Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 15/17/02. The protocol was agreed in January 2016. The assessment report began editorial review in June 2016 and was accepted for publication in February 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Saramago et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Pregnant women who have a rhesus blood group (D antigen) (RhD)-negative blood type may carry a RhD-positive fetus. The presence of fetal RhD-positive cells in the maternal circulation can cause a mother who is RhD negative to produce anti-D antibodies against the RhD antigen. This process, called sensitisation, can happen at any time during pregnancy, although it is most common in the third trimester and during childbirth. Sensitisation can follow events in pregnancy known to be associated with fetal–maternal haemorrhage (FMH). Potentially sensitising events include some medical interventions (e.g. chorionic villus sampling, amniocentesis or external cephalic version), terminations, late miscarriages, antepartum haemorrhage and abdominal trauma.
The process of sensitisation itself has no adverse effects to the mother and does not usually affect the pregnancy during which it occurs. However, in a subsequent pregnancy with a RhD-positive fetus in women who have been sensitised to the RhD antigen, the woman’s anti-D antibodies may respond to the presence of RhD-positive blood in the fetus, resulting in haemolytic disease of the fetus and newborn infant. This can cause severe fetal anaemia, which may lead to fetal heart failure, fluid retention and swelling (hydrops) and intrauterine death.
Prophylaxis with anti-RhD immunoglobulin can substantially reduce the risk of sensitisation in RhD-negative women and the prevalence of haemolytic disease of the fetus and newborn infant. 1 Before anti-D immunoglobulin was available, the incidence of RhD sensitisation in RhD-negative women following the birth of two RhD-positive babies was approximately 16%. Haemolytic disease of the fetus and newborn infant was a significant cause of morbidity and mortality, which occurred in approximately 1% of all births. Since the introduction of routine postnatal administration of anti-D immunoglobulin, the incidence of RhD sensitisation dropped to approximately 2%. The introduction of routine antenatal prophylaxis during the third trimester of pregnancy has led to a further reduction in the sensitisation rate to between 0.17% and 0.28%. This has led to a decrease in mortality associated with haemolytic disease of the fetus and newborn infant, from 46 in 100,000 births before 1969 to 1.6 in 100,000 births by 1991. 2
In England, there were 646,904 births from April 2013 to March 2014, of which approximately 15% (97,036 births) were to RhD-negative women. 3 Approximately 40% of these women will carry a RhD-negative fetus (around 39,000 per year) and therefore do not need administration of anti-D immunoglobulin. White populations of European descent have an approximately 15% incidence of RhD negativity; however, this is 3–5% in populations of African American ethnicity and is very rare in those of Eastern Asian origin. 4 Despite the mixing of genes, the majority of RhD-negative white people are RhD negative a result of gene deletion, and RHD gene variants are relatively rare in white people, who account for < 1% of all RhD-negative people. However, in people with black African ethnicity, an inactive RHD gene (known as the RHD pseudogene RHDψ), which is mostly the result of genes that contain RhD sequences but do not produce the D antigen, is present in 66% of RhD-negative people. The distribution of this gene varies between people with black African ethnicity and people with other African origins,5 with 24% of people with African American ethnicity and 17% of people with black South African ethnicity having the gene. 6
Current service provision and care pathway
The National Institute for Health and Care Excellence (NICE) guideline on antenatal care (2008)7 recommends that women should be offered testing for blood group and rhesus D status in early pregnancy. All women identified as RhD negative will be tested for the presence of RhD antibodies, regardless of whether or not they are known to be sensitised. In those identified as RhD negative, administration of anti-D immunoglobulin is recommended both as prophylaxis and following potential sensitising events to prevent sensitisation. Routine antenatal prophylaxis with anti-D immunoglobulin can be given as two doses at weeks 28 and 34 of pregnancy or as a single dose between 28 and 30 weeks. 7 Following potentially sensitising events, anti-D immunoglobulin should be administered within 72 hours of the event. 2
Anti-D immunoglobulin is produced from pooled plasma from large numbers of RhD-negative donors who have been transfused with RhD-positive red cells to stimulate the production of RhD antibodies. Thus, it carries a risk of transmission of human blood-borne viral and prion diseases. Despite this risk, the National Comparative Audit of Blood Transfusion from 20138 reports that of the women eligible for anti-D immunoglobulin, 99.0% received anti-D immunoglobulin.
