Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 15/17/07. The protocol was agreed in June 2016. The assessment report began editorial review in December 2016 and was accepted for publication in May 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Scotland et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Background and definition of the decision problem(s)
Condition(s) and aetiology(ies)
Brief statement describing the health problem
Chronic kidney disease (CKD) is a long-term condition in which the kidneys do not function effectively. There are many causes of CKD, including hereditary disease and autoimmune disorders, but the most common causes are high blood pressure or diabetes mellitus. 1 The progression of CKD can be measured according to five stages of severity. In the most severe stage of the disease, stage 5, the kidneys will be working at ≤ 15% of their normal function. At this point, the patient will need to start treatment in the form of conservative management, dialysis or kidney transplantation. 2
Collectively, these treatments are referred to as renal replacement therapy (RRT). Dialysis involves removing waste products and excess fluid from the bloodstream. 3 There are two types of dialysis treatment: haemodialysis (HD) and peritoneal dialysis (PD). To calculate the volume of fluid to be removed during dialysis, a person will be assigned a target weight, which is what they should weigh in the morning if they receive PD, or at the end of a HD session. Maintaining the correct volume of fluid in the body is essential for people receiving dialysis. 4 Multiple-frequency bioimpedance devices, which measure the fluid status of people receiving dialysis for CKD, have been proposed for the monitoring of fluid status and for assisting the decision about the optimum target weight for people receiving dialysis.
Aetiology, pathology and prognosis
The primary function of the kidneys is to remove waste products from the blood and expel them into the urine. The kidneys are also involved in maintaining blood pressure, regulating the levels of chemicals in the body, and producing vitamin D and erythropoietin. CKD is a long-term condition in which the ability of the kidney(s) to function is reduced,3,4 and is defined as either kidney damage (i.e. abnormalities of kidney function or structure; albuminuria) or glomerular filtration rate (GFR) of < 60 ml/minute/1.73 m2 for at least 3 months. 5–9 In healthy people, the level of GFR varies according to age, sex and body size. Normal GFR in young adults is approximately 120–130 ml/minute/1.73 m2 and declines with age. 6,10 Therefore, a GFR of < 60 ml/minute/1.73 m2 represents a loss of at least half of the normal adult kidney function and, below this level, the prevalence of CKD complications increases. 6 GFR is the ‘gold standard’ for assessment of kidney function, but its measurement is awkward and calculated creatinine clearance is often used as a proxy measure of GFR for practical purposes. 11
Risk factors for CKD lie within the following categories: (1) factors that increase the risk of kidney damage, for example age, diabetes mellitus, hypertension, family history; (2) factors that initiate kidney damage, for example diabetes mellitus, hypertension, autoimmune diseases, primary glomerulopathies; or (3) factors that cause progressive decline in renal function after onset of kidney disease, for example persistent activity of underlying disease, elevated blood pressure or blood glucose, diet including a high level of protein/phosphates, hyperlipidaemia, anaemia, cardiovascular (CV) disease, smoking. 6,11
Chronic kidney disease is classified into a continuum of five stages, based on renal function:5,6,11
-
normal or increased GFR
-
early renal insufficiency
-
moderate renal failure
-
severe renal failure
-
kidney failure.
In the early stages, kidney disease is often asymptomatic and can be reversible. Most diseases evolve slowly over time, but rapidly progressive diseases can result in kidney failure within months. 12 Kidney failure is considered to be the most serious outcome of CKD, with symptoms generally caused by reduced kidney function. Kidney failure is defined as GFR of < 15 ml/minute/1.73 m2, which is accompanied, in most cases, by signs and symptoms of uraemia, or the need to start kidney replacement therapy (dialysis or transplantation). 6,13–16
The two main types of dialysis that are available are (1) HD and (2) PD. The key factors in determining what type of dialysis people receive are patients’ preference, availability of options and clinical contraindications. 17
In HD, the patient is connected to a dialysis machine containing a semipermeable membrane and dialysis fluid. The patient’s blood is passed into the machine, in which electrolytes, water and metabolic waste products in the blood pass across the semipermeable membrane and the waste products are retained in the dialysis fluid. The most common HD prescription is for 4 hours, three times per week. HD can be given in hospital, in a satellite unit or at home. 18
Peritoneal dialysis involves dialysis fluid (usually containing glucose) being passed into the peritoneal cavity (via a permanent catheter), where blood vessels lining the cavity draw waste products and excess fluid from the blood into the dialysis fluid, which is then drained from the cavity. Changing the fluid takes around 30–40 minutes and is repeated four times daily (continuous ambulatory PD). Alternatively, the process of fluid exchange can be carried out by a machine overnight (automated PD). 3,4,19 It is also possible to have a combination of manual and automatic exchanges.
Incidence and/or prevalence
The UK Renal Registry 18th Annual Report indicates that the prevalence of patients receiving RRT in 2014 was 913 per million population. 20 Prevalence rates were observed to increase across the whole of the UK in 2014. The median age of prevalent patients was 59 years (HD, 67 years; PD, 64 years; and transplant, 53 years). It is worth noting that while half of all patients receiving RRT continued to be aged 40–69 years, the prevalent population is becoming more elderly, with 16% of patients aged > 75 years. For all ages, the prevalence rate in men exceeded that in women. The proportion of patients treated with PD, which has been falling since the early 1990s, was reported to be just 6% in 2014. In general, large variations in prevalence were observed between centres across the UK. This variation is likely to be explained by the proportion of patients requiring RRT, but also by the type and quality of clinical care delivered by renal centres. 20 In 2014, 21.5% of the prevalent UK population receiving RRT were from minority ethnic groups (23.7% in England). This figure represented an increase from 14.9% in 2007. 21 Ethnic origin has been shown to be associated with CV events and death in people with end-stage renal disease (ESRD) receiving dialysis. 22 In 2014, 917 children with established renal failure were receiving treatment, with 11.2% receiving HD and 9.5% receiving PD. Of these, 72 children were aged < 4 years. The body composition of children is different from that of adults23 and requires more frequent monitoring because of their rapid growth. 24
Impact of health problem: significance for patients in terms of ill health (burden of disease) and significance for the NHS
In replacing normal renal function, dialysis needs to remove any excess fluid. When HD is used, this is fluid that has accumulated in the body since the last dialysis session. In people receiving dialysis, it is vital to balance fluid status, as both overhydration (also referred to as hypervolaemia or fluid overload) and underhydration (also referred to as hypovolaemia) are associated with negative outcomes, such as mortality, intradialytic morbidity and long-term CV complications. 19,25–30 Removal of an appropriate volume of fluid is required to minimise complications caused by being either ‘overhydrated’ or ‘underhydrated’. Determining when a person is ‘overhydrated’ or ‘underhydrated’ varies depending on the parameter being used to determine fluid status, and also the cut-off points used to designate overhydration or underhydration, which differ between studies. When clinical assessment is used, fluid status is classified qualitatively. Individuals are classified as overhydrated or underhydrated if any corresponding symptoms are present, and normohydrated (or ‘euvolaemic’) when they are absent.
Overhydration resulting from removal of too little fluid during dialysis contributes to hypertension, CV complications, mortality, oedema and left-ventricular hypertrophy. 25,26,30–36 A negative association between higher diastolic blood pressure and residual renal function has also been reported. 37
Complications associated with overhydration can be asymptomatic. Oedema, for example, may not be detectable until interstitial fluid volumes rise to approximately 30% above normal. 32 The use of blood pressure as a surrogate measure for fluid status is not entirely reliable, as factors, such as age and comorbidities, may cause volume-independent hypertension.
Underhydration, which is caused by excessive volumes of fluid being removed during dialysis, can result in cramps, intradialytic hypotension and increased recovery time following dialysis. 38–41 In addition, there is an association between reduction of fluid volume in people commencing HD and loss of residual kidney function, along with a related increase in the risk of morbidity and mortality. 42,43
In the UK, on 31 December 2014, there were 58,968 adults receiving RRT (49,842 in England and 2842 in Wales). Of these, 27,804 patients were receiving dialysis (23,734 in England and 1308 in Wales). In particular, 86.9% received HD (38.6% in hospital, 44% in satellite units and 4.3% at home), 5.8% received continuous ambulatory PD and 7% received automated PD. 20,44 In addition, 190 children and young people aged < 18 years were receiving dialysis (103 receiving HD and 87 receiving PD). 20,44
The Hospital Episode Statistics for England for the 2014–15 period45 reported 40 finished consultant episodes and six outpatient attendances for renal dialysis (code X40.1), 2265 finished consultant episodes and 931 outpatient attendances for PD (code X40.2), 44,457 finished consultant episodes and 16,941 outpatient attendances for HD (code X40.3) and 570 finished consultant episodes and one outpatient attendance for automated PD (code X40.5). However, there is a possibility that the outpatient data are not complete, as procedure/intervention is not a mandated field in the outpatients’ data set and coverage within this field is poor.
Measurement of disease
To enable an assessment of the volume of fluid to be removed during dialysis (known as the ‘ultrafiltration volume’19), people are assigned a ‘dry weight’ or ‘target weight’ (i.e. euvolaemic), which is commonly defined as the lowest tolerated post-dialysis weight at which there are minimal signs or symptoms of underhydration or overhydration. This is achieved via gradual change in post-dialysis weight. 25,28,46,47 It can also be defined as how much a person should weigh in the morning, if receiving PD, or at the end of a HD session. 4 Although the terms ‘dry weight’ and ‘target weight’ are often used interchangeably in clinical practice and in the published literature, hereafter the term ‘target weight’ will be used in this report. Target weight is commonly estimated using methods, such as weight gain between dialysis sessions, pre-dialysis and post-dialysis blood pressure, and subjective symptoms. 38 However, methods for assessing target weight are not precise and it has been reported that approximately half of people who achieve their ‘ideal target weight’ are actually overhydrated. 48 Dialysis centres are now increasingly using measurement devices based on bioimpedance technology, as they are non-invasive, simple and inexpensive. 27,49,50
Description of technology(ies) under assessment
Summary of the multiple-frequency bioimpedance devices under assessment
Bioimpedance technology involves assessment of fat-free mass and total body water in people without significant fluid and electrolyte abnormalities. 51 Extracellular water (ECW) and intracellular water (ICW) contains ions and, therefore, conducts, so its volume measurement is based on its resistance, or impedance, as cell membranes may act as capacitors at low or intermediate frequencies. There are various bioimpedance methods, depending on the frequency of current involved and body site of measurement. Single-frequency bioimpedance analysis uses only one single current (e.g. 50 kHz), multiple-frequency bioimpedance analysis uses currents of multiple frequencies (e.g. 5, 50 and 100 kHz) and bioimpedance spectroscopy uses a range of frequencies (5–1000 kHz). 33,52 In particular, bioimpedance spectroscopy uses an electrical circuit of tissues with parallel resistances and a conductivity theory to take account of non-conducting elements to measure ECW and ICW volumes. 49 In a simple direct current electrical circuit, resistance is the determining factor of flow at a given voltage. However, when an alternating current is applied, there is a second factor causing resistance (or ‘reactance’) to flow and it is this factor that provides the additional metric to enable fluid compartments to be characterised. When an alternating current is applied to tissue, the resistance measurement is inversely proportional to the total content (ICW and ECW) between two electrodes on the skin; the reactance, a measure of electrical capacitance, is proportional to the cell mass in this tissue volume. The various methods of capturing and interpreting this information all obtain indirect measures of tissue water content and the proportion contained in the intracellular and extracellular spaces. 27,53 The limbs provide a disproportionate amount of information (> 80%), as compared with the trunk, by way of bioimpedance analysis, as a result of the neurovascular bundles and high muscle content in proportion to their cross-sectional area. As a result, measuring segments of the body, such as the lower leg54 or chest wall,55 is sometimes preferred. 27
The technologies relevant to this assessment are the Body Composition Monitor [(BCM) Fresenius Medical Care, Bad Homburg, Germany]; the MultiScan 5000 (Bodystat, Douglas, Isle of Man), the BioScan 920-II (Maltron International, Essex, UK), the BioScan touch i8 (Maltron International, Essex, UK), and the InBody S10 (InBody, Seoul, South Korea). Characteristics of these devices are reported below.
Body Composition Monitor
The BCM is a portable, stand-alone device, which uses bioimpedance spectroscopy to estimate a person’s fluid and nutritional status. The person is placed in a supine position and four electrodes are attached: two to the back of one hand and two to the foot on the same side of the body. The electrodes are connected to the BCM device via a cable. The device passes a painless alternating current at 50 different frequencies (5–1000 kHz) through the body and measures the impedance between the hand and foot, giving relative impedance values for each frequency. This range of measurements determines the electrical resistances of the total body water and ECW and allows distinction of ECW and ICW. 27,56 The software also calculates fluid overload using two physiological models. The volume of ECW that should be present based on the identified amounts of lean and adipose tissue is calculated and compared with the measured volume of extracellular fluid. 57,58 The resulting volume difference between predicted and actual extracellular fluid is used as a measure of a person’s overhydration volume and is reported by the device in litres.
The BCM is intended to be used as an objective measure of fluid imbalance, to complement clinical judgement. The associated software uses two validated physiological models to obtain the clinically relevant parameters: overhydration, lean tissue mass and adipose tissue mass. 4,56 There are no restrictions on the age of the person that this device can be used on. Results from the BCM are available within 2 minutes and are stored on a ‘PatientCard’ automatically, from which it can be loaded onto a database. Cards are reusable and can be reprogrammed for a new patient, or can have a patient’s data deleted if they become full, and remain programmed for that patient.
Good agreement has been shown between BCM assessment and current standard methods for measuring ECW and total body volumes, ICW volume, total fat, fat-free mass and fluid overload in adults and urea distribution volume in children. 24,59 The evidence of association between BCM assessment and improved patient outcomes is mixed. The Canadian Agency for Drugs and Technologies in Health (CADTH)’s rapid-response report, published in 2015,53 identified two randomised controlled trials (RCTs) of 131 and 189 participants, respectively60,61 and one observational study of 110 participants, which assessed the use of the BCM in people receiving HD. 62 The report concluded that there was improvement in some patient outcomes, such as decreased blood pressure and reduced fluid overload, with patient management guided by BCM assessments, but that the evidence base was limited. A study of people receiving PD compared assessment of overhydration status using the BCM with assessment using a standard protocol. Results showed that ECW volume and ECW-to-ICW volume ratio decreased steadily over the 3-month follow-up period in the group assessed using the BCM, but increased in the group assessed using standard methods. In addition, systolic blood pressure (SBP) decreased significantly in the group assessed using the BCM, but increased significantly in the group assessed using standard methods. 63
Further information on the BCM is available from the manufacturer’s website. 56
MultiScan 5000
The MultiScan5000 is a portable device that uses bioimpedance spectroscopy to measure at 50 frequencies (ranging from 5 kHz to 1000 kHz), which are used to calculate body composition and hydration by a mathematical model called Cole–Cole analysis (also used in the BCM models). Values for ECW, ICW, total body water, and volume of over/underhydration are obtained from similar physiological models as used in the BCM. 57,58
The volume of overhydration output is recommended for the assessment of hydration status in people aged 18–70 years. Outside this age range, this output can be used to track relative changes over time. In addition, the ratio of total body water to ECW volume calculated by the device (called the ‘prediction marker’) can be used as an additional marker to track hydration status over time in all age groups. The device can measure body segments, depending on the placement of the electrodes,64 and provides a bioelectrical impedance vector analysis. Additional parameters related to body composition, such as fat weight, lean weight, skeletal muscle mass and body cell mass, can also be estimated. These parameters can be used to estimate nutritional status and, therefore, help to identify malnutrition status in people with CKD who are treated with dialysis. Further information on the MultiScan 5000 device can be found on the product webpage. 64
BioScan 920-II
The BioScan 920-II is a portable multiple-frequency bioimpedance analysis device, which measures at 5, 50, 100 and 200 kHz. The eight electrodes allow monitoring of fluid changes in the whole body, thorax, trunk, legs or arms. All data are recorded and displayed immediately for analysis by the system. Alongside the standard output parameters related to hydration status [target water (minimum/maximum), target weight, target weight (minimum/maximum), extracellular fluid, ECW volume, ICW volume, total body water, ECW (%), ICW (%), total body water (%), ECW-to-ICW volume ratio, plasma fluid (intravascular), fat-free mass hydration], the device estimates additional parameters related to body composition [comprising body mass index (BMI), body density, body cell mass, protein mass, fat mass, fat-free mass and glycogen mass] and mineral content.
These parameters can be used to evaluate nutritional status and help to identify malnutrition in people with CKD receiving dialysis. Further information can be found on the product webpage. 65 The use of the BioScan 920-II is recommended for people aged 5–99 years. A version of the BioScan 920-II device (the BioScan 920-II-P) is also available for monitoring hydration status in preterm, neonatal and paediatric patients (for use from 23 weeks’ gestational age up to 18 years).
BioScan touch i8
According to the manufacturer, an updated version of the BioScan 920-II device, the BioScan touch i8 with an updated user interface, is due to be released during the course of this assessment. As with the BioScan 920-II, it is anticipated that there will be two versions: one suitable for people aged 0–18 years and one suitable for people aged 5–99 years.
InBody S10
The InBody S10 is a portable device that uses a direct multiple-frequency bioimpedance analysis method to provide measurements across six different frequencies (1, 5, 50, 250, 500 and 1000 kHz). Measurements of five segments of the body are available: right arm, left arm, trunk, right leg and left leg. Hydration-related outputs include water volumes (ECW, ICW), ratio of extracellular to total body water and history of body water condition.
These parameters are estimated along with a suggested standard range of values to facilitate identification of overhydrated or underhydrated individuals. In addition, the InBody S10 provides estimates related to body composition such as body cell mass, basal metabolic rate, bone mineral content, skeletal muscle mass, fat-free mass, and BMI. These parameters can be used to evaluate nutritional status and help to identify malnutrition in people with CKD who are on dialysis. A full list of outputs can be found on the product webpage. 66 The use of the InBody S10 device is recommended for people aged 3–99 years.
Identification of important subgroups
This assessment focuses on people with CKD who are treated with HD or PD.
Relevant patient subgroups may include:
-
people who are treated with HD
-
people who are treated with PD
-
people of different ethnic origins
-
people for whom recommended configurations of electrodes cannot be used or who cannot assume the required positions for measurements to be made
-
people at extremes of body composition measurements
-
children aged < 5 years who may require more frequent monitoring.
Current usage in the NHS
In the UK, multiple-frequency bioimpedance devices are used in some renal centres alongside clinical judgement to estimate fluid levels in patients receiving HD or PD. The Leeds Teaching Hospitals NHS Trust, for example, has prepared a standard operating procedure document for using the BCM in UK clinical practice. 4,67 However, there is currently no national guidance in England and Wales on the role and adoption of these devices in clinical practice.
Comparators
In UK clinical practice, standard clinical assessment (without the use of bioimpedance devices) is used to determine fluid status and set, or adjust, target weights for people with CKD who are treated with dialysis. This may include the consideration of clinical parameters such as blood pressure measurements, changes in weight, the presence of oedema, assessment of residual renal function, any pre-existing CV conditions, and any patient-reported symptoms, intradialytic or interdialytic, of overhydration or underhydration (e.g. cramps, fatigue, diarrhoea, nausea, dizziness, fainting, breathlessness, decreased appetite or visual disturbances).
It is worth pointing out that clinical assessment does not directly measure fluid levels in the body to identify if a person is over- or underhydrated, but rather relies on the presence of symptoms and signs of overhydration and underhydration. This approach could, therefore, miss individuals who are asymptomatic despite having an excess or deficit of body water. For example, symptoms such as oedema may not appear until individuals are substantially overhydrated and people with fluid overload do not always exhibit high blood pressure.
Additionally, some clinical features are only surrogate markers for fluid overload and can, therefore, be the result of other unrelated causes. This could lead to fluid levels being inappropriately adjusted. For example, a response to high blood pressure assumed to be caused by fluid overload (but actually caused by other factors) may involve the removal of increasing volumes of fluid during dialysis, which, in turn, may lead to underhydration with potential loss of residual renal function.
Care pathways
Figure 1 illustrates the management of stage 5 CKD currently recommended by the National Institute for Health and Care Excellence (NICE). 68
FIGURE 1.
Management of stage 5 CKD. Reproduced with permission from NICE. Management of Stage 5 Chronic Kidney Disease: NICE Pathway. London: NICE; 2017. 68

Chapter 2 Assessment of clinical effectiveness
Methods for systematic review of effectiveness
An objective synthesis of the evidence of the clinical effectiveness of multiple-frequency bioimpedance devices in comparison with standard clinical assessment for fluid management in people with CKD having dialysis was conducted. The evidence synthesis was conducted in accordance with the general principles of the Centre for Reviews and Dissemination guidance for conducting reviews in health care,69 the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.070 and the NICE Diagnostics Assessment Programme Manual. 71 The methods for this assessment were prespecified in a research protocol (www.crd.york.ac.uk/prospero/display_record.php?RecordID=41785; last accessed 12 December 2017).
Identification of studies
Comprehensive electronic searches were conducted to identify relevant reports of published studies. Highly sensitive search strategies were designed, including appropriate subject headings and text-word terms, to retrieve studies that assessed the selected bioimpedance devices for CKD patients receiving dialysis. Three facets were combined using the Boolean operator AND: CKD, RRT and devices. There were no date or language restrictions. MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Science Citation Index and Cochrane Central Register of Controlled Trials (CENTRAL) were searched for primary studies, while the Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE) and the Health Technology Assessment database were searched for reports of evidence syntheses. The searches were undertaken during the period of 27 June to 4 July 2016. The MEDLINE and EMBASE searches were rerun on 10 October 2016 to identify any recent reports. An additional search in MEDLINE and EMBASE was undertaken on 27 September 2016 to identify any published reports on validation of the devices that had not been identified by the main clinical effectiveness searches.
Reference lists of all included studies were perused in order to identify additional potentially relevant reports. The expert panel provided details of any additional potentially relevant citations.
Searches for recent conference abstracts (2014–16) were also undertaken and included the following annual conferences: European Renal Association – European Dialysis and Transplant Association (ERA-EDTA), Kidney Week (American Society of Nephrology) and the Annual Dialysis Conference.
Ongoing studies were identified through searching ClinicalTrials.gov, the European Union Clinical Trials Register and the World Health Organization’s International Clinical Trials Registry. Websites of professional organisations and health technology agencies were checked to identify additional reports. Full details of the search strategies used are presented in Appendix 1.
Inclusion and exclusion criteria
Studies fulfilling the following criteria were eligible for inclusion in this assessment.
Population
People with CKD treated with HD or PD.
Interventions
The multiple-frequency bioimpedance devices considered in this assessment were:
-
BCM
-
MultiScan 5000
-
BioScan 920-II and BioScan touch i8
-
InBody S10.
Comparator
The comparator considered in this assessment was standard clinical assessment, which takes account of the following parameters:
-
blood pressure
-
presence of oedema
-
changes in weight
-
residual renal function
-
pre-existing CV conditions
-
any patient-reported symptoms of overhydration or underhydration, for example cramps, fatigue, nausea, dizziness, breathlessness, decreased appetite or visual disturbances.
Outcomes
The following outcome measures were considered:
-
intermediate measures, including –
-
number and length of HD sessions
-
number of unplanned hospital visits/admissions as a result of fluid overload or dehydration
-
use of antihypertensive medication
-
incidence of anaemia
-
blood pressure
-
left ventricular hypertrophy
-
left ventricular mass index (LVMI)
-
arterial stiffness
-
incidence of overhydration or underhydration
-
changes of dialysis modality (from PD to HD) because of fluid overload
-
adherence with recommended fluid intake.
-
-
clinical outcomes, including –
-
incidence of CV events (including stroke and heart attack)
-
mortality
-
residual renal function
-
incidence of oedema
-
incidence of peritonitis
-
adverse effects associated with hypotensive episodes (including cramps, fatigue, diarrhoea, nausea, dizziness and fainting).
-
-
patient-reported outcomes, including –
-
post-dialysis recovery time and fatigue
-
health-related quality of life.
-
One further relevant outcome not specified in the scope or protocol was also considered because of its clinical importance: achievement of target weight.
Study design
Priority was given to RCTs assessing multiple-frequency bioimpedance devices versus standard clinical assessment and RCTs comparing the effectiveness of one device with that of another. To supplement the evidence provided by RCTs, we also included non-randomised evidence, solely consisting of observational/cohort studies. As there was a large body of non-randomised evidence, which was not manageable in the time frame of this assessment, we decided to focus exclusively on non-randomised studies with a sample size of at least 100 participants, which assessed the hydration status of people with CKD receiving dialysis.
Of the non-randomised studies, which were excluded based on these last criteria, three studies (published in four papers) with < 100 participants focused on paediatric populations. 24,72–74 Appendix 2 presents the characteristics of these studies. In the list of non-randomised studies that were not deemed suitable for inclusion based on the above criteria, no UK-based studies, studies that included any of the specified devices (other than the BCM) or studies reporting relevant outcomes not otherwise described in the report were identified.
The following types of studies were also excluded from this assessment:
-
narrative reviews, editorials and opinions
-
case reports
-
conference abstracts for which a full publication or further methodological information could not be found
-
non-English-language reports for which a translation could not be organised
-
studies reporting cross-sectional data only.
Data extraction strategy
One reviewer (MC) screened the titles and abstracts identified by the search strategies. A second reviewer (MB) independently screened a random sample of 10% of the titles and abstracts. Owing to time constraints, this strategy differed from that detailed in the protocol, which stated that two reviewers would independently screen all titles and abstracts.
A data extraction form was designed and piloted specifically for this assessment (see Appendix 3). One reviewer (MC or MS) extracted information on characteristics of studies and participants, details of interventions and comparators (when applicable) and relevant outcome measures. All extracted data were cross-checked by a second reviewer (DC, MC, MB or MS). Any disagreements were resolved by discussion between reviewers.
Assessment of risk of bias in included studies
The standard Cochrane risk-of-bias tool was used to assess the risk of bias in randomised trials (see Appendix 4). 70 One reviewer (MC) rated the risk of bias in each included RCT and the results of these assessments were cross-checked by a second reviewer (DC or MS). There were no disagreements between reviewers. Studies were not included or excluded based on the risk of bias rating. The Cochrane risk-of-bias tool incorporates the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting. Assessment of other sources of bias was based mainly upon the source of funding for the conduct of the study and potential links with the manufacturers of the devices under investigation. Individual risk-of-bias domains were rated as being at a high, low or unclear risk of bias.
Overall classification of studies was based on the assessment of three key domains: sequence generation, allocation concealment and blinding of outcome assessor. Studies were rated as being at a high risk of bias if one or more key domains were rated as being at a high risk of bias; an unclear risk of bias if one or more key domains were rated as being at an unclear risk of bias; or a low risk of bias if all key domains were rated as being at a low risk of bias.
Risk of bias of cohort studies was assessed using a modified version of a 17-item checklist previously developed by our research team (see Appendix 5). The checklist was originally adapted from several sources and developed through a partnership with the Review Body for Interventional Procedures (ReBIP) for NICE. The case series tool assessed the following domains: bias and generalisability, sample definition and selection, description of the intervention, outcome assessment, adequacy of follow-up and performance of statistical analyses. Individual items were rated as ‘yes’, ‘no’ or ‘unclear’. A rating of ‘yes’ indicated the study as being at a low risk of bias. When available, NCT records (published on clinicaltrials.gov) were checked for stated outcomes. We had originally intended to use the ROBINS-I (Risk Of Bias in Non-randomised studies of Interventions) tool75 to assess the risk of bias in the included non-randomised studies. However, as a result of time constraints, and the fact that many studies were non-comparative cohort studies, we opted for the use of the ReBIP tool.
Data analysis
The general approach recommended by the Cochrane Collaboration was used for data analysis and synthesis. 70 When possible, for binary outcomes, the DerSimonian and Laird method was used to pool hazard ratios (HRs) derived from each study, with the estimate of heterogeneity taken from the Mantel–Haenszel model. A random-effects model was used to calculate the pooled estimates of effect. For continuous outcomes, mean differences between groups were pooled.
The statistical analyses focused on the five separate outcome measures for which consistent data were reported by at least two studies and were suitable for combining across studies: mortality, SBP, arterial stiffness, absolute overhydration and relative overhydration (ROH). Other relevant outcomes that were reported, but not meta-analysed because they were inconsistently reported across studies, were achievement of target (dry) weight (reported as proportion of patients within 1.1 kg of bioimpedance-recommended dry weight, dry weight according to the BCM and proportion of patients reaching between 1 litre above and 2 litres below post-dialysis fluid overload specified by the BCM, respectively),60,61,76 hospitalisation (number of patients hospitalised at least once, all-cause hospitalisation events or hospitalisation events caused by new CV events, respectively),61,76,77 left ventricular hypertrophy,77 LVMI,77 incidence of CV events,76 adverse effects associated with hypotensive episodes (reported as hypotension/cramp events/patient/year, frequency of hypotensive events, hypotension as an intradialysis complication or frequency of intradialytic hypotensive events/1000 dialysis sessions, respectively)60,61,76,77 and fatigue. 76
Of the five outcome measures that were meta-analysed, mortality was reported in three trials. 60,61,76 Two trials60,76 reported the HR at 12 months and, for the trial by Ponce et al. ,61 this was computed by obtaining the probability of death in both the treatment group and the control group, and using the formula r = –ln(1 – p) to estimate the hazard rate in the two groups. The HR was then calculated from the estimated hazard rates. The standard error (SE) was estimated using the method described by Parmar et al. 78
The remaining four outcomes were all continuous measures, so mean differences between the treatment and control groups were pooled from the included trials and a 95% confidence interval (CI) was calculated to test whether or not the pooled summary effect showed a significant difference between treatment and control.
Heterogeneity across trials was explored by visual inspection of forest plots and assessed by means of the chi-squared test and I2-statistic.
There are five trials60,61,63,76,77 in the meta-analyses. Four of these trials60,63,76,77 randomised at the individual level, while Ponce et al. 61 randomised centres rather than individual patients. In order to include the trial by Ponce et al. 61 in our meta-analyses, the method described by Fawzi et al. 79 was used to inflate the SE.
In order to include a cluster randomised trial in a meta-analysis it is necessary to allow for the correlation of participants within clusters. This would be done by inflating the variance of the cluster randomised trial by the ‘design factor’. The design factor is 1 + (m – 1)ρ, in which m is the number of clusters and ρ is the intracluster correlation coefficient.
Many trials fail to report estimates of the design effect and, therefore, different strategies are used to obtain this required information.
The meta-analysis by Fawzi et al. 79 examined the relationship of vitamin A supplementation and child mortality. In Fawzi et al. ,79 there were four cluster randomised trials that did not adjust for clustering. The authors decided to increase the variance of the unadjusted trials by 30%. The Fawzi et al. 79 adjustment referred to in this report is therefore to increase the variances of the estimated intervention effects by an arbitrary amount of 30% as previously used.
A subgroup analysis was performed according to the type of dialysis: HD versus PD. Only the Luo et al. trial63 assessed PD, whereas the remaining four trials assessed HD. We were able to conduct subgroup analyses only for the following outcome measures: SBP and absolute hydration.
Results
Performance of multiple-frequency bioimpedance devices
A formal evaluation of the accuracy and validation of the multiple-frequency bioimpedance devices under assessment was beyond the scope of this assessment. However, information on the validation and accuracy of the specified devices was gathered from the available literature. Only information on the validation of the BCM was found in the current literature.
Wabel et al. 59 reviewed a number of studies on HD patients comparing the BCM against standard clinical methods for measuring extracellular and total body water, as well as ICW volume. The authors concluded that there was good agreement between the BCM and the standard clinical measurements of fluid overload.
Chen et al. 80 assessed the relationship between the dry weight determined by clinical evaluation and the ‘normally hydrated’ weight estimated by the BCM from serial follow-up data. The authors used serial measurements of six fluid parameters in the same HD patients to demonstrate that intraperson precision of the device was at an acceptable level of reliability for clinical use.
No studies have validated the BCM in people receiving PD. The BCM manufacturer maintains that the method used is valid across both forms of dialysis. 4
Quantity of evidence available
Records retrieved by the database searches totalled 4106. In addition, 18 conference abstracts were obtained by searching the selected recent conference abstracts, giving a total of 4124 records. After de-duplication, 2592 abstracts were screened for relevance. Of these, 129 were selected for full-text assessment, from which 15 met our inclusion criteria (Figure 2). All 15 studies involved use of the BCM and none enrolled paediatric populations. A list of all excluded studies is presented in Appendix 6 together with the main reasons for exclusion.
FIGURE 2.
Flow diagram outlining the study selection process.

Characteristics of the included studies
A total of five RCTs (published in six papers60,61,63,76,77,81) and eight non-randomised studies (published in nine papers30,50,82–88) were included in the review of clinical effectiveness. There was some question over whether or not the RCTs by Onofriescu et al. 81 and Onofriescu et al. 60 may be reporting the same trial or outcomes from an overlapping patient population. The principal investigators of each trial were contacted, but no replies were forthcoming. The decision was taken to include Onofriescu et al. 60 as the primary study and Onofriescu et al. 81 as a secondary publication. The Onofriescu et al. 60 study reports more relevant outcomes and is more recent. Similarly, there is a possibility that the non-randomised studies by O’Lone et al. 82 and Oei et al. 83 may involve an overlapping patient population, as both studies recruited at the same centre in the same time period. The corresponding authors of both studies were contacted for further clarification, but no replies were received. It is therefore unclear whether or not the studies are completely separate. Both studies have been included in the review, but only the results of the O’Lone et al. 82 study have been used for our cost-effectiveness analyses. Characteristics of the included studies are detailed in Appendix 7.
Randomised controlled trials
All five included RCTs were available in full-text format. 60,61,63,76,77 The BCM was the multiple-frequency device used in all five trials. One trial was conducted in Romania,60 one trial in Taiwan,76 one in Turkey77 and one in Portugal,61 and the remaining trial did not provide this information. 63 One trial recruited patients from 23 dialysis centres,61 one trial recruited from two dialysis centres,77 another trial from six dialysis centres76 and one trial recruited from a single dialysis centre. 60 In the remaining trial, it was unclear whether patients were recruited from a single dialysis centre or from multiple dialysis centres. 63 Four trials enrolled solely patients who were treated with HD,60,61,76,77 and one trial enrolled continuous ambulatory PD patients. 63 All five trials involved dialysis in a hospital setting. The multiple-frequency bioimpedance device used for assessment of fluid status by all five trials was the BCM. All five trials included only adults aged ≥ 18 years. 60,61,63,76,77 The main exclusion criteria reported in the trials, which assessed patients receiving HD, were coronary stents or pacemakers;76,77 metallic devices in the body, such as joint prostheses;60,61,76,77 limb amputations;60,76,77 and pregnancy. 60,61 One trial, which assessed PD patients,63 excluded those who had been on one or two exchanges per day because of economic limitation and those patients with acute infection and CV events in the month prior to enrolment.
The length of follow-up of the included trials ranged from 3 months61 to 2.5 years,60 with two trials reporting a follow-up period of 12 months. 61,77 In the case of the trial by Luo et al. ,63 the authors decided to terminate follow-up at 3 months rather than at 6 months, as originally planned, as the emerging differences between the groups and the adverse effect of fluid overload led the decision to extend the follow-up period to be considered unethical.
Three of the five trials had links to Fresenius Medical Care, the company that manufactures the BCM,60,61,77 albeit two of these trials reported that Fresenius had no involvement in the design or conduct of the trial. 60,77 Two trials were supported by grants from independent sources. 63,76
Non-randomised studies
The eight non-randomised studies were reported in nine full-text papers,30,50,82–88 with the study by O’Lone et al. 82 also reported in a secondary study with an additional 51 participants and a 21-month longer follow-up period. 84 The BCM was the multiple-frequency device used in all eight studies. None of these studies enrolled paediatric populations. Two studies were conducted in the UK,82,83 two in Seoul, South Korea,50,85 and one each in Spain,86 Poland,87 Romania88 and Europe. 30 Three studies were multicentred30,85,86 and the remaining five studies were conducted in single dialysis centres. 50,82,83,87,88 Six studies involved patients receiving HD30,50,85–88 and the remaining two studies involved solely patients treated with PD. 82,83
The length of follow-up in the eight non-randomised studies ranged from 16 weeks85 to 3.5 years. 30 Four studies reported median follow-up periods of 24 months,50 23.9 months,83 27 months82 and 66.2 months. 88 O’Lone et al. 82 further specified that patients were enrolled between January 2008 and March 2012 and followed up until September 2012, with follow-up continuing until June 2014. Three studies had no apparent links with Fresenius Medical Care50,83,87 and the other five studies reported either funding from Fresenius Medical Care85 or some form of connection with the company. 30,82,86,88
Two studies involved blood pressure being taken after 10 minutes’ rest or recumbence60,76 and one study after 5 minutes’ rest. 63 The study by Hur et al. 77 involved hourly ambulatory blood pressure measurement over a 48-hour period from the start of one dialysis session until the following session. The technique used to measure blood pressure in the remaining study is unclear. 61
Characteristics of participants
Table 1 summarises the baseline characteristics of the randomised and non-randomised studies included in this assessment.
| Characteristic | Included studies (N = 13) | |
|---|---|---|
| RCTs (N = 5) | NRS (N = 8) | |
| Enrolled | 1032 (n = 5) | 993 (n = 3) |
| Randomised | 939 (n = 5) | N/A |
| Analysed | 904 (n = 5) | 4915 (n = 8)a |
| Age (years): median (range) of means | 60 (51.7–66.3) (n = 5) | 61.9 (53.8–68.2) (n = 7) |
| Sex (male): median (range) % | 52.7 (46.3–76.2) (n = 5) | 62 (52.5–64.7) (n = 7) |
| Diabetes mellitus: median (range) % of participants | 27.5 (9.5–39.2) (n = 5) | 29.9 (10.4–37) (n = 6) |
| Dialysis vintage (months): median (range) of means | 61.9 (34.2–105.5) (n = 3) | 44.7 (10.7–66)b (n = 4) |
| Dialysis modality | ||
| HD | 867 (n = 4) | 4050 (n = 6) |
| Of which was haemodiafiltration | 218 (n = 1) | 1305 (n = 1) |
| PD | 165 (n = 1) | 865 (n = 2) |
Randomised controlled trials
The five RCTs60,61,63,76,77 randomised a total of 939 participants: 469 to bioimpedance measurements and 470 to standard clinical assessment.
