Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/211/02. The contractual start date was in December 2015. The draft report began editorial review in December 2016 and was accepted for publication in July 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Wade et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of underlying health problem
Hyperhidrosis is characterised by uncontrollable, excessive and unpredictable sweating that occurs at rest, regardless of temperature, and has a major impact on quality of life. It is caused by hyperfunction of the exocrine sweat glands, which are controlled by the sympathetic nervous system via postsynaptic cholinergic fibres.
Hyperhidrosis is a common condition that can be primary or secondary. Primary hyperhidrosis is excessive sweating without any discernible cause. It most commonly involves the axillae, palms and soles, but may also involve the face, groin or any area of the body. Secondary hyperhidrosis has an underlying cause, such as an endocrine disorder (e.g. hyperthyroidism), secretory tumour (e.g. phaeochromocytoma), sympathetic nervous system disorder, primary neurological condition (e.g. neuropathy), spinal disease or injury, or a psychiatric disorder. It is usually generalised over the entire body (i.e. not restricted to any specific areas of the body).
Primary hyperhidrosis is thought to affect approximately 1% of the UK population. 1 Firm estimates of prevalence for the UK or England, or England and Wales, are not available. Estimates of prevalence from a large US study2 reported a figure of 2.1%, although a recent much smaller study reported a much higher estimate of 4.8%. 3 A recent study of prevalence in Sweden reported a figure of 1.4% for primary hyperhidrosis [Hyperhidrosis Disease Severity Scale (HDSS) 3–4], whereas a study from Germany reported a figure of 6.1% with frequent or continuous disturbing sweating. 4,5 A study of two ethnically different cities reported prevalence rates of 14.5% in Shanghai and 12.3% in Vancouver. 6 It is unclear why the estimates are so varied, but reasons may include differences in study design and definition of primary hyperhidrosis. Primary hyperhidrosis normally develops in childhood and adolescence. In a few instances, it may improve with age, but it usually persists for the majority of life, although it may resolve spontaneously in the elderly. Although the cause of primary hyperhidrosis is unknown, it is likely that there is a genetic link, with one study reporting 65% of patients having a positive family history. 7
The symptoms of hyperhidrosis can significantly affect quality of life and can lead to social embarrassment, loneliness, anxiety and depression. Functional problems may arise from skin maceration and soreness. Severely affected patients also may have secondary microbial infections. Teenagers may be referred for treatment because problems holding a pen and sweating ruining paperwork lead to an inability to do schoolwork and examinations. Adults may find the condition affects employability and it may prevent individuals having personal relationships. The unpredictable and uncontrollable nature of the condition can make it very distressing for sufferers.
Description of current NHS service provision
Patients suffering from hyperhidrosis often have anxiety disorders or depression, which may exacerbate or can in some instances be the cause of their hyperhidrosis. 8 It is important to distinguish between primary and secondary hyperhidrosis as the treatment options are different. Treatment for secondary hyperhidrosis should be directed towards the underlying cause, rather than the hyperhidrosis itself.
The management of primary hyperhidrosis has been summarised in two non-systematic evidence reviews. 9,10 Therapy for primary hyperhidrosis differs depending on the site of the condition. The generally accepted treatment pathway with the various treatment options is summarised as follows.
Primary care
Patients have often tried various over-the-counter remedies before presenting to their general practitioner (GP). In primary care, an assessment of patients’ symptoms will include an assessment of the psychological and social effects of the condition to the patient.
As hyperhidrosis can be exacerbated by foods containing stimulants, especially caffeine and theobromine, dietary restriction of coffee, tea, caffeinated soft drinks and chocolate may improve mild cases of hyperhidrosis. Other lifestyle changes that may reduce symptoms include avoiding clothing that can make sweating worse, such as tight-fitting garments or man-made fibres, wearing clothing that absorbs sweat or disguises its appearance, or using devices such as armpit guards. 11 Hyperhidrosis can be associated with weight gain, and overweight people may benefit from advice about weight reduction. Drugs and physiological or disease-driven hormonal abnormalities may lead to secondary hyperhidrosis and should be excluded.
The first line of treatment for primary hyperhidrosis is topical pharmacological agents. 8 Most patients try a variety of topical antiperspirants and deodorants, but find no relief until they use 10% or 20% aluminium chloride applied daily to dry skin. This dose of aluminium chloride has been shown to be effective in clinical trials for mild to moderate hyperhidrosis. 10 It is hypothesised that the metallic antiperspirants enter the sweat gland duct and form an occlusive plug by combining with ductal keratin. Unfortunately, skin irritation is very common with these antiperspirants and often forces discontinuation of the treatment. 9 In UK clinical practice, for axillary hyperhidrosis, a 1-month trial of 20% aluminium chloride is the initial treatment. Treatment is similar for plantar hyperhidrosis except that a month’s trial of 3% formaldehyde solution to be applied to the soles can be offered. Failure of these treatments over the specified period may be followed by referral to a secondary care dermatologist.
The evidence base for the use of aluminium chloride in primary care is weak. The National Institute for Health and Care Excellence (NICE) Clinical Knowledge Summary found no placebo-controlled randomised controlled trials (RCTs) and identified a limited evidence base comprising two small poor-quality RCTs, an open-label trial, four small case series and expert opinion. 8 However, this low-cost therapy is used mainly as a first step, helping GPs discriminate between those who do and do not require referral for more specialised care. Patients whose apparent primary hyperhidrosis is actually secondary to an anxiety disorder, medication or a hormonal abnormality may also be identified in primary care and are referred for relevant treatments or investigation, rather than treatments specific to hyperhidrosis. Consequently, there is little decision uncertainty in relation to the treatment of hyperhidrosis in primary care, unlike the situation in secondary care.
Dermatology
Dermatologists may prescribe any of a number of treatments: iontophoresis, botulinum toxin (BTX) injections or systemic agents, such as anticholinergic (antimuscarinic) medications, depending on local prescribing policies. 8,10,12
Tap water iontophoresis is a process in which an electrical field drives the flow of ions in a medium and enables drug delivery through the near impenetrable barrier of the skin. 13 The technique involves immersion of the palms of the hands or soles of the feet in a shallow tray of water through which a weak electrical current is run. Sponges soaked in water can be used to treat the axillae. Iontophoresis can also be used with solutions of anticholinergics, although there is little evidence this these are any more effective than tap water. 9 The exact mechanism of action behind the therapeutic effect in hyperhidrosis is unknown and efficacy has been demonstrated only in small studies. 9 Adverse effects are minor, including a tingling ‘pins and needles’ sensation at the treatment site and dryness of the skin, although bruising or blisters can occur if the intensity of the current is too high.
Over the last few years, BTX injections have become an established licensed treatment for axillary hyperhidrosis. BTX blocks neuronal acetylcholine release at the neuromuscular junction and in cholinergic autonomic neurons, blocking the postganglionic sympathetic cholinergic nerve fibres to the sweat glands. 9 There is clinical trial evidence demonstrating the efficacy of BTX in hyperhidrosis, although this varies with site of hyperhidrosis, is only temporary (only 3–6 months) and may be technique dependent. 14 Potential drawbacks are the expense of the toxin, the discomfort associated with the injections and the need for repeated treatments. 9
Administration of anticholinergic (antimuscarinic) agents and beta-blockers may address symptoms in mild cases of hyperhidrosis. Oral propantheline (Pro-Banthine®, Kyowa Kirin Ltd) is licensed for this indication, but the unlicensed drug oral glycopyrronium bromide is often used. Occasionally other anticholinergics are used, such as oxybutynin and also methantheline bromide. 10 The doses of oral anticholinergic medication required to truly control abnormal sweating may cause significant adverse effects, including drowsiness, dry mouth, dilated pupils, photophobia, blurred vision, acute glaucoma, impaired micturition, reduced bronchial secretions, constipation, confusion, nausea, vomiting, giddiness, tachycardia, palpitations and arrhythmias. Thus, some patients are forced to discontinue this avenue of treatment, or titrate the dose up or down in accordance with tolerability in order to achieve a positive effect with minimal adverse effects.
The available medical treatments for primary hyperhidrosis are of uncertain efficacy and even when effective are not curative. Current recommendations are not underpinned by robust evidence and there are many areas of uncertainty. Importantly, the NICE Clinical Knowledge Summary on hyperhidrosis, updated in July 2013, was limited by poor-quality evidence: recommendations were often based on expert opinion in the absence of trial evidence. 8 In particular, the relative effectiveness of treatments prescribed by a dermatologist is uncertain.
Surgery
Thoracic sympathectomy involves interruption or ablation of the high thoracic sympathetic chain to decrease sympathetic tone to the upper extremity and/or face. 10 Open thoracic or cervical sympathectomy is now rarely performed and the less invasive technique of endoscopic thoracic sympathectomy (ETS) is preferred. 10 ETS is carried out under general anaesthesia through one or more small insertion incisions between the ribs. It is used for axillary, palmar, or facial hyperhidrosis. ETS can be performed at different levels of the thoracic sympathetic chain with varying efficacy and safety. 15 Adverse effects of thoracic sympathectomy can be serious, such as pneumothorax. A common adverse effect of thoracic sympathectomy is compensatory hyperhidrosis, whereby excessive sweating occurs in other parts of the body after treatment; reported in 80% of patients in one large survey. 16
Lower limb sympathectomy can also be performed as an open surgical procedure under general anaesthesia, but more minimally invasive procedures are now usually preferred. An alternative is endoscopic lumbar sympathectomy, which is less widely available but has been proposed to produce a more reliable interruption of the sympathetic chain. 17 Lower limb hyperhidrosis can be treated by chemical sympathectomy, which involves injecting the lumbar sympathetic chain with a chemical (phenol) to damage the nerve;18 however, it is rarely performed in the UK.
Guidance from NICE does specifically recommend ETS for primary hyperhidrosis of the upper limb (NICE interventional procedures guidance 487),19 but only for those ‘suffering from severe and debilitating primary hyperhidrosis that has been refractory to other treatments’. However, as for the dermatology treatment options, this recommendation for ETS was based on limited quality evidence: a non-systematic literature review, non-randomised comparative studies and case series; and focused on efficacy and safety more than quality of life.
Given the reluctance of patients to undergo ETS and its apparently limited effectiveness in terms of quality of life, alternative surgical options are required. Such procedures, for which guidance has not been issued, include removal of sweat glands. Traditionally, this was achieved through excision of sweat gland-containing skin (such as axillary skin), but now sweat gland clearance is more often done by subcutaneous curettage by open techniques or superficial liposuction, rather than skin resection:20 the inside layer of the skin (which contains the sweat glands) is scraped (curetted) and/or suctioned under general anaesthesia to remove the sweat glands but preserve skin integrity. 21–23 Adverse effects are not as serious as for thoracic sympathectomy, but can include wound breakdown or infection. These less invasive procedures are undertaken by only a few dermatology surgeons in the UK and are rarely available through the NHS.
Other emerging treatments for hyperhidrosis of the axilla include energy-based technologies that damage the sweat gland: laser, microwave, fractionated microneedle radiofrequency and ultrasound therapy. Laser treatment involves using a long-pulsed diode-powered laser, under local anaesthesia, to apply energy directly to the underside of the dermis to act on the hair follicle and surrounding sebaceous gland epithelium, causing necrosis and subsequent disruption of the exocrine gland. 24–26 The microwave device has been developed to heat target tissue at the interface between the skin and subcutaneous tissue, under local anaesthesia, causing irreversible thermolysis of apocrine and eccrine sweat glands that reside at that interface. 27 Fractionated microneedle radiofrequency uses energy to heat the tissue below the surface of the skin at a depth of 2–3 mm. 28 The microfocused ultrasound device uses high-intensity ultrasound to produce small (approximately 1 mm3) lesions or thermal coagulation points within the subcutaneous soft tissue layer of the dermis. At a depth of 4.5 mm within the subcutaneous tissue, the sweat glands can be treated without surface effects and once damaged the sweat glands are unable to regenerate. 29
Description of technologies under assessment
The technologies under assessment are those that are considered second-line treatments: iontophoresis, BTX, anticholinergic (antimuscarinic) agents and minor surgery (such as curettage, laser, microwave, fractionated microneedle radiofrequency and ultrasound).
As discussed in Description of current NHS service provision, topical aluminium chloride is a low-cost therapy, used mainly as a first step, helping GPs discriminate between those who do and do not require referral for more specialised care. Therefore, aluminium chloride has not been included in this assessment.
Sympathectomy is end-of-line treatment and NICE recommends ETS only for upper limb hyperhidrosis patients ‘suffering from severe and debilitating primary hyperhidrosis that has been refractory to other treatments’ (© NICE 2017, reproduced with permisison from NICE interventional procedures guidance 487). 19 As sympathectomy is unlikely to be considered at the same point in the treatment pathway as second-line treatments, it is not a comparator to the other treatments considered in this assessment.
Decision problem
Other than ETS, which is recognised as effective but to be reserved as a treatment of last resort, there is significant variation in the treatment for primary hyperhidrosis available in secondary care and the order in which they are prescribed. Current recommendations are not underpinned by robust evidence; there are many areas of uncertainty. In particular, the relative effectiveness of treatments prescribed by a dermatologist is uncertain and further research (both primary studies and evidence synthesis) may be required to resolve this. With regard to the minor surgical treatments available, guidance on which are the best alternative surgical options is needed, but the relative effectiveness of subcutaneous curettage and targeted sweat gland removal has not been researched or reviewed comprehensively.
Given the lack of clear research evidence to guide clinical practice, new RCTs may be warranted. However, RCTs can be difficult to conduct and extremely expensive to run. They are also demanding of both clinicians and patients and should not be undertaken without careful consideration. The need for further research is informed by both the clinical evidence and the cost-effectiveness of different treatments. The value of any future research is related to the cost of making suboptimal treatment decisions. Furthermore, in clinical practice a suite of interventions is available to patients; therefore, the decision problem also includes which treatment should be given to patients in the event that a treatment is not effective or a patient withdraws due to adverse effects. Therefore, the decision problem includes a comparison of treatment sequences.
This assessment included a systematic review to determine the relative clinical effectiveness and safety of interventions used in the management of refractory primary hyperhidrosis in secondary care (iontophoresis, BTX, anticholinergic agents and minor surgery), followed by a decision model to determine the most cost-effective treatment sequence and a value-of-information (VOI) analysis.
Aims and objectives of the research
The aim of this project was to establish the expected value of undertaking additional clinical studies (such as RCTs) to determine the most clinically effective and cost-effective interventions for the management of refractory primary hyperhidrosis (excluding patients with social anxiety disorder) in secondary care.
The key objectives were:
-
to undertake an evidence synthesis by systematic review to estimate clinical effectiveness and safety of treatments that would be available in secondary care and inform key clinical parameters for a decision model
-
to develop a decision model to estimate cost-effectiveness
-
using the decision model, to undertake a VOI analysis to determine the need for further research and to help inform the design of future clinical studies.
Chapter 2 Methods of the clinical effectiveness review
A systematic review was conducted to inform the clinical effectiveness and safety of second-line treatments that would be available for prescription by dermatologists and minor surgical treatments. The protocol included all treatments for hyperhidrosis prescribed in secondary care. However, screening and selecting the relevant literature revealed that ETS, although used as part of the treatment pathway for hyperhidrosis, could not be included in a comparative review as the position of ETS in the treatment pathway is uncontestable (ETS is considered only as an intervention of last resort because of its significant risks). Furthermore, the scoping searches identified that the rapid review of ETS for the NICE interventional procedures guidance 487 captured the relevant evidence on ETS. 19 They also revealed that because ETS is an established therapy, recent studies of ETS have focused on the details of the surgical procedure, addressing a question that is beyond the remit of the current project. This was included as a protocol amendment in our progress report.
The systematic review was conducted according to the general principles recommended in Centre for Reviews and Dissemination (CRD)’s guidance on the conduct of systematic reviews30 and is reported according to the general principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. 31 The protocol was written in accordance with the new Preferred Reporting Items for Systematic Review and Meta-Analysis – Protocols initiative32 and registered on PROSPERO, the international database of prospectively registered systematic reviews in health and social care (URL: www.crd.york.ac.uk/prospero/), as PROSPERO CRD42015027803.
Literature searches
To identify studies of effectiveness, an exhaustive systematic search of electronic databases was undertaken in January 2016 using the following databases: Allied and Complementary Medicine Database (AMED), British Nursing Index, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), EMBASE, Health Technology Assessment (HTA) database, MEDLINE, NHS Economic Evaluation Database (NHS EED), PsycINFO and PubMed.
The search strategy combined relevant search terms with indexed keywords (such as medical subject headings) and text terms that appear in the titles and/or abstracts of database records. Search strategies included appropriate search terms for ‘hyperhidrosis’ combined with search terms for treatment types (e.g. ‘botulinum toxin’, ‘iontophoresis’, ‘curettage’).
No date or language limits were applied.
Additional searches of EMBASE, MEDLINE and NHS EED were carried out to identify studies of cost-effectiveness. A recognised ‘costs’ search filter was used in conjunction with topic terms when the searches of the EMBASE and MEDLINE databases were undertaken.
Additional searches of AMED, British Nursing Index, CINAHL, CENTRAL, CDSR, DARE, EMBASE, HTA database, MEDLINE, NHS EED, PsycINFO and PubMed were carried out to identify quality-of-life studies. The search strategies used combined topic terms for hyperhidrosis with a recognised search filter for ‘quality of life’.
The search strategies are presented as Appendix 1.
Clinical advisors were consulted for additional potentially relevant studies; Julie Halford (nurse specialist and patient representative) provided an extensive bibliography of publications and articles relating to hyperhidrosis. Reference lists of relevant reviews were manually searched.
In addition, information on studies in progress, unpublished research or research reported in the grey literature was sought by searching relevant resources in July 2016 [including Conference Proceedings Citation Index: Science (ISI), ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform Portal].
Two researchers undertook the screening of titles and abstracts obtained through the search, although the library was split between the researchers, rather than each record being double screened. A sample of just over 10% of records was double screened in order to assess the level of agreement between the researchers; it was planned to undertake full double screening if the level of agreement was poor, but this was not necessary. Any record for which a researcher was unsure of their decision was marked and screened by an additional reviewer, in addition to the 10% of records double screened for quality assurance purposes.
Full manuscripts of potentially relevant studies were obtained and independently screened by two researchers, using pre-defined eligibility criteria. Disagreements were resolved through discussion and, when necessary, consultation with a third researcher. Relevant foreign language studies were translated and included in the reviews.
A separate library was created for the records identified by the searches of conference proceedings and trial registers (in July 2016) and all trials identified were deduplicated against the original library. The records were then assessed for relevance by two researchers and any selected for full-text screening were manually compared with the included studies from the original library to identify those that offered additional data. The extra studies were then separated by trial status as ongoing or completed. An online search of pharmaceutical websites and trial registration databases was performed for the completed studies to identify any published reports or trial data. When contact details were available, an e-mail was sent to the principal investigator to request research findings or published reports.
Inclusion and exclusion criteria
Population
Patients with primary hyperhidrosis (including adults and children). Patients with hyperhidrosis secondary to other conditions, such as overactive thyroid or spinal cord injury, or social anxiety disorder, were not eligible for inclusion.
Interventions
Treatments for hyperhidrosis that would be available for prescription by dermatologists and minor surgical treatments for hyperhidrosis.
Comparators
A different active treatment for hyperhidrosis, placebo or no treatment.
Outcomes
Any of the following:
-
disease severity (e.g. measured with the HDSS, or patient reported)
-
sweating (e.g. measured by gravimetry, or iodine starch test)
-
patient quality of life [e.g. assessed using Hyperhidrosis Impact Questionnaire (HHIQ), HDSS (which may be used to measure patient quality of life as well as disease severity) or the Dermatology Life Quality Index (DLQI)]
-
patient preference
-
patient satisfaction
-
patient compliance/adherence to treatment
-
social functioning (e.g. measured by Social Functioning Questionnaire)
-
adverse events [such as compensatory sweating (CS)]
-
resource use.
In addition, the duration of treatment effect was also assessed when adequate data were available.
Study designs
For each intervention, we planned to include good-quality, up-to-date, directly relevant systematic reviews, if they were available. In the absence of such a review, RCTs were included, when available. For interventions for which RCT evidence was lacking, non-RCTs were included. In the absence of controlled trials, large (> 100 patients) prospective case series (single-arm trials) were included.
Data extraction strategy
Data were extracted directly into a standard spreadsheet, which was initially piloted on a sample of studies and refined. Data extraction was conducted by one researcher and checked by a second researcher for accuracy, with any discrepancies resolved by discussion, or consultation with a third researcher, when necessary. Authors of studies were contacted for clarification and missing data, as necessary. In cases of multiple publications of the same study, the publication with the largest sample or longest follow-up was treated as the main source. Data extracted included details of study methods (including study design, country and year of publication), patient characteristics (including age, sex, body treatment site, previous treatments and baseline severity), interventions (including treatment type, dose, frequency and duration), relevant outcome measures (including outcome domain, measurement tool used and follow-up time points) and results. When possible, we sought to include intention-to-treat data. When intention-to-treat data were not available, we extracted and analysed the data as reported in the paper. When results data were missing or limited (e.g. conference abstracts), authors were contacted and, when relevant, manufacturer trials registers were consulted for further data. Where outcome data were presented only in graphical format, authors were contacted to provide further information. If the authors did not respond, data from graphs were extracted using Graph Grabber (version 1.5; Quintessa, Henley-on-Thames, UK) software. All data extraction was performed with Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA).
Quality assessment strategy
The quality assessment of studies was conducted as part of the data extraction process using criteria relevant to the study designs included. The quality of RCTs and non-RCTs was assessed using the Cochrane risk of bias tool, which focuses on the domains shown to have an impact on the trial results in particular (selection, performance and detection biases and attrition). 33 An additional question relating to the similarity of treatment groups at baseline was added. 34 In addition, a question about ‘within-patient’ study designs was added, owing to concerns about the validity of certain outcome measures in ‘within-patient’ study designs, in which patients receive different interventions on different sides of the body (i.e. the left axilla vs. the right axilla).
Studies without a control group were not formally quality assessed; however, study details are presented and their impact on the reliability of the results is discussed in the relevant sections of this report.
Each controlled trial was given an overall risk of bias judgement: trials that were rated as having a low risk of bias for all key domains (i.e. have a ‘yes’ response for each key domain) were judged to have a low overall risk of bias; trials that were rated as having a high risk of bias for one or more key domains (i.e. have a ‘no’ response) were judged to have a high overall risk of bias; and trials that were rated as having an unclear risk of bias (and no high risk of bias) for one or more key domains were judged to have an unclear overall risk of bias. When relevant, the overall risk of bias judgement was made separately for different outcomes, as certain outcomes are subjective (e.g. the HDSS), whereas other outcomes can be measured more objectively (e.g. gravimetry).
Sequence generation and allocation concealment were both considered to be ‘key domains’; therefore, non-randomised trials were all considered to have a high overall risk of bias. When prognostic factors were considered to differ between treatment groups at baseline, this was also considered to be a key source of bias. When prognostic factors were not reported, an ‘unclear’ response was not considered to be a key source of bias. It is not always possible to blind care providers to different hyperhidrosis interventions; therefore, blinding of care providers was not considered to be a ‘key domain’, nor was blinding of outcome assessors. Blinding of patients was considered to be a ‘key domain’; this is particularly relevant for subjective outcomes, which were assessed in many of the studies included in this review. Although it is acknowledged that it is not possible to blind patients in studies comparing minor surgical interventions with non-surgical interventions, or minor surgical interventions with more major surgery, blinding of participants was still considered a ‘key domain’ in these studies. Whether or not missing outcome data were balanced across groups or adjusted for was also considered to be a ‘key domain’. Whether or not the report appeared to be free from selective outcome reporting was not considered to be a key source of bias; the study protocol was unavailable for the majority of studies.
The final quality assessment question was about ‘within-patient’ study designs, in which patients received different interventions on different sides of the body. Clinical advisors to the project considered that this type of study was of limited use in hyperhidrosis research, particularly for outcomes, such as the HDSS, for which patients are asked to judge the tolerability and impact of their hyperhidrosis on different parts of their body separately. Therefore, ‘within-patient’ study designs were considered to be a key source of bias when the HDSS was used to assess disease severity or for assessments of quality of life. However, ‘within-patient’ study designs were not considered to be a key source of bias for more objective outcomes, such as gravimetry.
The quality of the body of evidence identified was classed according to a modified version of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) classification (very low, low, moderate, high or insufficient) for each intervention and comparison, taking into account the following criteria: study risk of bias, directness of evidence, heterogeneity, precision of effect estimates, risk of publication bias and magnitude of effect.
Data analysis
The results for each of the different treatment types are presented in separate sections within Chapter 3, Results of studies included in the review. Study characteristics and results are presented in a series of structured tables in Appendix 2. Quality assessment results are discussed and tabulated in Chapter 3, Quality of studies included in the review. The results were interpreted in the context of the quality of the individual studies.
Results were pooled in a pairwise meta-analysis if at least two studies reported the same outcome and were considered sufficiently similar for analysis to be appropriate and feasible. Otherwise, the results were summarised in a narrative synthesis. Although a network meta-analysis (NMA) was required to conduct the VOI analyses (see Chapter 6), the evidence was considered too heterogeneous and limited to perform a NMA to address the clinical review questions.
When meta-analyses were performed, dichotomous outcomes were combined to estimate pooled risk ratios (RRs) using standard random-effects DerSimonian–Laird meta-analyses35 and continuous outcomes were combined to estimate pooled mean differences (MDs) using standard random-effects inverse variance meta-analyses. Heterogeneity was assessed using the I2-statistic and visual inspection of forest plots.
For studies that included two intervention groups with two different doses and used one control group, data from each intervention group were entered separately to explore any dose response effect and the number of participants in the control group was divided by two to reduce the risk of double-counting data. 36 Although this approach may artificially reduce the power of the study in the meta-analysis and does not account for potential correlation between the two active treatment groups, a separate analysis combining the two arms showed no significant difference in results.
Studies using different units of analysis (i.e. axilla in half-side comparisons vs. patients in between-patient comparisons) were pooled where deemed appropriate and reported in separate subgroups. Meta-regressions and other subgroup analyses were considered inappropriate due to the small number of studies. All analyses were conducted using Review Manager 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark).
Chapter 3 Results of the clinical effectiveness review
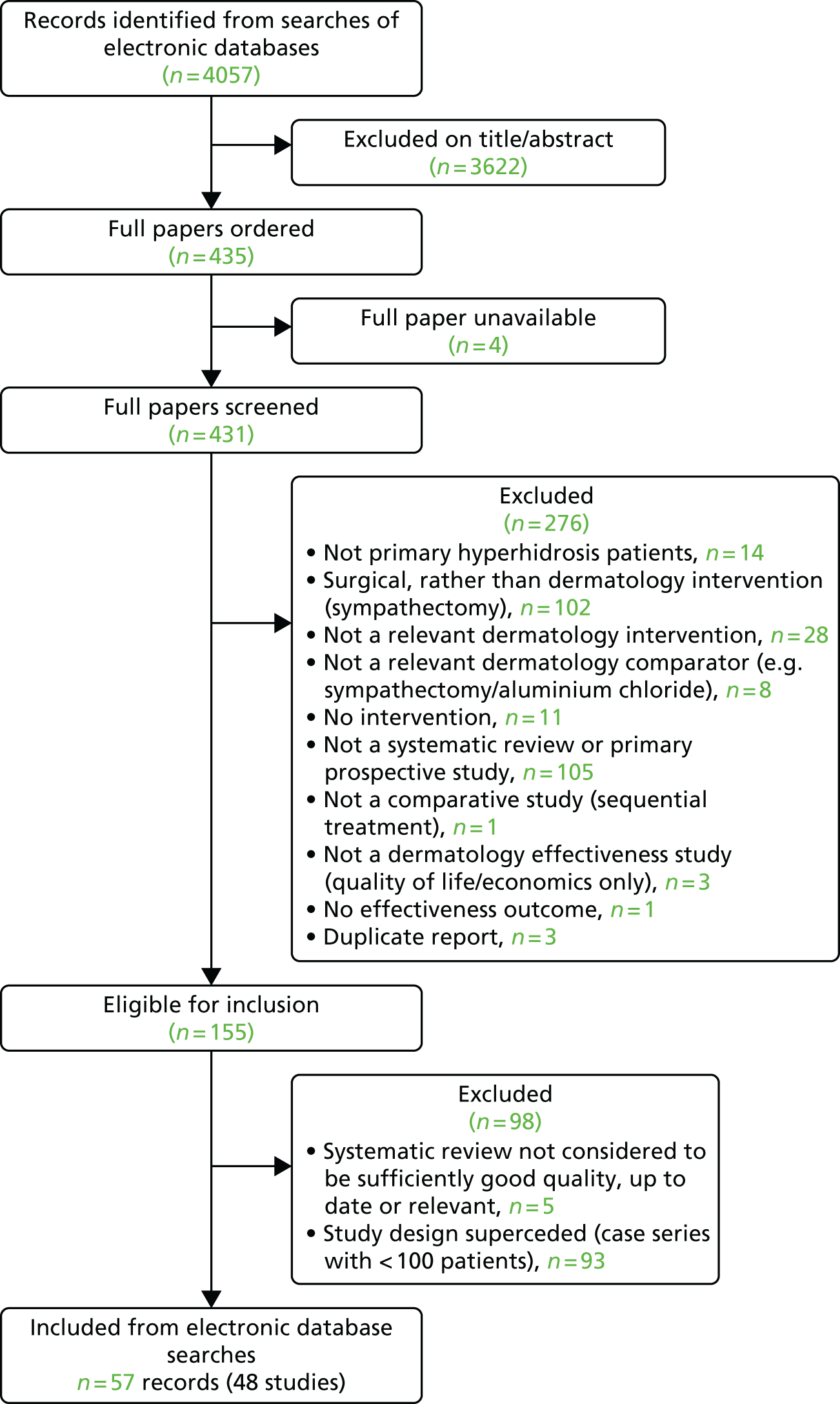
Flow of studies through the review of effectiveness
The electronic searches identified a total of 4057 records: 3572 records were identified from the clinical effectiveness searches, 337 records were identified from the quality-of-life searches and 148 records were identified from the cost-effectiveness searches. The 4057 records were inserted into an EndNote X8 library (Thomson Reuters, CA, USA). Just over 10% of the records (423/4057) were double screened and the level of agreement between the researchers was assessed. The level of agreement was 96.2% (407/423 records), which was considered adequate, and, therefore, the remaining records were single screened for study inclusion.
The full papers of 435 potentially relevant primary studies were ordered for inclusion screening. Four papers were unavailable and, therefore, 431 records were screened. A total of 276 studies were excluded at the full-paper stage. Details of these studies, along with the reason for their exclusion, are provided in Appendix 3.
Table 1 presents the 155 records that were eligible for inclusion in the systematic review to inform the clinical effectiveness and safety of second-line treatments that would be available for prescription by dermatologists and minor surgical treatments. For each specific intervention, we prioritised the more robust study designs; this resulted in 93 small (< 100 participants) case series studies being excluded because RCTs or non-randomised comparative studies were available for the specific intervention they assessed. Five additional studies were excluded because they were systematic reviews that were not considered to be of sufficiently good quality, up to date or directly relevant to be relied on.
| Study details | Intervention | Study design |
|---|---|---|
| Dahl and Glent-Madsen 1989 37 | Iontophoresis | RCT |
| Dolianitis et al. 2004 38 | Iontophoresis | Non-RCT |
| Karakoç et al. 2002 39 | Iontophoresis | Case series (n = 112) |
| Karakoc et al. 2004 40 | Iontophoresis | Non-RCT |
| Shimizu et al. 2003 41 | Iontophoresis | RCT |
| Stolman 1987 42 | Iontophoresis | RCT |
| Na et al. 2007 43 | Dry iontophoresis | Non-RCT |
| Choi et al. 2013 44 | Dry iontophoresis | Non-RCT |
| Akbar et al. 201345 | Iontophoresis | Case series |
| Chia et al. 201046 | Iontophoresis | Case series |
| Hölzle and Alberti 198747 | Iontophoresis | Case series |
| Ohshima et al. 200848 | Iontophoresis | Case series |
| Shams and Kavanagh 201149 | Iontophoresis | Case series |
| Wollina et al. 199850 | Iontophoresis | Case series |
| Hyun et al. 2015 51 | Glycopyrrolate | RCT |
| Mehrotra et al. 2014 52 | Glycopyrrolate | RCT (linked study to Mehrotra et al. 2015 53 ) |
| Mehrotra et al. 2015 53 | Glycopyrrolate wipes | RCT |
| Hale et al. 201454 | Glycopyrrolate | Case series |
| Lee et al. 201255 | Glycopyrrolate | Case series |
| Mackenzie et al. 201356 | Glycopyrrolate cream | Case series |
| Costa et al. 2014 57 | Oxybutynin | RCT |
| Costa et al. 2015 58 | Oxybutynin | RCT (linked study to Costa et al. 2014 57 ) |
| Schollhammer et al. 2015 59 | Oxybutynin | RCT |
| Wolosker et al. 2012 60 | Oxybutynin | RCT |
| Try et al. 201261 | Oxybutynin | Case series |
| Wolosker et al. 201162 | Oxybutynin | Case series |
| Wolosker et al. 201163 | Oxybutynin | Case series |
| Wolosker et al. 201364 | Oxybutynin | Case series |
| Wolosker et al. 201465 | Oxybutynin | Case series |
| Wolosker et al. 201466 | Oxybutynin | Case series |
| Wolosker et al. 201567 | Oxybutynin | Case series |
| Hund et al. 2004 68 | Methantheline bromide | RCT |
| Müller et al. 2013 69 | Methantheline bromide | RCT |
| Fuchslocher and Rzany 200270 | Methantheline bromide | Case series |
| Glogau 2007 71 | BTX (topical) | RCT |
| Balzani et al. 2001 72 | BTX | RCT |
| Baumann et al. 2005 73 | BTX | RCT |
| Baumann et al. 2005 74 | BTX | RCT |
| Heckmann et al. 2001 75 | BTX | RCT |
| Lowe et al. 2002 76 | BTX | RCT |
| Lowe et al. 2007 77 | BTX | RCT |
| Lowe et al. 2002 78 | BTX | RCT (linked study to Naumann and Lowe 2001 79 ) |
| Naumann and Lowe 2001 79 | BTX | RCT |
| Naumann et al. 2003 80 | BTX | RCT |
| Naver et al. 2000 81 | BTX | RCT |
| Odderson 2002 82 | BTX | RCT |
| Ohshima et al. 2013 83 | BTX | RCT |
| Schnider et al. 1997 84 | BTX | RCT |
| Schnider et al. 1999 85 | BTX | RCT |
| Naumann et al. 2002 86 | BTX | RCT (linked study to Naumann and Lowe 2001 79 ) |
| Ibrahim et al. 2013 21 | BTX vs. curettage | RCT |
| Ibrahim et al. 2013 87 | BTX vs. curettage | RCT (linked study to Ibrahim et al. 2013 21 ) |
| Rajagopal and Mallya 2014 88 | BTX vs. iontophoresis | RCT |
| Heckmann et al. 1999 89 | BTX | Non-RCT |
| Yamashita et al. 2008 90 | BTX | Non-RCT |
| Baker 2013 91 | BTX vs. glycopyrrolate | Non-RCT |
| Wachal et al. 2009 92 | BTX vs. iontophoresis | Non-RCT |
| Absar and Onwudike 200893 | BTX | Case series |
| Maltese et al. 201294 | BTX | SR |
| Naumann et al. 201314 | BTX | SR |
| Shayesteh and Nylander 201195 | BTX | SR |
| Aghaei 200796 | BTX | Case series |
| Baker and Hansen 201197 | BTX | Case series |
| Basciani et al. 201498 | BTX | Case series |
| Bechara et al. 200799 | BTX | Case series |
| Böger et al. 2000100 | BTX | Case series |
| Campanati et al. 2003101 | BTX | Case series |
| Campanati et al. 2011102 | BTX | Case series |
| Chattopadhyay et al. 2009103 | BTX | Case series |
| Chow and Wilder-Smith 2009104 | BTX | Case series |
| Coutinho dos Santos et al. 2009105 | BTX | Case series |
| D’Epiro et al. 2014106 | BTX | Case series |
| Glaser et al. 2007107 | BTX | Case series |
| Glaser et al. 2015108 | BTX | Case series |
| Glogau 1998109 | BTX | Case series |
| Gregoriou et al. 2010110 | BTX | Case series |
| Hanlon et al. 2006111 | BTX | Case series |
| Hasson et al. 2014112 | BTX | Case series |
| Heckmann et al. 1998113 | BTX | Case series |
| Ito et al. 2011114 | BTX | Case series |
| James et al. 2005115 | BTX | Case series |
| Karlqvist et al. 2014116 | BTX | Case series |
| Karlqvist et al. 2015117 | BTX | Case series |
| Kinkelin et al. 2000118 | BTX | Case series |
| Kouris et al. 2014119 | BTX | Case series |
| Kouris et al. 2014120 | BTX | Case series |
| Kouris et al. 2015121 | BTX | Case series |
| Krogstad et al. 2004122 | BTX | Case series |
| Krogstad et al. 2005123 | BTX | Case series |
| Lowe et al. 2003124 | BTX | Case series |
| Moffat et al. 2009125 | BTX | Case series |
| Naumann et al. 1998126 | BTX | Case series |
| Naver et al. 1999127 | BTX | Case series |
| Swartling et al. 2001128 | BTX | Case series |
| Nelson et al. 2005129 | BTX | Case series |
| Ogden et al. 2009130 | BTX | Case series |
| Paracka et al. 2015131 | BTX | Case series |
| Pérez-Bernal et al. 2005132 | BTX | Case series |
| Pinelli et al. 2000133 | BTX | Case series |
| Rosell et al. 2013134 | BTX | Case series |
| Salmanpoor and Rahmanian 2002135 | BTX | Case series |
| Scamoni et al. 2012136 | BTX | Case series |
| Schnider et al. 2001137 | BTX | Case series |
| Shelley et al. 1998138 | BTX | Case series |
| Solish et al. 2005139 | BTX | Case series |
| Solomon and Hayman 2000140 | BTX | Case series |
| Vadoud-Seyedi 2004141 | BTX | Case series |
| von Rhein et al. 2004142 | BTX | Case series |
| Weber et al. 2005143 | BTX | Case series |
| Wollina and Karamfilov 2001144 | BTX | Case series |
| Paracka et al. 2015145 | BTX | Case series |
| Wollina et al. 2002146 | BTX | Case series |
| Tronstad et al. 2014 147 | Liposuction curettage | RCT |
| Rompel and Scholz 2001 148 | Curettage vs. BTX | Non-RCT |
| Jemec 1975 149 | Curettage vs. excision | Non-RCT |
| Bechara et al. 2008 150 | Liposuction curettage vs. excision vs. Shelley’s procedure | RCT |
| Leclere et al. 2015 24 | Liposuction curettage vs. laser epilation | RCT |
| Darabaneanu et al. 2008151 | Curettage | Case series |
| Feldmeyer et al. 2015152 | Curettage | Case series |
| Proebstle et al. 2002153 | Curettage | Case series |
| Bechara et al. 2008 154 | Liposuction curettage | Non-RCT |
| Ottomann et al. 2007 155 | Liposuction curettage vs. BTX | Non-RCT |
| Hasche et al. 1997156 | Liposuction | Case series |
| Bechara et al. 2007157 | Liposuction curettage | Case series |
| Bechara et al. 2007158 | Liposuction curettage | Case series |
| Bechara et al. 2012 25 | Laser epilation | RCT |
| Letada et al. 2012 26 | Laser epilation | RCT |
| Caplin 2013159 | Laser | Case series |
| Chasin 2012160 | Laser | Case series |
| Fratila and Reckmeyer 2014161 | Laser | Case series |
| Goldman and Wollina 2008162 | Laser | Case series |
| Hoffmann 2012163 | Laser | Case series |
| Katz and Cangello 2014164 | Laser | Case series |
| Kim et al. 2010165 | Laser | Case series |
| Penrose et al. 2011166 | Laser | Case series |
| Yanes 2013167 | Laser | Case series |
| Canadian Agency for Drugs and Technologies in Health 2015168 | Laser | SR |
| Tataru and Avram 2013169 | Laser assisted lipolysis | Case series |
| Abtahi-Naeini et al. 2015 170 | Fractionated microneedle radiofrequency | Case series (linked study to Fatemi Naeini et al. 2015 28 ) |
| Fatemi Naeini et al. 2015 28 | Fractionated microneedle radiofrequency | Non-RCT |
| Kim et al. 2013171 | Fractionated microneedle radiofrequency | Case series |
| Glaser et al. 2012 27 | Microwave | RCT |
| Kilmer et al. 2011 172 | Microwave | RCT (linked study to Glaser et al. 2012 27 ) |
| Hong et al. 2012173 | Microwave | Case series |
| Lee et al. 2013174 | Microwave | Case series |
| Lupin et al. 2011175 | Microwave | Case series |
| Lupin et al. 2012176 | Microwave | Case series |
| Lupin et al. 2014177 | Microwave | Case series (linked study to Hong et al. 2012173) |
| Nestor and Park 2014 29 | Microfocused ultrasound | RCT (two RCTs reported in one publication) |
| Nestor and Park 2012 178 | Microfocused ultrasound | RCT (linked study to Nestor and Park 2014 29 ) |
| Nestor and Park 2013 179 | Microfocused ultrasound | RCT (linked study to Nestor and Park 2014 29 ) |
| Nestor and Park 2013 180 | Microfocused ultrasound | RCT (linked study to Nestor and Park 2014 29 ) |
| Pinson et al. 2014181 | Radiofrequency device | Case series |
| Commons and Lim 2009182 | VASER ultrasound | Case series |
| Nicholas et al. 2015183 | Dermatology and surgery | SR |
Forty-eight studies identified by electronic searching were included in the systematic review (reported in 57 papers); the 57 included records are highlighted in bold in Table 1.
No additional studies were identified by screening reference lists of relevant systematic reviews or from contact with clinical advisors. Figure 1 presents the flow of studies through the study selection process from the main searches.
FIGURE 1.
Flow diagram of the study selection process.
The separate searches of conference proceedings and trial registers, conducted in July 2016, identified a total of 306 records (80 of which were considered to be potentially relevant for the review). After the 80 records were de-duplicated against the original library, 31 were excluded as being duplicate reports, leaving 49 potentially relevant records. Ten were identified from searches of conference abstracts and 39 were identified from searches of trial registers.
Further details of the 10 conference abstracts were obtained, resulting in eight records being excluded: one had no intervention, one was found to be a duplicate report, three did not report an effectiveness outcome and three were small case series. Therefore, two additional records relating to completed studies, reported as conference abstracts, were included in the review. 184,185
Further details of the 39 records identified from trial registers were sought. One record was found to be a duplicate report, one was a study that was registered but later withdrawn (prior to recruiting participants), and an additional two studies were very small case series, so were rejected from the review. Thirteen of the records identified from trial registers were found to be ongoing trials; details are reported in Ongoing studies.
Pharmaceutical company websites and trial registration databases were searched for additional information on the remaining 22 studies and, when contact details were available, principal investigators were contacted to supply research findings or published reports. One study was found to have been terminated because of a high sham response, two studies were found to have been extended, with no data currently available, and a further two studies were found to be case series studies of an intervention for which several RCTs had already been identified. For eight records, no further information was found and principal investigators did not respond to e-mail requests (when contact details were identified). For four records, the investigators responded to e-mail requests, but did not offer any additional data. Five studies, relating to the same intervention (DRM04), were conducted by the same pharmaceutical company, which responded that their data were due to be presented at an upcoming conference. They agreed to share their results after the conference; however, data were not supplied in time to be included in the review.
A separate flow chart is presented with further details of the searches of conference proceedings and trial registers (Figure 2).
FIGURE 2.
Flow diagram of the study selection process for conference proceedings and trial register searches.

Characteristics of studies included in the review of effectiveness
There was substantial variation among the 32 RCTs, 17 non-RCTs and one case series study that were included in the review. Sample sizes ranged from 472 to 339,69 with most studies including < 50 patients.
Studies were conducted in a range of different countries: 23 studies were conducted in European countries [Germany (n = 925,68,69,75,89,148,150,154,155), the UK (n = 291,184), Denmark (n = 237,149), Austria (n = 284,85), Poland (n = 192), Sweden (n = 181), France (n = 159), Italy (n = 172), Norway (n = 1147) and multiple European countries (n = 324,79,80)], 15 studies were conducted in the Americas [USA (n = 1321,26,27,29,42,53,71,73,74,76,77,82) and Brazil (n = 257,60)], 11 studies were conducted in Asian countries [Japan (n = 441,83,90,185), South Korea (n = 343,44,51), Turkey (n = 239,40), India (n = 188) and Iran (n = 128)] and one study was conducted in Australia. 38 Therefore, many of the study populations may not be representative of hyperhidrosis patients in the UK, because of the differences in climate.
Studies were published between 1975149 and 2016;184 42 out of the 50 studies were published since 2000 and 19 of the 50 studies were published since 2010. Generally, studies of topical glycopyrrolate, oxybutynin and the newer minor surgical devices (laser epilation, fractionated microneedle radiofrequency, microwave and ultrasound) were the most recent (published after 2010), whereas some of the studies of iontophoresis, BTX and curettage were > 15 years old; therefore, some of the devices, formulations and procedures used in earlier studies may not be applicable to current practice.
When reported, all studies included adults, although 10 studies included a small number of participants aged < 18 years. 38–40,44,53,80,82,88,155,184 The majority of patients in the studies that reported the sex of participants were female; two studies included only female patients,57,72 while the rest of the studies included both male and female patients. Inclusion criteria relating to disease severity were reported in 31 studies; baseline severity was usually moderate to severe, with a HDSS score of 3 or 4 points and/or a sweat rate of at least 50 mg per 5 minutes.
As expected, the site of hyperhidrosis differed between studies of different interventions. Studies of iontophoresis all included patients with palmar hyperhidrosis, some of whom also had plantar hyperhidrosis. 38,41 All the studies of curettage, laser epilation, fractionated microneedle radiofrequency, microwave and ultrasound included only patients with axillary hyperhidrosis. All studies of BTX included patients with hyperhidrosis of the axilla except for seven74,76,81,84,88,90,92 that studied plantar hyperhidrosis. Studies of topical glycopyrrolate included patients with hyperhidrosis of the axilla53,91 or forehead. 51 Studies of methantheline bromide included patients with axillary and/or palmar hyperhidrosis. 68,69 Studies of oxybutynin included patients with hyperhidrosis of the axilla and palm,60 foot57 or generalised59 hyperhidrosis.
In the majority of comparative studies, the intervention was compared against a placebo/sham device or no treatment, although some studies compared two or more active treatments, such as BTX, compared with iontophoresis,88,92 BTX compared with glycopyrrolate,91 BTX compared with curettage,21,148,155,184 curettage compared with laser epilation,24 or curettage compared with more radical surgery. 149,150 Table 2 shows the comparisons made in the included comparative studies.
| Intervention | Iontophoresisa | Topical glycopyrrolate | Oxybutynin | Methantheline bromide | BTX | Curettageb | Laser epilation | Radical excision | Shelley’s procedure | Fractionated microneedle radiofrequency | Microwave | Ultrasound | Placebo | No treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iontophoresisa | 2c | 2 | 3 | 2 | ||||||||||
| Topical glycopyrrolate | 1 | 2 | 1 | |||||||||||
| Oxybutynin | 3 | |||||||||||||
| Methantheline bromide | 2 | |||||||||||||
| BTX | 2 | 1 | 4 | 13 | 4 | |||||||||
| Curettageb | 4 | 2d | 1e | 2 | 1 | |||||||||
| Laser epilation | 1e | 1 | 2 | |||||||||||
| Radical excision | 2 | |||||||||||||
| Shelley’s procedure | 1 | |||||||||||||
| Fractionated microneedle radiofrequency | 1 | |||||||||||||
| Microwave | 1 | |||||||||||||
| Ultrasound | 2 | |||||||||||||
| Placebo | 3 | 2 | 3 | 2 | 13 | 1 | 1 | 2 | ||||||
| No treatment | 2 | 1 | 4 | 2 |
Outcomes assessed in the studies included disease severity, sweat rate, patient-reported effectiveness, quality of life, satisfaction and adverse events. However, methods of assessing outcomes varied between studies. Disease severity was often assessed using the HDSS, although some studies also used a patient or physician assessment of disease severity or improvement. Sweat rate was assessed using gravimetry, iodine starch test, digitised ninhydrin-stained sheets, the Sakurai–Montagna sweating test, corneometry, evaporimetry or a bespoke sweat reduction questionnaire or visual analogue scale (VAS); some studies assessed sweat rate under stressful conditions, such as during a maths test. Some studies assessed patient-reported treatment effectiveness and willingness to undergo repeat treatment. Quality of life was assessed using a range of questionnaires, such as the DLQI, HHIQ and Short Form questionnaire-12 items (SF-12). HDSS was also used as a quality-of-life tool in some studies. Further details of quality-of-life tools used in hyperhidrosis studies are presented in Chapter 4. Patient satisfaction was assessed using questionnaires. Some studies of BTX used a dynamometer to assess grip strength. Some studies of minor surgical interventions also undertook histological analysis. Timing of the outcome assessment varied between the studies, ranging from around 1 week to > 1 year. Outcome assessment tended to be shorter for the shorter-lasting topical treatments and iontophoresis, and much longer for the longer-lasting BTX injections and minor surgical treatments, as might be expected.
Summary study characteristics and results for the separate interventions assessed are presented in Appendix 2. Interventions likely to be tried at the beginning of the treatment pathway are presented first (iontophoresis, systemic agents, BTX), with minor surgical procedures (curettage, laser, etc.) at the end of the tables.
Quality of studies included in the review
Thirty-two of the included studies were RCTs, 17 were non-RCTs and one study was a case series. The results of the quality assessment of the RCTs and non-RCTs, using the Cochrane risk of bias tool, are presented in Table 3. Each trial was given an overall risk of bias judgement, as described in Chapter 2, Quality assessment strategy.
| Study | Sequence generation adequate (key domain) | Allocation concealment adequate (key domain) | Prognostic factors similar at baseline (key domain) | Participants blind to treatment allocation (key domain) | Care providers blind to treatment allocation | Outcome assessors blind to treatment allocationa | Missing outcome data adequately addressed (key domain) | Report free from selective outcome reporting | Free from ‘within-patient’ study design | Overall risk of bias judgement |
|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al. 201344 | N/A | N/A | Unclear | No | No | Unclear (sweat rate); no (satisfaction) | Unclear | Unclear | No | High |
| Dahl and Glent-Madsen 198937 | Unclear | Unclear | Unclear | Yes | Yes | Unclear | Unclear | Unclear | No | Unclear |
| Na et al. 200743 | N/A | N/A | Unclear | No | No | Unclear (sweat rate); no (satisfaction) | Unclear | No | No | High |
| Rajagopal and Mallya 201488 | Unclear | Unclear | Unclear | Unclear | No | Unclear | No | Unclear | Yes | High |
| Stolman 198742 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | No | Unclear |
| Wachal et al. 200992 | N/A | N/A | Unclear | No | No | No | Unclear | Unclear | Yes | High |
| Dolianitis et al. 200438 | N/A | N/A | Unclear | Yes | No | Yes | Yes | Unclear | No | High |
| Karakoc et al. 200440 | N/A | N/A | Yes | Yes | No | No | Unclear | Unclear | No | High |
| Shimizu et al. 200341 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear |
| Baker 201391 | N/A | N/A | Unclear | Unclear | Unclear | Unclear | Unclear | No | Yes | High |
| Mehrotra et al. 201553 | Unclear | Unclear | No | Yes | Unclear | Unclear (sweat rate); yes (HDSS) | Yes | Unclear | Yes | High |
| Hyun et al. 201551 | Yes | Unclear | Unclear | Yes | Unclear | Unclear (sweat rate); yes (HDSS) | Yes | No | No | Unclear (gravimetry); high (HDSS) |
| Wolosker et al. 201260 | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | No | Yes | Unclear |
| Costa et al. 201457 and Costa et al. 201558 | Unclear | Unclear | Yes | Yes | Yes | Unclear (sweat rate); yes (quality of life) | Unclear | Unclear | Yes | Unclear |
| Schollhammer et al. 201559 | Yes | Unclear | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Unclear |
| Hund et al. 200468 | Unclear | Unclear | No | Unclear | Unclear | Unclear | Yes | No | Yes | High |
| Müller et al. 201369 | Yes | Unclear | Yesb (palm sweat rate differed between groups at baseline) | Yes | Yes | Unclear (sweat rate); yes (HDSS, quality of life) | Yes | No | Yes | Unclear (HDSS, DLQI, axilla gravimetry); high (palm gravimetry) |
| Balzani et al. 200172 | Unclear | Unclear | Unclear | Unclear | No | No | Yes | No | Yes | Unclear |
| Baumann et al. 200573 | Unclear | Unclear | No | Yes | Yes | Unclear (physician assessment); yes (quality of life) | No | No | Yes | High |
| Glogau 200771 | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Yes | Unclear | No | Unclear |
| Heckmann et al. 199989 | N/A | N/A | Unclear | No | No | No | Yes | Unclear | No | High |
| Heckmann et al. 200175 | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Unclear | No | Low |
| Ibrahim et al. 201321 and Ibrahim et al. 201387 | Yes | Yes | Yes | No c | No | Unclear (sweat rate); no (HDSS) | Yes | Yes | No | High |
| Lowe et al. 200777 | Unclear | Unclear | Yesb (DLQI scores differed between groups at baseline) | Yes | Yes | Unclear (sweat rate); yes (HDSS, quality of life) | Unclear | Unclear | Yes | Unclear (HDSS, gravimetry); high (DLQI) |
| Naumann and Lowe 2001;79 Naumann et al. 200286 and Lowe et al. 200278 | Yes | Unclear | Unclear | Yes | Unclear | Unclear (sweat rate); yes (quality of life, satisfaction) | Yes | Unclear | Yes | Unclear |
| Naumann et al. 200380 | N/A | N/A | Unclear | No | No | Unclear (sweat rate); no (satisfaction) | Unclear | Unclear | Yes | High |
| Odderson 200282 | Unclear | Unclear | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear |
| Ohshima et al. 201383 | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Unclear | Yes | Unclear |
| Rompel and Scholz 2001148 | N/A | N/A | Unclear | No c | No | No | No | Unclear | Yes | High |
| Schnider et al. 199985 | Unclear | Unclear | Unclear | Yes | Yes | Unclear (sweat rate); yes (patient-assessed sweating, satisfaction) | Unclear | Unclear | No | Unclear |
| Vakili and Baker 2016184 | N/A | N/A | Unclear | No c | Unclear | No | Unclear | Unclear | Yes | High |
| Wakugawa et al. 2001185 | N/A | N/A | Unclear | No | Unclear | Unclear | Unclear | No | No | High |
| Baumann et al. 200574 | Unclear | Unclear | No | Yes | Yes | Unclear (sweat rate); yes (quality of life) | No | No | Yes | High |
| Lowe et al. 200276 | Yes | Unclear | Unclear | Yes | Yes | Unclear (sweat rate); yes (patient-assessed sweating) | Unclear | Unclear | No | Unclear |
| Schnider et al. 199784 | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes | Unclear | No | Unclear |
| Yamashita et al. 200890 | N/A | N/A | Unclear | No | No | Unclear | No | Unclear | No | High |
| Naver et al. 200081 | N/A | N/A | Unclear | No | No | Yes (sweat rate); no (patient-assessed sweating) | Yes | Unclear | No | High |
| Bechara et al. 2008150 | Unclear | Unclear | Unclear | No c | No | Unclear (sweat rate); no (satisfaction) | Unclear | Unclear | Yes | High |
| Bechara et al. 2008154 | N/A | N/A | Unclear | Unclear | No | Unclear | Unclear | No | No | High |
| Jemec 1975149 | N/A | N/A | Unclear | No c | No | No | Unclear | Unclear | Yes | High |
| Leclere et al. 201524 | Unclear | Unclear | Unclear | No c | Unclear | Unclear (sweat rate); no (HDSS, satisfaction) | Yes | No | Yes | High |
| Ottomann et al. 2007155 | N/A | N/A | Unclear | No c | Unclear | Unclear (sweat rate); no (quality of life) | Unclear | Unclear | Yes | High |
| Tronstad et al. 2014147 | Unclear | Unclear | Unclear | Unclear | No | Unclear | Yes | No | No | Unclear (gravimetry); high (DLQI) |
| Bechara et al. 201225 | Unclear | Unclear | Unclear | No | No | Unclear (sweat rate); no (satisfaction) | Yes | Unclear | No | High |
| Letada et al. 201226 | Unclear | Unclear | Unclear | No | No | Unclear (sweat rate); no (patient-assessed sweating) | Yes | Unclear | No | High |
| Fatemi Naeini et al. 201528 and Abtahi-Naeini et al. 2015170 | N/A | N/A | Unclear | Yes | No | No (sweat rate); yes (HDSS, patient-assessed sweating, quality of life, satisfaction) | Yes | Unclear | No | High |
| Glaser et al. 201227 and Kilmer et al. 2011172 | Unclear | Unclear | No | Yes | No | Yes | Unclear | Yes | Yes | High |
| Nestor and Park 2014;29 Nestor and Park 2012;178 Nestor and Park 2013179 and Nestor and Park 2013180 | Study 1: unclear; study 2: unclear | Study 1: unclear; study 2: unclear | Study 1: unclear; study 2: unclear | Study 1: yes; study 2: yes | Study 1: unclear; study 2: unclear | Study 1: unclear; study 2: unclear (sweat rate); yes (HDSS, satisfaction) | Study 1: no; study 2: no | Study 1: no; study 2: unclear | Study 1: no; study 2: yes | Study 1: high; study 2: high |
| Total | Yes, n = 8; no, n = 17; unclear, n = 24 | Yes, n = 2; N/A, n = 17; unclear, n = 30 | Yes, n = 6; no, n = 5; unclear, n = 38 | Yes, n = 24; no, n = 17; unclear, n = 8 | Yes, n = 12; no, n = 21; unclear, n = 16 | Yes, n = 21 (for at least one outcome); no, n = 17 (for at least one outcome); unclear, n = 32 (for at least one outcome) | Yes, n = 21; no, n = 7; unclear, n = 21 | Yes, n = 3; no, n = 14; unclear, n = 32 | Yes, n = 26; no, n = 23; unclear, n = 0 | Low, n = 1; high, n = 30 (an additional 4 ‘unclear’ risk studies had a high risk of bias for one specific outcome each); unclear, n = 18 |
Generally, methods were poorly reported, with a high proportion of assessments having to be recorded as unclear. It was clear that the allocation sequence was adequately generated in only eight RCTs;21,51,59,69,75,76,79,83 methods of sequence generation were unclear in the other 24 RCTs. 24–27,29,37,41,42,53,57,60,68,71–74,77,82,84,85,88,147,150 Seventeen studies were non-RCTs;28,38,40,43,44,80,81,89–92,148,149,154,155,184,185 therefore, there was no randomised sequence generation. Concealment of allocation was also poorly reported; only two RCTs reported adequate methods, and methods were unclear in the remaining 30 RCTs. Again, this criterion was not relevant for the 17 non-RCTs. Study groups were generally similar at baseline in terms of most prognostic factors in only six studies,21,40,57,59,69,77 although, for two of these studies, one of the prognostic factors differed at baseline, which may have affected one of the outcomes reported. 69,77 There were differences between groups in important prognostic characteristics in five studies27,53,68,73,74 and insufficient data were available to assess similarity of baseline characteristics in 38 studies. 24–26,28,29,37,38,41–44,51,60,71,72,75,76,79–85,88–92,147–150,155,184,185
Twenty-four studies reported blinding of participants to treatment group,27–29,37,38,40,51,53,57,59,60,69,71,73–77,79,82–85 in 17 studies participants were not blinded,21,24–26,43,80,81,89,90,92,148–150,155,184,185 and in eight studies it was unclear whether or not participants were blinded. 41,42,68,72,88,91,147,154 It is not always feasible to blind patients in studies comparing minor surgical interventions with non-surgical interventions, or minor surgical interventions with more major surgery; such studies were considered to have a high overall risk of bias, owing to the potential effect on outcomes, many of which are subjective. Blinding of care providers was reported in only 12 studies;37,57,69,73–77,82–85 in 21 studies it was clear that care providers were not blinded,21,25–28,38,40,43,44,72,80,81,88–90,92,147–150,154 and in 16 studies it was unclear whether or not care providers were blinded. 24,29,41,42,51,53,59,60,68,71,79,91,155,184,185 Blinding of outcome assessors, for at least one outcome, was reported in 21 studies (for subjective outcomes, the patient was considered to be the outcome assessor),27–29,38,51,53,57,59,60,69,73–77,79,81–85 at least one outcome was assessed unblinded in 17 studies,21,25,26,28,40,43,44,72,80,81,89,92,148–150,155,184 and in 32 studies it was unclear whether or not outcome assessors were blinded, for at least one outcome. 21,24–26,29,37,41–44,51,53,57,68,69,71,74,76,77,79,80,85,88,90,91,147,150,154,155,185
Either outcome data were complete or incomplete outcome data were adequately addressed in 21 studies;21,24–26,28,38,51,53,59,60,68,69,71,72,75,79,81,83,84,89,147 seven studies did not adequately address missing outcome data,29,73,74,88,90,148 and in 21 studies it was unclear whether or not missing outcome data were adequately addressed. 27,37,40–44,57,76,77,80,82,85,91,92,149,150,154,155,184,185 Only three studies appeared to be free of the suggestion of selective outcome reporting21,27,59 and it was unclear whether or not 32 studies were free of the suggestion of selective outcome reporting; many studies did not have protocols available in order to make this assessment. 85,88–90,92,148–150,155,184 Twenty-three studies used a ‘within-patient’ study design,21,25,26,28,29,37,38,40,42–44,51,71,75,76,81,84,85,89,90,147,154,185 in which patients received different interventions on different sides of the body. Clinical advisors to the project considered that this type of study was of limited use in hyperhidrosis research, particularly for subjective outcome tools, such as the HDSS, in which patients are asked to judge the tolerability and impact of their hyperhidrosis on different sides of their body separately.
Overall, only one RCT,75 which compared botulinum toxin type A (BTX-A) with placebo in 145 patients with hyperhidrosis of the axilla, was rated as having a low overall risk of bias, scoring ‘yes’ for all four key domains. Although it was a ‘within-patient’ study design, the outcomes assessed were sweating (assessed using gravimetry), patient satisfaction and adverse events; therefore, the ‘within-patient’ study design is unlikely to have significantly biased these outcomes. 75
Five studies51,59,69,79,83 scored reasonably well on the risk-of-bias assessment, achieving a ‘yes’ response in three of the four ‘key’ domains and ‘unclear’ in the remaining key domain. All five studies were considered to have an unclear overall risk of bias, although they appeared to be of better quality than most of the other included studies. In all five studies, it was the method of allocation concealment that was not adequately reported. Two of the studies, involving 32079, and 15283 patients with hyperhidrosis of the axilla, compared BTX-A with placebo. One of the studies compared glycopyrrolate with placebo in 39 patients with facial hyperhidrosis. This study had a ‘within-patient’ study design and measured sweat rate using gravimetry and disease severity using the HDSS. Therefore, in this study, overall risk of bias was considered to be unclear for sweat rate, but high for the HDSS result, owing to the difficulty patients may have had scoring different sides of their face separately for tolerability and interference with daily activities. 51 One of the studies compared oxybutynin with placebo in 62 patients with mostly generalised hyperhidrosis. 59 The final study that scored reasonably well on the risk-of-bias assessment compared methantheline bromide with placebo in 339 patients with hyperhidrosis of the axilla and palm. In this study, most patient characteristics were comparable between groups at baseline (including HDSS score, DLQI score and axilla sweat rate), but palmar sweat rate (measured by gravimetry) was higher in the placebo group than in the treatment group; therefore, in this study, overall risk of bias was considered be unclear for most outcomes but high for the outcome palmar sweat rate. 69
An additional 13 studies had an unclear overall risk-of-bias judgement (with at least two ‘unclear’ key domain responses). 37,41,42,57,60,71,72,76,79,82–85 Thirty studies had a high overall risk-of-bias judgement (plus an additional four ‘unclear’ risk studies that had a high risk of bias for one specific outcome each,21,24–29,38,40,43,44,53,68,73,74,80,88–92,148–150,154,155,184,185 because of differences in baseline characteristics for one prognostic factor69,77 or because they were ‘within-patient’ studies that assessed HDSS or DLQI,51,147 as described above).
The case series study should be considered as having a high overall risk of bias,39 as this type of study is more susceptible to bias than a controlled trial. The study had a prospective study design. It was unclear whether patients were recruited consecutively or were a selected group. In general, the descriptions of patient characteristics, intervention and outcome assessment were adequate.
The quality of the studies for each of the individual interventions is summarised along with the results of studies in Results of studies included in the review.
Results of studies included in the review
Iontophoresis
Ten studies of iontophoresis were included. 37–44,88,92 Four studies were RCTs,37,41,42,88 five were non-RCTs38,40,43,44,92 and one was a case series. 39 Risk of bias was considered high in seven studies38–40,43,44,88,92 and unclear in three studies. 37,41,42
Two studies were conducted in South Korea43,44 and two in Turkey. 39,40 The other studies were conducted in Australia,38 Denmark,37 India,88 Japan,41 Poland92 and the USA. 42
Study sample size ranged from 1043 to 11239 participants. All studies included adults and some also recruited patients aged < 18 years. 38–40,44,88 All studies included males and females. The majority of participants were female in all studies except two. 43,88 Inclusion criteria relating to patients’ baseline disease severity were only reported in three studies; in one study patients had HDSS scores of 3–4,88 one study stated that patients had excessive sweating resulting in a social or occupational handicap42 and in one study patients rated their hyperhidrosis as ‘moderate to severe’. 38
There were a number of differences in the interventions that were used. Most studies used iontophoresis with tap water in at least one group,37–42 although two studies evaluated ‘dry-type’ hand-held devices. 43,44 Iontophoresis was combined with glycopyrrolate (in the iontophoresis tray) in one study38 and with oral oxybutynin in another. 41 Another study used iontophoresis in combination with topical aluminium chloride. 88 One study compared the efficacy of using alternative current (AC) against the more conventional direct current (DC). 41 The frequency of iontophoresis sessions varied across the studies, ranging from once daily to once weekly, and the electric current used ranged from 0 to 30 mA.
Iontophoresis was compared with BTX in two studies,88,92 with iontophoresis combined with an anticholinergic in two studies,38,41 with placebo in three studies37,40,42 and with no treatment in two studies. 43,44
Five studies treated hands only37,42–44,88 and four focused on both hands and feet. 38–41 Another study treated hands and it was not clear whether or not axillae were also treated with iontophoresis. 92 There were no studies identified that specifically treated hyperhidrosis of the axilla.
Iontophoresis compared with placebo
Three very small studies (two RCTs, one interrupted time series) compared tap water iontophoresis with a placebo (see Appendix 2, Table 43). 37,40,42
Dahl and Glent-Madsen37 was a half-side comparison RCT including 11 adult patients. Patients had one hand randomised to tap water iontophoresis and the other to a sham treatment. Initial treatment involved sessions of 15 minutes, repeated one to five times a week, and continued until ‘good subjective effect’ was reported. After the initial treatment (duration not reported), six patients continued on maintenance treatment every other week. Efficacy was evaluated with gravimetry, after the initial treatment and after 3 months of maintenance treatment. After the initial treatment, the median difference between treated and placebo hand in sweat reduction was 32%, favouring the treated hand (p < 0.01). The six patients who continued maintenance treatment had an 81% (median) reduction in sweating from baseline at 3 months (p < 0.05). No adverse events were observed.
Stolman42 was a half-side placebo-controlled randomised trial evaluating tap water iontophoresis to the palm delivered for 20 minutes, three times per week, for 3 weeks, in 18 adults. Sweating was evaluated with the iodine starch test. At the end of treatment, 83% patients experienced a ‘marked reduction’ in sweating in the treated hand, whereas no change was noticed in the untreated hand. No serious adverse events were reported.
Karakoc et al. 40 was an interrupted time series study that included 15 patients who received a low-intensity AC (designed as sham treatment) iontophoresis (with tap water) for 15 minutes, eight times over 28 days on both hands, followed by iontophoresis DC (with tap water) 1 week later. Sweating was evaluated by gravimetry up to 1 week after the end of each treatment. There was no reduction in sweating 1 week after the end of the sham treatment, but there was a statistically and clinically significant reduction in sweating 1 week following iontophoresis therapy (mean reduction of 88% in sweating from baseline, from 3.1 ± 0.4 g/hour to 0.4 ± 0.1 g/hour in the right hand and from 3.2 ± 0.3 g/hour to 0.4 ± 0.1 g/hour in the left hand). No data on adverse events were reported.
Iontophoresis compared with no treatment
Two small non-randomised trials compared a ‘dry-type’ iontophoresis device for the hand with no treatment (see Appendix 2, Table 44). 43,44
Na et al. 43 was a non-randomised study evaluating the effectiveness of a ‘dry’ hand-held iontophoresis device. Ten patients were instructed to hold the device with one hand for 30 minutes daily for 1 week, then every other day for another week. The other hand received no treatment. Results were assessed with gravimetry at up to 28 days from treatment initiation. There was a statistically significant difference (p < 0.001) in sweat reduction from baseline favouring the treated hand at the end of treatment (43% vs. 2%). At 2 weeks’ follow-up there was also a statistically significant difference in mean sweat reduction from baseline favouring the intervention (18% vs. 2%), although it is unclear if this result can also be considered clinically significant. The authors stated there were no serious adverse events.
Choi et al. 44 was a non-randomised study evaluating a similar ‘dry’ hand-held device. Twenty-three patients were instructed to hold the device with the left hand for 20 minutes daily for 4 weeks. The right hand received no treatment. Effectiveness was evaluated by gravimetry, Investigator Global Assessment (IGA), Patient Satisfaction Assessment and hydration capacitance. Improvement in IGA and hydration capacitance from 2 to 8 weeks’ follow-up and after 8 weeks was significantly greater in the treated palm than in the untreated hand (p < 0.05), but the clinical significance of this finding is unclear becuase of limited reporting. There was no statistically significant difference between the two palms in gravimetry and Patient Satisfaction Assessment. There were no serious adverse events.
Iontophoresis compared with botulinum toxin
Two studies (one RCT, one non-RCT) compared iontophoresis with BTX injections for palmar hyperhidrosis (see Appendix 2, Table 45). 88,92 It was not stated in the study reports whether tap water or anticholinergic drugs were used in the water bath; however, given the recency of the studies, it is assumed that tap water was used.
Rajagopal and Mallya 201488 compared the efficacy of iontophoresis with BTX (BTX-A formulation) for the palm in a RCT of 60 patients. Thirty participants were randomised to sessions of iontophoresis (2 × 10 minutes) combined with topical aluminium chloride (20% lotion) three times per week over 4 weeks, and 30 patients received injections of BTX-A [100 units (U) per palm]. Efficacy was assessed 4 weeks after the intervention using HDSS. Patients were also asked to score improvement in their symptoms at follow-up (either mild, good, excellent or no improvement). Participants without a HDSS score improvement from baseline at 4 weeks were crossed over to the other group for another 4 weeks and those with improvement in HDSS were followed up in the same group for 6 months. At 4 weeks, the proportion of reporting an improvement in HDSS of ≥ 2 points was 57% in the BTX-A group, compared with 27% in the iontophoresis group. This difference was clinically and statistically significant (p = 0.037). Similarly, the proportion of patients who achieved a reduction in HDSS of at least 1 point /was higher in the BTX-A group (80%) than in the iontophoresis group (47%). Among non-responders, the rate of improvement was significantly higher in those switching from iontophoresis to BTX-A than in those switching from BTX-A to iontophoresis. The study reported that the effect duration in responders was greater with BTX than with iontophoresis plus topical therapy. There was a statistically significant difference favouring BTX-A in the proportion of patients rating improvement as excellent or good (80% vs. 47%; p = 0.037). There were no severe adverse events. Mild to moderate pain (n = 8) and mild temporary motor weakness (n = 1) were reported in the BTX-A group.
Wachal et al. 92 carried out a non-randomised study of 86 adults with hyperhidrosis of the hand and other upper limb. Participants received one of three interventions: iontophoresis (n = 28), BTX-A (n = 22) or sympathectomy (n = 36). Iontophoresis patients received treatment for 20 minutes every 2–3 days. The total duration of treatment was not reported. Effectiveness and willingness to undergo retreatment were assessed by patients at 1, 6 and 12 months’ follow-up, using two VASs, both ranging from 1 to 10 points (with higher scores indicating greater effectiveness/willingness). The percentage of patients assessing treatment as either good/very good (5–10 points on the VAS) was higher in the BTX-A group than in the iontophoresis group at all follow-up points, but was statistically significant only at 1 month (90.9% vs. 35.7%). Similarly, the percentage of patients who were willing to undergo retreatment was greater in the BTX-A group than in the iontophoresis group, although the difference was statistically significant only at 1 month (81.8% vs. 42.8%). The authors reported that there were no adverse effects associated with iontophoresis and no adverse event data were reported for the BTX-A group.
Iontophoresis compared with iontophoresis with anticholinergics
Two studies (one RCT, one non-RCT) compared iontophoresis alone with iontophoresis combined with anticholinergic therapy (see Appendix 2, Table 46). 38,41
Shimizu et al. 41 carried out a RCT of 52 adult patients randomised to one of three treatments for palmoplantar hyperhidrosis: iontophoresis with AC, iontophoresis (AC) plus oral oxybutynin (4 mg/day) or iontophoresis with DC. Iontophoresis therapy was delivered for 30 minutes once a week to both hands and feet. Efficacy was measured by gravimetry and the results were presented after up to 15 treatment sessions. There was a statistically and clinically significant mean reduction from baseline in all three treatment groups from after the second treatment (p < 0.05), but no statistically significant difference in effectiveness between treatment groups at the end of follow-up (AC group from 0.73 to < 0.25 mg/cm2/minute; AC + oxybutynin from 0.81 to < 0.25 mg/cm2/minute; DC group not reported). No adverse events were reported other than small vesicles on palms in the DC group (n = 5) and dry mouth and eyes in the group receiving oxybutynin (n = 2).
Dolianitis et al. 38 conducted a non-randomised half-side comparison study in which patients acted as their own control, comparing iontophoresis plus a glycopyrrolate solution with standard iontophoresis plus tap water only. Of the 20 patients included, 19 had their palms treated and one had their soles treated. All patients underwent two treatment sessions. In the first treatment, patients were blinded as to which tray contained glycopyrrolate. Patients had one palm/foot treated with iontophoresis plus a 0.05% glycopyrrolate solution and the other side received iontophoresis plus tap water only, for 10 minutes. In a second visit, both palms or soles were treated for 10 minutes with 0.05% glycopyrrolate solution. The interval between both visits was between 2 and 3 weeks for most participants. Patient daily self-assessment was used to evaluate the total number of dry-hand days (total number of days during which hands were totally dry). The intervention resulting in the highest median number of dry-hand days was bilateral glycopyrrolate (11 days, range 0–31 days), followed by unilateral glycopyrrolate (5 days, range 0–17 days) and tap water (3 days, range 0–15 days). The incidence of dry/sore throat (n = 8) was higher after bilateral glycopyrrolate than after unilateral treatment. The authors reported that there were no other adverse events.
Non-comparative studies
The study by Karakoç et al. 39 was a case series involving 112 patients who received iontophoresis for both hands, eight times over 28 days. Sweating was evaluated by gravimetry 20 days after the end of treatment (see Appendix 2, Table 47). A statistically significant reduction in sweat from baseline was reported for 81% of participants and there were no serious adverse events.
Evidence summary for iontophoresis
In summary, 10 studies (four RCTs, five non-RCTs, one case series) of iontophoresis were included. All were rated as being at high or unclear risk of bias.
Three very small studies (two RCTs, one interrupted time series) compared iontophoresis with a placebo37,40,42 and found a positive effect of iontophoresis. Two studies reported a statistically significant difference in sweat reduction (measured by gravimetry) in the short term (approximately 1 to > 3 months from treatment initiation), favouring iontophoresis. 37,40 One RCT reported a marked reduction in hand sweating at 5 days post treatment (iodine starch test) when treatment with iontophoresis was compared with no treatment. 42
Two small non-randomised half-side comparisons with no treatment, in which patients acted as their own control, evaluated a hand-held ‘dry-type’ iontophoresis device;43,44 only one of them found a statistically significant reduction in sweating (gravimetry) and a significant improvement in the treated palm compared with the untreated palm at up to 28 days from treatment initiation.
Two studies (one RCT, one non-RCT) compared iontophoresis alone with iontophoresis combined with anticholinergic therapy. 38,41 One study found no significant difference in sweat reduction (gravimetry) of the hands and feet between iontophoresis alone and iontophoresis combined with oral oxybutynin by the end of the 6-week treatment period. Another study reported that iontophoresis with topical glycopyrrolate resulted in a longer duration of effect (as well as a higher incidence of dry/sore throat) than with iontophoresis alone.
Two studies (one RCT, one non-RCT)88,92 compared iontophoresis with BTX injections for palmar hyperhidrosis. One RCT found a statistically and clinically significant difference in treatment response (HDSS) and patient-reported symptoms between the two interventions favouring BTX at 4 weeks from baseline. One non-RCT92 reported a statistically and clinically significant improvement in patient-reported symptoms and willingness to undergo treatment at 1 month, but the difference was no longer statistically significant at 6 or 12 months. This may reflect a waning of the effect following a single treatment with BTX compared with regular use of iontophoresis, although the evidence is too limited to confirm this hypothesis. There was limited evidence suggesting that effect duration following end of therapy with iontophoresis was shorter than after BTX injection. Patients receiving BTX experienced pain more frequently than with iontophoresis.
One additional case series reported a statistically significant short-term reduction in sweating (gravimetry) for most patients undergoing standard tap water iontophoresis for the hand (20 days’ follow-up). 39
Overall, there is very low-quality but consistent evidence suggesting a beneficial effect of iontophoresis in the treatment of palmoplantar hyperhidrosis compared with placebo or no treatment and that the effect of iontophoresis after treatment discontinuation is short-lived. Compared with iontophoresis alone, the evidence for the effectiveness of combining anticholinergic therapy with iontophoresis is mixed and inconclusive.
There is very low-quality evidence to suggest that iontophoresis is less effective than BTX injections at reducing palmar hyperhidrosis symptoms in the short term and that the effect duration following end of treatment is shorter than with BTX.
Glycopyrrolate (topical)
Three studies of topical glycopyrrolate were included, including two RCTs51,53 and one non-RCT. 91 Sample sizes ranged from 3853 to 40. 91 The risk of bias was considered as high across all outcomes reported in two studies53,91 and unclear or high depending on the outcome reported in one study. 51
Studies were conducted in the UK,91 the USA53 and South Korea;51 therefore, the populations of two of the studies may not be representative of hyperhidrosis patients in the UK, notably because of differences in climate. Studies were recent, published between 201391 and 2015. 51,53
All of the studies included mostly adults, with ages ranging from 17 to 68 years. In two studies, the majority of patients were female,53,91 whereas one study included mostly male patients. 51 Inclusion criteria relating to patients’ baseline disease severity were reported in two of the studies: in one study patients had HDSS scores of 3–4 and a minimum sweat rate of 50 mg per 5 minutes,53 in the other study, patients had HDSS scores of 3–4 and a minimum sweat rate of 100 mg per 20 minutes on each side of the forehead. 51
Duration, dosage and intensity of treatment varied across the studies. Daily dosages ranged from 2% to 4%. Two of the studies compared two different dosages as well as comparing against a non-glycopyrrolate treatment group. 53,91 Glycopyrrolate was applied directly to the axillae in two studies53,91 and onto the face in one study. 51 There were no studies of oral glycopyrrolate.
Topical glycopyrrolate compared with placebo
Two small RCTs compared glycopyrrolate wipes with placebo, used for hyperhidrosis of the axilla for 4 weeks53 or the face for 10 days (see Appendix 2, Table 48). 51
Mehrotra et al. 53 carried out a three-arm randomised trial that compared 4 weeks of daily treatment glycopyrrolate wipes for axillary hyperhidrosis (4% group or 2% group) with placebo in 38 patients. Efficacy was evaluated with HDSS and gravimetry. At the end of week 4, patients receiving glycopyrrolate experienced a statistically and clinically significant reduction in sweating from baseline (glycopyrrolate 4%, 59% reduction; glycopyrrolate 2%, 48% reduction), and this was significantly greater than the reduction achieved in the placebo-treated group (16%). There was also a significantly greater proportion of responders (i.e. HDSS score reduction of ≥ 2 points) in the glycopyrrolate groups (glycopyrrolate 4%, 50%; glycopyrrolate 2%, 35%) than in the placebo group (9%). Adverse event rates were similar between groups, although it appears that blurred vision, dry mouth and application site discomfort were more frequent in the groups receiving active treatment.
In the study by Hyun et al. ,51 39 patients were treated with 2% topical glycopyrrolate on half of the forehead, while the other half of the forehead was treated with a placebo. Efficacy was evaluated with gravimetry and HDSS. At the end of the 10-day treatment, there was a statistically significant difference of 37% [standard deviation (SD) 11.4%] in reduction of sweat production rate favouring intervention versus placebo (p < 0.025). There was no difference in mean change from baseline in HDSS scores between treatment and placebo, although the risk of within-patient correlation cannot be excluded. One patient reported a transient headache following the intervention and no other adverse events were reported.
Topical glycopyrrolate compared with botulinum toxin type A
One study non-RCT compared topical glycopyrrolate with BTX (see Appendix 2, Table 49). Baker91 allocated patients (n = 40) with axillary hyperhidrosis to one of four groups: glycopyrrolate spray (2% or 1%), BTX-A injections or no treatment. Efficacy was evaluated using HDSS. At 6 weeks following treatment, patients who had received glycopyrrolate 1% had less improvement in HDSS scores than the BTX group; the difference was statistically significant (p < 0.05), although it was not clear if it was also clinically significant. There was no significant difference between glycopyrrolate 2% and BTX-A.
Evidence summary for topical glycopyrrolate
Three studies (two RCTs, one non-RCT) of topical glycopyrrolate were included. Two small, low-quality (rated as having high or unclear risk of bias) RCTs evaluated short-term treatment with glycopyrrolate wipes against placebo, used for hyperhidrosis of the axilla53 or the face. 51 Both studies found that the reduction in sweating (gravimetry) on the treated sides at the end of treatment was significantly greater in the active treatment group than in the placebo group. Compared with placebo, there was evidence of a significant short-term improvement in patient-reported disease severity (HDSS) in patients receiving treatment for axillary hyperhidrosis, but not for facial hyperhidrosis. There was limited and inconclusive evidence from one non-RCT91 regarding the relative effectiveness (HDSS) and safety of BTX injections compared with glycopyrrolate spray for axillary hyperhidrosis.
Overall, there is very low-quality evidence suggesting a short-term benefit of topical glycopyrrolate for axillary and facial hyperhidrosis. No evidence for other treatment sites was found.
Oxybutynin
Three placebo-controlled RCTs of oxybutynin were included (see Appendix 2, Table 50). 57,59,60 Risk of bias was considered unclear for all three studies. Sample sizes ranged from 3257 to 62. 59
Studies were conducted in France59 and Brazil57,60 and were recent, as they were published between 201260 and 2015. 59
When reported, studies included adult patients, with ages ranging from 18 to 62 years. One study included female patients only,57 with the other two studies including mostly female patients. 59,60 Inclusion criteria relating to patients’ baseline disease severity were reported in only one of the studies; patients had HDSS scores of ≥ 2. 59 However, one of the other studies reported that patients had persistent hyperhidrosis despite a previous sympathectomy. 57
In all three studies, oral oxybutynin was prescribed in progressively increasing doses (starting at 2.5 mg daily) throughout the treatment period, which lasted 30 days in one study57 and 6 weeks in two studies. 59,60 The maximum dose prescribed was 7.5 mg daily in one study59 and 10 mg daily in two studies. 57,60
The majority of patients in one study had generalised hyperhidrosis,59 whereas the other two studies focused on specific body parts (including axilla and palm60 and the foot57).
Schollhammer et al. 59 randomised 32 patients to oxybutynin and 30 patients to placebo. Efficacy was assessed using HDSS and quality of life with DLQI. At the end of the treatment period, the percentage of participants with an improvement in HDSS score of ≥ 2 points was higher in the treatment group (43%) than the placebo group (7%). This difference was relatively small but statistically significant. Mean improvement in DLQI score was small but statistically significantly higher in patients allocated to the oxybutynin group (6.9) than those allocated to the placebo group (2.3) (p < 0.01). Dry mouth was significantly more frequent in the oxybutynin group (41%) than in the placebo group (11%) (p < 0.01).
Wolosker et al. 60 randomised 25 patients to a oxybutynin group and 25 patients to a placebo group. Improvement in hyperhidrosis was assessed using a non-validated scale completed by patients ranging from 0 (no improvement) to 10 (absence of hyperhidrosis), with higher scores indicating greater improvements. Three outcomes, including improvement in palmar or axillary symptoms, improvement in plantar symptoms and improvement in quality of life at the end of treatment, were each assessed using a modified questionnaire by Amir et al. 186 using a summed total score ranging from 20 to 100, with lower scores indicating greater improvement from baseline. The study reported large and statistically significant differences in improvement favouring oxybutynin for all three outcomes at the end of treatment. However, the proportion of patients reporting moderate or severe dry mouth was significantly higher in the intervention group (35%) than in the placebo group (9%) (p = 0.038). Further results are reported in Appendix 2, Table 50.
Costa et al. 57 included patients with persistent plantar hyperhidrosis despite undergoing sympathectomy > 6 months earlier. The study randomised 16 patients to the oral oxybutynin group and 16 patients to the placebo group. Sweating was measured by evapometry, and improvement in quality of life at the end of treatment was assessed using a modified questionnaire by Amir et al. 186 and by imputing a summed total score ranging from 0 to 100, with lower scores indicating greater quality of life. There was an improvement from baseline measurements in sweating of the foot (38% reduction in the treated group vs. 9% in the placebo group) at the end of 30 days of treatment that was statistically significant only in the oxybutynin group, although the clinical relevance of these results may be limited. Statistically and clinically significant reductions in sweat from the hands, back and abdomen were also reported. Quality-of-life scores were significantly improved from baseline (p = 0.001) in the oxybutynin group (from ‘very good’ at baseline to ‘excellent’ at follow-up), but not in the placebo group, although the clinical relevance of these results is unclear. Dry mouth (100% vs. 44%), constipation (31% vs. 6%) and drowsiness (18% vs. 6%) were all more common in the oxybutynin group than in the placebo group.
Evidence summary for oxybutynin
Three studies, all rated as having an unclear risk of bias, evaluated the effectiveness and safety of oral oxybutynin against placebo for hyperhidrosis of the axilla and palm,60 foot57 and generalised hyperhidrosis. 59
One study reported statistically significant improvement in patient-reported symptoms compared with placebo at the end of treatment, in both the axilla and palm, at 6 weeks. 60 Another study reported a statistically significant difference in sweat reduction (gravimetry) in the foot and the hand at 30 days, although the reduction may be of limited clinical relevance. 57 A third study reported a small difference favouring active treatment compared with placebo in patient-reported disease severity improvement (HDSS) at the end of 6 weeks of treatment, although, again, this reduction was small and may be of limited clinical relevance. 59 All three studies reported a short-term quality-of-life improvement favouring oxybutynin, as well as a significantly higher incidence of dry mouth symptoms in patients receiving active therapy.
Overall, there is low-quality evidence suggesting a short-term small benefit of oxybutynin in hyperhidrosis symptoms and a short-term improvement in quality of life compared with placebo. There is insufficient evidence to indicate whether or not the effectiveness of oxybutynin differs according to target area. Oxybutynin is associated with a high incidence of adverse events, particularly dry mouth.
Methantheline bromide
Two placebo-controlled RCTs of oral methantheline bromide were included (see Appendix 2, Table 51). 68,69 Risk of bias was considered high across all outcomes in one study68 and mostly unclear (except for one outcome) in the other study. 69
Sample sizes were 4268 and 339. 69 Both studies were conducted in Germany. The studies were published in 200468 and 2013. 69
One study was conducted in adults, with an age range of 18–54 years, the majority of whom were female. 68 The other study did not report the age range or sex of the included patients. 69 Inclusion criteria relating to patients’ baseline disease severity was reported in both studies; in one study, patients had a minimum sweat rate of 50 mg per minute68 and, in the other study, patients had a minimum sweat rate of 50 mg per 5 minutes. 69 Both studies included patients with axillary and/or palmar hyperhidrosis.
In one study, oral methantheline bromide was prescribed at a dose of 50 mg twice daily for 4 weeks,68 whereas the other study prescribed oral methantheline bromide at a dose of 50 mg three times daily for 4 weeks. 69
Müller et al. 69 evaluated the efficacy of a 4-week course of 50 mg of methantheline three times daily, using gravimetry, HDSS and DLQI. Axillary sweat rates were significantly reduced at the end of treatment in the intervention group compared with placebo (intervention group: 41% reduction, from 168 ± 146 mg per 5 minutes at baseline to 99 ± 98 mg per 5 minutes at day 28; placebo group: 19% reduction, from 161 ± 119 mg per 5 minutes at baseline to 130 ± 119 mg per 5 minutes at day 28; p = 0.0013), but the study found no differences in palmar sweat. There was also a statistically significant difference in reduction in HDSS scores between groups, favouring methantheline bromide. A statistically significant difference in DLQI score reduction favouring the intervention group was also reported (intervention group: 6.9 points reduction, from 16.6 ± 5.3 at baseline to 9.7 ± 6.8 at day 28; placebo group: 4.2 points reduction, from 16.4 ± 5.6 to 12.2 ± 6.9 at day 28). However, both HDSS and DLQI score improvements were small when compared with placebo and may not be clinically significant. The most common adverse event was dry mouth, which occurred significantly more often in the intervention group (88 events) compared with the placebo group (28 events). Twenty-one events were recorded as severe, although the authors did not report rates by group or which events may have been related to the study medication.
Hund et al. 68 assessed the efficacy of a 4-week course of 50 mg of methantheline twice daily using gravimetry. They reported a statistically significant reduction in axillary sweating from baseline at the end of treatment in patients allocated to methantheline bromide [40% reduction, from 89.2 (SD 73.4) mg/minute to 53.3 (SD 48.7) mg/minute], compared with no change in the placebo group. The difference between methantheline and placebo was clinically significant. There were no differences in palmar sweating reduction between the intervention group and the placebo group at 4 weeks. The incidence of dry mouth symptoms was significantly greater in the intervention group than in the placebo group (intervention group: 47.8% at week 2 and 34.8% at week 4; placebo group: 11.1% at week 2 and 5.6% at week 4: between-group difference significant at p < 0.02).
Evidence summary for methantheline bromide
Two placebo-controlled RCTs of oral methantheline bromide were included. 68,69 Both studies were rated as being at a high or unclear risk of bias. They both reported relatively small reductions in axillary sweating (gravimetry) after 4 weeks of active treatment compared with placebo, but no difference in sweating of the palms. One study also reported small, non-clinically significant, differences in patient-reported disease severity (HDSS) and quality-of-life improvement favouring treatment against placebo. Both studies reported a statistically and clinically significant increase in dry mouth symptoms in patients receiving active treatment compared with those receiving placebo.
Overall, there is low-quality evidence that, compared with placebo, methantheline bromide has a short-term positive effect on axillary hyperhidrosis symptoms and quality of life, although this effect is small and may not be clinically significant. There is no evidence that methantheline bromide improves symptoms of the palm or any other body parts. There is evidence suggesting that methantheline bromide is associated with a high incidence of dry mouth symptoms.
Botulinum toxin (topical)
One very small study evaluated the efficacy of topically applied BTX for axillary hyperhidrosis (see Appendix 2, Table 52). 71 The risk of bias was considered unclear. In the study by Glogau,71 12 patients had one axilla randomly assigned to receive 200 U of BTX-A mixed with Cetaphil cream (Galderma, Fort Worth, TX, USA), which was massaged into the axilla and remained on the skin for 60 minutes. The other axilla received a placebo cream. Sweating outcomes were measured by gravimetry and iodine starch test at 4 weeks’ follow-up.
Two patients were excluded from the study analyses due to a significant imbalance in sweating (> 25%) at baseline. Among the remaining 10 patients, the reduction in sweating from baseline was 40% greater in the BTX-A-treated axillae than the placebo-treated side (65% vs. 25%) at 4 weeks’ follow-up. The difference was statistically significant. The authors stated that the results of the Minor’s iodine starch test were consistent with the gravimetry results. Four minor localised adverse events were reported in the treated axilla.
Evidence summary for topical botulinum toxin
Only one very small study (rated as having an unclear risk of bias) evaluated the efficacy of topically applied BTX for axillary hyperhidrosis. 71 There is insufficient evidence to conclude on the effectiveness and safety of topical BTX for primary hyperhidrosis.
Botulinum toxin (subcutaneous injection)
Twenty-three studies of BTX administered by subcutaneous injection were included. 21,72–77,80–85,88–92,148,155,184,185 Thirteen studies were randomised trials21,72–77,79,82–85,88 and 10 were non-randomised studies. 80,81,89–92,148,155,184,185
Risk of bias was considered to be high across all reported outcomes in 14 studies,21,73,74,80,81,88–92,148,155,184,185 unclear in seven studies72,76,79,82–85 and low in one study. 75 Another trial was rated as being at an unclear risk of bias for all assessed outcomes except one (DLQI), which was rated as high risk. 77
Two studies were conducted in multiple European countries. 79,80 Among the other studies, six were conducted in the USA,21,73,74,76,77,82 four in Germany,75,89,148,155 three in Japan,83,90,185 two in Austria,84,85 two in the UK91,184 and one each in India,88 Italy,72 Poland92 and Sweden. 81 Studies were published between 199784 and 2016;184 the BTX used in the earlier studies may not have the same effectiveness and adverse event profile as newer formulations.
When reported, all studies included adults; five studies also recruited a small number of participants aged < 18 years. 80,82,88,155,184 However, age range was not reported in seven studies. The majority of the participants in 12 of the studies were female; one study included only female patients. 72 The majority of the participants in seven studies were male. One study had 50% male participants and two studies did not report the sex of the participants.
Inclusion criteria relating to patients’ baseline disease severity was reported in 13 studies; ranging from a minimum sweat rate of 50 mg per 5 minutes77,79,80 to at least 100 mg per axilla per minute89 (when reported in terms of sweat rate). Other inclusion criteria were used for baseline disease severity, including HDSS score (generally patients had a score of 3–4), Haider 2005 criteria,187 described as ‘severely disabled in regard to occupation and social activities’ or ‘socially handicapped by condition’.
Most studies used BTX-A and only two used botulinum toxin type B (BTX-B). 73,74 Five studies used Botox® (Allergan Inc., Irvine, CA, USA),77,81,90,92,184 three studies used Dysport®,84,89,185 one study used both brands148 and 14 studies did not state which brand was used. 21,72–76,79,80,82,83,85,88,91,155 When stated, the most common dosage of BTX-A was 50 U,21,77,79,80,82,83,92,155,185 although some studies used dosages as high as 250 U. 72,89 Studies of BTX-B used dosages of 2500 U73 or 5000 U. 74
Twelve studies compared BTX with placebo exclusively72–76,79,82–85 (including two studies77,80 that compared different botulinum regimens with placebo) and four compared BTX with no treatment. 81,89,90,185 Of the seven studies that compared BTX with an active treatment, four studies compared it with curettage,21,148,155,184 two with iontophoresis88,92 and one with topical glycopyrrolate. 91
Most studies (n = 16) used BTX for treating axillary hyperhidrosis exclusively21,72,73,75,77,79,80,82,83,85,89,91,148,155,184,185 and five used it to treat palms only. 74,76,84,88,90 Two studies reported using the intervention for treating the palm and/or axilla. 81,92 None of the studies of BTX for treating axillary hyperhidrosis stated that anaesthesia was used, whereas all studies of BTX for treating hyperhidrosis of the palm, or palm and/or axilla, reported using local anaesthesia.
Axillary hyperhidrosis
By far the most studied intervention/site combination was BTX for axillary hyperhidrosis: there were many studies versus placebo or no treatment reporting various outcomes.
Botulinum toxin compared with placebo
Of the nine studies that compared subcutaneous injections of BTX with placebo for axillary hyperhidrosis,72,73,75,77,79,80,82,83,85 eight were RCTs and one80 was an open-label continuation study of an included RCT (see Appendix 2, Table 53). 79
Two studies reported using HDSS as a measure of efficacy,77,83 six measured sweating using gravimetry. 75,77,79,80,82,83 Five studies measured quality of life. 73,77,79,80,83 Patient satisfaction was assessed in three placebo-controlled BTX studies, using four different tools. 79,80,83 Three studies reported on duration of effect. 73,77,83 All studies reported data on adverse events.
Out of 10 studies, five were considered sufficiently similar to be pooled in a meta-analysis. 75,77,79,82,83 A range of outcomes were reported as presented in the following sections.
Two studies estimated treatment response in terms of a reduction in HDSS score of ≥ 2 points. 77,83 These studies were pooled in a meta-analysis. Lowe et al. 77 was a three-armed trial that compared two doses of BTX-A (50 U and 75 U) with placebo, and both active treatment arms were considered sufficiently homogeneous to be pooled. Figure 3 shows that there was a large and statistically significant difference in odds of response at 4 weeks favouring BTX-A [RR 3.30, 95% confidence interval (CI) 2.46 to 4.43]. There was no difference in response rates between the two BTX-A doses in Lowe et al. 77 (50 U and 75 U). There was no evidence of heterogeneity.
FIGURE 3.
Botulinum toxin vs. placebo. Reduction of ≥ 2 points in HDSS at 4 weeks. df, degrees of freedom; IV, inverse variance. In Lowe et al. ,77 the 75-U and 50-U groups were presented separately to explore any dose–response effect and total n/N in the placebo group was 27/108; events and total sample size were divided by two to avoid double counting. This artificially reduces the power of the study in the meta-analysis and does not account for correlation between the two active treatment groups. However, a separate analysis combining the two arms showed no significant difference in results.
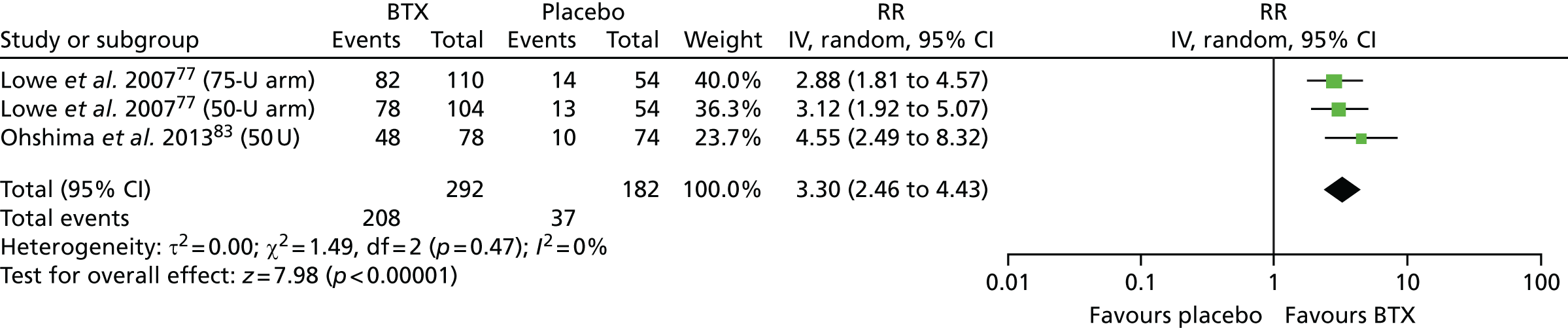
Ohshima et al. 83 found that HDSS response rates were maintained in the intervention group at 16 weeks and reported a similarly large difference compared with placebo at this time point (RR 5.96, 95% CI 2.87 to 12.39).
In the Ohshima et al. study,83 the initial treatment phase (which lasted 16–24 weeks) was followed by an open-label second treatment phase; the entire study lasted until 40 weeks after first treatment. Participants initially allocated to BTX received either a re-injection of BTX-A 50 U per axilla or no re-injection. Participants initially allocated to placebo received either one injection of BTX-A 50 U per axilla or no injection. Patients received a BTX-A injection during the second treatment phase if they met ‘re-injection criteria’: mean sweat production beyond 50% of baseline at any time between weeks 16 and 24 [of 78 patients allocated to BTX-A, 34 (44%) were included in the second treatment phase]. Treatment response was defined as ≥ 50% reduction in mean sweat from baseline and a reduction from baseline of ≥ 2 points on the HDSS. Rates of adverse events were higher in the intervention group (54%) than in the placebo group (30%). Duration of effect, defined as the number of days between the initial treatment and the first recording of > 50% of baseline sweat production, was 273 days (95% CI 171 days to a number of days that was not reported) for the BTX group compared with 35 days (95% CI 28 to 56 days) for placebo.
Lowe et al. 77 measured the median effect duration in the subgroup of responders, defined as time to return to a HDSS score of 3 or 4 points after treatment. The study reported a median effect duration of 205 days in the 50-U group, 197 days in the 75-U group and 96 days in the placebo group. The difference between each BTX-A group and placebo was statistically significant.
Five RCTs were included in at least one meta-analysis evaluating the effect of BTX on gravimetry against placebo75,77,79,82,83 and one unblinded continuation study of the Naumann et al. 80 RCT was only summarised narratively.
Figure 4 shows a pooled analysis of four studies75,79,82,83 reporting a reduction of ≥ 50% sweating from baseline at 2–4 weeks’ follow-up and suggests a large and statistically significant difference favouring BTX-A compared with placebo (RR 3.27, 95% CI 1.93 to 5.55).
FIGURE 4.
Botulinum toxin vs. placebo. Reduction in sweating from baseline to 2–4 weeks of ≥ 50%. df, degrees of freedom; IV, inverse variance. Follow-up duration was 2 weeks for Heckmann et al. 75 and 4 weeks for Naumann et al. 79 and Ohshima et al. 83 Median follow-up duration in Odderson82 was 2 weeks (range 1–8 weeks). Data for Odderson82 were extracted from figures.

