Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/57/32. The contractual start date was in October 2012. The draft report began editorial review in December 2016 and was accepted for publication in March 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Khan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background and rationale
Haemorrhage and caesarean section
Haemorrhage (excessive blood loss) is an important direct cause of maternal death. 1 Life-threatening blood loss is the primary indication for 95.6% of emergency hysterectomies in labour. 2 Haemorrhage is the most common cause for maternal critical care admission3–5 and places a profound health burden on the childbearing population during an important life event. Haemorrhage is more common in women who have caesarean sections,6 particularly when indicated for conditions such as placenta praevia (low-lying placenta) or when an emergency caesarean section is required. 7
Approximately 166,000 caesarean sections are performed annually in England. Almost two-thirds of these are performed as emergency procedures and the number of operations has been ever increasing. 8 Caesarean section currently accounts for 26.2% of deliveries (2013–14) and it is the most frequent major operation conducted by the NHS, with > 400 performed per day in England alone. 9 Major haemorrhage can occur without warning during caesarean section and the woman’s condition can quickly deteriorate during attempts to arrest blood loss. Rates of major obstetric haemorrhage vary in the literature according to the definition used; postpartum haemorrhage (PPH) occurs with a frequency of 2.93%6 but severe PPH of ≥ 2.5 is much less common, with a frequency of around 0.5–0.6%. 10 The likelihood of haemorrhage is increased by risk factors including previous caesarean section, low-lying or morbidly adherent placenta, emergency caesarean section for any indication, antepartum haemorrhage and pre-eclampsia. 6,10
Donor blood transfusion in obstetrics
The treatment for major haemorrhage involves allogeneic (donor) blood transfusion when the operative loss is life-threatening or when the mother has severe anaemia following arrest of the haemorrhage. Approximately 66,000 units of blood (known as packed red cells) are given annually in the UK maternity setting. 11 This equates to £7M per year12 without considering additional health-care costs involved in the administration of blood or the financial consequences of maternal acute illness. Thus, any reduction in the amount of blood required for obstetrics could significantly reduce the cost of blood transfusions.
Donor blood is a limited resource that needs to be used judiciously. Although national blood services are constantly improving their capacity to guarantee availability of blood for transfusion across all clinical requirements,13 it remains an expensive service to recruit and retain blood donors to minimise the risks of shortages, and new infective risks may arise in future. The availability of donor blood is an essential prerequisite for major procedures including joint replacement, cardiac surgery, organ transplantation, cancer care, obstetric emergencies and the management of trauma. This wide range of demands provides significant challenges to the NHS in the delivery of high-quality health care to all points of need simultaneously. All NHS hospitals are required to have policies for blood shortages, including cancellation of elective surgeries that may require transfusion.
There is an increasing focus on patient blood management, an international initiative promoting the use of transfusion alternatives including cell salvage, when feasible, and limiting the use of donor transfusion when avoidable. 14,15 Transfusion sparing strategies successful in other surgical populations, such as pre-donation and acute normovolaemic haemodilution, cannot be employed in caesarean section. The role of permissive anaemia and high transfusion threshold is potentially limited by maternal symptoms in the postnatal period (see Postnatal anaemia and its consequences).
In addition, there are major risks associated with donor blood transfusion, including death from transfusion error, acute transfusion reaction, fatal lung injury and infection transmission. 16 These risks are monitored by the UK haemovigilance scheme, Serious Hazards Of Transfusion (SHOT), with feedback of results via annual reports. 17 Despite improved safety mechanisms, these rates persist, although serious events are very rare, with mortality rates of 1 in 100,000. 18 Nevertheless, minimising unnecessary transfusion is an important strand in promoting patient safety.
Postnatal anaemia and its consequences
Concerns regarding transfusion safety together with changes in clinical practice, as highlighted in Donor blood transfusion in obstetrics, have led to a more overall restrictive approach to transfusion. The application of these principles to the obstetric setting with higher transfusion thresholds can result in significant postnatal maternal anaemia.
In addition to fatigue as a direct consequence, postoperative maternal anaemia has been associated with longer hospitalisation, increased wound infection rates and delayed time to mobility. 19 Anaemia prolongs hospital stay by one-third, with an overall 50% higher cost per hospitalisation. 20 The economic consequences of anaemia resulting from obstetric haemorrhage are therefore profound and any intervention that could reduce maternal morbidity and mortality is worthy of scrutiny.
Maternal morbidity resulting from anaemia crucially affects the mother’s capacity to provide care for the newborn. An intervention to relieve maternal anaemia is therefore highly relevant for the quality of life of this young, generally healthy population of mothers and that of their offspring.
Intraoperative cell salvage
Intraoperative cell salvage collects the patient’s own blood lost during an operation, processes it and returns it to their circulation. This way, cell salvage allows retransfusion of the patient’s own blood that would otherwise have been wasted.
Its use has been shown to reduce the amount of donor blood given in other operations. A Cochrane review and other meta-analyses of the use of cell salvage in non-obstetric settings demonstrated a significant reduction in patient exposure to donor blood. 21 A Health Technology Assessment (HTA) report put the relative risk (RR) of exposure to donor blood at 0.59 [95% confidence interval (CI) 0.48 to 0.73] for pooled trials of cell salvage. 22 However, this evidence did not include any trials examining caesarean section.
Given that cell salvage may reduce the need for a standard donor blood transfusion, there should be fewer transfusion reactions and infections that may be associated with donor blood. One potential complication associated with cell salvage in the non-obstetric setting arises owing to the use of leucocyte depletion filters (LDFs) during the return of salvaged blood. 17,23–26 LDFs are used in the retransfusion of salvaged blood with the aim of filtering out foreign cells, such as squamous cells contained within amniotic fluid. They have been the subject of scrutiny in the medical literature. There are some reports of unexplained hypotension associated with blood return and filters have been implicated as a potential source of most anaphylactoid responses (although this remains a contentious issue and, in rare cases, hypotension has been associated with cell salvage even when no LDF was used). 17 Moreover, the addition of a filter may restrict the rapid reinfusion of blood in the context of massive haemorrhage by slowing down the blood flow rate. Therefore, these filters are routinely omitted at the discretion of clinicians when rapid blood return is imperative.
Overall, cell salvage is a technology that may simultaneously reduce the need for donor blood transfusion and prevent anaemia. Therefore, it could avoid the serious morbidity associated with haemorrhage as well as achieve a significant reduction in costs. In recent years, cell salvage machines have been refined and have entered routine use in cardiac, orthopaedic, liver and vascular surgery for which there is a risk of major haemorrhage. Their use in caesarean section has not yet been adequately examined.
Cell salvage in caesarean section
Moderate blood loss is a normal expectation during uncomplicated caesarean section. By salvaging this blood, it may be returned to the patient, even when donor blood transfusion would not normally be considered for the reasons already discussed. This might further serve to reduce postnatal anaemia and its associated morbidity, thus benefiting mothers who lose only a moderate amount of blood during caesarean section and who would not normally be considered for a donor blood transfusion.
The use of cell salvage in the obstetric setting had previously been considered contraindicated as a result of theoretical concerns regarding the risk of amniotic fluid embolism (AFE), a serious but extremely rare (about 1 in 20,000) complication of pregnancy and childbirth. Its pathophysiology is more similar to anaphylaxis than to embolism. AFE is usually diagnosed at autopsy when fetal squamous cells are found in the maternal lungs, but fetal cells are also found in the circulation of labouring women who do not develop the typical clinical features of AFE. Although the term is controversial, the complications of AFE are attributed to multiorgan failure and maternal fatality. Studies examining the quality of blood that would be returned to the mother, if cell salvage had been used at caesarean section, have shown that there is no safety concern with modern equipment as amniotic fluid is both effectively and completely removed by cell salvage processing. 27,28 Despite concerns about AFE as a consequence of cell salvage having proven unfounded in research thus far,27,28 and evidence that the transfer of amniotic fluid into the maternal circulation is a common event that does not necessarily cause adverse effects,29–32 this issue remains of concern to clinicians.
Another potential risk associated with cell salvage in the obstetric setting is sensitisation to red cell antigens leading to haemolytic disease of the fetus and newborn (HDFN). 33,34 This occurs when there is an incompatibility between antigens carried on red blood cells (RBCs) of a woman and her infant, with the rhesus D (RhD) antigen being one of the most important. In a RhD-negative woman carrying a RhD-positive baby, fetal red cells entering the maternal circulation may provoke an immune response in the maternal immune system. These antibodies can then result in severe fetal and neonatal haemolytic disease in future pregnancies. All RhD-negative unsensitised women delivering a RhD-positive baby should be routinely offered a standard dose of anti-D immunoglobulin [at least 500 international units (IU)] as prophylaxis to minimise this risk of sensitisation.
A test for fetomaternal haemorrhage (FMH) is recommended to quantify the volume of fetal red cells that have entered the maternal circulation and determine if additional doses of anti-D immunoglobulin are indicated. The Kleihauer–Betke (hereafter referred to as Kleihauer) test is a manual test undertaken in hospital transfusion laboratories as an initial screen to assess the volume of FMH. As this test is associated with a high coefficient of variation, referral for more specialist testing with flow cytometry is recommended for accurate confirmation if the FMH is estimated to be ≥ 2 ml by the Kleihauer test.
The volume of fetal red cells in maternal blood following cell salvage is variable but can be relatively large. Accordingly, updated UK guidelines from the British Society of Haematology published in 201434 recommend a minimum anti-D immunoglobulin (Ig) dose of 1500 IU to be administered after reinfusion of salvaged red cells. FMH testing, as above, should indicate if any additional doses of anti-D Ig are required. Antibodies to other red cell antigens are also implicated in causing HDFN. 35 These may have consequences for future pregnancies or long-term blood transfusion. Although there is no evidence to suggest that cell salvage increases the risk of sensitisation, this topic has not been specifically addressed in studies to date,36,37 but merits further scrutiny.
The National Institute for Health and Care Excellence (NICE) currently only recommends cell salvage for obstetrics in the emergency management of massive haemorrhage in caesarean section, but has called for robust evidence from clinical trials to support its wider, routine use. 38 The guideline38 states that the technology may be of benefit with careful patient selection, for example caesarean or vaginal delivery in cases with known placenta praevia or placenta accreta. Selective use of cell salvage in obstetrics is also recommended by obstetric and anaesthetic professional bodies. 39,40
Cell salvage is beginning to enter routine use in caesarean section in some hospitals, with the aim of realising some of the benefits known from other settings. A national survey conducted in 2005–6 reported that 38% of UK maternity units had access to cell salvage and 12% included it in their major obstetric haemorrhage protocol. 41 By 2011, this had increased to 49% of UK maternity units having access to cell salvage. 42 However, use in this context remains unproven and is not supported by evidence for its clinical or economic effectiveness. Opinion had not yet solidified in the clinical community and clinicians who were engaged in preparation for the cell SALVage in Obstetrics (SALVO) trial showed that the need to launch a large multicentre randomised controlled trial (RCT) to generate reliable, valid, evidence was recognised.
Cost considerations
Caesarean section is a frequently performed operation and the cost per patient of consumables used in routine cell salvage is approximately the same as a single unit of blood. This must be set against the cost of blood transfusion, the care costs of prolonged hospital stay and the expense of treating adverse events (AEs) associated with transfusion. Cell salvage could realise the dual economic goals of earlier hospital discharge and enhanced maternal quality of life.
Existing evidence
We published43 and updated36 a systematic review that identified one small controlled trial of cell salvage in caesarean section in Italy, with 34 participants in each group, which reported a significant reduction in the number of participants requiring transfusion in the cell salvage group. 44 However, there were flaws in trial design and conduct, including no explanation of the randomisation method. Furthermore, the control group transfusion rate of 23.5% was at least four times greater than normal practice in the UK. The methodology employed in other studies, including a retrospective review,45 case series and isolated case reports,46–54 precluded definitive conclusions but supported the safety of cell salvage in obstetrics.
The above-mentioned NICE review of cell salvage38 focused on the lack of high-quality research and called for RCTs. The Royal College of Obstetricians and Gynaecologists (RCOG) guidelines of 200740 recognised that cell salvage in obstetrics remained controversial. The evidence was graded C as a result of the absence of robust trials on which to base recommendations.
An economic model, drawn from primary cost studies and randomised trials, concluded that cell salvage had lower costs and higher quality-adjusted life-years (QALYs) than all other alternative transfusion strategies, except acute normovolaemic haemodilution. 22 However, this model did not include caesarean section, limiting generalisability to the obstetric setting.
A pilot RCT of cell salvage in elective (planned) caesarean section was performed at one prospective SALVO participating centre55 to help refine the trial processes and assess feasibility. At closure, 57 women undergoing elective caesarean section had been randomised. The consent rate was 71% of women approached. The primary outcome data were collected for 100% of randomised women. The use of cell salvage was feasible and acceptable to staff and to the women who were randomised. Blood salvage and return was technically unproblematic, requiring minimal additional resource. Out of the 30 women who were randomised to cell salvage, it was set up and deployed in 28 women (93%), with sufficient blood collected to enable return of an average of 284 ml (SD 113 ml) of blood to five women. Adherence to the randomisation strategy was high with one case of use of cell salvage in the control group, following intraoperative haemorrhage due to undiagnosed placenta accreta. No woman in the cell salvage group required allogeneic transfusion but one woman (3.7%) in the routine treatment group, with an undiagnosed placenta accreta, received two units of allogeneic blood.
Objectives
The primary objective of the SALVO trial was to determine if the routine use of cell salvage during both elective and emergency caesarean section, in women at risk of haemorrhage, reduced the need for donor blood transfusion in comparison with current practice, for which salvage is not routinely used. In addition, we sought to assess the consistency of the effect across subgroups defined by indication for caesarean, and to determine the effect on secondary outcomes including the number of units of donor blood transfused, fall in haemoglobin level, maternal morbidity resulting from postoperative anaemia (time to first mobilisation, duration of hospital stay and postnatal fatigue), maternal exposure to fetal blood, and its cost-effectiveness in comparison with current practice.
Chapter 2 Methods
Trial design
The SALVO trial was a multicentre individually randomised controlled trial with cost-effectiveness analysis.
Setting
The trial was conducted in 26 hospitals with large obstetric units, in 23 NHS Trusts in England, Wales and Scotland (see Appendix 1 for a list of sites). These units each cared for between 3800 and 8000 births annually and performed between 900 and 2000 caesarean sections per year.
Participants
Eligibility criteria
Inclusion criteria
Women who were admitted to a participating labour ward and who fulfilled all of the following inclusion criteria were eligible to be randomised:
-
being aged ≥ 16 years
-
having the ability to provide informed consent
-
undergoing delivery by caesarean section with an identifiable increased risk of haemorrhage, defined as all emergency caesarean sections, and elective caesarean section for all indications other than maternal request or breech presentation.
A number of systems for classifying the urgency of caesarean section have been suggested,56 both to improve communication between health-care professionals and to assign maximum time intervals for audit purposes between decision for performing caesarean section and actually carrying out the delivery. 57,58 The classification system recommended by the RCOG57 was in use in the UK hospitals during the SALVO trial. For stratification purposes, the important distinction for our purposes was between elective (category 4: no maternal or fetal compromise and timing to suit the woman and maternity services), which has a lower incidence of haemorrhage and transfusion, and emergency categories. We use the term emergency to mean caesareans distinct from the elective category, in that early delivery was mandated clinically. In this category, the immediacy of threat to life of woman or fetus varies and accordingly the urgency to deliver varies too (category 1: immediate threat and timing immediate; categories 2 and 3: no immediate threat and timing flexible depending of assessment of maternal or fetal compromise). We have avoided the use of words such as crash, urgent and scheduled, as these have different meanings in different classification systems.
Abnormality of placentation was based on the degree of abnormal myometrial invasion (placenta accreta, increta and percreta) and the localisation of its insertion within the lower uterine segment (placenta praevia major or minor) as assessed by antenatal ultrasonography examination. In these circumstances, current guidelines suggest that cell salvage may be considered in women who are at a high risk of massive haemorrhage, especially in women who would refuse donor blood. Routine use in these cases is not recommended.
Exclusion criteria
-
Elective first caesarean section for maternal request or breech presentation, with no additional prognostic factor for haemorrhage. Maternal request included women with personal reasons for wishing to avoid vaginal delivery, such as previous traumatic delivery, or psychiatric or psychological problems. These indications do not put the mother at increased risk of haemorrhage. All other indications for caesarean sections, including all emergency cases, were considered an identifiable increased risk of haemorrhage.
-
Sickle cell disease or trait. Use of cell salvage may lead to the presence of abnormal RBCs, which can deform and block the microscopic blood vessels in the body, leading to a sickle cell ‘crisis’. Even if this is only in the trait form, there is an increased chance that this ‘sickling’ may occur while the blood is in the cell salvage collection reservoir awaiting processing owing to the low oxygen levels and, thus, a risk that a sickle cell crisis could be precipitated if this blood is returned to the woman.
-
Active malignancy contraindicated to caesarean section, especially cancer in the abdominal region, as there is a theoretical risk of spreading the cancer should cell salvage be used.
-
Cultural or religious beliefs contraindicating blood transfusion (e.g. Jehovah’s Witnesses), as donor RBC transfusion was the primary study outcome.
-
Significant antibodies making it difficult to find cross-matched blood for transfusion. This is because allogeneic blood for this group of patients is likely to be scarce or unavailable. We considered it appropriate to give these patients cell salvage from the start of their case.
-
Inability to understand written and spoken English.
In some circumstances, some of the participating sites applied clinical judgement not to recruit patients with a high risk of haemorrhage and instead preferred to use cell salvage a priori outside the study.
Screening and consent procedures
Screening and antenatal information
In addition to participant information sheets and informed consent forms, we provided sites with short patient pamphlets (which were used to provide information about the study during the antenatal period) as well as posters. All patient recruitment materials were approved by the Research Ethics Committee (REC) prior to use.
Information about the study was distributed to as many women as possible who were ‘booked’ to deliver at participating centres during their pregnancy and again on admission to delivery suite, whether they were intending a normal (vaginal) delivery or an elective caesarean section. This process was individualised at each participating centre depending on their routine practice to ensure that the maximum number of women were offered information well in advance of delivery. In some centres, women were provided with information about the trial at their routine anomaly scan appointment at 18–22 weeks’ gestation. The provision of study information was documented in the woman’s medical record or handheld notes and a sticker applied to indicate whether or not she was interested in taking part in the study. It was also documented at this point whether or not they would still be interested in taking part in the study in an emergency situation. Written informed consent was obtained by a trained health professional (obstetrician, anaesthetist or midwife) with delegated authority from the principal investigator. All women were assessed to ensure that they had the capacity to provide consent. The process and timing for obtaining written consent varied according to clinical urgency (see Figure 1).
FIGURE 1.
Consent procedure flow chart. CS, caesarean section; IOCS, intraoperative cell salvage.

Recruiting women undergoing elective caesarean section
Eligible women requiring elective caesarean section were provided with further information and the opportunity to ask questions at the time the operation was booked. They were then approached for written consent at the preoperative assessment clinic or on the day of surgery. Randomisation took place on the day of surgery.
Recruiting women undergoing emergency caesarean section
Women who were booked for delivery received information regarding the trial during their pregnancy so that there was sufficient time to consider participation in the trial, should an emergency caesarean section be required. On admission to delivery suite, women’s notes were checked to ensure this information had been supplied and the opportunity for further discussion provided.
After recruitment of half of the required target sample, a substantial protocol amendment was submitted and approved in order to facilitate recruitment of women undergoing emergency caesarean section. This allowed women to be approached for the first time on the delivery suite if they were found to be in the latent stage of labour (i.e. not yet in established first stage of labour according to NICE guidelines59,60) or were comfortable with epidural analgesia, provided that all of the following criteria were fulfilled.
-
They were willing to receive the trial information and were subsequently willing to discuss the participant information sheet and have any questions answered if desired.
-
They had either 0- to 3-cm cervical dilatation and were not contracting regularly (i.e. a maximum of one contraction in 10 minutes, with contraction lasting < 30 seconds) or they were comfortable with effective epidural analgesia in place.
-
They were given at least 1 hour to decide whether or not they would be interested in taking part, should they require a caesarean section. If their situation changed (i.e. labour became established during that hour or they were no longer comfortable under epidural analgesia, or required a caesarean section before the hour elapsed), they were not approached for inclusion. After 1 hour, the women were approached for further discussion and the opportunity for questions about the study. If the women had a contraction during the discussion, the health professional involved would pause and wait for the contraction to finish. Permission to continue with the discussion was then sought.
Women in established labour (i.e. 4-cm cervical dilatation and regular painful contractions), or who were not comfortable with epidural analgesia, were not approached for the first time on delivery suite. Women who were distressed and not in a position to absorb the information on the patient leaflet were not approached for the first time on delivery suite.
Consent was obtained if a decision for caesarean section was made.
Documenting written informed consent
Consent comprised a dated signature from the woman and the dated signature of the person who obtained informed consent. It was clearly stated that the participant was free to withdraw from the trial at any time, for any reason, without prejudice to future care and with no obligation to give the reason for withdrawal. A copy of the signed informed consent document was given to the woman. One copy was retained in the woman’s medical notes and another by the principal investigator in the investigator site file.
Verbal consent and timing of written informed consent
All participants undergoing elective caesarean section gave written informed consent before the intervention. Likewise, the majority of emergency caesarean sections in the absence of acute fetal distress were conducted in a controlled manner with ample time for regional anaesthesia to be established, and written consent was obtained at this stage once the decision for caesarean section had been made.
In some emergency situations, the urgency meant that there would be insufficient time for written consent to be obtained prior to the emergency caesarean section. Under these circumstances, if the woman had the capacity to consent and had previously indicated an interest in taking part in the trial, verbal consent was obtained by an authorised health professional as described above, and documented on the randomisation checklist. Written consent was then sought once the urgency of the situation was over and the caesarean section complete.
Intervention
All staff were sufficiently trained and familiar in the use of the cell salvage machine, in accordance with local procedures and requirements. The majority of sites used conventional cell-saver machines with separate set-up for collection and processing of shed blood, whereas some sites used continuous transfusion systems. To confirm eligibility for randomisation, investigators needed to verify that women met the inclusion/exclusion criteria for the trial as well as gaining informed consent. An eligibility checklist was completed prior to randomisation.
Women were randomly allocated to either:
-
caesarean section with cell salvage (intervention group), set up routinely with collection of shed blood from the outset of surgery and return of any processed blood obtained
-
caesarean section without cell salvage (control group), with transfusion of donor blood according to standard local guidelines.
The intervention group was treated as follows: blood was aspirated from the surgical field, the red cell component isolated by centrifugation and retransfused after washing and filtration. The ability to return salvaged blood is dependent on sufficient volume being collected and processed. Blood was uniformly returned to women in the cell salvage group if this volume threshold was reached and it was a protocol requirement that cell-saver machines were fully set up for both collection and processing upfront at commencement of surgery and that all available processed blood was retransfused regardless of volume. The use of a LDF for transfusion of salvaged blood was not mandated as part of the study intervention protocol, but left up to local guidance. Any reports of severe, unanticipated hypotension and the potential association with the presence of LDFs were monitored. Likewise, the use of one versus two suction devices, the latter having one dedicated to amniotic fluid only at uterotomy, as well as salvage machine ‘bowl size’, was at the discretion of the participating site. Swab washing was encouraged, as it was thought to increase the volume of blood available for processing and, thus, for retransfusion,61 but was ultimately left to the local investigator’s discretion.
The control group was treated as follows: participants received standard current practice (without cell salvage) with allogeneic donor blood transfusion as the standard treatment, if required. In life-threatening acute haemorrhage, women were managed at the discretion of attending clinicians in line with the standard of care for such an emergency,1,40 potentially including the use of cell salvage in the control group.
Follow-up
Participants were followed up until discharge or transfer from the participating hospital only. Postnatal investigations included assessment of postoperative haemoglobin levels, collection of multidimensional fatigue inventory (MFI)62 questionnaires completed by patients (with any missed MFI questionnaires followed up for completion up to 2 weeks after discharge), documentation of AEs, mobilisation and discharge times, and, for RhD-negative women with RhD-positive babies, assessment of exposure to fetal blood by Kleihauer tests and anti-D given.
We took the opportunity to undertake an observational study of practice around anti-D prophylaxis in RhD-negative women who gave birth to a RhD-positive baby. There are UK guidelines stating that all RhD-negative women giving birth to a RhD-positive baby should receive a minimum of 500 IU anti-D Ig as a standard dose following delivery to minimise the risk of RhD alloimmunisation. 34 These guidelines, published in 2014, also recommend that after cell salvage the minimum standard dose should be higher, at 1500 IU anti-D. The maternal sample should be tested after delivery to assess the level of FMH to guide if additional anti-D doses are required following the standard dose. In the majority of centres, the Kleihauer test is undertaken as an initial screening test but, as this is a manual test with a high coefficient of variation, the guidelines also make further recommendations. Given the crudeness of Kleihauer test results, these guidelines recommend flow cytometry tests to be performed for Kleihauer test results of ≥ 2 ml, and repeat administrations and repeat testing after 72 hours for any Kleihauer test results of > 4 ml. 63 All centres participating in the SALVO trial would have been expected to have local guidelines on anti-D prophylaxis. We aimed to collect data around anti-D prophylaxis and FMH testing in all RhD-negative women recruited to this study to assess current practice. We did not attempt to collect follow-up data on the development of red cell sensitisation either to the RhD or indeed other red cell antigens in either group, as this was outside the scope of this particular study.
Outcomes
The primary outcome was the use of donor blood transfusion. Reducing the proportion of women with this outcome should lead to fewer transfusion-related complications.