The mean age for each intervention group was reported in all five RCTs60,61,63,76,77 and ranged from 50.9 years77 to 65.8 years61 in the bioimpedance intervention group and from 52.5 years77 to 66.7 years61 in the standard clinical assessment group.
The five RCTs each reported the proportion of males and females for each intervention group. 60,61,63,76,77 Study populations tended to involve approximately equal proportions of men and women, with the exception of the studies by Hur et al. 77 (69% men) and Ponce et al. 61 (76.2% men). The proportion of men ranged from 43.6%63 to 71.3%61 in the bioimpedance intervention group, and from 48.8%63 to 81.8%61 in the standard clinical assessment group. The prevalence of diabetes mellitus among participants varied across trials. The proportion of participants with diabetes mellitus was reported by all five trials60,61,63,76,77 and ranged from 10%60 to 39.8%61 in the bioimpedance intervention group, and from 9%60 to 38.6%61 in the standard clinical assessment group. The mean dialysis vintage was reported in three RCTs60,63,77 and ranged from 35.2 months63 to 107 months57 in the bioimpedance assessment group, and from 33.2 months to 104 months60 in the control group.
Non-randomised studies
The eight included non-randomised cohort studies assessed a total of 4915 participants. 30,50,82,83,85–88 The studies were of two main types: in some studies, the BCM was used to classify patients into groups (e.g. overhydrated/non-overhydrated) and then outcomes were compared across the groups;30,88 and in other studies, the BCM was used as a basis for adjustment of dry weight50,82,85,86 or to obtain hydration parameters. 83,87 Six cohort studies reported the mean age of participants, which ranged from 53.8 to 68.2 years. 30,50,85–88 The two remaining cohort studies reported the median ages of participants of 57.9 years83 and 57 years. 82 Three studies reported the mean age for normohydrated and overhydrated groups. 30,50,85 The age range was 55.9 years85 to 66 years30 for the normohydrated groups and 58.4 years85 to 65.6 years50 for the overhydrated groups, respectively. The proportion of men in the seven studies reporting this information50,82,83,85–88 ranged from 52.5%88 to 64.7%87 and was, in general, higher than reported in the included RCTs. The proportion of participants with diabetes mellitus was reported by six of the observational studies30,82,83,86–88 and ranged from 10.4%88 to 37%. 83 The mean dialysis vintage was reported by half of the studies and ranged from 10.7 months87 to 66 months. 85 In the study by Hoppe et al. ,87 participants were split into those with a short-dialysis vintage (≤ 24 months) or a long-dialysis vintage (> 24 months), with mean dialysis vintage being 9.3 weeks and 76.2 weeks, respectively. The trial by Kim et al. 85 reported mean dialysis vintage separately for dehydrated, normohydrated and hyperhydrated participants, which was 6.0, 4.1 and 5.7 years, respectively.
Frequency of Body Composition Monitor measurements
Randomised controlled trials
The frequency of measurements using the BCM in the RCTs was at least every 3 months. The most frequent use of the device was twice monthly in the bioimpedance intervention group (and every 3 months in the control group). 77 Three-month assessments were reported by Onofriescu et al. ;60 monthly assessments by Huan-Sheng et al. 76 and Ponce et al. 61 and 6-week assessments by Luo et al. 63
Non-randomised studies
The frequency of BCM assessments in the non-randomised studies varied across studies: one study involved only one assessment within the first week of dialysis;50 two studies involved three assessments per week;30,88 another study involved weekly assessments;87 two other studies involved monthly assessments;85,86 one study involved quarterly assessments;82 and the remaining study did not report the frequency of the BCM use. 83
Standard clinical assessment: randomised controlled trials
In general, the type of standard clinical assessment in the included RCTs was not consistently reported across the included trials. Only one trial provided details of its control intervention; Onofriescu et al. 60 reported that:
The target dry weight was set according to clinical criteria by the attending physicians from the dialysis unit; i.e. target BP [blood pressure] equal to or less than 140/90 mm Hg, absence of oedema, and absence of intra-dialytic or inter-dialytic hypotension or other symptoms.
Onofriescu et al. 60
In the other four trials,61,63,76,77 details of the assessment in the control group were not reported. Bioimpedance analysis was carried out on both intervention and control groups of all studies at the frequencies reported in Frequency of Body Composition Monitor measurements (with the difference between the groups being that treated physicians in the control groups were blinded to the results). It was not explicitly stated by any of the studies whether or not standard clinical assessment was also carried out at these visits, and no further information on the frequency of standard clinical assessments was reported.
Risk of bias
Randomised controlled trials
Figure 3 presents the summary of risk-of-bias assessments for all included trials. Risk-of-bias assessments of individual studies are presented in Figure 4.
FIGURE 3.
Summary of risk-of-bias assessments for all included trials.
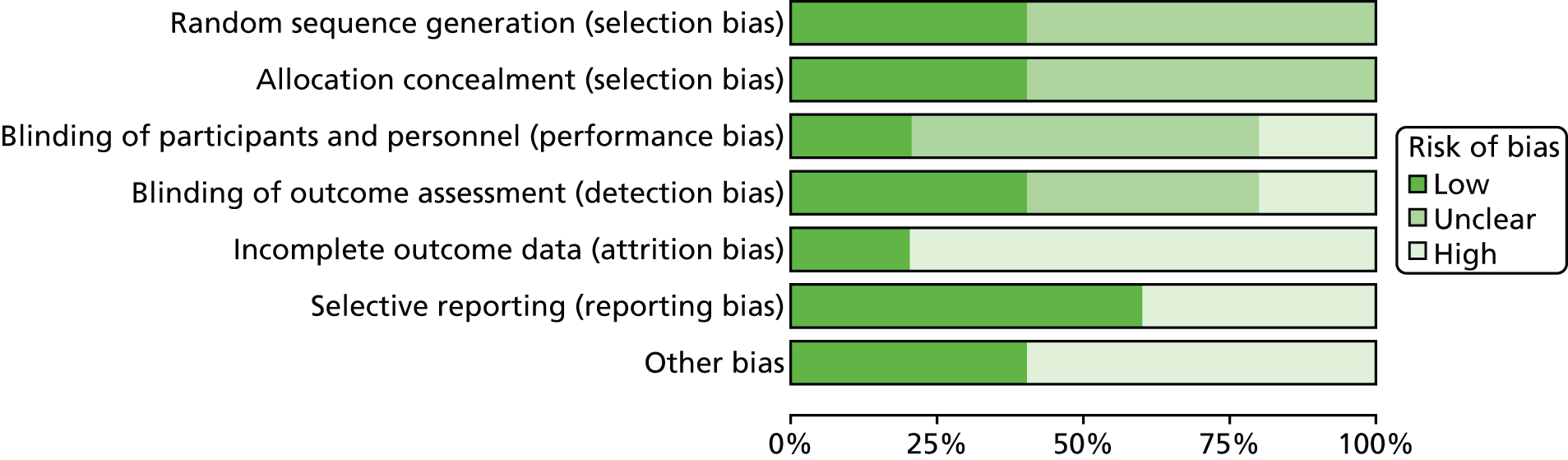
FIGURE 4.
Risk-of-bias assessments of individual studies.

According to the prespecified criteria for the assessment of the overall risk of bias, one of the five RCTs was rated as being at a high risk of bias,63 and the remaining four trials did not provide sufficient information on which to make a robust judgement. 60,61,76,77
Selection bias
Two trials reported sufficient information on which to make a full assessment of selection bias. Full details of allocation concealment were not reported, but the method of generation of sequence (i.e. random generation by computer) implies that the study personnel would be unable to predict the allocation, thus fulfilling the criterion of low risk. The trial by Ponce et al. 61 involved randomisation of centres, as opposed to randomisation of individuals within centres. No details of the randomisation process were reported. The remaining two trials merely stated that they were randomised trials, but provided no details of how randomisation was achieved. 60,63,77
Performance and detection bias
Only one trial reported that participants were blinded. 60 One trial reported that participants were not blinded. 63 In the remaining three trials, both the intervention group and the control group received BCM assessments but the measurements were used to assess the intervention group only. 61,76,77
Two trials reported that outcome assessors were blinded. 61,77 Luo et al. 63 reported that patients, investigators and dialysis staff were not blinded to treatment assignment; the trial was, therefore, rated as being at a high risk of bias for the blinding of outcome assessors domain and for overall risk. In the trials by Huan-Sheng et al. 76 and Onofriescu et al. ,60 it was unclear whether or not outcome assessors had been blinded.
Attrition bias
One trial reported a low number of dropouts63 and was, therefore, rated as being at a low risk of attrition bias. The remaining four studies were rated as being at a high risk of bias because of the high number of participants who dropped out. 60,61,76,77 It is worth noting that, in the Ponce et al. 61 trial, 29 out of 101 (28.7%) and 42 out of 88 (47.7%) discontinuations were observed in the intervention and control groups, respectively. The reasons given for terminating the trial prematurely were as follows: ‘no valid data available within the time frame, death, transplant or transfer to another clinic’. The proportion of participants within each of these categories and distribution of dropouts across centres were, however, not given.
Reporting bias
In four of the five included trials, the outcomes reported were in accordance with those specified in the respective methods section. 60,61,63,76 The trial by Hur et al. 77 was rated as being at a high risk of reporting bias, as some outcome measures that had not been previously specified were reported, such as iron dose, right ventricle end-diastolic diameter, urine output, triglyceride levels and cholesterol.
Other bias
Three RCTs reported links with Fresenius Medical Care (either in the form of funding, as an honorary speaker or through employment) and were rated as being at a high risk of ‘other bias’. 60,61,77 Two trials were supported by grants from independent sources. 63,76 No other sources of bias were apparent in the included trials.
Non-randomised studies
Figure 5 presents a summary of the risk-of-bias assessments for the included non-randomised cohort studies. The results of individual study-level assessments are presented in Appendix 8.
FIGURE 5.
Summary risk of bias for non-randomised cohort studies.

The majority of studies identified important prognostic factors, provided information on non-respondents/dropouts, included a sufficient length of follow-up, used objective outcome measures, considered important outcomes, delivered the intervention in an appropriate setting and by an experienced person, clearly defined the intervention, collected data prospectively, clearly defined the inclusion/exclusion criteria and involved a representative sample. None of the studies involved blinding of participants or study personnel. Two studies enrolled participants who entered the study at varying points in their disease progression. The study by Hoppe et al. 87 compared short- versus long-dialysis vintage and the study by O’Lone et al. 82 compared incident and prevalent patients. The majority of studies failed to provide information on the characteristics of participants who withdrew or did not complete follow-up. 30,50,82,83,85,88
Clinical effectiveness results
Data on the following relevant outcomes were not reported by any of the included studies: number and length of HD sessions, number of unplanned hospital visits/admissions as a result of fluid overload or dehydration, incidence of anaemia, incidence of overhydration or underhydration (although absolute overhydration and ROH were reported), changes of dialysis modality as a result of fluid overload, adherence with recommended fluid intake, incidence of oedema, incidence of peritonitis and health-related quality of life.
Evidence from randomised controlled trials: meta-analyses results
Meta-analyses of relevant clinical outcomes were performed, when appropriate, using random-effects models. As the trial by Ponce et al. 61 is a cluster randomised trial, the variance was inflated by the method used in Fawzi et al. 79 to allow it be included in the meta-analysis. The uninflated summary data for the Ponce et al. 61 trial are presented in Table 2.
| Outcome | Trial arm | |||
|---|---|---|---|---|
| Treatment | Control | |||
| Mean (SD) | n | Mean (SD) | n | |
| SBP (mmHg) | 134.6 (27.3) | 101 | 136.5 (24.7) | 88 |
| Absolute hydration (L) | 2.92 (1.47) | 101 | 3.36 (1.75) | 88 |
| Relative hydration (%) | 15.4 (6.36) | 101 | 16.26 (8.48) | 88 |
Full details of the relevant outcome measures extracted from the included RCTs are presented in Appendix 9.
Blood pressure
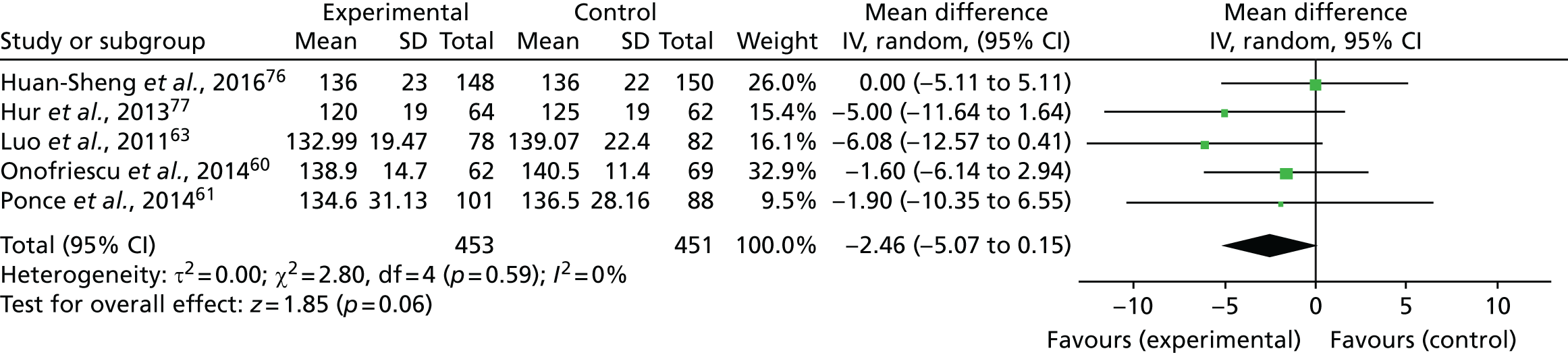
Five trials (one rated as being at a high risk of bias and four as being at an unclear risk of bias) reported SBP measurements, which were included in a meta-analysis. 60,61,63,76,77 Figure 6 shows that SBP was lower in participants who underwent bioimpedance measurements using the BCM device than in those assessed by standard clinical assessment, but the difference was not statistically significant (mean difference –2.46 mmHg, 95% CI –5.07 to 0.15 mmHg; p = 0.06, I2 = 0%).
FIGURE 6.
Meta-analysis for SBP. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
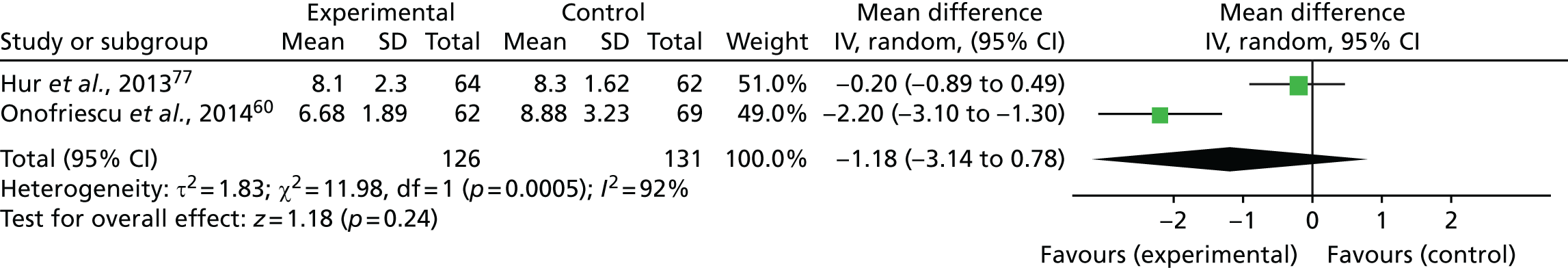
Arterial stiffness
Two trials (both rated as being at an unclear risk of bias) reported arterial stiffness results, which were included in a meta-analysis. 60,77 The measurement of pulse wave velocity (PWV) is generally accepted as the most simple, non-invasive, robust and reproducible method of determining arterial stiffness, with carotid–femoral PWV regarded as the gold standard. The PWV increases from 4–5 m/second in the ascending aorta to 5–6 m/s in the abdominal aorta and 8–9 m/s in the iliac and femoral arteries. 89 Normal values, using standardised calculation of PWV, are a mean of 7.2 m/s in people aged 40–49 years and 8.3 m/s in people aged 50–59 years. 90 Figure 7 shows that arterial stiffness (as assessed by carotid–femoral PWV) was lower, but not statistically significantly lower, in the bioimpedance assessment group than that in the standard clinical assessment group (mean difference –1.18 m/s, 95% CI –3.14 to 0.78 m/s; p = 0.24, I2 = 92%). Substantial statistical heterogeneity between trials was observed.
FIGURE 7.
Meta-analysis for arterial stiffness. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
Mortality
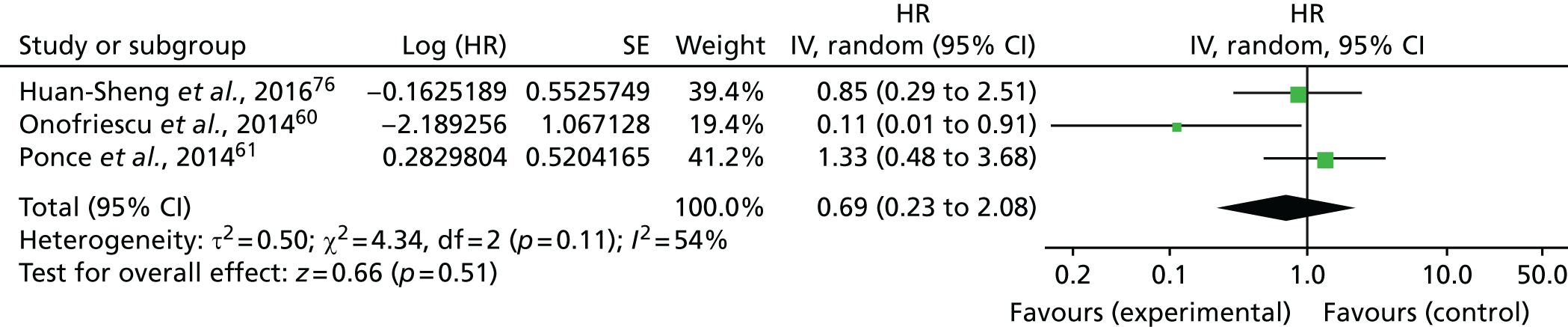
Three of the included trials (all rated as being at an unclear risk of bias) reported mortality data. 60,61,76 As mortality was reported with a HR, the log-HR and log-SE for the three trials were input manually (Table 3).
| First author of study and year of publication | Trial arm | Weight (%) | HR (95% CI) | |||
|---|---|---|---|---|---|---|
| Treatment | Control | |||||
| Number of events | Total number of events | Number of events | Total number of events | |||
| Huan-Sheng et al., 201676 | 6 | 148 | 7 | 150 | 39.4 | 0.850 (0.288 to 2.511) |
| Onofriescu et al., 201460 | 1 | 62 | 8 | 69 | 19.4 | 0.112 (0.014 to 0.907) |
| Ponce et al., 201461 | 12 | 101 | 8 | 88 | 41.2 | 1.327 (0.479 to 3.680) |
| Overall | 19 | 311 | 23 | 307 | 100.0 | 0.689 (0.228 to 2.084) |
A total of 19 out of 311 (6.1%) participants in the bioimpedance assessment group and 23 out of 307 (7.5%) participants in the standard clinical assessment group died. Figure 8 shows that, compared with standard clinical assessment, the use of the BCM had no significant effects on mortality (HR 0.69, 95% CI 0.23 to 2.08; p = 0.51, I2 = 54%). Moderate statistical heterogeneity was evident among trials.
FIGURE 8.
Meta-analysis for mortality. df, degrees of freedom; IV, inverse variance.
Absolute overhydration
Four trials (one rated as being at a high risk of bias and three rated as being at an unclear risk of bias) assessed absolute overhydration,61,63,76,77 which was defined as the difference between expected ECW and actual ECW. No data on underhydration were available. Figure 9 shows that absolute overhydration was significantly lower in the BCM assessment group than in the standard clinical assessment group [weighted mean difference (WMD) –0.44, 95% CI –0.72 to –0.15; p = 0.003, I2 = 49%]. Moderate statistical heterogeneity between trials was apparent.
FIGURE 9.
Meta-analysis for absolute overhydration. df, degrees of freedom; IV, inverse variance; SD, standard deviation.

Relative overhydration
Three trials (all rated as being at an unclear risk of bias) reported data on ROH,60,61,76 which was defined as the ratio of absolute fluid overload to ECW volume. Figure 10 shows that ROH was significantly lower in the BCM assessment group than in the standard clinical assessment group (WMD –1.84, 95% CI –3.65 to –0.03; p = 0.05, I2 = 52%). ROH was assessed by the BCM in both groups, therefore these results should be interpreted with caution.
FIGURE 10.
Meta-analysis of ROH. df, degrees of freedom; IV, inverse variance; SD, standard deviation.

Randomised controlled trial evidence: subgroup and sensitivity analyses
We had initially planned to perform subgroup analyses according to the type of dialysis (HD or PD), the type of population (children aged < 5 years) and ethnicity group, and according to certain characteristics of the patient population, that is, people for whom recommended configurations of electrodes could not be used, people who could not assume the required positions for measurements to be made or people at extremes of body composition measurements. However, because of a lack of available data, we were able to perform only subgroup analyses of SBP and absolute overhydration according to the type of dialysis utilised.
Figure 11 presents the forest plot of the subgroup analysis of SBP according to the type of dialysis. As there was only one trial in the PD group, we considered that testing for subgroup effects would have been statistically unsound. We considered that the comparison of the overall effect with the HD group effect (similar to a sensitivity analysis) was a better, more reliable approach. In this case, the effect on blood pressure was still not significant (WMD –1.76, 95% CI –4.61 to 1.08; z = 1.21, p = 0.22).
FIGURE 11.
Subgroup analysis for SBP according to the type of dialysis. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
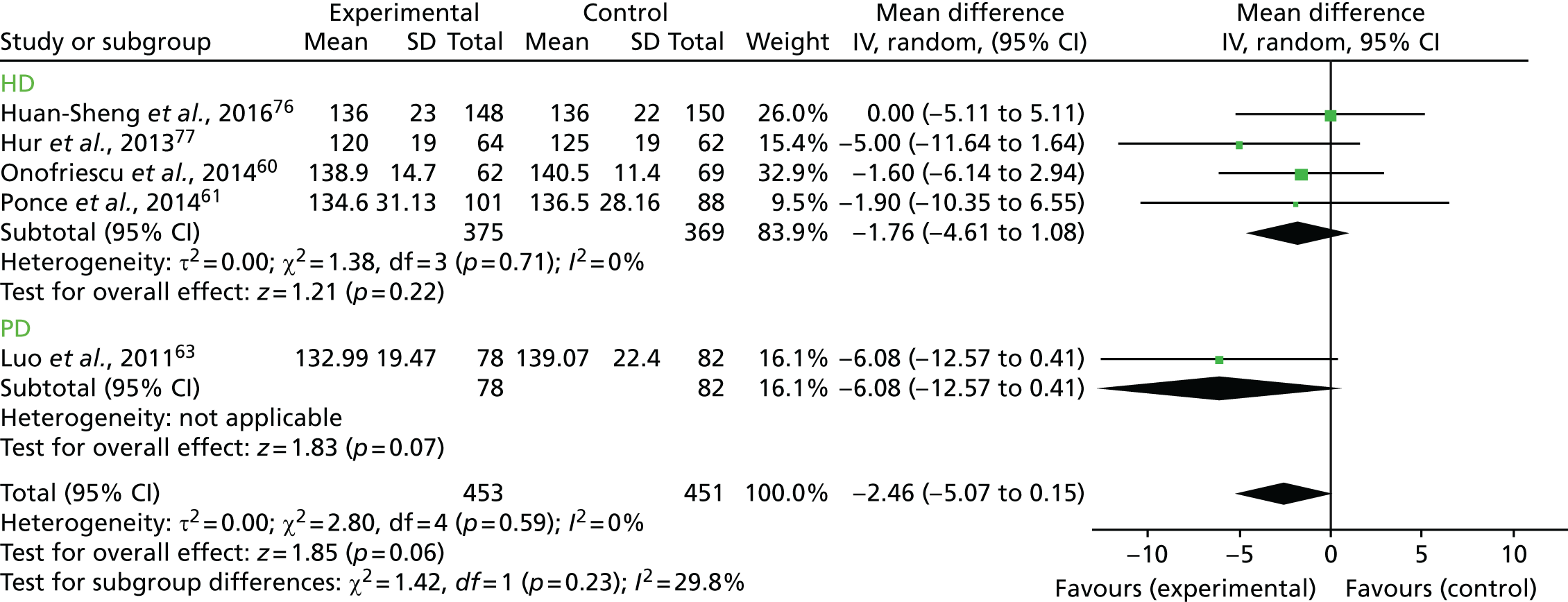
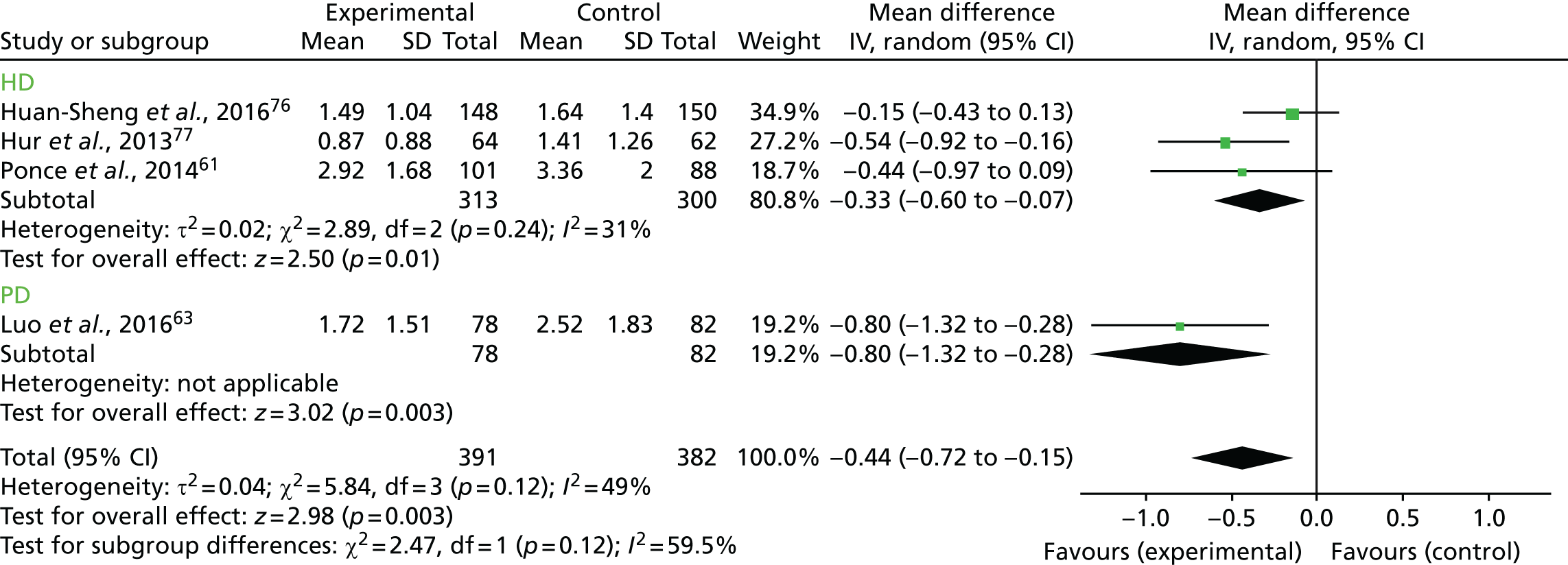
Figure 12 presents the subgroup analysis for absolute hydration according to the type of dialysis. As described above, we did not perform a test of subgroup effects. In the case of absolute overhydration, there is a difference between the overall effect compared with the HD subgroup effect (WMD –0.33, 95% CI –0.60 to –0.07; z = 2.50, p = 0.01), but this is not large enough to suggest a significant dialysis effect.
FIGURE 12.
Subgroup analysis for absolute overhydration according to the type of dialysis. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
We were unable to perform the planned sensitivity analyses (i.e. based on studies rated as being at a low risk of bias only or according to the type of multiple-frequency bioimpedance device), as only one trial was rated as being at a low risk of bias and only one device (the BCM) was used in all included trials.
Randomised controlled trial evidence: other outcomes
Intermediate reported outcomes
Hospitalisation
Three trials reported data on hospitalisation. 61,76,77 Huan-Sheng et al. 76 (at unclear risk of bias) reported 71 events of all-cause hospitalisation, with an incidence of 0.52 (95% CI 0.44 to 0.61) per patient-year, in the bioimpedance assessment group. In the standard clinical assessment group, there were 73 all-cause hospitalisation events, with an incidence of 0.54 (95% CI 0.46 to 0.63). The HR was 1.19 (95% CI 0.79 to 1.80). Hur et al. 77 (at unclear risk of bias) reported six participants in the bioimpedance assessment group hospitalised because of new CV events during the study period, with a hospitalisation rate/100 patient-years of 12.5. Four participants were hospitalised in the standard clinical assessment group, with a hospitalisation rate/100 patient-years of 30.9. The difference between the groups was not statistically significant. Ponce et al. 61 (at unclear risk of bias) reported that 39.6% of the bioimpedance assessment group and 31.8% of the standard clinical assessment group were hospitalised at least once.
Left ventricular hypertrophy
Hur et al. 77 (rated as being at an unclear risk of bias) reported presence of left ventricular hypertrophy at 12 months in 44% of the bioimpedance assessment group and 50% of the standard clinical assessment group. The difference from baseline, although not statistically significant, decreased in both groups (from 67% and 53%, respectively).
Left ventricular mass index
Hur et al. 77 2013 (rated as being at an unclear risk of bias) reported a significant reduction in LVMI in the bioimpedance assessment group from 131 [standard deviation (SD) 36] at baseline to 116 (SD 29) at 12 months (p < 0.001). In contrast, there was no change in LVMI in the standard clinical assessment group [from 121 (SD 35) at baseline to 120 (SD 30) at 12 months; p = 0.9].
Clinical outcomes
Incidence of cardiovascular events
One study reported a combination of acute fluid overload or CV-related events, which included hospitalisation related to CV or cerebrovascular events and episodes of acute fluid overload. Huan-Sheng et al. 76 (rated as being at an unclear risk of bias) reported 14 events in the bioimpedance assessment group, with an incidence rate of 0.10 (95% CI 0.06 to 0.17) per patient-year, and 28 events in the control group, with an incidence rate of 0.21 (95% CI 0.15 to 0.29) per patient-year. The overall incidence ratio was 0.50 (95% CI 0.26 to 0.94) per patient-year (p = 0.03).
Residual renal function
No trials reported residual renal function, but two studies reported urinary volume, which could be considered a surrogate measure thereof. Hur et al. 77 (rated as being at an unclear risk of bias) reported a significant increase in the proportion of anuric patients and a significant decrease in urine output in non-anuric patients at 12 months in the bioimpedance assessment group. By contrast, there was no change in the proportion of anuric patients in the control group and the decrease in urine output in non-anuric patients was not significant at follow-up. Luo et al. 63 (rated as being at a high risk of bias) reported non-significant decreases in urine volume in both the BCM group and the standard clinical assessment group at 12 weeks, although the bioimpedance assessment group showed a numerically larger decrease.
Adverse effects associated with hypotensive episodes
The top five intradialytic complications reported by Huan-Sheng et al. 76 (rated as being at an unclear risk of bias) were hypotension, cramping, skin itching, chest tightness and headache. There were significant differences between the bioimpedance assessment group and the standard clinical assessment group for all of these complications, but not in the same direction. In the bioimpedance assessment group, there was significantly more cramping, chest tightness and headaches, but significantly less hypotension and skin itching.
Frequency of intradialytic hypotensive events was reported by Hur et al. 77 (rated as being at an unclear risk of bias); there was no difference between groups at baseline (63.2 events/1000 dialysis sessions in the bioimpedance assessment group and 63.8 events/1000 dialysis sessions in the standard clinical assessment group; p = 0.9) or at 12 months (66.6 and 63.9 events/1000 dialysis sessions, respectively; p = 0.4). Similarly, Onofriescu et al. 60 (rated as being at an unclear risk of bias) reported no difference between groups in hypotension, cramps or patient-year (p = 0.6). Ponce et al. 61 (rated as being at an unclear risk of bias) defined hypotensive events as SBP reduced by at least 30 mmHg during dialysis or intradialytically below 90 mmHg, and reported no significant difference between groups at baseline (39 events in 17 patients in the bioimpedance assessment group, and 38 events in 12 patients in the standard clinical assessment group) or 12 months (48 events in 20 patients and 41 events in 15 patients, respectively).
No data were available on incidence of oedema or incidence of peritonitis.
Patient-reported outcomes
Fatigue
Only one trial reported details of any specified patient-reported outcomes. Huan-Sheng et al. 76 (rated as being at an unclear risk of bias) reported four events of intradialytic fatigue in the bioimpedance assessment group and five events in the standard clinical assessment group. The difference between groups was not statistically significant (p = 0.7).
Other relevant outcomes
Achievement of target weight
Three trials reported achievement of target weight. Huan-Sheng et al. 76 (rated as being at an unclear risk of bias) reported that post-dialysis target weight (PDTW) adjustment was performed in 816 months (out of a total of 1658 monthly assessments across the 148 participants in the intervention group over the 12-month follow-up period). PDTW was achieved in 650 of these months (80%). Of the 816 months, clinical signs and symptoms were comparable with the BCM results in 482 months (59%), of which PDTW was reached in 426 months (88%). The authors further reported that PDTW adjustments based on BCM results were not supported by firm and clear clinical evidence in up to 41% of occasions. Onofriescu et al. 60 (rated as being at an unclear risk of bias) stated that a significantly higher proportion of participants in the bioimpedance assessment group than in the control group maintained dry weight within 1.1 kg of the bioimpedance-recommended level. However, there is some uncertainty around the number of participants at each time point, and replicating the analysis was not possible. Ponce et al. 61 (rated as being at an unclear risk of bias) reported that, at 12 months, target weight was generally less overestimated in the BCM assessment group than in the standard clinical assessment group (0.67 vs. 1.00 kg).
Non-randomised evidence
Table 4 presents the relevant results reported by the eight included non-randomised cohort studies.
| Study outcomes relevant to this review | Study authors’ conclusions |
|---|---|
| Castellano et al., 201486 (Spain, cohort study, 6-month follow-up) | |
| Average ROH reduced within 6 months (n = 325) vs. average ROH not reduced within 6 months | Reduction in hyperhydration status related to better control of blood pressure and anaemia with fewer AHT drugs and ESAs |
Intermediate outcomes (n = 494), mean:
|
Maintained hyperhydrated patients, patients with diabetes mellitus with many comorbidities and young males with longer time on HD and non-adherence treatment may benefit from close monitoring of hydration state and individualised dialysis and drug treatments |
| Hoppe et al., 201587 (Poland, cohort study, 30-month follow-up) | |
| Short (n = 119) vs. long (n = 122) dialysis vintage subgroups | Longer dialysis vintage associated with CV dysfunction, overhydration and increased mortality, which may be predicted with overhydration percentage and cardiac troponin T |
Intermediate outcomes:
|
|
| Kim et al., 201285 (South Korea, interventional cohort study, 16-week follow-up) | |
| Dehydration (n = 18) vs. hyperhydration (n = 44) subgroups | BCM-guided optimisation of body fluid status may lead to improvement of inflammatory markers and anti-atherogenic adipokines as well as haemodynamic parameters in people receiving HD |
Intermediate outcomes:
|
|
| Kim et al., 201550 (South Korea, cohort study, median 24-month follow-up) | |
| Overhydrated group (n = 160) vs. non-overhydrated group (n = 80) | The ratio of overhydration to ECW volume measured with the BCM is related to the overall survival of ESRD patients who have started MHD |
Intermediate outcomes:
|
|
| Oei et al., 201683 (UK, cohort study, median 23.9-month follow-up) | |
| Death from cardiac vs. non-cardiac causes | Patients who were overhydrated had higher cTnT, and their deaths were more likely to be cardiac related. Reduction in overhydration correlated with lowering of cardiac troponin T |
Clinical outcomes:
|
|
| O’Lone et al., 201482 (UK, cohort study, median 27-month follow-up) | |
Intermediate outcomes:
|
BMI did not influence the hydration parameter of overhydration/ECW, which remained an independent predictor of mortality when BMI and lean tissue index were included in a multivariate model. However, it remains to be determined if correcting the overhydration status of a patient will lead to improvement in mortality |
| Onofriescu et al., 201588 (Romania, cohort study, median 66.2-month follow-up) | |
| RFO < 17.4% (n = 135) vs. RFO > 17.4% (n = 22) | Hydration status is associated with the mortality risk in a HD population, independently of cardiac morphology and function |
Intermediate outcomes:
|
|
| Wizemann et al., 200930 (Europe, cohort study, 3.5-year follow-up) | |
Clinical outcomes:
|
Hydration state is an important and independent predictor of mortality in chronic HD patients secondary only to the presence of diabetes mellitus. It is essential to measure hydration status objectively and quantitatively to obtain a more clearly defined assessment of HD patients’ prognosis |
Use of antihypertensive medication
Two studies reported the use of antihypertensive medication in specified patient subgroups. 85,86 Castellano et al. 86 reported significantly higher consumption of antihypertensive medications per month in the group with average ROH not reduced within 6 months than in those for whom average ROH was reduced within 6 months. 86 Kim et al. 85 reported no significant difference in the consumption of antihypertensive drugs between dehydrated and hyperhydrated patients, although the number of drugs used at week 16 was significantly lower than that at baseline or week 8 in the hyperhydrated group.
