Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/29/02. The contractual start date was in April 2010. The draft report began editorial review in November 2015 and was accepted for publication in February 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David Cunningham is funded by the National Institute for Health Research Biomedical Research Centre at the Royal Marsden and Institute of Cancer Research. Jon Deeks is on the Health Technology Assessment programme Commissioning Board and Systematic Reviews Programme Advisory Group Advisory Group.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Ghaneh et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Pancreatic cancer is one of the major causes of cancer death. In the UK in 2011 the incidence of pancreatic cancer was 8773 (15.7 per 100,000 in 2012) and in 2012 there were 8662 deaths from pancreatic cancer. 1 Over the past three decades considerable progress has been made towards understanding the biology of pancreatic cancer, refining imaging systems and improving surgical outcomes and more recently there has been a focus on biomarkers to enable targeted therapies. In spite of these advances the overall survival figures for pancreatic cancer remain bleak. The 5-year survival rate for all patients with pancreatic cancer persists at 7%. 2,3 Most patients present with advanced disease because of late presentation and difficulties in early diagnosis. Median survival for patients with advanced disease is between 3 and 6 months, but this can be improved with systemic chemotherapy. 4–6 The outlook for those patients who can undergo surgical resection is better. In specialised centres, resection rates of > 15% can be achieved. 7 Although surgery cannot guarantee a cure, the 5-year survival rate does improve to 10–15% following resection8,9 and increases to 20–30% with adjuvant chemotherapy. 10–12 The pattern of disease recurrence following resection includes both locoregional failure and distant metastases. 13 The biggest risk factors for pancreatic cancer are increasing age, smoking, new-onset diabetes mellitus, increased body mass index, ABO blood group, chronic pancreatitis (15- to 25-fold risk), hereditary pancreatitis and an inherited predisposition for pancreatic cancer (this may account for 10% of observed cases). Tobacco smoking is associated with a twofold increase and because of the prevalence may account for around 30% of all cases with pancreatic ductal adenocarcinoma (PDAC). 14,15
Standard diagnostic practice
The diagnosis of pancreatic cancer can be challenging. Patients with pancreatic cancer may be relatively asymptomatic during its early course, with vague presenting symptoms such as back and epigastric pain. 16,17 Until the systemic symptoms of weight loss, anorexia and obstructive jaundice appear, it can be a difficult diagnosis to achieve. The role of imaging in such patients is to identify a pancreatic lesion, determine its malignant potential and assess its resectability. At the same time, it must also correctly identify inoperable carcinomas so that patients can receive appropriate therapy as soon as possible and be spared unnecessary operations. Standard diagnostic practice [along with tumour marker carbohydrate antigen 19-9 (CA19.9) estimation] currently consists of:
-
Contrast-enhanced multidetector computed tomography (MDCT) (perhaps following an initial transabdominal ultrasound scan).
-
Endoluminal ultrasound (EUS) may be employed in cases in which further information is required. Histology may also be obtained.
-
Therapeutic endoscopic retrograde cholangiopancreatography (ERCP) (or percutaneous transhepatic cholangiography) to relieve jaundice and obtain cytological brushings.
-
Magnetic resonance imaging (MRI) may be used to evaluate equivocal liver lesions.
-
Laparoscopy and laparoscopic ultrasound may be used on a selective basis to stage a radiologically resectable tumour.
Carbohydrate antigen 19-9 is the most commonly used marker in everyday practice. CA19-9 has a sensitivity of 78% and specificity of 82% in discriminating between malignant and benign disease. 18 False-positive results are obtained in benign obstructive jaundice, chronic pancreatitis, cholangitis, cirrhosis and ascites. CA19-9 is most useful in assessing response to treatment in advanced cases and identifying early recurrence in resected cases. 19,20 Novel markers, for example urine panel biomarkers,21 may improve on the current standards in the future.
Initial imaging may include transabdominal ultrasound22 but the gold standard for pancreatic imaging is MDCT. This technology provides three-dimensional multiplanar reconstruction techniques enabling accurate determination of tumour involvement of the common bile duct, pancreatic duct and peripancreatic vasculature. The sensitivity and specificity of MDCT in detecting pancreatic malignancy may be between 97% and 81% and 72% and 66%, respectively. 23,24 The positive predictive value (PPV) for predicting unresectability (89–100%) is high but the PPV of computed tomography (CT) for predicting resectability (45–79%) is low. 25,26 Pancreatic carcinoma typically manifests as a hypoattenuating focal mass relative to the enhancing pancreatic parenchyma on contrast-enhanced CT. However, approximately 11% of carcinomas are isoattenuating with the pancreas and their detection relies on secondary signs such as interruption of the pancreatic duct, distal pancreatic atrophy and mass effect. 27 Chronic pancreatitis can show many of the features of adenocarcinoma on CT imaging, including having the appearance of a focal mass, appearing isodense or hypodense to the pancreatic parenchyma, pancreatic duct dilatation and pancreatic atrophy. This can lead to up to 10% of pancreatic resections being performed for benign disease. 28 Limitations of CT also include resolution to identify small tumours or differentiate a tumour in a diffusely enlarged or bulky pancreas. Bulky/diffuse enlargement on CT may be associated with malignancy in 8.7% of cases. 29 Furthermore, the sensitivity of CT for small hepatic and peritoneal metastases is also limited. MRI can be helpful as an adjunct to CT, particularly for evaluation of small hepatic lesions that cannot be fully characterised by CT. 30
Endoluminal ultrasound is employed to visualise the whole pancreas, the related vasculature and the associated lymph nodes and allows for EUS-guided fine-needle aspiration (FNA) of pancreatic lesions and suspicious lymph nodes. EUS can be superior to MDCT at detecting and determining the T stage of pancreatic tumours, with sensitivities ranging from 72% to 90%. 31,32 FNA with EUS is usually indicated when there is diagnostic uncertainty whether the lesion is inflammatory or malignant. The sensitivity and specificity of EUS and FNA in detecting pancreatic cancer are 85% and 98%, respectively. 33 ERCP is used therapeutically to relieve obstructive jaundice and obtain cytological brushings. Percutaneous biopsy is reserved only for patients with unresectable disease. Essentially, pancreatic biopsy (EUS or percutaneous) should not be performed on patients with resectable disease because of the risks of seeding, the false-negative rate, the complication rate and poor accuracy in cystic tumours, chronic pancreatitis and autoimmune pancreatitis. 34 Selective laparoscopy and laparoscopic ultrasound based on CA19.9 levels are used in patients with radiological resectable disease to identify distant metastases and avoid unnecessary laparotomy. The addition of platelet/lymphocyte ratio to the CA19.9 measurement has been useful in determining which patients should undergo laparoscopy. 35,36
18Fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography in pancreatic cancer
Positron emission tomography (PET) is a functional imaging technology that enables visualisation, characterisation and quantification of biological processes in vivo. By using positron-emitting radiotracers, PET provides unique information about the molecular and metabolic changes associated with disease. Glucose metabolism is often increased in malignant tumours resulting in increased cellular uptake of the glucose analogue 18fluorine-2-fluoro-2-deoxy-d-glucose (FDG). Imaging the metabolic activity of tumours provides sensitive and specific information about the extent of disease. PET imaging is a whole-body technique and therefore may be helpful in looking for metastases. The extent to which PET may influence diagnosis and management in solid tumours has been assessed in a large cohort study by the National Oncologic PET Registry (NOPR), which assessed 22,975 cases from 1178 centres. 37,38 These patients underwent FDG-PET scans for a diagnosis of suspected cancer, cancer staging, restaging and suspected recurrence. Prostate, pancreatic and ovarian cancers represented 30% of cases. The post-PET plan was threefold more likely to lead to treatment than non-treatment [28.3% vs. 8.2%; odds ratio 3.4, 95% confidence interval (CI) 3.2 to 3.6]. Overall, intended management was changed in 36.5% (95% CI 35.6% to 37.2%) of cases. An analysis of a further cohort demonstrated a similar management change in 33–35% of cases. FDG-PET scanning has been assessed in pancreatic cancer. There have been a variety of studies evaluating the accuracy of FDG-PET in pancreatic carcinoma; however, its usefulness at detecting early lesions remains unclear. 39 One of the larger studies assessed the role of FDG-PET in 112 patients with suspected pancreatic cancer40 and demonstrated a sensitivity and specificity for FDG-PET of 73% and 60% and for CT of 89% and 65%. FDG-PET had a similar accuracy to CT but did not provide any additional information in patients with equivocal CT findings. Pancreatic cancer is associated with a marked desmoplastic response and stromal inflammatory cells in and around the neoplasm may be responsible for the uptake of FDG. In the study by Lytras et al. ,40 10 of the 12 patients with false-positive results had chronic pancreatitis. Orlando et al. 24 conducted one of the first meta-analyses to compare FDG-PET with CT in studies of patients with pancreatic cancer. Sensitivity and specificity for CT were 81% (95% CI 72% to 88%) and 66% (95% CI 53% to 77%), respectively. The addition of PET to positive CT resulted in a sensitivity and specificity of 92% (95% CI 87% to 95%) and 68% (95% CI 51% to 81%). A further meta-analysis has shown that the role of the addition of FDG-PET in the diagnostic work-up of these patients remains to be proven and it cannot be recommended as standard practice. 41
Positron emission tomography/computed tomography in pancreatic cancer
Combined PET/CT was developed to add precise anatomical localisation to functional data. 42 PET and CT are acquired concurrently and co-registered, merging functional information from PET with the anatomical information from CT. Several studies/meta-analyses have demonstrated that FDG-PET/CT is more accurate than FDG-PET43–45 in solid tumours, including pancreatic tumours. In pancreatic cancer, a study by Heinrich et al. 46 found that FDG-PET/CT had a sensitivity of 89% for the detection of pancreatic cancer, altered treatment planning in 16% of 59 patients and was cost saving. Another study demonstrated that the sensitivity and specificity of FDG-PET/CT was 88% and 89%, respectively, in patients being assessed for pancreatic cancer and changed the management of six (11%) patients. 47 These patients were found to have extrapancreatic disease that prevented them from undergoing pancreatic resection. Another study assessed two groups of patients, a diagnosis and staging group and a screening group, for progressive or recurrent disease. 39 The accuracy rate for FDG-PET/CT for diagnosis and staging was 91.2% and 85.3%, respectively. In the restaging group FDG-PET/CT had a sensitivity of 90%. Management changes resulting from PET/CT have been demonstrated in a number of studies. 48–50 The additional feature of PET/CT is semiquantitative analysis of glucose uptake (FDG activity) in suspicious pancreatic lesions. Determination of FDG activity is obtained by calculating the standardised uptake value (SUV) in a given region of interest. An SUV of > 3.5 may indicate pancreatic malignancy; one study revealed a maximum SUV (SUVmax. ) in malignant lesions of 6.5 ± 4.6 and a SUVmax. in benign lesions of 4.2 ± 1.5. 51 Another study demonstrated an average SUVmax. for malignant lesions of 6.72 ± 3.84 and an average SUVmax. for benign disease of 2.56 ± 1.22 (p < 0.01). A definitive cut-off value is difficult to define for pancreatic malignancies and therefore qualitative data should also be included in clinical studies such as FDG tracer uptake patterns. 51,52 The use of contrast-enhanced PET/CT may represent a complete diagnostic staging procedure without the need for separate MDCT. One study found that contrast-enhanced FDG-PET/CT was superior to FDG-PET (p = 0.035) and there was a trend (p = 0.07) for contrast-enhanced FDG PET/CT to be superior to unenhanced PET/CT53 in assessing resectability. The sensitivity and specificity of contrast-enhanced PET/CT to detect malignancy ranges from 100% to 96% and from 94% to 90%, respectively, in several studies. 52,54 The use of radiopharmaceuticals such as 18fluorine-fluorothymidine (FLT) has been investigated in small numbers of patients. FLT-PET assesses the proportion of cells undergoing active proliferation and this process occurs before a change in glucose metabolism. This may be useful in monitoring response to therapy. The role of FLT-PET/CT in the diagnostic pathway of pancreatic cancer is not clear. 55 Fused PET and MRI has also been assessed in small numbers of patients with an accuracy of 96.6%. 56
Rationale for the study
The diagnosis of pancreatic cancer has improved with the use of MDCT, EUS and ERCP and the additional use of MRI. There are, however, up to 10–20% of patients in whom an accurate diagnosis is difficult. This proportion is increasing, in part because of the larger numbers of asymptomatic patients undergoing cross-sectional imaging. 57,58 Invasive methods of diagnosis such as EUS with or without FNA can add to the accuracy of MDCT but may require an inpatient stay and have a recognised complication rate (1–2%). 59 Currently, patients with chronic pancreatitis, autoimmune pancreatitis, cystic lesions, small tumours of < 2 cm, a bulky or diffusely enlarged pancreas on CT, a dilated pancreatic duct and no mass on CT, small-volume metastatic disease and suspected recurrent disease (with no mass on CT) following resection are the most challenging patients to diagnose. A major goal of accurate diagnosis and staging is to avoid major pancreatic resection in patients who will not benefit; about 10–15% of patients who have a pancreatic resection have benign disease on final histology28 and up to 20% of patients will develop recurrent disease 3–6 months post resection. 60 Functional imaging techniques such as PET/CT may add to staging of pancreatic cancer by diagnosing small-volume metastatic disease and differentiating between benign and malignant lesions and it is vital, therefore, that a well-designed prospective study answers this question. A number of studies have addressed the diagnostic accuracy of PET/CT and changes in management as a result of PET/CT. The main drawbacks of previous PET/CT studies tend to be that they have been single-centre studies with small numbers of patients and variability in the PET/CT imaging protocol used to assess suspected pancreatic cancer. This prospective multicentre study aims to address these issues in a large group of patients to identify whether there is a role for PET/CT in addition to standard diagnostic work-up in pancreatic cancer.
Chapter 2 Methods
Design and setting
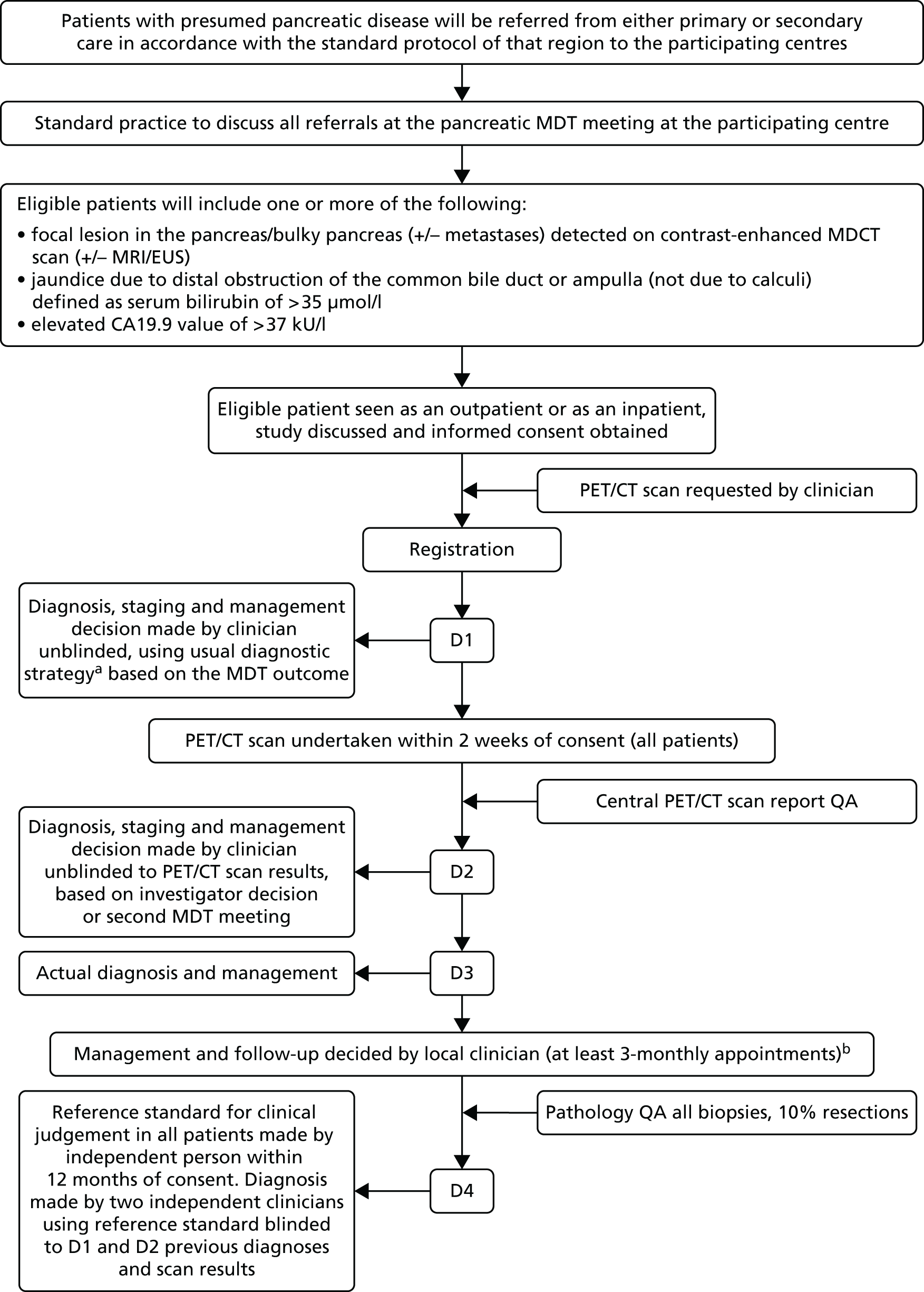
This study was a multicentre prospective diagnostic accuracy and clinical value study of PET/CT in suspected pancreatic malignancy (Figure 1). The study was carried out in 18 major pancreatic centres with annual referrals of > 120 pancreatic patients per year. To achieve the study objectives the case mix included pancreatic cancer, chronic pancreatitis and other benign and malignant neoplasms of the pancreas. For example, of 400 patients referred to the Liverpool pancreatic multidisciplinary team (MDT) each year, approximately 47% have pancreatic cancer and 53% have other tumours and chronic pancreatitis. The case mix for each centre incorporated a mix of benign and malignant cases and it was important that eligible patients were drawn from both groups to satisfy the aims of the study. The study was approved by the North West 1 Research Ethics Committee – Cheshire [following reorganisation this committee was superseded by National Research Ethics Service (now part of the Health Research Authority) Committee North West – Greater Manchester East (Cheshire)]. Following informed consent patients were registered and enrolled onto the study.
FIGURE 1.
Schematic of the study design. a, Usual diagnostic strategy defined as contrast-enhanced MDCT ± EUS ± MRI; b, follow-up clinical evaluation defined using information on improvement/deterioration in clinical symptoms ± CA19.9 ± CT ± EUS ± survival (does not include original PET/CT scan). D1, diagnosis, staging and management plan after MDCT; D2, diagnosis, staging and management plan after PET/CT; D3, actual diagnosis, staging and management; D4, diagnosis reference standard; QA, quality assurance.
Participants
Eligible patients for this study included those with suspected pancreatic malignancy as defined in the inclusion criteria.
Inclusion criteria
-
Patients with suspected pancreatic malignancy as defined by one or more of:
-
focal lesion in the pancreas/bulky pancreas/dilated pancreatic duct (± metastases) detected on MDCT scan [± MRI/EUS/ultrasound]
-
jaundice because of distal obstruction of the common bile duct or ampulla (not because of calculi) defined as serum bilirubin > 35 µmol/l
-
serum CA19.9 > 37 kU/l.
-
-
Able to attend for PET/CT scan.
-
Able to undergo MDCT scan.
-
Able to attend for 12 months of follow-up.
-
Fully informed written consent given.
Exclusion criteria
-
Patients aged < 18 years.
-
Pregnancy.
-
Patients with poorly controlled diabetes.
Interventions
18Fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography
Patients underwent FDG PET/CT scanning within a maximum of 2 weeks following informed consent. PET/CT was performed under carefully controlled conditions to ensure maximum accuracy of results. Patients fasted for 6 hours prior to the scan. To ensure accurate SUV measurements patients’ weight was obtained without shoes and coat and using a calibrated class III device that satisfied requirements defined in the Non-Automatic Weighing Instruments Directive 2003 [see http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32009L0023 (accessed 27 June 2016)] and blood glucose was recorded using a calibrated Boehringer Mannheim glucometer (Boehringer Ingelheim Ltd, Bracknell, UK). For diabetes mellitus patients only patients with a fasting blood glucose not exceeding 10.0 mmol/l were scanned to reduce false-negative FDG PET/CT results. Patients drank between two and three glasses of water before the PET/CT scan to ensure good hydration, which contributes to a good-quality scan. Metal denture and other metallic devices were removed whenever possible to reduce CT artefacts, which distort FDG uptake measurements including SUV measurements in the vicinity of the artefact. Tracer was injected via a butterfly cannula under quiet conditions. For two-dimensional scanning 350–530 MBq of FDG was injected. In patients requiring a larger dose because of a larger body weight Administration of Radioactive Substances Advisory Committee (ARSAC) certificate holder approval was obtained before giving the larger dose. For three-dimensional scanning 150–350 MBq was injected. Patients remained quiet and inactive during the uptake period in a warm room to avoid artefacts including skeletal muscle FDG uptake and brown adipose tissue uptake. Patients emptied their bladders just prior to positioning on the scanner bed to avoid artefacts from FDG activity in the urinary bladder. The PET/CT emission scan started at 90 minutes after FDG injection. Scanning was carried out on a standard PET/CT table top, beginning at the groin and ending at the base of the orbits and with arms up if a single whole-body scan was performed. Routine local acquisition parameters were used. Data were reconstructed using ordered subsets expectation maximisation reconstruction parameters with CT for attenuation correction. The PET/CT scan was first reported at the local participating centre.
Central positron emission tomography/computed tomography reporting
A central PET/CT reporting facility was established at the Paul Strickland Scanner Centre at Mount Vernon Hospital, Northwood, Middlesex. Data transfer was via secure FTP server from the National Cancer Research Institute (NCRI) PET Core Laboratory. All PET/CT scan reports were reviewed by an expert in clinical PET/CT who was independent of the local centre. The majority of PET/CT scans were reviewed at the Paul Strickland Scanner Centre using the agreed standard proforma. Central review of PET/CT scans was also performed at Aintree University Hospital (Liverpool), St James’s University Hospital (Leeds), the Royal Free Hospital (London) and Southampton General Hospital using the agreed standard proforma.
Objectives
Primary objective
-
To determine the incremental diagnostic accuracy and impact of PET/CT in addition to standard diagnostic work-up in patients with suspected pancreatic cancer.
Secondary objectives
-
To evaluate changes in diagnosis, staging and associated intended patient management as a result of the addition of PET/CT.
-
To determine the cost-effectiveness of the addition of PET/CT in the diagnosis, staging and management of pancreatic cancer.
-
To evaluate the impact of the addition of PET/CT in differentiating pancreatic malignancy from chronic pancreatitis.
-
To identify which groups of patients would most benefit from PET/CT.
-
To report the incremental diagnostic value of PET/CT for particular types of pancreatic tumour.
Outcome measures
The primary outcome measure was the incremental diagnostic value (sensitivity and specificity) of PET/CT in addition to standard diagnostic work-up with CT. The secondary outcome measures included (1) changes in patient management as a result of the addition of PET/CT; (2) changes in the costs of patient management as a result of the addition of PET/CT and effectiveness measured in terms of survival and/or health-related quality of life (HRQoL); (3) the incremental diagnostic value (sensitivity and specificity) of PET/CT findings in chronic pancreatitis; (4) the identification of groups of patients who would benefit the most from PET/CT based on clinical outcome; and (5) the incremental diagnostic value (sensitivity and specificity) of PET/CT findings in other pancreatic tumours.
Diagnostic pathway following multidetector computed tomography and positron emission tomography/computed tomography
Diagnosis, staging and management following MDCT (D1) and PET/CT (D2) were categorised by the investigator according to one or more of the following options.
-
Diagnosis:
-
PDAC
-
periampullary cancer
-
cholangiocarcinoma
-
benign cystic neoplasm
-
malignant cystic neoplasm
-
pancreatic pseudocyst
-
chronic pancreatitis
-
autoimmune pancreatitis
-
acute pancreatitis
-
neuroendocrine tumour
-
lymphoma
-
metastasis from non-pancreatic primary neoplasm
-
recurrent pancreatic cancer post resection
-
normal pancreas
-
other
-
-
Staging:
-
resectable [Union for International Cancer Control (UICC) tumour, node, metastasis (TNM) classification stage 0, IA, IB, IIA, IIB; see Appendix 1]61
-
borderline resectable, for example defined as up to 2 cm of portal/superior mesenteric vein involvement for 180o circumference
-
unresectable (UICC TNM stage III and IV)
-
other
-
-
Management:
-
resection (± previous laparoscopy)
-
biopsy (EUS/percutaneous)
-
drainage procedure, for example stent or surgical bypass
-
chemotherapy/trial
-
best supportive care
-
clinical follow-up ± further investigation
-
no further management required
-
other.
-
For all of the diagnosis, staging and management options listed in D1 and D2 a level of certainty was categorised as:
-
very certain
-
moderately certain
-
uncertain.
Planned treatment and follow-up
The patient then underwent planned management (D3). All patients were followed up for at least 12 months or until death if before 12 months. The patient completed a short quality of life questionnaire [European Quality of Life-5 Dimensions (EQ-5D)] [see www.euroqol.org/about-eq-5d.html (accessed 27 June 2016) and Appendix 2] after consent and at each 3-monthly outpatient review. The EQ-5D is a standardised instrument for use as a measure of health outcome and provides a simple descriptive profile and a single index value for health status that can be used in the clinical and economic evaluation of health care.
Target conditions
The analyses of test accuracy and staging considered the following target conditions:
-
pancreatic cancer (for the primary objective)
-
stage of pancreatic cancer (UICC TNM classification for resectable, borderline resectable and unresectable disease)
-
chronic pancreatitis
-
particular types of pancreatic tumour.
Reference standard(s)
The reference standard for diagnosis was a clinical judgement made by an independent expert based on histology (either biopsy or resection) or clinical outcome at the 12-month assessment. The expert agreed on the appropriate staging for each patient with pancreatic cancer and the appropriate management, to be used as reference diagnoses (D4). The process is described below:
-
Stage 1. The expert initially received a patient histology report for the target conditions (1–4) and information about the clinical status of the patient at 12 months (but excluding all information from investigations made at baseline and the PET/CT test results). This was according to the minimum data set of the Royal College of Pathologists,62 in a standard format for resection histology; this included pathological staging (pTNM) for tumours.
-
Stage 2. If the expert was unable to make a firm reference diagnosis based on the above information, results of baseline investigations were released but not the PET/CT investigation. The two-stage process was planned so that the expert’s initial decision was not contaminated by the standard work-up of either set of test results and never by the PET/CT scan results and to avoid incorporation bias. Finally, the expert was asked to judge the appropriateness of management for each patient and if a change prompted by PET/CT was appropriate.
Quality assurance
Radiology quality assurance
Central radiology review of 10% of the MDCT scans was carried out by Dr Jonathan Evans, Consultant Radiologist (Royal Liverpool and Broadgreen University Hospitals NHS Trust), for quality assurance. CT scans selected from each centre for central reporting were requested by the Liverpool Clinical Trials Unit (LCTU). Anonymised CT images in DICOM (Digital Imaging and Communications in Medicine) format were provided on disk and sent to the Department of Radiology, Royal Liverpool and Broadgreen University Hospitals NHS Trust. On receipt disks were loaded into an RA600 import workstation (GE Healthcare, Chalfont St Giles, UK). The scan was opened on the RA600 and the images exported from the RA600 to the Picture Archiving and Communication System (PACS; Carestream Health Inc., Hemel Hempstead, UK). Once all of the images had arrived on the PACS, they were post processed and the radiologist was able to report the images. MDCT scans were reported using the agreed standard proforma.
Pathology quality assurance
Central pathology review was carried out by Professor Fiona Campbell, Consultant Gastrointestinal Pathologist (Royal Liverpool and Broadgreen University Hospitals NHS Trust). The histology slides from all of the biopsies and approximately 10% of all resection specimens (with at least two specimens selected from each centre) were requested after completion of actual diagnosis and management (D3). Slides were sent directly to the Department of Pathology, Royal Liverpool and Broadgreen University Hospitals NHS Trust, and reported using the agreed standard proforma. Slides were returned to each centre when reviewed.
Positron emission tomography/computed tomography quality assurance
A PET/CT Core Laboratory facility was set up as part of the NCRI PET Research Network Clinical Trials Network at the PET imaging centre at St Thomas’s Hospital, London. PET/CT data were transferred in anonymised DICOM part 10 format. The submitted images were required to include the attenuation-corrected PET, the non-attenuation-corrected PET and the CT images. The recommended method for electronic data transfer from the NHS PET centres was via the NHS Secure File Transfer Service. All other sites with appropriate internet access used the NCRI Core Laboratory secure FTP server. The laboratory ensured that images acquired from participating centres were of comparable quality.
Sample size estimation and re-estimation
A previous meta-analysis24 reported a sensitivity of 81% and specificity of 66% for the diagnosis of pancreatic cancer with standard MDCT. The primary objective of this study was to investigate the incremental value of PET/CT. To be of clinical value to the diagnostic work-up the addition of PET/CT should increase the sensitivity from 81% to 90% and the specificity from 66% to 80%.
An appropriate sample size, accounting for the paired design, was then obtained using methodology from Alonzo et al. 63 For sensitivity it was based on:
where TPRB is the true positive rate (sensitivity) without PET/CT, TPPR is the proportion of diseased patients who test positive before and after PET/CT, α and β are the significance level and power of the study, respectively, π is the prevalence of disease and γ1 = TPRA/TPRB is the ratio of true positive rates with and without PET/CT.
For specificity we were interested in the true negative rates so the formula used was:
where TNRB is the true negative rate (specificity) without PET/CT, TNNR is the proportion of non-diseased patients who test negative before and after PET/CT, α and β are the significance level and power of the study, respectively, π is the prevalence of disease and γ2 = TNRA/TNRB is the ratio of true negative rates with and without PET/CT. The chosen sample size would be the larger of n1 and n2.
