema and patients with specific underlying autoimmune or inflammatory diseases.
-
A study of the long-term impacts of corticosteroids to gain further data on health and utility decrements and costs.
Acknowledgements
We would like to thank Dr Paul Tappenden [School of Health and Related Research (ScHARR)], Professor Eva Kaltenthaler (ScHARR), Dr Ewen Cummins (McMDC Ltd) and Professor Matt Stevenson (ScHARR) for providing comments on the draft report and Gill Rooney (Programme Manager, ScHARR) for providing administrative support and support in preparing and formatting the report.
This report was commissioned by the HTA programme of the National Institute for Health Research. The views expressed in this report are those of the authors and not necessarily those of the National Institute for Health Research. Any errors are the responsibility of the authors.
Contributions of authors
Hazel Squires (Senior Research Fellow, health economic modelling) was the AG lead and advised on the cost-effectiveness modelling.
Edith Poku (Research Fellow, systematic reviewing) undertook the clinical effectiveness review.
Inigo Bermejo (Research Associate, health economic modelling) undertook the cost-effectiveness review and developed the cost-effectiveness model.
Katy Cooper (Senior Research Fellow, systematic reviewing) undertook the clinical effectiveness review.
John Stevens (Reader, decision science) commented on statistical issues and the feasibility of NMA.
Jean Hamilton (Research Associate, statistics) commented on statistical issues and the feasibility of NMA.
Ruth Wong (Information Specialist) performed the literature searches.
Alastair Denniston (Consultant Ophthalmologist) provided clinical advice throughout the project.
Ian Pearce (Consultant Ophthalmologist) provided clinical advice throughout the project.
Fahd Quhill (University Senior Lecturer and Consultant Ophthalmologist) provided clinical advice throughout the project.
All authors were involved in drafting and commenting on the final report.
Data sharing statement
Data can be obtained from the corresponding author subject to them being non-confidential.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Pato E, Muñoz-Fernández S, Francisco F, Abad MA, Maese J, Ortiz A, et al. Systematic review on the effectiveness of immunosuppressants and biological therapies in the treatment of autoimmune posterior uveitis. Semin Arthritis Rheum 2011;40:314-23. https://doi.org/10.1016/j.semarthrit.2010.05.008.
- Brady CJ, Villanti AC, Law HA, Rahimy E, Reddy R, Sieving PC, et al. Corticosteroid implants for chronic non-infectious uveitis. Cochrane Database Syst Rev 2016;2. https://doi.org/10.1002/14651858.CD010469.pub2.
- Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol 2014;8:1891-911.
- Bell DS, Pandit R. North East Retinal Group: Treatment Protocol for the Use of Ozurdex (Dexamethasone Intravitreal Implant) in the Management of Uveitis 2012. http://ntag.nhs.uk/docs/Ozurdex%20uveitis%20NERG%20protocol%20-Feb2012.pdf (accessed 6 October 2017).
- NHS Southern Derbyshire Clinical Commissioning Group . Dexamethasone Intravitreal Implant (Ozurdex®) for the Treatment of Non-Infectious Posterior Uveitis With Royal Derby Hospital Foundation Trust 2015. www.derbyshiremedicinesmanagement.nhs.uk/assets/High_cost_drugs/Ozurdex_Commissioning_Policy.pdf (accessed 6 October 2017).
- Dunn JP. Uveitis. Prim Care 2015;42:305-23. https://doi.org/10.1016/j.pop.2015.05.003.
- Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol 1987;103:234-5. https://doi.org/10.1016/S0002-9394(14)74235-7.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group . Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509-16. https://doi.org/10.1016/j.ajo.2005.03.057.
- Zierhut M, Deuter C, Murray P. Classification of uveitis – current guidelines. Eur Ophthalmic Rev 2007:77-8.
- Mora P, Gonzales S, Ghirardini S, Rubino P, Orsoni JG, Gandolfi SA, et al. Perioperative prophylaxis to prevent recurrence following cataract surgery in uveitic patients: a two-centre, prospective, randomized trial. Acta Ophthalmol (Oxf) 2016;94:e390-4. https://doi.org/10.1111/aos.12955.
- Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol 2004;88:1159-62. https://doi.org/10.1136/bjo.2003.037226.
- Uveitis Group Factsheet. 2009.
- Levy RA, de Andrade FA, Foeldvari I. Cutting-edge issues in autoimmune uveitis. Clin Rev Allergy Immunol 2011;41:214-23. https://doi.org/10.1007/s12016-011-8267-x.
- Guly CM, Forrester JV. Investigation and management of uveitis. BMJ 2010;341. https://doi.org/10.1136/bmj.c4976.
- Rothova A, Buitenhuis HJ, Meenken C, Brinkman CJ, Linssen A, Alberts C, et al. Uveitis and systemic disease. Br J Ophthalmol 1992;76:137-41. https://doi.org/10.1136/bjo.76.3.137.
- Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, Papo T, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine 2001;80:263-70. https://doi.org/10.1097/00005792-200107000-00005.
- Jones NP. The Manchester Uveitis Clinic: the first 3000 patients, 2: uveitis manifestations, complications, medical and surgical management. Ocul Immunol Inflamm 2015;23:127-34. https://doi.org/10.3109/09273948.2014.968671.
- Tomkins-Netzer O, Taylor SRJ, Bar A, Lula A, Yaganti S, Talat L, et al. Treatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitis. Ophthalmology 2014;121:1649-54. https://doi.org/10.1016/j.ophtha.2014.02.003.
- Dick AD, Tundia N, Sorg R, Zhao C, Chao JD, Joshi A, et al. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology 2016;123:655-62. https://doi.org/10.1016/j.ophtha.2015.10.028.
- International Statistical Classification of Diseases, Injuries and Causes of Death, Tenth Revision. Geneva: WHO; 1993.
- Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:332-6. https://doi.org/10.1136/bjo.80.4.332.
- Williams GJ, Brannan S, Forrester JV, Gavin MP, Paterson-Brown SP, Purdie AT, et al. The prevalence of sight-threatening uveitis in Scotland. Br J Ophthalmol 2007;91:33-6. https://doi.org/10.1136/bjo.2006.101386.
- Patel AK, Newcomb CW, Liesegang TL, Pujari SS, Suhler EB, Thorne JE, et al. Risk of retinal neovascularization in cases of uveitis. Ophthalmology 2016;123:646-54. https://doi.org/10.1016/j.ophtha.2015.10.056.
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004;111:491-500. https://doi.org/10.1016/j.ophtha.2003.06.014.
- McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol 1996;121:35-46. https://doi.org/10.1016/S0002-9394(14)70532-X.
- Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol 1996;80:844-8. https://doi.org/10.1136/bjo.80.9.844.
- Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica 2004;218:223-36. https://doi.org/10.1159/000078612.
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. National Eye Institute Visual Function Questionnaire Field Test Investigators . Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050-8. https://doi.org/10.1001/archopht.119.7.1050.
- Hornbeak DM, Payal A, Pistilli M, Biswas J, Ganesh SK, Gupta V, et al. Interobserver agreement in clinical grading of vitreous haze using alternative grading scales. Ophthalmology 2014;121:1643-8. https://doi.org/10.1016/j.ophtha.2014.02.018.
- The EuroQoL Group . EuroQol – a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med 2009;103:975-94. https://doi.org/10.1016/j.rmed.2009.01.003.
- Rathinam SR, Babu M, Thundikandy R, Kanakath A, Nardone N, Esterberg E, et al. A randomized clinical trial comparing methotrexate and mycophenolate mofetil for noninfectious uveitis. Ophthalmology 2014;121:1863-70. https://doi.org/10.1016/j.ophtha.2014.04.023.
- Murphy CC, Greiner K, Plskova J, Duncan L, Frost NA, Forrester JV, et al. Cyclosporine vs. tacrolimus therapy for posterior and intermediate uveitis. Arch Ophthalmol 2005;123:634-41. https://doi.org/10.1001/archopht.123.5.634.
- Denniston AK, Holland GN, Kidess A, Nussenblatt RB, Okada AA, Rosenbaum JT, et al. Heterogeneity of primary outcome measures used in clinical trials of treatments for intermediate, posterior, and panuveitis. Orphanet J Rare Dis 2015;10. https://doi.org/10.1186/s13023-015-0318-6.
- Bailey IL, Lovie-Kitchin JE. Visual acuity testing. From the laboratory to the clinic. Vision Res 2013;90:2-9. https://doi.org/10.1016/j.visres.2013.05.004.
- Keane PA, Karampelas M, Sim DA, Sadda SR, Tufail A, Sen HN, et al. Objective measurement of vitreous inflammation using optical coherence tomography. Ophthalmology 2014;121:1706-14. https://doi.org/10.1016/j.ophtha.2014.03.006.
- Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 1985;92:467-71. https://doi.org/10.1016/S0161-6420(85)34001-0.
- Kempen JH, Daniel E, Gangaputra S, Dreger K, Jabs DA, Kacmaz RO, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the systemic immunosuppressive therapy for eye diseases (SITE) cohort study. Ophthalmic Epidemiol 2008;15:47-55. https://doi.org/10.1080/09286580701585892.
- Frick KD, Drye LT, Kempen JH, Dunn JP, Holland GN, Latkany P, et al. Associations among visual acuity and vision- and health-related quality of life among patients in the multicenter uveitis steroid treatment trial. Invest Ophthalmol Vis Sci 2012;53:1169-76. https://doi.org/10.1167/iovs.11-8259.
- Longworth L, Yang Y, Young T, Mulhern B, Hernández Alava M, Mukuria C, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18090.
- van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000;15:993-1000. https://doi.org/10.1359/jbmr.2000.15.6.993.
- Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis 2009;68:1119-24. https://doi.org/10.1136/ard.2008.092163.
- Allergan . Ozurdex (Dexamethasone Intravitreal Implant) for the Treatment of Posterior Segment Uveitis: NICE Multiple Technology Appraisal Submission 2016. www.nice.org.uk/guidance/ta460/documents/committee-papers-2 (accessed 1 June 2016).
- Electronic Medicines Compendium . Humira (Adalimumab), Summary of Product Characteristics 2016. www.medicines.org.uk/emc/medicine/31860 (accessed 1 October 2016).
- Electronic Medicines Compendium . Ozurdex, Summary of Product Characteristics 2015. www.medicines.org.uk/emc/medicine/23422 (accessed 18 January 2016).
- Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med 2016;375:932-43. https://doi.org/10.1056/NEJMoa1509852.
- Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 2016;388:1183-92. https://doi.org/10.1016/S0140-6736(16)31339-3.
- Lowder C, Belfort R, Lightman S, Foster CS, Robinson MR, Schiffman RM, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol 2011;129:545-53. https://doi.org/10.1001/archophthalmol.2010.339.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Zarranz-Ventura J, Carreno E, Johnston RL, Mohammed Q, Ross AH, Barker C, et al. Multicenter study of intravitreal dexamethasone implant in noninfectious uveitis: indications, outcomes, and reinjection frequency. Am J Ophthalmol 2014;158:1136-45.e5. https://doi.org/10.1016/j.ajo.2014.09.003.
- Pelegrín L, de la Maza MS, Molins B, Ríos J, Adán A. Long-term evaluation of dexamethasone intravitreal implant in vitrectomized and non-vitrectomized eyes with macular edema secondary to non-infectious uveitis. Eye 2015;29:943-50. https://doi.org/10.1038/eye.2015.73.
- AbbVie . Clinical Study Report for VISUAL-I: A Multicenter Study of the Efficacy and Safety of the Human Anti-TNF Monoclonal Antibody Adalimumab As Maintenance Therapy in Subjects Requiring High Dose Corticosteroids for Active Non-Infectious Intermediate Uveitis, Posterior Uveitis, or Panuveitis – Including a Sub-Study in Japanese Patients 2015. www.nice.org.uk/guidance/ta460/documents/committee-papers-2 (accessed 1 June 2016).
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Final Scope: Adalimumab and Dexamethasone for Treating Non-infectious Uveitis. London: NICE; 2016.
- Uveitis (Non-infectious) – Adalimumab: NICE Health Technology Appraisal Submission. London: NICE; 2016.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011.
- Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Louis TA, . Multicenter Uveitis Steroid Treatment Trial Research Group . Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology 2011;118:1916-26. https://doi.org/10.1016/j.ophtha.2011.07.027.
- Pavesio C, Zierhut M, Bairi K, Comstock TL, Usner DW. Fluocinolone Acetonide Study Group . Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmology 2010;117:567-75.e1.
- Shin JY, Yu HG. Intravitreal Triamcinolone injection for uveitic macular edema: a randomized clinical study. Ocul Immunol Inflamm 2015;23:430-6. https://doi.org/10.3109/09273948.2015.1025982.
- Ferrante PF, Ramsay A, Lightman SL. Posterior sub-tenon triamcinolone injection versus orbital floor methylprednisolone in posterior uveitis. Invest Ophthalmol Vis Sci 2000;41.
- Foster CS, Tufail F, Waheed NK, Chu D, Miserocchi E, Baltatzis S, et al. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol 2003;121:437-40. https://doi.org/10.1001/archopht.121.4.437.
- Yazici H, Pazarli H, Barnes CG, Tuzun Y, Ozyazgan Y, Silman A, et al. A controlled trial of azathioprine in Behçet’s syndrome. N Engl J Med 1990;322:281-5. https://doi.org/10.1056/NEJM199002013220501.
- de Vries J, Baarsma GS, Zaal MJ, Boen-Tan TN, Rothova A, Buitenhuis HJ, et al. Cyclosporin in the treatment of severe chronic idiopathic uveitis. Br J Ophthalmol 1990;74:344-9. https://doi.org/10.1136/bjo.74.6.344.
- Nussenblatt RB, Palestine AG, Chan CC, Stevens G, Mellow SD, Green SB. Randomized, double-masked study of cyclosporine compared to prednisolone in the treatment of endogenous uveitis. Am J Ophthalmol 1991;112:138-46. https://doi.org/10.1016/S0002-9394(14)76692-9.
- Bodaghi B. Luminate Investigator Group . Voclosporin: newer analyses of a randomized, controlled trial for noninfectious uveitis. Acta Ophthalmol 2012;90. https://doi.org/10.1111/j.1755-3768.2012.2274.x.
- Roesel M, Tappeiner C, Heiligenhaus A, Heinz C. Oral voclosporin: novel calcineurin inhibitor for treatment of noninfectious uveitis. Clin Ophthalmol 2011;5:1309-13. https://doi.org/10.2147/OPTH.S11125.
- Mackensen F, Jakob E, Springer C, Dobner BC, Wiehler U, Weimer P, et al. Interferon versus methotrexate in intermediate uveitis with macular edema: results of a randomized controlled clinical trial. Am J Ophthalmol 2013;156:478-86.e1. https://doi.org/10.1016/j.ajo.2013.05.002.
- Lightman S, Belfort R, Naik RK, Lowder C, Foster CS, Rentz AM, et al. Vision-related functioning outcomes of dexamethasone intravitreal implant in noninfectious intermediate or posterior uveitis. Invest Ophthalmol Vis Sci 2013;54:4864-70. https://doi.org/10.1167/iovs.12-10981.
- Jaffe GJ, Thorne JE, Scales D, Franco P, Tari SR, Camez A, et al. Adalimumab in patients with active, non-infectious uveitis requiring high-dose corticosteroids: the VISUAL-1 trial. Invest Ophthalmol Vis Sci 2015;56.
- Nguyen QD, Kurup SK, Merrill P, Sheppard J, Van Calster J, Dick AD, et al. Adalimumab in patients with inactive, non-infectious uveitis requiring systemic treatment. Arthritis Rheumatol 2015;67. http://acrabstracts.org/abstract/adalimumab-in-patients-with-inactive-non-infectious-uveitis-requiring-systemic-treatment/ (accessed 1 June 2016).
- Allergan . Clinical Study Report for HURON: An 8-Week, Multicenter, Masked, Randomized Trial (with an 18-Week Masked Extension) to Assess the Safety and Efficacy of 700 µg and 350 µg Dexamethasone Posterior Segment Drug Delivery System (DEX PS DDS) Applicator System Compared With Sham DEX PS DDS Applicator System in the Treatment of Non-Infectious Ocular Inflammation of the Posterior Segment in Patients With Intermediate or Posterior Uveitis 2009.