Primary outcome
The primary outcome was the proportion of women receiving donor blood transfusion to deal with haemorrhage and its consequences, either during caesarean section or between surgery and discharge.
Secondary outcomes
The secondary outcomes analysed included severity of events (quantified as units of donor blood transfused); time to first mobilisation after caesarean section (calculated as the time from delivery until documented first mobilisation, i.e. ability of the woman to walk unassisted); length of hospital stay (calculated as time from delivery until discharge of the mother); pre and postoperative serum haemoglobin; mean fall in haemoglobin level; maternal exposure to fetal blood (defined as FMH as quantified by Kleihauer test and defined as a result of ≥ 2 ml, and administration of anti-D antibody); maternal fatigue measured with the MFI62 [a 20-item self-report questionnaire covering five different dimensions of fatigue (general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity), in which each question is scored between 1 and 5, with each of the five fatigue dimensions yielding a maximum score of 20]; resources used intraoperatively and postoperatively (including cell salvage consumables and donor blood transfusions); AEs and serious adverse events (SAEs), including proportion of transfusion reactions associated with allogeneic blood transfusion; and costs of staff training, service procurement and provision of care, collected alongside clinical outcomes (for full details on health economics methods, see Chapter 4). In addition, we collected process outcomes including the volume of blood returned in cell salvage, the proportion of transfusion reaction associated with allogeneic donor blood transfusion and any episodes of technical failure of cell salvage.
Safety considerations
Adverse events were defined as any untoward medical occurrence in a participant receiving trial intervention, including occurrences that were not necessarily caused by, or related to, that intervention. An AE was therefore defined as any unfavourable and unintended sign (including an abnormal laboratory finding), symptom or disease temporarily associated with study activities.
A SAE was defined as an AE that fulfilled at least one of the following criteria: fatal, life-threatening, required prolongation of hospitalisation beyond 7 nights after caesarean section for maternal reasons, resulted in persistent or significant disability, was a congenital anomaly or birth defect, or was otherwise considered medically significant by the investigator.
The AEs and SAEs were documented if they occurred between randomisation and discharge. They were only reported if they related to the mother, except for SAEs that fulfilled the criteria of congenital anomaly above. The local principal investigator responsible for the care of the participant, or in his or her absence an authorised medic within the research team, was responsible for assessing the severity, causality and expectedness of an AE, and for assessing whether or not the event was serious according to the definitions given above.
If an AE was not defined as serious, the AE was documented in the participants’ medical notes (when appropriate) and on the case report form (CRF). All reported AEs were subject to a central medical review and coded and grouped by a clinician member of the trial team.
All SAEs occurring during the trial observed by the investigator or reported by the participant, whether or not attributed to the trial, were documented in the participants’ medical notes (when appropriate) and reported to the trials office within 24 hours of the site becoming aware of the event. All SAEs were followed up until resolution or the event being considered stable. The chief investigator, or a delegated clinical coapplicant, reviewed all SAE reports within 24 hours and raised any queries to be addressed to the sites. Locally, all serious incidents (such as maternal deaths) occurring at a UK NHS site were subject to root cause analyses. 64
Any SAEs considered both related to the intervention and unexpected were reported to the sponsor and the pragmatic clinical trials unit (PCTU) quality assurance manager within 24 hours, and to the main REC within 15 days. Although there were some known or theoretical potential risks associated with the trial (including maternal exposure to fetal blood, AFE, severe hypotension and transfusion reaction), none was considered to fulfil the criteria of being ‘expected’. Therefore, any SAEs that were at least possibly related to the trial intervention were reported as unexpected SAEs.
If applicable, it was the chief investigator’s responsibility to take any urgent safety measures to ensure the safety and protection of the clinical trial participants from any immediate hazard to their health and safety, in which case the REC was informed immediately by telephone and in writing within 3 days.
Annual progress reports to the REC included a listing of all related and unexpected SAEs. All SAEs were reported to the Data Monitoring Committee (DMC) and Trial Steering Committee (TSC) on the occasion of their meetings (i.e. every 6–12 months). The DMC viewed data with knowledge of treatment. In the event of a participant dying as a result of the study protocol or study interventions, any postmortem findings were to be provided to the chief investigator, who would report the findings to the DMC for continuous safety review.
Data collection and quality assurance
The SALVO study met the requirements of the Data Protection Act 1998,65 NHS Caldicott principles,66 the Research Governance Framework for Health and Social Care67 and REC approval. Identifiable information collected from participants, including name, date of birth, hospital number and contact details, was considered confidential and collected and stored only at the local NHS site. Study data were collected using paper CRFs, with all data being pseudonymised using a unique participant number and transmitted to the trials office by secure NHS e-mail transmission or post.
The following data were collected through CRFs.
-
Before surgery: eligibility, obstetric history, indication for caesarean section, prognostic factors for haemorrhage, demographics, due dates and labour data, preoperative haemoglobin and platelet count.
-
During surgery: time of delivery, time into and out of theatre, transfusion of donor blood products, set-up of cell salvage machine (if applicable), including consumables used and volume of blood returned, reasons for no return of salvaged blood, documentation of any technical failure of cell salvage, additional staff required in theatre owing to cell salvage.
-
Between surgery and discharge: transfusion of donor blood products, postoperative haemoglobin, FMH measured by Kleihauer test, anti-D administration, flow cytometry for Kleihauer results of > 2 ml, repeat Kleihauer and anti-D administration for initial Kleihauer results of > 4 ml, time of mobilisation and discharge, MFI, AEs including admission to higher level of care (HLC).
The CRF data were verified by the trials office and queries raised with individual sites for discrepancies identified. Data were input at the trials office by delegated staff into a bespoke Oracle Database [11g Release 2 (Oracle Corporation, Redwood Shores, CA, USA)] with a Java [SE 7 (Oracle Corporation, Redwood Shores, CA, USA)] user interface, set up and managed by the PCTU. Data quality was monitored through source data verification on samples of patient records during on-site monitoring and during remote self-monitoring activities, and through central statistical monitoring with discrepancies raised from database extracts, highlighting outliers and discrepancies. On-site and self-monitoring activities also included verification of eligibility, informed consent and completeness of local trial documents according to a predefined trial monitoring plan.
On 69 occasions, stratification factors were found to be entered incorrectly during the randomisation procedure, but these were corrected to the true values in the analysis, which adjusted for stratification factors.
Additional quality control measures undertaken included a cross-check of primary outcome data against local transfusion laboratory records on recommendation of the TSC. Manual data entry was also subject to quality control procedures according to predefined procedures, with 100% of primary outcome data being checked and 10% of all other data being checked with an allowable error threshold of 2% for non-primary outcome data.
Sample size
Establishing a baseline rate for the primary outcome was not straightforward because estimates in the published literature for blood transfusion in caesarean section varied widely (1.8–23.5%). 44,68 Factors influencing this figure include country of origin, indication for caesarean section (emergency or elective) and local transfusion policy. Our audits in two centres conducted at the time of study planning put transfusion rates for an unselected caesarean section population at around 5%. A detailed audit of donor blood use at the Royal Hallamshire Hospital Sheffield during the period 2009–10, without cell salvage in routine use, was performed by cross-reference of perioperative records, blood bank data and electronic records stored in cell salvage machines. It reported that in a recent series of 1647 caesarean sections over 10 months, 89 women were transfused with donor blood, giving a rate of 5.4% (Ian Wrench, Royal Hallamshire Hospital Sheffield, 2011, personal communication). A similar audit at Birmingham Women’s Hospital of all caesarean sections carried out in 2006 showed that, of 1674 women, 83 (5.0%) received a transfusion. 43 Both auditing units delivered approximately 7300 women per year with a comparable caesarean section rate and could thus be considered representative of UK tertiary obstetric unit practice. Our pilot sample55 was too small to assist in providing reliable information on sample size calculations. In the light of reported contemporary observations and audited data on transfusion rates, the assumption of a 5% event rate was used to base the main sample size calculation on.
The expected effect estimate was informed by the literature. Our systematic review43 and its most recent update36 showed only one small trial, published in 1998,44 which randomised a total of 68 participants to either cell salvage or standard care. The transfusion rate was 23.5% in the control group and 2.9% in the cell salvage group. The control event rate was considerably higher than that observed in current UK practice and inconsistent with literature from other sources. 21,22 This was probably due to a sample at an exceptionally high risk of haemorrhage. Weaknesses that raise the risk of bias (e.g. inadequate concealment of randomisation) precluded reliance on it alone to inform our calculations. Non-obstetric literature evaluating cell salvage in interventions with a moderate to high risk of transfusion had two high-quality systematic reviews: a HTA report citing a RR of exposure to allogeneic blood of 0.59 (95% CI 0.48 to 0.73) with salvage22 and a Cochrane review reporting a RR of 0.62 (95% CI 0.50 to 0.70) for transfusion with salvage compared with normal practice. 21 Detecting a smaller effect size would have been possible but the larger sample size required had to be balanced against the cost and practicability of undertaking such a trial. Based on the HTA report and Cochrane review cited,21,22 we assumed a relative risk for the intervention effect of 0.6 (at a control event rate of 5%, the intervention group would have a transfusion rate of 3%).
Therefore, the planned sample size was a total of 3050 women (1525 per group), to detect an absolute difference in the transfusion rate of 2% and given a power of 80% for a two-sided test, a type I error rate of 5% and event rate of 5% in the control group. Our sample size allowed for primary outcome data and follow-up loss of 1% of randomised cases.
The planned trial sample was also to represent an even split between elective and emergency caesarean sections, the rationale for which was as follows: the primary event rate in the control group was based on data representing caesarean section across ‘all-comers’ in obstetric units, including both emergencies and elective cases. It included all indications at increased risk of haemorrhage. Ideally, this distribution would be faithfully and proportionally represented in the trial population, but there was good reason to suspect that clinicians would find it much more difficult or be more reluctant to recruit those patients at higher risk of haemorrhage, such as emergency indications or in cases of placental abnormality. Equally, a decision to limit recruitment to these high-risk groups alone, while desirable to maximise the primary outcome event rate and reduce sample size, was likely to result in reluctance to take part in the study at all. Adoption of such narrow eligibility criteria may have restricted sites from ever gaining a sufficient rate of recruitment to become confident in the trial processes and rendered the conduct of the trial unviable. In addition, at the time the study was designed, there was an increasing trend for obstetric units to have started utilising cell salvage in the routine, uncomplicated elective caesarean section population to facilitate the generation of an effective skill base among clinical staff to support the deployment of the technology when deemed necessary, even though the majority of these would not suffer significant blood loss. Therefore, a pragmatic compromise to these conflicting requirements was to exclude those elective cases with the very lowest risk of haemorrhage (elective first caesarean section for breech or maternal request) while at the same time prespecifying a desired equal distribution across elective and emergency cases.
Between June 2013 and March 2014, the majority of the elective patient population was recruited relatively rapidly, exceeding our projected target accrual. The emergency patient population was recruited more slowly, along with high-risk elective cases (see Appendix 2, Figure 11). Although sites adapted to the more challenging recruitment of these participants, particularly once the changes to the consenting procedures that were introduced through a substantial protocol amendment had started to take effect, an extension of the projected recruitment duration by 11 months was necessary to allow completion of the target sample size.
Interim analyses
There were no planned interim analyses for this trial. In the lead-up to the recruitment extension request, the funding body recommended an interim futility analysis be presented to the unblinded DMC to assess the probability of achieving a significant result, should the trial be allowed to recruit to completion. This was performed in March 2015 but the DMC did not feel as though it was within its remit to make a decision on the future of the trial based on said analysis. The DMC made its recommendation without the use of the futility analysis results.
Randomisation
Randomisation to the allocated intervention (allocation ratio 1 : 1) was done using a bespoke web-based randomisation system hosted by the University of Bristol. Randomisation of participants was done on the delivery ward by local study staff. The randomisation used random permuted blocks of variable sizes to ensure that trial staff conducting randomisation could not reliably predict the next allocation. Randomisation was stratified by four criteria: centre, type of caesarean section (emergency vs. elective), presence of abnormal placentation versus normal placentation and multiple pregnancy (twins or more) versus singleton pregnancy.
Blinding
Allocation concealment with third-party randomisation helped minimise selection bias. However, given the nature of the intervention, it was not possible to blind local treatment staff and data entry staff to the allocation. Performance bias may cause transfusion rates to vary. This risk was minimised by ensuring that each centre had an intraoperative transfusion protocol for use in theatre and recovery to standardise operative transfusion triggers across both study groups in each centre. Some centres adopted an agreed haemoglobin threshold for transfusion, which was to be applied equally to both groups.
Sites were encouraged to blind postnatal carers to group allocation after caesarean section. The allocation was not recorded in routine case notes, but this did not represent formal blinding as theatre notes were available. The carers on postnatal wards were a different group of staff to the carers on labour wards and operating theatres, and it was on the postnatal wards that the decisions for postoperative donor blood transfusions were made, based on the postoperative haemoglobin level and maternal symptoms. In the event of the need for a donor blood transfusion, serum haemoglobin was measured by blood sample, and pre- and post-transfusion results recorded. This allowed monitoring of numeric transfusion thresholds between units and groups. In the event that between-group variations in haemoglobin transfusion triggers were indeed evident, consideration was given for adjusting for such differences in the final analysis.
The study statistician remained blinded until completion of data collection and sign off of the statistical analysis plan so as not to bias the analysis, and the chief investigator remained blinded until completion of the analysis. For interim reporting purposes to the DMC during the running of the trial, an independent statistician employed by the PCTU produced summaries of unblinded data for a closed report to the DMC.
Statistical methods
General considerations
A detailed analysis plan was developed and agreed by the TSC and the DMC prior to unblinding and data analysis. All coding and analyses were performed using Stata® version 12 (StataCorp LP, College Station, TX, USA). All analyses were intention to treat (ITT). When baseline covariates were missing, mean imputation of the covariate in adjusted analyses were used (note that epidemiological arguments against the use of a missing indicator do not apply in randomised trials). 69 An ITT approach does not dictate that all outcome data must have been collected,70 though pilot work for this trial suggested that all, or close to all, of the primary outcome data would be obtained. When outcome data were missing, we analysed the data for those who did have outcome data, adjusting for baseline covariates. This approach is unbiased if missingness for the outcome is related to observed covariates (‘missing at random’). If missingness in the primary outcome had been > 5% then a sensitivity analysis was to be conducted to explore the missing at random assumption. In this case, a pattern/mixture model estimated by a mean score approach would have been adopted. 70
Post-randomisation exclusions
Although analysis was by ITT, certain exclusions were made post randomisation. These included all women who were enrolled in error (e.g. who did not meet all eligibility criteria) or did not provide valid written informed consent.
Women who withdrew their consent were still analysed unless they specified that their data were not to be used, in which case the data were safely destroyed and excluded from the trial analysis. We also excluded women who experienced a vaginal delivery, as this was not applicable to the outcomes analyses in the sense of the trial, although their baseline characteristics remained available.
Post-randomisation exclusions were not replaced during the recruitment phase, as they were considered part of the 1% anticipated loss to follow-up (see Sample size).
Evaluation of demographics, baseline covariates and implementation of intervention
Demographic factors and clinical characteristics were summarised with counts (percentages) for categorical variables, mean with standard deviation (SD) for normally distributed continuous variables or median with interquartile range (IQR) for other continuous variables. The number of participants who were eligible, recruited and followed up were recorded in a CONSORT (Consolidated Standards of Reporting Trials) flow chart. We also included summaries detailing implementation of the intervention, for example whether or not swabs were washed.
Primary analysis
For the primary outcome measure of patient requirement of peripartum transfusion, differences in treatment effect between treatment groups were assessed using logistic regression. Univariate and multivariable logistic regression models were used to estimate crude and adjusted odds ratios (ORs) with 95% CIs. A two-sided p-value was reported in each case. The primary analysis was adjusted.
Adjusted analysis adjusts for a random effect of treatment centre and fixed effects of stratification variables and other baseline characteristics believed to be associated with the outcome measure of haemorrhage. The following are factors deemed to be associated antenatally with a substantial increase in the incidence of PPH, according to RCOG guidelines:71 known placenta praevia and pre-eclampsia/gestational hypertension.
Another factor believed to substantially increase risk of PPH is placental abruption. 32,71 As the number of individuals observed with this event was likely to be low, it was decided a priori that this covariate would not be adjusted for in the primary analysis. Instead, the analysis was redone excluding those who experienced placental abruption, as a sensitivity analysis.
Analysis of primary outcome: subgroup analysis
The following subgroup analyses were prespecified for the primary outcome:
-
analysis of treatment effect by indication for caesarean section
-
analysis of treatment effect by recruitment centre.
The first of these was analysed by statistically testing for an interaction term between treatment and indication for caesarean section, and the second was analysed by testing for a random slope for the effect of treatment at different treatment centres in addition to a random intercept.
Analysis of primary outcome: sensitivity analysis
The trial groups were compared according to this outcome on an ITT basis. However, because clinicians managing women in the control group had access to a cell salvage machine, it was anticipated that some women in the control group might receive cell salvage in place of a donor blood transfusion. As a sensitivity analysis, we therefore analysed the primary outcome assuming that all instances of the return of cell-salvaged blood in the control group would have been instances of donor blood transfusion had the cell salvage machine not been present.
As mentioned above, the primary analysis was redone excluding those participants who experienced placental abruption as an additional sensitivity analysis.
Analysis of other outcomes
Secondary outcome measures were compared between groups using appropriate methods. Linear regression was used to analyse quantitative outcomes when a symmetric unimodal distribution is expected (number of units transfused, postoperative serum haemoglobin, mean fall in serum haemoglobin level and MFI scales). We analysed five scales of fatigue (each the total score of four items from the 20 statements pertaining to a specific type of fatigue). The analysis of serum haemoglobin allowed for change from baseline by including the preoperative level as an additional covariate.
Time to event variables (i.e. time to first mobilisation, length of hospital stay) were analysed with Cox proportional hazard regression.
Fetomaternal haemorrhage was dichotomised into a Kleihauer test measurement of < 2 ml versus ≥ 2 ml and analysed using logistic regression. Other measures detailing FMH, such as dose of anti-D prophylaxis were summarised accordingly. In the analysis of FMH, we used a cut-off point of a Kleihauer result of ≥ 2 ml to dichotomise the measurement into a binary variable. 63 However, owing to the phrasing of our CRFs, certain measures, such as flow cytometry or repeat Kleihauer tests, were only taken in the event that the initial Kleihauer test results were > 2 ml or > 4 ml, in accordance with guidelines. 34 In addition, any results reported as, for example, < 4 ml could not be dichotomised as described above and were therefore classified as missing data.
Adverse events were analysed using logistic regression. Transfusion reaction associated with donor blood transfusion was not analysed as we only saw one event.
Crude and adjusted estimates of treatment effect were obtained for each outcome, using univariate and multivariable analyses with the same covariates as in the primary analysis.
Further exploratory analyses
Further to the prespecified subgroup analyses, an analysis of treatment effect on donor blood transfusion by abnormal placentation was undertaken for exploratory purposes. We also conducted further analysis to test for consistency of treatment effect in secondary outcomes across subgroups of elective and emergency caesarean section.
We conducted a further sensitivity analysis assuming that a donor blood transfusion would have been required, had salvaged blood not been returned in the control when the cell salvage machine was set up in an emergency situation only (as opposed to all cases of salvaged blood return in the control group). We included this further analysis as an amendment to our original prespecified sensitivity analysis as we recognised, with hindsight, that our assumptions about the erroneous return of salvaged blood in the control group were broad. Therefore, we only reclassified cases for which the blood was returned in an emergency as an attempt to more accurately reflect the truth.
There was also interest surrounding the effect of swab washing on the effectiveness of the intervention. We compared transfusion rates between participants who did and did not have swabs washed, within participants who had the cell salvage machine set up.
Governance and oversight
The SALVO trial was undertaken following clinical trials database registration (registry number ISRCTN66118656) and the required regulatory approvals and local NHS permissions (UK REC North West – Haydock, reference number 12/NW/0513). The study was funded by the National Institute for Health Research (NIHR) as part of the HTA programme (reference number 10/57/32).
A trial management group (TMG) was responsible for the day-to-day running of the trial, with support from the PCTU at Queen Mary University of London. The TMG reported to the TSC, which was composed of an obstetrician, an independent statistician and a consumer representative, and which convened every 6–12 months and provided overall supervision of the trial. This included giving advice on trial protocol and changes thereof, resolving problems brought to it by the TMG, monitoring the progress of the trial, protocol adherence and patient safety, considering new information and recommendations of the DMC and other authorities, and approving reports and papers for publication.
The DMC consisted of an independent statistician, obstetrician and anaesthetist. The DMC met approximately every 12 months during the running of the trial and reviewed accruing trial data in order to assess whether or not there were any ethics or safety issues as to why the trial should not continue. Interim reports were supplied to the DMC in strict confidence and included unblinded data provided by a PCTU statistician independent of the trial. The DMC formulated recommendations for the attention of the TSC. Both committees also monitored the pooled primary outcome event rate (i.e. across both arms) and formulated recommendations to encourage recruitment of the full spectrum of patients likely to benefit from the intervention.
Patient and public involvement
Working with organised consumer groups capable of identifying research priorities and introducing their ideas into research programmes was a crucial part of our activities leading to the trial. The National Childbirth Trust (NCT) significantly strengthened the project, being well placed to reflect on their experience in relation to avoiding the need for donor blood transfusion and to encourage participation. A volunteer for the NCT collaborated with the project from its inception, advised on the pilot protocol and agreed to provide representation on the TSC. An additional patient and public representative was identified through ‘Katie’s Team’, the Queen Mary University of London women’s health research advisory group, and included in the project at a later stage. This representative participated in TSC and clinical investigator group meetings, reviewed the plain English summary for this report, and advised on dissemination strategies.
In preparation for the trial, a survey was conducted among women who received cell salvage, showing that they perceived the intervention as reassuring, safe and preferable to donor blood transfusion (our primary outcome).
Summary of changes to the project protocol
No changes were made to the objectives, outcomes, eligibility criteria, sample size or statistical parameters during the course of the trial. Three substantial and four minor amendments to the protocol were implemented during the trial, which concerned changes to recruitment materials and strategies, clarifications and administrative changes to the protocol, and an extension of the overall recruitment period.
Chapter 3 Results
Participants
Between June 2013 and April 2016, 3054 participants requiring caesarean section from 26 participating hospitals were recruited (Figure 2). Of these, 26 participants had to be excluded owing to issues surrounding consent or eligibility, as follows.
-
Nine participants gave verbal consent as per protocol (PP), but written consent could not be obtained postoperatively.
-
Four participants gave written informed consent, but the consent form was destroyed or missing and consent could not be reobtained.
-
Four participants had not given consent and were randomised in error, but were not exposed to any trial intervention.
-
One participant was found to have given invalid consent owing to language issues.
-
One participant gave verbal consent but withdrew consent after surgery.
-
Seven participants were found not to have met the eligibility criteria.
FIGURE 2.
Participant enrolment and follow-up. Reproduced from © 2017 Khan et al. 72 This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
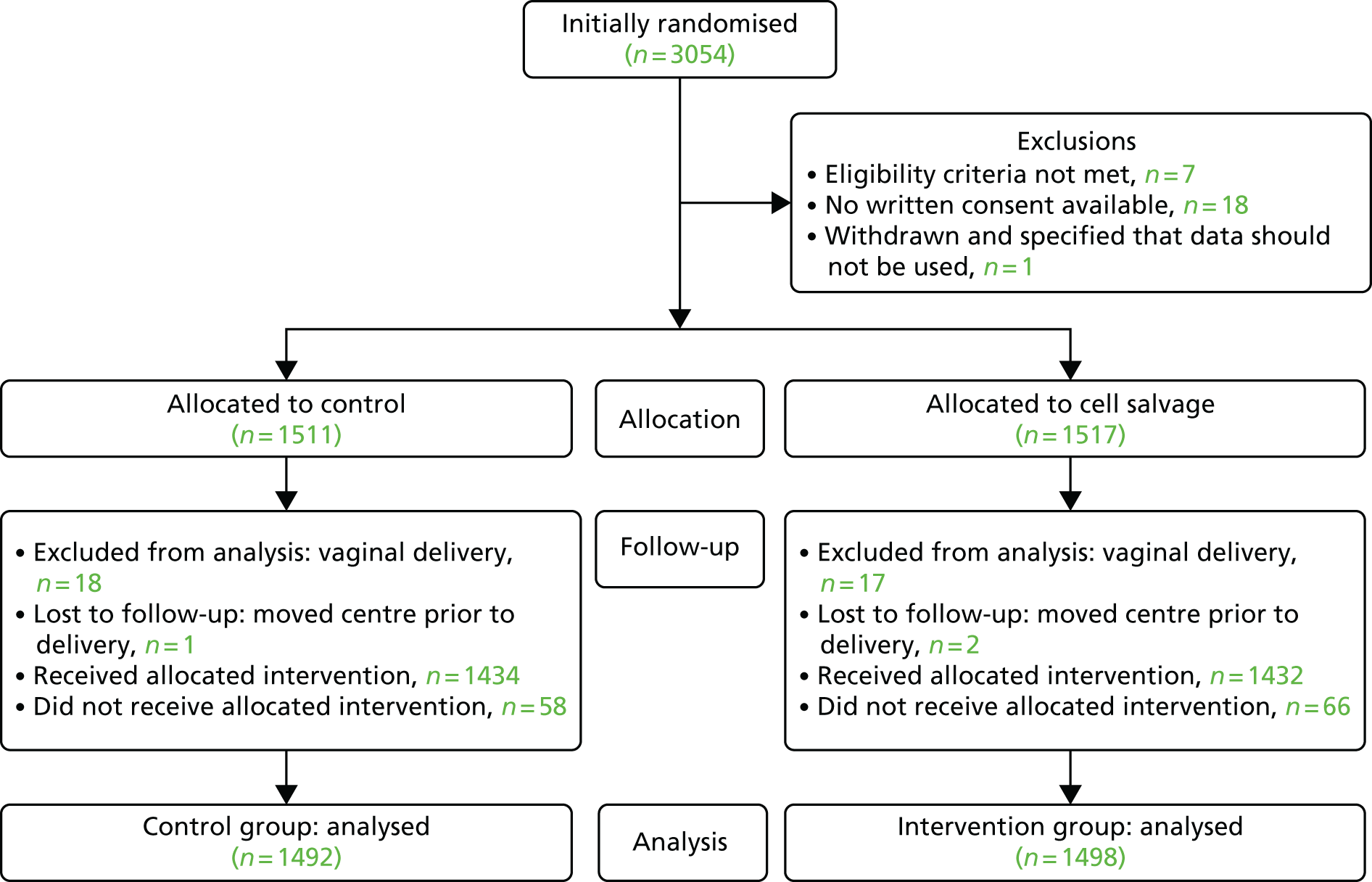
Therefore, 1517 participants were assigned to cell salvage (intervention) and 1511 to usual care (control). Pregnancies resulting in vaginal delivery after assignment (n = 17 in the cell salvage group and n = 18 in the control group) were excluded from the analysis, as were patients who were transferred to a different hospital prior to delivery (n = 2 in the cell salvage group and n = 1 in the control group) and who were therefore lost to follow-up. However, the baseline characteristics for these 38 patients are included in the tables of baseline characteristics. For the analysis, this left 2990 participants (n = 1498 in the cell salvage and n = 1492 in the control group, respectively).
