Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 15/17/05. The protocol was agreed in February 2016. The assessment report began editorial review in September 2016 and was accepted for publication in March 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Joanne Lord reports membership of the National Institute for Health Research Health Technology Assessment Commissioning Board from 2011 to 2016. Sophie Whyte reports personal fees from Southampton Health Technology Assessments Centre during the conduct of the study.
Note
The associated economic model in this report is protected by intellectual property rights, which are owned by the University of Southampton. Anyone wishing to modify, adapt, translate, reverse engineer, decompile, dismantle or create derivative work based on the economic model must first seek the agreement of the property owners.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Picot et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Colorectal polyps are small growths (usually < 1 cm in size) on the inner lining of the colon or rectum. They are common, affecting 15–20% of the general population, and they usually occur in people who are aged > 60 years. 1 Colorectal polyps do not usually cause symptoms, though some larger polyps are associated with rectal bleeding, diarrhoea, constipation and abdominal pain.
Colorectal polyps can be described in a variety of ways (e.g. by size, according to the type of cell or tissue they arise from within the colon or rectum, according to their shape and according to their histopathology). 2 Histopathological classification generally distinguishes between polyps that are adenomatous (known as adenomas or, less commonly, neoplastic polyps), hyperplastic or deep submucosal invasive cancers. Adenomas may eventually become cancerous if undiagnosed and untreated. Hyperplastic polyps usually do not carry a risk of developing into cancer; however, a subgroup of hyperplastic polyps, called sessile serrated polyps (polyps that have a slightly flattened shape with a saw tooth appearance), also have the potential to develop into cancer.
In terms of size, polyps measuring ≥ 10 mm are referred to as large, whereas those measuring 6–9 mm are considered small, and those ≤ 5 mm are classified as diminutive. It has been estimated that 80% of polyps detected at colonoscopy are diminutive. 3 A person can have more than one colorectal polyp and can have polyps of different sizes (e.g. diminutive polyps in addition to small polyps and large polyps). The morphology of a polyp can be described using the Paris endoscopic classification4 (Table 1). For the prediction of malignancy the Association of Coloproctology of Great Britain and Ireland (ACPGBI)5 recommends the use of the Paris endoscopic classification in conjunction with an estimation of the size of a polyp.
| The major variants of type 0 neoplastic lesions of the digestive tract | Type | Features |
|---|---|---|
| Protruded | Type 0–1p | Pedunculated (on a stalk) |
| Type 0–1sp | Subpedunculated | |
| Type 0–1s | Sessile | |
| Superficial elevated | Type 0–2a | Flat elevated |
| Type 0–2a + 2c | ||
| Type 0–2a + depression | ||
| Flat | Type 0–2b | Flat |
| Depressed | Type 0–2c | Slightly depressed |
| Type 0–2c + 2a | ||
| Excavated (ulcer) | Type 0–3 |
Colorectal polyps are usually detected during colonoscopy, a procedure involving examination of the rectum and the colon via a flexible tube called a colonoscope (a type of endoscope). The colonoscope is advanced inside the colon to the caecum (Figure 1), then slowly withdrawn by the endoscopist, who views images of the inner lining on a monitor. Patients might be referred for colonoscopy following an abnormal bowel screening result, or following referral from primary care as a result of symptoms suggestive of colorectal cancer or of inflammatory bowel disease (IBD), or as part of routine colonic surveillance [e.g. follow-up after previous polyp removal (a polypectomy) or for IBD] (see Care pathway for details of the care pathway).
FIGURE 1.
Illustration of the large intestine. Designua/Shutterstock.com. Image used under license from Shutterstock.com.
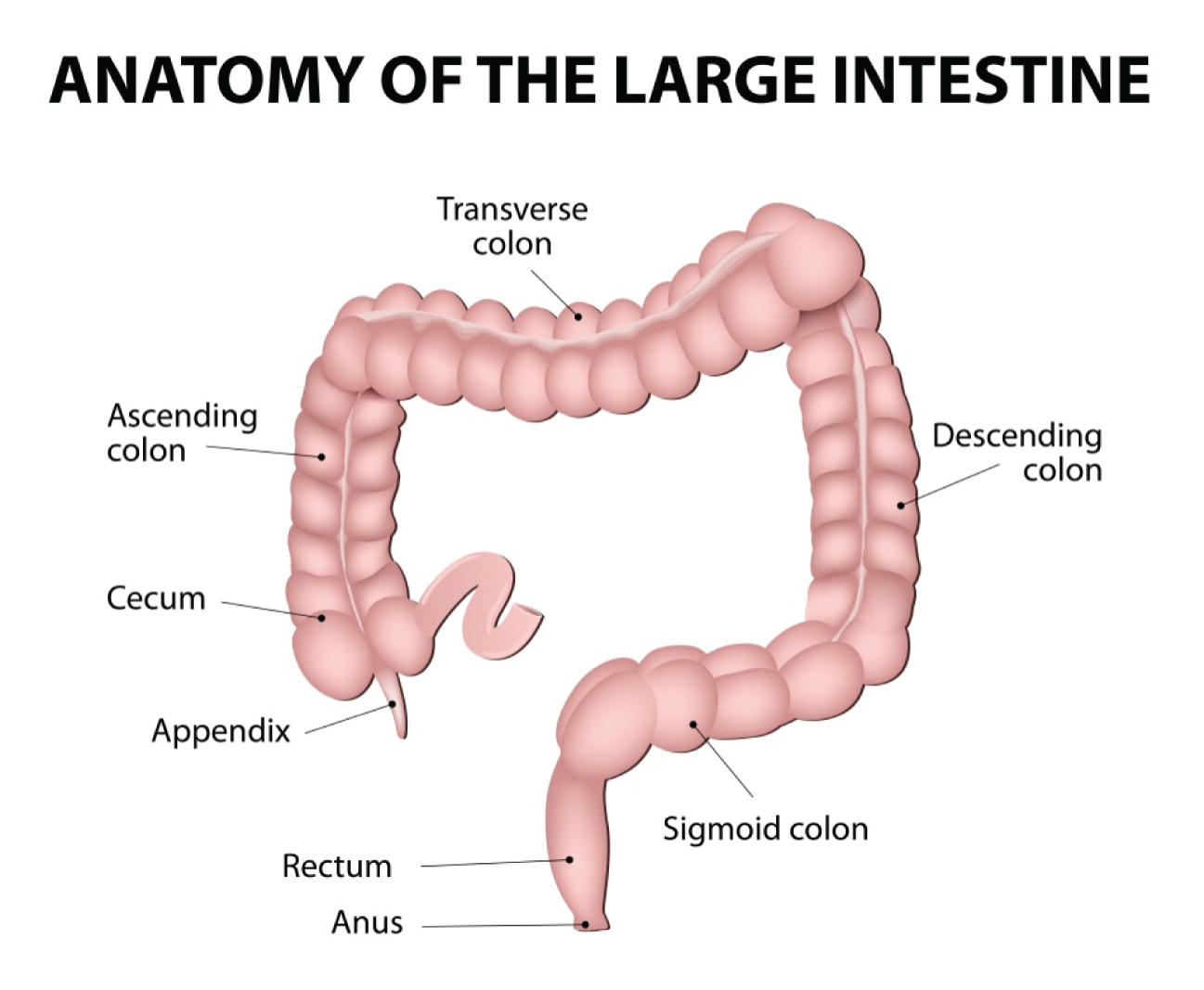
Colorectal cancer is one of the most common cancers in the UK after breast and lung cancer, with approximately 41,900 new cases registered each year. 6 The prevalence of colorectal cancer increases with age, with 99% of cases occurring in people aged > 40 years and 85% in those aged > 60 years. 7 A family history of bowel cancer is a key risk factor, with the risk increasing according to the greater number of first-degree relatives affected. 7 Familial adenomatous polyposis (FAP) and hereditary non-polyposis colorectal cancer (HNPCC) (also known as Lynch syndrome) are inherited genetic disorders that increase the risk of colorectal cancer, but are rare, accounting for only 5% of cancer cases. 7 Other factors thought to increase risk of colorectal cancer include diet (e.g. increased consumption of red and processed meat; lack of dietary fibre; lack of fruit and vegetables); obesity and lack of physical activity; consumption of alcohol and use of tobacco; and presence of longstanding IBD (e.g. Crohn’s disease or ulcerative colitis).
The NHS Bowel Cancer Screening Programme offers screening every 2 years to men and women aged 60–74 years. The programme invites eligible adults to carry out a faecal occult blood test (FOBT), which detects small amounts of blood in faeces. People with an abnormal FOBT result are referred for a colonoscopy to determine risk of colorectal cancer.
On diagnosis of colorectal cancer, patients will undergo staging and grading, with use of biopsy and imaging (e.g. computed tomography, endorectal ultrasonography or magnetic resonance imaging). The Dukes’ classification is a four-stage system (A–D), commonly used to determine the size and spread of the cancer. At Dukes’ A the cancer is only in the innermost lining of the bowel or slightly growing into the muscle layer, whereas at Dukes’ D the cancer has spread to other parts of the body such as the liver or the lungs. Treatment of the cancer will depend on the stage, but commonly includes surgical resection, combined with chemotherapy and radiotherapy where necessary, and, in some cases, biological therapies. 8 Bowel cancer survival rates in England vary according to stage, with rates for stage 1 patients (known as Dukes’ A colorectal cancer) in the range 95–100% at ≥ 5 years after diagnosis. 6 At stage 4 (Dukes’ D) survival rates at ≥ 5 years are just 5–10% (though this could be as high as 40%, if liver metastases can be successfully removed by surgery). 6 Generally, for people with colorectal cancer in England and Wales, almost 60% survive their cancer for 10 years or more following diagnosis (based on all stages). 6
Description of the diagnostic technologies under assessment
Current clinical practice is to detect colorectal polyps using conventional white-light endoscopy (WLE). This may be used in combination with dyes (chromoendoscopy) to enhance visualisation of tissues in the area being inspected. Detected polyps are then removed and each is sent for laboratory histopathological examination to determine whether it is an adenoma (therefore at a high cancer risk) or hyperplastic (at a low cancer risk). 1 (Note that in some centres some polyps may be left in situ if endoscopists are confident, on the basis of WLE, that they are hyperplastic.) The aim is to communicate the results to patients within 2 weeks. Histopathological examination is regarded as the reference standard method for characterising polyps, though it can be associated with errors of measurement and interpretation. For example, concerns have been raised about poor inter-rater reliability between gastrointestinal histopathologists. 9 Furthermore, some diminutive polyps may be damaged during resection (or cannot be resected at all), impairing the effectiveness of histopathological analysis. 3
Virtual chromoendoscopy (VCE) refers to electronic endoscopic imaging technologies that provide detailed contrast enhancement of the mucosal surface and blood vessels in the colon and rectum. A number of VCE technologies are available. All of these technologies use an endoscopy system typically consisting of an endoscope, a light source, a video processor and a visual display monitor. 10,11 The light source produces light that is transmitted to the distal end of the endoscope to illuminate the area under inspection. The video processor captures and processes electrical signals to enable an image of the inspected area to be displayed on the monitor. 11
The aim of VCE technologies is to provide enhanced visualisation of tissues without the need for dyes, enabling the endoscopist to differentiate between adenomatous and hyperplastic colorectal polyps in real time during colonoscopy. VCE technologies can be classed as optical or digital. In optical VCE, optical lenses are integrated into the endoscope’s light source, which selectively filters white light, resulting in narrow-band light. In digital chromoendoscopy, digital post-processing by the video processor is used to enhance the real-time image. 12
As discussed in Chapter 2, there are three commercial systems of relevance to this diagnostic assessment report:
-
narrow-band imaging (NBI), a type of optical chromoendoscopy
-
Flexible Spectral Imaging Colour Enhancement (FICE), a type of digital chromoendoscopy
-
i-scan, a type of digital chromoendoscopy.
Each of these will be described in turn.
Narrow-band imaging
Narrow-band imaging (Olympus Medical Systems Corp., Tokyo, Japan) is an optical image enhancement technology used in the Olympus endoscopic video imaging systems EVIS LUCERA ELITE,13 EVIS EXERA III14 (not available in the UK) and EVIS LUCERA SPECTRUM. 15 NBI is achieved by using a filter in the light source unit and a function on the video processor. The white light is filtered, resulting in narrow-band light, which consists of two wavelengths: 415-nm blue light and 540-nm green light. 12,15 These wavelengths are strongly absorbed by haemoglobin and thus NBI enhances the contrast between blood vessels and the surrounding mucosa in comparison with illumination by standard white light. The endoscopist can switch viewing mode from standard white light to NBI and vice versa at any time. The image quality achieved varies between the different endoscopy systems, as a result of differences in image sensors and video processors, with the newer EVIS LUCERA ELITE system offering the highest-quality images. Furthermore, within a class of endoscopy system, there will also be differences in image quality depending on the precise model of endoscope used. For example, within the EVIS LUCERA ELITE group, the EVIS LUCERA ELITE 290HQ high-definition (HD) endoscope offers the highest image quality, followed by the EVIS LUCERA ELITE 290H endoscope. The EVIS EXERA system is considered to be comparable to the EVIS LUCERA system in terms of diagnostic performance. The Olympus endoscopy system (including processor, endoscope and annual maintenance) is estimated to cost £87,385.
Flexible Spectral Imaging Colour Enhancement
Flexible Spectral Imaging Colour Enhancement [Aquilant Endoscopy/FujiFilm (Europe) GmbH, Willich, Germany] is a digital image processing function used in the Fuji video endoscopy systems EPX-4450HD, EPX-3500HD and EPX-4400. 16 White light illuminates the area of interest and the conventional images captured from the reflected light can be processed in real time by software into spectral images (images based on specific light wavelengths). FICE has 10 pre-set wavelength settings, which can also be manually altered to achieve the best enhancement of the image. 12,16 The endoscopist can switch between viewing conventional or FICE images at any time. The image quality achieved varies between the different systems, being higher on the EPX-4450HD and EPX-3500HD systems than on the EPX-4400 system. As well as being a feature of three Fuji endoscopy systems, the 500 series and 600 series endoscopes can also use FICE and it can be used in combination with magnifying endoscopes. The Aquilant Endoscopy/FujiFilm endoscopy system (including processor, endoscope and annual maintenance) is estimated to cost £59,312.
i-scan
i-scan (PENTAX Europe GmbH, Hamburg, Germany) is a digital image processing technology used with PENTAX endoscopy systems. 17 White light illuminates the area of interest and there are three different algorithms for real-time image processing:12,18
-
surface enhancement – helps to visualise the edges of anatomical structures by improving light–dark contrast
-
contrast enhancement – helps to visualise depressed areas by digitally adding blue colour to relatively dark areas
-
tone enhancement – modifies the colour contrast of the normal image to create an improved image with enhanced visibility of minute mucosal structures and subtle changes in colour.
The three different algorithms are then used in different combinations for three i-scan modes: (1) i-scan 1 for detection of lesions, (2) i-scan 2 for characterisation of lesions and (3) i-scan 3 for demarcation of lesions. The endoscopist can switch between the conventional image and the three i-scan modes at any time. If using equipment enabled with the capability (the EPK-i7000) it is possible to display a normal white-light image and an i-scan image simultaneously side by side. 18 The PENTAX endoscopy system (including processor, endoscope and annual maintenance) is estimated to cost £83,616.
Definition and magnification
The manufacturers of the technologies recommend that HD endoscopy systems are used to optimise the quality of the image. A HD system would be one in which the endoscope, the video processor, the display monitor and the cabling are, collectively, capable of producing an image corresponding to 650–720 lines of resolution. 19 The majority of monitors currently in use would be HD capable, although not all endoscopes would be HD. When equipment is due for replacement it will be upgraded to HD status.
Magnifying endoscopes (also sometimes referred to as near-focus or zoom endoscopes) can be used to enhance the clarity of images by magnifying up to 150 times. A movable lens can be fitted to the tip of the endoscope to provide optical zoom. However, magnifying endoscopes are largely unavailable in routine settings as they are not considered practical for day-to-day use. Most standard endoscopes can provide magnification of up to 35 times at the push of a button.
Classification schemes
Endoscopists make a general assessment of polyps based on observation of elements such as colour, blood vessels and surface pattern. There are several different classification schemes available, with particular schemes used with specific technologies. For example, the NBI International Colorectal Endoscopic scheme was devised specifically for use with NBI. 20 The novel classification system (NAC) has been developed for use with FICE. 21 Examples of classification schemes are shown in Table 2.
| Name of scheme | Basis for classification | Classification categories | ||
|---|---|---|---|---|
| NBI International Colorectal Endoscopic classification20 | Polyp histopathology (based on colour, vessels and surface pattern when viewed by NBI) | Type 1 | Hyperplastic | |
| Type 2 | Adenoma | |||
| Type 3 | Deep submucosal invasive cancer | |||
| Kudo classification22 | Pit pattern (fine surface structure of the mucosa when viewed by magnifying chromoendoscopy) | Round pits | Type I | Benign changes (e.g. normal, hyperplastic, inflammatory polyps) |
| Stellar or papillary pits | Type II | |||
| Large tubular or roundish pits | Type III L | Neoplastic and malignant changes | ||
| Small tubular or roundish pits | Type III s | |||
| Branch-like or gyrus-like pits | Type IV | |||
| Non-structural pits | Type V | |||
| Showa classification23 | Vascular pattern (pattern of microvessels surrounding the pit when viewed by NBI) | Normal | Characteristic of non-neoplasia | |
| Faint | ||||
| Network | Seen in neoplasia | |||
| Dense | ||||
| Irregular | Seen in neoplasia, useful for a diagnosis of cancer | |||
| Sparse | ||||
A classification system for endoscopic differentiation of small and diminutive adenomas, hyperplastic polyps and sessile serrated adenomas and polyps has recently been developed [the Workgroup serrAted polypS and Polyposis (WASP) classification]. 24
Training in the use of virtual chromoendoscopy
Training in the use of VCE is necessary to ensure adequate endoscopist performance in characterising polyps. Training methods vary and can involve endoscopists making ex vivo predictions based on still images previously taken using VCE as well as in vivo predictions in real time during colonoscopy, under supervision of an endoscopist more experienced in use of the technology. The duration of training may vary, with endoscopists subject to post-training key performance indicators and auditing. For example, the manufacturers of NBI estimate that a 1- to 2-day initial course would be sufficient. An online computer training application can be used as refresher training, in conjunction with audits and use of a validated classification scheme. The results of a recent study in England showed that a learning curve is observed in practice, even for endoscopists experienced in in vivo colorectal polyp characterisation. 25 A 90% threshold for diagnostic accuracy was achieved with use of HD WLE followed by i-scan once 200 polyps (< 10 mm in size) had been examined. This suggests that, following initial training, endoscopists should receive regular feedback on the accuracy of their diagnostic predictions (e.g. via histopathology on small batches of polyps), until an acceptable level of accuracy has been reached. This may take up to 6 months depending on the number of colonoscopies performed. Criteria for diagnostic performance of VCE have been proposed by international guidelines (see Care pathway), which specify the need for endoscopists to be adequately trained and audited. The Joint Advisory Group on gastrointestinal endoscopy has issued key performance indicators and quality assurance standards for colonoscopy26 and offers accreditation for colonoscopists, although there is no accreditation specifically for VCE.
Care pathway
Figure 2 provides an illustration of the care pathway showing indications for colonoscopy and subsequent management, reproduced from the National Institute for Health and Care Excellence scope for this diagnostic assessment. 27 As mentioned in Description of the health problem, patients may be referred for colonoscopy via a number of routes. For example, they may receive colonoscopy following an abnormal bowel cancer screening result or after referral from primary care as a result of having symptoms suggestive of colorectal cancer (e.g. rectal bleeding, pain or altered bowel habits).
FIGURE 2.
Care pathway before and after colonoscopy. Figure reproduced with permission from the National Institute for Health and Care Excellence’s scope for this appraisal. 27 © NICE 2017 Virtual Chromoendoscopy to Assess Colorectal Polyps during Colonoscopy. Available from https://www.nice.org.uk/guidance/dg28. All rights reserved. Subject to Notice of rights. BSG, British Society of Gastroenterology; ESGE, European Society of Gastrointestinal Endoscopy.
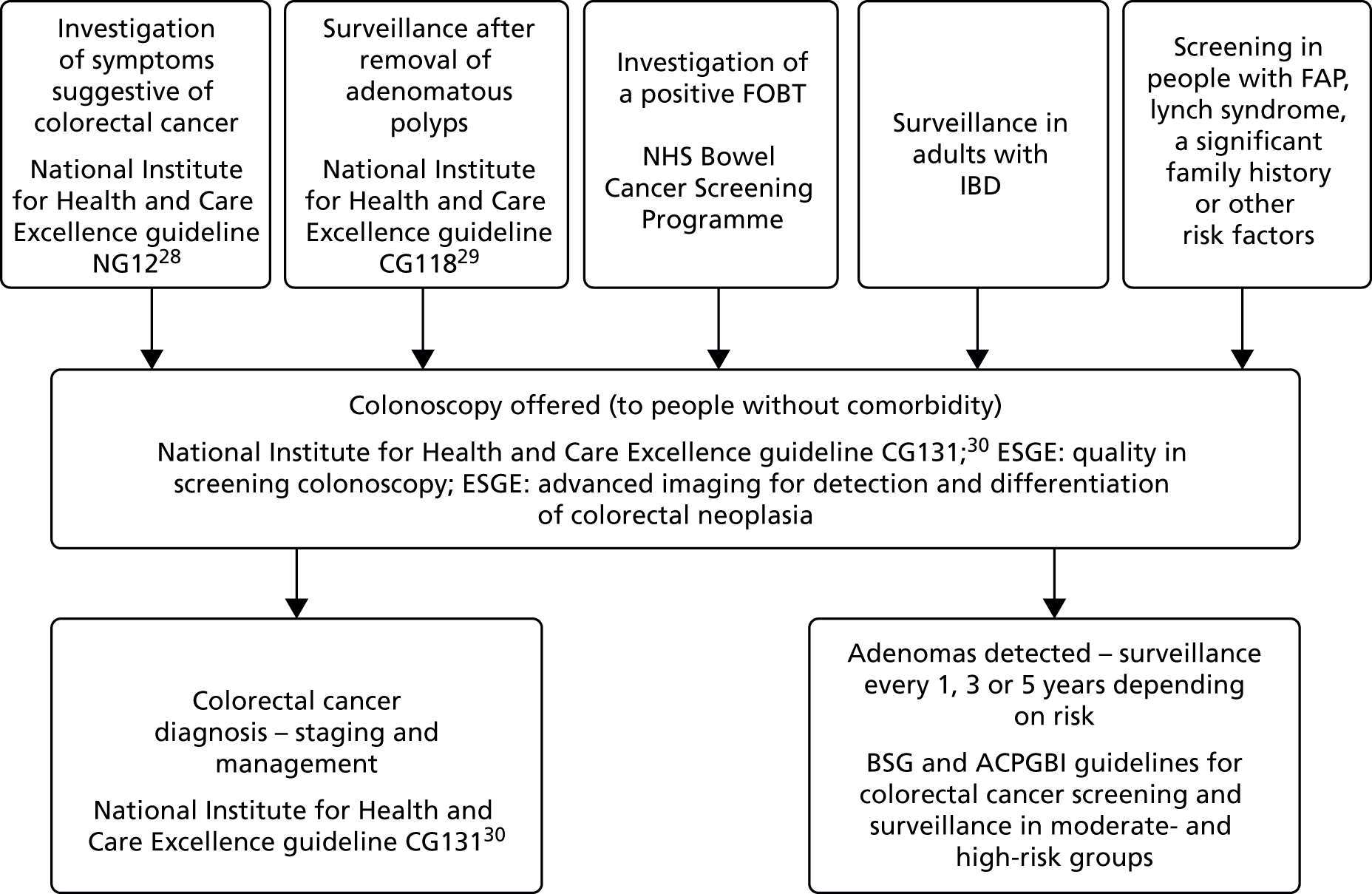
The risk of colorectal cancer varies between different patient groups. Patients with FAP and HNPCC (Lynch syndrome) have a high risk of colorectal cancer. Patients with an abnormal bowel cancer FOBT result may be at higher risk than patients undergoing surveillance for removal of adenomatous polyps.
Following the detection of colorectal adenomas by colonoscopy, a surveillance interval will be set, based on the size and number of adenomas found. The British Society of Gastroenterology (BSG) and the ACPGBI have issued guidelines for colorectal cancer screening and surveillance in moderate-risk and high-risk groups. 30 The following recommendations are made:
-
People with one or two small (< 1cm in size) adenomas are at low risk and need no colonoscopic surveillance or 5-yearly surveillance until one negative examination, following which surveillance should cease.
-
People with three or four small adenomas or at least one adenoma that is ≥ 1 cm are at intermediate risk and need 3-yearly surveillance until two consecutive examinations are negative.
-
People with five or more adenomas, or three or more adenomas at least one of which is ≥ 1 cm, are at high risk and an extra examination should be undertaken at 12 months before returning to 3-yearly surveillance.
The National Institute for Health and Care Excellence clinical guideline number 118 on colonoscopic surveillance in people with IBD or adenomas makes similar recommendations. 29
Virtual chromoendoscopy takes place in secondary or tertiary care at the same point in the care pathway as current clinical practice using conventional WLE or dye-based chromoendoscopy. It is likely that VCE technologies would be used alongside conventional WLE, as all the technologies relevant to this assessment allow the endoscopist to change viewing mode from standard white light to the VCE image in real time at the flick of a switch. For example, the endoscopist may begin examining the colon with WLE and then (in some cases) use dye to enhance visualisation of potential adenomas. They may then switch the endoscope to use VCE to further enhance visualisation. This practice is referred to as optical assessment of colorectal polyps. The care pathways would diverge when a diminutive polyp of ≤ 5 mm is detected. Under current clinical practice, a diminutive polyp identified by conventional WLE would be removed and sent for histopathological examination to determine whether it is adenomatous, hyperplastic or cancerous. 31 However, use of a VCE technology would enable the endoscopist to differentiate between adenomas and hyperplastic polyps during colonoscopy. When the endoscopist has high confidence in the polyp characterisation, adenomas would be removed and discarded, whereas hyperplastic polyps in the rectosigmoid colon would be left in situ (as these would be considered very low risk for colorectal cancer). This is referred to as the Detect, InSpect, ChAracterise, Resect and Discard (DISCARD) strategy (Figure 3). 3 When there is low confidence in determining whether a polyp is adenomatous or hyperplastic it should be resected and sent for histopathological examination. Any flat depressed polyps, polyps with a distorted shape and hyperplastic-appearing (serrated-appearing) polyps in the proximal colon should be sent for histopathological examination, irrespective of size. The level of confidence with which polyp classification is made is subjective and varies between endoscopists. Some endoscopists increase objectivity by referring to the relevant classification system [e.g. a high-confidence assessment made with NBI might be based on whether at least two of the NBI International Colorectal Endoscopic classification criteria apply to the particular polyp (i.e. based on polyp colour, vessels and surface pattern)].
FIGURE 3.
Flow chart for low-risk application of the DISCARD strategy for diminutive colorectal polyps (from Wang and East, 2015). 3 a, Appropriate patients are those aged > 50 years undergoing screening or surveillance colonoscopy. Less or inappropriate indications include positive FOBT, younger patients, patients with > 10 polyps or known or suspected familial cancer syndromes and IBD surveillance. SP, serrated polyps (hyperplastic polyps or sessile serrated polyps, but not traditional serrated adenoma). Reprinted from Gastrointestinal Endoscopy, 82/2, Wang LM and East JE, Diminutive polyp cancers and the DISCARD strategy: much ado about nothing or the end of the affair? pp. 385–8. Copyright (2015), with permission from Elsevier.

Advantages of the DISCARD strategy include the fact that real-time characterisation of polyps may potentially alleviate patient anxiety associated with waiting for histopathology results and reduce health service and patient costs associated with additional appointments. A surveillance interval can be set on the day of the procedure, rather than at a follow-up appointment following the results of histopathology, and savings may be made through reduced use of histopathology. It has been reported that histopathology accounts for up to 10% of the cost of colonoscopy,3 and that use of colonoscopy in the NHS is increasing each year.
There may be potential disadvantages associated with the use of VCE. For example, endoscopists will need to have sufficient experience with in vivo characterisation of polyps and adequate training in, and experience of, the particular VCE technology. This is a requirement of European and US endoscopy guidance (see Diagnostic thresholds and requirements for use of virtual chromoendoscopy). It has been noted that performance among community-based endoscopists may not necessarily meet these requirements. 3 Furthermore, there is the risk that a diminutive polyp cancer (incidence rates of which vary from 0% to 0.6%3) may inadvertently be characterised as an adenoma, resected and discarded without histopathological examination, with malignant cells left behind, and subsequent potential development of undiagnosed metastatic disease and death. 3 To attempt to address these concerns, international professional associations have issued guidance on the use of VCE as part of a DISCARD strategy, as discussed in the next section, Diagnostic thresholds and requirements for use of virtual chromoendoscopy.
Diagnostic thresholds and requirements for use of virtual chromoendoscopy
There are several different aspects to any decision to implement the new technology, and European31 and American guidance32 has been published.
The European guidance,31 produced by the European Society of Gastrointestinal Endoscopy (ESGE) in 2014, makes the recommendation that VCE (NBI, FICE, i-scan) and conventional chromoendoscopy can be used, under strictly controlled conditions, for real-time optical diagnosis of diminutive (≤ 5mm in size) colorectal polyps to replace histopathological diagnosis. The optical diagnosis has to be reported using validated scales, must be adequately photo-documented and can be performed only by experienced endoscopists who are adequately trained and audited (ESGE describe this as a weak recommendation based on high-quality evidence).
The American guidance32 on real-time endoscopic assessment of the histopathology of diminutive colorectal polyps is part of the Preservation and Incorporation of Valuable endoscopic Innovation programme (PIVI) of the American Society for Gastrointestinal Endoscopy (ASGE). The PIVI statement defines two requirements that new technologies for the real-time endoscopic assessment of the histopathology of diminutive colorectal polyps should meet before a ‘resect and discard’ strategy can be applied:
In order for colorectal polyps ≤ 5 mm in size to be resected and discarded without pathological assessment, endoscopic technology (when used with high confidence) used to determine histopathology of polyps ≤ 5 mm in size, when combined with the histological assessment of polyps > 5 mm in size, should provide a ≥ 90% agreement in assignment of post-polypectomy surveillance intervals when compared with decisions based on pathology assessment of all identified polyps.
In order for a technology to be used to guide the decision to leave suspected rectosigmoid colon hyperplastic polyps ≤ 5 mm in size in place (without resection), the technology should provide ≥ 90% negative predictive value (NPV) (when used with high confidence) for adenomatous histology.
If it is judged that the polyp cannot be confidently assessed using an endoscopic technology, then it should be resected and sent for histopathological diagnosis. The guidance also indicates that polyp images should be permanently stored and should be of sufficient resolution to support the endoscopists’ assessment and clinical decisions.
Current service provision
As stated above, current practice is to detect polyps using WLE, with additional dye-based chromoendoscopy used when necessary to provide additional information on polyp characteristics. All diminutive polyps detected are resected and undergo histopathological analysis to determine whether they are adenomatous or hyperplastic. A surveillance interval is then set based on the number and size of adenomas detected. The majority of existing endoscopy systems in use in NHS hospitals are thought to be capable of VCE. The technology is built into the light source and video processor and can be activated by the endoscopist by a switch at any time during colonoscopy. The lifecycle of an endoscopy system is estimated to be between 5 and 8 years, and all new systems are now equipped with VCE technology. However, VCE and the DISCARD strategy are not thought to be routinely used as a management protocol. However, in some centres diminutive polyps in the rectosigmoid colon are optically diagnosed using white light or VCE and left in place if there is high confidence the polyps are hyperplastic. Of the three technologies of relevance to this assessment, NBI is considered to be the most widely available, and it has the largest market share for electromedical service contracts in England.
Chapter 2 Definition of the decision problem
Under current clinical practice all diminutive polyps (1–5 mm in size) identified by conventional WLE would be removed and sent for histopathological examination to determine whether they are adenomas or hyperplastic, and the consequent colorectal cancer risk. Once histopathology results are available, a surveillance interval is set according to the number and size of adenomas detected. Use of a VCE technology would provide the endoscopist with enhanced visualisation to differentiate between adenomas, which could be resected and discarded (i.e. not sent for histopathological assessment), and hyperplastic polyps in the rectosigmoid colon, which could be left in situ. This can be done only when the endoscopist is highly confident in their characterisation of the polyp.
The potential benefits of VCE would be fewer resections (polypectomy) of low-risk hyperplastic polyps (with a resulting reduction in complications such as bleeding or perforation of the bowel); the provision of results more quickly, thus potentially reducing patient anxiety; a reduction in health resource use through fewer histopathological examinations; and quicker management (including surveillance) decisions. Guidelines recommend that VCE should be performed only under strictly controlled conditions by experienced endoscopists adequately trained in the use of the technology, using validated classification scales. 31
In order for VCE technologies to be incorporated into routine clinical practice for the real-time assessment of colorectal polyps during colonoscopy, there needs to be evidence that the new technology provides an appropriate and efficient standard of care compared with existing practice. Therefore, the decision question for this assessment is ’Does VCE for real-time assessment of diminutive colorectal polyps during colonoscopy represent a cost-effective use of NHS resources?’.
Populations and relevant subgroups
The population of relevance to this assessment is people referred for colonoscopy through the NHS Bowel Cancer Screening Programme because of an abnormal FOBT test result; people offered colonoscopic surveillance because they had adenomas previously removed; and people undergoing colonoscopy with diminutive colorectal polyps referred for colonoscopy by a general practitioner (GP) because of symptoms suggestive of colorectal cancer.
At the scoping stage of this assessment it was agreed that patients with IBD or conditions such as FAP or HNPCC would not be relevant, as these are distinct patient groups with increased risks of colorectal cancer in whom differentiation between adenomatous and non-adenomatous polyps during colonoscopy is more complicated (e.g. in patients with IBD because of factors such as increased number of microvessels). VCE with a DISCARD strategy would be unlikely to be used in these patients. 8 At the scoping stage it was also considered that small polyps (6–9 mm in size) would not be included in the scope of the assessment. 8
Index tests
Virtual chromoendoscopy is the index test, of which three technologies are considered relevant to this diagnostic assessment:
-
NBI
-
FICE
-
i-scan.
Each technology should be used with HD or high-resolution monitors and endoscopes without the use of magnification.
Reference standard
The reference standard for VCE is histopathological assessment of diminutive polyps.
Outcomes
A range of outcomes are relevant to this assessment, which can be classified as diagnostic test accuracy [e.g. accuracy (i.e. proportion of correctly classified polyps among all the polyps), sensitivity, specificity, accuracy, NPV and positive predictive value (PPV)]; intermediate outcomes (e.g. recommended surveillance intervals, time taken to perform colonoscopy); patient-reported outcome measures [e.g. health-related quality of life (HRQoL)]; clinical outcomes (e.g. adverse effects of polypectomy, incidence of colorectal cancer); and cost outcomes (e.g. endoscopy system costs, colonoscopy and related costs, training costs, histopathology costs).
Overall aims and objectives of assessment
The aim of this research is to assess the clinical effectiveness and cost-effectiveness of technologies that could aid the characterisation of diminutive colorectal polyps that have the potential to become cancerous.
Specific objectives are to determine, through a systematic review and economic evaluation, the clinical effectiveness and cost-effectiveness of the VCE technologies, NBI, FICE and i-scan, in the characterisation and management of diminutive colorectal polyps.
Chapter 3 Methods
We set out the methods for the systematic reviews of clinical effectiveness and cost-effectiveness a priori in a research protocol, which was published on the National Institute for Health and Care Excellence’s website (www.nice.org.uk/guidance/GID-DG10004/documents/final-protocol). The protocol was also registered with PROSPERO, a prospective register of systematic reviews (registration ID CRD42016037767). 33 Our Expert Advisory Group commented on a draft of the protocol. The reviews were undertaken following the general good practice approaches recommended by the Centre for Reviews and Dissemination,34 the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.9 and 1.035,36 and the National Institute for Health and Care Excellence’s Diagnostics Assessment Programme Manual. 37 Here, we outline the methods specified in the protocol and note minor modifications that were made during the review.
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Identification of studies
An experienced information specialist developed and tested a comprehensive search strategy. The strategy was designed to identify studies of the diagnostic accuracy of VCE and studies providing relevant clinical outcomes (morbidity, mortality, HRQoL) associated with VCE and histopathological diagnosis. The strategy was also designed to capture relevant cost-effectiveness studies to inform the economic evaluation (see Chapter 5).
The following databases were searched from inception to June 2016 for published research: MEDLINE, PREMEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Web of Science, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment database and NHS Economic Evaluation Database. (Note that the protocol for the systematic reviews stated that the Medion database of diagnostic studies would be searched; however, when the review commenced we found that this database had been discontinued.) Grey literature and ongoing studies were also identified, through searches of the following databases in March 2016: the UK Clinical Trials Gateway (UKCTG), the World Health Organization’s International Clinical Trials Registry Platform, International Standard Randomised Controlled Trials Number (ISRCTN; controlled and other trials), ClinicalTrials.gov and PROSPERO. [Note that the protocol for the systematic reviews stated that the UK Clinical Research Network Portfolio Database and the National Institute for Health Research (NIHR) Clinical Research Network Portfolio would be searched but these are now part of the UKCTG.] All searches were limited to the English language.
We additionally searched conference proceedings and the internet pages of relevant organisations for publications, both in April 2016. Proceedings from the following conferences were searched: the ACPGBI Annual Meeting; the Annual Meeting of the European Society of Coloproctology; the ASGE Digestive Disease Conference; the Digestive Disease Week Conference; and the United European Gastroenterology Week Conference. We searched the following organisations’ websites: the BSG, the ESGE, the ASGE and the American Gastrointestinal Association.
We also searched the bibliographies of the included studies and of relevant systematic reviews found during the searches to identify further references, and asked our Expert Advisory Group to identify additional published and unpublished studies. Information provided by the companies to the National Institute for Health and Care Excellence was also searched for additional studies that might meet the review inclusion criteria. A full list of databases searched, search dates and an example search strategy are provided in Appendix 1.
Inclusion and exclusion criteria
We screened all the publications identified from the searches against the prespecified eligibility criteria set out here to determine if they should be included in the reviews of clinical effectiveness and cost-effectiveness.
Study design
For the systematic review of clinical effectiveness, studies were eligible for inclusion if they were randomised controlled trials (RCTs), prospective longitudinal cohort studies or cross-sectional studies. Systematic reviews were not included and were retrieved only during screening to check their reference lists for potentially relevant primary research studies. Editorials and case reports were not included.
For the systematic review of cost-effectiveness, studies were included if they were full economic evaluations, assessing costs and consequences, of the specified VCE technologies.
Population
For both the reviews of clinical effectiveness and cost-effectiveness, studies had to include at least one of the following populations to be eligible for inclusion in the review:
-
people referred for colonoscopy following an abnormal bowel cancer screening result
-
people offered colonoscopic surveillance because they have had adenomas removed
-
people with symptoms that may be suggestive of colorectal cancer who are referred for colonoscopy by a GP.
As stated earlier (see Chapter 2, Populations and relevant subgroups), the target population in this assessment does not include people undergoing monitoring for IBD (e.g. Crohn’s disease) and people with polyposis syndromes such as HNPCC or FAP. Studies including these populations were therefore excluded.
Index test
Studies were included in both reviews if they evaluated one or more of the technologies of interest for the real-time diagnosis of colorectal polyps (as opposed to post-procedure image-based diagnosis):
-
NBI – EVIS LUCERA ELITE, EVIS LUCERA SPECTRUM or EVIS EXERA (Olympus Medical Systems). The EXERA system is not available in the UK, but expert advice to the External Assessment Group was that diagnostic outcomes are similar to the EVIS LUCERA series.
-
FICE (Fujinon/Aquilant Endoscopy).
-
i-scan (PENTAX Medical).
Studies of these technologies were included only if they used HD or high-resolution endoscopy systems without the use of magnification (in at least one study arm; in the case of RCTs, arms not meeting this criterion were excluded). These limitations were applied because, as explained in Chapter 1, Definition and magnification, the majority of endoscopy equipment used in practice is (or will be in the future) HD capable and because magnifying endoscopes are largely unavailable and not considered practical in routine care. During screening, the following decision rules were created to address uncertainty about inclusion of studies in the clinical effectiveness review when they used inbuilt or optional magnification or did not mention magnification:
-
Studies or study arms using inbuilt (close-focus) magnification (which is a low level of magnification, e.g. ×1.5) that did not require a zoom endoscope or any additional equipment were included.
-
When magnification was described as optional and no further details were provided or when magnification was not mentioned, we included the study (i.e. presumed no magnification).
In addition, if a standard-definition endoscope was used with a HD monitor in a study, we excluded the study as this type of monitor cannot compensate for lack of a HD endoscope. Studies or study arms using endoscopes with a push-button ‘near-focus’ capability were excluded, as these endoscopes use magnification, unless it was clear that the ‘near-focus’ function had not been used during polyp characterisation.
Reference test (comparator)
Only studies using histopathological assessment of resected diminutive (≤ 5 mm in size) colorectal polyps as the reference test were included. Studies of larger polyps were eligible if outcome data were given for a subgroup of diminutive polyps.
Outcomes
Studies had to measure and report results for at least one of the following outcomes to be included in the clinical effectiveness review (none were specified as primary or secondary outcomes for the review):
-
accuracy of VCE diagnosis of polyp (e.g. adenoma, hyperplastic)
-
number of polyps designated to be left in place
-
number of polyps designated to be resected and discarded
-
number of polyps designated to be resected and sent for histopathological examination
-
recommended surveillance interval
-
length of time to perform the colonoscopy
-
number of outpatient appointments or telephone consultations
-
HRQoL, including anxiety
-
adverse effects of the removal of polyps (i.e. of polypectomy)
-
incidence of colorectal cancer
-
mortality.
To be included in the cost-effectiveness review, studies needed to measure relevant outcomes including the incidence of colorectal cancer or life-years or quality-adjusted life-years (QALYs) gained.
Inclusion screening process
Reviewers selected studies for inclusion through a two-stage process using the predefined and explicit criteria specified above. Two reviewers independently assessed the titles and abstracts of the publications identified through the searches for potential relevance to the review. We then obtained the full texts of agreed potentially relevant publications for full-text screening. During full-text screening, one reviewer assessed each publication against the eligibility criteria, using a standardised inclusion flow chart, and another reviewer checked the first reviewer’s decision and a final decision regarding inclusion was agreed. Studies had to meet all of the eligibility criteria to be included in the review. At both stages any disagreements were resolved by discussion, with involvement of a third reviewer where necessary. The inclusion flow chart is shown in Appendix 2. The first item in the flow chart that the reviewers agreed would be a reason for exclusion was recorded as the primary reason for exclusion.
During full-text screening, we found that the population was unclear in some of the publications assessed (e.g. owing to lack of description). In these instances, we included the study in the review, unless there was evidence that it included a population not relevant to this assessment (e.g. IBD, polyposis syndromes). Studies published as abstracts or conference proceedings were included in the reviews only if they were published in 2014, 2015 or 2016 and if sufficient details were presented to allow appraisal of the methodology and assessment of results to be undertaken (as prespecified in the protocol).
Data extraction strategy
One reviewer extracted data from each included study, using a standardised and pilot-tested data extraction form, and a second reviewer checked the extracted data for accuracy. Reviewers resolved any discrepancies in the data extracted through discussion or, when necessary, arbitration by a third reviewer. Publications that reported the same primary study were data extracted together as one study, to avoid double counting information. Reviewers extracted data, when available, on the study and population characteristics; the endoscopic equipment used (including model numbers); the study endoscopists’ experience and training; the polyp classification system used; the sample size calculation; and results for all outcomes of interest in this review. When data were available, we extracted the results of subgroup analyses of diagnostic accuracy by the endoscopists’ level of expertise and experience in optical assessment of polyps; their level of confidence in their polyp assessment (i.e. high or low); and the location of the polyp. See Appendix 3 for the completed data extraction form for each study.
When we extracted the diagnostic accuracy results from each study, we used available data in the study publication(s) to populate a 2 × 2 contingency table showing how the index test results related to the histopathological analysis results, for each analysis or subgroup analysis of diminutive polyps. The contingency tables showed the number of true positives (TPs), false positives (FPs), true negatives (TNs) and false negatives (FNs). When these data were only partially reported in the study publications or not reported at all, reviewers imputed the data from other available results information, if possible. It was necessary to extract or impute these data, as we needed complete 2 × 2 tables to be able to include a study in a meta-analysis (see Method of data synthesis for further details about data synthesis). It was not always possible to impute these data (e.g. total number of diminutive polyps not reported and numbers of adenomas and hyperplastic polyps not reported). For five studies we asked the study contact author for the 2 × 2 table data. Two authors replied, but neither was able to supply data. Reviewers also calculated the accuracy (proportion of correctly classified polyps among all the polyps), clinical sensitivity, clinical specificity, PPV, NPV, positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio for each diagnostic accuracy analysis and subgroup analysis reported in each study. Reviewers compared the values they calculated with the study values and noted any discrepancies. If any of these outcomes had not been reported in the studies, the reviewer’s calculated values were used. We used an online calculator MedCalc (www.medcalc.org/calc/diagnostic_test.php; accessed 16 August 2016) to calculate clinical sensitivity, clinical specificity, PPV, NPV, and positive and negative likelihood ratios.
Quality assessment
The quality of studies reporting diagnostic accuracy was assessed using the Cochrane adaptation38 of the quality assessment of diagnostic accuracy studies (QUADAS) tool,39 which can be used to assess a variety of study designs (e.g. RCT, non-RCT, prospective cohort studies). Table 3 shows the types of bias assessed by the QUADAS tool. We assessed whether or not these types of bias were present in studies in this review. One reviewer assessed the methodological quality of each study and a second reviewer checked the first reviewer’s judgements, with any disagreements resolved by consensus or, if necessary, by arbitration by a third reviewer.
| QUADAS question | Type of bias | Explanation |
|---|---|---|
| 1 | Spectrum bias | The study population is not representative of those who will receive the index test (VCE, i.e. NBI, i-scan or FICE) in clinical practice |
| 2 | Verification bias | The reference standard (histopathology) does not accurately distinguish between adenomas and hyperplastic polyps |
| 3 | Disease progression bias | The time interval between the index (VCE) test and reference standard (histopathology) is long enough that the two tests may not have measured the same disease state |
| 4 and 5a | Differential verification bias | Diagnosis is inaccurate because not all patients receive the same reference standard |
| 6 | Incorporation bias | The index (VCE) test is not independent of the reference standard (e.g. if it was one of several tests used as the reference standard) |
| 7 | Diagnostic review bias | The index test (VCE) result influences interpretation of the reference standard result |
| 8 | Test review bias | The reference standard result influences interpretation of the index (VCE) test result |
| 9 | Clinical review bias | The information used when interpreting the index (VCE) test does not reflect that likely to be available in clinical practice |
| 10 | Test classification bias | If index test results classified as uninterpretable, intermediate or indeterminate are incorrectly included or excluded from the analysis, this may systematically influence sensitivity or specificity |
| 11 | Attrition bias | The exclusion of patients or test results from the analysis may systematically influence sensitivity or specificity if:
|
Method of data synthesis
The included studies were synthesised in a narrative review with tabulation of results. Meta-analysis was also conducted to provide pooled estimates of diagnostic sensitivity and specificity. The rationale for meta-analysis was to provide a more precise estimate of diagnostic accuracy than can be provided from single studies alone. In diagnostic test studies, sensitivity and specificity are often negatively correlated, sometimes because studies have used different thresholds for defining positive and negative test results. Furthermore, heterogeneity often exists between the studies in terms of patient characteristics, settings and tests used. These factors need to be taken into account in the choice of meta-analysis methods applicable to a given topic. A univariate meta-analysis pools sensitivity and specificity separately, failing to take into account the correlation. Hierarchical models include statistical distributions at the lower level (within-study variability in sensitivity and specificity) and at the higher level (between-study variability) and can therefore take into account correlation and heterogeneity. 40 In this systematic review it is likely that heterogeneity exists in factors (such as the endoscopist’s level of experience and training in VCE, the setting in which colonoscopy is performed and the patient’s indication for colonoscopy) and, therefore, risk of colorectal cancer. VCE does not require an explicit numerical threshold for a diagnostic prediction. Rather, the prediction is a binary one, of whether a polyp is an adenoma or hyperplastic. A hierarchical bivariate meta-analysis model was used in this assessment as it estimates summary sensitivity and specificity at various thresholds (in this case the threshold is the confidence and judgement with which the endoscopist makes their polyp characterisation). 41 Previously published meta-analyses of VCE for optical diagnosis of colorectal polyps have also used a bivariate model to estimate pooled sensitivity and specificity. 42–44
We conducted separate meta-analyses for the each of the three VCE technologies relevant to this report compared with histopathology. For each technology we produced individual meta-analyses according to the level of confidence with which the polyp characterisation had been made by the endoscopist in accordance with how the data were reported in the primary studies (high-confidence predictions; all predictions irrespective of confidence level). High-confidence predictions are of particular relevance to the DISCARD strategy and are used to inform the economic model in this assessment report (see Chapter 5, Independent economic evaluation). We also meta-analysed studies according to the area of the colon in which the polyps were located and thus characterised (e.g. whole colon, rectosigmoid colon), stratified according to level of endoscopist confidence in making characterisations. Again, this is relevant to the DISCARD strategy for decisions about whether or not hyperplastic polyps in the rectosigmoid colon can be left in situ (see Chapter 1, Care pathway). Where possible, we explored heterogeneity by conducting subgroup analyses for factors such as the level of experience of the endoscopist in the in vivo characterisation of polyps and in using the specific VCE (see Chapter 4, Quantity and quality of research available for a description of the studies included in the systematic review).
Consideration was given to meta-analysing NPVs from the included studies. A NPV of ≥ 90% is required for a high-confidence decision to leave a suspected hyperplastic diminutive polyp in place, as stated in the PIVI initiative32 (see Chapter 1, Care pathway). However, PPVs and NPVs vary with differences in disease prevalence, so pooling is not always advisable when it is suspected that there may be variation in prevalence between studies. 37 Because the prevalence of adenomas and hyperplastic polyps may vary between studies [e.g. as a result of differences in case mix (screening, surveillance and symptomatic populations) and patient characteristics (age, sex)], we chose not to pool NPVs across studies.
We used Stata software (Stata 14.0 IC, StataCorp LP, College Station, TX, USA) to conduct the meta-analysis, using the metandi Stata package, which has been specifically designed to perform bivariate meta-analyses of diagnostic studies. 45 The Stata package xtmelogit was also used where fewer than four studies were available in a meta-analysis, as metandi was not able to perform analyses on this number of studies. We used Stata programming code supplied by the Cochrane Screening and Diagnostic Tests Methods Group for bivariate meta-analysis models. 46 Four input variables were used by Stata to perform the meta-analysis: the number of TPs, FPs, FNs and TNs for each study (the unit of analysis is the individual polyp). These were taken from our data extraction forms for each included study and included in a spreadsheet from which Stata directly drew the data. We also used Cochrane Review Manager (RevMan, version 5.3; The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) to produce coupled forest plots of sensitivity and specificity and summary receiver operating characteristic (SROC) curve plots. The forest plots allow a visual interpretation of the individual study estimates, which can be informative in the assessment of heterogeneity. The SROC plots provide confidence and prediction regions around the summary estimate to enable joint inferences to be made about sensitivity and specificity. The confidence region is based on the confidence interval (CI) around the summary estimate. The prediction region indicated the area where we would expect results from a new study in the future to lie. 40 In the SROC plots, individual study estimate points are scaled to the sample size of the study (i.e. larger circles represent larger studies).
Chapter 4 Assessment of diagnostic studies
Results
Quantity and quality of research available
A total of 2068 references were identified by searches (after de-duplication) and two additional references were identified through other sources (Figure 4). We screened the titles and, where available, abstracts of the 2070 references and retrieved full copies of 125 references. We excluded 63 full-text references, the majority because either the intervention (n = 28) or comparator (n = 29) did not meet the inclusion criteria (a list of the excluded studies with reasons for exclusion is presented in Appendix 4). Twenty-four references were designated as ‘unclear’, all of which were conference abstracts (seven47–53 of these could be linked to full papers already either included or excluded and 17 appear to be ongoing or recently completed studies; see Ongoing studies). The remaining 32 references met the inclusion criteria of the systematic review and were included. These 32 references describe 30 separate studies.
FIGURE 4.
Flow chart for the identification of studies.

The majority of the 30 studies which met the inclusion criteria for this systematic review evaluated NBI (n = 24), with two of these also evaluating one of the other interventions of interest (NBI and i-scan, n = 1; and NBI and FICE, n = 1). A further four studies evaluated i-scan and a further two studies evaluated FICE. The final tally of included evidence is as shown in Table 4.
| Intervention | Number of studies |
|---|---|
| NBI | 2220,54–76 |
| NBI and i-scan | 177 |
| NBI and FICE | 178 |
| i-scan | 479–82 |
| FICE | 283,84 |
Narrow-band imaging
Twenty-four studies20,54–78 included in the systematic review provided data on the use of NBI for VCE of colorectal polyps. From here on in the report, Kaltenbach and colleagues57,72 and Gupta and colleagues68,73 will be identified by a single study reference to the main source of data (Kaltenbach and colleagues57 and Gupta and colleagues68). Two of these studies, a prospective cohort study by Lee and colleagues77 and a RCT by Kang and colleagues,78 also reported on i-scan and FICE, respectively, and so are also included in our report in the i-scan and FICE sections.
An overview of the characteristics of the included NBI studies is presented in Table 5 (more detailed information is available in the data extraction forms presented in Appendix 3). More than half of the studies were conducted in the USA (14 studies20,54,55,57,58,61,63,64,66,68,69,74–76). Five studies were conducted in Europe (one in the UK,70 two in Italy,59,60 one in Italy and the Netherlands62 and one in Spain65). The remaining five studies were conducted in Asia: two in Japan,56,71 two in South Korea77,78 and one in Australia. 67 Seven of the studies focused on diminutive polyps,55,57,59,67,68,76,77 nine focused on small polyps (< 10 mm in size)20,56,60,62,65,70,71,75,78 and eight included polyps of any size. 54,58,61,63,64,66,69,74 The studies that included polyps larger than diminutive polyps provided at least one outcome of interest for the subgroup of diminutive polyps. One study, by Hewett and colleagues,54 was restricted to polyps in the rectosigmoid colon.
| Study | Country | Centre(s) | Patient populationa | Patient characteristics | NBI processor | Endoscopists | Classification | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n or n/Nb | SCR (%) | SURV (%) | SYM (%) | Age (years), mean (SD) or median [range]c | Sex (M/F, %) | n | NBI experience | Training | |||||
| Aihara et al.66 | USA | NRd | NR/67 | Yese | NRe | NR | 54 (NR) | 64/36 | NR | 7 | Unclear | Yes | NBI International Colorectal Endoscopic-AS66 |
| Chandran et al.67 | Australia | 2 | 94 | 27 | 34 | 28 | 62 [19–84] | 97/3 | EXERA | 3 | Yes | Yes | Sano–Emura85 |
| Gupta et al.68 | USA | 2 | NR/410 | Yes | Yes | No | 62 (8)f | 90/10f | EXERA II | 6 | Yes | Yes (1/3 trials) | Authors73,86,87 |
| Henry et al.69 | USA | 1 | NR/52 | 29f | 42f | 27f | 60 [34–84]f | 63/37f | EXERA II | 1 | Unclear | Yes | Sano–Emura85,88 |
| Hewett et al.54 | USA | 1 | 31/255 | 29f | 45f | NR | 60 (10)f | 52/48f | EXERA II | 1 | Yes | No | Rex publication64 |
| Hewett et al.20 | USA | NR | NR/108 | Yes | Yes | Yesg | NR | NR | EXERA II | 2 | Unclear | Yes | NBI International Colorectal Endoscopic: no reference cited |
| Ignjatovic et al.70 | UK | 1 | NR/130 | 25 | 63 | 12 | 63 (11)f | 67/33f | LUCERA | 4 | Mixed | Of non-experts | Vascular pattern intensity |
| Ikematsu et al.71 | Japan | 2 | NR/37 | 100 | No | No | 67 (NR)f | 76/24f | LUCERA | 7 | Yes | No | NR |
| Iwatate et al.56 | Japan | 1 | NR/124 | NR | NR | NR | 56 (9)f | 58/42f | LUCERA | 5 | Mixed | No | NBI International Colorectal Endoscopic20,89 |
| Kaltenbach et al.57 | USA | 3 | NR/281 | 38f | 44f | 19f | 62 (9)f | 96/4f | EXERA II | 5 | Mixed | Yes | NBI International Colorectal Endoscopic20 |
| hKang et al.78 | South Korea | 1 | 203/399 | 100 | No | No | 55 (9) | 68/32 | LUCERA | 4 | No | Yes | Polyp colour, vessels and surface pattern64,90,91 |
| Ladabaum et al.58 | USA | NR | NR | NR | NR | NR | NR | NR | EXERA II | 12 | No | Yes | NBI International Colorectal Endoscopic92 |
| hLee et al.77 | South Korea | 1 | 70/142 | Yes | Yes | No | 58 (11) | 74/26 | LUCERA | 1 | Yes | No | Authors |
| Paggi et al.59 | Italy | 1 | NR/284 | 43f | 28f | 30f | 61 (18)f | 63/37f | EXERA | 4 | Yes | Yes | Based on published criteria20 |
| Paggi et al.60 | Italy | 1 | 197/286 | 37f | 26f | 36f | 60 (16)f | 56/44f | EXERA | 6 | Yes | Yes | Simplified NBI criteria, as proposed by Rex64 |
| Patel et al.55 | USA | 4 | 451 | Yes | Yes | Yes | NR | NR | EXERA II | 26 | No | Yes | Previously established NBI criteria73,87,93 |
| Pohl et al.61 | USA | 2 | 566/607 | 53i | 30i | 9i | 62 (8)i | 64/36i | NR | 10 | No | Yes | Polyp colour, vessels and mucosal pattern94 |
| Repici et al.62 | Italy and the Netherlands | 5 | 212/278 | 37f | 27f | 36f | 63 (10)f | 58/42f | NR | 5 | Yes | Yes | Criteria reported, but not attributed to any named system |
| Rex64 | USA | 1 | NR/136 | NR | NR | NR | NR | NR | EXERA HD 180 | 1 | Unclear | Yesj | Authors64 (also used by Hewett et al.54) |
| Rogart et al.74 | USA | 1 | NR/131 | 55 | 24 | 15 | 59 (10) | 65/35 | EXERA II | 4 | Unclear (without extensive experience) | Yes | Simplified Kudo pit pattern classification22 |
| Shahid et al.75 | USA | 1 | NR/65 | Yes | Yes | No | 69 [44–91]f | 62/38f | EXERA | 1 | Unclear | No | Kudo criteria, as modified by Sano et al.95 |
| Sola-Vera et al.65 | Spain | 1 | NR/195 | 38f | 16f | 25f | 64 (12)f | 56/44f | EXERA | 5 | 1/5 | Yes | NBI International Colorectal Endoscopic20,89 |
| Vu et al.76 | USA | 1 | 315 | 48 | 52 | No | 62 (9) | 51/49 | EXERA II | 6 | Unclear | Yes | Based on Rastogi et al.96 |
| Wallace et al.63 | USA | 1 | NR/264 | 46 | 43f | 10f | 60 [33–85]f | 58/42f | EXERA II | 7 | Unclear | Yes | Simplified NBI International Colorectal Endoscopic58 |
Half of the studies enrolled participants undergoing colonoscopy either for screening, surveillance or because of symptoms,20,57,59–63,65,67,69,70,74 with all but two (Hewett and colleagues20 and Patel and colleagues55) reporting the proportions of participants in each category. Five studies enrolled participants undergoing colonoscopy for either screening or surveillance reasons,54,68,75–77 but not because of symptoms, with one more study66 including participants presenting for elective screening or follow-up colonoscopy (reasons for the follow-up colonoscopy not provided). In two studies the entire sample of participants was drawn from a screening population. 71,78 In the remaining three studies the types of participants enrolled is not known because it was not reported in the publications. 56,58,64
The male-to-female ratio of participants in the included studies lay between 1 : 1 and 2 : 1 in 13 studies,54,56,59–63,65,66,69,74–76 and between 2 : 1 and 3 : 1 in three studies. 70,77,78 In the remaining four studies that reported the male-to-female ratio it was approximately 4 : 1,71 10 : 1,68 23 : 157 and, the highest reported male-to-female ratio, 35 : 1. 67 The male-to-female ratio of participants was not reported by four studies. 20,55,58,64
The mean age of participants, if it was reported, lay between 54 and 67 years (16 studies54,56,57,59–62,65,66,68,70,71,74,76–78) or the median age lay between 60 and 69 years (four studies63,67,69,75). The age of participants was not reported by the remaining four studies. 20,55,58,64
The majority of the studies were conducted in a single centre,54,56,59,60,63–65,69,70,74–78 four were conducted in two centres61,67,68,71 and one each at three centres,57 four centres55 and five centres. 62 The number of centres was not reported by three studies. 20,58,66
Study colonoscopies were undertaken by more than one endoscopist in most studies: one endoscopist in five studies,54,64,69,75,77 two in one study,20 three in one study,67 four in four studies,59,70,74,78 five in four studies,56,57,62,65 six in three studies,60,68,76 seven in three studies,63,66,71 10 in one study,61 12 in one study58 and, the largest number of endoscopists, 26 in one study. 55 In eight studies, all the endoscopists had prior experience of using NBI,54,59,60,62,67,68,71,77 and in four studies some of the endoscopists had prior experience of using NBI. 56,57,65,70 Only four studies stated that the endoscopists involved had no prior experience of using NBI to characterise colorectal polyps,55,58,61,78 but in a further eight studies it was not clear what experience of using NBI, if any, the endoscopist(s) may have had. 20,63,64,66,69,74–76 The majority of the studies included an element of training for the endoscopist(s) in the characterisation of colorectal polyps using NBI, either training all endoscopists20,55,57–67,69,74,76,78 or the non-experts. 70 In the study by Gupta and colleagues, which is a reanalysis of three earlier studies, training occurred in one of the three studies. 68 In five studies54,56,71,75,77 it was not stated if any training had taken place. In three of these, the endoscopists had prior experience of NBI. 54,71,77 In the Iwatate and colleagues study56 the five endoscopists had mixed levels of NBI experience, and it was unclear what NBI experience the single endoscopist in the Shahid and colleagues study had. 75
A variety of different systems were used to classify polyps as adenomas or hyperplastic polyps (see Table 5). The most commonly used systems were the NBI International Colorectal Endoscopic classification scheme or a version of this, which was cited by eight studies,20,56–59,63,65,66 and the criteria proposed by Rex,64 which were cited by four studies. 54,60,64,78 Two studies67,69 cited the Sano–Emura classification system, two74,75 based characterisations on modifications of the Kudo criteria and two55,68 on work by Rastogi and colleagues,73,86,87,93 with one further study76 also citing a Rastogi and colleagues publication,96 although it is not known in this case whether or not the criteria were the same. One study70 used vascular pattern intensity97 to classify polyps, one61 polyp colour, vessels and mucosal pattern,94 and one77 the author’s own system. In the final two studies either criteria were reported but not attributed to any named system62 or no criteria were reported or cited. 71
The QUADAS assessments of the NBI studies indicates that the studies were at a low risk of spectrum, verification, disease progression, incorporation, test review and clinical review biases (Table 6). Supporting information for the judgements shown in Table 6 is provided in the data extraction form for each study (see Appendix 3). Note that ‘yes’ answers to QUADAS questions 1–9 (see Table 3) imply a low risk of bias, whereas ‘yes’ answers to QUADAS questions 10 and 11 reflect adequacy of reporting and further supporting information is required to assess the risks of bias associated with these questions. For five studies55,56,58,64,66 the risk of spectrum bias (QUADAS question 1) was unclear because the reason(s) for patients having a colonoscopy were not reported. In two studies57,63 not all the polyps received verification by histopathology. In the Kaltenbach and colleagues study57 this was because, when two or more non-neoplastic polyps were identified in the rectosigmoid colon in any one patient, a ‘representative sample’ was resected for histopathological analysis. How often this circumstance arose was not reported. In the Wallace and colleagues study,63 10 polyps (from 321 polyps, therefore representing 3% of the total) were not assessed by histopathology (and whether or not one further polyp had been assessed by histopathology was unclear). Overall, it is our opinion that the risk of differential verification bias in these two studies was probably very low.
| Study | QUADAS item (questions are available in table footnotes) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | |
| Aihara et al.66 | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Chandran et al.67 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Gupta et al.68 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | n/a |
| Henry et al.69 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | No | Yes |
| Hewett et al.54 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Hewett et al.20 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Ignjatovic et al.70 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Ikematsu et al.71 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Iwatate et al.56 | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Kaltenbach et al.57 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Kang et al.78 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Ladabaum et al.58 | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Unclear |
| Lee et al.77 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Paggi et al.59 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | No | Yes |
| Paggi et al.60 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Patel et al.55 | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Pohl et al.61 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | No | Yes |
| Repici et al.62 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Rex64 | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Rogart et al.74 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Shahid et al.75 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Sola-Vera et al.65 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Vu et al.76 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Wallace et al.63 | Yes | Yes | Yes | No | Yes | Yes | Unclear | Yes | Yes | Unclear | Yes |
In all but four studies59,61,63,69 the risk of diagnostic review bias was rated as low (QUADAS question 7). The risk of bias was rated as unclear in the studies by Henry and colleagues,69 Paggi and colleagues,59 Pohl and colleagues61 and Wallace and colleagues63 because they did not report whether or not the histopathologist(s) were blinded to the NBI prediction for each polyp. The majority of studies did not report on uninterpretable/intermediate test results, probably because there were no uninterpretable/intermediate test results because of the nature of the NBI assessments (studies typically required a decision to be made, although this could be assigned as low confidence in some studies). In the studies by Gupta and colleagues and Iwatate and colleagues, there was evidence of uninterpretable or intermediate test results. 56,68 An optical diagnosis could not be determined for four polyps (0.3%) in the study by Gupta and colleagues,68 and Iwatate and colleagues56 excluded two patients with ‘unevaluable material’. Patel and colleagues55 reported that polyps were excluded from the analysis if a confidence level was not assigned or if histopathology was missing or ‘other’, or if the polyp could not be retrieved, so it seems likely that there were also some uninterpretable or intermediate test results in this study. The outcome for QUADAS item 10 was judged unclear for the Wallace and colleagues study because not all patients who were randomised completed the study, so it is possible that uninterpretable test results were the reason for the missing data. 63
For the final QUADAS item (question 11, attrition bias), the judgement was ‘yes’ for the majority of studies either because no withdrawals were apparent in the study20,54,56,59,60,64–67,69,71,74–77 or because withdrawals or other missing data were explained. 57,61–63,70,78 For two studies the judgement was ‘unclear’. 55,58 In the Ladabaum and colleagues study,58 the subjects of the study were endoscopists, and it was unclear whether or not any of them had dropped out of the study; there was little reporting on those undergoing colonoscopy. Patel and colleagues55 did not report the number of participants selected to take part or the number of patients included in the data analyses, so it was unclear whether or not there had been any withdrawals. For one study, by Gupta and colleagues,68 this question was not applicable because the included data were drawn from records of participants in three earlier trials that met the inclusion criteria for a retrospective analysis and, therefore, no participants were able to withdraw.
In addition to the assessment of the QUADAS items, the generalisability of each study was also briefly summarised during data extraction (the summary of reviewers’ comments can be seen in full in the data extraction forms in Appendix 3). The overall impression from the included NBI studies is that they enrolled participants likely to be representative of the types of participants who would receive colonoscopy in the UK for screening, surveillance or on account of symptoms experienced (in line with the inclusion criteria for this systematic review). However, only one study was conducted in the UK,70 and just four elsewhere in Europe,59,60,62,65 where it might reasonably be assumed that populations might be most similar to those in the UK. Most studies were conducted in a single centre,54,56,59,60,63–65,69,70,74–78 so inherently these results may not be transferable to other centres. In contrast, in most studies more than one endoscopist was involved in conducting colonoscopies and characterising polyps. 20,55–63,65–68,70,71,74,76,78 Across all the studies the experience of endoscopists covered the whole range from those who were less experienced in conducting colonoscopy generally and had little or no experience using NBI to very experienced endoscopists who also had extensive experience of using NBI. Training for endoscopists (which may have been to train those with no prior experience of NBI or to ensure that all endoscopists at a centre were characterising polyps to the same standard) formed a part of the majority of studies, but how relevant this training may have been to current UK practice is unknown. Finally, a variety of classifications systems were used to determine whether polyps were adenomas or hyperplastic. The assessment group understands that, in countries where polyp characterisation is conducted without magnification, such as the UK, the NBI International Colorectal Endoscopic classification is becoming widely accepted. It is unclear how generalisable the results obtained using other polyp classifications are to UK practice.
i-scan
Five studies77,79–82 included in the systematic review provided data on the use of i-scan for VCE of colorectal polyps. An overview of the characteristics of the included i-scan studies is presented in Table 7 (more detailed information is available in the data extraction forms presented in Appendix 3). Four of the studies were conducted in Europe (those by Basford and colleagues in the UK,79 Hoffman and colleagues80 and Rath and colleagues82 in Germany and Pigo and colleagues81 in Italy) and one, by Lee and colleagues,77 was conducted in South Korea. Basford and colleagues79 and Hoffman and colleagues80 enrolled all their participants from a screening population, whereas the other three studies77,81,82 enrolled participants receiving colonoscopy for screening or surveillance purposes, with one81 also including participants with gastrointestinal symptoms. In the three studies77,81,82 that enrolled different types of participants, the proportions of participants receiving colonoscopy for screening, surveillance or symptoms was not reported. The Pigo and colleagues study81 enrolled almost equal proportions of men and women, whereas more men than women were enrolled in the other four studies. Four studies77,80–82 reported the mean age of the participants, which ranged from 55 years to 66 years. The two studies conducted in Germany did not report data on polyp characterisation for the whole colon: Hoffman and colleagues80 reported on polyps only in the last 30 cm of colon, and Rath and colleagues82 characterised polyps in the distal colon (the descending colon, the sigmoid colon or the rectum). Three of the studies (i.e. those by Hoffman and colleagues,80 Lee and colleagues77 and Rath and colleagues82) focused on the characterisation of diminutive polyps, whereas Basford and colleagues79 focused on small polyps (< 10 mm) and Pigo and colleagues81 included polyps of all sizes (and their data on diminutive polyps were limited to the rectosigmoid colon). Consequently, for the three studies that focused on the characterisation of diminutive polyps, data are drawn from the whole patient population, whereas it is not clear what proportion of the patients contributed data on diminutive polyp characterisation in the Basford and colleagues79 and Pigo and colleagues81 studies. All the studies were conducted in single centres, and in all but one study a single endoscopist performed the study colonoscopies and characterised polyps. In the Hoffman and colleagues study,80 three endoscopists were involved. It was clearly reported in three of the five studies (i.e. by Basford and colleagues,79 Hoffman and colleagues80 and Lee and colleagues77) that the endoscopist(s) had prior experience using i-scan but, because of an absence of reported details, it is not clear whether or not study endoscopists underwent any specific training with i-scan prior to the start of the studies. Only two studies77,82 used the same system, which was developed for the Lee and colleagues study,77 to classify polyps as adenomas or hyperplastic polyps (see Table 7); the remainder all used different systems. One study81 cited the NBI International Colorectal Endoscopic classification system, one80 used surface pit pattern, citing studies by Kudo and colleagues among others, and Basford and colleagues79 developed their own system for their research.
| Study | Country | Centre(s) | Patient population | Patient | Endoscopists | Classification | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | SCR | SURV | SYM | Age (years), mean (SD) | Sex (M/F, %) | n | i-scan experience | Training | ||||
| Basford et al.79 | UK | 1 | 84a | 100% | n/a | n/a | NRb | 65 : 35 | 1 | Yes | Unclearc | Developed by the endoscopist for this study |
| dHoffman et al.80 | Germany | 1 | 69 | 100% | n/a | n/a | 55.9 | 62 : 38 | 3 | Yes | NR | Surface pit pattern |
| eLee et al.77 | South Korea | 1 | 72 | Yesf | Yesf | No | 55.4 (11.3) | 86 : 14 | 1 | Yes | NR | Developed by the endoscopist for this study |
| gPigo et al.81 | Italy | 1 | 78a | Yesh | Yesh | Yesh | 52 (9) | 51 : 49 | 1 | NR | NR | NBI International Colorectal Endoscopic |
| iRath et al.82 | Germany | 1 | 77 | Yesf | Yesf | No | 65.5 (14.4) | 64 : 36 | 1 | NRj | NR | Used that developed by Lee et al.77 |
The QUADAS assessments were conducted for each study and supporting information for the judgements shown in Table 8 is provided in the data extraction form for each study (see Appendix 3). Note that ‘yes’ answers to QUADAS questions 1–9 imply a low risk of bias whereas ‘yes’ answers to QUADAS questions 10 and 11 reflect adequacy of reporting and further supporting information is required to assess the risks of bias associated with these questions. The QUADAS assessments of the i-scan studies indicate that the studies were at a low risk of spectrum, verification, disease progression, differential verification, incorporation, diagnostic review, test review, clinical review and test classification biases (see Table 8). An exception is that, in the Hoffman and colleagues study,80 it was unclear how representative the patients were of those who would receive the test in practice because few details about the participants were reported, although it is known that they fulfilled the criteria for screening colonoscopy.
| Study | QUADAS item (questions are available in table footnotes) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | |
| Basford et al.79 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Hoffman et al.80 | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| aLee et al.77 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Pigo et al.81 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Unclear |
| Rath et al.82 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
None of the studies indicated that any uninterpretable or intermediate test results had been reported. Hoffman and colleagues80 reported results for normal mucosa in addition to adenomatous and hyperplastic polyps, but there is no indication in the paper that this was as a result of any difficulty in interpreting the index test.
No withdrawals (of patients or of polyps from the analysis) were apparent in the Hoffman and colleagues80 and Lee and colleagues77 studies. The exclusion of patients screened for inclusion was explained by Basford and colleagues. 79 Pigo and colleagues81 recruited 78 patients and 150 polyps were included in the analysis, but it was not clear whether or not the 150 polyps were from the full sample of 78 recruited participants. Rath and colleagues82 recruited 224 patients to their study, but the analysis included only 77 of these (all were described as having distal diminutive polyps). It is possible that the remaining patients in these studies had larger polyps located other than in the distal colon, but this is not explicitly stated. Therefore, the Pigo and colleagues81 and the Rath and colleagues82 studies are rated as being at possible risk of attrition bias.
In addition to the assessment of the QUADAS items, the generalisability of each study was also briefly summarised during data extraction (the summary of reviewers’ comments can be seen in full in the data extraction forms in Appendix 3). The overall impression from the included i-scan studies is they enrolled participants likely to be representative of the types of participants who would receive colonoscopy in the UK for screening or surveillance or on account of symptoms experienced. However, only one study was conducted in the UK,79 with three out of the remaining four conducted in Europe (two in Germany80,82 and one in Italy81), whereas the final study was conducted in South Korea. 77 Three of the five studies were conducted by endoscopists with prior experience of i-scan,77,79,80 and all took place in single centres often described as academic or specialist centres. The results of these studies may therefore not be applicable to less experienced endoscopists working in more generalist or community settings. Only one study used the NBI International Colorectal Endoscopic classification system (which is becoming widely accepted for polyp characterisation without magnification) to determine whether polyps were adenomas or hyperplastic. 81 It is unclear how generalisable the results obtained using other polyp classifications are to UK practice.
Flexible spectral imaging colour enhancement
Three studies included in the systematic review (Kang and colleagues,78 Longcroft-Wheaton and colleagues83,84) provided data on the use of FICE for VCE of colorectal polyps (Table 9). Two of the studies were conducted in the UK83,84 and the other was conducted in South Korea. 78 In all three of these studies, all the included participants were undergoing colonoscopy for screening purposes. The Longcroft-Wheaton and colleagues83 study enrolled a slightly higher proportion of women than men, whereas the other two studies enrolled a higher proportion of men than women. All three studies reported the mean age of participants, which ranged from 5478 to 65 years. 84 All three studies focused on the real-time diagnosis of colorectal polyps sized < 10 mm and provided subgroup analyses of diminutive polyps. All the studies were conducted in single centres. In the Kang and colleagues78 study, four endoscopists carried out the colonoscopies, whereas the other two studies each involved one endoscopist. Kang and colleagues78 reported that the study endoscopists had no prior experience with FICE, whereas Longcroft-Wheaton and colleagues83,84 reported that the endoscopist in each of these studies had previous experience of in vivo diagnosis of polyps, although the authors did not specify endoscopists’ experience with FICE. Longcroft-Wheaton and colleagues83 stated that the study endoscopist had had prior training in real-time diagnosis. In the other studies,78,84 the endoscopists’ prior training in both real-time diagnosis and, more specifically, the use of FICE was unclear. Kang and colleagues78 noted, however, that the endoscopists received feedback every 2 weeks during the study about the accuracy of their endoscopic predictions compared with the histopathological diagnosis. The study by Kang and colleagues78 (which also included a NBI arm), used a classification system for polyp characterisation based on colour, vascular density and vascular pattern. 64,90,91,98 The two studies by Longcroft-Wheaton and colleagues83,84 both used a characterisation system based on vascular patterns that was developed by Teixeira and colleagues. 99
| Study | Country | Centre(s) | Patient population | Patient characteristics | Endoscopists | Classification system for polyp characterisation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | SCR | SURV | SYM | Age (years), mean (SD) | Sex (M/F, %) | n | FICE experience | Training | ||||
| aKang et al.78 | South Korea | 1 | 196b | 100% | n/a | n/a | 54.3 (9.0) | 76/24 | 4 | No | Unclearc | Based on colour, vascular density and vascular pattern. Cites four references64,90,91,98 |
| Longcroft-Wheaton et al.83 | UK | 1 | 50b | 100% | n/a | n/a | 64 (4.2)d | 46/54e | 1 | Unclearf | Unclearf | Based on vascular patterns using a system developed by Teixeira et al.99 |
| Longcroft-Wheaton et al.84 | UK | 1 | 89b | 100% | n/a | n/a | 65 (6.7)g | 79/21g | 1 | Unclearf | Unclearf | System developed and validated by Teixeira et al.99 |
Table 10 shows the quality assessments of the three FICE studies. 78,83,84 Reviewers considered all three studies to be at a low risk of bias across most of the QUADAS items assessed. None of the studies, however, reported the number of uninterpretable test results, but reviewers believed this to be zero in two studies. 78,84 Two studies explained participant withdrawals. 78,83 Longcroft-Wheaton and colleagues84 did not state whether or not there were any withdrawals.
| Study | QUADAS item (questions are available in table footnotes) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | |
| aKang et al.78 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Longcroft-Wheaton et al.83 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Longcroft-Wheaton et al.83 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
In addition to the assessment of the QUADAS items, the generalisability of each study was also briefly summarised during data extraction (the summary of reviewers’ comments can be seen in full in the data extraction forms in Appendix 3). Reviewers noted that two of the studies were conducted in the UK83,84 and so are likely to be representative of a UK population (although it is noted that these studies included a small number of participants – 50 and 89 participants each). It was also noted that it is unclear how representative participants in the South Korea study78 would be of the UK population and how similar the endoscopists’ training in this study would be to endoscopists’ training in the NHS in the UK. As all the studies were conducted in single centres it is unclear how the results would generalise to other centres and settings.
Assessment of diagnostic accuracy (sensitivity, specificity, negative predictive value, accuracy)
Narrow-band imaging
Sensitivity and specificity of narrow-band imaging for the characterisation of diminutive colorectal polyps
All but one of the included NBI studies reported sensitivity74 or both sensitivity and specificity20,54–71,75,77,78 of NBI for the characterisation of diminutive colorectal polyps as adenomas or hyperplastic polyps compared with the characterisation verified by histopathological assessment of the resected polyps. Only Vu and colleagues76 did not report on either sensitivity or specificity (this study was included in the systematic review because it reported accuracy in terms of the proportion of correctly classified polyps and data on surveillance intervals). The way in which data were reported by the studies varied and is shown in Table 11. Some studies reported on all the polyp characterisations made by study endoscopists. In other studies, the endoscopist indicated how confident they were in their NBI characterisation of the polyp as adenomatous or hyperplastic, and results were reported separately for high- and low-confidence characterisations. Some studies reported data on all the characterisations and also the subsets of data for high- and low-confidence characterisations (data on low-confidence characterisations are available in the data extraction forms in Appendix 3). One study, by Hewett and colleagues,54 was restricted to the rectosigmoid colon. As can be seen in Table 11, several other studies also reported data for subsections of the colon as well as for the whole colon. One study, by Iwatate and colleagues,56 included a subgroup analysis by type of endoscopist (specialist or generalist).
| Location | Reported data | |
|---|---|---|
| All characterisations of polyps | Characterisations of polyps made with high confidence | |
| Whole colon | Aihara et al.66 (2 × 2 imputed) | a,bHewett et al.20 (unable to impute 2 × 2) |
| Chandran et al.67 | Iwatate et al.56 | |
| Gupta et al.68 (2 × 2 imputed) | bKaltenbach et al.57 (2 × 2 imputed) | |
| Henry et al.69 | aLadabaum et al.58 (unable to impute 2 × 2) | |
| Ignjatovic et al.70 | Lee et al.77 | |
| Ikematsu et al.71 (2 × 2 imputed) | bPaggi et al.60 | |
| Iwatate et al.56 | bPaggi et al.59 | |
| Kang et al.78 (2 × 2 imputed) | cPatel et al.55 (2 × 2 imputed) | |
| Ladabaum et al.58 (2 × 2 imputed) | Pohl et al.61 | |
| Lee et al.77 (2 × 2 imputed) | Repici et al.62 (2 × 2 imputed) | |
| cPatel et al.55 (2 × 2 imputed) | Rex et al.64 | |
| Repici et al.62 (2 × 2 imputed) | Sola-Vera et al.65 | |
| Rex et al.64 (2 × 2 imputed) | Wallace et al.63 | |
| aRogart et al.74 (unable to impute 2 × 2) | ||
| Shahid et al.75 | ||
| Sola-Vera et al.65 | ||
| Wallace et al.63 | ||
| Whole colon by colonoscopist type | Iwatate et al.56 (specialist and generalist colonoscopists) | |
| Right colon | Kaltenbach et al.57 (2 × 2 imputed) | |
| Proximal to splenic flexure | Pohl et al.61 | |
| Left colon | Gupta et al.68 (2 × 2 imputed) | Kaltenbach et al.57 (2 × 2 imputed) |
| Distal colon | Pohl et al.61 | |
| Rectosigmoid colon | cHewett et al.54 (2 × 2 imputed) | cHewett et al.54 (2 × 2 imputed) |
| Ladabaum et al.58 (2 × 2 imputed) | aPatel et al.55 (unable to impute 2 × 2) | |
| aPatel et al.55 (unable to impute 2 × 2) | Pohl et al.61 | |
| Wallace et al.63 | Repici et al.62 (2 × 2 imputed) | |
| Wallace et al.63 | ||
| Proximal to rectosigmoid colon | Ladabaum et al.58 (2 × 2 imputed) | aPatel et al.55 (unable to impute 2 × 2) |
| aPatel et al.55 (unable to impute 2 × 2) | ||
| Rectum | Kaltenbach et al.57 (2 × 2 imputed) | |
The subsections that follow report on the:
-
sensitivity and specificity of NBI for the characterisation of diminutive polyps in the whole colon (first, data on all characterisations, then the separate subset of data on the polyp characterisations made with high confidence by the endoscopists), with accompanying meta-analyses (including a post hoc analysis of high-confidence characterisations made by endoscopists with prior experience of NBI)
-
sensitivity and specificity of NBI for the characterisation of diminutive polyps in the rectosigmoid colon (again, for all characterisations and separately for the subset of high-confidence characterisations), with accompanying meta-analyses (including a post hoc analysis of high-confidence characterisations made by endoscopists with prior experience of NBI)
-
sensitivity and specificity of NBI for the characterisation of polyps in parts of the colon other than the rectosigmoid colon (too few studies to meta-analyse)
-
NPV of NBI for the characterisation of diminutive colorectal polyps; accuracy of NBI (proportion of correctly classified polyps).
Sensitivity and specificity of narrow-band imaging for the characterisation of diminutive colorectal polyps in the whole colon
Twenty-three studies20,54–71,74,75,77,78 reported on the characterisation of diminutive polyps within the whole colon, although five of these reported data only from high-confidence characterisations. 20,57,59–61
The results for all characterisations of diminutive polyps in the whole colon (i.e. not separated by confidence level), where 2 × 2 table data were reported or calculable, are shown in Figure 5.
FIGURE 5.
Accuracy of NBI for characterising diminutive colorectal polyps as either adenomas or hyperplastic polyps. a, The values for the 2 × 2 tables of these studies were imputed. For Patel and colleagues,55 values have been imputed by the reviewer, but it was not possible to find a solution that agreed with all the 2 × 2 table outcomes reported in the paper. The imputed values for Patel and colleagues55 (which should be regarded as illustrative) produce the reported sensitivity and specificity, but produce values for PPVs and NPVs that are lower than reported and an accuracy value (proportion of correctly classified polyps among all the polyps) that is higher; and b, Rogart and colleagues74 did not report a value for specificity and it was not possible to complete the 2 × 2 table from the information reported in the published paper.
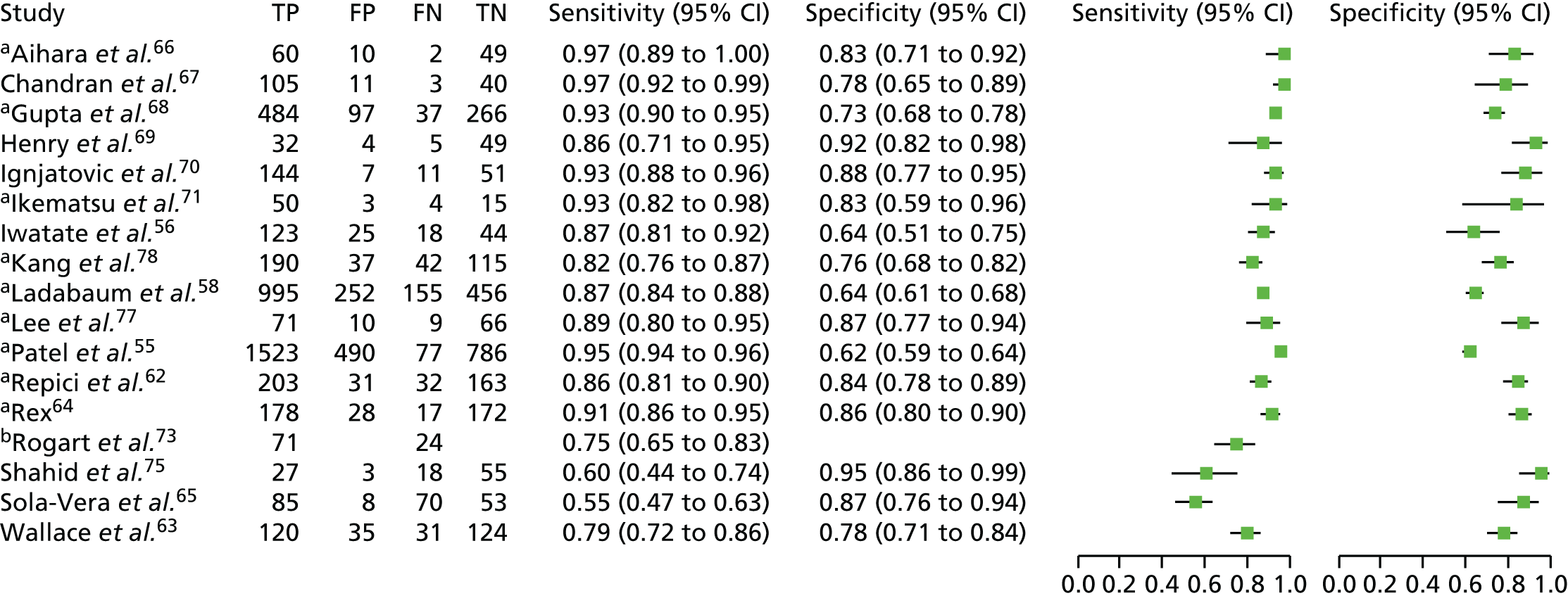
The ability of NBI to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) ranged from 0.55 to 0.97 (i.e. 55–97%) across the 17 studies that reported this outcome. Sensitivity was above 90% in seven studies55,64,66–68,70,71 (and in two of these it was ≥ 95%55,67) between 80% and 90% in six other studies56,58,62,69,77,78 and was < 80% in four studies. 63,65,74,75
The ability of NBI to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) was typically lower than the sensitivity of the test, ranging from 0.62 to 0.95 (i.e. 62% to 95%) across the 16 studies that reported this outcome. Specificity was above 90% in just two studies,69,75 between 80% and 90% in seven studies62,64–66,70,71,77 and was below 80% in seven studies. 55,56,58,63,67,68,78
It was possible to run a bivariate meta-analysis (using Stata/IC14 and metandi45) for the 16 studies that reported both sensitivity and specificity. This produced a summary value for sensitivity of 0.88 (95% CI 0.83 to 0.92) and for specificity of 0.81 (95% CI 0.75 to 0.85). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 6. The 95% confidence region around the summary point indicates where we have 95% confidence that the summary point lies. The 95% prediction region illustrates the extent of statistical heterogeneity among the studies. If the bivariate model for sensitivity and specificity is correct, we have 95% confidence that the true sensitivity and specificity of a new study in the future will lie within the 95% prediction region. As can be observed from Figure 6, the 95% prediction region is large.
FIGURE 6.
Summary receiver operating characteristic curve plot from the meta-analysis of NBI for all characterisations of polyps in the whole colon.