A complication with these formulae is that knowledge of the correlation between tests for a patient, driven by TPPR and TNNR, is generally required. However, it can be noted that TPPR ≥ (1 + γ1)TPRB – 1 and TNNR ≥ (1 + γ2)TNRB – 1 and that, moreover, the required sample size is largest when TPPR = (1 + γ1)TPRB – 1 and TNNR = (1 + γ2)TNRB – 1. These can then be used to find an upper bound on the required sample size.
Applying the formulae to ensure 80% power (β = 0.2) for a two-sided test at the 5% significance level (α = 0.05) with relative sensitivity γ1 = 10/9 and relative specificity γ2 = 40/33 yielded an upper bound of n = 600 as the initial sample size. However, as it was acknowledged that this would likely be an overestimate of the total number of patients required, an interim analysis after 200 patients was conducted to estimate the correlation between tests and estimate the disease prevalence so that the sample size could be re-estimated.
Interim analysis
The interim analysis used data from the 187 patients of the first 200 registered who had complete data (for D1–D3 diagnoses) by the cut-off date of 28 May 2012. Of these, 82 were deemed to have pancreatic cancer according to D3, giving a prevalence of 43.9% (which was consistent with the original assumption of 47%; p = 0.39).
Interim results (Tables 1 and 2) were used to estimate the correlation between tests and hence the estimated required total sample size, based on the formulae in the previous section from Alonzo et al. 63 From Table 1 (diseased patients), TPPR = 66/82 = 80.5% and TPRB = 69/82 = 84.1%. Thus, both quantities are larger than originally assumed. Applying these updated estimates into the sample size formula for n1 gives 341.6.
| Diseased patients | Pre PET/CT | ||
|---|---|---|---|
| Positive | Negative | ||
| Post PET/CT | Positive | 66 | 3 |
| Negative | 3 | 10 | |
| Non-diseased patients | Pre PET/CT | ||
|---|---|---|---|
| Positive | Negative | ||
| Post PET/CT | Positive | 21 | 4 |
| Negative | 11 | 69 | |
From Table 2 (non-diseased patients), TNNR = 69/105 = 65.7% and TNRB = 73/105 = 69.5%. Applying these updated estimates into the sample size formula for n2 gives 144.2.
Hence, taking the maximum of the two figures and rounding up, the estimated sample size is 342. However, it was noted that this estimated sample size was primarily driven by the numbers of discordant test results, both of which were small, and as a consequence there was considerable uncertainty in the estimate. The bootstrap 80% CI of the estimated sample size was 231 to 464. To ensure that there was a good chance that the study was adequately powered for the primary analysis, the upper value was chosen, with a specific target of 500 agreed on to account for possible dropouts (estimated at 5.5% after the first 200 registered patients).
Statistical methods
Primary analysis
The primary outcome measure was relative sensitivity and relative specificity of the PET/CT scan (in range as per protocol) compared with the baseline MDCT scan with respect to a diagnosis of PDAC. These were obtained by comparing each diagnosis with the reference diagnosis made at D4 for the target conditions (pancreatic malignancy, chronic pancreatitis, other tumour types) with standard errors accounting for pairing obtained using the formula from Alonzo et al. 63
The analyses were repeated both using patients who were outside the range for uptake time and/or blood glucose and excluding such patients to assess the impact on estimates.
The homogeneity of patients across sites was assessed by comparing disease prevalences using a funnel plot. 64 This involved plotting prevalence against sample size and observing whether the points lay outside of 95% or 99% control limits.
Estimation of incremental diagnostic benefit
The incremental accuracy of PET/CT over standard work-up was investigated using regression modelling following the Knottnerus approach summarised by Chan et al. 65 This approach allows the modelling of a sequence of tests through creative construction of indicator variables, which takes into account the non-independence of test results, but does not alter the value of previous test results when subsequent tests are added to the model. It also allows expression of the incremental diagnostic value as likelihood ratios.
Impact of positron emission tomography/computed tomography on certainty of diagnosis
Assessment of clinicians’ perceived certainty of diagnosis before and after the PET/CT scan was evaluated using both the clinicians’ qualitative assessment at D2 and quantitative (0–1 scale) assessments at D1 and D2. For the qualitative assessment a binomial test of whether the number of cases in which the uncertainty was perceived is significantly greater than the number of cases in which the uncertainty was perceived to increase was conducted. For the quantitative assessment a Wilcoxon signed-rank test was used to assess whether there was a significant change in perceived uncertainty from D1 to D2.
To assess whether clinicians’ perceptions were in line with the true benefit of PET/CT, the quantitative assessment (on a 0–1 scale) was treated as if it represented the clinicians’ estimated probability that their diagnosis (with respect to PDAC) was truly correct. The accuracy of these predictions was then assessed using a Brier score, defined as:
where pi is the clinicians’ certainty regarding the diagnosis for patient i, oi is an indicator variable for whether the diagnosis was eventually correct (taking value 1 if the diagnosis matches the D4 diagnosis and 0 otherwise) and N is the total number of patients. A smaller Brier score implies a better forecast.
The Brier score penalises both general poor predictive ability and also poor calibration. The worst possible Brier score for an exactly calibrated forecast is 0.25, occurring if an event has a (correctly determined) 50% chance of occurring. However, it is possible to obtain worse scores for poorly calibrated forecasts, for example estimating something with a 50% chance of occurring has a 100% chance gives an expected Brier score of 0.5.
Bootstrap resampling was used to assess whether or not the change in Brier score between D1 and D2 was statistically significant.
Note that the accuracy of this method depends on the assumption that the clinicians interpreted the certainty score as a probability with respect to the binary diagnosis of PDAC or not PDAC. If instead they interpreted it as the probability that the precise diagnosis (including either type of PDAC or precise other condition) was correct, or certainty measured on some scale other than probability, the Brier score results could be misleading.
Secondary analyses
Impact of positron emission tomography/computed tomography on diagnosis, staging and management
The impact of PET/CT on patient management was assessed by considering data from the D2 form on whether the PET/CT scan was perceived to have influenced patient management and also data from the D3 form on which management (D1, D2 or neither) the patient eventually followed. In the latter case a formal test was performed to assess whether a greater proportion of patients followed D2 than D1.
Counts of the most clinically important management changes occurring between D1 and D2 were collated (change from resection to no resection, change from no resection to resection, change from some form of chemotherapy to no chemotherapy, change from no chemotherapy to some form of chemotherapy, change from no further investigation to some form of further investigation). In addition, the number of cases in which PET/CT was perceived to have either directly identified a secondary malignancy or else suggested the need for further investigation in the case of a secondary malignancy was collated.
Benefit of positron emission tomography/computed tomography in chronic pancreatitis
The same general methodology was applied to assess the diagnostic accuracy of PET/CT in diagnosing chronic pancreatitis.
Benefit of positron emission tomography/computed tomography in subgroups
The benefit of PET/CT in relation to different patient groups [male vs. female, aged < 65 years vs. ≥ 65 years, World Health Organization (WHO) performance status > 0 vs. WHO performance status = 0, presence or absence of eligibility criteria] was assessed by fitting appropriate generalised estimating equation (GEE) models to test for differences in sensitivities and specificities and also relative sensitivities and specificities across different patient groups.
Benefit of positron emission tomography/computed tomography in other pancreatic tumours/disease
The same general methodology was applied to assess the diagnostic accuracy of PET/CT for malignant cystic neoplasm, cholangiocarcinoma, periampullary carcinoma and neuroendocrine tumour and also the diagnostic accuracy of PET/CT for malignant compared with benign pancreatic disease.
Additional analyses
Analysis of the standardised uptake value
Among PET/CT scans for which an apparent tumour was identified the distribution of SUVmax. in patients with and without pancreatic cancer was performed. A Mann–Whitney U-test was performed to compare the distributions in the two groups. In addition, the value of SUVmax. as a direct diagnostic tool for pancreatic cancer was assessed by estimating the receiver operating characteristic curve.
Survival analysis
Overall survival and survival for patients with or without pancreatic cancer and also with or without malignant disease was calculated using Kaplan–Meier estimates. To avoid issues of left truncation, survival times were taken from the date of the PET/CT scan as registered patients were required to have had a PET/CT scan to appear in the final set of patients. Withdrawal from the study before the end of 12 months’ follow-up was assumed to be non-informative censoring. Cox proportional hazards modelling was used to estimate hazard ratios (HRs) between these diagnostic groups, to evaluate the impact of other baseline demographic characteristics on patient survival and to assess the relationship between SUVmax. and survival among patients with PDAC.
Summary of changes to the protocol
Protocol version 1 (12 January 2010) to protocol version 2 (1 March 2011)
-
Alteration of inclusion criterion from ‘able to attend for 12 months’ follow-up’ to ‘able to attend for up to 12 months’ follow-up’ to reflect the potentially unpredictable and poor prognosis of patients with pancreatic malignancy.
-
Addition of the exclusion criterion ‘patients with poorly controlled diabetes’.
-
Inclusion of a random blood glucose test at baseline to the schedule of assessments.
-
Clarification that the post-PET/CT diagnosis (D2) could be conducted either at the MDT meeting or by the investigator because of capacity constraints at MDT meetings.
-
Confirmation that the NCRI PET Research Network PET Core Laboratory at St Thomas’s Hospital, London, would provide the core laboratory function and that central clinical reporting would be conducted at the Paul Strickland Scanner Centre.
-
Clarification that only serious adverse events as defined in the protocol should be reported to the co-ordinating centre within 24 hours of a site becoming aware of an event.
Protocol version 2 (1 March 2010) to protocol version 3 (1 September 2011)
-
Frequency of the Independent Safety and Data Monitoring Committee (ISDMC) meeting defined to be every 6 months to reflect the recruitment period.
-
Alteration to the PET/CT protocol:
-
suitable fasting blood glucose level of patients able to undergo a PET/CT scan increased from 7 mmol/l to 10 mmol/l to more adequately accommodate patients with diabetes mellitus, following a recommendation of the Trial Steering Committee
-
specific instructions included for research sites for preparation of patients with type I and type II diabetes mellitus, following a recommendation of the Trial Steering Committee
-
redefinition of injected activity to take account of variation in scanning equipment in scanning centres and redefinition of the position of the patient for scanning to prevent unnecessary exposure to radiation-sensitive tissues: ‘begin scanning at the groin and end at the vertex’ altered to ‘begin scanning at the groin and end at the base of the orbits’.
-
Protocol version 3 (1 September 2011) to protocol version 4 (10 September 2012)
-
Sample size redefined following the interim analysis.
-
Clarification on the requirement of the PET/CT scan to be performed within 2 weeks of the baseline assessment. The time limit was recommended to ensure that patients did not suffer unnecessary delays in diagnosis and management decisions because of participation in the study.
-
Clarification on the scheduling and format of follow-up visits.
Public and patient involvement
In this study patient involvement included (1) contribution to the study design, (2) consideration of the acceptability of extra-diagnostic tests for the patient pathway, (3) advice on the original grant application and study protocol and (4) membership of the Trial Steering Committee.
Chapter 3 Results
Recruitment
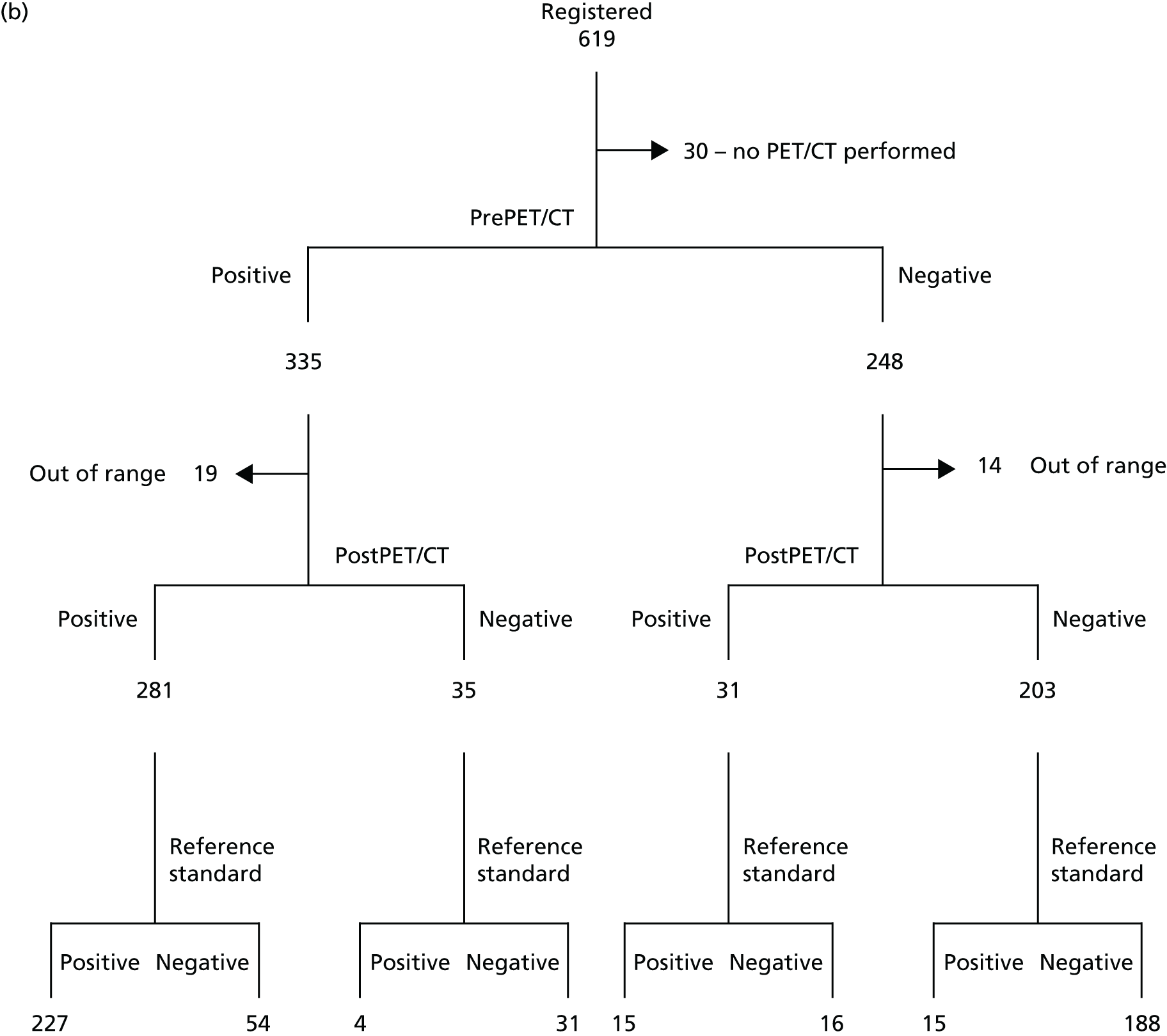
The first patient was recruited in January 2011 and recruitment was completed in April 2013. Follow-up was carried out until April 2014. Figure 2 describes the participant flow in the study. In total, 619 patients were registered and 589 patients underwent PET/CT scanning. Of these, 583 were included in the intention-to-treat (ITT) analysis and 550 were included in the in-range/per-protocol analysis; 33 patients either had a blood glucose level of > 10 mmol/l at the time of their PET/CT scan or underwent a PET/CT scan that was outside the 90-minute uptake time by ± 10 minutes. Diagnostic information was incomplete for six patients. A total of 316 patients completed the 12-month follow-up.
FIGURE 2.
(a) Participant flow in the study; and (b) Standards for the Reporting of Diagnostic Accuracy Studies (STARD) flow diagram. Positive, pancreatic cancer; negative, not pancreatic cancer.

Patients were registered from 18 sites in the UK. Table 3 shows the number of patients registered, number with a D4 diagnosis and number with a D4 diagnosis and within-range uptake time and blood glucose for each of the sites that had at least one registered patient. The level of attrition or screening failure between registration and D4 was broadly similar across all sites.
| Site | Patients registered | Patients with a D4 diagnosis | Patients with a D4 diagnosis and within-range PET/CT and blood glucose | Prevalence of PDAC (among per-protocol patients) (%) |
|---|---|---|---|---|
| Royal Liverpool Hospital | 226 | 224 | 212 | 44.3 |
| Royal Free Hospital, London | 11 | 11 | 9 | 22.2 |
| University College Hospital, London | 20 | 20 | 18 | 44.4 |
| St Bartholomew’s Hospital, London | 25 | 18 | 17 | 29.4 |
| Glasgow Royal Infirmary | 34 | 30 | 27 | 51.8 |
| Nottingham City Hospital | 3 | 3 | 3 | 0.0 |
| University Hospital Birmingham | 31 | 31 | 31 | 61.3 |
| Freeman Hospital, Newcastle upon Tyne | 4 | 3 | 3 | 100.0 |
| Royal Marsden Hospital, London | 21 | 21 | 18 | 77.8 |
| St James’s University Hospital, Leeds | 44 | 44 | 41 | 51.2 |
| Southampton General Hospital | 109 | 93 | 92 | 44.6 |
| Aberdeen Royal Infirmary | 1 | 1 | 0 | – |
| King’s College Hospital, London | 7 | 5 | 4 | 75.0 |
| Royal Blackburn Hospital | 10 | 9 | 9 | 88.9 |
| University Hospitals Coventry and Warwickshire NHS Trust | 10 | 9 | 7 | 71.4 |
| Portsmouth Hospitals NHS Trust | 51 | 49 | 47 | 44.7 |
| Ninewells Hospital, Dundee | 7 | 7 | 7 | 28.6 |
| Abertawe Bro Morgannwg University Health Board, Swansea | 5 | 5 | 5 | 20.0 |
Table 3 also shows the prevalence of PDAC, as judged via the D4 diagnosis, among those patients with a D4 diagnosis and a PET/CT scan within range. There was considerable variation in prevalence between sites, which may relate to the different numbers of patients recruited from sites. To investigate the variation in prevalence between sites a funnel plot of prevalence against number of patients recruited was constructed (Figure 3).
FIGURE 3.
Funnel plot of prevalence by site with 95% and 99% control limits.
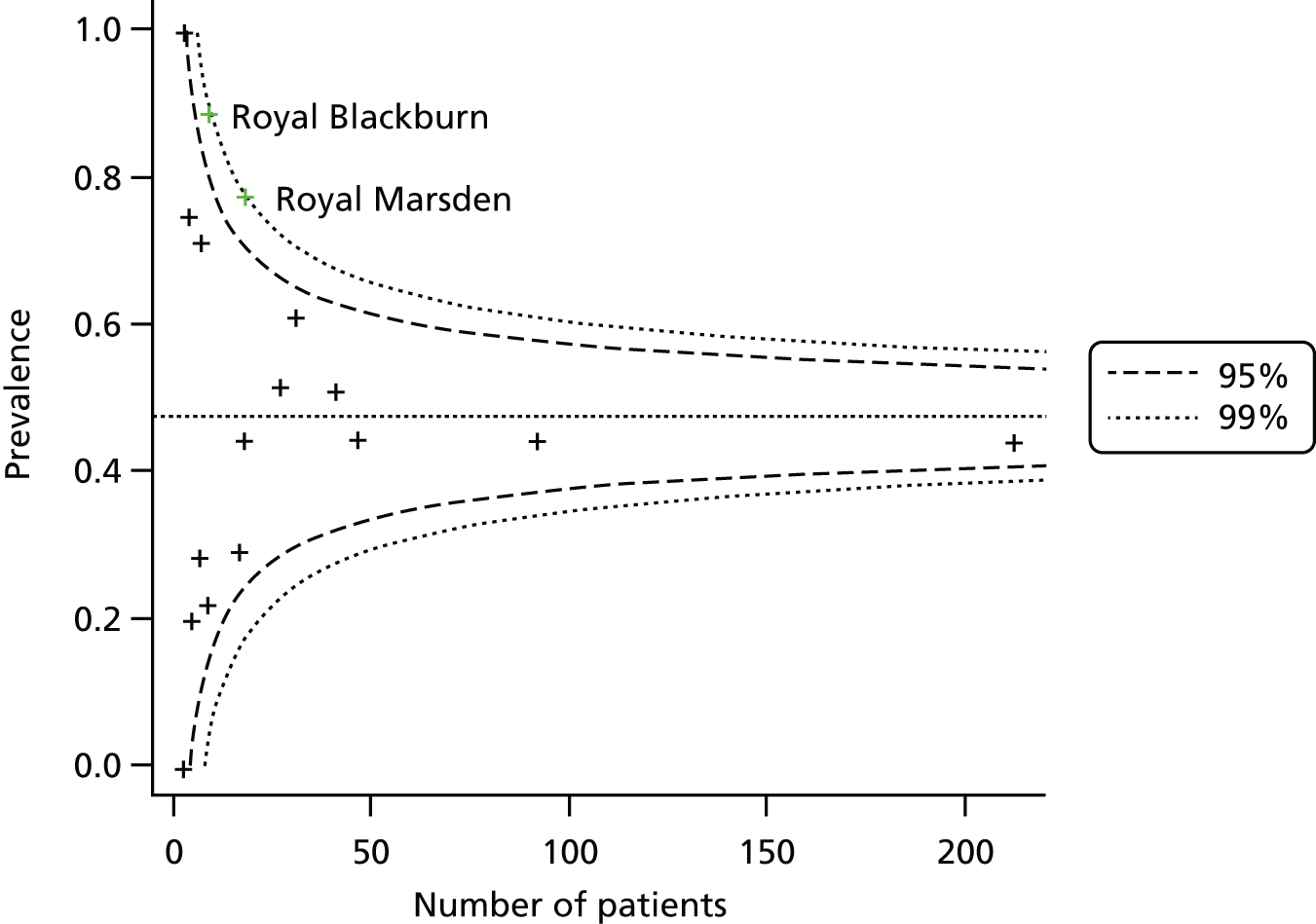
All but two of the centres lie within the 95% confidence limits of the funnel. The Royal Marsden Hospital and Royal Blackburn Hospital had prevalences that were higher than expected, although still within the 99% confidence limits. Overall, there was some evidence against an assumption of homogeneity across sites (p = 0.005).
Baseline characteristics
The baseline characteristics of all registered patients, patients with a D4 diagnosis and patients with a D4 diagnosis whose PET/CT uptake time was within range and whose blood glucose was < 10 mmol/l are detailed in Table 4. In general, there was little difference between the patient demographics of all patients and the patient demographics of those with a D4 diagnosis, suggesting that it was reasonable to assume that the patients for whom complete data were available are representative of the overall patient population. Some baseline characteristics (smoking status, height, weight, etc.) were determined at the baseline assessment rather than at registration and these were therefore available for < 619 patients.
| Characteristic | All registered patients (n = 619), n (%) | Patients with D4 (ITT) (n = 583), n (%) | Patients with D4 and in range (per protocol) (n = 550), n (%) |
|---|---|---|---|
| Age (years) | |||
| Median (IQR) | 66 (15) | 66 (15) | 66 (15) |
| Range | (21–87) | (21–87) | (21–87) |
| Sex | |||
| Male | 353 (57) | 328 (56) | 304 (54) |
| Female | 266 (43) | 255 (44) | 246 (46) |
| Height (cm), mean (SD)a | 167.3 (10.9) | 167.3 (10.9) | 167.1 (11.0) |
| Weight (kg), mean (SD)a | 74.9 (17.3) | 75.0 (17.3) | 75.2 (17.3) |
| WHO performance status at baseline | |||
| 0 | 294 (48) | 283 (49) | 264 (48) |
| 1 | 276 (46) | 253 (43) | 244 (44) |
| 2 | 40 (6) | 38 (7) | 35 (6) |
| 3 | 9 (1) | 9 (2) | 7 (1) |
| 4 | 0 (0) | 0 (0) | 0 (0) |
| Diabetes | |||
| None | 484 (78) | 476 (82) | 456 (83) |
| Type 1 | 12 (2) | 11 (2) | 10 (2) |
| Type 2 | 96 (16) | 90 (15) | 79 (14) |
| Missing | 27 (4) | 6 (1) | 5 (1) |
| Eligibility criteriab | |||
| Criterion 1 | 570 (92) | 538 (92) | 476 (87) |
| Criterion 2 | 172 (28) | 159 (27) | 148 (27) |
| Criterion 3 | 130 (21) | 127 (22) | 117 (21) |
| Previous resection | |||
| Yes (of pancreas) | 11 (2) | 11 (2) | 8 (1) |
| Yes (other) | 33 (5) | 33 (6) | 30 (5) |
| No | 575 (93) | 539 (92) | 512 (93) |
| Smoking statusc | |||
| Never | 221 (37) | 216 (37) | 205 (37) |
| Past | 223 (37) | 227 (39) | 216 (39) |
| Current | 132 (22) | 128 (22) | 118 (21) |
| Missing | 24 (4) | 12 (2) | 11 (2) |
| Concurrent medical conditionsc | |||
| Yes | 515 (86) | 501 (86) | 475 (86) |
| No | 81 (14) | 80 (14) | 73 (13) |
| Missing | 4 (1) | 2 (0) | 2 (0) |
| Concomitant medicationc | |||
| Yes | 546 (91) | 531 (91) | 500 (91) |
| No | 51 (9) | 51 (9) | 49 (9) |
| Missing | 3 (0) | 1 (0) | 1 (0) |
The majority of participants had a WHO performance status of at least 0 or 1. The main eligibility criterion for entry into the study was an abnormality of the pancreas found on CT scan. The incidence of diabetes was around 17% in this population.
Reference standard
The reference standard (D4) for diagnosis was a clinical judgement made by an independent expert based on histology (either biopsy or resection) or clinical outcome at the 12-month assessment. For the 583 patients who had PET/CT (ITT population), the reference standard was based on histology (resection) in 242 patients and biopsy in 249 patients, with 92 patients having clinical follow-up data. For the 550 patients who underwent PET/CT within range (per-protocol population), the reference standard was based on histology (resection) in 233 patients and biopsy in 230 patients, with 87 patients having clinical follow-up data.
Reference standard diagnosis (D4) of patients
The frequency of each confirmed diagnosis type (the D4 reference standard) is provided in Table 5. If patients had more than one diagnosis, the primary diagnosis was used. The largest single group of patients was those with pancreatic cancer. Other types of tumour included cholangiocarcinoma, periampullary carcinoma and neuroendocrine tumour. The other main groups consisted of those patients with benign cystic neoplasms and chronic pancreatitis. The overall disease frequencies represent the typical case mix found in a UK pancreatic MDT and reflect UK practice.
| Reference standard diagnosis (D4) | Number (%) of patients (ITT) (n = 583) | Number (%) of within-range patients (per protocol) (n = 550) |
|---|---|---|
| PDAC | 278 (48) | 261 (47) |
| Periampullary carcinoma | 39 (7) | 37 (7) |
| Cholangiocarcinoma | 43 (7) | 42 (8) |
| Benign cystic neoplasm | 64 (11) | 63 (11) |
| Malignant cystic neoplasm | 7 (1) | 7 (1) |
| Pancreatic pseudocyst | 4 (1) | 4 (1) |
| Chronic pancreatitis | 36 (6) | 32 (6) |
| Autoimmune pancreatitis | 10 (2) | 8 (1) |
| Acute pancreatitis | 27 (5) | 25 (5) |
| Neuroendocrine tumour | 26 (4) | 24 (4) |
| Lymphoma | 1 (0) | 1 (0) |
| Metastases from non-pancreatic primary neoplasm | 6 (1) | 6 (1) |
| Normal pancreas | 22 (4) | 21 (4) |
| Other | 20 (3) | 19 (3) |
Reference standard staging for patients with in-range positron emission tomography/computed tomography
For patients with a tumour the reference standard stagings are detailed in Table 6. All tumour types are included in the second column and patients with PDAC only are included in the third column. Overall, 20% of patients with pancreatic cancer had locally advanced or metastatic disease.
| Confirmed stagea at D4 | Number (%) of patients: all tumours (n = 550) | Number (%) of patients: PDAC (%) (n = 261) |
|---|---|---|
| 0 | 1 (0) | 0 (0) |
| IA | 15 (3) | 5 (2) |
| IB | 16 (3) | 5 (2) |
| IIA | 31 (6) | 20 (8) |
| IIB | 111 (20) | 79 (30) |
| III | 26 (5) | 17 (7) |
| IV | 63 (11) | 52 (20) |
| Other/unknown | 287 (52) | 83 (32) |
Reference standard management for patients with an in-range positron emission tomography/computed tomography scan
The management outcomes for patients are detailed in Table 7. Patients may have had more than one management outcome, for example resection plus adjuvant therapy. The majority of patients who underwent resection had a right-sided (standard or pylorus-preserving Whipple) procedure. Twenty-six patients were found to be inoperable at the time of surgery and underwent either surgical bypass or open and shut laparotomy.
| Outcome | n (%) |
|---|---|
| Resection | 216 (39) |
| Standard Whipple procedure | 60 (28) |
| Pylorus-preserving Whipple procedure | 102 (47) |
| Left pancreatectomy | 35 (16) |
| Total pancreatectomy | 8 (4) |
| Other | 11 (5) |
| Surgical bypass | 22 (4) |
| Laparotomy | 4 (1) |
| Biopsy | 135 (25) |
| EUS | 96 (71) |
| ERCP | 12 (9) |
| Percutaneous | 9 (7) |
| Other/unknown | 18 (13) |
| Chemotherapy | 249 (45) |
| Neoadjuvant | 7 (3) |
| Adjuvant | 100 (40) |
| Palliative | 142 (57) |
| Best supportive care | 28 (5) |
| Clinical follow-up | 52 (9) |
| No further investigation | 29 (5) |
Patient withdrawals
Out of the 619 registered patients a total of 280 patients withdrew from the trial. Of these, 187 patients died; the other reasons for withdrawal are detailed in Table 8. The median study duration for all patients who withdrew was 159 days.
| Patient withdrawals | n (%) |
|---|---|
| Patients withdrawn/died | 280 (45) |
| Reason for withdrawal | |
| Consent withdrawn | 20 (7) |
| Lost to follow-up | 26 (9) |
| Intercurrent illness preventing further follow-up | 5 (2) |
| Consultant decision following other changes in patient’s condition | 14 (5) |
| Death | 187 (67) |
| Other | 28 (10) |
| Study duration (days from registration to withdrawal from study) including deaths | |
| Median | 187 |
| IQR | 230 |
| Range | 0–466 |
| n | 280 |
| Study duration (days from registration to withdrawal from study) excluding deaths | |
| Median | 159 |
| IQR | 349 |
| Range | 0–538 |
| n | 92 |
Adverse events
There were no adverse events related to the study procedure. One adverse event was recorded as lymphangitis carcinomatosis, which was related to the patient’s original condition.