For pregnant women who are RhD negative and are sensitised to RhD antigen, the Royal College of Obstetricians and Gynaecologists has published guidance on the management of women with red cell antibodies during pregnancy. 9 This guideline recommends that all RhD-negative women who are sensitised to RhD antigen should attend pre-pregnancy counselling with a clinician who has knowledge and expertise of this condition, have their blood group and antibody status determined at the booking appointment (ideally by 10 weeks of gestation) and at 28 weeks of gestation and be offered non-invasive fetal RhD genotyping using maternal blood if maternal RhD antibodies are present. Once a RhD-positive fetus is identified, additional monitoring and treatment are required during the pregnancy.
Description of the technology under assessment
Summary of technologies (index tests)
The technology under assessment is high-throughput non-invasive prenatal testing (NIPT) for fetal rhesus D status (International Blood Group Reference Laboratory, NHS Blood and Transplant, Bristol, UK).
High-throughput NIPT of fetal RhD status uses a real-time quantitative polymerase chain reaction (PCR) method for predicting the fetal RhD genotype from fetal deoxyribonucleic acid (DNA) in the plasma of RhD-negative women. The test principle is based on the analysis of cell-free fetal DNA, that is, small fragments of fetal extracellular DNA shed from the placenta and circulating freely in the maternal plasma. The level of cell-free fetal DNA in maternal blood increases throughout the pregnancy. A woman who is RhD negative does not have a copy of the RHD gene; therefore, the presence of a RHD gene in a RhD-negative pregnant woman suggests a RhD-positive fetus.
High-throughput NIPT is performed using samples of maternal anticoagulated blood. DNA extraction is performed using an automated robotic platform, which can rapidly process samples. The robotic platform is used as a liquid handler to dispense samples and reagents. In the UK, primers and probes for specific exons of the RHD gene are used, with a number of controls being tested (such as RhD-positive DNA, RhD-negative DNA, RHD pseudogene positive DNA and no DNA). An algorithm is employed to determine the fetal RhD status. The samples can be tested in batches of between 32 and 88 samples. The time to complete the test from sample receipt to report generation is 5–6 hours.
High-throughput NIPT for fetal RhD status may enable anti-D immunoglobulin to be withheld from RhD-negative women who are carrying a RhD-negative fetus. These women could avoid unnecessary treatment with routine anti-D immunoglobulin, along with the potential risk associated with administration of blood products. In addition, these women may not need the provision of anti-D immunoglobulin following potentially sensitising events and there may no longer be a need for serological cord testing at birth.
Identification of important subgroups
There are potential challenges for the detection of fetal rhesus D status when performing NIPT in pregnant women. Dealing with the presence of RHD pseudogene poses a challenge. The majority of RhD-negative individuals with white European ethnicity have the pseudogene as a result of gene deletion; however, in people with African ethnicity the Rh-negative phenotype is mainly the result of genes that contain RhD sequences but do not produce D antigen (RHD pseudogene). 5 In the presence of the RHD pseudogene, prenatal determination of fetal Rh type from maternal blood would reveal a RhD-positive type, but this would be confirmed as RhD negative by serology because of the abundant maternal D gene sequences that are not expressed but are amplified. This may, therefore, lead to higher rates of false-positive results when performing NIPT in this population.
There is a diverse array of Rh variant genes and it is generally accepted that at least two exons of RHD should be targeted for accurate RhD status prediction. For instance, targeting only exon 7 (or exon 10) would not detect the presence of the RHD pseudogene and other variants and targeting only exon 10 would not detect the presence of the RHD pseudogene or the hybrid RHD-CE-D(s) gene, which are commonly present in people with African ethnicity.
Evidence suggests that the diagnostic accuracy of NIPT may vary according to different gestational ages at the time of sampling. Two meta-analyses found that the diagnostic accuracy of NIPT was higher in the first trimester than in the second and third trimester. 10,11 However, a recent UK cohort study found that fetal RhD genotyping was more accurate for the prediction of RhD status if it was performed after, rather than before, 11 weeks’ gestation. 12
In this assessment we aim to investigate findings of high-throughput NIPT from a number of subgroups, such as those based on different gestational ages and different ethnicities as well as on the usage of different exons of RHD, if data are available.