In order to investigate the heterogeneity between studies, a covariate for endoscopist experience with NBI was added to RevMan and separate SROC curves were drawn as shown in Figure 7. Although caution must be taken when interpreting this figure, because of the small number of studies for each subgroup, it nevertheless appears to support the hypothesis that endoscopists with prior experience of using NBI to characterise diminutive colorectal polyps achieve higher sensitivity and specificity than endoscopists who have had no prior experience of using NBI to characterise diminutive colorectal polyps (other than any training that they undertook at the start of the study).
FIGURE 7.
Summary receiver operating characteristic curve plots for all characterisations of polyps in the whole colon by endoscopists’ level of experience using NBI.

The results for studies that reported results from polyp characterisations using NBI that were designated as high-confidence decisions, and where 2 × 2 table data were reported or calculable, are shown in Figure 8.
FIGURE 8.
Accuracy of NBI high-confidence decisions for characterising diminutive colorectal polyps as either adenomas or hyperplastic polyps in the whole colon. a, It was not possible for us to impute the 2 × 2 table data necessary to plot these results within this figure. Hewett and colleagues’ study20 reported a value for sensitivity of 98% (no CI provided and specificity not reported) and Ladabaum and colleagues58 reported a sensitivity of 88.4% (95% CI 82.2% to 94.7%) and a specificity of 44.1% (26.5% to 61.6%); b, the values for the 2 × 2 tables of these studies were imputed.

The ability of high-confidence characterisations made with NBI to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) was ≥ 0.90 (i.e. ≥ 90%) in 9 of the 13 studies20,55–57,59,60,62,64,77 (in four of these it was ≥ 95%20,55,57,64) and between 80% and 90% in three other studies. 58,61,63 The lowest sensitivity value reported was 59%, by Sola-Vera and colleagues. 65 Some studies reported the sensitivity obtained from all characterisations and the sensitivity from only the high-confidence characterisations. In all studies in which both these values were reported, the sensitivity was higher when obtained from high-confidence decisions (difference ranging from an increase of 1.5% to 5.8%).
The ability of NBI to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) from high-confidence polyp characterisations was just > 90% (i.e. > 0.90) in three studies,64,65,77 but did not exceed 92% in any study. In just three studies, specificity lay between 80% and 90%,61–63 but in the majority of the studies it lay < 80%,55–60 with the lowest specificity just 44.1%, reported by Ladabaum and colleagues. 58 Specificity was higher when obtained from high-confidence decisions in seven of the eight studies that reported both the specificity obtained from all characterisations and the specificity from only the high-confidence characterisations, with the increase ranging from 3.5% to 7.3%. The one exception was the study by Ladabaum and colleagues58 in which the specificity calculated from high-confidence characterisations was lower than that obtained from all characterisations (44.1% vs. 64.4%, respectively).
A bivariate meta-analysis (using Stata/IC14 and metandi45) was run for the 11 studies that reported both sensitivity and specificity from polyp characterisations made with high confidence. This produced a summary value for sensitivity of 0.91 (95% CI 0.85 to 0.95) and for specificity of 0.82 (95% CI 0.76 to 0.87). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 9. The effect of reporting only on high-confidence characterisations rather than all polyp characterisations is to move the summary estimate up (increasing sensitivity) and slightly to the left (increasing specificity).
FIGURE 9.
Summary receiver operating characteristic curve plot showing the summary point on the summary curve from the meta-analysis of NBI for high-confidence characterisations of polyps in the whole colon. Note that two studies were not included in the meta-analysis: Hewett and colleagues’ study,20 with a sensitivity of 98%; and Ladabaum and colleagues’ study,58 with a sensitivity of 88.4% (95% CI 82.2% to 94.7%) and a specificity of 44.1% (26.5% to 61.6%).

The impact of restricting the analysis to high-confidence characterisations rather than including all characterisations can be observed in Figure 10, which shows both summary curves on the same plot. As already stated, the effect of reporting only on high-confidence characterisations rather than on all polyp characterisations is that the summary estimate moves up (increasing sensitivity) and slightly to the left (increasing specificity).
FIGURE 10.
Summary receiver operating characteristic curve for all NBI characterisations of polyps in the whole colon and SROC for only high-confidence NBI characterisations of polyps in the whole colon shown on the same plot. Note that for clarity the 95% prediction regions are not shown on this plot.

Seven studies55,56,58,62–65,77 reported both sensitivity and specificity from all diminutive polyp characterisations and separately for only high-confidence diminutive polyp characterisations, although for one of the these studies58 2 × 2 table data were not available for the high-confidence characterisations [which had a reported sensitivity of 88.4% (95% CI 82.2% to 94.7%) and specificity of 44.1% (95% CI 26.5% to 61.6%)]. The pairs of results from these studies are shown in Figure 11 and forest plots in Figure 12.
FIGURE 11.
Plot showing paired data from the studies that reported on all diminutive polyp characterisations and separately on high-confidence diminutive polyp characterisations.

FIGURE 12.
Accuracy of NBI in studies that reported on all diminutive polyp characterisations and separately on high-confidence diminutive polyp characterisations. a, The values for the 2 × 2 tables of these studies were imputed; b, it was not possible for us to impute the 2 × 2 table data necessary to plot these results within this figure [reported sensitivity of 88.4% (95% CI 82.2% to 94.7%) and specificity of 44.1% (26.5% to 61.6%)].

To obtain data for a scenario analysis within the economic model (see Chapter 5, Scenario analyses), a post hoc bivariate meta-analysis (using Stata/IC14 and metandi45) was run for a subgroup in which endoscopists experienced in the use of NBI characterised the polyps in the whole colon (Figure 13). Four such studies were included in this analysis. 59,60,62,77
FIGURE 13.
Accuracy of NBI high-confidence decisions for characterising diminutive colorectal polyps in the whole colon as either adenomas or hyperplastic polyps when made by endoscopists experienced in the use of NBI. a, The values for the 2 × 2 table of this study were imputed.
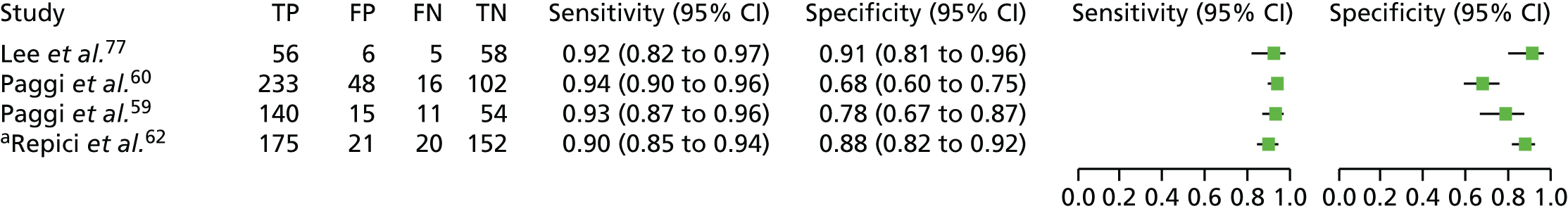
The meta-analysis produced a summary value for sensitivity of 0.92 (95% CI 0.89 to 0.94) and for specificity a value of 0.82 (95% CI 0.72 to 0.89). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 14. Restricting the meta-analysis from 11 studies reporting different levels of NBI experience (experienced, n = 4;59,60,62,77 mixed experience, n = 3;56,57,65 inexperienced, n = 2;55,61 and unclear, n = 263,64) to the four studies that reported endoscopists experienced in the use of NBI narrowed the 95% CI for sensitivity [11 studies with a variety of experience, 0.91 (95% CI 0.85 to 0.95); four studies with prior NBI experience, 0.91 (95% CI 0.89 to 0.94)] and widened the 95% CI for specificity [11 studies with a variety of experience, 0.82 (95% CI 0.76 to 0.87); four studies with prior NBI experience, 0.82 (95% CI 0.72 to 0.89)]. The changes in the 95% CIs are reflected in the change in the size and shape of the 95% confidence region and 95% prediction region in Figure 14 in comparison with Figure 9.
FIGURE 14.
Summary receiver operating characteristic plot showing the summary point on the summary curve from the meta-analysis of NBI for high-confidence characterisations of polyps in the whole colon when made by endoscopists experienced in the use of NBI.

Colonoscopies in one study, by Iwatate and colleagues,56 were conducted by five endoscopists. Two of the five endoscopists were described as specialists in colonoscopy and they had extensive experience in magnifying colonoscopy with NBI (> 1000 cases). The other three endoscopists were described as general endoscopists with limited experience in magnifying colonoscopy with NBI (≤ 1000 cases). As shown in Table 12, the two specialist endoscopists achieved higher sensitivity and specificity than the three general endoscopists, but the difference between the two was statistically significant only for specificity (p = 0.007).
| Accuracy | High-confidence characterisations of polyps 1–5 mm in size | |
|---|---|---|
| Specialist endoscopists | General endoscopists | |
| Sensitivity (95% CI) | 93.5% (78.58% to 99.21%)a | 92.9% (85.10% to 97.33%)a |
| Specificity (95% CI) | 87.0%b (66.41% to 97.22%)a | 51.7%b (32.53% to 70.55%)a |
Sensitivity and specificity of narrow-band imaging for the characterisation of diminutive colorectal polyps in the rectosigmoid colon
As shown in Table 11, four studies54,55,58,63 reported sensitivity and specificity following characterisation (any level of confidence) of diminutive polyps in the rectosigmoid colon, with three of these reporting sufficient data for a 2 × 2 table to be constructed for entry into the meta-analysis. 54,58,63
Three of the four studies54,55,63 that reported results for all characterisations also reported sensitivity and specificity following high-confidence characterisations of polyps in the rectosigmoid colon, with two further studies61,62 reporting only high-confidence characterisation data. Four of the five studies reporting on high-confidence characterisations provided sufficient data for 2 × 2 tables to be constructed for entry into the meta-analysis. 54,61–63
The results from the studies that used NBI to characterise polyps in the rectosigmoid colon, where 2 × 2 table data were reported or calculable, are shown in Figure 15. The results from Patel and colleagues55 are not represented in Figure 15 because it was not possible to impute values into a 2 × 2 table that provided a solution for the reported outcomes in the paper (accuracy, sensitivity, specificity, PPV and NPV).
FIGURE 15.
Accuracy of NBI for characterising diminutive colorectal polyps as either adenomas or hyperplastic polyps in the rectosigmoid colon. (a) NBI: characterisation of diminutive polyps in the rectosigmoid colon. (b) NBI: high-confidence characterisation of diminutive polyps in the rectosigmoid colon. a, The values for the 2 × 2 tables of these studies were imputed; b, it was not possible for us to impute the 2 × 2 table data necessary to plot these results within this figure. For characterisation of all diminutive polyps in the rectosigmoid colon, Patel and colleagues55 reported a sensitivity of 88.4% (95% CI 84.8% to 92.0%) and a specificity of 78.3% (95% CI 71.8% to 84.9%). The high-confidence polyp characterisations yielded a sensitivity of 90.9% (95% CI 87.4% to 94.4%) and a specificity of 88.6% (95% CI 81.0% to 96.1%).
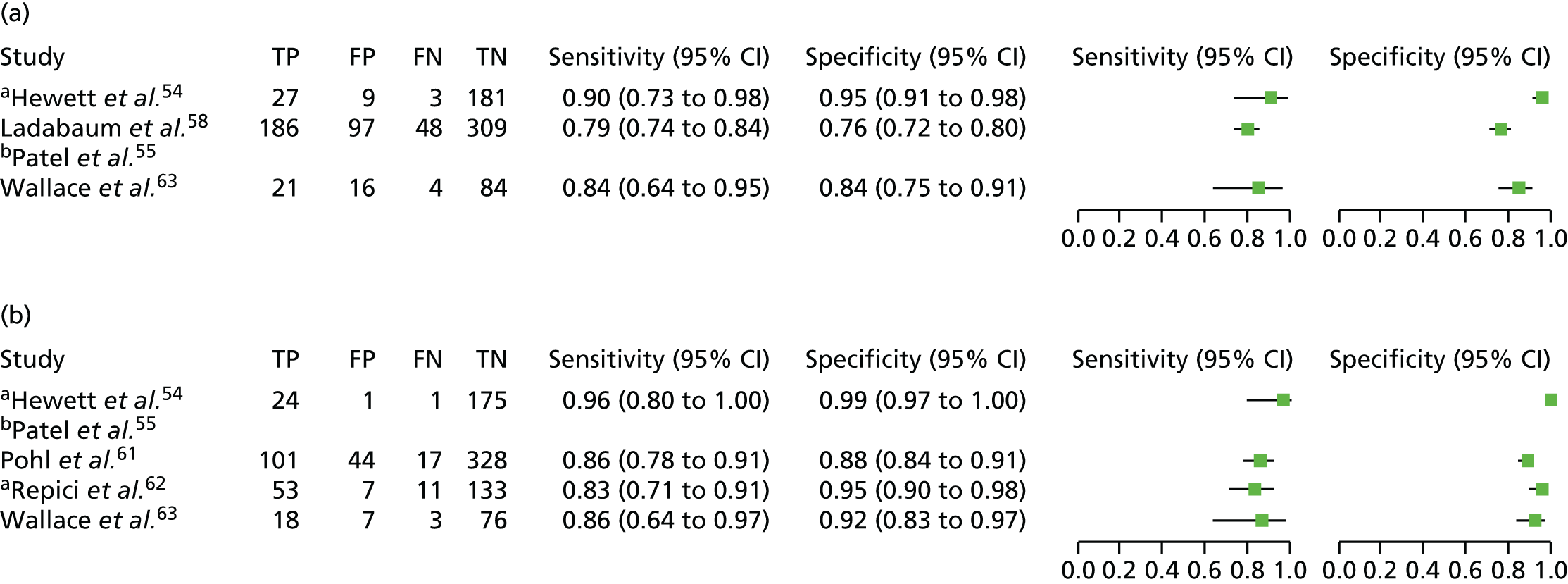
Bivariate meta-analyses were conducted (using Stata/IC14 and xtmelogit or using Stata/IC14 and metandi45) of the studies where 2 × 2 table data were available. For all characterisations of diminutive polyps in the rectosigmoid colon, the summary value for sensitivity is 0.85 (95% CI 0.75 to 0.91) and for specificity is 0.87 (95% CI 0.74 to 0.94). For high-confidence characterisations of diminutive polyps in the rectosigmoid colon, the summary value for sensitivity is 0.87 (95% CI 0.80 to 0.92) and for specificity is 0.95 (95% CI 0.87 to 0.98). The parameter estimates for the bivariate model from these two meta-analyses were entered into RevMan to produce the SROC plot shown in Figure 16. As seen with the results for the whole colon, the effect of reporting only high-confidence polyp characterisations rather than all polyp characterisations is to increase sensitivity and specificity (summary point moves up and to the left on the SROC plot).
FIGURE 16.
Summary receiver operating characteristic curve plot showing the summary points on the summary curves from the meta-analyses of NBI for all characterisations of polyps and for only high-confidence characterisations of polyps in the rectosigmoid colon.

Note that one study was not included in either meta-analysis, that is, Patel and colleagues,55 with all characterisations of polyps with a sensitivity of 88.4% (95% CI 84.8% to 92.0%) and a specificity of 78.3% (95% CI 71.8% to 84.9%) and high-confidence characterisations of polyps with a sensitivity of 90.9% (95% CI 87.4% to 94.4%) and a specificity of 88.6% (95% CI 81.0% to 96.1%). The large 95% confidence and a 95% prediction regions, which were generated for the high-confidence characterisation plot, are not shown on this figure and the software used to draw the SROC plot (RevMan) did not generate a 95% confidence region or a 95% prediction region for the other data set.
To obtain data for a scenario analysis within the economic model (see Chapter 5, Scenario analyses), a post hoc bivariate meta-analysis (using Stata/IC14 and xtmelogit) was run for a subgroup of studies in which the endoscopists were experienced in the use of NBI. Two such studies54,62 were included in the analysis (Figure 17).
FIGURE 17.
Accuracy of NBI high-confidence decisions, made by endoscopists with prior experience of NBI, for characterising diminutive colorectal polyps in the rectosigmoid colon as either adenomas or hyperplastic polyps. a, The values for the 2 × 2 tables of these studies were imputed.

The meta-analysis produced a summary value for sensitivity of 0.90 (95% CI 0.71 to 0.97) and for specificity of 0.98 (95% CI 0.91 to 1.00). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 18. Restricting the meta-analysis from the four studies reporting different levels of NBI experience (experienced, n = 2; inexperienced, n = 1; and unclear, n = 1) to only two studies in which endoscopists had experience in the use of NBI increased the summary value for sensitivity while widening the 95% CI [four studies with a variety of experience, 0.87 (95% CI 0.80 to 0.92); and two studies with prior NBI experience, 0.90 (95% CI 0.71 to 0.97)] and increased the summary value for specificity while narrowing the 95% CI [four studies with a variety of experience, 0.95 (95% CI 0.87 to 0.98); and two studies with prior NBI experience, 0.98 (95% CI 0.91 to 1.00)].
FIGURE 18.
Summary receiver operating characteristic curve plot showing the summary point on the summary curve from the meta-analyses of NBI for high-confidence characterisations of polyps in the rectosigmoid colon made by endoscopists with prior experience of NBI. Note that the software used to draw the SROC plot (RevMan) did not generate a 95% confidence region or a 95% prediction region for this meta-analysis. It is presumed that this is because of the small number of studies.
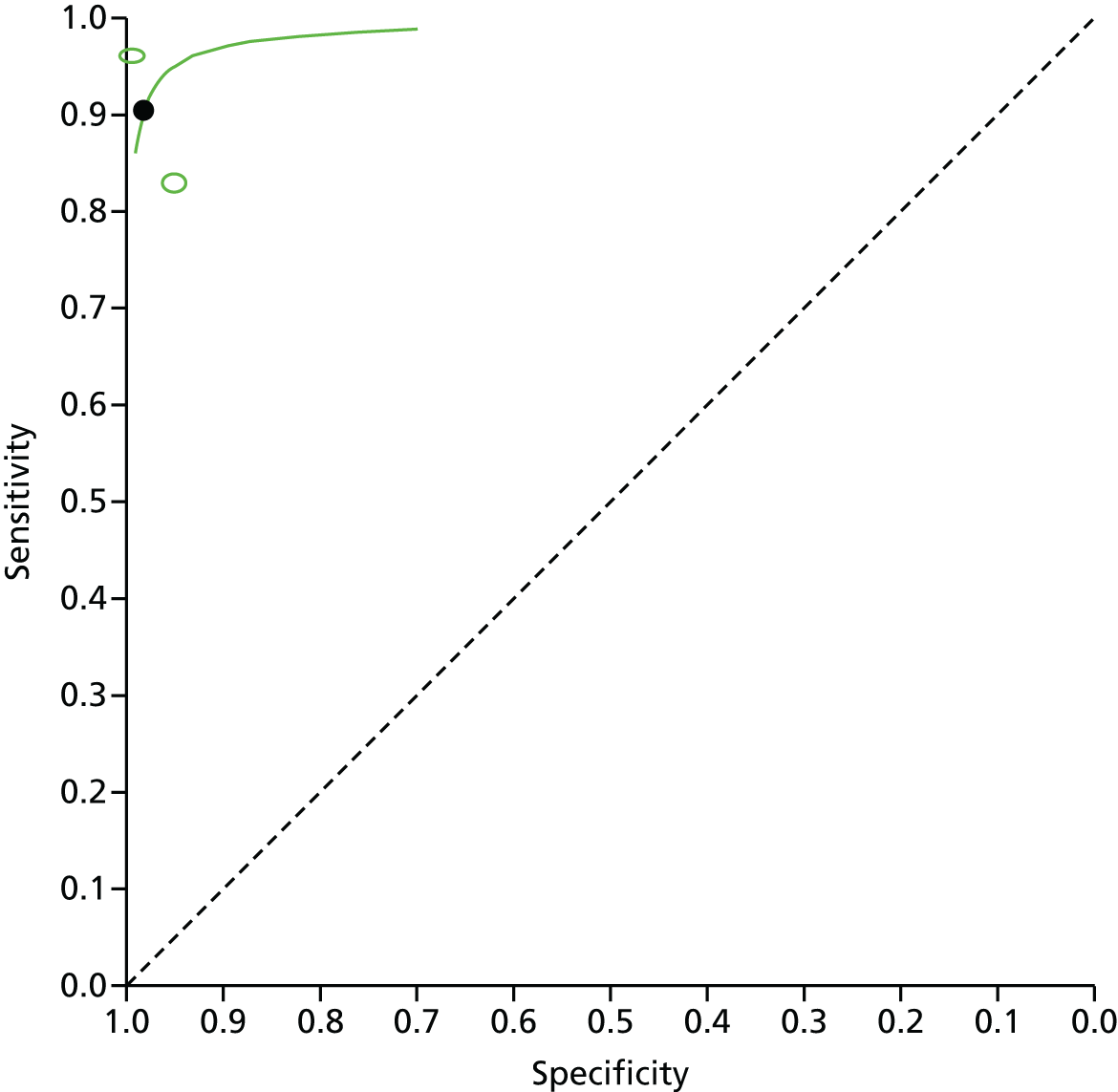
Sensitivity and specificity of narrow-band imaging for the characterisation of diminutive colorectal polyps in parts of the colon other than the rectosigmoid colon
Five studies55,57,58,61,68 provided data on the characterisation of diminutive polyps in regions of the colon other than the rectosigmoid colon (see Table 11). The results of these studies are summarised in Table 13.
| Colon region, type of characterisation | Study | Accuracy (95% CI) | |
|---|---|---|---|
| Sensitivity | Specificity | ||
| Right colon | |||
| High-confidence characterisations | Kaltenbach et al.57 | 96.4% (91.0% to 99.0%) | 61.4% (45.5% to 75.6%) |
| Proximal to splenic flexure | |||
| High-confidence characterisations | Pohl et al.61 | 82% (77.8% to 86.4%) | 62% (49.8% to 73.7%) |
| Left colon | |||
| All characterisations of polyps | Gupta et al.68 | 91.4% (86.8% to 94.8%) | 78.1% (73.0% to 82.6%) |
| High-confidence characterisations | Kaltenbach et al.57 | 95.5% (87.5% to 99.1%) | 83.6% (71.2% to 92.2%) |
| Distal colon | |||
| High-confidence characterisations | Pohl et al.61 | 84% (77.6% to 89.0%) | 87% (83.5% to 90.3%) |
| Proximal to rectosigmoid colon | |||
| All characterisations of polyps | Ladabaum et al.58 | 88.2% (82.2% to 94.2%) | 49.7% (34.7% to 64.6%) |
| Patel et al.55 | 91.0% (88.3% to 94.0%) | 36.9% (27.7% to 46.1%) | |
| High-confidence characterisations | Patel et al.55 | 96.2% (94.1% to 98.4%) | 34.9% (22.1% to 47.7%) |
| Patel et al.55 | 73.7% (65.8% to 81.5%) | 44.4% (37.3% to 51.1%) | |
| Rectum | |||
| High-confidence characterisations | Kaltenbach et al.57 | 77.8% (40.0% to 97.2%) | 81.1% (64.8% to 92.0%) |
Negative predictive value of narrow-band imaging for the characterisation of diminutive colorectal polyps
The NPV is the probability that subjects with a negative screening test (i.e. colorectal polyp is characterised as hyperplastic) truly do not have an adenoma. However, it must be borne in mind when viewing these results that the NPV is influenced by the prevalence of disease (i.e. in this case the prevalence of adenomas in the tested populations). When prevalence is increased, the result is a decrease in the NPV. Owing to the importance of NPV within the PIVI statement (see Chapter 1, Diagnostic thresholds and requirements for use of virtual chromoendoscopy), consideration was given to meta-analysing NPVs from the included studies even though this is not advised by either the National Institute for Health and Care Excellence’s Diagnostics Assessment Programme Manual37 or the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. 36 However, because it is clear that the prevalence of adenomas and hyperplastic polyps is likely to vary between studies [e.g. because of differences in case mix (screening, surveillance and symptomatic populations) and patient characteristics (age, sex)], we chose not to pool NPVs across studies. Instead, we have provided forest plots for these outcomes and marked the 90% threshold value on each plot.
For the characterisations of diminutive polyps in the whole colon (made with any level of confidence), the NPV ranged from 43% to 96.1% (Figure 19 and Table 14). The study by Sola-Vera and colleagues65 is noteworthy because this study reported the lowest NPV – far lower than in any other study. All the other studies reported NPVs of > 70%, with five studies reporting NPVs of ≥ 90%. 55,64,66,67,69 However, it should be noted that the lower limit of the 95% CI fell below 90% in every study except Patel and colleagues. 55
FIGURE 19.
Negative predictive values of NBI for all characterisations of diminutive polyps in the whole colon (made with any level of confidence).
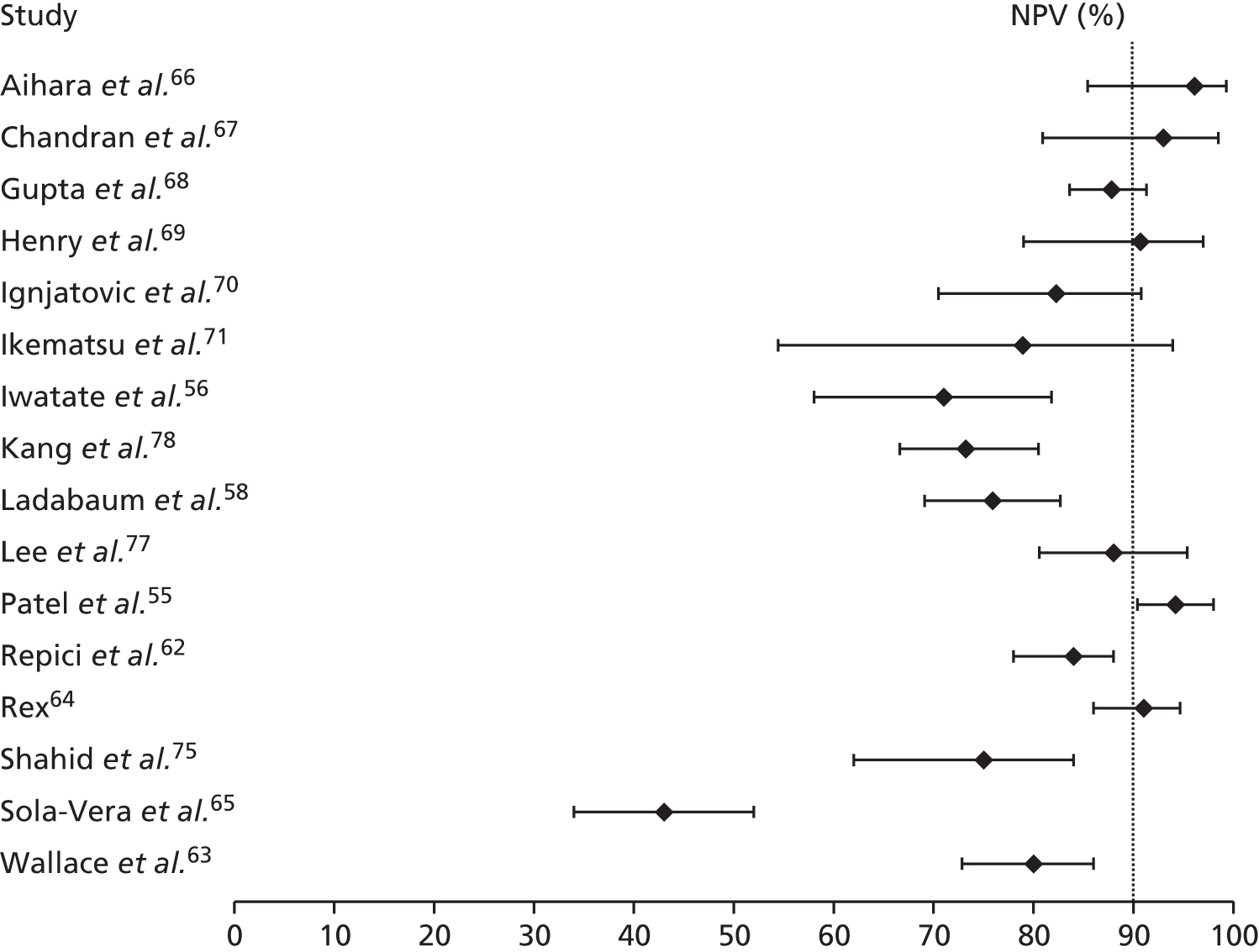
| Study | Characterisation, value (95% CI) | |
|---|---|---|
| All | High confidence | |
| Aihara et al.66 | 96.1% (85.4% to 99.3%) | NR (NR) |
| Chandran et al.67 | 93% (80.9% to 98.5%) | NR (NR) |
| Gupta et al.68 | 87.8%a (83.6 to 91.3)a | NR (NR) |
| Henry et al.69 | 90.7% (79% to 97%) | NR (NR) |
| Hewett et al.20 | NR (NR) | 95% (NR) |
| Ignjatovic et al.70 | 82.3%a (70.5% to 90.8%)a | NR (NR) |
| Ikematsu et al.71 | 78.9% (54.4% to 94.0%)a | NR (NR) |
| Iwatate et al.56 | 71.0% (58.1 to 81.8)a | 81.4% (66.6% to 91.6%)a |
| Kaltenbach et al.57 | NR (NR) | 92.0% (85.3% to 96.3%) |
| Kang et al.78 | 73.2% (66.6% to 80.5%) | NR (NR) |
| Ladabaum et al.58 | 75.9% (69.1% to 82.7%) | 78.3% (69.6% to 87.0%) |
| Lee et al.77 | 88.0% (80.6% to 95.4%) | 92.1%a (82.4% to 97.4%)a |
| Paggi et al.59 | NR (NR) | 83.1%a (71.7% to 91.2%)a |
| Paggi et al.60 | NR (NR) | 86.4%a (78.9% to 92.1%)a |
| Patel et al.55 | 94.2% (90.4% to 98.0%) | 98.3 (95.7% to 100.0%) |
| Pohl et al.61 | NR (NR) | 82.3 (78.6% to 85.6%) |
| Repici et al.62 | 84% (78% to 88%) | 89% (84% to 93%) |
| Rex et al.64 | 91.0%a (86.0% to 94.7%)a | 95.5%a (90.9% to 98.2%)a |
| Rogart et al.74 | NR (NR) | NR (NR) |
| Shahid et al.75 | 75% (62% to 84%) | NR (NR) |
| Sola-Vera et al.65 | 43% (34% to 52%) | 48% (37% to 59%) |
| Vu et al.76 | NR (NR) | NR (NR) |
| Wallace et al.63 | 80% (72.8% to 86.0%)a | 82% (74.4% to 88.1%)a |
| Assessed by specialists in colonoscopy (whole colon) | ||
| Iwatate et al.56 | NR (NR) | 90.9% (70.8% to 98.9%)a |
| Assessed by general endoscopists (whole colon) | ||
| Iwatate et al.56 | NR (NR) | 71.4% (47.8% to 88.7%)a |
Limiting the assessment of NPV to high-confidence polyp characterisations increased the NPVs, which ranged from 48% to 98.3% in the studies that reported this outcome (Figure 20 and Table 14). Again, the study by Sola-Vera and colleagues65 had the lowest NPV of any study by a considerable margin. All other studies reported NPVs for high-confidence assessments of > 78%, with five studies reporting NPVs of ≥ 90%. 20,55,57,64,77 Once again, however, inspection of the 95% CIs reveals that the lower limit of this fell below 90% in all but two studies. 55,64
FIGURE 20.
Negative predictive value of NBI for high-confidence characterisations of diminutive polyps in the whole colon. a, Note that no 95% CI was reported for the Hewett and colleagues study. 20

One study, by Iwatate and colleagues,56 compared differences in NPVs achieved by specialists in colonoscopy and general endoscopists. Specialists in colonoscopy achieved NPVs of > 90% (mean value 90.9%, 95% CI 70.8% to 98.9%), whereas the NPVs achieved by general endoscopists were lower, with a mean value of 71.4% (95% CI 47.8% to 88.8%); however, the difference between the groups was not statistically significant.
Seven studies54,55,58,61–63,68 reported on the NPVs for the characterisation of diminutive polyps in the rectosigmoid colon (top section, Table 15). Five of these studies54,55,58,63,68 reported data for all diminutive polyp characterisations in the rectosigmoid colon and NPVs ranged from 87.4% to 98.4%. In four54,55,63,68 of these five studies the NPVs were > 90%. Only in the study by Ladabaum and colleagues58 was the 90% threshold not reached.
| Study | Characterisation, value (95% CI) | |
|---|---|---|
| All | High confidence | |
| Rectosigmoid colon diminutive polyps | ||
| Gupta et al.68 | 95.4% (91.8% to 97.7%) | NR |
| Hewett et al.54 | 98.4% (95.3% to 99.7%) | 99.4% (96.9% to 100.0%) |
| Ladabaum et al.58 | 87.4% (82.5% to 92.4%) | NR |
| Patel et al.55 | 93.7% (91.8% to 95.7%) | 94.7% (92.6% to 96.8%) |
| Pohl et al.61 | NR | 95.1% (92.2% to 97.1%)a |
| Repici et al.62 | NR | 92% (88% to 96%) |
| Wallace et al.63 | 95% (88.8% to 98.8%)a | 96% (89.3% to 99.2%)a |
| Diminutive polyps located on the right side of the colon | ||
| Kaltenbach et al.57 | NR | 87.1% (70.2% to 96.4%) |
| Diminutive polyps located proximal to the splenic flexure | ||
| Pohl et al.61 | NR | 43.4% (33.5% to 53.8%)a |
| Diminutive polyps located on the left side of the colon | ||
| Gupta et al.68 | 93.0%a (89.2% to 95.8%)a | NR |
| Kaltenbach et al.57 | NR | 93.9% (83.1% to 98.7%) |
| Diminutive polyps located in the distal colon | ||
| Pohl et al.61 | NR | 92.6% (89.4% to 95.0%)a |
| Rectal diminutive polyps | ||
| Kaltenbach et al.57 | NR | 93.8% (79.2% to 99.2%) |
| Diminutive polyps proximal to rectosigmoid colon | ||
| Ladabaum et al.58 | 57.3% (38.4% to 76.2%) | NR |
| Patel et al.55 | 65.6% (59.2% to 71.9%) | 77.1% (67.9% to 86.2%) |
| Rectosigmoid colon diminutive polyps assessed by endoscopists with prior optical diagnosis experience in colonoscopy | ||
| bPohl et al.61 | NR | 96.6% (92.7% to 98.7%) |
| Rectosigmoid colon diminutive polyps assessed by endoscopists with no prior optical diagnosis experience in colonoscopy | ||
| bPohl et al.61 | NR | 93.5% (88.7% to 96.7%) |
Data for high-confidence characterisations of polyps in the rectosigmoid colon were reported by five of the seven studies (Figure 21). 54,55,61–63 In three of these five studies,54,55,63 the data on high-confidence characterisations were provided in addition to data on all polyp characterisations in the rectosigmoid colon. In these studies the high-confidence results led to NPVs that remained at > 90% and were slightly increased. Two studies61,62 provided high-confidence results only for the rectosigmoid colon and in both the NPV was over the 90% threshold. It is worth noting, however, that in two62,63 of the five studies that report NPVs for high-confidence characterisations of diminutive polyps in the rectosigmoid colon, the lower limit of the 95% CI falls below 90%.
FIGURE 21.
Negative predictive values of NBI for high-confidence characterisations of diminutive polyps in the rectosigmoid colon.
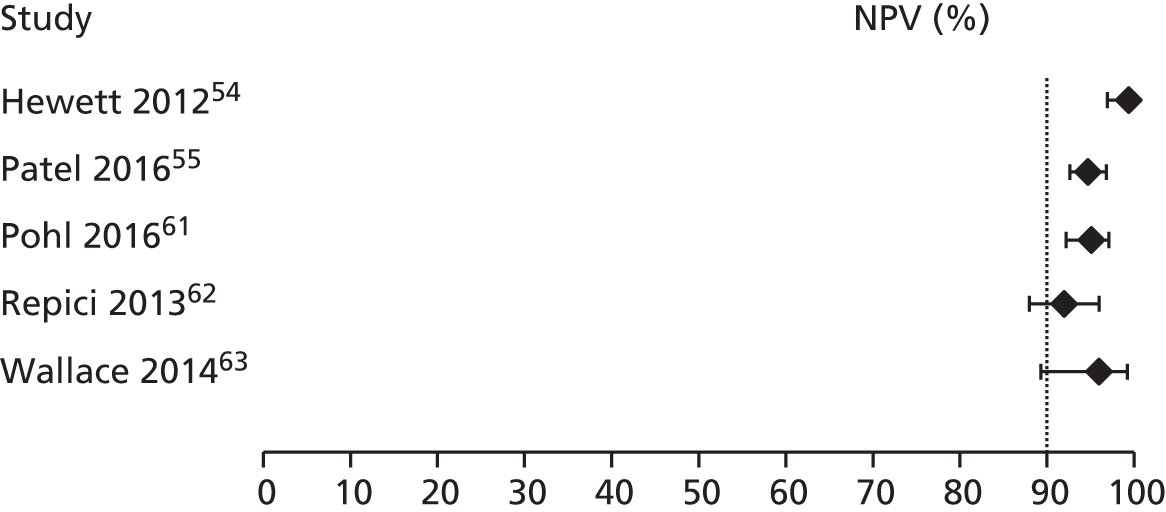
The NPVs of NBI for characterisation of diminutive polyps in other regions of the colon (where reported by studies) is also presented in Table 15. Although the mean NPV was above the 90% threshold in some instances, none of the lower limits of the 95% CI was > 90%.
One study61 reported the NPV for characterisations of diminutive polyps in the rectosigmoid colon achieved by endoscopists with prior optical diagnosis experience in colonoscopy and by endoscopists without prior optical diagnosis experience. Endoscopists with prior optical diagnosis experience achieved a NPV of 96.6% (95% CI 92.7% to 98.7%), whereas the NPV achieved by endoscopists without prior optical diagnosis experience was lower at 93.5% (95% CI 88.7% to 96.7%).
Accuracy of narrow-band imaging
As well as measures such as sensitivity, specificity and NPV reported above, another global measure, diagnostic accuracy, can be calculated from the 2 × 2 table data. This is expressed as the proportion of correctly classified polyps (the sum of the TP and TN results) among all the polyps (TP + TN + FP + FN). Like NPV, diagnostic accuracy is affected by disease prevalence such that at the same sensitivity and specificity diagnostic accuracy increases as disease prevalence decreases.
Accuracy of polyp characterisations in the whole colon was reported by, or could be calculated for, 16 studies (Table 16). 55,56,58,62–71,75,77,78 Accuracy was ≥ 90% in five studies,66,67,69–71 between 76% and 89% in 10 studies55,56,58,62–64,68,75,77,78 and only 63.9% in the final study. 65
| Study | Accuracy (95% CI) | |
|---|---|---|
| All | High confidence | |
| Whole colon | ||
| Aihara et al.66 | 90.1% (84.8% to 95.4%) | NR |
| Chandran et al.67 | 91.2%a | NR |
| Gupta et al.68 | 84.8% (82.3% to 87.1%) | NR |
| Henry et al.69 | 90.0% (82% to 95%) | NR |
| Hewett et al.20 | NR | 88% |
| Ignjatovic et al.70 | 92% | NR |
| Ikematsu et al.71 | 90.3% | NR |
| Iwatate et al.56 | 79.5% | 85.0% |
| Kaltenbach et al.57 | NR | 87.0% (82.8% to 90.5%) |
| Kang et al.78 | 79.4% (75.5% to 83.6%) | NR |
| Ladabaum et al.58 | 78.1% (73.7% to 82.5%) | 81.1% (75.8% to 86.3%) |
| Lee et al.77 | 87.8% (82.6% to 92.9%) | 91.2%a |
| Paggi et al.60 | NR | 84.0% |
| Paggi et al.59 | NR | 88.2% (83.9% to 92.5%) |
| Patel et al.55 | 76.7% (75.2% to 78.3%) | 84.8% (82.1% to 87.5%) |
| Pohl et al.61 | NR | 83.2% |
| Repici et al.62 | 85% | 89% (86% to 92%) |
| Rex et al.64 | 88.6%a | 93.0%a |
| Shahid et al.75 | 80% (70% to 87%) | NR |
| Sola-Vera et al.65 | 63.9% | 68.5% |
| Wallace et al.63 | 79% | 82% |
| Whole colon by colonoscopist type | ||
| Iwatate et al.56 | ||
| Specialist colonoscopists | NR | 90.7% |
| Generalist colonoscopists | NR | 82.3% |
| Right colon | ||
| Kaltenbach et al.57 | NR | 86.4% (80.0% to 91.4%) |
| Proximal to splenic flexure | ||
| Pohl et al.61 | NR | 78.8% |
| Left colon | ||
| Gupta et al.68 | 83.5% (80.0% to 86.6%) | NR |
| Kaltenbach et al.57 | NR | 90.2% (83.4% to 94.8%) |
| Distal colon | ||
| Pohl et al.61 | NR | 86.2% |
| Rectosigmoid colon | ||
| Hewett et al.54 | 94.5% (91.5% to 97.6%) | 99.0% (97.6% to 100%) |
| Ladabaum et al.58 | 77.4% (69.1% to 85.3%) | NR |
| Patel et al.55 | 80.9% (76.7% to 85.1%) | 88.1% (83.2% to 92.9%) |
| Repici et al.62 | NR | 91% (87% to 95%) |
| Pohl et al.61 | NR | 87.6% |
| Wallace et al.63 | 84% | 90% |
| Proximal to rectosigmoid colon | ||
| Ladabaum et al.58 | 79.3% (74.7% to 83.9%) | NR |
| Patel et al.55 | 78.8% (75.5% to 82.0%) | 84.7% (80.7% to 88.6%) |
| Rectum | ||
| Kaltenbach et al.57 | NR | 80.4% (66.1% to 90.6%) |
Thirteen studies20,55–65,77 reported on the accuracy of high-confidence polyp characterisations in the whole colon (see Table 16). Accuracy was ≥ 90% in two studies,64,77 between 81% and 90% in 10 studies20,55–63 and only 68.5% in the final study. 65
Accuracy of polyp characterisation was typically 3–5% higher among high-confidence characterisations than among all polyp characterisations in the eight studies55,56,58,62–65,77 that reported both values.
i-scan
Sensitivity and specificity of i-scan for the characterisation of diminutive colorectal polyps
Five studies77,79–82 provided data on the characterisation of diminutive polyps as adenomas or hyperplastic polyps using i-scan, with the characterisation verified by histopathological assessment of the resected polyps. The way in which data were reported by the studies varied. Two studies, by Basford and colleagues79 and Lee and colleagues,77 reported on the characterisation of diminutive polyps within the whole colon. Basford and colleagues79 presented data only from the polyp characterisations that the endoscopist had high confidence were correct, whereas Lee and colleagues77 provided data for all characterisations and then separately for characterisations made with either high or low confidence (data for low-confidence characterisations are available in Appendix 3). The other three studies presented data on the characterisation of diminutive polyps from within a part of the colon: the distal colon (Rath and colleagues82), the last 30 cm of colon (Hoffman and colleagues,80 who did not present a per-polyp analysis, only an analysis per patient) and the rectosigmoid colon (Pigo and colleagues81 and Rath and colleagues,82 although it was not possible to impute the 2 × 2 table data for the latter study). Rath and colleagues82 also provided data separately for the polyp characterisations they had made with high confidence.
The results for all characterisations (i.e. not separated by confidence level) are shown in Figure 22. The ability of i-scan to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) was > 90% in three of the four studies that reported results for all characterisations (i.e. Lee and colleagues,77 Pigo and colleagues81 and Rath and colleagues82), whereas sensitivity was only 82% in the per-patient analysis reported by Hoffman and colleagues. 80 The ability of i-scan to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) was more variable across the studies, ranging from 83% (Rath and colleagues,82 results for polyps in the distal colon) to 96% (Hoffman and colleagues80).
FIGURE 22.
Accuracy of i-scan for characterising diminutive colorectal polyps as either adenomas or hyperplastic polyps. (a) i-scan: polyps in the whole colon; (b) i-scan: polyps in the distal colon; (c) i-scan: polyps in the last 30 cm of colon (analysis by patient); and (d) i-scan: polyps in the rectosigmoid colon. a, 2 × 2 table data imputed; b, Rath and colleagues82 presented summary data for polyps in the rectosigmoid colon, but it was not possible for us to impute the 2 × 2 table data necessary to plot these results within this figure. The reported sensitivity was 90.3% (95% CI 73.1% to 97.5%) and specificity 87.5% (95% CI 74.1% to 94.8%).
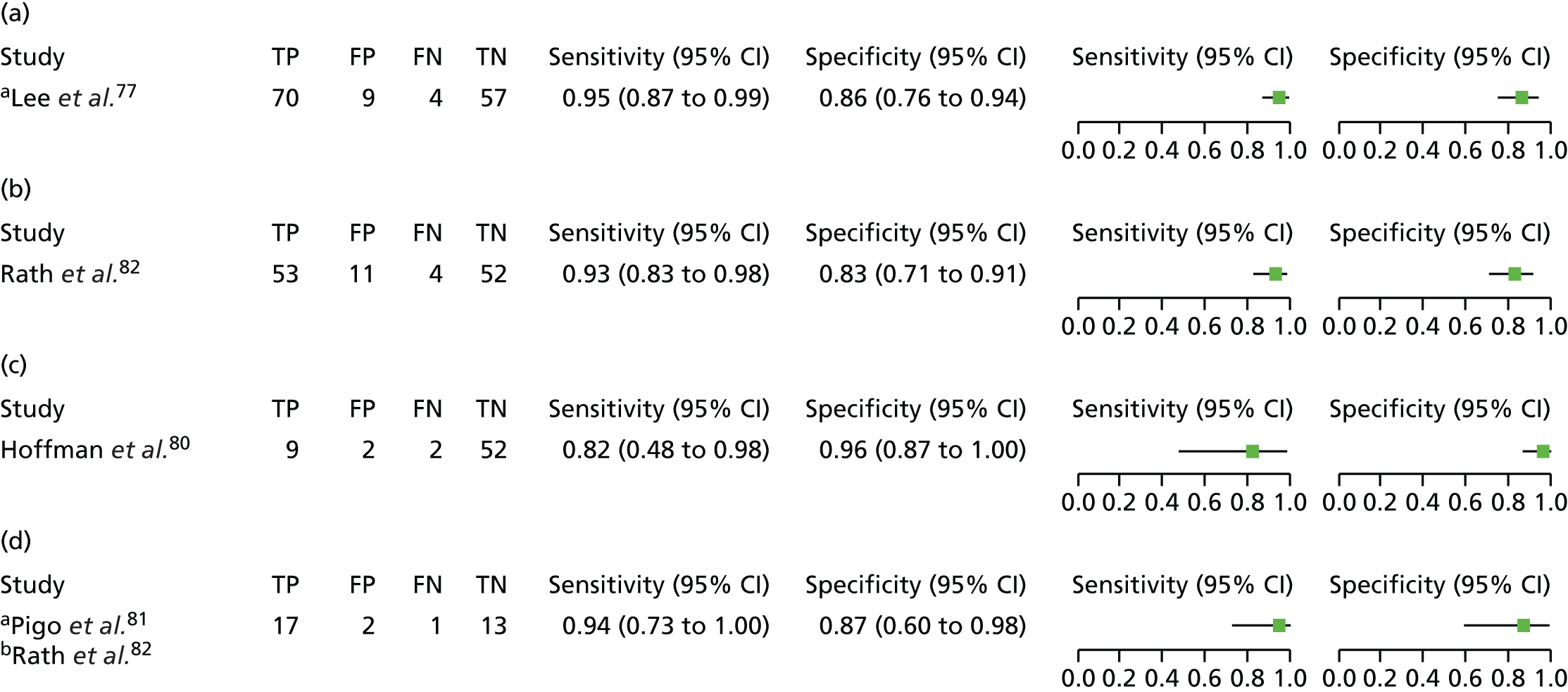
The results for studies that reported results from polyp characterisations with i-scan that were designated as high-confidence decisions are shown in Figure 23. The ability of high-confidence characterisations made with i-scan to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) in the three studies that provided data was 0.94 (i.e. 94%; Lee and colleagues77), 0.97 (97%; Basford and colleagues79) and, in the Rath and colleagues’ study,82 0.98 for distal polyps and 0.96 in the analysis limited to polyps in the rectosigmoid colon. In the Lee and colleagues study,77 the sensitivity achieved from high-confidence polyp characterisations was slightly lower than that obtained from all the polyp characterisations, 0.94 (95% CI 0.84 to 0.99) versus 0.95 (95% CI 0.87 to 0.99), whereas the reverse was true for the Rath and colleagues study82 for both the data set for distal polyps and that for rectosigmoid colon polyps (distal polyps: high confidence 0.98, 95% CI 0.90 to 1.00, vs. overall 0.93, 95% CI 0.83 to 0.98; rectosigmoid colon: high confidence 0.96, 95% CI 0.80 to 1.0, vs. overall 0.90, 95% CI 0.73 to 0.98). The ability of i-scan to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) when the characterisation was made with high confidence was ≥ 0.90 (i.e. 90%) in all three studies. Furthermore, the specificity of i-scan arising from high-confidence decisions was greater than the specificity observed when all the polyp characterisations were taken into account in the two studies that reported both sets of data (Lee and colleagues,77 92% vs. 86%; Rath and colleagues,82 distal polyps 95% vs. 83%, rectosigmoid colon polyps 95.5% vs. 87.5%). The 2005 Rath and colleagues82 study, which was conducted in Germany among patients attending for screening or surveillance colonoscopy and which reported on characterisation of distal polyps (polyps in the descending colon, the sigmoid colon or the rectum), achieved the best sensitivity (98%), which was coupled with the second highest value for specificity (95%). However, in common with the other studies providing data on i-scan, a single endoscopist working in what appears to be a specialist endoscopy centre achieved these results, so it is not clear how transferable these results would be to less experienced endoscopists working in less specialist settings.
FIGURE 23.
Accuracy of i-scan high-confidence characterisations of diminutive colorectal polyps as either adenomas or hyperplastic polyps. (a) i-scan: high-confidence characterisations of polyps in the whole colon; (b) i-scan: high-confidence characterisations of distal polyps; and (c) i-scan: high-confidence characterisations of polyps in the rectosigmoid colon. a, 2 × 2 table data imputed; b, Rath and colleagues82 presented summary data for high-confidence characterisations of polyps in the rectosigmoid colon, but it was not possible for us to impute the 2 × 2 table data necessary to plot these results within this figure. The reported sensitivity was 96.4% (95% CI 79.8% to 99.8%) and specificity 95.5% (95% CI 83.3% to 99.2%).
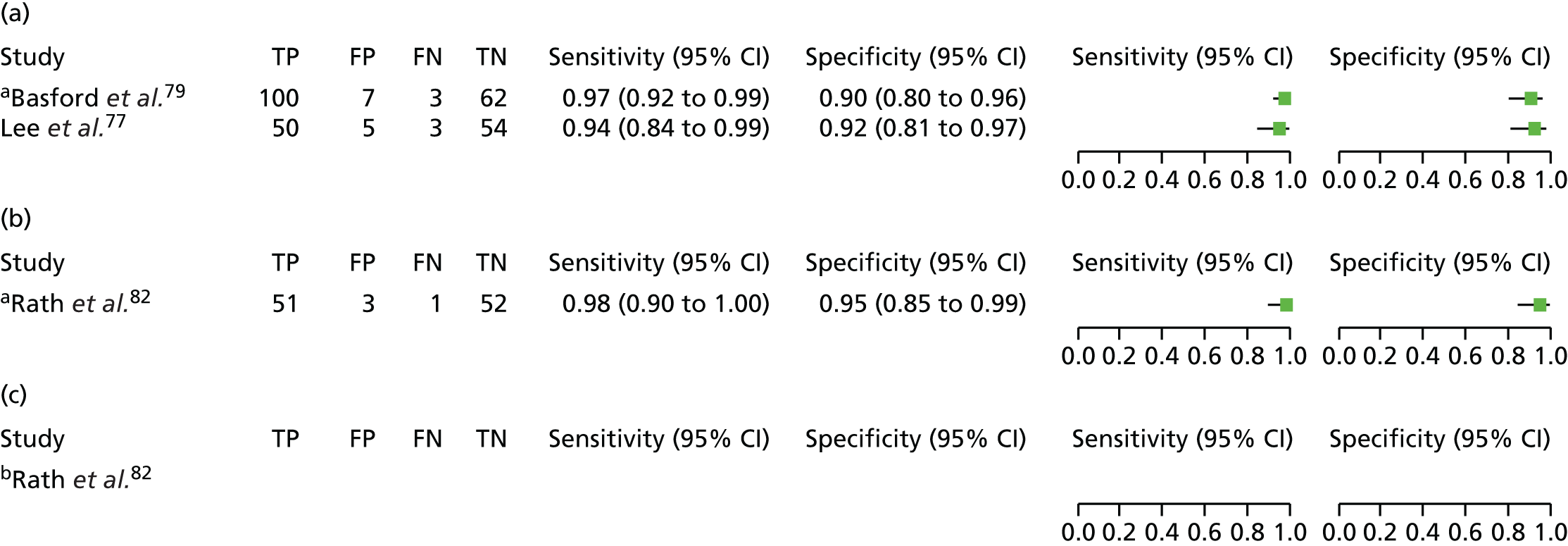
A bivariate meta-analysis was run (using Stata/IC14 and xtmelogit) to provide a summary estimate for the two studies that reported high-confidence characterisations of polyps in the whole colon, which could be used in the economic model. This produced a summary value for sensitivity of 0.96 (95% CI 0.92 to 0.98) and for specificity of 0.91 (95% CI 0.84 to 0.95). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 24.
FIGURE 24.
Summary receiver operating characteristic curve plot from the meta-analysis of i-scan for high-confidence characterisations of polyps in the whole colon. Note that the software used to draw the SROC plot (RevMan) did not generate a 95% confidence region or a 95% prediction region for this meta-analysis. It is presumed that this is because of the small number of studies.

Negative predictive value of i-scan for the characterisation of diminutive colorectal polyps
As previously stated, the NPV is the probability that subjects with a negative screening test (i.e. colorectal polyp is characterised as hyperplastic) truly do not have an adenoma. However, it must be borne in mind when viewing these results that the NPV is influenced by the prevalence of disease (i.e. in this case the prevalence of adenomas in the tested populations). When prevalence is increased, the result is a decrease in the NPV.
Two studies77,80 reported NPVs for the characterisations of diminutive polyps in the whole colon (made with any level of confidence), although one of these studies, by Hoffman and colleagues,80 reported only a per-patient analysis. Although the mean NPV was > 90%, the lower limit of the 95% CI fell below 90% in both studies (Table 17). High-confidence characterisation of polyps in the whole colon was reported by two studies. 77,79 Basford and colleagues79 reported a NPV of 95.4% (95% CI 87.1% to 99.0%) and Lee and colleagues77 a NPV of 94.7% (95% CI 85.4% to 98.9%).
| Study | Characterisation, value (95% CI) | |
|---|---|---|
| All | High confidence | |
| Whole colon | ||
| aBasford et al.79 | NR | 95.4% (87.1% to 99.0%) |
| Hoffman et al.80 (per-patient analysis) | 96.3%b (87.3% to 99.6%)b | NR |
| Lee et al.77 | 93.4% (87.2% to 99.7%) | 94.7%b (85.4% to 98.9%)b |
| Distal polyps | ||
| Rath et al.82 | 93.2% (82.7% to 97.8%) | 98.1% (88.4% to 99.1%) |
| Rectosigmoid colon polyps | ||
| Basford et al.79 | NR | 100% (93.4% to 100.0%) |
| Pigo et al.81 | 93% (81% to 100%) | NR |
| Rath et al.82 | 93.3% (80.1% to 98.3%) | 97.7% (86.2% to 99.9%) |
Three studies reported on the NPV for the characterisation of diminutive polyps in the distal portion of the colon82 or the rectosigmoid colon,79,81,82 with Rath and colleagues82 also reporting on high-confidence characterisations and Basford and colleagues79 reporting only on high-confidence characterisations. In all cases, although the point estimate for the NPV lay above the 90% threshold, the lower limit of the 95% CI fell below this.
Accuracy of i-scan
Diagnostic accuracy (the proportion of correctly classified polyps among all the polyps) was reported for all diminutive polyp characterisations,80,81 for only high-confidence polyp characterisations79 or for both77,82 (Table 18), with three studies providing data for the characterisations of polyps in the whole colon77,79,80 and a single study for polyps in the rectosigmoid colon81 or distal polyps. 82 Like NPV, diagnostic accuracy is affected by disease prevalence. At the same sensitivity and specificity, diagnostic accuracy increases as disease prevalence decreases.
| Study | Accuracy (95% CI) | |
|---|---|---|
| All | High confidence | |
| Whole colon | ||
| Basford et al.79 | NR | 94.2% (92.8% to 99.2%) |
| Hoffman et al.80 | 94% (per-patient analysis) | NR |
| Lee et al.77 | 90.7% (85.9% to 95.5%) | 92.9% |
| Rectosigmoid colon | ||
| Pigo et al.81 | 91%a | NR |
| Distal polyps | ||
| Rath et al.82 | 90.1%a | 96.3% |
Accuracy was ≥ 90% in all the studies77,79–82 and the accuracy of high-confidence polyp characterisations was higher than among all polyp characterisations in the two studies that reported both values. 77,82
Flexible spectral imaging colour enhancement
Sensitivity and specificity of flexible spectral imaging colour enhancement for the characterisation of diminutive colorectal polyps
Three studies 78,83,84 provided data on the characterisation of diminutive polyps as adenomas or hyperplastic polyps using FICE compared with characterisation verified by histopathological assessment of the resected polyps. In all three studies the characterisations were made on polyps in any part of the colon, and in all three the level of confidence with which the characterisation was made was not stated. The results of the polyp characterisations are shown in Figure 25.
FIGURE 25.
Accuracy of FICE for characterising diminutive colorectal polyps as either adenomas or hyperplastic polyps. a, 2 × 2 table data imputed.

The ability of FICE to correctly identify diminutive polyps as adenomas (i.e. the sensitivity of the test) ranged from 74% to 88% across the studies. The ability of FICE to correctly identify diminutive polyps as hyperplastic polyps (i.e. the specificity of the test) had a narrower range across the studies, from 82% to 88%.
It was possible to run a bivariate meta-analysis (using Stata/IC14 and xtmelogit) with data from the three studies. This produced a summary value for sensitivity of 0.81 (95% CI 0.73 to 0.88) and for specificity of 0.85 (95% CI 0.79 to 0.90). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 26.
FIGURE 26.
Summary receiver operating characteristic curve plot from the meta-analysis of FICE for all characterisations of polyps in the whole colon. Note that the software used to draw the SROC plot (RevMan) did not generate a 95% confidence region or a 95% prediction region for this meta-analysis. It is presumed that this is because of the small number of studies.
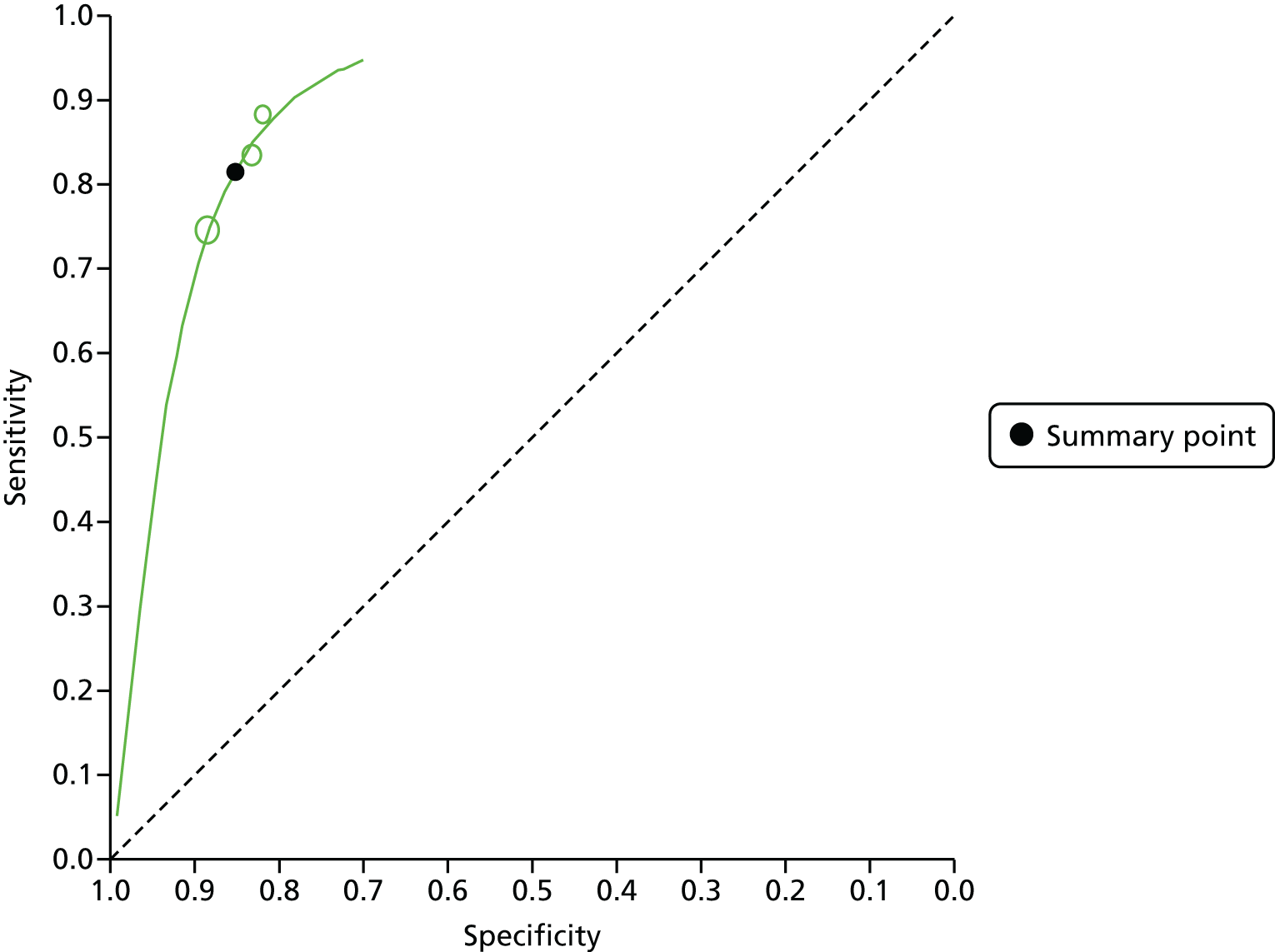
Negative predictive value of Flexible Spectral Imaging Colour Enhancement for the characterisation of diminutive colorectal polyps
Table 19 reports the NPVs for the three FICE studies. These ranged from 70% to 84%.
Accuracy of Flexible Spectral Imaging Colour Enhancement
The three studies that reported on the use of FICE provided diagnostic accuracy (the proportion of correctly classified polyps among all the polyps) for all diminutive polyp characterisations in the whole colon (Table 20). 78,83,84 The reported diagnostic accuracy values ranged from 80% to 85%.
Post hoc pooled analysis of all virtual chromoendoscopy technologies
The appropriateness of pooling evidence from different VCE technologies together is uncertain. The technologies certainly all aim to enhance surface vessel patterns, but the technologies use different methods to achieve this. We have therefore assumed that there is a ‘class effect’ and that evidence from different VCE technologies can be meaningfully pooled.
A pooled analysis of the studies included in this assessment for which 2 × 2 data were available was undertaken in order to inform a scenario analysis using the economic model (see Chapter 5, Sensitivity analyses). Data for high-confidence assessments of polyps in the whole colon were available from 11 NBI studies and two i-scan studies (note that Lee and colleagues77 contribute data on NBI and i-scan) (Figure 27). No FICE data were available to include in this analysis because the FICE studies did not report high-confidence polyp characterisations separately.
FIGURE 27.
Accuracy of VCE high-confidence decisions for characterising diminutive colorectal polyps as either adenomas or hyperplastic polyps in the whole colon.
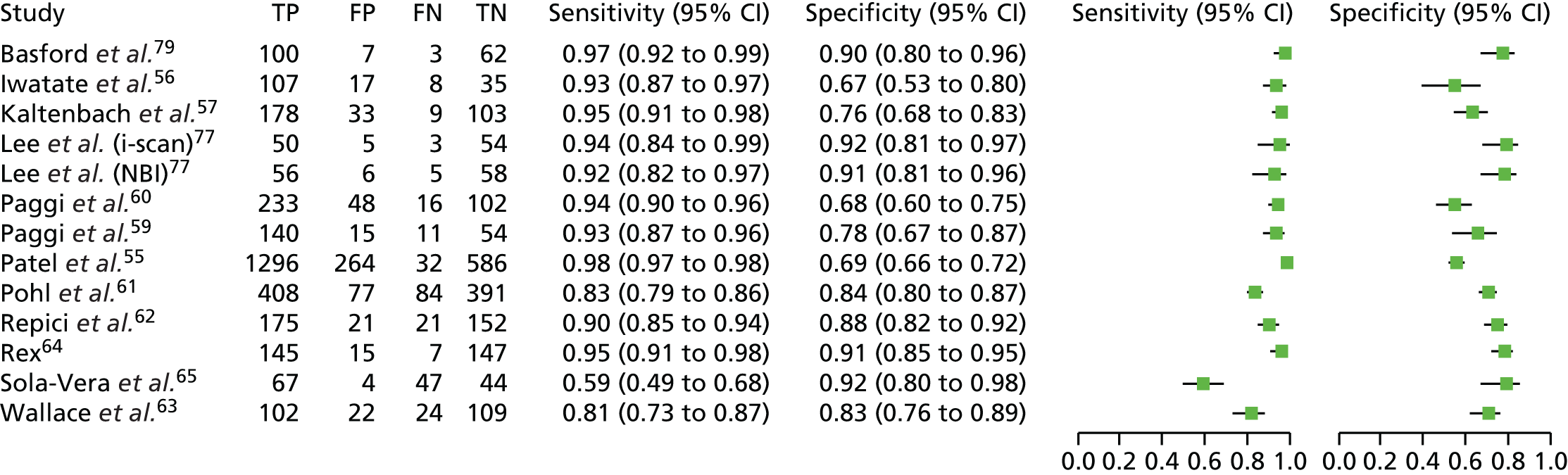
A bivariate meta-analysis (using Stata/IC14 and metandi45) was carried out, which produced a pooled summary estimate for sensitivity of 0.92 (95% CI 0.87 to 0.95) and for specificity of 0.83 (95% CI 0.78 to 0.87). The parameter estimates for the bivariate model were entered into RevMan to produce the SROC plot shown in Figure 28. The VCE pooled estimates for sensitivity and specificity do not differ greatly from the NBI pooled estimates (see Figure 9), which is unsurprising given that the bulk of the evidence comes from studies of NBI.
FIGURE 28.
Summary receiver operating characteristic curve plot showing the summary point on the summary curve from the meta-analysis of VCE high-confidence decisions for characterising diminutive colorectal polyps in the whole colon.
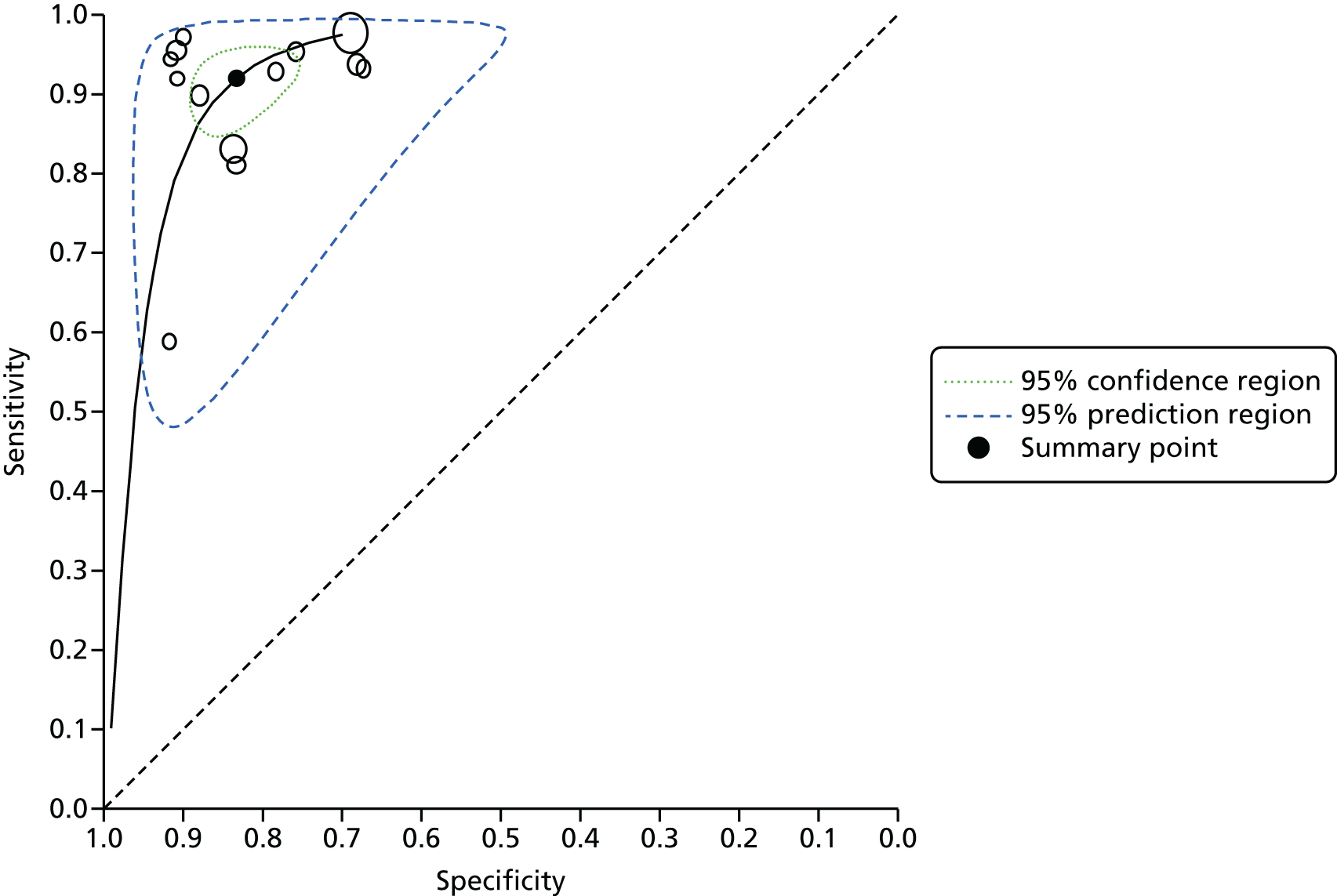
A pooled analysis of the virtual chromoendoscopy studies for high-confidence assessments of polyps in the rectosigmoid colon, equivalent to that above for the whole colon, has, in essence, already been presented earlier in this assessment. This is because the only data available for this analysis come from NBI studies and, thus, the results presented in Figures 15 and 16 represent all the available data on high-confidence assessments of polyps in the rectosigmoid colon; there are no equivalent data for i-scan or FICE.
Assessment of test impact on recommended surveillance intervals
Narrow-band imaging
Thirteen studies55,57,58,60–65,67,68,70,76 reported results on the impact that the use of NBI would have on recommended surveillance intervals (in comparison to surveillance intervals calculated following histopathology of all polyps) (Table 21). The agreement between the surveillance interval allocated using a NBI-based strategy and using the results of histopathology for all polyps ranged from 84%63,76 to 99%. 62 Eleven of the 13 studies reporting on this outcome achieved a level of agreement that was > 90%,55,57,58,61–65,67,68,70 although for three of these studies58,63,68 an agreement of > 90% was achieved by only one of the tested strategies (in two studies using a modified recommendation of colonoscopy in 10 years for patients with one or two small adenomas instead of 5 years,58,68 and in one study limiting the analysis to studies with high-confidence predictions for polyps ≤ 5 mm in size63). Where there were discrepancies between the surveillance interval assigned using the NBI-based strategy and the histopathology-only strategy, some studies reported whether the NBI strategy led to longer or shorter surveillance intervals being assigned. In the majority of studies in which a discrepancy in the surveillance interval was reported, the NBI-containing strategy led more often to shorter surveillance intervals being set (i.e. patients were recalled for a colonoscopy sooner than would have been the case with the histopathology-based surveillance interval) than to longer surveillance intervals. There were, however, some exceptions; in particular, in the study by Repici and colleagues,62 in the NBI-containing strategy, a difference between the surveillance intervals assigned was more likely to lead to the assignment of a longer interval (i.e. patients not recalled for repeat colonoscopy as early as they would have been with the histopathology-based surveillance interval) than to a shorter one.
| Study | Guideline used for determining surveillance interval (as cited by the study) | Surveillance interval | |
|---|---|---|---|
| Correctly allocated [95% CI] (n/N) | Shorter or longer intervals set with NBI, n (% of total allocations) | ||
| Chandran et al.67 | NHMRC, Australia, 2011100 | 98% (92/94) | 2 (2) shorter |
| Gupta et al.68 | US Multi-Society Task Force, 2008101
|
86.1% [95% CI 82.4% to 89.3%] | |
|
94.1% [95% CI 91.4 to 96.2] | ||
| Ignjatovic et al.70 | BSG guidelines 2002102 (and based on patients with no polyps > 10 mm in size) | 98% (80/82) | 2 (2) shorter |
| Kaltenbach et al.57 | US Multi-Society Task Force, 2012103 | ||
|
92.2% (259/281) | ||
|
95.2% (200/210)a | 7 (3.3) shorter; 3 (1.4) longer | |
| Ladabaum et al.58 | US Multi-Society Task Force, 2008101 | ||
|
88.4% [95% CI 86.8% to 89.9%] | ||
|
79.9% [95% CI 77.4% to 82.3%]a | 136 (13) shorter; 78 (7) longer | |
| Using modified recommendations 201268 (10 years for one or two small adenomas) | |||
|
98.4% [95% CI 97.6% to 98.9%] | ||
|
96.8% [95% CI 95.6% to 97.8%]a | 24 (2) shorter; 10 (1) longer | |
| Paggi et al.59 | US Multi-Society Task Force on Colorectal Cancer, 2006104 | 85.3% (168/197) | 22 (11) shorter; 7 (4) longer |
| Patel et al.55 | US Multi-Society Task Force, 2012103 | 91.2% [95% CI 89.67% to 92.65%] (1279/1403)a | 82 (5.8) shorter; 39 (2.8) longer |
| bPohl et al.61 | US Multi-Society Task Force guidelines103,105 | ||
|
96% | ||
|
93%a | 24 (4) shorter; 15 (3) longer | |
| Repici et al.62 | European guidelines 2010:106 one or more polyps ≤ 5 mm in size and characterised with high confidence | 99% [95% CI 97% to 100%]a | 3 (1) longer |
| US Multi-Society Task Force 2008101 | |||
|
92% [95% CI 88% to 96%]a | 5 (2) shorter; 12 (4) longer | |
|
99% [95% CI 97% to 100%]a | 3 (1) longer | |
| Rex64 | US Multi-Society Task Force on Colorectal Cancer, 2006104 | ||
|
94% (128/136)a | 4 (3) shorter; 4 (3) longer | |
|
98.5% (134/136)a | 2 (1) shorter; 1 (0.7) longer | |
| Sola-Vera et al.65 | European guideline, 2012107 | 97.8% (46/47) | NR |
| ESGE guideline108 | 97.8% (46/47) | NR | |
| Vu et al.76 | US Multi-Society Task Force, 2008;101 high-confidence predictions | 84.1%a | NR |
| Wallace et al.63 | Based only on number and size of adenomas109 | ||
|
84% [95% CIs 79% to 88%] (221/264) | 27 (10) shorter; 16 (6) longer | |
|
95% [95% CIs 91% to 97%] (250/264)a | 5 (2) shorter; 9 (3) longer | |
Nine studies clearly calculated the concordance of surveillance intervals between VCE and histopathology in line with the PIVI requirements. 57–59,61–64,67,76 The criterion of the PIVI statement, that agreement should be ≥ 90%, was met by all but one study,76 with one further study meeting the PIVI criterion in one of the two tested strategies. 58 When the agreement was ≥ 90%, the lower limit of the 95% CI (when reported) fell below 90% in two instances. 55,62
i-scan
Two studies79,82 examined the effect that the use of i-scan had on recommended surveillance intervals in comparison with those that were allocated based on histopathological assessment of all polyps (Table 22). Both studies79,82 used in vivo diagnosis of diminutive polyps to guide surveillance interval decisions in accordance with the PIVI requirements. Both studies79,82 also calculated agreement in surveillance intervals between i-scan and histopathology when using two different guidelines for determining the surveillance interval. Across these two studies, a surveillance interval agreement of > 90% was achieved regardless of the guideline used, with agreement ranging from 93.2%82 to 97.2%. 79 In the study by Basford and colleagues,79 identical results (an agreement of 97.2%) were achieved when using both the guidelines assessed. Both studies reported whether using i-scan resulted in a longer or shorter surveillance interval being allocated than that allocated by histopathology. In the Basford and colleagues study,79 two patients were set a shorter interval with i-scan and one patient a longer interval. In the Rath and colleagues study,82 i-scan tended to results in longer intervals being allocated than with histopathology, except in one case.
| Study | Guideline used for determining surveillance interval (as cited by the study) | Surveillance interval | |
|---|---|---|---|
| Correctly allocated [95% CI] (n/N) | Longer or shorter intervals set with i-scan, n (% of total allocations) | ||
| i-scan surveillance intervals based on high-confidence assessment of all diminutive polyps combined with histopathology of polyps > 5 mm | |||
| Basford et al.79 | ASGE103 and BSG guidelines30 | 97.2% [NR] (80/83) | 2 (2.4) shorter; 1 (1.2) longer |
| i-scan surveillance intervals based on high-confidence assessment of all distal polyps | |||
| aRath et al.82 | European guidelines107 | 94.5% [NR] (69/73) | 4 (5.5) longer |
| US guidelines103 | 93.2% [NR] (68/73) | 1 (1.4) shorter; 4 (5.5) longer | |
Flexible Spectral Imaging Colour Enhancement
Two studies83,84 reported results on the impact that the use of FICE would have on recommended surveillance intervals (in comparison with surveillance intervals calculated following histopathology of all polyps), although neither assessed this in accordance with the PIVI criteria. This analysis, in both of these studies, included polyps < 10 mm in size (i.e. neither was restricted to diminutive polyps). The agreement between the surveillance interval allocated using a FICE-based strategy and using the results of histopathology was 100% in one study83 and 97% in the other study,84 regardless of whether the BSG or ASGE guidelines were used to determine the surveillance intervals. In the single study for which there was a discrepancy for two participants between the surveillance interval assigned using the FICE-based strategy and the histopathology strategy, it is not known whether the FICE-based strategy led to a longer or a shorter surveillance interval being set (Table 23).
| Study | Guideline used for determining surveillance interval (as cited by the study) | Surveillance interval | |
|---|---|---|---|
| Correctly allocated [95% CI] (n/N) | Longer or shorter intervals set with FICE, n (% of total allocations) | ||
| aLongcroft-Wheaton et al.83 | BSG30 | 100% (38/38) | n/a |
| ASGE110 | 100% (38/38) | n/a | |
| aLongcroft-Wheaton et al.84 | BSG30 | 97% [89% to 100%] (67/69) | NR |
| ASGE110 | 97% [89% to 100%] (67/69) | NR | |
Assessment of other outcomes
In addition to the outcomes reported above on test accuracy and the impact on recommended surveillance intervals, the review also aimed to report data on the interpretability of the tests; interobserver agreement; intraobserver agreement; test acceptability (to patients and/or clinicians); adverse events; the number of polyps designated to be left in place; the number of polyps designated to be resected and discarded; the number of polyps designated for resection and histopathological examination; the length of time to perform the colonoscopy; the number of outpatient appointments; HRQoL; incidence of colorectal cancer; and mortality.
Narrow-band imaging
None of the studies reported on the interpretability of the test; test acceptability (to patients and/or clinicians), number of outpatients appointments, HRQoL, incidence of colorectal cancer or mortality.
One study, by Lee and colleagues,77 reported on interobserver agreement, although this was the agreement between the characterisation obtained during real-time assessment and that obtained by an independent reader who reviewed all recorded endoscopic images while blind to the real-time assessment and the histopathology results. The interobserver agreement was 86.5%, with a κ value of 0.730 (95% CI 0.623 to 0.837), which represents ‘substantial’ agreement. One other study, by Rogart and colleagues,74 reported interobserver agreement for 20 test images, but, as this did not include any real-time assessment these data were not extracted. Lee and colleagues77 were also the only researchers to report on intraobserver agreement. This was the agreement between the characterisation obtained during real-time assessment and that obtained by the same endoscopist who reviewed all recorded endoscopic images 1–3 months after the colonoscopy. The intraobserver agreement was 89.7%, with a κ value of 0.795 (95% CI 0.699 to 0.890), again representing ‘substantial’ agreement.
Adverse events were not reported by most studies. 20,54–56,58–71,74,76,78 Of the three studies that did make mention of potential adverse events,57,75,77 all indicated that no events had occurred. Kaltenbach and colleagues57 reported no post-polypectomy bleeding, coagulation syndrome, perforation or optical misdiagnosis of advanced histopathology, Lee and colleagues77 stated that participants did not experience any procedure-related complications and Shahid and colleagues75 stated that none of the patients experienced any endoscopic complications.
Ignjatovic and colleagues70 reported on the number of diminutive polyps that would have been left in place if the management strategy was to leave diminutive hyperplastic polyps in situ in the rectosigmoid colon. The endoscopists in this study made a high-confidence optical diagnosis for 323 polyps (< 10 mm in this study) and, of these, 33 would have been left in situ. All 33 were correctly predicted to be hyperplastic polyps and all were located in the sigmoid colon or the rectum. Repici and colleagues62 made a statement indicating that, in their study, a discard-type strategy would have reduced the need for polypectomy by 48%.
Two studies reported on the number of polyps that would have been resected and discarded if a resect and discard type of management strategy had been in place. Gupta and colleagues68 reported a hypothetical strategy in which, if all the 884 diminutive polyps in their study (in which the total number of polyps of any size was 1254) were resected and discarded, this would represent a 70.5% reduction in histopathology. Using this strategy, 13 adenomas with advanced histopathological features would have been discarded. However, it must be noted that this study did not record whether characterisations were made with high or low confidence and did not report how many diminutive polyps were in the rectosigmoid colon. Ignjatovic and colleagues70 reported that a high-confidence optical diagnosis was made for 323 polyps (< 10 mm in size in this study) and, of these, 290 would have been resected and discarded. The Ignjatovic and colleagues study70 was the only NBI study to ask endoscopists to identify polyps that they would have sent electively to histopathology, even if a policy of optical diagnosis had been in place. These were polyps where the optical diagnosis was made with low confidence or where no optical diagnosis could be made. For the subgroup of diminutive polyps in this study, 7.5% (22 of 293 polyps) would have been sent for elective histopathology.
The length of time taken to perform the withdrawal phase of the colonoscopy was reported by three studies. Kaltenbach and colleagues57 reported a mean withdrawal time of 10.3 minutes [standard deviation (SD) 5.7 minutes, range 3.3–58.0 minutes]. A procedure time of 12 seconds is reported, but a definition of procedure time is not provided in the study publication, so it is not clear what this comprises. In the Kang and colleagues78 study, the mean withdrawal time in the NBI group was 13.5 minutes (SD 7.3 minutes), whereas in the Wallace and colleagues study63 it was 16.1 minutes (SD 7.3 minutes). A fourth study, Shahid and colleagues,75 reported that the average withdrawal time at their centre was typically 8–10 minutes, but withdrawal time was not reported specifically for their study. However, they did report that NBI inspection time was typically < 1 minute.
i-scan
None of the studies reported on the interpretability of the test, test acceptability (to patients and/or clinicians), number of polyps designated to be left in place, number of polyps designated to be resected and discarded, number of polyps designated for resection and histopathological examination, number of outpatients appointments, HRQoL, incidence of colorectal cancer or mortality.
One study, by Lee and colleagues,77 reported on interobserver agreement, although this was the agreement between the characterisation obtained during real-time assessment and that obtained by an independent reader who reviewed all recorded endoscopic images while blind to the real-time assessment and the histopathology results. The interobserver agreement was 87.9%, with a κ value of 0.751 (95% CI 0.640 to 0.861), which represents ‘substantial’ agreement. One other study, by Pigo and colleagues,81 reported interobserver agreement but this was based on endoscopists’ assessments of still images so, because this did not include any real-time assessment, these data were not extracted. Two studies, by Lee and colleagues77 and Rath and colleagues,82 reported on intraobserver agreement. In the Lee and colleagues study77 this was the agreement between the characterisation obtained during real-time assessment and that obtained by the same endoscopist who reviewed all recorded endoscopic images 1–3 months after the colonoscopy. The intraobserver agreement was 86.4%, with a κ value of 0.729 (95% CI 0.616 to 0.841), again representing ‘substantial’ agreement. In the Rath and colleagues’ study82 it is not clear how intraobserver agreement was assessed because no details are reported in the paper. The authors state that agreement was achieved in 113 out of 121 polyps (93.4%), with a κ coefficient of agreement of 0.867 (95% CI 0.799 to 0.967), which indicated almost perfect agreement. In the Pigo and colleagues study81 intraobserver agreement was assessed based on the endoscopists’ assessment of still images rather than real-time assessment. Furthermore, the intraobserver agreement for the evaluation of diminutive polyps was not reported, so these data were not extracted.
As already stated in Narrow-band imaging, Lee and colleagues77 reported that participants did not experience any procedure-related complications. The other four i-scan studies79–82 made no reports of adverse events.
The length of time taken to perform the withdrawal phase of the colonoscopy was not reported in any of the studies. Basford and colleagues,79 however, commented that the in vivo assessment was performed in the time between finding a polyp and preparing for polypectomy and so did not cause a significant delay. Hoffman and colleagues,80 who examined only the last 30 cm of colon, reported that with surface enhancement with i-scan the total examination time was 5 minutes.
Flexible Spectral Imaging Colour Enhancement
None of the studies reported on the interpretability of test, interobserver agreement, intraobserver agreement, test acceptability (to patients and/or clinicians), adverse events, number of polyps designated to be left in place, number of polyps designated to be resected and discarded, number of polyps designated for resection and histopathological examination, length of time to perform the colonoscopy, number of outpatient appointments, HRQoL, incidence of colorectal cancer or mortality.
Head-to-head comparisons
Head-to-head comparisons of NBI, i-scan and FICE were not within the scope of this assessment; nevertheless, two studies met the inclusion criteria for the systematic review that did compare two technologies against each other. When NBI was compared with i-scan in a prospective cohort study of the real-time histopathological prediction of diminutive colonic polyps, Lee and colleagues77 found no statistically significant differences between the two technologies (sensitivity: NBI, 88.8% vs. i-scan 94.6%; specificity: NBI 86.8% vs. i-scan 86.4%; and accuracy: NBI 87.8% vs. i-scan 90.7%; p > 0.05). In the RCT that compared NBI with FICE, Kang and colleagues78 found that for polyps < 5 mm in size there was no statistically significant difference (p > 0.05) in accuracy (NBI 74.9% vs. FICE 80.1%) or sensitivity (NBI 81.9% vs. FICE 74.5%), but there was a statistically significant difference in specificity (NBI 75.7% vs. FICE 88.4%; p = 0.006). The authors concluded that better results should be achieved for both technologies before either are used for real-time optical biopsy of colorectal polyps in colorectal screening of the general population. 78 It is worth noting that in the study by Lee and colleagues77 a single endoscopist with experience of both NBI and i-scan undertook the study colonoscopies, whereas the four endoscopists in the Kang and colleagues study78 had no prior experience of either NBI or FICE.
Summary of diagnostic test performance evidence
-
Thirty studies met the inclusion criteria for the systematic review of test accuracy. These assessed NBI (24 studies20,54–78), i-scan (five studies77,79-82) and FICE (three studies78,83,84). Two of the included studies assessed two of the included interventions (NBI and i-scan;77 and NBI and FICE78). The way studies reported test accuracy outcomes (in terms of the region of the colon and the level of confidence assigned to the polyp characterisation) varied.
-
Most studies enrolled participants from more than one of the populations eligible for inclusion in this review (receiving colonoscopy for screening, surveillance or symptoms), but these studies did not report results separately for each participant type.
-
The included studies were rated as being likely to be at a low risk of bias.
Narrow-band imaging
-
A total of 23 studies reported either sensitivity (one study74) or both sensitivity and specificity (22 studies20,54–71,75,77,78).
-
In the whole colon, characterisations of diminutive polyps made with any level of confidence had a sensitivity ranging from 0.55 to 0.97 (17 studies55,56,58,62–71,74,75,77,78) and a specificity ranging from 0.62 to 0.95 (16 studies55,56. 58,62–71,75,77,78). A bivariate meta-analysis (16 studies55,56. 58,62–71,75,77,78) produced a summary sensitivity value of 0.88 (95% CI 0.83 to 0.92) and specificity of 0.81 (95% CI 0.75 to 0.85). For characterisations in the whole colon made with high confidence, summary sensitivity and specificity (11 studies55–57,59–65,77) were slightly higher [sensitivity 0.91 (95% CI 0.85 to 0.95) and specificity 0.82 (95% CI 0.76 to 0.87)] and limiting this analysis to studies in which the endoscopists were experienced in the use of NBI (four studies59,60,62,77) did not greatly alter these results [sensitivity 0.92 (95% CI 0.89 to 0.94) and specificity 0.82 (95% CI 0.72 to 0.89)].
-
In the rectosigmoid colon, characterisations of diminutive polyps made with any level of confidence (four studies54,55,58,63) had a sensitivity ranging from 0.84 to 0.90 and a specificity ranging from 0.76 to 0.95. A bivariate meta-analysis (three studies54,58,63) produced a summary estimate for sensitivity of 0.85 (95% CI 0.75 to 0.91) and for specificity of 0.87 (95% CI 0.74 to 0.94). For characterisations in the rectosigmoid colon made with high confidence (five studies54,55,61–63), sensitivity ranged from 0.83 to 0.96 and specificity from 0.88% to 0.99%. A bivariate meta-analysis (four studies54,61–63) produced a summary estimate for sensitivity of 0.87 (95% CI 0.80 to 0.92) and for specificity of 0.95 (95% CI 0.87 to 0.98). Limiting the analysis of high-confidence characterisations in the rectosigmoid colon to the two studies54,62 in which the endoscopists were experienced in the use of NBI increased the summary values for sensitivity and specificity [sensitivity 0.90 (95% CI 0.71 to 0.97) and specificity 0.98 (95% CI 0.91 to 1.00)].
-
Some studies that reported sensitivity and specificity were not included in meta-analysis because it was not possible to impute the required 2 × 2 table data. In two of three instances where this occurred, the sensitivity and specificity reported by the absent study lay within the 95% CI of the summary estimates of the meta-analysis. In one case (the meta-analysis of high-confidence polyp characterisations in the whole colon) the missing study, that by Ladabaum and colleagues,58 reported a sensitivity that lay within the 95% CI of the summary estimate but a specificity that lay outside the 95% CI of the summary estimate.
-
The NPV of NBI for the characterisation of diminutive polyps in the whole colon (made with any level of confidence) ranges from 43% to 96% (16 studies55,56,58,62–71,75,77,78). Five studies55,64,66,67,69 reported NPVs of ≥ 90%, but the lower limit of the 95% CI fell below 90% in every study except one. 55 When limited to high-confidence characterisations, NPVs ranged from 48% to 98% (13 studies20,55–65,77), with five studies20,55,57,64,77 reporting NPVs of ≥ 90%. However, the lower limit of the 95% CI remained above 90% in only two studies. 55,64
-
The NPVs of NBI for the characterisation of diminutive polyps in the rectosigmoid colon (made with any level of confidence) ranged from 87% to 98% and was > 90% in four out of the five studies that reported this outcome54,55,63,68 (but the lower limit of the 95% CI remained > 90% in only three studies54,55,68). When limited to high-confidence characterisations in the rectosigmoid colon (five studies54,55,61–63), the NPVs ranged from 92% to 99%, but the lower limit of the 95% CI fell below 90% in two studies. 62,63
-
Accuracy (the proportion of correctly classified polyps) of polyp characterisations in the whole colon was ≥ 90% in five studies and between 76% and 89% in 10 studies (16 studies reported this outcome55,56,58,62–71,75,77,78). High-confidence characterisations typically increased accuracy by 3–5% in studies reporting both overall and high-confidence data (eight studies55,56,58,62–65,77).
-
Agreement between the surveillance interval allocated using a NBI-based strategy, and using the results of histopathology, was > 90% in 11 of the 13 studies that reported this outcome. 55,57,58,61–65,67,68,70 When there was a discrepancy in surveillance intervals, the NBI-containing strategy more often led to an earlier recall for colonoscopy than would have occurred with the histopathology-based surveillance interval.
-
No outcome data were reported (interpretability of the test, test acceptability, number of outpatients appointments, HRQoL, incidence of colorectal cancer or mortality) or sparse outcome data (interobserver agreement, adverse events, polyps designated as ‘left in place’, polyps designated resect and discard, time taken to perform colonoscopy) were reported for other outcomes of interest to this review.
i-scan
-
Five studies77,79–82 provided sensitivity and specificity outcomes for the characterisation of diminutive polyps as adenomas or hyperplastic polyps using i-scan. Often only a single study provided data for a particular combination of the region of the colon and the level of confidence assigned to the polyp characterisation.
-
In the whole colon, or in regions of the colon, characterisations of diminutive polyps made with any level of confidence ranged in sensitivity from 0.82 to 0.95 and in specificity from 0.83 to 0.96. It was not possible to meta-analyse any of these results. For high-confidence characterisations in the whole colon, or in regions of the colon, sensitivity ranged from 94% to 98% and specificity from 90% to 95%. The only meta-analysis possible, which was conducted to inform the economic model, was for high-confidence characterisations of diminutive polyps in the whole colon. The summary value for sensitivity was 0.96 (95% CI 0.92 to 0.98) and for specificity was 0.91 (95% CI 0.84 to 0.95).
-
NPVs were > 90% (all five studies77,79–82); however, the lower limit of the 95% CI was > 90% in only one study. 79
-
Accuracy was ≥ 90% (all five studies) and higher for high-confidence polyp characterisations in the two studies that also reported accuracy for all polyp characterisations. 79,82
-
Surveillance interval agreement (two studies79,82) determined by i-scan and histopathology was > 90%. When surveillance intervals differed, longer intervals were more likely to be set with i-scan than histopathology.
-
No outcome data were reported (interpretability of the test, test acceptability, polyps designated as ‘left in place’, polyps designated resect and discard, number of outpatients appointments, HRQoL, incidence of colorectal cancer or mortality) or sparse outcome data (interobserver agreement, adverse events, time taken to perform colonoscopy) were reported for other outcomes of interest to this review.
Flexible Spectral Imaging Colour Enhancement
-
Three studies78,83,84 provided sensitivity and specificity, with all reporting on characterisations of diminutive polyps made with any level of confidence in the whole colon. Reported values for sensitivity range from 74% to 88% and for specificity from 82% to 88%.
-
None of the studies provided evidence on the high-confidence characterisation of diminutive polyps or restricted their analysis to a part of the colon (e.g. the rectosigmoid colon).
-
It was possible to run a bivariate meta-analysis that produced a summary estimate for sensitivity of 0.81 (95% CI 0.73 to 0.88) and for specificity of 0.85 (95% CI 0.79 to 0.90).
-
The NPVs of FICE (three studies78,83,84) ranged from 70% to 84%.
-
The accuracy of FICE (three studies78,83,84) ranged from 80% to 85%.
-
Surveillance interval agreement between FICE and histopathology was 100% (one study83) or 97% (one study84). When there was disagreement it was not reported whether the FICE-based strategy led to a longer or a shorter surveillance interval being set.
-
None of the other outcomes of interest to this review was reported.
Pooled analysis of virtual chromoendoscopy technologies
-
A pooled analysis of high-confidence decisions characterising diminutive polyps in the whole colon (NBI, 11 studies; and i-scan, two studies) was undertaken to inform a scenario analysis using the economic model. 54–57,59–62,64,65,77,79 This produced a pooled summary estimate for sensitivity of 0.92 (95% CI 0.87 to 0.95) and for specificity of 0.83 (95% CI 0.78 to 0.87).
Head-to-head comparisons
-
Head-to-head comparisons of the technologies were not within the scope for this assessment, but two of the included studies compared two technologies against each other. For the real-time histopathological prediction of diminutive colonic polyps, no statistically significant differences were found when a single endoscopist with experience of NBI and i-scan compared these technologies in a prospective cohort study. 77 A RCT conducted by endoscopists without experience of either NBI or FICE78 found no statistically significant difference in accuracy or sensitivity, but a statistically significant difference in specificity.
Table 24 provides a summary of the pooled sensitivity and specificity values from our bivariate meta-analysis, when available.
| Type of characterisation | Diminutive polyp location | Technology | |||||
|---|---|---|---|---|---|---|---|
| NBI | i-scan | FICE | |||||
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | ||
| All characterisationsa | Whole colon | 0.88 (0.83 to 0.92); 16 studies | 0.81 (0.75 to 0.85); 16 studies | 0.95 (0.87 to 0.99); single study | 0.86 (0.76 to 0.94); single study | 0.81 (0.73 to 0.88); 3 studies | 0.85 (0.79 to 0.90); 3 studies |
| High-confidence characterisations | 0.91 (0.85 to 0.95); 11 studies | 0.82 (0.76 to 0.87); 11 studies | 0.96 (0.92 to 0.98);b 2 studies | 0.91 (0.84 to 0.95);b 2 studies | No evidence | No evidence | |
| High-confidence characterisations by endoscopists with prior experience of the technologyc | 0.92 (0.89 to 0.94); 4 studies | 0.82 (0.72 to 0.89); 4 studies | 0.96 (0.92 to 0.98);b 2 studies | 0.91 (0.84 to 0.95);b 2 studies | No evidence | No evidence | |
| All characterisationsa | Rectosigmoid colon | 0.85 (0.75 to 0.91); 3 studies | 0.87 (0.74 to 0.94); 3 studies | Meta-analysis not possible; 2 studies | Meta-analysis not possible; 2 studies | No evidence | No evidence |
| High-confidence characterisations | 0.87 (0.80 to 0.92); 4 studies | 0.95 (0.87 to 0.98); 4 studies | 0.96 (0.80 to 1.00); single study | 0.96 (0.83 to 0.99); single study | No evidence | No evidence | |
| High-confidence characterisations by endoscopists with prior experience of the technologyc | 0.90 (0.71 to 0.97); 2 studies | 0.98 (0.91 to 1.00); 2 studies | No evidence | No evidence | No evidence | No evidence | |
| Pooled analysis of VCE technologies | |||||||
| Sensitivity (95% CI) | Specificity (95% CI) | ||||||
| High-confidence characterisationsc | Whole colon | 0.92 (0.87 to 0.95); 11 NBI studies and two i-scan studies | 0.83 (0.78 to 0.87); 11 NBI studies and two i-scan studies | ||||
Ongoing studies
We identified 19 potentially relevant ongoing studies on the use of NBI, i-scan or FICE to characterise diminutive colorectal polyps. Two were identified from searches of clinical trials databases (see Chapter 3, Identification of studies for details of these searches) and 17 were identified from conference abstracts found by the clinical effectiveness searches. Until further details are available it is not clear whether or not all would meet the eligibility criteria for this review, but they have the potential to do so. These studies are listed in Appendix 5.
Chapter 5 Economic analysis
This chapter consists of a systematic review of published cost-effectiveness analyses of VCE compared with histopathology and a de novo economic evaluation.
Systematic review of existing cost-effectiveness evidence
This section describes the systematic review of published cost-effectiveness analyses of VCE. The aim of the systematic review was to inform the development of the independent economic evaluation. The same search strategy that was used to identify diagnostic test studies was used to identify cost-effectiveness studies, as described in Chapter 3. Once the results of this search had been downloaded into our EndNote (X7.0.2, Thomson Reuters, CA, USA) bibliographic database, we searched for a subset of relevant cost-effectiveness studies using the keyword ‘cost’ in any field. (Note that the search strategy for our systematic review of diagnostic accuracy did use a study design filter, therefore it would not have excluded any relevant health economic studies.) Titles and abstracts were then screened by two health economists for relevance in accordance with the inclusion criteria. The inclusion criteria were for a full economic evaluation (cost-effectiveness, cost–utility, cost–benefit or cost–consequence analysis) that compared VCE with conventional (white light) colonoscopy for adults undergoing a colonoscopy for detection of colorectal polyps, that included long-term outcomes (such as life-years, incidence of colorectal cancer or QALYs). Full texts of references deemed relevant were then retrieved for further screening. The full texts of retrieved references were screened to identify those that met the inclusion criteria. Data from the included studies were extracted and evaluated for their quality and generalisability to the UK, based on criteria developed by Drummond and Jefferson. 111 The studies identified are described in more detail in the following section.
A total of 236 potentially relevant references from our database underwent title and abstract screening. Of these, the full-text versions (when available) of 10 references were retrieved for screening, and two of these met the inclusion criteria (Figure 29). 112,113 The characteristics of these studies are given in Table 25. Of the eight texts not included, four were abstracts with insufficient detail51,114–116 and four did not include long-term outcomes in their analysis67,70,84,117 (see Appendix 6). The full data extraction forms for both of the included studies are shown in Appendix 7.
FIGURE 29.
Flow chart of identification of studies for inclusion in the review of cost-effectiveness.
| Characteristic | Study | |
|---|---|---|
| Hassan et al.112 | Kessler et al.113 | |
| Publication year | 2010 | 2011 |
| Country | USA | USA |
| Funding source | Funding source not reported | National Institutes of Health grant |
| Study type | Cost-effectiveness analysis | Cost-effectiveness analysis |
| Perspective | Societal | Not stated (assumed to be payer) |
| Study population | Hypothetical cohort of 100,000 50-year-old people in the USA who underwent a colonoscopy for CRC screening | Patients receiving a colonoscopy at a single-institution tertiary centre who had at least one polyp removed during colonoscopy, irrespective of indication. Population characteristics taken from a database of 10,060 consecutive colonoscopies from 1999 to 2004 |
| Intervention(s) | NBI vs. colonoscopy vs. no screening | No pathological examination of diminutive polyps (resect and discard) vs. submitting all polyps for pathological examination (submit all) |
| Intervention effect | Feasibility of 84% for rate of high confidence in differentiating between hyperplastic and adenomatous diminutive polyps by using NBI without magnification. Sensitivity was 94% and specificity was 89% | Endoscopic sensitivity for non-adenoma: 90% |
| Endoscopic sensitivity for adenoma: 90% | ||
| Proportion of diminutive polyps with advanced histopathology: 0.6% | ||
| Pathology sensitivity for large adenoma: 100% | ||
| Pathology sensitivity for diminutive and small adenoma: 95% | ||
| Pathology sensitivity for non-adenoma: 100% | ||
| Currency base | US dollars | US dollars |
| Model type, health states | Markov model with health states for no colorectal neoplasia; diminutive (≤ 5 mm), small (6–9 mm) or large (≥ 10 mm) adenomatous polyps; localised, regional or distant CRC; and CRC-related death | Decision tree model |
| Time horizon | Lifetime horizon | Lifetime horizon |
| Base-case results | Compared with standard colonoscopy, colonoscopy with NBI was US$25 cheaper per person with no difference in life expectancy | The net cost saving from forgoing histopathological assessment was US$174.01. The expected increased benefit of the ‘submit-all’ strategy was 0.17 days of life and the cost-effectiveness of the ‘submit-all’ strategy compared with the resect and discard strategy was US$377,460 per life-year gained |
| The number needed to harm because of perforation, major bleed or missed cancer was 7979 (i.e. an absolute risk of 0.0125%) | ||
Critical appraisal of the studies
The Assessment Group’s critical appraisal of the identified studies by Hassan and colleagues112 and Kessler and colleagues113 is summarised in Table 26. Both studies report their methodology, assumptions and parameters clearly. Neither study included QALYs in their analysis and Kessler and colleagues113 did not include discounting. Hassan and colleagues112 did not present an incremental analysis, although it is possible to calculate this with the information provided.
| Item | Study | |
|---|---|---|
| Hassan et al.112 | Kessler et al.113 | |
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Yes | Yes |
| 2. Is the setting comparable to the UK? | Yes | Yes |
| 3. Is the analytical and modelling methodology appropriate? | Yes | Yes |
| 4. Are all the relevant costs and consequences for each alternative identified? | Yes | Yes |
| 5. Are the data inputs for the model described and justified? | Yes | Yes |
| 6. Are health outcomes measured in QALYs? | No | No |
| 7. Is the time horizon considered appropriate? | Yes | Yes |
| 8. Are costs and outcomes discounted? | Yesa | No |
| 9. Is an incremental analysis performed? | ?b | Yes |
| 10. Is uncertainty assessed? | Yes | Yes |
Hassan and colleagues
Hassan and colleagues112 developed a cost-effectiveness model to calculate the potential savings and drawbacks of a resect and discard approach using NBI in a simulated colorectal cancer screening cohort. In the resect and discard approach, diminutive colorectal lesions (≤ 5 mm in size) classified by endoscopy with high confidence were not analysed by a pathologist. A Markov model was constructed with health states for no colorectal neoplasia, diminutive (≤ 5 mm), small (6–9 mm) or large (≥ 10 mm) adenomatous polyps; localised, regional or distant colorectal cancer; and colorectal cancer-related death. The resect and discard policy was instituted for all the cases in which a high-confidence diagnosis was achieved by NBI. All diminutive polyps in which a high-confidence diagnosis was not possible were removed and sent for formal histopathological evaluation. The model assumed a screening strategy of colonoscopy every 10 years. After colonoscopy, patients received follow-up surveillance based on the size and classification of the polyp(s).
Feasibility and accuracy of NBI without optical magnification in differentiating between diminutive adenomas and hyperplastic polyps were derived from three published series. 64,70,73 Feasibility was defined as the rate of high confidence in differentiating between polyps. An 84% feasibility was assumed. The sensitivity and specificity for adenomas was 94% and 89%, respectively.
Costs were derived from Medicare reimbursement rates. No incremental cost for NBI was included because it was stated to be a standard feature in current-generation colonoscopes. The cost of colonoscopy was US$630, the cost of colonoscopy with polypectomy was US$925 and of pathological examination was US$102. Costs were also included for colorectal cancer treatment and adverse event costs, such as perforation and bleeding. Costs and life-years were discounted at 3% per annum.
The discounted cost for the no-screening strategy was US$3390 per person over their lifetime (Table 27). The colonoscopy screening strategy reduced costs by US$168 per person and the colonoscopy with resect and discard strategy reduced costs by a further US$25 per person. Colonoscopy with or without resect and discard improved life expectancy by an average of 51 days per person compared with no screening. The study also extrapolated the results to the US population.
| Cost and efficacy | Strategy | ||
|---|---|---|---|
| No screening | Colonoscopy | Colonoscopy with resect and discard | |
| Cost/person (US$) | 3390 | 3222 | 3197 |
| Relative efficacy | – | 51 days/person | 51 days/person |
Kessler and colleagues
Kessler and colleagues113 developed a decision tree model to quantify the expected costs and outcomes of removing diminutive polyps with or without subsequent pathological assessment. They compared two strategies: ‘submit all’ diminutive polyps (≤ 5 mm in size) to pathological examination and no pathological examination of diminutive polyps (resect and discard). All other polyps were submitted for pathological examination for both strategies.
The decision model was populated with polyp frequencies based on a database of 10,060 consecutive patients who underwent colonoscopy for screening, surveillance or diagnostic indications. The decision model evaluated the frequency with which the surveillance follow-up (based on the most advanced polyp) matched that of the actual follow-up interval for the two strategies. Patients in the endoscopy database were distributed among four groups based on the characteristics that form the basis for follow-up. Group 1 comprised people who had only one diminutive polyp. Group 2 comprised those with two polyps, at least one of which was diminutive and the other not a large adenoma (≥ 10 mm in size). Patients in group 3 people had at least three polyps, at least one of which was diminutive and the others were not large adenomas, while those in group 4 had at least one diminutive polyp, as well as one or more large adenoma(s) and could have any number of additional polyps. For each of the four groups, each patient’s most advanced polyp type was either an advanced adenoma, a non-advanced adenoma or a non-adenoma.
The sensitivity and specificity of endoscopic and pathological assessment were based on the published literature. Costs were included for pathology, colonoscopy and colorectal cancer treatment. The cost of sending a polyp to pathology was US$103.87. Costs of colonoscopy, colonoscopic perforation and cancer treatment were obtained from the literature. The colonoscopy costs were US$1329 for diagnostic and US$2038 for therapeutic colonoscopies. The downstream costs and outcomes after the colonoscopy were obtained from a published discrete event simulation model of colorectal cancer screening and surveillance intervals. 118 Discounting was not included in the model.
The submit-all strategy resulted in an incorrect surveillance interval 1.9% of the time, whereas the resect and discard strategy did so 11.8% of the time, with over half of the patients having only non-adenomatous polyps but scheduled for a 5-year, rather than a 10-year, surveillance examination. The cost saving from forgoing pathological assessment was US$210 per colonoscopy when diminutive polyps were removed, while the additional cost as a result of the incorrect surveillance interval was US$35.92. The net saving was US$174.01. The number needed to harm because of perforation, major bleed or missed cancer was 7979 (i.e. an absolute risk of 0.0125%).
The expected additional benefit of the submit-all strategy was 0.17 days of life over the lifetime horizon and the incremental cost-effectiveness ratio (ICER) of the submit-all strategy compared with the resect and discard strategy was US$377,460 per life-year gained.
Deterministic sensitivity analyses were conducted for the accuracy of the colonoscopy to detect adenomas and the proportion of diminutive polyps with advanced histopathology. The sensitivity analyses performed indicate that the error rate in assigning post-polypectomy surveillance intervals was most sensitive to the accuracy of endoscopic assessment of histopathology and to the proportion of diminutive polyps with advanced histopathology.
The authors concluded that endoscopic diagnosis of polyp histopathology during colonoscopy and forgoing pathological examination would result in substantial upfront cost savings. Downstream consequences of the resulting incorrect surveillance intervals appear to be negligible.
Summary of published economic evaluations
The cost-effectiveness review of published economic evaluation for VCE technologies found two relevant studies that were both published in the USA. 112,113 The patient population differed between the two studies: Hassan and colleagues112 simulated a screening population (i.e. included patients who had no polyps identified by the colonoscopy) and Kessler and colleagues’ population113 had at least one diminutive polyp identified. Both studies compared a resect and discard strategy with a ‘submit-all’ (to histopathology) strategy to the whole colon, although Kessler and colleagues113 assumed that the resect and discard strategy would be used for all polyps, whereas Hassan and colleagues113 assumed that it would not be feasible to resect and discard some polyps (i.e. those characterised with low confidence). Neither studies used surveillance intervals for follow-up screening that correspond to those used in the UK.
The model structure differed between the two studies: Hassan and colleagues113 used a Markov model and Kessler and colleagues113 used a decision tree model. We consider that both approaches are appropriate. The cost saved per person varied between US$25112 and US$174 over the patient’s lifetime. 113 Kessler considered the expected benefit of histopathology to be 0.17 days of life, whereas Hassan assumed that there was no difference in life expectancy between groups over the patient’s lifetime. The cost-effectiveness of the submit-all strategy compared with resect and discard was US$377,460 per life-year gained for Kessler and colleagues,113 whereas Hassan and colleagues112 were not able to calculate a value as there was no difference in the life expectancy between the submit-all and resect and discard strategies. It is unclear how generalisable these results are to the UK NHS, as they used non-UK resource costs and did not include QALYs.
Review of information provided by Olympus to the National Institute for Health and Care Excellence: economic evaluation
A budget impact model was supplied as part of the information provided by Olympus to the National Institute for Health and Care Excellence and the Assessment Group. The model has also recently been published by Solon and colleagues. 117 This study did not meet our inclusion criteria for cost-effectiveness models of VCE because it did not include long-term health outcomes. However, we have provided a critical review of it as a supplement to our systematic review of cost-effectiveness studies, as it has some relevance to the decision problem in this assessment.
Modelling approach
The analysis is a cost–consequence and budget impact model that follows cohorts of UK patients who attend colorectal cancer screening. The population includes patients identified through the national screening programme, as well as those attending for colonoscopic surveillance. The analysis is conducted from the perspective of the NHS in England. The model has a time horizon of 7 years and in each year there is a new incident cohort of patients who undergo an endoscopy. The model includes a discount rate of 3.5% per year for costs and health outcomes. The model has a starting population of 550,925 attending an endoscopy test per year, to reflect the number of procedures conducted in 2012, and assumes an annual increase of 20% in the population expected to attend endoscopy each year. It was assumed that 82% of the installed endoscopy systems in England were manufactured by Olympus.
After undergoing endoscopy, patients are classified in three outcomes according to the number and size of polyps identified (no polyps; one of more polyps ≤ 5 mm in size, but no polyps > 5 mm in size; and one or more polyps ≥ 5 mm in size). For WLE, all polyps are resected and sent for histopathological examination. With NBI, for polyps ≤ 5 mm in size, the diagnosis of a proportion of polyps is assumed to be predicted with low confidence and they are sent for histopathological examination, while polyps will be left in situ if there is high confidence that they are non-neoplastic, otherwise they will be resected and discarded. Where polyps are resected, there is a risk of adverse events of bleeding and bowel perforation. The model calculates the number of TNs, FNs, TPs and FPs, and the number of histopathological examinations, resects and adverse events for each cohort in each year.
Critical appraisal of the model
The Assessment Group’s critical appraisal of the Olympus economic model is summarised in Table 28. In general, the model is well reported, although some aspects were reported in the economic model provided by Olympus (see Appendix 8) rather than in Solon and colleagues. 117 The time horizon is 7 years but consists of 7-yearly cohorts and no longer-term outcomes, such as QALYs, were modelled.
| Item | Response |
|---|---|
| 1. Is there a clear statement of the decision problem? | Yes |
| 2. Is the comparator routinely used in UK NHS? | Yes |
| 3. Is the patient group in the study similar to those of interest in UK NHS? | Yes |
| 4. Is the health-care system comparable to UK? | Yes |
| 5. Is the setting comparable to the UK? | Yes |
| 6. Is the perspective of the model clearly stated? | Yes |
| 7. Is the study type appropriate? | Yes |
| 8. Is the modelling methodology appropriate? | Unclear |
| 9. Is the model structure described and does it reflect the disease process? | Yes |
| 10. Are assumptions about model structure listed and justified? | Yes |
| 11. Are the data inputs for the model described and justified? | Yes |
| 12. Is the effectiveness of the intervention established based on a systematic review? | Yes |
| 13. Are health benefits measured in QALYs? | No |
| 14. Are health benefits measured using a standardised and validated generic instrument? | No |
| 15. Are the resource costs described and justified? | Yes |
| 16. Have the costs and outcomes been discounted? | Yes |
| 17. Has uncertainty been assessed? | Yes |
| 18. Has the model been validated? | No |
Clinical effectiveness
The model parameters for the diagnostic accuracy of NBI, the feasibility of diagnosing diminutive polyps and adverse events were derived from a systematic literature review and are shown in Table 29.
| Parameter | Value (%) | Source |
|---|---|---|
| Patients with no polyps | 44 | Rastogi et al.120 |
| Patients with polyps ≤ 5 mm in size | 38 | Rastogi et al.120 |
| Patients with polyps > 5 mm in size | 18 | Rastogi et al.120 |
| Polyps that are adenomatous ≤ 5 mm in size | 17 | Butterly et al.121 |
| Polyps that are adenomatous > 5 mm in size | 10.1 | Butterly et al.121 |
| Diminutive polyp optical diagnosis feasibility rate | 75 | Kaltenbach et al.57 |
| Optical diagnosis sensitivity NBI | 93 | McGill et al.43 |
| Optical diagnosis specificity NBI | 83 | McGill et al.43 |
| Probability of hospitalisation for bleeding with polypectomy | 0.43 | Whyte et al.122 |
| Probability of perforation with polypectomy | 0.28 | Whyte et al.122 |
Estimation of costs
The model includes the costs incurred by the NHS, including equipment, maintenance, training, consumables, staff, endoscopy and histopathological examination costs, and hospital costs for managing adverse events. Unit costs of resources were taken from a variety of sources including NHS reference costs,123 Personal Social Services Research Unit (PSSRU)124 and the company’s own prices. The costs used in the model are shown in Table 30.
| Input parameter | Value | Source |
|---|---|---|
| Unit cost per system, NBI (£) | 40,395 | Olympus list price117 |
| Unit cost per scope, NBI (£) | 38,660 | Olympus list price117 |
| Training cost per year, NBI (£) | 2272 | Olympus list price117 |
| Maintenance cost of NBI system (£) | 3525 | Olympus list price117 |
| Maintenance cost of NBI scopes (£) | 4805 | Olympus list price117 |
| NHS tariff for colonoscopy: with biopsy (£) | 522 | Monitor 2014: HRG tariff FZ51Z123 |
| NHS tariff for colonoscopy: without biopsy (£) | 437 | Monitor 2014: HRG tariff FZ52Z123 |
| Cost per biopsy (£) | 82 | Unpublished data obtained from University College London Hospitals (2012), Plymouth Hospital NHS Trust (2014) and South Devon Healthcare NHS Foundation Trust (2012)117 |
| Number of biopsies per examination | 1.35 | Assumption based on data reported in Lee et al., 2012125 |
| Cost per hospital bleed (£) | 318 | Monitor 2015–16: HRG tariff FZ38F126 |
| Cost per perforation event (£) | 2211 | Monitor 2015–16: HRG tariff GB01B126 |
| Unit cost per hour for administration and support (£) | 23 | PSSRU’s Unit Costs of Health and Social Care 2014127 |
| Hours per test for administration and support | 0.30 | Modified from assumptions reported in Sharara et al., 2008128 |
| Unit cost per hour of nurse non-contact time (£) | 41 | PSSRU’s Unit Costs of Health and Social Care 2014127 |
| Hours per test for nurse non-contact time | 0.42 | Modified from assumptions reported in Sharara et al., 2008128 |
| Unit cost per hour of consultant time (£) | 142 | PSSRU’s Unit Costs of Health and Social Care 2014127 |
| Hours with consultant, excluding procedure | 0.50 | Modified from assumptions reported in Sharara et al., 2008128 |
| Length of procedure time in hours with NBI | 0.30 | Bisschops et al., 2012129 |
| Length of procedure time in hours with comparator | 0.30 | This input varies where options are selected |
| Unit cost per hour of nurse contact time (£) | 100 | PSSRU’s Unit Costs of Health and Social Care 2014127 |
| Snares: cost per pack (£) | 240 | Olympus list price117 |
| Snares: number per pack | 20 | Market data provided by Olympus117 |
| Forceps: cost per pack (£) | 210 | Olympus list price117 |
| Forceps: number per pack | 10 | Market data provided by Olympus117 |
The company’s list price for the NBI system is £40,395. The model assumes that at the start of the first year 82% of hospitals currently use Olympus systems, of which 95% are capable of NBI (i.e. 78% of hospitals use NBI). Of those hospitals with Olympus equipment, 50% that do not have NBI-capable systems will upgrade in year 1 and a similar number in each subsequent year. Of those hospitals with Olympus equipment, 50% have NBI-capable endoscopes in place in the first year. Of those hospitals with Olympus equipment that do not have NBI-capable endoscopes, 14% will upgrade in year 1 and a similar number will upgrade in each subsequent year. For NBI, 2 training days per endoscopist per year are required, whereas no additional training is required for WLE.
Staff costs for colonoscopy include costs for administration, nurse and consultant contact time and are based on a microcosting study of a Canadian hospital. 128 The consumables for biopsy are snares and forceps. The Assessment Group notes that consumables and staff costs would normally be included within the NHS reference costs and do not therefore need to be included separately.
Results
The results for the outcomes from the model are shown in Table 31. Over 7 years NBI reduced the incidence of colonoscopy-related adverse events by 32% and the frequency of histopathological examination by 39%.
| Outcome | VCE technology (number of people tested) | % change | |
|---|---|---|---|
| NBI | WLE | ||
| TN | 5,713,178 | 5,933,416 | –3.71 |
| FN | 1596 | – | n/a |
| TP | 148,296 | 149,893 | –1.07 |
| FP | 220,238 | – | n/a |
| Histopathological examination | 2,065,058 | 3,406,653 | –39.38 |
| Adverse event | 16,376 | 24,187 | –32.29 |
The cost over 7 years for NBI is predicted to be £3112M and for WLE is £3253M (i.e. a saving of £141M).
Deterministic sensitivity analyses were included in the model by varying the model parameters by ± 10%. The sensitivity analysis shows the effect of the parameters on the total difference in costs between NBI and WLE. The costs of colonoscopy with and without biopsy have the greatest impact on model results. The study also conducted an analysis reducing the cost of biopsy, which showed there was still a net cost saving with NBI, even when the biopsy cost was reduced to zero.
Independent economic evaluation
As described in Chapter 2, the decision problem for this diagnostic assessment is to assess the cost-effectiveness of real-time optical assessment of diminutive colorectal polyps in the English NHS.
We therefore conducted an economic evaluation to evaluate costs and outcomes of VCE. The economic evaluation takes the form of a cost–utility model informed by the systematic review of cost-effectiveness studies, the economic evaluation by Olympus, targeted literature searches and, where necessary, expert opinion. The economic evaluation uses the diagnostic accuracy for VCE from the meta-analyses reported in Chapter 4.
Methods for economic analysis
The decision problem
The patient population in our base-case analysis is people referred to colonoscopy after participating in a Bowel Cancer Screening Programme (referred to as the screening population). We included in scenario analyses two other patient populations of relevance to the decision problem for this assessment: people offered colonoscopic surveillance because they had previously had adenomas removed (surveillance population) and people referred for colonoscopy by a GP because of symptoms suggestive of colorectal cancer (symptomatic population).
For the purposes of the economic analysis, we include only patients with at least one diminutive polyp and exclude patients with one or more non-diminutive polyp. The scope for this assessment excludes the use of VCE for real-time assessment of non-diminutive polyps (> 5 mm in size), though VCE might be considered for use in the assessment of diminutive polyps in patients who also have non-diminutive polyps. In practice, patients do have a mixture of polyps of different sizes. Although most polyps are diminutive, patients are assigned to surveillance intervals according to their most advanced polyp. However, we could not identify data on the mix of different sized polyps in patients or how they affect the allocation to surveillance interval. In addition, all data in the model on adenoma and cancer risk are based on data that averages risk across adenoma sizes.
Furthermore, the model does not differentiate between the type of polyp, such as depressed polyps or sessile serrated polyps. Sessile serrated polyps are relatively uncommon and no diagnostic accuracy data were available for diminutive sessile serrated polyps from our systematic review of diagnostic studies (see Chapter 4).
For the base-case analysis in our economic evaluation, we compare strategies using VCE technologies (NBI, i-scan and FICE) with a histopathology assessment strategy. For the comparator histopathology strategy, we assume that all polyps are resected and sent to histopathology, and that subsequent screening and surveillance invitations are based on the histopathology results, which are assumed to be 100% accurate.
We refer to the VCE strategy used in our base-case analysis as the VCE strategy; it has the following characteristics:
-
Diminutive polyps in the whole colon are optically characterised using VCE.
-
Diminutive polyps characterised with high confidence as adenomas are resected and discarded.
-
Diminutive polyps characterised with high confidence as hyperplastic are left in situ.
-
Any polyps that cannot be characterised with high confidence are resected and sent to histopathology.
The VCE strategy is based on the DISCARD strategy described in Ignjatovic and colleagues70 and then subsequently adapted in the two economic models identified by our systematic review of economic evaluations. 112,113 Ignjatovic and colleagues’ study70 was one of the first to evaluate a resect and discard strategy, and they proposed that polyps < 10 mm in size should be characterised and, if appropriate, be discarded or left in situ without histopathology. Subsequent studies and guidance have modified the DISCARD strategy to apply to only diminutive polyps (≤ 5 mm in size). The National Institute for Health and Care Excellence scope, ESGE guidelines,31 both economic evaluations identified through our systematic review, and our model limit the VCE strategy to diminutive polyps.
Our VCE strategy does differ from the DISCARD strategy in the way that hyperplastic polyps are dealt with in the proximal colon (see Figure 3). In the base-case analysis, the model does not differentiate between diminutive hyperplastic polyps found in the rectosigmoid colon and those found in other parts of the colon, because the best-available diagnostic data from our systematic review were based on polyps in the whole colon. However, we have conducted scenario analyses (see Sensitivity analyses) using what we refer to as the DISCARD strategy, which has the following characteristics:
-
Any polyp assessed with low confidence is resected and sent to histopathology.
-
Diminutive polyps in the whole colon characterised with high confidence as adenomas are resected and discarded.
-
Diminutive polyps in the proximal colon characterised with high confidence as hyperplastic are resected and discarded.
-
Diminutive polyps in the rectosigmoid colon characterised with high confidence as hyperplastic are left in situ.
We assessed each of the VCE-based strategies (VCE and DISCARD) for each of the three technologies (NBI, i-scan and FICE). In addition, we conducted a scenario analysis using the post hoc pooled meta-analysis sensitivity and specificity estimates for the VCE technologies [see Chapter 4, Assessment of diagnostic accuracy (sensitivity, specificity, negative predictive value, accuracy)].
Following colonoscopy and receipt of histopathology results, patients are assigned a surveillance interval based on their estimated level of risk (Figure 30). The risk classification of patients used corresponds to British guidelines30 for determining surveillance intervals following identification of exclusively diminutive adenomas at colonoscopy: low risk (zero to two adenomas), intermediate risk (three or four adenomas) and high risk (five or more adenomas).
FIGURE 30.
NHS Bowel Cancer Screening Pathway (with endoscopy policies). Figure adapted under Open Government Licence v3.0 from Public Health Functions to be exercised by NHS England: Service Specification No. 26, Bowel Cancer Screening Programme. 130

There are four main implications of using a VCE strategy (VCE or DISCARD) rather than the histopathology strategy:
-
Initial costs: most hospitals already have equipment capable of VCE. There would be additional training costs for endoscopists to use this technology, but, conversely, the cost of polypectomies and histopathology tests would be reduced. Thus, the net effect on the cost of initial diagnosis and management (colonoscopy, polypectomy and histopathology) may be positive or negative.
-
Hyperplastic polyps resected: the number of hyperplastic polyps unnecessarily resected and hence the numbers of polypectomy-related adverse events, such as bleeding and bowel perforation, will be reduced. Some hyperplastic polyps will still be resected, because they are not assessed with high confidence or are mischaracterised as adenomas (FPs). Adverse events are associated with a mortality risk, reduced quality of life and costs to the health service.
-
Missed adenomas: some polyps, however, will be mischaracterised as hyperplastic when they are adenomas (FNs). Such errors will mean that some adenomas will be left in situ, leading to a small increase in the incidence of colorectal cancer, with associated QALY loss and health-care costs.
-
Incorrect follow-up: some patients may be assigned to the wrong follow-up interval (according to the Bowel Cancer Screening Pathway guidelines; see Figure 30): either too long an interval if one or more adenomas are missed (FNs) or too short an interval if one or more hyperplastic polyps are characterised as adenomas (FPs). In general, a shorter follow-up interval will be beneficial for the patient because of the reduced risk or earlier detection of cancer. However, for patients at very low risk of colorectal cancer, the potential harm from polypectomy-related adverse events could offset these benefits. The incremental cost to the health service of a shorter follow-up interval may, in principle, be positive or negative, as increased costs of screening or surveillance may, to some extent, be offset by cost savings from avoided cancer treatment.
The model estimates the proportion of patients likely to experience these various risks, and hence the expected costs and QALYs associated with the alternative colonoscopy strategies.
Model structure
The model consists of a decision tree for patients undergoing colonoscopy. The tree estimates the short-term costs and outcomes for the defined population under each decision strategy, from the time when patients are identified as potential candidates for use of VCE, up to the time when any polyps identified as adenomas have been removed and patients have been assigned to a follow-up policy. Long-term costs and QALY outcomes at the end points of the decision tree were estimated from an existing model, that is, the School of Health and Related Research’s bowel cancer screening (SBCS) model, developed by Whyte and colleagues. 122 We chose to use the SBCS model, rather than to develop a new one, because it is a long-standing model that has been validated, and which was used to inform the introduction of the National Bowel Cancer Screening Programme. The SBCS model was adapted for this current assessment, with updated parameters where possible. It was run independently, and the SBCS cost and QALY estimates for various subgroups of patients were entered as parameters at the end points of the decision tree model. The structures and assumptions of the decision tree and SCBS models are described below. Input parameters for both models are then discussed in Model parameters.
The decision tree
The decision tree model compares the VCE strategies (VCE with each of the technologies NBI, i-scan and FICE in the base case) with a histopathology strategy for a cohort of patients (the screening population in the base case). The model adopts a lifetime horizon and a NHS and Personal and Social Services perspective.
Patients enter the model at colonoscopy, having had at least one diminutive polyp, and no non-diminutive polyps, identified. The cohort is divided into four risk categories, based on the number of adenomas that they have:
-
no adenomas
-
low risk: one or two adenomas
-
intermediate risk: three or four adenomas
-
high risk: five or more adenomas.
The model then calculates the proportion of patients in each category expected to have the correct diagnosis and treatment, and the proportions expected to be diagnosed and treated incorrectly. There are essentially three types of error that can occur: patients might have one or more hyperplastic polyp misclassified as an adenoma and unnecessarily resected; they may have one or more adenoma misclassified as a hyperplastic polyp and left in situ; and/or they may be assigned to an incorrect surveillance interval – either too long or too short. The resulting permutations of diagnostic outcomes for patients are illustrated in Figure 31. It can be seen that there are six main patient outcomes, which are also defined in Table 32.
FIGURE 31.
Decision tree showing diagnostic outcomes for patient. CD, correct diagnosis; HPRC, hyperplastic polyp(s) resected correct surveillance; HPRI, hyperplastic polyp(s) resected incorrect surveillance; MAHPR, missed adenoma(s) and hyperplastic polyp(s) resected; MAI, missed adenoma(s) incorrect surveillance; Phe, probability that the patient is in the higher end of the risk classification; PHPR, probability of hyperplastic polyp being resected; Ple, probability that the patient is in the lower end of the risk classification; PMA, probability of missed adenoma.

| Patient outcome | Interpretation | Surveillance interval assigned | |
|---|---|---|---|
| ID | Description | ||
| CD | Correct diagnosis | All polyps correctly classified (as either adenomas or hyperplastic polyps) | Correct |
| MAC | Missed adenoma(s) correct surveillance | One or more adenomas identified incorrectly as hyperplastic polyps and left in situ | Correct |
| MAI | Missed adenoma(s) incorrect surveillance | One or more adenomas identified incorrectly as hyperplastic polyps and left in situ | Incorrect: too long |
| HPRC | Hyperplastic polyp(s) resected correct surveillance | One or more hyperplastic polyps identified incorrectly as adenomas and resected | Correct |
| HPRI | Hyperplastic polyp(s) resected incorrect surveillance | One or more hyperplastic polyps identified incorrectly as adenomas and resected | Incorrect: too short |
| MAHPR | Missed adenoma(s) and hyperplastic polyp(s) resected | One or more hyperplastic polyps identified incorrectly as adenomas and resected and one or more adenomas identified incorrectly as hyperplastic polyps and left in situ | Correcta |
The probability of these different outcomes depends on the number of polyps and adenomas that the patient has, the diagnostic accuracy of the colonoscopy technology and the policies for resecting polyps and assigning surveillance intervals.
In general, if the actual number of adenomas is at the higher end of the risk classification range, then a patient with one or more hyperplastic polyps identified incorrectly as adenomas may be given a shorter surveillance interval than is appropriate. Similarly, if the actual number of adenomas is at the lower end of the risk classification range, then if the patient has one or more adenomas identified incorrectly as hyperplastic polyps and left in situ, they may be given a longer surveillance interval than is appropriate.
Some outcomes are not possible for particular groups of patients; for example, a patient with one hyperplastic polyp and one adenoma (low risk) cannot be assigned an incorrect surveillance interval, as, even if the hyperplastic polyp is mistaken for an adenoma, they would still be placed in the low-risk group and be invited (correctly) for routine screening. Other outcomes will be very improbable for some patients; for example, a patient with nine adenomas (high risk) is very unlikely to be diagnosed with fewer than five adenomas, and so is unlikely to be assigned to a surveillance interval that is too long.
It is possible that patients could simultaneously have one or more hyperplastic polyp misdiagnosed as an adenoma (FP) and one or more adenoma misdiagnosed as a hyperplastic polyp (FN). If so, the patient would be at risk of harm from the unnecessary resection(s) and increased risk of cancer as a result of the adenoma(s) left in situ. However, it is unlikely that they would be assigned to an incorrect surveillance interval, as the errors for individual polyps would be likely to cancel out.
The mathematics behind the estimation of outcome probabilities for patients from polyp-level diagnostic accuracy estimates is explained in Estimating patient outcome probabilities from polyp-level diagnostic accuracy. First, we continue the overview of the decision tree model, and explain how it links to long-term outcomes from the SBCS model.
Under the histopathology strategy, all patients are assumed to receive the correct diagnosis (Table 33). All polyps including adenomas are resected, so no adenomas are left in situ, and patients are assigned to the correct follow-up strategy: routine invitation to screening for those with zero to two adenomas, 3-yearly surveillance for those with three or four adenomas and annual surveillance for those with five or more adenomas. The model calculates the resources required for histopathology and polypectomy and the number of adverse events that result from polypectomies, with associated treatment costs and disutilities. Long-term outcomes associated with each diagnostic outcome are taken from the SBCS model with no adenomas left in situ and all patients assigned to the correct follow-up. The SBCS model includes higher adenoma incidence rates for patients who have had adenomas resected than for patients who started without adenomas (normal epithelium), and the rate of recurrence of adenomas is higher for patients who were initially at higher risk. Cancer incidence, and hence costs and outcomes in the SBCS model, also depend on the surveillance interval assigned. A detailed description of the SBCS model is provided in The School of Health and Related Research’s bowel cancer screening Markov model.
| Initial risk (adenomas) | Patient outcome | Diagnostic outcome | Initial SBCS state | ||
|---|---|---|---|---|---|
| Hyperplastic resected | Adenomas missed | Surveillance interval | |||
| LR (0) | CD | All | None | Correct | Normal (screening) |
| LR (1 or 2) | CD | All | None | Correct | LR, all resected (screening) |
| IR (3 or 4) | CD | All | None | Correct | IR, all resected (3-yearly) |
| HR (5+) | CD | All | None | Correct | HR, all resected (annual) |
With VCE, errors in characterisation of polyps are possible, and hence patients may be left with one or more adenomas in situ (as a result of FNs) and/or have hyperplastic polyps unnecessarily resected (as a result of FPs). Errors in polyp characterisation with VCE might also cause patients to be allocated to the wrong follow-up strategy – with either too long or too short an interval. The diagnostic outcomes for patients under the VCE strategy are shown in Table 34. Outcomes that are impossible or very unlikely are omitted from this table.
| Initial risk (adenomas) | Patient outcome | Diagnostic outcome | Follow-up interval | Initial SBCS state | |
|---|---|---|---|---|---|
| Hyperplastic resected | Adenomas missed | ||||
| LR (0) | CD | None | – | Correct | Normal (screening) |
| HPRC | One or more | – | Correct | Normal (screening) | |
| LR (1 or 2) | CD | None | None | Correct | LR, all resected (screening) |
| MAC | None | One or more | Correct | LR, adenomas (screening) | |
| HPRC | One or more | None | Correct | LR, all resected (screening) | |
| HPRI | One or more | None | Too short | LR, all resected (3-yearly) | |
| MAHPR | One or more | One or more | Correct | LR, adenomas (screening) | |
| IR (3 or 4) | CD | None | None | Correct | IR, all resected (3-yearly) |
| MAC | None | One or more | Correct | LR, adenomas (3-yearly) | |
| MAI | None | One or more | Too long | LR, adenomas (screening) | |
| HPRC | One or more | None | Correct | IR, all resected (3-yearly) | |
| HPRI | One or more | None | Too short | IR, all resected (annual) | |
| MAHPR | One or more | One or more | Correct | LR, adenomas (3-yearly) | |
| HR (5+) | CD | None | None | Correct | HR, all resected (annual) |
| MAC | None | One or more | Correct | LR, adenomas (annual) | |
| MAI | None | One or more | Too long | LR, adenomas (3-yearly) | |
| HPRC | One or more | None | Correct | HR, all resected (annual) | |
| HPRI | One or more | None | Too short | HR, all resected (annual) | |
| MAHPR | One or more | One or more | Correct | LR, adenomas (annual) | |
For patients without any adenomas, there are only two possible outcomes: they may have a correct diagnosis and have no polyps resected (correct diagnosis); or they may have one or more hyperplastic polyps removed unnecessarily [hyperplastic polyp(s) resected correct surveillance]. In either case, patients with no adenomas are very unlikely to be assigned the wrong follow-up: the probability of the three or more FP results that would be required for them to be incorrectly assessed as intermediate risk is very low. Costs and outcomes for this group are therefore taken from the results for patients starting in SBCS model in the ‘normal epithelium’ health state and following routine screening. There are five possible diagnostic outcomes for patients with one or two adenomas. They may be correctly diagnosed; have one or more adenomas missed, but no resections of hyperplastic polyps and be assigned correctly to routine screening [missed adenoma(s) correct surveillance]; have no adenomas missed but one or more hyperplastic polyps resected, either with the correct follow-up of routine screening [hyperplastic polyp(s) resected correct surveillance] or unnecessary 3-yearly surveillance [hyperplastic polyp(s) resected incorrect surveillance]; or they may have one or more adenomas missed and also one or more hyperplastic polyps resected with the correct follow-up [missed adenoma(s) and hyperplastic polyp(s) resected]. Patients in this group start in the SBCS model in the ‘post-polypectomy (low-risk adenomas removed)’ health state or in the ‘low-risk adenomas’ health state (one or two diminutive adenomas in situ). All patients in this group will be invited for routine screening, except those with one or more FP results who are misclassified as intermediate risk. Finally, patients with three or more adenomas (intermediate risk or high risk) have all possible outcomes illustrated in Figure 31. We assume that patients in this group with one or more missed adenomas start in the ‘low-risk adenomas’ health state in the SCBS model, with one or two adenomas in situ; however, it is possible that patients could have three or more adenomas missed, but this is very unlikely.
Estimating patient outcome probabilities from polyp-level diagnostic accuracy
Probability of test results for an individual polyp
For the individual polyp, there are four possible VCE test results (TP, FP, FN and TN). The probability of these outcomes can be calculated as a function of the proportion of polyps that are adenomas (p), and the sensitivity (Se) and specificity (Sp) of the test, as shown in Table 35.
| Polyp result | Interpretation | Probability |
|---|---|---|
| TP | Adenoma correctly classified | P(TP) = p × Se |
| FP | Hyperplastic polyp identified incorrectly as an adenoma | P(FP) = (1 – p) × (1 – Sp) |
| FN | Adenoma identified incorrectly as a hyperplastic polyp | P(FN) = p × (1 – Se) |
| TN | Hyperplastic polyp correctly classified | P(TN) = (1 – p) × Sp |
Probability of test results for multiple polyps
For patients with more than one polyp, the probabilities of different combinations of test results can be estimated using the binomial distribution. For example, the probability that a patient with n polyps has k FP test results is:
This formula is used in the decision tree model to estimate the probability of the six main diagnostic outcomes shown in Figure 31 and Table 32. This approach does require an assumption that the test results for individual polyps within a patient are independent of one another: thus, for example, the probability that an individual polyp gives a FP test result is assumed to be constant, regardless of whether or not other polyps in the patient have given an FP result. In practice, the types of polyp within a patient are likely to be clustered; however, we have not identified any data to quantify the extent of any such clustering.
Probability that one or more hyperplastic polyps are misidentified as adenomas
The probability that one or more hyperplastic polyps are incorrectly identified as adenomas in a patient with n polyps is:
In the cases where one or more polyp is assessed with low confidence (lc is proportion of polyps assessed with low confidence), the above formula can be generalised to:
Probability that one or more adenomas are missed
In a similar way, the probability that one or more adenomas are incorrectly identified as hyperplastic polyps is:
Or, in the cases where the DISCARD strategy is used, and the proportion of polyps in the proximal region is px:
Probability of correct/incorrect follow-up intervals
Whether or not patients are given incorrect follow-up depends on their actual number of adenomas and the number of FP and FN results. Thus, a patient with five adenomas, who should be invited for annual surveillance, might be mistakenly invited for colonoscopy only once every 3 years if one or more adenoma was missed. Estimating the probabilities for every possible combination of adenomas, FP and FN results is complicated. However, the probability of being given the wrong surveillance interval is very low for some patients. For example, patients with no adenomas would need to have three more FP results than FN results before they would move into the range where they might be offered 3-yearly surveillance. Similarly, patients with seven adenomas would need three or more FN results than FP results to move from the annual to 3-yearly surveillance category. Given the multiplicative nature of the binomial formula, and relative rarity of FP and FN errors, such outcomes are very unlikely. We therefore made a simplifying assumption: that the probability of three or more errors in polyp characterisation (FP and/or FN) within a patient is negligible.
For each risk category, we estimated the proportion of patients who have the number of adenomas corresponding to the lower and higher ends of the classification range as:
The probability of patients having one or more missed adenomas and being assigned to an incorrect follow-up strategy (too long an interval) is:
Similarly, the probability of patients having one or more hyperplastic polyp(s) misclassified as an adenoma and being assigned to an incorrect strategy (too short an interval) is:
The probability calculations for the six patient outcomes are summarised in Table 36.
| Patient outcome ID | Interpretation | Follow-up interval | Probability |
|---|---|---|---|
| CD | Correct diagnosis | Correct | 1 – P(MAC) – P(MAI) – P(HPRC) – P(HPRI) – P(MAHPR) |
| MAC | Missed adenoma (correct surveillance) | Correct | (1 – le) × (1 – (1 – P(FN))n(1 – lc)(1 – px)) |
| MAI | Missed adenoma (incorrect surveillance) | Incorrect: too long | le × (1 – (1 – P(FN))n(1 – lc)(1 – px)) |
| HPRC | Hyperplastic polyp resected (correct surveillance) | Correct | (1 – he) × (1 – (1 – P(FP))n(1 – lc)) |
| HPRI | Hyperplastic polyp resected (incorrect surveillance) | Incorrect: too short | he × (1 – (1 – P(FP))n(1 – lc)) |
| MAHPR | Missed adenoma, hyperplastic polyp resected | Correct | (n!2!(n−2)!)P(FP) × P(FN) × (1 – P(FP) – P(FN))(n – 2) |
The School of Health and Related Research’s bowel cancer screening Markov model
The SBCS model122 describes the development of adenomas and colorectal cancer and subsequent disease progression for the general population of England eligible for bowel cancer screening. It was developed by the School of Health and Related Research for the NHS Bowel Cancer Screening Programme. The model is a ‘Markov-type’ health state transition model, that takes a cohort approach (rather than individual-level simulation). It estimates QALYs and costs for a cohort of 65-year-olds at risk of developing colorectal cancer over a lifetime horizon and using an annual cycle length. Costs were estimated from the perspective of the English NHS, and a discount rate of 3.5% was applied to costs and QALYs. The basic model structure consists of a natural history model and a screening and surveillance pathway.
The basic natural history model is illustrated in Figure 32. This shows the expected progression of adenomas and colorectal cancer in the absence of an active screening and surveillance programme.
FIGURE 32.
The School of Health and Related Research’s bowel cancer screening model: natural history model. Adapted from Whyte and colleagues. 131
Patients start in one of the pre-cancer health states: normal epithelium (no adenomas), low-risk adenomas or intermediate-/high-risk adenomas. Over time, they may progress through the adenoma–carcinoma route: from normal epithelium to low-risk adenomas, to intermediate-/high-risk adenomas and to preclinical Dukes’ stage A colorectal cancer. It is also possible for patients to transition directly from normal epithelium to preclinical stage A colorectal cancer (de novo cancers). Preclinical cancer progresses through the stages, from A to B to C to D, but at some time it is likely to be diagnosed, through chance detection or symptomatic presentation, at which time the patient moves to the related ‘clinical’ cancer stage. Progression through the clinical cancer stages is not modelled; instead a stage-specific cancer survival rate is applied. It is also possible for patients with undiagnosed stage D cancer to die. Patients can die from other causes in any of the health states.
The SBCS model was designed to evaluate alternative active screening and surveillance programmes. The post-screening surveillance pathway is illustrated in Figure 33.
FIGURE 33.
The School of Health and Related Research’s bowel cancer screening model: surveillance colonoscopy pathway. HR, high risk; IR, intermediate risk; LR, low risk.

This shows the assumptions built in to the SBCS model about how patients would be followed up under BSG guidelines, according to findings at an initial colonoscopy after a positive screening result, which reflects the starting point from the end of our decision tree for our base-case screening population. Patients assessed to be at low risk following an initial colonoscopy (zero to two diminutive adenomas in our population) and those with no adenomas at two successive 3-year surveillance colonoscopies are assumed to be invited for routine screening. The screening pathway in the version of the SBCS model used to generate cost and QALY estimates for the VCE model was chosen to reflect the current NHS Bowel Cancer Screening Programme, with the offer of a home FOBT every 2 years for all men and women aged 60–74 years, and invitation for colonoscopy for patients with an abnormal screening test.
In the SCBS model, colonoscopy is assumed to be standard colonoscopy without VCE. However, the model does assume less than perfect sensitivity of colonoscopy for detecting adenomas: 0.77 for low-risk adenomas and 0.98 for intermediate-risk/high-risk adenomas. It also assumes that the cost of histopathology is incurred only for adenomas, a mean of 1.9 per person undergoing colonoscopy. Thus, the cost and accuracy of colonoscopy in the SCBS model is possibly more reflective of VCE than with standard colonoscopy.
The simple natural history diagram in Figure 32 does not show all transitions in the SBCS model. In particular, it omits recurrence of adenomas and cancer incidence for patients who have had adenomas removed at colonoscopy. These additional transitions are illustrated in Figure 34.
FIGURE 34.
The School of Health and Related Research’s bowel cancer screening model: adenoma recurrence following polypectomy. HR, high risk; IR, intermediate risk; LR, low risk.

Following colonoscopy, patients enter the following health states in the SBCS model: patients who started with no adenomas go to the ‘normal epithelium’ state; patients with one or two adenomas left in situ go to ‘low-risk adenomas’; those with three or more adenomas left in situ go to ‘intermediate-risk/high-risk adenomas’; and patients who have all had adenomas resected go to the low-, intermediate- or high-risk adenomas removed states, depending on their initial risk level. Subsequently, patients who have had all adenomas removed may have a recurrence of low-risk or intermediate-/high-risk adenomas, and they also have a small chance of ‘de novo’ cancer, transitioning directly to preclinical Dukes’ stage A colorectal cancer.
Thus, the costs and QALYs for the end points of our decision tree were calculated by running the SBCS model with a cohort of 65-year-old patients starting in each of the post-colonoscopy health states (normal epithelium, low-risk adenomas removed, intermediate-risk adenomas removed, high-risk adenomas removed, low-risk adenomas and intermediate-/high-risk adenomas). The model was run for each possible post-colonoscopy state three times, assuming routine screening, 3-yearly surveillance and annual surveillance in turn. Several updates were made to the SBCS model for these analyses. The input parameters are described in Model parameters. Screening and treatment costs were inflated or updated where appropriate (see Tables 41 and 42). Analyses were run assuming that the average number of adenomas present in patients with at least one adenoma was 1.9, although the SBCS model does not explicitly simulate the number of polyps. The final cost and QALY estimates from the SBCS model that were used in our decision tree analysis are shown in Table 37.
| Initial risk (adenomas) | Patient outcome | Adenomas missed | Hyperplastic polyps resected | Surveillance interval | Cost | QALYs using quality-of-life estimates from | |
|---|---|---|---|---|---|---|---|
| Ara and Brazier143 | Färkkilä et al.145 | ||||||
| LR (0) | CD | None | None | Invited to screening | 109 | 11.26653 | 11.27254 |
| HPRC | None | One or more | Invited to screening | 109 | 11.26653 | 11.27254 | |
| LR (1 or 2) | CD | None | None | Invited to screening | 109 | 11.26653 | 11.27254 |
| HPRC | None | One or more | Invited to screening | 109 | 11.26653 | 11.27254 | |
| HPRI | None | One or more | 3-year surveillance | 1075 | 11.29947 | 11.30355 | |
| MAIa | One or more | None | Invited to screening | 250 | 11.26399 | 11.27027 | |
| MACa | One or more | None | Invited to screening | 250 | 11.26399 | 11.27027 | |
| MAHPRa | One or more | One or more | Invited to screening | 250 | 11.26399 | 11.27027 | |
| IR (3 or 4) | CD | None | None | 3-year surveillance | 1097 | 11.29934 | 11.30341 |
| HPRC | None | One or more | 3-year surveillance | 1097 | 11.29934 | 11.30341 | |
| HPRI | None | One or more | Annual surveillance | 1577 | 11.32057 | 11.30659 | |
| MAIc | One or more | None | Invited to screening | 250 | 11.26399 | 11.27027 | |
| MAC | One or more | None | 3-year surveillance | 1161 | 11.29891 | 11.30291 | |
| MAHPR | One or more | One or more | 3-year surveillance | 1161 | 11.29891 | 11.30291 | |
| HR (5+) | CD | None | None | Annual surveillance | 1584 | 11.30252 | 11.30654 |
| HPRC | None | One or more | Annual surveillance | 1584 | 11.30252 | 11.30654 | |
| HPRI | None | One or more | Annual surveillance | 1584 | 11.30252 | 11.30654 | |
| MAI | One or more | None | 3-year surveillance | 1161 | 11.29891 | 11.30291 | |
| MAC | One or more | None | Annual surveillance | 1681 | 11.30152 | 11.30553 | |
| MAHPR | One or more | One or more | Annual surveillance | 1681 | 11.30152 | 11.30553 | |
Evaluation of uncertainty
The evaluation of the cost-effectiveness of VCE technologies is based on uncertain information about variables, such as the diagnostic accuracy, polyp demographics, HRQoL and resource use. This uncertainty was evaluated using deterministic and probabilistic sensitivity analyses (PSAs). One-way deterministic sensitivity analyses were conducted to evaluate the influence of individual parameters on the model results and to test the robustness of the cost-effectiveness results to variations in the structural assumptions (see One-way deterministic sensitivity analyses).
Multiparameter uncertainty in the model was addressed using PSA (see Probabilistic sensitivity analysis). In the PSA, probability distributions are assigned to the point estimates used in the base-case analysis. The model is run for 5000 iterations, with a different set of parameter values for each iteration, by sampling parameter values at random from their probability distributions. The uncertainty surrounding the cost-effectiveness of each treatment is represented using a cost-effectiveness acceptability curve according to the probability that the intervention will be cost-effective at a particular willingness-to-pay threshold. Appendix 9 reports the parameters included in the PSA, the form of distribution used for sampling each parameter, and the upper and lower limits assumed for each variable.
The results of the PSA should be treated with some caution, however, as they do not reflect some important sources of uncertainty or correlations between model parameters. First, we note that the PSA does not integrate uncertainty over the long-term impact of diagnostic errors on patient outcomes and costs, as we could not obtain correlated samples of cost and QALY outputs from the SBCS model. The PSA also omits correlations between sensitivity and specificity estimates from our bivariate meta-analysis. Statistical advice to the team, indicated that if no threshold effect could be demonstrated between diagnostic sensitivity and specificity of VCE, then modelling these parameters as uncorrelated in PSA would have little effect on their uncertainty in comparison to modelling them allowing for correlation. In our meta-analyses [see Chapter 4, Assessment of diagnostic accuracy (sensitivity, specificity, negative predictive value, accuracy)], we found that there was no significant evidence of a threshold effect. Therefore, for the PSA we have varied sensitivity and specificity independently. It is most likely that the consequence of these omissions is that the PSA underestimates overall uncertainty over the cost-effectiveness of the VCE strategies. In addition, there are uncertainties over some structural assumptions that are not reflected in the PSA.
Model validation
The decision tree model was validated by checking its structure, calculations and data inputs for technical correctness. The model structure was reviewed by clinical experts for appropriateness for the disease and diagnosis. The model was checked for internal consistency by a second health economist. The robustness of the model to changes in input values was tested using sensitivity analyses to ensure that any changes to the input values produced changes to the results of the expected direction and magnitude.
The prediction of correct surveillance intervals was compared between the estimates from the model and those in the published literature. Three studies of NBI60,67,68 that reported both accuracy of diagnosing individual diminutive polyps and accuracy of assignment of patients to surveillance interval using data from diminutive polyps only were identified by our systematic review of diagnostic studies. In the study by Chandran and colleagues,67 the diagnostic accuracy was 91.2%, whereas the surveillance interval was correctly determined in 98% of patients. In the study by Gupta and colleagues,68 the diagnostic accuracy was 84.8%, whereas prediction of the surveillance interval was accurate in 86.1–94.1% of patients if only diminutive polyps were considered. In the study by Paggi and colleagues,60 diagnostic accuracy for diminutive polyps was 84.0%, whereas correct surveillance intervals were applied 85.3% of the time. None of the i-scan or FICE studies identified by our systematic review reported the accuracy of assignment of patients to a surveillance interval based on diminutive polyps only. The model predicted correct surveillance intervals in 93–98% of patients using the VCE technologies.
The majority of the estimates of correct surveillance interval prediction identified by our systematic review of diagnostic studies (see Chapter 4, Assessment of test impact on recommended surveillance intervals) were based on using VCE characterisations for polyps < 5 mm in size (or in some studies < 10 mm in size) combined with histopathological assessment of all other polyps (14/17 studies). In these 14 studies,55,57,58,61–65,70,76,79,82–84 the estimates of correct surveillance interval prediction ranged between 79.9% and 100% across all VCE technologies; only in three of the NBI studies58,63,76 did some agreements fall below 90.0%. The surveillance interval prediction from our model is broadly consistent with the systematic review findings.
Model parameters
The following subsections report parameters included in the model. The model parameters include polyp and adenoma demographics, diagnostic test accuracy, adverse event rates, health sector costs (such as cost of colonoscopy), HRQoL and long-term epidemiology (such as disease progression). The costs and adverse event parameters have been based on those previously used in the SBCS model122 and updated when necessary.
Prevalence of polyps and adenomas
The prevalence of patients presenting with different numbers of polyps and adenomas at colonoscopy was estimated from the literature for three populations: the screening population (base case) and the surveillance and symptomatic populations (used in scenario analyses).
Screening population
We searched for studies that described the distribution of polyps in patients in a bowel screening population. We identified one study, by Raju and colleagues,132 that reported data for the distribution of polyps and adenomas per patient. We analysed the distribution of polyps and adenomas to derive the average number of polyps and adenomas for low-risk, intermediate-risk and high-risk patients, and the frequency of patients in each risk category, assuming that all polyps are diminutive.
The study by Raju and colleagues132 is a retrospective analysis of data from a colon cancer screening programme in the USA. Three hundred and forty-three patients underwent colonoscopy between 2009 and 2011. In the study, 46 patients had no polyps and there were 882 polyps in the remaining 297 patients (2.97 polyps per patient). Of the patients who had polyps, 206 had a total of 422 adenomas (i.e. 1.4 adenomas per patient with a polyp or 2.04 per patient with an adenoma). Thirty per cent of patients who had polyps had no adenomas.
We used a graphical data extraction programme (XY Scan, version 4.1.0; New Haven, CT, USA) to extract the data from Raju and colleagues. This extraction resulted in a slight overestimation of the number of adenomas (426 instead of the reported 422) and the number of patients with adenomas (207 instead of 206) in order to keep polyp numbers correct at 882.
In order to calculate the number of polyps per patient in each risk category, we assumed that patients with adenomas were evenly distributed across the risk categories, where people had adenomas. The risk stratification was defined in accordance with the current BSG guidelines:30 people with one or two adenomas are low risk, those with three or four adenomas are intermediate risk and those with five or more adenomas are high risk. First, we calculated the proportion of patients with the number of adenomas that corresponded with the risk classification and then we calculated a weighted average of the number of polyps and adenomas in these patients. The derivation of the polyp demographics are shown in more detail in Appendix 10. Polyp demographics are shown in Table 38.
| Polyp demographics in patients with at least one polyp | Value | Source |
|---|---|---|
| Prevalence of patients with at least one adenoma | 0.698 | Raju et al.132 |
| Prevalence of patients with no adenomas | 0.302 | Raju et al.132 |
| Prevalence of patients with LR adenoma | 0.535 | Raju et al.132 |
| Prevalence of patients with IR adenoma | 0.107 | Raju et al.132 |
| Prevalence of patients with HR adenoma | 0.056 | Raju et al.132 |
| Average number of polyps | 2.97 | Raju et al.132 |
| Number of polyps, LR patients | 2.00 | Raju et al.132 |
| Number of polyps, IR patients | 4.78 | Raju et al.132 |
| Number of polyps, HR patients | 8.47 | Raju et al.132 |
| Number of adenomas, LR patients | 1.40 | Raju et al.132 |
| Number of adenomas, IR patients | 3.34 | Raju et al.132 |
| Number of adenomas, HR patients | 5.91 | Raju et al.132 |
Surveillance population
We were unable to identify any studies that reported the distribution of adenomas in a surveillance population, whereby all patients after colonoscopy had been followed up for the appropriate surveillance interval as defined by their risk classification. We found several studies that reported the distribution of adenomas at follow-up surveillance for specific subgroups. For example, Lee and colleagues133 reported the outcome of 12-month surveillance colonoscopy in high-risk patients (n = 1760) in the NHS Bowel Cancer Screening Programme. Martínez and colleagues134 reported a pooled analysis of eight prospective studies comprising 9167 people with previously resected colorectal adenomas during a median follow-up of 4 years. We found several other studies that reported the distribution of adenomas at various follow-up intervals for patients with more than one adenoma resected. 135,136 In the absence of data that fit our population group, we used these studies, together with an assumption, to calculate the distribution of adenomas in this population.
The proportion of patients with no adenomas at follow-up surveillance was similar for Lee and colleagues133 (49.2%) and Martínez and colleagues134 (53.3%). We chose the estimate from the study by Martínez and colleagues,134 as it was the larger study, and not only for high-risk patients. We stratified those patients who had low-risk, intermediate-risk and high-risk adenomas in the same proportion as for the screening population (see Table 38). The resulting distribution of adenomas for the surveillance population is shown in Table 39.
| Distribution of patients | Population | |
|---|---|---|
| Surveillance | Symptomatic | |
| No adenoma | 0.533 | 0.782 |
| LR | 0.358 | 0.125 |
| IR | 0.072 | 0.061 |
| HR | 0.037 | 0.032 |
Symptomatic population
We identified one relevant study by McDonald and colleagues137 that described the proportion of people who had adenomas in a group of consecutive patients referred from primary care for colonoscopic examination in the NHS. Patients were referred for symptoms including rectal bleeding, change in bowel habits and abdominal pain. Patients who had been referred as a result of the Bowel Cancer Screening Programme were not included. The distribution of adenomas for the symptomatic population is shown in Table 39.
The study also included a small number of patients with irritable bowel syndrome and we have excluded these from our calculation of the distribution of adenomas in the symptomatic population. The study reports the number of people who have no adenomas, low-risk adenomas and high-risk adenomas. The high-risk adenoma group was split between intermediate-risk and high-risk in the same proportion as for the screening population (see Table 39).
Diagnostic accuracy
The sensitivity and specificity of histopathology and the VCE technologies are taken from the meta-analyses conducted in this report, as described in Chapter 4. We have assumed that histopathology provides an accurate diagnosis of all polyps (i.e. 100% sensitivity and specificity). The diagnostic accuracy parameters are shown in Table 40 and are for high-confidence characterisations of polyps in the whole colon. The proportion of polyps assessed with low confidence is derived from those NBI studies in our systematic review that reported these data, and is assumed to be the same for FICE and i-scan.
| Parameter | Value | 95% CI | Source | |
|---|---|---|---|---|
| Lower | Upper | |||
| Histopathology sensitivity | 1 | Assumption | ||
| Histopathology specificity | 1 | Assumption | ||
| NBI sensitivity | 0.910 | 0.855 | 0.945 | Meta-analysis |
| NBI specificity | 0.819 | 0.760 | 0.866 | Meta-analysis |
| FICE sensitivity | 0.814a | 0.732 | 0.875 | Meta-analysis |
| FICE specificity | 0.850a | 0.786 | 0.898 | Meta-analysis |
| i-scan sensitivity | 0.962 | 0.917 | 0.983 | Meta-analysis |
| i-scan specificity | 0.906 | 0.842 | 0.946 | Meta-analysis |
| Proportion low confidence | 0.214 | 0.21 | 0.22 | NBI studies that reported these data in our review |
Scenario analyses were conducted for alternative diagnostic accuracy estimates derived from the systematic review and meta-analysis in Sensitivity analyses, as follows:
-
sensitivity and specificity for polyps characterised with high confidence in the rectosigmoid colon
-
sensitivity and specificity for polyps characterised with any confidence level in the rectosigmoid colon
-
sensitivity and specificity for polyps characterised with any confidence level in the whole colon
-
sensitivity and specificity for a pooled VCE analysis
-
sensitivity and specificity for endoscopists experienced in the use of NBI.
Adverse effects
There are small risks attached to polypectomy, such as bowel perforation and bleeding, which may lead to hospitalisation and, for those patients who experience perforation, a small risk of death. The probabilities of these adverse effects were taken from the published sources used in the SBCS model and are shown in Table 41.
| Parameter | Value | 95% CI | Source | |
|---|---|---|---|---|
| Lower | Upper | |||
| Probability of perforation with polypectomy | 0.003 | 0.00 | 0.01 | Whyte et al.122 |
| Probability of death, for patients with perforation during polypectomy | 0.052 | 0.01 | 0.11 | Gatto et al.138 |
| Probability of hospitalisation for bleeding with polypectomy | 0.003 | 0.00 | 0.01 | Atkin139 |
Estimation of costs
Costs were included for colonoscopy, polypectomy, adverse events and histopathology. The unit costs were taken from NHS Reference Costs 2014–2015. 123 A summary of the unit costs is shown in Table 42.
| Parameter | Value | 95% CI | Source123 | |
|---|---|---|---|---|
| Lower | Upper | |||
| Cost of colonoscopy without polypectomy | 518.36 | 340.89 | 695.83 | HRG 2014–15 FZ51Z, day case |
| Cost of colonoscopy with polypectomy | 600.16 | 406.24 | 794.08 | HRG 2014–15 FZ52Z, day case |
| Cost of treating bowel perforation (major surgery) | 2152.77 | 902.21 | 3403.33 | HRG 2014–15 FZ24E-J, weighted average, non-elective long stay |
| Cost of admittance for bleeding (overnight stay on medical ward) | 475.54 | 327.69 | 623.39 | HRG 2014–15 FZ38G-P, weighted average, non-elective short stay |
| Pathology cost per-polyp examination | 28.82 | 6.78 | 50.86 | HRG 2014–15 DAPS02 |
System costs
The equipment and maintenance costs for VCE technologies are shown in Appendix 11. These costs are not included in the base-case analysis for VCE versus histopathology as all equipment and maintenance costs are included within the national reference costs for colonoscopy and polypectomy (see Table 42). There are differences in the costs between the VCE technologies and these are explored in a scenario analysis (see Sensitivity analyses).
Colorectal cancer treatment costs
The SBCS model includes colorectal cancer treatment costs by patient age and Dukes’ colorectal cancer staging score. These costs were taken from the study by Pilgrim and colleagues140 and have been inflated to 2015 prices using The Hospital and Community Health Service index124 (Table 43).
| Age (years) at diagnosis | Dukes’ colorectal cancer stage at diagnosis | |||
|---|---|---|---|---|
| A | B | C | D | |
| 40–49 | 8871 | 8858 | 14,683 | 11,862 |
| 50–59 | 5789 | 7110 | 9821 | 8557 |
| 60–69 | 4686 | 5423 | 7357 | 6596 |
| 70–79 | 3220 | 3500 | 4546 | 4423 |
| 80–100 | 1398 | 1567 | 1581 | 818 |
Training costs
As discussed earlier (see Chapter 1, Training in the use of virtual chromoendoscopy), endoscopists will need to receive training to accurately use VCE. This may include training programmes in the form of video packages and/or supervision from endoscopists experienced in using VCE. Several studies have evaluated training packages that were developed to train endoscopists in the use of NBI. 72,94,141,142
For example, Ignjatovic and colleagues141 conducted a prospective education study of a computer-based training module in 21 individuals (novices, trainees and experienced gastroenterologists) with varying colonoscopy experience in the UK. There was significant improvement in the accuracy in characterisation of polyps after the training. Ignjatovic and colleagues141 commented that, although the NBI learning curve is thought to be relatively short, with an improvement in diagnostic accuracy after as few as 44 polyps, it is not clear how expertise is best transferred to community gastroenterologists and to trainees. McGill and colleagues72 showed that the performance of endoscopists could be sustained over time by repeating the training module at the mid-point of the study. Meads and colleagues142 suggest that ongoing training and assessment is necessary to sustain performance.
We assumed that the number of days training would be 2 days per year per endoscopist, in common with the NBI study by Solon and colleagues. 117 Using a daily rate for endoscopists of £1104 from PSSRU124 and assuming that each endoscopist completes 150 endoscopies per year gives a training cost per patient of £14.72.
Health-related quality of life
The SBCS model122 used a study by Ara and Brazier143 that reported utility values. Ara and Brazier143 pooled the data from four Health Surveys for England in order to compare self-reported health status and quality-of-life response for subjects with or without a specified list of health conditions. The mean EuroQol-5 Dimensions (EQ-5D) score for respondents was 0.697, whereas for those without cancer the mean EQ-5D score was 0.798. The mean age for respondents for this health state was 60.9 years.
We conducted a targeted search for other studies reporting the HRQoL for patients with colorectal cancer. The searches sought to identify studies reporting EQ-5D that described the HRQoL in general of patients with colorectal cancer, rather than a specific stage of colorectal cancer, such as metastatic cancer. The searches identified three potentially relevant studies, summarised in Table 44. One study was from the USA,144 one was from Finland145 and one was from the UK. 146
| Study | Year | Country | Study type | Population | EQ-5D values |
|---|---|---|---|---|---|
| Djalalov et al.144 | 2014 | USA | Systematic review and meta-analysis | 26 studies that reported utility weights for CRC health states. 6543 respondents (mean age 62 years) | 0.76 |
| Färkkilä et al.145 | 2013 | Finland | Cross-sectional study | 508 Finnish CRC patients (mean age 68 years). Patients were divided into five groups:
|
Remission 0.85; all patients 0.813 |
| Downing et al.146 | 2015 | UK | Population-level study | All individuals diagnosed with CRC in England in 2010 and 2011 who were alive 12–36 months after diagnosis were sent a questionnaire. 21,802 of 34,467 patients responded | Mean EQ-5D values not reported |
Djalalov and colleagues144 performed a systematic review of utility weights for colorectal cancer. They identified 26 studies providing unique utilities for colorectal cancer health states elicited from 6546 respondents. They included utility assessments including the EQ-5D, Health Utilities Index 3 and time trade-off. The colorectal cancer utility data were analysed using linear mixed-effects models for different variables including colorectal cancer type, stage and utility measure. They calculated the mean EQ-5D score of the population of people with colorectal cancer to be 0.76. It is unclear if this estimate captures the overall HRQoL for patients with colorectal cancer as the meta-analysis included more studies of patients with more severe disease, and the overall mean utility score reflects this.
Färkkilä and colleagues145 provide utility values for patients with colorectal cancer in Finland. In this study, patients diagnosed with colorectal cancer received a questionnaire by mail. A total of 508 patients assessed their HRQoL using the generic 15-dimensional and EQ-5D (with the UK tariff). Patients were divided into five groups: primary treatment, rehabilitation, remission, metastatic disease and palliative care. The patients’ HRQoL was compared with population reference values. The study reported an EQ-5D utility value of 0.813 for all patients with colorectal cancer and 0.85 for patients in cancer remission. The utility values were higher for patients in remission than for the standardised general population (non-significant difference). For the purposes of our analysis, we assumed that patients in remission have similar utility to the general population and, therefore, the mean decrement for colorectal cancer patients is 0.037.
Downing and colleagues146 sent a questionnaire to all individuals diagnosed with colorectal cancer in England in 2010 and 2011, who were alive 12–36 months after diagnosis, and 21,802 patients responded. The questionnaire included questions related to treatment, disease status and HRQoL (EuroQoL). However, Downing and colleagues146 did not provide mean EQ-5D values.
For our base-case analysis, we used HRQoL values from Ara and Brazier,143 for consistency with the SBCS model. We explored alternative quality-of-life values from Färkkilä and colleagues145 in a scenario analysis.
Disutility
Disutility values were sought for patients who experience adverse events during polypectomy, such as bowel perforation or bleeding. However, we were not able to identify values for disutilities for these events from the literature. As an alternative we estimated values for disutility for bleeding by assuming they would be similar to a major gastrointestinal bleed and used the value from Dorian and colleagues147 of 0.1511 for 2 weeks (i.e. a total QALY loss of 0.006). Values for perforation were assumed to be the same as for stomach ulcer/abdominal hernia/rupture taken from Ara and Brazier. 143 The disutility value was 0.118 for 1 month (i.e. a total QALY loss of 0.010).
Epidemiology of adenoma and cancer progression
Transition probabilities in the SBCS natural history model (progression between the adenoma states, preclinical colorectal cancer stages and from preclinical to clinical colorectal cancer stages) and screening test characteristics were estimated using a calibration approach. These parameters are not observable, so they were inferred based on available data on colorectal cancer incidence by age and stage in the absence of screening, and from colorectal cancer screening data sets. Results are presented in Whyte and colleagues. 122
The SBCS model uses cancer recurrence rates for people from the NHS Bowel Cancer Screening Programme with high-risk adenomas and data from a study by Martínez and colleagues134 for people with low-risk adenomas (Table 45). The proportion of people in the high-risk surveillance category who have had a polypectomy requiring annual surveillance is 0.29. Full details of the data and assumptions used are available in Whyte and colleagues. 122
| Description | Probability of transition to | Value |
|---|---|---|
| LR adenoma, all adenomas resected | LR adenomas health state | 0.100 |
| LR adenoma, all adenomas resected | HR adenomas health state | 0.040 |
| LR adenoma, all adenomas resected | CRC health state | a |
| HR adenoma (IR), all adenomas resected | LR adenomas health state | 0.163 |
| HR adenoma (IR), all adenomas resected | HR adenomas health state | 0.091 |
| HR adenoma (IR), all adenomas resected | CRC health state | a |
| HR adenoma (HR), all adenomas resected | LR adenomas health state | 0.188 |
| HR adenoma (HR), all adenomas resected | HR adenomas health state | 0.568 |
| HR adenoma (HR), all adenomas resected | CRC health state | a |
To ensure consistency between the model parameters, it is important that the post-polypectomy transition probabilities used align with the other natural history transition probabilities in the model. It was assumed that people who are undergoing surveillance post polypectomy are at higher risk of developing adenomas than people with a normal epithelium, and that polypectomy reduces the risk of developing colorectal cancer. Hence, restrictions were placed on the post-polypectomy transition probabilities, as described in Table 46.
| Post polypectomy (LR) to LR adenoma > normal epithelium to LR adenoma |
| Post polypectomy (HR) to LR adenoma > normal epithelium to LR adenoma |
| Post polypectomy (LR) to HR adenoma < LR adenoma to HR adenoma |
| Post polypectomy (LR) to HR adenoma > normal epithelium HR adenoma |
| Post polypectomy (HR) to HR adenoma > normal epithelium HR adenoma |
| Post polypectomy (LR) to CRC < LR adenoma to CRC |
| Post polypectomy (LR) to CRC > normal epithelium to CRC |
| Post polypectomy (HR) to CRC < HR adenoma to CRC |
| Post polypectomy (HR) to CRC > normal epithelium to CRC |
| Post polypectomy (LR) to LR adenoma < post polypectomy (HR) to LR adenoma |
| Post polypectomy (LR) to HR adenoma < post polypectomy (HR) to HR adenoma |
| Post polypectomy (LR) to CRC adenoma < post polypectomy (HR) to CRC adenoma |
Long-term estimates of costs and quality-adjusted life-years
Table 37 presents the results of the SBCS analyses, showing expected discounted costs and QALYs for patients at each of the diagnostic end points from the decision tree model (as listed in Table 34). Estimates are for one person aged 65 years in each diagnostic category, from the end of colonoscopy after a positive FOBT result with removal of polyps if indicated, and then modelled over a lifetime horizon. The costs presented here do not include costs for the initial colonoscopy, polypectomy, histopathology or adverse events, which are modelled in the decision tree. They do include costs for subsequent follow-up, including routine screening and surveillance, and for treatment of any incident cancers. Similarly, the QALY estimates do not include effects of any adverse events associated with the initial colonoscopy and polypectomies, but they do include adverse effects associated with subsequent rounds of screening or surveillance, and with incident cancers.
Results from the SBCS model were counterintuitive for patients with one or more adenomas missed and left in situ and routine screening follow-up. Estimated QALYs for this group (11.26730) were higher than for patients with all adenomas resected and the same follow-up interval (11.26653 for low risk). Similarly, long-term cost estimates for patients with routine screening were lower if adenomas were missed (£98) than if all adenomas had been successfully identified and removed (£109). This small inconsistency appears to result from the assumptions about direct (de novo) incidence of cancers from the ‘adenomas removed’ and ‘adenomas in situ’ health states (see Figure 34). In the low-risk group, if all adenomas are removed, the risk of progression to cancer through this direct route compensates for the reduced risk of cancer via the adenoma–carcinoma pathway. To compensate for this effect, we adjusted the estimated QALYs and costs for patients with adenomas left in situ and routine screening. We calculated the QALY loss of having adenomas left in situ compared with having all adenomas removed for the high-risk group with routine screening and similarly with 3-yearly surveillance. Then we calculated the ratio between the 3-year surveillance QALY loss and the routine screening QALY loss. This ratio was then assumed to be the same for the low-risk group. The same method was used to adjust the cost estimate for low-risk patients with adenomas left in situ and routine screening.
Results of the independent economic analysis
Base-case cost-effectiveness results
The base-case analysis patients in the model are those undergoing bowel cancer screening with a starting age of 65 years. The colonoscopy costs are derived from NHS reference costs and include the cost of the colonoscopy equipment and its maintenance in the base case, with all system costs (endoscope, system and maintenance) identical across interventions. A sensitivity analysis is conducted using costs system, scope and maintenance costs from each manufacturer in Scenario analyses.
Table 47 reports the clinical outputs produced by the decision tree model. In the histopathology strategy, all polyps are resected, whereas between 58% and 63% of polyps are resected for FICE and NBI, respectively. VCE reduces the number of hyperplastic polyps resected from 1.53 in the histopathology-alone strategy to between 0.06 (i-scan) and 0.14 (FICE), but leaves some adenomas in situ (between 0.04 for i-scan and 0.21 for FICE). VCE reduces adverse events as a result of bleeding and perforations, and deaths from perforations by roughly one-third. The incidence of colorectal cancer is about 3% for all technologies (see Appendix 12). The correct surveillance interval estimated in the model varies for the VCE technologies between 94% (FICE) and 97% (i-scan).
| Parameter | Histopathology | VCE technology | ||
|---|---|---|---|---|
| NBI | FICE | i-scan | ||
| Polypectomy (%) | 100.00 | 63.38 | 58.42 | 61.84 |
| Polyps resected (n) | 2.97 | 1.47 | 1.37 | 1.45 |
| Hyperplastic polyps resected (n) | 1.53 | 0.13 | 0.14 | 0.06 |
| Hyperplastic polyps left in situ (n) | 0 | 1.40 | 1.39 | 1.48 |
| Adenomas resected (n) | 1.44 | 1.33 | 1.22 | 1.39 |
| Adenomas left in situ (n) | 0 | 0.10 | 0.21 | 0.04 |
| Bleeding events (n) | 0.003 | 0.00190 | 0.00175 | 0.00186 |
| Perforations (n) | 0.003 | 0.00190 | 0.00175 | 0.00186 |
| Perforation deaths (n) | 0.000156 | 0.000099 | 0.000091 | 0.000096 |
| Adenomas left in situ (%) | 0.00 | 7.13 | 14.70 | 3.04 |
| Hyperplastic polyps resected (%) | 100.00 | 8.68 | 9.44 | 3.68 |
| Correct surveillance Interval (%) | 100 | 94.7 | 93.8 | 97.4 |
| Incidence of colorectal cancer (%) | 3.025 | 3.020 | 3.045 | 3.021 |
The incremental results of the base-case deterministic analysis with the long-term model are presented in Table 48. Where an intervention is dominated (more costly and less effective), the incremental costs for the next least costly intervention are compared with the costs for the next non-dominated intervention. Pairwise comparisons to histopathology are also presented for NBI, FICE and i-scan for full incremental costs, QALYs and ICERs.
| Comparator | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£ per QALY) |
|---|---|---|---|---|---|
| Full incremental results | |||||
| Histopathology | 988.95 | – | 11.2703 | – | Dominated |
| FICE | 901.25 | –87.70 | 11.2701 | –0.0001 | |
| i-scan | 909.74 | 8.49 | 11.2709 | 0.0008 | 10,465.74 |
| NBI | 915.85 | 6.11 | 11.2708 | –0.0001 | Dominated |
| Pairwise comparisons | |||||
| Histopathology | 988.95 | 11.2703 | |||
| NBI | 915.85 | –73.10 | 11.2708 | 0.0005 | Dominates |
| Histopathology | 988.95 | 11.2703 | |||
| FICE | 901.25 | –87.70 | 11.2701 | –0.0001 | 671,383a |
| Histopathology | 988.95 | 11.2703 | |||
| i-scan | 909.74 | –79.21 | 11.2709 | 0.0007 | Dominates |
In pairwise comparisons, NBI and i-scan and FICE are cost saving compared with histopathology. The QALYs for VCE and histopathology are similar, with very small differences between the technologies. Technically, NBI and i-scan dominate histopathology (i.e. they are cheaper and more effective). FICE is more cost-effective than histopathology, as the ICER for histopathology compared with FICE is > £30,000 per QALY. The difference between histopathology and i-scan, the most effective intervention, was 0.25 quality-adjusted days per individual. The differences in costs between the VCE technologies were < £15 over a patient lifetime. i-scan is £79 less costly than histopathology and produces 0.0007 more QALYs. VCE technologies have a cost saving of about £50 per-polyp resection avoided compared with histopathology.
Table 49 shows the costs and QALYs for the initial colonoscopy and for the long-term component for each risk group for NBI compared with histopathology. Most of the cost savings occur for the initial colonoscopy. For the low-risk group, the long-term costs are higher for NBI, as a result of the small proportion of patients who are assigned to a more frequent surveillance interval. Most of the QALY gains for NBI are from the reduction in deaths from perforation. There are QALY gains for NBI for patients assigned to more frequent surveillance interval, particularly for patients with low risk, and QALY losses for patients with adenomas left in situ and assigned to less frequent surveillance interval.
| Output | Cost | QALYs | ||||
|---|---|---|---|---|---|---|
| Histopathology | NBI | Difference | Histopathology | NBI | Difference | |
| Initial colonoscopy | 691.68 | 607.46 | 84.22 | –0.00005 | –0.00003 | –0.00002 |
| Zero adenomas | 32.88 | 32.88 | 0.00 | 3.3986 | 3.3990 | –0.0003 |
| Low-risk adenoma | 58.34 | 83.08 | –24.74 | 6.0298 | 6.0305 | –0.0007 |
| Intermediate-risk adenoma | 117.42 | 108.36 | 9.06 | 1.2095 | 1.2090 | 0.0005 |
| High-risk adenoma | 88.63 | 84.07 | 4.56 | 0.6324 | 0.6324 | 0.0000 |
| Total | 988.95 | 915.85 | 73.10 | 11.2703 | 11.2708 | –0.0005 |
Sensitivity analyses
One-way deterministic sensitivity analyses
Parameters were varied across a range of lower and upper values. The parameters that were varied in one-way sensitivity analyses are reported in Tables 50 and 51. Most of the one-way sensitivity analyses use 95% CIs from data identified during our systematic review and targeted parameter searches. However, some data were taken from different ranges, for example to show the variation between studies for these data. The prevalence of adenomas were varied across the possible range for each risk classification.
| Parameter | Mean | Lower | Upper | Range definition |
|---|---|---|---|---|
| NBI sensitivity | 0.910 | 0.855 | 0.945 | 95% CI |
| NBI specificity | 0.819 | 0.760 | 0.866 | 95% CI |
| FICE sensitivity | 0.814 | 0.732 | 0.875 | 95% CI |
| FICE specificity | 0.850 | 0.786 | 0.898 | 95% CI |
| i-scan sensitivity | 0.962 | 0.917 | 0.983 | 95% CI |
| i-scan specificity | 0.906 | 0.842 | 0.946 | 95% CI |
| Proportion of low-confidence assessments | 0.210 | 0.105 | 0.315 | Assumed range |
| Prevalence of adenomas in patients with polyps | 0.698 | 0.600 | 0.800 | Assumed range |
| Average adenomas in patients who have LR adenomas | 1.395 | 1.000 | 2.000 | Assumed range |
| Average adenomas in patients who have IR adenomas | 3.341 | 3.000 | 4.000 | Assumed range |
| Average adenomas in patients who have HR adenomas | 5.913 | 5.000 | 9.000 | Assumed range |
| Probability of perforation with polypectomy | 0.003 | 0.000 | 0.010 | 95% CI |
| Probability of perforation death | 0.052 | 0.010 | 0.110 | 95% CI |
| Probability of hospitalisation for bleeding | 0.003 | 0.000 | 0.010 | 95% CI |
| Cost of colonoscopy (without polypectomy) (£) | 518.36 | 340.89 | 695.83 | 95% CI |
| Cost of colonoscopy (with polypectomy) (£) | 600.16 | 406.24 | 794.08 | 95% CI |
| Cost of treating bowel perforation (major surgery) (£) | 2152.77 | 902.21 | 3403.33 | 95% CI |
| Cost of admittance for bleeding (overnight stay on medical ward) (£) | 475.54 | 327.69 | 623.39 | 95% CI |
| Pathology cost (£) | 28.82 | 6.78 | 50.86 | 95% CI |
| Training cost (£) | 14.72 | 10.30 | 19.14 | 95% CI = ± 30% of mean |
Data were not available for the uncertainty around the long-term outcomes. We included one-way sensitivity analyses for these outcomes but used arbitrary ranges. We included the long-term outcomes for patients with incorrect diagnoses (i.e. FNs and FPs in each risk category, for both costs and QALYs). The ranges used were calculated by adding or subtracting half the difference between a correct diagnosis and the false diagnosis in either costs or QALYs. The ranges used are reported in Table 51.
| Parameter | Mean | CI | Assumption | |
|---|---|---|---|---|
| Lower | Upper | |||
| Health state costs (£) | ||||
| LR hyperplastic polyps resected | 1075 | 592 | 1558 | CI = 50% of difference between HPR and CD |
| LR missed adenoma | 250 | 180 | 321 | CI = 50% of difference between MA and CD |
| IR hyperplastic polyps resected | 1577 | 1337 | 1817 | CI = 50% of difference between HPR and CD |
| IR missed adenoma | 250 | 0 | 674 | CI = 50% of difference between MA and CD |
| HR hyperplastic polyps resected | 1584 | 1584 | 1584 | CI = 50% of difference between HPR and CD |
| HR missed adenoma | 1161 | 950 | 1373 | CI = 50% of difference between MA and CD |
| Health state QALYs | ||||
| LR hyperplastic polyps resected | 11.2830 | 11.2830 | 11.3159 | CI = 50% of difference between HPR and CD |
| LR missed adenoma | 11.2627 | 11.2627 | 11.2653 | CI = 50% of difference between MA and CD |
| IR hyperplastic polyps resected | 11.3010 | 11.3010 | 11.3042 | CI = 50% of difference between HPR and CD |
| IR missed adenoma | 11.2463 | 11.2463 | 11.2817 | CI = 50% of difference between MA and CD |
| HR hyperplastic polyps resected | 11.3025 | 11.3025 | 11.3025 | CI = 50% of difference between HPR and CD |
| HR missed adenoma | 11.2971 | 11.2971 | 11.3007 | CI = 50% of difference between MA and CD |
The results of the one-way sensitivity analyses for each VCE technology [NBI, FICE and i-scan (Figures 35–37)] are presented as pairwise comparisons to histopathology.
FIGURE 35.
Tornado plot of one-way sensitivity analyses for NBI. MA, missed adenoma.

FIGURE 36.
Tornado plot of one-way sensitivity analyses for FICE. MA, missed adenoma.
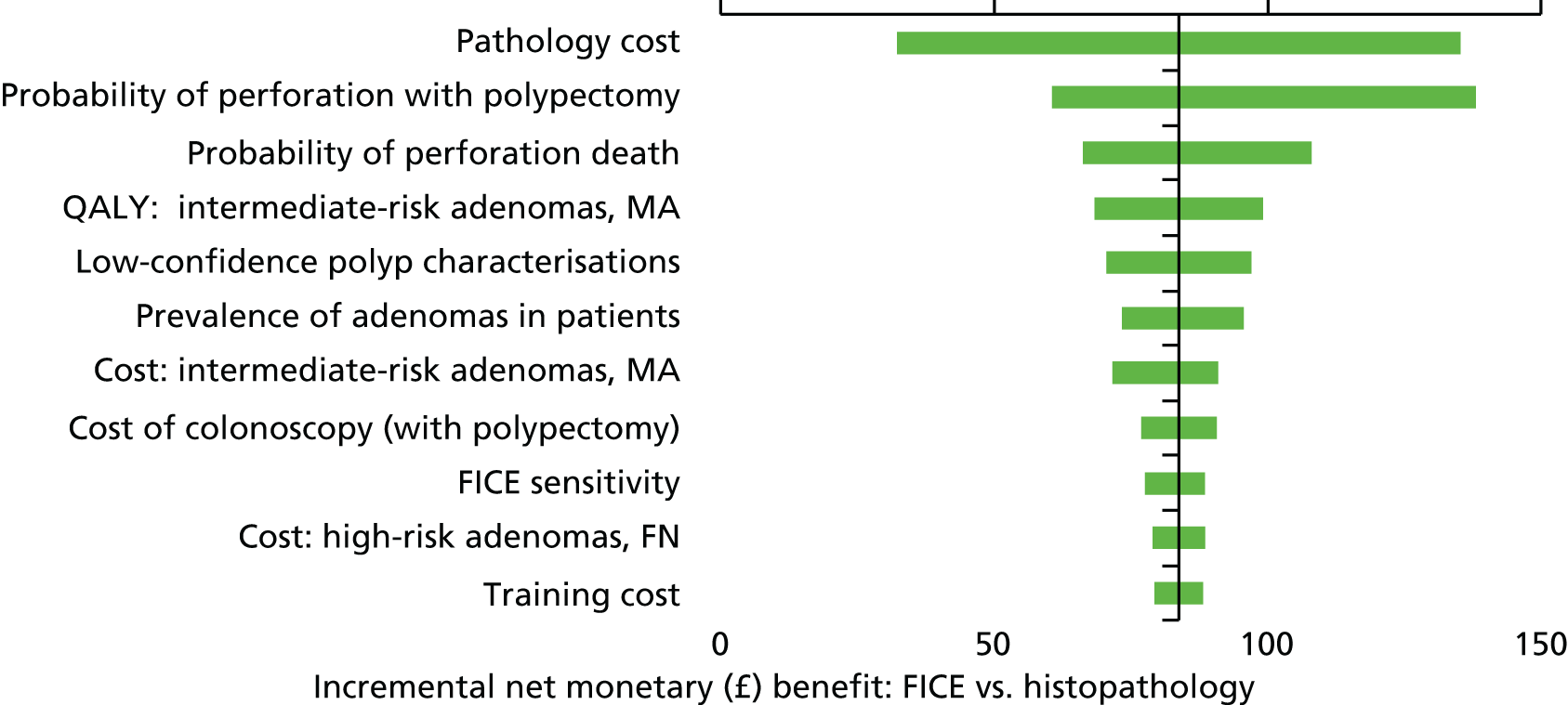
FIGURE 37.
Tornado plot of one-way sensitivity analyses for i-scan. MA, missed adenoma.

For each VCE technology, there were 25 parameters evaluated and the 11 most influential parameters on the model results are presented in the corresponding tables. The results show the changes in incremental net monetary benefits, rather than the change in ICERs. As the ICERs are negative, these values are more difficult to interpret.
For NBI compared with histopathology, NBI remained the dominant strategy for all sensitivity analyses. Figure 35 shows that, for NBI compared with histopathology, the most influential parameters on the model results are the pathology cost, the probability of perforation with polypectomy and the proportion of patients who die from perforation, and the long-term QALY estimate for intermediate patients with a missed adenoma.
Figure 36 shows that, for histopathology compared with FICE, the most influential parameters on the model results are the pathology cost, the probability of perforation with polypectomy and the proportion of patients who die from perforation, and the proportion of low-confidence characterisations. FICE remained more cost-effective than histopathology for all sensitivity analyses.
The most influential parameters on the model results for one-way analyses comparing i-scan with histopathology are the pathology cost, the probability of perforation with polypectomy and the proportion of polyp characterisations made at low confidence.
Scenario analyses
In this section, 12 scenario analyses are explored. The descriptions of the scenario analyses are provided in Table 52. Further description of the components of each analysis follow.
| Number | Analysis | Diagnostic accuracy (part of colon – confidence in characterisation)a | Other parameters changed |
|---|---|---|---|
| 0 | Base case | Whole colon – high | |
| 1 | Surveillance patients | Whole colon – high | Starting risk distributions changed |
| 2 | Symptomatic patients | Whole colon – high | Starting risk distributions changed |
| 3 | DISCARD strategy70 | Rectosigmoid colon – high | Only polyps in rectosigmoid colon may be left in situ |
| 4 | DISCARD strategy70 | Whole colon – high | Only polyps in rectosigmoid colon may be left in situ |
| 5 | DISCARD strategy70 | Whole colon – any | Only polyps in rectosigmoid colon may be left in situ |
| 6 | VCE strategy | Whole colon – any | |
| 7 | Costs calculated for each system (endoscope, system, maintenance) | Whole colon – high | Costs for each scope calculated as in Appendix 11 |
| 8 | Long-term QALYs derived from SBCS model use alternative utility values | Whole colon – high | Utility values for colorectal cancer derived from Färkkilä et al.145 and simulated using SBCS for long-term QALYs (see Table 49) |
| 9 | Pooled VCE base case | Whole colon – high | |
| 10 | NBI, experienced endoscopists | Whole colon – high | |
| 11 | NBI, experienced endoscopists | Rectosigmoid colon – high | Only polyps in rectosigmoid colon may be left in situ |
| 12 | Follow-up surveillance | Whole colon – high | Long-term costs and QALYs |
The population for the base-case analysis is for patients referred for colonoscopy following bowel cancer screening. Scenario analyses were used to explore two further populations: patients receiving surveillance colonoscopy following previous adenoma removal (referred to as surveillance patients) (scenario 1) and patients referred for colonoscopy for symptoms suggestive of colorectal cancer (symptomatic patients) (scenario 2). We performed scenario analyses using alternative starting distributions of patients between risk categories to conduct both of these analyses; the alternative values used in these analyses are reported in Prevalence of polyps and adenomas.
For our base-case analysis we used the VCE strategy. Three scenario analyses using the DISCARD strategy were conducted with different diagnostic accuracy data used for each. The differences between the VCE strategy and the DISCARD strategy are described in Methods for economic analysis. Scenario 3 uses diagnostic accuracy data derived from high-confidence characterisations in the rectosigmoid colon. Scenario 4 uses diagnostic accuracy data derived from high-confidence decisions in the whole colon. Scenario 5 uses diagnostic accuracy data from polyp characterisations made in the whole colon with any level of confidence.
We also conducted a scenario analysis in which the VCE strategy was applied to the whole colon (scenario 6), but with diagnostic accuracy data for any level of confidence characterisation instead of diagnostic accuracy from high-confidence characterisations in the whole colon (as in the base case). This analysis would represent a worst-case scenario on diagnostic accuracy. The diagnostic accuracy data used for scenarios 3–6 are reported in Table 53. All diagnostic accuracy data for NBI and FICE were derived from meta-analyses in Chapter 4, Assessment of diagnostic accuracy (sensitivity, specificity, negative predictive value, accuracy). For i-scan, diagnostic accuracy for the base case and scenario 4 was derived from our meta-analysis as reported in Chapter 4, Assessment of diagnostic accuracy (sensitivity, specificity, negative predictive value, accuracy), whereas diagnostic accuracy for scenario 3 was derived from Rath and colleagues,82 and scenarios 5 and 6 were derived from Lee and colleagues. 77
| Diagnostic accuracy (colon location – confidence in characterisation) | VCE technology, accuracy (%) | |||||
|---|---|---|---|---|---|---|
| NBI | FICE | i-scan | ||||
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| Rectosigmoid colon – high confidencea | 87.41 | 95.26 | 81.39 | 85.02 | 98.10 | 94.40 |
| Whole colon – high confidenceb | 90.97 | 81.88 | 81.39 | 85.02 | 94.34 | 91.53 |
| Whole colon – any confidence levelc | 88.17 | 80.74 | 81.39 | 85.02 | 96.05 | 88.15 |
In the base-case analysis, all VCE systems have the same cost, as the equipment and maintenance cost for the colonoscopy systems are included in the reference cost of colonoscopy. In this analysis, we investigated the effect on the model results of including the difference in the systems costs compared with the average costs of NBI, FICE and i-scan, using market share data. The net cost differences related to system costs (scope, system and maintenance) from average costs for colonoscopy techniques are reported in Table 54. The calculation of these parameter values is shown in Appendix 11.
| VCE technology | Cost difference | 95% CI | Standard error | |
|---|---|---|---|---|
| Lower | Upper | |||
| NBI | 19.36 | 5.08 | 33.64 | 7.29 |
| FICE | –61.93 | –81.22 | –42.63 | 9.84 |
| i-scan | –48.27 | –53.22 | –43.32 | 2.53 |
Scenario 8 investigates the effect of alternative utility values, derived through our literature review of quality-of-life studies, have on the model results. The utility values used to generate these long-term outcomes are reported in Table 55, whereas the long-term QALYs produced through by SBCS model for the alternative utility values are reported in Health-related quality of life.
| Health state | Analysis | |
|---|---|---|
| Base case143 | Scenario 8143,145 | |
| No cancer | 0.798 | 0.798 |
| Colorectal cancer | 0.697 | 0.761 |
Scenario 9 investigates the combined effect of VCE technologies compared with histopathology. The diagnostic accuracy data for this scenario were taken from our meta-analysis pooling all available studies from high-confidence characterisations in the whole colon (described in Chapter 4, Summary of diagnostic test performance evidence) and are shown in Table 56. This scenario is based on a post hoc meta-analysis used to illustrate a possible class effect of the VCE technologies. (Note that it features NBI and i-scan studies but there was insufficient evidence to include FICE.)
| Number | Scenario | Histopathology cost (£) | QALY | NBI cost (£) | QALY | ICER |
|---|---|---|---|---|---|---|
| 0 | Base case | 988.95 | 11.2703 | 915.85 | 11.2708 | Dominated |
| 1 | Surveillance patients | 925.66 | 11.2684 | 840.97 | 11.2692 | Dominated |
| 2 | Symptomatic patients | 910.75 | 11.2679 | 804.35 | 11.2687 | Dominated |
| 3 | DISCARD strategy, rectosigmoid colon: high confidence (diagnostic accuracy) | 988.95 | 11.2703 | 946.84 | 11.2703 | Dominated |
| 4 | DISCARD strategy, whole colon: high confidence (diagnostic accuracy) | 988.95 | 11.2703 | 962.08 | 11.2708 | Dominated |
| 5 | DISCARD strategy, whole colon: any confidence level (diagnostic accuracy) | 988.95 | 11.2703 | 962.38 | 11.2708 | Dominated |
| 6 | VCE strategy, whole colon: any confidence level (diagnostic accuracy) | 988.95 | 11.2703 | 914.29 | 11.2706 | Dominated |
| 7 | Costs calculated for each system | 988.95 | 11.2703 | 931.14 | 11.2708 | Dominated |
| 8 | Alternate utility values | 988.95 | 11.2759 | 915.85 | 11.2765 | Dominated |
Scenarios 10 and 11 use diagnostic accuracy data from studies that reported data for endoscopists experienced in the use of NBI. This scenario is informed by a post hoc meta-analysis of the subset of NBI studies in which endoscopists were experienced in the use of NBI for optical characterisation of polyps. This is in contrast to the base-case meta-analysis of NBI studies which included studies of experienced and non-experienced endoscopists. In the clinical review, experienced endoscopists had higher diagnostic accuracy, and the majority of i-scan studies were conducted exclusively with experienced endoscopists. Scenarios 10 and 11 were therefore conducted to provide a more comparable experience level across interventions. These data are shown in Table 57 and the meta-analysis to derive them is described in Chapter 4, Assessment of diagnostic accuracy (sensitivity, specificity, negative predictive value, accuracy).
| Number | Scenario | Accuracy (%) | |
|---|---|---|---|
| Sensitivity | Specificity | ||
| 9 | Pooled VCE base case | 91.82 | 83.20 |
| 10 | NBI, experienced endoscopists (whole colon) | 91.83 | 82.16 |
| 11 | NBI, experienced endoscopists (rectosigmoid colon) | 90.37 | 98.14 |
In the base case, the long-term cost and QALY outcomes derived from the SBCS model were estimated assuming the use of standard colonoscopy for any patients requiring follow-up surveillance (i.e. VCE was not used during follow-up colonoscopy). These long-term costs and QALY outcomes do not therefore show the true extent of the future colonoscopies. For example, we would expect there to be future cost savings for VCE in any future colonoscopies. We investigated the likely impact on the model results if all patients assigned to the VCE group were to receive VCE technologies for follow-up surveillance (scenario 12).
The long-term costs and QALYs for the histopathology group were adjusted by an estimate of the differences in costs and QALYs for a follow-up colonoscopy. These were calculated according to the numbers of patients receiving follow-up colonoscopy in each risk group and the additional costs and loss in QALYs at follow-up surveillance, taken from our analysis for the surveillance population (scenario 2, see Table 57). From this analysis, the additional cost for each patient receiving histopathology compared with NBI is £84.69 and the loss in QALYs is –0.0007.
We assumed that 20% of patients in the low-risk group would have a follow-up colonoscopy after 10 years, all intermediate-risk patients would have a follow-up colonoscopy after 3 years and all high-risk patients would have a follow-up colonoscopy after 1 year. Additional costs at colonoscopy were discounted according to how many years until the surveillance colonoscopy. The long-term costs and QALYs for histopathology for the low-risk, intermediate-risk and high-risk groups were then adjusted by the estimates shown in Table 58.
| Risk group | Proportion receiving follow-up colonoscopy (%) | Time (years) until surveillance colonoscopy | Additional cost (£), discounted at 3.5% p.a. | Additional discounted QALYs |
|---|---|---|---|---|
| Low | 20 | 10 | 12.01 | –0.00015 |
| Intermediate | 100 | 3 | 76.38 | –0.0007 |
| High | 100 | 1 | 81.82 | –0.0007 |
Results of scenario analyses
Pairwise results of the scenario analyses 1–8 are reported for histopathology compared with NBI (Table 56), FICE (Table 59) and i-scan (Table 60).
| Number | Scenario | Histopathology cost (£) | QALY | FICE cost (£) | QALY | ICER (£) |
|---|---|---|---|---|---|---|
| 0 | Base case | 988.95 | 11.2703 | 901.25 | 11.2701 | 671,383 |
| 1 | Surveillance patients | 925.66 | 11.2684 | 830.53 | 11.2687 | Dominated |
| 2 | Symptomatic patients | 910.75 | 11.2679 | 794.23 | 11.2684 | Dominated |
| 5 | DISCARD strategy, whole colon: any confidence level (diagnostic accuracy) | 988.95 | 11.2703 | 955.93 | 11.2705 | Dominated |
| 7 | VCE strategy, whole colon: any confidence level (diagnostic accuracy) | 988.95 | 11.2703 | 863.12 | 11.2701 | 963,335 |
| 8 | Alternate utility values | 988.95 | 11.2759 | 901.25 | 11.2759 | 1,273,941 |
| Number | Scenario | Histopathology cost (£) | QALY | i-scan cost (£) | QALY | ICER |
|---|---|---|---|---|---|---|
| 0 | Base case | 988.95 | 11.2703 | 909.74 | 11.2709 | Dominated |
| 1 | Surveillance patients | 925.66 | 11.2684 | 834.99 | 11.2693 | Dominated |
| 2 | Symptomatic patients | 910.75 | 11.2679 | 801.43 | 11.2689 | Dominated |
| 3 | DISCARD strategy, rectosigmoid colon: high confidence (diagnostic accuracy) | 988.95 | 11.2703 | 949.62 | 11.2706 | Dominated |
| 4 | DISCARD strategy, whole colon: high confidence (diagnostic accuracy) | 988.95 | 11.2703 | 954.70 | 11.2707 | Dominated |
| 5 | DISCARD strategy, whole colon: any confidence level (diagnostic accuracy) | 988.95 | 11.2703 | 958.58 | 11.2708 | Dominated |
| 6 | VCE strategy, whole colon: any confidence level (diagnostic accuracy) | 988.95 | 11.2703 | 913.85 | 11.2709 | Dominated |
| 7 | Costs calculated for each system | 988.95 | 11.2703 | 860.82 | 11.2709 | Dominated |
| 8 | Alternate utility values | 988.95 | 11.2759 | 909.74 | 11.2766 | Dominated |
The scenarios show that NBI dominates histopathology for all scenarios (i.e. NBI is less expensive and more effective).
Flexible spectral imaging colour enhancement has fewer scenario analyses because there is only one source of diagnostic accuracy, a meta-analysis of all FICE characterisations in the whole colon at any level of confidence, which eliminates the possibility of conducting scenario 3, 4 or 6. For subgroup analysis for surveillance and symptomatic patients and the DISCARD strategy (scenario 5), FICE dominates histopathology. For scenarios 7 and 8, FICE remains cost-effective compared with histopathology.
For all scenario analyses comparing i-scan to histopathology, i-scan was the dominant strategy.
Scenario 9 shows the analysis for pooled VCE compared with histopathology (Table 61). The results for this scenario are similar to the base-case analysis for NBI, and VCE dominates histopathology. For the analysis comparing NBI performed by an endoscopist with prior NBI experience to histopathology, the results are also similar to the base-case analyses for NBI and VCE.
| Number | Scenario | Cost (£) | QALYs | ICER (£/QALY) |
|---|---|---|---|---|
| 9 | Pooled VCE, whole colon, high confidence | |||
| Histopathology | 988.95 | 11.2703 | – | |
| All VCE | 914.96 | 11.2708 | Dominates | |
| 10 | Experienced endoscopists for NBI, whole colon | |||
| Histopathology | 988.95 | 11.2703 | – | |
| NBI | 916.49 | 11.2708 | Dominates | |
| 11 | Experienced endoscopists for NBI, rectosigmoid colon | |||
| Histopathology | 988.95 | 11.2703 | – | |
| NBI | 944.69 | 11.2703 | Dominates | |
The results for the surveillance scenario which included the differences in costs and QALYs between NBI and histopathology in a follow-up colonoscopy (scenario 12) are shown in Table 62. These results are not significantly different from the base-case analysis. Compared with the base-case analysis, there is an increase in cost savings for NBI of £20 and an increase in incremental QALYs of 0.0003.
| Comparator | Cost (£) | Incremental cost (£) | QALYs | Incremental QALY | ICER (£/QALY) |
|---|---|---|---|---|---|
| Histopathology | 1011.75 | – | 11.2700 | – | – |
| NBI | 915.85 | –95.91 | 11.2708 | 0.0008 | Dominates |
Probabilistic sensitivity analysis
A probabilistic sensitivity analysis was undertaken to provide estimates of cost-effectiveness and the likelihood of cost-effectiveness under joint uncertainty of parameters. In the probabilistic analysis, costs for colonoscopies are assumed to be identical between technologies. The PSA was undertaken using 5000 simulations. Cost-effectiveness acceptability curves were created using the net benefit method to represent the probabilities of interventions being the most cost-effective option across a range of cost-effectiveness thresholds. The parameters and the distributions used in the PSA are shown in Appendix 9. The choice of distributions used in the PSA is based on common practice.
Results
Table 63 and Figure 38 present the result of the base-case analysis using the VCE strategy (described in Methods for economic analysis).
| Comparator | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Histopathology | 987.07 | – | 11.2703 | – | Dominated |
| FICE | 899.74 | –87.33 | 11.2701 | –0.0001 | |
| i-scan | 908.07 | 8.34 | 11.2709 | 0.0008 | 10,298.72 |
| NBI | 914.19 | 6.12 | 11.2708 | –0.0001 | Dominated |
In the base-case analysis, i-scan was the most cost-effective technology in 85.2% of analyses at a cost-effectiveness threshold of £20,000 per QALY and in 99.5% of simulations at £30,000 per QALY.
FIGURE 38.
Cost-effectiveness acceptability curves (base case).
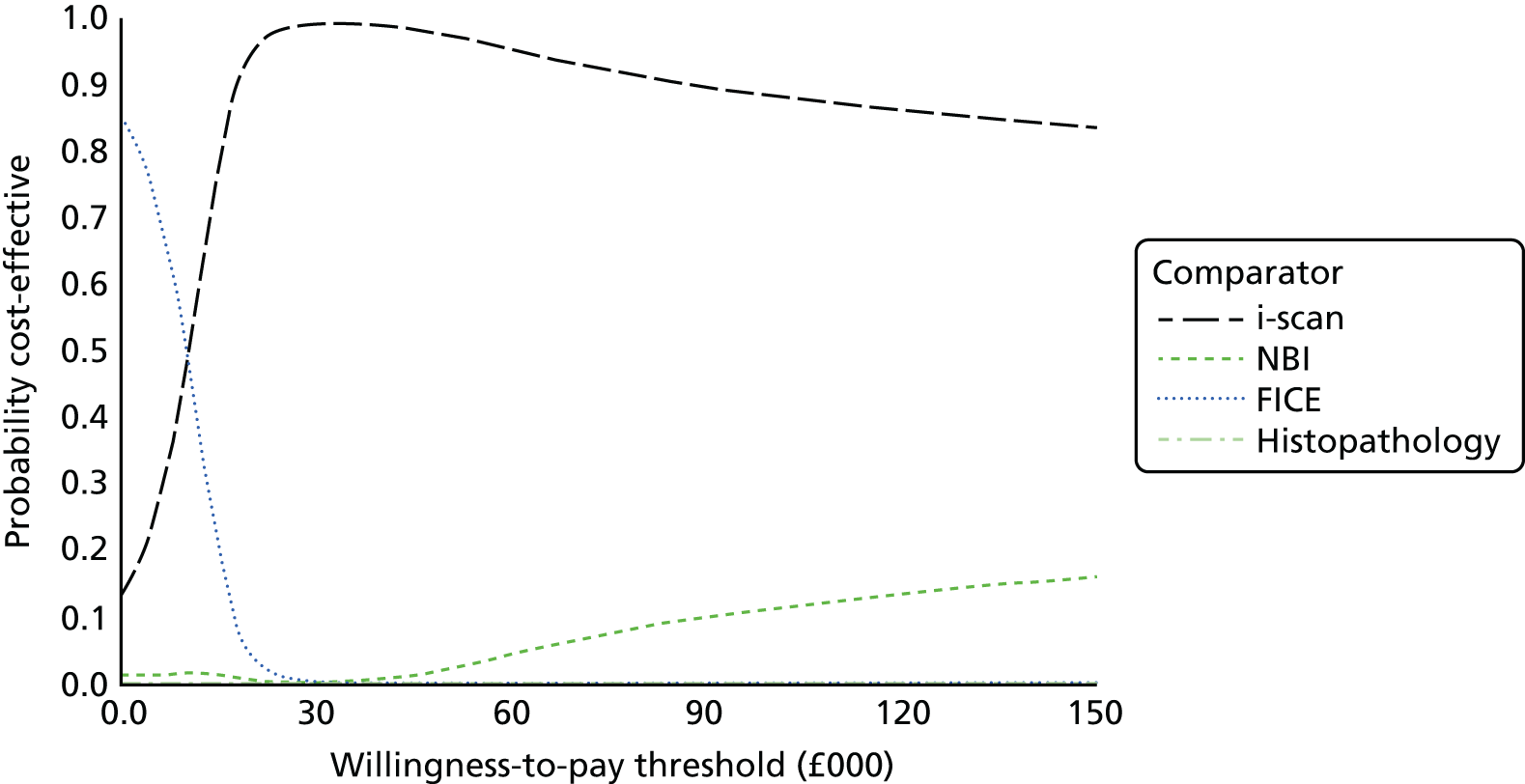
Comparison of the economic models
Our systematic review of cost-effectiveness identified two previous economic evaluations by Hassan and colleagues112 and Kessler and colleagues. 113 Comparing results from these evaluations with our model is difficult, given the differences in design and data used in these studies. Both previous economic evaluations used a similar strategy for VCE to that used in our model. They used a resect and DISCARD strategy in the whole colon. Furthermore, Hassan and colleagues112 included the whole screening population, whereas the population used for Kessler and colleagues113 and our analysis is for those who had one or more polyps identified. The two previous studies are for a different health-care system (USA), and so there are differences in the health state resource costs used between the models. In addition, the two previous studies have not presented the results in QALYs.
The proportion of low-confidence assessments and the diagnostic accuracy data used in the model are shown in Table 64. The sensitivity of NBI used in the model is similar between the studies, but we have used a lower specificity than the other models. Kessler and colleagues113 assumed that all patients would be assessed with high confidence, whereas we assume that only 79% of patients are assessed with high confidence.
| Study | Parameter, accuracy (%) | ||
|---|---|---|---|
| Low-confidence assessment | NBI | ||
| Sensitivity | Specificity | ||
| Hassan et al.112 | 16 | 94 | 89 |
| Kessler et al.113 | 0 | 90 | 90 |
| Current assessment | 21 | 91 | 82 |
All studies concluded that VCE would be cost saving compared with histopathology. The cost saved per person over the patient’s lifetime was US$174 in the model by Kessler and colleagues113 and £74 in our model.
The expected benefit of resect and DISCARD was 0.0005 years of life in Kessler and colleagues,113 compared with 0.0005 QALYs in our model, whereas Hassan and colleagues112 found no difference in life expectancy between groups over the patient’s lifetime. The data used for the disease progression to predict life expectancy were not fully reported in Kessler and colleagues. 113 The cost-effectiveness of the submit-all strategy compared with resect and DISCARD all polyps varied and was US$377,460 per life-year gained for Kessler and colleagues,113 whereas NBI dominated histopathology in our model. Hassan and colleagues112 were not able to calculate a value, as there was no difference in the life expectancy between the submit-all and the resect and DISCARD strategies.
Chapter 6 Assessment of factors relevant to the NHS and other parties
As discussed in Chapter 1, Current service provision, and Chapter 5, The decision problem, it is known that the majority of hospitals that perform endoscopy currently possess endoscopy systems capable of VCE. Implementation of the technology will therefore not require large-scale replacement of equipment. However, not all systems currently in use comprise fully HD components (i.e. endoscope, light source, video processor, visual display monitor, cabling). Optimum image quality will be attained by fully HD systems, and in some centres this may not be achieved until all equipment is routinely upgraded.
The PIVI statement requires that polyp images taken during VCE should be permanently stored and should be of sufficient resolution to support the endoscopists’ assessment and clinical decisions. 32 Therefore, hospitals would need to implement systems to permit adequate electronic storage of HD images linked to patients’ files to allow future re-examination if necessary.
In terms of patient issues and preferences, some patients find colonoscopy to be an uncomfortable experience and, therefore, may prefer that VCE is not used if it may potentially increase the time taken to do the procedure (e.g. the time needed for the endoscopist to inspect the image on the monitor before making a characterisation rather than just resecting it). However, there were very few data from the studies included in our systematic review on differences between procedure times between modes of polyp assessment to provide conclusive evidence.
It is possible that some patients may experience anxiety knowing that a polyp, even one characterised as hyperplastic, has not been resected. Some patients may prefer that all polyps are removed, even when there is negligible risk of them becoming cancerous (notwithstanding the fact that some endoscopists currently leave hyperplastic diminutive polyps in situ, as noted earlier, in Chapter 1 of this report). This would not prohibit VCE from being used as part of optical assessment, but would mean that a full DISCARD strategy (i.e. leaving in situ hyperplastic polyps in the rectosigmoid colon) would not be possible for such patients. If a DISCARD strategy is to be implemented there may be a requirement for patient information about the procedure, and the opportunity for discussion between patient and endoscopist before the colonoscopy.
Although VCE is currently used in some centres to characterise colorectal polyps, its more widespread use would require greater availability of training and auditing to ensure appropriate use. As discussed in Chapter 1, Training in the use of virtual chromoendoscopy, current training practices vary in terms of mode and duration, and studies have illustrated the presence of a learning curve to attain acceptable levels of diagnostic accuracy. The manufacturer of NBI suggests that training of up to 2 days in duration would be sufficient for initial training. However, expert clinical advice suggests that for some endoscopists allocating that amount of time for training might not be realistic because of busy work schedules.
Not all endoscopists may want to assume the responsibility for characterising colorectal polyps and leaving those considered to be hyperplastic in situ. If VCE is to be recommended in the NHS there may be a need for awareness raising and incentives to encourage greater acceptance and use of this technology in practice.
Chapter 7 Discussion
Statement of principal findings
Clinical effectiveness
Thirty studies met the inclusion criteria for the systematic review of test accuracy. These assessed NBI (24 studies20,54–78), i-scan (five studies77,79–82) and FICE (three studies78,83,84). Two of these studies assessed two of the technologies of interest in this diagnostic assessment (NBI and i-scan;77 and NBI and FICE79). Using the QUADAS criteria, we assessed that the results of the studies are likely to be at a low risk of bias. The evidence we identified meets the decision problem for this diagnostic assessment, but there is comparatively little evidence for two of the three technologies being considered (i-scan and FICE). Most of the available evidence evaluated the diagnostic accuracy of NBI for assessing diminutive colorectal polyps. The FICE evidence base was particularly limited. We did not identify any FICE studies that assessed the diagnostic accuracy of endoscopists’ real-time high-confidence evaluations of diminutive polyps, whereas we found evidence in relation to high-confidence assessments made with NBI and i-scan. Some of the included studies explicitly referred to a DISCARD strategy, whereas others did not.
Most of the included studies reported high sensitivity and specificity (with some exceptions), showing that endoscopists had a high probability of correctly identifying adenomas and hyperplastic polyps when using NBI, i-scan or FICE (sensitivity and specificity results are discussed in more detail below; see Table 65). NPV (that is, the probability that patients who are diagnosed by VCE as having a hyperplastic polyp truly do not have an adenoma) was more variable across the NBI studies than the FICE or i-scan studies. There was especially little variation in this outcome across the i-scan studies, in which NPV ranged from 93% to 96.30% for all characterisations and 94.74% to 100% for high-confidence characterisations. Of the three technologies, i-scan had the most consistently favourable results on this outcome. The greater heterogeneity found among the NBI studies may in part be explained by the larger pool of evidence available for NBI than for i-scan and FICE. In additional, two of the FICE studies were conducted by the same research group, which may have reduced heterogeneity. The heterogeneity in the NBI results may have also been as a result of variability in the prevalence of adenomas in the populations included in the studies. When prevalence is increased, the result is a decrease in the NPV. The more favourable NPV results found for i-scan and variability among the NBI studies may also be explained by the endoscopists’ experience in these studies. We note that a range of endoscopists was involved in the NBI studies; some were less experienced in conducting colonoscopy generally and had little or no experience using NBI, while others were very experienced endoscopists who also had extensive experience of using NBI. By contrast, three77,79,80 of the five77,79–82 i-scan studies included endoscopists with prior experience of i-scan and all the studies were conducted in single centres, often described as academic or specialist centres. The NPV results found in the i-scan studies may therefore not reflect the accuracy that might be achieved by endoscopists working in more generalist or community settings. On the other hand, the large evidence base for NBI may have captured the variability in this outcome that may be observed in practice, where it is likely endoscopists with a range of experience will carry out colonoscopy (although we note that the ESGE guidance recommends that only experienced and adequately trained endoscopists should undertake VCE for the real-time assessment of polyps31).
Table 65 summarises the key sensitivity and specificity results from the review and the meta-analyses, which we now discuss in more detail. Meta-analysis was conducted where possible, but the technologies were not assessed head to head in the meta-analyses (as this was not within the decision problem for the assessment, derived from the National Institute for Health and Care Excellence scope), so we cannot comment on how the technologies directly compare with each other statistically.
| Outcome | VCE technology | ||
|---|---|---|---|
| NBI | i-scan | FICE | |
| All characterisations in the whole colona | |||
| Sensitivity, range across all studies reporting outcome | 0.55–0.97 (17 studies) | 0.95b (one study) | 0.74–0.88 (three studies) |
| Sensitivity, bivariate meta-analysis summary value | 0.88 (95% CI 0.83 to 0.92) (16 studies) | Meta-analysis not possible | 0.81 (95% CI 0.73 to 0.88) (three studies) |
| Specificity, range across all studies reporting outcome | 0.62–0.95 (16 studies) | 0.86b (one study) | 0.82–0.88 (three studies) |
| Specificity, bivariate meta-analysis summary value | 0.81 (95% CI 0.75 to 0.85) (16 studies) | Meta-analysis not possible | 0.85 (95% CI 0.79 to 0.90) (three studies) |
| High-confidence characterisations in the whole colon | |||
| Sensitivity, range across all studies reporting outcome | 0.59–0.98 (13 studies) | 0.94–0.97c (two studies) | No evidence |
| Sensitivity, bivariate meta-analysis summary value | 0.91 (95% CI 0.85 to 0.95) (11 studies) | 0.96 (95% CI 0.92 to 0.98)d (two studies) | No evidence |
| Specificity, range across all studies reporting outcome | 0.44–0.92 (12 studies) | 0.90–0.92c (two studies) | No evidence |
| Specificity, bivariate meta-analysis summary value | 0.82 (95% CI 0.76 to 0.87) (11 studies) | 0.91 (95% CI 0.84 to 0.95) (two studies) | No evidence |
| High-confidence characterisations whole colon by endoscopists with prior experience of the technology (post hoc analysis) | |||
| Sensitivity, bivariate meta-analysis summary value | 0.92 (95% CI 0.89 to 0.94) (four studies) | 0.96 (95% CI 0.92 to 0.98)d (two studies) | No evidence |
| Specificity, bivariate meta-analysis summary value | 0.82 (95% CI 0.72 to 0.89) (four studies) | 0.91 (95% CI 0.84 to 0.95)d (two studies) | No evidence |
| All characterisations in the rectosigmoid colona | |||
| Sensitivity, range across all studies reporting outcome | 0.84–0.90 (four studies) | 0.90–0.94 (two studies) | No evidence |
| Sensitivity, bivariate meta-analysis summary value | 0.85 (95% CI 0.75 to 0.91) (three studies) | Meta-analysis not possible | No evidence |
| Specificity, range across all studies reporting outcome | 0.76–0.95 (four studies) | 0.87–0.88 (two studies) | No evidence |
| Specificity, bivariate meta-analysis summary value | 0.87 (95% CI 0.74 to 0.94) (three studies) | Meta-analysis not possible | No evidence |
| High-confidence characterisations in the rectosigmoid colon | |||
| Sensitivity, range across all studies reporting outcome | 0.83–0.96 (five studies) | 0.96 (one study) | No evidence |
| Sensitivity, bivariate meta-analysis summary value | 0.87 (95% CI 0.80 to 0.92) (four studies) | Meta-analysis not possible | No evidence |
| Specificity, range across all studies reporting outcome | 0.88–0.99 (five studies) | 0.96 (one study) | No evidence |
| Specificity, bivariate meta-analysis summary value | 0.95 (95% CI 0.87 to 0.98) (four studies) | Meta-analysis not possible | No evidence |
| High-confidence characterisations in the rectosigmoid colon by endoscopists with prior experience of the technology (post hoc analysis) | |||
| Sensitivity, bivariate meta-analysis summary value | 0.90 (95% CI 0.71 to 0.97) (two studies) | No evidence | No evidence |
| Specificity, bivariate meta-analysis summary value | 0.98 (95% CI 0.91 to 1.00) (two studies) | No evidence | No evidence |
| Post hoc pooled analysis of VCE technologies: high-confidence characterisations in the whole colon | |||
| Sensitivity, bivariate meta-analysis summary value | 0.92 (95% CI 0.87 to 0.95); 11 NBI studies and two i-scan studies | ||
| Specificity, bivariate meta-analysis summary value | 0.83 (95% CI 0.78 to 0.87); 11 NBI studies and two i-scan studies | ||
For all characterisations of polyps (regardless of confidence level) in the whole colon, the i-scan (one study77) and FICE (three studies78,83,84) results were in the same range of values obtained from the NBI studies (1755,56,58,62–71,74,75,77,78 and 16 studies55,56,58,62–71,75,77,78 for sensitivity and specificity, respectively). The summary values from bivariate meta-analysis for sensitivity and specificity of NBI and FICE for all characterisations in the whole colon did not reach 0.90 (i.e. 90%) in either case. Limiting the analysis to high-confidence characterisations of polyps in the whole colon increased the summary sensitivity and specificity values from bivariate meta-analysis; for i-scan (two studies77,79), both values were > 0.90; whereas for NBI (11 studies55–57,59–65,77) only the summary value for sensitivity was > 0.90. As mentioned above, none of the FICE studies analysed outcomes for high-confidence assessments of diminutive polyps. As with the NPV results, the higher sensitivity and specificity values seen for i-scan might be explained by the endoscopists in the two i-scan studies77,79 being experienced endoscopists working in specialist and academic centres. Therefore, we conducted a post hoc analysis restricting the meta-analysis to high-confidence characterisations in the whole colon obtained from studies that reported the endoscopists had prior experience with NBI (four studies59,60,62,77). The summary sensitivity and specificity results from this post hoc analysis of NBI were almost identical to those obtained from all the NBI studies.
Some NBI and i-scan studies provided data on characterisations of polyps in the rectosigmoid colon, but no evidence was available for FICE. For all characterisations of polyps (regardless of confidence level) in the rectosigmoid colon, the NBI (four studies54,55,58,63) and i-scan (two studies81,82) results were similar to those obtained from the whole colon. Limiting the analysis to high-confidence characterisations of polyps in the rectosigmoid colon increased the summary sensitivity and specificity values from bivariate meta-analysis of NBI, and the study estimates from i-scan were also higher (meta-analysis was not possible for i-scan). A post hoc analysis restricting the NBI meta-analysis to high-confidence characterisations in the rectosigmoid colon obtained from studies that reported the endoscopists had prior experience with NBI (two studies54,62) increased the summary sensitivity and specificity values further. However, there was no evidence for i-scan because the single study82 that reported on high-confidence characterisations in the rectosigmoid colon did not report on whether or not the endoscopist had prior experience using i-scan.
Overall, there is evidence showing that, in general, sensitivity and specificity estimates increase when only high-confidence characterisations of polyps are considered rather than all characterisations (i.e. not on the basis of high confidence). It is worth reiterating that the level of confidence with which polyp classifications are made is subjective and is likely to vary between endoscopists. Some endoscopists may refer to the relevant classification system to make a confident polyp characterisation. The studies included in our systematic review did not explicitly state how confidence was achieved. This creates possible uncertainty in the interpretation of diagnostic accuracy based on high-confidence characterisations.
We also generated SROC curves to explore the effect of endoscopist experience with NBI on sensitivity and specificity when characterising polyps in the whole colon. This confirmed that endoscopists with prior experience of using NBI to characterise diminutive colorectal polyps achieve higher sensitivity and specificity than endoscopists with no prior experience of using NBI to characterise diminutive colorectal polyps (other than any training that they undertook at the start of the study). It was not possible to discern this effect when comparing the post hoc meta-analysis of high-confidence characterisations in the whole colon made by endoscopists with prior experience of NBI with the meta-analysis of all high-confidence characterisations in the whole colon. This may be because, three studies in the pool of 11 NBI studies55–57,59–65,77 providing data on high-confidence characterisations in the whole colon included endoscopists with a mix of prior experience56,57,65 and two did not report on prior experience63,64 with NBI, which would probably have masked any difference between NBI-experienced (four studies59,60,62,77) and NBI-naive endoscopists (two studies55,61).
Finally, a post hoc bivariate meta-analysis pooling together all the available evidence for high-confidence characterisations of polyps in the whole colon was undertaken and yielded a sensitivity of 0.92 (95% CI 0.87 to 0.95) and a specificity of 0.83 (95% CI 0.78 to 0.87). There were differing opinions among the clinical experts we consulted regarding whether or not it was appropriate to pool evidence from different VCE technologies. The technologies have the same aim (to enhance surface vessel patterns), but achieve this either by filtering the light source (NBI) or by using digital post-processing software to convert white-light images such that they appear like narrow-band images (i-scan and FICE). This post hoc analysis should therefore be treated as illustrative because of the uncertainty regarding whether or not a class-effect can be assumed and also because the available evidence is predominantly from NBI (11 studies55–57,59–65,77) with only two i-scan studies77,79 and none for FICE.
In terms of the other outcomes of interest in this review, none of the studies measured HRQoL, anxiety, number of outpatient appointments or telephone consultations, incidence of colorectal cancer or mortality. Only three57,75,77 of the NBI studies and one77 of the i-scan studies reported AEs (e.g. complications of polypectomy such as bleeding). All studies reported that there were none. Thus, there are only limited data available on AEs in this review. This is an outcome that future studies should consider measuring. A few of the NBI studies reported on the number of polyps that would be resected and discarded if a resect and discard type of management strategy had been in place. 68,70 Given the limited evidence available, it is challenging to determine the number of polyps that would be designated to be left in place, the number of polyps that would be designated to be resected and discarded and the number of polyps that would be designated for resection and histopathological examination. Likewise, only limited data were available on the length of time to perform the colonoscopy, which means that no firm estimates can be made of the additional time it would take during colonoscopy to make real-time assessments of polyp histopathology.
Table 66 summarises the results of the studies included in this review in relation to the two PIVI requirements that new technologies for the real-time endoscopic assessment of the histopathology of diminutive colorectal polyps should meet, before a resect and discard strategy could be applied in practice. To reiterate, the criteria specify that, for colorectal polyps ≤ 5 mm in size to be resected and discarded without histopathological assessment, the endoscopic technology (when used with high confidence) should have a ≥ 90% agreement in assignment of post-polypectomy surveillance intervals when compared with decisions based on histopathology assessment of all identified polyps. The criteria also specify that, in order for a technology to be used to guide the decision to leave suspected rectosigmoid hyperplastic polyps ≤ 5 mm in size in place (without resection), the technology should provide ≥ 90% NPV (when used with high confidence) for adenomatous histopathology (see Chapter 1, Care pathway). Not all the studies that assessed surveillance intervals evaluated these in accordance with the PIVI criteria. We have therefore included here the results only of those studies that clearly calculated concordance of surveillance intervals between VCE and histopathology in line with the PIVI requirements. Neither of the two FICE studies that measured surveillance intervals used the PIVI requirements to do this. 83,84 None of the FICE studies examined the NPV for high-confidence assessments in the rectosigmoid colon either. This means that this review did not identify any evidence that enables us to assess how FICE meets the PIVI requirements.
| VCE technology | Assignment of surveillance intervals in accordance with PIVI | NPV (%) for high-confidence assessments of diminutive polyps in the rectosigmoid colon |
|---|---|---|
| NBI | Eight of the nine studies reporting on this outcome achieved a level of agreement that was ≥ 90% | 92.0–99.4 (range across five studies) |
| i-scan | Two of the two studies reporting this outcome achieved a level of agreement that was ≥ 90% | 97.7 (one study) |
| FICE | No evidence | No evidence |
As Table 66 shows, all but one76 of the NBI and i-scan studies that measured surveillance interval assignment in line with the PIVI criteria55,57,58,61–64,67,76,79,82 found a concordance of ≥ 90% between NBI or i-scan and histopathology and thus met this criterion of the PIVI statement (Ladabaum and colleagues58 achieved this for only one of the two guidelines used to determine surveillance interval). Most studies did not provide a CI, but where this was reported the lower limit fell below 90% in two of six cases. All the NBI and i-scan studies that measured the NPV of high-confidence assessments of diminutive polyps in the rectosigmoid colon found a ≥ 90% NPV, and thus met the second criterion of the PIVI statement. However, NPV and surveillance interval results for i-scan were provided by only one and two studies, respectively, and so the evidence in relation to how i-scan meets the PIVI requirements is limited. Our findings suggest that, on the whole, NBI appears to meet the PIVI criteria, supporting the use of NBI to carry out a resect and discard strategy in practice. We note that, in general, when there were discrepancies between the surveillance intervals set following NBI and histopathology, NBI surveillance intervals tended to be shorter than they would have been with histopathology (i.e. patients are seen again sooner).
It should be noted that our assessment here of the findings of the studies included in this review against the PIVI criteria does not take into account the settings of these studies (i.e. whether they were carried out in specialist, academic settings or routine practice). This could impact on whether or not VCE technologies meet the PIVI criteria. The DISCARD 2 study,148 which is a large, multicentre prospective UK study, concluded that NBI cannot be recommended for use in routine clinical practice, as when it is used by non-experts in this setting it does not result in a high enough concordance rate with histopathology for determining surveillance intervals. This study was not included in our systematic review as it did not meet the inclusion criteria as a result of only 22% of the colonoscopies being conducted using HD equipment. In this respect it differs from the studies included in this review and the decision problem for this assessment. It is possible that without HD equipment, diagnostic accuracy and appropriate allocation of surveillance intervals may be lower than that achieved when HD equipment is used.
The results of our systematic review have some similarities to those of previous systematic reviews of VCE for characterising colorectal polyps, notwithstanding certain differences between reviews in scope and study inclusion criteria. 42–44,149
For example, the ASGE Technology Committee conducted a systematic review to examine whether NBI, i-scan or FICE met the PIVI performance thresholds and, therefore, whether or not the evidence supported a ‘diagnosis-and-leave’ approach (ASGE Technology Committee, 2015, p. 1). 149 Literature searches were done on a number of standard health research databases, up to May 2014 (thus the search is around 2 years older than our literature search). For NBI the review included 19 studies giving estimates of NPVs and 10 studies giving estimates of agreement in post-polypectomy surveillance intervals. For i-scan there were eight studies of NPVs and one study of agreement in post-polypectomy surveillance intervals. For FICE there were eight NPV studies and two studies of agreement in post-polypectomy surveillance intervals. The majority of the studies used HD endoscopy systems, and some allowed use of magnification (in contrast to our systematic review).
In the ASGE systematic review149 the pooled random-effects NPV for studies in which an optical characterisation of diminutive polyps with NBI was made with high confidence was 93% (95% CI 90% to 96%). This increased to 95% (95% CI 92% to 98%) when high-confidence characterisations were made by endoscopists experienced in optical assessment of colorectal polyps. In our systematic review the majority of NBI studies reported NPVs for high-confidence assessments of > 78%, with five studies reporting NPVs of ≥ 90%20,55,57,64,77 (though note that the lower limit of the 95% CI fell below 90% in the majority of studies). The agreement in assignment of post-polypectomy surveillance intervals based on optical characterisation of diminutive colorectal polyps with high confidence using NBI was 91% (95% CI 88% to 95%). For i-scan there was no pooled NPV estimate given for high-confidence predictions. The overall pooled random-effects NPV (any level of confidence prediction) was 84% (95% CI 76% to 91%). A subgroup analysis based on endoscopist experience in performing and interpreting optical biopsies of colorectal polyps reported a pooled random-effects NPV of 96% (95% CI 94% to 98%) for experienced endoscopists compared with a pooled random-effects NPV of 72% (95% CI 69% to 76%) for novice endoscopists. As discussed earlier, our systematic review also found that diagnostic accuracy (in terms of sensitivity and specificity) increased in studies (of NBI) involving experienced endoscopists compared with those with less experience. The one i-scan study included in the ASGE review,149 which compared surveillance intervals based on optical assessment with histopathology, reported an agreement level of 69.5% (95% CI 63% to 75%), thus not meeting the PIVI threshold. The overall pooled random-effects NPV for FICE was 80% (95% CI 76% to 85%). This estimate did not improve when restricted to studies of endoscopists experienced in use of optical assessment of colorectal polyps.
Another systematic review, reported by Wanders and colleagues,42 assessed the diagnostic performance of VCE. This review assessed the sensitivity, specificity and NPV of NBI, FICE and i-scan for optical diagnosis of colonic polyps (in addition to autofluorescence imaging and confocal laser endomicroscopy, which are not within the scope of our systematic review). Key research databases were searched up to January 2013 (thus 3 years older than our systematic review). The inclusion criteria were broader than our review, permitting studies of diminutive and larger polyps, studies of real time as well as post-procedure image-based VCE, studies with or without magnification and studies with standard or HD endoscopy systems. However, subgroup analyses were presented based on these criteria, allowing a comparison more aligned to the scope of our systematic review to be made. Pooled bivariate meta-analysis sensitivity for the subgroup of five NBI studies with diminutive polyps where the prediction was made with high confidence was 87% (95% CI 78% to 93%) and corresponding pooled specificity was 85% (95% CI 74% to 92%). These estimates are reported to have been assessed in the context of the PIVI statement, which implies they are based on characterisations of polyps in the rectosigmoid colon. If this is the case then the corresponding NBI pooled sensitivity and specificity estimates for polyps characterised with high confidence in the rectosigmoid colon in our bivariate meta-analysis are 87% (95% CI 80% to 92%) and 95% (95% CI 87% to 98%), respectively (n = four studies). Thus, our estimates are similar in terms of sensitivity but not specificity. A pooled NPV of 83% (95% CI 75% to 88%) was reported for NBI, restricted to real-time studies (n = 35), but not further restricted in terms of diminutive polyps in the rectosigmoid colon based on high-confidence decisions (i.e. in accordance with the PIVI statement) or in terms of the definition status of the endoscopy systems used (standard or high) or magnification status (with or without). The authors suggest that studies of only rectosigmoid colon NPVs are likely to show a good diagnostic performance, as the prevalence of non-neoplastic polyps is increased in the rectosigmoid. For FICE, bivariate sensitivity and specificity are reported for diminutive polyps, though not stated to be for any particular confidence level (four studies). The estimates were 84% (95% CI 73% to 94%) and 87% (95% CI 79% to 94%), respectively, similar to our results (see Table 65). Owing to the lack of suitable studies, no diagnostic accuracy estimates were presented for diminutive polyps characterised with i-scan.
Also of note was that, in the review by Wanders and colleagues,42 sensitivity and specificity did not differ (statistically) significantly according to whether the EXERA or LUCERA NBI system was used. Even though only the LUCERA system is available for use in the UK, the inclusion criteria for our systematic review, based on the National Institute for Health and Care Excellence scope, allowed studies of both of these systems to be included. (Note that 16 of the NBI studies used EXERA, five used LUCERA and three did not report which system was used – see Table 5. ) We did not plan to conduct a formal subgroup analyses based on type of NBI system.
Cost-effectiveness
A systematic search of the literature found two economic evaluations112,113 of VCE compared with histopathology. Both studies compared the resect and discard strategy with current practice of submitting all polyps to histopathology. The evaluations were published in the USA. The studies found that there were cost savings for the resect and discard group between US$25 and US$174 per person.
A study by Olympus, the manufacturer of NBI, described a budget impact analysis of NBI in NHS England. The decision tree model has a time horizon of 7 years, and in each year there is a cohort of patients who undergo endoscopy. The study found that NBI offered cost savings of £141M over 7 years.
We developed an independent cost-effectiveness model comparing NBI, FICE and i-scan with histopathology. The base-case analysis uses a VCE strategy in a bowel screening population where diminutive polyps in the whole colon are optically characterised. The model uses estimates of diagnostic accuracy from our meta-analysis for diminutive polyps characterised with high confidence in the whole colon. The results from our economic model suggest that VCE is cost saving compared with histopathology, with a mean saving of between £73 and £87 per person over their lifetime. The QALYs are similar between the technologies with a very small increase in QALYs with NBI and i-scan compared with histopathology of between 0.0005 and 0.0007 QALYs per person, whereas FICE is associated with 0.0001 fewer QALYs per person than histopathology. VCE technologies have a cost saving of about £50 per polyp resection avoided compared with histopathology. The model estimates that the correct surveillance interval would be given to 95% of patients with NBI, 94% of patients with FICE and 97% of patients with i-scan. Results are most sensitive to the pathology cost, the probability of perforation with polypectomy and the proportion of patients who die from perforation. PSAs were conducted for pairwise and incremental comparisons for histopathology with VCE technologies. The probabilistic ICERs were similar to the base-case deterministic ICERs. At a willingness-to-pay threshold of £20,000 and £30,000, i-scan was most cost-effective in 95% and 33% of simulations, respectively.
Analyses were also conducted for a surveillance population of patients who had previously had one or more adenomas detected at an earlier colonoscopy and a symptomatic patient population that had been referred for colonoscopy with symptoms suggestive of colorectal cancer. These populations had a lower risk of adenomas than the screening population. All VCE technologies were less expensive and more effective than histopathology for the surveillance population and symptomatic population analyses.
Analyses were conducted for a DISCARD strategy in which diminutive polyps in the rectosigmoid colon are optically characterised. These analyses used the diagnostic accuracy from our meta-analysis for diminutive polyps characterised with high confidence in the rectosigmoid colon (see Figure 16). All VCE technologies were less expensive and more effective than histopathology. There were smaller differences in costs and QALYs between VCE and histopathology for this analysis than for the base-case analysis.
The base-case results show that the VCE technologies are associated with cost savings compared with histopathology and small gains in QALYs. Given the large number of colonoscopies performed every year, the potential cost savings for the NHS are substantial. The cost savings are a result of a reduction in the number of polypectomies performed (with a consequent reduction of adverse events from bleeding and perforation) and polyps sent for histopathological examination. Our base-case analysis estimated that there would be around 40% fewer polypectomies performed and this would result in between 3% and 15% of adenomas left in situ with VCE and ≥ 90% fewer hyperplastic polyps resected. The model estimates that VCE would lead to incorrect surveillance intervals for between 3% and 6% of patients. The QALY gains are attributable to the reduction in adverse events, such as perforation. The QALY losses are as a result of the long-term consequences of not resecting adenomas and patients receiving incorrect surveillance intervals.
The base-case analyses indicate that the cost-effectiveness of histopathology compared with VCE varies according to the VCE technology. The differences in cost-effectiveness between the VCE technology are largely attributable to the differences in the diagnostic sensitivity of the technologies, with our meta-analysis calculating sensitivity for i-scan of 0.96 and for FICE of 0.814. We urge caution when comparing between the results of different VCE technologies, given the differences in the diagnostic accuracy studies for these technologies in our meta-analyses.
Strengths and limitations of the assessment
Strengths of the assessment
The strengths of this assessment include that we carried out the systematic review and economic analysis independent of competing interests, and the methods we used were prespecified in a published protocol. We sought feedback from our Expert Advisory Group on the draft protocol and incorporated its comments into the final version. The protocol was published on the National Institute for Health and Care Excellence website and was discussed by experts in the topic area recruited by National Institute for Health and Care Excellence (specialist members of the Appraisal Committee). The protocol was also published on the PROSPERO prospective register of systematic reviews website.
We critically appraised all of the diagnostic test accuracy studies included in the review using recognised criteria38,39 to assess potential risks of bias and to assess the generalisability of the results. Our Expert Advisory Group commented on the protocol and a draft of this report, and we also sought specialist methodological input from the NIHR Complex Reviews Support Unit to conduct this assessment.
Our economic model is in line with current BSG109 and ESGE31 guidelines, unlike other models that have examined VCE. Hassan and colleagues112 assumed that all patients undergoing screening would have a repeat colonoscopy at 10 years, which is not the recommended surveillance interval in BSG or ESGE guidelines. In Kessler and colleagues,113 the polyp groups used are inconsistent with both guidelines. Kessler and colleagues113 divide patients into four groups by the types of polyps that patients have, whereas guidelines divide patients into risk groups by the number of adenomas that they have. Solon and colleagues117 did not examine surveillance intervals, so their study is not representative of UK practice.
Our model uses the SBCS model to generate long-term outcomes. The SBCS model was developed for the NHS Bowel Cancer Screening Programme. 122 Using long-term outcomes from the SBCS model allows guidance to be consistent across NHS evidence streams.
In line with National Institute for Health and Care Excellence methodological guidance,119 we derived as much of our evidence from systematic searches as feasible. The diagnostic accuracy data were obtained from a robust systematic review and meta-analysis using appropriate bivariate meta-analysis techniques, where possible. 41 Cost data were derived from appropriate NHS sources, and quality-of-life data were derived from EQ-5D and expressed in QALYs as the primary measure of benefit. Additionally, we conducted a wide variety of sensitivity analyses to explore uncertainty.
Limitations of the assessment
The evidence base for this assessment was particularly limited for FICE and to a lesser extent for i-scan. This limits the conclusions we can draw about the diagnostic accuracy of these technologies for assessing diminutive colorectal polyps in real time. None of the FICE studies we identified assessed surveillance intervals nor NPV in relation to the PIVI criteria, which meant that there was no evidence available to assess how use of FICE meets the PIVI requirements for a resect and discard strategy to be adopted using this technology in practice. Most of the studies included in this review evaluated NBI, but there was heterogeneity in the NBI studies in terms of the original purpose of the studies, country and settings, likely prevalence of adenomas (which can then impact NPV estimates), polyp classification systems used and experience of endoscopists. This makes it difficult to determine the diagnostic accuracy of NBI and to provide clear implications for practice. However, despite this heterogeneity, NBI appears to meet the PIVI requirements (with the caveat that, when reported, the lower limit of 95% CIs was sometimes below the 90% PIVI threshold), supporting its use for a resect and discard strategy in practice.
One limitation of this review is that we did not formally investigate the impact study setting has on diagnostic accuracy estimates. Some research has shown that studies conducted in academic or specialist centres tend to find better diagnostic accuracy outcomes than those conducted in generalist settings or community practice. 148 It is not possible to determine from this review how accurate NBI is for the real-time diagnosis of diminutive polyps when used in different settings. We also did not formally investigate the impact of the classification system used for characterising polyps in the studies. There was much variation in the reporting of the classification schemes used, which would have introduced uncertainty in assembling subgroups. Expert clinical advice suggested that diagnostic performance is unlikely to vary according to different schemes, as some of the classification schemes are derived from others (e.g. the NBI International Colorectal Endoscopic classification20 is based on the Kudo scheme22 among other schemes). Caution is also advised in the interpretation of our subgroup analysis based on endoscopist’s experience with VCE, as there was variation between studies in how experience was measured and also there were small numbers of studies in the subgroups.
In order to construct an economic model for histopathology compared with VCE technology, it was necessary to make several assumptions. First, it is not reported in the studies identified how the sensitivity and specificity for individual polyps relates to the surveillance intervals for patients. Although some studies in the systematic review of diagnostic accuracy examined correct assignment of surveillance intervals, the data from these studies were insufficient to incorporate in the model. Therefore, we assumed that diagnostic accuracy data for individual polyps were applicable to the entire patient, and assigned patients into risk categories a priori using data from Raju and colleagues. 132 When comparing our modelled outcomes with those found in the systematic review of diagnostic accuracy studies, the model’s correct prediction of surveillance intervals was similar to that found in the systematic review (see Chapter 4 for details). Furthermore, we assumed that the prevalence of adenomas was constant across risk groups with adenomas to predict the number of polyps that patients have. It may be that patients in different risk groups have different ratios of adenomas to polyps. If patients with low-risk adenomas have a higher number of polyps per adenoma than patients in the higher-risk categories, this would adversely affect the cost-effectiveness of histopathology compared with VCE, as more hyperplastic polyps would be resected and sent to histopathology.
The long-term cost and QALY outcomes derived from the SBCS model were estimated assuming use of standard colonoscopy for any follow-up surveillance. These long-term costs and QALY outcomes do not therefore show the true extent of the future colonoscopies; for example, we would expect there to be future cost savings for VCE in any future colonoscopies. It was not feasible to include our decision tree within the SBCS model. However, we included a sensitivity analysis to investigate the likely impact of including VCE, which had only a small effect on the model results. This was because the majority of patients were low risk (i.e. few of them would have repeat colonoscopy).
The economic analysis includes only diminutive polyps. Although the decision problem focuses on diminutive polyps, some people with diminutive polyps will also have larger polyps (falling into the ‘small’ and ‘large’ categories). We attempted to incorporate large and small polyps using data from studies identified in the systematic review and meta-analysis as well as targeted searches, but there were insufficient data to allow coherent analysis of larger polyps. In practice, large polyps would be assessed using only histopathology, and the effect would be an increase in the number of patients with intermediate- and high-risk adenoma (i.e. shorter surveillance intervals), and a decrease in the number of polyps characterised as adenomas in intermediate- and high-risk patients. It is this last feature of the analysis that made assessing large polyps infeasible as no data were available that indicated the number of polyps found in patients with large polyps at intermediate or high risk. Additionally, no information could be identified on what proportion of patients in the intermediate risk category had two or fewer adenomas with one adenoma being large. Including small polyps would affect only the proportion of patients assessed using only histopathology. Surveillance intervals for small polyps are identical to diminutive polyps.
Furthermore, the model does not differentiate between the type of polyp such as depressed polyps or sessile serrated polyps. No diagnostic accuracy data were identified, specifically, for either type of polyp. Additionally, sessile serrated polyps are rare and no diagnostic accuracy data were available for diminutive sessile serrated polyps from our systematic review of diagnostic studies (see Chapter 4). These polyps may be more likely to be given a low-confidence assessment, in which case they would therefore undergo histopathology.
In the absence of data on adverse events for diminutive polyps, we have used adverse event rates observed for all polyps. However, this overestimates the number of adverse events as adverse events for diminutive polyps are rarer than for larger polyps. Indeed, comments from our clinical advisors suggest that diminutive polyps are very unlikely to result in perforation. We have varied the adverse event rate in sensitivity analyses (see Table 50), where the lower estimate for adverse events for perforation and bleeding was set to zero. With these changes to the adverse event rates, the results are similar to reported in our base-case analyses.
Another uncertainty is the variation in diagnostic accuracy of VCE that would occur as a result of polyps that are unable to be successfully retrieved for histopathological analysis (e.g. as a result of fragmentation). We have noted earlier in this report (see Chapter 1, Description of the diagnostic technologies under assessment) that histopathology, despite being the accepted reference standard, is imperfect. Evidence shows that polyp retrieval failure increases significantly with smaller polyps, particularly those that are diminutive, even when resected by experienced colonoscopists. Lost polyps would be classified as adenomas, even though many would be hyperplastic. A retrospective analysis of 4383 polyps resected from 1495 patients undergoing colonoscopy in the Bowel Cancer Screening Programme reported a polyp retrieval failure rate of 6.1%. In our systematic review estimates of polyps not successfully resected for histopathological analysis, where reported, ranged from 0.5% (Basford and colleagues79) to 13% (DISCARD3), though in most studies estimates were < 5%. The effect of this is to reduce the diagnostic accuracy of histopathology relative to that of VCE. 3 We note that some polyps resected using the VCE strategy would also be sent to histopathology. We have not been able to incorporate this uncertainty into our economic analysis as a result of a lack of data to inform how this would affect all of the relevant input parameters. It may lead to a small reduction in the cost of histopathological assessment because of fewer polyps being sent to the laboratory.
The data on recurrence rates post polypectomy in the SBCS model have several limitations. The transition probabilities reported in Table 45 are not age dependent; however, the transition probabilities used in the model are age dependent. The study populations do not reflect the English bowel cancer screening population, are quite small in size, do not use the BSG surveillance guidelines to categorise adenomas, and report highly varying recurrence rates. The SBCS data on recurrence rates for people classified as intermediate risk or high risk and undergoing 1- or 3-yearly surveillance have not been updated with more recent data from the NHS Cancer Screening Programme.
The full uncertainty around the model results have not been explored in the PSA, as the long-term outcome parameters have not been varied. These data were not available from the SBCS model.
Uncertainties
We considered that the participants enrolled in the NBI, i-scan and FICE studies included in the systematic review of diagnostic accuracy are generally likely to be representative of the types of participants who would receive colonoscopy in the UK for screening, surveillance or on account of symptoms experienced. The majority of the studies were conducted in a single centre and so the results of these studies may not be transferable to other centres. The endoscopists who took part in the NBI studies had a range of experience with endoscopy and NBI across the studies, and it is unclear how this reflects the experience of endoscopists currently working in UK practice. Endoscopists underwent training in NBI in the majority of the NBI studies, but it is unclear how representative this training may be of any received in current UK practice. Relatedly, three77,79,80 of the five77,79–82 i-scan studies were conducted by endoscopists with prior experience of using i-scan, in single centres often described as academic or specialist centres. The results of these studies may therefore not be applicable to less experienced endoscopists working in more generalist or community settings. As we did not explore the effect of the study setting on the results from the NBI studies, it is unclear how generalisable the NBI findings are to specialist and generalist centres in the UK. The European (ESGE) guidance31 recommends that only experienced and adequately trained endoscopists should undertake VCE for the real-time assessment of diminutive colorectal polyps. Our review suggests that better diagnostic accuracy (i.e. sensitivity and specificity) outcomes are obtained by more experienced endoscopists, supporting the need for endoscopists to have adequate experience and training in these technologies to use them for real-time diagnosis.
Most of our studies reported diagnostic accuracy derived from expert endoscopists, so the results may not be generalisable to endoscopists with less experience with VCE technologies. It may be that the level of expertise in endoscopists is lower than in the studies, which would result in lower diagnostic accuracies seen in clinical practice.
The long-term outcomes from the SBCS model include disease progression for patients with small (6–9 mm) and large (> 10 mm) adenomas. It is likely that this overestimates the cancer rates in patients with diminutive polyps who would receive different management as a result of the use of VCE technology. It may be that cancer rates are lower in these patients than predicted by the SBCS model, which would result in lower QALY losses for people treated with VCE and, therefore, increase the cost-effectiveness of histopathology compared with VCE.
The FICE diagnostic accuracy data does not include data on polyp characterisations made with high confidence or polyp characterisations made in the rectosigmoid colon, so these cost-effectiveness results are not directly comparable with those of the other VCE technologies. More data on the diagnostic accuracy of FICE are necessary to adequately represent its cost-effectiveness.
We have not included within the analysis any benefits to patients in the case where they are informed of the results more quickly or do not have to attend follow-up consultation. There may also be a reduction in anxiety that some patients may experience while waiting for results. There was insufficient evidence on these factors to include within the economic analysis.
Chapter 8 Conclusions
Implications for service provision
This assessment found that VCE technologies (i.e. NBI, i-scan and FICE) using HD systems without magnification have potential for use in practice for the real-time assessment of diminutive colorectal polyps. The studies identified in this review suggest that, on the whole, NBI and i-scan (when used with high confidence) meet the PIVI requirements for these technologies to be used in practice to carry out a resect and discard strategy. Data for i-scan supporting this, however, were limited, and most data were from studies involving endoscopists with prior i-scan experience working in specialist or academic centres. It was unclear how generalisable the NBI results in relation to the PIVI were to UK routine practice settings, as we did not investigate the impact of study setting. Owing to limited evidence, it is unclear which of the three VCE technologies performs the best. NBI and i-scan had generally better diagnostic accuracy outcomes than FICE, but, again, a greater proportion of i-scan studies were known to involve endoscopists with prior experience of i-scan. Diagnostic accuracy results for NBI were more heterogeneous, but we found that endoscopists with prior experience of NBI achieved higher diagnostic accuracy results than endoscopists with no prior NBI experience and, in general, when polyp characterisations were made with high-confidence diagnostic accuracy was higher. Our findings suggest, as per the ESGE guidance,31 that VCE should be undertaken by experienced and adequately trained endoscopists. This has implications for practice in terms of the need to provide training. VCE technologies were cost saving compared with histopathology. NBI and i-scan were more effective than histopathology. FICE was cost-effective compared with histopathology.
Uptake of VCE for the assessment of diminutive polyps in practice will probably depend on the willingness of colonoscopists to take on the responsibility for characterising polyps and the provision of equipment for NBI, i-scan and FICE. We understand that most endoscopes used in the UK have this technology available, although not all centres may have HD equipment. We did not find any studies measuring patient HRQoL, anxiety or the acceptability of VCE to patients, so it is unclear how comfortable patients would be with VCE being used to assess their polyps. Some patients may experience anxiety knowing that a hyperplastic polyp has not been resected. Some patients may prefer that all polyps are removed, even when there is negligible risk of them becoming cancerous.
Suggested research priorities
More research is needed to assess the diagnostic accuracy performance of i-scan and FICE when used without magnification to assess diminutive colorectal polyps in real time, as there is currently only limited evidence available regarding these two technologies. Ideally any new evaluations of the performance of NBI, i-scan and FICE should be conducted in generalist, routine practice settings, particularly as the i-scan literature is currently limited, and most studies involved endoscopists with prior experience of i-scan working in specialist or academic centres. Multicentre studies, across a range of settings, would also be informative.
Further studies evaluating the effect of endoscopist experience and training on diagnostic accuracy outcomes when using these technologies would be useful. Endoscopist experience and training is an important consideration and we found few studies that compared the performance of endoscopists with different levels of training and experience, limiting the extent to which we could investigate the influence of this on outcomes in this review.
Future studies should measure adverse effects of polypectomy to provide clearer information about the potential harms of these technologies when used to carry out a resect and discard strategy compared with histopathological assessment of all polyps. We suggest that it would be ideal if future studies also included measures of HRQoL and patient anxiety, as it is currently unclear how patients will respond to the use of these technologies in practice.
Longitudinal data from studies following-up patients over time since their colonoscopy procedure was carried out are needed to quantify the impact of these technologies on colorectal cancer incidence, longer-term HRQoL and mortality.
Randomised head-to-head comparisons of NBI, FICE and i-scan would be useful to directly compare outcomes when these technologies are used without magnification to assess diminutive colorectal polyps in real time. We identified only two head-to-head studies in this review, and so we could only narratively comment on which technologies may perform better. (Note that head-to-head comparisons of the technologies were not within the decision problem for this assessment, but they may nonetheless be informative to endoscopists interested in using them.)
Acknowledgements
Thanks to Karen Welch, Information Specialist at the Southampton Health Technology Assessments Centre (SHTAC), for developing the search strategy, conducting the literature searches and downloading the search results into a bibliographic database. We are also grateful to Geoff Frampton (SHTAC) for acting as an internal editor for the draft report. We also thank the members of the project’s Expert Advisory Group who were contacted for clinical advice and comments on draft versions of the protocol, project report and economic model: Professor Pradeep Bhandari, Consultant Gastroenterologist and Professor of Gastrointestinal Endoscopy, Portsmouth Hospitals NHS Trust; Dr Philip Boger, Consultant in Gastroenterology and Advanced Endoscopy, University Hospitals Trust, Southampton; Professor Brian Saunders, Consultant Gastroenterologist and Specialist Gastrointestinal Endoscopist, Wolfson Unit for Endoscopy, St Mark’s Hospital and Academic Institute, London; and Professor John Schofield, Consultant Histopathologist, Maidstone and Tunbridge Wells NHS Trust.
In additional, the following National Institute for Health and Care Excellence Specialist Committee Members who provided comments on a draft version of the report: Dr James East, Consultant Gastroenterologist and Endoscopist, John Radcliffe Hospital; Mrs Susan McConnell, Nurse Endoscopist/Training Lead, County Durham & Darlington Foundation Trust; Dr Morgan Moorghen, Consultant Histopathologist, St Mark’s Hospital; and Dr Venkat Subramanian, Clinical Associate Professor and Consultant Gastroenterologist, Leeds Institute of Biomedical and Clinical Sciences/St James University Hospital.
This project was supported by the Complex Reviews Support Unit, which is funded by the NIHR (project number 14/178/29).
The views and opinions expressed by members of the Complex Reviews Unit are those of the individual members consulted and do not necessarily reflect those of the NIHR, NHS or Department of Health.
Contributions of authors
Dr Joanna Picot (Senior Research Fellow, evidence synthesis) project managed the study, developed the research protocol, assisted in the development of the search strategy, assessed test accuracy studies for inclusion, performed data extraction and critical appraisal of included test accuracy studies, synthesised evidence including conducting the meta-analyses, and drafted and edited the final report.
Mr Micah Rose (Research Fellow, health economics) developed the research protocol, assessed cost-effectiveness and HRQoL studies for inclusion, synthesised evidence, developed the economic model and drafted and edited the final report.
Dr Keith Cooper (Senior Research Fellow, health economics) developed the research protocol, assessed cost-effectiveness and HRQoL studies for inclusion, synthesised evidence, led the development of the economic evaluation and drafted and edited the final report.
Dr Karen Pickett (Research Fellow, evidence synthesis) developed the research protocol, assessed test accuracy studies for inclusion, performed data extraction and critical appraisal of included test accuracy studies, synthesised evidence and drafted and edited the final report.
Professor Joanne Lord (Professorial Fellow in Health Economics) contributed to discussions on the design of the economic model and drafted and edited the final report.
Ms Petra Harris (Research Fellow, evidence synthesis) performed data extraction and critical appraisal of included test accuracy studies, synthesised evidence and drafted and edited the final report.
Dr Sophie Whyte (Research Fellow, health economics) contributed to the development of the economic evaluation, provided data for the economic model and drafted and edited the final report.
Professor Dankmar Böhning (Professor in Medical Statistics) provided training and guidance in the conduct of meta-analyses of diagnostic studies and edited the final report.
Dr Jonathan Shepherd (Principal Research Fellow, evidence synthesis) developed the research protocol, assisted in the development of the search strategy, assessed test accuracy studies for inclusion, performed data extraction and critical appraisal of included test accuracy studies, synthesised evidence, drafted and edited the final report and acted as the project guarantor.
Data sharing statement
All data relevant to this technology assessment report are provided in the accompanying appendices or may be obtained on request from the corresponding author. Note that the current report does not include confidential data that were considered during the National Institute for Health and Care Excellence diagnostics assessment. Confidential data cannot be shared, but their implications for the conclusions of the diagnostic assessment are clearly stated in the current report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- NHS Choices . Bowel Polyps n.d. www.nhs.uk/Conditions/polyps-bowel/Pages/Introduction.aspx (accessed 13 January 2016).
- Kang YK. Diminutive and small colorectal polyps: the pathologist’s perspective. Clin Endosc 2014;47:404-8. https://doi.org/10.5946/ce.2014.47.5.404.
- Wang LM, East JE. Diminutive polyp cancers and the DISCARD strategy: much ado about nothing or the end of the affair?. Gastrointest Endosc 2015;82:385-8. https://doi.org/10.1016/j.gie.2015.02.036.
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-4. https://doi.org/10.1016/S0016-5107(03)02159-X.
- Williams JG, Pullan RD, Hill J, Horgan PG, Salmo E, Buchanan GN, et al. Management of the malignant colorectal polyp: ACPGBI position statement. Colorectal Dis 2013;15:1-38. https://doi.org/10.1111/codi.12262.
- Cancer Research UK . Bowel Cancer (Colorectal Cancer) n.d. www.cancerresearchuk.org/about-cancer/type/bowel-cancer/ (accessed 7 July 2016).
- Ballinger AB, Anggiansah C. Colorectal cancer. BMJ 2007;335:715-18. https://doi.org/10.1136/bmj.39321.527384.BE.
- Colorectal Cancer: Diagnosis and Management. Clinical Guideline CG131. Manchester: National Institute for Health and Care Excellence; 2014.
- van Putten PG, Hol L, van Dekken H, Han van Krieken J, van Ballegooijen M, Kuipers EJ, et al. Inter-observer variation in the histological diagnosis of polyps in colorectal cancer screening. Histopathology 2011;58:974-81. https://doi.org/10.1111/j.1365-2559.2011.03822.x.
- NHS Choices . Endoscopy n.d. www.nhs.uk/conditions/endoscopy/pages/introduction.aspx (accessed 13 January 2016).
- Varadarajulu S, Banerjee S, Barth BA, Desilets DJ, Kaul V, Kethu SR, et al. GI endoscopes. Gastrointest Endosc 2011;74:1-6.e6. https://doi.org/10.1016/j.gie.2011.01.061.
- Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, Gottlieb KT, Hwang JH, et al. Electronic chromoendoscopy. Gastrointest Endosc 2015;81:249-61. https://doi.org/10.1016/j.gie.2014.06.020.
- EVIS LUCERA ELITE Concept Brochure. Tokyo: Olympus Medical Systems Corp.; 2013.
- EVIS EXERA III Brochure. Center Valley, PA: Olympus America Inc.; 2012.
- EVIS LUCERA SPECTRUM Family Brochure. Center Valley, PA: Olympus America Inc.; 2009.
- Fujinon Video Endoscopes 500 Series Scope. Electronic Video Endoscopy System 4400. Saitama: Fujinon Corporation; 2009.
- PENTAX Medical . What Is I-Scan n.d. https://i-scanimaging.com/index.php?pg=What is i-scan?&l_id=1 (accessed 13 January 2016).
- PENTAX Medical . I-Scan Imaging n.d. www.pentaxmedical.com/pentax/en/95/1/i-scan-imaging/ (accessed 13 January 2016).
- American Society for Gastrointestinal Endoscopy (ASGE) Technology Committee . High-definition and high-magnification endoscopes. Gastrointest Endosc 2014;80:919-27. https://doi.org/10.1016/j.gie.2014.06.019.
- Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012;143:599-607.e1. https://doi.org/10.1053/j.gastro.2012.05.006.
- Longcroft-Wheaton GR, Bhandari P. A novel classification system (NAC) for the characterisation colonic polyps using FICE without optical magnification: a new classification system (NAC). Gastrointest Endosc 2012;75. https://doi.org/10.1016/j.gie.2012.03.1402.
- Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 1996;44:8-14. https://doi.org/10.1016/S0016-5107(96)70222-5.
- Wada Y, Kudo SE, Kashida H, Ikehara N, Inoue H, Yamamura F, et al. Diagnosis of colorectal lesions with the magnifying narrow-band imaging system. Gastrointest Endosc 2009;70:522-31. https://doi.org/10.1016/j.gie.2009.01.040.
- IJspeert JE, Bastiaansen BA, van Leerdam ME, Meijer GA, van Eeden S, Sanduleanu S, et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut 2016;65:963-70. https://doi.org/10.1136/gutjnl-2014-308411.
- Basford PJ, Longcroft-Wheaton GR, Bhandari P. The learning curve for in-vivo characterisation of small colonic polyps: number needed to train (NNT) is 200 polyps. Gastrointest Endosc 2013;1. https://doi.org/10.1016/j.gie.2013.03.886.
- Rees CJ, Thomas Gibson S, Rutter MD, Baragwanath P, Pullan R, Feeney M, et al. UK key performance indicators and quality assurance standards for colonoscopy. Gut 2016;65:1923-9. https://doi.org/10.1136/gutjnl-2016-312044.
- National Institute for Health and Care Excellence Diagnostics Assessment Programme . Virtual Chromoendoscopy for Real-Time Assessment of Colorectal Polyps During Colonoscopy – Final Scope n.d. www.nice.org.uk/guidance/indevelopment/gid-dg10004/documents (accessed 4 July 2016).
- Suspected Cancer: Recognition and Referral. Guideline NG12. Manchester: National Institute for Health and Care Excellence; 2015.
- Colorectal Cancer Prevention: Colonoscopic Surveillance in Adults with Ulcerative Colitis, Crohn’s Disease or Adenomas. Clinical Guideline CG119. Manchester: National Institute for Health and Care Excellence; 2011.
- Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. https://doi.org/10.1136/gut.2009.179804.
- Kaminski MF, Hassan C, Bisschops R, Pohl J, Pellise M, Dekker E, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2014;46:435-49. https://doi.org/10.1055/s-0034-1365348.
- Rex DK, Kahi C, O’Brien M, Levin TR, Pohl H, Rastogi A, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2011;73:419-22. https://doi.org/10.1016/j.gie.2011.01.023.
- Picot J, Cooper K, Pickett K, Rose M, Shepherd J. Virtual Chromoendoscopy for the Real-Time Assessment of Colorectal Polyps in Vivo n.d. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016037767 (accessed 14 April 2016).
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: York Publishing Services Ltd, CRD; 2009.
- Bossuyt P, Davenport C, Deeks J, Hyde C, Leeflang M, Scholten R, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.9. London: The Cochrane Collaboration; 2013.
- Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y, Deeks J, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. London: The Cochrane Collaboration; 2010.
- Diagnostics Assessment Programme Manual. Manchester: National Institute for Health and Care Excellence; 2011.
- Reitsma J, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. London: The Cochrane Collaboration; 2009.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. https://doi.org/10.1186/1471-2288-3-25.
- Takwoingi Y, Riley RD, Deeks JJ. Meta-analysis of diagnostic accuracy studies in mental health. Evid Based Ment Health 2015;18:103-9. https://doi.org/10.1136/eb-2015-102228.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Wanders LK, East JE, Uitentuis SE, Leeflang MM, Dekker E. Diagnostic performance of narrowed spectrum endoscopy, autofluorescence imaging, and confocal laser endomicroscopy for optical diagnosis of colonic polyps: a meta-analysis. Lancet Oncol 2013;14:1337-47. https://doi.org/10.1016/S1470-2045(13)70509-6.
- McGill SK, Evangelou E, Ioannidis JPA, Soetikno RM, Kaltenbach T. Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics (provisional abstract). Gut 2013;62:1704-13. https://doi.org/10.1136/gutjnl-2012-303965.
- Guo CG, Ji R, Li YQ. Accuracy of i-Scan for optical diagnosis of colonic polyps: a meta-analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0126237.
- Harbord R, Whiting P. metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J 2009;9:211-29.
- Takwoingi Y. Meta-Analysis of Test Accuracy Studies in Stata – A Bivariate Model Approach 2016. http://methods.cochrane.org/sdt/ (accessed 5 July 2016).
- Kang HY, Kim YS, Song JH, Yang SY, Lim SH. Comparison of narrow band imaging (NBI) and Fujinon intelligent color enhancement (FICE) in predicting small colorectal polyp histology. J Gastroenterol Hepatol 2014;29.
- Paggi S, Amato A, Rondonotti E, Spinzi G, Radaelli F. Do new generation narrow band imaging colonoscopes improve the prediction of histology for diminutive polyps? Preliminary results of an observational prospective study. Gastrointest Endosc 2014;1. https://doi.org/10.1016/j.gie.2014.02.712.
- Paggi S, Rondonotti E, Amato A, Spinzi G, Radaelli F. Narrow band imaging in the prediction of surveillance intervals after polypectomy in community practice: ready for (a European) prime time. Gastrointest Endosc 2014;1. https://doi.org/10.1016/j.gie.2014.02.159.
- Patel S, Schoenfeld P, Kim H, Kim Y, Ward E, Bansal A, et al. Learning curves for the real-time histologic characterization of diminutive colorectal polyps with narrow band imaging (NBI) using cumulative summation analysis (CUSUM): implications for resect and discard strategy. Am J Gastroenterol 2015;110:S598-S9.
- Patel SG, Rastogi A, Schoenfeld P, Bansal A, Hosford L, Myers A, et al. Cost-savings associated with the resect and discard strategy for diminutive polyps: results from a prospective multicenter study evaluating real-time characterization of diminutive colorectal polyp histology using narrow band imaging (NBI). Gastrointest Endosc 2016;1. https://doi.org/10.1016/j.gie.2016.03.512.
- Patel SG, Schoenfeld PS, Ward EK, Kim H, Bansal A, Hosford L, et al. A prospective multicenter study evaluating real-time characterization of diminutive colorectal polyp histology using narrow band imaging (NBI): implications for the resect and discard strategy. Gastrointest Endosc 2015;1. https://doi.org/10.1016/j.gie.2015.03.1239.
- Szura M, Bucki K, Pasternak A, Matyja A, Kulig J. Two-stage optical system for colorectal polyp assessment. Surg Endosc 2014;28.
- Hewett DG, Huffman ME, Rex DK. Leaving distal colorectal hyperplastic polyps in place can be achieved with high accuracy by using narrow-band imaging: an observational study. Gastrointest Endosc 2012;76:374-80. https://doi.org/10.1016/j.gie.2012.04.446.
- Patel SG, Schoenfeld P, Kim HM, Ward EK, Bansal A, Kim Y, et al. Real-time characterization of diminutive colorectal polyp histology using narrow-band imaging: implications for the resect and discard strategy. Gastroenterology 2016;150:406-18. https://doi.org/10.1053/j.gastro.2015.10.042.
- Iwatate M, Sano Y, Hattori S, Sano W, Hasuike N, Ikumoto T, et al. The addition of high magnifying endoscopy improves rates of high confidence optical diagnosis of colorectal polyps. Endosc Int Open 2015;3:E140-5. https://doi.org/10.1055/s-0034-1391362.
- Kaltenbach T, Rastogi A, Rouse RV, McQuaid KR, Sato T, Bansal A, et al. Real-time optical diagnosis for diminutive colorectal polyps using narrow-band imaging: the VALID randomised clinical trial. Gut 2015;64:1569-77. https://doi.org/10.1136/gutjnl-2014-307742.
- Ladabaum U, Fioritto A, Mitani A, Desai M, Kim JP, Rex DK, et al. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology 2013;144:81-9. https://doi.org/10.1053/j.gastro.2012.09.054.
- Paggi S, Rondonotti E, Amato A, Fuccio L, Andrealli A, Spinzi G, et al. Narrow-band imaging in the prediction of surveillance intervals after polypectomy in community practice. Endoscopy 2015;47:808-14. https://doi.org/10.1055/s-0034-1392042.
- Paggi S, Rondonotti E, Amato A, Terruzzi V, Imperiali G, Mandelli G, et al. Resect and discard strategy in clinical practice: a prospective cohort study. Endoscopy 2012;44:899-904. https://doi.org/10.1055/s-0032-1309891.
- Pohl H, Bensen SP, Toor A, Gordon SR, Levy LC, Anderson PB, et al. Quality of optical diagnosis of diminutive polyps and associated factors. Endoscopy 2016;48:817-22. https://doi.org/10.1055/s-0042-108432.
- Repici A, Hassan C, Radaelli F, Occhipinti P, De Angelis C, Romeo F, et al. Accuracy of narrow-band imaging in predicting colonoscopy surveillance intervals and histology of distal diminutive polyps: results from a multicenter, prospective trial. Gastrointest Endosc 2013;78:106-14. https://doi.org/10.1016/j.gie.2013.01.035.
- Wallace MB, Crook JE, Coe S, Ussui V, Staggs E, Almansa C, et al. Accuracy of in vivo colorectal polyp discrimination by using dual-focus high-definition narrow-band imaging colonoscopy. Gastrointest Endosc 2014;80:1072-87. https://doi.org/10.1016/j.gie.2014.05.305.
- Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology 2009;136:1174-81. https://doi.org/10.1053/j.gastro.2008.12.009.
- Sola-Vera J, Cuesta R, Uceda F, Morillo E, Pérez E, Picó MD, et al. Accuracy for optical diagnosis of colorectal polyps in clinical practice. Rev Esp Enferm Dig 2015;107:255-61.
- Aihara H, Kumar N, Ryou M, Burakoff R, Gergi MA, Ryan MB, et al. Prospective evaluation of a simplified narrowband imaging scoring system for a differential diagnosis of colorectal lesions. Surg Endosc 2016;30:3598-603. https://doi.org/10.1007/s00464-015-4660-5.
- Chandran S, Parker F, Lontos S, Vaughan R, Efthymiou M. Can we ease the financial burden of colonoscopy? Using real-time endoscopic assessment of polyp histology to predict surveillance intervals. Intern Med J 2015;45:1293-9. https://doi.org/10.1111/imj.12917.
- Gupta N, Bansal A, Rao D, Early DS, Jonnalagadda S, Edmundowicz SA, et al. Accuracy of in vivo optical diagnosis of colon polyp histology by narrow-band imaging in predicting colonoscopy surveillance intervals. Gastrointest Endosc 2012;75:494-502. https://doi.org/10.1016/j.gie.2011.08.002.
- Henry ZH, Yeaton P, Shami VM, Kahaleh M, Patrie JT, Cox DG, et al. Meshed capillary vessels found on narrow-band imaging without optical magnification effectively identifies colorectal neoplasia: a North American validation of the Japanese experience. Gastrointest Endosc 2010;72:118-26. https://doi.org/10.1016/j.gie.2010.01.048.
- Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol 2009;10:1171-8. https://doi.org/10.1016/S1470-2045(09)70329-8.
- Ikematsu H, Matsuda T, Osera S, Imajoh M, Kadota T, Morimoto H, et al. Usefulness of narrow-band imaging with dual-focus magnification for differential diagnosis of small colorectal polyps. Surg Endosc 2015;29:844-50. https://doi.org/10.1007/s00464-014-3736-y.
- McGill SK, Soetikno R, Rastogi A, Rouse RV, Sato T, Bansal A, et al. Endoscopists can sustain high performance for the optical diagnosis of colorectal polyps following standardized and continued training. Endoscopy 2015;47:200-6.
- Rastogi A, Keighley J, Singh V, Callahan P, Bansal A, Wani S, et al. High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: a prospective study. Am J Gastroenterol 2009;104:2422-30. https://doi.org/10.1038/ajg.2009.403.
- Rogart JN, Jain D, Siddiqui UD, Oren T, Lim J, Jamidar P, et al. Narrow-band imaging without high magnification to differentiate polyps during real-time colonoscopy: improvement with experience. Gastrointest Endosc 2008;68:1136-45. https://doi.org/10.1016/j.gie.2008.04.035.
- Shahid MW, Buchner AM, Heckman MG, Krishna M, Raimondo M, Woodward T, et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging for small colorectal polyps: a feasibility study. Am J Gastroenterol 2012;107:231-9. https://doi.org/10.1038/ajg.2011.376.
- Vu HT, Sayuk GS, Hollander TG, Clebanoff J, Edmundowicz SA, Gyawali CP, et al. Resect and discard approach to colon polyps: real-world applicability among academic and community gastroenterologists. Dig Dis Sci 2014;60:502-8. https://doi.org/10.1007/s10620-014-3376-z.
- Lee CK, Lee SH, Hwangbo Y. Narrow-band imaging versus i-scan for the real-time histological prediction of diminutive colonic polyps: a prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc 2011;74:603-9. https://doi.org/10.1016/j.gie.2011.04.049.
- Kang HY, Kim YS, Kang SJ, Chung GE, Song JH, Yang SY, et al. Comparison of narrow band imaging and Fujinon intelligent color enhancement in predicting small colorectal polyp histology. Dig Dis Sci 2015;60:2777-84. https://doi.org/10.1007/s10620-015-3661-5.
- Basford PJ, Longcroft-Wheaton G, Higgins B, Bhandari P. High-definition endoscopy with i-scan for evaluation of small colon polyps: the HiSCOPE study. Gastrointest Endosc 2014;79:111-18. https://doi.org/10.1016/j.gie.2013.06.013.
- Hoffman A, Kagel C, Goetz M, Tresch A, Mudter J, Biesterfeld S, et al. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis 2010;42:45-50. https://doi.org/10.1016/j.dld.2009.04.005.
- Pigo F, Bertani H, Manno M, Mirante V, Caruso A, Barbera C, et al. I-scan high-definition white light endoscopy and colorectal polyps: Prediction of histology, interobserver and intraobserver agreement. Int J Colorectal Dis 2013;28:399-406. https://doi.org/10.1007/s00384-012-1583-7.
- Rath T, Tontini GE, Nägel A, Vieth M, Zopf S, Günther C, et al. High-definition endoscopy with digital chromoendoscopy for histologic prediction of distal colorectal polyps. BMC Gastroenterol 2015;15. https://doi.org/10.1186/s12876-015-0374-3.
- Longcroft-Wheaton G, Brown J, Cowlishaw D, Higgins B, Bhandari P. High-definition vs. standard-definition colonoscopy in the characterization of small colonic polyps: results from a randomized trial. Endoscopy 2012;44:905-10. https://doi.org/10.1055/s-0032-1310004.
- Longcroft-Wheaton GR, Higgins B, Bhandari P. Flexible spectral imaging color enhancement and indigo carmine in neoplasia diagnosis during colonoscopy: a large prospective UK series (structured abstract). Eur J Gastroenterol Hepatol 2011;23:903-11. https://doi.org/10.1097/MEG.0b013e328349e276.
- Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, et al. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc 2009;69:278-83. https://doi.org/10.1016/j.gie.2008.04.066.
- Rastogi A, Pondugula K, Bansal A, Wani S, Keighley J, Sugar J, et al. Recognition of surface mucosal and vascular patterns of colon polyps by using narrow-band imaging: interobserver and intraobserver agreement and prediction of polyp histology. Gastrointest Endosc 2009;69:716-22. https://doi.org/10.1016/j.gie.2008.09.058.
- Rastogi A, Bansal A, Wani S, Callahan P, McGregor DH, Cherian R, et al. Narrow-band imaging colonoscopy-a pilot feasibility study for the detection of polyps and correlation of surface patterns with polyp histologic diagnosis. Gastrointest Endosc 2008;67:280-6. https://doi.org/10.1016/j.gie.2007.07.036.
- Sano Y, Emura F, Ikematsu H, Waye JD, Rex DK, Williams CB. Colonoscopy Principles and Practice. Hoboken, NJ: Wiley-Blackwell; 2009.
- Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc 2013;78:625-32. https://doi.org/10.1016/j.gie.2013.04.185.
- East JE, Suzuki N, Bassett P, Stavrinidis M, Thomas HJ, Guenther T, et al. Narrow band imaging with magnification for the characterization of small and diminutive colonic polyps: pit pattern and vascular pattern intensity. Endoscopy 2008;40:811-17. https://doi.org/10.1055/s-2008-1077586.
- Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, et al. Colorectal tumours and pit pattern. J Clin Pathol 1994;47:880-5. https://doi.org/10.1136/jcp.47.10.880.
- Oba S, Tanaka S, Sano Y, Oka S, Chayama K. Current status of narrow-band imaging magnifying colonoscopy for colorectal neoplasia in Japan. Digestion 2011;83:167-72. https://doi.org/10.1159/000321807.
- Rastogi A, Rao DS, Gupta N, Grisolano SW, Buckles DC, Sidorenko E, et al. Impact of a computer-based teaching module on characterization of diminutive colon polyps by using narrow-band imaging by non-experts in academic and community practice: a video-based study. Gastrointest Endosc 2014;79:390-8. https://doi.org/10.1016/j.gie.2013.07.032.
- Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc 2010;72:572-6. https://doi.org/10.1016/j.gie.2010.03.1124.
- Sano W, Sano Y, Iwatate M, Hasuike N, Hattori S, Kosaka H, et al. Prospective evaluation of the proportion of sessile serrated adenoma/polyps in endoscopically diagnosed colorectal polyps with hyperplastic features. Endosc Int Open 2015;3:E354-8. https://doi.org/10.1055/s-0034-1391948.
- Rastogi A, Early DS, Gupta N, Bansal A, Singh V, Ansstas M, et al. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc 2011;74:593-602. https://doi.org/10.1016/j.gie.2011.04.050.
- East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study. Gastrointest Endosc 2007;66:310-16. https://doi.org/10.1016/j.gie.2007.02.026.
- Kim YS, Kim D, Chung SJ, Park MJ, Shin CS, Cho SH, et al. Differentiating small polyp histologies using real-time screening colonoscopy with Fuji intelligent color enhancement. Clin Gastroenterol Hepatol 2011;9:744-9. https://doi.org/10.1016/j.cgh.2011.05.021.
- Teixeira CR, Torresini RS, Canali C, Figueiredo LF, Mucenic M, Pereira Lima JC, et al. Endoscopic classification of the capillary-vessel pattern of colorectal lesions by spectral estimation technology and magnifying zoom imaging. Gastrointest Endosc 2009;69:750-6. https://doi.org/10.1016/j.gie.2008.09.062.
- Clinical Practice Guidelines for Surveillance Colonoscopy – In Adenoma Follow-up; Following Curative Resection of Colorectal Cancer; and for Cancer Surveillance in Inflammatory Bowel Disease. Sydney, NSW: Cancer Council Australia; 2011.
- Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570-95. https://doi.org/10.1053/j.gastro.2008.02.002.
- Cairns S, Scholefield JH. Guidelines for colorectal cancer screening in high risk groups. Gut 2002;51:v1-2.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844-57. https://doi.org/10.1053/j.gastro.2012.06.001.
- Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006;130:1872-85. https://doi.org/10.1053/j.gastro.2006.03.012.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315-29. https://doi.org/10.1038/ajg.2012.161.
- Segnan N, Patnick J, von Karsa L. European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis – First Edition. Luxembourg: Publications Office of the European Union; 2010.
- Atkin WS, Valori R, Kuipers EJ, Hoff G, Senore C, Segnan N, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition – colonoscopic surveillance following adenoma removal. Endoscopy 2012;44:Se151-63.
- Hassan C, Quintero E, Dumonceau JM, Regula J, Brandao C, Chaussade S, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013;45:842-51. https://doi.org/10.1055/s-0033-1344548.
- Coe SG, Thomas C, Crook J, Ussui V, Diehl N, Wallace MB. Colorectal surveillance interval assignment based on in vivo prediction of polyp histology: impact of endoscopic quality improvement program. Gastrointest Endosc 2012;76:118-25. https://doi.org/10.1016/j.gie.2012.03.007.
- Davila RE, Rajan E, Baron TH, Adler DG, Egan JV, Faigel DO, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc 2006;63:546-57. https://doi.org/10.1016/j.gie.2006.02.002.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol 2010;8:865-869.e1. https://doi.org/10.1016/j.cgh.2010.05.018.
- Kessler WR, Imperiale TF, Klein RW, Wielage RC, Rex DK. A quantitative assessment of the risks and cost savings of forgoing histologic examination of diminutive polyps. Endoscopy 2011;43:683-91. https://doi.org/10.1055/s-0030-1256381.
- Longcroft-Wheaton G, Bhandari P. The cost impact of in vivo diagnosis of diminutive polyps: experience from a screening endoscopy programme. Gut 2011;60. https://doi.org/10.1136/gut.2011.239301.60.
- Longcroft-Wheaton GR, Bhandari P. The cost impact of in-vivo diagnosis of diminuitive colonic polyps in screening colonoscopy: results from a large prospective western study. Gastrointest Endosc 2011;1. https://doi.org/10.1016/j.gie.2011.03.113.
- McGill SK, Soetikno RM, Yokomizo L, Goldhaber-Fiebert JD, Owens D, Kaltenbach T. Optical diagnosis of small colorectal polyps with resect and discard strategy is cost saving. Gastrointest Endosc 2013;1. https://doi.org/10.1016/j.gie.2013.04.118.
- Solon C, Klausnitzer R, Blissett D, Ihara Z. Economic value of narrow band imaging versus white light endoscopy for the characterization of diminutive polyps in the colon: systematic literature review and cost-consequence model. J Med Econ 2016;19:1040-8. https://doi.org/10.1080/13696998.2016.1192550.
- Ness RM, Holmes AM, Klein R, Dittus R. Cost–utility of one-time colonoscopic screening for colorectal cancer at various ages. Am J Gastroenterol 2000;95:1800-11. https://doi.org/10.1111/j.1572-0241.2000.02172.x.
- Guide to the Methods of Technology Appraisal 2013. London: National Institute for Health and Care Excellence; 2013.
- Rastogi A, Bansal A, Rao DS, Gupta N, Wani SB, Shipe T, et al. Higher adenoma detection rates with cap-assisted colonoscopy: a randomised controlled trial. Gut 2012;61:402-8. https://doi.org/10.1136/gutjnl-2011-300187.
- Butterly LF, Chase MP, Pohl H, Fiarman GS. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol 2006;4:343-8. https://doi.org/10.1016/j.cgh.2005.12.021.
- Whyte S, Chilcott J, Halloran S. Reappraisal of the options for colorectal cancer screening in England. Colorectal Dis 2012;14:e547-61. https://doi.org/10.1111/j.1463-1318.2012.03014.x.
- Department of Health . NHS Reference Costs 2014–2015 2015. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_122803 (accessed 31 March 2016).
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit; 2015.
- Lee TJ, Rutter MD, Blanks RG, Moss SM, Goddard AF, Chilton A, et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut 2012;61:1050-5. https://doi.org/10.1136/gutjnl-2011-300651.
- Department of Health . NHS Reference Costs 2015–2016 n.d. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 31 March 2016).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Sharara N, Adam V, Crott R, Barkun AN. The costs of colonoscopy in a Canadian hospital using a microcosting approach. Can J Gastroenterol 2008;22:565-70. https://doi.org/10.1155/2008/854984.
- Bisschops R, Tejpar S, Willekens H, Hertogh G, Cutsem E. I-SCAN detects more polyps in lynch syndrome (HNPCC) patients: a prospective controlled randomized back-to-back study. Gastrointest Endosc 2012;75. https://doi.org/10.1016/j.gie.2012.03.857.
- Public Health Functions to be Exercised by NHS England: Service Specification No.26, Bowel Cancer Screening Programme. London: Department of Health; 2013.
- Whyte S, Chilcott J, Cooper K, Essat M, Stevens J, Wong R, et al. Re-appraisal of the Options for Colorectal Cancer Screening: Full Report. Sheffield: School of Health and Related Research (ScHARR); 2011.
- Raju GS, Vadyala V, Slack R, Krishna SG, Ross WA, Lynch PM, et al. Adenoma detection in patients undergoing a comprehensive colonoscopy screening. Cancer Med 2013;2:391-402. https://doi.org/10.1002/cam4.73.
- Lee TJ, Nickerson C, Goddard AF, Rees CJ, McNally RJ, Rutter MD. Outcome of 12-month surveillance colonoscopy in high-risk patients in the National Health Service Bowel Cancer Screening Programme. Colorectal Dis 2013;15:e435-42. https://doi.org/10.1111/codi.12278.
- Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009;136:832-41. https://doi.org/10.1053/j.gastro.2008.12.007.
- Laiyemo AO, Pinsky PF, Marcus PM, Lanza E, Cross AJ, Schatzkin A, et al. Utilization and yield of surveillance colonoscopy in the continued follow-up study of the polyp prevention trial. Clin Gastroenterol Hepatol 2009;7:562-7. https://doi.org/10.1016/j.cgh.2008.12.009.
- Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc 2006;64:614-26. https://doi.org/10.1016/j.gie.2006.06.057.
- McDonald PJ, Digby J, Innes C, Strachan JA, Carey FA, Steele RJ, et al. Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis 2013;15:e151-9. https://doi.org/10.1111/codi.12087.
- Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst 2003;95:230-6. https://doi.org/10.1093/jnci/95.3.230.
- Atkin WS. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet 2002;359:1291-300. https://doi.org/10.1016/S0140-6736(02)08268-5.
- Pilgrim HTP, Chilcott J, Bending M, Trueman P, Shorthouse AJ, Tappenden J. The costs and benefits of bowel cancer service developments using discrete event simulation. J Oper Res Soc 2009;10:1305-14. https://doi.org/10.1057/jors.2008.109.
- Ignjatovic A, Thomas-Gibson S, East JE, Haycock A, Bassett P, Bhandari P, et al. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc 2011;73:128-33. https://doi.org/10.1016/j.gie.2010.09.021.
- Mead RJ, Duku M, Longcroft-Wheaton G, Pearl D, Basford P, Bhandari P. Endoscopic training: colon polyp assessment. Gut 2011;60:A121-2. https://doi.org/10.1136/gut.2011.239301.258.
- Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health 2011;14:539-45. https://doi.org/10.1016/j.jval.2010.10.029.
- Djalalov S, Rabeneck L, Tomlinson G, Bremner KE, Hilsden R, Hoch JS. A review and meta-analysis of colorectal cancer utilities. Med Decis Making 2014;34:809-18. https://doi.org/10.1177/0272989X14536779.
- Färkkilä N, Sintonen H, Saarto T, Järvinen H, Hänninen J, Taari K, et al. Health-related quality of life in colorectal cancer. Colorectal Dis 2013;15:e215-22. https://doi.org/10.1111/codi.12143.
- Downing A, Morris EJ, Richards M, Corner J, Wright P, Sebag-Montefiore D, et al. Health-related quality of life after colorectal cancer in England: a patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol 2015;33:616-24. https://doi.org/10.1200/JCO.2014.56.6539.
- Dorian P, Kongnakorn T, Phatak H, Rublee DA, Kuznik A, Lanitis T, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J 2014;35:1897-906. https://doi.org/10.1093/eurheartj/ehu006.
- Rees CJ, Rajasekhar PT, Wilson A, Close H, Rutter MD, Saunders BP, et al. Narrow band imaging optical diagnosis of small colorectal polyps in routine clinical practice: the Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut 2017;66:887-95. https://doi.org/10.1136/gutjnl-2015-310584.
- Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, Chauhan SS, et al. ASGE technology committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2015;81:502-16. https://doi.org/10.1016/j.gie.2014.12.022.
- Snover DC, Ahnen DJ, Burt RW, Odze RD, Bostman FT, Carneiro F, et al. WHO Classification of Tumours of the Digestive System. Lyon: IARC; 2010.
- Rastogi A, Bansal A, Rao DS, Gupta N, Wani SB, Shipe T, et al. A prospective, randomized, controlled trial comparing cap assisted colonoscopy (CAC) and high definition white light colonoscopy (HDWL) for the detection of colon polyps. Gastrointest Endosc 2011;73:AB148-9. https://doi.org/10.1016/j.gie.2011.03.112.
- Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA 2008;299:1027-35. https://doi.org/10.1001/jama.299.9.1027.
- World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC press; 2000.
- Bosman FT. World Health Organization Classification of Tumours of the Digestive System. Lyon: IARC press; 2010.
- Belderbos TD, Van Oijen MG, Moons LM, Siersema PD. The accuracy of real-time probe based confocal LASER endomicroscopy for differentiation of colorectal polyps during colonoscopy. Gastrointest Endosc 2015;1. https://doi.org/10.1016/j.gie.2015.03.1549.
- Kaltenbach T, Rouse RV, McGill SK, Jayasakera CR, Motiwala A, Soetikno R, et al. Gastroenterology trainees can perform real time optical diagnosis of diminutive colorectal polyps using narrow band imaging. Gastroenterology 2014;1. https://doi.org/10.1016/S0016-5085(14)62778-5.
- Kheir AO, Sabanathan J, Hawken G, Dowsett JF, Singh S, Panetta J, et al. Optical diagnosis of diminutive colorectal polyps by non-academic general gastroenterologists using non-magnifying narrow band imaging (NBI): a prospective study. Gastrointest Endosc 2016;1. https://doi.org/10.1016/j.gie.2016.03.992.
- Klein A, Half EE, Chowers Y, Waterman M, Awadie H, Sabo E. Computerized, image analysis of diminutive polyps during colonoscopy-preliminary results of a feasibility study. Gastrointest Endosc 2014;1. https://doi.org/10.1016/j.gie.2014.02.891.
- Lee JY, Wiggins L, John S. Learning curve for optical biopsy using narrow band imaging – can real-time training improve accuracy?. J Gastroenterol Hepatol 2014;29:31-2.
- Lee JY, Wiggins L, John S. Learning curve for optical biopsy using narrow band imaging (NBI) – can real-time training improve accuracy?. Gastrointest Endosc 2015;1. https://doi.org/10.1016/j.gie.2015.03.1465.
- Madacsy L, Gyimesi G, Hritz I, Hausinger P, Velkei T, Gellert B, et al. Diagnostic Value of Fujinon Intelligent Color Enhancement (FICE) Technology With and Without Magnification to Differentiate Between Hyperplastic and Adenomatous Lesions According to the NICE Classification – A Prospective, Randomized, Controlled Study n.d.
- Maimone A, De Luca L, Bersani G, Buzzi A, Grillo A, Ricci G, et al. Real-time biopsy of colorectal polyps = 6 mm using FICE, I-scan and NBI technologies: experience of a young endoscopist. Dig Liver Dis 2015;47.
- Neumann H, Vieth M, Fry LC, Tontini GE, Grauer M, Neurath MF, et al. Development and validation of a simple classification system for in vivo diagnosis of colorectal polyps using digital chromoendoscopy – the visible study. Gastrointest Endosc 2015;1. https://doi.org/10.1016/j.gie.2015.03.1411.
- Paggi S, Rondonotti E, Amato A, Spini G, Radaelli F. Is it really so easy to learn histologic characterization of diminutive polyps by narrow band imaging? Preliminary results of endoscopists’ and nurses’ performances. Gastrointest Endosc 2014;1. https://doi.org/10.1016/j.gie.2014.02.673.
- Rastogi A, Gupta N, Sinh P, Sharma P, Wani S, Bansal A. Performance of gastroenterology (GI) trainees in real-time characterization of diminutive polyp (DP) histology with narrow band imaging (NBI)-results from a prospective trial. Gastroenterology 2014;1. https://doi.org/10.1016/S0016-5085(14)60773-3.
- Rastogi A, Gupta N, Sinh P, Sharma P, Wani S, Bansal A. Prediction time for characterizing diminutive (% 5mm) polyp (DP) histology with NBI during colonoscopy is a marker for high confidence (HC) diagnosis and accuracy. Gastrointest Endosc 2014;1. https://doi.org/10.1016/j.gie.2014.02.157.
- Rastogi A, Gupta N, Sinh P, Sharma P, Wani S, Bansal A. Gastroenterology (GI) trainees can achieve the PIVI benchmarks for real-time characterization of the histology of diminutive (% 5 mm) polyps (DP) – a prospective study. Gastrointest Endosc 2014;1. https://doi.org/10.1016/j.gie.2014.02.092.
- Rocha J, Lee S, Siegel L, Mathur N, Agarwal S, Sostre C, et al. In vivo diagnosis of colorectal polyps by GI endoscopists using HD narrow-band imaging. Am J Gastroenterol 2014;109.
- Staiano T, Grassia R, Savarese MF, Iiritano E, Bianchi G, Buffoli F. High-definition colonoscopy using i-scan in morphological characterization and real-time histological prediction of colonic neoplastic superficial lesion. A single Italian center pilot study, preliminary results. Dig Liver Dis 2016;48. https://doi.org/10.1016/S1590-8658(16)30077-9.
- Vleugels J, IJspeert J, Hazewinkel Y, Koens L, Fockens P, Dekker E. Incorporating sessile serrated polyps in optical diagnosis of diminutive polyps: what are the implications for the PIVI thresholds?. Gastroenterology 2016;1. https://doi.org/10.1016/S0016-5085(16)30221-9.
- Xu XB, Guo MX, Jin XX, Wang B, Li ML, Wu XW, et al. Significance of endoscopic mucosal surface features in diagnosing colorectal polyps. J Am Geriatr Soc 2015;63.
Appendix 1 Search strategy
The databases we searched for the clinical effectiveness and cost-effectiveness systematic reviews are listed below, along with the search dates.
| Database searched (host) | Clinical effectiveness and cost-effectiveness search dates |
|---|---|
| Combined search on MEDLINE (via Ovid) and MEDLINE In-Process & Other Non-Indexed Citations | MEDLINE: 1946–29 June 2016 |
| MEDLINE In-Process & Other Non-Indexed Citations: searched to 29 June 2016 | |
| EMBASE (via Ovid) | 1974–29 June 2016 |
| Web of Science (all databases) | Searched to 29 June 2016 |
| Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment database and NHS Economic Evaluation Database | Searched to 29 June 2016 |
| Searched for ongoing trials (all searched on either 12 March 2016 or 13 March 2016) |
|---|
| UKCTG |
| World Health Organization’s International Clinical Trials Registry Platform |
| ISRCTN (controlled and other trials) |
| ClinicalTrials.gov |
| PROSPERO |
The MEDLINE search strategy for identifying clinical effectiveness and cost-effectiveness publications is shown here. This strategy was adapted for other databases and the other strategies used are available on request.
MEDLINE search strategy
-
(virtual and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(“real time” and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(video and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(optical and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(digital and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(magnif* and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(“image enhanc*” and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(“post processing” and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(“high contrast” and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(“high performance” and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(“high definition” and (chromoendoscop* or chromo endoscop*)).tw.
-
(“high resolution” and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(electronic and (chromoendoscop* or “chromo endoscop*”)).tw.
-
(magnif* and zoom and imag*).tw.
-
“real time imag*”.tw.
-
“real time histology”.tw.
-
(“real time” and (chromoendoscop* or “chromo endoscop*”)).tw.
-
“narrow band”.tw.
-
NBI.tw.
-
“narrow* spectrum endoscop*”.tw.
-
“optical diagnosis”.tw.
-
“optical imaging”.tw.
-
“image enhancement”.tw.
-
“EVIS LUCERA”.mp.
-
“CV-290/CLV-290SL”.mp.
-
“CV-260SL/CLV-260SL”.mp.
-
“EVIS EXERA”.mp.
-
“dual focus”.tw.
-
(“290HQ/290H” and endoscop*).mp.
-
(“290HQ/290H” and Olympus).mp.
-
(“260Q/260H” and endoscop*).mp.
-
(“260Q/260H” and Olympus).mp.
-
FICE.mp.
-
flexible spectral imag* colo?r enhancement.tw.
-
flexible imag* colo?r enhancement.tw.
-
“white light”.tw.
-
“band limited white”.tw.
-
“Fuji* intelligent colo?r enhancement”.mp.
-
(Fuji* adj5 chromoendoscop*).mp.
-
(Fuji* adj5 endoscop*).mp.
-
“Fujinon/Aquilant Endoscop*”.mp.
-
Fuji* Aquilant Endoscop*.mp.
-
(“EPX-4450HD” or “EPX3500HD” or “EPX-4400”).tw.
-
((fuji* and “500 series”) or “600 series” or “600 CMOS”).tw.
-
“i-scan”.mp.
-
“image enhanced endoscop*”.tw.
-
“image enhanced chromoendoscop*”.tw.
-
“image enhanced chromo endoscop*”.tw.
-
(Pentax and endoscop*).mp.
-
(Pentax and chromoendoscop*).mp.
-
“EPK i5000”.mp.
-
“EPK i7000”.mp.
-
“EPK i7010”.tw.
-
(Pentax and (“i10” or “90i” or 90K)).mp.
-
(“high definition” and “video processing”).tw.
-
or/1-55
-
Colonoscopy/
-
Colonoscop*. tw.
-
Colonic Polyps/
-
(colon* adj5 polyp*).tw.
-
(colorectal adj5 polyp*).tw.
-
Intestinal Polyps/ or Intestinal Polyposis/ or Adenomatous Polyps/
-
(intestin* adj5 polyp*).tw.
-
(adenom* adj5 polyp*).tw.
-
(diminutive adj5 polyp*).tw.
-
(small adj5 polyp*).tw.
-
(hyperplas* adj5 polyp*).tw.
-
colo* lesion*.tw.
-
colo* mucosal lesion*.tw.
-
non neoplastic polyp*.tw.
-
Colorectal Neoplasms/
-
“colorectal cancer”.tw.
-
(colorectal adj2 neoplas*).tw.
-
“colon* cancer”.tw.
-
(colon adj5 neoplas*).tw.
-
or/57-75
-
56 and 76
-
((chromoendoscop* or “chromo endoscop*”) and polyp*).ti.
-
polyp*.tw.
-
nasal polyp*.tw.
-
Nasal Polyps/
-
80 or 81
-
79 not 82
-
56 and 83
-
77 or 78 or 84
-
limit 85 to animals
-
85 not 86
-
limit 87 to english language
-
remove duplicates from 88
Appendix 2 Study selection worksheet
Study selection took place in two stages.
For title/abstract screening the following criteria were used.
| PICO element | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population |
|
|
| Notes: if a mixed population (i.e. including one of the excluded groups), then retrieve because results may be presented separately for group(s) of interest | ||
| Intervention(s) | Real-time and HD assessment without magnification with one or more of:
|
Post-procedure assessment |
| Notes: it may not be clear from title or abstract whether or not the assessment has been done in real time, whether or not a HD system has been used and whether or not magnification has been used. If in doubt retrieve for assessment of the full paper | ||
| Comparator (reference standard) | Histopathological assessment of resected diminutive (≤ 5 mm in size) colorectal polyps. (Retrieve any studies stating that WLE was used as the comparator as this can mean that histopathology was used for diagnosis) | |
| Notes: abstract might not mention histopathology (e.g. might say biopsies taken but not indicate these were for histopathology). Studies of larger-sized polyps will be eligible if outcome data are given for the subgroup of diminutive polyps. If in doubt, retrieve for assessment of full-text paper | ||
| Outcomes | Any one of:
|
|
| Study design |
|
|
For full-text screening: same criteria as applied to titles and abstracts (also see Decision rules).


Decision rules
During the course of screening full papers issues arose and decision rules have been created to deal with these situations.
Population
-
When the population is unclear (i.e. because of a lack of description), err on the side of inclusion unless there is definite evidence that the population is one that we are not interested in (e.g. IBD, polyposis syndromes) (example papers are Hoffman et al. 80 and Rex64).
-
When population appears to be one we are interested in but paper does not specifically state that the groups we are excluding were not included, err on the side of inclusion (example papers are Basford et al. 79 and Rath et al. 82).
Intervention
-
Use of inbuilt (close-focus) magnification (which will be low level, e.g. × 1.5), that does not require a zoom endoscope or any other additional equipment can be included (example paper is Rex64).
-
When use of magnification is described as ‘optional’ but with no further details (i.e. about the level of magnification or the proportion of cases where it was used), err on the side of inclusion (example paper is Hoffman et al. 80).
-
When magnification is not mentioned and no zoom equipment is described, err on the side of inclusion (i.e. presume no magnification) (example papers are Basford et al. 79 and Rath et al. 82).
Appendix 3 Data extraction tables
Aihara et al.66
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: whether a polyp is neoplastic or non-neoplastic. The aim of study was to develop a scoring system for NBI classification of polyps, based on the NBI International Colorectal Endoscopic classification and to assess its performance First author: Aihara Publication year: 2015 Country: USA Study design: prospective cohort Number of centres: NR, but all authors were affiliated to the same hospital, so it is likely that this was a single centre study Funding: NR Competing interests: one author (CCT) was a consultant for Olympus. The other authors had no competing interests |
Index test: NBI. HD colonoscope (CF-H180AL, Olympus America Inc, Centre Valley, PA, USA) White light was used to initially diagnose the polyp, then the endoscopist switched to NBI to score the polyp (scores were compared with histopathological diagnoses to determine the threshold score) Reference standard: histopathology |
Number of participants: 203, of whom 67 were found to have polyps Sample attrition/dropout: not explicitly stated, but assumed to be zero Selection of participants: see inclusion criteria for study entry below Inclusion criteria for study entry: patients presenting for elective screening or follow-up colonoscopy (reason for follow-up colonoscopy not reported) Exclusion criteria for study entry: none stated |
Primary outcome of study: the threshold score on the polyp scoring system that provided the highest NPV Other relevant outcomes: diagnostic accuracy, sensitivity, specificity, PPV and NPV Recruitment dates: NR |
| Participant characteristics | |||
| Age (years) mean | 53.7 | ||
| Other key patient characteristics (list) | Patient characteristics of the 67 patients with detected polyps:
|
||
| Endoscopist experience and training | Seven endoscopists, described as ‘experienced’, carried out the colonoscopies. Before the study started, all the endoscopists took part in a training session on NBI interpretation and the scoring system. No further details of experience or training are reported | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
NBI polyp classification system: the Aihara score modification of the NBI International Colorectal Endoscopic classification (NBI International Colorectal Endoscopic-AS) system. Polyps were classified according to ‘lesion colour’, ‘surface pattern’ and ‘vessel pattern’. Polyps that were ‘light greenish’ or ‘brownish’ coloured, had ‘invisible’ or ‘small round’ surface pattern and ‘invisible’ or ‘slightly dilated’ vessel pattern, were classified as non-neoplastic. Polyps that were ‘deeper brownish’, had ‘dilated’, ‘elongated’ or ‘branched’ surface pattern and a ‘dilated’ vessel pattern, were classified as neoplastic. Polyps were scored on these factors and could receive a total score of between 0 and 3 (a score of 1 was assigned to each of ‘lesion colour’, ‘surface pattern’ and ‘vessel pattern’ if a feature suggestive of neoplasia was present) Pathological diagnoses of sessile serrated adenoma/polyp (SSA/P): the World Health Organization’s criteria. 150 SSA/Ps were classified as neoplastic in the final analysis. None of the three SSA/Ps was < 5 mm in size |
||
| Sample size calculation | It was calculated that 138 polyps were needed to allow a 95% confidence limit extend to 85%. This was based on data from a previous ex vivo study which found a diagnostic accuracy of 89% and an assumption that the true accuracy rate would be 90%. 156 polyps were included in the study | ||
| Results: for polyps sized < 5 mm (i.e. not including those 5 mm in size) when using a threshold score of ≥ 1 on the NBI International Colorectal Endoscopic-AS (indicating at least one feature of neoplasia was present) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 60a | (b) 10a | 70a |
| Index test negative | (c) 2a | (d) 49a | 51a |
| Total | 62a | 59a | 121 |
| Accuracy [(a + d)/(a + b + c + d)] | 90.1% (95% CI 84.8% to 95.4%) (109 of the 121 polyps were correctly classified) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 96.8% | 87.3% to 99.4% | |
| Clinical specificity d/(b + d) | 83.1% | 70.6% to 91.1% | |
| PPV a/(a + b) | 85.7% | 74.8% to 92.6% | |
| NPV d/(c + d) | 96.1% | 85.4% to 99.3% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 5.71a | 3.24 to 10.06a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.04a | 0.01 to 0.15a | |
| Diagnostic odds ratio (a × d)/(b × c) | 147.000a | 30.755 to 702.62a | |
| Reviewer calculated the same sensitivity, specificity, PPVs and NPVs as reported in the paper, but reviewer calculated CIs differed | |||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | NR | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Study included patients presenting for elective screening or follow-up colonoscopy, but no further information about the indications for colonoscopy were provided | Unclear |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polyps appeared to receive verification by histopathology | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Pathologists were blinded to the endoscopic findings | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Uninterpretable index test (NBI) results were not reported | No |
| 11 | Were withdrawals from the study explained? | There appeared to be no withdrawals in this study | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant studies identified | ||
Summary reviewer’s comments
The setting and population for this study were unclear, so it is unclear how generalisable the results are to the population of interest in this appraisal and the NHS setting in the UK. All the study endoscopists received training in NBI prior to the start of the study, so the results are applicable to those with some training in NBI. The authors point out that in this study the endoscopists did not diagnose the polyp as such, but scored it on the NBI International Colorectal Endoscopic-AS and point out that the scoring system requires further clinical validation. Different results may have been obtained if the endoscopists had diagnosed the polyp rather than using the scoring system, so the findings may not generalise to other contexts where diagnoses are made using other information or different classification systems.
Basford et al.79
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: Differentiation of adenomas from non-neoplastic polyps First author: Basford, the HiSCOPE study Publication year: 2014 Country: UK Study design: prospective cohort Number of centres: single academic hospital (Portsmouth Queen Alexandra) Funding: local departmental research budget Competing interests: stated none |
Index test: PENTAX EC-3890Li 1.2 megapixel HD+ colonoscopes, linked to an EPKi processor (PENTAX Medical, Montvale, NJ, USA) Each polyp assessed sequentially by using HD WLE followed by i-scan surface, contrast, and tone enhancement modes (i-scan 1 = surface enhancement +3, contrast enhancement +4; i-scan 2 = surface enhancement + 1, tone enhancement colon; i-scan 3 = surface enhancement + 3, contrast enhancement +2, tone enhancement colon) Reference standard: histopathology |
Number of participants: 84 Sample attrition/dropout: not stated Selection of participants: patients attending for colonoscopy through the UK Bowel Cancer Screening Programme were prospectively recruited Inclusion criteria for study entry: not explicitly stated, but appears to be people with a positive FOBT attending for colonoscopy as part of the UK Bowel Screening Programme Exclusion criteria for study entry: poor bowel preparation, polyposis syndrome, IBD. Polyps were not included in the study if they were ≥ 10 mm in diameter or if polyp tissue was not retrieved for histopathological analysis |
Primary outcome of study: overall diagnostic accuracy of high confidence in vivo assessment of small colon polyps (< 10 mm in size) Other relevant outcomes: specificity and sensitivity for adenomatous histopathology and the NPV for adenomatous histopathology of diminutive rectosigmoid colon polyps; the accuracy of prediction of polyp surveillance intervals based on high confidence in vivo assessment of all diminutive (< 5 mm in size) colon polyps combined with histopathology of polyps > 5 mm in size Recruitment dates: May 2011–May 2012 |
| Participant characteristics | |||
| Age (years), mean (SD) | Not stated, but the age range for the UK Bowel Screening Programme is 60–74 years | ||
| Other key patient characteristics |
55 (65%) male, 29 (35%) female (percentages calculated by reviewer) A total of 209 polyps (up to 10 mm in size) were included in the study. Of these, 172 (82%) were ≤ 5 mm in size (percentage calculated by reviewer) Mean polyp size was 4.3 mm, median 4.0 mm and SD 2.2 mm. Only 7 of the 209 polyps were pedunculated (0-Ip), with the remainder being sessile (0-Is, n = 90) or flat-raised (0-IIa, n = 112) in accordance with the Paris classification. A total of 75 of 209 polyps (35.9%) were non-neoplastic and 134 of 209 (64.1%) were neoplastic. A total of 43% of polyps included were found in the right side of the colon (transverse, ascending and caecum) |
||
| Endoscopist experience and training | All procedures were performed by a single endoscopist (one of the authors) with experience in in vivo characterisation of colon polyps. Before commencement of the study, the endoscopist underwent a period of familiarisation with the endoscope and imaging technology, including development of a NAC for assessment of colon polyps by using i-scan. It is also stated that the endoscopist was very familiar with the technology and had risen up any learning curve | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
The study used a NAC for assessment of colon polyps by using i-scan. This classification system was adapted from a previously described classification system (NAC) (note that NAC is not defined, but references to supporting publications are provided) that was developed for assessment of all colon mucosal lesions. A total of 100 polyps were assessed by the study endoscopist (senior author) documenting features predictive of neoplastic or non-neoplastic histopathology (as set out in table 1 and figure 5 of the journal article). It was validated on a further 100 polyps by two other investigators (co-authors) who recorded vascular and surface patterns, which were compared with the final histopathology The Paris classification system was used to assess polyp morphology |
||
| Sample size calculation | Prospective sample size calculations were performed with an expected HDWL accuracy of 75% and i-scan accuracy of 85%. When a power (1 – β) of 80% and a two-sided significance level (α) of 0.05 were used, a total of 198 polyps were required to demonstrate a significant difference between HD white light and i-scan. A 5% increase was made to allow for lost or non-retrieved specimens, giving a final target of 208 polyps. (Note that the comparison in accuracy between HDWL and i-scan is not directly relevant to this systematic review) | ||
| Results: subset of 172 polyps ≤ 5 mm in size all characterised with high confidence | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 100a | (b) 7a | 107a |
| Index test negative | (c) 3a | (d) 62a | 65a |
| Total | 103a | 69a | 172 |
| Accuracy [(a + d)/(a + b + c + d)] | 94.2% (95% CI 92.8% to 99.2%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 97.1% | 92.8% to 99.2% | |
| Clinical specificity d/(b + d) | 89.9% | 83.5% to 93.0% | |
| PPV a/(a + b) | 93.5%a | 87.0% to 97.3%a | |
| NPV d/(c + d)b | 100% | 93.4% to 100% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 9.57a | 4.74 to 19.33a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.03a | 0.01 to 0.10a | |
| Diagnostic odds ratio (a × d)/(b × c) | 295 | 73.6 to 1184.3 | |
| Interpretability of test | NR | ||
| Interobserver agreement | n/a | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | Only polyps characterised with high confidence were included in the analysis (n = 209). A total of 29 polyps were excluded from the original sample on the basis of low-confidence assessment | ||
| Low-confidence optical diagnosis | |||
| Number of polyps designated to be left in place | NR (but it is believed that all were left in place as authors state that in vivo assessment was performed in the time between finding a polyp and preparing for polypectomy, therefore implying that polypectomy was always done) | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
Assessed in accordance with ASGE and BSG guidelines for adenoma surveillance after colonoscopy. Predicted intervals were compared with those made with histopathology. The patient sample size was 83, as a result of one patient being excluded because a single polyp was not retrieved for histopathological analysis Surveillance intervals were in agreement with histopathology in 80 of 83 cases with i-scan (97.2%) in accordance with BSG guidelines, with identical results for ASGE guidelines. Under i-scan, two patients would return earlier and a single patient would have been brought back at 5 years rather than 3 years |
||
| Length of time to perform the colonoscopy | Not explicitly assessed as an outcome, but the authors report that in vivo assessment was performed in the time between finding a polyp and preparing for polypectomy and did not cause a significant delay | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Patients from the UK Bowel Cancer Screening Programme | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polyps received verification by histopathology (with the exception of one polyp which was not retrieved for histopathology) | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Predicted histopathology was subsequently compared with the final histopathological diagnosis as reported by a Bowel Cancer Screening Programme – accredited histopathologist who was not aware of the results of the in vivo assessment | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | Of 107 patients screened for inclusion, 23 were excluded (19 had no polyps, two had IBD and two had stricturing colorectal cancer) | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant studies cited | ||
Summary reviewer’s comments
The results are applicable to VCE with i-scan conducted in an academic hospital by a colonoscopist with extensive prior experience with in vivo polyp characterisation who was familiar with the i-scan technology and based only on high-confidence assessments. The patients were sampled from the UK Bowel Screening Programme and had apparently positive FOBT results. The authors acknowledge that the study was performed under optimised conditions for in vivo assessment and the high level of accuracy may not necessarily be found in studies without such conditions.
Chandran et al.67
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: the accuracy of real-time endoscopic assessment of diminutive polyps for predicting surveillance intervals First author: Chandran Publication year: 2015 Country: Australia Study design: prospective cohort Number of centres: two (a tertiary hospital and a private community hospital) Funding: none received Competing interests: the authors declared they had no conflicts |
Index test: polyps were identified using an adult or paediatric HD, variable stiffness colonoscopies (CF-H180AL or PCF-H180 AL; Olympus Inc., Tokyo, Japan). The study used the HD and NBI-compatible Exera processor (Olympus Inc.). Diminutive polyps were examined with NBI without magnification Reference standard: histopathology |
Number of participants: 94 Sample attrition/dropout: not explicitly reported, but assumed none (94 patients recruited and results reported for 159 polyps in 94 patients) Selection of participants: consecutive patients presenting to the endoscopists involved in the study, who fulfilled in the inclusion criteria below Inclusion criteria for study entry: aged ≥ 18 years; complete colonoscopy; satisfactory or good bowel preparation; at least one polyp sized ≤ 5 mm Exclusion criteria for study entry: IBD; primary sclerosing cholangitis; prior colon cancer; poor bowel preparation; and, incomplete colonoscopy |
Primary outcome of study: diagnostic accuracy of optical diagnosis of diminutive polyps compared with histopathology Other relevant outcomes: accuracy of surveillance intervals assigned following optical diagnosis compared with those assigned following histopathological assessment (stated secondary end point), as per the PIVI initiative. Assignment of surveillance intervals was based on NHMRC guidelines (abbreviation not defined in paper) (references provided in paper) Adverse events (recorded by study investigators) and costs (not included under outcomes). Costs data not extracted Recruitment dates: October 2012–July 2013 |
| Participant characteristics | |||
| Age (years), mean (SD) | Median 62 (range 19–84) | ||
| Other key patient characteristics (list) |
159 diminutive (≤ 5 mm) polyps. Median polyp size was 3 mm (range 1–5 mm) Female-to-male ratio of 1.35 (n and % of each gender not reported) Colonoscopy indications: previous polyps, 32/94 (34%); colon cancer screening, 25/94 (26.6%); altered bowel habit, 15/94 (16%); rectal bleeding, 11/94 (11.7%); and other, 11/94 (11.7%) Polyp location, n/N (%): caecum, 21/159 (13.2%); ascending colon, 27/159 (17%); transverse, 30/159 (18.9%); descending, 16/159 (10%); sigmoid, 40/159 (25.2%); and rectum, 25/159 (15.7%) |
||
| Endoscopist experience and training | Three endoscopists performed the colonoscopies and they had varying prior experience. One was an interventional endoscopist (ME), one a general community gastroenterologist (SL) and one an endoscopy fellow (SC). Prior to the study, only ME had routinely used NBI to assess polyps. All the endoscopists received training in the NBI/Sano–Emura classification system as part of the study. This was a self-study module created for the study, requiring the endoscopists to study an extensive photo library of polyps, a video on NBI classification of polyps and literature about the classification system prior to the study | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | A simplified version of the Sano–Emura classification system was used to classify diminutive polyps: non-adenomatous (type I, no meshed capillaries) and adenomatous (type II, IIIA and IIIB, with meshed capillaries) | ||
| Sample size calculation | A sample size of 146 polyps was calculated to demonstrate a sensitivity of 95% for adenoma detection with a two-sided 95% CI of ± 5%. This was based on an expected prevalence of adenomas of 50% | ||
| Results: NBI assessment of diminutive polyps (all study polyps, n = 159) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathologya | Total | |
| Index test positive | (a) 105 | (b) 11 | 116 |
| Index test negative | (c) 3 | (d) 40 | 43 |
| Total | 108 | 51 | 159 |
| Accuracy [(a + d)/(a + b + c + d)] | 91.2%b (145 of 159 polyps predicted accurately) | ||
| Diagnosisc | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 97.2% | 92.1% to 99.4% | |
| Clinical specificity d/(b + d) | 78.4% | 64.7% to 88.7% | |
| PPV a/(a + b) | 90.5% | 83.7% to 95.2% | |
| NPV d/(c + d) | 93% | 80.9% to 98.5% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 4.51 | 2.67 to 7.61 | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.0354 | 0.0115 to 0.109 | |
| Diagnostic odds ratio (a × d)/(b × c) | 127 | 35.3 to 450 | |
|
Reviewer calculated a diagnostic odds ratio of 127.3 (CI 33.7 to 480.0) Diagnostic accuracy results also reported for each of the three endoscopists, but not data extracted here |
|||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | Measured but NR | ||
| High-confidence optical diagnosis | NR | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | Using the current NHMRC guidelines, 92 out of 94 (98%) patients were correctly allocated to their repeat colonoscopy. The NPV for agreement in assignment of surveillance intervals was 95.7% (95% CI 78.1% to 99.9%). The results were also stratified by endoscopist, and one had a NPV of 88.2% (95% CI 63.6% to 98.5%), which is below the PIVI guidelines threshold | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes, the study included all three population groups relevant to this appraisal and who would receive the test in practice | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | Each polyp was resected for histopathological assessment | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Each polyp was assessed by a pathologist blinded to the real-time prediction of polyp histopathology | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | Withdrawals not explicitly reported, but believed to be zero | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes, no additional relevant studies identified | ||
Summary reviewer’s comments
The results reflect the use of NBI in a public and a private hospital setting in Australia, by three endoscopists with varying experience of colonoscopies and NBI, in patients undergoing screening and surveillance colonoscopies, and colonoscopies for symptoms suggestive of colorectal cancer. The population in this study is relevant to the population of interest in this appraisal and, although the reviewer is not aware of how practice in Australia differs to that in the UK, based on the population, the results are likely to be relevant to the UK context.
Gupta et al.68
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: the in vivo optical diagnosis of colon polyp histopathology (impact of novel imaging techniques on polyp detection and/or polyp histopathology prediction) First author: Gupta [linked publications: Rastogi et al. 2009;73 Rastogi et al. 2011;96 Rastogi et al. 2012. 120,151 The reviewer notes that Rastogi et al. 2012120,151 is not a study of NBI so the cited conference abstract151 (which is linked to a full paper120) may not be the correct reference] Publication year: 2012 Country: USA Study design: retrospective analysis of data from three prospective clinical trials73,96,120 Number of centres: in two studies, one centre (Veterans Affairs Medical Centre in Kansas City, Missouri). In one study, two centres (Veterans Affairs Medical Centre in Kansas City, MO, USA and Washington University, St Louis, MO, USA) Funding: not stated by Gupta et al.,68 but stated for the linked publications: Rastogi et al. 2009,73 the 2007 Midwest Biomedical research Foundation/Kansas City VA Medical Centre Research Award; Rastogi et al. 2011,96 research grant to the primary author from Olympus America Inc.; Rastogi et al. 2012,120,151 first author supported by Endoscopic Research Career Development award from the ASGE Competing interests (for Gupta et al. 68): Dr Jonnalagadda and Dr Edmundowicz provided consultant work for Olympus America Inc. Dr Sharma received previous research grants from Olympus America Inc. Dr Rastogi has received previous research grants from Olympus America Inc and has been supported by the Michael V. Sivak, Jr, MD, Endoscopic Research Award and Endoscopic Research Career Development Award from the ASGE. The other authors disclosed no financial relationships relevant to this publication |
Index test: histopathology predicted in real time using NBI without magnification In all three studies guessing or predicting the histopathology based on features other than surface patterns (as described under ‘Polyp classification system’ below) was not permitted Commercially available Olympus colonoscopes were used (CF-H180AL and PCF-H180AL) in conjunction with the Evis Exera II CV-180 video processor and a 19-inch HD monitor (OEV 191H, Olympus America Inc.) in all three studies Reference standard: histopathology |
Number of participants: 622 participants within the three original trials (total number of participants 1150) met the criteria for this retrospective analysis. Of these 622, 410 (65.95%) had a least one polyp detected and resected Total number of polyps: n = 1254 Sample attrition/dropout: an in vivo optical diagnosis could not be determined for four polyps (0.3%) (histopathology showed three to be adenomatous and the other one hyperplastic) Selection of participants: to identify data for this study the central database holding the data for all three trials was queried to identify all subjects who had colonoscopy with HD white light or NBI and who had in vivo prediction of polyp histopathology for every polyp detected by NBI. Participants with an endoscopically malignant-appearing mass or whose resected polyp could not be retrieved for histopathology were excluded Inclusion and exclusion criteria for the trials themselves were the same for all three trials Inclusion criteria for study entry: participants were referred, and scheduled, for screening or surveillance colonoscopy and the ability to provide informed consent Exclusion criteria for study entry: previous surgical resection of any part of the colon, history of colon cancer, history of IBD, use of antiplatelet agents or anticoagulants that would prevent removal of polyps, poor general condition or any other reason to avoid prolonged procedure time, history of polyposis syndrome or hereditary non-polyposis colon cancer, or the inability to give informed consent Potential participants with inadequate bowel preparation or in whom the caecum could not be reached during the procedure were excluded |
Primary outcome of study: accuracy in predicting colonoscopy surveillance intervals, NPV for diagnosing adenomatous histopathology in the rectosigmoid part of the colon Other relevant outcomes: sensitivity, specificity and overall accuracy of in vivo optical diagnosis in differentiating adenomas from non-adenomas, the reduction in the number of polyps sent to histopathology, cost savings Recruitment dates: November 2007–October 2010 (recruitment in one of three clinical trials) |
| Participant characteristics [for the 410/622 (65.9%) patients who had at least one polyp detected and resected] | |||
| Age (years), mean (SD) | 61.7 (8.1) | ||
| Other key patient characteristics (list) |
Male: n = 367 (89.5%) White: n = 314 (76.6%) History of polyps: n = 145 (35.4%) Family history of colon cancer: n = 23 (5.6%) |
||
| Endoscopist experience and training |
The colonoscopies in all three of the trials were performed by six experienced endoscopists (three at each centre). Each endoscopist had performed > 3000 colonoscopies and all had experience of HD WLE and NBI Rastogi et al. 200973 involved just one endoscopist (the lead author) described as ‘experienced’ In the Rastogi et al. 201196 study the lead investigator reviewed the surface mucosal and vascular patterns used for polyp prediction with NBI with the five other study endoscopists. Images of 50 polyps viewed with NBI were discussed in detail in a structured teaching session until all investigators were confident in their recognition |
||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | Location, size and morphology of each polyp detected were documented. Polyp location and size were characterised using the same method in each of the three studies. Polyp morphology was classified as follows:
|
||
| Sample size calculation | None provided for this retrospective analysis but provided for the primary outcome of each of the original clinical trials | ||
| Results: for subgroup of polyps ≤ 5 mm in size (n = 884) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | 484a (a) | 97a (b) | 581a (a + b) |
| Index test negative | 37a (c) | 266a (d) | 303a (c + d) |
| Total | 521a (a + c) | 363a (b + d) | 884 (a + b + c + d) |
| Accuracy [(a + d)/(a + b + c + d)] | 84.8% (95% CI 82.3% to 87.1%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 92.9% | 90.3 to 94.9 | |
| Clinical specificity d/(b + d) | 73.3% | 68.5 to 77.8 | |
| PPV a/(a + b) | 83.3%b | 80.0% to 86.3%b | |
| NPV d/(c + d) | 87.8%b | 83.6% to 91.3%b | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 3.48b | 2.93 to 4.13b | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.01b | 0.07 to 0.13b | |
| Diagnostic odds ratio (a × d)/(b × c) | 35.8b | 23.87 to 53.90b | |
| Results: for subgroup of polyps ≤ 5 mm and located on the left-side side of the colon | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | 191a (a) | 67a (b) | 258a (a + b) |
| Index test negative | 18a (c) | 240a (d) | 258a (c + d) |
| Total | 209a (a + c) | 307a (b + d) | 516 (a + b + c + d) |
| Accuracy [(a + d)/(a + b + c + d)] | 83.5% (95% CI 80.0% to 86.6%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 91.4% | 86.8 to 94.8 | |
| Clinical specificity d/(b + d) | 78.1% | 73.0 to 82.6 | |
| PPV a/(a + b) | 74.03%b | 68.23% to 79.27%b | |
| NPV d/(c + d) | 93.02%b | 89.20% to 95.81%b | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 4.19b | 3.37 to 5.20b | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.11b | 0.07 to 0.17b | |
| Diagnostic odds ratio (a × d)/(b × c) | 38.01b | 21.84 to 66.14 | |
| Results: for subgroup of polyps ≤ 5 mm and located in the rectosigmoid part of the colon | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | NR (a) | NR (b) | NR (a + b) |
| Index test negative | 11c (c) | 226c (d) | 237 (c + d) |
| Total | NR (a + c) | NR (b + d) | NR (a + b + c + d) |
| Accuracy [(a + d)/(a + b + c + d)] | NR | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | NR | NR | |
| Clinical specificity d/(b + d) | NR | NR | |
| PPV a/(a + b) | NR | NR | |
| NPV d/(c + d) | 95.4% | 91.8% to 97.7% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| Interpretability of test | NR | ||
| Interobserver agreement | n/a | ||
| Intraobserver agreement | n/a | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | NR | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | Table 4 in the paper provides values for the reduction in polyps requiring histopathology for various hypothetical predict, resect and discard strategies. One of these is for diminutive polyps (n = 884/1254 polyps discarded without histopathology, 70.5% reduction), but not limited to the rectosigmoid colon. The paper states:Using this strategy, 13 adenomas (1.5%) with advanced histological features (any villous component or high-grade dysplasia) would be discardedThe reviewer assumes the ‘this strategy’ referred to is a ‘predict, resect and discard’ strategy and from the values given this must relate to diminutive polyps only | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
The Joint Guidelines developed by the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer and the American College of Radiology were used to calculate surveillance intervals based on in vivo optical diagnosis and histopathology. Two surveillance interval groups (A and B) were calculated:Recommendations for surveillance intervals based on the in vivo optical diagnosis were generated only for the patients with at least one polyp. An analysis was conducted limited to the in vivo diagnosis of all diminutive polyps and surveillance intervals were predicted correctly in 86.1% (95% CI 82.4% to 89.3%) for surveillance interval A. For surveillance interval B, 94.1% (95% CI 91.4% to 96.2%) of surveillance interval predictions were correct Three hypothetical strategies led to higher accuracy rates than the predict, resect and discard strategy for diminutive polyps only. These three strategies were:Two other hypothetical predict, resect and discard strategies had higher accuracy rates for surveillance interval A (but not surveillance interval B), compared with the predict, resect and discard strategy for all diminutive polyps only. These two strategies were: |
||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | The three studies that provided data for this analysis enrolled participants referred and scheduled for screening or surveillance colonoscopy | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Reference standard was histopathology | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Polyps excised for histopathology at the time of index test | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | Whole sample | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | The pathologist was blinded to the optical diagnosis | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Histopathology results not available at time of index test | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | An optical diagnosis could not be determined for four polyps (0.3%) | Yes |
| 11 | Were withdrawals from the study explained? | This retrospective analysis included 622 of 1150 patients from three trials who met the inclusion criteria for the retrospective analysis therefore no participants were able to withdraw | n/a |
| Reference list of the included paper(s) checked? Yes/no | Yes (and for the two linked papers on NBI), no additional papers identified | ||
Summary reviewer’s comments
Each of the endoscopists involved was experienced, although it is not clear how experienced they were in the use of NBI. The participants were eligible for screening or surveillance and the majority were white men. The results may not be applicable to less experienced endoscopists and more diverse samples of participants.
Henry et al.69
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: efficacy of NBI without optical magnification for differentiating neoplastic from non-neoplastic colorectal polyps, using meshed capillary pattern First author: Henry Publication year: 2010 Country: USA Study design: retrospective comparison of prospectively collected data Number of centres: one (academic medical centre) Funding: not stated Competing interests: three authors disclosed consultant relationships with Olympus. One disclosed grant support from Boston Scientific, Alveolus, ConMed, and Cook Medical. The remaining authors disclosed no financial conflicts |
Index test: HD, adult or paediatric, variable-stiffness colonoscope (CF-H180AL or PCF-H180AL, Olympus America, Centre Valley, PA, USA). Processor capable of NBI and HD imaging (EVIS Exera II CV-180; Olympus America) Polyps had been previously identified with white-light HD colonoscopy and were examined with NBI and up to 1.5 × digital zoom (without true optical magnification) Reference standard: histopathology |
Number of participants: 33 (total sample; number of participants in the diminutive polyp subgroup analysis NR) Sample attrition/dropout: NR, but likely to be zero as this was a retrospective study of prospectively collected data and all participants that met the inclusion criteria were likely to have been included Selection of participants: a retrospective review of endoscopy logs identified consecutive patients who had undergone colonoscopy with NBI and polypectomy at the study centre for potential inclusion in the study Inclusion criteria for study entry: as above Exclusion criteria for study entry: no polyps identified; a polyp diagnosis was made before colonoscopy from a biopsy sample; and, active IBD |
Primary outcome of study: not described as primary, but main outcome measurements: sensitivity, specificity, PPV, NPV and diagnostic accuracy Other relevant outcomes: no other outcomes reported Recruitment dates: October 2008–March 2009 |
| Participant characteristics | |||
| Age (years), mean (SD) | Median 59.5 (range 34–84) (total sample) | ||
| Other key patient characteristics |
Male, n = 33/52 (63.5%) (total sample) Colonoscopy indications, n (%a): screening for colorectal adenoma and cancer, 15 (28.8); surveillance of patients with prior colorectal adenomas, 22 (42.3); prior colorectal cancer, one (1.9); symptoms suggestive of colorectal cancer, 14 (26.9) (total sample) A total of 126 polyps were identified (total sample). Median size 3 mm (range 2–30 mm). Location, n: caecum, 12; ascending colon, 24; hepatic flexure, 5; traverse colon, 17; descending colon, 11; sigmoid colon, 24; rectosigmoid colon, 12; and rectum, 21 Morphology (Paris type), n: 0-Is, 30; 0-Ip, 7; 0-IIa, 82; the remaining polyps were classified as 0-IIb, 0-IIc, 0-IIa + IIc, 0-IIa + Is, 1 and 3 (n = 7) – the exact number of polyps classified into the categories is provided in the paper but no data extracted here Histopathology, n (%a): neoplastic, 67 (53); and non-neoplastic, 59 (47). The neoplastic classification included the following histopathologies: adenoma (low grade), tubovillous adenoma, adenocarcinoma and squamous cell carcinoma. The non-neoplastic classification included the following histopathologies: hyperplastic, normal mucosa and inflammatory. The number of polyps classified into each histopathology subcategory is provided in the paper but no data extracted here 90 of the 126 polyps (71.4%a) were sized ≤ 5 mm Subgroup analyses by polyp size: ≤ 5 mm, 6–9 mm, ≥ 10 mm and ≥ 6 mm (which included the previous two size categories). Only data for polyps sized ≤ 5 mm extracted |
||
| Endoscopist experience and training | An endoscopist who had received training in NBI and chromoendoscopy either performed or supervised each colonoscopy. The endoscopist’s training consisted of lectures, self-study and a 1-week intensive course that involved performing or participating in > 50 NBI and chromoendoscopy examinations. No information is provided about the endoscopist’s previous experience in carrying out colonoscopies | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
During the colonoscopy and before polypectomy, the Sano–Emura classification (references provided in the paper85,88) was used to classify polyps as having neoplasia or as being non-neoplastic, based on the appearance of the meshed capillary vessels. Neoplasia (including Sano–Emura type II, IIIA and IIIB patterns) was denoted by a polyp being meshed capillary positive. Non-neoplastic polyps (including Sano–Emura type I pattern) were denoted by a polyp being meshed capillary negative After the colonoscopy, the Paris classification was used to classify the number, location and size of polyps found and resected, based on endoscopic photographs |
||
| Sample size calculation | NR | ||
| Results: NBI for polyps sized ≤ 5 mm | |||
| Adenomatousb polyps on histopathology | Hyperplasticc polyps on histopathology | Total | |
| Index test positive | (a) 32 | (b) 4 | 36 |
| Index test negative | (c) 5 | (d) 49 | 54 |
| Total | 37 | 53 | 90 |
| Accuracy [(a + d)/(a + b + c + d)] | 90.0% (95% CI 82% to 95%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 86.5% | 70% to 95% | |
| Clinical specificity d/(b + d) | 92.5% | 81% to 98% | |
| PPV a/(a + b) | 88.9% | 73% to 96% | |
| NPV d/(c + d) | 90.7% | 79% to 97% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 11.46d | 4.43 to 29.66d | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.15d | 0.06 to 0.33d | |
| Diagnostic odds ratio (a × d)/(b × c) | 78.400d | 19.563 to 314.198d | |
| Reviewer’s calculations of sensitivity, specificity, PPV and NPV generally agree with those reported in the paper, but some values and 95% CIs marginally differ: sensitivity = 86.49% (95% CI 71.23% to 95.46%); specificity = 92.45% (95% CI 81.79% to 97.91%); PPV = 88.89% (95% CI 73.49% to 96.89%); and NPV = 90.74% (95% CI 79.70% to 96.92%) | |||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | NR | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | All but one of the included patients were undergoing colonoscopy for screening for colorectal adenoma and cancer, for surveillance as a result of prior colorectal adenomas, or to investigate symptoms suggestive of colorectal cancer | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polyps received verification by histopathology | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | The paper does not provide information about whether or not the pathologist was blinded to the NBI prediction | Unclear |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | Not stated but believed to be zero | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant studies cited | ||
Summary reviewer’s comments
All but one of the included patients were undergoing colonoscopy for screening for colorectal adenoma and cancer, for surveillance as a result of prior colorectal adenomas, or to investigate symptoms suggestive of colorectal cancer. The findings from this study are therefore very relevant to the patient population of interest in this appraisal. However, patients were from the USA and it is unclear how representative of UK patients they are. In addition, the study included a small number of patients (n = 33) in the diminutive polyp subgroup analysis and it is unclear if a larger sample would give the same findings. No sample size calculation was reported, so it is unclear if the analysis was adequately powered. The study was carried out at one centre and one endoscopist was involved in the study colonoscopies. The endoscopist had received training in NBI, but it is unclear how experienced he was in carrying out colonoscopies. The results may not be applicable to a wider range of settings or endoscopists who have not received training in NBI.
Hewett et al.54
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: differentiation of adenomatous and hyperplastic polyps in the distal colon. Aim of study was to assess feasibility of leaving hyperplastic polyps in the distal colon in place First author: Hewett Publication year: 2012 Country: USA Study design: prospective cohort (described as a ‘prospective observational study’ by the authors) Number of centres: one (a university hospital and its affiliated ambulatory surgery centre, described by authors as a single centre) Funding: not stated Competing interests: two of the authors disclosed a consultant relationship with Olympus Medical Systems Corporation, Tokyo, Japan. The other author has received research support from Olympus America, Inc. |
Index test: HD NBI without optical magnification (CF180AL, Evis Exera II; Olympus America) When a polyp was detected in white light in the sigmoid colon or rectum, NBI was used to examine the surface characteristics. Electronic magnification (× 1.5) was used as needed Reference standard: histopathology |
Number of participants: 225 patients underwent colonoscopy; of these 31 had a total of 240 rectosigmoid colon polyps. A total of 235 polyps were included in the overall analyses; 220 (98%; reviewer calculates 93.6%, so this appears to be an error in the paper) polyps were included in the in the diminutive polyp (≤ 5 mm) subgroup analysis (number of patients not stated) Sample attrition/dropout: none reported Selection of participants: consecutive adult patients having elective screening or surveillance colonoscopy for ‘standard indications’ (p. 375) Inclusion criteria for study entry: as above Exclusion criteria for study entry: history of colectomy, IBD or polyposis syndrome |
Primary outcome of study: sensitivity and NPV of high-confidence predictions of histopathology Other relevant outcomes: diagnostic accuracy, specificity and predictive values Recruitment dates: not stated |
| Participant characteristics: total sample (n = 31,235 distal colorectal polyps) | |||
| Age (years), mean (SD), median | 59.6 (9.8), 59 | ||
| Other key patient characteristics (list) |
Gender, n/N (%): male 16/31 (52); female 15/31 (48) Indications, n/N (%): screening, 9/31 (29); surveillance, 14/31 (45); and other, 8/31 (26) Location of the 235 polyps, n (%): sigmoid, 125 (53); and rectum, 110 (47) Histopathology of the 235 polyps, n (%): adenoma, 38 (16); hyperplastic, 188 (80); and other, 9 (4) Size of the 235 polyps, n (%): ≤ 5 mm, 220 (97.8); 6–9 mm, 11 (4.9); and ≥ 10 mm, 4 (1.8). Median size of the polyps was 3 mm (range 1–20 mm, interquartile range 2) Morphology of the 235 polyps (Paris), n (%): 0–1p, 7 (3.1); 0–1s, 55 (24.4); and 0-IIa, 163 (72.4) |
||
| Endoscopist experience and training | One endoscopist carried out the colonoscopies. The endoscopist was described as having a special interest in colonoscopy and extensive experience in NBI. No further details were provided | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Paris classification. To describe the appearance of the polyp when using NBI, the endoscopist used established criteria (reference provided in paper64) Hyperplastic and ‘other’ histopathologies were classed as non-adenomatous. Other histopathologies included inflammatory polyps, lymphoid follicles and normal tissue |
||
| Sample size calculation | Authors state that the chosen sample size was 235 distal polyps, and that this would allow 95% CIs of ± 3%, based on an expected true accuracy rate of 93%. Subgroups < 235 and may be underpowered | ||
| Results: NBI assessment of distal polyps ≤ 5 mm (n = 220) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 27a | (b) 9a | 36a |
| Index test negative | (c) 3a | (d) 181a | 184a |
| Total | 30a | 190a | 220 |
| Accuracy [(a + d)/(a + b + c + d)] | 94.5% (95% CI 91.5% to 97.6%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 90.0% | 73.5% to 97.9% | |
| Clinical specificity d/(b + d) | 95.3% | 91.2% to 97.8% | |
| PPV a/(a + b) | 75.0% | 57.8% to 87.9% | |
| NPV d/(c + d) | 98.4% | 95.3% to 99.7% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 19.00%a | 9.93% to 36.35%a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.10%a | 0.04% to 0.31%a | |
| Diagnostic odds ratio (a × d)/(b × c) | 181.000a | 46.096 to 710.717a | |
| Reviewer’s calculations of sensitivity, specificity, PPV and NPV agree with the values reported in the paper | |||
| Results: NBI high-confidence predictions of histopathology of distal polyps ≤ 5 mm (n = 201) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 24a | (b) 1a | 25a |
| Index test negative | (c) 1a | (d) 175a | 176a |
| Total | 25a | 176a | 201 |
| Accuracy [(a + d)/(a + b + c + d)] | 99.0% (95% CI 97.6% to 100%) – 199 of 201 (99%) polyps accurately diagnosed | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 96.0% | 79.7% to 99.9% | |
| Clinical specificity d/(b + d) | 99.4% | 96.9% to 100% | |
| PPV a/(a + b) | 96.0% | 79.7% to 99.9% | |
| NPV d/(c + d) | 99.4% | 96.9% to 100% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 168.96a | 23.89 to 1194.79a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.04a | 0.01 to 0.27a | |
| Diagnostic odds ratio (a × d)/(b × c) | 4200.000a | 254.269 to 69375.426a | |
| Reviewer’s calculations of sensitivity, specificity, PPV and NPV agree with the values reported in the paper | |||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | Of the diminutive polyps located in the distal colon (n = 220), 201 (91.4%a) predictions were made with high confidence | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | The majority of the patients were undergoing screening or surveillance colonoscopy. The exact indications for colonoscopy were unclear, but described by the authors as standard indications | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polyps received verification by histopathology (with the exception of five polyps that were not retrieved for histopathology) | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | The pathologist who carried out the histopathology was blinded to the NBI prediction | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Five polyps from the total sample were not retrieved for histopathology and excluded from the analysis | Yes |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated | No |
| 11 | Were withdrawals from the study explained? | No withdrawals were reported, but there appear to be none | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant publications identified | ||
Summary reviewer’s comments
The study was conducted at one academic hospital and by one endoscopist, who was experienced in NBI and carried out the colonoscopies. The findings may not therefore be generalisable to less experienced endoscopists in other settings. Although a large number of diminutive polyps were included in the study (n = 220), these came from a small number of patients (≤ 31 patients; exact number of patients in the diminutive polyps subgroup is unclear), which may limit the generalisability of the findings. The majority of the participants were undergoing screening or surveillance colonoscopy for standard indications (not defined). It is unclear how relevant the findings of the study are to a UK patient population.
Hewett et al.20
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: differentiation of hyperplastic from adenomatous polyps (Note that the study was designed to develop and evaluate the validity of a NBI classification system – the NBI International Colorectal Endoscopic classification system. There were four phases, followed by a pilot clinical evaluation of the performance of the classification system. Only the last is relevant to this report) First author: Hewett Publication year: 2012 Country: USA Study design: prospective cohort Number of centres: not stated Funding: partially funded by Olympus Medical Systems Corporation (Japan) Competing interests: stated that authors are consultants to, or have received funding from, Olympus Medical Systems Corporation, Japan; Olympus Medical Systems Corporation; Olympus America, Inc, USA; Olympus KeyMed (Medical & Industrial Equipment) Ltd, UK; Olympus France S.A.S., and Olympus Europa Holding GmbH, Germany |
Index test: NBI, CF-H180AL HD colonoscope with Exera II CLV-180 light source, CV-180 processor and OEV-261H monitor (Olympus America, Inc.) Reference standard: histopathology |
Number of participants: 108 Sample attrition/dropout: of 220 enrolled patients, 108 had at least one polyp < 1 cm in size Selection of participants: patients undergoing routine screening, surveillance or diagnostic colonoscopy. Received real-time endoscopic diagnosis of all consecutive polyps measuring < 1 cm in size Inclusion criteria for study entry: not stated Exclusion criteria for study entry: not stated |
Primary outcome of study: not designated as primary outcomes, but reports diagnostic accuracy, sensitivity, specificity, NPV and PPV for the pilot clinical evaluation Other relevant outcomes: none Recruitment dates: not stated |
| Participant characteristics | |||
| Age (years), mean (SD) | Not stated | ||
| Other key patient characteristics (list) | Mean polyp size varied from 3.2 mm (range 1–8 mm) to 4.6 mm (range 1–9 mm), non-adenomas and adenomas, respectively. The vast majority were ≤ 5 mm in size (n = 192; 81%) | ||
| Endoscopist experience and training | Two colonoscopists completed a formal standardised training module in the use of NBI for real-time histopathology and achieved > 90% in post-test evaluation before study initiation | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | The study developed and evaluated the NBI International Colorectal Endoscopic classification system | ||
| Sample size calculation | A sample size calculation is reported for phases 1, 3 and 4, but not for the pilot clinical evaluation, which is the only part of the study relevant to this report | ||
| Results: high-confidence predictions for diminutive polyps (there were 192 diminutive polyps, but the number of high-confidence predictions made is not reported) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) NR | (b) NR | NR |
| Index test negative | (c) NR | (d) NR | NR |
| Total | NR | NR | NR |
| Accuracy [(a + d)/(a + b + c + d)] | 88% | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 98% | NR | |
| Clinical specificity d/(b + d) | NR | NR | |
| PPV a/(a + b) | NR | NR | |
| NPV d/(c + d) | 95% | NR | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| Due to the limited information reported for polyps measuring ≤ 5 mm, the reviewer was unable to calculate values for the 2 × 2 table | |||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | Of 236 polyps, diagnostic prediction was made in high confidence in 177 (75%) | ||
| Low-confidence optical diagnosis | Not explicitly stated, but can be assumed that 59 polyps were predicted with low confidence (177/236 were high confidence) | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Limited information given, but patients were undergoing routine screening, surveillance or diagnostic colonoscopy. However, it is possible that the last group might include patients with conditions (e.g. IBD) that are not relevant to the scope of this report | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | The whole sample | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | An independent pathologist blinded to the endoscopic prediction reported polyp histopathology | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated, but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | Not stated if there were any withdrawals, other than of 220 enrolled patients, 108 had at least one polyp < 1 cm in size | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional studies identified | ||
Summary reviewer’s comments
Limited information is given on the context of the study, but the results are based on the use of HD NBI using the NBI International Colorectal Endoscopic criteria in a general patient population (undergoing routine screening, surveillance or diagnostic colonoscopy) to characterise small (< 1 cm, predominantly < 5 mm in size) polyps. Predictions were made with high confidence by colonoscopists with formal standardised training in the use of NBI. The study appears to have been conducted in the USA, though one of the gastroenterologists was from the UK.
Hoffman et al.80
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: screening for colorectal cancer detection of lesions and characterisation of lesions < 5 mm in the last 30 cm of the colon The overall aim of the study was to compare three imaging modalities: HD+, HD+ with i-scan and HD+ with chromoendoscopy. All three modalities were used in each patient but to overcome potential bias based on sequential examination the order that HD+ alone and HD+ with i-scan were used was randomised. The last modality was always chromoendoscopy. Only data on imaging using HD+ with i-scan are relevant to this report, data on the other two modalities (HD+ only and chromoendoscopy) have not been extracted Note that aspects of the study relating to detection of polyps have not been data extracted First author: Hoffman Publication year: 2010 Country: Germany Study design: prospective cohort Number of centres: one (endoscopic unit at the Johannes Gutenberg University of Mainz) Funding: NR Competing interests: the authors disclosed no conflicts of interest |
Index test: identification of especially small lesions (< 5 mm in the last 30 cm of the colon) using the i-scan SE-mode and subsequent characterisation (using the i-scan p- and v-mode) of lesions to predict histopathology Used the PENTAX EPKi processor providing resolution of about 1.25 megapixels per image The optional use of magnification was allowed after a lesion had been detected, but how often this was used or what the level of magnification was is NR Reference standard: histopathology |
Number of participants: 69 Sample attrition/dropout: no participants appeared to drop out. The paper does not report whether any identified polyps were not characterised or whether any of the polyps characterised were not sent to histopathology Selection of participants: consecutive patients who fulfilled the criteria for screening colonoscopy Inclusion criteria for study entry: as above Exclusion criteria for study entry: none reported |
Primary outcomes of study: total amount of small lesions (< 5 mm in size) and the total amount of identified neoplastic lesions (< 5 mm in size) identified in the rectum and sigma (The number of lesions detected per patient has not been data extracted because they are not relevant to this review) Other relevant outcomes: characterisation of lesions (test performance characteristics) reported for polyps and patients Recruitment dates: study conducted between July 2007 and January 2008 |
| Participant characteristics | |||
| Age (years), mean (SD) | 55.9 (NR) | ||
| Other key patient characteristics (list) | Male, n = 43 (62%); female, n = 26 (38%) | ||
| Endoscopist experience and training | Three experienced colonoscopists performed the colonoscopies. The paper states that all were highly familiar with chromoendoscopy and HD+ endoscopy using the PENTAX EPKi processor. (Note that HD+ was not defined, but is described as allowing resolution above HDTV standard. Presume is HD+.) Discussion states examiners had a dedicated interest in colonoscopy and previous documentation of high adenoma detection rates using standard-definition colonoscopies in white light | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
All lesions were classified using the Paris classification and the surface pit pattern Intraepithelial neoplasia identified by histopathological diagnosis were divided into low and high grade using the new Vienna classification |
||
| Sample size calculation | A sample size of 20 patients was calculated. The probability for error (α) was set to 0.05 and a β-error was set to 0.1 (reflecting a power of 0.90). It was assumed that the detection rate of conventional colonoscopy was two small lesions in the colorectum, and a detection rate of seven small lesions was assumed after chromoendoscopy based on previous studies. It was assumed, in the absence of any comparative studies of HD+ and i-scan, that HD+ and i-scan would allow a fourfold increase in the detection rate of small polyps (compared with conventional colonoscopy) | ||
| Results: analysis by polyp | |||
| For patients investigated first with HD+ followed by i-scan (n = 54). Results available only for the additional 128 lesions identified with i-scan (results presented as a per-patient analysis are presented below) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | NR (a) | NR (b) | NR (a + b) |
| Index test negative | NR (c) | NR (d) | NR (c + d) |
| Total | 11 (a + c) | 117a (b + d) | 128 (a + b + c + d) |
| Accuracy [(a + d)/(a + b + c + d)] | NR and not possible to calculate | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | NR and not possible to calculate | ||
| Clinical specificity d/(b + d) | NR and not possible to calculate | ||
| PPV a/(a + b) | NR and not possible to calculate | ||
| NPV d/(c + d) | NR and not possible to calculate | ||
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR and not possible to calculate | ||
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR and not possible to calculate | ||
| Diagnostic odds ratio (a × d)/(b × c) | NR and not possible to calculate | ||
| Characterisation data by polyp for the 15 patients investigated firstly by i-scan followed by HD+ alone are NR | |||
| Results: analysis by patient | |||
| The table reporting these results is headed ‘Endoscopic prediction after i-scan’ and from the numbers of patients given it includes all 69 patients. However, because results include a third category ‘normal mucosa’ for the index test and histopathology, four patients with normal mucosa by both index test and histopathology are omitted from the 2 × 2 table below. For the 54 patients investigated first by HD+ and then by i-scan it is not clear whether the analysis includes only the 128 polyps additionally identified by i-scan or whether it also includes the 154 polyps identified with HD+ only | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps or normal mucosa on histopathology | Total | |
| Index test positive | 9 patients (a) | 2 patients (b) | 11 patients (a + b) |
| Index test negative | 2 patients (c) | 52 patients (41 hyperplastic and 11 normal mucosa on histopathology) (d) | 54 patients (c + d) |
| Total | 11 patients (a + c) | 54 patients (b + d) | 65 patients (a + b + c + d) |
| Accuracy [(a + d)/(a + b + c + d)] | 61/65 (94%)a | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 9/11 (82%) | 48.22% to 97.72%a | |
| Clinical specificity d/(b + d) | 52/54 (96%) | 87.25% to 99.55%a | |
| PPV a/(a + b) | 81.82%a | 48.22% to 97.72%a | |
| NPV d/(c + d) | 96.30%a | 87.25% to 99.55%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 22.09a | 5.51 to 88.54a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.19a | 0.05 to 0.66a | |
| Diagnostic odds ratio (a × d)/(b × c) | 117.00a | 14.56 to 940.08a | |
| Interpretability of test | Not commented on by the authors of the paper although results are presented, which included prediction of normal mucosa as well as hyperplasia and adenoma | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | NR | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | States total examination time for the last 30 cm of the colon did not differ significantly between the three groups (HD+, 4 minutes; surface enhancement with i-scan, 5 minutes; chromoendoscopy with methylene blue, 13 minutes). It is not clear whether these times are for detection only or include characterisation and/or polyp biopsies | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Patient description limited to a statement that they fulfilled the criteria for screening colonoscopy. Mean age and number of female participants reported but no other details | Unclear |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The i-scan assessment and polyp resection occurred during the same colonoscopy | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polyps were resected for histopathology. No exclusions or losses were reported | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All polyps were subject to histopathological diagnosis | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | One experienced pathologist who was blinded to the endoscopic findings classified the specimens | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Histopathology had not been performed at the time of the index test | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Although results were reported for normal mucosa in addition to adenomatous and hyperplastic polyps, there is no indication in the paper that this was as a result of any difficulty in interpreting the index test | No |
| 11 | Were withdrawals from the study explained? | No withdrawals were reported and none appeared to have occurred | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional studies identified | ||
Summary reviewer’s comments
The primary outcomes of this study were total number of small lesions (< 5 mm) and total number of identified neoplastic lesions (< 5 mm) identified in the rectum and sigma. Much of the reporting focuses on the detection of polyps and there is limited reporting on polyp characterisation. The three endoscopists involved in the study are described as experienced and with a particular interest in colonoscopy and, therefore, the results may not be applicable to less experienced endoscopists or those without a particular interest in polyp detection and characterisation.
Ignjatovic et al.70
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: differentiation of adenomas from non-neoplastic polyps First author: Ignjatovic Publication year: 2009 Country: UK Study design: prospective cohort Number of centres: one (St Mark’s Hospital, London, UK) Funding: Leigh Family Trust, London, UK Competing interests: stated none |
Index test: endoscopists were asked to predict a polyp type (hyperplastic, adenoma, carcinoma or other) using HD white light. If unable to make an optical diagnosis, NBI was activated. The polyp was assessed in vivo with both real-time and optimised freeze-frame NBI images. If colonoscopists were still unable to confidently predict polyp histopathology, chromoendoscopy was used NBI: HD monitors and non-magnifying Olympus CF-H260DL colonoscopes with LUCERA video processors (Olympus, Japan) Reference standard: histopathology |
Number of participants: 130 Sample attrition/dropout: n = 48 In 10 patients optical diagnosis not made; 17 patients with polyp > 10 mm; 15 patients polyp not retrieved; six patients polyp destroyed by diathermy Selection of participants: consecutive patients referred for a surveillance colonoscopy (for adenoma follow-up, but not polyposis syndrome) or who had a positive FOBT at St Mark’s Hospital (London, UK) Inclusion criteria for study entry: as above Exclusion criteria for study entry: patients with poor bowel preparation; surveillance for polyposis syndrome; presence of an obvious cancer; polyps ≥ 10 mm in size only; absence of polyps or polyps were seen but not retrieved; no optical diagnosis made |
Primary outcome of study: accuracy of optical diagnosis in differentiating adenomas from non-neoplastic polyps Other relevant outcomes: number of polyps assessed with confidence; recommended surveillance interval; costs Diagnostic threshold: n/a Recruitment dates: June 2008–June 2009 |
| Participant characteristics (based on 130 included patients, characteristics are not available for the subset of patients with diminutive ≤ 5 mm polyps) | |||
| Age (years), mean (SD) | 63.4 (10.6) | ||
| Other key patient characteristics (list) |
Male, n = 87 (67%a); and female, n = 43 (33%a) Indication for colonoscopy: |
||
| Endoscopist experience and training |
Colonoscopists referred to as experts or non-experts. Procedures were done by four colonoscopists: two experts who had previously done > 10,000 colonoscopies with experience of NBI in > 1000 cases, one trainee (< 500 colonoscopies, < 50 NBI colonoscopies) and one specialist nurse (> 3000 colonoscopies, < 10 NBI colonoscopies). All four colonoscopists were familiar with VPI classification, and the non-experts completed a training session on use of NBI in characterising polyps, using a library of images collected as part of a previous study Expert colonoscopists mainly examined patients were high risk and FOBT positive, as part of the national Bowel Cancer Screening Programme. Non-experts did routine surveillance colonoscopies Non-expert colonoscopists assessed 104 polyps in 64 patients and experts assessed 259 polyps in 66 patients, reflecting the fact that experts examined patients who were more likely to have a greater number of polyps |
||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | Polyp histopathology was classified in accordance with the VPI criteria. The location, size and shape of polyps was recorded with the Paris classification system | ||
| Sample size calculation | Total of 278 polyps needed to be prospectively assessed, assuming an accuracy for optical diagnosis of 93% (± 3%) | ||
| Results: subsample of polyps ≤ 5 mm | |||
| Number of neoplastic polyps on histopathology | Number of non-neoplastic polyps on histopathology | Total | |
| Index test positive | (a) 144 | (b) 7 | 151 |
| Index test negative | (c) 11 | (d) 51 | 62 |
| Total | 155 | 58 | 213 |
| Accuracy of index test [(a + d)/(a + b + c + d)] | 195/213 (92%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 92.90% | 87.66% to 96.40% | |
| Clinical specificity d/(b + d) | 87.93% | 76.70% to 95.01% | |
| PPV a/(a + b) | 95.36%a | 90.68% to 98.12% | |
| NPV d/(c + d) | 82.26%a | 70.47% to 90.80% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 7.70a | 3.84 to 15.44 | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.08a | 0.05 to 0.14 | |
| Diagnostic odds ratio (a × d)/(b × c) | 95.38a | 35.08 to 259.27 | |
|
278 polyps had both a high-confidence optical and histopathological diagnosis (overall sample of polyps). For the subsample of polyps ≤ 5 mm this figure was 213 Diagnostic accuracy estimates (sensitivity, specificity, etc.) are given for the overall sample of polyps (i.e. irrespective of polyp size) and stratified by whether an expert or non-expert performed the colonoscopy. These data are not extracted here, as they include polyp sizes larger than the scope of this assessment Sensitivity and specificity are similar for the overall sample and the diminutive (≤ 5 mm) polyp subgroup. Expert colonoscopists were more accurate than non-experts in optical diagnosis of adenomas (p = 0.04) 68 of 198 adenomas and 20 of 62 non-neoplastic lesions were correctly diagnosed using WLE alone (in the overall sample of polyps). The remaining polyps were diagnosed by a combination of white light and NBI, except for one adenoma and two hyperplastic lesions for which chromoendoscopy was also used Subgroup analyses were conducted for polyp size (6–9 mm vs. 5 mm) and for endoscopists’ experience. It is not explicitly stated whether or not these were pre-defined subgroups |
|||
| Interpretability of test | Not stated | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | n = 323/363 | ||
| Low-confidence optical diagnosisb | n = 37/363 | ||
| Low-confidence polyps ≤ 5 mm | n = 22/293 (8%) | ||
| Low-confidence polyps 6–9 mm | n = 15/67 (22%) | ||
| No diagnosis made | n = 3/363 (all ≤ 5 mm) | ||
| Number of polyps left in placec |
33/323 (high-confidence decision, for overall sample of polyps) All were hyperplastic polyps and located in the sigmoid colon or the rectum |
||
| Number of polyps resected and discardedc | 290/323 (high-confidence decision, for overall sample of polyps) | ||
| Number of polyps resected and sent for histopathological examination | 22/293 (8%) (subsample of polyps ≤ 5 mm in size) | ||
| Recommended surveillance interval | Given in 82/130 patients. Surveillance intervals based on histopathology and optical diagnosis were the same for 80/82 patients (98%) using BSG guidelines. Two patients had a longer interval recommended after histopathology. There was no difference between experts and non-experts in the accuracy of surveillance interval prediction [36 of 37 (97%) vs. 44 of 45 (98%); p = 1.00] | ||
| Total cost of histopathology (n = 363 polyps)d | £7623 | ||
| Total cost for optical diagnosis (n = 323 polyps)d | £840 | ||
| Total cost of follow-up appointments histopathologye | £10,400 | ||
| Total cost of follow-up appointments optical diagnosise | £3840 | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Two groups of patients were recruited: those indicated for colonoscopy based on surveillance and those referred from bowel screening (positive FOBT). These are relevant to the scope of the appraisal | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | The aim was to resect and submit all polyps for histopathology. Diagnostic accuracy results were reported for the sample of 278 polyps which had both an optical and histopathological diagnosis | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Experienced gastrointestinal histopathologists, who were blinded to endoscopic images and optical predictions, classified all specimens in accordance with the World Health Organization’s guidelines153 | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated, but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | Of 130 patients initially included, 48 appear to have been excluded from the analysis for a variety of reasons that are provided (e.g. no optical diagnosis was made; patients had polyps sized ≥ 10 mm in addition to polyps ≤ 10 mm; polyps not retrieved; polyps destroyed by diathermy) | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes | ||
Summary reviewer’s comments
This was a UK study and participants had been referred for colonoscopy following a positive FOBT or for surveillance (adenoma follow-up but not polyposis syndromes). These participants are likely to be representative of others in the UK. Colonoscopists were experts (n = 2) or non-experts (n = 2) and, although results were provided separately for all polyps by colonoscopist expertise, they were not provided separately for diminutive polyps.
Ikematsu et al.71
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: differentiation between adenomatous and hyperplastic polyps First author: Ikematsu Publication year: 2015 Country: Japan Study design: prospective cohort Number of centres: two (National Cancer Centre East Hospital and National Cancer Centre Hospital) Funding: NR Competing interests: stated that the 10 authors had no conflicts of interest or financial ties to disclose |
Index test: NBI without magnification to differentiate between adenomatous or hyperplastic polyps in real time. Endoscopists assigned a level of confidence (either high or low) to their prediction of polyp histopathology EVIS LUCERA ELITE, CV-290 (Olympus, Optical Co. Ltd, Tokyo, Japan) with a dual-focus colonoscope (CF-HQ290I) Reference standard: histopathological examination Note that the study also included white-light imaging and NBI with dual focus (magnification approximately 72-fold), but these data do not meet the inclusion criteria for this review so have not been extracted |
Number of participants: 37 (100 polyps, 72 polyps were ≤ 5 mm in size) Sample attrition/dropout: none reported Selection of participants: consecutive patients who underwent screening colonoscopy Inclusion criteria for study entry: NR Exclusion criteria for study entry: patients with polyps > 10 mm, with lesions previously evaluated by histopathology or colonoscopy, and patients with invasive carcinoma. Patients with IBD or FAP were also excluded |
Primary outcome of study: accuracy, sensitivity, specificity, NPV, PPV, level of confidence in each modality to differentiate between adenomatous and hyperplastic lesions and predict pathological findings (only NBI data extracted) Secondary outcome measure: ability of each modality to differentiate lesions based on their size (≤ 5 mm and 6–10 mm) (only NBI data extracted) Recruitment dates: July–December 2013 |
| Participant characteristics | |||
| Age (years), mean (SD) | 66.9 (range 39–82) | ||
| Other key patient characteristics |
Gender, male/female: 28/9 (ratio 3.1 : 1) Bowel preparation: excellent, n = 23; good, n = 13; fair, n = 1; and poor, n = 0 Paris classification type: 0-Is, n = 18; and 0-IIa, n = 82 Size of resected polyps (not stated but presume mean value): 4.6 mm (range 2–10 mm) Location of polyps: right colon, n = 51; left colon, n = 40; and rectum, n = 9 Histopathological findings: tubular adenoma with low-grade dysplasia, n = 74; tubular adenoma with high-grade dysplasia, n = 2; and hyperplastic polyp, n = 24 |
||
| Endoscopist experience and training | Seven endoscopists participated who had each performed > 1000 colonoscopies and > 500 NBI colonoscopies. No information provided regarding any endoscopist training | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
The Paris classification was used to describe macroscopic appearance of the polyps Histopathological results were determined in accordance with the World Health Organization’s criteria154 |
||
| Sample size calculation | NR | ||
| Results: for the subgroup of polyps ≤ 5 mm in size | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | a = 50a | b = 3a | a + b = 53a |
| Index test negative | c = 4a | d = 15a | c + d = 19a |
| Total | a + c = 54a | b + d = 18a | 72 |
| Accuracy [(a + d)/(a + b + c + d)] | 90.3% | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 92.6% | 82.11% to 97.94%a | |
| Clinical specificity d/(b + d) | 83.3% | 58.58% to 96.42%a | |
| PPV a/(a + b) | 94.3% | 84.34% to 98.82%a | |
| NPV d/(c + d) | 78.9% | 54.43% to 93.95%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 5.56a | 1.97 to 15.65a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.09a | 0.03 to 0.23a | |
| Diagnostic odds ratio (a × d)/(b × c) | 62.5a | 12.56 to 310.90a | |
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | For polyps ≤ 5 mm in size, 53 out of 72 (73.6%) predictions were made with high confidence | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Japanese patients attending for screening colonoscopy. No other inclusion criteria reported | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | Whole sample | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Histopathological diagnoses were performed by experienced gastrointestinal pathologists who were blinded to the prediction made during NBI colonoscopy | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Histopathology had not yet been performed at the time of the index test | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | No evidence of uninterpretable test results | No |
| 11 | Were withdrawals from the study explained? | No evidence of withdrawals from study | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional studies were identified | ||
Summary reviewer’s comments
It is not clear how representative these Japanese patients are to the UK population undergoing colonoscopy, in part because few details were provided about the included patients. The endoscopists involved were all experienced in the use of the technology, so the results might not be applicable to those new to NBI.
Iwatate et al.56
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: the impact of high ME vs. NME NBI-based optical diagnosis of colorectal polyps on rates of high-confidence assessment when differentiating neoplastic and non-neoplastic polyps First author: Iwatate Publication year: 2015 Country: Japan Study design: prospective study Number of centres: one (non-academic) Funding: not stated Competing interests: none |
Index test: NBI. Magnifying colonoscopes (H260AZI; maximum, × 80 optical zoom; Olympus, Tokyo, Japan) with LUCERA video processors (Olympus) and HD monitors All polyps detected by white-light imaging during colonoscopy were washed intensively and examined in two stages: first by NBI-NME and, second, by NBI-ME (the later data was not extracted). The polyp size was estimated with biopsy forceps [2.2 mm closed; EndoJaw, Olympus) or polypectomy snare (10 mm open; Dragonare S, Xemex, Tokyo, Japan)] Reference standard: histopathology |
Number of participants: 124 Sample attrition/dropout: no dropouts reported Selection of participants: consecutive adult patients scheduled for a high-magnifying (maximum, × 80) colonoscope colonoscopy Inclusion criteria for study entry: adults aged < 70 years scheduled to undergo colonoscopy with a magnifying colonoscope Exclusion criteria for study entry: polyps ≥ 11 mm; multiple (> 10) polyps (for ethical reasons, given the longer examination time); without polyps or whose polyp histopathology had not been evaluated; poor bowel preparation, melanosis, or a history of IBD, hereditary polyposis syndrome, or Lynch syndrome |
Primary outcome of study: not stated Other relevant outcomes: sensitivity, specificity, accuracy, PPV and NPV for high-confidence optical diagnosis by SCs and GEs; effect of NBI-ME on level of confidence with accuracy by NBI-NME (not data extracted) Recruitment dates: April and August 2012 |
| Participant characteristics (all n = 124,248 polyps) | |||
| Age (years), mean (SD) | 56.4 (8.7) | ||
| Other key patient characteristics | Male, %: 58 | ||
Polyps:
|
|||
Histopathology 1–5 mm (6–9 mm not data extracted), n:
|
|||
| Endoscopist experience and training | Five endoscopists: two SCs, with extensive experience in magnifying colonoscopy with NBI (> 1000 cases) and three GEs, with limited experience in magnifying colonoscopy with NBI (≤ 1000 cases). All five endoscopists were familiar with the NBI International Colorectal Endoscopic classification | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Paris classification for location, size and shape Polyp type: NBI International Colorectal Endoscopic classification [(1) non-neoplastic lesion, (2) adenoma and (3) deep submucosal invasive carcinoma]. Endoscopists had to assign their level of confidence (high or low) to the prediction Histopathological classification: World Health Organization Neoplastic lesions: adenoma, traditional serrated adenoma or carcinoma; others, including hyperplastic polyps or non-neoplastic lesions Sessile serrated adenomas/polyps: non-neoplastic lesions (stated that this was as a result of the endoscopic criteria to distinguish sessile serrated adenomas/polyps from hyperplastic polyps or a pathologic gold standard for diagnosis having not been fully established) |
||
| Sample size calculation | To detect a significant difference between a high-confidence rate of an 90% rate with a two-sided 5% significance level and 80% power with McNemar’s test for the NBI with NME and NBI-ME, a sample size of 250 consecutive polyps was required – 248 polyps were identified in the total sample of 124 patients | ||
| Results | |||
| NBI-NME 1- to 5-mm subgroup: all | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total |
| Index test positive | (a) 123 | (b) 25a | 148 |
| Index test negative | (c) 18a | (d) 44 | 62 |
| Total | 141 | 69 | 210 |
| Accuracy [(a + d)/(a + b + c + d)] | 79.5% (167/210) (CI not reported and not calculated by reviewer) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 87.2% | 80.58% to 92.26%a | |
| Clinical specificity d/(b + d) | 63.8% | 51.31% to 75.01%a | |
| PPV a/(a + b) | 83.1% | 76.08% to 88.76%a | |
| NPV d/(c + d) | 71.0% | 58.05% to 81.80%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 2.41a | 1.75 to 3.31a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.20a | 0.13 to 0.32a | |
| Diagnostic odds ratio (a × d)/(b × c) | 12.027a | 5.991 to 24.143a | |
| NBI-NME 1- to 5-mm subgroup: high confidence | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total |
| Index test positive | (a) 107 | (b) 17a | 124 |
| Index test negative | (c) 8a | (d) 35 | 43 |
| Total | 115 | 52 | 167 |
| Accuracy [(a + d)/(a + b + c + d)] | 85.0% (142/167) (CI not reported and not calculated by reviewer) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 93.0% | 86.75% to 96.95%a | |
| Clinical specificity d/(b + d) | 67.3% | 52.89% to 79.67%a | |
| PPV a/(a + b) | 86.3% | 78.96% to 91.81%a | |
| NPV d/(c + d) | 81.4% | 66.60% to 91.61%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 2.85a | 1.92 to 4.22a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.10a | 0.05 to 0.21a | |
| Diagnostic odds ratio (a × d)/(b × c) | 27.537a | 10.942 to 69.301a | |
| NBI-NME 1- to 5-mm subgroup: low confidence | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total |
| Index test positive | (a) 16 | (b) 8a | 24 |
| Index test negative | (c) 10 | (d) 9 | 19 |
| Total | 26 | 17 | 43 |
| Accuracy [(a + d)/(a + b + c + d)] | 58.1% (25/43) (CI not reported and not calculated by reviewer) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 61.5% | 40.57% to 79.77%a | |
| Clinical specificity d/(b + d) | 52.9% | 27.81% to 77.02%a | |
| PPV a/(a + b) | 66.7% | 44.68% to 84.37%a | |
| NPV d/(c + d) | 47.4% | 24.45% to 71.14%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 1.31 | 0.73 to 2.36 | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.73 | 0.38 to 1.41 | |
| Diagnostic odds ratio (a × d)/(b × c) | 1.800 | 0.522 to 6.204 | |
| Diagnostic accuracy rates of SCs for high-confidence predictions when using NBI-NME: 1- to 5-mm subgroup | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total |
| Index test positive | (a) 29 | (b) 3b | 32 |
| Index test negative | (c) 2b | (d) 20 | 22 |
| Total | 31 | 23 | 54 |
| Accuracy [(a + d)/(a + b + c + d)] | 90.7% (n/N: 49/54) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 93.5% | 78.58% to 99.21%b | |
| Clinical specificity d/(b + d) | 87.0%c | 66.41% to 97.22%b | |
| PPV a/(a + b) | 90.6% | 74.98% to 98.02%b | |
| NPV d/(c + d) | 90.9% | 70.84% to 98.88%b | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 7.17b | 2.49 to 20.69b | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.07b | 0.02 to 0.29b | |
| Diagnostic odds ratio (a × d)/(b × c) | 96.667b | 14.784 to 632.049b | |
| Diagnostic accuracy rates of GEs for high-confidence predictions when using NBI-NME: 1- to 5-mm subgroup | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total |
| Index test positive | (a) 78 | (b) 14b | 92 |
| Index test negative | (c) 6b | (d) 15 | 21 |
| Total | 84 | 29 | 113 |
| Accuracy [(a + d)/(a + b + c + d)] | 82.3% (n/N: 93/113) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 92.9% | 85.10% to 97.33%b | |
| Clinical specificity d/(b + d) | 51.7%c | 32.53% to 70.55%b | |
| PPV a/(a + b) | 84.8% | 75.79% to 91.42%b | |
| NPV d/(c + d) | 71.4% | 47.82% to 88.72%b | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 1.92b | 1.31 to 2.82b | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.14b | 0.06 to 0.32b | |
| Diagnostic odds ratio (a × d)/(b × c) | 13.929b | 4.615 to 42.034b | |
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis |
Endoscopists made a prediction with high confidence when they were 90% certain of the diagnosis (Hewett et al. 201220) and the diagnosis at each stage was recorded by an independent observer, who did not allow the prediction to be changed at subsequent steps Rates of high-confidence optical diagnosis with NBI-NME for 1- to 5-mm subgroup, % (n/N): 79.5 (167/210) Effect of NBI-ME on level of confidence with accuracy by NBI-NME: accuracy of high-confidence level for this outcome not data extracted |
||
| Low-confidence optical diagnosis |
Effect of NBI-ME on level of confidence with accuracy by NBI-NME: accuracy of low-confidence level not data extracted Rates of low-confidence optical diagnosis with NBI-NME for 1–5 mm subgroup, % (n/N): 20.5d (43/210) |
||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Patients aged ≤ 70 years scheduled to undergo a magnifying colonoscopy. Exact indication for colonoscopy was not provided | Unclear |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polyps in the prospective study were resected or biopsied for histopathological evaluation as the reference standard | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | An experienced gastrointestinal histopathologist blinded to the endoscopic diagnosis classified all specimens in accordance with the World Health Organization’s classification154 | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Two patients with ‘unevaluable material’ were excluded | Yes |
| 11 | Were withdrawals from the study explained? | While not specifically stated, there appear to have been no withdrawals | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant reverences were identified | ||
Summary reviewer’s comments
The population sample was based on patients from Japan and it is unclear how representative the population is of the patient population in the UK, and how similar endoscopists’ training is compared with training received in the NHS. Study was performed in a single centre, so the results may not be applicable to a wider range of settings. Patients were scheduled to undergo colonoscopy with a magnifying colonoscope, but the exact indication for colonoscopy was not provided. Therefore, it is unclear how relevant the patient population in this study is to the population of interest in this appraisal.
Kaltenbach et al.57
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: differentiating neoplastic and non-neoplastic diminutive colorectal polyps First author: Kaltenbach and McGill. All data without a reference number are extracted from Kaltenbach. Data extracted from McGill are clearly indicated by inclusion of the reference number and/or ‘McGill paper’ Publication year: 2015 (both Kaltenbach and McGill) Country: USA Study design: RCT (with one relevant arm: NBI standard view ×30 colonoscope) Number of centres: three Funding: study was partially funded by Olympus Medical America. Other funding sources not stated Competing interests: three of the authors have received research funding from Olympus Medical America and are consultants for Olympus Medical Systems Corporation. There were no other conflicts |
Index test: NBI standard-focus (× 30) colonoscope (CFH180AL, EVIS Exera II) HD monitors were used (OEV-261H) HD standard-view white light was initially used to examine a polyp. When a polyp was found, optical diagnosis was made using the NBI mode Participants could also be randomised to NBI dual-focus (×65) colonoscopy in this RCT: results from this arm have not been data extracted, as magnification was used Reference standard: histopathology |
Number of participants: 558 participants enrolled and randomised into the study (total sample); 281 participants included in the standard-focus arm and included in the analysis (missing data were imputed for two participants) Sample attrition/dropout: two patients did not have a complete colonoscopy as a result of poor bowel preparation quality or stricture in the standard-view arm. Missing data were imputed for these participants Selection of participants: consecutively recruited patients who were undergoing routine colonoscopy. 57 The McGill et al. paper72 states that patients were undergoing colonoscopy for screening, surveillance or symptoms Inclusion criteria for study entry: as above Exclusion criteria for study entry: referred for polypectomy; colitis; personal or family history of polyposis or hereditary colorectal cancer syndrome, or coagulopathy/thrombocytopenia. 57 The McGill et al. paper72 states that patients were also excluded if they needed an emergent endoscopy, had a known existing polyp or had poor or inadequate bowel preparation72 |
Primary outcome of study: proportion of accurate high-confidence optical diagnoses of neoplastic and non-neoplastic diminutive colorectal polyps57 The McGill et al. paper72 states that the main end points were NPV (for high-confidence diminutive polyps only) and surveillance interval agreement between optical diagnosis and histopathology (overall and by individual endoscopist) Other relevant outcomes: accuracy, sensitivity, specificity, PPV and NPV. Agreement in assignment of surveillance intervals between optical diagnosis and histopathology. Adverse events. Procedure and inspection time Recruitment dates: March 2011–May 2012 |
| Participant characteristics | |||
| Age (years), mean (SD) | Standard-view arm, mean ± SD years (range): 62.4 ± 8.7 (31–90) | ||
| Other key patient characteristics |
Standard-view arm, male, n/N (%): 269/281 (95.7) Standard-view arm, colonoscopy indication, n (%): screening, 106 (37.7%); surveillance, 123 (43.8%); and symptoms (anaemia, intermittent rectal bleeding, change in stool pattern, abdominal pain, weight loss), 52 (18.5%) 445 polyps from 281 patients were assessed in the standard-view arm. Three polyps were not retrieved for histopathological examination, resulting in a sample of 442 polyps. Of the 442 polyps, 252 (57.0%a) were neoplastic and 190 (43.0%a) were non-neoplastic. Exact pathology (i.e. cancer, high-grade dysplasia, villous adenoma, hyperplastic, other) is also provided in the paper, but not data extracted Polyp shape: of the 442 polyps, 381 were sessile, 59 flat and two depressed Polyp location of the 442 polyps, n: caecum, 30; ascending, 81; hepatic flexure, 10; transverse, 99; splenic flexure, 1; descending, 40; sigmoid, 117; and rectum, 64 The McGill et al. paper72 reports that of the 558 patients analysed, 219 (39.2%a) patients had diminutive polyps, 210 (37.6%a) had diminutive and other polyps, and 129 (23.1%) had no polyps. Overall, 975 diminutive polyps were assessed, of which 445 were diagnosed with high confidence in the standard-view arm (endoscopists made a high-confidence assessment for 72.6% of the polyps assessed in the standard view arm) Mean (SD, range) polyp size in standard-view arm, mm, by histopathology: neoplasia histopathology, 3.37 (1.13, 1–5); and non-neoplasia histopathology, 2.99 (1.16, 1–5) |
||
| Endoscopist experience and training |
Five endoscopists performed the colonoscopies. Before the study started, all took part in training in optical diagnosis of colorectal polyp histopathology using a learning management system, exceeding 90% accuracy. It is stated on page 1570 that ‘They used the NBI International Colorectal Endoscopic (NICE) classification’. No other information is provided about the endoscopists’ training or experience in the Kaltenbach paper57 In the McGill et al. paper,72 it is stated that the five endoscopists took part in a computer-based training module, based on the NBI International Colorectal Endoscopic criteria, and (as stated above) had to meet a minimum accuracy of 90%. They then carried out 10 real-time colonoscopies. The endoscopists’ histopathology predictions were compared with histopathology results. The endoscopists repeated the training module mid-way through the study. The endoscopists had 3–15 years’ clinical practice experience. Each endoscopist had annually performed between 500 and 1200 colonoscopies. All were based in an academic setting and all were familiar with NBI. Three were experts in the use of NBI |
||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
During optical diagnosis, the polyps were classified using the NBI International Colorectal Endoscopic classification. The Paris classification was used to estimate polyp size and morphology During histopathology, the polyps were defined as an adenoma or hyperplastic using the World Health Organization’s criteria |
||
| Sample size calculation |
It was assumed that 90% of polyps would be diagnosed with high confidence when using near focus and 80% when using standard focus. Based on the authors’ previously collected data, they assumed a 97% caecal intubation rate, 5% poor bowel preparation and that 60% of patients would have a colorectal lesion with a mean neoplasm of 0.85. This resulted in an estimated sample size needed of 279 patients in each study arm to provide a power of 80% with a two-sided level of 0.05 to detect a difference between the study arms in the proportions of accurate high-confidence optical diagnoses57 The reported sample size calculation in the McGill et al. paper72 differs to that reported in the Kaltenbach paper57 above. It was calculated that a sample size of 219 polyps in each study arm was needed to provide a power of 80% (at a two-sided alpha level of 0.05). This was based on the assumption, based on previous studies, that using the standard view and dual-focus NBI colonoscopes would each provide a 93% accuracy, with the standard view colonoscope and the dual-focus colonoscope predicting 80% and 90% of polyps with high confidence, respectively |
||
| Results: standard-view NBI, high-confidence diagnoses of all diminutive polyps (n = 323b) | |||
| Adenomatousc polyps on histopathology | Hyperplasticd polyps on histopathology | Total | |
| Index test positive | (a) 178a | (b) 33a | 211a |
| Index test negative | (c) 9a | (d) 103a | 112a |
| Total | 187a | 136a | 323 |
| Accuracy [(a + d)/(a + b + c + d)] | 87.0% (95% CI 82.8% to 90.5%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 95.2% | 90.8% to 97.6% | |
| Clinical specificity d/(b + d) | 75.7% | 67.5% to 82.5% | |
| PPV a/(a + b) | 84.4% | 78.7% to 89.0% | |
| NPV d/(c + d) | 92.0% | 85.3% to 96.3% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 3.92a | 2.91 to 5.29a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.06a | 0.03 to 0.12a | |
| Diagnostic odds ratio (a × d)/(b × c) | 61.731a | 28.412 to 134.121a | |
| Reviewer’s calculations of sensitivity, specificity, PPV and NPV agree with the values reported in the paper, but the reviewer’s calculations resulted in slightly differing 95% CIs: sensitivity, 95.19% (95% CI 91.06% to 97.78%); specificity, 75.74% (95% CI 67.64% to 82.67%); PPV, 84.36% (95% CI 78.74% to 88.98%); and NPV, 91.96% (95% CI 85.29% to 96.26%) | |||
| Results: standard-view NBI, high-confidence diagnoses of diminutive polyps located in the rectum (n = 46) | |||
| Adenomatousc polyps on histopathology | Hyperplasticd polyps on histopathology | Total | |
| Index test positive | (a) 7a | (b) 7a | 14a |
| Index test negative | (c) 2a | (d) 30a | 32a |
| Total | 9a | 37a | 46 |
| Accuracy [(a + d)/(a + b + c + d)] | 80.4% (95% CI 66.1% to 90.6%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 77.8% | 40.0% to 97.2% | |
| Clinical specificity d/(b + d) | 81.1% | 64.8% to 92.0% | |
| PPV a/(a + b) | 50.0% | 23.0% to 77.0% | |
| NPV d/(c + d) | 93.8% | 79.2% to 99.2% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 4.11a | 1.94 to 8.73a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.27a | 0.08 to 0.94a | |
| Diagnostic odds ratio (a × d)/(b × c) | 15.000a | 2.545 to 88.397a | |
|
Paper also reports that for a subgroup of diminutive polyps in the rectosigmoid colon, the NPV was 93.6% (95% CI 85.7% to 97.9%) when using standard view Reviewer’s calculations of values and 95% CIs match those reported in the paper |
|||
| Results: standard-view NBI, high-confidence diagnoses of diminutive polyps located in the right colon (n = 155) | |||
| Adenomatousc polyps on histopathology | Hyperplasticd polyps on histopathology | Total | |
| Index test positive | (a) 107a | (b) 17a | 124a |
| Index test negative | (c) 4a | (d) 27a | 31a |
| Total | 111a | 44a | 155 |
| Accuracy [(a + d)/(a + b + c + d)] | 86.4% (95% CI 80.0% to 91.4%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 96.4% | 91.0% to 99.0% | |
| Clinical specificity d/(b + d) | 61.4% | 45.5% to 75.6% | |
| PPV a/(a + b) | 86.3% | 79.0% to 91.8% | |
| NPV d/(c + d) | 87.1% | 70.2% to 96.4% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 2.49a | 1.72 to 3.36a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.06a | 0.02 to 0.16a | |
| Diagnostic odds ratio (a × d)/(b × c) | 42.485a | 13.211 to 136.631a | |
| Reviewer’s calculations of values and 95% CIs match those reported in the paper | |||
| Results: standard-view NBI, high-confidence diagnoses of diminutive polyps located in the left colon (n = 122) | |||
| Adenomatousc polyps on histopathology | Hyperplasticd polyps on histopathology | Total | |
| Index test positive | (a) 64a | (b) 9a | 73a |
| Index test negative | (c) 3a | (d) 46a | 49a |
| Total | 67a | 55a | 122 |
| Accuracy [(a + d)/(a + b + c + d)] | 90.2% (95% CI 83.4% to 94.8%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 95.5% | 87.5% to 99.1% | |
| Clinical specificity d/(b + d) | 83.6% | 71.2% to 92.2% | |
| PPV a/(a + b) | 87.7% | 77.9% to 94.2% | |
| NPV d/(c + d) | 93.9% | 83.1% to 98.7% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 5.84a | 3.20 to 10.63a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.05a | 0.02 to 0.16a | |
| Diagnostic odds ratio (a × d)/(b × c) | 109.037a | 27.973 to 425.024a | |
| Reviewer’s calculations of values and 95% CIs match those reported in the paper | |||
| Results: standard-view NBI, high-confidence diagnoses of diminutive polyps (n = 445e); data extracted from the McGill et al. paper72 | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) Incalculablef | (b) Incalculable | Incalculable |
| Index test negative | (c) Incalculable | (d) Incalculable | Incalculable |
| Total | Incalculable | Incalculable | 445 |
| Accuracy [(a + d)/(a + b + c + d)] | 87.0% of polyps correctly classified. (CIs not reported) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | Incalculable | Incalculable | |
| Clinical specificity d/(b + d) | Incalculable | Incalculable | |
| PPV a/(a + b) | Incalculable | Incalculable | |
| NPV d/(c + d) |
Overall: 92.6% NPV in first half of study: 88.0% NPV in second half of study: 95.8% |
Overall: CIs not reported First half of study: 75.7% to 95.5% Second half of study: 88.3% to 99.1% |
|
| Positive likelihood ratio [sensitivity/(1 – specificity)] | Incalculable | Incalculable | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | Incalculable | Incalculable | |
| Diagnostic odds ratio (a × d)/(b × c) | Incalculable | Incalculable | |
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | The authors report that there was no post-polypectomy bleeding, coagulation syndrome, perforation or optical misdiagnosis of advanced histopathology | ||
| High-confidence optical diagnosis | In the standard-view arm, the endoscopists made their histopathology prediction of 323 of the 445 (72.6%; 95% CI 68.2% to 76.7%) diminutive polyps with high confidence. Please see 2 × 2 table above | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
Surveillance intervals were assigned using the following guidelines (the Multi-Society guidelines):In the standard-view colonoscopy arm, 259 of 281 patients were (92.2%) were assigned the correct surveillance interval during optical diagnosis (i.e. this is the agreement with histopathology) When assigning surveillance intervals based on high-confidence optical diagnosis of diminutive polyps combined with histopathology results for all other polyps, of the 210 patients in the standard-view arm with polyps, 200 (95.2%) received the correct recommended interval. Seven (3.3%) were given an earlier recommended interval (told to return 2.4 ± 1.1 years earlier) and three (1.4%) were given a delayed recommended interval (delayed by 3.0 ± 1.7 years) Agreement in surveillance intervals assigned when using standard view also reported for each of the first and second halves of the study in the McGill et al. paper;72 these data are not extracted here |
||
| Length of time to perform the colonoscopy |
Procedure time: 12 seconds (standard view) (not stated if this is the mean or median) Mean inspection time (arm not stated): 10 minutes Withdrawal time (standard view) (reported in table 1, p. 1571), not stated if mean or median (±SD) minutes, range: 10.3 (5.7), 3.3–58 |
||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | All patients were colonoscopy for screening, surveillance or investigation of symptoms indicative of colorectal cancer | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | When multiple polyps (defined as two or more) were identified in the rectosigmoid colon in any one patient, a ‘representative sample’ (Kaltenbach paper57 p. 1570) was resected for histopathological analysis. Additionally, three polyps were not retrieved for histopathological examination (reasons not given). Otherwise, all polyps were subject to histopathological assessment (two patients did not undergo colonoscopy in the end, and missing data were imputed for these) | No |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | The pathologist was blinded to the endoscopic diagnosis | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | Of the 281 participants randomised to the standard-focus arm, 2 did not have a complete colonoscopy due to poor bowel preparation quality or stricture | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional, relevant publications identified | ||
Summary reviewer’s comments
The patients included in the study were undergoing colonoscopy for surveillance, screening and to investigate symptoms suggestive of colorectal cancer. More detailed information about the indications was not provided, but the patient population appears to be very relevant to the range of patients of interest in this appraisal. This study was conducted in three study centres. Five endoscopists carried out the colonoscopies and all received training in optical diagnosis before the study started. 57 Three were already experienced in using NBI. The authors point out that all the endoscopists had a history of performing high numbers of colonoscopies, and that they did not compare high and low-number endoscopists. 72 The findings may therefore not be generalisable to less experienced endoscopists. The authors imply on p. 1575 of the Kaltenbach paper57 that the three study centres were academic centres (this is not explicitly stated in the paper). The authors state that the literature shows that non-academic centres have not achieved high levels of diagnostic accuracy and that therefore the results of this study may not generalise to community practice.
Kang et al.78
| Reference and design | Diagnostic tests | Participants | Outcome measures | |
|---|---|---|---|---|
|
Condition being diagnosed/detected: comparison of the diagnostic performances of NBI and FICE in differentiating neoplastic from non-neoplastic colorectal polyps (< 10 mm) during real-time screening colonoscopy First author: Kang Publication year: 2015 Country: South Korea Study design: RCT Number of centres: one (Seoul National University Hospital Healthcare System Gangnam Centre) Funding: NR Competing interests: none |
Index test: endoscopists predicted histopathology in real time using NBI or FICE (adenoma or non-adenomatous polyp; also recorded the location, morphology and estimated size of polyp). After a polyp was detected in white light, either the NBI or FICE modes were used to predict the polyp histopathology Procedures were performed using either a colonoscope (CFH260ZI; Olympus, Optical, Tokyo, Japan) with a processor capable of NBI and HD imaging (EVIS 260 – LUCERA Spectrum Olympus Optical) or a high-resolution zoom endoscope (EC 590ZW; Fujinon, Inc., Saitama, Japan) with an EPX 4400 processor (Fujinon, Inc., FICE technology). The zoom function of the device was not used for this study Reference standard: histopathology |
Number of participants: 1005 (n = 50 excluded after randomisation. NBI: n = 28 poor bowel preparations; FICE: n = 20 poor bowel preparation, n = 2 failed colonoscopy) NBI: n = 475 FICE: n = 480 Sample attrition/dropout: excluded, n = 556 (calculated by reviewer) NBI: n = 262 lacking polyps, n = 10 polys measuring ≥ 10 mm in size FICE: n = 272 lacking polyps; n = 12 polys measuring ≥ 10 mm in size Used in analysis: n = 399 (with 851 colorectal polyps) NBI: n = 203 (463 polyps) FICE: n = 196 (388 polyps) Selection of participants: consecutive asymptomatic individual who attended the centre for colorectal cancer screening Inclusion criteria for study entry: as below Exclusion criteria for study entry: those with histories of IBD, polyposis syndrome, colorectal disease-related symptoms or signs (e.g. recent bowel habit change, unexplained weight loss, anaemia or lower GI tract bleeding not attributable to haemorrhoids), family history of colorectal cancer (at least one first-degree relative with colorectal cancer diagnosed at any age), history of colorectal cancer or polyps, surgical resection of colon or rectum, intestinal tuberculosis, coagulopathy, and incomplete examination of the entire colon because of failure to reach the caecum or inadequate bowel preparation |
Primary outcome of study: sensitivity, specificity, positive and NPVs and overall accuracy of differentiating neoplastic from non-neoplastic polyps using NBI and FICE Other relevant outcomes: effect of polyp size and location (subgroup analysis – subgroup analyses results by polys location NR); NBI and FICE system performances compared with the histopathology results Total examination time (all polyps) also reported Recruitment dates: August 2010–February 2011 |
|
| Participant characteristics | NBI (n = 203) | FICE (n = 196) | p-value | |
| Total sample: age (years), mean (SD) | 54.7 (8.9) | 54.3 (9.0) | 0.681 | |
| Other key patient characteristics | Total sample: male gender, n (%) | 139 (68.5) | 149 (76.0) | 0.093 |
| Total sample: polyp, n (%) | 0.899 | |||
| 1–2 | 148 (72.9) | 144 (73.5) | ||
| ≥ 3 | 55 (27.1) | 52 (26.5) | ||
| Polyps size 0–5 mm, n (%) | 384 (82.9) | 321 (82.7) | ||
| 0–5 mm subgroup: histopathology according to polyp size, n (%) | ||||
| Adenoma | 232 (60.4) | 192 (59.8) | 0.871 | |
| Non-adenoma | 152 (39.6) | 129 (40.2) | ||
| Total sample: average number of polyps per participant (range) | 2.2 (1–13) | |||
| 82.8% of all polyps were diminutive polyps measuring ≤ 5 mm in size | ||||
| Endoscopist experience and training | Four board-certified staff endoscopists, each having performed > 4000 colonoscopies. Endoscopists had no prior experience with NBI or FICE, but endoscopists performed a pilot study involving a minimum of 50 polyp examinations. Laminated reference sheets containing pictures and sketches were posted in each endoscopy room, showing the adenoma or non-adenomatous polyp classifications. During the study feedback was provided every 2 weeks on the accuracy of endoscopic predictions as compared with the histopathological diagnosis by the expert | |||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Polyp classification: presumed adenomatous if polyp was brown in colour, had increased vascular density or a round or tubulogyrus pattern was observed. Presumed non-adenomatous if surface showed normal or bland appearance, or if avascular or faint vascular patterns were observed. (Four supporting references for these criteria are provided in the paper) Histopathological classification: conducted by a single expert pathologist (blinded to the endoscopic images and optical predictions) classified all specimens in accordance with the World Health Organization guidelines and the serrated lesions in accordance with the diagnostic criteria proposed by Snover et al. 2011 (references provided in paper) |
|||
| Sample size calculation | The authors hypothesised that the diagnostic sensitivities of NBI and FICE were identical for identifying adenoma and calculated that a minimum sample size of 343 polyps in each group provided a statistically significant result with a difference in proportions of at least 5% (approximately 85% vs. 90%; 80% power and significance level 0.05) – planned enrolment a minimum of 430 participants per arm after consideration of the polyp detection rate and dropout rate from their previous data | |||
| Results | ||||
| NBI ≤ 5 mm subgroup | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 190a | (b) 37a | 227 | |
| Index test negative | (c) 42a | (d) 115a | 157 | |
| Total | 232 | 152 | 384 | |
| Accuracy [(a + d)/(a + b + c + d)] | 0–5 mm subgroup: 79.4% (95% CI 75.5% to 83.6%) | |||
| Diagnosis ≤ 5 mm subgroup | Value | 95% CI | ||
| Clinical sensitivity a/(a + c) | 81.9% | 77.1% to 87.0% | ||
| Clinical specificity d/(b + d) | 75.7% | 69.2% to 82.9% | ||
| PPV a/(a + b) | 83.7% | 79.0% to 88.7% | ||
| NPV d/(c + d) | 73.2% | 66.6% to 80.5% | ||
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 3.36a | 2.53 to 4.48a | ||
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.24a | 0.18 to 0.32a | ||
| Diagnostic odds ratio (a × d)/(b × c) | 14.1 | 8.5 to 23.2 | ||
| FICE ≤ 5 mm subgroup | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 143a | (b) 15a | 158 | |
| Index test negative | (c) 49a | (d) 114a | 163 | |
| Total | 192 | 129 | 321 | |
| Accuracy [(a + d)/(a + b + c + d)] | 0–5 mm subgroup: 80.1% (95% CI 75.8% to 84.6%) | |||
| Diagnosis ≤ 5 mm subgroup | Value | 95% CI | ||
| Clinical sensitivity a/(a + c) | 74.5% | 68.6% to 80.9% | ||
| Clinical specificity d/(b + d) | 88.4% | 82.9% to 94.2% | ||
| PPV a/(a + b) | 90.5% | 85.9% to 95.3% | ||
| NPV d/(c + d) | 69.9% | 63.2% to 77.3% | ||
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 6.41a | 3.95 to 10.38a | ||
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.29a | 0.22 to 0.37a | ||
| Diagnostic odds ratio (a × d)/(b × c) | 22.2 | 11.8 to 41.6 | ||
| Interpretability of test | NR | |||
| Interobserver agreement | NR | |||
| Intraobserver agreement | NR | |||
| Test acceptability (patients/clinicians) | NR | |||
| Adverse events | NR | |||
| High-confidence optical diagnosis | NR | |||
| Low-confidence optical diagnosis | NR | |||
| Number of polyps designated to be left in place | NR | |||
| Number of polyps designated to be resected and discarded | NR | |||
| Number of polyps designated for resection and histopathological examination | NR | |||
| Recommended surveillance interval | NR | |||
| Total sample: length of time (minutes) to perform the colonoscopy – mean (SD) | NBI, 18.6 (8.6); FICE, 18.6 (7.4); p = 0.947 | |||
| Number of outpatient appointments | NR | |||
| HRQoL | NR | |||
| Colorectal cancer | NR | |||
| Mortality | NR | |||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | The two groups of patients were based on average-risk adults undergoing screening colonoscopies | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis appear to be performed at the same time | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | The whole sample received verification using the intended reference standard | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Experienced gastrointestinal histopathologist, blinded to endoscopic images and optical predictions, classified all specimens in accordance with the World Health Organization’s guidelines154 | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Not stated, but believed to be none | Yes |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated, but believed to be none | No |
| 11 | Were withdrawals from the study explained? | Of 1005 patients randomised, 606 were excluded from the analysis for a variety of reasons, which were provided (i.e. poor bowel preparations, failed colonoscopy, lacked polyps and polys ≥ 10 mm) | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant reverences were identified | ||
Summary reviewer’s comments
Although the sample was based on average-risk adults undergoing screening colonoscopies, patients are from South Korea and it is unclear how representative the population is of the patient population in the UK, and how similar endoscopists’ training is compared with training received in the NHS. The study was performed in a single centre, so the results may not be applicable to a wider range of settings.
Ladabaum et al.58
| Reference and design | Diagnostic tests | Participants | Outcome measures | |||
|---|---|---|---|---|---|---|
|
Condition being diagnosed/detected: optical diagnosis of colorectal polyps as hyperplastic or adenoma or other (study also included an ex vivo computer training phase which has not been data extracted) First author: Ladabaum Publication year: 2013 Country: USA Study design: prospective cohort Number of centres: one (single-specialty practice, Ann Arbor, MI, USA) Funding: grant from division of Gastroenterology at Stanford University School of Medicine Competing interests: one of the eight authors had received research support and serves on the speaker’s bureau for Olympus Corp. The remaining authors disclosed no conflicts |
Index test: endoscopists predicted histopathology in real time using NBI (hyperplastic or adenoma; or other with explanation) and indicated level of confidence about their prediction (‘high’ if polyps had one or more features associated with one histopathology and no features associated with the other; and ‘low’ if there was uncertainty regarding features or if there were features of both histopathologies) NBI performed in endoscopy suites equipped with Evis Exera II CV-180 processors, CF-H180AL and PCF-H180AL colonoscopes (Olympus America, Centre Valley, PA) and HD monitors Reference standard: histopathology |
Number of participants: participants were considered to be the endoscopists n = 12 Sample attrition/dropout: unclear whether or not any endoscopists dropped out Fourteen polyps with missing size were excluded Selection of participants: endoscopists were community-based gastroenterologists. No details on how they were recruited to the study Colonoscopies included were any (including non-screening examinations) in which at least one polyp was removed Inclusion criteria for study entry: as above Exclusion criteria for study entry: none reported |
Primary outcome of study: the proportion of endoscopists achieving 90% accuracy in differentiating independent diminutive (≤ 5 mm) adenomas from non-adenomas Other relevant outcomes: nature of the learning curves, test performance characteristics, agreement between surveillance recommendations with vs. without the use of NBI Diagnostic threshold: n/a Recruitment dates: study took place March 2011–March 2012 |
|||
| Participant characteristics note that participants were considered to be the endoscopists in this study. No details provided regarding the patients | ||||||
| Age (years), mean (SD) | NR | |||||
| Other key patient characteristics (list) | NR | |||||
| Endoscopist experience and training |
Endoscopy practice experience in years, median (IQR): 12 (6–21) Colonoscopy volumea per year, median (IQR): 901 (803–1105) Adenoma detection rate,a median (IQR): 35% (30–38%) Prior to enrolment in this study no participants had formal training or significant experience with NBI. The first part (ex vivo phase) of the study consisted of three self-administered, computerised components that participants completed at their own pace during the first study week: a pre-test, a learning module on the NBI International Colorectal Endoscopic classification and a post-test. Results of the second part (in vivo phase) of the study therefore reflect the outcomes from endoscopists newly trained in NBI, the nature of their learning curves was a secondary outcome for the study (not data extracted) |
|||||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | Posters showing the NBI International Colorectal Endoscopic classification and photo examples present in endoscopy suites. The ex vivo study phase (not data extracted) included a learning module on the NBI International Colorectal Endoscopic classification | |||||
| Sample size calculation | The authors calculated a priori that with 12 participants their study design provided 79% power to detect an 80% success rate, based on a one-sided exact binomial test with an 8% type I error rate | |||||
| Results: subsample of diminutive polyps (≤ 5 mm) | ||||||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | ||||
| Index test positive | (a) 995b | (b) 252b | 1247b | |||
| Index test negative | (c) 155b | (d) 456b | 611b | |||
| Total | 1150 (62%)b | 708 (38%)b | 1858 | |||
| Accuracy, mean (95% CI) | 78.1% (73.7% to 82.5%) | |||||
| Diagnosis | Value | 95% CI | ||||
| Clinical sensitivity a/(a + c) | 86.5% | 80.9% to 92.1% | ||||
| Clinical specificity d/(b + d) | 64.7% | 54.9% to 74.6% | ||||
| PPV a/(a + b) | 79.8% | 74.3% to 85.3% | ||||
| NPV d/(c + d) | 75.9% | 69.1% to 82.7% | ||||
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 2.43b | 2.20 to 2.69b | ||||
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.21b | 0.18 to 0.24b | ||||
| Diagnostic odds ratio (a × d)/(b × c) | 11.62 | 9.24 to 14.60 | ||||
| The number of polyps identified by index test and reference test to populate the 2 × 2 table [i.e. values for (a), (b), (c) and (d)] are not reported in the paper, therefore the reviewer has imputed these. The imputed values provide the same sensitivity, PPV and NPV as reported in the paper, but the value for specificity (64.4%) differs slightly to that reported in the paper (64.7%) | ||||||
| Results: subsample of diminutive polyps (≤ 5 mm) in the rectosigmoid colon region | ||||||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | ||||
| Index test positive | (a) 186b | (b) 97b | 283b | |||
| Index test negative | (c) 48b | (d) 309b | 357b | |||
| Total | 234 | 406b | 640 | |||
| Accuracy, mean (95% CI) | 77.4% (69.1% to 85.3%) | |||||
| The number of polyps identified by index test and reference test to populate the 2 × 2 table [i.e. values for (a), (b), (c) and (d)] are not reported in the paper. The imputed values results in slightly different values for sensitivity (79.5% vs. 79.4% reported in paper), specificity (76.1% vs. 76.3% reported in paper), PPV (65.7% vs. 66.3% in paper), NPV (86.6% vs. 87.4% in paper) and accuracy (77.3% vs. 77.4% in paper) | ||||||
| Results: subsample of diminutive polyps (≤ 5 mm) in region proximal to the rectosigmoid colon | ||||||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | ||||
| Index test positive | (a) 806b | (b) 151b | 957b | |||
| Index test negative | (c) 108b | (d) 149b | 257b | |||
| Total | 914 | 300b | 1214 | |||
| Accuracy, mean (95% CI) | 79.3% (74.7% to 83.9%) | |||||
| The number of polyps identified by index test and reference test to populate the 2 × 2 table [i.e. values for (a), (b), (c) and (d)] are not reported in the paper. The imputed values results in slightly different values for PPV (84.2 vs. 85.0 in paper), NPV (58.0 vs. 57.3 in paper) and accuracy (78.7% vs. 79.3% in paper) | ||||||
| Results: comparison of the subsample of diminutive polyps (≤ 5 mm) in the rectosigmoid colon region versus proximal to rectosigmoid colon | ||||||
| Diagnosis | Rectosigmoid colon (n = 640) | Proximal to rectosigmoid colon (n = 1214) | Mean (SD) difference | p-value | ||
| Adenoma (% of polyps) | 234% (36.6%) | 914% (75.3%) | ||||
| Clinical sensitivity, mean (95% CI) | 79.4% (67.9% to 90.9%) | 88.2% (82.2% to 94.2%) | –8.8% (18.0%) | 0.121 | ||
| Clinical specificity, mean (95% CI) | 76.3% (66.1% to 86.6%) | 49.7% (34.7% to 64.6%) | 26.7% (22.8%) | 0.002 | ||
| PPV, mean (95% CI) | 66.3% (50.7% to 82.0%) | 85.0% (81.5% to 88.5%) | –18.7% (24.6%) | 0.024 | ||
| NPV, mean (95% CI) | 87.4% (82.5% to 92.4%) | 57.3% (38.4% to 76.2%) | 30.1% (30.7%) | 0.006 | ||
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 3.35b | 1.75b | NR | NR | ||
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.27b | 0.24b | NR | NR | ||
| Diagnostic odds ratio | NR | NR | NR | NR | ||
| Accuracy, mean (95% CI) | 77.4% (69.1% to 85.3%) | 79.3% (74.7% to 83.9%) | –1.9% (13.5%) | 0.628 | ||
| Results: subsample of diminutive polyps (≤ 5 mm) with low-confidence assessment | ||||||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | ||||
| Index test positive | (a) | (b) | ||||
| Index test negative | (c) | (d) | ||||
| Total | 210 | 158b | 368 | |||
| Accuracy, mean (95% CI) | 70.4% (58.9% to 82.0%) | |||||
| The number of polyps identified by index test and reference test to populate the 2 × 2 table [i.e. values for (a), (b), (c) and (d)] are not reported in the paper. The reviewer attempted to impute values, but it was not possible to find values that provide a close match to the data presented in the paper | ||||||
| Results: subsample of diminutive polyps (≤ 5 mm) with high-confidence assessment | ||||||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | ||||
| Index test positive | (a)b | (b)b | ||||
| Index test negative | (c)b | (d)b | ||||
| Total | 934 | 547b | 1481 | |||
| Accuracy, mean (95% CI) | 81.1% (75.8% to 86.3%) | |||||
| The number of polyps identified by index test and reference test to populate the 2 × 2 table [i.e. values for (a), (b), (c) and (d)] are not reported in the paper. The reviewer attempted to impute values, but it was not possible to find values that provide a close match to the data presented in the paper | ||||||
| Results: comparison of the subsample of diminutive polyps (≤ 5 mm) with low-confidence assessment vs. the subsample with a high-confidence assessment | ||||||
| Diagnosis | Low-confidence assessment (n = 368) | High-confidence assessment (n = 1481) | Mean (SD) difference | p-value | ||
| Adenoma (% of polyps) | 210% (57.1%) | 934% (63.1%) | ||||
| Clinical sensitivity, mean (95% CI) | 80.0% (72.7% to 87.4%) | 88.4% (82.2% to 94.7%) | –8.4% (13.1%) | 0.49 | ||
| Clinical specificity, mean (95% CI) | 88.4% (82.2% to 94.7%) | 44.1% (26.5% to 61.6%) | –24.2% (13.1%) | 0.17 | ||
| PPV, mean (95% CI) | 72.1% (59.0% to 85.3%) | 82.8% (77.0% to 88.6%) | –10.7% (21.3%) | 0.111 | ||
| NPV, mean (95% CI) | 51.8% (35.3% to 68.3%) | 78.3% (69.6% to 87.0%) | –26.5% (32.0%) | 0.15 | ||
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 6.90b | 1.58b | NR | NR | ||
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.23b | 0.26b | NR | NR | ||
| Diagnostic odds ratio | NR | NR | NR | NR | ||
| Accuracy, mean (95% CI) | 70.4 (58.9 to 82.0) | 81.1% (75.8% to 86.3%) | –10.6% (20.5%) | 0.100 | ||
|
The number of polyps identified by index test and reference test to populate the 2 × 2 table [i.e. values for (a), (b), (c) and (d)] are not reported in the paper and, therefore, the reviewer has not been able to check the reported values for sensitivity, specificity, etc. The paper also reports outcomes above for a comparison of first vs. last batch to explore learning effect and by polyp location (rectosigmoid colon vs. proximal to rectosigmoid colon). Outcomes for the last 20 polyps per endoscopist, all locations high confidence and for the last 20 polyps per endoscopist, rectosigmoid colon location, high confidence are also reported. These data have not been extracted. In addition, the paper contains data for small polyps (6–9 mm), which have also not been data extracted |
||||||
| Interpretability of test | NR | |||||
| Interobserver agreement | NR | |||||
| Intraobserver agreement | NR | |||||
| Test acceptability (patients/clinicians) | NR | |||||
| Adverse events | NR | |||||
| High-confidence optical diagnosis | ||||||
| Diminutive polyps (≤ 5 mm) | 1481/1858 (79.7%) | |||||
| Small polyps (6–9 mm) | 485/547 (88.7%) | |||||
| Low-confidence optical diagnosis | ||||||
| Diminutive polyps (≤ 5 mm) | 368/1858 (19.8%) | |||||
| Small polyps (6–9 mm) | 57/547 (10.4%) | |||||
| Number of polyps left in place | NR | |||||
| Number of polyps resected and discarded | NR | |||||
| Number of polyps resected and sent for histopathological examination | NR | |||||
| Recommended surveillance interval: all study colonoscopies | ||||||
| Recommended surveillance interval, n (%) | Agreement | |||||
| Using the US Multi-Society Task Force’s recommendations101 | 10 years | 5–10 years | 3 years | % agreement (95% CI) | κ-value | p-value |
| Diminutive polyps assessed by NBIc | 466 (28.3) | 957 (58.1) | 223 (13.6) | 88.4 (86.8 to 89.9) | 0.795 | < 0.001 |
| All polyps assessed by pathology | 507 (30.8) | 931 (56.6) | 208 (12.6) | |||
| Using modified recommendations (10 years for one or two small adenomas) | 10 years | 3 years | ||||
| Diminutive polyps assessed by NBI | 1423 (86.5) | 223 (13.6) | 98.4 (97.6 to 98.9) | 0.928 | < 0.001 | |
| All polyps assessed by pathology | 1438 (87.4) | 208 (12.6) | ||||
| Recommended surveillance interval: all study colonoscopies with at least one diminutive polyp characterised with high confidence | ||||||
| Recommended surveillance interval, Number (%) | Agreement | |||||
| Using the US Multi-Society Task Force’s recommendations101 | 10 years | 5–10 years | 3 years | % agreement (95% CI) | κ-value | p-value |
| Diminutive polyps assessed by NBIc | 357 (33.5) | 578 (54.3) | 130 (12.2) | 79.9 (77.4 to 82.3) | 0.654 | < 0.001 |
| All polyps assessed by pathology | 402 (37.8) | 547 (51.4) | 116 (10.9) | |||
| Using modified recommendations (10 years for one or two small adenomas) | 10 years | 3 years | ||||
| Diminutive polyps assessed by NBI | 935 (87.8) | 130 (12.2) | 96.8 (95.6 to 97.8) | 0.844 | < 0.001 | |
| All polyps assessed by pathology | 949 (89.1) | 116 (10.9) | ||||
|
Overall, there were 1673 study colonoscopies and 1646 contribute data to the surveillance intervals outcome for all study colonoscopies. The reason(s) for the absence of data for 27 colonoscopies is not provided. The total number of colonoscopies with at least one diminutive polyp characterised with high confidence is not reported, so it is not known whether or not any data are missing. For colonoscopies with at least one high-confidence diminutive polyp, NBI use would have led to 136 (13%) shorter and 78 (7%) longer recommended intervals than with histopathology alone using the US Multi-Society Task Force’s recommendations;101 using modified recommendations NBI use would have led to 24 (2%) shorter and 10 (1%) longer recommended intervals than with histopathology alone. When the presence of diminutive sessile serrated adenomas and traditional serrated adenomas informed surveillance intervals, the agreement between strategies was only minimally affected (data presented but not extracted) Surveillance interval recommendations reported for only the last 20 colonoscopies per endoscopist with at least one diminutive polyp characterised with high confidence have not been extracted |
||||||
| Length of time to perform the colonoscopy | NR | |||||
| Number of outpatient appointments | NR | |||||
| HRQoL | NR | |||||
| Colorectal cancer | NR | |||||
| Mortality | NR | |||||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Characteristics of those undergoing colonoscopy are not described. It is likely that many of the examinations were for screening, but it is specifically stated that non-screening examinations could be included | Unclear |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time NBI assessment and the polyp resection for histopathological analysis occurred at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polyps were resected for histopathology, although 14 polyps were excluded from the analysis as a result of missing information on size | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Three community-based fellowship-trained gastrointestinal pathologists interpreted all specimens as part of routine practice and were blinded to optical diagnosis | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference test results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | There is little reporting on withdrawals from the study. It is unclear whether or not any endoscopists dropped out. It is known that 14 polyps with missing size were excluded | Unclear |
| Reference list of the included paper(s) checked? Yes/no | Yes | ||
Summary reviewer’s comments
These results were obtained from 12 community gastroenterologists in the USA who had only just received training in the use of NBI and they were therefore not considered to be experts. Results may therefore not be applicable to endoscopists in other settings or with higher levels of experience.
Lee et al.77
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: NBI compared with i-scan to determine whether diminutive colonic polyps were adenomas or non-neoplastic polyps First author: Lee Publication year: 2011 Country: Korea Study design: prospective cohort Number of centres: one (academic hospital) Funding: NR Competing interests: the authors disclosed no financial relationships relevant to this publication |
Index test: endoscopists used HD white-light colonoscopy and then NBI or i-scan without magnification to predict the histopathology of diminutive polyps in real-time. (Purpose of the study was to compare NBI and i-scan) Confidence in the endoscopic prediction was recorded as high or low NBI: HD colonoscope CF-H260AL, EVIS LUCERA spectrum system, OEV-191H HDTV monitor, Olympus i-scan: HD colonoscope EC-3890, EPK-i system, PENTAX. Radiforce RS110 HDTV monitor, EIZO, Ishikawa, Japan. Used in the TE-c mode (tone enhancement for colonic lesions) Reference standard: histopathology |
Number of participants: 142 Sample attrition/dropout: none Selection of participants: consecutive patients undergoing screening or surveillance colonoscopy Inclusion criteria for study entry: as above Exclusion criteria for study entry: < 18 years old; pregnancy; currently using antiplatelet agents or anticoagulants; history of IBD, hereditary colorectal cancer or polyposis syndrome; and unable to provide informed consent |
Primary outcome of study: not stated Other relevant outcomes: accuracy of optical diagnosis in differentiating adenomas from non-neoplastic polyps; number of polyps assessed with high and low confidence; accuracy of diagnostic assessments made with high and low confidence; complications; Interobserver and intraobserver agreement (calculated using percentage agreement and values of κ statistics:Diagnostic threshold: n/a Recruitment dates: May–October 2010 |
| Participant characteristics (based on 142 patients; NBI, n = 70; and i-scan, n = 72) | |||
| Age (years), mean (SD) | NBI group: 57.98 (10.6); and i-scan group: 55.4 (11.3) | ||
| Other key patient characteristics (list) |
NBI: male, n = 52 (74.3%); and female, n = 18 (25.7%) (n and % calculated by reviewer) i-scan group: male n = 62 (86.1%); female n = 10 (13.9%) (n and % calculated by reviewer) Total number of diminutive polyps evaluated by NBI: n = 156 (from 70 patients) Total number of diminutive polyps evaluated by i-scan: n = 140 (from 72 patients) Note that the study solely focused on diminutive polyps |
||
| Endoscopist experience and training | One endoscopist described as ‘experienced’ carried out the colonoscopies. However, no details of the endoscopist’s experience or training are reported | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | One of the authors (the endoscopist carrying out the colonoscopies for the study), developed a classification system for use in this study. They developed it based on pilot work involving examination of the features of colon polyps based on images produced by NBI and i-scan, cross-referenced with histopathological findings | ||
| Sample size calculation | 76 diminutive polyps per group were needed for a power of 80% to demonstrate superiority of VCE in comparison to HD white light, assuming a diagnostic accuracy of 60% for HD white light and 90% for both NBI and i-scan | ||
| Results: NBI | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 71a | (b) 10a | 81 |
| Index test negative | (c) 9a | (d) 66a | 75 |
| Total | 80 (51.3%) | 76 (48.7%) | 156 |
| Accuracy | 87.8% (95% CI 82.6% to 92.9%) | ||
| Value | 95% CI | ||
| Clinical sensitivity a/(a + c) | 88.8% | 81.8% to 95.7% | |
| Clinical specificity d/(b + d) | 86.8% | 79.2% to 94.4% | |
| PPV a/(a + b) | 87.7% | 80.5% to 94.8% | |
| NPV d/(c + d) | 88.0% | 80.6% to 95.4% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 6.75a | 3.77 to 12.08a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.13a | 0.07 to 0.24 | |
| Diagnostic odds ratio (a × d)/(b × c) | 52.07a | 19.92 to 136.10a | |
| Reviewer has checked values reported for sensitivity, specificity, PPV and NPV using the reported index test-positive and test-negative results. The values agree, but different (slightly lower) 95% CIs were obtained | |||
| Results: NBI – high-confidence predictions | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 56 | (b) 6a | 62 |
| Index test negative | (c) 5a | (d) 58 | 63 |
| Total | 61a | 64a | 125a |
| Accuracy [(a + d)/(a + b + c + d)] |
High confidence overall: 91.2% (114/125)a For predicting adenomas: 90.3% (56/62) For predicting hyperplastic polyps: 92.1% (58/63) |
||
| Value | 95% CI | ||
| Clinical sensitivity a/(a + c) | 91.80%a | 81.90% to 97.28%a | |
| Clinical specificity d/(b + d) | 90.62%a | 80.70% to 96.48%a | |
| PPV a/(a + b) | 90.32%a | 80.12% to 96.37%a | |
| NPV d/(c + d) | 92.06%a | 82.44% to 97.37%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 9.79a | 4.55 to 21.05a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.09a | 0.04 to 0.21a | |
| Diagnostic odds ratio (a × d)/(b × c) | 108.27a | 31.26 to 375.00a | |
| Results: NBI – low-confidence predictions | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 15 | (b) 4a | 19 |
| Index test negative | (c) 4a | (d) 8 | 12 |
| Total | 19a | 12a | 31a |
| Accuracy [(a + d)/(a + b + c + d)] |
Low confidence overall: 74.2% (23/31)a For predicting adenomas: 79.0% (15/19) For predicting hyperplastic polyps: 66.7% (8/12) |
||
| Value | 95% CI | ||
| Clinical sensitivity a/(a + c) | 78.95%a | 54.43% to 93.95%a | |
| Clinical specificity d/(b + d) | 66.67%a | 34.89% to 90.08%a | |
| PPV a/(a + b) | 78.95%a | 54.43% to 93.95%a | |
| NPV d/(c + d) | 66.67%a | 34.89% to 90.08%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 2.37a | 1.03 to 5.45a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.32a | 0.12 to 0.82a | |
| Diagnostic odds ratio (a × d)/(b × c) | 7.50a | 1.47 to 38.28a | |
| The paper reports that there were no statistically significant differences in accuracy between high- and low-confidence predictions of adenomas with NBI (p = n.s.). In contrast, there were statistically significant differences in accuracy between high- and low-confidence predictions of hyperplastic polyps (p = 0.013) | |||
| Interpretability of test | NR | ||
| Interobserver agreement | % agreement = 86.5, κ-value (95% CI) = 0.730 (0.623 to 0.837), representing ‘substantial’ agreement | ||
| Intraobserver agreement | % agreement = 89.7, κ-value (95% CI) = 0.795 (0.699 to 0.890), representing ‘substantial’ agreement | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | It is stated that participants did not experience any procedure-related complications | ||
| High-confidence optical diagnosis |
High-confidence predictions, n/N (%) polyps = 125/156 (80.1) See 2 × 2 table above for results |
||
| Low-confidence optical diagnosis |
Low-confidence predictions, n/N (%) = 31/156 (19.9) See 2 × 2 table above for results |
||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
| Results: i-scan | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 70a | (b) 9a | 79 |
| Index test negative | (c) 4a | (d) 57a | 61 |
| Total | 74 (52.9%) | 66 (47.1%) | 140 |
| Accuracy | 90.7% (85.9% to 95.5%) | ||
| Value | 95% CI | ||
| Clinical sensitivity a/(a + c) | 94.6% | 89.4% to 99.7% | |
| Clinical specificity d/(b + d) | 86.4% | 78.1% to 94.6% | |
| PPV a/(a + b) | 88.6% | 81.6% to 95.6% | |
| NPV d/(c + d) | 93.4% | 87.2 to 99.7% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 6.94a | 3.77 to 12.76a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.06a | 0.02 to 0.16a | |
| Diagnostic odds ratio (a × d)/(b × c) | 110.83a | 32.44 to 378.66a | |
| The reviewer has checked values reported for sensitivity, specificity, PPV and NPV, using the reported index test-positive and test-negative results. The values agree, but slightly different 95% CIs were obtained | |||
| Results: i-scan – high-confidence predictions | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 50 | (b) 5a | 55 |
| Index test negative | (c) 3a | (d) 54 | 57 |
| Total | 53a | 59a | 112a |
| Accuracy [(a + d)/(a + b + c + d)] |
High confidence overall: 92.9% (104/112) For predicting adenomas: 90.9% (50/55) For predicting hyperplastic polyps: 94.7% (54/57) |
||
| Value | 95% CI | ||
| Clinical sensitivity a/(a + c) | 94.34%a | 84.34% to 98.82%a | |
| Clinical specificity d/(b + d) | 91.53%a | 81.32% to 97.19%a | |
| PPV a/(a + b) | 90.91%a | 80.05% to 96.98%a | |
| NPV d/(c + d) | 94.74%a | 85.38% to 98.90%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 11.13a | 4.80 to 25.82a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.06a | 0.02 to 0.19a | |
| Diagnostic odds ratio (a × d)/(b × c) | 180.00a | 40.89 to 792.43a | |
| Results: i-scan – low-confidence predictions | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 20 | (b) 4a | 24 |
| Index test negative | (c) 1a | (d) 3 | 4 |
| Total | 21a | 7a | 28a |
| Accuracy [(a + d)/(a + b + c + d)] |
Low confidence overall: 82.1% (23/28) For predicting adenomas: 83.3% (20/24) For predicting hyperplastic polyps: 75.0% (3/4) |
||
| Value | 95% CI | ||
| Clinical sensitivity a/(a + c) | 95.24%a | 76.18% to 99.88%a | |
| Clinical specificity d/(b + d) | 42.86%a | 9.90% to 81.59%a | |
| PPV a/(a + b) | 83.33%a | 62.62% to 95.26%a | |
| NPV d/(c + d) | 75.00%a | 19.41% to 99.37%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 1.67a | 0.87 to 3.19a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.11a | 0.01 to 0.90a | |
| Diagnostic odds ratio (a × d)/(b × c) | 15.00a | 1.23 to 183.63a | |
| The paper also reports that there were no statistically significant differences between the accuracy of high- and low-confidence predictions of adenomas or of hyperplastic polyps with i-scan (both p > 0.05) | |||
| Interpretability of test | NR | ||
| Interobserver agreement |
% agreement = 87.9, κ-value (95% CI) = 0.751 (0.640 to 0.861), representing ‘substantial’ agreement Reviewer note: these values are reported to be for NBI in the paper, but this appears to be a typo and that these values are for i-scan |
||
| Intraobserver agreement |
% agreement = 86.4, κ-value (95% CI) = 0.729 (0.616 to 0.841), representing ‘substantial’ agreement Reviewer note: these values are reported to be for NBI in the paper, but this appears to be a typo and that these values are for i-scan |
||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | It is stated that participants did not experience any procedure-related complications | ||
| High-confidence optical diagnosis |
High-confidence predictions, n/N (%) polyps = 112/140 (80.0) See 2 × 2 table above for results |
||
| Low-confidence optical diagnosis |
Low-confidence predictions, n/N (%) polyps = 28/140 (20.0) See 2 × 2 table above for results |
||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
| The paper reports that no significant difference (p > 0.05) was evident when NBI was compared with i-scan for the prediction of adenomas (based on reported sensitivity, specificity and accuracy of the two technologies) | |||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | The study included patients undergoing screening or surveillance colonoscopy and excluded those with a history of IBD, hereditary colorectal cancer or polyposis syndrome. These patients are relevant to the scope of this appraisal | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Reference standard was histopathology, the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polyps removed were sent for histopathological examination | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All polyps removed were sent for histopathological examination | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | VCE and histopathology were performed separately | Yes |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | An experienced gastrointestinal pathologist who was blinded to clinical information carried out the histopathological examination of the polyps. It is presumed the ‘clinical information’ means the results of the NBI and i-scan assessments | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Histopathological assessment was subsequent to the index test with NBI and i-scan | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | All polyps evaluated were diagnosed | No |
| 11 | Were withdrawals from the study explained? | The paper states that 142 consecutively recruited patients were included in the study. Results are reported for all 142 patients. Therefore, all selected participants appear to have been included in the analysis. No indication that any polyps were omitted from the analysis | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional references identified | ||
Summary reviewer’s comments
These results were obtained from a single endoscopist described as ‘experienced’. However, the level of experience was not described further or quantified. No details of training received for NBI and i-scan were provided. The study took place at an academic hospital in Korea. The results may therefore not be applicable to endoscopists with a differing level of experience and/or training working in other settings and/or countries.
Longcroft-Wheaton et al.84
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: in vivo diagnosis of colorectal polyps < 10 mm in size First author: Longcroft-Wheaton Publication year: 2011 Country: UK Study design: prospective series Number of centres: one Funding: not stated Competing interests: stated none |
Index test: EC-530 and EC-590 Fujinon colonoscopes and EPX 4400 processor (Fujinon Corporation, Saitama City, Saitama, Japan) without optical magnification. A flat-screen Sony 24-inch WUXGA LCD display was used (LMD-2450 MD) with a 1125 × 1080 resolution. FICE settings were preset at four (red channel, 520 nm; green channel, 500 nm; and blue channel, 405 nm) (Reviewer note: it is unclear whether or not the colonoscopies are HD, but the processor is ‘HD compatible’ and the resolution of the monitor appears to be HD) Polyps were assessed using WLE, followed by FICE, and then followed by VCE with indigo carmine dye. A diagnostic prediction was made with each technology. Only the diagnostic prediction for FICE is presented here Reference standard: histopathology |
Number of participants: 89 Sample attrition/dropout: 124 patients underwent colonoscopy in the UK BCSP, of which 89 had polyps < 10 mm in size (Reviewer note: it is assumed that these patients were a local population of patients from the national BCSP) Selection of participants: consecutive asymptomatic patients within the UK BCSP Inclusion criteria for study entry: positive FOBT Exclusion criteria for study entry: diagnosis of a familial polyp syndrome, a diagnosis of IBD, poor bowel preparation or melanosis coli |
Primary outcome of study: diagnostic accuracy (sensitivity; specificity; PPV; and NPV) Other relevant outcomes: surveillance intervals and costs Recruitment dates: September 2009–10 |
| Participant characteristics | |||
| Age (years), mean (SD) | 65 (6.7) | ||
| Other key patient characteristics |
Male, n = 70 (79%); and female, n = 19 (21%) Mean polyp size = 4.7 mm (range 2–9 mm; SD 2.7 mm) Polyps < 5 mm in size (diminutive), n = 155/232 (67%) Right-sided polyps, n = 79; left-sided polyps, n = 153 |
||
| Endoscopist experience and training | All assessments were conducted by a single endoscopist (one of the three co-authors) with expertise in in vivo diagnosis of polyps for > 8 years. It is not stated how much expertise or training the endoscopist had specifically with FICE | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Stated to be a previously developed and validated classification system developed by Teixeira et al. 99 Polyps were suspected to be non-neoplastic if they had a type I or II pattern. Polyps were suspected to be adenomatous if they had a type III or IV pattern and polyps were suspected of being cancers if a type V pattern was seen Serrated adenomas were treated as neoplastic for the purpose of calculating accuracy of in vivo histopathology prediction (i.e. the in vivo diagnosis was considered to be incorrect if the endoscopist called a serrated adenoma hyperplastic) The size, location and morphology of polyps were defined by the Paris classification system |
||
| Sample size calculation | The study was prospectively powered. The assumptions were made that 40% of polyps found are hyperplastic, that the true sensitivity for neoplasia with both FICE and indigo carmine would lie between 85% and 95%, and that the true specificity with FICE and indigo carmine lies between 75% and 90%. With 80% power (assuming a 5% significance level and ɸ coefficient of 0.2), 150 polyps would need to be assessed to achieve statistical significance. To demonstrate a 10% difference in the accuracy between FICE and indigo carmine, 200 polyps would need to be assessed to produce significant results. Note that the subgroup analysis of diminutive polyps may not be adequately statistically powered, though this relates to comparisons between white light, FICE and indigo carmine, which are not of direct relevance to this report | ||
| Results subset of diminutive polyps (n = 155) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | 75 | 11a | 86 |
| Index test negative | 15a | 54 | 69 |
| Total | 90 | 65 | 155 |
| Accuracy [(a + d)/(a + b + c + d)] | 129/155 (83%, 95% CI 77% to 88%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 83% | 78% to 88% | |
| Clinical specificity d/(b + d) | 83% | 75% to 89% | |
| PPV a/(a + b) | 87% | 81% to 91% | |
| NPV d/(c + d) | 78% | 70% to 84% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 4.92a | 2.85 to 8.51a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.20a | 0.12 to 0.32a | |
| Diagnostic odds ratio (a × d)/(b × c) | 24.5a | 10.5 to 57.6a | |
| The histopathology costs associated with three different protocols for histopathological assessment (traditional; proposed; futuristic) are reported, together with the savings that could be achieved from the last two. These have not been extracted here | |||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | NR | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
FICE correctly predicted rescope intervals for 67 of 69 (97% CI 89% to 100%) patients using BSG and ASGE guidelines (Note that 20 of the 89 patients were excluded from this analysis as they had additional larger polyps which would have influenced the rescope interval) |
||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Patients in the UK BCSP with a positive FOBT | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | Whole sample | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Consultant histopathologist was blinded to the diagnosis made by the endoscopist | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated, but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | Not stated whether or not there were any withdrawals | No |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional studies identified | ||
Summary reviewer’s comments
The results are based on FICE after white-light imaging by a single endoscopist with expertise with in vivo diagnosis of polyps in a single centre and in an English population of patients in the Bowel Cancer Screening Programme with a positive FOBT. It is not stated whether predictions were made with high or low confidence, but it is assumed that it was high confidence given that the endoscopist was experienced with in vivo diagnosis of polyps. The authors note that FICE is adequate for a resect and discard policy (i.e. as a result of ≥ 90% agreement in assignment of surveillance intervals), it is inadequate to guide the decision to leave suspected rectosigmoid colon polyps < 5 mm in size in place without resection, as the NPV fell below the 90% threshold in the PIVI criteria. The NPV only reached 90% when indigo carmine dye spray was used following FICE and WLE.
Longcroft-Wheaton et al.83
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: in vivo predicted diagnosis (non-neoplastic or adenomatous) of colorectal polyps < 10 mm in size First author: Longcroft-Wheaton Publication year: 2012 Country: UK Study design: prospective double-blind study Number of centres: one Funding: NR Competing interests: states ‘none’ |
Index test: diagnosis (neoplastic or hyperplastic) was made after both white-light imaging and reassessment using FICE. The maximum time allocated for examination with each modality was 30 seconds FICE assessments used setting 4 (red channel, 520 nm; green channel, 500 nm; and blue channel, 405 nm) Used Fujinon HD colonoscopes containing the Fujinon super CCD at 650,000 pixels (EC-530 and EC-590 colonoscopes) and an EPX-4400 processor. A flat-screen Sony 24-inch WUXGA LCD display with a 1125 × 1080 resolution was connected to the processor via a digital video interface connector This was a randomised trial but the other arm, which used standard-definition colonoscopes, does not meet the inclusion criteria for this review and data have not been extracted Reference standard: histopathology |
Number of participants: 143 polyps (103 of which were ≤ 5 mm) from 50 participants Sample attrition/dropout: none reported Selection of participants: positive FOBT and referred for bowel cancer screening colonoscopy on a standard screening list Inclusion criteria for study entry: as above Exclusion criteria for study entry: diagnosis of IBD, familial polyp syndromes and poor bowel preparation |
Primary outcome of study: to compare the accuracy of standard and HD colonoscopes in the diagnosis of neoplastic polyps of < 10 mm in size (Note that only the HD results for polyps ≤ 5 mm in size meet the inclusion criteria for this review, other data have not been extracted) Secondary outcomes: comparison of the accuracy of standard-definition and HD colonoscopes for the in vivo diagnosis of colonic polyps with white-light imaging. Comparison of the accuracy of standard-definition and HD colonoscopes for the in vivo diagnosis of colonic polyps with FICE when used after examination with white-light imaging Recruitment dates: NR |
| Participant characteristics for the HD group only (n = 85, n = 50 with polyps) | |||
| Age (years), mean (SD) | 64 (4.2). It is not clear if this is mean age for all 85 participants or only the 50 who had polyps | ||
| Other key patient characteristics (list) |
39/85 male (the proportion of males in the 50 participants with polyps is NR) Mean polyp size: 4.55 mm (range 2–10 mm) |
||
| Endoscopist experience and training | A single endoscopist who was trained and experienced in in vivo diagnostic methods assessed all the polyps. No further details | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Classification of polyps with FICE was based on vascular patterns and used the system developed by Teixeira et al. 99 which is a validated system. Polyps with a type I or II pattern were designated non-neoplastic. Polyps with a type III or IV pattern were designated adenomatous and if a type V pattern was observed a cancer was designated Histopathology reporting was done by an accredited colon cancer screening pathologist. In the analysis serrated adenomas were defined as neoplastic lesions |
||
| Sample size calculation | A sample size calculation was reported for the primary outcome (comparison of HD and standard-definition endoscopes in diagnosing neoplasia) and it was calculated that 218 polyps would be required | ||
| Results for the subgroup of polyps < 5 mm | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | a = 52 | b = 8a | a + b = 60a |
| Index test negative | c = 7a | d = 36 | c + d = 43a |
| Total | a + c = 59 | b + d = 44 | a + b + c + d = 103 |
| Accuracy [(a + d)/(a + b + c + d)] | 85% (95% CI 76 to 91) (n/N = 88/103) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 88% | 80% to 94% | |
| Clinical specificity d/(b + d) | 82% | 71% to 89% | |
| PPV a/(a + b) | 86.67%a | 75.41% to 94.06%a | |
| NPV d/(c + d) | 83.72%a | 69.30% to 93.19%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 4.85a | 2.57 to 9.14a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.15a | 0.07 to 0.29a | |
| Diagnostic odds ratio (a × d)/(b × c) | 33.43a | 11.13 to 100.40a | |
| The reviewer obtained different 95% CIs for sensitivity and specificity that those reported in the paper (77.07% to 95.09% and 67.29% to 91.81%, respectively) | |||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | n/a | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | NR | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
Predicted surveillance intervals used the BSG and ASGE guidelines and were performed on a per-patient basis. Patients in whom larger lesions were found that would require histopathological examination were excluded 12 patients in the HD group had additional lesions > 10 mm in size which would have required histopathology to set the surveillance interval so these were excluded from this analysis Correct surveillance interval using BSG guidelines = 100% (38/38) Correct surveillance interval using ASGE guidelines = 100% (38/38) Note that this analysis was not limited to patients with polyps ≤ 5 mm in size |
||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | UK-based study of patients with a positive FOBT and referred for bowel cancer screening colonoscopy | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | Whole sample | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Double-blind study. The consultant histopathologist was blinded to the diagnosis made by the endoscopist | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Histopathology takes place after FICE assessment | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | No results reported as being uninterpretable or intermediate | NO |
| 11 | Were withdrawals from the study explained? | All data used for 2 × 2 table, but 12 participants excluded from analysis of surveillance intervals because of the presence of lesion > 10 mm in size | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional papers identified | ||
Summary reviewer’s comments
The participants in this UK study are likely to be representative of participants in the UK generally (although only n = 50). Only a single endoscopist at a single centre was involved, so it is not clear how representative the results are to UK endoscopists and centres generally.
Paggi et al.59
| Reference and design | Diagnostic tests | Participants | Outcome measures | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Condition being diagnosed/detected: discriminating neoplastic from non-neoplastic polyps by NBI First author: Paggi Publication year: 2015 Country: Italy Study design: prospective observational study Number of centres: one (a community hospital) Funding: not stated Competing interests: none |
Index test: endoscopists used NBI to evaluate all diminutive polyps identified under white light. High-confidence categorisations of adenoma or non-adenoma were recorded Used standard HD colonoscopes (HDTV Olympus 180 Exera; Olympus, Tokyo, Japan) or dual-focus colonoscopes (HDTV Olympus 190 Exera) (Only one room of four was equipped with the 190 technology and use of the colonoscopes depended on scheduling issues. Results using the near-focus mode of the 190 colonoscopes do not meet the criteria for this review and have not been extracted) Reference standard: resection of all polyps into separate jars for pathological examination |
Number of participants: 284 participants with at least one diminutive polyp. A total of 465 diminutive polyps were identified from an overall total of 656 polyps. Of these, 446 were characterised with high confidence, 220 of these using the 180-HD colonoscope which meets the inclusion criteria for this review Sample attrition/dropout: none apparent Selection of participants: only patients with at least one diminutive polyp were included in the analysis Inclusion criteria for study entry: consecutive adult outpatients referred for colonoscopy categorised as screening, surveillance or symptoms with at least one diminutive polyp (< 5 mm in size) Exclusion criteria for study entry: medical history of colorectal cancer, IBD, hereditary polyposis syndromes, hereditary non-polyposis colorectal cancer; inadequate bowel preparation (used the Aronchick scale: more than 10% mucosa not visualised); caecal intubation not achieved or indicated; and polyps not resectable as a result of ongoing anticoagulation treatment or polyps not retrieved for pathological assessment |
Primary outcome of study: the agreement between endoscopy- and histopathology-directed surveillance strategies, by applying NBI-driven resect and discard strategy, in accordance with the PIVI statement32 (after the implementation of a retraining and monitoring initiative) Secondary outcomes: diagnostic performance (sensitivity, specificity, PPV, NBV, positive and negative likelihood ratios) of NBI for adenoma diagnosis of diminutive polyps; diagnostic performance of NBI in the rectosigmoid colon; evaluation of the impact of adopting ESGE guidelines on the adequacy of endoscopy-based post-polypectomy surveillance predictions108 Predefined subgroup analyses: operative characteristics of NBI for diminutive polyps according to the 180HD or 190HD technology; NBI diagnostic performances and agreement between endoscopy- and histopathology-based post-polypectomy surveillance predictions for individual endoscopists Recruitment dates: between October 2013 and February 2014 |
||||||||||||
| Participant characteristics (for the 284 participants with at least one diminutive polyp; number of participants assessed using the 180-HD colonoscope NR) | |||||||||||||||
| Age (years), mean (SD) | 61.3 (18.2) | ||||||||||||||
| Other key patient characteristics (list) |
Males, n (%) = 179 (63.0) Colorectal cancer family history, n (%) = 41 (14.4) Indication for colonoscopy, n (%): screening, 121 (42.6); surveillance, 79 (27.8); and symptoms, 84 (29.6) |
||||||||||||||
| Endoscopist experience and training |
Four endoscopists described as ‘highly experienced’ who had ‘used NBI technology regularly since 2009 (more than 200 exams per year per endoscopist)’. The four endoscopists had participated in an earlier study on NBI characterisation and had achieved different levels of performance (these are NR) Before the study all the endoscopists undertook a 1-hour training session with pre- and post-test assessments of a set of endoscopic images to standardise the classification of adenomatous and hyperplastic lesions. Every 2 months there were ‘refresh’ sessions regardless of performance level. The ‘refresh’ sessions included pre- and post-test performance evaluation and reference sets of 20 different endoscopic images or videos of NBI classified diminutive polyps (either adenomatous or hyperplastic). A collective discussion was held at the end of the session to evaluate cases where a disagreement between histopathology and NBI evaluation had occurred. All image sets were available to the endoscopists to consult at any time Each endoscopist received private monthly feedback on sensitivity and specificity of NBI for adenoma diagnosis in diminutive polys as part of the internal quality assurance programme, which also included other routinely monitored quality measured (e.g. caecal intubation and adenoma detection rates) |
||||||||||||||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
High-confidence categorisations of adenoma or non-adenoma were made based on published criteria20 and shown below: NBI featuresPredictive of adenomatous polypPredictive of hyperplastic polypColourBrowner than the backgroundSame or lighter than surrounding mucosaVascular patternBrown vessels surrounding white structuresNone or isolated lacy vessels coursing across the lesionSurface patternOval, tubular or branched white structures surrounded by brownHomogeneous absence of surface pattern, or dark or white spots of uniform size Diminutive polyps where only a low-confidence prediction could be made or in cases where the morphological features led to a suspicion of malignancy (e.g. depressed or ulcerated lesions) were not evaluated with NBI but were sent to pathology |
NBI features | Predictive of adenomatous polyp | Predictive of hyperplastic polyp | Colour | Browner than the background | Same or lighter than surrounding mucosa | Vascular pattern | Brown vessels surrounding white structures | None or isolated lacy vessels coursing across the lesion | Surface pattern | Oval, tubular or branched white structures surrounded by brown | Homogeneous absence of surface pattern, or dark or white spots of uniform size | ||
| NBI features | Predictive of adenomatous polyp | Predictive of hyperplastic polyp | |||||||||||||
| Colour | Browner than the background | Same or lighter than surrounding mucosa | |||||||||||||
| Vascular pattern | Brown vessels surrounding white structures | None or isolated lacy vessels coursing across the lesion | |||||||||||||
| Surface pattern | Oval, tubular or branched white structures surrounded by brown | Homogeneous absence of surface pattern, or dark or white spots of uniform size | |||||||||||||
| Sample size calculation | It was calculated that 280 patients with at least one diminutive polyp would be required based on an assumption of a 90% agreement between the endoscopy- and histopathology-directed strategies for surveillance and 3% precision of the estimates. Assuming an estimated prevalence of having at least one polyp of 63% resulted in the need to enrol 444 patients | ||||||||||||||
| Results [subgroup of 220 diminutive polyps assessed without magnification (i.e. 180-HD colonoscope, all high-confidence assessments)] | |||||||||||||||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |||||||||||||
| Index test positive | a = 140 | b = 15 | a + b = 155 | ||||||||||||
| Index test negative | c = 11 | d = 54 | c + d = 65 | ||||||||||||
| Total | a + c = 151 | b + d = 69 | a + b + c + d = 220 | ||||||||||||
| Accuracy [(a + d)/(a + b + c + d)] | 88.2% (95% CI 83.9% to 92.5%) | ||||||||||||||
| Diagnosis | Value | 95% CI | |||||||||||||
| Clinical sensitivity a/(a + c) | 92.7% | 89.3% to 96.2% | |||||||||||||
| Clinical specificity d/(b + d) | 78.2% | 72.7% to 83.7% | |||||||||||||
| PPV a/(a + b) | 90.32%a | 84.54% to 94.48%a | |||||||||||||
| NPV d/(c + d) | 83.08%a | 71.73% to 91.24%a | |||||||||||||
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 4.26a | 2.72 to 6.69a | |||||||||||||
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.09a | 0.05 to 0.17a | |||||||||||||
| Diagnostic odds ratio (a × d)/(b × c) | 45.8a | 19.8 to 106.02a | |||||||||||||
| Using the reported values for the 2 × 2 table, the reviewer obtained the same point estimates as reported but slightly different CIs. The results from the subgroup of polyps in the rectosigmoid colon have not been extracted because they are not presented separately for the 180-HD instrument | |||||||||||||||
| Interpretability of test | NR | ||||||||||||||
| Interobserver agreement | NR | ||||||||||||||
| Intraobserver agreement | NR | ||||||||||||||
| Test acceptability (patients/clinicians) | NR | ||||||||||||||
| Adverse events | NR | ||||||||||||||
| High-confidence optical diagnosis | Only high-confidence NBI characterisations were recorded, hence all the 180 colonoscope diagnoses were made from high-confidence characterisations | ||||||||||||||
| Low-confidence optical diagnosis |
NR separately for the 180 colonoscope However, it is known that 19 out of 465 (4.1%) diminutive polyps were categorised after evaluation by NBI with low confidence and were therefore sent directly for pathological evaluation (but this information was not broken down by the colonoscope used and may therefore include polyps assessed using the near-focus option of the 190-HD colonoscope) |
||||||||||||||
| Number of polyps designated to be left in place | NR | ||||||||||||||
| Number of polyps designated to be resected and discarded | NR | ||||||||||||||
| Number of polyps designated for resection and histopathological examination | NR | ||||||||||||||
| Recommended surveillance interval | High-confidence NBI histopathology predictions for diminutive polyps were merged with histopathological assessment of other polyps to generate an endoscopy-based surveillance interval. This was compared with the surveillance interval that would be recommended using pathological findings. Two guidelines (the European108 and the US Multi-Society Task Force American Cancer Society guideline103) were used to guide recommended follow-up intervals for each patient (i.e. a patient-level analysis). Results are reported only for the overall group, not separately for those patients examined with the 180-HD colonoscope (i.e. without near focus) and thus have not been extracted here | ||||||||||||||
| Length of time to perform the colonoscopy | NR | ||||||||||||||
| Number of outpatient appointments | NR | ||||||||||||||
| HRQoL | NR | ||||||||||||||
| Colorectal cancer | NR | ||||||||||||||
| Mortality | NR | ||||||||||||||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Patients receiving colonoscopy for screening, surveillance or symptoms | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Yes | |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | Whole sample | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Paper does not state whether or not the histopathologist(s) were blind to the NBI characterisation | Unclear |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | No | |
| 11 | Were withdrawals from the study explained? | No withdrawals or missing data apparent | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes | ||
Summary reviewer’s comments
This study included endoscopists who were described as ‘highly experienced’ and who also undertook training and regular review as part of the study. The results may therefore not be generalisable to less experienced endoscopists. The study took place in Italy and so participants might be reasonably similar to those who would receive this intervention in the UK.
Paggi et al.60
| Reference and design | Diagnostic tests | Participants | Outcome measures | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Condition being diagnosed/detected: assessment of NBI within a resect and discard strategy in routine clinical practice for small polyps (< 10 mm in size) on the accuracy of predicting post-polypectomy surveillance timing First author: Paggi Publication year: 2012 Country: Italy Study design: prospective cohort study Number of centres: one (community hospital) Funding: none reported Competing interests: none |
Index test: NBI. HD colonoscopes without additional magnification (HDTV Olympus 180 Exera; Olympus, Tokyo, Japan) After caecal intubation, the colonic mucosa was evaluated under white light during scope withdrawal and polyp size, location and morphology was documented (the size was estimated by comparison with open biopsy forceps or the sheath of a polypectomy snare placed against the polyp). Polyps identified under white light were further evaluated by NBI and categorised as adenoma or non-adenoma Reference standard: histopathology (assessed by two pathologists: one resident and one senior pathologist with long-standing experience in gastrointestinal pathology) |
Number of participants: 286 included in analysis (851 patients eligible of which 565 patients were excluded: 351 without polyps, 166 polyps ≥ 10 mm in size or cancerous, two failed polyp retrieval and 46 had low-confidence NBI evaluations) Sample attrition/dropout: no dropouts reported Selection of participants: consecutive adult outpatients undergoing colonoscopy for routine clinical indications Inclusion criteria for study entry: routine clinical indications for colonoscopy (screening, surveillance or symptoms) and at least one small polyp (< 10 mm in size) Exclusion criteria for study entry: surveillance interval not necessarily directed by endoscopic findings (history of colorectal cancer, IBD, hereditary polyposis syndromes, HNPCC); colonoscopy was performed without NBI technology; at least one lesion > 10 mm or < 10 mm and morphological features suspicious for malignancy (depressed or ulcerated lesions) was detected; bowel preparation was inadequate (Aronchick score 4, more than 10% of mucosa not visualised); caecal intubation was not accomplished; polyps could not be resected as a result of ongoing anticoagulation treatment or could not be retrieved for pathological assessment |
Primary outcome of study: not stated Other relevant outcomes: sensitivity, specificity, positive and negative likelihood ratios, of NBI for adenoma diagnosis in small and diminutive polyps, and left-sided polyps; accordance between endoscopy- and histopathology-directed surveillance strategies after polyp resection Subgroup analysis (pre-defined): operative characteristics of NBI for diminutive (≤ 5 mm) and left-sided (distal to splenic colonic flexure) polyps or the accordance between endoscopy- and histopathology-directed surveillance strategies for patients with diminutive polyps only Recruitment dates: February to May or June 2011 (there is a discrepancy in the reporting of the recruitment period in the paper) |
|||||||||||||||||||||||||||||||
| Participant characteristics are reported for the total sample (n = 286 with 511 small polyps). Participant characteristics for the subgroup of 197 participants with 399 diminutive polyps are NR | ||||||||||||||||||||||||||||||||||
| Age (years), mean (SD) | 60.3 (16.2) | |||||||||||||||||||||||||||||||||
| Other key patient characteristics | Male, n (%): 160 (55.9) | |||||||||||||||||||||||||||||||||
| First-degree colorectal cancer family history, n (%): 44 (15.4) | ||||||||||||||||||||||||||||||||||
Indication for colonoscopy, n (%)
|
||||||||||||||||||||||||||||||||||
| Endoscopist experience and training | Six highly experienced staff endoscopists, who regularly practised NBI technology (which was current practice at the Division of Gastroenterology where this study took place since 2009). All endoscopists underwent a re-training session with pro- and post-test assessments of a slide set of endoscopic pictures in order to standardise the classification of adenomatous and hyperplastic lesions prior to the start of the study | |||||||||||||||||||||||||||||||||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Each small polyp was categorised as adenoma or non-adenoma in accordance with simplified NBI criteria, as proposed by Rex,64 and summarised below: Predictive of adenomatous polypPredictive of hyperplastic polyp |
Predictive of adenomatous polyp | Predictive of hyperplastic polyp | |||||||||||||||||||||||||||||||
| Predictive of adenomatous polyp | Predictive of hyperplastic polyp | |||||||||||||||||||||||||||||||||
| Sample size calculation | The paper stated that given that the accuracy of histopathology in differentiating adenomas from non-adenomas is reported to range from 85% to 95% (reference provided in the paper) and that NBI could be competitive if reaching an accuracy of at least 90%, a sample size of 508 polyps was required – 511 small polyps were identified | |||||||||||||||||||||||||||||||||
| Results (all high-confidence characterisations) | ||||||||||||||||||||||||||||||||||
| Subgroup: diminutive polyps (n = 197) | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |||||||||||||||||||||||||||||||
| Index test positive | (a) 233 | (b) 48 | 281 | |||||||||||||||||||||||||||||||
| Index test negative | (c) 16a | (d) 102 | 118 | |||||||||||||||||||||||||||||||
| Total | 249 | 150 | 399 | |||||||||||||||||||||||||||||||
| Accuracy [(a + d)/(a + b + c + d)] | 84.0% (CI not reported and not calculated by reviewer) | |||||||||||||||||||||||||||||||||
| Diagnosis | Value | 95% CI | ||||||||||||||||||||||||||||||||
| Clinical sensitivity a/(a + c) | 93.9% | 89.77% to 96.28%b | ||||||||||||||||||||||||||||||||
| Clinical specificity d/(b + d) | 68.0% | 59.90% to 75.37%b | ||||||||||||||||||||||||||||||||
| PPV a/(a + b) | 82.9%b | 78.00% to 87.13%b | ||||||||||||||||||||||||||||||||
| NPV d/(c + d) | 86.4%b | 78.92% to 92.05%b | ||||||||||||||||||||||||||||||||
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 2.93 | 2.31 to 3.70b | ||||||||||||||||||||||||||||||||
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.09 | 0.06 to 0.15b | ||||||||||||||||||||||||||||||||
| Diagnostic odds ratio (a × d)/(b × c) | 30.945b | 16.784 to 57.054b | ||||||||||||||||||||||||||||||||
| Calculations agree with values reported in paper (although approximation of rounding differs) | ||||||||||||||||||||||||||||||||||
| Interpretability of test | NR | |||||||||||||||||||||||||||||||||
| Interobserver agreement | NR | |||||||||||||||||||||||||||||||||
| Intraobserver agreement | NR | |||||||||||||||||||||||||||||||||
| Test acceptability (patients/clinicians) | NR | |||||||||||||||||||||||||||||||||
| Adverse events | NR | |||||||||||||||||||||||||||||||||
| High-confidence optical diagnosis | Endoscopist defined the confidence level of the prediction (high vs. low) of polyp diagnosis. Patients with at least one polyp classified as low confidence were not included in the analysis | |||||||||||||||||||||||||||||||||
| Low-confidence optical diagnosis | A total of 46 (13.9%) patients were excluded from the analysis for having at least one polyp categorised with low confidence by the endoscopist | |||||||||||||||||||||||||||||||||
| Number of polyps designated to be left in place | NR | |||||||||||||||||||||||||||||||||
| Number of polyps designated to be resected and discarded | NR | |||||||||||||||||||||||||||||||||
| Number of polyps designated for resection and histopathological examination | NR | |||||||||||||||||||||||||||||||||
| Recommended surveillance interval |
Post-polypectomy surveillance interval on the basis of the number of polyps categorised as adenomas by NBI was assigned by the endoscopist after completion of the colonoscopy Patients with one or more polyps categorised as no adenoma were not given a specific follow-up indication (return to screening colonoscopy at 10 years):Post-polypectomy surveillance interval was re-assigned once the pathological report was complete (histopathology-directed strategy) according the US Multi-Society Task Force on Colorectal Cancer104 Practice guidelines for post-polypectomy surveillance:104 Patients with only one or two small (< 1 cm) tubular adenomas with only low-grade dysplasia (low-risk subjects)5–10 yearsPatients with 3–10 adenomas or any adenoma ≤ 1 cm in size or any adenoma with villous features or high-grade dysplasia (high-risk subjects)3 yearsPatients who have > 10 adenomas< 3 yearsPatients with small rectal hyperplastic polypsNo follow-up indication If based on by NBI endoscopic findings, surveillance would have been delayed in seven patients (4%) with diminutive polys and been too soon in 22 (11%) patients. Overall, concordance between endoscopy- and histopathology-directed surveillance intervals for patients with only diminutive polys occurred in 168/197 (85.3%) patients Accordance between endoscopy- and histopathology-directed post-polypectomy surveillance strategies in patients with diminutive polyps (n = 197): Endoscopy-directed surveillanceHistopathology-directed surveillance3 years5 years10 years3 years15a3c1c5 years0b112a18c10 years0b7b41aabc |
Patients with only one or two small (< 1 cm) tubular adenomas with only low-grade dysplasia (low-risk subjects) | 5–10 years | Patients with 3–10 adenomas or any adenoma ≤ 1 cm in size or any adenoma with villous features or high-grade dysplasia (high-risk subjects) | 3 years | Patients who have > 10 adenomas | < 3 years | Patients with small rectal hyperplastic polyps | No follow-up indication | Endoscopy-directed surveillance | Histopathology-directed surveillance | 3 years | 5 years | 10 years | 3 years | 15a | 3c | 1c | 5 years | 0b | 112a | 18c | 10 years | 0b | 7b | 41a | abc | |||||||
| Patients with only one or two small (< 1 cm) tubular adenomas with only low-grade dysplasia (low-risk subjects) | 5–10 years | |||||||||||||||||||||||||||||||||
| Patients with 3–10 adenomas or any adenoma ≤ 1 cm in size or any adenoma with villous features or high-grade dysplasia (high-risk subjects) | 3 years | |||||||||||||||||||||||||||||||||
| Patients who have > 10 adenomas | < 3 years | |||||||||||||||||||||||||||||||||
| Patients with small rectal hyperplastic polyps | No follow-up indication | |||||||||||||||||||||||||||||||||
| Endoscopy-directed surveillance | Histopathology-directed surveillance | |||||||||||||||||||||||||||||||||
| 3 years | 5 years | 10 years | ||||||||||||||||||||||||||||||||
| 3 years | 15a | 3c | 1c | |||||||||||||||||||||||||||||||
| 5 years | 0b | 112a | 18c | |||||||||||||||||||||||||||||||
| 10 years | 0b | 7b | 41a | |||||||||||||||||||||||||||||||
| abc | ||||||||||||||||||||||||||||||||||
| Length of time to perform the colonoscopy | NR | |||||||||||||||||||||||||||||||||
| Number of outpatient appointments | NR | |||||||||||||||||||||||||||||||||
| HRQoL | NR | |||||||||||||||||||||||||||||||||
| Colorectal cancer | NR | |||||||||||||||||||||||||||||||||
| Mortality | NR | |||||||||||||||||||||||||||||||||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Adult outpatients already undergoing colonoscopy for routine clinical indications, of which around 26% attended for surveillance, 37% for screening and 36% had symptoms | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | Each polyp was evaluated by pathologists after histopathology | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Two pathologists evaluated each polyp blindly and openly discussed all cases where discrepancy occurred (standard practice at the institution) | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | No | |
| 11 | Were withdrawals from the study explained? | Although not specifically stated, there appear to have been no withdrawals | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant publications were identified | ||
Summary reviewer’s comments
The population sample was based on patients from Italy, who were already undergoing colonoscopy for routine clinical indications (surveillance, symptoms and screening), and it is unclear how representative this sample is of the patient population in the UK, and how similar endoscopists training is compared with training received in the NHS. Study was performed in a single centre by highly experienced endoscopists who used NBI routinely, so the results may not be applicable to a wider range of settings or to less experienced endoscopists.
Patel et al.55
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: whether or not endoscopists without prior training can, when using NBI and having taken part in standardised training, achieve the ASGE PIVI thresholds for characterising diminutive polyps with high confidence: NPV ≥ 90% for adenomas in the rectosigmoid colon and a ≥ 90% agreement in surveillance intervals, compared with histopathology First author: Patel Publication year: 2016 Country: USA Study design: prospective cohort. Number of centres: four [two tertiary academic medical centres (University of Michigan and University of Colorado) and two Veterans Affairs hospitals (Ann Arbor, VA and Denver, VA)] Funding: ASGE Quality in Endoscopic Research Award Competing interests: two authors reported conflicts of interest. One was a consultant for and received a research grant from Olympus America. The other was supported by funding from the University of Colorado, Department of Medicine outstanding early scholars programme, AGA-Takeda Research Scholars Award in Barrett’s oesophagus and gastro-oesophageal reflux disease, educational grants from Covidien and Cook, and was a consultant for Covidien. The other authors reported that they had no conflicts |
Index test: NBI. The academic medical centres used Evis Exera II CV-180 processors with CF-H180AL and PCF-H180AL colonoscopes. The Veterans Affairs centres used Evis Exera III CV-190 processors and CF-H190AL and PCF-H190AL colonoscopes (Olympus America). HD monitors were used for all the colonoscopies Reference standard: histopathology |
Number of participants: 1451 colonoscopies in which a diminutive polyp was found Sample attrition/dropout: NR Selection of participants: participants undergoing colonoscopy for any indication between November 2013 and November 2014 and who had at least one diminutive polyp were included in the study. Specific indications not provided, but information on page 408 implies that patients with IBD and a history of colorectal cancer or familial cancer syndrome may have been included. Information in table 2, page 410, suggests that the study included six patients with familial syndrome and three with a history of IBD Inclusion criteria for study entry: as above Exclusion criteria for study entry: not stated |
Primary outcome of study: whether or not the endoscopists could achieve the PIVI thresholds for characterising diminutive polyps with high confidence: NPV ≥ 90% for adenomas in the rectosigmoid colon and a ≥ 90% agreement in surveillance intervals, compared with histopathology Other relevant outcomes: accuracy, sensitivity and NPV for characterising diminutive polyps using NBI by level of confidence and polyp location Recruitment dates: endoscopist training took place in October 2013. Study recruitment took place between November 2013 and November 2014 |
| Participant characteristics | |||
| Age (years), mean (SD) | NR | ||
| Other key patient characteristics (list) |
A total of 3012 diminutive polyps were included in the study, identified from 1451 colonoscopies. A total of 1088 (36%a) of the diminutive polyps were located in the rectosigmoid colon Patient characteristics: NR |
||
| Endoscopist experience and training |
26 endoscopists performed the colonoscopies. The endoscopists had no prior training in NBI and took part in a standardised training session at the start of the study in NBI interpretation, with structured performance feedback throughout the duration of the study The training session lasted approximately 2 hours. The endoscopists viewed a 20-minute audiovisual tool designed by one of the study authors, which described established NBI criteria for characterising polyps. They then viewed 80 videos of diminutive polyps taken when using HD white light and NBI. They predicted each polyp’s histopathology and recorded their confidence in their judgement (high or low). Then the histopathological diagnosis was revealed and the endoscopists received feedback where there was not consensus The endoscopists who completed this session then took part in a ‘study orientation’ and were introduced to the ‘characterise, resect, and discard’ strategy, the proposed PIVI thresholds and definitions of high- and low-confidence predictions Endoscopists who had annually performed < 200 colonoscopies were excluded from the study. A total of 57.7% of the endoscopists who took part in the study reported performing between 201 and 500 colonoscopies per year and the other participants reported performing > 500 colonoscopies per year. Eight (30.8%) had < 5 years’ experience, 10 (38.5%) had 5–10 years’ experience, four (15.4%) had 11–20 years’ experience and four (15.4%) had > 20 years’ experience |
||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
States used previously established NBI criteria and cites three references73,87,93 by Rastogi et al. Sessile serrated polyps were analysed as non-adenomas |
||
| Sample size calculation | It was calculated that approximately 2727 polyps and 1364 colonoscopies were needed to detect a NPV ≥ 90%, assuming that the true NPV would be 95% for rectosigmoid colon polyps characterised with high confidence. Calculations were based on an expected requirement of:336 total rectosigmoid non-adenomatous polyps characterised with high confidence . . . [and] 2 polyps per colonoscopy, 22% of all diminutive polyps located in the rectosigmoid, 70% with high confidence and 80% non-adenomasp. 408 | ||
| Results: Patel et al. report nine sets of diagnostic performance data (for three areas: all, proximal to the rectosigmoid colon, rectosigmoid colon, with an overall result for each region as well as results for high- and low-confidence characterisations). The reviewer has attempted to impute 2 × 2 table data to achieve the reported results, but it has not been possible to do this and match all the reported outcomes within a set of data. It has also not been possible to find values that are consistent between data sets (i.e. the 2 × 2 table values for high- and low-confidence assessments should sum to the 2 × 2 table for the overall results). Owing to the large size of this study illustrative 2 × 2 tables have been provided for possible use with meta-analysis for the overall data set, and for high-confidence assessments. A 2 × 2 table has also been imputed for the smallest data set (n = 238, low-confidence decisions in the rectosigmoid colon) | |||
| Results: NBI for characterising all diminutive polyps identified (n = 2876b) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 1523a | (b) 490a | 2013a |
| Index test negative | (c) 77a | (d) 786a | 863a |
| Total | 1600a | 1276a | 2876 |
| Accuracy [(a + d)/(a + b + c + d)] | 76.7% (95% CI 75.2% to 78.3%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 95.2% | 92.6% to 97.8% | |
| Clinical specificity d/(b + d) | 61.6% | 55.8% to 67.4% | |
| PPV a/(a + b) | 77.9% | 74.2% to 81.6% | |
| NPV d/(c + d) | 94.2% | 90.4% to 98.0% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| The reviewer was unable to find a solution for the 2 × 2 table that satisfies all the reported values. The values provided should be regarded as illustrative only because they produced the reported sensitivity and specificity, the values for PPV and NPV are lower than reported (75.7% and 91.1%, respectively), whereas the accuracy is higher than reported (80.3%). As the reviewer is not confident in the solution for the 2 × 2 table, these values have not been used to calculate positive and negative likelihood ratios or the diagnostic odds ratio | |||
| Results: NBI for characterising all diminutive polyps identified that were proximal to the rectosigmoid colon (n = 1818) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) Incalculable | (b) Incalculable | a + b |
| Index test negative | (c) Incalculable | (d) Incalculable | c + d |
| Total | a + c | b + d | 1818 |
| Accuracy [(a + d)/(a + b + c + d)] | 78.8% (95% CI 75.5% to 82.0%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 91.0% | 88.3% to 94.0% | |
| Clinical specificity d/(b + d) | 36.9% | 27.7% to 46.1% | |
| PPV a/(a + b) | 83.5% | 79.4% to 87.6% | |
| NPV d/(c + d) | 65.6% | 59.2% to 71.9% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a x d)/(b x c) | NR | NR | |
| The reviewer was unable to impute data for 2 × 2 table, as potential solutions did not provide outcomes that matched the reported values and, therefore, could not check if sensitivity, etc., values reported in the paper match the reviewer’s calculations | |||
| Results: NBI for characterising all diminutive polyps identified that were located in the rectosigmoid colon (n = 1058) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) Incalculable | (b) Incalculable | a + b |
| Index test negative | (c) Incalculable | (d) Incalculable | c + d |
| Total | a + c | b + d | 1058 |
| Accuracy [(a + d)/(a + b + c + d)] | 80.9% (76.7% to 85.1%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 88.4% | 84.8% to 92.0% | |
| Clinical specificity d/(b + d) | 78.3% | 71.8% to 84.9% | |
| PPV a/(a + b) | 56.8% | 51.1% to 62.4% | |
| NPV d/(c + d) | 93.7% | 91.8% to 95.7% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
|
The reviewer was unable to impute data for 2 × 2 table and, therefore, could not check if sensitivity, etc., values reported in the paper match the reviewer’s calculations Individually, 20 of the 26 endoscopists achieved ≥ 90% NPV in the rectosigmoid colon |
|||
| Results: NBI for characterising all diminutive polyps where predictions were made with high confidence (n = 2178) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 1296a | (b) 264a | 1560a |
| Index test negative | (c) 32a | (d) 586a | 618a |
| Total | 1328a | 850a | 2178 |
| Accuracy [(a + d)/(a + b + c + d)] | 84.8% (82.1% to 87.5%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 97.6% | 95.3% to 99.9% | |
| Clinical specificity d/(b + d) | 68.9% | 60.5% to 77.2% | |
| PPV a/(a + b) | 83.1% | 79.1% to 87.2% | |
| NPV d/(c + d) | 98.3% | 95.7% to 100.0% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| The reviewer has found a solution for the 2 × 2 table that provides the sensitivity, specificity and PPVs reported in the paper. However, the imputed 2 × 2 values produce a lower NPV (94.8%) in comparison to the value reported in the paper. This solution should be regarded as illustrative. As the reviewer is not confident in the solution for the 2 × 2 table, these values have not been used to calculate positive and negative likelihood ratios or the diagnostic odds ratio | |||
| Results: NBI for characterising all diminutive polyps proximal to the rectosigmoid colon where predictions were made with high confidence (n = 1360) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) Incalculable | (b) Incalculable | a + b |
| Index test negative | (c) Incalculable | (d) Incalculable | c + d |
| Total | a + c | b + d | 1360 |
| Accuracy [(a + d)/(a + b + c + d)] | 84.7% (80.7% to 88.6%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 96.2% | 94.1% to 98.4% | |
| Clinical specificity d/(b + d) | 34.9% | 22.1% to 47.7% | |
| PPV a/(a + b) | 85.2% | 80.9% to 89.5% | |
| NPV d/(c + d) | 77.1% | 67.9% to 86.2% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| The reviewer was unable to impute data for 2 × 2 table and, therefore, could not check if sensitivity, etc., values reported in the paper match the reviewer’s calculations | |||
| Results: NBI for characterising all diminutive polyps located in the rectosigmoid colon where predictions were made with high confidence (n = 818) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) Incalculable | (b) Incalculable | a + b |
| Index test negative | (c) Incalculable | (d) Incalculable | c + d |
| Total | a + c | b + d | 818 |
| Accuracy [(a + d)/(a + b + c + d)] | 88.1% (83.2% to 92.9%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 90.9% | 87.4% to 94.4% | |
| Clinical specificity d/(b + d) | 88.6% | 81.0% to 96.1% | |
| PPV a/(a + b) | 65.7% | 60.9% to 70.6% | |
| NPV d/(c + d) | 94.7% | 92.6% to 96.8% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| The reviewer was unable to impute data for 2 × 2 table and, therefore, could not check if sensitivity, etc., values reported in the paper match the reviewer’s calculations | |||
| Results: NBI for characterising all diminutive polyps where predictions were made with low confidence (n = 694) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) Incalculable | (b) Incalculable | a + b |
| Index test negative | (c) Incalculable | (d) Incalculable | c + d |
| Total | a + c | b + d | 694 |
| Accuracy [(a + d)/(a + b + c + d)] | 60.2% (55.4% to 65.1%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 74.6% | 65.9% to 83.4% | |
| Clinical specificity d/(b + d) | 50.6% | 45.6% to 55.7% | |
| PPV a/(a + b) | 55.3% | 45.6% to 64.9% | |
| NPV d/(c + d) | 80.8% | 67.9% to 93.7% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| The reviewer was unable to impute data for 2 × 2 table and, therefore, could not check if sensitivity, etc., values reported in the paper match the reviewer’s calculations | |||
| Results: NBI for characterising all diminutive polyps proximal to the rectosigmoid colon where predictions were made with low confidence (n = 456) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) Incalculable | (b) Incalculable | a + b |
| Index test negative | (c) Incalculable | (d) Incalculable | c + d |
| Total | a + c | b + d | 456 |
| Accuracy [(a + d)/(a + b + c + d)] | 61.3% (54.3% to 68.4%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 73.7% | 65.8% to 81.5% | |
| Clinical specificity d/(b + d) | 44.4% | 37.3% to 51.1% | |
| PPV a/(a + b) | 72.9% | 60.2% to 85.6% | |
| NPV d/(c + d) | 54.2% | 44.1% to 64.3% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| The reviewer unable to impute data for 2 × 2 table and, therefore, could not check if sensitivity, etc. values reported in the paper match the reviewer’s calculations | |||
| Results: NBI for characterising all diminutive polyps located in the rectosigmoid colon where predictions were made with low confidence (n = 238) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 34 | (b) 81 | 115 |
| Index test negative | (c) 12 | (d) 111 | 123 |
| Total | 46 | 192 | 238 |
| Accuracy [(a + d)/(a + b + c + d)] | 60.5% (52.5% to 68.5%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 73.9% | 61.2% to 86.6% | |
| Clinical specificity d/(b + d) | 57.8% | 46.9% to 68.8% | |
| PPV a/(a + b) | 29.1% | 20.8% to 37.3% | |
| NPV d/(c + d) | 90.1% | 84.8% to 95.4% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| The reviewer has imputed data for 2 × 2 table which broadly produces the same sensitivity, etc., values as reported in the paper | |||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis |
74.3% (n = 2293) of the diminutive polyp predictions were made with high confidence 74.4% (n = 844) of the diminutive polyp predictions of those in the rectosigmoid colon were made with high confidence |
||
| Low-confidence optical diagnosis |
24.3% (n = 731) of the diminutive polyp predictions were made with low confidence. Note that a classification of high or low confidence was missing for 1.4% (n = 42) of the diminutive polyps 22.1% (n = 251) of the diminutive polyp predictions of those in the rectosigmoid colon were made with low confidence |
||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
The following guidelines were used to determine surveillance intervals:The surveillance interval prediction was based on NBI predictions combined with histopathology outcome for low confidence and > 5-mm polyps There was a 91.2% (95% CI 89.67% to 92.65%; 1279 of 1403) agreement in surveillance intervals when using NBI to characterise polyps with high confidence in combination with histopathology for low-confidence characterisations and polyps > 5 mm There was a disagreement in surveillance interval in 124 colonoscopies. In 31.5% (n = 39) of these cases, endoscopists using NBI predicted a longer interval than histopathology. In 66.1% (n = 82) of these cases, endoscopists predicted a shorter interval than histopathology Overall, 97.0% [(1279 + 82)/1403] of the endoscopists’ predictions would bring patients back on time or early for surveillance follow-up examination Note that endoscopists made surveillance interval predictions for only high-confidence diminutive polyps. If there were one or two low-confidence characterisations, endoscopists were asked to predict the surveillance interval based on all the possible histopathological outcomes for the low-confidence characterisations. A surveillance interval prediction was not made if there were more than two low-confidence predictions. In addition, they did not predict surveillance intervals if there were > 10 polyps or if there was a reason to deviate from standard polyp surveillance guidelines (e.g. if a patient had IBD) or if the endoscopist was unable to retrieve all the polyps removed |
||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | This was a large study of 1451 colonoscopies, but no details were provided about the participants and the specific indications for carrying out the procedure | Unclear |
| 2 | Is the reference standard likely to classify the target condition correctly? | The reference standard was histopathology, the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | The investigators aimed to verify all polyps with histopathology | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | The index test result did not influence whether or not a polyp was resected and sent for histopathological assessment | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | The pathologists were blinded to study participation and NBI polyp prediction | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Histopathological assessment was subsequent to the index test with NBI | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Authors have reported that polyps were excluded from the analysis if a confidence level was not assigned or if histopathology was missing, ‘other’ (p. 411), or if the polyp could not be retrieved | Yes |
| 11 | Were withdrawals from the study explained? | Unclear if there were any withdrawals from the study, as the authors do not report this nor the number of participants selected to take part and the number of participants included in the data analyses | Unclear |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant studies identified | ||
Summary reviewer’s comments
This was a large study of 1451 colonoscopies that were carried out by 26 endoscopists with varying levels of experience in carrying out colonoscopies, but no prior training in NBI. The endoscopists were trained in NBI as part of the study. The findings may therefore be applicable to endoscopists of varying professional experience but with little training in NBI. The patient indications for colonoscopy were unclear and, therefore, it is unclear to which patient populations the findings of the study might generalise, but it is likely, given the large number of colonoscopies carried out, that a broad spectrum of patients were included.
Pigo et al.81
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: use of HD white-light i-scan for diagnosing the histopathology of colorectal polyps. Part of study aim was to also examine interobserver and intraobserver agreement regarding the histopathological diagnoses One endoscopist carried out a real-time assessment of all patients. Four other endoscopists then carried out a blinded assessment using only pictures generated from the colonoscopy to assess interobserver agreement. After 6 months, another assessment was carried out by these same four endoscopists to assess intraobserver agreement First author: Pigo Publication year: 2013 Country: Italy Study design: prospective cohort Number of centres: one (a hospital) Funding: NR Competing interests: NR |
Index test: endoscopists used HD white-light i–scan to predict the histopathology of colorectal polyps in real-time. EPK-i processor, HD colonoscope EC-3890i. 190-inch SXGA monitor. Surface enhancement SE4+ and TE-p or TE-c mode used (tone enhancement for colonic lesions) Reference standard: histopathology |
Number of participants: 78 Sample attrition/dropout: NR Selection of participants: consecutive patients, with at least one colorectal polyp, who met the inclusion criteria below Inclusion criteria for study entry: undergoing screening colonoscopy for colorectal cancer or for surveillance following polypectomy or colorectal cancer surgery; or, persistent gastrointestinal symptoms Exclusion criteria for study entry: aged < 18 years; IBD, HNPCC or FAP; currently using antiplatelet agents or anticoagulants; unable to provide informed consent |
Primary outcome of study: not stated, though aim of the study is stated as an evaluation of the diagnostic prediction of i-scan Other relevant outcomes: sensitivity, specificity and NPV for assessing histopathology of diminutive polyps located in the rectosigmoid colon. Accuracy, sensitivity and sensitivity also reported for assessment of all polyps, regardless of size. interobserver and intraobserver agreement reported, but was based on still picture evaluations rather than real-time assessment (so data not extracted) Recruitment dates: February–May 2011 |
| Participant characteristics | |||
| Age (years), mean (SD) | 52 (9) | ||
| Other key patient characteristics (list) |
Gender: male, n = 40 (51.3%a); and female, n = 38 (48.7%a) Indications for colonoscopy, n/N (%): positive FOBT, 51/78 (65.4a); polypectomy follow-up, 20/78 (25.6a); gastrointestinal symptoms, 7/78 (9.0a); and colorectal cancer familiarity, 9/78 (11.5a) Total number of polyps assessed: 150 Lesion size, n (%) polyps: ≤ 5 mm, 88 (58.7); > 5 mm, 62 (41.3) (% calculated by reviewer); mean polyp size, 6.8 mm (SD 5.5) and median polyp size, 5 mm (2–30) Note that the authors report diagnostic accuracy results (i.e. sensitivity, specificity and NPV) of real-time assessment of diminutive polyps located in the rectosigmoid colon only (n = 33 polyps). No other results relating to the assessment of diminutive polyps are reported |
||
| Endoscopist experience and training | The endoscopist who carried out all the first assessments had a history of undertaking > 1000 colonoscopies per year (although the number of years of experience are not provided). No details about the endoscopist’s training or experience in using i-scan are reported | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | Paris classification and NBI International Colorectal Endoscopic classification | ||
| Sample size calculation | NR | ||
| Results: i-scan – assessment of diminutive polyps located in the rectosigmoid colon (n = 33 polyps) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 17a | (b) 2a | 19a |
| Index test negative | (c) 1a | (d) 13a | 14a |
| Total | 18a | 15a | 33a |
| Accuracy [(a + d)/(a + b + c + d)] | 91%a (30 of 33 polyps accurately diagnosed) | ||
| Diagnosis: i-scan – assessment of diminutive polyps located in the rectosigmoid colon (n = 33 polyps) | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 94% | 83% to 100% | |
| Clinical specificity d/(b + d) | 87% | 72% to 100% | |
| PPV a/(a + b) | 89%a | 67% to 99%a | |
| NPV d/(c + d) | 93% | 81% to 100% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 7.08a | 1.94 to 25.86a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.06a | 0.01 to 0.44a | |
| Diagnostic odds ratio (a × d)/(b × c) | 110.500a | 9.01 to 1355.244a | |
| Interpretability of test | |||
| Interobserver agreement | Interobserver agreement was calculated for the assessment of diminutive polyps, but was based on endoscopists’ assessments of still images rather than real-time assessment. Data therefore not extracted | ||
| Intraobserver agreement | Intraobserver agreement assessed based on endoscopists’ assessment of still images rather than real-time assessment. Authors do not report intraobserver agreement for the evaluation of diminutive polyps. Data therefore not extracted | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | NR | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | The study included patients undergoing screening or surveillance colonoscopy and patients with persistent gastrointestinal symptoms suggestive of colorectal cancer. The study excluded patients with IBD, HNPCC or FAP. The patient population is therefore relevant to the scope of this appraisal | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Reference standard was histopathology, the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis were performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polyps removed were sent for histopathological examination | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All polyps removed were sent for histopathological examination | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | VCE and histopathology were performed separately | Yes |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | The pathologist who carried out the histopathology assessment was blinded to the endoscopist’s assessment | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Histopathological assessment was subsequent to the index test with i-scan | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated, but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | Unclear if there were any withdrawals from the study. A total of 78 patients were recruited and 150 polyps were included in the analysis, but the authors do not state if the 150 polyps were from the full sample of 78 recruited participants | Unclear |
| Reference list of the included paper(s) checked? Yes/no |
Yes – no additional relevant publications identified Note that paper cites paper by Lee (2001), but the date is incorrect and it is Lee (2011),77 which we have already identified through our searches |
||
Summary reviewer’s comments
The majority of patients had been screened for bowel cancer and had a positive FOBT. These results were obtained from an endoscopist who was experienced in carrying out colonoscopies, but no details were provided about the endoscopist’s experience or training in using i-scan. The study took place in one hospital in Italy. The results may therefore not be applicable to endoscopists with a differing level of experience and/or training working in other settings and/or countries.
Pohl et al.61
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: diagnosis of whether or not polyps were adenomas or not. Aim of study was to examine factors related to the quality of optical diagnosis of diminutive polyps using NBI First author: Pohl Publication year: 2016 Country: USA Study design: prospective cohort – participants who had previously taken part in a two-arm RCT were analysed as one group in this study. In the RCT, participants had been randomised to either cap-assisted or standard colonoscopy Number of centres: two (academic medical centres) Funding: NR Competing interests: none |
Index test: NBI. HD colonoscopes were used (models H-CF 180 or H-PCF 180, Olympus Inc., USA). Participants underwent either cap-assisted colonoscopy (4-mm Olympus cap) or standard colonoscopy. Whether magnification was used or not was not reported. Polyps were examined with white light and NBI Endoscopists rated their level of confidence in their prediction of polyp histopathology as high, low or do not know Reference standard: histopathology |
Number of participants: 1100 participants were eligible. 607 participants had at least one polyp; 566 participants had at least one diminutive polyp Sample attrition/dropout: of the 1113 participants randomised to the original RCT, 13 did not undergo optical diagnosis and so were not included in the prospective cohort study Selection of participants: see inclusion and exclusion criteria below Inclusion criteria for study entry: patients aged 50–89 years, presenting for an outpatient colonoscopy Exclusion criteria for study entry: patients with IBD, a coagulopathy or with an American Society of Anesthesiologists (ASA) class > 3. Patients who did not undergo real-time assessment were also excluded |
Primary outcome of study: the following outcomes were described as the ‘main’ outcomes of the study in the abstract: NPV for diminutive polyps diagnosed as adenomas in the rectosigmoid colon (stated later in the paper that this was to assess if the PIVI quality benchmark of at least 90% could be met); and, assessment of the endoscopist-related and procedural factors associated with the quality of optical diagnosis – the NPV for diminutive adenomas in the rectosigmoid colon and the concordance of surveillance intervals (effect of endoscopists’ prior experience data extracted, but findings for three other procedural factors investigated not data extracted) Other relevant outcomes: surveillance intervals – study also assessed the concordance of optical diagnosis surveillance recommendations with those from histopathology in accordance with the PIVI benchmark of 90%; sensitivity; specificity; and PPV Recruitment dates: NR |
| Participant characteristics | |||
| Age (years), mean (SD) | 61.8 (8.4) | ||
| Other key patient characteristics (list) |
The 607 patients had a total of 1650 polyps, of which 1311 (79%) were diminutive (defined as 1–5 mm in size). Location of all polyps also reported, but not data extracted. Of the 1650 polyps identified, 42 (2.6%) were not diagnosed because not being retrieved or there being insufficient material to make a diagnosis Characteristics of the 1100 eligible participants (characteristics for the 607 participants with polyps NR): Gender, n (%): male, 702 (63.8); and female, 398 (36.2) Indications, n (%): screening, 580 (52.7); surveillance, 332 (30.2); bleeding, anaemia, positive FOBT, 97 (8.8); and other, 91 (8.3) |
||
| Endoscopist experience and training | A total of10 endoscopists carried out the colonoscopies. None has had prior experience (beyond application of NBI in routine endoscopy practice) of optical diagnosis (although almost all had extensive colonoscopy experience), but two had been involved in other clinical studies on endoscopic imaging technologies. All the endoscopists took part in a NBI training course at the start of the study. Training was repeated when the study reached 50% of the enrolment target. The training course followed the structure of a validated programme published before the NBI International Colorectal Endoscopic classification was available (reference provided). The content of the training included a pre-test, a didactic session and a post-test. The course took 1 hour and was delivered to a group, enabling interaction and immediate feedback. As part of the training, the endoscopists also all had access at their units to a reference book containing images summarising all the polyp cases covered in the training | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
During real-time diagnosis, polyps were classified as adenomatous or non-adenomatous based on colour, the appearance of vessels, and mucosal pattern. 94 No formal method was used for evaluating SSP/A. If an endoscopist suspected that a polyp was a SSP/A, they categorised it as a neoplastic polyp During histopathology, polyps were classified as neoplastic and non-neoplastic. All adenomatous and SSP/As were classified as neoplastic, based on the World Health Organization’s classification of serrated polyps. 150 4.3% of polyps were SSP/As. All other polyps were classified as neoplastic |
||
| Sample size calculation | The sample size needed for the original RCT was calculated to be 1100 participants. It was expected that 45% of the participants would have a polyp and would therefore be included in the post-polypectomy surveillance interval analysis. Based on a surveillance interval recommendation concordance of at least 93%, it was expected that this sample size would provide a 95% CI with the lower margin above 90% (90.4% to 95.1%) | ||
| Results: polyps sized 1–5 mm diagnosed with high confidence | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 408 | (b) 77a | 485 |
| Index test negative | (c) 84a | (d) 391a | 475a |
| Total | 492 | 468a | 960 |
| Accuracy [(a + d)/(a + b + c + d)] | 83.2% (799a of 960 polyps correctly classified) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 83% | 79.30% to 86.15%a | |
| Clinical specificity d/(b + d) | 84% | 79.87% to 86.79%a | |
| PPV a/(a + b) | 84.1% | 80.56% to 87.26%a | |
| NPV d/(c + d) | 82.3% | 78.58% to 85.64%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 5.04a | 4.09 to 6.21a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.20a | 0.17 to 0.25a | |
| Diagnostic odds ratio (a × d)/(b × c) | 24.664a | 17.574 to 34.614a | |
| Reviewer’s calculations of sensitivity, specificity, PPV and NPV match the values reported in the paper. Paper did not report CIs | |||
| Results: polyps sized 1–5 mm located in the proximal colon diagnosed with high confidence | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 262 | (b) 26a | 288 |
| Index test negative | (c) 56a | (d) 43a | 99a |
| Total | 318 | 69a | 387 |
| Accuracy [(a + d)/(a + b + c + d)] | 78.8% (305a of 387 polyps correctly classified) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 82% | 77.75% to 86.41%a | |
| Clinical specificity d/(b + d) | 62% | 49.83% to 73.71%a | |
| PPV a/(a + b) | 91.0% | 87.05% to 94.02%a | |
| NPV d/(c + d) | 43.4% | 33.50% to 53.77%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 2.19a | 1.61 to 2.97a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.28a | 0.21 to 0.38a | |
| Diagnostic odds ratio (a × d)/(b × c) | 7.738a | 4.393 to 13.627a | |
| Reviewer’s calculations of sensitivity, specificity, PPV and NPV match the values reported in the paper. Paper did not report CIs | |||
| Results: polyps sized 1–5 mm located in the distal colon diagnosed with high confidence | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 146 | (b) 51a | 197 |
| Index test negative | (c) 28a | (d) 348a | 376a |
| Total | 174 | 399aa | 573 |
| Accuracy [(a + d)/(a + b + c + d)] | 86.2% (494a of 573 polyps correctly classified) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 84% | 77.59% to 89.03%a | |
| Clinical specificity d/(b + d) | 87% | 83.54% to 90.33%a | |
| PPV a/(a + b) | 74.1% | 67.41% to 80.08%a | |
| NPV d/(c + d) | 92.6% | 89.42% to 94.99%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 6.56a | 5.04 to 8.55a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.18a | 0.13 to 0.26a | |
| Diagnostic odds ratio (a × d)/(b × c) | 35.580a | 21.583 to 58.653a | |
| Reviewer’s calculations of sensitivity, specificity, PPV and NPV match the values reported in the paper. Paper did not report CIs | |||
| Results: polyps sized 1–5 mm located in the rectosigmoid colon diagnosed with high confidence | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 101 | (b) 44a | 145 |
| Index test negative | (c) 17a | (d) 328a | 345a |
| Total | 118 | 372a | 490 |
| Accuracy [(a + d)/(a + b + c + d)] | 87.6% (429a of 490 polyps correctly classified) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 86% | 77.94% to 91.38%a | |
| Clinical specificity d/(b + d) | 88% | 84.45% to 91.27%a | |
| PPV a/(a + b) | 69.7% | 61.48% to 77.01%a | |
| NPV d/(c + d) | 95.1% | 92.23% to 97.10%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 7.24a | 5.43 to 9.64a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.16a | 0.11 to 0.25a | |
| Diagnostic odds ratio (a × d)/(b × c) | 44.289a | 24.245 to 80.902a | |
| Reviewer’s calculations of sensitivity, specificity, PPV and NPV match the values reported in the paper. Paper did not report CIs | |||
| Effect of endoscopist prior experience on NPV for rectosigmoid colonb diminutive adenomas and the concordance of surveillance recommendations | |||
| Prior experience | NPV, % (95% CI) | Surveillance interval concordance, % (95% CI) | |
| Yes (n = 2 endoscopists) | 96.6 (92.7 to 98.7) | 94.4 (90.2 to 97.2) | |
| No (n = 8 endoscopists) | 93.5 (88.7 to 96.7) | 92.4 (89.2 to 94.9) | |
| Two endoscopists had prior experience and research interest in image-enhanced endoscopy. The eight endoscopists without prior experience had only experience of NBI in routine endoscopy practice | |||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | 960 of the 1311 (73.2%a) diminutive polyps (sized 1–5 mm) were diagnosed with high confidence | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
The study used two methods to determine surveillance intervals:The US Multi-Society Taskforce guidelines103,105 were used to assign surveillance intervals Among all patients who had a colonoscopy, the optical diagnosis assigned surveillance interval agreed with that of histopathology in 96% of the participants. Among the 566 participants with at least one diminutive polyp the surveillance interval assigned with optical diagnosis agreed with the interval assigned by histopathology in 93% of patients. In 24 cases, the optical diagnosis assigned surveillance interval was shorter than the one assigned by histopathology. In 15 cases it was longer. Eight of the 10 endoscopists reached the 90% PIVI threshold Surveillance intervals concordance according to endoscopist experience is data extracted above under ‘Effect of endoscopist prior experience on NPV for rectosigmoid colon diminutive adenomas and the concordance of surveillance recommendations’ |
||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Participants were undergoing surveillance and screening colonoscopy, and colonoscopy to investigate symptoms and a positive FOBT | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | Whole sample (where polyps could be retrieved/were materially sufficient enough to diagnose) | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear if the pathologists were blinded to the NBI diagnosis | Unclear |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | The authors did not report the number of polyps identified by NBI that could not be optically diagnosed. (The authors did report those that could not be retrieved or had insufficient material for a histopathological diagnosis) | No |
| 11 | Were withdrawals from the study explained? | 1113 participants were randomised in the original trial, of whom 13 were not included in this cohort study as they did not undergo optical diagnosis | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant references identified | ||
Summary reviewer’s comments
The colonoscopies were performed in this study by 10 endoscopists, in two academic study centres. None of the endoscopists has previous experience of optical diagnosis and all underwent a training session in NBI at the beginning of the study, which was repeated half-way through recruitment to the study. The results may therefore be applicable to endoscopists with relatively little experience of optical diagnosis and NBI.
Rath et al.82
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: distinguishing hyperplastic from adenomatous distal (located in the descending colon, the sigmoid colon or the rectum) diminutive polyps First author: Rath Publication year: 2015 Country: Germany Study design: prospective cohort Number of centres: one (Ludwig Demling Endoscopy Centre of Excellence at the University Hospital Erlangen) Funding: Deutsche Forschungsgemeinschaft (DFG) and Friedrich-Alexander-University Erlangen-Nuremberg (FAU) within the funding programme Open Access Publishing. Erlangen Interdisciplinary Centre for Clinical Research (IZKF). Italian Group for the study of IBD (IG-IBD) Competing interests: stated none |
Index test: real-time HD i-scan (PENTAX, Tokyo, Japan) (no information given on model number) Reference standard: histopathology |
Number of participants: 77 Sample attrition/dropout: 224 patients were included, but a subgroup of 77 patients with distal diminutive colorectal polyps (n = 121) was analysed. A further subgroup of 59 patients with polyps in the rectosigmoid colon area is also presented Selection of participants: patients identified during screening or surveillance colonoscopies Inclusion criteria for study entry: as above Exclusion criteria for study entry: history of IBD, poor bowel preparation, colectomy, anticoagulation or polyposis syndrome |
Primary outcome of study: sensitivity and NPV for prediction of adenomatous polyp histopathology in accordance with the PIVI statement Other relevant outcomes: diagnostic accuracy, specificity, PPV, surveillance intervals and intraobserver agreement Recruitment dates: not stated |
| Participant characteristics | |||
| Age (years), mean (SD) | 65.5 (14.4) | ||
| Other key patient characteristics (list) |
49 (63.6%) males and 28 (36.4%) females Polyp size: ≤ 3 mm, n = 75 (62%); 4–5 mm, n = 46 (38%); median = 3 mm; and mean = 3.3 mm Polyp location: descending colon, n = 42 (34.7%); sigmoid, n = 32 (26.5%); and rectum, n = 47 (38.8%) |
||
| Endoscopist experience and training | All colonoscopies were performed by a single experienced endoscopist | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Polyp histopathology classification based on previously published and validated criteria, assessing surface characteristics (pit pattern and mucosal vascular pattern morphology, colour, depression) (reference to a published study given). The Paris classification system was also used The endoscopist assigned a level of confidence (high or low) to their assessment of each polyp |
||
| Sample size calculation | The probability for error (α) was set to 0.05 and the β-error was set to 0.1 (reflecting a power of 0.90). For WLE, an expected accuracy of 74% and for i-scan an expected accuracy of 90% was assumed (citations are given for previous evaluations of VCE), resulting in a calculated sample size of 120 polyps | ||
| Results: all distal polyps, overall prediction (high and low confidence), n = 121 polyps | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathologya | Total | |
| Index test positive | (a) 53b | (b) 11 | 64b |
| Index test negative | (c) 4 | (d) 52b | 56b |
| Total | 57 | 63 | 120 |
| Accuracy [(a + d)/(a + b + c + d)] | 90.1% (109 of 121 polyps predicted accurately)c | ||
| Diagnosisd | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 93.3% | 82.7% to 97.8% | |
| Clinical specificity d/(b + d) | 88.7% | 77.5% to 95% | |
| PPV a/(a + b) | 88.7% | 77.5% to 95% | |
| NPV d/(c + d) | 93.2% | 82.7% to 97.8% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 5.33 | 3.10 to 9.15 | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.09 | 0.03 to 0.22 | |
| Diagnostic odds ratio (a × d)/(b × c) | 62.64 | 18.74 to 209.34 | |
| Results: all distal polyps, high-confidence prediction only, n = 107 polyps | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 51e | (b) 3e | 54e |
| Index test negative | (c) 1e | (d) 52e | 53e |
| Total | 52e | 55e | 107 |
| Accuracy [(a + d)/(a + b + c + d)] | 103/107 (96.3%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 98.1% | 88.6% to 99.9% | |
| Clinical specificity d/(b + d) | 94.4% | 83.7% to 98.6% | |
| PPV a/(a + b) | 94.5% | 83.9% to 98.6% | |
| NPV d/(c + d) | 98.1% | 88.4% to 99.1% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 17.98e | 5.98 to 54.07e | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.02e | 0.00 to 0.14e | |
| Diagnostic odds ratio (a × d)/(b × c) | 884.0e | 88.99 to 8781.07e | |
| Results: polyps in the rectosigmoid colon only, overall prediction (high and low confidence), n = 79 polyps | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | NRf | NRf | NRf |
| Index test negative | NRf | NRf | NRf |
| Total | 29 | 50 | 79 |
| Accuracy [(a + d)/(a + b + c + d)] | NRf | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 90.3% | 73.1% to 97.5% | |
| Clinical specificity d/(b + d) | 87.5% | 74.1% to 94.8% | |
| PPV a/(a + b) | 82.4% | 64.8% to 92.6% | |
| NPV d/(c + d) | 93.3% | 80.1% to 98.3% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| Results: polyps in the rectosigmoid colon only (high-confidence prediction only), n = 72 polyps | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | NRf | NRf | NRf |
| Index test negative | NRf | NRf | NRf |
| Total | NRf | NRf | 72 |
| Accuracy [(a + d)/(a + b + c + d)] | NRf | ||
| Diagnosis | |||
| Clinical sensitivity a/(a + c) | 96.4% | 79.8% to 99.8% | |
| Clinical specificity d/(b + d) | 95.5% | 83.3% to 99.2% | |
| PPV a/(a + b) | 93.1% | 75.8% to 98.8% | |
| NPV d/(c + d) | 97.7% | 86.2% to 99.9% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| Interpretability of test | NR | ||
| Interobserver agreement | n/a | ||
| Intraobserver agreement | Intraobserver agreement was achieved in 113 out of 121 polyps (93.4%). The κ coefficient of agreement was 0.867 (95% CI 0.799 to 0.967), indicating almost perfect agreement | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | A high-confidence prediction was made for 107 (88.4%) of the 121 polyps | ||
| Low-confidence optical diagnosis | A total of 14 (11.6) of the 121 polyps were predicted with low confidence | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
Surveillance based on European guidelines107 was predicted correctly in 69 out of 73 patients (94.5%); agreement was 68 out of 73 patients (93.2%) based on US guidelines. 103 (Surveillance intervals for polyps in the rectosigmoid colon area are reported but not extracted here) Discrepant surveillance intervals between digital chromoendoscopy and histopathology are reported at patient level but not extracted here. Intervals were longer for digital chromoendoscopy |
||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Yes, the study included patients from two of the population groups relevant for this appraisal and who would receive the test in practice. (Patients identified during screening or surveillance colonoscopies) | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | Each polyp was assessed by an experienced gastrointestinal pathologist | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Each polyp was assessed by an experienced gastrointestinal pathologist blinded to the real-time prediction of polyp histopathology | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | A total of 224 patients were included in the study, but the analysis included only 77 of these (all were described as having distal diminutive polyps). It is possible that the remaining patients had larger-sized polyps located other than in the distal colon, but this is not explicitly stated | No |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant studies identified | ||
Summary reviewer’s comments
Results reflect the use of i-scan in what appears to be a specialist endoscopy centre, by a single experienced endoscopist, to characterise diminutive polyps in the distal colon (i.e. descending colon, the sigmoid colon or the rectum) in patients undergoing screening or surveillance colonoscopy. The majority of predictions were made with high confidence. The authors suggest that studies are needed of less experienced and community physicians.
Repici et al.62
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: distal diminutive polyps First author: Repici Publication year: 2013 Country: Italy and the Netherlands Study design: prospective, multicenter study Number of centres: five Funding: states that software and website support were provided by Olympus. No other financial relationships relevant to this publication were disclosed Competing interests: not stated, but see Funding above |
Index test: available Olympus colonoscopes with HD and NBI were used in all the centres. Model number not stated. Electronic magnification (× 1.5) was allowed if needed. Polyps were detected with white light and then characterised using NBI Reference standard: histopathology |
Number of participants: 278 Sample attrition/dropout: 212 of 278 patients were included in the analysis of surveillance intervals (patients with at least one polyp ≤ 5 mm characterised with high confidence) (i.e. for analysis of PIVI surveillance interval agreement threshold of ≥ 90%). 128/278 patients with polyps ≤ 5 mm in size in the rectosigmoid colon area assessed with high confidence (i.e. for analysis of PIVI NPV threshold of ≥ 90%) Selection of participants: consecutive adult patients referred for elective outpatient colonoscopy (screening, surveillance or diagnostic workup) Inclusion criteria for study entry: detection and retrieval for histopathological examination at least one polyp < 10 mm in size Exclusion criteria for study entry: previous colon resection; IBD; personal history of polyposis syndrome; suspected chronic stricture potentially precluding complete colonoscopy; diverticulitis or toxic megacolon; previous radiation therapy to the abdomen or pelvis; severe cardiovascular, pulmonary, liver or renal disease; and coagulation disorders or use of anticoagulants; incomplete colonoscopy or inadequate bowel preparation |
Primary outcome of study: accuracy of prediction of surveillance intervals, and NPV for adenomatous histopathology in the rectosigmoid colon Other relevant outcomes: diagnostic accuracy (sensitivity, specificity, PPV) Recruitment dates: May 2011–May 2012 |
| Participant characteristics | |||
| Age (years), mean (SD) | 63 (10.4) | ||
| Other key patient characteristics (list) |
Males, n = 160 (58%); and females n = 118 (42%) Clinical indication429/574 (75%) polyps were ≤ 5 mm in size. 226/429 (53%) were located in the rectosigmoid colon tract |
||
| Endoscopist experience and training |
Five experienced endoscopists (one at each centre) performed all colonoscopies in the five selected centres. All had previous experience with HD WLE and NBI A library of endoscopic images and/or videos of NBI-classified hyperplastic and adenomatous polyps < 10 mm was created for endoscopist training. All the collected images and/or videos corresponded to histopathologically verified polyps. An online training course on the differential characteristics between hyperplastic and adenomatous lesions was provided to all endoscopists. At the end of the training, each endoscopist was required to complete a qualifying examination in which an accuracy rate of 80% for differentiating between hyperplastic and adenomatous polyps < 1 cm in size with NBI technology (20 cases) was required. If the operator accuracy was lower than the predefined threshold, the endoscopist had to repeat the training course and the qualifying examination |
||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Criteria are reported in the study publication (table 1), but are not attributed to any named system. For each < 10-mm polyp, the NBI criteria used to characterise the lesion were individually reported. Thereafter, each polyp was classified as type 1 (consistent with a hyperplastic polyp) or type 2 (consistent with an adenoma). The paper stated (p. 112) that most of the NBI individual criteria have been included in the NBI International Colorectal Endoscopic classification The Paris classification system was used to define polyp morphology |
||
| Sample size calculation | At an assumed threshold of 90% agreement for surveillance intervals, 280 patients were required to obtain a 3% precision of the estimates for the per-patient analysis. At an assumed NPV threshold of 90% for adenomatous histopathology of rectosigmoid colon diminutive lesions, 200 polyps were required, at an assumed 50% prevalence of adenomatous histopathology to obtain a 5% precision of the estimates for the per-polyp analysis | ||
| Results: polyps ≤ 5 mm in size (n = 429) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 203a | (b) 31a | 234a |
| Index test negative | (c) 32a | (d) 163a | 195a |
| Total | 235 | 194 | 429 |
| Accuracy [(a + d)/(a + b + c + d)] | 366a/429 (85%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 86% | 82% to 90% | |
| Clinical specificity d/(b + d) | 84% | 79% to 89% | |
| PPV a/(a + b) | 87% | 82% to 91% | |
| NPV d/(c + d) | 84% | 78% to 88% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 5.41a | 3.90 to 7.49a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.16a | 0.12 to 0.22a | |
| Diagnostic odds ratio (a × d)/(b × c) | 33.4a | 19.5 to 57.0a | |
| Results: polyps ≤ 5 mm in size predicted with high confidence (n = 368) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 175a | (b) 21a | 196a |
| Index test negative | (c) 20a | (d) 152a | 172a |
| Total | 195 | 173a | 368 |
| Accuracy [(a + d)/(a + b + c + d)] | 327a/368 (89%, 95% CI 86% to 92%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 90% | 86% to 94% | |
| Clinical specificity d/(b + d) | 88% | 83% to 93% | |
| PPV a/(a + b) | 89% | 85% to 94% | |
| NPV d/(c + d) | 89% | 84% to 93% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 7.39a | 4.94 to 11.07a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.12a | 0.08 to 0.18a | |
| Diagnostic odds ratio (a × d)/(b × c) | 63.3a | 33.1 to 121.3a | |
| Results: polyps ≤ 5 mm in size in the rectosigmoid colon region predicted with high confidence (n = 204) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 53a | (b) 7a | 61a |
| Index test negative | (c) 11a | (d) 133a | 144a |
| Total | 64 | 140a | 204 |
| Accuracy [(a + d)/(a + b + c + d)] | 186a/204 (91%, 95% CI 87% to 95%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 83% | 74% to 92% | |
| Clinical specificity d/(b + d) | 95% | 91% to 99% | |
| PPV a/(a + b) | 88% | 80% to 96% | |
| NPV d/(c + d) | 92% | 88% to 96% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 16.56a | 7.98 to 34.39a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.18a | 0.11 to 0.31a | |
| Diagnostic odds ratio (a × d)/(b × c) | 91.54a | 33.69 to 248.77a | |
| Results: polyps ≤ 5 mm in size predicted with low confidence (n = 61) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 27a | (b) 11a | 38a |
| Index test negative | (c) 13a | (d) 10a | 23a |
| Total | 40 | 21a | 61 |
| Accuracy [(a + d)/(a + b + c + d)] | 37a/61 (61%; 95% CI 49% to 73%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 68% | 54% to 82%b | |
| Clinical specificity d/(b + d) | 48% | 27% to 69%b | |
| PPV a/(a + b) | 71% | 57% to 86%b | |
| NPV d/(c + d) | 43% | 24% to 64%b | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 1.29a | 0.81 to 2.04a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.68a | 0.36 to 1.29a | |
| Diagnostic odds ratio (a × d)/(b × c) | 1.89a | 0.64 to 5.57a | |
| Results: polyps ≤ 5 mm in size in the rectosigmoid colon region predicted with low confidence (n = 22) | |||
| Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total | |
| Index test positive | (a) 5a | (b) 5a | 10a |
| Index test negative | (c) 7a | (d) 5a | 12a |
| Total | 12 | 10a | 22 |
| Accuracy [(a + d)/(a + b + c + d)] | 10a/22 (45%, 95% CI 25% to 66%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 42% | 14% to 70%b | |
| Clinical specificity d/(b + d) | 50% | 19% to 81% | |
| PPV a/(a + b) | 50% | 19% to 81% | |
| NPV d/(c + d) | 42% | 14% to 70%b | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 0.83a | 0.33 to 2.08a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 1.17a | 0.53 to 2.55a | |
| Diagnostic odds ratio (a × d)/(b × c) | 0.71a | 0.13 to 3.87a | |
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | 368/429 polyps ≤ 5 mm (86%) in size were predicted with high confidence | ||
| Low-confidence optical diagnosis | 61/429 polyps (14%) were predicted with low confidence | ||
| Number of polyps designated to be left in place | The discard strategy would have reduced by 48% the need for polypectomy | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
Of 278 patients there were 212 in whom a surveillance interval was able to be given, as a result of patients having at least one polyp ≤ 5 mm in size characterised with high confidence (i.e. simulation of the resect and discard strategy). (Note that this is therefore a lower number of patients than the 280 required in the sample size calculation) When a 5- or 10-year interval for non-advanced adenomas ≤ 2 mm in size was given by using the US guidelines, high-confidence NBI characterisation of polyps ≤ 5 mm predicted the correct surveillance interval in 92% of cases (95% CI 88% to 96%) and 99% of cases (95% CI 97% to 100%), respectively, and in 99% of cases (95% CI 97% to 100%) in accordance with the European guidelines. There were 17 patients with discrepancies between histopathology and NBI in prediction of surveillance intervals. According to the US guidelines (when we admitted a 5-year interval for non-advanced adenomas ≤ 2 mm in size), the NBI-recommended surveillance would have been inappropriately anticipated for 5 of 278 patients (2%) and delayed in 12 of 178c (4%) patients, whereas it would have been delayed in the 3 of 278 (1%) cases misclassified according to the US (with 10-year interval for ≤ 2 non-advanced adenomas) and European guidelines The observed agreement rate between endoscopic and pathological diagnosis appeared to be superior to the 90% threshold set by the ASGE (the PIVI criteria) The resect and discard strategy would have reduced the need for post-polypectomy pathological examination of the resected diminutive polyps by 86% US guidelines:European guidelines: |
||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Patients undergoing colonoscopy as part of screening, surveillance or investigation of symptoms | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | The whole sample | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | All patients were diagnosed with histopathology | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Each polyp was resected and reviewed by a pathologist blinded to the optical diagnosis | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated, but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | A per-patient analysis was performed for the estimation of surveillance intervals. The number of patients included in this analysis depended on whether or not a high-confidence prediction had been made, plus the size of polyps detected | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional studies identified | ||
Summary reviewer’s comments
Results are based on the use of HD NBI in a European (non-UK) population of patients undergoing colonoscopy as part of screening, surveillance or investigation of symptoms. Colonoscopy was performed by experienced endoscopists across five centres trained and qualified in the use of NBI. Predictions were made with high confidence, to inform surveillance intervals and decisions regarding whether or not to resect and discard diminutive polyps, and to leave hyperplastic polyps in the rectosigmoid colon area in situ (i.e. as per the PIVI statement). Surveillance intervals were predicted using US (ASGE) or European guidelines.
Rex64
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: determination of adenomatous vs. hyperplastic or other non-adenomatous polyps First author: Rex Publication year: 2009 Country: USA Study design: prospective cohort Number of centres: one Funding: NR Competing interests: the author disclosed receiving research support and being a member of the speakers bureau for Olympus America Corporation |
Index test: NBI with the Olympus Exera 180 HD colonoscope. Identified polyps were assigned a designation of high or low confidence. A high-confidence prediction was made if the polyp had one or more features associated with one histopathology (either adenomatous or hyperplastic) and no features associated with the other histopathology. A low-confidence prediction was made when there was uncertainty about the features or if there were features of both adenomatous and hyperplastic polyps The × 1.5 electronic magnification was not used if the prediction of polyp histopathology could be made with high confidence without magnification Reference standard: the attending pathologist’s report (histopathology) was accepted as the correct pathology. A subset of 30 polyps were reviewed by a specialist in gastrointestinal pathology who agreed with all the pathologists’ diagnoses |
Number of participants: 136 patients from whom 451 consecutively identified colorectal polyps were resected. The majority of the polyps (n = 395) were ≤ 5 mm in size Sample attrition/dropout: NR but none apparent so believed to be zero Selection of participants: NR Inclusion criteria for study entry: NR Exclusion criteria for study entry: NR |
Primary outcome of study: accuracy of high-confidence endoscopic predictions of adenoma vs. non-adenomatous histopathology for polyps ≤ 5 mm in size Other relevant outcomes: surveillance intervals Recruitment dates: NR |
| Participant characteristics | |||
| Age (years), mean (SD) | NR | ||
| Other key patient characteristics (list) | Total number of polyps = 451
|
||
| Endoscopist experience and training | A single endoscopist (the study author) who had a special interest in colonoscopy undertook the study. This endoscopist first created a library of 320 images that were used to determine polyp features consistently associated with adenomatous or hyperplastic histopathology. This could be considered to be the training received, although it is not described as such. The endoscopist experience in colonoscopy in general or the Olympus Exera HD 180 colonoscope in particular is not described | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | Size and shape (Paris classification) were recorded for each polyp. In addition, this study included an initial phase (not data extracted) in which a library of polyp photographs for 320 individual polyps photographed in both white and then blue light with the Olympus Exera HD 180 colonoscope was constructed. This library was used to determine which features were consistently associated with either adenomatous or hyperplastic polyps confirmed by histopathology. Five predictive features of adenoma are listed and three predictive features for hyperplastic polyps. The presence of these individual features was also recorded for each polyp | ||
| Sample size calculation | Details for a sample size calculation are provided. In the study, the authors report that 80% of polyps removed were ≤ 5 mm in size and approximately half of polyps ≤ 5 mm were adenomatous. It was estimated (based on a library of images from 320 polyps) that at least 80% of endoscopic determinations of polyp histopathology would be made with high confidence. The study author calculated that assuming accuracy of 93% for high-confidence interpretations, with a CI of ± 3%, a total of 278 polyps of ≤ 5 mm in size would need to be examined prospectively with high confidence or a total of 348 polyps ≤ 5 mm in size would need to be examined. The study authors also wanted to assess the association of accuracy with polyp size. For this, consecutive polyps (including those > 5 mm in size) were assessed and their histopathology estimated. It was calculated (based on knowing that 80% of polyps would be ≤ 5 mm in size) that a total sample size of 435 consecutive polyps would be required and a sample size of 450 polyps was chosen | ||
| Results for all polyps ≤ 5 mm in size | |||
| Adenomatous polyps on histopathology | Hyperplastic or other non-adenomatous polyps on histopathology | Total | |
| Index test positive | 178a (a) | 28a (b) | 206 (a + b) |
| Index test negative | 17a (c) | 172a (d) | 189 (c + d) |
| Total | 195 (a + c) | 200 (b + d) | 395 (a + b + c + d) |
| Accuracy [(a + d)/(a + b + c + d)] | 88.6%a | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 91.28%a | 86.41% to 94.84%a | |
| Clinical specificity d/(b + d) | 86.00%a | 80.41% to 90.49%a | |
| PPV a/(a + b) | 86.41%a | 80.96% to 90.77%a | |
| NPV d/(c + d) | 91.01%a | 85.99% to 94.67%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 6.52a | 4.61 to 9.22a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.10a | 0.06 to 0.16a | |
| Diagnostic odds ratio (a × d)/(b × c) | 64.32a | 33.98 to 121.74a | |
| Endoscopic predictions of hyperplastic polyps were scored as being correct if the polyps were histopathologically hyperplastic or other non-adenomatous tissue | |||
| Results for polyps ≤ 5 mm in size with high-confidence predictions | |||
| Adenomatous polyps on histopathology | Hyperplastic or other non-adenomatous polyps on histopathology | Total | |
| Index test positive | 145 (a) | 15a (b) | 160 (a + b) |
| Index test negative | 7a (c) | 147 (d) | 154 (c + d) |
| Total | 152a (a + c) | 162a (b + d) | 314a (a + b + c + d) |
| Accuracy [(a + d)/(a + b + c + d)] | 93.0%a | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 95.39%a | 90.74% to 98.13%a | |
| Clinical specificity d/(b + d) | 90.74%a | 85.19% to 94.72%a | |
| PPV a/(a + b) | 90.62%a | 85.01% to 94.66%a | |
| NPV d/(c + d) | 95.45%a | 90.86% to 98.15%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 10.30a | 6.35 to 16.71a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.05a | 0.02 to 0.10a | |
| Diagnostic odds ratio (a × d)/(b × c) | 203.0a | 80.41 to 512.46a | |
|
Endoscopic predictions of hyperplastic polyps were scored as being correct if the polyps were histopathologically hyperplastic or other non-adenomatous tissue For six (from a total of 15) polyps read with high confidence, but called normal tissue after histopathology, the tissue blocks were recut and two showed adenoma in the recut tissue |
|||
| Results for polyps ≤ 5 mm in size with low-confidence predictions | |||
| Adenomatous polyps on histopathology | Hyperplastic or other non-adenomatous polyps on histopathology | Total | |
| Index test positive | 33 (a) | 13a (b) | 46 (a + b) |
| Index test negative | 10a (c) | 25 (d) | 35 (c + d) |
| Total | 43a (a + c) | 38a (b + d) | 81a (a + b + c + d) |
| Accuracy [(a + d)/(a + b + c + d)] | 71.6%a | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 76.74%a | 61.37% to 88.24%a | |
| Clinical specificity d/(b + d) | 65.79%a | 48.65% to 80.37%a | |
| PPV a/(a + b) | 71.74%a | 56.54% to 84.01%a | |
| NPV d/(c + d) | 71.43%a | 53.70% to 85.36%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 2.24a | 1.40 to 3.59a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.35a | 0.20 to 0.64a | |
| Diagnostic odds ratio (a × d)/(b × c) | 6.346a | 2.395 to 16.817a | |
| Endoscopic predictions of hyperplastic polyps were scored as being correct if the polyps were histopathologically hyperplastic or other non-adenomatous tissue | |||
| Interpretability of test | NR | ||
| Interobserver agreement | n/a as only a single endoscopist | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | For all polyps and for the polyps ≤ 5 mm in size predicted to be adenomas, high-confidence predictions were more likely than low-confidence predictions to be accurate (p < 0.001, chi-squared test). High-confidence predictions of hyperplastic polyps were also more likely than low-confidence predictions to be accurate for all polyps and for polyps ≤ 5 mm in size (p < 0.001, chi-squared test) | ||
| High-confidence optical diagnosis: all polyps |
368/451 (81.6%)a predictions were made with high confidence 193/240 (80.4%) polyps predicted to be adenomas were predicted with high confidence 175/211 (82.9%) polyps predicted to be hyperplastic were predicted with high confidence |
||
| High-confidence optical diagnosis: polyps ≤ 5 mm |
314/395 (79.5%)a predictions were made with high confidence 160/206a (77.7%) polyps ≤ 5 mm predicted to be adenomas were predicted with high confidence 154/189 (81.5%) polyps ≤ 5 mm predicted to be hyperplastic were predicted with high confidence |
||
| Low-confidence optical diagnosis: all polyps |
83/451 (18.4%)a predictions were made with low confidence 47/240 (19.6%)a polyps predicted to be adenomas were predicted with low confidence 36/211 (17.1%)a polyps predicted to be hyperplastic were predicted low confidence |
||
| Low-confidence optical diagnosis: polyps ≤ 5 mm |
81/395 (20.5%)a predictions were made with low confidence 46/206a (22.3%)a polyps ≤ 5 mm predicted to be adenomas were predicted with low confidence 35/189 (18.5%)a polyps ≤ 5 mm predicted to be hyperplastic were predicted with low confidence |
||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | The US Multi-Society Task Force – American Cancer Society guideline104 was used to guide recommended follow-up intervals. The pathology-based recommendations used the pathologist’s report for each polyp. The endoscopic-based recommendations used the endoscopic prediction of histopathology for polyps of ≤ 5 mm if it was a high-confidence prediction. If the polyp was ≤ 5 mm, but endoscopically predicted histopathology was made with low confidence or if the polyp was > 5 mm in size, then the histopathological diagnosis was used. It was assumed that all polyps > 5 mm in size would be sent to histopathology | ||
| Assumption for recommended surveillance interval that clinical practice would be to perform colonoscopy in 5 years for the finding of one or two tubular adenomas < 1 cm in size |
For 128/136 (94%) of patients the recommendations for follow-up colonoscopy based on histopathology and endoscopic prediction were identical For the eight patients where the recommendations differed between histopathology diagnosis and endoscopic prediction of polyps, follow-up intervals, endoscopy-based recommendations were longer in four cases and shorter in four cases |
||
| Assumption for recommended surveillance interval that clinical practice would be to perform colonoscopy in 10 years for the finding of one or two tubular adenomas < 1 cm in size |
For 134 out of 136 (98.5%) of patients the recommendations for follow-up colonoscopy based on histopathology and endoscopic prediction were identical For the three patients where the recommendations differed between histopathology diagnosis and endoscopic prediction of polyps, follow-up intervals for endoscopy-based recommendations were longer in one case and shorter in two cases Reviewer note: there is a discrepancy in the paper, which reports 134/136 recommendations as identical but identifies three patients where the recommendation differs |
||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Basic patient details (e.g. age, sex) are not reported. The reason(s) for patients having a colonoscopy are also not reported, although the focus of the study appears to be on screening and surveillance | Unclear |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The VCE assessment and polyp resection for histopathology occurred during the same colonoscopy | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All resected polyps were assessed by histopathology | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Pathologists (number not stated) were blinded in all cases to the endoscopic prediction of histopathology | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The prediction of histopathology was made before the results of histopathology could be known | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | Not stated but believed to be zero | No |
| 11 | Were withdrawals from the study explained? | Not explicitly stated but believed to be zero | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional references found | ||
Summary reviewer’s comments
A single endoscopist with a special interest in colonoscopy obtained these results from a patient population that was not described. It is therefore not clear which patients these results apply to and whether or not the same results could be obtained by other endoscopists. Furthermore, the equipment used (Olympus Exera 180 HD colonoscope) was one of the first with HD and NBI capability, but may since have been superseded by newer instruments with increased capabilities.
Rogart et al.74
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: white light with NBI for the differentiation of adenomatous from non-adenomatous colorectal polyps during real-time colonoscopy First author: Rogart Publication year: 2008 Country: USA Study design: prospective study Number of centres: one (tertiary referral centre at Yale University) Funding: not stated Competing interests: none declared. |
Index test: white light with NBI Olympus CF-H180AL colonoscopes (Olympus Corp, Centre Valley, Pa) were used with Evis Exera II CV-180 processors (Olympus), with a xenon lamp as a light source and a colour charge-coupled device providing HD picture (1080 horizontal lines of resolution) when used with an HD monitor. Activation and deactivation of the double-band NBI filter (415 nm and 540 nm ± 30 nm) is by pushing a button on the handle of the colonoscope The processor is also equipped with a × 1.5 electronic magnification feature that can be activated with a separate button on the colonoscope and provides up to × 70 total magnification The location, size and shape of polyps were recorded and images were electronically magnified to × 1.5 the standard magnification. The endoscopist predicted the polyp type (adenoma, cancer or non-adenomatous) and level of confidence (low or high). Under the same magnification, NBI was activated, and the polyp was re-evaluated Reference standard: histopathology |
Number of participants: 131 (302 enrolled, of which 171 patients had no polyp) Sample attrition/dropout: selection of participants: consecutive individuals referred for routine colonoscopy to one of the study physicians Inclusion criteria for study entry: only inclusion criteria was individuals referred for routine colonoscopy Exclusion criteria for study entry: known or suspected familial polyposis syndromes; acute GI bleeding; international normalised ratio > 2.0 or platelets < 50,000/mm3 |
Primary outcome of study: not stated Other relevant outcomes (described as main outcomes): overall accuracy, sensitivity and specificity of endoscopic diagnosis by using white light alone and with NBI; improvement in endoscopists’ performance Also reported was interobserver agreement of 20 test images (not data extracted) Recruitment dates: August 2006 and July 2007 |
| Participant characteristics (n = 131; 265 polyps) | |||
| Age (years), mean (SD) [range] | 59 (10.0) [27–79] | ||
| Other key patient characteristics (list) | Male, n/N (%): 85/131 (65)
|
||
| Endoscopist experience and training |
Four experienced endoscopists (≥ 1000 colonoscopies previously performed, range 1000–10,000), without extensive experience with NBI or chromoendoscopy Before the study began, the endoscopists attended a 1-hour interactive lecture on NBI. They were also given an ‘atlas’ showing endoscopic images of polyps examined with both chromoendoscopy and NBI. Laminated reference sheets with classifications, pictures and sketches were posted in each endoscopy room. Each endoscopist completed a pre-test on a separate day, consisting of 20 unknown polyps photographed with the NBI system and received fortnightly feedback about the accuracy of their endoscopic predictions compared with the histopathological diagnosis throughout the study. After enrolment, the endoscopists completed a post-test involving the same 20 unknown polyps, which had been randomly re-ordered (mean score pre-test; mean score post-test 95%; p = 0.55) |
||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Simplified Kudo pit pattern classification (reference provided in paper; stated that it ‘cannot yet be validated for NBI’ as it has been for chromoendoscopy) and VCI grading Endoscopists classified polyps as modified Kudo A (Kudo pit pattern I or II, suggests non-adenomatous) or Kudo B (Kudo pit patterns III-V, suggests adenomatous polyp or cancer) and then specified a specific pit pattern (I–V). The VCI was graded by examining the mucosal hue of the polyp under NBI: light (same colour as surrounding mucosa), medium (mildly darker than surrounding mucosa, overall light-brown appearance) and dark (much darker than surrounding mucosa, dark brown or black in appearance). Image quality (good, fair or poor) was also recorded (not data extracted) Polyp classification system for histopathological classification not explicitly reported but authors refer to three references when describing the adenomatous and serrated categories |
||
| Sample size calculation | NR | ||
| Results | |||
| NBI subgroup: 1–5 mm | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total |
| Index test positive | 71a | NR | NRb |
| Index test negative | 24a | NR | NRb |
| Total | (a + d) 95 | (b + d) 126 | (a + b + c + d) 265 |
| Accuracy [(a + d)/(a + b + c + d)] | NR | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 75% | NR | |
| Clinical specificity d/(b + d) | NR | NR | |
| PPV a/(a + b) | NR | NR | |
| NPV d/(c + d) | NR | NR | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
| All endoscopists had approximately equal accuracy with NBI by the end of the study (improved by 13% from the first to the second half of the study; p < 0.05). However, also stated that three of the endoscopists showed significant improvements in diagnostic accuracies during the study, whereas one showed no change | |||
| Interpretability of test | NR | ||
| Interobserver agreement | Interobserver agreement was reported for 20 test images but not real-time assessment (not data extracted) | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | NR for the 1- to 5-mm subgroup | ||
| Low-confidence optical diagnosis | NR for the 1- to 5-mm subgroup | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Majority of patients were undergoing colonoscopy for screening, surveillance (history of polyps) or due to having symptoms suggestive of colorectal cancer | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All polys found were histopathologically assessed | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Two pathologists (with either expertise or special interest in gastrointestinal pathology) were blinded to the endoscopic images and predictions | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | No | |
| 11 | Were withdrawals from the study explained? | No withdrawal apparent | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant publications were identified | ||
Summary reviewer’s comments
The population sample was based on patients from the USA undergoing routine colonoscopy and it is unclear how representative this sample is of the patient population in the UK (age range 27–79 years), and how similar endoscopists training is compared with training received in the NHS. Study was performed in a single academic centre, so the results may not be applicable to a wider range of settings.
Shahid et al.75
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: comparison of pCLE and NBI for predicating histopathology of small colorectal polyps (< 10 mm), including combining both methods against histopathology First author: Shahid Publication year: 2012 Country: USA Study design: prospective cohort Number of centres: one (tertiary referral hospital) Funding: none reported. One of the authors receives research grant support from Mauna Kea Technologies, Olympus and Fujinon Corporations Competing interests: stated none, but see funding above |
Index test: HD colonoscope (CFH180 or PCF H 180, Olympus, Centre Valley, NY, USA), processor (CV 180 Excera, Olympus), HD monitor and 4-mm clear cap distal attachment (Olympus D-201–15004) pCLE details not data extracted, as not real time Polyps were first screened by white-light, HD colonoscopy. At first polyp (either during advancement or withdrawal, before or after caecal intubation), the mucus was washed away and the NBI mode was used to make a diagnosis, with the endoscopist blinded to pCLE imaging Reference standard: histopathology |
Number of participants: 65 Sample attrition/dropout: no dropouts reported Selection of participants: consecutive patients were recruited in a tertiary referral centre Inclusion criteria for study entry: aged ≥ 18 years, with polyps < 10 mm during surveillance or screening colonoscopies Exclusion criteria for study entry: non-corrected coagulopathy, pregnancy, breastfeeding, documented allergy to fluorescein, patients with no colorectal lesions found during a study colonoscopy, and any patient previously reported on by the authors |
Primary outcome of study: not stated Other relevant outcomes: sensitivity, specificity, accuracy, PPV, NPV, and positive and negative likelihood ratios of pCLE and NBI for predicting histopathology (neoplastic vs. non-neoplastic) Recruitment dates: April 2008 to April 2010 |
| Participant characteristics | |||
| Age (years), median (range) | 69 (44–91) | ||
| Other key patient characteristics (list) | Male, n/N (%): 40/65 (62) | ||
| Caucasians, n (%): 64 (98.5) | |||
| Number of colorectal lesions: 130 | |||
Number of polyps, n (%):
|
|||
| 103 polyps were sized 1–5 mm. Of these, 45 were neoplastic and 58 were non-neoplastic | |||
| Endoscopist experience and training | One endoscopist, who was an expert in advanced imaging methods and had performed ≥ 100 pCLE procedures, conducted all examinations. Unclear how experienced the endoscopist was with NBI | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
Surface pit pattern of the lesion was classified in accordance with Kudo criteria as modified by Sano et al. 85 for NBI (criteria were developed using magnification endoscopes, not used in this study). Round and stellate pit and vascular patterns represented benign, hyperplastic lesion, and villiform, gyrus-like irregular patterns represented neoplastic lesions. The anatomical site and morphological class of legions was recorded during the procedure according to the Paris classification Intraepithelial neoplasia was assessed by the pathologist using modified Vienna criteria |
||
| Sample size calculation | NR. A stated limitation of the study was the relatively small sample size. This meant that there was a lack of power to detect differences in accuracy, sensitivity and specificity between methods | ||
| Results | |||
| NBI subgroup (1–5 mm) | Adenomatous polyps on histopathologya | Hyperplastic polyps on histopathologyb | Total |
| Index test positive | (a) 27 | (b) 3c | 30 |
| Index test negative | (c) 18c | (d) 55 | 73 |
| Total | 45 | 58 | 103 |
| Accuracy [(a + d)/(a + b + c + d)] | 80% (95% CI 70% to 87%) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 60% | 45% to 73% | |
| Clinical specificity d/(b + d) | 95% | 85% to 98% | |
| PPV a/(a + b) | 90% | 72% to 96% | |
| NPV d/(c + d) | 75% | 62% to 84% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 11.60 | 3.76 to 35.82c | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.42 | 0.29 to 0.61c | |
| Diagnostic odds ratio (a × d)/(b × c) | 27.500c | 7.449 to 101.528c | |
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | Stated that none of the patients experienced any endoscopic complications. Most patients had transient yellow discoloration of the skin and urine, resolved within 1–2 hours for skin and 24 hours for urine | ||
| High-confidence optical diagnosis | NR | ||
| Low-confidence optical diagnosis | NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | NR | ||
| Length of time to perform the colonoscopy | Not specifically stated. NBI inspection time was typically < 1 minute. The average withdrawal time during most colposcopy procedures at the centre was 8–10 minutes (generally, not specifically in this study), making a procedure, at a minimum, > 11 minutes | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Patients were referred for screening and surveillance colonoscopies | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | The whole sample received verification using the intended reference standard | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Stated that all tissue specimens were examined by a gastrointestinal pathologist, blinded to the probe-based confocal laser endomicroscopy information. Presumed that this also applied to the results of the NBI | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Stated that per routine practice, only the site and anatomic location was provided | Yes |
| 10 | Were uninterpretable/intermediate test results reported? | No | |
| 11 | Were withdrawals from the study explained? | While not specifically stated, there appear to have been no withdrawals | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant publications were identified | ||
Summary reviewer’s comments
Patients were American with an age range of 44–91 years, recruited in a tertiary referral hospital. It is unclear how representative this US population is compared with a UK population, considering the age (median age 69 years) and ethnicity (98.5% Caucasian) of those included in the study. Included patients were undergoing screening and surveillance colonoscopies, but exact indication for colonoscopy were not provided. Therefore, it is unclear how relevant the patient population in this study is to the population of interest in this appraisal.
Sola-Vera et al.65
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: accuracy of optical diagnosis of diminutive colon polyps and of (secondary aims) < 10-mm polyps, and usefulness of optical diagnosis as a tool for predicting future colonoscopy surveillance interval First author: Sola-Vera Publication year: 2015 Country: Spain Study design: prospective cohort study Number of centres: one (endoscopic unit in medium-sized academic public hospital with 450 beds) Funding: none Competing interests: first author was collaborating with Olympus Iberia in training courses on optical diagnosis |
Index test: NBI. Exera II (Olympus Medical System, Tokyo, Japan) processor and HD monitors in three examination rooms; one room equipped with a processor not suitable for optical diagnosis (no HD processor). CF-H180AL (HD) and CF-Q180AL (high-resolution) Olympus colonoscopies were used (no statistical significant differences in results between endoscopes, p = 0.4) One photo with NBI and another with white light were taken of all the polyps. Endoscopists scored the polyps and registered the confidence level and if possible, recommended a surveillance interval at the end of the procedure, and for each polyp recorded the location, estimated the size (compared with open biopsy forceps or snare sheath) and the morphology (Paris classification). Polyp characteristics were evaluated in real time (i.e. not by using photos) Reference standard: histopathology |
Number of participants: 195 (822 patients submitted for colonoscopy, reasons for exclusion of 627 patients provided; 90/195 patients included for surveillance intervals, reasons for exclusions only provided for 101 patients) Sample attrition/dropout: none reported Selection of participants: consecutive adults patients referred for colonoscopy Inclusion criteria for study entry: patients aged > 18 years Exclusion criteria for study entry: patients examined in the room containing the equipment not suitable for optical diagnosis; rectosigmoidoscopy was requested; patients without polyps; patients with an obvious colon cancer without simultaneous polyps Exclusion criteria for the purposes of predicting future colonoscopy surveillance intervals were: preparation of the colon not adequate (poor or inadequate, Aronchick scale); incomplete colonoscopy, hereditary polyposis syndromes; personal history of IBD; obvious colorectal cancer detected without polyps; and some polyps not resected and/or not recovered |
Primary outcome of study (described as main outcomes): sensitivity, specificity, NPV and PPV, likelihood ratios and diagnostic odds ratio of diminutive and small adenomatous polyps, all predictions and those made with high confidence Other relevant outcomes (described as secondary outcomes): accuracy of optical diagnosis as a function of size and location of polyps, dedication of endoscopists and type of endoscope (not data extracted). The correlation between optical diagnosis and pathological diagnosis when recommending a follow-up interval after colonoscopy Recruitment dates: November 2013 and January 2014 |
| Participant characteristics (total sample) | |||
| Age (years), mean (SD) | 64.0 (12.4) | ||
| Other key patient characteristics (list) | Male, %: 55.9 | ||
Reason for colonoscopy, n (%):
|
|||
| Diminutive (≤ 5 mm) polyps, n/N (%): 219/401 (54.6) – three could not be recovered, final sample n = 216 | |||
| Endoscopist experience and training |
Five expert endoscopists were divided into two categories according to their dedication to endoscopy (two full time and three part time, i.e. < 30% of annual working time). All had completed > 5000 colonoscopies, but only one had experience in the characterisation of colon polyps with NBI. All endoscopists received training on the characterisation of colon polyps with NBI using the NBI International Colorectal Endoscopic classification on still images (a pre-test, a learning phase and a post-test) and all achieved 90% accuracy for optical diagnosis in the post-test. It was recommended that all parameters taken during the procedure were dictated to a nurse in real time During the study endoscopists were encouraged to compare the pathological diagnosis with their optical diagnosis prediction, in a continuous process of self-learning |
||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) |
NBI International Colorectal Endoscopic classification (type 1, hyperplastic polyp; type 2, adenomatous polyp; type 3, cancer with deep submucosal invasion). Paper stated that for purposes of analysis, all sessile serrated and traditional adenomas were considered as non-adenomatous in this study, as the NBI International Colorectal Endoscopic classification includes them in the same category as hyperplastic polyps During endoscopy polyp size, location and the morphology were determined according Paris classification Pathologist followed the World Health Organization’s154 classification for digestive tumours and the histopathological diagnosis was standardised in all cases |
||
| Sample size calculation | Stated that 239 polyps < 10 mm were needed, assuming a sensitivity of optical diagnosis of 91%. Assuming that 80% of the predictions would be made with high confidence, the total number of polyps < 10 mm needed was 299. This figure as increased by 5% to compensate for possible losses – 315 polyps < 10 mm were identified but 4 could not be recovered, leaving at total of 311 | ||
| Results | |||
| All predictions for the subgroup diminutive polys (n = 216) | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total |
| Index test positive | (a) 85 | (b) 8a | 93a |
| Index test negative | (c) 70a | (d) 53 | 123a |
| Total | 155 | 61 | 216 |
| Accuracy [(a + d)/(a + b + c + d)] | 63.9% (138/216) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 55% | 47% to 63% | |
| Clinical specificity d/(b + d) | 87% | 78% to 96% | |
| PPV a/(a + b) | 91% | 85% to 98% | |
| NPV d/(c + d) | 43% | 34% to 52% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 4.18 | 2.16 to 8.1 | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.52 | 0.43 to 0.63 | |
| Diagnostic odds ratio (a × d)/(b × c) | 8.04 | 3.59 to 18.05a | |
| High-confidence predictions for the subgroup diminutive polys (n = 166) | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total |
| Index test positive | 67 | 4a | 71a |
| Index test negative | 47a | 44 | 91a |
| Total | 114 | 48 | 162 |
| Accuracy [(a + d)/(a + b + c + d)] | 68.5% (111/162) | ||
| Diagnosis | Value | 95% CIb | |
| Clinical sensitivity a/(a + c) | 59% | 50% to 69% | |
| Clinical specificity d/(b + d) | 92% | 83% to 100% | |
| PPV a/(a + b) | 95% | 89% to 100% | |
| NPV d/(c + d) | 48% | 37% to 59% | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 7.12 | 2.75 to 18.41 | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.44 | 0.35 to 0.56 | |
| Diagnostic odds ratio (a × d)/(b × c) | 16.2 | 5.275 to 46.61a | |
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | High-confidence diagnosis if the polyps had one or more characteristics of one type and none of the other. 166/216 (76.9%) of the prediction of the histopathology of the diminutive polyps were made with high confidence | ||
| Low-confidence optical diagnosis | Although not specifically stated it can be deducted that 50 out of 216 (23.1%) of the prediction of the histopathology of the diminutive polyps were made with low confidence | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval | Surveillance intervals were based on histopathology and optical diagnosis using the European107 and ESGE108 guidelines and could only be made for 90/195 patients (i.e. 46% calculated by reviewer). Agreement of histopathology and optical diagnosis for diminutive polyps based on a possible 47 cases were the same for follow-up for 46/47 (97.8%) for both guidelines (European guidelines107 and ESGE guidelines108). Surveillance intervals are only provided for the total sample (n = 90) (not data extracted) and not reported separately for patients with diminutive polyps | ||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Majority of patients referred for screening, surveillance colonoscopy or colonoscopy to investigate symptoms suggestive of colorectal cancer | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | The whole sample received verification using the intended reference standard | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Stated that pathologist did not know the endoscopist prediction for each polyp | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Endoscopists would not have known the histopathology results for the polyp when they made their prediction | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Polyps were sent in a separate for histopathological analysis | Yes |
| 10 | Were uninterpretable/intermediate test results reported? | No | |
| 11 | Were withdrawals from the study explained? | Although not specifically stated, there appear to have been no withdrawals | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant reverences were identified | ||
Summary reviewer’s comments
The population sample was based on patients from Spain and it is unclear how representative the population is of the patient population in the UK, and how similar endoscopists training is compared with training received in the NHS. Only one of the five endoscopists in this study had experience in using NBI. The study was performed in a single centre, so the results may not be applicable to a wider range of settings. Patients were scheduled to undergo colonoscopy, but in over 20% of patients exact indication for colonoscopy was not provided. Around 80% of patients in the study had indication relevant to the appraisal.
Vu et al.76
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: comparison of surveillance interval recommendations and diagnostic performance between resect and discard and standard of care (histopathology) of diminutive polyps First author: Vu Publication year: 2015 Country: USA Study design: prospective cohort Number of centres: one (hospital outpatient endoscopy centre) Funding: none reported Competing interests: none for seven authors; GSS, grant (K23 DK84113); SAE, consultant and medical advisory board, Olympus corporation) |
Index test: HD white light or NB (at the discretion of the endoscopist) Real-time imaging using HD white light or NBI (polyps are resected and discarded rather than being sent for pathological review) The colonoscopes used were Olympus CF-H180 AL with HD white light and NBI capability in conjunction with the Evis Evera II CV-180 video processor and OEV 191H 19-inch HD monitor (Olympus America Inc, Centre Valley, PA) Reference standard: histopathology |
Number of participants: 315 (618 patients underwent colonoscopy, 303 excluded: 262 without diminutive polyps, 35 with poor bowl preparation and six with no histopathological diagnosis) Sample attrition/dropout: none reported Selection of participants: consecutive patients undergoing colonoscopy for CRC screening or surveillance indications Inclusion criteria for study entry: adults (no age criteria stated) identified with diminutive polyps (defined as ≤ 5 mm in size) at colonoscopy Exclusion criteria for study entry: colonoscopy was performed for an indication other than screening or surveillance; no diminutive polyps were found; an optical or histopathological diagnosis of the diminutive polyp could not be made; the polyp was resected but not retrieved for histopathology; a synchronous CRC was identified at the time of the colonoscopy; post hoc diagnoses of polyposis syndromes and IBD were made; colonoscopy was not complete to caecum; fair or poor bowel preparation (defined as a BBPS score) |
Primary outcome of study: concordance of recommended surveillance intervals [(1) endoscopist’ prediction of diminutive polyps by optical diagnosis using HDWL and/or NBI and (2) final histopathological diagnosis] Other relevant outcomes: accuracy, sensitivity, specificity, PPV and NPV of histopathology predictions by optical diagnosis using HD white light with/without NBI Subgroup analyses: diagnostic performance by level of confidence in prediction, type of endoscopist (academic vs. community), and use of NBI (not data extracted) Recruitment dates: October 2011 and October 2012 |
| Participant characteristics (n = 315; 606 diminutive polyps) | |||
| Age (years), mean (SD) | 62.4 (8.7) | ||
| Other key patient characteristics (list) | Male, n/N (%): 161/315 (51) (n calculated by reviewer) | ||
Indication, n (%):
|
|||
| Mean size polyp, mm (SD): 3.64 (1.04) | |||
Polyp location, %:
|
|||
| Endoscopist experience and training |
Four academic and two community gastroenterologists. All were highly experienced and had performed > 5000 colonoscopies each Endoscopists formally reviewed images of surface patterns of adenomatous and non-adenomatous polyps in HD white light and NBI using a validated study image set prior to the study (reference provided in paper) at study onset, as well as attending a formal structured teaching session led by the senior author (DSE) to review the polyp surface mucosal and vascular patterns and pit patterns of adenomatous and non-adenomatous lesions. HD white-light and NBI images of multiple polyps were then reviewed and discussed in detail until all endoscopists were confident in their recognition. The image set was also available to endoscopists at all times including in each procedure room for self-review throughout the study |
||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | None stated. All resected polyps were processed in standard fashion. Polyps were classified into adenoma or non-adenomatous polyp, which included hyperplastic polyps, inflammatory polyps or normal mucosa. For purposes of analysis, sessile serrated adenomas/polyps were grouped with adenomas given that surveillance recommendations for these lesions are similar to that of adenomas | ||
| Sample size calculation | Stated that testing the null hypothesis that the proportion positive was identical in the two populations, a proposed sample size of 300 patients was determined for the study to have power of 89.7% to yield a statistically significant result when the criterion for significance was set at alpha of 0.05 and a two-tailed testing was applied | ||
| Results | |||
| NBI | Adenomatous polyps on histopathology | Hyperplastic polyps on histopathology | Total |
| Index test positive | (a) | (b) | a + b |
| Index test negative | (c) | (d) | c + d |
| Total | a + c | b + d | 388 |
| Accuracy [(a + d)/(a + b + c + d)] | 73.9% | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | NR | NR | |
| Clinical specificity d/(b + d) | NR | NR | |
| PPV a/(a + b) | NR | NR | |
| NPV d/(c + d) | NR | NR | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | NR | NR | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | NR | NR | |
| Diagnostic odds ratio (a × d)/(b × c) | NR | NR | |
|
Histopathological prediction could be made for 580/606 (95.7%) of diminutive polyps, with high confidence in 74.2%. NBI was used in 64% of these predictions, but it is unclear if this refers to overall histopathological prediction made for diminutive polyps or those made with high confidence NBI failed to improve prediction accuracy in high-prediction confidence cases (78.6%) and in low-prediction confidence cases (60.8%) Variability in the use of NBI ranged from 3.4% to 88.4%, with lower NBI use among community than with academic endoscopists (13.2% vs. 75.8% of cases; p < 0.001) |
|||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis |
High-confidence accuracy was calculated using high-confidence predictions defined as visual analogue scale score ≥ 7 High-confidence accuracy with NBI: 78.6% |
||
| Low-confidence optical diagnosis | Low-confidence accuracy with NBI: 60.8% | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
Surveillance intervals (based on the US Multi-Society Task Force guidelines for colorectal surveillance101,103) for patients with:Confidence in NBI prediction (mean visual analogue scale score): 7.6 (SD 3.2) Concordance in surveillance interval recommendations: 84.1% with NBI (calculated using high-confidence predictions defined as visual analogue scale ≥ 7) |
||
| Length of time to perform the colonoscopy | NR | ||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Adult outpatients undergoing colonoscopy for colorectal cancer screening or surveillance indications | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | All resected polyps were processed in standard fashion and interpreted by histopathologists | Yes |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Yes | |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | Histopathologists were blinded to the polyp predictions | Yes |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | No | |
| 11 | Were withdrawals from the study explained? | Although not specifically stated, there appear to have been no withdrawals | Yes |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant publications were identified | ||
Summary reviewer’s comments
The population sample was based on patients from the USA who were undergoing colonoscopy for routine clinical indications (surveillance and screening). Endoscopists were a mixture of academic and community gastroenterologists and it is unclear how similar their training is compared with training received in the NHS. Study was performed in a single centre, so the results may not be applicable to a wider range of settings.
Wallace et al.63
| Reference and design | Diagnostic tests | Participants | Outcome measures |
|---|---|---|---|
|
Condition being diagnosed/detected: differentiation of neoplastic from non-neoplastic polyps. Aim of study was to compare dual-focus colonoscopy with standard colonoscopy with respect to the ASGE guidelines First author: Wallace Publication year: 2014 Country: USA Study design: RCT, with one arm relevant to our review Number of centres: one (an academic medical centre ambulatory surgical centre) Funding: Olympus Corporation of America Competing interests: one of the authors (MW) received research funding from Olympus, BSCI, Fujinon, Ninepoint Medical Rieger-Johnson, and Exact Sciences. CA received grants from Olympus Inc. AK received grants from GlaxoSmithKline and Gilead Sciences. JC received funding from Boston Scientific, Olympus, GI Supply and Masimo Corporation. EB received funding from Rhythm Pharmaceuticals Inc. Another from Abbott Laboratories. The final author received grants from Pfizer (MP) |
Index test: HD white-light imaging and NBI. Olympus CF-H180 and Exera II 180 colonoscopes, Olympus HD white-light imaging and NBI dual-focus colonoscopy (Olympus CF-HQ190 and Exera III 190 colonoscopes, Olympus) was used in the other study arm, but data have not been extracted from this arm as near focus (i.e. magnification) was used Reference standard: histopathology |
Number of participants: 264 study completers in the 180 arm (296 were randomised to this arm). Number of participants in the diminutive polyps subgroup analyses NR Overall in the study, 600 patients were enrolled and 593 were randomised Sample attrition/dropout: 32 (11%a) patients in the 180 arm were excluded after randomisation The most common reasons for exclusion post-randomisation in the total sample (n): scheduling difficulties (16) and lack of paediatric 190 colonoscope with anatomic issues (25). Breakdown of reasons not provided for each arm separately Selection of participants: patients at ‘average risk’ undergoing colonoscopy were considered for the study Inclusion criteria for study entry: as above Exclusion criteria for study entry: acute bleeding or active colitis; family or a personal history of polyposis syndrome; history of IBD; previous bowel surgery; inadequate bowel preparation |
Primary outcome of study: accuracy (neo-plastic vs. non-neoplastic) Other relevant outcomes: diagnostic sensitivity, specificity, NPV, PPV and surveillance intervals. Study also provides data on procedure times, confidence levels and subgroup analyses of ≤ 5-mm and rectosigmoid colon diminutive polyps Recruitment dates: NR |
| Participant characteristics | |||
| Age (years), median (minimum, 25th percentile, 75th percentile, maximum) | 60 (33, 55, 70, 85) | ||
| Other key patient characteristics (list) |
375 patients had at least one polyp identified, but of these patients, three had no histopathology = 372 patients in the overall final sample for analysis In total, 927 polyps (from 372 patients) were analysed, although table 4 states 963 polyps were characterised. Of the 488 polyps characterised, 321 (66%) diminutive polyps (≤ 5 mm) were characterised in the 180 NBI arm. Of these, 310 were included in the statistical analyses of diminutive polyps data. Data in table 5 (p. 1078) shows 10 diminutive polyps not assessed by histopathology, and footnote to table 6 (p. 1079) shows one patient missing predicted pathology for white-light imaging only (states polyp removed from analysis) Polyp shape: of the 321 identified diminutive polyps, 265 (83%) were sessile, 54 (17) flat and three other (< 1%). Histopathology: 159 (50%) were non-neoplastic and 152 (47%) were neoplastic Gender, female, n (%): 112 (42%) (180 NBI arm, all polyps) Reasons for this colonoscopy, n (%): routine 122 (46%), surveillance 114 (43%), diagnosis 27 (10%), and other 1 (< 1%) (180 NBI arm, all polyps) |
||
| Endoscopist experience and training | Seven endoscopists performed the colonoscopies. All of the study endoscopists underwent training on a simplified NBI International Colorectal Endoscopic before the study and had achieved > 90% accuracy rate when assessing ex vivo images. No other details about the endoscopists’ training or experience performing colonoscopies or using NBI are provided, although in the discussion, the authors state that the centre had already established expertise in endoscopy. Histopathological diagnosis by a clinical pathologist | ||
| Polyp classification system (including histopathological classification, e.g. NBI International Colorectal Endoscopic) | Not explicitly stated, but assumed to be the simplified NBI International Colorectal Endoscopic that the endoscopists were trained in before the study commenced | ||
| Sample size calculation | Based on preliminary data collected using the 180 colonoscope, a mean of 0.86 polyps and 0.51 adenomas per patient would need to be identified. This meant that it was likely that 59% of the polyps would be neoplastic. Previously collected data suggested that NBI has a sensitivity of 84%, a specificity of 75% and an overall accuracy of 80%. It was therefore calculated that 230 polyps per group (460 polyps in total) would be needed to detect an increase in accuracy from 80% to 90% between the two colonoscopy procedures, which would provide a power of 80% to find a statistical significance level of 5% | ||
| Results: NBI using 180 colonoscope to characterise polyps sized ≤ 5 mm (n = 310) | |||
| Adenomatous polyps on histopathologyb | Hyperplastic polyps on histopathologyc | Total | |
| Index test positive | (a) 120 | (b) 35d | 155 |
| Index test negative | (c) 31d | (d) 124 | 155d |
| Total | 151e | 159 | 310 |
| Accuracy [(a + d)/(a + b + c + d)] | 79% (244 of 310 polyps correctly diagnosed; CIs not reported) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 79% | 72.14% to 85.60%d | |
| Clinical specificity d/(b + d) | 78% | 70.74% to 84.16%d | |
| PPV a/(a + b) | 77% | 70.02% to 83.74%d | |
| NPV d/(c + d) | 80% | 72.83% to 85.99%d | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 3.61d | 2.66 to 4.89d | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.26d | 0.19 to 0.36d | |
| Diagnostic odds ratio (a × d)/(b × c) | 13.714d | 7.955 to 23.644d | |
| The reviewer’s calculations of accuracy, sensitivity, specificity, PPV and NPV agree with those reported in the paper. Note that CIs are not reported in the paper | |||
| Results: NBI using 180 colonoscope to characterise polyps sized ≤ 5 mm located in the rectosigmoid colon (n = 125) | |||
| Adenomatous polyps on histopathologyb | Hyperplastic polyps on histopathologyc | Total | |
| Index test positive | (a) 21 | (b) 16a | 37 |
| Index test negative | (c) 4a | (d) 84 | 88a |
| Total | 25 | 100a | 125 |
| Accuracy [(a + d)/(a + b + c + d)] | 84% (105 of 125 polyps accurately diagnosed; CIs not reported) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 84% | 63.92% to 95.46%a | |
| Clinical specificity d/(b + d) | 84% | 75.32% to 90.57%a | |
| PPV a/(a + b) | 57% | 39.49% to 72.90%a | |
| NPV d/(c + d) | 95% | 88.77% to 98.75%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 5.25a | 3.25 to 8.49a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.19a | 0.08 to 0.47a | |
| Diagnostic odds ratio (a × d)/(b × c) | 27.563a | 8.339 to 91.096a | |
| The reviewer’s calculations of accuracy, sensitivity, specificity, PPV and NPV agree with those reported in the paper. Note that CIs are not reported in the paper | |||
| Results: high-confidence predictions using NBI 180 colonoscope to characterise polyps sized ≤ 5 mm (n = 257) | |||
| Adenomatous polyps on histopathologyb | Hyperplastic polyps on histopathologyc | Total | |
| Index test positive | (a) 102 | (b) 22a | 124 |
| Index test negative | (c) 24a | (d) 109 | 133a |
| Total | 126 | 131a | 257 |
| Accuracy [(a + d)/(a + b + c + d)] | 82% (211 of 257 polyps accurately diagnosed) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 80.95%a | 73.00% to 87.40%a | |
| Clinical specificity d/(b + d) | 83.21%a | 75.69% to 89.17%a | |
| PPV a/(a + b) | 82% | 74.38% to 88.53%a | |
| NPV d/(c + d) | 82% | 74.35% to 88.08%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 4.82a | 3.26 to 7.12a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.23a | 0.16 to 0.33a | |
| Diagnostic odds ratio (a × d)/(b × c) | 21.057a | 11.121 to 39.871a | |
| The reviewer’s calculations of accuracy, PPV and NPV agree with those reported in the paper. Note that CIs are not reported in the paper | |||
| Results: low-confidence predictions using NBI 180 colonoscope to characterise polyps sized ≤ 5 mm (n = 53) | |||
| Adenomatous polyps on histopathologyb | Hyperplastic polyps on histopathologyc | Total | |
| Index test positive | (a) 18 | (b) 13a | 31 |
| Index test negative | (c) 7a | (d) 15 | 22a |
| Total | 25 | 28a | 53 |
| Accuracy [(a + d)/(a + b + c + d)] | 62% (33 of 53 polyps accurately diagnosed) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 72.00%a | 50.61% to 87.93%a | |
| Clinical specificity d/(b + d) | 53.57%a | 33.87% to 72.49%a | |
| PPV a/(a + b) | 58% | 39.08% to 75.45%a | |
| NPV d/(c + d) | 68% | 45.13% to 86.14%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 1.55a | 0.97 to 2.47a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.52a | 0.26 to 1.07a | |
| Diagnostic odds ratio (a × d)/(b × c) | 2.967a | 0.943 to 9.335a | |
| The reviewer’s calculations of accuracy, PPV and NPV agree with those reported in the paper. Note that CIs are not reported in the paper | |||
| Results: high-confidence predictions using NBI 180 colonoscope to characterise polyps sized ≤ 5 mm located in the rectosigmoid colon (n = 104) | |||
| Adenomatous polyps on histopathologyb | Hyperplastic polyps on histopathologyc | Total | |
| Index test positive | (a) 18 | (b) 7a | 25 |
| Index test negative | (c) 3a | (d) 76 | 79a |
| Total | 21 | 83a | 104 |
| Accuracy [(a + d)/(a + b + c + d)] | 90% (94 of 104 polyps accurately diagnosed) | ||
| Diagnosis | Value | 95% CI | |
| Clinical sensitivity a/(a + c) | 85.71%a | 63.66% to 96.95%a | |
| Clinical specificity d/(b + d) | 91.57%a | 83.39% to 96.54%a | |
| PPV a/(a + b) | 72% | 50.61% to 87.93%a | |
| NPV d/(c + d) | 96% | 89.30% to 99.21%a | |
| Positive likelihood ratio [sensitivity/(1 – specificity)] | 10.16a | 4.90 to 21.09a | |
| Negative likelihood ratio [(1 – sensitivity)/specificity] | 0.16a | 0.05 to 0.45a | |
| Diagnostic odds ratio (a × d)/(b × c) | 65.143a | 15.53 to 276.824a | |
| The reviewer’s calculations of accuracy, PPV and NPV agree with those reported in the paper. Note that CIs are not reported in the paper | |||
| Interpretability of test | NR | ||
| Interobserver agreement | NR | ||
| Intraobserver agreement | NR | ||
| Test acceptability (patients/clinicians) | NR | ||
| Adverse events | NR | ||
| High-confidence optical diagnosis | 257/310 (82.9%) diminutive polyps in the NBI 180 arm were predicted with high confidence. 104/125 (83.2%) diminutive polyps located in the rectosigmoid colon were predicted with high confidence. Percentages calculated by reviewer. 2 × 2 tables shown above | ||
| Low-confidence optical diagnosis | 53/310 (17.1%) diminutive polyps in the NBI 180 arm were predicted with low confidence. Percentage calculated by reviewer. 2 × 2 table shown above. The proportion of diminutive polyps located in the rectosigmoid colon which were predicted with low confidence is NR | ||
| Number of polyps designated to be left in place | NR | ||
| Number of polyps designated to be resected and discarded | NR | ||
| Number of polyps designated for resection and histopathological examination | NR | ||
| Recommended surveillance interval |
Assignment of surveillance intervals was based on the number and size of the adenomas: I, 0 adenomas (10 years); II, 1 or 2 adenomas < 10 mm in size (5 years); III, 3–5 adenomas < 10 mm in size or any adenomas 10–20 mm (3 years); IV, > 5 adenomas or any adenoma > 20 mm in size (3 months to 1 year) Agreement between histopathology and NBI 180 predictions, all polyps: 221/264 patients (84% CI 79% to 88%). Under NBI, 27 patients would have returned earlier and 16 later than assigned by histopathology Agreement between histopathology and NBI 180 predictions, when assignment of surveillance interval for polyps sized ≤ 5 mm predicted with high confidence is made with NBI 180, whereas histopathology is used for assignment of surveillance intervals in all other cases (as per the PIVI guidelines): 250/264 patients (95% CI 91% to 97%). Under NBI, five patients would have returned earlier and nine later than assigned by histopathology |
||
| Length of time to perform the colonoscopy |
Insertion time, minutes: mean 6.6 (SD 3.8); median 5.7 (IQR 3.9–8.2) Withdrawal time, minutes: mean 16.1 (SD 7.3); median 14.5 (IQR 11.0–19.2) Total procedure time, minutes: mean 22.7 (SD 8.3); median 20.8 (IQR 17.1–27.0) Note that the results are for all procedures and not just those in which diminutive polyps were identified |
||
| Number of outpatient appointments | NR | ||
| HRQoL | NR | ||
| Colorectal cancer | NR | ||
| Mortality | NR | ||
Critical appraisal criteria
Based on Reitsma and colleagues’38 adaptation of the QUADAS tool. 39
| Item | Description | Judgement | |
|---|---|---|---|
| 1 | Was the spectrum of patients representative of the patients who will receive the test in practice? | Few details provided about the indications for the colonoscopy. Of the patients, 46% were undergoing routine colonoscopy, 43% surveillance colonoscopy and 10% diagnostic colonoscopy – patients were described as being ‘at average risk’ (not further defined) | Yes |
| 2 | Is the reference standard likely to classify the target condition correctly? | Histopathology is considered to be the gold standard | Yes |
| 3 | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The real-time VCE assessment and the polyp resection for histopathological analysis would be performed at the same time (i.e. during the same colonoscopy) | Yes |
| 4 | Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? | 10 diminutive polyps were not assessed by histopathology and it is unclear whether or not another polyp was sent for histopathological examination | No |
| 5 | Did patients receive the same reference standard irrespective of the index test result? | Although 10 diminutive polyps were not assessed by histopathology there is no indication that they received a different reference standard or that it was the NBI result that caused them to be omitted from histopathological assessment | Yes |
| 6 | Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? | Yes | |
| 7 | Were the reference standard results interpreted without knowledge of the results of the index test? | It is unclear if the pathologist had knowledge of the colonoscopy result, as the authors do not report if she/he was blinded to this | Unclear |
| 8 | Were the index test results interpreted without knowledge of the results of the reference standard? | The reference standard results could not be known at the time of the index test result | Yes |
| 9 | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | |
| 10 | Were uninterpretable/intermediate test results reported? | The authors do not state if there were any uninterpretable results. Not all patients who were randomised completed the study, so it is possible that there might have been uninterpretable test results | Unclear |
| 11 | Were withdrawals from the study explained? | Yes | |
| Reference list of the included paper(s) checked? Yes/no | Yes – no additional relevant studies cited | ||
Summary reviewer’s comments
This study was carried out at one centre in the USA with established expertise in endoscopy and included a large number of diminutive polyps. It is unclear how generalisable these results are to practice (and the patient population of interest in this appraisal) as few details are provided about the patient population included in the study. Seven endoscopists performed the colonoscopies, meaning that the results came from a range of endoscopists, which enhances the generalisability of the findings. The authors comment, though, that the accuracy rates seen in established endoscopy centres may not apply to broader practice, so it is possible that the accuracy rates found in this study may not be found in other settings or among less experienced endoscopists.
Appendix 4 Table of excluded studies with rationale
| Authors and study reference | Reason for exclusiona |
|---|---|
| Adler A, Aschenbeck J, Yenerim T, Mayr M, Aminalai A, Drossel R, et al. Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: a prospective randomized trial. Gastroenterology 2009;136:410–6.e1 | Outcomes |
| Aminalai A, Roesch T, Aschenbeck J, Mayr M, Drossel R, Schroeder A, et al. Live image processing does not increase adenoma detection rate during colonoscopy: a randomized comparison between FICE and conventional imaging (Berlin Colonoscopy Project 5, BECOP-5). Am J Gastroenterol 2010;105:2383–8 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Bade K, MacPhail ME, Johnson CS, Kahi CJ, Rex DK. New colonoscope technology: impact on image capture and quality and on confidence and accuracy of endoscopy-based polyp discrimination. Endoscopy 2014;46:172–8 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Banks MR, Haidry R, Adil Butt M, Whitley L, Stein J, Langmead L, et al. High resolution colonoscopy in a bowel cancer screening program improves polyp detection. World J Gastroenterol 2011;17:4308–13 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Bowman EA, Pfau PR, Mitra A, Reichelderfer M, Gopal DV, Hall BS, et al. High definition colonoscopy combined with i-scan imaging technology is superior in the detection of adenomas and advanced lesions compared to high definition colonoscopy alone. Diagn Ther Endosc 2015;2015:167406 | Outcomes |
| Broek FJ, Fockens P, Eeden S, Kara MA, Hardwick JC, Reitsma JB, et al. Clinical evaluation of endoscopic trimodal imaging for the detection and differentiation of colonic polyps. Clin Gastroenterol Hepatol 2009;7:288–95 | Intervention (used magnification) |
| Buchner AM, Shahid MW, Heckman MG, Krishna M, Ghabril M, Hasan M, et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology 2010;138:834–2 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Burgess NG, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, et al. Sa1565 dysplasia impedes the correct endoscopic prediction of large sessile serrated polyp histology in a multicentre prospective cohort. Gastrointest Endosc 2015;81:AB263–AB4 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Bustamente M, Puchades L, Ponce M, Arguello L, Pons V. Olympus ‘Near Focus’ Narrow Band Imaging (NBI) Vs Conventional NBI for In Vivo Endoscopic Histology of Colonic Polyps: a Randomized Controlled Trial. United European Gastroenterology Journal (UEG) Week 2014 Poster Presentations, Amsterdam, the Netherlands, 1 October 2014. pp. A132–A605 | Abstract, insufficient details |
| Cha JM, Lee JI, Joo KR, Jung SW, Shin HP. A prospective randomized study on computed virtual chromoendoscopy versus conventional colonoscopy for the detection of small colorectal adenomas. Dig Dis Sci 2010;55:2357–64 | Outcomes |
| Chan JL, Lin L, Feiler M, Wolf AI, Cardona DM, Gellad ZF. Comparative effectiveness of i-SCAN (TM) and high-definition white light characterizing small colonic polyps. World J Gastroenterol 2012;18:5905–11 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Chernolesskiy A, Swain D, Lee JC, Corbett GD, Cameron EA. Comparison of Pentax HiLine and Olympus Lucera systems at screening colonoscopy. World J Gastrointest Endosc 2013;5:62–6 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Chiu H-M, Chang L-C, Shun C-T, Wu M-S, Wang H-P. Current management of diminutive colorectal polyps in Taiwan. Dig Endosc 2014;26:64–7 | Intervention |
| Chung SJ, Kim D, Song JH, Kang HY, Chung GE, Choi J, et al. Comparison of detection and miss rates of narrow band imaging, flexible spectral imaging chromoendoscopy and white light at screening colonoscopy: a randomised controlled back-to-back study. Gut 2014;63:785–91 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Chung SJ, Kim D, Song JH, Park MJ, Kim YS, Kim JS, et al. Efficacy of computed virtual chromoendoscopy on colorectal cancer screening: a prospective, randomized, back-to-back trial of Fuji Intelligent Color Enhancement versus conventional colonoscopy to compare adenoma miss rates. Gastrointest Endosc 2010;72:136–42 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Coe SG, Thomas C, Crook J, Ussui V, Diehl N, Wallace MB. Colorectal surveillance interval assignment based on in vivo prediction of polyp histology: impact of endoscopic quality improvement program. Gastrointest Endosc 2012;76:118–25.e1 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Gilani N, Stipho S, Panetta JD, Petre S, Young MA, Ramirez FC. Polyp detection rates using magnification with narrow band imaging and white light. World J Gastrointest Endosc 2015;7:555–62 | Intervention (not real-time assessment) |
| Gross SA, Buchner AM, Crook JE, Cangemi JR, Picco MF, Wolfsen HC, et al. A comparison of high definition-image enhanced colonoscopy and standard white-light colonoscopy for colorectal polyp detection. Endoscopy 2011;43:1045–51 | Intervention (no real-time characterisation) |
| Hoffman A, Loth L, Rey JW, Rahman F, Goetz M, Hansen T, et al. High definition plus colonoscopy combined with i-scan tone enhancement vs. high definition colonoscopy for colorectal neoplasia: a randomized trial. Dig Liver Dis 2014;46:991–6 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Hoffman A, Sar F, Goetz M, Tresch A, Mudter J, Biesterfeld S, et al. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy 2010;42:827–33 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Hong SN, Choe WH, Lee JH, Kim SI, Kim JH, Lee TY, et al. Prospective, randomized, back-to-back trial evaluating the usefulness of i-SCAN in screening colonoscopy. Gastrointest Endosc 2012;75:1011–21.e2 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Inoue T, Murano M, Murano N, Kuramoto T, Kawakami K, Abe Y, et al. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: a randomized, controlled trial. J Gastroenterol 2008;43:45–50 | Intervention (detection only, no characterisation) |
| Kąkol D, Frączek M, Banaszkiewicz A, Pertkiewicz J. Narrow-band imaging and white-light endoscopy for detection of colorectal polyps: a randomized study. Pol Arch Med Wewn 2013;123:519–25 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Kaltenbach T, Sano Y, Friedland S, Soetikno R. American gastroenterological association (AGA) institute technology assessment on image-enhanced endoscopy. Gastroenterology 2008;134:327–40 | Study design |
| Kim JJ, Hong KS, Kim JS, Jung HC. A randomized controlled clinical study comparing the diagnostic accuracy of the histological prediction for colorectal polyps depending on the use of either magnified or nonmagnified narrow band imaging. Clin Endosc 2015;48:528–33 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Kim WJ, Park SY, Park I, Lee WJ, Park J, Chon N, et al. Increased detection of colorectal polyps in screening colonoscopy using high definition i-scan compared with standard white light. Clin Endosc 2016;49:69–75 | Intervention (detection only, no characterisation) |
| Kim YS, Kim D, Chung SJ, Park MJ, Shin CS, Cho SH, et al. Differentiating small polyp histologies using real-time screening colonoscopy with Fuji Intelligent Color Enhancement. Clin Gastroenterol Hepatol 2011;9:744–9.e1. | Intervention (used magnification) |
| Kominami Y, Yoshida S, Tanaka S, Sanomura Y, Hirakawa T, Raytchev B, et al. Computer-aided diagnosis of colorectal polyp histology by using a real-time image recognition system and narrow-band imaging magnifying colonoscopy. Gastrointest Endosc 2016;83:643–9 | Intervention (used magnification) |
| Kuiper T, Broek FJ, Naber AH, Soest EJ, Scholten P, Mallant-Hent R, et al. Endoscopic trimodal imaging detects colonic neoplasia as well as standard video endoscopy. Gastroenterology 2011;140:1887–94 | Intervention (used magnification) |
| Kuiper T, Marsman WA, Jansen JM, van Soest EJ, Haan YC, Bakker GJ, et al. Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol 2012;10:1016–20 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Kuiper T, van den Broek FJ, van Eeden S, Fockens P, Dekker E. Feasibility and accuracy of confocal endomicroscopy in comparison with narrow-band imaging and chromoendoscopy for the differentiation of colorectal lesions. Am J Gastroenterol 2012;107:543–50 | Patient group (polyposis syndromes included) |
| Kumar S, Fioritto A, Mitani A, Desai M, Gunaratnam N, Ladabaum U. Optical biopsy of sessile serrated adenomas: do these lesions resemble hyperplastic polyps under narrow-band imaging? Gastrointest Endosc 2013;78:902–9 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Kuruvilla N, Paramsothy R, Gill R, Remedios M, Selby WS, Kaffes AJ. A prospective dual centre evaluation of narrow band imaging (NBI) with a fixed zoom function in real time prediction of polyp histology: can we resect and discard? J Gastroenterol Hepatol 2014;29(Suppl. 2):30 | Intervention (used magnification) |
| Kuruvilla N, Paramsothy R, Gill R, Selby WS, Remedios ML, Kaffes AJ. A prospective dual-center proof-of-principle study evaluating the incremental benefit of narrow-band imaging with a fixed zoom function in real-time prediction of polyp histology. Can we resect and discard? Gastrointest Endosc 2015;82:362–9 | Intervention (used magnification) |
| Lapalus MG, Helbert T, Napoleon B, Rey JF, Houcke P, Ponchon T. Does chromoendoscopy with structure enhancement improve the colonoscopic adenoma detection rate? Endoscopy 2006;38:444–8 | Intervention |
| Ljubicic N, Kujundzic M, Banic M, Roic G. The role of standard videochromocolonoscopy in distinguishing adenomatous from nonadenomatous diminutive colorectal polyps. Acta Clinica Croatica 2001;40:197–201 | Intervention |
| Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy 2004;36:1094–8 | Intervention (used magnification) |
| Mayr M, Treszl A, Balzer K, Wegscheider K, Aschenbeck J, Aminalai A, et al. Endoscopic versus histological characterisation of polyps during screening colonoscopy Guido Schachschal,1. Gut 2014;63:458–65 | Outcomes |
| Neumann H, Vieth M, Guenther C, Neurath MF. Improved detection of proximal colon adenomas with i-scan in comparison to high-definition white light endoscopy. J Gastroenterol Hepatol 2014;29:9–10 | Outcomes |
| Neumann H, Vieth M, Guenther C, Neurath MF. High-definition endoscopy with i-scan allows in vivo characterization of distal colorectal polyps according to the ASGE PIVI statement. J Gastroenterol Hepatol 2014;29:9 | Abstract, insufficient details |
| Notaristefano C, Viale E, Di Marco B, Maselli R, Testoni PA. High definition colonoscopy with I-SCAN and digital chromoendoscopy in the pit pattern analysis: a single center experience. Gastrointest Endosc 2015;1:AB384 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Paramsothy R, Kuruvilla NA, Gill RS, Selby W, Remedios M, Kaffes AJ. A prospective dual centre evaluation of narrow band imaging (NBI) with a fixed zoom function in real time prediction of polyp histology. Can we resect and discard? Gastrointest Endosc 2015;1:AB267–AB68 | Intervention (used magnification) |
| Patel SG, Schoenfeld P, Bansal A, Hosford L, Myers A, Wilson RH, et al. Low prevalence of advanced histological features in diminutive colon polyps: results from a prospective multicenter study evaluating real-time characterization of diminutive colorectal polyp histology using narrow band imaging (NBI). Gastrointest Endosc 2016;1:AB146 | Outcomes |
| Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, et al. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut 2009;58:73–8 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Rajasekhar PT, Mason J, Wilson A, Close H, Rutter MD, Saunders B, et al. Narrow Band Imaging Optical Diagnosis Of Small Colorectal Polyps In Routine Clinical Practice: The Detect Inspect Characterise Resect And Discard (Discard 2) Study. United European Gastroenterology Journal (UEG) Week 2015 Oral Presentations, Barcelona, 1 October 2015. pp. 1–145 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Rajasekhar PT, Mason J, Wilson A, Close H, Rutter M, Saunders B, et al. Detect inspect characterise resect and discard 2: are we ready to dispense with histology? Gut 2015;64:A13 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Ramirez-Ramirez MA, Mejia Cuan LA, Martinez C, Zamorano-Orozco Y, Vieyra SC. Prediction of colorectal polyp pathologic lesions with high definition and virtual chromoendoscopy with I-SCAN 2 in real time; a prospective study. Gastrointest Endosc 2015;1:AB265 | Abstract, insufficient details |
| Rastogi A, Early DS, Gupta N, Bansal A, Singh V, Ansstas M, et al. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc 2011;74:593–602 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Rees CJ, Rajasekhar PT, Wilson A, Close H, Rutter MD, Saunders BP, et al. Narrow band imaging optical diagnosis of small colorectal polyps in routine clinical practice: the Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut 2016;66:887–95 | Intervention (majority of colonoscopies not HD) |
| Rey JF, Tanaka S, Lambert R, Tajiri H. Evaluation of the clinical outcomes associated with EXERA II and LUCERA endoscopes. Dig Endosc 2009;21(Suppl. 1):S113–20 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Rotondano G, Bianco MA, Sansone S, Prisco A, Meucci C, Garofano ML, et al. Trimodal endoscopic imaging for the detection and differentiation of colorectal adenomas: a prospective single-centre clinical evaluation. Int J Colorectal Dis 2012;27:331–6 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Sakamoto T, Matsuda T, Aoki T, Nakajima T, Saito Y. Time saving with narrow-band imaging for distinguishing between neoplastic and non-neoplastic small colorectal lesions. J Gastroenterol Hepatol 2012;27:351–5 | Intervention (used magnification) |
| Sakatani A, Fujiya M, Tanaka K, Dokoshi T, Fujibayashi S, Ando K, et al. Usefulness of NBI for differentiating colon neoplasms from non-neoplasms: based on results of our institutional experience and a meta-analysis of comparative studies. Gastrointest Endosc 2014;1:AB442 | Intervention (not real-time assessment) |
| Seref Koksal A, Yildiz H, Taskiran I, Turhan N, Oztas E, Torun S, et al. Low magnification narrow band imaging by inexperienced endoscopists has a high accuracy in differentiation of colon polyp histology. Clin Res Hepatol Gastroenterol 2014;38:763–9 | Intervention (colonoscope not HD) |
| Sharma P, Frye J, Frizelle F. Accuracy of visual prediction of pathology of colorectal polyps: how accurate are we? ANZ J Surg 2014;84:365–70 | Intervention |
| Singh R, Cheong KL, Yeap SP, Ovenden A, Ruszkiewicz A, Dy F, et al. A prospective multicentre study assessing the utility of narrow band imaging with dual focus magnification in differentiating colorectal neoplasia using the nice and modified Sano’s classification. Gastrointest Endosc 2016;1:AB152 | Intervention (used magnification) |
| Singh R, Jayanna M, Navadgi S, Ruszkiewicz A, Saito Y, Uedo N. Narrow-band imaging with dual focus magnification in differentiating colorectal neoplasia. Dig Endosc 2013;25(Suppl. 2):16–20 | Intervention (used magnification) |
| Song LMWK, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, et al. Narrow band imaging and multiband imaging. Gastrointest Endosc 2008;67:581–9 | Study design |
| Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol 2006;101:2711–16 | Intervention (not real time) |
| Szura M, Pasternak A, Bucki K, Urbanczyk K, Matyja A. Two-stage optical system for colorectal polyp assessments. Surg Endosc 2016;30:204–14 | Intervention (used magnification) |
| Takeuchi Y, Hanafusa M, Kanzaki H, Ohta T, Hanaoka N. Proposal of a new ‘resect and discard’ strategy using magnifying narrow band imaging: pilot study of diagnostic accuracy. Dig Endosc 2014;26(Suppl. 2):90–7 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Takeuchi Y, Hanafusa M, Kanzaki H, Ohta T, Hanaoka N, Yamamoto S, et al. An alternative option for ‘resect and discard’ strategy, using magnifying narrow-band imaging: a prospective ‘proof-of-principle’ study. J Gastroenterol 2015;50:1017–26 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Tischendorf JJ, Schirin-Sokhan R, Streetz K, Gassler N, Hecker HE, Meyer M, et al. Value of magnifying endoscopy in classifying colorectal polyps based on vascular pattern. Endoscopy 2010;42:22–7 | Intervention (not real time) |
| Togashi K, Osawa H, Koinuma K, Hayashi Y, Miyata T, Sunada K, et al. A comparison of conventional endoscopy, chromoendoscopy, and the optimal-band imaging system for the differentiation of neoplastic and non-neoplastic colonic polyps. Gastrointest Endosc 2009;69:734–41 | Intervention (used magnification) |
| van Dam L, Wijkerslooth TR, Haan MC, Stoop EM, Bossuyt PM, Fockens P, et al. Time requirements and health effects of participation in colorectal cancer screening with colonoscopy or computed tomography colonography in a randomized controlled trial. Endoscopy 2013;45:182–8 | Intervention |
| Weigt J, Kandulski A, Malfertheiner P. New generation flexible spectral imaging color enhancement is useful to predict histology of small colorectal polyps. Gastrointest Endosc 2014;79(Suppl. 1):AB434 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Yeap SP, Singh R, Ovenden A, Ruszkiewicz A, Lau JY, Rerknimitr R, et al. A randomised controlled trial comparing the modified Sano’s versus the NICE classifications using narrow band imaging with near focus magnification in differentiating colorectal polyps Gastrointest Endosc 2015;81(Suppl. 1):AB259–AB60 | Intervention (used magnification) |
| Yoshida Y, Matsuda K, Sumiyama K, Kawahara Y, Yoshizawa K, Ishiguro H, et al. A randomized crossover open trial of the adenoma miss rate for narrow band imaging (NBI) versus flexible spectral imaging color enhancement (FICE). Int J Colorectal Dis 2013;28:1511–16 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
| Zhou QJ, Yang JM, Fei BY, Xu QS, Wu WQ, Ruan HJ. Narrow-band imaging endoscopy with and without magnification in diagnosis of colorectal neoplasia. World J Gastroenterol 2011;17:666–70 | Comparator (histopathology not compared with VCE separately for polyps ≤ 5 mm in size) |
Appendix 5 Ongoing studies
Tables 67 and 68 list the 19 potentially relevant ongoing studies identified from searches of clinical trials databases and identified from conference abstracts for recently completed and ongoing studies that have not been published in full yet. Reviewers decided during study selection that it was unclear if these conference abstracts met the inclusion criteria for the review. This as a result of the limitations in the information reported. For example, often the population was unclear, it was unclear whether or not optical diagnosis was performed using magnification and HD equipment, and, for studies not limited to diminutive polyps, it was unclear whether or not results will be presented separately for diminutive polyps only.
| Study identifier; location | Study title | Estimated completion date and enrolment |
|---|---|---|
| NCT02407925; the Netherlands | Implementation of optical diagnosis for diminutive polyps amongst accredited endoscopists for the Dutch bowel cancer screening program: training and long-term quality assurance (DISCOUNT2) | January 2017; n = 1500 |
| NCT02516748; Republic of Korea | Prospective study of real-time diagnosis of colorectal polyps using narrow-band imaging: Gangnam-ReaDi Study | August 2016; n = 5000 |
| Reference | Title |
|---|---|
| Belderbos et al., 2015155 | The accuracy of real-time probe based confocal LASER endomicroscopy for differentiation of colorectal polyps during colonoscopy |
| Kaltenbach et al., 2014156 | Gastroenterology trainees can perform real time optical diagnosis of diminutive colorectal polyps using narrow-band imaging |
| Kheir et al., 2016157 | Optical diagnosis of diminutive colorectal polyps by non-academic general gastroenterologists using non-magnifying narrow band imaging (NBI): a prospective study |
| Klein et al., 2014158 | Computerized, image analysis of diminutive polyps during colonoscopy-preliminary results of a feasibility study |
| Lee et al., 2014159 | Learning curve for optical biopsy using narrow band imaging-can real-time training improve accuracy? |
| Lee et al., 2015160 | Learning curve for optical biopsy using narrow band imaging (NBI) – can real-time training improve accuracy? |
| Madacsy et al., 2015161 | Diagnostic Value of Fujinon Intelligent Color Enhancement (FICE) Technology With and Without Magnification to Differentiate Between Hyperplastic and Adenomatous Lesions According to the NICE Classification – A Prospective, Randomized, Controlled Study. United European Gastroenterology Journal (UEG) Week 2015, Barcelona, Spain, 1 October 2015 |
| Maimone et al., 2015162 | Real-time biopsy of colorectal polyps = 6 mm using FICE, i-scan and NBI technologies: experience of a young endoscopist |
| Neumann et al., 2015163 | Development and validation of a simple classification system for in vivo diagnosis of colorectal polyps using digital chromoendoscopy – the visible study |
| Paggi et al., 2014164 | Is it really so easy to learn histologic characterization of diminutive polyps by narrow band imaging? Preliminary results of endoscopists’ and nurses’ performances |
| aRastogi et al., 2014165 | Performance of gastroenterology (GI) trainees in real-time characterization of diminutive polyp (DP) histology with narrow band imaging (NBI) – results from a prospective trial |
| aRastogi et al., 2014166 | Prediction time for characterizing diminutive (% 5 mm) polyp (DP) histology with NBI during colonoscopy is a marker for high confidence (HC) diagnosis and accuracy |
| aRastogi et al., 2014167 | Gastroenterology (GI) trainees can achieve the PIVI benchmarks for real-time characterization of the histology of diminutive (% 5 mm) polyps (DP) – a prospective study |
| Rocha et al., 2014168 | In vivo diagnosis of colorectal polyps by GI endoscopists using HD narrow-band imaging |
| Staiano et al., 2016169 | High-definition colonoscopy using i-scan in morphological characterization and real-time histological prediction of colonic neoplastic superficial lesion. A single Italian cente pilot study, preliminary results |
| Vleugels et al., 2016 170 | Incorporating sessile serrated polyps in optical diagnosis of diminutive polyps: what are the implications for the PIVI thresholds? |
| Xu et al., 2015171 | Significance of endoscopic mucosal surface features in diagnosing colorectal polyps |
Appendix 6 Studies excluded from the systematic review of cost-effectiveness studies
| Authors and study reference | Reason for exclusion |
|---|---|
| Longcroft-Wheaton GR, Higgins B, Bhandari P. Flexible spectral imaging color enhancement and indigo carmine in neoplasia diagnosis during colonoscopy: a large prospective UK series (Structured abstract). Eur J Gastroenterol Hepatol 2011;23:903–11 | Outcome |
| Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol 2009;10:1171–8 | Intervention/outcome |
| Chandran S, Parker F, Lontos S, Vaughan R, Efthymiou M. Can we ease the financial burden of colonoscopy? Using real-time endoscopic assessment of polyp histology to predict surveillance intervals. Intern Med J 2015;45:1293–9 | Outcome |
| Longcroft-Wheaton G, Bhandari P. The cost impact of in vivo diagnosis of diminutive polyps: experience from a screening endoscopy programme. Gut 2011;60:A30 | Abstract |
| Longcroft-Wheaton GR, Bhandari P. The cost impact of in-vivo diagnosis of diminuitive colonic polyps in screening colonoscopy: results from a large prospective western study. Gastrointest Endosc 2011;1:AB149 | Abstract |
| McGill SK, Soetikno RM, Yokomizo L, Goldhaber-Fiebert JD, Owens D, Kaltenbach T. Optical diagnosis of small colorectal polyps with resect and discard strategy is cost saving. Gastrointest Endosc 2013;1:AB168 | Abstract |
| Solon C, Klausnitzer R, Blissett D, Ihara Z. Economic value of narrow band imaging versus white light endoscopy for the characterization of diminutive polyps in the colon: systematic literature review and cost-consequence model. J Med Econ 2016;19:1040–8 | Outcome |
| Patel SG, Rastogi A, Schoenfeld P, Bansal A, Hosford L, Myers A, et al. Cost-savings associated with the resect and discard strategy for diminutive polyps: results from a prospective multicenter study evaluating real-time characterization of diminutive colorectal polyp histology using narrow band imaging (NBI). Gastrointest Endosc 2016;83:AB421 | Abstract |
Appendix 7 Data extraction forms of included economic evaluations
Hassan et al.112
| 1 | Study | Hassan et al. 2010 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Research question | To calculate the potential savings and drawbacks of a resect and discard policy for diminutive colorectal lesions in a simulated CRC screening cohort | ||||||||||||
| 3 | Country/setting | USA, secondary care | ||||||||||||
| 4 | Funding source | The funding source of the study is NR | ||||||||||||
| 5 | Analysis type | Cost-effectiveness analysis | ||||||||||||
| 6 | Study type | Markov model with health states for no colorectal neoplasia, diminutive (≤ 5 mm), small (6–9 mm) or large (≥ 10 mm) adenomatous polyps; localised, regional or distant CRC; and CRC-related death | ||||||||||||
| 7 | Perspective | Societal | ||||||||||||
| 8 | Time horizon | Trial, lifetime. Model cycle length: not stated (assumed to be yearly) | ||||||||||||
| 9 | Model assumptions | Resect and discard policy was instituted for all the cases in which a high-confidence diagnosis was achieved by NBI. All diminutive polyps in which a high-confidence diagnosis was not possible were removed and sent for formal histopathological evaluation | ||||||||||||
| 10 | Discounting (rate) | Future costs and life-years were discounted at 3% per year | ||||||||||||
| 11 | Costing year, currency | NR | ||||||||||||
| 12 | Population | Hypothetical cohort of 100,000 50-year-old persons in the USA who underwent a colonoscopy for CRC screening | ||||||||||||
| 13 | Intervention(s), comparator(s) | NBI vs. colonoscopy vs. no screening | ||||||||||||
| 14 | Intervention effect |
Feasibility refers to rate of high confidence in differentiating between hyperplastic and adenomatous diminutive polyps by using NBI without magnification. Feasibility of 84% was assumed as the average of Rex64 and Ignjatovic et al. 70 Accuracy was defined as the ability to correctly classify adenomatous (TP) and hyperplastic (TN) diminutive polyps Sensitivity was 94% and specificity was 89% based on the studies of Rex,64 Ignjatovic et al. 70 and Rastogi et al. 73 |
||||||||||||
| 15 | Health state utilities | HRQoL not included | ||||||||||||
| 16 | Intervention cost | The authors assumed that no additional costs were incurred for NBI as current-generation colonoscopes include this technology. No additional examination and training time or any other additional material costs were assumed. Cost of colonoscopy was US$630, cost of colonoscopy with polypectomy was US$925 and pathological examination was US$102. Costs were taken from Medicare reimbursement | ||||||||||||
| 17 | Indirect costs | None listed | ||||||||||||
| 18 | Results |
DiscountedNo screeningColonoscopyColonoscopy with resect and discardCost/personUS$3390U$3222US$3197Relative efficacy–51 days/person51 days/person When projecting the results on the US population, the undiscounted annual cost saving of colonoscopy screening with the resect and discard policy compared with the standard colonoscopy screening approach was estimated to be US$33M |
Discounted | No screening | Colonoscopy | Colonoscopy with resect and discard | Cost/person | US$3390 | U$3222 | US$3197 | Relative efficacy | – | 51 days/person | 51 days/person |
| Discounted | No screening | Colonoscopy | Colonoscopy with resect and discard | |||||||||||
| Cost/person | US$3390 | U$3222 | US$3197 | |||||||||||
| Relative efficacy | – | 51 days/person | 51 days/person | |||||||||||
| 19 | Sensitivity analysis |
PSAs were performed. The fifth and 95th percentiles of the undiscounted costs of the resect and discard policy were US$15M and US$54M, respectively. Deterministic sensitivity analyses were conducting, varying all parameters. Those results with most relevance were reported The feasibility rate of NBI was varied between 50% and 100% for differentiating between hyperplastic and adenomatous diminutive lesions, and the undiscounted benefit for the US population would be US$20M and US$40M, respectively. An increase in the cost of pathology examination from the baseline US$102 to US$150 resulted in an increase of the undiscounted benefit for the US population from the baseline US$33M to US$49M |
||||||||||||
| 20 | Author’s conclusions | A resect and discard strategy for diminutive polyps detected by screening colonoscopy resulted in a substantial economic benefit without an impact on efficacy |
Kessler et al.113
| 1 | Study | Kessler et al. 2011 |
|---|---|---|
| 2 | Research question | To quantify the expected costs and outcomes of removing diminutive polyps without subsequent pathological assessment |
| 3 | Country/setting | USA |
| 4 | Funding source | NIH grant |
| 5 | Analysis type | Cost-effectiveness analysis |
| 6 | Study type | Decision tree |
| 7 | Perspective | NR, but appears to be from payer perspective |
| 8 | Time horizon | Lifetime. The model has a decision tree for the colonoscopy followed by a long-term outcome derived from a discrete event simulation model of CRC screening and surveillance strategies (Ness et al.118) |
| 9 | Model assumptions | The two strategies did not have different impacts on the extent of the examination and preparation quality of the colonoscopy; there are no differences between strategies in respect of missed polyps, masses or other lesions; and for the resect and discard strategy the endoscopy would be unable to identify advance histopathology in adenomas ≤ 5 mm in size |
| 10 | Discounting (rate) | Costs not discounted. Unclear whether or not benefits discounted (NR) |
| 11 | Costing year, currency | US$ costing year 2009 |
| 12 | Population | Patients receiving a colonoscopy at a single-institution tertiary centre who had at least one polyp removed during colonoscopy, irrespective of indication. Population characteristic taken from a database of 10,060 consecutive colonoscopies from 1999 to 2004 |
| 13 | Intervention(s), comparator(s) | No pathological examination of diminutive polyps (resect and discard) vs. submitting all polyps for pathological examination (submit all) |
| 14 | Intervention effect |
Endoscopic sensitivity for non-adenoma: 90% Endoscopic sensitivity for adenoma: 90% Proportion of diminutive polyps with advanced histopathology: 0.6% Pathology sensitivity for large adenoma: 100% Pathology sensitivity for diminutive and small adenoma: 95% Pathology sensitivity for non-adenoma: 100% |
| 15 | Health state utilities | Not included |
| 16 | Intervention cost | Costs included for pathology, colonoscopy and CRC treatment. The cost of sending a polyp to pathology was US$103.87. Colonoscopy cost: diagnostic, US$1329; and therapeutic, US$2038. Major bleeding cost was US$4360 and perforation cost was US$13,000. CRC treatment cost: localised, US$51,800; regional, US$76,500; and distant US$80,000 |
| 17 | Indirect costs | Not included |
| 18 | Results |
The submit-all strategy results in an incorrect surveillance interval 1.9% of the time, whereas the resect and discard strategy does so 11.8% of the time, with over half of the patients having only non-adenomatous polyps and scheduled for a 5-year, rather than a 10-year, surveillance examination. The cost savings from forgoing pathological assessment is US$210 per colonoscopy when diminutive polyps are removed, whereas the additional cost attributable to the incorrect surveillance interval was US$35.92. The net savings was US$174.01. The number needed to harm because of perforation, major bleed or missed cancer is 7979 (i.e. an absolute risk of 0.0125%) The expected benefit of the submit-all strategy was 0.17 days and the cost-effectiveness of the submit-all strategy compared with the resect and discard was US$377,460 per life-year gained |
| 19 | Sensitivity analysis | Deterministic sensitivity analyses were conducted for the accuracy of the colonoscopy to detect adenomas and the proportion of diminutive polyps with advanced histopathology. The sensitivity analyses performed indicate that the error rate in assigning post-polypectomy surveillance intervals is most sensitive to the accuracy of endoscopic assessment of histology and to the proportion of diminutive polyps with advanced histopathology |
| 20 | Author’s conclusion | Endoscopic diagnosis of polyp histopathology during colonoscopy and forgoing pathological examination would result in substantial upfront cost savings. Downstream consequences of the resulting incorrect surveillance intervals appear to be negligible |
Appendix 8 Data extraction of the company’s economic evaluation
Reference
Solon117 and the company submission from Olympus.
Health technology
NBI.
Interventions and comparators
What interventions/strategies were included?
NBI was compared with HD WLE.
Was a no-treatment/supportive care strategy included?
No.
Describe interventions/strategies
All patients who enter the model undergo an endoscopy test using either NBI or HD WLE, which results in one or more polyp being identified.
Research question
What are the stated objectives of the evaluation?
To compare NBI with HD WLE (assumed to be the current standard of care in the UK).
Study type
Cost-effectiveness/cost–utility/cost–benefit analysis?
Cost–consequence.
Study population
What definition was used for (condition)? What are the characteristics of the baseline cohort for the evaluation?
The model cohort is an average-risk UK population attending colorectal cancer screening.
| Input | Proportion (%) | Source |
|---|---|---|
| Proportion of patients with no polyps | 44.0 | Rastogi et al., 201196 |
| Proportion of patients with polyps ≤ 5 mm in size | 38.0 | Rastogi et al., 201196 |
| Proportion of patients with polyps > 5 mm in size | 18.0 | Rastogi et al., 201196 |
| Proportion of polyps that are adenomatous ≤ 5 mm in size | 17.0 | Rastogi et al., 201196 |
| Proportion of polyps that are adenomatous > 5 mm in size | 10.1 | Rastogi et al., 201196 |
Institutional setting
Where is/are the intervention(s) being evaluated usually provided?
Secondary care.
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
UK pounds; costs are from 2014.
Funding source
Olympus.
Analytical perspective
What is the perspective adopted for the evaluation – health service, health and personal social services, third-party payer, societal (i.e. including costs borne by individuals and lost productivity)?
English NHS and individual UK hospital perspective.
Effectiveness
Were the effectiveness data derived from a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation
| Parameter | Value | Source |
|---|---|---|
| Diminutive polyp optical diagnosis feasibility rate | 75.00% | Kaltenbach et al., 201557 |
| Optical diagnosis sensitivity NBI | 93.00% | McGill et al., 201343 |
| Optical diagnosis specificity NBI | 83.00% | McGill et al., 201343 |
| Probability of hospitalisation for bleeding with polypectomy | 0.43% | Whyte et al., 2012122 |
| Probability of perforation with polypectomy | 0.28% | Whyte et al., 2012122 |
Intervention costs
Were the cost data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described?
| Input | Base case | Source |
|---|---|---|
| Unit cost per system NBI (£) | 40,395 | OLYMPUS list price117 |
| Unit cost per scope NBI (£) | 38,660 | OLYMPUS list price117 |
| Training cost per year NBI (£) | 2272 | OLYMPUS list price117 |
| Maintenance cost NBI system (£) | 3525 | OLYMPUS list price117 |
| Maintenance cost HD WLE system (£) | 3560 | Default value that varies with options selected |
| Maintenance cost NBI scopes (£) | 4805 | OLYMPUS list price117 |
| Maintenance cost HD WLE scopes (£) | 4438 | Default value that varies with options selected |
| NHS tariff for colonoscopy with biopsy (£) | 522 | Monitor 2014: HRG tariff FZ51Z123 |
| NHS tariff for colonoscopy without biopsy (£) | 437 | Monitor 2014: HRG tariff FZ52Z123 |
| Cost per histopathological examination (£) | 110.70 | Calculation |
| Cost per biopsy (£) | 82 | Unpublished data obtained from University College London Hospitals, Plymouth Hospital NHS Trust and South Devon Healthcare NHS Foundation Trust |
| Number of biopsies per examination | 1.35 | Assumption based on data reported in Lee et al.125 |
| Cost per hospital bleed (£) | 318 | Monitor 2015–16: HRG tariff FZ38F126 |
| Cost per perforation event (£) | 2211 | Monitor 2015–16: HRG tariff GB01B126 |
| Unit cost per hour for administration and support (£) | 23 | PSSRU’s Unit Costs of Health and Social Care 2014127 |
| Hours per test for administration and support | 0.30 | Modified from assumptions reported in Sharara et al.128 |
| Unit cost per hour nurse non-contact time (£) | 41 | PSSRU’s Unit Costs of Health and Social Care 2014127 |
| Hours per test for nurse non-contact time | 0.42 | Modified from assumptions reported in Sharara et al.128 |
| Unit cost per hour of consultant time (£) | 142 | PSSRU’s Unit Costs of Health and Social Care 2014127 |
| Hours with consultant, excluding procedure | 0.50 | Modified from assumptions reported in Sharara et al.128 |
| Length of procedure time in hours with NBI | 0.30 | Bisschops et al.129 |
| Length of procedure time in hours with comparator | 0.30 | This input varies where options are selected |
| Unit cost per hour nurse contact time (£) | 100 | PSSRU’s Unit Costs of Health and Social Care 2014127 |
| Staff and overhead cost NBI (£) | 167.58 | Calculation |
| Staff and overhead cost HD WLE (£) | 167.58 | Calculation |
| Snares: cost per pack (£) | 240 | OLYMPUS list price117 |
| Snares: number per pack | 20 | Market data provided by OLYMPUS117 |
| Forceps: cost per pack (£) | 240 | OLYMPUS list price117 |
| Forceps: number per pack | 10 | Market data provided by OLYMPUS117 |
| Cost consumables with resection | 36 | Calculation |
Indirect costs (costs as a result of lost productivity, unpaid inputs to patient care)
Were indirect costs included?
None.
Health state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described?
None.
List the utility values used in the evaluation
None.
Modelling
If a model was used, describe the type of model used. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model?
The model is a cost–consequence and budget impact model. The model begins with an at-risk cohort of 551,000 people and increases this population by 20% in each of the 7 years of the model. Each successive annual cohort undergoes colonoscopy to detect polyps. Colonoscopy identifies three mutually exclusive patient groups: patients with no polyps, patients with one or more polyps of ≤ 5 mm in size or patients with one or more polyps > 5 mm in size. For NBI, polyps ≤ 5 mm are visually diagnosed for adenomas, where there is high confidence that the polyps are hyperplastic the polyps are left in situ, where visual diagnosis has low confidence the polyps are resected and sent for histopathological examination. All polyps < 5 mm are resected and histopathologically examined. For WLE all polyps are resected and sent to histopathology.
The number of TNs, FNs, TPs and FPs, and the number of histopathological examination, resects and adverse events for each cohort in each year are calculated.
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text)
The model does not include disease progression.
What is the model time horizon?
Seven years.
What, if any, discount rates have been applied in the model?
3.5% per annum for costs and health outcomes.
If no economic evaluation was conducted, state the manufacturer’s reasons for this
Not applicable.
Results/analysis
What measure(s) of benefit were reported in the evaluation?
TPs correctly identified, histopathological tests avoided, adverse events avoided.
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
NBI reduced the incidence of colonoscopy-related adverse events by 32% over 7 years.
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
The cost over 7 years for NBI is £3112M and for HD WLE is £3253M (i.e. a saving of £141M).
Synthesis of costs and benefits: are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)?
No, costs and benefits reported separately.
Give results of any statistical analysis of the results of the evaluation
Not applicable.
Was any sensitivity analysis performed: if yes, what type(s)?
Deterministic sensitivity analysis was included in the model, varying the model parameters by ± 10%.
What scenarios were tested in the sensitivity analysis?
None.
Give a summary of the results of the sensitivity analysis: did they differ substantially from the base-case analysis? If so, what were the suggested causes?
The sensitivity analysis shows the effect of the parameters on the total difference in costs between NBI and HD WLE. The cost of colonoscopy and the cost of the histopathological exams have the greatest impact on model results.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis
The data presented underscore NBI’s cost-effectiveness related to HD WLE and establish it as a cost-effective diagnostic technology for colorectal cancer.
What are the implications of the evaluation for practice?
Implementation of NBI potentially leads to a reduction in histopathological tests and adverse events.
Appendix 9 Parameters and distributions used in the probabilistic sensitivity analysis
| Parameter | Mean value | Distribution | Alpha | Beta |
|---|---|---|---|---|
| NBI sensitivity | 0.910 | Beta | 145.80 | 14.47 |
| NBI specificity | 0.819 | Beta | 167.60 | 37.09 |
| FICE sensitivity | 0.814 | Beta | 91.44 | 20.90 |
| FICE specificity | 0.850 | Beta | 135.14 | 23.82 |
| i-scan sensitivity | 0.962 | Beta | 149.04 | 5.96 |
| i-scan specificity | 0.906 | Beta | 115.09 | 11.91 |
| Proportion low-confidence assessments | 0.210 | Fixed | – | – |
| Prevalence of adenomas, in patients with one or more polyps | 0.698 | Beta | 207.39 | 89.6 |
| Prevalence of 0 adenoma | 0.302 | Dirichlet | 89.61 | 207.4 |
| Prevalence of low-risk patients | 0.535 | Dirichlet | 158.98 | 138.0 |
| Prevalence of intermediate-risk patients | 0.107 | Dirichlet | 31.80 | 265.2 |
| Prevalence of high-risk patients | 0.056 | Dirichlet | 16.62 | 280.4 |
| Probability of perforation with polypectomy | 0.003 | Beta | 1.38 | 457.23 |
| Probability of perforation death | 0.052 | Beta | 4.00 | 73.00 |
| Probability of hospitalisation for bleeding | 0.003 | Beta | 1.38 | 457.23 |
| Bleeding adverse event | 0.006 | Gamma | 14.20 | 0.0004 |
| Perforation adverse event | 0.010 | Gamma | 49.12 | 0.0002 |
| Histopathology colonoscopy (no polypectomy) (£) | 518.36 | Gamma | 32.77 | 15.82 |
| Histopathology colonoscopy (polypectomy) (£) | 600.16 | Gamma | 36.80 | 16.31 |
| Expected polyps, 0 adenomas | 3.03 | Fixed | – | – |
| Expected polyps, low-risk adenomas | 2.00 | Fixed | – | – |
| Expected polyps, intermediate-risk adenomas | 4.78 | Fixed | – | – |
| Expected polyps, high-risk adenomas | 8.47 | Fixed | – | – |
| Average adenoma, low-risk patients | 1.40 | Fixed | – | – |
| Average adenoma, intermediate-risk patients | 3.34 | Fixed | – | – |
| Average adenoma, high-risk patients | 5.91 | Fixed | – | – |
| Cost of treating bowel perforation (£) | 2152.77 | Gamma | 11.38 | 189.10 |
| Cost of admittance for bleeding (£) | 475.54 | Gamma | 39.74 | 11.97 |
| Pathology cost (£) | 28.82 | Gamma | 6.57 | 4.39 |
| Training cost, per endoscopy (£) | 14.72 | Gamma | 42.68 | 0.34 |
Appendix 10 Derivation of the distribution of adenomas in patients undergoing colonoscopy
We searched for studies that described the distribution of polyps in patients in a screening population. We identified one study by Raju and colleagues,132 who reported data for the distribution of polyps and adenomas per patient. We analysed the distribution of polyps and adenomas to derive the average number of polyps and adenomas for low-risk, intermediate-risk and high-risk patients and the frequency of patients in each risk category, assuming all polyps are diminutive.
We used a graphical data extraction program (XY Scan) to extract the data from Raju and colleagues. 132 This extraction resulted in a slight overestimation of the number of adenomas (426 instead of the reported 422) and the number of patients with adenomas (207 instead of 206) in order to keep polyp numbers correct at 882.
The distribution of polyps for patients with one or more polyp is shown in Table 69 and the distribution adenomas for patients with more than one polyp is shown in Table 70. As seen in Table 70, the proportion of patients with one or more polyps and who have no adenomas is 30.2%.
| One or more polyps | Number of patients | |
|---|---|---|
| n | % | |
| 1 | 26.45 | 79 |
| 2 | 25.58 | 76 |
| 3 | 18.60 | 55 |
| 4 | 11.92 | 35 |
| 5 | 7.56 | 22 |
| 6 | 4.07 | 12 |
| 7 | 2.62 | 8 |
| 8 | 1.16 | 3 |
| 9 | 0.87 | 3 |
| 10 | 0.29 | 1 |
| 11 | 0.87 | 3 |
| Total | 100.00 | 297 |
| Adenomas | Number of patients | Number of adenomas | |
|---|---|---|---|
| n | % | ||
| 0 | 0.302 | 90 | 0 |
| 1 | 0.324 | 96 | 96 |
| 2 | 0.212 | 63 | 126 |
| 3 | 0.071 | 21 | 63 |
| 4 | 0.036 | 11 | 43 |
| 5 | 0.036 | 11 | 54 |
| 6 | 0.007 | 2 | 13 |
| 7 | 0.002 | 1 | 5 |
| 8 | 0.000 | 0 | 0 |
| 9 | 0.010 | 3 | 26 |
| 10 | 0.000 | 0 | 0 |
| 11 | 0.000 | 0 | 0 |
| Total | 1.0000 | 297 | 426 |
In order to calculate the number of polyps per patient in each risk category, we assumed that the overall prevalence of patients with adenomas was evenly distributed across the risk categories, in which people had adenomas. The risk stratification was defined in accordance with the current BSG guidelines in which people with one or two adenomas are low risk, those with three or four adenomas are intermediate risk and those with five or more adenomas are high risk. The proportion of patients in each risk category is shown in Table 71. The expected number of adenomas in each risk category is calculated as a weighted average. The expected number of polyps for each risk category is calculated by assuming a constant prevalence of 0.68 adenomas per polyp in each risk category.
| Risk category | Proportion of patients | Expected number of adenomas | Expected number of polyps |
|---|---|---|---|
| Low (0–2 adenomas) | 0.837 | 1.40 | 2.00 |
| Intermediate (3 or 4 adenomas) | 0.107 | 3.34 | 4.78 |
| High (5+ adenomas) | 0.056 | 5.91 | 8.47 |
Appendix 11 System costs (scope, system, maintenance)
The equipment and maintenance costs for VCE technologies have been supplied by the manufacturers of the systems (Table 72). These costs are not included in the base-case analysis for VCE compared with histopathology, as all equipment and maintenance costs are included within the national reference costs123 for colonoscopy and polypectomy.
| Item | NBI | FICE | i-scan |
|---|---|---|---|
| Processor/light source cost | 40,395.00 | 28,500.00 | Confidential information has been removed |
| Scope cost | 38,660.00 | 25,712.50 | Confidential information has been removed |
| Scope maintenance per year | 4805.00 | 2900.00 | Confidential information has been removed |
| System maintenance per year | 3525.00 | 2200.00 | Confidential information has been removed |
The costs of the VCE systems and scope were calculated assuming that systems lasted for 7 years and an equivalent discount rate of 3% per annum.
Assuming that payment is made in advance on the annuitisation, a useful life (n) of 7 years for a system and scope, and assuming that the discount rate (r) in National Institute for Health and Care Excellence appraisals (3.5%) represents social time preference, the annuity factor can be calculated using Equation 10:
Assuming annuitised costs, the annual cost of the system and scope per year is:
where the annualisation factor is 6.329, as calculated using the annuity factor equation above (see Equation 10).
The costs of the systems and scopes are calculated per endoscopy performed by dividing the cost per year by the number of endoscopies performed per system or scope. We used the Solon and colleagues117 estimates for the number of scopes and systems per year. They estimated that there would be 1071 systems and five scopes per system. We used the total number of colonoscopies from the national reference costs (302,422 per year).
Within the model, the average cost per year is calculated for VCE technologies by calculating the weighted average by market share, with an estimated market share, according to the companies’ submissions (NBI, 74%; FICE, 13%; and i-scan, 13%).
We calculated the cost for the VCE technologies per endoscopy to be £228.74.
The cost for the VCE technologies are shown in Table 73.
| VCE technique | Total cost per endoscopy | Difference compared with average cost |
|---|---|---|
| NBI | 232.85 | 20.55 |
| FICE | 146.99 | –65.31 |
| i-scan | 160.64 | –51.66 |
Appendix 12 Colorectal cancer clinical outcomes from the School of Health and Related Research bowel cancer screening model
The SBCS model provided estimates of colorectal cancer incidence for patients in each of the categories in the External Assessment Group model (i.e. by whether or not patients had all adenomas resected and what surveillance interval they were assigned to). These estimates ranged from 1.1% to 4.2%, as shown in Table 74. We then calculated the incidence of colorectal cancer for the total population by multiplying these estimates by the proportion in each group. The calculated incidence of lifetime risk of colorectal cancer is 3.025% for those receiving histopathology, 3.020% for those receiving NBI, 3.045% for those receiving FICE and 3.021% for those receiving i-scan.
| Underlying health state at colonoscopy | Status post polypectomy | Follow-up received | CRC deaths | CRC incidence |
|---|---|---|---|---|
| Normal epithelium | n/a | Invited to screening | 0.00575 | 0.01131 |
| LR adenomas | All adenomas resected | Invited to screening | 0.02145 | 0.04215 |
| HR adenomas (IR) | All adenomas resected | Invited to screening | 0.02141 | 0.04207 |
| HR adenomas (HR) | All adenomas resected | Invited to screening | 0.02140 | 0.04205 |
| LR adenomas or HR adenomas | LR adenomas remain post polypectomy | Invited to screening | 0.02132 | 0.04187 |
| LR adenomas or HR adenomas | HR adenomas remain post polypectomy | Invited to screening | 0.20775 | 0.43476 |
| Normal epithelium | n/a | 3-yearly surveillance | 0.00460 | 0.00955 |
| LR adenomas | All adenomas resected | 3-yearly surveillance | 0.01240 | 0.02689 |
| HR adenomas (IR) | All adenomas resected | 3-yearly surveillance | 0.01238 | 0.02685 |
| HR adenomas (HR) | All adenomas resected | 3-yearly surveillance | 0.01238 | 0.02684 |
| LR adenomas or HR adenomas | LR adenomas remain post polypectomy | 3-yearly surveillance | 0.01238 | 0.02677 |
| LR adenomas or HR adenomas | HR adenomas remain post polypectomy | 3-yearly surveillance | 0.02533 | 0.13572 |
| Normal epithelium | n/a | Annual surveillance | 0.00435 | 0.00913 |
| LR adenomas | All adenomas resected | Annual surveillance | 0.01123 | 0.02518 |
| HR adenomas (IR) | All adenomas resected | Annual surveillance | 0.01122 | 0.02514 |
| HR adenomas (HR) | All adenomas resected | Annual surveillance | 0.01121 | 0.02513 |
| LR adenomas or HR adenomas | LR adenomas remain post polypectomy | Annual surveillance | 0.01122 | 0.02513 |
| LR adenomas or HR adenomas | HR adenomas remain post polypectomy | Annual surveillance | 0.01193 | 0.03684 |
List of abbreviations
- ACPGBI
- Association of Coloproctology of Great Britain and Ireland
- ASGE
- American Society for Gastrointestinal Endoscopy
- BSG
- British Society of Gastroenterology
- CI
- confidence interval
- DISCARD
- Detect, InSpect, ChAracterise, Resect and Discard
- EQ-5D
- EuroQol-5 Dimensions
- ESGE
- European Society of Gastrointestinal Endoscopy
- FAP
- familial adenomatous polyposis
- FICE
- flexible spectral imaging colour enhancement
- FN
- false negative
- FOBT
- faecal occult blood test
- FP
- false positive
- GP
- general practitioner
- HD
- high definition
- HNPCC
- hereditary non-polyposis colorectal cancer
- HRQoL
- health-related quality of life
- IBD
- inflammatory bowel disease
- ICER
- incremental cost-effectiveness ratio
- ISRCTN
- International Standard Randomised Controlled Trials Number
- NAC
- novel classification system
- NBI
- narrow-band imaging
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- PIVI
- Preservation and Incorporation of Valuable endoscopic Innovation programme
- PPV
- positive predictive value
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QUADAS
- quality assessment of diagnostic accuracy studies
- RCT
- randomised controlled trial
- SBCS
- School of Health and Related Research’s bowel cancer screening
- SD
- standard deviation
- SROC
- summary receiver operating characteristic
- TN
- true negative
- TP
- true positive
- UKCTG
- UK Clinical Trials Gateway
- VCE
- virtual chromoendoscopy
- WLE
- white-light endoscopy
This monograph is based on the Technology Assessment Report produced for NICE (National Institute for Health and Care Excellence). The full report contained data in Appendix 11, Table 72 that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but data on the equipment and maintenance costs for i-scan have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.