Central review
The majority of the PET/CT scans were reviewed at the Paul Strickland Scanner Centre at Mount Vernon Hospital using the agreed standard proforma. Central review of the PET/CT scans was also performed at Aintree University Hospital, St James’s University Hospital, the Royal Free Hospital and Southampton General Hospital using the agreed standard proforma. There were 40 instances in which there was discordance between the site PET/CT report and the central PET/CT report. To date, two sites have been notified regarding abnormal uptake not seen in the local reports.
Quality assurance of computed tomography and histology
Review of pathological samples was completed on samples received from 12 of the recruiting research sites. Review of samples from the remaining six sites is currently still ongoing. Central pathology review has not produced any disparities that would have significantly affected patient management. Radiological review of baseline CT scans is currently ongoing and has been completed in one-third of cases.
Diagnostic accuracy and incremental benefit of positron emission tomography/computed tomography for pancreatic cancer
Patients with positron emission tomography/computed tomography and a D4 diagnosis (intention-to-treat population, n = 583)
Among the 583 patients with a D4 diagnosis the overall disease prevalence (pancreatic cancer) was 47.7% (95% CI 43.6% to 51.7%). Table 9 provides the 2 × 2 table for MDCT and PET/CT diagnosis by PDAC status (D4).
| Diagnosis | PDAC | No PDAC |
|---|---|---|
| MDCT positive | 243 | 92 |
| MDCT negative | 35 | 213 |
| PET/CT positive | 251 | 73 |
| PET/CT negative | 27 | 232 |
The sensitivity and specificity of the initial MDCT scan (D1) diagnosis were 87.4% (95% CI 83.5% to 91.3%) and 69.8% (95% CI 64.7% to 75.0%), respectively. The sensitivity and specificity of the subsequent PET/CT scan (D2) diagnosis were 90.3% (95% CI 86.8% to 93.7%) and 76.1% (95% CI 71.3% to 80.9%), respectively.
Table 10 shows the MDCT- and PET/CT-based diagnoses among patients with and without PDAC. Of main interest were the numbers of discordant patients (those whose diagnosis differed at each stage) as this drives the relative sensitivity and specificity of PET/CT compared with MDCT. In each case there was a greater number of cases in which the PET/CT diagnosis was correct than in which the MDCT diagnosis was correct, indicating that PET/CT performs better.
| Diagnosis | Patients with PDAC | Patients without PDAC | ||
|---|---|---|---|---|
| PET/CT positive | PET/CT negative | PET/CT positive | PET/CT negative | |
| MDCT positive | 234 | 7 | 58 | 35 |
| MDCT negative | 16 | 20 | 16 | 197 |
Table 11 provides the relative sensitivity and specificity for PET/CT compared with MDCT with the corresponding 95% CIs and two-sided p-values. Using all patients with a D4 diagnosis, including those out of range for either blood glucose or uptake time, the relative sensitivity did not attain statistical significance (1.03; p = 0.083). In contrast, the 9% improvement in specificity was statistically significant (p = 0.005). There were corresponding improvements in PPV and negative predictive value (NPV) but only the effect on PPV was statistically significant.
| Measure | MDCT | PET/CT | Relative | p-value |
|---|---|---|---|---|
| Sensitivity (95% CI) (%) | 87.4 (83.5 to 91.3) | 90.3 (86.8 to 93.7) | 1.03 (1.00 to 1.07) | 0.083 |
| Specificity (95% CI) (%) | 69.8 (64.7 to 75.0) | 76.1 (71.3 to 80.9) | 1.09 (1.03 to 1.15) | 0.005 |
| PPV (95% CI) (%) | 72.5 (67.8 to 77.3) | 77.5 (72.9 to 82.0) | 1.07 (1.00 to 1.14) | 0.037 |
| NPV (95% CI) (%) | 85.9 (81.6 to 90.2) | 89.6 (85.8 to 93.3) | 1.04 (0.99 to 1.10) | 0.101 |
Patients with positron emission tomography/computed tomography within the uptake range and a blood glucose of < 10 mmol/l (per-protocol population, n = 550)
Among the 550 patients with a D4 diagnosis and within-range uptake and blood glucose, the overall disease prevalence was 47.5% (95% CI 43.3% to 51.6%). Table 12 provides the 2 × 2 table for MDCT and PET/CT diagnosis by PDAC status (D4).
| Diagnosis | PDAC | No PDAC |
|---|---|---|
| MDCT positive | 231 | 85 |
| MDCT negative | 30 | 204 |
| PET/CT positive | 242 | 70 |
| PET/CT negative | 19 | 219 |
The sensitivity and specificity of the initial MDCT scan (D1) diagnosis were 88.5% (95% CI 84.6% to 92.4%) and 70.6% (95% CI 65.3% to 75.8%), respectively. The sensitivity and specificity of the subsequent PET/CT scan (D2) diagnosis were 92.7% (95% CI 89.5% to 95.9%) and 75.8% (95% CI 70.8% to 80.7%), respectively.
Table 13 shows the MDCT- and PET/CT-based diagnoses among patients with and without PDAC. The numbers of discordant patients (those whose diagnoses differed at each stage) drive the relative sensitivity and specificity of PET/CT compared with MDCT. In each case there was a greater number of cases in which the PET/CT diagnosis was correct than in which the MDCT diagnosis was correct, indicating that PET/CT performs better.
| Diagnosis | Patients with PDAC | Patients without PDAC | ||
|---|---|---|---|---|
| PET/CT positive | PET/CT negative | PET/CT positive | PET/CT negative | |
| MDCT positive | 227 | 4 | 54 | 31 |
| MDCT negative | 15 | 15 | 16 | 188 |
Excluding patients with out-of-range uptake or blood glucose leads to an improvement in the sensitivity of PET/CT (92.7% vs. 90.3%) and the analysis based only on these per-protocol patients shows a significant improvement in both relative sensitivity (1.05; p = 0.010) and specificity (1.07; p = 0.023) (Table 14). The PPV and NPV are specific to the prevalence of the disease in the population, which has to be estimated from the data. Here, the relative NPV was statistically significant (p = 0.031) but the relative PPV did not attain statistical significance.
| Measure | MDCT | PET/CT | Relative | p-value |
|---|---|---|---|---|
| Sensitivity (95% CI) (%) | 88.5 (84.6 to 92.4) | 92.7 (89.6 to 95.9) | 1.05 (1.01 to 1.09) | 0.010 |
| Specificity (95% CI) (%) | 70.6 (65.3 to 75.8) | 75.8 (70.8 to 80.7) | 1.07 (1.01 to 1.14) | 0.023 |
| PPV (95% CI) (%) | 73.1 (68.2 to 78.0) | 77.6 (72.9 to 82.2) | 1.06 (1.00 to 1.13) | 0.062 |
| NPV (95% CI) (%) | 87.1 (82.9 to 91.5) | 92.0 (88.6 to 95.5) | 1.06 (1.00 to 1.11) | 0.031 |
Assessment of incremental diagnostic benefit
The estimated incremental likelihood ratios demonstrate that the results of PET/CT significantly improve diagnostic accuracy in all scenarios (Figure 4). All results are significant with a bootstrap p-value of < 0.0002. A positive diagnosis from MDCT increased the odds of PDAC by 201% (95% CI 157% to 269%), whereas a negative diagnosis decreased the odds by 84% (95% CI 79% to 88%).
FIGURE 4.
Tree diagram of incremental likelihood ratios (LRs). The values of the LRs are given following positive or negative MDCT and then positive or negative PET/CT. Figures in parentheses represent 95% CIs.
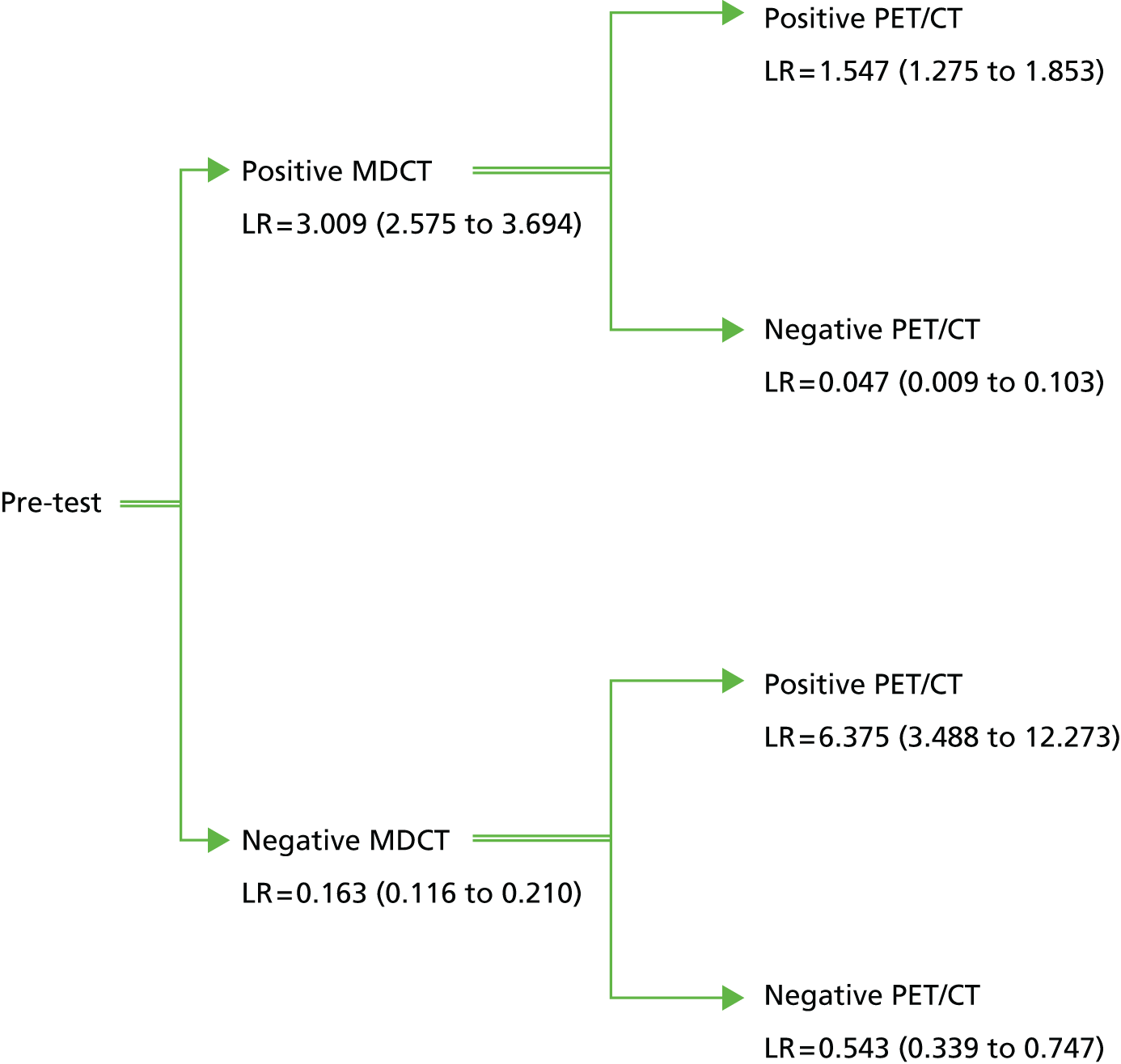
Following a positive diagnosis from MDCT, a positive diagnosis at PET/CT further increased the odds of PDAC by 55% (95% CI 28% to 85%). The most substantial incremental effect of PET/CT was a negative diagnosis following a positive diagnosis from MDCT. In this case the odds of PDAC decreased by 95% (95% CI 90% to 99%).
Following a negative diagnosis from MDCT, a positive diagnosis on PET/CT increased the odds of PDAC by 538% (95% CI 249% to 1127%). A second negative diagnosis on PET/CT following a negative diagnosis from MDCT further decreased the odds of PDAC by 46% (95% CI 25% to 66%).
These results suggest that there is a substantial diagnostic benefit for patients of receiving a PET/CT scan after a potential PDAC is detected on MDCT. Patients with no PDAC detected on PET/CT following a positive MDCT scan are unlikely to have PDAC (NPV of 89%). In contrast, although a positive diagnosis on PET/CT following a negative MDCT scan increases the odds of PDAC, the overall diagnosis is still highly uncertain (PPV of 50%). This latter result suggests that if both tests are administered there is benefit in combining the results rather than taking the PET/CT result alone. In particular, patients who were positive on MDCT and PET/CT are much more likely to have PDAC than patients who are positive only on PET/CT (PPV of 80% vs. 50%). The result of the MDCT is less important if the PET/CT result is negative (NPV of 93% if both tests are negative vs. NPV of 89% if the PET/CT scan is negative after a positive MDCT scan).
Certainty of diagnosis
Using the quantitative scale for assessing clinicians’ perceptions of diagnostic certainty (0 = no certainty and 1 = absolute certainty), there was a significant increase in the median certainty score after the PET/CT scan [0.80, interquartile range (IQR) 0.25] compared with before (0.68, IQR 0.34) (p < 0.0001) (Table 15). A box plot of the distributions of perceived certainty before and after the PET/CT scan is shown in Figure 5.
| Time point | Median (IQR)a | p-value |
|---|---|---|
| Before PET/CT | 0.68 (0.34) | < 0.0001 (Wilcoxon) |
| After PET/CT | 0.80 (0.25) |
FIGURE 5.
Distribution of perceived diagnostic certainty pre and post PET/CT.

A similar pattern was observed using a qualitative assessment of whether certainty after PET/CT was more, less or the same as that before PET/CT. In 322 (59%) cases clinicians were more certain following PET/CT and in 25 (5%) cases they were less certain. In 201 (37%) cases the level of certainty was the same before and after PET/CT. Clinicians were 12 times more likely to believe that they were more certain in their diagnosis than they were to be less certain (p < 0.0001) following PET/CT.
Assessing the accuracy of clinicians’ perceived certainty using the Brier score (Table 16) also indicated that clinicians’ predictions (interpreting their level of certainty as a probability) were significantly improved by the PET/CT scan result (reduction in Brier score, i.e. prediction error, of 0.045, 95% CI 0.024 to 0.066; p < 0.001).
| Time point | Brier score (PDAC diagnosis) | Improvement in Brier score |
|---|---|---|
| Before PET/CT | 0.218 | 0.045 (95% CI 0.024 to 0.066), p < 0.001 |
| After PET/CT | 0.173 |
Changes in diagnosis, staging and management following positron emission tomography/computed tomography
Changes in diagnosis following positron emission tomography/computed tomography
The discordance of diagnoses following MDCT and then PET/CT can be seen in Table 17. This table shows the MDCT- and PET/CT-based diagnoses among patients with and without PDAC. The numbers of discordant patients (those whose diagnoses differed at each stage) drives the relative sensitivity and specificity of PET/CT compared with MDCT. In each case there was a greater number of cases in which the PET/CT diagnosis was correct than in which the MDCT diagnosis was correct, indicating that PET/CT performs better.
| Diagnosis | Patients with PDAC | Patients without PDAC | ||
|---|---|---|---|---|
| PET/CT positive | PET/CT negative | PET/CT positive | PET/CT negative | |
| MDCT positive | 227 | 4 | 54 | 31 |
| MDCT negative | 15 | 15 | 16 | 188 |
Positron emission tomography/computed tomography changed the diagnosis correctly for both the diagnosis of pancreatic cancer and the diagnosis of not pancreatic cancer in 46 patients (8.3%). PET/CT incorrectly changed the diagnoses in 20 patients (3.6%). Considering the diagnosis of malignancy in general, PET/CT correctly changed the diagnosis in 48 patients (8.7%) and incorrectly changed the diagnosis in 11 patients (2%) (Table 18).
| Diagnosis | Patients with malignancy | Patients without malignancy | ||
|---|---|---|---|---|
| PET/CT positive | PET/CT negative | PET/CT positive | PET/CT negative | |
| MDCT positive | 369 | 5 | 46 | 42 |
| MDCT negative | 6 | 4 | 6 | 72 |
Changes in staging following positron emission tomography/computed tomography
For the purposes of examining change of stage, patients were grouped into four categories: (a) no tumour/IA/IB/IIA, (b) IIB, (c) III and (d) IV (for the breakdown of changes in staging according to the groups see Appendix 3). The accuracy of staging among patients in stages IA/IB/IIA was slightly worse under PET/CT than under MDCT, with more incorrect (eight patients) than correct (six patients) changes. However, the difference was not statistically significant (p = 0.79). Significantly more patients with stage IIB were correctly staged by PET/CT than by MDCT, with 22 patients (21%) moving to the correct stage compared with five patients (5%) moving to the wrong stage (p = 0.002). PET/CT had no significant effect on the accuracy of staging for patients in stage III. Significantly more patients in staging group IV were changed to the correct stage (27 patients) than moved to an incorrect stage (one patient) (43% vs. 2%; p < 0.001). A summary of stage changes is provided in Table 19.
| Change summary | n (%) |
|---|---|
| Remained correct | 221 (56) |
| Remained incorrect | 94 (24) |
| Changed to correct | 56 (14) |
| Changed from correct to incorrect | 14 (4) |
| Changed between incorrect groups | 8 (2) |
Overall, the effect of PET/CT was to change to the correct staging group significantly more often than to change from the correct group to an incorrect group (p < 0.001). However, the majority of the benefit was in correct changes to stage IIB or stage IV.
Changes in planned management
Using the question from the D2 (post-PET/CT scan) form, ‘Has the PET/CT scan influenced your planned management of this patient’s disease?’, the PET/CT scan was perceived to have changed the planned management in 250 (45%) patients. The proportion whose management was affected was slightly higher among patients whose final diagnosis was not pancreatic cancer [139 (48%) vs. 111 (43%) with pancreatic cancer] but the difference was not statistically significant (Table 20).
| Group | No, n (%) | Yes, n (%) | Missing, n (%) |
|---|---|---|---|
| All patients | 293 (53) | 250 (45) | 7 (1) |
| Patients with pancreatic cancer | 148 (57) | 111 (43) | 2 (1) |
| Patients without pancreatic cancer | 145 (50) | 139 (48) | 5 (2) |
Using the questions from the D3 (confirmed diagnosis) form, ‘Did the patient follow the course of treatment recommended at D1?’ and ‘Did the patient follow the course of treatment recommended at D2?’, 70% of patients did not undergo a change in management throughout the study Table 21). In total, 15% underwent a change in management from either that considered at D1 (pre PET/CT) or that considered at D2 (post PET/CT). A significantly higher proportion of patients (11% vs. 4%; p = 0.0002) followed the management plan recommended after PET/CT (and not that recommended after MDCT) than followed the MDCT management plan (and not that recommended after PET/CT).
| Management | D2 followed, n (%) | ||
|---|---|---|---|
| Yes | No | ||
| D1 followed | Yes | 376 (70) | 23 (4) |
| No | 58 (11) | 83 (15) | |
Clinically important changes resulting from positron emission tomography/computed tomography
Table 22 details the frequency of different types of clinically important management changes. The most common change was changing from resection to no resection, which occurred in 61 patients, representing 11% of all patients and 21% of patients scheduled for some kind of resection after MDCT. Changing from no further investigation to some form of further investigation/clinical follow-up occurred in 58 patients, representing 11% of all patients and 13% of those initially not scheduled for further investigation. In total, 13% of patients not thought to need surgical resection following MDCT were then planned for resection following PET/CT. Changes relating to the commencement or cessation of chemotherapy were less common.
| D1 change to D2 | n (%) | As a result of PET/CT, n (%) | Clinically significant, n (%) |
|---|---|---|---|
| Resection to no resection | 61 (21) | 58 (20) | 58 (20) |
| No resection to resection | 34 (13) | 19 (7) | 19 (7) |
| Chemotherapy to no chemotherapy | 8 (10) | 1 (1) | 1 (1) |
| No chemotherapy to chemotherapy | 41 (9) | 24 (5) | 24 (5) |
| No further investigation to further investigation | 58 (13) | 31 (7) | 31 (7) |
| PET/CT identified or perceived to have led to identification of a secondary malignancy | 5 (NA) | 5 (NA) | 5 (NA) |
Impact of positron emission tomography/computed tomography on the diagnosis of chronic pancreatitis
The prevalence of chronic pancreatitis in the patient cohort was 41 patients (7.5%, 95% CI 5.3% to 9.6%). The small number of patients diagnosed with chronic pancreatitis (Table 23) means that a statistical comparison of the diagnostic tests will have a low power to detect small or moderate effects.
| Diagnosis | Chronic pancreatitis | No chronic pancreatitis |
|---|---|---|
| MDCT positive | 15 | 8 |
| MDCT negative | 26 | 501 |
| PET/CT positive | 19 | 8 |
| PET/CT negative | 22 | 501 |
Table 24 shows the MDCT- and PET/CT-based diagnoses among patients with and without chronic pancreatitis. There was a greater number of cases in which the PET/CT diagnosis was correct than in which the MDCT diagnosis was correct for patients with a diagnosis of chronic pancreatitis.
| Diagnosis | Patients with chronic pancreatitis | Patients without chronic pancreatitis | ||
|---|---|---|---|---|
| PET/CT positive | PET/CT negative | PET/CT positive | PET/CT negative | |
| MDCT positive | 14 | 1 | 5 | 3 |
| MDCT negative | 5 | 21 | 3 | 498 |
Both MDCT and PET/CT had a relatively high specificity (98.4% in each case) but low sensitivity. The sensitivity of the PET/CT scan was higher than that of the MDCT scan (46.3% vs. 36.6%); however, this effect did not attain statistical significance (relative sensitivity 1.27; p = 0.066) (Table 25).
| Measure | MDCT | PET/CT | Relative | p-value |
|---|---|---|---|---|
| Sensitivity (95% CI) (%) | 36.6 (21.8 to 51.3) | 46.3 (31.1 to 61.6) | 1.27 (0.98 to 1.55) | 0.066 |
| Specificity (95% CI) (%) | 98.4 (97.3 to 99.5) | 98.4 (97.3 to 99.5) | 1.00 (0.99 to 1.01) | 1.000 |
| PPV (95% CI) (%) | 65.2 (45.8 to 84.7) | 70.4 (53.1 to 87.6) | 1.08 (0.82 to 1.41) | 0.582 |
| NPV (95% CI) (%) | 95.1 (93.2 to 96.9) | 95.8 (94.1 to 97.5) | 1.01 (0.99 to 1.03) | 0.436 |
Similarly, although the improvement in sensitivity for the PET/CT scan translated into an improvement in PPV, the effect was not significant. Although a positive diagnosis of chronic pancreatitis on PET/CT or MDCT greatly increases the odds of a patient having chronic pancreatitis, there was still considerable uncertainty about the diagnosis (PPV 65.2% for MDCT and 70.4% for PET/CT). Moreover, the NPVs were very close to the original prevalence, reflecting that the tests have poor negative likelihood ratios (0.65 and 0.56 for MDCT and PET/CT, respectively).
The role of PET/CT in distinguishing between pancreatic cancer and chronic pancreatitis was assessed. Very few patients were misdiagnosed as having chronic pancreatitis rather than PDAC. Two patients (with PDAC at D4) were suspected of having chronic pancreatitis after MDCT, which reduced to one after PET/CT (the breakdown of chronic pancreatitis diagnoses at D1 and D2 can be found in Appendix 4). One patient had confirmed PDAC and chronic pancreatitis and was diagnosed as PDAC only after both MDCT and PET/CT. The level of agreement between the diagnoses at D1 and D2 and the diagnosis at D4 was measured using Cohen’s kappa. Diagnosis of PDAC was grouped with the diagnosis of chronic pancreatitis and PDAC because of the very small number of patients with chronic pancreatitis and PDAC. At D1, the weighted Cohen’s kappa was 0.58 (95% CI 0.51 to 0.65), whereas at D2 this increased to 0.67 (95% CI 0.60 to 0.73). The increase in Cohen’s kappa was 0.083 (95% CI 0.021 to 0.134), indicating a statistically significant improvement in agreement with the D4 diagnosis (p = 0.004). It should be noted that most of this improvement was likely because of improvement in the diagnosis of PDAC rather than improvement in the diagnosis of chronic pancreatitis.
Subgroup analyses
Out-of-range patients
A GEE subgroup analysis (Table 26) comparing the patients within range for both uptake time and blood glucose with those out of range for either showed a statistically significant deterioration in sensitivity among out-of-range patients at just 52.9% (p < 0.0001). There was evidence that the relative sensitivity of PET/CT compared with MDCT was much lower among those patients, being worse than that of MDCT (relative sensitivity 0.75; p = 0.005). There was also some evidence of lower sensitivity of MDCT among this group. There was no evidence of a difference in PET/CT or MDCT specificity or relative specificity for this group.
| Measure | MDCT | PET/CT | Relative |
|---|---|---|---|
| Sensitivity (95% CI) (%) | 70.6 (45.8 to 87.2), p = 0.040a | 52.9 (30.3 to 74.5), p < 0.0001a | 0.75 (0.54 to 1.04), p = 0.005a |
| Specificity (95% CI) (%) | 56.3 (32.4 to 77.5), p = 0.230a | 81.3 (55.3 to 93.8), p = 0.190a | 1.44 (1.00 to 2.07), p = 0.097a |
Among patients who had an out-of-range uptake time (Table 27) there was some evidence of lower PET/CT sensitivity (71.4%), although this did not attain statistical significance. There was no evidence of differences in specificity.
| Measure | MDCT | PET/CT | Relative |
|---|---|---|---|
| Sensitivity (95% CI) (%) | 85.7 (41.9 to 98.0), p = 0.891a | 71.4 (32.7 to 92.8), p = 0.146a | 0.83 (0.58 to 1.19), p = 0.153a |
| Specificity (95% CI) (%) | 54.5 (26.8 to 79.7), p = 0.269a | 81.8 (49.3 to 95.4), p = 0.264a | 1.50 (0.95 to 2.38), p = 0.141a |
Among patients who had a blood glucose level of > 10 mmol/l (Table 28) there was evidence of considerably lower PET/CT sensitivity (40.0%; p = 0.0001); although the sensitivity of MDCT was also impacted, there was nevertheless also strong evidence of reduced relative sensitivity for these patients, with PET/CT performing worse than MDCT (relative sensitivity 0.67; p = 0.027). Again, there was no evidence of differences in specificity.
| Measure | MDCT | PET/CT | Relative |
|---|---|---|---|
| Sensitivity (95% CI) (%) | 60.0 (29.7 to 84.2), p = 0.016a | 40.0 (15.8 to 70.3), p = 0.0001a | 0.67 (0.38 to 1.17), p = 0.027a |
| Specificity (95% CI) (%) | 60.0 (20.0 to 90.0), p = 0.632a | 80.0 (30.9 to 97.3), p = 0.730a | 1.33 (0.76 to 2.35), p = 0.463a |
These results confirm the necessity for PET/CT to be administered after the correct uptake time and the unsuitability of PET/CT for patients with poorly controlled blood glucose.