Baseline data
The main characteristics of participants were similar at baseline (Table 1). The distribution of participants across the different sites is summarised in Table 2.
| Characteristics | Control (N = 1511)a | Cell salvage (N = 1517)a |
|---|---|---|
| Age at randomisation (years) | 31.8 (5.8) | 31.6 (5.7) |
| Preoperative haemoglobin (g/l)b | 118.1 (11.5) [19] | 118.4 (11.3) [11] |
| Type of caesarean | ||
| Elective | 687 (45.5) | 669 (44.1) |
| Emergency | 824 (54.5) | 848 (55.9) |
| Multiple births | ||
| Singleton | 1428 (94.5) | 1428 (94.1) |
| Twins or multiple | 83 (5.5) | 89 (5.9) |
| Placentation | ||
| Abnormalc | 135 (8.9) | 136 (9.0) |
| Normal | 1376 (91.1) | 1381 (91.0) |
| Placenta praevia | 130 (8.6) | 133 (8.8) |
| Placenta accreta | 8 (0.5) | 4 (0.3) |
| Pre-eclampsia | 74 (4.9) | 69 (4.5) |
| Previous emergency caesarean | 602 (39.8) | 633 (41.7) |
| Previous elective caesarean | 241 (15.9) | 231 (15.2) |
| Placental abruption | 3 (0.2) | 2 (0.1) |
| Ethnicity | ||
| White | 1213 (80.3) | 1219 (80.4) |
| Mixed | 23 (1.5) | 14 (0.9) |
| Asian or Asian British | 158 (10.5) | 173 (11.4) |
| Black or Black British | 67 (4.4) | 71 (4.7) |
| Other | 50 (3.3) | 40 (2.6) |
| Parity | ||
| 0 | 571 (37.8) | 583 (38.4) |
| 1 | 556 (36.8) | 562 (37.0) |
| 2 | 240 (15.9) | 238 (15.7) |
| ≥ 3 | 144 (9.5) | 134 (8.8) |
| Gravidity | ||
| 1 | 420 (27.8) | 441 (29.1) |
| 2 | 467 (30.9) | 465 (30.6) |
| ≥ 3 | 624 (41.3) | 611 (40.3) |
| Centre | Control (N = 1511)a | Cell salvage (N = 1517)a |
|---|---|---|
| Birmingham Heartlands Hospital | 41 (2.7) | 44 (2.9) |
| Birmingham Women’s Hospital | 7 (0.5) | 6 (0.4) |
| Croydon University Hospital | 48 (3.2) | 48 (3.2) |
| Derriford Hospital Plymouth | 57 (3.8) | 59 (3.9) |
| Hinchingbrooke Hospital | 83 (5.5) | 84 (5.5) |
| James Cook University Hospital | 109 (7.2) | 108 (7.1) |
| Leicester General Hospital | 5 (0.3) | 4 (0.3) |
| Leicester Royal Infirmary | 78 (5.2) | 75 (4.9) |
| Norfolk and Norwich University Hospital | 5 (0.3) | 5 (0.3) |
| Northwick Park Hospital | 13 (0.9) | 15 (1.0) |
| Nottingham City Hospital | 15 (1.0) | 16 (1.0) |
| Queens Hospital Romford | 60 (4.0) | 58 (3.8) |
| Queens Medical Centre Nottingham | 15 (1.0) | 13 (0.9) |
| Royal Hallamshire Hospital Sheffield | 138 (9.1) | 139 (9.2) |
| Royal London Hospital | 84 (5.6) | 87 (5.7) |
| Royal Stoke University Hospital, Stoke-on-Trent | 72 (4.8) | 73 (4.8) |
| Royal United Hospital Bath | 88 (5.8) | 87 (5.7) |
| Royal Victoria Infirmary Newcastle | 119 (7.9) | 116 (7.7) |
| Simpson Centre Edinburgh | 47 (3.1) | 51 (3.4) |
| Singleton Hospital Swansea | 84 (5.6) | 88 (5.8) |
| St. Michaels Hospital Bristol | 26 (1.7) | 21 (1.4) |
| Sunderland Royal Hospital | 192 (12.7) | 190 (12.5) |
| Torbay Hospital | 28 (1.9) | 30 (2.0) |
| West Middlesex University Hospital | 49 (3.2) | 52 (3.4) |
| Whipps Cross University Hospital | 29 (1.9) | 27 (1.8) |
| Whiston Hospital | 19 (1.3) | 21 (1.4) |
Implementation of cell salvage
In the intervention group, 1432 (95.6%) participants received their allocated treatment with the cell salvage machine set up. There were 24 cases (1.6%) for which the cell salvage machine was unavailable or out of order and 42 cases (2.8%) for which the machine was simply not set up in deviation of the protocol. In the group receiving salvage, 50.8% had salvaged blood returned averaging 259.9 ml (Table 3). In the control group, 1434 (96.1%) participants received their assigned intervention without the cell salvage machine set up. In 15 cases (1.0%) the cell salvage machine was used in an emergency and in 43 cases (2.9%) it was set up from the start of the operation, in deviation from the protocol.
| Measure | Control (N = 1492) | Cell salvage (N = 1498) |
|---|---|---|
| Cell salvage machine set up | ||
| Set up | 43 (2.9%) | 1432 (95.6%) |
| Emergency use | 15 (1.0%) | 0 (0.0%) |
| Not set up | 1434 (96.1%) | 42 (2.8%) |
| Unavailable/out of order | 0 (0.0%) | 24 (1.6%) |
| Received allocated treatment | 1434 (96.1%) | 1432 (95.6%) |
| If cell salvage set up (including emergency use) (n = 1490) | ||
| Suckers used | ||
| 1 | 27 (48.2%) | 829 (58.1%) |
| 2 | 29 (51.8%) | 598 (41.9%) |
| Missinga | 2 | 5 |
| Swabs washed | 21 (36.8%) [1] | 781 (54.8%) [6] |
| Size of centrifuge bowl used (ml)b | 183.2 (59.2) [2] | 177.1 (59.8) [37] |
| LDF used | 25 (43.9%) [1] | 782 (54.9%) [7] |
| Salvaged blood returned | 35 (60.3%) [0] | 726 (50.8%) [3] |
| If blood returned during cell salvage (n = 761) | ||
| Volume of blood returned to mother (ml) | 288.4 (198.3) | 259.9 (149.7) |
| If no blood returned during cell salvage (n = 726) | ||
| Reason for no return | ||
| No blood produced | 14 (63.6%) | 575 (88.9%) |
| Technical error | 0 (0.0%) | 25 (3.9%) |
| Otherc | 8 (36.4%) | 47 (7.3%) |
| Missing | 1 | 56 |
Primary outcome
Overall, the donor blood transfusion rate was 2.5% in the group assigned to cell salvage and 3.5% in the control group, but this result did not reach statistical significance [adjusted OR 0.65, 95% CI 0.42 to 1.01; p = 0.056 (Table 4)]. In a subgroup analysis exploring the consistency of treatment effects in procedures undertaken at different levels of urgency, the transfusion rate was 3.0% in women assigned to salvage and 4.6% in the control group among emergency caesareans (adjusted OR 0.58, 95% CI 0.34 to 0.99), whereas it was 1.8% in the intervention group and 2.2% in the control group among elective caesareans (adjusted OR 0.83, 95% CI 0.38 to 1.83), but the interaction was not statistically significant (p = 0.46, see Table 4).
| Analysis | Control (N = 1492), n (%) | Cell salvage (N = 1498), n (%) | Crude risk difference % (95% CI) | Crude intervention OR (95% CI) | p-value: crude analysis | Adjusteda intervention OR (95% CI) | p-value: adjusted analysis |
|---|---|---|---|---|---|---|---|
| Primary analysis | |||||||
| Overall | 52 (3.5) | 37 (2.5) | –1.0 (–2.2 to 0.2) | 0.70 (0.46 to 1.08) | 0.10 | 0.65 (0.42 to 1.01) | 0.056 |
| Subgroup analysis | |||||||
| Emergency caesarean (n = 1641) | 37 (4.6) | 25 (3.0) | 0.58 (0.34 to 0.99) | ||||
| Elective caesarean (n = 1349) | 15 (2.2) | 12 (1.8) | 0.83 (0.38 to 1.83) | ||||
| p-value for interaction | 0.46 | ||||||
| Sensitivity analysis | |||||||
| Assumption: return of cell-salvaged blood in the control group avoided transfusions | 83 (5.6) | 37 (2.5) | –3.1 (–4.5 to –1.7) | 0.43 (0.29 to 0.64) | < 0.001 | 0.39 (0.26 to 0.59) | < 0.001 |
| Excluding participants with placental abruption (cell salvage group: n = 2, control group: n = 3) | 51 (3.4) | 37 (2.5) | –1.0 (–2.2 to 0.3) | 0.72 (0.47 to 1.10) | 0.13 | 0.67 (0.43 to 1.03) | 0.071 |
There was no significant difference in the clinical effectiveness of the intervention between centres (p-value for random slope = 0.091). In a prespecified sensitivity analysis assuming that a donor blood transfusion would have been required, had salvaged blood not been returned in the control group (n = 31), the effect of cell salvage was significant [5.6% vs. 2.5%, adjusted OR 0.39, 95% CI 0.26 to 0.59; p < 0.001 (see Table 4)]. When excluding cases of placental abruption from the primary analysis, little difference in the results was seen, with one transfusion excluded in the control group as a result [3.4% vs. 2.5%, adjusted OR 0.67, 95% CI 0.43 to 1.03; p = 0.071 (see Table 4)].
We also reviewed primary outcome events against available transfusion guidelines in order to determine whether or not the lack of blinding introduced bias. When specific haemoglobin thresholds were defined in local guidelines, we compared these with participants’ reported postoperative haemoglobin values. We found 25 instances (cell salvage group n = 14, control group n = 11) in which donor blood was administered postoperatively without locally defined haemoglobin thresholds having been reached. The TMG did not deem the difference between intervention groups a cause for concern, also accounting for the fact that other less quantifiable factors may also be taken into account when deciding on donor blood transfusion. Therefore, we did not adjust our analysis accordingly.
Secondary outcomes
Allocation to cell salvage did not have an effect on the units of donor blood transfused [adjusted mean difference (MD) –0.12, 95% CI –0.80 to 0.57; p = 0.74 (Table 5)].
| Secondary outcomes | Control (n = 1492) | Cell salvage (n = 1498) | Crude intervention OR/MD/HR (95% CI) | p-value: crude analysis | Adjusteda intervention OR/MD/HR (95% CI) | p-value: adjusted analysis |
|---|---|---|---|---|---|---|
| Units of blood transfused,b mean (SD) | 2.65 (1.66) | 2.70 (1.70) | 0.05 (–0.67 to 0.76) | 0.89 | –0.12 (–0.80 to 0.57) | 0.74 |
| Time to mobilisation (days),c,d median (IQR) [n missing] | 0.74 (0.45) [49] | 0.72 (0.45) [61] | 1.07 (0.99 to 1.15) | 0.079 | 1.11 (1.03 to 1.19) | 0.006 |
| Length of hospital stay (days),c,e median (IQR) [n missing] | 2.13 (1.41) [24] | 2.13 (1.37) [12] | 1.04 (0.97 to 1.12) | 0.26 | 1.08 (1.00 to 1.16) | 0.050 |
| Safety outcomes | ||||||
| Postoperative haemoglobin level (g/l),f mean (SD) [n missing] | 103.1 (12.1) [47] | 103.8 (12.2) [61] | 0.74 (–0.15 to 1.63) | 0.10 | 0.63 (–0.09 to 1.35) | 0.085 |
| Fall in haemoglobin level (g/l),f mean (SD) [n missing] | 15.0 (11.2) [65] | 14.5 (11.1) [72] | –0.49 (–1.31 to 0.33) | 0.24 | –0.68 (–1.40 to 0.04) | 0.066 |
| Any AE experienced, n (%) [n missingg] | 191 (12.8) [0] | 199 (13.3) [1] | 1.04 (0.84 to 1.29) | 0.69 | 1.02 (0.81 to 1.29) | 0.84 |
| FMH,h n (%) [n missing] | 9 (10.5) [33] | 21 (25.6) [51] | 2.95 (1.26 to 6.89) | 0.013 | 5.63 (1.43 to 22.14) | 0.013 |
A small difference was detected between cell salvage and control groups for time to mobilisation [median 0.74 days vs. 0.72 days, adjusted hazard ratio (HR) 1.11 days, 95% CI 1.03 to 1.19 days; p = 0.006 (see Table 5)]. This represented a shorter absolute median time to mobilisation of 0.02 days, that is, approximately half an hour. A small difference was also observed in length of hospital stay [median 2.131 days vs. 2.126 days, adjusted HR 1.08 days, 95% CI 1.00 to 1.16 days; p = 0.050 (see Table 5)]. This represented a shorter absolute median hospital stay by 0.005 days, that is, approximately 10 minutes.
Analysis of postoperative haemoglobin levels showed no difference between cell salvage and control groups [adjusted MD 0.63, 95% CI –0.09 to 1.35; p = 0.085 (see Table 5)] and this was also the case for fall in haemoglobin level from baseline [adjusted MD –0.68, 95% CI –1.40 to 0.04; p = 0.066 (see Table 5)].
Among RhD-negative women giving birth to RhD-positive babies, allocation to cell salvage was associated with greater FMH, defined as a Kleihauer testing result of ≥ 2 ml [10.5% vs. 25.6%, adjusted OR 5.63, 95% CI 1.43 to 22.14; p = 0.013 (see Table 5)]. It should be noted that for 67 patients, Kleihauer testing was done but results could not be classified as they were reported as ‘< 4 ml’ or similar estimations, according to local guidelines; these results are therefore not available for analysis. Anti-D was routinely administered in the vast majority of RhD-negative mothers with RhD-positive babies (99.2% cases in control group, 98.6% cases in cell salvage group) (Table 6), although a total of three women across both groups did not seem to have received a minimum standard dose of anti-D following delivery, with a risk of RhD alloimmunisation. The dose of anti-D that was administered is summarised in Table 6, with further detail on management of large FMH detailed in Table 7. Out of the 140 RhD-negative mothers in the cell salvage group, only 40.6% received a dose of 1500 IU, with 56.5% receiving 500 IU. It is worth nothing that the updated guidance34 recommending a higher standard anti-D dose of 1500 IU after cell salvage was published in 2014; therefore, practice in some centres with doses of 500 IU may predate publication of these guidelines.
| Measure | Control (N = 1492) | Cell salvage (N = 1498) |
|---|---|---|
| RhD-negative mother with RhD-positive baby, n (%) | 130 (8.7) | 140 (9.3) |
| If mother negative and baby positive, n (%) (N = 270) | ||
| Anti-D prophylaxis administered? n (%) | 129 (99.2) | 138 (98.6) |
| Anti-D prophylaxis dose (IU), n (%) | ||
| 500 | 59 (46.1) | 78 (56.5) |
| 1500 | 67 (52.3) | 56 (40.6) |
| Othera | 2 (1.6) | 4 (2.9) |
| Missingb | 1 | 0 |
| Kleihauer test performed? n (%) [n missing] | 119 (92.2) [1] | 133 (95.0) [0] |
| FMH (Kleihauer test result of ≥ 2 ml) n (%) [n missing]c | 9 (10.5) [33] | 21 (25.6) [51] |
| Sample sent for flow cytometry, n (%)d | 1 (33.3) | 9 (75.0) |
| Repeat Kleihauer test performed? n (%)e | 1 (50.0) | 6 (100.0) |
| Further anti-D prophylaxis administered? n (%) [n missing]e | 1 (100.0) [1] | 1 (16.7) [0] |
| Further anti-D prophylaxis dose (IU), n (%) | ||
| 250 | 0 (0.0) | 1 (100.0) |
| 1500 | 1 (100.0) | 0 (0.0) |
| FMH by Kleihauer (ml) | Anti-D dose (IU) | Flow cytometry undertakena | Flow cytometry result (ml) | Repeat Kleihauer undertakenb | Blood returned during cell salvage? |
|---|---|---|---|---|---|
| Cell salvage group (n = 21) | |||||
| 26 | 4500 | Yes | 26 | Yes | Yes |
| 11 | 1500 | Yes | 9 | Yes | Yes |
| 10 | 1500 | Yes | 10 | Yes | Yes |
| 6 | 1000 | Yes | 5 | Yes | No |
| 6 | 1000 | Yes | 6 | Yes | Yes |
| 5 | 1500 | Yes | 12 | Yesc | Yes |
| 4 | 5000 | No | – | – | No |
| 4 | 500 | Yes | 2 | – | No |
| 3 | 500 | No | – | – | Yes |
| 3 | None | No | – | – | No |
| > 2 | 500 | Yes | 2 | – | Yes |
| > 2 | 1500 | Yes | 7 | – | Yes |
| 2 | 1500 | – | – | – | Yes |
| 2 | 1500 | – | – | – | No |
| 2 | 1500 | – | – | – | Yes |
| 2 | 500 | – | – | – | Yes |
| 2 | 500 | – | – | – | Yes |
| 2 | 500 | – | – | – | Yes |
| 2 | 500 | – | – | – | Yes |
| 2 | 500 | – | – | – | Yes |
| 2 | 500 | – | – | – | No |
| Control group (n = 9) | |||||
| 37 | 4000 | Yes | 37 | Yesc | Not set up |
| > 4 | 1500 | No | – | No | Not set up |
| 3 | 500 | No | – | – | Not set up |
| 2 | 500 | – | – | – | Not set up |
| 2 (5 patients) | 1500 | – | – | – | Not set up |
Kleihauer tests were undertaken on the majority of RhD-negative participants in both groups (92.2% cases in the control group and 95.0% cases in the cell salvage group, see Table 6). A total of 5% of women in cell salvage group and 7.8% women in the control group did not have Kleihauer tests undertaken following delivery. It should be noted that a dose of 500 IU of anti-D covers a FMH of 4 ml and, although a 1500-IU anti-D dose covers a FMH of ≈12 ml, a small proportion of women may have had an even higher fetomaternal bleed. A Kleihauer test is therefore recommended to determine if additional doses of anti-D are needed in addition to the standard dose.
Repeat Kleihauer tests were undertaken 72 hours post anti-D administration for the majority of applicable participants and further anti-D was administered for two participants (see Table 6). Overall, eight women (n = 2 in the control group and n = 6 in the intervention group) had larger instances of FMH with a Kleihauer test result of > 4 ml. Of the two in the control group, one woman had the largest observed FMH on the study, with 37 ml. She was managed as per guidelines with flow cytometry for confirmation of the FMH volume, additional anti-D dose administered and a repeat Kleihauer test undertaken. The other had an initial Kleihauer test result of > 4 ml and so was administered 1500 IU of anti-D but had no flow cytometry undertaken. Neither of these two women in the control group received cell salvage. Out of the six women in the intervention group, five had received cell-salvaged blood back. All six women had confirmatory flow cytometry done and repeat Kleihauer tests undertaken.
Breakdowns of FMH by sucker use and return of salvaged blood were also summarised (Table 8). On a descriptive level, sucker use appeared to have little effect on the proportion of participants experiencing FMH (28.3% when one sucker was used vs. 25.0% when two suckers were used). Return of salvaged blood appeared to increase FMH (13.0% in cases of no salvaged blood returned vs. 48.4% in cases for which salvaged blood was returned).
| One sucker used (N = 53) | Two suckers used (N = 24) | |
|---|---|---|
| FMHa | 15 (28.3) | 6 (25.0) |
| No blood returned (N = 46) | Blood returned (N = 31) | |
| FMHb | 6 (13.0) | 15 (48.4) |
Administration of other donor products (namely fresh-frozen plasma, platelets and cryoprecipitate) is summarised in Table 9.
| Measure | Control (N = 1492) | Cell salvage (N = 1498) |
|---|---|---|
| Intraoperative | ||
| Donor blood given, n (%) | 20 (1.3) | 12 (0.8) |
| Unitsa of blood, mean (SD) | 2.60 (1.27) | 2.08 (0.51) |
| FFP given, n (%) | 9 (0.6) | 3 (0.2) |
| Units of FFP, mean (SD) | 2.11 (0.33) | 3.00 (1.00) |
| Platelets given, n (%) | 2 (0.1) | 0 (0.0) |
| Units of platelets, mean (SD) | 1.50 (0.71) | – |
| Cryoprecipitate given, n (%) | 2 (0.1) | 0 (0.0) |
| Units of cryoprecipitate, mean (SD) | 2.00 (0.00) | – |
| Postnatal | ||
| Donor blood given, n (%) | 36 (2.4) | 30 (2.0) |
| Units of blood, mean (SD) | 2.39 (1.57) | 2.50 (1.68) |
| FFP given, n (%) | 8 (0.5) | 7 (0.5) |
| Units of FFP, mean (SD) | 3.25 (2.19) | 3.29 (0.95) |
| Platelets given, n (%) | 2 (0.1) | 1 (0.1) |
| Units of platelets, mean (SD) | 1.00 (0.00) | 1.00 (–) |
| Cryoprecipitate given, n (%) | 3 (0.2) | 0 (0.0) |
| Units of cryoprecipitate, mean (SD) | 2.00 (0.00) | – |
The MFI questionnaire was completed for 2408 (80.5%) participants. Analysis of MFI statement scores showed no significant differences between allocation groups for the fatigue categories of ‘general fatigue’, ‘physical fatigue’, ‘reduced motivation’ or ‘reduced activity’. There was a modest difference between the cell salvage and control groups for ‘mental fatigue’ [adjusted MD –0.30, 95% CI –0.59 to –0.01; p = 0.043 (Table 10)].
| MFI groupsa | Control (n = 1187) | Cell salvage (n = 1221) | Crude MD (95% CI) | p-value: crude analysis | Adjusted(b) MD (95% CI) | p-value: adjusted analysis |
|---|---|---|---|---|---|---|
| General fatigue | 12.7 (3.6) [52] | 12.5 (3.6) [39] | –0.18 (–0.47 to 0.12) | 0.24 | –0.18 (–0.47 to 0.11) | 0.22 |
| Physical fatigue | 12.3 (3.9) [22] | 12.3 (3.9) [31] | –0.05 (–0.36 to 0.26) | 0.75 | –0.06 (–0.37 to 0.25) | 0.69 |
| Reduced motivation | 9.6 (3.3) [36] | 9.8 (3.4) [46] | 0.12 (–0.15 to 0.40) | 0.37 | 0.13 (–0.14 to 0.40) | 0.36 |
| Reduced activity | 11.3 (3.8) [42] | 11.4 (3.6) [47] | 0.12 (–0.18 to 0.43) | 0.44 | 0.12 (–0.18 to 0.41) | 0.45 |
| Mental fatigue | 8.7 (3.6) [19] | 8.4 (3.6) [41] | –0.28 (–0.57 to 0.01) | 0.061 | –0.30 (–0.59 to –0.01) | 0.043 |
As for process outcomes, the cell salvage machine was set up for 1490 participants across both groups and salvaged blood was processed and returned for a total of 761 participants [35 out of 58 (60.3%) in the control group and 726 out of 1432 (50.8%) in the cell salvage group]. Volume of salvaged blood was recorded and summarised and reasoning behind no return of salvaged blood was also denoted, with the most common reason being that no blood was produced by the cell salvage machine. Further measures detailing implementation of the intervention, such as machine settings and equipment use, are summarised in Table 11.
