Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/192/10. The contractual start date was in March 2016. The draft report began editorial review in June 2017 and was accepted for publication in September 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Walters et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Introduction
Although many older people live well, the accumulation of health conditions over time can lead to increasing frailty for some. Frailty in later life is associated with greater risk of hospitalisation, functional decline, falls, worsening mobility and death1 and, consequently, a substantial increase in health and social care usage. Health-care costs over 3 months have been estimated as rising from €642 per person (£562 at the exchange rate in May 2017) for non-frail older adults to €3659 (£3201) for frail older adults, largely as a result of increases in inpatient care and medications. 2 The impact of frailty is likely to increase in the near future as the number of people aged ≥ 75 years is estimated to almost double by 2039 from 5.2 million to 9.9 million,3 with a well-recognised pressure on health and social care systems as well as family carers. Age UK (London, UK) suggests that UK public spending on social care will need to increase by at least £1.65B by 2020/21 to manage the impact of demographic and cost changes. 4
Although approximately 11% of people aged ≥ 65 years are frail, the proportion who are pre-frail is much higher, ranging from 19% to 53% across populations, with an average of 41%. 5 Pre-frailty (or early or mild frailty) is an intermediate stage at which individuals have some loss of physiological reserves but can recover after a stressor event6 and typically feel ‘slowed up’, with increasing dependency on others for assistance in instrumental activities of daily living (IADL), such as cooking, shopping and managing finances. 7 Box 1 outlines the different ways in which mild or pre-frailty is commonly defined and measured. Previous interventions have focused on preventing decline or reducing frailty in the highest-risk populations with moderate–severe frailty, with limited success. 11–13 Although moderate–severe frailty has a higher risk of decline than mild frailty, older people with mild frailty are also more likely to transition back to a robust state or remain stable than those who are more frail. 14 This suggests that health promotion interventions may be more effective when targeted at less frail populations. Indeed, positive outcomes of preventative home visits (multidimensional visits addressing medical, functional, psychosocial and/or environmental problems and resources) by nurses on mortality rates appear to be greater for younger-old rather than older-old populations. 15
Pre-frailty’ defined as one or two characteristics from the following: slow gait speed, weight loss, low physical activity, low energy and weakness. 10
Electronic Frailty Index‘Mild frailty’ defined as a score of > 0.12–0.24 on the electronic Frailty Index, which uses the cumulative deficit model to identify frailty according to a range of 30 deficits: symptoms, signs, diseases, disabilities and abnormal laboratory values. 8
Clinical Frailty Scale‘Mild frailty’ defined as people with more evident slowing who need help or support in higher order IADL (e.g. finances, heavy housework) and who have progressive impairment of outdoor mobility, shopping and housework. 7
Modified physical performance test‘Mild frailty’ defined as a score of 25–31 on the Physical Performance Test, assessing performance of nine functional tasks, including activities such as stair climbing or putting on a coat. 9
However, it is currently unclear which intervention components are most beneficial for older people with frailty. 16,17 Home-based interventions appear to be promising, with evidence suggesting that they can have beneficial effects on mortality, functioning and emergency department admissions, with neutral effects on costs. 16–19 Previous evidence supported interventions based on multidimensional geriatric assessment including follow-up visits. 15 However, this type of intervention, typically with involvement from a multidisciplinary team of health and social care professionals, is expensive and difficult to deliver at scale, particularly if targeted at the larger group of up to 41% of older people with mild or pre-frailty living at home. The most optimal content and delivery of services, in terms of clinical effectiveness and cost-effectiveness, targeted at this population are therefore unclear, and previous interventions have lacked rigorous development or stakeholder input. We aimed to develop a home-based health promotion intervention according to the Medical Research Council (MRC) framework for intervention development,20 targeted at older people with mild frailty and to assess the feasibility of delivering the intervention and of conducting a full-scale randomised controlled trial (RCT) to test clinical effectiveness and cost-effectiveness.
Objectives
Our study objectives were to:
-
Systematically review and synthesise existing evidence for home-based health promotion interventions for older people with mild frailty.
-
Explore how policy and practice in the health and social care system address health promotion with older people with mild frailty.
-
Explore key components for a new home-based health promotion intervention for older people with mild frailty using interviews and focus groups with older people and carers, home care workers and community health professionals.
-
Develop a new health promotion intervention for older people with mild frailty drawing on principles of ‘co-design’ in partnership with older people, carers, health/care professionals and other experts.
-
Test acceptability and feasibility for delivery in the NHS including testing recruitment, attrition, feasibility of individual randomisation for a future RCT, feasibility/acceptability of study procedures and suitability of outcome measures.
-
Determine the intervention costs and test the feasibility of collecting health economic data to calculate costs and effects. We will also determine the feasibility of calculating the cost-effectiveness for a full RCT from health and social care and societal perspectives, and of conducting a budget impact analysis, scaling up to Clinical Commissioning Group level, estimating where monetary costs and benefits will fall for the NHS and local authority.
-
Conduct a mixed-methods process evaluation exploring the context, potential mechanisms and pathways to impact of the intervention and identify issues to address in scaling up the intervention for a full RCT and/or implementation.
The latest version of the project protocol (V1.3) is available on the Health Technology Assessment website at www.journalslibrary.nihr.ac.uk/programmes/hta/1219210/#/. 21
The original commissioning brief is included in Report Supplementary Material 1.
Ethics and governance
The study was approved by the NHS Camden and King’s Cross Research Ethics Committee on 14 October 2014 (reference number 14/LO/1698). A substantial amendment was submitted and approved 16 September 2015 after initial development work to clarify the intervention content, for review of recruitment materials and to approve protocol changes required (see Chapter 3, Modifications to the trial protocol for details). A second substantial amendment (approved 26 May 2016) was submitted to review the process evaluation data collection materials (e.g. interview topic guides, questionnaires, information leaflets). No further protocol changes were made. All research and development approvals were sought and obtained for each site and amendment.
Public involvement and engagement
There was substantial public involvement across the whole project, from development of the initial protocol through to our dissemination processes. Two public members (JH and RE, see Acknowledgements) in particular advised on plain English summaries, study design (in particular approaches to engage older people to participate), study materials including summary leaflets and interview topic guides throughout. JH participated on the interview panel to appoint project staff and RE was an integral part of the process evaluation team, including analysis of qualitative data. Public members had a key role in the intervention development panels that served to shape the evidence base for the intervention into an acceptable and feasible service for older people with mild frailty. Two out of the three intervention development panels were cohosted with our third-sector partner, Age UK London, which helped involve 34 public members in this process (see Chapter 3, Service development).
Chapter 2 Intervention development: identifying effective content for a new service
In accordance with the MRC framework for intervention development,20 we undertook a series of systematic reviews to identify potentially effective components of a new service for mild frailty. These include two systematic reviews, a state-of-the-art review on six different areas of health promotion and a scoping review of policies targeted towards mild frailty. The systematic reviews are registered in PROSPERO (CRD42014010370) and reported below according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 22
Identifying components of effective interventions: systematic review of interventions targeted at community-dwelling older people with mild or pre-frailty
We reviewed the evidence for home- and community-based health promotion interventions targeted at populations with mild or pre-frailty. Original searches were conducted between December 2014 and February 2015 identifying studies from January 1990 to February 2015, prior to intervention development. Few studies were identified meeting our criteria in the original searches and, as this is a rapidly evolving field, these searches were updated in May 2016, reviewing evidence for the period January 1990 to May 2016. This yielded only a small number of extra studies. The findings of RCTs from the updated systematic review have been published in full elsewhere. 23 We have summarised these below in addition to our findings from observational and qualitative studies.
Objectives
This systematic review aimed to identify the evidence base for potential components of a new intervention targeted at older people with mild frailty. Our objectives were to systematically review:
-
RCTs of home- or community-based health promotion interventions aimed specifically at older people with mild frailty
-
qualitative studies with older people with mild or pre-frailty, exploring their experiences and perspectives on potential interventions
-
observational studies of people with mild or pre-frailty that identify modifiable risk factors for adverse outcomes (e.g. transitioning to frailty, worsening functioning) that could be potential new targets for intervention.
Methods
Data sources and search strategy
We searched the following bibliographic databases using the terms mild and pre-frailty and their synonyms to identify studies that have targeted this group (specific pre-frailty subject headings are currently unavailable; see Report Supplementary Material 2):
-
MEDLINE and MEDLINE In Process & Other Non-Indexed Citations
-
EMBASE
-
Scopus
-
Social Science Citation Index
-
Science Citation Index Expanded
-
Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Effective Practice and Organisation of Care Group, NHS Health Economic Evaluation Database, Cochrane Methodology Register, Cochrane Groups
-
Database of Abstracts of Reviews of Effects
-
PsycINFO
-
Cumulative Index to Nursing and Allied Health Literature
-
Evidence for Policy and Practice Information Centre Register of Health Promotion and Public Health Research
-
Sociological Abstracts
-
Social Care Online
-
Applied Social Sciences Index and Abstracts.
We also used the following data sources:
-
trials register searches – Health Technology Assessment database, the UK Clinical Research Network Portfolio Database and ClinicalTrials.gov.
-
screening reference lists of systematic reviews and included studies.
-
contacting authors of relevant protocols and conference abstracts to obtain study outcome reports where possible.
-
forward citation tracking for included studies.
Study selection
We stored and deduplicated references in an EndNote [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] database. One reviewer screened titles and abstracts of articles and two reviewers screened full texts according to the following criteria, with disagreements resolved through discussion between reviewers and by consultation with the wider team.
Inclusion/exclusion criteria
We included an English-language cohort, experimental and qualitative studies evaluating single or multiple domain health promotion interventions for community-dwelling older people aged ≥ 65 years with mild or pre-frailty identified using established criteria (including studies reporting separate outcomes for pre-frail subgroups). We excluded non-empirical studies and experimental studies of poor quality (e.g. before-and-after studies). Inpatient interventions and hospital or nursing home populations were excluded. We originally intended to focus on home-based interventions only; however, as a result of the paucity of these studies, we also included community-based interventions. We assessed the following outcomes: frailty or associated variables (e.g. gait speed), physical functioning, quality of life, physical activity and hospital admissions. We excluded studies assessing biological markers of frailty (e.g. inflammatory markers) as outcomes and those with an inadequate reference group (e.g. comparing pre-frail older adults plus a risk factor to non-frail older adults without the same risk factor).
Data extraction and quality assessment
Randomised controlled trials
One reviewer extracted data into a spreadsheet developed for this review on study design, sample size at baseline and follow-up, inclusion/exclusion criteria, frailty definition, intervention, control, outcomes assessed and results. Two independent reviewers assessed risk of bias within RCTs using the Cochrane Risk of Bias tool. 24 Participant blinding was excluded from our overall trial risk-of-bias ratings as a usual-care control is often appropriate in health promotion interventions, although active control treatments (e.g. a flexibility home exercise programme) were considered to be of a low risk of bias. Risk of bias was used descriptively and was not used to weight studies or as an inclusion criterion for meta-analysis.
Observational studies
One reviewer extracted data regarding sample size, independent and dependent variables, covariates controlled for, outcomes and key findings. Only data relating to the mild or pre-frail subgroup and potentially modifiable outcomes were extracted. Quality was assessed by one reviewer using the Newcastle–Ottawa Scale,25 which assesses the rigour of participant selection (up to a maximum of four stars), comparability (out of a maximum of two stars) and outcome assessments (out of a maximum of three stars).
Qualitative studies
We intended to extract data on sample characteristics, data collection methods, analysis methods and key findings, as well as evaluate study quality according to Social Care Institute for Excellence guidance. 26
Synthesis of data and data analysis
We inductively developed coding schemes to group types of interventions targeted to pre-frailty (e.g. exercise and nutrition) and summarised the evidence available within each type.
For RCTs, we used meta-analysis to combine the results for similar interventions assessing the same outcome. When studies assessed an outcome using two or more measures, we reviewed the literature and selected the most comprehensive, valid and reliable measure; when studies compared multiple interventions with the same control group, we pooled the mean and standard deviation (SD). 27 For crossover designs, we included first period data only (obtained from the authors). 28 Post-intervention end-point data were used for consistency across trials because intervention and follow-up durations differed. We combined continuous end-point scores using standardised mean difference (SMD) in a random-effects model and quantified heterogeneity using the I2-statistic. For outcome data that could not be synthesised (e.g. change scores rather than end-point scores27), interventions with only a single study and long-term follow-up data we used narrative synthesis. Authors were contacted when possible for further data.
For observational studies, we narratively summarised the data available relating to each modifiable risk factor for frailty progression/adverse outcomes to explore which factors have potential for improving outcomes to include in a new intervention. We intended to synthesise qualitative studies using meta-ethnography,29 but we did not find any qualitative studies meeting our inclusion criteria.
Results
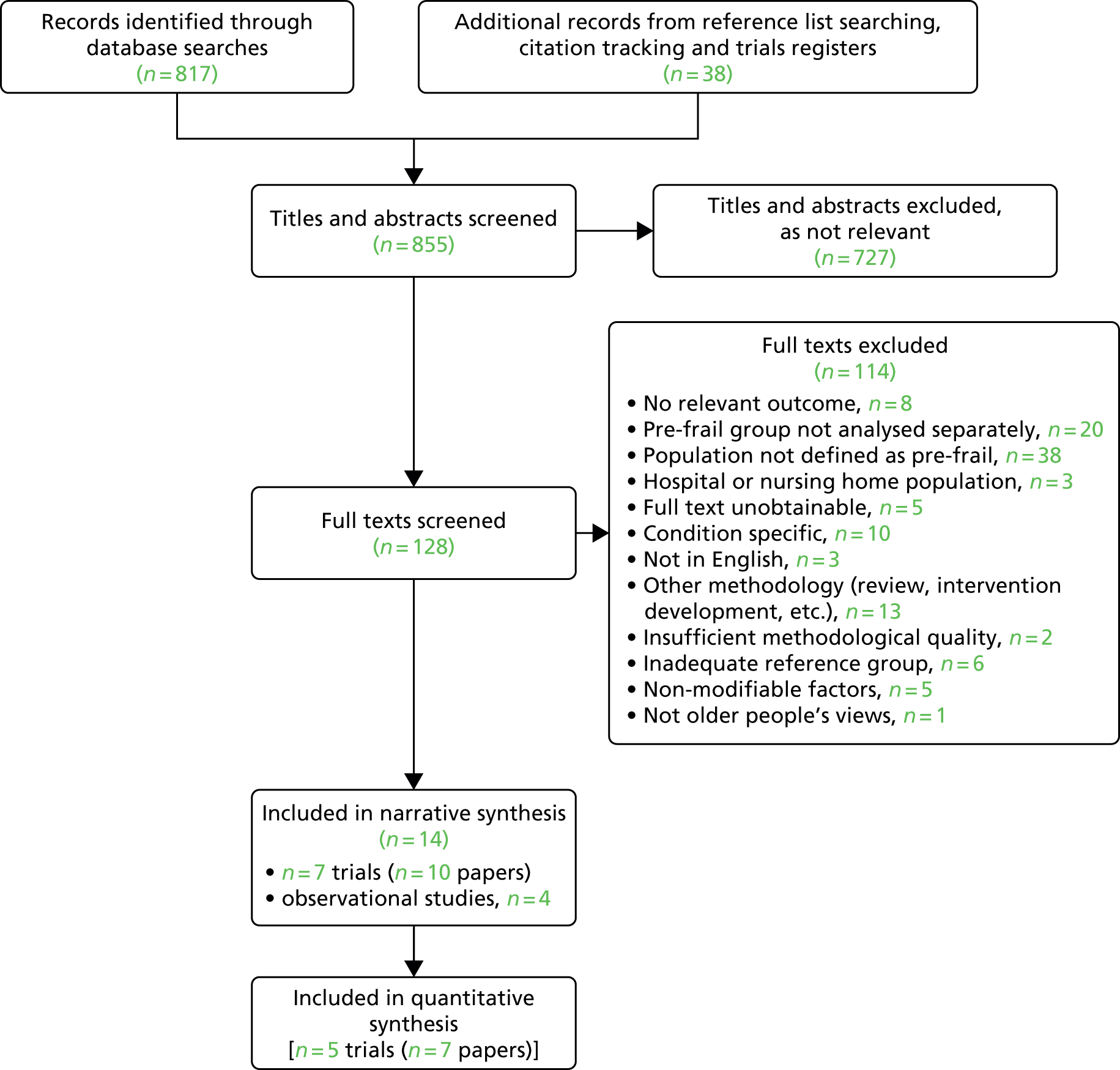
The flow diagram for this review is reported in Figure 1. We identified 855 unique references through database searches and citation tracking of eligible papers. We excluded 727 citations on title and abstract, screened 128 full texts and excluded 114, largely attributable to population (n = 71; e.g. combined outcome data for frailty and pre-frailty, frailty in a specific condition). Three were reported in Japanese and five were unobtainable. Therefore, the review included 10 papers reporting on seven RCTs and four observational studies. Of these, 13 were identified through database searches and one from citation tracking.
FIGURE 1.
The PRISMA flow diagram of studies included in the review.
Description of included randomised controlled trials
A full description of studies is included elsewhere23 and a brief summary of study details is outlined in Tables 1 and 2.
| Study ID (first author and year of publication) and country | Participants (n, frailty, inclusion/exclusion criteria) | Intervention | Control | Outcomes assessed | Main findings |
|---|---|---|---|---|---|
|
Binder et al. , 200230 USA |
119 adults aged ≥ 78 years with mild–moderate frailty (defined by modified PPT score) | Group balance, flexibility, co-ordination, reaction speed and strength exercises (9 months) | Low-intensity flexibility home programme (9 months) |
Performance-based physical functioning Self-reported functioning Balance Muscle strength Quality of life |
|
|
Brown et al. , 200031 USA |
87 adults aged > 78 years; adults with mild frailty (defined by PPT score) | Group flexibility, balance, body handling skills, speed of reaction, co-ordination and strength exercises (3 months) | Home range of motion exercises (3 months) |
Performance-based physical functioning Muscle strength Balance Gait speed |
|
|
Daniel, 201232 USA |
23 adults aged ≥ 65 years with pre-frailty (Fried criteria)10 |
1. Group Wii Fit™ (Nintendo® Nintendo Co., Ltd., Kyoto, Japan) basic games with weight vest (15 weeks) 2. Group seated upper and lower body strength and flexibility exercise (15 weeks) |
Usual activity (15 weeks) |
Self-reported functioning Physical activity Timed up and go |
|
|
Germany |
69 adults aged 65–94 years with pre-frailty (modified Fried criteria)10 |
1. Power training (upper and lower body), walking and balance exercises in groups (12 weeks) 2. Strength training (upper and lower body), walking and balance exercises in groups (12 weeks) |
Usual activity (12 weeks) |
Performance-based physical functioning Self-reported functioning Muscle strength |
|
|
Kwon et al. , 201535 Japan |
89 women aged ≥ 70 years with pre-frailty (modified Fried criteria)10 |
1. Group strength and balance training and cooking class focusing on protein- and vitamin D-rich foods (12 weeks) 2. Group strength and balance training alone (12 weeks) |
Monthly general health education sessions (12 weeks) |
Gait speed Balance Muscle strength Quality of life |
|
|
Lustosa et al. , 2011;36 and Lustosa et al. , 201337 Brazil |
32 women aged ≥ 65 years with pre-frailty (Fried criteria)10 | Group lower limb resistance exercise (10 weeks) | Usual activity (10 weeks) |
Gait speed Timed up and go Muscle strength |
|
|
Upatising et al. , 201338 USA |
87 adults aged ≥ 60 years with pre-frailty (modified Fried criteria)10 | Telemonitoring with individualised measurement (12 months) | Usual care (12 months) | Frailty state |
|
| Study ID (first author and year of publication) | Population | Independent variables | Dependent variable | Covariates | Follow-up duration (total number out of baseline number) | Findings | Qualitya (selection maximum 4*, comparability maximum 2*, outcome maximum 3*) |
|---|---|---|---|---|---|---|---|
| Jamsen et al., 201639 | Community-dwelling men aged ≥ 70 years in Australia (Concord Health and Ageing in Men Project),40 number of pre-frail men not reported |
Total number of medications (regular prescribed medications) Drug Burden Index (pharmacological risk assessment of cumulative exposure to sedative and anticholinergic medications) |
Frailty status (Fried phenotype)10 Death |
Age Baseline dementia or mild cognitive impairment diagnosis Comorbidity Education level Living status |
2 years (1366/1705) 5 years (954/1705) |
Number of medications had no effect on transitions from pre-frailty to other states (HR pre-frail-robust 0.99, pre-frail-frail 1.06, pre-frail-death 1.02) Drug Burden Index had no effect on transitions from pre-frailty to other states (HR pre-frail-robust 0.90, pre-frail-frail 1.03, pre-frail-death 1.18) |
Selection **** Comparability ** Outcome ** |
| Lee et al., 201441 | Community-dwelling men and women aged ≥ 65 years, equally distributed by age bracket and gender, in Hong Kong. A total of 48.7% of men and 52.5% of women pre-frail at baseline |
Smoking BMI MMSE score (Other non-modifiable characteristics also assessed) |
Frailty status (Fried phenotype)10 | Age | 2 years (1519/1745 men, 1499/1682 women) |
Pre-frail men: normal and overweight BMI was protective against worsening frailty compared with underweight [OR normal 0.47 (95% CI 0.23 to 0.99), overweight 0.36 (95% CI 0.16 to 0.81)], but no effect on odds of improving to robust. Obesity and smoking had no effects. Higher MMSE score predicted greater likelihood of improvement from pre-frail to robust [OR 1.10 (1.02–1.18)] Pre-frail women: BMI, smoking and MMSE scores had no effect on frailty transitions In multiple stepwise regression models (male: adjusted for age and stroke, female: adjusted for age, hospital admissions and stroke), higher MMSE score was a protective factor in both genders |
Selection **** Comparability * Outcome *** |
| Mohler et al., 201642 | Older adults aged ≥ 65 years from primary, secondary, and tertiary health-care settings, community providers, assisted living facilities, retirement homes, and ageing service organisations, without gait or mobility disorders in Arizona, USA (Arizona Frailty Cohort Study),43 n = 57 (48%) pre-frail |
Gait parameters (five inertial sensors worn walking 4.57-metre distance) Balance parameters (five sensors worn while standing with eyes open and closed) Physical activity parameters (all measured over 48 hours using triaxial accelerometer) |
Falls (yes/no, weekly log and telephone interview) | History of falls (use of assistive device, fear of falling, percentage body composition of muscle excluded from parsimonious model with sensitivity analysis) | 6 months (119/128) | Predictors of falls in pre-frail older adults (sensitivity analysis of one model only) included centre of mass sway (OR 8.8, p < 0.001), mean walking bout duration (OR 1.1, p = 0.02) and mean standing bout duration (OR 0.95, p = 0.03). Other predictors were not statistically significant and so were not included in the model |
Selection *** Comparability * Outcome ** |
| Shardell et al., 201244 | Random sample of residents aged ≥ 65 years in Italy (Invecchiare in Chianti Study),45 352 out of 1005 pre-frail | Vitamin D (serum 25(OH)D) classed as high or low (< 20 ng/ml) |
Frailty status (Fried phenotype)10 Mortality |
Alcohol consumption Calcium intake MMSE score Depression (CES-D) Comorbidities (congestive heart failure, diabetes mellitus, myocardial infarction, peripheral arterial disease, hypertension, angina, osteoarthritis, renal disease, chronic obstructive pulmonary disease) Age Education BMI Smoking Gender Blood collection season |
3 years 6 years (733/904) |
Lower serum 25(OH)D concentration significantly increased risk of mortality by 8.9% (95% CI 2.5% to 15.2%) and 5 ng/ml reductions were associated with greater odds of dying than becoming robust. Some non–significant associations with frailty state transitions |
Selection **** Comparability ** Outcome *** |
We included parallel-group RCTs,30,31,33,35 one pilot RCT,32 one randomised crossover trial36 and one secondary analysis of a RCT38 in our review, including 506 randomised participants (sample size range 23–194 participants) from a range of countries (see Table 1). Five trials used pre-frailty, defined through the Fried phenotype10 or modifications of this, to identify eligible older adults or women. 32,34–36,38 Two defined mild–moderate frailty according to the modified physical performance test,31 with two additional fitness and activities of daily living (ADL) criteria in one study. 30 Participants’ mean ages ranged from 72 to 83 years (inclusion criteria ranging from ≥ 60 to ≥ 78 years).
Most interventions were community-based group exercise (eight interventions reported in six studies30–32,34–36), with one three-arm study containing an additional exercise and nutrition group. 35 Exercise interventions were supervised by trained instructors, exercise physiologists or physiotherapists and delivered in 45- to 60-minute sessions one to three times per week over 12–36 weeks. Control groups were usual activity,32,34,36 a low intensity flexibility home exercise programme30,31 and monthly general health education sessions. 35 One study contained an 8-week run-in phase of vitamin D supplementation prior to randomisation. 34 Upatising et al. 38 carried out a subgroup analysis of a home individualised telemonitoring intervention compared with usual care.
Risk of bias was variable. One study was rated as having a low risk of bias,34 but three were unclear (two with some low-risk domains)30–32 and three were rated as having a high risk of bias. 35,36,38 The risk-of-bias plot can be found in the paper arising from this review. 23
Two further studies with poor methodological quality were identified in this review but not included. One compared 28 pre-frail elderly women with 28 non-randomly selected healthy controls undertaking a group exercise programme. 46 The other used a non-randomised trial design to assess the effects of a group compared with home control exercise programme on mild–moderately frail older women taking hormone replacement therapy; however, the only relevant outcome (muscle strength) was assessed in the exercise group alone. 47
Observational studies
We identified four observational studies that met our inclusion criteria (including reporting separate findings from a mild or pre-frail population and including potentially modifiable risk factors as the exposure; see Table 2). 39,41,42,44 They assessed the impact of medication burden on frailty status and death,39 the effects of smoking and body mass index (BMI) and Mini Mental State Examination score on frailty status,41 gait parameters on falls in pre-frail older adults,42 and the effects of high or low vitamin D on frailty and mortality. 44 Follow-up ranged from 6 months to 6 years. Total sample sizes ranged from 128 to 3018 at baseline and, within these, pre-frailty prevalence ranged from one-third to half of the sample. Study quality was good across all studies.
Qualitative studies
No qualitative studies of health promotion/behaviour change interventions from the perspectives of older people with mild or pre-frailty were identified.
Synthesis
Randomised controlled trials
Out of the six studies evaluating group exercise interventions, five contained sufficient intervention end-point data for meta-analysis across six outcomes. This is summarised in Table 3 reporting the SMD between exercise intervention and control groups for each trial.
| Outcome, study ID (first author and year of publication) | Outcome measure | SMD (95% CI) | Meta-analysis (95% CI) |
|---|---|---|---|
| Self-reported physical functioning | |||
| Binder et al., 200230 | Physical function subscale of Functional Status Questionnaire | 0.83 (0.44 to 1.21) |
0.19 (–0.57 to 0.95) p = 0.62 I2 = 80% |
| Daniel, 201232 | Late life function and disability index – function total | –0.74 (–1.79 to 0.32) | |
| Drey et al., 201234 | Short Form Late Life Function and Disability Instrument | 0.12 (–0.39 to 0.62) | |
| Performance-based (observed) physical function | |||
| Binder et al., 200230 | Modified physical performance test | 0.58 (0.20 to 0.96) |
0.37 (0.07 to 0.68) p = 0.02 I2 = 31% |
| Brown et al., 200031 | Physical Performance Test | 0.40 (–0.04 to 0.84) | |
| Drey et al., 201234 | SPPB | 0.03 (–0.47 to 0.54) | |
| Gait speed | |||
| Brown et al., 200031 | Preferred gait velocity (pressure-sensitive foot switches, m/min) | 0.27 (–0.16 to 0.69) |
–0.06 (–0.49 to 0.37) p = 0.79 I2 = 50% |
| Drey et al., 201234 | SPPB usual gait speed (3–4 m, points) | –0.10 (–0.61 to 0.40) | |
| Lustosa et al., 201136 | Usual speed (10 m, m/s) | –0.55 (–1.25 to 0.16) | |
| Balance | |||
| Binder et al., 200230 | Berg balance test | 0.40 (0.03 to 0.78) |
0.33 (0.08 to 0.57) p = 0.009 I2 = 0% |
| Brown et al., 200031 | Berg balance test | 0.39 (–0.03 to 0.82) | |
| Drey et al., 201234 | SPPB multicomponent static balance (points) | 0.09 (–0.42 to 0.60) | |
| Mobility | |||
| Daniel, 201232 | Timed up and go (8 feet) | 0.69 (–0.36 to 1.74) |
0.57 (–0.01 to 1.16) p = 0.06 I2 = 0% |
| Lustosa et al., 201136 | Timed up and go (3 m) | 0.52 (–0.19 to 1.22) | |
| Muscle strength | |||
| Binder et al., 200230 | Knee extension (Cybex isokinetic dynamometry) | 0.76 (0.38 to 1.14) |
0.44 (0.11 to 0.77) p = 0.009 I2 = 47% |
| Brown et al., 200031 | Knee extensors (Cybex isokinetic dynamometry) | 0.56 (0.13 to 0.99) | |
| Drey et al., 201234 | Sit-to-stand transfer power | 0.24 (–0.27 to 0.75) | |
| Lustosa et al., 201136 | Knee extensor (isokinetic dynamometer Byodex System) | –0.09 (–0.79 to 0.60) | |
Other outcomes that could not be meta-analysed as a result of insufficient number of studies were:
-
quality of life – no significant differences for exercise30,35 or exercise and nutrition35 interventions
-
physical activity – a WiiFit intervention showed a within-group increase in self-reported physical activity32
-
frailty – slightly fewer people transitioned to non-frail or frail from pre-frail over 6 months after telemonitoring compared with usual care. 38
Observational studies
The key findings from the four observational studies included in this review were as follows.
-
There is evidence that the number of medications and drug burden in pre-frail older adults have no effect on transitions to robustness, frailty or death. 39
-
There is evidence that a normal or overweight BMI in pre-frail men is protective against transitioning to frailty, with no evidence for the effects of smoking or obesity. In pre-frail women, there was no evidence for the effects of BMI, smoking or cognition scores on frailty transitions. When adjusting for key covariates, better cognition was protective in both genders. 41
-
In pre-frail older adults, low vitamin D levels significantly increase the risk of mortality by 8.9%. 44
-
Greater centre of mass sway and longer bouts of walking are associated with an increased falls risk, while longer bouts of standing are associated with reduced falls risk in pre-frail older adults. 42
Key findings
Currently, both observational and experimental research targeted at pre-frail or mildly frail older people is sparse. RCTs are mostly small and/or of poor quality and focused on group exercise to improve mobility and physical functioning, with mixed evidence that group exercise may have some effects on functioning. Observational studies were large, of good quality and suggested that BMI, cognition and vitamin D levels may influence future transitioning to frailty, but that the number and burden of medications is unlikely to have an effect. There were no qualitative studies exploring perspectives on health promotion interventions in populations with mild or pre-frailty.
Implications for intervention development
-
No interventions can currently be recommended for widespread use in pre-frail or mildly frail older people, but exercise may be an effective component for a new intervention. Including a nutritional or cognitive component may have potential in frailty prevention.
-
Broader, multidimensional interventions targeted to mildly frail populations have not previously been assessed.
-
Further qualitative work is needed to help clarify potential components for an intervention tailored for people with mild or pre-frailty. Current interventions for pre-frailty lack developmental input from service users or other stakeholders. Future interventions may benefit from this.
-
From observational studies, nutrition, including ensuring the use of vitamin D supplements (as per standard guidance for older people) and addressing low weight/weight loss, may be an important component of an intervention.
Using behavioural science to develop a new complex intervention: an exploratory review of the content of home-based behaviour change for older people with frailty or who are at risk of frailty
The effects of changing health behaviours to prevent frailty are mixed,12,48 which could arise from ineffectiveness in achieving behaviour change or the ineffectiveness of the behaviour change upon the outcome. Health behaviours can be defined as activities that may contribute to disease prevention, disease or disability detection, health promotion or protection from risk of injury. 49 Behaviour change interventions typically contain multiple interrelated components and so active ingredients may vary depending on the intervention target, the number of behaviours targeted and the amount of tailoring required. 50 Using behaviour change theory provides a systematic approach to identify a full range of mechanisms of change and potential causal associations. 50 To our knowledge, no previous review has described the behaviour change strategies used for health promotion in frailty and their impact on behaviour change and clinical outcomes.
Intervention functions
Michie et al. ’s51,52 behaviour change wheel and behaviour change technique (BCT) taxonomy identify and classify the content of interventions aimed at changing behaviour. Their systematic review of behaviour change frameworks identified nine intervention functions (broad, non-exclusive categories of means by which an intervention can change behaviour), namely:
-
education – increasing understanding or knowledge around the behaviour
-
persuasion – inducing positive or negative feelings or action through communication
-
incentivisation – creating the expectation of a reward
-
coercion – creating the expectation of cost or punishment
-
training – teaching skills
-
restriction – reducing the opportunity to engage in a target behaviour or reducing competing behaviours to increase engaging in a target behaviour, using rules
-
environmental restructuring – changing the social or physical context for a behaviour
-
modelling – providing an example for people to imitate or aspire to
-
enablement – reducing barriers, or increasing means, to improve capability or opportunity for a behaviour (beyond education, training or environmental restructuring).
Behaviour change techniques
Intervention functions describe broad mechanisms rather than specific ones. As such, the actual active components of effecting the changes needed are described as BCTs. BCTs are the active components of behaviour change interventions that are observable, replicable and irreducible. 52 Michie et al. ’s53 taxonomy of 93 BCTs was developed through a Delphi study in order to facilitate consistent description and replication of behaviour change interventions, as well as define mechanisms of action to use in intervention development and refinement.
Aims
In order to develop a new intervention from a rigorous theoretical basis, we undertook a systematic review to (1) identify behaviour change components used in home-based health promotion interventions in frail and pre-frail older adults and (2) explore how these components may be associated with intervention effectiveness.
This review is summarised in brief here – see Jovicic et al. 54 for the review protocol and Gardner et al. 55 for a comprehensive report of the findings.
Methods
Data sources and search strategy
We searched the following databases from January 1980 to September 2014 (see Report Supplementary Material 2 for search terms), using:
-
MEDLINE and MEDLINE In Process & Other Non-Indexed Citations
-
EMBASE
-
Scopus
-
Science Citation Index Expanded
-
Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, NHS Health Economic Evaluation Database
-
PsycINFO
-
Health Economics Evaluations Database
-
Cumulative Index to Nursing and Allied Health Literature
-
Evidence for Policy and Practice Information Centre Register of Health Promotion and Public Health Research
-
Bibliomap
-
Health Promis and Effective Practice and Organisation of Care (manual search as automated searches were unavailable).
We also used the following data sources:
-
trials register searches – Health Technology Assessment database, the UK Clinical Research Network Portfolio Database and ClinicalTrials.gov
-
backwards and forwards citation tracking of included trials and relevant systematic reviews
-
contacting authors for additional material.
Study selection
Two reviewers screened titles, abstracts and full texts, with disagreements resolved through consulting a third reviewer. We included peer-reviewed English-language RCTs of home-based interventions aiming to change health behaviours delivered in person by a health professional, but not requiring specialist expertise. The population was community-dwelling older adults aged ≥ 65 years who were either frail/at risk of frailty (including those defined as at risk of hospitalisation, with functional/mobility difficulties or aged ≥ 75 years with multiple comorbidities). Nursing home residents and hospital inpatients were excluded. We included interventions compared with no treatment or usual care, assessing behavioural, health or well-being outcomes relevant to frailty.
Data extraction and coding
Two reviewers extracted data into a spreadsheet developed for this review on study, sample and intervention characteristics; risk of bias (coded for descriptive purposes only); and behavioural, health or well-being measures at baseline and first follow-up. Using the definitions in Intervention functions and Behaviour change techniques, we coded behaviours targeted (when explicitly reported), intervention function and BCTs used. Practical, emotional and unspecified social supports were each divided into intervention provider support and family, friend or other caregiver (paid) support to give 96 possible BCTs.
Analysis and synthesis
We examined the relationship between intervention effectiveness and behavioural components. We classified outcomes into behavioural (behaviours or necessarily contingent outcome, e.g. medication adherence), health and social care use (e.g. hospital admission), mental health and functioning (e.g. depression), physical functioning (e.g. ADL), social functioning and well-being (e.g. loneliness) and generic (not captured by other clusters, e.g. quality of life). We defined an outcome as showing evidence of potential effectiveness when there was a statistically significant (p < 0.05) between-group change favouring the intervention in at least one outcome in the cluster. Intervention components were deemed to show potential effectiveness where the component was present in more effective than ineffective interventions (i.e. more than half of studies).
Results
Out of 1213 unique references identified from database searches and citation tracking, we screened 267 full texts and included 19 full texts describing 22 interventions (see Gardner et al. 55 for flow diagram). We excluded 248 full texts, largely attributable to an irrelevant population (n = 201).