Current usage in the NHS
Currently, all high-throughput NIPT for fetal RhD status determination in the UK is performed by the NHS Blood and Transplant International Blood Group Reference Laboratory in Bristol. If all pregnant RhD-negative women in England were to be tested, approximately 100,000 samples would be tested each year. An increased capacity would be required for the International Blood Group Reference Laboratory to be able to cope with this demand by employing additional staff and acquiring more analytical platforms. Beyond this, extending the testing service to other laboratories is an alternative option. Blood samples would need to be transported from local hospital laboratories to the International Blood Group Reference Laboratory in Bristol or other laboratories. The established NHS Blood and Transplant transport system would be used to deliver blood samples across the country. This would need to be achieved in reasonable time, although there is evidence to suggest that cell-free fetal DNA is very stable. 13 There would also need to be reporting systems in place to ensure the accurate transmission of test results back to the women and their physicians and midwives.
Expected costs associated with technology
The potential costs associated with high-throughput NIPT to the NHS comprise two components. First, there is the unit cost of the diagnostic test itself, which varies with the level of throughput and to which a royalty fee may be added. An estimated unit cost for high-throughput NIPT of (confidential information has been removed) and a royalty payment of (confidential information has been removed) were considered. It should be noted that these estimates were provided in confidence by the company with the underlying assumption that the International Blood Group Reference Laboratory in Bristol will be the sole provider of the test nationally. Second, the potential costs of incorporating the test into routine antenatal care must be considered, which may bring additional costs relating to the time for antenatal care appointments to provide information about the test, counselling and delivering test results and also relating to blood drawing and blood sample transportation.
Chapter 2 Definition of the decision problem
Decision problem
The clinical effectiveness and cost-effectiveness of high-throughput NIPT for assessing fetal rhesus D status in RhD-negative women not known to be sensitised to the RhD antigen for the NHS is uncertain. High-throughput NIPT for fetal RhD status may enable anti-D immunoglobulin to be withheld from RhD-negative women who are carrying a RhD-negative fetus. This subgroup of women could therefore avoid unnecessary prophylaxis with anti-D immunoglobulin during pregnancy, as well as the risk associated with exposure to blood products, which may have important resource implications for the NHS.
However, relying on NIPT to determine anti-D immunoglobulin use could lead to more women becoming sensitised, because women who incorrectly test negative on NIPT will not receive anti-D and so are at increased risk of sensitisation. This risk will be increased if cord blood testing is also withdrawn and postpartum anti-D given on the basis of the NIPT results. It is also unclear whether or not the cost of instituting NIPT screening will outweigh the savings from the reduced use of anti-D treatment.
This report, undertaken for the NICE Diagnostics Assessment Programme, examines the clinical effectiveness and cost-effectiveness of high-throughput NIPT. It considers the value of NIPT as a diagnostic test for RhD status, the clinical impact of using NIPT to determine anti-D immunotherapy use and the cost implications of implementing a NIPT screening programme. The report will allow NICE to make recommendations about how well the high-throughput NIPT works and whether or not the benefits are worth the cost of the tests for use in the NHS.
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Overall aims and objectives of the assessment
The purpose of this project was to assess the clinical effectiveness and cost-effectiveness of using high-throughput NIPT to identify fetal RhD status with any consequent changes in treatment management. In this assessment we addressed the following key objectives:
-
to perform a systematic review and meta-analysis of the diagnostic accuracy of high-throughput NIPT for fetal RhD status
-
to perform a systematic review of the clinical impacts of high-throughput NIPT, including incidence of sensitisation events, and adverse effects to the mother and fetus
-
to systematically review the cost-effectiveness evidence on high-throughput NIPT and its impact on the management of pregnant women
-
to produce a de novo cost-effectiveness model assessing the cost-effectiveness of high-throughput NIPT to identify fetal RhD status in RhD-negative women not known to be sensitised to the RhD antigen
-
to assess the impact of alternative scenarios related to the timing of the test and the impact of the test on the use of antenatal anti-D prophylaxis for sensitising events and postdelivery testing.
This report is divided into two sections: clinical effectiveness (covering objectives a and b) is discussed in Chapter 3; and cost-effectiveness (covering objectives c–e) is discussed in Chapter 4.
Chapter 3 Assessment of clinical effectiveness
The review of clinical effectiveness of high-throughput NIPT was broken down into the following three systematic reviews:
-
A review of the diagnostic accuracy of high-throughput NIPT for detecting RhD-positive fetuses.
