Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/24/03. The contractual start date was in January 2015. The draft report began editorial review in January 2017 and was accepted for publication in August 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Michael Steiner reports personal fees from Boehringer Ingelheim, non-financial support from Boehringer Ingelheim and GlaxoSmithKline plc, personal fees from Boehringer Ingelheim and GSK, grants from the Medical Research Council and grants from East Midlands CLAHRC, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Cox et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Acute exacerbation of chronic obstructive pulmonary disease
Epidemiology
Chronic obstructive pulmonary disease (COPD) affects > 3 million people in the UK and 210 million worldwide. 1,2 Many patients remain undiagnosed and attribute the symptoms to ageing or other medical conditions. 3 Up to 2 million people in the UK4 have no formal diagnosis of the condition and are unaware of the implications until the disease is in an advanced stage. Presentation typically occurs in the over-50s, with equal prevalence in men and women when adjusting for confounders such as smoking history. 5 Although some therapies reduce the rate of progression, many clinicians focus on relief of symptoms from the outset. Acute exacerbation of COPD (AECOPD) is defined as a sustained worsening of the patient’s symptoms from his or her usual stable state that is beyond normal day-to-day variation and is acute in onset. 1 It is typified by more marked breathlessness and a mucopurulent cough. Exacerbations frequently require hospital admission and AECOPD is the second most common reason for emergency hospital admission in the UK. 6
Aetiology
Acute exacerbation of COPD may be triggered by viral or bacterial infection but is also associated with greater background sputum production, a history of gastro-oesophageal reflux disease and varying underlying immunological responses. In those patients who experience frequent exacerbations, disease progression is associated more with exacerbation frequency than smoking status. 5,7,8
Pathology
During exacerbations of COPD, patients generally develop increased cough with change in sputum viscosity or colour, breathlessness, wheeze and fatigue. Airway inflammation and excess mucus production cause gas trapping and impaired gas exchange, resulting in hypoxaemia and tissue hypoxia. Decreased appetite, in addition to excess protein loss through increased sputum production, can result in nutritional deficiency. Systemic inflammation, tissue hypoxia and nutritional deficiency can cause changes to cardiac, cerebrovasculature and skeletal muscle function,9,10 elevating the risk of cardiovascular disease and skeletal muscle wasting. 11
Prognosis
Acute exacerbation of COPD is associated with accelerated disease progression and increased mortality;12 patients with frequent episodes have a more rapid decline in lung function13 and quality of life14 and decreased exercise performance. 15 Quadriceps force and muscle mass may fall by 5–10% between the third and eighth days of hospitalisation. 16 Loss of muscle function is, in turn, associated with poor exercise tolerance;17,18 quadriceps muscle strength and mass may be predictors of mortality, independent of change in lung function. 19,20 Muscle weakness is caused by physical inactivity,21 use of oral corticosteroids,22 systemic inflammation,16 negative nutritional balance,23,24 increased resting metabolism,23 hypoxia23,24 and hypercapnia. 25–27
Many patients with COPD underestimate the severity of their disease and do not present until the pathology is advanced. 28 As a result, AECOPD requiring hospitalisation may be the first presentation of their condition. Such patients have a much poorer prognosis and are more likely to have one or more comorbidities. 29 Exacerbation frequency is a significant prognostic factor determining future outcome. Previous exacerbations predict risk of future exacerbations and there is a direct relationship between exacerbation severity, exacerbation frequency and mortality. In addition, patients with frequent exacerbations are more likely to experience functional limitations and disabling symptoms, have more frequent contact with health-care professionals, have more comorbidities and have greater associated health-care utilisation and costs. 30
Burden
It is estimated that the direct costs to the NHS in England associated with COPD care are in excess of £800M per year,1 with AECOPD being the second most common reason for emergency admissions in the UK. 6 Reducing hospital admissions and improving patient outcomes following COPD exacerbations has been a major focus for the NHS. 31 Although quality of life and functional improvements have been shown following interventions in stable COPD patients, such assessment is poorly reported immediately following AECOPD. Some studies have shown a trend towards a reduction in mortality but not readmission frequency when patients receive enhanced support at home following hospitalisation, although the evidence is not consistent. 32 Therefore, the true benefit to patients of interventions during this time is difficult to estimate.
Current service provision
Pharmacological management and multidisciplinary care
During hospitalisation with AECOPD, patients will receive medical interventions such as bronchodilators, corticosteroids and antibiotics. Although physiotherapy is available, the focus is primarily on airway clearance and functional assessment rather than active rehabilitation. Aside from smoking cessation, few interventions have demonstrated reductions in exacerbation frequency, disease progression or mortality. 33 Many patients will receive a review post discharge, predominantly conducted by nursing staff. There is no standard model of exercise following discharge from hospital and the offer of active rehabilitation following AECOPD is not universal, although it is recommended. 34 For those who are offered active rehabilitation, the programme will often begin at least 1 month after the exacerbation. 32 The provision of in-home physiotherapy is uncommon and not nationally recommended at present.
Pulmonary rehabilitation
Pulmonary rehabilitation (PR) is defined as an interdisciplinary programme of care for patients with chronic respiratory impairment that is individually tailored and designed to optimise each patient’s physical and social performance and autonomy. Programmes consist of individualised exercise programmes and education. 35 Traditionally in the UK, PR following AECOPD is provided in a community setting; however, PR may also be provided in hospital.
Structured PR programmes following AECOPD should incorporate interval training and continuous exercise to increase exercise capacity while improving symptoms and quality of life. 34,36–40 The British Thoracic Society (BTS) guidelines34 recommend, at a minimum, twice-weekly supervised sessions over 6–12 weeks commencing within 1 month of hospital discharge. PR aims to restore the patient to the highest possible level of independent function through increased physical activity and the provision of education about their disease, different treatment options and coping strategies. 41 Systematic reviews have shown large and important clinical effects for those who adhere to PR programmes in terms of quality of life and daily functioning. 36,42 Despite the established benefits and widespread availability of PR, many patients are reluctant to attend because of misconceptions about the nature of the exercise training, social isolation or transportation difficulties. A UK audit found that < 10% of all hospital discharges for AECOPD complete early post-hospitalisation rehabilitation43 and completion of the full programme can be especially poor in patients with a recent hospitalisation and those receiving long-term oxygen therapy. 44,45
Theoretical basis for pulmonary rehabilitation
In healthy people, physical fitness improves when exercise is undertaken 3–5 days per week at an intensity above 40–85% of the oxygen uptake reserve (difference between resting and peak oxygen uptake) for > 20 minutes (or at lower intensity, preferably for 30 minutes), continuously or in intervals. 46 These principles are no different when applied to people with COPD; however, interventions delivered during PR must recognise and adapt to the limitations to exercise caused by the disease and physical training must be specific to an individual’s requirements. For exercise programmes to be effective, the training load must exceed the loads normally faced during activities of daily living to improve both ventilator capacity and peripheral muscle strength, with training loads being increased as improvement occurs. 37,45,46 PR programmes usually combine exercises that stimulate both the cardiovascular system and the peripheral muscles to reverse the peripheral muscle weakness that is seen in COPD. 47
Targeting individual peripheral muscle groups also helps to minimise the impact of exercise on the respiratory system, allowing patients to undertake exercise without intolerable increases in their respiratory symptoms. Resistance training has been demonstrated to be an effective method of exercise training in COPD. 48–52
In moderate COPD, aerobic training results in significant physiological effects. 53
Intervention methods and materials
In the UK, PR is generally offered in hospital outpatient or community settings, with a minimum of two supervised sessions per week;34 this frequency is not demonstrably optimal but is based on published studies that encompass two supervised sessions and either a third supervised or a formalised unsupervised PR session. 42,54 Programme duration is variable across Europe and globally. On cost grounds most UK programmes last between 6 and 8 weeks; there is debate over the efficacy of programmes lasting for < 6 weeks. 34 Educational components of PR are integral, appearing in every aspect of PR and in discrete educational sessions. They aim to support lifestyle change, behaviour change and self-management to promote decision-making and self-efficacy.
Early pulmonary rehabilitation
Introduction
The efficacy of PR following AECOPD is established, with current national guidelines recommending post-exacerbation rehabilitation commencing within 1 month of hospital discharge. 34 However, the detrimental effects of AECOPD on physical fitness and skeletal muscle function occur rapidly during the inpatient phase,16,55 suggesting that a rehabilitation intervention delivered at the time of the acute illness might have a role in preserving muscle strength and maintaining physical function.
Small-scale trials of PR during AECOPD suggest intervention feasibility and effectiveness. 56,57 However, a large trial (n = 389) in which patients were randomised to a 6-week rehabilitation programme, starting in hospital and continuing after discharge, failed to demonstrate significant improvements in muscle strength, function or quality of life. 58
Theoretical basis of early pulmonary rehabilitation
Acute exacerbation of COPD contributes to disease progression and has a significant systemic impact. Severe exacerbations are characterised by a hospital admission, during which significant skeletal muscle weakness has been observed,16,21 along with a negative protein balance. 59 Several mechanisms, including the presence of systemic inflammation,16 a negative nutritional balance,23 administration of oral corticosteroids22 and physical inactivity,21 may contribute to this acute muscle weakness. A number of potential interventions for maintaining muscle strength and physical function during and immediately after AECOPD, in a hospital, home or community setting, have been proposed. These interventions differ from traditional PR as they must be well tolerated by highly symptomatic patients with increased ventilatory limitation and respiratory symptoms.
Early pulmonary rehabilitation in hospital
Hospital admission for exacerbations should be kept as short as possible so interventions delivered in hospital during AECOPD should not lengthen overall the duration of hospital stay. 60
Exercise modalities delivered in a hospital setting during an inpatient stay for AECOPD need to be chosen carefully because of the markedly increased dyspnoea and fatigue experienced during exacerbations. To avoid excessive respiratory symptoms, demand on the respiratory system and air trapping within the lungs should be kept to a minimum. Exercise training during exacerbations may aggravate local inflammation and damage to peripheral muscle and high-intensity exercises performed until exhaustion are associated with increased muscle damage in stable COPD. 61 Candidates for suitable hospital-based exercise programmes include resistance training and non-volitional training.
Resistance training
Resistance training is effective in counteracting skeletal muscle deconditioning and weakness disuse atrophy62 and also has a relatively low demand on the ventilatory system. 62,63 Small-scale studies suggest that it is well tolerated and does not increase peripheral inflammation or muscle damage56 and successfully counteracts skeletal muscle deconditioning and weakness during AECOPD. 56,61
Non-volitional training
Neuromuscular electrical stimulation (NMES) is used for strengthening and maintenance of muscle mass during prolonged immobilisation, selective muscle retraining and the control of oedema;64 it also improves skeletal muscle strength and exercise capacity in stable patients with COPD. 65 NMES is also a potential strategy for use in those who experience intolerable symptoms during or after active (resistance) training. 60 The metabolic response to NMES is significantly lower than that to resistance training66 and it does not increase muscle oxidative stress. 67 NMES programmes of ≥ 16 sessions improve peripheral muscle strength, exercise capacity and health-related quality of life;68,69 it is safe and effective in frail patients with severe respiratory or cardiovascular impairment. 70,71 For those admitted to the intensive care unit with AECOPD, NMES conducted for 1 hour for 5 days per week for 6 weeks demonstrated enhanced effects on muscle force and 6-minute walk distance (6MWD) and allowed an increase in muscle fibres, without causing muscle damage. 67
Early pulmonary rehabilitation after hospital
A Cochrane review36 concluded that PR after the initial exacerbation recovery reduces hospital admissions, with follow-up in the reviewed studies ranging from 3 to 18 months.
Successful programmes in randomised controlled trials (RCTs) vary in terms of initiation, duration, setting and content. 72–74 Exercise training has mostly consisted of a combination of aerobic and resistance training, with intensities similar to programmes used in stable patients. 74–77 Two trials have confirmed that whole-body exercise training of appropriate intensity is feasible within days of an AECOPD. 73,77
Evidence for effectiveness
Evidence for early pulmonary rehabilitation in hospital
Resistance training in hospital is associated with a 10% increase in quadriceps strength, with muscle biopsies confirming the favourable impact of the intervention on the delicate balance between muscle damage and muscle strengthening. 56 The beneficial effects on muscle strength were still present 1 month after discharge. Training was well tolerated by most of the patients, reflected in the mean dyspnoea and fatigue symptom scores throughout the programme.
Resistance training delivered by cycle ergometer during the hospital stay of frail elderly patients with an AECOPD has also been shown to improve muscle strength, balance and exercise capacity. 61
Evidence for early pulmonary rehabilitation after hospital
Four [95% confidence interval (CI) 3 to 8] AECOPD patients need to be treated with PR to prevent one exacerbation-related admission, with considerable gains in health-related quality of life. 36 Rehabilitation provides pooled differences in 6MWD and the shuttle walking test that are significant and clinically relevant. 36 Two other trials have also reported benefits in terms of quadriceps strength. 74,75
One recent study58 combined in-hospital and home exercise programmes in a 6-week programme, randomising patients with an acute exacerbation of chronic respiratory disease (82% had COPD). The authors found that the programme resulted in enhanced physical fitness at 6 weeks but there was no significant difference in physical function or readmission rates over 12 months compared with usual care. Home rehabilitation was not directly supervised and patient-reported adherence to the programme was low (61%).
A Cochrane review36 reported data from 20 studies (1477 participants) that randomised patients to a PR programme within 3 weeks of hospital discharge following AECOPD. Overall evidence of high quality showed moderate to large effects of rehabilitation on health-related quality of life and exercise capacity in participants with COPD, which were clinically meaningful.
However, larger and more recent trials57,58,78,79 have shown smaller or no effects of PR after AECOPD compared with trials included in the Cochrane review. 36 This inconsistency has been attributed both to publication bias and to methodological shortcomings. The largest trial, which included 320 participants, showed no benefit of PR. 58 However, this trial has been criticised for not offering an extensive PR programme. Participants in the intervention group had, on average, 2.6 supervised sessions during the hospital admission and then received largely unsupervised training after discharge.
Rationale and objectives
Rationale
The National Institute for Health Research (NIHR) commissioning brief [HTA 13/24; see https://njl-admin.nihr.ac.uk/document/download/2009846 (accessed 14 December 2017)] requested a ‘feasibility study’ in ‘early pulmonary rehabilitation after an exacerbation of COPD’, to be started as soon as possible following an acute exacerbation. The brief requested that the control group receive usual care and that the study focus on the ability to recruit, randomise and deliver the intervention, leading us to interpret the brief as requiring an external pilot RCT, sometimes defined as ‘a version of the main study run in miniature to determine whether the components of the main study can all work together’. 80 In other words, we interpreted the brief primarily as requesting a study to understand the feasibility of carrying out a definitive trial. However, as the diffusion of innovations in health services is often difficult,81 and there was no clear front-running intervention to assess in a large-scale evaluation, we also assessed the feasibility of delivering early pulmonary rehabilitation (EPR) in NHS secondary care (in-hospital) and community (post-discharge) settings.
Primary objective
The primary objective was to assess the feasibility of carrying out a definitive RCT that would test the hypothesis that, compared with current practice, early initiation of PR is more clinically effective and cost-effective in AECOPD.
Secondary objectives
-
Carry out an external pilot RCT to determine:
-
the availability of eligible patients and the likely rates of participant recruitment and attrition
-
whether or not data of acceptable quality can be collected
-
whether or not the research interventions can be delivered per protocol
-
key design features including the best primary end point and sample size for the main trial.
-
-
Carry out fully integrated qualitative research to determine, in line with the Medical Research Council (MRC) framework:82
-
potential barriers to recruiting participating centres in the main trial
-
reasons for patient refusal of consent and obtain data on whether or not the baseline characteristics and adherence to routine treatment of non-recruited patients differ from those of consenting participants
-
the reasons for participant attrition
-
the acceptability of the research and intervention procedures to participants and health professionals.
-
-
Carry out a fully integrated health economic analysis and modelling to:
-
identify key drivers of NHS and social care costs
-
pilot data collection strategies in advance of the definitive trial
-
quantify the potential benefit of running the definitive trial.
-
Chapter 2 Methods
Treatment theory
The development of the intervention is presented in detail in Appendix 1 and a summary of the treatment theory for both interventions is shown in Figure 1.
FIGURE 1.
Treatment theory for the hospital and home EPR interventions.
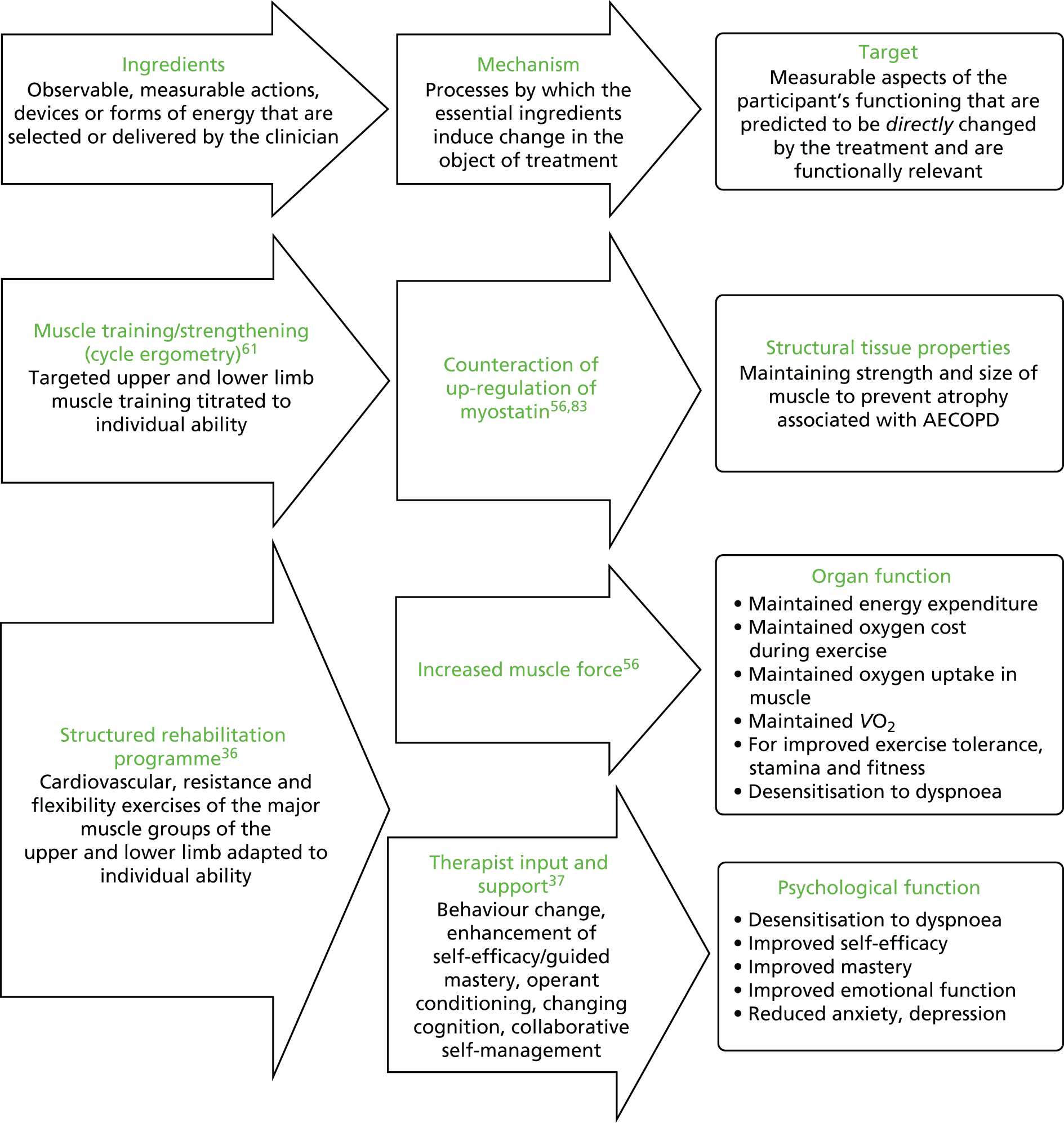
Based on the existing literature and team discussions, we developed the programme theory, which can be briefly described as follows:
-
Hospital EPR. Patients and physiotherapists will be willing and able to conduct hospital EPR using a cycle ergometer in the hospital setting and will allocate resources for this (inputs and activities). This will be manageable within the existing roles, interactions and relationships that characterise the management of AECOPD (context).
-
Home EPR. Patients and physiotherapists will be willing and able to conduct home EPR in patients’ homes and will allocate resources for this (inputs and activities). In some places this will involve reconfiguration of existing roles, interactions and relationships that characterise the management of AECOPD (context).
-
For both interventions. Delivery of the programme per protocol (immediate outcomes) will bring about a change in health behaviour and the physiological benefits described in the treatment theory (intermediate outcomes).
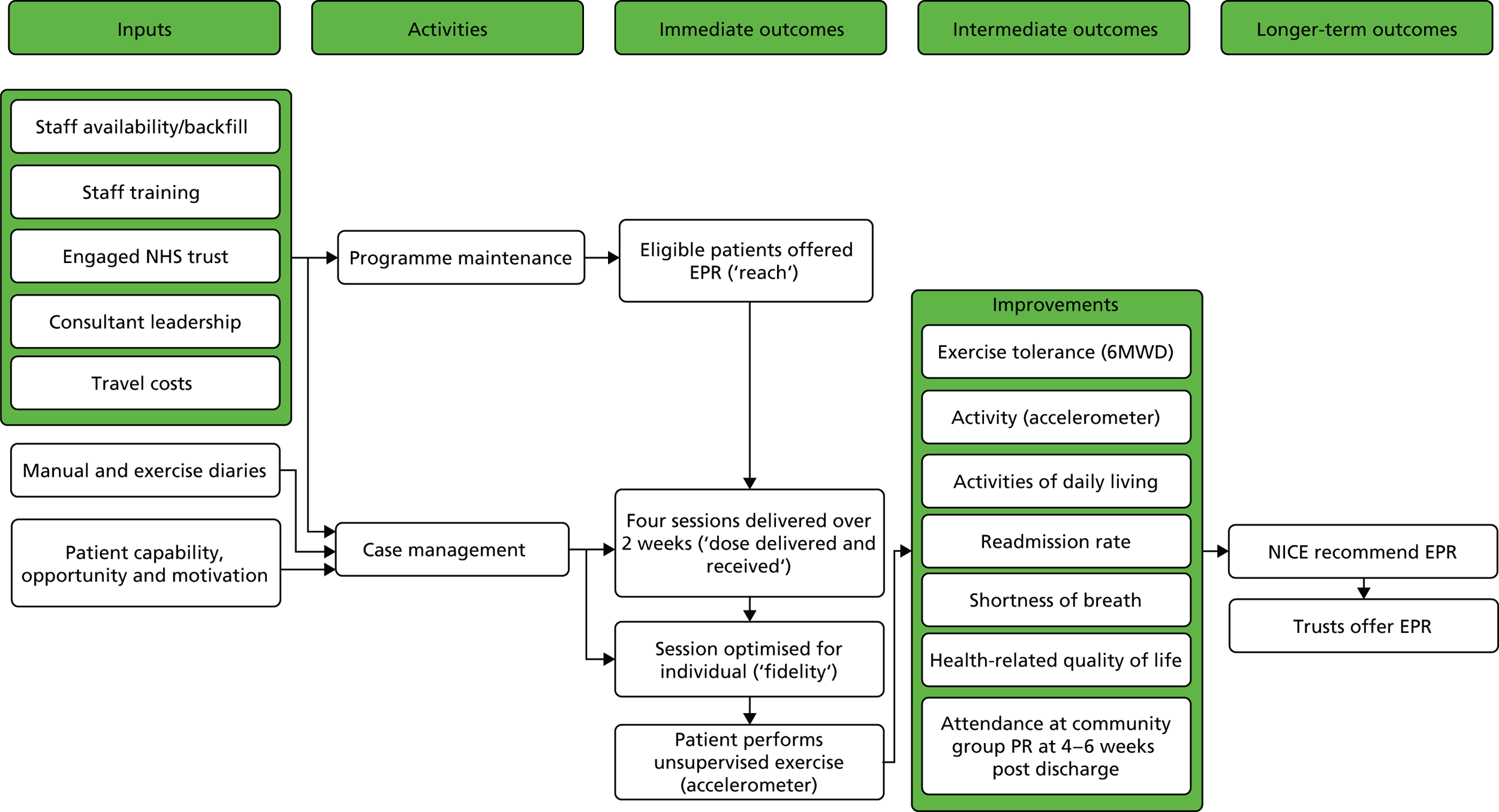
We developed a logic model for each intervention to illustrate how chains of events over time were to bring about the desired outcomes, in accordance with the programme theory (Figures 2 and 3). Contextual factors that can influence and be influenced by implementation are included.
FIGURE 2.
Logic model for hospital EPR. NICE, National Institute for Health and Care Excellence.

FIGURE 3.
Logic model for home EPR. NICE, National Institute for Health and Care Excellence.
The pilot trial
The pilot trial is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement84 and the pilot and feasibility trials extension. 85
Trial design
This was a parallel-group, randomised pilot 2 × 2 factorial trial (with equal allocation ratio for each of the four groups) comparing hospital EPR, home EPR, both hospital EPR and home EPR and usual care alone (delayed community-based group rehabilitation).
Participants
Participants had been admitted to collaborating centres with an acute onset and sustained increase in symptoms from a stable state outside the expected range of variation. Typical symptoms are were ‘worsening breathlessness, cough, and increased sputum production and change in sputum colour. The change in these symptoms often necessitates a change in medication’. 34
Inclusion criteria
-
Age ≥ 35 years with known COPD and admitted to hospital with a primary diagnosis of an AECOPD, clinically determined by the treating physician.
-
Blood pH (as measured by arterial blood gas) of > 7.35 (at the time of consent).
-
Maintaining blood oxygen saturation level (SpO2) within the prescribed target range (as prescribed by the treating physician) with or without controlled oxygen at rest. 35
-
Glasgow Coma Scale (GCS) score of 15.
Exclusion criteria
-
Acute myocardial infarction/heart failure within the last 6 weeks.
-
Suspected/confirmed pulmonary embolism within the last 6 weeks.
-
Known abdominal aortic aneurysm of > 5.5 cm (or > 4.5 cm if the ultrasound scan is > 3 months old).
-
Known cardiovascular instability: heart rate of > 120 beats per minute and/or systolic blood pressure of < 100 mmHg at the time of screening or the requirement for inotropic support or patients with an implantable cardioverter defibrillator.
-
Known extensive pulmonary fibrosis.
-
Absolute contraindications to exercise or musculoskeletal conditions limiting exercise capacity as assessed by a trained physiotherapist.
-
Unable to give full informed consent.
-
Non-English speaker (to allow fully informed consent and the completion of questionnaires).
Withdrawal criteria
Patients could withdraw from the trial; data collected up to that point were kept, in line with the Data Protection Act. 86 If participants were readmitted to hospital for a COPD- or intervention-related cause or underwent any inpatient stay of ≥ 48 hours, then they were withdrawn from trial treatment and managed under usual care pathways. Participants whose condition changed following randomisation so that they met the exclusion criteria or those whom the care team thought should discontinue with the intervention were also withdrawn from treatment. Outcome data were collected from these participants when possible.
Changes to eligibility following trial commencement
Changes made to the essential documentation, during the trial and following ethics approval on 13 August 2015, are provided in Appendix 2. Substantial amendment 1 (submitted October 2015) changed the exclusion criterion ‘predicted length of hospital stay < 5 days’ to ‘patients whose discharge is planned within 48 hours of admission’. In the first 2 weeks of recruitment, 28 screening failures as a result of the predicted length of stay (LOS) in hospital were found to be inaccurate by the Trial Management Group (TMG). Actual LOS was collected for use in any full-scale trial.
In substantial amendment 1, the existing exclusion criteria were clarified: ‘(at the time of consent)’ was added to the criterion blood pH of > 7.35; GCS score of ≥ 15 was amended to GCS = 15; ‘or patients with an implantable cardioverter defibrillator (ICD)’ was added to the cardiovascular instability exclusion criterion; and ‘known’ was added to ‘extensive pulmonary fibrosis’ to make it clear that this should be checked in the clinical notes and that specific tests were not required.
Substantial amendment 2 (submitted December 2015) removed the LOS criterion as it was still a barrier to recruitment. Clinical practice had changed since the study was designed, with patients having, or consultants predicting, shorter stays. The LOS criterion was related to intervention delivery and not patient suitability and so we enrolled patients to test the feasibility of the 5-day in-hospital intervention.
Substantial amendment 4 (submitted March 2016) changed the withdrawal criterion. A participant was admitted to hospital for 1 day with sickness and vomiting, leading to their withdrawal from home EPR, although the clinical team and the participant felt that they were able to continue. Following this, the withdrawal criteria were changed to COPD- or intervention-related hospital admissions or admissions lasting for ≥ 48 hours.
Settings and locations where the data were collected
Sheffield Teaching Hospitals (STH) NHS Foundation Trust (NHSFT) was the trial sponsor and the trust was the clinical co-ordinating centre. Co-ordination of the trial was undertaken by the Clinical Trials Research Unit (CTRU). Patients were approached and recruited at STHNHSFT and Aintree University Hospital (AUH) NHSFT, the two participating centres. Research interventions were delivered by hospital trust employees in participating hospitals (hospital EPR) and in homes in their service catchments (home EPR).
Both hospitals are large teaching hospitals: STHNHSFT serves a population of 550,000 in Sheffield and had 1194 COPD admissions in 2016; AUHNSFT serves a population of around 330,000 in North Liverpool, South Sefton and Kirkby and had 1486 COPD admissions in 2016.
Screening, assessment of eligibility and consent
Eligible patients were identified by physicians, physiotherapists and research nurses, who provided the participant information sheet and discussed the trial with patients. Patients were given a 1-hour cooling-off period to consider trial participation. Patients had to be entered into the trial within 48 hours of admission to allow enough time to complete the baseline assessments and start the in-hospital intervention. The cooling-off period therefore had to be kept short; however, if patients required more time to make a decision, this was accommodated within the 48-hour window when possible. Written informed consent was obtained from every participant by the Principal Investigator or a suitably trained delegate (physician, physiotherapist or research nurse). An eligibility form was completed for all participants.
Randomisation
Following consent and the baseline assessments, participants were allocated in equal proportions to one of four groups:
-
both hospital EPR and home EPR
-
hospital EPR but no home EPR
-
no hospital EPR but home EPR
-
usual care (neither hospital EPR nor home EPR).
All participants were invited to attend group rehabilitation as per usual care guidelines. However, those allocated to home EPR were to receive four fewer sessions to account for the four sessions delivered in the home.
The randomisation list was created using a computer-generated pseudo-random list, stratified by centre, with random permuted blocks of varying sizes; it was hosted by the Sheffield CTRU in accordance with standard operating procedures and held on a secure server. Access to the allocation sequence was restricted to those with authorisation. The sequence was concealed until recruitment, data collection and the analyses were complete. Randomisation was completed by the physician or physiotherapist (or central team member when they were not available), who then made the arrangements for the appropriate intervention(s) to be delivered. Participants were informed of their allocation in two stages: (1) they were told about hospital EPR following randomisation and (2) they were told about home EPR at discharge. Trial physiotherapists delivered both hospital EPR and home EPR (as well as aspects of usual care). Outcome data were collected by research nurses or other clinical staff, who were blind to the group allocation of the participants when possible; if blinded staff were not available or became unblinded, we still collected these data and recorded that the assessor was unblinded.
Interventions
Following randomisation the research physiotherapy team received a randomisation e-mail and arranged for the allocated treatment to be delivered. To arrange the home visit, the team delivering home EPR communicated with the hospital team to arrange the first home visit within 72 hours of discharge. Other visits were arranged directly with the participants. The interventions are described in the following sections according to guidance from Hoffmann et al. 87
Hospital early pulmonary rehabilitation
Materials
One cycle ergometer (‘bike’) was kept at each participating site; this could be moved to accommodate the location of the participants. Instructions for the cycle ergometer were available to all physiotherapists.
Procedures
A workload was set at the first session and at the two subsequent daily sessions. The protocol for determining the individual training workload was based on the maximal resistance against which participants were able to complete two pedal revolutions (2RM). Starting from the load at which participants could not move the pedals, the load was reduced by 1-kg decrements until participants were able to complete two pedal revolutions. To ensure that the selected load was effectively the maximum, participants were required to perform further attempts when the load was increased by 1-kg increments. The 2RM test was performed for both upper and lower limbs before the first exercise intervention on each day.
Following this assessment, patients completed 16 revolutions on the ‘bike’ with both the upper and lower limbs. During the intervention heart rate, SpO2 and symptoms of breathlessness or fatigue were monitored. Patients’ oxygen was adjusted as required to maintain the SpO2 within any prescribed target range.
Provider
A physiotherapist (band 6+) conducted the initial assessment each day to identify the workload required for the individual. Physiotherapists and physiotherapist assistants could deliver the remaining sessions.
Location and mode of delivery
The cycle ergometer was taken to the bedside by the physiotherapist delivering the session. Participants completed the revolutions on their own under instruction and supervision from the physiotherapist.
Schedule
Patients completed 16 revolutions for both sets of limbs, three times a day for 5 consecutive days.
Tailoring
The intervention was a largely inflexible experimental intervention. The only adjustments that could be made by physiotherapists during the intervention involved adjusting the load to maximise the number of repetitions undertaken while minimising symptoms of breathlessness or fatigue. Workload could be increased from session to session.
Modifications
The intervention was planned to be delivered over 5 consecutive days, although it was predicted that some patients might be discharged prior to receiving the full intervention. Patients who were discharged prior to the intervention being completed continued to receive all usual care and, when possible, the physiotherapist delivering the final intervention confirmed that the hospital EPR intervention was complete and addressed any concerns. During the pilot trial it was found that LOS was shorter than anticipated and an eligibility criterion was amended allowing patients with a predicted stay of < 5 days to be eligible for the trial, meaning that the intervention was often delivered for < 5 days.
Optimisation assessment
The physiotherapists recorded the workload, rotations completed at the session, any adjustments made and any adverse events (AEs). They also recorded participants’ Borg breathlessness scores88 before, during and after the sessions, the Borg rating of perceived exertion (RPE) score89 and session difficulty, as rated by participants at the end of the sessions, as well why a session did not go ahead when applicable.
Home early pulmonary rehabilitation
Materials
An exercise manual that included an exercise diary was provided to all participants and physiotherapists delivering the intervention. At the start of the exercise manual information was provided on exercise for patients with COPD as well as advice on breathing control, shortness of breath, mood and safety; the Borg breathlessness score was also described.
The exercise manual then provided instructions for a warm-up, the eight main exercises, a walking plan and a cool down. The eight exercises were marching on the spot, shoulder punches, sit to stand, arm lifts, wall push-up, step exercise, biceps curls and squats.
Weights (or suitable substitutions) were required for shoulder punches, arm lifts and biceps curls, a wall was needed for the wall push-up and the squats, a chair was required for the sit-to-stand exercises and a step was required for the step exercise.
Procedures
The 6MWD data were made available to the physiotherapists delivering the sessions to provide them with a functional assessment of participants. When this information was not available, physiotherapists conducted their own assessment of participants’ exercise capability, usually as assessment of the 2-minute walk distance.
Following this assessment, on the first visit the physiotherapists supervised and instructed participants through the exercises in the exercise manual. Exercise duration could be increased in line with the participants’ capability and the aim was to increase this over time. Visits could last from 20 minutes to 1 hour, depending on participant ability.
Provider
A senior physiotherapist conducted all of the exercise sessions. Because of safety concerns regarding EPR,58 the physiotherapists had a direct link to the care team in the hospital (usually the consultant respiratory physician) if there were any concerns about participants at the home visit.
Location and mode of delivery
Physiotherapists visited participants’ homes to deliver the intervention at a time suitable for the participants (09.00–17.00, Monday to Friday).
Schedule
Four sessions were delivered over 2 weeks, starting within 72 hours of discharge.
Tailoring
Physiotherapists could adapt the intervention to account for participants’ individual exercise capacity and limitations.
Modifications
The intervention did not change during the trial.
Optimisation assessment
The physiotherapists recorded the exercises undertaken, the duration of the exercises, any adjustments made and any AEs. They also recorded participants’ Borg scores before, during and after the sessions and difficulty, as rated by the participants, as well as why a particular session did not go ahead when applicable.
Usual care
Usual care is defined as the ‘best available alternative management strategy’,90 which in this pilot trial was group PR that is routinely offered to patients by the NHS trusts involved.
Participants were invited to attend 12 sessions of group exercise, four or six of which were supplemented with education. These sessions took place once participants were considered to be stable, 4–6 weeks post discharge, and were delivered at existing community venues in Sheffield and Liverpool. If these sessions were attended, participants were also asked to complete at least one prescribed unsupervised session per week34 and to complete an exercise diary91 and daily walking plan. The intervention was multifactorial (individual combination) and exercises were individualised depending on the ability of the participant; however, the intensity of the exercise session and major muscle groups exercised remained constant.
Feasibility criteria
Primary feasibility outcome
The primary feasibility outcome was the feasibility of recruitment to the main trial, defined as recruitment of 76 participants in a 7-month recruitment window at two centres (objective stop–go criterion). This is equivalent to 14 months of recruitment (7 months × two centres) and a recruitment rate of 5.4 participants per centre per month. See Sample size for the calculation of the feasibility recruitment target.
Other feasibility outcomes
-
Recruitment and attrition rates (CONSORT data92): number of patients assessed for eligibility, reasons for exclusion, number of physiotherapists in each group as well as the number of patients treated by each, numbers lost to follow-up, numbers discontinuing the interventions (with reasons) and numbers analysed and excluded from the analysis. Recruiting staff did not prompt for but recorded reasons for refusal of consent when volunteered by patients. We used a published conceptual framework to categorise non-participation in the trial. 93 No personal health identifiers were identified on the log. Research staff invited participants who withdrew from the intervention or research procedures to provide a reason.
-
Numbers of missing values/incomplete cases: acceptable rates of missing values for each questionnaire prospectively defined as 0.5%.
-
Intervention adherence: defined objectively as attendance at 80% of the sessions, which is 12 sessions for hospital EPR and (rounded to) three sessions for home EPR.
-
Intervention fidelity: subjective description of case notes by the study team. The co-applicant physiotherapists rated each physiotherapy session for optimisation using data from the study documentation. The data used for this assessment included the difficulty of the session, as assessed by the participant, whether or not any adaptations had been made and whether or not the session had been completed (see Optimisation assessment).
-
Participant views on acceptability of the interventions and trial procedures (see Chapter 5, Participant views of the interventions and Participant views of the trial procedures).
-
Therapist views on intervention/research protocol acceptability (see Chapter 5, Therapist views of the interventions analysed within normalisation process theory and Therapist comments on the trial procedures analysed within normalisation process theory).
-
Feasibility of recruiting participating centres: the Sheffield CTRU trial manager recorded problems with project approvals and set-up at participating sites; target sites for the main study have been screened for suitability by approaching potential principal investigators.
-
Decision on the primary end point for the main trial: a descriptive assessment based on the above as well as participant feedback on assessments (see Chapter 5, Participant views of the trial procedures, Six-minute walk distance and Questionnaires) and sample size estimation (see Chapter 3, Number of missing values/incomplete cases).
Clinical outcomes
Clinical outcome assessments are provided by time point in Table 1, with more detail provided in the following sections.
| Clinical outcome | Baseline | Prior to discharge | Discharge +7 days | 30 days | 90 days (3 months) |
|---|---|---|---|---|---|
| London Chest Activity of Daily Living scale | PSR | PSR | PSR | PSR | |
| EuroQol-5 Dimensions five-level version | PSR | PSR | PSR | PSR | |
| COPD Assessment Test score | PSR | PSR | PSR | PSR | |
| Dyspnoea, Eosinopenia, Consolidation, Acidaemia and atrial Fibrillation score | PSR | ||||
| Perceived Necessity and Concerns questionnaire | PSR | PSR | PSR | PSR | |
| Malnutrition Universal Screening Tool | PN | ||||
| Demographics | PSR and PN | ||||
| 6MWD | PC | PC | PC | ||
| MRC Dyspnoea Scale | PSR | PSR | PSR | ||
| Activity monitor | PC | ||||
| Activity diary (home EPR only) | PSF | ||||
| SAEs | PN | PN | PN | PN | |
| Health and social care resource use questionnaire | PSR | PSR | |||
| Record of the most recent FEV1 dataa | PN | ||||
| Exacerbations over the last 3 months | PN | ||||
| Readmission over the last 3 months | PN |
Primary clinical outcome
The primary clinical outcome was the 6MWD, a validated objective evaluation of functional exercise capacity. 94 The primary outcome was measured at 90 days post randomisation; this was also a secondary outcome, measured at 30 days post randomisation.
Secondary clinical outcomes
-
London Chest Activity of Daily Living scale (LCADL)95,96 – this is a standardised, reliable and validated assessment tool measuring the limitation in activities of daily living in patients with COPD, which is responsive to change after PR; the total score is computed as the sum of the single scores and can range from 0 to 75,97 with a higher score indicating a worse outcome.
-
EuroQol-5 Dimensions five-level version (EQ-5D-5L)98 – this is a generic health status measure for health economic analysis.
-
COPD Assessment Test (CAT)99 – this is a validated self-report multidimensional assessment of the global impact of COPD on health status (cough, sputum, dyspnoea, chest tightness); the total score can range from 0 to 40, with a higher score indicating a worse outcome.
-
MRC Dyspnoea Scale100 – this scale quantifies the disability associated with breathlessness by identifying that breathlessness occurs when it should not or by quantifying the associated exercise limitation; a higher score indicates a worse outcome.
-
Activity monitor – this is a sensitive and well-tolerated method of measuring energy expenditure and activity. Validated in people with COPD, the MoveMonitor (McRoberts B.V., The Hague, the Netherlands) was used in this study, with anonymised data uploaded and analysed using web-based software from McRoberts (DynaportManager version 1.1.5). Metabolic equivalent of task (MET), sedentary MET and steps were recorded for analysis. The following steps were used to build activity monitor-related outcomes:
-
only the days for which the accelerometer had been worn for at least 10 hours were considered
-
a participant was included in the analysis if he or she had at least 5 days of valid data (defined as above)
-
if he or she had > 5 days of valid data, only 5 days of data were used
-
the outcome considered was collapsed over the 5 days.
-
-
Written activity diary – a daily diary of activity was kept by patients allocated to home EPR.
-
Serious adverse events (SAEs) – death, hospitalisation (initial or prolonged), disability or permanent damage and other important medical events. These were elicited from participants, carers, health professionals or medical notes.
-
Health and social care resource use – a bespoke, study-specific questionnaire for health economic data was used, which also captured carer time, travel to appointments and time away from work or other usual activities. The questionnaire drew on data collection tools developed by the School of Health and Related Research (ScHARR) and those collated by the Database of Instruments for Resource Use Measurement. 101
-
Perceived Necessity and Concerns questionnaire – a questionnaire to measure COPD-specific self-reported beliefs regarding exercise that shape a person’s motivation to initiate and adhere to rehabilitation. 102 Validated for use in cardiac rehabilitation research103 and reliable for use in COPD. 102
-
Exacerbations – based on self-report and hospital records. Exacerbations were defined according to National Institute for Health and Care Excellence (NICE) 2010 criteria. 38 Various outcome definitions were proposed; however, given the way that the case report from was designed, it was not possible to ascertain the date on which the event started or ended (unless the event was serious).
-
Readmission and readmission LOS – based on self-report and hospital records. Readmission LOS (‘bed-days’) was analysed to establish if the intervention impacted on the severity of subsequent readmissions.
Baseline-only measures
-
Dyspnoea, Eosinopenia, Consolidation, Acidaemia and atrial Fibrillation (DECAF) score104 – a validated clinical tool for the prediction of mortality in patients hospitalised with an exacerbation of COPD.
-
Malnutrition Universal Screening Tool (MUST) (routinely in clinical use): Screening tool for identifying patients who are malnourished or at risk of malnutrition. 105
-
Demographics – data were collected on medical history, smoking history, previous exacerbations, age, sex, length of diagnosis and cognitive impairment.
-
Previous forced expiratory volume in 1 second (FEV1) (per cent predicted) – standardised spirometry as an overall marker of COPD severity106 (this was collected from the notes at 90 days to ensure that the most recent value was recorded).
Changes to trial outcomes after the trial commenced
Substantial amendment 2 (see Changes to eligibility following trial commencement) removed the LOS criterion and changed the time of the first follow-up to be prior to discharge. Substantial amendment 3 (submitted January 2016) removed the rectus femoris muscle cross-sectional area measurement, which had been difficult to collect and had produced data deemed unreliable by the TMG. Substantial amendment 4 (submitted March 2016) allowed unblinded assessors to collect data when it would otherwise not be collected. We recorded instances of unblinding to inform future trial design. Minor amendment 2 (submitted April 2016) allowed us to interview trial research nurses as well as the interventionists.
Sample size
The study was an external pilot trial intended to explore the feasibility of conducting a future definitive trial. The sample size for a feasibility study should be adequate to estimate the uncertain critical parameters [standard deviations (SDs) for continuous outcomes; consent rates, event rates and attrition rates for binary outcomes] needed to inform the design of the full RCT with sufficient precision. A sample size of 60 patients with 3-month outcome data (76 randomised with 20% dropout) would allow a SD to be estimated to within a precision of approximately ±19% of its true underlying value with 95% confidence. This estimate was synthesised with SDs observed in other published studies and ongoing trials to provide a robust estimate for use in the sample size calculation for the full trial.
Blinding
The care team and the participants were not blinded to the interventions. The research nurses (or clinicians) who collected the outcome data were blinded to treatment allocation although they recorded if they became unblinded for individual patients during the trial.
Statistical methods
Data are presented according to relevant guidance. 84,85 Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Analysis population
The intention-to-treat (ITT) population included all randomised participants who provided consent. 107,108 Treatment was dealt with ‘as randomised’. Demographics and baseline characteristics summaries were provided for the ITT population. Efficacy and safety analyses were performed on the ITT population.
The PEARL score is a simple tool that can effectively stratify patients’ risk of 90-day readmission or death. 109 Post hoc analysis compared the rates of exacerbation and hospital readmission with those predicted by the PEARL score for patients who completed the study or, when possible to ascertain, who had an event prior to withdrawal.
Baseline characteristics
Baseline characteristics are summarised using descriptive statistics. Continuous data are summarised using number of observations, mean and SD. Categorical data are summarised using total number of observations, total number of positive observations and percentage of positive observations among total observations. Summaries are reported overall and stratified by arm.
Feasibility outcomes
Descriptive statistics for the quantitative results are provided and the qualitative findings are presented.
Sample size calculations
Preliminary estimates suggest that the definitive RCT would need to have between 350 and 500 patients in total to detect a small standardised effect size of 0.35 at conventional levels of power (90%) and significance (5% two-sided).
Clinical outcomes
The following outcomes were analysed using linear regression with robust standard errors (SEs): 6MWD, LCADL and CAT pre discharge and at 30 and 90 days and accelerometer outcomes pre discharge and on discharge. Independent variables included were centre (stratification factor), treatment 1 (hospital EPR: usual care vs. experimental; reference: usual care), treatment 2 (home EPR: usual care vs. experimental; reference: usual care) and treatment 1*treatment 2 (interaction term). For the outcomes of CAT and LCADL at 30 and 90 days an additional analysis including baseline and previous independent variables was performed. Mean estimates and 95% CIs are reported for the variables treatment 1, treatment 2 and the interaction term.
The following outcomes were analysed using a logistic regression with robust SEs: having at least one COPD readmission between randomisation and 90 days from randomisation and having at least one exacerbation between randomisation and 90 days. Odds ratio (OR) estimates and 95% CIs are reported for the variables treatment 1, treatment 2 and the interaction term. Participants who withdrew from the trial were not included as we did not collect data on these patients at the follow-up time point after withdrawal.
The outcome of readmission bed-days was computed for participants having at least one COPD readmission. Participants who were not admitted were not included as the aim was to look at the severity of any readmission. If a participant experienced more than one readmission then the total bed stay for all readmissions was computed. If the number of bed-days for a particular readmission crossed the date ‘randomisation + 90 days’, then only days up to 90 days were counted. For example, if the readmission was between day 88 and day 92 from randomisation then, for this particular readmission, only 3 days would be counted (days 88–90). This outcome was analysed in the same way as for other continuous outcomes.
Sensitivity analyses were performed on the 6MWD outcome at 90 days. It should be noted that we were not interested in evaluating the robustness of the treatment effect given that this was a pilot trial but sensitivity analyses could be useful for evaluating the CIs in different scenarios. These included:
-
Physiotherapist effect – in the original statistical analysis plan, a mixed model using centre, treatment 1, treatment 2 and interaction as fixed factors with physiotherapist as a random term was specified; however, when the results were produced, there were difficulties with this estimation because of the low number of patients per physiotherapist and so this sensitivity analysis is reported.
-
Centre effect – two analyses (similar to the main analysis of the 6MWD but without the centre term) were carried out for STHNHSFT and AUHNHSFT.
-
Time effect – use of a generalised estimating equation with exchangeable correlation using 6MWD as the dependent variable at pre discharge, 30 days and 90 days and independent variables as for the main analysis. Time was included as a longitudinal effect.
-
No interaction – the main analysis was performed without the interaction term.
Safety outcomes are reported as descriptive statistics and are listed at a participant level (experiencing at least one of the event considered) and at an event level [according to the Medical Dictionary for Regulatory Activities (MedDRA)110 terms of classification, with further subgroups added within ‘respiratory, thoracic and mediastinal disorders’ and ‘musculoskeletal and connective tissue disorders’ to cover specific events relevant to this research]. Safety outcomes considered were AEs, SAEs and death. The intensity (only SAEs) and relationship (SAEs and deaths) to the intervention are reported.
Missing spurious and unused data
The frequency and percentage of forms completed by time point and site are reported.
Additional sensitivity analysis for dealing with missing data, multiple imputation (20 imputations) under a missing at random assumption, was used to evaluate CIs for the treatment effect under alternative scenarios. Imputed data sets were generated using the multivariate normal method. 111
Post hoc analysis
During the trial a new predictive measure for readmission was developed109 and we used the trial data to see if there was a relationship between the participants’ PEARL prediction score and readmission.
Optimisation assessment
Optimisation of prescription
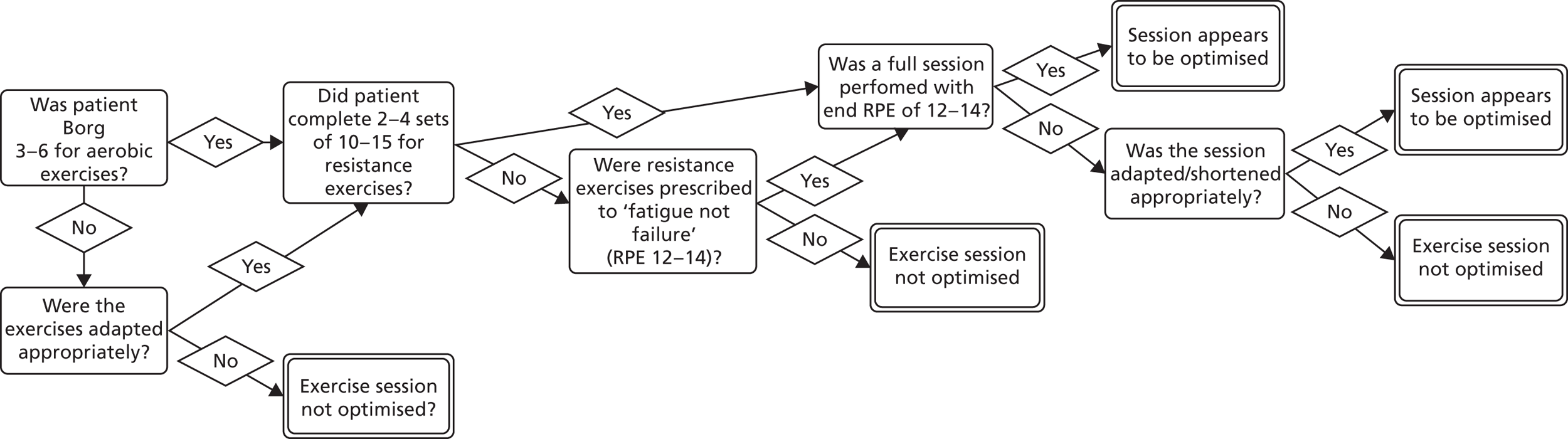
Fidelity is usually interpreted as the consistent delivery of intervention components. 112 However, physiotherapy interventions do not always benefit from consistency as they involve the revision of treatment plans to account for changing and uncertain experiences. 113 Evidence suggests that the fidelity to the treatment theory is more important114–116 than consistent delivery of the intervention. We therefore evaluated treatment optimisation to the patients’ needs and capabilities.
Decisions used to assess session optimisation are shown in Figure 4 (hospital EPR) and Figure 5 (home EPR). Assessment was undertaken by two co-applicant physiotherapists. Assessment was conducted on all participants allocated to hospital EPR (assessed by MC, hospital physiotherapist) and home EPR (assessed by CO’C, community physiotherapist). Decision rules were agreed with the TMG.
FIGURE 4.
Decisions used to assess clinical optimisation of hospital EPR for each session.
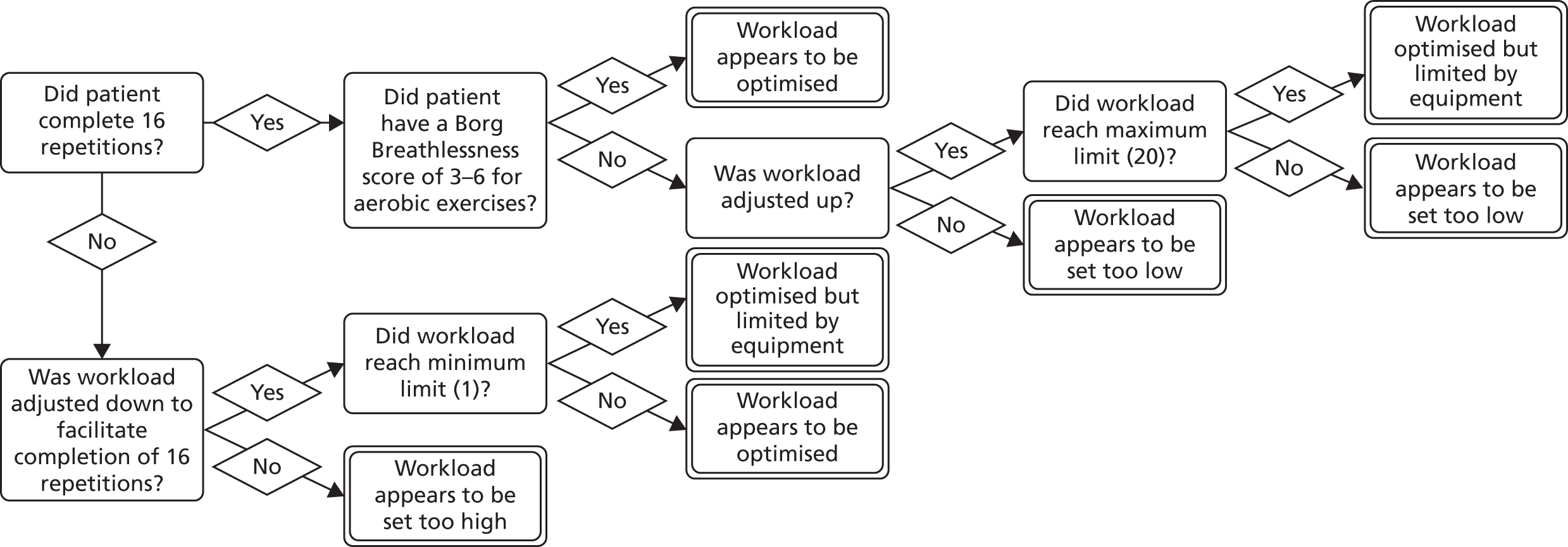
FIGURE 5.
Decisions used to assess clinical optimisation of home EPR for each session.
Adherence to the prescription
Physiotherapists who delivered the hospital EPR intervention recorded the number of rotations undertaken at each session and those who delivered the home EPR intervention recorded the exercises undertaken at each session. Physiotherapists recorded any AEs that occurred and, when possible, the reasons for not taking part in a session (e.g. patient choice, illness or at another appointment).