We included 19 studies of people who were frail or at risk of frailty: 16 RCTs,12,13,56–69 two clusters RCTs48,70 and one pseudocluster RCT71 (see Report Supplementary Material 3 for study characteristics). Most (n = 16) compared one intervention to control, with three three-arm trials. Overall, trials were fairly good quality. All were rated as having a low risk of bias on four out of seven criteria and three were rated as having an overall low risk of bias. Interventions largely took the form of a personalised assessment, focused on care or health needs (n = 15), compared with usual care or no treatment (n = 18) and delivered by nurses (n = 21/22). Some studies included more than one intervention. A small number of studies included other professionals such as physiotherapists or social workers. Physical health and functioning outcomes were most commonly assessed (n = 19), followed by mental health and functioning, health and social service use and generic health and well-being (n = 11). Social functioning and well-being was assessed in seven12,57,64–66,70,71 studies and behavioural outcomes in four. 58,65–67
Behavioural targets
Regarding behaviour, only three reported using behaviour change theories. 48,63,64 Most interventions (n = 11/22) targeted a single behaviour,12,61–64,66–68,70,71 while nine targeted two or three behaviours13,56–59,65,69 and two targeted four or more behaviours. 48,60 The behaviours most commonly targeted were medication adherence or management (n = 16),13,48,56,58–61,63–66,68,69 physical activity (n = 11)12,13,48,56,57,59,60,65,70 and diet (n = 8),13,48,56,57,60,69,71 with one or two studies addressing areas such as alcohol, sleep etc. Targeting a particular behaviour did not appear to be associated with positive results for any outcomes (Table 4).
| Number of trials out of total showing evidence of effectiveness (n/N) | ||||||
|---|---|---|---|---|---|---|
| Physical functioning outcomes | Behavioural outcomes | Health and social care use | Mental health and functioning | Social functioning and well-being | Generic health and well-being outcomes | |
| Behaviours targeted | ||||||
| Dietary consumption | 3/7 | – | 1/4 | – | – | 2/5 |
| Medication adherence or management | 5/13 | – | 2/9 | 2/7 | – | 3/8 |
| Physical activity | 3/11 | – | 1/5 | – | 0/4 | 2/5 |
| Intervention functions | ||||||
| Education | 5/6 | – | – | – | – | – |
| Enablement | 7/13 | – | 2/6 | 2/5 | – | 3/9 |
| Environmental restructuring | 2/5 | – | – | 1/4 | – | – |
| (None identified) | 1/5 | – | – | 1/6 | 0/3 | – |
| BCTs | ||||||
| Adding objects to the environment | 3/5 | – | – | – | – | – |
| Goal-setting (outcome) | 4/9 | – | – | 3/6 | 1/5 | 1/4 |
| Instruction on how to perform the behaviour | 3/4 | – | – | – | – | – |
| Monitoring of the behaviour by others without feedback | 2/4 | – | – | – | – | – |
| Monitoring of the outcomes of behaviour by others without feedback | 3/12 | 2/4 | 1/10 | 2/9 | 1/7 | 0/6 |
| Restructuring the physical environment | 3/5 | – | – | – | – | – |
| Social support from intervention provider (practical) | 5/10 | – | 2/5 | 2/4 | - | 3/6 |
| Social support from intervention provider (unspecified) | 4/11 | – | 2/9 | 2/7 | 1/6 | 0/7 |
Intervention functions
Intervention functions included:
-
enablement (n = 16/22) – increasing older adults’ means or reducing barriers to increase their capability (above and beyond education or training) or opportunity (above and beyond environmental restructuring) to undertake a behaviour
-
education (n = 7/22) – increasing knowledge or understanding
-
environmental restructuring (n = 4/22) – changing the context for a behaviour (including physical and social)
-
training (n = 2/22) – imparting skills
-
persuasion (n = 2/22) – inducing or stimulating positive or negative feelings or actions through communication.
No intervention used any other function. Functions could not be identified in five interventions. 12,66,68,69,71 More studies including education and enablement had positive results for physical functioning outcomes than those that did not including education and enablement (see Table 4); however, in other studies, all functions had less than half of the studies showing positive effects.
Behaviour change techniques
A minority, 21 out of 96 possible BCTs, were identified in at least one intervention (median 4.5, range 1–9). Social support (practical and unspecified) and monitoring outcomes of behaviour without feedback were used most commonly; however, only adding objects to the environment and instruction on how to perform the behaviour had a majority of studies with positive findings (see Table 4).
Key findings
-
Studies rarely assessed behavioural outcomes, used explicit theoretical approaches or reported intervention components clearly.
-
No single BCT or intervention function showed potential for outcomes other than physical functioning. Studies rarely measured specific behavioural outcomes.
-
Targeting a particular behaviour did not appear to lead to improvements in physical functioning.
-
No intervention functions or BCTs appear to be uniformly effective, but education and enablement and adding objects to the environment and instruction on how to perform the behaviour were more promising.
Implications for intervention development
In the light of the findings from this review, a new intervention for early frailty should be explicit about its theoretical basis, with clear development and definition of intervention components. Within a trial, behavioural outcomes (e.g. physical activity) should be assessed in addition to clinical outcomes in order to explore whether or not behaviour change led to better outcomes. The most promising components were the intervention functions education and enablement, which showed the most promise for improving physical functioning (a key frailty indicator), and the BCTs ‘adding objects to the environment’ and ‘instruction on how to perform the behaviour’. We found insufficient evidence to support the omission of other intervention functions or BCTs at this point.
What can we learn from effective single-domain health promotion interventions with frailer older populations? ‘State-of-the-art’ scoping review of systematic reviews
Our systematic reviews as outlined above located only limited evidence as to what components should be included in a multidomain health promotion service tailored specifically to those with mild or pre-frailty. In order to maximise the potential for intervention effectiveness, we scoped the evidence on single-domain home-based health promotion approaches that might work with older frailer populations more generally (e.g. those at risk of hospital admission), including six key domains: (1) exercises/physical activity, (2) falls prevention, (3) nutrition and diet, (4) social engagement and addressing loneliness, (5) mental health (depression and anxiety) and (6) memory (cognitive stimulation/memory adaption strategies in those with normal cognition or mild cognitive impairment). The purpose of this review was to identify evidence on which domains have the best evidence for inclusion in a new multidomain intervention.
This was not part of our original protocol and given time constraints it was not possible to conduct full systematic reviews in each of these areas. State-of-the-art reviews rapidly summarise the quantity and main characteristics of the evidence in a topic area, include comprehensive searches to identify the most relevant current evidence, followed by narrative synthesis without formal quality assessment. 72 Therefore, we conducted a state-of-the-art review using systematic search methods to identify key systematic reviews within each of the domains or topic areas listed above, to inform the development of the intervention. To rapidly appraise the evidence in each key area, we included only the strongest forms of evidence: systematic reviews of RCTs or, when the field was extensive, overviews of systematic reviews.
Objectives
To identify key systematic reviews of home- or community-based health promotion strategies, delivered by non-specialists, in the following domains and to extract key messages to inform components to include in a new health promotion service for mild frailty:
-
exercise/mobility/physical activity
-
falls prevention
-
nutrition and diet
-
social engagement
-
mental health
-
memory.
Methods
In the light of the paucity of studies focused on older adults with mild frailty, we took a broader approach to including reviews that could provide relevant evidence on which to base intervention components. Our inclusion criteria were:
-
Community-dwelling older adults who could be defined as ‘at risk’ or frailer than a general population (e.g. non-healthy, at risk of falls or hospitalisation, with frailty).
-
Interventions that could be delivered by non-specialists with or without minimal training, in the light of emerging findings from our qualitative work suggesting that a non-specialist support worker would be the most suitable professional (see Chapter 3, Person to deliver the service).
-
Any control treatment.
-
Outcomes relevant to each area of intervention (e.g. depression, social isolation, strength, frailty).
-
Systematic reviews of RCTs or overviews of systematic reviews. As we aimed to synthesise the most up-to-date evidence, we excluded earlier reviews that had been updated and superseded.
We searched three key databases (MEDLINE, EMBASE and Scopus) from inception to April 2015 (see Report Supplementary Material 2 for search terms) and one reviewer screened titles, abstracts and full texts and extracted key data from reviews meeting our eligibility criteria in each domain. Two reviewers then identified the main findings of relevance to the development of our new service from each review, with reference to the original articles. As this was a state-of-the-art review, quality assessment and formal synthesis were not carried out. 72 We summarise the key findings from the included reviews that informed intervention development below.
Key findings of relevance to design of our new intervention
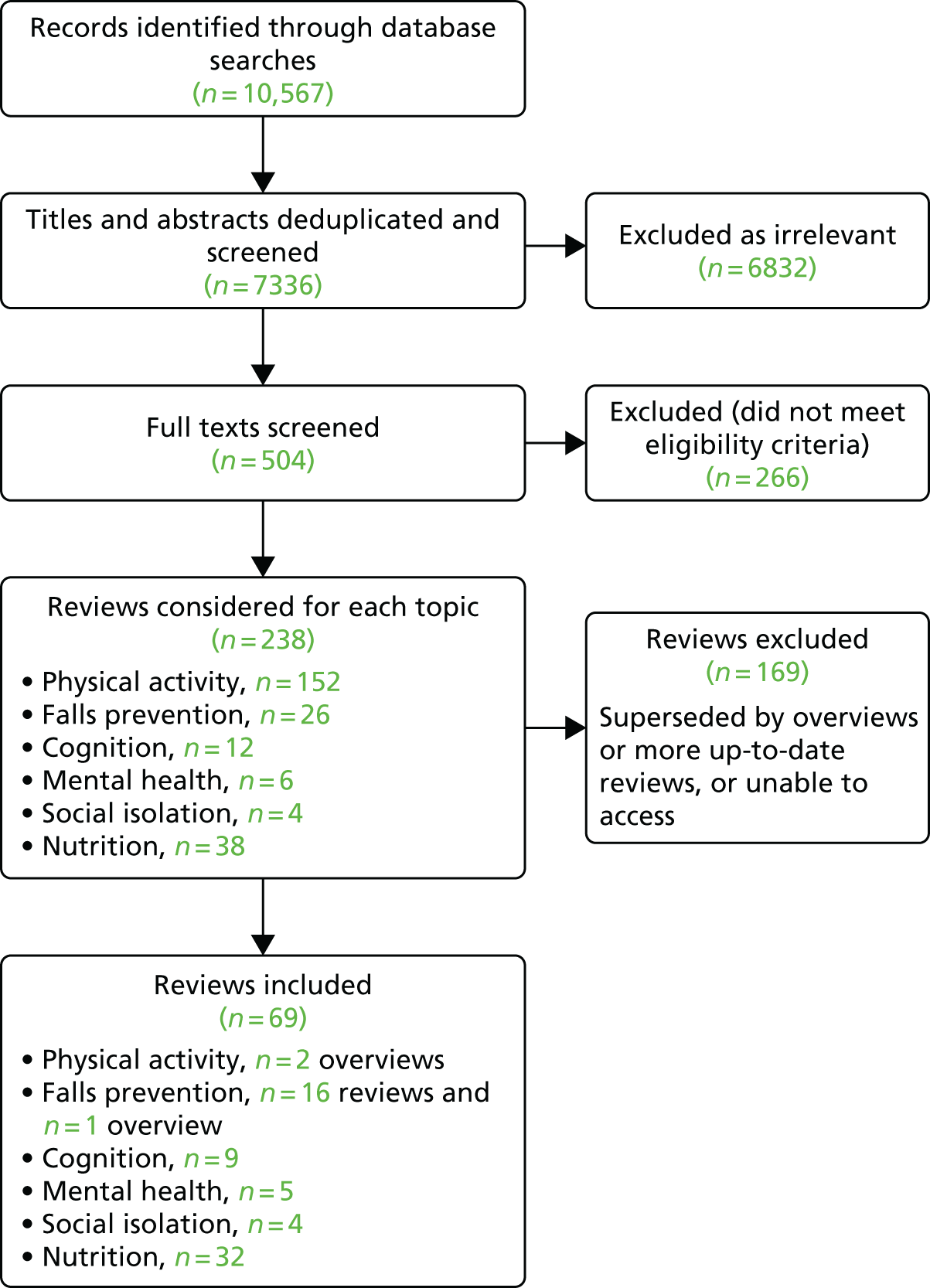
We extracted evidence from 66 reviews and three overviews of systematic reviews (Figure 2). We found the largest evidence base for physical activity and falls prevention (178 systematic reviews identified, including three overviews of reviews used for evidence extraction) and nutrition interventions (38 reviews), with limited evidence for social and mental health interventions and very limited evidence for cognitive interventions. Some reviews contained a mixture of systematic reviews, RCTs and observational studies.
FIGURE 2.
The PRISMA flow diagram of studies included in the state-of-the-art review.
Physical activity and falls
From two overviews of reviews encompassing evidence from numerous (n = 152) previous systematic reviews on exercise/physical activity interventions and other falls prevention reviews (containing 11–159 trials), we found moderate evidence that exercise, physical activity and falls prevention interventions can have positive effects on balance and physical functioning, but not in those with cognitive impairment. 73–76 We also found evidence that targeted supervised home strength and balance exercise programmes plus walking practice, delivered by a trained health professional, can prevent falls. 77 Untargeted group exercise (particularly balance) may also prevent falls. Combined group and individual approaches are most effective. 78 Individual prescriptions may be more important in frailer older adults. 77
Exercise may improve mental well-being, but the research quality was limited. Older adults should be encouraged to exercise at low-to-moderate intensity and walk for leisure. 79 Exercise does not appear to have an effect on depressive symptoms. 80
From 16 reviews including 11–159 trials and one overview regarding falls prevention,73–76,78,80–90 we found that exercise programmes assessing risk for falls and managing this, anti-slip devices in shoes, home safety assessment and modification and training in walking aid use, may help prevent falls. The strongest evidence related to exercise, which reduces the risk and rate of falls in community-dwelling older adults,73,75,78,81–85 though some authors advocate caution with using exercise interventions in frailer older adults. 81 Home assessment and modifications appear to be effective,84,86,87 especially in frailer older adults or previous fallers. 78,81
Falls risk assessment and management appears to be effective. 81,83 Falls prevention programmes may reduce falls risk by up to 10% and may also reduce the fear of falling and falls-related injuries;75,76,80,85,88–90 however, results are inconclusive in cognitively impaired older adults. 74 Walking aids should be correctly sized and adapted, recommended by a health professional and only used when necessary with training. 78 Fall-related injuries may be reduced by strength and balance training, vitamin D and calcium and hip protectors. 87
There was insufficient evidence to support the use of education alone or cognitive–behavioural therapy (CBT), and mixed evidence regarding vitamin D and calcium supplementation for falls prevention. 81,84,86,87
Social isolation and loneliness
We found limited evidence (four reviews, including 11–32 studies, both RCTs and quasi-experimental designs) summarising interventions for social isolation and loneliness. The most effective interventions were those that included a group component, particularly in which the older person was an active participant91–94 and those with a theoretical basis. 92 There was mixed evidence for home visits and befriending services93 and little evidence to support the use of telephone or internet support interventions. 93,94
Mental health
Five reviews including 5–69 studies assessed mental health interventions. 95–99 All reviewed interventions for preventing major depression in older adults with subthreshold depression symptoms (sometimes also including general older adult populations). There was evidence to support the use of psychotherapy interventions for preventing depression. 95 The effect of psychosocial interventions appeared to be weaker but still significant for depression and positive mental health;96,97 however, when evaluating intervention subgroups, only social activities had significant effects on depression,96,97 a component also highlighted as increasing the effectiveness for older people in another review. 98 Interventions using behavioural methods only98 or lasting < 3 months97 may be less effective in older adults.
Memory
Ten reviews including 7–35 studies assessed memory training strategies,100–109 but two of these were updated in a later review. The research was largely laboratory based and contained mixed evidence of varying quality. Reviews suggested that for healthy persons and persons with mild cognitive impairment, memory training can improve the type of memory being trained but that this may not transfer to other types of memory, everyday activities or functioning. 100–107 Strategies to compensate for mild memory loss and targeting learning-specific information were therefore recommended.
Nutrition
Evidence for nutritional advice and education interventions was mixed: positive effects were found in a review of 23 trials on diet, physical functioning and depression, but not anxiety, quality of life or service use. 110 However, within a nutritional counselling review of 15 trials, interventions containing active participation and collaboration with older adults showed greater promise for improving dietary behaviour changes. 111 Certain BCTs, including ‘barrier identification/problem-solving’, ‘plan social support/social change’, ‘goal-setting (outcome)’, ‘use of follow-up prompts’ and ‘provide feedback on performance’ were associated with greater intervention effects across 22 trials of increasing fruit and vegetable consumption. 112 There was evidence in a review of 22 studies that good adherence to a Mediterranean diet may reduce the risk of depression or cognitive impairment. 113 A further review of nine studies of carer interventions for malnutrition suggests these may also be effective. 114
Regarding individual supplements, vitamin D (with or without calcium) was the supplement most frequently evaluated. Twelve reviews including 8–42 trials indicated that vitamin D may reduce falls risk, increase muscle strength and improve function, though evidence was mixed. 115–126 Some individual reviews suggested that vitamin D may be more effective when calcium is added118,124 or in deficient populations,120 while evidence was mixed regarding the effects of dose. 121,122 Long-term supplementation may be required for effects on mortality. 115
Low-fat dairy products may benefit neurocognitive health,127 while protein and energy supplementation in malnourished people recovering from illness appears to reduce complications and hospital readmissions and increase grip strength. 128 Supplements not supported by the literature included multivitamins,129,130 B vitamins,131–133 omega-3 fatty acids134–137 or supplementing older people without malnutrition with amino acids or protein. 138 High doses of betacarotene, vitamin E and vitamin A are likely to be harmful and should be avoided in older adults. 139 The effects of carbohydrates on cognition or daily functioning are currently unclear. 140
Implications from the ‘state-of-the-art’ review
-
Physical activity is a beneficial intervention to improve functioning and reduce falls and should form part of a tailored intervention, with a focus on individualising activity to frailty status and signposting to group classes when appropriate.
-
Although promoting social activities has limited evidence, older people may benefit most from being signposted to local groups with a shared interest they can participate in, depending on their preferences.
-
The most beneficial ways to prevent depression in later life may be signposting to psychological services or encouraging socialising.
-
As there was very little evidence for effective methods to improve memory, this should not currently be included as part of an evidence-based intervention. Strategies to compensate for mild memory loss could be considered.
-
Owing to the supporting evidence for some nutritional interventions, it may be beneficial to include these within a new service.
Policy context: how does a new health promotion service for people with mild frailty fit with current policy and practice?
We completed a narrative health and social care policy analysis141 that investigated the extent to which health and social care policy in England addressed health promotion with older people with mild or pre-frailty, or frailty prevention. The purpose was to understand the policy context in which the intervention we developed could be implemented.
Background
Policy review and analysis helps explain past successes and failures, identify gaps and plan for future reforms. 142 In conducting this policy review we were informed by the work of Walt et al. 142 Our review was framed by theories of public policy as processes including problem analysis, formulation and implementation in which different interests, institutions and ideas interact. 143 These theories include recognition of the exercise of power by different interest groups, including the influence of ageism. 144
Public policy-makers internationally need to address increasing demand for health care and old-age support in the context of a declining labour force. 144 The Political Declaration and Madrid International Plan of Action on Ageing,145 agreed by the United Nations in 2002, addressed three priority areas: older persons and development, advancing health and well-being into old age, and ensuring enabling and supportive environments. Regional implementation strategies have been agreed at ministerial level, to work towards maintaining quality of life and promoting independence, health and well-being throughout the life course, underpinned by policies supporting health promotion and disease prevention. 146
In England, evidence of changing demography and potential impact on public spending costs has been known to governments for some decades and was recently requantified. 3 A ministerial commitment has translated into a range of policies that specify the promotion of well-being for older people as a strategic objective including those for longer working lives,147 for housing,148 and for transport. 149 We now summarise our analysis specifically of health and social care policies for frailty prevention.
Method
The design drew on the interpretative tradition in undertaking a narrative review using a method of documentary analysis. 141 It encompassed policy as created at three levels in the state:143
-
state laws
-
strategies and plans of government-mandated national bodies for the delivery of health and social care
-
government-mandated bodies at local administrative levels for health and social care.
The public policies for inclusion in our review had to be published and/or current to the period of the study (2014–17) and address one of the following:
-
a population of older people (without an age-specific definition)
-
public health and well-being for whole populations including older people
-
publicly funded health and social care services for whole populations, including older people.
Internet searches of government and a representative sample of local government and NHS commissioning websites were conducted in 2015 and updated in 2017. A snowball technique was used to follow linked policies. A total of 79 national level and 78 local level documents were included. Each document was scrutinised for key words of interest, such as ‘older people’, ‘elderly’, ‘frail’, ‘frailty’, ‘health promotion’ and ‘ageing well’. Relevant surrounding text on the problem analysis, the formulation of actions and stated intent as well as absence of attention to the question of interest, was noted. Iterative analysis was discussed within the research team meetings and a final narrative analysis written.
Findings
The findings are reported within the following themes: problem analysis, formulation, solutions and delivery mechanisms.
The policy problem analysis of the ageing population with a changing epidemiological profile and the consequences for society (national and local) was restated at the beginning of every policy document.
State policy solutions included directions for ‘preventative actions’ as one theme for all adults for local authorities,150 the NHS151 and for social care provision. 152 It is worth noting that the term health promotion, which featured in the English National Service Framework for Older People, published in 2001 and re-endorsed by the government in 2014,153 was not evident in relation to this age group. In recent years prevention of ill health has become a priority strategy for the health and social care system in addressing the changing population, as evidenced by policy commitments and subsequent legislation for the public health function within government, local authorities,150 the NHS153 and the care system. 152 For the first time in England, social care policy mandated the promotion of well-being and prevention or delaying the development of needs for care:152 domains previously associated with the responsibilities of the ‘health’ system. The associated guidance provides definitions of primary, secondary and tertiary prevention. 154 This policy shift reflected the wider policy aspiration of integration between the health and social care systems. 155
A shift in the target populations for prevention had also occurred. In 2001, three groups were identified within the older population: the well and healthy, the frail, and then a transition group (i.e. the pre-frail or mildly frail). 153 However, policies reviewed here give little explicit consideration of those with pre-frailty.
Policy solutions and delivery mechanisms
Within current public health policy, the aim for older people is to support prevention, promote active ageing and tackle inequalities while targeting depression, chronic loneliness, winter excess deaths of frail older people, vascular components of dementia and falls. 153 The government-mandated outcomes for the public health, NHS and adult social care in relation to an older adult age group are summarised in Box 2.
Improved older people’s perception of community safety.
Prevention of social isolation.
Prevention and improvement of excess weight in adults.
Improvement of the proportion of physically active and decrease in inactive adults.
Prevention and improvement in smoking prevalence – adults (> 18 years).
Prevention through uptake of national screening programmes (breast cancer screening for women aged 50–70 years, bowel cancer screening for men and women aged 60–74 years, and abdominal aortic aneurysm screening for all men aged ≥ 65 years).
Prevention through population vaccination coverage – flu (aged ≥ 65 years), shingles (70 years).
Prevention in premature mortality in those aged < 75 years from all cardiovascular diseases (including heart disease and stroke), cancer, liver disease, and respiratory diseases.
Prevention and reduction of excess mortality in adults aged < 75 years with serious mental illness.
Prevention of sight loss caused by age-related macular degeneration.
Prevention of injuries because of falls in people aged ≥ 65 years.
Prevention of hip fractures in people aged ≥ 65 years.
Prevention of excess winter deaths, with particular attention to those people aged > 85 years.
Improvement in the proportion of older people (≥ 65 years) who were still at home 91 days after discharge from hospital into reablement/rehabilitation services.
Improving health-related quality of life for people with multiple long-term conditions, and their carers.
In government health service policy, the term frail older people was only used in relation to improved integration of services for those most vulnerable, particularly for those with long-term conditions. 157
The mechanisms for policy delivery for prevention for older people are described in state and national agency policies as described in Box 3.
-
Preventative actions for older people included in all local authority areas of responsibilities (e.g. safe neighbourhoods, leisure and housing). 152
-
Voluntary sector creation of community agent roles and community groups to prevent social exclusion in those aged > 60 years. 151
-
Local authority duties to provide or arrange for resources to prevent, delay or reduce individual’s needs for care. 154
-
The NHS Health Check programme funded by the public health function of local authorities aimed to prevent heart disease, stroke, type 2 diabetes mellitus and kidney disease, and raise awareness of dementia for those aged 40–74 years. 158
-
The provision of primary prevention (e.g. recording smoking status in public health element of the Quality and Outcome Framework of general practice contracts). 159
-
The provision of a named and accountable GP for all those patients aged > 75 years,160 with an accompanying responsibility to provide a health check.
-
The proactive care programme as an enhanced service element of general practice contracts161 aimed at preventing hospital admissions in the frailest older patients.
GP, general practitioner.
The extent to which the preventative actions address those with mild or pre-frailty is debatable. The NHS Health Check is primarily a public health programme to reduce long-term condition risk, such as cardiovascular disease, in a younger population. 162 The provision of a health check for those aged > 75 years did not specify the activities or suggest that it presented the opportunity for prevention in those with mild frailty. The proactive care programme161 was targeted at the frailest with no consideration of those with mild or pre-frailty.
The extent to which the other mechanisms listed in Box 3 were visible in local strategies in 2015 varied. Most were described in more overarching language. A third of the nine local areas’ Joint Health and Wellbeing Strategies that we examined contained no specific priorities for older people. This was replicated in 2017 when we reviewed the corresponding areas’ Sustainability and Transformation Plans. All had priorities for preventative activities, only four specifically mentioned these with the older population. Four others discussed objectives in relation to frail older people but in relation to the medically unwell.
Policy iterations and outcomes
Quantifying the implementation and impact of preventative measures is challenging. 163 There has been no specific published evaluation of impact for this group. Explanations for this could be the inherent localism in implementation or a pervasive ageism resulting in lack of due attention. 164
In those delivery mechanisms without specified public funding, such as community agents, it is hard to judge the extent of implementation and outcomes. Those with public funding have some published evaluations. The NHS Health Check has had greater take-up by those aged 60–74 years than those < 60 years,165 but it is not aimed at preventing frailty or likely to include large numbers with mild frailty. The Proactive Care Programme focused on the frailest and by 2015 only 410 general practices of the 7841 in England were not providing this service. 166 Some local Clinical Commissioning Groups also promoted preventative activities with those with mild frailty. 167 The general practice contract for 2017–18 has changed. 168 The Proactive Care Programme has been replaced with a requirement for all general practices to identify and focus clinical attention on those with severe frailty. There are no explicit specific health promotion or prevention components to this contractual requirement.
Discussion and conclusion
This review has analysed contemporary health and social care policy for health promotion for older people with mild or pre-frailty in England. The review is time limited, our searches may not have been complete and the volume of material identified at the local level, and subject to amendments by changing governments, may have been incomplete. We have tried to mitigate this through our iterative processes.
We found that the older adult population was not always identified separately as a policy priority: the extent to which this represented a positive lack of age discrimination or a lack of attention to specific problems of some older people cannot be judged from the documentary evidence alone. Over time, the discourse changed to be more specific regarding prevention of ill health rather than promotion of health and was targeted either at the most frail and at risk of adverse outcomes or to those in mid-life (i.e. an ‘upstream’ public health solution169 and earlier in the life course). We found an absence of policy consideration for preventative actions for those on a pathway to frailty – a population that is predicted to grow in all countries. Publicly funded or supported services seeking to develop health promotion for older people with mild frailty may find it difficult to provide a policy ‘rationale’ amid other priorities. Addressing the individual and societal consequences of adverse experiences of those with the greatest frailty may require that some attention is paid further ‘upstream’ in the life course.
Evidence reviews: discussion
Although previous evidence suggests that interventions aimed at lower-risk populations may be more effective,15 our reviews found only limited evidence regarding interventions targeted at people with mild frailty and no qualitative work specific to this population. The evidence base largely consisted of exercise-based interventions, even when widening the remit to include a broader range of at-risk populations in our state-of-the-art review. Though there was some evidence for the effects of exercise on physical performance and falls prevention, evidence in other areas (e.g. socialising, mental health) was sparse. A focus on physical functioning alone has been criticised by stakeholders, some of whom have advocated addressing cognitive, social and psychological dimensions of frailty. 170
Our behaviour change review suggested that some BCTs and intervention functions as potentially effective components of a new service. There was a notable absence of local and national preventative policies targeted to older people on a pathway to frailty, despite wider European policies aimed at frailty prevention and improvement. 170
The findings from our reviews of the evidence are consistent with other frailty prevention literature. A scoping review of studies aimed at preventing or delaying frailty across robust, pre-frail and frail populations found mixed evidence of clinical effectiveness with a focus on physical activity, sometimes in comparison with a nutrition or cognition intervention. 170 There was some evidence that inclusion of physical activity can lead to reductions in frailty, but mixed effects for geriatric assessment and no effects of home modifications. Exercise may also have positive effects in frailer populations, but the most effective components of this are unclear. 171 Two further RCTs that have been published since our systematic review searches, of l-carnitnine supplementation172 and an exercise and nutrition intervention,173 found similarly mixed effects on frailty symptoms and physical functioning.
In spite of the lack of literature in some areas, we were able to scope and incorporate a large evidence base and identify potentially effective components to include in a new intervention, based on comprehensive database searches. Our behaviour change review included a novel methodology and enabled us to consider how interventions may be effective, as well as whether or not they were effective. The policy review appraised a range of current national and local policies, and included an iterative approach to maximise the documentation available. In combination with the mild frailty systematic review, these highlighted the lack of attention to and evidence for preventative interventions in people with mild frailty.
There were some limitations to the reviews. The first review found very little evidence and, for the evidence we retrieved, there were mixed results across outcomes (see Chapter 2, Results and Synthesis). 23 To our knowledge, this is the first review to focus solely on mild and pre-frail populations, rather than grouping them with frail or robust older adults. However, pre-frailty and mild frailty were defined inconsistently across studies and some possessed stringent exclusion criteria (e.g. history of orthopaedic fracture, mild cognitive impairment) that limited the generalisability of the results to the broader pre-frail population, in which multiple physical and cognitive comorbidities are fairly common.
Within our behaviour change review, we could only summarise clinical effectiveness using vote counting of effectiveness at first follow-up, which did not account for study quality or longer-term effects. Poor intervention reporting hindered assessment of the behavioural content of the treatment-as-usual (TAU) groups within studies. Reporting of underpinning theory of the interventions included was very sparse. In this review, all techniques present in effective interventions were also present in ineffective interventions, albeit to a greater or lesser extent. For the purposes of the review, we made an assumption that the BCTs were the sole active ingredients; however, effects are likely to depend on complex interactions between context, provider, individual, setting and behaviour, which we could not account for. Acceptability, affordability, safety, equity and practicability are also important considerations. 52
Within our state-of-the-art review, the rapid nature of the methodology used meant that we could not appraise the quality of included systematic reviews, but most included reviews discussed the strength of the evidence summarised within them.
In conclusion, the limited evidence base and policy focus for effective health promotion in mild frailty suggest that there is considerable future scope for rigorously developing and evaluating new multidimensional interventions targeted to older people with mild frailty.
Chapter 3 Intervention development with older people, carers and community health and social care professionals
Involving stakeholders in intervention development is paramount to ensure that interventions are relevant and acceptable to recipients and feasible in clinical practice. 20 Most studies that were identified in our reviews had limited or low service user involvement in intervention development. Although much research has been undertaken with general older populations exploring their perspectives on successful ageing,174 we did not identify any qualitative studies of older adults with mild frailty, eliciting their views on promoting health and well-being in the context of experiencing symptoms of frailty (e.g. lack of energy, feeling ‘slowed up’).
Alongside our systematic reviews we carried out qualitative work to explore ‘user requirements’ for a new service to promote health and well-being for older people with mild frailty, from the perspectives of older people, carers and community health and care professionals. We then synthesised findings from the evidence reviews and qualitative work and used the principles of co-design with multiple intervention development panels with stakeholders to develop the intervention, ‘the HomeHealth service’.
Exploring user perspectives on the aims, content and delivery of a new home-based health promotion service for older people with mild frailty
To ensure that the service is relevant, acceptable and feasible, it was important to understand the views of users, including both older people and carers using the service, and health and social care professionals who may be involved in providing or recommending the service. This included the aims of the service, the content, how it could be best delivered and anticipated problems in implementation and how these could be overcome. We used two sources of data to address this: a secondary analysis of data we had recently collected for a qualitative study on healthy ageing and health promotion for older people more generally, the Wellbeing Interventions for Social and Health needs in older people (WISH) study (see Wellbeing Interventions for Social and Health needs in older people study below),175 and a new empirical study exploring the aims, content and delivery of a new service more specifically for those with mild frailty (see Tailoring a new home-based health promotion service for older people with mild frailty: qualitative study of older people, carers and health and social care professionals below). 176
Wellbeing Interventions for Social and Health needs in older people study
The WISH study for health and well-being promotion in later life, funded by the MRC (Life Long Health & Wellbeing G1001822/1), immediately preceded this study (2012–13). The WISH study aimed to assess the feasibility and costs of embedding a computer-supported health and social risk appraisal system in primary care. The computer ‘expert system’ reviewed questionnaire responses to identify needs and provided personalised advice to older people who participated, with systematic follow-up in general practice. The ‘expert system’ was the ‘Multi-dimensional Risk Appraisal for Older People’ system, which included a breadth of health and well-being domains. 177,178
Methods and sample
The WISH study took place in two study sites: an urban and a semi-rural area comparable to the HomeHealth study sites. The methods for the quantitative feasibility study have been reported elsewhere. 175 Community-dwelling adults aged ≥ 65 years were recruited from five general practices. A total of 454 older people completed baseline Multidimensional Risk Appraisal for Older people (MRAO) assessments and received tailored feedback on their health and well-being. At 6 months, 348 (77%) completed follow-up questionnaires. As part of the evaluation, we interviewed a purposive sample of 30 older people. We sampled for diversity in age, gender, education, ethnicity, functional ability and location. Just over half of participants (17/30) were aged between 65 and 74 years, although a spread of older age bands were included (eight people aged 75–84 years and five people aged ≥ 85 years). Around half were male (17/30) and from the urban study site (18/30), two-thirds (20/30) had received only a basic level of education up to the age of 16 years, and the large majority (29/30) identified ethnically as white (white British or white other). In this community-dwelling population, one-third (10/30) needed assistance with one or more IADL (e.g. shopping and cleaning) and 1 in 10 needed assistance with one or more basic ADL (e.g. dressing and washing).
Within the semistructured interviews, we explored views on healthy ageing and identified barriers to and facilitators of health promotion in later life. Interviews were conducted in the participants’ homes and lasted 45–60 minutes. They were audio-recorded, transcribed and analysed for the WISH study using framework analysis.
The WISH study participants described perspectives on healthy ageing and health and well-being promotion in a comparable population to the current study. For pragmatic reasons, we conducted a secondary analysis of these data. 179 The charted data were reread by three members of the research team to identify themes relevant to the intervention development for the new HomeHealth service and to identify gaps in knowledge which could be supplemented by primary data collection in the current study (see Tailoring a new home-based health promotion service for older people with mild frailty: qualitative study of older people, carers and health and social care professionals).
Key findings of importance to service development for mild frailty
Three overarching themes described older people’s views on healthy ageing and health promotion in later life: ‘maintenance as well as change’, ‘recovery as well as decline’ and ‘social connectedness’.
There was a clear message that preventative activities should not focus on change alone, but that maintenance of current health and well-being status was more, or equally, important. Some people had no desire to become ‘healthier’ and prioritised staying as they currently were for as long as possible. To some extent, this reflected the awareness and practise of healthy ageing activities that many older people were already engaged in (i.e. they already felt that they were doing what they could to stay healthy). For others, their current status matched their age-related expectations. A further theme reflected the view that age-related changes were not necessarily about progressive decline; older people described the importance of addressing ‘recovery’ in response to periods of poor health that could be ameliorated, and that health promotion in later life should be able to be tailored to such circumstances.
Psychosocial aspects of healthy ageing dominated the discussions. These included acceptance of and adjustment to changes and maintenance of previous behaviours and roles, including within networks of friends and family, and the importance of staying connected and actively contributing to the world around them:
I think relationships, you know, friends, family, that certainly keeps me going . . . I’m in contact with my former wife and I’ve helped out when the kids needed help, like looking after her; she’s got Alzheimer’s I’m afraid. But that keeps me active and sort of within the family unit, and I think that helps a lot.
Male, 78 years
Facilitators and barriers to the implementation of a new service broadly fell into four key themes: knowledge, attitudes, health status and access, as described and illustrated with quotations in the next sections.
Knowledge
Many older people were already practising ‘healthy ageing’ to some extent but although they reported a good understanding of the broad concepts, there was often confusion or a lack of detail in their knowledge. For example, in terms of nutrition, participants were unclear about what constituted ‘good vs. bad fats’ and recommendations for exercise/activity levels:
I’m not sure about the fat foods and that, you know? I’m never quite sure what. I know it always seems to be the ones I like that I’m not supposed to have! [Slight laugh.] . . . They seem to be changing a bit now, you know?
Male, 77 years
Participants had a good knowledge of local services for health promotion but reported mixed views of these services including role of primary care.
Attitudes
Attitudes towards engagement in new services were shaped by personality and individual differences, and individual expectations for the future, reinforcing the need for tailored interventions. There were polar views on ‘individual expectations for the future’, with some older people wanting early diagnoses and planning for the future, to those who were more fatalistic in their thinking, stating ‘something might turn up’. Many reported that they did not want to live forever so the efforts involved in prevention seemed too much to bother with:
I mean, I don’t know how long I’ve got to live, I’m about to turn 70, just coming up to it, and I think, well does it matter?
Male, 69 years
However, despite this, fears of dependency and cognitive decline were widely reported motivators for action.
Health status
Two key factors were considered important for future healthy ageing: (1) maintaining independence, which included mobility, managing pain and living in one’s own home, and (2) avoiding cognitive impairment (memory loss and dementia). Fear of dependency and loss of cognitive function were motivating factors for engaging in health promoting activity:
Oh, God, don’t . . . just the thought of it. Well I always want to have a clear mind anyway. I don’t want anything, like people sort of have Alzheimer’s and things like that.
Female, 72 years
Other ‘triggers for change’ included changes in circumstances, such as the loss of carer, a health scare or new diagnosis, either for themselves or in others:
And then 6 or 7 years ago, I’ve been diagnosed with diabetes and that changed my life. I’ve changed my diet, I’ve changed my mobility, I am going out, I am walking, I am as much as I can, and that keeps me going.
Female, 70 years
A complexity of physical and mental health needs was reported by many, and prominent topics in these interviews included diminishing physical health, impaired functioning and pain control, and to some extent, symptoms of frailty, such as low energy levels and fatigue:
But also I get very, very tired. For probably about 4 days a week I go to bed in the afternoon and sleep like the dead . . . I can do 2 or 3 days in a row, but then I need to rest.