- AbbVie . Clinical Study Report for VISUAL-II: A Multicenter Study of the Efficacy and Safety of the Human Anti-TNF Monoclonal Antibody Adalimumab in Subjects With Inactive Non-Infectious Intermediate Uveitis, Posterior Uveitis, or Panuveitis – Including a Sub-Study in Japanese Patients 2015.
- Landewé R, van der Horst-Bruinsma I, Tari S, Florentinus S, Song A, Kron M, et al. Quiescence in active and inactive non-infectious, intermediate, posterior, or panuveitis in patients treated with adalimumab. Arthritis Rheumatol 2016;68.
- Snaith RP. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes 2003;1. https://10.1186/1477-7525-1-29.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 1993;4:353-65. https://doi.org/10.2165/00019053-199304050-00006.
- Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil 2002;83:193-200. https://doi.org/10.1053/apmr.2002.27467.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Ware J, Sherbourne C. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care 1992;30:473-83. https://doi.org/10.1097/00005650-199206000-00002.
- Miserocchi E, Modorati G, Pastore MR, Bandello F. Dexamethasone intravitreal implant: an effective adjunctive treatment for recalcitrant noninfectious uveitis. Ophthalmologica 2012;228:229-33. https://doi.org/10.1159/000343060.
- Palla S, Biswas J, Nagesha CK. Efficacy of Ozurdex implant in treatment of noninfectious intermediate uveitis. Indian J Ophthalmol 2015;63:767-70. https://doi.org/10.4103/0301-4738.171505.
- Lam WC, Albiani DA, Yoganathan P, Chen JC, Kherani A, Maberley DA, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol 2015;9:1255-68. https://doi.org/10.2147/OPTH.S80500.
- Pleyer U, Klamann M, Laurent TJ, Manz M, Hazirolan D, Winterhalter S, et al. Fast and successful management of intraocular inflammation with a single intravitreal dexamethasone implant. Ophthalmologica 2014;232:223-9. https://doi.org/10.1159/000368987.
- Nobre-Cardoso J, Champion E, Darugar A, Fel A, Lehoang P, Bodaghi B. Treatment of non-infectious uveitic macular edema with the intravitreal dexamethasone implant. Ocul Immunol Inflamm 2016:447-54.
- Tsang AC, Virgili G, Abtahi M, Gottlieb CC. Intravitreal dexamethasone implant for the treatment of macular edema in chronic non-infectious uveitis [published online ahead of print 18 May 2016]. Ocul Immunol Inflamm 2016. https://doi.org/10.3109/09273948.2016.1160130.
- Adán A, Pelegrín L, Rey A, Llorenc V, Mesquida M, Molins B, et al. Dexamethasone intravitreal implant for treatment of uveitic persistent cystoid macular edema in vitrectomized patients. Retina 2013;33:1435-40. https://doi.org/10.1097/IAE.0b013e31827e247b.
- Khurana RN, Porco TC. Efficacy and safety of dexamethoasone intravitreal implant for persistent uveitic cystoid macular edema. Retina 2015;35:1640-6. https://doi.org/10.1097/IAE.0000000000000515.
- NHS England . Clinical Commissioning Policy: Infliximab (Remicade) and Adalumimab (Humira) As Anti-TNF Treatment Options for Adult Patients With Severe Refractory Uveitis (NHS England D12 P A) 2015. www.engage.england.nhs.uk/consultation/specialised-services-consultation/user_uploads/uveitis-adults-policy.pdf (accessed December 2016).
- Frost NA, Sparrow JM, Durant JS, Donovan JL, Peters TJ, Brookes ST. Development of a questionnaire for measurement of vision-related quality of life. Ophthalmic Epidemiol 1998;5:185-210. https:// doi.org/10.1076/opep.5.4.185.4191.
- Nguyen QD, Sadiq MA, Soliman MK, Agarwal A, Do DV, Sepah YJ. The effect of different dosing schedules of intravitreal sirolimus, a mammalian target of rapamycin (mTOR) inhibitor, in the treatment of non-infectious uveitis (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2016;114.
- Mackensen F, Becker MD, Jakob E, Dobner BC, Heinz C, Lorenz HM, et al. Final results of an investigator initiated, multicenter randomised controlled trial of the efficacy of adalimumab in active uveitis refractoty to standard treatment (ADUR). Acta Ophthalmol 2012;90. https://doi.org/10.1111/j.1755-3768.2012.2276.x.
- Suhler EB, Jaffe GJ, Nguyen QD, Brezin AP, Zierhut M, Vitale A. Long-term safety and efficacy of adalimumab in patients with non-infectious intermediate, posterior, or panuveitis in an ongoing open-label study. Arthritis Rheumatol 2016;68. http://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-adalimumab-in-patients-with-non-infectious-intermediate-posterior-or-panuveitis-in-an-ongoing-open-label-study/ (accessed 1 June 2016).
- Bausch + Lomb . Prescribing Information: Retisert (Fluocinolone Acetonide Intravitreal Implant) 0.59 Mg 2012. www.bausch.com/ecp/our-products/rx-pharmaceuticals/rx-pharmaceuticals/retisert-fluocinolone-acetonide-intravitreal-implant-059-mg (accessed 1 January 2016).
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Eddy DM, Mosteller F. Assessing Medical Technologies. Washington, DC: National Academy Press; 1985.
- Canadian Agency for Drugs and Technologies in Health . Mycophenolate Mofetil Tablets for Uveitis: A Review of the Comparative Clinical and Cost-Effectiveness 2011. www.cadth.ca/media/pdf/htis/may-2011/RC0270_MMF_in_Uveitis_Final.pdf (accessed 1 August 2016).
- Ang M, Nguyen HV, Kiew SY, Chen S, Chee SP, Finkelstein E. Cost-effectiveness of alternative strategies for interferon-gamma release assays and tuberculin skin test in tuberculous uveitis. Br J Ophthalmol 2015;99:984-9. https://doi.org/10.1136/bjophthalmol-2014-306285.
- Ramanan AV, Dick AD, Benton D, Compeyrot-Lacassagne S, Dawoud D, Hardwick B, et al. A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE trial). Trials 2014;15. https://doi.org/10.1186/1745-6215-15-14.
- Ramanan AV, Dick AD, McKay A, Jones A, Williamson P, Compeyrot-Lacassagne S, et al. A Randomised Controlled Trial of the Clinical Effectiveness, Safety and Cost-Effectiveness of Adalimumab in Combination With Methotrexate for the Treatment of Juvenile Idiopathic Arthritis Associated Uveitis n.d.
- Nguyen QD, Harper SL, Medina CA, Uy HS, Foster CS. Cost analysis study of the management of patients with HLA-B27-associated uveitis. Invest Ophthalmol Vis Sci 1999;40.
- Padula WV, Yilmaz T, Cordero-Coma M. A cost-effectiveness analysis of off-label biologics to treat sarcoid posterior uveitis versus standard of care: comparing infliximab to methotrexate and systemic steroids. Value Health 2011;14:A505-6. https://doi.org/10.1016/j.jval.2011.08.1487.
- Sugar EA, Holbrook JT, Kempen JH, Burke AE, Drye LT, Thorne JE, et al. Cost-effectiveness of fluocinolone acetonide implant versus systemic therapy for noninfectious intermediate, posterior, and panuveitis. Ophthalmology 2014;121:1855-62. https://doi.org/10.1016/j.ophtha.2014.04.022.
- Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Sugar EA. Multicenter Uveitis Steroid Treatment Trial Research Group . The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol 2010;149:550-61.e10. https://doi.org/10.1016/j.ajo.2009.11.019.
- Dexamethasone Intravitreal Implant for the Treatment of Macular Oedema Secondary to Retinal Vein Occlusion. London: NICE; 2011.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Bressler NM, Chang TS, Suñer IJ, Fine JT, Dolan CM, Ward J, et al. Vision-related function after ranibizumab treatment by better- or worse-seeing eye: clinical trial results from MARINA and ANCHOR. Ophthalmology 2010;117:747-56.e4. https://doi.org/10.1016/j.ophtha.2009.09.002.
- Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-9.
- Perra D, Alba MA, Callejas JL, Mesquida M, Ríos-Fernández R, Adán A, et al. Adalimumab for the treatment of Behçet’s disease: experience in 19 patients. Rheumatology 2012;51:1825-31. https://doi.org/10.1093/rheumatology/kes130.
- Simonini G, Taddio A, Cattalini M, Caputo R, De Libero C, Naviglio S, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res 2011;63:612-18. https://doi.org/10.1002/acr.20404.
- Czoski-Murray C, Carlton J, Brazier J, Young T, Papo NL, Kang HK. Valuing condition-specific health states using simulation contact lenses. Value Health 2009;12:793-9. https://doi.org/10.1111/j.1524-4733.2009.00527.x.
- Burmester G, Panaccione R, Gordon KB, McIlraith MJ, Lacerda APM. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis 2012;72:517-24. https://doi.org/10.1136/annrheumdis-2011-201244.
- Dakin H. Review of studies mapping from quality of life or clinical measures to EQ-5D: an online database. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-151.
- Browne C, Brazier J, Carlton J, Alavi Y, Jofre-Bonet M. Estimating quality-adjusted life years from patient-reported visual functioning. Eye 2012;26:1295-301. https://doi.org/10.1038/eye.2012.137.
- Payakachat N, Summers KH, Pleil AM, Murawski MM, Thomas J, Jennings K, et al. Predicting EQ-5D utility scores from the 25-item National Eye Institute Vision Function Questionnaire (NEI-VFQ 25) in patients with age-related macular degeneration. Qual Life Res 2009;18:801-13. https://doi.org/10.1007/s11136-009-9499-6.
- Ara R, Brazier J. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc 1999;97:473-511.
- Aflibercept for Treating Visual Impairment Caused by Macular Oedema after Branch Retinal Vein Occlusion. London: NICE; 2016.
- Dexamethasone Intravitreal Implant for Treating Diabetic Macular Oedema. London: NICE; 2015.
- Aflibercept for Treating Visual Impairment Caused by Macular Oedema Secondary to Central Retinal Vein Occlusion. London: NICE; 2014.
- Ranibizumab for Treating Visual Impairment Caused by Macular Oedema Secondary to Retinal Vein Occlusion. London: NICE; 2013.
- Ranibizumab and Pegaptanib for the Treatment of Age-related Macular Degeneration. London: NICE; 2008.
- NHS Reference Costs 2014–15. London: Department of Health; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2016.
- Breeze PR, Thomas C, Squires H, Brennan A, Greaves C, Diggle PJ, et al. School for Public Health Research (SPHR) Diabetes Prevention Model: Detailed Description of Model Background, Methods, Assumptions and Parameters. Sheffied: University of Sheffield, School of Health and Related Research, Health Economics and Decision Science (HEDS); 2015.
- Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess 2008;12. https://doi.org/10.3310/hta12160.
- Meads C, Hyde C. What is the cost of blindness?. Br J Ophthalmol 2003;87:1201-4. https://doi.org/10.1136/bjo.87.10.1201.
- McCrone P, Dhanasir S, Patel A, Knapp M, Lawton-Smith S. Paying the Price. The Cost of Mental Health Care in England to 2026. London: The King’s Fund; 2008.
- Davis S, Martyn-St James M, Sanderson J, Stevens J, Goka E, Rawdin A, et al. A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20780.
- Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med 2015;32:459-66. https://doi.org/10.1111/dme.12647.
- National Life Tables, UK: 2013–15. Newport: Office for National Statistics; 2016.
- Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000;130:492-513. https://doi.org/10.1016/S0002-9394(00)00659-0.
- Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for Rheumatoid Arthritis Not Previously Treated with DMARDs or after Conventional DMARDs Only Have Failed. London: NICE; 2016.
- Public Summary of Opinion on Orphan Designation. Dexamethasone (Intravitreal Implant) for the Treatment of Non-infectious Uveitis Affecting the Posterior Segment of the Eye. London: European Medicines Agency; 2010.
- Population Estimates for UK, England and Wales, Scotland and Northern Ireland. Newport: Office for National Statistics; 2016.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Lee CF, Cheng ACO, Fong DYT. Eyes or subjects: are opthalmic randomized contolled trials properly designed and analyzed?. Ophtalmology 2012;119:869-72. https://doi.org/10.1016/j.ophtha.2011.09.025.
- Allegri P, Murialdo U, Peri S, Carniglia R, Crivelli MG, Compiano S, et al. Randomized, double-blind, placebo-controlled clinical trial on the efficacy of 0.5% indomethacin eye drops in uveitic macular edema. Invest Ophthalmol Vis Sci 2014;55:1463-70. https://doi.org/10.1167/iovs.13-13202.
- Alpsoy E, Durusoy C, Yilmaz E, Ozgurel Y, Ermis O, Yazar S, et al. Interferon alfa-2a in the treatment of Behçet disease: a randomized placebo-controlled and double-blind study. Arch Dermatol 2002;138:467-71. https://doi.org/10.1001/archderm.138.4.467.
- Biryukova A, Shirinsky I, Shirinsky V. Efficacy and safety of simvastatinin uveitis associated with HLAB27 and/or rheumatic diseases: a randomized, open-label study. Annals Rheum Dis 2015;74. https://doi.org/10.1136/annrheumdis-2015-eular.5421.
- Blumenkranz MS, Haller JA, Kuppermann BD, Williams GA, Ip M, Davis M, et al. Correlation of visual acuity and macular thickness measured by optical coherence tomography in patients with persistent macular edema. Retina 2010;30:1090-4. https://doi.org/10.1097/IAE.0b013e3181dcfaf3.
- Boscia F, Furino C, Dammacco R, Ferreri P, Sborgia L, Sborgia C. Intravitreal triamcinolone acetonide in refractory pseudophakic cystoid macular edema: functional and anatomic results. Eur J Ophthalmol 2005;15:89-95.
- Buggage RR, Levy-Clarke G, Sen HN, Ursea R, Srivastava SK, Suhler EB, et al. A double-masked, randomized study to investigate the safety and efficacy of daclizumab to treat the ocular complications related to Behçet’s disease. Ocul Immunol Inflamm 2007;15:63-70. https://doi.org/10.1080/09273940701299370.
- Dada T, Dhawan M, Garg S, Nair S, Mandal S. Safety and efficacy of intraoperative intravitreal injection of triamcinolone acetonide injection after phacoemulsification in cases of uveitic cataract. J Cataract Refract Surg 2007;33:1613-18. https://doi.org/10.1016/j.jcrs.2007.04.029.
- Davatchi F, Shahram F, Chams H, Akbarian M. Pulse cyclophosphamide in ocular manifestations of Behçet’s disease: a double blind controlled crossover study. Arch Iran Med 2004;7:201-5.
- Davatchi F, Sadeghi Abdollahi B, Tehrani Banihashemi A, Shahram F, Nadji A, Shams H, et al. Colchicine versus placebo in Behçet’s disease: randomized, double-blind, controlled crossover trial. Mod Rheumatol 2009;19:542-9. https://doi.org/10.3109/s10165-009-0200-2.
- Davatchi F, Shams H, Rezaipoor M, Sadeghi-Abdollahi B, Shahram F, Nadji A, et al. Rituximab in intractable ocular lesions of Behçet’s disease; randomized single-blind control study (pilot study). Int J Rheum Dis 2010;13:246-52. https://doi.org/10.1111/j.1756-185X.2010.01546.x.
- Foster CS, Alter G, DeBarge LR, Raizman MB, Crabb JL, Santos CI, et al. Efficacy and safety of rimexolone 1% ophthalmic suspension vs. 1% prednisolone acetate in the treatment of uveitis. Am J Ophthalmol 1996;122:171-82. https://doi.org/10.1016/S0002-9394(14)72008-2.
- Gupta A, Ram J, Gupta A, Gupta V. Intraoperative dexamethasone implant in uveitis patients with cataract undergoing phacoemulsification. Ocul Immunol Inflamm 2013;21:462-7. https://doi.org/10.3109/09273948.2013.822087.
- Kuppermann BD, Blumenkranz MS, Haller JA, Williams GA, Weinberg DV, Chou C, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol 2007;125:309-17. https://doi.org/10.1001/archopht.125.3.309.
- Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014;73:39-47. https://doi.org/10.1136/annrheumdis-2013-204231.