| Measure | Control (N = 1492) | Cell salvage (N = 1498) |
|---|---|---|
| If cell salvage set up (including emergency use) (n = 1490) | ||
| Collection sets useda | ||
| 0 | 0 (0.0) | 1 (0.1) |
| 1 | 36 (100.0) | 1240 (99.3) |
| 2 | 0 (0.0) | 7 (0.6) |
| 3 | 0 (0.0) | 1 (0.1) |
| Missingb | 0 | 3 |
| Processing packs useda | ||
| 0 | 2 (5.7) | 50 (4.0) |
| 1 | 32 (91.4) | 1179 (94.5) |
| 2 | 1 (2.9) | 13 (1.0) |
| 3 | 0 (0.0) | 4 (0.3) |
| 4 | 0 (0.0) | 2 (0.2) |
| Missing | 1 | 4 |
| Default settings used | 54 (94.7) [1] | 1287 (90.3) [6] |
Adverse events
There were 453 AEs reported in total across both groups, with 191 participants experiencing 220 AEs in total in the control group and 199 participants experiencing a total of 233 AEs in the cell salvage group. There was no significant difference between allocation groups for experiencing an AE (adjusted OR 1.02, 95% CI 0.81 to 1.29; p = 0.84, see Table 5). Table 12 shows details and descriptions of AEs. One case of transfusion reaction associated with allogeneic donor blood was observed in the control group. There was no case of AFE observed, with or without use of a LDF.
| Measure | Control (N = 1492) | Cell salvage (N = 1498) |
|---|---|---|
| Any AE experienced | 191 (12.8) [0] | 199 (13.3) [1] |
| Total AEs | 220 | 233 |
| Breakdowns per AE (n = 453) | ||
| AE severity | ||
| Mild | 89 (40.5) | 101 (43.3) |
| Moderate | 88 (40.0) | 92 (39.5) |
| Severe | 34 (15.4) | 35 (15.0) |
| Life-threatening | 9 (4.1) | 4 (1.7) |
| Fatal | 0 (0.0) | 1 (0.4) |
| AE relatedness to intervention (if cell salvage set up, including emergency use) (n = 238) | ||
| Unrelated | 8 (57.1) | 160 (71.4) |
| Unlikely | 5 (35.7) | 47 (21.0) |
| Possiblea | 1 (7.1) | 14 (6.3) |
| Probablea | 0 (0.0) | 2 (0.9) |
| Definitea | 0 (0.0) | 1 (0.4) |
| Is the AE seriousb | 20 (9.1) | 15 (6.4) |
| AE descriptionsc by system organ class | ||
| Blood and lymphatic system disorders | ||
| Thrombocytopenia | 0 (0.0) | 3 (1.3) |
| Anaemia | 2 (0.9) | 6 (2.6) |
| Cardiac disorders | ||
| Sinus tachycardia | 0 (0.0) | 5 (2.2) |
| Hypotension | 2 (0.9) | 2 (0.9) |
| Supraventricular tachycardia | 1 (0.5) | 1 (0.4) |
| Gastrointestinal disorders | ||
| Diarrhoea | 0 (0.0) | 1 (0.4) |
| Ileus | 4 (1.8) | 3 (1.3) |
| Incontinence | 0 (0.0) | 1 (0.4) |
| General disorders and administration site conditions | ||
| Pain | 4 (1.8) | 3 (1.3) |
| Non-cardiac chest pain | 1 (0.5) | 0 (0.0) |
| Immune system disorders | ||
| Reaction to cell-salvaged blood | 0 (0.0) | 5 (2.2) |
| Reaction to donor blood | 1 (0.5) | 0 (0.0) |
| Allergic reaction | 1 (0.5) | 0 (0.0) |
| Infections and infestations | ||
| Lung infection | 2 (0.9) | 0 (0.0) |
| Wound infection | 5 (2.3) | 6 (2.6) |
| Uterine infection | 1 (0.5) | 2 (0.9) |
| Sepsis | 11 (5.0) | 11 (4.7) |
| Unknown source | 12 (5.5) | 21 (9.0) |
| Injury, poisoning and procedural complications | ||
| Wound dehiscence | 1 (0.5) | 2 (0.9) |
| Metabolism and nutrition disorders | ||
| Hyperglycaemia | 0 (0.0) | 1 (0.4) |
| Musculoskeletal and connective tissue disorders | ||
| Pain in extremity | 0 (0.0) | 2 (0.9) |
| Back pain | 1 (0.5) | 0 (0.0) |
| Nervous system disorders | ||
| Presyncope | 2 (0.9) | 3 (1.3) |
| Seizure | 2 (0.9) | 1 (0.4) |
| Limb weakness | 0 (0.0) | 1 (0.4) |
| Pregnancy, puerperium and perinatal conditions – other | ||
| Hypertensive disease of pregnancy | 32 (14.6) | 34 (14.6) |
| Uterine atony | 1 (0.5) | 0 (0.0) |
| Placental abnormality | 2 (0.9) | 1 (0.4) |
| Maternal exposure to fetal blood | 1 (0.5) | 0 (0.0) |
| Renal and urinary disorders | ||
| Urinary retention | 3 (1.4) | 2 (0.9) |
| Oliguria | 3 (1.4) | 0 (0.0) |
| Chronic kidney disease | 0 (0.0) | 1 (0.4) |
| Prolonged catheterisation | 1 (0.5) | 3 (1.3) |
| Proteinuria | 1 (0.5) | 1 (0.4) |
| Haematuria | 1 (0.5) | 1 (0.4) |
| Reproductive system and breast disorders | ||
| Fibroids | 1 (0.5) | 0 (0.0) |
| Uterine haemorrhage | 106 (48.4) | 93 (39.9) |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough | 0 (0.0) | 1 (0.4) |
| Dyspnoea | 1 (0.5) | 0 (0.0) |
| Hypoxia | 1 (0.5) | 1 (0.4) |
| Pulmonary oedema | 0 (0.0) | 1 (0.4) |
| Sleep apnoea | 1 (0.5) | 0 (0.0) |
| Skin and subcutaneous tissue disorders | ||
| Pruritus | 0 (0.0) | 1 (0.4) |
| Surgical and medical procedures: other | ||
| Anaesthetic complication | 3 (1.4) | 1 (0.4) |
| Surgical complication | 5 (2.3) | 2 (0.9) |
| Wound haematoma | 1 (0.5) | 5 (2.2) |
| Vascular disorders | ||
| Venous eczema | 0 (0.0) | 1 (0.4) |
| Hypertension | 1 (0.5) | 3 (1.3) |
| Thromboembolic event | 1 (0.5) | 1 (0.4) |
| Missingd | 1 | 0 |
In 18 cases, AEs were considered to be related to cell salvage, with 15 events possibly related, two events probably related and one event definitely related to the intervention. Table 13 shows details of related AEs. Of the 18 AEs classed as related to cell salvage, the majority (n = 16) were also in the context of the use of a LDF. These included transient episodes of hypotension that might have been related to the return of cell-salvaged blood, as well as haemorrhagic and infective complications. As cell salvage removes clotting factors and platelets, it can theoretically lead to coagulopathy unless coagulation products are simultaneously given, and there is also a potential risk of returning infective agents such as bacteria in the salvaged blood. The ultimate judgement on whether or not these complications might have been caused by cell salvage lay with the local principal investigator.
| AE relatedness to intervention | Allocation | System organ class of AE | AE description |
|---|---|---|---|
| Possible | Control | Reproductive system and breast disorders | Uterine haemorrhage |
| Possible | Cell salvage | Cardiac disorders | Hypotension |
| Possible | Cell salvage | Immune system disorders | Reaction to cell-salvaged blood |
| Possible | Cell salvage | Immune system disorders | Reaction to cell-salvaged blood |
| Possible | Cell salvage | Infections and infestations | Sepsis |
| Possible | Cell salvage | Infections and infestations | Unknown source |
| Possible | Cell salvage | Infections and infestations | Unknown source |
| Possible | Cell salvage | Infections and infestations | Unknown source |
| Possible | Cell salvage | Infections and infestations | Unknown source |
| Possible | Cell salvage | Infections and infestations | Unknown source |
| Possible | Cell salvage | Infections and infestations | Unknown source |
| Possible | Cell salvage | Reproductive system and breast disorders | Uterine haemorrhage |
| Possible | Cell salvage | Reproductive system and breast disorders | Uterine haemorrhage |
| Possible | Cell salvage | Surgical and medical procedures – other | Wound haematoma |
| Possible | Cell salvage | Infections and infestations | Wound infection |
| Probable | Cell salvage | Immune system disorders | Reaction to cell-salvaged blood |
| Probable | Cell salvage | Immune system disorders | Reaction to cell-salvaged blood |
| Definite | Cell salvage | Immune system disorders | Reaction to cell-salvaged blood |
There were 36 SAEs reported during the SALVO trial. Of these, 32 are included in the AE table (Table 14), with one SAE having three AEs pertaining to it. There were four additional SAEs concerning the offspring (e.g. congenital anomalies), which did not require reporting for the main AE analysis. One fatal event was observed among trial participants. It was considered unrelated to the intervention. This maternal death occurred in a patient who died on the sixth day following her delivery.
| Descriptiona of SAE | Allocation | Reason for seriousness | SAE relatedness to intervention |
|---|---|---|---|
| Bladder damage during surgeryb | Control | Hospitalisation > 7 days | Unrelated |
| Concealed obstetric haemorrhage | Control | Life-threatening | Unrelated |
| HELLP syndrome | Control | Other | Unrelated |
| Infection of unknown origin | Control | Hospitalisation > 7 days | Unrelated |
| Massive obstetric haemorrhage | Control | Life-threatening | Unrelated |
| Massive obstetric haemorrhage | Control | Life-threatening | Unlikely |
| Massive obstetric haemorrhage | Control | Life-threatening | Unrelated |
| Massive obstetric haemorrhage | Control | Life-threatening | Unrelated |
| Massive obstetric haemorrhage | Control | Life-threatening | Unrelated |
| Massive obstetric haemorrhage | Control | Life-threatening | Unrelated |
| Massive obstetric haemorrhage | Control | Life-threatening | Unrelated |
| Pneumonia | Control | Hospitalisation > 7 days | Unrelated |
| Pre-eclampsia | Control | Hospitalisation > 7 days | Unrelated |
| Pre-eclampsia | Control | Hospitalisation > 7 days | Unrelated |
| Pulmonary embolism and obstetric haemorrhage | Control | Life-threatening | Unrelated |
| Sepsis | Control | Hospitalisation > 7 days | Unrelated |
| Vertebral disc prolapse | Control | Disability/incapacity | Unlikely |
| Wound complication | Control | Hospitalisation > 7 days | Unrelated |
| Bowel obstruction, caecal gangrene | Cell salvage | Hospitalisation > 7 days | Unrelated |
| Bowel perforation, sepsis, multi-organ failure | Cell salvage | Fatal | Unrelated |
| Fetal congenital abnormalityc | Cell salvage | Congenital abnormality/birth defect | Unrelated |
| Fetal congenital abnormalityc | Cell salvage | Congenital abnormality/birth defect | Unrelated |
| Fetal epidermolysis bullosac | Cell salvage | Congenital abnormality/birth defect | Unrelated |
| Hypertension | Cell salvage | Hospitalisation > 7 days | Unrelated |
| Massive obstetric haemorrhage | Cell salvage | Life-threatening | Unlikely |
| Massive obstetric haemorrhage | Cell salvage | Life-threatening | Unrelated |
| Palpitations and shortness of breath. Postpartum echocardiogram suggested mild left ventricular systolic dysfunction | Cell salvage | Hospitalisation > 7 days | Unlikely |
| Pre-eclampsia | Cell salvage | Hospitalisation > 7 days | Unrelated |
| Pre-eclampsia | Cell salvage | Hospitalisation > 7 days | Unrelated |
| Pre-existing atrial fibrillation and wound complication | Cell salvage | Hospitalisation > 7 days | Unrelated |
| Reaction to salvaged blood or LDF (hypotension) | Cell salvage | Life-threatening | Probably |
| Reaction to salvaged blood or LDF (tachycardia, dyspnoea) | Cell salvage | Life-threatening | Definitely |
| Sepsis | Cell salvage | Hospitalisation > 7 days | Unlikely |
| Sepsis | Cell salvage | Hospitalisation > 7 days | Unlikely |
| Stillbirthc | Cell salvage | Congenital abnormality/birth defect | Unrelated |
| Wound complication | Cell salvage | Hospitalisation > 7 days | Unrelated |
| Wound complication | Cell salvage | Hospitalisation > 7 days | Unrelated |
Two serious adverse reactions were reported in this trial (i.e. SAEs that were considered related to the intervention). The first was reported as a reaction to salvaged blood. The patient became tachycardic, flushed and had difficulty breathing, starting shortly after the start of the retransfusion and resolving completely once the transfusion was stopped. The event was classed by the local investigator as life-threatening and as most likely due to the use of a LDF. The second event was a sudden onset of hypotension, following retransfusion of 600 ml of cell-salvaged blood. The patient recovered fully. The event was also reported as life-threatening and as most likely secondary to the use of a LDF.
Further exploratory analyses
There was no significant difference in the clinical effectiveness of cell salvage on secondary outcomes between elective and emergency caesarean section (Table 15) or in the effect of cell salvage on reducing donor blood transfusion between participants with normal and abnormal placentation (p-value for interaction term = 0.28, Table 16).
| Secondary outcome | Elective caesarean section (N = 1349) | Emergency caesarean section (N = 1641) | p-value for interaction term | ||||
|---|---|---|---|---|---|---|---|
| Control (n = 684) | Cell salvage (n = 665) | Adjusteda intervention OR/MD/HR (95% CI) | Control (n = 808) | Cell salvage (n = 833) | Adjusted intervention OR/MD/HR (95% CI) | ||
| Units of blood transfused,b mean (SD) | 3.33 (2.53) | 2.92 (2.35) | –0.20 (–1.42 to 1.02) | 2.38 (1.06) | 2.60 (1.32) | –0.08 (–0.92 to 0.77) | 0.87 |
| Time to mobilisation (days),c median (IQR) [n missing] | 0.79 (0.43) [30] | 0.79 (0.46) [29] | 1.03 (0.92 to 1.15) | 0.69 (0.45) [19] | 0.66 (0.43) [32] | 1.18 (1.07 to 1.30) | 0.083 |
| Length of hospital stay (days),c median (IQR) [n missing] | 2.10 (1.21) [19] | 2.08 (1.00) [10] | 1.06 (0.95 to 1.18) | 2.20 (1.81) [5] | 2.18 (1.49) [2] | 1.09 (0.99 to 1.20) | 0.70 |
| Postoperative haemoglobin level (g/l),d mean (SD) [n missing] | 104.28 (11.19) [17] | 106.18 (12.05) [25] | 1.19 (0.12 to 2.25) | 102.03 (12.78) [30] | 101.91 (12.03) [36] | 0.17 (–0.80 to 1.14) | 0.17 |
| Fall in haemoglobin level (g/l),e mean (SD) [n missing] | 12.37 (9.41) [21] | 11.66 (9.92) [27] | –1.18 (–2.24 to -0.11) | 17.32 (12.17) [44] | 16.86 (11.53) [45] | –0.26 (–1.23 to 0.72) | 0.21 |
| Any AE experienced, n (%) [n missing]e | 48 (7.0) [0] | 48 (7.2) [0] | 1.08 (0.70 to 1.66) | 143 (17.7) [0] | 151 (18.1) [1] | 1.00 (0.77 to 1.31) | 0.78 |
| FMH,f n (%) [n missing] | 4 (10.3) [13] | 9 (26.5) [17] | 9.71 (1.11 to 85.11) | 5 (10.6) [20] | 12 (25.0) [34] | 4.08 (0.81 to 20.51) | 0.51 |
| Event | Placentation | p-value for interaction term | |||||
|---|---|---|---|---|---|---|---|
| Normal (n = 2720) | Abnormal (n = 270) | ||||||
| Control (N = 1357), n (%) | Cell salvage (N = 1363), n (%) | Adjusteda intervention OR (95% CI) | Control (N = 135), n (%) | Cell salvage (N = 135), n (%) | Adjusted intervention OR (95% CI) | ||
| Donor blood transfusion | 40 (2.9) | 24 (1.8) | 0.56 (0.34 to 0.94) | 12 (8.9) | 13 (9.6) | 0.98 (0.42 to 2.32) | 0.28 |
In a sensitivity analysis assuming that a donor blood transfusion would have been required had salvaged blood not been returned in the control when the cell salvage machine was set up in an emergency (n = 8), the effect of cell salvage on donor blood transfusion was significant [4.0% vs. 2.5%, adjusted OR 0.56, 95% CI 0.36 to 0.86; p = 0.008 (Table 17)].
| Event | Control (N = 1492), n (%) | Cell salvage (N = 1498), n (%) | Crude risk difference, % (95% CI) | Crude intervention OR (95% CI) | p-value: crude analysis | Adjusted(a) intervention OR (95% CI) | p-value: adjusted analysis |
|---|---|---|---|---|---|---|---|
| Donor blood transfusion: sensitivity analysis 3b | 60 (4.0) | 37 (2.5) | –1.6 (–2.8 to –0.3) | 0.60 (0.40 to 0.92) | 0.018 | 0.56 (0.36 to 0.86) | 0.008 |
We observed that swab washing greatly increased the proportion of participants who received salvaged blood (16.0% when swabs were not washed vs. 81.3% when swabs were washed) and that the volume of blood returned was higher when swabs were washed [mean (SD) = 32.8 (100.5) when swabs were not washed vs. 219.3 (169.8) when swabs were washed] (Table 18). In a comparison between participants who did and did not have swabs washed within those who had the cell salvage machine set up, no significant difference in the transfusion rates was observed [adjusted OR 0.79, 95% CI 0.39 to 1.57; p = 0.50 (Table 19)].
| Measure | Swab washing | |
|---|---|---|
| Not washed (n = 681) | Washed (n = 802) | |
| Salvaged blood returned, n (%) [n missinga] | 109 (16.0) [1] | 651 (81.3) [1] |
| Volume of blood returned to mother (ml), mean (SD) | 32.8 (100.5) | 219.3 (169.8) |
| Event | Swabs not washed (N = 681), n (%) | Swabs washed (N = 802), n (%) | Crude OR (95% CI) | p-value: crude analysis | Adjusteda OR (95% CI) | p-value: adjusted analysis |
|---|---|---|---|---|---|---|
| Donor blood transfusionb | 18 (2.6) | 18 (2.2) | 0.85 (0.44 to 1.64) | 0.62 | 0.79 (0.39 to 1.57) | 0.50 |
Chapter 4 Health economic evaluation
Introduction
This chapter reports the economic evaluation carried out alongside the SALVO trial. The primary objective of the study was to determine whether or not the routine use of cell salvage during caesarean section, in women at risk of haemorrhage, reduced the need for donor blood transfusion compared with standard care.
Methods
To compare the costs and outcomes of cell salvage and standard care in the SALVO trial, a decision-analytic model was deemed the most suitable method of presenting the alternative pathways and collating the data for analysis and sensitivity analysis. In a decision-analytic model, consequences are expressed as probabilities, weighted against costs and outcomes to derive an expected value for each alternative option. 73 The economic evaluation took the form of a cost-effectiveness analysis from the perspective of the health-care provider based on the principal clinical outcome of the trial. The main comparison is the use of cell salvage versus standard care. The results are reported in terms of the additional cost per donor blood transfusion avoided by using cell salvage compared with standard care. Standard care is defined for the purposes of the trial as ‘transfusion of donor blood according to standard local guidelines’. Costs were calculated in 2014–15 Great British pounds. Given the objectives of the trial and the duration of follow-up, only a within-trial economic analysis was carried out and outcomes beyond this point were not considered relevant.
Model structure
A decision tree model was developed in TreeAge Pro 2016 (TreeAge Software, Inc., Williamstown, MA, USA). The structure was informed by the objectives of the study and the pathways indicated by the clinical data. The model pathways (Figure 3) represent that of the trial in which patients undergoing a caesarean section were randomised to receive either cell salvage or standard care. Square boxes represent decision nodes, for which there is a choice to be made between strategies. Circles represent chance nodes, for which there are a number of subsequent events that could happen and each event is assigned a probability that it will occur. Triangles represent terminal nodes, signifying the last stage in the model.
FIGURE 3.
Decision tree structure.
Figure 3 shows that the model starts with the choice of transfusion strategies considered in the SALVO trial:
-
cell salvage
-
standard care.
Women allocated to either transfusion strategy have a possibility of receiving the treatment to which they were allocated or not. In both pathways, if the cell salvage machine was switched on, women have a possibility of receiving cell salvage, either on its own or in combination with donor blood transfusion. There is also a possibility that the woman may not require a transfusion.
The pathways of the model represent, as far as possible, the clinical procedures carried out in the study. The model combines the probability of a woman following a particular path and the associated costs. Probabilities, detailed in Table 20, were obtained from the trial and attached to each pathway. The cost and outcome measures that were incorporated into the model were collected prospectively during the SALVO trial using forms filled out at the pre-, intra- and postoperative phase and at the time of discharge from hospital. Intraoperative resource use and costs were estimated as the mean cost per caesarean section procedure conducted for each treatment pathway in the model and postoperative resource use and costs were estimated as the mean cost per patient in both treatment strategies represented in the model.
| Treatment pathway | Trial data (n/N) | Probability | Distribution |
|---|---|---|---|
| Cell salvage intended | |||
| Cell salvage intended → allocated treatment received (machine was on) | 1432/1498 | 0.96 | Beta |
| Allocated treatment received → salvaged blood returned | 726/1432 | 0.51 | Beta |
| Allocated treatment received → salvaged blood not returned | 703/1432 | 0.49 | Beta |
| Salvaged blood returned → donor blood transfusion given | 22/726 | 0.03 | Beta |
| Salvaged blood returned → donor blood transfusion not given | 704/726 | 0.97 | Beta |
| Salvaged blood not returned → donor blood transfusion given | 9/703 | 0.01 | Beta |
| Salvaged blood not returned → donor blood transfusion not given | 697/703 | 0.99 | Beta |
| Cell salvage intended → allocated treatment not received (machine was off) | 66/1498 | 0.04 | Beta |
| Allocated treatment not received → donor blood transfusion given | 6/66 | 0.09 | Beta |
| Allocated treatment not received → donor blood transfusion not given | 60/66 | 0.91 | Beta |
| Standard care intended | |||
| Standard care intended → allocated treatment received (machine was off) | 1434/1492 | 0.96 | Beta |
| Allocated treatment received → donor blood transfusion given | 47/1434 | 0.03 | Beta |
| Allocated treatment received → donor blood transfusion not given | 1387/1434 | 0.97 | Beta |
| Standard care intended → allocated treatment not received (machine was on) | 58/1492 | 0.04 | Beta |
| Allocated treatment not received → salvaged blood returned | 35/58 | 0.60 | Beta |
| Allocated treatment not received → salvaged blood not returned | 23/58 | 0.40 | Beta |
| Salvaged blood returned → donor blood transfusion given | 4/35 | 0.11 | Beta |
| Salvaged blood returned → donor blood transfusion not given | 31/35 | 0.89 | Beta |
| Salvaged blood not returned → donor blood transfusion given | 1/23 | 0.04 | Beta |
| Salvaged blood not returned → donor blood transfusion not given | 22/23 | 0.96 | Beta |
Data
Resource use and costs
The resource use for both groups of the trial was estimated by evaluating the individual components of these procedures (bottom-up costing). Unit cost data were then attached to the resource use. Data were collected on all major NHS resource use for each patient using the trial CRFs. The main resource use monitored included the following.
Intraoperative
-
Equipment and disposables required for the cell salvage procedure.
-
Additional staff called into theatre solely for the purposes of cell salvage.
-
Drugs used in the caesarean section procedure.
-
The use of donor blood transfusion to deal with haemorrhage and its consequences.
-
The use of salvaged blood transfusion to deal with haemorrhage and its consequences.
Postoperative
-
Length and type of hospital inpatient stay including additional treatment required attributed to the caesarean section procedure.
-
The use of donor blood transfusion to deal with haemorrhage and its consequences.