Male, 71 years
Access
Most participants had access to informal support and these friends and family enabled and supported ‘healthy living’. However, this could work both ways, for example, if something happened to one older person in a couple it often curtailed the other and created a dependency. Several people reported that it was daunting doing things on their own:
At the minute, thank goodness, he’s OK. But if [participant’s husband] got ill and he wasn’t able to do things, then I’d think what am I going to do? . . . I would be in big trouble.
Female, 73 years
Many older people talked about the importance of transport, both the availability of private transport and their confidence in (or lack of) public transport, to enable engagement, as well as the financial considerations of their choices.
Implications for service development
Key messages from the WISH study to inform the development of a new health and well-being promotion service are:
-
Maintenance of current health and well-being status may be as important as change, fitting an assets-based model of ageing. The language used for promoting the service should address this, as a service perceived as being aimed to promote being ‘healthy’ may deter some people from participating.
-
A tailored approach is appropriate to meet the diverse individual needs and expectations for health and well-being in later life. Time should be allowed to enable an understanding of individual circumstances (including their knowledge, attitudes, health status and accessibility concerns) and preferences.
-
A focus on maintaining independence and staying socially connected is important rather than ‘health and well-being promotion’ per se. This includes addressing mobility needs, concerns about memory loss and cognitive impairment, mood, social networks and identifying gaps in informal support, access including financial concerns and transport, and suitability of the home environment. Pain management and clarification of information on specific topics, such as nutrition and exercise, were raised.
Tailoring a new home-based health promotion service for older people with mild frailty: qualitative study of older people, carers and health and social care professionals
Although the views of older adults have been widely canvassed regarding successful ageing (e.g. Cosco et al. 174), health promotion (e.g. Lommi et al. 180, Menichetti and Graffigna181) and lived experiences of frailty (e.g. Birkeland and Natvig182, Lloyd et al. 183), to our knowledge none has focused on mild frailty or offered comprehensive practical recommendations for developing new services in this area. We report our qualitative study in brief here; further detail can be found in the associated paper reporting this study. 176
Aims
-
To explore experiences of current health promotion behaviours and support needs in older adults with mild frailty.
-
To identify potential components for a new home-based health promotion service for mildly frail older people.
Methods
We collected qualitative data from a range of stakeholders, including older people with mild frailty as potential new service recipients, carers as people who may support those involved in a new service, and community health and social care professionals (including home care workers) for their expertise in identifying potential content, training required and to ensure ‘fit’ with existing services. We undertook interviews and focus groups (Table 5), audio-recorded with consent and transcribed verbatim. Table 5 shows the sampling method, sample characteristics and data collection methods for each group. Recruitment of older adults and carers continued until the sample was sufficiently diverse and no new major themes emerged. All participants provided informed consent to participate. Topics explored in the interviews and focus groups are summarised in Table 5 (see Report Supplementary Material 4 for topic guides). We collated the data in NVivo 11 (QSR International, Warrington, UK) and used thematic analysis and constant comparison to analyse the data. 184 Further details of the data collection and analysis process are reported elsewhere. 176
| Views sought | Sampling method | Sample | Data collection method | Topics explored |
|---|---|---|---|---|
| Older people with mild frailty (n = 14) | Postal invitations and opportunistic referrals from one urban (London) and one semi-rural (Hertfordshire) practice to community-dwelling older adults aged ≥ 75 years judged to be ‘vulnerable’ or ‘mildly frail’ on the Rockwood Clinical Frailty Scale,7 sampled for maximum diversity with respect to age, gender, socioeconomic status and ethnicity | We sampled a range of ages between 75 and 94 years, predominantly white British (n = 11) and living alone (n = 9), with a range of educational levels (n = 6 educated up to the age of 16 years) and five receiving pension credits | Face-to-face semistructured interviews in participants’ homes |
|
| Carers (n = 12) | Recruited through asking mildly frail participants if they had someone who supported them that it was appropriate to speak to, and snowballing via carers’ groups in the same urban and semi-rural areas. | Current or former partner, family and other informal carers of people with frailty or dementia | Face-to-face semistructured interviews in participants’ homes (n = 3). Two focus groups (n = 3, n = 6) at carers’ groups |
|
| Community health and social care professionals (n = 27) | Community multidisciplinary frailty teams in the same urban and semi-rural areas, with purposive sampling of professionals who could not attend the focus groups; one urban home care organisation | GPs, geriatricians, nurses, physiotherapists, occupational therapists, social workers, care managers and co-ordinators, home care workers | Four focus groups (n = 8, n = 9, n = 2, n = 8) in their workplaces |
|
Results
Interviews included discussions of current health promotion behaviours, what helped and hindered them in achieving these and, more specifically, around what the content of a new service to promote well-being and independence in older people with mild frailty might include.
Current health promotion behaviours undertaken by older adults with mild frailty
Older people carried out a wide range of lifestyle behaviours, either consciously to promote their health and well-being, for enjoyment or as part of functional activities, including:
-
Mobility exercises, such as walking, specific exercises previously recommended by health-care professionals, avoiding long sedentary periods and for more robust participants, moderately intense exercise (e.g. dancing).
-
Following a healthy diet (described by most participants as a variety of meat, fish, fruit and vegetables), having a good meal at least once a day, avoiding weight gain and keeping energy up. Most followed lifetime habits rather than adjusting their diet to later life needs.
-
Social activities [e.g. meeting friends, shopping, attending social groups and using skype™ (Microsoft Corporation, Redmond, WA, USA)].
-
Employing strategies that they felt improved their memory, such as reading, crosswords, games, watching quiz programmes.
-
Developing ways to deal with low mood, including taking a philosophical attitude, waiting for it to pass, getting on with activities, talking to others, watching comedy.
-
Creating social or occupational behaviours, such as shopping and volunteering (this was mentioned by fewer people).
Older people with mild frailty and carers also reported modifying activities around frailty symptoms (e.g. fatigue and weakness) through pacing activities or arranging them at times when they felt more energetic. Many had ceased or reduced vigorous activities that they felt lowered their well-being (e.g. producing fatigue or pain) and some used assistive devices, for example hearing aids, walking aids or folding trolleys:
We try not to overdo things in the sense that we would try not to have a series of activities on the same day.
Carer 1, partner carer
Many mildly frail participants reported receiving support from adult children or grandchildren with activities such as transport, housework, finances, emotional support and encouraging and arranging professional support. Social support appeared to be highly valued – all older people spoke positively of this help, regardless of whether this was actively sought, more passively received or somewhat reciprocal (e.g. the older person helping with looking after grandchildren):
I can’t lift anything like, but everyday things are fine. If I get any difficulty, I just ring up one of the kids and they come down and help me.
OP12, female, 79 years
Several older people paid for private support services, mainly cleaners, which professionals mentioned only infrequently. Although professionals discussed a wide range of existing local services relevant to older people’s health and well-being (e.g. day centres and exercise classes) that a new service could complement, people with mild frailty rarely discussed or accessed these, suggesting a lack of awareness or desirability.
Barriers to and facilitators of health promotion behaviours
Prominent barriers discussed by many older people and carers included disabilities arising from physical health conditions (e.g. arthritis) or frailty, which affected more active behaviours such as exercise, hobbies and social activities. Those arising from cognitive impairment were also discussed, which was felt to mainly affect socialising:
I don’t think people want to come and see people who have no memory.
OP1, female, 93 years
Key resources for health promotion included a network of social support including partners, family, friends and occasionally neighbours, which was considered by older people to provide vital opportunities to socialise, contribute (e.g. volunteering, caring for grandchildren) or seek support to maintain independence (e.g. accompanying out). Adequate transport options (including walking in a good outdoor environment, taxis, driving and buses) were equally considered as vital to maintain freedom and independence and facilitate activities such as shopping or socialising:
Well, [husband] does every . . . thing! He does the shopping, he takes me to my hairdresser, hopefully! . . . thank heavens I have my partner. Oh, don’t you dare precede me to departure!
OP6, female, 86 years
Professionals, carers and several older people considered a lack of knowledge of local services and activities and the skills needed to access these (including computing, form-filling) to be crucial barriers to health promotion:
We didn’t have a ‘disabled’ badge [for parking]. I mean, you probably have to jump through hoops to get one of them, I’m not even sure how you’d go about it.
Carers focus group 1
A number of factors affected motivation for health promoting activities. There was an expectation across all older people of some physical, social and cognitive decline. However, though many tried to consciously accept this, there were strong fears of further decline and dependency that were compounded by fears of vulnerability to abuse and predominantly negative perceptions of social care. Consequently, behaviours which were associated with either independence or a sense of self identity developed over their life course were more likely to be undertaken than behaviours people associated with ‘being elderly’ or ‘giving up’. This varied across individuals.
I do try and keep myself active and not be a person that’s sitting in the armchair all the time, because I’m not that sort of person; I like to anticipate life.
OP14, female, 85 years
A positive mood facilitated problem solving and undertaking activities for most older people. Low mood and the effects of bereavement and loss were generally discussed in the third person as a possible barrier, though some people reported dealing with this by carrying on regardless and felt that low mood resulted from, rather than caused, an inability to do valued activities.
But [friend] gets very tearful, sometimes I go round and she gets really tearful; she says, ‘Remember what I was like!?’. . . and I do.
OP10, female, 78 years
The indoor environment (e.g. heating, stairs) and finances (e.g. for food and transport) were only discussed briefly by a few stakeholders.
Developing new service content
There was widespread stakeholder consensus that the domains covered by a new health promotion intervention would need to be tailored to the individual’s situation and preferences. However, there was strong support for focusing particularly on the domains of mobility and socialising:
There are people, many people who are extremely lonely and desperate for somebody to come in so they can talk and talk and talk, and [slight laugh] then there are others who would just like occasional, so they feel like they haven’t lost total contact with the outside world.
OP5, male, 91 years
Stakeholder views regarding mood and memory were mixed, partly as people found it difficult to envisage what support a service could provide:
I’d be willing to speak to someone [about low mood], yes . . . I don’t know what they would suggest.
OP9, female, 83 years
Although many older people felt that nutrition was not an area they would need support in, some carers and many professionals felt this was an important area to address:
I feel that I don’t know what they can say to me. My wife cooks and gets fruit every day and we’ve always got food in the cupboard.
OP8, male, 79 years
Accessing online shopping, or Wiltshire Farm Foods, or a food delivery service or something like that, can be very helpful for some people.
Community health and social care professionals focus group 1
Other supplementary topics supported by a small number of stakeholders included providing limited support regarding finances, form-filling, computing skills and environmental adjustments. Only carers felt that medicines management support would be helpful – older people and professionals felt this was within a general practitioner (GP) or pharmacist remit:
If we can make whoever was going in aware of if there was any issues with the medication, who to contact, which are the right services to contact [murmurs of agreement] whether it be the GP [general practitioner] or the pharmacist.
Community health and social care professionals focus group 1
A variety of ways a new service could address the above domains were suggested (Table 6), mainly identified by carers and professionals as older adults found it difficult to comment on this.
| Type of support | What to deliver | Example quotes |
|---|---|---|
| Information provision, education | Basic information regarding: what is normal in later life, health behaviours and options to address problems (e.g. different accessible transport schemes, range of local social groups), available support and how to access this (e.g. equipment) | I still think there’s an awful, awful lot of information out there and it doesn’t get through to peopleOP13, male, 75 years |
| Psychological and emotional support | Support around mood, memory, coping with decline and potential future changes, through social interaction and continuity of the intervention over time | You can’t always say what you’re feeling and to talk to someone else maybe, you know, it puts your mind at ease and that’s OKOP12, female, 79 yearsAdapted from Frost et al.176 This is an open access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY) license, which permits others to distribute, remix, adapt and build upon this work, provided the original work is properly cited2017The authors |
| Teaching skills | Low-level exercises to support mobility and balance | I think the exercise thing in the early stages is such a good idea, if you can manage to motivate someoneCarers focus group1 |
| Practical support and enablement | Fall prevention planning, providing equipment to assist mobility, assistance in accessing transport schemes | It’s practical stuff, giving that information, like someone to come in and have a look at their home and going. Actually if we take that rug down, that might helpCommunity health and social care professionals focus group 3 |
| Motivation | Ensuring proposed changes are relevant to the person, avoiding dictating what should be changed, building rapport and relationship over time, providing a wide range of information’ focusing on increasing independence | Something like a motivator, and somebody that would perhaps, not in a patronising or a challenging way, but may be able to persuade and encourage people to change behaviours or change health beliefsCommunity health and social care professionals focus group 2Adapted from Frost et al.176 This is an open access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY) license, which permits others to distribute, remix, adapt and build upon this work, provided the original work is properly cited2017The authors |
| Signposting | When higher levels of support were needed (e.g. medication or financial advice, psychological therapies), signposting to local organisations in order to keep the service distinct from others | So it’s not that they’re providing all the services; you know, there are people, like you say, who are coming with need and need a shopping service, but wouldn’t know where to go, where to start withCommunity health and social care professionals focus group 1 |
HomeHealth service delivery
Appointments
The majority of older people, carers and professionals felt that the frequency and duration of appointments needed to be individually tailored according to the health behaviour changes proposed. Carers and professionals favoured an intervention period of at least 3 months, with appointments one to eight times per month lasting 1–2 hours. Older people found this difficult to envisage and largely agreed with a possible structure proposed by the researcher (30 minutes per week over 6 weeks). Continuity of support from the same worker was emphasised as the most crucial aspect of service delivery, to encourage rapport, behaviour change and trust to discuss sensitive topics, though one professional focus group questioned whether this was feasible.
I would say at least a couple of months . . . a lot of people take a long while to learn to trust and to get to know people.
OP11, female, 79 years
There were mixed views regarding the involvement of a carer or family member in the service and although this was favoured by most carers, professionals felt that families could occasionally be a barrier to services or to building rapport. Although few older people commented on this, those who did were largely positive.
Person to deliver the service
Communication skills (e.g. empathy and listening skills) were much more highly prioritised across the vast majority of stakeholders than specific professional expertise or formal qualifications (with the exception of carers, who felt that a doctor, social worker or occupational therapist may be helpful), which were felt by most older people and professionals to be unnecessary or potentially detrimental (e.g. encouraging dependency on the professional and appearing too extreme for current issues):
I don’t think you need O levels [Ordinary levels] or A levels [Advanced levels] or anything like that to do that, as long as you’ve got the people skills.
OP10, female, 79 years
Professionals felt that a non-specialist support worker could be successful if they had general health knowledge, adequate support from local clinicians and a good knowledge of local services. Most people with mild frailty expressed preferences for a mature person with life experience who could relate more easily to their concerns, though some favoured a lively and young person.
Service promotion
Professionals felt people with mild frailty were underserved, but acknowledged potential difficulties in identifying and encouraging them to access a new health promotion service unless people were ‘on the radar’ because of previous events (e.g. a fall). Interest from older people was mixed. Although some were willing to try a hypothetical new service, many felt insufficient need to do so at present, as they had not experienced any significant health changes prompting them to seek help. Although several accessed support, such as private cleaners, this was deemed acceptable and many were afraid that a new service could encourage dependency and a reduced ability to cope. This was perhaps because there was some uncertainty on the purpose of such a potential service:
At the moment, I don’t need any help . . . you know, because it would stop me from coping, which would just make me worse, I think.
OP7, female, 88 years
Older people and professionals suggested ways of overcoming this, such as advertising in local non-health settings (e.g. local newspapers and shops) to increase awareness, using word of mouth and having a positive focus on maintaining health and independence, rather than frailty and decline:
Put it more as a health promotion rather than ‘you’re getting increasingly frail’.
Community health professionals 1 focus group
Maybe you have to sell it to people in a way that at the end of it, you will feel better for what you’ve achieved and not how bad you are.
OP13, male, 75 years
Summary of key findings
Within this qualitative study of older people with mild frailty, carers and health and social care professionals, we found that the service should aim to address a broad range of domains (in particular socialising and mobility) at a low level, delivered over a sustained period through mechanisms such as providing information and psychological support. Stakeholders preferred a single non-specialist worker with good communication skills to deliver the service, rather than a particular health-care professional. A focus on independence was advised to increase uptake of services.
Development of the HomeHealth service
As described in our protocol, based on our existing knowledge and work, we envisaged that a new home-based service would be a brief intervention (e.g. up to six sessions) and could include an initial assessment of a person’s personal strengths and further home visits to support them to maintain these using goal-setting, behaviour change, problem solving and signposting to relevant resources or services (e.g. mobility aids, low vision services). We thought the person delivering the service may have a similar role to a NHS Health Trainer,185 but would require further information regarding the necessary skills, experience and training. We refined and further developed these initial concepts through integrating this evidence with our theoretical approach, and by undertaking a synthesis of this with the evidence extracted from our reviews with the findings from our qualitative work. We then further developed the content and delivery of the service through a series of meetings with stakeholders and service development panels.
Theoretical framework
In addition to the evidence reviews and qualitative work described above, we considered relevant theories on successful ageing and behaviour change that could be applicable in later life. Three key theories were identified a priori to underpin intervention development and delivery:
-
Asset-based approaches, which aim to maximise and maintain health promoting factors and positive capability to enhance self-esteem, self-efficacy, problem-solving and coping,186 with the aim of reducing dependency on services and maintaining reserves and functioning. More commonly used deficit-based approaches may define individuals negatively and focus on addressing their problems.
-
Baltes’ theory, which suggests that successful ageing arises from focusing on maintaining activities prioritised by the older person and their environment (selection), optimising the performance of these (optimisation) and compensating for limitations where needed [e.g. using a walking stick for mobility (compensation)]. 187
-
Capability, Opportunity, Motivation – Behaviour (COM-B): a behavioural model developed by Michie et al. ,51 that states for a person to perform any behaviour, they must have the physical and psychological capacity (Capability), the physical and social opportunity (Opportunity) and the conscious or innate motivation (Motivation). 51 The model incorporates a wide range of constructs at a broader level and avoids prioritising certain factors (e.g. internal factors) over others. 50 Older adults with mild frailty are likely to encounter a variety of physical, psychological, social, environmental and motivational barriers to maintaining their ‘assets’ over time. COM-B provides a framework to consider these and can link to intervention functions and specific BCTs to address them.
In our evidence reviews we found that few interventions had explicitly described the theory that underpinned their approach. Our qualitative work in both the WISH study and the new empirical work with older people with mild frailty and health-care professionals strongly supported an asset-based approach, with selective goal optimisation and compensation. A range of factors within capability, opportunity and motivation domains was also identified as potential barriers to engagement, and supported an approach and structure that could consider these. Our findings were therefore supportive of incorporating these three theories in the design of the service.
Synthesis of key points from evidence reviews and qualitative work
We summarised the key points from the evidence reviews and qualitative work, and integrated these with our theory into a proposed framework for key components of the new service.
Key points from evidence reviews
-
Broader, multidimensional interventions targeted to mildly frail populations have not previously been assessed.
-
An explicit theoretical basis for behaviour change should be included within a new intervention.
-
A new service should include the intervention functions education and enablement and the BCTs adding objects to the environment and instruction on how to perform the behaviour.
-
No intervention functions or BCTs should be explicitly omitted at this point.
-
A new service should include the following domains:
-
Physical activity and exercise, to improve mobility and reduce falls. Exercise should be individualised, with signposting to group classes where necessary.
-
Support to encourage socialising.
-
Exploring mood and signposting to psychological services or encouraging socialising to improve mental well-being.
-
Nutritional advice/education, particularly relating to vitamin D and protein and energy intake. Other dietary supplements should not be advised.
-
Memory concerns should be addressed (e.g. signposting to GP memory assessments/memory services and strategies to compensate for milder cognitive impairments, for example use of memory aids); however, cognitive training should not be included in a new service as there is currently limited evidence for effective ways of improving this that have an impact on functioning.
-
Key points from qualitative studies
-
The service should cover a broad range of domains and be tailored to the individual. Socialising and mobility are the key areas to address, but aspects such as mood, memory, finances, form-filling, computing, nutrition and environmental adjustments may be important for some older people. Medication advice should not be within the remit of a new service.
-
The service should support the older person through providing information, practical and psychological support, building motivation and signposting to existing services.
-
The service should have continuity of a single support worker over a sustained period of time. A health professional qualification appears to be unnecessary to deliver this service and could be detrimental, but communication skills are vital.
-
To increase the uptake of a new service, it should be promoted in a way that emphasises independence, targets domains valued by older people and focuses on enabling individuals to carry out behaviours that improve their well-being and preserving assets.
Summary of core intervention components integrating theory with evidence from literature reviews and qualitative studies
-
Home-based ‘brief’ intervention, up to six sessions over up to 6 months.
-
Includes comprehensive assessment followed by individually tailored support/enablement, with goal-setting, monitoring, education, signposting, advocacy roles, with or without provision practical aids.
-
Tailored to individuals, ‘asset’-based maintenance of the things they like to do and overcoming barriers to achieving this.
-
Likely to encompass mobility, muscle strength/resistance training, falls prevention, home environment, nutrition, social engagement, mood, managing money, other (e.g. vision, hearing). Delivered by ‘mature’ non-specialist, non-health professional.
Service development
The intervention development process was conducted iteratively; the next phase included a series of individual meetings with key local stakeholders and three service development panels. The draft service outline developed in the earlier stages was debated and further refined in order to maximise the relevance, acceptability and feasibility from the perspectives of both potential recipients and service providers/commissioners working with this group.
Consultation with key stakeholders
To ensure that the new preventative service for older people with mild frailty would align with existing local services and structures, we arranged a series of one to one meetings with key stakeholders in the two study areas, including commissioners for older people’s services and managers and practitioners working across health, local authority and voluntary sectors. Although the new service was conceptualised as a NHS service, commissioners highlighted that the potential breadth of its remit meant that it could be funded from an integrated health and care budget, and delivered through third-sector providers. Commissioners shared useful information on contemporaneous local projects and services for their community-dwelling older population, both to help identify eligible older people, for example, implementation of the electronic Frailty Index (eFI) in GP practices, and to prevent any duplication or conflict with existing services for frail older people in each area.
Managers and practitioners were local frailty leads, from the Clinical Commissioning Groups and local authorities and third-sector providers of the local Care Navigation service (London, UK) and local Age UK providers of services, including dementia support workers. They clarified the eligibility criteria and remit of their services, shared information on the job specification, recruitment and caseload of their workers in similar fields (e.g. care navigation, complex case managers and reablement workers), discussed opportunities for sharing information on local resources and staff training, potential colocation and supervision of HomeHealth support worker(s) and referrals both during and after the end of the new service being developed. The discussions were important in terms of the practicalities of service delivery and helped to raise the profile of the new service and establish good local working relationships.
Service development panels
The purpose of the service development panels was to invite comment and debate within groups of key stakeholders and to flesh out and refine the new service under development. Facilitated group discussions share similarities with focus groups as a useful method to encourage participants to share experiences and elicit views that may be harder to express on a one-to-one basis, for example more negative views. 188 We asked for constructive criticism from panel members. Both separate and mixed panels were held for public representative groups to allow the research team to present information already gathered in a tailored manner, for example, using appropriate language, and to create an environment that allowed participants to speak freely. This was particularly important for the panel of older people with mild frailty, who had no prior experience of this type of group working.
Participants and settings
Three intervention development panels were held sequentially with the following groups. Panels 1 and 3 were co-hosted with Age UK London.
Panel 1: older people with mild frailty
Using their contact lists of service users of Age UK-provided services for older people (e.g. day centres) and residents of a sheltered housing facility, Age UK London identified and invited older people with characteristics similar to those who would be eligible to receive the service being developed (i.e. those who were largely independent but needed some help with IADLs or who were beginning to develop symptoms of frailty, e.g. feeling easily tired). Assistance with transport was available. Panel 1 was held in the common room of a sheltered housing facility from which several participants had been recruited. Participants had not previously engaged in research or other public engagement activities.
Panel 1 included 19 participants, who were predominantly female (n = 14), spread across older age bands (≤ 75 years, n = 9; > 75 years, n = 10), mainly British or Irish (n = 13) and with a self-identified disability (n = 11).
Panel 2: mixed public representatives and professionals
A multidisciplinary, cross-sector group was convened for panel 2, including frontline practitioners from health and social care, commissioners, policy-makers, public health/local authority and voluntary sector professionals, public and lay members, academic experts and Study Steering Committee members. Individuals were recruited using snowball sampling and internet-based searches to identify key informants from the two study sites and representatives from key national organisations. A certificate of attendance for Continuing Professional Development was given. Panel 2 was held at the central London campus of University College London, London, UK.
Panel 3: Age UK public representatives
A third panel was convened, to which Age UK London again identified and recruited participants, who brought expertise as older adults as well as, for some, being representatives of voluntary sector organisations working with older people with mild frailty. The 15 panel members were predominantly female (male, n = 5; female, n = 10) and from a diverse range of ethnic backgrounds (white British/white other, n = 7; black and minority ethnic, n = 8). In contrast to panel 1, participants in panel 3 had previous experience of being involved in research as public representatives. Panel 3 was held at Age UK London’s offices.
Service development panel meetings
All three panels were task orientated and interactive, with small group work that was led by facilitators.
In panels 1 and 2, tables were arranged in a ‘cabaret style’ with five or six participants and one facilitator per table. An outline of the potential content and delivery of the new service (as summarised in Synthesis of key points from evidence reviews and qualitative work), an overview of the associated evidence, and a potential example ‘person specification’ written as a job advert for the worker delivering the service, were presented to the whole group. This was interspersed with small group work which focused on service delivery, rather than its theoretical basis or overarching content. Views were sought on how the content could be refined to meet the needs of the target population and the skillset required of the person delivering the service, including education/qualifications, experience/knowledge and skills/ability. Vignettes of potential service recipients were used to aid discussion as necessary.
The third panel meeting focused in particular on motivating people to use the service and on the tailoring of study materials and service leaflets with this in mind. Motivation for older people with mild frailty to take on new activities was identified as a key issue in our qualitative work and the language used to describe the service was identified as a key area to address in more detail when this topic was raised in panels 1 and 2. Participants were asked to imagine that they had been identified as eligible to receive a new NHS service and take part in a research study by their GP. They were asked to review and comment on the draft covering letter, reply slip, patient information sheet and summary leaflet describing the new service.
All three groups were asked for their thoughts on the name of the new service and the person who would be delivering it. Panels were held as half-day meetings with refreshments and lunch provided. Demographic data were collected for panel 1 participants, a feedback form was given inviting further comments and providing research team contact details and the opportunity to discuss any of the issues raised further, as well as comments on the workshop itself. Meetings were not audio-recorded but contemporaneous field notes were taken by the researchers/facilitators during and after the meetings.
Outcomes
Service development panels 1 and 2
The findings from panel 1, the older people with mild frailty group, and panel 2, the mixed professionals group, are presented together to highlight the similarities and differences in their views.
Relevance and content
There was a consensus across the panels that the new service could usefully fill a gap in preventative care services and, in terms of content, the areas proposed were considered appropriate. Additional detail was suggested on the scope and delivery of some components as follows:
-
Administrative support (e.g. form-filling, how to deal with backlog of post/paperwork, managing finances, for example, arranging a bank appointment to set up direct debits/standing orders).
-
Information technology support (for computers and tablets, use of skype or e-mail, assistance with setting up/using on-line shopping to allow individuals to maintain control over their shopping choices).
-
Sensory impairments (routine enquiry about hearing and vision).
-
Pain (routine enquiry in particular if affecting function).
-
Medication management (help in making an inventory of stored medicines).
-
Support for accessing services (e.g. maintaining/cleaning the home and transport in particular for GP/hospital appointments).
-
Counselling/listening/social support (the opportunity to have a proper conversation for those with limited social support, the appointment length should allow time for ‘small talk’ to help build rapport, potential to discuss more sensitive issues that may be difficult to raise with other services, comparisons with advocacy services).
Boundaries of the service and safeguarding
There was debate that although the service should be person-led with potentially no subject outside the area of discussion, it did need to have clear boundaries so that clients understood the remit of what the service could provide and when it was appropriate to be signposted to other services. For example, for information technology support, the feasibility of on-going support compared with signposting to local information technology courses. For health-related issues, including pain and medication management, clients should be encouraged to talk to their GP/pharmacist for further clarification or care.
There was agreement that GPs should be made aware their patient was receiving the service and many felt that GPs needed to be involved but exactly how was debated. The professionals’ panel suggested that the inclusion of a ‘red flag’ system and using a checklist to determine whether or not referral to, for example, a GP or emergency service was needed. Within discussions about boundaries, the issue of safeguarding was also raised. Providing the service at the client’s home did allow opportunity for a broader of assessment including issues of concern related to the home environment and co-resident family/friends. Clarity about the level of financial support that could be provided was raised both in terms of the knowledge of the worker and sharing of sensitive information. There was agreement that clients should be referred onto specialist services once a need had been identified.
How can we encourage people to use the service?
Identifying what would encourage or motivate people to use a hypothetical new service was more difficult to answer although some useful points were raised in discussion.
There was strong agreement on emphasising independence in later life. Clarity of the remit of the new service was important too; the short-term nature of the service should be emphasised as a ‘positive’ in that engaging with the service would not lead to dependency. The older people’s panel considered that not all older people were interested in engaging with services if they consider themselves to be independent or managing; some felt it was ‘too early’ for them to be engaging with services.
This raised the importance of how the service was advertised. Participants suggested that using ‘positively’ framed language and specific words/phrases including well-being, empowering, motivation for living, healthy ageing, independent living, maintaining, encouraging, support, understanding, resilience, quality of life and the language of reablement, for example ‘getting you back to where you were before’. Participants were also clear about language to avoid, such as ‘help’ or ‘care’ to avoid potential confusion with social care, home care and other services, technical or ‘business speak’, for example, outcomes. In addition, the service should be careful not to be patronising by offering ‘common sense’ advice.
There was agreement on different avenues of advertisement at community venues and emphasising that there would be no cost to the participant was suggested. Professionals suggested additional referral routes via agencies with less scope to provide preventative care to this population such as, at ‘over 75s health checks’, those being seen by falls services, at discharge from hospital, through existing telephone support or signposting services (e.g. The Silver Line, UK) and accepting referrals by relatives, partners, friends and carers. Liaison of the new service with existing care navigation services was important and professionals wanted clarity on how referrals to and from the service would work in practice.
Who should deliver the service and how?
The older people’s panel agreed that ‘person skills’ (e.g. caring, empathy, good communication) and experience of working or volunteering with older people were a higher priority than specific professional skills. Knowledge of how to navigate and access local care and support systems and resources were also thought to be key. Some added that the worker should not be too young and the use of trained peers was debated and supported by some but not all. Participants found it difficult to think of a suitable job title for this role. Independent living officer/worker was debated but discounted because of the confusion with support for younger people with disabilities.
Structure of the service
The older people and professionals panels thought that six appointments may not be sufficient and a maximum of 12 sessions should be considered. In addition, appointments should be longer, in particular the first appointment should be up to 90 minutes to allow time to build rapport and 30 minutes to an hour for follow-up appointments, Most agreed that face-to-face home visits were best, in particular the first session to allow the worker to pick up on additional issues within the home environment. Group appointments and appointments in alternative community venues such as cafes were also considered appropriate if desired by the older person. The appointment frequency should be reduced towards the end of the period of time to avoid an abrupt end to the service.
What training and support would be needed? (Panel 2 only)
The professionals’ panel discussed where the worker delivering the service would be based as well as how a future service might be commissioned given the breadth of remit of the service. Although the service is badged as NHS, it has strong allies with social care and community social work.
The location base of the professional was not considered as important as the level of support the base could offer and options included voluntary sector organisations, integrated community health teams and GP surgeries with support from multidisciplinary frailty teams in the study sites where these exist. It was felt the service should be jointly funded and supervised by health and social care managers. In terms of commissioning, clarity on the outcomes of this service, that is, independence and well-being rather than reducing unplanned hospital admissions, was noted as important.
In terms of training, a comprehensive list was suggested and local courses available to the project team were identified. Specific training on communication skills (active listening, open questions, non-verbal communication) was suggested. Knowledge-based training, in addition to the core components of the intervention, included:
-
mental capacity assessment training
-
dementia, depression and delirium awareness
-
health psychology theory and BCTs
-
motivational interviewing
-
teaching skills on self-management
-
emotional intelligence around loss and age-related changes
-
safeguarding/raising alerts (awareness of procedures)
-
home safety assessment training
-
first aid
-
multiagency working and awareness of what is available in the local community
-
data protection and consent training
-
risk assessment for lone working.
This list highlights the potential breadth of the role with low-level support being provided across a range of topics. Clear professional boundaries would be necessary to avoid this becoming an overwhelming role.
Regular supervision was considered an essential component of the service structure, to be delivered by a trained, senior member of the team. In addition to issues related to knowledge and delivery of service described above, supervision should also offer emotional support given the potential of the service to address difficult and/or sensitive matters.
Service development panel 3
There was a particular focus in discussions on language that could/should be used to engage and motivate older people to use the service and join the study. The main recommendations from panel 3 related directly to the recruitment materials that had been drafted, which explained both the new service and the overall research study. Key points on which there was consensus within the group are outlined here.
Initially, one participant information sheet and summary leaflet had been drafted for potential participants. Panel members found it difficult to separate the research components from the intervention/service. Describing the intervention as a potential ‘new NHS service’ caused some confusion regarding the longevity and availability of the service. It was suggested that separate summary leaflets be written to describe (1) what involvement in the research would mean and (2) who the new service might be appropriate for and what they could expect from it.
They also suggested clarifying where this project ‘fitted’ with existing care, in particular, the role of the GP in the process. As the covering letter would come from the GP, there was some confusion on how active they would be in the project. The role of the worker providing the service needed more information in terms of their remit, accountability and line-management.
Further comments were made on emphasising the tailored nature of the intervention in the covering letter as a ‘selling point’ to potential participants and making the paperwork more eye-catching overall by use of more colour and pictures, while maintaining brevity of content.
Intervention name
Intervention development panels could not reach a consensus on the best name for a new mild frailty service or support worker, with concerns about differentiating it from other services. Following further discussions, the name ‘HomeHealth’ was chosen as a succinct name to reflect home-based health promotion and avoid connotations of dependency or decline. Accordingly, the term ‘HomeHealth Project Worker’ was used to describe the person delivering the service.
Summary
The key learning points from the consultation and intervention development panels are:
-
Confidence in our findings from phase 1 on approach and content.
-
Who should deliver the service.
-
Modified service delivery (e.g. length and number of sessions).
-
Boundary setting and clarity of service remit.
-
Fit to other services/resources: how it can integrate and be implemented.
-
Changes to study materials/leaflets (separating the research from the new service) and the explanations for participants.
HomeHealth: intervention description
Incorporating learning from our comprehensive development process as described above, we developed our logic model for the intervention, a comprehensive intervention manual, a study manual, a trial master file and a training programme for the HomeHealth project workers. Figure 3 gives a visual representation of the logic model for our intervention, incorporating underlying theory, the core components and how we anticipate that these might lead to changes in our outcomes. The intervention manual is available from the authors on request. The core elements of the intervention are described in the sections Service content, Behaviour change content and Delivery.
FIGURE 3.
Logic model of the HomeHealth intervention. AUDIT-C, Alcohol Use Disorders Identification Test – Consumption; CSRI, Client Services Receipt Inventory; EQ-5D, EuroQol-5 Dimensions; GHQ-12, 12-item General Health Questionnaire; IAPT, Increasing Access to Psychological Therapies; ICECAP-O, ICEpop CAPability measure for Older people; IPAQ-E, International Physical Activity Questionnaire for the Elderly; MoCA, Montreal Cognitive Assessment; WEMWBS, Warwick–Edinburgh Mental Wellbeing Scale.
Service content
HomeHealth aimed to be personalised to each individual, but cover four main domains: mobility, socialising, nutrition, and mood/psychological well-being. It addressed other domains raised by the individual as needed. Domains considered outside the service remit included detailed information regarding finances, medication management and nutritional or exercise advice for those with complex health needs for whom specialist support might be needed (e.g. diet advice in someone with renal failure).
Behaviour change content
HomeHealth project workers provided information (and signposting when necessary), skills (e.g. exercises), practical and emotional support and encouraged changes in behaviour. In terms of behaviour change content, the project workers delivered education, enablement, training and environmental restructuring as needed as the main intervention functions. These reflected the components derived from the qualitative work and our behaviour change review. In the light of the sparse evidence that we found in our systematic review of BCTs, we did not actively exclude any functions or BCTs from the intervention. As there was little consistent evidence about which BCTs to use, project workers were encouraged to tailor BCTs used to specific barriers that were highlighted by clients. Behaviour change content was primarily delivered through a core set of techniques: goal-setting, action-planning and monitoring progress, including maintenance of behaviours and developing habits. Goals were divided into three types in a hierarchy. From least to most specific, these were:
-
Outcome goal: the overarching goal that the person would like to achieve from the service. This may relate to an outcome of behaviour (e.g. ‘have more energy’). This is developed through discussion at the first appointment. This would then be reviewed according to progress and modified as needed.
-
Behavioural goal: the specific action(s) the older person wants to do to achieve their outcome goal (rather than what they would like to achieve). This goal relates to a specific behaviour, but phrased in general terms (e.g. for having more energy: ‘eat enough calories’).
-
A SMART goal (specific, measurable, achievable, relevant and timely): a detailed action plan that specifies key elements of the context in which the behavioural goal will be achieved (when, where, what and with whom), is measurable (recordable whether or not the goal was met), is achievable (to build motivation), relevant (important to the person) and timely (achievable within a target time).
If relevant, self-monitoring (i.e. recording behavioural progress towards goals), habit formation (repeated performance of the planned action in a specific context) and social and practical support (both from the project worker and through involvement of others, e.g. family members) were incorporated to assist in achieving goals or coping with setbacks.
Delivery
HomeHealth was delivered through approximately six (minimum of three and maximum of 12) appointments at clients’ homes over 6 months. Participants were told that there would be 3–6 appointments initially, with further appointments if needed (up to a maximum of 12 sessions) within 6 months, but an emphasis that this was a time-limited service. This would include:
-
First appointment:
-
learn about the person, focusing on mobility, nutrition, psychological well-being and socialising as key domains to address
-
build rapport and trust
-
identify their overall outcome goal from the service
-
if possible, identify a behavioural goal and a SMART goal and form an action plan, taking into account any barriers using the COM-B model or agree an action before the next meeting.