- Louis E, Lofberg R, Reinisch W, Schwartz DA, Maa JF, Berg S, et al. Adalimumab treatment reduces extraintestinal manifestations in patients with moderate to severe Crohn’s disease: a pooled analysis. Gastroenterology 2016;1. https://doi.org/10.1016/S0016-5085(16)32634-8.
- Parodi MB, Iacono P, Kontadakis DS, Zucchiatti I, Cascavilla ML, Bandello F. Bevacizumab vs. photodynamic therapy for choroidal neovascularization in multifocal choroiditis. Arch Ophthalmol 2010;128:1100-3. https://doi.org/10.1001/archophthalmol.2010.205.
- Perkins ES, Smith CH, Schofield PB. Treatment of uveitis with pyrimethamine (Daraprim). Br J Ophthalmol 1956;40:577-86. https://doi.org/10.1136/bjo.40.10.577.
- Perkins ES, MacFaul PA. Indomethacin in the treatment of uveitis. A double-blind trial. Trans Ophthalmol Soc UK 1965;85:53-8.
- Roesel M, Tappeiner C, Heinz C, Koch JM, Heiligenhaus A. Comparison between intravitreal and orbital floor triamcinolone acetonide after phacoemulsification in patients with endogenous uveitis. Am J Ophthalmol 2009;147:406-12. https://doi.org/10.1016/j.ajo.2008.09.011.
- Roesel M, Heinz C, Koch JM, Heiligenhaus A. Comparison of orbital floor triamcinolone acetonide and oral prednisolone for cataract surgery management in patients with non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol 2010;248:715-20. https://doi.org/10.1007/s00417-009-1269-1.
- Rosenbaum JT. Effect of etanercept on iritis in patients with ankylosing spondylitis. Arthritis Rheum 2004;50:3736-7. https://doi.org/10.1002/art.20754.
- Rudwaleit M, Landewé R, Marzo-Ortega H, Sieper J, van der Heijde D, Rosenbaum J, et al. Observed incidence rates of uveitis following certolizumab pegol treatment in patients with axial spondyloarthritis. Clin Exp Rheumatol 2014;32. https://doi.org/10.1136/annrheumdis-2014-eular.1806.
- Rudwaleit M, Rosenbaum JT, Landewé R, Marzo-Ortega H, Sieper J, van der Heijde D, et al. Observed incidence of uveitis following certolizumab pegol treatment in patients with axial spondyloarthritis. Arthritis Care Res 2016;68:838-44. https://doi.org/10.1002/acr.22848.
- Schlaegel TF, Weber JC. Double-blind therapeutic trial of isoniazid in 344 patients with uveitis. Br J Ophthalmol 1969;53:425-7. https://doi.org/10.1136/bjo.53.6.425.
- Sieper J, Koenig A, Baumgartner S, Wishneski C, Foehl J, Vlahos B, et al. Analysis of uveitis rates across all etanercept ankylosing spondylitis clinical trials. Ann Rheum Dis 2010;69:226-9. https://doi.org/10.1136/ard.2008.103192.
- Van Den Bosch F, Kruithof E, Baeten D, Herssens A, de Keyser F, Mielants H, et al. Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum 2002;46:755-65. https://doi.org/10.1002/art.511.
- Williams GA, Haller JA, Kuppermann BD, Blumenkranz MS, Weinberg DV, Chou C, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine–Gass syndrome. Am J Ophthalmol 2009;147:1048-54.e2. https://doi.org/10.1016/j.ajo.2008.12.033.
- Yates M, Hamilton LE, Elender F, Dean L, Doll H, MacGregor AJ, et al. Is etanercept 25 mg once weekly as effective as 50 mg at maintaining response in patients with ankylosing spondylitis? A randomized control trial. J Rheumatol 2015;42:1177-85. https://doi.org/10.3899/jrheum.141335.
- Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol 2008;126:1191-201. https://doi.org/10.1001/archopht.126.9.1191.
- Choi JY, Kafkala C, Foster CS. Pars plana vitrectomy versus immunomodulatory therapy in a prospective randomized trial for treatment of pars planitis. Invest Ophthalmol Vis Sci 2005;46.
- de Smet MD, Rubin BI, Whitcup SM, Lopez JS, Austin HA, Nussenblatt RB. Combined use of cyclosporine and ketoconazole in the treatment of endogenous uveitis. Am J Ophthalmol 1992;113:687-90. https://doi.org/10.1016/S0002-9394(14)74795-6.
- Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Liew SHM, Bezlyak V, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology 2013;120:777-87. https://doi.org/10.1016/j.ophtha.2012.09.040.
- Farber MD, Lam S, Tessler HH, Jennings TJ, Cross A, Rusin MM. Reduction of macular oedema by acetazolamide in patients with chronic iridocyclitis: a randomised prospective crossover study. Br J Opthalmol 1994;78:4-7. https://doi.org/10.1136/bjo.78.1.4.
- Haller JA, Dugel P, Weinberg DV, Chou C, Whitcup SM. Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema. Retina 2009;29:46-51. https://doi.org/10.1097/IAE.0b013e318188c814.
- Ibrahim MA, Sepah YJ, Watters A, Bittencourt M, Vigil EM, Do DV, et al. One-year outcomes of the SAVE study: Sirolimus as a therapeutic Approach for uVEitis. Transl 2015;4.
- Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T, et al. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology 2006;113:1020-7. https://doi.org/10.1016/j.ophtha.2006.02.021.
- Lashay AR, Rahimi A, Chams H, Davatchi F, Shahram F, Hatmi ZN, et al. Evaluation of the effect of acetazolamide on cystoid macular oedema in patients with Behçet’s disease. Eye 2003;17:762-6. https://doi.org/10.1038/sj.eye.6700464.
- Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL. AIN457A2208 Study Group . Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology 2015;122:939-48. https://doi.org/10.1016/j.ophtha.2014.12.033.
- Neri PG, Stagni E, Filippello M, Camillieri G, Giovannini A, Leggio GM, et al. Oral Echinacea purpurea extract in low-grade, steroid-dependent, autoimmune idiopathic uveitis: a pilot study. J Ocul Pharmacol Ther 2006;22:431-6. https://doi.org/10.1089/jop.2006.22.431.
- Nguyen QD, Ibrahim MA, Watters A, Bittencourt M, Yohannan J, Sepah YJ, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE study. J Ophthalmic Inflamm Infect 2013;3:1-15. https://doi.org/10.1186/1869-5760-3-32.
- Nussenblatt RB, Gery I, Weiner HL, Ferris FL, Shiloach J, Remaley N, et al. Treatment of uveitis by oral administration of retinal antigens: results of a Phase I/II randomized masked trial. Am J Ophthalmol 1997;123:583-92. https://doi.org/10.1016/S0002-9394(14)71070-0.
- Nussenblatt RB, Kim J, Thompson DJ, Davis MD, Chew E, Ferris FL, et al. Vitamin E in the treatment of uveitis-associated macular edema. Am J Ophthalmol 2006;141:193-4. https://doi.org/10.1016/j.ajo.2005.07.036.
- Quinones K, Choi JY, Yilmaz T, Kafkala C, Letko E, Foster CS. Pars plana vitrectomy versus immunomodulatory therapy for intermediate uveitis: a prospective, randomized pilot study. Ocul Immunol Inflamm 2010;18:411-17. https://doi.org/10.3109/09273948.2010.501132.
- Rahimi M, Shahrzad SS, Banifatemi M. Comparison of intravitreal injection of bevacizumab and triamcinolone acetonide in the treatment of uveitic macular edema. Iran J Immunol 2012;9:136-44. https://doi.org/IJIv9i2A7.
- Sangwan VS, Pearson PA, Paul H, Comstock TL. Use of the fluocinolone acetonide intravitreal implant for the treatment of noninfectious posterior uveitis: 3-year results of a randomized clinical trial in a predominantly Asian population. Ophthalmol Ther 2015;4:1-19. https://doi.org/10.1007/s40123-014-0027-6.
- Soheilian M, Rabbanikhah Z, Ramezani A, Kiavash V, Yaseri M, Peyman GA. Intravitreal bevacizumab versus triamcinolone acetonide for refractory uveitic cystoid macular edema: a randomized pilot study. J Ocul Pharmacol Ther 2010;26:199-206. https://doi.org/10.1089/jop.2009.0093.
- Soheilian M, Rabbanikhah Z, Ramezani A, Kiavash V, Yaseri M, Peyman GA. Bevacizumab vs. triamcinolone. Ophthalmology 2010;117:855-. https://doi.org/10.1016/j.ophtha.2009.10.012.
- Soheilian M, Eskandari A, Ramezani A, Rabbanikhah Z, Soheilian R. A pilot study of intravitreal diclofenac versus intravitreal triamcinolone for uveitic cystoid macular edema. Ocul Immunol Inflamm 2013;21:124-9. https://doi.org/10.3109/09273948.2012.745883.
- Soheilian M, Eskandari A, Ramezani A, Rabbanikhah Z, Esmaeilpour NF, Soheilian R. Intravitreal diclofenac versus intravitreal triamcinolone for the treatment of uveitic cystoid macular edema. Retina 2013;11. https://doi.org/10.1097/IAE.0b013e318285cdee.
- Tranos P, Scott R, Zambarakji H, Ayliffe W, Pavesio C, Charteris DG. The effect of pars plana vitrectomy on cystoid macular oedema associated with chronic uveitis: a randomised, controlled pilot study. Br J Ophthalmol 2006;90:1107-10. https://doi.org/10.1136/bjo.2006.092965.
- van Kooij B, Fijnheer R, de Boer J, ten Dam-van Loon N, Bartelink I, Roest M, et al. A randomized, masked, cross-over trial of lisinopril for inflammatory macular edema. Am J Ophthalmol 2006;141:646-51. https://doi.org/10.1016/j.ajo.2005.11.056.
- Vigil EM, Sepah YJ, Watters AL, Sadiq MA, Ansari M, Bittencourt MG, et al. Assessment of changes in quality of life among patients in the SAVE study – Sirolimus as therapeutic Approach to uVEitis: a randomized study to assess the safety and bioactivity of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis. J Ophthalmic Inflamm Infect 2015;5. https://doi.org/10.1186/s12348-015-0044-1.
- Whitcup SM, Csaky KG, Podgor MJ, Chew EY, Perry CH, Nussenblatt RB. A randomized, masked, cross-over trial of acetazolamide for cystoid macular edema in patients with uveitis. Ophthalmology 1996;103:1054-62. https://doi.org/10.1016/S0161-6420(96)30567-8.
- Goldstein DA, Godfrey DG, Hall A, Callanan DG, Jaffe GJ, Pearson PA, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol 2007;125:1478-85. https://doi.org/10.1001/archopht.125.11.ecs70063.
- Holbrook JT, Sugar EA, Burke AE, Vitale AT, Thorne JE, Davis JL, et al. Dissociations of the fluocinolone acetonide implant: the Multicenter Uveitis Steroid Treatment (MUST) trial and follow-up study. Am J Ophthalmol 2016;164:29-36. https://doi.org/10.1016/j.ajo.2015.12.028.
- Becker MD, Max R, Springer P, Wiehler U, Miller DW, Storch-Hagenlocher B, et al. First results of a randomized controlled clinical trial comparing interferon beta with methotrexate for the treatment of intermediate uveitis with macular edema. Invest Ophthalmol Vis Sci 2008;49.
- Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behçet’s disease. Lancet 1989;333:1093-6. https://doi.org/10.1016/S0140-6736(89)92381-7.
- Mercante AR, Huang SS, Reddy R. Cystoid macular edema in non-infectious uveitis treated with fluocinolone acetonide intravitreal implant: 3-year results of a multi-center clinical trial. Invest Ophthalmol Vis Sci 2007;48.
- Muller B, Orlic N, Pleyer U. Intravitreal fluocinolone-implant (Retisert) in posterior non-infectious uveitis – intermediate results after 34 weeks. Klin Monbl Augenheilkd 2004;221.
- Nussenblatt RB, de Smet MD, Rubin B, Freidlin V, Whitcup SM, Davis J, et al. A masked, randomized, dose–response study between cyclosporine A and G in the treatment of sight-threatening uveitis of noninfectious origin. Am J Ophthalmol 1993;115:583-91. https://doi.org/10.1016/S0002-9394(14)71454-0.
- Parekh A, Srivastava S, Bena J, Albini T, Nguyen QD, Goldstein DA. Risk factors associated with intraocular pressure increase in patients with uveitis treated with the fluocinolone acetonide implant. JAMA Ophthalmol 2015;133:568-73. https://doi.org/10.1001/jamaophthalmol.2015.51.
- Pavesio CE, Bairi K. Evaluation of an intravitreal fluocinolone acetonide implant vs. standard systemic therapy in noninfectious posterior uveitis. Am Acad Ophthalmol 2006. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/914/CN-00634914/frame.html (accessed 6 October 2017).
- Sheppard JD, Nguyen QD, Usner DW, Comstock TL. Post-cataract outcomes in patients with noninfectious posterior uveitis treated with the fluocinolone acetonide intravitreal implant. Clin Opthalmol 2012;6:79-85. https://doi.org/10.2147/OPTH.S24397.
- Soheilian M, Kiavash V, Rabbanikhah Z, Alizadeh Ghavidel L, Ramezani A, Ahmadieh H, et al. Intravitreal bevacizumab vs. triamcinolone for refractory cystoid macular edema in chronic uveitis: a randomized clinical trial. American Acad Ophthalmol 2007. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/394/CN-00717394/frame.html (accessed 6 October 2017).
- Waheed NK, Tufail F, Chu D, Miserocchi E, Foster C. A double-blind randomized controlled trial to assess the efficacy of enbrel versus placebo in preventing relapses of chronic or recurrent uveitis. Invest Ophthalmol Vis Sci 2002;43.
- Williams GA, Blumenkranz MS, Haller JA, Kuppermann BD. Posurdex Study Group . Treatment of persistent macular edema (PME) associated with uveitis or Irvine–Gass syndrome (IGS) with an intravitreal bioerodible sustained dexmethasone release implant; a prospective controlled multi-center clinical trial. Invest Ophthalmol Vis Sci 2003;44.
- Abu El-Asrar AM, Hemachandran S, Al-Mezaine HS, Kangave D, Al-Muammar AM. The outcomes of mycophenolate mofetil therapy combined with systemic corticosteroids in acute uveitis associated with Vogt–Koyanagi–Harada disease. Acta Ophthalmol 2012;90:e603-8. https://doi.org/10.1111/j.1755-3768.2012.02498.x.
- Barreiro-de-Acosta M, Lorenzo A, Dominguez-Munoz JE. Efficacy of adalimumab for the treatment of extraintestinal manifestations of Crohn’s disease. Rev Esp Enferm Dig 2012;104:468-72. https://doi.org/10.4321/S1130-01082012000900004.
- Benitez-del-Castillo JM, Martinez-de-la-Casa JM, Pato-Cour E, Méndez-Fernández R, López-Abad C, Matilla M, et al. Long-term treatment of refractory posterior uveitis with anti-TNFalpha (infliximab). Eye 2005;19:841-5. https://doi.org/10.1038/sj.eye.6701689.
- Bollinger KE, Smith SD. Prevalence and management of elevated intraocular pressure after placement of an intravitreal sustained-release steroid implant. Curr Opin Ophthalmol 2009;20:99-103. https://doi.org/10.1097/ICU.0b013e32831d7f3a.
- Callejas-Rubio JL, Sánchez-Cano D, Serrano JL, Ortego-Centeno N. Adalimumab therapy for refractory uveitis: a pilot study. J Ocul Pharmacol Ther 2008;24:613-14. https://doi.org/10.1089/jop.2008.0073.
- Castellino P, Pluvio C, Bianchi E, Vergani S, Carone M, de Pascale E, et al. Renal hemodynamic effects of cyclosporine treatment in patients with chronic uveitis and normal native kidneys. Transplantation 1994;58:958-61. https://doi.org/10.1097/00007890-199410270-00020.
- Chavis PS, Antonios SR, Tabbara KF. Cyclosporine effects on optic nerve and retinal vasculitis in Behçet’s disease. Doc Ophthalmol 1992;80:133-42. https://doi.org/10.1007/BF00161239.
- Chinchurreta Capote A, Requena Jiménez JM, Lorenzo Soto M, Romero Gómez C, García de Lucas MD. Effectiveness of adalimumab for refractory serpiginous choroiditis. Ocul Immunol Inflamm 2014;22:405-8. https://doi.org/10.3109/09273948.2013.859276.
- Coskun E, Celemler P, Kimyon G, Oner V, Kisacik B, Erbagci I, et al. Intravitreal dexamethasone implant for treatment of refractory Behçet posterior uveitis: one-year follow-up results. Ocul Immunol Inflamm 2015;23:437-43. https://doi.org/10.3109/09273948.2015.1042167.
- Davatchi F, Shahram F, Chams H, Jamshidi AR, Nadji A, Chams C, et al. High dose methotrexate for ocular lesions of Behçet’s disease. Preliminary short-term results. Adv Exp Med Biol 2003;528:579-84. https://doi.org/10.1007/0-306-48382-3_118.
- Denniston AK, Sen HN. VISUALising a new framework for the treatment of uveitis. Lancet 2016;388:1134-6. https://doi.org/10.1016/S0140-6736(16)31327-7.
- Ermertcan AT, Emre S, Öztürk F, Gençoğlan G, Gündüz K. Psoriatic uveitis responding to adalimumab therapy. Int J Dermatol 2014;53:e271-3. https://doi.org/10.1111/ijd.12194.
- Giardina A, Ferrante A, Ciccia F, Vadala M, Giardina E, Triolo G. One year study of efficacy and safety of infliximab in the treatment of patients with ocular and neurological Behçet’s disease refractory to standard immunosuppressive drugs. Rheumatol Int 2011;31:33-7. https://doi.org/10.1007/s00296-009-1213-z.
- Hamuryudan V, Ozyazgan Y, Hizli N, Mat C, Yurdakul S, Tüzün Y, et al. Azathioprine in Behçet’s syndrome: effects on long-term prognosis. Arthritis Rheum 1997;40:769-74. https://doi.org/10.1002/1529-0131(199704)40:4<769::AID-ART24>3.0.CO;2-E.
- Helveston WR, Gilmore R. Treatment of Vogt–Koyanagi–Harada syndrome with intravenous immunoglobulin. Neurology 1996;46:584-5. https://doi.org/10.1212/WNL.46.2.584.
- Jaffe GJ, Pearson PA, Ashton P. Dexamethasone sustained drug delivery implant for the treatment of severe uveitis. Retina 2000;20:402-3. https://doi.org/10.1097/00006982-200004000-00015.
- Jaffe GJ. Reimplantation of a fluocinolone acetonide sustained drug delivery implant for chronic uveitis. Am J Ophthalmol 2008;145:667-75. https://doi.org/10.1016/j.ajo.2007.11.008.
- Khalil HEM, Raafat HA, Azab NA, Haroun HE, Elgendi HA. The role of intraocular methotrexate in the management of uveitis and posterior segment involvement in Behçet’s disease patients. Egypt Rheumatol 2015;37:113-18. https://doi.org/10.1016/j.ejr.2014.08.001.
- Mehryar M, Samangooee S, Hosseini HJ, Kavian N, Mehdizadeh M, Safamanesh S. Clinical trial of sulfasalazine for treatment of uveitis in Behçet’s disease. Iran J Med Sci 2001;26:37-40.
- Mochizuki M, Wechsler B, Godeau P. Behçet’s Disease. Amsterdam: Excerpta Medica; 1993.
- Murphy CC, Greiner K, Plskova J, Frost NA, Forrester JV, Dick AD. Validity of using vision-related quality of life as a treatment end point in intermediate and posterior uveitis. Br J Ophthalmol 2007;91:154-6. https://doi.org/10.1136/bjo.2006.105528.
- Naik RK, Rentz AM, Foster CS, Lightman S, Belfort R, Lowder C, et al. Normative comparison of patient-reported outcomes in patients with noninfectious uveitis. JAMA Ophthalmol 2013;131:219-25. https://doi.org/10.1001/2013.jamaophthalmol.102.
- Nguyen QG, Callanan DG, Eliott D, Godfrey DG, Goldstein DA, Netland PA, et al. Clinical perspectives and considerations with Retisert (R) (fluocinolone acetonide intravitreal implant) 0.59 mg in the management of chronic non-infectious uveitis of the posterior segment: expertise beyond the 3-year follow-up clinical trial data. Retina 2009.
- Ozsahin M, Turan H, Ataoglu S, Baki AE, Celebi E. Anti-TNF-alpha therapy for concomitant Behçet’s disease and ankylosing spondylitis. Turk J Rheumatol 2012;27:214-16. https://doi.org/10.5606/tjr.2012.037.
- Ozyazgan Y, Yurdakul S, Yazici H, Tüzün B, Işçimen A, Tüzün Y, et al. Low dose cyclosporin A versus pulsed cyclophosphamide in Behçet’s syndrome: a single masked trial. Br J Ophthalmol 1992;76:241-3. https://doi.org/10.1136/bjo.76.4.241.
- Sakane T, Mochizuki M, Inaba G, Masuda K. A Phase II study of FK506 (tacrolimus) on refractory uveitis associated with Behçet’s disease and allied conditions. Ryumachi 1995;35:802-13.
- Sen HN, Drye LT, Goldstein DA, Larson TA, Merrill PT, Pavan PR, et al. Hypotony in patients with uveitis: the Multicenter Uveitis Steroid Treatment (MUST) trial. Ocul Immunol Inflamm 2012;20:104-12. https://doi.org/10.3109/09273948.2011.647228.
- Sen HN, Abreu FM, Louis TA, Sugar EA, Altaweel MM, Elner SG, et al. Cataract surgery outcomes in uveitis: the Multicenter Uveitis Steroid Treatment trial. Ophthalmology 2016;123:183-90. https://doi.org/10.1016/j.ophtha.2015.09.022.
- Suhler EB, Lowder CY, Goldstein DA, Giles T, Lauer AK, Kurz PA, et al. Adalimumab therapy for refractory uveitis: results of a multicentre, open-label, prospective trial. Br J Ophthalmol 2013;97:481-6. https://doi.org/10.1136/bjophthalmol-2012-302292.
- Tay-Kearney ML. Intravitreal triamcinolone acetonide injection for the treatment of posterior uveitis. Ocul Immunol Inflamm 2006;14:201-2. https://doi.org/10.1080/09273940600940926.
- Zlatanović G, Jovanović S, Veselinović D, Zivković M. Efficacy of TNF-alpha antagonist and other immunomodulators in the treatment of patients with ophthalmologic manifestations of Behçet’s disease and HLA B51 positive vasculitis. Vojnosanit Pregl 2012;69:168-74. https://doi.org/10.2298/VSP1202168Z.
- Anonymous . Erratum: randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the Multicenter Uveitis Steroid Treatment trial (Ophthalmology (2011) 118 (1916–1926)). Ophthalmology 2012;119. https://doi.org/10.1016/j.ophtha.2011.12.001.
- Cunningham ET, Zierhut M. TNF inhibitors for uveitis: balancing efficacy and safety. Ocul Immunol Inflamm 2010;18:421-3. https://doi.org/10.3109/09273948.2010.531176.
- Cunningham ET, Pasadhika S, Suhler EB, Zierhut M. Drug-induced inflammation in patients on TNFα inhibitors. Ocul Immunol Inflamm 2012;20:2-5. https://doi.org/10.3109/09273948.2011.644383.
- Farber MD, Tessler HH, Jennings TJ, Lam S, Cross A, Rusin MM. Acetazolamide reduction of macular edema in uveitis patients: a prospective crossover study. American Acad Ophthalmol 1992. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/910/CN-00483910/frame.html (accessed 6 October 2017).
- Fraser-Bell S, Pavesio C. Advances in the treatment of intermediate and posterior uveitis. Exp Rev Ophthalmol 2008;3:449-55. https://doi.org/10.1586/17469899.3.4.449.
- Goldstein DA. Uncovering the risks of immunosuppressive therapy in patients with uveitis. Arch Ophthalmol 2009;127:799-800. https://doi.org/10.1001/archophthalmol.2009.124.
- Gonzalez JM, Nader N. Uveitis recurrence rate lowered, adjunctive therapy reduced with implant. Ocular Surg News 2005;23:32-7.
- Hall J. Comment on: ‘Use of the fluocinolone acetonide intravitreal implant for the treatment of noninfectious posterior uveitis: 3-year results of a randomized clinical trial in a predominantly Asian population’. Ophthalmol Ther 2015;4:65-6. https://doi.org/10.1007/s40123-015-0029-z.
- Lai WW, Law RW, Lam DS. Efficacy of periocular corticosteroid injections in the management of posterior uveitis. Clin Exp Ophthalmol 2005;33.
- Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Ciclosporin treatment for Behçet’s disease – double-masked group comparative study. Rinsho Hyoka (Clinical Evaluation) 1986;14:437-51.
- Puchalska-Niedbał L. Evaluation of the effect of treatment with FIBS preparation on the clinical state and various parameters of the immune system in patients with uveitis. Ann Acad Med Stetin 1989;35:179-88.
- Rho D. Acetazolamide treatment of CME in patients with uveitis. Ophthalmology 1996;103. https://doi.org/10.1016/S0161-6420(96)30437-5.
- Shimakawa M, Yamamoto K, Tanaka M, Chen LL, Hori S. Corticosteroid therapy for sarcoid-associated uveitis [Japanese]. Folia Ophthalmol Jpn 2002;53:444-7.
- Wiederholt M, Kämpe C, Hansen LL, Hövener G. Treatment of posterior uveitis with cyclosporin or prednisolone – one-year report of a randomized prospective study. Fortschr Ophthalmol 1986;83:348-52.
- Wirostko WJ, Wirostko E. Scleritis-associated uveitis. Ophthalmology 1997;104:1207-8. https://doi.org/10.1016/S0161-6420(97)38953-2.
- Zhou D, Bi HS, Wei LJ, Xie XF. Clinical application of traditional Chinese medicine nourishing yin and promoting blood circulation in recurrent uveitis [Chinese]. Int J Ophthalmol 2010;10:966-7.
Appendix 1 Literature search strategies
Clinical effectiveness searches
MEDLINE, Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE(R) Daily and MEDLINE(R) (1946 to 2016) (via Ovid)
Date searched: 13 June 2016.
| # | Searches |
|---|---|
| 1 | exp Uveitis/ |
| 2 | uveiti*.mp. |
| 3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*).tw. |
| 4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*).tw. |
| 5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) adj (disease or syndrome)).tw. |
| 6 | (vogt koyanagi harada or triple symptom complex).tw. |
| 7 | (ophthalm* adj2 sympathetic).tw. |
| 8 | exp Retinitis/ |
| 9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis).tw. |
| 10 | or/1-9 |
| 11 | Meta-Analysis as Topic/ |
| 12 | meta analy$.tw. |
| 13 | metaanaly$.tw. |
| 14 | Meta-Analysis/ |
| 15 | (systematic adj (review$1 or overview$1)).tw. |
| 16 | exp Review Literature as Topic/ |
| 17 | or/11-16 |
| 18 | cochrane.ab. |
| 19 | embase.ab. |
| 20 | (psychlit or psyclit).ab. |
| 21 | (psychinfo or psycinfo).ab. |
| 22 | (cinahl or cinhal).ab. |
| 23 | science citation index.ab. |
| 24 | bids.ab. |
| 25 | cancerlit.ab. |
| 26 | or/18-25 |
| 27 | reference list$.ab. |
| 28 | bibliograph$.ab. |
| 29 | hand-search$.ab. |
| 30 | relevant journals.ab. |
| 31 | manual search$.ab. |
| 32 | or/27-31 |
| 33 | selection criteria.ab. |
| 34 | data extraction.ab. |
| 35 | 33 or 34 |
| 36 | Review/ |
| 37 | 35 and 36 |
| 38 | Comment/ |
| 39 | Letter/ |
| 40 | Editorial/ |
| 41 | animal/ |
| 42 | human/ |
| 43 | 41 not (41 and 42) |
| 44 | or/38-40,43 |
| 45 | 17 or 26 or 32 or 37 |
| 46 | 45 not 44 |
| 47 | Randomized Controlled Trials as Topic/ |
| 48 | randomized controlled trial/ |
| 49 | Random Allocation/ |
| 50 | Double Blind Method/ |
| 51 | Single Blind Method/ |
| 52 | clinical trial/ |
| 53 | clinical trial, phase i.pt. |
| 54 | clinical trial, phase ii.pt. |
| 55 | clinical trial, phase iii.pt. |
| 56 | clinical trial, phase iv.pt. |
| 57 | controlled clinical trial.pt. |
| 58 | randomized controlled trial.pt. |
| 59 | multicenter study.pt. |
| 60 | clinical trial.pt. |
| 61 | exp Clinical Trials as topic/ |
| 62 | or/47-61 |
| 63 | (clinical adj trial$).tw. |
| 64 | ((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or mask$3)).tw. |
| 65 | PLACEBOS/ |
| 66 | placebo$.tw. |
| 67 | randomly allocated.tw. |
| 68 | (allocated adj2 random$).tw. |
| 69 | or/63-68 |
| 70 | 62 or 69 |
| 71 | case report.tw. |
| 72 | letter/ |
| 73 | historical article/ |
| 74 | or/71-73 |
| 75 | 70 not 74 |
| 76 | 10 and (46 or 75) |
EMBASE (1974–2016) (via Ovid)
Date searched: 13 June 2016.
| # | Searches |
|---|---|
| 1 | exp uveitis/ |
| 2 | uveiti*.mp. |
| 3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*).tw. |
| 4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*).tw. |
| 5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) adj (disease or syndrome)).tw. |
| 6 | (vogt koyanagi harada or triple symptom complex).tw. |
| 7 | (ophthalm* adj2 sympathetic).tw. |
| 8 | exp retinitis/ |
| 9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis).tw. |
| 10 | or/1-9 |
| 11 | exp Meta Analysis/ |
| 12 | ((meta adj analy$) or metaanalys$).tw. |
| 13 | (systematic adj (review$1 or overview$1)).tw. |
| 14 | or/11-13 |
| 15 | cancerlit.ab. |
| 16 | cochrane.ab. |
| 17 | embase.ab. |
| 18 | (psychlit or psyclit).ab. |
| 19 | (psychinfo or psycinfo).ab. |
| 20 | (cinahl or cinhal).ab. |
| 21 | science citation index.ab. |
| 22 | bids.ab. |
| 23 | or/15-22 |
| 24 | reference lists.ab. |
| 25 | bibliograph$.ab. |
| 26 | hand-search$.ab. |
| 27 | manual search$.ab. |
| 28 | relevant journals.ab. |
| 29 | or/24-28 |
| 30 | data extraction.ab. |
| 31 | selection criteria.ab. |
| 32 | 30 or 31 |
| 33 | review.pt. |
| 34 | 32 and 33 |
| 35 | letter.pt. |
| 36 | editorial.pt. |
| 37 | animal/ |
| 38 | human/ |
| 39 | 37 not (37 and 38) |
| 40 | or/35-36,39 |
| 41 | 14 or 23 or 29 or 34 |
| 42 | 41 not 40 |
| 43 | Clinical trial/ |
| 44 | Randomized controlled trial/ |
| 45 | Randomization/ |
| 46 | Single blind procedure/ |
| 47 | Double blind procedure/ |
| 48 | Crossover procedure/ |
| 49 | Placebo/ |
| 50 | Randomi?ed controlled trial$.tw. |
| 51 | Rct.tw. |
| 52 | Random allocation.tw. |
| 53 | Randomly allocated.tw. |
| 54 | Allocated randomly.tw. |
| 55 | (allocated adj2 random).tw. |
| 56 | Single blind$.tw. |
| 57 | Double blind$.tw. |
| 58 | ((treble or triple) adj blind$).tw. |
| 59 | Placebo$.tw. |
| 60 | Prospective study/ |
| 61 | or/43-60 |
| 62 | Case study/ |
| 63 | Case report.tw. |
| 64 | Abstract report/ or letter/ |
| 65 | or/62-64 |
| 66 | 61 not 65 |
| 67 | 10 and (42 or 66) |
Web of Science® Core Collection, Science Citation Index Expanded (1900–2016), Conference Proceedings Citation Index – Science (1990–2016) (Thomson Reuters)
Date searched: 13 June 2016.
| # | Searches |
|---|---|
| #1 | TOPIC: (uveiti*) |
| #2 | TOPIC: ((panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*)) |
| #3 | TOPIC: ((iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*)) |
| #4 | TOPIC: (((vogt or harada or behcet* or blau* or jabs or reiter*) near (disease or syndrome))) |
| #5 | TOPIC: ((vogt koyanagi harada or triple symptom complex)) |
| #6 | TOPIC: ((ophthalm* near/2 sympathetic)) |
| #7 | TOPIC: ((retinitis or vitritis* or uveoretinitis or neuroretinitis) |
| #8 | #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 |
| #9 | TOPIC: ((“clinic* trial*” or “randomi* controlled trial*”)) OR TOPIC: (((singl* or doubl* or treb* or tripl*) and (blind* or mask*))) OR TOPIC: ((placebo*)) OR TOPIC: ((allocat* and random*)) |
| #10 | TOPIC: ((meta-analysis or meta analy* or metaanaly*)) OR TOPIC: ((“review literature” or “literature review”)) OR TOPIC: ((“systematic review*” or “systematic overview*”)) OR TOPIC: ((cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or cinhal or science citation index or bids or cancerlit)) OR TOPIC: ((“reference list*” or bibliograph* or hand-search* or “relevant journals” or “manual search*”)) OR TOPIC: (((“selection criteria” or “data extraction”) and review)) |
| #11 | #10 OR #9 |
| #12 | #11 AND #8 |
Cochrane Database of Systematic Reviews (CDSR) (1996–2016), Cochrane Central Register of Controlled Trials (CENTRAL) (1898–2016), Health Technology Assessment (HTA) database (1995–2016), Database of Abstracts of Reviews of Effects (DARE) (1995–2015), NHS Economic Evaluation Database (NHS EED) (1995–2015) (Wiley Online Library)
Date searched: 13 June 2016.
| # | Searches |
|---|---|
| #1 | MeSH descriptor: [Uveitis] explode all trees |
| #2 | uveiti*:ti,ab,kw |
| #3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*):ti,ab,kw |
| #4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*):ti,ab,kw |
| #5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) near (disease or syndrome)):ti,ab,kw |
| #6 | (vogt koyanagi harada or triple symptom complex):ti,ab,kw |
| #7 | (ophthalm* near/2 sympathetic):ti,ab,kw |
| #8 | MeSH descriptor: [Retinitis] explode all trees |
| #9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis):ti,ab,kw |
| #10 | #1 or #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 |
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982–2016) (EBSCOhost)
Date searched: 6 October 2016.
| # | Searches |
|---|---|
| S1 | (MH “Uveitis+”) |
| S2 | uveiti* |
| S3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*) |
| S4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*) |
| S5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) N1 (disease or syndrome)) |
| S6 | (vogt koyanagi harada or triple symptom complex) |
| S7 | (ophthalm* N2 sympathetic) |
| S8 | (MH “Retinitis+”) |
| S9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis) |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 |
| S11 | (MH “Meta Analysis”) |
| S12 | TI ( ( Meta analys* or Metaanaly* ) ) or AB ( ( Meta analys* or Metaanaly* ) ) |
| S13 | (MH “Literature Review+”) |
| S14 | systematic N2 review or systematic N2 overview |
| S15 | S11 or S12 or S13 or S14 |
| S16 | PT Commentary or PT Letter or PT Editorial |
| S17 | (MH “Animals”) |
| S18 | S16 or S17 |
| S19 | S15 not S18 |
| S20 | (MH “Clinical Trials+”) |
| S21 | PT Clinical trial |
| S22 | TI Randomi?ed control* trial* or AB Randomi?ed control* trial* |
| S23 | (MH “Random Assignment”) |
| S24 | (MH “Quantitative Studies”) |
| S25 | TI Allocat* random* or AB Allocat* random* |
| S26 | TI Random* allocat* or AB Random* allocat* |
| S27 | TI Placebo* or AB Placebo* |
| S28 | TI Placebos or AB Placebos |
| S29 | TI ( (singl* or doubl* or trebl* or tripl*) and (blind* or mask*) ) or AB ( (singl* or doubl* or trebl* or tripl*) and (blind* or mask*) ) |
| S30 | TI clinic* N1 trial* or AB clinic* N1 trial* |
| S31 | S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 |
| S32 | S10 and (S19 or S31) |
Cost-effectiveness studies
MEDLINE, Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE(R) Daily and MEDLINE(R) (1946–2016) (Ovid)
Date searched: 7 June 2016.
| # | Searches |
|---|---|
| 1 | exp Uveitis/ |
| 2 | uveiti*.mp. |
| 3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*).tw. |
| 4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*).tw. |
| 5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) adj (disease or syndrome)).tw. |
| 6 | (vogt koyanagi harada or triple symptom complex).tw. |
| 7 | (ophthalm* adj2 sympathetic).tw. |
| 8 | exp Retinitis/ |
| 9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis).tw. |
| 10 | or/1-9 |
| 11 | Economics/ |
| 12 | “costs and cost analysis”/ |
| 13 | Cost-benefit analysis/ |
| 14 | Cost control/ |
| 15 | Cost savings/ |
| 16 | Cost of illness/ |
| 17 | Cost sharing/ |
| 18 | “deductibles and coinsurance”/ |
| 19 | Medical savings accounts/ |
| 20 | Health care costs/ |
| 21 | Direct service costs/ |
| 22 | Drug costs/ |
| 23 | Employer health costs/ |
| 24 | Hospital costs/ |
| 25 | Health expenditures/ |
| 26 | Capital expenditures/ |
| 27 | Value of life/ |
| 28 | exp economics, hospital/ |
| 29 | exp economics, medical/ |
| 30 | Economics, nursing/ |
| 31 | Economics, pharmaceutical/ |
| 32 | exp “fees and charges”/ |
| 33 | exp budgets/ |
| 34 | (low adj cost).mp. |
| 35 | (high adj cost).mp. |
| 36 | (health?care adj cost*).mp. |
| 37 | (fiscal or funding or financial or finance).tw. |
| 38 | (cost adj estimate*).mp. |
| 39 | (cost adj variable).mp. |
| 40 | (unit adj cost*).mp. |
| 41 | (economic* or pharmacoeconomic* or price* or pricing).tw. |
| 42 | or/11-41 |
| 43 | 10 and 42 |
EMBASE (1974–2016) (Ovid)
Date searched: 7 June 2016.
| # | Searches |
|---|---|
| 1 | exp uveitis/ |
| 2 | uveiti*.mp. |
| 3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*).tw. |
| 4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*).tw. |
| 5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) adj (disease or syndrome)).tw. |
| 6 | (vogt koyanagi harada or triple symptom complex).tw. |
| 7 | (ophthalm* adj2 sympathetic).tw. |
| 8 | exp retinitis/ |
| 9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis).tw. |
| 10 | or/1-9 |
| 11 | Socioeconomics/ |
| 12 | Cost benefit analysis/ |
| 13 | Cost effectiveness analysis/ |
| 14 | Cost of illness/ |
| 15 | Cost control/ |
| 16 | Economic aspect/ |
| 17 | Financial management/ |
| 18 | Health care cost/ |
| 19 | Health care financing/ |
| 20 | Health economics/ |
| 21 | Hospital cost/ |
| 22 | (fiscal or financial or finance or funding).tw. |
| 23 | Cost minimization analysis/ |
| 24 | (cost adj estimate*).mp. |
| 25 | (cost adj variable*).mp. |
| 26 | (unit adj cost*).mp. |
| 27 | or/11-26 |
| 28 | 10 and 27 |
Web of Science® Core Collection, Science Citation Index Expanded (1900–2016), Conference Proceedings Citation Index – Science (1990–2016) (Thomson Reuters)
Date searched: 7 June 2016.
| # | Searches |
|---|---|
| #1 | TOPIC: (uveiti*) |
| #2 | TOPIC: ((panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*)) |
| #3 | TOPIC: ((iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*)) |
| #4 | TOPIC: (((vogt or harada or behcet* or blau* or jabs or reiter*) near (disease or syndrome))) |
| #5 | TOPIC: ((vogt koyanagi harada or triple symptom complex)) |
| #6 | TOPIC: ((ophthalm* near/2 sympathetic)) |
| #7 | TOPIC: ((retinitis or vitritis* or uveoretinitis or neuroretinitis)) |
| #8 | #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 |
| #9 | TOPIC: ((cost* and (effective* or utilit* or benefit* or minimi*))) OR TOPIC: (cost*) OR TOPIC: ((economic* or pharmacoeconomic* or pharmaco-economic*)) OR TOPIC: ((financial or finance or finances or financed)) OR TOPIC: ((fee or fees)) OR TOPIC: ((value and (money or monetary))) OR TOPIC: ((economic* and (hospital or medical or nursing or pharmaceutical))) OR TOPIC: ((“quality adjusted life year” or “quality adjusted life years”)) OR TOPIC: ((qaly or qalys)) OR TOPIC: (budget*) OR TOPIC: ((price* or pricing*)) |
| #10 | #9 AND #8 |
Cochrane Database of Systematic Reviews (CDSR) (1996–2016), Health Technology Assessment (HTA) database (1995–2016), Database of Abstracts of Reviews of Effects (DARE) (1995–2015), NHS Economic Evaluation Database (NHS EED) (1995–2015) (Wiley Online Library)
Date searched: 7 June 2016.
| # | Searches |
|---|---|
| #1 | MeSH descriptor: [Uveitis] explode all trees |
| #2 | uveiti*:ti,ab,kw |
| #3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*):ti,ab,kw |
| #4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*):ti,ab,kw |
| #5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) near (disease or syndrome)):ti,ab,kw |
| #6 | (vogt koyanagi harada or triple symptom complex):ti,ab,kw |
| #7 | (ophthalm* near/2 sympathetic):ti,ab,kw |
| #8 | MeSH descriptor: [Retinitis] explode all trees |
| #9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis):ti,ab,kw |
| #10 | #1 or #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 |
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982–2016) (EBSCOhost)
Date searched: 6 October 2016.
| # | Searches |
|---|---|
| S1 | (MH “Uveitis+”) |
| S2 | uveiti* |
| S3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*) |
| S4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*) |
| S5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) N1 (disease or syndrome)) |
| S6 | (vogt koyanagi harada or triple symptom complex) |
| S7 | (ophthalm* N2 sympathetic) |
| S8 | (MH “Retinitis+”) |
| S9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis) |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 |
| S11 | (MH “Costs and Cost Analysis+”) |
| S12 | (MH “Economics”) |
| S13 | (MH “Economics, Pharmaceutical”) |
| S14 | (MH “Fees and Charges+”) |
| S15 | (MH “Budgets”) |
| S16 | budget* |
| S17 | cost* |
| S18 | AB cost* and (effective* or utilit* or benefit* or minimi*) |
| S19 | TI economic* or pharmacoeconomic* or pharmaco-economic* |
| S20 | price* or pricing* |
| S21 | financial or finance or finances or financed |
| S22 | fee or fees |
| S23 | value and (money or monetary) |
| S24 | qaly or qalys |
| S25 | quality adjusted life year or quality adjusted life years |
| S26 | S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 |
| S27 | S10 AND S26 |
Quality-of-life studies
MEDLINE, Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations MEDLINE(R) Daily and MEDLINE(R) (1946–2016) (Ovid)
Date searched: 9 June 2016.
| # | Searches |
|---|---|
| 1 | exp Uveitis/ |
| 2 | uveiti*.mp. |
| 3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*).tw. |
| 4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*).tw. |
| 5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) adj (disease or syndrome)).tw. |
| 6 | (vogt koyanagi harada or triple symptom complex).tw. |
| 7 | (ophthalm* adj2 sympathetic).tw. |
| 8 | exp Retinitis/ |
| 9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis).tw. |
| 10 | or/1-9 |
| 11 | “Quality of Life”/ |
| 12 | (qol or (quality adj2 life)).ab,ti. |
| 13 | (value adj2 (money or monetary)).tw. |
| 14 | value of life/ |
| 15 | quality adjusted life year/ |
| 16 | quality adjusted life.tw. |
| 17 | (qaly* or qald* or qale* or qtime*).tw. |
| 18 | disability adjusted life.tw. |
| 19 | daly*.tw. |
| 20 | health status indicators/ |
| 21 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 22 | (sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 23 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 24 | (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen).tw. |
| 25 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 26 | (euroqol or euro qol or eq5d or eq 5d).tw. |
| 27 | (hql or hqol or h qol or hrqol or hr qol).tw. |
| 28 | (hye or hyes).tw. |
| 29 | health* year* equivalent*.tw. |
| 30 | health utilit*.tw. |
| 31 | (hui or hui1 or hui2 or hui3).tw. |
| 32 | disutilit*.tw. |
| 33 | rosser.tw. |
| 34 | (quality adj2 wellbeing).tw. |
| 35 | qwb.tw. |
| 36 | (willingness adj2 pay).tw. |
| 37 | standard gamble*.tw. |
| 38 | time trade off.tw. |
| 39 | time tradeoff.tw. |
| 40 | tto.tw. |
| 41 | letter.pt. |
| 42 | editorial.pt. |
| 43 | comment.pt. |
| 44 | 41 or 42 or 43 |
| 45 | or/11-40 |
| 46 | 45 not 44 |
| 47 | 10 and 46 |
EMBASE (1974–2016) (Ovid)
Date searched: 9 June 2016.
| # | Searches |
|---|---|
| 1 | exp uveitis/ |
| 2 | uveiti*.mp. |
| 3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*).tw. |
| 4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*).tw. |
| 5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) adj (disease or syndrome)).tw. |
| 6 | (vogt koyanagi harada or triple symptom complex).tw. |
| 7 | (ophthalm* adj2 sympathetic).tw. |
| 8 | exp retinitis/ |
| 9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis).tw. |
| 10 | or/1-9 |
| 11 | “Quality of Life”/ |
| 12 | (qol or (quality adj2 life)).ti,ab. |
| 13 | (value adj2 (money or monetary)).tw. |
| 14 | socioeconomics/ |
| 15 | quality adjusted life year/ |
| 16 | (qaly$ or qald$ or qale$ or qtime$).tw. |
| 17 | disability adjusted life.tw. |
| 18 | daly$.tw. |
| 19 | health survey/ |
| 20 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 21 | (sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 22 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 23 | (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen).tw. |
| 24 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 25 | (euroqol or euro qol or eq5d or eq 5d).tw. |
| 26 | (hql or hqol or h qol or hrqol or hr qol).tw. |
| 27 | (hye or hyes).tw. |
| 28 | health$ year$ equivalent$.tw. |
| 29 | health utilit$.tw. |
| 30 | (hui or hui1 or hui2 or hui3).tw. |
| 31 | disutilit$.tw. |
| 32 | rosser.tw. |
| 33 | (quality adj2 wellbeing).tw. |
| 34 | qwb.tw. |
| 35 | (willingness adj2 pay).tw. |
| 36 | standard gamble$.tw. |
| 37 | time trade off.tw. |
| 38 | time tradeoff.tw. |
| 39 | tto.tw. |
| 40 | letter.pt. |
| 41 | editorial.pt. |
| 42 | comment.pt. |
| 43 | 40 or 41 or 42 |
| 44 | or/11-39 |
| 45 | 44 not 43 |
| 46 | 10 and 45 |
Web of Science® Core Collection, Science Citation Index Expanded (1900–2016), Conference Proceedings Citation Index – Science (1990–2016) (Thomson Reuters)
Date searched: 9 June 2016.
| # | Searches |
|---|---|
| #1 | TOPIC: (uveiti*) |
| #2 | TOPIC: ((panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*)) |
| #3 | TOPIC: ((iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*)) |
| #4 | TOPIC: (((vogt or harada or behcet* or blau* or jabs or reiter*) near (disease or syndrome))) |
| #5 | TOPIC: ((vogt koyanagi harada or triple symptom complex)) |
| #6 | TOPIC: ((ophthalm* near/2 sympathetic)) |
| #7 | TOPIC: ((retinitis or vitritis* or uveoretinitis or neuroretinitis)) |
| #8 | #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 |
| #9 | TOPIC: ((qol or “quality of life” or “quality adjusted life”)) OR TOPIC: ((qaly* or qald* or qale* or qtime*)) OR TOPIC: ((“disability adjusted life” or daly*)) OR TOPIC: ((sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six)) OR TOPIC: ((sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six)) OR TOPIC: ((sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve)) OR TOPIC: ((sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen)) OR TOPIC: ((sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty)) OR TOPIC: ((euroqol or euro qol or eq5d or eq 5d)) OR TOPIC: ((hql or hqol or h qol or hrqol or hr qol)) OR TOPIC: ((hye or hyes)) OR TOPIC: ((“health* year* equivalent*”)) OR TOPIC: ((“health utilit*”)) OR TOPIC: ((hui or hui1 or hui2 or hui3)) OR TOPIC: ((disutilit* or rosser)) OR TOPIC: ((“quality of wellbeing” or qwb or “willingness to pay”)) OR TOPIC: ((“standard gamble*” or “time trade off” or “time tradeoff” or tto)) |
| #10 | #9 AND #8 |
Cochrane Database of Systematic Reviews (CDSR) (1990–2016), Cochrane Central Register of Controlled Trials (CENTRAL) (1898–2016), Health Technology Assessment (HTA) database (1995–2016), Database of Abstracts of Reviews of Effects (DARE) (1995–2015), NHS Economic Evaluation Database (NHS EED) (1995–2015) (Wiley Online Library)
Date searched: 9 June 2016.
| # | Searches |
|---|---|
| #1 | MeSH descriptor: [Uveitis] explode all trees |
| #2 | uveiti*:ti,ab,kw |
| #3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*):ti,ab,kw |
| #4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*):ti,ab,kw |
| #5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) near (disease or syndrome)):ti,ab,kw |
| #6 | (vogt koyanagi harada or triple symptom complex):ti,ab,kw |
| #7 | (ophthalm* near/2 sympathetic):ti,ab,kw |
| #8 | MeSH descriptor: [Retinitis] explode all trees |
| #9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis):ti,ab,kw |
| #10 | #1 or #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 |
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982–2016) (EBSCOhost)
Date searched: 6 October 2016.
| # | Searches |
|---|---|
| S1 | (MH “Uveitis+”) |
| S2 | uveiti* |
| S3 | (panuveitis or pars planitis or panophthalmitis or uveomeningoencephaliti*) |
| S4 | (iridocycliti* or heterochromic cycliti* or anterior scleritis or iriti* or choroiditi* or retinochoroiditi* or retinochoroidopath* or chorioretinitis or chorioretinopath*) |
| S5 | ((vogt or harada or behcet* or blau* or jabs or reiter*) N1 (disease or syndrome)) |
| S6 | (vogt koyanagi harada or triple symptom complex) |
| S7 | (ophthalm* N2 sympathetic) |
| S8 | (MH “Retinitis+”) |
| S9 | (retinitis or vitritis* or uveoretinitis or neuroretinitis) |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 |
| S11 | (MH “Quality of Life”) |
| S12 | TI ( qol or (quality N2 life) ) or AB ( qol or (quality N2 life) ) |
| S13 | TI value and TI ( money or monetary ) or AB value and AB ( money or monetary ) |
| S14 | (MH “Economic Value of Life”) |
| S15 | (MH “Quality-Adjusted Life Years”) |
| S16 | TI ( qaly* or qald* or qale* or qtime* ) or AB ( qaly* or qald* or qale* or qtime* ) |
| S17 | TI disability adjusted life or AB disability adjusted life |
| S18 | TI daly* or AB daly* |
| S19 | (MH “Health Status Indicators”) |
| S20 | TI ( sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six ) or AB ( sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six ) |
| S21 | TI ( sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six ) or AB ( sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six ) |
| S22 | TI quality adjusted life or AB quality adjusted life |
| S23 | TI ( sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve ) or AB ( sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve ) |
| S24 | TI ( sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen ) or AB ( sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen ) |
| S25 | TI ( sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty ) or AB ( sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty ) |
| S26 | TI ( euroqol or euro qol or eq5d or eq 5d ) or AB ( euroqol or euro qol or eq5d or eq 5d ) |
| S27 | TI ( hql or hqol or h qol or hrqol or hr qol ) or AB ( hql or hqol or h qol or hrqol or hr qol ) |
| S28 | TI ( hye or hyes ) or AB ( hye or hyes ) |
| S29 | TI health* year* equivalent* or AB health* year* equivalent* |
| S30 | TI health utilit* or AB health utilit* |
| S31 | TI ( hui or hui1 or hui2 or hui3 ) or AB ( hui or hui1 or hui2 or hui3 ) |
| S32 | TI disutilit* or AB disutilit* |
| S33 | TI rosser or AB rosser |
| S34 | TI quality N2 wellbeing or AB quality N2 wellbeing |
| S35 | TI qwb or AB qwb |
| S36 | TI willingness N2 pay or AB willingness N2 pay |
| S37 | TI standard gamble* or AB standard gamble* |
| S38 | TI time trade off or AB time trade off |
| S39 | TI time tradeoff or AB time tradeoff |
| S40 | TI tto or AB tto |
| S41 | PT letter |
| S42 | PT editorial |
| S43 | PT comment |
| S44 | S41 or S42 or S43 |
| S45 | S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 |
| S46 | S45 NOT S44 |
| S47 | S10 AND S46 |
Costs and utilities of blindness
MEDLINE, Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE(R) Daily and MEDLINE(R) (1946–2016) (Ovid)
Date searched: 20 October 2016.
| # | Searches |
|---|---|
| 1 | blindness.ti. |
| 2 | ((sight or visual or vision) adj1 loss).ti. |
| 3 | 1 or 2 |
| 4 | exp “costs and cost analysis”/ |
| 5 | costs.tw. |
| 6 | cost effective:.tw. |
| 7 | or/4-6 |
| 8 | 3 and 7 |
| 9 | limit 8 to yr=“2006 -Current” |
| 10 | “Quality of Life”/ |
| 11 | (qol or (quality adj2 life)).ab,ti. |
| 12 | (value adj2 (money or monetary)).tw. |
| 13 | value of life/ |
| 14 | quality adjusted life year/ |
| 15 | quality adjusted life.tw. |
| 16 | (qaly$ or qald$ or qale$ or qtime$).tw. |
| 17 | disability adjusted life.tw. |
| 18 | daly$.tw. |
| 19 | health status indicators/ |
| 20 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 21 | (sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 22 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 23 | (sf6D or sf 6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).tw. |
| 24 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 25 | (euroqol or euro qol or eq5d or eq 5d).tw. |
| 26 | (hql or hqol or h qol or hrqol or hr qol).tw. |
| 27 | (hye or hyes).tw. |
| 28 | health$ year$ equivalent$.tw. |
| 29 | health utilit$.tw. |
| 30 | (hui or hui1 or hui2 or hui3).tw. |
| 31 | disutilit$.tw. |
| 32 | rosser.tw. |
| 33 | (quality adj2 wellbeing).tw. |
| 34 | qwb.tw. |
| 35 | (willingness adj2 pay).tw. |
| 36 | standard gamble$.tw. |
| 37 | time trade off.tw. |
| 38 | time tradeoff.tw. |
| 39 | tto.tw. |
| 40 | letter.pt. |
| 41 | editorial.pt. |
| 42 | comment.pt. |
| 43 | 40 or 41 or 42 |
| 44 | or/10-39 |
| 45 | 44 not 43 |
| 46 | 3 and 45 |
Appendix 2 Table of excluded studies with reasons
| Reference | Intervention/conditions | Reason(s) for exclusion |
|---|---|---|
| Population not non-infectious uveitis (intermediate/posterior/panuveitis) (n = 28) | ||
| Allegri 2014136 | Indomethacin in macular oedema | Includes patients with anterior and postinfective uveitis (includes other ocular disease) |
| Alpsoy 2002137 | Interferon-alpha-2a in Behçet’s disease | Not a homogeneous group of patients with Behçet’s uveitis |
| Biryukova 2015138 | Simvastatin | Includes patients with anterior uveitis |
| Blumenkranz 2010139 | DEX for macular oedema | Most patients did not have uveitis; no separate data |
| Boscia 2005140 | Intravitreal triamcinolone for cystoid macular oedema | Not a RCT; not specific to uveitis |
| Buggage 2007141 | Daclizumab in Behçet’s disease | Uveitis or retinal vasculitis; no separate data. Daclizumab (anti-interleukin-2) not in scope |
| Dada 2007142 | Triamcinolone post cataract | n = 28/40 anterior uveitis; no separate data |
| Davatchi 2004143 | Cyclophosphamide in Behçet’s disease | Population uveitis or retinal vasculitis; no separate data. Posterior uveitis recorded as an outcome not a population |
| Davatchi 2009144 | Colchicine in Behçet’s disease | Not specific to uveitis |
| Davatchi 2010145 | Rituximab in Behçet’s disease | Posterior uveitis recorded as an outcome not a population |
| Foster 1996146 | Rimexolone vs. prednisolone | Anterior segment uveitis |
| Gupta 2013147 | DEX in cataract | Population included patients with tuberculous uveitis and anterior uveitis; DEX administered during cataract surgery |
| Kuppermann 2007148 | DEX in macular oedema | Patients (aged > 12 years) with macular oedema; not specific to uveitis |
| Landewé 2014149 | Certolizumab pegol in axial spondyloarthritis | Data for new cases of uveitis only |
| Louis 2016150 | ADA in Crohn’s disease | No data for uveitis only |
| Parodi 2010151 | Bevacizumab vs. photodynamic therapy | Neither treatment in scope; for treating neovascularisation. Population multifocal choroiditis |
| Perkins 1956152 | Pyrimethamine | Includes patients with anterior uveitis and infectious uveitis. Intervention not in scope |
| Perkins 1965153 | Indomethacin | Mostly anterior uveitis; some infectious uveitis |
| Roesel 2009154 | Triamcinolone, two routes in cataract surgery | Includes patients with anterior uveitis and patients undergoing cataract surgery |
| Roesel 2010155 | Triamcinolone vs. prednisolone in cataract surgery | Includes patients with anterior uveitis and patients undergoing cataract surgery |
| Rosenbaum 2004156 | Etanercept and iritis | Summary of iritis cases across trials of etanercept in ankylosing spondylitis |
| Rudwaleit 2014157 | Certolizumab pegol in axial spondyloarthritis | Not uveitis population. Data relate to uveitis flares (nine cases in total) |
| Rudwaleit 2016158 | Certolizumab pegol in axial spondyloarthritis | Not uveitis population. Data relate to uveitis flares (seven cases in total) |
| Schlaegel 1969159 | Isoniazid | Mostly infectious uveitis (tuberculosis) |
| Sieper 2010160 | Etanercept uveitis rates in trials of ankylosing spondylitis | Summary of uveitis cases across trials of etanercept in ankylosing spondylitis |
| Van den Bosch 2002161 | Infliximab | Uveitis reported only as an AE (in one patient) |
| Williams 2009162 | DEX | Patients with macular oedema as a result of uveitis or Irvine–Gass syndrome |
| Yates 2015163 | Etanercept in ankylosing spondylitis | Uveitis reported only as an AE (in three patients) |
| Intervention not relevant (n = 25) | ||
| Callanan 2008164 | Fluocinolone (two doses, USA) | Compared with non-licensed dose |
| Choi 2005165 | Vitrectomy vs. immunomodulatory treatment | Not in scope |
| de Smet 1992166 | Ciclosporin and ketoconazole | High-dose ciclosporin vs. lower-dose ciclosporin plus ketoconazole |
| Dick 2013167 | Secukinumab (three trials vs. placebo) | Not in scope |
| Farber 1994168 | Acetazolamide in macular oedema | Not in scope |
| Haller 2009169 | DEX | RCT comparing the effect of the insertion procedure |
| Ibrahim 2015170 | SAVE trial | Not in scope |
| Jaffe 2006171 | Fluocinolone (two doses, USA) | Compared with non-licensed dose |
| Lashay 2003172 | Acetazolamide in uveitic macular oedema in Behçet’s disease | Not in scope |
| Letko 2015173 | Secukinumab (one trial of three doses) | Not in scope |
| Neri 2006174 | Echinacea | Not in scope |
| Nguyen 2013175 | SAVE trial | Not in scope |
| Nussenblatt 1997176 | Retinal antigens | Not in scope |
| Nussenblatt 2006177 | Vitamin E | Not in scope |
| Quinones 2010178 | Vitrectomy | Not in scope |
| Rahimi 2012179 | Bevacizumab vs. triamcinolone in uveitic macular oedema | Not in scope |
| Sangwan 2015180 | Fluocinolone (two doses, Asia) | Compared with non-licensed dose; most data not from RCT |
| Soheilian 2010181 | Bevacizumab vs. triamcinolone in uveitic macular oedema | Not in scope |
| Soheilian 2010182 | Bevacizumab vs. triamcinolone in uveitic macular oedema | Not in scope |
| Soheilian 2013183 | Diclofenac vs. triamcinolone in uveitic macular oedema | Not in scope |
| Soheilian 2013184 | Diclofenac vs. triamcinolone in uveitic macular oedema | Not in scope |
| Tranos 2006185 | Vitrectomy | Not in scope |
| van Kooij 2006186 | Lisinopril | Not in scope |
| Vigil 2015187 | SAVE trial | Not in scope |
| Whitcup 1996188 | Acetazolamide in uveitic macular oedema | Not in scope |
| No relevant outcomes or data (n = 15) | ||
| Bodaghi 200116 | Various treatments | Retrospective analysis of causes of uveitis |
| Goldstein 2007189 | Fluocinolone | Analysis of the results of three RCTs of fluocinolone |
| Holbrook 2016190 | Fluocinolone (MUST trial) | Outcome was dissociation of the drug pellet |
| Mackensen 2008191 | Methotrexate vs. interferon in uveitic macular oedema | Secondary publication; intermediate results only |
| Masuda 1989192 | Ciclosporin vs. colchicine in Behçet’s disease | Outcomes were ‘frequency of ocular attack’ and ‘severity of ocular attack’ but these were not defined |
| Mercante 2007193 | Fluocinolone (two doses) | No comparison of data between groups |
| Muller 2004194 | Fluocinolone (two doses) | In German; duplicate publication. Same as Sangwan et al.180 |
| Multicenter Uveitis Steroid Treatment Trial Research Group 2010102 | Fluocinolone (MUST) study design | Secondary publication; no additional data |
| Nussenblatt 1993195 | Ciclosporin A and G | Compares two subtypes of same drug; cannot connect to network |
| Parekh 2015196 | Fluocinolone (IOP risk in three trials) | Analysis of the results of three RCTs of fluocinolone |
| Pavesio 2006197 | Fluocinolone | Secondary publication; preliminary data. Final data in Pavesio et al.58 |
| Sheppard 2012198 | Fluocinolone (two doses) | No comparison of data between groups. Secondary publication of Sangwan et al.180 |
| Soheilian 2007199 | Bevacizumab vs. triamcinolone in uveitic macular oedema | Secondary publication. Same as Soheilian et al.181,182 |
| Waheed 2002200 | Etanercept (abstract) | Secondary publication of Foster et al.61 |
| Williams 2003201 | DEX (Posurdex™, Oculex Pharmaceuticals, Sunnyvale, CA, USA) | Secondary publication of Kuppermann et al.;148 no results reported |
| Not a RCT (n = 33) | ||
| Abu El-Asrar 2012202 | Mycophenolate mofetil in VKH | Not a RCT |
| Barreiro-de-Acosta 2012203 | ADA for Crohn’s disease | Not a RCT |
| Benitez-del-Castillo 2005204 | Infliximab | Not a RCT |
| Bollinger 2009205 | Management of IOP with fluocinolone implant | Review of three RCTs reporting the adverse effects of fluocinolone acetonide |
| Callejas-Rubio 2008206 | ADA | Not a RCT (single-arm study) |
| Castellino 1994207 | Ciclosporin | Not a RCT |
| Chavis 1992208 | Ciclosporin | Not a RCT |
| Chinchurreta Capote 2014209 | ADA in serpiginous choroiditis | Letter |
| Coskun 2015210 | DEX in Behçet’s disease uveitis | Retrospective analysis of a single DEX implant (posterior uveitis as a result of Behçet’s disease) |
| Davatchi 2003211 | Methotrexate in Behçet’s disease | Not a RCT (controlled study) |
| Denniston 2016212 | ADA | News article |
| Ermertcan 2014213 | ADA | Case report of patients with psoriatic uveitis |
| Frick 201239 | Fluocinolone (MUST) | No RCT data, only baseline data. Reports visual acuity and quality of life |
| Giardina 2011214 | Infliximab in Behçet’s disease | Not a RCT |
| Hamuryudan 1997215 | Azathioprine in Behçet’s disease | Reanalysis of patients in RCT by Yazici et al.62 |
| Helveston 1996216 | Intravenous immunoglobulin | Case report |
| Jaffe 2000217 | DEX | Case report |
| Jaffe 2008218 | Fluocinolone | Not a RCT |
| Khalil 2015219 | Methotrexate in Behçet’s disease | Case series |
| Mehryar 2001220 | Sulfasalazine vs. cyclophosphamide in Behçet’s disease | Not a RCT |
| Mochizuki 1993221 | Tacrolimus (FK506) in Behçet’s disease | Not a RCT. Also compares doses only (no placebo/other group). Same as Sakane et al.227 |
| Multicenter Uveitis Steroid Treatment Trial Research Group 2014101 | Fluocinolone (MUST) | Not a RCT; cost-effectiveness analysis |
| Murphy 2007222 | Ciclosporin vs. tacrolimus | Not a RCT |
| Naik 2013223 | DEX (HURON) | Not a RCT. Comparison of PROMs using baseline data from the HURON trial and national data |
| Nguyen 2009224 | Fluocinolone | Not a RCT. Expert perspectives |
| Ozsahin 2012225 | TNF inhibitor | Case report |
| Ozyazgan 1992226 | Ciclosporin vs. cyclophosphamide | Not a RCT. Patients randomised but then could choose treatment |
| Sakane 1995227 | Tacrolimus (FK506) | Not a RCT. Also compares doses only (no placebo/other group) and non-English-language study (Japanese). Same as Mochizuki221 |
| Sen 2012228 | Fluocinolone (MUST) | Not a RCT. Prevalence of hypotony at baseline in the MUST trial |
| Sen 2016229 | Fluocinolone (MUST) | Not a RCT. Nested cohort study of visual acuity outcomes after cataract surgery |
| Suhler 2013230 | ADA | Single-arm study |
| Tay-Kearney 2006231 | Triamcinolone | Not a RCT. Clinical summary |
| Zlatanović 2012232 | TNF-alpha antagonist | Not a RCT. Non-English publication (Serbian) |
| Other (n = 16) | ||
| Anonymous 2012233 | Fluocinolone (MUST) | Letter to editor; erratum |
| Cunningham 2010234 | TNF inhibitors | Editorial |
| Cunningham 2012235 | TNF inhibitors | Editorial |
| Farber 1992236 | Acetazolamide | Clinical trial record |
| Fraser-Bell 2008237 | Various | Review of treatments in patients with uveitis |
| Goldstein 2009238 | TNF inhibitors | Letter |
| Gonzalez 2005239 | Fluocinolone | Editorial |
| Hall 2015240 | Fluocinolone | Letter to editor (difference between Retisert and Iluvien) |
| Lai 2005241 | Periocular corticosteroids | Letter |
| Masuda 1986242 | Ciclosporin | Non-English-language study (Chinese). Other report of this study192 was excluded as the outcomes were not sufficiently robust |
| Puchalska-Niedbał 1989243 | FIBS preparation (not defined) | Non-English-language study (Polish). Some patients with infectious uveitis; unlikely to be relevant intervention |
| Rho 1996244 | Acetazolamide | Letter |
| Shimakawa 2002245 | Corticosteroids (oral vs. topical) | Non-English-language study (Chinese). Likely non-RCT |
| Wiederholt 1986246 | Ciclosporin vs. prednisolone | Non-English-language study (German). Only eight patients and data difficult to interpret |
| Wirostko 1997247 | Scleritis-associated uveitis | Letter |
| Zhou 2010248 | Traditional Chinese medicine | Non-English-language study (Chinese). Intervention not in scope |
Appendix 3 Data extraction form
| Reviewer: | |||
| Study reference | Study name | Author year | Setting(s) |
| Study population | |||
|---|---|---|---|
| Inclusion and exclusion criteria: | |||
| Age: | Percentage males: | ||
| Sample size (number of patients randomised): | Sample size (number of eyes randomised): | ||
| Type of uveitis (intermediate uveitis, posterior uveitis, panuveitis/active, non-active/bilateral or unilateral): | |||
| Cause of uveitis (‘known systemic condition’, ‘no known systemic condition’, ‘not reported’, ‘unclear’): | |||
| State known systemic condition(s): | |||
| Prior treatment received (including treatment for any associated systemic condition)? Yes/no | |||
| List prior treatment(s): | |||
| Concomitant treatment(s) (‘ALL’ if treatment was received by all patients or ‘PRN’ if treatment was given as needed): | |||
| List concomitant treatment(s): | |||
| Baseline BCVA: | Baseline IOP: | ||
| Baseline VH grade: | Baseline central macular thickness: | ||
| Outcomes | |||
| Outcomes reported in the study: | Follow-up schedule for assessments: | ||
| Treatment arm and comparator arm | |||
| Allocated treatment (dosing routine and duration of treatment): | |||
| Number randomised (patients/eyes): | |||
| Number analysed (patients/eyes): | |||
| Details of any excluded/lost/withdrawn post randomisation and reasons: | |||
| Vision or visual acuity outcomes reported:a | |||
| Outcomes of intraocular inflammation activity (e.g. VH grade or AC cell grade) reported:a | |||
| Reported outcomes of uveitis-related tissue damage or complications (e.g. cataract, macular oedema):a | |||
| Other outcomes reported (e.g. composite outcomes):a | |||
| Patient-reported outcomes reported:a | |||
| Ocular and systemic adverse effects reported:a | |||
| Relevance for network meta-analysis | |||
| Clinically relevant? Yes/no | |||
| Connects relevant treatments via network? Yes/no | |||
Appendix 4 Criteria for assessment of the methodological quality of included studies
| Quality item | Reviewer’s judgement | Details |
|---|---|---|
| 1. Were participants assigned to study groups using an acceptable random method? | Yes | Use of centrally generated random numbers, random number tables, throwing dice |
| No | Use of case record numbers, date of birth or alternation or rotation | |
| Unclear | Insufficient details to assess quality item | |
| 2. Was allocation concealment adequately conducted? | Yes | Allocation to study arms achieved by using interactive or web-based system or sequentially numbered opaque envelopes |
| No | Allocation to study arms achieved without appropriate measures, e.g. unsealed, transparent envelopes, date of birth, alternation or rotation or other unconcealed methods | |
| Unclear | Insufficient details to assess quality item | |
| 3. Were eligibility criteria specified for selecting participants? | Yes | Eligibility criteria for study participants specified at study entry |
| No | Eligibility criteria for study participants not specified at study entry | |
| Unclear | Insufficient details to assess quality item | |
| 4. Was the study adequately powered? | Yes | Sample size considered to be adequate (i.e. ≥ 80%) based on the a priori sample size calculation and significance level to detect a minimally clinically significant difference in the primary outcome of interest |
| No | Sample size considered to be inadequate (i.e. < 80%) based on the a priori sample size calculation and significance level to detect a minimally clinically significant difference in the primary outcome of interest | |
| Unclear | Insufficient details to assess quality item | |
| 5. Were study groups comparable for most prognostic indicators at baseline? | Yes | Key prognostic variables (e.g. age, visual acuity, IOP) were reported to be similar in relevant treatment groups at baseline |
| No | Key prognostic variables (e.g. age, visual acuity, IOP) were reported to be different between relevant treatment groups at baseline | |
| Unclear | Insufficient details to assess quality item | |
| 6. Were patients and investigators/outcome assessors blinded to treatment allocation? | Yes | Patients, investigators and/or outcome assessors could not identify administered study treatments |
| No | Patients, investigators and/or outcome assessors may possibly identify administered study treatments | |
| Unclear | Insufficient details to assess quality item | |
| 7. Was follow-up adequate (≥ 70% randomised patients analysed)? | Yes | ≥ 70% of randomised patients (or eyes) were included in the analysis |
| No | <70% of randomised patients (or eyes) were included in the analysis | |
| Unclear | Insufficient details to assess quality item | |
| 8. Were reasons for attrition/exclusions stated? | Yes | Number of patients lost to follow-up (including withdrawals and those excluded from analysis) was reported to ensure completeness of data |
| No | Incomplete data reporting noted because number of patients lost to follow-up (including withdrawals and those excluded from analysis) was not reported | |
| Unclear | Insufficient details to assess quality item | |
| 9. Was an intention-to-treat analysis included? | Yes | Outcome data for all patients initially randomised to a specific study arm were included in the analysis of the specified outcome |
| No | Outcome data for selected patients initially randomised to a specific study arm were included in the analysis of the specified outcome | |
| Unclear | Insufficient details to assess quality item |
Appendix 5 Effectiveness data from non-randomised studies of the dexamethasone implant
| Study and design | Number of patients/eyes, follow-up and number of implants | BCVA | VH | CRT | Repeat implantations | Other |
|---|---|---|---|---|---|---|
| Adán 201385 retrospective study of DEX 700 after vitrectomy for uveitic macular oedema, Spain |
|
Median improvement in BCVA at 1 month was one line (range 0–3; n = 15 eyes; p < 0.01), increasing to two lines by 3 months; 52.9% of eyes improved by two or more lines (p < 0.01). Improvement was maintained in 5 eyes (29.4%) at 6 months. No eyes lost more than one line of BCVA from baseline (p = 0.003) | NR | Mean (SD) CRT at baseline was 461.6 (121.7) µm, decreasing to 277.2 (66.5) µm at 1 month (p < 0.01); at 3 and 6 months mean (SD) CRT was 349.9 (143.2) µm (p = 0.01) and 394.1 (138.4) µm (p = 0.14) respectively. Reduction in CRT of > 100 µm in 10 eyes (62%) at 1 month, 8 eyes (47.1%) at 3 months and 5 eyes (29.4%) at 6 months | NR | Duration of response: over follow-up (mean 9.6 months, range 6–17 months), relapse of CMO (CRT increase of > 150 µm from lowest level post implant) in 8/17 eyes (47.1%) after mean of 6.5 months (range 3–11 months) follow-up. These eyes had a repeat implant |
| Khurana 201586 retrospective review of DEX 700 for cystoid macular oedema secondary to non-infectious uveitis, single centre, USA |
|
Mean BCVA at baseline 0.449 logMAR (Snellen 20/60); improved to 0.238 logMAR (20/30) by 1 month. Significant improvement at 1 month (2.0 lines; p = 0.0016), 3 months (2.1 lines; p = 0.0051), 6 months (2.1 lines; p = 0.014) and 12 months (1.4 lines; p = 0.11). Improvement of two or more lines in 47% of eyes at 1 month and 50% at 3 months | Baseline VH was grade 1 in 33% of eyes and grade 2 in 11% of eyes. Mean VH was grade 0 at 1, 3, 6 and 12 months of follow-up | After first implant, complete resolution of CMO in 89% of eyes at 1 month and 72% of eyes at 3 months. In eyes without epiretinal membrane, CRT decreased at 1 month (190 µm; p = 0.00048) and 3 months (228 µm; p = 0.0039). In eyes with epiretinal membrane, mean change was not significant at 1 month (100 µm; p = 0.063) or 3 months (33 µm; p = 0.50). In all patients, median ± SE time to CMO recurrence was 201 ± 62 days | Repeat implantation in patients with recurrence of CMO and decrease in VA from previous visit. Number of implants per patient during follow-up ranged from one to four; 56% (10/18 eyes) needed two or more implants. Among those with a second implant, median time to retreatment was 300 ± 71 days | NR |
| Lam 201581 retrospective review of DEX 700 for macular oedema, multicentre, Canada |
|
After first implant, 17/21 eyes (81%) gained one or more line of vision, 13 (62%) gained two or more lines and 12 (57%) gained three or more lines | NR | 17/23 eyes showed improvement in CRT, with a mean (SE) peak improvement of 255.6 (43.6) µm. Eyes without prior pars plana vitrectomy showed a greater mean peak improvement than eyes with prior pars plana vitrectomy (295.1 ± 54.0 µm vs. 161.0 ± 20.4 µm) |
|
NR |
| Miserocchi 201279 retrospective study of DEX 700 for posterior uveitis, single centre, Italy |
|
Mean BCVA was 20/80 (0.6 logMAR) before implant and 20/40 (0.3 logMAR) at the end of follow-up (6–11 months). Mean improvement in BCVA of 3.3 lines at the end of follow-up (range 0–6 lines) | NR | CRT was 496 (123) µm at baseline, improving to 226 (66) µm by the end of follow-up | NR | Concomitant systemic immunosuppressants or corticosteroids: all patients were on systemic immunosuppressants or corticosteroids; 3/12 patients reduced corticosteroid dose after receiving DEX 700 |
| Nobre-Cardoso 201683 retrospective review of DEX 700 for non-infectious uveitic macular oedema, single centre, France |
|
Significant improvement in mean BCVA at 1 month after first implant, from 0.84 ± 0.81 logMAR (Snellen 20/140) at baseline to 0.74 ± 0.84 logMAR (20/110) (p < 0.01). Mean BCVA remained improved from baseline at 12 months | Percentage with VH = 0 increased from 51.2% at baseline to 71.1% at month 1 (p < 0.001) and 75.6% at month 3 (p < 0.01). Percentage with VH = 0 at month 12 was higher than at baseline (64.7%) | After first implant, significant improvement in mean CRT, from 461 ± 158 µm at baseline to 308 ± 93 µm at 1 month (p < 0.001). Mean CRT was 340 ± 110 µm at 3 months (p < 0.001) and 442 ± 172 µm at 6 months. After one implant, 6 eyes were free of relapse in MO at 12 months | In 13 eyes with relapse after a positive response to first implant, mean time to second implant was 7.1 ± 2.9 months after the first implant. Repeat implantations improved BCVA (+0.08 logMAR) and CRT (–304 µm) at 1 month post implant. After repeat implant, mean time to relapse was 5.0 ± 1.6 months, similar to that for first implant (p = 0.689) | Mean time to relapse: after first implant (increase of ≥ 50 µm in CRT from month 1) was 6.7 ± 3.7 months; at 12 months the overall relapse rate was 83.3% |
| Palla 201580 retrospective review of DEX 700 for non-infectious uveitis, single centre, India |
|
Mean BCVA improved from 0.666 logMAR (Snellen 20/93) at baseline to 0.479 logMAR (20/60) at 6 weeks (stated as statistically significant) | Proportion achieving VH = 0 was 60%, 45% and 30% at 6 weeks, 6 months and the last visit respectively | Mean CRT improved from 563.1 µm at baseline to 361.4 µm at 6 weeks. Trend continued at each follow-up. Two eyes with epiretinal membrane at baseline had minimal CRT improvement | NR | NR |
| Pelegrín 201551 retrospective review of DEX 700 for macular oedema secondary to non-infectious uveitis, single centre, Spain |
|
BCVA improved in vitrectomised and non-vitrectomised eyes. Maximum improvement at month 3 in both groups, maintained throughout follow-up. Difference between vitrectomised and non-vitrectomised eyes was statistically significant only at 24 months (favoured non-vitrectomised eyes; p = 0.04) | VH at baseline from +0.5 to +3.0 in 21 eyes (50%). Two-step improvement or change from +0.5 to 0 in 66.7% at 1 month, 62% at 3 months, 76.2% at 6 months and 80.1% at 12 months. Changes in maximum VH score were similar in non-vitrectomised and vitrectomised eyes at all follow-up points (p = 0.706) | Maximum decrease in CRT at month 1 in non-vitrectomised and vitrectomised eyes (251.2 and 229.9 µm respectively). Maintained throughout follow-up: at 24 months mean CRT improved by 189.1 and 273.8 µm in non-vitrectomised and vitrectomised eyes respectively (difference significant only at 24 months; p = 0.02) | Repeat implants required in 19 eyes (45.2%). No difference in frequency of repeat implants between non-vitrectomised and vitrectomised eyes. Median time to repeat implantation was 5 months (IQR 5–6 months). Twelve eyes (28.6%) required two implants, five (11.9%) required three implants and two (4.8%) received four implants | Concomitant systemic corticosteroid treatment: at baseline, 40.3% were receiving systemic prednisone and 53.1% second-line agents. Prednisone was reduced to < 10 mg/day in all patients at 1 month; dose reduction was maintained in 78% at 12 months. Prednisone was discontinued in 31.8% at 12 months |
| Pleyer 201482 prospective case series, single DEX 700 implant for intermediate or posterior uveitis, two centres, Germany |
|
Mean BCVA 0.68 ± 0.47 logMAR (Snellen 20/100) at baseline, improving to 0.53 ± 0.54 logMAR (20/63) by 4 weeks (p = 0.001) and 0.51 ± 0.49 logMAR (20/63) by 12 weeks (p < 0.001). BCVA improvement lost by week 24 (p = 0.999) | Percentage with VH = 0 increased from baseline to 4 weeks (19% vs. 61%; p < 0.001); percentage remained significantly above baseline throughout follow-up. Mean VH remained below baseline level (p < 0.001 at weeks 4, 12 and 24) | Mean CRT improved from 463 ± 165 µm at baseline to 300 ± 110 µm by week 4 (p < 0.001). Improvement remained significant throughout the follow-up period (p < 0.001 at 12 and 24 weeks) | NR | Concomitant systemic immunosuppressants or corticosteroids: 32 patients (38%) on systemic immunosuppressants (± corticosteroids) at baseline. Systemic corticosteroids discontinued in 8 (25%) and reduced (to < 10 mg) in a further 6 (19%) |
| Tomkins-Netzer 201418 retrospective review of treatment and retreatment with DEX 700 for non-infectious uveitis, two centres, UK |
|
Mean BCVA improved significantly after first implantation, from 0.47 (SEM 0.05) logMAR (Snellen 20/60) at baseline to 0.27 (0.07) logMAR (20/37) at 2 months (p < 0.001); deteriorated to 0.43 (0.12) logMAR (20/54) by 6 months | Significant improvement in the percentage of eyes with VH = 0 after first implantation, from 58% at baseline to 83% at 1 month (p = 0.03); remained until month 6 (85%; p = 0.02) but decreased by 12 months (53%) | Mean (SEM) CRT decreased significantly from 453 (34) µm at baseline to 263 (44) µm at 1 month after first implantation (p = 0.003). Macular oedema persisted in 50% of eyes but remaining eyes had a decrease in CRT of 127 (52) µm at 6 months (p = 0.01); improvement was maintained up to 12 months |
|
|
| Tsang 201684 retrospective review of DEX 700 for uveitic macular oedema, Canada |
|
BCVA improved in 20/25 eyes (80%). Significant improvement in mean BCVA at 3 months, from 0.614 ± 0.089 logMAR (Snellen 20/82) at baseline to 0.35 ± 0.10 logMAR (20/45) at month 3. Five of 25 eyes (20%) had worsening of VA during follow-up | NR | CRT improved in 32/35 eyes (91.4%), from 590 ± 28 µm at baseline to 380 ± 28 µm at 1 month and 370 ± 3 µm at 3 months (p < 0.001); maintained throughout follow-up | For 7 eyes with repeat implant: BCVA improvement at 1 month after first implant 0.069 ± 0.179 logMAR; improvement after second implant 0.184 ± 0.171 logMAR (difference not significant). CRT reduced by 268 ± 76 µm at 1 month after first implant and by 291 ± 74 µm at 1 month after repeat implant (difference not significant). Median time to treatment failure (increase in CRT > 10% and ≥ 50 µm or need for repeat implant) was 6 months | NR |
| Zarranz-Ventura 201450 retrospective review of DEX 700 for non-infectious uveitis, multicentre, UK and Spain |
|
Mean VA was 0.68 (SD 0.4) logMAR (Snellen 20/90) at baseline, improving to 0.59 (0.4) logMAR (20/78) after 2 weeks, 0.49 (0.4) logMAR (20/62) at 1 month, 0.49 (0.5) logMAR (20/62) at 3 months, 0.60 (0.5) logMAR (20/80) at 6 months and 0.52 (0.5) logMAR (20/66) at 12 months (all p < 0.01) | VH analysed in only 39 eyes with vitritis at baseline (VH ≥ +0.5). Probability of VH improvement (two-step improvement or change from +0.5 to 0) was 41% at 2 weeks, 63% at 1 month, 73% at 3 months, 79% at 6 months and 88% at 12 months. The median time to improvement in VH was 1 month (95% CI 0.6 to 1.3 months) | CRT analysed in only 59 eyes with CMO. Mean (SD) CRT at baseline was 469 (193) µm, improving to 326 (81) µm at 2 weeks, 267 (74) µm at 1 month, 318 (149) µm at 3 months, 366 (140) µm at 6 months and 355 (160) µm at 12 months (all p < 0.01) | Median time to second implant: 10 months (95% CI 6.3 to 13.6 months) | Concomitant systemic immunosuppressants or corticosteroids: probability of dose reduction (≥ 5 mg of steroids or any reduction in immunosuppressants) was 36% at 1 month, 42% at 3 months, 46% at 6 months and 62% at 12 months. Probability of steroid discontinuation: 8% at 1 and 3 months, 11% at 6 months and 36% at 12 months |
Appendix 6 Safety data from non-randomised studies of the dexamethasone implant
| Study | Design | Number of patients/eyes | Follow-up | Number of implants | Increased IOP | Cataracts | Other AEs |
|---|---|---|---|---|---|---|---|
| Adán 201385 | Retrospective study of DEX 700 after vitrectomy for uveitic macular oedema, Spain | 13 patients, 17 eyes | 12 months | Single: 9 eyes; repeat: 8 eyes |
|
|
|
| Khurana 201586 | Retrospective review of DEX 700 for cystoid macular oedema secondary to non-infectious uveitis, single centre, USA | 13 patients, 18 eyes | 3 months | 1: 8 eyes; 2–4: 10 eyes |
|
|
|
| Lam 201581 | Retrospective chart review of DEX 700 for macular oedema, multicentre, Canada | 101 patients, 120 eyes | 1–6 months | Mean implants: 1.7 |
|
|
|
| Miserocchi 201279 | Retrospective study of DEX 700 for chronic posterior non-infectious uveitis, single centre, Italy | 12 patients, 14 eyes | 11 months | 15 implants in 14 eyes |
|
|
|
| Nobre-Cardoso 201683 | Retrospective review of DEX 700 for non-infectious uveitic macular oedema, single centre, France | 31 patients, 41 eyes | 12 months | 1: 18 patients; 2: 10 patients; 3: 2 patients; 4: 1 patient |
|
|
|
| Palla 201580 | Retrospective review of DEX 700 for non-infectious uveitis, single centre, India | 15 patients, 20 eyes | 12 months | NR |
|
|
|
| Pelegrín 201551 | Retrospective review of DEX 700 for macular oedema secondary to non-infectious uveitis, single centre, Spain | 32 patients, 42 eyes | NR | 1: 23 eyes; 2: 12 eyes; 3: 5 eyes; 4: 2 eyes |
|
|
|
| Pleyer 201482 | Prospective case series of single DEX 700 implants for non-infectious intermediate or posterior uveitis, two centres, Germany | 84 patients, 84 eyes | 6 months | NR |
|
|
|
| Tomkins-Netzer 201418 | Retrospective review of treatment and retreatment with DEX 700 in non-infectious uveitis, two centres, UK | 27 patients, 38 eyes | 24 months | 1: 14 eyes; 2: 14 eyes; 3: 7 eyes; 4: 2 eyes; 6: 1 eye |
|
|
Implant migration in 1 eye that had undergone cataract extraction |
| Tsang 201684 | Retrospective review of DEX 700 for uveitic macular oedema, Canada | 15 patients, 25 eyes | 12 months | Single: 18 eyes; repeat: 7 eyes |
|
|
|
| Zarranz-Ventura 201450 | Retrospective review of DEX 700 for non-infectious uveitis, multicentre, UK and Spain | 63 patients, 82 eyes | 12 months | 1: 43 eyes; 2: 24 eyes; ≥ 3: 15 eyes |
|
|
|
Appendix 7 Breakdown of the cost-effectiveness analysis results for the base case
| Outcome | Sham | DEX | Increment |
|---|---|---|---|
| Life-years | |||
| On treatment | 18.669 | 18.703 | 0.034 |
| Blind | 1.859 | 1.826 | –0.034 |
| Total | 20.529 | 20.529 | 0.000 |
| QALYs | |||
| On treatment | 13.904 | 13.946 | 0.042 |
| Blind | 0.709 | 0.696 | –0.013 |
| Total | 14.613 | 14.641 | 0.029 |
| Costs (£) | |||
| Drugs | 2449.61 | 3324.03 | 874.42 |
| Administration and monitoring | 17,452.41 | 17,597.44 | 145.04 |
| AEs | 5186.39 | 5255.04 | 68.64 |
| Rescue therapy | 285.26 | 35.25 | –250.01 |
| Blindness | 14,281.54 | 14,023.09 | –258.46 |
| Total | 39,655.21 | 40,234.85 | 579.64 |
| ICER (£/QALY) | 20,057.73 | ||
| Outcome | Placebo | ADA | Increment |
|---|---|---|---|
| Life-years | |||
| On treatment | 0.620 | 2.081 | 1.460 |
| Failed treatment | 17.565 | 16.323 | –1.242 |
| Remission | 0.000 | 0.000 | 0.000 |
| Blind | 2.343 | 2.125 | –0.218 |
| Total | 20.529 | 20.529 | 0.000 |
| QALYs | |||
| On treatment | 0.524 | 1.799 | 1.274 |
| Failed treatment | 13.603 | 12.595 | –1.008 |
| Remission | 0.000 | 0.000 | 0.000 |
| Blind | 0.792 | 0.716 | –0.076 |
| Total | 14.919 | 15.110 | 0.191 |
| Costs (£) | |||
| Drugs | 2813.59 | 21,961.73 | 19,148.14 |
| Administration and monitoring | 18,352.07 | 18,811.80 | 459.73 |
| AEs | 8037.18 | 8338.60 | 301.43 |
| Blindness | 17,983.53 | 16,289.21 | –1694.32 |
| Total | 47,186.36 | 65,401.34 | 18,214.98 |
| ICER (£/QALY) | 95,505.74 | ||
| Outcome | Placebo | ADA | Increment |
|---|---|---|---|
| Life-years | |||
| On treatment | 2.937 | 4.223 | 1.286 |
| Failed treatment | 15.137 | 14.104 | –1.034 |
| Remission | 0.000 | 0.000 | 0.000 |
| Blind | 2.454 | 2.202 | –0.252 |
| Total | 20.529 | 20.529 | 0.000 |
| QALYs | |||
| On treatment | 2.458 | 3.519 | 1.061 |
| Failed treatment | 11.957 | 11.100 | –0.856 |
| Remission | 0.000 | 0.000 | 0.000 |
| Blind | 0.830 | 0.742 | –0.088 |
| Total | 15.244 | 15.361 | 0.116 |
| Costs (£) | |||
| Drugs | 4990.76 | 43,855.57 | 38,864.81 |
| Administration and monitoring | 19,944.05 | 20,708.76 | 764.71 |
| AEs | 4345.10 | 4002.68 | –342.42 |
| Blindness | 18,830.87 | 16,894.93 | –1935.94 |
| Total | 48,110.78 | 85,461.94 | 37,351.16 |
| ICER (£/QALY) | 321,405.45 | ||
Glossary
- Active systemic disease
- Systemic disease that is currently requiring symptomatic treatment (in these patients, systemic treatment may be more appropriate to treat both the uveitis and the underlying disease).
- Anterior chamber of the eye
- The fluid-filled space in the front part of the eye located between the iris and the inner surface of the cornea.
- Anterior segment of the eye
- The part of the eye composed of the cornea, iris, lens, ciliary body and front part of the sclera (white part of the eye). In general, it forms the anterior (front) one-third of the eye.
- Bilateral
- Uveitis affecting both eyes. For the purposes of this report, to avoid confusion, this does not relate to treatment for both eyes. In the case of local treatment, it may be for one or both eyes and will be referred to as such.
- Cataract
- A cloudiness of the lens of the eye.
- Corticosteroid-sparing therapy
- A single treatment or treatment regimen that allows the reduction or discontinuation of ongoing corticosteroids.
- Cycloplegic drug
- A drug that causes relaxation of the ciliary muscle of the eye.
- Extended dominance
- When the incremental cost-effectiveness ratio for a given treatment alternative is higher than that of the next more effective (non-dominated) comparator.
- Fluorescein angiography
- An eye test that uses a specialised camera and a fluorescent dye to examine the circulation of the retina and choroid.
- Glaucoma
- An eye condition characterised by damage to the optic nerve caused by intraocular pressure.
- Immunosuppression
- Reducing or lowering the immune response with the use of drugs.
- Indirect ophthalmoscope
- A magnifying instrument with a light source for examining the inside of the eye through the pupil, especially the space between the lens and the retina.
- Intraocular pressure
- Pressure exerted by fluid in the eye. The normal range is between 10 and 20 mmHg and may vary in an individual at different times of the day.
- Legal blindness
- best corrected visual acuity of ≤ 20/200 in the better-seeing eye and/or a visual field of ≤ 20°.
- Local treatment/local pathway
- Treatments that are local to the eye (may be given to one or both eyes; little effect on systemic disease).
- Macula
- The pigmented area or ‘yellow spot’ near the centre of the retina.
- Macular oedema
- Fluid collection in the region of the macula.
- Meta-analysis
- A statistical method by which the results of a number of studies are pooled to give a combined summary statistic.
- Mydriatic drug
- A drug instilled in the eye to dilate the pupil.
- No active systemic disease
- Either no systemic disease related to uveitis or systemic disease that is currently controlled (in these patients, treatment local to the eye may be more appropriate).
- Optic nerve
- A nerve that transmits visual information from the retina to the brain.
- Optical coherence tomography
- A non-invasive technique for cross-sectional imaging of the retina and light-sensitive areas of the eye.
- Posterior segment of the eye
- The part of the eye encompassing the vitreous, choroid, retina and optic nerve. It forms the posterior (back) two-thirds of the eye.
- Relative risk
- The ratio of the probability of an event occurring in an exposed group relative to the probability of an event occurring in a non-exposed or control group.
- Simple dominance
- When an intervention is less effective and more expensive than its comparator.
- Systemic disease
- Known underlying systemic disease related to the uveitis.
- Systemic treatment/systemic pathway
- Treatments that are given systemically (and by their nature treat both eyes and may also treat systemic disease).
- Unilateral
- Uveitis affecting one eye. For the purposes of this report, to avoid confusion, this does not relate to treatment for one eye.
- Visual acuity
- This refers to how well a person sees, that is, clarity of vision.
- Vitreous
- A clear jelly-like fluid that fills the middle of the eye, between the lens and the retina.
List of abbreviations
- AC
- anterior chamber
- ADA
- adalimumab
- AE
- adverse event
- AG
- Assessment Group
- AIC
- Akaike information criterion
- BCVA
- best corrected visual acuity
- BIC
- Bayesian information criterion
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CPCI
- Conference Proceedings Citation Index
- CP(M)
- current practice as provided in the MUST trial
- DEX
- dexamethasone
- DEX 350
- dexamethasone 0.35 mg
- DEX 700
- dexamethasone 0.7 mg
- EMA
- European Medicines Agency
- EQ-5D
- EuroQol-5 Dimensions
- ETDRS
- Early Treatment Diabetic Retinopathy Study
- HADS
- Hospital Anxiety and Depression Scale
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IOP
- intraocular pressure
- ITT
- intention to treat
- LCP(H)
- limited current practice based on the HURON trial
- LCP(VI)
- limited current practice based on the VISUAL I trial
- LCP(VII)
- limited current practice based on the VISUAL II trial
- LOCF
- last observation carried forward
- logMAR
- logarithm of the minimum angle of resolution
- MD
- mean difference
- MeSH
- medical subject heading
- MUST
- Multicenter Uveitis Steroid Treatment
- NEI
- National Eye Institute
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NSAID
- non-steroidal anti-inflammatory drug
- PROM
- patient-reported outcome measure
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SmPC
- Summary of Product Characteristics
- SUN
- Standardization of Uveitis Nomenclature
- TNF
- tumour necrosis factor
- VCM
- Vision Core Measure
- VFQ-25
- 25-item Visual Function Questionnaire
- VH
- vitreous haze
- VKH
- Vogt–Koyanagi–Harada disease
- WHO
- World Health Organization
- WPAI
- Work Productivity and Activity Impairment
- WTP
- willingness to pay