Intraoperative resource use and costs
For the analysis, intraoperative resource use data were obtained from the SALVO trial. Costs were estimated for each item to arrive at a mean cost per caesarean section procedure conducted for each treatment pathway in the model. To estimate the cost of a caesarean section procedure some costs were calculated at the patient level (e.g. swab washing) and some at the procedural level (e.g. drugs used in the caesarean section procedure). This is outlined in Table 21 and further detail is provided in the subsequent sections.
| Item | Resource use | Unit cost | Mean cost per procedure | Assumption/working | Source | ||
|---|---|---|---|---|---|---|---|
| Cell salvage (n = 1498) | Control (n = 1492) | Cell salvage (n = 1498) | Control (n = 1492) | ||||
| Running costs | 1432 | 58 | £6.14 | £6.14 | £6.14 | Based on annual maintenance costs for Haemonetics® Cell Saver® 5 (Haemonetics UK Ltd, Coventry, UK) machine and estimated annual usage | UHB, (Mr Scott Hancock, University Hospitals Birmingham, 2016, personal communication); NICE costing statement blood transfusion12 |
| Collection set | 1 | 1 | £41.71 | £41.71 | £41.71 | Based on the assumption that one collection set is used per procedure | NHS Supply Chain Catalogue.74 Autotransfusion reservoir 3 l |
| Processing pack | 1 | 1 | £77 | £77 | £77 | Based on the assumption that one processing pack is used per procedure | NHS Supply Chain Catalogue.74 Intraoperative autologous blood system cell saver 5+ bowl set 125 ml (Haemonetics UK Ltd, Coventry, UK) |
| LDF | 782 | 25 | n/a | n/a | n/a | Cost not included in the analysis as LDF included in the collection set for Haemonetics Cell Saver 5 machine | NHS Supply Chain Catalogue.74 Autotransfusion reservoir 3 l |
| Additional sucker | 598 | 29 | £15.41 | £6.43 | £7.70 | Mean cost based on the number of additional suckers used in each treatment group/total number of patients who received cell salvage | NHS Supply Chain Catalogue.74 Aspiration and anticoagulation line cell saver (Haemonetics UK Ltd, Coventry, UK). £308.02 for 20 |
| Swab washing | 781 | 21 | £0.80 | £0.44 | £0.29 | Mean cost based on the number of times swabs were washed in each treatment group/total number of patients who received cell salvage | ICS Factsheet 1 Swab Washing March 2015,75 based on the cost of 1 l of 0.9% sodium chloride, BNF76 |
| Staff | £0.72 (minute) | £11.57 | £12.03 | Based on the staff type most frequently called into theatre | Unit cost for hospital based nurse, band 5, PSSRU unit costs 2015 (costs include qualifications)77 | ||
| Saline (l) | 2 | 2 | £0.80 | £1.60 | £1.60 | Based on the assumption that 2 l of saline would be administered to all patients undergoing cell salvage prior to collection12 | Based on the cost of 1 l of 0.9% sodium chloride, BNF76 |
| Heparin sodium (30,000 IU) | 2 | 2 | £10.60 | £21.20 | £21.20 | Based on the assumption that 60,000 IU of heparin sodium would be administered to all patients undergoing cell salvage prior to collection12 | Based on the cost of a 1-ml amp of heparin sodium 25,000 IU/ml and a 1-ml amp of heparin sodium 5000 IU/ml, BNF76 |
| Anti-D (500 IU) | 1 | 1 | £33.75 | £3.04 | £3.04 | Based on the assumption that all RhD-negative women delivering a RhD-positive baby receive at least 500 IU of anti-D.34 Mean cost per procedure based on the probability of a woman requiring anti-D in each treatment group (0.09) | Based on the cost of 500-unit vial of anti-D immunoglobulin, BNF76 |
| Anti-D (1500 IU) | 1 | 1 | £58 | £5.22 | £5.22 | Based on the assumption that women who receive cell salvage are offered 1500 IU of anti-D.34 Mean cost per procedure based on the probability of a woman requiring anti-D in each treatment group (0.09) | Based on the cost of 1500-unit vial of anti-D immunoglobulin, BNF76 |
| RBC transfusion (units) | 3 | 3 | First unit: £194; subsequent units: £166 | £520 | £520 | Based on the assumption that all units transfused in each treatment group were RBCs12 | NICE costing statement for blood transfusion.12 Unit cost for RBCs obtained from NHSBT 2016/1778 |
Equipment and disposables required for the cell salvage procedure
Many centres reported that their cell salvage machines were obtained on lease and, as such, only the running costs and cost of consumables would be incurred. Therefore, the acquisition costs for a cell salvage machine were not included in the analysis but the addition of this cost was explored in a sensitivity analysis. The costs of materials used by participating centres varied. The acquisition cost and annual maintenance cost for a Haemonetics® Cell Saver® 5 (Haemonetics UK Ltd, Coventry, UK) machine was obtained from one centre. Costs for consumables were sourced from the NHS Supply Chain Catalogue (August 2016)74 and correspond to the consumables used with this machine. The annual number of procedures that would use the cell salvage machine was based on the NICE costing statement for blood transfusion published in November 2015. 12
The Haemonetics Cell Saver 5 machine uses two separate kits of consumables for collection and reinfusion. In the SALVO trial, some centres (n = 202 participants treated; see Table 11) used a continuous transfusion cell-saver machine that required the use of different consumables. However, for this analysis it was assumed that each centre used one set of consumables for collection and reinfusion. This was tested in a sensitivity analysis. The cost of a collection set and processing pack (used for reinfusion) with a 125 ml bowl for a Haemonetics Cell Saver 5 machine was obtained from the NHS Supply Chain Catalogue (August 2016). 74 For some cell salvage procedures in the study (n = 807), a LDF was used, but this filter was not used in the remaining 683 procedures. As this item is included in the collection set, no additional cost was incurred. Contained in the processing pack is one aspiration and anticoagulation line (sucker). For some caesarean section procedures in the study (n = 627), an additional sucker was used and this additional cost was apportioned across all caesarean section procedures for which cell salvage was conducted. The cost of an additional sucker was obtained from the NHS Supply Chain Catalogue (August 2016)74 and the total cost based on usage was divided by the number of patients in each group of the trial that set up the cell-saver machine to arrive at a mean cost of the procedure per patient in each group.
Blood loss can also be removed from the operative site by swabs. By washing swabs, the blood that is normally discarded can be collected and the overall efficiency of red cell recovery improved. 61 Swab washing occurred in 802 procedures in the SALVO trial, 781 procedures in the cell salvage group and 21 procedures in the control group. The UK Cell Salvage Action Group recommends that swabs are washed in 1 l of saline. 75 The cost of saline (0.9% sodium chloride) was obtained from the British National Formulary (BNF). 76 The total cost of swab washing in each group of the trial was apportioned by the number of patients who received cell salvage in that group of the trial.
Additional staff called into theatre solely for the purposes of cell salvage
The amount of time additional staff, called into theatre solely for the purposes of cell salvage, spent in the operating theatre was recorded in the SALVO trial. Staff grade was identified at a broad level [nurse, operating department practitioner (ODP) and doctor] and job band distinction was not recorded, but it was frequently included in the notes if a midwife was called into theatre. The analysis is based on the staff type most frequently called into theatre (ODP in both groups) and assumed the lowest possible cost within this job grade (hospital-based nurse, band 5). Staff unit costs were obtained from the Personal Social Services Research Unit (PSSRU) unit costs (2015). 77 The total cost of additional staff required for cell salvage was distributed by the number of times the cell salvage machine was set up in both groups of the trial.
Drugs used in the caesarean section procedure
Typically saline and an anticoagulant (e.g. heparin sodium) would be used for people undergoing cell salvage. The saline is required for collection of the blood (separate to saline used for swab washing) and the heparin sodium to stop the collected blood clotting. 12 It was assumed, as per the NICE costing statement, that 2 l of saline and 60,000 IU heparin sodium (30,000 IU per litre of saline) would be used for collection in any caesarean section procedure for which the cell salvage machine was turned on. 12 The cost of saline (0.9% sodium chloride) and heparin sodium were obtained from the BNF. 76 These costs were added to the average cost of the cell salvage procedure.
Guidelines suggest that all RhD-negative unsensitised women delivering a RhD-positive baby should be routinely offered a standard dose of anti-D immunoglobulin (at least 500 IU) as prophylaxis to minimise this risk of sensitisation, and all women who receive cell salvage should be offered a higher dose (1500 IU). 34 The probability of a woman requiring administration of anti-D (i.e. a RhD-negative mother with RhD-positive baby) was 0.09 in both groups of the SALVO trial. The cost of anti-D immunoglobulin was obtained from the BNF76 and the total cost based on usage was divided by the number of women in each arm of the model to arrive at a mean cost per patient in each treatment pathway.
Donor blood transfusion
The cost of donor blood transfusion used in the model was based on the costing methodology employed by NICE in which the resource use and costs for both blood bank and ward procedures were split to reflect the cost of transfusing the first unit and the cost of transfusing subsequent units. 12 For simplicity, the cost of transfusion of RBCs is used in the model. RBCs made up the largest proportion of the blood products transfused in the SALVO trial. Adjusting the cost of transfusion to reflect the different proportions of different blood products transfused is complex and unlikely to result in a significant cost difference. This approach is supported by NICE. 12 The unit cost of RBCs was taken from NHS Blood and Transfusion list price for 2016/17. 78 The mean number of units transfused per patient in each group of the trial was obtained and rounded up to represent the fact that any remaining blood in a unit would be disposed. The following approach was taken to calculate the mean cost:
All patients in the model required blood grouping and antibody screening, even if they did not end up requiring a donor blood transfusion. The cost of these procedures was obtained from the NICE costing statement12 and applied one time to people in the model who did not receive a donor blood transfusion. Note that for those who did receive a donor blood transfusion, this cost is incorporated into the cost of the first unit of blood.
Postoperative resource use and costs
For the analysis, postoperative resource use data were obtained from the SALVO trial. Costs were estimated for each item based on their occurrence in each branch of the model to arrive at a mean cost per patient for each branch. This is outlined in Table 22 and further detail is provided in the subsequent sections.
| Item | Resource use | Unit cost | Mean cost per patient | Assumption/working | Source | ||
|---|---|---|---|---|---|---|---|
| Cell salvage (n = 1498) | Control (n = 1492) | Cell salvage (n = 1498) | Control (n = 1492) | ||||
| Inpatient stay (normal days) | 3734.5 | 3852 | £431.45 | £1074 | £1113 | See Tables 23 and 25 | NHS Reference Costs 2014/2015 79 |
| Inpatient stay (HLC days) | 189.5 | 136 | See Table 24 | £78 | £56 | See Tables 24 and 25 | NHS Reference Costs 2014/2015.79 2016/17 National Prices80 |
| AEs | 3 | 0 | n/a | n/a | n/a | Based on the assumption that transfusion would be discontinued in the event of an adverse reaction | BCSH guidelines81 |
| Hospital transfer | 2 | 2 | £99 | £0.13 | £0.13 | n/a | PSSRU 201577 |
| Investigations | 6 | 10 | See Table 27 | £0.42 | £0.70 | n/a | NHS Reference Costs 2014/2015 79 |
| Additional surgery | 11 | 8 | See Table 28 | £13 | £9 | See Table 34 | NHS Reference Costs 2014/2015 79 |
| RBC transfusion (units) | 3 | 3 | First unit: £190. Subsequent units: £165 | £13 | £17 | Based on the assumption that all units transfused in each treatment group were RBCs | NICE costing statement for blood transfusion.12 Unit cost for RBCs obtained from NHSBT 2016/1778 |
| Total cost of postnatal care per patient | £1178.55 | £1195.83 | |||||
Length and type of hospital inpatient stay
Total time in hospital was recorded for each participant in the SALVO trial (cell salvage group mean = 2.64 days, standard care group mean = 2.72 days). Within the trial a HLC form was completed for 212 patients. This form indicated the number of days or partial days the patient received level 0, 1, 2 and 3 care. For this study, a partial day was costed as half a full day. Level 0 care is defined in the trial literature as ‘patients whose needs can be met through general ward care’. Admission to HLC for which level 0 care was administered was therefore costed as normal care. The total number of days spent receiving HLC (level 1–3) was deducted from the total time spent in hospital to arrive at the total number of days in normal care for each group. The weighted average cost per inpatient day was obtained from NHS Reference Costs 2014/201579 (Table 23) and was applied to arrive at a mean cost per patient of normal care.
| Currency code | Currency description | Activity | National average unit cost (£) |
|---|---|---|---|
| Elective inpatient excess bed-days | |||
| NZ50A | Planned caesarean section with a CC score of 4+ | 11 | 99.22 |
| NZ50B | Planned Caesarean Section with a CC score of 2 or 3 | 46 | 415.20 |
| NZ50C | Planned caesarean section with a CC score of 0 or 1 | 116 | 740.07 |
| NZ51A | Emergency caesarean section with a CC score of 4 + | 12 | 358.93 |
| NZ51B | Emergency caesarean section with a CC score of 2 or 3 | 83 | 231.87 |
| NZ51C | Emergency caesarean section with a CC score of 0 or 1 | 93 | 311.29 |
| Non-elective inpatient excess bed-days | |||
| NZ50A | Planned caesarean section with a CC score of 4+ | 2316 | 412.57 |
| NZ50B | Planned caesarean section with a CC score of 2 or 3 | 7670 | 438.31 |
| NZ50C | Planned caesarean section with a CC score of 0 or 1 | 6022 | 437.22 |
| NZ51A | Emergency caesarean section with a CC score of 4 + | 2840 | 408.49 |
| NZ51B | Emergency caesarean section with a CC score of 2 or 3 | 5388 | 417.82 |
| NZ51C | Emergency caesarean section with a CC score of 0 or 1 | 9806 | 440.84 |
| Weighted average cost per inpatient day | 431.00 | ||
The cost for a day receiving level 3 care was obtained from the 2016/17 National Prices80 maternity pathway, which gives costs for a day of intensive care. Information on the cost per day at level 1 and 2 was not available. For the purposes of this study it was assumed that level 1 care was 25% more expensive per day than level 0 care and level 2 care was 25% more expensive than level 1 care (Table 24). The following approach was taken to calculate the mean cost per patient in each arm of the model (Table 25):
| Level of care | Cost per day (£) | Assumption/working | Source |
|---|---|---|---|
| 0 | 431 | See Table 23 | NHS Reference Costs 2014/2015 79 |
| 1 | 539 | Based on the assumption that level 1 care is 25% more expensive than level 0 care | n/a |
| 2 | 674 | Based on the assumption that level 2 care is 25% more expensive than level 1 care | n/a |
| 3 | 848 | n/a | 2016/17 National Prices 80 |
| Item | Source/working | Cell salvage (n = 1498) | Control (n = 1492) |
|---|---|---|---|
| Normal days in hospital | |||
| Total days in hospital | Trial data | 3924 | 3988 |
| Total days in HLC | Trial data (level 1–3) | 189.5 | 136 |
| Total normal days | Total days in hospital minus total days in HLC | 3734.5 | 3852 |
| Cost per normal day (£) | See Table 23 | 431 | 431 |
| Total cost for normal days in hospital (£) | Total normal days × cost per day | 1,609,354 | 1,660,212 |
| Cost per patient (£) | Total cost/number in trial group | 1074 | 1113 |
| HLC days in hospital | |||
| Total days spent at level 1 care | Trial data | 96.5 | 67.5 |
| Cost per day at level 1 care (£) | See Table 24 | 539 | 539 |
| Total cost per treatment arm (£) | Total normal days × cost per day | 52,014 | 36,383 |
| Total days spent at level 2 care | Trial data | 84.5 | 67 |
| Cost per day at level 2 care (£) | 2016/17 National Prices 80 | 674 | 674 |
| Total cost per treatment arm (£) | Total days × cost per day | 56,953 | 45,158 |
| Total days spent at level 3 care | Trial data | 8.5 | 1.5 |
| Cost per day at level 3 care (£) | 2016/17 National Prices 80 | 848 | 848 |
| Total cost per treatment arm (£) | Total days × cost per day | 7208 | 1272 |
| Total cost of HLC per treatment arm (£) | Total cost level 1 + 2 + 3 | 116,175 | 82,813 |
| Cost per patient (£) | Total cost/number in trial group | 78 | 56 |
Adverse events
Although the occurrence of all AEs deemed relevant to the procedure were recorded as a categorical outcome (yes/no), only clinically defined SAEs that occurred within the trial and were deemed relevant to the procedure were considered potentially relevant to this analysis (n = 2). This is based on the assumption that non-SAEs deemed relevant to the procedure would have limited or zero resource impact. The two AEs considered potentially relevant to this analysis were recorded in the trial as life-threatening acute transfusion reactions. Guidelines recommend that for life-threatening acute transfusion reactions, the correct procedure is to discontinue transfusion. 81 Therefore, no additional costs were incurred and the occurrence of AEs is not included in this analysis.
Hospital transfer
Costs for hospital transfer were obtained from PSSRU. 77 The following approach was taken to calculate the mean cost per patient in each arm of the model (Table 26):
| Item | Source/working | Cell salvage (n = 1498) | Control (n = 1492) |
|---|---|---|---|
| Resource use | Trial data | 2 | 2 |
| Unit cost (£) | Based on cost per ambulance service. PSSRU 201577 | 99 | 99 |
| Total cost (£) | Resource use × unit cost | 198 | 198 |
| Number of patients requiring hospital transfer | Trial data | 2 | 2 |
| Probability of hospital transfer | Number of patients requiring hospital transfer/number in trial group | 0.0013 | 0.0013 |
| Cost per patient (£) | Total cost per patient × probability | 0.13 | 0.13 |
Investigations
Data on the number of X-rays, computed tomography (CT) scans and magnetic resonance imaging (MRI) scans were recorded in the SALVO trial. As stated in NHS Reference Costs 2014/2015,79 plain film X-rays that are part of an admission or outpatient attendance are not reported separately owing to their high volume and low cost. Therefore, occurrence of X-rays is not included in this analysis. Costs for CT scans and MRI scans were obtained from NHS Reference Costs 2014/2015. 79 The following approach was taken to calculate the mean cost per patient in each arm of the model (Table 27):
| Item | Source/working | Cell salvage (n = 1498) | Control (n = 1492) |
|---|---|---|---|
| CT scan | |||
| Resource use | Trial data | 6 | 9 |
| Unit cost (£) | NHS Reference Costs 2014/2015 79 | 94 | 94 |
| Total cost (£) | Resource use × unit cost | 564 | 846 |
| MRI scan | |||
| Resource use | Trial data | 0 | 1 |
| Unit cost (£) | NHS Reference Costs 2014/2015 79 | n/a | 138 |
| Total cost (£) | Resource use × unit cost | n/a | 138 |
| Total cost investigations (£) | Total cost CT + total cost MRI | 564 | 984 |
| Number of patients requiring investigations | Trial data | 4 | 7 |
| Probability of requiring investigations | Number of patients requiring investigations/number in trial group | 0.003 | 0.005 |
| Cost per patient (£) | Total cost per patient × probability | 0.42 | 0.70 |
Additional surgery
A total of 24 additional surgeries were recorded in the SALVO trial (cell salvage group n = 15, control group n = 9). The cost for each additional surgery was obtained from NHS Reference Costs 2014/2015. 79 The cost for time spent in hospital as a result of the additional surgery was subtracted from the NHS reference cost on the assumption that these costs would have been incorporated in the cost of hospital inpatient stay. The following approach was taken to calculate the mean cost per patient in each arm of the model (Table 28):
| Item | Resource use | Unit cost (£) | Total cost (£) | Source | ||
|---|---|---|---|---|---|---|
| Cell salvage (n = 1498) | Control (n = 1492) | Cell salvage (n = 1498) | Control (n = 1492) | |||
| Sutures | 2 | 2 | 2991 | 5982 | 5982 | NHS Reference Costs 2014/2015 79 |
| Hysterectomy | 3 | 2 | 1621 | 4863 | 3242 | NHS Reference Costs 2014/201579 (Table 29) |
| Laparotomy | 3 | 2 | 690 | 2070 | 1380 | NHS Reference Costs 2014/201579 (Table 30) |
| Evacuation | 2 | 2 | 1042 | 2084 | 2084 | NHS Reference Costs 2014/201579 (Table 31) |
| Colon procedure | 2 | 0 | 1088 | 2176 | n/a | NHS Reference Costs 2014/201579 (Table 32) |
| Bowel procedure | 0 | 1 | 399 | n/a | 399 | NHS Reference Costs 2014/201579 (Table 33) |
| Drainage | 3 | 0 | 690 | 2070 | n/a | NHS Reference Costs 2014/2015 79 |
| Total cost additional surgery | 19,245 | 13,087 | ||||
| Number of patients requiring additional surgery | 11 | 8 | ||||
| Probability of requiring additional surgery | 0.0073 | 0.0054 | ||||
| Cost per patient (£) | 13 | 9 | ||||
| Currency code | Currency description | Activity | National average | Average length of stay (days) | |
|---|---|---|---|---|---|
| Unit cost (£) | Elective | Non-elective | |||
| MAO7E | Major open upper genital tract procedures with a CC score of 5+ | 578 | 5909.25 | 5.37 | 11 |
| MAO7F | Major open upper genital tract procedures with a CC score of 3–4 | 1780 | 4387.14 | 3.49 | 6 |
| MAO7G | Major open upper genital tract procedures with a CC score of 0–2 | 24,190 | 3511.27 | 2.49 | 3 |
| MAO8A | Major, laparoscopic or endoscopic, upper genital tract procedures with a CC score of 2+ | 3076 | 3445.44 | 1.86 | 3 |
| MAO8B | Major, laparoscopic or endoscopic, upper genital tract procedures with a CC score of 0 or 1 | 16,845 | 2889.92 | 1.47 | 2 |
| Weighted average cost per procedure (£) | 3345 | ||||
| Average length of stay (days) | 4 | ||||
| Cost per day of care (level 0) (£) | 431 | ||||
| Total cost of hospital stay (£) | 1724 | ||||
| Average cost per procedure excluding cost of hospital stay (£) | 1621 | ||||
| Currency code | Currency description | Activity | National average | Average length of stay (days) | |
|---|---|---|---|---|---|
| Unit cost (£) | Elective | Non-elective | |||
| MA10Z | Minor, laparoscopic or endoscopic, upper genital tract procedures | 17,787 | 1341.48 | 1.02 | 2 |
| Average cost per procedure (£) | 1341 | ||||
| Average length of stay (days) | 1.51 | ||||
| Cost per day of care (level 0) (£) | 431 | ||||
| Total cost of hospital stay (£) | 651 | ||||
| Average cost per procedure excluding cost of hospital stay (£) | 690 | ||||
| Currency code | Currency description | Activity | National average | Average length of stay (days) | |
|---|---|---|---|---|---|
| Unit cost (£) | Elective | Non-elective | |||
| MA17D | Dilatation and evacuation, 14–20 weeks’ gestation | 763 | 2011.19 | 1.60 | 3 |
| Average cost per procedure (£) | 2011 | ||||
| Average length of stay (days) | 2.25 | ||||
| Cost per day of care (level 0) (£) | 431 | ||||
| Total cost of hospital stay (£) | 969 | ||||
| Average cost per procedure excluding cost of hospital stay (£) | 1042 | ||||
| Currency code | Currency description | Activity | National average | Average length of stay (days) | |
|---|---|---|---|---|---|
| Unit cost (£) | Elective | Non-elective | |||
| FZ75C | Proximal colon procedures, ≥ 19 years, with a CC score of 6+ | 809 | 8952.50 | 11.26 | 16 |
| FZ75D | Proximal colon procedures, ≥ 19 years, with a CC score of 3–5 | 2139 | 6751.80 | 7.18 | 11 |
| FZ75E | Proximal colon procedures, ≥ 19 years, with a CC score of 0–2 | 6430 | 5795.71 | 5.28 | 8 |
| Weighted average cost per procedure (£) | 6286 | ||||
| Average length of stay (days) | 10 | ||||
| Cost per day of care (level 0) (£) | 431 | ||||
| Total cost of hospital stay (£) | 5198 | ||||
| Average cost per procedure excluding cost of hospital stay (£) | 1088 | ||||
| Currency code | Currency description | Activity | National average | Average length of stay(days) | |
|---|---|---|---|---|---|
| Unit cost (£) | Elective | Non-elective | |||
| FZ67C | Major small intestine procedures, ≥ 19 years, with a CC score of 7+ | 860 | 9719.99 | 15.84 | 21 |
| FZ67D | Major small intestine procedures, ≥ 19 years, with a CC score of 4–6 | 1476 | 7127.58 | 8.86 | 14 |
| FZ67E | Major small intestine procedures, ≥ 19 years, with a CC score of 2 or 3 | 2751 | 5164.47 | 5.83 | 10 |
| FZ67F | Major small intestine procedures, ≥ 19 years, with a CC score of 0 or 1 | 4662 | 3561.35 | 4.66 | 7 |
| Weighted average cost per procedure (£) | 5097 | ||||
| Average length of stay (days) | 11 | ||||
| Cost per day of care (level 0) (£) | 431 | ||||
| Total cost of hospital stay (£) | 4703 | ||||
| Average cost per procedure excluding cost of hospital stay (£) | 399 | ||||
Donor blood transfusion
The cost of donor blood transfusion received postnatally is based on the same costs as those used for transfusion received intraoperatively in this model. For simplicity, the cost of transfusion of RBCs is used. The mean number of units transfused to each patient in each group of the trial was obtained and rounded up to represent the fact that any remaining blood in a unit would be disposed of. The following approach was taken to calculate the mean cost per patient in each arm of the model (Table 34):
| Item | Source/working | Cell salvage (n = 1498) | Control (n = 1492) |
|---|---|---|---|
| Resource use (mean units transfused) | Trial data | 3 | 3 |
| Unit cost: first unit | NHSBT 201678, NICE costing statement for blood transfusion12 | £190 | £190 |
| Unit cost: subsequent units | NHSBT 201678, NICE costing statement for blood transfusion12 | £165 | £165 |
| Total cost | Resource use × unit cost | £520 | £520 |
| Number of patients requiring transfusion | Trial data | 38 | 49 |
| Probability of transfusion | Number of patients requiring transfusion/number in trial group | 0.025 | 0.033 |
| Cost per patient | Total cost per patient × probability | £13 | £17 |
Outcomes
The outcome of interest in the trial was the use of donor blood transfusion in response to haemorrhage and its consequences.
Assumptions
It was necessary to make the following pragmatic assumptions before the analysis could be carried out:
-
Trial centres.
All of the centres involved in the trial were assumed to have the same expertise and to have followed similar protocols in the management of patients.
-
Equipment and disposables required for the cell salvage procedure.
It was assumed that all centres performing cell salvage used consumables and that one collection set and one processing pack were used per cell salvage procedure. Costs for equipment and disposables were obtained for a Haemonetics Cell Saver 5 machine. Variance in costs was explored in sensitivity analysis. When swab washing occurred, it was assumed that the swabs were washed in 1 l of saline. 75
-
Use of cell salvage machine.
When the cell salvage machine was switched on, it was assumed that running costs would be incurred and a collection set would be used even if no salvaged blood was returned to the patient. It was also assumed that heparin sodium and saline would be used prior to collection. 12
-
Additional staff called into theatre solely for the purposes of cell salvage.
We based our analysis on the staff type most frequently called into theatre in the trial and assumed the lowest possible cost within this job band distinction. Staff cost variance was explored through sensitivity analysis.
-
Salvaged blood.
The threshold setting on a cell salvage machine to process can be set to engage for salvaged blood above a certain volume and in this study trial centres displayed variance in the minimum volume threshold they selected. Trial guidance given to participating centres stated that all processed blood produced by the machines should be returned to the patient. This analysis assumed that all minimum threshold settings were disengaged. The cost of collection of all shed blood was considered, regardless of whether or not that blood was subsequently returned to the patient.
-
Donor blood.