Blood pressure
Four studies reported blood pressure among specified subgroups. 85–88 There were no statistically significant differences between groups in which average overhydration within 6 months was reduced versus not reduced,86 short- versus long-dialysis groups87 or groups in which relative fluid overload was < 17.4% versus > 17.4%. 88 Kim et al. 85 compared the blood pressure of dehydrated and hyperhydrated patients, and found that SBP was higher in the hyperhydrated group, although the statistical significance of the comparison was not reported.
Left ventricular hypertrophy
One study assessed left ventricular hypertrophy87 and showed that the thickness of the left ventricle wall (in mm) was not significantly different for short- versus long-dialysis vintage subgroups.
Hospitalisation
Two studies reported data on hospitalisation. 50,88 Kim et al. 50 reported a non-significant difference between overhydrated and non-overhydrated patients in the number of hospital-days per event. Onofriescu et al. 88 reported a significantly higher all-cause hospitalisation rate for patients classified as overhydrated according to a 17.4% cut-off point than for those classified as being not overhydrated. The value of 17.4% was proposed by the authors as a threshold for classifying a patient as overhydrated (i.e. relative fluid overload of at least 17.4%), as opposed to the value widely accepted in the literature of 15%. In contrast, there was no significant difference between all-cause hospitalisation rates for patients classified as overhydrated according to the traditional 15% threshold and those patients classified as being not overhydrated.
Hydration status
The majority of studies reported hydration status at follow-up, although not in a consistent way. Subgroups in which higher levels of overhydration at follow-up were reported were the subgroup whose average ROH was not reduced to < 15% in 6 months, as compared with the subgroup whose values were reduced to the desired level;86 the long versus short-dialysis vintage subgroup;87 patients with a cardiac cause of death, as opposed to those with a non-cardiac cause of death;83 and both absolute fluid overload and relative fluid overload in subgroups with a relative fluid overload of > 17.4%, as compared with subgroups with a relative fluid overload of < 17.4%. 88
Some studies reported the effects of hydration status on mortality. Kim et al. 50 reported a significant effect of overhydration as a risk factor for death; O’Lone et al. 82 reported a significant effect of absolute overhydration on mortality; Onofriescu et al. 88 reported that patients assessed as being overhydrated were at significantly increased risk for all-cause mortality; and Wizemann et al. 30 reported a significant risk of relative hydration status on mortality.
Cardiovascular events
Three studies reported data on CV events. 50,87,88 A non-significant difference in the incidence of acute myocardial infarction and stroke was observed between short- and long-dialysis vintage subgroups;87 no differences were found in the number of CV events per year between overhydrated and non-overhydrated subgroups;50 and no significant differences in the incidence of coronary heart disease, peripheral vascular disease, heart failure or stroke were detected between the subgroup with a relative fluid overload of < 17.4% and that with a relative fluid overload of > 17.4%. 88
Mortality
One study reported a significantly higher number of deaths in the long versus short-dialysis vintage subgroup. 87
Ongoing trials
Four relevant ongoing trials were identified. Table 5 summarises the main characteristics of the ongoing trials. More detailed study characteristics are presented in Appendix 10.
| Study name (trial acronym), ClinicalTrials.gov identifier or ISRCTN and country of study | Study aim | Primary outcome(s) |
|---|---|---|
|
Probing the Dry Weight (DW) by Bioimpedance (BIA): Which is the Gold Standard Between Clinical DW and BIA DW? NCT0244653591 Italy |
To verify if BIA-based DW control is truly superior to current volume management in HD patients | The definition for each patient of the gold standard DW when comparing the clinical and the BIA-guided DW |
|
Fluid Management Guided by Bioimpedance Analysis in Peritoneal Dialysis (PD) Patients NCT0200012892 China |
To investigate the effect of bioimpedance analysis-guided fluid management vs. experiential way on clinical outcomes in PD patients | All-cause mortality; CV-related mortality |
|
Control Of fluid balance guided by body composition Monitoring in Patients on PeritoneAl dialySiS (COMPASS) NCT0188726293 South Korea |
Bioimpedance-guided fluid management in PD patients may provide better protection of RRF over a 1-year period, compared with management guided by clinical information alone | Change of GFR from baseline to the twelfth month |
|
Bio-Impedance Spectroscopy To maintain Renal Output (BISTRO) ISRCTN1134200794 UK |
NIHR-funded open-label multicentre RCT to test whether or not taking regular measurements with a bioimpedance device improves outcomes for people aged > 18 years who have recently started HD treatment for kidney failure CKD stage 5. The target sample size is 516 patients from 30 UK dialysis units. The trial opened for recruitment 6 April 2017 | Time to anuria (loss of urine output), < 100 ml/day or 200 ml in the short interdialytic period confirmed by a further collection after 2 weeks to exclude temporary illness |
Summary of clinical effectiveness section
A total of five RCTs and eight non-randomised studies investigating the use of the BCM in adult populations were included in the review of clinical effectiveness. Taken together, evidence from randomised and non-randomised studies showed that using the BCM reduced SBP more than standard clinical practice, but not to the level of statistical significance. However, where the BCM was used, there was no difference in blood pressure between subgroups, such as long versus short vintage or normo- versus overhydrated. As compared with standard clinical practice, use of the BCM had no effect on mortality; however, the use of the BCM had a significant effect on mortality in long versus short-dialysis vintage subgroups. There was a non-significant effect on arterial stiffness of using the BCM versus standard clinical practice. A significant difference in the incidence of CV events was noted between the BCM assessment group and the standard clinical practice group, although there were no differences between subgroups using the BCM, such as long versus short-dialysis vintage and overhydrated versus non-overhydrated groups. Both absolute and ROH were significantly lower in the bioimpedance assessment group and subgroups, such as long versus short-dialysis vintage, and patients in whom ROH was not reduced to < 15% versus those whose overhydration was reduced to the desired level had higher levels of overhydration.
Overhydration was identified as a risk factor for mortality. Left ventricular hypertrophy was reported to decrease in both bioimpedance and standard clinical assessment groups, although not to the level of statistical significance; there was no difference in left ventricular hypertrophy between long and short-dialysis vintage subgroups. Findings regarding hospitalisation of the bioimpedance versus standard clinical assessment groups and overhydrated versus non-overhydrated subgroups were variable and inconclusive.
Chapter 3 Assessment of cost-effectiveness
The aim of the economic evaluation for this assessment was to assess the cost-effectiveness of using multiple-frequency bioimpedance technologies versus standard clinical assessment for fluid management in people with CKD receiving dialysis. The bioimpedance technologies considered were the BCM, MultiScan 5000, BioScan 920-II, BioScan touch i8 and the InBody S10.
The specific objectives were to:
-
review existing economic evaluations of multiple-frequency bioimpedance devices for fluid management in people with CKD receiving dialysis
-
develop a de novo economic model to assess the cost-effectiveness of using the identified multiple-frequency bioimpedance devices compared with standard clinical assessment alone to guide fluid management in people with CKD receiving dialysis, from a UK NHS and personal social services perspective.
Systematic review of existing cost-effectiveness evidence
Electronic searches were undertaken to identify reports of economic evaluations. The following bibliographic databases were included: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, National Institute for Health Research Economic Evaluations Database (NEED), the Health Technology Assessment database and the Research Papers in Economics (RePEC) database. No date or language restrictions were imposed, and searches were undertaken on 5 July 2016. Details of the search strategies are reproduced in Appendix 1. In addition, recent conference proceedings (over the period of 2014–16), including those of the European Renal Association, American Society of Nephrology, the Annual Dialysis Conferences and the International Society for Pharmacoeconomics and Outcomes Research, were also screened. Relevant websites of key professional organisations, registries and device manufacturers were checked for additional data and information. The searches identified no full economic evaluations of relevance to the scope of this assessment.
To help inform the design of the de novo economic model, broader searches were carried out to identify existing economic models in the area of CKD/ESRD, and NHS cost data applicable to relevant patient populations and health states were included in the model. A separate search was also developed for health state utility data relevant to the health states included in the economic model. Databases searched included MEDLINE, EMBASE, the Cost-effectiveness Analysis (CEA) Registry and ScHarrHUD (School of Health and Related Research Health Utilities Database). The searches were undertaken on 8 July 2016 and no date or language restrictions were imposed. The search strategies are reproduced in Appendix 1. Discussion of the potential data sources identified by these broader searches are provided under the relevant subheadings below.
Independent economic assessment
A de novo economic model was developed in TreeAge Pro (TreeAge Software, Williamstown, MA, USA). The model was designed to assess the cost-effectiveness using multiple-frequency bioimpedance testing to help guide fluid management decisions in people with CKD receiving dialysis.
The model structure was informed by the hypothesised benefits of bioimpedance testing and review of published models in the area of ESRD, with particular emphasis on models previously used to inform NHS policy surrounding the provision of dialysis. 17,95–98
The model was populated using data derived from focused reviews of the literature (to inform baseline mortality and hospitalisation risks in patients with ESRD), the systematic review of clinical effectiveness (to inform relative treatment effects) and other focused reviews to inform sources of cost and utility data. The model was built and analysed in accordance with the NICE reference case for the evaluation of diagnostic tests and devices. 17,98 It compares cumulative costs to the health service and quality-adjusted life-years (QALYs) gained for the alternative monitoring strategies.
Methods
Relevant patient population(s)
The model compared the alternative fluid management strategies for a prevalent cohort of people with ESRD receiving either HD or PD. The base-case analysis was conducted using the weighted average of the median age and sex distribution for the respective prevalent dialysis cohorts, as reported in the UK Renal Registry report:99 aged 67.2 years, 61% male for those receiving HD, and aged 64.2 years, 61% male for those receiving PD. Thus, the base-case analysis was run for a mixed cohort at the average age of 66 years, 61% male, with 87% receiving HD and 13% receiving PD. Separate subgroup analyses were also conducted for the PD and HD cohorts, applying the median ages for the respective subgroups. In addition, comorbidity burden is also used in the model in the estimation of baseline hospitalisation risks, and this was estimated from UK registry data. 99,100 Based on these sources, 63% of patients aged ≥ 65 years and 36% of patients aged < 65 years are modelled to have at least one comorbidity at baseline. The estimated mean number of comorbidities in those with any comorbidity is 1.6 and 2.0 for the PD and HD cohorts, respectively.
Monitoring strategies evaluated
Bioimpedance monitoring strategies, to help adjust target weight and guide fluid management, were compared with standard clinical assessment, in which target weight is set based on clinical signs and symptoms, including blood pressure, presence of oedema, changes in weight, residual renal function, pre-existing CV conditions and patient-reported symptoms of overhydration or underhydration. For the bioimpedance strategies, it was assumed that all patients would have their hydration status assessed every 3 months (four times per year), and have their target weight modified in line with the results if necessary. The above monitoring strategy is in line with clinical opinion regarding the necessary frequency of bioimpedance testing in an adult dialysis population, and is also consistent with the approach used in two of the trials included in the systematic review of clinical effectiveness. 60,81 It is less intensive than the testing strategies applied in the other RCTs included in the clinical effectiveness review, which varied from once per week101 to once every 6 weeks. 63 It is assumed in the base-case cost-effectiveness scenarios that quarterly testing can deliver effects in keeping with those observed across all the included randomised trials. The impact of increased testing frequency is addressed in sensitivity analysis.
The bioimpedance technologies included in the scope for this assessment were the BCM, MultiScan 5000, BioScan 920-II, BioScan touch i8 and the InBody S10. However, the review of existing literature only uncovered clinical effectiveness evidence relating to the BCM. Therefore, the economic modelling focused on assessing the cost-effectives of bioimpedance testing using the BCM device. For comparison, we include cost-per-test estimates using each of the other competitor devices, and assess the impact of applying these costs in a sensitivity analysis (assuming equivalent effects).
Framework (method of synthesis)
A discrete-time Markov cohort model was developed to assess the clinical effectiveness and cost-effectiveness of using multiple-frequency bioimpedance testing compared with standard clinical practice for guiding fluid management decisions in the dialysis cohort. This state-transition framework was chosen for its ability to capture the evolving disease process and recurrent event risks over time, while being relatively parsimonious in terms of data and computational requirements. Key states included in the model are ‘stable on HD’, ‘stable on PD’, ‘post-incident CV event – haemodialysis’, ‘post-incident CV event – peritoneal dialysis’, ‘post transplant’ ‘post transplant, post CV event’, ‘dialysis post transplant’, ‘dialysis post transplant, post CV event’ and ‘death’. The model also includes an option to dichotomise the ‘stable’ and ‘post CV event’ dialysis states by baseline ROH status into either severely overhydrated (> 15% ROH) or normohydrated (≤ 15% ROH), as measured by the BCM. This is to allow mortality and hospitalisation rates for the severely overhydrated portion of the prevalent cohort to be factored upwards, reflecting the observed adjusted association between hydration status and these outcomes. 26,30,60,82
Modelled transitions between the relative hydration states were then used to drive effects in an alternative scenario analysis (see Further adjustments to baseline risks for further details). States representing underhydration were not included in this alternative model structure because of a dearth of evidence on (1) the prevalence of underhydration, as measured by the BCM, in UK dialysis cohorts; (2) the impact of underhydration, as measured by the BCM, on the risk of adverse events and/or quality of life; and (3) the effectiveness of bioimpedance-guided fluid management on reducing the prevalence of underhydration. If underhydration (as measured by bioimpedance spectroscopy) is associated with adverse outcomes and quality of life, and bioimpedance-guided fluid management can reduce the prevalence of this, then this secondary model may fail to capture the associated benefits.
The model simulates mortality, hospitalisation events and transition to transplant over the lifetime of the modelled cohorts on a constant 3-monthly cycle (in keeping with the BCM testing cycle). All-cause hospitalisation events are disaggregated across CV events and other causes. It is assumed in the model that hospitalisation for incident CV events results in an increased comorbidity burden, which increases the risk of subsequent hospitalisations.
Costs of dialysis (by modality), background medication [blood pressure, erythropoiesis-stimulating agents (ESAs)], transplant, all-cause hospitalisation and outpatient attendances are included in the baseline model. Health state utility multipliers are applied to the dialysis states, and utility decrements are also incorporated for hospitalisations. These decrements are applied for an acute period for all hospitalisations. For hospitalisations caused by CV events, a long-term utility multiplier is also applied. This reflects the lasting impact that these events can have on health-related quality of life. A schematic of the model structure is provided in Figure 13. A simplifying assumption of the model precludes switching between dialysis modes. This is unlikely to have a significant impact on results since an equal baseline mortality rate is applied for patients on dialysis irrespective of modality, and the estimated costs of PD and HD were also found to be similar based on current reference costs (see Costs of renal replacement therapy). Furthermore, the clinical effectiveness evidence was insufficient to estimate bioimpedance effects by dialysis modality.
FIGURE 13.
Schematic of the baseline model structure.

The baseline model is replicated for the strategy of bioimpedance-guided fluid management, and correspondingly incorporates the additional cost of quarterly testing on top of standard practice. The bioimpedance model also allows for the incorporation of effects of bioimpedance monitoring on mortality, hospitalisation rates, background management costs (e.g. blood pressure medications) and within-state health state utility. The incorporation of these hypothesised benefits, in light of the available supporting evidence, is discussed in detail under the relevant headings below. The model can also capture downstream cost-savings and quality-of-life benefits associated with reduced hospitalisation rates and prolonged survival.
Modelled baseline risks
The baseline risks of mortality were derived from a number of sources. The UK Renal Registry report44 was first consulted as a source of population-based data. However, this report provides detailed data on survival only (by age) for the incident RRT cohort as a whole, without censoring for transplantation. This is not suited to the decision model structure (see Figure 13), in which mortality rates dependent on continuing to receive dialysis and on transitioning to transplant are required. Therefore, the ERA-EDTA annual report was consulted. 102 This report includes adjusted 5-year survival curves with censoring for transplantation in the dialysis survival estimates. The data are reported from day 91, with adjustment based on Cox regression for age, gender and primary diagnosis. The survival estimates on different modalities are expressed for a cohort of people aged 60 years and 60% male, with the following distribution for cause of renal disease: diabetes mellitus (20%), hypertension (17%), glomerulonephritis (15%) and other causes (48%). This distribution of characteristics is reasonably similar to that of the UK dialysis population, although age is slightly higher in the incident UK cohort at 63 years, and diabetes mellitus and hypertension are reported as the primary renal diagnosis in 26% and 6.5% of incident patients, respectively. 99 Although is preferable to reconstruct patient-level data from published Kaplan–Meier curves for the purpose of extrapolating survival in decision models,103 the adjusted nature of the reported data precluded estimation of numbers of events and censoring events. Therefore, a simple regression-based method was used to fit a Weibull distribution to the summary survival curve data. 104 Given limitations in the evidence base to support differences in survival by mode of dialysis, we based extrapolation on the survival curve for all dialysis modalities combined. The scale and shape parameters from the derived Weibull curves (Table 6) were incorporated in the model and used to extrapolate mortality risks out to 10 years. The scale parameter (λ) was further adjusted to reflect the starting age of the modelled cohort (67 years), using a published HR for mortality (beyond 91 days) associated with a 10-year increase in age:99
in which HRACM is the HR for all-cause mortality associated with a 10-year increase in age, and ‘start_age’ is the starting age of the modelled dialysis cohort.
| Clinical parameter | Value (95% CI) | Parameter distribution | Source |
|---|---|---|---|
| Mortality on dialysis (to 10 years) | |||
| Weibull scale parameter (cohort of people aged 60 years) | 0.114 | – | ERA-EDTA Registry annual report (2013)102 |
| Weibull shape parameter | 1.035 | – | ERA-EDTA Registry annual report (2013)102 |
| HR (10-year age increase on RRT) | 1.65 (1.56 to 1.75) | Log-normal | UK Renal Registry Report (2015)99 |
| Mortality year 1 post transplant (deceased donor) | |||
| Rate per patient-year (0–19) | 0.018 (0.006 to 0.029) | Log-normal | ERA-EDTA Registry annual report (2013)102 |
| Rate per patient-year (20–44) | 0.019 (0.016 to 0.022) | Log-normal | |
| Rate per patient-year (45–64) | 0.044 (0.040 to 0.048) | Log-normal | |
| Rate per patient-year (65–74) | 0.104 (0.090 to 0.120) | Log-normal | |
| Mortality year 1 post transplant (living donor) | |||
| Rate per patient-year (0–19) | 0.007 (0.004 to 0.010) | Log-normal | ERA-EDTA Registry annual report (2013)102 |
| Rate per patient-year (20–44) | 0.007 (0.004 to 0.010) | Log-normal | |
| Rate per patient-year (45–64) | 0.02 (0.014 to 0.026) | Log-normal | |
| Rate per patient-year (65–74) | 0.053 (0.028 to 0.079) | Log-normal | |
| Mortality 1–10 years post transplant | |||
| Weibull scale parameter (cohort of people aged 65 years) | 0.05 | Multivariate normal | Karim et al., 2014105 |
| Weibull shape parameter | 1.027 | Multivariate normal | |
| HR (10-year increase in transplant recipient age) | 1.766 (1.540 to 2.028) | Log-normal | Karim et al., 2014105 |
| HR for all-cause mortality with transplant vs. dialysis (applied beyond 10 years post transplant) | 0.42 (0.16 to 0.76) | Log-normal | Tonelli et al., 2011106 |
| Proportion of prevalent dialysis population on waiting list for transplant | |||
| Aged < 65 years | 0.346 (0.338 to 0.354) | Beta | Annual Report on Kidney Transplantation (2014);107 UK Renal Registry Report (2015)99 |
| Aged 65–75 years | 0.135 (0.128 to 0.142) | Beta | |
| Aged > 75 years | 0 | – | |
| Probability of transplant (3-monthly) among those on waiting list | 0.057 (0.055 to 0.058) | Beta | Annual Report on Kidney Transplantation (2014)107 |
| Probability of graft failure (3-monthly) | |||
| Deceased donors | 0.0075 (0.007 to 0.0081) | Beta | Annual Report on Kidney Transplantation (2014)107 |
| Living donors | 0.0047 (0.004 to 0.005) | Beta | |
| Proportion of transplants from deceased donors (aged 60–70 years) | 0.723 (0.706 to 0.40) | Beta | Karim et al., 2014;105 varies by age of recipient |
For those transitioning to renal transplant, survival data were derived from a combination of sources (see Table 6). In the first year following transplant, survival probabilities by age groups were taken from the ERA-EDTA Registry annual report. 102 The reported 1-year survival probabilities differ by donor type (deceased/living), and were weighted accordingly. Beyond 1 year, we used published 10-year Kaplan–Meier survival data from a UK population-based study of transplant recipients. 105 The individual patient data were reconstructed for 2887 subjects aged 60–69 years following the approach described by Hoyle et al. ,103 using reported numbers at risk and steps in the published Kaplan–Meier curve. Parametric survival models were then fitted using R statistical software, version 3.1 (The R Foundation, Vienna, Austria), and the best-fitting model selected based on the Bayesian information criterion. This was a Weibull model. The scale parameter of the derived Weibull curve is adjusted in the model for the recipient’s age at time of transplant using the HRs for age reported by Karim et al. 105 All of the parameters used to model survival are presented in Table 6.
To minimise uncertainty associated with the use of parametric curves to extrapolate survival beyond 10 years, we applied an alternative approach to model mortality in the longer term. Mortality rates on RRT were estimated by applying reported relative risks of mortality in the RRT population compared with the UK general population99 to general population mortality rates adjusted for age/sex from UK life tables. For those remaining in a post-transplant state beyond 10 years following transplant, an adjusted relative risk106 was applied to the modelled annual mortality rate of age-matched patients on dialysis. Tonelli et al. 106 conducted a systematic review of observational studies reporting an adjusted HR for all-cause mortality with renal transplant versus dialysis. 106 Although a formal meta-analysis of these data was not conducted because of diversity across the included observational studies, the central estimate (0.42) of the reported range (0.16–0.76) across 23 included studies was applied in the base-case model. The reported range was treated as a CI for the purposes of assigning a log-normal distribution to this parameter.
Three-monthly probabilities of renal transplantation for those on dialysis were derived from the percentage of dialysis patients on a waiting list for a transplant (aged < 65 and ≥ 65 years),99 combined with the median duration of time to transplant (1082 days). 107 The graft failure rate for those receiving a transplant was derived from the 5-year graft survival rates reported for grafts from living and deceased donors. 107
All-cause inpatient hospitalisation was modelled using the first part of a published two-part cost model developed by Li et al. 100 Li et al. 100 used a data set for a cohort of patients on the UK Renal Registry who started receiving dialysis or received a kidney transplant in England between 1 April 2003 and 31 December 2006. The data on these patients were linked to Health Episode Statistics (HES) data for inpatient hospital activity (excluding activity for maintenance dialysis or transplant surgery) up to 6 years following initiation of dialysis or transplant. Each hospital event was costed using the appropriate Healthcare Resource Group (HRG) Payment by Results tariff for the admission. The data were then analysed using a two-part model: logistic regression was used to predict the probability of a patient incurring any inpatient hospital costs in a given year on RRT (up to year 6), and a general linear model was used to predict total inpatient costs in those who had at least one hospital episode in a given year. The models were adjusted for age, gender, years receiving dialysis, mode of dialysis, comorbidities, transplant and year of death (to account for increased hospital resource use in the year of death and year preceding death). The published two-part models for dialysis and transplant patients are replicated in Tables 7 and 8.
| Term | Dialysis inpatient, OR (95% CI) | Mean annual cost (£) for dialysis patients (GLM), coefficient (95% CI) |
|---|---|---|
| Constant | 2.34 (2.18 to 2.51) | 7782 (7423 to 8140) |
| Age group (years) | ||
| < 50 | Reference | Reference |
| 50–64 | 0.98 (0.91 to 1.05) | –170 (–489 to 149) |
| 65–75 | 0.91 (0.85 to 0.97) | –181 (–513 to 151) |
| > 75 | 0.87 (0.81 to 0.94) | –444 (–806 to –83) |
| Sex | ||
| Male | Reference | Reference |
| Female | 1.1 (1.05 to 1.16) | 208 (–23 to 439) |
| Years on dialysis | ||
| 1 | Reference | Reference |
| 2 | 0.59 (0.56 to 0.62) | –1189 (–1487 to –891) |
| 3 | 0.5 (0.47 to 0.52) | –1434 (–1729 to –1140) |
| 4 | 0.58 (0.54 to 0.62) | –1848 (–2166 to –1530) |
| 5 | 0.61 (0.56 to 0.67) | –1709 (–2099 to –1319) |
| 6 | 0.65 (0.57 to 0.74) | –2270 (–2774 to –1767) |
| Dialysis modality | ||
| HD | Reference | Reference |
| PD | 0.83 (0.79 to 0.88) | –612 (–838 to –385) |
| Comorbidities | ||
| Myocardial infarction (17%) | 1.22 (1.14 to 1.31) | 390 (96 to 683) |
| Congestive heart failure (17%) | 1.11 (1.04 to 1.19) | 321 (58 to 584) |
| Peripheral vascular disease (16%) | 1.33 (1.24 to 1.42) | 721 (423 to 1019) |
| Cerebrovascular disease (11%) | 1.15 (1.07 to 1.24) | 506 (174 to 383) |
| Pulmonary (15%) | 1.26 (1.17 to 1.35) | 412 (128 to 696) |
| Liver (1%) | – | 1682 (–161 to 3524) |
| Diabetes mellitus (34%) | 1.27 (1.21 to 1.34) | 1191 (929 to 1453) |
| Cancer (8%) | 1.22 (1.11 to 1.33) | – |
| Hypertension (62%) | 1.09 (1.04 to 1.14) | – |
| Transplant | 1.11 (1.02 to 1.21) | –1863 (–1863 to –1585) |
| Recovered renal function | 0.82 (0.69 to 0.96) | 1293 (513 to 2073) |
| Death | 1.94 (1.81 to 2.07) | 2403 (2152 to 2654) |
| Death in the first half of the following year | 2.61 (2.34 to 2.92) | 4415 (3926 to 4904) |
| Term | Transplant inpatient, OR (95% CI) | Mean annual costs (£) for transplant patients (GLM), coefficient (95% CI) |
|---|---|---|
| Constant | 1.89 (1.65 to 2.16) | 4735 (4331 to 5138) |
| Age group (years) | ||
| < 35 | Reference | Reference |
| 36–45 | 0.81 (0.72 to 0.92) | –318 (–664 to 29) |
| 46–55 | 0.73 (0.64 to 0.82) | –310 (–676 to 56) |
| > 55 | 0.76 (0.67 to 0.87) | –91 (–487 to 306) |
| Sex | ||
| Male | Reference | Reference |
| Female | 1.35 (1.22 to 1.49) | 190 (–76 to 455) |
| Years following transplant | ||
| 1 | Reference | Reference |
| 2 | 0.21 (0.19 to 0.23) | –1576 (–1881 to –1271) |
| 3 | 0.18 (0.16 to 0.2) | –1919 (–2228 to –1611) |
| 4 | 0.19 (0.17 to 0.22) | –2138 (–2485 to –1790) |
| 5 | 0.19 (0.16 to 0.23) | –2061 (–2502 to –1620) |
| 6 | 0.18 (0.14 to 0.22) | –2654 (–3212 to –2096) |
| Transplant type | ||
| Deceased donor | Reference | Reference |
| Living donor | 0.82 (0.75 to 0.9) | –223 (–486 to 39) |
| Comorbidities | ||
| Myocardial infarction (8%) | 1.47 (1.24 to 1.73) | 641 (145 to 1138) |
| Congestive heart failure (6%) | 1.48 (1.22 to 1.73) | 1248 (646 to 1851) |
| Peripheral vascular disease (11%) | 1.87 (1.62 to 2.16) | 1222 (729 to 1715) |
| Cerebrovascular disease (6%) | 1.38 (1.16 to 1.65) | 898 (271 to 1524) |
| Pulmonary (13%) | 1.24 (1.09 to 1.4) | 264 (–87 to 616) |
| Liver (1%) | 2.18 (1.37 to 3.47) | 2093 (30 to 4155) |
| Diabetes mellitus (26%) | 1.62 (1.46 to 1.8) | 1046 (734 to 1395) |
| Cancer (4%) | 1.62 (1.31 to 2.01) | 485 (2 to 969) |
| Hypertension (74%) | 1.33 (1.21 to 1.46) | 324 (56 to 592) |
| Graft failure | – | 2438 (1723 to 3152) |
| Death | 1.62 (1.14 to 2.31) | 4924 (3726 to 6123) |
| Death in the first half of the following year | 4.55 (2.47 to 8.39) | 5725 (3350 to 8100) |
These models were incorporated in our decision model to predict the annual probability of hospitalisation each year based on the characteristics of the modelled cohort, and then to apply the associated inpatient hospitalisation costs. To keep the approach manageable in the context of a Markov cohort model, the odds ratios and cost coefficients associated with comorbidities were collapsed into a single weighted average for any one comorbidity, based on the reported frequency of each individual comorbidity. We then estimated the risk of hospitalisation at the cohort level by computing the weighted average of the risk for males and females, with and without comorbidities. The expected number of comorbidities among those in the cohort with any comorbidity was derived from the UK Renal Registry report,99 and the weighted average odds of hospitalisation associated with any one comorbidity was raised to this power in the calculation of hospitalisation risk in this segment of the cohort.
To fit the 3-month Markov cycle, the annual probabilities of hospital admission were converted to 3-monthly probabilities, assuming a constant inpatient hospitalisation rate over the year. Furthermore, the underlying rate was disaggregated into CV event- and other cause-related hospitalisation rates. To inform this process, we conducted a focused search of the literature for data on cause of hospitalisation in ESRD patients receiving dialysis. A number of studies were identified, suggesting that CV event-related hospitalisation rates account for ≈ 20% of all hospitalisations in dialysis cohorts. 108–110 The most relevant source of evidence to the UK RRT population reported that CV events made up 17.6% of the annual inpatient event rate in a cohort of 1226 UK HD patients. 110 This value was applied in the base case. We then further disaggregated expected CV hospitalisation events across types of CV events, in line with the reported relative frequency of CV event histories in the dialysis population (see Table 7). Although this is an uncertain assumption, we could not identify any better UK population-based data by which to model the relative frequency of different types of CV event in the dialysis population. A further limitation of the models used to predict annual hospitalisation risk, is the fact that these require extrapolation beyond the period of follow-up in the data sets used to develop them (i.e. beyond 6 years). We therefore had to assume that estimated probabilities of hospitalisation at 6 years on dialysis are generalisable across future years on dialysis.
Further adjustments to baseline risks
To allow for modelled scenarios in which effects are mediated through associations between hydration status and outcomes, the model was structured to enable mortality and hospitalisation rates to be adjusted upwards for proportions of the dialysis cohorts estimated to be severely overhydrated (ROH of > 15%). Modelled reductions in severe overhydration were then used to drive effects in scenarios using this version of the model.
The expected prevalence of severe overhydration (ROH of > 15%) was based on studies taking BCM measures at clinic visits (not necessarily first thing in the morning) for the PD cohort, and pre dialysis for the HD cohort. The threshold of ROH of > 15% was selected because, as noted in Non-randomised evidence, it has been associated with increased rates of mortality and hospitalisation in observational studies. 30,50,82,88 Time-averaged volume overload may give a more accurate estimate of the average exposure to fluid overload, but this measure has not been linked with mortality in observational studies. Limited data were identified regarding the prevalence of ROH of > 15% in UK dialysis cohorts. One observational study of 529 PD patients from a single UK centre82 reported that ≈ 31% of patients had ROH of > 10%. A multicentre European study, which included 734 patients from centres in Belgium, France, Romania, the UK (167 patients from two centres) and Switzerland, reported that 25.2% of the cohort were severely overhydrated (ROH of > 15%). 111 There were fewer published data available on the prevalence of severe overhydration in the UK HD population. However, a further multicentre European study matched PD patients from France, Romania and the UK with HD patients from the corresponding countries. 112 This study showed that pre-dialysis ROH in HD patients was similar to ROH in PD patients – although the time-averaged volume overload in HD patients was lower than the ROH value of PD patients. Based on these available data, the baseline prevalence of ROH of > 15% was set at 25% for both the HD and the PD cohorts.
The mortality rate for the severely overhydrated proportion of the HD cohort was increased using an adjusted HR of 1.87 (95% CI 1.12 to 3.13), as reported by Onofriescu et al. 88 The all-cause hospitalisation rate was also inflated upwards using an adjusted HR of 1.19 (95% CI 0.99 to 1.41), as reported by Onofriescu et al. 88 For the corresponding segment of the PD cohort, all-cause mortality was adjusted upwards using the HR of 1.83 (95% CI 1.19 to 2.82) reported by O’Lone et al. 82 No data were identified reporting the increased risk of all-cause hospitalisation in severely overhydrated PD patients, and so the same value as that used for HD patients was applied. It is plausible that any mortality/morbidity benefits associated with bioimpedance testing are also partly attributable to the avoidance of underhydration. However, no studies were identified linking underhydration, as measured using bioimpedance spectroscopy, to mortality and adverse events. Therefore, an underhydration state was not included in the model. As mentioned in Framework (method of synthesis), this could potentially underestimate the benefits if bioimpedance-guided fluid management can simultaneously reduce the proportion of patients that are seriously over- and underhydrated. Conversely, if the use of bioimpedance testing to guide fluid management decreases the proportion of patients who are overhydrated at the expense of increasing the proportion who are underhydrated, this model could potentially overestimate the benefits.
Incorporation of relative treatment effects
Alternative approaches to modelling effects of bioimpedance-guided fluid management on the baseline event rates were considered. Given the limitations in the existing evidence base for the clinical effectiveness of bioimpedance testing, combined with further limitations in the evidence base to inform certain baseline events, the modelled cost-effectiveness scenarios are subject to a significant degree of uncertainty.
With the availability of some trial evidence for the technology, the application of direct evidence for effects on final health outcomes was considered the preferred approach for modelling benefits. However, given the limitations in the trial evidence base, this was only possible for all-cause mortality. Of the three available BCM trials that included all-cause hospitalisation rates,61,76,77 these showed inconsistent and insignificant effects on this outcome. Therefore, we did not incorporate an effect on the overall hospitalisation rate in scenarios applying direct estimates of effects. Alternative approaches were explored in further scenario analyses to model plausible effects on CV event-related and non-CV event-related hospitalisation rates.
As a number of the trials reported effects on surrogate end points, including LVMI and PWV, we conducted a focused literature search to identify appropriate published sources of evidence to link changes in these surrogates to final health outcomes in the relevant patient population. A hierarchical approach was adopted to identify suitable sources of evidence, with priority given in descending order to the following types of evidence:
-
evidence linking intervention-induced changes in available surrogate outcomes to changes in the risk of final health outcomes
-
evidence linking non-intervention-induced longitudinal changes in surrogate outcomes to changes in the risk of final health outcomes
-
evidence from large UK or European cohort studies assessing the prognostic value of baseline measures of the surrogate measures for final health outcomes.
One systematic review, conducted in 2016, considered the value of LVMI as a treatment target in the area of ESRD, and concluded that there was no clear and consistent association between intervention-induced LVM change and all-cause or CV event-related mortality. 113 Furthermore, as only one of the BCM trials included this as an outcome, LVMI was considered no further. The search of available evidence did not identify any existing data showing a clear link between intervention-induced changes in PWV and final health outcomes in ESRD, but a large European observational study was identified. 114 This study assessed the prognostic value of baseline PWV on all-cause mortality and non-fatal CV events in a cohort of 1084 patients recruited from 47 European dialysis centres over a period of 2 years. It highlighted the importance of simultaneously considering abdominal aortic calcification (AAC) when assessing the prognostic value of PWV. Based on a multivariate Cox regression, both variables were found to be significant predictors of mortality and non-fatal CV events, but the effect of PWV was ameliorated at higher levels of aortic calcification (incorporated as tertiles), as a result of a significant negative interaction. The relevant HRs from the published Cox regression are provided in Table 9. Based on these estimates, and assuming that the UK dialysis cohort is similarly distributed across aortic calcification tertiles, we estimated an average effect on all-cause mortality and non-fatal CV events of a unit change in PWV, accounting for the interaction. This yielded a HR of 0.942 (95% CI 0.879 to 1.009) per unit reduction in PWV. We then explored the impact of scaling this effect to the magnitude of the pooled mean reduction in PWV (1.18 m/s) across the included BCM trials (see Figure 7), and applying it to the modelled proportion of all-cause hospitalisation events estimated to be attributable to CV events (assumes that a 1.18 m/s reduction in PWV is generalisable to the UK dialysis cohort). We also explored the impact of applying it to the all-cause mortality rate in the model. These analyses should be treated with caution, as they rely on cross-sectional associative evidence from an observational study to inform possible effects of bioimpedance monitoring. It should be further noted that the pooled estimate for the effect of bioimpedance monitoring on PWV is non-significant and based on results from only two trials, showing inconsistent results (see Figure 7). However, the point estimate is applied in the base-case model and the uncertainty surrounding it is propagated through the probabilistic analysis. Furthermore, the negative interaction between increasing AAC tertiles and the effect of baseline PWV on mortality and CV event-related hospitalisation, suggests that the relative effect of reductions in PWV may be greater in lower-risk groups (with lower AAC scores). On the other hand, evidence for an interaction in the prognostic value of baseline measures of these two variables does not necessarily mean that the AAC score would modify the effect of an intervention-induced reduction in PWV. Therefore, this model could potentially over- or underestimate the likely effects of the estimated reduction in PWV on final health outcomes. Better evidence on the effects of intervention-induced reductions in PWV are required to inform this issue.
| Variable | HR (95% CI) | Distribution | Source |
|---|---|---|---|
| PWV (m/s) | 1.154 (1.085 to 1.228) | – | Verbeke et al., 2011114 |
| PWV × lower AAC | 1 | – | – |
| PWV × middle AAC | 0.895 (0.828 to 0.968) | – | Verbeke et al., 2011114 |
| PWV × upper AAC | 0.865 (0.808 to 0.925) | – | Verbeke et al., 2011114 |
| Average effect per unit change in PWV across AAC tertiles = 1/{[(1.154 × 1) + (1.154 × 0.895) + (1.154 × 0.865)]/3} | 0.942 (0.879 to 1.009) | Log-normal | Assessment Group calculation |
| Inferred average effect for a 1.18 m/s reduction in PWV of 0.942^1.18 | 0.9318 (0.829 to 1.048) | Log-normal | Assessment Group calculation |
As an alternative approach to indirectly estimate possible effects of bioimpedance-guided fluid management on mortality and CV event-related hospitalisation, we considered linking the estimated pooled reduction in SBP (2.46 mmHg; see Figure 11) to effects on CV events and mortality using a meta-analysis on the effects of blood pressure-lowering medications in dialysis patients. Heerspink et al. 115 estimated pooled relative risks of 0.71 (0.55 to 0.92) for CV events and 0.8 (0.66 to 0.96) for all all-cause mortality across eight trials; corresponding to a mean reduction in SBP of 4.5 mmHg. Assuming a log-linear relationship between SBP reduction and the relative risk of events, these effects can be rescaled to the mean reduction in SBP across included BCM trials (2.46 mmHg):
Relative risk for CV events for a 2.46-mmHg reduction in SBP:
Relative risk for all-cause mortality for a 3.44-mmHg reduction in SBP:
These effects are substantially larger than the estimated effects using PWV above, and suggest a potentially larger effect on CV events than on all-cause mortality. However, it is uncertain if effects on SBP induced by blood pressure medication can be generalised to potential reductions in SBP induced by the management of fluid status, that is, some blood pressure medications are thought to have effects on CV events that are independent of their blood pressure-lowering effects. 116 Furthermore, there is a complex relationship between fluid management and blood pressure,117 which makes it difficult to generalise. Nevertheless, the effect of bioimpedance-guided fluid management on SBP (bordering on significance), suggests a possible beneficial effect on both CV events and mortality. Therefore, we explored the impact of applying larger and differential relative effects on these outcomes in further scenario analyses.
Finally, we also explored the impact of using associations between overhydration and all-cause mortality and hospitalisation rates to drive effects in the model. For this analysis, we used data from Chen et al. ,80 to estimate the proportion of patients who could be shifted from the overhydrated to the normally hydrated states in the bioimpedance assessment and standard care arms of the model. This analysis assumed that for everyone who is moved from the overhydrated (relative fluid overload > 15%) to the normally hydrated state, the increased risks associated with overhydration are completely reversed. This was an optimistic assumption, as, again, cross-sectional associations between baseline measures and final outcomes were used to drive the effects of bioimpedance-guided fluid management in the model. The increased risk associated with baseline overhydration may not be fully reversible for those that can be returned to normal hydration status (≤ 15%).
A further problem with this approach is the lack of reporting in the RCTs on the effect of bioimpedance-guided fluid management on the proportion of patients with pre-dialysis ROH of > 15% at baseline and follow-up. Onofriescu et al. 88 did report proportions of patients within, and > 1.1 kg above and below, the BCM-guided target weight, and this study suggested no real change in the average percentage of patients who were > 1.1 kg above target weight throughout the follow-up period. Yet, the study did demonstrate a significant effect on PWV and mortality, leading the authors to speculate that the mechanism for effect may be as much a result of the avoidance of chronic underhydration as overhydration. Huan-Sheng et al. 76 reported a significantly larger reduction in mean overhydration (per litre) in patients with ROH of > 15% at baseline. We used these data to approximate percentage reductions in ROH of > 15% (absolute overhydration of > 2.5 litres) over the follow-up period by (1) assuming normal distributions for absolute overhydration at follow-up up and (2) subtracting mean reported reductions in overhydration (per litre) from simulated gamma distributions of baseline overhydration of > 2.5 litres. This yielded plausible percentage reductions in ROH of > 15% from 28% to 38% with bioimpedance-guided management relative to control. These were applied in model scenarios utilising the change in ROH status to drive effects on all-cause mortality and all-cause hospitalisation.
Further hypothesised benefits of bioimpedance-guided fluid management that were not incorporated in the main analyses included changes in quality of life (independent of effects on hospitalisation and CV events), maintenance of residual renal function and effects on dialysis requirements (number and duration of sessions).
None of the identified BCM trials reported on health-related quality of life, and only one included any patient-reported outcomes. 80 One observational study was identified that reported an association between hydration status and quality of life in Korean PD patients, as measured by the Kidney Disease Quality of Life-Short Form (KDQOL-SF) questionnaire. This showed that reductions in absolute overhydration (per litre) between baseline and 12 months were associated with improvements in the physical component score (1.81 points, 95% CI 0.78 to 2.84 points), mental component score (0.92 points, 95% CI 0.2 to 1.65 points) and the kidney disease component score (0.9 points, 95% CI 0.36 to 144 points), as measured by the KDQOL-SF questionnaire. These analyses were adjusted for various potential confounders, including age, sex, dialysis vintage, haemoglobin level, baseline overhydration status and comorbidities (as measured by the Charlson Comorbidity Index). Although this study suggests that use of the BCM could lead to improvements in heath-related quality of life (independent of effects on adverse events), it is not clear how generalisable the reported changes are to the UK population. In addition, it is not possible to map from changes in the reported aggregate component scores of the KDQOL-SF questionnaire to changes in health state utility values. Furthermore, our model already captures QALY gains associated with prevention of hospitalisation events and increasing comorbidity, and, therefore, including a constant utility increment associated with the use of bioimpedance testing could lead to double-counting of QALY gains. Nevertheless, the impact of including a 2% and 5% improvement in health state utility as a result of improved interdialytic symptoms was assessed in a further scenario analysis.
The omission of residual renal function as an explicitly modelled state, and its knock-on effects on dialysis requirements and outcomes as potential benefits, is justified by a current lack of supporting evidence. The only BCM trial that assessed proxies for residual renal function in HD patients reported a significant increase in the proportion of anuric patients and a significant decrease in urine output in non-anuric patients at 12 months in the bioimpedance assessment group. 77 In the only other bioimpedance trial to report renal output, PD patients randomised to bioimpedance-guided fluid management showed a slightly larger reduction in mean urine volume at 12 weeks, although this was not statistically significant. 88 There is a recognised risk that aggressively pursuing lower target weights using a high ultrafiltration rate may in fact lead to increased morbidity/mortality as a result of hypoperfusion-induced ischaemic injury and accelerated loss of residual renal function. 118,119 Conversely, identifying patients that are severely underhydrated and adjusting their target weight upwards may help to preserve residual renal function. This question is currently being evaluated in two ongoing RCTs, one in PD patients120 and one in HD patients based in the UK (see Table 5). 94 If bioimpedance testing can preserve residual renal function in non-anuric patients, then our model may underestimate the average benefits in the population as a whole. Conversely, if the use of bioimpedance testing leads to decisions that accelerate the loss of residual renal function, this is a disbenefit that could have knock-on effects on dialysis costs and adverse outcomes.
Related to the above issue, there is also a lack of evidence on the effects of bioimpedance monitoring on the number, and the duration, of dialysis sessions required to achieve the prescribed target weight. A potential cost-saving could be achieved through a requirement for fewer dialysis sessions in some incident patients, if it is found to be effective in preserving residual renal function. As discussed above, this question remains currently unanswered. Conversely, the use on bioimpedance spectroscopy could result indirectly in increased dialysis costs through identification of patients that are severely overhydrated and require longer or additional dialysis sessions to achieve their new target weight without exceeding safe ultrafiltration rates and volumes. 118 Our cost-effectiveness model assumes that any effects of bioimpedance-guided fluid management on dialysis requirements are cost neutral.
Resource use estimation
The base-case cost-effectiveness model incorporates health service costs associated with maintenance dialysis, blood pressure medication and ESAs (on dialysis), all-cause inpatient hospitalisation, renal transplantation (including work-up, surgery and follow-up), post-transplantation immunosuppression and outpatient visits (all expressed in 2014–15 pounds sterling).
Costs of renal replacement therapy
It has previously been noted that dialysis treatment for CKD results in high costs to the health service, and that this can undermine the cost-effectiveness of technologies that prolong survival on dialysis. In some circumstances, a technology that prolongs survival for patients receiving dialysis may not be cost-effective at a price of zero. This has led to inconsistency across economic evaluations in the area of ESRD with respect to whether or not dialysis costs are included. Some have argued that they should not be included for interventions that aim to extend survival without impacting the need for dialysis. 121 A further argument for their exclusion is that a decision has already been made to fund dialysis on broader ethics/equity considerations that are not reflected in the cost-effectiveness of dialysis itself. It may then seem unfair to include dialysis costs when they act as an insurmountable barrier to demonstrating the cost-effectiveness of other technologies that prolong survival on dialysis. The alternative argument is that dialysis costs do represent a real opportunity cost associated with ongoing treatment for ESRD, and thus should be included in the analysis. The NICE Decision Support Unit has produced a report on assessing technologies that are not cost-effective at a price of zero, and this suggests that all NHS and Personal Social Services costs that differ between the technology being appraised and the comparator technologies should be included within the incremental cost-effectiveness ratio (ICER), as this provides the ICER that reflects the real opportunity cost of recommending the technology being appraised. 122 However, the report does also note that a case could have been made in a previous technology appraisal of cinacalcet123 for treating secondary hyperparathyroidism in ESRD, to exclude dialysis costs on the grounds that they are unrelated to the treatment of secondary hyperparathyroidism (the condition of interest in the appraisal). A similar argument was adopted in the modelling assessing the cost-effectiveness of alternative phosphate binders for people with stages 4 and 5 CKD with hyperphosphataemia. 98 In this example, the costs associated with dialysis were argued to be unaffected and unrelated to the choice of phosphate binder, with the target condition being hyperphosphataemia.
Considering the above, it is difficult to argue that dialysis costs are unrelated to a technology being used to guide fluid management decisions in patients receiving dialysis, and in theory the use of the technology could have an impact on dialysis costs in survivors, as well as prolong survival on dialysis. Therefore, dialysis costs are included in our base-case cost-effectiveness scenarios. However, it is a plausible argument that the cost-effectiveness of dialysis reflected in our model does not capture its broader value to society, relating to ethics and equity issues. Therefore, we also explored the impact of excluding dialysis costs.
Dialysis costs were taken from the current NHS reference costs. 124 For HD costs, we took the weighted average of the reference costs (per HD session) for the HRG codes LD01A to LD10A (at base and away from base), weighted by the relative proportion of overall activity reported against each code. This was multiplied by three sessions per week, and then by 52 to estimate the average annual cost of maintenance HD. For PD, we applied the weighted average of the reference costs (per day) for HRG codes LD11A to LD13A. These costs were multiplied by 365 to estimate total annual maintenance PD costs.
Transplantation costs were also taken from the reference costs, applying the average costs for HRG codes LA01A, LA02A and LA03A (elective inpatient). We also included costs for follow-up post transplantation. For year 1 this was derived from Treharne et al. 97 To this we added the costs of immunosuppressant in year 1, based on an initiation regimen in the first 2 weeks, and a maintenance regimen thereafter. For the initiation period, we costed basiliximab [(Simulect®, Novartis Pharmaceuticals UK Ltd, Frimley, UK) day 0 and day 4], prednisolone [(Concordia International, Oakville, ON, Canada) 60 mg per day], mycophenolate mofetil [(Cellcept®, Roche Products Limited, Welwyn Garden City, UK) 1 g twice daily] and tacrolimus [5 mg twice daily (we used the average list price of Adoport®, Sandoz Ltd, Frimley, Camberley, Surrey, UK; Prograf®, Astellas Pharma Ltd, Chertsey, UK; and Advagraf®, Astellas Pharma Ltd, Chertsey, UK)]. For the maintenance period, we costed prednisolone (7.5 mg per day), mycophenolate mofetil (1 g twice per day) and tacrolimus (5 mg twice daily). Beyond year 1 post transplant, we applied maintenance immunosuppression costs and added average outpatient costs observed in the transplant cohort (see Outpatient costs below). The maintenance dialysis and transplantation costs are provided in Table 10.
| Resource use item | Cost (£) | Quartile | Parameter distribution | Source | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| HD per session | 154 | 130 | 169 | Gamma | NHS Reference Costs 2014 to 2015 124 |
| PD per day | 69 | 50 | 69 | Gamma | NHS Reference Costs 2014 to 2015 124 |
| Transplant | 14,915 | 11,720 | 17,797 | Gamma | NHS Reference Costs 2014 to 2015 124 |
| Follow-up post transplant (year 1)a | 11,204 | Treharne et al., 201497 | |||
| Immunosuppressant costs (year 1) | 10,622 | NICE guidance (2015);125 expert opinion, BNF126 | |||
| Annual immunosuppressant costs (beyond year 1) | 9054 | ||||
Costs of unplanned inpatient hospitalisations
Annual inpatient hospital costs for patients on maintenance dialysis and post transplant were estimated using the two-part model developed by Li et al. ,100 as described above (see Modelled baseline risks and Tables 7 and 8). The second part of this model predicts annual inpatient costs conditional on experiencing any hospitalisation event(s) within a given year. Estimates are based on age, sex, years on dialysis (or years following transplant), dialysis modality and the comorbidity status of the modelled cohort. The model also accounts for increased costs in the year of death and the year preceding deaths that occur in the first half of the following year. This reflects the increasing level of morbidity experienced by patients towards the end of life.
To generate estimates of the cost incurred per hospitalisation event rather than costs per year, the predicted annual cost is divided by the expected number of events per patient-year in our model. The expected event rate is derived from the estimated annual probability of experiencing any hospital inpatient event, assuming a constant event rate across the year. This results in a cost per hospitalisation event that varies by the underlying characteristics of the modelled cohort, but comes to ≈ £4500 per event for patients receiving HD and £4300 per event for patients receiving PD. These estimates are substantially higher than costs per hospitalisation event that have been applied in previous models in the area of ESRD. 17,97,98 However, these previous models generally applied averages of aggregate reference costs. The estimates derived from the data reported by Li et al. 100 are based on a large data set of actual hospital episodes costed according to admission code and adjusting for length of stay. Thus, the estimates derived from Li et al. 100 are applied in the base-case analysis. Alternative estimates are applied in a sensitivity analysis. Furthermore, as the above approach makes a simplifying structural assumption of one hospitalisation event per quarter, it will actually slightly underestimate annual inpatient hospital costs as predicted by the published two-part model of Li et al. 100 Therefore, we also applied a structural sensitivity analysis in which the annual probability of hospitalisation and the conditional annual costs were applied only in the first cycle of whole years.
A further limitation of the inpatient cost models reported by Li et al. 100 is that they predict average costs across all causes of admission. Thus, our base-case model assumes that both CV and other cause-related hospitalisation events incur the same cost on average. A further uncertainty relates to the fact that some inpatient costs will be unrelated to ESRD. However, as Li et al. 100 noted, it is difficult to judge whether individual admissions are related or unrelated, as ESRD is associated with increased risks of hospitalisation across many major causes. The inclusion of all-cause hospitalisation as an outcome in a number of the bioimpedance trials further justifies the inclusion of all-cause hospitalisation events in the baseline model.
Outpatient costs
Total outpatient costs for dialysis and transplant patients were also included in the base-case model. These were taken simply as the observed annual outpatient costs on dialysis and transplant as reported by Li et al. :100 £1202 per year for dialysis patients, and £2388 per year for transplant patients. These were divided by four and applied per quarterly cycle in the model.
Costs of background medications for dialysis patients
Unit costs and the proportion of patients taking blood pressure medicine have been applied to provide the total cost of blood pressure medicine (Table 11). The percentage of patients taking different types of blood pressure medicine was taken from the baseline data of a RCT,127 which recruited dialysis patients from three UK dialysis centres: Stoke-on-Trent, Leeds and Sheffield. For the different classifications of drugs, prices for specific drug names commonly prescribed under each classification (informed by the clinical advisor of the assessment group) were taken from the British National Formulary (BNF). 126 Drugs were costed at the recommended dose as described in the BNF.
| Drug | Unit cost per year (£) | Proportion of patients under each classification | Total average cost (£) | Source |
|---|---|---|---|---|
| ACE inhibitor | 33.33 | 0.211 | 7.03 | Tan et al., 2016;127 BNF126 |
| ARBs | 98.19 | 0.156 | 15.29 | Tan et al., 2016;127 BNF126 |
| Calcium channel blockers | 258.95 | 0.219 | 56.75 | Tan et al., 2016;127 BNF126 |
| Diuretics | 10.82 | 0.487 | 5.26 | Tan et al., 2016;127 BNF126 |
| Beta blockers | 17.14 | 0.248 | 4.25 | Tan et al., 2016;127 BNF126 |
| Alpha blockers | 13.69 | 0.172 | 0.17 | Tan et al., 2016;127 BNF126 |
| Total average cost per year | 88.76 | |||
We further considered the potential impact of incorporating an effect of bioimpedance testing on the use/cost of blood pressure medication. Only two of the existing BCM trials reported on this outcome. 60,63 Luo et al. 63 reported no significant changes in the dose of blood pressure medication in either the control or BCM groups of their trial. Onofriescu et al. 60 reported that there was no statistically significant change from baseline to end of study in the percentage of patients taking blood pressure medication in the control group of their trial, but they did report a significant within-group reduction in the BCM arm (from 66% to 55%). No formal comparison was reported for this outcome. However, as an exploratory analysis, we assessed the impact of assuming a 10% reduction in blood pressure medication use in the bioimpedance assessment arm of our model. To estimate the associated cost reduction, we assumed 68.4% of the cohort would be taking at least one blood pressure medication,128 at an average cost of £129.81 (£88.76/£0.684) per year. The average cost reduction associated with an absolute 10% reduction in the proportion of patients on any blood pressure medication was then estimated:
The unit costs, units per week and proportion of patients taking ESAs were applied to provide an estimate of the total annual cost for ESAs for dialysis patients (Table 12). The proportion of patients taking an ESA was taken from the UK Renal Registry report,99 that is, 87% of those receiving HD and 68% of those receiving PD. The median dose for the corresponding population receiving HD and PD was 7400 international units (IUs) and 4500 IU per week, respectively. Based on opinion obtained from the clinical advisor of the assessment group, the unit cost per IU was derived as the average of the unit costs for epoetin beta (NeoRecormon®, Roche Diagnostics, Hertford, UK) and darbepoetin alfa (Aranesp®, Amgen, Thousand Oaks, CA, USA) as reported in the BNF (£0.00718 per IU). Thus, the total annual cost of ESAs was estimated to be £2403.69 ( = 0.87 × 7400 × 52 × 0.00718) for people receiving HD and £1142 ( = 0.68 × 4500 × 52 × 0.00718) for people receiving PD (see Table 12).
| Cost element | Dialysis | Source | |
|---|---|---|---|
| HD | PD | ||
| Proportion taking ESAs (%) | 87 | 68 | UK Renal Registry 18th Annual Report of the Renal Association, 201599 |
| Dose (IU) per week | 7400 | 4500 | UK Renal Registry 18th Annual Report of the Renal Association, 201599 |
| Unit cost per IU (£) | 0.00718 | 0.00718 | BNF, 2016126 [average price per IU for NeoRecormon® (Roche Diagnostics, Hertford, UK) and Aranesp® (Amgen, Thousand Oaks, CA, USA)] |
| Cost per year (£) | 2403.69 | 1142 | |
Costs of bioimpedance testing/monitoring
The costs of the devices, provided by the companies (Table 13), were annuitised over 5 years using an annual depreciation rate of 3.5%. The cost of the BCM was applied in the base-case analysis because of a lack of clinical effectiveness evidence for the alternative devices. For comparison, we also estimated the costs per patient-year and cost per test for the alternative devices, with identical assumptions about numbers of tests and staff time requirements per patient. Estimated costs of BCM equipment maintenance were provided at two levels: £250 for an annual maintenance contract, and £600 for annual maintenance, including parts and labour. We included the higher-cost maintenance contract in the base-case scenarios, but also assessed the impact of removing the maintenance costs in a sensitivity analysis.
| Device | Purchase price (£) | Expected service life (years) | Cost (£) | |||
|---|---|---|---|---|---|---|
| EAC | Quarterly | Maintenance | Maintenance, including parts and labour | |||
| BCM | 5750 | 5 | 1273.52 | 318.38 | 250 | 600 |
| MultiScan 5000 | 7600 | 5 | 1683.26 | 420.81 | 70a | – |
| BioScan 920-II | 4950 | 5 | 1096.33 | 274.08 | 333b | – |
| InBody S10 | 8100 | 5 | 1794.00 | 448.50 | – | – |
The unit costs of staff involved in bioimpedance testing were taken from the Unit Costs of Health and Social Care 2015 (Table 14). 129 These were applied to estimates of staff time required to conduct and interpret tests. They were also used to place a cost on staff time invested in training in the use of bioimpedance testing. The company responsible for the BCM device indicated that it takes, on average, 5–10 minutes to conduct a test. 4 In the base-case analysis, the time to perform the measurement was assumed to be 7 minutes. The company responsible for the BCM device indicated that they provide free training on its use, taking a half-day to attend. In the base-case analysis, the training was assumed to take 3.5 hours.
| Staff | Cost (£) | Source | ||
|---|---|---|---|---|
| Per patient contact hour | Per contracted hour | Per patient contact, assumed to be 7 minutes in duration | ||
| Grade 6 hospital nurse | 109.00 | 45.00 | 12.72 | PSSRU, 2015129 |
| Consultant medical | 139.65 | 105.00 | 16.29 | PSSRU, 2015; PSSRU, 2010129 |
| Clinical support workera | 52.47 | 21.19 | 6.12 | PSSRU, 2014129 |
| Registrar group (40 hour week)b | 65.17 | 49.00 | 7.60 | PSSRU, 2015129 |
| Hospital dietitian | 45.00 | 34.00 | 5.28 | PSSRU, 2010; PSSRU, 2015129 |
To gain a better understanding of the number of bioimpedance devices required to cover quarterly testing of the dialysis population, a brief questionnaire (see Appendix 11) was sent to the specialist members of the appraisal committee. Six different members responded, although three were from a single large centre covering adults and children. Therefore, we had information on testing practices from three centres covering adults, and two centres covering children (one exclusively). The adult centres were relatively large, with 538–942 dialysis patients in total, compared with the average in England of 456. 99 The impact of adopting lower extremes of device throughput was assessed in the sensitivity analysis.
The questionnaire included questions about centre size (number of HD and PD patients), number of satellite units, current practice with respect to fluid management decisions, and current practice regarding the use of bioimpedance testing. Questions were also included about the estimated level of resource that would be required to conduct quarterly testing of all HD and PD patients for whom the individual’s centre was responsible. Respondents from two of the centres described a situation in which the majority of their patients were already being monitored using bioimpedance testing at least every 3 months. For the third centre, it was noted that bioimpedance testing was not currently performed systematically, but was rather used for selected patients. Consequently, only the anticipated resource use required for quarterly bioimpedence testing was used for this centre.
Details of relevant resources and costs required for quarterly testing, based on the responses from the three adult centres, are summarised in Table 15. Total equipment costs were estimated by multiplying the equivalent annual cost per device by the estimated number of devices required for quarterly monitoring of all dialysis patients across the centres. This was then divided by the total number of patients to estimate the cost of equipment per patient per year, and then further divided by four to estimate the equipment costs per test performed. For example, centre A reported 15 bioimpedance devices to cover a total of 585 patients [(£1273.52 × 15)/585 = £32.65]. The maintenance costs also depended on the reported number of devices required by the centre to cover quarterly testing of its dialysis population. The total estimated annual maintenance cost, with and without parts and labour, was allocated across patients using the same approach as for equivalent annual costs of equipment. The larger centre latterly reported that it did not take out a maintenance contract on its machines, and so we also explored the impact of removing these costs completely.
| Resource use | Centre | ||
|---|---|---|---|
| A | B | C | |
| Patients/equipment | |||
| Number of patients receiving HD | 529 | 788 | 456 |
| Number of patients receiving HD | 56 | 154 | 82 |
| Total number of dialysis patients | 585 | 942 | 538 |
| Assumed number of tests per year | 4 | 4 | 4 |
| Estimated number of devices required | 15 | 5 | 6 |
| Estimated equipment cost per patient-year (£) | 32.65 | 6.76 | 14.20 |
| Estimated equipment cost per patient test, assuming four tests per year (£) | 8.16 | 1.69 | 3.55 |
| Estimated maintenance cost per patient (£) | 6.41 | 1.33 | 2.79 |
| Estimated maintenance cost per patient, including parts and labour (£) | 15.38 | 3.18 | 6.69 |
| Total staff cost (£)a | 12.72 | 12.72 | 12.72 |
| Total staff cost per year, assuming four tests per year at 7 minutes of band 6 nurse time | 51.00 | 51.00 | 51.00 |
| Total cost of interpreting results of test, assuming four tests per year at 5 minutes of consultant time | 9.00 | 9.00 | 9.00 |
| Staff for training, n | |||
| Consultant nephrologists | – | 2 | – |
| Trainee nephrologists | – | 8 | – |
| Nurses | 60 | 32 | 8 |
| Technicians | |||
| Dieticians | 2 | 2 | 5 |
| Others | 20 | – | – |
| Total training cost, assuming a 3.5-hour commitment (£) | 11,171.16 | 7385.00 | 1855.00 |
| Assumed average useful life of training (years) | 5 | 5 | 5 |
| Total EAC of training (£) | 2474.20 | 1635.64 | 410.85 |
| EAC of total training per patient (£) | 4.23 | 1.74 | 0.76 |
Staff costs associated with the time required to conduct each test were estimated based on 7 minutes of direct patient contact with a band 6 nurse [7 × (£109/60) = £12.72]. This was further multiplied by four to estimate the staff costs per patient per year (£12.72 × 4 = £51). The added consultant time required to interpret the findings of each bioimpedance test was assumed to be 5 minutes in the base-case analysis [5 × (105/60) = £8.75]. Total training costs for each centre were estimated based on the number of different grades of staff trained, multiplied by their costs per contract hour and the number of hours of training attended. This total initial investment was spread over 5 years, and the equivalent annual cost was divided by the number of patients in the centre to give a cost per patient per year. For example, for centre A the total training costs were estimated to be £11,171. Annuitised over 5 years, this comes to £2474 and £4.23 per patient per year (2474/585).
Finally, to estimate the total annual cost of adding bioimpedance testing to standard practice, the total cost of consumables (electrodes and patient cards), also based on quarterly testing (Table 16), was added to the estimated device, maintenance, staff time and training costs. The total estimated cost per patient-year for each adult centre, and the average cost per patient across centres, is reported in Table 17 for each device. For the base-case cost-effectiveness analysis, we applied the average cost per patient per year using the BCM, based on the higher maintenance costs (£101.41), and applied a distribution incorporating the lower and upper 95% confidence limits of £85 to £125. Given some uncertainty regarding the ongoing maintenance costs for each device, Table 17 also presents estimated costs per patient-year for each device, excluding all maintenance costs. The costs are very similar across the different devices. In addition to the estimates presented in Table 17, based on responses from dialysis units, we also estimated a cost per patient-year based on responses from two paediatric units. As a result of substantially lower throughput, the costs were significantly higher: £149–349 based on four tests per year and £243–451 based on 12 tests per year. However, these may be overestimated in situations where devices can be shared between adults and children.
| Consumable | Device | |||
|---|---|---|---|---|
| BCM | Multiscan 5000 | Inbody S10 | BioScan 920-II | |
| Electrodes (per test) | 3.00 | 1.10 | Reusable | 0.65 |
| Electrodes (per year) – assuming four tests annually | 12.00 | 4.40 | Reusable | 2.60 |
| Patient cards (per card) – 20 readings | 6.28 | N/A | N/A | N/A |
| Patient cards (per year) – assuming four tests annually | 1.26 | N/A | N/A | N/A |
| Results sheets (per year) – assuming four tests annually | N/A | N/A | 2.08 | N/A |
| Total | 13.26 | 4.40 | 2.08 | 2.60 |
| Device | Centre, annual cost per patient | Average cost per patient across all centres | ||
|---|---|---|---|---|
| A | B | C | ||
| BCM, including maintenance contract without parts and labour | 116 | 83 | 91 | 96.50 |
| BCM, including maintenance cost with parts and labour | 125 | 85 | 95 | 101.41 |
| Multiscan 5000 | 114 | 75 | 85 | 91.22 |
| Inbody S10 | 119 | 74 | 86 | 93.03 |
| Bioscan | 103 | 72 | 79 | 84.51 |
| BCM, excluding any maintenance costs | 110 | 81 | 88 | 92.99 |
| Multiscan 5000, excluding any maintenance costs | 112 | 75 | 84 | 89.88 |
| Inbody S10, excluding any maintenance costs | 114 | 73 | 83 | 90.36 |
| Bioscan, excluding any maintenance costs | 95 | 70 | 75 | 79.85 |
Health measurement and valuation
Health state utility values for patients on dialysis and post transplant were identified from a focused review of the literature. We first identified two systematic reviews of utility data in the context of ESRD incorporating studies relevant to the NICE reference case [reporting EuroQol-5 Dimensions (EQ-5D) data for UK patients]. 130,131 We focused our searches on identifying any more recent studies published following December 2010 (the end date of the search conducted for the most recent systematic review). This identified no further studies reporting EQ-5D values, specifically for UK patients. The systematic review conducted by Wyld et al. 131 included a random-effects metaregression to predict utility based on several factors: treatment (transplant, dialysis, pre-treatment, conservative management) and method of utility elicitation. This model predicted an EQ-5D utility value of 0.64 for patients receiving dialysis and 0.75 for transplant patients. However, a limitation of this study was that some of the EQ-5D scores were measured from mapping algorithms, and the age to which the mean utility estimates applied was not reported. The earlier systematic review by Liem et al. 130 restricted a meta-analysis to those studies using the EQ-5D index directly for each modality of RRT, and reported the pooled mean age and sex distribution for the corresponding pooled EQ-5D values. These are reported in Table 18. The age- and sex-matched EQ-5D UK population norms were calculated using an equation published by Ara and Brazier132 and used to derive age-/sex-adjusted utility multipliers from the raw pooled estimates. 133 The alternative utility values derived from Wyld et al. 131 were applied in a sensitivity analysis, assuming the same age and sex distributions, as reported by Liem et al. 130 for purposes of adjustment.
| Model state | Health state utility value | SE | Age of cohort (years) | Proportion of patients who were male | Age-related population norm | Age-adjusted multiplier for use in model | Adjusted SE | Source |
|---|---|---|---|---|---|---|---|---|
| Stable HD | 0.560 | 0.033 | 60.400 | 0.580 | 0.826 | 0.678 | 0.040 | Liem et al. 2008;130 Ara and Brazier, 2010132 |
| Stable PD | 0.580 | 0.043 | 57.900 | 0.550 | 0.836 | 0.694 | 0.052 | Liem et al. 2008;130 Ara and Brazier, 2010132 |
| Stable post transplant | 0.810 | 0.046 | 51.400 | 0.600 | 0.863 | 0.939 | 0.053 | Liem et al. 2008;130 Ara and Brazier, 2010132 |
| CV events | ||||||||
| MI within 12 months | 0.721 | 0.045 | 65.4 | 0.500 | 0.803 | 0.898 | 0.056 | HSE data (Ara and Brazier, 2010)132 |
| MI history | 0.742 | 0.02 | 65.1 | 0.500 | 0.804 | 0.923 | 0.025 | HSE data (Ara and Brazier, 2010)132 |
| Angina within 12 months | 0.615 | 0.019 | 68.8 | 0.500 | 0.787 | 0.782 | 0.024 | HSE data (Ara and Brazier, 2010)132 |
| Angina history | 0.775 | 0.015 | 68.0 | 0.500 | 0.790 | 0.981 | 0.019 | HSE data (Ara and Brazier, 2010)132 |
| Stroke within 12 months | 0.626 | 0.038 | 67.9 | 0.500 | 0.791 | 0.792 | 0.048 | HSE data (Ara and Brazier, 2010)132 |
| Stroke history | 0.668 | 0.018 | 66.8 | 0.500 | 0.796 | 0.839 | 0.023 | HSE data (Ara and Brazier, 2010)132 |
| Any new CV event within 12 months | – | – | – | – | – | 0.832 | 0.042 | Weighted average of parameters above |
| New CV event history | – | – | – | – | – | 0.931 | 0.022 | Weighted average of parameters above |
A significant proportion of inpatient hospitalisations are associated with CV events in the dialysis population, as assumed in the model. It is reasonable to assume that such events will be associated with short-term and lasting disutility. This is the assumption that is used in CV event models in non-dialysis populations, and the best-recognised source of English EQ-5D data for different CV event histories is the Health Survey for England, as reported by Ara and Brazier. 132 Therefore, these data were used to estimate age-adjusted utility multipliers during the first and subsequent years following different types of CV event. A weighted average of these multipliers for the first and subsequent years was then calculated (based on relative frequency of CV event histories in the dialysis population) and applied to the proportion of the cohort modelled to experience an incident CV event. For example, a cohort of 60-year-old patients, who were stable and receiving HD, would be assigned a utility value of 0.56, whereas a cohort of 60-year-old patients, receiving HD who experienced an incident CV event within 1 year, would be assigned a utility of 0.466 (0.56 × 0.832) and a cohort of 60-year-old patients, who had gone over 1 year since an incident CV event had occurred, would be assigned a utility value of 0.521 (0.56 × 0.931).
Finally, hospitalisations for any other reason were also assumed to incur an acute utility decrement. These were taken from the modelling used to inform the NICE guidelines on PD. 17 In the modelling for these guidelines, a 6% reduction was applied to any dialysis complication. 17 The same 6% reduction is applied in our model for the second half of the 3-month cycle in which complications occur.
Time horizon and discounting of costs and benefits
The modelling was analysed over the lifetime of patients: 30 years for a cohort of 66-year-old patients in the base-case analysis. The time horizon was extended in years for scenario analyses involving younger cohorts. The lifetime horizon was chosen to fully capture any survival or ongoing quality-of-life benefits associated with bioimpedance testing. All future costs and benefits were discounted at a rate of 3.5% per annum.
Analysis
The results of the model are presented in terms of a cost–utility analysis over the lifetime of the simulated cohorts. The bioimpedance-guided fluid management strategy is compared incrementally with standard care, to estimate its incremental costs and QALYs. This is expressed as the ICER. The net benefit framework is used to identify the optimal fluid management strategy at different threshold ratios of willingness to pay per QALY. To characterise the joint uncertainty surrounding point estimates of incremental costs and effects, probabilistic sensitivity analyses were undertaken. All costs were assigned either normal or gamma distributions, utility multipliers were assigned beta distributions and HRs were assigned log-normal distributions using the point estimates and CIs (or SEs) reported in Tables 6, 9, 10 and 18. The parameters of the derived Weibull survival functions were entered deterministically for the dialysis cohort, but as a multivariate normal distribution for post-transplant survival. Distributions for the computed hospitalisation rates and associated costs were assigned SDs set at 10% of the mean. The results of the probabilistic analyses are presented in the form of cost-effectiveness acceptability curves (CEACs). Further deterministic sensitivity analyses were used to address other forms of uncertainty.
The primary analysis was conducted for a mixed cohort of patients receiving HD or PD. Subgroup analyses were conducted to explore any differences in cost-effectiveness by mode of dialysis and, when data allowed, by characteristics of the patient population. The impact of applying different assumptions with respect to testing frequency and throughput was also explored through scenario analyses. Scenario analyses were also used to explore the impact on cost-effectiveness of other sources of uncertainty.
Cost-effectiveness results
The model was first set up to assess the cost-effectiveness of bioimpedance-guided fluid management versus standard care for a mixed cohort of HD (87%) and PD (13%) patients.
The key assumptions of the base model are as follows:
-
The starting age of the cohort is 66 years.
-
Survival with HD and PD treatment is equivalent, and patients do not switch between dialysis modes.
-
Survival to 10 years with dialysis treatment is based on parametric extrapolation of 5-year survival curves, reported for patients in the European renal registry. 102
-
Survival beyond 10 years is estimated by applying published, age-specific, relative risks of death to RRT cohorts compared with general population norms. 99
-
Fixed proportions of the cohort are on a waiting list for transplant, and wait a median of ≈ 3 years, conditional on survival. 107 No transplants occur beyond the age of 75 years.
-
Following graft failure, transplant patients incur costs of dialysis, that is, no further transplants are modelled.
-
Probabilities of all-cause inpatient hospitalisation are estimated by age band, time receiving RRT, RRT modality, sex and comorbidity status, using a published regression based on linked UK Renal Registry – HES data. 100
-
It is assumed that 17.6% of all inpatient hospitalisations are caused by CV events.
-
Health state utility decrements are applied in the acute period for all hospitalisation events, and ongoing health state utility decrements are also applied post CV event-related hospitalisation. 110
-
First-incident CV event-related hospitalisations increase the comorbidity burden on the cohort by one, resulting in an increased risk of hospitalisation in subsequent cycles.
-
Costs of dialysis, treatment for anaemia (ESA), blood pressure medication, all inpatient hospitalisations, all outpatient attendances, renal transplants, post-transplant hospitalisations, outpatient attendances and post-transplant immunosuppression are included in the base model.
-
Costs of CV event-related hospitalisation are assumed to be equal to the average cost across all hospitalisations in dialysis patients (i.e. CV events account for 17.6% of all hospitalisation costs).
-
The incremental cost of monitoring patients using bioimpedance testing is added in the bioimpedance assessment arm of the model (assuming four tests per year).
-
Effects of bioimpedance monitoring on all-cause mortality are applied for 10 years in the model.
-
Effects of bioimpedance monitoring on CV event-related or all-cause hospitalisation are applied over the lifetime of the cohort.
-
Costs and QALYs are discounted at a rate of 3.5% per annum.
The following set of results are based on several alternative base-case scenarios with respect to the possible effects of bioimpedance-guided fluid management on mortality, hospitalisation rates and blood pressure medication use. There is significant uncertainty surrounding the clinical effectiveness of bioimpedance monitoring, as highlighted in the clinical effectiveness chapter. Therefore, the point estimates of incremental cost-effectiveness should be treated with caution.
The main clinical effectiveness scenarios explored are described below and summarised in Table 19.
-
Only the pooled HR (0.689, 95% CI 0.228 to 2.084) for the effect of bioimpedance testing on mortality is applied to the base model. It should be noted that this pooled effect from the meta-analysis (see Figure 8) is not statistically significant, but directionally favours bioimpedance-guided fluid management. Given uncertainty regarding long-term effects, this effect is applied over 10 years in the model (up to cycle 40).
-
A possible effect of bioimpedance testing on non-fatal CV events is added to the effect on mortality in scenario 1. The applied HR (0.9318, 95% CI 0.829 to 1.048) was derived as described in Incorporation of relative treatment effects using published observational data on the prognostic value of PWV for CV events and mortality (see Table 9), combined with the pooled mean reduction in PWV (see Figure 7) observed across the bioimpedance trials included in our systematic review. This scenario is heavily caveated by the application of non-significant effects on PWV, combined with observational prognostic evidence, to model possible effects on health outcomes.
-
This scenario applies the same effect, derived through the pooled reduction in PWV, to both mortality and non-fatal CV events in the model, that is, a HR of 0.9318 is applied to both all-cause mortality and the CV event-related hospitalisation rate. This scenario comes with the same caveats as scenario 2.
-
Scenario 4 replicates scenario 3, but adds a possible effect of bioimpedance-guided fluid management on blood pressure medication use. As described under Costs of background medications for dialysis patients, a possible cost reduction of £12.98 per year was derived from existing trial evidence. Note, however, that this was only observed/reported in one of the RCTs,57 and was not based on a formal adjusted comparison.
-
Scenario 5 uses reported observational associations between baseline hydration status (as measured by the BCM) and mortality and all-cause hospitalisation. The effect of bioimpedance testing is modelled through a plausible reduction in the proportion of the cohort (25%) that is severely overhydrated (ROH of > 15%). 80 Using data reported by Huan-Sheng et al. ,76 it was estimated that the proportion of severely overhydrated patients could be reduced proportionally by 28–38% with bioimpedance-guided fluid management relative to control. This scenario applies a 28% proportional reduction in severe overhydration in the bioimpedance assessment arm of the model.
-
Scenario 6 replicates scenario 5, but applies a 38% proportional reduction in severe overhydration in the bioimpedance assessment arm of the model.
| Scenario | Relative effect on all-cause mortality, HR (95% CI) | Relative effect on non-fatal CV hospitalisation, HR (95% CI) | Effect on blood pressure medication costs (mean reduction), £ | Proportional reduction in severe overhydration (ROH of > 15%) |
|---|---|---|---|---|
| Scenario 1 | 0.689 (0.228 to 2.084) | 1 | 0 | N/A |
| Scenario 2 | 0.689 (0.228 to 2.084) | 0.932 (0.829 to 1.048) | 0 | N/A |
| Scenario 3 | 0.932 (0.829 to 1.048) | 0.932 (0.829 to 1.048) | 0 | N/A |
| Scenario 4 | 0.932 (0.829 to 1.048) | 0.932 (0.829 to 1.048) | –12.98 | N/A |
| Scenario 5 | N/A | N/A | N/A | 0.28 |
| Scenario 6 | N/A | N/A | N/A | 0.38 |
Table 20 presents the model-based cost-effectiveness findings for the main clinical effectiveness scenarios 1–6 (described above). Across the scenarios, bioimpedance-guided fluid management comes out as the more costly strategy, resulting in increased costs to the health service between £4519 and £35,680. These increased costs are accompanied by QALY gains under the alternative effectiveness scenarios between 0.07 and 0.58. The ICERs for bioimpedance testing range from £59,551 to £66,013 per QALY gained. It should be noted that the increased costs associated with bioimpedance-guided fluid management are primarily driven by the high dialysis costs during life-years gained. The cost of bioimpedance testing is modest, adding, on average, £101 per patient-year.
| Strategy | Cost (£) | QALYs | ICER (£) | NMB (£) | ||
|---|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | |||
| 1. Applying the point estimate for the pooled effect of BCM measurement on mortality only (HR = 0.689) | ||||||
| Standard care | 158,124 | – | 2.7014 | – | – | –104,097 |
| BCM | 193,805 | 35,680 | 3.2719 | 0.5706 | 62,532 | –128,366 |
| 2. Applying the point estimate for the pooled effect of BCM measurement on mortality (HR = 0.689), and a linked effect on non-fatal CV events through the pooled reduction in PWV (HR = 0.9318) | ||||||
| Standard care | 158,124 | – | 2.7014 | – | – | –104,097 |
| BCM | 193,497 | 35,373 | 3.2791 | 0.5777 | 61,228 | –127,916 |
| 3. Applying linked effects on mortality and non-fatal CV events through the pooled reduction in PWV (HR = 0.9318) | ||||||
| Standard care | 158,124 | – | 2.7014 | – | – | –104,097 |
| BCM | 165,077 | 6952 | 2.817 | 0.1157 | 60,095 | –108,736 |
| 4. Applying linked effects on mortality and non-fatal CV events through the pooled reduction in PWV (HR = 0.9318), and a 10% reduction in the use of blood pressure medications | ||||||
| Standard care | 158,124 | – | 2.7014 | – | – | –104,097 |
| BCM | 165,014 | 6889 | 2.817 | 0.1157 | 59,551 | –108,673 |
| 5. Modelling effects of bioimpedance testing through associations between severe overhydration and mortality and all cause-hospitalisation (assumes a 28% reduction in severe overhydration) | ||||||
| Standard care | 162,059 | – | 2.77 | – | – | –106,708 |
| BCM | 166,578 | 4519 | 2.84 | 0.07 | 66,013 | –109,858 |
| 6. Modelling effects of bioimpedance-guided fluid management through associations between severe overhydration and mortality and all cause-hospitalisation (assumes a 38% reduction in severe overhydration) | ||||||
| Standard care | 162,059 | – | 2.77 | – | – | –106,708 |
| BCM | 168,019 | 5960 | 2.86 | 0.09 | 64,157 | –110,810 |
As discussed in Costs of renal replacement therapy, others have argued for the exclusion of dialysis costs in the assessment of technologies that aim to extend survival of patients receiving dialysis without influencing the need for dialysis, as these technologies can act as an insurmountable hurdle to demonstrating cost-effectiveness. The results for effectiveness scenarios 1–6 with dialysis costs excluded are therefore provided for comparison in Table 21. It can be noted that this results in a large reduction in the ICERs for bioimpedance testing, ranging between £15,644 and £21,206 per QALY gained. Note, however, that these point estimates are based on uncertain effects incorporated as deterministic point estimates.
| Strategy | Cost (£) | QALYs | ICER (£) | NMB (£) | ||
|---|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | |||
| 1. Applying the point estimate for the pooled effect of BCM measurement on mortality only (HR = 0.689) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 55,579 | 9345 | 3.2719 | 0.5706 | 16,378 | 9859 |
| 2. Applying the point estimate for the pooled effect of BCM measurement on mortality (HR = 0.689), and a linked effect on non-fatal CV events through the pooled reduction in PWV (HR = 0.9318) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 55,272 | 9038 | 3.2791 | 0.5777 | 15,644 | 10,309 |
| 3. Applying linked effects on mortality and non-fatal CV events through the pooled reduction in PWV (HR = 0.9318) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 48,153 | 1919 | 2.817 | 0.1157 | 16,587 | 8188 |
| 4. Applying linked effects on mortality and non-fatal CV events through the pooled reduction in PWV (HR = 0.9318), and a 10% reduction in the use of blood pressure medications | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 48,090 | 1856 | 2.817 | 0.1157 | 16,044 | 8250 |
| 5. Modelling effects of bioimpedance testing through associations between severe overhydration and mortality and all-cause hospitalisation (assumes a 28% reduction in severe overhydration) | ||||||
| Standard care | 47,066 | – | 2.77 | – | – | 8285 |
| BCM | 48,517 | 1452 | 2.84 | 0.07 | 21,206 | 8203 |
| 6.Modelling effects of bioimpedance-guided fluid management through associations between severe overhydration and mortality and all-cause-hospitalisation (assumes a 38% reduction in severe overhydration) | ||||||
| Standard care | 47,066 | – | 2.77 | – | – | 8285 |
| BCM | 48,863 | 1798 | 2.86 | 0.09 | 19,350 | 8346 |
Markov traces
Figures 14 and 15 show the Markov traces for the standard care arm and the bioimpedance assessment arm under clinical effectiveness scenario 3. In the standard care arm, the 10-year mortality for the cohort of 66-year-old patients was 78.8%. This is consistent with the observed 10-year mortality in UK patients receiving RRT surviving beyond 90 days (≈ 68% in 56- to 64-year-olds and ≈ 88% in 65- to 74-year-olds). 99 Assuming a constant effect of bioimpedance-guided fluid management on mortality, the 10-year mortality in the bioimpedance assessment arm was 76.6%. Over the lifetime of the modelled cohort, the gain in undiscounted life expectancy was 0.29 years (6.29 vs. 6.0 years). The modelled lifetime cumulative incidence of any CV hospitalisation event was 46.9% in the bioimpedance assessment arm of the model, and 47.1% in the standard care arm. A total of 7.8% of patients in the bioimpedance assessment arm received a transplant during their lifetime, while the corresponding figure was 7.6% in the standard care arm.
FIGURE 14.
Markov cohort trace: standard care arm (one stage equals 3 months).

FIGURE 15.
Markov cohort trace: BCM measurement arm, under clinical effectiveness scenario 3 (one stage equals 3 months).
Table 22 provides a breakdown of the cumulative costs for the standard care and bioimpedance measurement arms, respectively, under clinical effectiveness scenario 3. The costs were higher across all categories in the bioimpedance measurement arm, as a result of the slight increase in survival. However, it can be noted that it was the additional dialysis costs in extra years that made up 74% of the total incremental cost of the bioimpedance-guided strategy. This same pattern was consistent across all the main clinical effectiveness scenarios (1–6). The actual increase in lifetime costs, as a result of bioimpedance testing, was small (£491 per patient in clinical effectiveness scenario 3).
| Cost category | Treatment arm, cost (£) | Difference in cost (£)between BCM measurement and standard care | |
|---|---|---|---|
| Standard care | BCM measurement | ||
| Cumulative inpatient hospital costs | 21,795 | 22,281 | 486 |
| Cumulative dialysis costs | 111,890 | 116,923 | 5033 |
| Cumulative medication costs | 10,792 | 11,277 | 485 |
| Cumulative outpatient costs | 6076 | 6349 | 273 |
| Cumulative acute transplant cost | 1066 | 1093 | 27 |
| Cumulative post-transplant follow-up costs | 6505 | 6663 | 158 |
| Bioimpedance testing costs | N/A | 491 | 491 |
| Cumulative cost | 158,124 | 165,077 | 6952 |
Deterministic sensitivity analysis
Figures 16 and 17 illustrate the effects of a one-way sensitivity analysis on key model input parameters, with dialysis costs included (see Figure 16) and excluded (see Figure 17). The reference ICERs for both these tornado diagrams reflected clinical effectiveness scenario 3, that is, a HR of 0.9318, inferred through the pooled reduction in PWV, applied to both all-cause mortality and CV event-related hospitalisation.
FIGURE 16.
One-way sensitivity analysis: BCM measurement vs. standard care (clinical effectiveness scenario 3, including dialysis costs). ACM, all-cause mortality; Bioimp, bioimpedance; c, cost; EV, expected value; ICHD, ischaemic coronary heart disease; p, probability; u, utility.
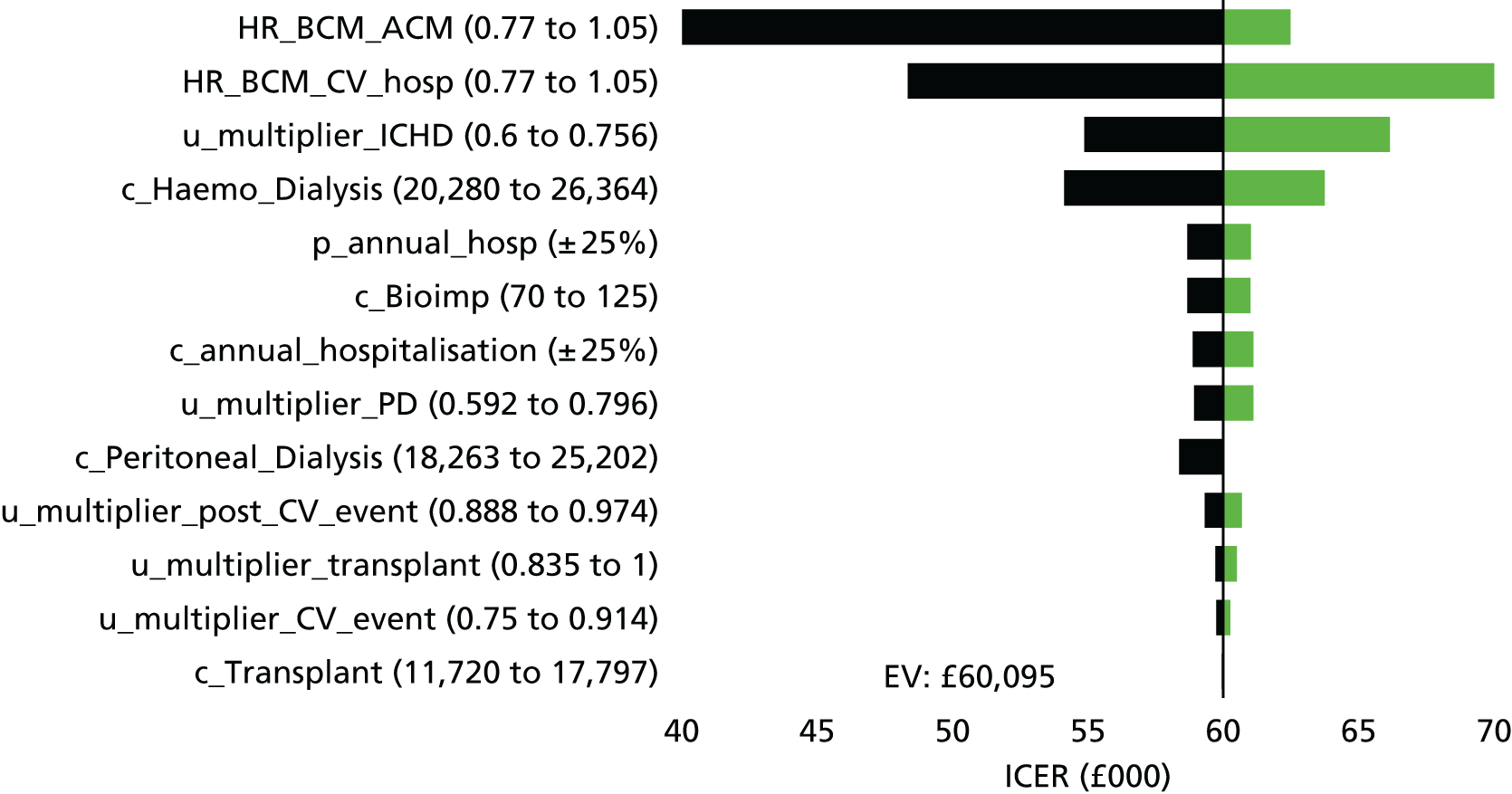
FIGURE 17.
One-way sensitivity analysis: BCM vs. standard care (clinical effectiveness scenario 3, excluding dialysis costs). ACM, all-cause mortality; Bioimp, bioimpedance; c, cost; EV, expected value; ICHD, ischaemic coronary heart disease; p, probability; u, utility.
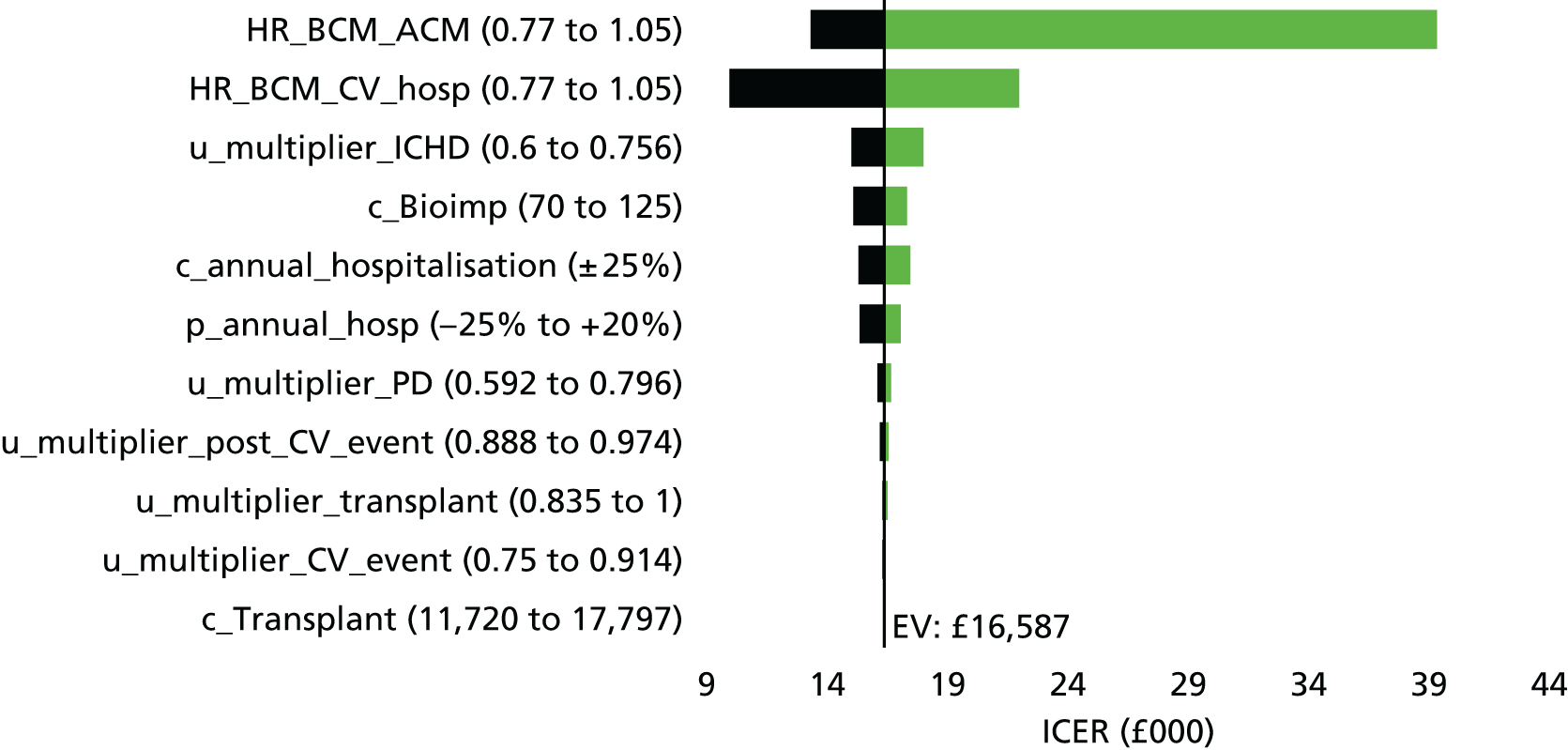
When dialysis costs were included, the ICER for bioimpedance-guided fluid management was most sensitive to changes in the HR for the effect on all-cause mortality. The most favourable ICER (£40,283) occurred when the HR on all-cause mortality was equal to one, as this equalised survival and eliminated the excess dialysis costs incurred in added years.
When dialysis costs were excluded, the ICER remained most sensitive to the HR on all-cause mortality. Results were also moderately sensitive to the utility multiplier for HD, the cost of HD and the HR for CV event-related hospitalisation. However, when dialysis costs were included, the ICER remained well above £30,000 when these parameters were varied within their ranges. Conversely, the ICERs all remained below £30,000 when the parameters were varied individually within their ranges (referent to clinical effectiveness scenario 3) with dialysis costs excluded.
Scenario analyses
Table 23 presents the results of further scenario analyses, referent to clinical effectiveness scenario 3 (HR of 0.9318 applied to all-cause mortality and CV event-related hospitalisation). Unless otherwise stated, these additional scenarios excluded dialysis costs to better illustrate sensitivity (around the cost-effectiveness threshold) when the exclusion of dialysis costs was considered to be appropriate for the purpose of decision-making. Under most of the scenarios with dialysis costs excluded, the ICER for bioimpedance monitoring remained below £30,000, and was most often below £20,000.
| Strategy | Cost (£) | QALYs | ICER (£) | NMB (£) | ||
|---|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | |||
| Base-case scenario 3: applying linked effects on mortality and non-fatal CV events, estimated through the pooled reduction in PWV (HR of 0.9318 applied to both all-cause mortality and CV event-related hospitalisation) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| Bioimpedance-guided fluid management | 48,153 | 1919 | 2.817 | 0.1157 | 16,587 | 8188 |
| Applying an increased cost of monitoring in adults by increasing the number of tests per patient to 12 annually (£229.65) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 48,774 | 2540 | 2.817 | 0.1157 | 21,953 | 7567 |
| 1. Applying the estimated costs of bioimpedance monitoring in paediatric centres with lower throughput (assuming four tests annually)a (£245.32) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 48,850 | 2616 | 2.817 | 0.1157 | 22,609 | 7491 |
| 2. Applying the estimated costs of bioimpedance monitoring in paediatric centres with lower throughput (assuming 12 tests annually)a (£347.06) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 49,342 | 3108 | 2.817 | 0.1157 | 26,866 | 6998 |
| 3. Applying the cost of BioScan for bioimpedance monitoring (£84.51) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BioScan | 48,071 | 1837 | 2.817 | 0.1157 | 15,880 | 8269 |
| 4. Applying the cost of Inbody S10 for bioimpedance monitoring (£90.36) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| Inbody S10 | 48,100 | 1865 | 2.817 | 0.1157 | 16,125 | 8241 |
| 5. Applying the cost of MultiScan 5000 for bioimpedance monitoring (£91.22) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| MultiScan 5000 | 48,104 | 1870 | 2.817 | 0.1157 | 16,161 | 8237 |
| 6. Applying the lowest estimated annual bioimpedance monitoring from Table 15 (£70) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 48,001 | 1767 | 2.817 | 0.1157 | 15,273 | 8340 |
| 7. Applying the highest estimated annual bioimpedance monitoring cost from 15 (£125) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 48,267 | 2033 | 2.817 | 0.1157 | 17,575 | 8073 |
| 8. Applying an alternative lower cost per CV event-related hospitalisation (£1386 per CV event) | ||||||
| Standard care | 44,136 | – | 2.7014 | – | – | 9891 |
| BCM | 46,110 | 1974 | 2.817 | 0.1157 | 17,063 | 10,231 |
| 9. Applying alternative age-adjusted utility multipliers for dialysis and post transplant131 | ||||||
| Standard care | 46,234 | – | 2.9813 | – | – | 13,392 |
| BCM | 48,153 | 1919 | 3.1108 | 0.1295 | 14,822 | 14,062 |
| 10. Assume bioimpedance-guided management results in a 2% improvement in the health state utility over the lifetime of patients receiving dialysis (including dialysis costs) | ||||||
| Standard care | 158,124 | – | 2.7014 | – | – | –104,097 |
| BCM | 165,077 | 6952 | 2.866 | 0.1646 | 42,230 | –107,757 |
| 11. Assume bioimpedance-guided management results in a 2% improvement in the health state utility over the lifetime of patients receiving dialysis (excluding dialysis costs) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 48,153 | 1919 | 2.866 | 0.1646 | 11,656 | 9166 |
| 12. Assume bioimpedance-guided management results in a 5% improvement in the health state utility over the lifetime of patients receiving dialysis (including dialysis costs) | ||||||
| Standard care | 158,124 | – | 2.7014 | – | – | –104,097 |
| BCM | 165,077 | 6952 | 2.9394 | 0.238 | 29,206 | –106,289 |
| 13. Assume bioimpedance-guided management results in a 5% improvement in the health state utility over the lifetime of patients receiving dialysis (excluding dialysis costs) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 48,153 | 1919 | 2.9394 | 0.238 | 8062 | 10,635 |
| 14. Assume bioimpedance-guided management results in a 10% reduction in dialysis costs over the lifetime of patients | ||||||
| BCM | 153,384 | – | 2.817 | – | – | –97,043 |
| Standard care | 158,124 | 4740 | 2.7014 | –0.1157 | Dominated | –104,097 |
| 15. Assume bioimpedance-guided management results in a 5% reduction in dialysis costs over the lifetime of patients | ||||||
| Standard care | 158,124 | – | 2.7014 | – | – | –104,097 |
| BCM | 159,230 | 1106 | 2.817 | 0.1157 | 9,560 | –102,890 |
| 16. Applying an effect only on non-fatal CV events (HR = 0.9318), excluding any effect on mortality (including dialysis costs) | ||||||
| Standard care | 158,124 | – | 2.7014 | – | – | –104,097 |
| BCM | 158,348 | 224 | 2.7069 | 0.0056 | 40,283 | –104,210 |
| 17. Applying a smaller effect on mortality and non-fatal CV events (HR = 0.95 for both) | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 47,757 | 1523 | 2.7853 | 0.084 | 18,135 | 7949 |
| 18. Applying a larger effect of bioimpedance monitoring on both CV events and mortality (0.844); consistent with the cross-sectional main effect of a unit change in PWV reported by Verbeke et al.114 | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 50,163 | 3929 | 2.9791 | 0.2777 | 14,145 | 9419 |
| 19. Applying differential effects on mortality (HR = 0.95) and non-fatal CV events (HR = 0.844), including dialysis costs | ||||||
| Standard care | 158,124 | – | 2.7014 | – | – | –104,097 |
| BCM | 162,903 | 4778 | 2.7946 | 0.0933 | 51,222 | –107,010 |
| 20. Applying differential effects on mortality (HR = 0.95) and non-fatal CV events (HR = 0.844), excluding dialysis costs | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 47,359 | 1125 | 2.7946 | 0.0933 | 12,054 | 8534 |
| 21. Excluding all non-CV event-related causes of hospitalisation form the analysis, including dialysis costs | ||||||
| Standard care | 144,951 | – | 2.7138 | – | – | –90,676 |
| BCM | 151,315 | 6364 | 2.83 | 0.1163 | 54,726 | –94,714 |
| 22. Applying no effects of bioimpedance monitoring beyond 3 years; HR of 0.9318 for all-cause mortality and CV event-related hospitalisation up to 3 years | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 47,531 | 1297 | 2.7663 | 0.065 | 19,963 | 7795 |
| 23. Applying no effects of bioimpedance monitoring beyond 3 years; HR of 0.95 for all-cause mortality and CV event-related hospitalisation up to 3 years | ||||||
| Standard care | 46,234 | – | 2.7014 | – | – | 7793 |
| BCM | 47,308 | 1074 | 2.7488 | 0.0474 | 22,642 | 7667 |
Under only a few scenarios did the ICER for bioimpedance monitoring fall close to or below £30,000 when dialysis costs were included, when assuming that bioimpedance testing would result in a 5% or 10% reduction in dialysis costs (scenarios 15 and 16) over the lifetime of patients and when it was assumed that bioimpedance-guided fluid management would result in a 5% increase in health state utility, maintained over the lifetime of all dialysis patients (scenario 13). However, there are very few data available to justify these possible scenarios.
Subgroup analysis
Table 24 presents the results of the analysis that considered key subgroups of the dialysis population.
| Strategy | Cost (£) | QALYs | ICER (£) | NMB (£) | ||
|---|---|---|---|---|---|---|
| Mean cost | Incremental | Mean | Incremental | |||
| 1. People receiving dialysis who have comorbidities and higher hospitalisation ratesa | ||||||
| Standard care | 47,021 | – | 2.6974 | – | – | 6927 |
| BCM | 48,961 | 1940 | 2.813 | 0.1156 | 16,780 | 7299 |
| 2. People receiving dialysis with no comorbidities and lower hospitalisation ratea | ||||||
| Standard care | 42,638 | – | 2.7166 | – | – | 11,693 |
| BCM | 44,456 | 1818 | 2.8325 | 0.116 | 15,675 | 12,195 |
| 3. People receiving HD (start age of 67 years; 3 years receiving dialysis) | ||||||
| Standard care | 45,833 | – | 2.5803 | – | – | 5773 |
| BCM | 47,763 | 1930 | 2.6933 | 0.113 | 17,078 | 6103 |
| 4. People receiving PD (start age of 64 years; 2 years receiving dialysis) | ||||||
| Standard care | 53,237 | – | 3.3991 | – | – | 14,745 |
| BCM | 55,021 | 1783 | 3.5183 | 0.1192 | 14,959 | 15,346 |
| 5. Mixed cohort of patients aged 55 years receiving HD/PD | ||||||
| Standard care | 80,080 | – | 4.7224 | – | – | 14,368 |
| BCM | 82,251 | 2171 | 4.8502 | 0.1278 | 16,986 | 14,753 |
| 6. Patients listed for a transplanta | ||||||
| Standard care | 87,370 | – | 4.1844 | – | – | –3682 |
| BCM | 89,563 | 2193 | 4.2891 | 0.1047 | 20,950 | –3781 |
| 7. Patients not listed for a transplanta | ||||||
| Standard care | 39,807 | – | 2.4696 | – | – | 9586 |
| BCM | 41,683 | 1876 | 2.587 | 0.1174 | 15,980 | 10,058 |
| 8. Chronically overhydrated patients only, at increased risk of mortality and all-cause hospitalisation, using modelling structure and assumptions of clinical effectiveness scenario 6 (38% reduction of chronic overhydration with bioimpedance monitoring relative to standard practice); dialysis costs included | ||||||
| Standard care | 119,413 | – | 2.04 | – | – | –78,613 |
| BCM | 168,019 | 48,606 | 2.86 | 0.82 | 59,382 | –110,819 |
| 9. Chronically overhydrated patients only, at increased risk of mortality and all-cause hospitalisation, using modelling structure and assumptions of clinical effectiveness scenario 6 (38% reduction of chronic overhydration with bioimpedance monitoring relative to standard practice); dialysis costs excluded | ||||||
| Standard care | 36,932 | – | 2.04 | – | – | 3868 |
| BCM | 48,863 | 11,931 | 2.86 | 0.82 | 14,576 | 8337 |
Separate analyses were considered by comorbidity status (none/at least one), dialysis modality (HD/PD), starting age of the cohort (55 years rather than 64 years) and transplant listing (yes/no). For comparability, all of these analyses were conducted with clinical effectiveness scenario 3 (HR of 0.912 for the effect of bioimpedance monitoring on mortality and CV hospitalisation). Finally, we also conducted a subgroup analysis using the overhydration states in the model (clinical effectiveness scenario 6), with the effect of bioimpedance testing modelled through a plausible proportional reduction in severe overhydration (ROH of > 15%), reducing the risk of all-cause mortality and CV event-related hospitalisation. This analysis focused on the subgroups that were identified as being severely overhydrated at baseline, and assumed a 38% reduction over the follow-up period (see Table 24, scenarios 8 and 9).
These analyses did not reveal any large differences in cost-effectiveness by subgroups. The ICER was slightly higher in the subgroup on a waiting list for a transplant, as the patients spent less time on dialysis and so benefited less from the modelled reduction in all-cause mortality and CV event-related hospitalisation conferred by bioimpedance-guided fluid management. In the scenario focusing on the severely overhydrated subgroup, the ICER was ≈ £5000 lower than in the corresponding base case for that clinical effectiveness scenario, but when dialysis costs are included the ICER remains well above the accepted thresholds (£59,318), as it does for all the subgroups (results not shown).
For comparison with the deterministic results in Tables 20 and 21, Tables 25 and 26 present the results for clinical effectiveness scenarios 1, 3 and 4 based on 1000 probabilistic iterations of the model, with dialysis costs included (see Table 25) and excluded (see Table 26). The point estimates of the ICERs are very similar to the deterministic ICERs. The final columns in Tables 25 and 26 indicate the probability of standard practice or bioimpedance testing being the preferred strategy, given a willingness to pay of £20,000 per QALY gained. With dialysis costs included, the probability of bioimpedance testing being cost-effective is ≈26% in scenario 1 and < 13% in scenarios 3 and 4.
| Strategy | Cost (£) | QALYs | ICER (£) | Probability of being cost-effective at the £20,000 threshold | ||
|---|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | |||
| 1. Clinical effectiveness scenario 1; applying the point estimate for the pooled effect of BCM on mortality only | ||||||
| Standard care | 159,712 | – | 2.6868 | – | – | 0.737 |
| BCM | 191,748 | 32,036 | 3.1875 | 0.5007 | 63,983 | 0.263 |
| 2. Clinical effectiveness scenario 3; applying linked effects on mortality and non-fatal CV events through the pooled reduction in PWV (HR = 0.9318 for both CV events and mortality) | ||||||
| Standard care | 157,558 | – | 2.6952 | – | – | 0.875 |
| BCM | 164,632 | 7074 | 2.8138 | 0.1186 | 59,666 | 0.125 |
| 3. Clinical effectiveness scenario 4; applying linked effects on mortality and non-fatal CV events through the pooled reduction in PWV (HR = 0.9318 for both CV events and mortality), and a 10% reduction in BP medications use | ||||||
| Standard care | 158,312 | – | 2.6887 | – | – | 0.87 |
| BCM | 165,217 | 6906 | 2.8038 | 0.1151 | 59,981 | 0.13 |
| Strategy | Cost (£) | QALYs | ICER (£) | Probability of being cost-effective at the £20,000 threshold | ||
|---|---|---|---|---|---|---|
| Mean | Incremental | Mean | Incremental | |||
| 1. Clinical effectiveness scenario 1, applying the point estimate for the pooled effect of BCM on mortality only | ||||||
| Standard care | 45,967 | – | 2.7003 | – | – | 0.328 |
| BCM | 53,907 | 7940 | 3.1884 | 0.4881 | 16,269 | 0.672 |
| 2. Clinical effectiveness scenario 3, applying linked effects on mortality and non-fatal CV events through the pooled reduction in PWV (HR = 0.9318 for both CV events and mortality) | ||||||
| Standard care | 45,966 | – | 2.6905 | – | – | 0.387 |
| BCM | 47,836 | 1871 | 2.8063 | 0.1158 | 16,150 | 0.613 |
| 3. Clinical effectiveness scenario 4, applying linked effects on mortality and non-fatal CV events through the pooled reduction in PWV (HR = 0.9318 for both CV events and mortality), and a 10% reduction in the use of blood pressure medications | ||||||
| Standard care | 46,190 | – | 2.6873 | – | – | 0.369 |
| BCM | 48,004 | 1814 | 2.8017 | 0.1144 | 15,859 | 0.631 |
With the dialysis costs excluded, the probability of bioimpedance testing being cost-effective at a threshold of £20,000 increased to ≈61–67% across effectiveness scenarios 1, 3 and 4 (see Table 26). There remains a high degree of uncertainty inherent in the approach required to link possible effects of bioimpedance monitoring on arterial stiffness (PWV) to effects on mortality and non-fatal CV events, which is not fully captured in the probabilistic model. Thus, the probability of cost-effectiveness in scenarios 3 and 4 may give a somewhat unrealistic impression of precision.
For further comparison, the incremental cost-effectiveness scatterplots for bioimpedance testing versus standard practice, and the corresponding CEACs, are presented in Figures 18–21 for scenarios 1 and 3 (including dialysis costs). The corresponding scatterplots and CEACs with dialysis costs excluded are presented in Figures 22–25.
FIGURE 18.
Incremental cost-effectiveness scatterplot: BCM vs. standard care (clinical effectiveness scenario 1, including dialysis costs).
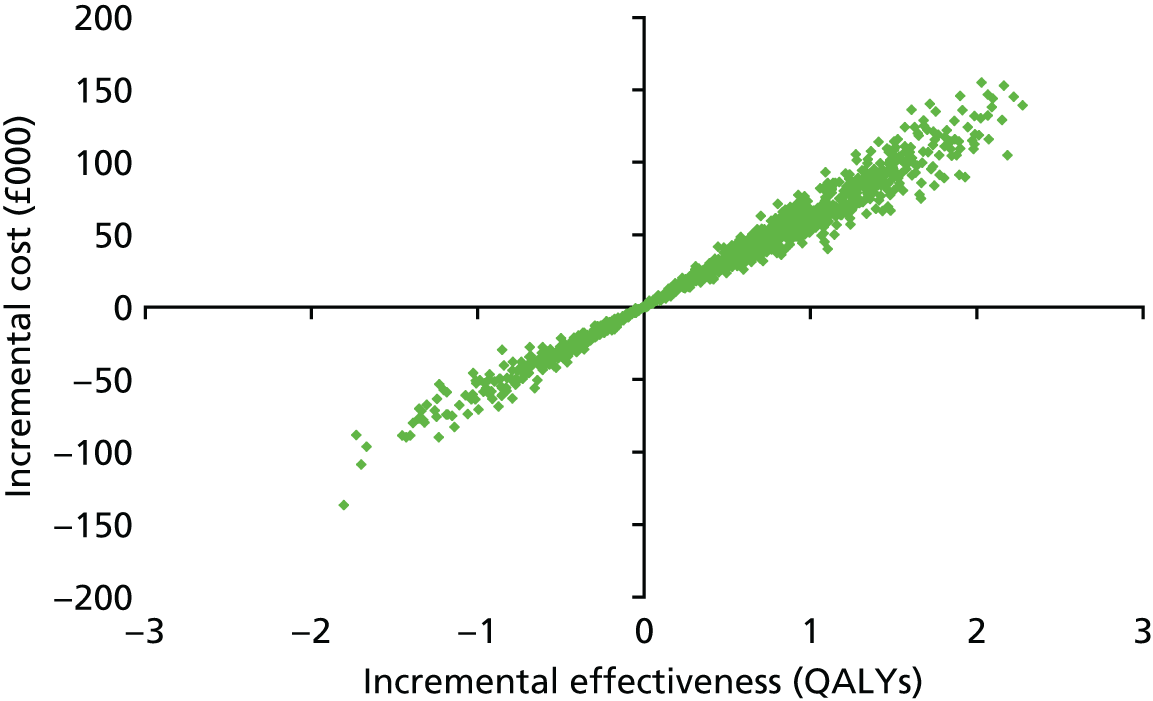
FIGURE 19.
Cost-effectiveness acceptability curves: BCM vs. standard care (clinical effectiveness scenario 1, including dialysis costs). WTP, willingness to pay.

FIGURE 20.
Incremental cost-effectiveness scatterplot: BCM vs. standard care (clinical effectiveness scenario 3, including dialysis costs).

FIGURE 21.
Cost-effectiveness acceptability curves: BCM vs. standard care (clinical effectiveness scenario 3, including dialysis costs). WTP, willingness to pay.
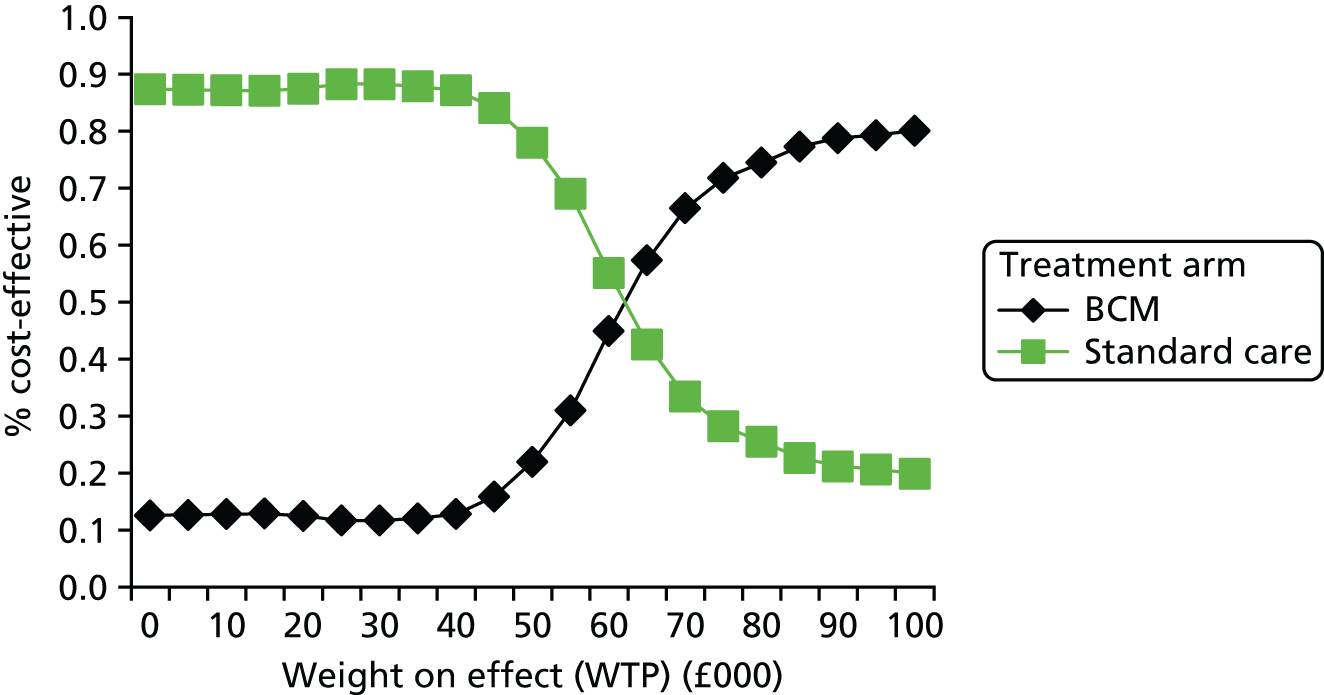
FIGURE 22.
Incremental cost-effectiveness scatterplot: BCM vs. standard care (clinical effectiveness scenario 1, excluding dialysis costs).

FIGURE 23.
Cost-effectiveness acceptability curves: BCM vs. standard care (clinical effectiveness scenario 1, excluding dialysis costs). WTP, willingness to pay.
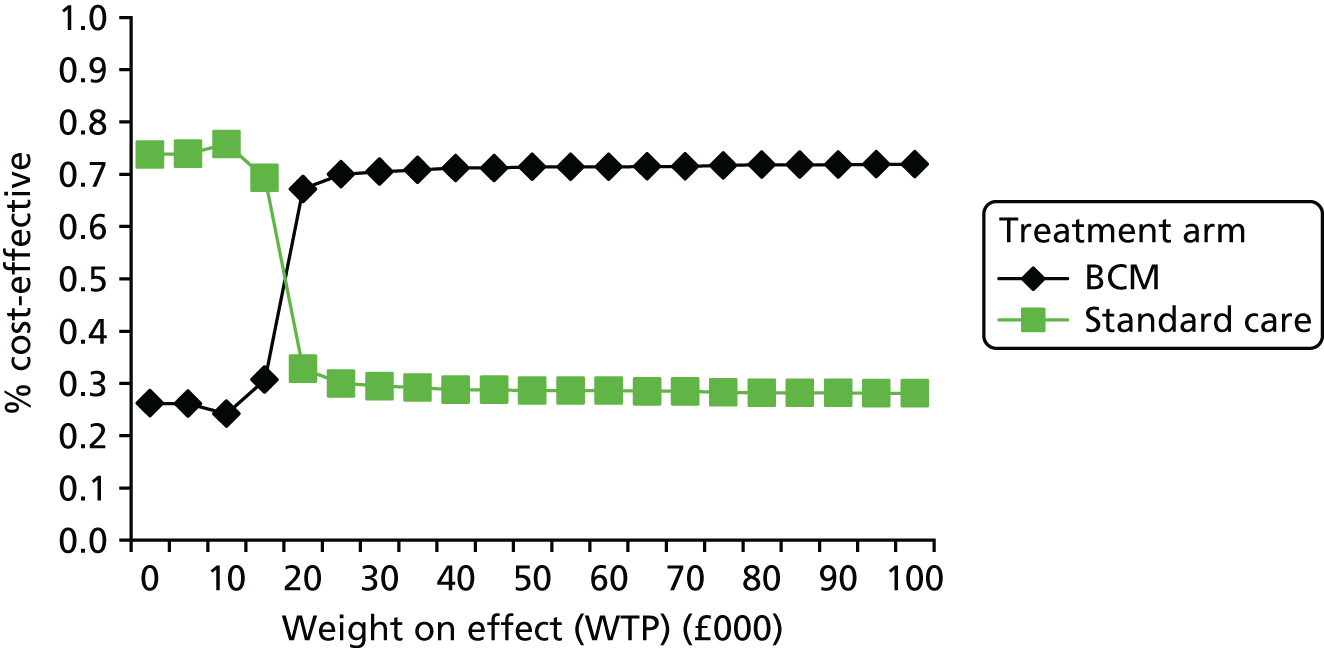
FIGURE 24.
Incremental cost-effectiveness scatterplot: BCM vs. standard care (clinical effectiveness scenario 3, excluding dialysis costs).

FIGURE 25.
Cost-effectiveness acceptability curves: BCM vs. standard care (clinical effectiveness scenario 3, excluding dialysis costs). WTP, willingness to pay.

Interpretation of the cost-effectiveness results
The cost-effectiveness results above are based on limited evidence for the effects of bioimpedance-guided fluid management on mainly surrogate end points (PWV and hydrations status). There is very limited high-quality evidence available by which to link intervention-induced changes in these surrogate end points to changes in health outcomes. Therefore, the indirect/linked modelling scenarios rely on observational associations to estimate possible effects of bioimpedance-guided fluid management on final health outcomes. It should also be noted that the pooled estimate of the effect on PWV is non-significant and based on data from only two trials, showing inconsistent results. As a consequence, the results of the cost-effectiveness modelling are somewhat speculative and subject to considerable uncertainty, which is not fully reflected in the probabilistic sensitivity analysis.
Nevertheless, the results reveal some useful insights. Given the high costs of dialysis, it is unlikely that bioimpedance-guided management will be cost-effective against the accepted thresholds (£20,000–30,000 per QALY gained) if it reduces mortality with these costs included in the model. Table 22 indicates that dialysis costs in additional years make up 74% of the incremental cost of bioimpedance-guided management under clinical effectiveness scenario 3 (a modest and equal effect on both mortality and CV event-related hospitalisation). Further scenario analyses suggest that the effect on mortality would have to be accompanied by a 5% reduction in dialysis costs over the lifetime of patients for the ICER to drop below £20,000 under clinical effectiveness scenario 3. Alternatively, with an accompanying 5% improvement in quality of life over the lifetime of patients, the ICER drops close to £30,000. With greater effects on mortality (and dialysis costs included), the magnitude of these accompanying effects would also have to increase to offset the greater increases in dialysis costs in extra years. The ICER for bioimpedance-guided fluid management also drops substantially, with dialysis costs included, when no effect on mortality is assumed, but an effect on the CV event-related hospitalisation rate is retained. This all but eliminates the incremental cost associated with the bioimpedance-guided strategy (reducing it to £224), but also greatly reduces the QALY gain that comes primarily from increased survival in the base-case clinical effectiveness scenarios. The plausibility of these additional scenarios is uncertain, given the available clinical evidence.
It can also be noted from the modelled scenarios that when dialysis costs are excluded from the model, the effects of bioimpedance-guided management do not need to be large for the ICER to remain below £20,000. The added cost of testing patients quarterly with bioimpedance spectroscopy is low (conservatively estimated to be ≈£100 per patient-year), and so relatively small effects on mortality and/or non-fatal CV events will compensate for this when dialysis costs in additional years are not included. That said, the modelled effects of bioimpedance monitoring are subject to considerable uncertainty, and so probabilities of cost-effectiveness at a willingness-to-pay threshold of £20,000 per QALY only reach ≈61–67%, even with dialysis costs excluded.
Chapter 4 Discussion
Clinical effectiveness
This assessment is based on six RCTs (analysing 1039 participants) and eight non-randomised studies (analysing 4915 participants) evaluating the use of the BCM for fluid management in people with CKD receiving dialysis. None of the studies involved paediatric populations or the other multiple-frequency bioimpedance devices specified in the protocol. The results of the assessment indicate that:
-
of the five RCTs, one was rated as being at a high risk of bias, and the remaining four trials were rated as being at an unclear risk of bias
-
four RCTs enrolled patients receiving HD and one RCT enrolled patients receiving PD
-
all five RCTs were conducted in countries other than the UK and all involved adult populations
-
absolute overhydration and ROH were significantly lower in the BCM group than in the standard clinical assessment group (WMD –0.44, 95% CI –0.72 to –0.15, p = 0.003, I2 = 49%; and WMD –1.84, 95% CI –3.65 to –0.03, p = 0.05, I2 = 52%, respectively)
-
compared with standard clinical methods, the use of the BCM reduced SBP, but the difference did not reach the level of statistical significance (mean difference –2.46, 95% CI –5.07 to 0.15; p = 0.06, I2 = 0%).
-
the pooled effects of bioimpedance monitoring on arterial stiffness (mean difference –1.18, 95% CI –3.14 to 0.78; p = 0.24, I2 = 92%) and mortality (HR 0.69, 95% CI 0.23 to 2.08; p = 0.51) were not statistically significant
-
there was a difference in absolute hydration at follow-up between patients receiving HD and patients receiving PD, but the difference was not large enough to suggest a significant effect of type of dialysis
-
no evidence was found regarding the use of the other devices specified for this assessment in the relevant clinical population
-
CV events and hospitalisation were reported by few studies and not in a consistent way
-
patient-reported outcomes were lacking in the included studies
-
evidence regarding adverse events associated with hypotensive episodes was mixed; some studies reported no difference between the groups at follow-up, but there was evidence that patients in the bioimpedance assessment group experienced significantly more cramping, chest tightness and headaches, but significantly less hypotension and itching, than those in the standard clinical methods group
-
caution should be applied in using bioimpedance to assess outcomes, such as absolute overhydration and ROH, as bias in favour of bioimpedance may be introduced
-
although none of the included studies directly assessed residual renal function as per the European Renal Association guidelines, two studies reported a tendency towards a greater decrease in urine output in patients randomised to bioimpedance-guided fluid management; this is a potential harm that requires further investigation
-
conversely, careful use of bioimpedance monitoring, to avoid underhydration in new dialysis patients, may reduce the loss of residual renal function; the ongoing BISTRO (Bio-Impedance Spectroscopy To maintain Renal Output) trial94 is currently investigating this relationship.
Comparison with other reviews
We have reinforced and extended the findings of the 2015 CADTH’s review,53 which included narrative descriptions of the studies by Onofriescu et al. 60 and Ponce et al. ,61 and concluded that the evidence base was limited, but there was a trend of decreased blood pressure and fluid overload in people whose management was guided by the BCM. The authors were unable to comment on the effects these changes would have on ‘hard end points’, such as hospitalisation and death. Furthermore, the original CADTH review, published in 2014,53 concluded that bioimpedance-based fluid management was associated with signs of better blood pressure control than the standard of care. Our study addressed some of these questions by conducting meta-analyses of both intermediate outcomes (SBP, arterial stiffness and absolute and relative fluid overload) and a clinical outcome (mortality). Notably, our assessment also included one study involving people receiving PD. Our study is also the first to explore the potential cost-effectiveness of bioimpedance-guided fluid management by using a decision-modelling framework to link estimated effects on surrogate end points to possible effects on health outcomes and health service costs. No other reviews or models were identified for comparison.
Cost-effectiveness
A cost-effectiveness Markov model was developed to simulate the progression of the prevalent dialysis cohort through a set of mutually exclusive health states capturing mortality, CV event-related and other causes of hospitalisation and transplantation (for those listed). The model included costs to the health service of providing dialysis treatment, inpatient and outpatient hospital costs, transplant costs, post-transplant follow-up and immunosuppressant costs and costs of dialysis following transplant graft failure. Health state utility multipliers were identified and incorporated for the dialysis and post-transplant states, allowing cumulative QALYs to be estimated. Further proportional reductions in health state utility were modelled in the short term for all hospitalisation events, and in the long term following incident CV hospitalisation events.
The added costs and possible effects of bioimpedance-guided fluid management were added to the baseline model, and the cumulative costs and QALYs were simulated over the lifetime of the cohorts under standard care and the bioimpedance-guided strategy. The base-case effectiveness scenarios modelled proportional reductions in all-cause mortality and CV event-related or all-cause hospitalisation with the bioimpedance-guided strategy. Given the limited direct evidence from the clinical effectiveness review, these effects were generally estimated by linking effects on surrogate end points [arterial stiffness (PWV), hydration status] to effects on the final outcomes using secondary published sources.
The costs and effects of the bioimpedance-guided strategy were compared incrementally to standard care under several possible clinical effectiveness scenarios. The impact of including and excluding dialysis costs was also explored for each of these.
Key findings from the analyses are as follows.
-
Under all of the main effectiveness scenarios, the ICER for bioimpedance-guided fluid management remained well above accepted thresholds for cost-effectiveness when dialysis costs were included in the model, and this was a result of the high costs of dialysis in the added years under the bioimpedance strategy.
-
For bioimpedance-guided management to appear cost-effective with dialysis costs included (assuming an effect on mortality), it would also have to provide a significant reduction in dialysis costs across the lifetime of patients or a constant percentage improvement in the health state utility of patients receiving dialysis.
-
There is little evidence to justify the modelled scenarios under which bioimpedance-guided fluid management becomes cost-effective (against standard thresholds) when dialysis costs are included in the model.
-
When dialysis costs are excluded from the model, the effects of bioimpedance-guided management do not need to be great for the ICER to remain below £20,000.
-
The added monitoring costs associated with the strategy are small (conservatively estimated to be ≈£100 per patient-year), and so relatively small effects on mortality and/or non-fatal CV events justify the added costs. That said, the costs in added years remain quite substantial, given the high background rates of other-cause hospitalisation.
-
With considerable uncertainty surrounding the modelled effect estimates, probabilities of cost-effectiveness at accepted thresholds remain low (≈61–67% at a willingness to pay of £20,000 per QALY gained).
Strength and limitations of the assessment
This assessment has been conducted in accordance with current standards and recommendations and the methods were specified a priori in a research protocol. Comprehensive literature searches of the major electronic databases were conducted, all potentially eligible studies were assessed for inclusion in the review and the methodological quality of all included studies was assessed using the recommended risk-of-bias tools. Despite these efforts, it is still possible that some relevant evidence may have been missed, although any omissions are likely to be minimal.
The economic model was able to draw on UK and European registry data to inform baseline mortality, all-cause hospitalisation rates and the likelihood of progression to transplant. Systematic searches were undertaken to identify suitable sources for other parameters in the model, such as the health state utility weights, and costs of RRT were based on standard NHS sources. A short survey of centres with expertise in using bioimpedance testing was carried out to get an accurate picture of the likely incremental cost of adopting it as an adjunct to standard clinical practice. There are limitations relating to the availability of evidence to inform clinical effects of bioimpedance testing in the model, and several simplifying assumptions had to be made in light of the data available to inform baseline probabilities.
The following limitations also need to be acknowledged:
-
We were able to include only studies involving the BCM because of a lack of published evidence of the effectiveness of the other specified bioimpedance devices. As the generalisability of the effects of bioimpedance devices has yet to be determined, we cannot generalise our findings across the devices beyond the BCM.
-
The longest follow-up period in the included RCTs was 2.5 years and the long-term effectiveness of the BCM in this population is yet to be established.
-
Overall risk of bias was rated as being unclear or high in the majority of included trials, with only one trial being rated as having a low risk of bias.
-
Units of measurement of some reported outcomes (e.g. hospitalisation) varied across trials and hampered the possibility of synthesising data.
-
Some clinically relevant outcomes (e.g. incidence of CV events, residual renal function, achievement of target weight) were lacking or not consistently reported.
-
We were unable to conduct the planned subgroup analyses, but were able to make some comparisons of the outcomes of people receiving HD and those receiving PD.
-
The majority of RCTs excluded patients with amputations, cardiac pacemakers and defibrillators. These exclusions further limit the generalisability of the current findings.
-
Frequency of assessment using the BCM varied across trials and the optimal frequency of assessment is yet to be determined.
-
With respect to the economic model, baseline risks of CV event-related hospitalisation had to be estimated as a set proportion of all-cause hospitalisation.
-
Plausible effects in the cost-effectiveness model had to be informed by linking effects on surrogate end points to effects on final health outcomes.
-
To keep the model manageable, and in keeping with the available data, some simplifying assumptions had to be made:
-
Mortality and hospitalisation rates could not be linked to certain explanatory variables and event histories in the model, limiting our ability to explore heterogeneity in cost-effectiveness.
-
It was difficult to capture the long-term health state utility impact of recurrent hospitalisation events, partly because of the constraints of the Markov modelling approach, and partly because of a lack of available data to inform the cost and utility impact of recurrent events.
-
-
With many differences between adults and paediatric dialysis patients, and a complete lack of evidence for the clinical effectiveness of bioimpedance-guided fluid management in children, we were not able to assess cost-effectiveness in children. As well as requiring data on clinical effectiveness in children, a different baseline cost-effectiveness model would also be required, including different mortality and hospitalisation rates, different costs and utilities and greater structural complexity, to allow for extrapolation over a much longer time horizon (e.g. allowing for multiple transplants over the lifetime of the cohort).
-
We were able to obtain a reasonable estimate of what it would cost to monitor children with bioimpedance spectroscopy, as a result of a lower throughput in paediatric centres and the need for more frequent testing. Although the estimated cost is substantially higher than in adults, the cost-effectiveness findings in adults were not found to be sensitive to increases in the monitoring cost to this level.
Uncertainties
-
Current evidence focuses exclusively on the use of BCM and not on other multiple-frequency bioimpedance devices.
-
The identified RCTs were all conducted outside the UK and the applicability of the results to the UK population is uncertain, with the greatest uncertainty relating to the comparability of the standard clinical assessments in these trials.
-
Included studies focused exclusively on adult populations. Therefore, our findings are not generalisable to paediatric populations.
-
The main uncertainty in the cost-effectiveness modelling relates to the plausibility of the modelled effects, which were extrapolated from mainly non-significant effects on surrogate end points [arterial stiffness (PWV) and blood pressure] using other external sources of evidence. Critically, there were no ideal sources of evidence to link intervention-induced changes in the relevant surrogates to effects on mortality and hospitalisation rates. Therefore, possible effects were informed by reference to cross-sectional prognostic studies, leading to great uncertainty in the robustness of the cost-effectiveness findings.
Acknowledgements
The authors are grateful to Lara Kemp for her secretarial support. The authors would also like to thank the members of the specialist committee assembled to support this assessment: Dr Andrew Davenport (Royal Free Hospital, London), Dr Simon Roe (Nottingham University Hospitals NHS Trust), Dr Elizabeth Lindley (St James’s University Hospital), Dr Wesley Hayes (Great Ormond Street Hospital), Ms Joanne Prince (Central Manchester University Hospitals NHS Foundation Trust), Mr Nick McAleer (Royal Devon & Exeter NHS Foundation Trust), Dr Kay Tyerman (Leeds General Infirmary), Dr Graham Woodrow (St James’s University Hospital) and Mr Paul Taylor (lay specialist committee member). The Health Services Research Unit, Health Economics Research Unit and Institute of Applied Health Sciences, University of Aberdeen are all core funded by the Chief Scientist Office of the Scottish Government Health Directorates.
Contributions of authors
Graham Scotland (Senior Research Fellow) developed the economic model and conducted the economic evaluation.
Moira Cruickshank (Research Fellow) reviewed and summarised the evidence on the clinical effectiveness of the bioimpedance devices.
Elisabet Jacobsen (Research Assistant) reviewed the evidence on the cost-effectiveness of the bioimpedance devices and contributed to the economic evaluation under the supervision of Graham Scotland (Senior Health Economist).
David Cooper (Research Fellow) double-checked the data extracted from the included randomised studies and conducted all statistical analyses.
Cynthia Fraser (Senior Information Specialist) developed and ran the literature searches and provided information support.
Michal Shimonovich (Research Assistant) contributed to the data extraction process and to the assessment of the risk of bias of included studies with assistance from Moira Cruickshank (Research Fellow) and from Miriam Brazzelli (Senior Research Fellow).
Angharad Marks (Clinician Scientist & Honorary Consultant Nephrologist) provided expert advice on the clinical aspects of this assessment.
Miriam Brazzelli (Senior Research Fellow) oversaw and co-ordinated all aspects of this assessment.
All authors contributed to the writing of this report and approved its final version.
Data sharing statement
Most technical data are included as appendices to this report. Additional data may be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- NHS Choices . Chronic Kidney Disease 2016. www.nhs.uk/conditions/Kidney-disease-chronic/Pages/Introduction.aspx (accessed November 2016).
- MyKidney . Choosing the Treatment Option that’s Right for You 2016. www.mykidney.org/TreatmentOptions/Your-Options-1-3.aspx (accessed November 2016).
- NHS Choices . Dialysis 2015. www.nhs.uk/conditions/Dialysis/Pages/Introduction.aspx (accessed November 2016).
- The BCM – Body Composition Monitor for Managing Fluid in People Having Dialysis. London: NICE; 2015.
- Chronic Kidney Disease in Adults: Assessment and Management. Clinical Guideline [CG182]. London: NICE; 2015.
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137-47. https://doi.org/10.7326/0003-4819-139-2-200307150-00013.
- Ley, Liu JJ, Wong DSM, Tan SHC, Tavintharan S, Sum CF, et al. Association of circulating irisin with renal function and body composition in type 2 diabetes mellitus. SHBC 2013;42.
- Stevens LA, Levey AS. Current status and future perspectives for CKD testing. Am J Kidney Dis 2009;53:17-26. https://doi.org/10.1053/j.ajkd.2008.07.047.
- Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis 2007;50:169-80. https://doi.org/10.1053/j.ajkd.2007.06.013.
- Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985;33:278-85. https://doi.org/10.1111/j.1532-5415.1985.tb07117.x.
- Parmar MS. Chronic renal disease. BMJ 2002;325:85-90. https://doi.org/10.1136/bmj.325.7355.85.
- Levey AS, Coresh J. Chronic kidney disease. Lancet 2012;379:165-80. https://doi.org/10.1016/S0140-6736(11)60178-5.
- Hailpern SM, Melamed ML, Cohen HW, Hostetter TH. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 2007;18:2205-13. https://doi.org/10.1681/ASN.2006101165.
- James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet 2010;376:2096-103. https://doi.org/10.1016/S0140-6736(10)61271-8.
- James MT, Quan H, Tonelli M, Manns BJ, Faris P, Laupland KB, et al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 2009;54:24-32. https://doi.org/10.1053/j.ajkd.2009.04.005.
- Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med 2009;122:664-71.e2. https://doi.org/10.1016/j.amjmed.2009.01.026.
- Chronic Kidney Disease (Stage 5): Peritoneal Dialysis. Clinical Guideline [CG125]. London: NICE; 2011.
- Guidance on Home Compared with Hospital Haemodialysis for Patients with End-Stage Renal Failure. Technology Appraisal Guidance [TA48]. London: NICE; 2002.
- Jaeger JQ, Mehta RL. Assessment of dry weight in hemodialysis: an overview. J Am Soc Nephrol 1999;10:392-403.
- MacNeill SJ, Casula A, Shaw C, Castledine C. UK Renal Registry 18th Annual Report: Chapter 2 UK Renal Replacement Therapy Prevalence in 2014: national and centre-specific analyses. Nephron 2016;132:41-68. https://doi.org/10.1159/000444816.
- Hamilton AJ, Braddon F, Casula A, Inward C, Lewis M, Mallett T, et al. UK Renal Registry 18th Annual Report: Chapter 4 Demography of Patients Receiving Renal Replacement Therapy in Paediatric Centres in the UK in 2014. Nephron 2016;132:99-110. https://doi.org/10.1159/000444818.
- Lash JP, Ricardo AC, Roy J, Deo R, Fischer M, Flack J, et al. Race/ethnicity and cardiovascular outcomes in adults with CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic CRIC studies. Am J Kidney Dis 2016;68:545-53. https://doi.org/10.1053/j.ajkd.2016.03.429.
- Milani GP, Groothoff JW, Vianello FA, Fossali EF, Paglialonga F, Edefonti A, et al. Bioimpedance and Fluid Status in Children and Adolescents Treated With Dialysis. Am J Kidney Dis 2017;69:428-35. https://doi.org/10.1053/j.ajkd.2016.10.023.
- Zaloszyc A, Fischbach M, Schaefer B, Uhlmann L, Salomon R, Krid S, et al. Body composition monitoring-derived urea distribution volume in children on chronic hemodialysis. Pediatr Nephrol 2016;31:991-9. https://doi.org/10.1007/s00467-015-3283-3.
- Agarwal R. Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension 2010;56:512-17. https://doi.org/10.1161/HYPERTENSIONAHA.110.154815.
- Chazot C, Wabel P, Chamney P, Moissl U, Wieskotten S, Wizemann V. Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol Dial Transplant 2012;27:2404-10. https://doi.org/10.1093/ndt/gfr678.
- Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int 2014;86:489-96. https://doi.org/10.1038/ki.2014.207.
- Kim JS, Yang JW, Chai MH, Lee JY, Park H, Kim Y, et al. Copeptin in hemodialysis patients with left ventricular dysfunction. Yonsei Med J 2015;56:976-80. https://doi.org/10.3349/ymj.2015.56.4.976.
- Paniagua R, Ventura MDJ, Avila-Diaz M, Hinojosa-Heredia H, Mendez-Duran A, Cueto-Manzano A, et al. NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol Dial Transplant 2010;25:551-7. https://doi.org/10.1093/ndt/gfp395.
- Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009;24:1574-9. https://doi.org/10.1093/ndt/gfn707.
- Aguiar PV, Santos O, Teixeira L, Silva F, Azevedo P, Vidinha J, et al. Overhydration prevalence in peritoneal dialysis – a 2 year longitudinal analysis. Nefrologia 2015;35:189-96. https://doi.org/10.1016/j.nefro.2015.05.020.
- Bozzetto S, Piccoli A, Montini G. Bioelectrical impedance vector analysis to evaluate relative hydration status. Pediatr Nephrol 2010;25:329-34. https://doi.org/10.1007/s00467-009-1326-3.
- Oei EL, Fan SL. Practical aspects of volume control in chronic kidney disease using whole body bioimpedance. Blood Purif 2015;39:32-6. https://doi.org/10.1159/000368953.
- Ozkahya M, Ok E, Cirit M, Aydin S, Akçiçek F, Başçi A, et al. Regression of left ventricular hypertrophy in haemodialysis patients by ultrafiltration and reduced salt intake without antihypertensive drugs. Nephrol Dial Transplant 1998;13:1489-93. https://doi.org/10.1093/ndt/13.6.1489.
- Ozkahya M, Ok E, Toz H, Asci G, Duman S, Basci A, et al. Long-term survival rates in haemodialysis patients treated with strict volume control. Nephrol Dial Transplant 2006;21:3506-13. https://doi.org/10.1093/ndt/gfl487.
- Tsai YC, Chiu YW, Tsai JC, Kuo HT, Hung CC, Hwang SJ, et al. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin J Am Soc Nephrol 2015;10:39-46. https://doi.org/10.2215/CJN.03610414.
- Jansen MA. Renal Function, Adequacy Parameters and Patient Outcomes in Pre-Dialysis and Dialysis Patients. Amsterdam: University of Amsterdam; 2007.
- Dasgupta I, Farrington K, Davies SJ, Davenport A, Mitra S. UK National Survey of Practice Patterns of Fluid Volume Management in Haemodialysis Patients: a need for evidence. Blood Purif 2016;41:324-31. https://doi.org/10.1159/000444246.
- Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 2015;26:724-34. https://doi.org/10.1681/ASN.2014020222.
- Stefánsson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, et al. Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 2014;9:2124-32. https://doi.org/10.2215/CJN.02680314.
- Zhou YL, Liu J, Sun F, Ma LJ, Han B, Shen Y, et al. Calf bioimpedance ratio improves dry weight assessment and blood pressure control in hemodialysis patients. Am J Nephrol 2010;32:109-16. https://doi.org/10.1159/000315135.
- Termorshuizen F, Dekker FW, Van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 2004;15:1061-70. https://doi.org/10.1097/01.ASN.0000117976.29592.93.
- Vilar E, Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial 2011;24:487-94. https://doi.org/10.1111/j.1525-139X.2011.00968.x.
- Steenkamp R, Rao A, Fraser S. UK Renal Registry 18th Annual Report (December 2015) Chapter 5: Survival and Causes of Death in UK Adult Patients on Renal Replacement Therapy in 2014: national and centre-specific analyses. Nephron 2016;132:111-44. https://doi.org/10.1159/000444819.
- NHS Digital . Hospital Episode Statistics. Hospital Outpatient Activity 2014–15 2015. http://content.digital.nhs.uk/article/2021/Website-Search?productid=19879&q=title%3a+%22hospital+outpatient+activity%22&sort=Most+recent&size=10&page=1&area=both#top (accessed November 2016).
- Henderson LW. Symptomatic hypotension during hemodialysis. Kidney Int 1980;17:571-6. https://doi.org/10.1038/ki.1980.67.
- Wystrychowski G, Levin NW. Dry weight: sine qua non of adequate dialysis. Adv Chronic Kidney Dis 2007;14:e10-6. https://doi.org/10.1053/j.ackd.2007.03.003.
- Spiegel DM, Bashir K, Fisch B. Bioimpedance resistance ratios for the evaluation of dry weight in hemodialysis. Clin Nephrol 2000;53:108-14.
- Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: a review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys 2008;30:1257-69. https://doi.org/10.1016/j.medengphy.2008.06.009.
- Kim YJ, Jeon HJ, Kim YH, Jeon J, Ham YR, Chung S, et al. Overhydration measured by bioimpedance analysis and the survival of patients on maintenance hemodialysis: a single-center study. Kidney Res Clin Pract 2015;34:212-18. https://doi.org/10.1016/j.krcp.2015.10.006.
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis – part I: review of principles and methods. Clin Nutr 2004;23:1226-43. https://doi.org/10.1016/j.clnu.2004.06.004.
- Abbas SR, Zhu F, Levin NW. Bioimpedance can solve problems of fluid overload. J Ren Nutr 2015;25:234-7. https://doi.org/10.1053/j.jrn.2014.10.014.
- Bioimpedance Devices for the Assessment of Body Fluid Volume for Patients Undergoing Dialysis: A Review of the Clinical Effectiveness, Cost-Effectiveness and Guidelines – An Update. Ottawa, ON: CADTH; 2015.
- Zhu F, Kuhlmann MK, Kaysen GA, Sarkar S, Kaitwatcharachai C, Khilnani R, et al. Segment-specific resistivity improves body fluid volume estimates from bioimpedance spectroscopy in hemodialysis patients. J Appl Physiol 2006;100:717-24. https://doi.org/10.1152/japplphysiol.00669.2005.
- Nescolarde L, Garcia-Gonzlez MA, Rosell-Ferrer J, Doate T, Querfeld U. Thoracic versus whole body bioimpedance measurements: the relation to hydration status and hypertension in peritoneal dialysis patients. Physiol Meas 2006;27:961-71. https://doi.org/10.1088/0967-3334/27/10/003.
- BCM – Body Composition Monitor. Bad Homburg vor der Höhe: Fresenius Medical Care; 2016.
- Chamney PW, Wabel P, Moissl UM, Müller MJ, Bosy-Westphal A, Korth O, et al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr 2007;85:80-9.
- Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 2006;27:921-33. https://doi.org/10.1088/0967-3334/27/9/012.
- Wabel P, Chamney P, Moissl U, Jirka T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif 2009;27:75-80. https://doi.org/10.1159/000167013.
- Onofriescu M, Hogas S, Voroneanu L, Apetrii M, Nistor I, Kanbay M, et al. Bioimpedance-guided fluid management in maintenance hemodialysis: a pilot randomized controlled trial. Am J Kidney Dis 2014;64:111-18. https://doi.org/10.1053/j.ajkd.2014.01.420.
- Ponce P, Pham J, Gligoric-Fuerer O, Kreuzberg U. Fluid management in haemodialysis: conventional versus body composition monitoring (BCM) supported management of overhydrated patients. Port J Nephrol Hypertens 2014;28:238-48.
- Di Gioia C, Gallar P, Cobo G, Garcia F, Oliet A, Rodrigues A, et al. Body composition changes in hemodialysis patients: implications for prognosis. Enliven: Nephrol Ren Stud 2014;1. https://doi.org/10.18650/2378-542X.11001.
- Luo YJ, Lu XH, Woods F, Wang T. Volume control in peritoneal dialysis patients guided by bioimpedance spectroscopy assessment. Blood Purif 2011;31:296-302. https://doi.org/10.1159/000322617.
- Bodystat . Multiscan 5000 Bioelectrical Impedance Spectroscopy 2016. www.bodystat.com/products/ (accessed November 2016).
- Maltron International Ltd . Bioscan 920-II 2016. http://maltronint.com/products/bioscan920-2.php (accessed November 2016).
- Comprehensive Body Water Analysis for Monitoring Nutritional Status. 2014.
- NHS Technology Adoption Centre . Assessment of Fluid Status Using Body Composition Monitoring (BCM) Implementation Pack 2011. http://webarchive.nationalarchives.gov.uk/20130701143131/http://www.ntac.nhs.uk/Publications/Assessment_of_fluid_status_using_Body_Composition_Monitoring_(BCM)_Implementation_Pack.aspx (accessed November 2016).
- Management of Stage 5 Chronic Kidney Disease: NICE Pathway. London: NICE; 2017.
- Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. www.cochrane-handbook.org/ (accessed November 2015).
- NICE Diagnostics Assessment Programme Manual. London: NICE; 2011.
- Allinovi M, Saleem MA, Burgess O, Armstrong C, Hayes W. Finding covert fluid: methods for detecting volume overload in children on dialysis. Pediatr Nephrol 2016;31:2327-35. https://doi.org/10.1007/s00467-016-3431-4.
- Canpolat N, Caliskan S, Sever L, Tasdemir M, Ekmekci OB, Pehlivan G, et al. Malnutrition and its association with inflammation and vascular disease in children on maintenance dialysis. Pediatr Nephrol 2013;28:2149-56. https://doi.org/10.1007/s00467-013-2527-3.
- Zaloszyc A, Schaefer B, Schaefer F, Krid S, Salomon R, Niaudet P, et al. Hydration measurement by bioimpedance spectroscopy and blood pressure management in children on hemodialysis. Pediatr Nephrol 2013;28:2169-77. https://doi.org/10.1007/s00467-013-2540-6.
- Sterne J, Hernan M, Reeves B, Savovic J, Berkman N, Viswanath AK, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J 2016;355. https://doi.org/10.1136/bmj.i4919.
- Huan-Sheng C, Yeong-Chang C, Ming-Hsing H, Fan-Lieh T, Chu-Cheng L, Tsai-Kun W, et al. Application of bioimpedance spectroscopy in Asian dialysis patients (ABISAD-III): a randomized controlled trial for clinical outcomes. Int Urol Nephrol 2016;48:1897-909. https://doi.org/10.1007/s11255-016-1415-8.
- Hur E, Usta M, Toz H, Asci G, Wabel P, Kahvecioglu S, et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis 2013;61:957-65. https://doi.org/10.1053/j.ajkd.2012.12.017.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. https://doi.org/10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8.
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality. A meta-analysis. JAMA 1993;269:898-903. https://doi.org/10.1001/jama.1993.03500070078033.
- Chen HS, Lee KC, Cheng CT, Hou CC, Liou HH, Lin CJ, et al. Application of Bioimpedance Spectroscopy in Asian Dialysis Patients (ABISAD): serial follow-up and dry weight evaluation. Clin Kidney J 2013;6:29-34. https://doi.org/10.1093/ckj/sfs155.
- Onofriescu M, Mardare NG, Segall L, Voroneanu L, Cusai C, Hogas S, et al. Randomized trial of bioelectrical impedance analysis versus clinical criteria for guiding ultrafiltration in hemodialysis patients: effects on blood pressure, hydration status, and arterial stiffness. Int Urol Nephrol 2012;44:583-91. https://doi.org/10.1007/s11255-011-0022-y.
- O’Lone EL, Visser A, Finney H, Fan SL. Clinical significance of multi-frequency bioimpedance spectroscopy in peritoneal dialysis patients: independent predictor of patient survival. Nephrol Dial Transplant 2014;29:1430-7. https://doi.org/10.1093/ndt/gfu049.
- Oei E, Paudel K, Visser A, Finney H, Fan SL. Is overhydration in peritoneal dialysis patients associated with cardiac mortality that might be reversible?. World J Nephrol 2016;5:448-54. https://doi.org/10.5527/wjn.v5.i5.448.
- Santhakumaran T, Samad N, Fan SL. Hydration status measured by BCM: a potential modifiable risk factor for peritonitis in patients on peritoneal dialysis. Nephrology 2016;21:404-9. https://doi.org/10.1111/nep.12622.
- Kim S, Sung J, Jung ES, Park HC, Lee H, Chin HJ, et al. Hemodynamic and biochemical benefits of the objective measurement of fluid status in hemodialysis patients. Tohoku J Exp Med 2012;228:125-33. https://doi.org/10.1620/tjem.228.125.
- Castellano S, Palomares I, Molina M, Perez-Garcia R, Aljama P, Ramos R, et al. Clinical, analytical and bioimpedance characteristics of persistently overhydrated haemodialysis patients. Nefrologia 2014;34:716-23.
- Hoppe K, Schwermer K, Klysz P, Radziszewska D, Sawatiuk P, Baum E, et al. Cardiac troponin T and hydration status as prognostic markers in hemodialysis patients. Blood Purif 2015;40:139-45. https://doi.org/10.1159/000376603.
- Onofriescu M, Siriopol D, Voroneanu L, Hogas S, Nistor I, Apetrii M, et al. Overhydration, cardiac function and survival in hemodialysis patients. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0135691.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588-605. https://doi.org/10.1093/eurheartj/ehl254.
- Reference Values for Arterial Stiffness’ Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010;31:2338-50. https://doi.org/10.1093/eurheartj/ehq165.
- Probing the Dry Weight (DW) by Bioimpedance (BIA): Which Is the Gold Standard Between Clinical DW and BIA DW (REST) NCT02446535 2015. https://clinicaltrials.gov/ct2/show/NCT02446535?term=NCT02446535&rank=1 (accessed November 2016).
- Fluid Management Guided by Bioimpedance Analysis in Peritoneal Dialysis(PD) Patients 2016. https://clinicaltrials.gov/ct2/show/NCT02000128?term=NCT02000128&rank=1 (accessed November 2016).
- Control Of Fluid Balance Guided by Body Composition Monitoring in Patients on PeritoneAl DialySiS (COMPASS) 2014. https://clinicaltrials.gov/ct2/show/NCT01887262?term=NCT01887262&rank=1 (accessed November 2016).
- Bio-Impedance Spectroscopy to Maintain Renal Output: A Randomised Controlled Trial. Geneva: WHO International Clinical Trials Registry Platform; 2016.
- Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012;27:iii73-80. https://doi.org/10.1093/ndt/gfs269.
- Kirby L, Vale L. Dialysis for end-stage renal disease. Determining a cost-effective approach. Int J Technol Assess Health Care 2001;17:181-9. https://doi.org/10.1017/S0266462300105045.
- Treharne C, Liu FX, Arici M, Crowe L, Farooqui U. Peritoneal dialysis and in-centre haemodialysis: a cost-utility analysis from a UK payer perspective. Appl Health Econ Health Policy 2014;12:409-20. https://doi.org/10.1007/s40258-014-0108-7.
- Chronic Kidney Disease (Stage 4 or 5): Management of Hyperphosphataemia. Clinical Guideline [CG157]. London: NICE; 2014.
- Caskey F, Castledine C, Dawnay A, Farrington K, Fogarty D, Fraser S, et al. UK Renal Registry 18th Annual Report of the Renal Association. Bristol: UK Renal Registry; 2015.
- Li B, Cairns J, Fotheringham J, Ravanan R. on behalf of the ATTOM Study Group . Predicting hospital costs for patients receiving renal replacement therapy to inform an economic evaluation. Eur J Health Econ 2016;17:659-68. https://doi.org/10.1007/s10198-015-0705-x.
- Moissl U, Arias-Guillén M, Wabel P, Fontseré N, Carrera M, Campistol JM, et al. Bioimpedance-guided fluid management in hemodialysis patients. Clin J Am Soc Nephrol 2013;8:1575-82. https://doi.org/10.2215/CJN.12411212.
- ERA-EDTA Registry Annual Report 2013. Amsterdam: Academic Medical Center, Department of Medical Informatics; 2015.
- Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-139.
- Tappenden P, Chilcott J, Ward S, Eggington S, Hind D, Hummel S. Methodological issues in the economic analysis of cancer treatments. Eur J Cancer 2006;42:2867-75. https://doi.org/10.1016/j.ejca.2006.08.010.
- Karim A, Farrugia D, Cheshire J, Mahboob S, Begaj I, Ray D, et al. Recipient age and risk for mortality after kidney transplantation in England. Transplantation 2014;97:832-8. https://doi.org/10.1097/01.TP.0000438026.03958.7b.
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011;11:2093-109. https://doi.org/10.1111/j.1600-6143.2011.03686.x.
- NHS Blood and Transplant . Annual Report on Kidney Transplantation 2014. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/1321/organ_specific_report_kidney_2014.pdf (accessed November 2016).
- Lafrance JP, Rahme E, Iqbal S, Elftouh N, Laurin LP, Vallee M. Trends in infection-related hospital admissions and impact of length of time on dialysis among patients on long-term dialysis: a retrospective cohort study. CMAJ Open 2014;2:E109-14. https://doi.org/10.9778/cmajo.20120027.
- Quinn RR, Ravani P, Zhang X, Garg AX, Blake PG, Austin PC, et al. Impact of modality choice on rates of hospitalization in patients eligible for both peritoneal dialysis and hemodialysis. Perit Dial Int 2014;34:41-8. https://doi.org/10.3747/pdi.2012.00257.
- Rayner HC, Pisoni RL, Bommer J, Canaud B, Hecking E, Locatelli F, et al. Mortality and hospitalization in haemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2004;19:108-20. https://doi.org/10.1093/ndt/gfg483.
- Van Biesen W, Williams JD, Covic AC, Fan S, Claes K, Lichodziejewska-Niemierko M, et al. Fluid status in peritoneal dialysis patients: the European Body Composition Monitoring (EuroBCM) study cohort. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0017148.
- van Biesen W, Claes K, Covic A, Fan S, Lichodziejewska-Niemierko M, Schoder V, et al. A multicentric, international matched pair analysis of body composition in peritoneal dialysis versus haemodialysis patients. Nephrol Dial Transplant 2013;28:2620-8. https://doi.org/10.1093/ndt/gft296.
- Badve SV, Palmer SC, Strippoli GF, Roberts MA, Teixeira-Pinto A, Boudville N, et al. The validity of left ventricular mass as a surrogate end point for all-cause and cardiovascular mortality outcomes in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis 2016;68:554-63. https://doi.org/10.1053/j.ajkd.2016.03.418.
- Verbeke F, Van Biesen W, Honkanen E, Wikstrom B, Jensen PB, Krzesinski JM, et al. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the calcification outcome in renal disease (CORD) study. Clin J Am Soc Nephrol 2011;6:153-9. https://doi.org/10.2215/CJN.05120610.
- Heerspink HJL, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet 2009;373:1009-15. https://doi.org/10.1016/S0140-6736(09)60212-9.
- Turnbull F, Neal B, Pfeffer M, Kostis J, Algert C, . Blood Pressure Lowering Treatment Triallists’ Collaboration . Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens 2007;25:951-8. https://doi.org/10.1097/HJH.0b013e3280bad9b4.
- Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, et al. Towards improved cardiovascular management: the necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant 2008;23:2965-71. https://doi.org/10.1093/ndt/gfn228.
- Flythe JE. Ultrafiltration rate clinical performance measures: ready for primetime?. Semin Dial 2016;29:425-34. https://doi.org/10.1111/sdi.12529.
- Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K. Preservation of residual kidney function in hemodialysis patients: reviving an old concept. Kidney Int 2016;90:262-71. https://doi.org/10.1016/j.kint.2016.02.037.
- Baek SH, Oh KH, Kim S, Kim DK, Joo KW, Oh YK, et al. Control of fluid balance guided by body composition monitoring in patients on peritoneal dialysis (COMPASS): study protocol for a randomized controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-432.
- Grima DT, Bernard LM, Dunn ES, McFarlane PA, Mendelssohn DC. Cost-effectiveness analysis of therapies for chronic kidney disease patients on dialysis: a case for excluding dialysis costs. PharmacoEconomics 2012;30:981-9. https://doi.org/10.2165/11599390-000000000-00000.
- Davis S. Assessing Technologies that Are Not Cost-Effective at a Zero Price. Sheffield: NICE Decision Support Unit; 2014.
- Cinacalcet for the Treatment of Secondary Hyperparathyroidism in Patients with End-Stage Renal Disease on Maintenance Dialysis Therapy. Technology Appraisal Guidance [TA117]. London: NICE; 2007.
- NHS Reference Costs 2014 to 2015. London: Department of Health; 2015.
- Kidney Transplantation (Adults) – Immunosuppressive Therapy (Review Of TA 85) [ID456]. London: NICE; 2015.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Tan BK, Yu Z, Fang W, Lin A, Ni Z, Qian J. Longitudinal bioimpedance vector plots add little value to fluid management of peritoneal dialysis patients. Kidney Int 2016;89:487-97. https://doi.org/10.1038/ki.2015.294.
- Stevens PE, O’Donoghue DJ, de Lusignan S, Van Vlymen J, Klebe B, Middleton R, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 2007;72:92-9. https://doi.org/10.1038/sj.ki.5002273.
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Liem YS, Bosch JL, Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health 2008;11:733-41. https://doi.org/10.1111/j.1524-4733.2007.00308.x.
- Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLOS Med 2012;9. https://doi.org/10.1371/journal.pmed.1001307.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Brazier J, Rowen D. NICE DSU Technical Support Document 11: Alternatives to EQ-5D for Generating Health State Utility Values. Sheffield: NICE Decision Support Unit; 2011.
Appendix 1 Search strategies
Multiple-frequency bioimpedance devices for fluid management in people with chronic kidney disease receiving dialysis
Clinical effectiveness
EMBASE Classic and EMBASE
Date range searched: 1947 to week 40 2016.
Epub ahead of print, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE(R) Daily (via Ovid) and MEDLINE(R) (via Ovid)
Date range searched: 1946 to 10 October 2016.
Ovid multifile search, URL: https://shibboleth.ovid.com/
Date searched: 10 October 2016.
Search strategy
-
exp Renal Insufficiency, Chronic/ use ppez
-
exp chronic kidney disease/ use emcz
-
exp chronic kidney failure/ use emcz
-
ckd.tw,kw.
-
(chronic adj3 (kidney or renal)).tw,kw.
-
or/1-5
-
exp renal dialysis/ use ppez
-
exp renal replacement therapy/ use emcz
-
(haemodialysis or hemodialysis or dialysis).kw,tw.
-
or/7-9
-
6 and 10
-
bioimpedance.tw,kw.
-
bioelectric$ impendance.tw,kw.
-
body composition monitor$.tw,kw.
-
bioscan$.tw,kw.
-
bio scan$.tw,kw.
-
multiscan$.tw,kw.
-
multi scan$.tw,kw.
-
inbody.tw,kw.
-
or/12-19
-
10 and 20
-
hypervol?emi?.tw,kw.
-
euvol?emi?.tw,kw.
-
hypovol?emi?.tw,kw.
-
(fluid adj3 (status or overload or monitor$ or level? or balance or imbalance)).tw,kw.
-
(hydration adj3 (status or monitor$)).tw,kw.
-
((under or over) adj3 hydration).tw,kw.
-
underhydrat$.tw,kw
-
overhydrat$.tw,kw.
-
normohydrat$.tw,kw.
-
((dry or target) adj weight).tw,kw.
-
ultrafiltration volume.tw,kw.
-
or/22-32
-
11 and 33
-
21 or 34
-
(editorial or comment or note or letter).pt.
-
35 not 36
-
exp animals/ not humans/ use ppez
-
nonhuman/ not human/ use emcz
-
37 not 38 use ppez
-
37 not 39 use emcz
-
40 or 41
-
remove duplicates from 42
Science Citation Index
Date range searched: 1970 to 27 June 2016.
Web of Knowledge URL: http://wok.mimas.ac.uk/
Date searched: 27 June 2016.
Search strategy
#1. TS=(haemodialysis or hemodialysis or dialysis)
#2. TS=bioimpedance
#3. TS=bioelectric* impendance
#4. TS=body composition monitor$*
#5. TS= (bioscan$* or bio scan*)
#6. TS=(multiscan* or multi scan*)
#7. TS=inbody
#8. #2 or #3 or #4 or #5 or #6 or #7
#9. #1 and #8
The Cochrane Library
Cochrane Database of Systematic Reviews: Issue 6 of 12, June 2016.
Cochrane Central Register of Controlled Trials: Issue 5 of 12, May 2016.
URL: www3.interscience.wiley.com/
Date searched: 27 June 2016.
Search strategy
#1. MeSH descriptor: [Renal Dialysis] explode all trees
#2. haemodialysis or hemodialysis or dialysis:ti,ab,kw (Word variations have been searched)
#3. #1 or #2
#4. bioimpedance.:ti,ab,kw (Word variations have been searched)
#5. bioelectric* impendance.ti,ab,kw
#6. body composition monitor*.ti,ab,kw
#7. (bioscan* or bio scan*) .ti,ab,kw.
#8. (multiscan* or multi scan*) .ti,ab,kw
#9. inbody.ti,ab,kw
#10. #4 or #5 or #6 or #7 or #8 or #9
#11. #3 and #10
Database of Abstracts of Reviews of Effects: December 2014
Centre for Reviews and Dissemination, URL: http://nhscrd.york.ac.uk/welcome.htm
Date searched: 27 June 2016.
Search strategy
#1. MeSH DESCRIPTOR Renal Dialysis EXPLODE ALL TREES IN DARE
#2. (haemodialysis) OR (hemodialysis) OR (dialysis)
#3. #1 OR #2
#4. (bioimpedance) OR (impedance)
#5. (body composition monitor*) OR (bioscan*) OR (bio scan*)
#6. (inbody) OR (multiscan*) OR (multi scan*)
#7. #4 OR #5 OR #6
#8. #3 AND #7
Additional conference proceedings
ERA-EDTA Congress 2014, Amsterdam, the Netherlands, 31 May to 3 June.
ERA-EDTA Congress 2015, London, UK, 28–31 May.
Kidney Week (Journal of the American Society of Nephrology) American Society of Nephrology 2014, Philadelphia, PA, USA, 11–16 November.
Kidney Week (Journal of the American Society of Nephrology) American Society of Nephrology 2015, San Diego, CA, USA, 3–8 November.
Annual Dialysis Conference 2014, Atlanta, GA, USA, 8–11 February.
Annual Dialysis Conference 2015, New Orleans, LA, USA, 31 January to 3 February.
Annual Dialysis Conference 2016, Seattle, WA, USA, 27 February to 1 March.
Clinical Trials (June 2016)
URL: http://clinicaltrials.gov/ct/gui/c/r
Date searched: 4 July 2016.
Search strategy
bioimpendance AND dialysis
or
bioimpendance AND hemodialysis
World Health Organization’s International Clinical Trials Registry Platform (June 2016)
Date searched: 4 July 2016.
Search strategy
bioimpendance AND dialysis
or
bioimpendance AND hemodialysis
European Union Clinical Trials Register (June 2016)
URL: www.clinicaltrialsregister.eu/
Date searched: 4 July 2016.
Search strategy
bioimpedance
Body Composition Monitor validation studies
EMBASE Classic and EMBASE
Date range searched: 1947 to week 39 2016.
Epub ahead of print, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE(R) Daily (via Ovid) and MEDLINE(R) (via Ovid)
Date range searched: 1946 to 2016.
OVID multifile search, URL: https://shibboleth.ovid.com/
Date searched: 27 September 2016.
Search strategy
-
exp Renal Insufficiency, Chronic/ use ppez
-
exp chronic kidney disease/ use emcz
-
exp chronic kidney failure/ use emcz
-
ckd.tw,kw.
-
(chronic adj3 (kidney or renal)).tw,kw.
-
or/1-5
-
exp renal dialysis/ use ppez
-
exp renal replacement therapy/ use emcz
-
(haemodialysis or hemodialysis or dialysis).kw,tw.
-
or/7-9
-
6 and 10
-
bioimpedance.tw,kw.
-
bioelectric$ impendance.tw,kw.
-
body composition monitor$.tw,kw.
-
bioscan$.tw,kw.
-
bio scan$.tw,kw.
-
multiscan$.tw,kw
-
multi scan$.tw,kw.
-
inbody.tw,kw.
-
or/12-19
-
10 and 20
-
hypervol?emi?.tw,kw.
-
euvol?emi?.tw,kw.
-
hypovol?emi?.tw,kw.
-
(fluid adj3 (status or overload or monitor$ or level? or balance or imbalance)).tw,kw
-
(hydration adj3 (status or monitor$)).tw,kw.
-
((under or over) adj3 hydration).tw,kw.
-
underhydrat$.tw,kw.
-
overhydrat$.tw,kw.
-
normohydrat$.tw,kw.
-
((dry or target) adj weight).tw,kw.
-
ultrafiltration volume.tw,kw.
-
or/22-32
-
validation studies/
-
measurement accuracy/
-
“reproducibility of results”/
-
(validation or validity).tw,kw.
-
(accuracy or accurate).tw,kw.
-
44 or 45 or 46 or 47 or 48
-
21 and 39
-
11 and 33 and 39
-
40 or 41
-
remove duplicates from 42
Cost-effectiveness
EMBASE Classic and EMBASE
Date range searched: 1947 to week 27 2016.
Epub ahead of print, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE(R) Daily (via Ovid) and MEDLINE(R) (via Ovid)
Date range searched: 1946 to 2016.
OVID multifile search, URL: https://shibboleth.ovid.com/
Date searched: 5 July 2016.
Search strategy
-
exp Renal Insufficiency, Chronic/ use ppez
-
exp *chronic kidney disease/ use emcz
-
exp *chronic kidney failure/ use emcz
-
ckd.tw,kw.
-
(chronic adj1 (kidney or renal)).tw,kw.
-
or/1-5
-
exp renal dialysis/ use ppez
-
exp renal replacement therapy/ use emcz
-
(haemodialysis or hemodialysis or dialysis).kw,tw.
-
or/7-9
-
6 and 10
-
exp “costs and cost analysis”/ use ppez
-
exp economic evaluation/ use emcz
-
economics/
-
health economics/ use emcz
-
exp health care cost/ use emcz
-
exp economics,hospital/ use ppez
-
exp economics,medical/ use ppez
-
economics,pharmaceutical/ use ppez
-
pharmacoeconomics/ use emcz
-
exp models, economic/ use ppez
-
exp decision theory/
-
monte carlo method/
-
markov chains/
-
exp technology assessment, biomedical/
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
-
economics model$.tw.
-
(economic$ or pharmacoeconomic$).tw.
-
price or prices or pricing).tw.
-
budget$.tw.
-
(value adj1 money).tw.
-
(expenditure$ not energy).tw.
-
markov$.tw.
-
monte carlo.tw.
-
decision$ adj2 (tree? or analy$ or model$)).tw.
-
or/12-35
-
(metabolic adj cost).tw.
-
((energy or oxygen) adj (cost or expenditure)).tw.
-
36 not (37 or 38)
-
(letter or editorial or note or comment).pt.
-
39 not 40
-
11 and 41
-
remove duplicates from 42
Health Technology Assessment, June 2016 and NHS Economic Evaluation Database, December 2014
Centre for Reviews and Dissemination, URL: http://nhscrd.york.ac.uk/welcome.htm
Date searched: 5 July 2016.
Search strategy
#1. MeSH DESCRIPTOR Renal Dialysis EXPLODE ALL TREES IN NHSEED
#2. (dialysis) OR (hemodialysis) OR (haemodialysis)
#3. #1 OR #2
#4. MeSH DESCRIPTOR Renal Insufficiency, Chronic EXPLODE ALL TREES IN NHSEED
#5. (ckd) OR (chronic renal) OR (chronic kidney)
#6. #4 OR #5
#7. #3 AND #6
Research Papers in Economics
URL: http://repec.org/
Search strategy
dialysis | hemodialysis |haemodialysis | CKD | renal | kidney
Quality of life/utilities
EMBASE Classic and EMBASE
Date range searched: 1947 to week 27 2016.
Epub ahead of print, MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE(R) Daily (via Ovid) and MEDLINE(R) (via Ovid)
Date range searched: 1946 to 2016.
Ovid multifile search, URL: https://shibboleth.ovid.com/
Date searched: 8 July 2016.
Search strategy
-
exp Renal Insufficiency, Chronic/ use ppez
-
exp chronic kidney disease/ use emcz
-
exp chronic kidney failure/ use emcz
-
ckd.tw,kw.
-
(chronic adj3 (kidney or renal)).tw,kw.
-
or/1-5
-
exp renal dialysis/ use ppez
-
exp renal replacement therapy/ use emcz
-
(haemodialysis or hemodialysis or dialysis).kw,tw.
-
or/7-9
-
6 and 10
-
quality adjusted life year/
-
“Value of Life”/ use ppez
-
(qaly? or qald? or qale? or qtime?).tw,kf.
-
(euro qual or euro qual5d or euro qol5d or eq-5d or eq5-d or eq5d or euroqual or euroqol or euroqual5d or euroqol5d).tw,kf.
-
(eq-sdq or eqsdq).tw,kf.
-
(hye or hyes).tw,kf.
-
health$ year$ equivalent$.tw,kf.
-
(hui or hui1 or hui2 or hui3).tw,kf.
-
(quality adjusted or adjusted life year$).tw,kf.
-
disability adjusted life.tw,kf.
-
daly?.tw,kf.
-
((index adj3 wellbeing) or (quality adj3 wellbeing) or qwb).tw,kf.
-
(multiattribute$ or multi attribute$).tw,kf.
-
(utility adj3 (score? or scoring or valu$ or measur$ or evaluat$ or scale? or instrument? or weight or weights or weighting or information or data or unit or units or health$ or life or estimat$ or elicit$ or disease$ or mean or cost$ or expenditure? or gain or gains or loss or losses or lost or analysis or index$ or indices or overall or reported or calculat$ or range$ or increment$ or state or states or status)).tw,kf.
-
utility.ab. /freq=2
-
utilities.tw,kf.
-
disutili$.tw,kf
-
(hsuv or hsuvs).tw,af.
-
(illness state$ or health state$).tw,kf.
-
(shortform$ or short form$).tw,kf.
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).tw,kf.
-
(sf6 or sf 6 or sf6d or sf 6d or sf six or sfsix or sf8 or sf 8 or sf eight or sfeight).tw,kf.
-
(sf12 or sf 12 or sf twelve or sftwelve).tw,kf.
-
(sf16 or sf 16 or sf sixteen or sfsixteen).tw,kf.
-
(sf20 or sf 20 or sf twenty or sftwenty).tw,kf.
-
(15d or 15-d or 15 dimension).tw,kf.
-
standard gamble$.tw,kf.
-
(time trade off$ or time tradeoff$ or tto or timetradeoff$).tw,kf.
-
(case report or editorial or letter).pt.
-
case report/
-
or/12-39
-
42 not (40 or 41)
-
11 and 43
-
remove duplicates from 44
Cost-effectiveness Analysis Registry: July 2016
URL: https://research.tufts-nemc.org/cear4/default.asp
Date searched: 8 July 2016.
Search strategy
Dialysis or hemodialysis or haemodialysis
School of Health and Related Research Health Utilities Database: July 2016
URL: www.scharrhud.org/
Date searched: 8 July 2016.
Search strategy
Dialysis or haemodialysis or haemodialysis
Websites consulted
Agency for Healthcare Research and Quality, URL: www.ahrq.gov/
American Society of Nephrology, URL: www.asn-online.org/
Belgian Health Care Knowledge Centre (KCE), URL: https://kce.fgov.be/
Bodystat, URL: www.bodystat.com/products/
CADTH, URL: www.cadth.ca/
ERA-ETDA, URL: http://era-edta.org/
French National Authority for Health (HAS), URL: www.has-sante.fr/
Fresenius Medical Care, URL: www.bcm-fresenius.com/
Health Information and Quality Authority, URL: www.hiqa.ie/
Inbody Co. Ltd, URL: www.inbody.com/eu
Institute for Clinical and Economic Review, URL: www.icer-review.org/
Institute for Quality and Efficiency in Health Care, URL: www.iqwig.de/
Maltron, URL: http://maltronint.com/industry/medical/dialysis.php
Medicines and Healthcare products Regulatory Agency, URL: www.mhra.gov.uk/
NICE, URL: www.nice.org.uk/
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), URL: www.niddk.nih.gov/
NHS Healthcare Improvement Scotland, URL: www.healthcareimprovementscotland.org/
US Food and Drug Administration, URL: www.fda.gov/default.htm
Appendix 2 Characteristics of excluded non-randomised studies that focused on a paediatric population
| Study details | Participant characteristics | Study aims | Main outcomes |
|---|---|---|---|
| Allinovi et al., 201672 |
|
To evaluate the accuracy of bioimpedance spectroscopy, echocardiographic assessment of inferior vena cava and lung ultrasound in detecting fluid overload in children with ESRD and to compare them with clinical measures, including weight, physical examination and SBP | The correlation of fluid overload by weight and the BCM measurement was reported as r = 0.43 (p = 0.2), although it is unclear which parameter(s) assessed by the device were used in the correlation |
| Country: UK | |||
| Number of centres: one | |||
| Study design: prospective observational study | |||
| Device used: BCM [in children aged > 2 years only (n = 11), as the authors stated that the technique was not validated with appropriate reference algorithms in children aged < 2 years] | |||
| Canpolat et al., 201373 |
|
To examine the prevalence of malnutrition and its possible associations with inflammation and vascular disease in children receiving chronic dialysis | Mean RRF was 0.41 (0.60) ml/min/1.73 m2 in patients receiving HD and 3.41 (2.52) ml/min/1.73 m2 in patients receiving PD (p < 0.001). Fat mass, as assessed by the BCM, was significantly lower in patients than in controls (20.6% vs. 24.6%; p = 0.048) |
| Country: Turkey | |||
| Number of centres: NR | |||
| Study design: cross-sectional | |||
| Device used: BCM | |||
| Zaloszyc et al., 2013;24 Zaloszyc et al., 201674 |
|
To assess the current practice of clinical estimate of hydration status and blood pressure control in children receiving long-term HD, the frequency of hypertension and its correlation with individual patient hydration status measured by means of the BCM were evaluated. In addition, the impact of dialysis prescription on blood pressure control, considering Napl, the prescribed NaD and the achieved dry weight, was evaluated. The urea distribution volume determined by the BCM was compared with that obtained from four different anthropometric formulas of which only the Morgenstern equation was validated in children receiving PD | Mean (SD) predialytic ROH was 6.3 (7.1%) (range: –6.1% to 21%). Of the total 463 dialysis sessions assessed, in 52 sessions (11.2%) the patients were assessed as being moderately overhydrated (i.e. mean ROH of > 15%), of which 5.6% of sessions showed pre- and post-HD hypertension; 21% of sessions were in the range 7–15%, with 26.8% of sessions showing pre- and post-HD hypertension; 62.4% of sessions were classed as normohydrated (i.e. mean ROH of –7% to 7%), of which 20% involved pre- and post-HD hypertension. Urea distribution volume, as determined by the BCM, was in agreement with the Morgenstern anthropometric equation |
| Country: Germany and France | |||
| Number of centres: three | |||
| Study design: retrospective | |||
| Device used: BCM |
Appendix 3 Data extraction form: details of outcomes extracted
| Data extraction section | Information provided in each section | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study characteristics 1 | Publication status | Study design | Country/ies | Number of centres | Recruitment method | Allocation method | Study dates | ||
| Study characteristics 2 | Secondary outcomes reported | Adverse events reported | Study power and statistical analysis | Funding source | |||||
| Intervention characteristics | Study ID | Intervention and comparator names (one per row) | Full details | Length of follow-up | |||||
| Participant characteristics | Study ID | Total/intervention/comparator (one per row) | Enrolled, n | Randomised, n | Analysed, n | Lost to follow-up, n | Lost to follow-up, reasons | Age (years), mean (SD); p-value if reported | Sex (male/female); p-value if reported |
| BMI (kg/m2) | Weight (kg), mean (SD) | Dialysis modality | Dialysis vintage (months), mean (SD) | Diabetes mellitus, n (%) | AHT medication, n (%) | Dry weight (kg), mean (SD) | SBP (mmHg), mean (SD) | Diastolic BP (mmHg), mean (SD) | |
| Cause of ESRD, n (%) | Presence of LVH | LVMI (g/m2) | OH (l), mean (SD) | TBW (l), mean (SD) | ECW (l), mean (SD) | ICW (l), mean (SD) | ECW/I, mean (SD) | Lean tissue index (kg/m2) | |
| Fat tissue mass | Comorbid conditions | ||||||||
| Intermediate outcomes | Study ID | Total/intervention/comparator (one per row) | Number of HD sessions | Length of HD sessions | Number of unplanned hospital visits/admissions as a result of FO or dehydration | Use of AHT medication | Incidence of anaemia | SBP (mmHg), mean (SD) | Diastolic BP (mmHg), mean (SD) |
| Presence of left ventricular hypertrophy | LVMI (g/m2), mean (SD) | Arterial stiffness PWV (m/s), mean (SD) | Incidence of overhydration | Incidence of underhydration | Change of dialysis modality as a result of FO | Adherence with recommended fluid intake | Hydration status | Relative hydration status | |
| Clinical outcomes | Study ID | Total/intervention/comparator (one per row) | Incidence of CV events (including stroke and heart attack) | Mortality | RRF | Incidence of oedema | Incidence of peritonitis | Adverse effects associated with hypotensive episodes (including cramps, fatigue, diarrhoea, nausea, dizziness, fainting) | |
| Patient-reported outcomes | Study ID | Post-dialysis recovery time | Fatigue | HRQoL | |||||
| NRS outcomes | Study ID | Summary of outcomes/conclusions | Any other information | ||||||
| Risk-of-bias RCT | Adequate sequence generation? | Allocation concealment? | Blinding: participants? | Blinding: outcome assessment? (Report each outcome separately) | Incomplete outcome data addressed? (Report each outcome separately) | Free of selective reporting? | Other sources of bias? | ||
| Risk-of-bias NRS | Were participants a representative sample selected from a relevant patient population? | Were the inclusion/exclusion criteria of participants clearly described? | Were participants entering the study at a similar point in their disease progression, i.e. disease severity? | Was selection of patients consecutive? | Was data collection undertaken prospectively? | Were the groups comparable on demographic characteristics and clinical features? | Was the intervention (and comparison) clearly defined? | Was the intervention undertaken by an experienced person? | Was the setting appropriate? |
| Were the staff, place and facilities where the patients were treated appropriate for performing the procedure? | Were any of the important outcomes considered? | Were objective (valid and reliable) outcome measures used? | Was the assessment of the main outcomes blind? | Was follow-up long enough to detect important effects on outcomes of interest? | Was information provided on non-respondents, dropouts, etc.? | Did the withdrawals, dropouts, etc., have similar characteristics as those who completed the study? | Was length of follow-up similar between comparison groups? | Were the important prognostic factors identified, for example age, duration of disease, disease severity? | |
| Were the analyses adjusted for confounding factors? | |||||||||
Appendix 4 Risk-of-bias form: randomised controlled trials (Cochrane risk-of-bias tool)
| Domain | Support for judgement | Review authors’ judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether or not it should produce comparable groups | Selection bias (biased allocation to interventions) as a result of inadequate generation of a randomised sequence |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether or not intervention allocations could have been foreseen in advance of, or during, enrolment | Selection bias (biased allocation to interventions) as a result of inadequate concealment of allocations prior to assignment |
| Performance bias | ||
| Blinding of participants and personnel: assessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind study participants and personnel to knowledge of which intervention a participant received. Provide any information relating to whether or not the intended blinding was effective | Performance bias as a result of knowledge of the allocated interventions by participants and personnel during the study |
| Detection bias | ||
| Blinding of outcome assessment: assessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind outcome assessors to knowledge of which intervention a participant received. Provide any information relating to whether or not the intended blinding was effective | Detection bias as a result of knowledge of the allocated interventions by outcome assessors |
| Attrition bias | ||
| Incomplete outcome data: assessments should be made for each main outcome (or class of outcomes) | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether or not attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions when reported, and any reinclusions in analyses performed by the review authors | Attrition bias as a result of the amount, nature or handling of incomplete outcome data |
| Reporting bias | ||
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors and what was found | Reporting bias as a result of selective outcome reporting |
| Other bias | ||
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were prespecified in the review protocol, responses should be provided for each question/entry | Bias as a result of problems not covered elsewhere in the table |
Appendix 5 Risk-of-bias checklist for non-randomised studies
| Criteria | Yes | No | Unclear | Comments |
|---|---|---|---|---|
| 1. Were participants a representative sample selected from a relevant patient population? | ||||
| 2. Were the inclusion/exclusion criteria of participants clearly described? | ||||
| 3. Were participants entering the study at a similar point in their disease progression, i.e. severity of disease? | ||||
| 4. Was selection of patients consecutive? | ||||
| 5. Was data collection undertaken prospectively? | ||||
| 6. Were the groups comparable on demographic characteristics and clinical features? | ||||
| 7. Was the intervention (and comparison) clearly defined? | ||||
| 8. Was the intervention undertaken by someone experienced at performing the procedure? | ||||
| 9. Were the staff, place, and facilities where the patients were treated appropriate for performing the procedure (e.g. access to back-up facilities in hospital or special clinic)? | ||||
| 10. Were any of the important outcomes considered, i.e. on clinical effectiveness, cost-effectiveness or learning curves? | ||||
| 11. Were objective (valid and reliable) outcome measures used, including satisfaction scale? | ||||
| 12. Was the assessment of main outcomes blind? | ||||
| 13. Was follow-up long enough to detect important effects on outcomes of interest? | ||||
| 14. Was information provided on non-respondents, dropouts? | ||||
| 15. Was length of follow-up similar between comparison groups? | ||||
| 16. Were the important prognostic factors identified, for example age, duration of disease, disease severity? | ||||
| 17. Were the analyses adjusted for confounding factors? |
Appendix 6 Excluded studies
Ineligible study design (N = 34)
Antlanger M, Hecking M, Haidinger M, Werzowa J, Kovarik JJ, Paul G, et al. Fluid overload in hemodialysis patients: a cross-sectional study to determine its association with cardiac biomarkers and nutritional status. BMC Nephrol 2013;14:266.
Bai Q, Zhang J, Zhang AH, Cheng LT, Duan JL, He L, et al. Role of arachidonoylethanolamine in blood pressure regulation in volume-resistant patients on peritoneal dialysis. Int Urol Nephrol 2012;44:1855–60.
Bai Q, Zhang J, Zhang AH, Cheng LT, He L, Fan MH, et al. Roles of human urotensin II in volume resistance hypertension in peritoneal dialysis patients. Ren Fail 2012;34:713–17.
Castellano S, Palomares I, Moissl U, Chamney P, Carretero D, Crespo A, et al. Risk identification in haemodialysis patients by appropriate body composition assessment. Nefrologia 2016;36:268–74.
Chen HS, Lee KC, Cheng CT, Hou CC, Liou HH, Lin CJ, Lim PS. Application of Bioimpedance Spectroscopy in Asian Dialysis Patients (ABISAD): serial follow-up and dry weight evaluation. Clin Kidney J 2013;6:29–34.
Davies SJ, Engel B, Chan C, Tan BK, Yu ZZ, Asghar R, et al. Breath analysis and the measurement of total body water using isotope dilution – applications in the dialysis clinic. Curr Anal Chem 2013;9:593–9.
Dekker MJ, Marcelli D, Canaud B, Konings CJ, Leunissen KM, Levin NW, et al. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: results from the international MONDO initiative. Eur J Clin Nutr 2016;20:20.
Di Gioia MC, Gallar Ruiz P, Cobo G, Garcia Lopez F, Agud Aparicio JL, Oliet A, et al. Body composition changes in hemodialysis patients: implications for prognosis. Enliven Arch Nephrol Ren Stud 2014;1:1–7.
Furusho M, Weng J, Mori T, Wang T. Impact of hydration and nutrition status on the Watson formula in peritoneal dialysis patients. Adv Perit Dial 2014;30:110–14.
Hassan MO, Duarte R, Dix-Peek T, Vachiat A, Dickens C, Grinter S, et al. Volume overload and its risk factors in South African chronic kidney disease patients: an appraisal of bioimpedance spectroscopy and inferior vena cava measurements. Clin Nephrol 2016;10:10.
Kalainy S, Reid R, Jindal K, Pannu N, Braam B. Fluid volume expansion and depletion in hemodialysis patients lack association with clinical parameters. Can J Kidney Health Dis 2015;2:54.
Kang SH, Cho KH, Park JW, Yoon KW, Do JY. Characteristics and clinical outcomes of hyponatraemia in peritoneal dialysis patients. Nephrology 2013;18:132–7.
Kaysen GA, Larive B, Painter P, Craig A, Lindsay RM, Rocco MV, et al. Baseline physical performance, health, and functioning of participants in the Frequent Hemodialysis Network (FHN) trial. Am J Kidney Dis 2011;57:101–12.
Kwan BCH, Szeto CC, Chow KM, Law MC, Cheng MS, Leung CB, et al. Bioimpedance spectroscopy for the detection of fluid overload in Chinese peritoneal dialysis patients. Perit Dial Int 2014;34:409–16.
Lu Q, Cheng LT, Wang T, Wan J, Liao LL, Zeng J, et al. Visceral fat, arterial stiffness, and endothelial function in peritoneal dialysis patients. J Ren Nutr 2008;18:495–502.
Marcelli D, Usvyat LA, Kotanko P, Bayh I, Canaud B, Etter M, et al. Body composition and survival in dialysis patients: results from an international cohort study. Clin J Am Soc Nephrol 2015;10:1192–200.
Marcelli D, Brand K, Ponce P, Milkowski A, Marelli C, Ok E, et al. Longitudinal changes in body composition in patients after initiation of hemodialysis therapy: results from an international cohort. J Ren Nutr 2016;26:72–80.
Mathew S, Abraham G, Vijayan M, Thandavan T, Mathew M, Veerappan I, et al. Body composition monitoring and nutrition in maintenance hemodialysis and CAPD patients – a multicenter longitudinal study. Ren Fail 2015;37:66–72.
Passauer J, Petrov H, Schleser A, Leicht J, Pucalka K. Evaluation of clinical dry weight assessment in haemodialysis patients using bioimpedance spectroscopy: a cross-sectional study. Nephrol Dial Transplant 2010;25:545–51.
Paudel K, Visser A, Burke S, Samad N, Fan SL. Can bioimpedance measurements of lean and fat tissue mass replace subjective global assessments in peritoneal dialysis patients? J Ren Nutr 2015;25:480–7.
Pérez-García R, Palomares I, Merello JI, Ramos R, Maduell F, Molina M, et al. Hyponatraemia, mortality and haemodialysis: an unexplained association. Nefrologia 2016;36:42–50.
Piccoli A, Italian CAPD-BIA Study Group. Bioelectric impedance vector distribution in peritoneal dialysis patients with different hydration status. Kidney Int 2004;65:1050–63.
Rosenberger J, Kissova V, Majernikova M, Straussova Z, Boldizsar J. Body composition monitor assessing malnutrition in the hemodialysis population independently predicts mortality. J Ren Nutr 2014;24:172–6.
Tsai YC, Chiu YW, Kuo HT, Chen SC, Hwang SJ, Chen TH, et al. Fluid overload, pulse wave velocity, and ratio of brachial pre-ejection period to ejection time in diabetic and non-diabetic chronic kidney disease. PLOS ONE 2014;9:e111000.
Unal A, Kavuncuoglu F, Duran M, Oguz F, Kocyigit I, Sipahioglu MH, et al. Inflammation is associated to volume status in peritoneal dialysis patients. Ren Fail 2015;37:935–40.
Van Biesen W, Williams JD, Covic AC, Fan S, Claes K, Lichodziejewska-Niemierko M, et al. Fluid status in peritoneal dialysis patients: the European body composition monitoring (EuroBCM) study cohort. PLOS ONE 2011;6:e17148.
Van Biesen W, Claes K, Covic A, Fan S, Lichodziejewska-Niemierko M, Schoder V, et al. A multicentric, international matched pair analysis of body composition in peritoneal dialysis versus haemodialysis patients. Nephrol Dial Transplant 2013;28:2620–8.
Vega A, Ruiz C, Abad S, Quiroga B, Velazquez K, Ampuero J, Lopez-Gomez JM. Body composition affects urea distribution volume estimated by Watson’s formula. J Ren Nutr 2015;25:420–5.
Vega A, Ruiz C, Abad S, Quiroga B, Velazquez K, Yuste C, et al. Body composition affects the response to erythropoiesis-stimulating agents in patients with chronic kidney disease in dialysis. Ren Fail 2014;36:1073–7.
Vega A, Quiroga B, Abad S, Ruiz C, López-Gómez JM. Study on overhydration in dialysis patients and its association with inflammation. Nefrologia 2014;34:579–83.
Voroneanu L, Cusai C, Hogas S, Ardeleanu S, Onofriescu M, Nistor I, et al. The relationship between chronic volume overload and elevated blood pressure in hemodialysis patients: use of bioimpedance provides a different perspective from echocardiography and biomarker methodologies. Int Urol Nephrol 2010;42:789–97.
Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, et al. Towards improved cardiovascular management: the necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant 2008;23:2965–71.
Wabel P, Chamney P, Moissl U, Jirka T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif 2009;27:75–80.
Yao YH, Fu CH, Ho SJ, Tsai SH, Ng YY, Chuang CL, et al. Peritoneal dialysis as compared with hemodialysis is associated with higher overhydration but non-inferior blood pressure control and heart function. Blood Purif 2012;34:40–7.
Ineligible device (N = 67)
Abreo AP, Chertow GM, Dalrymple LS, Kaysen GA, Johansen, KL. Association of bioimpedance spectroscopy-based volume estimation with postdialysis hypotension in patients receiving hemodialysis. Hemodial Int 2015;19:536–42.
Abreo AP, Herzog CA, Kutner NG, Lea J, Johansen KL. Estimated pulmonary artery systolic pressure and self-reported physical function in patients on hemodialysis. Am J Nephrol 2015;41:313–19.
Alijanian N, Naini AE, Shahidi S, Liaghat L, Samani RR. The comparative evaluation of patients’ body dry weight under hemodialysis using two methods: bioelectrical impedance analysis and conventional method. J Res Med Sci 2012;17:923–7.
Barros A, Costa BE, Mottin CC, d’Avila DO. Depression, quality of life, and body composition in patients with end-stage renal disease: a cohort study. Rev Bras Psiquiatr 2016;38:301–6.
Basile C, Vernaglione L, Di Iorio B, Bellizzi V, Chimienti D, Lomonte C, et al. Development and validation of bioimpedance analysis prediction equations for dry weight in hemodialysis patients. Clin J Am Soc Nephrol 2007;2:675–80.
Basile C, Vernaglione L, Bellizzi V, Lomonte C, Rubino A, D’Ambrosio N, Di Iorio B. Total body water in health and disease: Have anthropometric equations any meaning? Nephrol Dial Transplant 2008;23:1997–2002.
Beberashvili I, Sinuani I, Azar A, Yasur H, Feldman L, Averbukh Z, Weissgarten J. Longitudinal study of leptin levels in chronic hemodialysis patients. Nutr J 2011;10:68.
Beberashvili I, Azar A, Sinuani I, Shapiro G, Feldman L, Stav K, et al. Bioimpedance phase angle predicts muscle function, quality of life and clinical outcome in maintenance hemodialysis patients. Eur J Clin Nutr 2014;68:683–9.
Beberashvili I, Azar A, Sinuani I, Shapiro G, Feldman L, Sandbank J, et al. Geriatric nutritional risk index, muscle function, quality of life and clinical outcome in hemodialysis patients. Clin Nutr 2016;13:13.
Booth J, Pinney J, Davenport A. The effect of vascular access modality on changes in fluid content in the arms as determined by multifrequency bioimpedance. Nephrol Dial Transplant 2011;26:227–31.
Chen YC, Chen HH, Yeh JC, Chen SY. Adjusting dry weight by extracellular volume and body composition in hemodialysis patients. Nephron 2002;92:91–6.
Chen YC, Lin CJ, Wu CJ, Chen HH, Yeh JC. Comparison of extracellular volume and blood pressure in hemodialysis and peritoneal dialysis patients. Nephron Clin Pract 2009;113:c112–16.
Cheng LT, Chen W, Tang W, Wang T. Residual renal function and volume control in peritoneal dialysis patients. Nephron Clin Pract 2006;104:c47–54.
Cheng LT, Jiang HY, Tang LJ, Wang T. Seasonal variation in blood pressure of patients on continuous ambulatory peritoneal dialysis. Blood Purif 2006;24:499–507.
Cigarran S, Barril G, Cirugeda A, Bernis C, Aguilera A, Sanz P, et al. Hypoalbuminemia is also a marker of fluid excess determined by bioelectrical impedance parameters in dialysis patients. Ther Apher Dial 2007;11:114–20.
Cox-Reijven PL, Kooman JP, Soeters PB, Van der Sande FM, Leunissen KML. Role of bioimpedance spectroscopy in assessment of body water compartments in hemodialysis patients. Am J Kidney Dis 2001;38:832–8.
Davenport A, Willicombe M. Comparison of fluid status in patients treated by different modalities of peritoneal dialysis using multi-frequency bioimpedance. Int J Artif Organs 2009;32:779–86.
Davenport A, Willicombe MK. Does diabetes mellitus predispose to increased fluid overload in peritoneal dialysis patients? Nephron Clin Pract 2010;114:c60–6.
Davenport A, Sayed RH, Fan S. Is extracellular volume expansion of peritoneal dialysis patients associated with greater urine output? Blood Purif 2011;32:226–31.
Davenport A, Hussain Sayed R, Fan S. The effect of racial origin on total body water volume in peritoneal dialysis patients. Clin J Am Soc Nephrol 2011;6:2492–8.
Davenport A. Differences in prescribed Kt/V and delivered haemodialysis dose-why obesity makes a difference to survival for haemodialysis patients when using a ‘one size fits all’ Kt/V target. Nephrol Dial Transplant 2013;28(Suppl. 4):iv219–23.
Davison SN, Jhangri GS, Jindal K, Pannu N. Comparison of volume overload with cycler-assisted versus continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol 2009;4:1044–50.
de Araujo Antunes A, Vannini FD, de Arruda Silveira LV, Barretti P, Martin LC, Caramori JC. Associations between bioelectrical impedance parameters and cardiovascular events in chronic dialysis patients. Int Urol Nephrol 2013;45:1397–403.
Demirci C, Ozkahya M, Demirci MS, Asci G, Kose T, Colak T, et al. Effects of three times weekly eight-hour nocturnal hemodialysis on volume and nutritional status. Am J Nephrol 2013;37:559–67.
Demirci C, Asci G, Demirci MS, Ozkahya M, Toz H, Duman S, et al. Impedance ratio: a novel marker and a powerful predictor of mortality in hemodialysis patients. Int Urol Nephrol 2016;19:19.
Dumler F, Kilates C. Body composition analysis by bioelectrical impedance in chronic maintenance dialysis patients: comparisons to the National Health and Nutrition Examination Survey III. J Ren Nutr 2003;13:166–72.
Dumler F, Kilates MC. Body composition analysis in chronic dialysis patients: a longitudinal study. Hong Kong J Nephrol 2003;5:24–8.
Essig M, Escoubet B, de Zuttere D, Blanchet F, Arnoult F, Dupuis E, et al. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol Dial Transplant 2008;23:239–48.
Fagugli RM, Pasini P, Quintaliani G, Pasticci F, Ciao G, Cicconi B, et al. Association between extracellular water, left ventricular mass and hypertension in haemodialysis patients. Nephrol Dial Transplant 2003;18:2332–8.
Fan S, Davenport A. The importance of overhydration in determining peritoneal dialysis technique failure and patient survival in anuric patients. Int J Artif Organs 2015;38:575–9.
Fisch BJ, Spiegel DM. Assessment of excess fluid distribution in chronic hemodialysis patients using bioimpedance spectroscopy. Kidney Int 1996;49:1105–9.
Furstenberg A, Davenport A. Assessment of body composition in peritoneal dialysis patients using bioelectrical impedance and dual-energy X-ray absorptiometry. Am J Nephrol 2011;33:150–6.
Genctoy G, Arikan S, Eldem O. Pulmonary hypertension associates with malnutrition and body composition hemodialysis patients. Ren Fail 2015;37:273–9.
Guo Q, Lin J, Li J, Yi C, Mao H, Yang X, Yu X. The effect of fluid overload on clinical outcome in Southern Chinese patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int 2015;35:691–702.
Hung SC, Lin YP, Huang HL, Pu HF, Tarng DC. Aldosterone and mortality in hemodialysis patients: role of volume overload. PLOS ONE 2013;8:e57511.
Jha V, Jairam A, Sharma MC, Sakhuja V, Piccoli A, Parthasarathy S. Body composition analysis with bioelectric impedance in adult Indians with ESRD: comparison with healthy population. Kidney Int 2006;69:1649–53.
Kang SH, Cho KH, Park JW, Yoon KW, Do JY. Change in body composition in accordance with residual renal function in patients on peritoneal dialysis. J Ren Nutr 2013;23:438–44.
Katzarski K, Charra B, Laurent G, Lopot F, Divino-Filho JC, Nisell J, Bergstrom J. Multifrequency bioimpedance in assessment of dry weight in haemodialysis. Nephrol Dial Transplant 1996;11(Suppl. 2):20–3.
Katzarski KS, Charra B, Luik AJ, Nisell J, Divino Filho JC, Leypoldt JK, et al. Fluid state and blood pressure control in patients treated with long and short haemodialysis. Nephrol Dial Transplant 1999;14:369–75.
Koh KH, Wong HS, Go KW, Morad Z. Normalized bioimpedance indices are better predictors of outcome in peritoneal dialysis patients. Perit Dial Int 2011;31:574–82.
Kumar S, Khosravi M, Massart A, Davenport A. Is there a role for n-terminal probrain-type natriuretic peptide in determining volume status in haemodialysis patients? Nephron Clin Pract 2013;122:33–7.
Kumar S, Khosravi M, Massart A, Potluri M, Davenport A. Haemodiafiltration results in similar changes in intracellular water and extracellular water compared to cooled haemodialysis. Am J Nephrol 2013;37:320–4.
Kumar S, Khosravi M, Massart A, Potluri M, Davenport A. Are serum to dialysate sodium gradient and segmental bioimpedance volumes associated with the fall in blood pressure with hemodialysis? Int J Artif Organs 2014;37:21–8.
Lee SW, Song JH, Kim GA, Lee KJ, Kim MJ. Assessment of total body water from anthropometry-based equations using bioelectrical impedance as reference in Korean adult control and haemodialysis subjects. Nephrol Dial Transplant 2001;16:91–7.
Lin YP, Yu WC, Hsu TL, Ding PYA, Yang WC, Chen CH. The extracellular fluid-to-intracellular fluid volume ratio is associated with large-artery structure and function in hemodialysis patients. Am J Kidney Dis 2003;42:990–9.
Maggiore Q, Nigrelli S, Ciccarelli C, Grimaldi C, Rossi GA, Michelassi C. Nutritional and prognostic correlates of bioimpedance indexes in hemodialysis patients. Kidney Int 1996;50:2103–8.
McCafferty K, Fan S, Davenport A. Extracellular volume expansion, measured by multifrequency bioimpedance, does not help preserve residual renal function in peritoneal dialysis patients. Kidney Int 2014;85:151–7.
Nongnuch A, Panorchan K, Davenport A. Predialysis NTproBNP predicts magnitude of extracellular volume overload in haemodialysis patients. Am J Nephrol 2014;40:251–7.
Nongnuch A, Campbell N, Stern E, El-Kateb S, Fuentes L, Davenport A. Increased postdialysis systolic blood pressure is associated with extracellular overhydration in hemodialysis outpatients. Kidney Int 2015;87:452–7.
Ohashi Y, Tai R, Aoki T, Mizuiri S, Ogura T, Tanaka Y, et al. The associations of malnutrition and aging with fluid volume imbalance between intra- and extracellular water in patients with chronic kidney disease. J Nutr Health Aging 2015;19:986–93.
Omichi Y, Srivareerat M, Panorchan K, Greenhall GH, Gupta S, Davenport A. Measurement of muscle strength in haemodialysis patients by pinch and hand grip strength and comparison to lean body mass measured by multifrequency bio-electrical impedance. Ann Nutr Metab 2016;68:268–75.
Panorchan K, Nongnuch A, El-Kateb S, Goodlad C, Davenport A. Changes in muscle and fat mass with haemodialysis detected by multi-frequency bioelectrical impedance analysis. Eur J Clin Nutr 2015;69:1109–12.
Papakrivopoulou E, Booth J, Pinney J, Davenport A. Comparison of volume status in asymptomatic haemodialysis and peritoneal dialysis outpatients. Nephron Extra 2012;2:48–54.
Pillon L, Piccoli A, Lowrie EG, Lazarus JM, Chertow GM. Vector length as a proxy for the adequacy of ultrafiltration in hemodialysis. Kidney Int 2004;66:1266–71.
Santi Xavier P, Perez Vogt B, Cuadrado Martin L, Vaninni F, Araujo Antunes A, Ponce D, et al. Total body water and failure to control blood pressure by medication in hemodialysis patients. Nephron Extra 2014;4:95–100.
Segall L, Moscalu M, Hogas S, Mititiuc I, Nistor I, Veisa G, Covic A. Protein-energy wasting, as well as overweight and obesity, is a long-term risk factor for mortality in chronic hemodialysis patients. Int Urol Nephrol 2014;46:615–21.
Sezer S, Karakan S, Sasak G, Tutal E, Acar FNO. Body fat percentage as a risk factor for atherosclerosis but not for inflammation for hemodialysis patients: differences between genders. J Ren Nutr 2012;22:490–8.
Sivalingam M, Vilar E, Mathavakkannan S, Farrington K. The role of natriuretic peptides in volume assessment and mortality prediction in Haemodialysis patients. BMC Nephrol 2015;16:218.
Sugano N, Yokoyama K, Kato N, Hara Y, Endo S, Mitome J, et al. Monitoring of body water composition by the simultaneous use of bioelectrical impedance analysis and Crit-Line(®) during hemodialysis. Clin Exp Nephrol 2014;18:944–51.
Tan BK, Yu Z, Fang W, Lin A, Ni Z, Qian J, et al. Longitudinal bioimpedance vector plots add little value to fluid management of peritoneal dialysis patients. Kidney Int 2016;89:487–97.
Tian N, Guo QY, Yao FJ, Zhou Q, Yang X, Lin JX, Yu XQ. Bioimpedance-guided fluid management can improve clinical outcomes in peritoneal dialysis patients: a prospective randomized control trial. Hong Kong J Nephrol 2015;17(Suppl. 2):S123.
Tian N, Guo Q, Zhou Q, Cao P, Hong L, Chen M, et al. The impact of fluid overload and variation on residual renal function in peritoneal dialysis patient. PLOS ONE 2016;11:e0153115.
Unal A, Sipahioglu M, Oguz F, Kaya M, Kucuk H, Tokgoz B, et al. Pulmonary hypertension in peritoneal dialysis patients: prevalence and risk factors. Perit Dial Int 2009;29:191–8.
Wang X, Axelsson J, Lindholm B, Wang T. Volume status and blood pressure in continuous ambulatory peritoneal dialysis patients. Blood Purif 2005;23:373–8.
Yashiro M, Ochiai M, Fujisawa N, Kadoya Y, Kamata T. The evaluation of filtration coefficients of microvasculature for the assessment of fluid status in hemodialysis patients. Int J Artif Organs 2013;36:7–16.
Zhou YL, Liu J, Sun F, Ma LJ, Han B, Shen Y, Cui TG. Calf bioimpedance ratio improves dry weight assessment and blood pressure control in hemodialysis patients. Am J Nephrol 2010;32:109–16.
Zhou YL, Liu J, Ma L, Sun F, Shen Y, Huang J, Cui T. Impact of dry weight determined by calf bioimpedance ratio on carotid stiffness and left ventricular hypertrophy in hemodialysis patients. Artif Organs 2014;38:327–34.
Zimmerman DL, Ruzicka M, Hebert P, Fergusson D, Touyz RM, Burns KD. Short daily versus conventional hemodialysis for hypertensive patients: a randomized cross-over study. PLOS ONE 2014;9:e97135.
Ineligible participants (N = 3)
Keane DF, Lindley E. Use of hand-to-hand measurements for body composition monitoring in patients with inaccessible or amputated feet. J Ren Care 2015;41:28–32.
Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, Tarng DC. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int 2014;85:703–9.
Tsai YC, Tsai JC, Chiu YW, Kuo HT, Chen SC, Hwang SJ, et al. Is fluid overload more important than diabetes in renal progression in late chronic kidney disease? PLOS ONE 2013;8:e82566.
Ineligible outcomes (N = 8)
Broers NJH, Usvyat LA, Marcelli D, Bayh I, Scatizzi L, Canaud B, et al. Season affects body composition and estimation of fluid overload in haemodialysis patients: variations in body composition; a survey from the European MONDO database. Nephrol Dial Transplant 2015;30:676–81.
Ferrario M, Moissl U, Garzotto F, Signorini MG, Cruz D, Tetta C, et al. Study of the autonomic response in hemodialysis patients with different fluid overload levels. Ann Int Conf IEEE Eng Med Biol Soc 2010;2010:3796–9.
Gracia-Iguacel C, Gonzalez-Parra E, Perez-Gomez MV, Mahillo I, Egido J, Ortiz A, Carrero JJ. Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a centre in Spain. Nefrologia 2013;33:495–505.
Ronco C, Verger C, Crepaldi C, Pham J, De Los Rios T, Gauly A, et al. Baseline hydration status in incident peritoneal dialysis patients: the initiative of patient outcomes in dialysis (IPOD-PD study). Nephrol Dial Transplant 2015;30:849–58.
Santos PR, Monteiro DL, de Paula PH, Monte Neto VL, Coelho ML, Arcanjo CC, et al. Volaemic status and dyspepsia in end-stage renal disease patients. Nephrology 2015;20:519–22.
Schwermer K, Hoppe K, Radziszewska D, Klysz P, Sawatiuk P, Nealis J, et al. N-terminal pro-B-type natriuretic peptide as a marker of hypervolemia and predictor of increased mortality in patients on hemodialysis. Pol Arch Med Wewn 2015;125:560–9.
Siriopol D, Voroneanu L, Hogas S, Apetrii M, Gramaticu A, Dumea R, et al. Bioimpedance analysis versus lung ultrasonography for optimal risk prediction in hemodialysis patients. Int J Cardiovasc Imaging 2016;32:263–70.
Yuste C, Abad S, Vega A, Barraca D, Bucalo L, Pérez-de José A, López-Gómez JM. Assessment of nutritional status in haemodialysis patients. Nefrologia 2013;33:243–9.
Non-English language and unable to obtain translation (N = 2)
Castellano-Gasch S, Palomares-Sancho I, Molina-Nunez M, Ramos-Sanchez R, Merello-Godino JI, Maduell F. [New reliable methods for the diagnose of protein-energy wasting in hemodialysis patients]. Nutr Hosp 2014;30:905–10.
Diez GR, Del Valle E, Negri AL, Crucelegui S, Luxardo R, Zambrano L, et al. Hypovitaminosis D in patients on hemodialysis (HD): related factors and influence on muscle strength. Rev Nefrol, Dial Trasplant 2013;33:134–40.
Appendix 7 Characteristics of included studies
| Study details | Participant characteristics | Intervention characteristics | Aims and outcomes |
|---|---|---|---|
| RCTs (A = study group; B = control group; C = total, both groups) | |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| NRSs | |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Appendix 8 Risk-of-bias assessment: non-randomised studies
| ReBIP criteria | Study: first author and year of publication | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Castellano et al., 201486 | Hoppe et al., 201587 | Kim et al., 201285 | Kim et al., 201550 | Oei et al., 201683 | O’Lone et al., 201482 | Onofriescu et al., 201588 | Santhakumaran et al., 201684 | Wizemann et al., 200930 | |
| Representative sample | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Inclusion/exclusion criteria clearly defined | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Participants at a similar point in disease progression | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ |
| Consecutive selection of participants | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Prospective data collection | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Clearly defined intervention | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Intervention delivered by experienced person | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Intervention delivered in appropriate setting | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Important outcomes considered | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Objective outcome measured | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Blind assessment of main outcomes | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Long enough follow-up | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Information on non-respondents, dropouts | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ? | ✗ | ✓ |
| Withdrawals likely to introduce bias | ✓ | ✓ | ? | ✗ | ? | ? | ? | ? | ? |
| Important prognostic factors identified | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Appendix 9 Outcome measures extracted from the included randomised controlled trials
| Indicator/group assessed | Hospitalisation | AHT medication | SBP (mmHg), mean (SD) | Diastolic BP (mmHg), mean (SD) | Presence of left ventricular hypertrophy | LVMI (g/m2), mean (SD) | Arterial stiffness PWV (m/s), mean (SD) | Absolute hydration status | Relative hydration status |
|---|---|---|---|---|---|---|---|---|---|
| Huan-Sheng et al., 2016 76 | |||||||||
| Indicator | Incidence, IR ratio and HR (per patient-year) for all diabetes mellitus and non-diabetes mellitus patients, 95% CI | Pre-dialysis SBP | FO and FOpost for all patients; for patients with initial FO of > 2.5 l and for patients with initial FO of ≤ 2.5 l; change with baseline | FOR for all patients; for patients with initial FO of > 2.5 l and for patients with initial FO of ≤ 2.5 l; change with baseline | |||||
| Total | |||||||||
| Study (bioimpedance) | Overall: 71 events; IR = 0.52 (95% CI 0.44 to 0.61); diabetes mellitus, 30 events; IR = 0.58, 95% CI 0.46 to 0.73; non-diabetes mellitus, 41 events; IR = 0.48, 95% CI 0.39 to 0.60 | All: 136 (23)/FO of ≤ 2.5 l, 136 (23); FO of ≥ 2.5 l, 133 (21); p < 0.05 | All: FO = 1.49 (SD 1.04); FOpost, –0.50 (SD 1.21), p < 0.05; FO of ≤ 2.5 l, FO = 1.40 (SD 1.00), p < 0.01; FOpost = –0.56 (SD 1.21), p < 0.001; FO of ≥ 2.5 l, FO = 2.21 (SD 1.07), p < 0.001; FOpost = 0.02 (SD 1.07), p < 0.05 | All: 0.10 (SD 0.07); FO of ≤ 2.5 l, 0.09 (SD 0.06), p < 0.05; FO of ≥ 2.5 l, 0.14 (SD 0.006), p < 0.001 | |||||
| Control |
|
|
All: FO = 1.64 (SD 1.40); FOpost: –0.23 (SD 1.52); FO of ≤ 2.5 l: FO = 1.25 (SD 1.16); FOpost = –.53 (SD 1.39); p < 0.05; FO of ≥ 2.5 l: FO = 3.07 (SD 1.27); p < 0.05; FOpost = 0.89 (SD 1.48) | All: FOR: 0.11 (SD 0.09); FO of ≤ 2.5 l: FOR = 0.09 (SD 0.09); FO of ≥ 2.5 l: 0.19 (SD 0.07); p < 0.05 | |||||
| Luo et al. , 2011 63 | |||||||||
| Indicator | Total daily defined dose, mean (SD) at 12 weeks | OH | ECW/ICW | ||||||
| Total (N = 160) | |||||||||
| Bioimpedance (n = 78) | 2.33 (1.76) | 132.99 (19.47); p < 0.5; change with baseline and between groups | 77.63 (12.04); p < 0.5; change with baseline | 1.72 (SD 1.51); p < 0.05; change with baseline | 0.95 (SD 0.13); p < 0.05; change with baseline | ||||
| Control (n = 82) | 2.94 (1.87) | 139.07 (22.40); p < 0.5; change with baseline and between groups | 80.85 (14.15); p < 0.5; change with baseline | 2.52 (SD 1.83); p < 0.05; change with baseline and between groups | 1.00 (SD 0.14); p < 0.05; change with baseline | ||||
| Hur et al. , 2013 77 | |||||||||
| Indicator | Hospitalisation rate/100 patients | Pre and post dialysis; p-value – change from baseline | Pre and post dialysis; p-value – change from baseline | p-value – change from baseline | p-value – change from baseline | FOpre and FOpost, change with baseline | |||
| Totala | |||||||||
| Study (bioimpedance) | Hospitalised, n = 6; hospitalisation rate/100 patient-year, n = 12.5 | Pre dialysis: 120 (19); p < 0.001; post dialysis: 105 (18); p < 0.001 | Pre dialysis: 73 (9); p < 0.001; post dialysis: 65 (9); p < 0.001 | 28/64 (43.8%); p = 0.4 | 116 (29); p < 0.001 | –0.52 (1.38) | FOpre = 0.87 (SD 0.88); FOpost = –1.33 (SD 0.99); p < 0.001; change with baseline: FOpre = –0.6 (SD 0.8); FOpost = –0.5 (SD 0.9) | ||
| Control | Hospitalisation, n = 4; hospitalisation rate/100 patient-year: 30.9; p = NS, difference between groups | Pre dialysis: 125 (19); p = 0.006; post dialysis: 113 (21); p = 0.03 | Pre dialysis: 76 (9); p = 0.2; post dialysis: 70 (10); p = 0.07 | 31/62 (50%); p = 0.9 | 120 (30); p = 0.9 | 0.11 (1.31); difference between groups = –0.5, 95% CI –0.9 to –0.0; p = 0.04 | FOpre = 1.41 (SD 1.26); FOpost = –1.01 (SD 1.44); change: FOpre = 0.2 (SD 1.2); FOpost = 0.0 (SD 1.3) | ||
| Between-group changes (95% CI) | FOpre: –0.4 (95% CI –0.6 to –0.3); p < 0.001; FOpost: –0.5 (95% CI –0.8 to –0.1); p = 0.01 | ||||||||
| Onofriescu et al., 2014;60 did not report DBP60 | |||||||||
| Indicator | n = patients not treated with AHT medication, within-group change | Change with baseline | RFO, % (SD) change within groups (95% CI) | ||||||
| Bioimpedance | n = 45; p = 0.05 | 138.9 (14.7): –6.54 (95% CI –13.62 to –4.53); p = 0.04 | 7.46 (5.77), –2.05 (–5.70 to –1.10); p = 0.03 | ||||||
| Control | n = NR; p = NS | 140.5 (11.4) –4.00 (95% CI –10.83 to 2.63); p = 0.4 | 11.24 (7.62), 0.94 (–2.50 to 4.40); p = 0.9 | ||||||
| Between-group changes | Between-group mean difference (end of intervention): 1.67 (95% CI –5.24 to 8.60); p = 0.9; between-group mean difference (change from baseline to end of intervention): –2.43 (95% CI –7.70 to 2.84); p = 0.4 | End of intervention: 3.77 (2.20 – 7.35); p = 0.03; change from baseline to end of intervention: –2.99 (–5.00 to –0.89); p = 0.05 | |||||||
| Ponce et al. , 2014 61 | |||||||||
| Indicator | Hospitalised at least once | Pre and post dialytic | Pre and post dialytic | OH (l) (SD), compared with baseline | ROH, % (SD) compared with baseline | ||||
| Total | |||||||||
| Study (bioimpedance) | 40/101 (39.6%) | Predialytic SBP: 134.6 (27.3); post-dialytic SBP: 132.8 (28.6) | Predialytic DBP: 65.4 (15.8); post-dialytic DBP: 63.4 (15.0) | 2.92 (1.47); p < 0.0001 | 15.40 (6.36); p = NS | ||||
| Control | 28/88 (31.8%) | Predialytic SBP: 136.5 (24.7); post-dialytic SBP: 129.3 (24.0) | Predialytic DBP: 64.5 (16.2); post-dialytic DBP: 61.4 (12.9) | Mean OH: 3.36 (1.75); p = 0.0216 | 16.26 (8.48); p = NS | ||||
| Between-group difference | 0.4184 (95% CI –0.02 to 0.86); p = 0.0622 | p = NS | |||||||
Appendix 10 Characteristics of ongoing trials
| Study details | Participant characteristics | Aims and outcomes |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
Appendix 11 Questions for clinical experts on bioimpedance testing
List of abbreviations
- AAC
- abdominal aortic calcification
- BCM
- Body Composition Monitor
- BMI
- body mass index
- BNF
- British National Formulary
- CADTH
- Canadian Agency for Drugs and Technologies in Health
- CDSR
- Cochrane Database of Systematic Reviews
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CKD
- chronic kidney disease
- CV
- cardiovascular
- DARE
- Database of Abstracts of Reviews of Effects
- ECW
- extracellular water
- EQ-5D
- EuroQol-5 Dimensions
- ERA-EDTA
- European Renal Association – European Dialysis and Transplant Association
- ESA
- erythropoiesis-stimulating agent
- ESRD
- end-stage renal disease
- GFR
- glomerular filtration rate
- HD
- haemodialysis
- HES
- Health Episode Statistics
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- ICW
- intracellular water
- IU
- international unit
- KDQOL-SF
- Kidney Disease Quality of Life-Short Form (questionnaire)
- LVMI
- left ventricular mass index
- NICE
- National Institute for Health and Care Excellence
- PD
- peritoneal dialysis
- PDTW
- post-dialysis target weight
- PWV
- pulse wave velocity
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ReBIP
- Review Body for Interventional Procedures
- ROH
- relative overhydration
- RRT
- renal replacement therapy
- SBP
- systolic blood pressure
- SD
- standard deviation
- SE
- standard error
- WMD
- weighted mean difference