-
A review of the clinical effectiveness of high-throughput NIPT, including numbers of sensitisations, test compliance and incidence of adverse events.
-
A review of the implementation of high-throughput NIPT in countries or regions in which it has been used, examining feasibility, guidance or recommendations for practice and need for further research.
In addition to these three reviews, we searched for existing systematic reviews of antenatal anti-D prophylaxis, identifying numbers of sensitisations, compliance and incidence of adverse events. Data from these existing reviews then facilitated the modelling of the probable clinical impact of high-throughput NIPT and supported the subsequent cost-effectiveness analyses.
The methodology of these reviews is described in the following sections.
Methodology of the clinical effectiveness reviews
The methods for systematic reviews of the diagnostic accuracy and clinical impacts of high-throughput NIPT for fetal RhD status are provided in the following sections.
Searches
The literature search aimed to systematically identify studies relating to the clinical effectiveness and cost-effectiveness of high-throughput, non-invasive, prenatal blood testing to determine fetal rhesus D status.
The search strategy was developed in MEDLINE (via Ovid) and then adapted for use in the other resources searched. The strategy included terms for rhesus D status combined, using the Boolean operator AND, with terms for the test. No language, date or geographical limits were applied and study design search filters were not used. EndNote X7 software (Thomson Reuters, CA, USA) was used to manage the references for the project.
Search strategies were developed by an information specialist with input from the project team. The search strategy was checked by a second information specialist.
The following databases were searched for relevant clinical effectiveness or cost-effectiveness studies from inception to November 2015: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), EMBASE, Health Technology Assessment (HTA) database, Maternity and Infant Care, NHS Economic Evaluations Database (NHS EED), PubMed and the Science Citation Index.
In addition, the following resources were searched for ongoing, unpublished or grey literature: ClinicalTrials.gov, Conference Proceedings Citation Index: Science, EU Clinical Trials Register, PROSPERO and the World Health Organization’s International Clinical Trials Registry Platform portal.
The following websites were searched to identify any relevant guidelines: National Guidelines Clearinghouse, NICE, NHS Evidence, the Royal College of Obstetricians and Gynaecologists, the Turning Research into Practice database and the UK National Screening Committee. Reference lists of relevant reviews and included studies were checked to identify additional potentially relevant reports. The searches were updated in February 2016. A full search strategy can be found in Appendix 1.
Selection criteria
Types of studies
Diagnostic accuracy
Prospective cohort studies in which the index test (high-throughput NIPT) and reference standard test (cord blood sampling) were done independently in the same group of women to assess fetal RhD status were included. Included studies also had to report sufficient data to construct a 2 × 2 contingency table such that the cells in the table can be labelled as true positive, false positive, true negative and false negative.
Clinical effectiveness outcomes
Any experimental or observational study (controlled or non-controlled) was included provided that high-throughput NIPT was used to determine fetal RhD status and anti-D prophylaxis was given as required. Studies also had to report relevant clinical outcomes as listed in the following sections.
Implementation
Any publications discussing existing or experimental high-throughput NIPT screening programmes were included. Papers had to report issues related to the implementation of, or practical advice relating to, high-throughput NIPT as a screening tool to guide use of anti-D prophylaxis. This included publications that contained no numerical data but discussed practical issues of implementation, presented useful guidance or informed research recommendations.
Antenatal anti-D prophylaxis
Any systematic review reporting any aspect of the process of using routine antenatal anti-D to prevent sensitisation was included.
The following types of report were excluded: editorials and opinions, case reports and reports focusing only on technical aspects of the NIPT technology (such as technical descriptions of the testing process or specifications of machinery). Studies with a sample size of ≤ 10 were excluded. In the case of multiple reports for a given study or when the possibility of overlapping populations could not be excluded, the most recent or most complete reports were selected.
Population
For all reviews, the eligible population was pregnant women who were RhD negative and not known to be sensitised to RhD antigen.
Intervention
For all studies, high-throughput NIPT free-cell fetal DNA tests of maternal plasma used to determine fetal RhD status were eligible for inclusion. ‘High-throughput’ is a subjective concept and there is no clear consensus on its definition. For pragmatic reasons, we considered as high-throughput any NIPT that was conducted using an automated robotic platform (including automated DNA extraction and liquid handling) and that was able to process large numbers of samples rapidly for large-scale screening purposes. Studies in which this test was used for diagnosis (rather than screening) of sensitised women were excluded.