There was evidence of heterogeneity (I2-statistic = 94.7%), which seems to be due to the Heckmann et al. 75 study. Heckmann et al. 75 used a higher dose than the other studies (200 U vs. 50 U) and had a half-side comparison design. However, Figure 4 shows no evidence of a dose–response effect and suggests that the results in Heckmann et al. 75 were driven by a relatively lower overall response rate in the placebo group (15% in Heckmann et al. 75 vs. 40% overall in the other studies). The reason for the heterogeneity in response rates across placebo groups is unclear. Notwithstanding the heterogeneity the treatment effect favours BTX.
Figure 5 shows a meta-analysis of three studies75,77,82 and suggests a similarly large and statistically significant difference in the odds of patients experiencing a reduction of at least 75% in sweat volumes at 2–4 weeks’ follow-up (RR 6.74, 95% CI 2.84 to 16.03).
FIGURE 5.
Botulinum toxin vs. placebo. Reduction in sweating from baseline to 2–4 weeks of ≥ 75%. df, degrees of freedom; IV, inverse variance. Follow-up duration was 2 weeks for Heckmann et al. 75 and 4 weeks for Lowe et al. 77 Median follow-up duration in Odderson82 was 2 weeks (range 2–8 weeks). Data for Odderson82 were extracted from figures. In Lowe et al. ,77 total n/N in the placebo group was 20/108; events and total sample size were divided by 2 to avoid double-counting.
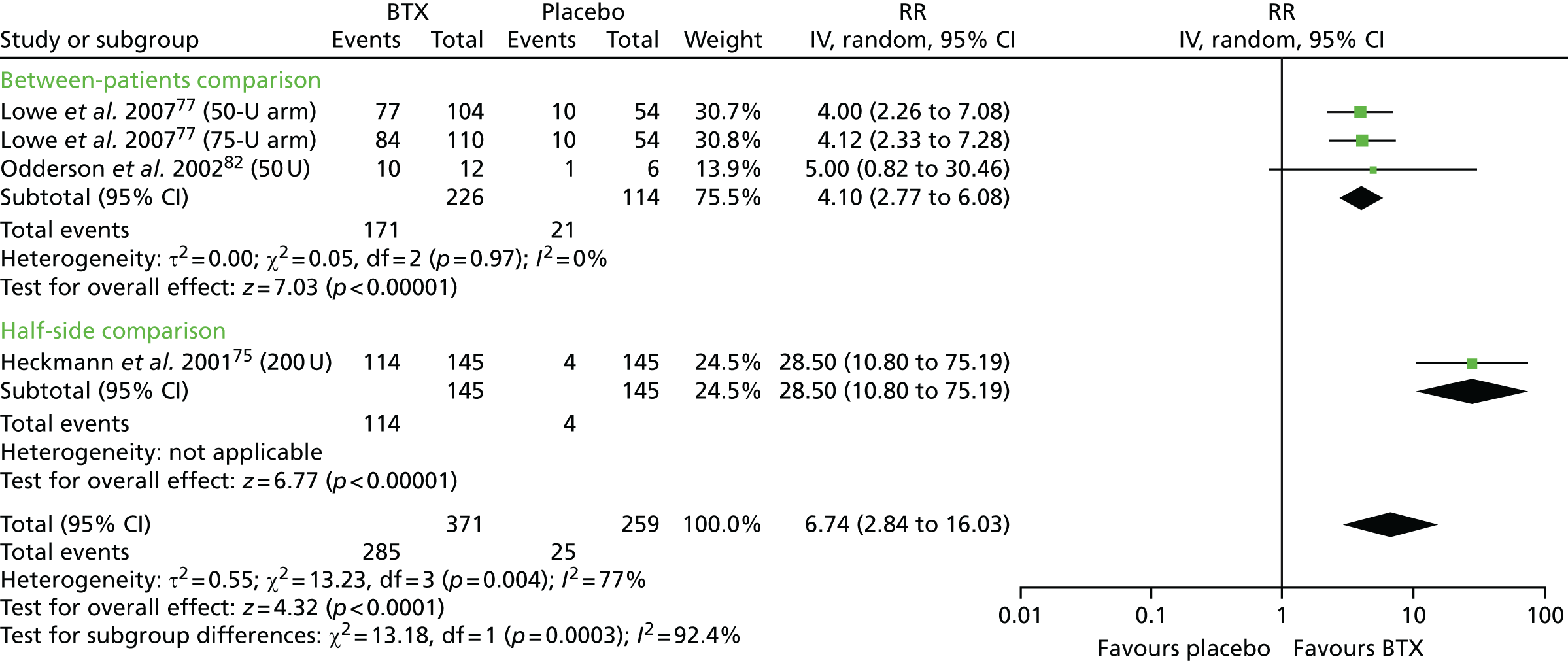
There was again evidence of heterogeneity (I2-statistic = 92.4%) due to the Heckmann et al. 75 study results, which had a higher RR than the other studies. As with Figure 4, Figure 5 shows no evidence of a dose–response effect and suggests that the results in Heckmann et al. 75 were driven by a relatively lower response rate in the placebo group than in other studies (3% in Heckmann et al. 75 vs. 18% overall in the other studies), rather than a higher response rate in the intervention group (79% in Heckmann et al. 75 vs. 76% overall in the other studies). As before, the reason for the heterogeneity in response rates across placebo groups is unclear.
Figure 6 shows a pooled analysis of three studies79,82,83 reporting a reduction of ≥ 50% sweating from baseline at 16 weeks’ follow-up and suggests a large and statistically significant difference favouring BTX-A (RR 2.87, 95% CI 1.94 to 4.26). There was no evidence of heterogeneity.
FIGURE 6.
Botulinum toxin vs. placebo. Reduction in sweating from baseline to 16 weeks of ≥ 50%. df, degrees of freedom; IV, inverse variance. Follow-up duration was 16 weeks for Naumann et al. 79 and Ohshima et al. 83 Median follow-up duration for Odderson82 was 16 weeks (range 10–21 weeks). Data for Odderson82 were extracted from figures.

Figure 6 suggests that the effect observed in patients undergoing BTX-A treatment at 2–4 weeks (see Figure 4) is largely maintained at 16 weeks, albeit slightly reduced: the absolute rate of BTX-A patients with ≥ 50% reduction in sweating at 2–4 weeks was 94% compared with 83% at 16 weeks.
Figure 7 shows a meta-analysis of four studies77,79,82,83 and suggests a large and statistically significant difference in mean percentage sweat reduction from baseline favouring BTX-A. Compared with placebo, patients receiving BTX-A had an approximately 57% greater reduction in sweating at 2–4 weeks’ follow-up (MD –56.83%, 95% CI –64.61% to –49.04%). There was no evidence of heterogeneity.
FIGURE 7.
Botulinum toxin vs. placebo. Mean per cent reduction in sweating from baseline to 2–4 weeks. df, degrees of freedom; IV, inverse variance. Follow-up duration was 4 weeks for Lowe et al.,77 Naumann et al. 79 and Ohshima et al. 83 Median follow-up duration in Odderson82 was 2 weeks (range 1–8 weeks). Data for Odderson82 were extracted and calculated from figures.

Figure 8 shows a pooled analysis of three studies,79,82,83 which suggests a slightly greater difference in mean percentage sweat reduction at 16 weeks’ follow-up. Compared with placebo, patients receiving BTX-A had an approximately 67% greater reduction in sweating (MD –66.93%, 95% CI –82.76% to –51.10%). There was no evidence of heterogeneity.
FIGURE 8.
Botulinum toxin vs. placebo. Mean per cent reduction in sweating from baseline to 16 weeks. df, degrees of freedom; IV, inverse variance. Follow-up duration was 16 weeks for Naumann et al. 79 and Ohshima et al. 83 Median follow-up duration for Odderson82 was 16 weeks (range 10–20 weeks). Data for Odderson82 were extracted and calculated from figures.

Naumann et al. 80 was a 16-month open-label continuation study of the Naumann et al. 79 RCT and was not included in the meta-analyses. After 4 months of the initial trial, in which patients were randomised to either BTX-A 50 U or placebo, participants could receive up to three further treatments with open-label BTX-A over 12 months. Of the 207 participants enrolled during the entire 16-month period, 80 received one treatment, 93 had two treatments, 30 received three treatments and four only received placebo during the initial phase of the trial. Outcomes assessed included treatment response (gravimetry), patient satisfaction and quality of life (bespoke questionnaires) and adverse events. The proportion of responders, defined as those achieving ≥ 50% reduction in spontaneous sweating, was higher in the BTX groups than in the placebo group both 4 weeks after treatments (BTX group: first treatment, 96.1%; second treatment, 91.1%; third treatment, 83.3%; placebo group: 34.7%) and 16 weeks after treatments (BTX group; first treatment, 85.7%; second treatment, 87.3%; third treatment, 80.5%; placebo group: 20.6%).
Two studies evaluated sweat outcomes using non-gravimetric methods. Schnider et al. 85 measured sweat production by digitising and analysing ninhydrin-stained sheets using standardised image analysis software. Balzani et al. 72 used the Minor’s iodine starch test. Both studies reported clinically relevant improvements in sweating at follow-up in the BTX group compared with the placebo group.
Of the five studies that measured quality-of-life outcomes, two used DLQI,77,83 one used SF-1279 and two used a bespoke questionnaire. 73,80
Two studies were combined in a meta-analysis. Figure 9 shows that there was a statistically significant difference in mean reduction in DLQI score from baseline to 4 weeks’ follow-up favouring BTX-A over placebo (MD –4.80, 95% CI –5.67 to –3.94). There was no evidence of significant heterogeneity. Ohshima et al. 83 reported further results at up to 16 weeks following initial treatment, which suggested that the effect was maintained over this period.
FIGURE 9.
Botulinum toxin vs. placebo. Mean reduction in DLQI score from baseline to 4 weeks. df, degrees of freedom; IV, inverse variance. In Lowe et al. ,77 the total sample size of the placebo group (n = 108) was divided by 2 to avoid double-counting.
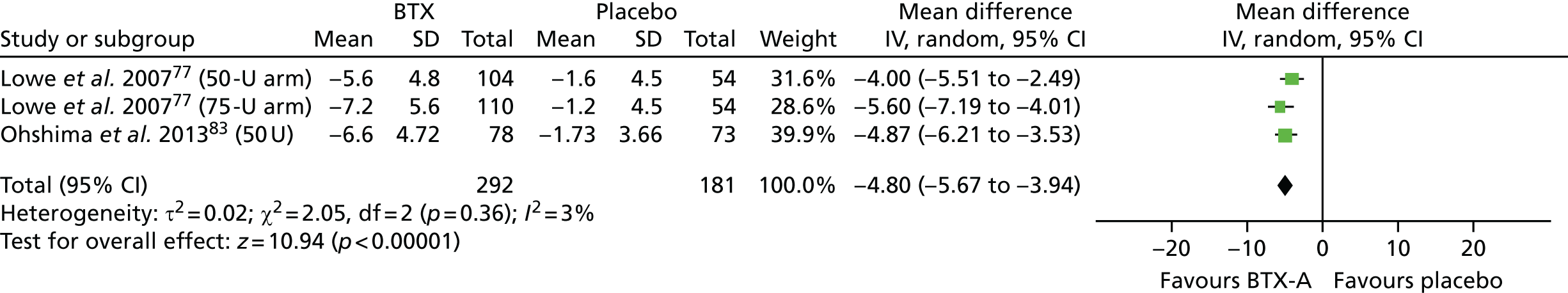
Naumann et al. 79 reported a statistically significant difference in mean change in quality of life, measured using SF-12 physical component summary (PCS) but not mental component summary (MCS) scores, from baseline to 16 weeks, favouring BTX-A. However, the improvement in PCS appeared small and may not be clinically significant. The two studies that used a bespoke unvalidated questionnaire73,80 both reported an improvement in quality of life following BTX-A treatment, although it is unclear whether or not this improvement was clinically significant.
Three studies reported data on patient satisfaction, using HHIQ79 and a global assessment of treatment satisfaction questionnaire. 79,80,83 Patients’ global assessment of treatment satisfaction was measured on a scale ranging from –4 to 4, with negative values indicating worsening and positive values indicating improvement. A score of 0 indicates no change from baseline, +1 indicates slight improvement, +2 moderate improvement, +3 substantial improvement and +4 complete abolition of signs and symptoms. Patient satisfaction, as measured by global assessment score, ranged from moderate (mean score of 2.6)79,83 to substantial (mean score of 3.5)80 in patients receiving BTX-A and from unchanged (mean score of 0.3)79,83 to slight (mean score of 1.4)80 in patients receiving placebo at 4–16 weeks’ follow-up.
None of the studies reported serious or severe adverse events related to the intervention. The most common treatment-related events reported included injection site pain and CS. Four studies reported injection site pain events,73,75,77,80 with incidence rates ranging from approximately 1%75 to 12%. 77 Six studies reported higher rates of non-axillary/CS in patients/axillae receiving BTX-A,77,79,80,82,83,85 with event rates ranging from under 1%82 to 15%. 85 One event of increased facial sweating was also reported in one study. 75 There was no evidence to suggest an increase in incidence or severity of treatment-related adverse events in the two studies in which patients received repeated injections. 80,83
Further study details and results not included in the meta-analysis are reported in Appendix 2, Table 53.
Botulinum toxin compared with no treatment
Three studies81,89,185 compared the effectiveness of BTX-A with no treatment (see Appendix 2, Table 54). All were small non-randomised half-side comparison trials (in which patients acted as their own control).
Heckmann et al. 89 included 12 patients. Participants initially received BTX-A 250 U in one axilla and the same treatment on the contralateral side 14 days later. Sweating outcomes were assessed by gravimetry and iodine starch test. Effect duration and patient satisfaction were assessed by patient questionnaire. The study found a significant reduction in sweating at 7 days’ follow-up, confirmed by iodine starch test. Most patients reported being symptom free for at least 9 months. Injection site stinging post intervention was the only adverse event reported.
Naver et al. 81 included 28 patients. Of those, 13 were treated for axillary hyperhidrosis with BTX-A. Sweating outcomes were evaluated using evaporimetry and iodine starch test at 1–2 weeks’ follow-up. The study found a significant reduction in sweating at follow-up with both measures. All patients reported a marked reduction or complete disappearance of sweating symptoms. Two patients reported intense pain from injection and reported that they would have preferred treatment under local anaesthesia.
Wakugawa et al. 185 included 20 patients. Thirteen patients received BTX-A in both axillae and seven patients received BTX-A (50 U) on one side only. Sweating was assessed by measuring sweating area with the Sakurai–Montagna paper sweating test at 1 and 3 months’ follow-up. 188 Test papers were digitised and sweating areas measured using image analysis software. In the seven patients who were treated on one side only, there was a statistically significant reduction in sweat area in the treated side, but not in the non-treated contralateral side at 1 and 3 months. There were no serious adverse events.
Botulinum toxin compared with curettage
Four studies (one RCT, three non-RCTs) compared curettage with BTX (see Appendix 2, Table 55). 21,148,155,184
Ibrahim et al. 21 was a half-side comparison RCT involving 20 patients and comparing tumescent suction curettage with injection of BTX-A (50 U). Efficacy was measured with HDSS and gravimetry. The reduction in mean HDSS score from baseline was statistically and clinically significantly greater in BTX-A-treated axillae than in curettage-treated axillae both at 3 months (MD 0.80; p = 0.0002) and at 6 months (MD 0.90; p = 0.0017). There was no statistically significant difference in sweat reduction between the two interventions at 3 months’ follow-up. There were few adverse events for curettage-treated axillae and none reported for BTX-A.
Ottomann et al. 155 carried out a non-randomised parallel-group study in 88 patients. Forty-one were assigned to curettage and 47 to BTX-A (50 U). Sweating was assessed with gravimetry. Quality of life was measured using a bespoke disease-specific questionnaire covering 10 items grouped under three categories (psychosocial symptoms, activities, subjective perspective/others). Both sweating and quality of life were measured at 2, 12 and 26 weeks’ follow-up. There were no significant differences between the two groups in sweating reduction from baseline at any of the follow-up times. There were also no significant differences between groups in quality-of-life improvement at 26 weeks (earlier follow-up results were not reported). There were no severe adverse events. The incidence of adverse events associated with treatment was higher in the curettage group (8.3%) than in the BTX-A group (1.7%).
Rompel and Scholz148 conducted a non-randomised parallel-group study in 113 patients. Ninety participants received curettage under general anaesthesia and 23 patients were treated with BTX-A injections. Sweating was measured with patient questionnaires at baseline, 6 months and the end of follow-up (median 28.2 months), when participants were asked to record the amount of axillary sweating based on a score ranging from 1 (no axillary secretion) to 6 (maximum axillary secretion). General satisfaction with the procedure was assessed using a four-item scale (from dissatisfied to very good). The study reported no significant differences in sweating. In the curettage group, the overall score of axillary sweating at rest was reduced to 40.0% of the baseline score after 6 months and to 45.7% of the baseline score at the end of follow-up (median 28.2 months). In the BTX-A group, sweating at rest was reduced to 48.5% of the baseline score after 6 months and to 68.8% at the end of follow-up (median 16.1 months). There was no statistically significant difference in general satisfaction score between the two interventions: satisfaction with treatment was rated as ‘very good’ or ‘good’ in 66% of curettage patients and 61% of BTX-A patients. The overall complication rate after curettage was 17.8%. The study reported that there were no adverse reactions after treatment with BTX-A except for minimal superficial haematoma. In the curettage group, 12 patients required surgical revision and additional suction drainage.
Vakili and Baker184 carried out a non-randomised parallel group study in 98 patients. The study was reported only as a conference abstract with limited details. Twenty-three participants were treated with microretrodermal curettage, and 75 received BTX-A. Efficacy was assessed using HDSS. The study reported significant and similar improvement from baseline in mean HDSS scores in both groups at 6 weeks’ follow-up. Improvements in other patient-reported outcomes (including physical effects and impact of psychological precipitating factors) were also equally significant in both groups at 6 weeks. No data on adverse events were reported.
Palmar hyperhidrosis
Seven studies evaluated the effectiveness of BTX for palmar hyperhidrosis. 74,76,81,84,88,90,92 Of those, five targeted the palm only74,76,84,88,90 and two targeted the palms and/or axillae. 81,92
Botulinum toxin compared with placebo
Three RCTs evaluated the effectiveness of BTX for palmar hyperhidrosis against placebo (see Appendix 2, Table 56). 74,76,84 Of those, two were half-side comparison trials. 76,84 All patients underwent local anaesthesia on both hands.
Baumann et al. 74 studied 20 patients. Fifteen patients were randomised to BTX-B (5000 U) treatment for both hands and five received a placebo. Sweating was assessed using iodine starch test, and the Palmar Hyperhidrosis Improvement (P-HI) questionnaires. The P-HI questionnaire evaluates patient-perceived improvement in symptoms. The lowest possible P-HI score is 2, representing great improvement in palmar hyperhidrosis, and the highest possible score is 8, representing worsening hyperhidrosis. Quality-of-life outcomes were evaluated using the Palmar Hyperhidrosis Quality of Life (P-HQOL) questionnaire. The P-HQOL assesses interference of hyperhidrosis with daily life, with scores ranging from 4 (no interference with daily life) to 15 (great deal of interference with daily life). Both questionnaires were unvalidated. At 30 days’ follow-up there was no statistically significant difference in iodine starch test results between the intervention and placebo groups. At 30 days, there was a statistically significant difference in mean change from baseline in P-HI scores (p = 0.002) and P-HQOL scores (p = 0.010), favouring BTX-B; however, owing to limited reporting (no mean scores or SDs), it is unclear whether or not these results were clinically significant. There were 83 adverse events that were considered to be definitely related to the intervention, including decreased grip strength (50% of participants), muscle weakness (60%), dry mouth (90%), excessively dry hands (60%) and indigestion/heartburn (60%). There was no statistically significant difference betwen groups in the incidence of injection site pain. The authors did not report on the rate of severe/serious events.
In the study by Lowe et al. ,76 19 patients were treated with BTX-A (100 U) in one hand. randomly assigned, and placebo in the other hand. Sweating outcomes were measured by gravimetry at 28 days’ follow-up. Hyperhidrosis severity was also assessed by physicians and patients. There was a reduction in gravimetric measurements favouring BTX-A over placebo (p = 0.0027). The difference with placebo was statistically and clinically significant (approximately 33% difference). The authors also reported significant improvements from baseline in iodine starch test results in BTX-A-treated palms compared with placebo-treated palms. Both physicians and patients considered the improvement in hyperhidrosis severity at 28 days to be significantly greater in the BTX-A-treated hand than in the placebo-treated palms (p < 0.01) . Four patients reported adverse events. There was no significant difference in grip strength between the BTX-A- and placebo-treated hands.
Schnider et al. 84 studied 11 patients in whom one hand was randomly assigned to BTX-A (120 U) treatment and the other hand to placebo treatment. Sweating was assessed by digitisation of ninhydrin-stained sheets, followed by image analysis to measure the stained area using a standardised algorithm. Subjective rating of symptoms was performed using a VAS for each hand, with scores ranging from 0 (no sweating) to 100 (most severe sweating). All outcomes were assessed at 3, 8 and 13 weeks. The study reported a statistically significant reduction in sweating area and a significant improvement in patient rating of symptoms in the BTX-A group, compared with no significant change in the placebo group. Three patients reported minor handgrip weakness in the BTX-A-treated hand and three patients reported that injections were more painful in hands receiving BTX-A than in the placebo-treated hands.
Botulinum toxin compared with no treatment
Two non-randomised half-side comparison studies (in which patients acted as their own control) compared BTX with no treatment (see Appendix 2, Table 57). 81,90
Naver et al. 81 studies 28 patients, of whom 19 are relevant to this review and were treated for palmar hyperhidrosis with BTX-A under local anaesthesia. Sweating outcomes were evaluated using evaporimetry and iodine starch test at 1–2 weeks’ follow-up. The study reported a significant reduction in evaporation in the treated side (57% reduction from baseline) and a significant reduction in area of colour reaction from the palms at 1–2 weeks (p = 0.0002). Fifteen out of 19 patients reported marked reduction or complete disappearance of sweating at 1–2 weeks. Twelve patients reported slight and transient reduction in power of the fingers and six reported intense dryness of the skin.
Yamashita et al. 90 administered BTX-A (60 U) to the right hand only in 27 patients. Sweating was assessed by gravimetry monthly from 1 to 6 months and using the iodine starch test. The study reported a statistically significant reduction from baseline in sweating in both groups at all follow-up points. Although the reduction in sweating was larger in the intervention group, it is unclear whether or not there was a statistically or clinically significant difference compared with the untreated hand. Larger decreases in sweating were also reported with the iodine starch test, although it is unclear if differences between BTX-A and no treatment were clinically significant. The study did not report whether or not the patients experienced any adverse events.
Botulinum toxin compared with iontophoresis
Two studies88,92 compared BTX with iontophoresis (see Appendix 2, Table 45). Results are reported in the Iontophoresis.
Summary of evidence for subcutaneous injection of botulinum toxin
Twenty-three studies of BTX used as subcutaneous injection were evaluated. More than two-thirds of the studies focused on treatment for axillary hyperhidrosis exclusively and about a third focused on palmar hyperhidrosis.
In the case of axillary hyperhidrosis, BTX was compared with placebo in nine studies (eight RCTs,72,73,75,77,79,82,83,85 one open-label continuation study80), no treatment in three studies81,89,185 (non-RCTs) and curettage in four studies21,148,155,184 (one RCT, three non-RCTs). There was evidence that BTX, compared with placebo, resulted in a clinically and stastically significant reduction in sweat production (gravimetry) (five pooled RCTs, one open-label continuation study) and improvement in patient-reported symptoms (HDSS) (two pooled RCTs). There was evidence to suggest that reductions in sweat production (three pooled RCTs) and improvements in HDSS score (one RCT) were largely sustained at 16 weeks’ follow-up. There was also evidence of a statistically significant improvement in some measures of quality of life in BTX-treated patients compared with placebo-treated patients at 4 weeks’ (four RCTs)73,77,80,83 and 16 weeks’ follow-up (three RCTs, one open-label continuation study). 73,79,80,83 None of the studies of BTX for axillary hyperhidrosis reported serious or severe adverse events that were considered to be related to the intervention by the investigators. The most common treatment-related adverse events reported included injection site pain and CS. Three non-randomised half-side comparison studies (in which patients acted as their own control) of BTX and no treatment reported broadly similar results. 81,89,185
Of the four studies (one RCT, three non-RCTs) that compared curettage with BTX,21,148,155,184 one small half-side RCT21 found a difference in HDSS score (at 3 and 6 months’ follow-up) and gravimetric sweat measurement improvements (3 months’ follow-up) favouring BTX, although the difference was statistically significant only for HDSS score. Three non-randomised studies found no evidence of a significant difference in gravimetric sweat measurements at up to 26 weeks (one non-RCT),155 patient-reported sweating or patient satisfaction at 6 months (one non-RCT),148 HDSS score improvement at 6 weeks (one non-RCT)184 or in quality of life up to 26 weeks (one non-RCT),155 between curettage and BTX. When reported, the incidence of adverse events was higher with curettage than BTX (one RCT,21 two non-RCTs148,155).
For the treatment of palmar hyperhidrosis, BTX was compared with placebo in three RCTs, no treatment in two non-RCTs and iontophoresis in two studies (one RCT, one non-RCT). Compared with placebo, two studies reported a small but statistically significant reduction in sweating outcomes at short-term follow-up (3–13 weeks), measured either by gravimetry (one RCT)76 or by sweat area tests (one RCT),84 although one study found no statistically significant difference compared with placebo using the iodine starch test. 74 There was very limited evidence from a single study of a significant improvement in quality of life at 30 days’ follow-up favouring BTX. 74 One study (BTX-B 5000 U) found a high incidence of treatment-related adverse events. 74 Two non-randomised half-side comparison studies (in which patients acted as their own control) compared BTX with no treatment. 81,90 Both studies reported a difference in at least one measure of sweat reduction favouring the intervention, although the clinical significance of these results is unclear. There were no serious adverse events.
As stated previously (iontophoresis evidence summary), two studies (one RCT, one non-RCT)88,92 compared iontophoresis with BTX injections for palmar hyperhidrosis. One RCT88 found a statistically and clinically significant difference in treatment response (HDSS) and patient-reported symptoms between the two interventions, favouring BTX, at 4 weeks from baseline. One non-RCT92 reported a statistically and clinically significant improvement in patient-reported symptoms and willingness to undergo treatment at 1 month, but the difference was no longer statistically significant at 6 or 12 months. There was limited evidence suggesting that effect duration following discontinuation from iontophoresis was shorter than after BTX. Patients receiving BTX experienced pain more frequently than with iontophoresis.
Overall, there is moderate-quality evidence of a large effect of subcutaneous BTX on symptoms of axillary hyperhidrosis in the short and medium term (up to 16 weeks), and of a small to moderate positive effect on quality of life in the short term, compared with placebo. There is low-quality evidence to suggest that BTX is associated with higher patient satisfaction in the short to medium term, as well as a higher incidence of non-severe adverse events, notably injection site pain and CS, compared with placebo. There is very low-quality evidence regarding the relative effectiveness of BTX injections to the axillae compared with curettage and no evidence of a difference in longer-term effectiveness, and low-quality evidence suggesting a higher incidence of adverse events in patients undergoing curettage.
There is very low-quality evidence suggesting that BTX injections have a small positive effect on palmar hyperhidrosis symptoms compared with placebo or no treatment, although there is some very low-quality evidence to suggest a high incidence of adverse events with BTX-B (5000 U). The evidence for an effect of BTX injections for palmar hyperhidrosis on quality of life is insufficient.
As stated previously, there is very low-quality evidence to suggest that iontophoresis is less effective than BTX injections at reducing palmar hyperhidrosis symptoms in the short term and that the duration of effect is shorter than with BTX.
Curettage
Nine studies evaluating curettage for axillary hyperhidrosis were included: four were RCTs21,24,147,150 and five were non-randomised comparative studies. 148,149,154,155,184 In one study, risk of bias was considered unclear for gravimetric outcomes and high for all other outcomes. 147 All other studies were considered to be at a high risk of bias.
Study size ranged from 4154 to 113148 participants (total 526 individuals). One of the studies was conducted in France, Germany and Spain. 24 The other studies were conducted in the UK,184 Germany,148,150,154,155 Denmark,149 Norway147 and the USA. 21 Studies were published between 1975149 and 2016,184 although most were published from 2007 onwards. The curettage procedures and equipment used in the later studies are likely to have progressed since the procedures used in the study dating back to 1975.
When reported, all studies included adults, although two studies also recruited a small number of participants aged < 18 years. 155,184 Ages ranged from 16 to 57 years, when reported; age range was not reported in four studies. 24,148,149,154 In most studies the majority of participants were female, although three studies did not report the sex of the participants. Inclusion criteria relating to patients’ baseline disease severity were reported in six studies: in two studies patients had a minimum sweat rate of 50 mg/minute,150,154 in one study patients had HDSS scores of ≥ 2,155 in one study patients had HDSS scores of 3–4,24 one study21 used Haider 2005 criteria187 and one study stated that patients were severely disabled in regard to occupation and social activities. 148
Most studies reported using curettage with a liposuction method. 21,147,150,154,155 When reported, the method of anaesthesia included both local and general anaesthesia, although the older studies were more likely than more recent studies to use general anaesthesia. 148,149 Curettage was compared with a range of therapies, including BTX-A (four studies21,148,155,184) and several surgical techniques, including Shelley’s procedure (skin-sparing technique) (one study150), radical skin excision (two studies149,150), laser therapy (with or without curettage) (one study24) and curettage with aggressive manual skin shaving (one study154). One study compared curettage with two interventions (skin sparing and radical excision)150 and one study compared curettage with tumescent suction curettage. 147
Curettage compared with botulinum toxin
Four studies compared BTX with curettage (see Appendix 2, Table 55). 21,148,155,184 Study results are reported in Botulinum toxin.
Comparison of curettage with other minor surgical interventions
Five studies (three RCTs, two non-RCTs)24,147,149,150,154 compared suction curettage with other types of minor surgery that are more radical (see Appendix 2, Table 58): radical skin excision, radical skin excision with Y-plasty closure, Shelley’s procedure (skin-sparing technique) and curettage with aggressive manual shaving.
Bechara et al. 150 included a total of 40 patients who were randomised to one of three surgical interventions: liposuction-curettage (n = 15), radical skin excision with Y-plasty closure (n = 14) and a skin-sparing technique (n = 11). At 1-year follow-up, all three groups experienced significant mean reductions in sweat rates from baseline and there were no statistically significant differences between the three interventions. Aesthetic outcome was assessed by patients on a scale of 1 (very good) to 5 points (not good at all). The mean aesthetic outcome score was 3.2 (range 2–5) in the skin excision group, 2.3 (range 1–3) in the skin-sparing technique group and 1.5 (range 1–2) in the curettage group. The mean aesthetic outcome score was significantly better in the curettage group than in the other groups (p < 0.05). The incidence of haematoma was similar across groups, although one moderate to severe hematoma occurred in the curettage group that resolved over 4 weeks. No other severe adverse events were reported.
Jemec149 carried out a non-randomised parallel group trial comparing curettage with radical skin excision in a total of 41 participants. Patient satisfaction and adverse events were the only outcomes reported. Follow-up data were reported between 6 and 9 months post intervention. Levels of patient satisfaction were comparable between the two interventions. It was reported that there were no abscesses, haematomas or wound defects. No other adverse event data were reported.
The study by Bechara et al. 154 was a non-randomised half-side comparison trial in which patients acted as their own control, comparing curettage with or without aggressive manual shaving. The trial was stopped early after treatment of only four patients because of extensive skin damage associated with aggressive shaving.
Tronstad et al. 147 compared tumescent suction curettage with curettage alone in a half-side randomised comparison involving 22 patients. Sweating was assessed using gravimetry and skin conductance tests. Patients also assessed their sweating using a VAS for each side at follow-up (no further details reported). Quality of life was assessed using the DLQI questionnaire. All measurements were performed at 3, 6 and 12 months’ follow-up. The reduction in sweating (both objective and subjective measurements) was statistically and clinically significant at 6 and 12 months compared with baseline in both treatment groups (p < 0.05), and significantly greater with tumescent suction curettage than with curettage alone (p < 0.05). Three-month follow-up results were similar, although the difference between interventions was not statistically significant for gravimetry. Quality-of-life results were not reported. One patient from the tumescent suction curettage group experienced postoperative neuropathic pain lasting through the observation period. There were no haematomas or infections requiring antibiotics at 1 week and no other adverse events were reported.
Curettage compared with energy-based sweat gland destroying (‘destructive’) technologies
Leclere et al. 24 (see Appendix 2, Table 59) conducted a RCT that included 100 patients and compared four interventions: laser alone with a radiation of 975 nm, laser alone with simultaneous wavelengths of 924 nm and 975 nm, laser (924/975 nm) followed by curettage, and curettage alone. Effectiveness was measured using the Starch-Iodine Scale Improvement, a four-point single-item questionnaire measuring changes in sweating, with scores ranging from 0 points (no evidence of sweating by starch test) to 3 points (big dark area without changes), and HDSS. At both 1 and 12 months’ follow-up, the largest reduction in mean HDSS and sweating scores was achieved in the the laser plus curettage group, followed by the laser 924/975 nm only group, the curettage only group and the laser 975 nm group. Although the results suggest a clinically relevant difference between laser plus curettage and the other interventions, the authors did not state whether or not differences between the interventions were statistically significant. Aesthetic outcomes were measured using the Global Aesthetic Improvement Scale (GAIS), a five-point scale rating global aesthetic improvement, compared with baseline, as judged by the investigator. At 1 and 12 months’ follow-up mean scores on the GAIS were highest in the laser plus curettage patients, followed by laser 924/975 nm, curettage alone and laser 975 nm groups. There were few adverse events in all groups, and all had resolved within 1 month of follow-up.
Evidence summary for curettage
In summary, nine studies (four RCTs,21,24,147,150 five non-RCTs148,149,154,155,184) evaluated curettage for axillary hyperhidrosis. All were rated as being at a high risk of bias.
Four studies (one RCT, three non-RCTs) compared curettage with BTX. 21,148,155,184 One small half-side RCT21 found a difference in HDSS score (at 3 and 6 months’ follow-up) and gravimetric sweat measurement improvements (3 months’ follow-up) favouring BTX compared with curettage, although the difference was statistically significant only for HDSS score. Three non-randomised studies found no evidence of a significant difference in gravimetric sweat measurements at up to 26 weeks (one non-RCT),155 patient-reported sweating or patient satisfaction at 6 months (one non-RCT),148 HDSS score improvement at 6 weeks (one non-RCT)184 or quality of life up to 26 weeks (one non-RCT),155 between curettage and BTX. When reported, the incidence of adverse events was higher with curettage than with BTX (one RCT, two non-RCTs).
Among the five studies (three RCTs, two non-RCTs) that compared suction curettage with other surgical interventions, one RCT150 found no difference in sweating (gravimetry) at 1 year of follow-up between liposuction curettage, radical skin excision and a skin-sparing technique (Shelley’s procedure), although patients undergoing curettage reported significantly greater satisfaction with aesthetic outcomes. One non-RCT149 reported comparable levels of patient satisfaction and safety between curettage and radical skin excision at 6–9 months’ follow-up. One small non-randomised half-side comparison trial (in which patients acted as their own control), evaluating curettage with and without aggressive manual shaving, was stopped early because of extensive skin damage associated with aggressive shaving. 154 One RCT147 evaluated tumescent suction curettage against curettage alone in a half-side comparison. Reduction in sweating (gravimetry, skin conductance and patient reported) was statistically significantly greater with tumescent suction curettage than with curettage alone at 6 and 12 months’ follow-up, although, owing to limited reporting, it is not clear whether or not these results were clinically significant. One event of neuropathic pain lasting throughout the 12 months’ follow-up was reported in the tumescent suction curettage group. One four arm RCT24 found a clinically significant benefit in mean HDSS and sweating, as well as better aesthetic outcomes, with laser plus curettage compared with two different regimens of laser alone, or curettage alone. Few adverse events were reported in all groups.
Overall, there is very low-quality evidence regarding the relative effectiveness and safety of curettage compared with other minor surgical interventions for axillary hyperhidrosis. Compared with the more radical excision techniques, there is no clear evidence of a difference in sweat reduction, patient satisfaction or safety. However, aggressive manual shaving was associated with extensive skin damage in one very small study, which was stopped early. There is insufficient evidence to conclude whether or not sweating symptoms are significantly more reduced with tumescent suction curettage than with curettage alone in the medium term (up to 12 months).
There is low-quality evidence suggesting that curettage combined with laser treatment is more effective at reducing hyperhidrosis symptoms and has better aesthetic outcomes than laser or curettage alone, and no evidence of a difference in safety between these therapies.
As stated previously, there is very low-quality evidence regarding the relative effectiveness of BTX injections to the axillae compared with curettage and no evidence of a difference in longer-term effectiveness, and low-quality evidence suggesting a higher incidence of adverse events with curettage than with BTX.
Radical skin excision
Two studies of radical skin excision were included (see Appendix 2, Table 58). Results are reported in the curettage results section. 149,150
Shelley’s procedure (skin-sparing technique)
One study evaluated the effectiveness and safety of Shelley’s skin-sparing procedure (see Appendix 2, Table 58). 150 Results are reported in Curettage.
Laser epilation
Three RCTs evaluated the efficacy and safety of laser epilation for axillary hyperhidrosis. 24–26 All three studies were considered as being at a high risk of bias.
Sample sizes ranged from 626 to 100. 24 Studies were conducted in Germany,25 the USA26 and France, Germany and Spain. 24 Studies were recent, published between 201225,26 and 2015. 24
Only one study reported the age range of participants, which was 24–66 years. 25 The majority of participants in two of the studies were female,25,26 whereas the other study did not report the sex of the participants. 24 Inclusion criteria relating to patients’ baseline disease severity were reported in two of the studies; patients had HDSS scores of 3–4 in one study24 and patients met Hornberger 2004 criteria189 in the other. 25
The wavelength used varied between the studies. Leclere et al. 24 compared four interventions: (1) laser alone with a wavelength of 975 nm, (2) laser alone with simultaneous wavelengths of 924 nm and 975 nm, (3) laser (924/975 nm) followed by curettage, and (4) curettage alone. Bechara et al. 25 and Letada et al. 26 both evaluated the efficacy of a long-pulsed laser using a half-side controlled trial; the wavelength was 800 nm, delivered in five treatments at 4-week intervals, in one study25 and 1064 nm, delivered in six treatments at monthly intervals, in the other. 26
Laser epilation compared with no treatment
Two RCTs comparing laser epilation with no treatment were included (see Appendix 2, Table 60).
The studies by Bechara et al. 25 and Letada et al. 26 were both half-side RCTs that evaluated the efficacy of a long-pulsed laser. Bechara et al. 25 found a significant reduction in sweat rate measured by gravimetry on the laser-treated side and the untreated contralateral side, but no significant difference in reduction between treated and non-treated sides at 12 months’ follow-up (p = 0.10). Participant satisfaction with reduction in sweating was measured using a VAS score ranging from 0 to 10 (0 = not satisfied at all, 10 = absolute satisfaction). Patients reported a mean score of 5.9 after the last laser treatment and 4.1 at follow-up. No serious adverse events were reported.
Letada et al. 26 reported visibly reduced sweating in the laser-treated axilla, as measured by iodine starch test, in all six patients compared with the contralateral side at 1 month follow-up. All patients at 1 month, and two out of the three patients with results at 3 months, reported good or excellent improvement in Global Assessment Questionnaire. No adverse events were reported.
Laser epilation compared with laser epilation with curettage
The results of the Leclere et al. study,24 which compared different laser interventions with curettage, are reported in Curettage and in Appendix 2, Table 58.
Evidence summary for laser epilation
Two small RCTs of half-side comparisons between laser epilation and no treatment were included; both were rated as being at high risk of bias. 25,26
One study25 found no significant difference in sweat reduction measured by gravimetry between the treated and non-treated sides at 12 months. The other study26 found that sweating was visibly more reduced at 1 month according to the iodine starch test in the treated axillae. Both studies reported no serious adverse events.
Overall, there is insufficient evidence to suggest that laser epilation alone is more effective than no treatment at reducing hyperhidrosis symptoms or improving quality of life. There was insufficient evidence regarding the safety of laser epilation, although no evidence was found to suggest the intervention was not safe. As stated previously, there is low-quality evidence suggesting that curettage combined with laser treatment is more effective at reducing hyperhidrosis symptoms and has better aesthetic outcomes than laser or curettage alone, and no evidence of a difference in safety between these therapies.
Fractionated microneedle radiofrequency
Only one study evaluating the efficacy of fractionated microneedle radiofrequency was included (see Appendix 2, Table 61). 28 Fatemi Naeini et al. 28 compared a therapy of three sessions of fractionated microneedle radiofrequency with a sham treatment in a half-side comparison trial in 25 patients with axillary hyperhidrosis (baseline severity HDSS 3–4) in Iran. The study was not randomised and considered as being at high risk of bias.
Fatemi Naeini et al. 28 reported significantly better results in mean HDSS scores (p < 0.001) and sweating intensity (p < 0.001) at 21 weeks’ follow-up in the treated axillae than on the sham-treated side. Most patients experienced transient side effects, but there were no severe adverse events. One patient experienced tingling and numbness in the treated group, which resolved after 2 months of discontinuing treatment.
Evidence summary for fractionated microneedle radiofrequency
Overall, there is insufficient evidence regarding the relative effectiveness and safety of fractionated microneedle radiofrequency for axillary hyperhidrosis.
Microwave
Only one study of a microwave device for axillary hyperhidrosis was included (see Appendix 2, Table 62). 27 Glaser et al. 27 conducted a RCT (risk of bias rated as high) that evaluated the effect of two sessions of microwave therapy for axillary hyperhidrosis under local anaesthesia, delivered over approximately 2 weeks, compared with a sham intervention. A third procedure was allowed for non-responders. The microwave-based device included integrated vacuum and cooling (DTS G2 System; Miramar Labs, Sunnyvale, CA, USA). Few other details were provided. The study included 120 patients and was conducted in the USA. Patients’ baseline disease severity was a HDSS score of 3 or 4 points and a minimum sweat rate of 50 mg per 5 minutes. The majority of participants were female.
Efficacy was evaluated using HDSS and gravimetry. Placebo group patients had follow-up visits at 30 days, 3 and 6 months and then exited the study. Active group participants had follow-up visits at 30 days and 3, 6, 9 and 12 months after treatment. The proportion of participants with reduction in HDSS score of ≥ 2 points was significantly higher in the microwave group at 30 days, 3 months and 6 months (p < 0.001). There was no statistically significant difference in the percentage of patients with ≥ 50% reduction in sweat rate between the intervention and control groups at any of the follow-up points. There was a statistically significant difference favouring microwave therapy in the percentage of patients with ≥ 75% reduction in sweat rate at 30 days only (p = 0.01). There were 45 procedure-related adverse events in 28% of active group participants and 13% of sham patients. None of these adverse events were classed as serious and all were considered transient except for one case of compensatory facial hyperhidrosis in a participant in the active group, which was still present after 6 months.
Evidence summary for microwave
Only one study of a microwave device for axillary hyperhidrosis was included. 27 Overall there is very low-quality evidence suggesting that microwave therapy is more effective than placebo at reducing patient-reported disease severity, although there was no evidence of a significant difference in sweat reduction at up to 6 months. The evidence regarding the safety of microwave therapy is insufficient.
Ultrasound
Two RCTs of microfocused ultrasound were included, reported in a single publication (see Appendix 2,Table 63). 29 Sample sizes were 14 and 20, and both studies were conducted in the USA. Both were rated as being at high risk of bias. In both studies, two sessions of microfocused ultrasound were given, 30 days apart, for axillary hyperhidrosis (baseline severity HDSS 3–4 and sweat rate at least 50 mg/5 minutes), and compared with a sham treatment.
The first study was a half-side comparison RCT that included 14 patients. Most participants were female. Only three patients received local anaesthesia. Treatment response was assessed with gravimetry (≥ 50% reduction in sweating from baseline). The study reported that all except one patient had a response in the treated side at 90 days’ follow-up, but did not state whether or not there were any differences with untreated axillae. Treated axillae were associated with significantly higher rates of adverse events (primarily axilla tenderness/soreness), although most were mild and transient and there were no serious adverse events.
In the second study, 12 patients were randomised to ultrasound therapy for both axillae and eight to placebo. All patients received local anaesthesia. Treatment response was assessed with HDSS (defined as reduction from 3 or 4 points at baseline to 1 or 2 points at follow-up) and gravimetry (≥ 50% sweat reduction from baseline), at multiple time points (30, 60, 90 and 335 days from the end of treatment). At 30 days’ follow-up, the HDSS-response rate was approximately 67% in the intervention group, compared with 27% in the placebo group. The effect of the intervention and superiority over placebo appeared to be maintained until 1 year from baseline. Gravimetric response (≥ 50% reduction from baseline) was achieved in 83% patients in the intervention group, compared with 0% in the placebo group, across all time points (p < 0.0001 at all follow-up points). Adverse events were more commonly reported in the treatment group. Most were transient and mild (most commonly axilla tenderness/soreness) and none were serious.
Evidence summary for ultrasound
Two small RCTs of microfocused ultrasound were included. 29 Overall, there was insufficient evidence regarding the safety and effectiveness of ultrasound therapy for axillary hyperhidrosis.
Ongoing studies
Thirteen ongoing studies were identified from the searches of trial registers in July 2016. Details of the studies are presented in Table 4.
| Study | Sample size and study location | Patient characteristic | Intervention | Comparator |
|---|---|---|---|---|
| NCT02673619, 2016,190 randomised placebo-controlled double-blind trial | 55; USA and Canada | Palm | Umeclidinium | Placebo |
| ACTRN12615000873527, 2015,191 randomised crossover study | 21; Austria | Axilla and/or palmar hyperhidrosis | THVD-102 (a combination of oxybutynin and pilocarpine) | Placebo |
| NCT02633371, 2015,192 single-group assignment, open label | 7; USA | Axilla, adolescents and young adults | Topical oxybutynin 3% gel | N/A |
| EUCTR2015-002163-42-DE, 2015,193 open-label long-term safety study | 660; USA and Germany | Axilla | Glycopyrronium topical wipes (DRM04) | Placebo |
| NCT02553798, 2015,194 single-group assignment, open label | 660; USA and Germany | Axilla | DRM04 topical wipes | N/A |
| JPRN-UMIN000015874, 2014,195 randomised placebo-controlled double-blind trial | 3; Japan | Hyperhidrosis | 0.2% rapamycin gel | Placebo base gel |
| JPRN-UMIN000020647, 2016,196 randomised, double-blind, placebo-controlled study | 45; Japan | Palmar | OSD-001 (0.2% sirolimus gel, 0.4% sirolimus gel) | Placebo |
| EUCTR2011-003132-30-SE, 2011,197 randomised, double-blind, placebo-controlled crossover study | 588; Sweden | Inguinal, palmar or plantar hyperhidrosis (BTX-A), truncal or craniofacial hyperhidrosis (BTX-B) | BTX-A/BTX-B | Placebo |
| CTRI/2015/06/005935, 2015,198 non-randomised comparative study | 20; India | Axilla | BTX-A injections | Suction curettage |
| NCT02105753, 2014,199 randomised efficacy study, single-group assignment within-patient control, open label | 10; USA | Axilla | 1210 nm diode laser treatments × 1 per axilla | 1210 nm diode laser treatments × 2 per axilla |
| NCT02823340, 2016,200 single-group assignment, open label | 20; China | Axilla | Fractionated microneedle radiofrequency | N/A |
| NCT02295891, 2014,201 non-randomised efficacy study, single-group assignment, open label | 24; USA | Axilla | MiraDry® (Miramar Labs, Inc., Santa Barbara, CA, USA) | N/A |
| NCT02286765, 2014,202 randomised clinical trial | 40; USA | Axilla | Ulthera ultrasound system treatment in a 3 × 4 grid, 12 treatment squares, 60 lines of treatment per square, at one treatment depth (2.0 mm), at 0.30 J of energy | Ulthera ultrasound system treatment in a 3 × 4 grid, 12 treatment squares, 40 lines of treatment per square, at one treatment depth (2.0 mm), at 0.30 J of energy |
Conclusions of the effectiveness of interventions for hyperhidrosis
The evidence for the effectiveness and safety of second-line treatments of primary hyperhidrosis is limited overall. Most studies were small, rated as being at high risk of bias and poorly reported. There was insufficient evidence to draw firm conclusions regarding the relative effectiveness and safety of any active second-line treatments for hyperhidrosis.
There is very low-quality but consistent evidence suggesting a short-term beneficial effect of tap water iontophoresis in the treatment of palmoplantar hyperhidrosis compared with placebo and of dry-type iontophoresis compared with no treatment. Compared with tap water iontophoresis alone, the evidence for the effectiveness of combining anticholinergic therapy with iontophoresis is mixed and inconclusive. There is low-quality evidence to suggest that iontophoresis (assumed to be tap water iontophoresis, although not specified in the studies) is less effective than BTX injections at reducing palmar hyperhidrosis symptoms in the short term and that the effect duration is shorter than with BTX. There were no studies assessing the clinical effectiveness of iontophoresis for hyperhidrosis of the axilla.
There is very low-quality evidence suggesting a short-term benefit of topical glycopyrrolate for axillary and facial hyperhidrosis. No evidence for other treatment sites was found. There were no studies assessing the clinical effectiveness of oral glycopyrrolate.
There is low-quality evidence suggesting a short-term small benefit of oral oxybutynin in reducing hyperhidrosis symptoms and a short-term improvement in quality of life compared with placebo, although there is insufficient evidence to determine whether or not the effectiveness of oxybutynin differs according to target area. There is low-quality evidence that, compared with placebo, oral methantheline bromide has a short-term positive effect on axillary hyperhidrosis symptoms and quality of life, although this effect is small and may not be clinically significant. There is no evidence that methantheline bromide improves symptoms of the palm or any other body parts. There is evidence suggesting that both oxybutynin and methantheline bromide are associated with a high incidence of dry mouth symptoms. There were no studies assessing the clinical effectiveness of propantheline bromide for hyperhidrosis.
There is insufficient evidence to draw conclusions on the effectiveness and safety of topical BTX for primary hyperhidrosis.
There is moderate-quality evidence of a large effect of subcutaneous BTX on symptoms of axillary hyperhidrosis in the short and medium term (up to 16 weeks), and of a small to moderate positive effect on quality of life in the short term (up to 4 weeks), compared with placebo. BTX may be associated with higher patient satisfaction in the short to medium term, as well as a higher incidence of adverse events, notably injection site pain and CS. The evidence regarding the effectiveness of BTX injections to the axillae compared with curettage is of very low quality and uncertain, although there is no evidence to suggest that curettage is more effective than BTX in the short to medium term and there is evidence to suggest a higher incidence of adverse events with curettage. Trials are too short term to explore the potential curative nature of curettage, compared with the retreatment needed with BTX.
There is very low-quality evidence suggesting that BTX injections had a small positive effect on palmar hyperhidrosis symptoms compared with placebo or no treatment, although there was some very low-quality evidence to suggest a high incidence of adverse events with BTX-B (5000 U). The evidence for the effect of BTX injections for palmar hyperhidrosis on quality of life is insufficient.
There is very low-quality evidence suggesting that curettage combined with laser treatment is more effective at reducing hyperhidrosis symptoms and has better aesthetic outcomes than laser or curettage alone, and no evidence of a difference in safety between these therapies. There is insufficient evidence to conclude whether or not sweating symptoms are significantly more reduced with tumescent suction curettage than with curettage alone in the medium term (up to 12 months). There is also very low-quality evidence suggesting that the use of aggressive skin shaving with curettage is associated with extensive skin damage.
There is very low-quality evidence regarding the relative effectiveness and safety of curettage compared with other minor surgical interventions for axillary hyperhidrosis. Compared with more radical excision techniques, there is no clear evidence of a difference in sweat reduction, patient satisfaction or safety. There is evidence suggesting that patients undergoing suction curettage are more satisfied with aesthetic outcomes than those undergoing radical skin excision or Shelley’s procedure, although the limited evidence precludes any firm conclusions.
There is low-quality evidence suggesting that microwave therapy is more effective than placebo at reducing patient-reported disease severity, although there was no evidence of a significant difference in sweat reduction at up to 6 months. The evidence regarding the safety of microwave therapy is insufficient. The limited evidence precludes any conclusions regarding the effectiveness and safety of laser epilation, fractionated microneedle radiofrequency or ultrasound.
Gaps in the clinical evidence
Botulinum toxin for hyperhidrosis of the axilla
There is sufficient evidence demonstrating the clinical effectiveness of BTX for hyperhidrosis of the axilla and, therefore, no more trials of BTX compared with placebo in the axilla are warranted. Future trials of interventions for hyperhidrosis of the axilla should use BTX as an active comparator.
Iontophoresis for palmar hyperhidrosis
There are three studies of tap water iontophoresis compared with placebo37,40,42 and two studies of dry-type iontophoresis compared with no treatment. 43,44 Although they have methodological limitations, they all consistently show that iontophoresis is more effective than placebo/no treatment for hyperhidrosis of the palm; therefore, no further trials of iontophoresis compared with placebo/no treatment are warranted. Iontophoresis is currently standard practice for palmar hyperhidrosis in many dermatology units.
Iontophoresis compared with botulinum toxin for palmar hyperhidrosis
There is limited evidence for the effectiveness of BTX for palmar hyperhidrosis. Although the studies of iontophoresis for palmar hyperhidrosis are of poor quality, there is sufficient evidence to indicate a real, albeit limited, effect of iontophoresis. To date there have been two reasonably small, poor-quality, studies88,92 comparing BTX with iontophoresis (one of which also prescribed topical aluminium chloride lotion to the iontophoresis group88) for palmar hyperhidrosis. BTX looks promising for palmar hyperhidrosis, but the current evidence is not sufficiently reliable to draw firm conclusions; therefore, further research is required. A well-conducted, adequately powered RCT of BTX (with anaesthesia) compared with iontophoresis for palmar hyperhidrosis is warranted. The cost of BTX plus anaesthesia is considerably higher than the cost of iontophoresis; therefore, cost-effectiveness should also be assessed.
Microwave, laser, fractionated microneedle radiofrequency and ultrasound
These new, energy-based, ‘destructive’ technologies offer the prospect of the benefits of curettage for hyperhidrosis of the axilla, but with fewer risks. Some small, poor-quality, studies have shown promising results for these interventions compared with placebo or no treatment; however, these studies cannot be considered reliable, so there may not be sufficient promise to warrant further research. Studies of microwave, laser, fractionated microneedle radiofrequency and ultrasound therapies are ongoing, but the numbers of participantsare low (≤ 40) and the interventions are not being ompared against other active treatments or placebo. If the results of this ongoing research are consistent with the existing study results, then a trial comparing these new energy-based technologies with BTX for hyperhidrosis of the axilla may be warranted.
Curettage compared with botulinum toxin for hyperhidrosis of the axilla
Botulinum toxin has been shown to be effective for hyperhidrosis of the axilla. However, the procedure has to be repeated at regular intervals (at least annually). Curettage offers the prospect of a single treatment. There are currently a few small and/or non-randomised trials comparing curettage with BTX for hyperhidrosis of the axilla. The short- to medium-term evidence suggests that these two interventions are comparable in terms of effectiveness, or that BTX may be superior to curettage. In view of the ongoing research into less invasive, energy-based, technologies (microwave, laser, fractionated microneedle radiofrequency and ultrasound), a trial comparing BTX with curettage for hyperhidrosis of the axilla may not be warranted at this time. When further evidence is available on the newer energy-based technologies, then it will be clearer whether or not further research on curettage is warranted.
Comparison of different oral/topical medications: propantheline bromide, glycopyrrolate, oxybutynin, methantheline bromide and newer medications
The oral medications have not been well studied in trials; there are no placebo-controlled trials of oral glycopyrrolate (thought to be the most effective) or of propantheline bromide (the cheapest available). There are no direct comparisons of the different oral/topical anticholinergic medications for hyperhidrosis. However, there are ongoing and recently completed trials of new oral and topical formulations; therefore, it may not be relevant to undertake further research of these specific drugs at this time. Although different medications may work better for some patients than others, it may be difficult to power a study to find any statistically significant differences between treatments.
Chapter 4 Review of quality-of-life measures/tools
Background
The aim of this review was to identify the tools commonly used to measure quality of life in studies of patients with hyperhidrosis. An assessment of the most appropriate tool for use in studies of hyperhidrosis is beyond the scope of this review, although a brief overview of data related to the reliability and validity of the tool has been provided, when such data were available.
Methods of the quality-of-life measures review
To locate quality-of-life measures/tools relevant to hyperhidrosis, studies were identified as part of the literature searching conducted for the review of clinical effectiveness of treatments for hyperhidrosis. The searches were undertaken in January 2016 and the full search methods are reported in Chapter 2, Literature searches. For the review of quality-of-life measures, the search strategies combined topic terms for hyperhidrosis with a recognised search filter for ‘quality of life’. The full list of search terms is presented in Appendix 1, Hyperhidrosis quality-of-life literature searching.
Inclusion and exclusion criteria
Study eligibility was not restricted to the interventions considered in the separate systematic review of effectiveness: all studies that reported measuring quality of life or described a quality-of-life measure/tool in the context of hyperhidrosis were included. These studies were identified at the abstract screening stage or from the full papers ordered for the review of effectiveness. It is acknowledged that some papers excluded from the effectiveness review at the abstract stage may have mentioned quality of life in the full paper and such studies will have been missed. However, we consider that it is unlikely that any important quality-of-life tools have been missed because of this, because of the large number of studies screened.
Population
Patients with primary hyperhidrosis (including adults and children). Patients with hyperhidrosis secondary to other conditions, such as overactive thyroid or spinal cord injury, or social anxiety disorder, were not eligible for inclusion.
Outcomes
Any quality-of-life-related outcome measure was included. Quality-of-life outcomes can be measured using a variety of tools: disease specific, such as the HDSS and HHIQ; discipline specific (dermatology), such as the DLQI or Skindex-29; or more general health or utility, such as the Short Form questionnaire-36 items (SF-36).
Study designs
Any study design was eligible for inclusion in this review.
Data extraction
All quality-of-life measures reported in the included studies were extracted into Microsoft Word® (2013; Microsoft Corporation, Redmond, WA, USA). The data extract comprised details of the quality-of-life tool or tools used, whether or not the tool was disease specific for hyperhidrosis, disease specific for skin disease or a generic tool for health-related quality of life (HRQoL), and any description of reliability and validity of the tool, when available.
Details of the identified studies (reference, country, study design and body part affected by hyperhidrosis) were extracted by two reviewers. Details of the quality-of-life outcome measures and tools used in studies were extracted by a single reviewer and checked by a second reviewer.
The quality-of-life studies were not quality assessed as they did not necessarily evaluate the effectiveness of interventions, and the data extracted from these studies were not effectiveness data. Although it has been suggested that the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist suggests it could be useful when selecting a measurement instrument’,203 it was found that it could not be readily used for the following reasons. First, the checklist requires a high level of detailed information about how a tool was developed, far more than was available for this review; the studies found did not provide sufficient information to enable such a detailed assessment of methodological quality. Second, in order to apply the COSMIN checklist, a level of expertise and understanding about tool development is required, which was beyond the remit of the project.
The information on the tools and their use in hyperhidrosis was summarised in a narrative synthesis describing the quality-of-life tool, the associated studies and the target of the tool.
Results of the quality-of-life measures review
The searches for studies or reviews that recorded quality-of-life outcomes identified 337 publications in total; the study selection process found 183 studies that were relevant for inclusion into the review of quality-of-life measures grouped as follows:
-
Dermatology (n = 75). Studies presenting a dermatology intervention for hyperhidrosis that included a measure of quality of life; interventions included iontophoresis, BTX injections, topical treatments and/or oral treatments.
-
Surgery (n = 82). Studies presenting a surgical intervention, including local surgical interventions, such as the removal of the sweat glands, or more major surgery, such as ETS.
-
Dermatology and surgery (n = 6). Studies involving both dermatological and surgical interventions.
-
Economics (n = 7). Studies excluded from the clinical effectiveness review, but which presented an economic analysis.
-
Quality-of-life measure only (n = 13). Studies that reviewed quality-of-life measures or reported the development of a quality-of-life tool and which were excluded from the systematic review of effectiveness but presented methods for the measurement of quality of life.
Some studies reported outcomes from more than one quality-of-life measure; therefore, the review recorded a total of 240 measures from the 183 studies: 202 measures from intervention studies, nine from economic analyses and 29 from reviews or reports that discussed quality of life only. The frequency of use for the most frequently used tools by type of study is shown in Figure 10.
FIGURE 10.
Most frequently used quality-of-life tools by treatment type: surgery, dermatology, surgery and dermatology, economics, or quality of life only. HQLQ, Hyperhidrosis Quality of Life Questionnaire.

Twenty-two individual tools for measuring quality of life were identified from the selected studies,; a further 32 studies reported quality-of-life outcomes, but the method used to measure quality of life was not reported or was unique to the single study. These are summarised in Table 5. As acknowledged in Methods of the quality-of-life measures review, this is not the result of a totally comprehensive search for all studies using these tools and, therefore, the data should be considered only indicative of the relative frequency of use of the identified tools, when tools were available. New tools recently designed and validated, such as the Hyperhidrosis Quality of Life Index (HidroQoL©) tool,204 are likely to be used in only a very few studies, if any, because of their relative novelty.
| Description | Tool | Surgery | Dermatology | Surgery and dermatology | Economics only | Quality of life only | Total |
|---|---|---|---|---|---|---|---|
| Hyperhidrosis Disease Severity Scale (Kowalski et al.205) | HDSS | 27 | 27 | 2 | 4 | 3 | 63 |
| Dermatology Life Quality Index (includes children’s version: Children’s Dermatology Life Quality Index) | DLQI | 11 | 28 | 2 | 2 | 5 | 48 |
| Hyperhidrosis Quality of Life Questionnaire | HQLQ | 17 | 13 | 0 | 0 | 1 | 31 |
| Short Form questionnaire-36 items | SF-36 | 6 | 3 | 1 | 0 | 3 | 13 |
| Keller Hyperhidrosis Scale | Keller scale | 7 | 1 | 0 | 0 | 2 | 10 |
| Hyperhidrosis Impact Questionnaire | HHIQ | 0 | 7 | 0 | 0 | 1 | 8 |
| Short Form questionnaire-12 items | SF-12 | 1 | 2 | 0 | 2 | 2 | 7 |
| Quality-of-life measure for people with skin disease | Skindex | 1 | 3 | 0 | 0 | 2 | 6 |
| A short disease-specific health-related questionnaire for people suffering from hyperhidrosis | Amir 2000186 | 2 | 1 | 0 | 0 | 2 | 5 |
| Hyperhidrosis Quality of Life Index | HidroQoL© | 0 | 0 | 0 | 1 | 2 | 3 |
| Hyperhidrosis Questionnaire | HQ | 0 | 0 | 0 | 0 | 2 | 2 |
| Illness Intrusiveness Rating Scale | IIRS | 0 | 0 | 0 | 0 | 2 | 2 |
| Leibowitz Social Anxiety Scale | Liebowitz 1987206 | 1 | 0 | 0 | 0 | 0 | 1 |
| A French-language scoring instrument validated for chronic skin diseases | VQ-Dermato scale | 1 | 0 | 0 | 0 | 0 | 1 |
| Questionnaire of Quality of Life (adapted from the Caregiver Questionnaire) | QQL | 0 | 1 | 0 | 0 | 0 | 1 |
| University of California, Los Angeles Loneliness Scale | UCLA V3 | 0 | 1 | 0 | 0 | 0 | 1 |
| Nottingham Health Profile | NHP | 0 | 0 | 0 | 0 | 1 | 1 |
| Freiburg Life Quality Assessment | FLQA | 0 | 0 | 0 | 0 | 1 | 1 |
| Patient Benefit Index | PBI | 0 | 0 | 0 | 0 | 1 | 1 |
| The Everyday Life Questionnaire | EDLQ | 1 | 0 | 0 | 0 | 0 | 1 |
| State–Trait Anxiety Inventory | STAI | 1 | 0 | 0 | 0 | 0 | 1 |
| International quality of life assessment | EQ-5D-5L | 0 | 0 | 0 | 1 | 0 | 1 |
| Tool used NR or unique to only one study | NR | 21 | 10 | 1 | 0 | 0 | 32 |
| Total | 97 | 97 | 7 | 9 | 30 | 240 | |
A brief description follows of the tools used to measure quality of life and the associated studies identified for the quality-of-life review. Each tool is described separately and grouped depending on the target patient group (disease specific for hyperhidrosis, disease specific for skin disease and generic tools to measure HRQoL). Any original validation work was also identified and is described and a reference cited when available. When the tool used was not described in the methods or the researchers designed their own method to evaluate quality of life, the data were recorded as ‘not reported’ (n = 32).
Characteristics of disease-specific tools identified to measure quality of life in patients with hyperhidrosis
Hyperhidrosis Disease Severity Scale
The HDSS was identified as the most commonly used tool. The HDSS was used in 63 studies in total: for surgical research in 27 studies,17,24,27–29,159–161,164,167,171,174–176,179,181,207–217 for dermatological research in 27 studies,2,45,51–53,59,61,77,91,97,102,106–108,119,125,131,136,139,145,180,218–223 for surgery and dermatology combined in two studies,21,224 for three quality-of-life-only studies225–227 and in four economic evaluation studies228–231 for measuring quality of life in patients with hyperhidrosis. The HDSS was often used in combination with the DLQI, with 18 studies using both tools (10 dermatology studies,61,77,102,106,107,119,139,221–223 five surgical studies,176,208,210,214,217 two quality of life studies225,227 and one economic assessment230).
Studies that were found to use the HDSS tool were conducted globally, including the USA and Canada (n = 2121,27,29,52,77,107,108,139,159,160,164,175,176,179,180,208,209,218,225,226,229), Spain (n = 6167,207,210,224,230,231), the UK (n = 591,97,125,222,232), Germany (n = 52,131,145,161,227), Italy (n = 5102,106,136,214,223), France (n = 359,61,211), South Korea (n = 351,171,174), Australia (n = 253,221), Egypt (n = 2213,219), Iran (n = 228,220), the Netherlands (n = 2215,217), Turkey (n = 2212,216), Austria (n = 117), Europe (n = 124), Greece (n = 1119), Pakistan (n = 145) and Russia (n = 1181). The majority of studies were case series (n = 2717,45,61,97,102,106,107,119,125,131,136,139,145,159–161,164,167,171,174–176,181,207–210). There were 15 RCTs,21,24,27,29,51–53,59,77,87,108,179,180,218,220 and the remainder were non-RCTs, retrospective observational studies or reviews (n = 222,28,91,211–217,219,221–227,229–232). The HDSS was used in studies to measure quality of life in patients with sweating in various areas of the body; studies of axillary sweating were most common (n = 362,21,24,27–29,52,53,77,91,97,107,108,125,136,139,159–161,164,167,171,174–176,179–181,209,212,214,218,219,221,223,229), with an additional seven studies102,106,119,131,145,207,213 examining sweating of the palm and axilla combined. Eleven studies17,45,61,208,211,215,222,224,225,230,231 were identified of the foot and/or palm combined and nine of the face, palm and/or foot or generalised hyperhidrosis. 51,59,210,216,217,220,226,227,232
The HDSS is a disease-specific tool considered important for diagnostic use in clinical practice and for research to identify and quantify the severity of disease in patients with hyperhidrosis and also to assess treatment effects over time. 12,205 The HDSS allows researchers to measure the impact of hyperhidrosis on those suffering from excessive sweating by using a four-point scale:
-
My sweating is never noticeable and never interferes with my daily activities.
-
My sweating is tolerable but sometimes interferes with my daily activities.
-
My sweating is barely tolerable and frequently interferes with my daily activities.
-
My sweating is intolerable and always interferes with my daily activities.
Information on the original design of the HDSS is lacking, although it was possibly created by Kowalski in 2004 (Lisa Pieretti, International Hyperhidrosis Society, 5 June 2016, personal communication). Efforts have been made to justify the use of the tool in hyperhidrosis research; for example, Kowalski et al. 205 presented work that examined the validity and reliability of the HDSS as a conference abstract in 2004. 205 The study presented data from a longitudinal clinical trial and a cross-sectional national survey of 150,000 US households. The HDSS score at 4 weeks post treatment was found to correlate well with the DLQI and relevant activity items from the HHIQ (r = 0.35 to 0.77; p < 0.001). Kowalski et al. 205 also presented data on construct analysis, which suggested that a reduction (improvement) in the HDSS score correlated well with a reduction in gravimetric results, a clinical test used to measure quantity of sweating in patients with hyperhidrosis, and that patients reported an increase in HDSS score with increases in limited daily activity due to sweating. The test–retest reliability coefficient was also found to be within an acceptable range (r = 0.82; p < 0.05). 205
Studies of hyperhidrosis were found to use the HDSS more frequently than any other method for measuring quality of life. The tool’s simple design has raised questions about its value as a tool to measure patient-reported quality of life (Lisa Pieretti, personal communication), and a consensus exercise by the Canadian Hyperhidrosis Advisory Committee considered the HDSS more appropriate as a measure of disease severity. 12 Furthermore, Panhofer et al. 233 published a survey and validation guide for tools used to measure quality of life in patients undergoing surgical interventions for hyperhidrosis and the HDSS was not considered as part of this work. This reflects the fact that the HDSS is not viewed purely as a quality-of-life tool; it is also possible the patient may complete the tool based on an improvement in quality of life or simply a perceived reduction in sweating (with an assumption that this will improve their quality of life).
Disease-specific health-related questionnaire for hyperhidrosis (Amir et al.186)
The disease-specific health-related questionnaire for hyperhidrosis was designed and validated by a research team in Israel, Amir et al. ,186 to assist with clinical decision-making and to measure the efficacy of surgical interventions in reducing sweat production in patients with hyperhidrosis. The questionnaire was designed and validated with a patient population who were considering surgery for the treatment of their excessive sweating.
The Amir et al. 186 questionnaire was found in five studies identified for the review of quality-of-life measures: the original development and validation work, integrated with a surgical study with a focus on quality of life,186 and four additional studies, all from the same team in Brazil (three surgical studies234–236 and one dermatology study237).
The Amir et al. 186 tool development was conducted in two stages. First, 10 patients aged between 15 and 35 years were interviewed by two psychology students using a semistructured interview method with questions that focused on the ways in which hyperhidrosis limits the daily life of patients. The responses were analysed by the same psychology students, a clinical psychologist and a surgeon. Participants were encouraged to discuss the effect on their leisure activities, social interactions, work or interpersonal relationships. From the responses, four domains were identified (functional, social, interpersonal and emotional). Functional included driving, writing and sports; social included shaking hands or being in public places; personal involved being intimate with a partner; and emotional was split between the patient’s own feelings about their condition (emotional self) and how they think others view them (emotional other). The authors note that not all patients would find all questions relevant (e.g. those who do not drive).
The questionnaire was designed with 35 questions separated into the five domains with a 7-point Likert scale for each response, where a score of 6–7 indicated a very low quality of life, 3–5 a medium level of quality of life and 1–2 a high level of quality of life. The validation found a high level of internal reliability (Cronbach’s α = 0.84).
The results suggest that patients felt impaired in many facets of life, both intimate and practical, and that the effect of heat and stress played a major part in the patients’ quality of life. The authors found no differences between the body area (hands, feet and axilla) and quality of life. Analysis of variance for the quality of life domains in relation to sex and duration since onset found that females reported a lower quality of life in all domains except emotional other. In addition, patients in whom onset of hyperhidrosis occurred during childhood had a lower quality of life. A limitation of the validation work noted by the authors is that only patients waiting for surgery were used in the survey and these may be patients whose symptoms are at the more severe end of the spectrum. 186 Another point to mention is that the tool was validated in Israel and reported in studies conducted in Brazil, both countries with a very hot climate that could have an impact on the patient population and subsequent outcome.
Hyperhidrosis Quality-of-Life Questionnaire
The Hyperhidrosis Quality of Life Questionnaire (HQLQ) was designed by de Campos et al. 238 in 2003 as a disease-specific tool to assess the effect of surgical intervention for patients with hyperhidrosis. The design built on the previous validation work of Amir et al. 186 and tested the tool on a large patient population (n = 378), with the aim of replacing more generic measures.
The HQLQ was used in 31 studies included in the quality-of-life review. Seventeen studies, including the validation work, had a surgical intervention,234,236,238–252 13 dermatology studies55,57,60,62–67,237,253–255 and one quality-of-life review. 227 The tool was used in Austria (n = 6240–242,248,249,251), Brazil (n = 2157,60–67,234,236–239,243,244,246,247,252–255), Germany (n = 1227), Italy (n = 1245), South Korea (n = 155) and India (n = 1250).
de Campos et al. 238 used their questionnaire to evaluate preoperative HRQoL and postoperative improvement in symptoms of hyperhidrosis in all patients undergoing surgical intervention between 1995 and 2002. The development study included 386 hyperhidrosis patients who underwent video-assisted thoracic surgery. 238 The survey response rate was 91% (344 patients). Patients included in the study suffered with either palmar and plantar (57.4%), palmar, axillary and plantar (25%), axillary (15.7%) or facial (6.5%) sweating.
The HQLQ consists of a single question to start ‘How would you rate your quality of life before and after treatment?’, to which the patient is asked to respond with a score between 1 and 5, with 5 being very poor/much worse. This is followed by 20 questions selected for relevance from the 36 items taken from the Amir et al. 186 questionnaire, again scored between 1 (excellent) and 5 (very poor), and further separated into domains (again as described previously by Amir et al. 186), including (1) functional or social life, (2) personal life, (3) emotional self and (4) special circumstances (such as climate or stress). Therefore, the final score for quality of life can range from 20 (excellent/much better after surgery) to 100 (very poor/much worse after surgery). No validation or reliability statistics or cross-validation with other tools was reported.
Keller Hyperhidrosis Scale
The Keller Hyperhidrosis Scale256 was cited in 10 studies in total: seven surgical studies,240–242,248,249,251,257 one dermatology study55 and two quality-of-life reviews. 227,256 The tool was designed by an American team, although, apart from the one South Korean dermatology study,55 all studies using the Keller tool appear to come from the same team in Austria and all studies except one257 used both the Keller and the de Campos et al. HQLQ238 methods combined to measure quality of life.
The Keller scale was designed by Keller et al. 256 to measure preoperative and postoperative quality of life in patients receiving bilateral ETS for palmar and plantar hyperhidrosis and appears to be a popular tool in the field. The validation work compared patient score against the SF-36 and validation work reported a strong level of reliability (Cronbach’s α = 0.89). The Keller Hyperhidrosis Scale measures quality of life on a scale of 0 (mild) to 10 (severe). A total of 71 patients were entered into the study with a mean preoperative Keller score of 6.85, which decreased significantly (p < 0.001) to 1.79, 1.53 and 1.91 at 2 weeks, 6 months and 1 year post surgery, respectively. Keller et al. 256 reported no significant MD between preoperative and postoperative scores for the SF-36 (p = 0.70) and concluded that standard quality-of-life instruments do not accurately assess the benefits of surgery to patients with palmar and plantar hyperhidrosis.
Hyperhidrosis Impact Questionnaire
The HHIQ was designed by Teale et al. 258 in 2002 to assess the impact of hyperhidrosis on the daily lives of patients and measure the effect of treatment. The HHIQ development and validation were performed by a team of researchers funded by Allergan Inc. (Irvine, CA, USA), a large international pharmaceutical company and significant producer of BTX-A for the treatment of hyperhidrosis. The relatively high frequency of use of this tool reflects its use in Allergan-sponsored research. The validation work was not available as a full publication, but does appear as a conference abstract presented at the ninth Annual Conference of the International Society for Quality of Life Research (ISOQOL) in 2002. 258
Eight studies selected for inclusion into the quality-of-life review used the disease-specific HHIQ for their quality-of-life assessment or review: the original validation work by Teale et al. 258 in 2002 and seven additional studies that examined the effect of BTX-A on axillary or palmar hyperhidrosis. 76,78,80,86,88,107,139 The study by Rajagopal and Mallya88 was conducted on behalf of the Indian armed forces and focused on the use of BTX to treat palmar hyperhidrosis and appears independent of the other six studies, all of which were conducted in either Europe or the USA/Canada and were funded or supported by Allergan Inc.
A review of the literature and interviews with key stakeholders (patients and physicians in the UK and Germany) informed the design of the tool; a pilot study with the same stakeholders then tested the validity and linguistic equivalence of the questionnaire. 258 The questionnaire contained four sections: (1) disease and treatment background; (2) direct impact on medical and non-medical resource utilisation; (3) indirect impact on employment and productivity; and (4) intangible impacts on emotional status. Forty-one questions measured baseline impact of the disease and a furtehr 10 questions were included in the follow-up assessments. The final design of the HHIQ was validated against the SF-12 health survey and the DLQI, using a population of 345 patients and 145 non-hyperhidrosis controls. A test–retest of the 10 follow-up questions using a cohort of clinical patients found consistent reliability and responsiveness of the HHIQ. The authors conclude that the tool is a valid and reliable instrument for measuring disease and treatment effects in patients with hyperhidrosis.
Hyperhidrosis Questionnaire
The Hyperhidrosis Questionnaire is a disease-specific instrument identified from a quality-of-life-only study published in 2004 by Kuo et al. 259 describing the tool’s design and validation. Although the development and design of this tool appears to be quite comprehensive, the tool has been used in only one other study selected for the quality-of-life review, a review paper of quality-of-life tools for hyperhidrosis by Rzany et al. 227
The development study reported a review of the literature, to inform the elements to include in the instrument’s design, followed by interviews with patients, nursing staff and clinicians. The pilot questionnaire content was then validated by a panel of patient experts before being tested. The pilot questionnaire contained 34 questions answered using a Likert scale of 1 (least disturbance) to 5 (most disturbance). The final questionnaire contained 29 questions across five factors (domains): (1) functional, (2) psychological, (3) social, (4) affective and (5) physical.
The study included 85 patients suffering from a combination of plantar, palmar, axilla or generalised hyperhidrosis attending a thoracic surgery outpatient clinic in southern Taiwan between April 2002 and March 2003. Internal reliability and construct validity was reported (Cronbach’s α = 0.95, range 0.71–0.94 across domains). There was no investigation of whether or not the scale reflects changes in disease severity, nor was any cross-validation with other scales reported.
Hyperhidrosis Quality of Life Index
The HidroQoL was not identified in any of the studies selected for the quality-of-life review apart from those published to describe its design and validation, probably because these are relatively recent (2012 and 2015). 204,260,261
The tool was developed as a disease-specific aid to both clinical practice and research to assist with hyperhidrosis patient and clinician communication. The HidroQoL was not developed for any specific body site or intervention. The HidroQoL tool was created using responses to interviews and focus group discussions. In 2012, Kamudoni et al. 204 recruited an online cohort of 71 patients from a number of social networking sites to participate in initial interviews. From this, a prototype of the HidroQoL was created. It contained 47 questions with a six-point patient response scale: (1) not at all, (2) a little, (3) somewhat, (4) quite a bit, (5) very much and (6) not relevant. A panel of experts, including patients suffering from focal or generalised hyperhidrosis (n = 7) and dermatology clinicians (n = 5), was recruited to validate the content of the prototype tool using a questionnaire administered by e-mail. Further work in 2015261 used modern test theory to examine differential item functioning, which allowed the researchers to assess if the tool would function in different ways for different groups or test-takers.
The second stage of validation involved a cross-sectional cohort of 595 patient volunteers recruited using prespecified inclusion criteria (age > 18 years, self-reported hyperhidrosis, HDSS score of ≥ 2 points and disease onset prior to early adult years). The participants completed a number of questionnaires for comparison (HDSS, DLQI and Skindex-17). Construct validity, reliability and responsiveness were assessed using an online longitudinal study (n = 260 patients), for which patients were required to complete the tool on three occasions over a period of time (7–21 days), using the final version of the questionnaire. Construct validation found that the HidroQoL correlated well with the DLQI (r = 0.6; p < 0.01) and HDSS (r = 0.59; p < 0.001), and showed correlation to the Skindex-16 scale, but to a lesser extent. The change in HDSS score recorded by participants over time was also indicated in the HidroQoL scores from the longitudinal study, suggesting that the tool could identify slight change or small responses to treatment over a period of time. Reliability, tested using baseline measures and a test–retest method, showed strong reproducibility (internal consistency, Cronbach’s α overall scale = 0.89; test–retest reliability, intraclass correlation = 0.93).
Characteristics of disease-specific tools identified to measure quality of life in patients with skin disease
Dermatology Life Quality Index (includes children’s version)
Described as a practical technique for routine clinical use, this questionnaire is a short and concise tool (10 questions) for use in the management of chronic skin disorders. 262 Developed by Finlay and Khan262 to provide a patient-centred method for comparison between different types of skin disease, the questionnaire records the impact each disease has on a patient’s quality of life and the relative effectiveness of treatment.
The DLQI was found in 48 studies for reporting quality-of-life measures in patients with hyperhidrosis. The study selection process found 28 dermatology studies,54,56,61,69,77,101–103,106,107,116,117,119–121,128,134,139,221–223,263–269 11 surgical studies,147,158,170,173,176,208,210,214,217,270,271 two dermatology/surgery combined,272,273 two economic assessments230,274 and five studies or reviews of quality of life. 225,227,261,262,275 As mentioned previously (see Hyperhidrosis Disease Severity Scale), the DLQI was often used alongside the HDSS in dermatology studies (n = 1061,77,102,106,107,119,139,221–223), surgical studies (n = 5176,208,210,214,217), studies of quality of life only (n = 2225,227) and also economic assessments (n = 1230).
The DLQI was identified in studies conducted globally, including in the UK (n = 9103,232,261,262,267,268,275), the USA and Canada (n = 877,107,139,173,176,208,222,263), Italy (n = 7101,102,106,214,223,266,272), Germany (n = 569,158,227,270,271), Sweden (n = 4114,116,117,128), Greece (n = 3119–121), Ireland (n = 2265,274), Iran (n = 2170,264), Spain (n = 2210,230), Australia (n = 1221), Austria (n = 1273), France (n = 161), India (n = 1269), the Netherlands (n = 1217) and Norway (n = 1147). The studies included 24 case series,54,56,61,101–103,106,107,114,116,117,119–121,128,139,158,170,173,176,208,210,214,266 nine retrospective observational studies,217,221,222,230,263,265,268,270,271 six quality-of-life or non-experimental studies227,232,261,262,274,275 and three RCTs;69,77,223 the remainder were non-RCTs (n = 3264,269,272) or surgical reviews or reviews of surgery compared with dermatology (n = 3147,267,273). The DLQI was used in studies to measure quality of life on various areas of the body; studies of axillary sweating combined with other parts of the body, such as palms or feet, were most common (n = 2654,56,69,77,101,103,106,107,114,119–121,139,147,158,170,173,176,214,221,223,263,266,273,274). Studies were identified of the palm or palm and foot combined (n = 1161,128,208,222,230,264,265,269–272) and of other combinations including the face, palm and/or foot or generalised hyperhidrosis (n = 11116,117,210,217,227,232,261,262,267,268,275).
The DLQI was validated by Finlay and Khan262 using a survey of 128 patients aged 15–70 years who attended a dermatology clinic. Patients were asked to document how certain aspects of their skin disease affected their life, for example any effects the condition had on their work life, social life or personal relationships. The results were combined and a questionnaire developed based on the 10 most commonly reported aspects of impaired quality of life. The answers to each of the 10 questions were scored from 0 to 3, where a total maximum score of 30 and minimum score of 0 could be achieved: the higher the score, the greater the impairment in quality of life. Once designed, the questionnaire was tested for use on 200 outpatients and 100 healthy volunteers and reliability was further validated using a cohort of 53 patients by means of measuring the correlation of test–retest results over a 10-day period.
A recent review of the DLQI reported that repeatability, internal consistency and sensitivity to change have all been demonstrated for this tool and it has been cross-validated against a number of other dermatology tools (though mainly for psoriasis and acne). 276 The DLQI instrument is licensed for use and, therefore, permission is required from the authors to use the tool.
Skindex: a quality-of-life measure for people with skin disease
The Skindex suite of tools includes the original Skindex questionnaire, Skindex-29, Skindex-17 and Skindex-16. The tools are dermatology-specific HRQoL instruments, originally based on the DLQI. The original tool was a 61-question survey refined to a 29-item questionnaire (Skindex-29),277 to reduce the time needed to complete the questions and to improve the tool’s evaluative properties. 278 Further refinement included using patients’ perspective responses to create a 16-item questionnaire (Skindex-16)279 for use in longitudinal research to measure changes over time in patient quality of life in addition to reducing the tool to one page. Finally, a study in 2006 created Skindex-17. A Rasch-reduced tool was created using a response theory model to address issues such as response order and differential item functioning,280 and resulted in a reduction of the Skindex-29 item tool to a 17-item instrument. The questions in the Skindex-16 and Skindex-17 differ; however, a simple comparison conducted by Nijsten et al. 280 found that the different reduction approaches (patient opinion vs. statistical modelling) resulted in very similar instruments.
The Skindex-29 tool was used in five of the six studies identified for the group of Skindex instruments by the hyperhidrosis quality-of-life review. 143,227,281–283 The Lessa et al. 284 study used Skindex-16. The studies included two quality-of-life reviews, a RCT, a single case series, a case report and a surgical observational study. Two studies were carried out in Germany and the other four studies in the Netherlands, Turkey, Spain and the USA.
The Skindex-61 item questionnaire was first created by Chren et al. 278 in 1996; the validation of the tool was later published in 1997. Cronbach’s α (0.71–0.94) was calculated to measure the reliability of the psychometric test. 285 The tool’s design was based on findings from a review of the literature and focus group interviews with patients and clinicians to construct the initial framework for the ways in which patients are affected by skin disease. The tool has eight scales (cognitive, social, discomfort, limitations, depression, fear, embarrassment and anger) within three domains (symptoms, emotions and functioning). Patients are asked to respond to the questions with one of five responses (never, rarely, sometimes, often, all the time), and the score is reported as three values, one for each domain, calculated by averaging the patient’s responses from each of the questions within each of the three domains.
In a study of 201 patients, scale scores were reproducible after 72 hours and were internally consistent (Cronbach’s α = 0.76–0.86). Construct validity was also demonstrated. However, physicians’ judgement of disease severity did not consistently correspond with Skindex scores and Skindex does not appear to have been cross-validated against other quality-of-life measures.
The authors suggest that the Skindex-29 tool is more suited to research for which a comprehensive overview of HRQoL is required and the goal may be to understand the response to treatment. 285 The Skindex-16 was designed to serve longitudinal data collection. As patients may be required to complete the same survey many times over the course of a study, it was considered that it would be better if this was a shorter exercise. The Skindex instruments are copyrighted and, therefore, permission is required from the authors to use either of the tools.
VQ-Dermato scale: a French-language scoring instrument validated for chronic skin diseases – Grob et al.286
The VQ-Dermato scale was designed and validated to provide a French-language dermatology-specific instrument for routine use to assess the quality of life of patients with ‘chronic skin disorders’. 286 The scale was identified in one article selected for inclusion into the quality-of-life review, a retrospective analysis of a 142 patient study to examine the long-term (> 5 years) effects of ETS on patient satisfaction and quality of life for patients with palmar hyperhidrosis between 1994 and 2005. 211
The VQ-Dermato scale is a self-administered 28-item instrument developed from interviews with patients. Grob et al. 286 tested the tool on a population of 231 hospital and private practice patients in France suffering from chronic skin conditions and reported the instrument was validated in terms of content, construct, reliability/reproducibility and internal consistency. A strong correlation was reported by authors between the VQ-Dermato and the SF-36 (Cronbach’s α = 0.67–0.88).
Freiburg Life Quality Assessment
The Freiburg Life Quality Assessment was designed and validated as a set of dermatology-specific modules. The first module addresses the core issues of all skin diseases. The additional questions are more specific to distinct diseases. The tool was found to have satisfactory discriminatory power and validation data were published in 2004. 287 This tool was found to be used in only one hyperhidrosis quality-of-life review. 288
Patient Benefit Index
The Patient Benefit Index created by Augustin et al. 289 is an instrument to identify patient-reported needs and benefits of dermatology research and treatment. Assessment is a two-step process; the first captures data on the patients’ needs prior to treatment, and this is followed by assessment of improvement after treatment. The result is an index of patient benefit in response to treatment. The measure was validated in 2009 by the authors using a large cohort of patients (n = 500) with many different skin diseases, including some with hyperhidrosis (n = 50). 289 Use of this tool was identified in only one quality-of-life review of patients with hyperhidrosis. 288
Characteristics of non-disease-specific tools
Illness Intrusiveness Rating Scale
The Illness Intrusiveness Rating Scale (IIRS) was developed by Devins290 in 1994 to measure the impact of illness based on its intrusion on valued lifestyle activities. Devins produced a follow-up publication in 2010 describing the use of the tool and validating how it can contribute to the understanding of HRQoL in chronic disease. 291 The IIRS was identified in only one other study from 1999. 292 This study of 68 patients found that total IIRS score correlated well with other disease severity measures (r = 0.61; p < 0.001) and reflected improvements post surgery. The tool is a 13-item survey scored on a Likert scale from 1 (not very much) to 7 (very much). The tool can be used as a 13-item scale or divided into three subsets with a score calculated for each subset. The subsets are relationships and personal development (may include family or social relationships), intimacy (with partner) and instrumental considerations (such as work).
The IIRS validation was informed by a qualitative review of literature examining the results of studies in which it has been used, and describes the value of the tool for studies measuring patient-reported quality of life in 36 chronic disease groups. 291 Hyperhidrosis was considered in the review, with data on 223 patients provided by Claudio Cina. 291 Cronbach’s α coefficient for the total scale was 0.97, for relationships and personal development was 0.94, for intimacy was 0.92 and for instrumental considerations was 0.93.
Although the results presented by Devins290 suggest that the IIRS is potentially useful and valid for measuring quality of life in patients with hyperhidrosis, it is possible that it is too general to be popular for measuring quality of life in studies of hyperhidrosis, as reflected by its limited presence in such studies since 1999.
Medical Outcomes Study short forms (Short Form questionnaire-36 items and Short Form questionnaire-12 items)
The Medical Outcomes Study (MOS) was a large US cross-sectional study (n = 22,462 patients) conducted in 1989 to examine the impact that different systems of care and clinician expertise may have on patient outcomes, with the aim of developing better tools to monitor patients in clinical care. 293 The study worked with a conceptual framework considering the structure of care, the process of care and the subsequent outcome of care. Patient-reported outcomes contributed significantly to the study and ‘short-form’ surveys (MOS short forms) were designed to facilitate data collection.
Short Form questionnaire-36 items
The SF-36 is a widely used generic health survey designed over a 7-year period using data gathered from the MOS. 294 The SF-36 tool was designed to address general health, such as basic daily functioning and emotional status, rather than disease-specific outcomes or any specific disease-related issues. The purpose of the survey was to measure patient-centred outcomes and examine the relative burden of disease and the benefits of treatment within diverse populations. The health concepts examined are common to other health surveys and include physical, social and role function; mental health; general health; and measures of pain and vitality, such as energy or fatigue. 295
Further work included the International Quality of Life Assessment project,296 a project to produce versions of the SF-36 into validated health questionnaires for patient-reported outcomes in multiple countries: Canada, France, Germany, Italy, Japan, the Netherlands, Sweden, Spain, the UK and the USA. Within each country the SF-36 was adapted and then validated using methods of forward and backward translation and focus groups. The aim was to conserve the validity of the questionnaire while providing a culturally relevant and standardised method of measuring HRQoL.
Thirteen studies identified for the review of measures of quality of life in patients with hyperhidrosis reported using the SF-36, including six studies of surgical interventions,251,297–301 three studies examining dermatology interventions,55,57,97 one study of dermatology and surgery combined272 and three quality-of-life-only studies. 225,256,288
Short Form questionnaire-12 items
The SF-12 tool302 was developed as a shorter version of the SF-36 form, and was designed to be used in large-scale UK and US health trials to reduce the number of data generated while still providing a practical and valid summary score for mental and physical health outcomes. The validation utilised regression models to select and score 12 items from the original MOS SF-36 and the authors performed cross-sectional and longitudinal validation tests to compare the results of the two surveys between patient groups. Cross-validation results suggest that downsizing reduced the length of time needed to complete the survey to 2 minutes while still maintaining the accuracy of the measures, which compared well with the SF-36 survey results. 295,302 The authors suggest that the survey may be better suited to large-scale studies particularly focused on physical and mental health outcomes.
Seven studies identified for the review of measures of quality of life in patients with hyperhidrosis reported using the SF-12: two economic assessments,303,304 one surgical intervention,305 two studies examining dermatology interventions80,86 and two quality-of-life-only studies. 258,288 Study designs included one case series,305 two RCTs,80,86 two economic studies303,304 and two of quality of life only. 227,256
Everyday Life Questionnaire or Fragebogen des täglichen Lebens
The Everyday Life Questionnaire (EDLQ) was designed by a German research team, Bullinger et al. 306 The EDLQ (or Fragebogen des täglichen Lebens) was created from responses to open-ended questions given to patients and healthy control subjects as a process to measure behavioural disease-specific quality of life. Questions consider issues such as mental health, physical well-being, social life and medical care. The authors suggest that it is more useful if the tool is used alongside the Munich Life quality Dimensions List. 307 The review found that the EDLQ was used in only one surgical study of palmar hyperhidrosis. 308
State–Trait Anxiety Inventory
The State–Trait Anxiety Inventory was originally developed in the early 1960s to measure anxiety in healthy populations, but then became useful as a tool for measuring anxiety disorders in patients. The review found use of the State–Trait Anxiety Inventory in only one surgical study of generalised hyperhidrosis. 305 The tool is designed to measure anxiety caused by certain situations (state anxiety) and also the tendency to perceive situations as threatening (trait anxiety). 309
International quality of life assessment (EuroQol-5 Dimensions, five-level version) or EuroQol-5 Dimensions
The international quality of life assessment (EuroQol-5 Dimensions, five-level version; EQ-5D-5L), or EuroQol-5 Dimensions (EQ-5D), is a HRQoL measure developed in 1990 by the EuroQoL group310 from the results of a postal survey conducted in England, the Netherlands and Sweden. The EuroQoL was designed to be used alongside other quality-of-life measures as a health status index for reference purposes. The group has since expanded from 23 academic contributors to 75 and has become an international multilingual and multidisciplinary team producing a suite of copyright-standardised generic instruments for describing HRQoL. Use of the EuroQoL tool was identified only in one study, an economic assessment of the burden of hyperhidrosis on the patient, by Kamudoni et al. 228 in 2014.
Leibowitz Social Anxiety Scale
The Leibowitz Social Anxiety Scale206 measures fear, anxiety and avoidance of social situations before and after treatment. 311 The questionnaire is completed by a clinician asking the patient a list of questions: 11 questions relating to social interaction and 13 questions related to public performance. It has also been validated in a modified version as a patient (self-) reported outcome measure. 312 Use of this tool was identified in only one study of hyperhidrosis, a surgical case series examining sweating problems in patients in Finland. 313
University of California, Los Angeles, Loneliness Scale
The University of California, Los Angeles, Loneliness Scale was developed in 1978 by Russell et al. 314 in the USA. The purpose of the scale is to measure loneliness in patients participating in social psychological research where they may suffer from limited social interaction or feel lonely as a result of their disease. The search identified its use in a single dermatology study from Greece,121 which used both the University of California, Los Angeles, Loneliness Scale and the DLQI together to measure quality of life in patients receiving BTX to treat palmar and axillary hyperhidrosis.
Nottingham Health Profile
The Nottingham Health Profile was first designed in 1975 at Nottingham University using survey responses from a cohort of 700 people asked to describe typical ill health. The tool was developed as a generic and standardised HRQoL tool to measure the health status of a population. The tool can also be used as an outcome measure for interventional management alongside a clinical assessment. 315 The Nottingham Health Profile was discussed in a review of quality of life by Rzany et al. 227 in 2012, along with 12 other tools for measuring quality of life in patients with hyperhidrosis, but was not found in any other studies.
The Questionnaire of Quality of Life adapted from the Caregiver Questionnaire
The Questionnaire of Quality of Life is an adaptation of the Caregiver Questionnaire which was developed by Geabler-Spira and adapted by Schneider et al. 316 The Caregiver Questionnaire is a disease-specific measure of HRQoL developed for children with spastic quadriplegic cerebral palsy who were to undergo treatment at the Rehabilitation Institute of Chicago, IL, USA. 316 Developed as a HRQoL and functional outcome measure for children, particularly those with disabilities, this tool was adapted by Coutinho dos Santos et al. 105 to measure quality of life in children and adolescents treated with BTX-A for palmar hyperhidrosis.
Studies identified where the tool used to measure quality of life was not described
Thirty-two additional studies of hyperhidrosis published between 1978 and 2009 mentioned the collection of quality-of-life data but did not describe how these data were captured, describe which tool was used or provide more than only minimal details (quality-of-life tool not reported) (Table 6). 58,73,74,81,95,111,118,137,155,182,317–336
| Number | Study | Year | Intervention | Body site | Country |
|---|---|---|---|---|---|
| 1 | Bachmann et al.317 | 2009 | Surgery | Palm and axilla | Germany |
| 2 | Bogokowsky et al.318 | 1983 | Surgery | Not stated | Israel |
| 3 | Boley et al.319 | 2007 | Surgery | All | USA |
| 4 | Cardoso et al.320 | 2009 | Surgery | Palm | Spain |
| 5 | Chan et al.321 | 2007 | Surgery | Palm, axilla and/or foot | China |
| 6 | Commons and Lim182 | 2009 | Surgery | Axilla | USA |
| 7 | Costa et al.58 | 2015 | Dermatology | Foot | Brazil |
| 8 | Currie et al.322 | 2011 | Surgery | Palm, axilla and face | UK |
| 9 | Divisi et al.323 | 2013 | Surgery | Generalised | Italy |
| 10 | Garcia et al.324 | 2011 | Surgery | Facial blushing | Spain |
| 11 | Hanlon et al.111 | 2006 | Dermatology | Axilla | Ireland |
| 12 | Jeganathan et al.325 | 2008 | Surgery | Palm, axilla and face | UK |
| 13 | Kinkelin et al.118 | 2000 | Dermatology | Forehead | Germany |
| 14 | Marić et al.326 | 2014 | Surgery | Face, palm and axilla | Unknown |
| 15 | Munia et al.327 | 2008 | Surgery | Axilla | Brazil |
| 16 | Munia et al.328 | 2007 | Surgery | Axilla | Brazil |
| 17 | Naver et al.81 | 2000 | Dermatology | Palm, axilla or palm plus axilla | Sweden |
| 18 | Ottomann et al.155 | 2007 | Dermatology and surgery | Axilla | Germany |
| 19 | Rathinam et al.329 | 2008 | Surgery | Not stated | UK |
| 20 | Schnider et al.137 | 2001 | Dermatology | Axilla and palm | Austria |
| 21 | Shalaby et al.330 | 2012 | Surgery | Palm and axilla | Egypt |
| 22 | Shayesteh and Nylander95 | 2011 | Dermatology | Not stated | Sweden |
| 23 | Shi et al.331 | 2015 | Surgery | Palm | China |
| 24 | Steiner et al.332 | 2007 | Surgery | Palm | Israel |
| 25 | Xiao et al.333 | 2015 | Surgery | Palm | China |
| 26 | Baumann et al.73 | 2005 | Dermatology | Axilla | USA |
| 27 | Baumann et al.74 | 2005 | Dermatology | Palm | USA |
| 28 | Baumann et al.73 | 2005 | Dermatology | Axilla | USA |
| 29 | Baumann et al.74 | 2005 | Dermatology | Palm | USA |
| 30 | Cheng et al.334 | 2015 | Surgery | Palm | USA |
| 31 | Flores335 | 2012 | Surgery | Palm and axilla | Brazil |
| 32 | Jaffer et al.336 | 2007 | Surgery | Palm and face | UK |
Summary of review of measures of quality of life
The aim of this review was to identify the tools commonly used to measure quality of life in patients suffering from hyperhidrosis. The studies were reviewed to identify measures of patient-reported HRQoL in studies of the treatment and/or management of hyperhidrosis.
The DLQI, the HDSS and the HQLQ were used more often than any other tool for measuring HRQoL in hyperhidrosis. The DLQI has a patient-centred approach, but it is criticised in the context of quality-of-life measures for hyperhidrosis for being too general and its inability to capture hyperhidrosis-specific problems or concerns. 261 The HDSS appears to have value for researchers of clinical effectiveness of treatments for hyperhidrosis as it is often used to measure response to treatment. It is unclear from the literature what measures were used to design or validate the tool, although attempts have been made to quantify the tool’s value as a measure of HRQoL in patients with hyperhidrosis. However, the HDSS is not highly regarded by all as a comprehensive tool for measuring of HRQoL, suggested by continued work on developing better tools. In the UK and the USA, the DLQI and HDSS are commonly used in combination in both surgical and dermatology studies. The HQLQ is designed specifically for surgical interventions of hyperhidrosis, making it a popular choice for surgical studies, although the majority of users are in Brazil, where the tool was originally developed, with none of the studies we identified being UK based.
Of interest is the newly developed HidroQoL tool, developed by UK researchers as a scoring system with more focus on patient-relevant measures than most quality-of-life tools used for hyperhidrosis research, for both research and clinical practice. 260,261 Use of this tool was not found in any studies identified for the review, although this may be because it is still relatively new.
In summary, the tools identified from studies of hyperhidrosis differ in their method and validation, with a significant degree of overlap. The tools appear to take the patients’ quality of life into consideration in most cases, although with the lack of standardisation in method or validation it is not clear from this review which tools should be used in studies of clinical efficacy and/or patient-reported quality of life in hyperhidrosis. The tools most commonly used, such as the HDSS, appear to lack any form of published validation during development. The combined use of two or more tools is common, but again there is a lack of clear standardisation for which combinations should be used or work best together. The type of intervention (surgery or dermatology) and geographical location may also be a factor in tool selection and it was not uncommon to find colleagues using the same tool. The HidroQoL is the most recent tool to be designed and validated for measuring the quality of life of patients with hyperhidrosis.
Chapter 5 Review of cost-effectiveness evidence
Methods
A review of full economic evaluations was conducted as part of the review of the clinical effectiveness of treatments for hyperhidrosis. The purpose of the review of economic evaluations was to identify economic evaluations that modelled the management of patients with hyperhidrosis in order to inform the model in this study.
Search strategy
The search strategy is outlined in Appendix 1, Hyperhidrosis cost-effectiveness literature searching.
Study selection
Two researchers undertook the screening of titles and abstracts obtained through the search, although the library was split between the researchers, rather than each record being double screened, as described in Chapter 2, Literature searches. All potentially relevant articles were obtained for scrutiny against the full selection criteria, with any disagreements resolved by discussion. The criteria were:
-
study design – full economic evaluations involving a decision model-based analysis
-
population – patients with primary hyperhidrosis (including adults and children)
-
intervention – treatments for hyperhidrosis available for prescription by dermatologists and defined surgical treatments for hyperhidrosis
-
comparator – alternative treatment or no treatment for primary hyperhidrosis
-
outcome – cost-effectiveness, cost estimates, utilisation estimates, quality-of-life estimates.
Data extraction and quality assessment strategy
Data on the following, when available, were extracted from included studies by one reviewer and checked by another:
-
study characteristics, such as study question, form of economic analysis, population, interventions, comparators, perspective, time horizon and form of modelling used
-
clinical effectiveness and cost parameters, such as effectiveness data, health state valuations (utilities), resource use data, unit cost data, price year, discounting and key assumptions
-
results and sensitivity analyses.
Studies were to be quality assessed using tools as part of the data extraction process, namely the Consensus on Health Economic Criteria list337 for economic evaluations and the checklist by Philips et al. 338 for model-based analyses.
Results
A total of eight records were identified as potentially relevant. 227–230,272,304,339,340 No duplicates were identified. Full copies were obtained for scrutiny against the inclusion criteria for the review. No records were found to meet the inclusion criterion of involving a decision model-based analysis. A flow diagram presenting the process of selecting studies can be found in Figure 11.
FIGURE 11.
Flow diagram showing study selection for the economic evaluations review.

Conclusion
No model-based economic evaluations of treatments for patients with primary hyperhidrosis were identified.
Chapter 6 Development of new cost-effectiveness model
Overview
The review did not identify any modelling studies, so a de novo cost-effectiveness model was developed to formally assess the cost-effectiveness of treatments offered by the NHS for the management of primary hyperhidrosis and to estimate the VOI to aid decisions about further research. VOI analysis can quantify the expected gain in net benefit from obtaining further information to inform a decision. The model was written in the R version 3.2 software (The R Foundation for Statistical Computing, Vienna, Austria). The model code is reported in Appendix 4. As outlined earlier (see Chapter 1, Description of underlying health problem), hyperhidrosis is an unpredictable, uncertain condition with unknown aetiology and an uncertain disease natural history. Given these uncertainties, when there was no, or very limited, effectiveness evidence, it was considered to be of little additional value to conduct VOI analysis. Therefore, although effectiveness evidence was sought for all hyperhidrosis body sites, a model was developed only for body sites for which there was sufficient effectiveness evidence identified to warrant a VOI analysis. From the clinical effectiveness review reported in Chapter 3, sufficient evidence was identified to warrant a model-based cost-effectiveness analysis (CEA) of the axilla body site only. A NHS and Personal Social Services (PSS) perspective was adopted for the analysis. The price year was 2015 and the costs used were in Great British pounds.
The structure of the model was developed based on the outcomes reported in the clinical studies identified in the review of the literature (see Chapter 3). The most common clinical outcomes were gravimetry sweat rates and HDSS response. The HDSS clinical outcome enabled a treatment response model using HDSS as a proxy for response in a clinical setting. Response was defined as at least a two-point decrease on the HDSS scale. EQ-5D utility data were available for HDSS levels, so a state-transition cohort model was developed with states defined by treatment and progression to different treatments in the treatment sequence determined by response. As the long-term costs and quality of life depend(s) on the treatments available after treatment failure, treatment sequences were modelled.
Costs and utilities were discounted by an annual discount rate of 3.5% per year, which is the current discount rate recommended by NICE. 341 The outcomes of the analysis were cost per quality-adjusted life-year (QALY) gained and the VOI. The QALYs were calculated using EQ-5D utility values. The benefit of hyperhidrosis is gain in quality of life and the EQ-5D index measures quality of life using health state preference values. The CEA met the NICE reference case. 342 All stages of the work were informed by discussions with clinical and patient advisors, who provided feedback on the model assumptions, including the prescribed treatments, resource use, referral decisions and treatment side effects.
Both deterministic and probabilistic models were developed. The deterministic model was used to perform sensitivity and scenario analyses on particular parameters, and groups of parameters, in order to identify the key drivers of the model. The probabilistic model was used to account for non-linearity in the model and correlations in parameters, and to characterise the decision uncertainty. The probabilistic model simulated a cohort of 5000 people with primary hyperhidrosis referred for secondary care. The probabilistic model also enabled the estimation of the expected value of perfect information (EVPI) in order to inform whether or not there is value in further research.
The following sections outline the key elements of the modelling and any associated assumptions:
-
population, treatment sequences and model structure
-
formulae of the transition probabilities and proportions of patients on different treatments
-
effectiveness data used in the model
-
utility data used in the model
-
cost data used in the model
-
EVPI methods
-
model results.
Population, treatment sequences and model structure
The decision problem investigated in this study is whether or not further research is required on interventions for hyperhidrosis. The need for further research is related to the cost of making suboptimal treatment decisions. This requires an evaluation of the cost-effectiveness of different treatment sequences and the identification of the optimal treatment sequence to be offered to patients with primary axillary hyperhidrosis. The decision question is not simply, ‘Which of the available treatments should be given to patients?’. It is also ‘which treatment should be given to patients in the event that a treatment is not effective or a patient has to withdraw from treatment due to adverse effects?’. As, in practice, patients are offered different treatments, modelling treatment sequences also accounts for the costs and effects of treatment after treatment failure. This section describes the population and the model structure developed to assess cost-effectiveness.
Population and time horizon
The population in the model is assumed to be people with primary axillary hyperhidrosis who have an equal mix of scores 3 and 4 on the HDSS scale. Patients are assumed to enter the model at 18 years of age. Hyperhidrosis is assumed to spontaneously resolve after the age of 65 years, so the model has a time horizon of 48 years. Resolution of the condition at the age of 65 years is a model assumption based on advice provided by the clinical experts on the project; however, it is worth noting that in clinical practice a significant number of patients may experience resolution later on in life.
Individual treatments and treatment sequences
The candidate treatments for each body site are listed in Table 7. Although treatments for each body site are listed, the economic model focuses solely on the axillae due to a lack of data associated with treatments for other body sites.
| Axillae | Hand | Foot | Craniofacial |
|---|---|---|---|
| Iontophoresis sponge | Iontophoresis liquid tray | Iontophoresis liquid tray | Oral medication |
| Oral medication | Oral medication | Oral medication | BTX |
| BTX | BTX | BTX | ETS |
| Curettagea | ETS | ||
| ETS |
Clinical expert advice was that curettage or ETS would not be offered before non-surgical treatments such as iontophoresis, oral medication or BTX. The reason for this was the invasive nature of the surgical interventions. It was also advised that ETS would not be offered before curettage for axillary hyperhidrosis. The rationale given for this was that there is a significant risk of CS from undergoing ETS, whereas the risk is believed to be minimal for curettage.
With these two constraints imposed on the possible treatment sequences, there were 64 possible treatment sequences for axillary hyperhidrosis, including aluminium chloride antiperspirant acting as a ‘no secondary care’ comparator. The full list of sequences is presented in Table 8. The interventions are abbreviated by their first letter: aluminium chloride antiperspirant (A), ETS (E), curettage (C), BTX (B), iontophoresis (I) and medication (M). For this analysis, medication is assumed to be oral medication, which is considered to be a class of treatment in terms of clinical effectiveness (i.e. the effectiveness of no one medication was incorporated in the model). The available data on topical medications were considered insufficient available and, thus, these medications were not included in the analysis.
| Number | Sequence | Number | Sequence | Number | Sequence | Number | Sequence |
|---|---|---|---|---|---|---|---|
| 1 | A | 17 | MB | 33 | IM | 49 | MIB |
| 2 | E | 18 | MBE | 34 | IME | 50 | MIBE |
| 3 | C | 19 | MBC | 35 | IMC | 51 | MIBC |
| 4 | CE | 20 | MBCE | 36 | IMCE | 52 | MIBCE |
| 5 | B | 21 | BM | 37 | MI | 53 | MBI |
| 6 | BE | 22 | BME | 38 | MIE | 54 | MBIE |
| 7 | BC | 23 | BMC | 39 | MIC | 55 | MBIC |
| 8 | BCE | 24 | BMCE | 40 | MICE | 56 | MBICE |
| 9 | M | 25 | IB | 41 | IMB | 57 | BIM |
| 10 | ME | 26 | IBE | 42 | IMBE | 58 | BIME |
| 11 | MC | 27 | IBC | 43 | IMBC | 59 | BIMC |
| 12 | MCE | 28 | IBCE | 44 | IMBCE | 60 | BIMCE |
| 13 | I | 29 | BI | 45 | IBM | 61 | BMI |
| 14 | IE | 30 | BIE | 46 | IBME | 62 | BMIE |
| 15 | IC | 31 | BIC | 47 | IBMC | 63 | BMIC |
| 16 | ICE | 32 | BICE | 48 | IBMCE | 64 | BMICE |
The number of treatment sequences was lower for the other body sites, as curettage is not used for the hand, foot or craniofacial sites, ETS is not used for the foot and iontophoresis is not used for the craniofacial site. However, as modelling was undertaken only for the axillae, only treatments for the axillae are presented here.
Minor surgery for the axilla
Minor surgery for the axilla includes curettage, microwave, fractionated microneedle radiofrequency, ultrasound and laser interventions. However, as there is insufficient evidence relating to microwave, fractionated microneedle radiofrequency, ultrasound and laser, only curettage is included in the model. If sufficient effectiveness evidence had been available, minor surgery would have been compared in a separate model. The treatment sequences evaluated would have included each of these minor surgery procedures (with or without ETS following failure of the minor surgery).
Treatment failure (failure to respond to any treatment)
Treatment response is defined as at least a two-point reduction on the HDSS scale. It is assumed in the model that a patient who achieves only a partial response (a reduction of one point on the HDSS scale) tries the next treatment because the level of response is not deemed satisfactory. However, having exhausted all available treatments (and if the patient is still deemed to be a non-responder), the patient returns to one of the treatments to which they exhibited a partial response. The section Derivation of different Hyperhidrosis Disease Severity Scale level responses describes the estimation of the treatment response and partial response.
If a patient fails to respond to any treatment, the treatment in the sequence chosen as the fall-back treatment depends on a hierarchy of treatments as follows: medication, iontophoresis, BTX, aluminium chloride.
Medication and iontophoresis sponge were chosen as the first fall-back treatment options because they afford clinicians potentially more flexibility in their use. They can be used at different doses/intensity or used only before specific occasions. Only non-surgical treatments are considered as fall-back options. This is to reflect the current clinical practice in which many patients never progress to surgery and to incorporate the assumption that any patient achieving a partial response to curettage would no longer have hyperhidrosis sufficiently severe to warrant having ETS. The effect on the results of changing the order to ‘iontophoresis, BTX, medication and aluminium chloride’ was tested in a scenario analysis. The treatment received as a fall-back option is the treatment in the particular sequence (at any point) that is highest up the hierarchy (medication, iontophoresis, BTX, aluminium chloride antiperspirant). For example, in the sequence:
the treatment received would be medication as it is the treatment in the sequence that is highest in the hierarchy. A patient who experienced no benefit from medication or stopped taking medication because of adverse effects would receive the next treatment in the hierarchy, which, in this case, is iontophoresis. If the patient experiences no benefit from iontophoresis or stops using iontophoresis because of side effects, they would then be offered BTX. The next treatment to try would be aluminium chloride perspirants. A patient who does not tolerate aluminium chloride antiperspirant would not receive any treatment.
This assumption was also maintained for shorter treatment sequences. For instance, in the sequence:
the first fall-back treatment option would be iontophoresis sponge. A patient who fails to respond to iontophoresis or stops because adverse effects would be offered aluminium chloride antiperspirants. A patient who does not tolerate aluminium chloride antiperspirant would not receive any treatment.
State transitions
During the first year of the model, patients try the different treatments in sequence until they find a treatment that works for them, or they enter the treatment failure state and retry an intervention that gave them a partial response. A patient may die at any time according to the general population mortality risk. In order to model the transitions between the treatments, a monthly cycle length is adopted during the first year of the model. The monthly treatment states during the first year associated with each treatment are reported in Table 9.
| Treatment | Monthly cycles associated with each intervention during the first year | ||||||
|---|---|---|---|---|---|---|---|
| Aluminium chloride | Monthly use aluminium chloride | ||||||
| Iontophoresis | 1-month iontophoresis trial | Continue on iontophoresis if responder | |||||
| Oral medication | Month 1 oral medication trial | Month 2 oral medication trial | Month 3 oral medication trial | Continue on medication if responder | |||
| BTX | First BTX injection | Second month after BTX injection | Third month after BTX injection | Fourth month after BTX injection | Fifth month after BTX injection | Sixth month after BTX injection | Have next set of BTX injection if respondera |
| Curettage | Undergo curettage in first month | Successful curettage | |||||
| ETS | Undergo ETS in first month | No CS | Mild CS | Moderate CS | Severe CS | Regret CS | |
Patients are entitled to a free 1-month trial of an iontophoresis device at a NHS clinic, so it is assumed that response can be determined within 1 month of starting iontophoresis treatment. If the patients who respond then move to the iontophoresis responder state and remain there. Clinical expert advice was that there are no long-term adverse effects from iontophoresis use, so it is also assumed that withdrawal because of adverse effects would also occur within the first month.
In the case of oral medication, clinical and patient group advice suggested that response can be determined within 3 months of starting medication. This is consistent with time to response reported by a small survey of dermatologists that was conducted to inform the model parameters, although there was considerable variation in response. The survey methods and results are outlined in Appendix 5. It was assumed that withdrawal because of adverse effects would also occur within 3 months, although the lack of evidence on long-term adverse effects from oral medication is an area of uncertainty.
In the case of BTX, it is assumed, based on expert clinical input, that response can be established within the first month following BTX injections and that withdrawal because of adverse effects would occur within the first month, as clinical expert advice was that there are no long-term adverse effects from BTX injections.
Curettage is a one-off treatment. If a patient is considered a responder, then the response is assumed to be permanent and the patient enters the successful curettage state; there is no long-term evidence to support this assumption. ETS is also a one-off treatment. ETS is assumed to be effective for treatment of hyperhidrosis in all cases but may lead to CS. Following ETS, a patient enters one of the CS states: no CS, mild CS, moderate CS, severe CS or CS so severe that the patient regrets having CS (regret CS).
From the second year onwards, patients are assumed to remain on a treatment for the remaining time in the model. The cycle length changed to 1 year.
The basic set of state transitions is presented in Figure 12. After trialling a treatment for a month in the case of iontophoresis and BTX, and 3 months in the case or oral medication, a patient responds to treatment and remains on the treatment, moves on to the next treatment or dies. If the patient does not respond to treatment and there are no more treatments in the sequence, then the patient enters the treatment failure state and retries a previous treatment that provided partial response. Patients progress to available surgical treatments only if they have received no benefit from any previous non-surgical treatment. If they achieved a partial response from a previous treatment, then they enter the treatment failure state and retry a treatment based on the hierarchy outlined previously. This assumption was made based on expert clinical advice regarding the level of severity required before a patient would advance to surgical treatment.
FIGURE 12.
The basic set of state transitions.
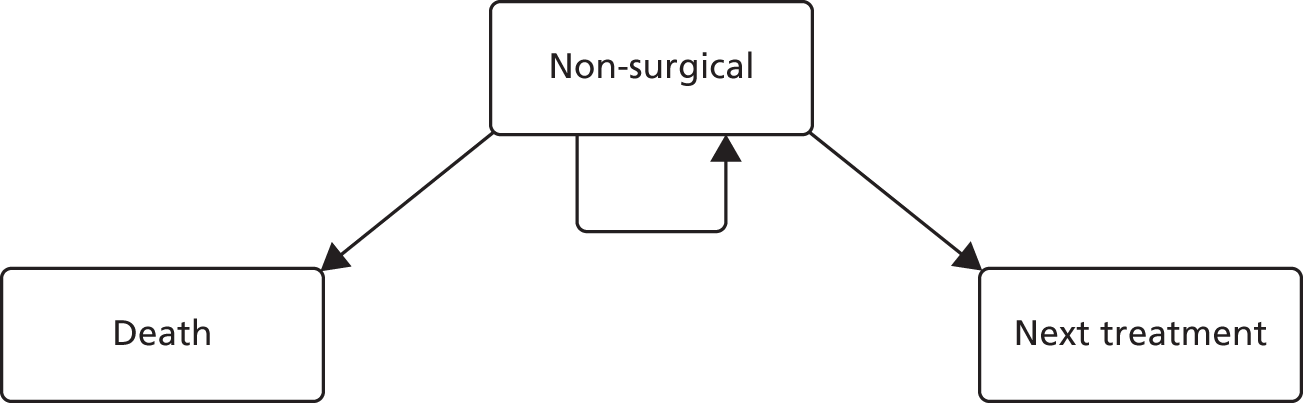
Following ETS, all patients enter one of the CS states as described above. If they have CS then they retry oral medication.
No half-cycle correction is required during the 1-month cycles as the cycle length is short compared to the model time horizon. It was also not applied to the annual cycles because patients receiving BTX continued to cycle through different BTX states despite the transition to annual cycles. The bias associated with this was considered to be minimal as the only transition out of BTX treatment in each cycle was general population mortality.
Summary of model data
This section provides a summary of the data included in the model, which are fully detailed in sections Derivation of transition probabilities and reuse of treatments to Resource use and unit costs. A description of the analyses conducted is then presented in Incremental cost-effectiveness analysis and scenario and sensitivity analyses and Value-of-information analysis, and the results are presented in Chapter 7.
The data included in the model can be categorised as treatment/clinical outcome data, utility data and cost data.
Treatment/clinical outcome data
A NMA was conducted to synthesise the HDSS outcome data from nine studies in order to provide consistent relative effectiveness data for the economic model. These studies were identified as being at risk of bias in the systematic review (see Chapter 3, Quality of studies included in the review). Change in HDSS was the selected outcome as it was one of the most prevalent outcomes, treatment response could be easily defined and utility data were available only for the HDSS outcome.
The key outcome variable required for the economic model was response, defined as a reduction in the HDSS score of at least 2 points. Half of the included studies reported response and half reported mean change from baseline. As there is no unique transformation from mean change to response, the following process was conducted: (1) a simulation was conducted to derive the likely numbers of responders and non-responders in each study that reported mean change, accounting for the uncertainty associated with the process; (2) a NMA was then conducted to estimate the relative odds ratio (OR) of response from the response numbers from each study; and (3) for the purpose of assigning utility values to different levels of response and for estimating the likelihood of retrying a previous treatment after exhausting the possible options (defined as a partial response of one HDSS point change), the likelihood of different levels of response was derived for different probabilities of response obtained from the NMA.
No distributions were specified for the relative effect parameters in the economic model. Instead, the model sampled from simulated results produced from the NMA. Summary statistics of the relative effects are reported in Table 10.
| Effectiveness (HDSS response) results utilised in the model | |||||
|---|---|---|---|---|---|
| Comparison | Median ORa | ||||
| Iontophoresis sponge vs. placebo | 3.03b (95% CI 1 to 9.2) | ||||
| Medication vs. placebo | 7.21 (95% CI 1.56 to 53.83) | ||||
| BTX vs. placebo | 9.207 (95% CI 4.73 to 18.10) | ||||
| Curettage vs. BTX | 6.391 (95% CI 1.20 to 27.61) | ||||
| ETS | 1 | ||||
| Probability of stopping treatment due to adverse effects | |||||
| Intervention | Mean | Distribution | |||
| Antiperspirants | 0.55 (95% CI 0.00 to 1.00) | α = 0.611, β = 0.505 | |||
| Medications | 0.37 (95% CI 0.01 to 0.89) | α = 0.962, β = 1.652 | |||
| Iontophoresis (tap water) | 0.16 (95% CI 0.00 to 0.63) | α = 0.511, β = 2.709 | |||
| Iontophoresis (glycopyrrolate) | 0.25 (95% CI 0.00 to 0.77) | α = 0.737, β = 2.212 | |||
| Long-term effectiveness: ETS CS event rates by severity | |||||
| No CS | Mild CS | Moderate CS | Severe CS | Incapacitating CS | n |
| 340 | 394 | 409 | 367 | 190 | 1700 |
| HDSS level utility estimates | |||||
| HDSS level | EQ-5D mean (SD) of sample distribution | Distribution (EQ-5D rescaled to 0–1 range) | |||
| HDSS 2 (n = 36) | 0.85 (0.13) | α = 416.6, β = 43.3 | |||
| HDSS 3 (n = 69) | 0.8 (0.15) | α = 391, β = 55.8 | |||
| HDSS 4 (n = 58) | 0.69 (0.2) | α = 276.2, β = 67 | |||
No evidence was identified on the long-term effectiveness of treatments for hyperhidrosis of the axillae. In the base case, it was assumed that effectiveness was permanently sustained. Probabilities of continued effectiveness were tested in sensitivity analyses. These are reported in Table 10.
Withdrawal rates due to side effects for different treatments were derived from a survey of UK dermatologists. These are reported in Table 10 along with the probability distributions used in the model. The likelihood of moving on to the next treatment in the sequence depends on both the probability of response and the probability of dropping out because of side effects.
The likelihood of CS following ETS was obtained from a published study. 16 These data and the probability distribution used in the model are reported in Table 10.
Mortality rates were obtained from the Office for National Statistics. 343
Utility data
Means and SDs for the sample distributions for HDSS levels 2–4 were obtained from a published abstract. 228 Sample sizes were obtained from the related thesis. 232 These were bootstrapped to obtain beta distributions on a transformed scale of 0–1. These data and the probability distributions used in the model are reported in Table 10.
Cost data
As stated in Overview, the analysis was undertaken from a NHS and PSS perspective. Costs relevant to the NHS and PSS were identified for the analysis. Information on the precise description of resources required for each individual treatment was partially based on data derived from the review, augmented when necessary by clinical experts in the study group and published economic literature. Unit costs were taken from appropriate routine sources, such as NHS Reference Costs 2014 to 2015,344 Personal Social Services Research Unit345 and the British National Formulary 2016346 for medication. The price year of the analysis was 2014/15. The monthly costs for each model state are reported in Table 11. See Resource use and unit costs for the unit costs and assumptions made to derive these monthly costs.
| Model state | Monthly cost (£) |
|---|---|
| Aluminium chloride (A) | 8.72 |
| Iontophoresis sponge (IS) | 198.64 |
| Iontophoresis sponge responder (ISR) | 0.00 |
| Medication month 1 (M1) | 73.34 |
| Medication month 2 (M2) | 28.13 |
| Medication month 3 (M3) | 28.13 |
| Medication responder (MR) | 39.55 |
| BTX month 1 (B1) | 201.21 |
| BTX 2 (B2) | 0.00 |
| BTX 3 (B3) | 0.00 |
| BTX 4 (B4) | 0.00 |
| BTX 5 (B5) | 0.00 |
| BTX 6 (B6) | 0.00 |
| BTX subsequent injections (BS) | 156.00 |
| Curettage (C) | 1194.62 |
| Curettage responder (CR) | 0.00 |
| ETS (E) | 5750.89 |
| ETS responder (ER) | 0.00 |
| ETS low CS (ELCS) | 39.55 |
| ETS moderate CS (EMCS) | 39.55 |
| ETS high CS (EHCS) | 39.55 |
| ETS regretful CS (ERCS) | 39.55 |
| Treatment failure (TF) | Based on methods described in Summary of model data |
| Dead (D) | 0.00 |
Derivation of transition probabilities and reuse of treatments
Transition probabilities
Each treatment is associated with a probability of response, defined as a reduction in the HDSS score of at least 2 points. Placebo is not a treatment option in the model; however, the baseline probability of response is derived from the placebo probability of response. The relative risk of response for each treatment is derived with placebo as the comparator. The derivation of the relative risk is described in Use of the odds ratio of response in the model. If Ppr is the placebo probability of response, RRmp is the relative risk of response for medication compared with placebo and Pmr is the medication probability of response, then:
The potential transitions from the third month on medication after the follow-up visit to determine response are presented in Figure 13. If there is another non-surgical treatment in the treatment sequence then the option to retry previous treatments does not apply. A patient dies due to causes prevalent in the general population, continues on medication or withdraws from the current treatment due to either lack of effectiveness or sufficiently serious adverse effects to warrant progression to the next treatment.
FIGURE 13.
Medication treatment state transitions.

There is a probability, Pmr, that a patient achieves adequate response and a probability, Pmae, that a patient experiences adverse effects that result in the patient stopping treatment. There is a probability, Pd, that a patient may die at any time from causes prevalent in the general population and the complement probability, Pa, that a patient remains alive. The probability, Pmc, that a patient continues on medication is:
The probability, Pnt, that a patient moves on to the next treatment is:
If medication was the last treatment in the sequence, then there is not another treatment to move on to and the patient retries previous treatments. The probability, Pmrt, that a patient retries a previous treatment is calculated in the same way as Pmnt in Equation 5.
If the next treatment in the sequence is a surgical intervention, it is assumed that patients who are non-responders, but have experienced a partial response and who have not experienced adverse effects sufficiently serious to withdraw from the treatment previously, retry those treatments. Only patients who have experienced no response at all from treatment, and those who have experienced a partial response but also serious adverse effects, move on to have surgery.
It is useful to break down the population into five categories of response, which are presented in Table 12. There are four possible response outcomes on the HDSS scale: from a zero-point reduction to a three-point reduction. The probability of a zero-point reduction for medication is Pm0 and the probability of a one-point reduction is Pm1.
| Category of response | Probability |
|---|---|
| Responders without adverse effects | Pmr(1 – Pmae) |
| Responders with adverse effects | P mr P mae |
| Partial responders without adverse effects | Pm1(1 – Pmae) |
| Partial responders with adverse effects | P m 1 P mae |
| Complete non-responders | P m 0 |
When there is only one treatment in the sequence, the probability, Pmpb, of a partial benefit with no adverse effects is:
And the probability of a patient having no benefit (i.e. either zero response or has adverse effects) is:
As:
Pmnb can also be expressed as:
If medication occurs before surgery in the sequence, accounting for mortality, the four transition probabilities are as presented in Table 13.
| Transition | Probability |
|---|---|
| Dies | P d |
| Continues on medication | Pmc = Pmr(1 – Pmae)Pa |
| Next treatment | Pmnt = (Pm0 + Pm1Pmae + PmrPmae)Pa |
| Retries previous treatments | Pmrt = 1 – Pd – Pmc – Pmnt |
The expression of Pmnb stated in Equation 10 is used when there are multiple treatments in the sequence before surgery. In the base case, it is assumed that treatment effects are independent. In the two treatment cases (e.g. medication followed by BTX), the probability, Pbnb, of no benefit from any of the previous treatments is the joint probability of no benefit from both treatments multiplied by the probability of not continuing on BTX treatment.
The frequency of treatment use after treatment failure
Treatment failure is when a patient has no adequate response to any of the treatments in the sequence. When there is no ETS in the treatment sequence, all patients who (1) have not had an adequate response of a reduction in HDSS score of at least 2 points and (2) have had an adequate response but have withdrawn from treatment because adverse effects enter a treatment failure state. Once in this state, a proportion of patients who achieved partial response on previous treatments retry those previous treatments in accordance with an assumed hierarchy.
Assuming that patients retry treatments in the order of medication, iontophoresis then BTX, then the proportions retrying each treatment is calculated as follows. The proportion, Pm, of patients in the treatment-failure group that is assumed to retry medication is:
The proportion, Pi, of patients assumed to retry iontophoresis is:
The proportion, Pb, of patients assumed to retry BTX is:
The remaining patients, Panti, are assumed to remain in the failed treatment state in which patients use antiperspirants:
When there is ETS in the sequence, then no patients who have ETS enter the treatment failure state. They either enter a successful ETS state or are in a state with different degrees of CS. Patients in the CS states are assumed to retry oral medication.
Effectiveness evidence
The clinical effectiveness review reported in Chapters 2 and 3 searched for studies of clinical effectiveness of treatments for different hyperhidrosis body sites. The two most common clinical outcomes were gravimetry sweat rates and HDSS outcomes. HDSS was chosen as the clinical outcome to inform the treatment outcomes in the model, as it enabled a treatment response model and estimates of EQ-5D utility values for HDSS levels were available in the literature.
Clinical evidence with Hyperhidrosis Disease Severity Scale outcome
From the clinical review, nine of the included studies had a HDSS outcome for the axilla body site. 21,24,27,28,53,69,77,83,184 One study had a HDSS outcome for the hand body site. 88 Intervention, comparator, body site, time point and event data for these studies are reported in Table 14. The Leclere et al. 24 study was a four-armed study of two intensities of laser, one curettage arm and one curettage followed by laser. The low-intensity laser was reported to be ineffectual and, therefore, only the results for the high-intensity laser and curettage are included. Half of these studies, indicated by footnote b in Table 14, reported mean HDSS data. Mean reduction in points on the HDSS scale cannot be easily converted into binary response data. There is no unique response rate associated with a specific mean reduction. The possible range of the number of events in each arm of each study was therefore derived through simulation. This is described in The frequency of treatment use after treatment failure.
| Author | Intervention | Comparator | Body site | Time point | Eventsa (intervention) | n (intervention) | Eventsa (comparator) | n (comparator) |
|---|---|---|---|---|---|---|---|---|
| Mehrotra et al.53 | Glycopyrrolate | Placebo | Axilla | 4 weeks | 10 | 24 | 1 | 14 |
| bMüller et al.69 | Methantheline bromide | Placebo | Axilla and palm | 4 weeks | 4 | 128 | 1 | 139 |
| bLeclere et al.24 | Laser | Curettage | Axilla | 1 month | 10 | 25 | 8 | 25 |
| Rajagopal and Mallya88 | Iontophoresis | BTX | Palm | 4 weeks | 17 | 30 | 8 | 30 |
| bIbrahim et al.21 | Curettage | BTX | Axilla | 3 months | 1 | 20 | 4 | 20 |
| Glaser et al.27 | Microwave | Placebo | Axilla | 3 months | 54 | 81 | 5 | 39 |
| Ohshima et al.83 | BTX | Placebo | Axilla | 3 months | 45 | 78 | 10 | 74 |
| bFatemi Naeini et al.28 | Fractionated microneedle radiofrequency | Placebo | Axilla | 9 weeks | 7 | 25 | 1 | 25 |
| Lowe et al.77 | BTX | Placebo | Axilla | 4 weeks | 161 | 214 | 27 | 108 |
| bVakili and Baker184 | Curettage | BTX | Axilla | 6 weeks | 7 | 23 | 21 | 75 |
These studies form a network of evidence presented in Figure 14; the thickness of the lines represents the relative weight of evidence for each comparison.
FIGURE 14.
The network of effectiveness evidence for the axilla body site. Graph produced using published Stata code. 347 Fraction, fractionated microneedle radiofrequency.
There are few studies, and most of these have small sample sizes. The quality assessment carried out as part of the systematic review indicates a high risk of bias and poor reporting across studies. There is, therefore, considerable uncertainty in the effectiveness evidence used in the NMA. One of the nine studies is a non-RCT. 184 It should be noted that a NMA was not included in the clinical evidence section (see Chapter 3) owing to the high risk of bias and heterogeneity of the trials. However, establishing relative effectiveness of the various treatments is essential for the economic model. The risk of bias should be considered in interpreting the results for both meta-analyses and NMA. See Between-study variance, inconsistency and bias for further discussion.
The advice provided by the project clinical experts was that time to response was short for iontophoresis and oral medication as these are interventions that are used continually and the clinical studies in general had short follow-up time points. Therefore, a time point closest to 4 weeks was selected as the optimal follow-up time point in the studies evaluating the effectiveness of medication and iontophoresis. Clinical studies investigating minor surgery generally had longer follow-up time points (as semi-permanent treatments) and so a time point closest to a 3-month follow-up time point was selected in studies with BTX and minor surgery, including curettage and laser. It is assumed that BTX is immediately effective so the time point at follow-up depends on the comparator.
Derivation of response rates from continuous outcomes
The derivation of response rates from continuous outcomes comprised three steps:
-
Derive the plausible ranges of events for each study arm that are consistent with the continuous outcomes reported in each study.
-
Derive the relative risk and standard error on the log-scale accounting for the uncertainty in the study arm events represented by the ranges of plausible events obtained in step 1.
-
Derive the study arm events that are consistent with the relative risk and standard error obtained in step 2.
These steps are described below in turn.
Step 1
The likely distribution of possible responses (a reduction in HDSS score of 0, 1, 2 or 3 points) depends on the skewness of the distribution across that range. If the population were evenly distributed across the possible responses, then the mean change would be 1.5. The distance of the mean change from 1.5 is an indication of the skewness of the distribution across the possible range of distributions.
There is no reason to think that there are differences for distinct populations in how they are likely to respond to treatment, so a unimodal distribution was assumed. This was imposed as a constraint in the simulation model by assuming that if the mean change is < 1.5 then:
where Ex represents the number of events with X points of reduction on the HDSS scale. If the mean change is > 1.5 then:
If the SD of the mean change was available, this would narrow down the possible range of events. All of the studies reporting mean HDSS data did not report mean change. Mean change was calculated from the mean at baseline and the mean at follow-up. The SD of the mean change could be calculated from the SD of the baseline scores and the SD of the follow-up scores if there was evidence for the correlation between baseline and follow-up scores. No evidence for the correlation was identified.
Therefore, the possible range of events was simulated using two constraints:
-
a unimodal distribution
-
the mean of the distribution should be the mean from the study treatment group to within rounding error in study reporting for one data point (e.g. 1.885 reported as 1.89).
In addition to rounding error in study reporting, a feasible mean may be different from the reported mean (1) because of cumulative rounding error from both baseline and follow-up scores; (2) because of misreporting by the study author; (3) because the reviewer misreported the study data; or (4) because the scale of a study graph was not adequate for a reviewer to correctly identify. The range of the possible means may therefore have had to be widened if no feasible mean was identified within the original range. This was the case for one study: for a calculated mean change of 0.17, the nearest feasible mean was 0.16. 28
The simulation required us to obtain 200,000 samples of combinations of numbers with responses of a reduction in HDSS score of 0, 1, 2 and 3 points. Results that did not meet the defined constraints were deleted. The R code is reported in Appendix 6.
Step 2
The relative risk was then derived for the studies reporting mean change in HDSS scores. It was assumed that each feasible number of events in each study arm had an equal probability of being the true number of events. The number of events was randomly sampled from a uniform distribution across these ranges of feasible numbers of events and the relative risk was calculated from the sample values. This was a reasonable assumption for many of the study arms. A total of 50,000 samples were drawn from the feasible range of event numbers for both the intervention and the comparator. The mean and standard error on the log-scale were also derived. Values of the log-RR were then sampled from a normal distribution and converted back to the relative risk scale. The relative risk and the standard error on the log-scale were derived to enable the event rates associated with these relative risk and standard errors to be derived through solving simultaneous equations in the next step. The R code is reported in Appendix 7.
Step 3
Finally, event rates were derived for each study arm; the uncertainty in the event rates was reflected by solving a pair of simultaneous equations. The standard error of the relative risk on the log-scale derived in Step 2 incorporates uncertainty in the event numbers for each study arm. The total number of patients in each arm is known, so event rates can be derived from each arm corresponding to the relative risk and the standard error of the relative risk on the log-scale, by solving the pair of simultaneous equations given by the formulae for the relative risk and the standard error of the relative risk on the log-scale.
The formulae for the mean relative risk and the variance on the log-scale provide two simultaneous equations to solve for a and b.
Solving these equations gives:
If b is < 1, b is set to 1 and a is recalculated. Results are rounded to the nearest integer.
The results incorporate the uncertainty in the unknown study arm events. As a lower number of events is associated with greater uncertainty in the effect estimate, the derived event numbers are quite low. The final events are included in Table 12.
Network meta-analysis methods
A NMA was performed in order to derive the relative effectiveness estimates for the economic model.
Analyses
Network meta-analyses were conducted for the network presented in Figure 14 using the event data for the axilla body site included in Table 14. The model code is reported in Appendix 8. The model adopted a binomial likelihood and logit model. The model was based on the code reported in NICE Technical Support Document 2. 348 Random-effects models were specified assuming a normal distribution on the log-OR scale for the between-study variance. Uninformative priors were given for the mean log-OR effects. Informative priors were given for the between-study variances (see Between-study variance, inconsistency and bias).
Convergence was assessed using the Brooks–Gelman–Rubin diagnostic along with visual inspection of the trace and density plots. 349 The initial 100,000 simulations were discarded and the results were based on a further sample of 50,000 simulations.
It is assumed that there is clinical and statistical heterogeneity in treatment effectiveness across the NHS for reasons such as varying medication use, surgical experience and hyperhidrosis severity. Therefore, it is the average treatment effect that is applicable to the decision problem rather than the treatment effect of specific treatments in specific contexts.
Between-study variance, inconsistency and bias
True variation in relative treatment effects across studies is known as between-study variance. There was insufficient evidence to estimate a between-study variance from the variation in relative risks among studies. However, a degree of between-study variance is highly likely. There is certainly heterogeneity across studies: the estimated mean response rate for curettage was 0.15 in Ibrahim et al. 21 compared with 0.64 in Leclere et al. 24
Accounting for between-study variance is especially important in an evidence network in which there are no loops (i.e. there is evidence for A vs. B and B vs. C but not for A vs. C, hence the effectiveness estimate for A vs. C is an indirect estimate from the evidence of A vs. B and B vs. C). For example, Figure 14 shows that the relative effectiveness of curettage compared with placebo is informed by an indirect chain of evidence. If there were a characteristic of the Leclere et al. 24 study that differs from the Ibrahim et al. 21 study that affects curettage response more than the comparator, then the relative effectiveness of curettage compared with placebo would be biased. Inconsistency cannot be quantified when direct and indirect evidence are not both available for the same comparison, but the relative significance of inconsistency is reduced if between-study variance is accounted for.
A priori, it is reasonable to expect that the between-study variance would differ between treatment effects that compare non-pharmacological interventions with pharmacological interventions and treatment effects that compare pharmacological interventions with placebo. For example, the experience and skill of a surgeon is an additional factor associated with curettage that may contribute to between-study variance. It is even reasonable to expect greater variation in treatment effects between placebo-controlled medication studies than in placebo-controlled BTX studies, as it would appear that there is greater variation in the types of medication than in BTX procedures. For example, the two medication studies included in the network investigate methantheline bromide and topical glycopyrrolate wipes.
Table 15 presents the log-normal prior distributions for the between-study variances utilised within the NMA. All treatment effects are assumed to be different. When there are no data to inform the between-study variance in the data set, then the posterior distribution will simply be the same as the prior distribution. The prior distributions for the quality-of-life outcomes were obtained from Turner et al. 350
| Generic comparison | Treatment effects | Log-normal distribution (mean, SD2) | Mean BSV on log-odds scale |
|---|---|---|---|
| Pharmacological vs. placebo | Medication vs. placebo | LN(–2.54, 1.542) | 0.245 |
| BTX vs. placebo | |||
| Non-pharmacological vs. placebo | Microwave vs. placebo | LN(–2.77, 1.732) | 0.324 |
| Fractionated microneedle radiofrequency vs. placebo | |||
| Pharmacological vs. non-pharmacological | Curettage vs. BTX | LN(–1.51, 1.272) | 0.487 |
| Non-pharmacological vs. non-pharmacological | Laser vs. curettage | LN(–2.1, 1.472) | 0.371 |
No estimate of inconsistency can be derived given the available evidence. This is because there are no loops of evidence in the network presented in Figure 14; that is, there are no treatment comparisons that are informed by both direct and indirect evidence. For example, if there were trials comparing A with B, B with C and A with C, then the trials A comparing with B and B with C provide indirect evidence of A compared with C, whereas the trial of A compared with C provides direct evidence for that comparison. Inconsistency refers to differences between direct and indirect evidence. It is not evident, based on the available evidence, that there is a significant difference, on average, in population severity in terms of HDSS score between pharmacological and non-pharmacological trials. The BTX response rates are similar in BTX and placebo trials and BTX and curettage trials. The placebo response rates are slightly higher, on average, in BTX and placebo trials than in minor surgery and placebo trials; however, this may be due to either different populations or different placebo effects. The important consideration in NMA is the difference in treatment effects. Given the considerable uncertainty within trials and in the between-study variances, and the indirect nature of the evidence, it is likely that the effect of uncertainty in study characteristics of different parts of the evidence chain on the relative effect estimates, and, therefore, in the economic model, is relatively insignificant.
Treatment effect estimates and inconsistency may be affected by bias. The quality assessment of studies included in the systematic review (see Chapter 3, Quality of studies included in the review) indicates that the risk of bias of all of the studies included in the NMA is rated as high. The presence of bias in favour of the intervention would mean that the effectiveness of the intervention compared with placebo may appear greater than it should be. In turn, the direction of effect on relative effectiveness derived indirectly is unclear. The effectiveness results have not been adjusted for bias in this NMA as further research would be required to estimate the degree of bias.
Network meta-analysis results
The OR results of each intervention compared with placebo are presented in Table 16, including the effects of different surgical procedures. The full set of results with every pairwise comparison is reported in Appendix 4. The estimated between-study variances are reported in Appendix 9. The results indicate that every treatment is effective compared with placebo, but there is considerable uncertainty as to how large the effect is. The intervention with greatest precision in its effectiveness compared with placebo is BTX. There was considerable uncertainty in the ORs comparing the interventions with each other. This was particularly true for the comparisons between different minor surgery procedures which were informed by longer indirect chains of evidence. For example, the OR of microwave compared with laser was 1.65 (95% CI 0.11 to 25.48).
| Intervention vs. placebo | Median | 95% CI |
|---|---|---|
| Medication | 7.211 | 1.56 to 53.83 |
| BTX | 9.207 | 4.73 to 18.10 |
| Curettage | 6.391 | 1.20 to 27.61 |
| Laser | 9.126 | 0.90 to 84.68 |
| Microwave | 14.68 | 3.74 to 68.99 |
| Fractionated microneedle radiofrequency | 12.64 | 1.32 to 401.80 |
Placebo response
The baseline probability of response in the model is the probability of response on placebo. The estimate was derived by conducting a random-effects meta-analysis of the placebo probabilities of response for the included studies. The meta-analysis was conducted in WinBUGS (1.4; MRC Biostatistics Unit, Cambridge, UK) using a logit model and transforming the log-odds back to a probability within the model. The mean was 0.13 and the SD was 0.05. The derived beta distribution was Beta (5.98, 38.4).
Use of the odds ratio of response in the model
The model utilises log-ORs of each intervention compared with placebo, specifically the Convergence Diagnostic and Output Analysis software output of the 50,000 simulation iterations used to estimate the results in WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). A total of 10,000 samples are then randomly drawn from these iterations across the treatment effects. The exponent is taken of these samples and converted to a relative risk as follows:
where assumed control risk (ACR) is the baseline risk.
The use of ORs in this way ensures that the probability of response for the intervention remains < 1.
The draw of random samples from the iterations across the treatment effects is important for two reasons. First, any correlations induced by network loops or shared between study variance data are accounted for. Second, a full Bayesian random-effects meta-analysis in WinBUGS results in a log-OR distribution that has excess kurtosis. As the ratio of the between-study variance uncertainty to the within-study effect estimate uncertainty increases from zero, the excess kurtosis increases from zero. The kurtosis of a standard normal distribution is 3 with excess kurtosis of 0 (as it is defined in relation to the standard normal distribution). For example, from the NMA the BTX effect estimate is the most precise, resulting in a high ratio of uncertainty with an excess kurtosis of 2.92. The medication effect estimate is the least precise, resulting in a low ratio of uncertainty with an excess kurtosis of 0.45.
Although log-ORs are utilised in the model, the OR estimates are summarised in Table 17 for axillae. There are no studies providing appropriate evidence for iontophoresis sponge, so in the model the OR is assumed to be 3.03, equivalent to half the log-OR of BTX compared with placebo. This is because clinical experts advised that iontophoresis is not as effective as BTX for the axillae.
| Comparison | Median OR | 95% CI |
|---|---|---|
| Iontophoresis sponge vs. placebo | 3.03a | Range 1 to 9.2 |
| Medication vs. placebo | 7.21 | 1.56 to 53.83 |
| BTX vs. placebo | 9.207 | 4.73 to 18.10 |
| Curettage vs. BTX | 6.391 | 1.20 to 27.61 |
| ETSb | 1 |
Treatment response
If the probability of response for placebo is Prp, then the probability of response for intervention i, Pri, with a relative risk with a placebo comparator, RRrip, is:
For curettage, which links to placebo via BTX in the network, the probability, Prc, of response is:
In the model, it is assumed that the placebo response is a sustained response, which is part of the treatment effect. The difference between a treatment sequence and no treatment includes the placebo effect.
The mean estimates of the probability of response are reported in Table 18. There is uncertainty in these estimates, which is accounted for in the analysis, but only the means are presented here to indicate the levels of response. For aluminium chloride, no response estimate was available for this population so it was assumed that patients using aluminium chloride would benefit from a 0.5-point reduction on the HDSS sale (see Effectiveness evidence).
| Intervention | Response probability |
|---|---|
| Placebo | 0.13 |
| Medication | 0.52 |
| BTX | 0.58 |
| Curettage | 0.49 |
| Iontophoresis sponge | 0.31a |
| ETS | 1 |
Derivation of different Hyperhidrosis Disease Severity Scale level responses
In Effectiveness evidence, it is explained that utility estimates are available for the different HDSS scores. The model also assumes that previous treatments would be retried only in patients with a partial response (a 1-point reduction on the HDSS). The outcome from the clinical studies is at least a 2-point reduction. In order to incorporate model features related to different levels of HDSS response, the likelihood of a reduction in the HDSS score of 2 or 3 points given a response of a reduction at least 2 points and the likelihood of a reduction in HDSS score of 0 or 1 points given no response need to be calculated. The NMA estimates the average response rate as a binary variable rather than a four-category variable; the binary response outcome is the most important outcome for the economic model. Furthermore, it is a simpler modelling process to convert all study outcomes into the same outcome first, incorporating the uncertainty associated with the conversion, then to conduct the NMA on binary response, before finally deriving the likelihood of different HDSS levels given response. Little information is lost because there were no data on individual response levels.
The likelihood of these different degrees of response, given the binary classifications of response or non-response, will depend on the skewness of the response distribution. There is no information on skewness for the studies that report binary response data. There is information on skewness for the studies that report mean HDSS data. For example, the BTX arm in Ibrahim et al. 21 has a mean change of 1.55, which is close to 1.5, and the ratio of the median number of patients with a reduction in HDSS score of 3 points to the median number of patients with a reduction in HDSS score of 2 points is 10 : 2. This is a much higher ratio than the 11 : 6 for the laser arm in Leclere et al. ,24 which has a mean change of 1.88, which is further from 1.5.
As there is no evidence on skewness for half of the studies, estimates were obtained using simulation methods. Both the ratio of the number of patients with a reduction in HDSS score of 3 points to the number of patients with a reduction in HDSS score of 2 points (HDSS 3/2 ratio) and the ratio of the number of patients with a reduction in HDSS score of 0 points to the number of patients with a reduction in HDSS score of 1 point (HDSS 0/1 ratio) were estimated by simulating the feasible combination of numbers across all possible levels of response.
The specific methods and graphic plots of the relationships between the HDSS response ratios and the probability of response are presented in Appendix 10.
The coefficients on the log-scale are reported in Table 19. Predicted values are compared with the simulated data in Table 20.
| Explanatory variable | Response > 0.5 for HDSS 3/2 ratio | Response < 0.5 for HDSS 0/1 ratio | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Intercept | –4.6407 | 0.3113 | 1.2076 | 0.1145 |
| Response probability | 5.8278 | 0.4091 | –5.8374 | 0.4069 |
| Model | HDSS ratio | Response rate | |||||
|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.4 | 0.6 | 0.8 | 0.9 | ||
| Original simulated data | 3/2 | 0.38 | 0.38 | 0.38 | 0.34 | 0.89 | 1.78 |
| 0/1 | 1.67 | 0.90 | 0.34 | 0.37 | 0.38 | 0.38 | |
| Predicted values | 3/2 | 0.38 | 0.38 | 0.38 | 0.32 | 1.02 | 1.83 |
| 0/1 | 1.87 | 1.04 | 0.32 | 0.38 | 0.38 | 0.38 | |
In the economic model, the sampled probability of response is an argument to one of the four linear regressions. If the HDSS 3/2 ratio is r and the probability of response is Pr, then the probability of a HDSS 2 response is:
The probability of a HDSS 3 response is:
The probability of a HDSS 1 response is:
The probability of a HDSS 0 response is:
The predicted distribution of HDSS response levels, given the mean probabilities of response presented in Table 18, is presented in Table 21.
| Intervention | Mean response | Reduction in HDSS | |||
|---|---|---|---|---|---|
| HDSS 0 | HDSS 1 | HDSS 2 | HDSS 3 | ||
| Medication | 0.52 | 0.13 | 0.35 | 0.43 | 0.09 |
| BTX | 0.58 | 0.12 | 0.3 | 0.45 | 0.13 |
| Curettage | 0.49 | 0.08 | 0.43 | 0.36 | 0.13 |
| Iontophoresis | 0.31 | 0.24 | 0.45 | 0.22 | 0.09 |
Withdrawal due to adverse effects
The probabilities of stopping treatment because of adverse effects were estimated for aluminium chloride antiperspirants, medications and iontophoresis based on the responses collected from a survey of UK dermatologists (see Appendix 5). The mean, µ, and SD, σ, of the survey responses were calculated. A beta distribution can appropriately represent the uncertainty around a probability as a beta distribution is bounded by 0 and 1. The parameters, α and β, of a beta distribution are calculated from the mean, µ, and SD, σ, according to the following equations:
The mean and SD of the responses are reported in Table 22. The associated parameters of a beta distribution are also presented. The 95% CI is derived through randomly sampling from the derived beta distributions and taking 2.5th and 97.5th percentiles. Unfortunately, the dropout rate due to adverse effects was not asked in the survey (see Appendix 5). However, the project clinical experts advised that there is negligible dropout due to pain, and that this is true with, or without, the aid of a commonly prescribed anaesthetic gel. As a result, the model assumes 0% dropout due to adverse effects. Some patients may drop out due to lack of effectiveness after 1–2 months, possibly because of blocking antibodies. A CEA of BTX compared with ETS based on an observational clinical study recorded 5 out of 77 patients dropped out because of adverse effects over 5 years. 230 This represents a withdrawal probability of 0.065. In order to test the impact on the results of withdrawal from BTX treatment because of side effects, probabilities of withdrawal of 0.1 probability and 0.2 were included in sensitivity analyses. A 0.2 probability is considered an extremely high estimate.
| Intervention | Mean | SD | 95% CI | Alpha | Beta |
|---|---|---|---|---|---|
| Antiperspirants | 0.55 | 0.3422 | 0.00 to 1.00 | 0.611 | 0.505 |
| Medications | 0.37 | 0.2537 | 0.01 to 0.89 | 0.962 | 1.652 |
| Iontophoresis (tap water) | 0.16 | 0.1779 | 0.00 to 0.63 | 0.511 | 2.709 |
| Iontophoresis (glycopyrrolate) | 0.25 | 0.2179 | 0.00 to 0.77 | 0.737 | 2.212 |
Long-term effectiveness
It is assumed that if iontophoresis and BTX are effective, then they remain effective in the long term. There is uncertainty over the long-term effectiveness for medication. Clinical opinion has suggested that there may be declining effectiveness while following the manufacturer’s dose regime, but there was no evidence to inform this.
In the base case, it is assumed that there is no decline in effectiveness. In sensitivity analysis, the impact of different rates of decline in effectiveness on cost-effectiveness were evaluated through applying a monthly probability of continued success. Figure 15 shows the decline in the proportion of originally successfully treated patients still on treatment over time, assuming monthly continued success probabilities of 0.9975, 0.995 and 0.99. Probabilities of 0.9975 and 0.99 are used in sensitivity analysis.
FIGURE 15.
Decline in oral medication effectiveness over time.

Different levels of compensatory sweating following endoscopic thoracic sympathectomy
Compensatory sweating outcomes following ETS were identified from studies in a rapid review of the literature for clinical studies of ETS. 351 Two studies had particularly useful classifications of CS. The first, reported in Table 23, was used in the base-case model. 16 This includes CS of such a degree that the patient regrets having had the ETS. The second, reported in Table 24, was used in sensitivity analysis. 252 Different utility assumptions associated with CS were made (see Network meta-analysis methods). The treatment following CS followed the same guideline as that for inadequate treatment response across all treatments.
| No CS | Mild CS | Moderate CS | Severe CS | Incapacitating/regret CS | n |
|---|---|---|---|---|---|
| 340 | 394 | 409 | 367 | 190 | 1700 |
| No CS | Mild CS | Moderate CS | Severe CS | n |
|---|---|---|---|---|
| 37 | 92 | 174 | 150 | 453 |
Utility
Utility for different Hyperhidrosis Disease Severity Scale levels
The literature was searched specifically for utility estimates for hyperhidrosis. One study with EQ-5D utility estimates for levels of HDSS response was identified from an abstract. 204,228 No applicable utility values were identified for other common hyperhidrosis outcomes. Means and SDs were reported for HDSS responses 2 to 4. No sample sizes were reported. Although these descriptive statistics were not reported in the PhD thesis on the development of the HidroQoL of Kamudoni et al. ,232,261 it is highly likely that the data originated from the study presented in chapter 8 of the thesis and these descriptive statistics were a byproduct of that study. The sample sizes from that chapter were utilised.
Beta distributions of rescaled utilities were derived from the means and SDs of the HDSS levels. First, the mean and SD were rescaled to the 0 to 1 scale from the –0.594 to 1 scale of the EQ-5D utility. Second, beta distributions were derived from the rescaled mean and SDs. Third, sample means were bootstrapped from the derived beta distributions, rescaling to the scale –0.594 to 1 for each sample. The bootstrap technique uses the sample size to calculate the mean at each iteration. The simulated distribution is the sampling distribution and its SD is the standard error of the mean. Fourth, beta distributions were specified for these sampling distributions rescaled. The utility estimates and beta distribution parameters of the rescaled utilities are reported in Table 25.
| EQ-5D distribution parameter | HDSS 2 (n = 36) | HDSS 3 (n = 69) | HDSS 4 (n = 58) |
|---|---|---|---|
| EQ-5D mean (SD) of sample distribution | 0.85 (0.13) | 0.8 (0.15) | 0.69 (0.2) |
| Beta parameters of sampling distribution | α = 416.6, β = 43.3 | α = 391, β = 55.8 | α = 276.2, β = 67 |
The sample and sampling distributions are presented in the Appendix 11.
Linking utility to treatment response
On the HDSS, 4 is the worst state and 1 is normal population health. The hypothetical model population is assumed to be split equally across HDSS levels 4 and 3. The utility data available are for HDSS levels 2–4. A patient who achieves a reduction in HDSS score of a specific number of points is assumed to have the utility of the HDSS score that he or she achieves after the improvement. As the hypothetical population is a mix of patients of HDSS levels 3 and 4, the final HDSS level is an average of two possible HDSS levels unless a reduction of 3 points on the HDSS is achieved. The utility assumptions for different reductions in HDSS scores are reported in Table 26.
| HDSS score reduction (points) | Utility |
|---|---|
| 3 | HDSS level 1 (normal population health) |
| 2 | (HDSS 1 + HDSS 2)/2 |
| 1 | (HDSS 2 + HDSS 3)/2 |
| 0 | (HDSS 3 + HDSS 4)/2 |
Utility for compensatory sweating, treatment failure and aluminium chloride
The different degrees of CS were reported in Different levels of compensatory sweating following endoscopic thoracic sympathectomy. The HDSS response rates assumed for the different degrees of CS are reported in Table 27. For mild CS, response was assumed to be a reduction in HDSS score of 1.5 points. For moderate CS, response was assumed to be a reduction in HDSS score of 1 point. For severe CS, response was assumed to be a reduction in HDSS score of 0 points, that is, no change. For the regret CS outcome, the 16th percentile was identified from the sample distribution of the HDSS 4 level. This 16th percentile corresponds to 1 SD from the mean for a normal distribution. However, a beta distribution is used here. The 16th percentile corresponds to a utility of 0.49. Sensitivity analyses were conducted using the 25th percentile (0.57 utility) and the 2.5th percentile (0.21 utility).
| Severity of CS | Assumed HDSS response |
|---|---|
| Mild | 1.5-point reduction |
| Moderate | 1-point reduction |
| Severe | 0-point reduction |
| Regret | 16th percentile from the sample distribution of the HDSS 4 level |
Having failed to achieve adequate response to all available treatments, treatments are retried if they have resulted in a partial response and no adverse events sufficiently serious to cause the patient to withdraw from treatment. A proportion of the patients in this treatment failure state who revert to previously tried treatments are allocated utility gain associated with a reduction in the HDSS score of 1 point.
As no treatment response estimate was available for aluminium chloride for this population, it was assumed that patients using aluminium chloride antiperspirants benefited from a 0.5-point reduction in the HDSS score.
Resource use and unit costs
Health service costs
As stated in Overview, the analysis was undertaken from a NHS and PSS perspective. Costs relevant to the NHS and PSS were identified for the analysis. Information on the precise description of resources required for each individual treatment was partially based on data derived from the review, augmented when necessary by clinical experts in the study group and published economic literature. Unit costs were taken from appropriate routine sources, such as NHS Reference Costs 2014 to 2015,344 Unit Costs of Health and Social Care 2015345 and the most recent British National Formulary346 for medication. The price year of the analysis was 2014/15. British National Formulary prices for 2015 were not available, so prices obtained from the British National Formulary 2016 were used. In addition, medical inflation indices beyond 2014/15 in the UK were unavailable.
Drug acquisition costs
The unit costs of aluminium chloride and medications were sourced from the British National Formulary. 346 Doses were calculated in accordance with their licences, unless otherwise stated. Medication costs included in the model were for propantheline bromide, based on expert clinical input. However, because multiple medications are available, alternative medication costs were explored in sensitivity analyses. Table 28 summarises the drug acquisition costs and the licensed dosage for hyperhidrosis patients.
| Medication | Dose | Cost (£) | Source |
|---|---|---|---|
| Aluminium chloride (Driclor 20% solution 75 ml, GlaxoSmithKline Consumer Healthcare) | One bottle per montha | 3.01 | BNF, November 2016346 |
| Propantheline bromide (Pro-Banthine 15 mg × 84 tablets, Kyowa Kirin Ltd) | 75 mg daily | 20.74 | BNF, November 2016346 |
| Oxybutynin (Ditropan 2.5 mg × 84 tablets, Sanofi-aventis) | 12.5 mg daily | 1.60 | BNF, November 2016346 |
| Glycopyrronium bromide 2 mg × 30 tablets | 2 mg dailyb | 186.50 | Significant Cost Changes – April 2016. NHS, Midlands and Lancashire Commissioning Support Unit352 |
Botulinum toxin procedure costs
The unit cost of a BTX procedure was based on the equivalent NHS reference cost (Healthcare Resource Group code JC42A, Intermediate Skin Procedures, 13 years and over, General Surgery category). 344 To support inclusion of this NHS reference cost, an additional cost of £170.45 for a BTX procedure was estimated based on the British National Formulary cost of 100 units of BTX and the cost of a nurse grade 5 delivering the procedure (as advised by clinical experts). Both the NHS reference cost and the estimated cost for BTX are presented in Tables 29 and 30. It is assumed that BTX injections are given every 6 months. This is because the clinical evidence suggests that the effectiveness of BTX may be sustained over a 6-month period.
| Procedure | Cost (£) | Source |
|---|---|---|
| BTX | 156 | HRG code JC42A, Intermediate Skin Procedures, 13 years and over, General Surgery category (NHS Reference Costs 2014 to 2015344) |
Iontophoresis procedure cost
The total cost of iontophoresis for the axillae included the cost of the iontophoresis device and the costs of associated electrodes and sponge pockets. Based on clinical advice, different types of iontophoresis device are used depending on whether the procedure is carried out in a clinical or home setting. The unit costs of iontophoresis devices for hospital and home use, as well as the costs of electrodes and sponge pockets, are summarised in Table 31.
| Setting | Type of device | Device cost (£) | Cost of electrodes (for sponge) (£) | Cost of one pair of sponge pockets (£) | Source |
|---|---|---|---|---|---|
| Hospital | Idrostar Pro pulse (Laboratories I2M, Caen, France) | 1290 | 47.23 | 1.98 | Idrostar354 |
| Home | Idrostar+ (Laboratories I2M, Caen, France) | 504 | 47.23 | 1.98 | Idrostar354 |
The monthly costs of iontophoresis at home and at hospital were estimated and are reported in Table 32. The cost of hospital iontophoresis was derived by summing the discounted monthly machine running cost, the cost of associated consumables and the cost of delivery and device maintenance. The main assumptions underlying this cost estimate are that the device is used by 10 patients per month, that the device lasts for 15 years and that in the NHS the treatment is delivered in seven sessions over a 1-month period. It was also assumed that sponge pockets are changed annually and that electrodes last for 15 years. It was assumed that the procedure takes 20 minutes (plus 10 minutes cleaning time) and is delivered by a nurse (band 5) (based on expert clinical advice).
| Setting | Lifetime of the device and electrodes (years) | Number of patients using the device every month | Discounteda device (including electrodes) running cost per patient per month (£) | Monthly cost of sponge (£) | Hourly cost of staff (job title of health professional) | Length of the procedure (minutes) | Number of procedures per month | Total cost per month (£) |
|---|---|---|---|---|---|---|---|---|
| Hospital | 15 | 10 | 0.95 | 1.98 | £43 (nurse, band 5 with qualifications)b | 20 + 10 minutes device cleaning time | 7 | 153.43 |
| Home | 20 | 1 | 3.20 | 0.17 | Procedure is self-administered | N/A | N/A | 3.37 |
The monthly cost of home iontophoresis includes the machine running costs and the cost of associated consumables, as the procedure is self-administered. All assumptions for deriving costs were based on clinical advice and are summarised in Table 32. In the base case, it is assumed that the patient has to pay for a home iontophoresis device. In a scenario analysis, it is assumed that the NHS pays for home iontophoresis. The effect of the cost to the patient on the uptake of iontophoresis is unclear. In both the base-case analysis and the scenario analysis, it is assumed that a patient who responds to iontophoresis continues to use it.
Surgery costs
Surgery for hyperhidrosis varies from minimally to fully invasive. The unit costs of minimally and fully invasive operations are reported in Tables 33 and 34, respectively. Curettage and fractionated microneedle radiofrequency costs were obtained from NHS Reference Costs 2014 to 2015. 344 For curettage, an estimate based on clinical advice was also calculated in order to support the relatively generic NHS reference cost. It was derived by summing the cost of utilising a surgical theatre at a dermatology unit for the duration of the procedure, the cost of nurse and anaesthesiologist time to deliver the procedure and also the cost of one overnight stay in hospital. The total estimated cost of curettage of £1460.25 was similar to the NHS reference cost of £1126.10.
| Minimally invasive surgery | Cost (£) | Source |
|---|---|---|
| Curettage | 1126.10 | HRG code JC42A, Intermediate Skin Procedures, 13 years and over, General Surgery category (NHS Reference Costs 2014 to 2015344) |
| Fractionated microneedle radiofrequency (outpatient) | 591 | NHS reference costs (AB157, Fractionated microneedle radiofrequency Ablation or Cryoablation for Pain Management) Outpatient, Service description: General Surgery (NHS Reference Costs 2014 to 2015344) |
| Fractionated microneedle radiofrequency (inpatient) | 1169.70 | NHS reference costs (AB157, Fractionated microneedle radiofrequency Ablation or Cryoablation for Pain Management) Inpatient (NHS Reference Costs 2014 to 2015344) |
| Laser | 3768 | The Whiteley Clinic355 |
| Microwave | 1495 | PHI Clinic356 |
| Ultrasound | 2749.41a | Commons and Lim182 |
| Fully invasive surgery | Cost (£) | Source |
|---|---|---|
| ETS | 2823.66 | NHS Reference Costs 2014 to 2015 (YQ407, ETS)344 |
For laser, microwave and ultrasound, no appropriate NHS reference costs were identified; hence, the prices for these procedures were obtained from UK private health-care providers.
The cost of ETS is reported in Table 34. According to clinical advice, owing to the risk of pneumothorax (collapse of lung), ETS is a unilateral procedure; hence patients will need two procedures.
Consultancy visits
Medication, BTX and iontophoresis are all associated with an initial consultation with a dermatologist, which is assumed to last 20 minutes. The associated resource use cost is £45 per visit. In the case of BTX and iontophoresis, it was assumed that no follow-up visits are required. It was assumed that, the treatment is unsuccessful, then the patient has another consultation with a dermatologist, which is considered to be the initial consultation for the next treatment. For aluminium chloride, follow-up visits were assumed to take place every 6, and for medication follow-up visits were assumed to take place every 3 months.
Minor invasive surgery was assumed to involve one visit before surgery and one visit after, both lasting 15 minutes and led by a surgeon. For ETS, two visits before treatment and one visit after were assumed. The associated resource use of both initial and follow-up visits is £34.50 per visit. All of these assumptions were based on clinical advice. Details of, and unit costs for, initial consultations and follow-up visits for all treatments are summarised in Tables 35 and 36.
| Type of treatment | Duration (minutes) | Job title of health professional | Unit cost (£)a |
|---|---|---|---|
| Medication | 20 | Consultant dermatologist | 45.21 |
| BTX | 20 | Consultant dermatologist | 45.21 |
| Iontophoresis | 20 | Consultant dermatologist | 45.21 |
| Minor invasive surgery | 15 | Consultant surgeon | 34.50 |
| ETS (two consultations) | 15 × 2 | Consultant surgeon | 69.00 |
| Type of treatment | Frequency of follow-up visits | Duration (minutes) | Job title of health professional | Unit cost (£)a |
|---|---|---|---|---|
| Aluminium chloride | 6 monthly | 15 | Consultant dermatologist | 34.25 |
| Medication | 3 monthly | 15 | Consultant dermatologist | 34.25 |
| Minor invasive surgery | Single | 15 | Consultant surgeon | 34.50 |
| ETS | Single | 15 | Consultant surgeon | 34.50 |
Adverse effects
Compensatory sweating
The cost of experiencing CS following curettage and ETS depends on the cost of the treatments used to treat it. These treatments depend on the treatment sequence. The proportion of patients trying each treatment is described in The frequency of treatment use after treatment failure.
Wound infections
Based on clinical advice, it was assumed that 0.1% of patients receiving ETS will experience a wound infection in month 1. A cost of £0.07 (based on 0.1% of the cost of ETS in month 1 prior to accounting for adverse events) was added to this state to account for the probability of experiencing an adverse event.
Model state costs
Resource use associated with individual treatments, associated consultation costs and the probability of experiencing adverse events for specific treatments were combined to estimate monthly costs for each state included in the base-case model. Monthly costs are outlined in Table 37. The states correspond to the states presented in Table 9.
| Model state | Monthly cost (£) |
|---|---|
| Aluminium chloride (A) | 8.72 |
| Iontophoresis sponge (IS) | 198.64 |
| Iontophoresis sponge responder (ISR) | 0.00 |
| Medication month 1 (M1) | 73.34 |
| Medication month 2 (M2) | 28.13 |
| Medication month 3 (M3) | 28.13 |
| Medication responder (MR) | 39.55 |
| BTX month 1 (B1) | 201.21 |
| BTX 2 (B2) | 0.00 |
| BTX 3 (B3) | 0.00 |
| BTX 4 (B4) | 0.00 |
| BTX 5 (B5) | 0.00 |
| BTX 6 (B6) | 0.00 |
| BTX subsequent injections (BS) | 156.00 |
| Curettage (C) | 1194.62 |
| Curettage responder (CR) | 0.00 |
| ETS (E) | 5750.89 |
| ETS responder (ER) | 0.00 |
| ETS low CS (ELCS) | 39.55 |
| ETS moderate CS (EMCS) | 39.55 |
| ETS high CS (EHCS) | 39.55 |
| ETS regretful CS (ERCS) | 39.55 |
| Treatment failure (TF) | Based on methods described in Summary of model data |
| Dead (D) | 0.00 |
Incremental cost-effectiveness analysis, and scenario and sensitivity analyses
Incremental cost-effectiveness analysis
Incremental CEA was conducted involving the following steps:
-
Estimate the total costs and QALYs for each treatment sequence.
-
Calculate the difference in cost and QALYs for each sequence compared with the sequence with the next highest cost.
-
Eliminate treatments/sequences where the incremental cost is positive and the incremental QALYs is negative; these interventions/sequences are said to be dominated (repeat steps 2 and 3 until no more are eliminated).
-
Calculate the ratio of incremental cost to incremental QALYs: this is the incremental cost-effectiveness ratio (ICER).
-
Eliminate treatments/sequences when the ICER is higher than the ICERs of treatments/sequences with higher costs; these interventions are said to be dominated by extension (repeat steps 4 and 5 until no more are eliminated).
The incremental CEA was calculated for both deterministic and probabilistic analyses. A deterministic analysis calculates the result using point estimates for all of the parameters in the model. A probabilistic analysis is described later in Scenario and sensitivity analyses.
Scenario and sensitivity analyses
The base-case model included the following assumptions:
-
The OR of response for iontophoresis sponge compared with placebo is 3.03 (half the OR of BTX vs. placebo).
-
There is no decline in medication effectiveness.
-
The cost of medication is the cost of propantheline bromide.
-
Patients pay for the iontophoresis sponge home device if successful.
-
BTX injections are given every 6 months and their effectiveness is sustained over 6 months.
-
No patients withdraw from BTX treatment because of adverse effects.
-
Only people who experienced no benefit from any of the previous non-surgical treatments take up the option of a surgical treatment.
-
Only people who experience no benefit from curettage go on to have ETS.
-
Patients retry medication, then iontophoresis sponge, then aluminium chloride, in the event of treatment failure if the treatments were in the strategy.
-
Patients remain on the treatment that worked best for them for the remainder of the time that they have hyperhidrosis.
A large number of the base-case model assumptions were varied in sensitivity analyses.
Deterministic sensitivity analysis
One-way, two-way and multiway sensitivity analyses were performed to determine the impact of changing key parameters on the model results. Sensitivity analyses were also carried out to test for the effect of assumptions and variability. Although deterministic sensitivity analyses do not precisely estimate the expected mean outcomes of a non-linear model, these were conducted initially in order to identify the parameter and assumption changes that had a significant effect on the results. Probabilistic sensitivity analyses (PSAs) were then conducted to evaluate scenarios of parameters and assumptions that had a particularly important effect on the results.
Scenario and sensitivity analyses have been introduced throughout this section. These are summarised here and listed in Box 1. For the iontophoresis sponge, threshold analysis was done around the response rate of iontophoresis sponge as there is no evidence of the effectiveness of iontophoresis. Current practice is for the NHS to provide a 1-month free trial of iontophoresis and then patients have to pay for a home iontophoresis device if they want to continue to use it. As the analysis is undertaken from a NHS and PSS perspective, the costs incurred by the patients paying for an iontophoresis device are not included in the analysis. A sensitivity analysis assuming that the NHS pays for iontophoresis home devices that patients can borrow free of charge was conducted.
-
Threshold analysis for the response rate of iontophoresis sponge.
-
Assume the NHS pays for the use of an iontophoresis device at home.
-
Glycopyrronium bromide medication cost.
-
Average cost of glycopyrronium bromide and propantheline bromide.
-
Moderate and considerable decline in medication effectiveness.
-
Use of medication only before important occasions (emergency use).
-
Increase the probability of withdrawal from BTX treatment due to adverse effects from 0 to 0.2.
-
Assume that BTX injections are given annually without any decline in effectiveness over the year.
-
Increase the probability of withdrawal from aluminium chloride from 0.55 to 0.9.
-
Change the distribution of severity of CS from that in Smidfelt and Drott16 to that of Wolosker et al. 252
-
Reduce the utility of people who regret having ETS due to severe CS from 0.49 to 0.21.
-
Halve the utility gained from an improvement (reduction) in the HDSS score of 1 point.
-
All non-responders to non-surgical treatments move on to minor surgery.
-
The order in which patients retry treatments after inadequate response to treatments is changed from medication/iontophoresis/BTX/aluminium chloride to iontophoresis/BTX/medication/aluminium chloride.
The base-case analysis assumes that the medication is propantheline bromide. Although a class effect is assumed for the effectiveness of medication (the two clinical studies investigated methantheline bromide and topical glycopyrrolate wipes), the cost of propantheline was used. Scenario analyses used the cost of glycopyrronium bromide or an average of the cost of glycopyrronium bromide and propantheline bromide instead. No cost of glycopyrronium wipes was identified. As a clinical expert advised that medication could be restricted to use only before particularly important occasions (emergency use), a scenario analysis was conducted in which tablets were taken only four times a week with quality-of-life benefit reduced on a pro rata basis (although that may not be the case). There is no evidence for the long-term effectiveness of medication, so monthly probabilities of continued success of 0.9975 and 0.99 were used in sensitivity analysis.
The base case assumes that the probability of withdrawal due to adverse effects from BTX is zero. Probabilities of 0.1 and 0.2 were used in a sensitivity analysis. There is very little evidence of withdrawal from BTX treatment because of adverse effects, and 0.2 is considered a high value. In the base case, BTX injections were given every 6 months. This was changed to annual injections, while assuming that there was no decline in effectiveness. The probability of withdrawal because of adverse effects was increased from 0.55 to 0.90 in another sensitivity analysis.
An alternative distribution of CS outcomes that represented a less severe outcome was evaluated. A more severe outcome was evaluated using a lower utility value for regretting ETS. In a separate sensitivity analysis, the utility gain for an improvement (reduction) in the HDSS score of 1 point was halved as a conservative estimate of the benefit of a small (1-point) response to treatment. The original utility for a 1-point gain improvement in HDSS was 0.825. The new, more conservative, utility was 0.8.
In the base case, only patients who experience no benefit at all from any of the non-surgical treatments go on to have a surgical intervention. In a scenario analysis, patients who were non-responders (less than a 2-point reduction in HDSS) but had a partial response (a 1-point reduction in HDSS) also went on to have a surgical intervention if there was one in the sequence. In another sensitivity analysis the order in which patients retried previous non-surgical treatments was changed from medication/iontophoresis/BTX/aluminium chloride to iontophoresis/BTX/medication/aluminium chloride to test if the order had a significant effect.
Probabilistic sensitivity analysis
When available, data were entered into the model as distributions in order to fully incorporate the uncertainty around model parameters so that a PSA could be undertaken. In decision modelling, many of the parameter values are often estimated with a degree of uncertainty. There is a need to propagate the joint parameter uncertainty in terms of decision uncertainty; to achieve this, distributions are assigned to input parameter values. Relevant distributions were informed by the systematic review, additional literature and expert opinion. The PSA was run with 3000 simulations. Estimation of costs and QALYs were calculated as the expectation over the joint distribution of the parameters. An incremental CEA was conducted based on the estimated costs and QALYs as described in Health service costs.
The probability of the treatment sequence with the highest net benefit being cost-effective across a range of willingness-to-pay (WTP) threshold values was estimated and presented through a cost-effectiveness frontier. First, the net benefit for each intervention was calculated, where:
The proportion of the simulations in which the treatment sequence has the highest net benefit represents the probability (p) that the treatment sequence is the most cost-effective. 1 – p is the error probability, the probability that one of the other treatment sequences is in fact the most cost-effective. This can be calculated at different thresholds.
This quantification of decision uncertainty also provided the starting point for assessing the value of additional research.
Value-of-information analysis
In addition to assessing the relative effectiveness and cost-effectiveness of the alternative treatment options, the economic model was used to quantify the main uncertainties facing decision-makers and to help inform decisions about the direction of future research. Within the economic component of this study, this was explored using variants of VOI analysis: EVPI and expected value of partial perfect information (EVPPI).
Value-of-information analysis can quantify the expected gain in net benefit from obtaining further information to inform a decision. A decision based on existing information will be uncertain and so may turn out to be incorrect if more information becomes available in the future. If the decision turns out to be incorrect, then there will be a cost in terms of lost health benefit and wasted resources. Quantifying the value of an incorrect decision, alongside the probability of making an incorrect decision, allows us to estimate EVPI. If the EVPI for a decision problem exceeds the cost of future research, additional investigation may be worthwhile.
As well as determining EVPI around the decision as a whole, VOI approaches can also be used for particular elements of the decision with the purpose of focusing research in areas where the elimination of uncertainty might have the most value. EVPPI analysis can be used to estimate the expected value of removing uncertainty surrounding specific parameters or groups of parameters to identify where future research should focus on identifying more precise and reliable estimates of specific pieces of information (e.g. relative effectiveness, costs or utilities). EVPI places an upper value on conducting further research overall, whereas EVPPI places an upper value on conducting further research on a specific area of information. Therefore, if the EVPI or EVPPI exceeds the expected costs of that additional research, then it is potentially cost-effective to acquire more information by undertaking this research. However, although this additional investigation may be worthwhile, calculation of the expected net gain of sample information (expected value of sample information – cost of sampling) would ultimately be required to confirm this. The expected value of sample information is computationally demanding and was not undertaken.
Population EVPI was calculated by multiplying the individual EVPI by the expected future population to benefit from the interventions:
No reliable estimates for the prevalence of primary hyperhidrosis and primary hyperhidrosis of the axilla in the UK were identified. 3–6 The Hyperhidrosis Support Group website quotes a prevalence of 1%, but no reference is provided. The largest survey of hyperhidrosis prevalence identified was conducted in the USA. 226 As hyperhidrosis is a chronic condition, it was assumed that the annual incidence was an average of the number of people with primary hyperdrosis for each 1-year age group in the age range 18–65 years. The estimated percentage of axillary hyperhidrosis among the US population aged 18–65 years was 2.1%. When adjusted for the entire population, resulting prevalence would be < 2.1%. The HSSS scores of the model population are assumed to be equally split between 3 and 4. The same study reported that 32.4% of patients with hyperhidrosis has a HDSS score of 3 or 4 points. The percentage of the population aged 18–65 years that has axillary hyperhidrosis and a HDSS score of 3 or 4 points was therefore calculated to be 0.67%. The annual incidence (5275) was therefore calculated to be the average population for each 1-year age group across the age range 18–64 years within England and Wales, multiplied by 0.0067.
Using the England and Wales population statistics from the Office for National Statistics,343 a discount rate of 3.5% and a 10-year useful life of the current interventions before a significant change in the treatment options, the applicable population was estimated to be 49,122 using the summation expression in Equation 32. This was multiplied by the EVPI estimates to obtain the population EVPI estimates.
For the EVPI, sensitivity analysis was performed around the incidence rate, varying the rate over the following values: 2%, 1.5%, 1%, 0.5% and 0.1%.
The EVPPI for specific parameters were calculated for eight different scenarios. It was assumed the NHS pays for home iontophoresis and the iontophoresis device has a life expectancy of 10 years. It is calculated assuming an OR of iontophoresis compared with placebo of 3.03 and of 1. Furthermore, it is calculated for the £20,000 and £30,000 cost-effectiveness thresholds and at a 2% axillary hyperhidrosis incidence and a 0.5% incidence.
The base-case model was structured with OR parameters for each treatment compared with placebo. In order to conduct EVPPI analysis on BTX compared with medication, and curettage compared with BTX, the model was recoded with the placebo-controlled OR replaced by an OR for the comparison of interest, and also for any other effectiveness parameter in the same indirect chain of evidence connected to placebo.
Chapter 7 Cost-effectiveness, expected value of perfect information and expected value of partial perfect information results
As stated in Chapter 6, given limited evidence for treatment effectiveness for different body sites, CEA and VOI analysis were limited to axillary hyperhidrosis. Sixty-four different treatment sequences were compared. The interventions included in the different sequences were iontophoresis sponge, medication, BTX, curettage, ETS and aluminium chloride antiperspirants. Aluminium chloride antiperspirants represent no secondary care treatment. In the base-case analysis, the medication was assumed to be propantheline bromide. Treatment sequences are represented by abbreviations where each letter represents the first letter of each treatment: iontophoresis (I), medication (M), BTX (B), curettage (C), ETS (E), aluminium chloride antiperspirants (A). For example, the treatment sequence MBICE represents the following order in which treatments would be offered by the NHS: medication, BTX, iontophoresis, curettage, ETS.
Base-case results
This section presents the results of the base-case CEA for the probabilistic model. The key assumptions of the base-case model are presented in Chapter 6, Model state costs. The analyses set out to investigate the value of further research in hyperhidrosis related to different body sites and different interventions. For the axillae body site, sufficient evidence was identified to model iontophoresis, medication, BTX, curettage and ETS in sequences. No other body sites were modelled.
Axilla probabilistic model
The probabilistic analysis, net benefit and cost-effectiveness acceptability frontier were calculated as described in Chapter 6, Scenario and sensitivity analyses.
The total costs and QALYs for each of the 64 treatment sequences are reported in Appendix 5. The base-case results for the probabilistic model shown in Table 38 indicate that the treatment sequence of I is the least expensive, with a mean cost of £900. Compared with I, the sequence of ICE has an ICER of £253. Although more expensive, the treatment sequence of IBMCE produces a higher QALY gain than ICE and has an ICER of £9304, below the NICE threshold of £20,000–30,000 per QALY gained. Although more costly than their rspective previous treatment sequences, the strategies of BMICE and MBICE also produce higher QALY gains than their comparators. However, the incremental QALY gains are so small that the ICERs for both strategies exceed the NICE threshold by a considerable margin. All other treatment strategies were either dominated or extendedly dominated.
| Strategy | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (cost per QALY) (£) |
|---|---|---|---|---|---|
| I | 900 | – | 18.47 | – | – |
| ICE | 1121 | 220 | 19.30 | 0.829 | 253 |
| IBMCE | 6091 | 4970 | 19.84 | 0.542 | 9304 |
| BMICE | 7468 | 1377 | 19.85 | 0.009 | 137,046 |
| MBICE | 8195 | 726 | 19.85 | 0.001 | 1,407,569 |
The probability that the treatment sequence with the highest net benefit is cost-effective across a range of WTP threshold values is presented in the cost-effectiveness acceptability frontier in Figure 16. ICE is the most cost-effective treatment up to a threshold of £9304, and IBMCE is the most cost-effective sequence when the threshold is between £10,000 and £50,000. The error probability that IBMCE is most cost-effective at NICE cost-effectiveness thresholds of £20,000 and £30,000 is around 0.2. The error probability is the probability that one of the treatments not identified as the most cost-effective is in fact the most cost-effective. When the pairwise net benefit of the most cost-effective sequence compared with every other sequence is calculated, then the comparator that has the highest probability of being the most cost-effective is the sequence IMBCE. This reflects the considerable uncertainty in the estimate of effectiveness for medication compared with placebo.
FIGURE 16.
Cost-effectiveness acceptability frontier: base-case analysis.

Scenario and sensitivity analysis results
Axilla deterministic model
Fourteen deterministic scenario/sensitivity analyses were undertaken in order to establish which parameters had a significant impact on the results. The PSAs that follow evaluate scenarios with the parameters that had the most impact. In each scenario, a key model parameter/assumption was varied with all other parameters fixed at base-case values. A number of these analyses had no significant impact on the base-case results. Each scenario is presented in turn, with either a statement of no significant impact or a short summary of the impact that the analysis had on the base-case results.
Scenario 1
No evidence was identified on the relative effectiveness of iontophoresis sponge. In the base case, the response rate of iontophoresis sponge was assumed to be 0.31, compared with a placebo response rate of 0.13 used in the model (this corresponds to a relative risk of 2.4 and an OR of 3.03 of iontophoresis sponge vs. placebo), half that of BTX compared with placebo. The response rate of iontophoresis sponge was reduced in a threshold analysis to identify how ineffective iontophoresis needs to be to significantly change the results. Reducing the iontophoresis response rate from 0.10 to 0.07 resulted in BMICE becoming most cost-effective. At a response rate of 0.07, the most cost-effective treatment was BMICE, with an ICER of £13,058. A response rate of 0.07 is less than the response rate for the placebo effect of medication and BTX, and so the threshold for changing the decision is low.
Scenario 2
Scenario 2 assumed that the NHS pays for the use of the iontophoresis device at home. As the analysis is undertaken from a NHS and PSS perspective, in the base case the costs incurred by the patients paying for an iontophoresis device are not included in the analysis. This analysis had no significant impact on the ICER results. The most cost-effective remained IBMCE, with an ICER of £9253.
Scenarios 3 and 4
The base-case analysis assumes that propantheline bromide is the first choice of oral medication and the cost of propantheline bromide is used in the model. When the cost of glycopyrronium bromide (£283.29 per month) is used instead of the cost of propantheline bromide (£28.13 per month), then IBCE becomes the most cost-effective sequence, rather than IBMCE. The result is the same when the average cost of propantheline bromide and glycopyrronium bromide was used. No cost of topical glycopyrrolate wipes was identified. The cost of 500 ml of 0.05% glycopyrrolate solution was £325.04. 357 It is likely that the monthly cost of using this solution would be somewhere between the monthly cost of propantheline bromide and that of glycopyrronium bromide. Using the cost of glycopyrronium bromide, the most cost-effective sequence was IBCE, with an ICER of £10,742.
Scenario 5
When it was assumed that there is a considerable decline in medication effectiveness over time, IBCE became the most cost-effective sequence at a threshold of £20,000, with an ICER of £10,742. At a threshold of £30,000, the most cost-effective sequence was IBMCE with an ICER of £26,518. A moderate decline in effectiveness did not have a significant effect.
Scenario 6
A clinical expert advised that oral medication may be taken sparingly in order to sustain long-term effectiveness. To model this scenario, the daily medication dose was reduced from the manufacturer’s recommendation to only four tablets a week to be taken at the most appropriate times. Cost and utility were reduced on a pro rata basis. In this scenario analysis, IBCE again became the most cost-effective sequence, with an ICER of £10,742. The relationship between utility and dose may not be linear, but is unknown. This means that this scenario potentially underestimates the cost-effectiveness of medication as the patients may get more benefit from the few occasions of medication use than assumed in this scenario.
Scenario 7
Scenario 7 showed an increase in the probability of withdrawal from BTX treatment because of adverse effects from 0 to 0.2. This analysis had no significant impact on the results. The most cost-effective scenario remained IBMCE, with an ICER of £11,381.
Scenario 8
Giving botulinum toxin injections given annually, rather than every 6 months, had no significant impact on the results. The most cost-effective secnario remained IBMCE, with an ICER of £11,978.
Scenario 9
Increasing in the probability of withdrawal from aluminium chloride treatment from 0.55 to 0.9 had no significant impact on the results. The most cost-effective scenario remained IBMCE, with an ICER of £10,242.
Scenario 10
Changing the distribution of severity of CS from that of Smidfelt and Drott16 to that of Wolosker et al. 252 had no significant impact on the results. The most cost-effective scenario remained IBMCE, with an ICER of £10,324.
Scenario 11
Reducing the utility of people who regret having ETS due to severe CS from 0.49 to 0.21 had no significant impact on the results. The most cost-effective scenario remained IBMCE, with an ICER of £9745.
Scenario 12
The utility gained from an improvement in one point on the HDSS score was halved. The original utility for a 1-point improvement was 0.825. The new more conservative utility was 0.8. This analysis had no significant impact on the results. The most cost-effective scenario remained IBMCE, with an ICER of £7318.
Scenario 13
The base-case model assumes that only people who receive no benefit at all from any of the previous treatments move on to minor surgery if minor surgery is an option in the treatment sequence. In a scenario analysis, it was assumed that all patients who did not respond to any of the previous non-surgical treatments in the sequence moved on to minor surgery. In this scenario, ICE was the most cost-effective treatment sequence, with an ICER of £330. This reflects the cost-effectiveness of the surgical interventions. In this scenario, more patients have surgery, and, as curettage is more cost-effective than medication and BTX given the assumptions made, curettage takes precedence over medication and BTX in the sequence. Based on clinical expert advice, the model assumes that surgery is offered only after non-surgical interventions available in the sequence, medication and BTX, are absent entirely from the optimal sequence.
Scenario 14
The order in which patients retry treatments after inadequate response to treatments was changed from MIBA to IBMA. This had no significant impact on the results. The most cost-effective scenario remained IBMCE, with an ICER of £5720.
Table 39 presents the optimal treatment sequence and ICER results of those one-way sensitivity scenarios in which the treatment decision changed as a result of the alternative assumption/data. Given a WTP threshold of £30,000 per QALY, the most cost-effective option for each of the seven scenarios is presented.
| Analysis | Optimal decision | ICER (£) | |
|---|---|---|---|
| Base case | IBMCE | 10,122 | |
| Scenario | Analyses | ||
| Scenario 1 | Response rate of 0.07 for iontophoresis sponge | BMICE | 13,058 |
| Scenario 3 | Glycopyrronium bromide medication cost | IBCE | 10,742 |
| Scenario 4 | Average cost of glycopyrronium bromide and propantheline bromide | IBCE | 10,742 |
| Scenario 5 | Considerable decline in medication effectiveness | IBMCE; IBCEa | 26,518; 10,742a |
| Scenario 6 | Medication utility and cost reduced to emergency medication use only | IBCE | 10,742 |
| Scenario 13 | All non-responders to non-surgical treatments move on to minor surgery | ICE | 330 |
Two-way and multiway sensitivity analyses were also undertaken to assess the impact of changing more than one key variable at the same time. Table 40 presents the results of the two-way and multiway sensitivity analyses of key model parameters. Given a WTP threshold of £30,000 per QALY, the most cost-effective option is presented following each analysis. When a NHS subsidy for using an iontophoresis sponge at home was included, along with an adjusted response rate for iontophoresis sponge of 0.07, the optimal strategy was BMICE. This held true when the duration of an iontophoresis was reduced to 10 years and this variation was combined with the NHS subsidy and adjusted relative risk.
| Analysis | Optimal decision | ICER (£) |
|---|---|---|
| Base case | IBMCE | 10,122 |
| Two-way sensitivity analyses | ||
| NHS subsidy and response rate of 0.07 for iontophoresis sponge | BMICE | 12,089 |
| NHS subsidy and response rate of 0.07 for iontophoresis sponge and reduced duration of iontophoresis device | BMICE | 12,221 |
Axilla probabilistic model
Finally, PSAs were undertaken to further explore the parameters that appeared to have the potential to have an impact on the overall treatment sequence decision. The parameters explored were the cost of using iontophoresis sponge at home (a NHS subsidy vs. no subsidy), the durability of an iontophoresis device (10 years instead of 15 years), the relative risk of iontophoresis sponge compared with placebo (1 instead of 2.4 with a placebo response of 0.13) and the decline in effectiveness of medication over time (from none to considerable decline; see Chapter 6, Long-term effectiveness). The four scenarios are as follows.
-
A NHS subsidy of iontophoresis at home and a 10-year durability of an iontophoresis device are assumed.
-
A relative risk of 1 for iontophoresis sponge compared with placebo is assumed.
-
A NHS subsidy of iontophoresis at home, a 10-year durability of an iontophoresis device and a relative risk of 1 for iontophoresis sponge compared with placebo are assumed.
-
A considerable decline in the effectiveness of medication over time is assumed.
For scenarios 1–3, IBMCE remained the most cost-effective treatment sequence at the conventional thresholds. The ICER ranged from £8352 to £10,210. The error probability for IBMCE in scenario 1 was 0.2, and this increased to 0.35 and 0.42 in scenarios 2 and 3, respectively, at a threshold of £20,000 per QALY. In scenario 1, the comparator with the next highest probability of being most cost-effective was the sequence IMBCE, reflecting the uncertainty in the effectiveness of medication. In scenarios 2 and 3, the reduced effectiveness of iontophoresis increased the uncertainty in the position of iontophoresis within the sequence. In theses scenarios, the comparator with the next highest probability of being most cost-effective was the sequence BMICE with an ICER of £41,837 in scenario 2 and an ICER of £36,796 in scenario 3. In scenario 4, IBCE was the most cost-effective sequence at conventional thresholds with an ICER of £10,058. The closest comparator was IBMCE, with an ICER of £30,170.
Expected value of perfect information results
Figure 17 presents population EVPI (number of patients) for all model parameters across a range of WTP thresholds. In the base case, at a WTP threshold of £20,000, based on an incidence of axillary hyperhidrosis in the adult population between the ages of 18 and 65 years of 2.1%, the cost of further research should not exceed £4.5M if it is to be worthwhile. This figure is unchanged if the NHS pays for iontophoresis at home, but increases to roughly £13.4M if the relative risk of iontophoresis compared with placebo is reduced to 1 from 2.4. The EVPI is very sensitive to the threshold value between thresholds of £5000 and £20,000 per QALY. If a considerable decline in effectiveness of oral medication is assumed, then the EVPI rises significantly between thresholds £20,000 and £30,000. At £30,000 the EVPI is £35.5M.
FIGURE 17.
Population EVPI for the base-case model and four sensitivity analyses.
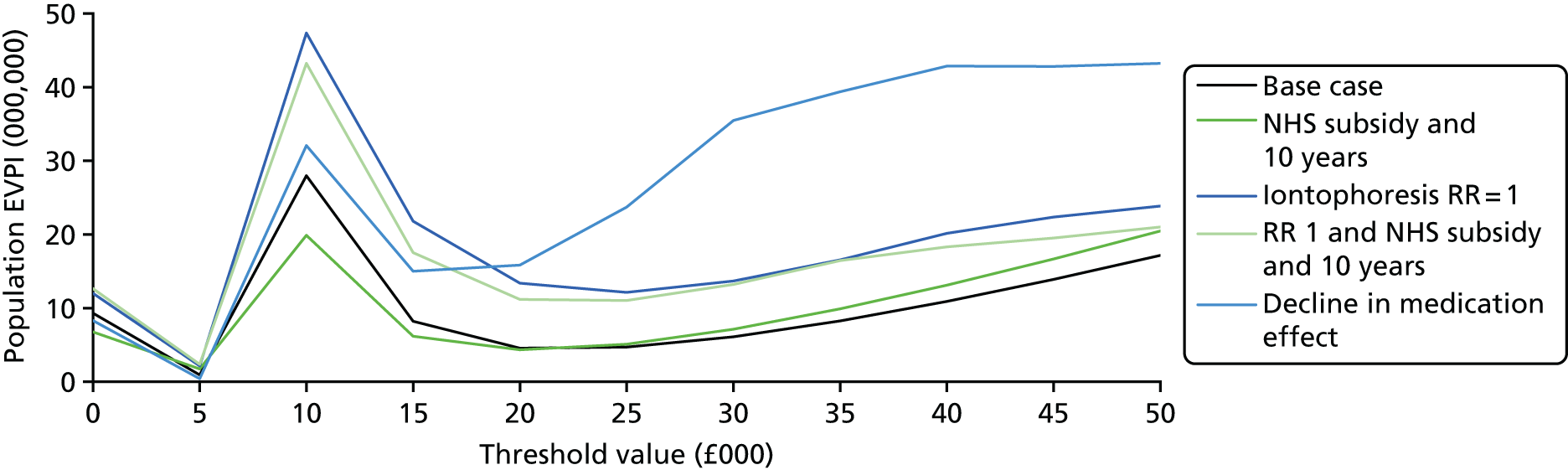
The population EVPI assuming different population annual incidences is presented in Figure 18. The population annual incidencess are 2%, 1.5%, 1%, 0.5% and 0.01%. At a threshold of £20,000 the population EVPI is £4.4M at an annual incidence of 2%, £3.3M at an annual incidence of 1.5%, £2.2M at an annual incidence of 1%, £1.1M at an annual incidence of 0.5% and £0.2M at an annual incidence of 0.1%.
FIGURE 18.
Population EVPI for different values of axilla hyperhidrosis incidence.
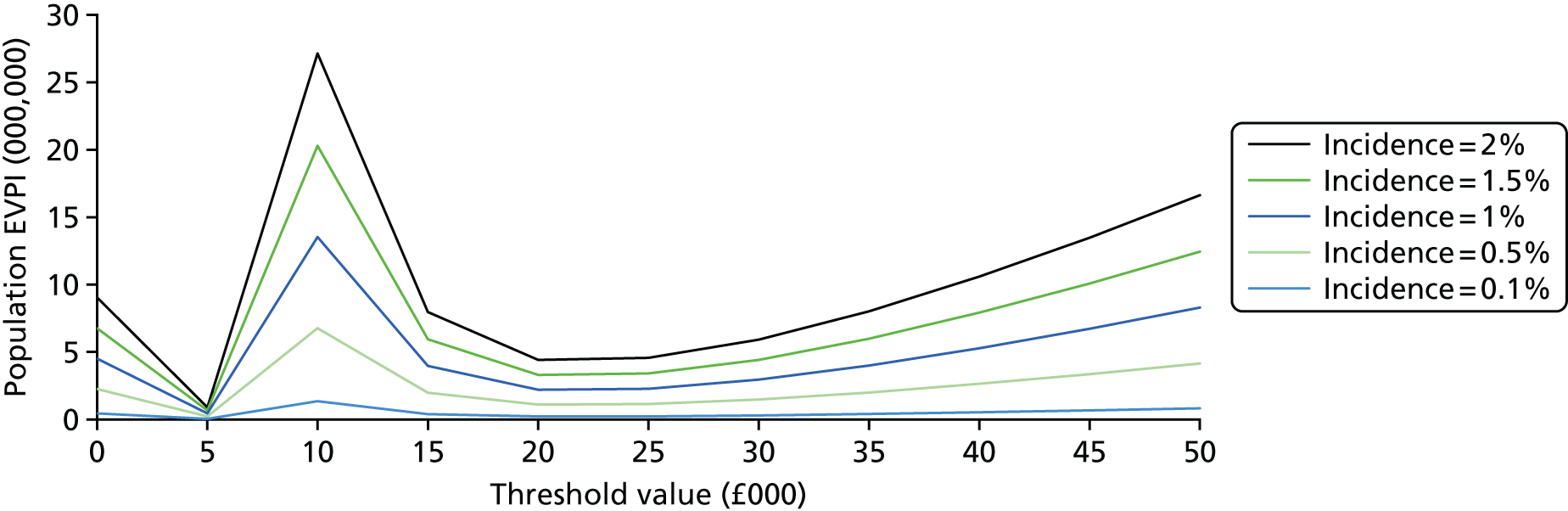
Population expected value of partial perfect information results
The population EVPPI of sets of parameters has been calculated based on the model assuming that the NHS pays for home iontophoresis and the iontophoresis device has a life expectancy of 10 years. It is calculated for two alternative ORs of iontophoresis compared with placebo: first, assuming OR = 3.03 and, second, assuming OR = 1. Furthermore, it is calculated for both the £20,000 and £30,000 cost-effectiveness thresholds and at a 2% axillary hyperhidrosis annual incidence and a 0.5% annual incidence. Combinations of these factors result in eight different scenarios. The results are presented in Table 41.
| Threshold (£/QALY) | Incidence (%) | IS effectiveness | PEVPI (£000) | Expected value of partial parameter information (£000) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | All effectiveness parameters | All utility parameters | All withdrawal due to adverse effects parameters | Botox vs. medication | Curettage vs. Botox | Medication vs. placebo | Botox vs. placebo | |||
| 20,000 | 2 | High | 4206 | 836 | 0 | 58 | 564 | 597 | 1040 | 0 |
| Low | 10,850 | 6109 | 21 | 972 | 2225 | 3137 | 1660 | 29 | ||
| 0.5 | High | 1048 | 208 | 0 | 14 | 141 | 149 | 259 | 0 | |
| Low | 2704 | 1523 | 5 | 242 | 555 | 782 | 414 | 7 | ||
| 30,000 | 2 | High | 6926 | 2901 | 0 | 137 | 3100 | 0 | 3628 | 63 |
| Low | 12,818 | 7250 | 183 | 2636 | 2711 | 2013 | 5786 | 374 | ||
| 0.5 | High | 1726 | 723 | 0 | 34 | 773 | 0 | 904 | 16 | |
| Low | 3195 | 1807 | 46 | 657 | 676 | 502 | 1442 | 93 | ||
Across all of the EVPPI analyses, the greatest VOI is associated with the parameter ‘medication versus placebo’. The population EVPPI ranges from £0.26M to £5.8M. In contrast, the EVPPI for Botox compared with placebo is negligible. This reflects the fact that the estimate of the effectiveness of BTX compared with placebo (OR 9.21, 95% CI 4.73 to 18.10) is much more precise than the effectiveness of medication compared with placebo (OR 7.21, 95% CI 1.56 to 53.83).
Reducing the annual incidence of axillary hyperhidrosis reduced the population EVPPI of all parameters because the population EVPPI is the result of multiplying the EVPPI for an individual by a factor related to the annual incidence of axillary hyperhidrosis.
The increase in the cost-effectiveness threshold from £20,000 per QALY to £30,000 per QALY increased the EVPPI for all parameters except for ‘curettage versus BTX’ effectiveness. ‘Curettage versus BTX’ is the exception because BTX and medication become more cost-effective relative to curettage as the threshold increases; BTX and medication are more costly than curettage because they involve recurring annual costs rather than the one-off cost of curettage. Therefore, there is less uncertainty about the position of curettage in the sequence. For the other parameters, as the threshold increases from £20,000 to £30,000 it becomes less certain that IBMCE is the most cost-effective sequence; overall decision uncertainty has increased and the value of further information has also correspondingly increased.
Reducing the effectiveness of iontophoresis sponge increases the EVPPI of all parameters because all of the other interventions become relatively more cost-effective than iontophoresis as the effectiveness of iontophoresis is reduced. Therefore, there is greater uncertainty as to which sequences are the most cost-effective.
To place the population EVPPI results into context, a clinical trial of a medication or surgical intervention may cost around £1.5M. This cost will vary considerably depending on a number of key factors, including the sample size of the trial required to achieve a statistically significant outcome and the interventions under investigation. An observational study of rates of withdrawal due to adverse effects can cost as little as £200,000; however, a large RCT may cost upwards of £1M.
The population EVPPI is a maximum VOI for that parameter or for a group of related parameters, such as effectiveness. Given that any further research is unlikely to achieve full perfect information – even the most robust trials produce estimates with some uncertainty – the real value of further research will necessarily be lower than the population EVPPI. Therefore, if a trial of two interventions cost £1.5M and we knew that it would only reduce our uncertainty by half, then our EVPPI estimate would need to be at least £3M to warrant conducting that study. Any further research needs to cost less than the EVPPI estimate; how much less will depend on the studies undertaken.
Population EVPPI analysis was conducted for seven different subsets of parameters. The results are summarised for each.
Effectiveness
This analysis investigated the EVPPI of the subset of parameters that inform the relative effectiveness of medication, BTX and curettage. The population EVPPI ranged from £208,000 to £7,250,000 across the different scenarios. As this subset of parameters covers three treatments and placebo, it is expected that the population EVPPI will be higher than the population EVPPI of a single relative effectiveness parameter for two interventions. For a threshold of £20,000 per QALY or £30,000 per QALY, the population EVPPI is only > £4M in the scenario of low effectiveness of iontophoresis sponge compared with placebo and an annual incidence of 2%. It would be useful to include the EQ-5D-5L instrument and a HDSS outcome, as well any preferred outcome in a future trial, in order to allow the trial results to be used in a future economic evaluation.
Medication compared with placebo effectiveness
This analysis investigated the EVPPI of the specific effectiveness parameter of medication compared with placebo. The population EVPPI ranged from £259,000 to £5,786,000 across the different scenarios. For a threshold of £20,000 per QALY, the population EVPPI is < £3M. For a threshold of £30,000 per QALY, the population EVPPI is only > £3M when the annual incidence is 2%.
Botulinum toxin compared with placebo effectiveness
This analysis investigated the EVPPI of the specific effectiveness parameter of BTX compared with placebo. The population EVPPI ranged from £0 to £374,000 across the different scenarios, so it is never > £3M.
Botulinum toxin compared with medication effectiveness
This analysis investigated the EVPPI of the specific effectiveness parameter of BTX compared with medication. The population EVPPI ranged from £141,000 to £3,100,000 across the different scenarios. For a threshold of £20,000 per QALY, the population EVPPI is < £3M. For a threshold of £30,000 per QALY, the population EVPPI is only > £3M in the scenario where the annual incidence of axillary hyperhidrosis is 2% and the OR of iontophoresis compared with placebo is assumed to be 3.03. The population EVPPI values for BTX compared with medication effectiveness are related to the population EVPPI value for medication compared with placebo effectiveness as the estimate of the effectiveness of BTX compared with medication was derived from indirect evidence using placebo-controlled trials.
Curettage compared with botulinum toxin effectiveness
This analysis investigated the EVPPI of the specific effectiveness parameter of curettage compared with BTX. The population EVPPI ranged from £0 to £3,137,000 across the different scenarios. For a threshold of £20,000 per QALY, the population EVPPI is > £3M only if the annual incidence is 2% and the OR of response of iontophoresis compared with placebo is 1. For a threshold of £30,000 per QALY, the population EVPPI is < £3M.
Withdrawal rates due to adverse effects
This analysis investigated the EVPPI of the subset of parameters that inform the rates of withdrawals due to adverse effects for medication and iontophoresis sponge. The population EVPPI ranged from £14,000 to £2,636,000 across the different scenarios. Assuming that future research would be an observational study, for a threshold of £20,000 per QALY the population EVPPI is > £400,000 in the scenario with an annual incidence of 2% and where the OR of response of iontophoresis compared with placebo is 1. For a threshold of £30,000 per QALY, the in which EVPPI is > £400,000 in the scenarios in which the OR of response of iontophoresis compared with placebo is 1.
Utility
This analysis investigated the EVPPI of the subset of parameters that inform the utilities of the different HDSS levels. The population EVPPI ranged from £0 to £183,000 across the different scenarios. Future research may be an observational study with a cost of £400,000. The population EVPPI is never > £400,000 for the utility parameters.
Summary of cost-effectiveness and value-of-information analysis
The base-case results indicated that IBMCE was the most cost-effective sequence with an ICER of £9304 per QALY. The first three treatments in this sequence are in order of annual cost: iontophoresis incurs the lowest annual expenditure and medication incurs the highest annual expenditure. Curettage and ETS come last because of the assumption that surgery would only be offered after a patient has tried the available non-surgical options. Fifty-nine out of the 64 treatment sequences were either strictly dominated or dominated by extension.
When pairwise net benefit comparisons between the most cost-effective sequence and the other sequences were made, the highest error probabilities were associated with comparisons with IMBCE (0.1 error probability) and then IBCE (0.05 error probability) at a threshold of £20,000 per QALY. At a threshold of £30,000 per QALY, they are IMBCE (0.17 error probability) and MIBCE (0.07 error probability). This indicates that the greatest uncertainty is associated with the effectiveness of medication compared with placebo and this reflects the clinical evidence. Despite the fact that the annual costs of medication are greater than the annual costs of BTX, the uncertainty in the effect estimate for medication means that it is possible that medication is sufficiently more effective than BTX to come before it in the sequence at a threshold of £20,000 per QALY. Equally, it may be sufficiently less effective than BTX so that it drops out of the sequence altogether.
Most sensitivity and scenario analyses had no effect on the results, with the exception of scenario analyses 1, 3, 4, 5, 6 and 13, which resulted in a significant change in the results.
Scenario 1
If the response rate of iontophoresis sponge is reduced to 0.07, BMICE becomes the most cost-effective sequence, with an ICER of £13,058.
Scenarios 3 and 4
If the cost of medication increases from the cost of propantheline bromide to an average of the cost of propantheline bromide and glycopyrrolate bromide, then it is no longer cost-effective to include medication in the treatment sequence at a threshold of £30,000 per QALY.
Scenario 5
If the effectiveness of medication declines over time then it becomes significantly less cost-effective to include medication in a treatment sequence.
Scenario 6
If the relative risk of iontophoresis sponge compared with placebo were only 0.5, then iontophoresis would come after medication in the sequence and BTX would be first.
Scenario 13
If partial responders to non-surgical options as well as patients with no response at all had curettage, instead of only those who had no response at all to previous treatments, then curettage comes after iontophoresis in the treatment sequence as it is relatively cost-effective compared with BTX and medication given the assumptions in the model.
The results of the EVPI and EVPPI analyses are very sensitive to the decision-maker’s cost-effectiveness threshold, to the annual incidence rate of axillary hyperhidrosis and to the effectiveness of iontophoresis sponge compared with placebo. There is uncertainty in the annual incidence rate because the study was based on a US population. The population EVPPI results also reflected the uncertainty in the estimate of effectiveness of medication compared with placebo. A conservative estimate of the expected VOI required for a future trial is assumed to be £3M and £400,000 for an observational study.
The population EVPPI for medication compared with placebo effectiveness is < £3M when the threshold is £20,000 per QALY, but is > £3M for a threshold of £30,000 per QALY and the annual incidence of axillary hyperhidrosis is 2%. The population EVPPI for BTX compared with medication effectiveness is > £3M when the OR of response of iontophoresis compared with placebo is 1, the annual incidence is 2% and the threshold is £30,000 per QALY. The population EVPPI for curettage compared with BTX effectiveness is > £3M when the OR of response of iontophoresis compared with placebo is 1, the annual incidence is 2% and the threshold is £20,000 per QALY. The population EVPPI for BTX compared with placebo effectiveness is very low in every scenario. The population EVPPI for withdrawal rates is > £400,000 when the relative risk of response of iontophoresis compared with placebo is 1, except when the threshold is £20,000 per QALY and the annual incidence of axillary hyperhidrosis is 0.5%. For utility parameters, the population EVPPI is never > £400,000.
Chapter 8 Patient and clinician perspective on research findings
Background
The patients’ perspective was collected at various points through the project, including at the initial team meetings and during protocol development (when Julie Halford acted as the patients’ representative). The patients’ perspective was also required to complement the narrative review of quality-of-life measures used in studies of hyperhidrosis and to guide the economic modelling by providing information on what is important to patients in terms of outcomes, both beneficial and adverse, and to advise on the various tools available for assessing HRQoL. The patients’ perspective was also required to help with the interpretation of the results of the systematic review and economic evaluation, and the resultant recommendations for research.
Methods
It should be noted that a ‘virtual workshop’ was originally planned to elicit the opinions of patients, dermatologists and surgeons through an online survey on items that should be captured in tools assessing HRQoL. However, our review of quality-of-life measures identified the literature describing the development of a new tool: the HidroQoL, developed by Kamudoni et al. 204,260,261 This development exercise was much more comprehensive than could be achieved by our planned virtual workshop. Therefore, the workshop was redesigned to be a smaller end-of-project event undertaken face to face with patients. This was submitted as a protocol amendment in the research progress report. In addition, patients were consulted on the structure and inputs of the economic model.
The end-of-project workshop was held at Harrogate District Hospital with four patient advisors and one dermatologist (AL). Julie Halford and the other clinical advisors provided their advice during telephone meetings or via e-mail.
Prior to the workshop the patient advisors were sent a short overview of the purpose of the project. They were also sent copies of four HRQoL tools together with a short list of questions about the tools and asked to consider these questions in preparation for the workshop (see Appendix 12).
At the workshop, an overview of the project was presented, along with a summary of findings from the review of clinical effectiveness of treatments for hyperhidrosis. Gaps in the evidence base that warranted further research were discussed and patients and the dermatologist attending the workshop were asked for their opinions and any further comments. The cost-effectiveness model and results were also described, along with results of VOI analyses relating to further research that would be cost-effective. Again, patients and the dermatologist attending the workshop were asked for their opinions and any further comments. Finally, the review of quality-of-life tools used in hyperhidrosis research was described and patients were asked to comment on the most commonly used tools (HDSS, DLQI, HQLQ) and the new HidroQoL tool, which patients had been sent in advance of the workshop.
The results of the clinical effectiveness review and cost-effectiveness modelling and the resultant recommendations for research were discussed further by teleconference with Dr Nick Levell (clinical advisor) and Julie Halford (nurse clinical advisor and patient representative).
The notes from the various discussions were collated and incorporated into our interpretation of the research findings and into the study’s conclusions and recommendations for research.
Results
Patient advisors
Patient advisors were not surprised by the finding of the review that there is evidence for effectiveness of BTX for hyperhidrosis of the axilla; there was a consensus among patient advisors that BTX for hyperhidrosis of the axilla was very effective. Interestingly, they stated that annual administration was adequate. The annual use of BTX compared very favourably with frequent use required with iontophoresis or frequent application of anticholinergic creams. BTX was considered to be a more effective treatment than iontophoresis and patients expressed an interest in receiving BTX to the hands, as well as the axilla.
Patient advisors agreed that a trial of BTX (plus an anaesthetic) compared with iontophoresis for palmar hyperhidrosis would be useful and that outcomes should include long-term impairment of hand sensitivity and pain of BTX administration. Patient advisors did not think that it would be worth conducting further research into iontophoresis for the axilla; they had limited knowledge of it but imagined it would be (even) less effective than water bath iontophoresis for the hands. The patient advisors agreed that future trials of treatments for hyperhidrosis of the axilla should compare new treatments against BTX, as an established effective treatment.
Patients advisors commented that topical glycopyrrolate (to the hand and other areas) had fewer adverse effects than oral anticholinergic medications. Oral medication was considered to have limited effectiveness, along with troublesome adverse effects. There are adverse effects associated with long-term use of anticholinergic medications; therefore, patients are often advised to use oral medications only when necessary (e.g. when going to public events), rather than on a daily basis. They did not think it was important to investigate which was the best anticholinergic medication: they were happy with the idea of trying one and switching to another if the first did not work. It would be worthwhile researching a new drug only if it had the potential to be effective and was associated with greatly reduced adverse effects compared with the current medications available.
Patient advisors expressed an interest in a more permanent solution for their hyperhidrosis, such as curettage or the newer, less invasive, energy-based ‘destructive’ technologies for hyperhidrosis of the axilla. However, they believed that patients would need assurance that it really was a ‘one-off’ treatment, otherwise they would rather continue with BTX. They also had significant concerns regarding scarring. They expressed an interest in research comparing newer, ‘destructive’, technologies with BTX.
Patient advisors were happy with the sequence of treatments identified as being cost-effective in the modelling exercise: iontophoresis, BTX, medication, curettage, ETS. They also considered that iontophoresis machines should be supplied by the NHS (if they were effective for a patient), rather than having to be purchased by patients.
When asked about the four quality-of-life tools they had been asked to consider, all patient advisors agreed that the HidroQoL tool was superior to the other tools commonly used in hyperhidrosis research (HDSS, DLQI, HQLQ) for assessing quality of life. They commented that it covers everything important to patients with hyperhidrosis and is easy to complete. The DLQI was considered to be too general and too focused on the skin, with questions that are not applicable to hyperhidrosis patients. The HDSS was considered to be too basic and, depending on different situations, patients could easily fluctuate between a HDSS score of 2 points and one of 3 points. They considered that measuring the actual amount of sweat produced (e.g. by gravimetry) was less important than measuring quality of life and it should be considered only a secondary outcome. Single measurements in time could give the wrong impression of the severity of hyperhidrosis and do not necessarily reflect the patient’s overall condition. The patient advisors considered that the HidroQoL tool should be the primary outcome in future studies.
Clinical advisors
It was clear that there is regional variation in the treatments available for patients with hyperhidrosis. Iontophoresis is widely used for hyperhidrosis of the hands and considered effective in a significant proportion of patients, but is used for hyperhidrosis of the axilla much less in NHS trusts that allow access to BTX. Clinical advisors commented that glycopyrrolate was more effective, with a better adverse event profile, than the cheaper anticholinergic medication propantheline bromide. Given the much higher cost of glycopyrrolate than propantheline bromide, it is reasonable to use the cheapest drug first. However, in reality many clinicians use glycopyrrolate, believing it to be superior. The clinical advisors agreed that curettage is not widely used and admitted that they had limited knowledge of the new sweat gland-destroying (‘destructive’) technologies.
Like the patient advisors, the clinical advisors agreed that a trial of BTX (plus an anaesthetic) compared with iontophoresis for palmar hyperhidrosis would be useful and that outcomes should include long-term impairment of hand sensitivity and pain of BTX administration. Given the cost and potential complexity of administering an anaesthetic, it was suggested that a CEA alongside such a trial would be warranted. The clinical advisors also did not think that it would be worth conducting further research into iontophoresis for the axilla and that future trials of treatments for hyperhidrosis of the axilla should compare new treatments against BTX, as an established effective treatment.
Unlike the patients, clinicians did not think that a trial comparing BTX with curettage or newer ‘destructive’ technologies for axillary hyperhidrosis was warranted, at least not until there is clear evidence of benefit with the new technologies, in terms of offering a permanent cure, with a low risk of scarring.
Regarding oral medications, it was considered by clinicians that it would be difficult to power a trial to find statistically significant differences between anticholinergic medications, as effectiveness is considered to be broadly similar between medications, although some medications work better for some patients than others.
Like the patient advisors, the clinical advisors were happy with the sequence of treatments identified as being cost-effective for hyperhidrosis of the axilla in the modelling exercise: iontophoresis, BTX, medication, curettage, ETS. Clinical advisors were happy to try BTX before medication; although this is not generally current standard practice, it reflects their belief in the order of effectiveness and acceptability of the treatments.
It was stated that there is anecdotal evidence of patients being unable to purchase iontophoresis machines because of the cost. In such cases, further clinic appointments are sometimes offered for further sessions of hospital administered iontophoresis.
Conclusions from patient and clinician perspective
Patients and clinicians favoured BTX for hyperhidrosis of the axilla and did not consider that further research on iontophoresis for the axilla would be worthwhile. A trial comparing the different anticholinergic medications currently available for hyperhidrosis was also not considered to be worthwhile. Patients and clinicians agreed that a trial of BTX (plus an anaesthetic) compared with iontophoresis for palmar hyperhidrosis would be useful.
Patients and clinicians were happy with the sequence of treatments identified as being cost-effective in the modelling exercise: iontophoresis, BTX, medication, curettage, ETS.
All patient advisors agreed that the HidroQoL tool was superior to the other tools commonly used in hyperhidrosis research (HDSS, DLQI, HQLQ) for assessing quality of life. Patients considered that the HidroQoL tool should be the primary outcome in future studies and that measuring the actual amount of sweat produced should be considered only as a secondary outcome.
Chapter 9 Discussion
Summary of findings
The aim of this research was to establish the expected value of undertaking additional clinical studies (such as RCTs) to determine the most clinically effective and cost-effective interventions for the management of refractory primary hyperhidrosis in secondary care. The key objectives were to undertake an evidence synthesis by systematic review to estimate clinical effectiveness and safety of treatments that would be available in secondary care, to inform key clinical parameters for a decision model, to develop a decision model to estimate cost-effectiveness and, using the decision model, to undertake a VOI analysis to determine the need for further research and to help inform the design of future clinical studies.
Clinical effectiveness and safety of second-line treatments for primary hyperhidrosis
Fifty studies were included in the systematic review of secondary care interventions for the treatment of primary hyperhidrosis. Despite the large number of studies, the evidence for the effectiveness and safety of second-line treatments of primary hyperhidrosis is limited overall: most studies were small, rated as being a high risk of bias and poorly reported. There were no studies assessing the clinical effectiveness of propantheline bromide, oral glycopyrrolate or iontophoresis applied to the axilla. Evidence was very limited regarding the newer, energy-based, ‘destructive’, technologies. Overall, there was insufficient evidence to draw firm conclusions regarding the relative effectiveness and safety of any active second-line treatments for hyperhidrosis.
There is moderate-quality evidence of a large effect of subcutaneous BTX on symptoms of axillary hyperhidrosis in the short and medium term (up to 16 weeks), and of a small to moderate positive effect on quality of life in the short term (4 weeks), compared with placebo. There is some limited evidence for BTX for palmar hyperhidrosis and very low-quality but consistent evidence of a small treatment effect with iontophoresis for palmar hyperhidrosis. Therefore, a trial of BTX compared with iontophoresis for palmar hyperhidrosis may be warranted.
The evidence regarding the effectiveness of BTX injections to the axillae compared with curettage is very low quality and uncertain, although there is no evidence to suggest that curettage is more effective than BTX in the short to medium term and there is evidence to suggest a higher incidence of adverse events with curettage. Trials are too short term to explore the potential curative nature of curettage, compared with the retreatment needed with BTX.
Review of quality-of-life measures/tools
The narrative review of quality-of-life measures/tools commonly used in hyperhidrosis research included 184 studies, many of which used two or more tools. Twenty-two individual tools for measuring quality of life were identified from the selected studies. In addition, 32 studies were identified that reported quality-of-life outcomes although the method used to measure quality of life was not reported. The DLQI, the HDSS and the HQLQ were used more often than any other tool for measuring quality of life in hyperhidrosis. The HidroQoL is the most recent tool to be designed and validated for measuring the quality of life of patients with hyperhidrosis.
Cost-effectiveness
The cost-effectiveness model considered only axillary hyperhidrosis, owing to a lack of evidence to support modelling other body sites.
The probabilistic base case suggested that IBMCE is the most cost-effective treatment sequence. A number of sensitivity analyses were conducted, and in general the model results were found to be quite robust.
Although no clinical studies were identified to inform the effectiveness of iontophoresis sponge for the axilla, sensitivity analysis for the base-case model demonstrated that iontophoresis needs to have very low effectiveness for it to be displaced as a first-line treatment choice; the response rate of iontophoresis sponge would need to be lower than 0.10, which is lower than the placebo response rate (0.13) for medication and BTX. The monthly cost of iontophoresis is substantially lower than that of either BTX or medication. In the base case, the patient has to pay for iontophoresis at home; however, even if the NHS paid for the iontophoresis device, the monthly cost is around £5.60, compared with £39.55 for propantheline bromide and £26 for BTX, assuming 6-monthly injections. Even if BTX injections were annual, the cost would be £13 per month.
The greatest value to the NHS is to keep patients on the most cost-effective treatments if the patients respond to that treatment. The most cost-effective treatments will be early in a treatment sequence. Reducing the number of patients who move on to more expensive treatments will result in cost savings. For the NHS, encouraging patients to remain on iontophoresis if they achieve a response will achieve this. The only negative consequence of experimenting with a cheaper treatment is that it is possible that a patient may not try a more cost-effective treatment later in the sequence. Current clinical practice, according to which the patient is expected to purchase an iontophoresis machine, and the base-case model presented do not account for patients being unable to pay for an iontophoresis device. In addition to the equity concerns of the ability to pay for an iontophoresis home device, there are also cost implications to the NHS of patients who elect not to purchase a machine, but rather move on to the next more costly treatment. Movement to the next treatment is a consequence of affordability, rather than lack of response to first-line treatment. Sensitivity analysis showed that in a scenario where the NHS loan individuals iontophoresis devices, rather than patients incurring the costs of purchase, iontophoresis remains a cost-effective first-line treatment option.
After iontophoresis, BTX is the next most cost-effective option, being effective and the next cheapest option.
Further to the affordability and equity issues, there is regional inequity in access to treatments. In a survey of dermatologists, 78% of respondents stated that iontophoresis was available and 58% of respondents stated that BTX was available.
Although the structure of the model ensured that non-invasive treatments were considered first, curettage was found to be cost-effective given the model assumptions. If future evidence demonstrates that newer techniques, such as laser, are equally effective as but less invasive than curettage, these other options may come to be considered a cost-effective option earlier in the treatment sequence offered to patients.
Value-of-information analysis and future research priorities
The lack of evidence available limited the modelling and subsequent VOI analysis to the axilla. The analysis was undertaken to determine the expected cost of decision uncertainty predicted by the model and the maximum value of further research undertaken to reduce that uncertainty. The population EVPI suggests that further research is of potential value, ranging from £4.5M to £15.8M; dependent on the WTP threshold.
Further population EVPPI analysis found that the greatest VOI was associated with the parameter ‘medication versus placebo’, ranging from £0.3M to £5.8M, reflecting the high level of uncertainty in the evidence base (OR 7.21, 95% CI 1.56 to 53.83).
The VOI analysis suggested that further research on medication compared with placebo effectiveness may be of value only if the cost-effectiveness threshold is £30,000 per QALY and the annual incidence of axillary hyperhidrosis is 2%. The impact that further research on medication compared with placebo effectiveness might have on the first- or second-line treatment options is unclear. It is likely that, even if a trial of medication compared with placebo were conducted, iontophoresis and BTX would retain their position before medication in any cost-effective treatment sequence. It would be useful to include the EQ-5D-5L instrument and a HDSS outcome, as well any preferred outcome in a future trial, in order to allow the trial results to be incorporated into the wider evidence base and to be used in a future economic evaluation.
The results of the population EVPI and EVPPI are very sensitive to the annual incidence rate of axilla hyperhidrosis and to the assumed effectiveness of iontophoresis sponge compared with placebo.
Patient and clinician perspective on research findings
In order to elicit the opinions of patients with hyperhidrosis and clinicians treating patients, an end-of-project workshop was held at Harrogate District Hospital with four patients and one dermatologist. Other clinicians provided advice during telephone meetings.
Patients and clinicians were unsurprised by the positive findings regarding BTX for hyperhidrosis of the axilla and did not consider that further research on iontophoresis for the axilla would be worthwhile. Despite the weak evidence, they believed that iontophoresis is effective in reducing palmar hyperhidrosis in some patients. Patients and clinicians agreed that a trial of BTX (plus an anaesthetic) compared with iontophoresis for palmar hyperhidrosis would be useful.
Patients and clinicians were satisfied with the sequence of treatments identified as being cost-effective in the modelling exercise: iontophoresis, BTX, medication, curettage, ETS.
A trial comparing the different anticholinergic medications currently available for hyperhidrosis was not considered to be worthwhile. Although there was interest in the new energy-based ‘destructive’ technologies as potential cures, patients and clinicians agreed that better evidence was needed before a comparative trial against BTX was warranted.
All patient advisors agreed that the HidroQoL tool is superior to the other tools commonly used in hyperhidrosis research (HDSS, DLQI, HQLQ) for assessing quality of life. Patients considered that the HidroQoL tool should be the primary outcome in future studies and that measuring the actual amount of sweat produced should be considered only as a secondary outcome.
Strengths and limitations of the review
Strengths
The systematic review of the clinical effectiveness of second-line treatments for primary hyperhidrosis used all of the best available evidence; 32 RCTs were included, as well as 17 non-RCTs and one case series for interventions where RCT evidence was lacking. Extensive searches were undertaken to identify all of the relevant research evidence. Five RCTs of subcutaneous injections of BTX compared with placebo for hyperhidrosis of the axilla were sufficiently similar to be pooled in a meta-analysis, with no evidence of significant statistical heterogeneity for most outcomes.
The clinical effectiveness results were combined with patient and clinical expertise in order to model the cost-effectiveness of the different interventions and undertake a VOI analysis. The results were presented to patients at an end-of-project workshop and their input was combined with the clinical and economic results to inform the conclusions and recommendations for further research.
A strength of the cost-effectiveness and EVPI analyses is that the clinical effectiveness evidence was based on studies identified through a systematic review of the literature. Sufficient data were available to model treatment sequences for the most common treatments for axillary hyperhidrosis in the UK and to explore the sensitivity of results to variation in parameters and assumptions for those based on little evidence. It was concluded that there was insufficient information to warrant cost-effectiveness and EVPI analyses for other body sites and for a comparison of minor surgery procedures (e.g. laser and microwave) with curettage.
Limitations
The protocol for this project included all treatments for hyperhidrosis prescribed in secondary care. However, screening and selecting the relevant literature for the clinical effectiveness review revealed that ETS, although used as part of the treatment pathway for hyperhidrosis, could not be included in a comparative review as the position of ETS in the treatment pathway is uncontestable (ETS is considered only as an intervention of last resort because of its significant risks). NICE recommends ETS for primary hyperhidrosis of the upper limb, but only for those ‘suffering from severe and debilitating primary hyperhidrosis that has been refractory to other treatments’. 19 Therefore, ETS was excluded from the systematic review of clinical effectiveness and safety of second-line treatments for hyperhidrosis, but remained in the cost-effectiveness model as the final treatment in the sequence. Recent studies of ETS have focused on the details of the surgical procedure. However, undertaking a systematic review of different specific techniques for undertaking ETS was beyond the remit of this project. Therefore, although further research on ETS may be warranted, recommendations for further research relating to ETS have not been made.
There was substantial variation among the 32 RCTs, 17 non-RCTs and one case series that were included in the systematic review of interventions for the treatment of primary hyperhidrosis. Studies were conducted in a range of different countries. Therefore, some study populations may not be representative of hyperhidrosis patients in the UK, notably because of differences in climate. In addition, the methods of outcome assessment varied between studies. Methods used to assess sweat rate varied, with some studies undertaking gravimetry under specific conditions, such as under stressful conditions or at a regulated room temperature. This may affect the generalisability and reliability of results relating to sweat rate. In addition, some studies used the iodine starch test as a primary measure of sweat rate; the iodine starch test is limited by the fact that it measures sweat area, rather than volume of sweat. Absolute and relative reductions in sweat rate may not necessarily correspond with improvement in a patient’s HRQoL. There was considerable variation in the tools used to assess HRQoL, as described in Chapter 4. Many studies used the HDSS for assessing treatment response, although there is limited evidence on the validity of this specific tool, or the various cut-off points used for responders (i.e. a 2-point reduction in HDSS score, or an improvement from HDSS 3 or 4 to HDSS 1 or 2). The evidence was considered too heterogeneous and limited to perform a NMA to address the clinical review question.
Most studies were small, rated as being at a high risk of bias and poorly reported. Only one RCT was judged to have a low overall risk of bias; 31 studies were judged to have a high overall risk of bias and for 18 studies the reporting was inadequate to judge the risk of bias.
There was a lack of long-term data for many of the interventions and, therefore, the long-term efficacy and safety of the interventions is unclear. This is particularly pertinent for interventions requiring repeated use over time and also for exploring the potential curative nature of minor surgical treatments, such as curettage.
For many of the interventions, the available data were very limited. In particular, for the newer, energy-based, ‘destructive’, technologies and new formulations of anticholinergic medications, studies were either ongoing or only recently completed. Research on two new medications, DRM04 and THVD-102, was presented at the European Academy of Dermatology and Venereology conference in Vienna in September/October 2016, but data were not available in time to be included in the review. 358 In addition, there were no studies assessing the clinical effectiveness of propantheline bromide, oral oxybutynin or iontophoresis applied to the axilla, which are used in standard NHS practice.
Where stated, most of the included studies restricted inclusion criteria to patients with moderate to severe hyperhidrosis (HDSS score of 3 or 4 points and/or a minimum sweat rate of 50 mg/5 minutes); therefore, the results may not be generalisable to patients with milder disease, who may be less willing to undergo more invasive or painful treatments.
The limitations of the evidence base for treatments for hyperhidrosis result in limitations for the modelling exercise. Only hyperhidrosis of the axilla could be modelled. In addition, HDSS and gravimetry sweating measurements are two of the most common outcomes in clinical studies of hyperhidrosis. Lack of evidence on the association between these two outcomes meant that, to facilitate modelling, the model had to be structured around HDSS measurements only.
Robust evidence for a number of key model parameters was not available: no evidence for the effectiveness of iontophoresis sponge, the long-term effectiveness of medication or the long-term toxicity of medication was identified. Assumptions regarding the effectiveness of iontophoresis sponge have an impact on the ICER results, although the ordering of treatment sequences remains the same. To inform some evidence gaps, such as the rates of withdrawal due to adverse effects and resource use, a survey of dermatologists was undertaken. It is possible that the findings of this survey are biased; however, it currently represents the best available evidence for these data.
The relative treatment effects in the model were derived from the NMA. There was considerable uncertainty in the effect estimates, which was reflected in the economic results. The quality assessment of studies included in the systematic review indicates that all of the studies included in the NMA are at high risk of bias. The presence of bias in favour of the intervention would mean that the effectiveness of the intervention compared with placebo would appear greater than it should be, but the direction of effect on relative effectiveness derived indirectly from medication and BTX placebo-controlled trials is unclear. The effectiveness results have not been adjusted for bias in this NMA, as further research would be required to estimate the degree of bias. As the direction of bias is unknown and there is already considerable uncertainty in the effect estimates, it is unlikely that the probabilistic results would change greatly by including an uninformative bias parameter. The potential for bias suggests that the EVPPI estimates for the relative effectiveness parameters are likely to be overestimates. The effect estimates used in the CEA ignored any effect of treatment order or failure on patient response to treatment as there was no evidence to inform this.
Finally, there is uncertainty in the estimate of the incidence of axillary hyperhidrosis, which was based on a US study. 226 The population EVPI and EVPPI are calculated using this statistic and this adds another layer of uncertainty, which was addressed by re-estimating the EVPI and EVPPI at different incidence rates.
Chapter 10 Conclusions
The evidence for the effectiveness and safety of second-line treatments for primary hyperhidrosis is limited overall. Most studies were small, rated as being at a high risk of bias and poorly reported. However, there is moderate-quality evidence of a large effect of BTX injections on symptoms of axillary hyperhidrosis compared with placebo. Evidence for other interventions is of low or very low quality. There was insufficient evidence to draw firm conclusions regarding the relative effectiveness and safety of second-line treatments for hyperhidrosis.
The narrative review of quality-of-life measures found that the DLQI, HDSS and HQLQ were used most frequently in hyperhidrosis research. All patients attending the end-of-project workshop preferred the new HidroQoL tool over the DLQI, HDSS or HQLQ and agreed that it captured all hyperhidrosis-related quality-of-life issues.
Although many of the data used to populate the model of axillary hyperhidrosis were derived from small studies at a high risk of bias and poorly reported, when augmented by clinical opinion and other literature the results are quite robust. Only when the effectiveness of iontophoresis sponge was reduced to less than that of placebo did iontophoresis cease to be the optimal first-line treatment choice in a cost-effective sequence at the £20,000 threshold. Only if medication were significantly more effective than BTX, would BTX be displaced from second-line treatment in cost-effective treatment sequences at the £20,000 threshold. VOI analysis showed that, although there is some value in undertaking further research to reduce decision uncertainty, it is unclear if the cost of the research would be offset by the reduction in that uncertainty.
Implications for practice
The findings of the research undertaken suggest that the treatment sequence for axillary hyperhidrosis (iontophoresis, BTX, medication, curettage, ETS) may be cost-effective within the NHS setting. When using medication, propantheline should be used first, before trying more expensive alternatives such as glycopyrrolate.
Implications for research
The VOI analysis showed that, although there is some value in undertaking further research to reduce decision uncertainty, it is unclear if the cost of the research would be offset by the reduction in that uncertainty. The VOI analysis also indicates that further research into the effectiveness of existing medications might be worthwhile, but it is unclear if such trials are of clinical importance. Research that established a robust estimate of the annual incidence of axillary hyperhidrosis in the UK population would reduce the uncertainty in future VOI analyses.
The implications from the systematic review of clinical evidence were as follows.
Botulinum toxin for hyperhidrosis of the axilla
There is sufficient evidence demonstrating the clinical effectiveness of BTX for hyperhidrosis of the axilla; therefore, there is little value in undertaking further studies of BTX compared with placebo for hyperhidrosis of the axilla. Future trials of interventions for hyperhidrosis of the axilla should use BTX as an active comparator.
Iontophoresis for palmar/plantar hyperhidrosis
Iontophoresis studies consistently show that iontophoresis is more effective than placebo/no treatment for hyperhidrosis of the palm; therefore, there is little value in undertaking further studies of iontophoresis compared with placebo/no treatment for hyperhidrosis of the palm. Iontophoresis is currently standard practice for palmar hyperhidrosis in many dermatology units.
Iontophoresis compared with botulinum toxin for palmar hyperhidrosis
A well-conducted, adequately powered, RCT of BTX (with anaesthesia) compared with iontophoresis for palmar hyperhidrosis may be warranted. The new HidroQoL tool appears appropriate for capturing hyperhidrosis-related quality-of-life issues. The cost of BTX plus anaesthesia is considerably higher than the cost iontophoresis; therefore, cost-effectiveness would also need to be assessed.
Microwave, laser, fractionated microneedle radiofrequency and ultrasound
There are ongoing studies of microwave, laser, fractionated microneedle radiofrequency and ultrasound therapies. If the results of this ongoing research are promising, then a trial comparing these new energy-based technologies with BTX for hyperhidrosis of the axilla may be warranted; patients expressed interest in a permanent treatment.
Curettage compared with botulinum toxin for hyperhidrosis of the axilla
In view of the ongoing research into less invasive energy-based technologies (microwave, laser, fractionated microneedle radiofrequency and ultrasound), a trial comparing BTX with curettage for hyperhidrosis of the axilla may not be warranted at this time. When further evidence is available on the newer energy-based technologies, then it will be clearer whether or not further research on curettage is warranted. Patients expressed interest in a permanent treatment, but were concerned about scar tissue resulting from curettage.
Comparison of different oral/topical medications: propantheline bromide, glycopyrrolate, oxybutynin, methantheline bromide and newer medications
There are ongoing/recently completed trials of new oral and topical anticholinergic medication formulations and, therefore, it is unlikely to be worthwhile undertaking further research of the anticholinergic medications currently available. Different medications may work better for some patients than others, therefore it may be difficult to power a study to find any statistically significant differences between treatments.
Acknowledgements
We would like to thank Ms Julie Halford, specialist nurse at The Hampshire Clinic, for clinical advice. In addition, we would like to thank the patient advisors who attended our workshop at Harrogate District Hospital for their comments and advice.
Contributions of authors
Ros Wade contributed to the protocol development, performed study selection, data extraction, validity assessment and synthesis of the included studies and took responsibility for writing the report.
Stephen Rice contributed to the protocol development, led the model development and undertook the network meta-analysis, cost-effectiveness and VOI analyses and contributed to the writing of the report.
Alexis Llewellyn undertook data extraction, validity assessment and synthesis of the included studies and contributed to the writing of the report.
Eoin Moloney undertook the model development, cost-effectiveness and VOI analyses and contributed to the writing of the report.
Julie Jones-Diette contributed to the protocol development, undertook study selection, data extraction and validity assessment of the included studies, took the lead in the evaluation of patient-relevant quality-of-life measures and contributed to the writing of the report.
Julija Stoniute undertook the cost-effectiveness and VOI analysis and contributed to the writing of the report.
Kath Wright contributed to the protocol development, developed the search strategies, conducted a range of searches to locate studies and wrote the sections of the report relating to the searches.
Alison M Layton contributed to the protocol development, provided advice throughout the project, contributed to the patient workshop and provided comments on drafts of the report.
Nick J Levell provided advice throughout the project and provided comments on drafts of the report.
Gerard Stansby provided advice throughout the project and provided comments on drafts of the report.
Dawn Craig contributed to the protocol development, the model development, cost-effectiveness and VOI analyses, took overall responsibility for the economic components, contributed to the writing of the report and provided comments on drafts of the report.
Nerys Woolacott is the principal investigator and led the application for funding. She took overall managerial responsibility for the project, contributed to the protocol development, undertook an advisory role in the clinical review section, contributed to the writing of the report and provided comments on all sections of the report.
Data sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Hyperhidrosis Support Group . What Is Hyperhidrosis? n.d. www.hyperhidrosisuk.org/ (accessed 21 July 2016).
- Dickmann LM, Dickmann JR. Quetiapine in the treatment of hyperhidrosis axillaris. Br J Dermatol 2010;163:1126-7. https://doi.org/10.1111/j.1365-2133.2010.09969.x.
- Doolittle J, Walker P, Mills T, Thurston J. Hyperhidrosis: an update on prevalence and severity in the United States. Arch Dermatol Res 2016;308:743-9. https://doi.org/10.1007/s00403-016-1697-9.
- Shayesteh A, Janlert U, Brulin C, Boman J, Nylander E. Prevalence and characteristics of hyperhidrosis in Sweden: a cross-sectional study in the general population. Dermatology 2016;232:586-91. https://doi.org/10.1159/000448032.
- Augustin M, Radtke MA, Herberger K, Kornek T, Heigel H, Schaefer I. Prevalence and disease burden of hyperhidrosis in the adult population. Dermatology 2013;227:10-3. https://doi.org/10.1159/000351292.
- Liu Y, Bahar R, Kalia S, Huang R, Phillips A, Su M, et al. Hyperhidrosis prevalence and demographical characteristics in dermatology outpatients in Shanghai and Vancouver. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0153719.
- Ro KM, Cantor RM, Lange KL, Ahn SS. Palmar hyperhidrosis: evidence of genetic transmission. J Vasc Surg 2002;35:382-6. https://doi.org/10.1067/mva.2002.119507.
- National Institute for Health and Care Excellence . CKS Clinical Knowledge Summary on Hyperhidrosis 2013. http://cks.nice.org.uk/hyperhidrosis (accessed 21 July 2016).
- Lee KY, Levell NJ. Turning the tide: a history and review of hyperhidrosis treatment. JRSM Open 2014;5. https://doi.org/10.1177/2042533313505511.
- Benson RA, Palin R, Holt PJ, Loftus IM. Diagnosis and management of hyperhidrosis. BMJ 2013;347. https://doi.org/10.1136/bmj.f6800.
- NHS Choices . Hyperhidrosis 2013. www.nhs.uk/conditions/Hyperhidrosis/Pages/Introduction.aspx (accessed 8 January 2015).
- Solish N, Bertucci V, Dansereau A, Hong HC, Lynde C, Lupin M, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg 2007;33:908-23. https://doi.org/10.1097/00042728-200708000-00003.
- Roustit M, Blaise S, Cracowski JL. Trials and tribulations of skin iontophoresis in therapeutics. Br J Clin Pharmacol 2014;77:63-71. https://doi.org/10.1111/bcp.12128.
- Naumann M, Dressler D, Hallett M, Jankovic J, Schiavo G, Segal KR, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of secretory disorders. Toxicon 2013;67:141-52. https://doi.org/10.1016/j.toxicon.2012.10.020.
- Cerfolio RJ, De Campos JR, Bryant AS, Connery CP, Miller DL, DeCamp MM, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 2011;91:1642-8. https://doi.org/10.1016/j.athoracsur.2011.01.105.
- Smidfelt K, Drott C. Late results of endoscopic thoracic sympathectomy for hyperhidrosis and facial blushing. Br J Surg 2011;98:1719-24. https://doi.org/10.1002/bjs.7682.
- Rieger R, Pedevilla S, Pöchlauer S. Endoscopic lumbar sympathectomy for plantar hyperhidrosis. Br J Surg 2009;96:1422-8. https://doi.org/10.1002/bjs.6729.
- Kim WO, Yoon KB, Kil HK, Yoon DM. Chemical lumbar sympathetic block in the treatment of plantar hyperhidrosis: a study of 69 patients. Dermatol Surg 2008;34:1340-5. https://doi.org/10.1111/j.1524-4725.2008.34286.x.
- National Institute for Health and Care Excellence . Endoscopic Thoracic Sympathectomy for Primary Hyperhidrosis of the Upper Limb. Interventional Procedures Guidance 487 2014. www.nice.org.uk/guidance/ipg487 (accessed 12 October 2016).
- Wollina U, Kostler E, Schonlebe J, Haroske G. Tumescent suction curettage versus minimal skin resection with subcutaneous curettage of sweat glands in axillary hyperhidrosis. Dermatol Surg 2008;34:709-16.
- Ibrahim O, Kakar R, Bolotin D, Nodzenski M, Disphanurat W, Pace N, et al. The comparative effectiveness of suction-curettage and onabotulinumtoxin-A injections for the treatment of primary focal axillary hyperhidrosis: a randomized control trial. J Am Acad Dermatol 2013;69:88-95. https://doi.org/10.1016/j.jaad.2013.02.013.
- Li Y, Li W, Lv X, Li X. A refined minimally invasive procedure for radical treatment of axillary osmidrosis: combined tumescent liposuction with subcutaneous pruning through a small incision. J Plast Reconstr Aesthet Surg 2012;65:e320-1. https://doi.org/10.1016/j.bjps.2012.05.020.
- Arneja JS, Hayakawa TE, Singh GB, Murray KA, Turner RB, Ross LL, et al. Axillary hyperhidrosis: a 5-year review of treatment efficacy and recurrence rates using a new arthroscopic shaver technique. Plast Reconstr Surg 2007;119:562-7. https://doi.org/10.1097/01.prs.0000246490.52593.ec.
- Leclere FM, Moreno-Moraga J, Alcolea JM, Vogt PM, Royo J, Cornejo P, et al. Efficacy and safety of laser therapy on axillary hyperhidrosis after one year follow-up: a randomized blinded controlled trial. Lasers Surg Med 2015;47:173-9. https://doi.org/10.1002/lsm.22324.
- Bechara FG, Georgas D, Sand M, Stücker M, Othlinghaus N, Altmeyer P, et al. Effects of a long-pulsed 800-nm diode laser on axillary hyperhidrosis: a randomized controlled half-side comparison study. Dermatol Surg 2012;38:736-40. https://doi.org/10.1111/j.1524-4725.2012.02339.x.
- Letada PR, Landers JT, Uebelhoer NS, Shumaker PR. Treatment of focal axillary hyperhidrosis using a long-pulsed Nd:YAG 1064 nm laser at hair reduction settings. J Drugs Dermatol 2012;11:59-63.
- Glaser DA, Coleman WP, Fan LK, Kaminer MS, Kilmer SL, Nossa R, et al. A randomized, blinded clinical evaluation of a novel microwave device for treating axillary hyperhidrosis: the dermatologic reduction in underarm perspiration study. Dermatol Surg 2012;38:185-91. https://doi.org/10.1111/j.1524-4725.2011.02250.x.
- Fatemi Naeini F, Abtahi-Naeini B, Pourazizi M, Nilforoushzadeh MA, Mirmohammadkhani M. Fractionated microneedle radiofrequency for treatment of primary axillary hyperhidrosis: a sham control study. Australas J Dermatol 2015;56:279-84. https://doi.org/10.1111/ajd.12260.
- Nestor MS, Park H. Safety and efficacy of micro-focused ultrasound plus visualization for the treatment of axillary hyperhidrosis. J Clin Aesthet Dermatol 2014;7:14-21.
- Systematic Reviews. CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91-2. https://doi.org/10.1016/j.jcms.2010.11.001.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4. https://doi.org/10.1186/2046-4053-4-1.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Synth Methods 2014;5:79-85. https://doi.org/10.1002/jrsm.1090.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011. www.cochrane-handbook.org (accessed 14 December 2016).
- Dahl JC, Glent-Madsen L. Treatment of hyperhidrosis manuum by tap water iontophoresis. Acta Derm Venereol 1989;69:346-8.
- Dolianitis C, Scarff CE, Kelly J, Sinclair R. Iontophoresis with glycopyrrolate for the treatment of palmoplantar hyperhidrosis. Australas J Dermatol 2004;45:208-12. https://doi.org/10.1111/j.1440-0960.2004.00098.x.
- Karakoç Y, Aydemir EH, Kalkan MT, Unal G. Safe control of palmoplantar hyperhidrosis with direct electrical current. Int J Dermatol 2002;41:602-5. https://doi.org/10.1046/j.1365-4362.2002.01473.x.
- Karakoc Y, Aydemir EH, Kalkan MT. Placebo-controlled evaluation of direct electrical current administration for palmoplantar hyperhidrosis. Int J Dermatol 2004;43:503-5. https://doi.org/10.1111/j.1365-4632.2004.02122.x.
- Shimizu H, Tamada Y, Shimizu J, Ohshima Y, Matsumoto Y, Sugenoya J. Effectiveness of iontophoresis with alternating current (AC) in the treatment of patients with palmoplantar hyperhidrosis. J Dermatol 2003;30:444-9. https://doi.org/10.1111/j.1346-8138.2003.tb00414.x.
- Stolman LP. Treatment of excess sweating of the palms by iontophoresis. Arch Dermatol 1987;123:893-6. https://doi.org/10.1001/archderm.1987.01660310061015.
- Na GY, Park BC, Lee WJ, Park DJ, Kim DW, Kim MN. Control of palmar hyperhidrosis with a new ‘dry-type’ iontophoretic device. Dermatol Surg 2007;33:57-61.
- Choi YH, Lee SJ, Kim DW, Lee WJ, Na GY. Open clinical trial for evaluation of efficacy and safety of a portable ‘dry-type’ iontophoretic device in treatment of palmar hyperhidrosis. Dermatol Surg 2013;39:578-83. https://doi.org/10.1111/dsu.12099.
- Akbar TM, Saqib MA, Fahim S, Nasir M, Nabi H. Efficacy and safety of tap water iontophoresis for palmoplantar hyperhidrosis. J Pakistan Assoc Dermatol 2013;23:304-9.
- Chia HY, Tey HL, Chong WS. Efficacy of using glycopyrronium bromide weekly for four weeks in the iontophoretic treatment of palmar hyperhidrosis. Ann Acad Med Singapore 2010;1.
- Hölzle E, Alberti N. Long-term efficacy and side effects of tap water iontophoresis of palmoplantar hyperhidrosis – the usefulness of home therapy. Dermatologica 1987;175:126-35. https://doi.org/10.1159/000248810.
- Ohshima Y, Shimizu H, Yanagishita T, Watanabe D, Tamada Y, Sugenoya J, et al. Changes in Na+, K+ concentrations in perspiration and perspiration volume with alternating current iontophoresis in palmoplantar hyperhidrosis patients. Arch Dermatol Res 2008;300:595-600. https://doi.org/10.1007/s00403-008-0877-7.
- Shams K, Kavanagh GM. Immediate reduction in sweat secretion with electric current application in primary palmar hyperhidrosis. Arch Dermatol 2011;147:241-2. https://doi.org/10.1001/archdermatol.2010.300.
- Wollina U, Uhlemann C, Elstermann D, Köber L, Barta U. Therapy of hyperhidrosis with tap water iontophoresis. Positive effect on healing time and lack of recurrence in hand-foot eczema. Hautarzt 1998;49:109-13. https://doi.org/10.1007/s001050050709.
- Hyun MY, Son IP, Lee Y, Choi HG, Park KY, Li K, et al. Efficacy and safety of topical glycopyrrolate in patients with facial hyperhidrosis: a randomized, multicentre, double-blinded, placebo-controlled, split-face study. J Eur Acad Dermatol Venereol 2015;29:278-82. https://doi.org/10.1111/jdv.12518.
- Mehrotra S, Schmith VD, Dumitrescu TP, Gobburu J. Longitudinal dose-response modeling for topical glycopyrrolate, an anti-hyperhidrosis agent. Clin Pharmacol Ther 2014;95:S13-14.
- Mehrotra S, Schmith VD, Dumitrescu TP, Gobburu J. Pharmacometrics-guided drug development of antihyperhidrosis agents. J Clin Pharmacol 2015;55:1256-67. https://doi.org/10.1002/jcph.536.
- Hale DR, MacKenzie AI, Kavanagh GM. Our experience of glycopyrrolate 2% cream for axillary hyperhidrosis. Br J Dermatol 2014;170. https://doi.org/10.1111/bjd.12857.
- Lee HH, Kim DW, Kim DW, Kim C. Efficacy of glycopyrrolate in primary hyperhidrosis patients. Korean J Pain 2012;25:28-32. https://doi.org/10.3344/kjp.2012.25.1.28.
- Mackenzie A, Burns C, Kavanagh G. Topical glycopyrrolate for axillary hyperhidrosis. Br J Dermatol 2013;169:483-4. https://doi.org/10.1111/bjd.12320.
- Costa Ada S, Leao LE, Succi JE, Perfeito JA, Filho Castelo A, Rymkiewicz E, et al. Randomized trial – oxybutynin for treatment of persistent plantar hyperhidrosis in women after sympathectomy. Clinics 2014;69:101-5. https://doi.org/10.6061/clinics/2014(02)05.
- Costa AS, Leao LEV, Miotto A. Variation in response with oral oxybutynin in women with persistent plantar hyperhidrosis after thoracoscopic sympathectomy. Am J Respir Crit Care Med 2015;191.
- Schollhammer M, Brenaut E, Menard-Andivot N, Pillette-Delarue M, Zagnoli A, Chassain-Le Lay M, et al. Oxybutynin as a treatment for generalized hyperhidrosis: a randomized, placebo-controlled trial. Br J Dermatol 2015;173:1163-8. https://doi.org/10.1111/bjd.13973.
- Wolosker N, de Campos JR, Kauffman P, Puech-Leão P. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg 2012;55:1696-700. https://doi.org/10.1016/j.jvs.2011.12.039.
- Try C, Messikh R, Elkhyat A, Aubin F, Humbert RP. Use of oral oxybutynin at 7.5 mg per day in primary hyperhidrosis. Rev Med Liege 2012;67:520-6.
- Wolosker N, de Campos JR, Kauffman P, Neves S, Yazbek G, Jatene FB, et al. An alternative to treat palmar hyperhidrosis: use of oxybutynin. Clin Auton Res 2011;21:389-93. https://doi.org/10.1007/s10286-011-0128-4.
- Wolosker N, de Campos JR, Kauffman P, Munia MA, Neves S, Jatene FB, et al. The use of oxybutynin for treating facial hyperhidrosis. An Bras Dermatol 2011;86:451-6. https://doi.org/10.1590/S0365-05962011000300005.
- Wolosker N, de Campos JR, Kauffman P, Yazbek G, Neves S, Puech-Leao P. Use of oxybutynin for treating plantar hyperhidrosis. Int J Dermatol 2013;52:620-3. https://doi.org/10.1111/j.1365-4632.2012.05746.x.
- Wolosker N, Teivelis MP, Krutman M, de Paula RP, de Campos JR, Kauffman P, et al. Long-term results of oxybutynin treatment for palmar hyperhidrosis. Clin Auton Res 2014;24:297-303. https://doi.org/10.1007/s10286-014-0264-8.
- Wolosker N, Teivelis MP, Krutman M, Campbell TP, Kauffman P, Campos JR, et al. Long-term results of oxybutynin use in treating facial hyperhidrosis. An Bras Dermatol 2014;89:912-16. https://doi.org/10.1590/abd1806-4841.20143272.
- Wolosker N, Teivelis MP, Krutman M, de Paula RP, Schvartsman C, Kauffman P, et al. Long-term efficacy of oxybutynin for palmar and plantar hyperhidrosis in children younger than 14 years. Pediatr Dermatol 2015;32:663-7. https://doi.org/10.1111/pde.12385.
- Hund M, Sinkgraven R, Rzany B. Randomized, placebo-controlled, double blind clinical trial for the evaluation of the efficacy and safety of oral methanthelinium bromide (Vagantin) in the treatment of focal hyperhidrosis. J Dtsch Dermatol Ges 2004;2:343-9. https://doi.org/10.1046/j.1439-0353.2004.04765.x.
- Müller C, Berensmeier A, Hamm H, Dirschka T, Reich K, Fischer T, et al. Efficacy and safety of methantheline bromide (Vagantin®) in axillary and palmar hyperhidrosis: results from a multicenter, randomized, placebo-controlled trial. J Eur Acad Dermatol Venereol 2013;27:1278-84.
- Fuchslocher M, Rzany B. Oral anticholinergic therapy of focal hyperhidrosis with methanthelinium bromide (Vagantin). Initial data on effectiveness. Hautarzt 2002;53:151-2. https://doi.org/10.1007/s00105-001-0328-2.
- Glogau RG. Topically applied botulinum toxin type A for the treatment of primary axillary hyperhidrosis: results of a randomized, blinded, vehicle-controlled study. Dermatol Surg 2007;33:S76-80. https://doi.org/10.1111/j.1524-4725.2006.32335.x.
- Balzani A, Lupi F, Pezza M, Amoruso F, Palese E, Panetta C, et al. Botulinum toxin type A in the treatment of axillary hyperhidrosis. Dermatol Clinica 2001;21:117-20.
- Baumann L, Slezinger A, Halem M, Vujevich J, Martin LK, Black L, et al. Pilot study of the safety and efficacy of Myobloc (botulinum toxin type B) for treatment of axillary hyperhidrosis. Int J Dermatol 2005;44:418-24. https://doi.org/10.1111/j.1365-4632.2004.02531.x.
- Baumann L, Slezinger A, Halem M, Vujevich J, Mallin K, Charles C, et al. Double-blind, randomized, placebo-controlled pilot study of the safety and efficacy of Myobloc (botulinum toxin type B) for the treatment of palmar hyperhidrosis. Dermatol Surg 2005;31:263-70. https://doi.org/10.1097/00042728-200503000-00002.
- Heckmann M, Ceballos-Baumann AO, Plewig G. Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med 2001;344:488-93. https://doi.org/10.1056/NEJM200102153440704.
- Lowe NJ, Yamauchi PS, Lask GP, Patnaik R, Iyer S. Efficacy and safety of botulinum toxin type a in the treatment of palmar hyperhidrosis: a double-blind, randomized, placebo-controlled study. Dermatol Surg 2002;28:822-7. https://doi.org/10.1097/00042728-200209000-00008.
- Lowe NJ, Glaser DA, Eadie N, Daggett S, Kowalski JW, Lai PY, et al. Botulinum toxin type A in the treatment of primary axillary hyperhidrosis: a 52-week multicenter double-blind, randomized, placebo-controlled study of efficacy and safety. J Am Acad Dermatol 2007;56:604-11. https://doi.org/10.1016/j.jaad.2007.01.009.
- Lowe NJ, Naumann MK, Hamm H. Effect of botulinum toxin type A on quality of life measures in patients with excessive anxillary sweating: a randomized controlled trial. J Invest Dermatol 2002;119.
- Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ 2001;323:596-9. https://doi.org/10.1136/bmj.323.7313.596.
- Naumann M, Lowe NJ, Kumar CR, Hamm H. Hyperhidrosis Clinical Investigators Group. Botulinum toxin type a is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol 2003;139:731-6. https://doi.org/10.1001/archderm.139.6.731.
- Naver H, Swartling C, Aquilonius SM. Palmar and axillary hyperhidrosis treated with botulinum toxin: one-year clinical follow-up. Eur J Neurol 2000;7:55-62. https://doi.org/10.1046/j.1468-1331.2000.00014.x.
- Odderson IR. Long-term quantitative benefits of botulinum toxin type A in the treatment of axillary hyperhidrosis. Dermatol Surg 2002;28:480-3.
- Ohshima Y, Tamada Y, Yokozeki H, Maeda T, Endo A, Senta T, et al. The efficacy and safety of botulinum toxin type a in patients with primary axillary hyperhidrosis. Nishinihon J Dermatol 2013;75:357-64. https://doi.org/10.2336/nishinihonhifu.75.357.
- Schnider P, Binder M, Auff E, Kittler H, Berger T, Wolff K. Double-blind trial of botulinum A toxin for the treatment of focal hyperhidrosis of the palms. Br J Dermatol 1997;136:548-52. https://doi.org/10.1111/j.1365-2133.1997.tb02139.x.
- Schnider P, Binder M, Kittler H, Birner P, Starkel D, Wolff K, et al. A randomized, double-blind, placebo-controlled trial of botulinum A toxin for severe axillary hyperhidrosis. Br J Dermatol 1999;140:677-80. https://doi.org/10.1046/j.1365-2133.1999.02769.x.
- Naumann MK, Hamm H, Lowe NJ. Botox Hyperhidrosis Clinical Study Group. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. Br J Dermatol 2002;147:1218-26. https://doi.org/10.1046/j.1365-2133.2002.05059.x.
- Ibrahim O, West D, Veledar E, Becker L, Alam M, Kakar R. Comparative effectiveness of suction-curettage and onabotulinumtoxin-A injections for the treatment of primary focal axillary hyperhidrosis. J Am Acad Dermatol 2013;1. https://doi.org/10.1016/j.jaad.2012.12.906.
- Rajagopal R, Mallya NB. Comparative evaluation of botulinum toxin versus iontophoresis with topical aluminium chloride hexahydrate in treatment of palmar hyperhidrosis. Med J Armed Forces India 2014;70:247-52. https://doi.org/10.1016/j.mjafi.2014.01.008.
- Heckmann M, Breit S, Ceballos-Baumann A, Schaller M, Plewig G. Side-controlled intradermal injection of botulinum toxin A in recalcitrant axillary hyperhidrosis. J Am Acad Dermatol 1999;41:987-90. https://doi.org/10.1016/S0190-9622(99)70258-6.
- Yamashita N, Shimizu H, Kawada M, Yanagishita T, Watanabe D, Tamada Y, et al. Local injection of botulinum toxin A for palmar hyperhidrosis: usefulness and efficacy in relation to severity. J Dermatol 2008;35:325-9. https://doi.org/10.1111/j.1346-8138.2008.00478.x.
- Baker D. Topical glycopyrrolate spray 2% reduces axillary hyperhidrosis to a similar extent as Botox injections. Br J Dermatol 2013;169.
- Wachal K, Bucko W, Staniszewski R, Majewska N, Blaszak M. Estimating the subjective efficiency after treatment of upper extremity hyperhidrosis using various methods. Postepy Dermatol Alergol 2009;26:501-5.
- Absar MS, Onwudike M. Efficacy of botulinum toxin type A in the treatment of focal axillary hyperhidrosis. Dermatol Surg 2008;34:751-5. https://doi.org/10.1111/j.1524-4725.2008.34142.x.
- Maltese K, Ryndel M, Alm-Dahlgren J, Bergh C, Ekelund AC, Elmer-Lans G, et al. Botulinum Toxin Treatment of Axillary and Palmar Hyperhidrosis. Göteborg, Sweden: RegionVästra Götaland, Sahlgrenska University Hospital, HTA-centre; 2012.
- Shayesteh A, Nylander E. Botulinum toxin helps against primary local hyperhidrosis. Good effect – few adverse effects according to a literature review. Lakartidningen 2011;108:2433-5.
- Aghaei S. Botulinum toxin therapy for palmar hyperhidrosis: experience in an Iranian population. Int J Dermatol 2007;46:212-14. https://doi.org/10.1111/j.1365-4632.2007.02928.x.
- Baker D, Hansen E. The psychological impact of axillary hyperhidrosis is not altered by Botox treatment. Br J Dermatol 2011;165.
- Basciani M, Di Rienzo F, Bizzarrini M, Zanchi M, Copetti M, Intiso D. Efficacy of botulinum toxin type B for the treatment of primary palmar hyperhidrosis: a prospective, open, single-blind, multi-centre study. Arch Dermatol Res 2014;306:497-503. https://doi.org/10.1007/s00403-014-1449-7.
- Bechara FG, Sand M, Achenbach RK, Sand D, Altmeyer P, Hoffmann K. Focal hyperhidrosis of the anal fold: successful treatment with botulinum toxin A. Dermatol Surg 2007;33:924-7. https://doi.org/10.1097/00042728-200708000-00004.
- Böger A, Herath H, Rompel R, Ferbert A. Botulinum toxin for treatment of craniofacial hyperhidrosis. J Neurol 2000;247:857-61. https://doi.org/10.1007/s004150070073.
- Campanati A, Penna L, Guzzo T, Menotta L, Silvestri B, Lagalla G, et al. Quality-of-life assessment in patients with hyperhidrosis before and after treatment with botulinum toxin: results of an open-label study. Clin Ther 2003;25:298-30. https://doi.org/10.1016/S0149-2918(03)90041-5.
- Campanati A, Sandroni L, Gesuita R, Giuliano A, Giuliodori K, Marconi B, et al. Treatment of focal idiopathic hyperhidrosis with botulinum toxin type A: clinical predictive factors of relapse-free survival. J Eur Acad Dermatol Venereol 2011;25:917-21. https://doi.org/10.1111/j.1468-3083.2010.03880.x.
- Chattopadhyay M, Fuller C, Morris-Jones R. Assessment of botulinum toxin a for primary axillary hyperhidrosis using a modified Dermatology Life Quality Index questionnaire. Br J Dermatol 2009;161.
- Chow A, Wilder-Smith EP. Effect of transdermal botulinum toxin on sweat secretion in subjects with idiopathic palmar hyperhidrosis. Br J Dermatol 2009;160:721-2. https://doi.org/10.1111/j.1365-2133.2008.09018.x.
- Coutinho dos Santos LH, Gomes AM, Giraldi S, Abagge KT, Marinoni LP. Palmar hyperhidrosis: long-term follow-up of nine children and adolescents treated with botulinum toxin type A. Pediatr Dermatol 2009;26:439-44. https://doi.org/10.1111/j.1525-1470.2009.00949.x.
- D’Epiro S, Macaluso L, Salvi M, Luci C, Mattozzi C, Marzocca F, et al. Safety and prolonged efficacy of botulin toxin A in primary hyperhidrosis. Clin Ter 2014;165:e395-e400.
- Glaser DA, Kowalski J, Ravelo A, Weng E. Functional and dermatology-specific quality of life benefits with repeated botulinum toxin type A treatment of primary axillary hyperhidrosis over 4 years. Abstract P569. American Academy of Dermatology 65th Annual Meeting, 2–6 February 2007. J Am Acad Dermatol 2007;56.
- Glaser DA, Pariser DM, Hebert AA, Landells I, Somogyi C, Weng E, et al. A prospective, nonrandomized, open-label study of the efficacy and safety of onabotulinumtoxinA in adolescents with primary axillary hyperhidrosis. Pediatr Dermatol 2015;32:609-17. https://doi.org/10.1111/pde.12620.
- Glogau RG. Botulinum A neurotoxin for axillary hyperhidrosis. No sweat Botox. Dermatol Surg 1998;24:817-19. https://doi.org/10.1111/j.1524-4725.1998.tb04257.x.
- Gregoriou S, Rigopoulos D, Makris M, Liakou A, Agiosofitou E, Stefanaki C, et al. Effects of botulinum toxin-a therapy for palmar hyperhidrosis in plantar sweat production. Dermatol Surg 2010;36:496-8. https://doi.org/10.1111/j.1524-4725.2010.01473.x.
- Hanlon L, Cahill R, Barry MC. Prospective evaluation of the efficacy of dermal botulinum toxin for primary axillary hyperhidrosis. Ir J Med Sci 2006;175:57-8. https://doi.org/10.1007/BF03169003.
- Hasson NA, Kam CS, Cataldo CK. Toxina botulinica en el tratamiento de la hiperhidrosis focal primaria. Dermatol Rev Mex 2014;58:331-8.
- Heckmann M, Breit S, Ceballos-Baumann A, Schaller M, Plewig G. Axillary hyperhidrosis: successful treatment with botulinum toxin A. Hautarzt 1998;49:101-3. https://doi.org/10.1007/s001050050707.
- Ito K, Yanagishita T, Ohshima Y, Tamada Y, Watanabe D. Therapeutic effectiveness of botulinum toxin type A based on severity of palmar hyperhidrosis. J Dermatol 2011;38:859-63. https://doi.org/10.1111/j.1346-8138.2011.01214.x.
- James R, Phillips D, Collin J. Durability of botulinum toxin injection for axillary hyperhidrosis. Br J Surg 2005;92:834-5. https://doi.org/10.1002/bjs.5001.
- Karlqvist M, Rosell K, Rystedt A, Hymnelius K, Swartling C. Botulinum toxin B in the treatment of craniofacial hyperhidrosis. J Eur Acad Dermatol Venereol 2014;28:1313-17. https://doi.org/10.1111/jdv.12278.
- Karlqvist M, Rosell K, Rystedt A, Hymnelius K, Swartling C. Botulinum neurotoxin B in the treatment of craniofacial hyperhidrosis. Toxicon 2015;93. https://doi.org/10.1016/j.toxicon.2014.11.120.
- Kinkelin I, Hund M, Naumann M, Hamm H. Effective treatment of frontal hyperhidrosis with botulinum toxin A. Br J Dermatol 2000;143:824-7. https://doi.org/10.1046/j.1365-2133.2000.03839.x.
- Kouris A, Armyra K, Stefanaki C, Christodoulou C, Karimali P, Kontochristopoulos G. Quality of life and social isolation in pediatric patients with primary focal hyperhidrosis treated with botulinum toxin type A. J Dtsch Dermatol Ges 2014;12.
- Kouris A, Armyra K, Christodoulou C, Karimali P, Karypidis D, Kontochristopoulos G. Quality of life in patients with focal hyperhidrosis before and after treatment with botulinum toxin A. ISRN Dermatol 2014;2014. https://doi.org/10.1155/2014/308650.
- Kouris A, Armyra K, Stefanaki C, Christodoulou C, Karimali P, Kontochristopoulos G. Quality of life and social isolation in Greek adolescents with primary focal hyperhidrosis treated with botulinum toxin type A: a case series. Pediatr Dermatol 2015;32:226-30. https://doi.org/10.1111/pde.12480.
- Krogstad AL, Skymne BS, Göran Pegenius BS, Elam M, Wallin BG. Evaluation of objective methods to diagnose palmar hyperhidrosis and monitor effects of botulinum toxin treatment. Clin Neurophysiol 2004;115:1909-16. https://doi.org/10.1016/j.clinph.2004.03.012.
- Krogstad AL, Skymne A, Pegenius G, Elam M, Wallin BG. No compensatory sweating after botulinum toxin treatment of palmar hyperhidrosis. Br J Dermatol 2005;152:329-33. https://doi.org/10.1111/j.1365-2133.2004.06255.x.
- Lowe PL, Cerdan-Sanz S, Lowe NJ. Botulinum toxin type A in the treatment of bilateral primary axillary hyperhidrosis: efficacy and duration with repeated treatments. Dermatol Surg 2003;29:545-8. https://doi.org/10.1097/00042728-200305000-00019.
- Moffat CE, Hayes WG, Nyamekye IK. Durability of botulinum toxin treatment for axillary hyperhidrosis. Eur J Vasc Endovasc Surg 2009;38:188-91. https://doi.org/10.1016/j.ejvs.2009.03.016.
- Naumann M, Hofmann U, Bergmann I, Hamm H, Toyka KV, Reiners K. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol 1998;134:301-4. https://doi.org/10.1001/archderm.134.3.301.
- Naver H, Swartling C, Aquilonius SM. Treatment of focal hyperhidrosis with botulinum toxin type A. Brief overview of methodology and 2 years’ experience. Eur J Neurol 1999;6:S117-20. https://doi.org/10.1111/j.1468-1331.1999.tb00028.x.
- Swartling C, Naver H, Lindberg M. Botulinum A toxin improves life quality in severe primary focal hyperhidrosis. Eur J Neurol 2001;8:247-52. https://doi.org/10.1046/j.1468-1331.2001.00207.x.
- Nelson L, Bachoo P, Holmes J. Botulinum toxin type B: a new therapy for axillary hyperhidrosis. Br J Plast Surg 2005;58:228-32. https://doi.org/10.1016/j.bjps.2004.07.003.
- Ogden E, Farley E, Schofield JK. Botulinum toxin service for axillary hyperhidrosis: patient outcomes. Br J Dermatol 2009;161:43-4.
- Paracka L, Kollewe K, Dengler R, Dressler D. Botulinum toxin therapy for hyperhidrosis: reduction of injection site pain by nitrous oxide/oxygen mixtures. J Neural Transm 2015;122:1279-82. https://doi.org/10.1007/s00702-015-1372-x.
- Pérez-Bernal AM, Avalos-Peralta P, Moreno-Ramírez D, Camacho F. Treatment of palmar hyperhidrosis with botulinum toxin type A: 44 months of experience. J Cosmet Dermatol 2005;4:163-6. https://doi.org/10.1111/j.1473-2165.2005.00304.x.
- Pinelli S, Beretta D, Signorini M. Botulinum toxin: a new tool in the treatment of focal hyperhidrosis. Eur J Plast Surg 2000;23:256-60. https://doi.org/10.1007/s002380000130.
- Rosell K, Hymnelius K, Swartling C. Botulinum toxin type A and B improve quality of life in patients with axillary and palmar hyperhidrosis. Acta Derm Venereol 2013;93:335-9. https://doi.org/10.2340/00015555-1464.
- Salmanpoor R, Rahmanian MJ. Treatment of axillary hyperhidrosis with botulinum-A toxin. Int J Dermatol 2002;41:428-30. https://doi.org/10.1046/j.1365-4362.2002.01448.x.
- Scamoni S, Valdatta L, Frigo C, Maggiulli F, Cherubino M. Treatment of primary axillary hyperhidrosis with botulinum toxin type a: our experience in 50 patients from 2007 to 2010. ISRN Dermatol 2012;2012. https://doi.org/10.5402/2012/702714.
- Schnider P, Moraru E, Kittler H, Binder M, Kranz G, Voller B, et al. Treatment of focal hyperhidrosis with botulinum toxin type A: long-term follow-up in 61 patients. Br J Dermatol 2001;145:289-93. https://doi.org/10.1046/j.1365-2133.2001.04349.x.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol 1998;38:227-9. https://doi.org/10.1016/S0190-9622(98)70242-7.
- Solish N, Benohanian A, Kowalski JW. Canadian Dermatology Study Group on Health-Related Quality of Life in Primary Axillary Hyperhidrosis . Prospective open-label study of botulinum toxin type A in patients with axillary hyperhidrosis: effects on functional impairment and quality of life. Dermatol Surg 2005;31:405-13. https://doi.org/10.1097/00042728-200504000-00006.
- Solomon BA, Hayman R. Botulinum toxin type A therapy for palmar and digital hyperhidrosis. J Am Acad Dermatol 2000;42:1026-9. https://doi.org/10.1067/mjd.2000.105156.
- Vadoud-Seyedi J. Treatment of plantar hyperhidrosis with botulinum toxin type A. Int J Dermatol 2004;43:969-71. https://doi.org/10.1111/j.1365-4632.2004.02304.x.
- von Rhein M, Bruck C, Jost WH. Botulinumtoxin Typ A: dosisfindung bei der behandlung der axillären hyperhidrose. Aktuelle Derm 2004;30:69-72. https://doi.org/10.1055/s-2004-814390.
- Weber A, Heger S, Sinkgraven R, Heckmann M, Elsner P, Rzany B. Psychosocial aspects of patients with focal hyperhidrosis. Marked reduction of social phobia, anxiety and depression and increased quality of life after treatment with botulinum toxin A. Br J Dermatol 2005;152:342-5. https://doi.org/10.1111/j.1365-2133.2004.06334.x.
- Wollina U, Karamfilov T. Botulinum toxin A for palmar hyperhidrosis. J Eur Acad Dermatol Venereol 2001;15:555-8. https://doi.org/10.1046/j.1468-3083.2001.00350.x.
- Paracka L, Kollewe K, Dressler D. Botulinum neurotoxin therapy for hyperhidrosis: reduction of injection site pain by nitrous oxide/oxygen mixtures. Toxicon 2015;93. https://doi.org/10.1016/j.toxicon.2014.11.154.
- Wollina U, Karamfilov T, Konrad H. High-dose botulinum toxin type A therapy for axillary hyperhidrosis markedly prolongs the relapse-free interval. J Am Acad Dermatol 2002;46:536-40. https://doi.org/10.1067/mjd.2002.118341.
- Tronstad C, Helsing P, Tønseth KA, Grimnes S, Krogstad AL. Tumescent suction curettage vs. curettage only for treatment of axillary hyperhidrosis evaluated by subjective and new objective methods. Acta Derm Venereol 2014;94:215-20. https://doi.org/10.2340/00015555-1671.
- Rompel R, Scholz S. Subcutaneous curettage vs. injection of botulinum toxin A for treatment of axillary hyperhidrosis. J Eur Acad Dermatol Venereol 2001;15:207-11. https://doi.org/10.1046/j.1468-3083.2001.00226.x.
- Jemec B. Abrasio axillae in hyperhidrosis. Scand J Plast Reconstr Surg 1975;9:44-6. https://doi.org/10.3109/02844317509022856.
- Bechara FG, Sand M, Hoffmann K, Boorboor P, Altmeyer P, Stuecker M. Histological and clinical findings in different surgical strategies for focal axillary hyperhidrosis. Dermatol Surg 2008;34:1001-9.
- Darabaneanu S, Darabaneanu HA, Niederberger U, Russo PA, Lischner S, Hauschild A. Long-term efficacy of subcutaneous sweat gland suction curettage for axillary hyperhidrosis: a prospective gravimetrically controlled study. Dermatol Surg 2008;34:1170-7. https://doi.org/10.1097/00042728-200809000-00002.
- Feldmeyer L, Bogdan I, Moser A, Specker R, Kamarashev J, French LE, et al. Short- and long-term efficacy and mechanism of action of tumescent suction curettage for axillary hyperhidrosis. J Eur Acad Dermatol Venereol 2015;29:1933-7. https://doi.org/10.1111/jdv.13078.
- Proebstle TM, Schneiders V, Knop J. Gravimetrically controlled efficacy of subcorial curettage: a prospective study for treatment of axillary hyperhidrosis. Dermatol Surg 2002;28:1022-6. https://doi.org/10.1097/00042728-200211000-00010.
- Bechara FG, Sand M, Hoffmann K, Altmeyer P. Aggressive shaving after combined liposuction and curettage for axillary hyperhidrosis leads to more complications without further benefit. Dermatol Surg 2008;34:952-3.
- Ottomann C, Blazek J, Hartmann B, Muehlberger T. Liposuction curettage versus Botox for axillary hyperhidrosis. A prospective study of the quality of life. Chirurg 2007;78:356-61. https://doi.org/10.1007/s00104-006-1288-y.
- Hasche E, Hagedorn M, Sattler G. Subcutaneous sweat gland suction curettage in tumescent local anesthesia in hyperhidrosis axillaris. Hautarzt 1997;48:817-19. https://doi.org/10.1007/s001050050666.
- Bechara FG, Sand M, Tomi NS, Altmeyer P, Hoffmann K. Repeat liposuction-curettage treatment of axillary hyperhidrosis is safe and effective. Br J Dermatol 2007;157:739-43. https://doi.org/10.1111/j.1365-2133.2007.08092.x.
- Bechara FG, Gambichler T, Bader A, Sand M, Altmeyer P, Hoffmann K. Assessment of quality of life in patients with primary axillary hyperhidrosis before and after suction-curettage. J Am Acad Dermatol 2007;57:207-12. https://doi.org/10.1016/j.jaad.2007.01.035.
- Caplin D. Clinical evaluation and quantitative analysis of axillary hyperhidrosis treated with a unique targeted laser energy delivery method with 1 year follow up. Lasers Surg Med 2013;45:277-8.
- Chasin M. Safety and efficacy of dual 924 and 975 nm wavelength laser for the treatment of primary axillary hyperhidrosis. Lasers Surg Med 2012;44:7-8.
- Fratila A, Reckmeyer M. Gravimetric analysis of hyperhidrosis treatment using the Nd: YAG 1440 nm laser with a sidefiring fibre sidelaze 800. Lasers Surg Med 2014;46.
- Goldman A, Wollina U. Subdermal Nd-YAG laser for axillary hyperhidrosis. Dermatol Surg 2008;34:756-62. https://doi.org/10.1111/j.1524-4725.2008.34143.x.
- Hoffmann K. Treatment of hyperhidrosis using a Nd: YAG 1440nm laser. Lasers Surg Med 2012;44.
- Katz B, Cangello D. Efficacy of a 1440 nm Nd: YAG laser with a targeted energy delivery system in the treatment of hyperhidrosis. Lasers Surg Med 2014;46.
- Kim IH, Lee KG, Kim JH, Yi SM, Choi JE, Ro KW. 1444 nm accusculpt laser: a new modality for treatment of axillary bromhidrosis and hyperhidrosis. Lasers Surg Med 2010;42:87-8.
- Penrose C, Marmur E, Emer J, Phelps R. Axillary sweating decreased by laser hair removal with the diode 810-nm system. J Am Acad Dermatol 2011;1.
- Yanes FD. Laser assisted minimally invasive surgery for primary hyperhidrosis. Lasers Surg Med 2013;45.
- Canadian Agency for Drugs and Technologies in Health . Laser Therapy for Hyperhidrosis: A Review of the Clinical Effectiveness and Guidelines 2015. www.ncbi.nlm.nih.gov/books/NBK304745/?term=hyperhidrosis%20AND%20collection_cadthrdcollect%5Bfilter%5D (accessed 29 April 2016).
- Tataru A, Avram A. Laser assisted lipolysis-a new treatment in axillary hyperhidrosis. Lasers Med Sci 2013;28.
- Abtahi-Naeini B, Naeini FF, Adibi N, Pourazizi M. Quality of life in patients with primary axillary hyperhidrosis before and after treatment with fractionated microneedle radiofrequency. J Res Med Sci 2015;20:631-5. https://doi.org/10.4103/1735-1995.166196.
- Kim M, Shin JY, Lee J, Kim JY, Oh SH. Efficacy of fractional microneedle radiofrequency device in the treatment of primary axillary hyperhidrosis: a pilot study. Dermatology 2013;227:243-9. https://doi.org/10.1159/000354602.
- Kilmer S, Coleman W, Fan L, Glaser DA, Kaminer M, Nossa R, et al. A randomized, blinded clinical study of a microwave device for treatment of axillary hyperhidrosis. Lasers Surg Med 2011;43.
- Hong HC, Lupin M, O’Shaughnessy KF. Clinical evaluation of a microwave device for treating axillary hyperhidrosis. Dermatol Surg 2012;38:728-35. https://doi.org/10.1111/j.1524-4725.2012.02375.x.
- Lee SJ, Chang KY, Suh DH, Jung Ryu H, Song KY. The efficacy of microwave device for treating axillary hyperhidrosis and osmidrosis in Asians: a preliminary study. Lasers Surg Med 2013;45:269-70. https://doi.org/10.3109/14764172.2013.807114.
- Lupin M, Hong CHH, O’Shaughnessy KF. A multi-center evaluation of the miradry system to treat subjects with axillary hyperhidrosis. Lasers Surg Med 2011;43.
- Lupin M, Hong HCH, O’Shaughnessy K. Long-term evaluation of microwave treatment for axillary hyperhidrosis. Lasers Surg Med 2012;44:6-7.
- Lupin M, Hong HC, O’Shaughnessy KF. Long-term efficacy and quality of life assessment for treatment of axillary hyperhidrosis with a microwave device. Dermatol Surg 2014;40:805-7.
- Nestor M, Park H. Evaluation of the efficacy and safety of micro-focused ultrasound for the treatment of axillary hyperhidrosis. Lasers Surg Med 2012;44.
- Nestor M, Park H. Randomized, double-blind, controlled pilot study of the efficacy and safety of micro-focused ultrasound for the treatment of axillary hyperhidrosis. Lasers Surg Med 2013;45.
- Nestor M, Park H. Randomized, double-blind, controlled pilot study of the efficacy and safety of microfocused ultrasound for the treatment of axillary hyperhidrosis [P7146]. J Am Acad Dermatol 2013;68.
- Pinson I, Olisova O, Verkhogliad I, Lepselter J. Long-term sweat reduction with noninvasive short wave radiofrequency device in patients with primary axillary hyperhidrosis: a preliminary study. Lasers Surg Med 2014;46.
- Commons GW, Lim AF. Treatment of axillary hyperhidrosis/bromidrosis using VASER ultrasound. Aesthetic Plast Surg 2009;33:312-23. https://doi.org/10.1007/s00266-008-9283-y.
- Nicholas R, Quddus A, Baker DM. Treatment of primary craniofacial hyperhidrosis: a systematic review. Am J Clin Dermatol 2015;16:361-70. https://doi.org/10.1007/s40257-015-0136-6.
- Vakili D, Baker DM. Surgical micro-retrodermal axillary curettage (mRAC) effectively reduces axillary hyperhidrosis. Br J Surg 2016;103:12-3.
- Wakugawa M, Fujiyama M, Yoshida T, Ando I, Takanashi M. Half-Side Comparison Study on the Efficacy of Botulinum Toxin Type A in Patients With Axillary Hyperhidrosis n.d.
- Amir M, Arish A, Weinstein Y, Pfeffer M, Levy Y. Impairment in quality of life among patients seeking surgery for hyperhidrosis (excessive sweating): preliminary results. Isr J Psychiatry Relat Sci 2000;37:25-31.
- Haider A, Solish N. Focal hyperhidrosis: diagnosis and management. CMAJ 2005;172:69-75. https://doi.org/10.1503/cmaj.1040708.
- Sakurai M, Montagna W. The skin of primates. XVII: pharmacological properties of the sweat gland of lemur mongoz. J Invest Dermatol 1964;42:411-14. https://doi.org/10.1038/jid.1964.89.
- Hornberger J, Grimes K, Naumann M, Glaser DA, Lowe NJ, Naver H, et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol 2004;51:274-86. https://doi.org/10.1016/j.jaad.2003.12.029.
- A Study to Evaluate Clinical Effect, Pharmacokinetics, Safety, and Tolerability of Umeclidinium in Palmar Hyperhidrosis Subjects 2016. https://clinicaltrials.gov/show/NCT02673619 (accessed 14 October 2016).
- The Safety and Efficacy of THVD-102, a Combination of Oxybutynin and Pilocarpine, in Subjects With Primary Focal Hyperhidrosis 2015. www.anzctr.org.au/ACTRN12615000873527.aspx (accessed 14 October 2016).
- A Pilot Study Exploring the Efficacy and Safety of Topical Oxybutynin 3% Gel for Primary Focal Hyperhidrosis in Adolescents and Young Adults 2015. https://clinicaltrials.gov/show/NCT02633371 (accessed 14 October 2016).
- A Study to Evaluate If Long Term Treatment With DRM04 Is Safe in Patients With Extreme Underarm Sweating 2015. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2015-002163-42 (accessed 14 October 2016).
- Long-Term Safety Study of DRM04 in Subjects With Primary Axillary Hyperhidrosis 2015. https://clinicaltrials.gov/show/NCT02553798 (accessed 14 October 2016).
- Pilot Study to Investigate the Efficacy of Rapamycin Topical Medicine Against Intractable Hyperhidrosis 2014. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000018187 (accessed 14 October 2016).
- A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Efficacy and Safety of OSD-001 in Primary Palmar Hyperhidrosis (Phase II Study) 2016. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000023830 (accessed 14 October 2016).
- Botulinum Toxin Treatment in Hyperhidrosis (Excessive Sweating) 2011. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2011-003132-30 (accessed 14 October 2016).
- Comparing Two Methods of Treatment for Arm Pit Sweating 2015. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=11353 (accessed 14 October 2016).
- Tx Axillary Hyperhidrosis 1210nm Diode Laser 2014. http://clinicaltrials.gov/show/NCT02105753 (accessed 14 October 2016).
- Fractionated Microneedle Radiofrequency for Treatment of Primary Axillary Hyperhidrosis n.d. https://ClinicalTrials.gov/show/NCT02823340 (accessed 17 July 2016).
- Miradry Treatment for Focal Axillary Hyperhidrosis 2014. http://clinicaltrials.gov/show/NCT02295891 (accessed 14 October 2016).
- Ulthera® System for Treating Axillary Hyperhidrosis 2014. https://clinicaltrials.gov/show/NCT02286765 (accessed 14 October 2016).
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement instruments: an international Delphi study. Qual Life Res 2010;19:539-49. https://doi.org/10.1007/s11136-010-9606-8.
- Kamudoni P, Salek SS, Mueller B, Mueller C. Hyperhidrosis quality of life index (Hidroqol©): a novel patient reported outcome measure in hyperhidrosis. J Invest Dermatol 2012;132.
- Kowalski JW, Eadie N, Dagget S, Lai P-Y. Validity and reliability of the hyperhidrosis disease severity scale (HDSS). J Am Acad Dermatol 2004;50. https://doi.org/10.1016/j.jaad.2003.10.202.
- Liebowitz MR, Klein DF. Anxiety. New York, NY: Karger Publishers; 1987.
- Fuentes MG, Zabaleta J, Aguinagalde B, Bazterargui N, Laguna S, Izquierdo JM, et al. Thoracoscopic sympathicotomy in primary hyperhidrosis: evaluation of patient satisfaction. Interact Cardiovasc Thorac Surg 2012;15.
- Andrade R, Briones E, Rueth N, D’Cunha J, Maddaus M. Objective assessment of primary palmoplantar hyperhidrosis and thoracoscopic sympathectomy. Interact Cardiovasc Thorac Surg 2012;15.
- Lupin M, Hong HCH. Microwave-based treatment for primary axillary hyperhidrosis: six months of follow-up. J Am Acad Dermatol 2012;66.
- Garcia Franco CE, Perez-Cajaraville J, Guillen-Grima F, España A. Prospective study of percutaneous radiofrequency sympathicolysis in severe hyperhidrosis and facial blushing: efficacy and safety findings. Eur J Cardiothorac Surg 2011;40:e146-51. https://doi.org/10.1016/j.ejcts.2011.05.010.
- Delaplace M, Dumont P, Lorette G, Machet L, Lagier L, Maruani A, et al. Factors associated with long-term outcome of endoscopic thoracic sympathectomy for palmar hyperhidrosis: a questionnaire survey in a cohort of French patients. Br J Dermatol 2015;172:805-7. https://doi.org/10.1111/bjd.13273.
- Yuncu G, Turk F, Ozturk G, Atinkaya C. Comparison of only T3 and T3-T4 sympathectomy for axillary hyperhidrosis regarding treatment effect and compensatory sweating. Interact Cardiovasc Thorac Surg 2013;17:263-7. https://doi.org/10.1093/icvts/ivt160.
- Ismail H, El Samadoni A. Bilateral simultaneous thoracoscopic sympathectomy in prone position for palmar hyperhydrosis: four years outcome and quality of life for university students. Surg Endosc 2015;29.
- Pastorelli F, Plasmati R. A randomized controlled trial comparing botulinum toxin type A xeomin and dysport for treatment of primary axillary hyperhidrosis. J Neurol Sci 2013;333:e454-5. https://doi.org/10.1016/j.jns.2013.07.1623.
- Coveliers HME, Abis GSA, Wisselink W. An objective assessment of palmar sweating after robot-assisted highly selective dorsal sympathectomy. Int J Med Robot 2011;7.
- Sanli A, Onen A, Karapolat S, Eyuboglu GM, Tasdogen A, Ulugun I, et al. Evaluation of quality of life after bilateral endoscopic thoracic sympathectomy for primary hyperhidrosis. Adv Clin Exp Med 2010;19:619-24.
- Amini M, Harmsze AM, Tupker RA. Patient’s estimation of efficacy of various hyperhidrosis treatments in a dermatological clinic. Acta Derm Venereol 2008;88:356-62.
- Flanagan KH, King R, Glaser DA. Botulinum toxin type a versus topical 20% aluminum chloride for the treatment of moderate to severe primary focal axillary hyperhidrosis. J Drugs Dermatol 2008;7:221-7.
- Attia A, Salah M. New topical photodynamic therapy for management of primary axillary hyperhidrosis. Lasers Surg Med 2011;43.
- Gorouhi F, Moghaddam NM, Davari P, Eghbalian R. Oxybutynin versus aluminum chloride on quality of life of generalized hyperhidrosis patients. J Am Acad Dermatol 2009;1.
- Marcella S, Goodman G, Cumming S, Foley P, Morgan V. Thirty-five units of botulinum toxin type A for treatment of axillary hyperhidrosis in female patients. Australas J Dermatol 2011;52:123-6. https://doi.org/10.1111/j.1440-0960.2010.00723.x.
- Sharpe C, Schofield JK, Hepburn NC. Iontophoresis for hyperhidrosis: effectiveness of treatment. Br J Dermatol 2013;169:49-50.
- Pastorelli F, Michelucci R, Plasmati R. A Randomized Controlled Trial Comparing Botulinum Toxin Type A Xeomin and Dysport for Treatment of Primary Axillary Hyperhidrosis n.d.
- Alvarez MA, Ruano J, Gómez FJ, Casas E, Baamonde C, Salvatierra A, et al. Differences between objective efficacy and perceived efficacy in patients with palmar hyperhidrosis treated with either botulinum toxin or endoscopic thoracic sympathectomy. J Eur Acad Dermatol Venereol 2013;27:e282-8. https://doi.org/10.1111/j.1468-3083.2012.04630.x.
- Tetteh HA, Groth SS, Kast T, Whitson BA, Radosevich DM, Klopp AC, et al. Primary palmoplantar hyperhidrosis and thoracoscopic sympathectomy: a new objective assessment method. Ann Thorac Surg 2009;87:267-74. https://doi.org/10.1016/j.athoracsur.2008.10.028.
- Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol 2004;51:241-8. https://doi.org/10.1016/j.jaad.2003.12.040.
- Rzany B, Muller C, Hund M. Focal hyperhidrosis. Quality of life, socioeconomic importance and use of internal medicinal therapy. Hautarzt 2012;63:456-61. https://doi.org/10.1007/s00105-012-2331-1.
- Kamudoni P, Salek MS, Mueller B. The burden of primary hyperhidrosis on the patient: EQ-5D-5l utilities, willingness to pay and daily time spent in managing the condition. Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.08.2141.
- Mehrotra S, Schmith VD, Dumitrescu TP, Gobburu J. Pharmacometrics guided design of a proof of concept (POC) study for topical glycopyrrolate, an anti-hyperhidrosis agent. Clin Pharmacol Ther 2014;95.
- Isla-Tejera B, Ruano J, Alvarez MA, Brieva T, Cardenas M, Baamonde C, et al. Economic evaluation of botulinum toxin versus thoracic sympathectomy for palmar hyperhidrosis: data from a real-world scenario. Dermatol Ther (Heidelb) 2013;3:63-72. https://doi.org/10.1007/s13555-013-0025-y.
- Alvarez M, Salvatierra A, Isla B, Casas E, Moreno-Gimenez JC, Ruano J, et al. Cost effectiveness analysis of botulinum toxin versus sympathectomy for palmar hyperhidrosis. J Am Acad Dermatol 2013;68.
- Kamudoni P. Development, Validation and Clinical Application of a Patient-reported Outcome Measure in Hyperhidrosis: The Hyperhidrosis Quality of Life Index (HidroQoL©). ORCA Online Research @ Cardiff; 2014.
- Panhofer P, Neumayer C, Zacherl J, Jakesz R, Bischof G. A survey and validation guide for health-related quality-of-life status in surgical treatment of hyperhidrosis. Eur Surg 2005;37:143-52. https://doi.org/10.1007/s10353-005-0148-1.
- Wolosker N, Yazbek G, de Campos JR, Munia MA, Kauffman P, Jatene FB, et al. Quality of life before surgery is a predictive factor for satisfaction among patients undergoing sympathectomy to treat hyperhidrosis. J Vasc Surg 2010;51:1190-4. https://doi.org/10.1016/j.jvs.2009.11.078.
- Yazbek G, Wolosker N, de Campos JR, Kauffman P, Ishy A, Puech-Leão P. Palmar hyperhidrosis – which is the best level of denervation using video-assisted thoracoscopic sympathectomy: T2 or T3 ganglion?. J Vasc Surg 2005;42:281-5. https://doi.org/10.1016/j.jvs.2005.03.041.
- Yazbek G, Wolosker N, Kauffman P, Campos JR, Puech-Leao P, Jatene FB. Twenty months of evolution following sympathectomy on patients with palmar hyperhidrosis: sympathectomy at the T3 level is better than at the T2 level. Clinics 2009;64:743-9. https://doi.org/10.1590/S1807-59322009000800006.
- Wolosker N, Schvartsman C, Krutman M, Campbell TP, Kauffman P, de Campos JR, et al. Efficacy and quality of life outcomes of oxybutynin for treating palmar hyperhidrosis in children younger than 14 years old. Pediatr Dermatol 2014;31:48-53. https://doi.org/10.1111/pde.12142.
- de Campos JR, Kauffman P, Werebe Ede C, Andrade Filho LO, Kusniek S, Wolosker N, et al. Quality of life, before and after thoracic sympathectomy: report on 378 operated patients. Ann Thorac Surg 2003;76:886-91. https://doi.org/10.1016/S0003-4975(03)00895-6.
- Ishy A, de Campos JR, Wolosker N, Kauffman P, Tedde ML, Chiavoni CR, et al. Objective evaluation of patients with palmar hyperhidrosis submitted to two levels of sympathectomy: T3 and T4. Interact Cardiovasc Thorac Surg 2011;12:545-8. https://doi.org/10.1510/icvts.2010.252015.
- Panhofer P, Ringhofer C, Gleiss A, Jakesz R, Prager M, Bischof G, et al. Quality of life after sympathetic surgery at the T4 ganglion for primary hyperhidrosis: clip application versus diathermic cut. Int J Surg 2014;12:1478-83. https://doi.org/10.1016/j.ijsu.2014.11.018.
- Panhofer P, Gleiss A, Eilenberg WH, Jakesz R, Bischof G, Neumayer C. Long-term outcomes after endothoracic sympathetic block at the T4 ganglion for upper limb hyperhidrosis. Br J Surg 2013;100:1471-7. https://doi.org/10.1002/bjs.9275.
- Panhofer TP, Schuch P, Kristo I, Jakesz R, Zacherl J, Bischof G, et al. Longterm outcome of endothoracic sympathetic block at T2, T3 and T4 for severe axillary hyperhidrosis. Surg Endosc 2011;25.
- Wolosker N, Ishy A, Yazbek G, de Campos JR, Kauffman P, Puech-Leão P, et al. Objective evaluation of plantar hyperhidrosis after sympathectomy. Clinics 2013;68:311-15. https://doi.org/10.6061/clinics/2013(03)OA05.
- Wolosker N, Munia MA, Kauffman P, Campos JR, Yazbek G, Puech-Leao P. Is gender a predictive factor for satisfaction among patients undergoing sympathectomy to treat palmar hyperhidrosis?. Clinics 2010;65:583-6. https://doi.org/10.1590/S1807-59322010000600004.
- Ibrahim M, Menna C, Andreetti C, Ciccone AM, D’Andrilli A, Maurizi G, et al. Bilateral single-port sympathectomy: long-term results and quality of life. Biomed Res Int 2013;2013. https://doi.org/10.1155/2013/348017.
- Loureiro Mde P, de Campos JR, Kauffman P, Jatene FB, Weigmann S, Fontana A. Endoscopic lumbar sympathectomy for women: effect on compensatory sweat. Clinics 2008;63:189-96. https://doi.org/10.1590/S1807-59322008000200006.
- Neves S, Uchoa PC, Wolosker N, Munia MA, Kauffman P, de Campos JR, et al. Long-term comparison of video-assisted thoracic sympathectomy and clinical observation for the treatment of palmar hyperhidrosis in children younger than 14. Pediatr Dermatol 2012;29:575-9. https://doi.org/10.1111/j.1525-1470.2012.01751.x.
- Panhofer P, Zacherl J, Jakesz R, Bischof G, Neumayer C. Improved quality of life after sympathetic block for upper limb hyperhidrosis. Br J Surg 2006;93:582-6. https://doi.org/10.1002/bjs.5304.
- Panhofer PT, Gleiss A, Eilenberg W, Jakesz R, Bischof G, Neumayer C. Long term outcome and quality of life after endoscopic sympathetic block at T4 ganglion for palmoaxillary hyperhidrosis. Eur Surg 2013;45.
- Prasad A, Ali M, Kaul S. Endoscopic thoracic sympathectomy for primary palmar hyperhidrosis. Surg Endosc 2010;24:1952-7. https://doi.org/10.1007/s00464-010-0885-5.
- Rieger R, Pedevilla S, Lausecker J. Quality of life after endoscopic lumbar sympathectomy for primary plantar hyperhidrosis. World J Surg 2015;39:905-11. https://doi.org/10.1007/s00268-014-2885-4.
- Wolosker N, de Campos JR, Kauffman P, de Oliveira LA, Munia MA, Jatene FB. Evaluation of quality of life over time among 453 patients with hyperhidrosis submitted to endoscopic thoracic sympathectomy. J Vasc Surg 2012;55:154-6. https://doi.org/10.1016/j.jvs.2011.07.097.
- Teivelis MP, Wolosker N, Krutman M, Kauffman P, Campos JR, Puech-Leão P. Treatment of uncommon sites of focal primary hyperhidrosis: experience with pharmacological therapy using oxybutynin. Clinics 2014;69:608-14. https://doi.org/10.6061/clinics/2014(09)06.
- Wolosker N, Krutman M, Kauffman P, Paula RP, Campos JR, Puech-Leão P. Effectiveness of oxybutynin for treatment of hyperhidrosis in overweight and obese patients. Rev Assoc Med Bras 2013;59:143-7. https://doi.org/10.1016/j.ramb.2012.11.002.
- Wolosker N, Teivelis MP, Krutman M, de Paula RP, Kauffman P, de Campos JR, et al. Long-term results of the use of oxybutynin for the treatment of plantar hyperhidrosis. Int J Dermatol 2015;54:605-11. https://doi.org/10.1111/ijd.12729.
- Keller S, Sekons D, Scher H, Homel P, Bookbinder M. A Novel Scale for Assessing Quality of Life Following Bilateral Endoscopic Thoracic Sympathectomy for Palmar and Plantar Hyperhidrosis n.d.
- Neumayer C, Panhofer P, Zacherl J, Bischof G. Effect of endoscopic thoracic sympathetic block on plantar hyperhidrosis. Arch Surg 2005;140:676-80. https://doi.org/10.1001/archsurg.140.7.676.
- Teale CW, Roberts G, Hamm H, Naumann M. Development, validity, and reliability of the Hyperhidrosis Impact Questionnaire (HHIQ). Qual Life Res 2002;11.
- Kuo CH, Yen M, Lin PC. Developing an instrument to measure quality of life of patients with hyperhidrosis. J Nurs Res 2004;12:21-30. https://doi.org/10.1097/01.JNR.0000387485.78685.1e.
- Salek S, Kamudoni P, Muller B. Interpretation of the constructs of Hyperhidrosis Quality of Life Index (HidroQoL©) by two different cultures same language using classical and modern techniques. Int J Clin Pharm 2015;37.
- Kamudoni P, Mueller B, Salek MS. The development and validation of a disease-specific quality of life measure in hyperhidrosis: the Hyperhidrosis Quality of Life Index (HidroQOL©). Qual Life Res 2015;24:1017-27. https://doi.org/10.1007/s11136-014-0825-2.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-16. https://doi.org/10.1111/j.1365-2230.1994.tb01167.x.
- Tan SR, Solish N. Long-term efficacy and quality of life in the treatment of focal hyperhidrosis with botulinum toxin A. Dermatol Surg 2002;28:495-9.
- Davarian S, Kalantari KK, Rezasoltani A, Rahimi A. Effect and persistency of botulinum toxin iontophoresis in the treatment of palmar hyperhidrosis. Australas J Dermatol 2008;49:75-9. https://doi.org/10.1111/j.1440-0960.2008.00441.x.
- McAleer MA, Rogers S, Collins P. Patients’ experience of hospital and home Iontophoresis. Br J Dermatol 2009;161.
- Micali G, Tedeschi A, Lacarrubba F, Umana M, Di Mauro P. A new aluminum sesquichlorhydrate topical foam for the treatment of axillary and/or palmar primary hyperhidrosis: a preliminary report. J Am Acad Dermatol 2013;68.
- Geddoa E, Balakumar AK, Paes TR. The successful use of botulinum toxin for the treatment of nasal hyperhidrosis. Int J Dermatol 2008;47:1079-80. https://doi.org/10.1111/j.1365-4632.2008.03517.x.
- Birnie A, Motley RJ. Treatment of scalp hyperhidrosis with intradermal botulinum toxin a results in a marked improvement of quality of life. Br J Dermatol 2009;161:106-7.
- Rameshkannan S, Sundar S, Sureshkumar, Muthuraj, Ayyadurai, Shanbougoe K. Efficacy of clonidine in the management of palmoplantar hyperhidrosis: a comparative study. Ann Indian Acad Neurol 2010;1:S28-9.
- Scheer F, Wiggermann P, Kamusella P, Wissgott C, Andresen R. CT-assisted sympathicolysis as an additional minimally invasive therapeutic option in primary focal plantar hyperhidrosis. Cardiovasc Intervent Radiol 2014;37:1554-8. https://doi.org/10.1007/s00270-013-0824-7.
- Scheer F, Kamusella P, Wissgott C, Vieweg H, Ludtke CW, Wiggermann P, et al. CT-guided paravertebral alcohol-installation in patients with focal plantar hyperhidrosis. Eur Spine J 2014;23:2561-62.
- Ambrogi V, Campione E, Mineo D, Paternò EJ, Pompeo E, Mineo TC. Bilateral thoracoscopic T2 to T3 sympathectomy versus botulinum injection in palmar hyperhidrosis. Ann Thorac Surg 2009;88:238-45. https://doi.org/10.1016/j.athoracsur.2009.04.003.
- Paul A, Kranz G, Schindl A, Kranz GS, Auff E, Sycha T. Diode laser hair removal does not interfere with botulinum toxin A treatment against axillary hyperhidrosis. Lasers Surg Med 2010;42:211-14. https://doi.org/10.1002/lsm.20891.
- Gibbons J, Nugent E, O’Donohoe N, Egan B, Feeley M, Tierney S. A 13 year experience with Botox treatment for axillary hyperhidrosis: cost effectiveness and quality of life analysis. Ir J Med Sci 2014:S241-2.
- Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008;159:997-1035. https://doi.org/10.1111/j.1365-2133.2008.08832.x.
- Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc 2004;9:169-80. https://doi.org/10.1111/j.1087-0024.2004.09113.x.
- Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol 1996;107:707-13. https://doi.org/10.1111/1523-1747.ep12365600.
- Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol 1997;133:1433-40. https://doi.org/10.1001/archderm.1997.03890470111018.
- Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg 2001;5:105-10. https://doi.org/10.1177/120347540100500202.
- Nijsten TE, Sampogna F, Chren MM, Abeni DD. Testing and reducing skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol 2006;126:1244-50. https://doi.org/10.1038/sj.jid.5700212.
- Aksu AEK, Saracoglu ZN, Sabuncu I. Treatment of frontal hyperhidrosis with botulinum toxin. Arch Turkish Dermatol Venerol 2011;45:161-2.
- Kuijpers M, Klinkenberg TJ, Bouma W, DeJongste MJ, Mariani MA. Single-port one-stage bilateral thoracoscopic sympathicotomy for severe hyperhidrosis: prospective analysis of a standardized approach. J Cardiothorac Surg 2013;8. https://doi.org/10.1186/1749-8090-8-216.
- Aguilar-Ferrandiz ME, Moreno-Lorenzo C, Mataran-Penarrocha GA, Castro-Sanchez AM, Peralta-Ramirez MI, Ruiz-Villaverde R. Effects of tap water iontophoresis and psychological techniques on psychosocial aspects of primary palmar hyperhidrosis. Eur J Dermatol 2011;21:256-8.
- Lessa Lda R, Luz FB, De Rezende RM, Duraes SM, Harrison BJ, De Menezes GB, et al. The psychiatric facet of hyperhidrosis: demographics, disability, quality of life, and associated psychopathology. J Psychiatr Pract 2014;20:316-23. https://doi.org/10.1097/01.pra.0000452570.69578.31.
- Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clin 2012;30:231-6. https://doi.org/10.1016/j.det.2011.11.003.
- Grob JJ, Auquier P, Martin S, Lançon C, Bonerandi JJ. Development and validation of a quality of life measurement for chronic skin disorders in French: VQ-Dermato. Dermatology 1999;199:213-22. https://doi.org/10.1159/000018250.
- Augustin M, Lange S, Wenninger K, Seidenglanz K, Amon U, Zschocke I. Validation of a comprehensive Freiburg Life Quality Assessment (FLQA) core questionnaire and development of a threshold system. Eur J Dermatol 2004;14:107-13.
- Rzany B, Hund M. Focal hyperhidrosis. Hautarzt 2003;54:767-78. https://doi.org/10.1007/s00105-003-0559-5.
- Augustin M, Radtke MA, Zschocke I, Blome C, Behechtnejad J, Schäfer I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res 2009;301:561-71. https://doi.org/10.1007/s00403-009-0928-8.
- Devins GM. Illness intrusiveness and the psychosocial impact of lifestyle disruptions in chronic life-threatening disease. Adv Ren Replace Ther 1994;1:251-63. https://doi.org/10.1016/S1073-4449(12)80007-0.
- Devins GM. Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. J Psychosom Res 2010;68:591-602. https://doi.org/10.1016/j.jpsychores.2009.05.006.
- Cinà CS, Clase CM. The Illness Intrusiveness Rating Scale: a measure of severity in individuals with hyperhidrosis. Qual Life Res 1999;8:693-8. https://doi.org/10.1023/A:1008968401068.
- Tarlov AR, Ware JE, Greenfield S, Nelson EC, Perrin E, Zubkoff M. Study. An Application of Methods for Monitoring the Results of Medical Care. JAMA. 1989.
- Stewart AL, Ware JE. Measuring Functioning and Well-being: the Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 1992;30:473-83. https://doi.org/10.1097/00005650-199206000-00002.
- Aaronson NK, Acquadro C, Alonso J, Apolone G, Bucquet D, Bullinger M, et al. International Quality of Life Assessment (IQOLA) Project. Qual Life Res 1992;1:349-51. https://doi.org/10.1007/BF00434949.
- Hekmat K, Czichelski J, Nemat A. Quality of Life After Thoracoscopic Sympathectomy for Palmar Hyperhidrosis n.d. https://doi.org/10.1055/s-0029-1246915.
- Young O, Neary P, Keaveny TV, Mehigan D, Sheehan S. Evaluation of the impact of transthoracic endoscopic sympathectomy on patients with palmar hyperhydrosis. Eur J Vasc Endovasc Surg 2003;26:673-6. https://doi.org/10.1016/S1078.
- Elia S, Guggino G, Mineo D, Vanni G, Gatti A, Mineo TC. Awake one stage bilateral thoracoscopic sympathectomy for palmar hyperhidrosis: a safe outpatient procedure. Eur J Cardiothorac Surg 2005;28:312-17. https://doi.org/10.1016/j.ejcts.2005.03.046.
- Rodriguez PM, Freixinet JL, Hussein M, Valencia JM, Gil RM, Herrero J, et al. Side effects, complications and outcome of thoracoscopic sympathectomy for palmar and axillary hyperhidrosis in 406 patients. Eur J Cardiothorac Surg 2008;34:514-19. https://doi.org/10.1016/j.ejcts.2008.05.036.
- Sayeed RA, Nyamekye I, Ghauri AS, Poskitt KR. Quality of life after transthoracic endoscopic sympathectomy for upper limb hyperhidrosis. Eur J Surg Suppl 1998;580:39-42. https://doi.org/10.1080/11024159850191139.
- Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Simonyi S, Jog M, Beauchamp R, Miller R, Bhogal M, Wein T. Impact of botulinum toxin type a (BOTOX) on health utility in patients receiving treatment for approved therapeutic indications: baseline characteristics and interim results of a large ongoing phase iv prospective observational cohort study (MDs on BOTOX utility mobility) in Canada. J Popul Ther Clin Pharmacol 2010;17.
- Simonyi S, Jog M, Wein T, Beauchamp R, Miller R, Ismail F, et al. Measuring the impact of previous treatment cycles on health utility in patients receiving botulinum toxin type a (BONTA) in a prospective observational cohort study: mobility study. Value Health 2011;14. https://doi.org/10.1016/j.jval.2011.02.831.
- Vazquez LD, Staples NL, Sears SF, Klodell CT. Psychosocial functioning of patients after endoscopic thoracic sympathectomy. Eur J Cardiothorac Surg 2011;39:1018-21. https://doi.org/10.1016/j.ejcts.2011.01.059.
- Bullinger M, Kirchberger I, Von Steinbüchel N. The questionnaire everyday life – a procedure for recording the health-related quality of life. Z Med Psychol 1993;3:121-31.
- Bullinger M, Kirchberger I, Steinbuechel N, Ravens-Sieberer U, Cieza A. Quality of Life and Health Economics. Concepts, Methods, Applications. Landsberg; 2000.
- Koskinen LO, Blomstedt P, Sjöberg RL. Predicting improvement after surgery for palmar hyperhidrosis. Acta Neurol Scand 2012;126:324-8. https://doi.org/10.1111/j.1600-0404.2012.01650.x.
- Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
- The EuroQoL Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Rytwinski NK, Fresco DM, Heimberg RG, Coles ME, Liebowitz MR, Cissell S, et al. Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depress Anxiety 2009;26:34-8. https://doi.org/10.1002/da.20503.
- Cox BJ, Ross L, Swinson RP, Direnfeld DM. A comparison of social phobia outcome measures in cognitive-behavioral group therapy. Behav Modif 1998;22:285-97. https://doi.org/10.1177/01454455980223004.
- Rantanen T, Telaranta T. Long-term results of endoscopic sympathetic block using the Lin-Telaranta classification. Surg Endosc 2013;27:3860-4. https://doi.org/10.1007/s00464-013-2995-3.
- Russell D, Peplau LA, Ferguson ML. Developing a measure of loneliness. J Pers Assess 1978;42:290-4. https://doi.org/10.1207/s15327752jpa4203_11.
- Hunt SM, McEwen J, McKenna SP. Measuring health status: a new tool for clinicians and epidemiologists. J R Coll Gen Pract 1985;35:185-8.
- Schneider JW, Gurucharri LM, Gutierrez AL, Gaebler-Spira DJ. Health-related quality of life and functional outcome measures for children with cerebral palsy. Dev Med Child Neurol 2001;43:601-8. https://doi.org/10.1017/S0012162201001098.
- Bachmann K, Standl N, Kaifi J, Busch P, Winkler E, Mann O, et al. Thoracoscopic sympathectomy for palmar and axillary hyperhidrosis: four-year outcome and quality of life after bilateral 5-mm dual port approach. Surg Endosc 2009;23:1587-93. https://doi.org/10.1007/s00464-009-0392-8.
- Bogokowsky H, Slutzki S, Bacalu L, Abramsohn R, Negri M. Surgical treatment of primary hyperhidrosis. A report of 42 cases. Arch Surg 1983;118:1065-7. https://doi.org/10.1001/archsurg.1983.01390090049011.
- Boley TM, Belangee KN, Markwell S, Hazelrigg SR. The effect of thoracoscopic sympathectomy on quality of life and symptom management of hyperhidrosis. J Am Coll Surg 2007;204:435-8. https://doi.org/10.1016/j.jamcollsurg.2006.12.007.
- Cardoso PO, Rodrigues KC, Mendes KM, Petroianu A, Resende M, Alberti LR. Evaluation of patients submitted to surgical treatment for palmar hyperhidrosis with regard to the quality of life and to the appearance of compensatory hyperhidrosis. Rev Col Bras Cir 2009;36:14-8. https://doi.org/10.1590/S0100-69912009000100005.
- Chan ACY, Ting ACW, Ho P, Poon JTC, Cheng SWK. Compensatory sweating after thoracoscopic sympathectomy for primary hyperhidrosis: single institute experience. Surg Pract 2007;11:98-101. https://doi.org/10.1111/j.1744-1633.2007.00355.x.
- Currie AC, Evans JR, Thomas PR. An analysis of the natural course of compensatory sweating following thoracoscopic sympathectomy. Int J Surg 2011;9:437-9. https://doi.org/10.1016/j.ijsu.2011.04.006.
- Divisi D, Ferrari V, De Vico A, Imbriglio G, Di Francescantonio W, Vaccarili M, et al. Electrocautery Versus Ultracision Versus Ligasure in Surgical Management of Hyperhidrosis n.d. https://doi.org/10.1093/icvts/ivt288.173.
- Garcia C, Perez-Cajaraville J, Guillen-Grima F, Espana A. Percutaneous radiofrequency sympathycolisis in severe hyperhidrosis and facial blushing: efficacy and quality of life. Interact Cardiovasc Thorac Surg 2011;13.
- Jeganathan R, Jordan S, Jones M, Grant S, Diamond O, McManus K, et al. Bilateral thoracoscopic sympathectomy: results and long-term follow-up. Interact Cardiovasc Thorac Surg 2008;7:67-70. https://doi.org/10.1510/icvts.2007.162479.
- Marić N, Stanić V, Ristanović A, Cvijanović V, Milisavljević S. A single incision transaxillary thoracoscopic sympathectomy. Vojnosanit Pregl 2014;71:432-7. https://doi.org/10.2298/VSP120122047M.
- Munia MA, Wolosker N, Kaufmann P, de Campos JR, Puech-Leão P. Sustained benefit lasting one year from T4 instead of T3-T4 sympathectomy for isolated axillary hyperhidrosis. Clinics 2008;63:771-4. https://doi.org/10.1590/S1807-59322008000600011.
- Munia MA, Wolosker N, Kauffman P, de Campos JR, Puech-Leão P. A randomized trial of T3-T4 versus T4 sympathectomy for isolated axillary hyperhidrosis. J Vasc Surg 2007;45:130-3. https://doi.org/10.1016/j.jvs.2006.09.011.
- Rathinam S, Nanjaiah P, Sivalingam S, Rajesh PB. Excision of sympathetic ganglia and the rami communicantes with histological confirmation offers better early and late outcomes in video assisted thoracoscopic sympathectomy. J Cardiothorac Surg 2008;3. https://doi.org/10.1186/1749-8090-3-50.
- Shalaby MS, El-Shafee E, Safoury H, El Hay SA. Thoracoscopic excision of the sympathetic chain: an easy and effective treatment for hyperhidrosis in children. Pediatr Surg Int 2012;28:245-8. https://doi.org/10.1007/s00383-011-2984-3.
- Shi H, Shu Y, Shi W, Lu S, Sun C. Single-port microthoracoscopic sympathicotomy for the treatment of primary palmar hyperhidrosis: an analysis of 56 consecutive cases. Indian J Surg 2015;77:270-5. https://doi.org/10.1007/s12262-015-1288-6.
- Steiner Z, Kleiner O, Hershkovitz Y, Mogilner J, Cohen Z. Compensatory sweating after thoracoscopic sympathectomy: an acceptable trade-off. J Pediatr Surg 2007;42:1238-42. https://doi.org/10.1016/j.jpedsurg.2007.02.015.
- Xiao P, Liu A, Liu W. Effect of T4 endoscopic thoracic sympathicotomy on life quality in patients with primary palmar hyperhidrosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2015;40:1126-31. https://doi.org/10.11817/j.issn.1672-7347.2015.10.012.
- Cheng A, Johnsen H, Chang MY. Patient satisfaction after thoracoscopic sympathectomy for palmar hyperhidrosis: do method and level matter?. Perm 2015;19:29-31.
- Flores LP. Long-term outcomes associated to video-assisted thoracic sympathotomy for palmar-axillar subtype of the hyperhidrosis. Arq Neuropsiquiatr 2012;70:398-403. https://doi.org/10.1590/S0004-282X2012000600003.
- Jaffer U, Weedon K, Cameron AE. Factors affecting outcome following endoscopic thoracic sympathectomy. Br J Surg 2007;94:1108-12. https://doi.org/10.1002/bjs.5792.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. PharmacoEconomics 2006;24:355-71. https://doi.org/10.2165/00019053-200624040-00006.
- Gibbons JP, Nugent E, O’Donohoe N, Maher B, Egan B, Feeley M, et al. Experience with botulinum toxin therapy for axillary hyperhidrosis and comparison to modelled data for endoscopic thoracic sympathectomy. A quality of life and cost effectiveness analysis. Surgeon 2016;14:260-4. https://doi.org/10.1016/j.surge.2015.05.002.
- Müller C, Augustin M. Willingness-to-pay and patient-defined benefits in the treatment of hyperhidrosis: results from the first German health services research study in hyperhidrosis. Br J Dermatol 2013;168:448-50. https://doi.org/10.1111/j.1365-2133.2012.11150.x.
- The Guidelines Manual. London: NICE; 2016.
- NICE Guide to the Methods of Technology Appraisal. NICE; 2013.
- MYE2 Population by Sex and Age for Local Authorities UK 2015. ONS; 2016.
- NHS Reference Costs 2014 to 2015. DH; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0076654.
- Dias S, Welton NJ, Sutton AJ, Ades A. A General Linear Modelling Framework for Pair-wise and Network Meta-analysis of Randomised Controlled Trials (Last Updated September 2016). NICE; 2011.
- Brooks S, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graphical Stat 1998;7:434-55.
- Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JP. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med 2015;34:984-98. https://doi.org/10.1002/sim.6381.
- Endoscopic Thoracic Sympathectomy for Primary Hyperhydrosis of the Upper Limb. NICE; 2010.
- Significant Cost Changes – April 2016. NHS Midlands and Lancashire Commissioning Support Unit; 2016.
- Walling HW, Swick BL. Treatment options for hyperhidrosis. Am J Clin Dermatol 2011;12:285-95. https://doi.org/10.2165/11587870-000000000-00000.
- Idrostar . Treatment for Hyperhirdosis. Accessories n.d. www.iontophoresis.info/shop/accessories (accessed 1 August 2016).
- The Whiteley Clinic . Laser Sweat Ablation n.d. www.thewhiteleyclinic.co.uk/procedures/laser-sweat-ablation/ (accessed 1 July 2016).
- PHI Clinic . MiraDry Underarm Sweat Treatment n.d. www.phiclinic.com/non-surgical/treatments/miradry/ (accessed 1 July 2016).
- NHS Business Services Authority . Prescription Services. Drug Tariff. Preface Amendments to the Drug Tariff June 2015 2015. www.nhsbsa.nhs.uk/sites/default/files/2017-04/June_2015.pdf (accessed 13 October 2016).
- International Hyperhidrosis Society . Results Are IN! Promising Data Re: Two New Hyperhidrosis Treatments n.d. www.sweathelp.org/sweatsolutions-newsletter/news-blog/371-results-are-in-promising-data-re-two-new-hyperhidrosis-treatments.html (accessed 5 December 2016).
- GlaxoSmithKline . A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study Including an Open-Label Phase to Evaluate the Efficacy and Safety of GSK1358820 (Botulinum Toxin Type A) in Patients With Axillary Hyperhidrosis n.d. www.gsk-clinicalstudyregister.com/study/114078?search=study&#rs (accessed 9 August 2016).
- Abrams SB, Hallett M. Clinical utility of different botulinum neurotoxin preparations. Toxicon 2013;67:81-6. https://doi.org/10.1016/j.toxicon.2012.11.024.
- Adar R, Kurchin A, Zweig A, Mozes M. Palmar hyperhidrosis and its surgical treatment: a report of 100 cases. Ann Surg 1977;186:34-41. https://doi.org/10.1097/00000658-197707000-00006.
- Ak M, Dincer D, Haciomeroglu B, Akarsu S, Cinar A, Lapsekili N. Temperament and character properties of primary focal hyperhidrosis patients. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-5.
- Ak M, Dinçer D, Haciomeroglu B, Akarsu S, Lapsekili N, Ada S. The evaluation of primary idiopathic focal hyperhidrosis patients in terms of alexithymia. J Health Psychol 2013;18:704-10. https://doi.org/10.1177/1359105312454908.
- Andrews BT, Rennie JA. Predicting changes in the distribution of sweating following thoracoscopic sympathectomy. Br J Surg 1997;84:1702-4. https://doi.org/10.1002/bjs.1800841215.
- Anonymous . Transthoracic sympathectomy and human thermoregulatory problems. J Thermal Biol 2004;29:63-4. https://doi.org/10.1016/j.jtherbio.2003.10.005.
- Araujo CA, Azevedo IM, Ferreira MA, Ferreira HP, Dantas JL, Medeiros AC. Compensatory sweating after thoracoscopic sympathectomy: characteristics, prevalence and influence on patient satisfaction. J Bras Pneumol 2009;35:213-20. https://doi.org/10.1590/S1806-37132009000300004.
- Atkinson L, George S, Shergill B. Botulinum toxin A for focal hyperhidrosis of the face and amputation stump. Br J Dermatol 2010;163.
- Awad MS, Elzeftawy A, Mansour S, Elshelfa W. One stage bilateral endoscopic sympathectomy under local anesthesia: is a valid, and safe procedure for treatment of palmer hyperhidrosis?. J Minim Access Surg 2010;6:11-5. https://doi.org/10.4103/0972-9941.62529.
- Aydemir EH, Kalkan MT, Karakoç Y. Quantitative effect of anodal current in the treatment of primary hyperhidrosis by direct electrical current. Int J Dermatol 2006;45:862-4. https://doi.org/10.1111/j.1365-4632.2006.02909.x.
- Bandeira COP, Sarrao BD, Santos EQ, Souza LP, Bandeira IF. The influence of compensatory sweating on the satisfaction of patients subjected to thoracoscopic sympathectomy. Acta Sci Health Sci 2009;31:65-70. https://doi.org/10.4025/actascihealthsci.v31i1.4845.
- Baumgartner FJ, Toh Y. Severe hyperhidrosis: clinical features and current thoracoscopic surgical management. Ann Thorac Surg 2003;76:1878-83. https://doi.org/10.1016/S0003-4975(03)01069-5.
- Baumgartner FJ, Bertin S, Konecny J. Superiority of thoracoscopic sympathectomy over medical management for the palmoplantar subset of severe hyperhidrosis. Ann Vasc Surg 2009;23:1-7. https://doi.org/10.1016/j.avsg.2008.04.014.
- Bechaux S. Axillary hyperhidrosis and botulinum toxin. Nouvelles Dermatologiques 2009;28:325-7.
- Bell D, Jedynak J, Bell R. Predictors of outcome following endoscopic thoracic sympathectomy. ANZ J Surg 2014;84:68-72. https://doi.org/10.1111/ans.12098.
- Benohanian A, Dansereau A, Bolduc C, Bloom E. Localized hyperhidrosis treated with aluminum chloride in a salicylic acid gel base. Int J Dermatol 1998;37:701-3. https://doi.org/10.1046/j.1365-4362.1998.00543.x.
- Bischof G, Zacherl J, Fugger R, Neumayer C. Endoscopic transthoracic sympathectomy: current indications and techniques. Eur Surg 2005;37:121-6. https://doi.org/10.1007/s10353-005-0145-4.
- Bisseriex H, Thefenne L, Compere S, Rogez D, Dochez F, Mercier H, et al. The treatment of the hyperhidrosis of the residual limb, the contribution of the botulinum toxin: about a series of case at the military hospital Percy. Ann Phys Rehabil Med 2012;55:e84-7. https://doi.org/10.1016/j.rehab.2012.07.210.
- Boas RA. What price success? Ask the patient. J Pain 2000;1:258-60. https://doi.org/10.1054/jpai.2000.14258.
- Bryan H, Alster T, Tanzi E. Treatment of axillary hyperhidrosis using a novel non-invasive electromagnetic device. Lasers Surg Med 2012;44.
- Bygstad E, Terkelsen AJ, Pilegaard HK, Hansen J, Molgaard H, Hjortdal VE. Thoracoscopic sympathectomy increases efferent cardiac vagal activity and baroreceptor sensitivity. Eur J Cardiothorac Surg 2013;44:e193-9. https://doi.org/10.1093/ejcts/ezt356.
- Cameron AE, Ojimba TA. Evaluation of the impact of transthoracic endoscopic sympathectomy on patients with palmar hyperhidrosis. Eur J Vasc Endovasc Surg 2004;27. https://doi.org/10.1016/j.ejvs.2004.03.011.
- Chen HJ, Shih TY, Cheng MH. Transthoracic endoscopic sympathectomy for primary palmar hyperhidrosis in children. Pediatr Surg Int 1996;11:119-22. https://doi.org/10.1007/BF00183741.
- Chen HJ, Lu K, Liang CL. Transthoracic endoscopic T-2, 3 sympathectomy for facial hyperhidrosis. Auton Neurosci 2001;93:91-4. https://doi.org/10.1016/S1566-0702(01)00341-1.
- Chen W, Zhu L, Yang S, Li D, Wang W, Chen L. Embryonic-notes thoracic sympathectomy for palmar hyperhidrosis: results of a novel technique and comparison with the conventional vats procedure. Surg Endosc 2013;27.
- Chia HY, Tan AS, Chong WS, Tey HL. Efficacy of iontophoresis with glycopyrronium bromide for treatment of primary palmar hyperhidrosis. J Eur Acad Dermatol Venereol 2012;26:1167-70. https://doi.org/10.1111/j.1468-3083.2011.04197.x.
- Cho NJ, Lee YJ, Lew Y, Kim DK, Lee SH. Evaluation of treatment effect of primary hyperhidrosis using skin surface hydrometer. [Korean]. Korean J Dermatol 1994;32:369-75.
- Clerico C, Fernandez J, Camuzard O, Chignon-Sicard B, Ihrai T. Axillary hyperhidrosis, botulinium A toxin treatment: review. Ann Chir Plast Esthet 2014;61:60-4. https://doi.org/10.1016/j.anplas.2014.11.006.
- Coelho MDS, Kuenzer Caetano Da Silva RF, Francisco AN, Guimaraes PF, Santos AFR. T3 sympathectomy versus T3 sympathicotomy for palmar hyperhidrosis: evolution of plantar hyperhidrosis. Interact Cardiovasc Thorac Surg 2014;19. https://doi.org/10.1093/icvts/ivu276.160.
- Copleymerriman C, Zelt S, Clark M, Gnanasakthy A. Benefits of patient-reported outcomes in dermatology drug development. Value Health 2015;18. https://doi.org/10.1016/j.jval.2015.03.1065.
- de Campos JR, Wolosker N, Takeda FR, Kauffman P, Kuzniec S, Jatene FB, et al. The body mass index and level of resection: predictive factors for compensatory sweating after sympathectomy. Clin Auton Res 2005;15:116-20. https://doi.org/10.1007/s10286-005-0259-6.
- Deng B, Tan QY, Jiang YG, Zhao YP, Zhou JH, Ma Z, et al. Optimization of sympathectomy to treat palmar hyperhidrosis: the systematic review and meta-analysis of studies published during the past decade. Surg Endosc 2011;25:1893-901. https://doi.org/10.1007/s00464-010-1482-3.
- Doolabh N, Horswell S, Williams M, Huber L, Prince S, Meyer DM, et al. Thoracoscopic sympathectomy for hyperhidrosis: indications and results. Ann Thorac Surg 2004;77:410-14. https://doi.org/10.1016/j.athoracsur.2003.06.003.
- Dressler D, Adib Saberi F, Benecke R. Botulinum toxin type B for treatment of axillar hyperhidrosis. J Neurol 2002;249:1729-32. https://doi.org/10.1007/s00415-002-0929-4.
- Drott C, Göthberg G, Claes G. Endoscopic transthoracic sympathectomy: an efficient and safe method for the treatment of hyperhidrosis. J Am Acad Dermatol 1995;33:78-81. https://doi.org/10.1016/0190-9622(95)90015-2.
- Drott C, Claes G. Hyperhidrosis treated by thoracoscopic sympathicotomy. Cardiovasc Surg 1996;4:788-90. https://doi.org/10.1016/S0967-2109(96)00048-8.
- Duarte JB, Kux P, Castro CH, Cruvinel MG, Costa JR. Fast track endoscopic thoracic sympathicotomy. Clin Auton Res 2003;13:I63-5. https://doi.org/10.1007/s10286-003-1117-z.
- Dumont P, Hamm A, Skrobala D, Robin P, Toumieux B. Bilateral thoracoscopy for sympathectomy in the treatment of hyperhidrosis. Eur J Cardiothorac Surg 1997;11:774-5. https://doi.org/10.1016/S1010-7940(97)01151-2.
- Ebrahim KS. Percutaneous chemical dorsal sympathectomy for hyperhidrosis. Minim Invasive Neurosurg 2011;54:29-32. https://doi.org/10.1055/s-0030-1269859.
- Edmondson RA, Banerjee AK, Rennie JA. Endoscopic transthoracic sympathectomy in the treatment of hyperhidrosis. Ann Surg 1992;215:289-93. https://doi.org/10.1097/00000658-199203000-00015.
- Ellis H. Axillary hyperhidrosis: failure of subcutaneous curettage. Br Med J 1977;2:301-2. https://doi.org/10.1136/bmj.2.6082.301.
- Ellis H, Scurr JH. Axillary hyperhidrosis – topical treatment with aluminium chloride hexahydrate. Postgrad Med J 1979;55:868-9. https://doi.org/10.1136/pgmj.55.650.868.
- Ellis H. Surgery of the sweat glands. J R Soc Med 1982;75:585-7.
- Farahmand M, Toolabi KA. Minimal invasive surgery for the treatment of palmar hyperhydrosis; a suturless surgery with mini thoracoscopic bilateral cervical sympathectomy. Surg Endosc 2011;25.
- Fatemi Naeini F, Pourazizi M, Abtahi-Naeini B, Nilforoushzadeh MA, Najafian J. A novel option for treatment of primary axillary hyperhidrosis: fractionated microneedle radiofrequency. J Postgrad Med 2015;61:141-3. https://doi.org/10.4103/0022-3859.153111.
- Flørenes T. Thoracoscopic sympathectomy – surgery for palmar hyperhidrosis and facial blushing. Tidsskr Nor Laegeforen 2003;123:463-4.
- Furlan AD, Mailis A, Papagapiou M. Are we paying a high price for surgical sympathectomy? A systematic literature review of late complications. J Pain 2000;1:245-57. https://doi.org/10.1054/jpai.2000.19408.
- Galati DM, Raposio E. A new sympathectomy technique for the treatment of palmar and axillary hyperhydrosis. Eur Surg Res 2010;45.
- George SM, Atkinson LR, Farrant PB, Shergill BS. Botulinum toxin for focal hyperhidrosis of the face. Br J Dermatol 2014;170:211-13. https://doi.org/10.1111/bjd.12568.
- Ghisletta N, Habicht J, Stulz P. Video-assisted thoracosopic sympathectomy: spectrum of indications and our own results (1995–1997). Schweiz Med Wochenschr 1999;129:985-92.
- Glent-Madsen L, Dahl JC. Axillary hyperhidrosis. Local treatment with aluminium-chloride hexahydrate 25% in absolute ethanol with and without supplementary treatment with triethanolamine. Acta Derm Venereol 1988;68:87-9.
- Goh CL. Aluminum chloride hexahydrate versus palmar hyperhidrosis. Evaporimeter assessment. Int J Dermatol 1990;29:368-70. https://doi.org/10.1111/j.1365-4362.1990.tb04766.x.
- Goh CL, Yoyong K. A comparison of topical tannic acid versus iontophoresis in the medical treatment of palmar hyperhidrosis. Singapore Med J 1996;37:466-8.
- Goh PM, Cheah WK, De Costa M, Sim EK. Needlescopic thoracic sympathectomy: treatment for palmar hyperhidrosis. Ann Thorac Surg 2000;70:240-2. https://doi.org/10.1016/S0003-4975(00)01489-2.
- Goldman A. Treatment of axillary and palmar hyperhidrosis with botulinum toxin. Aesthetic Plast Surg 2000;24:280-2. https://doi.org/10.1007/s002660010046.
- Gossot D, Kabiri H, Caliandro R, Debrosse D, Girard P, Grunenwald D. Early complications of thoracic endoscopic sympathectomy: a prospective study of 940 procedures. Ann Thorac Surg 2001;71:1116-19. https://doi.org/10.1016/S0003-4975(01)02422-5.
- Grabham JA, Raitt D, Barrie WW. Early experience with day-case transthoracic endoscopic sympathectomy. Br J Surg 1998;85. https://doi.org/10.1046/j.1365-2168.1998.00817.x.
- Greenhalgh RM, Rosengarten DS, Martin P. Role of sympathectomy for hyperhidrosis. Br Med J 1971;1:332-4. https://doi.org/10.1136/bmj.1.5744.332.
- Grimalt R, Domínguez Tordera P, Callejas MA. Topical atropine sulfate for the treatment of axillary hyperhidrosis. J Cosmet Dermatol 2006;5:294-6. https://doi.org/10.1111/j.1473-2165.2006.00273.x.
- Grootens KP, Hartong EGTM. Oxybutynin for antidepressant-induced hyperhidrosis. Eur Neuropsychopharmacol 2011;21. https://doi.org/10.1016/S0924-977X(11)70618-6.
- Guérin JC, Demolombe S, Brudon JR. Thoracic sympathectomy by thoracoscopy. Apropos of 15 cases. Rev Mal Respir 1990;7:327-30.
- Hashmonai M. Endoscopic lumbar sympathectomy following thoracic sympathectomy in patients with palmoplantar hyperhidrosis. World J Surg 2011;35:54-5. https://doi.org/10.1007/s00268-010-0809-5.
- Hecht MJ, Birklein F, Winterholler M. Successful treatment of axillary hyperhidrosis with very low doses of botulinum toxin B: a pilot study. Arch Dermatol Res 2004;295:318-19. https://doi.org/10.1007/s00403-003-0440-5.
- Heidemann E, Licht PB. A comparative study of thoracoscopic sympathicotomy versus local surgical treatment for axillary hyperhidrosis. Ann Thorac Surg 2013;95:264-8. https://doi.org/10.1016/j.athoracsur.2012.08.103.
- Henriques M, Costa J, Domingues S, Alves M. Botulinum toxin type a in palmar hyperhidrosis: the role of iontophoresis. Ann Phys Rehabil Med 2014;57. https://doi.org/10.1016/j.rehab.2014.03.669.
- Henteleff HJ, Kalavrouziotis D. Evidence-based review of the surgical management of hyperhidrosis. Thorac Surg Clin 2008;18:209-16. https://doi.org/10.1016/j.thorsurg.2008.01.008.
- Hoorens I, Ongenae K. Primary focal hyperhidrosis: current treatment options and a step-by-step approach. J Eur Acad Dermatol Venereol 2012;26:1-8. https://doi.org/10.1111/j.1468-3083.2011.04173.x.
- Hsu CP, Chen CY, Hsia JY, Shai SE. Resympathectomy for palmar and axillary hyperhidrosis. Br J Surg 1998;85:1504-5. https://doi.org/10.1046/j.1365-2168.1998.00905.x.
- Ibrahim M, Menna C, Andreetti C, Ciccone AM, D’Andrilli A, Maurizi G, et al. Two-stage unilateral versus one-stage bilateral single-port sympathectomy for palmar and axillary hyperhidrosis. Interact Cardiovasc Thorac Surg 2013;16:834-8. https://doi.org/10.1093/icvts/ivt039.
- Innocenzi D, Ruggero A, Francesconi L, Lacarrubba F, Nardone B, Micali G. An open-label tolerability and efficacy study of an aluminum sesquichlorohydrate topical foam in axillary and palmar primary hyperhidrosis. Dermatol Ther 2008;21:S27-30. https://doi.org/10.1111/j.1529-8019.2008.00199.x.
- Jani K. Retroperitoneoscopic lumbar sympathectomy for plantar hyperhidrosis. J Am Coll Surg 2009;209:e12-15. https://doi.org/10.1016/j.jamcollsurg.2009.04.015.
- Jog M, Wein T, Beauchamp R, Miller R, Bhogal M, Simonyi S. Botulinum toxin type a (BOTOX) improves health utility in patients treated for approved therapeutic indications: interim analysis of a large ongoing phase IV prospective observational cohort study (MDS on BOTOX utility-mobility) in Canada. Mov Disord 2010;25.
- Kavanagh GM, Oh C, Shams K. BOTOX delivery by iontophoresis. Br J Dermatol 2004;151:1093-5. https://doi.org/10.1111/j.1365-2133.2004.06250.x.
- Kavanagh GM, Shams K. Botulinum toxin type A by iontophoresis for primary palmar hyperhidrosis. J Am Acad Dermatol 2006;55:115-17. https://doi.org/10.1016/j.jaad.2005.07.017.
- Khademi Kalantari K, Zeinalzade A, Kobarfard F, Nazary Moghadam S. The effect and persistency of 1% aluminum chloride hexahydrate iontophoresis in the treatment of primary palmar hyperhidrosis. Iran J Pharm Res 2011;10:641-5.
- Kim DH, Paik HC, Lee DY. Video assisted thoracoscopic re-sympathetic surgery in the treatment of re-sweating hyperhidrosis. Eur J Cardiothorac Surg 2005;27:741-4. https://doi.org/10.1016/j.ejcts.2005.01.054.
- Kim WO, Kil HK, Yoon KB, Yoon DM. Topical glycopyrrolate for patients with facial hyperhidrosis. Br J Dermatol 2008;158:1094-7. https://doi.org/10.1111/j.1365-2133.2008.08476.x.
- Kopelman D, Hashmonai M, Ehrenreich M, Bahous H, Assalia A. Upper dorsal thoracoscopic sympathectomy for palmar hyperhidrosis: improved intermediate-term results. J Vasc Surg 1996;24:194-9. https://doi.org/10.1016/S0741-5214(96)70093-9.
- Kravarusic D, Freud E. Thoracoscopic sympathectomy ganglia ablation in the management of palmer hyperhidrosis: a decade experience in a single institution. Afr J Paediatr Surg 2012;9:143-7. https://doi.org/10.4103/0189-6725.99402.
- Krogstad AL, Mork C, Piechnik SK. Daily pattern of sweating and response to stress and exercise in patients with palmar hyperhidrosis. Br J Dermatol 2006;154:1118-22. https://doi.org/10.1111/j.1365-2133.2006.07212.x.
- Kumar MG, Foreman RS, Berk DR, Bayliss SJ. Oral glycopyrrolate for refractory pediatric and adolescent hyperhidrosis. Pediatr Dermatol 2014;31:e28-30. https://doi.org/10.1111/pde.12236.
- Lakraj AA, Moghimi N, Jabbari B. Hyperhidrosis: anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins 2013;5:821-40. https://doi.org/10.3390/toxins5040821.
- Lau R, Ng C, Wong R, Yeung E, Kwok M, Wan I, et al. Single Port Video-assisted Thoracic Surgery Vasoview Sympathectomy for Palmar Hyperhidrosis. Chest.: CHEST World Congress; 2014.
- Law NW, Ellis H. Transthoracic sympathectomy for palmar hyperhidrosis in children under 16 years of age. Ann R Coll Surg Engl 1989;71:70-1.
- Lawrence CM, Lonsdale Eccles AA. Selective sweat gland removal with minimal skin excision in the treatment of axillary hyperhidrosis: a retrospective clinical and histological review of 15 patients. Br J Dermatol 2006;155:115-18. https://doi.org/10.1111/j.1365-2133.2006.07320.x.
- Leão LE, de Oliveira R, Szulc R, Mari Jde J, Crotti PL, Gonçalves JJ. Role of video-assisted thoracoscopic sympathectomy in the treatment of primary hyperhidrosis. Sao Paulo Med J 2003;121:191-7. https://doi.org/10.1590/S1516-31802003000500003.
- Lecouflet M, Leux C, Fenot M, Celerier P, Maillard H. Duration of efficacy increases with the repetition of botulinum toxin A injections in primary palmar hyperhidrosis: a study of 28 patients. J Am Acad Dermatol 2014;70:1083-7. https://doi.org/10.1016/j.jaad.2013.12.035.
- Lee KS, Chuang CL, Lin CL, Tsai LC, Hwang SL, Howng SL. Percutaneous CT-guided chemical thoracic sympathectomy for patients with palmar hyperhidrosis after transthoracic endoscopic sympathectomy. Surg Neurol 2004;62:501-5. https://doi.org/10.1016/j.surneu.2004.02.016.
- Lee MR, Ryman WJ. Liposuction for axillary hyperhidrosis. Australas J Dermatol 2005;46:76-9. https://doi.org/10.1111/j.1440-0960.2005.00145.x.
- Lee D, Cho SH, Kim YC, Park JH, Lee SS, Park SW. Tumescent liposuction with dermal curettage for treatment of axillary osmidrosis and hyperhidrosis. Dermatol Surg 2006;32:505-11. https://doi.org/10.1097/00042728-200604000-00004.
- Lee HC, Chen CC, Lee WY, Chuang HU, Kao MC. Axillary hyperhidrosis and osmidrosis treated by ultrasonic surgical aspiration compared with transthoracic endoscopic sympathectomy. Surg Neurol 2008;70:64-8. https://doi.org/10.1016/j.surneu.2008.08.077.
- Lewis DR, Irvine CD, Smith FC, Lamont PM, Baird RN. Sympathetic skin response and patient satisfaction on long-term follow-up after thoracoscopic sympathectomy for hyperhidrosis. Eur J Vasc Endovasc Surg 1998;15:239-43. https://doi.org/10.1016/S1078-5884(98)80183-4.
- Licht PB, Pilegaard HK. Severity of compensatory sweating after thoracoscopic sympathectomy. Ann Thorac Surg 2004;78:427-31. https://doi.org/10.1016/j.athoracsur.2004.02.087.
- Lin TS, Fang HY. Transthoracic endoscopic sympathectomy in the treatment of palmar hyperhidrosis--with emphasis on perioperative management (1,360 case analyses). Surg Neurol 1999;52:453-7. https://doi.org/10.1016/S0090-3019(99)00111-1.
- Lin TS, Fang HY, Wu CY. Repeat transthoracic endoscopic sympathectomy for palmar and axillary hyperhidrosis. Surg Endosc 2000;14:134-6. https://doi.org/10.1007/s004649900084.
- Lin TS. Video-assisted thoracoscopic ‘resympathicotomy’ for palmar hyperhidrosis: analysis of 42 cases. Ann Thorac Surg 2001;72:895-8. https://doi.org/10.1016/S0003-4975(01)02852-1.
- Little AG. Video-assisted thoracic surgery sympathectomy for hyperhidrosis. Arch Surg 2004;139:586-9. https://doi.org/10.1001/archsurg.139.6.586.
- Lladó A, León L, Valls-Solé J, Mena P, Callejas MA, Peri JM. Changes in the sympathetic skin response after thoracoscopic sympathectomy in patients with primary palmar hyperhidrosis. Clin Neurophysiol 2005;116:1348-54. https://doi.org/10.1016/j.clinph.2005.02.009.
- Malmivaara A, Kuukasjarvi P, Autti-Ramo I, Kovanen N, Makela M. Effectiveness and safety of endoscopic thoracic sympathectomy for excessive sweating and facial blushing: a systematic review. Int J Technol Assess Health Care 2007;23:54-62. https://doi.org/10.1017/S0266462307051574.
- Malone PS, Cameron AE, Rennie JA. Endoscopic thoracic sympathectomy in the treatment of upper limb hyperhidrosis. Ann R Coll Surg Engl 1986;68:93-4.
- Masters A, Rennie JA. Endoscopic transthoracic sympathectomy for idiopathic upper limb hyperhidrosis. Clin Auton Res 1992;2:349-52. https://doi.org/10.1007/BF01824306.
- Menna C, Ibrahim M, Andreetti C, Ciccone AM, D’Andrilli A, Poggi C, et al. Two-stage unilateral versus one-stage bilateral single-port sympathectomy for palmar and axillary hyperhidrosis. Interact Cardiovasc Thorac Surg 2012;15.
- Misiak P, Jablonski S, Rzepkowska-Misiak B, Piskorz L, Brocki M, Wcislo S, et al. Evaluation of the effectiveness of thoracic sympathectomy in the treatment of primary hyperhidrosis of hands and armpits using the measurement of skin resistance. Wideochir 2012;7:147-55. https://doi.org/10.5114/wiitm.2011.26843.
- Montaser-Kouhsari L, Zartab H, Fanian F, Noorian N, Sadr B, Nassiri-Kashani M, et al. Comparison of intradermal injection with iontophoresis of abobotulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat 2014;25:337-41. https://doi.org/10.3109/09546634.2012.739679.
- Mordant P, Castier Y, Maury JM, Karsenti A, Paraskevas N, Cerceau P, et al. Management of invalidating primary hyperhidrosis. Sang Thrombose Vaisseaux 2010;22:302-10.
- Moya J, Ramos R, Morera R, Villalonga R, Perna V, Macia I, et al. Thoracic sympathicolysis for primary hyperhidrosis: a review of 918 procedures. Surg Endosc 2006;20:598-602. https://doi.org/10.1007/s00464-005-0557-z.
- Muhammad M, Allam A. Thoracoscopic sympathectomy using single port vs. multiple ports as a treatment for palmar hyperhidrosis. Innovations 2014;9.
- Nakamura H, Haruki T, Adachi Y, Fujioka S, Miwa K, Taniguchi Y. Patient satisfaction after endoscopic thoracic sympathectomy for palmar hyperhidrosis. Yonago Acta Medica 2008;51:55-60.
- Naumann M, Hamm H. Treatment of axillary hyperhidrosis. Br J Surg 2002;89:259-61. https://doi.org/10.1046/j.0007-1323.2001.02017.x.
- Neidhardt S, Rocha-Unold M, Sommer S, Neureuther C, Jost W. Botulinumtoxin in the treatment of axillary hyperhidrosis – longitudinal analysis. Klin Neurophysiol 2010;41. https://doi.org/10.1055/s-0030-1250999.
- Neumayer C, Zacherl J, Holak G, Jakesz R, Bischof G. Experience with limited endoscopic thoracic sympathetic block for hyperhidrosis and facial blushing. Clin Auton Res 2003;13:I52-7. https://doi.org/10.1007/s10286-003-1113-3.
- Neumayer C, Panhofer P, Jakesz R, Zacherl J, Bischof G. Surgical treatment of facial hyperhidrosis and blushing: mid-term results after endoscopic sympathetic block and review of the literature. Eur Surg 2005;37:127-36. https://doi.org/10.1007/s10353-005-0146-3.
- Nicholson ML, Dennis MJ, Hopkinson BR. Endoscopic transthoracic sympathectomy: successful in hyperhidrosis but can the indications be extended?. Ann R Coll Surg Engl 1994;76:311-14.
- Nieuwenhuis F. The effectiveness of oxybutynin for hyperhidrosis. Huisarts Wet 2015;58. https://doi.org/10.1007/s12445-015-0176-4.
- Odderson IR. Axillary hyperhidrosis: treatment with botulinum toxin A. Arch Phys Med Rehabil 1998;79:350-2. https://doi.org/10.1016/S0003-9993(98)90020-X.
- Ojimba TA, Cameron AE. Drawbacks of endoscopic thoracic sympathectomy. Br J Surg 2004;91:264-9. https://doi.org/10.1002/bjs.4511.
- Oncel M, Sudam GS, Karabagli H, Kara I, Celik JB. Palmar skin temperature importance during transthoracic endoscopic sympathectomy for palmar hyperhidrosis. J Neurol Sci Turk 2012;29:285-90.
- Penna V, Iblher N, Stark BG. Histologic findings in axillary hydradenosuction. Aesthetic Plast Surg 2007;31:16-8. https://doi.org/10.1007/s00266-006-0192-7.
- Phadke VA, Joshi RS, Khopkar US, Wadhwa SL. Comparison of topical methenamine, glutaraldehyde and tap water Iontophoresis for palmoplantar hyperhidrosis. Indian J Dermatol Venereol Leprol 1995;61:346-8.
- Ponce-Olivera RM, Armas A, Tirado-Sanchez A, Fierro-Arias L. Craniofacial hyperhidrosis treatment with botulinum toxin type A. Dermatol Rev Mex 2014;58:514-22.
- Purtuloglu T, Deniz S, Atim A, Tekindur S, Gurkok S, Kurt E. A new target of percutaneus sympathic radiofrequency thermocoagulation for treatment of palmar hyperhidrosis: T4. Agri Derg 2013;25:36-40. https://doi.org/10.5505/agri.2013.09226.
- Purtuloglu T, Atim A, Deniz S, Kavakli K, Sapmaz E, Gurkok S, et al. Effect of radiofrequency ablation and comparison with surgical sympathectomy in palmar hyperhidrosis. Eur J Cardiothorac Surg 2013;43:e151-4. https://doi.org/10.1093/ejcts/ezt024.
- Raja SN, Campbell JN. Risk-benefit ratio for surgical sympathectomy: dilemmas in clinical decision making. J Pain 2000;1:261-4. https://doi.org/10.1054/jpai.2000.14261.
- Ramos R, Moya J, Morera R, Masuet C, Perna V, Macia I, et al. An assessment of anxiety in patients with primary hyperhidrosis before and after endoscopic thoracic sympathicolysis. Eur J Cardiothorac Surg 2006;30:228-31. https://doi.org/10.1016/j.ejcts.2006.05.018.
- Ramos R, Moya J, Macia I, Morera R, Escobar I, Perna V, et al. Anatomical redistribution of sweating after T2-T3 thoracoscopic sympathicolysis: a study of 210 patients. Surg Endosc 2007;21:2030-3. https://doi.org/10.1007/s00464-007-9262-4.
- Ramos R, Masuet C, Badia M, Perna V, Macia I, Escobar I, et al. Quantification of eccrine sweat glands with acetylcholine sweat-spot test and anatomical redistribution of sweating after T2-T3 thoracoscopic sympathicolysis. Surg Endosc 2009;23:321-6. https://doi.org/10.1007/s00464-008-9922-z.
- Ramos R, Ureña A, Rivas F, Macia I, Rosado G, Pequeño S, et al. Impact of T3 thoracoscopic sympathectomy on pupillary function: a cause of partial Horner’s syndrome?. Surg Endosc 2012;26:1146-52. https://doi.org/10.1007/s00464-011-2022-5.
- Ramsaroop L, Singh B, Moodley J, Partab P, Satyapal KS. Anatomical basis for a successful upper limb sympathectomy in the thoracoscopic era. Clin Anat 2004;17:294-9. https://doi.org/10.1002/ca.10238.
- Rayner CR, Ritchie ID, Stark GP. Axillary hyperhidrosis, 20% aluminum chloride hexahydrate, and surgery. Br Med J 1980;280. https://doi.org/10.1136/bmj.280.6224.1168.
- Rezai K. Suction curettage of the sweat glands – an update. Dermatol Surg 2009;35:1126-9. https://doi.org/10.1111/j.1524-4725.2009.01198.x.
- Rezende RM, Luz FB. Surgical treatment of axillary hyperhidrosis by suction-curettage of sweat glands. An Bras Dermatol 2014;89:940-54. https://doi.org/10.1590/abd1806-4841.20142873.
- Rieger R, Pedevilla S. Retroperitoneoscopic lumbar sympathectomy for the treatment of plantar hyperhidrosis: technique and preliminary findings. Surg Endosc 2007;21:129-35. https://doi.org/10.1007/s00464-005-0690-8.
- Rieger R, Loureiro Mde P, Pedevilla S, de Oliveira RA. Endoscopic lumbar sympathectomy following thoracic sympathectomy in patients with palmoplantar hyperhidrosis. World J Surg 2011;35:49-53. https://doi.org/10.1007/s00268-010-0801-0.
- Roddy SP. Evaluation of quality of life over time among 453 patients with hyperhidrosis submitted to endoscopic thoracic sympathectomy. J Vasc Surg 2012;55. https://doi.org/10.1016/j.jvs.2011.11.046.
- Rodriguez-Lopez JM, Sastre-Rincon JA, Del Barrio E, Martinez-Ragues G, Vargas-Fajardo C, Muriel C. Outpatient video-thorascopic sympathectomy for palmar and axillary hyperhidrosis. J Cardiothorac Vasc Anesth 2010;24.
- Saadia D, Voustianiouk A, Wang A, Kaufmann H. Treatment of primary palmar hyperhidrosis with botulinum toxin type A. A single-blind, two-dose, parallel group trial. Neurology 2001;56:A470-1.
- Schestatsky P, Callejas M, Valls-Sole J. Temperature-induced electrodermal activity in patients with primary palmar hyperhidrosis. J Neurol 2009;256.
- Scholes KT, Crow KD, Ellis JP, Harman RR, Saihan EM. Axillary hyperhidrosis treated with alcoholic solution of aluminium chloride hexahydrate. Br Med J 1978;2:84-5. https://doi.org/10.1136/bmj.2.6130.84.
- Sciuchetti JF, Corti F, Ballabio D, Angeli MC. Results, side effects and complications after thoracoscopic sympathetic block by clamping. The monza clinical experience. Clin Auton Res 2008;18:80-3. https://doi.org/10.1007/s10286-008-0460-5.
- Shen JL, Lin GS, Li WM. A new strategy of iontophoresis for hyperhidrosis. J Am Acad Dermatol 1990;22:239-41. https://doi.org/10.1016/0190-9622(90)70031-C.
- Simonyi S, Jog M, Beauchamp R, Miller R, Bhogal M, Wein T. Interim analysis of a large ongoing phase IV prospective observational cohort study (MDS on BOTOX utility mobility) of botulinum toxin type a on health utility in patients receiving treatment for approved therapeutic indications in Canada. Value Health 2010;13. https://doi.org/10.1016/S1098-3015(10)72683-8.
- Solish N. Long-term efficacy of subcutaneous sweat gland suction curettage for axillary hyperhidrosis: a prospective gravimetrically controlled study: commentary. Dermatol Surg 2008;34.
- Stefaniak T, Pirski MI, Oseka T, Kobiela J, Proczko-Markuszewska M, Horyd R, et al. Simultaneous bilateral transthoracic sympathectomy through posterior access in Lin-Telaranta modification for primary hyperhidrosis. Wideochir 2009;4:47-52.
- Stefaniak TJ. Ćwigoń M. Long-term results of thoracic sympathectomy for primary hyperhidrosis. Pol Przegl Chir 2013;85:247-52. https://doi.org/10.2478/pjs-2013-0038.
- Stefaniak T, Ćwigoń M, Vingerhoets AJ, Dobosz L, Kaczor M, Cwaliński T, et al. Influence of thoracoscopic sympathectomy on tendency to cry – case-controlled study. Wideochir Inne Tech Maloinwazyjne 2013;8:315-20. https://doi.org/10.5114/wiitm.2011.35134.
- Streker M, Reuther T, Verst S, Kerscher M. Axillary hyperhidrosis – efficacy and tolerability of an aluminium chloride antiperspirant. Prospective evaluation on 20 patients with idiopathic axillary hyperhidrosis. Hautarzt 2010;61:139-44. https://doi.org/10.1007/s00105-009-1841-y.
- Streker M, Reuther T, Hagen L, Kerscher M. Hyperhidrosis plantaris – a randomized, half-side trial for efficacy and safety of an antiperspirant containing different concentrations of aluminium chloride. J Dtsch Dermatol Ges 2012;10:115-19. https://doi.org/10.1111/j.1610-0387.2011.07750.x.
- Suh DH, Lee SJ, Kim K, Ryu HJ. Transient median and ulnar neuropathy associated with a microwave device for treating axillary hyperhidrosis. Dermatol Surg 2014;40:482-5. https://doi.org/10.1111/dsu.12425.
- Swaile DF, Elstun LT, Benzing KW. Clinical studies of sweat rate reduction by an over-the-counter soft-solid antiperspirant and comparison with a prescription antiperspirant product in male panelists. Br J Dermatol 2012;166:22-6. https://doi.org/10.1111/j.1365-2133.2011.10786.x.
- Swinehart JM. Treatment of axillary hyperhidrosis: combination of the starch-iodine test with the tumescent liposuction technique. Dermatol Surg 2000;26:392-6. https://doi.org/10.1046/j.1524-4725.2000.00604.x.
- Tang H, Xue L, Xu Z, Li B, Zhao X, Wu B. A new surgical procedure for palmar hyperhidrosis: is it possible to perform endoscopic sympathectomy under intravenous anaesthesia without intubation?. Interact Cardiovasc Thorac Surg 2013;17. https://doi.org/10.1093/icvts/ivt372.44.
- Toolabi K, Ahmadi H, Elyasinia F, Parsaei R. Thoracoscopic sympathicotomy for treatment of hyperhidrosis. Arch Neurosci 2015;2:1-3. https://doi.org/10.5812/archneurosci.19514.
- Umezawa A, Seki Y, Kasama K, Negishi Y, Kurokawa Y. Outcome comparing t3-t4 with t2-t4 sympathectomy for palmer hyperhidrosis. Surg Endosc 2011;25.
- Ureña A, Ramos R, Masuet C, Macia I, Rivas F, Escobar I, et al. An assessment of plantar hyperhidrosis after endoscopic thoracic sympathicolysis. Eur J Cardiothorac Surg 2009;36:360-3. https://doi.org/10.1016/j.ejcts.2009.02.062.
- Van Schil PEY. Awake one stage bilateral thoracoscopic sympathectomy for palmar hyperhidrosis: a safe outpatient procedure. Eur J Cardiothorac Surg 2005;28. https://doi.org/10.1016/j.ejcts.2005.03.025.
- Vorkamp T, Foo FJ, Khan S, Schmitto JD, Wilson P. Hyperhidrosis: evolving concepts and a comprehensive review. Surgeon 2010;8:287-92. https://doi.org/10.1016/j.surge.2010.06.002.
- Wein T, Jog M, Beauchamp R, Miller R, Bhogal M, Simonyi S. Baseline characteristics of patients enrolled in a large ongoing Canadian phase IV prospective observational cohort study (MDs on BOTOX UTILITY - MOBILITY) of botulinum toxin type A (BOTOX) for approved therapeutic indications. Can J Neurol Sci 2010;1.
- Weiss D, Tseng T, Stoller M. Transperitoneal laparoscopic bilateral lumbar sympathectomy: a treatment option for plantar and genital hyperhidrosis. J Urol 2010;183:e127-8. https://doi.org/10.1016/j.juro.2010.02.387.
- Wollina U, Konrad H. About finding the right dose, or, is there no evidence outside prospective controlled trials?. J Am Acad Dermatol 2004;50. https://doi.org/10.1016/S0190-9622(03)02086-3.
- Wolosker N, Yazbek G, Milanez de Campos JR, Kauffman P, Ishy A, Puech-Leao P. Evaluation of plantar hyperhidrosis in patients undergoing video-assisted thoracoscopic sympathectomy. Clin Auton Res 2007;17:172-6. https://doi.org/10.1007/s10286-007-0420-5.
- Woolery-Lloyd H. Aluminum chloride 15% a hexahydrate in a salicylic acid 2% gel: a novel topical agent for hyperhidrosis with decreased irritation. J Am Acad Dermatol 2009;1.
- Woolery-Lloyd H, Valins W. Aluminum chloride hexahydrate in a salicylic acid gel: a novel topical agent for hyperhidrosis with decreased irritation. J Clin Aesthet Dermatol 2009;2:28-31.
- Wörle B, Rapprich S, Heckmann M. Definition and treatment of primary hyperhidrosis. J Dtsch Dermatol Ges 2007;5:625-8. https://doi.org/10.1111/j.1610-0387.2007.06409.x.
- Yanagishita T, Tamada Y, Ohshima Y, Ito K, Akita Y, Watanabe D. Histological localization of aluminum in topical aluminum chloride treatment for palmar hyperhidrosis. J Dermatol Sci 2012;67:69-71. https://doi.org/10.1016/j.jdermsci.2012.02.016.
- Youssef T, Soliman M. Unilateral sequential endoscopic thoracic sympathectomy for palmar hyperhidrosis: a proposed technique to overcome compensatory hyperhidrosis and improve plantar hyperhidrosis. J Laparoendosc Adv Surg Tech A 2015;25:370-4. https://doi.org/10.1089/lap.2014.0620.
- Zaba R, Samborski W, Zaba Z, Talaga-Kieronczyk E. The treatment of hyperhidrosis of hands and feet using iontophoresis. Abstract P20-26 The 12th Congress of the European Academy of Dermatology and Venereology. Barcelona, Spain 15–18th October 2003. J Eur Acad Dermatol Venereol 2003;17.
- Zackrisson T, Eriksson B, Hosseini N, Johnels B, Krogstad AL. Patients with hyperhidrosis have changed grip force, coefficient of friction and safety margin. Acta Neurol Scand 2008;117:279-84. https://doi.org/10.1111/j.1600-0404.2007.00938.x.
Appendix 1 Literature search strategies
Hyperhidrosis effectiveness literature searching
| 3572 records identified after deduplication | |
|---|---|
| Databases searched | |
| AMED | 17 records |
| British Nursing Index | 35 records |
| CINAHL | 276 records |
| CENTRAL | 206 records |
| CDSR | 1 record |
| DARE | 4 records |
| EMBASE | 3122 records |
| HTA database | 4 records |
| MEDLINE | 2162 records |
| NHS EED | 2 records |
| PsycINFO | 53 records |
| PubMed | 1989 records |
Allied and Complementary Medicine Database (via OvidSP)
Date range searched: 1985 to January 2016.
Date searched: 12 January 2016.
Search strategy
-
exp Hyperhidrosis/ (24)
-
hyperhidrosis.ti,ab. (31)
-
hyperhydrosis.ti,ab. (2)
-
(excess$ adj2 sweat$).ti,ab. (15)
-
(primary HH or secondary HH).ti,ab. (0)
-
1 or 2 or 3 or 4 or 5 (45)
-
(aluminum adj2 (chloride or hydrochloride)).ti,ab. (2)
-
(aluminium adj2 (chloride or hydrochloride)).ti,ab. (4)
-
(antiperspirant$ or deodorant$).ti,ab. (4)
-
driclor.ti,ab. (0)
-
anhydrol forte.ti,ab. (0)
-
7 or 8 or 9 or 10 or 11 (9)
-
6 and 12 (1)
-
Iontophoresis/ (45)
-
(iontophoresis or iontophoreses).ti,ab. (74)
-
14 or 15 (79)
-
6 and 16 (6)
-
exp Botulinum Toxins/ (391)
-
botulinum toxin$.ti,ab. (415)
-
botox.ti,ab. (55)
-
18 or 19 or 20 (467)
-
6 and 21 (6)
-
Cholinergic Blocking Drugs.ti,ab. (0)
-
cholinergic receptor blocking agent$.ti,ab. (0)
-
muscarinic antagonist$.ti,ab. (3)
-
muscarinic receptor blocking agent$.ti,ab. (0)
-
(quaternary ammonium adj (compound$ or derivative$)).ti,ab. (4)
-
glycopyrronium bromide.ti,ab. (1)
-
robinul.ti,ab. (1)
-
glycopyrrolate.ti,ab. (4)
-
propantheline.ti,ab. (0)
-
anticholinergic$.ti,ab. (79)
-
pro-banthine.ti,ab. (0)
-
oxybutynin.ti,ab. (4)
-
methantheline.ti,ab. (0)
-
vagantin.ti,ab. (0)
-
methantheliniumbromide.ti,ab. (0)
-
atropine.ti,ab. (134)
-
23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 (219)
-
6 and 39 (0)
-
sympathectom$.ti,ab. (21)
-
sympathicotom$.ti,ab. (0)
-
sympathotom$.ti,ab. (0)
-
(sympathetic adj2 (ablation or surgery or block$ or excision$)).ti,ab. (19)
-
endoscopic thoracic sympathectom$.ti,ab. (1)
-
ETS.ti,ab. (14)
-
41 or 42 or 43 or 44 or 45 or 46 (53)
-
6 and 47 (5)
-
curettage.ti,ab. (63)
-
curretage.ti,ab. (2)
-
49 or 50 (65)
-
6 and 51 (0)
-
Lasers/ (285)
-
laser$.ti,ab. (906)
-
53 or 54 (940)
-
6 and 55 (0)
-
Microwaves/ (26)
-
microwave$.ti,ab. (86)
-
57 or 58 (89)
-
6 and 59 (0)
-
Ultrasonic therapy/ (259)
-
ultrasound.ti,ab. (1482)
-
61 or 62 (1533)
-
6 and 63 (0)
-
(lipectom$ or liposuction).ti,ab. (6)
-
6 and 65 (0)
-
miraDry.ti,ab. (0)
-
bilateral axillae aspiration.ti,ab. (0)
-
shelley$ procedure$.ti,ab. (0)
-
((remove$ or removal or removing) adj2 sweat gland$).ti,ab. (0)
-
6 and 70 (0)
-
(clonidine or diltiazem or benzodiazepine$).ti,ab. (236)
-
6 and 72 (1)
-
13 or 17 or 22 or 40 or 48 or 52 or 56 or 60 or 64 or 66 or 67 or 68 or 69 or 71 or 73 (17)
British Nursing Index (via ProQuest)
Date searched: 13 January 2016.
Search strategy
(Hyperhidrosis OR Hyperhydrosis) OR (excess* N2 sweat*) OR (“primary HH” OR “secondary HH”)
Cumulative Index of Nursing and Allied Health Literature (via EBSCOhost)
Date searched: 12 January 2016.
Search strategy
S1 hyperhidrosis OR hyperhidrosis OR excess* N2 sweat* OR primary HH OR secondary HH (564)
S2 ( aluminum N2 (chloride or hydrochloride) ) OR ( aluminium N2 (chloride or hydrochloride) ) OR ( antiperspirant* or deodorant* ) OR driclor OR anhydrol forte (222)
S3 iontophoresis or iontophoreses (509)
S4 botulinum toxin* OR botox (4080)
S5 cholinergic receptor blocking agent* OR muscarinic antagonist* OR muscarinic receptor blocking agent* OR quaternary ammonium compound* OR quaternary ammonium derivative* OR glycopyrronium bromide OR robinul OR glycopyrrolate* OR propantheline OR anticholinergic* OR pro-banthine OR oxybutynin (3093)
S6 methantheline OR vagantin OR methantheliniumbromide OR atropine (1493)
S7 sympathectom* OR sympathicotom* OR sympathotom* OR ( sympathetic N2 (ablation or surgery or block* or excision*) ) OR endoscopic thoracic sympathectom* OR ETS (1386)
S8 curettage OR curretage OR laser* OR microwave* OR ultrasound OR ultrasonic (47,577)
S9 ( lipectom* or liposuction ) OR miraDry OR bilateral axillae aspiration OR shelley* procedure* OR ( (remove* or removal or removing) N2 (sweat gland*) ) (530)
S10 Clonidine OR diltiazem OR Benzodiazepine* (6750)
S11 S1 AND S2 (24)
S12 S1 AND S3 (46)
S13 S1 AND S4 (119)
S14 S1 AND S5 (27)
S15 S1 AND S6 (2)
S16 S1 AND S7 (114)
S17 S1 AND S8 (22)
S18 S1 AND S9 (4)
S19 S1 AND S10 (10)
S20 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 (276)
The Cochrane Library (via John Wiley)
Date searched: 12 January 2016.
Note, the same search strategy was used to search CDSR, DARE, CENTRAL, HTA database and NHS EED.
Search strategy
#1 MeSH descriptor: [Hyperhidrosis] explode all trees
#2 hyperhidrosis:ti,ab,kw (Word variations have been searched)
#3 hyperhydrosis:ti,ab,kw (Word variations have been searched)
#4 excess* near/2 sweat*:ti,ab,kw (Word variations have been searched)
#5 primary HH or secondary HH:ti,ab,kw (Word variations have been searched)
#6 #1 or #2 or #3 or #4 or #5
#7 MeSH descriptor: [Antiperspirants] explode all trees
#8 MeSH descriptor: [Deodorants] explode all trees
#9 MeSH descriptor: [Aluminum Compounds] explode all trees
#10 aluminum near/2 (chloride or hydrochloride):ti,ab,kw or aluminum near/2 (chloride or hydrochloride):ti,ab,kw or antiperspirant* or deodorant*:ti,ab,kw or driclor:ti,ab,kw or anhydrol forte:ti,ab,kw (Word variations have been searched)
#11 #7 or #8 or #9 or #10
#12 #6 and #11
#13 MeSH descriptor: [Iontophoresis] explode all trees
#14 iontophoresis or iontophoreses:ti,ab,kw (Word variations have been searched)
#15 #13 or #14
#16 #6 and #15
#17 MeSH descriptor: [Botulinum Toxins] explode all trees
#18 botulinum toxin:ti,ab,kw or botox:ti,ab,kw (Word variations have been searched)
#19 #17 or #18
#20 #6 and #19
#21 MeSH descriptor: [Cholinergic Antagonists] explode all trees
#22 MeSH descriptor: [Muscarinic Antagonists] explode all trees
#23 MeSH descriptor: [Quaternary Ammonium Compounds] explode all trees
#24 MeSH descriptor: [Glycopyrrolate] explode all trees
#25 glycopyrronium bromide:ti,ab,kw or “Robinul”:ti,ab,kw or “glycopyrrolate”:ti,ab,kw or “propantheline”:ti,ab,kw or anticholinergic*:ti,ab,kw (Word variations have been searched)
#26 pro-banthine:ti,ab,kw or “oxybutynin”:ti,ab,kw or “methantheline”:ti,ab,kw or vagantin:ti,ab,kw or methantheliniumbromide:ti,ab,kw (Word variations have been searched)
#27 “atropine”:ti,ab,kw (Word variations have been searched)
#28 #21 or #22 or #23 or #24 or #25 or #26 or #27
#29 #6 and #28
#30 MeSH descriptor: [Sympathectomy] explode all trees
#31 sympathectom*:ti,ab,kw or sympathicotom*:ti,ab,kw or sympathotom*:ti,ab,kw or sympathetic near/2 (ablation or surgery or block* or excision*):ti,ab,kw or (endoscopic thoracic sympathectom*) or ETS:ti,ab,kw (Word variations have been searched)
#32 #30 or #31
#33 #6 and #32
#34 MeSH descriptor: [Curettage] explode all trees
#35 “curettage”:ti,ab,kw or curretage:ti,ab,kw (Word variations have been searched)
#36 #34 or #35
#37 #6 and #36
#38 MeSH descriptor: [Lasers] explode all trees
#39 laser*:ti,ab,kw (Word variations have been searched)
#40 #38 or #39
#41 #6 and #40
#42 MeSH descriptor: [Microwaves] explode all trees
#43 microwave*:ti,ab,kw (Word variations have been searched)
#44 #42 or #43
#45 #6 and #44
#46 MeSH descriptor: [Ultrasonic Therapy] explode all trees
#47 “ultrasound”:ti,ab,kw (Word variations have been searched)
#48 #46 or #47
#49 #6 and #48
#50 MeSH descriptor: [Lipectomy] explode all trees
#51 lipectom* or liposuction:ti,ab,kw (Word variations have been searched)
#52 #50 or #51
#53 #6 and #52
#54 miraDry:ti,ab,kw or bilateral axillae aspiration:ti,ab,kw or shelley* procedure*:ti,ab,kw or (remove* or removal or removing) near/2 (sweat gland*):ti,ab,kw (Word variations have been searched)
#55 MeSH descriptor: [Clonidine] explode all trees
#56 MeSH descriptor: [Diltiazem] explode all trees
#57 MeSH descriptor: [Benzodiazepines] explode all trees
#58 #55 or #56 or #57
#59 #6 and #58
#60 #12 or #16 or #20 or #29 or #33 or #37 or #41 or #45 or #49 or #53 or #59
EMBASE
Date range searched: 1974 to 8 January 2016.
Date searched: 12 January 2016.
Search strategy
-
Hyperhidrosis/ (6531)
-
hyperhidrosis.ti,ab. (3530)
-
hyperhydrosis.ti,ab. (352)
-
(excess$ adj2 sweat$).ti,ab. (778)
-
(primary HH or secondary HH).ti,ab. (13)
-
1 or 2 or 3 or 4 or 5 (7562)
-
antiperspirant agent/ or deodorant agent/ (1077)
-
Aluminum Derivative/ (4496)
-
(aluminum adj2 (chloride or hydrochloride)).ti,ab. (1106)
-
(aluminium adj2 (chloride or hydrochloride)).ti,ab. (704)
-
(antiperspirant$ or deodorant$).ti,ab. (846)
-
driclor.ti,ab. (1)
-
anhydrol forte.ti,ab. (0)
-
7 or 8 or 9 or 10 or 11 or 12 or 13 (7499)
-
6 and 14 (260)
-
Iontophoresis/ (9004)
-
(iontophoresis or iontophoreses).ti,ab. (4755)
-
16 or 17 (10,143)
-
6 and 18 (361)
-
Botulinum Toxin A/ (15,690)
-
botulinum toxin$.ti,ab. (14,121)
-
botox.ti,ab. (2489)
-
20 or 21 or 22 (21,445)
-
6 and 23 (1011)
-
exp Cholinergic Receptor Blocking Agent/ or exp Muscarinic Receptor Blocking Agent/ (158,851)
-
exp Quaternary Ammonium Derivative/ (69,462)
-
Glycopyrrolate/ (5067)
-
Glycopyrronium Bromide/ (5067)
-
glycopyrronium bromide.ti,ab. (140)
-
robinul.ti,ab. (18)
-
glycopyrrolate.ti,ab. (988)
-
propantheline.ti,ab. (451)
-
anticholinergic$.ti,ab. (14,310)
-
pro-banthine.ti,ab. (31)
-
oxybutynin.ti,ab. (1551)
-
methantheline.ti,ab. (54)
-
vagantin.ti,ab. (3)
-
methantheliniumbromide.ti,ab. (2)
-
atropine.ti,ab. (30,771)
-
25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 (221,161)
-
6 and 40 (630)
-
exp Sympathectomy/ (9187)
-
sympathectom$.ti,ab. (7408)
-
sympathicotom$.ti,ab. (148)
-
sympathotom$.ti,ab. (26)
-
(sympathetic adj2 (ablation or surgery or block$ or excision$)).ti,ab. (3191)
-
endoscopic thoracic sympathectom$.ti,ab. (196)
-
ETS.ti,ab. (10895)
-
42 or 43 or 44 or 45 or 46 or 47 or 48 (25,151)
-
6 and 49 (1398)
-
Curettage/ (10,938)
-
curettage.ti,ab. (11,284)
-
curretage.ti,ab. (139)
-
51 or 52 or 53 (16,384)
-
6 and 54 (88)
-
exp Laser/ (96,883)
-
laser$.ti,ab. (201,279)
-
56 or 57 (217,779)
-
6 and 58 (138)
-
Microwave Radiation/ (17,993)
-
microwave$.ti,ab. (28,836)
-
60 or 61 (31,972)
-
6 and 62 (28)
-
exp Ultrasound Therapy/ (19,942)
-
ultrasonic therapy.ti,ab. (489)
-
ultrasound.ti,ab. (251,254)
-
64 or 65 or 66 (264,491)
-
6 and 67 (61)
-
Lipectomy/ (1690)
-
(lipectom$ or liposuction).ti,ab. (3524)
-
69 or 70 (4289)
-
6 and 71 (58)
-
miraDry.ti,ab. (2)
-
bilateral axillae aspiration.ti,ab. (0)
-
shelley$ procedure$.ti,ab. (1)
-
((remove$ or removal or removing) adj2 sweat gland$).ti,ab. (14)
-
6 and 76 (9)
-
Clonidine/ (38,187)
-
Diltiazem/ (26,350)
-
Benzodiazepine/ (22,631)
-
78 or 79 or 80 (84,820)
-
6 and 81 (206)
-
15 or 19 or 24 or 41 or 50 or 55 or 59 or 63 or 68 or 72 or 73 or 74 or 75 or 77 or 82 (3122)
MEDLINE(R) In-Process & Other Non-Indexed Citations [Ovid MEDLINE(R)]
Date range searched: 1946 to present.
Date searched: 12 January 2016.
Search strategy
-
exp Hyperhidrosis/ (3163)
-
hyperhidrosis.ti,ab. (2736)
-
hyperhydrosis.ti,ab. (181)
-
(excess$ adj2 sweat$).ti,ab. (526)
-
(primary HH or secondary HH).ti,ab. (10)
-
1 or 2 or 3 or 4 or 5 (4434)
-
antiperspirants/ or deodorants/ (442)
-
Aluminum Compounds/ (4302)
-
(aluminum adj2 (chloride or hydrochloride)).ti,ab. (776)
-
(aluminium adj2 (chloride or hydrochloride)).ti,ab. (357)
-
(antiperspirant$ or deodorant$).ti,ab. (582)
-
driclor.ti,ab. (1)
-
anhydrol forte.ti,ab. (0)
-
7 or 8 or 9 or 10 or 11 or 12 or 13 (5773)
-
6 and 14 (173)
-
Iontophoresis/ (6927)
-
(iontophoresis or iontophoreses).ti,ab. (3962)
-
16 or 17 (8461)
-
6 and 18 (201)
-
exp Botulinum Toxins/ (13,444)
-
botulinum toxin$.ti,ab. (10,541)
-
botox.ti,ab. (1499)
-
20 or 21 or 22 (16,204)
-
6 and 23 (643)
-
exp Cholinergic Antagonists/ or exp Muscarinic Antagonists/ (75,966)
-
Quaternary Ammonium Compounds/ (22,384)
-
Glycopyrrolate/ (764)
-
glycopyrronium bromide.ti,ab. (67)
-
robinul.ti,ab. (17)
-
glycopyrrolate.ti,ab. (798)
-
propantheline.ti,ab. (382)
-
anticholinergic$.ti,ab. (10,401)
-
pro-banthine.ti,ab. (31)
-
oxybutynin.ti,ab. (1148)
-
methantheline.ti,ab. (58)
-
vagantin.ti,ab. (3)
-
methantheliniumbromide.ti,ab. (2)
-
atropine.ti,ab. (26,989)
-
25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 (116,077)
-
6 and 39 (229)
-
Sympathectomy/ (8186)
-
sympathectom$.ti,ab. (6642)
-
sympathicotom$.ti,ab. (122)
-
sympathotom$.ti,ab. (27)
-
(sympathetic adj2 (ablation or surgery or block$ or excision$)).ti,ab. (2523)
-
endoscopic thoracic sympathectom$.ti,ab. (165)
-
ETS.ti,ab. (9160)
-
41 or 42 or 43 or 44 or 45 or 46 or 47 (22,526)
-
6 and 48 (1158)
-
Curettage/ (3791)
-
curettage.ti,ab. (9465)
-
curretage.ti,ab. (89)
-
50 or 51 or 52 (11,530)
-
6 and 53 (70)
-
Lasers/ (33,355)
-
laser$.ti,ab. (205,410)
-
55 or 56 (210,678)
-
6 and 57 (69)
-
Microwaves/ (14,078)
-
microwave$.ti,ab. (24,745)
-
59 or 60 (27,361)
-
6 and 61 (15)
-
Ultrasonic therapy/ (8596)
-
ultrasound.ti,ab. (176,119)
-
63 or 64 (180,393)
-
6 and 65 (33)
-
Lipectomy/ (3579)
-
(lipectom$ or liposuction).ti,ab. (3378)
-
67 or 68 (4803)
-
6 and 69 (67)
-
miraDry.ti,ab. (0)
-
bilateral axillae aspiration.ti,ab. (0)
-
shelley$ procedure$.ti,ab. (1)
-
((remove$ or removal or removing) adj2 sweat gland$).ti,ab. (12)
-
6 and 74 (9)
-
Clonidine/ (13,027)
-
Diltiazem/ (6062)
-
Benzodiazepines/ (19,607)
-
76 or 77 or 78 (38,568)
-
6 and 79 (27)
-
15 or 19 or 24 or 40 or 49 or 54 or 58 or 62 or 66 or 70 or 71 or 72 or 73 or 75 or 80 (2162)
PsycINFO (via OvidSP)
Date range searched: 1806 to January week 1 2016.
Date searched: 12 January 2016.
Search strategy
-
hyperhidrosis.ti,ab. (145)
-
hyperhydrosis.ti,ab. (26)
-
(excess$ adj2 sweat$).ti,ab. (89)
-
(primary HH or secondary HH).ti,ab. (1)
-
1 or 2 or 3 or 4 (236)
-
Aluminum/ (155)
-
(aluminum adj2 (chloride or hydrochloride)).ti,ab. (21)
-
(aluminium adj2 (chloride or hydrochloride)).ti,ab. (8)
-
(antiperspirant$ or deodorant$).ti,ab. (38)
-
driclor.ti,ab. (0)
-
anhydrol forte.ti,ab. (0)
-
6 or 7 or 8 or 9 or 10 or 11 (206)
-
5 and 12 (1)
-
(iontophoresis or iontophoreses).ti,ab. (172)
-
5 and 14 (0)
-
exp Botulinum Toxin/ (794)
-
botulinum toxin$.ti,ab. (1052)
-
botox.ti,ab. (158)
-
16 or 17 or 18 (1177)
-
5 and 19 (26)
-
exp Cholinergic Blocking Drugs/ (14,427)
-
cholinergic receptor blocking agent$.ti,ab. (1)
-
muscarinic antagonist$.ti,ab. (350)
-
muscarinic receptor blocking agent$.ti,ab. (1)
-
(quaternary ammonium adj (compound$ or derivative$)).ti,ab. (13)
-
glycopyrronium bromide.ti,ab. (4)
-
robinul.ti,ab. (0)
-
glycopyrrolate.ti,ab. (40)
-
propantheline.ti,ab. (4)
-
anticholinergic$.ti,ab. (2470)
-
pro-banthine.ti,ab. (1)
-
oxybutynin.ti,ab. (52)
-
methantheline.ti,ab. (0)
-
vagantin.ti,ab. (0)
-
methantheliniumbromide.ti,ab. (0)
-
atropine.ti,ab. (1746)
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 (17,317)
-
5 and 37 (12)
-
Sympathectomy/ (77)
-
sympathectom$.ti,ab. (289)
-
sympathicotom$.ti,ab. (3)
-
sympathotom$.ti,ab. (1)
-
(sympathetic adj2 (ablation or surgery or block$ or excision$)).ti,ab. (124)
-
endoscopic thoracic sympathectom$.ti,ab. (2)
-
ETS.ti,ab. (536)
-
39 or 40 or 41 or 42 or 43 or 44 or 45 (949)
-
5 and 46 (15)
-
curettage.ti,ab. (33)
-
curretage.ti,ab. (0)
-
48 or 49 (33)
-
5 and 50 (0)
-
laser$.ti,ab. (2561)
-
5 and 52 (0)
-
microwave$.ti,ab. (207)
-
5 and 54 (0)
-
Ultrasound/ (1198)
-
ultrasound.ti,ab. (2502)
-
ultrasonic therapy.ti,ab. (4)
-
56 or 57 or 58 (2865)
-
5 and 59 (0)
-
(lipectom$ or liposuction).ti,ab. (29)
-
5 and 61 (0)
-
miraDry.ti,ab. (0)
-
bilateral axillae aspiration.ti,ab. (0)
-
shelley$ procedure$.ti,ab. (0)
-
((remove$ or removal or removing) adj2 sweat gland$).ti,ab. (0)
-
5 and 66 (0)
-
Clonidine/ (1307)
-
diltiazem.ti,ab. (127)
-
Benzodiazepines/ (4610)
-
68 or 69 or 70 (6025)
-
5 and 71 (3)
-
13 or 15 or 20 or 38 or 47 or 51 or 53 or 55 or 60 or 62 or 63 or 64 or 65 or 67 or 72 (53)
PubMed
URL: www.ncbi.nlm.nih.gov/pubmed
Date searched: 14 January 2016.
Search strategy
#1 Search (((((Hyperhidrosis[MeSH Terms]) OR hyperhidrosis[Title/Abstract]) OR hyperhydrosis[Title/Abstract]) OR “excess* sweat*”[Title/Abstract]) OR “primary HH”[Title/Abstract]) OR “secondary HH”[Title/Abstract] (4147)
#2 Search ((((((((((Antiperspirants[MeSH Terms]) OR Deodorants[MeSH Terms]) OR Aluminum Compounds [MeSH Terms]) OR “aluminum chloride”[Title/Abstract]) OR “aluminum hydrochloride”[Title/Abstract]) OR “aluminium chloride”[Title/Abstract]) OR “aluminium hydrochloride”[Title/Abstract]) OR antiperspirant* [Title/Abstract]) OR deodorant*[Title/Abstract]) OR driclor[Title/Abstract]) OR “anhydrol forte” [Title/ Abstract] (27,492)
#3 Search (#1 AND #2) (180)
#4 Search ((Iontophoresis[MeSH Terms]) OR iontophoresis[Title/Abstract]) OR iontophoreses[Title/Abstract] (8441)
#5 Search (#1 AND #4) (201)
#6 Search ((Botulinum Toxins[MeSH Terms]) OR “botulinum toxin*”[Title/Abstract]) OR botox[Title/
Abstract] (15,884)
#7 Search (#1 AND #6) (624)
#8 Search ((((((((((((((Cholinergic Antagonists[MeSH Terms]) OR Muscarinic Antagonists[MeSH Terms]) OR Quaternary Ammonium Compounds[MeSH Terms]) OR Glycopyrrolate[MeSH Terms]) OR “glycopyrronium bromide”[Title/Abstract]) OR robinul[Title/Abstract]) OR glycopyrrolate[Title/Abstract]) OR propantheline [Title/Abstract]) OR anticholinergic*[Title/Abstract]) OR pro-banthine[Title/Abstract]) OR oxybutynin[Title/ Abstract]) OR methantheline[Title/Abstract]) OR vagantin[Title/Abstract]) OR methantheliniumbromide[Title/ Abstract]) OR atropine[Title/Abstract] (156,869)
#9 Search (#1 AND #8) (221)
#10 Search (((((((((Sympathectomy/[MeSH Terms]) OR sympathectom*[Title/Abstract]) OR sympathicotom* [Title/Abstract]) OR sympathotom*[Title/Abstract]) OR “sympathetic ablation”[Title/Abstract]) OR “sympathetic surgery”[Title/Abstract]) OR “sympathetic block*”[Title/Abstract]) OR “sympathetic excision*”[Title/Abstract]) OR “endoscopic thoracic sympathectom*” [Title/Abstract]) OR ETS[Title/ Abstract] (17,469)
#11 Search (#1 AND #10) (993)
#12 Search ((Curettage[MeSH Terms]) OR curettage[Title/Abstract]) OR curretage[Title/Abstract] (13,080)
#13 Search (#1 AND #12) (67)
#14 Search (((Lasers[MeSH Terms]) OR Microwaves[MeSH Terms]) OR laser*[Title/Abstract]) OR
microwave*[Title/Abstract] (241,816)
#15 Search (#1 AND #14) (80)
#17 Search (Search ((((Ultrasonic therapy[MeSH Terms]) OR ultrasound[Title/Abstract]) OR Lipectomy [MeSH Terms]) OR lipectom*[Title/Abstract]) OR liposuction[Title/Abstract]) (4668)
#18 Search (#1 AND #17) (49)
#19 Search ((((bilateral axillae aspiration[Title/Abstract]) OR “shelley* procedure*”[Title/Abstract]) OR
“remove* sweat gland*”[Title/Abstract]) OR “removal sweat gland*”[Title/Abstract]) OR “removing
sweat gland*”[Title/Abstract] (6)
#20 Search ((Clonidine[MeSH Terms]) OR Diltiazem[MeSH Terms]) OR Benzodiazepines[MeSH Terms] (78,025)
#21 Search (#1 AND #20) (34)
#22 Search (#3 OR #5 OR #7 OR #9 OR #11 OR #13 OR #15 OR #18 OR #19 OR #21) (1989)
Hyperhidrosis quality-of-life literature searching
The searches for hyperhidrosis and quality of life combined the six initial lines of the effectiveness search strategy with a published MEDLINE search filter (URL: www.yhec.co.uk/yhec-content/uploads/2015/06/Poster-374-Sensitivity-Of-A-Search-Filter.pdf) designed to retrieve studies reporting health state utility values. The search filter was adapted as required for each of the databases searched.
| Databases searched | |
|---|---|
| AMED | 5 records |
| British Nursing Index | 2 records |
| CINAHL | 55 records |
| EMBASE | 646 records |
| MEDLINE | 99 records |
| PsycINFO | 19 records |
| PubMed | 197 records |
Three hundred and thirty-seven records identified after deduplication (against the other quality-of-life search results and the effectiveness search results).
Allied and Complementary Medicine Database (via OvidSP)
Date range searched: 1985 to January 2016.
Date searched: 21 January 2016.
Search strategy
-
exp Hyperhidrosis/ (24)
-
hyperhidrosis.ti,ab. (31)
-
hyperhydrosis.ti,ab. (2)
-
(excess$ adj2 sweat$).ti,ab. (15)
-
(primary HH or secondary HH).ti,ab. (0)
-
1 or 2 or 3 or 4 or 5 (45)
-
“Quality of Life”/ (7722)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab,kf. (71)
-
(quality adjusted or adjusted life year$).ti,ab,kf. (112)
-
disability adjusted life.ti,ab,kf. (13)
-
daly$1.ti,ab,kf. (10)
-
((index adj3 wellbeing) or (quality adj3 wellbeing) or qwb).ti,ab,kf. (46)
-
(multiattribute$ or multi attribute$).ti,ab,kf. (19)
-
(utility adj3 (score$1 or scoring or valu$ or measur$ or evaluat$ or scale$1 or instrument$1 or weight or weights or weighting or information or data or unit or units or health$ or life or estimat$ or elicit$ or disease$ or mean or cost$ or expenditure$1 or gain or gains or loss or losses or lost or analysis or index$ or indices or overall or reported or calculat$ or range$ or increment$ or state or states or status)).ti,ab,kf. (571)
-
utility.ab. /freq=2 (228)
-
utilities.ti,ab,kf. (123)
-
disutili$.ti,ab,kf. (5)
-
(HSUV or HSUVs).ti,ab,kf. (0)
-
health$1 year$1 equivalent$1.ti,ab,kf. (0)
-
(hye or hyes).ti,ab,kf. (0)
-
(hui or hui1 or hui2 or hui3).ti,ab,kf. (67)
-
(illness state$1 or health state$1).ti,ab,kf. (145)
-
(euro qual or euro qual5d or euro qol5d or eq-5d or eq5-d or eq5d or euroqual or euroqol or euroqual5d or euroqol5d).ti,ab,kf. (302)
-
(eq-sdq or eqsdq).ti,ab,kf. (0)
-
(short form$ or shortform$).ti,ab,kf. (1525)
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).ti,ab,kf. (1524)
-
(sf6 or sf 6 or sf6d or sf 6d or sf six or sfsix or sf8 or sf 8 or sf eight or sfeight).ti,ab,kf. (55)
-
(sf12 or sf 12 or sf twelve or sftwelve).ti,ab,kf. (177)
-
(sf16 or sf 16 or sf sixteen or sfsixteen).ti,ab,kf. (0)
-
(sf20 or sf 20 or sf twenty or sftwenty).ti,ab,kf. (7)
-
(15D or 15-D or 15 dimension).ti,ab,kf. (37)
-
(standard gamble$ or sg).ti,ab,kf. (87)
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).ti,ab,kf. (53)
-
7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 (9833)
-
6 and 34 (5)
-
dermatology life quality index.ti,ab. (3)
-
DLQI.ti,ab. (2)
-
Skindex$.ti,ab. (2)
-
Hyperhidrosis Disease Severity Scale.ti,ab. (0)
-
HDSS.ti,ab. (0)
-
Hyperhidrosis Scale.ti,ab. (0)
-
Hyperhidrosis Quality of Life Questionnaire.ti,ab. (0)
-
HQLQ.ti,ab. (1)
-
Illness Intrusiveness Rating Scale.ti,ab. (2)
-
IIRS.ti,ab. (2)
-
36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 (9)
-
6 and 46 (1)
-
35 or 47 (5)
British Nursing Index (via ProQuest)
Date searched: 21 January 2016.
Search strategy
(((SU.EXACT.EXPLODE(“Health and Quality of Life”) OR (qaly* OR qald* OR qale* OR qtime*) OR ((quality adjusted) OR (adjusted life year*)) OR (disability adjusted life OR daly*) OR ((index N3 wellbeing) OR (quality N3 wellbeing) OR qwb) OR (multiattribute* OR multi attribute*) OR (utility N3 (score* OR scoring OR valu* OR measur* OR evaluat* OR scale* OR instrument* OR weight OR weights OR weighting OR information OR data OR unit OR units OR health* OR life OR estimat* OR elicit* OR disease* OR mean OR cost* OR expenditure* OR gain OR gains OR loss OR losses OR lost OR analysis OR index* OR indices OR overall OR reported OR calculat* OR range* OR increment OR state OR states OR status)) OR (utilities OR disutili*)) OR ((HSUV OR HSUVs) OR (health* year* equivalent*) OR (hye OR hyes) OR (hui OR hui1 OR hui2 OR hui3) OR ((illness state*) OR (health state*))) OR (“euro qual” OR “euro qual5D” OR “euro qol5d” OR eq-5d OR eq5-d OR eq5d OR euroqual OR euroquol OR euroqual5d OR euroqol5d) OR ((eq-sdq OR eqsdq) OR (“short form*” OR shortform*) OR (sf36* OR “sf 36*” OR “sf thirtysix” OR “sf thirty six”) OR (sf6 OR “sf 6” OR sf6d OR “sf 6d” OR “sf six” OR sfsix OR sf8 OR “sf 8” OR “sf eight” OR sfeight) OR (sf12 OR “sf 12” OR “sf twelve” OR sftwelve) OR (sf16 OR “sf 16” OR “sf sixteen” OR sfsixteen)) OR ((sf20 OR “sf 20” OR “sf twenty” OR sftwenty) OR (15D OR 15-D OR “15 dimension”) OR (“standard gamble*” OR sg) OR (“time trade off*” OR timetradeoff* OR tto))) OR (“dermatology life quality index” OR DLQI OR (Skindex* OR HQLQ) OR (“Hyperhidrosis Disease Severity Scale” OR HDSS) OR (“Hyperhidrosis Scale” OR “Hyperhidrosis Quality of Life Questionnaire”) OR (“Illness Intrusiveness Rating Scale” OR IIRS))) AND (Hyperhidrosis OR Hyperhydrosis OR (excess* N2 sweat*) OR (“primary HH” OR “secondary HH”))
Cumulative Index of Nursing and Allied Health Literature (via EBSCOhost)
Date searched: 21 January 2016.
Search strategy
S1 hyperhidrosis OR hyperhidrosis OR excess* N2 sweat* OR primary HH OR secondary HH
S2 (MH “Quality of Life”)
S3 (MH “Quality-Adjusted Life Years”)
S4 TX ( (qaly* or qald* or qale* or qtime*) ) OR TX ( (quality adjusted or adjusted life year*) ) OR TX
disability adjusted life OR TX daly* OR TX ( (index N3 wellbeing) or (quality N3 wellbeing) or qwb ) OR TX ( (multiattribute* or multi attribute*) ) OR TX ( utility N3 (score* or scoring or valu* or measur* or evaluat* or scale* or instrument* or weight or weights or weighting or information or data or unit or units or health* or life or estimat* or elicit* or disease* or mean or cost* or expendi . . .
S5 TX ( (hui or hui1 or hui2 or hui3) ) OR TX ( (illness state* or health state*) ) OR TX ( (euro qual or euro qual5d or euro qol5d or eq-5d or eq5-d or eq5d or euroqual or euroqol or euroqual5d or euroqol5d) ) OR TX ( (eq-sdq or eqsdq) ) OR TX ( (short form* or shortform*) ) OR TX ( (sf36* or sf 36* or sf thirtysix or sf thirty six) ) OR TX ( (sf6 or sf 6 or sf6d or sf 6d or sf six or sfsix or sf8 or sf 8 or sf eight or sfeight) ) OR TX ( (sf12 or sf 12 or sf twelve or sftwelve) ) OR TX ( (sf16 or . . .
S6 TX (time trade off* or time tradeoff* or tto or timetradeoff*)
S7 “dermatology life quality index” OR DLQI OR Skindex* OR “Hyperhidrosis Disease Severity Scale” OR HDSS OR “Hyperhidrosis Scale” OR “Hyperhidrosis Quality of Life Questionnaire” OR HQLQ OR “Illness Intrusiveness Rating Scale” OR IIRS (175)
S8 S2 OR S3 OR S4 OR S5 OR S6 OR S7 (186,197)
S9 S1 AND S8 Search modes - Boolean/Phrase (55)
EMBASE (via OvidSP)
Date range searched: 1974 to 20 January 2016.
Date searched: 21 January 2016.
Search strategy
-
exp Hyperhidrosis/ (6552)
-
hyperhidrosis.ti,ab. (3538)
-
hyperhydrosis.ti,ab. (352)
-
(excess$ adj2 sweat$).ti,ab. (782)
-
(primary HH or secondary HH).ti,ab. (13)
-
1 or 2 or 3 or 4 or 5 (7583)
-
Quality of Life/ (305,322)
-
Quality-Adjusted Life Year/ (15,384)
-
Quality of Life Index/ (2010)
-
exp Short Form 36/ (17,697)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab,kf. (11,511)
-
(quality adjusted or adjusted life year$).ti,ab,kf. (13,879)
-
disability adjusted life.ti,ab,kf. (2068)
-
daly$1.ti,ab,kf. (2103)
-
((index adj3 wellbeing) or (quality adj3 wellbeing) or qwb).ti,ab,kf. (687)
-
(multiattribute$ or multi attribute$).ti,ab,kf. (728)
-
(utility adj3 (score$1 or scoring or valu$ or measur$ or evaluat$ or scale$1 or instrument$1 or weight or weights or weighting or information or data or unit or units or health$ or life or estimat$ or elicit$ or disease$ or mean or cost$ or expenditure$1 or gain or gains or loss or losses or lost or analysis or index$ or indices or overall or reported or calculat$ or range$ or increment$ or state or states or status)).ti,ab,kf. (31,947)
-
utility.ab. /freq=2 (16,325)
-
utilities.ti,ab,kf. (7320)
-
disutili$.ti,ab,kf. (499)
-
(HSUV or HSUVs).ti,ab,kf. (35)
-
health$1 year$1 equivalent$1.ti,ab,kf. (39)
-
(hye or hyes).ti,ab,kf. (98)
-
(hui or hui1 or hui2 or hui3).ti,ab,kf. (1508)
-
(illness state$1 or health state$1).ti,ab,kf. (7072)
-
(euro qual or euro qual5d or euro qol5d or eq-5d or eq5-d or eq5d or euroqual or euroqol or euroqual5d or euroqol5d).ti,ab,kf. (9732)
-
(eq-sdq or eqsdq).ti,ab,kf. (0)
-
(short form$ or shortform$).ti,ab,kf. (27,842)
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).ti,ab,kf. (25,379)
-
(sf6 or sf 6 or sf6d or sf 6d or sf six or sfsix or sf8 or sf 8 or sf eight or sfeight).ti,ab,kf. (2979)
-
(sf12 or sf 12 or sf twelve or sftwelve).ti,ab,kf. (4775)
-
(sf16 or sf 16 or sf sixteen or sfsixteen).ti,ab,kf. (35)
-
(sf20 or sf 20 or sf twenty or sftwenty).ti,ab,kf. (298)
-
(15D or 15-D or 15 dimension).ti,ab,kf. (4859)
-
(standard gamble$ or sg).ti,ab,kf. (9779)
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).ti,ab,kf. (1878)
-
7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 (400,530)
-
6 and 37 (594)
-
dermatology life quality index.ti,ab. (1620)
-
DLQI.ti,ab. (1480)
-
Skindex$.ti,ab. (488)
-
Hyperhidrosis Disease Severity Scale.ti,ab. (67)
-
HDSS.ti,ab. (284)
-
Hyperhidrosis Scale.ti,ab. (0)
-
Hyperhidrosis Quality of Life Questionnaire.ti,ab. (0)
-
HQLQ.ti,ab. (22)
-
Illness Intrusiveness Rating Scale.ti,ab. (31)
-
IIRS.ti,ab. (75)
-
39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 (2767)
-
6 and 49 (121)
-
38 or 50 (646)
MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) (via OvidSP)
Date range searched: 1946 to present.
Date searched: 21 January 2016.
Search strategy
-
exp Hyperhidrosis/ (3213)
-
hyperhidrosis.ti,ab. (2673)
-
hyperhydrosis.ti,ab. (181)
-
(excess$ adj2 sweat$).ti,ab. (518)
-
(primary HH or secondary HH).ti,ab. (10)
-
1 or 2 or 3 or 4 or 5 (4375)
-
Quality-Adjusted Life Years/ (7896)
-
Value of Life/ (5469)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab,kf. (6327)
-
(quality adjusted or adjusted life year$).ti,ab,kf. (9728)
-
disability adjusted life.ti,ab,kf. (1716)
-
daly$1.ti,ab,kf. (1600)
-
((index adj3 wellbeing) or (quality adj3 wellbeing) or qwb).ti,ab,kf. (434)
-
(multiattribute$ or multi attribute$).ti,ab,kf. (569)
-
(utility adj3 (score$1 or scoring or valu$ or measur$ or evaluat$ or scale$1 or instrument$1 or weight or weights or weighting or information or data or unit or units or health$ or life or estimat$ or elicit$ or disease$ or mean or cost$ or expenditure$1 or gain or gains or loss or losses or lost or analysis or index$ or indices or overall or reported or calculat$ or range$ or increment$ or state or states or status)).ti,ab,kf. (21,810)
-
utility.ab. /freq=2 (10,938)
-
utilities.ti,ab,kf. (4656)
-
disutili$.ti,ab,kf. (275)
-
(HSUV or HSUVs).ti,ab,kf. (22)
-
health$1 year$1 equivalent$1.ti,ab,kf. (40)
-
(hye or hyes).ti,ab,kf. (57)
-
(hui or hui1 or hui2 or hui3).ti,ab,kf. (1051)
-
(illness state$1 or health state$1).ti,ab,kf. (4369)
-
(euro qual or euro qual5d or euro qol5d or eq-5d or eq5-d or eq5d or euroqual or euroqol or euroqual5d or euroqol5d).ti,ab,kf. (5345)
-
(eq-sdq or eqsdq).ti,ab,kf. (0)
-
(short form$ or shortform$).ti,ab,kf. (21,115)
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).ti,ab,kf. (15,765)
-
(sf6 or sf 6 or sf6d or sf 6d or sf six or sfsix or sf8 or sf 8 or sf eight or sfeight).ti,ab,kf. (2242)
-
(sf12 or sf 12 or sf twelve or sftwelve).ti,ab,kf. (2820)
-
(sf16 or sf 16 or sf sixteen or sfsixteen).ti,ab,kf. (19)
-
(sf20 or sf 20 or sf twenty or sftwenty).ti,ab,kf. (310)
-
(15D or 15-D or 15 dimension).ti,ab,kf. (4004)
-
(standard gamble$ or sg).ti,ab,kf. (7088)
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).ti,ab,kf. (1349)
-
7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 (95,049)
-
6 and 35 (27)
-
dermatology life quality index.ti,ab. (914)
-
DLQI.ti,ab. (676)
-
Skindex$.ti,ab. (271)
-
Hyperhidrosis Disease Severity Scale.ti,ab. (40)
-
HDSS.ti,ab. (239)
-
Hyperhidrosis Scale.ti,ab. (0)
-
Hyperhidrosis Quality of Life Questionnaire.ti,ab. (0)
-
HQLQ.ti,ab. (10)
-
Illness Intrusiveness Rating Scale.ti,ab. (19)
-
IIRS.ti,ab. (51)
-
37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 (1544)
-
6 and 47 (74)
-
36 or 48 (99)
PsycINFO (via OvidSP)
Date range searched: 1806 to January week 3 2016.
Date searched: 21 January 2016.
Search strategy
-
hyperhidrosis.ti,ab. (145)
-
hyperhydrosis.ti,ab. (26)
-
(excess$ adj2 sweat$).ti,ab. (89)
-
(primary HH or secondary HH).ti,ab. (1)
-
1 or 2 or 3 or 4 (236)
-
“Quality of Life”/ (31,012)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab,kf. (785)
-
(quality adjusted or adjusted life year$).ti,ab,kf. (1212)
-
disability adjusted life.ti,ab,kf. (269)
-
daly$1.ti,ab,kf. (470)
-
((index adj3 wellbeing) or (quality adj3 wellbeing) or qwb).ti,ab,kf. (309)
-
(multiattribute$ or multi attribute$).ti,ab,kf. (885)
-
(utility adj3 (score$1 or scoring or valu$ or measur$ or evaluat$ or scale$1 or instrument$1 or weight or weights or weighting or information or data or unit or units or health$ or life or estimat$ or elicit$ or disease$ or mean or cost$ or expenditure$1 or gain or gains or loss or losses or lost or analysis or index$ or indices or overall or reported or calculat$ or range$ or increment$ or state or states or status)).ti,ab,kf. (6903)
-
utility.ab. /freq=2 (5182)
-
utilities.ti,ab,kf. (1544)
-
disutili$.ti,ab,kf. (158)
-
(HSUV or HSUVs).ti,ab,kf. (2)
-
health$1 year$1 equivalent$1.ti,ab,kf. (4)
-
(hye or hyes).ti,ab,kf. (13)
-
(hui or hui1 or hui2 or hui3).ti,ab,kf. (439)
-
(illness state$1 or health state$1).ti,ab,kf. (1170)
-
(euro qual or euro qual5d or euro qol5d or eq-5d or eq5-d or eq5d or euroqual or euroqol or euroqual5d or euroqol5d).ti,ab,kf. (1298)
-
(eq-sdq or eqsdq).ti,ab,kf. (0)
-
(short form$ or shortform$).ti,ab,kf. (9682)
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).ti,ab,kf. (3645)
-
(sf6 or sf 6 or sf6d or sf 6d or sf six or sfsix or sf8 or sf 8 or sf eight or sfeight).ti,ab,kf. (281)
-
(sf12 or sf 12 or sf twelve or sftwelve).ti,ab,kf. (807)
-
(sf16 or sf 16 or sf sixteen or sfsixteen).ti,ab,kf. (0)
-
(sf20 or sf 20 or sf twenty or sftwenty).ti,ab,kf. (42)
-
(15D or 15-D or 15 dimension).ti,ab,kf. (169)
-
(standard gamble$ or sg).ti,ab,kf. (750)
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).ti,ab,kf. (308)
-
6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 (53,974)
-
5 and 33 (14)
-
dermatology life quality index.ti,ab. (23)
-
DLQI.ti,ab. (14)
-
Skindex$.ti,ab. (13)
-
Hyperhidrosis Disease Severity Scale.ti,ab. (3)
-
HDSS.ti,ab. (30)
-
Hyperhidrosis Scale.ti,ab. (0)
-
Hyperhidrosis Quality of Life Questionnaire.ti,ab. (0)
-
HQLQ.ti,ab. (1)
-
Illness Intrusiveness Rating Scale.ti,ab. (12)
-
IIRS.ti,ab. (22)
-
35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 (100)
-
5 and 45 (10)
-
34 or 46 (19)
PubMed
URL: www.ncbi.nlm.nih.gov/pubmed
Date searched: 21 January 2016.
Search strategy
#11 Search (#4 and #10) (197)
#10 Search (#5 or #6 or #7 or #8 or #9) (687,290)
#9 Search (((((((((((((((((((((((“utility overall”) OR “utility reported”) OR “utility calculate*”) OR “utility range*”) OR “utility increment*”) OR “utility state*”) OR “utility status”) OR (utilities OR disutilities)) OR (hsuv OR hsu s)) OR “health* year* equivalent*”) OR ((hye OR hyes))) OR ((hui OR hui1 OR hui2 OR hui3))) OR (“illness state*” OR “health state*”)) OR (“euro qual” OR “euro qual5d” OR “euro qol5d” OR eq-5d OR eq5-d OR eq5d OR euroqol OR euroqol OR euroqual5d OR euroqol5d)) OR (eq sd OR eedq)) OR (“short form*” OR shortform*)) OR (sf36* OR “sf 36*” OR “sf thirtysix” OR “sf thirty six”)) OR (sf6 OR “sf 6” OR sf6d OR “sf 6d” OR “sf six” OR sifsix OR sf8 OR “sf 8” OR “sf eight” OR speight)) OR (sf12 OR “sf 12” OR “sf twelve” OR twelve)) OR (sf16 OR “sf 16” OR “sf sixteen” OR s sixteen)) OR (sf20 OR “sf 20” OR “sf twenty” OR twenty)) OR (15D OR 15-D OR 15 dimension)) OR standard gamble*) OR (“time trade off*” OR “time tradeoff*” OR tto OR “timetradeoff*”) (662,265)
#8 Search (((((((((((((((((((((((“utility overall”) OR “utility reported”) OR “utility calculate*”) OR “utility range*”) OR “utility increment*”) OR “utility state*”) OR “utility status”) OR (utilities or disutilities)) OR (HSUV or HSUVs)) OR “health* year* equivalent*”) OR ((hye or hyes))) OR ((hui or hui1 or hui2 or hui3))) OR (“illness state*” or “health state*”)) OR (“euro qual” or “euro qual5d” or “euro qol5d” or eq-5d or eq5-d or eq5d or euroqual or euroqol or euroqual5d or euroqol5d)) OR (eq-sdq or eqsdq)) OR (“short form*” or shortform*)) OR (sf36* or “sf 36*” or “sf thirtysix” or “sf thirty six”)) OR (sf6 or “sf 6” or sf6d or “sf 6d” or “sf six” or sfsix or sf8 or “sf 8” or “sf eight” or sfeight)) OR (sf12 or “sf 12” or “sf twelve” or sftwelve)) OR (sf16 or “sf 16” or “sf sixteen” or sfsixteen)) OR (sf20 or “sf 20” or “sf twenty” or sftwenty)) OR (15D or 15-D or 15 dimension)) OR standard gamble*) OR (“time trade off*” or “time tradeoff*” or tto or “timetradeoff*”) (219,267)
#7 Search (((((((((((((((“utility information”) OR “utility data”) OR “utility unit*”) OR “utility health*”) OR “utility life”) OR “utility estimate*”) OR “Utility elicit*”) OR “utility disease*”) OR “utility mean*”) OR “utility cost*”) OR “utility expenditure*”) OR “utility gain*”) OR “utility loss*”) OR “utility analysis”) OR “utility index”) OR “utility indices” (68,284)
#6 Search (((((((((((((((((qaly* or qald* or qale* or qtime*))) OR “quality adjusted”) OR “adjusted life year*”) OR “disability adjusted life”) OR Daly*) OR “index of wellbeing”) OR “quality of wellbeing”) OR qwb) OR (multiattribute* or “multi attribute*”)) OR “utility score*”) OR “utility scoring”) OR “Utility valu*”) OR “Utility measure*”) OR “Utility scale*”) OR “Utility instrument*”) OR “Utility weight*” (53,715)
#5 Search (Quality-Adjusted Life Years[MeSH Terms]) OR value of life[MeSH Terms] (13,132)
#4 Search (Hyperhidrosis[MeSH Terms]) OR hyperhidrosis[Title/Abstract]) OR hyperhydrosis[Title/Abstract]) OR “excess* sweat*”[Title/Abstract]) OR “primary HH”[Title/Abstract]) OR “secondary HH”[Title/Abstract]) (4148)
Hyperhidrosis cost-effectiveness literature searching
The searches for hyperhidrosis and cost-effectiveness combined the six initial lines of the effectiveness search strategy with a published search (URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp), designed to retrieve studies reporting economic evaluations.
One hundred and forty-eight records identified after deduplication (against the effectiveness search results and the quality-of-life search results).
| Databases searched | |
|---|---|
| EMBASE | 167 records |
| MEDLINE | 22 records |
| NHS EED | 18 records |
EMBASE (via OvidSP)
Date range searched: 1974 to 25 January 2016.
Date searched: 26 January 2016.
Note, the EMBASE search retrieved 321 records and of these 167 were downloaded (those not identified by the effectiveness searches).
Search strategy
-
Hyperhidrosis/ (6556)
-
hyperhidrosis.ti,ab. (3538)
-
hyperhydrosis.ti,ab. (352)
-
(excess$ adj2 sweat$).ti,ab. (782)
-
(primary HH or secondary HH).ti,ab. (13)
-
1 or 2 or 3 or 4 or 5 (7587)
-
antiperspirant agent/ or deodorant agent/ (1078)
-
Aluminum Derivative/ (4515)
-
(aluminum adj2 (chloride or hydrochloride)).ti,ab. (1117)
-
(aluminium adj2 (chloride or hydrochloride)).ti,ab. (711)
-
(antiperspirant$ or deodorant$).ti,ab. (846)
-
driclor.ti,ab. (1)
-
anhydrol forte.ti,ab. (0)
-
7 or 8 or 9 or 10 or 11 or 12 or 13 (7535)
-
6 and 14 (260)
-
Iontophoresis/ (9022)
-
(iontophoresis or iontophoreses).ti,ab. (4768)
-
16 or 17 (10164)
-
6 and 18 (362)
-
Botulinum Toxin A/ (15,762)
-
botulinum toxin$.ti,ab. (14,205)
-
botox.ti,ab. (2515)
-
20 or 21 or 22 (21,564)
-
6 and 23 (1014)
-
exp Cholinergic Receptor Blocking Agent/ or exp Muscarinic Receptor Blocking Agent/ (159,313)
-
exp Quaternary Ammonium Derivative/ (69,657)
-
Glycopyrrolate/ (5099)
-
Glycopyrronium Bromide/ (5099)
-
glycopyrronium bromide.ti,ab. (142)
-
robinul.ti,ab. (18)
-
glycopyrrolate.ti,ab. (995)
-
propantheline.ti,ab. (455)
-
anticholinergic$.ti,ab. (14,385)
-
pro-banthine.ti,ab. (31)
-
oxybutynin.ti,ab. (1557)
-
methantheline.ti,ab. (55)
-
vagantin.ti,ab. (3)
-
methantheliniumbromide.ti,ab. (2)
-
atropine.ti,ab. (30,840)
-
25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 (221,819)
-
6 and 40 (634)
-
exp Sympathectomy/ (9206)
-
sympathectom$.ti,ab. (7419)
-
sympathicotom$.ti,ab. (148)
-
sympathotom$.ti,ab. (26)
-
(sympathetic adj2 (ablation or surgery or block$ or excision$)).ti,ab. (3198)
-
endoscopic thoracic sympathectom$.ti,ab. (196)
-
ETS.ti,ab. (10,936)
-
42 or 43 or 44 or 45 or 46 or 47 or 48 (25,220)
-
6 and 49 (1398)
-
Curettage/ (10,955)
-
curettage.ti,ab. (11,307)
-
curretage.ti,ab. (139)
-
51 or 52 or 53 (16,411)
-
6 and 54 (88)
-
exp Laser/ (97,333)
-
laser$.ti,ab. (202,208)
-
56 or 57 (218,773)
-
6 and 58 (138)
-
Microwave Radiation/ (18,060)
-
microwave$.ti,ab. (28,965)
-
60 or 61 (32,105)
-
6 and 62 (28)
-
exp Ultrasound Therapy/ (20,005)
-
ultrasonic therapy.ti,ab. (489)
-
ultrasound.ti,ab. (252,978)
-
64 or 65 or 66 (266,249)
-
6 and 67 (62)
-
Lipectomy/ (1698)
-
(lipectom$ or liposuction).ti,ab. (3544)
-
69 or 70 (4312)
-
6 and 71 (58)
-
miraDry.ti,ab. (2)
-
bilateral axillae aspiration.ti,ab. (0)
-
shelley$ procedure$.ti,ab. (1)
-
((remove$ or removal or removing) adj2 sweat gland$).ti,ab. (14)
-
6 and 76 (9)
-
Clonidine/ (38,239)
-
Diltiazem/ (26,392)
-
Benzodiazepine/ (22,687)
-
78 or 79 or 80 (84,970)
-
6 and 81 (207)
-
15 or 19 or 24 or 41 or 50 or 55 or 59 or 63 or 68 or 72 or 73 or 74 or 75 or 77 or 82 (3131)
-
health economics/ (34,997)
-
exp economic evaluation/ (237,188)
-
exp health care cost/ (228,071)
-
exp pharmacoeconomics/ (176,482)
-
or/84-87 (525,121)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (696,142)
-
(expenditure$ not energy).ti,ab. (27,775)
-
(value adj2 money).ti,ab. (1645)
-
budget$.ti,ab. (27,781)
-
(or/89) or 90 or 91 or 92 (723,587)
-
88 or 93 (1,013,374)
-
letter.pt. (921,279)
-
editorial.pt. (497,990)
-
note.pt. (625,236)
-
95 or 96 or 97 (2,044,505)
-
94 not 98 (918,417)
-
(metabolic adj cost).ti,ab. (1047)
-
((energy or oxygen) adj cost).ti,ab. (3454)
-
((energy or oxygen) adj expenditure).ti,ab. (23,336)
-
100 or 101 or 102 (26,950)
-
99 not 103 (912,855)
-
exp animal/ (21,031,187)
-
exp animal experiment/ (1,903,901)
-
nonhuman/ (4,670,037)
-
(rat or rats or mouse oor mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (4,193,562)
-
105 or 106 or 107 or 108 (22,606,867)
-
exp human/ (16,657,067)
-
exp human-experiment/ (346,578)
-
110 or 111 (16,658,513)
-
109 not (109 and 112) (5,949,324)
-
104 not 113 (841,578)
-
6 and 114 (321)
-
115 not 83 (167)
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) (via Ovid SP)
Date range searched: 1946 to present.
Date searched: 26 January 2016.
The MEDLINE search retrieved 79 records and of these 22 were downloaded (those not identified by the effectiveness searches).
Search strategy
-
exp Hyperhidrosis/ (3213)
-
hyperhidrosis.ti,ab. (2674)
-
hyperhydrosis.ti,ab. (181)
-
(excess$ adj2 sweat$).ti,ab. (520)
-
(primary HH or secondary HH).ti,ab. (10)
-
1 or 2 or 3 or 4 or 5 (4378)
-
antiperspirants/ or deodorants/ (473)
-
Aluminum Compounds/ (4356)
-
(aluminum adj2 (chloride or hydrochloride)).ti,ab. (778)
-
(aluminium adj2 (chloride or hydrochloride)).ti,ab. (352)
-
(antiperspirant$ or deodorant$).ti,ab. (584)
-
driclor.ti,ab. (1)
-
anhydrol forte.ti,ab. (0)
-
7 or 8 or 9 or 10 or 11 or 12 or 13 (5816)
-
6 and 14 (173)
-
Iontophoresis/ (7056)
-
(iontophoresis or iontophoreses).ti,ab. (3924)
-
16 or 17 (8381)
-
6 and 18 (199)
-
exp Botulinum Toxins/ (12,983)
-
botulinum toxin$.ti,ab. (10,074)
-
botox.ti,ab. (1412)
-
20 or 21 or 22 (15,668)
-
6 and 23 (618)
-
exp Cholinergic Antagonists/ or exp Muscarinic Antagonists/ (78,592)
-
Quaternary Ammonium Compounds/ (22,711)
-
Glycopyrrolate/ (774)
-
glycopyrronium bromide.ti,ab. (65)
-
robinul.ti,ab. (17)
-
glycopyrrolate.ti,ab. (786)
-
propantheline.ti,ab. (378)
-
anticholinergic$.ti,ab. (10,252)
-
pro-banthine.ti,ab. (33)
-
oxybutynin.ti,ab. (1131)
-
methantheline.ti,ab. (66)
-
vagantin.ti,ab. (3)
-
methantheliniumbromide.ti,ab. (2)
-
atropine.ti,ab. (26,905)
-
25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 (118,080)
-
6 and 39 (231)
-
Sympathectomy/ (8586)
-
sympathectom$.ti,ab. (6783)
-
sympathicotom$.ti,ab. (121)
-
sympathotom$.ti,ab. (29)
-
(sympathetic adj2 (ablation or surgery or block$ or excision$)).ti,ab. (2509)
-
endoscopic thoracic sympathectom$.ti,ab. (162)
-
ETS.ti,ab. (8822)
-
41 or 42 or 43 or 44 or 45 or 46 or 47 (22,433)
-
6 and 48 (1132)
-
Curettage/ (3988)
-
curettage.ti,ab. (9354)
-
curretage.ti,ab. (88)
-
50 or 51 or 52 (11,399)
-
6 and 53 (64)
-
Lasers/ (32,487)
-
laser$.ti,ab. (201,104)
-
55 or 56 (206,239)
-
6 and 57 (68)
-
Microwaves/ (14,045)
-
microwave$.ti,ab. (24,617)
-
59 or 60 (27,181)
-
6 and 61 (15)
-
Ultrasonic therapy/ (8490)
-
ultrasound.ti,ab. (172,434)
-
63 or 64 (176,744)
-
6 and 65 (32)
-
Lipectomy/ (2921)
-
(lipectom$ or liposuction).ti,ab. (2868)
-
67 or 68 (4008)
-
6 and 69 (62)
-
miraDry.ti,ab. (0)
-
bilateral axillae aspiration.ti,ab. (0)
-
shelley$ procedure$.ti,ab. (1)
-
((remove$ or removal or removing) adj2 sweat gland$).ti,ab. (11)
-
6 and 74 (9)
-
Clonidine/ (12,706)
-
Diltiazem/ (5969)
-
Benzodiazepines/ (19,158)
-
76 or 77 or 78 (37,710)
-
6 and 79 (27)
-
15 or 19 or 24 or 40 or 49 or 54 or 58 or 62 or 66 or 70 or 71 or 72 or 73 or 75 or 80 (2110)
-
economics/ (26,624)
-
exp “costs and cost analysis”/ or Cost Allocation/ or Cost-Benefit Analysis/ or Cost Control/ or Cost of Illness/ or Cost Sharing/ or Health Care Costs/ or Health Expenditures/ (193,082)
-
economics, dental/ (1874)
-
exp “economics, hospital”/ or Hospital Charges/ or Hospital Costs/ (20,991)
-
economics, medical/ (8839)
-
economics, nursing/ (3931)
-
economics, pharmaceutical/ (2597)
-
(economic$ or cost$ or price or prices or pricing or pharmacoeconomic$).tw. (559,861)
-
(expenditure$ not energy).tw. (20,734)
-
(value adj1 money).tw. (27)
-
budget$.tw. (21,258)
-
82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 (691,881)
-
((energy or oxygen) adj cost).ti,ab. (3160)
-
(metabolic adj cost).ti,ab. (953)
-
((energy or oxygen) adj expenditure).ti,ab. (18,671)
-
or/94-96 (21,995)
-
93 not 97 (686,861)
-
letter.pt. (898,943)
-
editorial.pt. (391,868)
-
historical article.pt. (325,476)
-
99 or 100 or 101 (1,600,318)
-
98 not 102 (655,996)
-
exp animals/ not humans/ (4,173,052)
-
103 not 104 (609,814)
-
6 and 105 (79)
-
106 not 81 (22)
NHS Economic Evaluation Database
Via CRD website URL: www.crd.york.ac.uk/CRDWeb/HomePage.asp
Date searched: 26 January 2016.
Search strategy
-
MeSH DESCRIPTOR Hyperhidrosis EXPLODE ALL TREES (18)
-
(hyperhidrosis) OR (hyperhydrosis) IN NHSEED (2)
-
(“excessive sweating”) IN NHSEED (0)
-
(primary HH) OR (secondary HH) IN NHSEED (0)
-
#1 OR #2 OR #3 OR # 4 (18)
Ongoing and/or unpublished studies
To identify ongoing and/or unpublished studies we searched the Conference Proceedings Citation Index: Science (ISI), the ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform Portal trials registries. Details of the search strategies and results are as follows:
Conference Proceedings Citation Index: Science (ISI) via Web of Science
Date searched: 12 July 2016.
Records retrieved n = 335.
Search strategy
#1 253 TOPIC: (Hyperhidrosis)
Indexes=CPCI-S Timespan=All years
#2 17 TOPIC: (hyperhydrosis)
Indexes=CPCI-S Timespan=All years
#3 31 TOPIC: (excess* NEAR/2 sweat*)
Indexes=CPCI-S Timespan=All years
#4 51 TOPIC: (primary HH) OR TOPIC: (secondary HH)
Indexes=CPCI-S Timespan=All years
#5 335 #4 OR #3 OR #2 OR #1
Indexes=CPCI-S Timespan=All years
Searching for “hyperhidrosis” using ClinicalTrials.gov (URL: https://clinicaltrials.gov/) identified 66 records, whereas using the World Health Organization International Clinical Trials Registry Platform (URL: www.who.int/ictrp/en/) the same search term identified 106 records. Both searches were conducted on 12 July 2016.
Appendix 2 Study details and results tables
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention 1 | Intervention 2 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Dahl and Glent-Madsen 1989;37 RCT (half-side comparison) | 11; Denmark |
Age range: 18–44 years Male: 27% |
NR | Palm | Iontophoresis (DC administration, tap water), 0–20 mA (median 4 mA), 15 minutes, one to five times per week until ‘good subjective effect’ was reported. After the initial treatments (length NR), six patients continued on maintenance treatment (n = 11) | Sham iontophoresis (circuit disconnected) (n = 11) | Sweating (gravimetry): median reduction in sweat production in iontophoresis-treated hand: 38% (p < 0.01) (baseline sweating NR). Median difference between treated and placebo hand: 32% (p < 0.01). Six patients continued maintenance treatment every second week and achieved an 81% (median) reduction at 3 months (p < 0.05). AEs: none observed | Unclear |
| Karakoc et al. 2004;40 interrupted time series | 15; Turkey |
Age range: 15–26 years Male: 47% |
NR | Palm | Sham iontophoresis, low AC current (9–12 mA, 10–15 V and 8–10 Hz), 15 minutes, eight times over 28 days, followed by iontophoresis (DC administration with tap water), DC: 18–22 mA and 40–60 V, 15 minutes, eight times over 28 days (n = 15) | N/A | Sweating (gravimetry): reduced by mean 88% in both hands (from mean 3.1 ± 0.4 g/hour to 0.4 ± 0.1 g/hour in the right hand and from 3.2 ± 0.3 g/hour to 0.4 ± 0.1 g/hour in the left hand); p < 0.001 at 1 week. No significant change at 1 week for placebo | High |
| Stolman 1987;42 RCT (half-side comparison) | 18; USA |
Age range: 20–46 years Male: 44% |
Excessive sweating resulting in a social or occupational handicap | Palm | Iontophoresis (tap water), 12–20 mA, 20 minutes (polarity reversed after 10 minutes), three times per week for 3 weeks (n = 18) | Placebo (tap water tray without electrode) (n = 18) |
Sweating (iodine starch test): 15/18 patients experienced a marked reduction in sweating in the treated hand 5 days after treatment. There was no change in the untreated hand. Two patients did not improve subjectively or by iodine starch test AEs: one patient dropped out because of transient erythema of the foot. Three patients reported slight transient vesiculation of the skin of the hand, 12 patients noticed redness of the skin for a number of hours after treatment, two patients reported intermittent tingling in the treated hand |
Unclear |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention 1 | Intervention 2 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Choi et al. 2013;44 non-RCT (half-side comparison) | 23; South Korea |
Age range: 13–64 years Male: 30% |
NR | Palm | Iontophoresis (dry type), 20 mA, 20 minutes daily for 4 weeks (left palm; n = 23) | No treatment (right palm; n = 23) |
IGA and hydration capacitance: statistically significant difference favouring treatment group in improvement from baseline (data NR) from 2 to 8 weeks’ follow-up and after 8 weeks (p < 0.05) Gravimetry and patient satisfaction: no statistically significant differences between hands after 4 weeks of treatment AEs: mild local events in two patients in treated palm [pruritic erythematous macules (n = 1), hyperpigmentation (n = 1)] |
High |
| Na et al. 2007;43 non-RCT (half-side comparison) | 10; South Korea |
Age range: 18–34 years Male: 70% |
NR | Palm | Iontophoresis (dry type), 5–25 mA/30 minutes daily for 1 week, then every other day for 1 week (one palm; n = 10) | No treatment (contralateral palm; n = 10) |
Sweating (gravimetry): after-treatment treatment group: mean 42% reduction from baseline (from mean 4.3 ± 0.4 g/hour to 2.5 ± 0.4 g/hour); control group: 2% reduction from baseline (from 4.2 ± 0.3 g/hour to 4.0 ± 0.3 g/hour). At 2 weeks after treatment discontinuation treatment group: mean 19% reduction from baseline (3.5 ± 0.4 g/hour); control group: 2% reduction from baseline (4.1 ± 0.4 g/hour). All differences with control group were statistically significant (p < 0.001) Patient satisfaction: 9/10 patients were satisfied with the therapy AEs: erythema, mild local burning in treated hands (number NR) |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention 1 | Intervention 2 | Intervention 3 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Rajagopal and Mallya 2014;88 RCT (crossover) | 60; India |
Age range: 10–43 years Male: 65% |
HDSS 3–4 | Palm | Iontophoresis, 2 × 10 minutes + topical aluminium chloride (20% lotion) three times per week for 4 weeks (n = 30) | BTX-A (100 U) with local anaesthesia (n = 30) | N/A |
HDSS: response (≥ 2 points reduction): 57% of BTX-A patients vs. 27% in the iontophoresis group (p = 0.037) at 4 weeks from baseline. Improvement (≥ 1-point reduction): higher in BTX-A group (80%) than in iontophoresis group (47%) and higher in more severe cases (HDSS 4 at baseline) with BTX-A than with iontophoresis (p = 0.005). In non-responders, significantly higher rates of improvement in those switching from iontophoresis to BTX-A compared with those switching from BTX-A to iontophoresis Sweating (patient reported): statistically significant difference favouring BTX-A in proportion of patients rating improvement as excellent or good (80% vs. 47%; p = 0.037) Effect duration: maintained in all 24 BTX-A responders for approximately ≥ 4 months and all 11 iontophoresis responders approximately ≥ 1 month after end of treatment AEs: none severe; mild-to-moderate pain (n = 8) and mild temporary motor weakness (n = 1) in BTX-A group |
High |
| Wachal et al. 2009;92 non-RCT | 86; Poland |
Age range: 18–43 years Male: 28% |
NR | Palm and possibly other upper limb | Iontophoresis DC, 12–25 mA, 20 minutes every 2–3 days (n = 28) | BTX-A (50 U Botox), topical anaesthesia (EMLA) (n = 22) | Sympathectomy (n = 36) |
Sweating (patient reported, VAS): percentage of patients assessing as good/very good, VAS score: higher in BTX group vs. iontophoresis at 1 month (90.9% vs. 35.7%, statistically significant), 6 months (36.3% vs 28.5%, NS) and 12 months (14.2% vs. 7.1%, NS) Willingness to undergo retreatment: higher for BTX vs. iontophoresis at 1 month (81.8% patients vs. 42.8%, statistically significant), 6 months (54.4% vs. 21.4%, NS) and 12 months (18.1% vs. 7.1%, NS) AEs: iontophoresis: none; BTX-A: NR |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention 1 | Intervention 2 | Intervention 3 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Shimizu et al. 2003;41 RCT | 52; Japan |
Age range: NR Male: 44% |
NR | Palm and feet | Iontophoresis (AC) with tap water, 0–20 V, 4.3 kHz, 30 minutes, once per week (n = 24) | Iontophoresis (AC) + oxybutynin, 0–20 V, 4.3 kHz, 30 minutes, once per week. Oxybutynin oral 4 mg/day (n = 19) | Iontophoresis (DC), 5–10 mA, 30 minutes, once a week (n = 9) |
Sweating (gravimetry): statistically significant mean reduction from baseline in all three treatment groups from after the second treatment (p < 0.05); no significant difference in effectiveness between treatment groups. AC group: reduced by > 32%a (from mean 0.73 ± 0.06 mg/cm2/minute at baseline to below 0.5 mg/cm2/minute) by treatment 2 and reduced by > 66%a (from baseline and to below 0.25 mg/cm2/minute) by treatment 12. AC + oxybutynin group: reduced by > 38%a (from mean 0.81 ± 0.07 mg/cm2/minute at baseline to below 0.5 mg/cm2/minute) by treatment 2 and reduced by > 69%a (from baseline to below 0.25 mg/cm2/minute) by treatment six. DC group: NR AEs: small vesicles on palms in DC group (n = 5), dry mouth and eyes in oxybutynin group (n = 2) |
Unclear |
| Dolianitis et al. 2004;38 non-RCT (half-side comparison) | 20; Australia |
Age range: 12–50 years Male: 30% |
Moderate to severe (patient rated) | Palm (n = 20), feet (n = 1) | Iontophoresis (glycopyrrolate 0.05%), 3–20 mA (median 10 mA), once weekly for 10 minutes (interval based on response). Unilateral (n = 20) | Iontophoresis (tap water), 3–20 mA (median 10 mA), once weekly for 10 minutes. Unilateral (n = 20) | Iontophoresis (glycopyrrolate 0.05%), 3–20 mA (median 10 mA), once weekly for 10 minutes. Unilateral, then other side treated 2–3 weeks after (n = 20) |
Median number of dry hand days: following treatment: tap water 3 days (range 0–15 days), unilateral glycopyrrolate 5 days (range 0–17 days) and bilateral glycopyrrolate 11 days (range 0–31 days). Unilateral vs. tap water, bilateral vs. unilateral, bilateral vs. tap water all statistically significant (p ≤ 0.001) AEs: dry/sore throat (n = 8) with glycopyrrolate (increased in bilateral vs. unilateral) |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention 1 | Intervention 2 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Karakoç et al. 2002;39 case series | 112; Turkey |
Age range: 8–32 years Male: 45% |
NR | Palm | Iontophoresis (DC administration, tap water), DC: 0–30 mA and 0–90 V, 15 minutes each side, eight times over 28 days (n = 112) | N/A |
Sweating (gravimetry): 81% with statistically significant reduction in sweat from baseline. Right hand: baseline 3.0 ± 1.2 g/hour, final for responders (n = 91) 0.4 ± 0.1 g/hour, final for non-responders (n = 21) 2.8 ± 1.0 g/hour. Left hand: baseline 3.0 ± 1.3 g/hour, final for responders (n = 91) 0.5 ± 0.2 g/hour, final for non-responders (n = 21) 2.9 ± 1.0 g/hour. Difference between baseline and final sweat intensity for responders was statistically significant in both hands (p < 0.001) Effect duration: mean first remission period 35 ± 6 days AEs: erythema (n = 12), vesicle (n = 8), discomfort from burning (n = 20) |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention details | Comparators | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Mehrotra et al. 2015;53 RCT | 38; USA |
Age range: 17–68 years Male: 42% |
HDSS 3–4; ≥ 50 mg/5 minutes | Axilla |
Glycopyrrolate wipes 4%; once daily for 4 weeks (n = 12) Glycopyrrolate wipes 2%; once daily for 4 weeks (n = 12) |
Placebo (n = 14) |
Gravimetry: mean reduction in sweat rate was larger with 4% glycopyrrolate (59% reduction,a from mean 384 mg/5 minutes to 157 mg/5 minutes) than 2% glycopyrrolate (48% reduction,a from mean 370 mg/5 minutes to 191 mg/5 minutes) or placebo (16% reduction,a from mean 367 mg/5 minutes to 310 mg/5 minutes) at the end of treatment (data extracted from graph) HDSS: the proportion of patients with reduction of ≥ 2 points was greater in patients receiving glycopyrrolate 4% (50%) than glycopyrrolate 2% (35%) and placebo (9%) at the end of treatment (data extracted from graph) AEs: rates were similar between groups. Three AE were reported in ≥ 2 patients per group: blurred vision (n = 2) in glycopyrrolate 4% group, dry mouth and application site discomfort in at least one glycopyrrolate group (group and n NR) |
High |
| Hyun et al. 2015;51 RCT (half-side) | 39; South Korea |
Age range: 20–66 years Male: 77% |
HDSS 3–4 and ≥ 100 mg/20 minutes on each side of forehead | Face | Glycopyrrolate wipes (2%), nine times over 10 days (n = 39) | Placebo (n = 39) |
Gravimetry: statistically significant difference of 37% (± 11.4) in reduction of sweat production rate favouring intervention vs. placebo at day 10 (p < 0.025). Baseline sweat production NR HDSS: mean (SD) change from baseline on day 10 in HDSS was 1.08 (0.98) in intervention group vs. 0.90 (0.97) in the control group (NS, p > 0.025) AEs: transient headache after intervention (n = 1), no other AE |
Unclear (gravimetry); high (HDSS) |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention details | Comparators | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Baker 2013;91 non-RCT (abstract only) | 40; UK |
Age range: 20–41 years Male: 20% |
NR | Axilla |
Glycopyrrolate 1% spray; twice daily (n = 10) Glycopyrrolate 2% spray; twice daily (n = 10) |
BTX-A (dose NR) (n = 10) No treatment (n = 10) |
HDSS: glycopyrrolate 1% group had significantly less improvement than BTX-A group (p < 0.05); no significant difference between glycopyrrolate 2% and BTX-A at 6 weeks’ follow-up. Differences with no treatment and mean HDSS scores NR AEs: NR |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention | Comparator | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Schollhammer et al. 2015;59 RCT | 62; France |
Age range: 18–62 years Male: 43% |
HDSS ≥ 2 | Generalised (83%); localised (17%) | Oxybutynin, starting dose 2.5 mg daily, increased to maximum 7.5 mg daily, for 6 weeks (n = 32) | Placebo for 6 weeks (n = 30) |
HDSS: ≥ 2-point reduction in HDSS score oxybutynin: 43%, placebo, 7% at 6 weeks (p-value NR). ≥ 1-point reduction in HDSS score: oxybutynin: 60%, placebo, 27% (p < 0.01) at 6 weeks DLQI: mean improvement: oxybutynin 6.9 points (from baseline 11.4 ± 4.1 to 4.5 ± NR),a placebo 2.3 points (from 10.8 ± 4.7 to 8.5 ± NR)a at 6 weeks. Statistically significant difference between groups (p < 0.01) AEs: dry mouth (43% oxybutynin patients vs. 11% placebo patients) (p < 0.01), ‘severe intensity’ in two oxybutynin patients |
Unclear |
| Wolosker et al. 2012;60 RCT | 50; Brazil |
Age range: 18–50 years Male: 27% |
NR | Axilla and palmb | Oxybutynin, starting dose 2.5 mg daily, increased to maximum 10 mg daily, for 6 weeks (n = 25) | Placebo for 6 weeks (n = 25) |
Sweating (bespoke questionnaire): moderate or great improvement in palmar or axillary symptoms: 74% oxybutynin patients vs. 27% placebo patients (difference p < 0.001). Moderate or great improvement in plantar symptoms: 92% oxybutynin patients vs. 13% placebo patients (difference p < 0.001) Quality of life: improved in 74% oxybutynin patients vs. 14% placebo patients (p < 0.001) AEs: moderate or severe dry mouth: 35% oxybutynin patients vs. 9% placebo patients (p = 0.038, measured during 10-mg dose phase) |
Unclear |
| Costa et al. 201457 and Costa et al. 2015;58 RCT | 32; Brazil |
Age range: NR Male: 0% |
Persistent plantar hyperhidrosis despite sympathectomy ≥ 6 months earlier | Feetb | Oxybutynin, starting dose 2.5 mg daily, increased to maximum 10 mg daily, for 30 days (n = 16) | Placebo for 30 days (n = 16) |
Sweating (evaporimetry): significantly improved from baseline (p = 0.01) in the oxybutynin group (right foot: mean 38%a reduction, from 140.3 ± 40.3 g/m2/hour to 87.6 ± 70.2 g/m2/hour after treatment; right hand: 54%a reduction, from 61.7 ± 43.9 g/m2/hour to 28.6 ± 20.5 g/m2/hour; back: 72%a reduction, from 38.2 ± 64.3 g/m2/hour to 10.8 ± 8.7 g/m2/hour; abdomen: 58%a reduction, from 39.7 ± 46.0 g/m2/hour to 16.5 ± 19.2 g/m2/hour), but not the placebo group (right foot: 9%a reduction, from 112.6 ± 49.3 g/m2/hour to 102.2 ± 55.9 g/m2/hour after placebo; right hand: 14%a reduction, from 58.3 ± 39.3 g/m2/hour to 50.4 ± 37.8 g/m2/hour; back: 4%a increase, from 18.2 ± 19.0 g/m2/hour to 19.0 ± 27.9 g/m2/hour; abdomen: 12%a increase, from 24.0 ± 18.1 g/m2/hour to 26.8 ± 31.4 g/m2/hour) Quality of life: significantly improved in the oxybutynin group from baseline from ‘very good’ (adjusted quality-of-life mean 40.4 ± 14.4) at baseline to ‘excellent’ (17.5 ± 11.9) at follow-up (p = 0.001), but not the placebo group (‘very good’ at both time points) AEs: dry mouth (100% vs. 44%), constipation (31% vs. 6%) and drowsiness (18% vs. 6%) more common with oxybutynin than placebo |
Unclear |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention | Comparator | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Müller et al. 2013;69 RCT | 339; Germany |
Age range: NR Male: NR |
≥ 50 mg/5 minutes | Axilla and/or palm | Methantheline bromide, 3 × 50 mg daily for 28 days (n = 171) | Placebo, three times per day for 28 days (n = 168) |
Sweating (gravimetry, axillae): reduced by 41% (from 168 ± 146 mg/5 minutes at baseline to 99 ± 98 mg/5 minutes on day 28) in intervention group vs. 19% (161 ± 119 mg at baseline to 130 ± 119 mg/5 minutes on day 28) in placebo group (p = 0.0013) Sweating (gravimetry, palms): no significant difference between groups at follow-up HDSS: statistically significant reduction in intervention group vs. placebo (0.8 vs. 0.5 points reduction, p = 0.002) at day 28 DLQI: statistically significant improvement from baseline favouring intervention: 6.9 pointsa (± NR) reduction, from 16.6 ± 5.3 at baseline to 9.7 ± 6.8 at day 28; placebo: 4.2 pointsa (± NR) reduction, from 16.4 ± 5.6 to 12.2 ± 6.9 at day 28. Difference between groups statistically significant (p = 0.003) AEs: more frequent in intervention group: 147 events in 101 patients in the treatment group vs. 61 events in 50 patients in the placebo group. Most common: dry mouth, 88 events in treatment group vs. 28 events in placebo group. 19 methantheline patients dropped out of study because of AEs or were lost to follow-up. 17 placebo patients dropped out of the study |
Unclear (HDSS, DLQI, axilla gravimetry); high (palm gravimetry) |
| Hund et al. 2004;68 RCT | 42; Germany |
Age range: 18–54 years Male: 25% |
≥ 50 mg/minute | Axilla and/or palm | Methantheline bromide, 2 × 50 mg daily for 4 weeks (n = 23) | Placebo for 4 weeks (n = 19) |
Sweating (gravimetry, axillae): intervention group: 40%a reduction in sweating from baseline [from 89.2 ± 73.4 mg/minute to 53.3 ± 48.7 mg/minute, (p = 0.02)], compared with no change from baseline for the placebo group at 4 weeks Sweating (gravimetry, palms): no differences between intervention and placebo in palmar hyperhidrosis at 4 weeks. NS difference in number of patients with overall sweat reduction from baseline to < 50 mg/minute at 4 weeks (treatment: 16/23, placebo 9/18) AEs: dry mouth (47.8% at week 2 and 34.8% at week 4 in intervention group vs. 11.1% and 5.6% in placebo group; significant between group difference p < 0.02). No other differences in AEs reported |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Body site | Intervention 1 | Intervention 2 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Glogau 2007;71 RCT (half-side comparison) | 12; USA |
Age range: NR Male: 50% |
≥ 50 mg/5 minutes | Axilla | Topical BTX-A (200 U Botox), 60 minutes (n = 12) | Placebo (n = 12) |
Sweating (gravimetry): 40% difference in mean sweat reduction between groups favouring treated axillae at 4 weeks: 65% (± 22%) reduction from baseline in the BTX-A group (from 89.8 mg/5 minutes to 31.4 mg/5 minutes) vs. 25% (± 66%) reduction in the placebo group (from 96.8 mg/5 minutes to 72.6 mg/5 minutes); p < 0.05. Results confirmed by iodine starch test AEs: all local, none severe (n = 4; all in placebo group) |
Unclear |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Intervention 1 | Intervention 2 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|
| Balzani et al. 2001;72 RCT | 4; Italy |
Age range: 23–65 years Male: 0% |
NR | BTX-A (250 U) (n = 2) | Placebo (n = 2) |
Sweating (minor test): BTX group: near total reduction from baseline at 26–28 weeks; placebo: no effect AE: none |
Unclear |
| Baumann et al. 2005;73 RCT | 20; USA |
Age range: NR Male: 35% |
NR | BTX-B (2500 U) (n = 15) | Placebo (n = 5) |
Quality of life (Axillary Hyperhidrosis Quality of Life): score reduction from baseline across groups statistically significant up to 180 days’ follow-up. Differences between groups NR AE: 16 events definitely or probably related to BTX-B, including bruising (four events), pain at injection site (one event), dry eyes (three events), dry mouth (five events), indigestion (three events). All were mild–moderate events |
High |
| Heckmann et al. 2001;75 RCT (half-side comparison) | 145; Germany |
Age range: NR Male: 52% |
≥ 50 mg/minute | BTX-A (200 U) (n = 145) | Placebo (n = 145) + BTX-A (100 U) 2 weeks later |
Sweating (gravimetry): statistically significant reduction in sweat production from baseline (192 ± 136 mg/minute) in treated axilla compared with control at 2 weeks (24 ± 27 mg/minute in the axillae treated with BTX-A vs. 144 ± 113 mg/minute with placebo, MD between groups: 111 mg/minute; 95% CI 91 to 132 mg/minute; p < 0.001). At 2 weeks, 86.9% of BTX-A group had ≤ 50 mg/minute vs. 4.8% of placebo group AEs: none major at 14 weeks’ follow-up. Headache (four patients), soreness at injection site (two patients), increased facial sweating (one patient). Further longer-term results (post crossover) reported. Tolerance: patient rated as excellent (81.4%), good (17.2%), fair (1.4%) |
Low |
| Lowe et al. 2007;77 RCT | 322; USA |
Age range: 18–69 years Male: 55% |
HDSS 3–4, ≥ 50 mg/5 minutes room temperature |
BTX-A (50 U Botox) (n = 104) BTX-A (75 U Botox) (n = 110) |
Placebo (n = 108) |
HDSS: median effect duration (time to return to HDSS score of 3 or 4 points) after first treatment (responders subgroup): 75-U group – 197 days; 50-U group – 205 days; placebo group – 96 days; p < 0.001 for both treatment groups vs. placebo Sweating: gravimetry (% with ≥ 90% reduction in sweat at 4 weeks post first treatment) – 75-U group, 60%; 50-U group, 59%; placebo group, 9%; p = 0.001 for both treatment groups vs. placebo Quality of life: all reported in Figure 9 AEs: none significant; most common: injection site pain (75-U group 9%, 50-U group 12%, placebo group 8%); injection site bleeding (75-U group 6%, 50-U group 5%, placebo group 3%); non-axillary sweating (75-U group 6%, 50-U group 10%, placebo group 4%) |
Unclear (HDSS, gravimetry); high (DLQI) |
| Naumann and Lowe 2001,79 Naumann et al. 200286 and Lowe et al. 2002;78 RCT | 320; Germany, Belgium and the UK |
Age range: 17–74 years Male: 46% |
≥ 50 mg/5 minutes | BTX-A (50 U) (n = 242) | Placebo (n = 78) |
Sweating: all reported in Figure 6 Quality of life (SF-12): statistically significant difference in mean change from baseline in quality of life in physical (PCS) but not mental (MCS) scores at 16 weeks favouring BTX-A-treated patients. PCS: improvement from baseline (52.2 points) by 0.9 points in BTX-A group vs. decrease from baseline (52.8 points) by 1.2 points in placebo group (p = 0.019). MCS: improvement from baseline (49.1 points) by 1.7 points in BTX-A group vs. improvement from baseline (46.4 points) by 0.89 points in placebo group (p = 0.247) Patient satisfaction (HHIQ): BTX-A treatment resulted in a greater level of overall treatment satisfaction than previous (mostly failed) hyperhidrosis treatments (p < 0.001) Patient satisfaction (Patients’ Global Assessment of Treatment Satisfaction Scale): significant difference favouring BTX group in mean (SD) scores at 4 weeks (3.3 ± 0.9 vs. 0.8 ± 1.4) and 16 weeks (2.6 ± 1.6 vs. 0.3 ± 1.2); p < 0.001 AEs: increase in treatment-related non-axillary sweating in BTX group (5% patients) vs. none in placebo. No clinically important changes in vital signs or findings on physical examination observed. No other treatment-related AEs |
Unclear |
| Naumann et al. 2003;80 open label extension of Naumann and Lowe 200179 | 207; Germany, Belgium and the UK |
Age range: 17–74 years Male: NR |
≥ 50 mg/5 minutes |
BTX-A (50 U) (n = 80) BTX-A (50 U, two treatments, spaced by at least 16 weeks) (n = 93) BTX-A (50 U, three treatments, spaced by at least 16 weeks) (n = 30) |
Placebo (n = 4) |
Sweating (gravimetry): significantly greater difference in mean (SD) % change from baseline in BTX groups at 4 weeks and at 16 weeks after each treatment and placebo; p < 0.047. Higher rate of responders (≥ 50% reduction in spontaneous sweating, gravimetry) in BTX groups vs. placebo at 4 weeks after first treatment (96.1%), second treatment (91.1%) and third treatment (83.3%) vs. placebo (34.7%), and at 16 weeks after first treatment (85.7%), second treatment (87.3%) and third treatment (80.5%) vs. placebo (20.6%) (data from graph) Quality of life: percentage very or somewhat satisfied with ability to perform daily tasks) at 4 and 16 weeks higher in treatment groups; first treatment (4 weeks 94%, 16 weeks 92%); second treatment (4 weeks 95%, 16 weeks 89%); third treatment (4 weeks 85%, 16 weeks 78%) vs. placebo (4 weeks 44%, 16 weeks 18%) Patient satisfaction (Patients’ Global Assessment of Treatment Satisfaction Scale): higher scores in BTX groups in mean (SD) scores at 4 and 16 weeks after first treatment (3.5 ± 0.9 at 4 weeks; 2.8 ± 1.3 at 16 weeks), second treatment (3.4 ± 0.8 at 4 weeks; 2.9 ± 1.1 at 16 weeks) and third treatment (3.3 ± 1.0 at 4 weeks; 1.9 ± 2.3 at 16 weeks) compared with placebo (1.4 ± 1.5 at 4 weeks; 0.4 ± 1.1 at 16 weeks). Statistical significance NR. Proportion of patients satisfied with BTX-A compared with prior treatments was consistently higher in treatment groups (83% to 99%) compared with placebo (21% to 40%) at 4 and 16 weeks AEs (treatment related): 9.9% of patients after first treatment, 4.9% after second treatment, 3.3% after third treatment, 4.1% after placebo. None serious. Most common: non-axillary sweating (3.4% patients after first treatment, 1.6% after second treatment, 0 post third treatment and placebo), pain at injection site (1.5% after first treatment, 0.8% after second treatment, 0 post third treatment and placebo) |
High |
| Odderson 2002;82 RCT | 18; USA |
Age range: 16–50 years Male: 61% |
NR | BTX-A (50 U) (n = 12) | Placebo (n = 6) |
Sweating (gravimetry): average reduction in sweating in BTX-A group of 91.6% at 2 weeks and 88.2% over 5 months. Placebo group experienced a smaller average reduction in sweating (NR) AEs: one event (mild compensatory hyperhidrosis of thighs) |
Unclear |
| Ohshima et al. 2013;83,359 RCTa | 152; Japan |
Age range: NR Male: 24% |
HDSS 3–4 | BTX-A (50 U) (n = 78) | Placebo (n = 74) |
HDSS response (HDSS ≥ 2-point reduction from baseline): proportion of responders was higher for BTX-A group at all time points: 8 weeks – 66.7% vs. 12.3%, difference 54.3% (95% CI 41.4% to 67.2%); 12 weeks – 57.9% vs. 13.7%, difference 44.2% (95% CI 30.6% to 57.8%); 16 weeks – 57.9% vs. 9.6%, difference 48.3% (95% CI 35.3% to 61.3%) Mean change from baseline in HDSS (values < 0 indicate greater response for BTX-A group): week 8, –1.7 (SD 0.8) vs. –0.5 (SD 0.8), MD –1.1; week 12, –1.6 (SD 0.8) vs. –0.7 (SD 0.8), MD –0.9; week 16, –1.6 (SD 0.75) vs. –0.6 (SD 0.71) Sweating (gravimetry): mean weight (mg/5 minutes) of axillary sweating was lower in the BTX-A group compared with placebo at all time points (LOCF). Baseline: mean 125.2 (± 85.4) for BTX group, 137.5 (± 128.2) for placebo group; week 8 – 15.5 (24.5) vs. 80.4 (121.2), MD –65.0; week 12 – 18.0 (28.4) vs. 68.3 (67.5); week 16 – 18.6 (26.8) vs. 115.4 (157.7), MD –96.8 Duration of effect (from first treatment to first recording of > 50% of baseline sweat production): median (95% CI) 273.0 (95% CI 171.0 to NR) days for BTX-A group vs. 35.0 (95% CI 28.0 to 56.0) days for placebo DLQI: greater mean reduction from baseline in DLQI scores for BTX-A group at all time points. 8 weeks: –6.9 (SD 4.7) vs. –2.0 (SD 3.9), MD –4.9 (SD NR). 12 weeks: –6.9 (SD 4.4) vs. –2.5 (SD 4.3), MD –4.4 (SD NR). 16 weeks: –6.9 (SD 4.5) vs. –2.5 (SD 4.3), MD –4.4 (SD NR). Baseline scores NR Patient satisfaction (Global Assessment Treatment Satisfaction): greater improvement for BTX-A group. Week 4: 2.6 ± 1.1 vs 0.5 ± 1.1, MD 2.2. Week 8: 2.7 ± 1.2 vs.0.3 ± 1.0. Week 12: 2.6 ± 1.3 vs 0.4 ± 0.9, MD 2.2. Week 16: 2.6 ± 1.2 vs. 0.4 ± 0.8, MD 2.2 AEs: 54% AEs in BTX group vs. 30% in placebo group. Similar overall incidence of treatment-related AEs with BTX-A and placebo (3% in both groups). CS 3% in BTX-A group vs. 0% in placebo. No treatment-related SAEs in either groups. No patients withdrew due to AEs. Further data from open-label second treatment phase reported |
Unclear |
| Schnider et al. 1999;85 RCT (half-side comparison) | 13; Austria |
Age range: 21–55 years Male: 31% |
Socially handicapped by condition | BTX-A (200 U) (n = 13) | Placebo (n = 13) |
Sweating (digitised ninhydrin-stained sheets): statistically significant MD in sweating favouring BTX-A vs. placebo at 3 weeks (–34.5%), 8 weeks (–36.9%) and 13 weeks (–28.4%) (p < 0.001) Sweating (subjective rating, VAS): statistically significant MD in sweating favouring BTX-A vs. placebo at 3 weeks (–56.5%), 8 weeks (–67.4%) and 13 weeks (–62.5%) (p < 0.001) AEs: no SAEs reported, transient increase in palm sweating for 1 week (15%) |
Unclear |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Intervention | Control | Results | Risk of bias |
|---|---|---|---|---|---|---|---|
| Heckmann et al. 1999;89 non-RCT (half-side comparison) | 12; Germany |
Age range: 21–42 years Male: 42% |
≥ 100 mg/axilla/minute | BTX-A (250 U Dysport) (n = 12) | No treatment (n = 12), followed by BTX-A (250 U) 14 days later |
Sweating (gravimetry): treated axillae had ≤ 10% of the untreated axilla at 7 days and ≤ 50 mg/minute in each patient Iodine starch test: sweat secretion only observed on untreated side Patient satisfaction: 10/12 ‘completely satisfied’; 2/12 ‘almost completely satisfied’ Effect duration (patient reported): patients were symptom free for 12 months (seven patients), 9 months (three patients) or between 3 and 6 months (two patients) AEs: stinging on first day after treatment (n = 4). No other AEs reported |
High |
| Naver et al. 2000;81 non-RCT (half-side comparison) | 28; Sweden |
Age range: 19–57 years Male: 38% |
NR | BTX-A [Botox, mean 104 U (axilla), 56 U (palm) once or twice], local anaesthesia (palmar hyperhidrosis only) (n = 28; palmar, n = 19; axillary, n = 13) | No treatment (n = 28) |
Sweating (iodine starch test): NR (not quantifiable by investigators) Sweating (evaporimetry): significant evaporation reduction in treated side vs. untreated. Reduced by 46% in treated axillae at 1–2 weeks Sweating (patient reported): 13/13 axilla patients reported marked reduction or complete disappearance of sweating at 1–2 weeks AE: intense pain from injection (n = 2) with axillary hyperhidrosis |
High |
| Wakugawa et al. 2001;185 non-RCT (half-side comparison) | 20; Japan |
Age range: NR Male: NR |
NR |
BTX-A (50 U Dysport) one side only (n = 7) BTX-A (50 U Dysport) both sides (n = 13) |
No treatment (n = 7) |
Sweating: Sakurai–Montagna sweating paper test:188 Reduced to 16.4% (± 11.7%) of the pre-treatment level at 1 month and 45.8% (± 39.4%) of the pre-treatment level at 3 months in the treated group (p < 0.05) vs. 94.9% (± 19.8) at 1 month and 93.6 (± 38.1) in the untreated side (NS) Need for retreatment: further BTX-A administrations were needed in two of seven cases after 3 months AEs: none serious |
High |
| Study details | Sample size; study location | Patient characteristic | Hyperhidrosis severity inclusion criteria | Intervention 1 | Intervention 2 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|
| Ibrahim et al. 201321 and Ibrahim et al. 2013;87 RCT (half-side comparison) | 20; USA |
Age range: 19–50 years Male: 65% |
Haider 2005 criteria187 | Tumescent suction curettage (tumescent anaesthesia) (n = 20) | BTX-A (50 U) injection (n = 20) |
HDSS: significantly greater reduction in mean HDSS score in BTX-A-treated axillae compared with curettage at 3 months [BTX-A: from mean 3 points at baseline to 1.45 points at 3 months and 1.85 points at 6 months (p < 0.0001); curettage: from mean 3.05 points at baseline to 2.25 points at 3 months and 2.75 points at 6 months (p = 0.047)]. MD between groups: 0.80 (p = 0.0002) at 3 months and 0.90 (p = 0.0017) at 6 months Sweating (gravimetry): 72.1% sweat reduction with BTX-A (from 27.77 to 7.75 mg/minute) vs. 60.4% (from 28.42 to 11.25 mg/minute) with suction curettage at 3 months (non-significant difference of 11.7% between groups) AEs: BTX-A none reported; curettage soreness for ≤ 1 week; hyperpigmentation (n = 3); dysaesthesia (n = 1) |
High |
| Ottomann et al. 2007;155 non-RCT | 88; Germany |
Age range: 17–39 years Male: 16% |
HDSS ≥ 2 | Suction curettage (n = 41); anaesthesia NR | BTX-A (50 U) (n = 47) |
Sweating (gravimetry): non-significant differences between groups in change from baseline (mean 171 ± 92 mg/minute across groups) at 2, 12 and 26 weeks Quality of life: no significant differences between groups at 26 weeks (earlier follow-up NR) AEs: higher rate of patients in curettage group [8.3%, including epidermolysis (n = 2), scarring (n = 1) and haematoma (n = 1)] than BTX-A [1.7%, hypoesthesia (n = 1)]. None long term |
High |
| Rompel and Scholz 2001;148 non-RCT | 113; Germany |
Age range: NR Male: 36% |
‘Severely disabled in regard to occupation and social activities’ | Suction curettage (subcutaneous) under general anaesthesia (n = 90) | BTX-A (40–50 U Botox or 200–250 U Dysport per axilla) (n = 23) |
Sweating (patient reported): curettage – reduced to mean 40% of baseline score at 6 months and mean 46% at end of follow-up (median 28 months, n = 77a); BTX-A – reduced to mean 49% of baseline score at 6 months and mean 69% at end of follow-up (median 16 months, n = 23). Mean duration of effect of BTX-A: 7.6 months Patient satisfaction: curettage – 36% ‘very good’, 30% ‘good’, 17% ‘satisfactory’, 16% ‘dissatisfied’ (n = 77);a BTX-A – 39% ‘very good’, 22% ‘good’, 9% ‘satisfactory’, 30% ‘dissatisfied’ (n = 23); no significant difference between treatment groups AEs: curettage – 17.8% events [wound infection (n = 2), partial epidermal necrosis (n = 2), bleeding/haematoma requiring surgical revision (n = 12)]; BTX-A – transient minimal superficial haematoma (n = NR). 4% curettage patients judged the surgical scar to be ‘bothersome’ |
High |
| Vakili and Baker 2016;184 non-RCT (abstract only) | 98; UK |
Age range: 16–56 years Male: 26% |
NR | mRAC (n = 23) | BTX-A (Botox) (n = 75) |
HDSS: significant improvement from baseline in mean HDSS score in both groups (p < 0.01) and no significant difference between groups. mRAC group: reduced by 47%b (from 3.6 at baseline to 1.9) at 6 weeks. BTX-A group: reduced by 48%b (from 3.3 at baseline to 1.7) at 6 weeks Physical effects: (wearing bright clothes): significant improvement from baseline (p < 0.01). mRAC group reduced from 4.0 at baseline to 1.7 at 6 weeks, BTX-A group reduced from 4.0 at baseline to 1.9 at 6 weeks. The influence of psychological precipitating factors, such as public speaking, were significantly and equally improved in both groups (p < 0.05 from baseline) AEs: NR |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Intervention | Control | Results | Risk of bias |
|---|---|---|---|---|---|---|---|
| Baumann et al. 2005;74 RCT | 20; USA |
Age range: 20–60 years Male: 50% |
NR | BTX-B (5000 U), local anaesthesia (n = 15) | Placebo + local anaesthesia (n = 5) |
Sweating (iodine starch test): no statistically significance difference at 30 days. Mean time to return of baseline sweating levels: 3.8 months (range 2.3–4.9 months) Sweating (patient reported): statistically significant difference in mean change from baseline in P-HI scores (z = –4.1; p = 0.002) at 30 days favouring BTX-B vs. placebo. Mean (SD) for each group NR Quality of life: statistically significant difference in mean change from baseline in P-HQOL scores (z = –2.6; p = 0.010) at 30 days favouring BTX-B vs. placebo. Mean (SD) for each group NR AEs: 83 events definitely related, including decreased grip strength (dynamometer) (50% of participants), muscle weakness (60%), dry mouth (90%), excessively dry hands (60%), indigestion/heartburn (60%). No statistically significant difference with placebo in injection pain. Number of severe AEs NR |
High |
| Lowe et al. 2002;76 RCT (half-side comparison) | 19; USA |
Age range: NR Male: 53% |
≥ 40 mg/minute | BTX-A (100 U), anaesthesia: Lidocaine/Prilocaine Cream and Cold pack (n = 19) | Placebo (saline), anaesthesia: Lidocaine/Prilocaine Cream and Cold pack (n = 19) |
Sweating (gravimetry): mean reduction of approximately 66%a (from approximately 290 mg/5 minutes at baseline to approximately 100 mg/5 minutes at 28 days) in BTX-A hand and mean reduction of approximately 30%a (from approximately 300 mg/5 minutes at baseline to approximately 210 mg/5 minutes at 28 days) in placebo hand; difference between hands: 33%a (p = 0.0027) Physician assessment, patient assessment: significantly greater improvement in BTX-A hand at 28 days (p < 0.01) in physician (five-point scale) and patient assessment (VAS). 17/17 patients (two lost to follow-up) rated the treatment as successful in the BTX-A-treated palm compared with 2/17 with the placebo-treated palm (p < 0.0001) AEs: transient minor thumb and finger weakness (n = 1 BTX-A), tingling/numbness in fingers (n = 1 BTX-A), weakness (n = 1, placebo), pain (n = 1, both hands). No significant difference between hands in grip strength (dynamometer) |
Unclear |
| Schnider et al. 1997;84 RCT (half-side comparison) | 11; Austria |
Age range: 23–54 years Male: 64% |
NR | BTX-A (120 U Dysport), anaesthesia: cold packs for 30 minutes (n = 11) | Placebo (saline), anaesthesia: cold packs for 3 minutes (n = 11) |
Sweating (digitised ninhydrin-stained sheets): BTX-A – statistically significant mean reduction from baseline of 26% at 3 weeks and 8 weeks and 31% at 13 weeks (p ≤ 0.002); placebo – NS reduction from baseline (0.2% to 1.2%) Subjective rating (VAS): BTX-A – mean improvement from baseline 38–40% at 3, 8 and 13 weeks (p = 0.002); placebo – NS improvement at any time point AEs: minor handgrip weakness in the BTX-A-treated hand, lasting 2–5 weeks (n = 3). Greater pain in BTX-A hand at injection site vs. placebo (n = 3) |
Unclear |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Intervention | Control | Results | Risk of bias |
|---|---|---|---|---|---|---|---|
| Naver et al. 2000;81 non-RCT (half-side comparison) | 28; Sweden |
Age range: 19–57 years Male: 38% |
NR | BTX-A [Botox, mean 104 U (axilla), 56 U (palm) once or twice], local anaesthesia (palmar hyperhidrosis only) (n = 28; palmar n = 19; axillary n = 13) | No treatment (n = 28) |
Sweating (iodine starch test): significant reduction in area of colour reaction from the palms at 1–2 weeks (p = 0.0002), axilla could not be quantified. 27/28 had marked reduction in treated side vs. none in control side Sweating (evaporimetry): significant evaporation reduction in treated side vs. untreated. Reduced by 57% in treated palm at 1–2 weeks Sweating (patient reported): 15/19 palm patients reported marked reduction or complete disappearance of sweating at 1–2 weeks AEs: intense pain from injection (n = 2) with axillary hyperhidrosis; intense skin dryness (n = 6) treated with palmar hyperhidrosis; transient slight reduction in power of fingers (n = 12) palmar patients |
High |
| Yamashita et al. 2008;90 non-RCT (half-side comparison) | 27; Japan |
Age range: NR Male: 22% |
14 severe (> 1 mg/cm2/minute), 13 mild (< 1 mg/cm2/minute) | BTX-A (60 U Botox), local anaesthesia: ice packs (n = 27) | No treatment (n = 27) |
Sweating (gravimetry): statistically significant reduction from baseline in both groups. Larger reduction from baseline in right hand (BTX-A group) compared with left hand (no treatment) monthly at 1–6 months. Statistical significance of between group difference NR Iodine starch test: decreases observed approximately 2 cm around each injection site AE: NR |
High |
| Study details | Sample size; study location | Patient characteristic | Hyperhidrosis severity inclusion criteria | Intervention 1 | Intervention 2 | Intervention 3 | Intervention 4 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Bechara et al. 2008;150 RCT | 40; Germany |
Age range: 19–57 years Male: 45% |
≥ 50 mg/minute | Curettage (liposuction), local anaesthesia (n = 15) | Shelley (SST), local anaesthesia (n = 11) | Radical skin excision (modified Bretteville–Jensen technique with Y-plasty closure), local anaesthesia (n = 14) | N/A |
Sweating (gravimetry): significantly reduced sweat rates from baseline (ranging from mean 76.1 mg/minute to 81 mg/minute across groups) to 12 months in all groups [reduction of: LC 66.4%; SST 62.9%; RSE 65.3% (p < 0.05)], no significant difference between groups Aesthetic outcome (patient reported from 1 ‘very good’ to 5 ‘not good at all’): mean score was 3.2 (range 2–5) in the RSE group, 2.3 (range 1–3) in the SST group and 1.5 (range 1–2) in the LC group. Significantly better for LC compared with other interventions (p < 0.05) (follow-up duration NR) AEs: haematoma (LC, n = 3, including 1 moderate to severe, resolved over 4 weeks; SST, n = 2; RSE, n = 3); paraesthesia (LC, n = 4; SST, n = 3; RSE, n = 5); focal hair loss (LC, n = 9; SST, n = 11; RSE, n = 14); subcutaneous fibrotic bridles (LC, n = 8; SST, n = 3; RSE, n = 0); seroma (LC, n = 1; SST, n = 3; RSE, n = 0); skin erosion (LC, n = 3; SST, n = 4; RSE, n = 0); flap necrosis (LC, n = 1; SST, n = 2; RSE, n = 0); infection (LC, n = 0; SST, n = 1; RSE, n = 2); and suture dehiscence (LC, n = 0; SST, n = 1; RSE, n = 2). No signs of movement impairment. Other outcomes (histology) were reported |
High |
| Jemec 1975;149 non-RCT | 41; Denmark |
Age range: NR Male: NR |
NR | Curettage (liposuction), local or general anaesthesia (n = 20) | Radical excision (complete excision and relieving Z-plasty), local or general anaesthesia (n = 21) | N/A | N/A |
Patient satisfaction: curettage – 12/20 patients were entirely satisfied, four were partly satisfied, three were dissatisfied and one lost to follow-up. No patients reported troublesome scars; excision – 10/21 patients were entirely satisfied, six were partly satisfied, three were dissatisfied and two lost to follow-up. Four patients reported troublesome scars. Follow-up 6–9 months AEs: there were no abscesses, haematomas or wound defects |
High |
| Bechara et al. 2008;154 non-RCT (half-side comparison) | 4; Germany |
Age range: NR Male: NR |
≥ 50 mg/minute | Curettage (liposuction) (n = 4) | Curettage (liposuction) + aggressive manual shaving (n = 4) | N/A | N/A |
Sweating (gravimetry): no significant difference in sweat reduction between surgical methods at 15 weeks AEs: 4/4 in aggressive shaving group had moderate to severe complications, extensive skin damage, not tolerable. Trial stopped early due to complications |
High |
| Tronstad et al. 2014;147 RCT (half-side comparison) | 22; Norway |
Age range: 20–44 years Male: 18% |
NR | Curettage (anaesthesia: 50/50 mixture of lidocaine 1% with adrenaline and saline, maximum volume 40 ml) (n = 22) | Tumescent suction curettage (anaesthesia: 200 ml 0.9% saline with 8 ml lidocaine 2% and 0.2 mg adrenaline) (n = 22) | N/A | N/A |
Sweating (gravimetry, skin conductance and VAS score): reduced significantly at 6 and 12 months compared with baseline in both treatment groups (p < 0.05) and was significantly more reduced with tumescent suction curettage than curettage alone (p < 0.05) DLQI: NR AEs: postoperative neuropathic pain lasting through the observation period (n = 1 in tumescent suction curettage group). No infection requiring antibiotics, haematoma or scarring |
Unclear (gravimetry); high (DLQI) |
| Leclere et al. 2015;24 RCT | 100; France, Germany and Spain |
Age range: NR Male: NR |
HDSS 3–4 (mean 3.86, SD 0.35 across groups) | Laser alone (924/975 nm simultaneous) once (n = 25), LA | Laser (924/975 nm) + curettage once (n = 25), LA | Laser alone (975 nm) once (n = 25), LA | Suction curettage alone once (n = 25), LA |
HDSS: laser + curettage group had the greatest reduction in mean score at 12 months, with 3.4 points reduction from baseline (baseline 3.88 ± 0.33; 1 month 1.24 ± 0.44; 12 months 0.48 ± 0.51), followed by laser 924/975 nm only, with 1.88 points reduction (baseline 3.84 ± 0.37; 1 month 1.96 ± 0.68; 12 months 1.96 ± 0.61), curettage only (1.52 points reduction, baseline 3.84 ± 0.37; 1 month 2.20 ± 0.41; 12 months 2.32 ± 0.48) and laser 975 nm (0.44 points reduction, baseline 3.88 ± 0.33; 1 month 3.40 ± 0.50; 12 months 3.44 ± 0.51) Starch-Iodine Scale improvement: largest mean change from baseline at 12 months with laser + curettage (–2.2),a followed by laser 924/975 nm only (–1.1),a curettage (–0.8)a and laser 975 nm (increase of 0.2)a GAIS: highest mean (SD) scores at 12 months in laser + curettage group (3.8 ± 0.4), followed by laser 924/975 nm [2.7 (SD 0.5)], curettage [2.6 (SD 0.5)] and laser 924/975 nm [0.9 (SD 0.3)]. Results at 1 month follow-up were similar to the 12-month result for all three outcomes ANOVA results comparing all interventions NR AEs: transient burns (n = 2, laser 975 nm group), bruising (n = 3, curettage group) and temporary loss of sensation (n = 1, laser 924/975 nm) |
High |
| Study details | Sample size; study location | Patient characteristic | Hyperhidrosis severity inclusion criteria | Intervention 1 | Intervention 2 | Intervention 3 | Intervention 4 | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Leclere et al. 2015;24 RCT | 100; France, Germany and Spain |
Age range: NR Male: NR |
HDSS 3–4 (mean 3.86, SD 0.35 across groups) | Laser alone (924/975 nm simultaneous) once (n = 25), LA |
Laser (924/975 nm) + curettage once (n = 25), LA |
Laser alone (975 nm) once (n = 25), LA | Suction curettage alone once (n = 25), LA |
HDSS: laser + curettage group had the greatest reduction in mean score at 12 months, with 3.4 points reduction from baseline (baseline 3.88 ± 0.33; 1 month 1.24 ± 0.44; 12 months 0.48 ± 0.51), followed by laser 924/975 nm only, with 1.88 points reduction (baseline 3.84 ± 0.37; 1 month 1.96 ± 0.68; 12 months 1.96 ± 0.61), curettage only, 1.52 points reduction (baseline 3.84 ± 0.37; 1 month 2.20 ± 0.41; 12 months 2.32 ± 0.48) and laser 975 nm, with 0.44 points reduction (baseline 3.88 ± 0.33; 1 month 3.40 ± 0.50; 12 months 3.44 ± 0.51) Starch-Iodine Scale improvement: largest mean change from baseline at 12 months with laser + curettage (–2.2),a followed by laser 924/975 nm only (–1.1),a curettage (–0.8)a and laser 975 nm (increase of 0.2)a GAIS: highest mean (SD) scores at 12 months in laser + curettage group (3.8 ± 0.4), followed by laser 924/975 nm [2.7 (SD 0.5)], curettage [2.6 (SD 0.5)] and laser 975 nm [0.9 (SD 0.3)]. Results at 1 month follow-up were similar to the 12-month result for all three outcomes ANOVA results comparing all interventions NR AEs: transient burns (n = 2, laser 975 nm group), bruising (n = 3, curettage group) and temporary loss of sensation (n = 1, laser 924/975 nm) |
High |
| Study details | Sample size; study location | Patient characteristic | Hyperhidrosis severity inclusion criteria | Intervention details | Comparator | Results | Risk of bias |
|---|---|---|---|---|---|---|---|
| Bechara et al. 2012;25 RCT (half-side comparison) | 21; Germany |
Age range: 24–66 years Male: 24% |
Hornberger 2004189 |
Long-pulsed laser (800 nm). Five treatments at 4-week intervals Anaesthesia: NR |
No treatment |
Sweat rate (gravimetry): significant reduction in both axillae: treated axillae from median 89 mg/minute, range 42–208 mg/minute at baseline to 48 mg/minute, range 17–119 mg/minute after treatment (p < 0.001); untreated axillae from median 78 mg/minute, range 25–220 mg/minute at baseline to median 65 mg/minute, range 24–399 mg/minute after treatment (p = 0.04); no significant difference in reduction between treated and non-treated side after treatment (p = 0.1) Patient satisfaction (VAS): 5.9/10 after treatment and 4.1/10 at 12 months Histology: no significant changes in sweat glands AEs: none serious |
High |
| Letada et al. 2012;26 RCT (half-side comparison) | 6; USA |
Age range: NR Male: 17% |
NR |
Long-pulsed laser (1064 nm). Six treatments at monthly intervals Anaesthesia: NR |
No treatment |
Sweating (iodine starch test): ‘visibly reduced’ in 6/6 vs. control at 1 month Sweating (GAQ): 6/6 reported good or excellent improvement in GAQ at 1 month. 2/3 reported good or excellent and 1/3 reported fair to good at 3 months Histology: no difference between groups. AEs: none |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Intervention | Comparator | Results | Risk of bias |
|---|---|---|---|---|---|---|---|
| Fatemi Naeini et al. 201528 and Abtahi-Naeini et al. 2015;170 non-RCT (half-side comparison | 25; Iran |
Age range: NR Male: 32% |
HDSS 3–4 |
FMR (1 MHz of radiofrequency current), three sessions at 3-week intervals (n = 25) Local topical anaesthesia |
Sham FMR (n = 25) Local topical anaesthesia |
HDSS: mean score at 21 weeks – FMR reduced by 1.59 pointsa (from 3.46 ± 0.5 to 1.87 ± 0.61); placebo reduced by 0.08 pointsa (from 3.46 ± 0.5 to 3.38 ± 0.49) (p < 0.001) Sweating intensity (iodine starch test, VAS): mean sweating intensity score at 21 weeks (out of 10, higher scores = greater intensity) – FMR from 9 ± 1.55 to 3.92 ± 1.31; placebo from 9 ± 1.55 to 8.44 ± 1.55 (p < 0.001) Patient satisfaction: 80% of patients reported > 50% satisfaction at 21 weeks AEs: most patients experienced temporary swelling (% NR), pain (% NR), erythema (68%) and pin-point bleeding (56%) in the treated areas. Transient post-inflammatory hyperpigmentation (44%), resolved in 2 months. Tingling/numbness in the group (4%), discontinued and resolved after 2 months |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Intervention details | Comparator | Results | Risk of bias |
|---|---|---|---|---|---|---|---|
| Glaser et al. 201227 and Kilmer et al. 2011;172 RCT | 120; USA |
Age range: NR Male: 43% |
HDSS 3–4; ≥ 50 mg/5 minutes |
Microwave, two sessions approximately 2 weeks apart. Third procedure allowed for 10 non-responders (total n = 81) Local anaesthesia |
Sham microwave (n = 39) Local anaesthesia |
HDSS: percentage with reduction from baseline by ≥ 2 points significantly greater for microwave vs. placebo at 30 days (67% vs. 13%; p < 0.001), 3 months (57% vs. 13%; p < 0.001) and 6 months (47% vs. 13%; p < 0.001) Sweating (gravimetry): 50% reduction in sweat rate: no statistically significant difference between groups at 30 days, 3 or 6 months. ≥ 75% reduction in sweat rate: 62% for treatment group vs. 39% for placebo at 30 days (p = 0.01), but not significant difference between groups at 3 or 6 months AEs (treatment related): 28% microwave patients and 13% placebo patients; mostly mild; most frequent were transient altered sensation in skin of upper group (10% for microwave vs. 3% placebo) and pain (6% vs. 5%). Persistent compensatory facial hyperhidrosis at 6 months (n = 1, microwave group). Seven patients did not receive second microwave treatment due to AEs: two dropped out due to pain, five had ongoing side effects (swelling, pustules or blisters) |
High |
| Study details | Sample size; study location | Patient characteristics | Hyperhidrosis severity inclusion criteria | Intervention | Comparator | Anaesthesia | Results | Risk of bias |
|---|---|---|---|---|---|---|---|---|
|
Nestor and Park 201429 and Nestor and Park 2012;178 RCT (half-side comparison n = 11)a Study 1 |
14; USA |
Age range: NR Male: 21% |
HDSS 3–4; ≥ 50 mg/5 minutes | Microfocused ultrasound (4 MHz, 4.5-mm depth; then 7 MHz, 3.0-mm depth), two treatments 30 days apart (n = 14) | Placebo, two treatments 30 days apart (n = 14) | Local (only for subgroup n = 3)a |
Response (gravimetry): 13/14 had ≥ 50% reduction in sweating from baseline (difference between groups NR) at 90 days’ follow-up AEs: 93% intervention group had ≥ 1 treatment related vs. 9% for placebo. Most AEs were mild. No serious AEs. Most common axilla tenderness/soreness (86% in intervention group). Less discomfort in axilla pre-treated with lidocaine + adrenaline compared with lidocaine alone (n = 3) |
High |
|
Nestor and Park 201429 and Nestor and Park 2012;178 RCT Study 2 |
20; USA |
Age range: 21–52 years Male: 65% |
HDSS 3–4; ≥ 50 mg/5 minutes | Microfocused ultrasound (4 MHz, 4.5-mm depth; then 7 MHz, 3.0-mm depth), two treatments 30 days apart (n = 12) | Placebo, two treatments 30 days apart (n = 8) | Local |
Response (HDSS): from 3 or 4 at baseline to 1 or 2 – 30 days’ follow-up 67% vs. 27% in placebo group (p = 0.005); 60 days 59% vs. 17%; 90 days 73% vs. 24%; 335 days 81% vs. 7%b Response (gravimetry): 83% patients in intervention group had ≥ 50% sweat reduction from baseline vs. 0% in placebo group across all time points (p < 0.0001 at all follow-up points) AEs: 92% intervention group had ≥ 1 AE vs. 50% for placebo. Most AEs were mild and none serious. Most common AE was axilla tenderness/soreness (83% in intervention group) |
High |
Appendix 3 Studies excluded at full-paper stage with rationale
| Study details | Reason for exclusion |
|---|---|
| Abrams and Hallett 2013360 | Not a SR or primary prospective study |
| Adar et al. 1977361 | Not a SR or primary prospective study |
| Aguilar-Ferrandiz et al. 2011283 | Not relevant comparator (psychological techniques) |
| Ak et al. 2013362 | No intervention |
| Ak et al. 2013363 | No intervention |
| Aksu et al. 2011281 | Not a SR or primary prospective study |
| Alvarez et al. 2013231 | Not a SR or primary prospective study |
| Alvarez et al. 2013224 | Not a SR or primary prospective study |
| Ambrogi et al. 2009272 | Not relevant comparator (sympathectomy) |
| Amini et al. 2008217 | Not a SR or primary prospective study |
| Amir et al. 2000186 | No intervention |
| Andrade et al. 2012208 | Not dermatology study: sympathectomy |
| Andrews and Rennie 1997364 | Not dermatology study: sympathectomy |
| Anonymous 2004365 | Not a SR or primary prospective study |
| Araujo et al. 2009366 | Not dermatology study: sympathectomy |
| Atkinson et al. 2010367 | Not a SR or primary prospective study |
| Attia and Salah 2011219 | Not relevant intervention (photodynamic therapy) |
| Awad et al. 2010368 | Not dermatology study: sympathectomy |
| Aydemir and Toh 2006369 | Not relevant intervention (comparison of specific techniques) |
| Bachmann et al. 2009317 | Not a SR or primary prospective study |
| Bandeira et al. 2009370 | Not a SR or primary prospective study |
| Basra et al. 2008275 | Not primary hyperhidrosis |
| Baumgartner and Toh 2003371 | Not a SR or primary prospective study |
| Baumgartner et al. 2009372 | Not a comparative study (treatments undertaken sequentially) |
| Bechaux 2009373 | Not a SR or primary prospective study |
| Bell et al. 2014374 | Not a SR or primary prospective study |
| Benohanian et al. 1998375 | Not relevant intervention (aluminium chloride) |
| Birnie and Motley 2009268 | Not a SR or primary prospective study |
| Bischof et al. 2005376 | Not dermatology study: sympathectomy |
| Bisseriex et al. 2012377 | Not primary hyperhidrosis |
| Boas 2000378 | Not a SR or primary prospective study |
| Boley et al. 2007319 | Not dermatology study: sympathectomy |
| Bryan et al. 2012379 | Not a SR or primary prospective study |
| Bygstad et al. 2013380 | Not dermatology study: sympathectomy |
| Cameron and Ojimba 2004381 | Not a SR or primary prospective study |
| Cardoso et al. 2009320 | Not a SR or primary prospective study |
| Cerfolio et al. 201115 | Not a SR or primary prospective study |
| Chan et al. 2007321 | Not dermatology study: sympathectomy |
| Chen et al. 1996382 | Not a SR or primary prospective study |
| Chen et al. 2001383 | Not dermatology study: sympathectomy |
| Chen et al. 2013384 | Not dermatology study: sympathectomy |
| Cheng et al. 2015334 | Not dermatology study: sympathectomy |
| Chia et al. 2012385 | Not relevant intervention (glycopyrronium bromide iontophoresis) |
| Cho et al. 1994386 | Not relevant comparator (aluminium chloride and sympathectomy) |
| Cinà and Clase 1999292 | No intervention |
| Clerico et al. 2014387 | Not a SR or primary prospective study |
| Coelho et al. 2014388 | Not dermatology study: sympathectomy |
| Copleymerriman et al. 2015389 | Not primary hyperhidrosis |
| Coveliers et al. 2011215 | Not dermatology study: sympathectomy |
| Currie et al. 2011322 | Not a SR or primary prospective study |
| Davarian et al. 2008264 | Not relevant intervention (BTX iontophoresis vs. saline) |
| de Campos et al. 2003238 | Not dermatology study: sympathectomy |
| de Campos et al. 2005390 | Not dermatology study: sympathectomy |
| Delaplace et al. 2015211 | Not dermatology study: sympathectomy |
| Deng et al. 2011391 | Not dermatology study: sympathectomy |
| Dickmann and Dickmann 20102 | Not a SR or primary prospective study |
| Divisi et al. 2013323 | Not dermatology study: sympathectomy |
| Doolabh et al. 2004392 | Not a SR or primary prospective study |
| Dressler et al. 2002393 | Not relevant intervention (comparing different doses of BTX) |
| Drott et al. 1995394 | Not a SR or primary prospective study |
| Drott and Claes 1996395 | Not a SR or primary prospective study |
| Duarte et al. 2003396 | Not dermatology study: sympathectomy |
| Dumont et al. 1997397 | Not dermatology study: sympathectomy |
| Ebrahim 2011398 | Not dermatology study: sympathectomy |
| Edmondson et al. 1992399 | Not dermatology study: sympathectomy |
| Elia et al. 2005299 | Not dermatology study: sympathectomy |
| Ellis 1977400 | Not a SR or primary prospective study |
| Ellis and Scurr 1979401 | Not relevant intervention (aluminium chloride) |
| Ellis 1982402 | Not a SR or primary prospective study |
| Farahmand and Toolabi 2011403 | Not dermatology study: sympathectomy |
| Fatemi Naeini et al. 2015404 | Not a SR or primary prospective study |
| Flanagan et al. 2008218 | Not relevant comparator (aluminium chloride) |
| Flørenes 2003405 | Not dermatology study: sympathectomy |
| Flores 2012335 | Not a SR or primary prospective study |
| Fuentes et al. 2012207 | Not dermatology study: sympathectomy |
| Furlan et al. 2000406 | Not primary hyperhidrosis |
| Galati and Raposio 2010407 | Not a SR or primary prospective study |
| Garcia Franco et al. 2011210 | Not dermatology study: sympathectomy |
| Geddoa et al. 2008267 | Not a SR or primary prospective study |
| George et al. 2014408 | Not a SR or primary prospective study |
| Ghisletta et al. 1999409 | Not dermatology study: sympathectomy |
| Gibbons et al. 2014274 | Not a SR or primary prospective study (CEA) |
| Glent-Madsen and Dahl 1988410 | Not primary hyperhidrosis |
| Goh 1990411 | Not relevant intervention (aluminium chloride) |
| Goh and Yoyong 1996412 | Not relevant comparator (tannic acid) |
| Goh et al. 2000413 | Not dermatology study: sympathectomy |
| Goldman 2000414 | Not a SR or primary prospective study |
| Gorouhi et al. 2009220 | Not relevant comparator (aluminium chloride) |
| Gossot et al. 2001415 | Not a SR or primary prospective study |
| Grabham et al. 1998416 | Not dermatology study: sympathectomy |
| Gracia et al. 2011324 | Not dermatology study: sympathectomy |
| Greenhalgh et al. 1971417 | Not a SR or primary prospective study |
| Grimalt et al. 2006418 | Not relevant intervention (atropine) |
| Grootens and Hartong 2011419 | Not primary hyperhidrosis |
| Guérin et al. 1990420 | Not dermatology study: sympathectomy |
| Hasche et al. 1997156 | Duplicate report of Hasche et al. 1997156 |
| Hashmonai 2011421 | Not a SR or primary prospective study |
| Hecht et al. 2004422 | Not relevant intervention (comparing different doses of BTX) |
| Heidemann and Licht 2013423 | Not a SR or primary prospective study |
| Hekmat et al. 2010297 | Not a SR or primary prospective study |
| Henriques et al. 2014424 | Not a SR or primary prospective study |
| Henteleff and Kalavrouziotis 2008425 | Not dermatology study: sympathectomy |
| Hoorens and Ongenae 2012426 | Not a SR or primary prospective study |
| Hornberger et al. 2004189 | Not a SR or primary prospective study |
| Hsu et al. 1998427 | Not a SR or primary prospective study |
| Ibrahim et al. 2013245 | Not dermatology study: sympathectomy |
| Ibrahim et al. 2013428 | Not a SR or primary prospective study |
| Innocenzi et al. 2008429 | Not relevant intervention (aluminium sesquichlorhydrate foam) |
| Ishy et al. 2011239 | Not dermatology study: Ssympathectomy |
| Isla-Tajera et al. 2013230 | Not a dermatology study: quality of life/economics only |
| Ismail and El Samadoni 2015213 | Not a SR or primary prospective study |
| Jaffer et al. 2007336 | Not dermatology study: sympathectomy |
| Jani 2009430 | Not dermatology study: sympathectomy |
| Jeganathan et al. 2008325 | Not dermatology study: sympathectomy |
| Jog et al. 2010431 | Not primary hyperhidrosis |
| Kamudoni et al. 2012204 | No intervention |
| Kamudoni et al. 2014228 | No intervention |
| Kamudoni et al. 2015261 | No intervention |
| Kavanagh et al. 2004432 | Not a SR or primary prospective study |
| Kavanagh and Shams 2006433 | Not relevant intervention (BTX iontophoresis vs. saline) |
| Khademi Kalantari et al. 2011434 | Not relevant intervention (aluminium chloride) |
| Kim et al. 2005435 | Not dermatology study: sympathectomy |
| Kim et al. 200818 | Not a SR or primary prospective study |
| Kim et al. 2008436 | Not primary hyperhidrosis |
| Kopelman et al. 1996437 | Not a SR or primary prospective study |
| Koskinen et al. 2012308 | Not dermatology study: sympathectomy |
| Kravarusic and Freud 2012438 | Not a SR or primary prospective study |
| Krogstad et al. 2006439 | No intervention |
| Kuijpers et al. 2013282 | Not dermatology study: sympathectomy |
| Kumar et al. 2014440 | Not a SR or primary prospective study |
| Kuo et al. 2004259 | No intervention |
| Lakraj et al. 2013441 | Not a SR or primary prospective study |
| Lau et al. 2014442 | Not dermatology study: sympathectomy |
| Law and Ellis 1989443 | Not a SR or primary prospective study |
| Lawrence and Lonsdale Eccles 2006444 | Not a SR or primary prospective study |
| Leão et al. 2003445 | Not a SR or primary prospective study |
| Lecouflet et al. 2014446 | Not a SR or primary prospective study |
| Lee et al. 2004447 | Not dermatology study: sympathectomy |
| Lee and Ryman 2005448 | Not a SR or primary prospective study |
| Lee et al. 2006449 | Not primary hyperhidrosis |
| Lee et al. 2008450 | Not a SR or primary prospective study |
| Lessa et al. 2014284 | No intervention |
| Lewis et al. 1998451 | Not a SR or primary prospective study |
| Li et al. 201222 | Not primary hyperhidrosis |
| Licht and Pilegaard 2004452 | Not a SR or primary prospective study |
| Lin and Fang 1999453 | Not a SR or primary prospective study |
| Lin et al. 2000454 | Not a SR or primary prospective study |
| Lin 2001455 | Not dermatology study: sympathectomy |
| Little 2004456 | Not a SR or primary prospective study |
| Lladó et al. 2005457 | Not dermatology study: sympathectomy |
| Loureiro et al. 2008246 | Not dermatology study: sympathectomy |
| Lupin and Hong 2012209 | Duplicate report Lupin et al. 2011175 |
| Malmivaara et al. 2007458 | Not dermatology study: sympathectomy |
| Malone et al. 1986459 | Not dermatology study: sympathectomy |
| Marcella et al. 2011221 | Not a SR or primary prospective study |
| Masters and Rennie 1992460 | Not a SR or primary prospective study |
| McAleer et al. 2009265 | Not a SR or primary prospective study |
| Mehrotra et al. 2014229 | Not a SR or primary prospective study |
| Menna et al. 2012461 | Not dermatology study: sympathectomy |
| Micali et al. 2013266 | Not relevant intervention (aluminium chloride) |
| Misiak et al. 2012462 | Not dermatology study: sympathectomy |
| Montaser-Kouhsari et al. 2014463 | Not relevant intervention (BTX iontophoresis) |
| Mordant et al. 2010464 | Not a SR or primary prospective study |
| Moya et al. 2006465 | Not dermatology study: sympathectomy |
| Muhammad and Allam 2014466 | Not dermatology study: sympathectomy |
| Muller and Augustin 2013340 | Not dermatology study: quality of life/economics only |
| Munia et al. 2007328 | Not dermatology study: sympathectomy |
| Munia et al. 2008327 | Not dermatology study: sympathectomy |
| Nakamura et al. 2008467 | Not a SR or primary prospective study |
| Naumann and Hamm 2002468 | Not a SR or primary prospective study |
| Neidhardt et al. 2010469 | Not a SR or primary prospective study |
| Neumayer et al. 2003470 | Not dermatology study: sympathectomy |
| Neumayer et al. 2005471 | Not dermatology study: sympathectomy |
| Neumayer et al. 2005257 | Not dermatology study: sympathectomy |
| Neves et al. 2012247 | Not dermatology study: sympathectomy |
| Nicholson et al. 1994472 | Not dermatology study: sympathectomy |
| Nieuwenhuis 2015473 | Not a SR or primary prospective study |
| Odderson 1998474 | Not a SR or primary prospective study |
| Ojimba and Cameron 2004475 | Not a SR or primary prospective study |
| Oncel et al. 2012476 | Not dermatology study: sympathectomy |
| Panhofer et al. 2005233 | Not a SR or primary prospective study |
| Panhofer et al. 2006248 | Not dermatology study: sympathectomy |
| Panhofer et al. 2011242 | Not dermatology study: sympathectomy |
| Panhofer et al. 2013249 | Not dermatology study: sympathectomy |
| Panhofer et al. 2013241 | Not dermatology study: sympathectomy |
| Panhofer et al. 2014240 | Not dermatology study: sympathectomy |
| Pastorelli and Plasmati 2013214 | Not relevant intervention (comparison of BTX brands) |
| Pastorelli et al. 2014223 | Not relevant intervention (comparison of BTX brands) |
| Paul et al. 2010273 | Not relevant intervention (effect of laser hair removal on effectiveness of BTX) |
| Penna et al. 2007477 | No effectiveness or quality-of-life outcome |
| Phadke et al. 1995478 | Not relevant comparator (topical methenamide and glutaraldehyde) |
| Ponce-Olivera et al. 2014479 | Not a SR or primary prospective study |
| Prasad et al. 2010250 | Not dermatology study: sympathectomy |
| Purtuloglu et al. 2013480 | Not a SR or primary prospective study |
| Purtuloglu et al. 2013481 | Not a SR or primary prospective study |
| Raja and Campbell 2000482 | Not a SR or primary prospective study |
| Rameshkannan et al. 2010269 | Not relevant intervention (clonidine vs. propranolol) |
| Ramos et al. 2006483 | Not dermatology study: sympathectomy |
| Ramos et al. 2007484 | Not a SR or primary prospective study |
| Ramos et al. 2009485 | Not dermatology study: sympathectomy |
| Ramos et al. 2012486 | Not dermatology study: sympathectomy |
| Ramsaroop et al. 2004487 | Not dermatology study: sympathectomy |
| Rantanen and Telaranta 2013313 | Not dermatology study: sympathectomy |
| Rathinam et al. 2008329 | Not a SR or primary prospective study |
| Rayner et al. 1980488 | Not relevant intervention (aluminium chloride) |
| Rezai 2009489 | Not a SR or primary prospective study |
| Rezende and Luz 2014490 | Not a SR or primary prospective study |
| Rieger and Pedevilla 2007491 | Not dermatology study: sympathectomy |
| Rieger et al. 200917 | Not dermatology study: sympathectomy |
| Rieger et al. 2011492 | Not a SR or primary prospective study |
| Rieger et al. 2015251 | Not dermatology study: sympathectomy |
| Roddy 2012493 | Not a SR or primary prospective study |
| Rodriguez et al. 2008300 | Not dermatology study: sympathectomy |
| Rodriguez-Lopez et al. 2010494 | Not dermatology study: sympathectomy |
| Rzany et al. 2012227 | Not a dermatology study: quality of life/economics only |
| Saadia et al. 2001495 | Not relevant intervention (comparison of BTX doses) |
| Salek et al. 2015260 | Not primary hyperhidrosis |
| Sanli et al. 2010216 | Not a SR or primary prospective study |
| Scheer et al. 2014271 | Not a SR or primary prospective study |
| Scheer et al. 2014270 | Not dermatology study: sympathectomy |
| Schestatsky et al. 2009496 | Not dermatology study: sympathectomy |
| Scholes et al. 1978497 | Not relevant intervention (aluminium chloride) |
| Sciuchetti et al. 2008498 | Not dermatology study: sympathectomy |
| Shalaby et al. 2012330 | Not dermatology study: sympathectomy |
| Sharpe et al. 2013222 | Not a SR or primary prospective study |
| Shen et al. 1990499 | Not relevant intervention (aluminium chloride iontophoresis vs. tap water) |
| Shi et al. 2015331 | Not dermatology study: sympathectomy |
| Simonyi et al. 2010500 | Not primary hyperhidrosis |
| Simonyi et al. 2010303 | Not a SR or primary prospective study |
| Simonyi et al. 2011304 | Not a SR or primary prospective study |
| Solish et al. 200712 | Not a SR or primary prospective study |
| Solish 2008501 | Not a SR or primary prospective study |
| Stefaniak et al. 2009502 | Not dermatology study: sympathectomy |
| Stefaniak and Ćwigoń 2013503 | Not dermatology study: sympathectomy |
| Stefaniak and Ćwigoń 2013504 | Not dermatology study: sympathectomy |
| Steiner et al. 2007332 | Not dermatology study: sympathectomy |
| Streker et al. 2010505 | Not relevant intervention (aluminium chloride) |
| Streker et al. 2012506 | Not relevant intervention (aluminium chloride) |
| Strutton et al. 2004226 | No intervention |
| Suh et al. 2014507 | Not a SR or primary prospective study |
| Swaile et al. 2012508 | Not primary hyperhidrosis |
| Swinehart 2000509 | Not a SR or primary prospective study |
| Tan and Solish 2002263 | Not a SR or primary prospective study |
| Tang et al. 2013510 | Not dermatology study: sympathectomy |
| Teivelis et al. 2014253 | Not a SR or primary prospective study |
| Toolabi et al. 2015511 | Not dermatology study: sympathectomy |
| Umezawa et al. 2011512 | Not dermatology study: sympathectomy |
| Ureña et al. 2009513 | Not a SR or primary prospective study |
| Van Schil 2005514 | Not dermatology study: sympathectomy |
| Vazquez et al. 2011305 | Not dermatology study: sympathectomy |
| Vorkamp et al. 2010515 | Not a SR or primary prospective study |
| Wein et al. 2010516 | Not primary hyperhidrosis |
| Weiss et al. 2010517 | Not dermatology study: sympathectomy |
| Wollina et al. 2002146 | Not relevant intervention (comparing different doses of BTX) |
| Wollina and Konrad 2004518 | Not a SR or primary prospective study |
| Wollina et al. 200820 | Not a SR or primary prospective study |
| Wolosker et al. 2007519 | Not dermatology study: sympathectomy |
| Wolosker et al. 2010244 | Not dermatology study: sympathectomy |
| Wolosker et al. 2010234 | Not dermatology study: sympathectomy |
| Wolosker et al. 2012252 | Not dermatology study: sympathectomy |
| Wolosker et al. 2013254 | Not a SR or primary prospective study |
| Wolosker et al. 2013243 | Not dermatology study: sympathectomy |
| Wolosker et al. 2014237 | Not a SR or primary prospective study |
| Wolosker et al. 2015255 | Duplicate report of Wolosker et al. 201567 |
| Woolery-Lloyd and Valins 2009520 | Not a SR or primary prospective study |
| Woolery-Lloyd and Valins 2009521 | Not a SR or primary prospective study |
| Wörle et al. 2007522 | Not a SR or primary prospective study |
| Xiao et al. 2015333 | Not dermatology study: sympathectomy |
| Yanagishita et al. 2012523 | Not relevant intervention (aluminium chloride) |
| Yazbek et al. 2005235 | Not dermatology study: sympathectomy |
| Yazbek et al. 2009236 | Not dermatology study: sympathectomy |
| Young et al. 2003298 | Not dermatology study: sympathectomy |
| Youssef and Soliman 2015524 | Not dermatology study: sympathectomy |
| Yuncu et al. 2013212 | Not dermatology study: sympathectomy |
| Zaba et al. 2013525 | Not relevant comparator (aluminium chloride and sodium chloride) |
| Zackrisson et al. 2008526 | Not relevant intervention (comparing different doses of BTX) |
Appendix 4 The results of the network meta-analysis
The full NMA results of all pairwise comparisons are reported in Table 63.
| Intervention | Placebo | Medication | Botox | Curettage | Laser | Microwave | Radiofrequency | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median OR | 95% CI | Median OR | 95% CI | Median OR | 95% CI | Median OR | 95% CI | Median OR | 95% CI | Median OR | 95% CI | Median OR | 95% CI | |
| Placebo | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Medication | 7.21 | 1.56 to 53.83 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Botox | 9.21 | 4.73 to 18.10 | 1.26 | 0.15 to 6.88 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Curettage | 6.39 | 1.20 to 27.61 | 0.85 | 0.07 to 7.52 | 0.70 | 0.15 to 2.54 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Laser | 9.13 | 0.90 to 84.68 | 1.20 | 0.06 to 19.50 | 0.99 | 0.11 to 8.25 | 1.45 | 0.28 to 7.62 | N/A | N/A | N/A | N/A | N/A | N/A |
| Microwave | 14.68 | 3.74 to 68.99 | 2.01 | 0.18 to 17.98 | 1.60 | 0.34 to 18.60 | 2.36 | 0.30 to 21.79 | 1.65 | 0.11 to 25.48 | N/A | N/A | N/A | N/A |
Appendix 5 Survey of UK dermatologists
Introduction
The purpose of the survey was to obtain information on current clinical practice to help inform the economic modelling assumptions. The survey was conducted by means of an online survey tool: ‘Qualtrics’ version 2016 (Provo, UT, USA). It was circulated to > 1000 dermatologists in various NHS units across the UK. The questionnaire dealt with treatments typically administered, effectiveness of treatments, adverse events related to treatments and resource use associated with each individual treatment.
Forty-five respondents from 42 different dermatology units completed the survey at least partially (i.e. not all respondents answered every question and, therefore, most of the questions had different response rates).
Methods
Each type of treatment (medication, Botox, iontophoresis, curettage and alternative minor non-invasive surgery) was assigned a separate section in the survey. It was assumed that all dermatologists prescribe medication, including aluminium chloride, for hyperhidrosis and so respondents were asked to list all prescribed medications, their dosages and recommended frequencies. For all other treatments, a filter question was included whereby respondents were simply asked to choose ‘yes’ or ‘no’ depending on the availability of that treatment at their individual unit. If they chose ‘yes’, they were asked to answer further questions on the effectiveness of, and resource use associated with, that treatment. If they chose ‘no’, respondents were asked to move to the next section. The full survey can be seen at the end of Appendix 5.
NB, questions about ETS were not included in the survey as sufficient evidence for the model had already been obtained.
Results
Treatment availability
Medications
When asked what medications were available at their units, 42 dermatologists (93% of the total sample) mentioned at least one type of medication. The total number of responses, or medications listed (n = 114), was greater than the total number of respondents (n = 42), as the majority of clinicians (83%) listed more than one type of medication. Seventy-one per cent of these included oxybutynin and 55% included propantheline bromide.
Table 64 shows the medications prescribed by dermatologists and the percentage delivering each type of medication.
| Medication | Number of respondents | % of respondents |
|---|---|---|
| Aluminium chloride | 10 | 24 |
| Oxybutynin | 30 | 71 |
| Propantheline bromide | 23 | 55 |
| Propranolol | 4 | 10 |
| Clonidine | 2 | 5 |
| Oral glycopyrrolate | 12 | 29 |
| Topical glycopyrrolate | 8 | 19 |
In terms of combinations of the medications outlined in Table 64, 22 different combinations were reported by the 42 respondents. This suggests that there is no unique combination of medications that is prescribed. However, 19 out of the 22 combinations (86%) included oxybutynin and/or propantheline bromide. Use of these two medications only combined was also notably more frequent than use of any other combination (reported by 19% of respondents). All medication combinations are presented in Table 65.
| Aluminium chloride | Oxybutynin | Propantheline | Topical glycopyrrolate | Oral glycopyrrolate | Propranolol | Clonidine | None | Number of combinations | % of respondents |
|---|---|---|---|---|---|---|---|---|---|
| ✓ | ✓ | 8 | 19.0 | ||||||
| ✓ | 6 | 14.0 | |||||||
| ✓ | ✓ | ✓ | 4 | 10.0 | |||||
| ✓ | 3 | 7.0 | |||||||
| ✓ | ✓ | ✓ | 2 | 5.0 | |||||
| ✓ | 2 | 5.0 | |||||||
| ✓ | ✓ | ✓ | 2 | 5.0 | |||||
| ✓ | ✓ | 1 | 5.0 | ||||||
| ✓ | ✓ | ✓ | ✓ | 1 | 2.4 | ||||
| ✓ | ✓ | 1 | 2.4 | ||||||
| ✓ | ✓ | ✓ | ✓ | 1 | 2.4 | ||||
| ✓ | ✓ | ✓ | 1 | 2.4 | |||||
| ✓ | ✓ | 1 | 2.4 | ||||||
| ✓ | ✓ | 1 | 2.4 | ||||||
| ✓ | ✓ | ✓ | 1 | 2.4 | |||||
| ✓ | ✓ | ✓ | ✓ | 1 | 2.4 | ||||
| ✓ | ✓ | 1 | 2.4 | ||||||
| ✓ | ✓ | ✓ | ✓ | 1 | 2.4 | ||||
| ✓ | ✓ | ✓ | ✓ | 1 | 2.4 | ||||
| ✓ | ✓ | ✓ | ✓ | ✓ | 1 | 2.4 | |||
| ✓ | ✓ | ✓ | 1 | 2.4 | |||||
| ✓ | ✓ | 1 | 2.4 | ||||||
| Total | 42 | 100.0 | |||||||
Other treatments
Regarding the availability of other treatments (Botox, iontophoresis, curettage and alternative minor non-invasive surgery), all 45 dermatologists (100%) indicated the availability of one or more procedures. Responses are summarised in Table 66.
| Procedure | Number of respondents who said that the procedure was available | % of respondents |
|---|---|---|
| Iontophoresis (tap water) | 35 | 78 |
| Botox | 26 | 58 |
| Iontophoresis (glycopyrrolate) | 10 | 22 |
| Curettage | 2 | 4 |
| Laser/microwave/ultrasound/radiofrequency | 0 | 0 |
As shown in Table 66, the vast majority of respondents (84%) indicated that iontophoresis (tap water) was available at their dermatology unit, and over half of the sample (58%) stated that their unit offered Botox, but no respondents indicated that alternative minor non-invasive surgery procedures were available.
In terms of combinations of the available treatments, the most commonly offered combination was Botox and iontophoresis (tap water) – reported by 33% of the sample. Slightly fewer dermatologists (22%) indicated iontophoresis (tap water) only. The remaining combinations of treatments varied across the sample, but most included Botox and/or iontophoresis (tap water). All combinations of other treatments can be seen in Table 67.
| Botox | Iontophoresis (tap water) | Iontophoresis (glycopyrrolate) | Curettage | Number of combinations | % of respondents |
|---|---|---|---|---|---|
| ✓ | ✓ | 15 | 33 | ||
| ✓ | 10 | 22 | |||
| ✓ | ✓ | ✓ | 7 | 16 | |
| 5 | 11 | ||||
| ✓ | ✓ | 3 | 7 | ||
| ✓ | 3 | 7 | |||
| ✓ | 1 | 2 | |||
| ✓ | ✓ | 1 | 2 | ||
| Total | 45 | 100 | |||
It can be concluded that there are a variety of medications and other treatments available for hyperhidrosis from dermatologists in the UK. The most prevalent medications are oxybutynin and propantheline bromide and the most common clinical procedures are Botox injections and iontophoresis (tap water), according to the survey results. No respondents indicated availability of the alternative minor non-invasive surgery procedures, suggesting that they are not yet available on the NHS.
Resource use associated with treatments
The survey respondents were asked to provide additional information about available treatments. For medications, they were asked to indicate dosage, frequency and details about follow-up visits. For other treatments, they were asked to indicate duration of the procedure, job title of the treatment provider and details about monitoring visits. Dermatologists that provided iontophoresis (tap water) were additionally asked to indicate the type of machine that would be used in the clinic, the type of machine that would be used at home and the proportion of patients that would continue this treatment at home.
Medications
Nineteen out of 30 dermatologists who prescribe oxybutynin indicated the dose. Responses ranged from 2.5 to 30 mg daily, but the most common minimum and maximum values were 5 mg and 15 mg daily, indicated by eight and six respondents respectively. This is largely consistent with the British National Formulary recommendation, which is, on average, 12.5 mg per day. For propantheline bromide, 21 out of 23 respondents indicated the dose. Responses ranged from 15 to 125 mg daily. The most frequently indicated value was 45 mg daily, which is lower than the British National Formulary recommendation of 75 mg daily. Only 6 out of 12 respondents indicated the dose for glycopyrronium bromide, out of which the most common recommendation was 1–4 mg daily and four indicated the dose for propranolol (120 mg per day).
Thirty-three dermatologists out of 42 who prescribe medication specified the frequency of, length of and the title of health professional present at monitoring visits. More than a third of them (n = 12) stated that follow-up visits take place every 3 months, over a half of them (n = 18) said it would last for 10 minutes and the majority of respondents (n = 25) indicated that it would be led by a consultant dermatologist. According to four clinicians, there are no follow-up visits; patients would be discharged back to their GP.
Botox
According to 13 out of 26 dermatologists who indicated that Botox was available at their unit, the procedure is delivered by a consultant dermatologist. The other half responded that it would be carried out by specialist nurse. In terms of duration of the procedure, 22 out of 24 dermatologists who responded to this question stated values of < 1 hour (mostly 30 minutes). Only 5 of the 26 respondents who indicated that they provide the procedure reported frequency of follow-up visits. In the majority of cases (four), these were every 6 months.
Iontophoresis (tap water)
The treatment details for iontophoresis (tap water) are summarised in Table 68 (the most frequent responses are shown), based on the responses from the 35 dermatologists who indicated that they provide this treatment.
| Details provided | Iontophoresis (tap water) | Number of respondents |
|---|---|---|
| Type of machine used at hospital | ‘Not sure’ | 18/24 |
| Type of machine for home use | ‘Not sure’ | 17/19 |
| Proportion of patients who would carry out the treatment at home | ‘Not sure’ (other responses vary between 5% and 100%) | 8/28 |
The results presented in Table 68 highlight the uncertainty and variability in responses among dermatologists in relation to iontophoresis (tap water) treatment. This could potentially be explained by the fact that the survey was completed by dermatologists, whereas the procedure is normally carried out by a nurse.
Iontophoresis (glycopyrrolate)
Eight of the 10 respondents that indicated that they provide iontophoresis (glycopyrrolate) reported the job title of the health professional. According to all eight, the procedure is delivered by a specialist nurse. Six of the 10 clinicians stated that the procedure could be done at home. Five of the six respondents who reported the length of the procedure said that the procedure would last for up to 1 hour (varied between 15 and 60 minutes).
Curettage
Only one dermatologist provided treatment details about curettage surgery. According to the respondent, the procedure would last for 30 minutes, it would be delivered by a consultant dermatologist and there would be one follow-up visit that would last for 5 minutes.
Adverse events and treatment effectiveness
Dropout rates due to adverse events and lack of effectiveness
The survey respondents were asked to indicate the dropout rates for each type of treatment due to both lack of effectiveness and adverse events. The percentage of who drop out, according to the respondents, was recorded. Results are presented in Tables 69 and 70.
| Treatment type | Percentage of patients who drop out | SD (%) | Number of respondents |
|---|---|---|---|
| Antiperspirants | 68.57 | 26.13 | 21 |
| Medications | 36.46 | 25.09 | 15 |
| Iontophoresis (tap water) | 47.17 | 15.58 | 15 |
| Iontophoresis (glycopyrrolate) | 36.67 | 10.33 | 6 |
| Treatment type | Percentage of patients who drop out | SD (%) | Number of respondents |
|---|---|---|---|
| Aluminium chloride | 54.76 | 34.22 | 22 |
| Medications | 36.80 | 25.37 | 18 |
| Iontophoresis (tap water) | 15.88 | 17.79 | 18 |
| Iontophoresis (glycopyrrolate) | 25 | 21.79 | 5 |
In the case of aluminium chloride and iontophoresis (tap water and glycopyrrolate), dropout rates due to lack of effectiveness were higher than dropout rates due to side effects. For medications, the dropout rates were similar in both categories. Overall, the dropout rates are relatively high for both of the reasons.
Time to effectiveness
As sufficient clinical advice on time to effectiveness had already been obtained for all types of treatment except for medications, data were only collected for medications. The responses varied significantly, from ‘immediate’ to ‘up to 3 months’. The most common response was ‘variable’ (stated by 8 out of 38 respondents).
Survey questions
-
What is the name of the town/city to which your responses relate?
-
What is the name of the hospital trust(s) to which your responses relate?
-
What medications, and with what dose and frequency, do you prescribe to hyperhidrosis patients?
-
What is the time to effectiveness of medication for hyperhidrosis?
-
If a hyperhidrosis patient is on medication:
-
How frequently would a monitoring visit (follow-up visit following the prescription of medication) be required?
-
What is the duration of one monitoring visit? (In minutes.)
-
Job title of the health professional(s) that is present at the monitoring visit.
-
-
Is there an ongoing risk of dropout due to adverse events related to medication use beyond 1 month?
-
Is Botox procedure available at your dermatology unit?
-
What is the job title of the health professional(s) that delivers the procedure?
-
What is the duration of the procedure (in minutes)?
-
How many monitoring visits following Botox treatment and how often do they occur?
-
Is curettage surgery (for hyperhidrosis) available at your dermatology unit?
-
What is the duration of the surgery? (In minutes.)
-
What is the job title of the health professional(s) that delivers the procedure?
-
What is the duration of monitoring visits? (In minutes.)
-
What is the frequency of monitoring visits?
-
What are the side effects?
-
What are the treatments of side effects?
-
Is laser surgery available at your dermatology unit?
-
What is the duration of the surgery? (In minutes.)
-
What is the job title of the health professional(s) that delivers the procedure?
-
What is the duration of monitoring visits? (In minutes.)
-
What is the frequency of monitoring visits?
-
What are the side effects?
-
What are the treatments of side effects?
-
Is microwave surgery available at your dermatology unit?
-
What is the duration of the surgery? (In minutes.)
-
What is the job title of the health professional(s) that delivers the procedure?
-
What is the duration of monitoring visits? (In minutes.)
-
What is the frequency of monitoring visits?
-
What are the side effects?
-
What are the treatments of side effects?
-
Is ultrasound treatment of hyperhidrosis available in your unit/hospital?
-
What is the duration of the surgery? (In minutes.)
-
What is the job title of the health professional(s) that delivers the procedure?
-
What is the duration of monitoring visits? (In minutes.)
-
What is the frequency of monitoring visits?
-
What are the side effects?
-
What are the treatments of side effects?
-
Is radiofrequency treatment of hyperhidrosis available in your unit/hospital?
-
What is the duration of the surgery? (In minutes.)
-
What is the job title of the health professional(s) that delivers the procedure?
-
What is the duration of monitoring visits? (In minutes.)
-
What is the frequency of monitoring visits?
-
What are the side effects?
-
What are the treatments of side effects?
-
Is iontophoresis (with tap water) available at your dermatology unit?
-
What is the model of the iontophoresis machine used at the hospital?
-
Approximately, what is the proportion (%) of patients who would carry on with the treatment at home?
-
What is the model of the iontophoresis machine recommended for home use?
-
What are the side effects?
-
What are the treatments of the side effects?
-
Is iontophoresis (with glycopyrrolate) available at your dermatology unit?
-
What is the job title of the health professional(s) that delivers the procedure?
-
What is the duration of the procedure? (In minutes.)
-
What are the side effects?
-
What are the treatments of the side effects?
-
Can the patient have the procedure at home?
-
Approximately what proportion of patients (%) would carry on with the treatment at home?
-
Approximately, please indicate the dropout rates (%) due to lack of effectiveness for the following treatments:
-
antiperspirants
-
medication
-
iontophoresis (tap water)
-
iontophoresis (glycopyrrolate).
-
-
Approximately, please indicate the dropout rates (%) due to side effects for the following treatments:
-
antiperspirants
-
medication
-
iontophoresis (tap water)
-
iontophoresis (glycopyrrolate).
-
Appendix 6 R code for deriving event ranges for continuously reported outcome measure for the Hyperhidrosis Disease Severity Scale
Appendix 7 Derivation of the relative risk from the event ranges
Appendix 8 Network meta-analysis assuming individual surgery effects
Appendix 9 Between-study variance estimates
Mean between-study variance estimates assuming individual surgery effects (Table 71).
| Comparison | Mean between-study variance |
|---|---|
| Medication vs. placebo | 0.13 |
| Botox vs. placebo | 0.13 |
| Curettage vs. Botox | 0.48 |
| Laser vs. curettage | 0.37 |
| Microwave surgery vs. placebo | 0.29 |
| Radiofrequency vs. placebo | 0.29 |
Appendix 10 Methods and graphic plots of the relationships between the Hyperhidrosis Disease Severity Scale level response ratios and the probability of response
Specifically, 200,000 random combinations of numbers of different responses were drawn. These were categorised into 21 response categories: < 2.5%, ≥ 97.5% and nineteen 5% bins in between. The ratio of the numbers with a HDSS 3-point reduction to the numbers with a HDSS 2-point reduction, and of the numbers with a HDSS 0-point reduction to the numbers with a HDSS 1-point reduction, were calculated for each category for each simulation and then the average calculated. The lowest and highest categories and the middle category were discarded. The relationship between the response rate and each of the ratios was explored for a response rate < 0.5 and > 0.5.
For the ratio of numbers with a HDSS 3-point reduction to the numbers with a HDSS 2-point reduction, the ratio is plotted against response levels for response > 0.5 in Figure 19a. The HDSS 3/2 ratio for response > 0.5 is log-transformed before fitting a linear regression in Figure 19b.
FIGURE 19.
(a) Ratio of HDSS 3 to HDSS 2 at high response; and (b) ratio log-transformed with regression line.

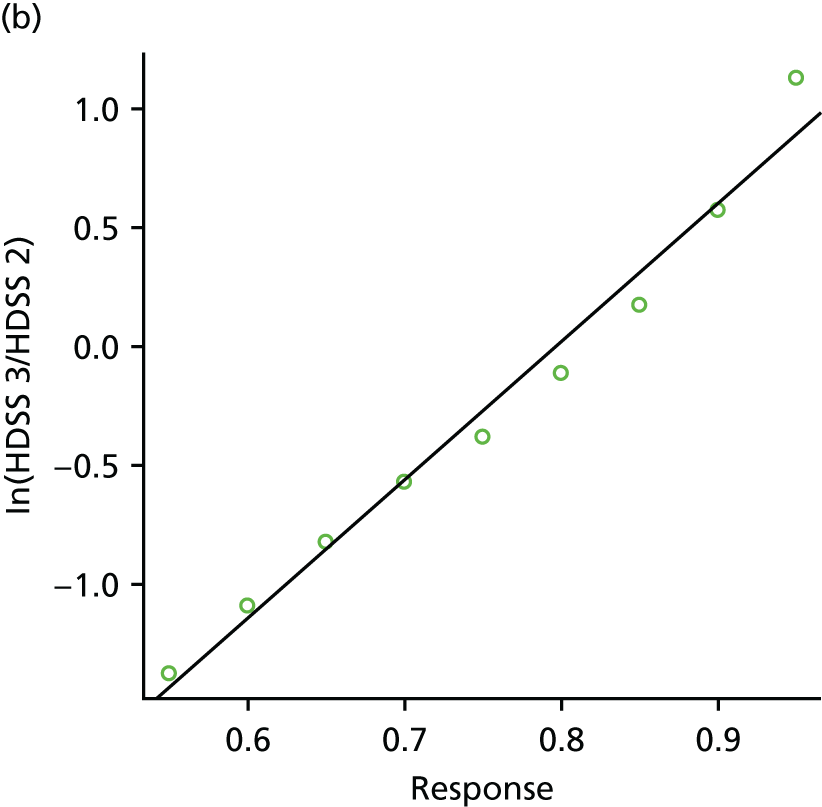
For the ratio of numbers with a HDSS 0 reduction to the numbers with a HDSS 1-point reduction, the ratio is plotted against response levels for response < 0.5 in Figure 20a. The HDSS 0/1 ratio for response < 0.5 is log-transformed before fitting a linear regression in Figure 20b.
FIGURE 20.
(a) Ratio of HDSS 0 to HDSS 1 at low response; and (b) ratio log-transformed with regression line.
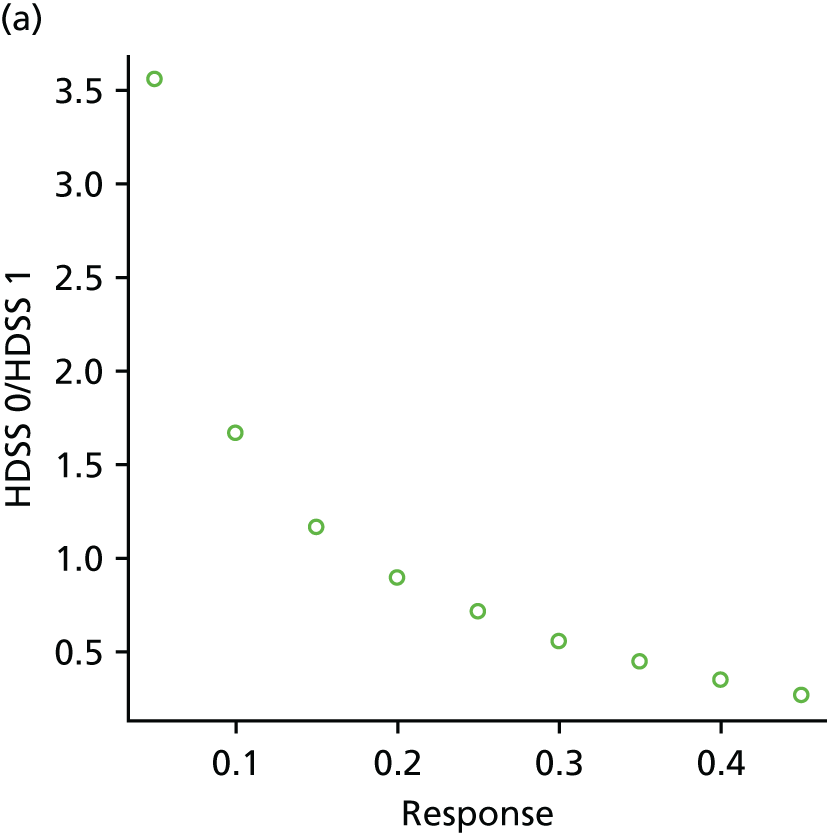
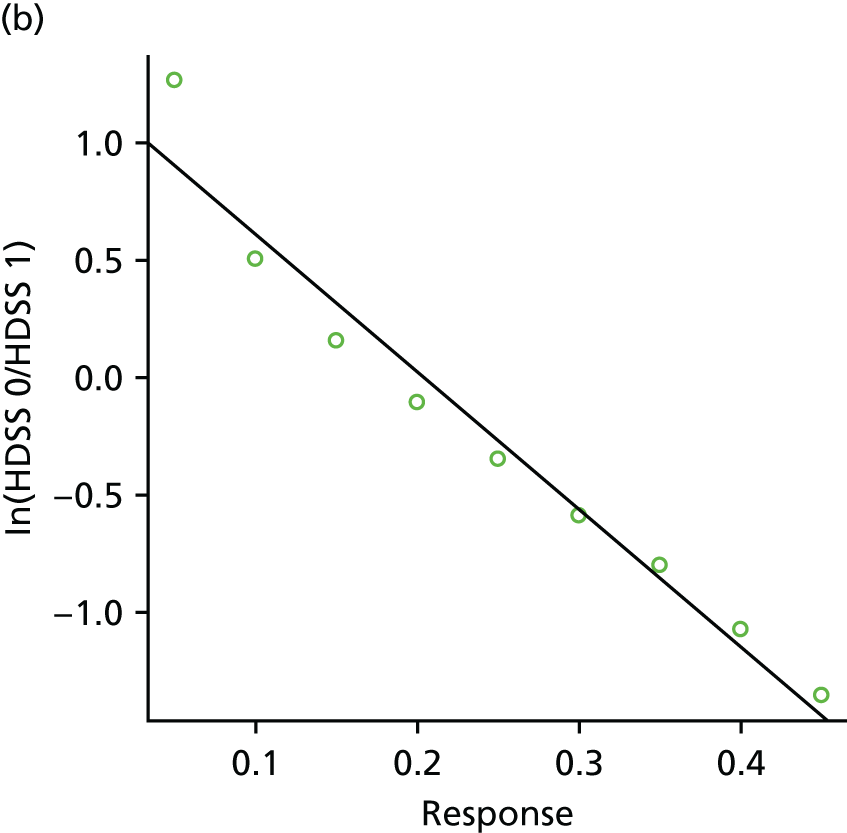
The HDSS 3 : 2 ratio is plotted against response levels for response < 0.5 in Figure 21a. There is no discernible relationship between the HDSS 3 : 2 ratio and response when response is < 0.5. The average ratio is taken to be 0.38. The HDSS 0 : 1 ratio is plotted against response levels for response > 0.5 in Figure 21b. Again, there is no discernible relationship between the HDSS 0 : 1 ratio and response when response is < 0.5 and the average ratio is taken to be 0.38.
FIGURE 21.
(a) Ratio of HDSS 3 to HDSS 2 at low response; and (b) ratio of HDSS 0 to HDSS 1 at high response.


Appendix 11 Sample and sampling distributions for Hyperhidrosis Disease Severity Scale-level EuroQol-5 Dimensions index
Figure 22a presents the sample distribution of the utilities for the HDSS level 2 on the EQ-5D range (–0.594 to 1) simulated from a beta distribution derived from EQ-5D index mean and SDs rescaled to the range of 0 to 1. Figure 22b presents the sampling distribution of the EQ-5D index estimates rescaled as a beta distribution. Figures 23 and 24 present the same results for HDSS levels 3 and 4 respectively.
FIGURE 22.
(a) HDSS 2 sample distribution; and (b) sampling distribution.

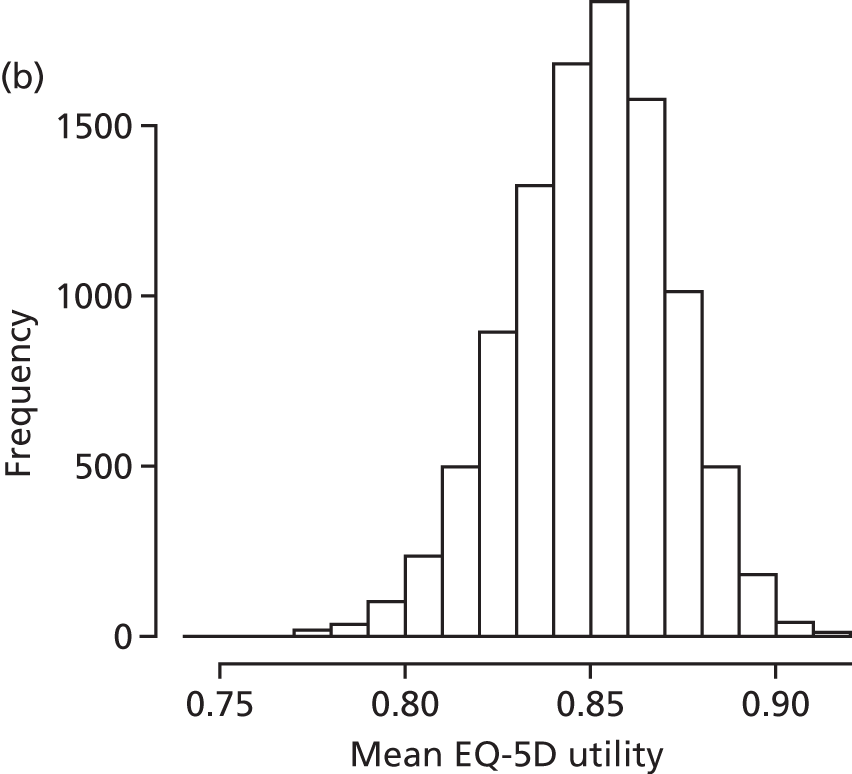
FIGURE 23.
(a) HDSS 3 sample distribution; and (b) sampling distribution.

FIGURE 24.
(a) HDSS 4 sample distribution; and (b) sampling distribution.


Appendix 12 Summary cost and quality-adjusted life-year estimates for each treatment sequence
Table 72 presents the mean QALYs and costs for each of the 64 treatment sequences from the base-case probabilistic model. In the results in Chapter 7, the treatment sequences that were either dominated or dominated by extension were not presented.
| Strategy | Mean QALYs | Mean cost (£) | Strategy | Mean QALYs | Mean cost (£) |
|---|---|---|---|---|---|
| I | 18.48 | 1457 | IMBE | 19.82 | 6898 |
| IC | 18.84 | 1640 | BIMC | 19.81 | 7082 |
| ICE | 19.30 | 1813 | BIM | 19.80 | 7111 |
| A | 14.33 | 1914 | BIMCE | 19.85 | 7248 |
| C | 16.42 | 2056 | MIE | 19.36 | 7308 |
| CE | 16.81 | 2667 | BIME | 19.83 | 7325 |
| IE | 18.89 | 3467 | BMIC | 19.82 | 7424 |
| IMC | 19.27 | 5020 | BMI | 19.80 | 7461 |
| IM | 19.16 | 5096 | MIBC | 19.81 | 7535 |
| IBC | 19.64 | 5271 | MIB | 19.80 | 7576 |
| IB | 19.61 | 5337 | BMICE | 19.85 | 7593 |
| E | 16.20 | 5406 | BMIE | 19.83 | 7693 |
| IBCE | 19.71 | 5583 | MIBCE | 19.85 | 7704 |
| IMCE | 19.53 | 5622 | MIBE | 19.82 | 7813 |
| IBE | 19.66 | 5800 | MBIC | 19.82 | 8151 |
| MIC | 19.32 | 5935 | MBI | 19.81 | 8188 |
| MI | 19.17 | 6012 | MBICE | 19.85 | 8320 |
| IBMC | 19.80 | 6130 | BC | 19.45 | 8330 |
| IBM | 19.79 | 6159 | MBIE | 19.83 | 8420 |
| IME | 19.39 | 6177 | BMC | 19.68 | 8680 |
| BIC | 19.65 | 6231 | B | 19.36 | 8720 |
| BI | 19.62 | 6289 | BM | 19.65 | 8800 |
| IBMCE | 19.84 | 6297 | BMCE | 19.76 | 9288 |
| IBME | 19.82 | 6373 | MBC | 19.69 | 9344 |
| MC | 18.79 | 6442 | BME | 19.72 | 9491 |
| BICE | 19.72 | 6543 | MB | 19.66 | 9527 |
| MICE | 19.56 | 6564 | BCE | 19.61 | 9630 |
| IMBC | 19.81 | 6620 | MCE | 19.39 | 9657 |
| IMB | 19.80 | 6661 | MBCE | 19.77 | 9934 |
| M | 18.42 | 6706 | MBE | 19.71 | 10,240 |
| BIE | 19.67 | 6741 | BE | 19.47 | 10,250 |
| IMBCE | 19.84 | 6788 | ME | 19.02 | 10,733 |
Appendix 13 Economic model
Appendix 14 End-of-project workshop documents
Research project to investigate the management and treatment of hyperhidrosis
Health Technology Assessment project: 14/211/02 – interventional management of hyperhidrosis: an evidence synthesis and value-of-information analysis
The emotional, psychosocial and physical impact of hyperhidrosis is substantial and the optimal management of patients with this condition is essential. There is substantial variation in which treatments are available in the NHS and access to treatments is limited for many patients. In addition, decisions about which treatments to use are often made in a context of uncertainty.
The National Institute for Health Research have provided funding to thoroughly review the evidence for the different treatments for hyperhidrosis. A team of researchers from the University of York and Newcastle University have come together with clinicians in dermatology and surgery to carry out this research. The focus of the project is to undertake a review of studies on all potentially useful treatments, supplemented by patients’ view regarding important treatment effects, to establish which treatments are most potentially effective and value for money to both the NHS and public. The results will help inform further research.
The research project started on 1 December 2015 and will be completed by 14 December 2016.
We will report the results of the project to our patient advisors at a workshop on 18 November 2016. We would appreciate any feedback you can offer at that time regarding our findings and recommendations.
Workshop 18 November 2016
Outline
-
Introduction and welcome.
-
Short presentation of the project and its purpose.
-
Review results:
-
which treatments have been studied
-
which ones definitely work (good evidence)
-
which ones definitely do not work (good evidence)
-
which look promising but more research needed.
-
-
Economic modelling methods and results.
-
Discussion of trials that might be recommended.
-
Discussion of tools used for measuring quality of life.
Quality-of-life questionnaires
We would be very interested in hearing your thoughts and opinions regarding the attached quality-of-life questionnaires and the ways quality of life are measured in patients with hyperhidrosis.
Please could you consider the following questions and we look forward to discussing your views at the workshop.
-
Which quality-of-life tool do you find the easiest to complete?
-
Which tool is the most appropriate to capture quality-of-life issues for patients with hyperhidrosis?
-
Do you feel a combination of tools or questions would be best to address quality of life in patients with hyperhidrosis?
-
In your opinion do the questions in the new HidroQoL© tool address all the quality-of-life issues a patient with hyperhidrosis may have? Is there anything you feel is lacking from the HidroQoL© tool?
-
Do you have any further comments you wish to share?
Thank you for taking the time to consider these questions.
Links to the quality-of-life questionnaires
Dermatology Life Quality Index
URL: http://sites.cardiff.ac.uk/dermatology/quality-of-life/dermatology-quality-of-life-index-dlqi/ (accessed 18 November 2016).
Hyperhidrosis Disease Severity Scale
URL: www.sweathelp.org/pdf/HDSS.pdf (accessed 18 November 2016).
Hyperhidrosis Quality of Life Questionnaire
URL: www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-35862003000400003#at (accessed 18 November 2016).
Hyperhidrosis Quality of Life Index
URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC4366556/figure/Fig3/ (accessed 18 November 2016).
List of abbreviations
- AC
- alternative current
- AMED
- Allied and Complementary Medicine Database
- BTX
- botulinum toxin
- BTX-A
- botulinum toxin type A
- BTX-B
- botulinum toxin type B
- CDSR
- Cochrane Database of Systematic Reviews
- CEA
- cost-effectiveness analysis
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COSMIN
- COnsensus-based Standards for the selection of health Measurement INstruments
- CRD
- Centre for Reviews and Dissemination
- CS
- compensatory sweating
- DARE
- Database of Abstracts of Reviews of Effects
- DC
- direct current
- DLQI
- Dermatology Life Quality Index
- EDLQ
- Everyday Life Questionnaire
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ETS
- endoscopic thoracic sympathectomy
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- GAIS
- Global Aesthetic Improvement Scale
- GP
- general practitioner
- HDSS
- Hyperhidrosis Disease Severity Scale
- HHIQ
- Hyperhidrosis Impact Questionnaire
- HidroQoL©
- Hyperhidrosis Quality of Life Index
- HQLQ
- Hyperhidrosis Quality of Life Questionnaire
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IGA
- Investigator Global Assessment
- IIRS
- Illness Intrusiveness Rating Scale
- MCS
- mental component summary
- MD
- mean difference
- MOS
- Medical Outcomes Study
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- OR
- odds ratio
- PCS
- physical component summary
- P-HI
- Palmar Hyperhidrosis Improvement
- P-HQOL
- Palmar Hyperhidrosis Quality of Life
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- risk ratio
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- U
- unit
- VAS
- visual analogue scale
- VOI
- value of information
- WTP
- willingness to pay