-
-
Subsequent appointments:
-
review goals and progress
-
address problem-solving, coping with setbacks and motivation
-
modify or create new goals as needed and form an action plan
-
maintain changes in behaviour (if applicable).
-
-
Final appointment
-
reinforce self-efficacy
-
reinforce new things they have learned (e.g. exercises)
-
advise on strategies to maintain motivation and continue behaviours after the HomeHealth service stops
-
provide information on further help or support (e.g. Care Navigators, GP, local Age UK).
-
HomeHealth project workers
The HomeHealth project worker role was created for this project. In line with the recommendations from the developmental phase of the study outlined above, our main criteria were excellent communication skills rather than a specific professional background, previous experience of working with older people in community settings and experience of engagement and person-centred planning (ideally with older people).
Training
The workers were briefed on the development of the HomeHealth service and the rationale and evidence base for it. They received an initial pre-intervention training programme delivered over four half-day training sessions by members of the research team and external experts as appropriate:
-
communication skills, low mood and depression, safeguarding
-
physical activity, strength and balance exercises
-
nutrition in older adults
-
delivering and personalising BCTs.
All sessions were led by an expert in that field. This training supplemented the intervention manual and resource packs developed for the HomeHealth service and was designed to provide basic knowledge in the key intervention areas, with signposting and referral recommended in cases that required specialist input. Training was interactive, with role-play, vignettes and demonstration and participation in exercise training, including the use of weights and resistance bands.
After commencing intervention delivery, one further session 2 months later was given by the health psychologist to reinforce the behaviour change content of the intervention, with a focus on overcoming setbacks and maintenance of behaviour change. It further allowed case-based discussion of applying this in a mildly frail population.
Supervision
In line with recommendations from the professionals within the qualitative study and intervention development panels, the project workers were supervised weekly/once every 2 weeks by an expert in older people and communication skills, with interim contact as needed. Clinical input from GPs and experts in nutrition, exercise and behaviour change were available as necessary to answer queries. Project workers were trained in identifying ‘red flags’ (health or social concerns or potential risks to the individual or others) and given protocols for associated action and follow-up in the intervention manual. This normally involved liaison with their accountable GP in the first instance.
Modifications to the trial protocol
Following our recruitment experiences in the qualitative study, consultation with stakeholders and recommendations from our Study Steering Committee, we made the following changes to the protocol (approved by our local Research Ethics Committee on 16 September 2015) to address issues that arose:
-
In line with the commissioning brief, we originally had an eligibility criterion requiring participants to have received at least one home visit in the previous 6 months. We removed this criterion following agreement with our Study Steering Committee, as this drastically reduced the potential participants (e.g. in one practice the initial pool of eligible patients reduced from 284 to 26 patients), which would have missed a large number of people with mild frailty who would be eligible in practice. Stakeholders also thought this criterion was an unsuitable one for identifying a population with mild frailty.
-
Use of the Clinical Frailty Scale7 as a practical tool to define eligibility for the new service as those with ‘mild frailty’ on this scale that could be used in clinical practice (recommended by our Study Steering Committee, and because the eFI was not available for use in most practices at study set-up). This was used successfully in recruitment for the qualitative study.
-
In the light of the importance of maintaining cognitive health highlighted by older people in the first phase of the study, we added a measure of cognitive function, the Montreal Cognitive Assessment (MoCA). 189
-
Include the ICEpop CAPability measure for Older people (ICECAP-O),190 a measure of capability for older people recommended by our health economists. Capability aligned with the values raised in the service development panels with stakeholders and older people.
-
Blood pressure measurement was removed, as the fully developed intervention did not include content that would have a direct impact on this (e.g. medication adherence).
Discussion
In combination with our comprehensive evidence reviews, our extensive qualitative work, stakeholder consultation and intervention development panels facilitated the development of a new intervention, ‘HomeHealth’. Building on the evidence base, we were able to identify what a new service should contain and how it could best be delivered, and refine this iteratively in consultation with older people, professionals, commissioners and providers.
Although previous qualitative work has canvassed older people’s experiences of transitioning to frailty or general health promotion in later life,180,182,191,192 none to date has focused specifically on older people’s views of health promotion in mild frailty. We incorporated a wide range of views from a range of older people with and without mild frailty, carers, community health and social care professionals, policy-makers and commissioners and were able to make practical recommendations on the content of a new service. However, in our qualitative study, older people generally found it difficult to comment in detail on a hypothetical new service, and the majority of our older adult sample was white British, which may limit the applicability of the developed intervention to minority ethnic groups.
The components included within our service are supported in the wider literature. Consistent with our findings, communication skills and building a positive relationship are seen by older people as a vital part of their services191,193,194 and have been emphasised in other participatory design processes around frailty prevention. 195 Addressing cognitive, social, psychological and home environment dimensions within frailty prevention services have been advocated by stakeholders170 and physical and occupational therapists. 196 Although memory was also highlighted as an important component by our stakeholders, the limited evidence base available for maintaining memory meant that this could not be a major focus within the new service. The emphasis placed on mobility by older people in our study aligns well with the current evidence base for health promotion (see Chapter 2). In other studies, physical and occupational therapists have suggested that the additional social dimension of group exercise programmes means that they may be more suitable for pre-frail older people than home-based exercise;196 however, the effort involved in attending an exercise group on a regular basis may be a significant barrier in those with mild frailty. In previous qualitative work, older people have reported activities, socialising and being physically active as key ways to resist ‘feeling frail’ and, similarly to our study, felt that self-identifying as frail and old had negative effects on attitudes and behaviour. 192,196 Occupational therapists and physiotherapists have also highlighted that pre-frail older people felt less at risk and so were more challenging to engage, particularly in using mobility aids. 196
There are some previous community care navigation or home-based nursing services that have covered the domains found within our work and previous work, using care co-ordination, signposting or providing psychosocial support. 197,198 However, although older people felt that this was valuable,199 few of these services were targeted at a mildly frail population, were sustained over time and focused on motivating people to maintain or change health-promoting behaviours, which was raised as a particular challenge in this population. Therefore, the newly developed HomeHealth service has the potential to fill these gaps within health promotion services for frailty prevention.
Chapter 4 Phase II: feasibility randomised controlled trial – methods
Chapter 4 outlines the methods used to evaluate the feasibility and acceptability of the intervention and trial methods for a full RCT. The methods of our linked process evaluation are reported separately in Chapter 6.
Objectives
This trial aimed to evaluate the feasibility and acceptability of the HomeHealth intervention for delivery in the NHS and for a full-scale RCT, specifically:
-
recruitment and attrition
-
feasibility and acceptability of individual randomisation and study procedures
-
suitability of outcome measures for a full trial
-
feasibility and acceptability of the intervention
-
determine intervention costs
-
assess the feasibility of calculating cost-effectiveness in a full RCT from health and social care and societal perspectives
-
assess the feasibility of conducting a budget impact analysis for Clinical Commissioning Groups.
Overview of trial design
This was a two-arm, pilot and feasibility parallel-group 1 : 1 single-blind RCT of the HomeHealth intervention in community-dwelling older people with mild frailty.
The trial was supported by the PRIMENT™ clinical trials unit (CTU; University College London, London, UK).
No changes were made to the trial design after commencement.
Trial registration
The trial was prospectively registered as ISRCTN11986672 on 2 February 2015.
Trial setting
Intervention and study procedures were carried out in participants’ homes. The trial site was designated as University College London with four general practices as participant identification centres (two in the London Borough of Camden and two in Hertfordshire), selected to capture a range of urban and semi-rural communities with diverse socioeconomic and ethnic backgrounds and access differences in health, transport and associated services. General practices were not involved in intervention delivery, apart from liaison with the HomeHealth project worker, if necessary, and as part of usual care for a participant.
Study participants
Participants were community-dwelling older adults with mild frailty, defined according to the criteria below.
Inclusion criteria
-
Older people aged ≥ 65 years registered with a participating general practice
-
Scoring as ‘mildly frail’ on the Rockwood Clinical Frailty Scale7
-
Community dwelling (including extra care housing)
-
A life expectancy of > 6 months
-
Capacity to consent to participate (including those with dementia or communication difficulties who retained capacity).
We included people unable to speak English, with the provision of translated materials and translators, if required.
Exclusion criteria
Those people who:
-
were living in care homes
-
had moderate to severe frailty or who are not frail (according to the Rockwood Clinical Frailty Scale)7
-
were on the GP register for palliative care or dementia
-
were housebound
-
were already case managed
-
were lacking capacity to consent
-
it would be inappropriate to have an invitation to participate for at this time (e.g. because of recent bereavement), as judged by their GP.
Interventions
Treatment group
The HomeHealth intervention was a manualised home-based behaviour change intervention, targeted at increasing independence and well-being through addressing key areas of mobility, nutrition, psychological well-being and social isolation (see Chapter 3, HomeHealth: intervention description). The service was tailored to each participant, who developed goals and strategies to achieve them in conjunction with the HomeHealth project worker. Each participant received up to six sessions over 6 months, with a further six if considered necessary following a joint discussion with the participant and project worker. HomeHealth project workers were non-specialists recruited specifically for the project, who underwent training in communication skills, behaviour change, safeguarding, nutrition and exercises for mobility. Each participant also received TAU. We have summarised the intervention according to the template for intervention description and replication (TIDieR)200 checklist in Table 7.
| TIDieR item | Application in HomeHealth |
|---|---|
| 1. Brief name: provide the name or a phrase that describes the intervention | HomeHealth |
| 2. Why: describe any rationale, theory, or goal of the elements essential to the intervention | Asset-based approach, Baltes’ theory of successful ageing, behavioural science approach using the COM-B model (see Chapter 3, HomeHealth: intervention description) |
| 3. What materials: describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (such as online appendix, URL) |
HomeHealth intervention manual for provider training (available from the authors on request) Resource packs (educational information for the service providers, local services to signpost to and resources to hand out to clients, such as leaflets) within key areas (e.g. mobility and nutrition) Equipment (e.g. weights and resistance bands) to supply to clients when necessary Forms to be completed with the client: goal-setting, action-planning, making contingency plans |
| 4. Procedures: describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities | Tailored intervention (see Chapter 3, HomeHealth: intervention description) focused on mobility, nutrition, psychological well-being and socialising. Behaviour change functions included education, training, enablement and environmental restructuring. BCTs included goal-setting, action-planning and problem-solving, reviewing progress, providing feedback with other BCTs (e.g. encouraging self-monitoring) used, when applicable |
| 5. Who provided: for each category of intervention provider (such as psychologist, nursing assistant), describe their expertise, background, and any specific training given | Trained non-specialist support workers with experience in working with older adults (see Chapter 3, HomeHealth: intervention description). Training included in communication skills, BCTs, nutrition and strength and balance exercises (based on the Otago home-exercises model) |
| 6. How: describe the modes of delivery (such as face to face or by some other mechanism, such as the internet or telephone) of the intervention and whether or not it was provided individually or in a group | Face-to-face individual appointments (with carer present when there was significant cognitive impairment), with telephone support if needed. Project workers also liaise with GPs, therapists or other services as needed |
| 7. Where: describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features | The participants’ homes |
| 8. When and how much: describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose | Planned as six sessions over 6 months (minimum of three and maximum of 12), with a longer first appointment of approximately 1–2 hours and subsequent appointments of 30–60 minutes |
| 9. Tailoring: if the intervention was planned to be personalised, titrated or adapted, describe what, why, when and how | The intervention was tailored according to the needs and goals of the participant. The goals set, number and duration of appointments, involvement of others, and BCTs used were tailored through an in-depth baseline assessment and discussion of each client’s issues and progress at each appointment |
| 10. Changes: if the intervention was modified during the course of the study, describe the changes (what, why, when and how) | There were no changes during the course of the study. Minor modifications are suggested in our process evaluation (see Chapter 6, Process evaluation) |
| 11. How well – planned: if intervention adherence or fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them | Intervention adherence/fidelity was assessed with mixed methods, including documentation of attendance, content of sessions, goals set and progress towards goals by the service providers and questionnaires and interviews with service recipients. We further audio-recorded intervention appointments and these were assessed against a fidelity checklist by the process evaluation lead |
| 12. How well – actual: if intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned | The intervention was largely delivered as intended (see Chapter 6, Process evaluation) |
Comparator group
The control participants received TAU, which consisted of primary and secondary care treatment that the person would usually receive and any social care, private or family support as needed. No services or therapeutic interventions were prohibited in either group and participants were not asked to maintain particular behaviours. A supplementary objective of the study was to document ‘treatment as usual’ to define this for a full-scale trial. At the end of the study period (6-month outcome assessment) all study participants received four Independent Age201 guides (Your health and the NHS; Advice for later life; Extra help at home; Healthy, happy, connected), an Age UK and NHS England booklet202 (A Practical Guide To Healthy Ageing) and a list of local services collated by the project worker in each area.
Measurements
Feasibility and acceptability success criteria
The primary objective for this study was to determine feasibility and acceptability, according to the following success criteria:
-
A minimum recruitment rate of 70% of our target of 50 people within 6 months.
-
A retention rate of 80% at 6 months, excluding people with outcomes measured as part of the study (i.e. deaths).
-
A positive evaluation of acceptability to older people (uptake of the service, satisfaction with service).
-
At least no difference between groups on analyses of the candidate primary outcomes (i.e. no negative effects).
Clinical outcomes
We assessed a range of outcome measures for feasibility of data collection at baseline at 6 months. The outcomes collected at each stage are summarised in Table 8.
| Outcome | Time point (months) | ||
|---|---|---|---|
| Baseline | 3 months | 6 months | |
| Demographics | ✓ | ||
| Modified Barthel index | ✓ | ✓ | |
| Grip strength | ✓ | ✓ | |
| Gait speed | ✓ | ✓ | |
| IPAQ-E | ✓ | ✓ | |
| WEMWBS | ✓ | ✓ | |
| GHQ-12 | ✓ | ✓ | |
| EQ-5D-5L | ✓ | ✓ | |
| ICECAP-O | ✓ | ✓ | |
| Weight | ✓ | ✓ | |
| Height | ✓ | ✓ | |
| AUDIT-C | ✓ | ✓ | |
| Smoking | ✓ | ✓ | |
| MoCA | ✓ | ✓ | |
| CSRI | ✓ | ✓ | ✓ |
| Falls | ✓ | ✓ | ✓ |
| NHS service use (NHS GP records) | ✓ | ||
| Long-term conditions (NHS GP records) | ✓ | ||
| Prescribed medication (NHS GP records) | ✓ | ||
Self-report questionnaires were administered verbally in a minority of cases for which participants had impairments preventing self-completion (e.g. visual impairment) or for which participants refused to self-complete but were happy for the questionnaire to be interviewer administered. Participants were given £10 vouchers for each assessment.
Our candidate primary outcomes for a full trial included generic measures of well-being and functioning (ADL), health-related quality of life, capability, well-being and psychological distress. In addition, we collected data to calculate the Fried frailty phenotype score (including gait speed, grip strength, physical activity, weight loss and exhaustion). 10 This is a key outcome of interest, but as it has not been validated as an outcome measure it would therefore not be our primary outcome in a full trial.
Functioning
We assessed functioning, a key outcome in frailty trials,55,203 using the Modified Barthel Index, which scores patients between 0 and 100 according to the level of assistance required with 10 ADLs, including personal hygiene, bathing, feeding, going to the toilet, stair climbing, dressing, bowel control, bladder control, ambulation (or use of wheelchair) and chair/bed transfers. 204 It correlates significantly with the need for different types of home care support in older people. 205 In this scale, 100 indicates full independence, 91–99 indicates slight dependence and 61–90 indicates moderate dependence. 204 The Modified Barthel Index was interviewer administered at baseline and 6 months, and was based on participant self-report.
Quality of life
We assessed quality of life using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), a commonly used and low-burden five-domain utility measure that is valid and reliable for assessing economic outcomes in community-dwelling older adults. 206,207 We used the five-level version of the questionnaire as a result of concerns about ceiling and floor effects in the three-level version. 208 Tariffs are weighted according to societal valuations of health, for which 1 is perfect health and 0 is equivalent to the state of death. 209 The EQ-5D-5L was administered by the research assistant at baseline and 6 months.
Capability
We also assessed broader quality of life using the ICECAP-O, a valid measure of capability specific to older people that assesses attachment, security, role, enjoyment and independence on four ordinal levels. 207 It performs well in those with mild cognitive impairment. 207 The ICECAP-O was self-completed at baseline and 6 months.
Well-being
We measured well-being using the Warwick–Edinburgh Mental Wellbeing Scale (WEMWBS), a 14-item scale assessing well-being and positive mental health on Likert scales, including positive effect, satisfying interpersonal relationships and positive functioning. 210 WEMWBS has good validity and reliability in UK populations210 and is responsive to change. 211 The WEMWBS was self-completed by participants at baseline and 6 months.
Psychological distress
We assessed psychological distress at baseline and 6 months using the 12-item General Health Questionnaire (GHQ-12), a self-report questionnaire assessing domains of anxiety and depression, social impairment and loss of confidence. 212 The GHQ-12 has high sensitivity and specificity for detecting depression across a range of countries, translations, older people and people with mild cognitive impairment. 213,214
Frailty measurements
There are a range of potential approaches to defining and measuring frailty, with some consensus around the importance of ‘physical frailty’. 215 The most widely used measurement of physical frailty is the Fried phenotype, which is composed of five frailty characteristics: weakness, slowness, exhaustion, shrinking and low physical activity. 10
Mobility: gait speed
Gait speed is a component of the Fried frailty phenotype10 and is a reliable and valid measure of physical performance in community-dwelling older adults,216 with predictive validity for disability216 and mortality. 217 As assessments were carried out at participants’ homes, which had differing amounts of available space, the research assistant mapped out as long a distance as possible (between 1.5 and 5 metres) using card markers, with 0.5–1 metres either side (depending on total distance available) marked out for speeding up and slowing down. We recorded distance walked (metres) and time taken (seconds) using a stopwatch and used the mean of two attempts.
Grip strength
Grip strength is a frailty phenotype component,10 which predicts mortality218 and ADL disability. 219 Handheld dynamometry is a valid, reliable and acceptable method for assessing grip strength in community-dwelling older adults,216 and was measured at baseline and 6 months using a Lafayette 5030L1 dynamometer (Lafayette Instruments Europe Ltd, Loughborough, UK). We followed Roberts et al. ’s220 protocol, although as measurement was carried out in participants’ homes it was not possible to use the same chair for all participants. Participants were seated with their forearm resting on a chair arm or table at 90° and instructed to grip the dynamometer as hard as possible then let go. Three readings were taken for each hand, alternating hands between readings. The highest recorded reading was used in the analysis.
Exhaustion
The question ‘I’ve had energy to spare’ (the responses to which were ‘none of the time’ or ‘rarely’) from the WEMWBS scale was used to represent the exhaustion component of the Fried frailty phenotype (see Fried phenotype definition). 10
Weight loss
Frailty is associated with weight loss as a result of sarcopenia (decline of skeletal muscle tissue with age) and forms part of the Fried frailty phenotype. 10 We assessed weight (seca 875 Class III digital floor scales; seca United Kingdom, Birmingham, UK) and height (portable Leicester height measure; Chasmors Ltd, London, UK) at baseline and 6 months to calculate:
-
weight loss as a continuous measure in participants not obese at baseline (BMI of < 30 kg/m2)
-
number of participants losing ≥ 3 kg over the study
-
number of participants with a BMI of ≤ 18.5 kg/m2 at baseline and 6 months.
Participants were asked to remove shoes for both measures and wore light clothing. Data on whether or not weight loss was intentional was not collected, so weight loss could not be used as in the original Fried phenotype. 10 We used low body weight to define this criterion, as did many other previous studies. 221
Physical activity
Low physical activity is a component of the Fried phenotype10 and may have some effects on functioning. 23,55 We assessed physical activity using the International Physical Activity Questionnaire (IPAQ), which includes time spent sitting, walking and in moderate and vigorous physical activity over the previous week. It has excellent reliability and fair to moderate validity across a range of countries. 222 We used the version adapted for older people [International Physical Activity Questionnaire for the Elderly (IPAQ-E), with reversed question order and older adult-specific examples], which has similar validity to the IPAQ. 223 The IPAQ-E was research assistant-administered at baseline and 6 months. We summarised physical activity in categories using IPAQ guidance thresholds224 at baseline but, as these were unlikely to change over 6 months, we also summarised physical activity continuously in metabolic equivalent of task-minutes/week, using the IPAQ guidance formulae. 224
Fried phenotype definition
Using the outcome measures collected in the trial, we defined the Fried phenotype10 within the HomeHealth study as in Table 9. As our sample was selected for ‘mild frailty’ we were not able to use the internal population distribution to calculate the required cut-off points (lowest quintile) for slowness and weakness. Therefore, we used data from the English Longitudinal Study of Ageing cohort (own data) as potentially the most applicable to our population to identify the relevant cut-off points for slowness and weakness. 225
| Fried phenotype criteria10 | Measure | Definition used in HomeHealth | |
|---|---|---|---|
| Weakness | Grip strength (kg), lowest grip strength quintiles in the ELSA database, stratified according to BMI quartiles | For a man with a BMI of (kg/m2): | The cut-off point for weak grip strength is (kg): |
|
≤ 25.1 25.2–27.1 27.2–29.8 > 29.8 |
≤ 33 ≤ 34 ≤ 39 ≤ 45 |
||
| For a woman with a BMI of (kg/m2): | The cut-off point for weak grip strength is (kg): | ||
|
≤ 24.3 24.4–27.1 27.2–30.7 > 30.7 |
≤ 19 ≤ 20 ≤ 24 ≤ 28 |
||
| Slowness | Gait speed (m/second), lowest quintile for gait speed stratified according to height (above or below median height) and gender | For a man with a height of (cm): | The cut-off point for slow gait speed (m/second): |
|
≥ 171.9 < 171.9 |
≤ 0.75 ≤ 0.71 |
||
| For a woman with a height of (cm): | The cut-off point for slow gait speed (m/second): | ||
|
≥ 158.6 < 158.6 |
≤ 0.72 ≤ 0.59 |
||
| Exhaustion | WEMWBS item 5 ‘I’ve had energy to spare’ | Answers ‘none of the time’ or ‘rarely’ | |
| Shrinking | BMI | BMI of ≤ 18.5 kg/m2 | |
| Low physical activity | IPAQ-E | ‘Inactive’ category: no activity is reported or some activity is reported but not enough to meet ‘minimally active’ or ‘HEPA active’ categories224 | |
Participants were categorised according to presence of absence of each frailty characteristic and categorised as robust (no frailty characteristics), pre-frail (1 or 2 characteristics) and frail (≥ 3 characteristics). 10 To compare frailty level at baseline and 6 months, we used the number of Fried frailty phenotype characteristics.
Other frailty measures: cumulative deficits
In the original protocol, we intended to collect eFI8 scores for participants from their GP medical records if available. The eFI is an index of cumulative deficits that totals the presence or absence of 36 frailty-related deficits out of the total, based on data already collected in GP medical records. The eFI scoring algorithm was planned to be introduced into GP electronic software systems by the time the feasibility study commenced; however, at the time of baseline data collection, it was not yet available in three out of our four study practices. Therefore, we were unable to use this score within our study.
In replacement of this, as a proxy measure of frailty, we collected the number of long-term conditions at baseline from problem lists in participants’ medical records. A long-term condition was defined as one requiring ongoing care, impacting upon the person’s life and is likely to last longer than a year. 226 We developed a list of relevant conditions, using a combination of relevant items from the Charlson comorbidity index,227 the Quality Outcomes Framework228 and other conditions and impairments relevant to this population (e.g. incontinence, hearing impairment).
We included these data as a baseline covariate to describe the population in terms of their comorbidities, but did not assess changes in the number of long-term conditions as this outcome was not an intervention target.
Other outcomes
We assessed a range of other potential secondary or intermediate outcomes for a full RCT, including other health behaviours (smoking, alcohol), cognition and falls.
Smoking
Past, never or current smoking was assessed using a single, research assistant-administered categorical question.
Alcohol consumption
Alcohol intake was assessed at baseline and 6 months using the Alcohol Use Disorders Identification Test – Consumption (AUDIT-C), a brief three-item screening questionnaire that gives a 0–12 score regarding the typical frequency, number of drinks and episodes of binge drinking (six or more drinks on one occasion). The AUDIT-C has good sensitivity and specificity for hazardous and harmful drinking in the elderly. 229 We summarised the AUDIT-C as a continuous variable.
Cognition
We assessed cognitive function using the MoCA version 7.1, a 30-item interviewer-administered assessment of visuoconstructional skills, executive functions, naming, attention and concentration, calculations, language, conceptual thinking, memory and orientation. 230 The MoCA is scored continuously out of 30, with ≥ 26 considered normal230 and has good sensitivity and specificity for detecting mild cognitive impairment and mild Alzheimer’s disease. 183 The MoCA was research assistant administered at baseline and 6 months. We did not exclude participants on the basis of their MoCA scores, only if they lacked capacity to consent.
Falls
Falls were defined using the Prevention of Falls Network Europe (ProFANE) consensus criteria as ‘a slip or trip in which you lost your balance and landed on the floor or ground or lower level’. 231 At baseline, 3 months and 6 months, we collected retrospective self-reported falls data over the preceding 3-month period, including:
-
total number of falls
-
number of falls when an ambulance was called
-
number of falls when participants visited accident and emergency (A&E).
Falls were summarised categorically as zero, one, two, or three or more falls.
Health economic outcomes
As part of the feasibility study, we aimed to determine intervention costs and the feasibility of our planned health economic analyses in a full RCT.
Intervention costs
The costs of delivering the intervention were collected throughout intervention delivery, including:
-
training costs (including costs of trainers, including oncosts and overheads)
-
supervision costs (hourly wage and hours of supervision needed)
-
client contact time (self-reported log from project workers of time spent at each client appointment and travel time from their ‘base’ general practice)
-
consumables supplied to clients (e.g. weights).
Quality- and capability-adjusted life-years
Quality-adjusted life-years (QALYs) were collected using the EQ-5D-5L (see Quality of life) and calculating the area under the curve adjusting for baseline differences. 232 Capability-adjusted life-years (CALYs) were calculated from the ICECAP-O (see Capability) and applying index values from Coast et al. 190 to calculate the area under the curve adjusting for baseline.
Service use
We collected service use data from participants’ medical records for the 3 months preceding baseline and the 6 months following baseline assessment, including:
-
overnight inpatient stays
-
outpatient appointments
-
A&E attendance
-
general practice service use
-
medication use.
We collected additional self-reported health service data through a Client Services Receipt Inventory (CSRI) adapted for the study from the Institute for Medical Technology Assessment Valuation of Informal Care Questionnaire (see Report Supplementary Material 5). 175,233,234 This was administered retrospectively for the preceding 3 months by the research assistant at baseline and 6 months, and by post at 3 months and included:
-
additional health services (e.g. physiotherapy, podiatry)
-
over-the-counter medicines and supplements
-
residential, nursing and respite care
-
personal care and help at home [participants were asked whether or not they received help with a list of specific tasks (e.g. cleaning, shopping), whether or not this was state funded, privately funded or from friends or family (unpaid), and the frequency and duration of this. This was recalled over the previous week, with fractions used for less frequent regular help (e.g. 0.5 per week)]
-
local transport services
-
benefits (as this is a population likely to receive a range of range of benefits, e.g. pension credit, attendance allowance, carers allowance, which may potentially change as result of this intervention given that some patients received assistance with financial related matters)
-
receipt of direct payments and individual or personal budgets from the local authority
-
social outings.
Descriptive summaries and mean cost per patient were calculated for each treatment group. Unpaid carers were costed using the proxy good method at the hourly rate of a home care worker. 235 This is based on the assumption that reductions in social care provision result in an increase in unpaid carer time undertaking personal care and help at home.
Demographics
At baseline, we collected date of birth, gender, living arrangements, marital status, current housing, educational level, employment, volunteering, receipt of state/employer/private pension and receipt of state benefits. We determined the local area deprivation based on the participant’s postcode, using the Index of Multiple Deprivation (IMD) in deciles. 236
Feasibility and acceptability of trial procedures
We assessed feasibility and acceptability of running a full-scale RCT using a range of sources of information. This included:
-
recruitment and retention rates
-
completion of clinical outcome measures and completion of individual measures including missing data
-
completion of health economic data
-
questionnaires with study participants (intervention and TAU arms) on their perspectives of study processes, including the length and burden of research assessments.
A postal questionnaire was sent to all participants completing the 6 months’ follow-up, which included questions on the content of research assessments and the acceptability of the assessments (see Report Supplementary Material 6). The control arm was additionally asked about ‘treatment as usual’ (see Defining ‘treatment as usual). Those in the intervention arm were additionally asked about their views regarding the intervention (see Chapter 6, Process evaluation).
Defining ‘treatment as usual’
Our developmental work (see Chapters 2 and 3) demonstrated that ‘treatment as usual’ for this population is ill-defined and heterogeneous. We attempted to quantify ‘treatment as usual’ for our TAU arm of our RCT in order to better define this for a full RCT. For this we used two sources of data:
-
questionnaires for TAU arm participants that included questions on any activities or lifestyle changes undertaken during the study period
-
CSRI data on support services accessed by TAU arm participants, including NHS services, local authority and third-sector/voluntary provided services and privately funded services.
Harms
Adverse events (AEs) that were potentially related to the intervention and all serious adverse events (SAEs) were recorded throughout the study. We categorised AEs in accordance with PRIMENT CTU standard operating procedures and reported severe unexpected serious adverse reactions to the sponsor. After discussion between the participant and project worker, the intervention would be temporarily ceased, if appropriate.
Participant timeline
Participant recruitment took place between 16 December 2015 and 13 May 2016. The first participant was recruited on 4 January 2016 (randomised 5 January 2016) and the final outcome assessment was on 14 November 2016. Recruitment was stopped when the target sample size was reached. Recruitment procedures are summarised in Figure 4.
FIGURE 4.
Participant flow throughout the feasibility study.
Sample size calculation
As this was a feasibility study, a sample size calculation was not undertaken. We selected 50 as a sufficient and realistic number to test intervention feasibility and acceptability, pilot recruitment, retention and randomisation processes for a full RCT and recruit sufficient numbers per practice and for intervention delivery in the short time frame available. 237,238 We aimed to recruit four general practices, assuming that recruiting 12–14 people from each would be feasible.
Methods for participant recruitment
We undertook a two-stage screening process. 203 An initial list of participants was generated through GP database searches of patients aged ≥ 65 years, using practice-specific database tools to remove clearly ineligible persons (e.g. on the dementia register, housebound code, case managed). GPs at each participating practice screened the resulting list and used their clinical knowledge to remove those who did not fit the inclusion criteria. A random sample of remaining participants (100–150 per practice) was approached by post to take part in the study using materials developed through consultation with intervention panels. Practices were also given information packs for GPs or nurses to opportunistically refer patients to the study, and posters were displayed in the study general practice waiting rooms and other areas (e.g. toilets) with a contact number for self-referral.
Within the original protocol (version 1.2) we aimed to use the eFI8 to identify potentially eligible patients. This tool was only available in one of our study general practices at the time of recruitment. For this practice, they identified a random sample of participants who were classed as mildly frail (0.12–0.24 on the eFI8) for postal recruitment, to test the feasibility of using this approach in a larger trial at a later date when the eFI was available at all practices.
Interested participants returned a reply slip or contacted the research assistant by telephone. Non-responders were to receive a single telephone reminder from the practice if needed. The research assistant screened interested participants for eligibility by telephone using a set of questions based on the Fried frailty criteria10 (asking about symptoms of slowness, weakness, etc.) and whether or not they required support for IADLs or ADLs. During this telephone call, the research assistant also explained the study purpose, answered any questions and, if the person was eligible, arranged a baseline assessment (see Figure 4).
Baseline assessments were carried out at home by the research assistant and informed consent was sought. Consenting and eligible people were entered into the study.
Randomisation
We randomised participants in a 1 : 1 ratio to intervention or TAU, in random blocks of four or six and stratified by practice to balance differences in geographic location or therapist. Independent of the research team and study and intervention delivery, PRIMENT CTU developed a randomisation list and held this in a secure location. A study team member not involved in participant recruitment (KK) contacted PRIMENT for the person’s allocation when an eligible person entered the study.
Blinding
The research assistant who screened older people, conducted baseline and outcome assessments and collected medical notes data was blinded to intervention status. They were unaware of the allocation sequence and participants were informed of their allocation by telephone. The research assistant was not involved in intervention delivery discussions and participants were asked to avoid discussing experiences of being in the study during the outcome assessment. Incidents of unblinding were documented throughout the trial and the research assistant completed a researcher perception form following the outcome assessment. In this the researcher recorded which study arm they thought the participant was in, according to the following classification:
-
I do not know which group the participant is in.
-
I have guessed the participant is in the intervention group.
-
I have guessed the participant is in the TAU group.
-
I know that the participant is in the intervention group.
-
I know that the participant is in the TAU group.
Statisticians and health economists were blind to group allocation when checking and analysing the database. Owing to the nature of the HomeHealth and control interventions, participants could not be blinded to group allocation.
Data entry
Pseudonymised data were entered into a database using REDCap version 7.4.9 (Research Electronic Data Capture tools; Vanderbilt University, Nashville, TN, USA) hosted by PRIMENT at University College London. 239 REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources. Data entry rules were developed for CSRI data between Rachael Frost and Rachael Hunter, as some of these data were returned by post or were considered difficult to quantify by participants. A random sample of 10% (n = 5 participants) of all assessment data were checked between the paper case report form and the RedCap by a second team member. We considered < 1% an acceptable margin of error. Percentage error was zero for the majority of case report forms (total error 0.30%). The trial database was reviewed and checked by blinded statisticians and health economists and issues discussed with the research assistant prior to database lock (23 January 2017). Personal and sensitive data (names, addresses, identification number, dates of birth, ethnicity) were stored separately in a secure location (the Internet Data Safe Haven) with restricted permissions according to University College London guidelines. The University College London Data Safe Haven is a secure environment, designed to store and process sensitive, identifiable research data. The Data Safe Haven comprises a technical solution and an information security management system, which includes policies and procedures to govern the use of the Data Safe Haven. The Data Safe Haven meets the NHS Information Governance Toolkit criteria and is certified to the International Standards Organisation (ISO) 27001 information security standard. The Data Safe Haven technical solution has been built using standard, commercial components including HP storage area network (SAN) storage and blade servers (Hewlett Packard Enterprise, London, UK), Sophos unified threat management (UTM) security appliance (version 9.2; Sophos Ltd, Abingdon, UK) and Citrix remote desktop technology (XenDesktop 7; Citrix, Fort Lauderdale, FL, USA). The Data Safe Haven provides a remote desktop user experience with all data held within the secure environment and controls in place to prevent data being saved onto any local devices. Intervention delivery data were also stored in the Internet Data Safe Haven in a separate folder inaccessible to the blinded research assistant, as this included personally identifiable information.
Statistical methods
Analyses were conducted by Federico Ricciardi, John Wood and Rachael Hunter using Stata® 14 (StataCorp LP, College Station, TX, USA) and R, version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria) according to the statistical analysis plan developed by John Wood, Federico Ricciardi and Rachael Hunter (version 2.2, 26 October 2016).
Feasibility outcomes
We descriptively summarised recruitment, participant characteristics and loss to follow-up to ascertain whether or not our success criteria were met and assessed the acceptability of the intervention through questionnaires and interviews as part of the process evaluation (see Chapter 6, Participant questionnaires). We also assessed the feasibility of trial procedures (see Chapter 4, Feasibility and acceptability of trial procedures).
Outcome measures
Demographic characteristics, baseline variables and outcome variables were descriptively summarised, overall and by group. For continuous measures, we assessed outcomes using the following regression models:
-
model 1 – baseline value of the outcome measure, area (therapist effect) and treatment group
-
model 2 – baseline value of the outcome measure, area (therapist effect), treatment group, age and gender
-
model 3 – baseline value of the outcome measure, area (therapist effect), treatment group, age, gender, deprivation (assessed by proxy by receipt of extra pension above state pension) and number of long-term conditions (as a frailty indicator).
For the majority of outcomes, model 2 provided the best fit, particularly as gender was unbalanced between groups at baseline. Results from model 2 are therefore presented in this report. Model assumptions (normality, homoscedasticity and linearity) were checked using histograms and Q–Q plots. We calculated 95% two-sided confidence intervals (CIs) and reported p-values using a 5% (two-sided) significance level. Number of frailty characteristics was compared using Poisson regression, adjusted for the same variables. Categorical variables were analysed descriptively.
All analyses were conducted using intention to treat with all available data. We intended to use established conventions for imputing missing data; however, given the low number of missing data, this was unnecessary. Sensitivity analyses were planned to define unintentional weight loss by restricting weight loss assessment to non-obese participants and to identify participants losing ≥ 3 kg. Other subgroup analyses were not possible because of the small sample size.
Health economic analysis
In line with the statistical analysis, the primary health economic evaluation was complete case analysis.
Intervention costs
The mean costs of the intervention were calculated per patient, based on a range of assumptions on the case-load, staff turnover and training requirements for scaling up to a full trial or implementation. For the calculation of costs, we assumed that the person delivering the service would be employed under NHS Agenda for Change band 6 (equivalent to the band project workers were employed as within this study), with a caseload of 50 people per year and an average employment duration of 12 months (as high staff turnover is possible). We also calculated costs if the project worker was employed at NHS Agenda for Change band 5 or 7 to inform service commissioners. All costs are in 2015/16 Great British Pounds and costed based on Personal Social Services Research Unit calculations of mean salary, oncosts and overheads by NHS band. 240
We calculated the hourly costs of specialist trainers and supervision at the equivalent level of the providers in this study. Supervision was costed as an hourly rate for a senior staff member (manager) and training was costed based on consultancy rates of £350 per session charged by our external training providers. As the training was delivered to only two staff members in this small feasibility study and this would be scaled up to larger numbers in practice (in both a full RCT and if implemented), we assumed that four service providers would be participating in a single training session for the cost calculations. We combined these data with the average number of minutes per client, average travel time per client and cost of consumables to calculate the total intervention cost per client.