Further generalised estimating equation subgroup analyses
In the remaining analyses the subgroup of patients with the per-protocol uptake time and within-range blood glucose was considered. Tables 29–34 present the results of univariate GEE analyses for the effect of sex, age (dichotomised at < 65 years or ≥ 65 years), WHO performance status (0 vs. 1, 2 or 3; see Appendix 5) and eligibility criteria. There was no evidence of any differences in sensitivity or specificity for either test with respect to sex (see Table 29) or age (see Table 30). However, both MDCT and PET/CT had significantly higher sensitivity among patients with a WHO status of ≥ 1 than among those with a WHO status of 0, but conversely had significantly lower specificity (see Table 31). Both MDCT and PET/CT had significantly lower specificity among patients with jaundice because of distal obstruction of the common bile duct or ampulla (see Table 33). Moreover, there was also evidence that PET/CT was not more effective than MDCT for this group. There was no evidence of differences in sensitivity or specificity in relation to the other eligibility criteria (see Tables 32 and 34).
| Measure | MDCT | PET/CT | Relative | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Sensitivity (95% CI) (%) | 88.1 (81.7 to 92.5) | 89.0 (81.9 to 93.5) | 92.7 (84.2 to 96.8) | 93.2 (87.0 to 96.6) | 1.05 (0.99 to 1.12) | 1.05 (0.99 to 1.11) |
| p = 0.826 | p = 0.958 | p = 0.905 | ||||
| Specificity (95% CI) (%) | 66.4 (58.8 to 73.3) | 75.8 (67.6 to 82.4) | 74.5 (67.2 to 80.7) | 77.3 (69.3 to 83.8) | 1.12 (1.00 to 1.25) | 1.02 (0.94 to 1.11) |
| p = 0.085 | p = 0.191 | p = 0.215 | ||||
| Measure | MDCT | PET/CT | Relative | |||
|---|---|---|---|---|---|---|
| < 65 years | ≥ 65 years | < 65 years | ≥ 65 years | < 65 years | ≥ 65 years | |
| Sensitivity (95% CI) (%) | 89.3 (82.1 to 93.8) | 87.9 (81.6 to 92.3) | 92.0 (85.3 to 95.7) | 93.3 (88.0 to 96.4) | 1.03 (0.98 to 1.09) | 1.06 (1.01 to 1.12) |
| p = 0.732 | p = 0.701 | p = 0.404 | ||||
| Specificity (95% CI) (%) | 73.3 (65.3 to 80.1) | 68.2 (60.4 to 75.0) | 77.0 (69.2 to 83.4) | 74.7 (67.2 to 80.9) | 1.05 (0.98 to 1.12) | 1.10 (1.01 to 1.19) |
| p = 0.338 | p = 0.629 | p = 0.630 | ||||
| Measure | MDCT | PET/CT | Relative | |||
|---|---|---|---|---|---|---|
| WHO 0 | WHO ≥ 1 | WHO 0 | WHO ≥ 1 | WHO 0 | WHO ≥ 1 | |
| Sensitivity (95% CI) (%) | 84.2 (76.3 to 89.8) | 91.8 (86.2 to 95.3) | 86.8 (79.3 to 91.9) | 97.3 (93.0 to 99.0) | 1.03 (0.91 to 1.17) | 1.06 (1.01 to 1.11) |
| p = 0.059 | p = 0.012 | p = 0.069 | ||||
| Specificity (95% CI) (%) | 78.0 (70.7 to 83.9) | 62.6 (54.3 to 70.2) | 82.0 (75.0 to 87.4) | 69.1 (60.9 to 76.2) | 1.05 (0.99 to 1.12) | 1.10 (1.00 to 1.22) |
| p = 0.004 | p = 0.009 | p = 0.882 | ||||
| Measure | MDCT | PET/CT | Relative | |||
|---|---|---|---|---|---|---|
| Criterion 1 not met | Criterion 1 met | Criterion 1 not met | Criterion 1 met | Criterion 1 not met | Criterion 1 met | |
| Sensitivity (95% CI) (%) | 76.5 (51.4 to 90.9) | 89.3 (84.1 to 92.6) | 88.2 (63.2 to 97.0) | 93.0 (89.1 to 95.6) | 1.15 (1.07 to 1.25) | 1.04 (1.00 to 1.08) |
| p = 0.119 | p = 0.277 | p = 0.543 | ||||
| Specificity (95% CI) (%) | 76.9 (57.2 to 89.2) | 70.0 (64.1 to 75.2) | 73.1 (53.3 to 86.6) | 76.0 (70.5 to 80.8) | 0.95 (0.89 to 1.01) | 1.09 (1.02 to 1.16) |
| p = 0.459 | p = 0.387 | p = 0.171 | ||||
| Measure | MDCT | PET/CT | Relative | |||
|---|---|---|---|---|---|---|
| Criterion 2 not met | Criterion 2 met | Criterion 2 not met | Criterion 2 met | Criterion 2 not met | Criterion 2 met | |
| Sensitivity (95% CI) (%) | 87.6 (81.2 to 91.8) | 90.1 (82.1 to 94.8) | 91.1 (85.9 to 94.6) | 95.6 (88.9 to 98.3) | 1.04 (0.95 to 1.14) | 1.06 (1.00 to 1.13) |
| p = 0.553 | p = 0.436 | p = 0.336 | ||||
| Specificity (95% CI) (%) | 73.7 (67.7 to 79.0) | 57.9 (44.8 to 69.9) | 81.5 (75.9 to 86.0) | 52.6 (40.0 to 65.1) | 1.11 (0.98 to 1.24) | 0.91 (0.73 to 1.14) |
| p = 0.020 | p < 0.0001 | p = 0.024 | ||||
| Measure | MDCT | PET/CT | Relative | |||
|---|---|---|---|---|---|---|
| Criterion 3 met | Criterion 3 not met | Criterion 3 met | Criterion 3 not met | Criterion 3 met | Criterion 3 not met | |
| Sensitivity (95% CI) (%) | 89.7 (84.4 to 93.3) | 85.7 (76.0 to 91.9) | 92.9 (88.2 to 95.9) | 92.2 (83.7 to 96.5) | 1.04 (0.99 to 1.09) | 1.08 (1.01 to 1.15) |
| p = 0.362 | p = 0.580 | p = 0.505 | ||||
| Specificity (95% CI) (%) | 71.5 (65.6 to 76.7) | 65.0 (49.2 to 78.1) | 76.3 (70.6 to 81.2) | 72.5 (56.8 to 84.1) | 1.07 (0.94 to 1.22) | 1.12 (0.95 to 1.31) |
| p = 0.404 | p = 0.704 | p = 0.730 | ||||
Impact of positron emission tomography/computed tomography on the diagnosis of pancreatic tumours
Malignant cystic neoplasm
The prevalence of malignant cystic neoplasm in the patient cohort was 1.5% (95% CI 0.5% to 2.5%). The small number of patients diagnosed with malignant cystic neoplasm (Table 35) means that statistical tests comparing the diagnostic tests have low power to detect small or moderate effects.
| Diagnosis | Malignant cystic neoplasm | No malignant cystic neoplasm |
|---|---|---|
| MDCT positive | 6 | 39 |
| MDCT negative | 2 | 503 |
| PET/CT positive | 6 | 21 |
| PET/CT negative | 2 | 521 |
Both MDCT and PET/CT had a sensitivity of 75.0% (95% CI 45.0% to 99.9%). The specificity of PET/CT was higher than that of MDCT [96.1% (95% CI 94.5% to 97.8%) vs. 92.8% (95% CI 90.6% to 95.0%)]. The 3.6% (95% CI 1.8% to 5.4%) improvement in specificity between tests was statistically significant (p < 0.001) (Table 36). The very small number of cases of malignant cystic neoplasm meant that the significant difference in specificity did not translate into a significant difference in either PPV or NPV.
| Measure | MDCT | PET/CT | Relative | p-value |
|---|---|---|---|---|
| Sensitivity (95% CI) (%) | 75.0 (45.0 to 99.9) | 75.0 (45.0 to 99.9) | 1.00 (0.54 to 1.46) | 1.0 |
| Specificity (95% CI) (%) | 92.8 (90.6 to 95.0) | 96.1 (94.5 to 97.8) | 1.04 (1.02 to 1.05) | < 0.001 |
| PPV (95% CI) (%) | 13.3 (3.4 to 23.3) | 22.2 (6.5 to 37.9) | 1.67 (0.97 to 2.87) | 0.066 |
| NPV (95% CI) (%) | 99.6 (99.1 to 99.9) | 99.6 (99.1 to 99.9) | 1.00 (0.99 to 1.01) | 0.967 |
Cholangiocarcinoma
The prevalence of cholangiocarcinoma in the patient cohort was 8.0% (95% CI 5.7% to 10.3%). The small number of patients diagnosed with cholangiocarcinoma (Table 37) means that statistical tests comparing the diagnostic tests have low power to detect small or moderate effects.
| Diagnosis | Cholangiocarcinoma | No cholangiocarcinoma |
|---|---|---|
| MDCT positive | 11 | 11 |
| MDCT negative | 33 | 495 |
| PET/CT positive | 11 | 6 |
| PET/CT negative | 33 | 500 |
Both MDCT and PET/CT had a relatively high specificity (97.8% and 98.8%, respectively) but low sensitivity (25% in each case). There were no significant differences in either sensitivity or specificity (Table 38).
| Measure | MDCT | PET/CT | Relative | p-value |
|---|---|---|---|---|
| Sensitivity (95% CI) (%) | 25.0 (12.2 to 37.8) | 25.0 (12.2 to 37.8) | 1.00 (0.64 to 1.36) | 1.000 |
| Specificity (95% CI) (%) | 97.8 (96.6 to 99.1) | 98.8 (97.8 to 99.8) | 1.01 (1.00 to 1.02) | 0.094 |
| PPV (95% CI) (%) | 50.0 (29.1 to 70.9) | 64.7 (42.0 to 87.4) | 1.29 (0.86 to 1.94) | 0.214 |
| NPV (95% CI) (%) | 93.8 (91.7 to 95.8) | 93.8 (91.8 to 95.9) | 1.00 (0.98 to 1.02) | 0.954 |
Similarly, although the improvement in sensitivity (for cholangiocarcinoma) for the PET/CT scan translated into an improvement in PPV, the effect was not significant. Although a positive diagnosis of cholangiocarcinoma on PET/CT or MDCT greatly increases the odds of a patient having cholangiocarcinoma, there was still considerable uncertainty about the diagnosis (PPV 50% for MDCT and 65% for PET/CT). Moreover, the NPVs were very close to the original prevalence reflecting that the tests have poor negative likelihood ratios (0.77 and 0.76 for MDCT and PET/CT, respectively).
Neuroendocrine tumours
The prevalence of neuroendocrine tumours in the patient cohort was 4.9% (95% CI 3.1% to 6.7%). The small number of patients diagnosed with neuroendocrine tumour (Table 39) means that statistical tests comparing the diagnostic tests have low power to detect small or moderate effects.
| Diagnosis | Neuroendocrine tumour | No neuroendocrine tumour |
|---|---|---|
| MDCT positive | 12 | 3 |
| MDCT negative | 15 | 520 |
| PET/CT positive | 12 | 7 |
| PET/CT negative | 15 | 516 |
Both MDCT and PET/CT had a very high specificity (99.4% and 98.7%, respectively) but low sensitivity (44.4% in both cases). The relative specificity and relative sensitivity of MDCT and PET/CT were not significantly different (Table 40).
| Measure | MDCT | PET/CT | Relative | p-value |
|---|---|---|---|---|
| Sensitivity (95% CI) (%) | 44.4 (25.7 to 63.2) | 44.4 (25.7 to 63.2) | 1.00 (0.67 to 1.33) | 1.000 |
| Specificity (95% CI) (%) | 99.4 (98.8 to 99.9) | 98.7 (97.7 to 99.6) | 0.99 (0.98 to 1.00) | 0.159 |
| PPV (95% CI) (%) | 80.0 (59.8 to 99.9) | 63.2 (41.5 to 84.8) | 0.79 (0.55 to 1.13) | 0.191 |
| NPV (95% CI) (%) | 97.2 (95.8 to 98.6) | 97.2 (95.8 to 98.6) | 1.00 (0.98 to 1.01) | 0.977 |
The slightly lower specificity for PET/CT meant that the PPV was estimated to be lower for PET/CT. However, the difference was not statistically significant. Similarly, the NPV was slightly lower but again the difference was not significant.
Periampullary carcinoma
The prevalence of periampullary carcinoma in the patient cohort was 6.9% (95% CI 4.8% to 9.0%). The small number of patients diagnosed with periampullary carcinoma (Table 41) means that statistical tests comparing the diagnostic tests have low power to detect small or moderate effects.
| Diagnosis | Periampullary carcinoma | No periampullary carcinoma |
|---|---|---|
| MDCT positive | 27 | 21 |
| MDCT negative | 11 | 491 |
| PET/CT positive | 25 | 14 |
| PET/CT negative | 13 | 498 |
Both MDCT and PET/CT had a relatively high specificity (95.9% and 97.2%, respectively) but relatively low sensitivity (71.1% and 65.8%, respectively) (Table 42). The relative specificity of PET/CT compared with MDCT was statistically significant (p = 0.034) but the practical improvements were outweighed by the observed, although non-significant, reduction in sensitivity.
| Measure | MDCT | PET/CT | Relative | p-value |
|---|---|---|---|---|
| Sensitivity (95% CI) (%) | 71.1 (56.6 to 85.5) | 65.8 (50.7 to 80.9) | 0.92 (0.74 to 1.11) | 0.432 |
| Specificity (95% CI) (%) | 95.9 (94.2 to 97.6) | 97.2 (95.9 to 98.7) | 1.01 (1.00 to 1.03) | 0.034 |
| PPV (95% CI) (%) | 56.3 (42.2 to 70.3) | 64.1 (49.0 to 79.2) | 1.14 (0.90 to 1.44) | 0.278 |
| NPV (95% CI) (%) | 97.8 (96.5 to 99.1) | 97.5 (96.1 to 98.8) | 1.00 (0.98 to 1.01) | 0.633 |
Although PET/CT gave an improved PPV, the relative PPV was not significantly different from 1. Moreover, the estimated NPV was worse for PET/CT than for MDCT, although this differences was also non-significant.
Malignant compared with benign disease
The prevalence of any malignant disease among the patient population was 69.8% (95% CI 66.0% to 73.7%). Table 43 provides the 2 × 2 table for MDCT and PET/CT diagnosis by malignant or benign disease status (D4).
| Diagnosis | Malignant | Benign |
|---|---|---|
| MDCT positive | 374 | 88 |
| MDCT negative | 10 | 78 |
| PET/CT positive | 375 | 52 |
| PET/CT negative | 9 | 114 |
Both MDCT and PET/CT scans had high sensitivity with respect to detecting any malignancy (97.4% and 97.7%, respectively) but there was no evidence that PET/CT had better sensitivity (p = 0.763) (Table 44).
| Measure | MDCT | PET/CT | Relative | p-value |
|---|---|---|---|---|
| Sensitivity (95% CI) (%) | 97.4 (95.8 to 99.0) | 97.7 (96.1 to 99.2) | 1.00 (0.99 to 1.02) | 0.763 |
| Specificity (95% CI) (%) | 47.0 (39.4 to 54.6) | 68.7 (61.6 to 75.7) | 1.46 (1.32 to 1.61) | < 0.0001 |
| PPV (95% CI) (%) | 80.9 (77.3 to 84.5) | 87.8 (84.7 to 90.9) | 1.08 (1.04 to 1.13) | 0.0002 |
| NPV (95% CI) (%) | 88.6 (82.0 to 95.2) | 92.7 (88.1 to 97.3) | 1.05 (0.97 to 1.13) | 0.240 |
In contrast, the specificity of PET/CT was considerably higher than that for MDCT (68.7% vs. 47.0%, relative sensitivity 1.46; p < 0.0001). The improvement in specificity also corresponded with a statistically significant improvement in PPV, increasing from 80.9% for MDCT to 87.8% for PET/CT (p = 0.0002). The NPV also improved but by a non-significant margin (relative NPV 1.05; p = 0.24).
Incremental diagnostic benefit for malignancy versus benign
Using the Knottnerus method, estimates of the incremental likelihood ratios corresponding to a positive or negative PET/CT diagnosis with respect to whether the tumour was malignant or benign were obtained. In each case, the results of the PET/CT scan had a statistically significant impact on the odds of malignancy. The results suggest particular benefits of administering PET/CT after a positive MDCT scan. A positive PET/CT assessment following a positive MDCT assessment was estimated to increase the odds of malignancy by a further 88.7% (95% CI 55.4% to 134.8%) whereas a negative PET/CT assessment following a positive MDCT assessment reduced the odds of malignancy by 97.2% (95% CI 94.4% to 99.5%), meaning that malignancy is very unlikely for these patients (NPV 89.4%). A positive PET/CT assessment following a negative MDCT assessment increased the odds of malignancy by 680% (95% CI 106% to 2735%). However, there was still considerable uncertainty in the diagnosis of these patients (PPV 50.0%). A negative PET/CT assessment following a negative MDCT assessment decreased the odds of malignancy by 60.6% (95% CI 25.6% to 90.3%) and these patients were very unlikely to have a malignancy (NPV 94.7%) (Figure 6).
FIGURE 6.
Tree diagram of incremental likelihood ratios (LRs). The values of the LRs are given following positive or negative MDCT and then positive or negative PET/CT. Figures in parentheses represent 95% CIs.

Additional analyses
Analysis of maximum standardised uptake value
A SUVmax. measurement for the primary tumour was available only if a primary tumour was identified. In addition, in a further 49 cases no SUVmax. was recorded despite identification of a primary tumour (Table 45).
| D4 diagnosis | No primary tumour identified | Primary tumour identified | |
|---|---|---|---|
| SUVmax. missing | SUVmax. reported | ||
| Positive | 12 | 20 | 229 |
| Negative | 127 | 29 | 133 |
The following analyses use only the data from the 362 patients for whom a primary tumour was identified and the SUVmax. was recorded.
Distribution of maximum standardised uptake value
The distribution of the SUVmax. was positively skewed, with a median of 7.0 (Table 46).
| Group | Percentiles | ||||
|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | |
| All | 1.4 | 4.9 | 7.0 | 10.1 | 55.0 |
| D4 positive | 2.2 | 5.8 | 7.5 | 10.3 | 55.0 |
| D4 negative | 1.4 | 3.7 | 5.7 | 9.5 | 33.1 |
The median SUVmax. was higher for patients who were confirmed to have pancreatic cancer at D4 (median 7.5) than for patients who did not have pancreatic cancer (median 5.7) diagnosis (Wilcoxon test, p < 0.0001).
Diagnostic accuracy of the maximum standardised uptake value for pancreatic ductal adenocarcinoma
Although the level of SUVmax. provided statistically significant discrimination between patients with and without pancreatic cancer, the level of discrimination achieved was quite poor. Figure 7 demonstrates that the area under the curve (AUC) is 0.64 (95% CI 0.57 to 0.70). The optimal cut-off point, if the aim is to minimise the sum of the error probabilities of the test, is 6.2, meaning that all tumours with a SUVmax. > 6.2 would lead to a positive diagnosis of PDAC. However, such a test has an estimated sensitivity of only 70.3% and specificity of only 59.4%.
FIGURE 7.
Receiver operating characteristic curve for the diagnosis of PDAC based on SUVmax.

Diagnostic accuracy of the maximum standardised uptake value for chronic pancreatitis
Patients with chronic pancreatitis for whom a primary tumour was identified on the PET/CT scan tended to have a lower SUVmax. than those without chronic pancreatitis. However, note that only 20 of the 41 patients with chronic pancreatitis had an identified primary tumour with a measured SUVmax.
The SUVmax. level was a statistically significant indicator of the presence of chronic pancreatitis; however, the level of discrimination was relatively modest. The estimated AUC was 0.75 (95% CI 0.64 to 0.85) (Figure 8). The optimal cut-off point, if the aim is to minimise the sum of the error probabilities of the test, is 6.4, meaning that all tumours with a SUVmax. < 6.4 would lead to a positive diagnosis of chronic pancreatitis. Such a test has an estimated sensitivity of 85% and specificity of 60%. This cut-off value is also very similar to the optimal value for assessing PDAC, suggesting that an approach of assuming PDAC for a SUVmax. of > 6.3 and chronic pancreatitis for a SUVmax. of < 6.3 has some merit.
FIGURE 8.
Receiver operating characteristic curve for the diagnosis of chronic pancreatitis based on SUVmax.

Survival analysis
In total, 186 patients (among the 550 with a per-protocol PET/CT scan and a D4 diagnosis) were observed to die. Assuming non-informative dropout, estimated 6-month survival was 82.8% (95% CI 79.7% to 86.0%) whereas 12-month survival was 69.0% (95% CI 65.1% to 73.1%). Survival was substantially lower in patients with a confirmed diagnosis of PDAC, with 6-month survival being 71.4% (95% CI 66.0% to 77.2%) and 12-month survival being 50.9% (95% CI 44.9% to 57.6%) compared with 92.6% (95% CI 89.6% to 95.7%) and 84.9% (95% CI 80.8% to 89.2%), respectively, for patients without PDAC. Survival was lower in patients with any malignant disease than in patients with benign disease (Table 47 and Figures 9 and 10).
| Group | Estimated survival (95% CI) (%) | |
|---|---|---|
| 6 months | 12 months | |
| All patients | 82.8 (79.7 to 86.0) | 69.0 (65.1 to 73.1) |
| Pancreatic cancer | 71.4 (66.0 to 77.2) | 50.9 (44.9 to 57.6) |
| No pancreatic cancer | 92.6 (89.6 to 95.7) | 84.9 (80.8 to 89.2) |
| Malignant disease | 76.3 (72.1 to 80.7) | 57.7 (52.8 to 63.0) |
| Benign disease | 97.6 (95.3 to 100.0) | 94.5 (91.0 to 98.0) |
FIGURE 9.
Kaplan–Meier estimate of survival for all patients.

FIGURE 10.
Kaplan–Meier estimates of survival for patients with and without PDAC.
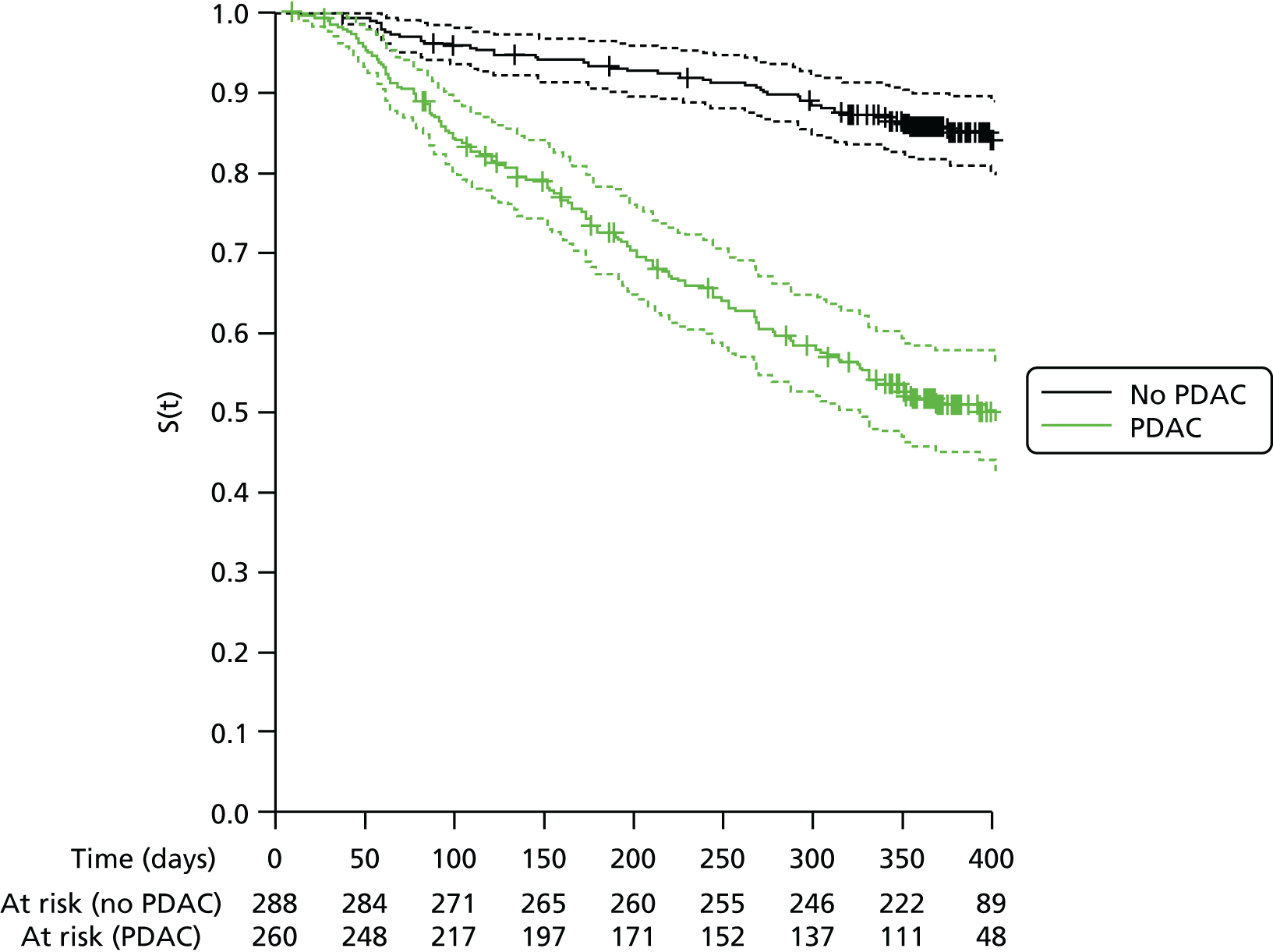
Survival by stage of disease
Among patients with a D4 diagnosis of PDAC, 95% had staging information at D2. Patients assessed to be in stage IA/IB at D2 or not assigned a stage had similar 12-month survival to those assessed to be in stage IIA or IIB (68.3% vs. 65.5%) (Table 48 and Figure 11). Patients in stages III and IV had poorer 12-month survival (44.8% and 21.0%, respectively).
| Stage (determined at D2) | No. of patients | 12-month survival (95% CI) (%) |
|---|---|---|
| IA/IB/no tumour identified | 87 | 68.3 (59.9 to 79.2) |
| IIA/IIB | 66 | 65.5 (54.5 to 78.7) |
| III | 29 | 44.8 (29.3 to 68.7) |
| IV | 79 | 21.0 (13.4 to 33.1) |
FIGURE 11.
Estimated survival among PDAC patients grouped by diagnosed stage at D2.

Effects of baseline variable on survival
Estimated HRs for a Cox proportional hazards model for survival in patients with pancreatic cancer are given in Table 49. Only WHO status at baseline was significantly associated with survival, with patients with a WHO status of 1 and a WHO status of 2 or 3 having significantly worse survival than those with a WHO status of 0.
| Variable | HR (95% CI) | p-value |
|---|---|---|
| WHO status 0 | 1 | 0.03 |
| WHO status 1 | 1.62 (1.09 to 2.40) | |
| WHO status 2/3 | 1.79 (1.01 to 3.17) | |
| Age (per 10 years) | 1.10 (0.91 to 1.31) | 0.32 |
| Diabetes (type 1 or 2) | 0.64 (0.37 to 1.10) | 0.11 |
| Female | 0.99 (0.69 to 1.43) | 0.96 |
| Never smoked | 1 | 0.94 |
| Past smoker | 1.01 (0.68 to 1.52) | |
| Current smoker | 1.10 (0.66 to 1.81) |
Chapter 4 Health economic analysis
Introduction
Positron emission tomography/computed tomography is widely used across the UK, although the economic case for its benefits over MDCT alone is scant. The aim of the health economics component of this study, using data from the Impact of combined modality positron emission tomography with computed tomography scanning (PET/CT) in the diagnosis and management of pancreatic cancer (PET-PANC) cohort, was to model the incremental cost-effectiveness of PET/CT compared with MDCT alone in the diagnosis and management of patients with pancreatic cancer. The PET-PANC study was not a randomised controlled trial but a cohort study, with the aim of collecting information on how PET/CT affects the diagnosis and management of patients and HRQoL data, which were previously unavailable. The key point of our analysis was whether PET/CT changed the diagnosis and management of patients over and above how they would have been managed on the basis of MDCT alone. To date, there have been few published studies internationally that could inform the modelling of PET/CT in the diagnosis and management of patients with pancreatic cancer in a UK setting. This was the challenge for the PET-PANC health economists based at the Centre for Health Economics and Medicines Evaluation in Bangor.
Existing economic evidence
Evidence on the cost-effectiveness of PET/CT in oncology generally is mixed but largely skewed towards the procedure not being cost-effective. Some sensitivity analysis brings the incremental cost-effectiveness ratio (ICER) below National Institute for Health and Care Excellence (NICE) thresholds of £20,000–30,000 per quality-adjusted life-year (QALY), but largely it is found not to be cost-effective in a UK context. This seems to depend on the type of cancer screened for and the availability of reliable data. More research is needed as and when reliable data become available.
A rapid review electronic database search for relevant economic evaluations concerning the use of PET/CT imaging in oncology was undertaken, with searches undertaken in The Cochrane Library [Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA) database and NHS Economic Evaluation Database (NHS EED)], EMBASE, PubMed and Web of Science. Studies were eligible for inclusion if they included full health economic evaluations (cost-effectiveness, cost–utility, cost–benefit analyses) comparing PET/CT (i.e. integrated PET and MDCT scan) with different invasive and non-invasive diagnostic strategies in the clinical work-up of cancer patients. Databases were searched between March 2010 and May 2012 (see Appendix 12 for an example of the search strategies).
Our inclusion criteria identified four studies66–69 (see Appendix 6). Two of these were from the UK,68,69 one was from Denmark66 and one was from Germany. 67 One study was a cost-effectiveness analysis,66 one was a cost-effectiveness and cost–utility analysis67 and two were cost–utility analyses. 68,69 Two studies used decision-analytical models68,69 and the other two used Cox proportional hazards and generated cost-effectiveness acceptability curves (CEACs). 66,67 Cost-effectiveness was measured in terms of cost per avoided thoracotomy66 and cost per QALY. 67–69 The evidence on the cost-effectiveness of PET/CT technology in oncology was mixed. Cost-effectiveness varied according to the type of cancer screened for and varied further with the different assumptions made about the relevant costs and benefits.
Methods
Outcome measures
The outcome measure for the PET-PANC economic analysis was the QALY. Both NICE and the National Coordinating Centre for Health Technology Assessment (NCCHTA) support the use of QALYs as an outcome measure in technology assessment. 70,71 QALYs are an index of health gain, combining survival and HRQoL. 72 We calculated QALYs using the AUC approach by weighting survival by HRQoL weights (HRQoL utilities values) over the 12-month follow-up period of the PET-PANC study. When possible, the AUC was calculated in four parts representing each 3-month follow-up period. For each participant the area under the HRQoL curve gives the total QALYs. HRQoL utilities were generated using European Quality of Life-5 Dimensions three-level version (EQ-5D-3L)73 data (n = 452) collected in the PET-PANC study. The EQ-5D is a validated generic health-related preference-based measure developed by the European Quality of Life group. The EQ-5D provides a single index (utility) value for health status for each patient. In our model, we calculated the difference in mean QALYs associated with the change in patient management resulting from the use of PET/CT (over and above those for MDCT alone).
Life-years gained
When patients were alive at 12 months post baseline, life-days were assumed as 365; otherwise, life-days were calculated by subtracting date of death from date of entry into the study. Life-years were calculated as life-days divided by 365.
Resource use and costs in secondary and primary care
In the PET-PANC study we measured costs and outcomes from a NHS perspective. We recorded the type and frequency of patient contacts with NHS secondary and primary care. These included investigations, treatment and palliation and other elements of secondary, primary and pharmaceutical care. Within cancer care, we focused on the costs of PET/CT, surgery, chemotherapy, radiotherapy, other drugs, outpatient appointments and day care and inpatient stays. We collected this information as part of the case report form (CRF). We took special care to check and maintain the quality of these data. This led to a complete set of secondary and primary care use data for the 279 participants for whom cost data were available for use in our economic model.
Sources of unit costs
We drew costs from a number of sources including the Unit Costs of Health and Social Care 201374 and NHS reference costs for 2012–13. 75 The Prescription Cost Analysis (PCA) 201376 was used for costing drugs. Estimates of the costs of PET/CT were obtained from NHS reference costs. All costs are in 2012–13 pounds sterling unless stated otherwise. A table referencing unit costs for each resource use category for secondary and primary care is provided in Appendix 7.
Missing data
No formal imputation was carried out on missing data; however, assumptions were made following the death of a patient. For patients who died during the course of the study, the follow-up period in which death occurred was calculated based on each follow-up period being 365/4 days long. EQ-5D utility data were assumed to be equal to zero from the end of the follow-up period in which the patient died. No assumption was made about costs for the follow-up period during which the patient died; however, costs for subsequent follow-ups were assumed to be zero.
Cost-effectiveness analysis
Our cost-effectiveness analysis was in three parts: model 1, in which we calculated the marginal cost per additional correct diagnosis of PDAC using PET/CT and MDCT alone; model 2, in which we calculated the budget impact of use of PET/CT; and model 3, in which we modelled the change in management of patients as a result of the use of PET/CT in diagnosis. All health economic modelling was performed in Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA).
Model 1: marginal costs of using positron emission tomography/computed tomography per additional correct diagnosis
Our analysis presents a model in which we calculated the marginal cost per additional correct diagnosis of PDAC using PET/CT and MDCT alone using national average unit costs for PET/CT and MDCT scans. 75 Primary analyses consider the diagnosis of PDAC in the per-protocol (within-range uptake and blood glucose) cohort, whereas subgroup analyses consider the impact of PET/CT in the diagnoses of chronic pancreatitis and malignancy. This analysis was based purely on the cost of PET/CT diagnostics compared with the cost of MDCT alone. These analyses extend only to the point of diagnosis and exclude any costs beyond the initial scan pertaining to the ongoing care and management of the patient.
Model 2: budget impact of use of positron emission tomography/computed tomography
We calculated the wider budget impact for commissioners for various scales of use of PET/CT. Based on the results from model 1 and published figures for PDAC prevalence (8773 new diagnoses in 2011) [see www.cancerresearchuk.org/cancer-info/cancerstats/ (accessed 24 May 2016)], we estimated the annual cost of routine use of PET/CT and the number of additional correct diagnoses, based on a range of percentages of patients with access to the service. As with model 1, we note that these analyses extend only to the point of diagnosis.
Model 3: change in management of patients as a result of use of positron emission tomography/computed tomography in diagnosis – incremental decision model
For each management strategy (e.g. resection, chemotherapy), patients in the PET-PANC study were prescribed either strategy X or strategy no X following CT scanning at time point D1. In some cases this management strategy was changed following PET/CT scanning (D2). Figure 12 presents a schematic of this change for the management strategy of resection, where a, b, c and d represent the numbers of patients following each branch of the schematic.
FIGURE 12.
Study schematic (example of resection).

We mapped the data from diagnoses at D1 and D2 into two arms of a decision model, representing hypothetical management if diagnosis was via CT alone or PET/CT. This is represented in Figure 13. To assess the cost-effectiveness of the addition of PET/CT, only incremental costs and effects require consideration. From Figure 13 it can be seen that these incremental effects are represented entirely by the patients who experienced a change of management following D2.
FIGURE 13.
Decision-analytical model (example of resection).

The impact of change in management strategies on costs, QALYs and life-years was modelled with regression models using data gathered in the PET-PANC study. Regression inputs reflect the categories of data collected on the CRF. To account for multiple treatments, QALYs and life-years gained (LYG) were modelled as decrements (1 – QALY) and (1 – LYG) and combined additively. Costs were also combined additively. When patients were moved from resection to no resection, this change indicated that the cancer had progressed beyond a state where resection would be beneficial, and bypass surgery or an open and shut laparotomy may have been performed. To adjust for this in the primary model, resection data were entered into the regression model as ‘resection: no metastases’ and ‘resection: with metastases’, the latter reflecting the case in which resection was not the optimal management.
In-house data indicated that bypass surgery is associated with increased mortality and a longer recovery phase than resection. A structural sensitivity analysis adjusted for this. Patients who were indicated a change of ‘stop resection’ were clinically assessed based on D3 and D4 and categorised whether the planned surgery would have been a bypass, open and shut laparotomy or resection, with costs and utilities adjusted accordingly in the model. Cost and utility adjustments for bypass and open and shut laparotomy were extracted from the study by a further breakdown of patient notes in the ‘resection’ and ‘drainage procedure’ categories of the CRF. For this model, ‘resection’ was entered as a single variable in the regression to avoid double counting.
Cost-effectiveness was assessed according to the ICER for the intervention, calculated as:
An intervention with a lower ICER is deemed to be more cost-effective than one with a higher ICER. NICE considers an ICER of < £20,000–30,000 per QALY to mean that a health technology represents good value for money for the NHS in the UK.
Primary analysis was based on QALYs for the within-range cohort of patients (n = 550). As there are varying costs for PET/CT by department (nuclear medicine, clinical oncology or medical oncology), sensitivity analysis was carried out using alternative costing assumptions. Analyses are also presented for LYG. Secondary subgroup analyses were also carried out on all patients (n = 583), patients with a pancreatic cancer diagnosis at D1 (n = 316) and patients with a pancreatic cancer diagnosis at D1 who were suitable for resection (n = 217). For all models to retain power, costs, QALYs and LYG were based on those for the within-range patients. For the ‘within-range’ and ‘all patient’ groups, the cost of PET/CT was presumed to be instead of the cost of MDCT. For the PDAC and PDAC with resection groups, the cost of PET/CT was presumed to be in addition to the cost of MDCT.
A probabilistic sensitivity analysis (PSA) assessing the simultaneous uncertainty of model parameters is more informative than univariate deterministic sensitivity analysis. Correlation between regression coefficients is reflected in the PSA by use of Cholesky decomposition on the covariance matrix. Uncertainty in the cost of the PET/CT scan was represented by a normal distribution, with an assumed standard deviation of £50 to reflect uncertainty. For the PSA, when no patients experienced a given change in management, 0.1 patients were modelled as experiencing the change, to generate a distribution. This is to reflect changes in management that may occur but did not necessarily occur in the PET-PANC study. PSA was accomplished by drawing iteratively (10,000 times) from the generated distributions. CIs for incremental costs and QALYs were generated from the bootstrap simulations. A CEAC was constructed to depict the probability of the intervention being cost-effective for a given cost-effectiveness or payer threshold.
Results
Quality-adjusted-life-years
Figure 14 shows the progression of the EQ-5D data over time based on diagnosis. Health state is seen to be worse for patients with PDAC, double cancer (pancreatic cancer plus another primary cancer) and malignant disease than for patients with benign cysts. We note that the numbers with double cancer are small (n = 15, 7, 9, 8 and 10 at the respective time points) and so we interpret these results, in particular the ‘peak’ at 3 months, with extreme caution. For all other diagnoses and time points, n > 40. These progressions are in line with what would be expected in the different disease areas and support the conclusion that patients within the study are representative of the wider population.
FIGURE 14.
European Quality of Life-5 Dimensions utility data over time by disease status.

Table 50 shows the QALY breakdown by age as calculated by the EQ-5D AUC. It seems that younger people have higher QALYs; however, care must be taken when interpreting these data as the numbers of younger people in the sample are very low.
| Age (years) | EQ-5D AUC (QALYs) | ||
|---|---|---|---|
| n | Mean | Standard deviation | |
| All | 452 | 0.554 | 0.30 |
| < 25 | 2 | 0.656 | 0.23 |
| 25–34 | 3 | 0.813 | 0.15 |
| 35–44 | 17 | 0.526 | 0.26 |
| 45–54 | 62 | 0.540 | 0.28 |
| 55–64 | 108 | 0.554 | 0.30 |
| 65–74 | 165 | 0.565 | 0.31 |
| 75+ | 95 | 0.539 | 0.31 |
Unit costs
In a UK NHS setting the cost of a standard CT scan as required to diagnose pancreatic cancer is £86 (lower quartile £79, upper quartile £95). 75 The corresponding cost for a PET/CT scan is £795 (lower quartile £793, upper quartile £795). This cost was sourced from nuclear medicine service costs and was the most conservative estimate (most costly). Costs pertaining to the different service descriptions are summarised in Table 51.
| Service | Point estimate (£) | Lower quartile (£) | Upper quartile (£) | |
|---|---|---|---|---|
| Nuclear medicine | CT | 86 | 79 | 95 |
| PET/CT | 795 | 793 | 795 | |
| Clinical oncology | CT | 121 | 84 | 162 |
| PET/CT | 563 | 92 | 793 | |
| Medical oncology | CT | 136 | 84 | 174 |
| PET/CT | 602 | 547 | 793 | |
A cost of £709 can therefore be derived as the additional cost per patient of scanning with PET/CT in place of scanning with CT alone. If PET/CT is to be used for diagnosis subsequent to CT, then the additional cost per patient is equivalent to the cost of the PET/CT scan, that is, £795. For all analyses, when PET/CT and CT costs are considered alongside each other, both costs are taken from the same departmental source for consistency.
Comparing the PET/CT lower quartile with the CT upper quartile gives a lower bound on the marginal cost of £698. Comparing the PET/CT upper quartile with the CT lower quartile gives an upper bound on the marginal cost of £714.
Frequency of resource use
The mean frequency of use of primary and secondary care services over the 12 months of the study is shown in Table 52. The table in Appendix 7 provides the 3-monthly breakdown showing the number of patients in each follow-up period. It can be seen that the most frequent resource use in primary care is for community nurses, followed by practice nurses and general practitioners (GPs). For secondary care the most frequent resource use is for outpatient visits.
| Resource use category | Mean frequency of service use over 12 months |
|---|---|
| Primary care | |
| Cancer nurse | 0.44 |
| GP | 0.47 |
| Practice nurse (GP clinic) | 0.56 |
| Community nurse | 0.72 |
| Health visitor | 0.00 |
| Psychologist | 0.01 |
| Counsellor | 0.02 |
| Physiotherapist | 0.05 |
| Occupational health therapist | 0.01 |
| Care manager | 0.04 |
| Social worker | 0.01 |
| Home care worker | 0.16 |
| Care attendant | 0.02 |
| Carer’s support worker | 0.01 |
| Chiropodist | 0.03 |
| Dietitian | 0.04 |
| Self-help group | 0.00 |
| Other | 0.22 |
| Secondary care | |
| Oncology inpatient ward (bed-days) | 0.67 |
| Medical inpatient ward (bed-days) | 1.43 |
| Continuing care/respite inpatient ward (bed-days) | 0.05 |
| Assessment/rehabilitation inpatient ward (bed-days) | 0.13 |
| Other inpatient ward (bed-days) | 1.08 |
| Intensive care inpatient ward (bed-days) | 0.10 |
| Inpatient consultations (including PAMs) (bed-days) | 0.32 |
| Outpatient visits (including consultations) (attendance) | 3.00 |
| Accident and emergency (attendance) | 0.27 |
| Day hospital (attendance) | 0.39 |
Costs over the 12-month study period
Table 53 shows the sources of unit costs used in the economic model. Appendix 8 provides further information on resource use data and unit costs.
| Item of resource use | Source of resource usea | Source of unit cost |
|---|---|---|
| Primary care | ||
| Cancer nurse | CSRI | Unit Costs of Health and Social Care 2013 74 |
| GP | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Practice nurse (GP clinic) | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Community nurse | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Health visitor | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Psychologist | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Counsellor | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Physiotherapist | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Carer’s support worker | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Occupational health therapist | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Care manager | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Social worker | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Home care worker | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Care attendant | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Chiropodist | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Dietitian | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Self-help group | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Other | CSRI | Unit Costs of Health and Social Care 2013 74 |
| Secondary care | ||
| Oncology inpatient ward | CSRI | Department of Health75 |
| Medical inpatient ward | CSRI | Department of Health75 |
| Continuing care/respite inpatient ward | CSRI | www.stlukes-hospice.co.uk/about-us/financial-breakdown/ (accessed December 2014) |
| Assessment/rehabilitation inpatient ward | CSRI | Department of Health75 |
| Other inpatient ward | CSRI | Department of Health75 |
| Intensive care inpatient ward | CSRI | Department of Health75 |
| Inpatient consultations (including PAMs) | CSRI | Department of Health75 |
| Outpatient visits (including consultations) | CSRI | Department of Health75 |
| Accident and emergency | CSRI | Department of Health75 |
| Day hospital | CSRI | Department of Health75 |
| Other | CSRI | Department of Health75 |
The resultant mean total costs of service use by patients in the PET-PANC cohort are shown in Table 54, with the full table including the 3-month breakdown provided in Appendix 10. These costs are in UK pounds sterling for the year 2012–13. It can be seen that for primary care the highest cost was for GPs, followed by community nurses and practice nurses. For secondary care the highest cost was for inpatient stays.
| Resource use category | Mean cost (£) |
|---|---|
| Primary care | |
| Cancer nurse | 13.21 |
| GP | 77.89 |
| Practice nurse (GP clinic) | 26.22 |
| Community nurse | 49.48 |
| Health visitor | 0.34 |
| Psychologist | 0.58 |
| Counsellor | 1.04 |
| Physiotherapist | 1.89 |
| Occupational health therapist | 0.33 |
| Care manager | 1.66 |
| Social worker | 0.69 |
| Home care worker | 3.27 |
| Care attendant | 0.67 |
| Carer’s support worker | 0.19 |
| Chiropodist | 0.78 |
| Dietitian | 1.26 |
| Self-help group | 0.03 |
| Other | 12.79 |
| Secondary care | |
| Oncology inpatient ward | 433.53 |
| Medical inpatient ward | 1015.59 |
| Continuing care/respite inpatient ward | 21.13 |
| Assessment/rehabilitation inpatient ward | 46.04 |
| Other inpatient ward | 589.37 |
| Intensive care inpatient ward | 149.05 |
| Inpatient consultations (including PAMs) | 80.04 |
| Outpatient visits (including consultations) | 444.14 |
| Accident and emergency | 41.73 |
| Day hospital | 77.53 |
| Other | 274.53 |
Cost-effectiveness analysis
Model 1: marginal costs of using positron emission tomography/computed tomography for diagnosis
All patients (intention to treat)
The PET-PANC study has shown that CT (D1) had a sensitivity of 87.4% and a specificity of 69.8%, with a diagnostic accuracy of 78.2% for the 583 ITT patients. PET/CT (D2) had a sensitivity of 90.3% and a specificity of 76.1%, with an overall diagnostic accuracy of 82.8%. The increased accuracy corresponds to an additional 27 correct diagnoses, corresponding to an increment of 5.9% (100 × 27/456) in accuracy rate.
The incremental cost per additional accurate diagnosis was £15,309 (955 CI £15,072 to £15,460), corresponding to £69,810 (95% CI £68,727 to £70,499) per additional percentage gained in diagnostic accuracy rate.
All in-range patients (per protocol)
In the per-protocol analysis, CT alone had a sensitivity of 88.5% and a specificity of 70.6%, with a diagnostic accuracy of 79.1%. PET/CT had a sensitivity of 92.7% and a specificity of 75.8%, with an overall diagnostic accuracy of 83.8%. The increased accuracy corresponds to an additional 26 correct diagnoses out of 550 patients, corresponding to an increment of 6.0% in accuracy rate.
For the per-protocol cohort, the incremental cost per additional accurate diagnosis was £14,998 (95% CI £14,765 to £15,146), corresponding to £65,242 (95% CI £64,229 to £65,886) per additional percentage gained in diagnostic accuracy rate.
Chronic pancreatitis
Considering chronic pancreatitis, CT alone had a diagnostic accuracy of 93.8% whereas PET/CT had a diagnostic accuracy of 94.5%. An additional four patients in 550 were accurately diagnosed with PET/CT, with an increase in diagnostic accuracy rate of 0.775%. This corresponds to an incremental cost per additional accurate diagnosis of £97,488 (95% CI £95,975 to £98,450) or a cost of £503,035 (95% CI £495,231 to £508,002) per additional percentage gained in diagnostic accuracy rate.
Malignant compared with benign disease
Considering malignant compared with benign disease, CT alone had a diagnostic accuracy of 82.2% whereas PET/CT had a diagnostic accuracy of 88.9%. An additional 37 patients in 550 were diagnosed correctly with PET/CT, with an increase in diagnostic accuracy rate of 8.2%. This corresponds to an incremental cost per additional accurate diagnosis of £10,539 (95% CI £10,376 to £10,643) or a cost of £47,637 (95% CI £46,898 to £48,107) per additional percentage gained in diagnostic accuracy rate.
Malignant cystic neoplasm
Considering malignant cystic neoplasm, CT alone had a diagnostic accuracy of 92.8% whereas PET/CT had a diagnostic accuracy of 95.8%. An additional 18 patients in 550 were diagnosed correctly with PET/CT, with an increase in diagnostic accuracy rate of 3.5%. This corresponds to an incremental cost per additional accurate diagnosis of £21,664 (95% CI £21,328 to £21,878) or a cost of £110,269 (95% CI £108,558 to £111,358) per additional percentage gained in diagnostic accuracy rate.
These analyses extend only to the point of diagnosis and exclude any costs beyond the initial scan pertaining to the ongoing care and management of patients. To explore beyond this point, capturing the costs and effects of differences in changes of management, a more complex economic model is required.
Model 2: budget impact of use of positron emission tomography/computed tomography
We calculated the wider budget impact for commissioners for various scales of use of PET/CT.
In the UK in 2011 the incidence of pancreatic cancer was 9.7 per 10,000, corresponding to 8773 new diagnoses per year, making pancreatic cancer the 10th most common cancer in the UK (see www.cancerresearchuk.org/cancer-info/cancerstats/).
Table 55 shows the breakdown of costs to the NHS and additional diagnoses based on 10%, 25%, 50% and 100% of these patients theoretically having had access to PET/CT.
| Patients with access to PET/CT (%) | Additional cost (£), mean (LH, HL) | Additional accurate diagnoses, n | |||
|---|---|---|---|---|---|
| PDAC | Chronic pancreatitis | Malignancy | Malignant cystic neoplasm | ||
| 10 | 622,006 (612,355, 628,147) | 41 | 6 | 59 | 29 |
| 25 | 1,555,014 (1,530,889, 1,570,367) | 102 | 16 | 148 | 72 |
| 50 | 3,110,029 (3,061,777, 3,140,734) | 203 | 32 | 295 | 144 |
| 100 | 6,220,057 (6,123,554, 6,281,468) | 406 | 64 | 590 | 287 |
Model 3: modelling the change in management of patients as a result of use of positron emission tomography/computed tomography in diagnosis
Model inputs
Incremental costs and outcomes were generated for the per-protocol population using a regression analysis. The results of these regressions are presented for the primary model in Table 56 and for the structural sensitivity analysis in Table 57. It should be noted that in many cases patients would experience more than one management strategy. Negative coefficients for incremental costs for neoadjuvant chemotherapy, further investigations, biopsy and follow-up do not reflect negative costs but suggest that patients following these pathways generate less costs than average. Likewise, negative coefficients for QALY decrements for adjuvant chemotherapy, resection (no metastases) and further investigations do not indicate a QALY gain but a reduced QALY decrement compared with the average patient. Note that the coefficient for resection (no metastases) is negative whereas the coefficient for resection (with metastases) is positive, indicating a larger QALY loss. In Table 57, resection is treated as a single input regardless of metastases. This is because there is some overlap in the recording of some resection and bypass patients and we wished to avoid any double counting of increased costs and QALY decrements associated with bypass surgery. The patients entered into the regression as ‘resection’ in this case were those who had only a resection procedure and not a bypass.
| Management | Incremental costs (n = 255) | QALY decrements (n = 421) | Life-year decrements (n = 550) | |||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Neoadjuvant chemotherapy | –3 | 7807 | 0.0276 | 0.1606 | –0.0698 | 0.1000 |
| Adjuvant chemotherapy | 4980.71 | 2568 | –0.0686 | 0.0410 | –0.0477 | 0.0351 |
| Palliative chemotherapy | 4199.50 | 2500 | 0.1121 | 0.0386 | 0.1236 | 0.0315 |
| Resection (no metastases) | 5090 | 2313 | –0.0662 | 0.0373 | –0.0319 | 0.0306 |
| Resection (with metastases) | 15645 | 9521 | 0.1477 | 0.1237 | 0.1537 | 0.1153 |
| Further investigations | –1207 | 2070 | –0.0636 | 0.0369 | –0.0776 | 0.0298 |
| Biopsy | –1652 | 2018 | 0.0273 | 0.0339 | 0.0245 | 0.0274 |
| Drainage procedure | 4361 | 2721 | 0.0926 | 0.0449 | 0.0468 | 0.0381 |
| Best supportive care | 1321 | 2971 | 0.2693 | 0.0470 | 0.3262 | 0.0399 |
| Follow-up, no further investigations | –5618 | 7629 | 0.0053 | 0.1223 | –0.1006 | 0.1144 |
| Other | 3739 | 2209 | 0.0037 | 0.0321 | 0.0123 | 0.0260 |
| Constant | 8337 | 228 | 0.4230 | 0.0374 | 0.1171 | 0.0305 |
| Management | Incremental costs (n = 255) | QALY decrements (n = 421) | Life-year decrements (n = 550) | |||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Neoadjuvant chemotherapy | –99 | 7811 | 0.0305 | 0.1608 | –0.0684 | 0.1001 |
| Adjuvant chemotherapy | 5135 | 2566 | –0.0709 | 0.0415 | –0.0491 | 0.0352 |
| Palliative chemotherapy | 4120 | 2501 | 0.1154 | 0.0393 | 0.1256 | 0.0315 |
| Resection | 5135 | 2314 | –0.0590 | 0.0396 | –0.0269 | 0.0305 |
| Further investigations | –1324 | 2068 | –0.0613 | 0.0372 | –0.0762 | 0.0299 |
| Biopsy | –1693 | 2019 | 0.0261 | 0.0343 | 0.0237 | 0.0275 |
| Drainage procedure | 4313 | 2722 | 0.0904 | 0.0521 | 0.0450 | 0.0381 |
| Best supportive care | 1278 | 2972 | 0.2703 | 0.0472 | 0.3269 | 0.0400 |
| Follow-up, no further investigations | –5809 | 7632 | 0.0041 | 0.1224 | –0.1015 | 0.1146 |
| Other | 3672 | 2210 | 0.0305 | 0.1608 | 0.0131 | 0.0260 |
| Constant | 8461 | 2283 | 0.4213 | 0.0375 | 0.1160 | 0.0306 |
Table 58 shows the adjustments in costs and utility for the structural sensitivity analysis based on data from patients who underwent bypass or open and shut laparotomy in the PET-PANC study. As there were no cost observations for open and shut laparotomy, no adjustment was made and the cost of care in this instance was treated as equivalent to that for resection. Note that these values represent the total costs and utilities of all treatments for these patients as we were interested in the differences between resection and bypass and between resection and open and shut laparotomy. The differences are listed in the third column (‘Adjustment from resection’); these were then applied to the ‘resection’ coefficients from Table 57 to calculate the values used in the model, which are presented in the final column of Table 58.
| Outcome measure | Point estimate | Adjustment from resection | Values used in the model | ||||
|---|---|---|---|---|---|---|---|
| Resection (n) | Open and shut laparotomy (n) | Bypass (n) | Open and shut laparotomy | Bypass | Open and shut laparotomy | Bypass | |
| Cost (£) | 15,863 (115) | No observations | 16,697 (13) | 0 | 834 | 5135 | 5969 |
| QALY decrement | 0.3432 (187) | 0.8141 (3) | 0.5370 (19) | 0.4708 | 0.1938 | 0.4118 | 0.1348 |
| Life-year decrement | 0.0769 (232) | 0.3486 (4) | 0.2689 (22) | 0.2718 | 0.1920 | 0.2449 | 0.1651 |
Table 59 presents the change in management model inputs for the per-protocol primary analysis (column 2) and subgroup analyses of all patients (column 3), patients diagnosed with PDAC (column 4) and patients diagnosed with PDAC for whom resection was the indicated treatment (column 5). Subgroup analyses related to the 550 within-range patients. The model assumes that the only changes in management are those deemed to be attributable to PET and that all costs and utilities not relating to a change of management remain the same. Table 60 shows the change in management model inputs for the structural sensitivity analysis, in which clinical opinion was used to judge whether patients would have likely undergone a resection, bypass or open and shut laparotomy following the D1 diagnosis.
| Change | Within range (N = 550), n | All patients (N = 583), n | PDAC (N = 316 of 550), n | PDAC and resection (N = 217 of 550), n |
|---|---|---|---|---|
| Stop resection | 59 | 64 | 35 | 35 |
| Start resection | 20 | 22 | 8 | 8 |
| Stop palliative chemotherapy | 1 | 1 | 0 | 0 |
| Start palliative chemotherapy | 27 | 28 | 23 | 21 |
| Stop neoadjuvant chemotherapy | 1 | 1 | 1 | 1 |
| Start further investigations | 28 | 29 | 14 | 11 |
| Start best supportive care | 1 | 1 | 1 | 1 |
| Change | Within range (N = 550), n | All patients (N = 583), n | PDAC (N = 316 of 550), n | PDAC and resection (N = 217 of 550), n |
|---|---|---|---|---|
| Stop open and shut laparotomy | 2 | 3 | 1 | 1 |
| Stop bypass | 37 | 39 | 26 | 26 |
| Stop resection | 20 | 22 | 8 | 8 |
| Start resection | 20 | 22 | 8 | 8 |
| Stop palliative chemotherapy | 1 | 1 | 0 | 0 |
| Start palliative chemotherapy | 27 | 28 | 23 | 21 |
| Stop neoadjuvant chemotherapy | 1 | 1 | 1 | 1 |
| Start further investigations | 28 | 29 | 14 | 11 |
| Start best supportive care | 1 | 1 | 1 | 1 |
The results from the basic change of management model are presented in Table 61. For within-range patients the incremental cost of PET/CT was estimated as being –£645 (95% CI –£2743 to £1314) for nuclear medicine and –£912 (95% CI –£2927 to £1045) for clinical oncology and the mean QALY gain associated with PET/CT was 0.0157 (95% CI –0.0101 to 0.0430). PET/CT dominates CT as PET/CT is both less costly and more effective. Similar results were seen for the subgroup analyses, with the lowest cost and highest QALY gain seen for the PDAC with resection group.
| Outcome measure | Within range | All patients | PDAC | PDAC and resection | ||||
|---|---|---|---|---|---|---|---|---|
| Nuclear medicine | Clinical oncology | Nuclear medicine | Clinical oncology | Nuclear medicine | Clinical oncology | Nuclear medicine | Clinical oncology | |
| Incremental costs (£) | –645 | –912 | –680 | –947 | –639 | –906 | 1275 | –1542 |
| Incremental QALYs | 0.0157 | 0.0163 | 0.0119 | 0.0175 | ||||
| Cost per QALY gained (£) | PET/CT dominates | |||||||
| Incremental life-years | 0.0150 | 0.0155 | 0.0110 | 0.0161 | ||||
| Cost per LYG (£) | PET/CT dominates | |||||||
The results of the model that considers bypass surgery or open and shut laparotomy are presented in Table 62. For within-range patients the incremental cost of PET/CT was estimated as being £419 (95% CI –£138 to £930) for nuclear medicine and £152 (95% CI –£399 to £688) for clinical oncology and the mean QALY gain associated with PET/CT was 0.0078 (95% CI 0.0012 to 0.0172). For nuclear medicine, the ICERs were £53,677 per QALY gained and £45,423 per LYG. Based on the upper end of the NICE threshold, £30,000 per QALY, these estimates suggest that PET/CT is not cost-effective. Although the ICER was lower for all patients, PET/CT was not cost-effective for any subgroup using nuclear medicine costs for PET/CT. Using clinical oncology costs within the model, for within-range patients the ICERs were £19,445 per QALY and £16,455 per LYG, which are borderline cost-effective at the lower NICE threshold of £20,000 per QALY and cost-effective at the upper end of the NICE threshold. The most cost-effective subgroup was again the PDAC with resection subgroup, with the ICER ranging from £4626 to £34,654.
| Outcome measure | Within range | All patients | PDAC | PDAC and resection | ||||
|---|---|---|---|---|---|---|---|---|
| Nuclear medicine | Clinical oncology | Nuclear medicine | Clinical oncology | Nuclear medicine | Clinical oncology | Nuclear medicine | Clinical oncology | |
| Incremental costs (£) | 419 | 152 | 411 | 144 | 447 | 180 | 308 | 41 |
| Incremental QALYs | 0.0078 | 0.0084 | 0.0060 | 0.0089 | ||||
| Cost per QALY gained (£) | 53,677 | 19,445 | 48,683 | 17,027 | 75,069 | 30,252 | 34,654 | 4626 |
| Incremental life-years | 0.0092 | 0.0096 | 0.0073 | 0.0108 | ||||
| Cost per LYG (£) | 45,423 | 16,455 | 42,786 | 14,964 | 60,947 | 24,561 | 28,556 | 3812 |
Probabilistic sensitivity analysis results
Probabilistic sensitivity analysis was performed as described in the methods section. The results of the 10,000 simulations for both nuclear medicine PET/CT costs and clinical oncology PET/CT costs for both model structures are plotted on cost-effectiveness planes in Figure 15. The planes illustrate the joint distributions of the incremental costs and QALYs. Blue and black lines indicate £20,000 per QALY and £30,000 per QALY thresholds, respectively. Points below the lines are considered cost-effective at the given threshold. An ‘×’ marks the point estimate of the ICER. For the conservative estimate associated with nuclear medicine in the primary model, 24% of simulations are in the north-east quadrant (higher cost, higher QALYs), 3% are in the north-west quadrant (higher cost, lower QALYs), 64% are in the south-east quadrant (lower cost, higher QALYs) and 9% are in the south-west quadrant (lower cost, lower QALYs). For clinical oncology costs in the primary model, 17% of simulations are in the north-east quadrant, 2% are in the north-west quadrant, 71% are in the south-east quadrant and 10% are in the south-west quadrant.
FIGURE 15.