All units transfused were assumed to be RBCs. 12 The mean number of units transfused per patient was rounded up to account for the fact that any remaining blood in a bag would be disposed of.
-
Length and type of inpatient stay.
For those patients who received level 0 care when admitted to HLC, it was assumed that their needs could be met through general ward care. It was assumed that level 1 care was 25% more expensive per day than level 0 care and level 2 care was 25% more expensive than level 1 care.
-
Additional surgeries.
Additional surgeries were included in this analysis with the cost of inpatient bed-days excluded to avoid double counting. Inpatient bed-days were assumed to be the equivalent of level 0 care.
-
AEs.
It was assumed that non-SAEs deemed relevant to the procedure would have limited or zero resource impact. It was assumed that in the case of an acute transfusion reaction, the transfusion would be discontinued. 11
-
Infant health.
In this study, the health of the infant was not considered relevant to the intervention. Information relating to the clinical status and care of the infant was therefore not included in the analysis.
Analysis
Given the objectives of the trial and the duration of follow-up, a within-trial economic analysis was carried out. The analysis took the perspective of the NHS following current recommendations from NICE. 82 The main economic analysis was a cost-effectiveness analysis with results expressed as cost per donor transfusion avoided.
We carried out three main analyses on the trial data. In analysis 1, the base case was based on the ITT principle. In this method, patients are compared within the treatment groups to which they were originally randomised irrespective of the treatment received. 83 This method of analysis allows the estimates to follow real-life scenarios in which patients may not always receive the planned treatment. Not using ITT analysis can often exaggerate the benefits of a given intervention. 83
Analysis 2 was based on the treatment received by patients irrespective of randomisation (the PP analysis). Within the SALVO trial, equal numbers of patients were randomised to either the cell salvage or control group. However, because some clinicians managing women in the control group had access to a cell salvage machine, it was possible that women in the control group could receive cell salvage in place of a donor blood transfusion. A PP analysis was carried out to look at the effect of treatment received on the outcome estimates. Therefore, in analysis 2, all patients who received cell salvage were compared with those who received standard care, irrespective of the treatment to which they were randomised.
Analysis 3 considered only patients who underwent an emergency caesarean section. This analysis was considered necessary as the SALVO trial found that numerically, there was a greater reduction in rate of transfusion within the emergency patient group than the elective patient group. This analysis followed the same methodology as analyses 1 and 2. Probabilities were obtained from the trial and attached to each pathway in the existing model. For the analysis, intra- and postoperative resource use data were obtained from the SALVO trial. Intraoperative costs were estimated for each item to arrive at a mean cost per caesarean section procedure for each treatment pathway in the model. Postoperative costs were estimated for each item based on their occurrence in each branch of the model to arrive at a mean cost per patient for each branch (Tables 35–37).
| Treatment pathway | Trial data (n/N) | Probability | Distribution |
|---|---|---|---|
| Cell salvage intended | |||
| Cell salvage intended → allocated treatment received (machine was on) | 794/833 | 0.953 | Beta |
| Allocated treatment received → salvaged blood returned | 390/794 | 0.491 | Beta |
| Allocated treatment received → salvaged blood not returned | 401/794 | 0.509 | Beta |
| Salvaged blood returned → donor blood transfusion given | 5/390 | 0.013 | Beta |
| Salvaged blood returned → donor blood transfusion not given | 385/390 | 0.987 | Beta |
| Salvaged blood not returned → donor blood transfusion given | 2/401 | 0.005 | Beta |
| Salvaged blood not returned → donor blood transfusion not given | 399/401 | 0.995 | Beta |
| Cell salvage intended → allocated treatment not received (machine was off) | 39/833 | 0.047 | Beta |
| Allocated treatment not received → donor blood transfusion given | 1/39 | 0.026 | Beta |
| Allocated treatment not received → donor blood transfusion not given | 38/39 | 0.974 | Beta |
| Standard care intended | |||
| Standard care intended → allocated treatment received (machine was off) | 780/808 | 0.965 | Beta |
| Allocated treatment received → donor blood transfusion given | 9/780 | 0.012 | Beta |
| Allocated treatment received → donor blood transfusion not given | 771/780 | 0.988 | Beta |
| Standard care intended → allocated treatment not received (machine was on) | 28/808 | 0.035 | Beta |
| Allocated treatment not received → salvaged blood returned | 14/28 | 0.5 | Beta |
| Allocated treatment not received → salvaged blood not returned | 14/28 | 0.5 | Beta |
| Salvaged blood returned → donor blood transfusion given | 1/14 | 0.071 | Beta |
| Salvaged blood returned → donor blood transfusion not given | 13/14 | 0.929 | Beta |
| Salvaged blood not returned → donor blood transfusion given | 0/14 | 0 | Beta |
| Salvaged blood not returned → donor blood transfusion not given | 14/14 | 1 | Beta |
| Item (units) | Resource use | Unit cost (£) | Mean cost per procedure (£) | Assumption/working | Source | ||
|---|---|---|---|---|---|---|---|
| Cell salvage (n = 833) | Control (n = 808) | Cell salvage (n = 833) | Control (n = 808) | ||||
| Running costs | 6.14 | 6.14 | 6.14 | Based on annual maintenance costs for Haemonetics Cell Saver 5 machine and estimated annual usage | UHB, (Mr Scott Hancock, University Hospitals Birmingham, 2016, personal communication). NICE costing statement blood transfusion12 | ||
| Collection set | 1 | 1 | 41.71 | 41.71 | 41.71 | Based on the assumption that one collection set is used per procedure | NHS Supply Chain Catalogue 2015:74 autotransfusion reservoir 3 litre |
| Processing pack | 1 | 1 | 77.00 | 77.00 | 77.00 | Based on the assumption that one processing pack is used per procedure | NHS Supply Chain Catalogue 2015:74 intraoperative autologous blood system cell saver 5+ bowl set 125 ml |
| Filter | n/a | n/a | n/a | n/a | n/a | No usage of LDF recorded in the trial | n/a |
| Additional sucker | 321 | 14 | 15.41 | 6.23 | 7.70 | Mean cost based on the number of additional suckers used in each treatment arm/total number of patients who received cell salvage | NHS Supply Chain Catalogue 2015:74 aspiration and anticoagulation line cell saver. £308.02 for 20 |
| Swab washing | 444 | 11 | 0.80 | 0.45 | 0.31 | Mean cost based on the number of times swabs were washed in each treatment arm/total number of patients who received cell salvage | ICS Factsheet 1 Swab Washing March 2015,75 based on the cost of 1 l 0.9% of sodium chloride, BNF76 |
| Staff | 82.21 (minutes) | 30 (minutes) | 0.72 (minutes) | 11.70 | 1.54 | Based on the staff type most frequently called into theatre | Unit cost for hospital based nurse, band 5, PSSRU unit costs 2015 (costs include qualifications)77 |
| Saline (l) | 2 | 2 | 0.80 | 1.60 | 1.60 | Based on the assumption that 2 l of saline would be administered to all patients undergoing cell salvage prior to collection | Based on the cost of 1 l of 0.9% sodium chloride, BNF76 |
| Heparin sodium (30,000 IU) | 2 | 2 | 10.60 | 21.20 | 21.20 | Based on the assumption that 60,000 IU heparin sodium would be administered to all patients undergoing cell salvage prior to collection | Based on the cost of a 1-ml amp of heparin sodium 25,000 IU/ml and a 1-ml amp of heparin sodium 5000 IU/ml, BNF76 |
| Anti-D (500 IU) | 1 | 1 | 33.75 | 3.04 | 3.04 | Based on the assumption that all RhD-negative women delivering a RhD-positive baby receive at least 500 IU of anti-D. Mean cost per procedure based on the probability of a woman requiring anti-D in each treatment arm (0.09) | Based on the cost of 500-unit vial of anti-D immunoglobulin, BNF76 |
| Anti-D (1500 IU) | 1 | 1 | 58.00 | 5.22 | 5.22 | Based on the assumption that women who receive cell salvage are offered 1500 IU of anti-D. Mean cost per procedure based on the probability of a woman requiring anti-D in each treatment arm (0.09) | Based on the cost of 1500-unit vial of anti-D immunoglobulin, BNF76 |
| RBC transfusion | 2 | 3 | First unit: 190 Subsequent units: 165 |
355 | 520 | Based on the assumption that all units transfused in each treatment arm were RBCs | NICE costing statement for blood transfusion.12 Unit cost for RBCs obtained from NHSBT 2016/1778 |
| Item | Resource use | Unit cost (£) | Mean cost per patient (£) | Assumption/working | Source | ||
|---|---|---|---|---|---|---|---|
| Cell salvage (n = 833) | Control (n = 808) | Cell salvage (n = 833) | Control (n = 808) | ||||
| Inpatient stay (normal days) | 2212 | 2246 | 431 | 1147 | 1205 | See Tables 23 and 25 | NHS Reference Costs 2014/2015 79 |
| Inpatient stay (HLC) | 135 | 99.5 | See Table 24 | 100 | 78 | See Tables 24 and 25 | NHS Reference Costs 2014/2015.79 National tariff payment system 2016/1780 |
| AEs | n/a | n/a | n/a | n/a | n/a | No AEs recorded in the trial | |
| Hospital transfer | 1 | 2 | 99 | 0.12 | 0.25 | n/a | PSSRU 201577 |
| Investigations | 2 | 6 | See Table 27 | 0.23 | 0.75 | n/a | NHS Reference Costs 2014/2015 79 |
| Additional surgery | 8 | 3 | See Table 28 | 11 | 3 | See Table 34 | NHS Reference Costs 2014/2015 79 |
| RBC transfusion (units) | 3 | 3 | First unit: 190. Subsequent units: 165 | 13 | 19 | Based on the assumption that all units transfused in each treatment arm were RBCs | NICE costing statement for blood transfusion (November 2015).12 Unit cost for RBCs obtained from NHSBT 2016/1778 |
| Total cost of postnatal care per patient | 1271 | 1306 | |||||
Sensitivity analysis
Deterministic sensitivity analyses and probabilistic sensitivity analyses (PSAs) were carried out for each analysis to explore the effects of the inherent uncertainty in parameter estimates on model results. Deterministic sensitivity analysis involves varying one or more parameters while keeping the others at their baseline value. Although deterministic sensitivity analyses can be helpful to identify which model inputs are important in driving a decision or to identify threshold values, comprehensive representation can be obtained by undertaking a probabilistic sensitivity analysis (PSA), in which the uncertainty around a parameter is represented with a probability distribution. Monte Carlo simulation was used to sample from these distributions to allow the effect of parameter uncertainty to be evaluated. This involved 1000 repeated random draws from the distributions to indicate how variation in the model parameters would affect the results and hence illustrate the decision uncertainty. Beta distributions were used for probability data and Gamma distributions for costs. 84,85
The results of the analyses are presented in terms of incremental cost-effectiveness ratios (ICERs), which reflect the additional cost per donor blood transfusion avoided of cell salvage compared with standard care. The results of the cost-effectiveness analysis are presented using scatterplots and cost-effectiveness acceptability curves (CEACs) to reflect sampling variation and uncertainties in the appropriate threshold cost-effectiveness value.
Deterministic sensitivity analyses
A number of deterministic sensitivity analyses were conducted in each analysis:
-
Equipment and disposables required for the cell salvage procedure.
The main analyses used costs for consumables based on a particular model of cell-saver machine. To assess the difference that variation in these estimates would make, the unit costs were replaced with unit costs obtained from the NICE costing statement for blood transfusion. 12 We then explored the impact of the inclusion of acquisition costs for a cell salvage machine and the impact of using a continuous transfusion cell salvage machine that requires a suction set and reservoir (prices sourced from participating unit) when the machine is only set up for processing in patients having blood returned and when swab washing was not conducted.
-
Staff.
The main analyses used the mean length of time additional staff were present in theatre in each group solely for the purposes of cell salvage. We explored the impact of not calling an additional member of staff into theatre.
-
Donor blood.
To facilitate robust evaluation in cost-effectiveness analyses relating to donor blood, a comprehensive estimate for the cost of a unit of donor blood is required. The NHS Blood and Transplant Authority has valued the cost of RBCs to be £120 per unit based on direct costs to the health-care services;78 however, there is significant uncertainty surrounding this figure. We conducted a study (submitted for publication; McLoughlin C, Roberts T, Jackson L, University of Birmingham, 2017) parallel to the SALVO trial that aimed to dissect the current price of blood. We explored what elements are contributing to the current cost of blood and what elements are missing. Our study concluded that the current costing approach of assuming there will always be an adequate supply of donor blood must be replaced with including provisions for the continued shrinking of the donor pool and the impact that future shocks to the blood supply system could have. The sensitivity analysis assessed the difference that variation in the estimated cost of blood made to the overall cost-effectiveness of cell salvage.
Results
Analysis 1: intention to treat
The results of the analysis are shown in Table 38. The strategy in which standard care was intended was the least costly, with the average cost per patient estimated at £1244. However, the cell salvage intended group was only slightly more expensive, with the average cost per patient estimated at £1327. The cell-salvage-intended strategy was the most effective at avoiding a transfusion. The estimated incremental cost-effectiveness ratio (ICER) for the cell-salvage-intended strategy compared with standard care was £8110 per donor blood transfusion avoided. This means that it would cost an additional £8110 to avoid a donor blood transfusion through cell salvage compared with standard care.
| Transfusion strategy | Average cost per patient (£) | Effectiveness: donor blood transfusion avoided | ICER (£) |
|---|---|---|---|
| Standard care intended | 1244 | 0.965 | |
| Cell salvage intended | 1327 | 0.975 | 8110 |
The scatterplot (Figure 4) shows the modelled uncertainty in the cost and effectiveness of the cell salvage intended strategy compared with the standard care intended strategy from 1000 Monte Carlo simulations. In this, the ICER of each simulation is plotted on the cost-effectiveness plane providing information about the joint density of the differences in cost and effectiveness between the two strategies. From Figure 4, it is evident that although cell salvage is a more effective transfusion strategy, it is uncertain whether it is more or less costly than standard care. The CEAC (Figure 5) shows that the probability that cell salvage is cost-effective increases as the willingness to pay for a donor blood transfusion avoided increases. Given that there is not a prespecified threshold of willingness to pay for a blood transfusion avoided, as in the case of QALYs of £20,000–30,000 are the recommended cut-off points by NICE, the identification of the probability of cell salvage being cost-effective is less straightforward. However, the CEAC shows that if the maximum willingness to pay for a donor blood transfusion avoided was, for example, £50,000, the probability of cell salvage being cost-effective would be 62%.
FIGURE 4.
Incremental cost-effectiveness scatterplot for donor blood transfusion avoided (ITT).

FIGURE 5.
Cost-effectiveness acceptability curve for donor blood transfusion avoided (ITT).
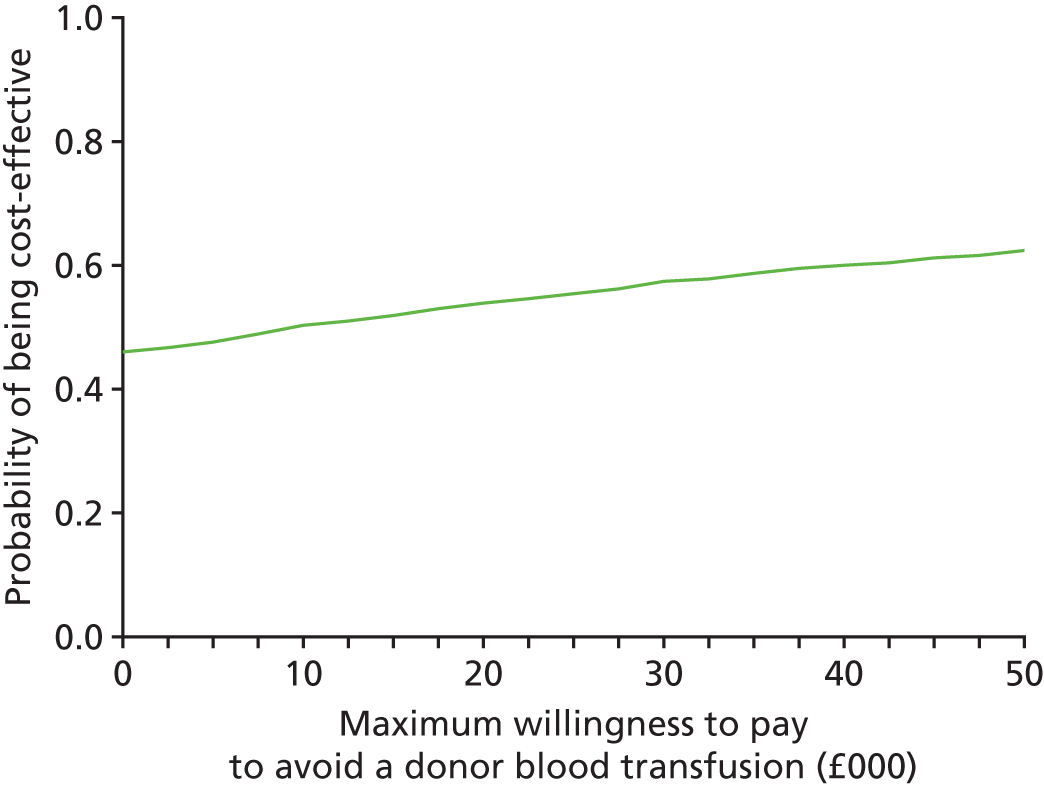
Analysis 2: per protocol
The results of the analysis are shown in Table 39. In terms of cost, standard care is, again, the least costly strategy with a mean cost per patient estimated at £1238. The cell salvage strategy was the most effective at avoiding a transfusion. The estimated ICER for the cell salvage strategy compared with standard care was £8252 per donor blood transfusion avoided.
| Transfusion strategy | Average cost per patient (£) | Effectiveness donor blood transfusion avoided | ICER (£) |
|---|---|---|---|
| Standard care | 1238 | 0.967 | |
| Cell salvage | 1330 | 0.978 | 8252 |
The scatterplot (Figure 6) shows that although cell salvage is a more effective transfusion strategy, it is again uncertain whether it is more or less costly than standard care. This uncertainty has been graphed in the CEAC (Figure 7). The graph shows that if the maximum willingness to pay for a donor blood transfusion avoided was £50,000, the probability that cell salvage was cost-effective would be 63%.
FIGURE 6.
Incremental cost-effectiveness scatterplot for donor blood transfusion avoided (PP).

FIGURE 7.
Cost-effectiveness acceptability curve for donor blood transfusion avoided (PP).

Analysis 3: emergency caesarean
The results of the analysis are shown in Table 40. The strategy in which standard care was followed remains the least costly, but higher in comparison with the previous two analyses, with the average cost per patient estimated at £1352. The cell salvage group had an average cost per patient estimated at £1407, which was also slightly higher than the previous analyses. The cell salvage strategy continues to be the most effective at avoiding a transfusion. The estimated ICER for the cell salvage strategy compared with standard care was £13,713 per donor blood transfusion avoided.
| Transfusion strategy | Average cost per patient (£) | Effectiveness donor blood transfusion avoided | ICER (£) |
|---|---|---|---|
| Standard care | 1352 | 0.986 | |
| Cell salvage | 1407 | 0.990 | 13,713 |
The scatterplot (Figure 8) shows again that although cell salvage is a more effective transfusion strategy, it remains uncertain whether it is less or more costly than standard care. The CEAC (Figure 9) shows that the probability that cell salvage is cost-effective remains between 47% and 55% as the willingness to pay for a donor blood transfusion avoided increases.
FIGURE 8.
Incremental cost-effectiveness scatterplot for donor blood transfusion avoided (emergency only).

FIGURE 9.
Cost-effectiveness acceptability curve for donor blood transfusion avoided (emergency only).

Sensitivity analysis
Deterministic sensitivity analysis
As demonstrated in Table 41, the results of the deterministic sensitivity analysis were as follows:
-
Varying the cost of consumables to those used by NICE had a marginal impact on the ICER in each analysis. Similarly, including acquisition costs in the analysis had only a minimal impact on the ICER. In the trial, 202 centres used a continuous transfusion cell-saver machine that required different consumables to the ones included in the main analyses. The impact of including costs for the consumables used by this machine, when the machine is only set up for processing in patients having blood returned and when swab washing is not conducted, resulted in an ICER of £1022 in analysis 1, £1184 in analysis 2 and a dominant ICER in analysis 3 (i.e. cell salvage was considered less costly and more effective than standard care).
-
In this sensitivity analysis, the cost of additional staff called into theatre solely for the purposes of cell salvage was removed. This reduced the ICER to £7065 in the ITT analysis, £7210 in the PP analysis and £10,932 in the emergency-caesareans analysis.
-
Raising the cost of a three-unit transfusion of RBCs to £1500 reduced the ICER by £974 in both analyses 1 and 2 and it reduced the ICER by over £1000 in analysis 3. Threshold analysis showed that for cell salvage to be considered cost-effective, the cost of a transfusion would have to be £8637 in analysis 1, £8778 in analysis 2 and £13,186 in analysis 3.
| Deterministic sensitivity analysis | Original value | Revised value | Original result | |||
|---|---|---|---|---|---|---|
| Cost per donor blood transfusion avoided | ||||||
| Analysis 1 | Analysis 2 | Analysis 3 | ||||
| £8110 | £8252 | £13,713 | ||||
| Revised result | ||||||
| 1. Equipment and disposables required for the cell salvage procedure | ||||||
| Applying the cost of consumables (collection set + processing pack) used by NICE | £118.71 | £119.75 | £8205 | £8346 | £13,952 | |
| Including acquisition costs based on Haemonetics Cell Saver machine | – | £22.13 | £10,114 | £10,246 | £18,805 | |
| Using a continuous transfusion cell saver when the machine is only set up for processing in patients having blood returned and swab washing is not conducted | Cell salvage | Control | ||||
| £125.14 | £126.40 | £34.73 | £1022 | £1184 | Dominates | |
| 2. Staff | ||||||
| No additional member of staff being called into theatre solely for the purposes of cell salvage | Cell salvage | Control | £0 | £7065 | £7210 | £10,932 |
| £11.57 | £12.03 | |||||
| 3. Donor blood | ||||||
| Variation in the estimate of the cost of a three-unit RBC transfusion | £520 | £750 | £7886 | £8028 | £13,330 | |
| £1000 | £7636 | £7778 | £13,080 | |||
| £1250 | £7386 | £7528 | £12,830 | |||
| £1500 | £7136 | £7278 | £12,580 | |||
| For cell salvage to be considered cost-effective in this model the price of a three-unit RBC transfusion would have to be: | £8637 | £8778 | £13,186 | |||
Discussion
Principal findings
The results of this economic evaluation suggest that routine cell salvage is more costly than standard care with the average cost per patient estimated at £1327 compared with £1,244. The ICER of this strategy compared with standard care is approximately £8110 to avoid a donor blood transfusion. The PSA suggests that at a willingness-to-pay threshold of £50,000, the probability of routine cell salvage being cost-effective is 62%. The results of this analysis were shown to be robust for the majority of deterministic sensitivity analyses with one exception: using a cell salvage machine that required different consumables to those included in the main analyses, when the machine is only set up for processing in patients having blood returned and when swab washing is not conducted, resulted in a significant effect on the ICER, reducing it to £1022 per donor blood transfusion avoided.
A PP analysis produced an ICER of £8252 per transfusion avoided, but this result should be considered with caution as the population in this analysis is a subset of the ITT population who completed the study without any major protocol violations. 83 In clinical practice, uptake of cell salvage is unlikely to be ‘PP’. In a third analysis, looking at emergency caesareans only, cell salvage appears to be more effective than standard care for avoiding a donor blood transfusion but the resulting ICER of £13,713 is driven by the increased probability that these patients will require a higher level of postoperative care.
Strengths and limitations of the economic evaluation
The strength of this model-based economic evaluation is that it was based on a rigorously conducted RCT. The cost and outcome data measures that were incorporated into the model were collected prospectively during the RCT using forms filled out at the pre, intra- and postoperative phases and at the time of discharge from hospital. In addition, the economic evaluation benefited from significant clinical and statistical input throughout its design and development. All assumptions used in the model were agreed with the trial team before the analysis was carried out and without knowledge of how these assumptions would affect the results.
In terms of limitations, not all potential outcomes have been included because of the limited time scale in our model and the lack of long-term data, for example, we could not account for long-term implications relating to FMH as data relating to this were not available from the trial. In addition, information relating to the clinical status and care of the infant was not included in the analysis. A further limitation of the evaluation is that outcomes were expressed in terms of clinical effectiveness rather than in terms of a standard unit of benefit: the QALY. Finally, the use of platelets and other blood products has not been included in the evaluation. However, the results of the sensitivity and threshold analyses demonstrated that including these costs would not have had an impact on the cost-effectiveness results.
Strengths and weaknesses in relation to other studies
To date, and to our knowledge, there has only been one, small, RCT looking at the elective use of cell salvage at caesarean section44 and this study did not include an economic element. A Cochrane review of cell salvage in adult elective surgery assessed the clinical effectiveness and cost-effectiveness of cell salvage and other autologous transfusion strategies in elective surgery. 21 It suggested that cell salvage may be an ‘effective and cost-effective alternative to the allogeneic blood transfusion strategy’. However, no obstetric papers were identified for this review.
Meaning of the economic evaluation
The results of the economic evaluation suggest that although cell salvage is a marginally more effective strategy than standard care in avoiding a donor blood transfusion, it is unlikely that cell salvage would be considered cost-effective. However, the results in natural units of cost per transfusion avoided are difficult to interpret and are very subjective. They will vary depending on the context. The lack of long-term data on the health and quality of life of patients in both groups of the trial means that further research is needed to fully understand the cost implications of both strategies. For patients undergoing an emergency caesarean section, when cell salvage is performed using a continuous transfusion cell-saver machine, the machine is only set up for processing in patients having blood returned; and when swab washing is not conducted, cell salvage would be considered less costly and more effective than standard care. However, this scenario is not necessarily generalisable; therefore, this result should be interpreted with caution.