Feasibility and acceptability of collecting relevant health economic data
We report the proportion of missing data at baseline, 3-month (for CSRI only) and 6-month follow-up for EQ-5D-5L, ICECAP-O and CSRI and the completeness of data extracted from medical notes.
Service use
We report the percentage of patients and mean number of contacts for patients who used the service for each type of health, social care and out-of-pocket health-care costs for baseline, 3 and 6 months. These were costed for each patient using costs from the most recent Unit Costs of Health and Social Care 2016 published by the Personal Social Services Research Unit240 and Reference Costs 2015–2016. 241 All costs are reported in 2015/16 Great British Pounds.
We calculated mean cost per patient plus 95% CIs for the HomeHealth intervention and TAU groups by type of service use at 3 and 6 months, adjusted by baseline service use. As the aim of the study is to assess the feasibility of collecting data for a full trial of HomeHealth and given the small sample size, we have not reported incremental cost-effectiveness ratios or cost-effectiveness curves as this would imply hypothesis testing of the cost-effectiveness of HomeHealth compared with TAU, which is not recommended for feasibility trials. 242
Quality- and capability-adjusted life-years
Mean and SDs for utility scores for the HomeHealth and TAU arms are reported based on response to the EQ-5D-5L and the formula developed by EuroQoL. 243 QALYs are calculated as the area under the curve, adjusting for baseline differences using regression analysis. 232
We also calculated mean capability indexes and CALYs using the ICECAP-O and the index values reported by Coast et al. 190
Societal perspective
Societal costs were also included to capture out-of-pocket costs and the impact on unpaid carers. Unpaid carers (family and close others) often provide essential support and care to frail older patients. Their contribution to care needs to be recognised and valued. If not, this can represent an undervaluing of the total cost of care if an unpaid carer provides a significant amount of care for a patient. This has been costed as equivalent to the cost of an hour of face-to-face time of a home care worker of £24 per hour. 240 There was some missing data for how many times people received help per week and how long a typical visit was but, so that a total cost could be calculated if a value was missing, it was replaced with the mean for completed variables greater than zero at that time point.
Budget impact analysis
Budget impact analysis can provide information to commissioners of public services regarding where costs for new services are likely to be incurred and who will see any of the cost savings. We report costs by different commissioning sectors to demonstrate the potential impact on health and social care budgets of commissioning the HomeHealth service.
Safety
We descriptively summarised per group:
-
number and type of severe unexpected serious adverse reactions
-
number and type of SAEs
-
AEs.
Adherence
Within the HomeHealth intervention, appointment number and duration were individually tailored and adherence to the personalised goals and behaviours set by participants was reviewed by project workers. Therefore, in order to assess adherence, we collected data regarding number and duration of appointments per person and progress towards goals set in order to develop future recommendations for a larger trial regarding intervention dosage. These are summarised as part of the process evaluation (see Chapter 6, Process evaluation), alongside further assessment of intervention fidelity.
Chapter 5 Feasibility randomised controlled trial: results
In this chapter, we report our findings for the feasibility RCT, including feasibility data, clinical outcomes, our health economic analysis and acceptability of trial processes. In line with the Consolidated Standards of Reporting Trials (CONSORT) feasibility study statement,242 we outline the flow of participants through the study and baseline and outcome data for each trial group. We report a definition of ‘treatment as usual’ from our control arm for use in a future RCT in this chapter, and the acceptability of the intervention itself later in the report (see Chapter 6, Process evaluation).
Participant flow
We recruited 51 older adults to the feasibility study (Figure 5). A total of 26 were allocated to the intervention arm and 25 to the TAU arm. A total of 48 participants completed the study (n = 25 intervention, n = 23 TAU). All participants received the intended intervention as allocated, though levels of engagement with the intervention varied (see Chapter 6, HomeHealth service documentation).
FIGURE 5.
Flow diagram of participants through the trial.
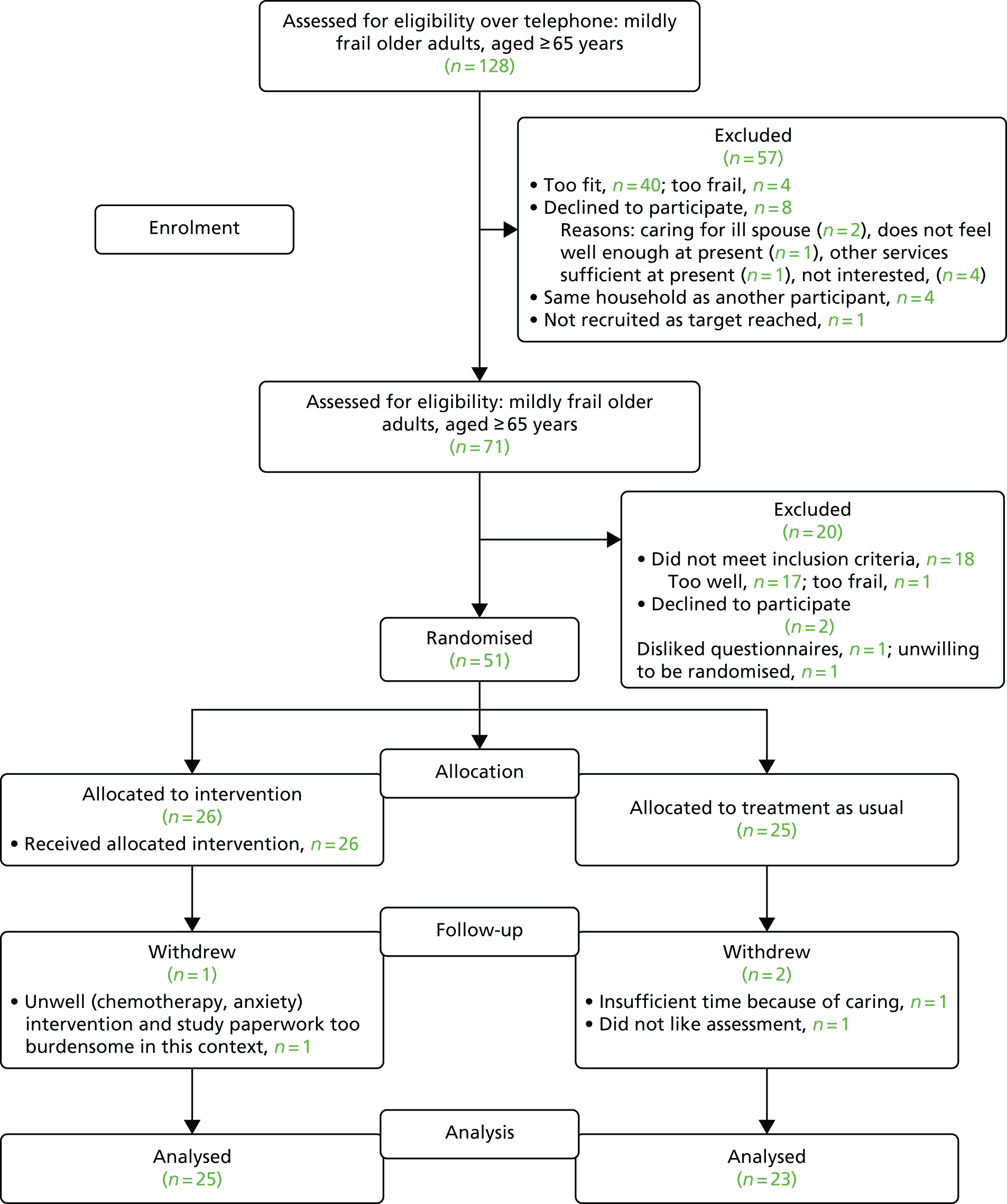
Recruitment
The trial was feasible in terms of recruitment. We successfully met our recruitment criterion of a minimum recruitment rate of 70% of our target of 50 people within 6 months. We recruited 51 people within a 5-month period, between 16 December 2015 and 13 May 2016, and most were recruited within 3 months (Figure 6). During our peak recruitment period, we were limited by researcher unavailability to undertake baseline assessments, and higher recruitment rates (in a full RCT) could be achieved with more researcher time. We met our target of recruiting 11–14 people per practice, with 25 from our urban site and 26 from our semi-rural site. The first participant was randomised on 5 January 2016 and the final outcome assessment was on 14 November 2016.
FIGURE 6.
Monthly recruitment for the HomeHealth trial.
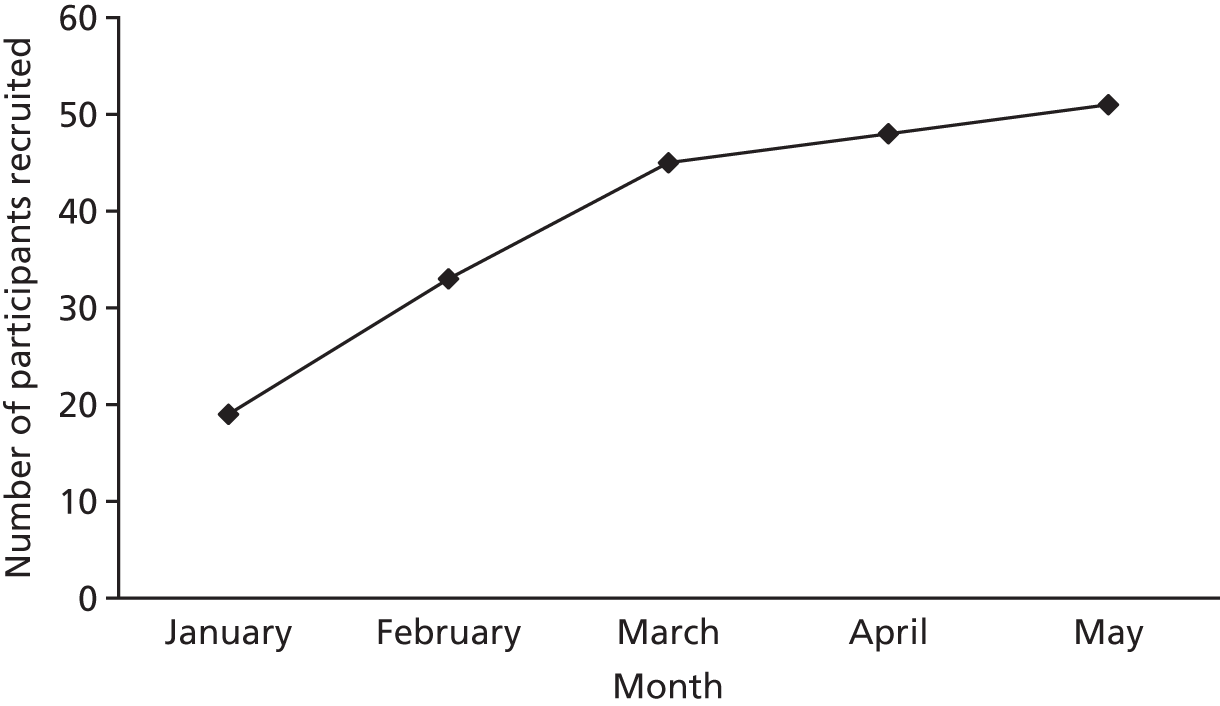
We sent out a total of 586 recruitment packs from four general practices. Two of these practices sent a single large mailing to 145 people and two practices sent these out in stages. Approximately one-third (33%) of those approached responded, of which two-thirds were positive response (22% of total mailed) and one-third declined to participate (11%). Many of the group that declined reported on reply slips that this was because they had no health concerns/felt too well. After telephone screening by the research assistant, just over half were eligible for a baseline assessment (55%). As seen in Figure 5, the majority of these did not fit the criteria as they were too fit to participate. A small number declined to participate, owing to caring responsibilities, not feeling well enough for the study, felt other services were sufficient or were simply not interested. A small number (n = 4) lived in the same house as another study participant and so, because of the potential for contamination across trial groups, could not be enrolled into the study. Out of the total approached by post, 8.4% were recruited to the study. Two people (both eligible and randomised) were recruited through clinician referral and one person self-referred, responding to a poster within the practice but was too frail to take part.
After baseline assessment, 20 were excluded, mostly as they did not fit the criteria (n = 17 were too fit). Only two people declined to participate: one disliked the baseline questionnaires and one was unwilling to be randomised.
Retention
The trial was feasible in terms of retention. Our retention success criteria (80% at 6 months) were exceeded, as 48 (94%) participants completed the 3-month and 6-month outcome assessments. Three people withdrew from the study, one in the intervention arm and two in the TAU arm. Withdrawals in the TAU arm were reportedly because of caring responsibilities and a dislike of the assessment questionnaires (the person who disliked the assessment questionnaires consented to medical notes data to be extracted for health economic analyses). One participant withdrew from the intervention arm as they were too unwell (having chemotherapy and anxiety disorder) and found the research and intervention paperwork too burdensome in this context.
Baseline data
Participant characteristics
Tables 10 and 11 show the demographic characteristics of participants at baseline.
| Characteristic | Trial group | Total | |
|---|---|---|---|
| Intervention | TAU | ||
| Age (years) | |||
| Mean (SD) | 80.38 (6.89) | 79.68 (6.36) | 80.04 (6.58) |
| Range | 67–91 | 69–91 | 67–91 |
| Gender, n (%) | |||
| Female | 19 (73.1) | 11 (44) | 30 (58.8) |
| Male | 7 (26.9) | 14 (56) | 21 (41.2) |
| Locality, n (%) | |||
| Area A (urban) | 13 (52) | 12 (48) | 25 (49) |
| Area B (semi-rural) | 13 (50) | 13 (50) | 26 (51) |
| Ethnicity, n (%) | |||
| White British | 22 (84.6) | 23 (92) | 45 (88.2) |
| Other white | 3 (11.5) | 1 (4) | 4 (7.8) |
| African | 1 (3.9) | 0 (0) | 1 (2) |
| Other Asian | 0 (0) | 1 (4) | 1 (2) |
| Country of birth, n (%) | |||
| UK | 21 (80.8) | 22 (88) | 43 (84.3) |
| Another country | 5 (19.2) | 3 (12) | 8 (15.7) |
| Educational level, n (%) | |||
| < 15 years | 4 (15.4) | 4 (16) | 8 (15.7) |
| Aged 15–16 years | 10 (38.5) | 9 (36) | 19 (37.3) |
| Aged 17–20 years | 3 (11.5) | 2 (8) | 5 (9.8) |
| > 21 years | 9 (34.6) | 10 (40) | 19 (37.3) |
| Characteristic | Trial group | Total | |
|---|---|---|---|
| Intervention | TAU | ||
| Living status, n (%) | |||
| Alone | 14 (53.8) | 12 (48) | 26 (51) |
| With spouse or partner | 9 (34.6) | 11 (44) | 20 (39.2) |
| With another family member | 3 (11.5) | 2 (8) | 5 (9.8) |
| Marital status, n (%) | |||
| Single | 2 (7.7) | 5 (20) | 7 (13.7) |
| Cohabiting | 0 (0) | 1 (4) | 1 (2) |
| Married/civil partnership | 9 (34.6) | 10 (40) | 19 (37.3) |
| Divorced | 4 (15.4) | 4 (16) | 8 (15.7) |
| Widowed | 11 (42.3) | 5 (20) | 16 (31.4) |
| Pension status, n (%) | |||
| State pension only | 7 (26.9) | 5 (20) | 12 (23.5) |
| Additional private pension | 5 (19.2) | 10 (40) | 15 (29.4) |
| Additional employer pension | 15 (57.7) | 15 (60) | 30 (58.8) |
| Housing, n (%) | |||
| Owner occupied | 18 (69.2) | 18 (72) | 36 (70.6) |
| Council rented | 2 (7.7) | 4 (16) | 6 (11.8) |
| Housing association rented | 1 (3.8) | 0 (0) | 1 (2) |
| Private rented | 2 (7.7) | 1 (4) | 3 (5.9) |
| Sheltered housing | 2 (7.7) | 0 (0) | 2 (3.9) |
| Other | 1 (3.8) | 2 (8) | 3 (5.9) |
| Deprivation by postcode (IMD decile),236 n (%) | |||
| 1–2 (most deprived) | 1 (3.8) | 1 (4) | 2 (4) |
| 3–4 | 4 (15.4) | 4 (16) | 8 (15.7) |
| 5–6 | 8 (30.7) | 4 (16) | 12 (23.5) |
| 7–8 | 10 (38.4) | 10 (40) | 20 (39.2) |
| 9–10 (least deprived) | 3 (11.5) | 6 (24) | 9 (17.6) |
| Current employment, n (%) | |||
| None | 24 (92.3) | 25 (100) | 49 (96.1) |
| Part-time | 2 (7.7) | 0 (0) | 2 (3.9) |
| Volunteering, n (%) | |||
| Yes | 4 (15.4) | 4 (16) | 8 (15.7) |
| No | 22 (84.6) | 21 (84) | 43 (84.3) |
| Carer for another person, n (%) | |||
| Yes | 5 (19.2) | 6 (24) | 11 (21.6) |
| No | 21 (80.8) | 19 (76) | 40 (78.4) |
The sample was predominantly of white ethnic origin, with a mean age of 80 years and with more women (59%) than men. It appeared more affluent than the general population, with fewer people living in more deprived areas, which reflected the deprivation levels of the study practice areas. The majority owned their own homes (70%) and had an additional pension to the state pension (76.5%). Around half (51%) lived alone and just under half (47%) had an education past 16 years of age.
Apart from a considerably higher proportion of women in the treatment arm, there were no other evident imbalances between the two groups. Between the urban and the semi-rural areas, we did not observe any differences apart from a greater number of participants who had been born outside the UK in the urban area.
Baseline functional, physical, psychological and lifestyle measures
Table 12 shows the baseline clinical data for participants. Baseline measurements demonstrate that the sample was largely independent in ADL on the Modified Barthel Index, but were demonstrating some signs of frailty with a lower grip strength and slower gait speed than population averages244,245 and had an average of three or four long-term health conditions. Mental well-being scores were slightly lower than age-adjusted population norms of 51.6 points246 and levels of psychological distress were high, with the mean GHQ-12 score at baseline (13.4) being higher than the accepted threshold for a ‘case’ of psychological distress of 11/12. 246,247 Quality of life (EQ-5D-5L) was slightly lower than population norms248 and capability (ICECAP-O) was similar to population norms. 190,249 In keeping with other data on older populations in high-income countries,250–252 they were relatively sedentary, with low physical activity levels, and were, on average, overweight (mean BMI of 28 kg/m2). There was only one smoker in the sample, but 29% had a high alcohol consumption, with an AUDIT-C score of ≥ 5 points. 253 The baseline MoCA scores suggest that many of those in the sample were mildly cognitively impaired, with a mean score of 23.6 points (a score of ≥ 26 points is considered normal cognition and < 19 points considered indicative of dementia). 189,254 Most variables were similar in the treatment and TAU arms.
| Clinical outcome | Trial group | Overall (N = 51) | |
|---|---|---|---|
| Intervention (n = 26) | TAU (n = 25) | ||
| Modified Barthel Index, mean (SD) | 98.58 (1.75) | 98.04 (2.32) | 98.31 (2.04) |
| Maximum grip strength (kg), mean (SD) | 19.46 (6.91) | 23.12 (10.38) | 21.25 (8.89) |
| Gait speed (ms/second), mean (SD) | 0.66 (0.25) | 0.65 (0.26) | 0.66 (0.25) |
| Sitting time per week (minutes), mean (SD) | 3117.69 (996.9) | 3094.56 (1041.41) | 3106.35 (1008.77) |
| Activity time per week (MET minutes), mean (SD) | 3288.04 (2707.87) | 3095.04 (3759.21) | 3193.43 (3234.03) |
| Days per activity category (n), mean (SD) | |||
| Walking | 6.15 (1.62) | 5.88 (1.96) | 6.02 (1.78) |
| Moderate | 4.28 (2.54) | 4.27 (2.15) | 4.27 (2.34) |
| Vigorous | 1 (0) | 2.5 (3) | 1.86 (2.27) |
| WEMWBS, mean (SD)a | 50.28 (9.19) | 47.68 (8.25) | 48.98 (8.75) |
| GHQ-12, mean (SD) | 13.46 (6.52) | 13.4 (5.66) | 13.43 (6.05) |
| EQ-5D-5L, mean (SD) | 0.68 (0.19) | 0.71 (0.19) | 0.70 (0.19) |
| ICECAP-O, mean (SD) | 0.80 (0.15) | 0.83 (0.11) | 0.82 (0.13) |
| Height (m), mean (SD) | 1.58 (0.09) | 1.64 (0.1) | 1.61 (0.1) |
| Weight (kg), mean (SD) | 69.27 (12.57) | 79.07 (18.51) | 74.07 (16.37) |
| BMI (kg/m2), mean (SD) | 27.59 (3.83) | 28.99 (4.77) | 28.28 (4.33) |
| AUDIT-C, median (IQR) | 2 (0.25–4) | 4 (2–5) | 3 (1–5) |
| Smoking | |||
| Never | 13 (50%) | 13 (52%) | 26 (51%) |
| Past | 12 (46.2%) | 12 (48%) | 24 (47.1%) |
| Current | 1 (3.8%) | 0 (0%) | 1 (2%) |
| MoCA, mean (SD)b | 23.76 (3.61) | 23.38 (3.91) | 23.57 (3.72) |
| Long-term conditions, n (SD)b | 3.52 (2.37) | 3.71 (1.57) | 3.61 (2) |
| Falls, n (%) | |||
| 0 | 20 (76.9) | 17 (68) | 37 (72.5) |
| 1 | 2 (7.7) | 5 (20) | 7 (13.7) |
| 2 | 2 (7.7) | 2 (8) | 4 (7.8) |
| ≥ 3 | 2 (7.7) | 1 (4) | 3 (5.9) |
Baseline Fried frailty phenotype
Table 13 shows the Fried frailty phenotype10 of participants at baseline. Although all participants met the criteria for ‘mild frailty’ at baseline, using the Fried phenotype21 and applying cut-off points for the bottom quintile of grip strength and gait speed from the English Longitudinal Study of Ageing (own data),225,255 we found that just over of half the sample were frail and only 43% were ‘pre-frail’. This was largely explained by poor grip strength, low physical activity and low energy levels in the sample. No participants were underweight.
| Frailty phenotype | Trial group, n (%) | Total (N = 51) | |
|---|---|---|---|
| Intervention (n = 26) | TAU (n = 25) | ||
| Frailty phenotype status | |||
| Robust (score of 0) | 0 (0) | 1 (4) | 1 (2) |
| Pre-frail (score of 1–2) | 15 (57.7) | 7 (28) | 22 (43.1) |
| Frail (score of ≥ 3) | 11 (42.3) | 17 (68) | 28 (54.9) |
| Frailty components | |||
| Low grip strength | 22 (84.6) | 22 (88) | 44 (86.3) |
| Slow gait speed | 10 (38.5) | 13 (52) | 23 (45.1) |
| Low BMI | 0 (0) | 0 (0) | 0 (0) |
| Low physical activity | 15 (57.7) | 19 (76) | 34 (66.7) |
| No energy to spare | 13 (50) | 19 (76) | 32 (62.7) |
Caring responsibilities
In the total sample, just over one-fifth (n = 11) of participants were carers themselves, mostly for their spouses, with an average of nearly 8 hours a day spent caring. This was causing a degree of carer strain, but most (> 80%) felt able to continue longer term (Table 14).
| Caring outcome | Trial group | Total (n/N = 11/51, 21.6%) | |
|---|---|---|---|
| Intervention (n/N = 5/26, 19.2%) | TAU (n/N = 6/25, 24%) | ||
| Average hours per day, mean (SD) | 12.6 (10.8) | 3.8 (6.1) | 7.8 (9.3) |
| Burden of caring (mean, SD) (0 = ‘not at all straining’, 10 = ‘much too straining’) | 4.8 (3.6) | 3.2 (3.1) | 3.9 (3.2) |
| Able to continue caring, n (%) | |||
| < 1 month | 0 (0) | 0 (0) | 0 (0) |
| Between 1 month and 1 year | 2 (40) | 0 (0) | 2 (18.2) |
| > 1 year | 3 (60) | 6 (100) | 9 (81.8) |
Clinical outcomes at 6 months
Functional, physical, psychological and lifestyle outcomes
Table 15 shows the participant outcome data at 6 months. Those receiving the HomeHealth service had significantly better functioning (Modified Barthel Index score), better grip strength and improved psychological distress (GHQ-12 score) than the TAU arm. There were no significant differences in any other outcome.
| Clinical outcome | Time point, unadjusted analyses | Effecta | p-value | |||
|---|---|---|---|---|---|---|
| Baseline | 6-month follow-up | |||||
| Intervention (n = 26) | TAU (n = 25) | Intervention (n = 25) | TAU (n = 23) | |||
| Modified Barthel Index, mean (SD) | 98.58 (1.75) | 98.04 (2.32) | 98.84 (1.77) | 97.04 (3.32) | 1.684 | 0.004 |
| Maximum grip strength (kg), mean (SD) | 19.46 (6.91) | 23.12 (10.38) | 28.72 (14.35) | 26.87 (11.05) | 6.480 | 0.023 |
| Gait speed (m/second), mean (SD) | 0.66 (0.25) | 0.65 (0.26) | 0.69 (0.35) | 0.67 (0.27) | 0.001 | 0.988 |
| Sitting time (minutes), mean (SD) (missing = 1) | 3117.69 (996.9) | 3094.56 (1041.41) | 2808.75 (1206.85) | 3212.09 (994.94) | –212.7 | 0.473 |
| Activity time (MET minutes), mean (SD) | 3288.04 (2707.87) | 3095.04 (3759.21) | 2653.52 (1908.88) | 2511.98 (1681.29) | –8.511 | 0.986 |
| WEMWBS, mean (SD) | 50.28 (9.19) | 47.68 (8.25) | 54.36 (9.21) | 49.74 (9.06) | 2.449 | 0.277 |
| GHQ-12, mean (SD) | 13.46 (6.52) | 13.4 (5.66) | 9.52 (5.75) | 13.17 (4.76) | –3.923 | 0.010 |
| Weight (kg), mean (SD) | 69.27 (12.57) | 79.07 (18.51) | 67.27 (13.07) | 79.69 (18.66) | 0.003 | 0.998 |
| BMI (kg/m2), mean (SD) | 27.59 (3.83) | 28.99 (4.77) | 26.82 (3.83) | 28.92 (4.77) | 0.029 | 0.941 |
| AUDIT-C, mean (SD) | 2.85 (3.15) | 3.56 (2.12) | 2.92 (3.12) | 3.52 (2.37) | –0.141 | 0.669 |
| MoCA, mean (SD) (missing = 2) | 23.76 (3.61) | 23.38 (3.91) | 24.61 (3.82) | 24.7 (4.18) | –0.460 | 0.527 |
| Falls, n (%) | ||||||
| 0 | 20 (76.9) | 17 (68) | 19 (76) | 16 (69.6) | 0.619b | 0.522 |
| ≥ 1 | 6 (23.1) | 8 (32) | 6 (24) | 7 (30.4) | – | – |
Frailty status
Frailty status improved slightly in both groups compared with baseline scores, largely explained by differences in grip strength and energy levels (Table 16).
| Frailty phenotype | Time point, n (%) | ||||
|---|---|---|---|---|---|
| Baseline | 6 months | ||||
| Intervention (N = 26) | TAU (N = 25) | Intervention (N = 25) | TAU (N = 23) | Total (N = 48) | |
| Frailty phenotype status | |||||
| Robust | 0 (0) | 1 (4) | 2 (8) | 2 (8.7) | 4 (8.3) |
| Pre-frail | 15 (57.7) | 7 (28) | 14 (56) | 8 (34.8) | 22 (45.8) |
| Frail | 11 (42.3) | 17 (68) | 9 (36) | 13 (56.5) | 22 (45.8) |
| Frailty components | |||||
| Low grip strength | 22 (84.6) | 22 (88) | 15 (60) | 17 (73.9) | 32 (66.7) |
| Slow gait speed | 10 (38.5) | 13 (52) | 12 (48) | 11 (47.8) | 23 (47.9) |
| Low BMI | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Low physical activity | 15 (57.7) | 19 (76) | 16 (64) | 13 (56.5) | 29 (60.4) |
| No energy to spare | 13 (50) | 19 (76) | 8 (32) | 15 (65.2) | 23 (47.9) |
There were no differences in the number of frailty characteristics between groups at 6 months (relative risk 1.008, 95% CI 0.67 to 1.54; p = 0.968).
Caring responsibilities
During the 6 months’ follow-up, the number of carers reduced in the treatment arm (as a result of one spouse dying and one with dementia moving to a care home) and increased in the TAU arm. Overall, at 6 months’ follow-up, the majority felt able to continue caring longer term. The number involved (n = 11) is too small to report on changes from baseline or differences between the treatment and TAU arms (Table 17).
| Caring outcome | Trial group | Total (n/N = 11/48, 22.9%) | |
|---|---|---|---|
| Intervention (n/N = 3/25, 12%) | TAU (n/N = 8/23, 34.8%) | ||
| Average hours per day, mean (SD) | 3.1 (3.5) | 4.2 (8.1) | 3.9 (7.0) |
| Burden of caring (0 = not at all straining, 10 = much too straining), mean (SD) | 3.7 (3.2) | 2.75 (2.9) | 3 (2.9) |
| Able to continue caring, n (%) | |||
| < 1 month | 0 (0) | 0 (0) | 0 (0) |
| Between 1 month and 1 year | 0 (0) | 1 (12.5) | 1 (9.1) |
| > 1 year | 3 (100) | 7 (87.5) | 10 (90.9) |
Supplementary analyses
We undertook a planned sensitivity analysis to explore the effects of excluding obese people from weight loss calculations. No participant had a BMI of < 18.5 kg/m2 at baseline, therefore no analyses were possible for this subgroup. When excluding obese participants (BMI ≥ 30 kg/m2, n = 16) from the analysis (as weight loss in this group may be intentional), there was no significant difference in weight loss between baseline and follow-up in the treatment arm compared with the TAU arm.
Health economic outcomes
In this section, we report the findings of the health economic analysis, including intervention costs, feasibility of collecting health economic data for the full trial, EQ-5D-5L and ICECAP-O as outcomes, QALYs and CALYs, health service use and the societal perspective.
Intervention costs
The costs of the intervention are summarised in Table 18, using the assumptions outlined in the methods (see Chapter 4, Intervention costs) and based on the scaling up of the service as delivered in our feasibility service. The total average cost of the intervention per patient is £307, assuming the service is delivered by a NHS band 6 employee, when four staff members are trained together and using conservative assumptions that they stay in post for an average of 12 months, with a caseload of 50 people per year (assuming each person has 6 months of contact with the service, so caseload at any given point would be approximately 25 people).
| Type of cost | Pay scale, cost (£) | Other | ||
|---|---|---|---|---|
| NHS band 5 | NHS band 6 | NHS band 7 | ||
| Total training costs per staff member (assuming four staff members attend, and including: pre-training preparation, communication skills, BCTs, nutrition training, physical activity training, BCTs follow-up training) | 859 | 1009 | 1159 | – |
| Average staff training cost per patienta | 17 | 20 | 23 | – |
| Service delivery costs – contact time per patient, including appointment time, administration (average of 322 minutes in total per patient) and travel time to appointments for service provider (average of 54 minutes in total per patient) | 201 | 263 | 326 | – |
| Consumables (per patient, including paperwork, instruction booklets, theraband/ankle weights/grip strength balls) | – | – | – | 7 |
| Total intervention appointment costs per patient, including appointment contact time, administration, travel time and consumables | 208 | 270 | 333 | – |
| Supervision costs (per patient, weekly/once every 2 weeks clinical supervision meetings)a | 14 | 16 | 18 | – |
| Total average cost per patienta | 239 | 307 | 374 | – |
Quality- and capability-adjusted life-years
The QALYs and CALYs are shown in Table 19. At a p-value of < 0.05, adjusting for baseline differences, CALYs were significantly higher in the intervention arm than in the TAU arm (mean difference 0.017, 95% CI 0.001 to 0.031). There were no significant differences in QALYs between the intervention and TAU arms.
| Outcome | Time point | |||
|---|---|---|---|---|
| Baseline | 6 months | |||
| Intervention (n = 26) | TAU (n = 25) | Intervention (n = 25) | TAU (n = 23) | |
| EQ-5D-5L | 0.68 (0.19) | 0.71 (0.19) | 0.73 (0.16) | 0.70 (0.21) |
| QALYs (95% CI) | – | – | 0.354 (0.321 to 0.387) | 0.354 (0.312 to 0.380) |
| Adjusted QALYsa (95% CI) | – | – | 0.362 (0.349 to 0.374) | 0.347 (0.334 to 0.360) |
| Intervention (n = 26) | TAU (n = 24) | Intervention (n = 25) | TAU (n = 22) | |
| ICECAP-O | 0.80 (0.15) | 0.83 (0.11) | 0.83 (0.11) | 0.79 (0.13) |
| CALYs (95% CI) | – | – | 0.410 (0.385 to 0.434) | 0.404 (0.379 to 0.429) |
| Adjusted CALYsa (95% CI) | – | – | 0.415 (0.405 to 0.424) | 0.398 (0.388 to 0.408) |
Service use
The number of participants using each service is summarised in Report Supplementary Material 7. The total cost of service use per participant in each group is outlined in Table 20. Total 6-month costs were lower in the intervention arm than in the TAU arm (£1650 vs. £2575), largely explained by differences in secondary care costs incurred by participants; a small number of participants had some expensive treatments during follow-up (e.g. pacemaker fitting, coronary bypass surgery), which were included in the analysis. Therefore, costs incurred were highly skewed with a large range, which coupled with the small sample size resulted in wide CIs in both groups. Given the small sample size, rare expensive events that were unrelated to the intervention have the ability to significantly distort the analysis and, hence, limited conclusions can be drawn from this analysis.
| Type of service | Time point, cost (£) | |||
|---|---|---|---|---|
| Baseline | 6 month | |||
| Intervention (n = 25) | TAU (n = 24) | Intervention (n = 25) | TAU (n = 24) | |
| Community care, mean (SD) | 253 (220) | 220 (204) | 650 (571) | 666 (678) |
| Secondary care, mean (SD) | 679 (1479) | 289 (487) | 1038 (2269) | 1945 (5228) |
| Total cost, mean (SD) | 933 (1528) | 508 (597) | 1732 (2410) | 2490 (5759) |
| Baseline adjusted costs | ||||
| Mean (standard error) | – | – | 1650 (908) | 2575 (927) |
| 95% CIs | – | – | –179 to 3478 | 707 to 4445 |
Societal perspective
From baseline to 6-month follow-up there was little change to benefits (Table 21). In the intervention arm, one person began receiving carers allowance at 3 months and one person started receiving attendance allowance at 6 months. One person in the intervention arm moved into sheltered accommodation 1 week before the end of the study, therefore the total additional cost of accommodation was £297 in the intervention arm.
| Type of benefit | Time point, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | ||||
| Intervention (n = 26) | TAU (n = 25) | Intervention (n = 25) | TAU (n = 23) | Intervention (n = 25) | TAU (n = 23) | |
| Received pension credits | 7 (27) | 6 (24) | 7 (28) | 6 (26) | 7 (28) | 6 (26) |
| Received attendance allowance (previously DLA) | 2 (8) | 5 (20) | 2 (8) | 5 (22) | 3 (12) | 5 (22) |
| Received housing benefit | 4 (15) | 4 (16) | 4 (16) | 4 (17) | 4 (16) | 4 (17) |
| Received council tax benefit | 5 (19) | 8 (32) | 5 (20) | 8 (35) | 5 (20) | 8 (35) |
| Received carers allowance | 0 (0) | 1 (4) | 0 (0) | 1 (4) | 0 (0) | 1 (4) |
| Received winter fuel allowance | 26 (100) | 24 (96) | 25 (100) | 23 (100) | 25 (100) | 23 (100) |
| Received carers allowance | 0 (0) | 1 (4) | 1 (4) | 1 (4) | 1 (4) | 1 (4) |
| Received universal credit | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Received direct payments | 0 (0) | 1 (4) | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Mean payment | – | £65 | – | £200 | – | – |
| Had individual budget | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mean payment | – | £50 | – | – | – | – |
There were some missing data for impact on carers with 66 out of the 252 items (26%) across the three time points having at least one value missing. The majority of the missing data were for estimation of unpaid carer time with 52% of items missing data (44/84 variables were missing at least one response). After this was identified at baseline, the wording was changed for four items from ‘how much help do you receive each week’ to ‘how much help have you received over the past 3 months’ at the 3- and 6-month follow-ups with the aim of making the questionnaire easier to complete. Owing to the high number of missing data, mean values have been imputed for missing responses.
Use of care and support services is summarised in Table 22. Unpaid (e.g. family or friends) and private (e.g. cleaners, ironing services) services were more frequently used than state services (including voluntary sector services, such as British Red Cross or Age UK). Care and support were largely accessed for activities such as shopping and cleaning, rather than basic ADLs (e.g. eating, moving around the house and personal care), in line with a mildly frail population. Total care and support costs were similar between the two groups at baseline. The total cost of help from carers at 6 months was £1563 in the intervention arm and £3632 in the TAU arm, with unpaid help accounting for the majority of the costs (£768 in the intervention arm and £2849 in the TAU arm) (Table 23 shows costs). At 6 months, the cost and use of care and support services increased for all private, voluntary and unpaid services across both groups except for unpaid help, which reduced in the intervention group.
| Type of service | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | ||||
| Intervention | TAU | Intervention | TAU | Intervention | TAU | |
| State-funded services | ||||||
| Yes, n (%) | 2 (8) | 1 (4) | 4 (16) | 4 (17) | 6 (24) | 3 (13) |
| Mean (hours per week) (SD) for those who used the service | 2.04 (0.06) | 1.5 (–) | 1.94 (1.13) | 6.59 (12.33) | 0.97 (1.25) | 0.47 (0.47) |
| Maximum | 2.08 | 1.5 | 2.5 | 25.1 | 3.0 | 1.0 |
| Privately funded services | ||||||
| Yes, n (%) | 9 (35) | 13 (52) | 11 (44) | 10 (43) | 11 (44) | 15 (65) |
| Mean (hours per week) (SD) for those who used the service | 2.64 (2.38) | 2.36 (3.18) | 2.53 (2.55) | 1.53 (0.82) | 2.76 (2.66) | 1.66 (1.48) |
| Maximum | 7.5 | 11.75 | 9.0 | 3.08 | 8.83 | 6.0 |
| Unpaid help | ||||||
| Yes, n (%) | 14 (54) | 13 (52) | 10 (38) | 12 (48) | 12 (48) | 15 (65) |
| Mean (hours per week) (SD) for those who used the service | 5.49 (5.09) | 5.69 (6.26) | 5.2 (4.42) | 11.4 (9.67) | 1.45 (1.81) | 7.37 (9.51) |
| Maximum | 15.67 | 18.55 | 13.28 | 25.83 | 5.79 | 29.75 |
| Type of care | Time point, cost (£) | |||
|---|---|---|---|---|
| Baseline | 6 months | |||
| Intervention (n = 25) | TAU (n = 24) | Intervention (n = 25) | TAU (n = 24) | |
| State-funded help | ||||
| Mean (SD) | 67 (172) | 17 (86.4) | 150 (408) | 320 (1441) |
| Minimum–maximum | 0–600 | 0–432 | 0–1588 | 0–7224 |
| Privately funded help | ||||
| Mean (SD) | 263 (535) | 353 (734) | 645 (1196) | 463 (559) |
| Minimum–maximum | 0–2160 | 0–3384 | 0–5136 | 0–1728 |
| Unpaid help | ||||
| Mean (SD) | 852 (1329) | 852 (1525) | 768 (1217) | 2849 (4292) |
| Minimum–maximum | 0–4512 | 0–5342 | 0–4116 | 0–14376 |
| Total, mean | 1182 | 1222 | 1563 | 3632 |
Budget impact analysis
We have developed a tool that estimates the cost per patient dependent on a range of assumptions. This could be used in a full RCT to estimate costs and savings for the NHS and local authorities, respectively. To illustrate this tool, we have used data from the feasibility RCT; however, please note that this is presented as an example and not definitive findings because of our small sample size:
If the NHS was to commission the HomeHealth service, and assuming that the intervention is delivered by a band 6 staff member, based on our feasibility study findings, the total cost per patient of HomeHealth would be £307 with savings of £907 per patient in secondary care and £16 in community care. This would equal a net saving to a NHS commissioner of £616 per patient. This is dependent on the number of patients the service is delivered to, with greater numbers reducing the per-patient cost.
If the local government was to commission a HomeHealth service, and assuming the same cost of £307 per patient of the HomeHealth service, the total cost saving of home-based social care is £170 and a total additional cost of £297 for accommodation. This results in a total additional cost to local government of £434 per patient.
As with all figures in this analysis it is important to note that these are based on small patient numbers and hence are very sensitive to costly outliers. For example, removing the one patient in the intervention arm who moved into sheltered accommodation towards the end of the study would make the total cost to the local government of commissioning a HomeHealth service to be £137 per patient. Similarly, there were a small number of patients in the TAU arm who received costly inpatient care, which influenced these findings.
Feasibility and acceptability of trial processes
We further assessed the feasibility and acceptability of trial processes, including data collection methods and participants’ perspectives on trial processes.
Data collection
Research assessments
Baseline interviews took 50–155 minutes to complete and the 6-month outcome assessments took 40–150 minutes. Assessments generally took longer in participants who were frailer, but all participants were able to complete the assessments in one sitting. Both self-report and interviewer-administered data collection was acceptable to participants, but a small number preferred self-report questionnaires to be interviewer administered. Participants typically required greater interviewer assistance with questions on the IPAQ-E and CSRI, as they found quantifying activity and carer help difficult. Missing data were minimal (< 1%) or there was no missing data for most measures. One person declined to complete the MoCA, as they did not want their memory assessed.
Health economic data
One of the three participants who withdrew was willing for medical notes data to be extracted (the other two were not asked at the point of withdrawal and were not contacted again). The interviewer-administered EQ-5D-5L and the self-reported ICECAP-O was completed by all participants at baseline and by all who completed follow-up at 6 months (n = 48). One item on the ICECAP-O was missing for one participant in the TAU arm at baseline. The EuroQol-5 Dimensions (EQ-5D) and ICECAP-O therefore appear to be feasible and acceptable measures to use in a full trial. Greater number of missing data were found in the CSRI, particularly regarding reporting the frequency and duration of formal and informal care (see Societal perspective). This was greater for the 3-month postal version of the CSRI, as when the questionnaire was research assistant administered (at baseline and our main 6-month follow-up) the data could be recorded more easily in the format of the questionnaire (e.g. as a fraction if less frequently than weekly help). However, missing data for the CSRI were still minimal, with a maximum of 26% missing data for the caring section. In this question, the main difficulty was informal carers (e.g. spouses, living with the person) in specifying the number of hours per day they spent caring for the person they lived with. We will work further with public representatives to improve the wording of these questions and to simplify the CSRI when possible for a full RCT. Completion of these data by telephone or face to face could also be considered, but this has implications for researcher time.
Participants’ views on trial processes
Questionnaire response rates
In total, 48 out of 51 participants completed the outcome assessment and were sent an evaluation form. Out of these, 42 (88%) replied, with similar response rates in the intervention (22/25, 88%) and TAU (20/23, 87%) arm. Response rates were slightly lower in the urban area (18/23, 78%) than in the semi-rural area (24/25, 96%). Including the three withdrawals, the overall response rate was 42/51 (82%).
Content of research assessment
Breadth
Most participants (14/18, 78% of the TAU arm; 20/21, 95% of the intervention arm) did not think that any additional questions were needed. Four participants suggested possible additional questions, related to mental health, medication, family and spare time interests including religious/spiritual practices.
‘Difficult’ questions
Most respondents (36/42, 86%) did not report any difficulty with questions. Of the six people who reported questions that they found difficult or disliked, most were in relation to the memory test (four people), one reported not liking questions that require a like or dislike or a scale of numbers, one reported not understanding some questions and one did not like one of the questions within the GHQ-12.
Length of research assessment
When asked ‘How would you describe the length of the visit?’ (Response options: too long/length of time was OK/not long enough), almost all participants (41/42, 98%) reported that the length of the assessment was acceptable, but one person reported that it was too long.
Improvements
When asked how the study processes could be improved, most participants did not have any suggestions to make. In the TAU arm, eight participants made suggestions; however, these were not directly related to the study processes and included comments such as wanting more information about transport, to be able to meet someone regularly, and follow-up telephone calls to isolated people or to ‘contact them again’.
Defining ‘treatment as usual’
We used data from two sources to assess the content of ‘treatment as usual’ in order to define this for a full RCT.
Questionnaires with treatment-as-usual arm
During the 6 months of the trial, study participants in the TAU arm reported the following lifestyle changes:
-
none (12/20, 60%)
-
dietary changes because of medical reasons (1/20)
-
starting balance exercises (1/20)
-
starting walking daily (1/20)
-
joining an exercise group (2/20)
-
joining a day centre following GP advice (1/20).
One participant said that seeing the researcher was ‘uplifting’.
Data from Client Services Receipt Inventory for treatment-as-usual arm
We used self-reported CSRI data obtained at 3 and 6 months and GP medical records at 6 months, to assess TAU in the TAU arm with regard to recent use of NHS services, private health services, local authority and third-sector provided services and other privately funded services. Data for both research arms are summarised in Report Supplementary Material 7 and those regarding the TAU arm are briefly discussed below.
Over the study period, TAU participants mainly used primary care GP services and attended outpatient appointments. Within primary care, TAU participants attended an average of four clinic appointments with a GP over 6 months’ follow-up and received an average of 0.7 GP telephone appointments and 0.3 GP home visits. Participants attended an average of 1.5 practice nurse appointments, 0.5 appointments with other practice staff (e.g. a health-care assistant) and 0.26 appointments with a specialist nurse over 6 months. A minority (13%) received a home visit from an older person community team member. Primary and community care mental health service use was rare (maximum of one person for various services).
Within secondary care, participants attended an average of 2 or 3 outpatient appointments over the intervention period. A minority (17%) had an unplanned admission or a day-patient admission. Planned admissions were rare (n = 1). The A&E/out-of-hours urgent care centres were attended by almost one-third of people, usually not by ambulance, usually one or two times over 6 months (a maximum of four times).
Self-report data for other community health services indicated that NHS optician (43%), private podiatry (39%) and NHS dental services (22%) were most commonly used. On average, these were used one or two times in 6 months for those that used it. A few attended a single hearing clinic (17%) or a NHS podiatry appointment (17%). A minority of participants (n = 1 or 2) used each of the NHS psychological therapy services, private and NHS physiotherapy services, private hearing clinic services, private nail cutting services and NHS osteopathy/chiropractic services; when used, physiotherapy and psychological services were used most intensively (an average of four or five times). No participants used NHS counselling or nail cutting services, or private counselling, psychological therapy, opticians or osteopath/chiropractic services. One-quarter took over-the-counter medicines, only 17% took vitamin D and 43% took other vitamins or supplements.
Regarding care and support services, private and unpaid care and support was most commonly accessed over the 3 months preceding outcome assessment. Privately funded care or support services (e.g. cleaners and ironing services) were used by 65% of TAU arm participants for an average of 1.66 hours per week at 6 months. This might reflect the relative affluence of the study practice areas and participants. Unpaid help from friends or family was used by 65% of participants, for an average of 7.4 hours per week. Only 13% had used state care and support services (e.g. Age UK services), for an average of 0.47 hours per week. It should be noted that high SDs were common in these data and may reflect the difficulty that participants had in accurately recording care or support time received, particularly for unpaid care.
Adverse events
Serious adverse events
A total of 10 SAEs in five participants (one SAE, n = 1; two SAEs, n = 3; three SAEs, n = 1) were reported or collected from medical notes throughout the intervention period. In six cases, it was resolved and four were under ongoing management. In the intervention arm, one participant experienced two SAEs, a chest infection leading to hospitalisation and fall within the home arising from dizziness on the stairs during normal activity. These events were judged to be unrelated to the intervention by the principal investigator (a practising GP). No serious unexpected AEs occurred in the intervention arm. Eight SAEs were reported in the TAU arm, including three falls leading to hospital admittance (n = 1 participant), fainting episodes leading to hospital admittance (n = 1), myocardial infarction (n = 1) and chest pain (n = 3 events, n = 2 participants). No deaths occurred in either arm.
Adverse events
Six AEs were reported. AEs in the intervention arm included two falls leading to injury but not hospital admission; these were not considered to be related to the intervention. In one case, the intervention was temporarily interrupted while the participant recovered. In one further participant, the intervention was temporarily interrupted while they were investigated by their cardiologist for an episode of dizziness and chest pain. In one participant, there was exacerbation of knee arthritis considered possibly related to exercises recommended by the project worker (intervention temporarily interrupted).
In the TAU arm, there was one fall leading to injury but not hospitalisation and one episode of chest pain causing a visit to A&E, subsequently diagnosed as gastro-oesophageal reflux.
Researcher blinding
Researcher blinding had only limited success. Unblinding occurred in 20 participants before or at the 6-month outcome assessment visit, mainly attributable to accidental unblinding by participants. This occurred more often in the intervention arm, in which participants called the researcher in error (e.g. to rearrange appointments for the intervention) or mentioned the name of the project worker during contacts with the researcher.
For the remaining 31 participants, the researcher made the following guesses:
-
unable to guess – seven participants (TAU, n = 4; intervention, n = 3)
-
intervention – three out of three (100%) guessed correctly
-
TAU – 16 out of 21 (76%) guessed correctly, 5 out of 21 (24%) incorrectly.
Discussion
The feasibility study demonstrated that a RCT of the HomeHealth intervention is feasible; we exceeded our success criteria for recruitment and retention. Around one-third of people we contacted by letter from their GP expressed an interest in participating; however, many of these were ineligible as they did not meet criteria for mild frailty, generally as they were too fit. This is unsurprising as Rockwood et al. 7 report the prevalence of mild frailty in the Canadian Study of Health and Ageing, using the same definition, in 305 out of 2297 participants (13.3%). As the invitation letter requested that they reply if they had one of a list of symptoms of mild frailty, we would anticipate that many more of those not replying would also be ineligible. Only one person, to our knowledge, was not willing to be randomised, indicating that an individually randomised controlled trial is feasible. There was minimal loss to follow-up [n = 3 (6%)] over 6 months, and very little missing data, indicating that our data collection methods were also feasible. The large number of outcome measures were lengthy to complete and the burden on participants needs to be considered in a full trial, especially for the more frail older people. However, completing all outcome measures was possible in a single session and only one person felt that the session was too long.
There was difficulty in maintaining blinding of our researcher conducting outcome assessments, mainly as a result of to accidental unblinding by our research participants themselves. However, the majority of measures used within the trial were self-reported/self-completed or objective measures rather than researcher-observed judgements, reducing the possibility of outcome assessor bias. Further measures to improve assessor blinding would be desirable within a full trial, but this is not likely to have a large impact on the data collected.
The sample we recruited all had ‘mild frailty’ assessed by the Clinical Frailty Scale; however, using a modification of the Fried frailty phenotype,10 around half of these participants scored as ‘frail’ and just under half as ‘pre-frail’. Participants also had high levels of cognitive impairment and psychological distress. The sample was diverse but relatively more affluent and predominantly of white ethnic origin (white British or white other), reflecting the practice areas of study general practices. Our findings may not apply in other more diverse areas and a full RCT is needed to ensure that these other areas are captured.
The clinical outcomes show some promising early findings, with significant benefits observed for the intervention in three outcomes, including a small but significant effect for the Modified Barthel Index [an important outcome that measures functioning and independence, but which is difficult to change with ceiling effects (our sample was relatively independent at baseline)]. There were also significant improvements in grip strength but not gait speed, both measuring different aspects of muscle strength. Our sample had high levels of psychological distress at baseline, and there was a significant and clinically meaningful improvement in this in the intervention compared with the TAU arm at 6 months. There were no significant differences in other outcomes, but most showed small positive (but non-significant) effects favouring the intervention. No previous study targeting those with mild frailty identified in our systematic review showed significant benefits for either functioning or psychological distress (see Chapter 2, Key findings).
Health economic outcomes also show some promising findings, but with small sample sizes there are wide CIs and inferences are therefore limited. There was a significant improvement in CALYs but not QALYs. As our developmental work with older people suggested that independence and capability was a key priority for them as they age, and our intervention’s main aim was to promote independence and well-being, CALYs are an important outcome measure for this study. Intervention costs are modest at £307 per participant (based on delivery by a NHS band 6 worker or equivalent), which is lower than those reported for an Australian interdisciplinary multifactorial intervention256 aimed at reducing frailty, which costed an average of AU$1528.52 per person (£910 at 2017 exchange rate). This is also a lower cost than a course of CBT delivered across England through the current increasing access to psychological therapies programme of £105 per session with a median number of five sessions per patient. 240,257 NHS costs were lower in the intervention arm, but there was large variability and this may be a chance finding driven by a small number of people in the TAU arm receiving expensive hospital care. As the data on health-care use was extracted from medical records, it was not possible to determine whether or not it was as a result of participation in the study. The cost to society of unpaid carers was also less in the intervention arm than in the TAU arm, although, again, caution is required when interpreting these results. We did not measure quality-of-life improvements for carers to minimise responder burden. As a result, there may be additional benefits to carers that may not have been captured. There was one patient in the intervention arm who was admitted to sheltered accommodation, one patient who received carers allowance and one who received attendance allowance increasing the costs in the intervention arm. However, a larger trial would be required to assess if this is a chance finding.
This is a small feasibility study and we should be cautious when interpreting findings based on this small sample size. Our results are nonetheless encouraging and indicate that the intervention shows promise, has the potential to be cost-effective and a larger, adequately powered definitive RCT is now needed.
Chapter 6 Process evaluation
In this chapter, we report our mixed-methods process evaluation of the feasibility RCT, based on MRC guidance for the evaluation of complex interventions. 258 It reports the experiences of receiving and delivering the new HomeHealth service, aiming to identify modifications for future implementation. It explores the domains covered by the intervention, whether or not it was delivered as intended, the dose and reach of the intervention, how it may work, and factors that may have had an impact on this.
Objectives
The objectives for our process evaluation were to:
-
explore the feasibility and acceptability of the intervention, identifying any modifications required
-
assess the implementation (reach, dose and fidelity) of the intervention
-
explore how the intervention may work and the context of the intervention.
Methods
We conducted a mixed-methods process evaluation collecting data from a range of sources:
-
recruitment and retention data (objectives 1 and 2)
-
HomeHealth service provider documentation (objective 2)
-
audio-recording of appointments with the service (objective 2)
-
questionnaires with trial participants (objective 1)
-
semistructured interviews with participants who received the HomeHealth service and service providers (objectives 1 and 3).
Recruitment and retention data
In order to determine the ‘reach’ of the intervention, we documented the characteristics of participants for the RCT and, when possible, the characteristics of those who were excluded as ineligible to take part (see Chapter 5, Participant flow and Baseline data). We do not have data on those who did not respond to the letter of invitation from their GP.
HomeHealth service documentation
Our service providers collected a range of data on intervention appointments in order to assess the ‘dose’ of the intervention received, the content of the intervention delivered and their perceptions prospectively on progress of participants during the intervention delivery.
The following data were collected by the project workers.
-
Number and duration of appointments (both face to face and telephone), rescheduled or cancelled appointments, interim contact with the service.
-
Topics covered and goals (outcome goal, behavioural goal, SMART objective).
-
Project workers’ assessment of progress towards achievement of behavioural goals at each appointment. The project worker assessed the individual’s progress towards achieving their behavioural goals at each visit according to the scale: 2 = goal achieved; 1 = some progress made towards achieving the goal; 0 = no progress made.
Using the above data, the process evaluation team (led by CA) calculated the following:
-
Number of appointments per participant and per area and the overall appointment attendance rate, accounting for cancelled and missed appointments (number of completed appointments/total number of scheduled appointments).
-
Number of participants who received fewer than three appointments (minimum intended to be delivered) and explored reasons for this by reviewing the project worker’s notes.
-
The mean rating by the project worker of progress towards participants’ behavioural goals. This was calculated as follows:
Mean progress towards behavioural goals =(1)[(n of behavioural goals score=2)×2]+[(n of behavioural goals score=1)×1]+[(n of behavioural goals score=0)×0]n of behavioural goals set across all appointments for the participant.
The overall mean behavioural goal rating score had values of between 0 and 2. We created three categories to classify participants by level of progress towards goals:
-
mean behavioural goal rating of 1.33–2.00 – good progress
-
mean behavioural goal rating of 0.66–1.32 – moderate progress
-
mean behavioural goal rating of ≤ 0.65 – poor progress.
Participant questionnaires
Questionnaires (see Chapter 4, Feasibility and acceptability of trial procedures, and Report Supplementary Material 6) were posted to participants with a return envelope for self-completion immediately after their 6-month outcome assessment in the feasibility RCT, with one telephone reminder.
We conducted a descriptive analysis of quantitative questionnaire data on the acceptability/satisfaction with the intervention components and a thematic analysis of open questions.
Semistructured interviews with participants
We conducted interviews with intervention recipients and service providers to explore the acceptability and feasibility of the service, contextual factors, content and how the intervention might work, modifications needed and potential barriers to implementation.
Design and sampling
We invited all intervention participants and service providers (the two project workers and their supervisor) to take part in a semistructured interview to explore their experiences with HomeHealth.
Data collection and informed consent
Along with the evaluation form for the intervention arm, all participants who received the service were sent an invitation to take part in an interview. Those who gave a positive reply were contacted by telephone, posted an information sheet and an interview was arranged.
The interview took place in the participant’s home, with informed consent. The topic guide is attached in Report Supplementary Material 8. The mean length of the interview was 49 minutes (range 23–87 minutes). Interviews were audio-recorded, transcribed and data anonymised. Participants were given a £20 high-street shopping voucher as thanks.
Service provider interviews were conducted in a private room in university offices and were audio-recorded as well as transcribed.
Data analysis
Data were analysed using a thematic analysis with constant comparison using a similar approach as described for the intervention development qualitative interviews (see Chapter 3, Methods). 184 We collated data in NVivo 11. Members of the multidisciplinary analysis team (CA, KW, KK, RF, AL and public representative RE) independently read transcripts and identified a preliminary thematic framework, which was refined and agreed by consensus across a series of meetings. This thematic framework was applied to a selection of transcripts by CA, further refined with the team and then applied by CA to all transcripts. The themes generated were then considered and interpreted by the team.
Intervention fidelity: audio-recording of intervention appointments
We requested that all intervention appointments were audio-recorded by the project worker for the purposes of checking fidelity of delivery of the intervention (i.e. that it was delivered as intended in our service manual), with consent of the participant.
Data collection
During the delivery of the intervention, the service providers were asked to use appointment checklists (see Report Supplementary Material 9) to self-report the delivery of key tasks included in the intervention. Checklists were specific to the appointment number (first, interim, final). These were used as the basis for fidelity checklists for independent verification by the process evaluation team.
The fidelity of delivery of the intervention was assessed by analysing a stratified random 10% sample of recorded appointments against a prespecified fidelity checklist of key components of the intervention (see Report Supplementary Material 9). We randomly sampled 10% of first, interim and final audio-recorded appointments across the two areas, according to a randomisation algorithm developed by PRIMENT CTU. The randomly selected recorded appointments were transcribed and anonymised.
Fidelity assessments
After the end of the trial, researchers rated the completion of tasks as per fidelity checklists, specific to the appointment number (first, interim, final). In the first instance, two coders independently read and coded three appointment transcripts (one first, one interim and one final appointment) using the fidelity checklist. The researchers met and discussed their individual findings, resolved any disagreements by discussion and came to a consensus regarding applying the rating checklist. The two researchers then coded independently the remaining appointments.
Tasks were initially coded as one of four categories: completed, completed to some extent, not done and not appropriate to be done. Tasks that could not be assessed from the transcripts of audio-recording (e.g. items regarding completion of intervention paperwork such as recording goals in ‘My Health and Well-being Plan’) were not included in the calculation of fidelity scores. We calculated an overall score for intervention fidelity (tasks completed or completed to some extent/expected tasks as described in the checklist). The discrimination between ‘not done’ and ‘not appropriate to be done’ in any given appointment was difficult to assess. Therefore, we took a conservative approach and considered tasks rated by the researchers under ‘not appropriate to be done’ as ‘not done’.
Results
Recruitment and retention data
The representativeness of the sample included in the RCT was reported earlier (see Chapter 5, Participant flow and Discussion). We have no specific information on those who were approached by letter from their GP but did not respond. However, we can assume that, as the prevalence of mild frailty on the Clinical Frailty Scale is only 13% in older people7 and we were unable to accurately determine frailty status before approaching participants, a large proportion of those not responding would not be eligible, largely as they would not be frail. Therefore, although only 8.4% of those approached by letter were recruited for the study, it may be that only a small proportion of those not responding would have been eligible. Only one person declined to take part as they did not want to be randomised, but it is possible that more people did not respond because they realised only half would receive the service. Of those who agreed to take part, most of those randomised to receive the intervention went on to engage with the service. Reasons for non-engagement are outlined in Number of appointments and appointment attendance rate.
HomeHealth service documentation
Number of appointments and appointment attendance rate
In total, there were 126 completed appointments with the HomeHealth service and 12 appointments that were missed or cancelled and rescheduled. The overall appointment attendance rate was 126 out of 138 (91.3%).
The median number of appointments across the two areas was five (range 1–8 appointments). A difference was noted between areas, with participants in area A (urban) receiving slightly more appointments than those in area B (semi-rural). The median number of appointments was six and five, respectively. Most (22/26, 85%) received our minimum ‘dose’ (three out of six potential appointments). One participant in area A received fewer than three appointments, compared with three participants in area B. Reasons for non-engagement included being too fit (n = 1), concurrent medical problems (n = 1), their partner restricting involvement (n = 1), and not being interested (n = 1).
Appointment length and location
The average length of appointments with the service was 62 minutes and it varied depending on the number of the appointment (first, interim, final). The median length of first appointments was 122 minutes (range 45–195 minutes), that of interim appointments was 45 minutes (range 5–145 minutes) and the median length of final appointments was also 45 minutes (range 2–63 minutes). The majority (116/126, 92%) were conducted in participants’ homes, with a few (10/126, 8%) conducted by telephone.
Topics covered by the HomeHealth service
Nearly all (25/26, 96%) participants identified at least one outcome goal in their first or subsequent appointments. One participant did not identify any goals and withdrew from the study after the first appointment. More than half of participants (16/26, 62%) identified more than one outcome goal/topic to work on during the service.
Mobility and physical activity was the most popular goal, chosen by 19 out of 26 (73%) participants. Other domains covered were environment (e.g. home adaptions, decluttering their home), psychological well-being, socialising, finances, diet, transport, memory, supporting carers, and dealing with incontinence or sensory impairment. The number of goals set for each domain are summarised in Figure 7.
FIGURE 7.
Distribution of topics covered by the HomeHealth service (over total number of outcome goals/topics covered).
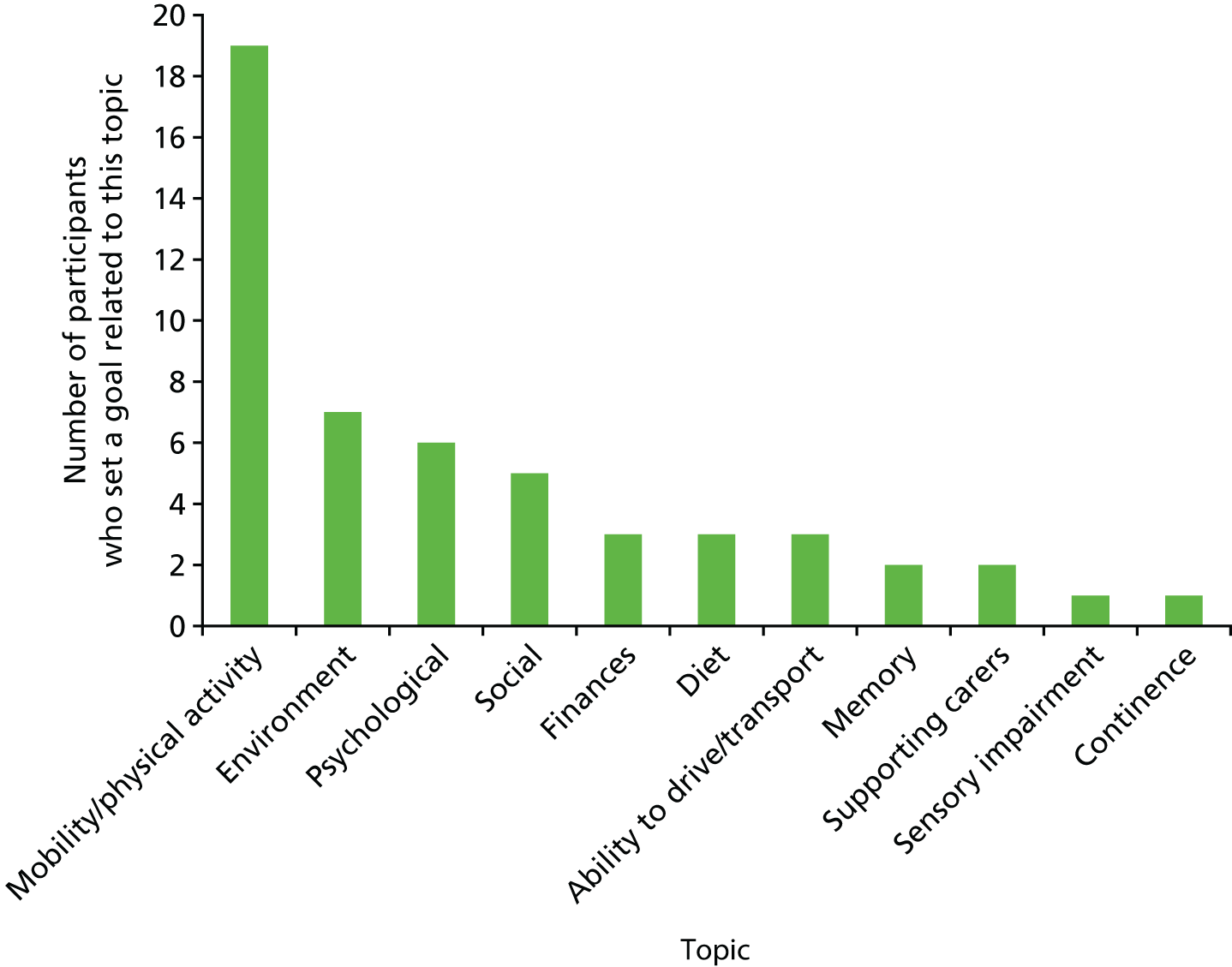
Progress towards behavioural goals
Based on average behavioural goal progress rating scored by the project workers, we estimate that 13 out of 26 (50%) participants made good progress, 8 out of 26 (31%) participants made moderate progress, and 5 out of 26 (19%) participants made poor progress. This last group includes the four people who received fewer than three appointments (see Number of appointments and appointment attendance rate for reasons for non-engagement) and a further person who received three appointments but made limited progress on their goals because of medical problems.
Fidelity of delivery: analysis of audio-recorded appointments
Analysis of a random 10% sample (13/126) of appointments with the HomeHealth service showed that, in total, 119 out of 165 (72.1%) of items on the checklists that could be assessed by review of appointment transcripts were completed during appointments. By chance, 3 out of 13 randomly selected audio-recorded appointments belonged to a single participant with cognitive impairment, for whom using a behaviour change goal-setting approach was very difficult and the project worker chose instead to use a different, more open and case-management approach. There was one other appointment with low fidelity from a different participant, which was a brief telephone final appointment.
Stratified analysis of this small random sample of transcribed appointments showed that the fidelity of delivery was moderate to high (77%) (range 30–100%) excluding the individual with cognitive impairment, and it was modest (53%) (range 38.9–70%) in the person with cognitive impairment across the three recorded appointments selected.
Many of the items that we have conservatively recorded as ‘not done’ in our fidelity analysis above were originally scored as ‘not appropriate to be done’, but these two categories were merged in analysis as differentiation between them for several items was difficult and subjective. A limitation of the fidelity assessment method that we used is that some items that were not delivered in one randomly selected appointment may have already been covered in other appointments. As we randomly sampled appointments and not participants, we were unable to track if items were covered over a series of appointments; therefore, our estimated fidelity score above may underestimate the true fidelity.
These findings indicate that further refinement of the appointment and fidelity checklists should be considered in order to reflect the flexibility in approach in which a goal-setting behaviour change approach is less applicable. For fidelity analysis in a full trial it may be more suitable to randomly sample participants not appointments, and assess fidelity across all appointments for each randomly selected individual.
Questionnaire data
Questionnaires sent to all intervention arm participants completing the study assessed overall acceptability of the HomeHealth service. Questionnaires also assessed acceptability of the study procedures (see Chapter 5).
Overall attitudes
Most (19/21, 90%) of the participants reported that they found the HomeHealth service helpful or very helpful. Among the reasons they gave for this were that the service provider raised a number of subjects that they had not dealt with before, encouraged them to do things that they should have done long ago, made them aware of all that was available, made them feel comfortable and helped them with goals that were realistic.
The majority (19/21, 90%) of respondents were satisfied/very satisfied with the progress they made towards their goals. Similarly, most reported that the HomeHealth service met their expectations (18/20, 90%) and would recommend the service to a friend or family member (20/21, 95%).
Service delivery
All respondents (100%) felt they had about the right number of appointments and were happy with the home-based location of the appointments. Most considered the appointment length to be ‘about right’ (19/21, 90%), although one respondent said it was too long and one respondent said that it was too short.
Strengths of the service
All respondents (100%) said they could talk to their service provider freely and openly. When asked in an open question what they liked about the service, participants reported liking that it gave them an insight into available services, encouragement to do things and the home-based location.
Limitations of the service
When asked in an open question what they did not like about the service, more than half of respondents (11/21, 52%) reported that there was nothing to dislike. Two respondents reported it useful/OK, one said that they did not know what goals to go for, one that they felt inundated with various simultaneous medical appointments, one said that some of the questions did not seem relevant, one found the questionnaire disconcerting sometimes, and one said that there was too much focus on improvement.
Improvements to the service
When asked how we could improve the service, the majority of respondents could not think of any improvements, but one respondent said that they would have liked more focus on acceptance, and another said they would have liked appointments to be more spread out over time.
Semistructured interview findings
In this section, we report the findings of the semistructured interviews that aimed to explore participants’ motivations for participating in the study, their experiences of the service and acceptability, potential processes of behaviour change and the context in which the service was delivered, as well as providers’ experiences of delivering the service and how it could be modified.
Sample characteristics
In total, 19 semistructured interviews were conducted (older people, n = 16; HomeHealth project workers, n = 2; supervisors, n = 1). The demographic characteristics of service recipients are shown in Table 24.
| Characteristic | Number |
|---|---|
| Area | |
| Area A: urban | 11 |
| Area B: semi-rural | 5 |
| Gender | |
| Male | 4 |
| Female | 12 |
| Age group (years) | |
| 70–74 | 2 |
| 75–79 | 4 |
| 80–84 | 4 |
| 85–89 | 3 |
| ≥ 90 | 3 |
| Ethnicity | |
| White British | 12 |
| Any other white | 3 |
| African | 1 |
| Years of education | |
| Before 15 years | 2 |
| Between the age of 15 and 16 years | 4 |
| Between the age of 17 and 20 years | 2 |
| After 21 years of age | 8 |
| Progress towards goals | |
| Good | 8 |
| Moderate | 7 |
| Poor | 1 |
Motivation and expectations of participating
Motivations
The majority of people participated because they acknowledged they had unmet needs related to their health and well-being, and were hoping they could benefit from a health promotion intervention. Poor mobility, problems with balance, low mood and loneliness were the main issues identified by participants:
And I said, ‘Well I’ve got nothing to lose and if it’s going to be of help to me, then that’s fine’, [. . .] I was having problems with my balance and knees [. . .]. And I’m very happy that I put my name down and was chosen.
Male, 87 years
Other reasons reported for joining the study included a desire to remain independent, fear of losing independence in the future, hope to improve access to GP consultations, curiosity and altruistic reasons such as contribution to the community and the NHS:
Well I think it’s such a good idea, you know, if, if it can help to save money for the NHS, then, you know, I’m all for it. And um, I thought it was a very good idea to do that.
Female, 92 years
Expectations
Most participants were uncertain about what exactly to expect from the study. Some of them mentioned that they were hoping to get some help in managing problems such as low mood, anxiety, memory loss, chronic pain and reduced mobility.
Delivery of the HomeHealth service
Appointment length, timing and location
In line with quantitative data collected from the questionnaire, most participants reported being happy with the length of the appointments. One participant with cognitive impairment felt that the first appointment was too long, and they informed the project worker, who modified this for further appointments.
Most people were happy with the time arrangements of the appointments, but one participant with complex needs said they found it difficult to keep up with attending concurrent medical appointments over the same period of time, after being recently discharged from hospital. Most participants were happy with the frequency of the appointments with the service as it allowed them sufficient time in between to work on the goals they had set with the service provider.
All participants were happy with the home-based location of the appointments for many reasons, including convenience, feeling at ease, confidentiality, difficulty mobilising outdoors and having a home assessment (allowing for environment modifications, mentioned by one participant).
HomeHealth service provider attributes
Participants in both areas were positive regarding the communication skills of the service providers. They described the project worker as a good listener, relaxed, friendly and easy to talk to. Personal attributes, such as being kind, punctual and consistent, were valued by participants:
Yes, she was very good and very friendly. We got on extremely well. And um, I think she’s the perfect person to go and meet older people and talk to them and support them and I think it’s wonderful, um, when people realise that there is that support.
Female, 82 years
Participants were unsure what particular skills the service provider was meant to have, but were happy with a trained non-specialist professional delivering the intervention. Although most people reported enjoying a younger person visiting them as the project worker, one participant who did not engage very well with the service thought it might have been different with a person closer to their age, with more life experience.
Topics covered by the HomeHealth service
Participants and service providers (project workers and supervisor) described in detail what was covered within their contacts with the HomeHealth service, and their experiences in addressing each aspect.
Getting out and about: mobility and physical activity
Most HomeHealth participants identified getting out and about as a priority in order to maintain their independence. Many participants reported being encouraged to increase their physical activity, by walking or doing muscle strengthening or balance exercises. Overall, exercise was highly valued by HomeHealth participants, as it helped them increase their energy levels and had a positive impact on their mood. Some participants joined classes as a result of receiving the service, whereas others undertook exercise individually, facilitated by the service provider who gave them leaflets or booklets demonstrating exercises:
. . . she actually got me walking in the afternoons. Um, it was only my laziness that stopped me normally walking in the afternoons since the tendonitis. But um, I think she did a good job there.
Male, 82 years
Getting out and about: transport
Dealing with transport-related issues was reported by HomeHealth participants as equally important in getting out and about, particularly for people with difficulty mobilising outdoors. The project workers gave information and assistance regarding available options [e.g. blue badges (car parking for disabled people) and taxi cards (subsidy for travel for disabled people)].
I got a lot of help one way or another, often from people that [project worker] put me in touch with, … the right experts. And . . . that was . . . really helpful. I mean I didn’t know about the taxi card, . . . which if you don’t mind it being a bit late, is brilliant.
Male, 78 years
Mood and psychological well-being
Dealing with low mood, anxiety and bereavement was a key area identified by many participants. People appreciated that somebody was there to listen to them and signpost them to the right services. The project workers were reported by HomeHealth participants to have encouraged them to socialise, increase their physical activity, create time for themselves, and raise their self-esteem.
I felt really down and . . . somebody said that they help you inasmuch as they can, . . . suggest places to go and things to do, so you’re not so isolated, . . . you need somebody to talk to and give you confidence to go out there and do it, which is what [project worker] did.
Female, 73 years
Social activity
Physical health and transport were described as important determinants of social activity for most interviewees. Participants reported project workers providing information about locally available socialising opportunities and encouraged them to engage in leisure activities.
Memory
For two of the participants, concerns regarding significant memory problems became apparent during the delivery of the intervention and they were both referred to the memory clinic (one diagnosed with mild cognitive impairment and one with dementia). Project workers reported using a case-management approach with these two participants, signposting and co-ordinating access to a range of support services, which was felt to be successful by family carers:
. . . My mum has found that she has some memory problems [. . .] [project worker] she’s been very helpful in putting in touch with, . . . various . . . support that is available. [. . .] So, it was quite nice to have somebody who was able to just point us in the right direction, different services.
Family carer of participant with dementia
Diet
Diet and ensuring adequate nutrition was one of the core topics within the HomeHealth service remit that project workers discussed in initial assessments with all participants. However, this was infrequently identified by HomeHealth participants as a topic to address within the ongoing service. Losing weight was an outcome goal set by some overweight participants and one interviewee reported becoming more aware of their diet as a result of receiving the service. However, project workers reported difficulties in dealing with the opposite, that is, unintentional weight loss. One participant with complex needs who was losing weight was offered screening for malnutrition, which showed they were at risk, but the client found it difficult to accept that:
You know, it’s really, really difficult trying to get somebody to want to gain weight, because it’s so unknown. People really don’t understand the consequences of malnutrition and often don’t think they’re malnourished.
Project worker, area A
Supporting carers
When applicable, participants reported that the project workers supported them in caring for those close to them (e.g. spouses) with disability, by signposting them to available services for provision of equipment, giving them information about benefits, and offering respite solutions:
And you’ve got to start thinking and generally chatting with [project worker] it made me think more positive . . . we changed the car, got an estate. He’s got a mobility scooter. He’s got a wheelchair . . . And it made us think, ‘Well, hang on, we can, I can cope with this,’ . . .
Female, 72 years
Finances
One participant reported that the service supported them in dealing with financial matters, such as claiming benefits that they were entitled to. Project workers felt that finance could be a sensitive topic and dealing with it generally required an initial period of establishing rapport and trust:
So, once we’d built up this trust, we then started talking about finances.
Project worker, area A
How does it work?
The intended functions of the HomeHealth intervention were education and enablement, training, and restructuring the environment. This section explores how participants understood its purpose and which elements of the intervention they valued and responded to, giving examples of the methods used within these intervention functions.
Education and enablement
Education was provided in many ways, including the introduction of new ideas. Project workers gave information on locally available services and signposted or referred participants when appropriate. Many interviewees said that the new service increased awareness of local service provision.
And sort of, um, and you know, and opened my eyes to the sort of help you can get from, from the council. And the local NHS, you know.
Male, 78 years
Project workers enabled participants, optimising their capability. For example, they supported a client who was caring for her husband with disability to get equipment such as wheelchair and mobility scooter, encouraged people with poor physical health to seek medical advice, and so on:
And then we went into things . . . I didn’t know I needed and, . . . I we sort of discovered a few things that I could be helped with and she helped. And she did put me in touch with people . . . it’s all been a very good outcome for me really.
Female, 92 years
Training
Participants were provided training in home-based muscle strengthening exercises and balance exercises, as well as problem-solving skills. Participants responded well to practical elements of the intervention and reported feeling motivated to put exercises into practice.
My balance is much improved, because she gave me or suggested exercises I could do. She gave me some literature. She covered most exercises I should do and I did. And um, I felt much more positive, yes. Those were a tremendous, tremendous help.
Male, 87 years
This was supported by the use of exercise aids, which were popular with many:
. . . [Project worker] brought things for me to exercise. I have to do weights with my feet; to exercise walking, . . . I did lots of exercises and she brought all the things for me. And it got me better and better and better.
Female, 78 years
Restructuring the environment
For some, making environment modifications was an important part of the intervention by providing support to remain independent at home (e.g. occupational therapy referrals to fit grab rail and bathroom modifications) or, in one instance, improving mental well-being by supporting home decluttering in a participant with anxiety:
. . . mainly clearing out cupboards. Getting rid of things that you didn’t need. [. . .] I’ve got rid of four bags . . . a settee . . . a chest of drawers that I didn’t need . . . Once I’d done it, it felt good, oh that’s lovely, my life’s not so cluttered now.
Female, 77 years
Counselling and reflective listening
Counselling and reflective listening appeared to be an essential part of the intervention. Project workers reported this as a valuable tool used in the initial comprehensive assessment as well as continuously throughout the delivery of the service. Many participants clearly valued this aspect:
I felt that I could talk to her, that I’d known her a long while. There was no um, I don’t know what the word is, no I felt that, um, I could talk to her. [. . .] And that she listened and suggested.
Female, 77 years
Goal-setting
The majority of participants found it helpful to have a goal to work on and their progress being monitored. Goal-setting was thought to increase motivation:
The goals we set was . . . she used to say to me, ‘Could you go for a walk for half an hour?’ Then we increased it to three quarters of an hour and then we increased it to an hour. And I found it very therapeutic.
Female, 80 years
People had different aspirations as to what extent they wanted the project worker to ‘push’ them to achieve their goals. Most of the participants were happy with what they received, described as ‘gentle encouragement’ or somebody ‘gently giving them a push’, although one participant felt that they might have wanted to be pushed a bit further.
Monitoring, including self-monitoring of behaviour/outcomes and giving feedback
The project workers monitored the participants’ progress towards their goals and gave them feedback about the progress made. They also encouraged people to self-monitor their behaviour, by keeping diaries or using technology (e.g. recording walking activity on a smartphone):
Well we were talking about diet and things like that and [project worker] suggested doing a weekly planner of food for the week and see how I get on like that.
Female, 72 years
Social support (emotional and practical)
Practical support was an essential part of the intervention and was particularly useful in people with complex needs, for whom the project workers, for example, undertook a case-management role by signposting or referring them to appropriate services, helped them to arrange appointments to address their unmet needs (e.g. psychological, hearing impairment, memory issues), liaised with third-party organisations with their consent, and/or involved a family member in the care of a client who was newly diagnosed with dementia.
Emotional support from the project worker was reported by participants as particularly helpful in people experiencing loneliness, low mood, anxiety or memory loss. Participants with loneliness explored strategies to improve their social activity (e.g. those who were fit enough were encouraged to engage in voluntary social activities in the community or share their previous experiences as carers).
She was encouraging, she was telling me that I was doing well, improving. It’s very nice to be encouraged, but I was – actually when she was with me, I was well because she is so nice. And I was enjoying her visits.
Female, 87 years
Factors affecting engagement with the HomeHealth service
The main factors affecting engagement with the service were contextual factors, physical health, cognitive impairment, personality of the individual, personal interaction, level of need, mismatch of expectations and suitability of the approach to the individual.
Contextual factors
What was going on in people’s lives at the time that the service was delivered had an impact on their engagement with the service. For some of them it coincided with recent deterioration in their physical or mental health, bereavement, cognitive decline, disability of the partner among other things, and people found it beneficial to receive support during that phase of their lives. For others, the sheer number of appointments they had outside the service, given the complexity of their needs, could be overwhelming and they were reluctant to add more.
Rather than thinking, ‘Oh this is the end of life, we’re just going to sit here until it’s over’ . . . You start thinking, ‘Right, this is not me, we’re not letting things go, I’ve got to pick myself up.’ . . . it all came at the right time really for me.
Female, 72 years
Physical health
The level of physical health did not affect appointment attendance as a result of the home-based location, but in some cases it had an impact on the participant’s progress towards their goals. Symptoms such as breathlessness, joint pain or swelling were mentioned by some interviewees as factors limiting their ability to undertake or sustain exercise:
Yes, because still I do have my spine, my back and my hip, arthritis is there. That is not going away. Yes, the arthritis is still there. And it’s on my shoulders as well, yes. But I am otherwise – the exercise, the walking is doing me well.
Female, 78 years
Cognitive impairment
In two participants, problems with memory impairment affected their ability to retain information sufficiently to progress towards their goals and, in both cases, the project worker reported modifying the approach to be focused more on the facilitation and co-ordination of care.
[Project worker] looked into it. And that was, that was something she sent me through the post actually, was the different exercise classes that were available. And then we – we decided that that was the best one for my mum. So, my sister takes her there every Monday.
Family carer of participant with dementia
Involvement of family carers with the client’s consent was also important.
To get around that [goal-setting], it was actually, in this case . . . it was luck that her daughter came in just half way through the second appointment. And we were able to sort of have an agreement that we would all work together . . . And that worked.
Project worker, area A
Suitability of a behaviour change approach
Two participants reported that a goal-setting approach was not relevant to them. One of them thought that goal-setting applies to younger people and another came from psychoanalytic background and disliked the language and approach of goal-setting:
I mean, like I was asked how would I like to be better. And, of course, there are ways I would like to be better than I am . . . But I felt then under pressure with letters coming, ‘You have undertaken to . . .’ And I really objected to that.
Female, 82 years
The project workers thought that BCTs worked generally well for most, but not all, participants, and were concerned that some participants may have found it pressuring to be asked questions in the process of identifying goals to achieve.
Complexity of needs and mismatch of expectations
In one person with complex health needs (multiple health conditions and disabilities limiting capability), dealing with unrealistic expectations was a challenge for both the project worker and participant:
Yes, I mean the main goal was getting me walking again and that’s not happened . . . that was the main thing, which, you know, with arthritis, was asking too much, because, you know, you can’t do it . . .
Male, 78 years
Service provider perspectives: training, supervision and roles
Project workers and their supervisor reported them gradually gaining confidence to deliver the intervention, as it was a newly developed multidimensional service. They felt that more training on nutritional advice for older people and approaching the issue of weight loss would be beneficial.
. . . everything was new to start with. And there was a lot about getting them confident to deliver the service. And it was a service that we had just developed . . . So there was a lot to grapple with in the beginning.
Supervisor
Project workers enjoyed this new role of being a counsellor and a ‘motivator’ for older people:
And I think it comes down to change. And so are you a change agent, . . . you’re a bit of a life coach in some respects. You’re a bit of a counsellor. You’re a bit of a change agent, you know, you’re a bit of a motivator.
Project worker, area B
The project workers thought that they had good support throughout the delivery of the new service. The content of their supervision was about their engagement with the individuals, communication skills and use of BCTs. If any clinical issues arose from participants, they were able to discuss them with clinical members of the research team or the participant’s own GP.
Strengths of the HomeHealth service
Overall strengths of the new service from the accounts of both participants and the project workers appeared to be its comprehensive approach, enablement and reinforcing self-efficacy, sense of achievement leading to increasing confidence and sustainability.
Comprehensive approach, enablement and sense of achievement
Most of the participants thought that the service covered all areas, had a positive impact on their confidence and made them more capable of managing their everyday activities. Many of them said that the achievement of goals made them feel good about themselves:
That’s what I wait for and the programme has helped me to overcome all my fears and things. And I am managing myself better now. Although the body is not 100% strong, but I am able to manage myself now.
Female, 78 years
Filling a gap in services
For many participants, their participation in the study was offered at a time when they were facing increasing needs not covered by existing services. The new service filled a gap in services for older people who did not know where to seek help from, especially those who were physically or socially isolated:
. . . I really, I needed that help, definitely, I needed someone to sort of push me . . . would I have done them without their help? I really don’t know, I really don’t, because I didn’t have any numbers, any telephone numbers.
Female, 73 years
Sustainability
Many participants reported seeing some change (e.g. in physical health, mood and outlook) as a result of receiving the service. Many of the participants also said they carried on with activities or behaviours adopted through the service even after the end of it, and this view was also held by the project workers:
. . . and it was important and a lot of people made huge, huge progress. But it was some simple things, but it was still, it was big things to them. And it has made a difference. It’s made a difference that I think is sustainable for them.
Project worker, area B
Limitations of the HomeHealth service
Few limitations were mentioned in accounts of experiences of the service by participants. As mentioned above, a behaviour change approach was not felt suitable for a minority of participants. Further limitations of the new service mentioned in the interviews were mostly related to limitations in other services that the HomeHealth service could signpost to (e.g. long waits for NHS physiotherapy, issues with Increasing Access to Psychological Therapies (IAPT) telephone assessment for a participant with anxiety disorder). One family carer of a participant with dementia felt that longer-term support should be available to support people with dementia living on their own.
Discussion
The service was overall feasible to deliver and highly acceptable to the majority of recipients (objective 1).
The ‘reach’ of the service (objective 2) is hard to determine accurately for this feasibility study, with no information available to determine the eligibility of non-responders. It is likely that not all of those who were potentially eligible responded to a postal invitation from their GP, and few were referred from other sources (e.g. via clinicians). Although our sample included some social and educational diversity, the study practices were in relatively more affluent areas and there was little ethnic diversity. Our findings might not apply to other populations and this should be addressed in a larger-scale evaluation.
The ‘dose’ of service received (objective 2) varied between individuals, as expected with a tailored service, with a median of five appointments (range 1–8 appointments). Four participants received fewer than three appointments (and did not engage with the service), for a variety of reasons. The original intended service was for six appointments, and up to 12 if needed, but our data suggest that a lower-intensity service may be sufficient for most.
The service was delivered mostly as intended, with generally high-intervention fidelity (objective 2). As planned, the service covered a range of domains, with goals identified tailored to the individual. Mobility and physical activity were by far the most popular type of goals chosen, but goals also included addressing psychological concerns, social isolation and modifying their home environment among others. Diet and nutrition was rarely identified as a goal by participants, in particular how to prevent weight loss and maintain nutrition. This is consistent with our qualitative study in the development phase (see Chapter 3), in which older people did not perceive this to be a problem to address.
Most participants were able to set outcome and behavioural goals and most were reported by project workers as making good or moderate progress towards meeting these. For a minority of participants, a goal-setting behaviour change approach was less suitable, including those that did not like the accompanying implied need for change (albeit in order to maintain an asset for longer) and those with more significant cognitive impairment. In the latter group, involvement from a carer was needed from the outset. A case-management/co-ordination of care approach was used alongside goal-setting for participants who were frailer with more complex needs or cognitive impairment.
Objective 3 was to explore how the intervention might work. In line with the behaviour change approach used, it appeared that the main mechanisms of the impact of the service included a combination of increasing motivation to take action through goal-setting, monitoring and feedback with a counselling/reflective listening and emotionally supportive role. In conjunction with goal-setting, there was an education and skills training component, particularly regarding muscle strengthening and balance exercises to increase mobility. There was also an ‘enablement’ practical support component in particular for overcoming barriers in capability and opportunity to make changes, including signposting (e.g. on local support/services available), providing aids (e.g. exercise aids) and supporting environmental modifications. Overall, our findings support the processes outlined in our logic model (see Chapter 3, HomeHealth: intervention description and Figure 3).
The strengths of this process evaluation include drawing on data from a range of sources and a good response to the end-of-study evaluation questionnaires, including from those who had low engagement with the service. Interviews with both those who delivered and received the HomeHealth service helped us to identify potential modifications for a full-scale trial. Limitations include that data are drawn from a small sample participating in the feasibility RCT and that not all agreed to be interviewed. As this includes some of those who also had low engagement, it may be that there are other limitations of the service that might have emerged on further probing at interview. Regarding fidelity assessment, it is challenging to transform qualitative data from audio-recordings to quantitative measures and inevitably involved a level of subjectivity. Furthermore, the lack of consensus on what fidelity thresholds should be adopted makes it more difficult to quantify the size of fidelity. 259 Our conservative approach of including tasks ‘not appropriate to be done’ with those ‘not done’ (as the differentiation between these was in many instances difficult) will underestimate fidelity as not all tasks could be expected to be achieved in any given appointment.
From our process evaluation, we identified some potential modifications that might improve service delivery including:
-
greater clarity for potential recipients on what the service includes in the initial approach
-
inclusion in the manual of specified alternative approaches to using a goal-setting behaviour change approach for those who do not want to engage with this, or for those for whom this might not be suitable (e.g. those with dementia and no carer to support them)
-
inclusion in the manual of a section on involving carers
-
consider expanding training to include more on nutritional advice (which was infrequently addressed), cognitive impairment (as this was common and required adaptations to intervention delivery) and potentially more advanced counselling skills (as this was a core intervention component).
Chapter 7 Discussion
Summary of findings
Within this project, we conducted two systematic reviews, a state-of-the-art review, a policy-scoping review, a qualitative study, intervention development panels and a feasibility RCT with process evaluation.
Evidence reviews
Our systematic reviews identified only limited evidence for interventions targeted at mildly frail older people and these appeared to mostly be single domain (exercise/physical activity). Past interventions did not appear to be based on qualitative developmental work incorporating ‘user’ needs or include explicit behaviour change theory and content. There was a limited evidence base for which BCTs have most potential for effectiveness in an older, frailer population. The most promising components we identified were the intervention functions of education and enablement and the BCTs ‘adding objects to the environment’ (e.g. providing aids) and ‘instruction on how to perform the behaviour’. Our state-of-the-art review suggested that physical activity and nutrition had the largest evidence base, whereas home-based social and mental health interventions for frailer older people had limited evidence. Evidence of effective ways of improving cognition that made a difference to clinical outcomes was lacking. Our policy review found that although there was widespread recognition of the problem of the needs of an ageing population, there was little explicit policy on how to address the needs of older people with mild frailty, or prevent worsening frailty in those who were starting to become frail.
Qualitative studies
Qualitative interviews and focus groups suggested that a range of domains needed to be addressed in a new service, including mobility and social activities as core areas but also addressing others tailored to the individual. It further suggested that it should be delivered through low-level support over a sustained period by a trained non-specialist worker with good communication skills. The importance of maintenance of current assets was noted and not necessarily becoming ‘healthier’. The priority for older people was remaining independent and actively contributing. A range of barriers and facilitators was noted, including accumulating health problems (physical, psychological, memory), resources and social capital, perceptions of ageing and willingness/ability to adapt to health declines. Our theoretical framework incorporates these issues, with an assets-based (vs. deficits) model aiming to promote maintenance of independence,186 and the selection of goals to optimise while addressing barriers to compensate for,187 considering the person’s capability, opportunity and motivation to make changes. 51 Our qualitative work further identified potential difficulties in engagement of older people with mild frailty, some of whom felt that accepting help of any kind may indicate the start of dependency and precipitate a decline.
Intervention development
Using evidence from our reviews, qualitative work and theoretical framework we developed our intervention, through a series of intervention development panels that refined the content, and specified the model of service delivery. We also set out to address approaches to engagement of older people with mild frailty, with a focus on language that should be used. From this, we developed a home-based health promotion service, targeted to mildly frail older people and delivered through 3–12 appointments over a 6-month period by a trained non-specialist support worker with good communication skills and previous experience of working with older adults. The service encompassed addressing mobility, socialising, mood and nutrition, and other domains as identified by participants. It would use an explicit behaviour change approach, with goals identified by the individual, focusing on assets that they would like to preserve as well as maintaining independence.
Feasibility randomised controlled trial
We completed a feasibility RCT, testing the feasibility and acceptability of this new service and the feasibility of undertaking a full-scale RCT. This demonstrated that an individually randomised controlled trial was feasible, with recruitment exceeding our targets and very high retention (94%) over 6 months despite this being an older, frailer population. Our battery of outcome measures took some time to complete for frailer participants though was feasible and data were of good quality with very little missing data. It was also feasible to collect health economic data from both NHS and societal perspectives. There was no evidence of contamination of the TAU arm, suggesting that individual randomisation is feasible; however, maintaining blinding for the outcome assessor was difficult in this population, as many had some degree of cognitive impairment and accidentally disclosed their status.
Our study population all had ‘mild frailty’, defined using the Clinical Frailty Scale7 (i.e. they may increasingly need some support with IADL, but are otherwise largely independent in personal care); however, they were classified as a mixture of ‘pre-frail’ and ‘frail’, measured using the Fried frailty phenotype at baseline. 10 They had low gait speed and grip strength for their age, high baseline levels of psychological distress and many had some degree of cognitive impairment. These findings suggest that although only 8.4% of those approached by letter from their GP to take part were eligible and randomised, it had successfully identified a group potentially in need of some support and not the ‘worried well’.
Clinical outcomes for our RCT at 6 months showed no harmful effects and there were no intervention-related SAEs during the course of the study. The intervention shows some promise, in that there were significant differences demonstrated favouring the intervention in our main functioning measure, the Modified Barthel index, in grip strength (a measure of muscle strength and frailty) and in psychological distress, measured by the GHQ-12. All other outcomes showed no significant differences. These findings should be interpreted with caution as the sample size was small and not powered to show differences, but they do provide support for the need to now evaluate this intervention in a large definitive RCT.
Our health economic analysis showed a significant difference in CALYs favouring the intervention, but not in QALYs. The costs of the intervention were modest, at around £307 per patient, using assumptions on the scaling-up of the service, which is slightly lower than a course of CBT currently in the NHS. There were lower NHS costs and carer support costs in the intervention arm, but a large variability and this may be a chance finding. We did not conduct a full health economic analysis, as this is a feasibility study with a small sample size, but we demonstrated this would be feasible alongside a full trial. Further consideration may also need to be given to the time horizon of the analysis given that delaying or reducing a decline in functioning in frail adults potentially has lifelong quality of life, capability and cost implications.
Process evaluation
The process evaluation demonstrated that the intervention was acceptable to participants, who, in general, engaged well with the service, found it helpful, made good or moderate progress towards their goals, and would recommend it to a friend or family. A minority did not engage with the service, for a range of reasons including being ‘too fit’, concurrent complex medical/psychological problems or not liking the approach of goal-setting. The intervention was delivered as intended for most people, incorporating a behaviour change approach, which was well received by most and was endorsed for being motivating and enabling progress and a sense of achievement. However, for some this was less suitable, for example, the small minority who did not like a goal-setting approach and those with significant cognitive impairment. A counselling, reflective listening and emotionally supportive role was also considered an important feature of the service, as was an enablement function, which included practical support in overcoming barriers and promoting capability and opportunity for change. Potential improvements to the service included greater clarity on what it includes at recruitment/initial engagement, more explicit tailoring/alternative approaches to goal-setting when this is less suitable, further carer involvement when there is cognitive impairment, and suggestions for additional training for project workers. An important finding was that the HomeHealth service filled a gap in services for many older people participating who were beginning to encounter increasing difficulties and who did not know where to seek help from.
Implications for practice, service delivery and commissioning
Our evidence reviews demonstrated that although the problem of preventing frailty and maintaining independence and well-being in later life is widely acknowledged, there is little current evidence to inform the design and implementation of services to address this for those who are starting to become frail. From the perspectives of older people, such services should be multidomain, encompassing mobility, social engagement and other areas tailored to the individual. The evidence base supports the inclusion of exercise/physical activity at group or individual levels, but is far less clear for other domains. This is mainly as high-quality research in these domains in older people with mild frailty and is yet to be undertaken.
Our qualitative work suggested that a service need not be delivered by a highly trained qualified health or social care professional and, in fact, seeing a non-specialist was preferred by some. This was supported by the success of our feasibility study, which showed that it was feasible to deliver this type of service with trained non-specialist support workers. The key attributes were communication skills and some experience of working (and empathising) with older people. This model is already being used widely in the voluntary sector under contract with the statutory sector, with a similar workforce providing Care Navigation services and related roles in different parts of the UK. It is likely to be easier to implement than a service delivered by nurses, occupational therapists or social workers, with a wider pool of people with capacity to deliver the service, although this would be geographically variable.
A further interesting finding from our qualitative and developmental work was the importance of language and how a service is framed. It was clear that the language of becoming ‘healthier’ was off-putting for many, who felt this was not relevant (or possibly achievable) to them in this stage of their life. For many, the goal was not to become healthier, but to maintain their current state and, in particular, maintain their independence for as long as possible. This has more general implications for preventative services for older people and how they are marketed.
Although our feasibility RCT showed some promising findings in terms of the potential impact of the service and its potential for cost-effectiveness, this was a small study and it would be premature to recommend more widespread implementation, except as part of a larger-scale evaluation. The budget impact assessment provides preliminary information to Clinical Commissioning Groups and/or local authorities on the potential costs and benefits to their local budget should they wish to implement this service. Health and social care costs and EQ-5D data were collected in a way so as to comply with National Institute for Health and Care Excellence (NICE) recommendations for health technology assessments. 260 We also assessed the feasibility of collecting information beyond a health-care perspective given that this work falls within the remit of public health interventions and, hence, data from this study would be suitable for informing NICE Public Health Guidance. Societal costs and outcomes including capability were collected to test the feasibility of providing advice to national decision-makers, such as the Department of Health or Public Health England, on the total impact to society of implementing this service. The results provide an indication to decision-makers of the benefits of further research in this area.
Implications for research
It was very clear from our evidence reviews that high-quality large-scale studies evaluating multidomain interventions to maintain independence and well-being for older people with mild frailty are lacking. Given the ageing population and accompanying potential strain on health and social care systems, these are urgently needed.
We particularly identified gaps in the evidence for effective interventions tailored to older people with mild frailty to improve nutrition, adapt to or reduce cognitive decline, and promote mental well-being and social engagement. In these areas, further qualitative work is needed to understand the needs of those with mild frailty better and, in particular, how the interventions can work in a population who are increasingly struggling with low energy, weakness and undertaking ADL. We have been funded by the National Institute for Health Research (NIHR) School of Primary Care Research to undertake this work, which is under way (2017).
In this report, we have demonstrated that our new service was feasible and acceptable to older people with mild frailty and the feasibility RCT was successful, indicating that it is possible to now scale-up to a full RCT. This should be undertaken in diverse sites to ensure that findings are widely generalisable. A robust definitive RCT to determine the clinical effectiveness and cost-effectiveness of this promising approach is now urgently needed, to provide timely results to inform practice.
Acknowledgements
The authors would like to acknowledge the contributions of the following.
The Study Steering Committee for their oversight of the project, particularly Dr Andy Clegg for chairing the group, and his contribution and support throughout. The group members were Marie Johnston, Samantha Mauger, Kath Parson, Jane Hopkins, Rekha Elaswarapu, Visva Sathasivam, Sylvie Silver, Karen Timperley and Christina Victor. We would like to add particular thanks to our public representatives Rekha Elaswarapu and Jane Hopkins for additional input on study materials, project design and evaluation, and in recruiting project staff. In addition, Sam Mauger, with Ben Donovan and staff at Age UK London for cohosting the intervention development panels with older people, and Marie Johnston for her expertise in health psychology in developing the intervention.
We would like to thank Sheena Gawler and Sue Taylor for their professional expertise and training in exercise and nutrition, respectively, for the HomeHealth Project Workers.
We would also like to thank Eleanor Tildesley and Anne-Marie Taylor for delivering the newly developed intervention; Celia Belk for her work on the systematic review of interventions for older people with mild or pre-frailty; Lorna Hobbs for her work on the state-of-the-art review and on the intervention development panels; Fiona Giles for her research administration; and Natalia Lago, Shabana Khan, Jo Bagshaw, Nick Beckley and Ella Zomer from PRIMENT CTU for their trial management, database, statistical and health economic expertise.
We also thank the four study practices for their interest and support in recruiting participants, two of which were involved throughout the study in both the intervention development and feasibility trial phases; the Clinical Commissioning Group, local authority and voluntary sector representatives and professionals in Camden and Hertfordshire for their valued insights on local service planning and delivery, in particular Sharn Elton, Chris Badger, Sharleen Rudolf and Karen Timperley; and the multidisciplinary professionals who participated in focus groups, interviews and one-to-one meetings to inform the development of the new service.
Finally, we thank North Thames and East Hertfordshire Clinical Research Networks for their support with participant recruitment and the older people who participated in the study.
Contributions of authors
Kate Walters was chief investigator for the study, led the application for the funding and took overall responsibility for design, conduct, analysis and writing up of the project. She also wrote sections of the final report.
Rachael Frost was the research assistant on the study during the feasibility trial and led data collection for this phase. She was also jointly responsible for the systematic review of interventions for older people with mild or pre-frailty, analysis of qualitative data from the intervention development. She also wrote sections of the final report.
Kalpa Kharicha was a coapplicant for funding and project manager for the study. She contributed to data collection and analysis for the intervention development and process evaluation, was jointly responsible for the intervention development panels, delivered training and led the supervision of intervention delivery staff. She also wrote sections of the final report.
Christina Avgerinou led the design and conduct of the process evaluation and analysis. She was involved in the analysis of qualitative data in the intervention development phase and the design and conduct of the feasibility trial. She also wrote sections of the final report.
Benjamin Gardner was a coapplicant for funding, provided health psychology expertise, in particular on BCT, and led the review of behaviour change components in home-based health promotion interventions in frail and pre-frail older adults. He also provided training to the HomeHealth project workers on BCTs. He also contributed to drafts of the final report and approved the final version.
Federico Ricciardi conducted the statistical analysis as part of PRIMENT CTU. He also contributed to drafts of the final report and approved the final version.
Rachael Hunter was a coapplicant for funding, provided health economic expertise throughout and conducted the health economic analysis as part of PRIMENT CTU. She also wrote sections of the final report.
Ann Liljas contributed to the systematic review of interventions for older people with mild frailty, the intervention development panels and data collection and analysis of the intervention development and process evaluation data. She also contributed to drafts of the final report and approved the final version.
Jill Manthorpe was a coapplicant for funding and provided critical oversight and guidance for the study throughout. She also contributed to drafts of the final report and approved the final version.
Vari Drennan was a coapplicant for funding, led on the policy review and provided critical oversight and guidance for the study throughout. She also wrote sections of the final report.
John Wood was a coapplicant for funding and advised on statistical analysis as part of PRIMENT CTU. He also contributed to drafts of the final report and approved the final version.
Claire Goodman was a coapplicant for funding and provided critical oversight and guidance for the study throughout. She also contributed to drafts of the final report and approved the final version.
Ana Jovicic was the research assistant on the study during the intervention development and led data collection for this phase. She was also jointly responsible for the systematic review of interventions for older people with mild or pre-frailty and the review of behaviour change components in home-based health promotion interventions in frail and pre-frail older adults. She also contributed to drafts of the final report and approved the final version.
Steve Iliffe was a coapplicant for funding and provided critical oversight and guidance for the study throughout. He also contributed to drafts of the final report and approved the final version.
Publications
Published
Jovicic A, Gardner B, Belk, Kharicha K, Iliffe S, Manthorpe J, et al. Identifying the content of home-based health behaviour change interventions for frail older people: a systematic review protocol. Systematic Reviews 2015;4:151.
Gardner B, Jovicic A, Belk C, Kharicha K, Iliffe S, Manthorpe J, et al. Specifying the content of home-based health behaviour change interventions for older people with frailty or at risk of frailty: an exploratory systematic review. BMJ Open 2017;7:e014127.
Frost R, Belk C, Jovicic A, Ricciardi F, Kharicha K, Gardner B, et al. Health promotion interventions for community-dwelling older people with mild or pre-frailty: a systematic review and meta-analysis. BMC Geriatrics 2017;17:157.
Frost R, Kharicha K, Jovicic A, Liljas A, Iliffe S, Manthorpe J, et al. Identifying key components of a new home-based health promotion service for older people with mild frailty: a qualitative study. Health Soc Care Community 2017; in press. 176
Submitted (under review)
Drennan V, Walters K, Avgerinou C, Gardner B, Goodman C, Frost R, et al. Moving upstream in health promoting policies for older people with mild frailty in England? A policy analysis. Health Serv Res Policy.
Data sharing statement
All available anonymised data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752-62. https://doi.org/10.1016/S0140-6736(12)62167-9.
- Bock JO, König HH, Brenner H, Haefeli WE, Quinzler R, Matschinger H, et al. Associations of frailty with health care costs – results of the ESTHER cohort study. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1360-3.
- Future of an Ageing Population. London: Government Office for Science; 2016.
- Briefing: Health and Care of Older People in England 2017. London: Age UK; 2017.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487-92. https://doi.org/10.1111/j.1532-5415.2012.04054.x.
- Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology 2009;55:539-49. https://doi.org/10.1159/000211949.
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489-95. https://doi.org/10.1503/cmaj.050051.
- Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016;45:353-60. https://doi.org/10.1093/ageing/afw039.
- Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci 2000;55:M350-5. https://doi.org/10.1093/gerona/55.6.M350.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-57. https://doi.org/10.1093/gerona/56.3.M146.
- Puts MTE, Toubasi S, Atkinson E, Ayala AP, Andrew M, Ashe MC, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing 2017;43:386-92. https://doi.org/10.1093/ageing/afw247.
- Kono A, Kanaya Y, Fujita T, Tsumura C, Kondo T, Kushiyama K, et al. Effects of a preventive home visit program in ambulatory frail older people: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2012;67:302-9. https://doi.org/10.1093/gerona/glr176.
- Gustafsson S, Wilhelmson K, Eklund K, Gosman-Hedström G, Zidén L, Kronlöf GH, et al. Health-promoting interventions for persons aged 80 and older are successful in the short term – results from the randomized and three-armed Elderly Persons in the Risk Zone study. J Am Geriatr Soc 2012;60:447-54. https://doi.org/10.1111/j.1532-5415.2011.03861.x.
- Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166:418-23. https://doi.org/10.1001/archinte.166.4.418.
- Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA 2002;287:1022-8. https://doi.org/10.1001/jama.287.8.1022.
- Tappenden P, Campbell F, Rawdin A, Wong R, Kalita N. The clinical effectiveness and cost-effectiveness of home-based, nurse-led health promotion for older people: a systematic review. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16200.
- Liimatta H, Lampela P, Laitinen-Parkkonen P, Pitkala KH. Effects of preventive home visits on older people’s use and costs of health care services: a systematic review. Eur Geriatr Med 2016;7:571-80. https://doi.org/10.1016/j.eurger.2016.08.006.
- McCusker J, Verdon J. Do geriatric interventions reduce emergency department visits? A systematic review. J Gerontol A Biol Sci Med Sci 2006;61:53-62. https://doi.org/10.1093/gerona/61.1.53.
- Elkan R, Kendrick D, Dewey M, Hewitt M, Robinson J, Blair M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ 2001;323:719-25. https://doi.org/10.1136/bmj.323.7315.719.
- Craig P, Dieppe P, MacIntyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. Medical Research Council; 2009.
- Walters K, Kharicha K, Iliffe S, Drennan V, Goodman C, Manthorpe J, et al. Home-based Health Promotion for Vulnerable Older People. Research Protocol V1.3. London; 2015.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (reprinted from Annals of Internal Medicine). Phys Ther 2009;89:873-80.
- Frost R, Belk C, Jovicic A, Ricciardi F, Kharicha K, Gardner B, et al. Health promotion interventions for community-dwelling older people with mild or pre-frailty: a systematic review and meta-analysis. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0547-8.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute; 2014.
- Coren E, Fisher M. The Conduct of Systematic Research Reviews for SCIE Knowledge Reviews. London: Social Care Institute for Excellence; 2006.
- Higgins PT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2011.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2008.
- Noblit G, Hare R. Meta-Ethnography: Synthesizing Qualitative Studies. London: Sage Publications Ltd; 1988.
- Binder EF, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc 2002;50:1921-8. https://doi.org/10.1046/j.1532-5415.2002.50601.x.
- Brown M, Sinacore DR, Ehsani AA, Binder EF, Holloszy JO, Kohrt WM. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil 2000;81:960-5. https://doi.org/10.1053/apmr.2000.4425.
- Daniel K. Wii-hab for pre-frail older adults. Rehabil Nurs 2012;37:195-201. https://doi.org/10.1002/rnj.25.
- Zech A, Drey M, Freiberger E, Hentschke C, Bauer JM, Sieber CC, et al. Residual effects of muscle strength and muscle power training and detraining on physical function in community-dwelling prefrail older adults: a randomized controlled trial. BMC Geriatr 2012;12. https://doi.org/10.1186/1471-2318-12-68.
- Drey M, Zech A, Freiberger E, Bertsch T, Uter W, Sieber CC, et al. Effects of strength training versus power training on physical performance in prefrail community-dwelling older adults. Gerontology 2012;58:197-204. https://doi.org/10.1159/000332207.
- Kwon J, Yoshida Y, Yoshida H, Kim H, Suzuki T, Lee Y. Effects of a combined physical training and nutrition intervention on physical performance and health-related quality of life in prefrail older women living in the community: a randomized controlled trial. J Am Med Dir Assoc 2015;16:263.e1-8. https://doi.org/10.1016/j.jamda.2014.12.005.
- Lustosa LP, Silva JP, Coelho FM, Pereira DS, Parentoni AN, Pereira LSM. Impact of resistance exercise program on functional capacity and muscular strength of knee extensor in pre-frail community-dwelling older women: a randomized crossover trial. Rev Bras Fisioter 2011;15:318-24. https://doi.org/10.1590/S1413-35552011000400010.
- Lustosa LP, Maximo Pereira LS, Coelho FM, Pereira DS, Silva JP, Parentoni AN, et al. Impact of an exercise program on muscular and functional performance and plasma levels of interleukin 6 and soluble receptor tumor necrosis factor in prefrail community-dwelling older women: a randomized controlled trial. Arch Phys Med Rehabil 2013;94:660-6. https://doi.org/10.1016/j.apmr.2012.11.013.
- Upatising B, Hanson GJ, Kim YL, Cha SS, Yih Y, Takahashi PY. Effects of home telemonitoring on transitions between frailty states and death for older adults: a randomized controlled trial. Int J Gen Med 2013;6:145-51. https://doi.org/10.2147/IJGM.S40576.
- Jamsen KM, Bell JS, Hilmer SN, Kirkpatrick CMJ, Ilomäki J, Le Couteur D, et al. Effects of changes in number of medications and drug burden index exposure on transitions between frailty states and death: the Concord Health and Ageing in Men Project cohort study. J Am Geriatr Soc 2016;64:89-95. https://doi.org/10.1111/jgs.13877.
- Cumming RG, Handelsman D, Seibel MJ, Creasey H, Sambrook P, Waite L, et al. Cohort profile: the Concord Health and Ageing in Men Project (CHAMP). Int J Epidemiol 2009;38:374-8. https://doi.org/10.1093/ije/dyn071.
- Lee JS, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc 2014;15:281-6. https://doi.org/10.1016/j.jamda.2013.12.002.
- Mohler MJ, Wendel CS, Taylor-Piliae RE, Toosizadeh N, Najafi B. Motor performance and physical activity as predictors of prospective falls in community-dwelling older adults by frailty level: application of wearable technology. Gerontology 2016;62:654-64. https://doi.org/10.1159/000445889.
- Schwenk M, Mohler J, Wendel C, D’Huyvetter K, Fain M, Taylor-Piliae R, et al. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: Baseline results of the arizona frailty cohort study. Gerontology 2015;61:258-67. https://doi.org/10.1159/000369095.
- Shardell M, D’Adamo C, Alley DE, Miller RR, Hicks GE, Milaneschi Y, et al. Serum 25-hydroxyvitamin D, transitions between frailty states, and mortality in older adults: the Invecchiare in Chianti Study. J Am Geriatr Soc 2012;60:256-64. https://doi.org/10.1111/j.1532-5415.2011.03830.x.
- Ferrucci L, Bandinelli S, Benvenuti E, . Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618-25. https://doi.org/10.1111/j.1532-5415.2000.tb03873.x.
- Sugimoto H, Demura S, Nagasawa Y, Shimomura M. Changes in the physical functions of pre-frail elderly women after participation in a 1-year preventative exercise program: pre-frail elderly. Geriatr Gerontol Int 2014;14:975-82. https://doi.org/10.1111/ggi.12198.
- Villareal DT, Binder EF, Yarasheski KE, Williams DB, Brown M, Sinacore DR, et al. Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women. J Am Geriatr Soc 2003;51:985-90. https://doi.org/10.1046/j.1365-2389.2003.51312.x.
- Boult C, Leff B, Boyd CM, Wolff JL, Marsteller JA, Frick KD, et al. A matched-pair cluster-randomized trial of guided care for high-risk older patients. J Gen Intern Med 2013;28:612-21. https://doi.org/10.1007/s11606-012-2287-y.
- Steptoe A, Gardner B, Wardle J, French D, Kaptein A, Vedhara K, et al. Health Psychology. Chichester: Blackwell; 2010.
- Michie S, West R, Campbell R, Brown J, Gainforth H. ABC of Behaviour Change Theories. Surrey: Silverback Publishing; 2014.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide to Designing Interventions. Surrey: Silverback Publishing; 2014.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Jovicic A, Gardner B, Belk C, Kharicha K, Iliffe S, Manthorpe J, et al. Identifying the content of home-based health behaviour change interventions for frail older people: a systematic review protocol. Syst Rev 2015;4.
- Gardner B, Jovicic A, Belk C, Kharicha K, Iliffe S, Manthorpe J, et al. Specifying the content of home-based health behaviour change interventions for older people with frailty or at risk of frailty: an exploratory systematic review. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014127.
- Avlund K, Jepsen E, Vass M, Lundemark H. Effects of comprehensive follow-up home visits after hospitalization on functional ability and readmissions among old patients. A randomized controlled study. Scand J Occup Ther 2002;9:17-22. https://doi.org/10.1080/110381202753505827.
- Bouman A, van Rossum E, Ambergen T, Kempen G, Knipschild P. Effects of a home visiting program for older people with poor health status: a randomized, clinical trial in the Netherlands. J Am Geriatr Soc 2008;56:397-404. https://doi.org/10.1111/j.1532-5415.2007.01565.x.
- Dalby DM, Sellors JW, Fraser FD, Fraser C, van Ineveld C, Howard M. Effect of preventive home visits by a nurse on the outcomes of frail elderly people in the community: a randomized controlled trial. CMAJ 2000;162:497-500.
- Favela J, Castro LA, Franco-Marina F, Sánchez-García S, Juárez-Cedillo T, Espinel Bermudez C, et al. Nurse home visits with or without alert buttons versus usual care in the frail elderly: a randomized controlled trial. Clin Interv Aging 2013;8:85-9. https://doi.org/10.2147/CIA.S38618.
- Hall N, De Beck P, Johnson D, Mackinnon K, Gutman G, Glick N. Randomized trial of a health promotion program for frail elders. Can J Aging Rev Can Vieil 1992;11:72-91. https://doi.org/10.1017/S0714980800014537.
- Levine S, Steinman BA, Attaway K, Jung T, Enguidanos S. Home care program for patients at high risk of hospitalization. Am J Manag Care 2012;18:e269-76.
- Luck T, Motzek T, . Luppa, Matschinger, Fleischer, Sesselmann . Effectiveness of preventive home visits in reducing the risk of falls in old age: a randomized controlled trial. Clin Interv Aging 2013;8:697-702. https://doi.org/10.2147/CIA.S43284.
- Marek KD, Stetzer F, Ryan PA, Bub LD, Adams SJ, Schlidt A, et al. Nurse care coordination and technology effects on health status of frail older adults via enhanced self-management of medication: randomized clinical trial to test efficacy. Nurs Res 2013;62:269-78. https://doi.org/10.1097/NNR.0b013e318298aa55.
- Markle-Reid M, Weir R, Browne G, Roberts J, Gafni A, Henderson S. Health promotion for frail older home care clients. J Adv Nurs 2006;54:381-95. https://doi.org/10.1111/j.1365-2648.2006.03817.x.
- Markle-Reid M, Browne G, Gafni A, Roberts J, Weir R, Thabane L, et al. The effects and costs of a multifactorial and interdisciplinary team approach to falls prevention for older home care clients ‘at risk’ for falling: a randomized controlled trial. Can J Aging 2010;29:139-61. https://doi.org/10.1017/S0714980809990377.
- Siu AL, Kravitz RL, Keeler E, Hemmerling K, Kington R, Davis JW, et al. Postdischarge geriatric assessment of hospitalized frail elderly patients. Arch Intern Med 1996;156:76-81. https://doi.org/10.1001/archinte.1996.00440010094012.
- Stuck AE, Minder CE, Peter-Wüest I, Gillmann G, Egli C, Kesselring A, et al. A randomized trial of in-home visits for disability prevention in community-dwelling older people at low and high risk for nursing home admission. Arch Intern Med 2000;160:977-86. https://doi.org/10.1001/archinte.160.7.977.
- van Hout HPJ, Jansen APD, van Marwijk HWJ, Pronk M, Frijters DF, Nijpels G. Prevention of adverse health trajectories in a vulnerable elderly population through nurse home visits: a randomized controlled trial [ISRCTN05358495]. J Gerontol A Biol Sci Med Sci 2010;65A:734-42. https://doi.org/10.1093/gerona/glq037.
- Williams EI, Greenwell J, Groom LM. The care of people over 75 years old after discharge from hospital: an evaluation of timetabled visiting by health visitor assistants. J Public Health Med 1992;14:138-44.
- Metzelthin SF, van Rossum E, de Witte LP, Ambergen AW, Hobma SO, Sipers W, et al. Effectiveness of interdisciplinary primary care approach to reduce disability in community dwelling frail older people: cluster randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f5264.
- Melis RJF, van Eijken MIJ, Teerenstra S, van Achterberg T, Parker SG, Borm GF, et al. A randomised study of a multidisciplinary programme to intervene on geriatric syndromes in vulnerable older people who live at home (Dutch EASYcare Study). J Gerontol Biol Sci Med Sci 2008;63:283-90. https://doi.org/10.1093/gerona/63.3.283.
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26:91-108. https://doi.org/10.1111/j.1471-1842.2009.00848.x.
- Chase CA, Mann K, Wasek S, Arbesman M. Systematic review of the effect of home modification and fall prevention programs on falls and the performance of community-dwelling older adults. Am J Occup Ther 2012;66:284-91. https://doi.org/10.5014/ajot.2012.005017.
- Winter H, Watt K, Peel NM. Falls prevention interventions for community-dwelling older persons with cognitive impairment: a systematic review. Int Psychogeriatr 2013;25:215-27. https://doi.org/10.1017/S1041610212001573.
- Kwan E, Straus S, Holroyd-Leduc J, Reddy M. Evidence-Based Geriatric Medicine: A Practical Clinical Guide. Chichester: John Wiley & Sons Ltd; 2012.
- Beswick A, Gooberman-Hill R, Smith A, Wylde V, Ebrahim S. Maintaining independence in older people. Rev Clin Gerontol 2010;20:128-53. https://doi.org/10.1017/S0959259810000079.
- Sherrington C, Lord SR, Finch CF. Physical activity interventions to prevent falls among older people: update of the evidence. J Sci Med Sport 2004;7:43-51. https://doi.org/10.1016/S1440-2440(04)80277-9.
- Gschwind YJ, Wolf I, Bridenbaugh SA, Kressig RW. Basis for a Swiss perspective on fall prevention in vulnerable older people. Swiss Med Wkly 2011;141. https://doi.org/10.4414/smw.2011.13305.
- Windle G. Exercise, physical activity and mental well-being in later life. Rev Clin Gerontol 2014;24:319-25. https://doi.org/10.1017/S0959259814000173.
- Sjösten N, Vaapio S, Kivelä SL. The effects of fall prevention trials on depressive symptoms and fear of falling among the aged: a systematic review. Aging Ment Health 2008;12:30-46. https://doi.org/10.1080/13607860701366079.
- Balzer K, Bremer M, Schramm S, Lühmann D, Raspe H. Falls prevention for the elderly. GMS Health Technol Assess 2012;8. https://doi.org/10.3205/hta000099.
- Carpenter CR. Evidence-based emergency medicine/systematic review abstract. Preventing falls in community-dwelling older adults. Ann Emerg Med 2010;55:296-8. https://doi.org/10.1016/j.annemergmed.2009.06.014.
- Bazian Ltd . Fall prevention programmes in older people. Evid Based Healthc Public Health 2005;9:343-8. https://doi.org/10.1016/j.ehbc.2005.07.003.
- Medical Advisory Secretariat . Prevention of falls and fall-related injuries in community-dwelling seniors: an evidence-based analysis. Ont Health Technol Assess Ser 2008;8:1-78.
- Hill-Westmoreland EE, Soeken K, Spellbring AM. A meta-analysis of fall prevention programs for the elderly: how effective are they?. Nurs Res 2002;51:1-8. https://doi.org/10.1097/00006199-200201000-00002.
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD007146.pub3.
- Kannus P, Sievänen H, Palvanen M, Järvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet 2005;366:1885-93. https://doi.org/10.1016/S0140-6736(05)67604-0.
- Choi M, Hector M. Effectiveness of intervention programs in preventing falls: a systematic review of recent 10 years and meta-analysis. J Am Med Dir Assoc 2012;13:188.e13-21. https://doi.org/10.1016/j.jamda.2011.04.022.
- Goodwin VA, Abbott RA, Whear R, Bethel A, Ukoumunne OC, Thompson-Coon J, et al. Multiple component interventions for preventing falls and fall-related injuries among older people: systematic review and meta-analysis. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-15.
- Marks R, Allegrante JP. Falls-prevention programs for older ambulatory community dwellers: from public health research to health promotion policy. Soz Praventivmed 2004;49:171-8. https://doi.org/10.1007/s00038-004-3040-z.
- Heaven BEN, Brown LJ, White M, Errington L, Mathers JC, Moffatt S. Supporting well-being in retirement through meaningful social roles: systematic review of intervention studies. Milbank Q 2013;91:222-87. https://doi.org/10.1111/milq.12013.
- Dickens AP, Richards SH, Greaves CJ, Campbell JL. Interventions targeting social isolation in older people: a systematic review. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-647.
- Cattan M, White M, Bond J, Learmouth A. Preventing social isolation and loneliness among older people: a systematic review of health promotion interventions. Ageing Soc 2005;25:41-67. https://doi.org/10.1017/S0144686X04002594.
- Medical Advisory Secretariat . Social isolation in community-dwelling seniors: an evidence-based analysis. Ont Health Technol Assess Ser 2008;8:1-49.
- Lee SY, Franchetti MK, Imanbayev A, Gallo JJ, Spira AP, Lee HB. Non-pharmacological prevention of major depression among community-dwelling older adults: a systematic review of the efficacy of psychotherapy interventions. Arch Gerontol Geriatr 2012;55:522-9. https://doi.org/10.1016/j.archger.2012.03.003.
- Forsman AK, Schierenbeck I, Wahlbeck K. Psychosocial interventions for the prevention of depression in older adults: systematic review and meta-analysis. J Aging Health 2011;23:387-416. https://doi.org/10.1177/0898264310378041.
- Forsman AK, Nordmyr J, Wahlbeck K. Psychosocial interventions for the promotion of mental health and the prevention of depression among older adults. Health Promot Int 2011;26:i85-107. https://doi.org/10.1093/heapro/dar074.
- Jané-Llopis E, Hosman C, Jenkins R, Anderson P. Predictors of efficacy in depression prevention programmes. Meta-analysis. Br J Psychiatry 2003;183:384-97. https://doi.org/10.1192/bjp.183.5.384.
- Cole MG. Brief interventions to prevent depression in older subjects: a systematic review of feasibility and effectiveness. Am J Geriatr Psychiatr 2008;16:435-43. https://doi.org/10.1097/JGP.0b013e318162f174.
- Gross AL, Parisi JM, Spira AP, Kueider AM, Ko JY, Saczynski JS, et al. Memory training interventions for older adults: a meta-analysis. Aging Ment Health 2012;16:722-34. https://doi.org/10.1080/13607863.2012.667783.
- Stott J, Spector A. A review of the effectiveness of memory interventions in mild cognitive impairment (MCI). Int Psychogeriatr 2011;23:526-38. https://doi.org/10.1017/S1041610210001973.
- Kurz AF, Leucht S, Lautenschlager NT. The clinical significance of cognition-focused interventions for cognitively impaired older adults: a systematic review of randomized controlled trials. Int Psychogeriatr 2011;23:1364-75. https://doi.org/10.1017/S1041610211001001.
- Jean L, Bergeron M-È, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010;18:281-96. https://doi.org/10.1097/JGP.0b013e3181c37ce9.
- Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry 2009;17:179-87. https://doi.org/10.1097/JGP.0b013e3181953b57.
- Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res Rev 2013;12:263-75. https://doi.org/10.1016/j.arr.2012.07.003.
- Simon SS, Yokomizo JE, Bottino CM. Cognitive intervention in amnestic Mild Cognitive Impairment: a systematic review. Neurosci Biobehav Rev 2012;36:1163-78. https://doi.org/10.1016/j.neubiorev.2012.01.007.
- Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev 2014;15:28-43. https://doi.org/10.1016/j.arr.2014.02.004.
- Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cochrane Database Syst Rev. 2011.
- Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimers Dement 2009;5:50-6. https://doi.org/10.1016/j.jalz.2008.10.008.
- Young K, Bunn F, Trivedi D, Dickinson A. Nutritional education for community dwelling older people: a systematic review of randomised controlled trials. Int J Nurs Stud 2011;48:751-80. https://doi.org/10.1016/j.ijnurstu.2011.03.007.
- Bandayrel K, Wong S. Systematic literature review of randomized control trials assessing the effectiveness of nutrition interventions in community-dwelling older adults. J Nutr Educ Behav 2011;43:251-62. https://doi.org/10.1016/j.jneb.2010.01.004.
- Lara J, Evans E, O’Brien N, Moynihan P, Meyer T, Adamson A, et al. Association of behaviour change techniques with effectiveness of dietary interventions among adults of retirement age: a systematic review and meta-analysis of randomised controlled trials. BMC Med 2014;12. https://doi.org/10.1186/s12916-014-0177-3.
- Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol 2013;74:580-91. https://doi.org/10.1002/ana.23944.
- Marshall S, Bauer J, Capra S, Isenring E. Are informal carers and community care workers effective in managing malnutrition in the older adult community? A systematic review of current evidence. J Nutr Health Aging 2013;17. https://doi.org/10.1007/s12603-013-0341-z.
- Zheng Y, Zhu J, Zhou M, Cui L, Yao W, Liu Y. Meta-analysis of long-term vitamin D supplementation on overall mortality. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0082109.
- Bolland MJ, Grey A, Davidson JS, Cundy T, Reid IR. Should measurement of vitamin D and treatment of vitamin D insufficiency be routine in New Zealand?. N Z Med J 2012;125:83-91.
- Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 2011;59:2291-300. https://doi.org/10.1111/j.1532-5415.2011.03733.x.
- Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011;96:2997-3006. https://doi.org/10.1210/jc.2011-1193.
- Rejnmark L. Effects of vitamin d on muscle function and performance: a review of evidence from randomized controlled trials. Ther Adv Chronic Dis 2011;2:25-37. https://doi.org/10.1177/2040622310381934.
- Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int 2011;22:859-71. https://doi.org/10.1007/s00198-010-1407-y.
- Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews DC. Vitamin D treatment for the prevention of falls in older adults: systematic review and meta-analysis. J Am Geriatr Soc 2010;58:1299-310. https://doi.org/10.1111/j.1532-5415.2010.02949.x.
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 2009;339. https://doi.org/10.1136/bmj.b3692.
- Jackson C, Gaugris S, Sen SS, Hosking D. The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: a meta-analysis. QJM 2007;100:185-92. https://doi.org/10.1093/qjmed/hcm005.
- Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc 2003;51:1219-26. https://doi.org/10.1046/j.1532-5415.2003.51405.x.
- Zheng YT, Cui QQ, Hong YM, Yao WG. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0115850.
- Dierkes J, Ranhoff A, Lehmann U, Gjessing H. Clin Nutr. 2014.
- Camfield DA, Owen L, Scholey AB, Pipingas A, Stough C. Dairy constituents and neurocognitive health in ageing. Br J Nutr 2011;106:159-74. https://doi.org/10.1017/S0007114511000158.
- Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev 2012;11:278-96. https://doi.org/10.1016/j.arr.2011.12.008.
- Macpherson H, Pipingas A, Pase MP. Multivitamin-multimineral supplementation and mortality: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2013;97:437-44. https://doi.org/10.3945/ajcn.112.049304.
- Manders M, De Groot LC, Van Staveren WA, Wouters-Wesseling W, Mulders AJ, Schols JM, et al. Effectiveness of nutritional supplements on cognitive functioning in elderly persons: a systematic review. J Gerontol A Biol Sci Med Sci 2004;59:M1041-9. https://doi.org/10.1093/gerona/59.10.M1041.
- Clarke R, Bennett D, Parish S, Lewington S, Skeaff M, Eussen SJ, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr 2014;100:657-66. https://doi.org/10.3945/ajcn.113.076349.
- Malouf R, Evans JG. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev 2008;4. https://doi.org/10.1002/14651858.CD004514.pub2.
- Jia X, McNeill G, Avenell A. Does taking vitamin, mineral and fatty acid supplements prevent cognitive decline? A systematic review of randomized controlled trials. J Hum Nutr Diet 2008;21:317-36. https://doi.org/10.1111/j.1365-277X.2008.00887.x.
- Übeda N, Achón M, Varela-Moreiras G. Omega 3 fatty acids in the elderly. Br J Nutr 2012;107:137-51. https://doi.org/10.1017/S0007114512001535.
- Sydenham E, Dangour AD, Lim Wee-Shiong. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev 2012;13.
- Dangour AD, Andreeva VA, Sydenham E, Uauy R. Omega 3 fatty acids and cognitive health in older people. Br J Nutr 2012;107:152-8. https://doi.org/10.1017/S0007114512001547.
- Lim W-S, Gammack JK, Van Niekerk JK, Dangour A. Cochrane Database Syst Rev. 2006.
- Xu ZR, Tan ZJ, Zhang Q, Gui QF, Yang YM. Clinical effectiveness of protein and amino acid supplementation on building muscle mass in elderly people: a meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0109141.
- Bjelakovic G, Nikolova D, Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm?. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0074558.
- Ooi CP, Loke SC, Yassin Z, Hamid TA. Carbohydrates for improving the cognitive performance of independent-living older adults with normal cognition or mild cognitive impairment. Cochrane Database Syst Rev 2011;4. https://doi.org/10.1002/14651858.CD007220.pub2.
- Petticrew M, Roberts H. Systematic Reviews in the Social Sciences: A Practical Guide. London, Oxford: Blackwell Publishing; 2006.
- Walt G, Shiffman J, Schneider H, Murray S, Brugha R, Gilson L. ‘Doing’ health policy analysis: methodological and conceptual reflections and challenges. Health Policy Plan 2008;23:308-17. https://doi.org/10.1093/heapol/czn024.
- Hill M. The Public Policy Process. Oxon: Routledge; 2013.
- World Economic and Social Survey. New York, NY: United Nations; 2007.
- Political Declaration and Madrid International Plan of Action on Ageing. New York, NY: United Nations; 2002.
- Regional Implementation Strategy For The Madrid International Plan Of Action On Ageing. Geneva: United Nations Economic Commission for Europe; 2002.
- Fuller Working Lives – A Framework for Action. London: Department of Work and Pensions; 2014.
- Fixing our Broken Housing Market. London: The Stationery Office; 2017.
- Creating Growth, Cutting Carbon: Making Sustainable Local Transport Happen. London: The Stationery Office; 2011.
- Equity and Excellence: Liberating the National Health Service. London: The Stationery Office; 2010.
- Healthy Lives, Healthy People: Our Strategy for Public Health in England. London: The Stationery Office; 2012.
- Caring for our Future: Reforming Care and Support. London: The Stationery Office; 2010.
- Five Year Forward View. London: NHS England; 2014.
- Care and Support Statutory Guidance. London: Department of Health; 2017.
- Integrated Care and Support: Our Shared Commitment. London: Department of Health; 2013.
- Government response to the consultation Refreshing the Public Health Outcomes Framework (2015). London: Department of Health; 2016.
- Government Response to the House of Commons Health Select Committee Report into Long-term Conditions. London: Department of Health; 2014.
- Local Authorities (Public Health Functions and Entry to Premises by Local Healthwatch Representatives) Regulations 2013 (SI 2013/351). London: HMSO; 2013.
- 2014/15 General Medical Services Contract Quality and Outcomes Framework. London: NHS Employers; 2014.
- Transforming Primary Care. London: NHS England; 2014.
- Proactive Care Programme: CCG Support for Implementation. London: NHS England; 2014.
- NHS Health Check. London: Public Health England; 2017.
- White H. A contribution to current debates in impact evaluation. Evaluation 2010;16:153-64. https://doi.org/10.1177/1356389010361562.
- Oliver D, Foot C, Humphries R. Making Our Health and Care Systems Fit for an Ageing Population. London: The King’s Fund; 2014.
- Robson J, Dostal I, Sheikh A, Eldridge S, Madurasinghe V, Griffiths C, et al. The NHS Health Check in England: an evaluation of the first 4 years. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-008840.
- NHS Payments to General Practice, England, 2015/16. London: NHS Digital; 2016.
- Two Year Delivery Plan 2014 to 2016. Poole: Dorset Clinical Commissioning Group; 2014.
- Outcome of 2017/18 GMS Contract Negotiations. London: NHS England; 2017.
- McKinlay J, Conrad P, Leiter V. The Sociology of Health and Illness: Critical Perspectives. New York, NY: Worth Publishing; 2012.
- Puts MTE, Toubasi S, Atkinson E, Ayala AP, Andrew M, Ashe MC, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a protocol for a scoping review of the literature and international policies. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010959.
- Giné-Garriga M, Roqué-Fíguls M, Coll-Planas L, Sitjà-Rabert M, Salvà A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch Phys Med Rehabil 2014;95:753-69. https://doi.org/10.1016/j.apmr.2013.11.007.
- Badrasawi M, Shahar S, Abd Manaf Z, Rajab NF, Kas D. Efficacy of L-carnitine supplementation on frailty status and its biomarkers, nutritional status, and physical and cognitive function among prefrail older adults: a double-blind, randomized, placebo-controlled clinical trial. Clin Interv Aging 2016;11:1675-86. https://doi.org/10.2147/CIA.S113287.
- Serra-Prat M, Sist X, Domenich R, Jurado L, Saiz A, Roces A, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing 2017. https://academic.oup.com/ageing/article-lookup/doi/10.1093/ageing/afw242 (accessed 16 May 2017).
- Cosco TD, Prina AM, Perales J, Stephan BC, Brayne C. Lay perspectives of successful ageing: a systematic review and meta-ethnography. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002710.
- Walters K, Kharicha K, Goodman C, Handley M, Manthorpe J, Cattan M, et al. Promoting independence, health and well-being for older people: a feasibility study of computer-aided health and social risk appraisal system in primary care. BMC Fam Pract 2017;18.
- Frost R, Kharicha K, Jovicic A, Liljas A, Iliffe S, Manthorpe J, et al. Identifying key components of a new home-based health promotion service for older people with mild frailty: a qualitative study. Health Soc Care Community 2017.
- Iliffe S, Kharicha K, Harari D, Swift C, Goodman C, Manthorpe J. User involvement in the development of a health promotion technology for older people: findings from the SWISH project. Health Soc Care Community 2010;18:147-59. https://doi.org/10.1111/j.1365-2524.2009.00882.x.
- Manthorpe J, Kharicha K, Goodman C, Harari D, Swift C, Iliffe S. Smarter working in social and health care: professional perspectives on a new technology. Br J Soc Work 2010;40:1829-46. https://doi.org/10.1093/bjsw/bcp100.
- Ziebland S, Hunt K. Using secondary analysis of qualitative data of patient experiences of health care to inform health services research and policy. J Health Serv Res Policy 2014;19:177-82. https://doi.org/10.1177/1355819614524187.
- Lommi M, Matarese M, Alvaro R, Piredda M, De Marinis MG. The experiences of self-care in community-dwelling older people: a meta-synthesis. Int J Nurs Stud 2015;52:1854-67. https://doi.org/10.1016/j.ijnurstu.2015.06.012.
- Menichetti J, Graffigna G. How older citizens engage in their health promotion: a qualitative research-driven taxonomy of experiences and meanings. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010402.
- Birkeland A, Natvig GK. Coping with ageing and failing health: a qualitative study among elderly living alone. Int J Nurs Pract 2009;15:257-64. https://doi.org/10.1111/j.1440-172X.2009.01754.x.
- Lloyd A, Kendall M, Starr JM, Murray SA. Physical, social, psychological and existential trajectories of loss and adaptation towards the end of life for older people living with frailty: a serial interview study. BMC Geriatr 2016;16. https://doi.org/10.1186/s12877-016-0350-y.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Choosing Health: Making Healthy Choices Easier. London: Department of Health; 2004.
- Morgan A, Ziglio E. Revitalising the evidence base for public health: an assets model. Promot Educ 2007:17-22. https://doi.org/10.1177/10253823070140020701x.
- Baltes P, Baltes M, Baltes P, Baltes M. Successful Ageing: Perspectives from the Behavioural Sciences. Cambridge: Cambridge University Press; 1990.
- Finch H, Lewis J, Turley C, Ritchie J, Lewis J, Mcnaughton Nichols C, et al. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2014.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
- Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, et al. Valuing the ICECAP capability index for older people. Soc Sci Med 2008;67:874-82. https://doi.org/10.1016/j.socscimed.2008.05.015.
- Behm L, Ivanoff SD, Zidén L. Preventive home visits and health – experiences among very old people. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-378.
- Warmoth K, Lang I, Phoenix C, Abraham C, Andrew M, Hubbard R, et al. ‘Thinking you’re old and frail’: a qualitative study of frailty in older adults. Ageing Soc 2016;36:1483-500. https://doi.org/10.1017/S0144686X1500046X.
- van Kempen JA, Robben SH, Zuidema SU, Olde Rikkert MG, Melis RJ, Schers HJ. Home visits for frail older people: a qualitative study on the needs and preferences of frail older people and their informal caregivers. Br J Gen Pract 2012;62:e554-60. https://doi.org/10.3399/bjgp12X653606.
- Lagerin A, Törnkvist L, Hylander I. District nurses’ experiences of preventive home visits to 75-year-olds in Stockholm: a qualitative study. Prim Health Care Res Dev 2016;17:464-78.
- van Velsen L, Illario M, Jansen-Kosterink S, Crola C, Di Somma C, Colao A, et al. A community-based, technology-supported health service for detecting and preventing frailty among older adults: a participatory design development process. J Aging Res 2015;2015:1-9. https://doi.org/10.1155/2015/216084.
- Roland KP, Theou O, Jakobi JM, Swan L, Jones GR. Exploring frailty: community physical and occupational therapists’ perspectives. Phys Occup Ther Geriatr 2011;29:270-86. https://doi.org/10.3109/02703181.2011.616986.
- Manderson B, McMurray J, Piraino E, Stolee P. Navigation roles support chronically ill older adults through healthcare transitions: a systematic review of the literature. Health Soc Care Community 2012;20:113-27. https://doi.org/10.1111/j.1365-2524.2011.01032.x.
- Tøien M, Bjørk IT, Fagerström L. Older users’ perspectives on the benefits of preventive home visits. Qual Health Res 2015;25:700-12. https://doi.org/10.1177/1049732314553595.
- Williams V, Smith A, Chapman L, Oliver D. Community matrons – an exploratory study of patients’ views and experiences. J Adv Nurs 2011;67:86-93. https://doi.org/10.1111/j.1365-2648.2010.05458.x.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ n.d.;348.
- Independent Age . Advice Guides, Factsheets &Amp; Leaflets n.d. www.independentage.org/information/advice-guides-factsheets-leaflets (accessed 13 June 2017).
- A Practical Guide To Healthy Ageing. Age UK & NHS England; 2015.
- Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc 2004;52:625-34. https://doi.org/10.1111/j.1532-5415.2004.52174.x.
- Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 1989;42:703-9. https://doi.org/10.1016/0895-4356(89)90065-6.
- Fortinsky RH, Granger CV, Seltzer GB. The use of functional assessment in understanding home care needs. Med Care 1981;19:489-97. https://doi.org/10.1097/00005650-198105000-00002.
- Haywood KL, Garratt AM, Fitzpatrick R. Quality of life in older people: a structured review of generic self-assessed health instruments. Qual Life Res 2005;14:1651-68. https://doi.org/10.1007/s11136-005-1743-0.
- Bulamu NB, Kaambwa B, Ratcliffe J. A systematic review of instruments for measuring outcomes in economic evaluation within aged care. Health Qual Life Outcomes 2015;13. https://doi.org/10.1186/s12955-015-0372-8.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care 1989;5:559-75. https://doi.org/10.1017/S0266462300008461.
- Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5. https://doi.org/10.1186/1477-7525-5-63.
- Maheswaran H, Weich S, Powell J, Stewart-Brown S. Evaluating the responsiveness of the Warwick Edinburgh Mental Well-Being Scale (WEMWBS): group and individual level analysis. Health Qual Life Outcomes 2012;10. https://doi.org/10.1186/1477-7525-10-156.
- Bun Cheung Y. A confirmatory factor analysis of the 12-item General Health Questionnaire among older people. Int J Geriatr Psychiatry 2002;17:739-44. https://doi.org/10.1002/gps.693.
- Papassotiropoulos A, Heun R, Maier W. Age and cognitive impairment influence the performance of the General Health Questionnaire. Compr Psychiatry 1997;38:335-40. https://doi.org/10.1016/S0010-440X(97)90929-9.
- Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, et al. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med 1997;27:191-7. https://doi.org/10.1017/S0033291796004242.
- Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392-7. https://doi.org/10.1016/j.jamda.2013.03.022.
- Mijnarends DM, Meijers JM, Halfens RJ, ter Borg S, Luiking YC, Verlaan S, et al. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: a systematic review. J Am Med Dir Assoc 2013;14:170-8. https://doi.org/10.1016/j.jamda.2012.10.009.
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA 2011;305:50-8. https://doi.org/10.1001/jama.2010.1923.
- Cooper R, Kuh D, Hardy R. teams MRG on behalf of the Falc and Halc study . Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 2010;341. https://doi.org/10.1136/bmj.c4467.
- Vermeulen J, Neyens JC, Rossum E, van, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr 2011;11. https://doi.org/10.1186/1471-2318-11-33.
- Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423-9. https://doi.org/10.1093/ageing/afr051.
- Theou O, Cann L, Blodgett J, Wallace LMK, Brothers TD, Rockwood K. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Res Rev 2015;21:78-94. https://doi.org/10.1016/j.arr.2015.04.001.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB.
- Hurtig-Wennlöf A, Hagströmer M, Olsson LA. The International Physical Activity Questionnaire modified for the elderly: aspects of validity and feasibility. Public Health Nutr 2010;13:1847-54. https://doi.org/10.1017/S1368980010000157.
- The IPAQ Group . Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) - Short Form 2004. https://sites.google.com/site/theipaq/scoring-protocol (accessed 3 June 2017).
- English Longitudinal Study of Ageing . Insight into a Maturing Population n.d. www.elsa-project.ac.uk (accessed 30 May 2017).
- Measuring Long-term Conditions in Scotland. Edinburgh: NHS National Services Scotland; 2008.
- Formiga F, Ferrer A, Sanz H, Marengoni A, Alburquerque J, Pujol R. Octabaix study members. Patterns of comorbidity and multimorbidity in the oldest old: the Octabaix study. Eur J Intern Med 2013;24:40-4. https://doi.org/10.1016/j.ejim.2012.11.003.
- Quality and Outcomes Framework – Prevalence, Achievements and Exceptions Report England 2015–16. Leeds: NHS Digital; 2016.
- Berks J, McCormick R. Screening for alcohol misuse in elderly primary care patients: a systematic literature review. Int Psychogeriatr 2008;20:1090-103. https://doi.org/10.1017/S1041610208007497.
- Nazreddine Z. Montreal Cognitive Assessment (MoCA) Administration and Scoring Instructions 2010. www.mocatest.org/pdf_files/instructions/MoCA-Instructions-English_2010.pdf (accessed 7 November 2017).
- Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Prevention of Falls Network Europe and Outcomes Consensus Group. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618-22. https://doi.org/10.1111/j.1532-5415.2005.53455.x.
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Hoefman R, van Exel N, Brouwer W. iMTA Valuation of Informal Care Questionnaire (iVICQ). Rotterdam: iBMG/iMTA; 2011.
- Koopmanschap MA, van Exel JN, van den Berg B, Brouwer WB. An overview of methods and applications to value informal care in economic evaluations of healthcare. PharmacoEconomics 2008;26:269-80. https://doi.org/10.2165/00019053-200826040-00001.
- Department For Communities and Local Government . English Indices of Deprivation 2015 2015. http://imd-by-postcode.opendatacommunities.org/ (accessed 14 February 2017).
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j.2002.384.doc.x.
- Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012;65:301-8. https://doi.org/10.1016/j.jclinepi.2011.07.011.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit; 2016.
- Reference Costs 2015–2016. London: Department of Health; 2016.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0105-8.
- Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. London: Office of Health Economics; 2016.
- Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: normative data from twelve British studies. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0113637.
- Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy 2011;97:182-9. https://doi.org/10.1016/j.physio.2010.12.004.
- Ng Fat L, Scholes S, Boniface S, Mindell J, Stewart-Brown S. Evaluating and establishing national norms for mental wellbeing using the short Warwick–Edinburgh Mental Well-being Scale (SWEMWBS): findings from the Health Survey for England. Qual Life Res 2017;26:1129-44. https://doi.org/10.1007/s11136-016-1454-8.
- Schmitz N, Kruse J, Tress W. Psychometric properties of the General Health Questionnaire (GHQ-12) in a German primary care sample. Acta Psychiatr Scand 1999;100:462-8. https://doi.org/10.1111/j.1600-0447.1999.tb10898.x.
- Janssen B, Szende A, Szende A, Janssen B, Cabases J. Self-reported Population Health: An International Perspective Based on the EQ-5D. London: Springer; 2013.
- Flynn TN, Chan P, Coast J, Peters TJ. Assessing quality of life among British older people using the ICEPOP CAPability (ICECAP-O) measure. Appl Health Econ Health Policy 2011;9:317-29. https://doi.org/10.2165/11594150-000000000-00000.
- Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act 2015;23:471-87. https://doi.org/10.1123/japa.2014-0164.
- Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-449.
- Houston DK, Ding J, Nicklas BJ, Harris TB, Lee JS, Nevitt MC, et al. Overweight and obesity over the adult life course and incident mobility limitation in older adults: the health, aging and body composition study. Am J Epidemiol 2009;169:927-36. https://doi.org/10.1093/aje/kwp007.
- AUDIT-C (updated June 2017). Public Health England; 2017.
- Nasreddine Z. MoCA: Montreal Cognitive Assessment 2017. www.mocatest.org/ (accessed 22 June 2017).
- Liljas AEM, Carvalho LA, Papachristou E, Oliveira CD, Wannamethee SG, Ramsay SE, et al. Self-reported hearing impairment and incident frailty in english community-dwelling older adults: a 4-year follow-up study. J Am Geriatr Soc 2017;65:958-65. https://doi.org/10.1111/jgs.14687.
- Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Howard K, et al. Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. J Am Med Dir Assoc 2015;16:41-8. https://doi.org/10.1016/j.jamda.2014.07.006.
- Psychological Therapies, Annual Report on the use of IAPT services: England 2013/14. NHS Digital; 2014.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Noell GH, Gresham FM, Gansle KA. Does treatment integrity matter? A preliminary investigation of instructional implementation and mathematics performance. J Behav Educ 2002;11:51-67. https://doi.org/10.1023/A:1014385321849.
- Guide to the Methods of Technology Appraisals. London: NICE; 2013.
List of abbreviations
- A&E
- accident and emergency
- ADL
- activities of daily living
- AE
- adverse event
- AUDIT-C
- Alcohol Use Disorders Identification Test – Consumption
- BCT
- behaviour change technique
- BMI
- body mass index
- CALY
- capability-adjusted life-year
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- COM-B
- Capability, Opportunity Motivation – Behaviour
- CSRI
- Client Services Receipt Inventory
- CTU
- clinical trials unit
- eFI
- electronic Frailty Index
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GHQ-12
- 12-item General Health Questionnaire
- GP
- general practitioner
- IADL
- instrumental activities of daily living
- ICECAP-O
- ICEpop CAPability measure for Older people
- IMD
- Index of Multiple Deprivation
- IPAQ
- International Physical Activity Questionnaire
- IPAQ-E
- International Physical Activity Questionnaire for the Elderly
- MoCA
- Montreal Cognitive Assessment
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SMART
- specific, measurable, achievable, relevant and timely
- SMD
- standardised mean difference
- TAU
- treatment as usual
- TIDieR
- template for intervention description and replication
- WEMWBS
- Warwick–Edinburgh Mental Wellbeing Scale
- WISH
- Wellbeing Interventions for Social and Health needs in older people