Cost-effectiveness planes. (a) Nuclear medicine costs in the primary model; (b) clinical oncology costs in the primary model; (c) nuclear medicine costs in the bypass and open and shut laparotomy model structure; and (d) clinical oncology costs in the bypass and open and shut laparotomy model structure.
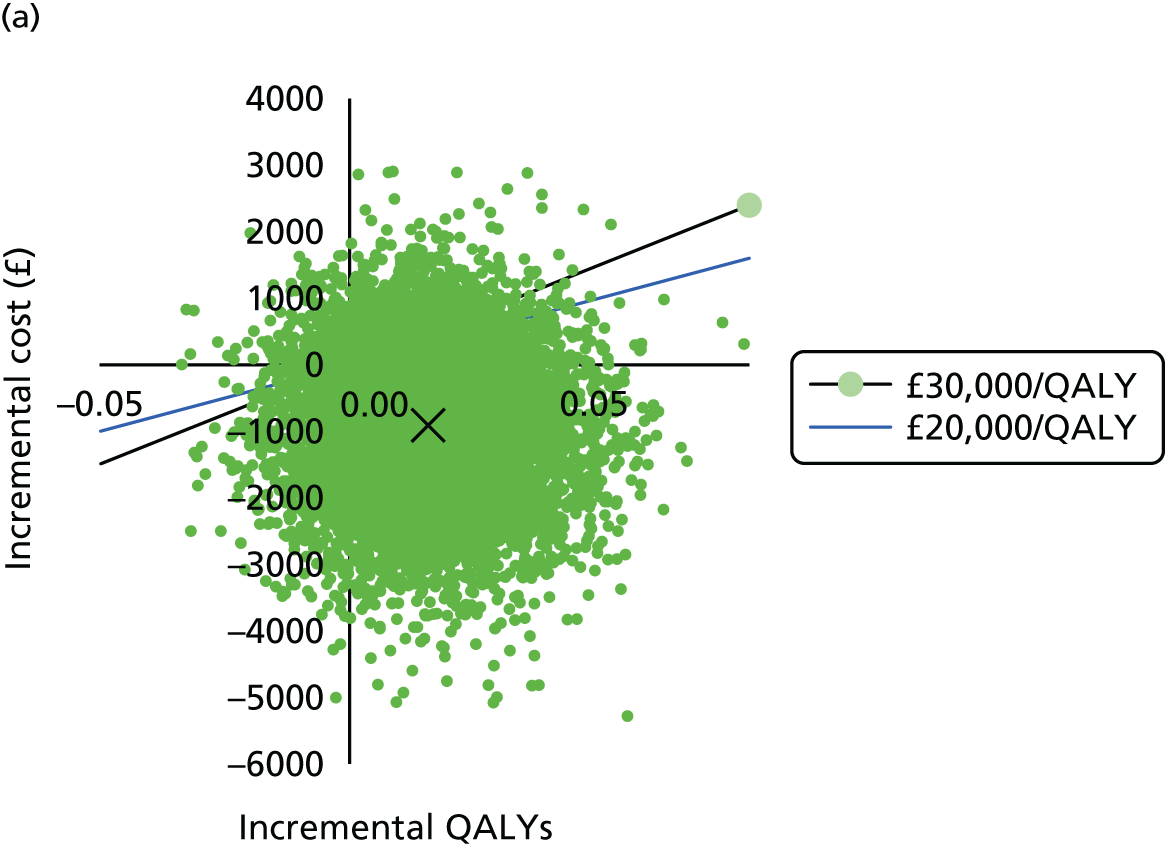

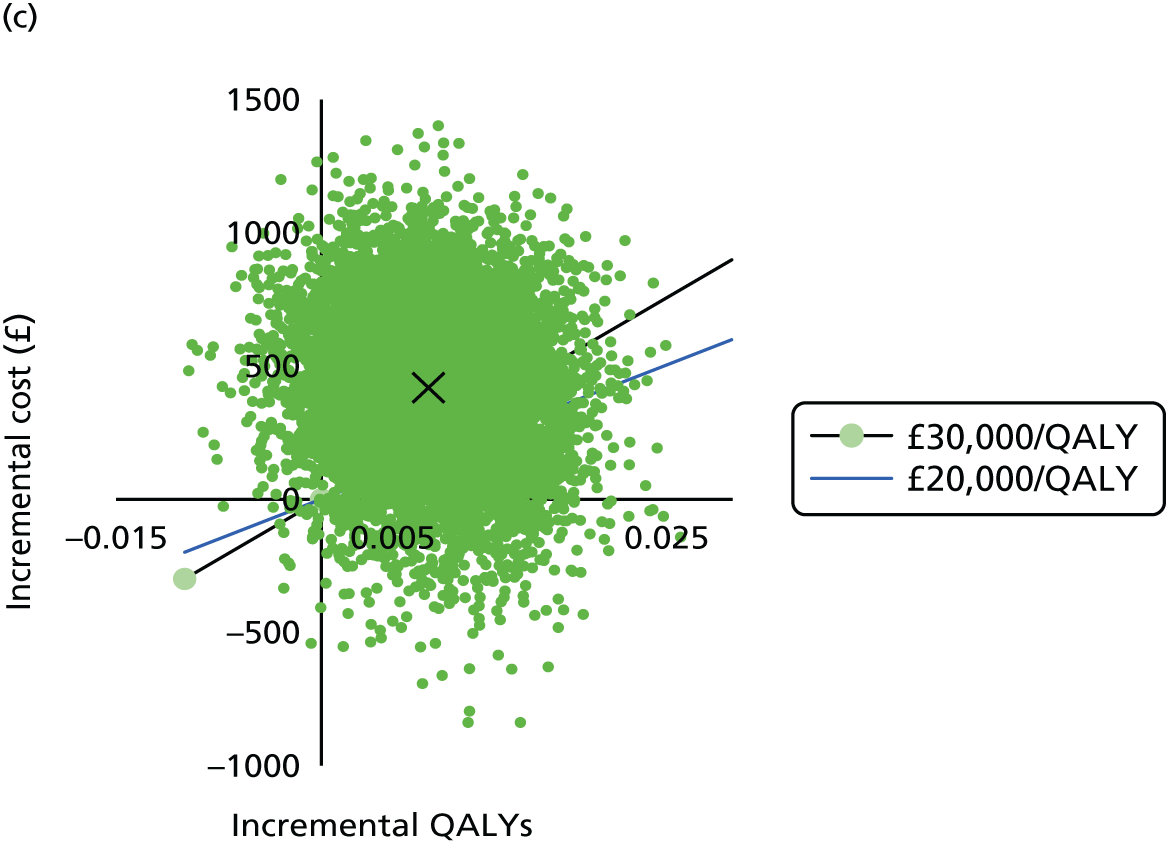

Modelling bypass and open and shut laparotomy associated with nuclear medicine costs, 90% of simulations are in the north-east quadrant, 4% are in the north-west quadrant, 6.19% are in the south-east quadrant and 0.3% are in the south-west quadrant. For clinical oncology costs, 68% of simulations are in the north-east quadrant, 4% are in the north-west quadrant, 27% are in the south-east quadrant and 1% are in the south-west quadrant.
Cost-effectiveness acceptability curves were generated, indicating the probability that PET/CT is cost-effective over a variety of cost-effectiveness thresholds. The CEACs are illustrated in Figure 16 and show that, for the primary model, for nuclear medicine costs the probability of being cost-effective at the £20,000 per QALY threshold is 82% and at the £30,000 per QALY threshold is 85%. For clinical oncology costs the probabilities are 88% at the £20,000 per QALY threshold and 90% at the £30,000 per QALY threshold; it is likely from this model that PET/CT would be cost-effective for the UK NHS. For the structural sensitivity analysis and conservative nuclear medicine costs, the probability of PET/CT being cost-effective at the £30,000 per QALY threshold is 28% and at the £20,000 per QALY threshold is 18%. At these costs it is unlikely that PET/CT would be cost-effective from the perspective of the UK NHS. Using clinical oncology as the source of PET/CT costs, the probability of PET/CT being cost-effective at the £30,000 per QALY threshold is 60% and at the £20,000 per QALY threshold is 50%. Based on these costs, it is likely that PET/CT would be cost-effective from the perspective of the UK NHS.
FIGURE 16.
Cost-effectiveness acceptability curves. (a) Nuclear medicine costs in the primary model; (b) clinical oncology costs in the primary model; (c) nuclear medicine costs in the bypass and open and shut laparotomy model structure; and (d) clinical oncology costs in the bypass and open and shut laparotomy model structure.

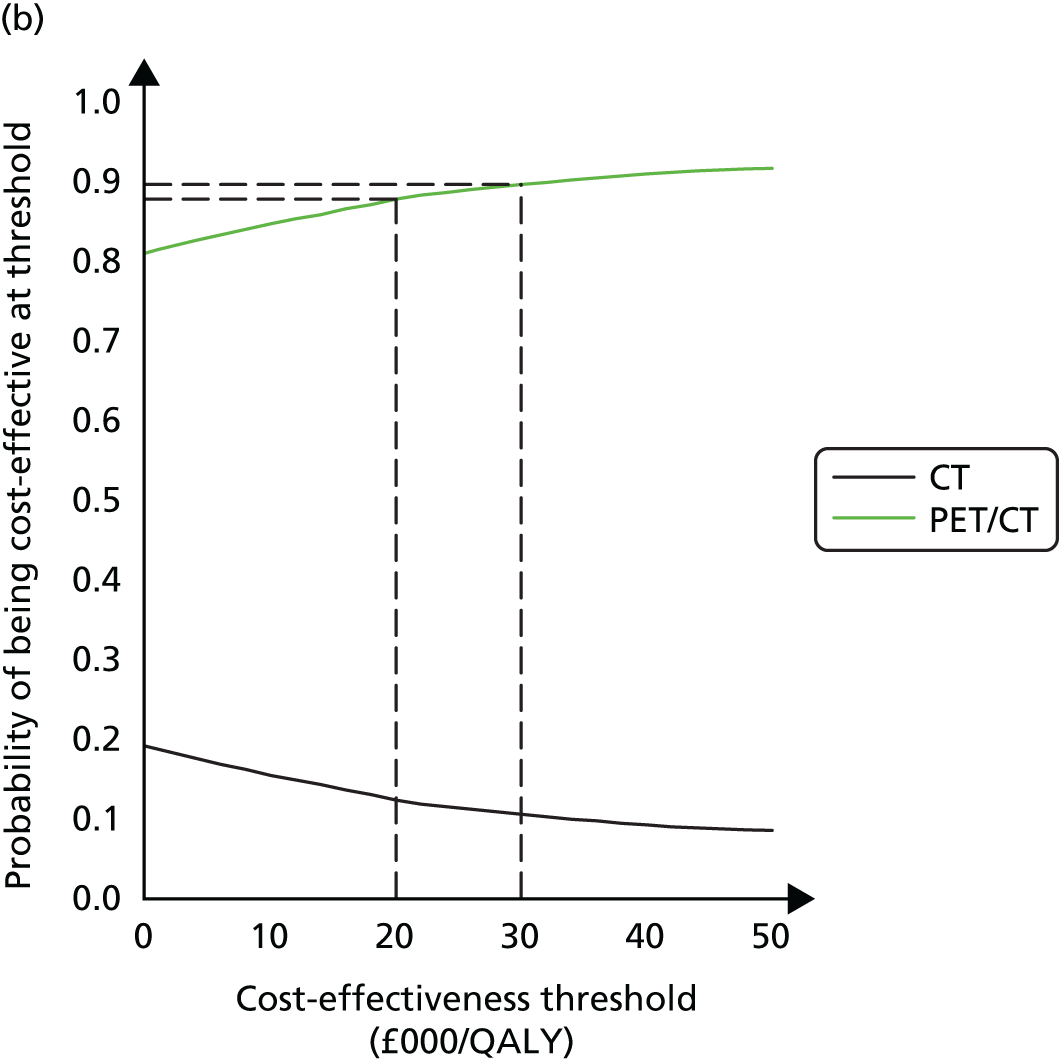


Summary
Model 3 (change of management model, primary model) indicates that PET/CT dominates management based on CT alone. This is largely because of the number of unnecessary surgeries avoided. The probability of cost-effectiveness at a threshold of £20,000 per QALY ranges from 82% to 90%, according to the source of PET/CT costs used. Our structural sensitivity analysis considers the likely prognoses of patients who undergo contraindicated resection surgery. The ICER ranges from £19,445 per QALY to £53,557 per QALY depending on the source of PET/CT costs used. The corresponding probabilities of being cost-effective range from 18% to 50% at the lower NICE threshold of £20,000 per QALY and from 28% to 60% at the upper NICE threshold of £30,000 per QALY. For both models, PET/CT is seen to be most cost-effective for the PDAC with resection subgroup. The ICER point estimate depends on the source of PET/CT costs used and it is a matter of judgement which is chosen for analysis. We chose the most conservative (and highest costs) for our base-case analysis.
Chapter 5 Discussion
Introduction
The accurate diagnosis and staging of pancreatic cancer is essential to ensure that the most appropriate management is planned for patients in a timely and efficient manner. The standard diagnostic pathway for pancreatic cancer relies on contrast-enhanced CT as the mainstay for diagnosis with the addition of MRI and EUS in patients with equivocal liver and pancreatic lesions, respectively. The role of PET or PET/CT in the diagnostic algorithm for pancreatic cancer has been under review for many years. The benefits in terms of diagnosis and management have been reported mainly in single-centre studies. The lack of multicentre prospective confirmatory studies has meant that widespread take-up of PET/CT as a standard diagnostic tool in pancreatic cancer diagnosis has not happened. The lack of cost-effectiveness data has also hampered the widespread use of PET/CT in health-care systems. The PET-PANC study is the first large-scale, multicentre diagnostic accuracy study of PET/CT in pancreatic cancer, which also modelled the cost-effectiveness of this approach.
Principal findings
Diagnostic accuracy and incremental benefit of positron emission tomography/computed tomography
Between 2011 and 2013, 583 patients with suspected pancreatic cancer from 18 UK sites underwent PET/CT scans, of whom 550 had PET/CT scans completed according to protocol. The primary outcome measure was diagnostic accuracy and incremental benefit of PET/CT for pancreatic cancer. Adherence to the PET/CT protocol was essential to ensure that scans were at an accepted level for quality assurance. This would mean that the results would be applicable across multiple sites. There were 33 scans that were out of range, because of either abnormal blood glucose levels or PET uptake times; therefore, analyses were undertaken on the whole population with PET/CT scans and the per-protocol population. Sensitivity and specificity for the ITT and per-protocol populations were 90.3% and 76.1% and 92.7% and 75.8%, respectively. The relative sensitivity and specificity of PET/CT compared with MDCT was significantly higher in the per-protocol group, confirming that PET/CT was more accurate than standard MDCT. For the whole population, only the relative sensitivity (not the specificity) of PET/CT compared with MDCT was significantly higher. This highlights how the PET/CT protocol can affect accuracy in terms of the specificity of PET/CT. Out-of-range scans in this study would lead to higher false-negative rates. Part of the reason for out-of-range scans was high blood glucose levels at the time of scanning. In this study 17% of patients were diabetic and this prevalence would very likely be replicated in pancreatic MDT populations across the country. Good diabetic control is therefore essential for accurate PET/CT scans in this population. Overall, these results confirm the significant superiority of PET/CT compared with MDCT in the diagnosis of pancreatic cancer.
The incremental diagnostic benefit of PET/CT in addition to MDCT was quantified using incremental likelihood ratios. These demonstrated that PET/CT significantly increased diagnostic accuracy in all scenarios. Following a positive diagnosis of pancreatic cancer on MDCT, a positive PET/CT scan increased the odds of pancreatic cancer by 55% and a negative PET/CT scan decreased the odds of pancreatic cancer by 95%. Following a negative diagnosis of pancreatic cancer on MDCT, a positive PET/CT scan increased the odds of pancreatic cancer by 538% and a negative PET/CT scan decreased the odds of pancreatic cancer by 46%.
Patients with no pancreatic cancer detected on PET/CT following a positive MDCT scan were unlikely to have pancreatic cancer (NPV of 89%). In contrast, although a positive diagnosis on PET/CT following a negative MDCT scan increased the odds of PDAC, the overall diagnosis was still highly uncertain (PPV of 50%). This latter result suggests that if both tests are performed there is benefit in combining the results rather than taking the PET/CT result alone. In particular, patients who were positive on MDCT and PET/CT were much more likely to have PDAC than patients who were positive only for PET/CT (PPV of 80% vs. 50%). The result of MDCT is less important if the PET/CT result is negative (NPV of 93% if both tests are negative vs. NPV of 89% if the PET/CT scan is negative after a positive MDCT scan). These results demonstrate that there is a substantial diagnostic benefit for patients of receiving a PET/CT scan after a potential pancreatic cancer is detected on MDCT.
We also assessed clinician certainty following PET/CT using both quantitative and qualitative measurements. With both methods there was a significant increase in clinicians’ perceived certainty of diagnosis after PET/CT (p = 0.0001). Clinicians were 12 times more likely to believe that they were more certain in their diagnosis than they were to be less certain. Assessing the accuracy of the clinicians’ perceived certainty also indicated that the clinicians’ predictions (interpreting their level of certainty as a probability) were significantly improved by the PET/CT scan result (p < 0.001). These results suggest that the use of PET/CT increases the level of certainty and the predictions of the clinicians, which may contribute to better planned management overall.
Impact of positron emission tomography/computed tomography on diagnosis, staging and management
The difference in diagnoses following MDCT and then PET/CT drives the relative sensitivity and specificity of PET/CT compared with MDCT. For pancreatic cancer diagnosis, there was a greater number of cases in which the PET/CT diagnosis was correct than in which the MDCT diagnosis was correct, indicating that PET/CT performs better. This was considered for patients with pancreatic cancer and patients with a diagnosis of malignancy. PET/CT changed the diagnosis correctly for diagnoses of both pancreatic cancer and not pancreatic cancer in 46 patients (8.3%). PET/CT incorrectly changed the diagnosis in 20 patients (3.6%). Considering the diagnosis of malignancy in general, PET/CT correctly changed the diagnosis in 48 patients (8.7%) and incorrectly changed the diagnosis in 11 patients (2%). Therefore, in terms of relative sensitivity and specificity, PET/CT has a significant impact on diagnosis compared with MDCT.
The accuracy of staging among patients with early-stage disease (lymph node-negative disease) was slightly worse under PET/CT than under MDCT, with more incorrect (eight patients) than correct (six patients) changes. However, the difference was not statistically significant (p = 0.79). Significantly more patients with stage IIB disease (lymph node-positive disease) were correctly staged by PET/CT than by MDCT, with 22 patients (21%) moving to the correct stage compared with five patients (5%) moving to the wrong stage (p = 0.002). PET/CT had no significant effect on the accuracy of staging for patients in stage III. Significantly more patients in staging group IV (metastatic disease) were changed to the correct stage (27 patients) than moved to an incorrect stage (one patient) (43% vs. 2%; p < 0.001). The effect of PET/CT was to change to the correct staging group significantly more often than to change from the correct group to an incorrect group (p < 0.001). This change in stage was limited to stage IIB and IV patients; there was no significant impact of PET/CT in staging larger tumours invading surrounding structures (stage III). Overall, PET/CT more accurately staged patients in terms of identifying lymph node disease in smaller tumours and metastatic disease.
The changes in diagnosis and staging also have implications for changes in the management of patients. Anticipated management was documented after MDCT and PET/CT and actual management was then recorded. Clinicians documented that PET/CT had influenced their management decisions for 45% of patients. Overall, 30% of patients underwent a management change throughout the study. PET/CT changed the management of 11% of patients in the overall study population.
The types of management changes resulting from the use of PET/CT were then analysed. The most common alteration in management was the change from resection to no resection, which occurred in 60 patients, representing 11% of all patients and 21% of patients scheduled for surgery after MDCT. In total, 13% of patients not thought to need surgical resection following MDCT were then planned for resection following PET/CT. Changes relating to the commencement or cessation of chemotherapy were less common. The biggest impact of PET/CT was on those patients who were identified as having resectable tumours on MDCT and who were then deemed not suitable for resection following PET/CT. This would place PET/CT as an integral component of the staging process for patients who are thought to have resectable tumours at MDCT, to identify patients who would not benefit from surgery because of more advanced disease.
Impact of positron emission tomography/computed tomography on the diagnosis of chronic pancreatitis
The prevalence of chronic pancreatitis was quite low in the patient population (41 patients; 7.5%). Therefore, the power to assess the diagnostic technologies was limited. Both MDCT and PET/CT had relatively high specificity (98.4% in each case) but low sensitivity for the diagnosis of chronic pancreatitis. The sensitivity of PET/CT was higher than that for MDCT (45.2% vs. 36.6%); however, this effect did not attain statistical significance (relative sensitivity 1.27; p = 0.066). Although a positive diagnosis of chronic pancreatitis from the PET/CT or MDCT scan result greatly increased the odds of a patient having chronic pancreatitis, there was still considerable uncertainty about the diagnosis. The main diagnostic dilemma facing clinicians is when a patient already has chronic pancreatitis and pancreatic cancer is suspected. In this population only one patient had chronic pancreatitis and pancreatic cancer. The sensitivity and specificity of PET/CT in this study do not support the routine use of PET/CT in the diagnosis and assessment of patients with chronic pancreatitis. The use of PET/CT to differentiate pancreatic cancer in patients with chronic pancreatitis cannot be supported based on the findings of this study.
Benefits of positron emission tomography/computed tomography for different subgroups
A GEE subgroup analysis was performed for all those patients who had out-of-range PET/CT scans either because of an out-of-range uptake time or elevated blood glucose. The biggest effect was on the sensitivity of PET/CT, which was reduced to 52.9% and was significantly worse than the sensitivity of MDCT (70.6%) (p = 0.005). Taking each factor individually, patients with an out-of-range uptake time had a PET/CT sensitivity of 71.4% and those with elevated blood glucose (> 10 mmol/l) had a PET/CT sensitivity of 40%. These values confirm that it is essential that PET/CT be performed after the correct uptake time and that PET/CT is unsuitable for patients with poorly controlled blood glucose.
Further subgroup analyses were undertaken for age, sex and WHO performance status. There was no evidence of any differences in the sensitivity or specificity of either test with respect to sex or age. However, both MDCT and PET/CT had a significantly higher sensitivity among patients with a WHO status of ≥ 1 than among those with a WHO status of 0, but conversely had significantly lower specificity. In terms of the eligibility criteria (pancreatic mass, jaundice and serum CA19.9 > 37 kU/l), both MDCT and PET/CT had a significantly lower specificity among patients with jaundice because of distal obstruction of the common bile duct or ampulla. Moreover, there was also evidence that PET/CT was not more effective than MDCT in this group. There was no evidence of differences in sensitivity or specificity for the other eligibility criteria. It can be noted that, based on pancreatic mass on CT, PET/CT sensitivity and specificity were 93% and 76%, respectively, for diagnosing pancreatic cancer; based on jaundice, PET/CT sensitivity and specificity were 95.6% and 52.6%, respectively; and based on elevated CA19.9, PET/CT sensitivity and specificity were 92.2% and 72.5%, respectively. There were no major differences between these criteria (apart from the specificity in jaundiced patients).
Impact of positron emission tomography/computed tomography on the diagnosis of pancreatic tumours
The value of PET/CT in other pancreatic tumours was evaluated. The most frequent tumour types were assessed.
Malignant cystic neoplasm
The prevalence of malignant cystic neoplasm in the patient cohort was 1.5% (95% CI 0.5% to 2.5%). The small number of patients diagnosed with malignant cystic neoplasm means that statistical tests comparing the diagnostic tests have low power to detect small or moderate effects. Both MDCT and PET/CT had a sensitivity of 75.0% (95% CI 45.0% to 99.9%). The specificity of PET/CT (96.1%, 95% CI 94.5% to 97.8%) was higher than that of MDCT (92.8%, 95% CI 90.6% to 95.0%). The 3.6% (95% CI 1.8% to 5.4%) improvement in specificity between tests is statistically significant (p < 0.001). PET/CT may have a role in the diagnosis of malignant cystic neoplasms and would have a low false-positive rate. The numbers of patients in this study are low and therefore larger studies would be needed.
Cholangiocarcinoma
The prevalence of cholangiocarcinoma in the patient cohort was 8.0% (95% CI 5.7% to 10.3%). The small number of patients diagnosed with cholangiocarcinoma means that statistical tests comparing the diagnostic tests have low power to detect small or moderate effects. Both MDCT and PET/CT had a relatively high specificity (97.8% and 98.8%, respectively) but low sensitivity (25% in each case). There were no significant differences in either sensitivity or specificity. Although a positive diagnosis of cholangiocarcinoma from the PET/CT or MDCT scan result greatly increased the odds of a patient having cholangiocarcinoma, there is still considerable uncertainty about the diagnosis. These results would not support the use of PET/CT in this patient group.
Neuroendocrine tumour
The prevalence of neuroendocrine tumour in the patient cohort was 4.9% (95% CI 3.1% to 6.7%). The small number of patients diagnosed with neuroendocrine tumour means that statistical tests comparing the diagnostic tests have low power to detect small or moderate effects. Both MDCT and PET/CT had a very high specificity (99.4% and 98.7%, respectively) but low sensitivity (44.4% in both cases). The relative specificity and sensitivity are not significantly different. These results would not support the use of PET/CT in this patient group.
Periampullary carcinoma
The prevalence of periampullary carcinoma in the patient cohort was 6.9% (95% CI 4.8% to 9.0%). The small number of patients diagnosed with periampullary carcinoma means that statistical tests comparing the diagnostic tests have low power to detect small or moderate effects. Both MDCT and PET/CT had relatively high specificity (95.9% and 97.3%, respectively) but relatively low sensitivity (71.1% and 65.8%). The relative specificity of PET/CT compared with MDCT is statistically significant (p = 0.034) but the practical improvements are outweighed by the observed, although non-significant, reduction in sensitivity. There is some evidence for the use of PET/CT in these tumours.
Malignant compared with benign disease
The prevalence of the different tumour types was low and therefore we undertook a global analysis of all malignant disease compared with benign disease. The prevalence of any malignant disease among the patient population was 69.8% (95% CI 66.0% to 73.7%). Both MDCT and PET/CT had a high sensitivity with respect to detecting any malignancy (97.4% and 97.7%, respectively). The specificity of PET/CT was considerably higher than that for MDCT (69.7% vs. 47.0%, relative sensitivity 1.46; p < 0.0001). PET/CT had a statistically significant impact on the odds of malignancy. The results suggest particular benefits of administering a PET/CT scan after a positive MDCT scan. These results confirm that PET/CT in addition to MDCT is useful in the diagnosis of malignant compared with benign disease.
Additional analyses
Maximum standardised uptake value
The median SUVmax. for patients with pancreatic cancer was 7.5 and for patients who did not have pancreatic cancer was 5.7. The SUVmax. optimal cut-off for a diagnosis of pancreatic cancer would be 6.2 according to the AUC. The drawback is that this test has an estimated sensitivity of only 70.3% and a specificity of only 59.4%. The optimum cut-off for a diagnosis of chronic pancreatitis was 6.4. This cut-off value was similar to the optimal value for assessing PDAC, suggesting that an approach of assuming PDAC for a SUVmax. of > 6.3 and chronic pancreatitis for a SUVmax. of < 6.3 has some merit.
Survival analysis
The 6- and 12-month survival rates for the overall patient population were 82.8% (95% CI 79.7% to 86.0%) and 69.0% (95% CI 65.1% to 73.1%), respectively. Patients who had a diagnosis of pancreatic cancer had 6- and 12-month survival rates of 71.4% (95% CI 66.0% to 77.2%) and 50.9% (95% CI 44.9% to 57.6%), respectively. The survival times observed in the study were as expected for this type of pancreatic MDT population.
Health economic evaluation
Health economic analysis was conducted from a NHS perspective. Our cost-effectiveness analysis was in three parts: model 1, in which we calculated the marginal cost per additional correct diagnosis of PDAC using PET/CT and MDCT alone; model 2, in which we calculated the budget impact of the use of PET/CT; and model 3, in which we modelled the change in management of patients over a 1-year time horizon as a result of the use of PET/CT for diagnosis. We undertook sensitivity analysis to explore uncertainty in costs (univariate) and model structure (structural). PSA assessed the likelihood that PET/CT is cost-effective at thresholds of £20,000 per QALY and £30,000 per QALY. We found evidence for the cost-effectiveness of PET/CT in terms of change in management for patients with suspected pancreatic cancer. In the change of management model, the primary model dominated management based on MDCT alone. This is largely because of the number of unnecessary surgeries avoided. Structural sensitivity analysis involved varying our base-case assumption that all patients received a resection so that some patients who were clinically indicated received bypass or open and shut laparotomy. Alongside our main finding from the base-case analysis that PET/CT dominated, the ICER in our structural sensitivity analysis ranged from £19,445 per QALY to £53,557 per QALY depending on the source of PET/CT costs chosen. For both change of management models, PET/CT was seen to be most cost-effective for the PDAC with resection subgroup. Overall, our base-case analysis showed that PET/CT dominated MDCT alone, in particular for patients who were suspected of having pancreatic cancer after standard diagnostic work-up and who were planned for surgery. However, we note that QALY gains were small and our analysis was sensitive to our source of published costs and to structural assumptions in the model.
Overall evidence
Many studies have evaluated PET or PET/CT in the diagnosis and management of pancreatic cancer. However, these have been small single-centre studies that lack the applicability of multicentre prospective series of patients. In general, PET/CT demonstrates better accuracy in the diagnosis of pancreatic cancer, with particular benefit for detecting distant disease and altering management. Five meta-analyses have compared PET with PET/CT and a variety of other imaging modalities. 24,41,44,45,77 The sensitivities and specificities in these meta-analyses are provided in Table 63. The sensitivity and specificity of 92.7% and 75.8% for PET/CT from the PET-PANC study are similar to those found in the latest meta-analysis by Rijkers et al. 41
| Study | Studies, n | Sensitivity/specificity (%) | ||||
|---|---|---|---|---|---|---|
| PET | PET/CT | CT | DW MRI | EUS | ||
| Rijkers 201441 | 35 | 90/76 | 90/76 | –/– | –/– | –/– |
| Wang 201344 | 39 | 91/80 | 90/85 | –/– | –/– | –/– |
| Wu 201277 | 16 | –/– | 87/83 | –/– | 85/91 | –/– |
| Tang 201145 | 51 | 88/83 | 90/80 | –/– | –/– | 81/93 |
| Orlando 200424 | 17 | 92/68 | –/– | 81/66 | –/– | –/– |
There is a lack of comparison between PET/CT and MDCT in these meta-analyses. There are individual series that have compared MDCT and PET/CT; generally, PET/CT is superior to MDCT when detecting the primary tumour (Table 64).
| Study | n | Sensitivity/specificity (%) | |
|---|---|---|---|
| MDCT | PET/CT | ||
| Zhang 201554 | 70 | 82/65 | 92/65 |
| Ergul 201478 | 52 | 92/50 | 100/89 |
| Zhang 201252 | 56 | 87.5/75 | 89.7/88.2 |
| Kauhanen 200979 | 38 | 85/67 | 85/94 |
| Casneuf 200739 | 46 | Accuracy 88.2% | Accuracy 91.2% |
| Heinrich 200546 | 59 | 93/21 | 89/69 |
| Lemke 200480 | 104 | 76.6/63.9 | 89.1/63.9 |
It is apparent that there is variability in the reported sensitivities and specificities of PET/CT in individual studies; however, again, the number of patients per study remains low.
The role of PET/CT in staging pancreatic tumours has been investigated in a number of studies and meta-analyses. 44,79,81 In general, the findings have been that PET/CT may be useful in staging primary tumours (and also small pancreatic tumours82) and distant disease. Sensitivity for detecting metastases varies between 76% and 88%. 44,79,81 The role of PET/CT in staging lymph node disease is more uncertain, with sensitivity for nodal status of between 30% and 81%,44,79,81 although it is useful in detecting distant lymph node disease. 79 There is a lack of evidence for a role of PET/CT in assessing local resectability of pancreatic cancer in terms of vessel involvement83 and MDCT remains the mainstay of imaging for this. The PET-PANC study was most useful in accurately staging patients with stage IIb and stage IV pancreatic cancer, illustrating a role for PET/CT in identifying locoregional lymph node disease and distant metastases.
The role of PET/CT in the management of patients with pancreatic cancer has been evaluated in studies from single centres and in meta-analyses. 44,46–48,78,79,84 The effect size with regard to change in management ranges from 41% to 10%. 44,46–48,78,79,84 The largest study was a retrospective series of 285 patients over a period of 7 years. The addition of PET/CT changed management in 31 (10%) patients. Metastases were found in 19 patients. Both this study and another recent review recommended that ‘these findings will be required to be validated in a prospective study along with cost-effective analysis’ (p. e505) and ‘the effects of PET/CT on patient survival and cost-effectiveness need to be further investigated’ (p. 149). 83 In this study PET/CT influenced the management of 45% of patients. The most common change was changing from resection to no resection, which occurred in 60 patients, representing 11% of all patients and 21% of patients scheduled for some kind of resection after MDCT.
There are no prospective studies of the cost-effectiveness of PET/CT in pancreatic cancer. One study of a series of 59 patients undergoing PET/CT for pancreatic cancer demonstrated a 16% change in management. 46 The use of PET/CT was found to be cost-effective in this limited study. The planned economic analysis in the PET-PANC study found evidence of cost-effectiveness for PET/CT in terms of management changes for patients. The most cost-effective use of PET/CT was in the subgroup of patients who had resectable disease and who were planned for surgery.
The PET-PANC study has demonstrated that there is a clinical benefit from the use of PET/CT in the diagnosis and management of pancreatic cancer and that it is cost-effective for patients who are being considered for surgery.
Strengths
The PET-PANC study is the largest, prospective, multicentre study of the use of PET/CT in patients with suspected pancreatic cancer to date. The study demonstrated that it is possible for multiple sites to adhere to a PET/CT protocol and attain a high level of quality assurance in the majority of PET/CT scans. The prospective data collected have been vital in establishing the accuracy of PET/CT in the diagnosis of pancreatic cancer. They have also been invaluable in providing an insight into patient populations treated in the major UK pancreatic centres and into variations in practice. The multicentre nature of the study enabled the results to be interpreted and applied in a pragmatic way. The PET/CT quality assurance was an excellent resource that was invaluable for this study and we were very fortunate that this was available at the time of our research. The study recruited patients in a reasonable time frame.
We believe that, internationally, this is the first study to generate QALY data for the purposes of robust economic evaluation in this patient group, using patient-level data from a UK cohort. This study generated rich QALY data for patients over time, which were stratified by diagnosis: PDAC, double cancer, malignant disease, benign disease and cysts. Analysis of the data and construction of economic models required close collaboration between the principal investigator as a clinician familiar with modes of cancer treatment in this population, the study statisticians and the health economists. The health economists were responsible for designing data collection from the PET-PANC cohort, building the economic model and analysing service use, costs and HRQoL data and undertaking the health economic analysis and sensitivity analysis. The challenging part of the study was, collaboratively, how to tease out how PET/CT had changed the management of a patient and the associated resource and outcome implications in a cohort rather than randomised controlled trial study design.
Limitations
This was not a randomised controlled trial and therefore we do not have information from patients who would have undergone only MDCT for comparison. There were some missing data for patients and there were also issues with regard to estimating costs for PET/CT. What became clear was the significant impact that variations in the cost of PET/CT had on estimates of cost-effectiveness, something to be explored by the NHS with manufacturers to find a way of reducing purchase and operating costs. The health economists responsible for building the economic model struggled with the lack of a control arm in the PET-PANC study. This required significant clinical input about alternative management modes of patients within the cohort. This engendered a particularly close working relationship between clinicians, statisticians and health economists. We were surprised to find such a wide range of published cost estimates for PET/CT (inconsistency across departments). Our analysis can be viewed as conservative as we chose the highest cost estimate in our base-case analysis. We undertook a complete-case analysis of cost data and did not impute QALY data other than to assume a zero value for the EQ-5D for patients who had died over the 12-month follow-up period. To isolate the additional role of PET/CT in decisions about the management of patients the change of management model accounted only for changes in management that were deemed clinically to be the result of PET/CT. Subsequent changes in management were not modelled. Our base-case model did not consider the negative impacts of surgery where it is contraindicated. We explored this in a structural sensitivity analysis. In our base-case analysis PET/CT dominated CT alone; however, in our structural sensitivity analysis our findings were comparable to estimates of cost-effectiveness in the diagnosis of other cancers, that is, borderline cost-effective with respect to NICE thresholds. 66–69
The PET-PANC study showed that the use of PET/CT has the potential to reduce unnecessary surgery, by changing patient management, and provide quicker more accurate diagnoses, with associated patient benefits and benefits to the NHS. The bypass and open and shut laparotomy data were considered too weak (exceptionally small number of patients) for the primary model; however, we included it in a structural sensitivity analysis. In the PET-PANC study there were also very few patients with metastases who underwent a resection procedure. It is believed that the pathway of these patients is typical and representative of such a patient group; however, with such a small number of patients the results need to be interpreted with caution and the strength of the conclusion may be affected by the quality of these data. Regardless of model structure, if the cost of PET/CT is taken from clinical oncology, it is likely that the ICER lies below the NICE threshold of £20,000–30,000 per QALY. When costs are taken from nuclear medicine, only the primary model indicates cost-effectiveness.
Implications for practice
This study was designed to evaluate the diagnostic accuracy of PET/CT and its effects on management and cost-effectiveness in patients with suspected pancreatic cancer in a prospective, multicentre manner with the aim of helping to address the issues around widespread application of this technology and the cost implications for health care. Patients with suspected pancreatic cancer present unique challenges and may have complicated patient journeys. Based on the evidence from the PET-PANC study, PET/CT adds significant benefit to patients in terms of diagnosis, staging and management of pancreatic cancer. The most cost-effective use of PET/CT would be in the subgroup of patients who are suspected of having pancreatic cancer on MDCT and who are planned for surgery. Evidence is lacking for the use of PET/CT in patients with chronic pancreatitis, other pancreatic tumours and pancreatic cysts.
Recommendations for future research
-
The role of PET/CT in the diagnosis and management of intraductal papillary mucinous neoplasm is an important topic as the number of patients diagnosed with incidental pancreatic cysts is increasing year on year and their management and follow-up is challenging.
-
The role of alternative radiopharmaceuticals for PET/CT should be assessed in terms of the diagnosis and prognosis of pancreatic cancer.
-
The role of PET/CT as a response marker in the treatment of pancreatic cancer should be evaluated.
-
A randomised controlled trial comparing standard work-up (MDCT) with PET/CT in addition to standard work-up would provide more data on outcomes associated with MDCT standard work-up alone. More data are needed on the prognosis of MDCT-alone patients. We would like to suggest three routes for further enquiry:
-
Where possible, scrutiny of how patients fare with MDCT alone when PET/CT is not available. These data would be useful for future economic modelling exercises.
-
Extrapolation of what happens to patients beyond the 12-month follow-up used in the PET-PANC study. Resources were not available to allow us to extend follow-up in this study.
-
Stronger data on unnecessary surgery would add to the strength of the conclusion. Stronger data on the impact of bypass surgery or open and shut laparotomy would add strength to the conclusions of our structural sensitivity analysis.
-
Conclusions
-
This study was the first multicentre, prospective, large-scale study of PET/CT in the diagnosis, staging and management of patients with suspected pancreatic cancer.
-
PET/CT demonstrated significantly increased relative sensitivity and specificity compared with MDCT and a significant incremental diagnostic benefit in addition to MDCT in the diagnosis of pancreatic cancer.
-
PET/CT corrected the staging of pancreatic cancer in a significant proportion of patients. PET/CT influenced management in 45% of patients and prevented resection in 21% of patients scheduled for surgery.
-
PET/CT has limited use in chronic pancreatitis and other pancreatic tumours.
-
Internationally, as the first cost-effectiveness study using robust QALY data from this patient group, the PET-PANC study demonstrated that PET/CT is cost-effective for the UK NHS at current costs of PET/CT, although we note that the QALY gains were small and the results were sensitive to the source of the cost of PET/CT.
-
The PET-PANC study demonstrated that, at current costs of PET/CT to the UK NHS, PET/CT is most cost-effective for patients with suspected pancreatic cancer who are planned to undergo resection.
Acknowledgements
Contributions
All of the patients and their carers who took part in the study.
Trial Steering Committee: Professor P Clark (Chairperson), members of the Trial Management Group, Mr R Carter, Professor T Jones, Mr P Morris, Professor J Deeks, Dr Wai Lup Wong, Mr Rob Hanson, Dr G Lancaster, Dr A Titman, Professor R Tudor Edwards and Mr H Lloyd-Williams.
Independent Safety and Data Monitoring Committee: Dr I Chau (Chairperson), Dr S Chua and Mrs L Kilburn.
Trial Management Group: Mr Rob Hanson, Dr G Lancaster, Dr A Titman, Professor R Tudor Edwards and Mr H Lloyd-Williams.
PET/CT quality assurance – PET/CT Core Laboratory: Ms L Pike and Mr D Sinclair.
Reference standard expert panel: Mr C Halloran and Professor C Imrie.
Central PET/CT reporting: Dr WL Wong, Dr B Sanghera, Dr H Weishmann, Dr A Scarsbrook, Dr C Patel, Dr T Wagner, Dr S Navalkissoor and Dr F Sundram.
Pathology quality assurance: Dr Fiona Campbell.
Radiology (MDCT) quality assurance: Dr J Evans.
Specialist nurse support: Phil Whelan, Jo Garry and Faye Hughes.
Health Technology Assessment programme of the National Institute for Health Research: Dr Kate Fenton and all of the staff at the National Institute for Health Research who provided support and advice throughout.
Co-investigator: Professor David Berry.
Colleagues and research staff at recruiting centres: Royal Liverpool and Broadgreen University Hospitals NHS Trust; University Hospital Southampton NHS Foundation Trust; Portsmouth Hospitals NHS Trust; Leeds Teaching Hospitals NHS Trust; Glasgow Royal Infirmary – NHS Greater Glasgow and Clyde; University Hospitals Birmingham NHS Foundation Trust; Barts Health NHS Trust; Royal Marsden NHS Foundation Trust; University College London Hospitals NHS Foundation Trust; Royal Free London NHS Foundation Trust; Royal Blackburn Hospital – East Lancashire Hospitals NHS Trust; University Hospitals Coventry and Warwickshire NHS Trust; Ninewells Hospital – NHS Tayside; King’s College Hospital NHS Foundation Trust; Morriston Hospital – Abertawe Bro Morgannwg University Health Board; Newcastle Hospitals NHS Foundation Trust; Aberdeen Royal Infirmary – NHS Grampian; Nottingham University Hospitals NHS Trust.
The study protocol can be accessed by application to the LCTU [see www.LCTU.org.uk (accessed 27 June 2015)].
Contributions of authors
Paula Ghaneh (Chief Investigator and Consultant Surgeon) contributed to the conception and design of all aspects of the project, the conduct of the trial, the interpretation of the results and the writing and editing of the report.
Robert Hanson (Trial Co-ordinator) contributed to the conduct and co-ordination of the study, collation of the data and interpretation of the results.
Andrew Titman (Trial Statistician) contributed to the design of the trial, conducted the statistical analyses, prepared the results and contributed to the writing of the report.
Gill Lancaster (Senior Statistician) contributed to the design of the trial, supervised the statistical analyses and interpretation of the results and contributed to the writing of the report.
Catrin Plumpton (Health Economist) conducted and prepared the results of the economic model and contributed to the writing of the report.
Huw Lloyd-Williams (Health Economist) conducted and prepared the costing of the economic analysis and contributed to the writing of the report.
Seow Tien Yeo (Health Economist) conducted and prepared the costing of the economic analysis and contributed to the writing of the report.
Rhiannon Tudor Edwards (Professor of Health Economics) contributed to the design of the trial, the economic analysis and writing of the report.
Colin Johnson (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Mohammed Abu Hilal (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Antony P Higginson (Consultant Radiologist and Nuclear Medicine Physician) contributed to recruitment, data acquisition and the conduct of the study.
Tom Armstrong (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Andrew Smith (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Andrew Scarsbrook (Consultant Radiologist and Nuclear Medicine Physician) contributed to recruitment, data acquisition and the conduct of the study.
Colin McKay (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Ross Carter (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Robert P Sutcliffe (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Simon Bramhall (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Hemant M Kocher (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
David Cunningham (Consultant Oncologist) contributed to recruitment, data acquisition and the conduct of the study.
Stephen P Pereira (Consultant Gastroenterologist) contributed to recruitment, data acquisition and the conduct of the study.
Brian Davidson (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
David Chang (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Saboor Khan (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Ian Zealley (Consultant Radiologist) contributed to recruitment, data acquisition and the conduct of the study.
Debashis Sarker (Consultant Medical Oncologist) contributed to recruitment, data acquisition and the conduct of the study.
Bilal Al Sarireh (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Richard Charnley (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Dileep Lobo (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Marianne Nicolson (Consultant Medical Oncologist) contributed to recruitment, data acquisition and the conduct of the study.
Christopher Halloran (Consultant Surgeon) contributed to expert review of the reference standard.
Michael Raraty (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Robert Sutton (Consultant Surgeon) contributed to recruitment, data acquisition and the conduct of the study.
Sobhan Vinjamuri (Professor of Nuclear Medicine) contributed to the design and conduct of the study and advised on the PET/CT and nuclear medicine aspects of the study.
Jonathan Evans (Expert Consultant Radiologist) contributed to the design and conduct of the study and advised on the radiology aspects of the study.
Fiona Campbell (Professor of Pathology) contributed to the design and conduct of the study and advised on the pathology aspects of the study.
Jon Deeks (Professor of Biostatistics) contributed to the design of the study and advised on the diagnostic aspects of the study.
Bal Sanghera (Medical Physics Expert) contributed to the design of the study and advised on the PET/CT aspects of the study.
Wai-Lup Wong (Expert Consultant Radiologist) contributed to the design of the study and advised on the PET/CT aspects of the study.
John P Neoptolemos (Professor of Surgery) contributed to the design and conduct of the study.
Data sharing statement
Data sharing is covered by the LCTU data sharing policy, which can be accessed via the LCTU website [see www.lctu.org.uk/LCTU_NET/Frontend/Default.aspx?Data=W1tRMjl1ZEdWdWRFbEVdXVtNekkzXVtbYkc5allXeGxdXVtNUT09XQ%3d%3d (accessed October 2015)]. Potential requesters should contact the study investigator or corresponding author in the first instance to discuss their reasons for requesting the data and their study proposal. If data sharing is a possibility the proposal will be sent to the LCTU Senior Information Systems Developer and Chief Investigator for consideration. Proposals will then be forwarded to the LCTU Senior Management Team and Trial Adoption Committee for decision making. If the proposal is granted approval the LCTU information systems team is responsible for providing a copy of the data set.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Cancer Research UK . Cancer Statistics for the UK n.d. www.cancerresearchuk.org/health-professional/cancer-statistics (accessed 2015).
- National Cancer Institute . SEER Stat Fact Sheets: Pancreas Cancer n.d. http://seer.cancer.gov/statfacts/html/pancreas.html (accessed 25 May 2016).
- Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, et al. EUROCARE-5 Working Group . Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: results of EUROCARE-5 [published online ahead of print 5 September 2015]. Eur J Cancer 2015. http://dx.doi.org/10.1016/j.ejca.2015.07.034.
- Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513-18. http://dx.doi.org/10.1200/JCO.2009.24.2446.
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. http://dx.doi.org/10.1056/NEJMoa1011923.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. http://dx.doi.org/10.1056/NEJMoa1304369.
- Gooiker GA, Lemmens VE, Besselink MG, Busch OR, Bonsing BA, Molenaar IQ, et al. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg 2014;101:1000-5. http://dx.doi.org/10.1002/bjs.9468.
- Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a US population-based study. Am J Gastroenterol 2007;102:1377-82. http://dx.doi.org/10.1111/j.1572-0241.2007.01202.x.
- Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586-94. http://dx.doi.org/10.1002/bjs.4484.
- Neoptolemos J, Stocken D, Freiss H, Bassi C, Dunn J, Hickey H, et al. A randomised trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. http://dx.doi.org/10.1056/NEJMoa032295.
- Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. http://dx.doi.org/10.1001/jama.2010.1275.
- Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. http://dx.doi.org/10.1001/jama.2013.279201.
- Smeenk H, van Eijck C, Khe T, Erdmann J, Tran KC, Debois M, et al. Long-term survival and metastatic pattern of pancreatic cancer after adjuvant chemoradiation or observation: long-term results of EORTC-trial 40891. Ann Surg 2007;246:734-40. http://dx.doi.org/10.1097/SLA.0b013e318156eef3.
- Raimondi S, Maisonneuve P, Lowenfels A. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699-708. http://dx.doi.org/10.1038/nrgastro.2009.177.
- Rizzato C, Campa D, Pezzilli R, Soucek P, Greenhalf W, Capurso G, et al. ABO blood groups and pancreatic cancer risk and survival: results from the PANcreatic Disease ReseArch (PANDoRA) consortium. Oncol Rep 2013;29:1637-44.
- Porta M, Fabregat X, Malats N, Guarner L, Carrato A, de Miguel A, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005;7:189-97. http://dx.doi.org/10.1007/BF02712816.
- Gobbi PG, Bergonzi M, Comelli M, Villano M, Pozzoli D, Vanolli L, et al. The prognostic role of time to diagnosis and presenting symptoms in patients with pancreatic cancer. Cancer Epidemiol 2013;37:186-90. http://dx.doi.org/10.1016/j.canep.2012.12.002.
- Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, et al. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med 2013;13:340-51. http://dx.doi.org/10.2174/1566524011313030003.
- Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008;9:132-8. http://dx.doi.org/10.1016/S1470-2045(08)70001-9.
- Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg 2008;12:1422-8. http://dx.doi.org/10.1007/s11605-008-0554-3.
- Radon TP, Massat NJ, Jones R, Alrawashdeh W, Dumartin L, Ennis D, et al. Identification of a three-biomarker panel in urine for early detection of pancreatic adenocarcinoma. Clin Cancer Res 2015;21:3512-21. http://dx.doi.org/10.1158/1078-0432.CCR-14-2467.
- Minniti S, Bruno C, Biasiutti C, Tonel D, Falzone A, Falconi M, et al. Sonography versus helical CT in identification and staging of pancreatic ductal adenocarcinoma. J Clin Ultrasound 2003;4:175-82. http://dx.doi.org/10.1002/jcu.10156.
- Tamm EP, Loyer EM, Faria SC, Evans DB, Wolf RA, Charnsangavej C. Retrospective analysis of dual-phase MDCT and follow-up EUS/EUS-FNA in the diagnosis of pancreatic cancer. Abdom Imaging 2007;32:660-7. http://dx.doi.org/10.1007/s00261-007-9298-x.
- Orlando LA, Kulasingam SL, Matchar DB. Meta-analysis: the detection of pancreatic malignancy with positron emission tomography. Aliment Pharmacol Ther 2004;20:1063-70. http://dx.doi.org/10.1111/j.1365-2036.2004.02266.x.
- Wong JC, Lu DS. Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol 2008;6:1301-8. http://dx.doi.org/10.1016/j.cgh.2008.09.014.
- Klauss M, Mohr A, von Tengg-Kobligk H, Friess H, Singer R, Seidensticker P, et al. A new invasion score for determining the resectability of pancreatic carcinomas with contrast-enhanced multidetector computed tomography. Pancreatology 2008;8:204-10. http://dx.doi.org/10.1159/000128557.
- Rafique A, Freeman S, Carroll N. A clinical algorithm for the assessment of pancreatic lesions: utilization of 16- and 64-section multidetector CT and endoscopic ultrasound. Clin Radiol 2007;62:1142-53. http://dx.doi.org/10.1016/j.crad.2007.05.006.
- Sasson AR, Gulizia JM, Galva A, Anderson J, Thompson J. Pancreaticoduodenectomy for suspected malignancy: have advancements in radiographic imaging improved results?. Am J Surg 2006;192:888-93. http://dx.doi.org/10.1016/j.amjsurg.2006.08.064.
- Horwhat JD, Gerke H, Acosta RD, Pavey DA, Jowell PS. Focal or diffuse ‘fullness’ of the pancreas on CT. Usually benign, but EUS plus/minus FNA is warranted to identify malignancy. J Pancreas (Online) 2009;10:37-42.
- Birchard KR, Semelka RC, Hyslop WB, Brown A, Armao D, Firat Z, et al. Suspected pancreatic cancer: evaluation by dynamic gadolinium-enhanced 3D gradient-echo MRI. Am J Roentgenol 2005;185:700-3. http://dx.doi.org/10.2214/ajr.185.3.01850700.
- Li H, Hu Z, Chen J, Guo X. Comparison of ERCP, EUS, and ERCP combined with EUS in diagnosing pancreatic neoplasms: a systematic review and meta-analysis. Tumour Biol 2014;35:8867-74. http://dx.doi.org/10.1007/s13277-014-2154-z.
- Li JH, He R, Li YM, Cao G, Ma QY, Yang WB. Endoscopic ultrasonography for tumor node staging and vascular invasion in pancreatic cancer: a meta-analysis. Dig Surg 2014;31:297-305. http://dx.doi.org/10.1159/000368089.
- Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012;75:319-31. http://dx.doi.org/10.1016/j.gie.2011.08.049.
- Hartwig W, Schneider L, Diener MK, Bergmann F, Büchler MW, Werner J. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg 2009;96:5-20. http://dx.doi.org/10.1002/bjs.6407.
- Halloran CM, Ghaneh P, Connor S, Sutton R, Neoptolemos JP, Raraty MG. Carbohydrate antigen 19.9 accurately selects patients for laparoscopic assessment to determine resectability of pancreatic malignancy. Br J Surg 2008;95:453-9. http://dx.doi.org/10.1002/bjs.6043.
- Smith RA, Bosonnet L, Ghaneh P, Sutton R, Evans J, Healey P, et al. The platelet–lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery 2008;143:658-66. http://dx.doi.org/10.1016/j.surg.2007.12.014.
- Hillner BE, Siegel BA, Liu D, Shields AF, Gareen IF, Hanna L, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol 2008;26:2155-61. http://dx.doi.org/10.1200/JCO.2007.14.5631.
- Hillner BE, Siegel BA, Hanna L, Shields AF, Duan F, Gareen IF, et al. Impact of 18F-FDG PET used after initial treatment of cancer: comparison of the national oncologic PET registry 2006 and 2009 cohorts. J Nucl Med 2012;53:831-7. http://dx.doi.org/10.2967/jnumed.112.103911.
- Casneuf V, Delrue L, Kelles A, Van Damme N, Van Huysse J, Berrevoet F, et al. Is combined 18F-fluorodeoxyglucose-positron emission tomography/computed tomography superior to positron emission tomography or computed tomography alone for diagnosis, staging and restaging of pancreatic lesions?. Acta Gastroenterol Belg 2007;70:331-8.
- Lytras D, Connor S, Bosonnet L, Jayan R, Evans J, Hughes M, et al. Positron emission tomography does not add to computed tomography for the diagnosis and staging of pancreatic cancer. Dig Surg 2005;22:55-61. http://dx.doi.org/10.1159/000085347.
- Rijkers AP, Valkema R, Duivenvoorden, van Eijck CHJ. Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: a meta-analysis. Eur J Surg Oncol 2014;40:794-80. http://dx.doi.org/10.1016/j.ejso.2014.03.016.
- Kalra MK, Blake MA, Saini S. Role of dual PET/CT scanning in abdominal malignancies. Cancer Imaging 2004;4:121-3. http://dx.doi.org/10.1102/1470-7330.2004.0019.
- Javery O, Shyn P, Koenraad Mortele K. FDG PET or PET/CT in patients with pancreatic cancer: when does it add to diagnostic CT or MRI?. Clin Imaging 2013;37:295-301. http://dx.doi.org/10.1016/j.clinimag.2012.07.005.
- Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: a meta-analysis. World J Gastroenterol 2013;19:4808-17. http://dx.doi.org/10.3748/wjg.v19.i29.4808.
- Tang S, Huang G, Liu J, Liu T, Treven L, Song S, et al. Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol 2011;78:142-50. http://dx.doi.org/10.1016/j.ejrad.2009.09.026.
- Heinrich S, Goerres GW, Schäfer M, Sagmeister M, Bauerfeind P, Pestalozzi BC, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005;242:235-43. http://dx.doi.org/10.1097/01.sla.0000172095.97787.84.
- Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol 2008;15:2465-71. http://dx.doi.org/10.1245/s10434-008-9992-0.
- Barber T, Kalff V, Cherk MH, Yap KSK, Evans, Kelly MJ. 18F-FDG PET/CT influences management in patients with known or suspected pancreatic cancer. Intern Med J 2011;41:776-83. http://dx.doi.org/10.1111/j.1445-5994.2010.02257.x.
- Kim R, Prithviraj G, Kothari N, Springett G, Malafa M, Hodul P, et al. PET/CT fusion scan prevents futile laparotomy in early stage pancreatic cancer. Clin Nucl Med 2015;40:e501-5. http://dx.doi.org/10.1097/RLU.0000000000000837.
- Yao J, Gan G, Farlow D, Laurence JM, Hollands M, Richardson A, et al. Impact of F18-fluorodeoxyglycose positron emission tomography/computed tomography on the management of resectable pancreatic tumours. ANZ J Surg 2012;82:140-4. http://dx.doi.org/10.1111/j.1445-2197.2011.05972.x.
- Schick V, Franzius C, Beyna T, Oei ML, Schnekenburger J, Weckesser M, et al. Diagnostic impact of 18F-FDG PET-CT evaluating solid pancreatic lesions versus endosonography, endoscopic retrograde cholangio-pancreatography with intraductal ultrasonography and abdominal ultrasound. Eur J Nucl Med Mol Imaging 2008;35:1775-85. http://dx.doi.org/10.1007/s00259-008-0818-x.
- Zhang M, Zhang M, Wang L, Hu J, Li B. Combined 18F-FDG PET/CT with enhanced CT perform one-stop shop imaging for assessing pancreatic carcinoma. J Cancer Ther 2012;3:546-52. http://dx.doi.org/10.4236/jct.2012.35070.
- Strobel K, Heinrich S, Bhure U, Soyka J, Veit-Haibach P, Pestalozzi BC, et al. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J Nucl Med 2008;49:1408-13. http://dx.doi.org/10.2967/jnumed.108.051466.
- Zhang J, Zuo C-J, Jia N-Y, Wang J-H, Hu S-P, Yu Z-F, et al. Cross-modality PET/CT and contrast-enhanced CT imaging for pancreatic cancer. World J Gastroenterol 2015;21:2988-96. http://dx.doi.org/10.3748/wjg.v21.i10.2988.
- Herrmann K, Erkan M, Dobritz M, Schuster T, Siveke JT, Beer AJ, et al. Comparison of 3′-deoxy-3′-[(1)(8)F]fluorothymidine positron emission tomography (FLT PET) and FDG PET/CT for the detection and characterization of pancreatic tumours. Eur J Nucl Med Mol Imaging 2012;39:846-51. http://dx.doi.org/10.1007/s00259-012-2061-8.
- Nagamachi S, Nishii R, Wakamatsu H, Mizutani Y, Kiyohara S, Fujita S, et al. The usefulness of (18)F-FDG PET/MRI fusion image in diagnosing pancreatic tumor: comparison with (18)F-FDG PET/CT. Ann Nucl Med 2013;27:554-63. http://dx.doi.org/10.1007/s12149-013-0719-3.
- Ghaneh P, Neoptolemos JP. A new approach to managing intraductal papillary mucinous pancreatic neoplasms. Gut 2007;56:1041-4. http://dx.doi.org/10.1136/gut.2006.113068.
- Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2016;65:305-12. http://dx.doi.org/10.1136/gutjnl-2015-309638.
- Al-Haddad M, Wallace MB, Woodward TA, Gross SA, Hodgens CM, Toton RD, et al. The safety of fine-needle aspiration guided by endoscopic ultrasound: a prospective study. Endoscopy 2008;40:204-8. http://dx.doi.org/10.1055/s-2007-995336.
- Smith RA, Dajani K, Dodd S, Whelan P, Raraty M, Sutton R, et al. Preoperative resolution of jaundice following biliary stenting predicts more favourable early survival in resected pancreatic ductal adenocarcinoma. Ann Surg Oncol 2008;15:3138-46. http://dx.doi.org/10.1245/s10434-008-0148-z.
- Sobin LH, Wittekind CH. TNM: Classification of Malignant Tumours. New York, NY: John Wiley; 2009.
- Campbell F, Bennett M, Foulis AJ. Minimum Dataset for Histopathological Reporting of Pancreatic, Ampulla of Vater and Bile Duct Carcinoma 2002. www.rcpath.org (accessed 2015).
- Alonzo TA, Pepe MS, Moskowitz CS. Sample size calculations for comparative studies of medical tests for detecting presence of disease. Stat Med 2002;21:835-52. http://dx.doi.org/10.1002/sim.1058.
- Spiegelhalter D. Funnel plots for comparing institutional performance. Stat Med 2005;24:1185-202. http://dx.doi.org/10.1002/sim.1970.
- Chan S, Deeks JJ, Macaskill P, Irwig L. Three methods to construct predictive models using logistic regression and likelihood ratios to facilitate adjustment for pretest probability give similar results. J Clin Epidemiol 2008;61:52-63. http://dx.doi.org/10.1016/j.jclinepi.2007.02.012.
- Søgaard R, Fischer BMB, Mortensen J, Højgaard L, Lassen U. Preoperative staging of lung cancer with PET/CT: cost-effectiveness evaluation alongside a randomized controlled trial. Eur J Med Mol Imaging 2010;38:802-9. http://dx.doi.org/10.1007/s00259-010-1703-y.
- Schreyögg J, Weller J, Stargardt T, Herrmann K, Bluemel C, Dechow T, et al. Cost-effectiveness of hybrid PET/CT for staging of non-small cell lung cancer. J Nucl Med 2010;51:1668-75. http://dx.doi.org/10.2967/jnumed.109.072090.
- Auguste P, Barton P, Hyde C, Roberts TE. An economic evaluation of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15180.
- Brush J, Boyd K, Chappell F, Crawford F, Dozier M, Fenwick E, et al. The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15350.
- Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al. What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? Multi-Institution Nurse Endoscopy Trial (MINuET). Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10400.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Measuring Effectiveness and Cost-Effectiveness: The QALY. London: NICE; 2010.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2012.
- National Reference Costs 2012 to 2013. London: Department of Health; 2013.
- Prescription Cost Analysis: England 2013. Leeds: HSCIC; 2014.
- Wu LM, Hu JN, Hua J, Liu MJ, Chen J, Xu JR. Diagnostic value of diffusion-weighted magnetic resonance imaging compared with fluorodeoxyglucose positron emission tomography/computed tomography for pancreatic malignancy: a meta-analysis using a hierarchical regression model. J Gastroenterol Hepatol 2012;27:1027-35. http://dx.doi.org/10.1111/j.1440-1746.2012.07112.x.
- Ergul N, Gundogan C, Tozlu M, Toprak H, Kadioglu H, Aydin M, et al. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in diagnosis and management of pancreatic cancer; comparison with multidetector row computed tomography, magnetic resonance imaging and endoscopic ultrasonography. Rev Esp Med Nucl 2014;33:159-64. http://dx.doi.org/10.1016/j.remn.2013.08.005.
- Kauhanen SP, Komar G, Seppanen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957-63. http://dx.doi.org/10.1097/SLA.0b013e3181b2fafa.
- Lemke AJ, Niehues SM, Hosten N, Amthauer H, Boehmig M, Stroszczynski C, et al. Retrospective digital image fusion of multidetector CT and 18F-FDG PET: clinical value in pancreatic lesions – a prospective study with 104 patients. J Nucl Med 2004;45:1279-86.
- Yoneyama T, Tateishi U, Endo I, Inoue T. Staging accuracy of pancreatic cancer: comparison between non-contrast-enhanced and contrast-enhanced PET/CT. Eur J Radiol 2014;83:1734-9. http://dx.doi.org/10.1016/j.ejrad.2014.04.026.
- Kawada N, Uehara H, Hosoki T, Takami M, Shiroeda H, Arisawa T, et al. Usefulness of dual-phase 18F-FDG PET/CT for diagnosing small pancreatic tumors. Pancreas 2015;44:655-9. http://dx.doi.org/10.1097/MPA.0000000000000313.
- Nunna P, Sheikhbahaei S, Ahn S, Young B, Subramaniam RM. The role of positron emission tomography/computed tomography in management and prediction of survival in pancreatic cancer. J Comput Assist Tomogr 2016;40:142-51. http://dx.doi.org/10.1097/RCT.0000000000000323.
- Burge ME, O’Rourke N, Cavallucci D, Bryant R, Francesconi A, Houston K, et al. A prospective study of the impact of fluorodeoxyglucose positron emission tomography with concurrent non-contrast CT scanning on the management of operable pancreatic and peri-ampullary cancers. HPB (Oxford) 2015;17:624-31. http://dx.doi.org/10.1111/hpb.12418.
- Sobin L, Gospodarowicz M, Wittekind C. The TNM Classification of Malignant Tumours. Chichester: Wiley-Blackwell; 2009.
- Beecham J, Knapp M, Thornicroft G, Brewin CR, Wing J. Measuring Mental Health Needs 1992:163-83.
Appendix 1 Union for International Cancer Control TNM classification, 7th edition (pancreas section extract)
Reproduced with permission from Union for International Cancer Control. The TNM Classification of Malignant Tumours. L Sobin, M Gospodarowicz, C Wittekind, editors. 7th edition. Chichester: Wiley-Blackwell; 2009. 85
Appendix 2 Quality of life questionnaire: European Quality of Life-5 Dimensions
Appendix 3 Changes in staging of pancreatic cancer
D4 stage: no tumour/IA/IB/IIA
| D1 | D2 | |||
|---|---|---|---|---|
| No tumour/IA/IB/IIA | IIB | III | IV | |
| No tumour/IA/IB/IIA | 171 | 3 | 1 | 4 |
| IIB | 4 | 4 | 0 | 0 |
| III | 2 | 0 | 4 | 1 |
| IV | 0 | 0 | 0 | 2 |
D4 stage: IIB
| D1 | D2 | |||
|---|---|---|---|---|
| No tumour/IA/IB/IIA | IIB | III | IV | |
| No tumour/IA/IB/IIA | 50 | 21 | 1 | 3 |
| IIB | 3 | 19 | 0 | 2 |
| III | 0 | 1 | 3 | 0 |
| IV | 2 | 0 | 0 | 2 |
D4 stage: III
| D1 | D2 | |||
|---|---|---|---|---|
| No tumour/IA/IB/IIA | IIB | III | IV | |
| No tumour/IA/IB/IIA | 9 | 0 | 0 | 0 |
| IIB | 1 | 6 | 0 | 0 |
| III | 0 | 0 | 10 | 0 |
| IV | 0 | 0 | 1 | 0 |
D4 stage: IV
| D1 | D2 | |||
|---|---|---|---|---|
| No tumour/IA/IB/IIA | IIB | III | IV | |
| No tumour/IA/IB/IIA | 10 | 0 | 0 | 15 |
| IIB | 0 | 4 | 0 | 5 |
| III | 0 | 0 | 0 | 7 |
| IV | 1 | 0 | 0 | 21 |
Appendix 4 Distinguishing between pancreatic ductal adenocarcinoma and chronic pancreatitis at D1 and D2
D4: patients with neither pancreatic ductal adenocarcinoma nor chronic pancreatitis
| D1 | D2 | |||
|---|---|---|---|---|
| Neither | CP only | PDAC only | CP and PDAC | |
| Neither | 158 | 1 | 12 | 1 |
| CP only | 0 | 4 | 1 | 0 |
| PDAC only | 24 | 1 | 47 | 0 |
| CP and PDAC | 0 | 0 | 0 | 0 |
There was an improvement in diagnosis for patients without either PDAC or chronic pancreatitis through a reduction in those misdiagnosed with PDAC at D2 compared with D1. There was no reduction in the diagnosis of chronic pancreatitis.
D4: patients with chronic pancreatitis only
| D1 | D2 | |||
|---|---|---|---|---|
| Neither | CP only | PDAC only | CP and PDAC | |
| Neither | 10 | 2 | 0 | 0 |
| CP only | 0 | 13 | 1 | 1 |
| PDAC only | 3 | 3 | 7 | 0 |
| CP and PDAC | 0 | 0 | 0 | 0 |
Among patients with chronic pancreatitis, 13 were misdiagnosed as having PDAC at D1, which reduced to eight at D2.
D4: patients with pancreatic ductal adenocarcinoma only
Very few patients were misdiagnosed as having chronic pancreatitis rather than PDAC. Two patients at D1 with PDAC were suspected of having chronic pancreatitis, which reduced to one at D2.
| D1 | D2 | |||
|---|---|---|---|---|
| Neither | CP only | PDAC only | CP and PDAC | |
| Neither | 13 | 0 | 15 | 0 |
| CP only | 1 | 1 | 0 | 0 |
| PDAC only | 4 | 0 | 225 | 0 |
| CP and PDAC | 0 | 0 | 1 | 0 |
Appendix 5 World Health Organization performance status
-
Able to carry out all normal activity without restriction.
-
Restricted in physically strenuous activity but ambulatory and able to carry out light work.
-
Ambulatory and capable of all self care but unable to carry out any work; up and about more than 50% of waking hours.
-
Capable only of limited self care; confined to bed or chair more than 50% of waking hours.
-
Completely disabled; cannot carry out any self care; totally confined to bed or chair.
Appendix 6 Economic evaluation studies of positron emission tomography/computed tomography in oncology
| First author | Year | Country and perspective | Title | Sample | Type of cancer | Type of analysis | Aim of study | Tree diagram | Model | Outcomes | Software used | Comment/notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Søgaard66 | 2010 | Denmark; health-care sector perspective | Preoperative staging of lung cancer with PET/CT: cost-effectiveness evaluation alongside a randomised controlled trial | Randomised controlled trial of 189 patients aged 18–80 years allocated to conventional staging (n = 91) or conventional staging plus PET/CT (n = 98) | NSCLC | Cost-effectiveness based on cost per avoided futile thoracotomy | To assess the cost-effectiveness of PET/CT as an adjunct to conventional work-up for preoperative staging of NSCLC | No | CEACs for alternative scenarios | CEACs were generated. Mean cost per avoided futile thoracotonomy €19,314 with comorbidities included or €4495 with comorbidities excluded and cost saving of €899 | Not specifed | First economic evaluation (as far as the authors are aware) of PET/CT as part of a randomised controlled trial |
| Schreyögg67 | 2010 | Germany; payer’s perspective | Cost-effectiveness of hybrid PET/CT for staging of non-small cell lung cancer | 172 NSCLC patients (132 men, 40 women) from a prospective clinical study who underwent diagnostic, contrast-enhanced helical CT and integrated PET/CT | NSCLC | (a) Cost-effectiveness based on correctly staged patient (for those undergoing surgery = 77/172); (b) cost–utility was modelled | To evaluate the cost-effectiveness of staging NSCLC with CT alone compared with PET/CT | No | Cox proportional hazards model for cost–utility analysis | Cost of diagnosis per patient with PET/CT was $783 compared with $100 for CT alone. (a) ICER was $3508 per correctly staged patient and $4784 according to resectability; (b) ICER was $79,878 per QALY gained | Not specifed | |
| Auguste68 | 2011 | UK; NHS perspective | An economic evaluation of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence | Modelling study, Monte Carlo simulation | Recurrent breast cancer | Cost–utility. Mean cost per patient: (a) conventional work-up £534, (b) PET £1101, (c) PET/CT £1333, (d) conventional work-up + PET/CT £1831 | With respect to recurrent breast cancer: (a) review published economic studies and (b) undertake model-based economic evaluation of PET/CT compared with conventional work-up | Yes; model structure p. 10 | Decision tree. Estimated mean cost associated with each procedure and assumed that patients entering the model were aged 50–75 years | ICER for PET/CT: £29,300 per QALY compared with conventional work-up, £31,000 per QALY compared with PET and £42,100 per QALY compared with conventional work-up + PET/CT | TreeAge | |
| Brush69 | 2011 | UK; NHS perspective | The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation | Modelling study, Monte Carlo simulation | CRC | Cost–utility | Ascertain (a) whether FDG PET/CT is likely to be cost-effective as an add-on test for preoperative staging in CRC, (b) in which patient groups it is likely to be cost-effective and (c) under what circumstances it is likely to be cost-effective | Yes; staging primary CRC p. 84, staging recurrent CRC p. 94, staging metastatic recurrence p. 102 | Probabilistic decision-analytic modelling; five models developed | Conventional imaging modalities for primary CRC in terms of cost per correct diagnosis and cost per QALY were favoured. Recurrent models: (a) rectal model ICER: £21,409 per QALY, (b) colon model ICER: £6189 and (c) metastatic model ICER: £21,434 per QALY | Not specified |
Appendix 7 Mean costs over 12 months for the intention-to-treat cohort
| Resource use category | Mean cost (£) | |||
|---|---|---|---|---|
| 3 months’ follow-up (n = 311) | 6 months’ follow-up (n = 272) | 9 months’ follow-up (n = 233) | 12 months’ follow-up (n = 243) | |
| Primary care | ||||
| Cancer nurse | 10.94 | 8.63 | 25.70 | 7.58 |
| GP | 89.39 | 92.09 | 70.79 | 59.28 |
| Practice nurse (GP clinic) | 77.53 | 18.00 | 4.67 | 4.69 |
| Community nurse | 62.61 | 30.72 | 76.76 | 27.82 |
| Health visitor | 0.00 | 0.00 | 0.00 | 1.36 |
| Psychologist | 0.00 | 0.11 | 2.22 | 0.00 |
| Counsellor | 0.00 | 0.00 | 1.01 | 3.16 |
| Physiotherapist | 0.48 | 3.55 | 3.04 | 0.47 |
| Occupational health therapist | 0.26 | 0.56 | 0.00 | 0.51 |
| Care manager | 6.30 | 0.34 | 0.00 | 0.00 |
| Social worker | 2.55 | 0.00 | 0.04 | 0.16 |
| Home care worker | 7.72 | 0.66 | 0.26 | 4.44 |
| Care attendant | 0.14 | 2.10 | 0.43 | 0.00 |
| Carer’s support worker | 0.00 | 0.00 | 0.19 | 0.56 |
| Chiropodist | 0.34 | 0.83 | 1.00 | 0.95 |
| Dietitian | 1.23 | 1.90 | 1.35 | 0.55 |
| Self-help group | 0.00 | 0.11 | 0.00 | 0.00 |
| Other | 2.16 | 6.19 | 6.96 | 9.58 |
| Secondary care | ||||
| Oncology inpatient ward | 776.22 | 375.85 | 260.81 | 321.22 |
| Medical inpatient ward | 2032.06 | 794.64 | 586.99 | 648.65 |
| Continuing care/respite inpatient ward | 72.32 | 8.44 | 0.00 | 3.78 |
| Assessment/rehabilitation inpatient ward | 145.82 | 27.83 | 0.00 | 10.53 |
| Other inpatient ward | 1283.76 | 522.46 | 136.71 | 414.56 |
| Intensive care inpatient ward | 388.65 | 94.10 | 54.93 | 58.52 |
| Inpatient consultations (including PAMs) | 131.71 | 42.51 | 10.47 | 135.45 |
| Outpatient visits (including consultations) | 433.75 | 484.13 | 467.04 | 391.64 |
| Accident and emergency | 48.21 | 60.76 | 35.25 | 22.69 |
| Day hospital | 71.00 | 129.43 | 80.61 | 29.08 |
| Other | 500.09 | 324.47 | 177.04 | 96.50 |
| Total cost | 6145.26 | 3030.41 | 2004.27 | 2253.73 |
Appendix 8 Sources of resource use data and unit costs
| Health-care resource | Unit | Unit cost (£) | Details and source |
|---|---|---|---|
| Primary care | |||
| Cancer nurse | Home/clinic visits | 30/30 | Per patient contact lasting 60 minutesa |
| GP | Home/clinic visits | 292/147 | Per patient contact lasting 60 minutesa |
| Practice nurse (GP clinic) | Home/clinic visits | 52/40 | Per patient contact lasting 60 minutesa |
| Community nurse | Home/clinic visits | 70/48 | Per patient contact lasting 60 minutesa |
| Health visitor | Home/clinic visits | 71/49 | Per patient contact lasting 60 minutesa |
| Psychologist | Home/clinic visits | 59/59 | Per patient contact lasting 60 minutesa |
| Counsellor | Home/clinic visits | 59/59 | Per patient contact lasting 60 minutesa |
| Physiotherapist | Home/clinic visits | 36/36 | Per patient contact lasting 60 minutesa |
| Occupational health therapist | Home/clinic visits | 34/34 | Per patient contact lasting 60 minutesa |
| Carer’s support worker | Home/clinic visits | 30/25 | Per patient contact lasting 60 minutesa |
| Care manager | Home/clinic visits | 62/40 | Per patient contact lasting 60 minutesa |
| Social worker | Home/clinic visits | 79/57 | Per patient contact lasting 60 minutesa |
| Home care worker | Home/clinic visits | 20/19 | Per patient contact lasting 60 minutesa |
| Care attendant | Home/clinic visits | 30/30 | Per patient contact lasting 60 minutesa |
| Chiropodist | Home/clinic visits | 30/30 | Per patient contact lasting 60 minutesa |
| Dietitian | Home/clinic visits | 35/35 | Per patient contact lasting 60 minutesa |
| Self-help group | Home/clinic visits | 30/25 | Per patient contact lasting 60 minutesa |
| Other | Home/clinic visits | Various | |
| Secondary care | |||
| Oncology inpatient ward | Inpatient day | Various | Department of Health75 |
| Medical inpatient ward | Inpatient day | Various | Department of Health75 |
| Continuing care/respite inpatient ward | Inpatient day | Various | www.stlukes-hospice.co.uk/about-us/financial-breakdown/ (accessed December 2014) |
| Assessment/rehabilitation inpatient ward | Inpatient day | Various | Department of Health75 |
| Other inpatient ward | Inpatient day | Various | Department of Health75 |
| Intensive care inpatient ward | Inpatient day | Various | Department of Health75 |
| Inpatient consultations (including PAMs) | Appointment | Various | Department of Health75 |
| Outpatient visits (including consultations) | Appointment | Various | Department of Health75 |
| Accident and emergency | Attendance | Various | Department of Health75 |
| Day hospital | Day attendance | Various | Department of Health75 |
| Other | Various | Various | Department of Health75 |
Appendix 9 Client Service Receipt Inventory86 (version 3, 01/03/11)
Adapted from Beecham J, Knapp M. Costing Psychiatric Interventions. In Thornicroft G, Brewin CR, Wing J. Measuring Mental Health Needs. London: Gaskell; 1992. pp. 163–183.
Appendix 10 Frequency of contacts with primary and secondary care health services by 583 patients up to 12 months
| Resource use category | Mean frequency of service use at | |||
|---|---|---|---|---|
| 3 months’ follow-up (n = 311) | 6 months’ follow-up (n = 272) | 9 months’ follow-up (n = 233) | 12 months’ follow-up (n = 243) | |
| Primary care | ||||
| Cancer nurse | 0.365 | 0.288 | 0.857 | 0.253 |
| GP | 0.548 | 0.514 | 0.443 | 0.382 |
| Practice nurse (GP clinic) | 1.566 | 0.449 | 0.117 | 0.113 |
| Community nurse | 0.929 | 0.447 | 1.098 | 0.408 |
| Health visitor | 0.000 | 0.000 | 0.000 | 0.019 |
| Psychologist | 0.000 | 0.002 | 0.038 | 0.000 |
| Counsellor | 0.000 | 0.000 | 0.017 | 0.053 |
| Physiotherapist | 0.013 | 0.099 | 0.084 | 0.013 |
| Occupational health therapist | 0.008 | 0.017 | 0.000 | 0.015 |
| Care manager | 0.158 | 0.006 | 0.000 | 0.000 |
| Social worker | 0.033 | 0.000 | 0.001 | 0.002 |
| Home care worker | 0.386 | 0.033 | 0.013 | 0.222 |
| Care attendant | 0.005 | 0.070 | 0.014 | 0.000 |
| Carer’s support worker | 0.000 | 0.000 | 0.006 | 0.019 |
| Chiropodist | 0.011 | 0.028 | 0.033 | 0.032 |
| Dietitian | 0.035 | 0.054 | 0.039 | 0.016 |
| Self-help group | 0.000 | 0.004 | 0.000 | 0.000 |
| Other | 0.082 | 0.245 | 0.198 | 0.349 |
| Secondary care | ||||
| Oncology inpatient ward (bed-days) | 1.38 | 0.49 | 0.48 | 0.33 |
| Medical inpatient ward (bed-days) | 3.14 | 1.08 | 0.65 | 0.83 |
| Continuing care/respite inpatient ward (bed-days) | 0.16 | 0.02 | 0.00 | 0.01 |
| Assessment/rehabilitation inpatient ward (bed-days) | 0.36 | 0.10 | 0.00 | 0.06 |
| Other inpatient ward (bed-days) | 2.63 | 1.16 | 0.20 | 0.32 |
| Intensive care inpatient ward (bed-days) | 0.27 | 0.07 | 0.04 | 0.04 |
| Inpatient consultations (including PAMs) (attendance) | 0.68 | 0.17 | 0.07 | 0.39 |
| Outpatient visits (including consultations) (attendance) | 2.91 | 3.25 | 3.20 | 2.65 |
| Accident and emergency (attendance) | 0.32 | 0.38 | 0.24 | 0.14 |
| Day hospital (attendance) | 0.37 | 0.65 | 0.48 | 0.07 |
Appendix 11 List of study research sites and principal investigators
| Institution | Principal investigator | Institution address |
|---|---|---|
| Royal Liverpool University Hospital NHS Trust | JP Neoptolemos | Prescot Street, Liverpool, L7 8XP |
| University Hospital Southampton NHS Foundation Trust | CD Johnson | Tremona Road, Southampton, SO16 6YD |
| Portsmouth Hospitals NHS Trust | M Abu Hilal | Queen Alexandra Hospital, Southwick Hill Road, Portsmouth, Hampshire, PO6 3LY |
| Leeds Teaching Hospitals NHS Trust | A Smith | Beckett Street, Leeds, LS9 7TF |
| Glasgow Royal Infirmary – NHS Greater Glasgow and Clyde | C McKay | 84 Castle Street, Glasgow, G4 0SF |
| University Hospitals Birmingham NHS Foundation Trust | R Sutcliffe (S Bramhall, Wye Valley NHS Trust) | Edgbaston, Birmingham, B15 2TH |
| Barts Health NHS Trust | HM Kocher | Whitechapel, London, E1 1BB |
| Royal Marsden NHS Foundation Trust | D Cunningham | Fulham Road, London, SW3 6JJ |
| University College London Hospitals NHS Foundation Trust | SP Pereira | 69–75 Chenies Mews, London, WC1EHX |
| Royal Free London NHS Foundation Trust | B Davidson | Pond Street, London, NW3 2QG |
| Royal Blackburn Hospital – East Lancashire Hospitals NHS Trust | D Chang | Haslingden Road, Blackburn, BB2 3HH |
| University Hospitals Coventry and Warwickshire NHS Trust | S Khan | Clifford Bridge Road, Coventry, West Midlands, CV2 2DX |
| Ninewells Hospital – NHS Tayside | I Zealley | Dundee, DD1 9SY |
| King’s College Hospital NHS Foundation Trust | D Sarker | Denmark Hill, London, SE5 9RS |
| Morriston Hospital – Abertawe Bro Morgannwg University Health Board | B Al Sarireh | Heol Maes Eglwys, Morriston, Swansea, SA6 6NL |
| Newcastle Hospitals NHS Foundation Trust | R Charnley | High Heaton, Newcastle upon Tyne, NE7 7DN |
| Nottingham University Hospitals NHS Trust | D Lobo | QMC campus, Derby Road, Nottingham, NG7 2UH |
| Aberdeen Royal Infirmary – NHS Grampian | M Nicholson | Foresterhill, Aberdeen, AB25 2ZN |
Appendix 12 Sample search strategy
The following search terms were used: economic, cost analysis, positron emission tomography, PET, cancer, oncology.
Figure 17 captures our literature selection process.
FIGURE 17.
Literature selection process.
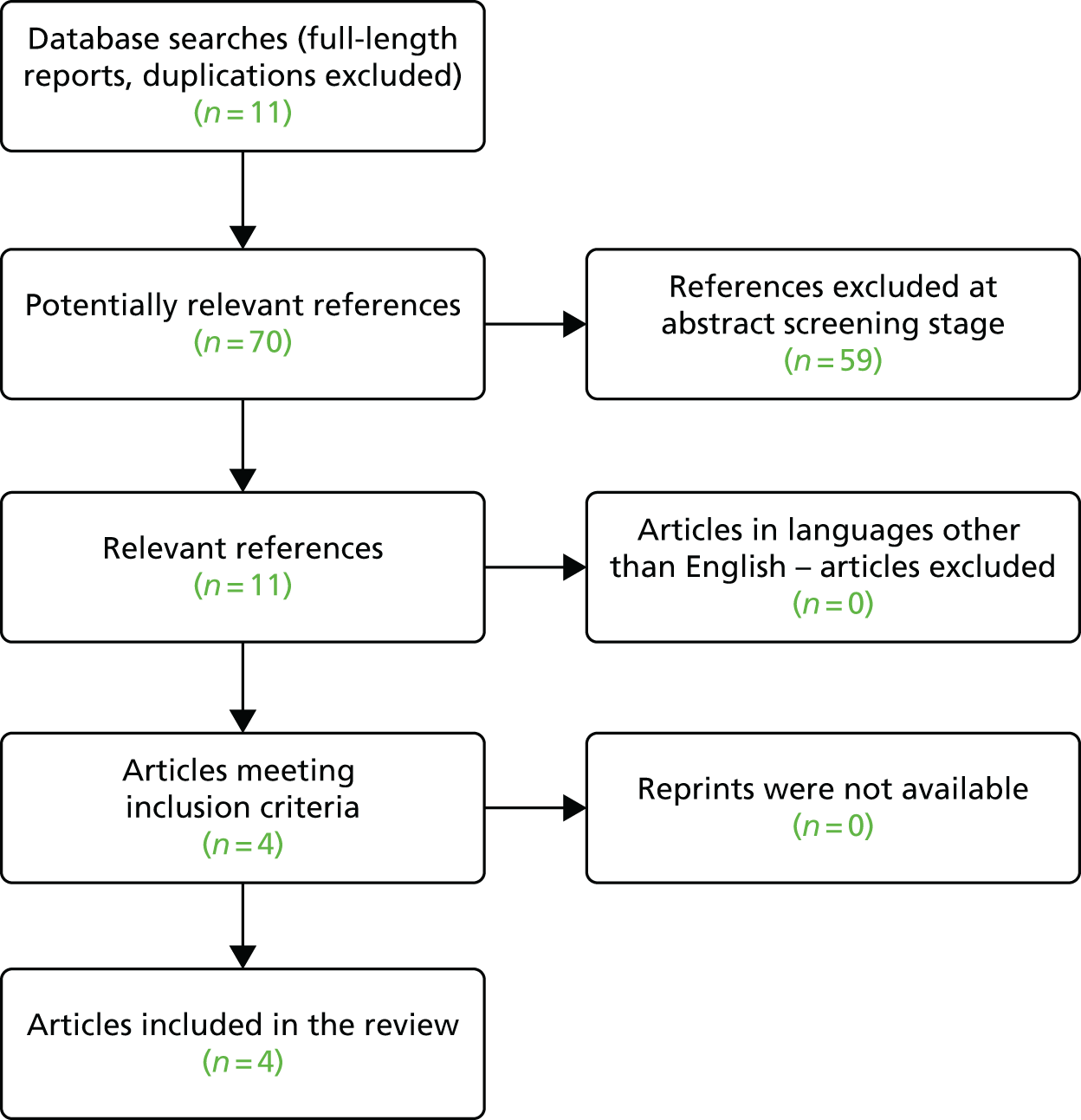
List of abbreviations
- AUC
- area under the curve
- CA19.9
- carbohydrate antigen 19-9
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CRF
- case report form
- CT
- computed tomography
- D1
- diagnosis, staging and management plan after MDCT
- D2
- diagnosis, staging and management plan after PET/CT
- D3
- actual diagnosis, staging and management
- D4
- diagnosis reference standard
- DICOM
- Digital Imaging and Communications in Medicine
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions three-level version
- ERCP
- endoscopic retrograde cholangiopancreatography
- EUS
- endoluminal ultrasound
- FDG
- 18fluorine-2-fluoro-2-deoxy-d-glucose
- FLT
- 18fluorine-fluorothymidine
- FNA
- fine-needle aspiration
- GEE
- generalised estimating equation
- GP
- general practitioner
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- LCTU
- Liverpool Clinical Trials Unit
- LYG
- life-years gained
- MDCT
- multidetector computed tomography
- MDT
- multidisciplinary team
- MRI
- magnetic resonance imaging
- NCRI
- National Cancer Research Institute
- NICE
- National Institute for Health and Care Excellence
- NPV
- negative predictive value
- PACS
- Picture Archiving and Communication System
- PDAC
- pancreatic ductal adenocarcinoma
- PET
- positron emission tomography
- PET/CT
- positron emission tomography with computed tomography
- PET-PANC
- The impact of combined modality positron emission tomography with computed tomography scanning (PET/CT) in the diagnosis and management of pancreatic cancer
- PPV
- positive predictive value
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- SUV
- standardised uptake value
- SUVmax.
- maximum standardised uptake value
- TNM
- tumour, node, metastasis
- UICC
- Union for International Cancer Control
- WHO
- World Health Organization