Assessment of overall treatment optimisation
An overall assessment of optimisation for each intervention by participant was obtained using the assessment of each session. The following rules were used:
-
count the number of exercises completed (1 for completed, 0 otherwise) to provide a value for each session (0–8)
-
count the number of exercises optimised (1 for optimised, 0 otherwise) to provide a value for each session (0–8)
-
for each session, divide the number of exercises optimised by the number of exercises completed to give a ‘single optimised’ score
-
if fewer than five exercises were completed no optimised score is calculated
-
the previous steps are carried out at the ‘session level’; the mean score from all sessions is used as the overall score for each participant.
The qualitative research
-
Participant interviews. A subsample of participants was invited to take part in interviews for the qualitative research. The interviews were detailed in the participant information sheet and an optional tick box was included on the consent form to identify those willing to participate. These participants were followed up by the research team to discuss their participation further and make arrangements for the interviews to take place. Carrying out interviews at 7 days post discharge and at 90 days aimed to provide a longitudinal component.
-
Health professional interviews. Physiotherapists involved in delivering the interventions were given the interview information sheet by the two co-applicant physiotherapists; research nurses were sent the interview information sheet by the trial manager. All health professionals were asked to contact the trial manager if they were willing to take part in the interviews. This was so their colleagues would not know whether they had opted to take part or not; one reminder e-mail was sent by the trial team when required.
-
Non-recruited patient interviews. Patients who did not consent to participate in the trial were asked whether or not they would be willing to receive information about a non-recruited patient qualitative study. Previous studies of this nature have indicated that the circumstances of recruitment will necessitate an opportunistic approach to sampling. 117 Those who agreed to be interviewed for the non-recruited patient qualitative study signed a consent form or provided formal verbal consent prior to their interview. Consent forms included optional tick boxes to allow the research team to collect baseline data from the care team and to contact the patient and his or her care team 3 months after discharge to assess take-up of community-based PR (usual care). These data would be available to the interviewer and the research team and would be combined with feasibility data for designing the main trial.
Interviewer characteristics
Interviews were conducted by DH, a male graduate anthropologist and researcher, KB, a female graduate psychologist and researcher, and CO’C, a female physiotherapist and clinical research graduate.
Relationship with participants
No relationship was established with any participant prior to or outside the interviews; some participants were interviewed twice by the same researcher. Participants were fully informed of the purpose of the interview at the time of consent; this was checked at the start of each interview.
Theoretical and thematic framework
Rationale and worldview118/epistemology119
We employed qualitative research to help understand the implementation of, and response to, the interventions and research protocols,120–122 thereby better understanding causal pathways to their success or failure. 120–123 The pragmatic rationale124 was to provide a basis for ‘organising future observations and experiences’ (p. 33),125 to understand the ‘conceivable practical consequences’ (p. 494)126 of future decisions, rather than to advance, build or test social science theory. 123,127
Research design,118 methodology119 and approach128
The qualitative research had a multiple case design129 with the unit of analysis variably at the participant level and at the level of the two experimental intervention programmes (n = 11 staff interviews). For the participant case studies (n = 27 participants), embedded units of analysis were (1) interview at 7 days post discharge (n = 17), (2) interview at 90 days post randomisation (n = 18) and (3) quantitative case report forms, especially the Perceived Necessity and Concerns questionnaire. 102 Data were available for all three embedded units in eight participants.
Theory
A conceptual framework for describing the context for implementation was provided by the International Classification of Functioning, Disability and Health (ICF). 130 Two published, generic determinant frameworks131 guided the initial design of the study. We used normalisation process theory (NPT)132–135 to understand barriers to intervention implementation by the health system. We used the theoretical domains framework (TDF)136 to more fully understand uptake of and adherence to EPR by patients. The logic models (Figures 2 and 3) provided the elements of specific programme theory to be tested. NPT was also used to understand whether or not wider implementation of the trial would be feasible. 134
Participant selection
Participant interviews
We assumed that the study population was not overly diverse because of the nature of the inclusion criteria and the demographic data. The study protocol was well defined and participants within each group should have experienced similar treatments. We therefore felt that data saturation might be achieved with lower numbers and that it was appropriate to interview eight participants from each randomised group. This would probably be adequate to understand common perceptions of the 12 people who experienced hospital EPR, the 12 people who experienced home EPR and the eight who received neither, achieving thematic saturation137 (as distinct from other forms of saturation138) for both the interventions and the study procedures. Participants were approached face to face by the research nurse or clinician in hospital when they consented to take part in the trial.
Of the 58 trial participants, only two declined an interview but, because of withdrawals, readmissions, patient choice and timing, we conducted interviews with 27 participants in total (Table 2). We interviewed 17 participants around 1 week after discharge, eight of whom were interviewed again around 90 days post randomisation. A further 10 participants (with no 7-day interview) were interviewed at 90 days.
| Interview data | Randomisation group | |||
|---|---|---|---|---|
| Usual care | Hospital EPR | Home EPR | Hospital and home EPR | |
| 7-day interviews | ||||
| Number of interviews | 5 | 7 | 3 | 2 |
| Minimum length (minutes) | 4 | 12 | 10 | 15 |
| Maximum length (minutes) | 15 | 37 | 77 | 28 |
| Average length (minutes) | 11.4 | 22.43 | 40.33 | 21.5 |
| 90-day interviews | ||||
| Number of interviewsa | 2 | 6 | 4 | 6 |
| Minimum length (minutes) | 10 | 6 | 5 | 9 |
| Maximum length (minutes) | 19 | 32 | 14 | 30 |
| Average length (minutes) | 15 | 18 | 10 | 19 |
Health professional interviews
We invited all physiotherapists and research nurses working on the trial to be interviewed. We did not formally assess whether or not saturation occurred or employ stopping criteria. 139 Eleven staff interviews were conducted with two physiotherapists and one research nurse from AUHNHSFT and seven physiotherapists and one research nurse from STHNHSFT.
Non-recruited patient interviews
We aimed to interview six non-recruited patients and conduct further interviews until data saturation had been reached, prospectively defined as six interviews since the last new theme arose (minimum n = 12) or until we recruited a maximum of 24 individuals. Participants were approached face to face by the research nurse or clinician in hospital following non-entry to the trial. Eight non-recruited patients consented; two were interviewed at 7 and 90 days and six changed their mind when contacted to arrange the interview.
Interviewees did not receive any incentivisation or recompense for interview time.
Setting
Semistructured interviews took place between 15 December 2015 and 11 August 2016 for participants and between 29 April 2016 and 26 August 2016 for physiotherapists and research nurses. Participants chose the setting for data collection: most were interviewed by telephone, with six interviewed in their home. In general, interviews were conducted in quiet and private settings to reduce distractions; one interview was conducted with the participant’s daughter present. Two physiotherapists were interviewed in person, one at their place of work and one at the university; other health professionals were interviewed by telephone.
Data collection
In addition to the a priori themes identified in Table 3, semistructured interview guides for participants contained questions about the acceptability of intervention and research protocols.
| COM-B domains | TDF domains | Questions in the topic guide |
|---|---|---|
| Physical capability | Physical skills | What skills do you already have to do the exercises? |
| Can you tell me what you didn’t like about it [PR]? | ||
| Were some exercises better or worse than others? | ||
| How did you feel after each session? | ||
| Psychological capability | Knowledge | Have you done (community) pulmonary rehabilitation before? |
| What do you know about how exercise can help your COPD? | ||
| Cognitive and interpersonal skills | ||
| Memory, attention and decision processes | How do you assess if you are well enough to exercise? | |
| Is exercise something you normally do? | ||
| Behavioural regulation | ||
| Physical opportunity | Environmental context and resources | Do you have the space (to exercise at home)? |
| Can you get to the classes? | ||
| How did you find balancing the rehabilitation with other things? | ||
| How long were the sessions? Was that OK? | ||
| Social opportunity | Social influences (norms) | What support do you have to do exercise? |
| What did you think of the physiotherapist? | ||
| Reflective motivation | Professional/social role and identity | |
| Beliefs about capabilities | Are you confident in your ability to exercise? How confident are you that you will exercise (or continue to exercise) on your own? | |
| When do you think the rehabilitation should take place after you are admitted to hospital? | ||
| Optimism | ||
| Beliefs about consequences | What do you think exercise will achieve? Do you think there are reasons that you should exercise – to get better quicker? To stay healthy (for longer)? | |
| Intentions | Do you intend to attend community pulmonary rehabilitation? | |
| Do you intend to do exercises on your own? | ||
| How would you feel about doing the exercises at hospital/in your home if you ever had another flare-up? | ||
| How do you feel now it’s the end of the course? | ||
| Goals | Do you have any goals regarding exercising? | |
| Do you have any goals regarding your COPD? | ||
| Automatic motivation | Reinforcement | Are there any incentives for exercise? |
| What motivates you to exercise? Is there anything that would help motivate you? How important is exercise to you? | ||
| Can you tell me what you liked about it? | ||
| Emotion | Have you got any concerns about the exercising? What are they? |
The interview guide for health professionals adapted questions suggested by the NPT developers. 134 Interview guides were not piloted; eight participants were interviewed at two time points but otherwise no repeat interviews were undertaken and transcripts were not returned to participants for correction.
All interviews were recorded on an encrypted digital recorder and fully transcribed, with transcriptions anonymised; notes were not taken by the interviewers. Participant interviews at 7 days lasted a median of 16 (range 4–77) minutes and at 90 days lasted a median of 14.5 (range 5–32) minutes, with the duration of the interviews related to the participants’ length of responses to the questions. Physiotherapist interviews lasted a median of 52 (range 44–81) minutes. Although we did not interview eight participants from each group, a total of 27 participant interviews were carried out in total and new content did not seem to appear in later interviews. There was, for the most part, consistency in messages across the groups.
Data analysis
CO’C, KB and DH independently coded samples of transcripts before conferring with each other and the study patient representatives to agree the working coding tree. The coding frameworks are provided in Figure 6. Umbrella themes and subthemes of a priori interest were identified deductively through recourse to the three frameworks: ICF, TDF and NPT (see Theory). The comprehensive core set of the ICF for obstructive pulmonary disease130 includes 17 functions, with a brief core set of 14 functions, and interview transcripts were coded according to these functions. The TDF consists of 14 domains used in behaviour change psychology; each domain of the TDF maps to a COM-B (capability, opportunity, motivation and behaviour) component. 136,140 Skills, although considered a single domain in the TDF, maps to both physical and psychological capability and so appears twice in Figure 6 and Table 3. To understand the perspectives of physiotherapists delivering the two interventions, we used NPT to assess how complex interventions are implemented and maintained in routine practice. NPT categorises the various tasks involved in embedding interventions into services as sense-making, relational, operational and appraisal; each construct contains four subthemes. 132,141 Implementation failure can arise if there are problems in any of these categories.
FIGURE 6.
Coding frameworks.
To assess the conflicting attitudes of participants to EPR and group PR (usual care), the following intervention dimension categories were not derived from a framework but were derived inductively by identifying the differences between EPR and usual care: timing of PR, location of PR, flexibility of visits, one-to-one therapy and introduction to PR (without committing to a PR group programme). These were reported alongside the data for group PR attendance.
Analysis of participant themes took place in NVivo 11 (QSR International, Warrington, UK). Quotations are presented to illustrate the themes. Narrative vignettes about selected individual participant cases are provided for illustrative purposes; they express maximum variation in participant characteristics and findings and combine triangulated data from different embedded units of analysis. A cross-case synthesis142 is provided aggregating findings across a series of individual cases to explore similarities and differences in the findings.
The findings were not discussed with participants for feedback.
Health economics
Overview
An economic evaluation was undertaken to compare the potential incremental cost per quality-adjusted life-year (QALY) of three interventions (home EPR, hospital EPR and home EPR and hospital EPR) compared with usual care over a 90-day trial time horizon. Because of the nature of a pilot study, these cost-effectiveness results are provided to (1) suggest if there is the potential for the interventions to be cost-effective, which could be assessed further in a larger trial; (2) assess the uncertainty around the cost and effect (QALY) estimates and the incremental cost-effectiveness ratios (ICERs) produced; and (3) quantify the expected value of perfect information (EVPI) from obtaining more information from a larger study. The perspective of the economic evaluation was that of the NHS and social care.
Measurement of health status and effectiveness
The EQ-5D-5L data were collected at baseline, pre discharge (before patients were discharged from their index hospital admission; the timing of this varied between patients) and 30 and 90 days post discharge. The EQ-5D-5L was assigned a preference weight based on the UK tariff scores produced by Devlin et al. 143 to calculate QALYs. This is the method recommended by NICE144 for economic evaluation and preference-weighted measurement.
Measurement of resource use
There were three main sources of resource use information: (1) physiotherapist-recorded time, relating to how much time physiotherapists spent on a hospital or home EPR session; data recorded by physiotherapists on case report forms included whether or not a planned session was started and completed and the session time; (2) a person-reported modified Client Service Receipt Inventory (CSRI) collected at both 30 days (describing patients’ resource use between baseline and 30 days) and 90 days (describing patients’ resource use between 30 days’ and 90 days’ follow-up) post discharge; and (3) hospital ward notes, which included the dates that patients were admitted and discharged from hospital for the index hospital admission and any readmissions within the 90-day trial period. Only events or hospital stays that occurred within the 90-day trial period were included in the analysis. The types of resource use parameters from a NHS perspective included in the CSRI and used in this analysis were the number of contacts with a general practitioner (GP) (in the surgery, in the home or by telephone), physiotherapist, occupational therapist, social worker, home care worker and health visitor.
Unit costs
The price year was set to 2015/16. For the interventions, three types of resource use were identified for which a unit cost needed to be sourced: (1) the physiotherapists’ time (hospital EPR and home EPR), (2) the bike purchased for the hospital EPR sessions (hospital EPR only) and (3) a booklet describing the exercises that the patient should carry out in-between physiotherapist visits in the home (home EPR only).
Other resources for which a unit cost was sourced included GP contacts, therapy services and hospital visits. All unit costs are presented in Appendix 3; a summarised description of these unit costs is provided below.
The unit cost of the physiotherapists’ time was based on a mid-point band wage estimate;145 additional associated staff costs (employee national insurance, salary on-costs, overheads and capital overheads) were included based on the calculations used in Curtis and Burns. 146
The cost of the bike used for hospital EPR (MOTOmed viva 2;147 RECK-Technik, Betzenweiler, Germany), including value-added tax (VAT), was £5520. The equivalent yearly cost of the bike was estimated using a annuitisation procedure. 148 For this 90-day study, the equivalent cost of the bike was £295, which is attributed to the intervention cost for those people within the hospital EPR arm and the hospital EPR and home EPR arm.
The home EPR costs consisted of the physiotherapists’ time to deliver the intervention, physiotherapists’ travel time to the home of participants and back to their base of operation and the printing of a 20-page A4 information booklet (it was assumed that each patient could keep the booklet even after the intervention was over).
For GP contacts and therapy services, unit costs were sourced from Curtis and Burns. 146 Hospital visit unit costs were sourced from the NHS reference costs for 2014/15. 149
Economic analysis
The health economic analysis was restricted to those in the ITT population who completed the study. The rationale for basing the economic analysis on this group is that the model-based economic evaluation is based on the observed data from this patient group (because of the small sample size in this pilot study, multiple imputation to account for missing data was not deemed appropriate) and therefore those who withdrew or who were lost to follow-up would automatically not contribute data at specific time points of interest (in particular, at 90 days’ follow-up for the EQ-5D-5L and CSRI). The average cost per patient was calculated by combining the resource use estimates with unit costs.
It was assumed for the purpose of this analysis that all participants across all trial arms received usual care. The incremental intervention cost was therefore estimated to consist of only the cost of the intervention, omitting the cost of usual care; this is because, when using this assumption within this incremental analysis, the cost of usual care cancels out across all trial arms. However, not all participants received usual care in the intervention arms, with some receiving only the intervention without usual care [see Chapter 3, Attendance at group pulmonary rehabilitation (usual care)]. This means that we may have overestimated the cost within the intervention arms compared with the usual care arm (thus underestimating the potential cost-effectiveness, as the effect on health status is still captured in the same way using the EQ-5D-5L). However, this assumption avoids the need to estimate the cost of usual care directly and avoids complicating the exact cost estimation of the interventions with and without usual care between individuals, within and between trial arms.
The economic evaluation for this pilot study is based on an economic decision tree model over a 90-day time horizon, with incremental cost-effectiveness based on the intervention compared with usual care (the model is presented in Figure 7). The rationale for using an economic model rather than a trial-based evaluation was to utilise all of the observed data from the study and then parameterise the uncertainty around the observed data to conduct a probabilistic sensitivity analysis over 10,000 iterations to generate cost-effectiveness acceptability curves (CEACs) and to subsequently conduct an EVPI analysis150 (the EVPI analysis is described in more detail in Expected value of perfect information analysis). A one-way sensitivity analysis could also then be conducted to determine the key outcome drivers in the model and partial EVPI could be conducted to assess the value of collecting more data around specific model parameters. The information from the economic model and subsequent EVPI analysis can be used to inform the design of data collection methods and the potential economic value of a future larger trial. ICERs and cost-effectiveness planes are reported and health status, resource use and cost profiles are described as part of the analysis to provide descriptive statistics around the changes in health and resource use of this patient group between trial arms; missing data are also a focus of these descriptive statistics to assess how well the data collection methods worked in this pilot study.
FIGURE 7.
Illustration of the decision tree model used for the analysis. See Economic model: decision tree for description of terms.

Quality-adjusted life-years were calculated using the area under the curve (AUC) method. 148 The EQ-5D-5L data collected pre discharge were not used in the calculation because of the variable time points at which these data were collected (however, the EQ-5D-5L tariff scores at this time point are reported for descriptive purposes). There are two ways to quantify the AUC based on when the EQ-5D-5L data were collected: (1) use the data collected at baseline and 90 days’ follow-up and (2) use the data collected at baseline and 30 days’ and 90 days’ follow-up. The benefit of using option (1) is that it requires completion of the EQ-5D-5L at only two time points rather than three and therefore there is the potential for fewer missing data points. The benefit of using option (2) is that data from three time points should enable a better health status profile to be elicited over the trial period by accounting for an intermediate change in health status at 30 days rather than assuming a linear change between baseline and 90 days. Given the design of the economic model, option (2) is preferred to option (1) to best reflect the change in health over the 90-day period rather than focusing on the issues around missing data. However, the difference in QALYs using the two methods was assessed using a t-test for two unpaired samples and assuming unequal variance because of the likely difference in sample sizes and variability caused by the inclusion of patients who did not complete the EQ-5D-5L at 30 days.
The statistical analysis was conducted using Stata® version 14 (StataCorp LP, College Station, TX, USA), the economic decision tree model was built and the analysis performed in TreeAge Pro 2015 (TreeAge Software, Inc., Williamstown, MA, USA), the EVPI analysis was conducted using the Sheffield Accelerated Value of Information Tool151 and all figures for the economic analysis (e.g. cost-effectiveness planes and CEACs) were generated within Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA).
Economic model: decision tree
A simple decision tree was used for the model-based economic evaluation, based on the design shown in Figure 7. In this diagram there are four arms related to the four trial arms of the study. Each arm has two types of end point: (1) the cost associated with being in that trial arm (e.g. cUsualCare) and (2) effectiveness in terms of a QALY value associated with being in that trial arm (e.g. uUsualCare). The costs and QALY values are provided by the data collected within this study and then parameterised as part of the economic model. The final parameters used within the model are presented in Chapter 6 (see Summary of physiotherapist time and intervention costs). The time horizon of the model was 90 days and the model was run over 10,000 iterations.
Expected value of perfect information analysis
Cost-effectiveness estimates are subject to uncertainty relating to values of the input parameters for clinical effectiveness, costs and health outcomes. In this instance, the cost-effectiveness analysis was informed by an economic model populated by data from a small sample size as part of a pilot study, which is a particular source of uncertainty. This uncertainty is a genuine concern because any decision based on the evidence provided could be incorrect and this may lead to a loss in health benefits because of an investment in a treatment that is not cost-effective. The value of eliminating all uncertainty, such that there is no risk of an incorrect decision, is called the EVPI. 150 The EVPI provides an estimate of the upper-bound cost of any additional research that would reduce uncertainty and therefore is equivalent to the upper-bound cost of a future larger trial when the analysis is informed by a pilot study. The partial EVPI was also determined to enable identification of those parameters in the economic mode that contribute particularly highly to decision uncertainty and for which there may therefore be a favourable economic return from investing in collecting more information about them.
Triangulation protocol
Rationale
We used different methods in this study and compared their findings using a formal framework, to investigate different components of the research question, to increase the confidence of patients and professionals in our findings and to provide a platform for their feedback.
Design
The qualitative and quantitative methods were used concurrently, with priority granted to neither. We used a modified triangulation protocol152 to relate the qualitative and quantitative findings (methodological triangulation of data sets) in five steps: sorting, convergence coding, convergence assessment, completeness assessment and feedback (resource constraints meant that researcher comparison was not undertaken).
Each data set was reviewed to identify whether or not examples of intervention logic model components (Figures 2 and 3) were present (‘sorting’). We summarised similarities for each logic model component in a matrix using the following codes: ‘agreement’ – coherent interpretation of the data sets; ‘partial agreement’ – some disagreement between data sets; ‘silence’ – the logic mode component is covered by only one data set; and ‘dissonance’ – disagreement between data sets or sources (‘convergence coding’). The level of agreement between data sets was quantified (‘convergence assessment’) and differences in their contribution to the evaluation were highlighted (‘completeness comparison’). The triangulated results were shared with team members and the Trial Steering Committee (TSC) at a face-to-face meeting on 9 November 2016 and further by e-mail communication and discussion to enable ‘feedback’ to be provided, so that points of disagreement could be discussed and any changes in interpretation could be made.
Ethical aspects
The protocol and patient-facing study documentation were reviewed and approved by Sheffield Research Ethics Committee (REC) on 13 August 2015 (reference 15/YH/0259). All substantial amendments were approved by Sheffield REC and all amendments were reviewed and approved by the research and development department at both sites before implementation.
Patient and public involvement
The chief investigator has regular contact with the Breathe Easy group in Sheffield and members of this group collaborated on the development of the interventions and have been involved in consultation at all stages of the research, from design to completion. One group member was a co-applicant, attended TMG meetings and contributed to study management. Another member of the group was on the TSC and attended meetings. The chief investigator and co-applicant (C’OC) presented the results to the group on 9 November 2016 and their feedback has been included in our discussion (see Chapter 8, Implications for policy makers, health professionals and people with chronic obstructive pulmonary disease).
Trial registration and protocol
The trial is registered as ISRCTN18634494, UKCRN 19145 and IRAS 163228 and the protocol is available on the Health Technology Assessment (HTA) programme project webpage. 153
Chapter 3 Results of the pilot trial
Implementation of the interventions and the trial
Implementation summary
The trial was due to start recruitment at the beginning of September but it did not start in STHNHSFT until 28 September 2015 and in AUNNHSFT until 14 October 2015; to keep to the 7-month recruitment window, we extended recruitment to the end of April 2016. There were a number of issues with regard to recruitment, data collection and delivery of the interventions, which are discussed below. Follow-up was completed in August 2016.
NHS treatment costs
Excess treatment costs (ETCs) for delivery of the hospital EPR intervention were provided by the two acute hospital trusts: STHNHSFT in Sheffield and AUHNHSFT in Liverpool. ETCs for home EPR were agreed by Sheffield Clinical Commissioning Group (CCG) in Sheffield. In Liverpool ETCs needed to be agreed by three CCGs but they were only agreed by two, which restricted the population that we could recruit from.
Problems with the delivery of the intervention
Physiotherapist funding
In both centres, the ETCs, research funding and service support costs provided funding for trial physiotherapist time, which allowed delivery of hospital EPR in both centres and home EPR in Liverpool. The funding for the community physiotherapists in Sheffield was agreed in the preliminary discussions for the trial but in the time between bid submission and delivering the intervention there had been cuts in funding and it was not possible to fund the time of any of the physiotherapists. This meant that they had to deliver the intervention on top of their normal workload. Great efforts were taken to deliver the intervention and no sessions were missed as a result of physiotherapists not being available, but recruitment was paused when it was known that the intervention could not be delivered and therefore recruitment stopped for 2 weeks at both sites because of annual leave.
Length of stay
The LOS affected the delivery of the hospital EPR intervention, with 131 sessions missed because of patients being discharged. As discussed in Chapter 2 (see Changes to trial outcomes after the trial commenced), the trial started with an exclusion criterion of a LOS of < 5 days so that the intervention could be delivered over 5 consecutive days.
Problems with recruitment and data collection
Research and service support costs provided funding for research nurse time; this was provided at both sites by a team of research nurses who also worked on other studies, with a dedicated research nurse for the trial. Having a dedicated research nurse who could fully understand and give time to the study was imperative. There were times when the research nurse resource was reduced because of staff sickness and staff not being replaced when leaving their posts, which affected recruitment and data collection, although this cannot be fully appreciated from the non-recruited patient data as these instances may not have been recorded.
Recruitment and participant flow (feasibility outcomes)
Recruitment to the trial
Of 449 patients screened for the trial, 58 were recruited to the trial. Target and actual recruitment figures are shown in Figure 8 and recruitment data by the two centres by month are shown in Table 4. The two centres (Sheffield and Aintree) recruited for approximately 7 months each, giving 14 centre-months of recruitment. It is clear that recruitment started slowly and did not meet the target of 76 but it ended on a rate similar to the target rate. The overall recruitment rate was 4.1 participants per centre per month compared with the target rate of 5.4 participants per centre per month. The overall recruitment rate for Sheffield was 6.4 participants per month and for Aintree it was 1.8 participants per month.
FIGURE 8.
Recruitment graph.
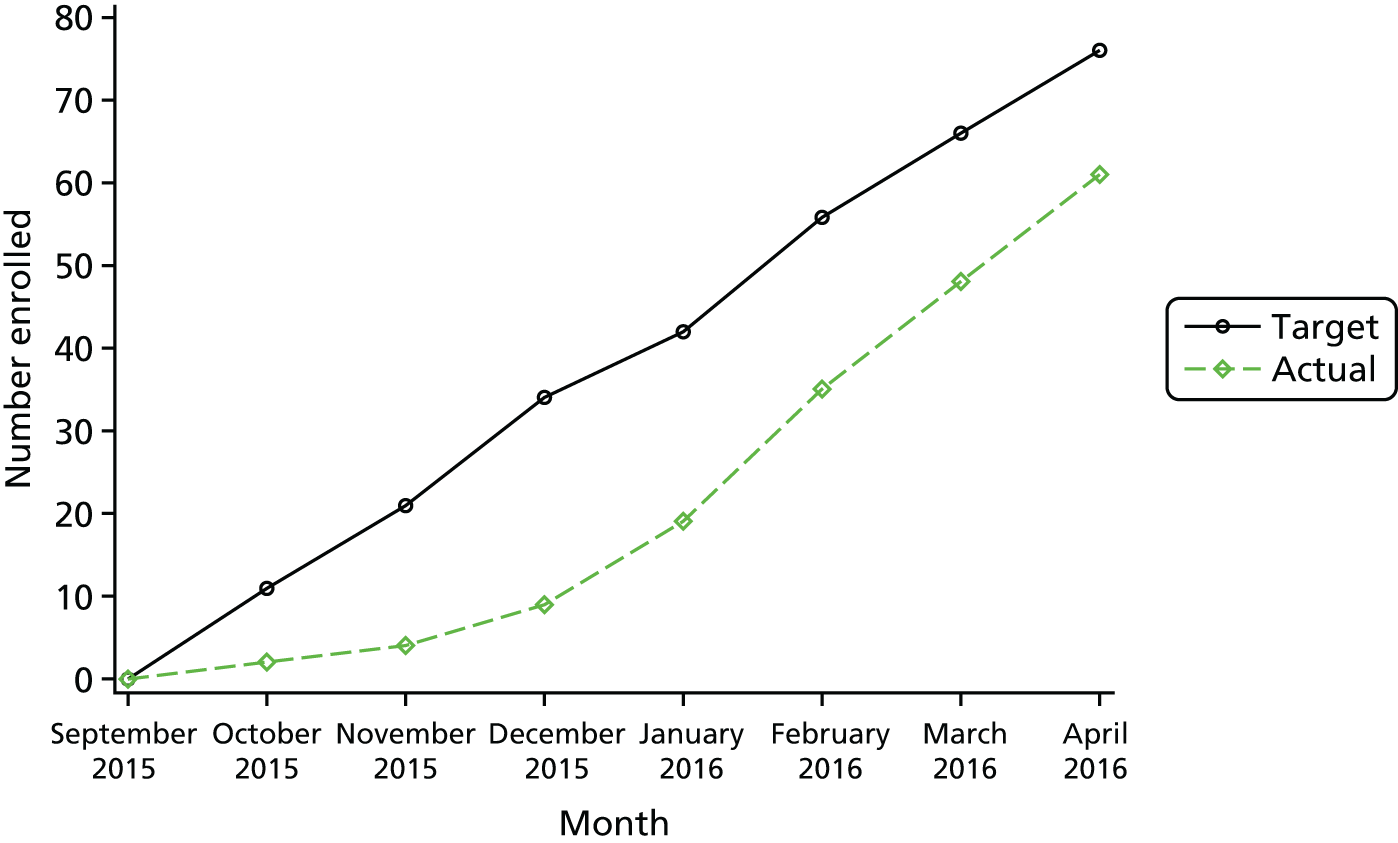
| Month | Sheffield | Aintree | Total | Rate per centre per month |
|---|---|---|---|---|
| September 2015 | 0 | N/A | 0 | |
| October 2015 | 1 | 0 | 1 | 0.5 |
| November 2015 | 2 | 0 | 2 | 1.0 |
| December 2015 | 1 | 4 | 5 | 2.5 |
| January 2016 | 6 | 2 | 8 | 4.0 |
| February 2016 | 14 | 2 | 16 | 8.0 |
| March 2016 | 11 | 2 | 13 | 6.5 |
| April 2016 | 10 | 3 | 13 | 6.5 |
| Total | 45 | 13 | 58 | 4.1 |
In the last 3 months of recruitment, the target recruitment rate was exceeded, although it should be noted that the majority of participants were recruited from the Sheffield centre. The recruitment rate in this period for Sheffield was 11.6 participants per month and for Aintree was 2.3 participants per month.
As discussed in Implementation of the interventions and the trial, in the first few months of recruitment the TMG realised that the LOS exclusion criterion was the main factor affecting recruitment so the LOS criterion was reduced from 5 days to 48 hours (implemented at sites in November 2015). This did improve recruitment at both sites (recruitment rate from December 2015 to April 2016 for Sheffield was 8.4 participants per month and for Aintree was 2.6 participants per month) but was still an issue and so this criterion was removed completely (implemented at sites in January 2016), which improved recruitment in Sheffield.
Figure 9 presents the participant flow for the trial, with the follow-up figures presented overall and for the 6MWD outcome (at relevant time points). Further details of questionnaire completion for all participant-reported measures can be found in Table 10.
FIGURE 9.
Participant flow. a, n = the number of participants with some data collected at this time point. Numbers of participants completing the 6MWD outcome at associated time points are also provided.

Table 5 provides the reasons for non-recruitment to the trial, which are also categorised according to Kanarek et al. 93 The table shows that the eligibility criteria were the main barrier to recruitment, with 233 patients being ineligible; 106 eligible patients did not want to take part in the study. As mentioned previously, the criterion relating to LOS was removed during the trial, but no other criteria could be removed as they related to patient safety with regard to the interventions. Outside the eligibility criteria, the most common reason for non-participation was patient choice relating to feeling too ill to take part.
| Reason | Aintree | Sheffield | Total | Kanarek category |
|---|---|---|---|---|
| Inclusion criteria not met | 79 | 36 | 115 | Eligibility |
| Met exclusion criteria | 72 | 46 | 118 | Eligibility |
| Detoxing | 2 | 0 | 2 | Physician triage |
| Palliative care | 3 | 0 | 3 | Physician triage |
| Patient unavailable | 2 | 2 | 4 | Scientific oversight |
| Discharged | 5 | 4 | 9 | Scientific oversight |
| Outside 48-hour window | 3 | 1 | 4 | Scientific oversight |
| Prisoner | 1 | 0 | 1 | Scientific oversight |
| Recruited to different study | 4 | 0 | 4 | Scientific oversight |
| Staffing | 17 | 5 | 22 | Discussion of trials |
| Patient too ill | 24 | 17 | 41 | Patient consent |
| Not interested | 0 | 1 | 1 | Patient consent |
| Unknown | 2 | 11 | 13 | Patient consent |
| Patient choice | 27 | 24 | 51 | Patient consent |
| Not randomised | 1 | 2 | 3 | Patient enrolment |
Protocol non-compliance
There were 11 cases of non-compliance reported in the trial related to trial documentation, two related to the recruitment window, five related to data collection and one related to eligibility. Non-compliance related to recruitment documentation included instances of using the incorrect consent form or information sheet during consent, although all participants were fully informed of the trial prior to participation. In relation to the recruitment window, one person was recruited outside the 48-hour window and one could not be randomised because of passing the recruitment window. Data collection non-compliance related to follow-up being completed over the telephone and an investigator collecting an outcome (prior to amending the protocol to allow this). Eligibility non-compliance related to an interview participant who did not receive any intervention. None of the reported cases of non-compliance led to a participant being withdrawn. Six cases were reported in Aintree (0.5 per participant) and 13 were reported in Sheffield (0.3 per participant). Cases of non-compliance related to the activity diaries and treatment delivery are reported in Intervention non-compliance as these are all feasibility outcomes.
In addition to the reports of non-compliance, instances of unblinding of the outcome assessors were recorded. In total, there were eight instances in which research nurses were unblinded, relating to seven of the 13 participants recruited in Aintree and one of the 45 participants recruited in Sheffield. Unblinding occurred because of recording in patient notes (n = 2), patient reports (n = 2), a lack of blinded staff (n = 1), the presence of the bike on the ward (n = 1) and a database error (n = 2).
Losses and exclusions after randomisation
Table 6 presents the number of participants who withdrew from the trial. The main reason for withdrawal of participant consent was because the participant felt too ill to continue (n = 7); this was followed by no longer having time (n = 3), being unwilling to complete the follow-up (n = 3), being unhappy with intervention allocation (n = 2), because of the presence of comorbidities (n = 1) and because of personal/family issues (n = 1), with one further participant withdrawing with no reason being given. More than one reason may have been given by participants. One participant was withdrawn by the investigator after receiving a new diagnosis of lung cancer.
| Reason for withdrawal | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Not randomised | Total |
|---|---|---|---|---|---|---|
| Before randomisation | ||||||
| Withdrew consent | N/A | N/A | N/A | N/A | 3 | 3 |
| After randomisation before discharge | ||||||
| Withdrew consent | 1 | 0 | 1 | 2 | N/A | 4 |
| Investigator decision | 1 | 0 | 0 | 0 | N/A | 1 |
| After discharge | ||||||
| Withdrew consent | 3 | 0 | 3 | 2 | N/A | 8 |
| Lost to follow-up | 1 | 0 | 0 | 0 | N/A | 1 |
| Total | 6 | 0 | 4 | 4 | 3 | 17 |
Baseline data
Table 7 shows the characteristics of the 57 participants (the 58 randomised participants minus the one participant who did not complete the baseline assessment); data were collected at baseline with the exception of the FEV1, which was collected at 90 days (and so was not collected for withdrawn participants).
| Measure | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR |
|---|---|---|---|---|---|
| Age (years) | |||||
| n | 57 | 15 | 15 | 13 | 14 |
| Mean (SD) | 67.8 (11.12) | ||||
| Sex | |||||
| Male, n (%) | 22 (39) | 5 (33) | 6 (40) | 6 (46) | 5 (36) |
| Female, n (%) | 35 (61) | 10 (67) | 9 (60) | 7 (54) | 9 (64) |
| COPD severity | |||||
| FEV1 (l) | |||||
| n | 38 | 9 | 11 | 7 | 11 |
| Mean (SD) | 1 (–0.51) | 1.2 (–0.73) | 0.9 (–0.43) | 0.9 (–0.32) | 1.1 (–0.49) |
| FEV1% predicted | |||||
| n | 38 | 9 | 11 | 7 | 11 |
| Mean (SD) | 46.1 (18.59) | 55.6 (22.74) | 36.5 (14.90) | 44.0 (14.51) | 49.4 (17.91) |
| Using oxygen on admission, n (%) | 18 (31) | 6 (40) | 7 (47) | 3 (23) | 2 (14) |
| Comorbidities, n (%) | |||||
| Ischaemic | 11 (19) | 3 (20) | 2 (13) | 4 (31) | 2 (14) |
| Stroke | 5 (9) | 1 (7) | 2 (13) | 1 (8) | 1 (7) |
| Vascular | 3 (5) | 0 (0) | 2 (13) | 0 (0) | 1 (7) |
| Diabetes | 11 (19) | 2 (13) | 2 (13) | 3 (23) | 4 (29) |
| Smoking historya | |||||
| Not reported, n (%) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 0 (0.0) |
| Non-smoker, n (%) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (7) |
| Current smoker, n (%) | 18 (32) | 3 (20) | 7 (47) | 3 (23) | 5 (36) |
| Pack-years | |||||
| n | 17 | 3 | 6 | 3 | 5 |
| Mean (SD) | 36.6 (–19.98) | 35 (–18.25) | 26.8 (–13.2) | 60 (–2) | 35.2 (–25.56) |
| Previous smoker, n (%) | 38 (67) | 12 (80) | 8 (53) | 10 (77) | 8 (57) |
| Pack-years | |||||
| n | 35 | 10 | 8 | 9 | 8 |
| Mean (SD) | 36 (–28.52) | 25.5 (–29.27) | 40.1 (–16.94) | 36.7 (–22.73) | 44.4 (–41.42) |
Table 8 presents the results of the baseline assessments by group. The MUST and extended MRC Dyspnoea Scale data show the number (%) of participants with each score; other outcomes are presented as averages across the groups.
| Measure | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR |
|---|---|---|---|---|---|
| DECAF score | |||||
| n | 57 | 15 | 15 | 13 | 14 |
| Mean (SD) | 1.50 (1.00) | 1.60 (1.12) | 1.53 (0.92) | 1.50 (0.52) | 1.21 (1.31) |
| Dichotomised DECAF score, n (%) | |||||
| 0 | 8 (14) | 1 (7) | 2 (13) | 0 (0) | 5 (36) |
| ≥ 1 | 49 (86) | 14 (93) | 13 (87) | 13 (100) | 9 (64) |
| MUST score, n (%) | |||||
| 0 | 39 (68) | 9 (60) | 9 (60) | 9 (69) | 12 (86) |
| 1 | 5 (9) | 1 (7) | 1 (7) | 1 (8) | 2 (14) |
| 2 | 7 (12) | 3 (20) | 2 (13) | 2 (15) | 0 (0) |
| 3 | 4 (7) | 1 (7) | 2 (13) | 1 (8) | 0 (0) |
| 4 | 1 (2) | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
| LCADL score | |||||
| n | 43 | 10 | 12 | 11 | 10 |
| Mean (SD) | 43.3 (15.4) | 45.5 (17.9) | 41.4 (16.8) | 45.7 (14.9) | 40.5 (12.9) |
| EQ-5D-5L score | |||||
| n | 55 | 13 | 15 | 13 | 14 |
| Mean (SD) | 0.51 (0.27) | 0.46 (0.30) | 0.52 (0.26) | 0.45 (0.29) | 0.60 (0.25) |
| CAT score | |||||
| n | 54 | 14 | 14 | 12 | 14 |
| Mean (SD) | 26.1 (8.1) | 29.4 (7.8) | 24.9 (8.2) | 27.0 (7.5) | 23.3 (8.5) |
| Extended MRC Dyspnoea Scale score, n (%) | |||||
| 1 | 4 (7) | 2 (13) | 1 (7) | 0 (0) | 1 (7) |
| 2 | 11 (19) | 3 (20) | 4 (27) | 1 (8) | 3 (21) |
| 3 | 8 (14) | 2 (13) | 2 (13) | 2 (15) | 2 (14) |
| 4 | 19 (33) | 2 (13) | 4 (27) | 7 (54) | 6 (43) |
| 5a | 11 (19) | 2 (13) | 4 (27) | 3 (23) | 2 (14) |
| 5b | 4 (7) | 4 (27) | 0 (0) | 0 (0) | 0 (0) |
Feasibility outcomes
The primary feasibility outcome, recruitment and attrition, has been discussed earlier in Recruitment and participant flow (feasibility outcomes). Intervention fidelity is discussed in Chapter 4. Participant views on the interventions and trial procedures and therapist views are presented in Chapter 5. All other feasibility outcomes are presented in this section.
Number of missing values/incomplete cases
Data completion
Table 9 presents the percentage of data completion for the indicated measures across all time points when data were collected; forms collected at baseline only are not included.
| Measure | % complete |
|---|---|
| CAT | 99.2 |
| EQ-5D-5L | 99.0 |
| Extended MRC Dyspnoea Scale | 100 |
| LCADL | 97.1 |
| Perceived Necessity and Concerns questionnaire | 98.7 |
| Total for these measures | 98.8 |
Table 10 details questionnaire completion rates at each time point by group. The percentages relate to the number of completed questionnaires for participants who had not withdrawn by that time point. The 6MWD outcome was the measure missed most at each relevant time point.
| Measure | Overall, n (%) | Usual care, n (%) | Home EPR, n (%) | Hospital EPR, n (%) | Hospital and home EPR, n (%) |
|---|---|---|---|---|---|
| Baseline expected,a n | 58 | 15 | 15 | 14 | 14 |
| MRC Dyspnoea Scale | 57 (98.3) | 15 (100.0) | 15 (100.0) | 13 (92.9) | 14 (100.0) |
| DECAF | 57 (98.3) | 15 (100.0) | 15 (100.0) | 13 (92.9) | 14 (100.0) |
| MUST | 56 (96.6) | 14 (93.3) | 15 (100.0) | 13 (92.9) | 14 (100.0) |
| AMT | 3 (5.2) | 1 (6.7) | 1 (6.7) | 0 | 1 (7.1) |
| Smoking pack-years | 52 (89.7) | 13 (86.7) | 14 (93.3) | 12 (85.7) | 13 (92.9) |
| LCADL | 43 (74.1) | 10 (66.7) | 12 (80.0) | 11 (78.6) | 10 (71.4) |
| EQ-5D-5L | 55 (94.8) | 13 (86.7) | 15 (100.0) | 13 (92.9) | 14 (100.0) |
| EQ-5D health today | 55 (94.8) | 15 (100.0) | 15 (100.0) | 12 (85.7) | 13 (92.9) |
| CAT | 54 (93.1) | 14 (93.3) | 14 (93.3) | 12 (85.7) | 14 (100.0) |
| Perceived Necessity and Concerns questionnaire | 57 (98.3) | 15 (100.0) | 15 (100.0) | 13 (92.9) | 14 (100.0) |
| Pre discharge expected,a n | 54 | 14 | 15 | 13 | 12 |
| MRC Dyspnoea Scale | 32 (59.3) | 8 (57.1) | 9 (60.0) | 9 (69.2) | 6 (50.0) |
| LCADL | 27 (50.0) | 6 (42.9) | 9 (60.0) | 7 (53.8) | 5 (41.7) |
| EQ-5D-5L | 31 (57.4) | 7 (50.0) | 9 (60.0) | 9 (69.2) | 6 (50.0) |
| EQ-5D health today | 31 (57.4) | 7 (50.0) | 9 (60.0) | 9 (69.2) | 6 (50.0) |
| CAT | 31 (57.4) | 8 (57.1) | 8 (53.3) | 9 (69.2) | 6 (50.0) |
| Perceived Necessity and Concerns questionnaire | 30 (55.6) | 8 (57.1) | 9 (60.0) | 7 (53.8) | 6 (50.0) |
| 6MWD | 20 (37.0) | 7 (50.0) | 7 (46.7) | 4 (30.8) | 2 (16.7) |
| 7-day follow-up expected,a n | 52 | 12 | 15 | 13 | 12 |
| Perceived Necessity and Concerns questionnaire | 32 (61.5) | 7 (58.3) | 10 (66.7) | 9 (69.2) | 6 (50.0) |
| 30-day follow-up expected,a n | 50 | 11 | 15 | 13 | 11 |
| MRC Dyspnoea Scale | 44 (88.0) | 11 (100.0) | 14 (93.3) | 9 (69.2) | 10 (90.9) |
| LCADL | 36 (72.0) | 8 (72.7) | 11 (73.3) | 8 (61.5) | 9 (81.8) |
| EQ-5D-5L | 43 (86.0) | 10 (90.9) | 14 (93.3) | 9 (69.2) | 10 (90.9) |
| EQ-5D health today | 43 (86.0) | 11 (100.0) | 14 (93.3) | 8 (61.5) | 10 (90.9) |
| CAT | 42 (84.0) | 10 (90.9) | 14 (93.3) | 9 (69.2) | 9 (81.8) |
| Perceived Necessity and Concerns questionnaire | 39 (78.0) | 10 (90.9) | 12 (80.0) | 8 (61.5) | 9 (81.8) |
| 6MWD | 33 (66.0) | 9 (81.8) | 11 (73.3) | 5 (38.5) | 8 (72.7) |
| 90-day follow-up expected,a n | 46 | 10 | 15 | 10 | 11 |
| MRC Dyspnoea Scale | 40 (87.0) | 8 (80.0) | 14 (93.3) | 9 (90.0) | 9 (81.8) |
| LCADL | 33 (71.7) | 8 (80.0) | 12 (80.0) | 6 (60.0) | 7 (63.6) |
| EQ-5D-5L | 38 (82.6) | 8 (80.0) | 14 (93.3) | 8 (80.0) | 8 (72.7) |
| EQ-5D health today | 36 (78.3) | 7 (70.0) | 13 (86.7) | 8 (80.0) | 8 (72.7) |
| CAT | 36 (78.3) | 8 (80.0) | 14 (93.3) | 8 (80.0) | 6 (54.5) |
| 6MWD | 21 (45.7) | 5 (50.0) | 6 (40.0) | 5 (50.0) | 5 (45.5) |
Follow-up windows
In addition to the missing forms detailed in the previous section, the date of completion was recorded for all of the forms that were completed. In total, 68 forms were completed outside the follow-up window of ± 2 days. The 30-day follow-up was the hardest to complete in this time frame (33 forms completed outside the follow-up window), followed by the 90-day follow-up (19 forms), the 7-day follow-up (13 forms) and the baseline assessment (three forms).
Delivery and receipt of the interventions
Delivery of the intervention is detailed for each intervention separately in the following sections; optimisation of the interventions is presented in Chapter 4. The expected number of sessions was calculated for each participant allocated to the interventions and session attendance is detailed along with reasons for sessions not taking place.
Delivery of hospital early pulmonary rehabilitation
Table 11 presents the hospital EPR attendance data and reasons for non-attendance, indicating that delivery of this intervention was difficult, with only 34.1% of sessions going ahead overall. Of the sessions that were started, all were completed, showing 100% adherence to individual sessions. Optimisation of the intervention sessions is presented in Chapter 4 (see Hospital early pulmonary rehabilitation optimisation). As discussed earlier (see Length of stay), the main issue with the delivery of the hospital intervention was that participants had a shorter LOS than expected and this accounted for 112 (54%) of the missed sessions in Sheffield and 19 (41%) of the missed sessions in Aintree.
| Variable | Aintree | Sheffield | Total |
|---|---|---|---|
| Expected number of sessionsa | 84 | 300 | 384 |
| Number (%) of sessions attended | 38 (45.2) | 93 (31.0) | 131 (34.1) |
| Number of sessions completed | 38 | 93 | 131 |
| Number (%) of sessions not attended | 46 (54.8) | 207 (69.0) | 253 (65.9) |
| Reason for non-attendance,b n | |||
| Participant illness | 0 | 4 | 4 |
| Change in participant’s availability | 1 | 5 | 6 |
| Change in therapist’s availability | 19 | 17 | 36 |
| Participant declined session | 0 | 43 | 43 |
| Intervention not started within window (72 hours from admission) and remaining sessions not delivered | 0 | 24 | 24 |
| Missed window for starting intervention because of weekend | 0 | 6 | 6 |
| Participant discharged | 19 | 112 | 131 |
| Sessions started in the afternoon, only time for one session | 4 | 2 | 6 |
Adherence to the hospital EPR sessions that were available (i.e. patients were in hospital) was 51.8% (131 out of 253 sessions).
Delivery of home early pulmonary rehabilitation
The home EPR intervention had a better level of adherence than the hospital EPR intervention, with 78.3% of the expected sessions going ahead overall. Only two sessions were missed in Aintree, because of participant illness and choice (Table 12).
| Variable | Aintree | Sheffield | Total |
|---|---|---|---|
| Expected number of sessionsa | 20 | 72 | 92 |
| Number (%) of sessions attended | 18 (90.0) | 54 (75.0) | 72 (78.3) |
| Number of sessions completed | 16 | 49 | 65 |
| Number (%) of sessions not attended | 2 (10.0) | 18 (25.0) | 20 (21.7) |
| Reason for non-attendance,b n | |||
| Participant illness | 1 | 10 | 11 |
| Change in participant’s availability | 1 | 2 | 3 |
| Change in therapist’s availability | 0 | 0 | 0 |
| Participant declined session | 0 | 4 | 4 |
| Other | 0 | 6 | 6 |
| 2-minute walk distance performance showed not fit to exercise | 0 | 2 | 2 |
| Administrative error | 0 | 1 | 1 |
| Intervention withdrawal because of readmission | 0 | 3 | 3 |
Intervention non-compliance
Activity diaries
Participants allocated to receive home EPR should have all returned an activity diary detailing the exercises completed at the home EPR visits and any other activity carried out over the 2-week intervention period. The physiotherapists were to collect these diaries at the end of the sessions. Of the 29 participants allocated to home EPR, 18 returned their activity diaries: six participants completed more than the four visit entries, six completed the four visit entries and six completed fewer than the four visit entries.
There were several reasons why the activity diaries were not returned: participant withdrew prior to home EPR (n = 3), participant withdrew from the intervention (n = 4), participant wanted to keep the diary (n = 1) and unknown reason/presumed lost (n = 3).
Treatment windows
In addition to the cases of non-attendance recorded earlier, there were other cases of non-compliance relating to the treatment windows. For hospital EPR, the intervention should have started within 72 hours of admission and there were two instances when this did not occur. For home EPR, the first session should have taken place within 72 hours of discharge and sessions should not have been more than 2 days apart; there were 12 instances when these windows were missed.
Table 13 provides the reasons for withdrawal from the intervention. The number of participants recorded as being readmitted and withdrawn does not match the number of missed sessions because of withdrawal reported in Table 12, which is because of blinding of the research nurse. Some participants were reported as being withdrawn from the intervention on admission to hospital by the research nurse even if they had not been allocated to the intervention or if they had completed the sessions.
| Reason for withdrawal | n |
|---|---|
| Patient request | 4 |
| Did not want further PR in home or group | 1 |
| Does not feel like doing it – too unwell | 1 |
| Participant has no time | 2 |
| Other | 7 |
| Participant readmitted and withdrawn from home EPR as per protocol | 7 |
Feasibility of recruiting participating centres
The chief investigator discussed the proposal for a full trial with a number of respiratory consultants in the UK, with eight centres identified as being willing to take part in a large-scale RCT.
Decision on the primary end point and sample size for a full-scale trial of early pulmonary rehabilitation in patients following acute exacerbation of chronic obstructive pulmonary disease
The candidate clinical primary outcome was 6MWD at 90 days. However, this measure had a low completion rate in the pilot trial, with the outcome being available for only 21 out of 58 (36%) participants. In hindsight, COPD readmission or exacerbation may have been an easier outcome to collect as it could have been taken from patient notes.
Readmission was a more reliable measure than exacerbation as the former was based on hospital data whereas the latter was based on patient self-reported events. COPD readmission was used as a continuous variable (mean number of COPD readmissions) rather than a categorical variable (i.e. proportion of participants having at least one COPD readmission during follow-up) to reduce the sample size estimation. It was unclear how to use time to readmission to define the period at risk (i.e. how to count days of hospitalisation) and whether to count only the first or all readmissions. Moreover, much of the literature on pilot trials is focused on continuous and categorical outcomes154 rather than incidence and time-to-event outcomes.
Overall, there were 34 readmissions in 48 patients within the 90-day follow-up period, giving a mean of 0.7 readmissions with a SD of 1.2 (see Table 24). To compute the sample size for a full-scale trial, the SD of the mean number of readmissions was used from the pilot trial. However, it should be noted that there is a considerable degree of uncertainty in this estimate of variability because:
-
the outcome is likely to be skewed, hence SD may not be the best statistic for summarising dispersion
-
the estimate is based on 48 participants.
The upper 95% confidence limit of the SD is 1.5. We decided to use 1.2 as the likely SD. Assuming a variability (SD) of 1.2, Table 14 provides the likely sample size required based on various target differences in the mean number of readmissions between groups, assuming a two-sided significance level of 5% and 90% power.
| Target difference in mean number of readmissions at 90 days | Standardised effect size | n (total) with 10% dropout | n (total) |
|---|---|---|---|
| 0.24 | 0.20 | 1052 | 1169 |
| 0.30 | 0.25 | 674 | 749 |
| 0.36 | 0.30 | 468 | 520 |
| 0.42 | 0.35 | 344 | 383 |
Clinical outcomes and estimation
Key pilot trial outcomes
Tables 15–17 present the data for the 6WMD, EQ-5D-5L and activity monitor outcomes as these are considered the key outcomes of the pilot trial. The numbers of participants included in the 6MWD analysis are lower than anticipated, with only 21 individuals completing the 6MWD at 90 days. Because of the small numbers reported in this pilot trial, the CIs are large and we cannot draw conclusions. The results for the 6MWD outcome without the interaction term are reported in Appendix 4.
| Time point | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR: usual care vs. experimental | Treatment 2 effect: Home EPR: usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Pre discharge | n | 20 | 7 | 7 | 4 | 2 | 1.3 (95% CI –148.6 to 151.2) | Value = –47.1 | Value = –70.7 |
| Mean (SD) | 143.6 (92.76) | 168.9 (80.60) | 121.9 (80.26) | 172.8 (146.54) | 73.0 (15.56) | 95% CI = (–138.4 to 44.2) | 95% CI = (–249.3 to 107.8) | ||
| 30 days | n | 33 | 9 | 11 | 5 | 8 | 3.8 (95% CI –164.1 to 171.7) | Value = –26.3 | Value = 62.9 |
| Mean (SD) | 231.9 (130.87) | 231.2 (138.51) | 206.0 (109.02) | 230.2 (151.47) | 269.5 (153.63) | 95% CI = (–145.8 to 93.2) | 95% CI = (–148.8 to 274.7) | ||
| 90 daysb | n | 21 | 5 | 6 | 5 | 5 | 44.0 (95% CI –162.0 to 250.0) | Value = 144.9 | Value = –54.7 |
| Mean (SD) | 278.9 (150.15) | 199.6 (146.80) | 328.7 (108.02) | 267.4 (160.90) | 310.0 (194.29) | 95% CI = (–7.9 to 297.8) | 95% CI = (–350.6 to 241.1) |
| Time point | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR: usual care vs. experimental | Treatment 2 effect: home EPR: usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | n | 55 | 13 | 15 | 13 | 14 | –0.0 (95% CI –0.2 to 0.2) | 0.1 (95% CI –0.2 to 0.3) | 0.1 (95% CI –0.2 to 0.4) |
| Mean (SD) | 0.5 (0.27) | 0.5 (0.30) | 0.5 (0.26) | 0.4 (0.29) | 0.6 (0.25) | ||||
| Pre discharge | n | 31 | 7 | 9 | 9 | 6 | –0.1 (95% CI –0.4 to 0.3) | 0.2 (95% CI –0.1 to 0.4) | –0.0 (95% CI –0.5 to 0.5) |
| Mean (SD) | 0.6 (0.32) | 0.6 (0.34) | 0.7 (0.27) | 0.5 (0.35) | 0.6 (0.36) | ||||
| 30 days | n | 43 | 10 | 14 | 9 | 10 | –0.2 (95% CI –0.5 to 0.1) | –0.0 (95% CI –0.3 to 0.2) | 0.3 (95% CI –0.1 to 0.7) |
| Mean (SD) | 0.6 (0.30) | 0.6 (0.34) | 0.6 (0.28) | 0.4 (0.32) | 0.7 (0.27) | ||||
| 90 days | n | 38 | 8 | 14 | 8 | 8 | –0.1 (95% CI –0.5 to 0.2) | –0.1 (95% CI –0.4 to 0.2) | 0.3 (95% CI –0.2 to 0.7) |
| Mean (SD) | 0.6 (0.33) | 0.6 (0.36) | 0.6 (0.29) | 0.5 (0.49) | 0.7 (0.23) |
| Outcome | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR: usual care vs. experimental | Treatment 2 effect: home EPR: usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Sedentary METsa | n | 21 | 6 | 6 | 5 | 4 | –87,108.4 (95% CI –151,523 to –22,693.9) | –44,895.9 (95% CI –115,176 to 25,384.7) | 78,525.1 (95% CI –47,508.3 to 204,558.5) |
| Mean (SD) | 358,959.2 (68,344.86) | 402,713.2 (19,179.07) | 357,817.3 (79,047.60) | 315,604.8 (67,121.68) | 349,234.0 (83,758.90) | ||||
| METsb | n | 21 | 6 | 6 | 5 | 4 | 440.0 (95% CI –207.4 to 1087.3) | –153.6 (95% CI –653.1 to 345.9) | 131.6 (95% CI –1216.8 to 1480.1) |
| Mean (SD) | 815.8 (638.10) | 675.3 (484.34) | 521.7 (315.69) | 1115.2 (532.57) | 1093.3 (1146.56) | ||||
| Daily step countb | n | 21 | 6 | 6 | 5 | 4 | 379.8 (95% CI –10,451.5 to 11,211.1) | –3189.8 (95% CI –11,627.3 to 5247.7) | 7570.5 (95% CI –12,858.4 to 27,999.5) |
| Mean (SD) | 9778.8 (9382.11) | 9693.0 (8737.25) | 6503.2 (4361.47) | 10,072.8 (8375.35) | 14,453.5 (16,840.59) |
Because of issues with the timing of discharge and patient choice, it was not always possible to ensure that participants left hospital with an activity monitor. Eleven participants did not receive an activity monitor because of the timing of discharge, three participants did not want to wear the activity monitor, four participants withdrew before being given an activity monitor and one activity monitor was lost. Of the participants who received an activity monitor (n = 39), 21 (54%) provided 10 hours of data for 5 days, which were used in the analysis.
Other clinical outcomes
The LCADL, CAT and MRC Dyspnoea Scale scores are reported in Appendices 5–7 respectively.
Attendance at group pulmonary rehabilitation (usual care)
Table 18 shows the numbers of participants who had attended group PR or an alternative to PR at the 90-day follow-up point. The mean number of sessions of group PR attended for these individuals was 2.8 (SD 2.35), with five people attending the minimum of one session and one person attending eight sessions. The reasons for not attending group PR and for stopping group PR are shown in Table 19; the main reason for non-attendance at group PR (when a reason was identified) was that it was scheduled to start outside the 90-day trial period.
| Randomisation group | n | Number attending group PR | Number receiving alternative PR |
|---|---|---|---|
| Hospital and home EPR | 14 | 1 | 1 |
| Hospital EPR | 14 | 0 | 0 |
| Home EPR | 15 | 7 | 3 |
| Usual care | 15 | 5 | 0 |
| Reason | n |
|---|---|
| Feeling too ill/readmitted to hospital | 6 |
| No time | 2 |
| Transport issues | 2 |
| Moved out of area | 1 |
| Unable to contact | 3 |
| Completing other exercises on own | 8 |
| Not referred/not eligible | 3 |
| Scheduled outside the trial period | 12 |
| No reason identified | 13 |
Baseline-adjusted analyses
Similar results were found for baseline-adjusted analyses of CAT (30 and 90 days) and LCADL (30 and 90 days) scores. These results are provided in Appendix 8.
Sensitivity analyses
Similar results were observed in the sensitivity analyses using no interaction, a time effect and multiple imputation. The results of the sensitivity analyses are provided in Appendix 9.
It was not possible to calculate a coefficient for Aintree (hospital: standard of care vs. experimental) when evaluating the centre (analyses constrained in each centre) and the interaction term because of the low number of participants (five participants overall).
Because of the low number of participants and high number of variables included, sensitivity analysis of baseline imbalances was not conducted. For instance, analyses could not be run using Stata version 14 when the centre effect was included (low number of participants in Aintree); hence, it was not appropriate to add additional variables in the models.
Physiotherapist effect was not analysed as there were too few patients per physiotherapist.
Adverse events
Adverse events at the participant level
Most of the participants randomised experienced at least one AE, with the lowest number of AEs reported in the group allocated to both hospital EPR and home EPR (Table 20).
| Variable | Usual care (n = 15) | Home EPR (n = 15) | Hospital EPR (n = 14) | Hospital and home EPR (n = 14) | Overall (n = 58) |
|---|---|---|---|---|---|
| Number (%) of patients experiencing at least one AE | 14 (93) | 15 (100) | 13 (93) | 11 (79) | 53 (91) |
Adverse events at the event level
Patients admitted to hospital with AECOPD often have significant comorbidities in addition to a high risk of COPD exacerbation and readmission. The AEs observed within the study were predominantly related to the respiratory and cardiovascular systems and would be consistent with those expected for COPD patients more generally (Table 21).
| AE | Usual care (n = 15) | Home EPR (n = 15) | Hospital EPR (n = 14) | Hospital and home EPR (n = 14) | Overall (n = 58) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants, n (%) | Events, n | Participants, n (%) | Events, n | Participants, n (%) | Events, n | Participants, n (%) | Events, n | Participants, n (%) | Events, n | |
| Overall | 14 (93) | 29 | 15 (100) | 51 | 13 (93) | 28 | 11 (79) | 34 | 53 (91) | 142 |
| Aggravation of respiratory symptoms | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 1 (7) | 1 | 2 (3) | 2 |
| Increased shortness of breath | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 1 (7) | 1 | 2 (3) | 2 |
| Increased cough | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 1 (7) | 1 | 2 (3) | 2 |
| Increased sputum | 0 (0) | 0 | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 1 (2) | 1 |
| Cardiac disorders | 2 (13) | 2 | 2 (13) | 2 | 0 (0) | 0 | 1 (7) | 2 | 5 (9) | 6 |
| Ear and labyrinth disorders | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Exacerbation of COPD | 8 (53) | 12 | 8 (53) | 22 | 7 (50) | 13 | 5 (36) | 8 | 28 (48) | 55 |
| Eye disorders | 0 (0) | 0 | 2 (13) | 2 | 0 (0) | 0 | 1 (7) | 2 | 3 (5) | 4 |
| Fall | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Gastrointestinal disorders | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 2 (14) | 3 | 3 (5) | 4 |
| General disorders | 2 (13) | 3 | 6 (40) | 8 | 3 (21) | 3 | 2 (14) | 3 | 13 (22) | 17 |
| Infections and infestations | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 1 (7) | 1 | 2 (3) | 2 |
| Influenza | 1 (7) | 1 | 1 (7) | 1 | 1 (7) | 1 | 1 (7) | 1 | 4 (7) | 4 |
| Investigations | 3 (20) | 3 | 0 (0) | 0 | 0 (0) | 0 | 1 (7) | 1 | 4 (7) | 4 |
| Metabolism and nutrition disorders | 0 (0) | 0 | 0 (0) | 0 | 1 (7) | 2 | 1 (7) | 2 | 2 (3) | 4 |
| Muscle pain | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Muscle soreness | 0 (0) | 0 | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 1 (2) | 1 |
| Musculoskeletal and connective tissue disorders | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 2 (14) | 2 | 3 (5) | 3 |
| Muscle fatigue/physical fatigue | 0 (0) | 0 | 0 (0) | 0 | 1 (7) | 3 | 0 (0) | 0 | 1 (2) | 3 |
| Muscle pain | 0 (0) | 0 | 3 (20) | 3 | 0 (0) | 0 | 0 (0) | 0 | 3 (5) | 3 |
| Nervous system disorders | 1 (7) | 1 | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 2 (3) | 2 |
| Pneumonia | 1 (7) | 2 | 0 (0) | 0 | 1 (7) | 1 | 1 (7) | 1 | 3 (5) | 4 |
| Renal and urinary disorders | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Respiratory, thoracic and mediastinal disorders | 2 (13) | 2 | 3 (20) | 4 | 1 (7) | 1 | 3 (21) | 4 | 9 (16) | 11 |
| Surgical and medical procedures | 0 (0) | 0 | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 1 (2) | 1 |
| Vascular disorders | 0 (0) | 0 | 1 (7) | 1 | 1 (7) | 1 | 1 (7) | 1 | 3 (5) | 3 |
There were no unexpected AEs related to the interventions. In particular, there did not appear to be evidence of significant musculoskeletal events reported in association with the exercise-based intervention arms.
Serious adverse events at the participant level
A lower proportion of patients experiencing at least one SAE was observed in the group receiving both home EPR and hospital EPR than in the other arms (Table 22). No events related to the interventions or deaths were observed. The assessment of expectedness was problematic as readmission with COPD is related to and expected in COPD, but it is thought to be expected in only about one-third of patients; therefore, some individuals completing the assessment of expectedness events as unexpected.
| Variable | Usual care (n = 15) | Home EPR (n = 15) | Hospital EPR (n = 14) | Hospital and home EPR (n = 14) | Overall (n = 58) |
|---|---|---|---|---|---|
| At least one SAE, n (%) | |||||
| Patients | 8 (53) | 9 (60) | 6 (43) | 3 (21) | 26 (45) |
| Intensity – mild | 1 (7) | 1 (7) | 0 (0) | 1 (7) | 3 (5) |
| Intensity – moderate | 6 (40) | 6 (40) | 6 (43) | 3 (21) | 21 (36) |
| Intensity – severe | 2 (13) | 3 (20) | 0 (0) | 0 (0) | 5 (9) |
| Related to intervention | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Expected | 3 (20) | 5 (33) | 3 (21) | 3 (21) | 14 (24) |
| Deaths, n (%) | |||||
| Patients | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Related to intervention | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Expected | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Serious adverse events at the event level
The majority of SAEs were respiratory in nature and represented worsening or exacerbation of the patients’ underlying COPD (Table 23). The rate of subsequent readmission was similar to that expected for this cohort, based on their PEARL prediction score (see Post hoc analysis).
| SAE | Usual care (n = 15) | Home EPR (n = 15) | Hospital EPR (n = 14) | Hospital and home EPR (n = 14) | Overall (n = 58) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants, n (%) | Events, n | Participants, n (%) | Events, n | Participants, n (%) | Events, n | Participants, n (%) | Events, n | Participants, n (%) | Events, n | |
| Aggravation of respiratory symptoms – increased shortness of breath | 1 (7) | 1 | 0 (0%) | 0 | 0 (0%) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Cardiac disorders | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Ear and labyrinth disorders | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Exacerbation of COPD | 4 (27) | 7 | 4 (27) | 9 | 5 (36) | 8 | 3 (21) | 4 | 16 (28) | 28 |
| Fall | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Gastrointestinal disorders | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 1 (7) | 2 | 2 (3) | 3 |
| Influenza | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Investigations | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 | 1 (7) | 1 | 1 (2) | 1 |
| Nervous system disorders | 1 (7) | 1 | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 2 (3) | 2 |
| Pneumonia | 1 (7) | 2 | 0 (0) | 0 | 1 (7) | 1 | 1 (7) | 1 | 3 (5) | 4 |
| Renal and urinary disorders | 0 (0) | 0 | 1 (7) | 1 | 0 (0) | 0 | 0 (0) | 0 | 1 (2) | 1 |
| Respiratory, thoracic and mediastinal disorders | 1 (7) | 1 | 1 (7) | 1 | 1 (7) | 1 | 0 (0) | 0 | 3 (5) | 3 |
| Vascular disorders | 0 (0) | 0 | 1 (7) | 1 | 1 (7) | 1 | 1 (7) | 1 | 3 (5) | 3 |
Despite the high incidence of reported exacerbations in the home EPR group, the readmission rate was subsequently lower, suggesting a combination of over-reporting and perhaps more effective intervention of other health-care professionals (e.g. GP calls).
Chronic obstructive pulmonary disease-related adverse events (including readmissions)
Chronic obstructive pulmonary disease readmission
Proportions
The proportions of participants experiencing at least one COPD readmission are presented by randomised group in Table 24. Forty-eight participants are included in this table: 44 participants who completed the study, two participants who withdrew after 90 days from randomisation and two participants who experienced the event before withdrawal. A lower proportion of events was observed in the group undergoing home EPR. However, care should be used when interpreting these results because the:
-
frequency of events is low and a positive result could have been seen by chance
-
study was not designed to detect a minimum clinically important difference for this outcome
-
choice of the denominator (and therefore how proportions are calculated) could be disputed.
| Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR: usual care vs. experimental | Treatment 2 effect: home EPR: usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|
| Completers, n | 48 | 10 | 15 | 12 | 11 | 1.044 (95% CI 0.201 to 5.426) | 0.385 (95% CI 0.064 to 2.296) | 1.006 (95% CI 0.075 to 13.571) |
| Patients with events, n (%) | 18 (38) | 5 (50) | 4 (27) | 6 (50) | 3 (27) | |||
| Patients with no events, n (%) | 30 (63) | 5 (50) | 11 (73) | 6 (50) | 8 (73) | |||
| Readmissions, n | 34 | 10 | 10 | 9 | 5 | |||
| Mean (SD) number of readmissions per participant | 0.7 (1.20) | 1.0 (1.33) | 0.7 (1.59) | 0.8 (0.87) | 0.5 (0.82) |
Mean number of events per participant
The mean number of COPD readmissions per participant is reported in Table 24. This post hoc analysis was run to evaluate the SD of this outcome to see whether or not this would be a suitable primary outcome for the main trial. The SD ranges from 0.80 (both treatments) to 1.6 (home EPR).
Time to event
The Kaplan–Meier curve for the time to first COPD readmission is reported in Figure 10. Overall, 38% of participants experienced at least one readmission related to COPD.
FIGURE 10.
Kaplan–Meier plot of time to first readmission. Participants who are not readmitted are censored at day 90.

Exacerbation
Proportions
The proportions of participants experiencing at least one exacerbation by group are presented in Table 25. Forty-six participants are included in this table: 44 patients who completed the study plus two participants who withdrew after 90 days from randomisation. Similar proportions were observed across arms.
| Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR: usual care vs. experimental | Treatment 2 effect: home EPR: usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|
| Completers, n | 46 | 10 | 15 | 10 | 11 | 1.147 (95% CI 0.160 to 8.238) | 0.858 (95% CI 0.131 to 5.635) | 0.637 (95% CI 0.047 to 8.559) |
| Patients with events, n (%) | 25 (54) | 6 (60) | 8 (53) | 6 (60) | 5 (45) | |||
| Patient with no events, n (%) | 21 (46) | 4 (40) | 7 (47) | 4 (40) | 6 (55) | |||
| Exacerbations, n | 51 | 9 | 22 | 12 | 8 | |||
| Mean (SD) number of exacerbations per participant | 1.1 (1.43) | 0.9 (0.88) | 1.5 (1.96) | 1.2 (1.40) | 0.7 (1.01) |
Mean number of events per participant
The mean number of exacerbations per participant is reported in Table 25. This post hoc analysis was run to evaluate the SD of this outcome to determine whether or not it could be a suitable primary outcome for the main trial. The SD ranges from 0.88 (usual care) to 1.96 (home EPR).
The participants included in Table 24 are different from the participants included in the safety analysis. This explains why the number of exacerbations differs (here we report 51 exacerbations in 46 participants whereas in Table 21 we report 55 exacerbations in 58 participants).
A slightly higher number of exacerbations per participant was reported by participants receiving home EPR alone than by the other subgroups, although this was not associated with an increased rate of readmission (see Table 24) and may represent ascertainment bias in those receiving more intensive post-discharge care.
Post hoc analysis
PEARL prediction scores
The mean (SD) PEARL prediction score was 3.0 (2.2) whereas the median [interquartile range (IQR)] score was 3 (1–5). These statistics were calculated for 48 participants who completed the study, underwent a readmission before withdrawal or withdrew after 90 days from randomisation. An increase in PEARL prediction score was associated with an increase in readmission (OR 1.57, 94% CI 1.17 to 2.11).
Chronic obstructive pulmonary disease readmission bed-days
This analysis included 48 participants (i.e. the same number of participants who experienced a COPD readmission). The median (IQR) LOS in days was 1.5 (0.0–15.0) for the usual care group, 0.0 (0.0–1.0) for the home EPR group, 0.5 (0.0–7.5) for the hospital EPR group and 0.0 (0.0–5.0) for the group receiving both interventions. The SD ranged from 6.0 (both interventions) to 13.6 (hospital EPR). A similar LOS was observed across the arms.
Perceived Necessity and Concerns questionnaire data
These data are presented in Appendix 10 and are discussed in conjunction with the qualitative data in Chapter 5 (see Case study vignettes).
Chapter 4 Intervention optimisation study results
Introduction
The number of hospital EPR sessions started per participant (out of a possible 15) ranged from 0 to 14, with 131 (40.88%) out of a possible 321 sessions started in total (see Table 11). All of the 131 sessions started were completed successfully. Data on the optimisation of all 131 hospital EPR sessions were available.
Data on the optimisation of individual exercise components of home EPR were available for all patients who undertook the intervention.
Optimisation of hospital early pulmonary rehabilitation
Table 26 shows the number of sessions that were optimised for the hospital EPR intervention according to the decision rules detailed in Chapter 2 (see Optimisation of prescription). In total, 106 out of 131 sessions were optimised. When the sessions were not optimised the workload was either not set high enough for the participant (‘workload too low’) or the maximum workload had been used and could not be increased (‘limited by equipment’).
| Participant ID | Hospital EPR records | Session started | Session optimised | Workload too low | Limited by equipment |
|---|---|---|---|---|---|
| P1/059 | 15 | 9 | 9 | 0 | 0 |
| P1/090 | 15 | 12 | 12 | 0 | 0 |
| P1/098 | 15 | 6 | 2 | 0 | 4 |
| P1/099 | 15 | 9 | 9 | 0 | 0 |
| P1/115 | 15 | 2 | 2 | 0 | 0 |
| P1/117 | 15 | 11 | 7 | 4 | 0 |
| P1/123 | 15 | 8 | 7 | 0 | 1 |
| P1/124 | 15 | 9 | 7 | 0 | 2 |
| P1/126 | 15 | 5 | 0 | 2 | 3 |
| P1/150 | 15 | 0 | 0 | 0 | 0 |
| P1/173 | 15 | 0 | 0 | 0 | 0 |
| P1/178 | 15 | 6 | 4 | 0 | 2 |
| P1/200 | 15 | 1 | 1 | 0 | 0 |
| P1/210 | 15 | 3 | 3 | 0 | 0 |
| P1/211 | 15 | 9 | 8 | 1 | 0 |
| P1/227 | 15 | 3 | 3 | 0 | 0 |
| P2/075 | 15 | 4 | 4 | 0 | 0 |
| P2/079 | 15 | 8 | 8 | 0 | 0 |
| P2/130 | 6 | 4 | 2 | 2 | 0 |
| P2/176 | 15 | 4 | 4 | 0 | 0 |
| P2/233 | 15 | 14 | 14 | 0 | 0 |
| P2/259 | 15 | 4 | 0 | 4 | 0 |
| Total | 321 | 131 | 106 | 13 | 12 |
Table 26 also presents data on adherence, with no participant receiving the full intervention (15 sessions) and only two individuals (9.1%) meeting the prespecified criterion of completing 12 out of 15 sessions.
Optimisation of home early pulmonary rehabilitation
Table 27 presents the optimisation of the eight exercises in the home EPR intervention for all participants allocated to home EPR. The numbers shown are the numbers of sessions in which the exercises were optimised (a Borg score of 3–6 or an appropriate modification) (maximum of four sessions). The single optimisation score is the number of optimised aerobic and resistance sessions divided by the number of optimised and non-optimised sessions, providing the proportion of sessions that were optimised.
| Participant ID | Number of aerobic exercises completed with a Borg score of 3–6 | 2–4 sets of 10–15 repetitions (Borg RPE 12–14) | Single optimisation score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Marching on the spot | Shoulder punches | Sit to stand | Arm lifts | Wall push-up | Step | Bicep curls | Squats/wall slides | ||
| P1/052 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0.13 |
| P1/059 | 2 | 4 | 4 | 4 | 4 | 4 | 0 | 0 | 0.77 |
| P1/077 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P1/092 | 1 | 1 | 3 | 3 | 3 | 4 | 4 | 4 | 0.72 |
| P1/098 | 2 | 2 | 3 | 3 | 3 | 3 | 4 | 3 | 0.73 |
| P1/099 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P1/100 | 0 | 0 | 0 | 1 | 2 | 2 | 2 | 1 | 0.50 |
| P1/115 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P1/118 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P1/121 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0.25 |
| P1/123 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0.21 |
| P1/150 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 0.33 |
| P1/161 | 2 | 3 | 3 | 2 | 1 | 4 | 4 | 4 | 0.72 |
| P1/173 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 0.25 |
| P1/210 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.13 |
| P1/215 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0.20 |
| P1/220 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 2 | 0.50 |
| P1/224 | 0 | 0 | 3 | 1 | 1 | 1 | 1 | 1 | 0.46 |
| P1/226 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 0.25 |
| P2/083 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P2/092 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P2/176 | 2 | 1 | 3 | 1 | 2 | 4 | 0 | 0 | 0.54 |
| P2/212 | 1 | 0 | 4 | 0 | 0 | 2 | 0 | 0 | 0.29 |
| P2/259 | 0 | 1 | 4 | 0 | 4 | 1 | 0 | 0 | 0.42 |
The single optimisation scores show that none of the participants achieved optimisation of ≥ 0.8 (≥ 80%) over all of the sessions; four participants achieved > 70% optimisation of their sessions, three achieved ≤ 70% and ≥ 50% optimisation of their sessions and the remainder achieved < 50% optimisation of their sessions.
In addition to assessing intervention delivery using the optimisation rules, adherence to the intervention was assessed. The number of sessions undertaken and completed and the number of exercises completed in each session are shown in Table 28. The feasibility criteria set out in Chapter 2 (see Other feasibility outcomes) defined adherence as completing three out of four sessions. Table 28 shows that 15 out of 24 (62.5%) participants completed three or more sessions. Of these participants, 14 out of 15 completed six or more exercises at these sessions.
| Participant ID | Sessions attended | Full sessions completed | 3+ sessions completed | Number of exercises completed | Number of sessions in which six exercises were completed |
|---|---|---|---|---|---|
| P1/052 | 4 | 4 | Yes | 31 | 4 |
| P1/059 | 4 | 4 | Yes | 29 | 4 |
| P1/077 | 1 | 1 | No | 0 | 0 |
| P1/092 | 4 | 4 | Yes | 32 | 4 |
| P1/098 | 4 | 4 | Yes | 31 | 4 |
| P1/099 | 0 | 0 | No | 0 | 0 |
| P1/100 | 2 | 2 | No | 16 | 2 |
| P1/115 | 0 | 0 | No | 0 | 0 |
| P1/118 | 1 | 1 | No | 0 | 0 |
| P1/121 | 3 | 3 | Yes | 24 | 3 |
| P1/123 | 4 | 3 | Yes | 28 | 3 |
| P1/150 | 3 | 3 | Yes | 18 | 3 |
| P1/161 | 4 | 4 | Yes | 32 | 4 |
| P1/173 | 4 | 4 | Yes | 32 | 4 |
| P1/210 | 3 | 3 | Yes | 24 | 3 |
| P1/215 | 1 | 0 | No | 6 | 0 |
| P1/220 | 2 | 2 | No | 16 | 2 |
| P1/224 | 3 | 2 | No | 18 | 2 |
| P1/226 | 4 | 4 | Yes | 32 | 4 |
| P2/083 | 3 | 3 | Yes | 0 | 0 |
| P2/092 | 3 | 1 | No | 0 | 0 |
| P2/176 | 4 | 4 | Yes | 24 | 4 |
| P2/212 | 4 | 4 | Yes | 24 | 4 |
| P2/259 | 4 | 4 | Yes | 24 | 4 |
Summary
Summary of hospital early pulmonary rehabilitation
Resistance training during AECOPD has been shown to be effective at counteracting the catabolic effects of myostatin, improving quadriceps strength. 56 During each intervention session physiotherapists were able to increase or decrease the load based on patient symptoms. Breathlessness was measured during and after the intervention using the Borg score and participants were asked to rate the difficulty of the intervention on a scale of 1 (very easy) to 5 (very difficult) after each session. In 13 of the 131 sessions (9.9%) the load was considered to be set too low for the participant; although the load was set at 80% of the 2RM, participants reported the intervention to be very easy (difficulty rated at < 2) and had very little increase in breathlessness (Borg score of < 2). In 12 of the 131 sessions (9.2%) the equipment used was felt to be a limiting factor. The MOTOmed viva 2 cycle ergometer had a maximum resistance of 20 kg and some individuals were able to reach this maximal level. In total, 106 of the 131 sessions (80.9%) were considered to be optimised.
Summary of home early pulmonary rehabilitation
Problems associated with the Borg 0–10 breathlessness score and the lack of use of the Borg RPE score led to lower than expected optimisation scores (see Chapter 8, Evidence of feasibility, Intervention). Only 5 out of 18 people achieved an optimisation score of ≥ 50%; this is partly because of missing data. The results were shared and discussed with staff delivering the intervention and the results were reported by these staff to be predominantly related to inadequacies in study documentation and also a time lapse between training in the study protocol and delivery of the intervention.
In contrast to the optimisation data, adherence data were more positive: 69 sessions were attended out of a possible 96 and 64 of these sessions were fully completed, as recorded on the trial data entry forms; however, because of issues with the return of paper diaries it could be shown only that 58 of the 96 sessions included at least six of the core exercises. Recurrent exacerbation was the core reason for non-completion of the sessions. Only 10 out of 24 participants received all four sessions. The reasons for non-attendance are shown in Table 12.
Chapter 5 Qualitative research results
Context understood through the International Classification of Functioning130
Impairment and activity limitation
Participants in the study had difficulty moving around (d455) because of their respiration functions (b440) and exercise tolerance functions (b455):
Well yeah, yeah, can’t do it [exercise] now as much as I’d liked to do obviously with COPD but I do what I can and that’s it.
P1/092
Yeah, but it’s breathlessness that really . . . that stops you from doing anything more active?
Yes it does.
P1/107
This had an effect on carrying out their daily routine (d230) and doing housework (d640):
I was hoovering before I could go through it no problem but now I just can’t I’ve gotta stop and start, stop and start all the time.
P1/101
In general, participants said that walking (d450) was their way of getting exercise, although some participants struggled to walk much because of their breathlessness:
Well, I mean, I can’t walk a long way but I like to walk generally [mm] but every so often I have to stop you know what I mean [yeah] but I’m just across the road I’ve got fields and what have you, I usually go across there [yeah] you know.
P1/178
In addition to the functions in the brief core set, participants clearly had motivation issues related to energy and drive functions (b130) and some of the participants mentioned comorbidities that also limited their activity, for example pain in other body parts (b2801) and their weight (b530).
Er no energy, I’m always drained [yeah] so it’s always a struggle. Even the warming up exercises it takes it out of me [right] so, yeah.
P2/092
I’ve got arthritis in both me knees, so I don’t go very far.
P1/128
Participation restriction
Participants were often frustrated at not being able to be as active as they used to be. Some stated that they became breathless doing housework (d640) and participants often struggled on the stairs, causing some to have moved their bedroom downstairs. In Sheffield the majority of participants stated that the hills in the city made it very difficult to walk around (d450) and could affect their breathing (b440):
because I can walk onto end of road but then it’s an hill [yeah] I know it’s, it’s only like 10, 10 yards up and then I walk on flat again and then walk down same again 10 yards back to my house but that going up 10 yards then coming down other end 10 yards it, that kills me.
P1/098
Personal factors
Individuals found that their breathing was the main factor limiting their daily activity and in general they lacked the confidence to undertake activity because of their breathlessness (b440):
Yeah you don’t know what to don’t know what to do, you know when you have trouble breathing [yeah] you don’t know if you should take it easy or try and motivate yourself a bit more to be quite honest.
P1/223
Environmental factors
A number of participants said that they used oxygen to aid their breathing and some participants said that carrying oxygen limited what they could do and where they could go:
But I can’t you know with the oxygen and I’ve got my stuff upstairs, how can we go? So, I mean we don’t drive so I’d be you know it’s a bit awkward to try and get anywhere.
With the oxygen?
Yeah, because I have to have that beep out machine on at night and I’ve got my oxygen generator upstairs and this is my mobile one. So really we’re a bit lumbered.
P1/220
A few participants mentioned that they keep antibiotics for self-administration (e110) to control their COPD and avoid exacerbations:
Yeah because I’ve only been into the hospital once in what about year I think [mmhm] whereas really because I self-administer antibiotics and stuff for myself, I have them here, so if I know that I need them I’ll take them.
P1/161
Only one person mentioned products for daily living, having had a bathroom put in downstairs (e115), with no participants mentioning technology. Eighteen of the participants interviewed mentioned that family (e310) and friends (e320) help them with shopping or housework or act as motivators to carry out activity.
Participant views of the interventions
Acceptability of hospital early pulmonary rehabilitation
Of the participants who attended any hospital EPR sessions, all of them found hospital EPR to be more or less acceptable (Table 29), with three participants finding it very highly acceptable and three finding it highly acceptable:
In the hospital I was doing it three times a day [yeah] you do it with your arms and your legs, and you sit in a chair and do it [yeah] I says to her, I wish I could bring that home.
P1/059
| Participant ID | Number of available sessionsa | Number of sessions attended | Number of completed sessions | % attended of available sessions | % completed of attended sessions | Acceptability rating based on qualitative data |
|---|---|---|---|---|---|---|
| P1/059 | 11 | 5 | 5 | 45.5 | 100 | Very high |
| P1/098 | 6 | 6 | 6 | 100 | 100 | Very high |
| P1/107 | 15 | 0 | 0 | 0 | 0 | Cannot tell from interview |
| P1/121 | 0 | 0 | 0 | 0 | 0 | High |
| P1/124 | 9 | 9 | 9 | 100 | 100 | High |
| P1/150 | 15 | 0 | 0 | 0 | 0 | None |
| P1/178 | 7 | 6 | 6 | 85.7 | 100 | High |
| P1/200 | 4 | 1 | 1 | 25 | 100 | None |
| P1/211 | 12 | 9 | 9 | 75 | 100 | Mixed acceptability |
| P2/176 | 6 | 4 | 4 | 66.7 | 100 | Mixed acceptability |
| P2/233 | 15 | 14 | 14 | 93.3 | 100 | Mixed acceptability |
| P2/259 | 9 | 4 | 4 | 44.4 | 100 | Very high |
Some commented on the fact that carrying out the exercises made them feel more capable and more confident to go home following their AECOPD:
How would you feel about doing exercises in the hospital?
Yes, yeah . . . because of being part of the recovery programme . . . they’d indicate also that I am well enough to actually go home erm, and I cater for myself alright.
P1/124
One hospital EPR participant declined all of the sessions because of feeling too unwell and another hospital EPR participant completed only one of four available sessions because of not finding the intervention acceptable. One individual did not have the chance to complete the sessions because of being discharged but indicated that hospital EPR would be highly acceptable. One participant received no sessions because of an administration error and so did not discuss acceptability. Three people appeared to show some acceptability of hospital EPR and this was because they felt unwell and thought it may have been too early to carry out the exercises:
It was a bit strenuous, yeah, yeah . . . Um, a little bit, er, a little bit heavy. You know I mean I done it but I found it a little bit, you know . . . I just, I just couldn’t seem to getting meself better.
P2/233
Participants who were not allocated to hospital EPR (n = 15) were mixed in their opinions: eight felt that it would be acceptable, one said that they felt that their bad knees would prohibit engagement and the remaining six thought that it was too early to begin exercising following an exacerbation, although one thought that they might have still tried the exercises:
Oh yeah if you go in with an exacerbation you just wanna get on the meds and feel a bit better before you do anything.
P2/092
Acceptability of home early pulmonary rehabilitation
Those who received home EPR generally found the intervention to be acceptable (Table 30), with four participants expressing a high level of acceptability and five indicating a high level of acceptability:
But the physio I had at home when they came to me after I’d been in hospital [yeah] for the erm, that was fantastic!
P1/161
| Participant ID | Number of sessions attended | Number of completed sessions | % of sessions attendeda | % completed of attended sessions | Acceptability rating based on qualitative data |
|---|---|---|---|---|---|
| P1/059 | 4 | 4 | 100 | 100 | Very high |
| P1/092 | 4 | 4 | 100 | 100 | High |
| P1/098 | 4 | 4 | 100 | 100 | High |
| P1/100 | 2 | 2 | 50 | 100 | Mixed acceptability |
| P1/118 | 1 | 1 | 25 | 100 | Very high |
| P1/121 | 3 | 3 | 75 | 100 | Mixed acceptability |
| P1/150 | 3 | 3 | 75 | 100 | Little to none |
| P1/161 | 4 | 4 | 100 | 100 | Very high |
| P1/211 | 0 | 0 | 0 | 0 | None |
| P1/215 | 3 | 2 | 75 | 66.7 | High |
| P1/220 | 2 | 2 | 50 | 100 | Mixed acceptability |
| P1/226 | 4 | 4 | 100 | 100 | High |
| P2/092 | 3 | 1 | 75 | 33.3 | Mixed acceptability |
| P2/176 | 4 | 4 | 100 | 100 | Mixed acceptability |
| P2/212 | 4 | 4 | 100 | 100 | High |
| P2/259 | 4 | 4 | 100 | 100 | Very high |
Some participants found it a little too soon after leaving hospital to receive the intervention and others did not attend all of their sessions. One participant found home EPR to be unacceptable and had no sessions; another indicated little or no acceptability, declining the final session because of poor health and the perceived burden of health-care visitors:
Yeah, they were alright be nah I can’t be bothered.
P1/150
When participants indicated some acceptability they found the sessions to be acceptable when they completed them but found them difficult, felt tired after the sessions or felt that the intervention was provided too soon following discharge:
Well, I thought they might have been a bit early really because I was, I think she came the day after I came out of hospital, and I really wasn’t feeling up to it much for the first ones.
P2/176
Interview participants who were not allocated to home EPR (n = 11) said that they would find it acceptable, with one exception, who preferred not to wait in for visitors.
Participant views of the interventions: capability, opportunity, motivation and behaviour analysis
Data on participant capability, opportunity and motivation to attend group PR (usual care) are presented in Table 31; the same data are presented in Table 32 in terms of the study interventions. Further data are available in Appendix 11.
| Participant ID | Trial arm | Capability | Opportunity | Motivation | Attendance at group PR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical | Psychological | Social | Physical | Reflective | Automatic | ||||||||||||
| Skills | Knowledge | Cognitive and interpersonal skills | Memory, attention and decision | Behavioural regulation | Social influences | Environmental context and resources | Beliefs about capabilities | Beliefs about consequences | Identity | Intentions | Optimism | Goals | Reinforcement | Emotion | |||
| P1/059 | Hospital and home EPR | Barrier | Enabler | Enabler | Barrier | Enabler | Barrier | Barrier | Enabler | Enabler | – | Enabler | Enabler | – | Enabler | Enabler | Received standard home EPR (STHNHSFT only) |
| P1/98 | Hospital and home EPR | Barrier | Enabler | Enabler | Barrier | – | Barrier | Barrier | Enabler | Enabler | – | – | Barrier | Barrier | Enabler | Enabler | Did not attend PR |
| P1/121 | Hospital and home EPR | Barrier | Enabler | Enabler | Enabler | Enabler | Enabler | Mixed response | Enabler | Enabler | – | – | Enabler | Mixed response | Enabler | Enabler | Attended PR |
| P1/150 | Hospital and home EPR | Barrier | Enabler | Enabler | Barrier | – | Barrier | Barrier | Barrier | Barrier | – | Barrier | Barrier | Barrier | Barrier | Mixed response | Did not attend PR |
| P1/211 | Hospital and home EPR | Barrier | Barrier | Enabler | Barrier | – | Mixed response | Barrier | Barrier | Mixed response | – | Mixed response | – | Enabler | Barrier | Barrier | Did not attend PR |
| P2/176 | Hospital and home EPR | Barrier | Enabler | Enabler | Barrier | – | Enabler | Enabler | Barrier | Barrier | – | Barrier | Barrier | Barrier | Barrier | – | Did not attend PR |
| P1/092 | Home EPR | Barrier | Enabler | Enabler | Enabler | – | Enabler | Mixed response | Enabler | Enabler | – | Enabler | Enabler | Enabler | Enabler | Enabler | Attended PR |
| P1/100 | Home EPR | Barrier | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | – | Enabler | Enabler | Enabler | Enabler | Enabler | Attended PR |
| P1/118 | Home EPR | Barrier | Barrier | Enabler | – | – | Enabler | Enabler | Enabler | Enabler | – | Barrier | – | Barrier | Enabler | Enabler | PR postponed because of illness |
| P1/161 | Home EPR | Barrier | Barrier | Enabler | Enabler | – | Barrier | Barrier | Mixed response | Enabler | – | – | – | – | – | – | Did not attend PR |
| P1/215 | Home EPR | Barrier | Mixed response | Enabler | Enabler | – | Mixed response | Barrier | Enabler | Enabler | Enabler | Barrier | Enabler | – | Enabler | Enabler | Received standard home EPR (STH only) |
| P1/220 | Home EPR | Barrier | Mixed response | Enabler | Barrier | Mixed response | Barrier | Barrier | Enabler | Mixed response | – | Barrier | Enabler | Enabler | Barrier | Barrier | Received standard home EPR (STH only) |
| P1/226 | Home EPR | Barrier | Enabler | Enabler | Enabler | Enabler | – | Enabler | Enabler | Enabler | – | Mixed response | – | Enabler | – | Enabler | Attended PR |
| P2/092 | Home EPR | Barrier | Enabler | Enabler | – | – | Mixed response | Mixed response | Enabler | Enabler | – | Enabler | Enabler | Enabler | – | Barrier | Attended PR |
| P2/212 | Home EPR | Barrier | Enabler | Enabler | Enabler | Enabler | Barrier | – | Enabler | Enabler | – | Barrier | Enabler | – | Enabler | Mixed response | Did not attend PR |
| P1/107 | Hospital EPR | Barrier | Barrier | Enabler | Enabler | – | – | – | – | – | – | – | – | Barrier | – | – | Did not attend PR |
| P1/124 | Hospital EPR | Barrier | Enabler | Enabler | Mixed response | – | Barrier | Mixed response | Enabler | Enabler | – | Mixed response | Enabler | Enabler | Enabler | Mixed response | Attended PR |
| P1/178 | Hospital EPR | Barrier | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | – | Barrier | Mixed response | Mixed response | Enabler | Barrier | Received standard home EPR (STH only) |
| P1/200 | Hospital EPR | Barrier | Mixed response | Enabler | Enabler | – | Barrier | Mixed response | Mixed response | Mixed response | Barrier | Mixed response | Enabler | Enabler | Enabler | Barrier | Did not attend PR |
| P2/233 | Hospital EPR | Barrier | Enabler | Enabler | Barrier | – | Enabler | Mixed response | Barrier | – | – | Enabler | Enabler | Mixed response | Enabler | Barrier | PR postponed because of illness |
| P1/101 | Usual care | Barrier | Barrier | Enabler | Mixed response | Barrier | Barrier | Barrier | Mixed response | Enabler | – | Enabler | – | Enabler | Enabler | Enabler | Attended PR |
| P1/116 | Usual care | Barrier | Barrier | Enabler | Mixed response | – | Enabler | Mixed response | Enabler | Enabler | – | Mixed response | Enabler | Enabler | Enabler | Enabler | Attended PR |
| P1/128 | Usual care | Barrier | Enabler | Enabler | Mixed response | Barrier | Enabler | Mixed response | Enabler | Enabler | – | Enabler | Enabler | Enabler | Enabler | Enabler | Attended PR |
| P1/223 | Usual care | Barrier | Barrier | Enabler | Barrier | – | Mixed response | – | Barrier | Barrier | – | Barrier | – | Barrier | – | – | Did not attend PR |
| P2/246 | Usual care | Mixed response | Enabler | Enabler | Mixed response | – | Mixed response | Barrier | Mixed response | Enabler | – | Enabler | – | – | Mixed response | – | Did not attend PR |
| Participant ID | Trial arm | Capability | Opportunity | Motivation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical | Psychological | Social | Physical | Reflective | Automatic | |||||||||||
| Skills | Knowledge | Cognitive and interpersonal skills | Memory, attention and decision | Behavioural regulation | Social influences | Environmental context and resources | Beliefs about capabilities | Beliefs about consequences | Identity | Intentions | Optimism | Goals | Reinforcement | Emotion | ||
| P1/059 | Hospital and home EPR | Mixed response | Enabler | Enabler | Barrier | – | Enabler | Mixed response | Enabler | Enabler | – | Enabler | – | – | Enabler | Enabler |
| P1/098 | Hospital and home EPR | Mixed response | Barrier | Enabler | Barrier | – | Enabler | Enabler | Enabler | Enabler | – | – | Barrier | Barrier | Enabler | Enabler |
| P1/121 | Hospital and home EPR | Mixed response | Enabler | Enabler | – | Enabler | Mixed response | Barrier | Enabler | Enabler | – | – | Enabler | – | Enabler | Enabler |
| P1/150 | Hospital and home EPR | Mixed response | Enabler | Barrier | Barrier | – | Barrier | Barrier | Barrier | Barrier | – | Barrier | Barrier | Barrier | Barrier | Mixed response |
| P1/211 | Hospital and home EPR | Barrier | Barrier | Enabler | Mixed response | – | Enabler | Enabler | Barrier | Mixed response | – | Mixed response | – | Enabler | Enabler | Barrier |
| P2/176 | Hospital and home EPR | Mixed response | Enabler | Enabler | Barrier | – | Enabler | Enabler | Barrier | Mixed response | – | Barrier | Barrier | Mixed response | Barrier | – |
| P1/092 | Home EPR | Enabler | Enabler | Enabler | Enabler | – | Mixed response | Enabler | Enabler | Enabler | – | Enabler | Enabler | Enabler | Enabler | Enabler |
| P1/100 | Home EPR | Mixed response | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | – | Enabler | Enabler | – | Enabler | Enabler |
| P1/118 | Home EPR | Barrier | Barrier | Enabler | – | – | Enabler | Enabler | Barrier | Enabler | – | Barrier | – | – | Enabler | Enabler |
| P1/161 | Home EPR | Enabler | Enabler | Enabler | Enabler | – | Mixed response | Enabler | Enabler | Enabler | – | Enabler | Enabler | Enabler | Enabler | Mixed response |
| P1/215 | Home EPR | Mixed response | – | Enabler | Enabler | – | Enabler | Enabler | Enabler | Enabler | Enabler | Barrier | Enabler | – | Enabler | Enabler |
| P1/220 | Home EPR | Enabler | – | Enabler | Mixed response | – | Enabler | Enabler | Enabler | Mixed response | – | Barrier | Enabler | Enabler | Mixed response | Enabler |
| P1/226 | Home EPR | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | – | Enabler | Enabler | Enabler | Enabler | Enabler |
| P2/092 | Home EPR | Barrier | Enabler | Enabler | – | – | Enabler | Enabler | Enabler | Enabler | – | Enabler | Enabler | Enabler | – | – |
| P2/212 | Home EPR | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | – | Enabler | Enabler | – | Enabler | Enabler |
| P1/107 | Hospital EPR | – | Barrier | Enabler | Enabler | – | – | Barrier | – | – | – | – | – | Barrier | – | – |
| P1/124 | Hospital EPR | Enabler | Enabler | Enabler | Mixed response | – | Enabler | Enabler | Enabler | Enabler | – | Enabler | Enabler | – | Mixed response | Enabler |
| P1/178 | Hospital EPR | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | Enabler | – | Barrier | Mixed response | Mixed response | Enabler | Enabler |
| P1/200 | Hospital EPR | Barrier | Enabler | Enabler | Mixed response | – | Enabler | Barrier | Mixed response | Mixed response | Barrier | Mixed response | Enabler | Enabler | Enabler | Barrier |
| P2/233 | Hospital EPR | Barrier | Enabler | Enabler | Barrier | – | – | – | Barrier | Mixed response | – | Enabler | Enabler | Mixed response | Enabler | Barrier |
Participants were less likely to attend PR (Table 31) if they (1) did not believe that the exercises were beneficial (reflective motivation; beliefs about consequences); (2) had low self-efficacy or did not feel that they had the capability to exercise (reflective motivation; beliefs about capabilities); (3) did not enjoy exercise (automatic; reinforcement); or (4) were not usually active (psychological capability; memory, attention and decision processes). Those with no access to transport (physical opportunity) were also less likely to attend group PR (see Appendix 11).
Although physical capability is a barrier to attending group PR, participants receiving EPR generally had the skills to perform the intervention (see Tables 31 and 32). Social opportunity was a greater barrier to group PR than to the EPR intervention and tended to be related to reluctance to exercise in a group. Physical opportunity to receive the EPR intervention was also much greater than for attendance at group PR. Those people who did not have the opportunity to receive hospital EPR (see Table 32) were discharged from hospital before treatment could be commenced or completed.
Intervention dimension categories are mapped to the COM-B theoretical framework and TDF using case study vignettes (see Case study vignettes). Hospital EPR and home EPR overcame a number of the barriers to group PR reported by participants who were unable or unwilling to attend a group programme. However, limitations of hospital EPR and home EPR were also evident.
The following sections describe the barriers and enablers associated with the intervention functions of EPR in relation to the COM-B theoretical framework.
Timing of pulmonary rehabilitation
Standard group PR usually takes place 4–6 weeks after discharge from hospital, whereas in this study the intervention commenced earlier following AECOPD, either while in hospital or within 72 hours of discharge. STHNHSFT routinely offers PR within the first 2 weeks following discharge for those on the early supported discharge (ESD) scheme; however, it is rare that a service user would be seen within the first 72 hours post hospital discharge. AUHNHSFT does not offer a home PR service. Therefore, breathlessness, muscle wastage and inflammatory processes associated with AECOPD may have a greater effect on a participant’s capability to undertake activity. It was anticipated that ‘physical capability, skills’ may be a barrier to EPR; however, feedback from participants was conflicting. Exercising in hospital was perceived to be too early by some, because of illness:
When you’re in hospital there’s no way you can exercise.
P1/161
It [the bike in hospital] was a bit strenuous . . . a little bit [early], er, a little bit heavy. You know I mean I done it but I found it a little bit, you know . . . I just, I just couldn’t seem to getting meself better . . . This time when I went in, I had pneumonia, so . . . I think that if I’d have been fitter . . . I would have liked it.
P2/233
However, most participants found that they were able to manage the in-hospital exercises:
I tell them my legs were feeling a lot better, I could carry on and do more and they said ‘No you’ve got to keep it to this, so many times’ . . . But that were on that bike [hospital EPR].
P1/059
Well to me, I could do it [hospital EPR] . . . I weren’t fighting for breath and to me it was quite simple, quite easy . . . and I told . . . you know physio’s like, he’s say ‘You alright?’ and I say ‘I could do another half dozen’, kinda thing you know what I mean, said ‘Well we’ll come down in another 20 minutes have another go’. Which were fine, which were fine . . . might have been a little bit too easy.
P1/178
Oh no, didn’t push me hard, not hard just maybe a little bit too much in first I suppose it’s like trial and error. They didn’t push me, push me like go on make it thin . . . I think he said I was 11 or something I don’t know. They’re nice people they’re only trying to help I know they are.
P1/200
Participants also found that the home EPR exercises were within their ‘physical capability’:
[Home EPR was] quite straightforward yeah, it’s only about 20 minutes, 25 minutes or something like that I do like y’know what I mean . . . that just does me like y’know what I mean.
P2/259
Recurrent readmissions to hospital with AECOPD in the follow-up period were a barrier to exercise in terms of ‘physical capability, skills’:
I’m still, I still find meself catching me breath and that.
P2/233
In clinical practice service users are often anxious about exercise-induced breathlessness and fears may potentially be amplified during and following AECOPD (‘automatic motivation, emotion’):
I think I do [worry about breathlessness when exercising] sometimes. I mean, I, I wish I could be different. I wish I could, you know, I, I want to get better and, and, and I know I will get better but I wanna be able to breathe a bit better.
P2/233
Just getting out of bed really, you know, I’m frightened of bringing that breathlessness on . . . I think, you know, the more I do and it’s making me a bit more breathless, you don’t know whether to stop or not.
P1/233
Enabling participants to be in control of their activity level enhances autonomy and thus intrinsic motivation (‘reflective motivation, beliefs about capabilities’) and may have augmented the positive reactions to EPR in addition to overcoming fears (‘automatic motivation, emotion’) and working within ‘physical capability’:
Oh they ask me while I’m doing the exercises do you want to rest, do you wanna give up, you can give up if you want and all this, that and the other. We’re not pushing you it’s you know.
P1/059
Acute exacerbation of COPD may affect participants’ ability to concentrate or follow instructions surrounding the exercise programme (‘psychological capability, memory, attention and decision processes and cognition’). However, most of the participants found the hospital and home EPR exercises easy to learn:
They’re all really simple, yeah.
P1/220
This may have been because of the reported levels of supervision and support (‘social opportunity’). All of the hospital EPR sessions and four home EPR sessions were supervised. Participants receiving home EPR also had a booklet to follow for the unsupervised in-home sessions, which aided memory and also helped establish a routine (‘psychological capability, behavioural regulation’):
My programme is still there out . . . I got a table. I’m doing all my timing . . . I need to do it really tidy because otherwise I’m losing interest.
P1/121
A negative experience of home EPR associated with delivery of the intervention at an earlier time point post AECOPD involved the large number of health professionals involved during the early stages following discharge from hospital. In Sheffield, a team of health professionals including physiotherapists, nurses and therapy assistants was visiting a participant to assist with self-care and mobility post discharge at the same time as the participant was receiving home EPR for the trial. This was too much of a burden for the participant in terms of time (‘physical opportunity, environmental context and resources’) and intrusion (‘social opportunity, social influences’) and led to withdrawal from the intervention. Service users have declined early in-home PR in clinical practice in the past for these reasons.
Most participants felt that EPR would enable them to get fitter more quickly and that exercise would help them stay healthy for longer. Access to EPR was facilitated by the location of the intervention.
Location
The location of group PR may affect participants’ ability to attend (‘physical opportunity, environmental context and resources’), as reflected in Tables 31 and 32. Venues may be too far away, travel time may be excessive, there may be issues with public transport, such as there not being a suitable bus route or the venue being too far from the bus stop, there may be negative perceptions of community transport options or there may be issues surrounding the cost of travel:
It would cost me ‘bout £4/£5 in taxi, taxi fare . . . each way.
P1/116
Even those participants with access to a car were concerned about parking or having to walk up a hill to reach the venue once they had parked. Travelling to a venue can be tiring and difficult, particularly for those carrying ambulatory oxygen or those with comorbidities affecting their mobility and exercise capacity:
It [previous group PR] was good but it took up too much of my time . . . my husband used to take me up in the car. Now I can’t go this time erm, apart from I’ve got a bad shoulder, I can’t go it’s too far, I can’t get transport up there . . . I mean I wouldn’t even know where to start because I can’t get to the bus stop . . . because of pulmonary arterial hypotension I can’t walk anywhere and I can’t afford taxis up and down.
P1/161
Issues such as access may also be a barrier to attending group PR:
There’s 13 steps to get up from my house so for transport . . . I’ve no chance you know what I mean . . . I’d have to go by ambulance, there’s no way I can walk.
P1/092
Negating the need to travel therefore facilitates participation in terms of ‘physical capability, skills’ and ‘physical opportunity, environmental context and resources’.
Receiving PR within the home may potentially cause less anxiety than the prospect of travelling to an unknown venue (‘automatic motivation, emotion’). It may also cause less anxiety for those who find it difficult to leave their home because they suffer from depression (‘automatic motivation, emotion’):
I don’t like going out a lot . . . I’ve gotta be honest about that you know. I had to be pushed really to even to go to my daughter to be honest. I like my own company, since me wife died obviously and I like it in here.
P1/178
Others found it difficult to attend a group session because of carer commitments, the time that attending a group session entails (taking into account the length of the session and the travel time) or inclement weather (‘physical opportunity, environmental context and resources’):
I’m only going on a Wednesday. I can’t, I can’t do Monday and Wednesday because I’m, I’m not prepared to tie two mornings up a week.
P1/128
It’s [home EPR] actually easier in many respects erm, than going into the, the COPD clinic [PR venue] being one to one, but also it’s cutting down the amount of time of driving over there and all the rest of it.
P1/226
if you’ve got to be there for 9, then I’d have to get up at like 6 o’clock, half-past 6 in the morning to get myself organised to get over there y’know and then it’d finished like I say 12 by the time you’ve got back home and you’d had your lunch you were just . . . that [home EPR] was a lot easier, it’s all expense and it can’t be done these days that’s why I do it myself.
P1/161
Receiving hospital EPR and/or home EPR enabled the commencement of exercise while negating a number of problems associated with group PR attendance. The limitations of exercising at home may be associated with ‘physical opportunity, environmental context and resources’ in terms of there being insufficient space or equipment:
If I’m being honest I’d like a bike in my house myself . . . And being able to go on it.
P1/200
However, the majority of participants reported that they had the space to exercise at home and utilised features and objects within the home to compensate for lack of equipment (‘physical opportunity’):
Well as I say I do it all at home which is wonderful you know and all the feet and I use the stairs and my walls and window ledges [OK] you know it does help.
P1/161
Lack of privacy when exercising at home (‘social opportunity, social influences’ and ‘physical opportunity, environmental context and resources’) was easily addressed by drawing the curtains or exercising elsewhere within the home.
A limitation of delivering hospital EPR was that some participants did not have the ‘physical opportunity’ to receive the full number of bike sessions because of discharge from hospital:
I said to him ‘Friend I’m not being funny here but I’ve got someone coming to pick me up and I don’t wanna be doing this in case he wants to get off’.
P1/200
A limitation of home EPR compared with group PR is the lack of social interaction with people suffering from the same condition. However, home EPR did not prohibit attendance at group PR and attendance at group PR was encouraged. Those participants who had previously attended group PR and reported that they really enjoyed the social aspects were keen to attend again. However, those isolated at home and unable to access group PR appreciated the input from physiotherapy staff who visited them (‘social opportunity’):
It’s nice to meet other people you know that are like that are cheerful, strangers that are cheerful if you know what I mean and by the time they’ve finished they’re not strangers anymore . . . You’ve gained a friend.
P1/161
Sheffield Active Programmes has operated an in-home PR service as standard for a number of years as an alternative to group PR for those who struggle to attend. However, it is appreciated that the Liverpool site and a number of other hospital trusts do not have this facility. In-home PR has the potential to be more flexible in terms of the individual needs of participants.
Flexibility
Although participants may have the option of attending the group classes once a week if twice a week attendance is unattainable, the PR group class times are fixed because of venue constraints. This can lead to barriers to attending in terms of ‘physical capability, physical skills’, particularly if sessions take place in the morning. Other barriers to attending group PR are associated with the opportunity to attend, for example because of other commitments such as work or social activities (‘physical opportunity, environmental context and resources’). In contrast, home visits and in-hospital sessions may be negotiated with participants to fit in with their abilities and schedule, reduce the burden of treatment and enhance the opportunity for them to receive the intervention (‘physical opportunity’):
Well, they asked me what time [to visit] . . . Yeah, they do it to like to what I asked them.
P1/059
She always says that will that time be alright. It’s always late afternoon . . . but she said if you wanna change it or anything, I said no whatever suits you I’m only sitting here you know what I mean my social programme’s not that good [both laugh] yeah.
P2/092
They come in the afternoon, it’s roughly 2 o’clock . . . one of them must of got her in said ‘You don’t like morning ones?’ I said ‘I don’t like if they’re too early, if they come about 11 or 12 o’clock if they can something like that’ . . . once I’ve come round a bit.
P1/220
A criticism of group PR from one participant who had attended prior to the study was the inflexibility of the group sessions themselves (‘physical capability’):
in the class everybody were doing the same thing . . . you know and you can’t say well you do that and I’ll do that but they’re all doing the same thing.
P1/178
A flexible, more individualised approach is facilitated by one-to-one therapy.
One-to-one therapy
Both the hospital EPR and the home EPR interventions were delivered individually to participants. One-to-one therapy may be preferential for those who do not like exercising in a group (‘social opportunity, social influences’) as reflected in Tables 31 and 32:
I said no to that [group PR] . . . Because it was an 8-week course and I think no I didn’t want to commit myself to anything like that. That’s a group session I don’t want that, I’m quite happy doing what I do in my own exercises.
P2/212
Individual therapy may also reduce anxiety and safety concerns for those who feel that they need close monitoring during exercise (‘automatic motivation, emotion’):
It was a one-to-one approach which is where the recognition of the problem with the knees err, was appreciated because they could work a little bit closer with me to suggest alternatives.
P1/226
One-to-one therapy enables those who may have declined group PR or who may not have previously considered exercising to have a ‘taster’ of exercise.
Introduction to pulmonary rehabilitation
Those participants randomised to an intervention arm of the study were able to experience a short introduction to PR within the hospital and/or domiciliary setting prior to committing to a group programme. This may influence a number of aspects within the COM-B theoretical framework that impact on subsequent attendance at the PR group or the decision to receive ongoing standard in-home PR (Sheffield only).
An introduction to PR enabled some participants to experience the benefits of exercise, providing an incentive to continue exercising, or to find that they enjoyed exercising, enhancing the intrinsic motivation to continue (‘automatic motivation, reinforcement’):
Oh yeah, yes I really enjoy it as well as the benefits, I enjoy doing it yeah.
P1/092
the bike was brilliant . . . and y’know with hurting my left hand shoulder it was bit stiff but y’know with doing that bike it, it seemed to cure it y’know what I mean . . . it’s not too bad at all.
P2/259
Education by the PR team led to participants recognising the importance of exercise (‘automatic motivation, reinforcement’) and altering their beliefs about outcome expectancies (‘reflective motivation, beliefs about consequences’):
They said how important it is and all that.
P1/098
Not knowing what to expect at a PR class is a common concern among service users in clinical practice and was highlighted by one participant who did not receive the intervention as part of the study:
I know nothing about it, I know nothing about it . . . Once I know what it entails I can make a decision then . . . I don’t know enough about it love . . . I’m in the dark, I’m in the dark . . . I know nothing about it.
P1/116
A short trial of PR combined with techniques to manage breathlessness may increase knowledge about what to expect (‘psychological capability, knowledge’), potentially reduce anxieties about exercise (‘automatic motivation, emotion’, ‘reflective motivation, beliefs about capabilities’) and give participants the confidence to continue, thus promoting self-efficacy, self-esteem and self-empowerment (‘reflective motivation, beliefs about capabilities’):
I thought it was very, very good to have [name] come out to the house to start motivating me and letting me know I could breathe and exercise and you know I can do certain. At first it was like I’ve never had anything like that happen [name] and it was ‘Oh my god what happened? Can I do this?’. And she come and go ‘Yeah you can’ you know this that and the other and it motivated me again and got me thinking ‘You can do that’.
P2/212
I’ve never actually exercised before to be honest so it was all new to me and it made me think about things, just turning my head from side to side you think yeah it’s exercise, and it did make me think about exercising . . . in a different way yeah.
P2/212
Yes, yeah [it gave me confidence]. Plus it encouraged me to do a little bit more than possibly I might have been inclined to do if they hadn’t been here.
P1/226
For some, despite having a taster of EPR, their motivation to continue exercising was not affected (‘reflective motivation, optimism’):
They [home EPR exercises] were alright be nah I can’t be bothered . . . Well when I were younger it would have been alright but now . . . next month I’m 74 . . . and I get outta breath easy and no, I just sit down and breathe and I’m alreight.
P1/150
I didn’t particularly wanna do it but my husband said I needed exercise, so I did it . . . I’m not really mad about going [to group PR] to be quite honest . . . I’m probably too lazy.
P2/276
Intervention characteristics linked to the COM-B theoretical framework and the theoretical domains framework
It can be seen that the EPR interventions were generally well received and that participants predominantly had the capability, opportunity and motivation to take part in the interventions (Table 33). However, despite this, a number of factors limit ongoing commitment to exercise, particularly if this means attending a structured, venue-based PR programme.
| Intervention characteristics | Barrier | Enabler |
|---|---|---|
| Timing – earlier post AECOPD than standard group PR |
|
|
| Location – in hospital or at home; standard group PR takes place at a community venue |
|
|
| Flexibility – visits can be arranged at a mutually convenient time; standard group PR times are fixed | No barrier |
|
| One-to-one therapy – home EPR/hospital EPR is one-to-one PR rather than group PR |
|
|
| Introduction to PR – allows people to commit to a short number of exercise sessions | No barrier |
|
Case study vignettes
The case studies in Box 1 present three individuals, allocated to both hospital EPR and home EPR, who differed in their uptake of usual care but who all experienced barriers relating to physical opportunity (for hospital EPR) and physical capability. P1/059 and P1/121 did not report further barriers and had high levels of motivation according to the Perceived Necessity and Concerns questionnaire. P1/059 and P1/121 commenced home PR and group PR respectively. P2/176 reported additional barriers related to reflective motivation, with scores on the necessity questions of the Perceived Necessity and Concerns questionnaire reducing over time, indicating reduced motivation; this participant did not attend group PR (or any other form of PR). Training and enablement intervention functions could help reduce barriers in all three of these cases, but further intervention functions may be required for individuals with lower levels of motivation.
P1/059 was a 75-year-old male who, in interviews, acknowledged PR to be necessary and that he would benefit physically and socially from attending group PR (‘reflective motivation, beliefs about consequences’). A high level of motivation was indicated quantitatively by a high necessity score (see Figure 18) and decreased concerns (see Figure 24) at discharge. He completed nine out of 11 hospital EPR sessions (he was discharged early so four sessions were not available; see Table 45) and completed six to eight exercises at all four home EPR sessions (see Table 28). He did not attend group PR, but commenced a non-trial home PR intervention, routinely available in Sheffield. Principal barriers to uptake and maintenance of hospital EPR (see Table 32) were comorbidities (‘physical capability, skills’) because of a fall and hip/back pain and early discharge (‘physical opportunity, environmental context and resources’). In this case, barriers of physical capability could be addressed through intervention functions including training and enablement. 155
P1/121 – growing motivation; engaged with home EPR and group PR but not hospital EPR because of comorbiditiesP1/121 was a 51-year-old female who initially reported quantitatively no clear picture of how PR could help her lung condition or what it could achieve (‘reflective motivation, beliefs about consequences’); during the qualitative interview she appeared to have knowledge of PR. The quantitative necessities score increased (indicating motivation) over time (see Figure 18) and her concerns had also lessened by 30 days (see Figure 24). She did not attend any hospital EPR sessions as she was discharged (see Table 29), but completed three out of four home PR sessions (see Table 30) and attended group PR. Her barriers to PR were related to early discharge with regard to hospital EPR (‘physical opportunity, environmental context and resources’), which cannot be overcome using intervention functions, and physical skills (‘physical capabilities, skills’), which could be improved using training and enablement intervention functions in the intervention. 155
P2/176 – not motivated; engaged with hospital and home EPR but did not attend group PRP2/176 was a 76-year-old female who gave mixed responses over time with regard to necessities and concerns. Agreement in relation to necessities generally reduced over time (see Figure 18) but response to concerns was mixed with no clear pattern (see Figure 24). She completed four out of four sessions of home EPR (see Table 30) and all hospital EPR sessions (see Table 29), but only four were available because she was discharged (‘physical opportunity, environmental context and resources’); she did not attend group PR. She expressed barriers around comorbidities (‘physical capability, skills’), ‘laziness’ (‘reflective motivation, beliefs about capabilities’), a lack of belief that PR could aid recovery (‘reflective motivation, beliefs about consequences’) and accessing PR in certain environments (‘physical opportunity, environmental context and resources’). To overcome opportunity and motivation barriers intervention functions should include training, education, persuasion, modelling and enablement. 155
All participants reported barriers relating to physical skills, which could be helped with training and enablement intervention functions. The three participants in Box 2 did not complete the 15 sessions of hospital EPR as they were discharged and this was the case for the majority of participants allocated to receive the hospital intervention. This is a barrier relating to physical opportunity but cannot be avoided by adopting further intervention functions as the intervention is aimed at patients admitted with AECOPD and discharge will not be delayed to enable the intervention to be delivered. P1/200 declined some sessions as he was too unwell, in line with his reported barriers of COPD and comorbidities. He did not believe that exercise would help when he was feeling unwell, which is a barrier related to reflective motivation. Reflective motivation was also a barrier for P1/178 as he did not intend to attend group PR based on a previous experience; he did not attend group PR at the end of the trial but wwa on the waiting list for home PR (usual care in Sheffield). The response of P1/124 to the Perceived Necessity and Concerns questionnaire suggests that she did not have any barriers relating to reflective motivation and she did attend group PR. She also reported being socially isolated, which may have been an enabler for attending group PR rather than a barrier.
P1/124 was a 66-year-old female who somewhat disagreed that some aspects of rehabilitation are harmful and somewhat agreed that she understood how PR can improve lung function (‘reflective motivation, beliefs about consequences’); otherwise, she neither agreed or disagreed with the statements on the Perceived Necessities and Concerns questionnaire at 30 days (agreed with all necessities statements at baseline). Motivation was varied over time and this is demonstrated quantitatively, reducing from baseline to pre discharge, increasing at 7 days and reducing again at 30 days (see Figures 18 and 24). In relation to hospital EPR, she completed all nine available sessions [discharged for six sessions (‘physical opportunity, environmental context and resources’)] (see Table 29) but disliked being disturbed when resting to exercise in hospital. She did attend group PR but when interviewed presented barriers in relation to a chest infection (‘physical capability, skills’) and reported her social isolation as an incentive to engage (‘social opportunity, social influences’). Intervention functions including training, modelling and enablement could be incorporated to help overcome these barriers. 155
P1/178 – moderate motivation; completed available hospital early pulmonary rehabilitation; general practitioner advised against group pulmonary rehabilitationP1/178 was an 80-year-old male who generally agreed with the necessities statements, although at 30 days he disagreed that PR would help him resume activities more quickly (‘reflective motivation, beliefs about consequences’). Quantitatively, the mean necessities score remained the same until 30 days, when it decreased (see Figure 18), and this pattern was also evident for concerns, which increased at 30 days (see Figure 24). He completed six of the seven available hospital EPR sessions [discharged for eight sessions (‘physical opportunity, environmental context and resources’) (see Table 29); he did not attend group PR but was on the waiting list for standard home PR at the end of the trial. Barriers to PR were related to physical skills (‘physical capability, skills’) and intentions (‘reflective motivation, intentions’) as previously PR had led to dizziness and he did not want to go again based on that experience. Education, persuasion, incentivisation, coercion and modelling are intervention functions that could conquer barriers related to intentions and reflective motivation and training intervention functions could aid barriers around physical capability. 155
P1/200 – declining motivation; declined hospital early pulmonary rehabilitation and group pulmonary rehabilitation sessionsP1/200 was a 62-year-old male who agreed with the necessity of PR, which reduced by 30 days (see Figure 18). He provided mixed responses over time with regard to concerns but agreed that he might become tired from exercising (‘reflective motivation, beliefs about consequences’) (see Figure 24). He found the bike difficult because of breathlessness (‘physical capability, skills’). He completed one out of four of the available sessions, declining three; for the rest of the sessions he was discharged (see Table 29) (‘physical opportunity, environmental context and resources’). He did not attend group PR. His reported barriers to attending PR were related to COPD and comorbidities (‘physical capability, skills’), social support (‘social opportunity, social influences’) and driving/parking (‘physical opportunity, environmental context and resources’). He was driven to get better but did not think that exercise would help when he was feeling unwell (‘reflective motivation, beliefs about consequences’). Apart from physical opportunity, this participant’s barriers may have been reduced if the intervention functions of training, education, persuasion, modelling, enablement, restriction and environmental restructuring were adopted. 155
All of the individuals described in Box 3, who were allocated to home EPR, reported barriers to PR relating to their physical capability, specifically mentioning breathlessness. P1/092 was admitted during the home EPR, meaning that physical opportunity was a barrier, but when not acutely unwell she did not have any other barriers. Motivation increased over the trial period and she attended group PR.
P1/092 was a 64-year-old female who agreed with the necessity of PR at baseline, with agreement strengthening at 30 days. She initially thought that PR may be harmful but had changed her mind by 30 days (‘reflective motivation, beliefs about consequences’). This increase in motivation is presented quantitatively, as the necessities scores increased and concern scores decreased over time (see Figures 18 and 24). She completed all of the home EPR sessions (see Table 30) and attended group PR. She had previously been limited by a chest infection and breathlessness (‘physical capability, skills’) and readmissions (‘physical opportunity’) but no other barriers were indicated, suggesting that there is not a need to introduce additional intervention functions for this participant if she is well enough to attend PR.
P1/100 – decreasing motivation; declined some home early pulmonary rehabilitation sessions but attended group pulmonary rehabilitationP1/100 was a 70-year-old female who agreed with the necessity of PR, although this reduced over time (see Figure 18). From baseline to 30 days her concerns increased around PR in relation to some elements being harmful, that she may not feel fit enough to do it and that she may become tired from it (‘reflective motivation, beliefs about consequences’) (see Figure 24). She declined two out of four home EPR sessions (see Table 30) because of feeling unwell and attended group PR. Her barriers to exercise were related to breathlessness and comorbidities (‘physical capability, skills’) and becoming tired after exercise (reflective motivation; beliefs about consequences), suggesting that her experience could be improved through intervention functions including training, education, persuasion and modelling. 155
P1/161 – increasing motivation; completed home early pulmonary rehabilitation but not group pulmonary rehabilitationP1/161 was a 63-year-old female who agreed with the necessity of PR, although she did think that some aspects were unnecessary and she disagreed with the concerns presented (‘reflective motivation, beliefs about consequences’). Her increase in motivation over time is indicated quantitatively, with the necessities scores increasing and the concerns scores decreasing (see Figures 18 and 24). She completed all four home EPR sessions (see Table 30) and did not go to group PR but said that she was continuing with in-home exercises on their own. Her barriers to group PR were related to breathlessness and comorbidities (‘physical capability, skills’) and being able to get out and about (‘social opportunity’ and ‘physical opportunity’), although these barriers were not present for home EPR. It appears that home EPR removed these barriers for this participant, but the following intervention functions could be included to improve the uptake and maintenance of group PR: training, restriction, environmental restructuring, modelling and enablement. 155
P2/212 – declining motivation; completed home early pulmonary rehabilitation but did not engage with group pulmonary rehabilitationP2/212 was a 65-year-old female whose agreement with the necessity of PR reduced over time; at 30 days she neither agreed or disagreed with any statement except agreeing that rehabilitation could help her return to normal activities (previously agreed strongly) (‘reflective motivation, beliefs about consequences’). This reduction in motivation is presented quantitatively by necessity scores that reduce over time, with no change in the concerns scores (see Figures 18 and 24). She completed all four home EPR sessions (see Table 30) and did not attend group PR. The barriers to group PR (see Table 31) were related to breathlessness (‘physical capability, skills’), carrying oxygen and limitations with regard to getting out of the house (‘social opportunity’ and ‘physical opportunity’). These barriers were not present for home EPR as she felt that she could go ‘at own pace’ and ‘needed one to one’, but, as above, intervention functions could be introduced to improve the uptake and maintenance of PR.
Reflective motivation was presented as a barrier for P1/100, whose concerns were around harmful aspects of PR, not being fit enough to take part or becoming tired from exercising; however, although she was not well enough to complete all of the home EPR sessions, she did attend group PR, suggesting that these barriers were not fixed and would vary with how she felt. P1/161 and P2/212 did not attend group PR but P1/161 said that she still carried out the exercises and P2/212 indicated that her barriers related to opportunity were removed in the home PR intervention.
More individuals allocated to home EPR attended group PR than those allocated to hospital EPR or the usual care group, suggesting that the home EPR intervention helped to remove some of the barriers for uptake of group PR as it incorporates training and enablement functions. Other barriers that appeared to inhibit the uptake of group PR related to reflective motivation, which could be overcome by the physiotherapists providing education at the home EPR sessions. However, this should be interpreted with caution because of the small numbers involved.
Summary of the cross-case synthesis
-
In contrast to expectations, the majority of participants had the ‘physical capability’ to perform EPR exercises.
-
Concerns about ‘psychological capability’ during and early post AECOPD were negated as the activity diary both aided memory and provided a source of behavioural regulation.
-
Intrusion associated with numerous health professionals attending participants at home may occur, negatively affecting ‘social opportunity’ for home EPR.
-
Early pulmonary rehabilitation did not enable ‘social opportunity’ for interaction with peers in a group setting.
-
Early pulmonary rehabilitation provided ‘social opportunity’ for interaction and support from health professionals.
-
Early pulmonary rehabilitation enabled commencement of exercise for those unable to attend group PR, for which ‘physical capability’, ‘physical opportunity’ and ‘social opportunity’ were barriers.
-
Home EPR did not use the same equipment as group PR or hospital EPR; however, this was not always considered a barrier to ‘physical opportunity’.
-
Home EPR enabled ‘physical opportunity’ flexibility in terms of times of visits.
-
Discharge (‘physical opportunity’) and illness (‘physical capability’) were barriers for completion of hospital EPR.
-
Home EPR provided a more individualised intervention, potentially enhancing ‘automatic motivation’.
-
Early pulmonary rehabilitation felt safe, potentially enhancing ‘automatic motivation’.
-
Early pulmonary rehabilitation enhanced confidence, highlighted the importance of exercise and informed participants about what to expect from standard/group PR, thus potentially influencing both ‘automatic motivation’ and ‘reflective motivation’.
Participant views of the trial procedures
Overall, the participants who were interviewed found the trial procedures to be acceptable. Transcripts were coded for trial acceptability and six categories emerged: approach, randomisation, 6MWD, activity monitor, questionnaires and burden.
Approach
In general, participants found that the way that they were approached was acceptable and did not mind being approached when they were feeling acutely unwell. However, one of the non-participants interviewed did not take part because of how they felt in hospital and four of the participants did think that it was a bit too early in their admission to be approached, even though they still took part in the trial:
Do you remember how you found out? How you were approached?
No, I don’t [no] I can’t remember at all.
Were you quite poorly at the time?
Yeah.
So I was gonna ask questions about the information provided but you, do you, you don’t remember?
No, no.
No, so do you think it was probably just that you felt a bit poorly at the time that you . . .
. . . I think it must have been.
P1/157
Related to the approach was the information provided when introducing participants to the trial; 12 of the interviewed participants indicated that there was a lot of information to take in when they were not feeling well:
Just that when you’re in hospital, you’ve not been very well, you’ve not been able to breathe and your mind’s all over the place to be honest and I don’t think you take everything in at the time.
No I think and those information sheets we give you are quite long aren’t they.
Yeah, yeah. I told them and then afterwards I thought oh crikey did I fill that question in right.
P1/223
Randomisation
Of the 14 participants who discussed randomisation, half were happy with this part of the trial, being happy with their allocation, not having a preference or showing an understanding of the process. Some individuals seemed not to fully understand randomisation but were happy with being in the trial and with the group that they were allocated to or they had no preference. Two people expressed their disappointment when they were not allocated to the interventions, with one person withdrawing from the trial during the interview for this reason and one person expressing that they would have liked to use the equipment. One person was not happy with being allocated to both of the interventions, but still completed them both:
Yeah and how did you feel that you could have got the hospital EPR, you could have got in-home and that was sort of done randomly did that worry you at all?
I was disappointed [yeah] yeah disappointed that I didn’t get any of the equipment.
P1/161
Six-minute walk distance
Most of the participants interviewed did not have any issues with carrying out the walk test but two did not complete it while in hospital and some missed their follow-up appointments so could not complete the test at these time points. A few participants were worried prior to the walk test but felt that it was fine to complete and two participants felt good after doing the test as they felt more capable and thought that the test was good at showing them that they could do more than they thought:
Then I thought oh god will I be able to walk properly [yeah] you panic, you know, you’re not sure what you can do. I think I surprised myself how I could walk [yeah] once I got going. I don’t know whether it was because somebody else was there and you got a bit more confidence, I don’t know but once I started walking I surprised myself I think.
So do you initially, you didn’t feel very, maybe not very safe but you were happy to do it.
Yeah a bit insecure really I think, nerves.
P1/223
One person expressed that they did not believe that the test was a good measure, especially for individuals who need to walk on hills most of the time:
How you felt about the walk test, how did you find doing that in hospital?
It’s just, I think the, I think that’s about my only quibble, it’s fine doing it [yeah] but unfortunately it’s not a representative of walking in general, particularly if you live in [city name].
No that is true [laughs].
It’s all on the flat [it’s all flat] and I can walk on the flat erm, particularly on a hospital ward or a corridor [mmm] I can walk a bit and for quite a long time no problem at all [yeah]. If I walk out of my house, by the time I’ve gone 15 metres I’m out of breath if I go in one direction and I’m out of breath if I go in the other direction because it’s on a hill.
P1/226
Activity monitor
Of those who received the activity monitor only one person expressed that it bothered them, with most participants not noticing it very much:
How have you found it wearing the monitor?
Err [pause] a little bit uncomfy [yeah?] but it were only for a week so I didn’t mind.
Yeah? So did you, have you worn it quite a lot of the time, you haven’t taken it off much?
Er, just for a shower [yeah?] or a bath yeah.
Oh brilliant, so yeah I had to wear one for a week before we started it off [laughs].
I think it’s that Velcro.
Yeah the strap.
That snaps you sometimes.
P1/223
However, because of the timing of discharge, not all participants received the activity monitor (see Chapter 3, Key pilot trial outcomes).
Questionnaires
Some participants thought that the questionnaires were too long, especially when they were feeling acutely ill, but all generally saw the point of the questions. A few individuals indicated a little confusion over the questions, particularly the Borg scale (clinical measure of effort).
Burden
Three individuals expressed that the follow-up visits, intervention visits or questionnaires were burdensome as they had a number of other health visitors or people asking them questions while in hospital:
And what did you think about the number of questions you were asked?
There was a lot. A lot, yeah and I didn’t think there was all these questions and that because there were other people that was in the ward that was asking me questions as well and then I went down there it was all questions, just a lot, the whole leaflet was more than I expected to be honest with you.
Do you think it was too much?
A little bit because I think because I was getting better as well I didn’t want to be annoyed with it as well.
P1/101
Therapist views of the interventions analysed within normalisation process theory
Making sense of the intervention
Staff distinguished the hospital EPR intervention from usual care by its novel equipment, strength training component and greater protocolisation (‘differentiation’). It was also delivered earlier, even than in existing ESD schemes. The home EPR intervention was similar to current practice in Sheffield, but was delivered earlier – within 72 hours compared with 2 weeks; staff said that it had the potential to include sicker people who had been in hospital for longer. At Aintree the in-home service was completely novel. For both experimental interventions, higher-grade staff [Agenda for Change (AfC) bands 5–7), normally confined to assessment, took on the supervision and monitoring of exercise, typically delegated to assistants (AfC band 3 or 4). This meant better continuity of care. Physiotherapists could describe the aims of the interventions (‘individual specification’). They shared a sense of those aims with their colleagues if not always the participants (‘communal specification’), for whom they framed information, to increase engagement:
I feel some of the patients needed that additional information from us . . . they’d had all the paperwork . . . but there were some patients that needed that reinforcing during our visits.
Physiotherapist 103, AfC band 6, home EPR
The purpose is to rehabilitate them quicker and get them on a pathway of self-management. That’s what the professionals and patients see. But I think the professionals also see the bigger picture, which is, if we get these patients on this pathway of exercise and education and self-management that ultimately it’s going to lead to fewer exacerbations and hospital admissions . . . it’s cost saving. For the patient the reason is quality of life, they don’t wanna be continually having antibiotics and they don’t want numerous hospital admissions. So I think the ultimate goal is the same but for slightly different reasons.
Physiotherapist 104, AfC band 7, home EPR
I don’t think the patients fully understood what was expected of them . . . I think as soon as you mention the word exercise, patients don’t want to know.
Physiotherapist 107, AfC band 6, hospital EPR
Most physiotherapists constructed potential value for the hospital EPR intervention (‘internalisation’) in terms of maintaining strength and function without impacting on the cardiovascular system, thereby improving readmission and survival rates. They also believed that the intervention improved knowledge of the disease process, self-management and the benefits of exercise, instilling confidence to exercise. One dissenting voice doubted that the hospital EPR intervention would deliver functional gains, but acknowledged that there was a strong link between exercising and chest clearance (see Operational work around the intervention, ‘skill-set workability’). Further benefits were identified for home EPR, including improvements in general post-discharge support and case management. Professional satisfaction arose from the resulting improvements in communication between the in-hospital teams and the community teams.
Relational work around the intervention
Agenda for Change band 7 physiotherapists drove the interventions forward (‘initiation’). As well as engaging other physiotherapists and assistants to implement the interventions (‘enrolment’), they persuaded nurses to identify patients who could benefit. Many patients felt too ill or insufficiently motivated to engage with hospital EPR, unless approached by a consultant rather than an allied health professional. Resistance to the hospital EPR intervention focused around competing priorities and insufficient resources; there were concerns about both hospital EPR and home EPR interventions around the selection of sufficiently well patients (a problem of ‘legitimation’), although senior physiotherapists were less concerned than junior staff:
The sooner we can start them the better I think really . . . I think it’s beneficial because I think it helps alleviate some of their fears as well.
Physiotherapist 108, AfC band 7, hospital EPR and home EPR
Physiotherapists continued to support the interventions (‘activation’) but stressed that ‘staffing was really, really tight’ (physiotherapist 102, AfC band 6, home EPR). Patients were often discharged before hospital EPR was complete or were unavailable concurrently with the physiotherapists or bike because of family visits/clinical consultations. Patients sometimes declined in-home sessions because of competing priorities but, on the whole, remained well engaged:
That’s reflected in the diaries as well . . . they had taken the time to do the exercises in their own time, when I wasn’t there supervising them and actually document that.
Physiotherapist 108, AfC band 7, referring to home EPR
Operational work around the intervention
Staff translated or adapted instructions from manuals and training to enact intervention procedures (‘interactional workability’). For the in-hospital intervention, there was mixed satisfaction with the training and instructions; many described the bike as complicated and there being a learning curve. Some were unclear how to gauge whether or not patients could tolerate higher levels of exercise:
So the protocol that I was taught and work was to set your two repetition max and then figure out your 80% load and that is the working load; however, for the more able people they would be able to manage . . . the two repetition max for the actual intervention, and [investigator’s name] encouraged us to do that whereas, myself, I was unsure so I stuck to the protocol to make sure that nothing changed.
Physiotherapist 101, AfC band 5, hospital EPR
Logistically, hospital EPR was difficult to implement because of the competing demands on the patients’ time, the availability of the bike and positioning the equipment on the ward:
You’ve got to plan it around lunches and breakfasts and things . . . if you’ve got two patients in 1 day it’s trying to fit them around.
Physiotherapy assistant 105, AfC band 3, hospital EPR
Just things like the charging unit and where the plug sockets were . . . you were moving furniture out of the way or you had cables kind of obstructing the patient . . . you might get some patients really extending and leaning forward in the chair then you might get others that are laid back and it’s in their face so they aren’t getting a full extension . . . there wasn’t any [guidance on] how far it should be from the patient . . . it’s quite pivotal for what muscles you’re gonna be engaging.
Physiotherapy assistant 106, AfC band 3, hospital EPR
Physiotherapists reported knowledge work to maintain confidence in the intervention and each other (‘relational integration’). In-hospital staff expressed concern about the face validity of the Borg score to gauge breathlessness, before, during and after bike use. They also questioned aspects of the intervention protocol, such as the load ceiling being too low for some patients and the absence of a cool-down component:
I think that understanding of Borg and peak exertion that was like the epitome for me . . . getting them to recall back to their most breathlessness during that session, was quite difficult.
Physiotherapy assistant 106, AfC band 3, hospital EPR
At Aintree, where home EPR delivery was novel, the physiotherapists were wary of rehabilitating acutely unwell patients without a crash team being available. The Sheffield in-home team had to communicate with each other more, to ensure per-protocol delivery and intervention optimisation. They had to repeat tests, required to tailor the intervention, the results of which were unavailable from the hospital team. Although communication between the in-hospital and the community teams could be improved, community teams appreciated the increased interaction generated by the intervention. In-hospital and community teams acknowledged that respiratory consultants had to be involved to sustain implementation of either intervention:
It was good for us to be able to link up with other disciplines like the GPs and the district nurses and the consultants and the physios in the hospital . . . the link with the people at the hospital was absolutely paramount . . . we can’t do it without them.
Physiotherapist 104, AfC band 7, home EPR
Physiotherapists and their assistants were generally confident in their expertise while enacting it (‘skill-set workability’). Some comments (see Approach) revealed a poor understanding of the background theory of how exercise affects physiology immediately after an exacerbation, which could be addressed in training. Although hospital EPR could be passed on to physiotherapy assistants after the first session, continuation of home EPR could not be delegated:
I did have some tricky ones . . . there was only really one chap who I might have been able to pass on . . . on my fourth visit he was actually quite poorly and I had to get the GP to come and see him . . . it probably did need a qualified member of staff on nearly all of the visits.
Physiotherapist 102, AfC band 6, home EPR
‘Contextual integration’ of the new interventions was incomplete. Although NHS trusts were enthusiastic to participate in the research, ETCs were insufficient to create new posts or backfill old ones for the purposes of delivering the study interventions. Delivery and documentation of hospital EPR was constrained by staffing levels, the numbers trained to deliver/document the intervention and regular clinical work. Fitting the three short daily sessions around meal times and consultations was a challenge. When the staffing level was good, the ward caseload could be covered while hospital EPR was delivered. However, patients were spread out over the hospital and had to be tracked, from assessment units to respiratory or outline wards, meaning that the single available bike had to be constantly transported around the hospital. Continuity of delivery at weekends was ‘difficult’ and reliant on voluntary additional hours. Interviewees thought that hospital EPR could be integrated into usual care if a full-scale trial demonstrated efficacy.
For home EPR, staffing levels and the timescales for participant contact, post discharge, were a constraint on the ability of staff to integrate the intervention; the service was fragile in the face of leave or sickness. Interviewees believed that the service could be sustained or scaled up only if commissioners, consultant physicians, inpatient physiotherapists, therapy assistants and nurses could be engaged and extra physiotherapist capacity could be funded. To plan and deliver the service, extra hours were worked. The four home EPR visits required qualified physiotherapists, whereas Sheffield’s ESD service, which aims to see AECOPD patients within 2 weeks of discharge, delegates most of that work to physiotherapy assistants. Most of the Aintree districts did not have a community physiotherapy team. As procedures allowed little flexibility or patient choice in the timing of visits, visits were sometimes declined. Routine client visits had to be cancelled or moved to accommodate the experimental service, causing an increase in waiting times, from a typical 6–8 weeks.
At the time it was very stressful because we hadn’t got the staffing for it and it was quite labour intensive compared to what we normally do . . . It was the routine home rehabs, that haven’t had a recent hospital admission, but generally can’t get to a group because they’re housebound, or they’ve got transport issues . . . those were the ones that were neglected. And we don’t have targets for when we should see those in. And we’re still trying to catch up the waiting list for those at the moment.
Physiotherapist 104, AfC band 7, home EPR
The difficulties are delivering it at weekends and bank holidays. We obviously have a completely different set of priorities at a weekend . . . it’s not a true 7-day service . . . but certainly during the week it definitely fits and is deliverable.
Physiotherapist 109, AfC band 7, hospital EPR
Appraisal of the intervention
The extent to which physiotherapists were able to access the results of the interventions (‘systematisation’) was limited. Some believed that they could observe minor differences in function as a result of the interventions. However, evidencing gains in strength or mobility or a reduction/delay in readmissions would require a collaborative routine data collection effort by consultants, nursing staff and therapists:
Certainly with some of these chronic patients, uhm, yeah there was a strong link between exercising and chest clearance.
Physiotherapist 107, AfC band 6, hospital EPR
We’re very bad at following patients up . . . we don’t do any follow-up; we need to look at 6 months post rehab, are they still engaging with physical activity. And I’m sure most of them aren’t, because we see them come back again and again through rehab, and we don’t really want that . . . So you know, long-term stats and data is so important, and we certainly don’t have it at the moment.
Physiotherapist 104, AfC band 7, home EPR
There was little opportunity for ‘communal appraisal’; individually, resourcing aside (see previous section), physiotherapists assessed the effects of the rehabilitation programmes on their workplace in positive terms (‘individual appraisal’). They reported that many patients enjoyed the higher intensity and frequency of in-hospital rehabilitation; one assistant reported that patients thanked her for pushing them to exercise, because they felt the benefits afterwards. On the other hand, patients in hospital who were more unwell and those with longer, more complicated hospital stays or comorbidities at home could find PR too much, depending on how their chest was feeling:
I loved the fact that I had more contact . . . we’d normally see them for one or two visits and then pass them on and we’d never find out how they got on or whether they ended up coming to group rehab . . . So the continuity was really nice . . . four sessions over 2 weeks, you could really see them improving. Or if they weren’t improving, because you’d see them regularly, you could do something to help them, they weren’t on their own, you could get the GP to review them, I could ring [the consultant] which was absolutely invaluable.
Physiotherapist 104, AfC band 7, home EPR
I think a number of patients were either too breathless or too generally unwell to undertake the intervention but I think those that felt well enough, benefitted from it and could see the benefit . . . It’s basically understood that hospital admission is not good for patients’ functional mobility and that that needs to be addressed at some point but I’m not sure hospital is the right place to address it.
Physiotherapist 109, AfC band 7, hospital EPR
Physiotherapists reported having the confidence to make and document adjustments to procedures, when necessary, and proposed modifications to treatment protocols (‘reconfiguration’):
I did any adaptations I did document it and if I was unsure as to whether it would still fit into the protocol I could get in touch with [the trial physiotherapists] and just run that through them. So I didn’t feel concerned about those adaptations that I’d made.
Physiotherapist 103, AfC band 6, home EPR
For the in-hospital intervention this included allowing the use of higher settings on the bike, when patients’ exercise capacity permitted, with an additional aerobic component. For home EPR, physiotherapists suggested more of an emphasis on goal setting, that documentation follow patients from the ward into the community and that the community team carry out their own walk tests rather than relying on the in-hospital team to perform and document functional tests.
Therapist comments on the trial procedures analysed using normalisation process theory
Making sense of the trial procedures
Health professionals who were involved in other trials (most were not) could distinguish the trial from others (‘differentiation’):
Quite often we get given trials to do and they’re very prescriptive and they’re very inflexible, to the patients’ detriment . . . what I’m trying to say is, it’s more patient focused, definitely than all of the other trials that I work on . . . some of them are probably more, as you say, dangerous to patients.
Research nurse
Physiotherapists understood the feasibility purpose of the study (‘individual specification’). Collectively, health professionals agreed on that purpose with each other (‘communal specification’) but were careful to frame details to often frail inpatients in ways that would not discourage recruitment overly:
You had to be very clear, it’s not running upstairs, it’s not doing anything that’s beyond what is your capacity. . . . the explanation was crucial.
Research nurse
Physiotherapists believed that, by enabling a large-scale trial, our study could increase understanding of the harm-to-benefit ratio of early rehabilitation58 and whether or not it should be part of the patient pathway (‘internalisation’). Many patients who were approached about the trial did not construct value for it; when they did so, it included benefits for other patients:
Of those that were eligible and said no . . . some of them just can’t see any further than the fact that they can’t breathe.
Research nurse
Very highly valued . . . patients were saying you know, if this is going to help other people then that’s what I want to do it for . . . I think they know how distressing it is for themselves so they obviously don’t want other people to feel like that.
Physiotherapist 102, AfC band 6, home EPR
Relational work around trial procedures
The importance of respiratory consultants and senior physiotherapists to drive the research forward was noted (‘initiation’). With persuasion and appropriate support for participant recruitment from research nurses, physiotherapists were happy to support implementation of the research (‘enrolment’), but this was made easier by contextual knowledge. In particular, involving staff in the medical assessment unit (MAU) helped in the early identification of patients. Comorbidities in those who were eligible, together with the 48-hour recruitment window, prevented higher levels of participant recruitment:
I’m pretty lucky because I came from MAU where a lot of these people were recruited . . . I knew the way that they were working, that we had a new computer system rolled out last year . . . And it saved me a lot of time because [someone with access] was able to look through and see quite quickly who would be eligible . . . in outpatients I had to take biscuits and things, because they’re really, really busy, and you’re asking them to do something that isn’t part of their job . . . there’s no real benefit for them . . . I made quite a lot of small talk.
Research nurse
Health professionals bought into the research, because of interest or belief in the treatment hypothesis and because of the involvement of consultants (‘legitimisation’). Keeping the research nurses and physiotherapists engaged in supporting the research was challenging (‘activation’). Continued engagement with research procedures was problematic for patients, many of whom were too ill to attend further study visits:
And yeah, it required a lot more flexible input than a lot of other studies . . . knowing when people are being discharged, and communicating with the wards.
Research nurse
We did have you know quite a few that wouldn’t turn up for their appointments because, they were re-exacerbating . . . I can understand why they didn’t want to come back when they’re feeling rotten.
Research nurse
Operational work around the trial procedures
Health professionals generally found the training and protocol to be straightforward and delivered the research per protocol (‘interactional workability’). However, ensuring the correct allocation of study interventions and on-schedule assessments was challenging. Assessors were sometimes unblinded by information in hospital notes/GP summaries or encounters with hospital interventionists working with participants. Research nurses had difficulty conducing ultrasonography (rectus femoris muscle cross-sectional area), which was later abandoned, and programming activity monitors because of restrictions in NHS wi-fi access when using project laptops. Almost all physiotherapists lost confidence in what the Borg scores actually represented (‘relational integration’) and research nurses were concerned that they might miss in-hospital AEs, although this should have been picked up in the discharge summaries. In-home physiotherapists had to communicate more, to ensure that participants were seen per protocol. Hand-offs occurred between senior physiotherapists rather than between physiotherapists and assistants, as was normal practice. In Sheffield, staff relied on SystmOne (an electronic patient record for community services) for scheduling visits and determining their content. Getting paperwork to clinicians in multisite trusts was challenging. Health professionals felt that delivery of the trial was appropriate to their role (‘skill-set workability’). In general, the research was adequately supported by host organisations (‘contextual integration’), although local research nurse staffing at one centre was unable to meet the demand for some of the trial because of leave and staff turnover. The fact that the research nurse services were essentially unavailable at weekends impacted on the ability of research nurses to recruit participants and made some data collection components difficult to undertake. Physiotherapists also felt that more capacity was needed and that study implementation was barely robust to staff being on leave. In-home staff, especially, described study involvement as ‘stressful’ and expressed relief at the closure of the trial:
A few weeks ago, I was the only person in the team that could administer the intervention, and I think we had two patients randomised for the exercise trial and I had another ward to run as well.
Physiotherapist 101, AfC band 5, hospital EPR
Having that short window and the four sessions to come within a certain time frame . . . the effect that that has on . . . our overall caseload and our working week.
Physiotherapist 103, AfC band 6, home EPR
The amount of SAEs and the amount of contact you try and get people to come back or to get people to answer the phones for the 7-day follow-up. A lot of it is small but bitty jobs that if you don’t do them it just makes everything a lot harder. And it works quite differently to a lot of our other trials, which are either tied in with clinic visits or are regimented to a particular day.
Research nurse
Appraisal work around the trial procedures
As already noted, those involved with the trial sometimes found the procedures burdensome (‘communal appraisal’), although some also commented ‘that it definitely got easier over time’ (physiotherapist 103, AfC band 6, home EPR). At the same time, the opportunity to be able to carry out a more systematic follow-up of patients was appreciated (‘systematisation’) as was the opportunity that the full-scale trial presented to test the hypothesis and participate in research (‘individual appraisal’):
I think probably for a period it did create some unhappiness and stress within the team [laughs]. But I think, if I’m speaking from my own perspective then I’m quite pleased that I’ve been involved . . . it was quite rewarding actually following the patients, whereas normally I would just be doing that sort of assessment and screening and passing them on.
Physiotherapist 103, AfC band 6, home EPR
Staff proposed changes to the study procedures (‘reconfiguration’), which included simplifying and integrating the AEs form; improving intersite communication/documentation and co-ordination between research nurses and physiotherapists; implementing procedures to identify patients and track them once recruited, including using routine data systems; simplifying the information sheet and adding pictures; removing unsuccessful procedures, such as the use of predicted LOS as an eligibility criterion and the research nurse-led measurement of the rectus femoris muscle cross-sectional area; providing more training in the outcome assessments; recording oxygen use; and providing training to research nurses on the 6MWD test and the circumstances under which participants should not complete it.
Non-participant interviews
Although eight patients agreed to take part in the non-recruited patient study, interviews with only two patients were conducted at 7 days following discharge and 90 days post randomisation. The main reason for the interviews not going ahead was participant choice.
One of these participants wanted to enter the trial but met an exclusion criterion and so could not take part, although he was very keen to be interviewed. This participant undertakes his own exercise plan and appears to be extremely knowledgeable in relation to COPD and the effects of exercise. Although he identified that feeling unwell was his only barrier to exercise, he believed that key barriers for other COPD patients were related to their psychological capabilities, especially in those with depression, which he thought was common in COPD patients.
The other patient felt too ill at the time of being approached to take part in the study but may have taken part if asked later. This patient has since completed in-home EPR (as part of usual care in Sheffield) and found it useful, but did not intend to go to group PR.
Qualitative summary
The interviews with patients and staff involved in the trial were analysed and reported in a number of ways. Context was reviewed by looking at aspects of COPD that affected participants using ICF codes, which indicated that participants’ main concerns were related to them becoming breathless. We summarised participant views on intervention acceptability and used the COM-B/TDF framework to understand participant responses to the interventions.
The qualitative interviews identified that the interventions were acceptable. However, issues were raised surrounding the timing of exercise, with some participants feeling that it may have been conducted too early. This could be addressed in future research by providing education surrounding the purpose of maintenance strengthening exercises in the post-exacerbation phase and ensuring that exercises are tailored appropriately.
Non-attendance at follow-up group PR sessions appeared to be associated with beliefs that exercises were not beneficial and with low self-efficacy. These issues could be addressed by providing education, goal setting and behaviour change interventions. Not enjoying exercise and a lower baseline activity level are more difficult to address. Reluctance to exercise in a group demonstrates the need for alternative formats of PR. Home PR programmes are an alternative to group PR for those who cannot or who prefer not to attend group PR, but not all NHS trusts offer these. The use of technology to support PR is increasing; however, levels of supervision must be considered given that programmes with less input have been found to be less effective. 36
Being discharged before the hospital exercise sessions could be completed was a limitation of the equipment (bike) being available only in hospital. An approach that was more accessible to staff on the ward and that could have been continued at home may be more appropriate for future research.
Chapter 6 Economic analysis and cost-effectiveness
Descriptive statistics of the patient groups for analysis
Sixty-seven patients were eligible for the study, of whom 61 (91%) consented to take part in the trial; of these 61 patients, 58 (95%) were randomised to one of four trial arms:
-
both (hospital EPR followed by home EPR) (n = 14)
-
hospital EPR followed by usual care (n = 14)
-
no hospital EPR followed by home EPR (n = 15)
-
usual care (no hospital EPR followed by usual care) (n = 15).
At the end of the 90-day study period, 44 (76%) patients had completed the study; 12 patients withdrew their consent, one patient was lost to follow-up and one patient was removed from the study (investigator decision). No patients died during the study period. Based on these 44 patients who completed the study and therefore for whom economic data were potentially available for analysis, the remaining patient numbers in each trial arm were:
-
both, n = 10 (71% of those randomised)
-
hospital EPR, n = 10 (71% of those randomised)
-
home EPR, n = 15 (100% of those randomised)
-
usual care, n = 9 (60% of those randomised).
A summary of the baseline characteristics (age and sex) of the 44 participants who completed the study are provided in Table 34.
| Trial arm | Age (years), mean (median, SD, range) | Sex, n (%) female |
|---|---|---|
| Hospital and home EPR (n = 10) | 70 (74, 11, 49–85) | 5 (50) |
| Hospital EPR (n = 10) | 66 (65, 11, 49–81) | 6 (60) |
| Home EPR (n = 15) | 65 (66, 8, 40–78) | 9 (60) |
| Usual care (n = 9) | 69 (69, 8, 57–80) | 5 (56) |
| Across all trial arms (n = 44) | 67 (68, 9, 40–85) | 25 (57) |
Summary of physiotherapist time and intervention costs
The time and costs associated with the interventions are presented in Table 35; costs are dependent on the session time and wage band of the physiotherapist. For the hospital EPR intervention, an additional cost of the bike is included, which is £14.25 per patient. This cost is based on a 90-day equivalent cost of the bike, which is £295 based on the calculations presented in Table 45 (see Appendix 3), distributed across the 20 people randomised to both hospital and home EPR and hospital EPR. For the home EPR intervention, the cost of the booklet is included (£0.50), which is assumed to have been printed for all participants (only those who completed the study for this analysis), and the cost of travel time between the patients’ homes and the physiotherapists’ base of operation (hospital or community physiotherapist office).
| Intervention | Total physiotherapist time (minutes), mean (SD, range) | Total cost of the intervention (£), mean (SEM, range) |
|---|---|---|
| Home EPR (n = 15) | 262 (154, 0–547) | 209 (30, 0.5–426) |
| Hospital EPR (n = 10) | 168 (138, 0–380) | 103 (23, 14–206) |
| Hospital and home EPR (n = 10) | 383 (84, 213–426) | 301 (27, 145–373) |
For the hospital EPR intervention, the mean (SD, range) physiotherapist time associated with the intervention is 168 (138, 0–380) minutes, with a mean [standard error of the mean (SEM), range] cost of £103 (£23, £14–206). For the home EPR intervention, the mean (SD, range) physiotherapist time associated with the intervention is 262 (154, 0–547) minutes, which includes travel time; the mean (SEM, range) cost is £209 (£30, £0.5–426). For those receiving both interventions, the mean (SD, range) physiotherapist time associated with the intervention is 383 (84, 213–426) minutes, which includes travel time; the mean (SEM, range) cost is £301 (£27, £145–373).
Model parameters
All parameters used in the decision tree are presented in Table 36. These parameters are based on the observed statistics from the four trial arms of the pilot study for those 44 people in the ITT group who completed the study. These statistics are all described in detail in Appendix 12, which provides descriptive statistics of resource use, costs and health status by trial arm and across all four trial arms.
| Parameter | Distribution | Usual care | Home | Hospital | Hospital and home EPR | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| Effects | |||||||||
| QALYsb | Beta | 0.1386 | 0.0328 | 0.1489 | 0.0183 | 0.1449 | 0.0296 | 0.1739 | 0.0178 |
| Costs (£) | |||||||||
| Interventionb | Gamma | N/A | N/A | 209 | 30 | 103 | 23 | 301 | 27 |
| Secondary care | |||||||||
| Hospitalb | Gamma | 4784 | 1257 | 4198 | 1127 | 3508 | 846 | 3232 | 1015 |
| GP | |||||||||
| GP surgeryb | Gamma | 47 | 27 | 34 | 9 | 119 | 28 | 42 | 15 |
| GP homeb | Gamma | 5 | 5 | 30 | 17 | 0 | 0 | 18 | 18 |
| GP telephoneb | Gamma | 6 | 4 | 14 | 6 | 4 | 4 | 6 | 6 |
| Therapy | |||||||||
| Physiotherapistb | Gamma | 8 | 5 | 77 | 35 | 32 | 22 | 75 | 24 |
| Occupational therapistb | Gamma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Social workerb | Gamma | 0 | 0 | 10 | 10 | 0 | 0 | 0 | 0 |
| Home care worker (£)b | Gamma | 0 | 0 | 7 | 7 | 0 | 0 | 5 | 5 |
| Health visitor (£)b | Gamma | 66 | 24 | 180 | 61 | 176 | 43 | 6 | 6 |
Economic evaluation: exploratory cost per quality-adjusted life-year analysis
Summary of key results from the cost-effectiveness analysis
A summary of the key cost-effectiveness statistics is presented in Table 37. Mean baseline utility values were highest in the trial arm receiving both hospital and home EPR (0.74, SD 0.17), followed by hospital EPR (0.65, SD 0.27), usual care (0.58, SD 0.19) and home EPR (0.44, SD 0.32) (see Table 74), suggesting that those in trial arm receiving both interventions had the better health status at baseline. In contrast, between baseline and 90 days, only the home and usual care trial arms showed a mean increase in utility values (see Table 74). Mean QALYs were lower and mean costs were higher in the usual care trial arm relative to any other intervention trial arm; however, neither costs nor QALYs were statistically significantly different in any of the intervention trial arms relative to usual care. The home intervention produced the lowest mean cost saving at £145 [95% credible interval (CrI): cost saving of £3519 up to an additional cost of £3067] and the trial arm receiving both interventions produced the highest mean cost saving of £1203 (95% CrI: cost saving of £4467 up to an additional cost of £1842). In comparison, the hospital intervention produced the lowest QALY gain of 0.062 (95% CrI: a QALY loss of 0.082 up to a QALY gain of 0.092), but the trial arm receiving both interventions also produced the highest QALY gain of 0.0353 (95% CrI: a QALY loss of 0.042 up to a QALY gain of 0.104). The mean QALY gain produced by being in the trial arm receiving both interventions is equivalent to almost an extra 13 days in perfect health compared with the usual care group. Each of the three interventions therefore dominate usual care (they cost less and are more effective) at the mean point estimate.
| Care strategy | Mean cost | Incremental cost (95% CrI) | Mean QALYs | Incremental QALYs (95% CrI) | ICER (strategy vs. usual care) (£) | Probability more effective | Probability cost-effective at a WTP of | ||
|---|---|---|---|---|---|---|---|---|---|
| QALYs | £0K | £20K | £30K | ||||||
| Usual care | £4902 | N/A | 0.1388 | N/A | N/A | N/A | N/A | N/A | N/A |
| Home | £4757 | –145 (–3519 to 3067) | 0.1488 | 0.0101 (–0.068 to 0.079) | Dominant | 0.62 | 0.53 | 0.58 | 0.59 |
| Hospital | £3961 | –941 (–4080 to 1882) | 0.1450 | 0.0062 (–0.082 to 0.092) | Dominant | 0.56 | 0.73 | 0.73 | 0.71 |
| Hospital and home EPR | £3699 | –1203 (–4467 to 1842) | 0.1740 | 0.0353 (–0.042 to 0.104) | Dominant | 0.83 | 0.78 | 0.87 | 0.88 |
However, it is important to note the uncertainty surrounding these cost and QALY point estimates and the small sample size associated with the pilot study used to inform this analysis. For example, the 95% CrIs indicate that for both interventions compared with usual care there is a 95% chance that the intervention could lead to anywhere between a QALY loss of 0.042 and a QALY gain of 0.104 and between a reduction in cost to the NHS of £4467 and an additional cost of £1842 per person. The cost-effectiveness planes, which indicate the difference in costs and QALYs of the home (Figure 11), hospital (Figure 12) and both (Figure 13) interventions, visually show the uncertainty around these point estimates and the dispersion of the ICERs. The CEACs (Figure 14; results are summarised in Table 37) suggest that, compared with usual care, the probabilities of the interventions being cost-effective at a willingness-to-pay (WTP) threshold of £20,000 and £30,000 per QALY gained are 58% and 59%, respectively, for home EPR, 73% and 71%, respectively, for hospital EPR and 87% and 88%, respectively, for both interventions.
FIGURE 11.
Cost-effectiveness plane for the home intervention compared with usual care.
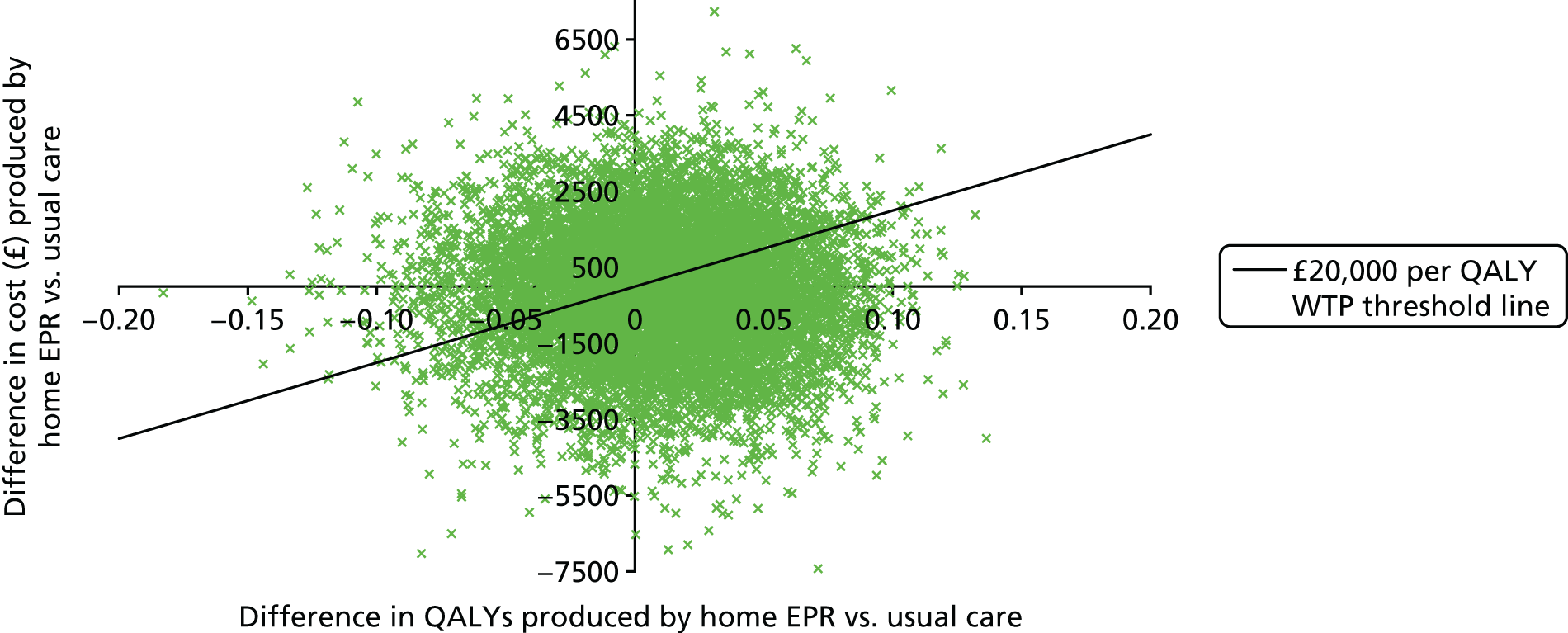
FIGURE 12.
Cost-effectiveness plane for the hospital intervention compared with usual care.

FIGURE 13.
Cost-effectiveness plane for both intervention compared with usual care.

FIGURE 14.
Cost-effectiveness acceptability curves for each of three interventions compared with usual care. Note: this graph demonstrates the probability of cost-effectiveness at a range of decision-maker ceiling WTP values for each of the three interventions compared with usual care.

Interestingly, when the home and hospital interventions are compared against usual care, the home intervention has a higher probability of being more effective than the hospital intervention (62% vs. 56% respectively), but the hospital intervention has a higher probability of being cost saving than the home intervention (73% vs. 53% respectively) (see Table 37).
Expected value of perfect information analysis
The results presented in Table 38 are a selection of the key results from the EVPI analysis. The EVPI analysis compares the decision uncertainty around investing in a particular intervention relative to usual care at a £20,000 WTP per QALY threshold and therefore the decision uncertainty is assessed on an intervention-by-intervention compared with usual care basis.
| Analysis | Home EPR vs. usual care (£) | Hospital EPR vs. usual care (£) | Hospital and home EPR vs. usual care (£) |
|---|---|---|---|
| Overall EVPI (£) | |||
| Per person affected per year | 576 | 276 | 123 |
| People affected per year: 100,000 | 57.6M | 27.6M | 12.3M |
| People affected over 5 years: 100,000 | 288M | 138M | 61.5M |
| People affected per year: 3 million | 1728M | 828M | 369M |
| Partial EVPI per person (£) | |||
| QALYs, intervention (usual care) | 32 (142) | 3 (26) | 0 (2) |
| Hospital costs, intervention (usual care) | 311 (330) | 49 (108) | 23 (13) |
| Partial EVPI for parameter groups per person (£) | |||
| QALYs, intervention and usual care | 171 | 54 | 4 |
| Hospital costs, intervention and usual care | 508 | 193 | 85 |
| QALYs and hospital costs, intervention and usual care | 575 | 275 | 123 |
Based on the cost-effectiveness results, the most interesting EVPI results are associated with the analysis around being allocated to both interventions compared with usual care. In this instance, the EVPI is £123 per person per year, which, if we assume that a hypothetical cohort of 100,000 people would be affected by the intervention, results in an overall EVPI of £12.3M per year (this is the upper-bound value of investing in a future trial comparing both interventions with usual care assuming that the period of time that this decision would be relevant for is 1 year and that the affected population consists of 100,000 people; the overall EVPI increases if the assumed effected population or time period of interest increases, as shown in Table 38). The reason that the EVPI is lower than for the other two interventions is because the probability of this intervention being cost-effective is much higher (there is less uncertainty around the benefit of this intervention) and therefore reducing uncertainty in any of the input parameters for this analysis is less likely to change the outcome than for the other two interventions. The partial EVPI of collecting more QALY information for the both interventions group is zero whereas there is a small (£2 per person) partial EVPI of collecting more QALY information from the usual care group (the relatively higher partial EVPI for the parameters associated with the usual care group suggests that it is the uncertainty around the outcomes in this group that is contributing particularly highly to the decision uncertainty). In contrast, there is more uncertainty around the hospital costs of the both interventions group (£23 per person) than the usual care group (£13 per person).
Sensitivity analysis and key cost and effectiveness drivers
Based on the partial EVPI analysis, there is a suggestion that the QALYs and hospital costs contribute particularly highly to the decision uncertainty. The effect on costs and outcomes can be explored by performing one-way sensitivity analysis by altering the QALYs and hospital costs to the upper and lower bounds of the 95% CIs around the mean point estimates. For example, in the base-case analysis, all interventions dominated usual care based on the mean point estimates, which were used to determine the ICERs; therefore, this one-way sensitivity analysis focused on (1) increasing the mean QALYs to the upper bound of the 95% CI and reducing mean hospital costs to the lower bound of the 95% CI for usual care (making usual care less costly and more effective within the bounds of the 95% CI) and (2) increasing the mean hospital cost to the upper bound of the 95% CIs and reducing the mean QALYs to the lower bound of the 95% CIs for each of the intervention arms (making the interventions more costly and less effective within the bounds of the 95% CIs). All other values were kept equal in the model. The results from this sensitivity analysis are presented in Table 39 for a change in mean hospital costs and Table 40 for a change in mean QALYs.
| Care strategy | Base case | Usual care (hospital cost = £1885) | Home (hospital cost = £6615) | Hospital (hospital cost = £5422) | Hospital and home EPR (hospital cost = £5528) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean cost (£) | Incremental cost (£) | Mean QALYs | Incremental QALYs | ICER (£) | Incremental cost (£) | ICER (£) | Incremental cost (£) | ICER (£) | Incremental cost (£) | ICER (£) | Incremental cost (£) | ICER (£) | |
| Usual care | 4902 | – | 0.1388 | – | – | – | – | – | – | – | – | – | – |
| Home | 4757 | –145 | 0.1488 | 0.0101 | Dominant | 2754 | 272,673 | 2272 | 224,950 | – | – | – | – |
| Hospital | 3961 | –941 | 0.1450 | 0.0062 | Dominant | 1958 | 315,806 | – | – | 973 | 156,935 | – | – |
| Hospital and home EPR | 3699 | –1203 | 0.1740 | 0.0353 | Dominant | 1696 | 48,045 | – | – | – | – | 1093 | 30,963 |
| Care strategy | Base case | Usual care (QALYs = 0.2098) | Home (QALYs = 0.1150) | Hospital (QALYs = 0.0931) | Hospital and home EPR (QALYs = 0.1403) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean cost (£) | Incremental cost (£) | Mean QALYs | Incremental QALYs | ICER (£) | Incremental QALYs | ICER (£) | Incremental QALYs | ICER (£) | Incremental QALYs | ICER (£) | Incremental QALYs | ICER (£) | |
| Usual care | 4902 | – | 0.1388 | – | – | – | – | – | – | – | – | – | – |
| Home | 4757 | –145 | 0.1488 | 0.0101 | Dominant | –0.061 | 2377 | –0.0238 | 6092 | – | – | – | – |
| Hospital | 3961 | –941 | 0.1450 | 0.0062 | Dominant | –0.0648 | 14,522 | – | – | –0.0457 | 20,591 | – | – |
| Hospital and home EPR | 3699 | –1203 | 0.1740 | 0.0353 | Dominant | –0.0358 | 33,603 | – | – | – | – | 0.0015 | Dominant |
To summarise the results in Tables 39 and 40, the change in mean hospital costs resulted in the ICERs for the home and hospital interventions being substantially higher than the £30,000 WTP threshold when the comparison group was usual care; however, the both interventions trial arm remained almost potentially cost-effective at a £30,000 WTP threshold relative to usual care, with an ICER of £30,963 when the mean hospital cost associated with using both interventions was increased to £5528. The change in mean QALY values meant that the home and hospital interventions resulted in a mean QALY loss and produced an ICER representing cost savings per QALY forgone (rather than the more common cost per QALY gained associated with ICERs); however, when the mean QALY value for the both interventions trial arm was reduced to a mean value of 0.1403 (from 0.1740), using both interventions still dominated usual care in this sensitivity analysis. These results are indicative of how the mean cost-effectiveness estimates based on ICERs can change as a result of a change in hospital costs and QALY values in the model and also of how the both interventions trial arm remained relatively or almost cost-effective at the mean point estimates under these scenarios compared with the other interventions assessed relative to usual care.
Summary of results from the economic analysis
In terms of data collection, the relevant CSRI sections for this analysis were completed at 30 days and 90 days by 75% of those participants who completed the study. Only 61% of participants completed the EQ-5D-5L pre discharge, compared with 89% at 30 days, 86% at 90 days and 100% at baseline; only 45% of participants completed the measure at all four time points. There were also issues with the physiotherapists recording the times of the sessions, which meant that not all sessions had a time that could be assessed, and a number of planned sessions did not start or were not completed (these results are described in detail in Appendix 12).
The mean (SEM) incremental intervention cost relative to usual care was £209 (£30) for home EPR, £103 (£23) for hospital EPR and £301 (£27) for both interventions. The highest cost associated with the resource use of this patient group was for hospital care, with the cost of the index hospital admission and readmission ranging from £671 to £17,801 (mean £3942) across all four trial arms (see also Appendix 12). The cost-effectiveness results suggest that the both interventions trial arm has the highest probability of being cost-effective (87% at a WTP threshold of £20,000 and 88% at a WTP threshold of £30,000), cost saving (78%) and more effective based on QALYs gained (83%) than any other intervention relative to usual care; however, all interventions dominated usual care (were less costly and more effective) at the mean point estimate. The aforementioned cost-effectiveness results are interesting, particularly because of the number of missed sessions associated with the per protocol-defined interventions, with the suggestion being that, even if the per-protocol intervention is considered not to be feasible, the number of sessions that were started or/and completed across the study sample was enough to produce a cost-effective outcome relative to usual care in this analysis. It should be noted that this analysis is based on a small sample size and therefore more research with a larger sample should be carried out to confirm or refute this result, with decision uncertainty being driven by the QALY and hospital cost estimates. The EVPI analysis suggests that the overall EVPI per year of conducting a larger trial comparing the use of both interventions (the intervention with the highest probability of being cost-effective in this analysis) with the use of usual care is £123 per person. If we assume that a hypothetical cohort of 100,000 people is eligible to receive both interventions, the upper-bound cost of conducting a larger trial is £12.3M per year for as long as the decision would remain relevant (e.g. usual care does not change).
Chapter 7 Triangulation exercise
Convergence assessment
Tables 41 and 42 show the convergence coding matrix for hospital EPR and home EPR, respectively, in line with the logic model constructs.
| Theme | Quantitative findings | Qualitative findings | Convergence code |
|---|---|---|---|
| Resources | |||
| Staff availability/backfill | 11 physiotherapists delivered at least one session of hospital EPR; 36 sessions were missed because of staffing issues; 36 sessions were missed because of weekend or evening staffing issues (see Chapter 3, Delivery of hospital early pulmonary rehabilitation) | Physiotherapists had the right skillset (see Chapter 5, Operational work around the intervention). Level of extra resource requested was insufficient to deliver the intervention over 7 days alongside existing caseload (see Chapter 5, Operational work around the intervention) | Agreement: lack of staff availability for intervention delivery, explained in terms of resource scarcity/opportunity cost |
| Staff training | – | Mixed satisfaction with training and instructions (see Chapter 5, Operational work around the intervention) | Silence: issue appropriate only for qualitative investigation |
| Engaged NHS trust | ETC funding provided (see Chapter 3, NHS treatment costs) | Organisations supportive (see Chapter 5, Operational work around the intervention) | Agreement: NHS trusts funded interventions and were viewed by staff as being engaged |
| Consultant leadership | – | Was found to be important for professionals (see Chapter 5, Relational work around the intervention) | Silence: issue appropriate only for qualitative investigation |
| Manual and bike | No recorded issues (AEs, non-compliance) with the bike during the trial; all sessions that were started were completed (see Chapter 3, Delivery of hospital early pulmonary rehabilitation) | Use of the bike was not straightforward for staff (see Chapter 5, Operational work around the intervention); most participants found the exercises acceptable (see Chapter 5, Acceptability of hospital early pulmonary rehabilitation) | Partial agreement: some qualitative data suggest that current levels of training are insufficient for complex intervention delivery |
| Patient capability | Baseline data indicate that participants were acutely unwell; four potential sessions were missed as a result of participant illness and 43 were missed as a result of participant choice; 131 initiated sessions were completed (see Chapter 3, Delivery of hospital early pulmonary rehabilitation) | Most patients had the physical capability to complete hospital EPR (see Chapter 5, Participant views of the interventions: COM-B analysis) | Partial agreement: although patients were acutely unwell and declined some sessions, both data sources reported that sessions could be completed |
| Patient opportunity | Six sessions were missed because of patient availability; 36 were missed because of weekend or evening staffing; 131 were missed because of patient discharge (see Table 11) | Participants were sometimes discharged before treatment commenced/was completed (see Chapter 5, Relational work around the intervention) | Agreement: rapid discharge was highlighted as a major barrier to opportunity for hospital EPR in both the quantitative and the qualitative data sets |
| Patient motivation | Participants generally agreed with perceived necessities although scores did not increase over time; participants also generally agreed with perceived concerns (indicating concern) but scores decreased over time (see Appendix 10) | Perceived importance of the intervention and beliefs about consequences and capabilities were generally positive (see Table 32). Few concerns were raised – hospital EPR felt safe and enhanced confidence in/safety to exercise (see Chapter 5, Intervention characteristics linked to the COM-B theoretical framework and the theoretical domains framework) | Partial agreement: perceived necessity of PR was indicated in the quantitative and qualitative data but concerns appeared greater in the quantitative data as few concerns were raised by individuals |
| Activities | |||
| Programme maintenance | – | Insufficient resources and competing priorities made continued engagement hard (see Chapter 5, Relational work around the intervention) | Silence: issue appropriate only for qualitative investigation |
| Case management | 224 missing items (not key variables) in the hospital EPR documentation (see Table 9) | The logistics of bike access, patient tracking and documentation were constrained by resources (see Chapter 5, Operational work around the intervention). Physiotherapists could not access individual outcome data (see Chapter 5, Appraisal of the intervention) | Agreement: high levels of missing data in hospital EPR forms were confirmed by qualitative findings |
| Immediate outcomes | |||
| Eligible patients offered EPR (‘reach’) | 158 patients were eligible but were not recruited; 41 patients were reported to be ‘too ill’and 51 were not recruited because of ‘patient choice’ (see Table 5) | Patients often felt too ill or insufficiently motivated to enrol in the trial (see Chapter 5, Relational work around the intervention) | Agreement: feeling too ill was the most common reason for non-participation other than eligibility |
| Three sessions delivered over 5 consecutive days (‘dose delivered and received’) | 131/384 (34.1%) expected sessions were completed (see Chapter 3, Delivery of hospital early pulmonary rehabilitation) | Fitting in three daily sessions and weekend delivery were difficult (see Chapter 5, Operational work around the intervention) | Agreement: qualitative data helped explain the numbers of missed sessions |
| Session optimised for individual (‘fidelity’) | 106/321 sessions were optimised; 12 sessions (four participants) were not optimised because of limitations of the equipment (see Chapter 4, Optimisation of hospital early pulmonary rehabilitation) | Some patients found the exercises too easy, even on the hardest setting. Junior staff were not confident with regard to optimising the intervention (see Chapter 5, Operational work around the intervention) | Agreement: the quantitative data showed that four participants found hospital EPR too easy, as mentioned in the staff interviews |
| Intermediate outcomes | |||
| Muscle strength, exercise tolerance, activity, activities of daily living, readmission rate and health-related quality of life | The study was not powered to detect important differences | The intervention period was considered too short by staff to have an effect on intermediate outcomes (see Chapter 5, Appraisal of the intervention) | Silence: unable to triangulate the qualitative findings reliably with the quantitative data |
| Attendance at community PR | 1/22 participants allocated to hospital EPR attended group PR; 1/22 attended an alternative to PR [see Chapter 3, Attendance at group pulmonary rehabilitation (usual care)] | A number of factors limited ongoing commitment to exercise, particularly if this meant attending a structured, venue-based PR programme (see Chapter 5, Participant views of the interventions: COM-B analysis) | Agreement: both data sets highlight that access to community PR is difficult |
| Theme | Quantitative findings | Qualitative findings | Convergence code |
|---|---|---|---|
| Inputs | |||
| Staff availability/backfill | No sessions were missed because of staffing issues; however, recruitment was paused on occasion when there were no physiotherapists to deliver the intervention (see Chapter 3, Physiotherapist funding) | Staffing levels were fragile in the face of leave or sickness (see Chapter 5, Operational work around the intervention) | Partial agreement: the service seems quite stable based on quantitative data; qualitative data show instability and reasons for this |
| Staff training | – | When no community service exists, physiotherapists are unused to working with such acutely ill patients in their own homes (see Chapter 5, Operational work around the intervention) | Silence: issue appropriate only for qualitative investigation |
| Engaged NHS trust | ETCs were initially agreed in Sheffield but the trust was unable to buy out the extra resource; two out of three CCGs in Liverpool agreed to fund ETCs (see Chapter 3, NHS treatment costs) | NHS trusts were reported to be enthusiastic (see Chapter 5, Operational work around the intervention); ETCs were insufficient to create new posts/backfill old posts for the purposes of intervention delivery (see Chapter 3, NHS treatment costs) | Agreement: trusts wish to support research but the treatment cost system makes it difficult to do so |
| Consultant leadership | – | Consultants were cited as key to maintaining the service and stimulating patient uptake (see Chapter 5, Relational work around the intervention) | Silence: issue appropriate only for qualitative investigation |
| Travel costs | Average of 33 minutes of travel per visit, with a cost of £27 (see Appendix 12) | – | Silence: staff did not discuss travel in the interviews |
| Manual and exercise diaries | 18 activity diaries were returned; six participants completed more than the four visit entries, five completed the four visit entries and the remaining participants completed fewer than four visit entries (see Chapter 3, Intervention non-compliance) | Participants reported that the manual helped with memory and behavioural regulation (see Chapter 5, Timing of pulmonary rehabilitation) | Agreement: the qualitative research confirms the value of the diaries, the use of which was documented qualitatively |
| Patient capability | Baseline data indicated that participants were acutely unwell, with 16/92 sessions missed because of participant illness or participants not being fit to exercise (see Chapter 3, Delivery of home early pulmonary rehabilitation) | Most interviewed participants were not too breathless (see Chapter 5, Timing of pulmonary rehabilitation); the protocol could be adapted with regard to physical limitations (see Chapter 5, Appraisal of the intervention) | Partial agreement: baseline data and decliners suggest that patients were acutely unwell, but most patients undertaking the exercises were capable, even if they had limitations |
| Patient opportunity | One session was missed not because of patient choice or illness (see Chapter 3, Delivery of home early pulmonary rehabilitation) | Participants liked the flexibility and convenience of the visits (see Chapter 5, Flexibility). Home EPR provided an opportunity for interaction with/to receive support from professionals (see Chapter 5, One-to-one therapy) | Agreement: both data sets showed that barriers to opportunity were less of a problem than for hospital EPR |
| Patient motivation | Participants in the home EPR group showed improved engagement and motivation over time, with a reduction in concerns (see Appendix 10) | Participants felt that exercise was important/beneficial. Physiotherapists provided most participants with the confidence to exercise. Participants liked the social interaction, convenience, low cost, location and privacy (see Chapter 5, Participant views of the interventions: COM-B analysis). Beliefs about capabilities were generally positive (see Table 32). No concerns were raised | Agreement: both the quantitative and the qualitative data suggest that patients were motivated to undertake home EPR and this improved over time |
| Activities | |||
| Programme maintenance | – | Because of resource constraints, the service displaced client-facing activity for other client groups (see Chapter 5, Operational work around the intervention) | Silence: issue appropriate only for qualitative investigation |
| Case management | 46 items were missing in relation to the home EPR paperwork (see Table 9); 10/24 participants received all four sessions (see Chapter 4, Optimisation of home early pulmonary rehabilitation) | Physiotherapists welcomed the increased communication with the hospital team and among their own team and the continuity of the service for clients (see Chapter 5, Operational work around the intervention) | Partial agreement: paperwork was well completed by all staff, indicating good communication, although only 42% of the participants received all four sessions of the intervention |
| Immediate outcomes | |||
| Eligible patients offered EPR (‘reach’) | 158 patients were eligible but were not recruited; 41 were reported to be ‘too ill’ and 51 were not recruited because of ‘patient choice’ (see Table 5) | Patients often felt too ill or were insufficiently motivated to enrol in the study (see Chapter 5, Relational work around the intervention) | Agreement: feeling too ill was the most common reason for non-participation other than eligibility |
| Four sessions delivered over 2 weeks (‘dose delivered and received’) | 72/92 (78.3%) of the sessions were delivered. The main reason for session non-attendance was participant illness (see Chapter 3, Delivery of home early pulmonary rehabilitation) | Patient illness/comorbidities and staff annual leave were given as reasons for sessions not going ahead (see Chapter 5, Operational work around the intervention) | Partial agreement: the qualitative work showed additional reasons for non-delivery of the sessions; the quantitative data did not show missed sessions as a result of annual leave as recruitment was paused to account for annual leave |
| Session optimised for individual (‘fidelity’) | 207/990 (21%) sessions were optimised (see Chapter 4, Optimisation of home early pulmonary rehabilitation); 15/24 (62.5%) participants completed three out of four sessions (see Table 28) | Optimisation required senior staff in contrast to existing ESD programmes (see Chapter 5, Operational work around the intervention) | Partial agreement: sessions were not optimised but senior staff could assess patients and deliver what was appropriate |
| Patient performs unsupervised exercise | Six patients reported in their activity diary performing additional exercises (see Chapter 3, Delivery of home early pulmonary rehabilitation) | Some patients claimed to be doing daily unsupervised rehabilitation, but others were wary (see Chapter 5, Introduction to pulmonary rehabilitation) | Agreement: some individuals completed unsupervised exercises following the completion of home EPR sessions |
| Intermediate outcomes | |||
| Exercise tolerance, activity, activities of daily living, readmission rate, shortness of breath, health-related quality of life | Study not powered to detect important differences | Some patients felt that their exercise tolerance and activity levels had improved over the 2 weeks of the home EPR intervention (see Chapter 5, Introduction to pulmonary rehabilitation) | Silence: unable to triangulate the qualitative findings reliably with the quantitative data |
| Attendance at community PR | 8/26 participants allocated to home EPR attended group PR; 4/26 attended an alternative to PR [see Chapter 3, Attendance at group pulmonary rehabilitation (usual care)] | A number of factors limited ongoing commitment to exercise, particularly if this meant attending a structured, venue-based PR programme (see Chapter 5, Participant views of the interventions: COM-B analysis) | Agreement: both data sets highlight that access to community PR is difficult |
In relation to hospital EPR, there was agreement on eight components, partial agreement on three components and silence on four components (all expected areas of silence as they were amenable only to qualitative assessment). Partial agreement was found for the patient capability, patient motivation and manual and bike components of the intervention; these are all components of the resources needed. In relation to patient capability, partial agreement arose as the patients were acutely unwell but were still able to undertake hospital EPR in most cases. With regard to patient motivation, more concerns were reported in the quantitative data than in the qualitative data. With regard to the manual and bike component, the physiotherapists reported that the bike was not straightforward to use, but there were no reported issues in relation to AEs, which could indicate that the issues identified by the physiotherapists were not severe enough to cause issues for the patients.
In relation to home EPR, there was agreement on seven components, partial agreement on five components and silence on five components (again, these were all amenable only to qualitative assessment). Partial agreement was found for staff availability and backfill, patient capability, case management, four sessions delivered over 2 weeks and session optimised for the individual, which are components of the resources and the activities required for intervention delivery. The reasons for partial agreement with regard to staff availability, case management and four sessions delivered over 2 weeks are related: physiotherapists reported issues with delivery because of annual leave and sickness but this is not evident in the quantitative data. This discrepancy occurred because the trial team paused recruitment when it was aware that there would be issues with delivering the intervention. The partial agreement related to patient capability is the same as for hospital EPR: patients were acutely unwell but were still able to undertake home EPR in most cases. Partial agreement with regard to optimisation arose because physiotherapists believed that senior staff were required to deliver optimised home EPR, but the optimisation assessment indicated that the programme was not optimised. This is discussed in more detail in Chapter 4, suggesting that the optimisation rules that were used may not be appropriate for assessing optimisation in EPR following an AECOPD.
Completeness assessment
For hospital EPR, the qualitative research contributed information to 15 out of 15 logic model constructs, whereas the quantitative data contributed to 11 of the components. Qualitative information contributed to 16 out of 17 components of the home EPR intervention and quantitative data contributed to 13 out of 17 components. This was expected as some components were not amenable to investigation in quantitative terms; as the trial was a pilot trial, triangulation was not possible for some of the components.
Summary
Summary of the triangulation of data for hospital early pulmonary rehabilitation
Figure 15 provides a summary of the key points relating to the implementation of hospital EPR. Participants were capable and willing to undertake the exercises while in hospital, but it was difficult to deliver three sessions on 5 consecutive days, particularly during the evenings and at weekends, because of staff availability. Discharge was the main barrier to delivery of the hospital EPR intervention, as patients were often discharged within 5 days of recruitment. Some of the participants found the intervention too easy, so for these patients the exercises were suboptimal, although in general the intervention was optimised for individual patients. Optimisation may have been improved by providing enhanced training in relation to the cycle ergometer, although the workload could not be increased for some patients because of the limitations of the equipment.
FIGURE 15.
Hospital EPR summary of triangulation.

Summary of the triangulation of data for home early pulmonary rehabilitation
Figure 16 summaries the triangulation of data relating to the implementation of home EPR in this trial. Although the NHS trusts were engaged, because of changes in funding since the agreement of ETCs, this support did not translate into increased capacity to deliver home EPR in Sheffield. This in turn affected the delivery of the intervention (by pausing recruitment) and the delivery of existing services.
FIGURE 16.
Home EPR summary of triangulation.

As with hospital EPR, participants were capable and willing to take part in the intervention sessions. The participants liked the flexibility and convenience of the sessions taking place in the home at a time that suited them and 90% of the sessions that were started were completed. Both participants and physiotherapists found the activity diaries useful and participants appreciated the case management by physiotherapists, although the quantitative data suggest that case management was not successful.
The home EPR sessions were not optimised according to the guidelines for PR in stable patients, although this may not be a suitable assessment, and it was thought that senior physiotherapists were required to deliver the intervention in this group of acutely ill patients. Consultant leadership was thought to be important for both interventions and a link to a consultant was thought to be necessary for physiotherapists delivering home EPR.
Chapter 8 Discussion
Summary of findings
Feasibility outcomes
Recruitment
Over 7 months at two centres, 449 patients were screened and 58 were randomised to the trial (76% of target). The overall recruitment rate was 4.1 participants per centre per month (5.1 participants per centre per month) after protocol amendments, compared with the target of 5.4 participants per centre per month.
Attrition and data collection
Fourteen participants withdrew from the trial following randomisation, with 40 participants providing some data at 90 days. Only 36% (21 out of 58) contributed to the primary clinical outcome (6MWD).
Intervention adherence
Adherence to hospital EPR was poor: only 131 out of 384 (34.1%) expected sessions were completed, with sessions not completed because of discharge, participant refusal or therapist availability.
Adherence to home EPR was better, with 72 out of 92 (78.3%) sessions taking place. Sessions were missed because of participant illness/readmission or refusal. At least three out of four sessions were completed by 15 out of 24 (62.5%) participants, of whom 14 completed six out of eight exercises in each session.
Optimisation
Of the hospital EPR sessions that were not optimised, generally being too easy for the participant, half could have been optimised with enhanced training but the other half could not be optimised because of limitations of the equipment. This intervention may not provide the resistance training required for those who manage easily at the highest level on the cycle ergometer.
No participants achieved optimisation in the home EPR sessions. Clinical reasoning led to suboptimal aerobic exercise assessment in the first session and gradual introduction to exercise in the three subsequent sessions. Optimisation was hampered by inappropriate scoring and inadequate documentation of Borg RPE values for resistance exercises.
Clinical outcomes
The primary outcome assessment was undertaken in fewer than half of the participants. The small number of participants involved did suggest better outcomes in the home EPR group and the group allocated to both hospital and home EPR.
Secondary clinical outcomes for this study were extensive, with several being exploratory. The small numbers in the pilot trial do not allow us to draw inferences from the data and the focus was on safety data, especially in light of recent evidence. 58
No deaths were recorded in any of the intervention arms during the 90-day follow-up period, which, given the severity of illness of the patients recruited (see Chapter 3, Baseline data, and previous studies32,58,156,157), is perhaps surprising.
Attendance at group PR (usual care) was poor for the participants in our trial at the 90-day follow-up period. This was partly a result of the length of the follow-up as the majority of participants had these sessions scheduled outside the follow-up period.
The observation of a potential reduction in exacerbation rates in those patients who received home EPR may be a promising outcome for both patients and health-care providers. Exacerbation frequency is a strong predictor of adverse outcomes and poorer prognosis, quality of life and function and contributes to the risk of hospital admission and mortality. 32,45,58 The rates in our trial are lower than those found in another study. 158
Readmission to hospital within 30 days is a crude indicator of rate of recovery following AECOPD, but is considered a significant marker of the effectiveness of clinical management strategies within the NHS. 38 In the small number of participants in this trial, we observed a slight reduction in readmission to hospital in those who received home EPR, in line with a recent review. 159 This outcome is dependent on the severity of patients included and can be predicted utilising tools such as the PEARL score. 109 Our analysis suggests that the overall readmission rates were similar to those predicted by the PEARL score and are representative of the wider COPD population.
After seeing the results, COPD readmission was chosen as the candidate primary outcome. Among the motivations for this were:
-
Readmission is a clinically important outcome for both patients and the health-care system.
-
As a safety outcome, readmission data could potentially be collected from withdrawn participants (with the appropriate permissions), resulting in a low proportion of missing outcome data.
-
Readmission is an ‘objective’ outcome as it can be retrieved from medical records and is not influenced by reporting.
Among the challenges found were:
-
Specification to be chosen (continuous, categorical, time to event). Continuous data were chosen as this could lead to a smaller sample size estimation. It was unclear how to define an incidence/survival outcome (and there is less literature on pilot studies using this kind of data).
-
Estimation of the SD. Given the skewed outcome it could be debated whether or not choosing a continuous outcome is an efficient way of computing the sample size. A potential solution is to evaluate the outcome on the log scale (adding 1) when performing statistical inference and then report transformed and untransformed estimates.
-
Denominator and participants to include. This issue will be mitigated in the main trial as we will collect data from patient notes on all patients.
Given these uncertainties and challenges, sensitivity analyses should be carried out in the main trial to evaluate the robustness of the results. 160
Qualitative research
The acceptability of both interventions was high for participants and physiotherapists and most participants were capable of undertaking the interventions.
Barriers to EPR were participants concerns about breathlessness, participants believing that they were too ill and did not have the necessary skills to undertake exercise and participants not believing that the exercises were beneficial. There were insufficient resources available to deliver both interventions without affecting existing services. Similar results have been found previously, although we found no sex differences. 161,162
In relation to the trial protocol, participants found most aspects acceptable, with mixed views about the burden of the interventions and outcome measures. The Borg score was difficult to complete and the study documentation and training may not have been sufficient for physiotherapists. Some aspects of organising the participant pathway were challenging.
Health economic analysis
Resource use data at 30 and 90 days were available for 75% of study participants who completed the study; hospital data were available for 100% of these participants. The percentage of people completing the EQ-5D-5L was 100% at baseline, 61% at pre discharge, 89% at 30 days and 86% at 90 days. Exploratory cost-effectiveness analysis suggested that the hospital EPR and both interventions trial arms had a > 70% probability of being cost-effective at a £20,000 per QALY threshold (73% and 87% respectively); this probability was 58% for the home EPR trial arm. Key drivers of uncertainty in the EVPI analysis were hospital costs and QALYs.
Strengths and limitations
The pilot trial
External pilot trials are not designed or powered to generate estimates of clinical effect that should be used for decision-making. 160,163 Instead, we have measured process variables160 and understood intervention optimisation and reach;164 the trial was not powered to test mechanisms of impact or contextual moderators. 123 Although we did not reach our prespecified recruitment target, the data are adequate for sample size estimation. 165,166 Study administration issues can be used as a learning tool for the management of a full-scale trial.
Recruitment
Recruitment was initially restricted to those who we believed would be admitted for a period of time that was sufficient to necessitate hospital EPR. At the proposal stage, we estimated that at least 50% of patients would fulfil this criterion based on known LOS data. However, we did not anticipate two key factors that would influence eligibility:
-
We relied on estimates of LOS by the attending physician at the time of admission, which may not have been accurate.
-
Changes were made to service provision that reduced average LOS for many patients (enhanced ESD).
After discussion with the TSC, it was decided to reduce and then remove the 5-day estimated LOS exclusion criteria.
Interventions
We modified portable cycle ergometer protocols from other cardiopulmonary diseases and developed a new customisable in-hospital training protocol for patients with AECOPD. This protocol proved to be very acceptable to patients, with a high rate of completed sessions delivered with no safety concerns. The intervention was also acceptable to staff and could be delivered by appropriately trained staff within the standard physiotherapy session time. However, it was not possible to deliver all of the anticipated sessions, mainly because of early discharge and patient condition.
Similarly, the home EPR programme was a modifiable, exercise prescription, developed specifically for this study and supervised by a physiotherapist. Patient acceptance was high, with a high rate of completed sessions and no safety concerns. The physiotherapists adjusted the prescription according to the condition of patients on the day and, if there were any concerns, there was an opportunity for discussion with and review by the clinical team. Patients expressed feeling of enhanced support during the period of the intervention.
Participants were informed of their allocation in two stages: (1) immediately after randomisation for hospital EPR and (2) at discharge for home EPR. This was to try not to influence adherence to hospital EPR if a participant knew that they would be receiving further exercise at home. There is a possibility that some patients were disheartened by this two-stage process if they had not received hospital EPR; however, this did not seem to be the case.
Outcomes
Participants did not consider the outcome assessments to be arduous or difficult to complete. The primary clinical outcome, 6MWD, is well validated and widely used. 36,167 However, completion proved challenging, with many participants feeling too unwell to undertake this test. Unpredictable discharge times and difficulty undertaking assessments outside standard working hours also contributed to poor completion rates for the pre-discharge measures and to a low distribution rate for the activity monitors. The assessment of rectus femoris muscle cross-sectional area proved difficult to standardise between study personnel and was removed to reduce unnecessary participant burden. When worn, accelerometers were acceptable to patients but, by setting a minimum requirement of 10 hours of data for at least 5 days,168 we restricted the use of this measure, producing results that may not be reliable. The reporting of SAEs may have been subject to some ascertainment bias, with participants receiving home EPR being visited and monitored more regularly by health-care professionals.
It was noted that the assessment of expectedness of SAEs differed between those completing the assessment because of the expectation by the principal investigator at Aintree that, on average, 30% of patients would experience a readmission; in contrast, the principal investigator at Sheffield assessed all readmissions as expected. This was not rectified during the trial as we did not want to dictate the assessment carried out at each site, but this could be clarified in future reporting protocols.
Setting an acceptable threshold for missing data is challenging169 and ours – 0.5% of values missing – now seems unduly stringent. It is now more common for trial teams to use a ‘traffic light system for criteria used to judge feasibility’85 and, under this system, it is unlikely that our missing value rates of < 3% would be deemed sufficient to prevent progression to a full-scale trial.
The qualitative research
Through the use of qualitative methods we have successfully described implementation problems and perspectives on the intervention123 and so can make recommendations about intervention feasibility and changes to the trial design. Through the use of logic models and social science theory we have ensured that we addressed key uncertainties and important questions. 123 We used longitudinal interviews to capture changes in experience over time. 123,170
Data saturation was not formally assessed and stopping criteria were not employed. Future research could address this to ensure that themes and/or conflicting views are not missed.
One member of the qualitative research team (one of those conducting the interviews and analyses) was a physiotherapist with experience of the management of AECOPD in both hospital and community settings. Reflexivity may have been influenced by these experiences.
Semistructured interviews in health care typically last for 20–60 minutes,171 meaning that ours were generally short, although there was a considerable variation in length. The point has been made that the length of interviews can be as important as the number of interviews. 138,172 For some qualitative researchers, the length of the interviews is a marker of rigour. 173 Others understand it as a function of purpose,171 as in our case when we are identifying barriers to intervention uptake using existing theoretical frameworks, rather than attempting to advance social science theory. But short interviews, especially when conducted longitudinally, can produce rich data and are justifiable in vulnerable populations,174 in our case people who are very sick and easily tired. Data were gathered to the point of redundancy – new data yielded no new information175 – and were adequate to identify whether or not individual barriers to uptake were modifiable problems of capability, opportunity and motivation. 140
Optimisation
As physiotherapy involves making sense of uncertain, ambiguous and continually emergent phenomena, revising subjective impressions and accommodating shifting objectives, the specialty resists standardisation. 113 It follows that optimisation or ‘fidelity of function’ is more an appropriate construct for assessing implementation quality than ‘fidelity of form’,114 ensuring congruence with the intervention theory. 116,176,177 The use of an experienced physiotherapist to rate the delivery of each intervention could have been improved by having independence from the study team and the use of a second rater, blind to the ratings of the first. Optimisation assessment of home EPR, but not hospital EPR, was discussed with the practitioners and feedback was elicited, adding nuance to the initial ratings.
Issues apparent in the use of the modified Borg RPE score suggest that difficulty ratings provided by the patients and physiotherapists may be a more reliable assessment in future.
Current rehabilitation adherence and optimisation measures are limited. 178
Optimisation of hospital early pulmonary rehabilitation
Optimisation of hospital EPR may have been improved by enhanced training in relation to the cycle ergometer, although the workload could not be increased for some patients because of the limitations of the equipment, which had a maximum resistance of 20 kg. Future research would be required to determine if this maximum load is sufficient to provide a positive training effect for patients.
Optimisation of home early pulmonary rehabilitation
Few participants exercised within the target range of moderate to severe/very severe (Borg breathlessness score of 3–6). This was attributed to suboptimal exercise intensity and difficulties with using Borg scores. Clinical decision-making was considered appropriate. Sessions were assessed and adapted to reduce any detrimental impact and promote long-term commitment by increasing confidence and enabling success.
Resistance exercises were considered optimised if two to four sets of exercises with 10–15 repetitions were performed until fatigue not failure. 179 However, fatigue and RPE scores were not reported. This was attributed to inadequate documentation and information retention and insufficient time to review the training manual prior to sessions.
Some resistance exercises relied on household goods and were potentially suboptimal with regard to weight and closed-chain resistance exercises relied on the weight of the participant. The use of repetitions, sets and speed was flexible to allow for exercise to fatigue; however, because of inadequate reporting this cannot be assumed.
Of the overall session RPE scores recorded, 22 out of 63 were within the target range (12–14),37 35 were below the target range and six were above the target range. Difficulties using the RPE score as an overall measure of fatigue were reported. Current PR guidelines34,37 are aimed at standard PR and not PR post AECOPD and it is recommended that this be taken into account during exercise prescription.
Health economics
To collect data for the economic analysis, participant-reported methods were used at multiple time points as well as utilising routinely collected data; these data collection methods have strengths and limitations. With regard to the strengths of these methods, first, the CSRI was collected at 30 days (focusing on resource use over the last 30 days) and 90 days (focusing on resource use over the last 60 days) to try and reduce the implications of recall bias for the patient group (the assumption being that people can recall information more reliably over shorter time periods. 180,181 Second, the EQ-5D-5L was collected at four time points (baseline, pre discharge, 30 days and 90 days) to enable a more accurate health status profile to be determined over the 90-day trial period [i.e. the minimum number of time points required to elicit a QALY is two (baseline and 90 days), but this may assume a linear change in health between baseline and 90 days]. Third, hospital resource use was based on patients’ paper-based hospital records, which reduced the cognitive burden on participants of having to self-report their hospital resource use and avoided recall bias associated with self-reported methods.
With regard to the limitations of the approach used, first, the inclusion of more outcomes that need to be collected, particularly usually self-reported methods, can lead to a higher chance that there will be missing data. Second, collection of EQ-5D-5L data pre discharge required prior knowledge that a patient was being discharged, which was problematic as discharge did not necessarily occur at a consistent time point post baseline and was probably a contributing factor to the low completion rate of this measure at this time point (interpretation of the results was difficult compared with interpretation of other data that was collected at consistent time points, although it did provide an indication of the health status of patients at the point of discharge). Third, the CSRI had to be collected at both 30 days and 90 days to provide enough information to infer participant resource use patterns across the 90-day trial period. Fourth, paper-based hospital records provide only certain levels of information that can be utilised for the purpose of health economic assessment; although they might be considered superior to self-reported methods because of relieving the burden on patients, they may not contain sufficient information for microcosting exercises [such as costing per episode based on Healthcare Resource Group (HRG) codes182].
We may have underestimated the cost-effectiveness of the interventions relative to usual care when assuming that usual care was used by all participants across all trial arms for the purpose of the exploratory economic evaluation (which was not the case in reality). To more appropriately understand usual care and account for the resource use costs, we may want to collect this detailed information as part of a larger trial and consideration needs to be given to the optimal method for the collection of information on this aspect of resource use.
The trade-off is between collecting more detailed information for the purpose of analysis and compensating for missing data, which is a common issue in any study. A strength of this pilot study was that an attempt was made to collect a lot of detailed information, but the levels of missing data were quite high for certain data collection points, such as for the EQ-5D-5L at pre discharge. For a larger trial, the decision would have to be made whether to (1) improve the data collection method at this time point (assuming that this is possible); (2) accept the level of missing data and rely on multiple imputation methods, assuming that the data are considered to be missing at random (but not completely at random); or (3) not collect the data at this time point.
Although an economic model and evaluation was performed utilising the observed data, a limitation is that this evaluation was informed by a small sample size, normally associated with a pilot study and the reason that missing data were not assessed as part of this evaluation. Baseline adjustments and the correlation between costs and QALYs were not accounted for in the model, also because of the small sample size; however, these factors should be considered as part of any future analysis alongside a larger study and associated larger data set. It was assumed that the observed data are representative of this patient population, particularly at the mean point, and this could bias the results (e.g. people may have been too ill to complete the self-reported questionnaires, which could have resulted in lower costs and higher QALY values in certain trial arms then if patients with poor health status and potentially higher resource use had completed the measures, which could have affected the results of the economic evaluation). Particular attention should be paid to the uncertainty around the economic evaluation results and the EVPI results, which suggest that the value of obtaining information from a larger trial would be beneficial if the interventions are deemed to be feasible, effective and cost-effective and that the cost of a larger trial would be lower than the overall EVPI value (dependent on whether the larger trial focuses on all three or only one of the intervention strategies).
Triangulation protocol
A range of methods, appropriate to the commissioning brief, were selected,123 but these methods were not implemented independently, as is sometimes recommended. 183 The use of a formal mixed-methods approach, using logic models, enabled the use of qualitative findings to better understand the quantitative data.
Patient and pubic involvement
Patients had input to the development stages of the trial protocol and to both interventions. Patients reviewed and approved the MOTOmed viva 2 bike for use in hospital EPR and, through discussion, modified the design of home EPR; patients suggested that it should be delivered by a senior physiotherapist because of concerns over safety.
There were patient and pubic involvement (PPI) representatives on the TMG and TSC and these individuals fed back on issues during the trial. The results of the study were shared with the PPI representatives and their feedback was incorporated into the report. Further to this, the Chief Investigator (RH) and co-applicant physiotherapist (CO’C) presented the results to another group of patients, who provided their feedback and contributed to the discussion regarding future protocol development.
Generalisability
Population
Initially, participant accrual was more difficult and those screened had more severe symptoms than anticipated (see Chapter 2, Participants). After changing the admission duration criterion, patients with lower levels of COPD severity were screened and recruited, as reflected in the DECAF (see Table 8) and PEARL (see Chapter 3, PEARL prediction scores) scores. On the basis of these markers of prognosis and subsequent readmission rate, participants were representative of those admitted to our institutions. Those recruited were self-selecting and perhaps more motivated to undertake the physiotherapy interventions. However, our dropout rate was as expected and comparable to that in a similar study. 58 Research in non-English speakers would be useful in the future.
Intervention implementation
The provision of an in-hospital physiotherapy service using a cycle ergometer is not widespread and would be difficult for most organisations to deliver. However, in-hospital physiotherapists can provide a vital role in assessing patients who might benefit from a home-based therapy approach and earlier discharge from hospital. The community respiratory physiotherapy model is more widely available but at present is poorly standardised. 38 By providing individualised exercise prescription, based on a standardised set of exercises and supervised by a trained physiotherapist, we believe that in-home exercise may be a more acceptable and effective intervention for patients and health-care providers. By incorporating this into the clinical discharge programmes already widely available in many NHS trusts there is the potential to provide more integrated and personalised in-home care for patients.
Evidence of feasibility
Population/recruitment
Our original recruitment target for this pilot study was 76 patients (a target of 5.4 participants per centre per month), whereas, in total, we recruited 58 participants (4.1 participants per centre per month). We were able to screen a large number of potential patients. The predominant reason for screening failure during the initial 3 months of the trial was the difficulty in accurately predicting those patients who would have a LOS in excess of 5 days. In the last 3 months of recruitment, the recruitment rate exceeded 5.1 patients per centre per month, although it should be noted that the majority of participants were recruited from the Sheffield centre. After changing the LOS eligibility criterion, recruitment improved significantly and proceeded at the rate that was expected. The recruitment target may have been achieved with a slightly longer recruitment period and with the LOS criterion removed.
There was a significant difference in recruitment between the two centres. To some extent, this reflects the different sizes of the institutions. However, recruitment also seemed to be dependent on the health-care professional who made the initial approach to the patient. Patients seemed to be more likely to consider consent if the study was offered by a doctor as part of their overall clinical care. As the chief investigator was based at the Sheffield centre it is prudent to assume that the recruitment rate at Aintree is more indicative of a ‘real’ recruitment rate.
An additional consideration is that one of the centres appeared to initially approach a greater proportion of patients with confusion, dementia and other comorbidities; these were not listed as exclusion criteria, although these patients were excluded for these reasons. This may reflect differences in patient populations and demographics between the participating centres, but could have been more clearly defined within the screening criteria.
Refusal of consent for many patients appears to have been based on a misperception of the requirements of the exercise programme (particularly hospital EPR) and their functional capability at that time. Once the exercises were started, the majority of patients were able to perform them both in hospital and at home. We had to recruit patients within 48 hours of them being admitted to deliver hospital EPR and we believe that approaching patients at a later time point, once their clinical condition was improving, may have resulted in further improvements in recruitment.
Intervention
Despite the high level of acceptance of hospital EPR once patients were recruited to the trial, this intervention proved difficult to provide reliably during the first 5 days of their admission. Despite the participation of highly motivated physiotherapists, completing three sessions per day proved logistically difficult as patients were often not available to undertake the intervention (patients were resting, were not on the ward, were receiving other treatments such as nebulisers or were being visited by relatives). Most of these reasons were seen in Sheffield, where there were more participants and more physiotherapists delivering the intervention.
We initially considered offering patients hospital EPR for the duration of their hospital stay or until they were deemed ‘medically fit for discharge’. However, this would have resulted in significant variation in intervention duration, a lack of standardisation between individuals and further resource requirements for staffing.
Additionally, the capital cost of the exercise bike equipment, although possibly useful for other patient populations, may prove prohibitive.
It was therefore difficult to demonstrate a definitive benefit of this intervention because of poor adherence, with the intervention proving to be resource intensive. The fact that many patients were discharged before completing 5 days of the intervention further limits interpretation of the results. Although we cannot conclude that hospital EPR is ineffective, particularly for those patients with a more prolonged LOS, we believe that it is not deliverable in its current format more widely across the NHS unless a reliable method of predicting hospital LOS is identified, allowing those with a longer LOS to be specifically targeted by this intervention.
Furthermore, there is an increasing trend within the NHS to offer patients with severe AECOPD more intensive management within the home (so-called ‘hospital at home’ approaches). In the future, only very severe patients may require hospitalisation and these represent a different patient population from the patient population included in the current study.
On the contrary, not only was home EPR by a qualified physiotherapist highly acceptable to the patients, but also the provision of the intervention was perceived to be simple and the intervention was deemed to be effective and sustainable for the wider population. There are resource implications regarding the provision of physiotherapist time that need to be considered carefully, but this approach appears to be appropriate for further investigation and is preferred by patients.
Physiotherapists are not typically policy-makers within their own workplace. Physiotherapists told us that these interventions can be implemented and sustained if there is the political will from consultants and commissioners to reallocate resources, but also that there would be considerable opportunity costs as the interventions are currently configured (see Chapter 5, Operational work around the intervention).
Outcomes
It has been difficult to establish a physiological outcome measure to assess the efficacy of the interventions and the poor completion of the 6MWD measure indicates that it is not an ideal primary outcome for this patient population.
The use of activity monitors in this study was equally problematic. Although acceptance of the monitors was high, the total data recording times were insufficient in many patients to obtain meaningful results. The reasons for this require further exploration; although this may be a useful secondary outcome,168,184–186 it is difficult to recommend this marker as a primary outcome.
The functional and quality of life assessment questionnaires were more acceptable to patients and completion rates were high across visits.
Time to readmission and severe exacerbation frequency appear to be clinical outcomes that are important to patients and health-care providers. Given the nature of the intervention, promoting behavioural change related to exercise and health seeking may result not only in functional improvement but also a reduced susceptibility to or severity of subsequent exacerbations. This is one component of the in-home EPR approach that is significantly different from the PR approach used in previous studies. 36
To address the lack of data required for the optimisation assessment of home EPR, future recommendations include the use of prompts on data entry forms to improve reporting of exercise prescription and deviations from target prescription and the reporting of RPE scores and enhanced training in the intervention protocol, such as increased training time, proximity to delivery and role play.
Implications for policy makers, health professionals and people with chronic obstructive pulmonary disease
Although some of the outcomes from the home EPR group are promising, this is a feasibility study and should not be used to guide service provision or policy at this time. A larger study to more clearly define outcomes and safety is required before any recommendations can be made.
Of major importance is that the interventions offered to patients with AECOPD in this study appeared to be safe and generally acceptable. There was an observation of delayed readmission in patients who received home EPR, which should be further investigated as this outcome was valued highly during discussions with our patient representative group, who indicated that they would be willing to undertake such an intervention for this reason alone. Home EPR may also be delivered as part of a package of service and professional integration, which has been shown to reduce readmissions. 187–189 This may benefit COPD patients when primary care is not sufficient. 190
The patient representatives were also encouraged by patients’ acceptance of the interventions once recruited. One representative suggested that he would be willing to assist with recruitment to any further studies and present a patient perspective to assist potential participants.
The patient representatives were disappointed, but not surprised, by the conversion of patients from acute exercise training to subsequent group-based rehabilitation. They believed that any future studies should attempt to immediately follow home EPR with group-based sessions, to ‘strike while the iron was hot’. They believed that there was sufficient potential in reducing admission rates and encouraging ongoing rehabilitation to support further studies using this approach.
Equally, our discussions with local commissioners indicated that confirmation of a delay and a reduction in readmissions to hospital for patients with AECOPD, and a reduction in the associated health-care costs, would be considered a clinically significant result of any intervention and would be sufficient to provide consideration for support and commissioning.
A revised programme theory
As hospital EPR was difficult to deliver because of patient discharge, we propose a revised programme theory for home EPR:
Patients and physiotherapists will be willing and able to conduct home EPR in patients’ homes and will allocate resources for this (inputs and activities). This will involve reconfiguration of existing roles, interactions and relationships that characterise the management of AECOPD (context). Delivery of the programme per protocol (immediate outcomes) will bring about a change in health behaviour and the physiological benefits described in the treatment theory (intermediate outcomes).
Uptake and maintenance of this physical therapy intervention would be enhanced by a behaviour change intervention targeting the theoretical domains physical capability and reflective motivation (barriers), with training and enablement intervention functions.
Assessment of optimisation will be improved by additional training for physiotherapists, improved documentation and a clearer definition of ‘dose’.
Chapter 9 Further research
The following sections detail suggestions for future research based on the findings from the pilot trial.
Full-scale pragmatic randomised controlled trial
A full-scale RCT is required to assess the efficacy of EPR following AECOPD. Both the acute nature of the condition and the hospital exercise were barriers to recruitment; the hospital intervention was also logistically difficult to deliver. Therefore, we propose removing the hospital intervention from the full-scale RCT:
-
Population – patients admitted to hospital with AECOPD.
-
Intervention – 2 weeks of home PR immediately after a hospital admission followed by early community PR.
-
Comparator – delayed community PR. This will be usual care as it is currently offered in the participating centres: ‘“Usual practice” or the best available alternative management strategy, offering practitioners considerable leeway in deciding how to apply it’. 90
-
Outcomes: We propose that the primary outcome for this study should be the rate of readmission at 6 months, collected from participant notes (to improve completion rates and reduce burden), with secondary outcomes of exacerbation frequency and severity, functional assessments (LACDL), exercise capacity (1 week of accelerometry), mortality, health-care resource utilisation and quality of life (EQ-5D-5L, CAT).
A tentative CONSORT diagram outlining the proposed approach is presented in Figure 17. Based on a sample size of 750 participants and the recruitment rate in the feasibility study, we believe that it would be possible to recruit sufficient participants at 10 centres over an 18-month period, with a 6-month follow-up period. Eight UK acute hospital trusts have been approached and have indicated a willingness to contribute to such a trial. A separate clinical trial in a more defined group of patients with very severe AECOPD with an extended hospital stay (perhaps those requiring acute non-invasive ventilation, with a high DECAF score or with associated pneumonia) may be indicated to establish the efficacy of hospital EPR approaches. However, we believe that this would be a difficult patient population to recruit given the reluctance of many participants to participate in our feasibility study.
FIGURE 17.
Consolidated Standards of Reporting Trials (CONSORT) diagram for the proposed full RCT. MI, myocardial infarction; PE, pulmonary embolism.
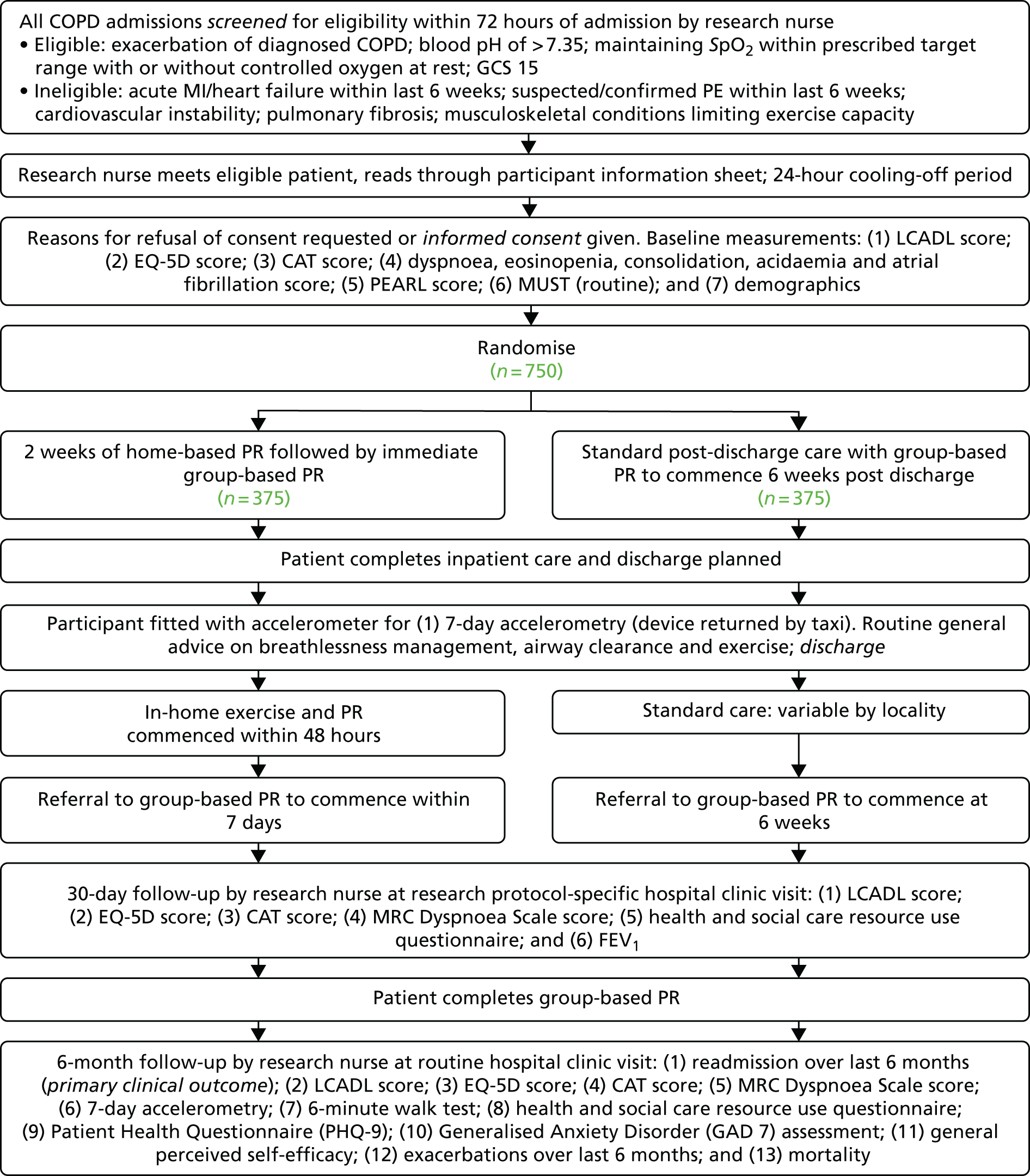
Behaviour modification to increase the uptake of and adherence to pulmonary rehabilitation
Our study has systematically defined the problem of engagement with PR in behavioural terms, selected and specified target behaviours and identified what needs to change. These are the first four stages in a stepwise approach to the design of behaviour change interventions. 155 Subsequent research should involve a behaviour change specialist and service users to identify intervention functions, policy categories, behaviour change techniques and modes of delivery that are affordable, practicable, effective, acceptable, safe and equitable (the APEASE criteria155). Research should be carried out to finalise the content of a behaviour change intervention for testing in subsequent research and use in clinical practice. Research components might include:
-
systematic review of interventions to increase the uptake of PR, with papers coded against the TDF136
-
qualitative research to elicit ideas from health professionals and service users for further potential components
-
a two-round Delphi exercise in which health professionals and service users independently rate proposed components against the APEASE criteria to define a package.
Markers of response to treatment after exacerbation
Evidence from existing prognosis research about treatment response after AECOPD should be systematically reviewed. 191,192 When necessary, candidate prognostic factors should be included in future interventional and observational studies, with a minimum of 10 events per predictor variable. 193,194
Chapter 10 Conclusions
This pilot study assessed the feasibility of evaluating EPR in a cohort of patients with AECOPD in hospital and immediately post discharge. The hospital exercise intervention discouraged many potential participants from consenting and was difficult to accommodate within locally available physiotherapy resources.
In-home rehabilitation, immediately post discharge, supervised by a trained physiotherapist, proved to be more acceptable to patients, was deliverable by local services and provided similar potential outcome benefits. It was provided safely with no harms associated with the intervention.
Many of the outcome assessments used proved to be challenging: the 6-minute walk test was unacceptable and measurement of ultrasound quadriceps muscle mass was unreliable. Exploratory assessment of home-based accelerometry may prove to be a more useful marker of effectiveness. Further evaluation, in a larger study, of whether or not EPR delays readmission is warranted.
Acknowledgements
We gratefully acknowledge the hard work of, and support and advice from, the following: Paul Albert, Julie Channell and Lynne Keogan (AUHNHSFT) and Rodney Hughes, Matt Cox and Alexandra Mudd (STHNHSFT) for participant screening and data collection; Tim Chater, Saleema Rex, Emily Turnton and Ella Baldwin (University of Sheffield) for data management and administrative and clerical support; Jim Lithgow (Respiratory Directorate Research Co-ordinator, STHNHSFT) and Nana Theodorou (Research Co-ordinator, STHNHSFT) for support on ethics and governance; and Lisa Leivesley, Joseph Byrne, Paula Cowley, Alan Price, Jenny Pierpoint, Kerry Warburton and Tracy Blanchard (AUHNHSFT) and Carl Page, Andrew Wortham, Ian Hardman, Ursula Freeman, Laura Turner, Kerry Howkins, Helen Lawrence, Jo Adams and Sarah Lee (STHNHSFT) for delivering the intervention, with administrative support from Jane O’Connell and Gemma Hale (STHNHSFT).
We offer special thanks to the members of our TSC and Data Monitoring and Ethics Committee (DMEC). The members of the TSC were Dr Jon Miles (Chairperson, Rotherham NHSFT), Mr David McWilliams (University Hospitals Birmingham NHSFT), Mr Tom O’Brien (PPI representative), Dr Moe Kyi (Doncaster and Bassetlaw Hospitals NHSFT) and Dr Stephen Bourke (Northumbria Healthcare NHSFT). The members of the DMEC were Dr Michael Steiner (Chairperson, University Hospitals of Leicester NHS Trust), Dr Brenda O’Neill (University of Ulster) and Dr Martyn Lewis (Keele University).
Contributions of authors
Matthew Cox (Co-applicant Physiotherapist) conceived of or designed the study, was involved in the acquisition of data and the analysis and interpretation of the data and produced the first draft of the report.
Catherine O’Connor (Co-applicant Physiotherapist) conceived of or designed the study, was involved in the analysis and interpretation of the data and produced the first draft of the report.
Katie Biggs (Trial Manager) was involved in the analysis and interpretation of the data and produced the first draft of the report.
Daniel Hind (Co-applicant/Assistant Director CTRU) conceived of or designed the study, was involved in the analysis and interpretation of the data and produced the first draft of the report.
Oscar Bortolami was involved in the analysis and interpretation of the data.
Matthew Franklin (Health Economist) was involved in the analysis and interpretation of the data and produced the first draft of the report.
Barbara Collins was involved in the analysis and interpretation of the data.
Stephen Walters (Co-applicant/Statistician) conceived of or designed the study, was involved in the analysis and interpretation of the data and produced the first draft of the report.
Allan Wailoo was involved in the analysis and interpretation of the data.
Julie Channell (Co-applicant Physiotherapist) conceived of or designed the study and produced the first draft of the report.
Paul Albert (Co-applicant/Principal Investigator) conceived of or designed the study, was involved in the acquisition of data and produced the first draft of the report.
Ursula Freeman provided feedback on the qualitative results, contributed to the management and conduct of the trial and was involved in key decisions leading to improved recruitment.
Stephen Bourke contributed to the management and conduct of the trial and was involved in key decisions leading to improved recruitment.
Michael Steiner contributed to the management and conduct of the trial and was involved in key decisions leading to improved recruitment.
Jon Miles contributed to the management and conduct of the trial and was involved in key decisions leading to improved recruitment.
Tom O’Brien provided feedback on the qualitative results, contributed to the management and conduct of the trial and was involved in key decisions leading to improved recruitment.
David McWilliams contributed to the management and conduct of the trial and was involved in key decisions leading to improved recruitment.
Terry Schofield (Co-applicant/PPI Representative) conceived of or designed the study, provided feedback on the qualitative results, contributed to the management and conduct of the trial, was involved in key decisions leading to improved recruitment and produced the first draft of the report.
John O’Reilly contributed to the management and conduct of the trial and was involved in key decisions leading to improved recruitment.
Rodney Hughes (Chief Investigator) conceived of or designed the study, was involved in the acquisition of data and the analysis and interpretation of the data and produced the first draft of the report.
All authors revised the work critically for important intellectual content and were involved in the final approval of the version to be published.
All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data sharing statement
Requests for patient-level data and statistical code should be made to the corresponding author. Although specific consent for data sharing was not obtained, the management group will consider the release of data on a case-by-case basis following published guidelines. 195 The presented data do not contain any direct identifiers; we will minimise indirect identifiers and remove free-text data to minimise the risk of identification.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- O’Reilly J, Jones MM, Parnham J, Lovibond K, Rudolf M. Management of stable chronic obstructive pulmonary disease in primary and secondary care: summary of updated NICE guidance. BMJ 2010;340. https://doi.org/10.1136/bmj.c3134.
- World Health Organization . Burden of COPD n.d. www.who.int/respiratory/copd/burden/en/index.html (accessed 23 November 2017).
- Shahab L, Jarvis MJ, Britton J, West R. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative population sample. Thorax 2006;61:1043-7. https://doi.org/10.1136/thx.2006.064410.
- British Lung Foundation . The Battle for Breath – the Impact of Lung Disease in the UK 2016. www.blf.org.uk/what-we-do/our-research/the-battle-for-breath-2016 (accessed 23 November 2017).
- Rodriguez-Roisin R, Anzueto A, Bourbeau J, DeGuia T, Hui D, Jenkins C. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ (accessed 6 December 2017).
- Hunter LC, Lee RJ, Butcher I, Weir CJ, Fischbacher CM, McAllister D, et al. Patient characteristics associated with risk of first hospital admission and readmission for acute exacerbation of chronic obstructive pulmonary disease (COPD) following primary care COPD diagnosis: a cohort study using linked electronic patient records. BMJ 2016;6. https://doi.org/10.1136/bmjopen-2015-009121.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. https://doi.org/10.1056/NEJMoa0909883.
- Rennard SI, Vestbo J. Natural histories of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:878-83. https://doi.org/10.1513/pats.200804-035QC.
- Barker BL, McKenna S, Mistry V, Pancholi M, Patel H, Haldar K, et al. Systemic and pulmonary inflammation is independent of skeletal muscle changes in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014;9:975-81. https://doi.org/10.2147/COPD.S63568.
- Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis 2014;9:871-88. https://doi.org/10.2147/COPD.S49621.
- Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010;137:1091-7. https://doi.org/10.1378/chest.09-2029.
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003;124:459-67. https://doi.org/10.1378/chest.124.2.459.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. https://doi.org/10.1136/thorax.57.10.847.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22. https://doi.org/10.1164/ajrccm.157.5.9709032.
- Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev 2010;19:113-18. https://doi.org/10.1183/09059180.00002610.
- Spruit MA, Gosselink R, Troosters T, Kasran A, Gayan-Ramirez G, Bogaerts P, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003;58:752-6. https://doi.org/10.1136/thorax.58.9.752.
- Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centered outcomes. Chest 2007;131:696-704. https://doi.org/10.1378/chest.06-1610.
- Schols AM, Mostert R, Soeters PB, Wouters EF. Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax 1991;46:695-9. https://doi.org/10.1136/thx.46.10.695.
- Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:809-13. https://doi.org/10.1164/rccm.2107031.
- Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007;62:115-20. https://doi.org/10.1136/thx.2006.062026.
- Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006;129:536-44. https://doi.org/10.1378/chest.129.3.536.
- Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med 1994;150:11-6. https://doi.org/10.1164/ajrccm.150.1.8025735.
- Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, Dentener MA, Schols AM. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:1239-45. https://doi.org/10.1164/ajrccm.162.4.9912016.
- Vermeeren MA, Schols AM, Wouters EF. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J 1997;10:2264-9. https://doi.org/10.1183/09031936.97.10102264.
- Couillard A, Prefaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J 2005;26:703-19. https://doi.org/10.1183/09031936.05.00139904.
- Gertz I, Hedenstierna G, Hellers G, Wahren J. Muscle metabolism in patients with chronic obstructive lung disease and acute respiratory failure. Clin Sci Mol Med 1977;52:396-403. https://doi.org/10.1042/cs0520395.
- Pouw EM, Schols AM, van der Vusse GJ, Wouters EF. Elevated inosine monophosphate levels in resting muscle of patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:453-7. https://doi.org/10.1164/ajrccm.157.2.9608064.
- Rennard S, Decramer M, Calverley PM, Pride NB, Soriano JB, Vermeire PA, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J 2002;20:799-805. https://doi.org/10.1183/09031936.02.03242002.
- Hersh CP, Make BJ, Lynch DA, Barr RG, Bowler RP, Calverley PM, et al. Non-emphysematous chronic obstructive pulmonary disease is associated with diabetes mellitus. BMC Pulm Med 2014;14. https://doi.org/10.1186/1471-2466-14-164.
- Huber MB, Wacker ME, Vogelmeier CF, Leidl R. Excess costs of comorbidities in chronic obstructive pulmonary disease: a systematic review. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0123292.
- Department of Health . An Outcomes Strategy for Chronic Obstructive Pulmonary Disease (COPD) and Ashtma in England 2011. www.gov.uk/government/uploads/system/uploads/attachment_data/file/216139/dh_128428.pdf (accessed 14 December 2017).
- Echevarria C, Brewin K, Horobin H, Bryant A, Corbett S, Steer J, et al. Early supported discharge/hospital at home for acute exacerbation of chronic obstructive pulmonary disease: a review and meta-analysis. COPD 2016;13:523-33. https://doi.org/10.3109/15412555.2015.1067885.
- Scott DA, Woods B, Thompson JC, Clark JF, Hawkins N, Chambers M, et al. Mortality and drug therapy in patients with chronic obstructive pulmonary disease: a network meta-analysis. BMC Pulm Med 2015;15. https://doi.org/10.1186/s12890-015-0138-4.
- Bolton CE, Bevan-Smith EF, Blakey JD, Crowe P, Elkin SL, Garrod R, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013;68:ii1-30. https://doi.org/10.1136/thoraxjnl-2013-203808.
- O’Driscoll BR, Howard LS, Davison AG. BTS guideline for emergency oxygen use in adult patients. Thorax 2008;63:vi1-68. https://doi.org/10.1136/thx.2008.102947.
- Puhan M, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016;12. https://doi.org/10.1002/14651858.CD005305.pub4.
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. https://doi.org/10.1164/rccm.201309-1634ST.
- Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management. London: NICE; 2010.
- Spielmanns M, Gloeckl R, Schmoor C, Windisch W, Storre JH, Boensch M, et al. Effects on pulmonary rehabilitation in patients with COPD or ILD: a retrospective analysis of clinical and functional predictors with particular emphasis on gender. Respir Med 2016;113:8-14. https://doi.org/10.1016/j.rmed.2016.02.006.
- Lahham A, McDonald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis 2016;11:3121-36. https://doi.org/10.2147/COPD.S121263.
- Ries AL. Pulmonary rehabilitation executive summary. Chest J 2007;131:1S-3S. https://doi.org/10.1378/chest.07-0892.
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;4. https://doi.org/10.1002/14651858.CD003793.pub2.
- Jones SE, Green SA, Clark AL, Dickson MJ, Nolan AM, Moloney C, et al. Pulmonary rehabilitation following hospitalisation for acute exacerbation of COPD: referrals, uptake and adherence. Thorax 2014;69:181-2. https://doi.org/10.1136/thoraxjnl-2013-204227.
- Hayton C, Clark A, Olive S, Browne P, Galey P, Knights E, et al. Barriers to pulmonary rehabilitation: characteristics that predict patient attendance and adherence. Respir Med 2013;107:401-7. https://doi.org/10.1016/j.rmed.2012.11.016.
- Stone RA, Holzhauer-Barrie J, Lowe D, Searle L, Skipper E, Welham S, et al. COPD: Who Cares Matters. National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: Clinical Audit of COPD Exacerbations Admitted to Acute Units in England and Wales 2014. London: Royal College of Physicians; 2015.
- ACSM’s Guidelines for Exercise Testing and Prescription. Baltimore, MD: American College of Sports Medicine; 2009.
- Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:19-38. https://doi.org/10.1164/rccm.200408-1109SO.
- Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Bérubé C, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:896-901. https://doi.org/10.1164/ajrccm.159.3.9807034.
- Ortega F, Toral J, Cejudo P, Villagomez R, Sánchez H, Castillo J, et al. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:669-74. https://doi.org/10.1164/rccm.2107081.
- Clark CJ, Cochrane L, Mackay E. Low intensity peripheral muscle conditioning improves exercise tolerance and breathlessness in COPD. Eur Respir J 1996;9:2590-6. https://doi.org/10.1183/09031936.96.09122590.
- Panton LB, Golden J, Broeder CE, Browder KD, Cestaro-Seifer DJ, Seifer FD. The effects of resistance training on functional outcomes in patients with chronic obstructive pulmonary disease. Eur J Appl Physiol 2004;91:443-9. https://doi.org/10.1007/s00421-003-1008-y.
- Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis MI, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:870-8. https://doi.org/10.1164/rccm.200305-617OC.
- Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis 1991;143:9-18. https://doi.org/10.1164/ajrccm/143.1.9.
- Griffiths TL, Burr ML, Campbell IA, Lewis-Jenkins V, Mullins J, Shiels K, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000;355:362-8. https://doi.org/10.1016/S0140-6736(99)07042-7.
- Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007;297:1772-4. https://doi.org/10.1001/jama.297.16.1772-b.
- Troosters T, Probst VS, Crul T, Pitta F, Gayan-Ramirez G, Decramer M, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:1072-7. https://doi.org/10.1164/rccm.200908-1203OC.
- Eaton T, Young P, Fergusson W, Moodie L, Zeng I, O’Kane F, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology 2009;14:230-8. https://doi.org/10.1111/j.1440-1843.2008.01418.x.
- Greening NJ, Williams JEA, Hussain SF, Harvey-Dunstan TC, Bankart MJ, Chaplin EJ, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ 2014;349. https://doi.org/10.1136/bmj.g4315.
- Saudny-Unterberger H, Martin JG, Gray-Donald K. Impact of nutritional support on functional status during an acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;156:794-9. https://doi.org/10.1164/ajrccm.156.3.9612102.
- Burtin C, Decramer M, Gosselink R, Janssens W, Troosters T. Rehabilitation and acute exacerbations. Eur Respir J 2011;38:702-12. https://doi.org/10.1183/09031936.00079111.
- Torres-Sánchez I, Valenza MC, Cabrera-Martos I, López-Torres I, Benítez-Feliponi Á, Conde-Valero A. Effects of an exercise intervention in frail older patients with chronic obstructive pulmonary disease hospitalized due to an exacerbation: a randomized controlled trial. COPD J Chronic Obstr Pulm Dis 2017;14:37-42. https://doi.org/10.1080/15412555.2016.1209476.
- Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, et al. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 1998;84:157-63.
- Probst VS, Troosters T, Pitta F, Decramer M, Gosselink R. Cardiopulmonary stress during exercise training in patients with COPD. Eur Respir J 2006;27:1110-18. https://doi.org/10.1183/09031936.06.00110605.
- Lake DA. Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports Med 1992;13:320-36. https://doi.org/10.2165/00007256-199213050-00003.
- Latimer L, Greening N, Morgan M, Singh S, Bradding P, Steiner M. Unilateral neuromuscular electrical stimulation (NMES) of the quadriceps muscles in stable COPD. Eur Respir J 2013;42.
- Sillen MJH, Janssen PP, Akkermans MA, Wouters EFM, Spruit MA. The metabolic response during resistance training and neuromuscular electrical stimulation (NMES) in patients with COPD, a pilot study. Respir Med 2008;102:786-9. https://doi.org/10.1016/j.rmed.2008.01.013.
- Abdellaoui A, Préfaut C, Gouzi F, Couillard A, Coisy-Quivy M, Hugon G, et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J 2011;38:781-8. https://doi.org/10.1183/09031936.00167110.
- Vivodtzev I, Lacasse Y, Maltais F. Neuromuscular electrical stimulation of the lower limbs in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2008;28:79-91. https://doi.org/10.1097/01.HCR.0000314201.02053.a3.
- Sillen MJH, Speksnijder CM, Eterman RA, Janssen PP, Wagers SS, Wouters EFM, et al. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: a systematic review of the English-language literature. Chest 2009;136:44-61. https://doi.org/10.1378/chest.08-2481.
- Zanotti E, Felicetti G, Maini M, Fracchia C. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest 2003;124:292-6. https://doi.org/10.1378/chest.124.1.292.
- Vivodtzev I, Pépin JL, Vottero G, Mayer V, Porsin B, Lévy P, et al. Improvement in quadriceps strength and dyspnea in daily tasks after 1 month of electrical stimulation in severely deconditioned and malnourished COPD. Chest 2006;129:1540-8. https://doi.org/10.1378/chest.129.6.1540.
- Kirsten DK, Taube C, Lehnigk B, Jörres RA, Magnussen H. Exercise training improves recovery in patients with COPD after an acute exacerbation. Respir Med 1998;92:1191-8. https://doi.org/10.1016/S0954-6111(98)90420-6.
- Behnke M, Jörres RA, Kirsten D, Magnussen H. Clinical benefits of a combined hospital and home-based exercise programme over 18 months in patients with severe COPD. Monaldi Arch Chest Dis 2003;59:44-51.
- Murphy N, Bell C, Costello RW. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respir Med 2005;99:1297-302. https://doi.org/10.1016/j.rmed.2005.02.033.
- Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010;65:423-8. https://doi.org/10.1136/thx.2009.124164.
- Clini EM, Crisafulli E, Costi S, Rossi G, Lorenzi C, Fabbri LM, et al. Effects of early inpatient rehabilitation after acute exacerbation of COPD. Respir Med 2009;103:1526-31. https://doi.org/10.1016/j.rmed.2009.04.011.
- Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004;329. https://doi.org/10.1136/bmj.38258.662720.3A.
- Carr SJ, Hill K, Brooks D, Goldstein RS. Pulmonary rehabilitation after acute exacerbation of chronic obstructive pulmonary disease in patients who previously completed a pulmonary rehabilitation program. J Cardiopulm Rehabil Prev 2009;29:318-24. https://doi.org/10.1097/HCR.0b013e3181ac7bb8.
- Ko FW, Dai DL, Ngai J, Tung A, Ng S, Lai K, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology 2011;16:617-24. https://doi.org/10.1111/j.1440-1843.2010.01921.x.
- Whitehead AL, Sully BG, Campbell MJ. Pilot and feasibility studies: is there a difference from each other and from a randomised controlled trial?. Contemp Clin Trials 2014;38:130-3. https://doi.org/10.1016/j.cct.2014.04.001.
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581-629. https://doi.org/10.1111/j.0887-378X.2004.00325.x.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and Evaluating Complex Interventions: New Guidance. London: MRC; 2008.
- De Brandt J, Spruit MA, Derave W, Hansen D, Vanfleteren LEGW, Burtin C. Changes in structural and metabolic muscle characteristics following exercise-based interventions in patients with COPD: a systematic review. Expert Rev Respir Med 2016;10:521-45. https://doi.org/10.1586/17476348.2016.1157472.
- Schulz KF, Altman DG, Moher D. CONSORT group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726-32. https://doi.org/10.7326/0003-4819-152-11-201006010-00232.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0105-8.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377-81.
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 1970;2:92-8.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62:464-75. https://doi.org/10.1016/j.jclinepi.2008.12.011.
- Behnke M, Taube C, Kirsten D, Lehnigk B, Jörres RA, Magnussen H. Home-based exercise is capable of preserving hospital-based improvements in severe chronic obstructive pulmonary disease. Respir Med 2000;94:1184-91. https://doi.org/10.1053/rmed.2000.0949.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Methods and processes of the CONSORT group: example of an extension for trials assessing nonpharmacologic treatments. Ann Intern Med 2008;148:W60-6. https://doi.org/10.7326/0003-4819-148-4-200802190-00008-w1.
- Kanarek NF, Kanarek MS, Olatoye D, Carducci MA. Removing barriers to participation in clinical trials, a conceptual framework and retrospective chart review study. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-237.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-17. https://doi.org/10.1164/ajrccm.166.1.at1102.
- Garrod R, Paul EA, Wedzicha JA. An evaluation of the reliability and sensitivity of the London Chest Activity of Daily Living Scale (LCADL). Respir Med 2002;96:725-30. https://doi.org/10.1053/rmed.2002.1338.
- Garrod R, Bestall JC, Paul EA, Wedzicha JA, Jones PW. Development and validation of a standardized measure of activity of daily living in patients with severe COPD: the London Chest Activity of Daily Living scale (LCADL). Respir Med 2000;94:589-96. https://doi.org/10.1053/rmed.2000.0786.
- Muller JP, Gonçalves PA, Fontoura FF, Mattiello R, Florian J. Applicability of the London Chest Activity of Daily Living scale in patients on the waiting list for lung transplantation. J Bras Pneumol 2013;39:92-7. https://doi.org/10.1590/S1806-37132013000100013.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J 2011;38:29-35. https://doi.org/10.1183/09031936.00177210.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. https://doi.org/10.1136/thx.54.7.581.
- Database of Instruments for Resource Use Measurement (DIRUM) n.d. www.dirum.org (accessed 23 November 2017).
- Fischer MJ, Scharloo M, Abbink J, van’t Hul A, van Ranst D, Rudolphus A, et al. Concerns about exercise are related to walk test results in pulmonary rehabilitation for patients with COPD. Int J Behav Med 2012;19:39-47. https://doi.org/10.1007/s12529-010-9130-9.
- Cooper AF, Weinman J, Hankins M, Jackson G, Horne R. Assessing patients’ beliefs about cardiac rehabilitation as a basis for predicting attendance after acute myocardial infarction. Heart 2007;93:53-8. https://doi.org/10.1136/hrt.2005.081299.
- Steer J, Gibson J, Bourke SC. The DECAF score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax 2012;67:970-6. https://doi.org/10.1136/thoraxjnl-2012-202103.
- Elia M. The ‘MUST’ Report: Nutritional Screening for Adults: a Multidisciplinary Responsibility. Development and Use of the ‘Malnutrition Universal Screening Tool’ (‘MUST’) for Adults. Redditch: British Association for Parenteral and Enteral Nutrition (BAPEN); 2003.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. https://doi.org/10.1183/09031936.05.00034805.
- Gravel J, Opatrny L, Shapiro S. The intention-to-treat approach in randomized controlled trials: are authors saying what they do and doing what they say?. Clin Trials 2007;4:350-6. https://doi.org/10.1177/1740774507081223.
- Bell ML, Fiero M, Horton NJ, Hsu CH. Handling missing data in RCTs; a review of the top medical journals. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-118.
- Echevarria C, Steer J, Heslop-Marshall K, Stenton S, Hickey P, Hughes R, et al. The PEARL score predicts 90-day readmission or death after hospitalisation for acute exacerbation of COPD. Thorax 2017;72:686-93. https://doi.org/10.1136/thoraxjnl-2016-209298.
- MedDRA . Medical Dictionary for Regulatory Activities n.d. www.meddra.org/ (accessed 4 January 2017).
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 2004.
- Dusenbury L, Brannigan R, Falco M, Hansen WB. A review of research on fidelity of implementation: implications for drug abuse prevention in school settings. Health Educ Res 2003;18:237-56. https://doi.org/10.1093/her/18.2.237.
- Wears RL. Standardisation and its discontents. Cogn Technol Work 2015;17:89-94. https://doi.org/10.1007/s10111-014-0299-6.
- Hawe P, Shiell A, Riley T. Complex interventions: how ‘out of control’ can a randomised controlled trial be?. BMJ 2004;328:1561-3. https://doi.org/10.1136/bmj.328.7455.1561.
- Hawe P. Minimal, negligible and negligent interventions. Soc Sci Med 2015;138:265-8. https://doi.org/10.1016/j.socscimed.2015.05.025.
- Haynes A, Brennan S, Redman S, Williamson A, Gallego G, Butow P, et al. Figuring out fidelity: a worked example of the methods used to identify, critique and revise the essential elements of a contextualised intervention in health policy agencies. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0378-6.
- Sanders C, Rogers A, Bowen R, Bower P, Hirani S, Cartwright M, et al. Exploring barriers to participation and adoption of telehealth and telecare within the Whole System Demonstrator trial: a qualitative study. BMC Health Serv Res 2012;12. https://doi.org/10.1186/1472-6963-12-220.
- Creswell JW. Research Design. London: Sage; 2014.
- Carter SM, Little M. Justifying knowledge, justifying method, taking action: epistemologies, methodologies, and methods in qualitative research. Qual Health Res 2007;17:1316-28. https://doi.org/10.1177/1049732307306927.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J. Maximising the value of combining qualitative research and randomised controlled trials in health research: the QUAlitative Research in Trials (QUART) study – a mixed methods study. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18380.
- O’Cathain A, Goode J, Drabble SJ, Thomas KJ, Rudolph A, Hewison J. Getting added value from using qualitative research with randomized controlled trials: a qualitative interview study. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-215.
- O’Cathain A, Hoddinott P, Lewin S, Thomas KJ, Young B, Adamson J, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0026-y.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Cherryholmes CH. Notes on pragmatism and scientific realism. Educ Res 1992;21:13-7. https://doi.org/10.3102/0013189X021006013.
- Dewey J, Thayer H. Pragmatism: the Classic Writings. Indianapolis, IN: Hackett; 1989.
- Peirce CS. Meaning and Action: A Critical History of Pragmatism. Indianapolis, IN: Hackett; 1984.
- Eakin JM. Educating critical qualitative health researchers in the land of the randomized controlled trial. Qual Inq 2016;22:107-18. https://doi.org/10.1177/1077800415617207.
- O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med 2014;89:1245-51. https://doi.org/10.1097/ACM.0000000000000388.
- Yin RK. Case Study Research: Design and Methods. London: Sage; 2014.
- Stucki A, Stoll T, Cieza A, Weigl M, Giardini A, Wever D, et al. ICF core sets for obstructive pulmonary diseases. J Rehabil Med 2004;36:114-20. https://doi.org/10.1080/16501960410016794.
- Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0242-0.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Finch TL, Rapley T, Girling M, Mair FS, Murray E, Treweek S, et al. Improving the normalization of complex interventions: measure development based on normalization process theory (NoMAD): study protocol. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-43.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- May CR, Finch T, Ballini L, MacFarlane A, Mair F, Murray E, et al. Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-245.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-37.
- Guest G. How many interviews are enough?: An experiment with data saturation and variability. Field Methods 2006;18:59-82. https://doi.org/10.1177/1525822X05279903.
- O’Reilly M, Parker N. ‘Unsatisfactory saturation’: a critical exploration of the notion of saturated sample sizes in qualitative research. Qual Res 2013;13:190-7. https://doi.org/10.1177/1468794112446106.
- Francis JJ, Johnston M, Robertson C, Glidewell L, Entwistle V, Eccles MP, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25:1229-45. https://doi.org/10.1080/08870440903194015.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- Yin RK. Case Study Research: Design and Methods. London: Sage; 2014.
- Devlin N, Shah K, Mulhern B, Van Hout B. Valuing Health-related Quality of Life: an EQ-5D-5L Value Set for England. London: Office of Health Economics; 2016.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- NHS Health Education England . Agenda for Change – Pay Rates n.d. www.healthcareers.nhs.uk/about/careers-nhs/nhs-pay-and-benefits/agenda-change-pay-rates (accessed 20 December 2016).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit (PSSRU), University of Kent; 2015.
- RECK . MOTOmed Viva2 n.d. www.motomed.com/en/models/motomed-viva2.html (accessed 4 January 2017).
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- NHS Reference Costs 2014 to 2015. London: Department of Health; 2015.
- Felli JC, Hazen GB. Sensitivity analysis and the expected value of perfect information. Med Decis Making 1998;18:95-109. https://doi.org/10.1177/0272989X9801800117.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26. https://doi.org/10.1177/0272989X13505910.
- Farmer T, Robinson K, Elliott SJ, Eyles J. Developing and implementing a triangulation protocol for qualitative health research. Qual Health Res 2006;16:377-94. https://doi.org/10.1177/1049732305285708.
- National Institute for Health Research . Pulmonary Rehabilitation and ACTIvity After COPD Exacerbations – the PRACTICE Trial n.d. www.journalslibrary.nihr.ac.uk/programmes/hta/132403/#/ (accessed 12 January 2017).
- Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012;65:301-8. https://doi.org/10.1016/j.jclinepi.2011.07.011.
- Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide To Designing Interventions. Sutton: Silverback Publishing; 2014.
- Molinari N, Briand C, Vachier I, Malafaye N, Aubas P, Georgescu V, et al. Hospitalizations for COPD exacerbations: trends and determinants of death. COPD 2015;12:621-7. https://doi.org/10.3109/15412555.2015.1007931.
- Esteban C, Arostegui I, Garcia-Gutierrez S, Gonzalez N, Lafuente I, Bare M, et al. A decision tree to assess short-term mortality after an emergency department visit for an exacerbation of COPD: a cohort study. Respir Res 2015;16. https://doi.org/10.1186/s12931-015-0313-4.
- Rubinsztajn R, Przybyłowski T, Maskey-Warzęchowska M, Karwat K, Chazan R. Exacerbations of chronic obstructive pulmonary disease and quality of life of patients. Adv Exp Med Biol 2016;884:69-74. https://doi.org/10.1007/5584_2015_178.
- Moore E, Palmer T, Newson R, Majeed A, Quint JK, Soljak MA. Pulmonary rehabilitation as a mechanism to reduce hospitalizations for acute exacerbations of COPD: a systematic review and meta-analysis. Chest 2016;150:837-59. https://doi.org/10.1016/j.chest.2016.05.038.
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-1.
- Witcher CS, McGannon KR, Hernandez P, Dechman G, Ferrier S, Spence JC, et al. A qualitative exploration of exercise among pulmonary rehabilitation participants: insight from multiple sources of social influence. Respir Care 2015;60:1624-34. https://doi.org/10.4187/respcare.04120.
- Thorpe O, Johnston K, Kumar S. Barriers and enablers to physical activity participation in patients with COPD: a systematic review. J Cardiopulm Rehabil Prev 2012;32:359-69. https://doi.org/10.1097/HCR.0b013e318262d7df.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j.2002.384.doc.x.
- Linnan L, Steckler A, Linnan L, Steckler A. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- Browne RH. On the use of a pilot sample for sample size determination. Stat Med 1995;14:1933-40. https://doi.org/10.1002/sim.4780141709.
- Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287-91. https://doi.org/10.1002/pst.185.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD003793.pub3.
- Byrom B, Rowe DA. Measuring free-living physical activity in COPD patients: deriving methodology standards for clinical trials through a review of research studies. Contemp Clin Trials 2016;47:172-84. https://doi.org/10.1016/j.cct.2016.01.006.
- Katz TL. Missing data in clinical trials forum. Clin Investig (Lond) 2015;5:681-5. https://doi.org/10.4155/cli.15.35.
- Murray SA, Kendall M, Carduff E, Worth A, Harris FM, Lloyd A, et al. Use of serial qualitative interviews to understand patients’ evolving experiences and needs. BMJ 2009;339. https://doi.org/10.1136/bmj.b3702.
- May K, Morse JM. Qualitative Nursing Research: a Contemporary Dialogue. Newbury Park, CA: Sage; 1991.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol 2005;52:250-60. https://doi.org/10.1037/0022-0167.52.2.250.
- Tracy SJ. Qualitative quality: eight ‘big-tent’ criteria for excellent qualitative research. Qual Inq 2010;16:837-51. https://doi.org/10.1177/1077800410383121.
- Deatrick J, Faux S, Morse JM. Qualitative Nursing Research: a Contemporary Dialogue. Newbury Park, CA: Sage; 1991.
- Lincoln Y, Guba E. Naturalistic Inquiry. Los Angeles, CA: Sage; 1985.
- Weiss CH. Theory-based evaluation: past, present, and future. New Dir Eval 1997;1997:41-55. https://doi.org/10.1002/ev.1086.
- Saunders RP, Evans MH, Joshi P. Developing a process-evaluation plan for assessing health promotion program implementation: a how-to guide. Health Promot Pract 2005;6:134-47. https://doi.org/10.1177/1524839904273387.
- Frost R, Levati S, McClurg D, Brady M, Williams B, Organization WH, et al. What adherence measures should be used in trials of home-based rehabilitation interventions? A systematic review of the validity, reliability, and acceptability of measures. Arch Phys Med Rehabil 2017;98:1241-1256.e45. https://doi.org/10.1016/j.apmr.2016.08.482.
- Ferguson B. ACSM’s guidelines for exercise testing and prescription. J Can Chiropr Assoc 2014;58.
- Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev 2006;63:217-35. https://doi.org/10.1177/1077558705285298.
- Thorn JC, Coast J, Cohen D, Hollingworth W, Knapp M, Noble SM, et al. Resource-use measurement based on patient recall: issues and challenges for economic evaluation. Appl Health Econ Health Policy 2013;11:155-61. https://doi.org/10.1007/s40258-013-0022-4.
- Geue C, Lewsey J, Lorgelly P, Govan L, Hart C, Briggs A. Spoilt for choice: Implications of using alternative methods of costing hospital episode statistics. Health Econ 2012;21:1201-16. https://doi.org/10.1002/hec.1785.
- Caracelli VJ, Riggin L. Mixed-method evaluation: developing quality criteria through concept mapping. Am J Eval 1994;15:139-52. https://doi.org/10.1177/109821409401500204.
- Dhillon SS, Sima CA, Kirkham AR, Syed N, Camp PG. Physical activity measurement accuracy in individuals with chronic lung disease: a systematic review with meta-analysis of method comparison studies. Arch Phys Med Rehabil 2015;96:2079-88.e10. https://doi.org/10.1016/j.apmr.2015.05.015.
- Berendsen BA, Hendriks MR, Meijer K, Plasqui G, Schaper NC, Savelberg HH. Which activity monitor to use? Validity, reproducibility and user friendliness of three activity monitors. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-749.
- Demeyer H, Dueñas-Espín I, De Jongh C, Louvaris Z, Hornikx M, Gimeno-Santos E, et al. Can health status questionnaires be used as a measure of physical activity in COPD patients?. Eur Respir J 2016;47:1565-8. https://doi.org/10.1183/13993003.01815-2015.
- Ospina MB, Mrklas K, Deuchar L, Rowe BH, Leigh R, Bhutani M, et al. A systematic review of the effectiveness of discharge care bundles for patients with COPD. Thorax 2017;72:31-9. https://doi.org/10.1136/thoraxjnl-2016-208820.
- Howcroft M, Walters EH, Wood-Baker R, Walters JA. Action plans with brief patient education for exacerbations in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016;12. https://doi.org/10.1002/14651858.CD005074.pub4.
- Kayyali R, Odeh B, Frerichs I, Davies N, Perantoni E, D’arcy S, et al. COPD care delivery pathways in five European Union countries: mapping and health care professionals’ perceptions. Int J Chron Obstruct Pulmon Dis 2016;11:2831-8. https://doi.org/10.2147/COPD.S104136.
- Sandelowsky H, Hylander I, Krakau I, Modin S, Ställberg B, Nager A. Time pressured deprioritization of COPD in primary care: a qualitative study. Scand J Prim Health Care 2016;34:55-6. https://doi.org/10.3109/02813432.2015.1132892.
- Hemingway H, Croft P, Perel P, Hayden JA, Abrams K, Timmis A, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ 2013;346. https://doi.org/10.1136/bmj.e5595.
- Riley RD, Hayden JA, Steyerberg EW, Moons KG, Abrams K, Kyzas PA, et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLOS Med 2013;10. https://doi.org/10.1371/journal.pmed.1001380.
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373-9. https://doi.org/10.1016/S0895-4356(96)00236-3.
- Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503-10. https://doi.org/10.1016/0895-4356(95)00048-8.
- Smith CT, Hopkins C, Sydes MR, Woolfall K, Clarke M, Murray G, et al. How should individual participant data (IPD) from publicly funded clinical trials be shared?. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0532-z.
- Couillard A, Koechlin C, Cristol JP, Varray A, Prefaut C. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J 2002;20:1123-9. https://doi.org/10.1183/09031936.02.00014302.
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007;131:4S-42S. https://doi.org/10.1378/chest.06-2418.
- Mesquita R, Donária L, Genz IC, Pitta F, Probst VS. Respiratory muscle strength during and after hospitalization for COPD exacerbation. Respir Care 2013;58:2142-9. https://doi.org/10.4187/respcare.02393.
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006;173:1390-413. https://doi.org/10.1164/rccm.200508-1211ST.
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. London: Guilford Press; 2013.
- Coventry PA, Hind D. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstructive pulmonary disease: systematic review and meta-analysis. J Psychosom Res 2007;63:551-65. https://doi.org/10.1016/j.jpsychores.2007.08.002.
- GOV.UK . National Insurance Rates and Categories n.d. www.gov.uk/national-insurance-rates-letters/contribution-rates (accessed 4 January 2017).
- Kaur G, English C, Hillier S. Physiotherapists systematically overestimate the amount of time stroke survivors spend engaged in active therapy rehabilitation: an observational study. J Physiother 2013;59:45-51. https://doi.org/10.1016/S1836-9553(13)70146-2.
- Renforth P, Yapa RS, Forster DP. Occupational therapy predischarge home visits: a study from a community hospital. Br J Occup Ther 2004;67:488-94. https://doi.org/10.1177/030802260406701104.
- Ball J, Philippou J, Pike G, Sethi J. Survey of District and Community Nurses in 2013: Report to the Royal College of Nursing. London: National Nursing Research Unit; 2014.
Appendix 1 Intervention development
Introduction
We hypothesised that the hospital EPR resistance training would improve muscle power during AECOPD,56 counteracting skeletal muscle dysfunction and functional decline. Resisted quadriceps exercises performed daily during an exacerbation are safe, result in a significant, sustained increase in muscle force and increase functional exercise tolerance after discharge. 56 Hospital EPR was based on national and international guidance34,38 and was developed through focus group discussions and pilot work at STHNHSFT. We hypothesised that the home EPR intervention incorporating interval training and continuous exercise would increase exercise capacity and improve symptoms and quality of life following AECOPD. 34,36,37 Both interval and continuous exercise have been shown to improve function under stable conditions. 34 A summary of the treatment theory for both interventions is shown in Figure 1.
The development of treatment manuals and theory
Hospital early pulmonary rehabilitation intervention
The hospital EPR manual was developed by the co-author and specialist physiotherapist MC based on published evidence that resistance training during AECOPD is safe and successfully counteracts skeletal muscle dysfunction. The modality and intensity of training needed to suit patients with increased dyspnoea and fatigue and to avoid excessive respiratory symptoms, ventilatory requirements and dynamic hyperinflation had to be kept to a minimum. 60 Exercise training during or following exacerbations may aggravate local inflammatory and oxidative stress to the muscles and high-intensity exercises performed until exhaustion are associated with increased muscle oxidative stress in stable patients with COPD. 196 However, high-intensity resistance training is effective at counteracting the effects of skeletal muscle disuse atrophy, has a relatively low ventilatory burden and is well tolerated during AECOPD. 56
Thrice-daily quadriceps resistance training is known to improve muscle strength and function. 56 We developed our intervention based on this format, using a portable cycle ergometer (MOTOmed viva 2) at the patient’s bedside to train both upper and lower limbs while the patient remained seated on a chair or the edge of a hospital bed. This allowed patients who were frail and suffering increased respiratory symptoms to receive the intervention.
The manualised intervention was piloted by a group of volunteers from a local COPD support group who had previously been admitted to hospital with AECOPD. They were invited to undertake the intervention and comment on its acceptability.
Home early pulmonary rehabilitation intervention
The home EPR manual was developed by the co-author and community physiotherapist CO’C based on current PR guidance, which was adapted for post-AECOPD patients. Current recommendations are that rehabilitation should begin no later than 1 month post discharge from hospital;34 however, moving this intervention closer to the point of discharge could prevent muscle wasting before PR begins. Delivery in the participants’ own home with close supervision accommodates acutely ill participants, who are likely to remain symptomatic.
The home EPR intervention offers a combination of interval training and continuous exercise training in a structured rehabilitation programme and a daily walking plan. The interval training incorporates cardiovascular, resistance and flexibility exercises of the major muscle groups of the upper and lower limb in keeping with current guidelines. 34,37,197 Traditionally, early rehabilitation following AECOPD has taken place in a hospital inpatient or outpatient setting, rather than a domiciliary setting. 36,37 The advantage of the domiciliary setting for the acutely unwell is that participants do not have to travel while recovering from an exacerbation. This may be more convenient and less distressing for participants and may promote adherence. The intervention is structured in such a way as to initiate exercise training within 72 hours of hospital discharge, thereby limiting the physical deconditioning that occurs post exacerbation198 while also allowing participants to take part in group rehabilitation 4–6 weeks post discharge so that training may be optimised while stable. The components of the intervention are based on current BTS34 and American and European197,199 PR guidelines. The protocol is in routine use by community physiotherapists working from STHNHSFT and close to that delivered at AUHNHSFT.
Although no formal behavioural change techniques were prescribed as part of the intervention, some of the physiotherapists were trained in motivational interviewing techniques. 200 Guided mastery and goal setting are part of routine care and therefore it was anticipated that confidence and self-efficacy could be enhanced. 37 PR has also been associated with a reduction in anxiety and depression. 201
Details of both interventions in line with the Template for Intervention Description and Replication (TIDieR) checklist87 can be found in Chapter 2 (see Interventions).
Appendix 2 Changes to the protocol
| Changes to protocol | Progress report | Date | Approved by |
|---|---|---|---|
|
Protocol version 1.3, substantial amendment 1, approved 17 November 2015 1. Amended the exclusion criteria ‘Predicted length of hospital stay < 5 days’ to ‘Patients whose discharge is planned within 48 hours of admission’ 2. Clarified existing eligibility criteria: a. ‘pH > 7.35’ now includes ‘(at the time of consent)’ b. Changed ‘Glasgow Coma Scale (GCS) ≥ 15’ to ‘Glasgow Coma Scale (GCS) = 15’ c. Added ‘or patients with an implantable cardioverter defibrillator (ICD)’ to the cardiovascular instability exclusion criteria d. Added ‘known’ to ‘extensive pulmonary fibrosis’ to make it clear that this should be checked in the clinical notes and that specific tests are not required 3. Clarified in the protocol that the hospital EPR should be started within 72 hours of admission 4. Clarified that only those allocated to home EPR will receive the activity diary 5. Added the TDF for use in understanding the qualitative data; this is reflected in the protocol and the topic guides for participants and health professionals. We have also added some prompts to these topic guides 6. Added ‘acute medical units (AMUs)’ as a place for recruitment |
2 | January 2016 | Yorkshire & The Humber – Sheffield REC |
|
Protocol version 2.0, substantial amendment 2, approved 11 January 2016 1. Removed exclusion criteria ‘Patients whose discharge is planned within 48 hours of admission’ completely 2. Amended the time point of the 5-day follow-up to be taken prior to discharge 3. Added that other blinded clinical staff can collect data from the participants in addition to the research nurse |
2 | January 2016 | Yorkshire & The Humber – Sheffield REC |
|
Protocol version 2.2, substantial amendment 3, approved 16 February 2016 1. Removed the rectus femoris muscle cross-sectional area measurement 2. Updated to reflect when the activity monitor is given to participants in the trial |
3 | July 2016 | Yorkshire & The Humber – Sheffield REC |
|
Protocol version 2.3, minor amendment 1 Added 2-minute walk distance (usual practice for community physiotherapists) for safety for the first in-home session when required |
Not in progress report, uploaded to HTA programme monitoring system 1 March 2016 | Not applicable as non-substantial amendment | |
|
Protocol version 2.4, substantial amendment 4, approved 15 April 2016 1. The withdrawal criteria were amended to withdraw individuals readmitted for COPD or intervention-related issues and if admitted for ≥ 48 hours (rather than all admissions leading to withdrawal) 2. Amended to allow unblinded assessors to collect data in the event that a blinded assessor is not available, to avoid losing the data |
3 | July 2016 | Yorkshire & The Humber – Sheffield REC |
|
Protocol version 2.5, minor amendment 2 Expanded the interviews with staff to include ‘health professionals’ rather than only ‘physiotherapists’ |
Not in progress report, uploaded to HTA programme monitoring system 19 October 2016 | Not applicable as non-substantial amendment |
Appendix 3 Health economics unit costs
The cost of the physiotherapists’ time was based on a mid-point band salary estimate as outlined within the AfC pay rates;145 additional associated staff costs (employee national insurance, salary on-costs, overheads, and capital overheads) were included based on the calculations used within Curtis and Burns146 to estimate the applied unit costs. A generalised working calendar of 42.4 weeks per year and 37.5 hours per week was assumed. 146 The unit costs and calculations are provided in Table 44. It should be noted that the interventions did involve a variety of physiotherapists on different salary bands:
-
13 physiotherapists were involved in the delivery of hospital EPR: one on salary band 2, seven on band 3, one on band 5, two on band 6, one on band 7 and one on band 8
-
seven physiotherapists were involved in the delivery of home EPR: four on band 6 and three on band 7.
| Band wage | (a) Mid-point salary (£)a | (b) Salary on-costs (£)b | (c) Employer’s national insurance contribution (£)c | (d) Overheads (inclusive staff) (£)d | (e) Overheads (non-staff) (£)e | (f) Capital overheads (£)f | (g) Cost per year (£)g | (h) Cost per hour (£)h |
|---|---|---|---|---|---|---|---|---|
| 1 | 15,384 | 2154 | 1003 | 3875 | 9066 | 6179 | 37,661 | 23.69 |
| 2 | 16,372 | 2292 | 1140 | 4139 | 9684 | 6179 | 39,806 | 25.04 |
| 3 | 18,152 | 2541 | 1386 | 4614 | 10,797 | 6179 | 43,669 | 27.46 |
| 4 | 21,052 | 2947 | 1786 | 5389 | 12,609 | 6179 | 49,962 | 31.42 |
| 5 | 24,801 | 3472 | 2303 | 6390 | 14,952 | 6179 | 58,097 | 36.54 |
| 6 | 30,357 | 4250 | 3070 | 7874 | 18,424 | 6179 | 70,154 | 44.12 |
| 7 | 36,250 | 5075 | 3883 | 9448 | 22,107 | 6179 | 82,942 | 52.16 |
| 8a | 43,871 | 6142 | 4935 | 11,484 | 26,869 | 6179 | 99,479 | 62.57 |
The relative costs of a physiotherapist on salary bands 1–8a are shown in Table 44.
The cost of the bike used for hospital EPR (a MOTOmed viva 2),147 including VAT, was £5520. The equivalent yearly cost of the bike when estimated using a annuitisation procedure148 is shown in Table 45. For this study it was assumed that the capital life of the bike would be 5 years (alternative capital life assumptions from 1 to 10 years are also shown in Table 5) and that the bike was paid for in advance rather than assuming that annuity was in arrears. A discount rate of 3.5% (as recommend by NICE)144 was applied as a depreciation rate, which is assumed to be equal to the maintenance cost of the bike per year and so is paid as an equivalent interest rate from owning the bike. The equivalent annual cost of the bike, therefore, assuming that it was paid for in advance, that it had a capital life cycle of 5 years and that the interest rate (maintenance cost) was 3.5% per year, was £1181; for this 90-day study, the equivalent cost of the bike was £295, which is attributed to the intervention cost of those people within the combined hospital and home EPR and hospital EPR trial arms.
| Annuitisation calculation variables | Life of capital (years, t) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| (a) Annuity factor per yeara | 1 | 0.9662 | 0.9335 | 0.9019 | 0.8714 | 0.8420 | 0.8135 | 0.7860 | 0.7594 | 0.7337 |
| (b) Cumulative annuity factor | 1 | 1.9662 | 2.8997 | 3.8016 | 4.673 | 5.515 | 6.3285 | 7.1145 | 7.8739 | 8.6076 |
| (c) Equivalent annual cost (£)b | 5520 | 2807 | 1904 | 1452 | 1181 | 1001 | 872 | 776 | 701 | 641 |
The home EPR costs included the physiotherapists’ time for delivering the intervention, travel time to and from their base of operation and the cost of printing a 20-page A4 information booklet (it was assumed that each patient could keep the booklet even after the intervention was over). No other travel costs (e.g. petrol costs) were assumed in the calculation and travel time was based on a time obtained from Google Maps during the midweek period and the middle of the day. The information booklet when printed double-sided and in black and white cost £0.50 per booklet [the double-sided printing cost per page at standard printing cost is £0.05; £0.05 times 10 pages (20 sides) is £0.50, which is the cost of the booklet].
For GP contacts and therapy services, unit costs were sourced from Curtis and Burns146 (Table 46). Time assumptions about how long a contact would normally last (in minutes) were also sourced from Curtis and Burns146 if available; otherwise, time assumptions were sourced from the empirical literature (see also the ‘comment’ column in Table 46).
| Type | Costing metric | Unit cost (£) | Time assumption (minutes) | Applied unit cost (£) | Comments | References |
|---|---|---|---|---|---|---|
| GP contacts | ||||||
| GP surgery visit | Per minute | 3.20 | 11.7 | 37.44 | None | Curtis and Burns (pp. 176–7)146 |
| GP home visit | Per minute | 3.20 | 11.4 | 36.48 | None | Curtis and Burns (pp. 176–7)146 |
| GP telephone consultation | Per minute | 3.20 | 7.1 | 22.72 | None | Curtis and Burns (pp. 176–7)146 |
| Therapy services | ||||||
| Physiotherapist | Per hour | 34 | 55.6 | 31.51 | Assumed hospital physiotherapist salary (excluding qualification costs); time assumption based on Australian stroke survivors | Curtis and Burns (p. 217);146 bKaur et al.203 |
| Occupational therapist | Per hour | 41 | 49 | 33.48 | Assumed community occupational therapist salary (excluding qualification costs) | Curtis and Burns (p. 191);146 bRenforth et al.204 |
| Social worker | Per hour | 55 | 60 | 55.00 | Time assumption from expert opinion | Curtis and Burns (p. 188);146 bexpert option (social workers) |
| Home care worker | Per hour | 24 | 30 | 12.00 | None | Curtis and Burns (p. 192)146 |
| Health visitor | Per hour | 66 | 40 | 44.00 | Unit cost based on patient-based work. Time assumption the same as for a district nurse, based on commentary from Curtis and Burns146 | Curtis and Burns (p. 171);146 bBall et al.205 |
Hospital unit costs were sourced from NHS Reference Costs 2014 to 2015. 149 Index admissions and readmissions that involved an overnight inpatient stay were assumed to be non-elective; any hospital visit that did not involve an overnight stay was classified as day-case care. A pooled and weighted unit cost was estimated based on the HRG code for ‘COPD or Bronchitis’. The calculation of the pooled and weighted unit costs for inpatient stays (including the unit costs of a standard inpatient stay and excess bed-days and LOS) and for day cases is shown in Table 47 (including the costing algorithm for inpatient stays in the table notes) and Table 48 respectively.
| HRG code | HRG description | (a) Number of FCEs | (b) National average unit cost (£) | (c) Average LOS (days) | (d) EBDs | (e) National average unit cost per EBD (£) | (f) Total stay cost across all FCEs (£)a | (g) Total EBDs across all FCEsb | (h) Total EBD cost (per EBD) (£)c |
|---|---|---|---|---|---|---|---|---|---|
| DZ65A | COPD or Bronchitis, with Multiple Interventions, with CC Score 9+ | 424 | 5826 | 17 | 377 | 235 | 2,470,255 | 7149 | 88,589 |
| DZ65B | COPD or Bronchitis, with Multiple Interventions, with CC Score 0–8 | 622 | 3376 | 9 | 334 | 249 | 2,099,680 | 5528 | 83,156 |
| DZ65C | COPD or Bronchitis, with Single Intervention, with CC Score 9+ | 1844 | 3706 | 10 | 770 | 243 | 6,834,699 | 18,506 | 86,950 |
| DZ65D | COPD or Bronchitis, with Single Intervention, with CC Score 5–8 | 3410 | 2668 | 7 | 1361 | 268 | 9,096,341 | 24,073 | 365,161 |
| DZ65E | COPD or Bronchitis, with Single Intervention, with CC Score 0–4 | 3222 | 2153 | 6 | 1362 | 268 | 6,935,804 | 17,729 | 364,926 |
| DZ65F | COPD or Bronchitis, without Interventions, with CC Score 13+ | 1699 | 3503 | 10 | 1184 | 229 | 5,951,927 | 17,441 | 270,681 |
| DZ65G | COPD or Bronchitis, without Interventions, with CC Score 9–12 | 11,081 | 2565 | 7 | 7429 | 254 | 28,425,049 | 78,345 | 1,888,385 |
| DZ65H | COPD or Bronchitis, without Interventions, with CC Score 5–8 | 34,706 | 1953 | 5 | 17,784 | 254 | 67,798,169 | 183,203 | 4,523,965 |
| DZ65J | COPD or Bronchitis, without Interventions, with CC Score 0–4 | 49,122 | 1552 | 4 | 22,757 | 249 | 76,216,962 | 198,712 | 5,674,097 |
| DZ65K | COPD or Bronchitis, with length of stay 1 day or less, discharged home | 1250 | 1218 | 4 | 1 | 269 | 1,522,116 | 4570 | 269 |
| N/A | Total | 107,380 | N/A | N/A | 53,359 | N/A | 207,351,002 | 555,256 | 13,446,180 |
| Weighted average cost per stay (£)d | Weighted average LOSe | Weighted average EBD cost (per EBD) (£)f | |||||||
| N/A | Weighted values | 1931 | 5 | 252 |
| HRG code | HRG description | (a) Number of FCEs | (b) National average unit cost (£) | (c) Total cost for day cases (£)a |
|---|---|---|---|---|
| DZ65D | COPD or Bronchitis, with Single Intervention, with CC Score 5–8 | 6 | 232 | 1394 |
| DZ65H | COPD or Bronchitis, without Interventions, with CC Score 5–8 | 1 | 870 | 870 |
| DZ65J | COPD or Bronchitis, without Interventions, with CC Score 0–4 | 9 | 577 | 5197 |
| DZ65K | COPD or Bronchitis, with length of stay 1 day or less, discharged home | 909 | 490 | 445,506 |
| N/A | Total | 925 | N/A | 452,968 |
| Weighted average day-case costb | ||||
| N/A | Weighted values | 490 | ||
Appendix 4 Six-minute walk distance results without the interaction term
| Time point | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental difference (95% CI)a | Treatment 2 effect: home EPR – usual care vs. experimental difference (95% CI)a | Interaction between the interventionsb |
|---|---|---|---|---|---|---|---|---|---|
| Pre discharge | n | 20 | 7 | 7 | 4 | 2 | –24.9 (95% CI –119.6 to 69.8) | –66.3 (95% CI –152.9 to 20.4) | –70.7 (95% CI –249.3 to 107.8) |
| Mean (SD) | 143.6 (92.8) | 168.9 (80.6) | 121.9 (80.3) | 172.8 (146.5) | 73.0 (15.6) | ||||
| 30 days | n | 33 | 9 | 11 | 5 | 8 | 41.3 (95% CI –60.5 to 143.1) | –2.4 (95% CI –100.9 to 96.2) | 62.9 (95% CI –148.8 to 274.7) |
| Mean (SD) | 231.9 (130.9) | 231.2 (138.5) | 206.0 (109.0) | 230.2 (151.5) | 269.5 (153.6) | ||||
| 90 daysc | n | 21 | 5 | 6 | 5 | 5 | 15.1 (95% CI –118.7 to 148.9) | 120.2 (95% CI –20.0 to 260.5) | –54.7 (95% CI –350.6 to 241.1) |
| Mean (SD) | 278.9 (150.1) | 199.6 (146.8) | 328.7 (108.0) | 267.4 (160.9) | 310.0 (194.3) |
Appendix 5 London Chest Activity of Daily Living results
| Time point | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | n | 43 | 10 | 12 | 11 | 10 | |||
| Mean (SD) | 43.3 (15.40) | 45.5 (17.95) | 41.4 (16.77) | 45.7 (14.93) | 40.5 (12.94) | ||||
| Pre discharge | n | 27 | 6 | 9 | 7 | 5 | 5.0 (95% CI –10.0 to 20.0) | –12.7 (95% CI –30.3 to 4.8) | –5.6 (95% CI –28.9 to 17.6) |
| Mean (SD) | 39.9 (15.71) | 43.7 (19.34) | 33.0 (16.06) | 50.1 (7.01) | 33.2 (13.39) | ||||
| 30 days | n | 36 | 8 | 11 | 8 | 9 | 3.8 (95% CI –11.2 to 18.9) | –3.1 (95% CI –18.5 to 12.3) | –6.9 (95% CI –26.7 to 13.0) |
| Mean (SD) | 40.1 (14.12) | 42.9 (17.48) | 38.8 (14.47) | 44.4 (11.88) | 35.3 (12.90) | ||||
| 90 days | n | 33 | 8 | 12 | 6 | 7 | 1.2 (95% CI –12.3 to 14.8) | 2.9 (95% CI –11.5 to 17.3) | –6.0 (95% CI –26.4 to 14.4) |
| Mean (SD) | 40.6 (15.21) | 40.6 (15.87) | 41.9 (16.62) | 41.3 (16.93) | 37.6 (13.43) |
Appendix 6 COPD Assessment Test results
| Time point | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | n | 54 | 14 | 14 | 12 | 14 | |||
| Mean (SD) | 26.1 (8.14) | 29.4 (7.78) | 24.9 (8.24) | 27.0 (7.46) | 23.3 (8.50) | ||||
| Pre discharge | n | 31 | 8 | 8 | 9 | 6 | 1.7 (95% CI –6.8 to 10.2) | 0.2 (95% CI –9.3 to 9.7) | –4.0 (95% CI –18.6 to 10.6) |
| Mean (SD) | 22.8 (9.02) | 22.0 (9.58) | 23.5 (9.72) | 24.2 (8.26) | 21.0 (10.41) | ||||
| 30 days | n | 42 | 10 | 14 | 9 | 9 | 6.5 (95% CI –0.8 to 13.9) | 3.7 (95% CI –2.4 to 9.9) | –10.9 (95% CI –20.5 to –1.3) |
| Mean (SD) | 22.8 (7.95) | 20.8 (9.67) | 24.0 (6.25) | 26.1 (8.74) | 19.7 (6.91) | ||||
| 90 days | n | 36 | 8 | 14 | 8 | 6 | 6.4 (95% CI –3.7 to 16.5) | 5.3 (95% CI –3.2 to 13.9) | –11.9 (95% CI –22.9 to –0.9) |
| Mean (SD) | 25.1 (8.91) | 22.6 (12.66) | 26.4 (6.91) | 27.8 (9.74) | 22.0 (6.16) |
Appendix 7 Medical Research Council Dyspnoea Scale results
| Time point | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Pre discharge | n | 32 | 8 | 9 | 9 | 6 | 0.0 (95% CI –1.6 to 1.6) | –0.4 (95% CI –2.2 to 1.4) | –0.5 (95% CI –3.1 to 2.0) |
| Mean (SD) | 3.5 (1.57) | 3.8 (1.91) | 3.3 (1.41) | 3.8 (0.97) | 2.8 (2.14) | ||||
| 30 days | n | 44 | 11 | 14 | 9 | 10 | 0.5 (95% CI –0.9 to 1.8) | –0.4 (95% CI –1.7 to 0.9) | –0.8 (95% CI –2.5 to 0.9) |
| Mean (SD) | 3.5 (1.41) | 3.7 (1.79) | 3.4 (1.22) | 4.2 (1.09) | 3.0 (1.33) | ||||
| 90 days | n | 40 | 8 | 14 | 9 | 9 | 1.2 (95% CI –0.0 to 2.4) | 0.7 (95% CI –0.6 to 2.0) | –1.5 (95% CI –3.2 to 0.2) |
| Mean (SD) | 3.8 (1.32) | 3.1 (1.46) | 3.9 (1.46) | 4.3 (0.87) | 3.6 (1.24) |
Appendix 8 Analyses adjusted for baseline variables
| Time point | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Pre discharge | n | 29 | 7 | 7 | 9 | 6 | 1.7 (95% CI –3.8 to 7.1) | 0.6 (95% CI –5.2 to 6.5) | –4.4 (95% CI –15.4 to 6.5) |
| Mean (SD) | 23.3 (8.89) | 24.0 (8.35) | 23.6 (10.50) | 24.2 (8.26) | 21.0 (10.41) | ||||
| 30 days | n | 40 | 10 | 13 | 8 | 9 | 5.6 (95% CI –1.5 to 12.7) | 4.0 (95% CI –1.0 to 9.1) | –8.1 (95% CI –16.8 to 0.6) |
| Mean (SD) | 22.4 (7.91) | 20.8 (9.67) | 23.8 (6.44) | 25.1 (8.79) | 19.7 (6.91) | ||||
| 90 days | n | 35 | 8 | 14 | 7 | 6 | 6.2 (95% CI –3.4 to 15.8) | 7.2 (95% CI –1.6 to 16.0) | –10.3 (95% CI –20.9 to 0.4) |
| Mean (SD) | 25.0 (9.00) | 22.6 (12.66) | 26.4 (6.91) | 27.4 (10.47) | 22.0 (6.16) |
| Time point | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Pre discharge | n | 22 | 4 | 7 | 7 | 4 | 2.6 (95% CI –12.2 to 17.4) | –12.5 (95% CI –33.6 to 8.5) | –3.6 (95% CI –29.6 to 22.5) |
| Mean (SD) | 42.1 (15.63) | 48.3 (19.97) | 35.4 (17.00) | 50.1 (7.01) | 33.8 (15.39) | ||||
| 30 days | n | 27 | 6 | 9 | 6 | 6 | 0.1 (95% CI –16.7 to 16.9) | –3.1 (95% CI –20.6 to 14.3) | –3.4 (95% CI –26.1 to 19.2) |
| Mean (SD) | 42.0 (14.33) | 45.2 (18.30) | 41.4 (14.80) | 46.8 (11.96) | 35.0 (11.82) | ||||
| 90 days | n | 23 | 4 | 10 | 5 | 4 | 0.3 (95% CI –12.4 to 13.1) | –0.8 (95% CI –16.7 to 15.1) | –12.1 (95% CI –31.8 to 7.6) |
| Mean (SD) | 39.5 (15.95) | 47.3 (13.60) | 39.1 (16.71) | 42.4 (18.70) | 29.3 (11.87) |
| Time point | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Pre discharge | n | 31 | 7 | 9 | 9 | 6 | –0.1 (95% CI –0.2 to 0.0) | 0.2 (95% CI –0.0 to 0.4) | –0.2 (95% CI –0.4 to 0.0) |
| Mean (SD) | 0.6 (0.32) | 0.6 (0.34) | 0.7 (0.27) | 0.5 (0.35) | 0.6 (0.36) | ||||
| 30 daysa | n | 42 | 9 | 14 | 9 | 10 | –0.2 (95% CI –0.4 to 0.0) | –0.0 (95% CI –0.2 to 0.2) | 0.2 (95% CI –0.1 to 0.5) |
| Mean (SD) | 0.6 (0.30) | 0.6 (0.34) | 0.6 (0.28) | 0.4 (0.32) | 0.7 (0.27) | ||||
| 90 daysa | n | 38 | 8 | 14 | 8 | 8 | –0.1 (95% CI –0.4 to 0.2) | –0.1 (95% CI –0.3 to 0.1) | 0.1 (95% CI –0.2 to 0.5) |
| Mean (SD) | 0.6 (0.33) | 0.6 (0.36) | 0.6 (0.29) | 0.5 (0.49) | 0.7 (0.23) |
Appendix 9 Sensitivity analyses
| Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|
| n | 41 | 10 | 13 | 9 | 9 | 2.3 (95% CI –143.3 to 147.9) | 29.9 (95% CI –101.4 to 161.3) | –6.3 (95% CI –202.6 to 190.0) |
| Mean (SD) | 245.3 (137.82) | 223.3 (130.82) | 256.3 (127.43) | 234.0 (139.51) | 265.3 (152.86) |
| Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental |
|---|---|---|---|---|---|---|---|
| n | 21 | 5 | 6 | 5 | 5 | 15.1 (95% CI –117.8 to 148.0) | 120.2 (95% CI –15.9 to 256.3) |
| Mean (SD) | 278.9 (150.15) | 199.6 (146.80) | 328.7 (108.02) | 267.4 (160.90) | 310.0 (194.29) |
| Centre | Statistic | Overall | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental | Interaction term |
|---|---|---|---|---|---|---|---|---|---|
| Aintree | n | 5 | 1 | 2 | 0 | 2 | 200.0 (95% CI –157.0 to 557.0) | ||
| Mean (SD) | 216.0 (132.54) | 85.0 (.) | 285.0 (162.63) | 212.5 (130.81) | |||||
| Sheffield | n | 16 | 4 | 4 | 5 | 3 | 39.1 (95% CI –187.8 to 266.1) | 122.3 (95% CI –68.4 to 312.9) | –14.6 (95% CI –375.5 to 346.2) |
| Mean (SD) | 298.6 (153.76) | 228.3 (152.52) | 350.5 (93.40) | 267.4 (160.90) | 375.0 (226.05) |
| Statistic | Overall | Time point | Treatment | Treatment 1 effect: hospital EPR – usual care vs. experimental | Treatment 2 effect: home EPR – usual care vs. experimental | Interaction term | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre discharge | 30 days | 90 days | Usual care | Home EPR | Hospital EPR | Hospital and home EPR | |||||
| n | 74 | 20 | 33 | 21 | 21 | 24 | 14 | 15 | 14.7 (95% CI –123.9 to 153.2) | 5.1 (95% CI –89.1 to 99.4) | 12.8 (95% CI –170.4 to 196.1) |
| Mean (SD) | 221.4 (136.31) | 143.6 (92.76) | 231.9 (130.87) | 278.9 (150.15) | 202.9 (121.25) | 212.1 (124.20) | 227.1 (146.68) | 256.8 (168.92) | |||
Appendix 10 Perceived Necessity and Concerns questionnaire data
Perceived necessities
Figure 18 shows that, in general, the scores for interviewed participants indicate agreement (score of 5–7) rather than disagreement (score of 1–3) with the statements in the questionnaire.
FIGURE 18.
Overall perceived necessity scores over four time points by randomised group. 1 = strongly disagree (reflects lack of engagement with PR); 7 = strongly agree (reflects engagement with PR). The bold red line indicates the mean score. B, baseline.

The mean scores show that, over time, those receiving home EPR mostly increased their beliefs that engagement with PR was necessary, whereas participants in the other groups did not.
Figure 19 shows that, over time, the home EPR group was the only group that had an increasingly clear picture of how engagement with PR would improve their condition.
FIGURE 19.
Graph showing responses to the question, ‘I have a clear picture of how pulmonary rehabilitation will improve the condition of my lungs’. 1 = strongly disagree (reflects lack of engagement with PR); 7 = strongly agree (reflects engagement with PR). B, baseline.
Figure 20 shows that, over time, the home EPR group was the only group that had an increasingly clear picture of what they wanted to achieve through engagement with PR.
FIGURE 20.
Graph showing responses to the question, ‘I have a clear picture of what I want to achieve by doing pulmonary rehabilitation exercises’. 1 = strongly disagree (reflects lack of engagement with PR); 7 = strongly agree (reflects engagement with PR). B, baseline.

Figure 21 shows that, over time, those receiving home PR were the only group that increased their belief that engagement with PR would improve their condition.
FIGURE 21.
Graph showing responses to the question, ‘My physical condition will improve considerably if I do the rehabilitation exercises’. 1 = strongly disagree (reflects lack of engagement with PR); 7 = strongly agree (reflects engagement with PR). B, baseline.
Figure 22 shows that, over time, the largest increases in engagement were visible in the home EPR group and the combined hospital and home EPR group.
FIGURE 22.
Graph showing responses to the question, ‘Some aspects of the rehabilitation exercises are unnecessary for me’. 1 = strongly disagree (reflects engagement with PR); 7 = strongly agree (reflects lack of engagement with PR). B, baseline.
Figure 23 shows that, of all of the groups, the home EPR group maintained optimism that engagement with PR would lead to resumed activities of daily living.
FIGURE 23.
Graph showing responses to the question, ‘I hope that doing pulmonary rehabilitation exercises may help me resume my activities more quickly’. 1 = strongly disagree (reflects lack of engagement with PR); 7 = strongly agree (reflects engagement with PR). B, baseline.
Perceived concerns
Figure 24 shows that on average, concerns about engagement with PR declined in all groups over time.
FIGURE 24.
Overall perceived concerns scores over four time points by randomised groups. 1 = strongly disagree that I have concerns (reflects engagement with PR); 7 = strongly agree that I have concerns (reflects lack of engagement with PR). The bold red line indicates the mean score. B, baseline.
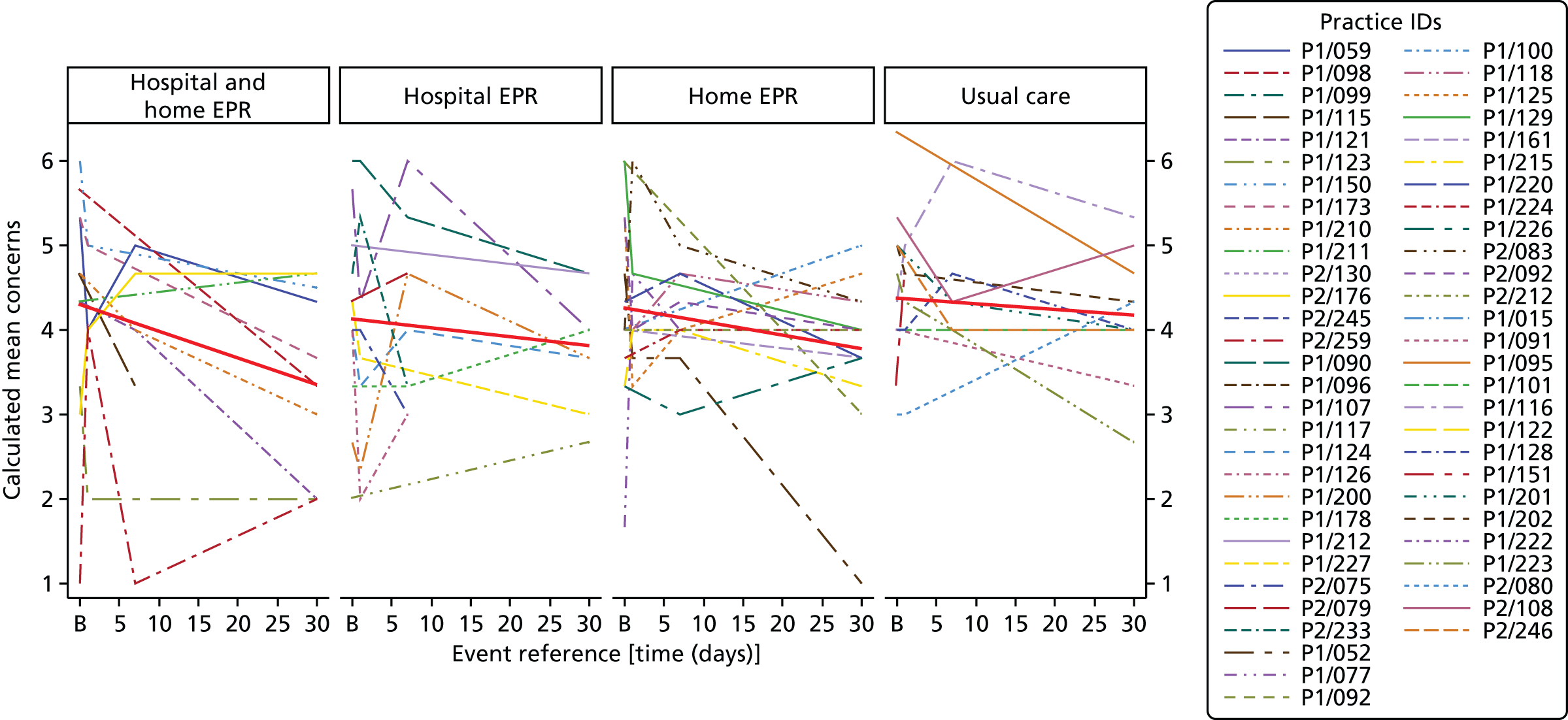
Figure 25 shows that all groups improved their engagement with PR over time, increasingly accepting that exercise was not harmful.
FIGURE 25.
Graph showing responses to the question, ‘Some aspects of the rehabilitation exercises may be harmful to me’. 1 = strongly disagree (reflects engagement with PR); 7 = strongly agree (reflects lack of engagement with PR). B, baseline.

Figure 26 shows that, over time, those receiving home EPR increased their belief that they were fit enough to participate in PR; that belief decreased in the other groups.
FIGURE 26.
Graph showing responses to the question, ‘I may not be physically fit enough to participate in the rehabilitation exercises’. 1 = strongly disagree (reflects engagement with PR); 7 = strongly agree (reflects lack of engagement with PR). B, baseline.
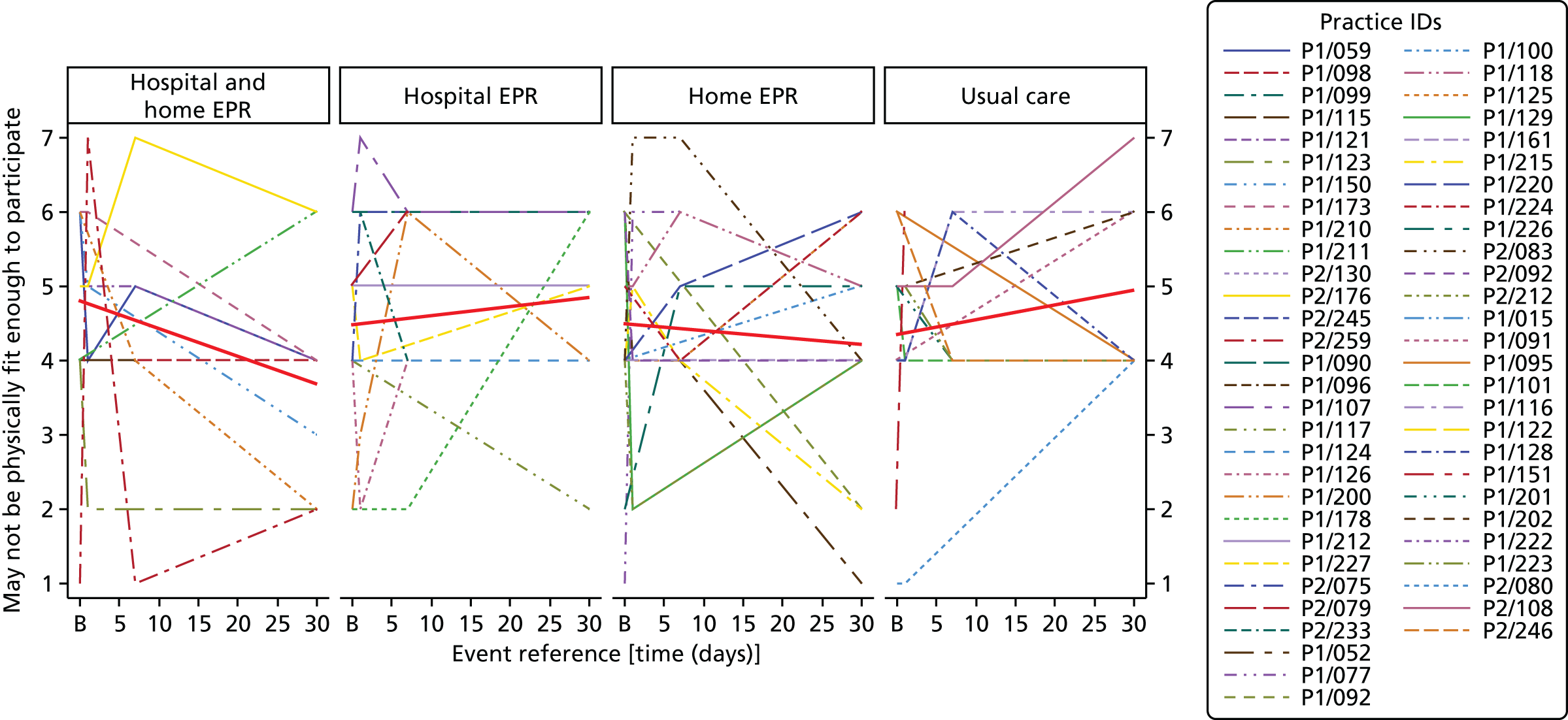
Figure 27 shows that, over time, all groups were increasingly concerned about fatigue after exercise.
FIGURE 27.
Graph showing responses to the question, ‘In the days in between the rehabilitation sessions, I am probably very tired from exercising’. 1 = strongly disagree (reflects engagement with PR); 7 = strongly agree (reflects lack of engagement with PR). B, baseline.
Appendix 11 Full COM-B/theoretical domains framework analysis
| Participant | Capability | Opportunity | Motivation |
|---|---|---|---|
|
P1/059 Hospital EPR and home EPR + usual care Male, 75 years MRC: 5a LTOT: no AO: yes DECAF: 3 CAT: 3 LCADL: 59 Baseline N: 5.6 Baseline C: 5.3 30-day N: 5.2 30-day C: 4.3 |
Physical: Skills: Comorbidities, reduced mobility (Zimmer frame), severe COPD (nebuliser and oxygen). Feels that the home EPR exercises were tiring Psychological: Memory, attention and decision processes: No problem cognitively with learning skills. Previously inactive |
Physical: Environmental context and resources: Did not feel that he was in hospital long enough to exercise on the bike. Would have liked to have a bike at home. Has the space and time to exercise and is able to get to the group programme Social: Social Influences: Does not go out much; widower, dependent on family to go out and for support. Felt supported by the home EPR and hospital physiotherapists and family to exercise |
Reflective: Beliefs about capabilities: High self-efficacy despite poor physical ability. Happy to put trust in physiotherapists who are advising yet putting him in control of the level of exercise (empowered) Beliefs about consequences: Feels will benefit physically and socially from attending the group Intentions: Intending to go to group Automatic: Emotion: No concerns raised surrounding exercise that has been advised but unsure of the safety of performing exercises that have not been sanctioned. Feels previous level of inactivity is own fault Reinforcement: Felt that has benefited from hospital and in-home exercise so far |
| P1/059, 90-day follow-up |
Physical: Skills: Current problem with sciatica not related to study, limiting ability to drive car. Previously found that was pushed the right amount during the PR sessions, very tired afterwards, relieved by sitting in car Knowledge: Been to PR before |
Physical: Environmental context and resources: Car user, but currently limited in terms of attending the group by capability – unable to drive. Felt that had limited opportunity to use the bike in hospital and would have liked a bike at home; cites cost of equipment to the NHS as a barrier Social: Social influences: Previously found that PR provided an avenue to meet people. Feels that pushes himself more when observed |
Reflective: Beliefs about consequences: Exercise makes his lungs feel better. Preference would be for group treatment if he did not have sciatica Goals: Wants to get better Intentions: Unable to attend group Automatic: Emotion: Felt safe when did group PR previously. Some concerns about breathing in general, not just related to exercise. Concerned about current back/leg (?sciatic) pain Reinforcement: Feels pushes self more when under supervision – creation of a dependent relationship |
|
P1/092 Home EPR + usual care Female, 64 years MRC: 4 LTOT: yes AO: yes DECAF: 0 CAT: 0 LCADL: 26 Baseline N: 5.4 Baseline C: 6 30-day N: 7 30-day C: 3 |
Physical: Skills: No problems with previous exercises at previous group PR or this episode of home EPR. Exercise level suitable for her, tired after sessions but no pain. Uses oxygen during exercise and adapts to individual capacity – exercises gently. Limited by chest infection and breathlessness (although will persevere once breathlessness subsides) Psychological: Knowledge: Attended PR three times |
Physical: Environmental context and resources: Has to wait for the opportunity to attend again because of guidelines. Feels that cannot get enough PR. Has time, equipment (tins of beans) and space in the home required to exercise. Prepared to get community transport (ambulance) to the group venue – problems with waiting times for the transport but feels that this is acceptable Social: Social influences: Lives alone, no support. Appreciated cup of tea at the end of previous session of PR. Thought that the PR staff at the PR group were professional |
Reflective: Beliefs about capabilities: Confident to exercise on own Beliefs about consequences: Wishes to get better and stronger – previously good experiences. Believes exercises keeps you fit and keeps chest clear and group PR motivates you to keep you going Optimism: Flexible (in terms of transport and adapting exercise depending on capability) Goals: Wants to get better and stronger Intentions: Intending to attend group PR Automatic: Emotion: Loved PR previously. No concerns. Felt safe and reassured at previous PR that was being monitored Reinforcement: Previous performance was rewarding in terms of walking test score and certificate. Feels brilliant and energised after group PR. PR keeps ‘you’ motivated. Weight management? |
| P1/092 90-day |
Physical: Currently breathless but used to it and adapts. Multiple admissions, readmitted during study period. Limited by hip pain but feels has skills to exercise Psychological: Memory attention and decision processes: Has always tried to be active |
Physical: Environmental context and resources: Waiting to go to PR, was postponed as was readmitted. Summer usually a good time to go. Has the time to attend. Planning to get taxi to PR venue. PR venue has equipment that is not available at home. Wishes classes were more frequent, perceives only able to attend every 2 years Social: Social influences: Felt that in-home PR staff were supportive and grandson now supporting exercise. Liked social aspect of group PR and ‘having a laugh’, especially as lives alone. Balances time at PR well with home life, has a short rest after and then gets on with activities of daily living |
Reflective: Beliefs about consequences: Feels that exercise is very, very beneficial. Feels the benefits help the lungs, the legs and everything Beliefs about capabilities: Happy to exercise alone Optimism: Adapts exercises according to ability. Cannot wait to go to rehabilitation. Wants to keep going and not give up Goal: Wants to keep active Intentions: Intending to attend PR Automatic: Emotion: Not concerned about breathlessness. Sad that home PR has finished Reinforcement: Enjoys group PR and feels better after has been. Feels that exercise is important |
|
P1/098, 90-day follow-up Hospital EPR and home EPR Male, 62 years MRC: 4 LTOT: No AO: No DECAF: 1 CAT: 1 LCADL: 0 Baseline N: 6.2 Baseline C: 5.7 30-day N: 6 30-day C: 3.3 |
Physical: Skills: Multiple comorbidities including diabetes and cerebrovascular accident. Difficulty on hills, cannot bend or walk far. Felt tired after sessions but no other adverse symptoms. Felt that the exercises were easy to do Psychological: Knowledge: Not aware of the benefits of PR Memory, attention and decision processes: Usually walks only a short distance when goes outside house to smoke. Walks more in the hospital because it is flat. Used to be very strong |
Physical: Environmental context and resources: Attended PR over a year ago but was banned from driving, would have attended if transport paid for. Would not use the bus. Has the space at home to exercise. Has the time to exercise as no longer has car or motorbike Social: Social influences: Lives alone, has brother but worse than him. Had good relationship with physiotherapists and trusts the health service |
Reflective: Beliefs about consequences: Gets tired with exercise but believes this is beneficial. Believes anything is better than sitting all day, physically and to relieve boredom Beliefs about capabilities: Nothing would stop him exercising. Confident to exercise alone but felt more confident to push himself under supervision from physiotherapist. States that he is ‘useless’ Optimism: Unable to address the barrier to walking in terms of hills Goals: No goals Intentions: None stated Automatic: Reinforcement: Feels that it is important to exercise. Feels better for exercising Emotion: Does not feel that arm/wrist pain is related to exercises |
|
P1/100, 90-day follow-up Home EPR Female, 70 years MRC: 5a LTOT: yes AO: no DECAF: 1 CAT: 1 LCADL: 50 Baseline N: 5.8 Baseline C: 4 30-day N: 5.2 30-day C: 5 |
Physical: Skills: Limited by osteoporosis and chest infections. Sometimes tired after exercise. Avoided home exercises that ‘put you out’. Felt that it takes a while to build up capability Psychological: Knowledge: Went to PR 3 years ago Memory attention and decision processes: Finds exercises straightforward. Uses PR booklet for reference. Previously walked a lot. Been doing exercises following early home PR. Exercise booklet aids memory of what she needs to do Behavioural regulation: Uses booklet to look at what she should be doing |
Physical: Environmental context and resources: No problems with transport, has own car. Venue is not far. Early home PR team was flexible with timing of visits. Timing of PR previously was difficult as was winter mornings Social: Social influences: Feels that staff and family are supportive. Finds it encouraging to exercise with others and share experiences. Busy at the moment but this viewed positively |
Reflective: Beliefs about consequences: Feels that exercise has benefits for lungs, circulation and mobility Beliefs about capabilities: Feels that staff are flexible and accommodate varying ability to exercise. Feels confident to exercise. Usually goes out accompanied Optimism: Persevered even when tired after previous sessions of PR. Is sure that PR will be good for her Intentions: Waiting to start PR Automatic: Reinforcement: Felt good that had exercised when previously attended PR. Feels that it is important to exercise Emotion: No concerns with supervised exercises. Felt safe to undertake EPR and previous group PR because of monitoring and access to consultant. Would be concerned to exercise unsupervised in a gym and lost confidence going out on own |
|
P1/101 Usual care only Female, 75 years MRC: 4 LTOT: yes AO: no DECAF: 2 CAT: 2 LCADL: 0 Baseline N: 5.2 Baseline C: 4 30-day N: 5 30-day C: 4 |
Physical: Skills: Unable to undertake PR previously because of twisted bowels. Main symptoms breathlessness and fatigue – breathlessness a bigger problem. Stops and starts whilst performing activities of daily living. Nothing other than this limited exercise Psychological: Knowledge: Not undertaken PR or any formal exercise before. Unaware of venue location, Sheffield community transport or portable oxygen cylinder availability Memory, attention and decision processes: Not currently exercising, does go shopping and feels was previously active Behavioural regulation: Not good at reading, therefore all regulation done mentally. Does not use any behavioural regulation methods |
Physical: Environmental context and resources: Has the time to attend PR. Considering getting treadmill exercise machine, but feels may be for daughter’s benefit not hers. Travel to venue may be an issue as dependent on bus and harder in winter Social: Social influences: Does not have support to exercise. Not keen on wearing oxygen outside, would not want people looking at her |
Reflective: Beliefs about capability: Willing to give it a ‘go’ to learn skills to exercise – level of confidence 5/10, not confident with anything Beliefs about consequences: Feels that exercise keeps you fit and helps keeps the mind going. Feels that exercise helps you get better quicker as you are working muscles and getting fit and may help you live longer Goals: Wants to get fit again and be less dependent Intentions: Intending to go to group but would have preferred to be seen at home and undertake hospital exercise Automatic: Emotion: Not keen on wearing oxygen outside, would not want people looking at her. No worries about exercise itself. Has become frustrated that cannot do things as is less independent Reinforcement: Feels important to exercise because of her age |
|
P1/107, 90-day follow-up Hospital EPR Male, 49 years MRC: 4 LTOT: N AO: N DECAF: 1 CAT: 1 LCADL: 51 Baseline N: 5 Baseline C: 5.7 30-day N: 6.2 30-day C: 4 |
Physical: Been given a stick to aid balance, does not do any other exercise, limited mainly by breathlessness Psychological: Knowledge: Unaware of PR Memory, attention and decision processes: Walks to the chemist every day (half-hour) |
Physical: Environmental context and resource: Not been offered PR, in hospital or group Social: Social influences: Not specified |
Reflective: Beliefs about capabilities: Stick has aided confidence Goal: Goal now to give up smoking, no exercise goals Automatic: Emotion: None specified Reinforcement: None specified |
|
P1/116 Usual care Male, 67 years MRC: 2 LTOT: no AO: no DECAF: 1 CAT: 1 LCADL: 0 Baseline N: 5.6 Baseline C: 4.3 30-day N: 5.4 30-day C: 5.3 |
Physical: Still recovering from pneumonia. Has hip problems. Gets out of breath easily Psychological: Knowledge: Has no knowledge about PR, reports that has not been informed. Feels ‘in the dark’ Memory, attention and decision processes: Cannot make a decision until has more knowledge of the PR group. Been walking since discharge |
Physical: Environmental context and resources: Has space inside (mainly walks outside), uses bus to travel Social: Social influences: No support |
Reflective: Beliefs about capabilities: Confident walking Beliefs about consequences: Wants to improve lung function and fight illness. Believes fresh air improves lung health Optimism: Despite hip problems will continue walking when rested Intentions: When finds out what PR entails and where it is will make decision about attending Automatic: Emotion: No concerns about exercising |
|
P1/116, 90-day follow-up Male, 67 years MRC: 2 LTOT: N AO: N DECAF: 1 CAT: 1 LCADL: 0 Baseline N: 5.6 Baseline C: 4.3 30-day N: 5.4 30-day C: 5.3 |
Physical: Fully recovered now from exacerbation. The bike and session of PR leaves him out of breath Psychological: Knowledge: First time at PR Memory, attention and decision processes: Attending PR weekly and exercising at home daily |
Physical: Taxi would cost a lot of money. Gets the bus to the sessions, which is an easy thing to do. An hour in length is acceptable for the sessions. Would struggle to attend if not on Monday or Thursday (therefore attending one session per week not two as other session at Shirecliffe is on a Wednesday) Social: Likes the social aspect and feels that class is ‘good company’ |
Reflective: Beliefs about capabilities: Goes at own pace and stops if needs to. Happy to monitor self Beliefs about consequences: Believes ‘in theory, it’s doing me good’, but is unsure Goals: To keep healthy and to keep lungs going Intentions: Intending to carry on exercising Automatic: Emotion: Dislikes the bike as makes him breathless. No concerns about exercising Reinforcement: Thinks things are doing him good |
|
P1/118 Home EPR and usual care Male, 78 years MRC: 3 LTOT: no AO: no DECAF: 1 CAT: 1 LCADL: 31 Baseline N: 4.4 Baseline C: 4 30-day N: 4.8 30-day C: 4.3 |
Physical: Had a bad cold during home EPR and three admissions in 3 months. Previously active when younger Psychological: Knowledge: Not undertaken group PR before, but has been invited |
Physical: Environmental context and resources: Has space inside, tins of beans and time to exercise. Has car but no intention of going to class. Weather limits outside activity Social: Social influences: Supportive wife. Physiotherapists were flexible in terms of fitting home EPR sessions into routine |
Reflective: Beliefs about capabilities: Not motivated or confident. Describes self as ‘lazy’ Beliefs about consequences: Hopes exercise will help with breathing Goals: None Intentions: Makes ‘big plans’ to do things and then prefers to sit at home. Not intending going to group PR Automatic: Emotion: No real concerns about the exercises themselves – previous experience of circuits when younger. Embarrassed to exercise in a group Reinforcement: Wants ‘kicking up the bum’ |
| P1/118, 90-day follow-up |
Physical: Suffering with chest and hay fever. Has the skills to exercise Psychological: Knowledge: Has experience of PR at home |
Physical: Environmental context and resources: No barriers raised, has the space at home to exercise Social: Social influences: Wife supports exercise. Feels supported by health staff |
Reflective: Beliefs about consequences: Found home PR to be good. Has helped him walk further and perform more activities of daily living. Does not believe that COPD will improve but feels that exercises will help Beliefs about capabilities: Confident to exercise and knows own limits Goals: Keeping going and doing best that he can Intentions: Intending to carry on with home exercise and not attend group PR Automatic: Reinforcement: Believes that exercise is important. Although describes self as lazy the benefits of PR have reinforced the continuation of home exercises Emotion: No concerns about exercising |
|
P1/121, 90-day follow-up Hospital EPR and home EPR Female, 57 years MRC: 3 LTOT: N AO: N DECAF: 0 CAT: 0 LCADL: 28 Baseline N: 3.6 Baseline C: 4.3 30-day N: 5.8 30-day C: 2 |
Physical: Sometimes feels too poorly to exercise. Does not like the step. Gets related muscle pain post exercise but feels that this is acceptable Psychological: Knowledge: Knows ‘everything’ about PR, it has been explained Behavioural regulation: Has programme on the fridge and is following it and filling it in |
Physical: Environmental context and resources: Did not get chance to go on the bike in hospital as was discharged. Has recently become carer to mother and her husband. No longer has the time to exercise. Lives in a hilly area, which limits walking. Has no step at home. Has the space to exercise at home. Has access to transport to class Social: Social influences: Did not want to exercise on the landing as was in view of neighbours. Happy to exercise at PR group class Staff support: Felt that it was good to have someone to talk to straight after discharge |
Reflective: Beliefs about consequences: Believes exercise can help COPD. PR gets you out of the house, allowing you to meet people with the same problem, and is good for your sense of humour Beliefs about capabilities: Confident in ability to exercise Goals: Prefers not to ‘think big’ in case gets disappointed Optimism: Despite being busy trying to fit exercise in at home Automatic: Reinforcement: Exercise is important. Gets endorphin release from exercise. Feels better in the long term. Felt sense of achievement after attending PR session Emotion: Trying not to let self down. No concerns about exercise. Felt safer with supervision. Looking after family causing stress |
|
P1/124 Hospital EPR and usual care Female, 66 years MRC: 4 LTOT: no AO: no DECAF: 1 CAT: 1 LCADL: 43 Baseline N: 5 Baseline C: 4 30-day N: 4.2 30-day C: 3.7 |
Physical: Skills: Recurrent chest infection, still on antibiotics. Has skills to exercise – previously attended PR and gym. Bit achy after bike exercise but felt that the study bike was more comfortable than a standard exercise bike as used in a chair rather than using a hard seat, which hurt bottom Psychological: Memory, attention and decision processes: Potters around the house since discharge, not been far since discharge |
Physical: Environmental context and resources: Has a stepper but this may be upstairs, which makes it difficult to use whilst unwell. Has own car, would prefer venue to be local, with parking, for PR maintenance, e.g. Concorde rather than Ponds Forge, which is further and difficult to park at. Previously was limited by work commitments, which is no longer a problem as retired – now has ‘too much free time’. Had 3 days each with three sessions of the bike, then discharged. Hospital bike better and more comfortable than a standard exercise bike. Finds cold weather difficult. States that groups may have a limit on numbers, which may affect opportunity. Felt that a 12-week course was not long enough – wanted ongoing motivational support. Does not have home monitoring, e.g. telehealth. Prefers group PR as group uses monitoring equipment Social: Social Influences: Wants and needs the support of supervised exercise. Felt positive about hospital EPR staff. Socially isolated. Family are busy with their own lives – tries ‘not to be a burden’. Finance limits gym attendance. Disliked being disturbed from sleep on the ward to do exercise |
Reflective: Beliefs about capabilities: Found previous PR exercises easy Beliefs about consequences: Believes exercise will help – has previous positive experience of PR Intentions: Intending to go to maintenance PR rather than PR itself (? realises cost implications as this is stated as a barrier) Automatic: Emotion: Concerned that 12 sessions of PR would not be enough and wants to join an ongoing course. Has safety concerns about exercising alone – lack of monitoring. Does not push herself as far if not monitored. Concerned about needing oxygen in the future Reinforcement: Feels that she needs external support to provide motivation to exercise. Believes that exercise is important |
| P1/124, 90-day follow-up |
Physical: Skills: Has had ongoing chest infection since discharge, limiting function. Managed PR previously Psychological: Knowledge: Aware of PR, done it before |
Physical: Environmental context and resources: Previously had problems attending because of work but retired now. Has own car – would have preferred to do course nearer home at Concorde, but has to attend PR group prior to maintenance. House is full of gym equipment that she does not use as does not have the motivation. Difficult to go walking as lives on a hill Social: Social influences: Does not want to spend the money on the gym and then be too ill to go |
Reflective: Beliefs about consequences: Feels that PR benefits breathing. Feels better when exercises Beliefs about capabilities: Aware that needs reinforcement of group PR Optimism: ‘I can’t just give my life up to it because I know my limitations’ Intentions: Intending to attend PR, date set Automatic: Reinforcement: Prefers to be pushed to exercise and the staff and appointments support this. Wants ongoing support Emotion: Stops when gets breathless. Feels panicky when sputum gets stuck in throat. Feels safer exercising under supervision |
|
P1/128, 90-day follow-up Usual care Male, 80 years MRC: 3 LTOT: N AO: N DECAF: 1 CAT: 1 LCADL: 23 Baseline N: 5.4 Baseline C: 4 30-day N: 4 30-day C: 4 |
Physical: Skills: Limited because of arthritis in knee. Avoids bike and wall squat because of knees. Cannot do heavy physical things, has to be steady. Stops when gets breathless. Finding PR exercises easier now, Borg scores not as high. PR might not be taxing him sufficiently Psychological: Knowledge: Knew about hospital exercise and group PR. When in hospital was told about PR and thought ‘I would be silly not to go’. Attending PR. Was not told about the benefits of exercise Memory, attention and decision processes: Goes to PR class once a week. Goes to Meadowhall and Morrisons to shop and performs activities of daily living. Does not walk because of knee arthritis Behavioural regulation: A piece of paper on it’s own (from rheumatology physiotherapist) will not change behaviour |
Physical: Environmental context and resources: Did not receive hospital exercise. Declined rheumatology physiotherapy as feels that PR is of more benefit and that additional rheumatology sessions would be a burden in terms of the time and the journey. Only going to PR once a week as feels that twice a week is too much of a commitment. Feels that 12 weeks of PR is not long enough Social: Social influences: Family support him to exercise (although reports that they do not have much success). Feels that attending PR is of more benefit. Feels benefit of PR staff monitoring sessions. Not prepared to exercise if only a sheet of exercises is provided |
Reflective: Beliefs about consequences: Believes that PR is helping with breathing. Although wants PR to help reduce readmissions is aware of the possibility of recurrence Beliefs about capabilities: Confident in ability to exercise and knows when to stop. Does not worry that would push self too hard. Feels will be capable of performing chair aerobics because of similarity to chair-based PR exercises and warm-up. Is motivated to attend the class. Reports that is too idle to exercise without group support (rheumatology physiotherapist) Optimism: Although had some pain after session took painkiller Exercises are adapted because of arthritis Goals: Hopes COPD will not get any worse. Hopes to stay out of hospital Intentions: Intending to continue with PR and to commence local chair aerobics when has completed PR Automatic: Reinforcement: Feels that he is getting value for money and that it does him good. Has improved on the 6MWT – this has reinforced that he is doing better Emotion: Not concerned. Feels safe being monitored |
|
P1/150, 90-day follow-up Male, 74 years MRC: 4 LTOT: no AO: no DECAF: 2 CAT: 2 LCADL: 47 Baseline N: 3.8 Baseline C: 6 30-day N: 3.25 30-day C: 4.5 |
Physical: Skills: Had a bad exacerbation when was admitted. Gets out of breath when exercising. At risk of falling. Legs give way when sitting down and standing up. Problems with pacing. Gets tired. Found the sessions to be okay Psychological: Knowledge: Did not receive any hospital EPR sessions. Has been told about the benefits of PR and underwent early PR. The in-home EPR physiotherapist visited the participant but it is unclear from the qualitative data whether or not the participant exercised within the sessions Memory, attention and decision processes: Usually walks only to toilet and back in the house. Daughter takes him out in a wheelchair Cognitive: Was very ill when made the decision to become involved and would have said yes to anything (daughter) |
Physical: Environmental context and resources: Felt that it was inappropriate timing as was giving up smoking. It was too much. Told physiotherapist not to come anymore. Would not have had physiotherapy at another time. Weather (showers) limits going out of house. Used to attend a walking group but route became too difficult. Reports that was not asked to do any exercise in hospital Social: Social influences: Does not like mixing with people he does not know (daughter). Felt that too many people were coming into the house – ‘Open all hours, that’s what that was’ |
Reflective: Beliefs about consequences: Does not believe that the exercises were helpful Beliefs about capabilities: Feels that he is nearly 74 years old and gets out of breath, which limits capability Optimism: ‘I can’t be bothered’ Goals: None specified – focusing on giving up smoking Intentions: Not intending to go to PR Automatic: Reinforcement: Felt that exercise was important when he was younger but not now aged 74 years. Feels that inhalers are important. Does not like exercise Emotion: No concerns about exercising. Gets out of breath and worried about falling |
|
P1/161, 90-day follow-up Home EPR Female, 63 years MRC: 5a LTOT: yes AO: yes DECAF: 2 CAT: 2 LCADL: 46 Baseline N: 5.4 Baseline C: 4 30-day N: 5.8 30-day C: 3.7 |
Physical: Skill: Currently has a bad shoulder and difficulty with carrying oxygen would make it difficult to attend group PR. Breathing is a bigger limitation than shoulder. Finds local hills make it difficult to go out walking. Has pulmonary arterial hypertension as well as COPD. Tires after exercise. Found the exercises at home quite easy and not painful but felt that muscles had worked Psychological: Knowledge: Does not appear to realise that class actually takes place twice a week and that could attend once if preferred (states three times a week would be too much). Does not appear to be aware of Sheffield community transport. Aware of PR – has done it before, had home EPR after admission and saw the bike on the ward Memory, attention and decision processes: Exercises every day since receiving EPR. Has been exercising for about 6 months. Has a chairlift but now sometimes does not use this and prefers to walk upstairs. Does sit-ups. Rolls out pastry to exercise arms. Sometimes uses the bike machine at Stocksbridge |
Physical: Environmental context and resources: Thought that PR group was too far way to attend. Cannot get the bus as too far to walk to bus stop with condition and cannot afford a taxi. Does not have the time to go to the class – would have to get up at 06.30 to make the 9 o’clock class and would not be back until dinner time. Attending three days a week would be too much commitment. Has to go to visit husband twice a week so does not have time to attend PR. Lives on a hill, which limits opportunity to walk outside. Limited in terms of swimming because of oxygen canister. Uses tins at home. Has the space at home to exercise – uses sink, kitchen units, walls, window ledges and stairs. Found the home EPR exercise sessions fit in with routine – was easier and less expensive than group PR. Prefers to walk in a quiet supermarket where it is flat Social: Social influences: Husband used to take her in the car but is no longer able to since has had brain injury. Felt that some people complained about their illness unnecessarily at Jordanthorpe. Does not have or need anyone to support her to exercise. Shuts the blinds so that neighbours cannot see her exercising. Feels that there needs to be more support groups for pulmonary arterial hypertension – wants to share experiences with people with the same condition. Does not want her husband to see her ill so keen to keep well. Thought that the physiotherapists were brilliant – will miss the company. Feels has made a friend. Has good friends that will take her out and sees importance of friendships and family relationships |
Reflective: Beliefs about consequences: Feels that exercise helps – will lead to a longer life, open airways and improve mobility: ‘the more mobile you are the better you are’. Exercise will aid weight loss and when loses weight breathing is better Beliefs about capabilities: Competent and confident in COPD management regime. Previously lost weight by diet and exercise pending lung transplant and felt healthier. Confident to exercise. Postpones if not up to it and tries later. Knows limitations. Would exercise unless broke her legs! Does not feel capable of walking any distance Optimism: Positive. Pushes herself. Feels has enough motivation. ‘You’ve just gotta be positive and don’t give in and fight it all you know and do what you’re told’ Goals: Wants to lose weight. Wants to be fit for grandchildren Intentions: Intends to carry on exercising at home Automatic: Reinforcement: Thought that EPR was fantastic. Likes the ball exercise because can choose speed. Feels that exercise helps. Felt ‘exhilarated’ after the exercise sessions and ‘wonderful’ after the 6MWT. Has always liked exercising Emotion: Gets frustrated by weight. No concerns about exercising. Does not exercise if down in the dumps but as soon as this passes she recommences exercising |
|
P1/178 Hospital EPR + usual care Male, 80 years MRC: 4 LTOT: no AO: no DECAF: 1 CAT: 1 LCADL: 30 Baseline N: 5.6 Baseline C: 3.3 30-day N: 4.4 30-day C: 4 |
Physical: Skills: Stopped group PR previously because of dizziness (one episode). Felt that had the skills to learn the bike exercises. Felt that the bike exercises did not trigger breathlessness or tiredness. Felt that the bike exercises may have been too easy Psychological: Memory, attention and decision processes: Goes out walking in the field opposite and round Chrystal Peaks. Goes into the garden. Felt that the bike exercises were simple to learn Behavioural regulation: Prepared to exercise with a routine at a certain time each day |
Physical: Environmental context and resources: Has the time and space at home to exercise. Has a car to get to PR. Would have liked an exercise bike at home, similar to the hospital bike, i.e. no resistance. Was willing to travel back to the hospital for the bike exercises. Would not have stayed in hospital longer to undertake the bike exercises Social: Social influences: Okay about exercising in a group. Goes out alone and out to the park with family. Felt supported by the physiotherapists in the hospital and also by the physiotherapists when previously attended PR in the community. Does not feel that family would be able to support home exercise. Wife has died, has to be pushed to go out – prefers being alone and not leaving the house. Was dependent on availability of the physiotherapists in hospital |
Reflective: Beliefs about capability: Felt that he was ‘back to normal’ at the time of the 30-day interview. Felt that the hospital EPR bike exercises were too easy but this is what he wanted it to be like – 15–20 minutes, three times a day was fine for him but he felt that he could have exercised for longer. Liked group PR. Feels confident to exercise – felt that the dizziness was a one-off. Would not have stopped PR himself but was told to stop by a health professional. Felt that age might limit his capacity to get fitter. Did not feel that PR exercises were adapted in the past when attended Beliefs about consequences: Wished to be fitter, felt that exercise might help circulation. Felt that gradual exercise might aid recovery and help him stay healthy for longer Goals: No hobbies but likes to walk Optimism: Less motivation since wife died. Does not want to ‘sit and mope’ Intentions: Not intending to attend group PR because of previous episode of dizziness and being told to stop Automatic: Emotions: May have been feeling low when attended previous PR Reinforcement: Felt that by doing the study he was helping others. Would do the exercises if told to do so. Felt that exercise was important |
|
P1/200 Hospital EPR + usual care Male, 62 years MRC: 5a LTOT: N AO: N DECAF: 2 CAT: 2 LCADL: 60 Baseline N: 6 Baseline C: 2.3 30-day N: 4.6 30-day C: 3.7 |
Physical: Skills: No problems learning the exercises. Has had ongoing problems for 10 weeks because of infection and is limited by breathlessness, fatigue, sputum retention and comorbidities. Fitter in younger life, blames unhealthy lifestyle for current state. Thought that the bike exercises in hospital were too hard and that he was asked to do too much – was limited by breathlessness. Travelling is tiring and complicated by urgency for the toilet Psychological: Knowledge: Previously been to Jordanthorpe for assessment and unhappy with manner of a staff member. Had home PR previously and happy with this Memory, attention and decision processes: Tiredness is a limiting factor |
Physical: Environmental context and resources: Has the time to exercise. Has own car. Needs to be able to transport himself in case he needs to use the toilet. Finds driving tiring. Finds it difficult to get a disabled parking spot near to appointments. Burden of treatment limits opportunity in terms of multiple appointments, rescheduling appointments missed because of hospitalisation, treatment programmes and difficulty accessing the GP and medications. Does not feel that he has the space at home to exercise. Would like to have a study bike in his home. Only had the opportunity to exercise once on the bike in hospital as was discharged home Social: Social influences: Has family but no familial support to exercise. Divorced. Found the hospital physiotherapist to be alright and when previously undertook home PR liked the physiotherapy assistant |
Reflective: Professional? – lost job because of ill health Beliefs about consequences: Feels exercise will help him feel better and keep well for longer. Believes will help him lose weight. Feels that exercise would not help him get better quicker and at current point would make him feel worse Beliefs about capabilities: Managed to give up smoking and drinking. Feels strong-willed. Not lazy. Confident to exercise if has carbocisteine. Feels that cannot exercise because of his physical limitations – thinks needs another week to recover. Feels lack of energy is limiting Optimism: Feels driven to get better and not be beaten Goals: Wants to lose weight and keep everything ‘going’. Would like to get a motorbike but has lost confidence Intentions: Intending to attend PR at a venue furthest from home (wants a fresh start) Automatic: Reinforcement: Feels exercise is important. Felt like he had achieved something when had exercised previously. Feels that he should leave the car and walk but does not do it. Feels he lets people down Emotion: Frustrated at health-care system and anxious about home situation. Panics. Only concern about exercise is that leg might give way |
|
P1/211 Hospital EPR and home EPR (did not receive home EPR as was readmitted) Female, 85 years MRC: 4 LTOT: no AO: no DECAF: 0 CAT: 0 LCADL: 0 Baseline N: 5.4 Baseline C: 4.3 30-day N: 4 30-day C: 4.7 |
Physical: Skills: Currently bloating is main problem as well as lack of energy. Found that hospital bike exercises hurt her shoulder (upper-limb exercises). Felt that she was trying to do the bike exercises too quickly. Goes to bingo in taxi with friend Psychological: Knowledge: Had not attended PR previously Memory, attention and decision processes: Son and grandson perform activities of daily living. Used to be more active and walk more – not done in last 1.5 years. Usually pretty active, likes to go to bingo. Used to ride a bike |
Physical: Environmental context and resources: Underwent bike exercises in hospital, which continued despite changing ward. Has space to exercise at home. Does not have a car – would take a taxi but does not want to travel too far Social: Social influences: Son has told her to take it easy and does everything for her. Likes social interaction/ having a joke. Felt that the in-hospital physiotherapist was sociable and enthusiastic |
Reflective: Beliefs about consequences: Feels still recovering so would not benefit much at the moment, but more positive about benefits when recovered more. ‘It makes you go’ and get around more Beliefs about capabilities: States that at 85 years you would not expect to do much activity. ‘I’m not idle, please don’t think that’ Goals: Would like to be more energetic and walk further Intentions: Wants to wait until she has had the results of her aortic aneurysm scan before making a decision about attending group PR Automatic: Reinforcement: Enjoyed the bike exercises. Activities of daily living are tiring and therefore not doing additional exercise Emotion: Scared to exercise because of aortic aneurysm. Afraid of not being able to breathe properly |
|
P1/215 Home EPR + usual care Male, 72 years MRC: 5a LTOT: yes AO: no DECAF:-3 CAT: 3 LCADL: 0 Baseline N: 3 Baseline C: 3.3 30-day N: 5.8 30-day C: 3.3 |
Physical: Skills: Exacerbating during home EPR. Low oxygen – has LTOT, difficulty with activities of daily living. Breathlessness is a limiting factor. No problems with the exercises. Has the skills – exercises not complicated. Legs give way when stands Psychological: Knowledge: Previously been offered rehabilitation Memory, attention and decision processes: Regularly exercises at home. Does not find the exercises complicated |
Physical: Environmental context and resources: Has the space. No direct buses to Shirecliffe Social: Social influences: Has wife at home but does not influence motivation to exercise. Felt supported by the physiotherapists. Felt that the physiotherapists were flexible around routine |
Reflective: Beliefs about capabilities: Confident to exercise, tries to exercise despite limitations Beliefs about consequences: Exercise makes him feel stronger Optimism: Tries to exercise despite breathlessness and illness Professional identity:? Previously active job Intentions: Not intending to go to group PR because of transport issues. Prefers to exercise at home Automatic: Emotion: No concerns about exercising Reinforcement: Feels that exercise is important because legs give way and limited – exercise makes him feel stronger |
|
P1/220 Home EPR + usual care Female, 61 years MRC: 4 LTOT: yes AO: yes DECAF: 0 CAT: 0 LCADL: 34 Baseline N: 5.6 Baseline C: 4.3 30-day N: 5.6 30-day C: 3.7 |
Physical: Skills: Has learnt the skills okay. Capability varies and therefore felt unable to commit to group PR but happy to be seen at home. Felt a bit achey after exercise but that the exercises were not too strenuous. The walk to the group PR sessions from the bus would be too exhausting. Back issues can limit exercise Psychological: Knowledge: Was not aware of Sheffield community transport Memory, attention and decision processes: Found exercises simple. Not exercised for 3 years. Usually just potters around house and does not go out alone Behavioral regulation: Has exercise book to follow but has not set a routine |
Physical: Environmental context and resources: No direct bus to the PR group, which limits attendance. Uses portable oxygen cylinder. Has the time and space at home to exercise Social: Social influences: Happy with support from physiotherapists. Son is carer – does not go out alone. Has good neighbour and husband. Would prefer to do exercise without support of family, does not feel that it is necessary. Prefers to exercise alone rather than in a group. Felt that Sheffield community transport would have made her feel old |
Reflective: Beliefs about capabilities: Describes self as determined. Feels in control of exercise session – would tell physiotherapists if struggling or would stop herself. Confident to exercise and aware of own limitations Beliefs about consequences: Feels more relaxed after exercise and feels that exercises help with breathlessness and tiredness. Feels that exercise may help you stay healthy for longer by exercising muscles and improving muscle tone but a little unsure. Benefits may be transient Optimism: States is flexible Goals: Would like to become more mobile and go away for a weekend with her husband Intentions: Not intending to go to group PR but happy to have home EPR Automatic: Emotion: Felt that community transport had an age stigma Reinforcement: Felt that nurses had wanted her to go to PR |
|
P1/223 Usual care only Female, 67 years MRC: 1 LTOT: no AO: no DECAF: 0 CAT: 0 LCADL: 0 Baseline N: 5.2 Baseline C: 4.7 30-day N: 5.2 30-day C: 2.7 |
Physical: Barrier to exercise is getting out of bed – gets breathless. Feels has the skills to exercise. Walking more since discharge Psychological: Knowledge: Not previously done PR. Unsure of how much to push herself when breathless Memory, attention and decision processes: Does not usually exercise |
Physical: Environmental context and resources: Has space to exercise Social: Social influences: Husband is a keen walker and encourages her to go too. Not keen on classes – prefers to do things on her own. Potentially felt more confident in the 6MWT test because of walking under supervision |
Reflective: Beliefs about capabilities: Would have said no to hospital exercise as would not have felt capable. Surprised self during the 6MWT test about how much could do Beliefs about consequences: Feels exertion makes her breathless. Describes not being motivated to exercise. Since discharge has felt should walk more Intentions: Not intending to attend group. Feels should walk more Automatic: Emotion: Frightened of bringing on the breathlessness when gets out of bed. Had been worried about the 6MWT test. Insecure initially. No other concerns about exercising |
|
P1/226 Home EPR + usual care Male, 69 years MRC: 2 LTOT: no AO: no DECAF: 2 CAT: 2 LCADL: 30 Baseline N: 5.8 Baseline C: 3.3 30-day N: 5.4 30-day C: 3.7 |
Physical: Skills: COPD limits exercise, knee replacements would have limited bike exercises. Borg scores been fine with home EPR Psychological: Knowledge: Been to PR before Behavioural regulation: Refers to exercise routine as a work schedule. Had devised own schedule prior to admission Memory, attention and decision processes: Is exercising alone since starting home EPR |
Physical: Environmental context and resources: Has the time and space at home to exercise. Hand weights were supplied. Has car so can get to community venue. Less happy to drive to hospital because of parking issues or to get the bus because of the distance from the stop and the hill Social: Social influences: Physiotherapist has supported exercises and is aware of limits |
Reflective: Beliefs about capability: Knows own limits. Feels self-motivated. Had devised self-exercise programme prior to admission Beliefs about consequences: Aids mobility and reduces breathlessness when active Goals: To go out, get fit and live a normal lifestyle, to go walking and not be as breathless walking up hills and to get back to doing sufficient exercise to be reasonably mobile Intentions: Not mentioned whether or not intends to attend group PR. Had intended to do more exercise prior to admission. Intends to walk more to increase stamina Automatic: Emotion: No concerns about exercising. COPD progression at retirement has hit psychologically |
| P1/226, 90-day follow-up |
Physical: Skills: Painful knees do not stop him exercising but do reduce the amount of exercise being undertaken. Some exercises are painful, therefore does some alternative exercises. Psychological: Knowledge: States that has not been to PR when actually has attended course – refers to PR as COPD clinic. Refers to maintenance PR as community PR. Later, confirms has been to PR 1 year ago and after recent AECOPD Memory, attention and decision processes: Forgets to exercise |
Physical: Environmental context and resources: Has a busy life. PR (maintenance) is ‘not exactly local’. Reported that the PR class took up half a day and so it would be difficult to attend maintenance PR because of looking after grandchildren during the summer holidays. Felt that home EPR was easier than attending a class as it cuts down travel time. PR visits fitted in with own schedule Social: Social influences: Liked the one-to-one approach of home EPR |
Reflective: Beliefs about consequences: Found a benefit to the breathing exercises. Doing the exercises in hospital might indicate readiness for discharge Beliefs about capabilities: Motivated to exercise alone despite being limited by knee. Home EPR physiotherapist gave him confidence to do more than he would have done if not there Optimism: Able to adapt exercises accordingly to overcome barrier of knee pain Goals: Get fit so can go out walking again as well as can, partly for the exercise and partly for the fresh air and to keep weight down Intentions: Intending to attend gym for maintenance PR Automatic: Reinforcement: Found it provided a benefit for his breathing. Relationship with physiotherapist encouraged him to do more and push further Emotion: Concerned that might forget to exercise. Concerned that will end up a ‘couch potato’. Not concerned that exercise will make knees worse. Felt safer to push himself further with the physiotherapists but did not feel unsafe to exercise on own |
|
P2/092 Home EPR + usual care Male, 68 years MRC: 4 LTOT: no AO: no DECAF: 2 CAT: 2 LCADL: 57 Baseline N: 5.2 Baseline C: 5.3 30-day N: 4.8 30-day C: 4 |
Physical: Skills: Breathlessness and fatigue main barriers. Struggles with co-ordination. Did not find group PR challenging previously but was younger. More breathless during home EPR exercises this time Psychological: Knowledge: Undertaken PR before. Given appropriate knowledge to continue exercising |
Physical: Environmental context and resources: Has the time and space at home to exercise. Prepared to get taxi to group. No car Social: Social influences: Support from family and physiotherapist. Thought that physiotherapist was ‘smashing’. Previously thought that the classes were for old people |
Reflective: Beliefs about capability: Confident to exercise, relates this to previous experience. Always been independent. Stubbornness makes him push himself Beliefs about consequences: Positive – feels that exercise will prevent deterioration Goal: Wants breathlessness to improve and to be able to go out again Optimism: Optimistic about the way forwards – will continue post-home EPR rehabilitation Intentions: Intending to go to group PR Automatic: Emotion: Some embarrassment about condition. Does not like being watched when breathless. Scared to go out in case he cannot get back |
|
P2/176 Hospital EPR and home EPR + usual care Female, 76 years MRC: 4 LTOT: no AO: no DECAF: 1 CAT: 1 LCADL: 36 Baseline N: 4.8 Baseline C: 3 30-day N: 4.4 30-day C: 4.7 |
Physical: Skills: Arthritis in leg makes it difficult to stand or walk. Breathlessness not main problem. Found the bike exercises at the hospital okay as was sitting. Thought that home EPR exercises may have been started a bit too early – was not feeling up to it Psychological: Knowledge: Undertaken PR before Memory, attention and decision processes: Uses mobility scooter |
Physical: Environmental context and resources: Has the time to exercise. Able to access the group. Has a car and there is a lift at the venue. However, would find stairs at the group venue difficult because of leg pain Social: Social influences: Has social support. Husband would support her to exercise |
Reflective: Beliefs about capability: Low self-efficacy – too ‘lazy’ to attend, husband instigated involvement. Felt that home EPR started too early Beliefs about consequences: To prevent muscles stiffening and to be fitter, probably helps stay healthy for longer. As did not feel that breathlessness limited her did not feel that the exercises were needed. Does not think that exercise would aid recovery Goals: To be fitter. Feels already does activities that wants to do Intentions: Not intending to go to group PR Automatic: Reinforcement: Did not like any of the exercises at home – leg exercises affected knee. Feels a bit more ‘bendy’ since home EPR rehabilitation |
|
P2/212, 90-day follow-up Home EPR Female, 65 years MRC: 2 LTOT: no AO: no DECAF: 3 CAT: 3 LCADL: 61 Baseline N: 5.4 Baseline C: 4 30-day N: 4.4 30-day C: 4 |
Physical: Skills: Stops activity at the point of breathlessness. Avoids wall slide as does not like it. Felt fine after the home EPR sessions. Thought that they were manageable as went at own pace Psychological: Knowledge: Has read up on the benefits of exercise for COPD. Has booklet for reference Memory, attention and decision processes: Walks for hours with dog and cleans. Had never exercised formally before. Continuing to exercise alone Behavioural regulation: Has booklet for reference for continued exercise and found it helpful |
Physical: Environmental context and resources: Has the space to exercise at home Social: Social influences: Did not want to commit to an 8-week group programme. Prefers to exercise on own. Managed to fit in the exercise at home okay. Walks with husband and the dog. Felt very supported by the home EPR physiotherapist, felt that she gave the time to explain things – ‘needed one-to-one’ and felt that she had made a friend |
Reflective: Beliefs about consequences: Will help keep healthier and help control weight. Does not feel that there is a benefit to the lungs (but does not get that breathless). Helps avoid smoking cravings. Felt that exercise got her going quicker Beliefs about capabilities: Confident to exercise, knows own limits and does not push them. Prefers to exercise ‘under own steam’. Feels capable of continuing alone. Never been a person who would just sit around Goals: None specified Optimism: Overcame the barrier of a new skill – ‘it was all new to me’ Intentions: Not intending to go to the group, intending to carry on with home exercise Automatic: Reinforcement: Felt that the home EPR exercises did him good. Exercise helps reduce cigarette cravings. Idolises dog so committed to walking the dog. Was motivated by the physiotherapist to exercise and feels that it would have taken longer to recover without support Emotions: No concerns about exercising. Did not want to waste time of the group PR session in case had a bad day and was not able to exercise |
|
P2/233, 90-day follow-up Female, 72 years MRC: 3 LTOT: no AO: no DECAF: 2 CAT: 2 LCADL: 53 Baseline N: 5.4 Baseline C: 4.7 30-day N and C not completed |
Physical: Skills: Was very unwell at admission, limiting ability on the bike. Has had multiple admissions since. Feels unable to exercise yet because of breathlessness, still recovering Psychological: Knowledge: Aware of PR, is awaiting appointment Memory, attention and decision processes: Activity has been very limited in the last year, previously could go to the shop and into the garden |
Physical: Environmental context and resources: Been unable to attend because of multiple readmissions. Prepared to get taxi to the PR venue. Has the time to exercise Social: Social influences: Happy to attend group exercise sessions |
Reflective: Beliefs about consequences: See emotional concerns Beliefs about capabilities: Does not feel confident about exercising at the moment because of breathlessness. Aware of limitations, has not felt well enough to attend PR Optimism: ‘I know I will get better’ Goals: Wants to be able to breathe better prior to exercise Intentions: Intending to go to PR Automatic: Reinforcement: Feels safer if exercise supervised Emotion: Concerns about exercising related to COPD |
|
P2/246 Female, 74 years MRC: 1 LTOT: no AO: yes DECAF: 1 CAT: 1 LCADL: 0 Baseline N: 5.2 Baseline C: 5 30-day N: 6 30-day C: 4 |
Physical: Skills: Now feels well. Managed well at group PR in past, had the skills – was previously a dancer. Has been unwell over the last year Psychological: Knowledge: Been to group PR before Memory, attention and decision processes: Walks a lot. Does not exercise usually |
Physical: Environmental context and resources: Liked one of the machines at PR. Has no problem getting to the classes at the hospital (does not have a car). Works and does not have the time to exercise Social: Social influences: Feels that gyms are full of posers. Lives alone, has no support. Felt that the PR physiotherapists in the past were good. Nurse supports her. Does not want to wait in at home for physiotherapist, prefers to have an appointment |
Reflective: Beliefs about capabilities: Feels that she is good at the exercises. Confident to exercise. Feels may be lazy, cannot be bothered to do the exercises Beliefs about consequences: Felt that had achieved something after PR last time, felt that the PR was good and helpful Intentions: Intending to go to group PR. Feels that ought to exercise alone more Automatic: Emotion: Feels that exacerbation was frightening Reinforcement: Felt refreshed after PR previously. Benefitted for a few months after previous PR. Does not have the motivation to exercise alone. Nurse motivated her to attend last time. Feels that breathing exercises are important for the chest. Does not feel that physical exercise is important |
Appendix 12 Descriptive statistics for resource use, costs and health status
Intervention resource use and costs
Number of sessions started or completed and time and associated costs of the sessions: hospital early pulmonary rehabilitation
A total of 20 patients were randomised to receive the hospital EPR intervention in some form and completed the study (both hospital and home EPR, n = 10; hospital EPR, n = 10). However, three participants (both, n = 1; hospital EPR, n = 2) did not receive hospital EPR at all because they were discharged from hospital; therefore, there are no intervention records for these participants. The remaining 17 participants could have feasibly started each of the 15 sessions planned as per the protocol (three sessions a day on 5 consecutive days). It should be noted that the denominator patient group in the following descriptive statistics focuses on the 17 people who were in hospital to receive the intervention.
Of the 17 people who feasibly could have started each of the 15 sessions, no patient in either trial arm completed all 15 sessions; the percentage completion rates for all sessions that started are presented in Table 61. The mean times of these sessions are presented in Table 62. It should be noted that every session that was started was classified as being fully completed.
| Day | Session 1, n (%) | Session 2, n (%) | Session 3, n (%) | All sessions, n (%) | Sessions per day, n/N (%) |
|---|---|---|---|---|---|
| Hospital and home EPR (n = 9) | |||||
| 1 | 5 (56)b | 2 (22) | 2 (22) | 2 (22) | 9/27 (33) |
| 2 | 6 (67) | 6 (67) | 6 (67) | 4 (44) | 18/27 (67) |
| 3 | 4 (44) | 3 (33) | 3 (33) | 3 (33) | 10/27 (37) |
| 4 | 4 (44) | 4 (44) | 3 (33) | 3 (33) | 11/27 (41) |
| 5 | 1 (11) | 0 (0) | 0 (0) | 0 (0) | 1/27 (4) |
| Hospital EPR (n = 8) | |||||
| 1 | 6 (75) | 6 (75) | 6 (75) | 6 (75) | 18/24 (75) |
| 2 | 8 (100) | 7 (88) | 6 (75) | 6 (75) | 21/24 (88) |
| 3 | 3 (38) | 3 (38) | 3 (38) | 3 (38) | 9/24 (38) |
| 4 | 4 (50) | 4 (8) | 3 (38) | 3 (8) | 11/24 (46) |
| 5 | 2 (25) | 0 (0) | 3 (38) | 0 (0) | 5/24 (21) |
| Both arms (n = 17) | |||||
| 1 | 11 (65) | 8 (47) | 8 (47) | 8 (47) | 27/51 (53) |
| 2 | 14 (82) | 13 (76) | 12 (71) | 10 (59) | 39/51 (76) |
| 3 | 7 (41) | 6 (35) | 6 (35) | 6 (35) | 19/51 (37) |
| 4 | 8 (47) | 8 (47) | 6 (35) | 6 (35) | 22/51 (43) |
| 5 | 3 (18) | 0 (0) | 3 (18) | 0 (0) | 6/51 (12) |
| Trial arm | Across all 5 days, n/N (%) | Across all 5 days and all sessions, n/N (%) | |||
| Session 1 | Session 2 | Session 3 | |||
| Hospital and home EPR | 20/45 (44)c | 15/45 (33) | 14/45 (31) | 49/135 (36) | |
| Hospital EPR, n/N (%) | 23/40 (58) | 20/40 (50) | 21/40 (53) | 64/120 (53) | |
| Both arms, n/N (%) | 43/85 (51) | 35/85 (41) | 35/85 (41) | 113/255 (44)d | |
| Day | Time per session, mean (n; SD, range) | |||
|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Across all sessions | |
| Hospital and home EPR (n = 9) | ||||
| 1 | 27 (5; 6, 20–35) | 23 (2; 4, 20–25) | 13 (2; 11, 5–20) | 23 (9; 8, 5–35) |
| 2 | 24 (6; 2, 20–26) | 16 (6; 3, 10–20) | 16 (6; 12, 5–35) | 19 (18; 8, 5–35) |
| 3 | 38 (4; 14, 25–50) | 23 (2; 4, 20–25)b | 45 (2; 28, 25–65)b | 36 (8; 17, 20–65)b |
| 4 | 39 (4; 34, 20–90) | 23 (4; 5, 15–25) | 25 (3; 5, 20–30) | 29 (11; 21, 15–90) |
| 5 | . (1;.,. to.)b | 0 (0; 0, 0 to 0) | 0 (0; 0, 0 to 0) | . (1;.,. to.)b |
| Hospital EPR (n = 8) | ||||
| 1 | 34 (6; 12, 20–55) | 28 (6; 12, 10–40) | 26 (6; 11, 15–45) | 29 (18; 11, 10–55) |
| 2 | 30 (8; 10, 20–45) | 26 (7; 11, 10–40) | 27 (6; 12, 20–52) | 28 (21; 11, 10–52) |
| 3 | 20 (4; 7, 15–30) | 20 (4; 6, 15–25) | 20 (3; 9, 15–30)b | 20 (11; 6, 15–30)b |
| 4 | 28 (2; 4, 25–30)b | 25 (3; 10, 15–35) | 38 (2; 11, 30–45) | 29 (7; 9, 15–45)b |
| 5 | 35 (2; 7, 30–40) | 0 (0; 0, 0 to 0) | 25 (3; 5, 20–30) | 29 (5; 7, 20–40) |
| Both arms (n = 17) | ||||
| 1 | 31 (11; 10, 20–55) | 27 (8; 10, 10–40) | 23 (8; 12, 5–45) | 27 (27; 11, 5–55) |
| 2 | 28 (14; 8, 20–45) | 21 (13; 10, 10–40) | 22 (12; 13, 5–52) | 24 (39; 11, 5–52) |
| 3 | 29 (8; 14, 15–50) | 21 (6; 5, 15–25)b | 30 (5; 21, 15–65)b | 27 (19; 14, 15–65)b |
| 4 | 35 (6; 27, 20–90)b | 24 (7; 7, 15–35) | 30 (5; 9, 20–45) | 29 (18; 17, 15–90)b |
| 5 | 35 (2; 7, 30–40)b | 0 (0; 0, 0 to 0) | 25 (3; 5, 20–30) | 29 (5; 7, 20–40)b |
| Trial arm | Across all 5 days, mean (n; SD, range) | Across all sessions, mean (n; SD, range) | ||
| Session 1 | Session 2 | Session 3 | ||
| Time | ||||
| Hospital and home EPR | 31 (19; 17, 20–90)b | 20 (14; 5, 10–25)b | 22 (13; 16, 5–65)b | 25 (46; 15, 5–90)b |
| Hospital EPR | 30 (22; 10, 15–55)b | 25 (20; 10, 10–40) | 26 (20; 10, 15–52)b | 27 (62; 10, 10–55)b |
| Both arms | 30 (41; 14, 15–90)b | 23 (34; 9, 10–40)b | 25 (33; 13, 5–65)b | 26 (108; 12, 5–90)b |
| Costs (£)c | ||||
| Hospital and home EPR | 19 (19; 2, 9–41) | 10 (14; 1, 5–18) | 11 (13; 2, 2–30) | 14 (46; 1, 2–41) |
| Hospital EPR | 17 (22; 1, 9–33) | 13 (20; 1, 5–21) | 14 (20; 1, 7–27) | 14 (62; 1, 5–33) |
| Both arms | 18 (41; 1, 9–41) | 12 (34; 1, 5–21) | 13 (33; 1, 2–30) | 14 (108; 1, 2–41) |
Across both trial arms, the highest completion rate occurred for session 1, with 58% of these sessions starting and being completed; this dropped to 41% across both trial arms for sessions 2 and 3, with the lowest completion rates occurring in the both interventions arm across all three sessions. Across both trial arms, of the 255 sessions that were planned as per the protocol (three sessions a day for 5 consecutive days across 17 patients who were still in hospital), 51% (113) were started and completed. For the both interventions trial arm, 33% (49/135) of the sessions were completed; for the hospital EPR intervention trial arm the completion rate was slightly higher, with 53% (64/120) of the sessions being completed.
Of the 113 sessions that were started and completed, a session time was classified as missing for five of these sessions. The mean (SD, range) session time for the 108 sessions for which a relatively reliable time could be inferred was 26 (13, 5–90) minutes. For these 108 sessions, the associated mean (SEM, range) cost per session was £14 (£1, £2–41).
Numbers of sessions started or completed and time and associated costs of the sessions: home early pulmonary rehabilitation
Of the 25 patients randomised to receive both interventions (n = 10) or the home EPR intervention (n = 15), 23 (both, n = 9; home EPR, n = 14) were eligible to receive the home EPR intervention. One person was admitted to hospital before the intervention started and one person withdrew from the intervention but was still classified as having completed the study. The denominator patient group in the following descriptive statistics focuses on the 23 people who were not readmitted to hospital or who did not withdraw from the intervention and so could relatively feasibly receive the intervention.
Of the 23 participants who could have feasibly started the four home EPR intervention visits, 14 (61%) started and 11 (48%) completed all four sessions. The numbers of participants who started and completed the four sessions are presented in Table 63. The mean times and costs of these sessions are presented in Table 64. It should be noted that a session time could not be inferred for all sessions that were classified as having been started or completed and therefore the number of sessions with an estimated time is not the same as the number of sessions that were classified as having been started or completed.
| Trial arm | Visits over 2 weeks | Sessions started per day, n (%) | Sessions completed per day, n (%) |
|---|---|---|---|
| Hospital and home EPR (n = 9) | 1 | 7 (78) | 7 (78) |
| 2 | 8 (89)b | 7 (78)b | |
| 3 | 8 (89) | 8 (89) | |
| 4 | 7 (78) | 7 (78) | |
| Home EPR (n = 14) | 1 | 9 (64) | 8 (57) |
| 2 | 11 (79) | 9 (64) | |
| 3 | 10 (71) | 9 (64) | |
| 4 | 9 (64) | 7 (50) | |
| Both arms (n = 23) | 1 | 16 (70) | 15 (65) |
| 2 | 19 (83) | 16 (70) | |
| 3 | 18 (78) | 17 (74) | |
| 4 | 16 (70) | 14 (61) | |
| Trial arm | Visits over 2 weeks | Participants starting all 4 days of visits, n (%) | Participants completing all 4 days of visits, n (%) |
| Hospital and home EPR | All visits | 7 (78) | 6 (67) |
| Home EPR | All visits | 7 (50) | 5 (36) |
| Both arms | All visits | 14 (61)c | 11 (48)c |
| Trial arm | Visits over 2 weeks | Sessions started (across all 4 days), n (%) | Sessions completed (across all 4 days), n (%) |
| Hospital and home EPR | All visits | 30/36 (83) | 29/36 (81) |
| Home EPR | All visits | 39/56 (70) | 33/56 (59) |
| Both arms | All visits | 69/92 (75)d | 62/92 (67)d |
| Trial arm | Visits over 2 weeks | Length of started visits per day (minutes), mean (n; SD, range) | Length of completed visits per day (minutes), mean (n; SD, range) |
|---|---|---|---|
| Hospital and home EPR (n = 9) | 1 | 63 (5; 14, 40–75)b | 63 (5; 14, 40–75)b |
| 2 | 53 (8; 8, 45–65) | 53 (7; 8, 45–65) | |
| 3 | 61 (8; 19, 43–90) | 61 (8; 19, 43–90) | |
| 4 | 54 (6; 14, 40–80)b | 54 (6; 14, 40–80)b | |
| Home EPR (n = 14) | 1 | 77 (9; 31, 40–140) | 81 (8; 30, 40–140) |
| 2 | 62 (9; 17, 40–90)b | 65 (7; 19, 40–90)b | |
| 3 | 60 (9; 15, 35–80)b | 61 (8; 16, 35–80)b | |
| 4 | 66 (8; 17, 45–90)b | 67 (6; 20, 45–90)b | |
| Both arms (n = 23) | 1 | 72 (14; 26, 40–140)b | 74 (13; 26, 40–140)b |
| 2 | 58 (17; 14, 40–90)b | 59 (14; 15, 40–90)b | |
| 3 | 60 (17; 17, 35–90)b | 61 (16; 17, 35–90)b | |
| 4 | 61 (14; 16, 40–90)b | 61 (12; 18, 40–90)b | |
| Trial arm | Visits over 2 weeks | Length of started visits across all 4 days (minutes), mean (n; SD, range) | Length of completed visits across all 4 days (minutes), mean (n; SD, range) |
| Hospital and home EPR | All visits | 58 (27; 14, 40–90)b | 57 (26; 14, 40–90)b |
| Home EPR | All visits | 66 (35; 21, 35–140)b | 68 (29; 22, 35–140)b |
| Both arms | All visits | 62 (62; 19, 35–140)b | 63 (55; 20, 35–140)b |
| Trial arm | Visits over 2 weeks | Cost of started visits across all 4 days (£), mean (n; SEM, range)c | Cost of completed visits across all 4 days (£), mean (n; SEM, range)c |
| Hospital and home EPR | All visits | 47 (27; 2, 35–66) | 47 (26; 2, 35–66) |
| Home EPR | All visits | 52 (35; 2, 30–103) | 54 (29; 3, 30–103) |
| Both arms | All visits | 50 (62; 2, 30–103) | 50 (55; 2, 30–103) |
Of the 92 sessions planned across both trial arms (23 people undertaking four sessions each), 69 (75%) were started and 62 (67%) were completed; therefore, only seven sessions that were started could not be completed. A session time was available for 62 sessions that were started and 55 sessions that were completed (see Table 64). The mean (SD, range) session time for those sessions that were started was 62 (19, 35–140) minutes and for those sessions that were completed was 63 (20, 35–140) minutes. The mean (SEM, range) cost associated with the 62 sessions that were started and the 55 sessions that were completed for which a time could be estimated was £50 (£2, £30–103) in both cases.
Summary statistics for travel times and associated costs are presented in Table 65. In general, across both trial arms, the mean (SD, range) travel time for the 84 sessions was 33 (17, 8–74) minutes, which included the time for the physiotherapists to travel to the patients’ homes and back; the associated mean (SEM, range) cost was £27 (£2, £6–64), which is dependent on the travel time and salary band of the physiotherapists. When accounting for the time of travel and the time for the session (which need not have started), the mean (SD, range) time for the 84 sessions was 79 (36, 14–178) minutes, with an associated mean (SEM, range) cost of £64 (£3, £12–131).
| Trial arm | Visits over 2 weeks | Travel time per day (minutes), mean (n; SD, range) | Session and travel time per day (minutes), mean (n; SD, range) |
|---|---|---|---|
| Hospital and home EPR (n = 9) | 1 | 32 (9; 16, 10–54) | 67 (9; 35, 14–109) |
| 2 | 32 (9; 16, 10–54) | 79 (9; 28, 16–114) | |
| 3 | 32 (9; 16, 10–54) | 86 (9; 36, 16–144) | |
| 4 | 34 (8; 16, 10–54) | 74 (8; 33, 34–134) | |
| Home EPR (n = 14) | 1 | 35 (13; 17, 10–74) | 88 (13; 48, 18–178) |
| 2 | 32 (12; 19, 8–74) | 79 (12; 23, 34–128) | |
| 3 | 32 (12; 19, 8–74) | 77 (12; 38, 22–149) | |
| 4 | 32 (12; 19, 8–74) | 76 (12; 45, 18–164) | |
| Both arms (n = 23) | 1 | 34 (22; 17, 10–74) | 79 (22; 43, 14–178) |
| 2 | 32 (21; 18, 8–74) | 79 (21; 25, 16–128) | |
| 3 | 32 (21; 18, 8–74) | 81 (21; 36, 16–149) | |
| 4 | 33 (20; 18, 8–74) | 75 (20; 40, 18–164) | |
| Trial arm | Visits over 2 weeks | Travel time of visits across all 4 days (minutes), mean (n; SD, range) | Session and travel time of visits per day (minutes), mean (n; SD, range) |
| Hospital and home EPR | All visits | 32 (35; 15, 10–54) | 77 (35; 33, 14–144) |
| Home EPR | All visits | 33 (49; 18, 8 –74) | 80 (49; 39, 18–178) |
| Both arms | All visits | 33 (84; 17, 8–74) | 79 (84; 36, 14–178) |
| Trial arm | Visits over 2 weeks | Cost of travel time across all 4 days (£), mean (n; SEM, range) | Cost of session and travel time across all 4 days (£), mean (n; SEM, range) |
| Hospital and home EPR | All visits | 27 (35; 2, 7–47) | 63 (35; 4, 12–106) |
| Home EPR | All visits | 27 (49; 2, 6–64) | 64 (49; 4, 13–131) |
| Both arms | All visits | 27 (84; 2, 6–64) | 64 (84; 3, 12–131) |
Health and social care resource-use and costs
The CSRI must have been completed at both the 30-day and the 90-day time points to assess participants’ resource use over the 90-day period. A total of 33 participants (75% of the 44 participants who completed the study) completed the CSRI sections describing their use of primary care (specifically, consultations with a GP at the surgery, at home or by telephone) and therapy services (such as a physiotherapist, occupational therapist, social worker, home care worker and health visitor) at both 30 days [41 (93%) participants completed this section of the CSRI at this time point] and 90 days [35 (80%) participants completed this section of the CSRI at this time point]. Resource use and costs for the aforementioned services between baseline and 30 days and between 30 days and 90 days for all 33 patients are presented in Table 66; for resource users (i.e. those people who utilised the specific services), their resource use and costs for the same time period are presented in Table 67. Resource use and costs are described by trial arm for all patients in Table 68 and for resource users in Table 69.
| Resource | 30 days, mean (SEM, range) | 30–90 days, mean (SEM, range) | Total (90 days), mean (SEM, range) | |||
|---|---|---|---|---|---|---|
| Resource use | Cost (£) | Resource use | Cost (£) | Resource use | Cost (£) | |
| Primary care | ||||||
| GP surgery | 0.58 (0.21, 0–6) | 22 (8, 0–225) | 0.88 (0.19, 0–4) | 33 (7, 0–150) | 1.45 (0.28, 0–6) | 54 (11, 0–225) |
| GP home | 0.18 (0.11, 0–3) | 7 (4, 0–109) | 0.24 (0.11, 0–2) | 9 (4, 0–73) | 0.42 (0.2, 0–5) | 15 (7, 0–182) |
| GP telephone | 0.24 (0.1, 0–2) | 6 (2, 0–45) | 0.12 (0.06, 0–1) | 3 (1, 0–23) | 0.36 (0.11, 0–2) | 8 (3, 0–45) |
| All GP contacts | 1 (0.23, 0–6) | 34 (8, 0–225) | 1.24 (0.22, 0–4) | 44 (8, 0–150) | 2.24 (0.32, 0–6) | 78 (12, 0–225) |
| Therapy services | ||||||
| Physiotherapist | 1.48 (0.38, 0–7) | 47 (12, 0–221) | 0.15 (0.12, 0–4) | 5 (4, 0–126) | 1.64 (0.45, 0–10) | 52 (14, 0–315) |
| Occupational therapist | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) |
| Social worker | 0.06 (0.06, 0–2) | 3 (3, 0–110) | 0 (0, 0–0) | 0 (0, 0–0) | 0.06 (0.06, 0–2) | 3 (3, 0–110) |
| Home care worker | 0.09 (0.09, 0–3) | 1 (1, 0–36) | 0.18 (0.18, 0–6) | 2 (2, 0–72) | 0.27 (0.2, 0–6) | 3 (2, 0–72) |
| Health visitor | 1.7 (0.4, 0–8) | 75 (18, 0–352) | 0.79 (0.32, 0–7) | 35 (14, 0–308) | 2.48 (0.57, 0–12) | 109 (25, 0–528) |
| All therapy contacts | 3.33 (0.54, 0–11) | 126 (21, 0–434) | 1.12 (0.45, 0–12) | 42 (16, 0–336) | 4.45 (0.77, 0–17) | 168 (29, 0–578) |
| Total contacts | ||||||
| Primary care and therapy services | 4.33 (0.52, 0–11) | 160 (21, 0–434) | 2.36 (0.54, 0–15) | 86 (18, 0–446) | 6.7 (0.83, 0–23) | 246 (31, 0–798) |
| Resource | 30 days, mean (SEM, range) | 30–90 days, mean (SEM, range) | Total (90 days), mean (SEM, range) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RN | Resource use | Cost (£) | RN | Resource use | Cost (£) | RN | Resource use | Cost (£) | |
| Primary care | |||||||||
| GP surgery | 11 | 1.73 (0.47, 1–6) | 65 (18, 37–225) | 17 | 1.71 (0.22, 1–4) | 64 (8, 37–150) | 21 | 2.29 (0.32, 1–6) | 86 (12, 37–225) |
| GP home | 3 | 2 (0.58, 1–3) | 73 (21, 36–109) | 5 | 1.6 (0.24, 1–2) | 58 (9, 36–73) | 6 | 2.33 (0.71, 1–5) | 85 (26, 36–182) |
| GP telephone | 6 | 1.33 (0.21, 1–2) | 30 (5, 23–45) | 4 | 1 (0, 1–1) | 23 (0, 23–23) | 9 | 1.33 (0.17, 1–2) | 30 (4, 23–45) |
| All GP contacts | 17 | 1.94 (0.31, 1–6) | 65 (12, 23–225) | 21 | 1.95 (0.23, 1–4) | 70 (8, 23–150) | 26 | 2.85 (0.32, 1–6) | 99 (12, 23–225) |
| Therapy services | |||||||||
| Physiotherapist | 13 | 3.77 (0.53, 1–7) | 119 (17, 32–221) | 2 | 2.5 (1.5, 1–4) | 79 (47, 32–126) | 13 | 4.15 (0.7, 1–10) | 131 (22, 32–315) |
| Occupational therapist | 0 | 0 (N/A) | 0 (N/A) | 0 | 0 (N/A) | 0 (N/A) | 0 | 0 (N/A) | 0 (N/A) |
| Social worker | 1 | 2 (0, 2–2) | 110 (0, 110–110) | 0 | 0 (N/A) | 0 (N/A) | 1 | 2 (0, 2–2) | 110 (0, 110–110) |
| Home care worker | 1 | 3 (0, 3–3) | 36 (0, 36–36) | 1 | 6 (0, 6–6) | 72 (0, 72–72) | 2 | 4.5 (1.5, 3–6) | 54 (18, 36–72) |
| Health visitor | 15 | 3.73 (0.51, 1–8) | 164 (23, 44–352) | 6 | 4.33 (0.76, 2–7) | 191 (33, 88–308) | 17 | 4.82 (0.76, 1–12) | 212 (33, 44–528) |
| All therapy contacts | 24 | 4.58 (0.55, 1–11) | 173 (22, 32–434) | 7 | 5.29 (1.23, 3–12) | 196 (34, 120–336) | 25 | 5.88 (0.84, 1–17) | 221 (32, 32–578) |
| Total contacts | |||||||||
| Primary care and therapy services | 30 | 4.77 (0.5, 1–11) | 176 (20, 23–434) | 24 | 3.25 (0.65, 1–15) | 118 (22, 23–446) | 32 | 6.91 (0.83, 1–23) | 253 (31, 37–798) |
| Resource | Hospital and home EPR (n = 8), mean (SEM, range) | Hospital EPR (n = 6), mean (SEM, range) | Home EPR (n = 11), mean (SEM, range) | Usual care (n = 8), mean (SEM, range) | ||||
|---|---|---|---|---|---|---|---|---|
| Resource use | Cost (£) | Resource use | Cost (£) | Resource use | Cost (£) | Resource use | Cost (£) | |
| Primary care | ||||||||
| GP surgery | 1.13 (0.4, 0–3) | 42 (15, 0–112) | 3.17 (0.75, 1–6) | 119 (28, 37–225) | 0.91 (0.25, 0–2) | 34 (9, 0–75) | 1.25 (0.73, 0–5) | 47 (27, 0–187) |
| GP home | 0.50 (5, 0–4) | 18 (18, 0–146) | 0 (0, 0–0) | 0 (0, 0–0) | 0.82 (0.46, 0–5) | 30 (17, 0–182) | 0.13 (0.13, 0–1) | 5 (5, 0–36) |
| GP telephone | 0.25 (0.25, 0–2) | 6 (6, 0–45) | 0.17 (0.17, 0–1) | 4 (4, 0–23) | 0.64 (0.24, 0–2) | 14 (6, 0–45) | 0.25 (0.16, 0–1) | 6 (4, 0–23) |
| All GP contacts | 1.88 (0.58, 0–4) | 66 (20, 0–146) | 3.33 (0.8, 1–6) | 122 (29, 37–225) | 2.36 (0.56, 0–6) | 78 (19, 0–220) | 1.63 (0.68, 0–5) | 57 (26, 0–187) |
| Therapy services | ||||||||
| Physiotherapist | 2.38 (0.75, 0–5) | 75 (24, 0–158) | 1 (0.68, 0–4) | 32 (22, 0–126) | 2.45 (1.11, 0–10) | 77 (35, 0–315) | 0.25 (0.16, 0–1) | 8 (5, 0–32) |
| Occupational therapist | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) |
| Social worker | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0 (0, 0–0) | 0.18 (0.18, 0–2) | 10 (10, 0–110) | 0 (0, 0–0) | 0 (0, 0–0) |
| Home care worker | 0.38 (0.38, 0–3) | 5 (5, 0–36) | 0 (0, 0–0) | 0 (0, 0–0) | 0.55 (0.55, 0–6) | 7 (7, 0–72) | 0 (0, 0–0) | 0 (0, 0–0) |
| Health visitor | 0.13 (0.13, 0–1) | 6 (6, 0–44) | 4 (0.97, 0–7) | 176 (43, 0–308) | 4.09 (1.38, 0–12) | 180 (61, 0–528) | 1.5 (0.53, 0–4) | 66 (24, 0–176) |
| All therapy contacts | 2.88 (0.77, 0–5) | 85 (22, 0–158) | 5 (1.48, 0–11) | 208 (58, 0–434) | 7.27 (1.78, 0–17) | 274 (67, 0–578) | 1.75 (0.56, 0–4) | 74 (24, 0–176) |
| Total contacts | ||||||||
| Primary care and therapy services | 4.75 (0.73, 2–9) | 151 (23, 75–278) | 8.33 (1.36, 4–13) | 330 (51, 169–509) | 9.64 (1.86, 2–23) | 352 (70, 60–798) | 3.38 (0.78, 0–7) | 131 (32, 0–275) |
| Resource use | Hospital and home EPR (n = 8), mean (SEM, range) | Hospital EPR (n = 6), mean (SEM, range) | Home EPR (n = 11), mean (SEM, range) | Usual care (n = 8), mean (SEM, range) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RN | Resource use | Cost | RN | Resource use | Cost | RN | Resource use | Cost | RN | Resource use | Cost | |
| Primary care | ||||||||||||
| GP surgery | 5 | 1.8 (0.37, 1–3) | 67 (14, 37–112) | 6 | 3.17 (0.75, 1–6) | 119 (28, 37–225) | 7 | 1.43 (0.2, 1–2) | 53 (8, 37–75) | 3 | 3.33 (1.2, 1–5) | 125 (45, 37–187) |
| GP home | 1 | 4 (0, 4–4) | 146 (0, 146–146) | 0 | 0 (N/A) | 0 (N/A) | 4 | 2.25 (0.95, 1–5) | 82 (35, 36–182) | 1 | 1 (0, 1–1) | 36 (0, 36–36) |
| GP telephone | 1 | 2 (0, 2–2) | 45 (0, 45–45) | 1 | 1 (0, 1–1) | 23 (0, 23–23) | 5 | 1.4 (0.24, 1–2) | 32 (6, 23–45) | 2 | 1 (0, 1–1) | 23 (0, 23–23) |
| All GP contacts | 6 | 2.5 (0.56, 1–4) | 88 (19, 37–146) | 6 | 3.33 (0.8, 1–6) | 122 (29, 37–225) | 9 | 2.89 (0.54, 1–6) | 96 (19, 36–220) | 5 | 2.6 (0.81, 1–5) | 91 (33, 23–187) |
| Therapy services | ||||||||||||
| Physiotherapist | 5 | 3.8 (0.49, 2–5) | 120 (15, 63–158) | 2 | 3 (1, 2–4) | 95 (32, 63–126) | 4 | 6.75 (1.25, 4–10) | 213 (39, 126–315) | 2 | 1 (0, 1–1) | 32 (0, 32–32) |
| Occupational therapist | 0 | 0 (N/A) | 0 (N/A) | 0 | 0 (N/A) | 0 (N/A) | 0 | 0 (N/A) | 0 (N/A) | 0 | 0 (N/A) | 0 (N/A) |
| Social worker | 0 | 0 (N/A) | 0 (N/A) | 0 | 0 (N/A) | 0 (N/A) | 1 | 2 (0, 2–2) | 110 (0, 110–110) | 0 | 0 (N/A) | 0 (N/A) |
| Home care worker | 1 | 3 (0, 3–3) | 36 (0, 36–36) | 0 | 0 (N/A) | 0 (N/A) | 1 | 6 (0, 6–6) | 72 (0, 72–72) | 0 | 0 (N/A) | 0 (N/A) |
| Health visitor | 1 | 1 (0, 1–1) | 44 (0, 44–44) | 5 | 4.8 (0.66, 3–7) | 211 (29, 132–308) | 6 | 7.5 (1.36, 4–12) | 330 (60, 176–528) | 5 | 2.4 (0.51, 1–4) | 106 (22, 44–176) |
| All therapy contacts | 6 | 3.83 (0.6, 1–5) | 113 (16, 44–158) | 5 | 6 (1.34, 3–11) | 249 (50, 132–434) | 8 | 10 (1.52, 4–17) | 377 (57, 176–578) | 6 | 2.33 (0.56, 1–4) | 99 (24, 32–176) |
| Total contacts | ||||||||||||
| Primary care and therapy services | 8 | 4.75 (0.73, 2–9) | 151 (23, 75–278) | 6 | 8.33 (1.36, 4–13) | 330 (51, 169–509) | 11 | 9.64 (1.86, 2–23) | 352 (70, 60–798) | 7 | 3.86 (0.7, 1–7) | 150 (29, 37–275) |
To summarise some of the key results, 26 (79%) participants had a contact with a GP over the 90-day period, 13 (39%) participants had a contact with a physiotherapist; one (3%) participant had a contact with a social worker, two (6%) participants had a contact with a home care worker, 17 (52%) participants had a contact with a health visitor and no participants had a contact with an occupational therapist. Across all participants over the 90-day period, there was a mean (SEM, range) of 2.24 (0.32, 0–6) contacts with a GP by telephone, at the surgery or at home, which equated to a mean (SEM, range) cost of £78 (£12, £0–225); the highest number of mean contacts was with a GP at the surgery (1.45), which equated to a mean cost of £55. For resource users over the 90-day period, there was a mean (SEM, range) of 2.85 (0.32, 1–6) contacts with a GP by telephone, at the surgery or at home, which equated to a mean (SEM, range) cost of £99 (£12, £23–225); participants who required a GP contact required on average 2.29 contacts at the surgery, 2.33 contacts at home and 1.33 contacts by telephone.
When focusing on resource use across trial arms (see Tables 68 and 69), only those receiving the home intervention reported seeing a social worker (one participant reported seeing a social worker in this trial arm) and only those receiving both interventions or the home intervention reported seeing a home care worker (one participant in each of these trial arms reported seeing a home care worker). Across all four trial arms, the only professionals that participants consistently reported having contact with were GPs, physiotherapists and health visitors. The highest mean costs across all trial arms were associated with seeing a health visitor (17 participants reported seeing a health visitor, with resource users having a mean of 5.88 contacts, associated with a mean cost of £212) and a physiotherapist (13 participants reported seeing a physiotherapist, with resource users having a mean of 4.15 contacts, associated with a mean cost of £131). Across all health and social care contacts in this cohort, there was a mean of 6.7 contacts with the health professionals included in the CSRI, with an associated mean cost of £246.
Hospital resource use and costs: index admissions and readmissions
Of the 44 participants who completed the study, 21 (48%) had a readmission to hospital within the 90-day trial period; the mean (SD, range) LOS for these resource users was 13 (12, 1–48) days. Across all 44 participants, the mean (SD, range) LOS after the index admission was 6 (2, 0–48) days, which was associated with a mean (SEM, range) cost of £2148 (£509, £0–16,122).
For the index admission, the mean (SD, range) LOS from the point of randomisation was 4 (4, 0–23) days, which was associated with a mean (SEM, range) cost of £1794 (£152, £671–6467); it should be noted that those participants who were discharged on the day of randomisation still incurred a cost associated with the last day of being in hospital. The mean (SD, range) time spent in hospital during the 90-day trial period across participants who completed the study was 11 (11, 0–52) days, which was associated with a mean (SEM, range) cost of £3942 (£540, £671–17,801). Those receiving usual care spent on average more time in hospital during the trial period than those in any other trial arm (13 days), although they spent on average no longer in hospital than those in any other trial arm for their index admission (5 days).
| Trial arm | Whole cohort (n = 44) | Resource users | ||
|---|---|---|---|---|
| Cost (£), mean (SEM, range) | LOS (days), mean (SD, range) | Cost (£), mean (n; SEM, range) | LOS (days), mean (n; SD, range) | |
| Hospital and home EPR | 1402 (839, 0–7809) | 4 (2, 0–23) | 4674 (3; 2959, 1931–7809) | 12 (3; 9, 5–23) |
| Hospital EPR | 1628 (735, 0–5711) | 4 (2, 0–20) | 4069 (4; 1725, 1679–5711) | 11 (4; 7, 4–20) |
| Home EPR | 2536 (1101, 0–16,122) | 7 (3, 0–48) | 4755 (8; 4929, 923–16,122) | 14 (8; 15, 1–48) |
| Usual care | 2909 (1227, 0–10,737) | 9 (4, 0–38) | 4364 (6; 3748, 923–10,737) | 13 (6; 14, 1–38) |
| All arms | 2148 (509, 0–16,122) | 6 (2, 0–48) | 4501 (21; 3662, 923–16,122) | 13 (21; 12, 1–48) |
| Trial arm | Index admission (n = 44) | Index admission and readmission (n = 44) | ||
|---|---|---|---|---|
| Cost (£), mean (SEM, range) | LOS (days), mean (SD, range) | Cost (£), mean (SEM, range) | LOS (day), mean (SD, range) | |
| Hospital and home EPR | 1830 (534, 671–6467) | 5 (7, 0–23) | 3232 (1015, 671–9992) | 8 (10, 0–29) |
| Hospital EPR | 1881 (243, 671–2939) | 5 (3, 0–9) | 3508 (846, 671–8146) | 9 (8, 0–27) |
| Home EPR | 1662 (175, 923–3443) | 4 (3, 1–11) | 4198 (1127, 923–17,801) | 11 (14, 1–52) |
| Usual care | 1875 (287, 923–3947) | 5 (3, 1–13) | 4784 (1257, 1427–11,912) | 13 (13, 2–40) |
| All arms | 1794 (152, 671–6467) | 4 (4, 0–23) | 3942 (540, 671–17,801) | 11 (11, 0–52) |
Health status and quality-adjusted life-years
Data describing the completeness of the data for the EQ-5D-5L at the domain and tariff score level for all trial arms are presented in Table 72; the EQ-5D-5L tariff scores by trial arm are presented in Table 73. At baseline, across all four trial arms (n = 44), all five domains of the EQ-5D-5L were completed by all 44 (100%) participants, which enabled the tariff score to be elicited. At pre discharge, 30 days and 90 days, all five domains of the EQ-5D-5L were completed by 27 (61%), 39 (89%) and 38 (86%) participants respectively.
| Time point | Participants completing domain, n (%) | |||||
|---|---|---|---|---|---|---|
| Mobility | Self-care | Usual activities | Anxiety/depression | Pain/discomfort | Tariff score | |
| (1) Baseline | 44 (100) | 44 (100) | 44 (100) | 44 (100) | 44 (100) | 44 (100) |
| (2) Pre discharge | 27 (61) | 27 (61) | 27 (61) | 27 (61) | 27 (61) | 27 (61) |
| (3) 30 days | 40 (91) | 39 (89) | 40 (91) | 40 (91) | 40 (91) | 39 (89) |
| (4) 90 days | 38 (86) | 38 (86) | 38 (86) | 38 (86) | 38 (86) | 38 (86) |
| Trial arm | EQ-5D-5L tariff score, mean (SD, range) | |||
|---|---|---|---|---|
| (1) Baseline | (2) Pre discharge | (3) 30 days | (4) 90 days | |
| Hospital and home EPR (n = 5) | 0.74 (0.17, 0.58–0.94) | 0.68 (0.3, 0.31–1) | 0.77 (0.14, 0.57–0.89) | 0.69 (0.3, 0.27–0.92) |
| Hospital EPR (n = 4) | 0.65 (0.27, 0.29–0.87) | 0.66 (0.24, 0.31–0.82) | 0.60 (0.29, 0.19–0.83) | 0.56 (0.52, –0.22–0.84) |
| Home EPR (n = 7) | 0.44 (0.32, 0.07–0.9) | 0.63 (0.29, 0.22–1) | 0.63 (0.33, 0.14–1) | 0.57 (0.32, 0.14–0.95) |
| Usual care (n = 4) | 0.58 (0.19, 0.33–0.74) | 0.64 (0.23, 0.29–0.77) | 0.65 (0.34, 0.15–0.87) | 0.63 (0.49, –0.10–0.89) |
The EQ-5D-5L must be completed at least two time points to calculate the change in QALYs using the AUC method over the time horizon of the trial (in this case baseline and 90 days) using the observed data; this analysis could be performed on a maximum of 38 (86%) participants within the trial (Table 74). If the AUC analysis was based on the EQ-5D-5L tariff scores from all four time points for which the data were collected, the AUC analysis could be conducted on 20 (45%) participants. However, if the AUC analysis was based on the EQ-5D-5L tariff scores from three of the four time points (baseline, 30 days and 90 days), this analysis could be conducted on 34 (77%) participants.
| Trial arm | EQ-5D-5L tariff scores completed, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| (1) Baseline | (2) Pre discharge | (3) 30 days | (4) 90 days | (1) and (4) | (1), (3) and (4) | (1)–(4) | |
| Hospital and home EPR (n = 10) | 10 (100) | 6 (60) | 9 (90) | 8 (80) | 8 (80) | 8 (80) | 5 (50) |
| Hospital EPR (n = 10) | 10 (100) | 7 (70) | 8 (80) | 8 (80) | 8 (80) | 6 (60) | 4 (40) |
| Home EPR (n = 15) | 15 (100) | 9 (60) | 14 (93) | 14 (93) | 14 (93) | 13 (87) | 7 (47) |
| Usual care (n = 9) | 9 (100) | 5 (56) | 8 (89) | 8 (89) | 8 (89) | 7 (78) | 4 (44) |
| Total | 44 (100) | 27 (61) | 39 (89) | 38 (86) | 38 (86) | 34 (77) | 20 (45) |
Mean (SD, range) EQ-5D-5L tariff scores by trial arm at all four time points based on those participants for whom data were collected at all four time points (n = 20) are presented in Table 74. Mean (SD; trial arm) EQ-5D-5L tariff scores ranged from 0.44 (0.32; home EPR) to 0.74 (0.17; hospital and home EPR) at baseline. At pre discharge, mean tariff scores were reasonably consistent across all four trial arms, with a utility value ranging from 0.63 (0.29; home EPR) to 0.68 (0.3; hospital and home EPR). At 30 days the tariff scores ranged from 0.60 (0.29; hospital EPR) to 0.77 (0.14; hospital and home EPR). At 90 days the tariff scores ranged from 0.56 (0.52; hospital EPR) to 0.69 (0.3; hospital and home EPR). Those in the both interventions trial arm consistently reported the highest mean utility score at all four time points. The lowest mean utility score was reported in the home EPR arm at baseline and pre discharge and in the hospital EPR arm at 30 days and 90 days.
Quality-adjusted life-years were calculated using the AUC method. Two methods of calculating QALYs were used, based on using EQ-5D-5L data at two time points (baseline and 90 days) and three time points (baseline, 30 days and 90 days); the QALY values are presented in Table 75. A two-sample t-test for unpaired samples assuming unequal variance was used to assess if there was any statistical difference between the two methods of eliciting QALYs. The t-test suggests that there are no statistically significant differences between the QALY values obtained using the two methods (see Table 75). However, it should be noted that, when using data at two time points, both the hospital EPR and home EPR intervention arms have lower mean QALYs than the usual care trial arm, whereas when using data at three time points the opposite is true and both the hospital EPR and home EPR arms have higher mean QALYs than the usual care arm. This is a point for discussion in relation to which method should be used for the exploratory analysis; however, for this study a decision was made to include the third time point as this allows a more detailed method to be used for calculating the AUC, despite the slightly smaller sample of people who reported this information.
| Trial arm | QALYs: two time points (n = 38), mean (SD, range) | QALYs: three time points (n = 34), mean (SD, range) | p-valuea |
|---|---|---|---|
| Hospital and home EPR | 0.1724 (0.05, 0.10–0.23) | 0.1739 (0.05, 0.07–0.22) | 0.9553 |
| Hospital EPR | 0.1243 (0.09, –0.00–0.21) | 0.1449 (0.07, 0.05–0.20) | 0.6451 |
| Home EPR | 0.1338 (0.06, 0.01–0.22) | 0.1489 (0.07, 0.03–0.23) | 0.5360 |
| Usual care | 0.1361 (0.07, 0.03–0.20) | 0.1386 (0.07, 0.02–0.23) | 0.9524 |
List of abbreviations
- 2RM
- maximal resistance against which participants were able to complete two pedal revolutions
- 6MWD
- 6-minute walk distance
- AE
- adverse event
- AECOPD
- acute exacerbation of chronic obstructive pulmonary disease
- AfC
- Agenda for Change
- AUC
- area under the curve
- AUH
- Aintree University Hospital
- BTS
- British Thoracic Society
- CAT
- COPD Assessment Test
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COM-B
- capability, opportunity, motivation and behaviour
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CrI
- credible interval
- CSRI
- Client Service Receipt Inventory
- CTRU
- Clinical Trials Research Unit
- DECAF
- Dyspnoea, Eosinopenia, Consolidation, Acidaemia and atrial Fibrillation
- DMEC
- Data Monitoring and Ethics Committee
- EPR
- early pulmonary rehabilitation
- EQ-5D-5L
- EuroQol-5 Dimensions five-level version
- ESD
- early supported discharge
- ETC
- excess treatment cost
- EVPI
- expected value of perfect information
- FEV1
- forced expiratory volume in 1 second
- GCS
- Glasgow Coma Scale
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICF
- International Classification of Functioning, Disability and Health
- IQR
- interquartile range
- ITT
- intention to treat
- LCADL
- London Chest Activity of Daily Living
- LOS
- length of stay
- MAU
- medical assessment unit
- MET
- metabolic equivalent of task
- MRC
- Medical Research Council
- MUST
- Malnutrition Universal Screening Tool
- NHSFT
- NHS Foundation Trust
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMES
- neuromuscular electrical stimulation
- NPT
- normalisation process theory
- OR
- odds ratio
- PPI
- patient and pubic involvement
- PR
- pulmonary rehabilitation
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RPE
- rating of perceived exertion
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SEM
- standard error of the mean
- SpO2
- blood oxygen saturation level
- STH
- Sheffield Teaching Hospitals
- TDF
- theoretical domains framework
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAT
- value-added tax
- WTP
- willingness to pay