Unanswered questions and future research
The current evaluation has used data from a large, multicentre RCT that demonstrated modest evidence that routine use of cell salvage during caesarean section reduced the need for donor blood transfusion. The main cause of uncertainty relates to the cost implications of adopting cell salvage in routine practice. Future studies should explore the long-term health and economic impacts associated with both transfusion strategies. In addition, evidence on the preferences of women needs to be considered. For example, hospitals may wish to have the option of cell salvage available for Jehovah’s Witness patients, for whom there is no option to use donor blood.
Finally, and related, the issue of donor blood as a limited resource needs to be considered. It is important to remember that transfusion with cell salvage can always exist, but we have not considered this in our evaluation. Although there is an expectation that donor blood will always be there when needed, transfusion using donor blood cannot be guaranteed. In such a scenario, when the option of donor blood is limited or not available, the routine use of cell salvage would dominate (less costly and more effective) standard care.
The routine use of cell salvage during caesarean section reduces, to some extent, the need for donor blood. Whether or not this treatment strategy is also cost-effective will depend on one hand, on the cost of a donor blood transfusion and, on the other hand, the operating cost of the cell salvage procedure. Implementation and more efficient operation of cell salvage machines in routine care could reduce the associated costs. At the current price level of donor blood and operating costs of cell salvage, cell salvage appears to be a more costly strategy to reduce the use of donor blood and only when the price of a blood transfusion increases to levels beyond £8637 does cell salvage potentially become a cost-effective strategy.
Chapter 5 Discussion
Aim and overview
The provision of a reliable donor blood transfusion service has critical implications for maternal health. In health-care systems in which this is available, maternal mortality due to haemorrhage is almost one thousand fold less than in those in which it is not. 86,87 Donor blood transfusion is a safe intervention with remarkably few associated AEs, although these may be serious and even, although rarely, fatal. In the face of such a proven clinical intervention, any new technique seeking to further reduce mortality would have to be extremely effective and require an unfeasibly large trial in order to demonstrate it. Despite these limitations to evaluation of a new technology, for many clinicians this would be intuitively considered the aim of introducing cell salvage into obstetric clinical practice. More realistically, cell salvage might reduce reliance on donor blood, the production and delivery of which remains relatively expensive (£120 per unit78) and while representing a ‘pooled’ national resource, may suffer from local hospital shortages when consumption is increased by a case of unexpected severe haemorrhage. However, cell salvage is a technology with its own costs and, therefore, it was clear from the outset of the SALVO trial that the health economic evaluation would be a crucial aspect of the trial, in order to show a worthwhile reduction in spend on donor blood units that was greater than the cost of the intervention. With an increasing reluctance to transfuse donor blood to even quite substantially anaemic women, owing to the possible adverse effects, cell salvage offered the possibility of safely increasing postoperative haemoglobin levels, leading to additional savings in patient care and hospital stay. These again, required rigorous health economic evaluation to provide meaningful conclusions, particularly as other clinical interventions88 for optimising preoperative haemoglobin levels (e.g. iron therapy) or reducing intraoperative blood loss (e.g. tranexamic acid or interventional radiology) may be cheaper and more efficacious.
A number of end points were considered as candidates for a primary outcome. The selection of transfusion rate was ultimately a pragmatic one, based on objectiveness, ease of collection and the existence of pre-existing data. In addition, a patient questionnaire given to women who had undergone the procedure highlighted the reassuring nature of receiving one’s own blood instead of donor blood [see original SALVO protocol (URL: www.journalslibrary.nihr.ac.uk/programmes/hta/105732#/ (accessed August 2017)].
Main findings
This large, multicentre RCT demonstrated modest evidence that routine, prophylactic use of cell salvage during caesarean section reduced the need for donor blood transfusion. It was associated with increased maternal exposure to fetal blood among Rh-negative mothers. Small differences were observed between groups for time to mobilisation and length of hospital stay but not in other secondary outcomes. Although numerically there appeared to be a greater effect in the emergency group compared with the elective group, the difference in effect between subgroups was not statistically significant. Exploratory analysis did not suggest a subgroup benefit in women with abnormal placentation. Although it appeared to increase the volume of salvaged blood returned, the use of ‘swab washing’ when conducting cell salvage did not appear to affect the need for donor blood transfusion.
Health economic analysis demonstrated that it would cost £8110 to avoid a blood transfusion with the use of cell salvage as used in the study. This cost could potentially be reduced by varying both the indication for cell salvage in caesarean section and by changing the technique used. Set-up for a continuous cell-saver machine, with ‘collection’ only, until sufficient volume was obtained for processing, could save the cost of the processing consumables. Swab washing could be relinquished as a technique, as it did not appear to have any effect on donor blood transfusion rate.
Strengths and limitations of the trial
To our knowledge, the SALVO trial is the largest multicentre evaluation of cell salvage in caesarean section to date. The RCT was prospectively registered, robustly conducted, independently monitored, rigorously analysed and transparently reported (see Figure 2). This should provide for confidence in validity and reliability of the findings. The study sample was diverse, spread across more than 20 UK centres. Only two indications for caesarean section were considered exclusions: first elective caesarean section for either breech or maternal request. The very low probability that these cases might require donor blood meant that excluding them left only women with a recognisable increased risk of haemorrhage and, thus, potential need for transfusion, increasing the power of the study to detect a difference. This broad base for inclusion adds to the generalisability of the findings.
The trial recruited to target and had comparability at baseline and compliance with assignment, minimal loss on follow-up and primary outcome. A substantial challenge to the conduct of an individually randomised trial is obtaining consent from women in labour who require emergency caesarean. 89,90 Therefore, meeting the target for recruitment in a challenging clinical context was a major achievement. We considered this group to be the one most likely to derive specific benefit from this health technology. Another challenge was the promotion of equipoise among participating clinicians, who were keen to adopt the technology, without robust evidence in cases for which anxiety for life-threatening haemorrhage was high, particularly for cases of abnormal placentation.
Owing to the nature of the intervention and because mothers are usually awake for caesarean section, it was considered impractical to formally blind either clinicians or patients to the group allocation. In view of this and the local variation in transfusion practices, the trial collected transfusion policies from each unit and then reviewed transfusion decisions in light of these. Although clinicians were found to commonly give donor blood in deviation to local policy, no difference was found in the rate with which this non-adherence to local policy occurred between intervention and control groups.
A criticism of sample size and power with presumption of likelihood of type II error could risk erroneous conclusions. We highlight that a p-value that is in the region of a 0.05, regardless of the side of the threshold in which it lies, deserves careful consideration with respect to use of the evidence for guiding practice. The failure to achieve statistical significance cannot be attributed to insufficient data when the study is completed to the planned size with independent monitoring. We would like to propose the following considerations in interpretation of our main finding: (1) addition of new data does not guarantee that the p-value threshold for significance will be reached91 and (2) the point estimate is the most plausible estimate of the true effect. This being the case, we believe that our main finding meets the criteria for accurate decision-making.
From the outset, we were aware that we were investigating the effect of an intervention in a heterogeneous population in terms of baseline risk. This heterogeneity is principally derived from the indication for caesarean section. This situation guided the use of covariate-adjusted and subgroup analyses as an integral part of trial planning, analysis and inference. The credibility of findings in subgroup analysis depends on a number of factors. We planned these a priori and limited the number of subgroup analyses to the bare minimum in order to limit the risk of spurious significance associated with multiple hypothesis testing. Caution must be exercised in the conduct and interpretation of evidence derived from subgroup analysis; however, not investigating or ignoring results of subgroup analyses could also lead to incorrect inferences. Although interaction proved statistically insignificant, suggesting no evidence for an inconsistent effect of cell salvage between elective and emergency cases, taking into account the observed point estimates and CIs from subgroup analyses for the primary outcome measure, we believe that our findings concerning the effect in emergency caesareans merit consideration.
The role of our sensitivity analysis was to evaluate the integrity of the primary analysis conducted based on the ITT principle. The design and analysis was predicated on adherence to assignment, whether control or cell salvage. We sought for consistency between the results of primary analysis and the results of sensitivity analysis to examine the credibility of the main finding. We planned a priori to assess if the erroneous return of cell-salvaged blood in the control group could potentially avert the use of donor transfusion. In the throes of a developing surgical emergency, it may be thought of as a useful intervention to deal with ongoing haemorrhage by clinicians handling cases in the control group. We could not prevent such a clinical intervention in an ethically consistent trial policy. Therefore, we proposed to reclassify such cases as having experienced the primary outcome. Study structures and team members strove to promote adherence to assignment but protocol deviations are common in pragmatic trials and several cases assigned to the control group did indeed receive salvage, even in the absence of such an acute emergency situation. One proposal to handle this problem could include alternative trial designs, such as cluster randomisation, but this approach does not necessarily guarantee avoidance of performance bias and generally reduces statistical power. Our approach to sensitivity analysis maintained the ITT principle, avoiding PP and as-treated analyses that have a tendency to produce spurious significance. Our sensitivity analyses confirmed the main finding for the primary outcome.
The SALVO trial studied both emergency and elective (planned) caesarean sections, despite the fact that owing to a higher incidence of haemorrhage in the former, a significant result might be more likely to be detected if the trial had excluded electives. There were two reasons for this. First, some centres were known to have already commenced using cell salvage routinely for elective cases in the absence of any evidence, so the question of evaluating effectiveness in this group remained pertinent. Second, emergencies represented a population much more challenging to recruit. The elective sample gave centres the opportunity to deploy and prove the trial processes in a much more straightforward population. Despite this, two groups of electives were known to have extremely low rates of haemorrhage and were therefore excluded (first caesareans for either breech or maternal request). The effect of cell salvage in the emergency group, while non-significant with regards to interaction with the elective group, will be interesting to clinicians. On the other hand, the finding of no effect in the placental abnormalities subgroup is less relevant for policy as guidance already exists for use of salvage in these high-risk cases. 40
At the time that the SALVO trial was conceived and designed, cell salvage techniques used in obstetrics were markedly heterogeneous and dependent on local attitudes and expertise. It was clear from the outset that a robust trial would require close to optimal use of the intervention in order for the results to be accepted by the clinical community. Optimal use in this context maximises the volume of salvaged blood returned to the mother. Therefore, a number of technical aspects of the machine use were made mandatory for trial patients to achieve this aim. Some other aspects that might increase blood return were left to local preference, as it was felt that it would be difficult to commission enough recruiting centres to complete the study if these technical elements of cell salvage management were made mandatory. Subgroup analysis of swab washing failed to show any effect on the primary outcome. We conclude that there are no other identifiable mechanisms, utilising current cell salvage technology, which might significantly increase the return of salvaged blood to the patient compared with trial practices capable of casting any doubt on the validity of our results.
External validity and generalisability
We consider the external validity of the SALVO trial to be very robust. It was conducted in a broad group of obstetric units, ranging from large, tertiary teaching hospitals to small district general hospitals. It recruited from a diverse range of indications for caesarean section, with few exclusion criteria. The nature of the intervention was maintained as pragmatic as possible, consistent with efficacy and adequate recruitment, yet delivered in a predictable manner that is easy to emulate outside the context of a trial. The adherence to protocol was higher than expected for an intervention that had already begun to enter routine practice with a consequent potential loss of equipoise when the trial commenced.
Red cell immunisation
The UK SHOT haemovigilance scheme has repeatedly highlighted suboptimal practice in relation to the management of anti-D prophylaxis in cases of caesarean section with rhesus incompatibility. 92 They have stressed the need for improved awareness of national guidelines, supported by education and training among all health-care professionals involved. 92 We had the novel opportunity to observe practice around anti-D prophylaxis, in relation to cell salvage in particular.
Although total omissions of anti-D prophylaxis after delivery occurred in only a small number of cases in either group (total n = 3), there are many other opportunities for improved adherence to national guidelines, in particular regarding the recommended minimum anti-D dose of 1500 IU following cell salvage34 and the need for FMH testing to assess if further anti-D is needed beyond the standard dose. This highlights a need for close communication between clinicians and laboratory teams to ensure that relevant testing is undertaken followed by subsequent appropriate management to minimise the risk of RhD sensitisation.
Although secondary analysis indicated a significantly higher risk of FMH of ≥ 2 ml based on Kleihauer testing in the cell salvage group, the clinical implications of this result are unclear. We have incomplete data on flow cytometry results to confirm FMH volumes. Further analysis of available results indicate that the majority of women with bleeds > 4 ml did receive appropriate doses of anti-D with further follow-up testing to check for fetal red cell clearance as per guidelines, but there were some omissions. We are unable to comment on the overall efficacy of anti-D prophylaxis and the subsequent risk of RhD sensitisation. The SHOT scheme is currently collecting data on all pregnant women who have produced immune anti-D detected for the first time, to better understand the reasons underlying RhD sensitisation.
Although we are unable to comment on the risk of alloimmunisation to other red cell antibodies following cell salvage as opposed to standard care, our data support the assertion that the use of cell salvage significantly increases the risk of FMH. When just the women receiving cell-salvaged blood are considered, the rate of FMH, based on the definition used in the study, is more than four times that of the control group. The implications of this may go beyond the increased cost of a larger anti-D dose required in women receiving cell-salvaged blood. Although we did not specifically study other red cell antigens, it is plausible and likely that this increased FMH would cause increased rates of introduction of these antigens into the maternal circulation. As no anti-D equivalent is available for these antigens, maternal antibody formation will not be avoided and these antibodies (e.g. anti-Kell) may make cross matching blood for these women significantly more difficult in the future, incurring additional health-care costs. As the rate and severity of these potential complications is completely unknown, we were unable to incorporate it into our health economic analysis.
Adverse and serious adverse events
The AEs and SAEs were spread evenly across the two groups. An increase in the rate of AFE has long been considered a potential AE of returning cell-salvaged blood at caesarean section, even though research indicates it is removed by the cell saver. It is reassuring that we did not observe any cases of AFE in either group, but particularly not in the subgroup who actually received salvaged blood back, whether or not a LDF was used. It is notable that all the AEs definitely or probably related to cell salvage occurred when a LDF was in use, consisting of acute haemodynamic and respiratory reactions to the return of salvaged blood. These reactions have been well reported in the literature and are thought to be due to an effect the filter induces on the salvaged blood, rather than due to the blood per se. 18,23–26 We did not observe any definitely or probably cell-salvage-related AEs when a filter was not used. Out of the 15 possibly related AEs, a filter had been used in 13. These included a range of AEs, including infective and haemorrhagic complications that could easily have been unrelated to the use of cell salvage. Ultimately, it was for the local principal investigator to make this judgement on relatedness. There was one case of reaction to donor blood in the control group, from which the mother made a full recovery. This is in keeping with the known rates of reaction. 18 One maternal death occurred in a trial participant, who had been allocated to the cell salvage intervention group. A local case review was carried out by the trust involved and did not find any link to the use of cell salvage.
Adverse events from donor blood transfusion are potentially serious but are also very rare, with a rate of 1 in 16,000. 18 Mortality associated with donor blood transfusion is even more uncommon, with a rate of 1 in 100,000. 18 We have demonstrated that the cost to avoid a transfusion event when routinely using cell salvage in caesarean section is £8110. When considered with the observed increase in FMH and potential long-term effects of this, which currently remain uncharacterised, it remains unclear whether or not cell salvage is beneficial in this patient group. The exception to this is in cases such as for Jehovah’s Witnesses, for whom donor blood cannot be used and cell salvage represents the only therapeutic option. We are also unable to comment on the specific benefit in the group at a high risk of torrential haemorrhage such as placenta accreta, as we had insufficient recruits in this group to come to any meaningful conclusions.
Conclusions
Implications for health-care service
-
Cell salvage may reduce the need for donor blood transfusion. It is unlikely to be considered cost-effective when routinely set up for use in caesarean section. The cost-effectiveness varies by indication for caesarean section and cost of cell-saver technique used.
-
In RhD-negative mothers having RhD-positive babies, there appears to be an increased chance of FMH when cell-salvaged blood is returned to the mother, which needs to be taken into consideration with regard to applying and updating guidance on the use of anti-D prophylaxis. Our findings highlight the need for increased vigilance and appropriate prevention of the risk of RhD-isoimmunisation among RhD-negative mothers.
-
If cell salvage continues to be used in groups such as Jehovah’s Witnesses and those with placenta accreta, women should be counselled about the balance of risks in using cell salvage.
Recommendations for further research
-
The effect of increased FMH associated with cell salvage on the incidence of rarer, non-RhD red cell antigens needs to be characterised and quantified in the long term.
-
Investigation is needed to determine if greater amounts of routine anti-D administration are required when cell salvage has been used on RhD-negative mothers.
-
Additional factors (e.g. swab washing or number of suckers used) that may increase the likelihood of blood return during use of cell salvage should be investigated.
-
The effectiveness of cell salvage in specific subgroups, such as those with placenta accreta, remains to be investigated.
-
The role of cell salvage in low- to middle-income countries, where caesarean rates are rising and blood transfusion services are not well developed, should be investigated.
-
If new, cheaper or more efficient cell salvage technology becomes available, the conclusions of the SALVO trial may need to be revisited. The same is true if donor blood shortages should become extreme and acute.
-
Recent and ongoing research into the use of tranexamic acid and other strategies to prevent or manage maternal anaemia to make caesarean section safer will merit consideration in practice and future research alongside our findings. 88
Acknowledgements
Acknowledgements for contributions to the project
The team would like to thank the following people:
Rebecca Harmston and Fleur Parker for lay advice.
Harry Gee (retired, Clinical Academic Obstetrician), Pollyanna Hardy (National Perinatal Epidemiology Unit, University of Oxford), Richard Gray (Medical Research Council Population Health Research Unit, University of Oxford), Mike Grocott (NIHR Respiratory Biomedical Research Unit, University of Southampton) and Andrew Shennan (Department of Women’s Health, King’s College London) for their advice and support as part of the TSC and DMC.
Sarah Weist and Felipe Castro Cardona at Barts Health NHS Trust for lead research midwife support. Aoife Harvey for trial management support.
Anna Placzek, Malika Barakat and James Heighway at the Queen Mary University of London Women’s Health Research Unit for data management and administrative support.
Anitha Manivannan, Natasha Stevens, Jeanette Hansen, Amy Hoon, Irene Simmonds, and Zuhur Balayah at PCTU for monitoring, quality assurance and early trial coordination support. Nadine Marlin, Lauren Bell, Lauren Greenberg and Miland Joshi at PCTU for statistical support. Mike Waring, Tom Power and Sandy Smith at PCTU for database and IT support. Glenn Poon at PCTU for logo design.
Angela Devine and Mhairi Harkness for input into the original trial application.
All the women who agreed to participate in the SALVO trial.
Contributions of authors
Khalid S Khan (Professor, Women’s Health, Queen Mary University of London) was the chief investigator. He contributed to the study design and protocol writing, study management, oversight of study conduct and the initial writing and final editing of the report.
Philip Moore (Consultant Anaesthetist) was a coapplicant, contributed to study design and writing the protocol, provided day-to-day clinical advice, oversight as a member of the TMG, medical review of clinical study data, contributed to study management and the writing and final editing of the report.
Matthew Wilson (Consultant Anaesthetist) was a coapplicant, contributed to study design and writing the protocol, contributed to study management as a member of the TMG and to the writing and final editing of the report.
Richard Hooper (Senior Statistician) contributed to the design of the study, provided statistical supervision and advice as a member of the TMG, and contributed to the writing and final editing of the report.
Shubha Allard (Consultant Haematologist) contributed to the design of the study, to study management as a member of the TMG, provided transfusion-related supervision and advice, and contributed to the final report.
Ian Wrench (Consultant Anaesthetist) contributed to the design of the study, study management as a member of the TMG, provided clinical advice and contributed to the final report.
Tracy Roberts (Professor of Health Economics) contributed to the design and planning of the health economic analyses, provided health economic supervision and advice throughout the study, and contributed to the final report.
Carol McLoughlin (Health Economist) contributed to the health economic analysis plan, conducted the health economic analysis and contributed to the final report.
Lee Beresford (Statistician) wrote the statistical analysis plan, provided statistical support, performed the statistical analysis of the study and contributed to the writing and final editing of the report.
James Geoghegan (Consultant anaesthetist) contributed to the study design, led on the pilot study, provided clinical advice and contributed to the final report.
Jane Daniels (Senior Research Fellow) contributed to the study design and the final report.
Sue Catling (Consultant Anaesthetist) contributed to the study design, provided clinical advice and contributed to the final report.
Vicki A Clark (Consultant Anaesthetist) contributed to the study design, provided clinical advice and contributed to the final report.
Paul Ayuk (Consultant Obstetrician) contributed to the study design, provided clinical advice and contributed to the final report.
Stephen Robson (Professor of Fetal Medicine) contributed to the study design, provided clinical advice and contributed to the final report.
Fang Gao-Smith (Professor in Anaesthesia) contributed to the study design, provided clinical advice and contributed to the final report.
Matthew Hogg (Consultant in Obstetrics and Gynaecology) contributed to study conduct and oversight as a member of the TMG and contributed to the final report.
Louise Jackson (Health Economist) contributed to the health economic analysis plan, provided health economic analysis support and reviewed the final report.
Doris Lanz (Senior Clinical Trial Coordinator) supervised the general management and day-to-day conduct of the trial, supervised data collection and contributed to the writing of the final report.
Julie Dodds (Senior Research Manager) supervised the running of the trial, provided direction and support as a member of the TMG and contributed to the final report.
Publication
Khan KS, Moore PAS, Wilson MJ, Hooper R, Allard S, Wrench I, et al. Cell salvage and donor blood transfusion during cesarean section: a pragmatic, multicentre randomised controlled trial (SALVO). PLOS Med 2017;14:e1002471.
Data sharing statement
We shall make data available to the scientific community with as few restrictions as feasible, while retaining exclusive use until the publication of major outputs. Requests for anonymised data should be sent to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Centre for Maternal and Child Enquiries . Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. BJOG 2011;118:1-203. https://doi.org/10.1111/j.1471-0528.2010.02847.x.
- Bodelon C, Bernabe-Ortiz A, Schiff MA, Reed SD. Factors associated with peripartum hysterectomy. Obstet Gynecol 2009;114:115-23. https://doi.org/10.1097/AOG.0b013e3181a81cdd.
- Intensive Care National Audit & Research Centre . Female Admissions (Aged 16–50 Years) to Adult, General Critical Care Units in England, Wales and Northern Ireland Reported As ‘Currently Pregnant’ or ‘Recently Pregnant’ 2013.
- Lataifeh I, Amarin Z, Zayed F, Al-Mehaisen L, Alchalabi H, Khader Y. Indications and outcome for obstetric patients’ admission to intensive care unit: a 7-year review. J Obstet Gynaecol 2010;30:378-82. https://doi.org/10.3109/01443611003646298.
- Saravanakumar K, Davies L, Lewis M, Cooper GM. High dependency care in an obstetric setting in the UK. Anaesthesia 2008;63:1081-6. https://doi.org/10.1111/j.1365-2044.2008.05581.x.
- Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg 2010;110:1368-73. https://doi.org/10.1213/ANE.0b013e3181d74898.
- Holm C, Langhoff-Roos J, Petersen KB, Norgaard A, Diness BR. Severe postpartum haemorrhage and mode of delivery: a retrospective cohort study. BJOG 2012;119:596-604. http://dx.doi.org/10.1111/j.1471-0528.2011.03267.x.
- Betran AP, Torloni MR, Zhang JJ, Gülmezoglu AM. WHO Working Group on Caesarean Section . WHO Statement on Caesarean Section Rates. BJOG 2016;123:667-70. https://doi.org/10.1111/1471-0528.13526.
- NHS Maternity Statistics – England 2013–14. 2015.
- Healthcare Improvement Scotland . Scottish Confidential Audit of Severe Maternal Morbidity: Reducing Avoidable Harm 2014. www.healthcareimprovementscotland.org/our_work/reproductive,_material_child/programme_resources/scasmm.aspx (accessed 18 September 2017).
- Tinegate H, Pendry K, Murphy M, Babra P, Grant-Casey J, Hopkinson C, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139-45. https://doi.org/10.1111/trf.13342.
- Costing Statement: Blood Transfusion. Implementing the NICE Guideline on Blood Transfusion (NG24). London: NICE; 2015.
- NHS Blood and Transplant . Saving and Improving Lives. Strategic Plan 2015–20 2015. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/1593/strategic_plan_2015_20.pdf (accessed 18 September 2017).
- Shander A, Isbister J, Gombotz H. Patient blood management: the global view. Transfusion 2016;56:94-102. https://doi.org/10.1111/trf.13529.
- Mehra T, Seifert B, Bravo-Reiter S, Wanner G, Dutkowski P, Holubec T, et al. Implementation of a patient blood management monitoring and feedback program significantly reduces transfusions and costs. Transfusion 2015;55:2807-15. http://dx.doi.org/10.1111/trf.13260.
- Catling S. Intraoperative cell salvage in obstetrics. Clinical Risk 2008;14:14-7. http://dx.doi.org/10.1258/cr.2007.070009.
- Bolton-Maggs P, Poles D, Watt A, Thomas D. The Annual SHOT Report 2013. Manchester: SHOT; 2014.
- Bolton-Maggs P, Poles D, . on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group . The 2015 Annual SHOT Report 2016. www.shotuk.org/wp-content/uploads/SHOT-2015-Annual-Report-Web-Edition-Final-bookmarked.pdf (accessed 18 September 2017).
- Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med 2004;116:58-69. https://doi.org/10.1016/j.amjmed.2003.12.013.
- James AH, Patel ST, Watson W, Zaidi QR, Mangione A, Goss TF. An assessment of medical resource utilization and hospitalization cost associated with a diagnosis of anemia in women with obstetrical bleeding in the United States. J Womens Health (Larchmt) 2008;17:1279-84. http://dx.doi.org/10.1089/jwh.2007.0605.
- Carless PA, Henry DA, Moxey AJ, O’Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2010;4. http://dx.doi.org/10.1002/14651858.CD001888.pub3.
- Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10440.
- Sreelakshmi TR, Eldridge J. Acute hypotension associated with leucocyte depletion filters during cell salvaged blood transfusion. Anaesthesia 2010;65:742-4. http://dx.doi.org/10.1111/j.1365-2044.2009.06190.x.
- Kessack LK, Hawkins N. Severe hypotension related to cell salvaged blood transfusion in obstetrics. Anaesthesia 2010;65:745-8. https://doi.org/10.1111/j.1365-2044.2010.06301.x.
- Waldron S. Hypotension associated with leucocyte depletion filters following cell salvage in obstetrics. Anaesthesia 2011;66:133-4. https://doi.org/10.1111/j.1365-2044.2010.06588.x.
- Zoon KC, Jacobson ED, Woodcock J. Hypotension and bedside leukocyte reduction filters. Int J Trauma Nurs 1999;5:121-2. https://doi.org/10.1016/S1075-4210(99)90002-0.
- Sullivan I, Faulds J, Ralph C. Contamination of salvaged maternal blood by amniotic fluid and fetal red cells during elective Caesarean section. Br J Anaesth 2008;101:225-9. https://doi.org/10.1093/bja/aen135.
- Waters JH, Biscotti C, Potter PS, Phillipson E. Amniotic fluid removal during cell salvage in the cesarean section patient. Anesthesiology 2000;92:1531-6. https://doi.org/10.1097/00000542-200006000-00008.
- Mulder JI. Amniotic fluid embolism: an overview and case report. Am J Obstet Gynecol 1985;152:430-5. https://doi.org/10.1016/S0002-9378(85)80153-8.
- Clark SL, Pavlova Z, Greenspoon J, Horenstein J, Phelan JP. Squamous cells in the maternal pulmonary circulation. Am J Obstet Gynecol 1986;154:104-6. https://doi.org/10.1016/0002-9378(86)90402-3.
- Lee W, Ginsburg KA, Cotton DB, Kaufman RH. Squamous and trophoblastic cells in the maternal pulmonary circulation identified by invasive hemodynamic monitoring during the peripartum period. Am J Obstet Gynecol 1986;155:999-1001. https://doi.org/10.1016/0002-9378(86)90334-0.
- Kuhlman K, Hidvegi D, Tamura RK, Depp R. Is amniotic fluid material in the central circulation of peripartum patients pathologic?. Am J Perinatol 1985;2:295-9. https://doi.org/10.1055/s-2007-999974.
- White J, Qureshi H, Massey E, Needs M, Byrne G, Daniels G, et al. British Committee for Standards in Haematology . Guideline for blood grouping and red cell antibody testing in pregnancy. Transfus Med 2016;26:246-63. https://doi.org/10.1111/tme.12299.
- Qureshi H, Massey E, Kirwan D, Davies T, Robson S, White J, et al. BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfus Med 2014;24:8-20. https://doi.org/10.1111/tme.12091.
- Royal College of Obstetricians and Gynaecologists . The Management of Women With Red Cell Antibodies During Pregnancy 2014.
- Dhariwal SK, Khan KS, Allard S, Wilson M, Moore P. SALVO study group . Does current evidence support the use of intraoperative cell salvage in reducing the need for blood transfusion in caesarean section?. Curr Opin Obstet Gynecol 2014;26:425-30. https://doi.org/10.1097/GCO.0000000000000116.
- Ralph CJ, Sullivan I, Faulds J. Intraoperative cell salvaged blood as part of a blood conservation strategy in Caesarean section: is fetal red cell contamination important?. Br J Anaesth 2011;107:404-8. https://doi.org/10.1093/bja/aer168.
- Intraoperative Blood Cell Salvage in Obstetrics. London: NICE; 2005.
- Association of Anaesthetists of Great Britain and Ireland . Blood Transfusion and the Anaesthetist: Intra-Operative Cell Salvage 2009.
- Royal College of Obstetricians and Gynaecologists . Blood Transfusion in Obstetrics (Green-Top Guideline No. 47) 2007.
- Teig M, Harkness M, Catling S, Clark V. Survey of Cell-Salvage Use in Obstetrics in the UK n.d.
- Evans H, Lewis E. UK survey of the availability of cell salvage and interventional radiological services for the management of obstetric haemorrhage. Int J Obstet Anesth 2013;22:355-6. http://dx.doi.org/10.1016/j.ijoa.2013.05.006.
- Geoghegan J, Daniels JP, Moore PA, Thompson PJ, Khan KS, Gülmezoglu AM. Cell salvage at caesarean section: the need for an evidence-based approach. BJOG 2009;116:743-7. https://doi.org/10.1111/j.1471-0528.2009.02129.x.
- Rainaldi MP, Tazzari PL, Scagliarini G, Borghi B, Conte R. Blood salvage during caesarean section. Br J Anaesth 1998;80:195-8. https://doi.org/10.1093/bja/80.2.195.
- Rebarber A, Lonser R, Jackson S, Copel JA, Sipes S. The safety of intraoperative autologous blood collection and autotransfusion during cesarean section. Am J Obstet Gynecol 1998;179:715-20. https://doi.org/10.1016/S0002-9378(98)70070-5.
- Catling SJ, Freites O, Krishnan S, Gibbs R. Clinical experience with cell salvage in obstetrics: 4 cases from one UK centre. Int J Obstet Anesth 2002;11:128-34. https://doi.org/10.1054/ijoa.2001.0914.
- Keeling MM, Gray LA, Brink MA, Hillerich VK, Bland KI. Intraoperative autotransfusion. Experience in 725 consecutive cases. Ann Surg 1983;197:536-41. https://doi.org/10.1097/00000658-198305000-00006.
- Jackson SH, Lonser RE. Safety and effectiveness of intracesarean blood salvage. Transfusion 1993;33. https://doi.org/10.1046/j.1537-2995.1993.33293158054.x.
- Potter PS, Waters JH, Burger GA, Mraović B. Application of cell-salvage during cesarean section. Anesthesiology 1999;90:619-21. https://doi.org/10.1097/00000542-199902000-00038.
- Rees SG, Boheimer NO. Autologous blood transfusion. Br J Anaesth 1998;80. https://doi.org/10.1093/bja/80.4.563.
- Waters JH, Lukauskiene E, Anderson ME. Intraoperative blood salvage during cesarean delivery in a patient with beta thalassemia intermedia. Anesth Analg 2003;97:1808-9. https://doi.org/10.1213/01.ANE.0000087046.91072.E8.
- Grimes DA. A simplified device for intraoperative autotransfusion. Obstet Gynecol 1988;72:947-50. https://doi.org/10.1097/00006250-198812000-00030.
- Katz AR, Walker WA, Ross PJ, Held B. Autologous transfusion in obstetrics and gynecology. Int J Gynaecol Obstet 1978;16:345-7. https://doi.org/10.1002/j.1879-3479.1979.tb00461.x.
- Zichella L, Gramolini R. Autotransfusion during cesarean section. Am J Obstet Gynecol 1990;162. https://doi.org/10.1016/0002-9378(90)90880-G.
- Geoghegan J, Middleton L, Moore P, Subseson G, Khan K, Daniels J. Routine cell salvage during elective caesarean section: a pilot randomised trial. Int J Obstet Anesth 2015;24:86-7. https://doi.org/10.1016/j.ijoa.2014.08.003.
- Torloni MR, Betran AP, Souza JP, Widmer M, Allen T, Gulmezoglu M, et al. Classifications for cesarean section: a systematic review. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0014566.
- Royal College of Obstetricians and Gynaecologists . The Royal College of Anaesthetists. Classification of Urgency of Caesarean Section – A Continuum of Risk 2010.
- Lucas DN, Yentis SM, Kinsella SM, Holdcroft A, May AE, Wee M, et al. Urgency of caesarean section: a new classification. J R Soc Med 2000;93:346-50. https://doi.org/10.1177/014107680009300703.
- National Institute for Health and Care Excellence . Intrapartum Care: Care of Healthy Women and Their Babies During Childbirth. Clinical Guideline CG55 2007.
- National Institute for Health and Care Excellence . Intrapartum Care for Healthy Women and Babies. Clinical Guideline CG190 2014.
- Haynes SL, Bennett JR, Torella F, McCollum CN. Does washing swabs increase the efficiency of red cell recovery by cell salvage in aortic surgery?. Vox Sang 2005;88:244-8. https://doi.org/10.1111/j.1423-0410.2005.00631.x.
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995;39:315-25. https://doi.org/10.1016/0022-3999(94)00125-O.
- Guidelines for the Estimation of Fetomaternal Haemorrhage. London: British Society for Haematology; 2009.
- NHS England Patient Safety Domain . Serious Incident Framework. Supporting Learning to Prevent Recurrence 2015.
- Data Protection Act. London: The Stationery Office; 1998.
- The Caldicott Committee. Report on the Review of Patient-Identifiable Information. London: Department of Health; 1997.
- Research Governance Framework for Health and Social Care, Second Edition. London: The Stationery Office; 2005.
- Fong J, Gurewitsch ED, Kang HJ, Kump L, Mack PF. An analysis of transfusion practice and the role of intraoperative red blood cell salvage during cesarean delivery. Anesth Analg 2007;104:666-72. https://doi.org/10.1213/01.ane.0000253232.45403.e5.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. https://doi.org/10.1136/bmj.d40.
- Royal College of Obstetricians and Gynaecologists . Prevention and Management of Postpartum Haemorrhage (Green-Top Guideline No. 52) 2009.
- Khan KS, Moore PAS, Wilson MJ, Hooper R, Allard S, Wrench I, et al. Cell salvage and donor blood transfusion during cesarean section: a pragmatic, multicentre randomised controlled trial (SALVO). PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002471.
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- NHS Supply Chain . NHS Supply Chain Catalogue 2015 n.d. https://my.supplychain.nhs.uk/Catalogue (accessed August 2016).
- UK Cell Salvage Action Group . ICS Technical Factsheet 1: Swab Washing 2015. http://www.transfusionguidelines.org/transfusion-practice/uk-cell-salvage-action-group/technical-factsheets-and-frequently-asked-questions-faq (accessed 18 September 2017).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- NHS Blood and Transplant . Blood and Components Price List 2016 2017 2016. http://hospital.blood.co.uk/media/28230/component-price-list-2016-2017.pdf (accessed 18 September 2017).
- Department of Health . NHS Reference Costs 2014 2015 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/477919/2014-15_Reference_costs_publication.pdf (accessed 18 September 2017).
- NHS National Tariff Payment System . 2016/17/National/Prices 2016. www.gov.uk/government/publications/national-tariff-payment-system-2014-to-2015 (accessed August 2016).
- Tinegate H, Birchall J, Gray A, Haggas R, Massey E, Norfolk D, et al. Guideline on the investigation and management of acute transfusion reactions. Prepared by the BCSH Blood Transfusion Task Force. Br J Haematol 2012;159:143-53. https://doi.org/10.1111/bjh.12017.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011;2:109-12. http://dx.doi.org/10.4103/2229-3485.83221.
- Briggs AH. A Bayesian approach to stochastic cost-effectiveness analysis. Health Econ 1999;8:257-61. https://doi.org/10.1002/(SICI)1099-1050(199905)8:3<257::AID-HEC427>3.0.CO;2-E.
- Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 1999;3:1-134.
- Alkema L, Chou D, Hogan D, Zhang S, Moller A-B, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet n.d.;387:462-74. http://dx.doi.org/10.1016/S0140-6736(15)00838-7.
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323-33. https://doi.org/10.1016/S2214-109X(14)70227-X.
- Jardine JE, Law P, Hogg M, Murphy D, Khan KS. C-SAFETY . Haemorrhage at caesarean section: a framework for prevention and research. Curr Opin Obstet Gynecol 2016;28:492-8. https://doi.org/10.1097/GCO.0000000000000328.
- Royal College of Obstetricians and Gynaecologists . Obtaining Valid Consent to Participate in Perinatal Research Where Consent Is Time Critical 2016.
- George RT, Butcher M, Yentis SM. Pregnant women’s views on informed consent for research in labour. Int J Obstet Anesth 2014;23:233-7. https://doi.org/10.1016/j.ijoa.2014.03.004.
- Wood J, Freemantle N, King M, Nazareth I. Trap of trends to statistical significance: likelihood of near significant P value becoming more significant with extra data. BMJ 2014;348. https://doi.org/10.1136/bmj.g2215.
- SHOT . SHOT Annual Reports and Summaries 2017. www.shotuk.org/shot-reports (accessed 6 September 2017).
Appendix 1 List of participating sites and the SALVO research staff
-
Barts Health Trust, London:
-
Royal London Hospital
-
Whipps Cross University Hospital
-
Matthew Hogg (principal investigator), Sajith Philip, Sarah Weist, Felipe Castro Cardona, Heike Bojahr, Lilith Loncke and Prudence Jones.
-
-
Royal United Hospitals Bath NHS Foundation Trust:
-
Royal United Hospital, Bath
-
Chris Marsh (principal investigator), Sara Burnard, Wendy Duberry, Elly Doyle, Karen Patrick, Catherine Bressington, Jenny Pullen, Mel Rich, Jess Withers and Amy Lloyd.
-
-
Heart of England NHS Foundation Trust:
-
Birmingham Heartlands Hospital
-
Nicky Osborn (principal investigator), Ahmed Mesbah, Katie Trowman, Linda Bradley, Katie Atterbury and Teresa Melody.
-
-
Birmingham Women’s NHS Foundation Trust:
-
Birmingham Women’s Hospital
-
James Geoghegan (principal investigator), Philip Moore, Chloe O’Hara and Elizabeth Ewer.
-
-
University Hospitals Bristol NHS Foundation Trust:
-
St Michael’s Hospital, Bristol
-
Isobel Gardner (principal investigator), Carole Shahin, Alison Kirby, Mariethel Gudaca, Eirini Troupkou, Colleen Hunt, Claire Dowse, Nicola Harvey, Nicolas Wharton and Mark Scrutton.
-
-
Chelsea and Westminster Hospital NHS Foundation Trust:
-
West Middlesex University Hospital, Isleworth
-
Dominika Dabrowska (principal investigator), Marie O’Connell, Bernadette Tilley, Catherine Sheehan, Philip Barclay, Christine Adamson, Mehari Teklay, Sherif Omran, Sujatha Vishvanath, Amanpreet Sarna, Sheldon Zhang, Rafiu Ojo, Lisa Takab, Tina Brough, Emma Fox, Sarah Barker, Ano Rathambegoda, Edward Twumasi, Jacob Sheen, Belinda White, Wilky Ian Nunal and Emmanuel Espiritu.
-
-
Croydon Health Services NHS Trust:
-
Croydon University Hospital
-
Bini Ajay (principal investigator), Dhileepan Srinivasan, Temilola Doherty, Valerie Fuller, Tony Hewitt, Ramandeep Sharma, Ajeet Kumar, Rebecca Byrne and Vana Wardley.
-
-
NHS Lothian:
-
Simpson Centre for Reproductive Health, Edinburgh
-
Vicki Clark, Arlene Wise (principal investigators), Ida Hassing and Karen Edgar.
-
-
Hinchingbrooke Health Care NHS Trust:
-
Hinchingbrooke Hospital
-
Sangeeta Pathak (principal investigator), Tara Pauley, Charlotte Clayton and Aarti Bahirat.
-
-
Leicester University Hospitals NHS Trust:
-
Leicester Royal Infirmary
-
Leicester General Hospital
-
Tommy Mousa (principal investigator), Molly Patterson, Sharon Bates, Jo Dickens, Katie Peck, Anna Muggleton, Claire Dodd, Asma Rabab, Tina Evans, Tracey Bryan, Magda Kierzenkowska, Margaret Weston, Sarah Clarke, Katie Warwick, C Elton, P Sharpe, A Morris, P Ramasamy, E Hart, R Leighton, O Navti and O Joseph.
-
-
South Tees Hospitals NHS Foundation Trust:
-
James Cook University Hospital, Middlesbrough
-
Sanjay Rao (principal investigator), Aethele Khunda and the research team, Hazel Alexander, Sarah Croft, Obstetric Consultants and Anaesthetists, Speciality Trainees, Labour Ward Midwifery team and Theatre team.
-
-
The Newcastle upon Tyne Hospitals NHS Foundation Trust:
-
Royal Victoria Infirmary, Newcastle
-
Paul Ayuk (principal investigator), Sophia Webster, Jill Sturt, Celia McKee, Angela Yulia, Andrea Fenn, Michelle Perkins, MaCassie Galeon, Jill Riches, Cat Rowney, Erica Del Prete, Sue Harbertson, Terri Brosnan, Sharon Chilton, Victoria Murtha, Jenna Wall, Emma Schultz, Alison Bates and Nicola King.
-
-
Norfolk and Norwich University Hospitals NHS Foundation Trust:
-
Norfolk and Norwich University Hospital
-
Maria del Rocio Ochoa-Ferraro (principal investigator), Elizabeth Turner, Jonathon Francis, David Thornton, Carole Winstanley, Jeremy Corfe and Rachel Appleton.
-
-
London North West Healthcare NHS Trust:
-
Northwick Park Hospital
-
Parijat Bhattacharjee (principal investigator).
-
-
Nottingham University Hospitals NHS Trust:
-
Queens Medical Centre, Nottingham
-
Nottingham City Hospital
-
Lesley Woods (principal investigator), Jim Thornton (a member of the NIHR HTA Editorial Board), George Bugg, Sujata Handa, Arani Pillai, Yvette Davis, Yvonne Toomassi, Yvette Gunn, Denise Lochrie and Carys Smith.
-
-
Plymouth Hospitals NHS Trust:
-
Derriford Hospital, Plymouth
-
Darryl Thorp-Jones (principal investigator), Heidi Hollands, Jocelyn Watson, Alison Stolton and Amanda Carney.
-
-
Barking, Havering and Redbridge University Hospitals NHS Trust:
-
Queen’s Hospital, Romford
-
Vinod Patil (principal investigator), Annemarie McGregor, Rebecca Murray, Dorothy Sutton, Theresa McCluskey, Julie Wright, Molly Murwira, Sue Rogers and Mark Beaufond.
-
-
Sheffield Teaching Hospitals NHS Foundation Trust:
-
Royal Hallamshire Hospital, Sheffield
-
Ian Wrench (principal investigator), Siobhan Gillespie, Carolyn Clark, Emma Steel, Sarah Senbeto, Paula Woodcock, Tessa Bonnett, Nicola Cawley and Hannah Yeeles.
-
-
University Hospitals of North Midlands NHS Trust:
-
Royal Stoke University Hospital, Stoke-on-Trent
-
Jules Allt (principal investigator), Charlotte Howell, Siby Sebastian, A Rajashanker, Angela Rooney, Sara Mountford, Suzanne Jerreat, Amanda Redford, Anna Fleming, Donna Brayford, Wendy Dudley, Sarah Elson, Rachel Sparkes, Andrea Vickers, Chris Hollins, N Butler, S Scally, Theresa Webbon, Susan Bell, Andrea Morgan, Brett Beasley and MJ Newton.
-
-
City Hospitals Sunderland NHS Foundation Trust:
-
Sunderland Royal Hospital
-
Aarti Ullal (principal investigator), Kim Hinshaw, Helen Cameron, Kirsten Herdman, Eileen Walton, Gill Campbell, Lesley Hewitt, Deborah Bonney, Kathleen Hubbard, Karen Armstrong, Judith Ormonde, Joanne Knight, Kathryn Witte, Dawn Edmundson, Sonia Thompson, Denise Mace, Sharon Morrell, Suzanne Stelling, Marion Collings, Julie Harris, Amanda Bargh, Judith Holland, Chris Field and Catherine Parkinson.
-
-
Abertawe Bro Morgannwg University Health Board:
-
Singleton Hospital, Swansea
-
Susan Williams (principal investigator), Sue Catling, Sharon Jones, Trudy Smith, Helen Worrell and Sarah Fox.
-
-
Torbay and South Devon NHS Foundation Trust:
-
Torbay Hospital
-
David Portch (principal investigator), Richard Hughes, Shakila Sudhaker, Jeremy Ackers, Pauline Fitzell and Janet Palmer.
-
-
St Helens and Knowsley Teaching Hospitals NHS Trust:
-
Whiston Hospital, Prescot
-
Peter Yoxall (principal investigator) and Zoe Grindley.
-
Appendix 2 Recruitment graphs
FIGURE 10.
Overall recruitment graph.

FIGURE 11.
Recruitment graph by caesarean section type.
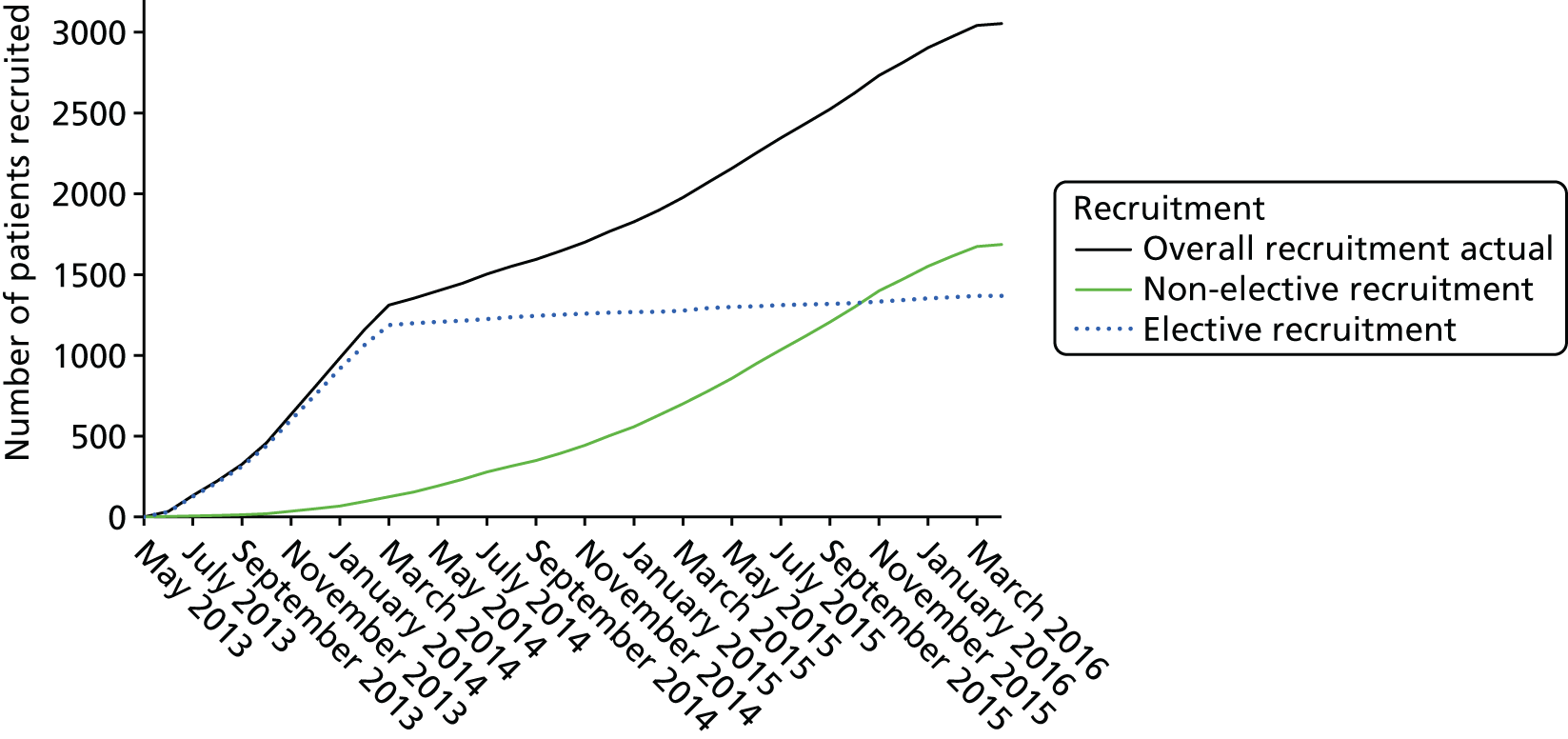
List of abbreviations
- AE
- adverse event
- AFE
- amniotic fluid embolism
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CRF
- case report form
- CT
- computed tomography
- DMC
- Data Monitoring Committee
- FMH
- fetomaternal haemorrhage
- HDFN
- haemolytic disease of the fetus and newborn
- HLC
- higher level of care
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- Ig
- immunoglobulin
- IQR
- interquartile range
- ITT
- intention to treat
- IU
- international unit
- LDF
- leucocyte depletion filter
- MD
- mean difference
- MFI
- multidimensional fatigue inventory
- MRI
- magnetic resonance imaging
- NCT
- National Childbirth Trust
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- ODP
- operating department practitioner
- OR
- odds ratio
- PCTU
- pragmatic clinical trials unit
- PP
- per protocol
- PPH
- postpartum haemorrhage
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RBC
- red blood cell
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RhD
- rhesus D
- RR
- relative risk
- SAE
- serious adverse event
- SALVO
- cell SALVage in Obstetrics
- SD
- standard deviation
- SHOT
- Serious Hazards Of Transfusion
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee