Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/17/01. The contractual start date was in February 2015. The draft report began editorial review in October 2016 and was accepted for publication in April 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Wailoo et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Rheumatoid arthritis (RA) is a common chronic inflammatory disease that typically affects the joints of the body that are lined with synovium, such as those in the hands and feet. It is characterised by progressive, irreversible joint damage, impaired joint function and pain and tenderness caused by swelling of the synovial lining of joints (synovitis), and manifests as increasing disability and reduced quality of life. 1 As RA is a systemic disease, it affects much more than the joints. The primary symptoms are pain, morning stiffness, swelling, tenderness, loss of movement, fatigue and redness of the peripheral joints. 2,3 Fever, sweats and weight loss may be experienced. More significant inflammatory manifestations may lead to serious pathology. 4 RA has long been reported as being associated with increased mortality,5,6 particularly as a result of cardiovascular events,7 which may result from reduced mobility and ongoing inflammation. For example, a 50-year-old woman with RA is expected to die 4 years earlier than a 50-year-old woman without RA. 4
The costs of RA are substantial. Treatment costs associated with drug acquisition and hospitalisation are major components of this. Reduced work productivity is also a significant cost burden in this patient group. 3 The total costs of RA in the UK, including indirect costs and work-related disability, have been estimated at between £3.8B and £4.75B per year. 8
Epidemiology
Symmons et al. 9 estimated a prevalence of 0.8% in a study of the population in Norfolk. This leads to an estimated 400,000 people in England and Wales with RA,10 with approximately 10,000 incident cases per year. 11 The disease is about 2–4 times more common in women (1.16%) than in men (0.44%),11 with the majority of cases being diagnosed when patients are aged between 40 and 80 years,12 with peak incidence among those in their seventies. 11 Thus, a large proportion of people affected by RA are of working age.
Significance for the NHS
The treatment of RA is costly to the NHS and the wider economy. Many of the treatment options for patients are based on drug therapy, some of which (particularly biologic therapies) are extremely costly. The cost of treating patients whose disease is not well controlled from an early stage is also driven substantially by the need for joint surgery and hospitalisation.
Treat to target (TTT) has the potential to allow more effective treatment strategies to be given to patients. Given in early disease, this may result in better disease control. Remission has been shown to correlate strongly with long-term structural damage identifiable on radiographs and with patient function. Disease control may also allow the tapering of drug doses over time and avoid the need to progress to costlier biologic therapies.
Current service provision
A series of drug-based treatments have contributed to the rapid improvement in the care of patients with RA over the last 20 years. Traditionally, patients have been treated with conventional disease-modifying antirheumatic drugs (cDMARDs), which include methotrexate (MTX), sulfasalazine (SSZ), hydroxychloroquine (HCQ), leflunomide (LEF), ciclosporin and gold injections, as well as corticosteroids, analgesics and non-steroidal anti-inflammatory drugs (NSAIDs). More recently, a group of drugs has been developed consisting of monoclonal antibodies and soluble receptors that specifically modify the disease process by blocking key protein messenger molecules (such as cytokines) or cells (such as B lymphocytes). 4 Such drugs have been labelled as biologic disease-modifying antirheumatic drugs (bDMARDs).
Given the number of treatments available, there is a vast number of potential treatment strategies for patients in both early and established disease.
Clinical guidelines
For people with newly diagnosed RA, the National Institute for Health and Care Excellence (NICE) clinical guideline number 794 recommends a combination of cDMARDs [including MTX and at least one other disease-modifying antirheumatic drug (DMARD) plus short-term glucocorticoids (GCs)] as a first-line treatment, ideally beginning within 3 months of the onset of persistent symptoms. Where combination therapies are not appropriate (e.g. where there are comorbidities or pregnancy), DMARD monotherapy is recommended, this was considered a more standard treatment approach prior to the NICE guidelines.
Current National Institute for Health and Care Excellence Technology Appraisal guidance
The NICE guidance [Technology Appraisal (TA) number 37513] recommends the use of adalimumab (ADA), etanercept (ETN), infliximab (IFX), certolizumab pegol, golimumab, tocilizumab (TOC) and abatacept, in combination with MTX, in people with RA only if:
-
disease is severe, that is, Disease Activity Score, 28 joints (DAS28) is > 5.1
-
disease has not responded to intensive therapy with a combination cDMARDs
-
the manufacturers provide certolizumab pegol, golimumab, abatacept and TOC as agreed in their patient access schemes.
Adalimumab, ETN, certolizumab pegol or TOC can be used as monotherapy for people who cannot take MTX, because it is contraindicated or because of intolerance, when the above criteria are met.
At least a moderate European League Against Rheumatism (EULAR) response must be achieved at 6 months for treatment to continue.
The NICE has also issued guidance on the treatment of RA after the failure of a tumour necrosis factor inhibitor (TNFi) (TA195,14 TA22515 and TA24716).
Description of technology under assessment
Dramatic improvements in the treatment of RA have been seen in the last 20 years (see Smolen et al. 17), particularly through the development of more targeted bDMARDs and non-bDMARDs. A more recent development that has consolidated these improvements is the concept of TTT. Rather than referring to a single, precise technology or treatment strategy, TTT in rheumatology can be better described as a treatment ‘paradigm’17 encompassing a range of broad features. The American College of Rheumatology (ACR)/EULAR have issued a series of recommendations on TTT based on analysis of a series of trials that test varying treatment strategies. There is therefore no single set of interventions constituting a TTT treatment approach.
Treat to target has a strong history in the treatment of other chronic diseases, such as diabetes mellitus and hypertension. Long-term outcome data supporting the TTT approach in these conditions have motivated the drive for successful TTT approaches in RA.
The central component of the TTT concept is the setting of a treatment target. Recommendations typically specify low disease activity (LDA) or remission as appropriate targets. This was the case in EULAR’s 2010 recommendations (see Smolen et al. 18) and EULAR’s 2016 updated recommendations (see Smolen et al. 17), both of which recommended clinical remission as the primary treatment goal. The ACR/EULAR’s 2011 definition of remission was originally designed specifically to be a predictor of good patient outcomes in terms of a later lack of radiography-detected joint damage and good, stable function and quality of life for patients,19 more so than other disease activity states (including LDA). 17 These goals have become realistic targets with the availability of newer drugs.
The ACR/EULAR definition of remission is:
-
Boolean-based definition: at any time point, a patient must satisfy all of the following: a tender joint count (TJC) of ≤ 1, a swollen joint count (SJC) of ≤ 1, a C-reactive protein (CRP) concentration of ≤ 1 mg/dl and a patient global assessment of ≤ 1 (on a scale of 0–10) or
-
index-based definition: at any time point, a patient must have Simple Disease Activity Index (SDAI) of ≤ 3.3.
Low disease activity as an acceptable, alternative therapeutic goal, particularly in long-standing disease, is a recommendation intended to imply that there are situations where remission may not be a feasible outcome.
However, TTT, as a concept, is also often described as comprising more than just a treatment goal. Different commentators refer to different elements of the TTT strategy that aim to take action to reach and maintain the treatment goal. Common to most is the concept of regular treatment adaptation in response to the assessment of a patient in comparison to the target. Sometimes treatment adaptation is described in broad terms;17,18 in other instances reference is made to ‘aggressive treatment’. 20 Terminology also varies, with some referring to ‘tight control’. 21
Solomon et al. 20 also refers to TTT as a ‘proactive’ treatment approach. In many studies of TTT this is evident through more frequent assessment of patients than would normally be the case in standard practice. Assessments are often made as frequently as monthly under TTT principles. The ACR/EULAR’s recommendations state that assessments should be made ‘as frequently as monthly’ and drug therapy should be altered at least every 3 months, until the target is reached. Less frequent (6-monthly) monitoring of patients is required once the target is reached.
Treat to target can therefore be composed of a range of different features that leads to a continuum of ‘weak’ to ‘strong’ TTT principles. We define the entirety of those features as the ‘treatment strategy’. The inclusion of a treatment target is a necessary condition for a strategy to be considered a TTT strategy. Additional components of the strategy include the frequency with which patients are assessed against the target and the provision of a treatment protocol that specifies how treatments are to be changed in response to assessments. Thus, the weakest TTT strategy would be one where a treatment target is specified, but no further instruction is provided on which treatment protocols should be used to reach that goal.
Treat-to-target studies have often focused on the optimal strategy for patients with newly diagnosed RA. This links to the concept of there being a ‘window of opportunity’ that has emerged in the RA literature, which posits that the earlier DMARD therapy is introduced, the greater the impact on long-term health outcomes. 21
Additional costs associated with TTT depend on the nature of the overall treatment strategy. Of course, the setting of a target itself incurs no additional resources, but the increased frequency of visits to a rheumatologist and the potential for a more intense use of drug treatments can make TTT a more resource-intensive option for the initial treatment period.
A simplified schematic of operationalising a TTT strategy has been provided in Figure 1.
FIGURE 1.
A simplified conceptual model of TTT.

Chapter 2 Definition of the decision problem
We aim to systematically review evidence on the use of TTT strategies for the treatment of RA compared with standard care with non-TTT strategies.
Decision problem
Interventions
The intervention of interest is a TTT approach to care. At a minimum, for a treatment strategy to be considered TTT in this review it must contain the explicit setting of a target, assessment of that target and use of that information by the treating clinician. TTT strategies may also include additional elements; for example, in the frequency of assessment or in providing a treatment protocol that provides instruction to the treating clinician on treatment changes in the light of assessments. Our review will seek to distinguish these different types of TTT.
Population including subgroups
Adult patients aged ≥ 18 years with a diagnosis of RA were eligible for the study. We will consider two groups of patients separately: (1) those with early disease (≤ 3 years since diagnosis) and (2) those with established disease (> 3 years since diagnosis). We used definitions of early and established RA as defined in the trials. When no definition was provided, a cut-off point of 3 years was used, which is generally consistent across the literature (e.g. Scott et al. 22), bearing in mind that no fixed consensus exists. Examining definitions and mean disease durations across trials, a 3-year cut-off point seems appropriate.
Relevant comparators
The comparator is treatment of patients without a TTT strategy. This includes sequential non-bDMARD therapy in early disease, clinician preference and any treatment protocol that lacks an explicit treatment target.
Outcomes
A number of outcomes were assessed. Primarily, we examined the proportion of patients achieving target, remission and LDA. We also examined changes in DAS28/ Disease Activity Score, 44 joints (DAS44), SJC, TJC, Health Assessment Questionnaire (HAQ), joint erosion and quality of life, as well as EULAR and ACR response.
Overall aims and objectives of assessment
The aim of this review is to identify and evaluate the evidence for the clinical effectiveness and cost-effectiveness of TTT strategies compared with routine care for adult patients with RA.
Patient and public involvement
Two members of the public who have RA were asked to peer review the following sections of the report: the abstract; plain English summary; scientific summary; discussion; and conclusions. The full report was provided to supply further information and the patient and public involvement representatives could comment on other sections too. The report was then amended based on the comments received to improve the clarity and readability of the text.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing clinical effectiveness
The search for evidence of clinical effectiveness was undertaken systematically following the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (URL: www.prisma-statement.org/; accessed 30 October 2017). Search strategies are described in Appendix 1.
Identification of studies
There were two search phases conducted for this review.
Phase I scoping searches were carried out to identify the extent of potentially relevant literature, the range and types of TTT strategies that have been subject to clinical study, the types of clinical study and the relevance of these strategies to UK NHS practice.
Four databases and one clinical trials registry were searched (Table 1). Terms for RA (see Appendix 2, statements 1 and 2) were combined with TTT terms (see Appendix 2, statements 4–18). Terms for TTT were obtained and adapted from the review by Schoels et al. 23
| Source, date searched from | Phase (date of search) | |
|---|---|---|
| I (May 2015) | II (January 2016 and August 2016) | |
| MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (via Ovid), from 1948 | ✓ | ✓ |
| EMBASE (via Ovid), from 1980 | ✓ | ✓ |
| CDSR (via Wiley Online Library), from 1996 | ✓ | ✗ |
| CENTRAL (via Wiley Online Library), from 1898 | ✓ | ✓ |
| NHS EED (via Wiley Online Library), from 1995 to 2015 | ✓ | ✓ |
| HTA database (via Wiley Online Library), from 1995 | ✓ | ✗ |
| DARE (via Wiley Online Library), from 1995 | ✓ | ✗ |
| WoS Citation Index Expanded (Thomson Reuters), from 1900 | ✓ | ✓ |
| WoS Citation Index and Conference Proceedings Index (Thomson Reuters), from 1990 | ✓ | ✓ |
| BIOSIS Previews (Thomson Reuters), from 1969 | ✗ | ✓ |
| CINAHL (via EBSCOhost), from 1982 | ✗ | ✓ |
| EconLit (via Ovid), from 1886 | ✗ | ✓ |
| EULAR (via Web of Science, Thomson Reuters) | ✓ | ✗ |
| ACR (via Web of Science, Thomson Reuters)) | ✓ | ✗ |
| ClinicalTrials.gov (US National Institutes of Health) (www.clinicaltrials.gov) | ✓ | ✓ |
| ICTRP (www.who.int/ictrp/en/) | ✗ | ✓ |
| NICE Evidence (www.nice.org.uk/) | ✗ | ✓ |
The search was combined with specific search filters for randomised controlled trials (RCTs) (see Appendix 2, statements 21 and 22), systematic reviews (see Appendix 2, statements 26–28) and economic evaluations (see Appendix 2, statements 32–34). The RCT and systematic reviews search was limited by date from 2008 until May 2015, whereas for recent cost-effectiveness studies, the last 2 years were searched (2013–15).
The Phase II search was informed by the literature identified from the Phase I searches to refine and carry out a full systematic search of the evidence.
Additional free-text terms for TTT were added to the Phase I search strategies to increase the sensitivity of the search (see Appendix 2). Only RCT and economic evaluations were searched by combining the strategies with sensitive search filters. Three further databases, one trials registry and a search engine were searched (see Table 1). No date or language limits were applied in the search. Records retrieved from the search were combined and duplicate titles were removed from the Phase I search.
The number of records retrieved from the various sources in Phase I and II searches can be found in Table 2.
| Source | Phase | |||||
|---|---|---|---|---|---|---|
| I | II | |||||
| RCTs (specific and 2008–) | SRs (specific and 2008–) | Cost-effectiveness (2013–) | RCTs | RCT update (August 2016) | Cost-effectiveness | |
| MEDLINE | 475 | 168 | 73 | 3778 | 294 | 417 |
| EMBASE | 1315 | 302 | 290 | 7633 | 423 | 923 |
| CDSR | – | 81 | – | – | – | – |
| DARE | – | 79 | – | – | – | – |
| CENTRAL | 1588 | – | – | 2243 | 45 | 0 |
| HTA database | – | 7 | – | – | – | – |
| NHS EED | – | – | 9 | 0 | 0 | 45 |
| WoS Citation Index Expanded and WoS Citation Index and Conference Proceedings Index | 2179 | 641 | 199 | 5435 | 245 | 444 |
| BIOSIS | – | – | – | 1753 | 32 | 0 |
| CINAHL | – | – | – | 789 | 66 | 642 |
| EconLit | – | – | – | 0 | – | 6 |
| EULAR | 650 | – | – | – | – | – |
| ACR | 792 | – | – | – | – | – |
| ClinicalTrials.gov | 122 | – | – | 26 | 6 | 0 |
| ICTRP | – | – | – | 0 | 0 | 0 |
| NICE Evidence | – | – | – | 0 | – | 2 |
| Total retrieved | 7121 | 1278 | 571 | 21,657 | 1115 | 2479 |
| Unique | 4937 | 946 | 436 | 10,093a | 635b | 913a |
Inclusion and exclusion criteria
Population
The population was adults with clinically diagnosed RA, with or without prior cDMARDs or bDMARDs, commencing or currently undergoing treatment anywhere on the RA treatment pathway.
Intervention
The intervention is the use of TTT strategies to guide treatment decisions for individual patients, as defined in Methods for reviewing clinical effectiveness. Sufficient description of the intervention needed to be reported for a study to be included in the review.
Comparators
The following comparators were permitted: (1) usual care, in which TTT strategies were not used, (2) a different TTT strategy, in which an alternative target was used and (3) a different TTT strategy, in which an alternative treatment protocol was used.
Outcomes
A number of outcomes were assessed. Primarily, we examined the proportion of patients achieving target, remission and LDA. We also examined score changes in the DAS28/DAS44, SJC, TJC and HAQ, joint erosion and quality of life, as well as EULAR and ACR response. According to the TTT ACR/EULAR international task force, TTT strategies should use composite measures that include joint counts to assess disease activity as related to the target. 17
Study design
Randomised controlled trials were included. The reference lists of any systematic reviews identified through the searches were checked for potentially relevant trials.
Exclusion criteria
-
Animal models.
-
Pre-clinical and biological studies.
-
Narrative reviews, editorials, opinions.
-
Non-English-language papers.
-
Reports published as meeting abstracts only, where insufficient methodological details are reported to allow critical appraisal of study quality.
-
Trials of personalised medicine.
-
Non-randomised clinical trials and other study types, such as cohort studies.
-
Trials designed to test an active drug versus placebo (PBO) where both arms pursue the same target and treatment strategy.
Study selection
Titles and abstracts were examined by one reviewer and 5% were checked by another reviewer. Study selection based on full texts was decided by two reviewers, with discrepancies resolved by discussion.
Data extraction strategy
Data relevant to the decision problem were extracted from all studies by one reviewer using a standardised data extraction form. Data on the study characteristics (population, type of comparison, study type, method of RA diagnosis, sample size, treatment arms relevant to the review, duration of RCT phase, duration of follow-up, primary outcome, geographical location and funding source); population characteristics (mean age, number and percentage female, number and percentage rheumatoid factor positive, mean disease duration, mean baseline DAS28, mean baseline SJC, mean baseline TJC, mean baseline pain score, mean baseline HAQ score); TTT characteristics (target, treatment protocol, frequency of assessment); and key outcomes (number and percentage completing randomised phase, reasons for withdrawal, number and percentage meeting study target, number and percentage attaining LDA, number and percentage attaining remission, treatment adaptations, total dose of each drug given over the trial period, mean DAS28, mean DAS44, mean SJC, mean TJC, EULAR response, ACR 20/50/70 response, mean HAQ, joint erosion, quality of life), including adverse events (AEs) [proportion of patients experiencing any AE, any serious adverse event (SAE), death, withdrawal as a result of an AE, musculoskeletal AEs, endocrine and metabolic AEs, cardiovascular AEs, dermatological AEs, ophthalmological AEs, gastrointestinal AEs, infectious AEs, psychological AEs and other AEs] were extracted.
All data extracted were checked thoroughly by a second reviewer, who checked the first reviewer’s extraction against the article(s). Data were extracted without blinding to authors or journal. Discrepancies were discussed and an agreement was reached. We planned that a third reviewer would be consulted when no consensus could be reached; however, this was not necessary in any instance.
Quality assessment strategy
The methodological quality of each included study was assessed by one reviewer, and checked by a second reviewer, who checked the first reviewer’s quality assessment against the article(s). Discrepancies were discussed and an agreement was reached. We planned that a third reviewer would be consulted when no consensus could be reached; however, this was not necessary in any instance.
The methodological quality of RCTs, identified from the literature search for inclusion, was assessed using the Cochrane Collaboration risk-of-bias assessment criteria. This tool addresses specific domains, namely sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; and selective outcome reporting. 24 Judgements for domains are made as low, high, or unclear risk of bias. For cluster RCTs we also included three additional domains for recruitment bias (were participants recruited prior to clusters being randomised), risk of baseline differences between clusters and attrition of clusters. We classified RCTs as being at overall ‘low risk’ of bias if they were rated as ‘low’ for each of three key domains – allocation concealment, blinding of outcome assessment and completeness of outcome data (> 10% attrition25). RCTs judged as being at ‘high risk’ of bias for any of these domains were judged as overall ‘high risk’. RCTs not judged as being at ‘high risk’ for any of these domains, or ‘low risk’ for all of these domains, were judged as overall ‘unclear risk’.
Methods of analysis/synthesis
Evidence examining the clinical effectiveness of TTT was reported according to the TTT comparison, namely (1) TTT compared with usual care, (2) a comparison of different targets against each other and (3) a comparison of different treatment protocols against each other. Two trials did not fit into this framework and so were examined separately, under ‘other comparisons’. Within these comparisons, trials were further grouped according to whether they used early RA populations or established RA populations, as patients with early and established RA may respond to treatment differently. 17,18,22 We used definitions of early and established RA as defined in the trials, and, when no definition was provided, a cut-off point of 3 years was used, which is generally consistent across the literature (e.g. Scott et al. 22), bearing in mind that no fixed consensus exists. Examining definitions and mean disease durations across trials, a 3-year cut-off point seems appropriate. Two trials used populations with both early and established RA patients, so these trials were reported separately from those with early RA and those with established RA populations. Statistical meta-analysis was planned where the evidence allowed. Because of the heterogeneity in treatment protocols, the data from trials in comparison 3 were not narratively combined and examined by outcome, as with the previous two comparisons, and results were reported separately by trial.
Results
Quantity and quality of research available
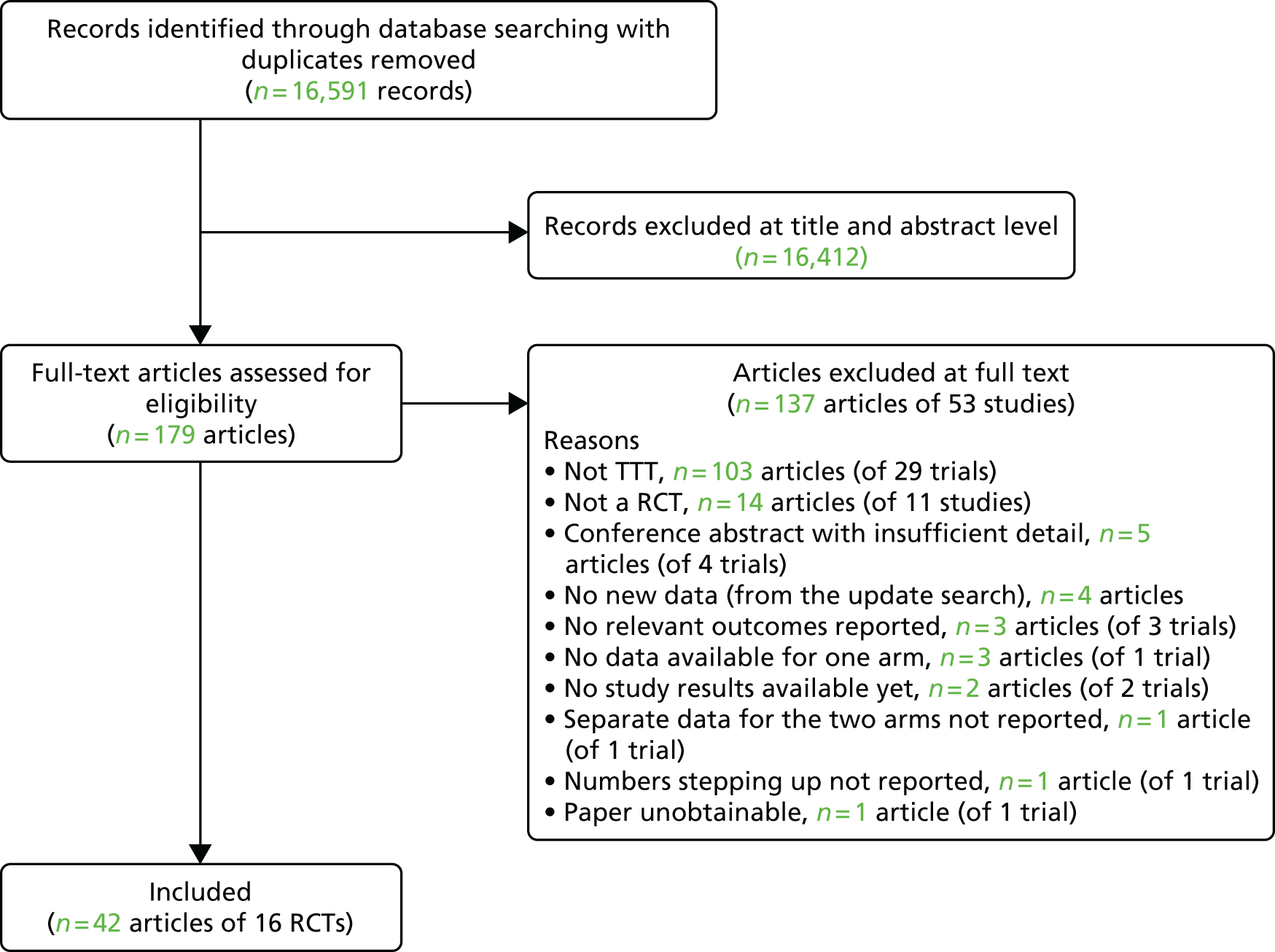
A total of 16,591 records were identified from electronic databases for the clinical effectiveness systematic review, following deletion of duplicates. The study selection process is represented as a PRISMA flow diagram (Figure 2). A total of 16,412 records were excluded at title and abstract level and 137 articles (reporting 53 separate studies) were excluded in the full-paper sift (see Appendix 3 for a comprehensive list with rationale for exclusion). A total of 42 articles describing 16 trials were included in the review (Table 3).
FIGURE 2.
Flow diagram of study inclusion.
| Trial acronym or first author and year of publication | RA population | Type of comparison | Study type | Trial start date | RA diagnosis | Sample size (randomised) | Treatment arms for which data extraction performed | Duration of RCT phase | Duration of follow-up | Primary outcome | Geographical location | Funding source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BeSt26–34 | Early RA | Comparison of different treatment protocols | RCT | April 2000 | ACR 1987 | 508 |
|
12 months | 10 years | Functional ability (D-HAQ), and radiographic joint damage (modified SHS) | The Netherlands | Dutch College of Health Insurances, Schering-Plough B.V. (Houten, the Netherlands) and Centocor Inc. (Horsham, PA, USA) |
| BROSG trial9 | Established RA | Other comparisons | RCT | 1997 | ACR 1987 | 466 |
|
3 years | 3 years | HAQ score | UK | HTA |
| CAMERA35–37 | Early RA | Other comparisons | RCT | 1999 | ACR 1987 | 299 |
|
2 years | 2 years | Sustained remission (no swollen joints and any two of number of painful joints ≤ 3, ESR of ≤ 20 mm/hour, VAS general well-being ≤ 20 mm for ≥ 3 months) | The Netherlands | NR |
| CareRA trial38–43 | Early RA | Comparison of different treatment protocols | RCT | January 2009 | ACR 1987 | 289 high-risk patients; 90 low-risk patients | High-risk patients:
|
16 weeks | 16 week | Proportion of patients in remission (DAS28-CRP of < 2.6) at week 16 | Flemish countries | Flemish governmental grant |
Low-risk patients:
|
||||||||||||
| COBRA-light44,45 | Early RA | Comparison of different treatment protocols | RCT | March 2008 | ACR 1987 | 164 |
|
12 months | 24 months | Change in DAS44 after 26 weeks of treatment compared with baseline (ΔDAS44) | The Netherlands | The Dutch Top Institute Pharma (TIPharma, Leiden, the Netherlands) and Pfizer (New York City, NY, USA) |
| FIN-RACo46–49 | Early RA | Comparison of different treatment protocols | RCT | April 1993 | ACR 1987 | 199 |
|
2 years | 11 years | Remission – modified version of ACR 1981 definition (no swollen or tender joints) | Finland | Finnish Society for Rheumatology, Rheumatism Research Foundation in Finland, Medical Research Foundation of Turku University Central Hospital and the Finnish Office for Health Care Technology Assessment, Finland |
| Fransen et al., 200550 | Established RA | TTT vs. usual care | Cluster RCT | March 2000 | ACR (date NR) | 384 (142 in subsample reporting DAS) (24 clusters) |
|
24 weeks | 24 weeks | Proportion reaching LDA (DAS28 of ≤ 3.2) at week 24 in a subgroup of patients; DMARD treatment changes during 24 weeks, in all patients | The Netherlands | Pfizer |
| Hodkinson et al., 201551 | Early RA | Comparison of different targets | RCT | April 2011 | ACR 2010 | 102 |
|
12 months | 12 months | Proportion of patients achieving at least LDA by DAS28 at 12 months | South Africa | Carnegie Corporation (New York, NY, USA) and the Connective Tissue Fund of the University of Witwatersrand, Johannesburg, South Africa |
| Optimisation of Adalimumab study52,53 | Established RA | TTT vs. usual care; comparison of different targets | Cluster RCT | August 2006 | NR | 308 (31 clusters) |
|
18 months | 18 months | Change in DAS28 between baseline and 12 months of treatment | Canada | Abbott Canada (Abbott Laboratories, Québec, QC, Canada) |
| Saunders et al., 200854 | Early RA | Comparison of different treatment protocols | RCT | February 2003 | NR | 96 |
|
12 months | 12 months | Mean decrease in the DAS28 at 12 months | UK | Chief Scientist Office of the Scottish Executive (Edinburgh, UK) |
| STREAM55 | Early RA | TTT vs. usual care | RCT | July 2004 | 2–5 swollen joints and SHS of < 5 | 82 |
|
2 years | 2 years | Progression of radiographic joint damage at 2 years | The Netherlands | Abbott (Hoofddorp, the Netherlands) |
| T-4 study56,57 | Early RA | TTT vs. usual care; comparison of different targets | RCT | August 2008 | ACR 1987 | 243 |
|
56 weeks | 56 weeks | Proportion of patients in clinical remission (DAS28 of < 2.6) | Japan | NR |
| TEAR58–60 | Early RA | Comparison of different targets; comparison of different treatment protocols | RCT | May 2004 | ACR 1987 | 755 |
|
102 weeks | 102 weeks | Change in DAS28-ESR between week 48 and week 102 | USA | Amgen, NIH [study drugs provided by Amgen (Thousand Oaks, CA, USA), Barr Pharmaceuticals (Montvale, NJ, USA) and Pharmacia (Kalamazoo, MIUSA)] |
| TICORA61 | Both | TTT vs. usual care | RCT | August 1999 | Criteria NR (all patients had a DAS44 of > 2.4) | 111 |
|
18 months | 18 months | Mean fall in DAS, and the proportion of patients with a EULAR good response | UK | Chief Scientist’s Office, Scottish Executive (Edinburgh, UK) |
| U-Act-Early62 | Early RA | Comparison of different treatment protocols | RCT | January 2010 | ACR 1987/2010 or EULAR | 317 |
|
2 years | 2 years | Number of patients achieving sustained remission | The Netherlands | Hoffmann-La Roche/Roche Nederland BV |
| van Hulst et al., 201063 | Both | TTT vs. usual care | Cluster RCT | June 2001 | NR | 248 (7 clusters) |
|
18 months | 18 months | Change in DAS28 and the number of medication changes over 18 months | The Netherlands | CVZ/VAZ doelmatigheidsprojecten ‘Doelmatigheid Academische Ziekenhuizen’ (efficiency projects in academic hospitals) |
Table 3 displays the characteristics of the studies included in this review. Eleven trials26–36,38–49,51,54–60,62,64–67 examined an early RA population (defined as disease duration of < 3 years; see Chapter 4, Inclusion and exclusion criteria) [i.e. BehandelStrategieën in Reumatoïde Artritis (BeSt),26–34,64–66 the Computer-Assisted Management in Early Rheumatoid Arthritis (CAMERA) studies,35,36,67 Care in early Rheumatoid Arthritis (CareRA) trial,38–43 the COmBination theRApy with rheumatoid arthritis (COBRA)-light trial,44,45 FINnish Rheumatoid Arthritis Combination therapy (FIN-RACo) trial,46–49 Hodkinson et al. ,51 Saunders et al. ,54 the STRategies in Early Arthritis Management (STREAM) trial,55 the TreaTing to Twin Targets (T-4) study,56,57 the Treatment of Early Aggressive Rheumatoid arthritis (TEAR) trial58–60 and the early rheumatoid arthritis treated with tocilizumab, methotrexate or their combination (U-Act-Early) trial62], three trials9,50,52,53 examined an established RA population [i.e. British Rheumatoid Outcome Study Group (BROSG) trial,9 Fransen et al. 50 and the Optimisation of Adalimumab study52,53] and two trials61,63 examined populations that include both patients with early RA and those with established RA [i.e. the TIght COntrol for RA (TICORA) trial61 examined patients with a disease duration of < 5 years and van Hulst et al. 63 reported including both newly diagnosed patients and those with established disease (and did not report on the findings for these patients separately at all)]. Six studies50,52,53,55–57,61,63 compared one or more TTT approaches with usual care (i.e. Fransen et al. ,50 the Optimisation of Adalimumab study,52,53 the STREAM trial,55 the T-4 study,56,57 the TICORA trial61 and van Hulst et al. 63), four studies51–53,56–60 compared different targets against each other (Hodkinson et al. ,51 the Optimisation of Adalimumab study,52,53 the T-4 study56,57 and the TEAR trial58–60) and six studies26–34,38–42,44–49,54,58–60 compared different treatment protocols against each other (BeSt,26–34 the CareRA trial,38–42 the COBRA-light trial,44,45 the FIN-RACo trial,46–49 Saunders et al. 54 and the TEAR trial58–60). Two studies9,35,36,67 made other comparisons which did not seem to fit with any of the above comparisons (i.e. the BROSG trial9 and the CAMERA trial35,36,67). The BROSG trial9 had two arms: intensive management, which used a DAS44 of ≤ 2.4 target and employed a seven-step treatment protocol based on the target, where patients were seen in a hospital setting; and a routine management arm, which had no target and patients were seen in the community as well as in the clinic, with treatment escalation based on clinical opinion. The CAMERA35,36,67 trials employed a conventional strategy group and an intensive strategy group, which used (different) compound improvement-based targets, different frequencies of assessment and the same treatment protocol.
Most studies specified a RA diagnosis based on ACR 1987 or 2010 criteria; however, the STREAM trial55 required patients to have 2–5 swollen joints (and did not require an ACR diagnosis), the TICORA trial61 required a DAS44 of > 2.4 and RA diagnosis was not reported in the Optimisation of Adalimumab study,52,53 Saunders et al. 54 or van Hulst et al. 63 Sample sizes ranged from 82 to 755 participants and study durations (randomised phase) ranged from 24 weeks to 3 years. Six trials took place in the Netherlands,26–37,44,45,50,55,62,63 three in the UK,9,54,61 one in Flemish countries (actual countries were not stated),38–43 one in Finland,46–49 one in South Africa,51 one in Canada,52,53 one in Japan56,57 and one in the USA. 58–60
Risk-of-bias assessment
Risk-of-bias judgements for included non-cluster randomised controlled trials
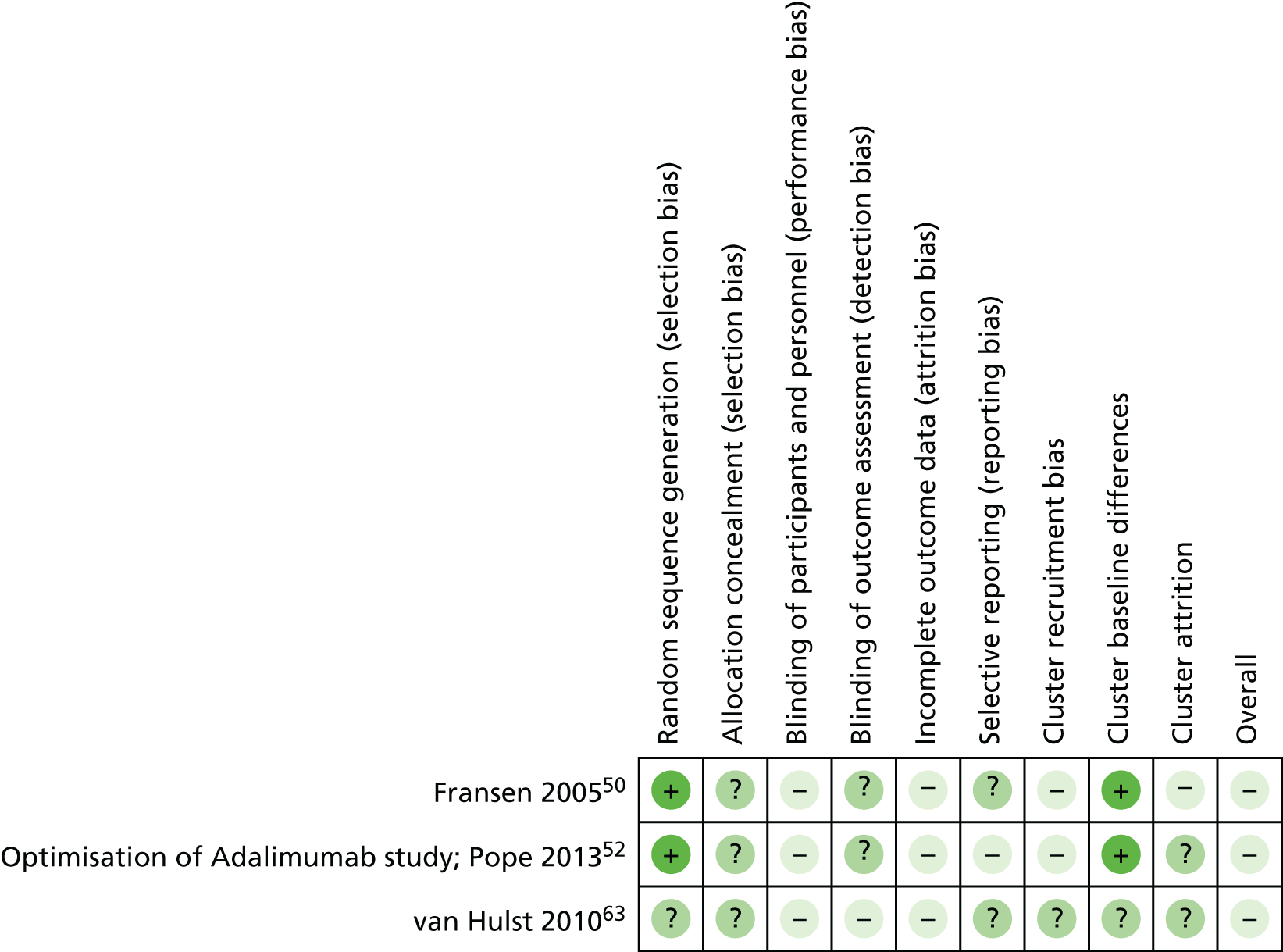
A summary of the risk-of-bias judgements for the included non-cluster RCTs is presented in Figure 3, with details presented in Table 4.
FIGURE 3.
Risk-of-bias judgements for included non-cluster RCTs. +, low risk of bias; –, high risk of bias; ?, unclear risk of bias.

| Trial acronym; first author and year of publication | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Overall rating and reason |
|---|---|---|---|---|---|---|---|
| BeSt; Goekoop-Ruiterman et al., 200530 | Unclear: method of random sequence generation not reported | Unclear risk: closed envelopes used but not reported if opaque and sequentially numbered | Unclear risk: blinding of participants and personnel not reported | Low risk: assessors were blinded | Low risk: < 10% withdrew from each group | Low risk: all outcomes in online protocol (reported in van der Kooij 200968) reported | Unclear: allocation method not reported |
| BROSG trial; Symmons et al., 20059 | Low risk: using a minimisation computer program | Unclear: concealment of allocation not reported | High risk: not blinded | Low risk: reported as observer blinded | High risk: > 10% withdrew from both arms | Unclear risk: protocol details ‘Not provided at time of registration’ from the ISRCTN | Unclear: allocation method not reported |
| CAMERA; Verstappen et al., 200736 | Unclear: method of random sequence allocation not reported | Unclear: reports allocation performed by an independent person, but method not reported | High: patients and clinicians not blinded (not possible, two different monitoring/treatment strategies) | Unclear: radiographs scored independently by blinded assessors, unclear on other outcomes | High: 31% withdrew | Unclear: no protocol available | High risk: attrition bias |
| CareRA; Verschueren et al., 201542 | Unclear: method of random sequence generation not reported | Unclear: concealment of allocation not reported | High risk: reports that no blinding was implemented | High risk: reports that no blinding was implemented | Low risk: < 10% withdrew from each group | Low risk: all outcomes in online protocol reported | High risk: outcome assessment not blinded |
| COBRA-light; den Uyl et al., 201444 | Low risk: online randomisation software was used | Unclear risk: sequentially numbered envelopes were used, but not reported if sealed and opaque | High risk: reported as open label | Low risk: reports that to minimise influence performed by trained research nurses uninvolved in the routine care | Low risk: < 10% withdrew from each group | High risk: radiological progression reported as an outcome in the protocol, but not reported in publication. Publication states that this outcome will only be reported at 12 months | Unclear: allocation method not reported |
| Fin-RACo; Mottonen et al., 199946 | Unclear: method of sequence generation not reported | Unclear risk: centrally organised, numbered envelopes, but not reported if sequentially numbered and opaque | High risk: open label | High risk: clinical measures not blinded | Low risk: < 10% lost from each group | Unclear: no protocol available | High risk: outcome assessment not blinded |
| Hodkinson et al., 201551 | Low: computer-generated sequence | Unclear: concealment of allocation not reported | Unclear: blinding of patients and personnel not reported | High: outcome assessment not blinded | Low risk: < 10% lost from each group | Unclear: no protocol available | High risk: outcome assessment not blinded |
| Saunders et al., 200854 | Low risk: randomisation software used | Unclear: concealment of allocation not reported | High risk: single-blind study (only outcome assessment blind) | Low risk: metrologist was blinded to treatment allocation; also radiologists assessing Sharp score were blinded | Low risk: < 10% attrition both groups | Low risk: outcomes in online protocol reported | Unclear: allocation method not reported |
| STREAM; van Eijk et al., 201255 | Unclear: method of random sequence allocation not reported | Unclear: concealment of allocation not reported | High risk: described as single blind | Low: DAS and radiographs assessed by blinded assessors | Low risk: < 10% lost from each group | High risk: QoL in protocol but not in paper; everything else as per protocol | Unclear: allocation method not reported |
| T-4 study; Urata et al., 201257 | Unclear risk: sequence generation not reported | Unclear: concealment of allocation not reported | High risk: reported limitation that study was ‘open’ | High risk: primary outcome DAS assessed by physicians not radiologists | High risk: > 10% withdrew from groups 2 and 4; reasons not stated | Unclear risk: trial registration not reported, unable to locate protocol | High risk: attrition bias |
| TEAR; Moreland et al., 201258 | Low risk: treatment was allocated via a computerised data entry system | Low risk: treatment was allocated via a computerised data entry system | Low risk: all subjects and site personnel (including the treating rheumatologists) were blinded (for the duration of the trial) to treatment assignment and change to active medication at the step-up period | Low risk: all subjects and site personnel (including the treating rheumatologists) were blinded (for the duration of the trial) to treatment assignment and change to active medication at the step-up period | High risk: > 10% withdrew from all groups | High risk: methods state SF-12 used to assess HRQoL, but no results presented | High risk: attrition bias |
| TICORA; Grigor et al., 200461 | Low risk: randomisation software used | Unclear risk: treating doctor telephoned an administrative co-ordinator, but not reported if the allocation was concealed | High risk: only the assessors were blind ‘single blind’ | Low risk: a metrologist assessed patients from both groups, unaware of participants’ assigned treatment groups | Low risk: < 10% lost from each group | Unclear: no protocol available | Low risk: allocation, assessor blinding and attrition all low risk |
None of the included non-cluster RCTs was rated as being at high risk of bias for random sequence generation. Only one RCT was rated as being at low risk of bias for allocation concealment. 58 The remaining RCTs were rated as having an unclear risk for this domain. 9,30,36,41,44,46,51,54,55,57,61 Nine RCTs were rated as having a high risk of bias for blinding of participants and personnel,9,36,41,44,46,54,55,57,61 and one RCT was rated as having an unclear risk for this domain. 30 Only one RCT was rated as having a low risk for this domain. 58 It should, however, be borne in mind that in most cases it would be difficult for patients to be blinded, given the differences in treatment approach between trial arms in most trials. Seven RCTs were rated as having a low risk of bias for blinded outcome assessment,9,30,44,54,55,58,61 four RCTs were rated as having a high risk of bias41,46,51,57 and one RCT36 was rated as having an unclear risk for this domain. Four RCTs reported withdrawals of > 10% and were rated as having a high risk of attrition bias. 9,36,57,58 The remaining RCTs were rated as having a low risk of bias for this domain. 9,36,57,58 Trial protocols were unavailable for six RCTs that were rated as having an unclear risk of selective reporting. 9,36,46,51,57,61 Three RCTs were rated as having a low risk of bias for this domain30,41,54 and three were rated as having a high risk of bias, as some outcomes were not reported in either the protocol44,55 or the methods section of the trial report58. Based on the judgements for allocation concealment, blinded outcome assessment and attrition domains (see Quality assessment strategy), six RCTs were rated as having an overall high risk of bias36,41,46,51,57,58 and five RCTs were rated as having an overall unclear risk of bias. 9,30,44,54,55 Only one RCT was rated as having an overall low risk of bias. 61
Risk-of-bias judgements for included cluster randomised controlled trials
A summary of the risk-of-bias judgements for the included cluster RCTs is presented in Figure 4, with the details presented in Table 5.
FIGURE 4.
Risk-of-bias judgements for included cluster RCTs. +, low risk of bias; –, high risk of bias; ?, unclear risk of bias.
| Trial acronym; first author and year of publication | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Cluster recruitment bias | Cluster risk of baseline differences | Cluster attrition | Overall rating and reason |
|---|---|---|---|---|---|---|---|---|---|---|
| Fransen et al., 200550 | Low risk: random number generator used to allocate the centres randomly to DAS (12 centres) or UC (12 centres) | Unclear risk: not reported if clusters were randomised at the same time | High risk: single blind | Unclear risk: reports as the participating physicians could not be blinded, it was necessary to measure the DAS28 independently. However, unclear if the independent assessors were blind to treatment allocation | High risk: > 10% withdrew from both groups | Unclear risk: study protocol not available (Dr Jaap Fransen, Radboud University Medical Center, 2016, personal communication) | High risk: centre allocation remained concealed until the start of patient recruitment | Low risk: randomisation took place in two strata: one stratum comprised the participating centres in the predetermined region; the other of all other participating centres. The data were analysed using linear regression with random coefficients (mixed models), correcting for clustering of the data in centres | High risk: only three DAS and four UC centres provided DAS assessments | High risk of recruitment bias, performance bias and participant attrition bias |
| Optimisation of Adalimumab study; Pope et al., 201352 | Low risk: using a computer-generated, site-stratified blocked schedule that assigned physicians from the same geographic region to one of the three groups | Unclear risk: not reported if clusters were randomised at the same time | High risk: only patients blinded | Unclear: not reported | High risk: > 10% withdrew from all groups | High risk: AEs classified according to MedDRA as in the NCT protocol not reported | High risk: physician randomisation took place prior to initiation of enrolment | Low risk: reports clusters were randomised using a site-stratified blocked schedule and models were used with shared frailty to account for the clustered design considering baseline covariates | Unclear risk: loss of clusters not reported | High risk of recruitment bias, performance bias, participant attrition bias and selective reporting |
| van Hulst et al., 201063 | Unclear: method of random sequence allocation not reported | Unclear risk: not reported if clusters were randomised at the same time | High risk: clinicians not blinded | High risk: DAS28 assessment not blinded, HAQ blinding unclear, medication changes blinded | High: attrition was 4% in intervention group, but 12% in usual-care group | Unclear: no protocol available | Unclear risk: not reported if rheumatologists recruited patients before or after randomisation | Unclear: baseline comparability of clusters not reported | Unclear risk: loss of clusters not reported | High as a result of selection bias, differential attrition, unclear blinding and unclear randomisation |
Two of the included cluster RCTs were rated as being at low risk of bias for random sequence generation50,52 and one63 was rated as being at unclear risk for this domain. All three cluster RCTs were rated as being at unclear risk of bias for allocation concealment. 50,52,63 All three cluster RCTs were rated as being at high risk of bias for blinding of participants and personnel. 50,52,63 One cluster RCT was rated as being at high risk of bias for blinded outcome assessment,63 whereas the other two cluster RCTs were rated as being at unclear risk for this domain. 50,52 All three cluster RCTs were rated as being at high risk of attrition bias (> 10% participants withdrawing). 50,52,63 A trial protocol was not available for two cluster RCTs that were rated as being at unclear risk of bias for selective reporting. 50,63 One cluster RCT was rated as being at high risk of bias for this domain, as AEs that were reported as an outcome in the trial protocol were not included in the trial report. 52 Two RCTs reported that participant recruitment occurred after clusters were randomised to treatment allocation, thus these trials were rated as being at high risk of bias for cluster recruitment bias. 50,52 The remaining cluster RCT was rated as being at unclear risk of bias for this domain. 63 Two cluster RCTs reported statistical methods to accommodate for baseline differences across clusters and rated as being at low risk of bias for cluster baseline differences. 50,52 The remaining cluster RCT was rated as being at unclear risk for this domain. 63 One cluster RCT reported that DAS28 outcomes were available for only a subset of the original clusters that were randomised. This RCT was rated as being at high risk of bias for cluster attrition. 50 The other two cluster RCTs were rated as being at unclear risk for this domain. 52,63 All three included cluster RCTs rated as being at an overall high risk of bias. 50,52,63
Assessment of characteristics of individual study arms
We considered the features of each arm of the included studies, with a view to drawing out common features across them. If strategies in different study arms are considered sufficiently similar, that would allow a grouping of TTT ‘types’ and subsequent analysis at this group level, drawing on evidence from more than one study.
Tables 6–13 display information about each of the study types. We report information on (1) the treatment target, (2) the treatment protocol and (3) the frequency of clinician contacts with the patient. For point 3, the focus is on the frequency of visits during the period while the patient has not achieved the target, as less frequent assessment is often required once target is achieved. These are considered to be the key distinguishing features of ‘TTT’ strategies as a whole.
In addition, study arms were grouped together using a broad categorisation of the treatment protocol. This approach was taken by the NICE clinical guideline for RA,4 where the focus was purely on treatment protocols using conventional DMARDs in early disease. We used the following eight classification categories.
Sequential monotherapy without a treatment target
Study arms in this classification would typically be control arms in studies compared with other TTT strategies. The arms identified within this classification category are included in Table 6.
| Trial acronym or first author and year of publication | Description of target | Protocol | Frequency of assessments |
|---|---|---|---|
| BeSt26–34 | Treatment adjustments were made every 3 months in an effort to obtain a DAS44 of ≤ 2.4. Remission was defined as a DAS44 of < 1.6 |
|
3 months |
| Optimisation of Adalimumab study52,53 | A DAS28 of < 2.6 in the DAS-targeted arm and a SJC of 0 in the SJC-targeted arm |
|
0, 6, 12 and 18 months. Assessments at 2, 4 and 9 months were also recommended |
| Symmons et al., 20059 | To control joint pain, stiffness and related symptoms | NSAIDs, intra-articular steroid injections (up to a maximum of one per month), DMARDs (antimalarials, SSZ, intramuscular gold, penicillamine, azathioprine, MTX, LEF) and low-dose steroids (≤ 7.5 mg daily). Non-drug modalities, such as physiotherapy referral, were also used as the GP felt appropriate. DMARD therapy was monitored using the standard guidelines in current use in the five centres | Every 4 months |
| Fransen et al., 200550 | A DAS28 of ≤ 3.2 | Systematic monitoring of disease activity by assessment of the DAS28 by the treating rheumatologist. According to the study guidelines the aim was to reach a DAS28 of ≤ 3.2 (LDA) by changing DMARD treatment if the score was > 3.2 | Systematic monitoring of disease activity was carried out at weeks 0, 4, 12 and 24 |
| van Hulst et al., 201063 | A DAS28 of ≤ 3.2 | None | 3 months |
Sequential disease-modifying antirheumatic drug monotherapy, or no treatment protocol, with a treatment target
This refers to study arms that could be seen as comprising only a limited component of ‘TTT’, the weakest form of TTT strategy feasible under our definition of TTT. The arms identified within this classification category are included in Table 7.
| Trial acronym or first author and year of publication | Target | Protocol | Frequency of assessments |
|---|---|---|---|
| TICORA61 | A DAS44 of ≤ 2.4 | DMARD monotherapy was given in patients with active synovitis. Failure of treatment (because of toxic effects or lack of effect) resulted in a change to alternative monotherapy, or addition of a second or third drug at the discretion of the attending rheumatologist. Intra-articular injections of corticosteroid were given to patients assigned routine care with the same restrictions as those in the intensive group | 3 months |
| T-4 study56,57 | A DAS28 of < 2.6 (DAS-targeted arm);a MMP-3 concentration of < 121 ng/ml (men)/< 59.7 ng/ml (women) (MMP-3-targeted arm); and a DAS28 of < 2.6 and a MMP-3 concentration of < 121 ng/ml (men)/ < 59.7 ng/ml (women) (combined DAS- and MMP-3-targeted arm) |
|
20, 24, 28, 32, 36, 40, 44, 48, 52 and 56 weeks |
| CAMERA35,36,67 |
|
3 months | |
| STREAM55 | The following order of drugs was suggested to the treating rheumatologist: HCQ, SSZ, MTX and LEF | 3 months | |
| Fransen et al., 200550 | No guideline to adapt treatment strategy was supplied | ||
| van Hulst et al., 201063 | 3 months | ||
| FIN-RACo46–49 |
|
Baseline and at months 1, 3, 4, 5, 6, 9, 12, 18 and 24 |
Steroid step-down, disease-modifying antirheumatic drug step-up combination
Study arms classified in this group involved initial combination therapy with steroids and non-bDMARDs (either alone or in combination), with the option of increasing the dose or adding in DMARDs. The steroid dose is tapered downwards over time. The arms identified within this classification category are included in Table 8.
| Trial acronym or first author and year of publication | Description of target | Protocol | Frequency of assessments |
|---|---|---|---|
| den Uyl et al., 201369 | A DAS44 of < 1.6 | 60 mg/day prednisolone (tapered to 7.5 mg/day in 6 weeks), 7.5 mg/week of MTX and 1 g/day of SSZ (increased to 2 g/day after 1 week). MTX increased to 25 mg/week after 13 weeks of treatment if the DAS44 was ≥ 1.6 | 3 months |
| A DAS44 of < 1.6 |
|
3 months | |
| CareRA42 | A DAS28-CRP of < 3.2 | 15 mg of MTX weekly, 2 g of SSZ daily and a weekly step-down scheme of oral GCs (60 mg to 40 mg to 25 mg to 20 mg to 15 mg to 10 mg to 7.5 mg PDN) | Baseline, 4, 8 and 16 weeks. If a treatment adjustment was required at week 8, an optional visit was held at week 12 |
| A DAS28-CRP of < 3.2 | 15 mg of MTX weekly with a weekly step-down scheme of oral GCs (30 mg to 20 mg to 12.5 mg to 10 mg to 7.5 mg to 5 mg PDN) | ||
| A DAS28-CRP of < 3.2 | 15 mg of MTX weekly, 10 mg of LEF daily and a weekly step-down scheme of oral GCs (30 mg to 20 mg to 12.5 mg to 10 mg to 7.5 mg to 5 mg PDN) |
Step-up disease-modifying antirheumatic drugs to biologics
In this classification the treatment protocols start patients on non-bDMARDs and outline increases in dose and/or combinations with other DMARDs, with the option of switching to bDMARDs as part of the sequence. The arms identified within this classification category are included in Table 9.
| Trial acronym | Description of target | Protocol | Frequency of assessments |
|---|---|---|---|
| T-4 study57,57 | A DAS28 of < 2.6 |
1. 1 g/day of SSZ plus oral 4 mg/week of MTX – the dosage of was not changed if the patient had responded compared with the previous visit otherwise |
Variable, but approximately monthly |
| A MMP-3 concentration of < 121 ng/ml for men and < 59.7 ng/ml for women |
2. The dosage of oral MTX was increased in a stepwise manner to a maximum of 8 mg/week if patient had not responded. Change of therapy based on improvement in the number of tender joints (0–28), swollen joints (0–26) and concentration of CRP from pre-assessment values, without access to current DAS28 and MMP-3 values |
||
| A DAS28 of < 2.6 and a MMP-3 concentration of < 121 ng/ml for men and < 59.7 ng/ml for women |
3. If the maximum tolerable dose of MTX that introduced a dose-dependent side effect was reached and the patient still did not fulfil a sustained response, TNF blockers were allowed. If patients on the administration of TNF blockers did not show improvement compared with the previous measurement, TNF blockers were changed to another biological agent, or the TNF blocker dose increased, or the interval for TNF administration was shortened. Maximum dosages were 1 g/day of SSZ, 20 mg/day of LEF, 300 mg/day of bucillamine,a 25 mg/week of gold sodium thiomalate, 3 mg/day of tacrolimus hydrate, 10 mg/kg bimonthly or 6 mg/kg monthly of IFX, 50 mg/week of ETN, 40 mg of ADA fortnightly and 8 mg/kg of TOC monthly. DMARDs including bucillamine,a gold sodium thiomalate, tacrolimus and LEF were given, as allowed by the rheumatologist at all times. Combination therapy with DMARDs other than MTX was allowed for two kinds of agents. Intra-articular GC (to a maximum of 10 mg of triamcinolone acetonide on a single visit) was permitted for persistently swollen and tender joints |
||
| STREAM55 | Remission (a DAS44 of < 1.6) | Treatment was started with 15 mg/week of oral MTX. If the DAS was ≥ 1.6 at a given time point:
|
|
| TEAR58–60 | A DAS28-ESR of ≥ 3.2 | Oral MTX, escalated to a dosage of 20 mg/week or to a lower dosage if treatment resulted in no active tender/painful or swollen joints by week 12. Corticosteroid use at entry tapered. If the DAS28-ESR was ≥ 3.2 at week 14, patients were stepped up to MTX plus SSZ at a dosage of 500 mg twice daily, escalated (if tolerated) to 1000 mg twice daily at 6 weeks, plus HCQ at a dosage of 200 mg twice daily |
Step-up combinations not including to biologics
Similar to the previous set of treatment protocols, study arms in this category start patients on a non-bDMARD and subsequently allow increases in dose and/or combinations with other non-bDMARDs and steroids. The arms identified within this classification category are included in Table 10.
| Trial acronym or first author and year of publication | Description of target | Protocol | Frequency of assessments |
|---|---|---|---|
| BeSt26–34 | Treatment adjustments were made every 3 months in an effort to obtain a DAS44 of ≤ 2.4. Remission was defined as a DAS44 of < 1.6 |
|
3 months |
| TICORA61 | A DAS44 of ≤ 2.4 | Escalation of DMARDs in patients with persisting disease activity:
|
Monthly |
| FIN-RACo46–49 | ACR remission | Combination therapy started with:
|
Baseline and at months 1, 3, 4, 5, 6, 9, 12, 18 and 24 |
| Saunders et al., 200854 | A DAS28 of < 3.2 |
|
Monthly |
| CAMERA35,36,67 | 20%, 50% responses based on ESR, SJC, TJC and VAS of overall well-being |
|
|
| Symmons et al., 20059 | The goal was to control joint pain, stiffness and related symptoms and to suppress clinical and laboratory evidence of inflammation. CRP concentrations below twice the upper limit of normal | NSAIDs, intra-articular steroid injections (up to a maximum of one per month), DMARDs (antimalarials, SSZ, intramuscular gold, penicillamine, azathioprine, MTX, LEF) and low-dose steroids (≤ 7.5 mg daily) plus, if necessary, ciclosporin, parenteral steroids, medium-dose oral steroids (up to 10 mg daily) and cyclophosphamide | Every 4 months |
| TEAR58–60 | A DAS28-ESR of ≥ 3.2 | Oral MTX, escalated to a dosage of 20 mg/week or to a lower dosage if treatment resulted in no active tender/painful or swollen joints by week 12. Corticosteroid use at entry tapered. If the DAS28-ESR was ≥ 3.2, patients were stepped up to triple therapy (MTX + SSZ + HCQ) | Every 6 weeks for 48 weeks, then every 12 weeks |
| Hodkinson et al., 201551 | A SDAI of ≤ 11 (LDA) | 15 mg/week of MTX, increased to 20 mg and then 25 mg | Monthly (3 months) then every 3 months |
| A CDAI of ≤ 10 (LDA) | If this failed, triple therapy of 1000 mg/day of SSZ and 200 mg/day of chloroquine in combination with 25 mg/week of MTX was introduced |
Combination disease-modifying antirheumatic drugs plus steroids
Patients are treated from the outset with two non-bDMARDs and steroids. Adjustments to treatments do not allow bDMARDs. The arms identified within this classification category are included in Table 11.
| Trial acronym | Protocol | Frequency of assessments | |
|---|---|---|---|
| BeSt26–34 | Treatment adjustments were made every 3 months in an effort to obtain a DAS44 of ≤ 2.4. Remission was defined as a DAS44 of < 1.6 |
|
3 months |
Disease-modifying antirheumatic drug and biologic combinations
Treatments for patients from the beginning of the protocol include both bDMARDs and non-bDMARDs in combination. The arms identified within this classification category are included in Table 12.
| Trial acronym | Description of target | Protocol | Frequency of assessments |
|---|---|---|---|
| BeSt26–34 | Treatment adjustments were made every 3 months in an effort to obtain a DAS44 of ≤ 2.4. Remission was defined as a DAS44 of < 1.6 |
|
3 months |
| Optimisation of Adalimumab study52,53 | A DAS28 of < 2.6 in the DAS-targeted arm and a SJC of 0 in the SJC-targeted arm |
|
0, 6, 12 and 18 months. Assessments at 2, 4 and 9 months were also recommended |
| A SJC of 0 |
|
Triple disease-modifying antirheumatic drug therapy
Three non-bDMARDs are used in combination from the outset. The arms identified within this classification category are included in Table 13.
| First author and year of publication | Description of target | Protocol | Frequency of assessments |
|---|---|---|---|
| Saunders et al., 200854 | A DAS28 of < 3.2 (to correspond with a EULAR good response) |
|
Monthly |
Even with this relatively large number of TTT treatment strategy classifications (which are designed to differ in terms of the treatment protocol, except for the control arms of studies), there is substantial variation. Tables 6–13 show that, within each classification group, study arms exhibit significant differences. These can be seen within each grouping in terms of the treatment target and the frequency of assessment. For example, within the group of study arms classified as ‘step-up non-biologic combination therapy’, the frequency of assessments was monthly in some studies (e.g. the TICORA trial,61,63 Saunders et al. 54) and every 3 months in others (e.g. BeSt26–34). Within the same classification group, treatment targets varied enormously. A DAS44 of ≤ 2.4 was used in two studies (BeSt26–34,65,68 and TICORA61,63). A range of different measurement scales [ACR, SDAI, Clinical Disease Activity Index (CDAI), DAS28 and other multifaceted measures] and cut-off points (remission, other measures of response) were used in the other study arms.
Differences are also apparent in the details of the treatment protocols within each broad grouping. Study arm treatment protocols exhibit substantial variation in the study drugs used, the combinations in which they may be used, the doses used (both initially and when changing dose in response to assessment of treatment response), and the ordering of study drugs in the sequence of the protocol.
This variation would be present even if an alternative classification approach were to be adopted. For these reasons, it is not considered desirable to conduct analyses that synthesise results quantitatively across different sets of studies. The extent of variation between study arms would make interpretation of results difficult and potentially result in misleading conclusions.
Assessment of effectiveness
Population characteristics and treat-to-target characteristics
Trials examining early rheumatoid arthritis populations
Eleven trials reported on early RA populations (BeSt,26–34,64–66 CAMERA,35,36,67 CareRA,38–43 COBRA-light,44,45 FIN-RACo,47–49,70 Hodkinson et al. ,51 Saunders et al. ,54 STREAM,55 T-4 study,56,57 TEAR58–60 and U-Act-Early62). Population characteristics are presented in Table 14. The mean age of trial participants ranged from 46 to 62 years and trial arm samples ranged from 58% to 84% female and from 23.4% to 91.7% rheumatoid factor positive. Mean disease duration at baseline ranged from just < 2 weeks to just > 1 year. The mean DAS28 at baseline ranged from 4.4 to 6.9, indicating moderate to severe RA at baseline among the early RA population. The mean SJC (on 66 joints) ranged from 10.0 to 14.0 and the mean TJC (0–68 joints) ranged from 12.3 to 20.0, across all trial arms. The mean HAQ score at baseline ranged from 0.92 to 2.0.
| Trial acronym or first author and year of publication | Treatment arm | Number of participants | Characteristic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age, years (SD) | Female, n/N (%) | Rheumatoid factor positive, n/N (%) | Mean disease duration (months) (SD) | Mean DAS28 at baseline (SD) (ESR or CRP where stated) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | Mean pain score (100-mm VAS) (SD) | Mean HAQ score (SD) | |||
| BeSt26–34,64–66 | Sequential monotherapy | 126 | 54 (13) | 86/126 (68) | 84/126 (67) | 2 (1–5) weeksa,b | DAS44, 4.5 (0.9) | NR | NR | NR | D-HAQ, 1.4 (0.7) |
| Symptom duration 23 (14–54) weeksa | |||||||||||
| Step-up combination therapy | 121 | 54 (13) | 86/121 (71) | 77/121 (64) | 2 (1–4) weeksa,b | DAS44, 4.5 (0.8) | NR | NR | NR | D-HAQ, 1.4 (0.6) | |
| Symptom duration 26 (14–56) weeksa | |||||||||||
| Initial combination therapy with PDN | 133 | 55 (14) | 86/133 (65) | 86/133 (65) | 2 (1–4) weeksa,b | DAS44, 4.4 (0.9) | NR | NR | NR | D-HAQ, 1.4 (0.7) | |
| Symptom duration 23 (15–53) weeksa | |||||||||||
| Initial combination therapy with IFX | 128 | 54 (14) | 85/128 (66) | 82/128 (64) | 3 (1–5) weeksa,b | DAS44, 4.3 (0.9) | NR | NR | NR | D-HAQ, 1.4 (0.7) | |
| Symptom duration 23 (13–46) weeksa | |||||||||||
| CAMERA35,36,67 | Intensive strategy group | 151 | 54 (14) | 104/151 (69) | 89/151 (66) | NR | 5.6 (1.2), n = 102 | 14 (6) | 15 (7) | 51 (26) | 1.2 (0.7) |
| Conventional strategy group | 148 | 53 (15) | 97/148 (66) | 77/148 (62) | NR | 5.6 (1.0), n = 103 | 14 (6) | 14 (7) | 47 (25) | 1.2 (0.7) | |
| CareRA: high-risk patients40,42,43 | COBRA Classic | 98 | 53.2 (11.9) | 64/98 (65) | 78/98 (80) | 1.8 (3.1) weeks | DAS28 (ESR), 5.4 (1.3); DAS28 (CRP), 5.0 (1.2) | 11.9 (8.9); DAS28 joints 7.9 (6.0) | 14.7 (9.5); DAS28 joints 9.5 (6.0) | 59.5 (23.6) | 1.2 (0.7) |
| Symptom duration 33.8 (35.5) weeks | |||||||||||
| COBRA Slim | 98 | 51.8 (131) | 63/98 (64) | 82/98 (84) | 2.6 (3.3) weeks | DAS28 (ESR), 5.2 (1.2); DAS28 (CRP), 4.9 (1.1) | 10.8 (6.5); DAS28 joints 7.1 (4.6) | 13.7 (8.2); DAS28 joints 8.5 (5.5) | 56.5 (21.9) | 0.98 (0.69) | |
| Symptom duration 33.2 (38.2) weeks | |||||||||||
| COBRA Avant-Garde | 93c | 51.2 (12.8) | 64/93 (69) | 70/93 (75) | 3.1 (6.4) weeks | DAS28 (ESR), 5.0 (1.3); DAS28 (CRP), 4.7 (1.2) | 10.6 (6.7); DAS28 jointsc,e 7.0 (5.1) | 14.1 (9.0); DAS28 jointsc,e 8.2 (5.5) | 57.5 (23.8) | 1.0 (0.6) | |
| Symptom durationd 44.3 (65.9) weeks | |||||||||||
| CareRA: low-risk patients40,43 | MTX-TSU | 47 | 51.0 (14.0) | 38/47 (81) | 11/47 (23) | 3.17 (6.62) weeks | DAS28 (ESR), 4.83 (1.68); DAS28 (CRP), 4.55 (1.63) | 10.00 (6.98); SJC28g 6.89 (6.11) | 14.06 (8.61); TJC28g 9.49 (7.46) | Pain (range 0–100) 52.09 (23.23); Nocturnal pain (yes)g 34/47 (72.3%) | 0.99 (0.67) |
| Symptom durationf 33.11 (62.21) weeks | |||||||||||
| COBRA Slim | 43 | 51.4 (14.4) | 33/43 (77) | 11/43 (26) | 1.86 (2.70) weeks; | DAS28 (ESR), 4.88 (1.64); DAS28 (CRP), 4.50 (1.63) | 10.93 (7.55); SJC28g 7.79 (6.0) | 13.14 (10.70); TJC28g 8.51 (7.80) | Pain (range: 0 to 100) 48.23 (31.19) nocturnal pain (yes)g 21/43 (48.8%) | 0.92 (0.85) | |
| Symptom durationg 34.42 (68.16) weeks | |||||||||||
| COBRA-light44,45 | COBRA | 81 | 53 (13) | 54/81 (67) | 47/81 (58) | 16 (9–28) weeksa | 5.67 (1.13) | 13 (10–17)a | 17 (12–24)a | NR | 1.36 (0.66) |
| COBRA-light | 83 | 51 (13) | 58/81 (70) | 48/83 (58) | Median 17 (8–33) weeksa | 5.45 (1.29) | 11 (9–14)a | 16 (10–23)a | NR | 1.37 (0.71) | |
| FIN-RACo47–49,70 | Combination treatment | 99 (97 in ITT) | 47 (23–65)h | 56 (58) | 68 (70) | 7.3 (2–22)d | NR | 13 (6) | 18 (8) | NR | NR |
| Single-drug treatment | 100 (98 in ITT) | 48 (20–65)h | 65 (66) | 65 (66) | 8.6 (2–23)d | NR | 14 (7) | 20 (10) | NR | NR | |
| Hodkinson et al., 201551 | SDAI arm | 42 | 50.1 (13.4)c | 34/42 (81) | 36/42 (86) | Symptom duration 2.6 (3.1) years | 6.1 (1.2) | 11 (5.7) | 12.5 (8.5) | 66.7 (22.6) | HAQ-DI, 1.7 (0.8) |
| CDAI arm | 60 | 46.7 (13.0)c | 50/60 (83) | 54/60 (90) | Symptom duration 3.3 (4.2) years | 6.3 (1.2) | 10.0 (5.7) | 12.3 (7.3) | 59.2 (26.0) | HAQ-DI, 1.7 (0.7) | |
| Saunders et al., 200854 | Parallel triple therapy | 49 | 55 (15) | 37/49 (76) | 34/49 (69) | 10 (9) | 6.8 (0.9) | 12 (4) | 18 (6) | 71 (26) | 1.9 (0.7) |
| Step-up therapy | 47 | 55 (11) | 37/47 (79) | 34/47 (72) | 13 (12) | 6.9 (0.9) | 13 (5) | 18 (6) | 65 (22) | 2.0 (0.7) | |
| STREAM55 | Aggressive group | 42 | 48 (13) | 24/42 (58) | 20/42 (48) | 6 (3–10)a | DAS44, 2.2 (0.5) | NR | NR | NR | 0.50 (0.25–0.88)a |
| Conventional care | 40 | 46 (12) | 32/40 (79) | 13/40 (33) | 6 (4–9)a | DAS44, 2.4 (0.7) | NR | NR | NR | 0.69 (0.32–1.06)a | |
| T-4 study56,57 | Routine care | 62 | 62 (12); n = 55 | 42/55 (76) | NR | 1.5 (1.1) years; n = 55 | 4.4 (1.1); n = 55 | NR | NR | NR | mHAQ score, 0.3 (0.4); n = 55 |
| DAS28-driven therapy | 60 | 60 (11); n = 56 | 42/56 (77) | NR | 1.3 (1.1) years; n = 56 | 4.6 (1.3); n = 56 | NR | NR | NR | mHAQ score, 0.4 (0.6); n = 56 | |
| MMP-3-driven therapy | 60 | 62 (13); n = 53 | 44/53 (83) | NR | 1.3 (1.2) years; n = 53 | 4.8 (1.3); n = 53 | NR | NR | NR | mHAQ score, 0.4 (0.8); n = 53 | |
| DAS28 and MMP-3-driven therapy | 61 | 56 (13); n = 58 | 49/58 (84) | NR | 1.3 (1.2) years; n = 58 | 4.6 (1.3); n = 58 | NR | NR | NR | mHAQ score, 0.3 (0.4); n = 58 | |
| TEAR58–60 | Immediate ETN | 244 | 50.7 (13.4) | 181/244 (74) | 216/244 (89) | 3.5 (6.4) | DAS28-(ESR), 5.8 (1.1) | 12.7 (5.8) | 14.3 (6.6) | mHAQ pain score 5.3 (2.6); n = 243 | mHAQ score, 1.1 (0.4); n = 227 |
| Immediate triple therapy | 132 | 48.8 (12.7) | 101/132 (77) | 121/132 (92) | 4.1 (7.2) | DAS28-(ESR), 5.8 (1.1) | 12.1 (5.8) | 14.1 (6.8) | mHAQ pain score 5.3 (2.5); n = 131 | mHAQ score, 1.0 (0.4); n = 125 | |
| Step-up ETN | 255 | 48.6 (13.0) | 176/255 (69) | 232/255 (91) | 2.9 (5.6) | DAS28-(ESR), 5.8 (1.1) | 13.1 (6.2) | 14.2 (6.9) | mHAQ pain score 5.2 (2.4) | mHAQ score, 1.0 (0.4); n = 237 | |
| Step-up triple therapy | 124 | 49.3 (12.0) | 87/124 (70) | 108/124 (87) | 4.5 (7.6) | DAS28-(ESR), 5.9 (1.1) | 13.1 (6.1) | 14.6 (7.0) | mHAQ pain score 5.1 (2.5) | mHAQ score, 1.0 (0.4); n = 117 | |
| U-Act-Early62 | TOC + MTX | 106 | 53.0 (46.0–60.0)a | 65/106 (61) | RF, 75 (71); CCP, 72 (68); both, 79 (75) | Symptom duration: 24.5 (16.0–41.5)a days | 5.2 (1.1) | 9 (6–15)a (44 joints) | 10 (7–17)a (44 joints) | NR | 1.1 (0.67) |
| TOC + PBO–MTX | 103 | 55.0 (47.0–63.0)a | 78/103 (76) | RF, 68 (66); CCP, 67 (65); both, 77 (75) | Symptom duration: 25.5 (18.0–45.0)a days | 5.3 (1.1) | 11 (7–16)a (44 joints) | 11 (7–18)a (44 joints) | NR | 1.3 (0.66) | |
| MTX + PBO–TOC | 108 | 53.5 (44.5–62.0)a | 69/108 (64) | RF, 86 (80); CCP, 84 (78); both, 93 (86) | Symptom duration: 27.0 (15.0–46.0)a days | 5.1 (1.2) | 9 (5–15)a (44 joints) | 10 (5.5–17)a (44 joints) | NR | 1.1 (0.59) | |
Characteristics of the TTT features of the early RA trials are presented in Table 15. Targets included LDA [a DAS44 of ≤ 2.4 (BeSt26–34,64–66); a Disease Activity Score, 28 joints with C-reactive protein concentration (DAS28-CRP) of ≤ 3.2 (CareRA38–43); a SDAI of ≤ 11 (Hodkinson et al. 51); a CDAI of ≤ 10 (Hodkinson et al. 51); a DAS28 of < 3.2 (Saunders et al. 54); a Disease Activity Score, 28 joints with erythrocyte sedimentation rate (DAS28-ESR) of < 3.2 (TEAR58–60)], remission [a DAS44 of < 1.6 (COBRA-light44,45 and STREAM55); modified ACR 1981 definition (FIN-RACo46–49); a DAS28 of < 2.6 (T-4 study56,57 and Optimisation of Adalimumab study52,53); a DAS28 of < 2.6 and a SJC (28 joints) of ≤ 4 (U-Act-Early62)] response [defined by thresholds of SJC, TJC, erythrocyte sedimentation rate (ESR) and general well-being visual analogue scale (VAS) (CAMERA35,36,67)]: and matrix metalloproteinase 3 (MMP-3) concentration (< 121 ng/ml for men or < 59.7 ng/ml for women; T-4 study56,57). Treatment protocols for attaining the target varied considerably across studies in terms of the number of steps in the treatment protocol and the treatments used at each step, the only similar ones being in the COBRA Classic arms of the CareRA40,42,43 and COBRA-light44,45 trials (which were similar, but not exactly alike). Assessments were made every 3 months or less in all trials with an early RA population.
| Trial acronym or first author and year of publication | Type of comparison | Treatment arm | Number of participants | Target | Treatment protocol | Frequency of assessment |
|---|---|---|---|---|---|---|
| BeSt26–34,64–66 | Comparison of different treatment protocols | Sequential monotherapy | 126 | A DAS44 of ≤ 2.4 |
|
Every 3 months |
| Step-up combination therapy | 121 |
|
||||
| Initial combination therapy with PDN | 133 |
|
||||
| Initial combination therapy with IFX | 128 |
|
||||
| CAMERA35,36,67 | Other comparisons | Conventional strategy group | 151 | Response (> 20% improvement in SJC, > 20% improvement in any two of ESR, TJC and VAS general well-being), avoiding inadequate response (≤ 50% improvement from baseline for SJC and ≤ 50% improvement from baseline for two of ESR, TJC and VAS general well-being) |
|
Every 3 months |
| Intensive strategy group | 148 | Response (decrease in SJC, if SJC unchanged, assessors’ judgement looking at TJC, ESR and VAS general well-being), avoiding inadequate response (SJC ≥ 6, number of painful joints ≥ 3, ESR ≥ 28 mm/hour and a morning stiffness of ≥ 45 minutes) | Every 4 weeks | |||
| CareRA: high-risk patients40,42,43 | Comparison of different treatment protocols | COBRA Classic | 98 | DAS28-CRP ≤ 3.2 | 15 mg of MTX weekly, 2 g of SSZ daily and a weekly step-down scheme of oral GCs (60 mg to 40 mg to 25 mg to 20 mg to 15 mg to 10 mg to 7.5 mg of PDN), then:
|
Screening: baseline, weeks 4, 8 and 16. If a treatment adjustment was required at week 8, an optional visit was held at week 12 |
| COBRA Slim | 98 | 15 mg of MTX weekly with a weekly step-down scheme of oral GCs (30 mg to 20 mg to 12.5 mg to 10 mg to 7.5 mg to 5 mg of PDN), then:
|
||||
| COBRA Avant-Garde | 93a | 15 mg of MTX weekly, 10 mg of LEF daily and a weekly step-down scheme of oral GCs (30 mg to 20 mg to 12.5 mg to 10 mg to 7.5 mg to 5 mg of PDN), then:
|
||||
| CareRA: low-risk patients40,43 | Comparison of different treatment protocols | MTX-TSU | 47 | A DAS28-CRP of ≤ 3.2 | 15 mg of MTX weekly, no oral steroids allowed, then:
|
Screening: baseline, weeks 4, 8 and 16. If a treatment adjustment was required at week 8, an optional visit was held at week 12 |
| COBRA Slim | 43 | 15 mg of MTX weekly with a step-down scheme of daily oral GCs (30-20-12.5-10-7.5-5 mg of PDN). From week 28, GCs were tapered on a weekly basis by leaving out one daily dose each week over a period of 6 weeks until complete discontinuation, then:
|
||||
| COBRA-light44,45 | Comparison of different treatment protocols | COBRA | 81 | A DAS44 of < 1.6 |
|
Every 3 months |
| COBRA-light | 83 |
|
||||
| FIN-RACo47–49,70 | Comparison of different treatment protocols | Combination treatment | 99 (97 in ITT) | Remission – a modified version of ACR 1981 defined remission (Pinals et al.71) – on any drug treatment, no swollen or tender joints (modified by excluding fatigue and duration definition) |
|
Baseline and at months 1, 3, 4, 5, 6, 9, 12, 18 and 24 |
| Single-drug treatment | 100 (98 in ITT) |
|
||||
| Hodkinson et al., 201551 | Comparison of different targets | SDAI arm | 42 | A SDAI of ≤ 11 (LDA) | 15 mg/week of MTX, then if not achieving a SDAI of ≤ 11:
|
Monthly for the first 3 months then every 3 months until the end of 12 months |
| CDAI arm | 60 | A CDAI of ≤ 10 (LDA) | 15 mg/week of MTX, then if not achieving a CDAI of ≤ 10:
|
|||
| Saunders et al., 200854 | Comparison of different treatment protocols | Parallel triple therapy | 47 | A DAS28 of < 3.2 |
|
Every 3 months by an independent, blinded assessor |
| Step-up therapy | 49 |
|
||||
| STREAM55 | TTT vs. usual care | Aggressive group | 42 | Remission (a DAS44 of < 1.6) | Treatment was started with 15 mg/week of oral MTX. If the DAS was ≥ 1.6 at a given time point:
|
Every 3 months |
| Conventional care | 40 | Not prompted to make treatment decisions based on DAS. The treating physician could only change therapy if the DAS was > 2.4 at the 3-month assessment | The rheumatologist had access to the DAS, but was not prompted to make treatment decisions based on the DAS. The following order of drugs was suggested to the treating rheumatologist: HCQ (or MTX after 2005 with 25 patients recruited), SSZ, MTX and LEF | |||
| T-4 study56,57 | TTT vs. usual care; comparison of different targets | Routine care | 62 | No target | All groups received 1 g/day of SSZ. Change of therapy in the routine care group was based on the treating physician’s clinical judgement according to the improvement in the number of tender joints (0–28), swollen joints (0–26) and value of serum CRP from pre-assessment values, without access to current DAS28 and MMP-3 values | Weeks 0, 2, 4, 6, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 52 and 56 |
| DAS28-driven therapy | 60 | A DAS28 of < 2.6 |
|
|||
| MMP-3-driven therapy | 60 | A MMP-3 concentration of < 121 ng/ml for men or < 59.7 ng/ml for women | ||||
| DAS28 and MMP-3-driven therapy | 61 | A DAS28 of < 2.6 and a MMP-3 concentration of < 121 ng/ml for men or 59.7/< ng/ml for women | ||||
| TEAR58–60 | Comparison of different targets; comparison of different treatment protocols | Immediate ETN | 244 | No target | Oral MTX, escalated to 20 mg/week or to a lower dosage if treatment resulted in no active tender/painful or swollen joints by week 12, plus subcutaneous 50 mg/week of ETN. Corticosteroid use at entry tapered | Every 6 weeks during the first 48 weeks and every 12 weeks thereafter (joint assessments) |
| Immediate triple therapy | 132 | Oral MTX, escalated to 20 mg/week or to a lower dosage if treatment resulted in no active tender/painful or swollen joints by week 12, plus 500 mg twice daily of SSZ, escalated (if tolerated) to 1000 mg twice daily at 6 weeks, plus 200 mg twice daily of HCQ. Corticosteroid use at entry tapered | ||||
| Step-up ETN | 255 | A DAS28-ESR of < 3.2 | Oral MTX, escalated to 20 mg/week or to a lower dosage if treatment resulted in no active tender/painful or swollen joints by week 12 plus an ETN PBO. Corticosteroid use at entry tapered. If DAS28-ESR was ≥ 3.2 at week 24, patients were stepped up to MTX plus subcutaneous 50 mg/week of ETN | |||
| Step-up triple therapy | 124 | Oral MTX, escalated to 20 mg/week or to a lower dosage if treatment resulted in no active tender/painful or swollen joints by week 12 plus a triple-therapy PBO. Corticosteroid use at entry tapered. If DAS28-ESR was ≥ 3.2 at week 24, patients were stepped up to MTX plus 500 mg twice daily of SSZ, escalated (if tolerated) to 1000 mg twice daily at 6 weeks, plus 200 mg twice daily of HCQ | ||||
| U-Act-Early62 | Comparison of different treatment protocols | TOC + MTX | 105 | A DAS28 of < 2.6 and a SJC28 of ≤ 4 |
|
There was no fixed number of weeks or visits per patient. SJC, TJC, pain, general health, ESR and CRP assessed at baseline and every 4 weeks thereafter up to 104 weeks including baseline |
| TOC + PBO–MTX | 103 |
|
||||
| MTX + PBO–TOC | 108 |
|
Trials examining established rheumatoid arthritis populations
Three trials9,50,52,53 reported on established RA populations (i.e. BROSG trial,9 Fransen et al. 50 and the Optimisation of Adalimumab study52,53). Population characteristics are presented in Table 16. The mean age of trial participants ranged from 51.5 to 60.8 years, and trial arm samples ranged from 62% to 83.5% female and from 62% to 93.5% rheumatoid factor positive. The mean disease duration at baseline ranged from 4 to 12.6 years. The mean DAS28 at baseline ranged from 4.5 to 5.8, indicating moderate to severe RA at baseline among the established RA population. The mean SJC (on 66 joints) ranged from 10.5 to 11.1 and mean TJC (0–68 joints) ranged from 11.3 to 13.5, across all trial arms. The mean HAQ score at baseline ranged from 1.25 to 1.6.
| Trial acronym or first author and year of publication | Treatment arm | Number of participants | Characteristic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age, years (SD) | Female, n/N (%) | Rheumatoid factor positive, n/N (%) | Mean disease duration (months) (SD) | Mean DAS28 at baseline (SD) (ESR or CRP where stated) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | Mean pain score (100-mm VAS) (SD) | Mean HAQ score (SD) | |||
| BROSG trial9 | Symptomatic treatment (shared care) | 233 | 60.4 (11.1) [61.8 (27.1–61.8)]a | 159/233 (68) | NR | 12.6 (6.7) years | NR | 28 joint count: 4.5 (4.5); n = 228 | 28 joint count: 5.7 (6.3); n = 228 | 41.7 (23.1); n = 221 | 1.25 (0.68); n = 233 |
| Aggressive therapy (hospital) | 233 | 60.8 (11.3) [62.5 (30.1–87.4)]a | 158/233 (68) | NR | 12.5 (6.8) years | NR | 28 joint count: 3.9 (3.8); n = 233 | 28 joint count: 4.6 (5.4); n = 232 | 42.6 (23.2); n = 230 | 1.31 (0.72); n = 233 | |
| Fransen et al., 200550 | DAS28 | 205 |
Main sample, n = 205: 57 (11), 58 (52–65)b Subsample, n = 61: 57 (10), 57 (51–65)b |
Main sample, 138/205 (67) Subsample: 38/61 (62) |
Main sample, n = 205: 172 (84) Subsample, n = 61: 53 (87) |
Main sample, n = 205: 6 (3–14) yearsb Subsample, n = 61: 4 (2–10) yearsb |
Subsample, n = 61: 4.6 (1.2) | NR | NR | NR | NR |
| Usual care | 179 |
Main sample, n = 179: 59 (13), 58 (50–70)a Subsample n = 81: 59 (12), 58 (51–68)b |
Main sample: 132/179 (74) Subsample: 62/81 (77) |
Main sample, n = 179: 132 (74) Subsample, n = 81: 55 (68) |
Main sample, n = 179: 7 (3–14) yearsb Subsample, n = 81: 5 (2–12) yearsb |
Subsample, n = 81: 4.5 (1.2) | NR | NR | NR | NR | |
| Optimisation of Adalimumab study52,53 | Routine care | 109 | 56.0 (12.9) | 91/109 (84) | NR | NR | 5.7 (1.0) | 10.6 (6.0) | 11.3 (6.9) | NR | 1.6 (0.6) |
| DAS28 target | 100 | 55.3 (13.7) | 82/100 (82) | NR | NR | 5.7 (1.1) | 11.1 (5.3) | 13.0 (7.9) | NR | 1.4 (0.7) | |
| SJC target | 99 | 51.5 (13.2) | 77/99 (78) | NR | NR | 5.8 (1.3) | 10.5 (5.7) | 13.5 (7.3) | NR | 1.5 (0.6) | |
Characteristics of the TTT features of the established RA trials are presented in Table 17. Targets included LDA (DAS28 of ≤ 3.250), remission (DAS28 of < 2.652,53), SJC of 0,52,53 control of joint pain, stiffness and related symptoms, and suppression of clinical and laboratory evidence of inflammation, plus CRP below twice the upper limit of normal. 9 Two9,50 of the three trials examining an established RA population (BROSG9 trial and Fransen et al. 50) did not employ a specific treatment protocol for reaching the target. The Optimisation of Adalimumab study52,53 used the same treatment protocol for both the DAS28 of < 2.6- and the SJC of 0-targeted arms. Only the Fransen et al. 50 trial had assessments every 3 months or less (0, 4, 12 and 24 weeks), whereas the BROSG9 trial had assessments every 4 months and the Optimisation of Adalimumab study52,53 had assessments every 6 months (although visits every 2 months until 6 months and then every 3 months until 12 months were recommended for the two targeted arms).
| Trial acronym or first author and year of publication | Type of comparison | Treatment arm | Number of participants | Target | Treatment protocol | Frequency of assessment |
|---|---|---|---|---|---|---|
| BROSG trial9 | Other comparisons | Symptomatic treatment (shared care) | 233 | To control joint pain, stiffness and related symptoms | Managed predominantly in the primary care setting. NSAIDs, intra-articular steroid injections (up to a maximum of one per month), DMARDs (antimalarials, SSZ, intramuscular gold, penicillamine, azathioprine, MTX and LEF) and low-dose steroids (≤ 7.5 mg daily). Non-drug modalities, such as physiotherapy referral, were also used as the GP felt appropriate. DMARD therapy was monitored using the standard guidelines in current use in the five centres | Seen at home every 4 months by a rheumatology specialist nurse and annually by the rheumatologist, encouraged to visit the GP if developed any new or deteriorating symptoms |
| Aggressive therapy (hospital) | 233 | To control joint pain, stiffness and related symptoms and to suppress clinical and laboratory evidence of inflammation. CRP concentrations below twice the upper limit of normal | Managed predominantly in the hospital clinic setting. Symptomatic treatment group drugs plus, if necessary, ciclosporin, parenteral steroids, medium-dose oral steroids (up to 10 mg daily) and cyclophosphamide | Every 4 months (or more often if clinically indicated) | ||
| Fransen et al., 200550 | TTT vs. usual care | DAS28 | 205 | A DAS28 of ≤ 3.2 (for subsample of 142 patients with DAS assessment) | Systematic monitoring of disease activity by assessment of DAS28 by the treating rheumatologist. The aim was to reach a DAS28 of ≤ 3.2 (LDA) by changing DMARD treatment if the score was > 3.2 | 0, 4, 12 and 24 weeks |
| Usual care | 179 | No target | No systematic monitoring of disease activity was done and no guideline to adapt treatment strategy was supplied | |||
| Optimisation of Adalimumab study52,53 | TTT vs. usual care; comparison of different targets | RC | 109 | No target | No treatment protocol | 0, 6, 12 and 18 months |
| DAS28 target | 100 | A DAS28 of < 2.6 |
|
0, 6, 12 and 18 months. Assessments at 2, 4 and 9 months were also recommended | ||
| SJC target | 99 | SJC of 0 |
Trials examining both early and established rheumatoid arthritis populations
Two trials61,63 examined both early and established RA populations combined (TICORA61 and van Hulst et al. 63). Population characteristics are presented in Table 18. The mean age of trial participants ranged from 51 to 60 years, and trial arm samples ranged from 60% to 71% female and 73% to 79% rheumatoid factor positive. The mean disease duration at baseline ranged from 19 months to 8.9 years. The mean DAS28 at baseline ranged from 3.9 to 4.2 (and DAS44 ranged from 4.6 to 4.9). The mean SJC (on 66 joints) ranged from 11 to 12 and the mean HAQ score at baseline ranged from 1.1 to 2.0.
| Trial acronym or first author and year of publication | Treatment arm | Number of participants | Characteristic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age, years (SD) | Female, n/N (%) | Rheumatoid factor positive, n/N (%) | Mean disease duration (months) (SD) | Mean DAS28 at baseline (SD) (ESR or CRP where stated) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | Mean pain score (100-mm VAS) (SD) | Mean HAQ score (SD) | |||
| TICORA61 | Intensive management | 55 | 51 (15) | 39/55 (71) | 41/55 (75) | 19 (16) | DAS44: 4.9 (0.9) | 12 (4) (0–44) | NR | 62 (20) | 2.0 (0.8) |
| Routine management | 55 | 54 (11) | 38/55 (69) | 40/55 (73) | 20 (16) | DAS44: 4.6 (1.0) | 11 (4) (0–44) | NR | 59 (20) | 1.9 (0.7) | |
| van Hulst et al., 201063 | Intervention group | 144 | 59 (13) | 87/144 (60) | 105/144 (75) | 8.9 (0–32) yearsa | 4.2 (1.3) | NR | NR | NR | 1.3 (0.7) |
| Usual-care group | 104 | 60 (13) | 71/104 (68) | 80/144 (79) | 5.8 (0–40) yearsa | 3.9 (1.3) | NR | NR | NR | 1.1 (0.7) | |
The characteristics of the TTT features of the trials with populations containing both patients with early RA and those with established RA are presented in Table 19. LDA targets were used by both trials (a DAS44 of ≤ 2.461 and a DAS28 of ≤ 3.263) (with no target in the usual-care arms). The van Hulst et al. 63 trial did not employ a specific treatment protocol for reaching the target. The TICORA61 trial used a specific treatment protocol for the intensive management arm, but not for the routine management arm. Both trials had assessments every 3 months.
| Trial acronym or first author and year of publication | Type of comparison | Treatment arm | Number of participants | Target | Treatment protocol | Frequency of assessment |
|---|---|---|---|---|---|---|
| TICORA61 | TTT vs. usual care | Intensive management | 55 | A DAS44 of ≤ 2.4 | Escalation of DMARDs in patients with persisting disease activity:
|
Every 3 months |
| Routine management | 55 | No target | Clinical decision-making not aided by formal composite measures of disease activity | |||
| DMARD monotherapy was given in patients with active synovitis, and failure of treatment (because of toxic effects or lack of effect) resulted in a change to alternative monotherapy, or addition of a second or third drug at the discretion of the attending rheumatologist. Intra-articular injections of corticosteroid were given with the same restrictions as those in the intensive group | ||||||
| van Hulst et al., 201063 | TTT vs. usual care | Intervention group | 144 | A DAS28 of ≤ 3.2 | No protocol: DAS28 provided on paper to the rheumatologist. Rheumatologist advised to aim for their patients to reach a DAS28 of ≤ 3.2 | Every 3 months (more if needed) |
| Usual-care group | 104 | No target | No protocol |
Effectiveness of treat to target compared with usual care
Heterogeneity in the treatment protocols used, and outcomes reported, precluded statistical meta-analysis and, therefore, the findings of the trials examining TTT compared with usual care were combined and examined narratively by outcome. Where it was possible, some comparisons were examined using forest plots, with odds ratios (ORs) and confidence intervals (CIs) calculated.
Trials examining early rheumatoid arthritis populations
Table 20 summarises the TTT outcomes for the comparison of TTT with usual care for the early RA population. Details of treatment adaptations and dose of drugs given are summarised in Appendix 4, Table 51. Both trials (i.e. STREAM55 and T-4 study57) reported the proportion of patients meeting the target for at least one of the study arms (although the T-4 study reported only the number and proportion of patients meeting the DAS28 target and not the MMP-3 target or the combined DAS28 and MMP-3 target). The number meeting the target was 38% in the DAS28 of < 2.6 arm of the T-4 study. 57 The number meeting the target in the STREAM trial was slightly higher in the conventional care group (65%) than in the aggressive group (54%) at 1 year and then slightly higher in the aggressive group (66%) than in the conventional care group (49%) at 2 years, although the statistical significance of these comparisons was not reported [our calculated OR is 1.66 (95% CI 0.67 to 4.12) at 1 year and 0.52 (95% CI 0.21 to 1.28) at 2 years]. Neither trial reported the proportion of patients attaining LDA at follow-up. In the STREAM trial, the proportion of patients in remission was slightly higher in the usual-care arm (65%) than in the TTT arm (54%) at 1 year and slightly higher in the TTT arm (66%) than the usual-care arm (49%) at 2 years, although the statistical significance of these comparisons was not reported. 55 In the T-4 study, the proportion of patients attaining DAS28 remission was highest in the combined DAS28 and MMP-3 target group (56%; OR 0.21, 95% CI 0.10 to 0.47) vs. routine care], still significantly higher in the DAS28 of < 2.6 target group (38%; OR 0.43, 95% CI 0.19 to 0.95) vs. routine care] than the MMP-3 target group (13%), which in turn was significantly lower than in the routine care arm (21%;57 OR 1.72, 95% CI 0.66 to 4.52). Visualised data in Figure 5 show some evidence of a benefit of TTT on remission at 1 year, when some targets may be more beneficial than others.
| Trial name or acronym | Treatment arm | n a | Duration of randomised phase | Follow-up time point | Number completing, n/N (%) (randomised phase) | Definition of study target | Number (%) | OR (95% CI) for remission | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Meeting study target | Attaining LDA (criterion) | Attaining remission (criterion) | ||||||||
| STREAM55 | Aggressive group | 42 | 2 years | 1 year | 41/42 (98) | Remission (DAS44 of < 1.6) | 22b (54) | NR | 22b (54) (DAS44 of < 1.6) | 1.66 (0.67 to 4.12) |
| Conventional care | 40 | 1 year | 38/40 (95) | No target | 21b (65)c | NR | 21b (65) (DAS44 of < 1.6) | |||
| Aggressive group | 42 | 2 years | 41/42 (98) | Remission (DAS44 of < 1.6) | 27b (66) | NR | 27b (66) (DAS44 of < 1.6) | 0.52 (0.21 to 1.28) | ||
| Conventional care | 40 | 2 years | 38/40 (95) | No target | 19b (49)c | NR | 19b (49) (DAS44 of < 1.6) | |||
| T-4 study56,57 | Routine care | 62 | 56 weeks | 56 weeks | 55/62 (89) available for analysis | No target | NA | NR | 13/62 (21) (DAS28 of < 2.6);d,e 9/62 (15) (SDAI of ≤ 3.3)d | – |
| DAS28-driven therapy | 60 | 56 weeks | 56/60 (93) available for analysis | A DAS28 of < 2.6 | 23/60 (38%) | NR | 23/60 (38) (DAS28 of < 2.6);d 19/60 (32) (SDAI of ≤ 3.3)f | 0.43 (0.19 to 0.95)g | ||
| MMP-3-driven therapy | 60 | 56 weeks | 53/60 (8%) available for analysis | A MMP-3 concentration of < 121 ng/ml for men or < 59.7 ng/ml for women | NR | NR | 8/60 (13) (DAS28 of < 2.6);d,h 8/60 (13) (SDAI of ≤ 3.3) | 1.72 (0.66 to 4.52)g | ||
| DAS28 and MMP-3-driven therapy | 61 | 56 weeks | 58/61 (95) available for analysis | A DAS28 of < 2.6 and a MMP-3 concentration of < 121 ng/ml for men or < 59.7 ng/ml for women | NR | NR | 34/61 (56) (DAS28 of < 2.6);e,h 28/61 (46) (SDAI of ≤ 3.3)d,f | 0.21 (0.10 to 0.47)g | ||
FIGURE 5.
Forest plot for TTT vs. usual care in the early RA population on remission at 1 year. M–H, Mantel–Haenszel.
Specific disease activity outcomes are summarised in Table 21. In the T-4 study,57 the mean decrease in DAS28 score between baseline and 56 weeks was significantly greater in one of the three TTT arms {the DAS28-targeted arm: –2.5 [standard deviation (SD) 3.1]} than in the usual care arm [–1.3 (SD 2.7)], although there was no significant difference between either of the other arms (the MMP-3-targeted arm or the combined DAS28 and MMP-3-targeted arm) and the usual-care arm. Nor in the STREAM trial55 was there any difference in the DAS44 score between the TTT arm and the usual-care arm at 2 years. In neither study was there any significant difference in mean change from baseline in HAQ score/modified Health Assessment Questionnaire (mHAQ) score between the targeted arm and the usual-care arm (measured at 2 years in the STREA trial55 and at 56 weeks in the T-4 study57). In the T-4 study,57 the mean change in erosion score from baseline to 56 weeks was significantly better in the combined DAS28 and MMP-3 target group [in which erosion score was reduced by –0.8 (SD 4.8)] than in the routine care group [in which the erosion score increased by 0.8 (SD 1.4)], but differences in the change in erosion score between either of the other arms (the DAS28-targeted arm or MMP-3-targeted arm) and usual care were not significant. Similarly, the increase in joint space narrowing (JSN) score at 56 weeks was significantly lower in the combined DAS28 and MMP-3 target group [0.3 (SD 2.1)] than in the routine care group [1.4 (SD 2.7)]. In addition, in the T-4 study,57 the mean change in Sharp/van der Heijde score (SHS) from baseline to 56 weeks was better in the combined DAS28 and MMP-3 target group [a reduction in total score of –0.6 (SD 5.9)] than in the routine care group [an increase in total score [2.0 (SD 2.1)]. In the STREAM trial, however, there was no significant difference in the proportion of patients without erosions at baseline who developed new erosions over the course of the trial, or in the median increase in SHS from baseline at 2 years. 55 SJC, TJC, EULAR response, ACR response and quality of life were not reported in any trial comparing TTT with usual care in an early RA population.
| Trial name or acronym | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean DAS28 (SD) | DAS44 (SD) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | EULAR good/moderate/none | ACR 20/50/70 | Mean HAQ score (SD) | Joint erosion | Quality of life | |||||
| STREAM55 | Aggressive group | 42 | 2 years | 2 years | NR | 1.4 (0.7) | NR | NR | NR | NR | 0.09 (0.50)b,c | New erosions in 5 out of 39 (13%) patients without erosions at baseline | NR |
| SHS: 0 (0–1.0)c,d | |||||||||||||
| Conventional care | 40 | 2 years | NR | 1.7 (0.8) | NR | NR | NR | NR | 0.25 (0.59)b,c | New erosions in 8 out of 34 (24%) patients without erosions at baseline | NR | ||
| SHS: 0.25 (0–2.5)c,d | |||||||||||||
| T-4 study56,57 | Routine care | 62 | 56 weeks | 56 weeks | –1.3 (2.7);c,e n = 55 | NR | NR | NR | NR | NR | 0.0 (0.7)c (mHAQ); n = 55 | SHS: erosion score, 0.8 (1.4)c,f | NR |
| JSN score: 1.4 (2.7)c,g | |||||||||||||
| Total score: 2.0 (2.1)c,h | |||||||||||||
| DAS28-driven therapy | 60 | 56 weeks | –2.5 (3.1);c,e n = 56 | NR | NR | NR | NR | NR | 0.0 (1.0)c (mHAQ); n = 56 | SHS: erosion score, 0.2 (3.1)c | NR | ||
| JSN score: 1.4 (2.8)c,i | |||||||||||||
| Total score: 1.6 (4.3)c,f | |||||||||||||
| MMP-3-driven therapy | 60 | 56 weeks | –1.3 (2.4);c n = 53 | NR | NR | NR | NR | NR | –0.1 (0.8)c (mHAQ); n = 53 | SHS: erosion score, 0.0 (2.0)c | NR | ||
| JSN score: 0.7 (2.0)c | |||||||||||||
| Total score: 0.7 (2.4)c | |||||||||||||
| DAS28 and MMP-3-driven therapy | 61 | 56 weeks | –2.0 (2.2);c n = 58 | NR | NR | NR | NR | NR | 0.0 (0.6)c (mHAQ); n = 58 | SHS: erosion score, –0.8 (4.8)c,f | NR | ||
| JSN score: 0.3 (2.1)c,g,i | |||||||||||||
| Total score: –0.6 (5.9)c,f,h | |||||||||||||
In summary, among trials examining an early RA population, there is no clear evidence either in favour of or against the clinical effectiveness of a TTT approach, in comparison with usual care, overall. There is some evidence to suggest that TTT may be more effective than usual care in terms of remission at 1 year. The STREAM55 trial found usual care to be more effective at 1 year, but TTT to be more effective at 2 years, in terms of the proportion of patients meeting the target and attaining remission. The T-4 study57 found usual care to be more effective than the MMP-3 target, but the combined DAS28 and MMP-3-targeted arm to be more effective than usual care, in terms of the proportion of patients attaining remission. There were mixed findings in relation to DAS28/DAS44 (the T-4 study57 found greater benefit for the DAS28-targeted arm than for usual care, but no difference between the MMP-3 or combined targeted arms and usual care; the STREAM55 trial also found no difference), and in relation to joint erosion (the T-4 study57 found greater benefit for the combined target arm than with usual care in terms of SHS, but no difference between the DAS28- or MMP-3-targeted arms and usual care; the STREAM55 trial also found no difference). There was no difference between TTT arms and usual care on HAQ score (assessed in the STREAM trial55 and the T-4 study57).
Among trials with early RA populations that used a remission target (DAS44 of < 1.655 and DAS28 of < 2.657), there was mixed evidence in terms of the proportion of patients meeting the target and the proportion attaining remission (the STREAM trial55 found usual care to be more effective at 1 year, but TTT to be more effective at 2 years) and DAS28/DAS44 (the T-4 study57 found TTT to be more effective than usual care, but the STREAM trial55 found no difference), with no difference on HAQ score55,57 or joint erosion. 55
The one trial examining a MMP-3 normalisation target in an early RA population found evidence in favour of usual care in terms of the proportion of patients attaining remission,57 but no difference between the MMP-3 normalisation-targeted arm and usual care on HAQ score progression. 57
The one trial examining a combined remission and MMP-3 normalisation target in an early RA population found evidence in favour of TTT in terms of the proportion of patients attaining remission and joint erosion,57 but no difference between the combined DAS28 remission and MMP-3 normalisation-targeted arm and usual care on HAQ score progression. 57
In summary, comparing trials by target, there is no clear evidence in favour of any specific target being more effective than usual care in an early RA population.
Trials examining established rheumatoid arthritis populations
Table 22 summarises the TTT outcomes for the comparison of TTT with usual care for the established RA population. Details of treatment adaptations and doses of drugs given are summarised in Appendix 4, Table 65. In the Fransen et al. 50 trial, the proportion of patients who met the study target at 24 weeks was significantly greater in the DAS28-targeted arm (31%) than in the usual-care arm (16%), as was the proportion who attained LDA at 24 weeks (remission was not reported). In the Optimisation of Adalimumab study there were no significant differences between the usual-care arm and each of the two targeted arms at 6, 12 or 18 months in terms of the proportion of patients meeting the target (for both a DAS28 of < 2.6 and SJC of 0), attaining LDA or attaining remission. 52,53 The data depicted in Figure 6 show some evidence of a benefit of TTT on LDA at 6 months, in that some targets may be more beneficial than others. This became more evident at 12 and 18 months, certainly for the DAS28-targeted arm compared with routine care [OR 0.74 (95% CI 0.42 to 1.31) at 12 months and OR 0.34 (95% CI 0.19 to 0.61) at 18 months].
| Trial name or first author and year of publication | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Number completing, n/N (%) (randomised phase) | Definition of study target | Number (%) | OR (95% CI) for remission | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Meeting study target | Attaining LDA (criteria) | Attaining remission (criteria) | ||||||||
| Fransen et al., 200550 | DAS28 | 205 | 24 weeks | 4 weeks | NA | A DAS28 of ≤ 3.2 | NR | NR | NR | – |
| Usual care | 179 | 4 weeks | NA | No target | NR | NR | NR | – | ||
| DAS28 | 205 | 12 weeks | NA | A DAS28 of ≤ 3.2 | NR | NR | NR | – | ||
| Usual care | 179 | 12 weeks | NA | No target | NR | NR | NR | – | ||
| DAS28 | 205 | 24 weeks | 189/205 (82) | A DAS28 of ≤ 3.2 | 19/61 (31)b | 19/61 (31) (DAS28 of ≤ 3.2)b | NR | 0.42 (0.19 to 0.94) | ||
| Usual care | 179 | 24 weeks | 159/179 (89) | No target | 13/81 (16)b,c | 13/81 (16) (DAS28 of ≤ 3.2)b | NR | |||
| Optimisation of Adalimumab study52,53 | Routine care | 109 | 18 months | 6 months | NA | No target | 17% (DAS28 of < 2.6);c 22% (SJC = 0)c | 28% (DAS28 of < 3.2) | 17% (DAS28 of < 2.6) | – |
| DAS28 target | 100 | 6 months | NA | A DAS28 of < 2.6 | 24% | 33% (DAS28 of < 3.2) | 24% (DAS28 of < 2.6) | 0.81 (0.45 to 1.45)d | ||
| SJC target | 99 | 6 months | NA | A SJC of 0 | 24% | 30% (DAS28 of < 3.2) | 16% (DAS28 of < 2.6) | 0.91 (0.50 to 1.66)d | ||
| Routine care | 109 | 12 months | NA | No target | 21% (DAS28 of < 2.6);c 23% (SJC = 0)c | 32% (DAS28 of < 3.2) | 21% (DAS28 of < 2.6) | – | ||
| DAS28 target | 100 | 12 months | NA | A DAS28 of < 2.6 | 28% | 39% (DAS28 of < 3.2) | 28% (DAS28 of < 2.6) | 0.74 (0.42 to 1.31)d | ||
| SJC target | 99 | 12 months | NA | A SJC of 0 | 26% | 31% (DAS28 of < 3.2) | 26% (DAS28 of < 2.6) | 1.04 (0.58 to 1.86)d | ||
| Routine care | 109 | 18 months | 52/109 (47.7) | No target | 16% (DAS28 of < 2.6);c 21% (SJC = 0)c | 23% (DAS28 of < 3.2) | 16% (DAS28 of < 2.6) | – | ||
| DAS28 target | 100 | 18 months | 73/100 (73) | A DAS28 of < 2.6 | 38% | 47% (DAS28 of < 3.2) | 38% (DAS28 of < 2.6) | 0.91 (0.50 to 1.66)d | ||
| SJC target | 99 | 18 months | 77/99 (77.8) | A SJC of 0 | 26% | 27% (DAS28 of < 3.2) | 22% (DAS28 of < 2.6) | 0.79 (0.42 to 1.49)d | ||
FIGURE 6.
Forest plot for TTT vs. usual care in the established RA population on LDA at 6 months. M–H, Mantel–Haenszel.

Table 23 summarises the disease activity outcomes for the comparison of TTT with usual care for the established RA population. There were no significant differences in mean DAS28 at 6, 12 or 18 months between either of the targeted arms and the routine care arm in the Optimisation of Adalimumab study,52,53 nor between the targeted and usual-care arms of the Fransen et al. 50 trial at 24 weeks. Similarly, there were no significant differences in mean SJC or TJC at 6, 12 or 18 months between either of the targeted arms and the routine care arm in the Optimisation of Adalimumab study52,53 (the Fransen et al. 50 trial did not report SJC or TJC). The proportion of patients with a good or moderate EULAR response was significantly different across the three arms of the Optimisation of Adalimumab study, with higher proportions in the DAS28-targeted arm (63.0%) and SJC-targeted arm (53.5%) than in the usual-care arm at 18 months, but not at 6 or 12 months. 52,53 There were no significant differences in mean change from baseline in the HAQ score at 6, 12 or 18 months between either of the targeted arms and the routine care arm in the Optimisation of Adalimumab study52,53 (the Fransen et al. 50 trial did not report HAQ scores). The DAS44, ACR 20/50/70 responses, joint erosion and quality of life were not reported by either trial examining TTT compared with usual care in an established RA population.
| Trial name or first author and year of publication | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean DAS28 (SD) | DAS44 (SD) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | EULAR good/moderate/none | ACR 20/50/70 | Mean HAQ score (SD) | Joint erosion | Quality of life | |||||
| Fransen et al., 200550 | DAS28 | 205 | 24 weeks | 24 weeks | –0.40 (1.0)b,c | NR | NR | NR | NR | NR | NR | NR | NR |
| Usual care | 179 | 24 weeks | –0.14 (1.2)b,c | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Optimisation of Adalimumab study52,53 | Routine care | 109 | 18 months | 6 months | 3.26d | NR | –6.4 (0.7)c,d | –7.8 (0.9)c,d | Good/moderate response: 57% | NR | –0.47 (0.06)c,d | NR | NR |
| DAS28 target | 100 | 6 months | 3.72d | NR | –7.6 (0.8)c,d | –9.0 (1.0)c,d | Good/moderate response: 62% | NR | –0.44 (0.06)c,d | NR | NR | ||
| SJC target | 99 | 6 months | 3.49d | NR | –6.9 (0.7)c,d | –7.6 (0.9)c,d | Good/moderate response: 63% | NR | –0.45 (0.05)c,d | NR | NR | ||
| Routine care | 109 | 12 months | 3.12d | NR | –6.5 (0.7)c,d | –8.4 (0.9)c,d | Good/moderate response: 51% | NR | –0.57 (0.06)c,d | NR | NR | ||
| DAS28 target | 100 | 12 months | 3.38d | NR | –8.3 (0.8)c,d | –9.4 (1.0)c,d | Good/moderate response: 61% | NR | –0.47 (0.06)c,d | NR | NR | ||
| SJC target | 99 | 12 months | 3.18d | NR | –7.2 (0.7)c,d | –7.8 (0.9)c,d | Good/moderate response: 51% | NR | –0.39 (0.06)c,d | NR | NR | ||
| Routine care | 109 | 18 months | 3.27d | NR | –6.5 (0.8)c,d | –8.0 (1.0)c,d | Good/moderate response: 36%e | NR | –0.49 (0.07)c,d | NR | NR | ||
| DAS28 target | 100 | 18 months | 3.40d | NR | –8.3 (0.8)c,d | –9.3 (1.0)c,d | Good/moderate response: 63%e | NR | –0.51 (0.07)c,d | NR | NR | ||
| SJC target | 99 | 18 months | 3.16d | NR | –6.4 (0.8)c,d | –7.4 (1.0)c,d | Good/moderate response: 54%e | NR | –0.41 (0.06)c,d | NR | NR | ||
In summary, among trials examining an established RA population, there is no clear evidence either in favour of or against the clinical effectiveness of a TTT approach, in comparison with usual care, overall. There is some evidence to suggest that TTT may be more effective than usual care in terms of LDA at 6 months. There was evidence in favour of a TTT approach compared with usual care in the Fransen et al. trial50 in terms of the proportion of patients meeting the target and attaining LDA, but no difference between TTT and usual care in the Optimisation of Adalimumab study52 in terms of the proportion of patients meeting the target and attaining LDA and remission. There was evidence in favour of a TTT approach compared with usual care in terms of EULAR response in the Optimisation of Adalimumab study52 (both the DAS28- and SJC-targeted arms were found to be more effective than usual care in terms of attaining a good or moderate EULAR response at 18 months, although there was no difference at 6 or 12 months). There was, however, no difference between TTT and usual care in terms of DAS28 in the Fransen et al. 50 trial and the Optimisation of Adalimumab study,52 nor in terms of SJC, TJC or HAQ response in the Optimisation of Adalimumab study. 52
The one trial examining a LDA target (DAS28 of ≤ 3.2), in an established RA population, found evidence in favour of TTT in terms of the proportion of patients meeting the target and the proportion attaining remission. 50
The one trial examining a remission target (DAS28 of < 2.6) in an early RA population found evidence in favour of TTT in terms of EULAR good/moderate response,52 but no difference between the DAS28 remission-targeted arm and usual care on the proportion of patients meeting the target, the proportion of patients attaining LDA,52 the proportion of patients attaining remission, DAS28, SJC, TJC or HAQ score progression. 52
The one trial examining a SJC of zero target in an early RA population found evidence in favour of TTT in terms of EULAR good/moderate response,52 but no difference between the SJC of zero-targeted arm and usual care on the proportion of patients meeting the target, the proportion of patients attaining LDA, the proportion of patients attaining remission, DAS28, SJC, TJC or HAQ score progression. 52
In summary, when the evidence is examined by target there is evidence in favour of TTT [using a LDA target (DAS28 of ≤ 3.2)], compared with usual care in an established RA population. 50 However, the evidence is mixed with regard to remission (DAS28 of < 2.6) and SJC (SJC of 0) targets,52 although only one trial used each of these targets and this related only to one outcome reported in the case of the LDA target. 50 The remission and SJC targets impacted on the same outcomes in the same way, although findings were from the same (single) trial. 52
Trials examining both early and established rheumatoid arthritis populations
Table 24 summarises the TTT outcomes for the comparison of TTT with usual care for the trials with populations containing both patients with early RA and those with established RA. Details of treatment adaptations and dose of drugs given are summarised in Appendix 4, Table 53. Neither the TICORA61 nor the van Hulst et al. 63 trial reported the proportion of patients meeting the study target or the proportion of patients attaining LDA, and the van Hulst et al. 63 trial did not report the proportion of patients attaining remission. In the TICORA trial, the proportion of patients who attained remission at 18 months was significantly greater in the intensive management arm (65%) than in the routine management arm (16%). 61
| Trial acronym or first author and year of publication | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Number completing, n/N (%) (randomised phase) | Definition of study target | Number (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Meeting study target | Attaining LDA (criterion) | Attaining remission (criterion) | |||||||
| TICORA61 | Intensive management | 55 | 18 months | 18 months | 53/55 (96) | A DAS44 of ≤ 2.4 | NR | NR | 36 (65)b (EULAR remission – a DAS44 of < 1.6) |
| Routine management | 55 | 18 months | 50/55 (91) | No target | NR | NR | 9 (16)b (EULAR remission – a DAS44 of < 1.6) | ||
| van Hulst et al., 201063 | Intervention group | 144 | 18 months | 18 months | 138/144 (96) | A DAS28 of ≤ 3.2 | NR | NR | NR |
| Usual-care group | 104 | 18 months | 92/104 (88) | No target | NR | NR | NR | ||
Table 25 summarises the disease activity outcomes for the comparison of TTT with usual care for the trials with populations containing both patients with early RA and those with established RA. In the van Hulst et al. 63 trial there was no significant difference in mean DAS28 between the intervention group and usual-care group at 3, 6, 9, 12, 15 or 18 months, or in the mean change from baseline in DAS28 at 18 months. The TICORA61 trial did not report the DAS28. At 3, 6, 9, 12, 15 and 18 months, the mean DAS44 was significantly lower in the intensive management arm than the routine management arm. In addition, there was a significantly greater mean change from baseline in the DAS44 in the intensive management arm [–3.5 (SD 1.1)] than in the routine management arm [–1.9 (SD 1.4)] of the TICORA trial at 18 months. 61 The DAS44 was not reported in the van Hulst et al. trial. 63 There was a significantly greater mean change from baseline in the SJC in the intensive management arm [–11 (SD 5)] than in the routine management arm [–8 (SD 5)] at 18 months in the TICORA trial; however, there was no significant difference in mean change from baseline in the SJC between the intervention group and usual-care group at 18 months in the van Hulst et al. trial. 63 There was a significantly greater mean change from baseline in the TJC in the intensive management arm [–20 (SD 5)] than in the routine management arm [–12 (SD 12)] at 18 months in the TICORA trial; however, there was no significant difference in mean change from baseline in the TJC between the intervention group and usual-care group at 18 months. 61
| Trial acronym or first author and year of publication | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean DAS28 (SD) | DAS44 (SD) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | EULAR good/moderate/none | ACR 20/50/70 | Mean HAQ score (SD) | Joint erosion | Quality of life | |||||
| TICORA61 | Intensive management | 55 | 18 months | 3 months | NR | 2.67 (1.38)b,c | NR | NR | NR | NR | NR | NR | NR |
| Routine management | 55 | 3 months | NR | 3.65 (1.40)b,c | NR | NR | NR | NR | NR | NR | NR | ||
| Intensive management | 55 | 6 months | NR | 2.29 (1.43)b,c | NR | NR | NR | NR | NR | NR | NR | ||
| Routine management | 55 | 6 months | NR | 3.32 (1.70)b,c | NR | NR | NR | NR | NR | NR | NR | ||
| Intensive management | 55 | 9 months | NR | 2.07 (1.30)b,c | NR | NR | NR | NR | NR | NR | NR | ||
| Routine management | 55 | 9 months | NR | 3.08 (1.48)b,c | NR | NR | NR | NR | NR | NR | NR | ||
| Intensive management | 55 | 12 months | NR | 1.77 (1.13)b,c | NR | NR | NR | NR | NR | NR | NR | ||
| Routine management | 55 | 12 months | NR | 2.78 (1.46)b,c | NR | NR | NR | NR | NR | NR | NR | ||
| Intensive management | 55 | 15 months | NR | 1.50 (0.96)b,c | NR | NR | NR | NR | NR | NR | NR | ||
| Routine management | 55 | 15 months | NR | 2.66 (1.36)b,c | NR | NR | NR | NR | NR | NR | NR | ||
| Intensive management | 55 | 18 months | NR | 1.33 (0.96);b,c change from baseline –3.5 (1.1)b,d,e | –11 (5)d,e,f | –20 (9)d,e,g | Good response: 45 (82%)b,h | ACR 20: 50 (91%)b,h | –0.97 (0.8)d,e,i | SHS: erosion score, 0.5 (0–3.4)e,j,k | SF-12 Physical Summary: 9.3 (12.0)d,e,l | ||
| ACR 50: 46 (84%)b,h | JSN score: 3.25 (1.1–7.5)e,j | SF-12 Mental Health Summary: 10.9 (16.0)d,e | |||||||||||
| ACR 70: 39 (71%)b,h | Total score: 4.5 (1 to 9.9)e,j,m | ||||||||||||
| Routine management | 55 | 18 months | NR | 2.68 (1.25);b,c –1.9 (1.4)b,d,e | –8 (5)d,e,f | –12 (12)d,e,g | Good response: 24 (44%)b,h | ACR 20: 35 (64%)b,h | –0.47 (0.9)b,e,i | SHS: erosion score, 3 (0.5–8.5)e,j,k | SF-12 Physical Summary: 4.0 (11.0)d,e | ||
| ACR 50: 22 (40%)b,h | JSN score: 4.5 (1.5–9.0)e,j | SF-12 Mental Health Summary: 6.0 (18.0)d,e | |||||||||||
| ACR 70: 10 (18%)b,h | Total score: 8.5 (2.0 to 15.5)e,j,m | ||||||||||||
| van Hulst et al., 201063 | Intervention group | 144 | 18 months | 3 months | 4.06 (3.84 to 4.28)c,n | NR | NR | NR | NR | NR | NR | NR | NR |
| Usual-care group | 104 | 3 months | 3.87 (3.66 to 4.11)c,n | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Intervention group | 144 | 6 months | 3.87 (3.65 to 4.08)c,n | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Usual-care group | 104 | 6 months | 3.60 (3.35 to 3.87)c,n | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Intervention group | 144 | 9 months | 3.68 (3.45 to 3.86)c,n | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Usual-care group | 104 | 9 months | 3.40 (3.17 to 3.64)c,n | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Intervention group | 144 | 12 months | 3.74 (3.54 to 3.98)c,n | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Usual-care group | 104 | 12 months | 3.53 (3.28 to 3.80)c,n | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Intervention group | 144 | 15 months | 3.61 (3.37 to 3.81)c,n | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Usual-care group | 104 | 15 months | 3.39 (3.13 to 3.66)c,n | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Intervention group | 144 | 18 months | 3.55 (3.33 to 3.76);c,n –0.66e,o | NR | –3.11e,o | –2.10e,o | Good response 21.5% | NR | –0.19 (NR)e,o | NR | NR | ||
| Moderate response 22.9% | |||||||||||||
| No response 55.6% | |||||||||||||
| Usual-care group | 104 | 18 months | 3.24 (2.99 to 3.49);c,n –0.69e,o | NR | –1.52e,o | 1.24f,o | Good response 18.3% | NR | –0.15 (NR)e,o | NR | NR | ||
| Moderate response 26.9% | |||||||||||||
| No response 54.8% | |||||||||||||
In the TICORA61 trial at 18 months, the proportion of patients who attained a EULAR good response was significantly greater in the intensive management arm (82%) than in the routine management arm (44%); however, in the van Hulst et al. trial63 at 18 months, the proportion of patients who attained a EULAR good response, a moderate response and no response were similar in the intervention group and usual-care group (statistical significance not reported). In the TICORA trial,61 the proportions of patients who attained an ACR 20, ACR 50 and ACR 70 at 18 months were significantly greater in the intensive management arm (90%, 84% and 71%, respectively) than in the routine management arm (64%, 40% and 18%, respectively); in the van Hulst et al. trial,63 however, ACR response was not reported. In the TICORA trial,61 at 18 months, mean change in the HAQ score from baseline was a significantly greater in the intensive management arm [–0.97 (SD 0.8)] than in the routine management arm [–0.47 (SD 0.9)];61 in the van Hulst et al. trial,63 however, there was no significant difference at 18 months in mean change from baseline in HAQ score between the intervention group and usual-care group.
In the TICORA61 trial, median increase from baseline in the SHS erosion score and total SHS were significantly lower in the intensive management arm [SHS erosion score: 0.5, interquartile range (IQR) 0–3.4; total SHS: 4.5, IQR 1–9.9] than in the routine management arm (SHS erosion score: 3, IQR 0.5–8.5; total SHS: 8.5, IQR 2.0–15.5), but no significant difference in SHS JSN score between the arms was found at 18 months. Erosion was not reported in the van Hulst et al. trial. 63 Mean change from baseline in the Short Form questionnaire-36 items (SF-36) Physical Summary score was significantly greater in the intensive management arm [9.3 (SD 12.0)] than in the routine management arm [4.0 (SD 11.0)]. Furthermore, at 18 months, there was no significant difference in mean change from baseline in the SF-36 Mental Health Summary score between the TTT and usual-care arms. 61 Quality of life was not reported in the van Hulst et al. trial. 63
We identified an abstract also relating to the TICORA trial (i.e. Porter et al. 72); however, there is a discrepancy between the data reported in the abstract and those reported in the Grigor et al. 61 paper in terms of EULAR good response, EULAR remission, ACR 20, ACR 70, CRP and SHS erosion score. We used the data from the Grigor et al. 61 paper in our synthesis.
In summary, among trials with populations including both patients with early RA and those with established RA, there is no clear evidence either in favour of or against the clinical effectiveness of a TTT approach in comparison with usual care. TICORA,61 which was the only trial in the systematic review that was rated as having a low risk of bias, demonstrated evidence in favour of a TTT approach in terms of the proportion of patients attaining remission. There was also evidence in favour of a TTT approach rather than usual care in terms of ACR 20/50/70 response in the TICORA61 trial. The evidence, however, was equivocal for DAS28/DAS44 score, SJC, TJC, EULAR response and HAQ score progression, with the TICORA61 trial reporting TTT to be more effective than usual care and the van Hulst et al. 63 trial reporting no difference between TTT and usual care. In terms of joint erosion and quality of life, evidence from the TICORA61 trial was ambiguous, with some subscales showing benefit for TTT over usual care and some demonstrating no difference.
Both trials examining populations including both patients with early RA and those with established RA61,63 examined a LDA target (DAS44 of ≤ 2.461 or DAS28 of ≤ 3.263). Thus, the findings described and summarised above relate only to a LDA target for this comparison and this population group.
Comparison of different targets
Heterogeneity in the treatment protocols used, and outcomes reported, precluded statistical meta-analysis and, therefore, the findings of the trials comparing different targets were combined and examined narratively by outcome.
Trials examining early rheumatoid arthritis populations
Table 26 summarises the TTT outcomes for the comparison of different targets for the early RA population. Details of treatment adaptations and doses of drugs given are summarised in Appendix 4, Table 54. Only one of the three trials (i.e. TEAR58) reported the proportion of patients meeting the target (while Hodkinson et al. 51 and the T-4 study57 did not). This was lower for both DAS28 of < 3.2-targeted arms combined (28%) than for the immediate ETN and immediate triple-therapy arms without a target (41% and 43%, respectively) at 24 weeks. 58 Similar proportions of patients attained LDA and remission in the Hodkinson et al. trial,51 whereas in the TEAR trial a significantly greater proportion of patients in each of the immediate ETN (41%) and immediate triple-therapy (43%) arms attained LDA according to DAS28 criteria than both step-up arms combined at 24 weeks (28%); however, the proportions of participants attaining remission at 102 weeks were not significantly different between the two DAS28 < 3.2 targeted arms and the two immediate therapy arms without a target. 58 In the T-4 study, the proportion of patients attaining DAS28 remission was highest in the combined DAS28 and MMP-3-targeted group (56%). The proportion of patients attaining DAS28 remission was significantly higher than in the DAS28 of < 2.6 target group (38%) or the MMP-3 target group (13%), the proportion in this last group being significantly lower than in the routine care arm (21%). 57
| Trial acronym or first author and year of publication | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Number completing, n/N (%) (randomised phase) | Definition of study target | Number (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Meeting study target | Attaining LDA (criterion) | Attaining remission (criterion) | |||||||
| Hodkinson et al., 201551 | SDAI arm | 42 | 12 months | 12 months | 41/42 (98) | A SDAI of ≤ 11 (LDA) | NR | 13(32) (DAS28b) | 14 (34) (DAS28b) |
| CDAI arm | 60 | 12 months | 57/60 (95) | A CDAI of ≤ 10 (LDA) | NR | 17 (30) (DAS28b) | 19 (33) (DAS28b) | ||
| T-4 study56,57 | Routine care | 62 | 56 weeks | 56 weeks | 55/62 (89) available for analysis | No target | NA | NR | 13/62 (21) (DAS28 of < 2.6)c,d |
| 9/62 (15) (SDAI of ≤ 3.3)e | |||||||||
| DAS28-driven therapy | 60 | 56 weeks | 56/60 (93) available for analysis | A DAS28 of < 2.6 | 23/60 (38) | NR | 23/60 (38) (DAS28 of < 2.6)f | ||
| 19/60 (32) (SDAI of ≤ 3.3)g | |||||||||
| MMP-3-driven therapy | 60 | 56 weeks | 53/60 (88) available for analysis | A MMP-3 concentration of < 121 ng/ml for men or < 59.7 ng/ml for women | NR | NR | 8/60 (13) (DAS28 of < 2.6)h | ||
| 8/60 (13) (SDAI of ≤ 3.3) | |||||||||
| DAS28 and MMP-3-driven therapy | 61 | 56 weeks | 58/61 (95) available for analysis | A DAS28 of < 2.6 and a MMP-3 concentration of < 121 ng/ml for men or < 59.7 ng/ml for women | NR | NR | 34/61 (56) (DAS28 of < 2.6) | ||
| 28/61 (46) (SDAI of ≤ 3.3) | |||||||||
| TEAR58–60 | Immediate ETN | 244 | 102 weeks | 24 weeks | See below | No target | 100/244 (41) | 100/244 (41) (DAS28-ESR of ≤ 3.2) | NR |
| Immediate triple therapy | 132 | 24 weeks | See below | 65/152 (43) | 65/152 (43) (DAS28-ESR of ≤ 3.2) | NR | |||
| Step-up ETN | 255 | 24 weeks | See below | A DAS28-ESR of < 3.2 | 105/376 (28) | 105/376 (28)i (DAS28-ESR of ≤ 3.2) | NR | ||
| Step-up triple therapy | 124 | 24 weeks | See below | NR | |||||
| Immediate ETN | 244 | 102 weeks | 168/244 (69) (159 with DAS28) | No target | 90/159 (57)d | NR | 90/159 (57)j (DAS28-ESR of ≤ 2.6) | ||
| Immediate triple therapy | 132 | 102 weeks | 82/132 (62) (76 with DAS28) | 45/76 (59)d | NR | 45/76 (59)j (DAS28-ESR of ≤ 2.6) | |||
| Step-up ETN | 255 | 102 weeks | 182/255 (71) (166 with DAS28) | A DAS28-ESR of < 3.2 | 88/166 (53)d | NR | 88/166 (53)j (DAS28-ESR of ≤ 2.6) | ||
| Step-up triple therapy | 124 | 102 weeks | 81/124 (65) (75 with DAS28) | 42/75 (57)d | NR | 42/75 (57)j (DAS28-ESR of ≤ 2.6) | |||
Table 27 summarises the disease activity outcomes for the comparison of different targets for the early RA population. There were no significant differences in DAS28 or decrease in DAS28 between arms with different targets in any of the three trials to examine the comparison of different targets in an early RA population. In the TEAR trial,58 the mean SJC was slightly higher in the two targeted step-up arms [4.4 (SD 3.1) and 4.4 (SD 2.8) for step-up the ETN and step-up triple-therapy arms, respectively] than in either immediate treatment arm with no target [2.2 (SD 3.9) and 2.3 (SD 3.3) for the immediate ETN and immediate triple-therapy arms, respectively] at 102 weeks (although the statistical significance between groups was not reported). In the Hodkinson et al. trial,51 however, there was no significant difference in the mean SJC at 12 months between the SDAI- and CDAI-targeted arms. There was little difference in the mean TJC between the two DAS28-targeted (step-up) arms and the two immediate treatment arms with no target, at 102 weeks, in the TEAR trial. 58 In addition, there was no significant difference in the mean TJC at 12 months between the SDAI- and CDAI-targeted arms in the Hodkinson et al. trial. 51
| Trial acronym or first author and year of publication | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean DAS28 (SD) | DAS44 (SD) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | EULAR good/moderate/none | ACR 20/50/70 | Mean HAQ score (SD) | Joint erosion | Quality of life | |||||
| Hodkinson et al., 201551 | SDAI arm | 42 | 12 months | 12 months | 3.0 (1.2) | NR | 1.3 (2.6) | 1.4 (2.4) | EULAR good response: 23 (56%) | NR | HAQ-DI: 1.0 (0.7) | NR | NR |
| CDAI arm | 60 | 12 months | 3.3 (1.2) | NR | 1.4 (2.4) | 1.7 (2.5) | EULAR good response: 29 (51%) | NR | HAQ-DI: 1.0 (0.7) | NR | NR | ||
| T-4 study56,57 | Routine care | 62 | 56 weeks | 56 weeks | –1.3 (2.7);b n = 55 | NR | NR | NR | NR | NR | 0.0 (0.7)b (mHAQ); n = 55 | SHS erosion score: 0.8 (1.4)b,c | NR |
| JSN score: 1.4 (2.7)b,d | |||||||||||||
| Total score: 2.0 (2.1)b,c | |||||||||||||
| DAS28-driven therapy | 60 | 56 weeks | –2.5 (3.1);b n = 56 | NR | NR | NR | NR | NR | 0.0 (1.0)b (mHAQ); n = 56 | SHS erosion score: 0.2 (3.1)b | NR | ||
| JSN score: 1.4 (2.8)b,e | |||||||||||||
| Total score: 1.6 (4.3)b,f | |||||||||||||
| MMP-3-driven therapy | 60 | 56 weeks | –1.3 (2.4);b n = 53 | NR | NR | NR | NR | NR | –0.1 (0.8)b (mHAQ); n = 53 | SHS erosion score: 0.0 (2.0)b | NR | ||
| JSN score: 0.7 (2.0)b | |||||||||||||
| Total score: 0.7 (2.4)b | |||||||||||||
| DAS28 and MMP-3-driven therapy | 61 | 56 weeks | –2.0 (2.2);b n = 58 | NR | NR | NR | NR | NR | 0.0 (0.6)b (mHAQ); n = 58 | SHS erosion score: –0.8 (4.8)b,c | NR | ||
| JSN score: 0.3 (2.1)b,d,e | |||||||||||||
| Total score: –0.6 (5.9)b,f,g | |||||||||||||
| TEAR58–60 | Immediate ETN | 244 | 102 weeks | 6 months | NR | NR | NR | NR | NR | ACR 20: 56.28%h,i | NR | NR | NR |
| ACR 50: 32.14%h,i | |||||||||||||
| ACR 70: 11.13%h,i | |||||||||||||
| Immediate triple therapy | 132 | 6 months | NR | NR | NR | NR | NR | ACR 20: 55.88%h,i | NR | NR | NR | ||
| ACR 50: 31.33%h,i | |||||||||||||
| ACR 70: 7.97%h,i | |||||||||||||
| Step-up ETN | 255 | 6 months | NR | NR | NR | NR | NR | ACR 20: 40.12%h,i | NR | NR | NR | ||
| ACR 50: 19.51%h,i | |||||||||||||
| ACR 70: 2.84%h,i | |||||||||||||
| Step-up triple therapy | 124 | 6 months | NR | NR | NR | NR | NR | ACR 20: 39.32%h,i | NR | NR | NR | ||
| ACR 50: 17.53%h,i | |||||||||||||
| ACR 70: 3.62%h,i | |||||||||||||
| Immediate ETN | 244 | 2 years | NR | NR | NR | NR | NR | ACR 20: 51.10%h | NR | NR | NR | ||
| ACR 50: 37.18%h | |||||||||||||
| ACR 70: 20.52%h | |||||||||||||
| Immediate triple therapy | 132 | 2 years | NR | NR | NR | NR | NR | ACR 20: 45.97%h | NR | NR | NR | ||
| ACR 50: 31.27%h | |||||||||||||
| ACR 70: 10.66%h | |||||||||||||
| Step-up ETN | 255 | 2 years | NR | NR | NR | NR | NR | ACR 20: 49.11%h | NR | NR | NR | ||
| ACR 50: 32.44%h | |||||||||||||
| ACR 70: 15.77%h | |||||||||||||
| Step-up triple therapy | 124 | 2 years | NR | NR | NR | NR | NR | ACR 20: 47.92%h | NR | NR | NR | ||
| ACR 50: 37.15%h | |||||||||||||
| ACR 70: 11.43%h | |||||||||||||
| Immediate ETN | 244 | 102 weeks | 3.0 (1.4); n = 159 (DAS28-ESR) | NR | 2.2 (3.9); n = 159 | 3.3 (5.5) | NR | NR | mHAQ: 1.0 (0.3); n = 15 | SHS erosion score: 3.6 (7.4); n = 159 | NR | ||
| JSN score: 3.7 (9.8); n = 159 | |||||||||||||
| Total SHS: 7.0 (16.6); n = 159 | |||||||||||||
| Immediate triple therapy | 132 | 102 weeks | 2.9 (1.5); n = 76 (DAS28-ESR) | NR | 2.3 (3.3); n = 76 | 2.6 (4.5); n = 76 | NR | NR | mHAQ: 1.0 (0.3); n = 73 | SHS erosion score: 3.3 (3.9); n = 76 | NR | ||
| JSN score: 3.9 (10.6); n = 76 | |||||||||||||
| Total SHS: 7.3 (13.3); n = 76 | |||||||||||||
| Step-up ETN | 255 | 102 weeks | 3.0 (1.4); n = 166 (DAS28-ESR) | NR | 4.4 (3.1); n = 166 | 3.6 (5.8); n = 166 | NR | NR | mHAQ: 0.9 (0.3); n = 154 | SHS erosion score: 3.0 (3.9); n = 166 | NR | ||
| JSN score: 2.1 (4.4); n = 166 | |||||||||||||
| Total SHS: 4.8 (7.2); n = 166 | |||||||||||||
| Step-up triple therapy | 124 | 102 weeks | 2.8 (1.3); n = 75 (DAS28-ESR) | NR | 4.4 (2.8); n = 75 | 2.6 (4.4); n = 75 | NR | NR | mHAQ: 0.9 (0.3); n = 71 | SHS erosion score: 3.3 (4.4); n = 75 | NR | ||
| JSN score: 2.6 (5.0); n = 75 | |||||||||||||
| Total SHS: 6.2 (8.9); n = 75 | |||||||||||||
There was no significant difference in the proportion of patients between the CDAI- and SDAI-targeted arms with EULAR good response at 12 months in the Hodkinson et al. trial51 (the T-4 study57 and TEAR trial58 did not report EULAR response). In the TEAR trial,58 the proportion of patients achieving ACR 20/50/70 response at 6 months was significantly higher in the immediate treatment arms with no target (56.28%, 32.14% and 11.13%, respectively, and 55.88%, 31.33% and 7.97%, respectively, for the immediate ETN and immediate triple-therapy arms) thanin the two DAS28-targeted (step-up) arms (40.12%, 19.51% and 2.84%, respectively, and 39.32%, 17.53% and 3.62%, respectively, for the step-up ETN and step-up triple-therapy arms). There was no significant difference in ACR 20/50/70 response between the two DAS28-targeted (step-up) arms and the immediate treatment arms with no target at 2 years. ACR response was not reported in the Hodkinson et al. 51 trial or the T-4 study. 56,57 There were no significant differences in the mean HAQ score or change in HAQ score between arms with different targets in the Hodkinson et al. trial,51 T-4 study57 or TEAR trial58 at 1 or 2 years.
In the T-4 study,57 mean change from baseline in the SHS JSN score and total SHS were significantly lower in the combined DAS28 and MMP-3-targeted group [0.3 (SD 2.1) and –0.6 (SD 5.9), respectively] than in the DAS28-targeted group [1.4 (SD 2.8) and 1.6 (SD 4.3), respectively] at 56 weeks. There was no significant difference between the DAS28-targeted, MMP-3-targeted and combined DAS28 and MMP-3-targeted arms in the mean change from baseline in SHS erosion score at 56 weeks. Similarly, in the TEAR trial, there was no significant difference in mean SHS erosion score, SHS JSN score or total SHS between both DAS28-targeted (step-up) arms and either immediate treatment arm with no target at 102 weeks. 58
The DAS44 and quality of life were not reported in any trial examining TTT compared with usual care in an early RA population.
In summary, there was mainly no difference in the clinical effectiveness of different targets on the proportion of patients meeting the target,58 attaining LDA51,58 and attaining remission. 51 Only the T-4 study57 (early RA) found differences between targets: the DAS28 of < 2.6 target was more effective than the MMP-3 target, and the combined DAS28 of < 2.6 and MMP-3 target was more effective than both the DAS28 of < 2.6 target and the MMP-3 target, in terms of the proportion of patients in remission. There was no difference in the clinical effectiveness of different targets on DAS28/DAS44,51,57,58 TJC,51,58 EULAR response51 and HAQ response. 51,57,58 Findings in other outcomes were equivocal. In terms of SJC, a LDA target (DAS28 of < 3.2) was found to be more effective than immediate treatment with no target in the TEAR trial;58 however, there was no difference between the two LDA-targeted arms (a CDAI of ≤ 10 and a SDAI of ≤ 11) in the Hodkinson et al. trial. 51 In terms of ACR 20/50/70 response, the immediate treatment arm was more effective than the LDA arm in the TEAR trial58 at 6 months; however, there were no differences between targets in the TEAR trial58 at 2 years. In terms of joint erosion, the T-4 study57 found the combined target arm to be more effective than the DAS28 of < 2.6-targeted arm on SHS JSN score and total score; however, there was no difference between the combined target arm, DAS28 of < 2.6 arm and MMP-3 normalisation arm on the SHS erosion score,57 and no difference between the DAS28 of < 3.2 arm and the immediate treatment arm with no target on SHS erosion score, JSN score and total score in the TEAR trial. 58
Trials examining established rheumatoid arthritis populations
Table 28 summarises the TTT outcomes for the comparison of different targets for the established RA population. Details of treatment adaptations and dose of drugs given are summarised in Appendix 4, Table 55. Only one trial with an established RA population made comparisons between different treatment targets. The Optimisation of Adalimumab study found no differences between arms with different targets in terms of the proportions of patients meeting the target, attaining LDA or attaining remission. 52,53
| Trial name | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Number completing, n/N (%) (randomised phase) | Definition of study target | Number (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Meeting study target | Attaining LDA (criterion) | Attaining remission (criterion) | |||||||
| Optimisation of Adalimumab study52,53 | Routine care | 109 | 18 months | 6 months | NA | No target | NR (17) (DAS28 of < 2.6); NR (22) (SJC = 0) | NR (28) (DAS28 of < 3.2) | NR (17) (DAS28 of < 2.6) |
| DAS28 target | 100 | 6 months | NA | A DAS28 of < 2.6 | 24% | NR (33) (DAS28 of < 3.2) | NR (24) (DAS28 of < 2.6) | ||
| SJC target | 99 | 6 months | NA | A SJC of 0 | 24% | 30% (DAS28 of < 3.2) | NR (16) (DAS28 of < 2.6) | ||
| Routine care | 109 | 12 months | NA | No target | NR (21) (DAS28 of < 2.6); NR (22.9) (SJC = 0) | NR (32) (DAS28 of < 3.2) | NR (21) (DAS28 of < 2.6) | ||
| DAS28 target | 100 | 12 months | NA | A DAS28 of < 2.6 | 28% | NR (39) (DAS28 of < 3.2) | NR (28) (DAS28 of < 2.6) | ||
| SJC target | 99 | 12 months | NA | A SJC of 0 | 26% | NR (31) (DAS28 of < 3.2) | NR (26) (DAS28 of < 2.6) | ||
| Routine care | 109 | 18 months | 52/109 (48) | No target | NR (16) (DAS28 of < 2.6); NR (21.1) (SJC = 0) | NR (23) (DAS28 of < 3.2) | NR (16) (DAS28 of < 2.6) | ||
| DAS28 target | 100 | 18 months | 73/100 (73) | A DAS28 of < 2.6 | NR (38.0) | NR (47) (DAS28 of < 3.2) | NR (38) (DAS28 of < 2.6) | ||
| SJC target | 99 | 18 months | 77/99 (78) | A SJC of 0 | NR (26.3) | NR (27) (DAS28 of < 3.2) | NR (22) (DAS28 of < 2.6) | ||
Table 29 summarises the TTT outcomes for the comparison of different targets for the established RA population. The one trial that compared different targets in an established RA population was the Optimisation of Adalimumab study. 52,53 There were no significant differences in mean DAS28 at 6, 12 or 18 months between the DAS28 of < 2.6- and SJC of 0-targeted arms. 52,53 The proportion of patients with a good or moderate EULAR response was significantly different across the three trial arms, with a slightly higher proportion in the DAS28 of < 2.6-targeted arm (63.0%) than the SJC of 0-targeted arm (53.5%). 52,53 There were no significant differences in mean change from baseline in HAQ score at 6, 12 or 18 months between the DAS28 of < 2.6- and SJC of 0-targeted arms. 52,53 DAS44, ACR 20/50/70 responses, joint erosion and quality of life were not reported in the Optimisation of Adalimumab study. 52,53
| Trial name | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean DAS28 (SD) | DAS44 (SD) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | EULAR good/moderate/none | ACR 20/50/70 | Mean HAQ score (SD) | Joint erosion | Quality of life | |||||
| Optimisation of Adalimumab study52,53 | Routine care | 109 | 18 months | 6 months | 3.26b | NR | –6.4 (0.7)c,d | –7.8 (0.9)c,d | Good/moderate response: 56.9% | NR | –0.47 (0.06)c,d | NR | NR |
| DAS28 target | 100 | 6 months | 3.72b | NR | –7.6 (0.8)c,d | –9.0 (1.0)c,d | Good/moderate response: 62.0% | NR | –0.44 (0.06)c,d | NR | NR | ||
| SJC target | 99 | 6 months | 3.49b | NR | –6.9 (0.7)c,d | –7.6 (0.9)c,d | Good/moderate response: 62.5% | NR | –0.45 (0.05)c,d | NR | NR | ||
| Routine care | 109 | 12 months | 3.12b | NR | –6.5 (0.7)c,d | –8.4 (0.9)c,d | Good/moderate response: 51.4% | NR | –0.57 (0.06)c,d | NR | NR | ||
| DAS28 target | 100 | 12 months | 3.38b | NR | –8.3 (0.8)c,d | –9.4 (1.0)c,d | Good/moderate response: 61.0% | NR | –0.47 (0.06)c | NR | NR | ||
| SJC target | 99 | 12 months | 3.18b | NR | –7.2 (0.7)c,d | –7.8 (0.9)c,d | Good/moderate response: 50.5% | NR | –0.39 (0.06)c,d | NR | NR | ||
| Routine care | 109 | 18 months | 3.27b | NR | –6.5 (0.8)c,d | –8.0 (1.0)c,d | Good/moderate response: 35.8%e | NR | –0.49 (0.07)c,d | NR | NR | ||
| DAS28 target | 100 | 18 months | 3.40b | NR | –8.3 (0.8)c,d | –9.3 (1.0)c,d | Good/moderate response: 63.0%e | NR | –0.51 (0.07)c,d | NR | NR | ||
| SJC target | 99 | 18 months | 3.16b | NR | –6.4 (0.8)c,d | –7.4 (1.0)c,d | Good/moderate response: 53.5%e | NR | –0.41 (0.06)c,d | NR | NR | ||
In summary, there was no difference in the clinical effectiveness of different targets on the proportion of patients meeting the target, attaining LDA and attaining remission. 52 In addition, there was mainly no difference in the clinical effectiveness of different targets on disease activity outcomes. 52 Only the Optimisation of Adalimumab study52 demonstrated the benefit of a DAS28 of < 2.6 target over a SJC of 0 target and this was only for the proportion of patients with a EULAR moderate/good response.
Comparison of different treatment protocols
Heterogeneity in the treatment protocols used and outcomes reported precluded statistical meta-analysis. Because of the heterogeneity in treatment protocols, the data from trials in the comparison of different treatment protocols were not narratively combined and examined by outcome as with the previous two comparisons; instead, results are reported separately by trial.
Trials examining early rheumatoid arthritis populations
Appendix 5, Table 75 summarises the TTT outcomes for the comparison of different treatment protocols for the early RA population. All seven trials reported the proportion of patients meeting the target. In the BeSt trial, significantly higher proportions of patients met the target in the initial combination therapy with prednisone (PDN) (71%) and initial combination therapy with IFX (74%) arms than in the sequential monotherapy arm (53%) at 12 months. 30 However, at the 7- and 8-year follow-ups, the numbers meeting the target were similar across the four arms. 28,29 In CareRA trial, the proportion of patients meeting the target was not significantly different between the COBRA Classic, COBRA Slim and COBRA Avant-Garde arms among the high-risk patients,42,43 nor between the methotrexate tight step-up (MTX-TSU) and COBRA Slim arms40,43 at 16 or 52 weeks. In COBRA-light, there was no significant difference in the proportion meeting the target between the different treatment protocol arms at 13 weeks and 6 or 12 months. 44,45 In the FIN-RACo trial, the proportion of participants meeting the target was significantly higher in the combination treatment arm (37%) than in the single-drug arm (18%) at 246 and 11 years47 (37% and 19%, respectively), but not at 5 years (29% and 22%, respectively). 47 In the Saunders et al. 54 trial, the proportions of patients meeting the target in the step-up therapy and parallel triple-therapy arms at 12 months were not significantly different. In the TEAR trial, the proportion of patients meeting the target was similar for the step-up ETN (52.9%) and step-up triple-therapy (56.5%) arms at 12 weeks, but lower for both the step-up etanercept and step-up triple-therapy arms combined (28%) than the immediate etanercept and immediate triple-therapy arms (41% and 43%, respectively) at 24 weeks. 58 There was no significant difference in patients attaining the LDA target among arms using different treatment protocols in the high- or low-risk patient populations in the CareRA trial at 52 weeks. 43 In the U-Act-Early trial, a significantly greater proportion of patients met the target in the TOC plus MTX (86%) and TOC plus PBO–MTX (88%) arms than the MTX plus PBO–TOC arm (77%) at 104 weeks. 62
Five trials reported the proportion of patients with LDA at follow-up in each arm. In the BeSt trial, significantly higher proportions of patients attained LDA in the initial combination therapy with PDN (71%) and initial combination therapy with IFX (74%) arms than in the sequential monotherapy arm (53%) at 12 months,30 but at 7- and 8-year follow-up the proportions attaining LDA were similar across the four arms. In the CareRA trial, the proportion of patients attaining LDA was not significantly different between the COBRA Classic, COBRA Slim and COBRA Avant-Garde arms among the high-risk patients,42,43 nor between the MTX-TSU and COBRA Slim arms among the low-risk patients40,43 at 16 and 52 weeks. In the Saunders et al. 54 trial, there was no significant difference between the proportions of patients attaining LDA in the step-up therapy and parallel triple-therapy arms at 12 months. In the TEAR trial, a significantly greater proportion of patients in each of the immediate ETN (41%) and immediate triple-therapy (43%) arms attained LDA according to DAS28 criteria than both step-up arms combined at 24 weeks (28%). 58
All seven trials reported the proportion of patients attaining remission at follow-up in each arm. In the BeSt trial, the proportions of patients attaining remission in the four arms were not significantly different at 7 or 8 years. 28,29 Among the high-risk patients in the CareRA trial, the proportions of patients attaining remission at 4, 8, 16 and 52 weeks were similar among the different treatment protocol arms. 42,43 Similarly, among the low-risk patients in the CareRA trial, the proportion of patients attaining remission at 16 and 52 weeks was not significantly different between the COBRA Slim and MTX-TSU arms. 40,43 We noted some discrepancies between the data reported in an abstract and the full text for the CareRA trial. In the De Cock et al. 38 abstract, the percentage of patients attaining remission is different from that reported in the Verschueren et al. 42 paper for the COBRA Classic and COBRA Avant-Garde arms among the high-risk patients. We have used data from the full text42 in our synthesis. In the COBRA-light trial, there was no significant difference between the different treatment protocol arms in the proportion of patients attaining remission at 13 weeks and at 6 or 12 months. 44,45 In the FIN-RACo trial, the proportion of participants attaining remission was significantly higher in the combination treatment arm than in the single-drug arm at 246 (37% vs. 18%) and 11 years47 (37% vs. 19%), but not at 5 years (29% vs. 22%). 47 In the Saunders et al. 54 trial, there was no significant difference between the proportions of patients attaining remission in the step-up therapy and parallel triple-therapy arms at 12 months. In the TEAR trial, the proportions of participants attaining remission at 102 weeks were not significantly different between the step-up etanercept, step-up triple-therapy, immediate etanercept and immediate triple-therapy arms. 59 In the U-Act-Early trial, the proportion of patients attaining remission at 104 weeks was significantly greater in the TOC plus MTX (86%) arm and TOC plus PBO–MTX (88%) arm than in the MTX plus PBO–TOC arm (77%). 62
The disease activity outcomes for the trials comparing different treatment protocols for the early RA population are summarised in Appendix 5, Table 73.
In the BeSt trial, the proportion of patients who attained ACR 20 and ACR 70 response was greater in the initial combination therapy with PDN and initial combination therapy with IFX arms than in the sequential monotherapy and step-up combination therapy arms at 3, 6 and 12 months; however, the statistical significance of this comparison was not reported. 30 The Dutch version of the Health Assessment Questionnaire (D-HAQ) mean score was significantly lower in the initial combination with PDN and initial combination with IFX arms than in the sequential monotherapy and step-up combination therapy arms at 3, 6 and 9 months, and significantly lower in the initial combination with PDN and initial combination with IFX arms than in the sequential monotherapy arm at 12 months. 30 At 5 years, the mean HAQ scores were significantly lower in the initial combination therapy with IFX arm than in the three other arms, and also in the initial combination with PDN arm than in the sequential monotherapy and step-up combination therapy arms. 31 Similarly, at 7 years, HAQ scores were significantly lower in the initial combination with IFX arm than in the sequential monotherapy and step-up combination therapy arms. 29 In addition, at 8 years, the mean HAQ scores were significantly lower in the initial combination therapy with IFX arm than in the step-up combination therapy arm. 28 There were no significant differences between HAQ scores at 10 years. 64 The SHS erosion score and total SHS at 12 months were significantly lower in the initial combination with PDN [0.9 (SD 1.9) and 2.0 (SD 3.6), respectively] and initial combination with IFX arms [0.7 (SD 2.1) and 1.3 (SD 4.0), respectively] than in the sequential monotherapy [3.5 (SD 8.2) and 7.1 (SD 15.4), respectively] and step-up combination therapy arms [2.6 (SD 4.7) and 4.3 (SD 6.5), respectively)] . 30 However, there were no differences among the arms in median change in SHS or mean SHS estimates corrected for baseline SHS at 10 years. 64 The mean improvement from baseline in the SF-36 Physical Components score at 3 and 6 months was significantly greater in the initial combination therapy with PDN (11.2 and 12.5, respectively) and the initial combination therapy with IFX (9.6 and 12.4, respectively) arms than in the sequential monotherapy (5.8 and 8.0, respectively) and the step-up combination therapy (3.9 and 8.5, respectively) arms, with no significant differences between groups in SF-36 Mental Components score at 3 or 6 months and no significant differences between groups in either SF-36 score at 12 months30 or 2 years. 26 DAS28, DAS44, SJC, TJC and EULAR response were not reported in the BeSt trial.
In the CareRA trial, there was no significant difference in change from baseline in DAS28-CRP between the COBRA Classic, COBRA Slim and COBRA Avant-Garde arms among the high-risk patients42 and in the MTX-TSU and COBRA Slim arms among the low-risk patients40 at 16 or 52 weeks. 43 There were no significant differences in the proportion of patients with a EULAR good response and EULAR moderate response between the COBRA Classic, COBRA Slim and COBRA Avant-Garde arms among the high-risk patients42 and between the MTX-TSU and COBRA Slim arms among the low-risk population40 at 16 or at 52 weeks. 43 We noted some discrepancies between the data reported in an abstract and the full text. In the De Cock et al. 38 abstract, the percentage of patients with a good EULAR response is different from the percentage reported in the Verschueren et al. 42 paper in all high-risk arms. We have used data from the full texts in our synthesis. Among the high-risk patients, similar proportions of patients in the COBRA Classic, COBRA Slim and COBRA Avant-Garde arms had a clinically meaningful HAQ response and a HAQ of 0 at 16 weeks. 42 Among the low-risk patients, the proportion of patients who had a HAQ score of 0 at 16 weeks was significantly greater in the MTX-TSU arm (51.2%) than in the COBRA Slim arm (23.4%), although there were no significant differences between arms in mean HAQ change at 16 or 52 weeks40,43 or in the proportion of patients with clinically meaningful HAQ change at 16 weeks. 40 The change in SHS was not significantly different between arms using different treatment protocols in the high- and low-risk populations at 52 weeks. 43 The DAS44, SJC, TJC, ACR 20/50/70 response and quality of life were not reported in the CareRA trial.
In the COBRA-light trial, there was no significant difference in mean DAS28 at 12 months between the COBRA and COBRA-light arms. There was no significant difference in mean DAS44 scores, change from baseline in DAS44 or change from baseline in the Disease Activity Score, 44 joints with C-reactive protein concentration (DAS44-CRP) between the COBRA and COBRA-light arms at 6 or 12 months. 44,45 There was no significant difference in SJC or TJC at 12 months between the COBRA and COBRA-light arms. 45 There was no significant difference in the proportion of patients attaining a EULAR good response or EULAR non-response between the COBRA and COBRA-light arms at 13 weeks or 6 months. 44 There was no significant difference in the proportion of patients with ACR 70 in the COBRA and COBRA-light arms at 12 months. 45 There was no significant difference in mean HAQ scores between the COBRA and COBRA-light arms at 3, 6 or 12 months. 44,45 The difference in change in SHS erosion score between the COBRA [0.18 (SD 0.4)] and COBRA-light [0.30 (SD 0.8)] arms at 12 months approached statistical significance (p = 0.05). 45 There were no significant differences in mean SHS JSN scores or total SHSs at 12 months between the COBRA and COBRA-light arms. 45 Quality of life was not reported in the COBRA-light trial.
In the FIN-RACo trial,46–49 there was a significant treatment effect over time (baseline to 11-year follow-up) for DAS28 in the combination treatment arm compared with the single-drug arm, although the difference between arms at 11 years was not statistically significant. 49 There was no significant difference in mean change from baseline in the SJC between the combination treatment and single-drug treatment arms at 2 years, or in median SJC at 11 years. 49 There was no significant difference in mean change from baseline in the TJC between the combination treatment and single-drug arms at 2 years,46 or in median TJC at 11 years. 49 There was no significant difference between the combination treatment and single-drug arms in the proportion of patients meeting ACR 20 response criteria at 6 months or 2 years;46 however, the proportion of patients who met ACR 50 response criteria at 2 years was significantly greater in the combination treatment arm than in the single-drug arm. 46 There was no significant difference between the combination treatment and single-drug arms in the mean HAQ score at 2,46 547 or 1149 years, the mean change from baseline in Stanford HAQ score at 2 years46 or the proportion of patients with a HAQ score of 0 or a HAQ score of > 1 at 11 years. 47 There was a significantly lower median number of eroded joints (2 eroded joints, IQR 0–5 eroded joints) and a significantly lower median Larsen score (4, IQR 0–4) in the combination treatment arm than in the single-drug arm (4 eroded joints, IQR 2–7 eroded joints; Larsen score 12, IQR 4–20) at 2 years. 46 There was also a significantly greater mean increase from baseline in Larsen score in the single-drug arm (27, 95% CI 22 to 33) than in the combination treatment arm (17, 95% CI 12 to 26) at 11 years,49 with no significant difference in the proportion of patients in both treatment arms having no erosive changes in large joints at this time point. The DAS44, EULAR response and quality of life were not reported in the FIN-RACo trial.
In the Saunders et al. 54 trial, there was no significant difference in mean change from baseline in DAS28 between the parallel triple-therapy and step-up therapy arms at 12 months. There was no significant difference in the proportion of patients with a EULAR good response between the parallel triple-therapy and step-up therapy arms at 12 months. There was no significant difference in the proportion of patients meeting the criteria for ACR 20/50/70 response between the parallel triple-therapy and step-up therapy arms at 12 months. There was no significant difference in mean change from baseline in HAQ score between the parallel triple-therapy and step-up therapy arms at 12 months. There was no significant difference in mean change from baseline in SHS erosion score, SHS JSN score or total SHS between the parallel triple-therapy and step-up therapy arms at 12 months. There was no significant difference in mean change from baseline in Short Form questionnaire-12 items (SF-12) score between the parallel triple-therapy and step-up therapy arms at 12 months. The DAS44, SJC and TJC were not reported in the Saunders et al. 54 trial.
In the TEAR trial, there were no significant differences in the DAS28-ESR between the immediate ETN, immediate triple-therapy, step-up ETN and step-up triple-therapy arms at 102 weeks. 58 The mean SJC was slightly higher in the step-up ETN [4.4 (SD 3.1)] and step-up triple-therapy [4.4 (SD 2.8)] arms than in the immediate ETN [2.2 (SD 3.9)] and immediate triple-therapy [2.3 (SD 3.3)] arms at 102 weeks, although the statistical significance between groups was not reported. 58 The mean TJC was similar in the immediate ETN, immediate triple-therapy, step-up ETN and step-up triple-therapy arms at 102 weeks. 58 The proportion of patients achieving ACR 20/50/70 response at 6 months was significantly higher in the immediate ETN (56.28%, 32.14% and 11.13%, respectively) and immediate triple-therapy (55.88%, 31.33% and 7.97%, respectively) arms than in the step-up ETN (40.12%, 19.51% and 2.84, respectively) and step-up triple-therapy (39.32%, 17.53% and 3.62, respectively) arms. 58 There was no significant difference in ACR 20/50/70 response between the four different treatment arms at 2 years (although there was a significant effect of drug, in favour of ETN over triple-therapy in terms of ACR 70 response at 2 years). 58 There was no significant difference in mean mHAQ scores between the immediate ETN, immediate triple-therapy, step-up ETN and step-up triple-therapy arms at 102 weeks. 58 There was no significant difference in mean SHS erosion score, SHS JSN score or total SHS between the immediate ETN, immediate triple-therapy, step-up ETN and step-up triple-therapy arms at 102 weeks. 58 The DAS44, EULAR response and quality of life were not reported in the TEAR trial. 58
There were no significant differences in the DAS28 change from baseline across the study arms in the U-Act-Early trial at 24, 52 or 104 weeks. 62 Patients in the MTX plus PBO–TOC arm had a significantly higher mean SJC [3.0 (SD 4.4)] and TJC [3.7 (SD 4.8)] than in the TOC plus MTX arm [1.0 (SD 2.1) and 2.8 (SD 4.9), respectively] and the TOC plus PBO–MTX arm [1.6 (SD 2.8) and 3.0 (SD 3.9), respectively] at 24 weeks, but there were no significant differences between the arms with different treatment protocols at 52 or 104 weeks. 62 A significantly greater proportion of patients in the TOC plus MTX (89%) and TOC plus PBO–MTX (87%) arms attained a EULAR good response at 24 weeks than in the MTX plus PBO–TOC arm (49%). In addition, the proportion of patients who attained a EULAR moderate response at 24 weeks was significantly greater in the MTX plus PBO–TOC arm (32%) than in the TOC plus MTX (5%) and TOC plus PBO–MTX (11%) arms. 62 The proportion of patients who attained a EULAR good response at 52 weeks was significantly greater in the TOC plus PBO–MTX arm (88%) than in the TOC plus MTX (75%) arm or in the MTX plus PBO–TOC (72%) arm; however, at 104 weeks there were no significant differences between arms receiving different treatment protocols. 62 The proportions of patients who attained ACR 20/50/70/90 responses at 24 weeks and an ACR 90 response at 52 weeks was significantly greater in the TOC plus MTX and TOC plus PBO–MTX arms than in the MTX plus PBO–TOC arm; however, there were no significant differences across arms in terms of ACR 20/50/70/90 at 104 weeks. 62 The three groups were significantly different on mean HAQ score at 24 weeks, with a lower score in the TOC plus MTX arm [0.50 (SD 0.55)] than in the TOC plus PBO–MTX arm [0.63 (SD 0.66)] or the MTX plus PBO–TOC [0.65 (SD 0.54)] arm, with no significant differences at 52 or 104 weeks. 62 There was a significantly smaller mean increase in SHS in the TOC plus MTX arm [0.50 (SD 1.50)] than in the MTX plus PBO–TOC arm [0.96 (SD 2.87)] at 52 weeks, and a significantly smaller mean increase in SHS in the TOC plus MTX arm [1.18 (SD 3.92)] and the TOC plus PBO–MTX arm [1.45 (SD 4.27)] than in the MTX plus PBO–TOC arm [1.53 (SD 2.42)] at 104 weeks. 62 The DAS44 and quality of life were not reported in the U-Act-Early trial. 62
Other comparisons
Trials examining early rheumatoid arthritis populations
The CAMERA35,36,67 trial employed a conventional strategy group and an intensive strategy group, which used (different) compound improvement-based targets, different frequencies of assessment and the same treatment protocol. The CAMERA35,36,67 trial did not report the proportions of patients attaining the target or attaining LDA at 1- or 2-year follow-up points (Table 30). The proportions of patients attaining remission at 1 and 2 years were significantly higher in the intensive strategy group (35% and 50%, respectively) than in the conventional strategy group (14% and 37%, respectively). 36
| Trial acronym | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Number completing, n/N (%) (randomised phase) | Definition of study target | Number (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Meeting study target | Attaining LDA (criterion) | Attaining remission (criterion) | |||||||
| CAMERA35,36,67 | Intensive strategy group | 151 | 2 years | 1 year | NA | Clinical response (see Table 15) | NR | NR | 53/151 (35)b (≥ 1 period of remission)c |
| Conventional strategy group | 148 | 1 year | NA | Clinical and laboratory response (see Table 15) | NR | NR | 21/148 (14)b (≥ 1 period of remission)c | ||
| Intensive strategy group | 151 | 2 years | 92/151 (61) | Clinical response (see Table 15) | NR | NR | 76/151 (50)d (≥ 1 period of remission)c | ||
| Conventional strategy group | 148 | 2 years | 113/148 (76) | Clinical and laboratory response (see Table 15) | NR | NR | 55/148 (37)d (≥ 1 period of remission)c | ||
The mean SJC and TJC were slightly lower in the intensive strategy group than in the conventional strategy group at 6 months, 1 and 2 years, although the statistical significance of these comparisons was not reported (Table 31). 36 The proportion of patients with a good EULAR response was a significantly higher in the intensive strategy group (48%) than in the conventional strategy group (25%) and the proportion of patients with no EULAR response was significantly smaller in the intensive strategy group (12%) than in the conventional strategy group (32%), with no significant difference between groups in the proportion of patients with a moderate EULAR response at 6 months. 67 The proportion of patients who attained an ACR 50 response at 1 year was significantly greater in the intensive strategy group (58%) than in the conventional strategy group (43%), although there was no significant difference in the proportion of patients with an ACR 50 response at 2 years between the intensive strategy group and the conventional strategy group. 36 There were similar mean changes from baseline in HAQ score in the intensive strategy group and the conventional strategy group at 1 and 2 years. 36 The Disease Activity Score (DAS), joint erosion and quality of life were not reported.
| Trial acronym | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean DAS28 (SD) | DAS44 (SD) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | EULAR good/moderate/none | ACR 20/50/70 | Mean HAQ score (SD) | Joint erosion | Quality of life | |||||
| CAMERA35,36,67 | Intensive strategy group | 151 | 2 years | 6 months | NR | NR | 3.76b,c | 4.83b,c | Good 67 (48%);d moderate 55 (40%); none 16 (12%);d n = 151 | NR | 0.82b,c | NR | NR |
| Conventional strategy group | 148 | 6 months | NR | NR | 7.44b,c | 7.93b,c | Good 34 (25%);d moderate 58 (43%); none 43 (32%);d n = 148 | NR | 0.92b,c | NR | NR | ||
| Intensive strategy group | 151 | 1 year | NR | NR | 3.11;b,c –11 (8)e | 3.96;b,c –11 (7)e | NR | ACR 50: 87/151 (58%)f | 0.78;b,c –0.44 (0.59)e | NR | NR | ||
| Conventional strategy group | 148 | 1 year | NR | NR | 5.29;b,c –9 (7)e | 5.74;b,c –8 (8)e | NR | ACR 50: 64/151 (43%)f | 0.83;b,c –0.39 (0.66)e | NR | NR | ||
| Intensive strategy group | 151 | 2 years | NR | NR | 6.87;b,c –11 (8)e | 4.87;b,c –0 (9)e | NR | ACR 50: 69/148 (46%) | 0.82;b,c –0.41 (0.64)e | NR | NR | ||
| Conventional strategy group | 148 | 2 years | NR | NR | 6.87;b,c –11 (8)e | 4.39;b,c –9 (8)e | NR | ACR 50: 67/148 (45%) | 0.78;b,c –0.42 (0.76)e | NR | NR | ||
Trials examining established rheumatoid arthritis populations
The BROSG9 trial had two arms: intensive management, which used a DAS44 of ≤ 2.4 target and employed a seven-step treatment protocol based on the target, and in which patients were seen in a hospital setting; and a routine management arm, which had no target and patients were seen in the community as well as in the clinic, with treatment escalation based on clinical opinion. The BROSG trial did not report the proportions of patients attaining the target or attaining LDA or remission (Table 32). By the end of the trial (3 years’ follow-up), 77.1% of patients in the aggressive therapy arm had some change in disease suppressive treatment, compared with 55.9% in the symptomatic treatment arm (significance not reported). 9
| Trial | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Number completing, n/N (%) (randomised phase) | Definition of study target | Number (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Meeting study target | Attaining LDA (criteria) | Attaining remission (criteria) | |||||||
| BROSG trial9 | Symptomatic treatment (shared care) | 233 | 3 years | 3 years | 197/233 (85) | To control joint pain, stiffness and related symptoms | NR | NR | NR |
| Aggressive therapy (hospital) | 233 | 3 years | 202/233 (87) | To control joint pain, stiffness and related symptoms, and to suppress clinical and laboratory evidence of inflammation | NR | NR | NR | ||
| CRP concentration below twice the upper limit of normal | |||||||||
The mean SJC, TJC and HAQ scores were similar in the symptomatic treatment arm and aggressive therapy arm at 3 years in the BROSG trial (Table 33). 9 No other disease activity outcomes were reported.
| Trial | Treatment arm | Number of participantsa | Duration of randomised phase | Follow-up time point | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean DAS28 (SD) | Mean DAS44 (SD) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | EULAR good/moderate/none | ACR 20/50/70 | Mean HAQ score (SD) | Joint erosion | Quality of life | |||||
| BROSG trial9 | Symptomatic treatment (shared care) | 233 | 3 years | 3 years | NR | NR | 28 joint count: 3.2 (3.8) | 28 joint count: 5.0 (5.9) | NR | NR | 1.40 (0.73) | Larsen score: 78.6 (39.4)b | NR |
| Eroded joint count 13.1 (7.8);b n = 171 | |||||||||||||
| Aggressive therapy (hospital) | 233 | 3 years | NR | NR | 28 joint count: 2.7 (2.9) | 28 joint count: 4.4 (5.7) | NR | NR | 1.45 (0.76) | Larsen score 77.9 (42.4)b | NR | ||
| Eroded joint count 13.4 (8.2);b n = 176 | |||||||||||||
Adverse events
Comparison of treat to target with usual care
Two studies compared TTT with usual care in the early RA population (Table 34). In the STREAM trial,55 there were significantly more AEs at 2 years in the aggressive group (62) than in the conventional care group (35). However, there was no significant difference between the TTT and usual-care arms in the proportion of patients who experienced any AE at 2 years. 55 The proportion of patients with any AE was similar in the routine care and targeted arms of the T-4 study at 56 weeks. 57 The proportion of patients with SAEs was not significantly different between the usual-care and TTT arms in the T-4 study, and was similar in terms of number of deaths and withdrawals as a result of AEs at 56 weeks. 57
| Trial acronym or first author and year of publication | Treatment arm | Safety population, n | Follow-up time point | AE, n/N (%) | |||
|---|---|---|---|---|---|---|---|
| Any AEa | Any SAEa,b | Death | Withdrawal as a result of AEsa | ||||
| Early RA population | |||||||
| STREAM55 | Aggressive group | NR | 2 years | 59% patients had 62 AEsc | Five SAEs, including one patient hospitalised once and one hospitalised twice for drug-related SAEs | None reported | 0 |
| Conventional care | NR | 2 years | 42% patients had 35 AEsc | Three SAEs, including one patient hospitalised once for a drug-related SAE | None reported | 0 | |
| T-4 study56,57 | Routine care | 61 | 56 weeks | 41/NR (67) | Malignancy, n = 1 (1.6); serious infection, n = 0 (0) | 0 (0) | 6 |
| DAS28-driven therapy | 59 | 56 weeks | 49/NR (81) | Death, n = 0 (0); malignancy, n = 0 (0); serious infection, n = 0 (0) | 0 (0) | 3 | |
| MMP-3-driven therapy | 59 | 56 weeks | 46/NR (78) | Malignancy, n = 0 (0); serious infection, n = 1 (1.7) | 2 (3.4) | 6 | |
| DAS28 and MMP-3-driven therapy | 61 | 56 weeks | 42/NR (69) | Malignancy, n = 1 (1.6); serious infection, n = 0 (0) | 0 (0) | 2 | |
| Established RA population | |||||||
| Fransen et al., 200550 | DAS28 | 205 | 24 weeks | NR | NR | NR | NR |
| Usual care | 179 | 24 weeks | NR | NR | NR | NR | |
| Optimisation of Adalimumab study52,53 | Routine care | 100 | 18 months | NR | NR | NR | 10 (10) |
| DAS28 target | 109 | 18 months | NR | NR | NR | 12 (9.08)d | |
| SJC target | 99 | 18 months | NR | NR | NR | 4 (4) | |
| Combined early and established population | |||||||
| TICORA61 | Intensive management | 55 | 18 months | 32/55 (58) patients had 46 AEs | NR | 1/55 (2) | 20/129 (16), new DMARD courses were stopped because of toxic effects |
| Routine management | 55 | 18 months | 42/55 (76) patients had 85 AEs | NR | 3/55 (5) | 38/89 (43), new DMARD courses were stopped because of toxic effects | |
| van Hulst et al., 201063 | Intervention group | AEs NR | NR | NR | NR | NR | NR |
| Usual-care group | AEs NR | NR | NR | NR | NR | NR | |
Appendix 5, Table 74 summarises the specific AEs reported in trials of TTT compared with usual care in the early RA population. There was no difference in the proportion of patients with serious infection between any of the TTT arms and usual care in the T-4 study at 56 weeks. 57 No other specific AEs were reported in the T-4 study57 or STREAM trial. 55
In summary, among trials examining an early RA population, there was no difference in the proportion of patients experiencing any AE,55,57 SAE,57 death,57 withdrawals as a result of AEs57 or specific AEs;57 however, more events (any AE) were experienced in the TTT arm than in the usual-care arm in the STREAM trial. 55
Impact of target among trials comparing treat to target with usual care in an early rheumatoid arthritis population
Among trials with early RA populations that used a remission target (a DAS44 of < 1.655 and a DAS28 of < 2.657), there was mixed evidence in terms of the proportion of patients with any AE, with no difference in the proportion of patients with a SAE,57 deaths,57 withdrawals as a result of AEs57 and specific AEs (serious infection57). The STREAM55 trial found fewer AEs in the usual-care arm at 2 years, but no difference between arms in the proportions of patients with any AE at 2 years. The T-4 study57 also found no difference between the remission-targeted arm and usual care at 56 weeks in the proportions of patients with any AE.
One trial, the T-4 study,57 examining a MMP-3 normalisation target in an early RA population, found no difference between the MMP-3 normalisation-targeted arm and usual care at 56 weeks in the proportions of patients with any AE, the proportion of patients with a SAE, death, withdrawals as a result of AEs and specific AEs (serious infection).
Similarly, the T-4 study,57 examining a combined remission and MMP-3 normalisation target in an early RA population, found no difference between the MMP-3 normalisation-targeted arm and usual care at 56 weeks in the proportions of patients with any AE, the proportion of patients with a SAE, deaths, withdrawals as a result of AEs and specific AEs (serious infection).
In summary, comparing trials by target on AEs, there is no clear evidence in favour of any target being more or less safe than usual care in an early RA population.
Table 34 summarises the AEs among trials of patients with established RA comparing TTT with usual care. The proportions of patients with any AE or a SAE or who died were not reported in either the Fransen et al. 50 trial or the Optimisation of Adalimumab study. 52 In the Optimisation of Adalimumab study, the proportion of patients who withdrew because of AEs was lower in the SJC-targeted arm(4%) than in the routine care arm (10%), although statistical significance was not reported. 52
Appendix 6, Table 75 summarises the specific AEs reported in trials of TTT compared with usual care in an early RA population. The Fransen et al. 50 trial reported that the proportion of patients with dermatological AEs (rash or itching) was significantly greater in the usual-care arm (11%) than in the TTT arm (4%). In addition, the proportion of patients who at 24 weeks were reported to have experienced a gastrointestinal AE was significantly greater in the usual-care arm (9%) than in the TTT arm (4%). Specific AEs were not reported in the Optimisation of Adalimumab study. 52,53
In summary, the findings of trials examining established RA populations suggest that TTT may be more beneficial to patients than usual care in terms of AE outcomes. A smaller proportion of patients withdrew because of AEs52 and experienced specific AEs (dermatological and gastrointestinal AEs50) in the TTT arm than in the usual-care arm.
Impact of target among trials comparing treat to target with usual care in an established rheumatoid arthritis population
One trial, Fransen et al. ,50 examining a LDA target (DAS28 of ≤ 3.2) in an established RA population, found evidence in favour of TTT in terms of specific AEs at 24 weeks (dermatological and gastrointestinal AEs).
One trial, the Optimisation of Adalimumab study,52 examining a remission target in an established RA population, found no difference in the number and proportion of patients who withdrew from the trial because of AEs between the remission-targeted arm (DAS of < 2.6) and the usual-care arm, although the statistical significance of this comparison was not reported.
The one trial, the Optimisation of Adalimumab study,52 examining a SJC of 0 target in an established RA population found evidence in favour of TTT in terms of withdrawals as a result of AEs, although the statistical significance of this comparison was not reported.
In summary, there is evidence in favour of the safety of TTT, using a LDA target, compared with usual care in an established RA population,50 but of a difference between targeted arms and usual care with regard to remission (DAS28 of < 2.6) and SJC (SJC of 0) targets,52 although only one trial used each of these targets and this related to only one outcome reported in the case of the LDA target. 50 The remission target and SJC target impacted on the same outcomes in the same way, although findings were from the same (single) trial (i.e. the Optimisation of Adalimumab study52).
Table 34 summarises the AEs among trials with a population of patients with early and established RA comparing TTT with usual care. In the TICORA trial,61 the proportion of patients who experienced any AE was greater in the usual-care arm (76% experienced 85 AEs) than in the TTT arm (58% experienced 46 AEs; statistical significance not reported), and at 18 months the proportion in each arm who had died was similar to that in the TTT arm. AEs were not reported for the van Hulst et al. trial. 63
Appendix 6, Table 76 summarises the specific AEs reported in trials of TTT compared with usual care in the early RA population. The TICORA61 trial reported only numbers of AEs and not the numbers and/or proportions of patients with AEs. There were slightly more dermatological, gastrointestinal and infectious AEs in the usual-care arm than in the TTT arm, although the statistical significance of this comparison was not reported. Specific AEs were not reported in the van Hulst et al. trial. 63
In summary, the one trial61 reporting on AE outcomes in a population including both patients with early RA and those with established RA reported mixed findings in terms of AE outcomes. The TICORA trial,61 which was the only trial rated as being at a low risk of bias, reported that a smaller proportion of patients reported any AE and specific AEs (dermatological, gastrointestinal and infectious AEs, significance not reported) in the TTT arm than in the usual-care arm; however, there was no difference in the number of deaths between the TTT and usual-care arms. 61
Impact of target among trials comparing treat to target with usual care in a population containing both early and established rheumatoid arthritis patients
The one trial61 reporting on AE outcomes in a population including both patients with early RA and those with established RA examined a LDA target (DAS44 of ≤ 2.4). Thus, the findings described and summarised above relate only to a LDA target for this comparison and this population group.
Comparison of different targets
The Hodkinson et al. 51 trial did not report AEs. In the two trials comparing different targets in an early RA population, the T-4 study57 and TEAR58 (Table 35), the proportions of patients with AEs or SAEs and of deaths and withdrawals due to AEs were similar across arms of different targets, including a DAS28 of < 2.6; a MMP-3 concentration of < 121 ng/ml for men and < 59.7 ng/ml for women; a DAS28 of < 2.6 and a MMP-3 concentration of < 121 ng/ml for men or < 59.7 ng/ml for women; no target; and a DAS28-ESR of < 3.2.
| Trial acronym or first author and year of publication | Treatment arm | Safety population, n | Follow-up time point | AE in source, n/N (%) | |||
|---|---|---|---|---|---|---|---|
| Any AEa | Any SAEa,b | Death | Withdrawal as a result of AEsa | ||||
| Early RA population | |||||||
| Hodkinson et al., 201551 | SDAI arm | AEs NR | NR | NR | NR | NR | NR |
| CDAI arm | AEs NR | NR | NR | NR | NR | NR | |
| T-4 study56,57 | Routine care | 61 | 56 weeks | 41 (67.2) | Death, n = 0 (0); malignancy, n = 1 (1.6); serious infection, n = 0 (0) | 0 (0) | 6 |
| DAS28-driven therapy | 59 | 56 weeks | 49 (81.4) | Death, n = 0 (0); malignancy, n = 0 (0); serious infection, n = 0 (0) | 0 (0) | 3 | |
| MMP-3-driven therapy | 59 | 56 weeks | 46 (78.0) | Death, n = 2 (3.4); malignancy, n = 0 (0); serious infection, n = 1 (1.7) | 2 (3.4) | 6 | |
| DAS28 and MMP-3-driven therapy | 61 | 56 weeks | 42 (68.9) | Death, n = 0 (0); malignancy, n = 1 (1.6); serious infection, n = 0 (0) | 0 (0) | 2 | |
| TEAR58–60 | Immediate ETN | 244 | 102 weeks | 193 (79.1) | 35 (14.3) | 1 | 12 (4.9): SAE, n = 8; AE, n = 3; death, n = 1 |
| Immediate triple therapy | 132 | 102 weeks | 101 (76.5) | 18 (13.6) | 1 | 7 (5.3): SAE, n = 2; AE, n = 4; death, n = 1 | |
| Step-up ETN | 255 | 102 weeks | 187 (73.3) | 32 (12.5) | 2 | 9 (3.5): SAE, n = 2; AE, n = 5; death, n = 2 | |
| Step-up triple therapy | 124 | 102 weeks | 92 (74.2) | 16 (12.9) | 0 | 4 (3.2): SAE, n = 2; AE, n = 2; death, n = 0 | |
| Established RA population | |||||||
| Optimisation of Adalimumab study52,53 | Routine care | 100 | 18 months | NR | NR | NR | 10 (10) |
| DAS28 target | 109 | 18 months | NR | NR | NR | 12 (9.08)c | |
| SJC target | 99 | 18 months | NR | NR | NR | 4 (4) | |
Appendix 5, Table 72 summarises the specific AEs reported in trials comparing different targets in the early RA population. The T-4 study57 reported no difference in the proportion of patients with serious infection between the DAS28-, MMP-3- and combined DAS28 and MMP-3-targeted arms at 56 weeks. No significant differences in the number of specific AEs were reported in the TEAR trial at 102 weeks. 58
In summary, among trials comparing different targets in an early RA population, different targets made no difference to the proportions of patients with any AE,57,58 SAEs,57,58 deaths,57,58 withdrawals as a result of AEs57,58 and specific AEs. 57,58
Table 35 summarises the AEs among trials of patients with established RA comparing different targets. In the Optimisation of Adalimumab study,52 the proportion of patients who withdrew because of an AE was greater in the DAS28 of < 2.6-targeted arm (9%) than in the SJC-targeted arm (4%), although statistical significance was not reported. The proportions of patients with any AE, a SAE or who died were not reported. No specific AEs were reported.
Comparison of different treatment protocols
Tables 73 and 74 in Appendix 5 examine AEs among trials of patients with early RA comparing different treatment protocols.
In the BeSt trial, the proportions of patients across the four arms (varying in treatment protocol) who had experienced any AE or a SAE were similar at 1 year30 and 5 years. 31 Similar proportions of patients died in each arm at 531 and 10 years,33 although this proportion was slightly greater in the two initial combination therapy arms (15.8% and 15.6%), than in the sequential monotherapy and step-up combination therapy arms (12.7% and 12.4%, respectively; statistical significance not reported). Withdrawals as a result of AEs were not reported. Similar proportions of patients had specific AEs in each treatment arm of the BeSt trial at 1 year (see Appendix 5, Table 74; significance not reported). 30 The proportion of patients with specific AEs were not significantly different across treatment arms at 5 years. 31
Among the high-risk patients in the CareRA trial, the proportions of patients with any AE at 16 weeks were significantly higher in the COBRA Classic (61.2%) and COBRA Avant-Garde (69.9%) arms than in the COBRA Slim (46.9%) arm. 42 Similar numbers of SAEs were reported in the three trial arms and similar proportions of deaths and withdrawals as a result of AEs across all arms were reported at 16 weeks. 42 Among the low-risk patients, there were no significant differences in the proportion of patients with any AE across arms at 16 weeks, with no SAEs in either arm. 40
The proportion of patients with any AE was not significantly different between arms using different treatment protocols in the high- and low-risk populations of the CareRA trial at 52 weeks; however, the mean number of AEs per patient differed significantly across groups in the high-risk population of the CareRA trial, with a higher mean number of AEs per patient in the COBRA Classic [1.9 (SD 2.0)] and COBRA Avant-Garde [1.9 (SD 1.6)] arms than in the COBRA Slim arm [1.3 [SD 1.4)] at 52 weeks. 43 The number of therapy-related SAEs was similar across arms in the high- and low-risk populations of the CareRA trial at 52 weeks. 43 There were no deaths in either arm of the low-risk population and similar proportions of patients died in the high-risk population. There were no withdrawals as a result of AEs in the high- or low-risk population of the CareRA trial. 43
Specific AEs were reported only in terms of numbers of events in the CareRA trial for the high-risk patients at week 16 and for the high- and low-risk patients at week 52 (rather than the proportion of patients experiencing each AE), and the statistical significance of comparisons across groups was not reported at 16 weeks. There were more dermatological AEs in the COBRA Classic and COBRA Avant-Garde arms than in the COBRA Slim arm at 16 weeks among the high-risk patients. 42 Among the low-risk patients, there were similar proportions of patients with gastrointestinal and infectious AEs at 16 weeks. 40 The numbers of dermatological, ophthalmological and infectious AEs were similar across trial arms in the high- and low-risk populations; however, there were more gastrointestinal AEs in the COBRA Avant-Garde arm (67) than in the COBRA Classic (45) and COBRA Slim (48) arms among the high-risk population at 52 weeks. 43 No other specific AEs were reported.
In the COBRA-light trial, similar proportions of patients had any AE (≥ 1) across the COBRA and COBRA-light arms at 6 months. 44 Twice as many patients in the COBRA-light arm as in the COBRA arm experienced a SAE (6 vs. 3) at 6 months44 and 12 months (9 vs. 16),45 although the statistical significance was not reported. Numbers of withdrawals as a result of AEs across arms were similar at 6 months. 44 In terms of specific AEs, in the COBRA-light trial, the proportion of patients who had dermatological AEs at 6 months was slightly greater in the COBRA-light arm (43%) than in the COBRA arm (37%), although statistical significance was not reported. 44 All other specific AEs were similar across trial arms.
In the FIN-RACo46 trial, the proportion of patients who had any AE or SAEs were similar in the combination treatment and single-drug arms at 2 years, with no deaths or withdrawals as a result of AEs in either arm at 2 years. There were no significant differences in the proportions of patients with specific AEs in the combination treatment and single-drug arms at 2 years.
In the Saunders et al. 54 trial, AEs were reported only in terms of number of AEs rather than as number and/or proportion of patients with AEs. Similar numbers of AEs were reported in the parallel triple-therapy and step-up therapy arms at 12 months, with similar numbers of drug withdrawals and no withdrawals from the trial in either arm. Numbers of specific AEs were similar in the parallel triple-therapy and step-up therapy arms.
In the TEAR trial,58 AEs were reported only in terms of numbers of AEs rather than as number and/or proportion of patients with AEs. Similar proportions of patients experienced any AE, a SAE, death or withdrawal as a result of AEs or specific AEs in the four trial arms (immediate ETN, immediate triple therapy, step-up ETN and step-up triple therapy) at 102 weeks.
The proportion of patients with any AE was not significantly different between arms using different treatment protocols in the U-Act-Early trial at 104 weeks. 62 The proportion of patients with a SAE was not significantly different between arms at 104 weeks, and there were no deaths in either arm. 62 There was no significant difference in withdrawals as a result of AEs across groups in the U-Act-Early trial. 62 The proportion of patients with specific AEs was similar across arms at 104 weeks. 62
Other comparisons
Trials examining early rheumatoid arthritis populations There were similar proportions of patients with any AE in the intensive strategy group and conventional strategy group in the CAMERA trial (Table 36). 36 More SAEs (graded as severe by the rheumatologist) were reported in the intensive strategy arm (n = 35) than in the conventional strategy arm (n = 10), although the statistical significance of this comparison was not reported. 35 Likewise, there was a greater proportion of withdrawals as a result of AEs in the intensive strategy arm (18%) than in the conventional strategy arm (7%), although the statistical significance was not reported. 36 In terms of specific AEs (see Appendix 6, Table 75), a significantly greater proportion of patients in the intensive strategy group experienced dermatological AEs (53.7%), gastrointestinal AEs (66.4%), central nervous system AEs (59.1%), hepatic AEs (55.0%) and haematological AEs (25.5%) than in the conventional strategy group (40.0%, 53.6%, 38.6%, 35.0% and 10.7%, respectively) at 2 years. 35
| Trial acronym | Treatment arm | Safety population, n | Follow-up time point | AE, n/N (%) | |||
|---|---|---|---|---|---|---|---|
| Any AEa | Any SAEa,b | Death | Withdrawal as a result of AEsa | ||||
| CAMERA35,36,67 | Intensive strategy group | 149 | 2 years | 142 (95) | 35 severe AEs reportedc | NR | 27/151 (18) |
| Conventional strategy group | 140 | 2 years | 126 (90) | 10 severe AEs reportedc | NR | 11/148 (7) | |
AEs were not reported for the BROSG trial. 9
Discussion
This section focuses on the main outcomes of the proportion of patients meeting the target, attaining LDA and attaining remission. Overall, across populations, there is no clear evidence either in favour of or against the clinical effectiveness of a TTT approach, in comparison with usual care, in terms of the proportion of patients meeting the target, attaining LDA or attaining remission. However, there does seem to be some limited support for TTT among the early RA population, in terms of remission, among the established RA population, in terms of LDA, and among trials that included populations of patients with both early and established RA, in terms of remission. Similarly, there is no clear evidence either in favour of or against the clinical effectiveness of a TTT approach, in comparison with usual care, in terms of the DAS28/DAS44 response, SJC, TJC, EULAR response, HAQ score, erosion and quality of life across populations, when overall findings are considered;50,52,55,57,61,63 however, there does seem to be some limited support for TTT on some clinical outcomes. Similarly, comparing trials by target, there is no clear evidence in favour of any target being more or less effective than usual care across population groups, as measured by the proportion of patients meeting the target, attaining LDA and attaining remission. There is also no clear evidence in favour of one particular target over all others in relation to DAS28/DAS44 response, SJC, TJC, EULAR response, HAQ score, erosion and quality of life across populations, when overall findings are considered. Specifically, we found no difference in the clinical effectiveness of different targets on DAS28/DAS44,51,57,58 TJC51,58 and HAQ score response51,58 among the trials reviewed and equivocal evidence for SJC,51,58 EULAR response,51,52 ACR 20/50/70 response58 and joint erosion. 57,58 All trials comparing different targets were rated as being at high risk of bias.
Adverse events appear to have been experienced differently by different population groups in the comparison of TTT with usual care. Among trials examining an early RA population, evidence was mixed, favouring the usual-care arm. Among trials examining established RA populations, the evidence favoured the TTT arm and was mixed in the only trial reporting on these outcomes in a population containing both early and established RA patients (i.e. the TICORA trial61), slightly favouring TTT.
Overall, when the evidence comparing trials by target on AEs is considered across all population groups, there is no clear evidence in favour of any target being more or less safe than usual care, or more or less safe than any other target. Caution is warranted, however, in interpreting these findings, given the small number of studies reporting AE data in each population group. In addition, the same AEs were not considered in all studies and AEs were recorded/reported seemingly erratically in the original papers, with little systematic consideration of AEs. Future trials should systematically record and report on an agreed range of AEs, to build the evidence base of AEs within TTT. This is an important issue to address, given that the intensity of TTT as a treatment plan is typically greater than that usual care.
The quality of the included studies will have inevitably had an impact on the findings of our review. The risk of bias, as assessed with the Cochrane risk-of-bias instrument,24 was rated as being high in six RCTs and three cluster RCTs, and unclear in five RCTs; this was generally because of the judgements for allocation concealment, blinded outcome assessment and attrition domains. Only one RCT (i.e. the TICORA trial61) was rated as being at low risk of bias.
In trials rated as being at high or unclear risk of bias, findings were generally equivocal or demonstrated no difference between arms. The one trial rated as being at low risk of bias,61 which compared TTT against usual care in a population that included both patients with early RA and those with established RA, by the criterion of this review (patients with a disease duration of < 5 years) demonstrated evidence that was generally in favour of a TTT approach, with favourable results in terms of the proportion of patients attaining remission, DAS44 response, change in SJC and TJC, EULAR good response, ACR 20/50/70 response, HAQ score progression, change in joint erosions (as measured by the SHS erosion score and total SHS) and SF-36 Physical Summary score (and no difference between TTT and usual care in terms of SHS JSN score and SF-36 Mental Health Summary score). 61
There are, however, some elements of the design of the TICORA trial61 that require consideration in interpreting findings. The TTT arm had more intensive assessments, more frequent visits and a higher dose of steroids than the usual-care arm; therefore, it is unclear whether the effects on outcomes were because of TTT or the increased assessment or escalation of therapy. The issue of unbalanced steroid use also applies to the FIN-RACo trial,46–49 in which more patients in the intensive treatment arm received prednisolone; as a result, it is difficult to untangle the effects of TTT from the effects of the steroid. Future trials are needed in which TTT is the only variable that differs between arms and in which all other aspects of care are held constant, as far as is practicable. In addition, the STREAM trial,55 which appears frequently in the current review because of the large number of outcomes it contributed, had a small sample size and uneven distribution of some baseline variables, such as rheumatoid factor (which was also relatively low at baseline).
The current systematic review is the most comprehensive review to date to examine the clinical effectiveness of TTT. We have synthesised findings on TTT compared with usual care and a comparison of different targets. Synthesis of different treatment protocols was precluded (even in terms of a narrative integration of findings) by heterogeneity and lack of comparability between treatment protocols, although we have provided a comprehensive summary of the findings of trials that compare different treatment protocols. The current review is also the only systematic review to examine findings by population, which is important in this context as the recommendations for TTT differ slightly for early and established RA patients, as the treatment prognosis and implications of TTT may be different in these populations. 17,18 Another strength of the current review is the focus on RCTs, which reduces the impact of selection bias on the review findings.
The main limitation of the current review is the small number of trials within each comparison, in each population group. Trials showed much heterogeneity in targets, treatment protocols, frequency of contact, outcomes and follow-up time points, even within the same population group, in each comparison. Risk of bias was rated to be high in the majority of included trials. Another consideration is that many trials used a DAS28 of < 2.6 to assess clinical remission as an outcome; however, doubts have been raised as to whether or not this is true remission, as it is now known that active disease and radiological progression can be present in patients with a DAS28 of < 2.6. 73–78
The systematic reviews by Schoels et al. 23 (initial review) and Stoffer et al. 79 (update of the Schoels et al. review), designed to inform the international task force recommendations for treating to target,17,18 both concluded that TTT was more effective than usual care. Similarly, Jurgens et al. 21 examined the effectiveness of TTT on remission rates and found results in favour of TTT, Schipper et al. 80 compared the effectiveness of TTT with usual care on mean change in DAS28 and found TTT to be more effective than usual care and Bakker et al. 81 found evidence of the effectiveness of TTT in terms of remission, but did not synthesise the evidence (findings were reported individually by study). The Knevel et al. 82 review, which also analysed RCTs and non-randomised studies, found no evidence to recommend one particular target over others, similar to the findings of the current review. The Knevel et al. 82 review also reported no particular benefit of TTT among patients with established RA, but an overall benefit of TTT among early RA patients. It is unclear whether or not the reviews by Schoels et al. ,23 Stoffer et al. 79 and Knevel et al. 82 reported on all outcomes reported in all included studies; the current review provides an explicit and comprehensive overview of all measured outcomes. All five previous reviews examined data from RCTs and non-randomised studies together, whereas the current review focused on RCTs only, to reduce the possibility of selection bias.
Although the search strategy for this assessment report was comprehensive, the possibility of a publication bias cannot be discounted. It was not possible to undertake a formal assessment of publication bias, as there were too few trials per comparison to assess funnel plot asymmetry.
From the evidence reviewed here, we consider that the evidence for TTT is mixed but, in early RA, there does seem to be some limited support for TTT, particularly in terms of the numbers of patients achieving remission. This is particularly true if the TICORA trial,61 which was the only trial in the review rated as being at low risk of bias, is interpreted as providing evidence relevant to the early RA population (inclusion criterion for the TICORA trial was disease duration of < 5 years). We also found no clear evidence to support the clinical effectiveness of one particular target over others. There is also no clear evidence of a greater likelihood of AEs in a TTT approach compared with usual care (or of the use of one target in particular), suggesting that such an approach is no more harmful (and may, indeed, be less harmful, as some of the evidence suggests). Caution is warranted in interpreting this conclusion, however, as there were only small numbers of trials within each comparison, in each population group, and there was much heterogeneity in terms of targets, treatment protocols, frequency of contact, outcome and follow-up time points. In addition, only one trial (i.e. the TICORA trial61) was rated as being at low risk of bias; the rest were rated as being unclear or high risk of bias, mainly because of the lack of blinding in outcome assessment and attrition bias (and, in addition, elements of design make the attribution of the TICORA trial’s61 findings to TTT less clear).
According to the current review findings, there is a weak basis for recommending TTT in the routine care of RA and TTT does not appear to confer any significant harms, although AEs were not examined in a consistent way across trials. Despite this uncertainty, it is not immediately clear that additional research on TTT should be conducted because there are many different aspects to the TTT concept. If such research is to be conducted, there should be a focus on well-conducted trials comparing TTT with usual care and/or different TTT targets, which are adequately blinded (in terms of participants, study personnel and outcome assessment), with low rates of attrition and adequate allocation concealment, designed to control for all other variables than TTT/different targets, reporting on the proportion of patients meeting the target and being in remission.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
The objective of this review was to identify and evaluate studies exploring the cost-effectiveness of TTT strategies in the treatment of RA.
Identification of studies
Electronic databases
Studies were identified by searching the following electronic databases on the 14 January 2016:
-
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (via Ovid) 1948 to present
-
EMBASE (via OvidSP) 1980 to present
-
Web of Science Science Citation Index Expanded (via Thomson Reuters) from 1900 to present
-
Web of Science Conference Proceedings Index (via Thomson Reuters) from 1990 to present
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost) 1982 to present
-
NHS Economic Evaluation Database (via The Cochrane Library) 1995 to 2015
-
American Economic Association’s electronic bibliography (via Ovid) from 1886 to present.
Sensitive keyword strategies using free text and, where available, subject headings or thesaurus terms for RA and TTT were combined in the electronic database searches. Searches in MEDLINE, EMBASE, Web of Science and CINAHL were limited by study design by combining the results with economic- and cost-related search terms (see Appendix 2 for full search strategies). Date limits or language restrictions were not used on any of the database searches.
Inclusion and exclusion criteria
Studies were selected according to predetermined inclusion and exclusion criteria. Studies were included if they reported the cost-effectiveness of TTT strategies in RA estimating the health-related benefits in terms of quality-adjusted life-years (QALYs) gained; life-years gained; DAS; SHS; percentage of patients achieving remission; percentage of patients achieving LDA; percentage of patients achieving treatment response; change in patient utility (VAS or SF-36 scores); or any other clinical measure of disease progression/activity or health-related quality of life. Studies that did not report costs but in which the treatment protocols were described in sufficient detail to allow the incremental cost between treatment arms to be assumed or approximated were also included. Papers not published in the English language were excluded.
One reviewer (AR) independently screened all titles and abstracts. When there was uncertainty in the decision, a second reviewer (MDS) was used and a consensus through discussion was obtained. Full papers were obtained for any titles/abstracts that were considered relevant or where the title/abstract information was not sufficient to make a decision.
Quality assessment strategy
The quality of the economic evaluation studies that met the inclusion criteria was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist. 83,84 The use of this checklist ensures a consistent approach to assessing the quality of each economic evaluation. This can be found in Appendix 5.
Results of the cost-effectiveness review
The systematic searches identified 1231 potentially relevant citations. Of the titles and abstracts screened, two relevant full-text papers were retrieved and assessed in detail. A PRISMA flow diagram describing the process of identifying relevant literature can be found in Appendix 4.
Two eligible studies were found to be relevant: Vermeer et al. 85 and van den Hout et al. 86 Vermeer et al. 85 conducted an economic evaluation of TTT compared with usual care in patients in the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry: the DREAM remission induction cohort (TTT) and the Nijmegen early-RA-inception cohort. Adult patients with an ACR classification criterion for RA, with symptom duration of < 1 year and who had had no previous DMARDs were included.
The TTT strategy included regular assessment of patients (weeks 0, 8, 12, 20, 24, 36 and 52, and every 3 months thereafter) against the target of remission (DAS28< 2.6). A treatment protocol of DMARD monotherapy, followed by a step-up to combination DMARD therapy, followed by anti-tumour necrosis factor (TNF), was adopted. The usual-care group did not have the DAS28 released to the treating rheumatologist. Treatment was not provided in accordance with a protocol, but left to the discretion of the rheumatologist. In general, patients were treated with step-up or sequential monotherapy with cDMARDs and/or a biologic, notably anti-TNF.
The costs used were those applicable in 2011 in the Netherlands. Results were expressed in terms of cost per remission and cost per QALY using 2-year follow-up data and an extended analysis using 3-year follow-up for a smaller proportion of patients with available data.
The authors found that TTT was associated with an incremental cost of €3591 per remission at 2 years and after 3 years was dominant (cost saving and more patients in remission). Similarly, at 2 years the cost per QALY for TTT compared with usual care was €19,410 and it was dominant at 3 years (more QALYs, cost saving). This suggests that TTT has higher costs in the short term (as the strategy requires more intensive drug therapy and more frequent assessment of patients), but it is more effective and, in the longer term, this greater effectiveness offsets some of the initial extra costs and may more than offset them. For example, patients in remission are less likely to progress to costly biologic therapies or require hospitalisation. However, it may be the case that TTT simply delays rather than avoids these cost-incurring events completely. This would become apparent only with longer follow-up.
van den Hout et al. 86 conducted an economic evaluation as part of the BeSt study. 26–34 This was a randomised trial of patients (n = 508) with early-onset RA randomised to (1) DMARD monotherapy, (2) step-up combination therapy (including to treatment with IFX), (3) DMARD and steroid combination therapy stepped up (including to treatment with IFX) or (4) initial IFX and DMARD combination therapy. DASs were measured every 3 months and used to change treatments according to the protocol the patient was randomised to. Costs and utilities were measured over 2 years to allow cost per QALY estimates to be made from a Dutch perspective. Productivity costs were included using both friction cost and human capital approaches.
The primary analysis used British tariffs to generate utility scores for the EuroQol-5 Dimensions (EQ-5D) and the friction cost method for costing productivity losses. The most effective strategy in terms of QALYs generated was strategy 4, but the additional cost meant that this was unlikely to be considered cost-effective: the incremental cost-effectiveness ratio (ICER) compared with the next most effective strategy was €130,000. Strategy 2 was most likely to be cost-effective for willingness-to-pay values up to €74,000 per QALY and strategy 3 for willingness-to-pay values between €74,000 and €130,000 per QALY. These general findings did not change in sensitivity analysis, except when the human capital approach to valuing productivity losses was taken. Then, strategy 4 became highly cost-effective.
Independent economic assessment
Methods
We considered how economic modelling could best be undertaken to be of use to decision-makers in relation to TTT strategies. As noted above, TTT does not represent a specific intervention or technology, but is a term that encompasses a range of different treatment strategies. Various aspects of TTT – the setting of a treatment target, frequent assessment of that target, changes to therapy informed by patient performance against the target and protocols for switching or amending drug therapy according to patient performance – have been tested in clinical trials but not in a consistent manner.
As all trials vary in terms of the aspects of TTT they test, one means of providing generalisable results is to pool similar studies together. In Chapter 3, Assessment of characteristics of individual study arms, an illustrative grouping of studies was provided on the basis of the treatment protocols used to adjust drug therapies according to the patient assessments. A similar approach to this was taken in the economic modelling undertaken to help inform the NICE clinical guideline in RA. 4 The NICE guideline considered the optimal use of non-bDMARDs and steroids in patients with early RA. Trial arms were categorised by treatment protocol and a similar approach was taken here for studies identified as TTT trials. It can be seen that, even within studies that used treatment protocols that were broadly similar within TTT approaches, there remains substantial heterogeneity. The precise details of the treatment protocols differ: cDMARDs used are different, doses vary and the timings with which treatment changes are made also are different. There are also differences between studies in terms of the frequency of assessment and the treatment target. For these reasons it was not considered feasible to pool study arms together in the assessment of clinical effectiveness. Those same concerns remain valid when considering the appropriate method for assessing cost-effectiveness.
There are additional issues to be considered in relation to cost-effectiveness methods. To conduct an economic evaluation, even within the follow-up period of any of the single trials identified, requires substantial information about the experiences of patients. For example, unlike standard technologies, TTT trials typically have a complex treatment protocol that specifies how drug treatments are to be amended, and how the frequency of visits can be amended, by taking the assessment of patients into account. To estimate resource use that will vary by patient because of these factors requires a level of reporting of results that is typically absent from published papers. Indeed, in most cases such data will not have been collected.
The importance of extrapolation beyond the trial study period is critical in this area. Vermeer et al. 85 show how cost-effectiveness estimates vary substantially between their 2- and 3-year analyses. Extrapolation is critical in this area because TTT typically involved higher upfront costs – patients are treated in a more intense fashion and assessments are made more frequently. The expectation is that better control of patients gained from this more intense management will provide benefits both to patients and to the health system in the longer term. This can be achieved because it is often the case that patients in continued remission of LDA can have their drug treatments reduced or eliminated entirely, even if only for a short period. 87 This may not be apparent within the short follow-up periods of clinical trials. It is also the case that better disease control is likely to reduce or eliminate the requirement for joint replacement surgery and hospitalisations. 88 Finally, and critically, the lifetime cost-effectiveness of any treatment strategy is likely to be dominated by the extent to which biologic drugs are used. Current eligibility for biologic drug treatment in the UK NHS requires the failure of at least two cDMARDs. It is not feasible to estimate the rate at which drug failures occur within clinical trials in order to then construct a model that moves patients to biologic drugs in the extrapolation period. Even if it was, such is the importance of the use of biologics on cost-effectiveness that it is entirely feasible that this would dominate any influence of the TTT element of the treatment strategy. In the extreme case, one could imagine a case where biologics themselves were not considered to be cost-effective. In this case, treatment strategies in early RA that resulted in the greatest delay in patients failing two DMARDs (and, therefore, moving to biologics) would become the optimal strategy. This could lead to perverse results.
Owing to the combination of these factors, a standard economic analysis conducted in terms of cost per QALY was not deemed feasible or useful for informing decision-making. Owing to a lack of validity in synthesising the data, an approach of a single model that compares all identified treatment strategies simultaneously was not considered appropriate. Instead, each study identified within the clinical effectiveness review was considered in isolation, with an ICER being extracted if the authors provided a measure of cost-effectiveness, or estimated by the authors of this report, if possible. Where insufficient data were provided to allow an estimate of the ICER, a narrative account of the relative cost-effectiveness of the treatment arms contained within each study is provided. The data provided in the papers were often insufficient to allow a robust estimate of cost–utility. However, cost-effectiveness ratios such as incremental cost per additional patient in remission could typically be calculated and presented. In contrast to results generated by cost–utility analyses, there is no stated threshold to determine whether or not treatments are likely to be deemed to be cost-effective using different metrics. Where the results are sufficiently robust to allow conclusions on likely cost-effectiveness, the authors have done so. Although a more limited approach to cost-effectiveness, our aim was to provide evidence that portrays the limited evidence base in its most useful form.
We adopted a pragmatic approach to costing health resources consumed by patients in the identified studies. Studies rarely report data in a form that would allow an estimate of the timing or dosages of drug change for cDMARDs, steroids, immunosuppressants and anti-inflammatory painkillers. For these types of drugs, it was assumed that the costs would be approximately equal in each treatment strategy arm and, therefore, not included in the cost analysis. This is not believed to introduce significant inaccuracy as a result of the relatively inexpensive unit costs for the drugs, but will be favourable to those treatments that start with combination treatment rather than monotherapy. Where there were differences reported between study arms in terms of hospital inpatient and outpatient care, appointments with rheumatologists and other health-care professionals, or other aids and applications, these were costed at 2014/15 values. 89,90 For studies which allowed the use of bDMARDs, a more precise estimation of the additional drug costs was undertaken as these drugs are markedly more expensive, having been estimated to incur an annual cost of £9200:91 exact costs could not be used because of the commercial-in-confidence patient access schemes for some bDMARDs.
The following section details the TTT studies identified and the cost-effectiveness ratios reported in the original papers or calculated by the authors of this report. These studies are presented in chronological order of the last paper within the study, with papers relating to the same study grouped together. To avoid repetition, the exact components of the treatment arm have not been detailed. Readers are referred to Independent economic assessment for further information.
Results
Estimation of cost-effectiveness for each of the 16 TTT studies identified within the literature review of clinical evidence is provided here. We report results separately for studies considering patients with early, established disease or mixed populations.
Early rheumatoid arthritis populations
Fin-RACo (Mottonen et al., 1999)
The paper by Mottonen et al. 46 reports on a 2-year multicentre RCT set in Finland. A total of 199 early RA patients were randomly allocated to one of two treatment arms: four patients did not start treatment. A total of 97 patients, 58% female, with a mean age of 47 year received combination drug therapy. The remaining 98 patients, 66% female, with a mean age of 48 years, received single-drug therapy. The target was remission.
The primary outcomes were the percentages of patients achieving remission after 1 and 2 years. After 1 year, 25% of patients receiving combination drug therapy and 11% of patients receiving single-drug therapy achieved remission (p = 0.011). After 2 years, both of these values increased: to 37% of patients receiving combination drug therapy and 18% of patients receiving single-drug therapy (p = 0.003). Both the results at 1 year and the results at 2 years are statistically significant.
This study presents the percentage of patients who experienced a drug-induced AE. Seventy per cent of those patients on combination therapy experienced an AE, compared with 71% of those on single therapy (p = 0.840). There was a statistically significant difference only in the percentage of patients with alanine aminotransferase and alkaline phosphatase levels double that of normal patients, with fewer events in the combination therapy arm (p = 0.026).
From the evidence contained in this paper, it would appear that combination drug therapy is more effective than single-drug therapy in inducing remission without having a significant effect on costs. Thus, in terms of cost-effectiveness it would appear, from the evidence of this study, that combination drug therapy either dominates single-drug therapy or would be highly likely to have a cost per QALY below the values published by NICE,92 of £20,000–30,000, compared with monotherapy.
CAMERA (Verstappen et al., 2007)
The paper by Verstappen et al. 36 reports on a 2-year randomised open-label prospective trial, which was set in the Netherlands. A total of 299 patients were randomly allocated to one of two treatment arms, with one patient dropping out after randomisation. A total of 151 patients, 69% female, with a mean age of 54 years received intensive therapy. A total of 148 patients, 66% female, with a mean age of 53 years, received routine therapy. During the 2-year duration of the study, 94 patients withdrew, 59 in the intensive therapy arm and 35 in the conventional therapy arm, including 10 shortly after inclusion (two in the intensive therapy arm and eight in the conventional therapy arm). The target was complex and multidimensional in both treatment arms.
Patients receiving conventional therapy attended appointments with their treating rheumatologist every 3 months and were treated in accordance the study protocol, whereas patients receiving intensive therapy attended appointments with their treating rheumatologist every 4 weeks. Rheumatology visits are assumed to cost £12893 in the UK; thus, intensive therapy will be associated with an additional cost of £2315 per patient completing the study compared with conventional therapy. Intensive therapy also includes performing specific clinical measurements, although these costs were assumed small enough to omit.
The primary outcome was the number of patients who achieved and maintained remission for a period of 3 months during the trial. During the first year, 53 patients (35%) in the intensive therapy arm and 21 patients (14%) in the conventional therapy arm achieved, and maintained, at least one 3-month period of remission (p < 0.001). Over 2 years these numbers increased to 76 patients (55%) in the intensive therapy arm and 55 patients (37%) in the conventional therapy arm (p = 0.029). The mean duration patients spent in the study until remission was 10.4 months for patients receiving intensive therapy and 14.3 months for patients receiving conventional therapy (p < 0.001). The duration of remission was also significantly longer for patients receiving intensive therapy (11.6 months) than for patients receiving conventional therapy (9.1 months) (p = 0.025).
After 1 year, 87 patients (58%) receiving intensive therapy and 64 patients (43%) receiving conventional therapy had achieved an ACR 50 response (p = 0.018). At 2 years, these numbers were 69 patients (46%) in the intensive therapy arm and 67 patients (45%) in the conventional therapy arm (p = 1.00). Results for ESR, morning stiffness, SJC, TJC, VAS scores for general well-being and pain and functional disability (as measured using the HAQ) for participants on an intention-to-treat basis using a last observation carried forward methodology basis are also presented. Patients receiving intensive therapy score significantly better in all measures after 3 months of therapy (p < 0.01), except functional disability (p = 0.8). After 1 year of therapy statistical significant differences are maintained for SJC, TJC and VAS scores for general well-being and pain. After 2 years of therapy a statistical difference is shown only for the VAS scores for general well-being and pain, with the mean differences in these categories diminishing. Thus, it appears that intensive therapy produces a more rapid response than conventional therapy.
Broadly similar mean AEs per appointment are presented: 0.745 in the intensive therapy arm and 0.771 in the conventional therapy arm (p-value not reported).
If it is assumed that all patients completed the study, then the estimated cost per incremental patient in remission would be £36.18 at the end of year 1, increasing to £110.26 at the end of year 2. Dropouts from the study mean that these values should be taken as indicative rather than definitive. However, given the potential for increased patient utility and a reduction in costs for a patient being in remission, we believe that the intensive therapy is likely to have a cost per QALY gained below thresholds published by NICE. 92
Saunders et al., 200854
The paper by Saunders et al. 54 reports on a 12-month RCT set in the UK. A total of 96 patients with early RA were randomly allocated to one of two treatment arms. A total of 49 patients, 76% female, with a mean age of 55 years, received parallel triple therapy. The remaining 47 patients, 79% female, with a mean age of 55 years, received step-up therapy. The target in both arms was a DAS28 of < 3.2.
The primary study outcome was the mean decrease in DAS28 at 12 months. Secondary outcomes, also measured at 12 months, were based on the percentage of patients in each arm achieving good response (EULAR definition) and the percentage of patients achieving remission (EULAR definition) together with the percentage of patients experiencing an ACR 20, ACR 50 and ACR 70 response. These outcomes are presented in Table 37.
| Outcome | Therapy | p-value | |
|---|---|---|---|
| Parallel triple | Step-up | ||
| Number of participants | 49 | 47 | – |
| Mean reduction in the DAS28 | –3.3 | –4.0 | 0.16 |
| EULAR good response | 41% | 60% | 0.47 |
| EULAR remission | 33% | 45% | 0.6 |
| ACR 20 response | 76% | 77% | 0.9 |
| ACR 50 response | 51% | 60% | 0.7 |
| ACR 70 response | 20% | 30% | 0.6 |
These study outcomes indicate that there is no statistically significant difference in the effectiveness of parallel triple therapy compared with step-up therapy in terms of the mean reduction in the DAS28, the percentage of patients achieving a good EULAR response or the percentage of patients achieving remission (as defined by EULAR). The results also indicate that there is no statistically significant difference between the two therapies in terms of the percentage of patients experiencing an ACR 20, ACR 50 or ACR 70 response. The step-up therapy approach has a numerical advantage compared with parallel triple therapy: it is possible that step-up therapy is better, but this was not observed because of the small sample size.
Saunders et al. 54 acknowledged in the paper that their results were at odds with previous studies which had demonstrated the superiority of triple therapy in achieving clinical improvement among patients with early RA. However, they state that these results were seen mainly in studies which compared triple therapy with single or dual cDMARD therapy in non-intensive treatment strategies. The authors hypothesise that the outcomes could also be as a result of using a relatively low dose of MTX (7.5 mg per week), SSZ (500 mg twice a day) and HCQ (200 mg daily) in the triple-therapy arm compared with the dose of SSZ used as the first treatment in the step-up therapy arm (40 mg/kg/day or maximally tolerated dose). The authors also state that the outcomes of the FIN-RACo trial,46 which was the only other published study comparing a triple-therapy treatment regimen with a monotherapy treatment regimen in early RA patients, were measured after 24 months of treatment rather than after just 12 months of treatment. Patients in the triple-therapy arm of the FIN-RACo study46 were also prescribed oral prednisolone, which was not part of the treatment regimen in this study.
From the data in this paper there is no evidence of statistically significant differences in the effectiveness of the two therapies and only a minor difference in terms of the costs of the two therapies. Therefore, no firm conclusion can be drawn between parallel triple-therapy and step-up therapy in terms of cost-effectiveness or cost–utility.
STREAM (van Eijk et al., 2011)
The paper by van Eijk et al. 55 reports on a 2-year RCT set in the Netherlands. A total of 82 patients were randomly allocated to one of two treatment arms. Forty-two patients, 58% female, with a mean age of 48 years, received aggressive therapy under a tight control treatment regimen. Forty patients, 79% female, with a mean age of 46 years, received conventional therapy. Patients assigned to aggressive therapy were able to receive ADA; however, patients assigned to the conventional therapy were not. The target was remission, defined as a DAS44 score of < 1.6.
The primary outcome was the Sharp/van der Heijde radiographic score. There was a numerical advantage favouring aggressive therapy, although this was not statistically significant at 2 years (p = 0.17).
Secondary outcomes, which were again measured at 2 years, included differences in the DAS28; the percentage of patients in clinical remission (a DAS28 of < 1.6); the percentage of patients in pharmaceutical-free clinical remission; HAQ score; and AEs. A total of 66% of patients in the aggressive therapy group and 49% of patients in the conventional therapy group achieved remission; the authors report that this is not statistically significant, but do not report a p-value. In the aggressive therapy group, 17.9% of patients achieved a period of pharmaceutical-free remission, compared with 15.8% of patients in the conventional therapy group (p = 0.08). The mean duration of pharmaceutical-free remission in the aggressive therapy group was 6 months and the mean duration of pharmaceutical-free remission in the conventional therapy group was 7.5 months. Analysis of HAQ score changes did not show a clear benefit of either treatment arm.
AE occurrence was recorded, with 62 events among patients receiving aggressive therapy patients, compared with 35 events in the conventional therapy patients within the follow-up period (p = 0.034). Thus, there is a statistically significant difference in the AE rate, with patients receiving aggressive therapy more likely to experience an AE than patients receiving conventional therapy.
Based on the evidence contained in this study, aggressive therapy is associated with greater costs than conventional therapy as a result of the use of a bDMARD in 19 out of 42 patients in the aggressive arm and the need to record the DAS28. There is not a statistically significant difference in the efficacy of the two therapies, although a beneficial effect may not have been observed because of the small sample sizes. Therefore, it is possible that the aggressive therapy is dominated by conventional therapy as a result of achieving the same outcomes at a considerably higher cost. However, there remains some uncertainty because of the numerical advantage of patients reaching remission in the aggressive arm: it was not possible to calculate a robust cost per incremental patient reaching remission as a result of the lack of data provided by van Eijk et al. 55 on the treatment duration with bDMARDs. Therefore, no definitive conclusion on the cost-effectiveness of the aggressive therapy arm can be made.
The T-4 study (Urata et al., 2011; and Urata et al., 2012)
The more recent paper by Urata et al. 57 reports on a 56-week trial set in Japan and supersedes an earlier abstract. 56 A total of 243 patients with early RA were randomly allocated to one of four treatment arms. A total of 62 patients were allocated to routine care, 60 patients were allocated to a regimen in which treatment decisions were made based on DAS28, 60 patients were allocated to a regimen in which treatment decisions were based on MMP-3 scores and 61 patients were allocated to a regimen in which treatment decisions were based on both the DAS28 and MMP-3 scores.
A total of 21 patients dropped out during the trial as a result of AEs. Seven patients dropped out of routine care, leaving 55 patients in the routine care group, 76% female, with a mean age of 62 years. Four patients dropped out of the DAS therapy group, leaving 56 patients in this group, 77% female, with a mean age of 60 years. Seven patients dropped out of the MMP-3 therapy group, leaving 53 patients, 83% female, and a mean age of 62 years. The remaining three patients dropped out of the combination therapy group, leaving 58 patients, 84% female, with a mean age of 56 years. Baseline patient characteristics were reported for only those patients who completed the study. There was no target for routine care; the target for the DAS28-driven therapy was a DAS28 of < 2.6. The target for the MMP-3-driven therapy was a MMP-3 concentration of < 121 ng/ml in men and < 59.7 ng/ml in women and the targets for both the DAS28 and MMP-3-driven therapies were those for the individual components.
Patients in all the arms of the trial could receive bDMARD pharmaceuticals, which included a first-line anti-TNF, a second-line anti-TNF and TOC. The times of bDMARD initiation in each of the four treatment arms is presented in table 3 of Urata et al. ,57 although the numbers provided for each arm (55 for routine care, 59 for DAS28-driven therapy, 59 for MMP-3-driven therapy and 61 for both the DAS28 and MMP-3-driven therapy) do not match either the intention-to-treat population or the numbers who completed the trial. We have assumed that those patients who would be included in the intention-to-treat analysis did not receive a bDMARD.
A number of primary and secondary outcomes were presented for the study; these include clinical remission (defined by a DAS28 of < 2.6), radiographic non-progression [defined by a change in modified total Sharp score (mTSS) of ≤ 0.5], normal physical function (as defined by a mHAQ = 0), a combination of all three of the above conditions and clinical remission (as defined by a SDAI of ≤ 3.3). These results are presented in Table 38.
| Therapy | Outcome (%) | ||||
|---|---|---|---|---|---|
| Clinical remission, a DAS28 of < 2.6 | Radiographic non-progression | Normal physical functioning | Combination of the first three | Clinical remission, a SDAI of ≤ 3.3 | |
| Routine care | 21 | 27 | 44 | 6 | 15 |
| DAS28-driven therapy | 38 | 42 | 60 | 15 | 32 |
| MMP-3-driven therapy | 13 | 62 | 43 | 7 | 13 |
| Both DAS28- and MMP-3-driven therapy | 56 | 59 | 72 | 34 | 46 |
There appear to be errors in the marking of statistical significance between groups in figure 2 of the Urata et al. 57 paper. The correct markings are not easy to interpret, so no further comment will be made on these. However, it is reported in the results section of the paper’s abstract that clinical remission at 56 weeks was achieved by more patients in the DAS28 and MMP-3-driven group than in either the routine therapy group (p < 0.0005) or the MMP3-driven group (p < 0.0005).
From the numbers in Table 38 it is seen that the DAS28 and MMP-3-driven group is the most efficacious treatment in all categories bar prevention of radiographic progression.
Table 39 presents the estimated total treatment duration with bDMARD received by patients in each arm of the trial. From this the estimated average cost per patient associated with bDMARDs in each arm of the study could be calculated. It was assumed that the annual cost of all bDMARDs was £9200, which will result in a daily cost of bDMARD treatment of £25.19, in line with a recent publication. 91 The exact costs could not be used because of the commercial-in-confidence patient access schemes. It has been assumed that any costs associated with providing MMP-3-driven therapy and/or DAS28-driven therapy are not excessive and can be ignored for simplicity.
| Intervention | Number of patients | Days of bDMARD treatment | Cost of arm per patient (£) | |||
|---|---|---|---|---|---|---|
| First TNF | Second TNF | TOC | Total | |||
| Routine care | 62 | 5950 | 0 | 0 | 5950 | 2417 |
| DAS28-driven therapy | 60 | 5432 | 560 | 756 | 6748 | 2833 |
| MMP-3-driven therapy | 60 | 5012 | 0 | 588 | 5600 | 2351 |
| Combined DAS28 and MMP-3-driven therapy | 61 | 10,136 | 784 | 784 | 11,704 | 4833 |
From our calculations the most expensive treatment is combined DAS28 and MMP-3-driven therapy, which has a per-patient cost of £4833, followed by DAS28-driven therapy, which has a per-patient cost of £2833, followed by routine care, which has a per-patient cost of £2417, with MMP-3-driven therapy being the cheapest, at £2351 per patient.
The cost per additional patient in remission is approximately £167 when combined DAS28 and MMP-3-driven therapy is used (Table 40). Given the long-term benefits associated with remission (in terms of both costs incurred and health-related quality of life accrued), we believe that it is highly likely that the cost per QALY of the strategy would be below those quoted by NICE as thresholds for cost-effectiveness. 92
| Therapy arm | Patients achieving clinical remission | Cost (£) | ICER (£)a | ||
|---|---|---|---|---|---|
| Number | Incremental | Per patient | Incremental | ||
| MMP-3-driven therapy | 8 | – | 2351 | – | – |
| Routine care | 13 | 5 | 2417 | 66 | 13 |
| DAS28-driven therapy | 22 | 9 | 2833 | 416 | 46 |
| Combined DAS28 and MMP-3-driven therapy | 34 | 12 | 4833 | 2000 | 167 |
COBRA-light (den Uyl et al., 2013)
The paper by den Uyl et al. 69 reports on a 6-month randomised controlled non-inferiority study set in the Netherlands. A total of 164 early RA patients were randomly allocated to one of two treatment arms. A total of 81 patients, 67% female, with a mean age of 53 years, were allocated to COBRA therapy. A total of 83 patients, 70% female, with a mean age of 51 years, were allocated to COBRA-light therapy. 44,45 The target was a DAS44 score of < 1.6.
A summary of key outcomes from the study is presented in Table 41.
| Outcome | Therapy | p-value | |
|---|---|---|---|
| COBRA | COBRA-light | ||
| Number of participants | 81 | 83 | – |
| Change in the DAS44 | 2.50 | 2.18 | 0.19a |
| Percentage of patients achieving ACR response | |||
| ACR 20 response | 74 | 72 | NRb |
| ACR 50 response | 57 | 62 | NRb |
| ACR 70 response | 38 | 49 | NRb |
| Percentage of patients achieving EULAR response | |||
| Good response | 75 | 65 | NRb |
| Moderate response | 19 | 24 | NRb |
| Non-response | 6 | 11 | NRb |
| Percentage of patients achieving remission (ACR/EULAR definition) | 16 | 20 | NRb |
| Percentage of patients achieving minimal disease activity (a DAS44 of < 1.6) | 49 | 41 | NRb |
There were no significant differences in any outcome measure presented in Table 41 and there was no clear indication that one treatment strategy was better than the other. Therefore, no firm conclusion can be made regarding which strategy is the more cost-effective.
The TEAR study
The TEAR study,58–60 set in the Netherlands, evaluated two treatment options [ETN plus MTX and triple therapy (MTX + SSZ + HCQ)] and two timings regarding the initiation of therapy (immediately or step-up from MTX monotherapy if the DAS28-ESR was ≥ 3.2 at week 24). A total of 755 patients with early RA (symptom duration of < 3 years) were randomly allocated to one of four treatment arms. A total of 245 patients, 74.2% female, with a mean age of 50.7 years, received immediate MTX plus ETN. A total of 132 patients, 76.5% female, with a mean age of 48.8 years, received immediate triple therapy. A total of 255 patients, 69.0% female, with a mean age of 48.6 years, received step-up therapy from MTX monotherapy to MTX plus ETN. The remaining 124 patients, 70.2% female, with a mean age of 49.3 years, received step-up therapy from MTX monotherapy to triple therapy.
The TEAR study: results after 102 weeks (Moreland et al., 2010)
This abstract by Moreland et al. 59 provided preliminary data on radiographic scores by treatment type and by the timing of treatment initialisation; these data came from 297 participants. At week 102, the baseline-adjusted change in mTSSs in the ETN plus MTX group was 0.6 (SD 3.3) and for the triple-therapy group was 2.4 (SD 10.1), which was significantly different (p = 0.0180). No significant differences were found when analysing the timing of treatment (immediate vs. step-up; p = 0.8059), the percentage of patients with no damage (p = 0.30 by treatment group), JSN (p = 0.15 by treatment group) or the number of patients with no erosions (p-value not reported). It is unlikely that the benefit in the reduced progression of mTSSs would warrant the increased costs associated with ETN.
The TEAR study: 2-year results (Moreland et al., 2012; and O’Dell et al., 2013)
At 2 years, 67.9% of participants completed the study, with the authors reporting that there was no differential dropout rate across the treatment arms (p = 0.73) or according to the timing of intensive treatment (p = 0.61) or medication type (ETN plus MTX or triple therapy) (p = 0.18). Of those completing the study, only 476 had sufficient data for the DAS28-ESR to be determined at week 102.
At week 24, 72% of the patients in the step-up group had a DAS28-ESR of ≥ 3.2 and treatment was intensified. In contrast, the proportion of patients with a DAS28-ESR of ≥ 3.2 in the immediate-intensified treatment groups was 59% for those receiving ETN plus MTX and 57% for those receiving triple therapy. Combining the two immediate treatment groups showed a statistically significant benefit in the DAS28-ESR reduction compared with those in the step-up arms (p < 0.0001). However, by week 48 the DAS28-ESR was similar in all groups and there was no significant difference across the four treatment groups between week 48 and week 102 (p = 0.28). This conclusion held when the results were analysed by treatment type (p = 0.48) and timing of intensified treatment (p = 0.55).
There was no difference in those patients achieving clinical remission (defined as a DAS28-ESR of < 2.6) between groups (p = 0.93), by timing of intensification (p = 0.36) or by type of treatment received (p = 0.43). A similar pattern was seen with ACR responses, with both immediate treatment groups achieving better ACR 20/50/70 responses than the step-up groups (p < 0.0001), but few results were statistically significant at week 102. The only significant comparison from all tested was an improvement in ACR 70 responses between ETN plus MTX and triple-therapy groups (p = 0.01).
With respect to mTSSs, there was no difference in the change between baseline and week 102 when comparing immediate with step-up treatment (p = 0.74), although those allocated to ETN plus MTX had better results than those allocated to triple therapy (p = 0.047). Using a definition of progression of a radiographic score of > 0.5, 33.6% of patients showed progression, although this did not differ significantly across the groups (p = 0.33) or according to the timing of intensified treatment (p = 0.56). However, there was a difference between those allocated to ETN plus MTX and those receiving triple therapy (p = 0.02), although this became non-significant when an outlier (with a mTSS increase of 78.5) was removed (p = 0.069). No significant differences across treatment groups were reported for GC use or for those experiencing AEs or SAEs.
The evidence presented in this study prompted the authors to state that:
Initial use of MTX monotherapy with the addition of SSZ plus HCQ (or ETN, if necessary, after 6 months) is a reasonable therapeutic strategy for patients with early RA.
The authors of this report would concur and additionally point out that the use of ETN is considerably more expensive than SSZ or HCQ, and it is highly unlikely that the costs could justified an improvement in radiographic damage.
O’Dell et al. 60 presented very similar results to those provided in Moreland et al. 58 It was reported that no statistically significant difference was observed between the percentage of patients in each treatment modality (immediate combination treatment vs. step-up treatment) who discontinued treatment by week 24 (p = 0.84). Nor was any statistically significant difference observed between the percentage of patients in each treatment modality (immediate combination treatment vs. patients who stepped up treatment at week 24 vs. patients who remained on MTX monotherapy at week 24) who discontinued treatment between weeks 24 and 102 (p = 0.86). At 102 weeks, 159 patients remained in the immediate MTX plus ETN treatment arm, 76 patients remained in the immediate triple-therapy treatment arm, 166 patients remained in the step-up MTX plus ETN treatment arm and 75 patients remained in the step-up triple-therapy treatment arm.
The authors conclude that radiographic outcomes for those who started on MTX monotherapy and then stepped up to combination therapy if the DAS28-ESR was ≥ 3.2 at 24 weeks were ‘indistinguishable from week 48 to week 102’. The authors further report that the advantage of initial combination of ETN plus MTX compared with the addition of MTX in those that did not meet the goal of a DAS28-ESR of < 3.2 had ‘no clinical or radiographic advantage’. The authors discuss the economic consequences of the results, stating that 28% of those patients started on MTX monotherapy had a very good outcome and thus the expense of ETN need not be incurred. We concur with this view and believe that the use of ETN before intensive treatment with cDMARDs would not be justified economically and the ICER for such a strategy would be significantly higher than published NICE thresholds. 92
The BehandelStrategieën in Reumatoïde Artritis trial
The BeSt trial30,33,34,68 is a multicentre RCT set in the Netherlands. A total of 508 patients were randomly allocated to one of four treatment arms. A total of 126 patients, 68% female, with a mean age of 54 years, received sequential monotherapy; 121 patients, 71% female, with a mean age of 54 years, received step-up combination therapy; 133 patients, 65% female, with a mean age of 55 years, received initial combination therapy with PDN; and 128 patients, 66% female, with a mean age of 54 years, received initial combination therapy with IFX. The target was a DAS44 score of ≤ 2.4.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 1 year (Goekoop Ruiterman et al., 2005)
Study outcomes measured at 1 year included the D-HAQ, a lower score on which indicates better patient functioning. 30 Radiological damage was assessed in terms of mean total SHS, mean erosion score and mean JSN. These outcomes are presented in Table 42.
| Outcome | Therapy | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination therapy | Initial combination with PDN | Initial combination with IFX | ||
| Percentage of patients achieving LDAa | 53 (50) | 64 (60) | 71 (65) | 74 (70) | b |
| Mean D-HAQ score | |||||
| Baseline | 1.4 | 1.4 | 1.4 | 1.4 | – |
| 3 months | 1.0 | 1.0 | 0.6 | 0.6 | < 0.001c |
| 6 months | 0.9 | 0.9 | 0.5 | 0.5 | < 0.001c |
| 9 months | 0.8 | 0.8 | 0.6 | 0.5 | < 0.001c |
| 12 months | 0.7 | 0.7 | 0.5 | 0.5 | < 0.009d |
| Progression of radiological damage | |||||
| Mean total SHS | 7.1 | 4.3 | 2.0 | 1.3 | < 0.001c |
| Mean erosion score | 3.5 | 2.6 | 0.9 | 0.7 | < 0.001c |
| Mean JSN score | 3.6 | 1.6 | 1.0 | 0.6 | < 0.001e |
These outcomes show a statistically significant difference between patients receiving initial combination therapy with PDN and patients receiving initial combination therapy with IFX compared with patients receiving sequential monotherapy and patients receiving step-up combination therapy for mean total SHS at every time point; mean erosion score at every time point; and mean D-HAQ score at 3, 6 and 9 months. At 12 months, D-HAQ was statistically significant between patients receiving initial combination therapy with PDN and patients receiving initial combination therapy with IFX compared with patients receiving sequential monotherapy.
A statistically significant difference in mean JSN was observed between patients receiving initial combination therapy with PDN or IFX and patients receiving sequential monotherapy and also between patients receiving combination therapy with IFX and patients receiving step-up combination therapy.
A statistically significant difference was observed in the D-HAQ scores at 3, 6 and 9 months between patients receiving initial combination therapy with PDN or IFX and patients receiving step-up combination therapy or sequential monotherapy.
Table 43 provides data on AEs observed in the first year of the BeSt trial. 26–34,65,68 No statistically significant difference was observed in the rate of AEs. However, numerically the combination strategies had fewer patients with AEs, although the combination strategy with PDN had the numerically largest number of SAEs.
| AE | Therapy (%) | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination | Initial combination with PDN | Initial combination with IFX | ||
| SAEs | 6 | 7 | 13 | 5 | 0.438 |
| At least one AE | 43 | 47 | 37 | 39 | 0.367 |
| Gastrointestinal AEs | 16 | 15 | 8 | 11 | NR |
| Dermatological AEs | 10 | 12 | 9 | 6 | NR |
| Upper RTI AEs | 4 | 7 | 8 | 8 | NR |
| Vascular AEs | 2 | 2 | 6 | 2 | NR |
| Reaction in infusion | 0 | 0 | 0 | 8 | NR |
| Latent tuberculosis | 0 | 0 | 0 | 7 | NR |
Given the statistically significant better efficacy results for the strategy of combination therapy with PDN compared with sequential monotherapy and with step-up combination therapy, it is plausible that the first strategy would have a cost per QALY ratio compared with the last two strategies that was below published NICE thresholds,92 although this would be dependent on the D-HAQ gains being translated into clinically meaningful outcomes.
Given the similar efficacy data observed for the combination with PDN and the combination with IFX therapies, it is anticipated that, because of the relatively large costs of IFX, the cost per QALY gained associated with the use of IFX rather than PDN would be markedly higher than the published NICE thresholds. 92
The BehandelStrategieën in Reumatoïde Artritis trial: results at 2 years (Allaart et al., 2006)
The results presented in Allaart et al. 27 included clinical data at 2 years for each of the four study arms. These data are summarised in Table 44.
| Outcome | Therapy | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination therapy | Initial combination with PDN | Initial combination with IFX | ||
| HAQ improvement compared with baseline: mean (SD) | |||||
| 6 months | 0.5 (0.7) | 0.5 (0.7) | 0.9 (0.7) | 0.8 (0.6) | < 0.001 |
| 12 months | 0.7 (0.7) | 0.7 (0.7) | 0.9 (0.7) | 0.9 (0.7) | 0.03 |
| 18 months | 0.7 (0.7) | 0.8 (0.7) | 0.8 (0.8) | 0.9 (0.7) | 0.26 |
| 24 months | 0.7 (0.7) | 0.8 (0.7) | 0.9 (0.7) | 0.9 (0.7) | 0.26 |
| Progression of SHS compared with baseline at 2 years: mean (SD) | |||||
| Total SHS | 9.0 (17.9) | 5.2 (8.1) | 2.6 (4.5) | 2.5 (4.6) | < 0.001 |
| Erosion score | 4.7 (9.0) | 3.1 (5.0) | 1.1 (2.2) | 1.3 (2.7) | < 0.001 |
| JSN score | 4.3 (9.8) | 2.1 (3.8) | 1.5 (3.2) | 1.2 (2.9) | 0.07 |
| Relative risk for SHS progression of radiographic joint at 2 years: mean (95% CI) | |||||
| Relative risk | Referent | 0.91 (073 to 1.12) | 0.74 (0.61 to 0.89) | 0.73 (0.61 to 0.88) | – |
| Patients achieving a DAS44 of < 2.4 | |||||
| Percentage | 75 | 81 | 78 | 82 | NS |
| Patients achieving a DAS44 of < 1.6 | |||||
| Percentage | 46 | 38 | 41 | 42 | NS |
The results indicate that at 2 years many of the key clinical outcomes are not significantly different between the four arms. The two initial combination arms, however, had a quicker effect on the HAQ score and had less radiographic progression at 2 years than the two remaining arms. Given the significant costs associated with bDMARDs it is anticipated that the initial combination therapy with IFX arm would have a cost per QALY in excess of that published by NICE92 when compared with initial combination therapy with PDN.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 3 years (Allaart et al., 2007)
The paper by Allaart et al. 26 provided information additional to that of the previous year. 27 Data were provided on the HAQ scores, DAS28, ESR and SHSs for the sequential monotherapy arm and the step-up combination therapy arm at 1 year, with a conclusion that the benefits of DAS-adjusted treatment was clear, with statistically significant improvements for all comparisons. Drug-free clinical remission at 3 years was reported: 11% for those started on sequential monotherapy, 6% for those started with step-up combination therapy, 7% for those started with initial combination therapy with PDN and 16% for those started with combination therapy with IFX. The comparison between the IFX combination and both the step-up therapy and PDN combination was statistically significant. Radiological damage progression was significantly lower in the initial combination therapy arms than in the remaining arms. The data provided in this paper are more favourable to an initial IFX combination therapy arm, but would not change our conclusions regarding the likely cost-effectiveness of initial IFX combination therapy compared with initial PDN combination therapy.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 2 years (van der Kooij et al., 2009)
Study outcomes measured at 2 years were patient-reported outcomes, including changes in the McMaster–Toronto Arthritis Patient Preference Disability Questionnaire (MACTAR), SF-36 Physical Component score, SF-36 Mental Component score and the VAS scores for pain, disease progression and global general health. 68 These results are presented in Table 45.
| Outcome | Therapy | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination | Initial combination with PDN | Initial combination with IFX | ||
| SF-36 Physical Component score | |||||
| Baseline | 32.9 | 32.9 | 32.8 | 33.4 | 0.93 |
| 6 months | 8.0 | 8.5 | 12.5 | 12.4 | < 0.001a |
| 1 year | 8.9 | 11.2 | 11.9 | 12.0 | 0.10 |
| 2 years | 11.9 | 12.3 | 12.3 | 12.7 | 0.95 |
| SF-36 Mental Component score | |||||
| Baseline | 47.5 | 46.3 | 47.6 | 47.6 | 0.73 |
| 6 months | 3.1 | 3.5 | 1.2 | 4.1 | 0.17 |
| 1 year | 4.3 | 4.4 | 3.2 | 4.3 | 0.83 |
| 2 years | 4.3 | 4.6 | 4.6 | 4.0 | 0.97 |
| VAS pain | |||||
| Baseline (0–100) | 53.1 | 53.4 | 54.1 | 54.1 | 0.98 |
| 6 months | –17.4 | –25.5 | –30.3 | –30.2 | < 0.001b |
| 1 year | –21.3 | –26.0 | –28.9 | –30.5 | 0.05c |
| 2 years | –28.2 | –27.3 | –26.9 | –32.6 | 0.33 |
| VAS disease activity | |||||
| Baseline (0–100) | 59.2 | 59.4 | 59.5 | 61.8 | 0.77 |
| 6 months | –22.3 | –28.0 | –32.0 | –35.9 | 0.003b |
| 1 year | –27.5 | –29.8 | –32.8 | –38.3 | 0.03c |
| 2 years | –33.2 | –33.0 | –31.5 | –39.0 | 0.19 |
| VAS global health | |||||
| Baseline (0–100) | 51.9 | 51.9 | 50.6 | 55.0 | 0.36 |
| 6 months | –17.7 | –21.3 | –21.5 | –28.4 | 0.01c |
| 1 year | –21.7 | –23.2 | –22.7 | –30.0 | 0.06 |
| 2 years | –26.4 | –25.6 | –23.9 | –31.8 | 0.10 |
| MACTAR | |||||
| Baseline | 47.5 | 47.1 | 47.3 | 47.0 | 0.77 |
| 6 months | 12.6 | 15.4 | 16.4 | 19.1 | < 0.001d |
| 1 year | 15.2 | 16.3 | 16.9 | 19.3 | 0.02c |
At 2 years there was no significant difference in SF-36 Physical Component (p = 0.95), SF-36 Mental Component (p = 0.97), VAS pain (p = 0.33), VAS disease activity (p = 0.19) and VAS global health (p = 0.10), although some scores were statistically significant at 1 year, as was the MACTAR score. However, there is a difference in costs, with those patients in the combination therapy with IFX anticipated to be more expensive and thus unlikely to be cost-effective compared with the remaining arms.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 7 years (Dirven et al. 2010)
This abstract, by Dirven et al. ,29 presented clinical results at 7 years. These are summarised in Table 46.
| Analyses | Therapy | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination | Initial combination with PDN | Initial combination with IFX | ||
| Number recruited | 126 | 121 | 133 | 128 | – |
| Completers | 83 | 72 | 79 | 97 | – |
| Completers analyses | |||||
| A DAS of ≤ 2.4 (%) | 82 | 76 | 82 | 76 | 0.64 |
| A DAS of < 1.6 (%) | 49 | 39 | 53 | 45 | 0.35 |
| A DAS of < 1.6 drug free (%) | 16 | 18 | 17 | 17 | 0.96 |
| SHS progression over 7 years: median (mean) | 3.8 (15.1) | 3.5 (10.7) | 2.0 (8.4) | 2.0 (5.5) | 0.205 |
| Intention-to-treat analyses | |||||
| Use of IFX at 7 years | 14 | 6 | 11 | 21 | < 0.05 |
| Mean HAQ score over 7 years | 0.70 | 0.71 | 0.63 | 0.57 | < 0.001 |
| Non-completers (%) | 26 | 33 | 26 | 17 | 0.04 |
It is seen that there is a statistically significant difference neither in the DAS categories nor in SHS progression. Although there has been a statistically significant reduction in mean HAQ score, equating to approximately one HAQ level, this is not believed to provide sufficient clinical benefit to make the use of initial combination with IFX cost-effective given that the number of people using IFX at 7 years is significantly higher in the initial IFX combination therapy arm.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 8 years (Dirven et al. 2011)
This abstract, by Dirven et al. ,28 presented clinical results at 8 years and was very similar to the abstract presented in 2010. 29 The results are summarised in Table 47. It is not stated how the number of completers was higher in the 8-year results than in the 7-year results.
| Analyses | Therapy | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination | Initial combination with PDN | Initial combination with IFX | ||
| Completers analyses | |||||
| Number recruited | 126 | 121 | 133 | 128 | – |
| Completers | 85 | 78 | 86 | 98 | – |
| A DAS of ≤ 2.4 (%) | 79 | 76 | 84 | 76 | 0.49 |
| A of DAS < 1.6 (%) | 49 | 56 | 57 | 47 | 0.48 |
| A of DAS < 1.6 drug free (%) | 18 | 19 | 17 | 15 | 0.90 |
| SHS progression over 8 years: median (mean) | 3.0 (14.6) | 4.3 (13.9) | 2.0 (8.5) | 2.0 (8.3) | 0.567 |
| Intention-to-treat analyses | |||||
| Use of IFX at 8 years | 21 | 10 | 13 | 24 | 0.06 |
| Mean HAQ score over 8 years | 0.69 | 0.71 | 0.63 | 0.57 | < 0.05 |
| Non-completers (%) | 33 | 36 | 35 | 23 | 0.13 |
The conclusions remain the same as for the 7-year results. Namely, it is seen that there is a statistically significant difference neither in the DAS categories nor in SHS progression. Although there has been a statistically significant reduction in mean HAQ score, equating to approximately one HAQ level, this is not believed to provide sufficient clinical benefit to make the use of initial combination with IFX cost-effective given that the number of people using IFX at 7 years is significantly higher in the initial IFX combination arm.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 5 years (Klarenbeek et al., 2011)
The paper by Klarenbeek et al. 31 presented clinical and radiological outcomes at 5 years and focused on functional status, quality of life, joint damage, AEs and the percentage of patients in remission (DAS44 of < 1.6). As previously reported, the beneficial impacts of the two initial combination arms were quicker than for the remaining arms, but these gains did not persist over time. The results are presented in Table 48.
| Outcome | Therapy | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination | Initial combination with PDN | Initial combination with IFX | ||
| Drug-free remission (%) | 14 | 16 | 10 | 19 | 0.18 |
| SHS progression over 5 years: median (mean) | 3.5 (14.0) | 2.5 (11.0) | 1.0 (7.6) | 1.0 (6.0) | NR |
| Mean HAQ score over 5 years | 0.70 | 0.70 | 0.62 | 0.54 | NR |
| Mean SF-36 Physical Component score over 5 years: area under the curve per month | 43.5 | 43.3 | 44.1 | 45.0 | NS |
| Mean SF-36 Mental Component score over 5 years: area under the curve per month | 51.8 | 51.0 | 50.9 | 51.2 | NS |
At 5 years there was no significant difference in HAQ score (mean value 0.58), although there was a significant difference in mean HAQ score over the 5 years for the combination therapy arms compared with the sequential monotherapy and the step-up combination arms (p < 0.001) and for the initial combination therapy with IFX arm compared with initial combination therapy with PDN. There was no significant difference in either of the quality of life measurements recorded. Radiological progression was least in the initial combination arms (p < 0.01), although after the first year the authors reported no difference between the arms. The authors report that 48% of patients were in clinical remission and were equally distributed between arms, although the initial combination therapy with IFX arm had the greatest proportion (81%) achieving this on the treatment to which they were initially allocated, with the lowest value being that for sequential monotherapy (46%). No significant difference was observed in the proportion with drug-free remission (p = 0.18). The authors report that 86% of patients had at least one AE and 30% of patients had SAEs, which were equally distributed across treatment groups.
The data from this paper do not alter the conclusions that the use of initial IFX treatment is unlikely to be cost-effective.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 7 years (van den Broek et al., 2012)
The paper by van den Broek et al. 65 reports clinical results at 7 years of follow-up. It was reported that there was no statistically significant difference between the four treatment arms after the first year, although 36% of patients in the initial combination therapy with PDN arm and 53% of patients in the initial combination therapy with IFX arm had been tapered to monotherapy after year 2 because of persistent low DAS44 score. A statistically significant difference was observed between the initial combination therapy with IFX or PDN groups and the remaining two groups. At the end of the seventh year the percentages of patients across the groups with drug-free remission were similar: 13% for sequential monotherapy; 16% for step-up combination treatment; 16% for initial combination with PDN; and 14% with initial combination therapy with IFX.
The data from this paper do not alter the conclusions that the use of initial IFX treatment is unlikely to be cost-effective.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 5 years (Koevoets et al., 2013)
The paper by Koevoets et al. 32 focused on the relationship between erosions and JSN with the D-HAQ. The analysis used generalised estimating equations regression models, as these are relatively robust to violations of normality. Covariates of the DAS44, sex, treatment arm and body mass index were used. In univariate analyses, the effects of erosions and JSN were similar (erosions: β = 0.003, 95% CI –0.001 to 0.006; JSN: β = 0.004, 95% CI 0.001 to 0.008), although erosions were not statistically significant whereas JSN was significant. The variables that have the greatest influence on HAQ score when analysed univariately were DAS44, female sex and DAS44 at baseline. Compared with the initial combination treatment with IFX, sequential monotherapy (β 0.176, 95% CI 0.047 to 0.578) and step-up combination treatment (β 0.148, 95% CI 0.017 to 0.279) were associated with significantly worse HAQ scores, although initial combination therapy with PDN was not (β 0.076, 95% CI –0.035 to 0.188). When a multivariate model was used, the impact of both erosions and JSN was reduced and neither was a statistically significant predictor of HAQ score.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 8 years (van den Broek et al., 2013)
The study outcomes measured at 8 years were the percentage of patients achieving LDA (as defined as a DAS28 of ≤ 2.4), the percentage of patients achieving remission (as defined as a DAS28 of < 1.6), the percentage of patients maintaining remission while pharmaceutical free, the percentage of patients in each treatment arm who are still on their initial treatment, the mean HAQ score over the 8-year period of the study, the percentage of patients in each arm who were receiving IFX after 8 years (patients in all arms could progress to treatment with IFX during the 8-year period of the study) and the mean SHS over the 8-year period of the study. 34 These results are presented in Table 49.
| Outcome | Therapy | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination | Initial combination with PDN | Initial combination with IFX | ||
| A DAS28 of ≤ 2.4 (%) | 79 | 76 | 84 | 76 | 0.5 |
| A DAS28 of < 1.6 (%) | 49 | 56 | 57 | 47 | 0.5 |
| A DAS28 of < 1.6 pharmaceutical free (%) | 18 | 19 | 17 | 15 | 0.9 |
| Still on initial treatment step (%) | 29 | 22 | 45 | 66 | < 0.001 |
| Mean HAQ score over the study period (8 years) | 0.69 | 0.71 | 0.63 | 0.57 | < 0.05a |
| Current use of IFX (%) | 21 | 10 | 13 | 24 | 0.06 |
| Mean SHS progression (years 0–8) | 14.6 | 13.9 | 8.5 | 8.3 | 0.6 |
No statistically significant difference between the groups was observed in any of the DAS28 outcomes or in mean SHS. The mean HAQ score over the 8-year period was statistically significantly different across all groups when analysed simultaneously, with the authors additionally reporting that initial combination therapy with IFX group had statistically significant better results against the step-up combination therapy group and had borderline statistically significantly better results than the sequential monotherapy group. None of the other comparisons were statistically significantly different. Current use of IFX analysed across all groups approached statistical significance, with the greatest use being in the initial combination therapy with IFX group and in the sequential monotherapy group.
Based on van den Broek et al.,34 there is no evidence to suggest that the initial combination with PDN group has worse outcomes than the initial combination with IFX group. Owing to the relatively high price of IFX, it is assumed that the initial combination with IFX group would either be dominated by the initial combination therapy with PDN group or have a cost per QALY ratio greater than those published by NICE. 92
The use of IFX in the sequential monotherapy group is numerically higher (21%) than for those who began on combination therapy with PDN (13%), although this finding did not reach statistical significance. There was, however, a significant difference in the mean D-HAQ score and radiological progression in the first year (see Table 42). No firm conclusions can be made on the relative cost-effectiveness of the sequential monotherapy and the initial combination therapy with PDN group, although it is plausible that initiating combination therapy with PDN is more cost-effective than initiating sequential monotherapy.
The results of those in the step-up combination group and those in the initial combination therapy with PDN were broadly comparable, although there was a significantly quicker change in D-HAQ score at 3 months in the combination therapy with PDN group (see Table 42). No firm conclusions can be made regarding which out of the initial step-up combination therapy group and the initial combination therapy with PDN group is more cost-effective.
The BehandelStrategieën in Reumatoïde Artritis trial: results at 10 years (Markusse et al., 2014)
The results at 10 years report only mortality rates33 and these rates are presented in Table 50.
| Outcome | Therapy | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination | Initial combination with PDN | Initial combination with IFX | ||
| Number of patients | 126 | 121 | 133 | 128 | – |
| Number of deaths | 16 | 15 | 21 | 20 | – |
| Percentage mortality at the years | 12.70 | 12.40 | 15.79 | 15.63 | 0.805 |
It can be seen that there is a numerical advantage for patients who receive initial combination therapy with PDN and patients who receive initial combination therapy with IFX compared with patients who receive sequential monotherapy and patients who receive step-up combination therapy. However, these differences are not statistically significant (p = 0.805) and are contrary to the mean HAQ score over the initial 8 years (see Table 49), so this difference is likely to be by chance.
For this reason, the results presented by Markusse et al. 33 would not influence the conclusions on cost-effectiveness generated by the data from van den Broek et al. 34
The BehandelStrategieën in Reumatoïde Artritis trial: results at 10 years (Markusse et al., 2016)
The paper by Markusse et al. 64 reports on the results at 10 years and comments that there was a significant difference in dropout rates among the four arms, with 28% dropping out of the initial combination therapy with IFX arm, compared with 40–45% in the remaining arms (p = 0.031). Of those patients dropping out, 39% were in clinical remission. No significant differences were observed in the number of AEs (p = 0.159), SAEs (p = 0.47) or deaths (p = 0.81). Survival was stated to be comparable to that of the Dutch population. The 10-year results have been included in Table 51.
| Outcome | Therapy | p-value | |||
|---|---|---|---|---|---|
| Sequential monotherapy | Step-up combination | Initial combination with PDN | Initial combination with IFX | ||
| Drug-free remission: study completers (%) | 8.7 | 9.1 | 9.0 | 10.2 | NR |
| Remission (%) | 51 | 49 | 53 | 53 | 0.94 |
| A DAS28 of ≤ 2.4 (%) | 84 | 77 | 83 | 84 | 0.72 |
| Use of initial treatment at 10 years: intention to treat (%) | 17 | 11 | 25 | 41 | NR |
| Use of IFX at 10 years: study completers (%) | 18 | 12 | 13 | 25 | NR |
| Mean HAQ score over 10 years | 0.69 | 0.72 | 0.64 | 0.58 | 0.12 |
These 10-year results do not change the previously reached conclusions that no firm conclusions can be made regarding which of sequential monotherapy, initial step-up combination therapy and initial combination therapy with PDN group is the most cost-effective. However, initiation with IFX is not likely to be cost-effective.
Hodkinson et al., 2015
The paper by Hodkinson et al,51 reports on a prospective 12-month study set in South Africa. A total of 102 patients, 94% black Africans, 83% female, with a mean symptom duration of 3.0 years and a mean DAS28 of 6.2, were randomly allocated to one of two monitoring methodologies: CDAI or SDAI. This study cites post hoc analyses,94–96 which have shown both indexes to be valid instruments in monitoring disease activity in patients receiving treatment under a tight control strategy. The target in the SDAI arm was a score of ≤ 11 and the target in the CDAI arm was a score of ≤ 10, both of which represented LDA.
The authors state that SDAI is based on a summation of five variables and that CDAI is based on a similar summation. However, SDAI requires an acute-phase reactant test at each monitoring visit, whereas CDAI does not. Thus, SDAI will be associated with a marginally greater cost than CDAI.
Two patients were lost to follow-up and two patients died during the study period. The analyses presented by the authors were based predominantly on 98 patients: 57 patients receiving CDAI monitoring and 41 patients receiving SDAI monitoring.
The study outcomes at 12 months are presented in Table 52. However, the definitions for remission, LDA, moderate disease activity and high disease activity are not provided. It can be seen from the p-values that there is no statistically significant difference between the treatments in terms of the percentage of patients in the four disease categories or the percentage of patients achieving a good response, as defined by EULAR. The p-values of 1.00 appear to have been miscalculated, but this would not affect the broad conclusion.
| Outcome | Monitoring methodology (%) | p-value | |
|---|---|---|---|
| SDAI | CDAI | ||
| Percentage of patients in remission | 34.1 | 33.3 | 1.00 |
| Percentage of patients with LDA | 31.7 | 29.8 | 1.00 |
| Percentage of patients with moderate disease activity | 31.7 | 31.6 | 1.00 |
| Percentage of patients with high disease activity | 2.4 | 5.3 | 0.64 |
| EULAR good response | 56.1 | 50.9 | 0.68 |
No firm conclusion can be made regarding the cost-effectiveness of the treatments; however, current evidence suggests that the treatment strategies are broadly comparable, but that SDAI will cost more than CDAI. These data would suggest that, in the absence of further data, CDAI would be preferable.
The Care in early Rheumatoid Arthritis trial
The CareRA study38–43 was a 52-week randomised, pragmatic, open-label, superiority trial set in Belgium. Patients were assigned to either the high- or low-risk group based on the presence of erosions, disease activity, rheumatoid factor and anti-citrullinated protein antibody. The risk for which patients were reported to be high or low was not stated, but we presume it is of RA progression. The target for all patients in all arms was LDA, defined as a DAS28-CRP of ≤ 3.2.
The Care in early Rheumatoid Arthritis trial: results at 16 weeks (De Cock et al., 2013)
The results from this abstract38 were also reported in a full paper by Verschueren et al. ,42 although slight differences were noted. It is assumed that the later, full, paper would contain the correct results. For this reason, no further comment will be provided on this abstract.
The Care in early Rheumatoid Arthritis trial: results at 16 weeks (Verschueren et al., 2014)
The results from this abstract41 were also reported in a full paper by Verschueren et al. 42 We have focused on the full paper.
The Care in early Rheumatoid Arthritis trial: results at 16 weeks (Verschueren et al., 2015)
The results provided in this paper, by Verschureren et al. ,42 compared the use of COBRA Classic with COBRA Slim and COBRA Avant-Garde in patients at high risk following 16 weeks of treatment. A total of 98 patients were allocated to COBRA Classic, 65.3% female, with a mean age of 53.2 years; 98 patients were allocated to COBRA Slim, 64.3% female, with a mean age of 51.8 years; and 94 patients were allocated to COBRA Avant-Garde, 69.1% female, with a mean age of 51.2 years. Four outcomes were assessed: DAS28-CRP remission defined as a score of < 2.6, cumulative disease activity, HAQ scores and AEs. These results are reported in Table 53.
| Outcome | Therapy | p-value | ||
|---|---|---|---|---|
| COBRA Classic (n = 98) | COBRA Slim (n = 98) | COBRA Avant-garde (n = 94) | ||
| Change in DAS28-CRP | 2.80 | 2.60 | 2.40 | 0.140 |
| Area under the curve of DAS28-CRP in weeks 0–16 | 10.66 | 11.05 | 10.72 | 0.521 |
| Remission (%) | 70.4 | 73.5 | 68.1 | 0.713 |
| LDA (%) | 84.7 | 86.7 | 87.2 | 0.863 |
| Good EULAR response (%) | 79.6 | 79.6 | 76.6 | 0.844 |
| Moderate or good EULAR response (%) | 98.0 | 95.9 | 93.6 | 0.320 |
| Reduction in HAQ score | 0.8 | 0.6 | 0.7 | 0.081 |
| HAQ clinically meaningful change (%) | 84.7 | 76.5 | 76.6 | 0.271 |
| HAQ = 0 | 45.9% | 42.9% | 48.9% | 0.700 |
| (n = 91) | (n = 96) | (n = 91) | ||
| Patients with therapy-related AEs (%) | 61.2 | 46.9 | 69.1 | 0.006 |
| Number of therapy-related AEs per ITT patient | 1.63 | 0.73 | 1.43 | NR |
| Patients with therapy-related SAEs (%) | 2.2 | 1.0 | 3.3 | NR |
There was only one statistically significant difference among the three groups at 16 weeks, which related to therapy-related AEs with COBRA Slim having fewer events than the remaining arms. Given the comparable costs and efficacy it is likely that COBRA Slim would be the most cost-effective of the three strategies given the 16-week data.
The Care in early Rheumatoid Arthritis trial: results at 16 weeks for low-risk patients (De Cock et al., 2014)
The results from this abstract39 were also reported in a full paper by Verschueren et al. 40 For this reason, no further comment will be provided on this abstract.
The Care in early Rheumatoid Arthritis trial: results at 16 weeks for low-risk patients (Verschueren et al., 2015)
The results provided in the paper40 by Verschueren et al. compared the use of MTX-TSU with COBRA Slim in patients at low risk following 16 weeks of treatment. A total of 47 patients were allocated to MTX-TSU, 80.9% female, with a mean age of 51.02 years, and 43 patients were allocated to COBRA Slim, 76.7% female, with a mean of 51.42 years. Four outcomes were assessed: DAS28-CRP remission defined as a score of < 2.6, cumulative disease activity, HAQ scores and AEs. These results are reported in Table 54.
| Outcome | Therapy | p-value | |
|---|---|---|---|
| MTX-TSU (n = 47) | COBRA Slim (n = 43) | ||
| Change in DAS28-CRP | 1.76 | 2.12 | 0.192 |
| Area under the curve DAS28-CRP in weeks 0–16 | 13.84 | 11.18 | 0.006 |
| Remission (%) | 46.8 | 65.1 | 0.081 |
| LDA (%) | 72.3 | 79.1 | 0.458 |
| Good EULAR response (%) | 44.7 | 58.1 | 0.202 |
| Moderate or good EULAR response (%) | 72.3 | 86.0 | 0.111 |
| Reduction in HAQ score | 0.40 | 0.58 | 0.267 |
| Clinically meaningful reduction in HAQ (%) | 53.2 | 62.8 | 0.357 |
| HAQ score of 0 (%) | 23.4 | 51.2 | 0.006 |
| Patients with therapy-related AEs (%) | 44.7 | 39.5 | 0.622 |
| Number of therapy-related AEs per ITT patient | 0.681 | 0.698 | NR |
| Patients with therapy-related SAEs (%) | 0 | 0 | NR |
COBRA Slim was observed to have a statistically significant benefit in terms of both the area under the curve of DAS28-CRP in the 16-week period and the proportion of patients with a HAQ score of zero. Although not reaching significance, there were numerical advantages for COBRA Slim in key clinical outcomes such as remission, change in DAS28-CRP, change in HAQ score and EULAR response.
Given the likely comparability of costs for the two arms, but the superior efficacy for the COBRA Slim arm, it is likely that COBRA Slim would be more cost-effective than MTX-TSU given the 16-week data.
The Care in early Rheumatoid Arthritis trial: 52-week results (Verscheuren et al., 2015)
The results from this abstract, by Verscheuren et al. ,40 were also reported in a full paper by Verschueren et al. ,43 although slight differences were noted. It is assumed that the later, full, paper, would contain the correct results and, therefore, no further comment will be provided on this abstract.
The Care in early Rheumatoid Arthritis trial: 52-week results (Verschueren et al., 2016)
Two analyses were described within this paper by Verscheuren et al. :43 one assessed three interventions for high-risk patients and one assessed two interventions for low-risk patients.
A total of 289 high-risk patients were randomly allocated to one of three treatment strategies. Ninety-eight patients, 65.3% female, with a mean age of 53.2 years, received COBRA Classic therapy; 98 patients, 64.3% female, with a mean age of 51.8 years, received COBRA Slim therapy; and 93 patients, 68.8% female, with a mean age of 51.1 years, received COBRA Avant-Garde therapy. Note that this number of patients is one fewer in the COBRA Avant-Garde arm than previously reported. 38,42
Study outcomes, measured at 54 weeks, were the percentage of patients achieving LDA, the change in DAS28-CRP, the percentage of patients achieving remission (defined as DAS28-CRP < 2.6), the percentage of patients achieving a good EULAR response and the percentage of patients achieving a moderate EULAR response. The results for these outcomes are presented in Table 55.
| Outcome | Therapy | p-value | ||
|---|---|---|---|---|
| COBRA Classic | COBRA Slim | COBRA Avant-Garde | ||
| Number of participants | 98 | 98 | 93 | – |
| DAS28-CRP reduction | 2.5 | 2.3 | 2.3 | 0.329 |
| Remission (%) | 64.3 | 60.2 | 62.4 | 0.840 |
| LDA (%) | 74.5 | 75.5 | 79.6 | 0.684 |
| Good EULAR response (%) | 67.3 | 68.4 | 67.7 | 0.995 |
| Moderate or good EULAR response (%) | 84.7 | 88.8 | 88.2 | 0.654 |
| Patients with therapy-related AEs (%) | 67.3 | 66.3 | 78.5 | 0.125 |
| Number of therapy-related AEs per ITT patient | 1.9 | 1.3 | 1.9 | 0.028 |
| Patients with therapy-related SAEs (%) | 2.0 | 2.0 | 2.2 | NR |
Study outcomes indicate that there are no statistically significant differences in the efficacy of the three interventions. Indeed, each treatment possesses a numerical advantage in at least one outcome measure.
No firm conclusions can be made regarding the cost-effectiveness of the three treatment strategies. In the absence of other data, it is noted that COBRA Slim was associated with significantly fewer AEs. This conclusion is consistent with the 16-week results.
A total of 90 low-risk patients were randomly assigned to one of two treatment strategies. Forty-seven patients, 80.9% female, with a mean age of 51.0 years, received MTX-TSU; and 43 patients, 76.7% female, with a mean age of 51.4 years, received COBRA Slim (low-risk) therapy. The results for low-risk patients are presented in Table 56.
| Outcome | Therapy | p-value | |
|---|---|---|---|
| MTX-TSU | COBRA Slim | ||
| Number of participants | 47 | 43 | – |
| DAS28-CRP change | 2.1 | 2.1 | 0.990 |
| Remission (%) | 57.4 | 67.4 | 0.329 |
| LDA (%) | 76.6 | 81.4 | 0.577 |
| Good EULAR response (%) | 57.4 | 60.5 | 0.771 |
| Moderate or good EULAR response (%) | 78.7 | 76.7 | 0.822 |
| Patients with therapy-related AEs (%) | 63.8 | 51.2 | 0.224 |
| Number of therapy-related AEs per ITT patient | 1.2 | 1.2 | 0.737 |
| Patients with therapy-related SAEs (%) | 0.0 | 4.3 | NR |
There were no statistically significant differences in the efficacy of the two interventions. However, COBRA Slim therapy does possess a numerical advantage in all outcomes except that of obtaining at least a moderate EULAR response. The possibility that a true difference was not observed because of a small sample size cannot be discounted. However, there were two SAEs in the COBRA Slim arm and zero in the MTX-TSU group.
No firm conclusions can be made regarding which treatment strategy was more cost-effective. COBRA Slim has a numerical advantage on key clinical outcomes and AEs, but not SAEs; however, this was not statistically significant. This conclusion differs from that which was formed when only the results at 16 weeks were known.
The U-Act-Early trial (Bijlsma et al., 2016)
The paper by Bijlsma et al. 62 reports on a 2-year RCT which was set in the Netherlands. A total of 317 patients with early RA were randomly allocated to one of three treatment arms. One hundred and six patients, 61% female, with a mean age of 53.0 years, received TOC plus MTX; 103 patients, 76% female, with a mean age of 55 years, received TOC monotherapy; and the remaining 108 patients, 64% female, with a mean age of 53.5 years, received MTX monotherapy. The target was to achieve sustained remission defined as a period of at least 24 weeks with a DAS28 of < 2.6 and a SJC of < 5.
The primary study outcome was the percentage of patients achieving sustained remission at 104 weeks. Secondary outcomes, measured at 24, 52 and 104 weeks, included the percentage of patients achieving a good or moderate EULAR response, the percentage of patients achieving an ACR 20/50/70/90 response and the mean physical function score (the D-HAQ adjusted for centre and baseline). A measure of the progression of radiological joint damage using the SHS was also assessed at 52 and 104 weeks. These outcomes are presented in Table 57.
| Outcome | Therapy | p-value by therapy | ||||
|---|---|---|---|---|---|---|
| TOC and MTX | TOC monotherapy | MTX monotherapy | TOC and MTX vs. MTX | TOC vs. MTX | TOC and MTX vs. TOC | |
| Sustained remission at 104 weeks on initial treatment, n (%) | 91 (86) | 81 (83) | 48 (44) | < 0.0001/2.0a | < 0.0001/1.86a | 0.62/1.03a |
| Week 24 | ||||||
| Good EULAR response (%) | 89 | 87 | 49 | < 0.0001 | < 0.0001 | 0.43 |
| Moderate EULAR response (%) | 5 | 11 | 32 | |||
| ACR 20 response (%) | 75 | 75 | 59 | 0.0099 | 0.0343 | NS |
| ACR 50 response (%) | 64 | 59 | 34 | < 0.0001 | 0.0009 | NS |
| ACR 70 response (%) | 44 | 37 | 15 | < 0.0001 | 0.0003 | NS |
| ACR 90 response (%) | 18 | 12 | 5 | 0.0027 | NS | NS |
| Mean physical function score | 0.50 | 0.63 | 0.65 | 0.0275 | ||
| Week 52 | ||||||
| Good EULAR response (%) | 75 | 88 | 72 | 0.26 | 0.0074 | 0.06 |
| Moderate EULAR response (%) | 6 | 4 | 7 | |||
| ACR 20 response (%) | 75 | 72 | 69 | NS | NS | NS |
| ACR 50 response (%) | 62 | 59 | 51 | NS | NS | NS |
| ACR 70 response (%) | 44 | 44 | 33 | NS | NS | NS |
| ACR 90 response (%) | 19 | 21 | 7 | 0.0045 | 0.0026 | NS |
| Mean physical function score | 0.46 | 0.48 | 0.55 | 0.14 | ||
| SHSb | 0.50 | 0.79 | 0.96 | 0.0164 | 0.06 | 0.49 |
| Week 104 | ||||||
| Good EULAR response (%) | 66 | 76 | 68 | 0.87 | 0.13 | 0.10 |
| Moderate EULAR response (%) | 8 | 8 | 8 | |||
| ACR 20 response (%) | 63 | 65 | 61 | NS | NS | NS |
| ACR 50 response (%) | 49 | 55 | 48 | NS | NS | NS |
| ACR 70 response (%) | 36 | 39 | 35 | NS | NS | NS |
| ACR 90 response (%) | 21 | 20 | 14 | NS | NS | NS |
| Mean physical function score | 0.48 | 0.61 | 0.62 | 0.06 | ||
| SHSb | 1.18 | 1.45 | 1.53 | 0.0207 | 0.0381 | 0.53 |
Patients were allowed to add HCQ to their initial allocated treatment. If sustained remission was not achieved, then subsequent treatment regimens were allowed. These included alternative TNFis for those who were initially on TOC treatment, and TOC and an alternative TNFi for those patients who were allocated to MTX monotherapy.
No statistically significant difference was observed between TOC plus MTX and TOC monotherapy in terms of the percentage of patients achieving sustained remission. However, statistically significant differences were observed between both TOC arms compared with patients receiving MTX monotherapy. The same pattern exists in terms of EULAR response and ACR 20/50/70 responses at week 24. However, by week 52 most of these differences had become non-significant and by week 104 no statistically significant differences existed between treatment groups. In terms of physical function, a statistically difference between treatment groups as present at week 24 (p = 0.0275). However, again by week 52 this difference had become non-significant (week 52, p = 0.14; week 104, p = 0.06). In terms of the progression of radiological joint damage, measured by the SHS, there was a statistically significant difference at week 52 (patients receiving TOC + MTX vs. patients receiving MTX monotherapy; p = 0.0164) and week 104 [patients receiving TOC + MTX vs. patients receiving MTX monotherapy (p = 0.0207) and patients receiving TOC monotherapy vs. patients receiving MTX monotherapy (p = 0.0381)].
The study presents the percentage of patients who experienced AEs, SAEs and serious infections. However, there appear to be no statistically significant differences in AE frequency between treatment arms.
The study also reports results for patients at the end of the study, when patients could move on to subsequent treatments. At the end of the study, 88% of the TOC arm, 86% of the TOC plus MTX arm and 77% of the MTX arm had achieved sustained remission, with only the comparison between TOC monotherapy and MTX monotherapy being significant (p = 0.0356). It is stated that ‘roughly 50%’ of patients in the MTX monotherapy arm received TOC.
Without further details on the costs for each patient in each of the allocated treatment arms, it is not possible to estimate a robust cost per QALY for the interventions. What is known is that the use of TOC produces a statistically significantly higher percentage of patients who achieve sustained remission and that TOC is markedly more expensive than MTX. Given that 44% of patients on MTX monotherapy achieved sustained remission without the use of a bDMARD, there can be considerable savings by not starting patients on bDMARDs and in potentially increasing the intensity of cDMARDs before progressing to biologic use. It is the opinion of the authors of this report that the use of bDMARDs initially would produce a cost per QALY greater than the thresholds published by NICE. 92
Established disease rheumatoid arthritis populations
Fransen et al., 2005
The paper by Fransen et al. 50 reports on a 24-week multicentre cluster RCT set in the Netherlands. A total of 384 RA patients were included with a subgroup of 142 patients who had DAS28 assessed were included. Twenty-four rheumatology outpatient centres were randomly allocated to provide DAS28-guided care (61 patients) or usual care (81 patients). A summary of the patients’ characteristics are tabulated in Table 58. The target in the systematic therapy arm was a DAS28 of ≤ 3.2: there was no target in the usual-care arm.
| Characteristic | Therapy | |
|---|---|---|
| Systematic monitoring | Usual care | |
| Mean age (years) | 57 | 58 |
| Patients who are female (%) | 62 | 77 |
| Baseline | ||
| Mean DAS28 | 4.6 | 4.5 |
| Patients with LDA (%) | 13 | 12 |
| 24 weeks | ||
| Mean DAS28 | 4.2 | 4.4 |
| Patients with LDA (%) | 31 | 16 |
The frequency of appointments with treating rheumatologists was no different between patients receiving systematic therapy and patients receiving usual-care therapy. However, rheumatologists would perform a joint count and calculate the DAS28 during appointments with patients in the systematic monitoring arm, which are likely to be associated with a cost. However, this cost is unlikely to be significant and has been omitted from this analysis.
Outcomes included the percentage of patients achieving LDA (defined as a DAS28 of ≤ 3.2) at 24 weeks and the mean DAS28. These results are also presented in Table 58.
The authors state that the difference between the results in terms of the percentage of patients achieving LDA was statistically significant at the 5% level (p = 0.028). However, the difference in DAS28 was not statistically significant at the 5% level (p = 0.36).
It was found that, in terms of treatment-induced adverse reactions, a statistically significant difference between the treatment arms was observed only for rash or itching, with 4% of patients in the systematic monitoring therapy arm and 11% of patients in the usual-care therapy arm reporting this adverse reaction to treatment. However, it was assumed that this difference would not have a substantial effect on costs.
The evidence in the study appears to indicate that the systematic monitoring of patients is more effective without having a significant effect on costs. Thus, in terms of cost-effectiveness, the evidence in this paper would appear to indicate that usual-care therapy is dominated by the systematic monitoring of patients.
The BROSG trial (Symmons et al., 2005)
This Health Technology Assessment (HTA) report9 presents the methodology and results of the BROSG RCT set in the UK. This study compared a shared-care treatment methodology, with patients treated predominantly within a primary care setting, with a hospital-based treatment modality, with patients treated predominantly within a hospital clinical setting. In the shared-care treatment methodology, the aims were to reduce joint pain, stiffness and related symptoms, whereas in the hospital-based methodology these aims were supplemented by an additional aim, to reduce clinical and laboratory evidence of inflammation.
A total of 466 patients were randomly allocated to one of the two treatment arms. Two hundred and thirty-three of these patients, 68.2% female, with a mean age of 60.4 years and mean duration of symptomatic RA of 12.6 years received shared care. The remaining 233 patients, 67.8% female, with a mean age of 60.8 years and mean duration of symptomatic RA of 12.5 years, received hospital-based care. The target was controlling joint pain, stiffness and related symptoms.
Health-related quality of life values were evaluated using the EQ-5D instrument at baseline and every 4 months, finishing at 36 months. The discounted QALYs were accrued by patients in each cohort of the study for three time periods: baseline to 12 months, 12–24 months and 24–36 months. The QALYs are presented in Table 59 together with the total QALYs accrued by patients in both arms of the study across the entire study period.
| Assessment period | Treatment | |
|---|---|---|
| Shared care | Hospital based | |
| Baseline to 12 months | 0.60 | 0.57 |
| 12–24 months | 0.55 | 0.53 |
| 24–36 months | 0.52 | 0.50 |
| Entire study period | 1.67 | 1.60 |
Thus, according to the authors, the incremental QALYs accrued by patients in the shared-care cohort compared with patients in the hospital-based treatment cohort is 0.07 QALYs. A sensitivity analysis was conducted, which adjusted the calculation for differences in baseline utilities between arms.
The undiscounted costs incurred by patients in the shared-care cohort and the hospital-based treatment cohort across the 36-month period of the study are presented in Table 60. The authors report an incremental discounted cost of the shared-care treatment compared with hospital-based treatment of £106.
| Cost category | Treatment (£) | |
|---|---|---|
| Shared care | Hospital based | |
| Inpatient care | 1575 | 1261 |
| Outpatient care | 997 | 1369 |
| Primary care | 502 | 395 |
| Other health care | 98 | 90 |
| Drug therapy | 1475 | 1403 |
| Aids and appliances | 68 | 76 |
| Total | 4700 | 4581 |
The authors conclude that the ICER of shared-care compared with hospital-based treatment is £1517 per QALY. A sensitivity analysis was conducted in which the calculation was adjusted for baseline utility values and this produced an ICER of £7571.
From the evidence presented in this study, shared-care treatment appears cost-effective when compared with hospital-based care.
The Optimisation of Adalimumab study (Pope et al., 2010; and Pope et al., 2013)
Both of the documents by Pope et al. 52,53 report results from the Optimisation of Adalimumab study. The Optimisation of Adalimumab study is an 18-month cluster RCT set in Canada that investigated whether or not TTT produced better results than routine care in established RA. Pope et al. 53 was an abstract that has been superseded by a full paper. 52 A total of 308 patients were randomly allocated to one of three treatment arms. One hundred and nine patients, 83.5% female, with a mean age of 56.0 years and mean baseline DAS28 of 5.7 received routine care with no target. One hundred patients, 82.0% female, with a mean age of 55.3 years and mean baseline DAS28 of 5.7 received treatment in which the target was a DAS28 of < 2.6. The remaining 99 patients, 77.8% female, with a mean age of 51.5 years and mean baseline DAS28 of 5.8 received treatment in which the target was to achieve a SJC of 0.
Study outcomes measured at 6, 12 and 18 months were the DAS28, the percentage of patients achieving LDA (defined as a DAS28 of < 3.2), the percentage of patients achieving remission (defined as a DAS28 of < 2.6), the percentage of patients achieving a good/moderate EULAR response (undefined) and the percentage of patients achieving a SJC of 0. Analyses were undertaken using an intention-to-treat basis. These outcomes are presented in Table 61.
| Outcome by time point | Treatment group | p-value | ||
|---|---|---|---|---|
| Routine care | Target of | |||
| DAS28 of < 2.6 | SJC of 0 | |||
| Mean DAS28a | ||||
| 6 months | 3.26 | 3.72 | 3.49 | 0.460 |
| 12 months | 3.12 | 3.38 | 3.18 | 0.619 |
| 18 months | 3.27 | 3.40 | 3.16 | 0.273 |
| Intention-to-treat analyses | ||||
| Percentage of patients achieving remission (DAS28 of < 2.6) | ||||
| 6 months | 17.4 | 24.0 | 16.2 | 0.564b |
| 12 months | 21.1 | 28.0 | 26.3 | 0.697b |
| 18 months | 15.6 | 38.0 | 22.2 | 0.027b |
| Percentage of patients achieving LDA (DAS28 of < 3.2) | ||||
| 6 months | 28.4 | 33.0 | 30.3 | 0.880b |
| 12 months | 32.1 | 39.0 | 31.3 | 0.672b |
| 18 months | 22.9 | 47.0 | 27.3 | 0.022b |
| Percentage of patients achieving a good or moderate EULAR response | ||||
| 6 months | 56.9 | 62.0 | 62.5 | 0.634b |
| 12 months | 51.4 | 61.0 | 50.5 | 0.508b |
| 18 months | 35.8 | 63.0 | 53.5 | 0.018b |
| Percentage of patients achieving an SJC of 0 | ||||
| 6 months | 22.0 | 27.0 | 24.2 | 0.839b |
| 12 months | 22.9 | 29.0 | 26.3 | 0.780b |
| 18 months | 21.1 | 34.0 | 26.3 | 0.331b |
At all of the time points the strategy of treating to a target of a DAS28 of < 2.6 had a numerical advantage in terms of the percentage of patients achieving the following criteria: achieving remission; LDA; good or moderate EULAR response; and no swollen joints. Statistical significance was not reached given an adjustment in the significance level as a result of multiple testing, but was borderline at 18 months for EULAR response and approached significance at 18 months for remission and for LDA. Thus, there may be a true beneficial effect that has not been observed as a result of the small sample size.
The dose of ADA was not changed across arms, although the paper reports that there were more intensifications of other drugs in the targeted arms. The incremental cost of treatment between the arms of the study was therefore assumed to be negligible. However, the paper reports that there were more physician visits in the targeted groups, 4.3 in the routine care arm and 6.5 in each of the targeted arms. The additional 2.2 visits were assumed to cost £128,93 resulting in additional costs of £281.60 for the two targeted arms.
Given the numerical advantage of the targeted treatment strategies, which may have failed to reach reached significance only because of small sample sizes, no firm conclusion on the cost-effectiveness or cost–utility of the treatment strategies can be made.
Mixed, early and established disease populations
TIght COntrol for Rheumatoid Arthritis trial (Grigor et al., 2004)
The paper by Grigor et al. 61 reports on an 18-month single-blind RCT set in the UK. A total of 111 RA patients were randomly allocated to one of two treatment arms, with one patient dropping out after randomisation. Fifty-five patients, 71% female, with a mean age of 51 years, received intensive therapy. The remaining 55 patients, 69% female, with a mean age of 54 years, received routine therapy. The target in the intensive management arm was a DAS44 score of ≤ 2.4: there was no target in the routine therapy arm.
The primary outcome was the mean reduction in DAS and the proportion of patients with a good EULAR response (a DAS44 of < 2.4 and a fall from baseline of > 1.2) over the 18-month study period. Secondary outcomes included the percentage of patients achieving remission, defined as a DAS28 of < 1.6. These results are presented in Table 62. The results indicate that there are statistically significant differences in the effectiveness of intensive care compared with routine care.
| Outcome | Treatment group | p-value | |
|---|---|---|---|
| Routine care | Intensive care | ||
| Number in study arm | 55 | 55 | – |
| Mean reduction in DAS44 | –1.9 | –3.5 | < 0.0001 |
| Patients achieving a good EULAR response (%) | 44 | 82 | < 0.0001 |
| Number achieving remission (%) | 16 | 65 | < 0.0001 |
Costs, using 2001/2 prices, were taken directly from the paper (Table 63). All costs except prescription costs were uplifted to 2014/15 values using inflation indices from Unit Costs for Health & Social Care 201589 and Unit Costs for Health & Social Care 201090 (as the 2015 version did not include the inflation index for 2001/2). It was assumed that the annual increase in the cost of pharmaceuticals is not in line with the inflation indices given in the Unit Costs for Health & Social Care 2015. 89 Ideally, the total pharmaceutical cost for each arm would have been calculated using current costs; however, the paper did not specify either the total or the individual pharmaceutical use in each arm and so this was not possible. These values were left at their 2001/2 values assuming that the incremental pharmaceutical or prescription cost would not differ considerable between 2001/2 and 2014/15.
| Cost category | Cost year calculation (£) | |||
|---|---|---|---|---|
| Original paper (2001/2) | Uplifted to current costs (2014/15) | |||
| Routine care | Intensive care | Routine care | Intensive care | |
| Outpatient cost | 401 | 698 | 569 | 991 |
| Inpatient cost | 1611 | 571 | 2287 | 810 |
| Prescription costs | 452 | 649 | 452 | 649 |
| Health professional visits | 1249 | 859 | 1773 | 1219 |
| Diagnostic tests | 341 | 568 | 484 | 806 |
| Total | 4054 | 3345 | 5565 | 4476 |
The incremental mean savings per patient, using 2014/15 values, was £1089. There were 55 participants in both the intensive care arm and the routine care arm of this study and the total savings associated with intensive care were £59,892.
Given that the intensive care arm was associated with better patient outcomes and cost savings, intensive care is estimated to dominate routine care.
van Hulst et al., 2010
The paper by van Hulst et al. 63 reports on an 18-month randomised pilot study set in the Netherlands. A total of 248 patients were randomly allocated to one of two treatment arms. One hundred and forty-four patients, 68% female, with a mean age of 60 years, were allocated to a nurse-led intervention. In this intervention, treating rheumatologists were informed of a patient’s DAS28 before each consultation with a target of reducing a patient’s DAS28 to ≤ 3.2 (mean age 60 years and 68% were female). One hundred and four patients, 60% female, with a mean age of 59 years, were allocated to a usual-care arm. These patients had their DAS28 calculated at each consultation, to allow a comparison of efficacies between arms to be evaluated, but the DAS28 was not communicated to the treating rheumatologists. The target in the intensive management arm was a DAS28 of ≤ 3.2: there was no target in the routine therapy arm.
In this study, participants underwent an assessment by a rheumatology nurse each time they were seen by their treating rheumatologist. In practice, usual-care patients would not be assessed by a rheumatology nurse at each consultation and, therefore, it is anticipated that the nurse-led intervention would be associated with a higher cost.
Study outcomes that were measured at 18 months were based on the change in DAS28 and the percentage of patients exhibiting a good, moderate or no EULAR response. These outcomes are presented in Table 64.
| Outcome | Therapy | p-value | |
|---|---|---|---|
| Intervention | Usual care | ||
| Number of participants | 144 | 104 | – |
| Change in DAS28 | –0.69 | –0.66 | 0.7 |
| Good EULAR response (%) | 21.5 | 18.3 | NR |
| Moderate EULAR response (%) | 22.9 | 26.9 | NR |
| No EULAR response (%) | 55.6 | 54.8 | NR |
There was no evidence of a statistically significant difference in the effectiveness of a nurse-led intervention compared with usual care. Given the anticipated higher costs, it is highly likely that the nurse-led approach would be dominated by usual care or have an estimated cost per QALY greater than those published by NICE. 92
Discussion
Literature relating to 16 studies was found. In papers relating to six of these studies [i.e. Saunders et al.,54 van Eijk et al.,55 Pope et al.,52,53 den Uyl et al.,69 Hodkinson et al. 51 and CareRA39,40,43 (low risk, presumably of progression, patient subgroup)], no clear conclusions could be made regarding comparative cost-effectiveness. In the remaining 10 studies and for the high-risk patient subgroup in the CareRA trial,39,42,43 the authors believe that conclusions could be made with some confidence. In Mottonen et al.,46 combination drug therapy was estimated to be more cost-effective than single-drug therapy; in Grigor et al.,61 intensive care was associated with both better outcomes and lower costs than routine care; in Fransen et al.,50 systematic monitoring produced significantly more patients with LDA than usual care for similar costs; in Symmons et al.,9 the paper concludes that the ICER of shared-care treatment compared with hospital-based treatment is £1517 per QALY or £7571 per QALY when accounting for different baseline utility; in Verstappen et al.,36 it was concluded that intensive therapy would be more cost-effective than conventional therapy; in van Hulst et al.,63 usual care was deemed more cost-effective than a nurse-led approach as the additional resources required were not translated into health benefits; in Urata et al,57 a policy of using both DAS28 and MMP-3 to drive treatment decisions has been estimated by the authors of this report to be < £170 per additional person in remission compared with strategies of MMP-3-driven treatment or DAS28-driven treatment alone or routine care; in the TEAR58–60 study, the additional costs of immediate ETN, or the use of ETN before triple therapy, would not be justified by any clinical gain; whereas in the BeSt trial,26–34,42,64,65,68 the additional costs of initial combination therapy with IFX has not been shown to produce health benefits above initial combination therapy with PDN despite the markedly higher costs; in the CareRA trial,38,42,43 for high-risk (presumably of progression) patients, efficacy was similar across the three arms but there were significantly fewer AEs in the COBRA Slim arm; and in Bijlsma et al.,62 although TOC produced a statistically significantly higher percentage of people who achieved sustained remission, it would likely be associated with markedly higher costs than MTX and it is believed that the cost per QALY of using bDMARDs prior to treatment with intensive cDMARDs would have a cost per QALY greater than those published by NICE. 92
There were too few studies in established RA to discern a pattern regarding cost-effectiveness: in two studies it was believed that a clear conclusion on cost-effectiveness could be made,9,50 and in one no clear conclusion could be drawn. 52,53
As previously stated, the regimens used within the studies were too heterogeneous to draw conclusions that compared all of the tested strategies simultaneously. Furthermore, it is unclear how routine care has changed over time and setting; for example, recent data97 have shown that the intensity of cDMARD treatment has increased since the publication of results from the TICORA trial,61 which indicated that an intensive approach was both more beneficial for patients and saved money. However, there appears to be a pattern that treating intensively with cDMARDs was likely to be more cost-effective than treating with routine practice: the extent and degree to which this result was attributable to treating to a specific target is uncertain. A further pattern was that treating with bDMARDs prior to intensive treatment with cDMARDs would not produce sufficient benefit to justify the additional costs, given published NICE thresholds. 92 Of course, caution in the interpretation of these results is required because of the fact that cost estimates for some papers had to be undertaken on the basis of very limited data and outcomes could not always be expressed in standard metrics.
Chapter 5 Assessment of factors relevant to the NHS and other parties
For people with newly diagnosed RA, NICE clinical guideline number 79,4 published in 2009, recommends a combination of cDMARDs (including MTX and at least one other DMARD plus short-term GCs) as first-line treatment, ideally beginning within 3 months of the onset of persistent symptoms. In early disease, this element of more intensive treatment of patients is often part of TTT strategies that have been tested in clinical studies. It is therefore the case that some elements of TTT are likely to be already in widespread NHS practice. It is not clear the extent to which other elements of TTT (the setting of explicit treatment goals and more frequent assessments of patients) are practised in the NHS or if such approaches are consistently followed in all areas of the UK.
Treat to target is typically more resource intensive during the early management of disease, until LDA or remission is achieved. There is a requirement for more frequent assessments and monitoring. Therefore, if there was a more widespread adoption of this form of TTT than is currently the case, this may place demands on rheumatology services.
It is also feasible that some patients whose disease progresses rapidly will be identified earlier via TTT management approaches and move to bDMARD therapies more rapidly than would otherwise be the case.
Chapter 6 Discussion
Statement of principal findings
Treat to target refers not to a single concept, but to a spectrum of broad approaches to the treatment of patients. TTT requires, at a minimum, the specification of a treatment objective. However, TTT commonly combines the specifying of a patient target with more frequent assessment of the target and adjustments to treatments by the clinician in response to those assessments. Those treatment adjustments can be entirely at the discretion of the clinician or may be protocolised. Clinical studies included in this report exhibit marked heterogeneity in each of the aforementioned aspects of TTT. Studies also differ in terms of the patient populations they examine, with the distinction between early and established disease being particularly important. Owing to these differences, it was not possible to quantitatively synthesise evidence across the studies and the number of studies where valid comparisons can be made, even qualitatively, is reduced.
Compared with usual care, there is no clear evidence either in favour of, or against, the clinical effectiveness of a TTT approach, in terms of the proportion of patients meeting the target, attaining LDA and attaining remission. In early RA, two studies found evidence in favour of the TTT approach. TTT was more effective in terms of the proportion of patients meeting the target and attaining remission. The T-4 study56,57 found usual care to be more effective than the MMP-3-targeted arm, but the combined DAS28 of < 2.6- and MMP-3-targeted arm to be more effective than usual care [OR 0.21 (95% CI 0.10 to 0.47) at 1 year in the T-4 study56,57], in terms of the proportion of patients attaining remission. In trials with an established RA population, there was evidence in favour of a TTT approach compared with usual care in the Fransen et al. trial50 in terms of the proportion of patients meeting the target or attaining LDA, but no difference between TTT and usual care in the Optimisation of Adalimumab study52 in terms of the proportion of patients meeting the target or attaining LDA or remission. The TICORA trial61 demonstrated evidence in favour of a TTT approach in a population containing both early and established RA patients in terms of the proportion of patients attaining remission.
The evidence is also mixed when comparing TTT to usual care in terms of other outcomes (DAS44 response, SJC, TJC, EULAR response, HAQ, erosions and quality of life). In early RA, there were mixed findings for DAS28/DAS4455,57 and joint erosion,55,57 and no difference between TTT arms and usual care on HAQ score. 55,57 In established RA, there was evidence in favour of in terms of EULAR response in the Optimisation of Adalimumab study. 52 There was, however, no difference between TTT and usual care in terms of DAS28,50,52 SJC,52 TJC52 or HAQ response. In a mixed population, a TTT approach was favoured compared with usual care in terms of ACR 20/50/70 response in the TICORA61 trial. The evidence, however, was equivocal for DAS28/DAS44 score,61,63 SJC,61,63 TJC,61,63 EULAR response,61,63 HAQ response,61,63 joint erosion61 and quality of life. 61
Few differences in outcomes were found in relation to different targets within TTT strategies. Only the T-4 study56,57 (early RA) found differences between targets: the DAS28 of < 2.6 target was more effective than the MMP-3 target, and the combined DAS28 of < 2.6 and MMP-3 target was more effective than both the DAS28 of < 2.6 target and the MMP-3 target, in terms of the proportion of patients in remission. Among trials examining an early RA population, there was no difference in the clinical effectiveness of different targets on DAS28/DAS44,51,57,58 TJC,51,58 EULAR response51 and HAQ score response. 51,57,58 Findings relating to SJC,51,58 ACR 20/50/70 response58 and joint erosion57,58 were equivocal. In established RA populations, there is evidence in favour of TTT, using a LDA target (DAS28 of ≤ 3.2),50 but evidence is mixed with regard to remission (DAS28 of < 2.6) and SJC (SJC of 0) targets. 52 For other outcomes, only the Optimisation of Adalimumab study52 demonstrated the benefit of a DAS28 of < 2.6 target over a SJC of 0 target. Both trials examining populations containing both early and established RA patients (i.e. the TICORA trial61 and van Hulst et al. 63) examined a LDA target (DAS44 of ≤ 2.461 or DAS28 of ≤ 3.263), and demonstrated evidence in favour of a TTT approach on most outcomes, with ambiguous evidence on some clinical outcomes. It is also important to note that all trials comparing different targets were rated as being at high risk of bias.
Only a small number of studies report data on AEs. Among trials examining an early RA population, there was no difference in the proportion of patients experiencing any AE, SAE, death, withdrawals as a result of AEs or specific AEs, although more events were experienced in the TTT arm compared with the usual-care arm in the STREAM trial. 55 Among trials examining established RA populations, a smaller proportion of patients withdrew as a result of AEs52 and experienced specific AEs (dermatological and gastrointestinal AEs50) in the TTT arm, compared with usual care. The only trial reporting on these outcomes in a population containing both early and established RA patients, the TICORA trial,61 which was also the only trial examining TTT compared with usual care rated as being at a low risk of bias, reported that a smaller proportion of patients reported any AE and specific AEs (dermatological, gastrointestinal and infectious AEs, significance not reported) in the TTT arm than in the usual-care arm. Overall, comparing trials by target on AEs, there is no clear evidence in favour of any target being more or less safe, compared with usual care.
Overall, we consider that the evidence for TTT is mixed but, in early RA, there does seem to be some limited support for TTT, in general, on some clinical outcomes. This is particularly true if the TICORA trial results, which was the only study in the review considered at low risk of bias, are interpreted as providing evidence relevant to the early RA population. The inclusion criterion for the TICORA trial was that patients had to have a disease duration of < 5 years.
There is also evidence to suggest that, in early RA, the components of care that together constitute TTT are likely to form a cost-effective approach. Conclusions relating to cost-effectiveness could be drawn for 10 studies, and for the high-risk patient subgroup in the CareRA trial. Almost all of the estimates from these studies indicated that TTT would be considered cost-effective other than when the TTT strategy included the use of bDMARDs in early disease. No conclusions could be made in relation to TTT in established disease.
Patient and public involvement representatives stated that if a TTT strategy were to be implemented, it would be beneficial that the patient was explicitly informed of this and made aware of the planned escalation of medication and the proposed target.
Strengths and limitations of the assessment
The current systematic review is the most comprehensive review to date to examine the clinical effectiveness of TTT. We have synthesised findings on TTT compared with usual care and a comparison of different targets. However, TTT is a broad term covering a spectrum of different treatment strategies. Clinical studies test TTT strategies that are heterogeneous. It was not felt justified to pool these strategies and make comparisons based on a synthesis of the evidence base.
Synthesis of different treatment protocols was precluded (even in terms of a narrative synthesis) by heterogeneity and lack of comparability between treatment protocols, although we have provided a comprehensive summary of the findings of trials that compare different treatment protocols. The current review is also the only systematic review to examine findings by population, which is important in this context as the recommendations for TTT differ slightly for early and established RA patients, as the treatment prognosis and implications of TTT may be different in these populations. 17,18 Another strength of the current review is the focus on RCTs, which reduces the impact of selection bias on the review findings.
A further strength of the work undertaken is in assessing the likely cost-effectiveness implications of each study identified in the clinical review. These results, which help inform the conclusions on the cost-effectiveness of TTT, also serve as a source of information for fellow researchers.
The main limitation of the current review is the small number of trials within in each comparison, in each population group. There was much heterogeneity in terms of targets, treatment protocols and frequency of contact between trials, even within each population group, in each comparison. Risk of bias was rated as being high in the majority of included trials.
The heterogeneity in identified studies, inter alia, placed additional constraints on the cost-effectiveness analysis. We adopted a pragmatic approach to estimate costs and outcomes from data reported alongside each clinical study. The required assumptions to optimise the use of limited resource use data and often also required the expression of outcomes in non-standard terms.
Uncertainties
It is unclear from the current review if there are specific elements of TTT that drive clinical effectiveness and cost-effectiveness, as TTT strategies tested in the identified clinical studies are varied and variously include the formal assessment of patients, clear protocols for drug treatment changes in response to patient assessments and more frequent assessment of patients. Existing NICE guidelines, based on systematic review of the literature, already recommend combination non-biologic drug treatment in early disease. This review is unable to establish if the adding in of other elements of TTT represents a cost-effective approach to care.
There is more limited evidence relating to TTT as a concept in established disease.
Chapter 7 Conclusions
Implications for service provision
For people with newly diagnosed RA, NICE Clinical Guideline 794 recommends a combination of cDMARDs (including MTX and at least one other DMARD plus short-term GCs) as first-line treatment. For patients with early disease, this element of more intensive treatment for patients is often part of TTT strategies that have been tested in clinical studies. Furthermore, it is likely that rheumatologists would monitor patients more frequently if treating with a more intense drug strategy. It is therefore likely that many rheumatologists, if compliant with NICE guidance, would experience little impact on their services were they to switch to a formal TTT strategy. It is also likely that many are already providing TTT in some form.
It is likely that more widespread adoption of TTT principles, particularly those TTT approaches that combine a treatment target with intensive drug protocols and frequent assessment of patients, would require more intense management of patients with early disease during their initial treatment period. It is also feasible that some patients whose disease progresses rapidly will be identified earlier via TTT management approaches, and move to bDMARD therapies more rapidly than would otherwise be the case.
Suggested research priorities
We highlight substantial uncertainty in the evidence base for TTT in RA. In part, this stems from the relatively low quality of the clinical trials identified. Only one study, the TICORA trial,61 was rated as having a low risk of bias. However, there is also significant uncertainty that stems from the fact that TTT describes a broad concept, used to describe a spectrum of ways to treat patients, rather than a clearly defined, individual technology. Clinical studies reflect this and exhibit a wide degree of heterogeneity in the types of TTT they seek to examine. This results in a series of studies that cannot feasibly be pooled quantitatively, diminishing the strength of any conclusions. This heterogeneity also means that it is difficult to disentangle the effect of different elements of TTT strategies: the target itself, whether or not there is a treatment protocol to be followed in the light of assessment of the target and the frequency of patient assessment.
The design of any future clinical trials needs to be carefully assessed to ensure that aspects of these uncertainties will be resolved by the data they generate. If such research is to be conducted, there should be a focus on well-conducted trials comparing TTT with usual care and/or different TTT targets, which are adequately blinded (in terms of participants, study personnel and outcome assessment), with low rates of attrition and adequate allocation concealment, reporting on the proportion of patients meeting the target and being in remission. It is imperative that the design of such trials is mindful of the contribution results may make to the overall body of existing evidence on the subject. This requires comparability between trials. For example, remission, defined in a consistent manner, should be the target of choice for future studies. This extends to considerations of cost-effectiveness. As well as conducting cost-effectiveness analyses alongside the trial, methods for ensuring long-term extensions studies, that allow some of the key uncertainties in this area to be addressed, are critical (e.g. the rate at which patients move to bDMARD therapies in the long term).
Patient and public involvement representatives stated that future research into the fatigue that is associated with RA would be beneficial, as it was not only swollen joints and pain that were of concern to the patient.
Acknowledgements
We would like to thank John Stevens, Reader in Decision Science, School of Health and Related Research (ScHARR), for advice regarding the decision problem; Eva Kaltenthaler, Professor of HTA, ScHARR, and Dr Chris Skedgel, Senior Lecturer in Applied Health Economics, University of East Anglia, for providing comments on the draft report; and Andrea Shippam, Programme Manager, ScHARR, for providing administrative support, and in preparing and formatting the report.
Contributions of authors
Allan Wailoo (Professor, Health Economics) oversaw and contributed to all aspects of the project.
Emma S Hock (Research Fellow, Systematic review) undertook the literature review relating to clinical effectiveness, detailed the identified studies and provided the summary of evidence found.
Matt Stevenson (Professor, HTA) undertook the review of cost-effectiveness papers and produced the estimates of cost-effectiveness related to each study identified in the clinical effectiveness review.
Marrissa Martyn St James (Research Fellow, Systematic review) undertook the literature review relating to clinical effectiveness, detailed the identified studies and provided the summary of evidence found.
Andrew Rawdin (Research Assistant, Health Economics) undertook the review of cost-effectiveness papers and produced the estimates of cost-effectiveness related to each study identified in the clinical effectiveness review.
Emma Simpson (Research Fellow, Systematic review) undertook the literature review relating to clinical effectiveness, detailed the identified studies and provided the summary of evidence found.
Ruth Wong (Information Specialist) devised and ran the search strategies.
Naila Dracup (Information specialist) devised and ran the search strategies.
David L Scott (Professor, Clinical rheumatology) provided clinical advice to the project and commented on specific aspects of the clinical evidence.
Adam Young (Consultant Rheumatologist) provided clinical advice to the project and commented on specific aspects of the clinical evidence.
All authors were involved in drafting and commenting on the final report.
Data sharing statement
There are no new data generated from this project beyond those reported in the project report. Further information on any aspects of the study can be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Scott DL, Steer S. The course of established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:943-67. https://doi.org/10.1016/j.berh.2007.05.006.
- Pincus T, Fuchs HA, Callahan LF, Nance EP, Kaye JJ. Early radiographic joint space narrowing and erosion and later malalignment in rheumatoid arthritis: a longitudinal analysis. J Rheumatol 1998;25:636-40.
- Drossaers-Bakker KW, Kroon HM, Zwinderman AH, Breedveld FC, Hazes JM. Radiographic damage of large joints in long-term rheumatoid arthritis and its relation to function. Rheumatology 2000;39:998-1003. https://doi.org/10.1093/rheumatology/39.9.998.
- Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults. London: Royal College of Physicians; 2009.
- Naz SM, Symmons DP. Mortality in established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:871-83. https://doi.org/10.1016/j.berh.2007.05.003.
- Dadoun S, Zeboulon-Ktorza N, Combescure C, Elhai M, Rozenberg S, Gossec L, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine 2013;80:29-33. https://doi.org/10.1016/j.jbspin.2012.02.005.
- Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology 2009;48:1309-13. https://doi.org/10.1093/rheumatology/kep252.
- Pugner KM, Scott DI, Holmes JW, Hieke K. The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum 2000;29:305-20. https://doi.org/10.1016/S0049-0172(00)80017-7.
- Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL. The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9340.
- Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800. https://doi.org/10.1093/rheumatology/41.7.793.
- Symmons DP, Barrett EM, Bankhead CR, Scott DG, Silman AJ. The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. Br J Rheumatol 1994;33:735-9. https://doi.org/10.1093/rheumatology/33.8.735.
- Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 2005;4:130-6. https://doi.org/10.1016/j.autrev.2004.09.002.
- Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for Rheumatoid Arthritis Not Previously Treated With DMARDs or After Conventional DMARDs Only Have Failed. London: NICE; 2016.
- Adalimumab, Etanercept, Infliximab, Rituximab and Abatacept for the Treatment of Rheumatoid Arthritis After the Failure of a TNF Inhibitor. London: NICE; 2010.
- Tocilizumab for the Treatment of Rheumatoid Arthritis (Rapid Review of Technology Appraisal Guidance 198). London: NICE; 2012.
- Golimumab for the Treatment of Rheumatoid Arthritis After the Failure of Previous Disease-Modifying Anti-Rheumatic Drugs. London: NICE; 2011.
- Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3-15. https://doi.org/10.1136/annrheumdis-2015-207524.
- Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631-7. https://doi.org/10.1136/ard.2009.123919.
- Felson DT, Smolen JS, Wells G, Zhang B, Van Tuyl LHD, Funovits J, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011;63:573-86. https://doi.org/10.1002/art.30129.
- Solomon DH, Bitton A, Katz JN, Radner H, Brown EM, Fraenkel L. Review: Treat to Target in Rheumatoid Arthritis: Fact, Fiction, or Hypothesis? 2014;66:775-82. https://doi.org/10.1002/art.38323.
- Jurgens MS, Welsing PM, Jacobs JW. Overview and analysis of treat-to-target trials in rheumatoid arthritis reporting on remission. Clin Exp Rheumatol 2012;30:56-63.
- Scott DL, Ibrahim F, Farewell V, O’Keeffe AG, Ma M, Walker D, et al. Randomised controlled trial of Tumour necrosis factor inhibitors Against Combination Intensive Therapy with conventional disease-modifying antirheumatic drugs in established rheumatoid arthritis: the TACIT trial and associated systematic reviews. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18660.
- Schoels M, Knevel R, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas DT, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search. Ann Rheum Dis 2010;69:638-43. https://doi.org/10.1136/ard.2009.123976.
- Higgins JPT, Altman DG, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 510 [updated March 2011]: The Cochrane Collaboration. n.d.
- Wright CC, Sim J. Intention-to-treat approach to data from randomized controlled trials: a sensitivity analysis. J Clin Epidemiol 2003;56:833-42. https://doi.org/10.1016/S0895-4356(03)00155-0.
- Allaart CF, Breedveld FC, Dijkmans BA. Treatment of recent-onset rheumatoid arthritis: lessons from the BeSt study. J Rheumatol Suppl 2007;80:25-33.
- Allaart CF, Geokoop-Ruiterman YPM, de Vries-Bouwstra JK, Breedveld FC, Dijkmans BAC. Aiming at low disease activity in rheumatoid arthritis with initial combination therapy or initial monotherapy strategies: The BeSt study. Clin Exp Rheumatol 2006;24:S77-82.
- Dirven L, Bek M, Klarenbeek NB, Han KH, Ronday HK, Kerstens P, et al. Eight year results of disease activity steered treatment in a large recent rheumatoid arthritis cohort: clinical and radiological outcomes. Arthritis Rheum 2011;63.
- Dirven L, van den Broek M, Klarenbeek NB, Van Krugten MV, Van Der Lubbe PAHM, Kerstens PJSM, et al. Seven year results of DAS steered treatment in the best study: clinical and radiological outcomes. Arthritis Rheum 2010;62.
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381-90. https://doi.org/10.1002/art.21405.
- Klarenbeek NB, Güler-Yüksel M, van der Kooij SM, Han KH, Ronday HK, Kerstens PJ, et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis 2011;70:1039-46. https://doi.org/10.1136/ard.2010.141234.
- Koevoets R, Dirven L, Klarenbeek NB, van Krugten MV, Ronday HK, van der Heijde DM, et al. ‘Insights in the relationship of joint space narrowing versus erosive joint damage and physical functioning of patients with RA’. Ann Rheum Dis 2013;72:870-4. https://doi.org/10.1136/annrheumdis-2011-201191.
- Markusse IM, Dirven L, Van Groenendael JH, Han KH, Ronday HK, Kerstens PJSM, et al. Mortality in a large cohort of patients with early rheumatoid arthritis that were treated-to-target for 10 years. Arthritis Rheumatol 2014;66.
- van den Broek M, Dirven L, Klarenbeek N, Krugten M, Ronday H, Kerstens P, et al. Clinical and radiological outcomes of four disease activity driven treatment strategies: 8-year results of the best study. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.1837.
- Verstappen SM, Bakker MF, Heurkens AH, van der Veen MJ, Kruize AA, Geurts MA, et al. Adverse events and factors associated with toxicity in patients with early rheumatoid arthritis treated with methotrexate tight control therapy: the CAMERA study. Ann Rheum Dis 2010;69:1044-8. https://doi.org/10.1136/ard.2008.106617.
- Verstappen SM, Jacobs JW, Veen MJ, Heurkens AH, Schenk Y, ter Borg EJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis 2007;66:1443-9. https://doi.org/10.1136/ard.2007.071092.
- Bakker MF, Jacobs JWG, Welsing PMJ, Verstappen SMM, Tekstra J, Ton E, et al. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med 2012;156:329-39. https://doi.org/10.7326/0003-4819-156-5-201203060-00004.
- De Cock D, Meyfroidt S, Joly J, Van der Elst K, Westhovens R, Verschueren P. For remission induction with glucocorticoid bridging, methotrexate monotherapy is as effective as a combination with other DMARDs, with fewer reported side effects: 4 month primary outcome of care, a randomized induction strategy and treat to target trial in early rheumatoid arthritis. Arthritis Rheum 2013;65:3325-6.
- De Cock D, Westhovens R, Corluy L, Joos R, Langenaken C, Taelman V, et al. Comparison of MTX therapy with or without a moderate dose glucocorticoid bridging scheme in early rheumatoid arthritis patients lacking classical poor prognostic markers: week 16 results from the randomized multicenter carera trial. Ann Rheum Dis 2014;73. https://doi.org/10.1136/annrheumdis-2014-eular.2144.
- Verschueren P, Cock D, Corluy L, Joos R, Langenaken C, Taelman V, et al. Patients lacking classical poor prognostic markers might also benefit from a step-down glucocorticoid bridging scheme in early rheumatoid arthritis: week 16 results from the randomized multicenter CareRA trial. Arthritis Res Ther 2015;17. https://doi.org/10.1186/s13075-015-0611-8.
- Verschueren P, Cock D, Corluy L, Joos R, Langenaken C, Taelman V, et al. Associated with a glucocorticoid bridging scheme, methotrexate is as effective alone as in combination with other DMARDs for early rheumatoid arthritis, with fewer reported side effects: 16 weeks remission induction data from the carera trial. Ann Rheum Dis 2014;73. https://doi.org/10.1136/annrheumdis-2014-eular.2137.
- Verschueren P, Cock D, Corluy L, Joos R, Langenaken C, Taelman V, et al. Methotrexate in combination with other DMARDs is not superior to methotrexate alone for remission induction with moderate-to-high-dose glucocorticoid bridging in early rheumatoid arthritis after 16 weeks of treatment: the CareRA trial. Ann Rheum Dis 2015;74:27-34. https://doi.org/10.1136/annrheumdis-2014-205489.
- Verschueren P, De Cock D, Corluy L, Joos R, Langenaken C, Taelman V, et al. Effectiveness of methotrexate with step-down glucocorticoid remission induction (COBRA Slim) versus other intensive treatment strategies for early rheumatoid arthritis in a treat-to-target approach: 1-year results of CareRA, a randomised pragmatic open-label superiority trial. Ann Rheum Dis 2017;76:511-20. https://doi.org/10.1136/annrheumdis-2016-209212.
- den Uyl D, ter Wee M, Boers M, Kerstens P, Voskuyl A, Nurmohamed M, et al. A non-inferiority trial of an attenuated combination strategy (‘COBRA-light’) compared to the original COBRA strategy: clinical results after 26 weeks. Ann Rheum Dis 2014;73:1071-8. https://doi.org/10.1136/annrheumdis-2012-202818.
- ter Wee MM, den Uyl D, Boers M, Kerstens P, Nurmohamed M, van Schaardenburg D, et al. Intensive combination treatment regimens, including prednisolone, are effective in treating patients with early rheumatoid arthritis regardless of additional ETN: 1-year results of the COBRA-light open-label, randomised, non-inferiority trial. Ann Rheum Dis 2015;74:1233-40. https://doi.org/10.1136/annrheumdis-2013-205143.
- Mottonen T, Hannonen P, Leirisalo-Repo M, Nissila M, Kautiainen H, Korpela M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. Lancet 1999;353:1568-73. https://doi.org/10.1016/S0140-6736(98)08513-4.
- Rantalaiho V, Korpela M, Hannonen P, Kautiainen H, Järvenpää S, Leirisalo-Repo M, et al. The good initial response to therapy with a combination of traditional disease-modifying antirheumatic drugs is sustained over time: the eleven-year results of the Finnish rheumatoid arthritis combination therapy trial. Arthritis Rheum 2009;60:1222-31. https://doi.org/10.1002/art.24447.
- Rantalaiho V, Korpela M, Laasonen L, Kautiainen H, Järvenpää S, Hannonen P, et al. Early combination disease-modifying antirheumatic drug therapy and tight disease control improve long-term radiologic outcome in patients with early rheumatoid arthritis: the 11-year results of the Finnish Rheumatoid Arthritis Combination Therapy trial. Arthritis Res Ther 2010;12. https://doi.org/10.1186/ar3060.
- Rantalaiho V, Puolakka K, Korpela M, Hannonen P, Mottonen T. Long-term results of the FIN-RACo trial; treatment with a combination of traditional disease-modifying anti-rheumatic drugs is an excellent option in early rheumatoid arthritis. Clin Exp Rheumatol 2012;30:S27-31.
- Fransen J, Moens HB, Speyer I, van Riel PL. Effectiveness of systematic monitoring of rheumatoid arthritis disease activity in daily practice: a multicentre, cluster randomised controlled trial. Ann Rheum Dis 2005;64:1294-8. https://doi.org/10.1136/ard.2004.030924.
- Hodkinson B, Musenge E, Tikly M. Tight control of rheumatoid arthritis in a resource-constrained setting: A randomized controlled study comparing the clinical disease activity index and simplified disease activity index. Rheumatol 2015;54:1033-8. https://doi.org/10.1093/rheumatology/keu443.
- Pope JE, Haraoui B, Rampakakis E, Psaradellis E, Thorne C, Sampalis JS. Treating to a target in established active rheumatoid arthritis patients receiving a tumor necrosis factor inhibitor: results from a real-world cluster-randomized adalimumab trial. Arthritis Care Res 2013;65:1401-9. https://doi.org/10.1002/acr.22010.
- Pope JE, Thorne JC, Haraoui B, Sampalis J. Treating to target with TNFi in active established rheumatoid arthritis results in longer drug survival than routine care with TNFi: results from the optimization of Humira RCT. Arthritis Rheum 2010;62.
- Saunders SA, Capell HA, Stirling A, Vallance R, Kincaid W, McMahon AD, et al. Triple therapy in early active rheumatoid arthritis: a randomized, single-blind, controlled trial comparing step-up and parallel treatment strategies. Arthritis Rheum 2008;58:1310-17. https://doi.org/10.1002/art.23449.
- van Eijk IC, Nielen MM, van der Horst-Bruinsma I, Tijhuis GJ, Boers M, Dijkmans BA, et al. Aggressive therapy in patients with early arthritis results in similar outcome compared with conventional care: the STREAM randomized trial. Rheumatol 2012;51:686-9. https://doi.org/10.1093/rheumatology/ker355.
- Urata Y, Uesato R, Tanaka D, Nakamura Y, Motomura S. Treating to target matrix metalloproteinase 3 normalisation together with disease activity score below 2.6 yields better effects than each alone in rheumatoid arthritis patients: treating to twin targets; t-4 study. Arthritis Rheum 2011;63:534-40.
- Urata Y, Uesato R, Tanaka D, Nakamura Y, Motomura S. Treating to target matrix metalloproteinase 3 normalisation together with disease activity score below 2.6 yields better effects than each alone in rheumatoid arthritis patients: T-4 study. Ann Rheum Dis 2012;71:534-40. https://doi.org/10.1136/annrheumdis-2011-200108.
- Moreland LW, O’Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheum 2012;64:2824-35. https://doi.org/10.1002/art.34498.
- Moreland LW, O’Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. Two-year radiographic results from the TEAR trial. Arthritis Rheum 2010;62.
- O’Dell JR, Curtis JR, Mikuls TR, Cofield SS, Bridges SL, Ranganath VK, et al. Validation of the methotrexate-first strategy in patients with early, poor-prognosis rheumatoid arthritis: Results from a two-year randomized, double-blind trial. Arthritis Rheum 2013;65:1985-94. https://doi.org/10.1002/art.38012.
- Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet (North American Edition) 2004;364:263-9. https://doi.org/10.1016/S0140-6736(04)16676-2.
- Bijlsma JW, Welsing PM, Woodworth TG, Middelink LM, Petho-Schramm A, Bernasconi C, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016;388:343-55. https://doi.org/10.1016/S0140-6736(16)30363-4.
- van Hulst LT, Creemers MC, Fransen J, Li LC, Grol R, Hulscher ME, et al. How to improve DAS28 use in daily clinical practice? – a pilot study of a nurse-led intervention. Rheumatology 2010;49:741-8. https://doi.org/10.1093/rheumatology/kep407.
- Markusse IM, Akdemir G, Dirven L, Goekoop-Ruiterman YP, van Groenendael JH, Han KH, et al. Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann Int Med 2016;164:523-31. https://doi.org/10.7326/M15-0919.
- van den Broek M, Lems WF, Allaart CF. BeSt practice: the success of early-targeted treatment in rheumatoid arthritis. Clin Exp Rheumatol 2012;30:35-8.
- van der Kooi E, Klarenbeek NB, Guler-Yuksel M, Kerstens P, van der Lubbe P, Westedt ML, et al. A decrease in disease activity score (DAS) level is associated with a decrease in health assessment questionnaire (HAQ) score, independent of follow-up duration, during 5 years of tightly controlled treatment: results from the BeSt study. Ann Rheum Dis 2011;70:168-71. https://doi.org/10.1136/ard.2010.133132.
- Bakker MF, Jacobs JW, Welsing PM, Vreugdenhil SA, van Booma-Frankfort C, Linn-Rasker SP, et al. Early clinical response to treatment predicts 5-year outcome in RA patients: follow-up results from the CAMERA study. Ann Rheum Dis 2011;70:1099-103. https://doi.org/10.1136/ard.2010.137943.
- van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YP, Ewals JA, Han KH, Hazes JM, et al. Patient-reported outcomes in a randomized trial comparing four different treatment strategies in recent-onset rheumatoid arthritis. Arthritis Rheum 2009;61:4-12. https://doi.org/10.1002/art.24367.
- den Uyl D, Ter Wee MM, Boers M, Voskuyl AE, Kerstens PJ, Nurmohamed MT, et al. Cobra-light therapy is clinically non-inferior to original cobra therapy in the treatment of early rheumatoid arthritis. Ann Rheum Dis 2013;71:104-5. https://doi.org/10.1136/annrheumdis-2012-eular.1834.
- Rantalaiho V, Kautiainen H, Korpela M, Mottonen T. Targeted treatment and combination DMARD therapy are additively important for reaching remission in early rheumatoid arthritis. Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-eular.1752.
- Pinals RS, Masi AT, Larsen RA. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum 1981;24. https://doi.org/10.002/art.1780241012.
- Porter DR, Grigor C, Stirling A, Capell HA. A randomised controlled trial of a strategy of tight control of disease activity in rheumatoid arthritis – outcome over 18 months. Arthritis Rheum 2003;48.
- Felson D. Defining remission in rheumatoid arthritis. Ann Rheum Dis 2012;71:i86-8. https://doi.org/10.1136/annrheumdis-2011-200618.
- Lillegraven S, Prince FH, Shadick NA, Bykerk VP, Lu B, Frits ML, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis 2012;71:681-6. https://doi.org/10.1136/ard.2011.154625.
- Lisbona MP, Solano A, Ares J, Almirall M, Salman-Monte TC, Maymó J. ACR/EULAR definitions of remission are associated with lower residual inflammatory activity compared with DAS28 remission on hand MRI in rheumatoid arthritis. J Rheumatol 2016;43:1631-6. https://doi.org/10.3899/jrheum.150849.
- Thiele K, Huscher D, Bischoff S, Späthling-Mestekemper S, Backhaus M, Aringer M, et al. Performance of the 2011 ACR/EULAR preliminary remission criteria compared with DAS28 remission in unselected patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:1194-9. https://doi.org/10.1136/annrheumdis-2012-201821.
- Van Tuyl LHD, Britsemmer K, Wells GA, Smolen JS, Zhang B, Funovits J, et al. Remission in early rheumatoid arthritis defined by 28 joint counts: limited consequences of residual disease activity in the forefeet on outcome. Ann Rheum Dis 2012;71:33-7. https://doi.org/10.1136/ard.2011.153742.
- Zufferey P, Möller B, Brulhart L, Tamborrini G, Scherer A, Finckh A, et al. Persistence of ultrasound synovitis in patients with rheumatoid arthritis fulfilling the DAS28 and/or the new ACR/EULAR RA remission definitions: results of an observational cohort study. Joint Bone Spine 2014;81:426-32. https://doi.org/10.1016/j.jbspin.2014.04.014.
- Stoffer MA, Schoels MM, Smolen JS, Aletaha D, Breedveld FC, Burmester G, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016;75:16-22. https://doi.org/10.1136/annrheumdis-2015-207526.
- Schipper LG, van Hulst LT, Grol R, van Riel PL, Hulscher ME, Fransen J. Meta-analysis of tight control strategies in rheumatoid arthritis: protocolized treatment has additional value with respect to the clinical outcome. Rheumatol 2010;49:2154-64. https://doi.org/10.1093/rheumatology/keq195.
- Bakker MF, Jacobs JW, Verstappen SM, Bijlsma JW. Tight control in the treatment of rheumatoid arthritis: efficacy and feasibility. Ann Rheum Dis 2007;66:iii56-60. https://doi.org/10.1136/ard.2007.078360.
- Knevel R, Schoels M, Huizinga TWJ, Aletaha D, Burmester GR, Combe B, et al. Current evidence for a strategic approach to the management of rheumatoid arthritis with disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2010;69:987-94. https://doi.org/10.1136/ard.2009.126748.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- Vermeer M, Kievit W, Kuper HH, Braakman-Jansen LM, Bernelot Moens HJ, Zijlstra TR, et al. Treating to the target of remission in early rheumatoid arthritis is cost-effective: results of the DREAM registry. BMC Musculoskelet Disord 2013;14. https://doi.org/10.1186/1471-2474-14-350.
- van den Hout WB, Goekoop-Ruiterman YP, Allaart CF, de Vries-Bouwstra JK, Hazes JM, Kerstens PJ, et al. Cost–utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2009;61:291-9. https://doi.org/10.1002/art.24169.
- Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky DS, Naredo E, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis 2016;75:1428-37. https://doi.org/10.1136/annrheumdis-2016-209201.
- Barnabe C, Nguyen Xuan T, Ohinmaa A, Homik J, Barr SG, Martin L, et al. Healthcare service utilisation costs are reduced when rheumatoid arthritis patients achieve sustained remission. Ann Rheum Dis 2013;72:1664-8. https://doi.org/10.1136/annrheumdis-2012-201918.
- Curtis L, Burns A. Unit Costs for Health & Social Care 2015. Canterbury: Personal Social Services Research Unit; 2015.
- Curtis L. Unit Costs for Health & Social Care 2010. Canterbury: Personal Social Services Research Unit; 2010.
- Simpson E, Hock E, Stevenson M, Wong R, Dracup N, Wailoo A, et al. What is the added value of ultrasound joint examination for monitoring synovitis in rheumatoid arthritis and can it be used to guide treatment decisions? A systematic review and cost-effectiveness analysis;. Health Technol Assess 2017.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess 2016;20:1-672.
- Gaujoux-Viala C, Mouterde G, Baillet A, Claudepierre P, Fautrel B, Le Loet X, et al. Evaluating disease activity in rheumatoid arthritis: which composite index is best? A systematic literature analysis of studies comparing the psychometric properties of the DAS, DAS28, SDAI and CDAI. Joint Bone Spine 2012;79:149-55. https://doi.org/10.1016/j.jbspin.2011.04.008.
- Salaffi F, Cimmino MA, Leardini G, Gasparini S, Grassi W. Disease activity assessment of rheumatoid arthritis in daily practice: validity, internal consistency, reliability and congruency of the Disease Activity Score including 28 joints (DAS28) compared with the Clinical Disease Activity Index (CDAI). Clin Exp Rheumatol 2009;27:552-9.
- Singh H, Kumar H, Handa R, Talapatra P, Ray S, Gupta V. Use of clinical disease activity index score for assessment of disease activity in rheumatoid arthritis patients: an Indian experience. Arthritis 2011;2011. https://doi.org/10.1155/2011/146398.
- Mian A, Ibrahim F, Scott I, Bahadur S, Filkova M, Pollard L, et al. Changing clinical patterns in rheumatoid arthritis management over two decades: sequential observational studies. BMC Musculoskelet Disord 2016;17. https://doi.org/10.1186/s12891-016-0897-y.
- Dougados M, Kissel K, Conaghan PG, Mola EM, Schett G, Gerli R, et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann Rheum Dis 2014;73:803-9. https://doi.org/10.1136/annrheumdis-2013-204761.
- Huizinga TW, Conaghan PG, Martin-Mola E, Schett G, Amital H, Xavier RM, et al. Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann Rheum Dis 2015;74:35-43. https://doi.org/10.1136/annrheumdis-2014-205752.
- Alemao EA, Joo S, Banerjee S, Emery P, Weinblatt M. Impact of rapid attainment of stringent measures of efficacy in rheumatoid arthritis on patient-reported outcomes. Arthritis Rheumato 2014;66.
- Haavardsholm EA, Aga AB, Olsen IC, Hammer HB, Uhlig T, Fremstad H, et al. Aiming for remission in rheumatoid arthritis: Clinical and radiographic outcomes from a randomized controlled strategy trial investigating the added value of ultrasonography in a treat-to-target regimen. Arthritis Rheumatol 2015;67.
- Bijlsma JW, Welsing PM, Woodworth TG, Middelink LM, Bernasconi C, Borm ME, et al. Rapid and sustained remission in early rheumatoid arthritis (RA) treated to target with tocilizumab, methotrexate, or their combination: The U-ACT-early strategy study. Ann Rheum Dis 2015;74:77-8. https://doi.org/10.1136/annrheumdis-2015-eular.2287.
- Bakker MF, Jacobs JWG, Welsing PMJ, Verstappen SMM, Tekstra J, Ton E, et al. Double-blind randomized CAMERA-II trial: better control of disease and erosive joint damage with inclusion of low-dose prednisone into a MTX-based tight control strategy for early rheumatoid arthritis. Arthritis Rheum 2011;1.
- Bakker MF, Jacobs JWG, Welsing PMJ, Verstappen SMM, Tekstra J, Ton E, et al. Double-blind randomized CAMERA-II trial: better control of disease and erosive joint damage with inclusion of low-dose prednisone into a MTX-based tight control strategy for early rheumatoid arthritis. Arthritis Rheum 2011;63:S663-S4.
- De Hair M, Ijff N, Jacobs J, Van Laar J. Long-term adverse events after daily concomitant treatment with 10mg prednisone in the 2-year computer assisted management in early rheumatoid arthritis trial-ii. Arthritis Rheumat 2015;67.
- Jurgens M, Welsing PM, Geenen MJ, Bakker MF, Lafeber FP, Bijlsma JW, et al. Effect of prednisone on quality of life domains in early rheumatoid arthritis-additional analyses of the CAMERA II trial. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.315.
- Carubbi F, Zugaro L, Cipriani P, Conchiglia A, Gregori L, Danniballe C, et al. Safety and efficacy of intra-articular anti-tumor necrosis factor alpha agents compared to corticosteroids in a treat-to-target strategy in patients with inflammatory arthritis and monoarthritis flare. Int J Immunopathol Pharmacol 2016;29:252-66. https://doi.org/10.1177/0394632015593220.
- Charles-Schoeman C, Wang X, Lee YY, Shahbazian A, Navarro-Millan I, Yang S, et al. Association of triple therapy with improvement in cholesterol profiles over two-year follow-up in the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheum 2016;68:577-86. https://doi.org/10.1002/art.39502.
- Hetland ML, Horslev-Petersen K. The CIMESTRA study: intra-articular glucocorticosteroids and synthetic DMARDs in a treat-to-target strategy in early rheumatoid arthritis. Clin Exp Rheumatol 2012;30:S44-9.
- Hetland ML, Ostergaard M, Ejbjerg B. Short- and long-term efficacy of intra-articular injections with betamethasone as part of a treat-to-target strategy in early rheumatoid arthritis: impact of joint area, repeated injections, MRI findings, anti-CCP, IgM-RF and CR. Ann Rheum Dis 2012;71. https://doi.org/10.1136/annrheumdis-2011-200632.
- Hetland ML, Stengaard-Pedersen K, Junker P, Lottenburger T, Hansen I, Andersen LS, et al. Aggressive combination therapy with intra-articular glucocorticoid injections and conventional disease-modifying anti-rheumatic drugs in early rheumatoid arthritis: second-year clinical and radiographic results from the CIMESTRA study. Ann Rheum Dis 2008;67:815-22. https://doi.org/10.1136/ard.2007.076307.
- Boers M, Verhoeven AC, Markusse HM, Laar MA, Westhovens R, Denderen JC, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997;350:309-18. https://doi.org/10.1016/S0140-6736(97)01300-7.
- Korthals-de Bos I, Tulder M, Boers M, Verhoeven AC, Adèr HJ, Bibo J, et al. Indirect and total costs of early rheumatoid arthritis: a randomized comparison of combined step-down prednisolone, methotrexate, and sulfasalazine with sulfasalazine alone. J Rheumatol 2004;31:1709-16.
- Landewé RB, Boers M, Verhoeven AC, Westhovens R, van de Laar MA, Markusse HM, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum 2002;46:347-56. https://doi.org/10.1002/art.10083.
- van Tuyl LH, Boers M, Lems WF, Landewé RB, Han H, van der Linden S, et al. Survival, comorbidities and joint damage 11 years after the COBRA combination therapy trial in early rheumatoid arthritis. Ann Rheum Dis 2010;69:807-12. https://doi.org/10.1136/ard.2009.108027.
- Verhoeven AC, Bibo JC, Boers M, Engel GL, Van Der Linden S. Cost-effectiveness and cost-utility of combination therapy in early rheumatoid arthritis: Randomized comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone. B J Rheumatol 1998;37:1102-9. https://doi.org/10.1093/rheumatology/37.10.1102.
- Dale J, Stirling A, Grigor C, Saunders S, Porter D. Comparison of outcomes between studies of intensive treatment strategies in early RA from a single centre over 20 years. Ann Rheum Dis 2015;74. https://doi.org/10.1136/annrheumdis-2015-eular.1980.
- De Cock D, Meyfroidt S, Vanderschueren G, Lateur L, Joly J, Westhovens R, et al. Two year radiological follow-up of early rheumatoid arthritis patients treated with initial step up monotherapy or initial step down therapy with glucocorticoids, followed by a tight control approach. Ann Rheum Dis 2013;72:211-12. https://doi.org/10.1136/annrheumdis-2013-eular.672.
- De Cock D, Vanderschueren G, Meyfroidt S, Joly J, Westhovens R, Verschueren P. Two-year clinical and radiologic follow-up of early RA patients treated with initial step up monotherapy or initial step down therapy with glucocorticoids, followed by a tight control approach: lessons from a cohort study in daily practice. Clin Rheumatol 2014;33:125-30. https://doi.org/10.1007/s10067-013-2398-9.
- De Cock D, Corluy L, Joos R, Langenaken C, Taelman V, Raeman F, et al. Low-risk patients also benefit from remission induction treatment in early rheumatoid arthritis: Week 52 results from the carera trial. Ann Rheum Dis 2015;74:236-7. https://doi.org/10.1136/annrheumdis-2015-eular.3984.
- den Broeder AA, van Herwaarden N, van der Maas A, van den Hoogen FH, Bijlsma JW, van Vollenhoven RF, et al. Dose reduction strategy of subcutaneous TNF inhibitors in rheumatoid arthritis: design of a pragmatic randomised non inferiority trial, the DRESS study. BMC Musculoskelet Dis 2013;14. https://doi.org/10.1186/1471-2474-14-299.
- Dumitru RB, Horton S, Hodgson R, Wakefield RJ, Hensor EM, Emery P, et al. A prospective, single-centre, randomised study evaluating the clinical, imaging and immunological depth of remission achieved by very early versus delayed etanercept in patients with rheumatoid arthritis (VEDERA). BMC Musculoskelet Dis 2016;17. https://doi.org/10.1186/s12891-016-0915-0.
- Emery P, Mease PJ, Rubbert-Roth A, Curtis JR, Muller-Ladner U, Gaylis NB, et al. Retreatment with rituximab (RTX) based on a treatment to target (TT) approach provides better disease control than treatment as needed (PRN) in patients (PTS) with rheumatoid arthritis (RA). Arthritis Rheum 2009;60.
- Emery P, Mease PJ, Rubbert-Roth A, Curtis JR, Muller-Ladner U, Gaylis N, et al. Comparison of two retreatment strategies with rituximab in patients with rheumatoid arthritis (RA). Rheumatol 2010;49.
- Emery P, Olszynski W, Mease P. Retreatment with rituximab (RTX) based on a treatment to target (TT) approach provides better disease control than treatment as needed (PRN) in patients with rheumatoid arthritis (RA). J Rheumatol 2010;37:1302-3.
- Emery P, Mease PJ, Rubbert-Roth A, Curtis JR, Muller-Ladner U, Gaylis NB, et al. Retreatment with rituximab based on a treatment-to-target approach provides better disease control than treatment as needed in patients with rheumatoid arthritis: a retrospective pooled analysis. Rheumatol 2011;50:2223-32. https://doi.org/10.1093/rheumatology/ker253.
- Fedorenko E, Lukina G, Sigidin Y. Remission as the main therapeutic target: comparative efficacy of four treatment regimens in early rheumatoid arthritis (RA) patients (PTS). Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-203223.15.
- Ferraccioli GF, Gremese E, Tomietto P, Favret G, Damato R, Di Poi E. Analysis of improvements, full responses, remission and toxicity in rheumatoid patients treated with step-up combination therapy (methotrexate, cyclosporin A, sulphasalazine) or monotherapy for three years. Rheumatology 2002;41:892-8. https://doi.org/10.1093/rheumatology/41.8.892.
- Fiehn C, Jacki S, Heilig B, Lampe M, Wiesmüller G, Richter C, et al. Eight versus 16-week re-evaluation period in rheumatoid arthritis patients treated with leflunomide or methotrexate accompanied by moderate dose prednisone. Rheumatol Int 2007;27:975-9. https://doi.org/10.1007/s00296-007-0347-0.
- Galloway JB, Kingsley G, Ma M, Lorente-Canovas B, Cope A, Ibrahim F, et al. Optimising treatment with TNF inhibitors in rheumatoid arthritis with different dose tapering strategies: the OPTTIRA trial. Ann Rheum Dis 2015;74. https://doi.org/10.1136/annrheumdis-2015-eular.4684.
- Giacomelli R, Cipriani P, Cerinic MM, Fulminis A, Barattelli G, Pingiotti E, et al. Combination therapy with cyclosporine and methotrexate in patients with early rheumatoid arthritis soon inhibits TNF alpha production without decreasing TNF alpha mRNA levels. An in vivo and in vitro study. Clin Exp Rheumatol 2002;20:365-72.
- Harrold LR, Reed GW, Harrington JT, Barr CJ, Saunders KC, Gibofsky A, et al. A cluster-randomized trial of a behavioral intervention to incorporate a treat-to-target approach in the clinical care of rheumatoid arthritis patients. Ann Rheum Dis 2015;74:988-9. https://doi.org/10.1136/annrheumdis-2015-eular.2424.
- Hørslev-Petersen K, Hetland ML, Junker P, Podenphant J, Ellingsen T, Ahlqvist P, et al. Adalimumab added to methotrexate and intra-articular glucocorticoid increases remission rates at one year in early, DMARD-nave patients with rheumatoid arthritis an investigator-initiated randomized, controlled, double-blinded study. Arthritis Rheum 2011;63.
- Hwang YG, Balasubramani GK, Metes ID, Levesque MC, Bridges SL, Moreland LW. Differential response of serum amyloid A to different therapies in early rheumatoid arthritis and its potential value as a disease activity biomarker. Arthritis Res Ther 2016;18. https://doi.org/10.1186/s13075-016-1009-y.
- Nam JL, Villeneuve E, Hensor EM, Conaghan PG, Keen HI, Buch MH, et al. Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: a double-blind, randomised, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the IDEA study). Ann Rheum Dis 2014;73:75-8. https://doi.org/10.1136/annrheumdis-2013-203440.
- Nam JL, Villeneuve E, Hensor EMA, Conaghan PG, Keen HI, Gough AK, et al. Inhibition of structural damage with two intensive treatment strategies using infliximab or high dose intravenous steroid followed by treat to target in DMARD naive rheumatoid arthritis (the IDEA study) – a preliminary report. Ann Rheum Dis 2012;71:106-7.
- Moller-Bisgaard S, Horslev-Petersen K, Ejbjerg BJ, Boesen M, Hetland ML, Christensen R, et al. Impact of a magnetic resonance imaging-guided treat-to-target strategy on disease activity and progression in patients with rheumatoid arthritis (the IMAGINE-RA trial): study protocol for a randomized controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0693-2.
- Jalal H, O’Dell JR, Bridges SL, Cofield S, Curtis JR, Mikuls TR, et al. Cost-effectiveness of triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis. Arthritis Care Res 2016;68:1751-7. https://doi.org/10.1002/acr.22895.
- Kume K, Amano K, Hatta K, Ohta H. Treating to target ultrasound with clinical remission better effects than clinical remission in RRP. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.2074.
- Kuusalo L, Puolakka K, Kautiainen H, Blåfield H, Eklund KK, Ilva K, et al. Impact of physicians’ adherence to treat-to-target strategy on outcomes in early rheumatoid arthritis in the NEO-RACo trial. Scand J Rheumatol 2015;44:449-55. https://doi.org/10.3109/03009742.2015.1043142.
- Li R, Zhao JX, Su Y, He J, Chen LN, Gu F, et al. High remission and low relapse with prolonged intensive DMARD therapy in rheumatoid arthritis (PRINT): A multicenter randomized clinical trial. Medicine 2016;95. https://doi.org/10.1097/MD.0000000000003968.
- Marchesoni A, Battafarano N, Arreghini M, Pellerito R, Cagnoli M, Prudente P, et al. Step-down approach using either cyclosporin A or methotrexate as maintenance therapy in early rheumatoid arthritis. Arthritis Rheum 2002;47:59-66. https://doi.org/10.1002/art1.10245.
- Markusse I, Dirven L, Han H, Ronday K, Speyer I, Kerstens P, et al. Survival in early rheumatoid arthritis patients after 10 years of targeted treatment. Ann Rheum Dis 2015;74:84-5. https://doi.org/10.1136/annrheumdis-2015-eular.1638.
- Markusse IM, Dirven L, Han KH, Ronday HK, de Sonnaville PB, Kerstens PJ, et al. Evaluating adherence to a treat-to-target protocol in recent-onset rheumatoid arthritis: reasons for compliance and hesitation. Arthritis Care Res 2016;68:446-53. https://doi.org/10.1002/acr.22681.
- Capell HA, Madhok R, Porter DR, Munro RA, McInnes IB, Hunter JA, et al. Combination therapy with sulfasalazine and methotrexate is more effective than either drug alone in patients with rheumatoid arthritis with a suboptimal response to sulfasalazine: results from the double-blind placebo-controlled MASCOT study. Ann Rheum Dis 2007;66:235-41. https://doi.org/10.1136/ard.2006.057133.
- Hampson R, Madhok R, McInnes I, Munro R, Porter D, Tierney A, et al. Methotrexate and sulphasalazine combination therapy (MASCOT) is more effective than either drug alone in patients with a sub-optimal response to sulphasazine monotherapy a double blind placebo randomised controlled trial (RCT). Ann Rheum Dis 2005;64.
- Menon N, Kothari SY, Gogna A, Sharma R. Comparison of intra-articular glucocorticoid injections with DMARDs versus DMARDs alone in rheumatoid arthritis. J Assoc Physicians India 2014;62:673-6.
- Moller-Bisgaard S, Ejbjerg BJ, Eshed I, Horslev-Petersen K, Jurik AG, Vallo J, et al. Effect of methotrexate and intra-articular betamethasone with or without additional cyclosporine on magnetic resonance imaging (MRI)-determined inflammatory and destructive changes in very early rheumatoid arthritis-results from a 24-months’ randomised double blind placebo controlled trial. Ann Rheum Dis 2015;74. https://doi.org/10.1136/annrheumdis-2015-eular.2808.
- Montecucco C, Todoerti M, Sakellariou G, Scire CA, Caporali R. Low-dose oral prednisone improves clinical and ultrasonographic remission rates in early rheumatoid arthritis: results of a 12-month open-label randomised study. Arthritis Res Ther 2012;14. https://doi.org/10.1186/ar3838.
- Kuusalo L, Rantalaiho V, Kautiainen H, Korpela M, Puolakka K, Leirisalo-Repo M. Impact of physicians’ activity and adherence to treat-to-target strategy on outcomes in early rheumatoid arthritis: a subanalysis of the Neo-RACo trial. Scand J Rheumatol 2014;43:1-2.
- Leirisalo-Repo M, Kautiainen H, Laasonen L, Mottonen T, Hannonen P, Korpela M, et al. A randomized double-blind placebo-controlled study on addition of 6-month induction therapy with infliximab to triple DMARD plus prednisolone therapy in patients with early active rheumatoid arthritis: High remission rates and no joint destruction during first 2 years. The NEO-RACo study. Rheumatol 2009;48:i11-i2.
- Rantalaiho V, Kautiainen H, Korpela M, Hannonen P, Kaipiainen-Seppänen O, Möttönen T, et al. Targeted treatment with a combination of traditional DMARDs produces excellent clinical and radiographic long-term outcomes in early rheumatoid arthritis regardless of initial infliximab. The 5-year follow-up results of a randomised clinical trial, the NEO-RACo trial. Ann Rheum Dis 2014;73:1954-61. https://doi.org/10.1136/annrheumdis-2013-203497.
- Rantalaiho V, Kautiainen H, Korpela M, Leirisalo-Repo M. Strict ACR-remission within 6 months predicts sustained long-term very low disease activity in actively treated rheumatoid arthritis patients, 5-year results of the NEO-RACo trial. Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-eular.1754.
- Rantalaiho V, Korpela M, Hannonen P, Kaipiainen-Seppanen O, Mottonen T, Kauppi M, et al. Targeted treatment with combination dMARDs produces excellent clinical and radiographic long-term outcomes in early rheumatoid arthritis regardless of initial infliximab. the 5-year follow-up results of the NEO-RACo trial. Ann Rheum Dis 2012;71.
- Rantalaiho V, Martikainen J, Kautiainen H, Puolakka K, Leirisalo-Repo M. Initial infliximab is not cost-effective added on to a remission-targeted combination therapy in early rheumatoid arthritis: the 2-year results of the randomized clinical NEO-RACo trial. Scand J Rheumatol 2014;43.
- Rantalaiho V, Martikainen J, Kautiainen H, Puolakka K, Leirisalo-Repo M. Initial infliximab is not cost-effective in otherwise actively treated early rheumatoid arthritis. The 2-year follow-up results of the randomized clinical NEO-RACo trial. Ann Rheum Dis 2014;73. https://doi.org/10.1136/annrheumdis-2014-eular.3265.
- Rantalaiho V, Puolakka K, Martikainen J, Kautiainen H, Leirisalo-Repo M. Adding an initial six-month course of infliximab to an active combination treatment is cost saving in working-aged early rheumatoid arthritis patients. Arthritis Rheumatol 2014;66:S1083-S4.
- Axelsen MB, Eshed I, Horslev-Petersen K, Steengaard-Petersen K, Hetland ML, Moller JM, et al. A treat-to-target strategy with methotrexate and intra-articular triamcinolone with or without added adalimumab reduces synovitis, osteitis and tenosynovitis and halts structural damage progression in early rheumatoid arthritis: the opera magnetic resonance imaging sub-study. Arthritis Rheum 2013;65.
- Axelsen MB, Eshed I, Horslev-Petersen K, Stengaard-Pedersen K, Hetland ML, Moller J, et al. A treat-to-target strategy with methotrexate and intra-articular triamcinolone with or without adalimumab effectively reduces MRI synovitis, osteitis and tenosynovitis and halts structural damage progression in early rheumatoid arthritis: results from the OPERA randomised controlled trial. Ann Rheum Dis 2015;74:867-75. https://doi.org/10.1136/annrheumdis-2013-204537.
- Hørslev-Petersen K, Hetland ML, Junker P, Pødenphant J, Ellingsen T, Ahlquist P, et al. Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann Rheum Dis 2014;73:654-61. https://doi.org/10.1136/annrheumdis-2012-202735.
- Hørslev-Petersen K, Hetland ML, Junker P, Podenphant J, Ellingsen T, Ahlqvist P, et al. Very high remission rates are achieved by methotrexate and intraarticular glucocorticoids independent of induction therapy with adalimumab; year 2 clinical results of an investigator-initiated randomised, controlled clinical trial of early, rheumatoid arthritis (opera). Arthritis Rheum 2013;65.
- Hetland ML, Junker P, Poodenphant J, Ellingsen T, Ahlquist P, Lindegaard H, et al. Remission rates increase substantially by adding adalimumab to methotrexate and intra-articular glucocorticoid in patients with early rheumatoid arthritis-1-year results of investigator-initiated, double-blinded randomized clinical trial (OPERA). Ann Rheum Dis 2013;71.
- Emery P, Smolen JS, Fleischmann R, Van Vollenhoven R, Florentinus S, Santra S, et al. Achieving long-term comprehensive disease control with adalimumab and methotrexate in patients with early rheumatoid arthritis in the optima study. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.2628.
- Kavanaugh A, Emery P, Fleischmann R, Van Vollenhoven R, Pavelka K, Durez P, et al. Withdrawal of adalimumab in early rheumatoid arthritis patients who attained stable low disease activity with adalimumab plus methotrexate: results of a phase 4, double-blind, placebo-controlled trial. Rheumatol 2012;51:iii29-iii30.
- Kavanaugh A, Emery P, Fleischmann R, Van Vollenhoven RF, Pavelka K, Durez P, et al. Withdrawal of adalimumab in early rheumatoid arthritis patients who attained stable low disease activity with adalimumab plus methotrexate: results of a phase 4, double-blind, placebo-controlled trial. Arthritis Rheum 2011;63.
- Kavanaugh A, Van Vollenhoven RF, Emery P, Shaw JW, Cifaldi MA, Florentinus S, et al. Patient-reported outcomes in early rheumatoid arthritis patients failing to achieve stable low disease activity: comparing addition of adalimumab to methotrexate monotherapy with maintenance on adalimumab plus methotrexate. Arthritis Rheum 2012;64:S162-S3.
- Smolen J, Fleischmann RM, Emery P, Van Vollenhoven RF, Guerette B, Santra S, et al. Treating rheumatoid arthritis to target: outcomes and predictors in early rheumatoid arthritis patients treated with adalimumab plus methotrexate, methotrexate alone, or methotrexate plus subsequent adalimumab. Arthritis Rheum 2011;63.
- Wolff M, Zhou ZY, Signorovitch J, Shaw JW, Ganguli A. Cost-effectiveness of different treatment sequences including adalimumab in the treat-to-target framework for early rheumatoid arthritis in Germany. Arthritis Rheum 2013;65.
- Edmonds J, Lassere M, Carlton K. OSRA – a therapeutic target-driven RCT: update on safety during combination DMARD & biological therapies. Ann Rheum Dis 2005;64:451-2.
- Edmonds J, Lassere M, Sharp JT, Bird P. Objectives study in RA (OSRA): a RCT defining the best clinical target for disease activity control in RA. Ann Rheum Dis 2007;66.
- Pablos J, Navarro F, Blanco F, Roman-Ivorra J, Alonso A, Martin-Mola E, et al. Maintenance of response in patients with rheumatoid arthritis (RA) after switching to TCZ administered alone compared to the combination of TCZ and MTX. Ann Rheum Dis 2015;74:476-7. https://doi.org/10.1136/annrheumdis-2015-eular.4501.
- Pavelka K, Akkoc N, Al-Maini M, Zerbini CA, Bao C, Karateev DE, et al. Impact of combination etanercept-DMARD induction therapy in active rheumatoid arthritis: interim results of an international treat-to-target study conducted in regions with limited biologic access. Ann Rheum Dis 2015;74. https://doi.org/10.1136/annrheumdis-2015-eular.1689.
- Pavelka K, Akkoc N, Al-Maini M, Zerbini C, Bao CD, Karateev D, et al. Maintenance of remission with combination etanercept-DMARD therapy versus DMARDs alone in active rheumatoid arthritis: results of an international treat-to-target study with a double-blind, placebo-controlled maintenance phase. J Clin Rheumatol 2016;22:127-8.
- Aletaha D, Funovits J, Breedveld FC, Sharp J, Segurado O, Smolen JS. Rheumatoid arthritis joint progression in sustained remission is determined by disease activity levels preceding the period of radiographic assessment. Arthritis Rheum 2009;60:1242-9. https://doi.org/10.1002/art.24433.
- Breedveld FC, Keystone E, Heijde D, Landewe R, Smolen J, Guerette B, et al. Initial combination therapy with adalimumab plus methotrexate leads to better long-term outcomes than with either monotherapy in patients with early rheumatoid arthritis: 8-year results of an open-label extension of a phase 3 trial. Arthritis Rheum 2011;63.
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study – a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26-37. https://doi.org/10.1002/art.21519.
- Emery P, Genovese MC, van Vollenhoven R, Sharp JT, Patra K, Sasso EH. Less radiographic progression with adalimumab plus methotrexate versus methotrexate monotherapy across the spectrum of clinical response in early rheumatoid arthritis. J Rheumatol 2009;36:1429-41. https://doi.org/10.3899/jrheum.081018.
- Keystone E, Weinblatt, Haraoui B, Guerette B, Mozaffarian N, Patra K, et al. Clinical and radiographic implications of time to treatment response in patients with early rheumatoid arthritis. Reumatol Clin Supl 2011;7.
- Keystone EC, Breedveld FC, van der Heijde D, Landewé R, Florentinus S, Arulmani U, et al. Longterm effect of delaying combination therapy with tumor necrosis factor inhibitor in patients with aggressive early rheumatoid arthritis: 10-year efficacy and safety of adalimumab from the randomized controlled PREMIER trial with open-label extension. J Rheumatol 2014;41:5-14. https://doi.org/10.3899/jrheum.130543.
- Keystone EC, Breedveld FC, Kavanaugh A, Van Riel P, Patra K, Pangan A, et al. Sustained clinical remission and response for early RA patients with adalimumab and methotrexate initial combination therapy: 5-year results of the PREMIER trial. J Rheumatol 2009;36.
- Keystone EC, Breedveld FC, Van Der Heijde D, Landewe R, Florentinus S, Arulmani U, et al. Long-term impact of delaying combination therapy with adalimumab plus methotrexate by 2 years in patients with early rheumatoid arthritis: final 10-year results of the premier trial. Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-eular.718.
- Keystone EC, Breedveld FC, Van Der Heijde D, Landewe R, Florentinus S, Arulmani U, et al. Long-term impact of delaying combination therapy with adalimumab plus methotrexate by 2 years in patients with early rheumatoid arthritis: final 10-year results of the premier trial. Arthritis Rheum 2013;65. http://onlinelibrary.wiley.com/doi/10.1002/art.38216/full.
- Keystone EC, Van Der Heijde D, Landewe R, Patra K, Perez JL, Pangan AL. Better long-term inhibition of radiographic progression in early RA with adalimumab and methotrexate initial combination therapy: 5-year results of the PREMIER trial. J Rheumatol 2009;36:2573-4.
- Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health-related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. J Rheumatol 2012;39:63-72. https://doi.org/10.3899/jrheum.101161.
- van der Heijde D, Breedveld FC, Kavanaugh A, Keystone EC, Landewe R, Patra K, et al. Disease activity, physical function, and radiographic progression after longterm therapy with adalimumab plus methotrexate: 5-year results of PREMIER. J Rheumatol 2010;37:2237-46. https://doi.org/10.3899/jrheum.100208.
- Keystone EC, Weinblatt ME, Haraoui B, Guerette B, Mozaffarian N, Patra K, et al. Clinical and radiographic implications of time to treatment response in patients with early rheumatoid arthritis. Arthritis Rheum 2010;62.
- Bananis E, Smolen J, Van Vollenhoven R, Pederson R, Szumski A, Koenig A. Relationship between clinical response and radiographic outcomes in patients with moderate rheumatoid arthritis in the preserve trial. Int Med 2013;43:15-6.
- Kobelt G. Treating to target with etanercept in rheumatoid arthritis: cost-effectiveness of dose reductions when remission is achieved. Value Health 2014;17:537-44. https://doi.org/10.1016/j.jval.2014.04.005.
- Pavelka K, Burgos-Vargas R, Miranda P, Guzman R, Yen JH, Izzi MA, et al. Etanercept in moderate rheumatoid arthritis: PRESERVE study results from central/eastern Europe, Latin America and Asia. Int J Clin Rheumatol 2014;9:415-30. https://doi.org/10.2217/ijr.14.27.
- Pavelka K, Khalil A, Koenig AS, Szumsk A, Youseif EA, Bananis E, et al. Maintenance of response with conventional- or reduced-dose etanercept therapy or biologic free after induction of response with etanercept -methotrexate therapy in patients with moderately active rheumatoid arthritis participating in the PRESERVE study in Europe, Latin America, and Asia. Int J Rheum Dis 2012;15:54-5.
- Pavelka K, Szekanecz Z, Damjanov N, Majdan M, Nasonov E, Mazurov V, et al. Induction of response with etanercept-methotrexate therapy in patients with moderately active rheumatoid arthritis in Central and Eastern Europe in the PRESERVE study. Clin Rheumatol 2013;32:1275-81. https://doi.org/10.1007/s10067-013-2240-4.
- Smolen J, Szumski A, Marshall L, Koenig AS. Baseline predictors of remission with combination etanercept -methotrexate therapy in moderately active rheumatoid arthritis: interim results of the preserve trial. Arthritis Rheum 2011;63.
- Smolen JS, Collier D, Szumski A, Jones H, Marshall L. Characteristics of responding versus non-responding moderate rheumatoid arthritis patients treated with etanercept plus methotrexate. Arthritis Rheum 2014;66:S1091-S2.
- Smolen JS, Collier D, Szumski A, Jones H, Marshall L. Effect of disease duration on clinical outcomes in moderate rheumatoid arthritis patients treated with etanercept plus methotrexate in the preserve study. Arthritis Rheumatol 2014;66:S812-S3.
- Smolen JS, Ilivanova E, Hall S, Irazoque-Palazuelos F, Park MC, Kotak S, et al. Low disease activity or remission induction with etanercept 50 mg and methotrexate in moderately active rheumatoid arthritis: Maintenance of response and safety of etanercept 50 mg, 25 mg, or placebo in combination with methotrexate in a randomized double-blind study. Arthritis Rheum 2011;63.
- Smolen JS, Nash P, Durez P, Hall S, Ilivanova E, Irazoque-Palazuelos F, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918-29. https://doi.org/10.1016/S0140-6736(12)61811-X.
- Smolen JS, Szumski A, Koenig AS, Jones TV. Predictors of loss of clinical remission with etanercept 50 mg, 25 mg or placebo combined with methotrexate in moderate rheumatoid arthritis patients: results of the preserve trial. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.2621.
- Strand V, Jones TV, Li W, Koenig AS, Kotak S. Factors that impact work productivity in the preserve trial: a randomized controlled trial of combination etanercept-methotrexate therapy in patients with moderately active rheumatoid arthritis. Arthritis Rheum 2012;64.
- Strand V, Jones TV, Li W, Koenig AS, Kotak S. Predictors of work impairment in a randomized controlled trial of etanercept-methotrexate therapy in patients with moderate rheumatoid arthritis. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.1385.
- Szekanecz Z, Yen JH, Miranda P, Szumski A, Bananis E, Kotak S, et al. Impact of etanercept-methotrexate therapy on patient-reported outcomes in moderately active rheumatoid arthritis (RA) patients of Europe, Latin America, and Asia. Value Health 2012;15. https://doi.org/10.1016/j.jval.2012.08.419.
- Proudman SM, Conachan PG, Richardson C, Griffiths B, Green MJ, McGonagle D, et al. Treatment of poor-prognosis early rheumatoid arthritis – a randomized study of treatment with methotrexate, cyclosporin A, and intraarticular corticosteroids compared with sulfasalazine alone. Arthritis Rheum 2000;43:1809-19. https://doi.org/10.1002/1529-0131(200008)43:8<1809::AID-ANR17>3.0.CO;2-D.
- Scott DL, Kowalczyk A. Clinical trials: tight control in early RA pays off in the long run. Nat Rev Rheumatol 2010;6:623-4. https://doi.org/10.1038/nrrheum.2010.174.
- Scott IC, Ibrahim F, Scott DL, Lewis CM. The impact of intensive treatment and remission on health-related quality of life in rheumatoid arthritis. Lancet 2016;387. https://doi.org/10.1016/S0140-6736(16)00477-3.
- Solomon DH, Lee SB, Zak A, Corrigan C, Agosti J, Bitton A, et al. Implementation of treat-to-target in rheumatoid arthritis through a learning collaborative: rationale and design of the TRACTION trial. Semin Arthritis Rheum 2016;46:81-7. https://doi.org/10.1016/j.semarthrit.2016.02.009.
- Danre A, Fautrel B, Alfaiate T, Pham T, Morel J, Labous ED, et al. What discriminates best flares in rheumatoid arthritis (RA)? A subanalysis of the strass treatment tapering in RA study. Arthritis Rheum 2014;66:S610-S1.
- Danre A, Gossec L, Pham T, Morel J, Alfaiate T, Dernis E, et al. Effects of TNF alpha-blockers tapering on occurrence of patient-perceived flares in rheumatoid arthritis: a subanalysis of strass. Ann Rheum Dis 2014;73.
- Fautrel B, Pham T, Alfaiate T, Gandjbakhch F, Foltz V, Morel J, et al. Step-down strategy of spacing TNF-blocker injections for established rheumatoid arthritis in remission: results of the multicentre non-inferiority randomised open-label controlled trial (STRASS: Spacing of TNF-blocker injections in Rheumatoid ArthritiS Study). Ann Rheum Dis 2016;75:59-67. https://doi.org/10.1136/annrheumdis-2014-206696.
- Fautrel B, Gandjbakhch F, Foltz V, Pham T, Morel J, Alfaiate T, et al. Targeting the lowest efficacious dose for rheumatoid arthritis patients in remission: Clinical and structural impact of a step-down strategy trial based on progressive spacing of TNF-blocker injections (STRASS trial). Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-eular.271.
- Fautrel B, Pham T, Morel J, Alfaiate T, Dernis E, Gaudin P, et al. Results of the STRASS trial regarding impact of progressive spacing of TNF-blocker injections in rheumatoid arthritis patients in DAS28 remission: Is there a difference between drugs – adalimumab and etanercept – or their mode of use-monotherapy or combination?. Arthritis Rheum 2013;65.
- Fautrel B, Pham T, Morel J, Alfaiate T, Dernis E, Gaudin P, et al. Impact of progressive spacing of TNF-blocker injections on signs and symptoms of rheumatoid arthritis patients in DAS28 remission: the STRASS randomized controlled trial. Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-eular.708.
- Fautrel B, Pham T, Tubach F, Alfaiate T, Morel J, Dernis E, et al. Tapering TNF-blockers in established rheumatoid arthritis patients in DAS28 remission: results of a DAS28-driven step-down strategy randomized controlled trial. Arthritis Rheum 2012;64:4169-70.
- Pham T, Morel J, Alfaiate T, Dernis E, Gaudin P, Brocq O, et al. Predictive factors of relapse or persistent stable remission for rheumatoid arthritis (RA) patients in remission in a TNF blocker-spacing strategy trial (STRASS trial). Arthritis Rheum 2013;65.
- Vanier A, Tubach F, Alfaiate T, Mariette X, Fautrel B. Step-down strategy of spacing TNF-blockers injections for established rheumatoid arthritis in remission: a within randomized control trial based cost-utility analysis. Arthritis Rheum 2015;67.
- Dale J, Purves D, McConnachie A, McInnes I, Porter D. Tightening up? Impact of musculoskeletal ultrasound disease activity assessment on early rheumatoid arthritis patients treated using a treat to target strategy. Arthritis Care Res 2014;66:19-26. https://doi.org/10.1002/acr.22218.
- Dale J, Purves D, McConnachie A, Porter D, McInnes IB. Tightening up: musculoskeletal ultrasound could further individualise treatment decisions in early rheumatoid arthritis patients treated by a step-up DMARD escalation regimen. Arthritis Rheum 2012;64.
- Dale J, Stirling A, McInnes IB, Porter D. Targeting ultrasound remission in early rheumatoid arthritis – results of the TaSER study. Arthritis Rheum 2013;65:S338-S9.
- Todoerti M, Scirè CA, Boffini N, Bugatti S, Montecucco C, Caporali R. Early disease control by low-dose prednisone comedication may affect the quality of remission in patients with early rheumatoid arthritis. Ann N Y Acad Sci 2010;1193:139-45. https://doi.org/10.1111/j.1749-6632.2009.05367.x.
- Claessen SJ, Hazes JM, Huisman MA, van Zeben D, Luime JJ, Weel AE. Use of risk stratification to target therapies in patients with recent onset arthritis; design of a prospective randomized multicenter controlled trial. BMC Musculoskelet Dis 2009;10. https://doi.org/10.1186/1471-2474-10-71.
- de Jong PH, Hazes JM, Barendregt PJ, Huisman M, van Zeben D, van der Lubbe PA, et al. Induction therapy with a combination of DMARDs is better than methotrexate monotherapy: first results of the tREACH trial. Ann Rheum Dis 2013;72:72-8. https://doi.org/10.1136/annrheumdis-2011-201162.
- de Jong PH, Hazes JM, Han HK, Huisman M, van Zeben D, van der Lubbe PA, et al. Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann Rheum Dis 2014;73:1331-9. https://doi.org/10.1136/annrheumdis-2013-204788.
- de Jong PH, Quax RA, Huisman M, Gerards AH, Feelders RA, de Sonnaville PB, et al. Response to glucocorticoids at 2 weeks predicts the effectiveness of DMARD induction therapy at 3 months: post hoc analyses from the tREACH study. Ann Rheum Dis 2013;72:1659-63. https://doi.org/10.1136/annrheumdis-2012-202152.
- De Jong PHP, Hazes J, Barendregt PJ, Huisman AM, Van Zeben D, Van Der Lubbe PA, et al. Combination of disease-modifying antirheumatic drugs prohibits the need of early initiation of biologicals, independent of glucocorticosteroids. A clinical trial to investigate different induction therapies in early rheumatoid arthritis. Arthritis Rheum 2011;63.
- De Jong PHP, Hazes JMW, Han KH, Huisman AM, Van Zeben D, Van Der Lubbe PA, et al. Initial triple DMARD therapy is more efficient than methotrexate monotherapy in recent onset rheumatoid arthritis; 1-year data of a randomized clinical trial (tREACH). Arthritis Rheum 2013;65.
- De Jong PHP, Weel AEAM, Luime JJ, Barendregt PJ, Gerards AH, Van Der Lubbe PA, et al. Better cost-effectiveness and worker productivity in triple DMARD therapy versus methotrexate monotherapy in early rheumatoid arthritis; cost-utility analysis of the tREACH trial. Arthritis Rheum 2013;65:S753-S4.
- Van der Elst K, De Cock D, Vecoven E, Arat S, Meyfroidt S, Joly J, et al. Are illness perception and coping style associated with the delay between symptom onset and the first general practitioner consultation in early rheumatoid arthritis management? An exploratory study within the CareRA trial. Scand J Rheumatol 2016;45:171-8. https://doi.org/10.3109/03009742.2015.1074278.
- van Tuyl LHD, Lems WF, Voskuyl AE, Kerstens P, Garnero P, Dijkmans BAC, et al. Tight control and intensified COBRA combination treatment in early rheumatoid arthritis: 90% remission in a pilot trial. Ann Rheum Dis 2008;67:1574-7. https://doi.org/10.1136/ard.2008.090712.
- Verschueren P, Esselens G, Westhovens R. Daily practice effectiveness of a step-down treatment in comparison with a tight step-up for early rheumatoid arthritis. Rheumatology 2008;47:59-64. https://doi.org/10.1093/rheumatology/kem288.
- Verschueren P, De Cock D, Corluy L, Joos R, Langenaken C, Taelman V, et al. Remission induction with DMARD combinations and glucocorticoids is not superior to remission induction with MTX monotherapy and glucocorticoids: week 52 results of the high-risk group from the carera trial. Ann Rheum Dis 2015;74. https://doi.org/10.1136/annrheumdis-2015-eular.4018.
- Yoo DH, Racewicz A, Brzezicki J, Yatsyshyn R, Arteaga ET, Baranauskaite A, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther 2016;18. https://doi.org/10.1186/s13075-016-0981-6.
- Bansback N, Marra C, Tsuchiya A, Anis A, Guh D, Hammond T, et al. Using the health assessment questionnaire to estimate preference-based single indices in patients with rheumatoid arthritis. Arthritis Rheum 2007;57:963-71. https://doi.org/10.1002/art.22885.
- Oostenbrink JB, Koopmanschap MA, Rutten FF. Standardisation of costs: the Dutch Manual for Costing in economic evaluations. PharmacoEconomics 2002;20:443-54. https://doi.org/10.2165/00019053-200220070-00002.
- Oostenbrink JB, Koopmanschap MA, Rutten FF. Manual for Cost Analyses, Methods and Standard Prices for Economic Evaluations in Health Care. Amstelveen: Dutch Health Insurance Executive Board; 2004.
- Koopmanschap MA, Rutten FF, van Ineveld BM, van Roijen L. The friction cost method for measuring indirect costs of disease. J Health Econ 1995;14:171-89. https://doi.org/10.1016/0167-6296(94)00044-5.
- van den Hout WB, Tijhuis GJ, Hazes JM, Breedveld FC, Vliet Vlieland TP. Cost effectiveness and cost utility analysis of multidisciplinary care in patients with rheumatoid arthritis: a randomised comparison of clinical nurse specialist care, inpatient team care, and day patient team care. Ann Rheum Dis 2003;62:308-15. https://doi.org/10.1136/ard.62.4.308.
- van den Berg B, Brouwer W, van Exel J, Koopmanschap M. Economic valuation of informal care: the contingent valuation method applied to informal caregiving. Health Econ 2005;14:169-83. https://doi.org/10.1002/hec.893.
- van Roijen L, Essink-Bot ML, Koopmanschap MA, Bonsel G, Rutten FF. Labor and health status in economic evaluation of health care. The Health and Labor Questionnaire. Int J Technol Assess Health Care 1996;12:405-15. https://doi.org/10.1017/S0266462300009764.
Appendix 1 Cost-effectiveness review: Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart
Appendix 2 Literature search strategies
Phase I search strategies
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R)
Date searched: 19 May 2015.
Search strategy
| 1. | exp Arthritis, Rheumatoid/ |
| 2. | rheumatoid arthritis.tw. |
| 3. | or/1-2 |
| 4. | Remission Induction/ |
| 5. | (strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or intens$ or control$).ti. |
| 6. | ((strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or control$) adj2 (treat$ or therap$)).mp. |
| 7. | (optim$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 8. | (adapt$ or titrat$ or adjust$ or response-based).tw. |
| 9. | 7 or 8 |
| 10. | *Disease Progression/ |
| 11. | *Disease Management/ |
| 12. | *Disease Outbreaks/ |
| 13. | Disease/ |
| 14. | 10 or 11 or 12 or 13 |
| 15. | 9 and 14 |
| 16. | ((strateg$ or proced$ or consequ$ or therap$ or halt$ or stop$ or revers$ or dela$ or arrest$ or detain$ or slow$ or preven$ or retard$ or avoid$) adj3 (structural or functional or erosi$ or progre$ or disabilit$ or invalidity or impediment or disablement or radiograph$ or radiolog$)).mp. |
| 17. | (remission adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).mp. |
| 18. | (((low$ or moderate or medium or high) and activity) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).mp. |
| 19. | 4 or 5 or 6 or 15 or 16 or 17 or 18 |
| 20. | 3 and 19 |
| 21. | randomized controlled trial.pt. |
| 22. | randomized controlled trial.mp. |
| 23. | 21 or 22 |
| 24. | 20 and 23 |
| 25. | limit 24 to yr=‘2008 -Current’ |
| 26. | MEDLINE.tw. |
| 27. | systematic review.tw. |
| 28. | meta analysis.pt. |
| 29. | or/26-28 |
| 30. | 20 and 29 |
| 31. | limit 30 to yr=‘2008 -Current’ |
| 32. | ec.fs. |
| 33. | cost.tw. |
| 34. | health care costs.sh. |
| 35. | or/32-34 |
| 36. | 20 and 35 |
| 37. | limit 36 to yr=‘2013 -Current’ |
EMBASE
Date searched: 19 May 2015.
Search strategy
| 1. | exp rheumatoid arthritis |
| 2. | rheumatoid arthritis.tw. |
| 3. | 1 or 2 |
| 4. | remission/ |
| 5. | (strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or intens$ or control$).ti. |
| 6. | ((strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or control$) adj2 (treat$ or therap$)).tw. |
| 7. | (optim$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 8. | (adapt$ or titrat$ or adjust$ or response-based).tw. |
| 9. | 7 or 8 |
| 10. | *disease course/ |
| 11. | *disease management/ |
| 12. | *epidemic/ |
| 13. | diseases/ |
| 14. | or/10-13 |
| 15. | 9 and 14 |
| 16. | ((strateg$ or proced$ or consequ$ or therap$ or halt$ or stop$ or revers$ or dela$ or arrest$ or detain$ or slow$ or preven$ or retard$ or avoid$) adj3 (structural or functional or erosi$ or progre$ or disabilit$ or invalidity or impediment or disablement or radiograph$ or radiolog$)).tw. |
| 17. | (remission adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 18. | (((low$ or moderate or medium or high) and activity) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 19. | 4 or 5 or 6 or 15 or 16 or 17 or 18 |
| 20. | 3 and 19 |
| 21. | double-blind:.mp. |
| 22. | placebo:.tw. |
| 23. | blind:.tw. |
| 24. | or/21-23 |
| 25. | 20 and 24 |
| 26. | limit 25 to yr=‘2008 -Current’ |
| 27. | meta-analysis.tw. |
| 28. | systematic review.tw. |
| 29. | 27 or 28 |
| 30. | 20 and 29 |
| 31. | limit 30 to yr=‘2008 -Current’ |
| 32. | cost.tw. |
| 33. | costs.tw. |
| 34. | 32 or 33 |
| 35. | 20 and 34 |
| 36. | limit 35 to yr=‘2013 -Current’ |
Cochrane Database of Systematic Reviews; Cochrane Central Register of Controlled Trials; Health Technology Assessment Database; Database of Abstracts of Reviews of Effects; and NHS Economic Evaluation Database
Date searched:19 May 2015.
Search strategy
| #1 | MeSH descriptor: [Arthritis, Rheumatoid] explode all trees |
| #2 | rheumatoid arthritis:ti,ab,kw |
| #3 | #1 or #2 |
| #4 | MeSH descriptor: [Remission Induction] explode all trees |
| #5 | (strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control*):ti,ab,kw |
| #6 | ((strateg* or aim* or goal* or target* or tight* or aggressiv* or control*) next/2 (treat* or therap*)):ti,ab,kw |
| #7 | (optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*):ti,ab,kw |
| #8 | (adapt* or titrat* or adjust* or response-based):ti,ab,kw |
| #9 | #7 or #8 |
| #10 | MeSH descriptor: [Disease Progression] this term only |
| #11 | MeSH descriptor: [Disease Management] this term only |
| #12 | MeSH descriptor: [Disease Outbreaks] this term only |
| #13 | MeSH descriptor: [Disease] explode all trees |
| #14 | #10 or #11 or #12 or #13 |
| #15 | #9 and #14 |
| #16 | ((strateg* or proced* or consequ* or therap* or halt* or stop* or revers* or dela* or arrest* or detain* or slow* or preven* or retard* or avoid*) next/3 (structural or functional or erosi* or progre* or disabilit* or invalidity or impediment or disablement or radiograph* or radiolog*)):ti,ab,kw |
| #17 | (remission next/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)):ti,ab,kw |
| #18 | (((low* or moderate or medium or high) and activity) next/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)):ti,ab,kw |
| #19 | #4 or #5 or #6 or #15 or #16 or #17 or #18 |
| #20 | #3 and #19 |
Web of Science Citation Index Expanded and Web of Science Citation Index and Conference Proceedings Index
Date searched: 19 May 2015.
Search strategy
| # 19 | #18 AND #13 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2013-2015 | |
| # 18 | TOPIC: ((cost* and (effective* or utilit* or benefit* or minimi*))) OR TITLE: (costs) OR TOPIC: (((economic* or pharmacoeconomic* or pharmaco-economic*))) OR TITLE: (economic*) |
| # 17 | #16 AND #13 |
| # 16 | TS=((meta-analysis or meta analy* or metaanaly*)) OR TS=(systematic review*) |
| # 15 | #14 AND #13 |
| # 14 | TS=(randomi* controlled trial*) OR TS=(placebo*) |
| # 13 | #12 AND #1 |
| # 12 | #11 OR #10 OR #9 OR #8 OR #3 OR #2 |
| # 11 | TS=(((low* or moderate or medium or high) and activity)) AND TS=((strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)) |
| # 10 | TS=(((remission NEAR/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)))) |
| # 9 | TS=(((strateg* or proced* or consequ* or therap* or halt* or stop* or revers* or dela* or arrest* or detain* or slow* or preven* or retard* or avoid*) NEAR/3 (structural or functional or erosi* or progre* or disabilit* or invalidity or impediment or disablement or radiograph* or radiolog*))) |
| # 8 | #7 AND #6 |
| # 7 | TOPIC: (disease) |
| # 6 | #5 OR #4 |
| # 5 | TOPIC: ((adapt* or titrat* or adjust* or response-based)) |
| # 4 | TITLE: ((optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*)) |
| # 3 | TS=(((strateg* or aim* or goal* or target* or tight* or aggressiv* or control*) NEAR/2 (treat* or therap*))) |
| # 2 | TITLE: ((strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control*)) |
| # 1 | TOPIC: (rheumatoid arthritis*) |
ClinicalTrials.gov: US National Institutes of Health
URL: http://clinicaltrials.gov/.
Date searched:19 May 2015.
Search strategy
ultrasound | arthritis
ultrasonography | arthritis
sonography | arthritis
echography | arthritis
European League Against Rheumatism Abstract Archive; published in the Annals of the Rheumatic Diseases and searched via the Web of Science
URL: www.abstracts2view.com/eular/sessionindex.php
Date searched: 21 May 2015.
Search strategy
| # 15 | #14 AND #13 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2008-2015 | |
| # 14 | PUBLICATION NAME: (ann rheum dis) |
| # 13 | #12 AND #1 |
| # 12 | #11 OR #10 OR #9 OR #8 OR #3 OR #2 |
| # 11 | TS=(((low* or moderate or medium or high) and activity)) AND TS=((strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)) |
| # 10 | TS=(((remission NEAR/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)))) |
| # 9 | TS=(((strateg* or proced* or consequ* or therap* or halt* or stop* or revers* or dela* or arrest* or detain* or slow* or preven* or retard* or avoid*) NEAR/3 (structural or functional or erosi* or progre* or disabilit* or invalidity or impediment or disablement or radiograph* or radiolog*))) |
| # 8 | #7 AND #6 |
| # 7 | TOPIC: (disease) |
| # 6 | #5 OR #4 |
| # 5 | TOPIC: ((adapt* or titrat* or adjust* or response-based)) |
| # 4 | TITLE: ((optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*)) |
| # 3 | TS=(((strateg* or aim* or goal* or target* or tight* or aggressiv* or control*) NEAR/2 (treat* or therap*))) |
| # 2 | TITLE: ((strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control*)) |
| # 1 | TOPIC: (rheumatoid arthritis*) |
American College of Rheumatology and Association of Rheumatology Health Professionals; published in Arthritis and Rheumatology and searched via the Web of Science
Date searched: 21 May 2015.
Search strategy
| # 15 | #14 AND #13 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2008-2015 | |
| # 14 | SO=(ARTHRITIS ‘AND’ RHEUMATISM) |
| # 13 | #12 AND #1 |
| # 12 | #11 OR #10 OR #9 OR #8 OR #3 OR #2 |
| # 11 | TS=(((low* or moderate or medium or high) and activity)) AND TS=((strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)) |
| # 10 | TS=(((remission NEAR/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)))) |
| # 9 | TS=(((strateg* or proced* or consequ* or therap* or halt* or stop* or revers* or dela* or arrest* or detain* or slow* or preven* or retard* or avoid*) NEAR/3 (structural or functional or erosi* or progre* or disabilit* or invalidity or impediment or disablement or radiograph* or radiolog*))) |
| # 8 | #7 AND #6 |
| # 7 | TOPIC: (disease) |
| # 6 | #5 OR #4 |
| # 5 | TOPIC: ((adapt* or titrat* or adjust* or response-based)) |
| # 4 | TITLE: ((optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*)) |
| # 3 | TS=(((strateg* or aim* or goal* or target* or tight* or aggressiv* or control*) NEAR/2 (treat* or therap*))) |
| # 2 | TITLE: ((strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control*)) |
| # 1 | TOPIC: (rheumatoid arthritis*) |
Phase II randomised controlled trials search strategies
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R)
Date searched: 8 January 2016.
Search strategy
| 1 | exp Arthritis, Rheumatoid/ |
| 2 | rheumatoid arthritis.tw. |
| 3 | or/1-2 |
| 4 | Remission induction/ |
| 5 | (remission adj2 induc$).ti,ab. |
| 6 | (strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or intens$ or control$ or optim$ or adapt$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 7 | ((strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or control$) adj2 (treat$ or therap$)).tw. |
| 8 | (ttt or t2t).tw. |
| 9 | (treat$ and target$).ti. |
| 10 | ((disease activity score or das28) adj3 (driven or step$ or strateg$ or therap$ or treat$)).tw. |
| 11 | (optim$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 12 | (adapt$ or titrat$ or adjust$ or response-based).tw. |
| 13 | or/11-12 (1662622) |
| 14 | *Disease Progression/ |
| 15 | *Disease Management/ |
| 16 | *Disease Outbreaks/ |
| 17 | Disease/ |
| 18 | or/14-17 |
| 19 | 13 and 18 |
| 20 | ((strateg$ or proced$ or consequ$ or therap$ or halt$ or stop$ or revers$ or dela$ or arrest$ or detain$ or slow$ or preven$ or retard$ or avoid$) adj3 (structural or functional or erosi$ or progre$ or disabilit$ or invalidity or impediment or disablement or radiograph$ or radiolog$)).tw. |
| 21 | ((remission or activ$) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 22 | (((low$ or moderate or medium or high) and activity) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 23 | 4 or 5 or 6 or 7 or 8 or 9 or 10 or 19 or 20 or 21 or 22 |
| 24 | 3 and 23 |
| 25 | Randomized controlled trials/ |
| 26 | Randomized controlled trial.pt. |
| 27 | Controlled clinical trial.pt. |
| 28 | Random Allocation/ |
| 29 | Double blind method/ |
| 30 | Single Blind Method/ |
| 31 | Clinical trial.pt. |
| 32 | exp Clinical Trial/ |
| 33 | (clin$ adj25 trial$).ti,ab. |
| 34 | ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. |
| 35 | Placebos/ |
| 36 | Placebo$.ti,ab. |
| 37 | Random$.ti,ab. |
| 38 | or/25-37 |
| 39 | 24 and 38 |
| 40 | Comment.pt. |
| 41 | Letter.pt. |
| 42 | Editorial.pt. |
| 43 | case report.tw. |
| 44 | Historical article.pt. |
| 45 | Animal/ |
| 46 | Human/ |
| 47 | 45 not (45 and 46) |
| 48 | or/40-44,47 |
| 49 | 39 not 48 |
EMBASE
Date searched: 8 January 2016.
Search strategy
| 1 | exp rheumatoid arthritis/ |
| 2 | rheumatoid arthritis.tw. |
| 3 | or/1-2 |
| 4 | remission/ |
| 5 | (remission adj2 induc$).ti,ab. |
| 6 | (strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or intens$ or control$ or optim$ or adapt$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 7 | ((strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or control$) adj2 (treat$ or therap$)).tw. |
| 8 | (treat$ and target$).ti. |
| 9 | ((disease activity score or das28) adj3 (driven or step$ or strateg$ or therap$ or treat$)).tw. |
| 10 | (optim$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 11 | (adapt$ or titrat$ or adjust$ or response-based).tw. |
| 12 | 10 or 11 |
| 13 | *disease course/ |
| 14 | *disease management/ |
| 15 | *epidemic/ |
| 16 | diseases/ |
| 17 | or/13-16 |
| 18 | 12 and 17 |
| 19 | ((strateg$ or proced$ or consequ$ or therap$ or halt$ or stop$ or revers$ or dela$ or arrest$ or detain$ or slow$ or preven$ or retard$ or avoid$) adj3 (structural or functional or erosi$ or progre$ or disabilit$ or invalidity or impediment or disablement or radiograph$ or radiolog$)).tw. |
| 20 | ((remission or activ$) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 21 | 4 or 5 or 6 or 7 or 8 or 9 or 18 or 19 or 20 |
| 22 | 3 and 21 |
| 23 | Randomized controlled trial/ |
| 24 | clinical trial/ |
| 25 | Randomization/ |
| 26 | Single blind procedure/ |
| 27 | Double blind procedure/ |
| 28 | Crossover procedure/ |
| 29 | Placebo/ |
| 30 | randomi?ed controlled trial$.tw. |
| 31 | (clin$ adj25 trial$).ti,ab. |
| 32 | ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. |
| 33 | placebo$.ti,ab. |
| 34 | random$.ti,ab. |
| 35 | or/23-34 |
| 36 | 22 and 35 |
| 37 | Case study/ |
| 38 | case report.tw. |
| 39 | Abstract report/ |
| 40 | letter/ |
| 41 | Animal/ |
| 42 | Human/ |
| 43 | 41 not (41 and 42) |
| 44 | 37 or 38 or 39 or 40 or 43 |
| 45 | 36 not 44 |
Cochrane Central Register of Controlled Trials
Date searched: 14 January 2016.
Search strategy
| #1 | MeSH descriptor: [Arthritis, Rheumatoid] explode all trees |
| #2 | rheumatoid arthritis:ti,ab,kw |
| #3 | #1 OR #2 |
| #4 | MeSH descriptor: [Remission Induction] explode all trees |
| #5 | (remission next/2 induc*):ti,ab,kw |
| #6 | (strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control* or optim* or adapt* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*):ti |
| #7 | (strateg* or aim* or goal* or target* or tight* or aggressiv* or control*) next/2 (treat* or therap*):ti,ab,kw |
| #8 | (ttt or t2t):ti,ab,kw |
| #9 | (treat* and target*):ti |
| #10 | ((disease activity score or das28) near/3 (driven or step* or strateg* or therap* or treat*)):ti,ab,kw |
| #11 | (optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*):ti |
| #12 | (adapt* or titrat* or adjust* or response-based):ti,ab,kw |
| #13 | #11 or #12 |
| #14 | MeSH descriptor: [Disease Progression] this term only |
| #15 | MeSH descriptor: [Disease Management] this term only |
| #16 | MeSH descriptor: [Disease Outbreaks] this term only |
| #17 | MeSH descriptor: [Disease] this term only |
| #18 | #14 or #15 or #16 or #17 |
| #19 | #13 and #18 |
| #20 | (strateg* or proced* or consequ* or therap* or halt* or stop* or revers* or dela* or arrest* or detain* or slow* or preven* or retard* or avoid*) n3 (structural or functional or erosi* or progre* or disabilit* or invalidity or impediment or disablement or radiograph* or radiolog*):ti,ab,kw |
| #21 | ((remission or activ*) near/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)):ti,ab,kw |
| #22 | (((low* or moderate or medium or high) and activity) near/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)):ti,ab,kw |
| #23 | #4 or #5 or #6 or #7 or #8 or #9 or #10 or #19 or #20 or #21 or #22 |
| #24 | #3 and #23 |
Web of Science Citation Index Expanded and Web of Science Citation Index and Conference Proceedings Index
Date searched: 26 January 2016.
Search strategy
| S1 | TOPIC=(Rheumatoid arthritis) |
| S2 | TOPIC=(remission near/2 induc*) |
| S3 | TITLE=(strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control* or optim* or adapt* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*) |
| S4 | TS=((strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control*) near/2 (treat* or therap*)) |
| S5 | TOPIC=(ttt or t2t) |
| S6 | TITLE=(treat* and target) |
| S7 | TOPIC=(‘disease activity score’ or das28) |
| S8 | TI=(optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*) |
| S9 | TOPIC=(adapt* or titrat* or adjust* or response-based) |
| S10 | S9 OR S8 |
| S11 | TOPIC=Disease Progression |
| S12 | TOPIC=Disease Management |
| S13 | TOPIC=Disease Outbreaks |
| S14 | TOPIC=Disease |
| S15 | S14 OR S13 OR S12 OR S11 |
| S16 | S15 AND S10 |
| S17 | TOPIC=((strateg* or proced* or consequ* or therap* or halt* or stop* or revers* or dela* or arrest* or detain* or slow* or preven* or retard* or avoid*) near/3 (structural or functional or erosi* or progre* or disabilit* or invalidity or impediment or disablement or radiograph* or radiolog*)) |
| S18 | TOPIC=((remission or activ*) near/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)) |
| S19 | TOPIC=((low* or moderate or medium or high) and (activity) near/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)) |
| S20 | S19 OR S18 OR S17 OR S16 OR S7 OR S6 OR S5 OR S4 OR S3 or S2 |
| S21 | S20 AND S1 |
| S22 | TOPIC=(randomi?ed controlled trial) |
| S23 | TOPIC=(rct) |
| S24 | TOPIC=(random allocation) |
| S25 | TOPIC=(allocated near/2 random*) |
| S26 | TOPIC=single blind* |
| S27 | TOPIC=(double blind*) |
| S28 | TOPIC=((treble or triple) near/1 (blind*)) |
| S29 | TOPIC=(placebo*) |
| S30 | S29 OR S28 OR S27 OR S26 OR S25 OR S24 OR S23 OR S22 |
| S31 | S30 AND S21 |
Bioscience Information Service Previews
Date searched: 14 January 2016.
Search strategy
| S1 | TOPIC=Rheumatoid arthritis |
| S2 | TOPC=(remission near/2 induc*) |
| S3 | TITLE=(strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control* or optim* or adapt* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*) |
| S4 | TOPIC=((strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control*) near/2 (treat* or therap*)) |
| S5 | TOPIC=(ttt or t2t) |
| S6 | TITLE=(treat* and target) |
| S7 | TOPIC=(‘disease activity score’ or das28) |
| S8 | TITLE=(optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*) |
| S9 | TOPIC=(adapt* or titrat* or adjust* or response-based) |
| S10 | #9 OR #8 |
| S11 | TOPIC=Disease Progression |
| S12 | TOPIC=Disease Management |
| S13 | TOPIC=Disease Outbreaks |
| S14 | TOPIC=Disease |
| S15 | #14 OR #13 OR #12 OR #11 |
| S16 | #15 AND #10 |
| S17 | TOPIC=((strateg* or proced* or consequ* or therap* or halt* or stop* or revers* or dela* or arrest* or detain* or slow* or preven* or retard* or avoid*) near/3 (structural or functional or erosi* or progre* or disabilit* or invalidity or impediment or disablement or radiograph* or radiolog*)) |
| S18 | TOPIC=((remission or activ*) near/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)) |
| S19 | TOPIC=((low* or moderate or medium or high) and (activity) near/3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)) |
| S20 | #19 OR #18 OR #17 OR #16 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 |
| S21 | #20 AND #1 |
| S22 | TOPIC=(randomi?ed controlled trial |
| S23 | TOPIC=(rct) |
| S24 | TOPIC=(random allocation) |
| S25 | TOPIC=(allocated near/2 random*) |
| S26 | TOPIC=(single blind*) |
| S27 | TOPIC=(double blind*) |
| S28 | TOPIC=((treble or triple) near/1 (blind*)) |
| S29 | TOPIC=(placebo*) |
| S30 | S29 OR S28 OR S27 OR S26 OR S25 OR S24 OR S23 OR S22 |
| S31 | S30 AND S21 |
Cumulative Index to Nursing and Allied Health Literature
Date searched: 13 January 2016.
Search strategy
| S1 | (Mesh Heading ‘Arthritis, Rheumatoid+’) |
| S2 | TX rheumatoid arthritis |
| S3 | (S1 OR S2) |
| S4 | TI (remission n2 induc*) or AB (remission n2 induc*) |
| S5 | TI (strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control* or optim* or adapt* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*) |
| S6 | TX (strateg* or aim* or goal* or target* or tight* or aggressiv* or control*) n2 (treat* or therap*) |
| S7 | TX (ttt or t2t) |
| S8 | TITLE (treat* and target*) |
| S9 | TX (disease activity score or das28) n3 (driven or step* or strateg* or therap* or treat*) |
| S10 | TITLE (optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*) |
| S11 | TX (adapt* or titrat* or adjust* or response-based) |
| S12 | S10 OR S11 |
| S13 | (Major concept ‘Disease Progression’) |
| S14 | (Major concept ‘Disease Outbreaks’) |
| S15 | (Mesh Heading ‘Disease’) |
| S16 | S13 OR S14 OR S15 |
| S17 | S12 AND S16 |
| S18 | TX (strateg* or proced* or consequ* or therap* or halt* or stop* or revers* or dela* or arrest* or detain* or slow* or preven* or retard* or avoid*) n3 (structural or functional or erosi* or progre* or disabilit* or invalidity or impediment or disablement or radiograph* or radiolog*) |
| S19 | TX ((remission or activ*) n3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing) |
| S20 | TX (((low* or moderate or medium or high) and activity) n3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)) |
| S21 | S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S17 OR S18 OR S19 OR S20 |
| S22 | S3 AND S21 |
| S23 | (Mesh Heading ‘Randomized Controlled Trials’) |
| S24 | Publication Type Randomized controlled trial |
| S25 | TX clinic* n1 trial* |
| S26 | TX (Singl* n1 blind*) OR TX (singl* n1 mask*) OR TX (doubl* n1 blind*) OR TX (doubl* n1 mask*) OR TX (tripl* n1 blind*) OR TX (tripl* n1 mask*) OR TX (trebl* n1 blind*) OR TX (trebl* n1 mask*) |
| S27 | (Mesh Heading ‘Clinical Trials+’) |
| S28 | Publication Type Clinical trial |
| S29 | TX Randomi* control* trial* |
| S30 | (MeSH heading ‘Random Assignment’) |
| S31 | TX Random* allocat* |
| S32 | TX Placebo* |
| S33 | (MeSH heading ‘Placebos’) |
| S34 | (MeSH heading ‘Quantitative Studies’) |
| S35 | (S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34) |
| S36 | S22 AND S35 |
| S37 | Publication Type commentary |
| S38 | Publication Type Letter |
| S39 | Publication Type Editorial |
| S40 | TX Case report |
| S41 | (MH ‘Animals’) |
| S42 | S37 OR S38 OR S39 OR S40 OR S41 |
| S43 | S36 NOT S42 |
ClinicalTrials.gov: US National Institutes of Health
URL: http://clinicaltrials.gov/
Date searched: 18 January 2016.
Search strategy
Remission induction | rheumatoid arthritis
‘TTT’ | rheumatoid arthritis
International Clinical Trials Registry Platform (URL: www.who.int/ictrp/en/)
Date searched: 18 January 2016.
Search strategy
Remission induction | rheumatoid arthritis
‘TTT’ | rheumatoid arthritis
National Institute for Health and Care Excellence Evidence (URL: www.nice.org.uk/)
Date searched: 18 January 2016.
Search strategy
‘TTT’ filtered by type of information – commissioning guide
‘TTT’ filtered by type of information – ongoing trials
‘Remission induction’ rheumatoid arthritis filtered by type of information – ongoing trials
‘Remission induction’ rheumatoid arthritis filtered by type of information – commissioning guide
Phase II cost-effectiveness search strategies
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R)
Date searched: 14 January 2016.
Search strategy
| 1 | exp Arthritis, Rheumatoid/ |
| 2 | rheumatoid arthritis.tw. |
| 3 | or/1-2 |
| 4 | Remission induction/ |
| 5 | (remission adj2 induc$).ti,ab. |
| 6 | (strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or intens$ or control$ or optim$ or adapt$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 7 | ((strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or control$) adj2 (treat$ or therap$)).tw. |
| 8 | (ttt or t2t).tw. |
| 9 | (treat$ and target$).ti. |
| 10 | ((disease activity score or das28) adj3 (driven or step$ or strateg$ or therap$ or treat$)).tw. |
| 11 | (optim$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 12 | (adapt$ or titrat$ or adjust$ or response-based).tw. |
| 13 | or/11-12 |
| 14 | *Disease Progression/ |
| 15 | *Disease Management/ |
| 16 | *Disease Outbreaks/ |
| 17 | Disease/ |
| 18 | or/14-17 |
| 19 | 13 and 18 |
| 20 | ((strateg$ or proced$ or consequ$ or therap$ or halt$ or stop$ or revers$ or dela$ or arrest$ or detain$ or slow$ or preven$ or retard$ or avoid$) adj3 (structural or functional or erosi$ or progre$ or disabilit$ or invalidity or impediment or disablement or radiograph$ or radiolog$)).tw. |
| 21 | ((remission or activ$) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 22 | (((low$ or moderate or medium or high) and activity) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 23 | 4 or 5 or 6 or 7 or 8 or 9 or 10 or 19 or 20 or 21 or 22 |
| 24 | 3 and 23 |
| 25 | Economics/ |
| 26 | “Costs and cost analysis”/ |
| 27 | Cost allocation/ |
| 28 | Cost-benefit analysis/ |
| 29 | Cost control/ |
| 30 | Cost savings/ |
| 31 | Cost of illness/ |
| 32 | Cost sharing/ |
| 33 | “Deductibles and coinsurance”/ |
| 34 | Health care costs/ |
| 35 | Direct service costs/ |
| 36 | Drug costs/ |
| 37 | Employer health costs/ |
| 38 | Hospital costs/ |
| 39 | Health expenditures/ |
| 40 | Capital expenditures/ |
| 41 | Value of life/ |
| 42 | exp Economics, hospital/ |
| 43 | exp Economics, medical/ |
| 44 | Economics, nursing/ |
| 45 | Economics, pharmaceutical/ |
| 46 | exp "fees and charges"/ |
| 47 | exp budgets/ |
| 48 | (low adj cost).mp. |
| 49 | (high adj cost).mp. |
| 50 | (health?care adj cost$).mp. |
| 51 | (fiscal or funding or financial or finance).tw. |
| 52 | (cost adj estimate$).mp. |
| 53 | (cost adj variable).mp. |
| 54 | (unit adj cost$).mp. |
| 55 | (economic$ or pharmacoeconomic$ or price$ or pricing).tw. |
| 56 | exp models, economic/ |
| 57 | or/25-56 |
| 58 | 24 and 57 |
| 59 | Comment.pt. |
| 60 | Letter.pt. |
| 61 | Editorial.pt. |
| 62 | case report.tw. |
| 63 | Historical article.pt. |
| 64 | Animal/ |
| 65 | Human/ |
| 66 | 64 not (64 and 65) |
| 67 | or/59-63,66 |
| 68 | 58 not 67 |
EMBASE
Date searched: 14 January 2016.
Search strategy
| 1 | exp rheumatoid arthritis/ |
| 2 | rheumatoid arthritis.tw. |
| 3 | or/1-2 |
| 4 | remission/ |
| 5 | (remission adj2 induc$).ti,ab. |
| 6 | (strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or intens$ or control$ or optim$ or adapt$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 7 | ((strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or control$) adj2 (treat$ or therap$)).tw. |
| 8 | (treat$ and target$).ti. |
| 9 | ((disease activity score or das28) adj3 (driven or step$ or strateg$ or therap$ or treat$)).tw. |
| 10 | (optim$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 11 | (adapt$ or titrat$ or adjust$ or response-based).tw. |
| 12 | 10 or 11 |
| 13 | *disease course/ |
| 14 | *disease management/ |
| 15 | *epidemic/ |
| 16 | diseases/ |
| 17 | or/13-16 |
| 18 | 12 and 17 |
| 19 | ((strateg$ or proced$ or consequ$ or therap$ or halt$ or stop$ or revers$ or dela$ or arrest$ or detain$ or slow$ or preven$ or retard$ or avoid$) adj3 (structural or functional or erosi$ or progre$ or disabilit$ or invalidity or impediment or disablement or radiograph$ or radiolog$)).tw. |
| 20 | ((remission or activ$) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 21 | 4 or 5 or 6 or 7 or 8 or 9 or 18 or 19 or 20 |
| 22 | 3 and 21 |
| 23 | Socioeconomics/ |
| 24 | Cost benefit analysis/ |
| 25 | Cost effectiveness analysis/ |
| 26 | Cost of illness/ |
| 27 | Cost control/ |
| 28 | Economic aspect/ |
| 29 | Financial management/ |
| 30 | Health care cost/ |
| 31 | Health care financing/ |
| 32 | Health economics/ |
| 33 | Hospital cost/ |
| 34 | (fiscal or financial or finance or funding).tw. |
| 35 | cost minimization analysis/ |
| 36 | (cost adj estimate$).mp. |
| 37 | (cost adj variable$).mp. |
| 38 | (unit adj cost$).mp. |
| 39 | or/23-38 |
| 40 | 22 and 39 |
| 41 | Letter.pt. |
| 42 | Editorial.pt. |
| 43 | Animal/ |
| 44 | Human/ |
| 45 | 43 not (43 and 44) |
| 46 | or/41-42,45 |
| 47 | 40 not 46 |
NHS Economic Evaluation Database
Date searched: 14 January 2016.
Search strategy
| #1 | MeSH descriptor: [Arthritis, Rheumatoid] explode all trees |
| #2 | rheumatoid arthritis:ti,ab,kw |
| #3 | #1 OR #2 |
| #4 | MeSH descriptor: [Remission Induction] explode all trees |
| #5 | (remission next/2 induc*):ti,ab,kw |
| #6 | (strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control* or optim* or adapt* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*):ti |
| #7 | (strateg* or aim* or goal* or target* or tight* or aggressiv* or control*) next/2 (treat* or therap*):ti,ab,kw |
| #8 | (ttt or t2t):ti,ab,kw |
| #9 | (treat* and target*):ti |
| #10 | ((disease activity score or das28) near/3 (driven or step* or strateg* or therap* or treat*)):ti,ab,kw |
| #11 | (optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*):ti |
| #12 | (adapt* or titrat* or adjust* or response-based):ti,ab,kw |
| #13 | #11 or #12 |
| #14 | MeSH descriptor: [Disease Progression] this term only |
| #15 | MeSH descriptor: [Disease Management] this term only |
| #16 | MeSH descriptor: [Disease Outbreaks] this term only |
| #17 | MeSH descriptor: [Disease] this term only |
| #18 | #14 or #15 or #16 or #17 |
Cumulative Index to Nursing and Allied Health Literature
Date searched: 14 January 2016.
Search strategy
| S1 | (MeSH Heading “Arthritis, Rheumatoid+”) |
| S2 | TX rheumatoid arthritis |
| S3 | (S1 OR S2) |
| S4 | TI (remission n2 induc*) OR AB (remission n2 induc*) |
| S5 | TITLE (strateg* or aim* or goal* or target* or tight* or aggressiv* or intens* or control* or optim* or adapt* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*) |
| S6 | TX (strateg* or aim* or goal* or target* or tight* or aggressiv* or control*) n2 (treat* or therap*) |
| S7 | TX (ttt or t2t) |
| S8 | TI (treat* and target*) |
| S9 | TX (disease activity score or das28) N3 (driven or step* or strateg* or therap* or treat*) |
| S10 | TI (optim* or switch* or add* or chang* or expand* or step* or combin* or intensif* or escalat*) |
| S11 | TX (adapt* or titrat* or adjust* or response-based) |
| S12 | S10 OR S11 |
| S13 | (MeSH descriptor “Disease Progression”) |
| S14 | (MeSH descriptor "Disease Outbreaks") |
| S15 | (Mesh Heading “Disease”) |
| S16 | S13 OR S14 OR S15 |
| S17 | S12 AND S16 |
| S18 | TX (Strateg* or proced* or consequ* or therap* or halt* or stop* or revers* or dela* or arrest* or detain* or slow* or preven* or retard* or avoid*) N3 (structural or functional or erosi* or progre* or disabilit* or invalidity or impediment or disablement or radiograph* or radiolog*) |
| S19 | TX ((Remission or activ*) N3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing) |
| S20 | TX (((Low* or moderate or medium or high) and activity) N3 (strateg* or optimi* or adapt* or control* or frequency or dose* or dosing)) |
| S21 | S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S17 OR S18 OR S19 OR S20 |
| S22 | S3 AND S21 |
| S23 | (MeSH Heading “Economics+”) |
| S24 | (MeSH Heading "Financial Management+") |
| S25 | (MeSH Heading “Financing, Organized+”) |
| S26 | (MeSH Heading "Business+") |
| S27 | S24 OR S25 OR S26 |
| S28 | S23 NOT S27 |
| S29 | MeSH Heading Health resource allocation |
| S30 | MeSH Heading Health resource utilization |
| S31 | S29 OR S30 |
| S32 | S28 OR S31 |
| S33 | TX (cost or costs or economic* or pharmacoeconomic* or price* or pricing*) |
| S34 | S32 OR S33 |
| S35 | S22 AND S34 |
| S36 | Publication Type Editorial |
| S37 | Publication Type letter |
| S38 | (MeSH Heading “Animal Studies”) |
| S39 | S36 OR S37 OR S38 |
| S40 | S35 NOT S39 |
American Economic Association’s electronic bibliography
Date searched: 14 January 2016.
Search strategy
| 1 | Rheumatoid arthritis.tw. |
| 2 | (remission adj2 induc$).ti,ab. |
| 3 | (strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or intens$ or control$ or optim$ or adapt$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 4 | ((strateg$ or aim$ or goal$ or target$ or tight$ or aggressiv$ or control$) adj2 (treat$ or therap$)).tw. |
| 5 | (ttt or t2t).tw. |
| 6 | (treat$ and target$).ti. |
| 7 | ((disease activity score or das28) adj3 (driven or step$ or strateg$ or therap$ or treat$)).tw. |
| 8 | (optim$ or switch$ or add$ or chang$ or expand$ or step$ or combin$ or intensif$ or escalat$).ti. |
| 9 | (adapt$ or titrat$ or adjust$ or response-based).tw. |
| 10 | 8 or 9 |
| 11 | disease progression.tw. |
| 12 | disease management.tw. |
| 13 | disease outbreaks.tw. |
| 14 | disease.tw. |
| 15 | or/11-14 |
| 16 | 10 and 15 |
| 18 | ((Remission or activ$) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 19 | (((low$ or moderate or medium or high) and activity) adj3 (strateg$ or optimi$ or adapt$ or control$ or frequency or dose$ or dosing)).tw. |
| 20 | 2 or 3 or 4 or 5 or 6 or 7 or 16 or 17 or 18 or 19 |
| 21 | 1 and 20 |
Appendix 3 Table of excluded studies with rationale
| Study | Reason |
|---|---|
| ACT-RAY98,99 | Not a trial of TTT |
| Alemao et al., 2014100 | Conference abstract: insufficient detail |
| ARCTIC101 | Conference abstract: insufficient detail |
| Bijlsma et al., 2015102 | No new data (from update search): U-Act-Early62 |
| CAMERA II37,103–106 | Not a trial of TTT |
| Carubbi et al., 2016107 | Not a trial of TTT |
| Charles-Schoeman 2016108 | No relevant outcomes (from update search): TEAR58–60 |
| CIMESTRA109–111 | Not a trial of TTT |
| COBRA112–116 | Not a trial of TTT |
| Dale 2015117 | Not a RCT |
| De Cock et al., 2013;118 and 2014119 | Not a RCT |
| De Cock et al., 2015120 | No new data (from update search): CareRA40,42,43 |
| DRESS121 | Not a trial of TTT |
| Dumitru et al., 2016122 | No study results yet |
| Emery et al., 2009;123 2010;124,125 and 2011126 | Not a RCT |
| Fedorenko et al., 2013127 | Not a trial of TTT |
| Ferraccioli et al., 2002128 | Numbers stepping up not reported |
| Fiehn et al., 2007129 | Not a trial of TTT |
| Galloway et al., 2015130 | Not a trial of TTT |
| Giacomelli et al., 2002131 | Not a trial of TTT |
| Harrold et al., 2015132 | No relevant outcomes |
| Hørslev-Petersen et al., 2011133 | Not a trial of TTT |
| Hwang et al., 2016134 | No relevant outcomes (from update search): TEAR58–60 |
| IDEA135,136 | Not a trial of TTT |
| IMAGINE-RA137 | No study results yet |
| Jalal et al., 2016138 | Not a RCT |
| Korthals-de Bos et al., 2004113 | Unobtainable |
| Kume et al., 2013139 | Conference abstract: insufficient detail |
| Kuusalo et al., 2015140 | Not a trial of TTT: Neo-RACo (from update search) |
| Li et al., 2016141 | Not a trial of TTT |
| Marchesoni et al., 2002142 | Not a trial of TTT |
| Markusse et al., 2015143 | No new data (from update search): BeSt26–34,64–66 |
| Markusse et al., 2016144 | Not a RCT |
| MASCOT145,146 | Not a trial of TTT |
| Menon et al., 2014147 | Not a trial of TTT |
| Moller-Bisgaard et al., 2015148 | Not a trial of TTT: CIMESTA (from update search) |
| Montecucco et al., 2012149 | Not a trial of TTT |
| Neo-RACo140,150–157 | Not a trial of TTT |
| OPERA158–162 | Not a trial of TTT |
| OPTIMA163–168 | Not a trial of TTT |
| OSRA169,170 | Conference abstract: insufficient detail |
| Pablos et al., 2015171 | Not a trial of TTT |
| Pavelka et al., 2015172 | Not a RCT |
| Pavelka et al., 2016173 | Not a trial of TTT |
| PREMIER174–186 | Not a trial of TTT |
| PRESERVE187–200 | Not a trial of TTT |
| Proudman et al., 2000201 | Not a trial of TTT |
| Scott and Kowalczyk, 2010202 | Not a RCT |
| Scott et al., 2016203 | Not a RCT |
| Solomon et al., 2016204 | Not a RCT |
| STRASS205–213 | Not a trial of TTT |
| TaSER214–216 | No data available for the DAS28-targeted group |
| Todoerti et al., 2010217 | Not a trial of TTT |
| tREACH218–224 | Not a trial of TTT |
| Van der Elst et al., 2016225 | Not a RCT |
| van Tuyl et al., 2008226 | Separate data not reported for the two arms |
| Verschueren et al., 2008227 | Not a RCT |
| Verschueren et al., 2015228 | No new data (from update search): CareRA40,42,43 |
| Yoo et al., 2016229 | Not a trial of TTT |
Appendix 4 Tables of treatment adaptations and drug dosing
| Trial acronym | Treatment arms | Treatment adaptations | Total dose of each drug given over trial period |
|---|---|---|---|
| STREAM55 | Aggressive group | NR | NA |
| Conventional care | NR | NA | |
| Aggressive group |
29 patients stepped up to 25 mg/week of MTX 19 patients stepped up to 40 mg/2 weeks of ADA + MTX 25 mg/week 15 patients stepped up to 40 mg/week of ADA + 25 mg/week of MTX 11 patients converted to triple cDMARDs 3 patients stepped up to triple cDMARDs + PDN 1 patient converted to LEF |
NR | |
| Conventional care | 24 patients started on HCQ:
|
Mean dose of MTX among MTX users was 19 mg/week | |
| T-4 study56,57 | Routine care | Number initiating:
|
NR |
| (N = 55) | |||
| DAS28-driven therapy | Number initiating:
|
NR | |
| (N = 59) | |||
| MMP-3-driven therapy | Number initiating:
|
NR | |
| (N = 59) | |||
| DAS2- and MMP-3-driven therapy | Number initiating:
|
NR | |
| (N = 61) |
| Trial acronym; first author and year of publication | Treatment arm | Treatment adaptations | Total dose (mg/week) of each drug given over trial period |
|---|---|---|---|
| Fransen et al., 200550 | DAS28 | % patients with changes to DMARDs: 25.0%a | MTX: 14.2b |
| SSZ: 2011.83b | |||
| PDN: 48.93b | |||
| Usual care | % patients with changes to DMARDs: 6.8%a | MTX: 12.4b | |
| SSZ: 1940.83b | |||
| PDN: 45.30b | |||
| DAS28 | % patients with changes to DMARDs: 25.9%a | MTX: 14.9b | |
| SSZ: 2047.34c | |||
| PDN: 46.42c | |||
| Usual care | % patients with changes to DMARDs: 12.9%a | MTX: 12.1c | |
| SSZ: 1869.82c | |||
| PDN: 40.46c | |||
| DAS28 | % patients with changes to DMARDs: 18.0%a | MTX: 15.3c | |
| SSZ: 1923.08c | |||
| PDN: 44.64c | |||
| Usual care | % patients with changes to DMARDs: 8.8%a | MTX: 12.4c | |
| SSZ: 1754.44c | |||
| PDN: 39.70c | |||
| Optimisation of Adalimumab study52,53 | Routine care | NR | NR |
| DAS28 target | NR | NR | |
| SJC target | NR | NR | |
| Routine care | NR | NR | |
| DAS28 target | NR | NR | |
| SJC target | NR | NR | |
| Routine care | Changes per 100 patient-months: 5.1c,d | NR | |
| DAS28 target | Changes per 100 patient-months: 6.2b,c | NR | |
| SJC target | Changes per 100 patient-months: 3.3c | NR |
| Trial acronym; first author and year of publication | Treatment arm | Treatment adaptations | Total dose of each drug given over trial period |
|---|---|---|---|
| TICORA61 | Intensive management | Combination DMARD initiation: 37 (67)a | MTX: 17.6 mg/weekb |
Numbers receiving by 18 months:
|
SSZ: 2.9 g/dayb | ||
| Triamcinolone acetonide: 28 mg/monthb | |||
| Routine management | Combination DMARD initiation: 6 (11)a | MTX: 13.6 mg/weekb | |
Numbers receiving by 18 months:
|
SSZ: 3.0 g/dayb | ||
| Triamcinolone acetonide: 8 mg/monthb | |||
| van Hulst et al., 201063 | Intervention group | Medication changed in 35% (263/760) clinic visits:
|
NR |
| Usual-care group | Medication changed in 33% (133/406) clinic visits:
|
NR |
| Trial acronym; first author and year of publication | Treatment arm | Treatment adaptations | Total dose of each drug given over trial period |
|---|---|---|---|
| Hodkinson et al., 201551 | SDAI arm | NR | NR |
| CDAI arm | NR | NR | |
| T-4 study56,57 | Routine care | Number initiating:
|
NR |
| (N = 55) | |||
| DAS28-driven therapy | Number initiating:
|
NR | |
| (N = 59) | |||
| MMP-3-driven therapy | Number initiating:
|
NR | |
| (N = 59) | |||
| DAS28 and MMP-3-driven therapy | Number initiating:
|
NR | |
| (N = 61) | |||
| TEAR58–60 | Immediate ETN | NR | NR |
| Immediate triple therapy | NR | NR | |
| Step-up ETN | NR | NR | |
| Step-up triple therapy | NR | NR | |
| Immediate ETN | NR | For all four groups, the mean MTX dosage: 19.1 mg/week (for the whole sample; no significant differences between groups) | |
| Immediate triple therapy | NR | ||
| Step-up ETN | NR | ||
| Step-up triple therapy | NR |
| Trial name | Treatment arm | Treatment adaptations | Total dose of each drug given over trial period |
|---|---|---|---|
| Optimisation of Adalimumab study52,53 | Routine care | NR | NR |
| DAS28 target | NR | NR | |
| SJC target | NR | NR | |
| Routine care | NR | NR | |
| DAS28 target | NR | NR | |
| SJC target | NR | NR | |
| Routine care | Changes per 100 patient-months: 5.1a,b | NR | |
| DAS28 target | Changes per 100 patient-months: 6.2a,c | NR | |
| SJC target | Changes per 100 patient-months: 3.3a | NR |
| Trial acronym | Treatment arm | Treatment adaptations | Total dose of each drug given over trial period |
|---|---|---|---|
| CAMERA35,36,67 | Intensive strategy group | NR | NR |
| Conventional strategy group | NR | NR | |
| Intensive strategy group | 55 patients converted to subcutaneous MTX as a result of reaching the maximum dose of MTX or toxicity. Ciclosporin given to 38 patients after the maximum MTX dose reached | MTX: 16.1 (14.8 to 17.3) mg/weeka (in completers) | |
| Conventional strategy group | 12 patients converted to subcutaneous MTX as a result of reaching the maximum dose of MTX or toxicity. Ciclosporin given to four patients after the maximum MTX dose reached | MTX: 14.0 (13.1 to 14.8) mg/weeka (in completers) |
| Trial | Treatment arm | Treatment adaptations | Total dose of each drug given over trial period |
|---|---|---|---|
| BROSG trial9 | Symptomatic treatment (shared care) | 131/234 (56%) of patients had some change in their disease-suppressive treatment [including patients who had the dose changed, but not those who only had joint injection(s)] | NR |
| Aggressive therapy (hospital) | 179/232 (77%) of patients had some change in their disease-suppressive treatment [including patients who had the dose changed, but not those who only had joint injection(s)] | NR |
Appendix 5 Quality assessment of the economic papers
As some of the authors of this report were key members of the team undertaking the HTA of bDMARDs, it was deemed that a review of economic models that did not focus on TTT was unnecessary. Systematic reviews that were identified had references checked to see if any of the included studies were assessments of TTT.
| Section/item | Item number | Recommendation |
|---|---|---|
| Title and abstract | ||
| Title | 1 | Identify the study as an economic evaluation or use more specific terms, such as ‘cost-effectiveness analysis’, and describe the interventions compared |
| Abstract | 2 | Provide a structured summary of objectives, perspective, setting, methods (including study design and inputs), results (including base-case and uncertainty analyses) and conclusions |
| Background and objectives | ||
| Background and objectives | 3 | Provide an explicit statement of the broader context for the study |
| Present the study question and its relevance for health policy or practice decisions | ||
| Methods | ||
| Target population and subgroups | 4 | Describe characteristics of the base-case population and subgroups analysed, including why they were chosen |
| Setting and location | 5 | State relevant aspects of the system(s) in which the decision(s) need(s) to be made |
| Study perspective | 6 | Describe the perspective of the study and relate this to the costs being evaluated |
| Comparators | 7 | Describe the interventions or strategies being compared and state why they were chosen |
| Time horizon | 8 | State the time horizon(s) over which costs, and consequences, are being evaluated and say why appropriate |
| Discount rate | 9 | Report the choice of discount rate(s) used for costs and outcomes and say why appropriate |
| Choice of health outcomes | 10 | Describe what outcomes were used as the measure(s) of benefit in the evaluation and their relevance for the type of analysis performed |
| Measurement of effectiveness | 11a | Single study-based evaluation: describe fully the design features of the single effectiveness study and why the single study was a sufficient source of clinical effectiveness data |
| 11b | Synthesis-based estimates: describe fully the methods used for identification of included studies and synthesis of clinical effectiveness data | |
| Measurement and valuation of preference-based outcomes | 12 | If applicable, describe the population and methods used to elicit preferences for outcomes |
| Estimating resources and costs | 13a | Single study-based evaluation: describe the approaches used to estimate resource use associated with the alternative interventions. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs |
| 13b | Model-based evaluation: describe the approaches and data sources used to estimate resource use associated with model health states. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | |
| Currency, price date and conversion | 14 | Report the dates of the estimated resource quantities and unit costs. Describe methods for adjusting estimated unit costs to the year of reported costs if necessary. Describe methods for converting costs into a common currency base and the exchange rate |
| Choice of model | 15 | Describe and give reasons for the specific type of decision-analytical model used. Providing a figure to show model structure is strongly recommended |
| Assumptions | 16 | Describe all structural or other assumptions underpinning the decision-analytical model |
| Analytical methods | 17 | Describe all analytical methods supporting the evaluation. This could include methods for dealing with skewed, missing or censored data; extrapolation methods; methods for pooling data; approaches to validate or make adjustments (such as half cycle corrections) to a model; and methods for handling population heterogeneity and uncertainty |
| Results | ||
| Study parameters | 18 | Report the values, ranges, references and, if used, probability distributions for all parameters. Report reasons or sources for distributions used to represent uncertainty where appropriate. Providing a table to show the input values is strongly recommended |
| Incremental costs and outcomes | 19 | For each intervention, report mean values for the main categories of estimated costs and outcomes of interest, as well as mean differences between the comparator groups. If applicable, report ICERs |
| Characterising uncertainty | 20a | Single study-based economic evaluation: describe the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters, together with the impact of methodological assumptions (such as discount rate, study perspective) |
| Incremental costs and outcomes | 20b | Model-based economic evaluation: describe the effects on the results of uncertainty for all input parameters and uncertainty related to the structure of the model and assumptions |
| Characterising heterogeneity | 21 | If applicable, report differences in costs, outcomes, or cost-effectiveness that can be explained by variations between subgroups of patients with different baseline characteristics or other observed variability in effects that are not reducible by more information |
| Discussion | ||
| Discussion | 22 | Summarise key study findings and describe how they support the conclusions reached. Discuss limitations and the generalisability of the findings and how the findings fit with current knowledge |
| Other | ||
| Source of funding | 23 | Describe how the study was funded and the role of the funder in the identification, design, conduct and reporting of the analysis. Describe other non-monetary sources of support |
| Conflicts of interest | 24 | Describe any potential for conflict of interest of study contributors in accordance with journal policy. In the absence of a journal policy, we recommend authors comply with the recommendations of the International Committee of Medical Journal Editors |
| Item number | Response |
|---|---|
| 1 | The study is identified in the title as an economic evaluation. However, the names of the interventions being compared were not identified in the title |
| 2 | The study includes a structured summary or abstract |
| 3 | The study provides a rationale and context for the research question |
| The study identifies and clearly states the research question to be answered, but does not state the relevance that answering the research question will have in health policy or practice decisions | |
| 4 | The cost-effectiveness analysis was based on the results achieved by patients entered into the DREAM registry which began in 2006. Patients who received TTT care (n = 261), 61.7% female, had a mean age of 57.9 years (SD = 13.8 years) and a mean DAS28 of 5.0 (SD = 1.1). Patients who received usual care over the same period (n = 69), 62.3% female, had a mean age of 53.9 years (SD = 13.0 years) and a mean DAS28 of 4.8 (SD = 1.3). However, the number of patients entered into the usual-care cohort was considered insufficient and thus patients who had received usual care from the year 2000 were entered into a third cohort (n = 213). These patients, 62% of whom were female, had a mean age of 56.6 years (SD = 13.4 years) and a mean DAS28 of 4.8 (SD = 1.2). All patients had a symptom duration of < 1 year and had received no previous DMARD treatment |
| 5 | The study was set in Denmark |
| 6 | The perspective of the study was direct health-care costs |
| 7 | The TTT strategy included a standardised, protocol-based treatment regimen targeted at achieving remission. Patients were assessed at weeks 0, 8, 12, 20, 36 and 52 and every subsequent 3 months with doctors informed of each patient’s DAS28 measured by a rheumatology nurse. Treatment consisted of initial MTX monotherapy followed by MTX/SSZ combination therapy followed by MTX/anti-TNF combination therapy. Patients achieving remission were maintained on their current treatment for 6 months; if remission persisted beyond 6 months treatment was gradually reduced and discontinued. NSAID medications, prednisolone and intra-articular corticosteroid injections were allowed at the discretion of the doctor |
| The usual-care strategy was a non-standard, non-protocol-based regimen. Patients were assessed every 3 months by a rheumatology nurse, but doctors were not informed of a patient’s DAS28. Treatment regimen was at the discretion of the doctor, although the usual pathway is MTX monotherapy followed by either SSZ monotherapy, or combination MTX/SSZ therapy followed by two conventional DMARDs and finally an anti-TNF agent. NSAID medications and prednisolone were allowed at the discretion of the doctor | |
| 8 | The reporting of cost-effectiveness analysis was poor; time horizon and cycle length were not reported |
| 9 | The annual discount rate used to discount future costs and benefits was not reported |
| 10 | The study used DAS28 to assess if a patient has achieved remission (i.e. a DAS28 of < 2.6). The HAQ was used to assess the heath-related quality of life of registry patients rather than a preference-based measure such as the EQ-5D. However, HAQ scores were converted to EQ-5D scores using model 5 of the mapping methodology derived by Bansback et al.230 |
| 11a | The study was based on the DREAM registry, a quasi-experimental study performed at multiple locations in eastern Denmark. Patients were allocated to the TTT cohort or the usual-care cohort based on their home address. Patient characteristics and resource use were recorded for all patients, with the interim results (at the time of publication the study was ongoing) used to inform this cost-effectiveness analysis |
| 11b | N/A |
| 12 | See response to item 4 |
| 13a | See response to item 11a |
| 13b | N/A |
| 14 | The study states that a cost year of 2011 was used. Costs from other sources were uplifted to 2011 values, if necessary, using the Dutch price index rate |
| 15 | The reporting of cost-effectiveness analysis was poor, no model specification or schematic is reported |
| 16 | Not reported |
| 17 | Missing data were dealt with through single imputation using a regression method or linear interpolation using the trapezoid method conditional on missing data occurring at random |
| 18 | The reporting of cost-effectiveness analysis was poor, the nature and value of the input parameters is not specified |
| 19 |
The economic results were provided for data after a 2-year follow-up period and data after a 3-year follow-up period 2-year follow-up period: |
3-year follow-up period:
|
|
| 20a | The economic answers were based running the model on 1000 bootstrap replicates. However, there is no reporting of the data behind this bootstrapping |
| 20b | N/A |
| 21 | A subgroup analysis was performed using usual-care data collected on patients entering since 2006 (see response to item 4). After 2 years’ follow-up the ICER was €8709 per patient in remission and after 3 years’ follow-up TTT dominates. However, the 3-year results were based on data for only 45 patients in the usual-care cohort) |
| 22 | A comprehensive discussion of the conclusion, strengths and limitations of the study was provided |
| 23 | The study was funded by an unrestricted education grant from Abbott |
| 24 | All 10 authors state that they had no competing interests |
| Item number | Response |
|---|---|
| 1 | The study is identified in the title as a cost–utility appraisal. However, the names of the interventions being compared are not identified in the title |
| 2 | The study includes a structured summary or abstract |
| 3 | The study provides a rationale and context for the research question |
| The study identifies, and clearly states, the research question to be answered, but does not state the relevance that answering the research question will have in health policy or practice decisions | |
| 4 | The cost–utility appraisal was based on the results achieved by patients with recent-onset RA who entered into treatment at the 20 included sites in the Netherlands between March 2000 and August 2002. Patients were at least 18 years of age, fulfilled the 1987 criteria of the ACR and had a disease duration of, at most, 2 years. The details of the randomisation groups is as follows:
|
| 5 | The study was set in the Netherlands |
| 6 | The primary perspective of the study was a societal cost. However, a secondary analysis was conducted with a health-care cost perspective |
| 7 | The study compared four treatment strategies:
|
| The actual treatment a patient received was based on their disease activity score, which was measured every 3 months by an experienced research nurse who was unaware of the randomisation group. In addition, all patients receiving IFX had their disease activity score measured 1 week before infusion. If a patient’s DAS was > 2.4, the next treatment regimen in their treatment strategy was started; otherwise, patients remained on their current treatment regimen. After 6 months with a disease activity score of ≤ 2.4, treatment was tapered until the treatment regimen contained only a single DMARD | |
| Parallel treatment with NSAID containing corticosteroids | |
| 8 | The time horizon of the analysis was 2 years and the cycle length was 3 months |
| 9 | The annual discount rate used to discount future costs and benefits was 3% |
| 10 | The study used QALYs to measure the outcome in terms of health-related benefit which was appropriate for a cost–utility appraisal |
| 11a | The study was based on the BeSt trial, which was designed to determine the best treatment regimen for patients with RA, and was populated to ensure a power of 80% at a significance level of 0.05 |
| 11b | N/A |
| 12 | The study used a number of methods and instruments to elicit health outcomes from the entire study population, as described in item 4. These included measuring patients’ quality of life using the British EQ-5D instrument, the Dutch EQ-5D instrument and the SF-6D every 3 months. The time trade-off method was also used to measure patients’ quality of life at baseline, and at 6, 12 and 24 months |
| 13a | Resources used, including pharmaceuticals, were obtained from patient’s case notes with pharmaceutical and associated unit costs being taken from the Dutch Health Insurance Executive Board. Other health-care unit costs were based on Dutch standard prices, which were designed to reflect societal costs231,232 |
| The societal cost of absence from paid work was costed using an age- and sex-appropriate standard hourly rate and ranged between €17 and €41 per hour. The friction method233 was used in the initial 6-month study period and the human capital method over the entire study period, but did not include the cost of lost production or the cost of replacement staff | |
| Patients’ out-of-pocket expenses were valued using costs reported by patients in their quarterly cost diaries. If costs were not specified in the diary, expenses were costed using published cost prices234,235 or current market prices | |
| Extra time spent by patients on household and voluntary work was estimated by subtracting the average time taken by an age- and gender-matched population for a task from the time spent on that task by patients. This extra time was valued at the cost of informal care taken from van Roijen et al.236 | |
| 13b | N/A |
| 14 | A cost year of 2008 was used with all costs converted to euros using the general Dutch price index rate |
| 15 | Not reported |
| 16 | Not reported |
| 17 | A multiple imputation by chained equations method was employed to reduce any bias attributable to missing data |
| 18 | In this instance, all that was required was a statistical analysis of study data rather than health economics modelling |
| 19 | Results of the primary analysis: in the primary analysis, QALY estimates were based on the British EQ-5D instrument and a societal perspective was taken, with the friction method used to estimate cost of absence from work. Strategy 4 resulted in the highest number of QALYs but at a considerably higher cost, indeed the cost–utility ratio of strategy 4 compared with the next best strategy (strategy 3) is €130,000 (95% CI €27,000 to €3,000,000) per QALY. Strategy 3 had the greatest chance of being optimal at willingness-to-pay thresholds between €74,000 per QALY and €130,000 per QALY, with strategy 2 having the greatest change of being optimal at willingness-to-pay thresholds < €74,000 per QALY. Strategy 1 was dominated by strategies 2 and 3 |
Results of the secondary analysis: the cost–utility ratio of strategy 4 compared with strategy 3 using the other utility estimates were:
|
|
Costs of pharmaceuticals: the following costs are the mean pharmaceutical costs (SD) for the four strategies over the 2-year study period:
|
|
| 20a | No deterministic or stochastic sensitivity analyses were performed |
| 20b | N/A |
| 21 | No subgroup analyses were performed |
| 22 | A comprehensive discussion of the conclusion was provided |
| 23 | The study was funded by a grant from the Dutch Health Insurance Board. However, additional funding was also provided by Schering-Plough B.V. and by Centocor Inc. |
| 24 | A number of the authors state that they have competing interests |
Appendix 6 Tables of treat to target and disease activity outcomes for the comparison of different treatment protocols
| Trial acronym; first author and year of publication | Treatment arm | Number of participantsa | Follow-up time point | Number completing, n/N (%) (randomised phase) | Reasons for withdrawal | Definition of study target | Number (%) | Treatment adaptations | Total dose of each drug given over trial period | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meeting study target | Attaining LDA (criteria) | Attaining remission (criteria) | |||||||||
| BeSt26–34,64–66 (12-month randomised phase) | Sequential monotherapy | 126 | 12 months30 | 122/126 (97) |
|
A DAS44 of ≤ 2.4 | 63/118 (53)b,c | 63/118 (53)b,c (DAS44 of ≤ 2.4) | NR | NR | NR |
| Step-up combination therapy | 121 | 12 months | 115/121 (95) |
|
72/112 (64) | 72/112 (64) (DAS44 of ≤ 2.4) | NR | NR | NR | ||
| Initial combination therapy with PDN | 133 | 12 months | 128/133 (96) |
|
87/122 (71) | 87/122 (71) (DAS44 of ≤ 2.4) | NR | NR | NR | ||
| Initial combination therapy with IFX | 128 | 12 months | 126/128 (98) |
|
89/121 (74) | 89/121 (74) (DAS44 of ≤ 2.4) | NR | NR | NR | ||
| Sequential monotherapy | 126 | 5 years31 | 111/126 (88) |
|
NR | NR | NR | 25% still on initial treatment; 41% started delayed IFX and 21% were still on it at 5 years | NR | ||
| Step-up combination therapy | 121 | 5 years | 94/121 (78) |
|
NR | NR | NR | 21% still on initial treatment; 12% started delayed IFX and 5% were still on it at 5 years. 26% started PDN and 6% were still on it at 5 years | NR | ||
| Initial combination therapy with PDN | 133 | 5 years | 113/133 (85) |
|
NR | NR | NR | 45% still on initial treatment; 21% started delayed IFX and 11% were still on it at 5 years; 46% had successfully tapered and stopped PDN | NR | ||
| Initial combination therapy with IFX | 128 | 5 years | 116/128 (91) |
|
NR | NR | NR | 65% still on initial treatment; 19% were still taking delayed IFX at 5 years; 50% had permanently discontinued IFX | NR | ||
| Sequential monotherapy | 126 | 7 years29 | 83/126 (66) | NR | 68/83 (82) | 68/83 (82) (DAS44 of ≤ 2.4) | 41/83 (49) (DAS44 of < 1.6) | Still on initial treatment step, 17/83 (21%); IFX current use, 12/83 (14%) | NR | ||
| Step-up combination therapy | 121 | 7 years | 72/121 (60) | NR | 55/72 (76) | 55/72 (76) (DAS44 of ≤ 2.4) | 28/72 (39) (DAS44 of < 1.6) | Still on initial treatment step, 12/72 (16%); IFX current use, 4/72 (6%) | NR | ||
| Initial combination therapy with PDN | 133 | 7 years | 79/133 (59) | NR | 59/79 (82) | 59/79 (82) (DAS44 of ≤ 2.4) | 42/79 (53) (DAS44 of < 1.6) | Still on initial treatment step, 16/79 (20%); IFX current use, 9/79 (11%) | NR | ||
| Initial combination therapy with IFX | 128 | 7 years | 97/128 (76) | NR | 74/97 (76) | 74/97 (76) (DAS44 of ≤ 2.4) | 44/97 (45) (DAS44 of < 1.6) | Still on initial treatment step, 53/97 (55%) p < 0.001; IFX current use, 20/97 (21%) | NR | ||
| Sequential monotherapy | 126 | 8 years28 | 85/126 (67) | NR | 67/85 (79) | 67/85 (79) (DAS44 of ≤ 2.4) | 42/85 (49) (DAS44 of < 1.6) | Still on initial treatment step, 25/85 (29%); IFX current use, 18/85 (21%) | NR | ||
| Step-up combination therapy | 121 | 8 years | 78/121 (64) | NR | 59/78 (76) | 59/78 (76) (DAS44 of ≤ 2.4) | 44/78 (56) (DAS44 of < 1.6) | Still on initial treatment step, 17/78 (22%); IFX current use, 8/78 (10%) | NR | ||
| Initial combination therapy with PDN | 133 | 8 years | 86/133 (65) | NR | 72/86 (84) | 72/86 (84) (DAS44 of ≤ 2.4) | 49/86 (57) (DAS44 of < 1.6) | Still on initial treatment step, 39/86 (45%); IFX current use, 11/86 (13%) | NR | ||
| Initial combination therapy with IFX | 128 | 8 years | 98/128 (77) | NR | 74/98 (76) | 74/98 (76) (DAS44 of ≤ 2.4) | 46/98 (47) (DAS44 of < 1.6) | Still on initial treatment step, 65/98 (66%) p < 0.001; IFX current use, 24/98 (24%) | NR | ||
| CareRA: high-risk patients40,42,43 (52-week randomised phase) | COBRA Classic | 98 | 4 weeks | NR | NR | A DAS28-CRP of ≤ 3.2 | NR | NR | 63/98 (64) (DAS28-CRP < 2.6) | NR | NA |
| COBRA Slim | 98 | 4 weeks | NR | NR | NR | NR | 60/98 (61) (DAS28-CRP < 2.6) | NR | NA | ||
| COBRA Avant-Garde | 94 | 4 weeks | NR | NR | NR | NR | 60/94 (70) (DAS28-CRP < 2.6) | NR | NA | ||
| COBRA Classic | 98 | 8 weeks | NR | NR | NR | NR | 64/98 (65) (DAS28-CRP < 2.6) | NR | NA | ||
| COBRA Slim | 98 | 8 weeks | NR | NR | NR | NR | 61/98 (62) (DAS28-CRP < 2.6) | NR | NA | ||
| COBRA Avant-Garde | 94 | 8 weeks | NR | NR | NR | NR | 65/94 (74) (DAS28-CRP < 2.6) | NR | NA | ||
| COBRA Classic | 98 | 16 weeks | 91/98 (93) |
|
83/98 (84.7) | 83/98 (85) (DAS28-CRP ≤ 3.2) | 69/98 (70) (DAS28-CRP < 2.6) | Adaptations in 19 out of 98 (19.4%) patients over the first 16 weeks | NR | ||
| COBRA Slim | 98 | 16 weeks | 96/98 (98) |
|
85/98 (86.7) | 85/98 (87) (DAS28-CRP ≤ 3.2) | 72/98 (74) (DAS28-CRP < 2.6) | Adaptations in 22 out of 98 (22.4%) patients over the first 16 weeks | NR | ||
| COBRA Avant-Garde | 94 | 16 weeks | 91/94 (97) |
|
82/94 (87.2) | 82/94 (87) (DAS28-CRP ≤ 3.2) | 64/94 (68) (DAS28-CRP < 2.6) | Adaptations in 14 out of 94 (14.9%) patients over the first 16 weeks | NR | ||
| COBRA Classic | 98 | 52 weeks43 | 90/98 (92) |
|
73/98 (74.5) | 73/98 (75) (DAS28-CRP ≤ 3.2) | NR (64%) (63/98). No treatment failures, 9/62 (79%); treatment failures, 12/28 (43%); DAS28-ESRe remission, NR (58%); SDAI remission, NR (38%); Boolean remission, NR (27%) | One treatment adaptation: 21 (21.4%) | Cumulative PDN dose (mg) mean 2597.2 (SD 666.8) | ||
| COBRA Slim | 98 | 52 weeks | 89/98 (91) |
|
74/98 (76) | 74/98 (76) (DAS28-CRP ≤ 3.2) | NR (60%) (59/98). Not treatment failures 52/75 (69%); treatment failures 2/14 (14%); DAS28-ESRe remission NR (52%); SDAI remission NR (31%); Boolean remission NR (17%) | Two treatment adaptations: 3 (3.1%) | Average daily PDN dose (mg) mean 6.5 (SD 2.7) | ||
| COBRA Avant-Garde | 93 | 52 weeks | 85/93 (91) |
|
74/93 (80) | 74/93 (80) (DAS28-CRP ≤ 3.2) | 62% (58/93). Not treatment failures, 45/60 (75%); treatment failures, 11/25 (44%); DAS28-ESRe remission, NR (55%); SDAI remission, NR (45.2%); Boolean remission, 30.1% | One treatment adaptation: 38 (39%) | Patients with GC injections: 15 (15%) | ||
| CareRA: low-risk patients40,43 (52-week randomised phase) | MTX-TSU | 47 | 8 weeks | NA | NR | DAS28-CRP ≤ 3.2 | NR | NR | NR | Adaptations in 16 out of 47 (34%) patients over the first 8 weeks | NR |
| COBRA Slim | 43 | 8 weeks | NA | NR | NR | NR | NR | Adaptations in 10 out of 43 (23%) patients over the first 8 weeks | NR | ||
| MTX-TSU | 47 | 16 weeks | Unclear |
|
34/47 (72) | 34/47 (72) (DAS28-CRP ≤ 3.2) | 22/47 (47) (DAS28-CRP < 2.6) | Adaptations in 11 out of 47 (21%) patients over the first 16 weeksi | NR | ||
| Intra-articular GC injections in 10 out of 47 (21%) patients (1 patient received 2 injections) | |||||||||||
| COBRA Slim | 43 | 16 weeks | Unclear |
|
34/43 (79) | 34/43 (79) (DAS28-CRP ≤ 3.2) | 28/43 (65) (DAS28-CRP < 2.6) | Adaptations in 7 out of 43 (16%) patients over the first 16 weeksi | NR | ||
| One patient considered as efficacy failure. Intra-articular GC injections in 3 out of 43 (7.0%) patients | |||||||||||
| MTX-TSU | 47 | 52 weeks43 | 44/47 (93.6) |
|
36/47 (77) | 36/47 (77) (DAS28-CRP ≤ 3.2) | 27/47 (57%). Not considered treatment failures 24/36 (67%); treatment failures 3/8 (38%); DAS28-ESRe remission NR (55%); SDAI remission NR (30%); Boolean remission NR (21)% | Two treatment adaptations:16 (16%) | Cumulative PDN dose (mg) mean 36.3 (SD 49.6) | ||
| Average daily PDN dose (mg) mean 0.1 (SD 0.1) | |||||||||||
| Patients with GC injections: 17 (36%) | |||||||||||
| COBRA Slim | 43 | 52 weeks | 38/43 (88.4) |
|
33/43 (77) | 33/43 (77) (DAS28-CRP ≤ 3.2) | 29/43 (67%). Not treatment failures, 25/30 (83%); treatment failures, 4/8 (50%); DAS28-ESR remission,e 63%; SDAI remission, NR (44%); Boolean remission, 37% | One treatment adaptation: 22 (24%) | Cumulative PDN dose (mg) mean 1554.0 (SD 307.6) | ||
| Average daily PDN dose (mg) mean 3.8 (SD 1.6) | |||||||||||
| Patients with GC injections: 6 (14%) | |||||||||||
| COBRA-light44,45 (12-month randomised phase) | COBRA | 81 | 13 weeks | NA | NR | A DAS44 of < 1.6 | 35/81 (43) | NR | 35/81 (43) (DAS44 of < 1.6) | Treatment intensified in 46/81 (57%) patientsf | NR |
| COBRA-light | 83 | 13 weeks | NA | NR | 36/81 (44) | NR | 36/81 (44) (DAS44 of < 1.6) | Treatment intensified in 45/81 (56%) patientsf | NR | ||
| COBRA | 81 | 6 months | 80/81 (99) |
|
NR (49)e | NR | NR (49)e (DAS44 of < 1.6) | NR | Mean MTX dose: 15.6 mg/week | ||
| COBRA-light | 83 | 6 months | 78/81 (96%) 2/83 did not start treatment (withdrew informed consent immediately after randomisation), not included in ITT population, n = 81 |
|
NR (41)e | NR | NR (41)e (DAS44 of < 1.6) | NR | Mean MTX dose: 24.0 mg/week | ||
| COBRA | 81 | 12 months | 78/81 (96) |
|
38/81 (47) | NR | 38/81 (47) (DAS44 of < 1.6) | Started ETN: 27/81 (57%)f | NR | ||
| 12/81 (15) (ACR/Boolean remission) | 16 patients received ETN for 26 weeks | ||||||||||
| COBRA-light | 83 | 12 months | 77/81 (95) |
|
31/81 (38) | NR | 31/81 (38) (DAS44 of < 1.6) | Started ETN: 40/81 (66%)f | NR | ||
| 14/81 (17) (ACR/Boolean remission) | 30 patients received ETN for 26 weeks | ||||||||||
| FIN-RACo47–49,70 (2-year randomised phase) | Combination treatment | 99 (97 in ITT) | 2 years46 | 87/97 (90) |
|
Remission: modified version of ACR 1981 defined remission | 36/NR (37)g | NR | 36/NR (37)g (modified ACR 1981 criteria) | NR | NR |
| Single-drug treatment | 100 (98 in ITT) | 2 years | 91/98 (93) |
|
18/NR (18)g | NR | 18/NR (18)g (modified ACR 1981 criteria) | NR | NR | ||
| Combination treatment | 99 (97 in ITT) | 5 years47 | 78 (80) |
|
18, 41j/NR (29)h | NR | 18, 41j/NR (29)h (modified ACR 1981 criteria) | NR | NR | ||
| Single-drug treatment | 100 (98 in ITT) | 5 years | 82/NR (84) |
|
13, 33j/NR (22)h | NR | 13, 33j/NR (22)h (modified ACR 1981 criteria) | NR | |||
| Combination treatment | 99 (97 in ITT) | 11 years47 | 68/NR (70) |
|
26, 49j/NR (37)k | NR | 26, 49j/NR (37)k (modified ACR 1981 criteria); NR (57) (DAS28 of < 2.6) | At some time between the 2- and 11-year visits, a combination-DMARD strategy had been used by 62 (91%) patients | NR | ||
| 22 (32%) patients had been able to discontinue all DMARDs, at least temporarily, from year 2 to year 11 | |||||||||||
| Single-drug treatment | 100 (98 in ITT) | 11 years | 70/NR (71) |
|
11, 29j/NR (19%)l | NR | 11, 29j/NR (19)k (modified ACR 1981 criteria); NR (49) (DAS28 of < 2.6) | At some time between the 2- and 11-year visits, a combination-DMARD strategy had been used by 56 (80%) of patients | NR | ||
| 27 (39%) of patients had been able to discontinue all DMARDs, at least temporarily, from year 2 to year 11 | |||||||||||
| Saunders et al., 200854 (12-month randomised phase) | Parallel triple therapy | 49 | 12 months | 47/49 (96) |
|
A DAS28 of < 3.2 | 20/49 (41) | 20/49 (41) (EULAR good response, defined by a DAS28 of < 3.2) | 16/49 (33) (EULAR remission) | 15/49 (31%) drug withdrawals as a result of AEs:
|
NR |
| Step-up therapy | 47 | 12 months | 44/47 (94) |
|
28/47 (60) | 28/47 (60) (EULAR good response, defined by a DAS28 of < 3.2) | 21/47 (45) (EULAR remission) | 18/47 (39%) drug withdrawals as a result of AEs:
|
NR | ||
| TEAR58–60 (102-week randomised phase) | Immediate ETN | 244 | 24 weeks | See below | See below | No target | 100/244 (41) | 100/244 (41) (DAS28-ESR of ≤ 3.2) | NR | NR | NR |
| Immediate triple therapy | 132 | 24 weeks | 65/152 (43) | 65/152 (43) (DAS28-ESR of ≤ 3.2) | NR | NR | NR | ||||
| Step-up ETN | 255 | 24 weeks | A DAS28-ESR of < 3.2 | 105/376 (28) | 105/376 (28) (DAS28-ESR of ≤ 3.2) | NR | NR | NR | |||
| Step-up triple therapy | 124 | 24 weeks | NR | NR | NR | ||||||
| Immediate ETN | 244 | 102 weeks | 168/244 (69) (159 with DAS28) |
|
No target | 90/159 (57)l | NR | 90/159 (57%)l (DAS28-ESR of ≤ 2.6) | NR | Mean MTX dosage: 19.1 mg/week (for the whole sample; no significant differences between groups) | |
| Immediate triple therapy | 132 | 102 weeks | 82/132 (62) (76 with DAS28) |
|
45/76 (59)l | NR | 45/76 (59)l (DAS28-ESR of ≤ 2.6) | NR | |||
| Step-up ETN | 255 | 102 weeks | 182/255 (71) (166 with DAS28) |
|
A DAS28-ESR of < 3.2 | 88/166 (53)l | NR | 88/166 (53)l (DAS28-ESR of ≤ 2.6) | NR | ||
| Step-up triple therapy | 124 | 102 weeks | 81/124 (65) (75 with DAS28) |
|
42/75 (57)l | NR | 42/75 (57)l (DAS28-ESR of ≤ 2.6) | NR | |||
| U-Act-Early62 (104-week randomised phase) | TOC + MTX | 106 | 24 weeks | 100/106 (94) |
|
A DAS28 of < 2.6 and a SJC of ≤ 4 of the 28 joints assessed | NR | NR | NR | NR | NR |
| TOC + PBO–MTX | 103 | 24 weeks | 101/103 (98) |
|
NR | NR | NR | NR | NR | ||
| MTX + PBO–TOC | 108 | 24 weeks | 98/108 (91) |
|
NR | NR | NR | NR | NR | ||
| TOC + MTX | 106 | 52 weeks | 87/106 (82) | Weeks 24–52:
|
NR | NR | NR | NR | NR | ||
| TOC + PBO–MTX | 103 | 52 weeks | 92/103 (89) | Weeks 24–52:
|
NR | NR | NR | NR | NR | ||
| MTX + PBO–TOC | 108 | 52 weeks | 92/108 (85) | Weeks 24–52:
|
NR | NR | NR | NR | NR | ||
| TOC + MTX | 106 | 104 weeks | 78/106 (74) | Weeks 52–104:
|
91/106 (86)m | NR | 91/106 (86)m [a DAS28 of < 2.6 and a SJC of (28 joints) ≤ 4] | Switch to subsequent regimen, 9/106 (8.5%) (by week 104) | NR | ||
| TOC + PBO–MTX | 103 | 104 weeks | 81/103 (79) | Weeks 52–104
|
91/103 (88)m | NR | 91/103 (88)m [a DAS28 of < 2.6 and a SJC of (28 joints) ≤ 4] | Switch to subsequent regimen, 13/103 (13%) (by week 104) | NR | ||
| MTX + PBO–TOC | 108 | 104 weeks | 78/108 (72) |
|
83/108 (77)m | NR | 83/108 (77)m [a DAS28 of < 2.6 and a SJC (28 joints) of ≤ 4] | Switch to subsequent regimen, 50/108 (46%) (by week 104) | NR | ||
| Trial acronym or first author and year of publication | Treatment arm | Number of participantsa | Follow-up time point | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean DAS28 (SD) | DAS44 (SD) | Mean SJC (0–66) (SD) | Mean TJC (0–68) (SD) | EULAR good/moderate/none | ACR 20/50/70 | Mean HAQ score (SD) | Joint erosion | Quality of life | ||||
| BeSt26–34,64–66 (12-month randomised phase) | Sequential monotherapy | 126 | 3 months30 | NR | NR | NR | NR | NR | ACR 20: 29.2%b | D-HAQ: 1.0 (0.7)c,d | NR | SF-36 PCS improvement: 5.8e,f |
| ACR 70: 24.2%b | SF-36 MCS improvement: 2.1e | |||||||||||
| Step-up combination therapy | 121 | 3 months | NR | NR | NR | NR | NR | ACR 20: 36.9%b | D-HAQ: 1.0 (0.6)c,d | NR | SF-36 PCS improvement: 3.9e,f | |
| ACR 70: 24.2%b | SF-36 MCS improvement: 2.5e | |||||||||||
| Initial combination therapy with PDN | 133 | 3 months | NR | NR | NR | NR | NR | ACR 20: 70.1%b | D-HAQ: 0.6 (0.6)c,d | NR | SF-36 PCS improvement: 11.2e,f | |
| ACR 70: 20.2%b | SF-36 MCS improvement: 0.4e | |||||||||||
| Initial combination therapy with IFX | 128 | 3 months | NR | NR | NR | NR | NR | ACR 20: 60.2 %b | D-HAQ: 0.6 (0.6)c,d | NR | SF-36 PCS improvement: 9.6e,f | |
| ACR 70: 19.3%b | SF-36 MCS improvement: 3.1e | |||||||||||
| Sequential monotherapy | 126 | 6 months30 | NR | NR | NR | NR | NR | ACR 20: 49.4%b | D-HAQ: 0.9 (0.7)c,d | NR | SF-36 PCS improvement: 8.0e,f | |
| ACR 70: 15.7%b | SF-36 MCS improvement: 3.1e | |||||||||||
| Step-up combination therapy | 121 | 6 months | NR | NR | NR | NR | NR | ACR 20: 60.2%b | D-HAQ: 0.9 (0.7)c,d | NR | SF-36 PCS improvement: 8.5e,f | |
| ACR 70: 11.8%b | SF-36 MCS improvement: 3.5e | |||||||||||
| Initial combination therapy with PDN | 133 | 6 months | NR | NR | NR | NR | NR | ACR 20: 70.6%b | D-HAQ: 0.5 (0.5)c,d | NR | SF-36 PCS improvement: 12.5e,f | |
| ACR 70: 26.6%b | SF-36 MCS improvement: 1.2e | |||||||||||
| Initial combination therapy with IFX | 128 | 6 months | NR | NR | NR | NR | NR | ACR 20: 74.2%b | D-HAQ: 0.5 (0.5)c,d | NR | SF-36 PCS improvement: 12.4e,f | |
| ACR 70: 31.2%b | SF-36 MCS improvement: 4.1e | |||||||||||
| Sequential monotherapy | 126 | 9 months30 | NR | NR | NR | NR | NR | ACR 20: 62.8%b | D-HAQ: 0.8 (0.7)c,d | NR | NR | |
| ACR 70: 16.3%b | ||||||||||||
| Step-up combination therapy | 121 | 9 months | NR | NR | NR | NR | NR | ACR 20: 72.0%b | D-HAQ: 0.8 (0.7)c,d | NR | NR | |
| ACR 70: 18.4%b | ||||||||||||
| Initial combination therapy with PDN | 133 | 9 months | NR | NR | NR | NR | NR | ACR 20: 70.2%b | D-HAQ: 0.6 (0.6)c,d | NR | NR | |
| ACR 70: 23.6%b | ||||||||||||
| Initial combination therapy with IFX | 128 | 9 months | NR | NR | NR | NR | NR | ACR 20: 77.7%b | D-HAQ: 0.5 (0.6)c,d | NR | NR | |
| ACR 70: 34.4% | ||||||||||||
| Sequential monotherapy | 126 | 12 months30 | NR | NR | NR | NR | NR | ACR 20: 63.4%b | D-HAQ: 0.7 (0.7)c,g | Progression of SHS: | SF-36 PCS improvement: 8.9e | |
| ACR 70: 19.6%b | SF-36 MCS improvement: 4.3e | |||||||||||
| Step-up combination therapy | 121 | 12 months | NR | NR | NR | NR | NR | ACR 20: 63.3%b | D-HAQ: 0.7 (0.6)c | Progression of SHS: | SF-36 PCS improvement: 11.2e | |
| ACR 70: 22.0%b | SF-36 MCS improvement: 4.4e | |||||||||||
| Initial combination therapy with PDN | 133 | 12 months | NR | NR | NR | NR | NR | ACR 20: 77.3%b | D-HAQ: 0.5 (0.5)c,g | Progression of SHS: | SF-36 PCS improvement: 11.9e | |
| ACR 70: 29.9%b | SF-36 MCS improvement: 3.2e | |||||||||||
| Initial combination therapy with IFX | 128 | 12 months | NR | NR | NR | NR | NR | ACR 20: 79.1%b | D-HAQ: 0.5 (0.5)c,g | Progression of SHS: | SF-36 PCS improvement: 12.0e | |
| ACR 70: 40.2%b | SF-36 MCS improvement: 4.3e | |||||||||||
| Sequential monotherapy | 126 | 2 years26,68 | NR | NR | NR | NR | NR | NR | NR | Progression of SHS from baseline: | SF-36 PCS improvement: 11.9e | |
| SF-36 MCS improvement: 4.3e | ||||||||||||
| Step-up combination therapy | 121 | 2 years | NR | NR | NR | NR | NR | NR | NR | Progression of SHS from baseline: | SF-36 PCS improvement: 12.3e | |
| SF-36 MCS improvement: 4.6e | ||||||||||||
| Initial combination therapy with PDN | 133 | 2 years | NR | NR | NR | NR | NR | NR | NR | Progression of SHS from baseline: | SF-36 PCS improvement: 12.3e | |
| SF-36 MCS improvement: 4.6e | ||||||||||||
| Initial combination therapy with IFX | 128 | 2 years | NR | NR | NR | NR | NR | NR | NR | Progression of SHS from baseline: | SF-36 PCS improvement: 12.7e | |
| SF-36 MCS improvement: 4.0e | ||||||||||||
| Sequential monotherapy | 126 | 3 years26 | NR | NR | NR | NR | NR | NR | NR | Increase in total SHS: 3.8k,l,m | NR | |
| Step-up combination therapy | 121 | 3 years | NR | NR | NR | NR | NR | NR | NR | Increase in total SHS: 3.0k,n | NR | |
| Initial combination therapy with PDN | 133 | 3 years | NR | NR | NR | NR | NR | NR | NR | Increase in total SHS: 1.8k | NR | |
| Initial combination therapy with IFX | 128 | 3 years | NR | NR | NR | NR | NR | NR | NR | Increase in total SHS: 1.5k,l,m,n | NR | |
| Sequential monotherapy | 126 | 5 years31 | NR | NR | NR | NR | NR | NR | 0.70o,s | SHS progression:i 14.0p/3.5k | NR | |
| Step-up combination therapy | 121 | 5 years | NR | NR | NR | NR | NR | NR | 0.70o,p | SHS progression:i 11.0p/2.5k | NR | |
| Initial combination therapy with PDN | 133 | 5 years | NR | NR | NR | NR | NR | NR | 0.62o,p | SHS progression:i 7.6p/1.0k | NR | |
| Initial combination therapy with IFX | 128 | 5 years | NR | NR | NR | NR | NR | NR | 0.54o,p | SHS progression:i 6.0p/1.0k | NR | |
| Sequential monotherapy | 126 | 7 years29 | NR | NR | NR | NR | NR | NR | 0.70;p,q n = 83 | SHS progression: 15.1p/3.8,k n = 83 | NR | |
| Step-up combination therapy | 121 | 7 years | NR | NR | NR | NR | NR | NR | 0.71;p,q n = 72 | SHS progression: 10.7p/3.5;k n = 72 | NR | |
| Initial combination therapy with PDN | 133 | 7 years | NR | NR | NR | NR | NR | NR | 0.63;p n = 79 | SHS progression: 8.4p/2.0;k n = 79 | NR | |
| Initial combination therapy with IFX | 128 | 7 years | NR | NR | NR | NR | NR | NR | 0.57;p,q n = 97 | SHS progression: 5.5p/2.0;k n = 97 | NR | |
| Sequential monotherapy | 126 | 8 years28 | NR | NR | NR | NR | NR | NR | 0.69;p n = 85 | SHS progression: 14.6p/3.0;k n = 85 | NR | |
| Step-up combination therapy | 121 | 8 years | NR | NR | NR | NR | NR | NR | 0.71;p,r n = 78 | SHS progression: 13.9p/4.3;k n = 78 | NR | |
| Initial combination therapy with PDN | 133 | 8 years | NR | NR | NR | NR | NR | NR | 0.93;p n = 86 | SHS progression: 8.5p/2.0;k n = 86 | NR | |
| Initial combination therapy with IFX | 128 | 8 years | NR | NR | NR | NR | NR | NR | 0.57;p,r n = 98 | SHS progression: 8.3p/2.0;k n = 86 | NR | |
| Sequential monotherapy | 126 | 10 years64 | NR | NR | NR | NR | NR | NR | 0.69 (during 10 years) | Change in SHS: 2.0 (0–11.0)s | NR | |
| SHS estimate corrected for baseline SHS: 14.2p | ||||||||||||
| Step-up combination therapy | 121 | 10 years | NR | NR | NR | NR | NR | NR | 0.72 (during 10 years) | Change in SHS: 2.5 (0–13.5)s | NR | |
| SHS estimate corrected for baseline SHS: 14.1p | ||||||||||||
| Initial combination therapy with PDN | 133 | 10 years | NR | NR | NR | NR | NR | NR | 0.64 (during 10 years) | Change in SHS: 3.0 (0.3–11.3)s | NR | |
| SHS estimate corrected for baseline SHS: 14.6p | ||||||||||||
| Initial combination therapy with IFX | 128 | 10 years | NR | NR | NR | NR | NR | NR | 0.58 (during 10 years) | Change in SHS: 1.5 (0.0–6.0)s | NR | |
| SHS estimate corrected for baseline SHS: 8.9p | ||||||||||||
| CareRA: high-risk patients40,42,43 (52-week randomised phase) | COBRA Classic | 98 | 16 weeks | Change from baseline (DAS28-CRP): 2.8 (1.2) | NR | NR | NR | Good response: 78/98 (79.6%) | NR | Clinically meaningful HAQ response: 83/98 (84.7%) | NR | NR |
| Moderate response: 96/98 (98.0%) | HAQ = 0: 45/98 (45.9%) | |||||||||||
| COBRA Slim | 98 | 16 weeks | Change from baseline (DAS28-CRP): 2.6 (1.2) | NR | NR | NR | Good response: 78/98 (79.6%) | NR | Clinically meaningful HAQ response: 85/98 (86.7%) | NR | NR | |
| Moderate response: 94/98 (95.9%) | HAQ = 0: 42/98 (42.9%) | |||||||||||
| COBRA Avant-Garde | 94 | 16 weeks | Change from baseline (DAS28-CRP): 2.4 (1.3) | NR | NR | NR | Good response: 72/94 (76.6%) | NR | Clinically meaningful HAQ response: 72/94 (76.6%) | NR | NR | |
| Moderate response: 87/94 (93.6%) | HAQ = 0: 46/94 (48.9%) | |||||||||||
| COBRA Classic | 98 | 52 weeks43 | DAS28-CRP change: 2.5 (1.5) | NR | NR | NR | Good response: 67.3% | NR | HAQ change: 0.7 (0.7)j | Change in SHS: 0.3 (0.5), (n) X-ray pairs = 74 | NR | |
| Clinically meaningful HAQ change: 68.4% | ||||||||||||
| Mean (SD) AUC from baseline 35.0 (11.6) | Moderate response: 84.7% | HAQ = 0: 37.8% | ||||||||||
| COBRA Slim | 98 | 52 weeks | DAS28-CRP change: 2.3 (1.4) | NR | NR | NR | Good response: 68.4% | NR | HAQ change: 0.5 (0.7) | Change in SHS: 0.4 (1.1), (n) X-ray pairs = 68 | NR | |
| Clinically meaningful HAQ change: 70.4% | ||||||||||||
| Mean (SD) AUC from baseline 35.3 (10.6) | Moderate response: 88.8% | HAQ = 0: 36.7% | ||||||||||
| COBRA Avant-Garde | 93 | 52 weeks | DAS28-CRP change: 2.3 (1.5) | NR | NR | NR | Good response: 67.7% | NR | HAQ change: 0.6 (0.7)j | Change in SHS: 0.3 (0.6), (n) X-ray pairs = 68 | NR | |
| Clinically meaningful HAQ change: 71.7% | ||||||||||||
| Mean (SD) AUC from baseline 33.9 (8.6) | Moderate response: 88.2% | HAQ = 0: 44.1% | ||||||||||
| CareRA: low-risk patients40,43 (52-week randomised phase) | MTX-TSU | 47 | 16 weeks | Change from baseline (DAS28-CRP): 1.76 (1.68) | NR | NR | NR | Good response: 21/47 (44.7%) | NR | HAQ change: 0.40 (0.62) | NR | NR |
| Clinically meaningful HAQ change: 25/47 (53.2%) | ||||||||||||
| Moderate response: 34/47 (72.3%) | HAQ = 0: 11/47 (23.4%)t | |||||||||||
| COBRA Slim | 43 | 16 weeks | Change from baseline (DAS28-CRP): 2.12 (1.41) | NR | NR | NR | Good response: 25/43 (58.1%) | NR | HAQ change: 0.58 (0.64) | NR | NR | |
| Clinically meaningful HAQ change: 27/43 (62.8%) | ||||||||||||
| Moderate response: 34/43 (86.0%) | HAQ = 0: 22/43 (51.2%)t | |||||||||||
| MTX-TSU | 47 | 52 weeks43 | DAS28-CRP change: 2.1 (1.7) | NR | NR | NR | Good response: 57.4% | NR | HAQ change: 0.5 (0.6) | Change in SHS: 0.2 (0.3), (n) X-ray pairs = 31 | NR | |
| Clinically meaningful HAQ change: 59.6% | ||||||||||||
| Mean (SD) AUC from baseline to week 52: 42.0 (13.1)u | Moderate response: 78.7% | HAQ = 0: 29.8% | ||||||||||
| COBRA Slim | 43 | 52 weeks | DAS28-CRP change: 2.1 (1.9) | NR | NR | NR | Good response: 60.5%; | NR | HAQ change: 0.6 (0.7) | Change in SHS 0.3 (0.5), (n) X-ray pairs = 28 | NR | |
| Clinically meaningful HAQ change: 55.8% | ||||||||||||
| Mean (SD) AUC from baseline to week 52: 5.8 (14.1)u | Moderate response: 76.7% | HAQ = 0: 48.8% | ||||||||||
| COBRA-light44,45 (12-month randomised phase) | COBRA | 81 | 13 weeks | NR | NR | NR | NR | Good response: 63%i | NR | 0.52b,o | NR | NR |
| Non-response: 4%i | ||||||||||||
| COBRA-light | 83 | 13 weeks | NR | NR | NR | NR | Good response: 47%i | NR | 0.62b,o | NR | NR | |
| Non-response: 11%i | ||||||||||||
| COBRA | 81 | 6 months | NR | 1.62 (0.96)h | NR | NR | Good response: 75%i | NR | 0.47b,o | NR | NR | |
| Change from baseline: –2.50 (1.21)h | ||||||||||||
| DAS44-CRP change from baseline: −2.15 (1.09)h | Non-response: 6%i | |||||||||||
| COBRA-light | 83 | 6 months | NR | 1.78 (1.13)h | NR | NR | Good response: 65%i | NR | 0.56b,o | NR | NR | |
| Change from baseline: –2.18 (1.10)h | ||||||||||||
| DAS44-CRP change from baseline: −2.10 (1.09)h | Non-response: 11%i | |||||||||||
| COBRA | 81 | 12 months | 2.49 (1.3) | 1.70 (1.0) | 2.6 (3.6) | 5.3 (7.2) | NR | ACR 70: 25/81 (31%) | 0.57 (0.5) | 6% erosive disease | NR | |
| DAS44-CRP: 1.71 (1.2) | ||||||||||||
| DAS44-CRP change from baseline: −2.41 (1.2)h | SHS: | |||||||||||
| COBRA-light | 83 | 12 months | 2.71 (1.3) | 1.88 (1.0) | 2.3 (2.6) | 5.0 (6.0) | NR | ACR 70: 28/81 (35%) | 0.61 (0.6) | 5% erosive disease | NR | |
| DAS44-CRP: 1.69 (1.1) | ||||||||||||
| DAS44-CRP change from baseline: −2.02 (1.1) | SHS: | |||||||||||
| FIN-RACo47–49,70 (2-year randomised phase) | Combination treatment | 99 (97 in ITT) | 3 months46 | NR | NR | NR | NR | NR | ACR 50: 60.6% (95% CI 49.7% to 70.2%)b | NR | NR | NR |
| Single-drug treatment | 100 (98 in ITT) | 3 months | NR | NR | NR | NR | NR | ACR 50: 40.2% (95% CI 30.8% to 51.2%)b | NR | NR | NR | |
| Combination treatment | 99 (97 in ITT) | 6 months46 | NR | NR | NR | NR | NR | ACR 20: (95% CI 71 to 88) | NR | NR | NR | |
| ACR 50: 69.6% (95% CI 58.9% to 78.1%)b | ||||||||||||
| Single-drug treatment | 100 (98 in ITT) | 6 months | NR | NR | NR | NR | NR | ACR 20: 78% (95% CI 69% to 86%) | NR | NR | NR | |
| ACR 50: 55.4% (95% CI 45.2% to 65.6%)b | ||||||||||||
| Combination treatment | 99 (97 in ITT) | 12 months46 | NR | NR | NR | NR | NR | ACR 50: 74.7% (95% CI 64.0% to 82.9%)b | NR | NR | NR | |
| Single-drug treatment | 100 (98 in ITT) | 12 months | NR | NR | NR | NR | NR | ACR 50: 58.6% (95% CI 48.6% to 69.7%)b | NR | NR | NR | |
| Combination treatment | 99 (97 in ITT) | 2 years46 | 2.23 (0.33)b,w,x | NR | Change from baseline: –10 (95% CI –12 to –9)y | Change from baseline: –13 (95% CI –15 to –11)y | NR | ACR 20: 78% (95% CI 69/% to 80%) | 0.27 (0.11)b,w | Number of eroded joints: 2 (IQR 0–5)s,t | NR | |
| ACR 50: 57.1% (95% CI 46.3% to 67.3%)b | Stanford HAQ score change from baseline: –0.6 (95% CI –0.7 to –0.4)y | Larsen score: 4 (IQR 0–4)s,z | ||||||||||
| Single-drug treatment | 100 (98 in ITT) | 2 years | 3.11 (0.28)b,w,x | NR | Change from baseline: –10 (95% CI –12 to –8)y | Mean change from baseline: –14 (95% CI –16 to –12)y | NR | ACR 20: 84% (95% CI 75% to 90%) | 0.30 (0.10)b,w | Number of eroded joints: 4 (IQR 2–7)s,t | NR | |
| ACR 50: 76.0% (95% CI 65.5%–84.4%)b,p | Stanford HAQ change from baseline: –0.6 (95% CI –0.8 to –0.5)y | Larsen score: 12 (IQR 4–20)s,z | ||||||||||
| Combination treatment | 99 (97 in ITT) | 5 years47 | 2.51 (0.28)b,w,x | NR | NR | NR | NR | NR | 0.27 (0.11)b,w | NR | NR | |
| Single-drug treatment | 100 (98 in ITT) | 5 years | 2.94 (0.28)b,w,x | NR | NR | NR | NR | NR | 0.33 (0.12)b,w | NR | NR | |
| Combination treatment | 99 (97 in ITT) | 11 years48,49 | 2.48 (1.22)x | NR | 0 (0–3)s | 1 (0–5)s | NR | NR | 0.34 (0.54) | Change from baseline in Larsen score: 17 (95% CI 12–26);y,aa 87% (95% CI 74% to 94%) patients in had no erosive changes in large joints | NR | |
| HAQ score of 0: 56% | ||||||||||||
| HAQ score of > 1: 10% | ||||||||||||
| Single-drug treatment | 100 (98 in ITT) | 11 years | 2.73 (1.23)x | NR | 2 (IQR 0–4)s | 2 (IQR 0–5)s | NR | NR | 0.38 (0.58) | Change from baseline in Larsen score: 27 (95% CI 22–33);y,aa 72% (95% CI 58% to 84%) patients in had no erosive changes in large joints | NR | |
| HAQ score of 0: 43% | ||||||||||||
| HAQ score of > 1: 9% | ||||||||||||
| Saunders et al., 200854 (12-month randomised phase) | Parallel triple therapy | 47 | 12 months | Change from baseline: –3.3 (1.6) | NR | NR | NR | Good response: 20/49 (41%) | ACR 20: 37/49 (76%) | Change from baseline: –0.8 (0.7) | SHS change from baseline: | SF-12 change from baseline: 9 (SD 13)h |
| ACR 50: 25/49 (51%) | ||||||||||||
| ACR 70: 10/49 (20%) | ||||||||||||
| Step-up therapy | 49 | 12 months | Change from baseline: –4.0 (1.8) | NR | NR | NR | Good response: 28/47 (60%) | ACR 20: 36/47 (77%) | Change from baseline: –0.9 (0.7) | SHS change from baseline: | SF-12 change from baseline: 10 (SD 11)h | |
| ACR 50: 28/47 (60%) | ||||||||||||
| ACR 70: 14/47 (30%) | ||||||||||||
| TEAR58–60 (102-week randomised phase) | Immediate ETN | 244 | 6 months | NR | NR | NR | NR | NR | ACR 20: 56.28%b,ab | NR | NR | NR |
| ACR 50: 32.14%b,ab | ||||||||||||
| ACR 70: 11.13%b,ab | ||||||||||||
| Immediate triple therapy | 132 | 6 months | NR | NR | NR | NR | NR | ACR 20: 55.88%b,ab | NR | NR | NR | |
| ACR 50: 31.33%b,ab | ||||||||||||
| ACR 70: 7.97%b,ab | ||||||||||||
| Step-up ETN | 255 | 6 months | NR | NR | NR | NR | NR | ACR 20: 40.12%b,ab | NR | NR | NR | |
| ACR 50: 19.51%b,ab | ||||||||||||
| ACR 70: 2.84%b,ab | ||||||||||||
| Step-up triple therapy | 124 | 6 months | NR | NR | NR | NR | NR | ACR 20: 39.32%b,ab | NR | NR | NR | |
| ACR 50: 17.53%b,ab | ||||||||||||
| ACR 70: 3.62%b,ab | ||||||||||||
| Immediate ETN | 244 | 2 years | NR | NR | NR | NR | NR | ACR 20: 51.10%b | NR | NR | NR | |
| ACR 50: 37.18%b | ||||||||||||
| ACR 70: 20.52%b | ||||||||||||
| Immediate triple therapy | 132 | 2 years | NR | NR | NR | NR | NR | ACR 20: 45.97%b | NR | NR | NR | |
| ACR 50: 31.27%b | ||||||||||||
| ACR 70: 10.66%b | ||||||||||||
| Step-up ETN | 255 | 2 years | NR | NR | NR | NR | NR | ACR 20: 49.11%b | NR | NR | NR | |
| ACR 50: 32.44%b | ||||||||||||
| ACR 70: 15.77%b | ||||||||||||
| Step-up triple therapy | 124 | 2 years | NR | NR | NR | NR | NR | ACR 20: 47.92%b | NR | NR | NR | |
| ACR 50: 37.15%b | ||||||||||||
| ACR 70: 11.43%b | ||||||||||||
| Immediate ETN | 244 | 102 weeks | 3.0 (1.4); n = 159 (DAS28-ESR) | NR | 2.2 (3.9); n = 159 | 3.3 (5.5) | NR | NR | mHAQ: 1.0 (0.3); n = 15 | SHS: | NR | |
| Immediate triple therapy | 132 | 102 weeks | 2.9 (1.5); n = 76 (DAS28-ESR) | NR | 2.3 (3.3); n = 76 | 2.6 (4.5); n = 76 | NR | NR | mHAQ: 1.0 (0.3); n = 73 | SHS: | NR | |
| Step-up ETN | 255 | 102 weeks | 3.0 (1.4); n = 166 (DAS28-ESR) | NR | 4.4 (3.1); n = 166 | 3.6 (5.8); n = 166 | NR | NR | mHAQ: 0.9 (0.3); n = 154 | SHS: | NR | |
| Step-up triple therapy | 124 | 102 weeks | 2.8 (1.3); n = 75 (DAS28-ESR) | NR | 4.4 (2.8); n = 75 | 2.6 (4.4); n = 75 | NR | NR | mHAQ: 0.9 (0.3); n = 71 | SHS: | NR | |
| U-Act-Early62 (104-week randomised phase) | TOC + MTX | 108 | 24 weeks | Decrease from baseline: median 3.6 (range 0.75–7.48)ac | NR | 1.0 (2.1); n = 98 | 2.8 (4.9); n = 98ad | Good response: 93/105 (89%)ae | ACR 20: 79/105 (75%)af | D-HAQ: 0.50 (0.55); n = 94ag | NR | NR |
| ACR 50: 67/105 (64%)af | ||||||||||||
| Moderate response: 5/105 (5%)ae | ACR 70: 46/105 (44%)af | |||||||||||
| ACR 90: 19/105 (18%)af | ||||||||||||
| TOC + PBO–MTX | 106 | 24 weeks | Decrease from baseline: 3.6 (range 0.45–7.64)ac,ah | NR | 1.6 (2.8); n = 96ac | 3.0 (3.9); n = 96ad | Good response: 84/97 (87%)ae | ACR 20: 77/102 (75%)af | D-HAQ: 0.63 (0.66); n = 96ag | NR | NR | |
| ACR 50: 60/102 (59%)af | ||||||||||||
| Moderate response: 11/97 (11%)ae | ACR 70: 38/102 (37%)af | |||||||||||
| ACR 90: 12/102 (12%)af | ||||||||||||
| MTX + PBO–TOC | 103 | 24 weeks | Decrease from baseline: 2.1 (range 1.67–5.11)ac,ah | NR | 3.0 (4.4); n = 96ac | 3.7 (4.8); n = 96ad | Good response: 50/103 (49%)ae | ACR 20: 63/106 (59%)af | D-HAQ: 0.65 (0.54); n = 87ag | NR | NR | |
| ACR 50: 36/106 (34%)af | ||||||||||||
| Moderate response: 33/103 (32%)ae | ACR 70: 16/106 (15%)af | |||||||||||
| ACR 90: 5/106 (5%)af | ||||||||||||
| TOC + MTX | 108 | 52 weeks | Decrease from baseline: 3.3 (range 1.02–7.48)ah | NR | 0.6 (1.5); n = 84 | 2.4 (4.3); n = 84 | Good response: 75/100 (75%) | ACR 20: 74/99 (75%) | D-HAQ: 0.46 (0.50); n = 83ag | Change in SHS: 0.50 (1.495)h,j | NR | |
| ACR 50: 61/99 (62%) | ||||||||||||
| Moderate response: 6/100 (6%)ai | ACR 70: 44/99 (44%) | |||||||||||
| ACR 90: 19/99 (19%)XI | ||||||||||||
| TOC + PBO–MTX | 106 | 52 weeks | Decrease from baseline: 3.4 (range 0.28–7.66)ah | NR | 1.0 (1.7); n = 91 | 1.9 (3.6); n = 91 | Good response: 85/96 (88%) | ACR 20: 71/99 (72%) | D-HAQ: 0.48 (0.55); n = 82% | Change in SHS: 0.79 (3.242)h,j | NR | |
| ACR 50: 58/99 (59%) | ||||||||||||
| Moderate response: 4/96 (4%)ai | ACR 70: 44/99 (44%) | |||||||||||
| ACR 90: 21/99 (21%)ak | ||||||||||||
| MTX + PBO–TOC | 103 | 52 weeks | Decrease from baseline: 3.3 (range 0.74–6.13)ah | NR | 0.8 (1.6); n = 84 | 2.7 (4.2); n = 84 | Good response: 71/99 (72%) | ACR 20: 71/103 (69%) | D-HAQ:al 0.55 (0.51); n = 84% | Change in SHS: 0.96 (2.870)h,j | NR | |
| ACR 50: 53/103 (51%) | ||||||||||||
| Moderate response: 7/99 (7%)ai | ACR 70: 34/103 (33%) | |||||||||||
| ACR 90: 7/103 (7%)ak | ||||||||||||
| TOC + MTX | 108 | 104 weeks | Decrease from baseline: 3.3 (range 0.73–6.07)ah | NR | 0.8 (1.6), median 0; n = 76 | 2.5 (5.0); n = 76 | Good response: 63/96 (66%) | ACR 20: 61/96 (63%) | D-HAQ:al 0.48 (0.55); n = 70an | Change in SHS: 1.18 (3.919)h,o | NR | |
| ACR 50: 47/96 (49%) | ||||||||||||
| Moderate response: 8/96 (8%)am | ACR 70: 35/96 (36%) | |||||||||||
| ACR 90: 20/96 (21%) | ||||||||||||
| TOC + PBO–MTX | 106 | 104 weeks | Decrease from baseline: 3.3 (range 0.1–6.8)ah | NR | 0.7 (1.7); n = 82 | 2.0 (3.3); n = 82 | Good response: 72/95 (76%) | ACR 20: 62/95 (65%) | D-HAQ:al 0.61 (0.61); n = 77an | Change in SHS: 1.45 (4.272)h,o | NR | |
| ACR 50: 52/95 (55%) | ||||||||||||
| Moderate response: 8/95 (8%)am | ACR 70: 37/95 (39%) | |||||||||||
| ACR 90: 19/95 (20%) | ||||||||||||
| MTX + PBO–TOC | 103 | 104 weeks | Decrease from baseline: 3.2 (range 0.79–7.52)ah | NR | 1.1 (2.3); n = 75 | 2.5 (4.4); n = 75 | Good response: 65/96 (68%) | ACR 20: 60/99 (61%) | D-HAQ:al 0.62 (0.50); n = 73an | Change in SHS: 1.53 (2.421)h,o | NR | |
| ACR 50: 48/99 (48%) | ||||||||||||
| Moderate response: 8/96 (8%)am | ACR 70: 35/99 (35%) | |||||||||||
| ACR 90: 14/99 (14%) | ||||||||||||
Appendix 7 Tables of specific adverse events
| Trial acronym | Treatment arm | Safety population, n | Follow-up time point | AEs, n/N (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Musculoskeletal | Endocrine and metabolic | Cardiovascular | Dermatological | Ophthalmological | Gastrointestinal | Infectious | Psychological | Other | ||||
| STREAM55 | Aggressive group | NR | 2 years | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Conventional care | NR | 2 years | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| T-4 study56,57 | Routine care | 61 | 56 weeks | NR | NR | NR | NR | NR | NR | 0 (0)b | NR | NR |
| DAS28-driven therapy | 59 | 56 weeks | NR | NR | NR | NR | NR | NR | 0 (0)b | NR | NR | |
| MMP-3-driven therapy | 59 | 56 weeks | NR | NR | NR | NR | NR | NR | 1 (1.7)b | NR | NR | |
| DAS28 and MMP-3-driven therapy | 61 | 56 weeks | NR | NR | NR | NR | NR | NR | 0 (0)b | NR | NR | |
| Trial acronym or first author and year of publication | Treatment arm | Safety population, n | Follow-up time point | AEs, n/N (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Musculoskeletal | Endocrine and metabolic | Cardiovascular | Dermatological | Ophthalmological | Gastrointestinal | Infectious | Psychological | Other | ||||
| Fransen et al., 200550 | DAS28 | 205 | 24 weeks | NR | NR | NR | Rash or itching 4%b | NR | Nausea or vomiting 4%b | NR | NR | NR |
| Usual care | 179 | 24 weeks | NR | NR | NR | Rash or itching 11%b | NR | Nausea or vomiting 9%b | NR | NR | NR | |
| Optimisation of Adalimumab study52,53 | Routine care | 100 | 18 months | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| DAS28 target | 109 | 18 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| SJC target | 99 | 18 months | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Trial acronym or first author and year of publication | Treatment arm | Safety population, n | Follow-up time point | AEs, n/N (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Musculoskeletal | Endocrine and metabolic | Cardiovascular | Dermatological | Ophthalmological | Gastrointestinal | Infectious | Psychological | Other | ||||
| TICORA61 | Intensive management | 55 | 18 months | NR | NR | NR | 10 AEs | NR | 18 AEs | 5 AEs | NR |
|
| Routine management | 55 | 18 months | NR | NR | NR | 15 AEs | NR | 25 AEs | 7 AEs | NR |
|
|
| Van Hulst et al., 201063 | Intervention group | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Usual-care group | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Trial acronym or first author and year of publication | Treatment arm | Safety population, n | Follow-up time point | AEs, n/N (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Musculoskeletal | Endocrine and metabolic | Cardiovascular | Dermatological | Ophthalmological | Gastrointestinal | Infectious | Psychological | Other | ||||
| Hodkinson et al., 201551 | SDAI arm | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| CDAI arm | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| T-4 study56,57 | Routine care | 61 | 56 weeks | NR | NR | NR | NR | NR | NR | 0 (0)b | NR | NR |
| DAS28-driven therapy | 59 | 56 weeks | NR | NR | NR | NR | NR | NR | 0 (0)b | NR | NR | |
| MMP-3-driven therapy | 59 | 56 weeks | NR | NR | NR | NR | NR | NR | 1 (1.7)b | NR | NR | |
| DAS28 and MMP-3-driven therapy | 61 | 56 weeks | NR | NR | NR | NR | NR | NR | 0 (0)b | NR | NR | |
| TEAR58–60 | Immediate ETN | 244 | 102 weeks | 1 AE | 0 |
|
1 AE | 0 | 2 AEsc | 9 AEsc | 3 AEsc | |
| Immediate triple therapy | 132 | 102 weeks | 0 | 0 | 0 | 0 | 5 AEsc | 4 AEsc | 0 |
|
||
| Step-up ETN | 255 | 102 weeks | 4 AEsc | 0 | 2 AEsc | 0 | 0 | 7 AEsc | 0 | |||
| Step-up triple therapy | 124 | 102 weeks | 0 | 2 AEsc |
|
0 | 1 AE | 0 | 3 AEsc | 1 AE |
|
|
| Trial | Treatment arm | Safety population, n | Follow-up time point | AEs, n/N (%) | |||
|---|---|---|---|---|---|---|---|
| Any AEa | Any SAEa,b | Death | Withdrawals as a result of an AEa | ||||
| BeSt30,31,33 | Sequential monotherapy | 126 | 1 year30 | ≥ 1 AE: 54 (43%) |
8/126 (6.3) defined as life-threatening condition or death, a significant or permanent disability, a malignancy, hospitalisation or prolongation of hospitalisation, a congenital abnormality, or a birth defect Hospitalised, n = 8: |
NR | NR |
| Step-up combination therapy | 121 | 1 year | ≥ 1 AE: 57 (47%) |
9/121 (7.4) Hospitalised, n = 10: |
NR | NR | |
| Initial combination therapy with PDN | 133 | 1 year | ≥ 1 AE: 49 (37%) |
17/133 (12.8) Hospitalised, n = 20: |
NR | NR | |
| Initial combination therapy with IFX | 128 | 1 year | ≥ 1 AE: 50 (39%) |
6/128 (4.7) Hospitalised, n = 6: |
Transient cardiac ischaemia, pulmonary embolism NR | NR | |
| Sequential monotherapy | 126 | 5 years31 | 110 (87%) | 42 (33) | 3/126 (2.38%):
|
NR | |
| Step-up combination therapy | 121 | 5 years | 103 (85) | 34 (28) | 3/121 (2.48):
|
NR | |
| Initial combination therapy with PDN | 133 | 5 years | 112 (84) | 37 (28) | 2/133 (1.5):
|
NR | |
| Initial combination therapy with IFX | 128 | 5 years | 112 (88) | 37 (31) | 4/128 (3.1):
|
NR | |
| Sequential monotherapy | 126 | 10 years33 | NR | NR | 16 (12.7) | NR | |
| Step-up combination therapy | 121 | 10 years | NR | NR | 15 (12.4) | NR | |
| Initial combination therapy with PDN | 133 | 10 years | NR | NR | 21 (15.8) | NR | |
| Initial combination therapy with IFX | 128 | 10 years | NR | NR | 20 (15.6) | NR | |
| CareRA: high-risk patients42,43 | COBRA Classic | 91 | 16 weeks | 56/91 (61.2)c (therapy related) | 2 AEsd | 0/91 (0) | 2/91 (2) |
| COBRA Slim | 96 | 16 weeks | 45/96 (46.9)c (therapy related) | 1 AE | 1/96 (1) | 0/96 (0) | |
| COBRA Avant-Garde | 91 | 16 weeks | 63/91 (69.1)c (therapy related) | 3 AEsd | 0/91 (0) | 0/91 (0) | |
| COBRA Classic | 98 | 52 weeks | 66/98 (67.3) (therapy related); mean n AEs per patient = 1.9 (2.0)e | 2 AEs (therapy related)d | 1/98 (1) | 0 | |
| COBRA Slim | 98 | 52 weeks | 65/98 (66.3) (therapy related); mean n AEs per patient = 1.3 (1.4)e | 2 AEs (therapy related)d | 1/98 (1) | 0 | |
| COBRA Avant-Garde | 93 | 52 weeks | 73/93 (78.5) (therapy related); mean n AEs Per patient = 1.9 (1.6)e | 2 AEs (therapy related)d | 0/93 (0) | 0 | |
| CareRA: low-risk patients40,43 | MTX-TSU | 47 | 16 weeks | 32 (68.1) (therapy related) | 0/47 | NR | NR |
| COBRA Slim | 43 | 16 weeks | 30 (69.8) (therapy related) | 0/43 | NR | NR | |
| MTX-TSU | 47 | 52 weeks | 30/47 (63.8) (therapy related); mean n AEs per patient 1.2 (1.2) | 0 | 0/47 (0) | 0 | |
| COBRA Slim | 43 | 52 weeks | 22/43 (51.2) (therapy related); mean n AEs per patient 1.2 (1.5) | 2 AEs (therapy related)d | 0/43 (0) | 0 | |
| COBRA-light44,45 | COBRA | 81 | 6 months44 | ≥ 1 AE: 94% | n = 3:
|
NR | 1 manic episode |
| COBRA-light | 81 | 6 months | ≥ 1 AE: 90% |
|
NR | 1 myocardial infarction | |
| COBRA | 81 | 12 months45 | NR | n = 9:
|
NR | NR | |
| COBRA-light | 81 | 12 months | NR | n = 16:
|
NR | NR | |
| FIN-RACo46 | Combination treatment | 97 | 2 years | 68 (70) | SAEs (necessitating hospital admission, life-threatening, fatal or malignant disease), n = 3 (3%) | 0 | 0 |
| Single-drug treatment | 98 | 2 years | 70 (71) | SAEs (necessitating hospital admission, life-threatening, fatal or malignant disease), n = 5 (5%) | 0 | 0 | |
| Saunders 200854 | Parallel triple therapy | NR | 12 months | 141 AEs, n patients not reported | NR | NR | 0 withdrawal from trial (15 drug withdrawals) |
| Step-up therapy | NR | 12 months | 135 AEs, n patients not reported | NR | NR | 0 withdrawal from trial (18 drug withdrawals) | |
| TEAR58 | Immediate ETN | 244 | 102 weeks | 193 (79.1) | 35 (14.3) | 1 | 12 (4.9):
|
| Immediate triple therapy | 132 | 102 weeks | 101 (76.5) | 18 (13.6) | 1 | 7 (5.3):
|
|
| Step-up ETN | 255 | 102 weeks | 187 (73.3) | 32 (12.5) | 2 | 9 (3.5):
|
|
| Step-up triple therapy | 124 | 102 weeks | 92 (74.2) | 16 (12.9) | 0 | 4 (3.2):
|
|
| Trial acronym or first author and year of publication | Treatment arm | Safety population, n | Follow-up time point | AEs, n/N (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Musculoskeletal | Endocrine and metabolic | Cardiovascular | Dermatological | Ophthalmological | Gastrointestinal | Infectious | Psychological | Other | ||||
| BeSt30,31,33 | Sequential monotherapy | 126 | 1 year30 | NR | NR | 3 (2) | 12 (10) | NR | 20 (16) | 5 (4) | NR | NR |
| Step-up combination therapy | 121 | 1 year | NR | NR | 2 (2) | 15 (12) | NR | 18 (15) | 8 (7) | NR | NR | |
| Initial combination therapy with PDN | 133 | 1 year | NR | NR | 8 (6) | 12 (9) | NR | 11 (8) | 10 (8) | NR | NR | |
| Initial combination therapy with IFX | 128 | 1 year | NR | NR | 2 (2) | 8 (6) | NR | 14 (11) | 10 (8) | NR | NR | |
| Sequential monotherapy | 126 | 5 years31 | NR | NR | 20 (16) | Dermal/mucosal: 34 (27) | NR | 56 (44) | 56 (44). Serious infection: 13 (10.3) | NR | Malignancies: 5 (4) | |
| Neurological: 27 (21) | ||||||||||||
| Infusion reactions 3/52 infusions | ||||||||||||
| Step-up combination therapy | 121 | 5 years | NR | NR | 21 (17) | Dermal/mucosal: 36 (30) | NR | 55 (46) | 51 (42). Serious infection: 5 (4.1) | NR | Malignancies: 4 (3.3) | |
| Neurological: 30 (25) | ||||||||||||
| Infusion reactions: 0/15 infusions | ||||||||||||
| Initial combination therapy with PDN | 133 | 5 years | NR | NR | 36 (27) | Dermal/mucosal: 39 (29) | NR | 48 (36) | 53 (40). Serious infection: 7 (5.3) | NR | Malignancies: 6 (4.5) | |
| Neurological: 22 (17) | ||||||||||||
| Infusion reactions: 3/28 infusions | ||||||||||||
| Initial combination therapy with IFX | 128 | 5 years | NR | NR | 26 (20) | Dermal/mucosal: 24 (19) | NR | 57 (45) | 61 (48). Serious infection: 9 (7.0) | NR | Malignancies: 4 (3.1) | |
| Neurological: 25 (20) | ||||||||||||
| Infusion reactions: 11/120 infusions | ||||||||||||
| CareRA: high-risk patients42,43 | COBRA Classic | 91 | 16 weeks | NR | NR | NR | Discomfort: 111 AEsb | NR | NR | 5 AEsb | NR | Toxicity: 27 AEsb |
| Others: 4 AEsb | ||||||||||||
| Surgery: 1 AE | ||||||||||||
| COBRA Slim | 96 | 16 weeks | NR | NR | NR | Discomfort: 50 AEsb | NR | NR | 3 AEsb | NR | Toxicity: 10 AEsb | |
| Others: 7 AEsb | ||||||||||||
| COBRA Avant-Garde | 91 | 16 weeks | NR | NR | NR | Discomfort: 96 AEsb | NR | NR | 5 AEsb | NR | Toxicity: 23 AEsb | |
| Others: 6 AEsb | ||||||||||||
| COBRA Classic | 98 | 52 weeks | NR | NR | NR | Itch and rash: 4 AEsb | 0 | 45 AEsb | 11 AEsb | NR | Malaise: 52 AEsb | |
| Liver function abnormalities: 17 AEsb | ||||||||||||
| Hair loss: 7 AEsb | ||||||||||||
| Changed in appetite related: 18 AEsb | ||||||||||||
| GC related: 9 AEsb | ||||||||||||
| Blood level related: 7 AEsb | ||||||||||||
| Diabetes related: 2 AEsb | ||||||||||||
| Visual impairment related: 0 AEsb | ||||||||||||
| Kidney function abnormalities: 1 AEb | ||||||||||||
| Miscellaneous: 11 AEsb | ||||||||||||
| COBRA Slim | 98 | 52 weeks | NR | NR | NR | Itch and rash: 3 AEsb | Visual impairment related: 1 AEb | 48 AEsb | 8 AEsb | NR | Malaise: 22 AEsb | |
| Liver function abnormalities: 17 AEsb | ||||||||||||
| Hair loss: 10 AEsb | ||||||||||||
| Changed appetite related: 3 AEsb | ||||||||||||
| GC related: 5 AEsb | ||||||||||||
| Blood level related: 3 AEsb | ||||||||||||
| Diabetes related: 1 AEb | ||||||||||||
| Visual impairment related: 1 AEb | ||||||||||||
| Kidney function abnormalities: 0 AEs | ||||||||||||
| Miscellaneous: 10 AEsb | ||||||||||||
| COBRA Avant-Garde | 93 | 52 weeks | NR | NR | NR | Itch and rash: 1 AE b | 0 | 67 AEsb | 7 AEsb | NR | Malaise: 30 AEsb | |
| Liver function abnormalities: 22 AEsb | ||||||||||||
| Hair loss: 16 AEsb | ||||||||||||
| Change in appetite related: 9 AEsb | ||||||||||||
| GC related: 7AEsb | ||||||||||||
| Blood level related: 4 AEsb | ||||||||||||
| Diabetes related: 2 AEsb | ||||||||||||
| Visual impairment related: 0 AEsb | ||||||||||||
| Kidney function abnormalities: 0 AEsb | ||||||||||||
| Miscellaneous: 10 AEsb | ||||||||||||
| CareRA: low-risk patients40,43 | MTX-TSU | 47 | 16 weeks | NR | NR | NR | NR | NR | 11/47 (23.4) | 1/47 (2) | NR | NR |
| COBRA Slim | 43 | 16 weeks | NR | NR | NR | NR | NR | 10/43 (23.3) | 0/43 (0) | NR | NR | |
| MTX-TSU | 47 | 52 weeks | NR | NR | NR | Itch and rash: 3 AEsb | Visual impairment related: 1 AEb | 20 AEsb | 7 AEsb | NR | Malaise: 11 AEsb | |
| Liver function abnormalities: 6 AEsb | ||||||||||||
| Hair loss: 3 AEsb | ||||||||||||
| Change in appetite in related: 0 AEsb | ||||||||||||
| GC related: 0 AEsb | ||||||||||||
| Blood level related: 0 AEsb | ||||||||||||
| Diabetes related: 1 AEb | ||||||||||||
| Visual impairment related: 1 AEb | ||||||||||||
| Kidney function abnormalities: 2 AEsb | ||||||||||||
| Miscellaneous: 0 AEsb | ||||||||||||
| COBRA Slim | 43 | 52 weeks | NR | NR | NR | Itch and rash: 2 AEsb | Visual impairment related: 2 AEsb | 15 AEsb | 2 AEsb | NR | Malaise: 12 AEsb | |
| Liver function abnormalities: 3 AEsb | ||||||||||||
| Hair loss: 5 AEsb | ||||||||||||
| Change in appetite related: 4 AEsb | ||||||||||||
| GC related: 0 AEsb | ||||||||||||
| Blood level related: 2 AEsb | ||||||||||||
| Diabetes related: 0 AEsb | ||||||||||||
| Visual impairment related: 2 AEsb | ||||||||||||
| Kidney function abnormalities: 0 AEsb | ||||||||||||
| Miscellaneous: 4 AEsb | ||||||||||||
| COBRA-light44,45 | COBRA | 81 | 6 months | NR | NR | 0 | 37% | NR | 42% | 42% | NR | ≥ 5-kg gain in weight, reported as n = 7 (9%) (suggests safety n of 78). New diagnosis of type 2 diabetes, reported as n = 2. Hypertension reported as n = 1 |
| COBRA-light | 81 | 6 months | NR | NR | 1 (myocardial infarction) | 43% | NR | 42% | 40% | NR | ≥ 5-kg gain in weight, reported as n = 14 (18%) (suggests safety n of 78)
|
|
| FIN-RACo46 | Combination treatment | 97 | 2 years | 23 (24) | NR | NR | NR | NR | 29 (30) | NR | NR |
|
| Single-drug treatment | 98 | 2 years | 16 (16) | NR | NR | NR | NR | 30 (31) | NR | NR |
|
|
| Saunders et al., 200854 | Parallel triple therapy | NR | 12 months | NR | NR | NR | 19 mucocutaneous AEsb | NR | 52 gastrointestinal AEs;b 5 abnormal liver function testsb | 29 infective AEsb | NR | |
| Step-up therapy | NR | 12 months | NR | NR | NR | 16 mucocutaneous AEsb | NR | 48 gastrointestinal AEs;b and 6 abnormal liver function testsb | 27 infective AEsb | NR | ||
| TEAR58 | Immediate ETN | 244 | 102 weeks | 1 AE | 0 |
|
1 AE | 0 | 2 AEsb | 9 AEsb | 3 AEsb | |
| Immediate triple therapy | 132 | 102 weeks | 0 | 0 | 0 | 0 | 5 AEsb | 4 AEsb | 0 |
|
||
| Step-up ETN | 255 | 102 weeks | 4 AEsb | 0 | 2 AEsb | 0 | 0 | 7 AEsb | 0 | |||
| Step-up triple therapy | 124 | 102 weeks | 0 | 2 AEsb |
|
0 | 1 AE | 0 | 3 AEsb | 1 AE |
|
|
| Trial acronym | Treatment arm | Safety population, n | Follow-up time point | AEs, n/N (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Musculoskeletal | Endocrine and metabolic | Cardiovascular | Dermatological | Ophthalmological | Gastrointestinal | Infectious | Psychological | Other | ||||
| CAMERA35 | Intensive strategy group | 149 | 2 years | NR | NR |
|
|
NR |
|
NR | NR |
|
| Conventional strategy group | 140 | 2 years | NR | NR | 0 |
|
NR |
|
NR | NR |
|
|
List of abbreviations
- ACR
- American College of Rheumatology
- ADA
- adalimumab
- AE
- adverse event
- bDMARD
- biologic disease-modifying antirheumatic drug
- BeSt
- BehandelStrategieën in Reumatoïde Artritis
- BROSG
- British Rheumatoid Outcome Study Group
- CAMERA
- Computer-Assisted Management in Early Rheumatoid Arthritis
- CareRA
- Care in early Rheumatoid Arthritis
- CDAI
- Clinical Disease Activity Index
- cDMARD
- conventional disease-modifying antirheumatic drug
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- COBRA
- COmBination theRApy with rheumatoid arthritis
- CRP
- C-reactive protein
- D-HAQ
- Dutch version of the Health Assessment Questionnaire
- DAS
- Disease Activity Score
- DAS28
- Disease Activity Score, 28 joints
- DAS28-CRP
- Disease Activity Score, 28 joints with C-reactive protein concentration
- DAS28-ESR
- Disease Activity Score, 28 joints with erythrocyte sedimentation rate
- DAS44
- Disease Activity Score, 44 joints
- DAS44-CRP
- Disease Activity Score, 44 joints with C-reactive protein concentration
- DMARD
- disease-modifying antirheumatic drug
- DREAM
- Dutch Rheumatoid Arthritis Monitoring
- EQ-5D
- EuroQol-5 Dimensions
- ESR
- erythrocyte sedimentation rate
- ETN
- etanercept
- EULAR
- European League Against Rheumatism
- FIN-RACo
- FINnish Rheumatoid Arthritis Combination therapy
- GC
- glucocorticoid
- HAQ
- Health Assessment Questionnaire
- HCQ
- hydroxychloroquine
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IFX
- infliximab
- IQR
- interquartile range
- JSN
- joint space narrowing
- LDA
- low disease activity
- LEF
- leflunomide
- MACTAR
- McMaster Toronto Arthritis Patient Preference Disability Questionnaire
- mHAQ
- modified Health Assessment Questionnaire
- MMP-3
- matrix metalloproteinase 3
- mTSS
- modified total Sharp score
- MTX
- methotrexate
- MTX-TSU
- methotrexate tight step-up
- NICE
- National Institute for Health and Care Excellence
- NSAID
- non-steroidal anti-inflammatory drug
- OR
- odds ratio
- PBO
- placebo
- PDN
- prednisone
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RA
- rheumatoid arthritis
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SDAI
- Simple Disease Activity Index
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SHS
- Sharp/van der Heijde score
- SJC
- swollen joint count
- SSZ
- sulfasalazine
- STREAM
- STRategies in Early Arthritis Management
- T-4
- TreaTing to Twin Targets
- TA
- Technology Appraisal
- TEAR
- Treatment of Early Aggressive Rheumatoid arthritis
- TICORA
- TIght COntrol for Rheumatoid Arthritis
- TJC
- tender joint count
- TNF
- tumour necrosis factor
- TNFi
- tumour necrosis factor inhibitor
- TOC
- tocilizumab
- TTT
- treat to target
- U-Act-Early
- early rheumatoid arthritis treated with tocilizumab, methotrexate or their combination
- VAS
- visual analogue scale