For clinical effectiveness studies, high-throughput NIPT had to be used to enable targeted anti-D prophylaxis.
Reference standard
For diagnostic accuracy studies, the reference standard considered was serological cord blood testing at birth or any other suitable postnatal blood test of the infant.
Outcomes
The following outcomes were included:
-
test accuracy, including sensitivity and specificity
-
number of inconclusive results, with reasons (e.g. no DNA detected)
-
number of pregnant women who accept the test
-
number of doses of anti-D immunoglobulin given (routine antenatal, following potentially sensitising events and postnatal)
-
uptake of anti-D (antenatal and postnatal) immunoglobulin
-
number of infections from anti-D immunoglobulin
-
number of sensitisations
-
number of cases of haemolytic disease of the fetus and newborn infant in subsequent pregnancies
-
adverse effects of testing
-
health-related quality of life.
At least two reviewers independently screened the titles and abstracts (if available) of all reports identified by the search strategy. Full-text copies of all studies deemed to be potentially relevant were obtained and two reviewers independently assessed them for inclusion. Any disagreements were resolved by consensus or by a third reviewer.
Data extraction
We selected the most recent or most complete report in cases of multiple reports for a given study or when we could not exclude the possibility of overlapping populations.
The data extraction forms were developed and piloted. One reviewer independently extracted details from full-text studies of study design, participants, index, comparator and reference standard tests and outcome data. The data extraction was checked by another reviewer. Any disagreements were resolved by consensus or by recourse to a third reviewer.
For studies reporting diagnostic data, we extracted the number of true positives, true negatives, false positives and false negatives for each index test evaluated in each study to construct 2 × 2 tables. If such data were not provided by the study authors, we attempted to contact them to construct the 2 × 2 table for the study population or the prespecified subgroups. Otherwise, we calculated the number of true positives, true negatives, false positives and false negatives from the summary estimates of sensitivity and specificity of the index test, if available. If reported, we extracted data on the number of undetermined or uninterpretable results. For studies in which only a subgroup of patients was included in the review, we extracted, analysed and presented data for this subgroup only. If some data were unclear or missing, we attempted to contact study authors to obtain additional data.
For studies reporting clinical outcomes, we extracted data as the numbers of women or fetuses experiencing the specified outcome. Mean differences, relative risks (RRs) or odds ratios [with 95% confidence intervals (CIs)] were extracted from comparative studies, when reported as unadjusted data.
For the implementation review, we summarised the findings and conclusions of the included publications using the following broad categories: study results and findings, issues for implementation, practical guidance and recommendations for research.
For the review of anti-D prophylaxis, we extracted summary results from syntheses or meta-analyses of studies on each clinical outcome reported. Mean differences, RRs or odds ratios with 95% CIs were extracted, when reported.
Critical appraisal
One reviewer independently assessed the quality of all included studies in terms of risk of bias. Risk of bias from diagnostic accuracy studies was assessed using a modified version of the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) checklist. 14 The QUADAS-2 tool was adapted to ensure that it is applicable to assessing the quality of studies of non-invasive prenatal tests for detecting rhesus D status. The QUADAS-2 tool consists of four key domains: (1) patient selection, (2) index test, (3) reference standard and (4) flow of patients through the study and timing of the index test(s) and reference standard. Each domain was assessed in terms of the risk of bias. The first three domains were also assessed for concerns regarding their applicability in terms of whether or not the participants and setting; the index test, its conduct or interpretation; and the target condition, as defined by the reference standard, were applicable to nationwide screening in the UK.
A Cochrane Risk Of Bias Assessment Tool: for Non-Randomised Studies of Interventions (ACROBAT-NRSI) was used to assess risk of bias for each outcome of all comparative studies reporting other eligible clinical outcomes. The quality assessment was checked by another reviewer. Any disagreements were resolved by consensus or by recourse to a third party.
The quality of the studies in the implementation review was not assessed, as there is no validated tool for assessing the quality of studies on the implementation of health interventions.
Methods of data synthesis
Using extracted diagnostic accuracy data from the 2 × 2 tables, estimates of sensitivity, specificity, false-positive rates (FPRs) and false-negative rates (FNRs) were calculated and presented on forest plots and in receiver operating characteristic (ROC) space to examine the variability in diagnostic test accuracy within and between studies. In the primary analysis, undetermined or uninterpretable results were counted as being test positive, in accordance with current practice.
The hierarchical bivariate model described by Reitsma et al. 15 was fitted, which calculates summary estimates of sensitivity, specificity, FPRs, FNRs and the associated 95% CIs. The hierarchical summary receiver operating characteristic (HSROC) model16 was fitted to produce summary ROC curves. Results of both models were presented in ROC plots.
Other eligible clinical outcomes were pooled if at least two studies reported on the same outcome and if data were reported consistently enough for analysis to be feasible. Otherwise, results were synthesised narratively. When meta-analyses were performed, data were pooled using standard random-effects DerSimonian and Laird meta-analyses. Analyses were conducted in R version 3 (The R Foundation for Statistical Computing, Vienna, Austria) and/or Stata® version 14 (StataCorp LP, College Station, TX, USA) software, as appropriate.
Investigation of heterogeneity
For diagnostic accuracy data, forest plots and ROC space were inspected to check for heterogeneity between study results. Subgroup analyses were conducted, when feasible, by performing separate bivariate and HSROC models in defined subgroups of studies.
If sufficient studies were available, we considered the following factors as potential sources of heterogeneity:
-
gestational age at time of NIPT
-
type of NIPT (e.g. test as used in Bristol vs. other)
-
ethnicity (e.g. European vs. African).
For other clinical outcomes, when possible, heterogeneity was assessed using the I2-statistic value and visual inspection of forest plots. Subgroup analyses and metaregression were used when feasible. Possible sources of heterogeneity were discussed and accounted for in the interpretation of the results.
Sensitivity analyses
We conducted sensitivity analyses (SAs) to explore:
-
the impact of including and excluding undetermined or uninterpretable NIPT results on the pooled test accuracy estimates
When participants from several studies were recruited from the same cohorts and significant overlap was suspected, data from only one study, with the most reliable reporting, were included in the main analyses.
Narrative synthesis
When quantitative synthesis and meta-analysis were not feasible, results for each study or systematic review were tabulated, categorised by outcome. For the review of implementation, we performed a narrative review of the findings of each included study, summarising their conclusions in terms of study findings, issues for implementation, practical guidance and recommendations for research.
Simulation study of clinical effectiveness
During the course of this report we found very little evidence on the probable clinical effectiveness of high-throughput NIPT and its impact on future sensitisation rates and adverse events. In order to investigate these issues, we opted to perform a simulation study to simulate possible outcomes of high-throughput NIPT in the UK, based on results from the diagnostic accuracy review and the results of published systematic reviews of antenatal anti-D prophylaxis and relevant audit data identified through additional literature searches.
The simulation sought to estimate the following in the UK population:
-
rates of women with a RhD-positive fetus
-
rates of women with positive/negative/inconclusive NIPT results
-
rates of women who receive NIPT and/or antenatal anti-D prophylaxis
-
number of sensitisations
-
number of adverse effects on fetuses in subsequent pregnancies.
Data were extracted from the diagnostic accuracy review, existing systematic reviews of antenatal anti-D prophylaxis and other primary sources, when necessary.
We considered the following clinical scenarios:
-
no antenatal anti-D and postpartum anti-D based on cord blood serology only (control)
-
antenatal anti-D offered to all RhD-negative women (current practice)
-
antenatal anti-D offered based on NIPT and postpartum anti-D based on cord blood test for all RhD-negative women
-
antenatal and postpartum anti-D offered based on NIPT only. No cord blood testing.
Scenario 3 is equivalent (in clinical outcomes) to performing cord blood testing on women with negative NIPT but offering postpartum anti-D to all test-positive women without cord blood testing. Scenario 4 is equivalent (in clinical outcomes) to withdrawing cord blood testing and postpartum anti-D for women with negative NIPT but offering cord blood testing and postpartum anti-D (if needed) to all test-positive women.
A Monte Carlo simulation of 10 million women was performed in R. Monte Carlo analysis is a modelling method that uses random number generation to simulate the running of multiple scenarios to define all potential outcomes of an event. We compared the amount of antenatal anti-D prescribed, the level of unnecessary anti-D use and the relative numbers of sensitisations and other adverse outcomes for each scenario.
Clinical effectiveness results
This chapter is structured as follows. The next section provides information on the quantity of research available, including characteristics and risk of bias of the included studies. This is then followed by the results sections with diagnostic accuracy, clinical effectiveness and implementation of high-throughput NIPT presented separately.
Quantity and quality of research available
Number of studies included
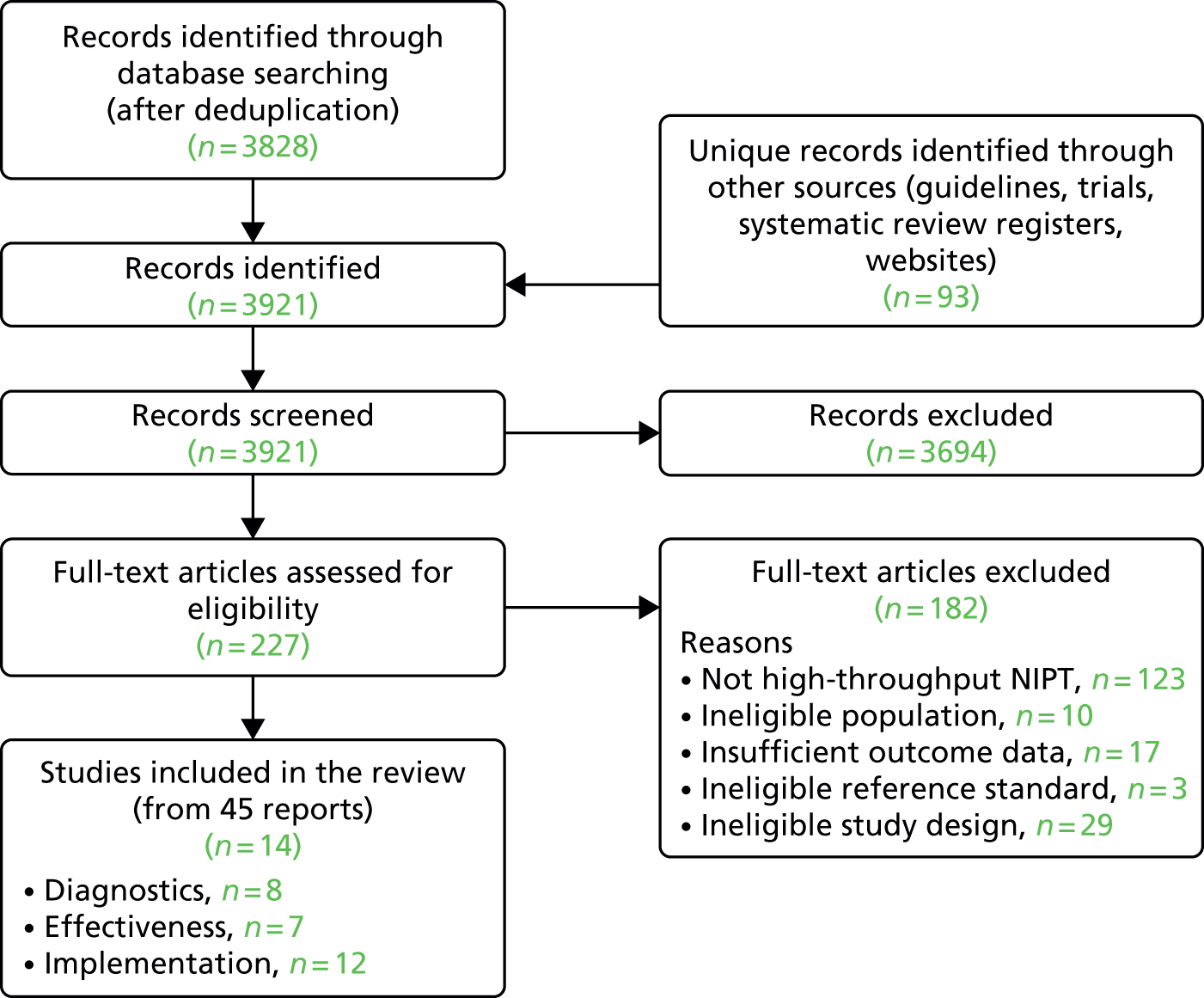
The literature searches of bibliographic databases identified 3921 references. After initial screening of titles and abstracts, 227 were considered to be potentially relevant and were ordered for full-text paper screening. In total, eight studies12,17–23 were included in the diagnostic review of high-throughput NIPT, seven studies18,20,22,24–27 were included in the clinical effectiveness review and 12 studies13,17,18,20–28 were included in the review of implementation of high-throughput NIPT (with some overlap between studies). Figure 1 shows a flow diagram outlining the screening process with reasons for exclusion of full-text papers.
FIGURE 1.
Flow diagram: study selection process.
All studies except two8,28 were cohort studies. Most cohorts were reported in several papers and abstracts, with considerable overlaps in data and reporting. For each cohort and each review we selected the paper with the most up-to-date and complete data. Consequently, some papers were included in more than one review and some papers (mostly conference abstracts with limited or outdated data) were not included in any analysis. Table 1 presents an overview of these cohort studies, the publications associated with each cohort and in which review the publications were included. Appendix 2 presents a list of all included references.
| Cohort (country) | Number of full-text papers | Number of conference abstracts | Papers included in review | |||
|---|---|---|---|---|---|---|
| Diagnostic accuracy (full-text paper) | Clinical effectiveness (full-text paper) | Implementation (full-text paper) | Linked conference abstracts | |||
| UK (Bristol) | 3 | 6 | Chitty et al., 2014;12 Finning et al., 2008;17 and Soothill et al., 201518 | Soothill et al., 201518 | Finning et al., 2008;17 and Soothill et al., 201518 | Chitty et al., 2011;29 Chitty et al., 2012;30 Daniels et al., 2012;31 Finning et al., 2015;32 Finning et al., 2014;33 and Ford and Soothill, 201634 |
| UK (London) | 2 | 0 | Akolekar et al., 201119 | None | Oxenford et al., 201328 | None |
| Denmark | 4 | 5 | Banch Clausen et al., 201420 | Banch Clausen et al., 2014;20 Banch Clausen et al., 2012;24 and Damkjaer et al., 201227 | Banch Clausen et al., 2014;20 Banch Clausen et al., 2012;24 Clausen et al., 2013;13 and Damkjaer et al., 201227 | Banch Clausen 2012;35,36 Dziegiel 2012;37 Banch Clausen et al., 2011;38 and Steffensen et al., 201239 |
| The Netherlands | 2 | 10 | Thurik et al., 201521 | de Haas et al., 201225 | de Haas et al., 2012;25 and Thurik et al., 201521 | Veldhuisen et al., 2014;40 Veldhuisen et al., 2013;41 Thurik et al., 2014;42,43 Scheffer et al., 2013;44 van der Schoot et al., 2005;45 de Haas et al., 2012;46 de Haas et al., 2013;47 Grootkerk-Tax et al., 2006;48 and van der Ploeg et al., 201549 |
| Spain | 1 | 0 | Grande et al., 201322 | Grande et al., 201322 | Grande et al., 201322 | None |
| Sweden | 2 | 10 | Wikman et al., 201223 | Tiblad et al., 201326 | Wikman et al., 2012;23 and Tiblad et al., 201326 | Wikman et al., 2012;50 Wikman et al., 2011;51 Wikman 2013;52 Wikman et al., 2010;53 Tiblad et al., 2010;54 Tiblad et al., 2012;55 Neovius et al., 2014;56 Tiblad 2012;57 and Neovius et al., 201658 |
| Total | 8 | 7 | 12 | 31 | ||
Excluded studies
A list of full-text papers that were excluded, along with the reasons for their exclusion, is given in Appendix 3. These papers were excluded because they failed to meet one or more of the inclusion criteria in terms of the type of study, participants, test, reference standard or outcomes reported.
Results: assessment of diagnostic accuracy
Characteristics of the included studies
Table 2 presents the summary information of characteristics of the included diagnostic accuracy studies. There were eight studies12,17–23 for the diagnostic review. All the studies were prospective studies and were conducted in European countries. Four studies were conducted in England,12,17–19 three of which were based in Bristol. 12,17,18
| Study | Location | DNA extraction tool | Gestational age (weeks) at time of NIPT, median (range) | Sample sizea | RhD-positive fetuses | RhD-negative fetuses | Inconclusive test results |
|---|---|---|---|---|---|---|---|
| Akolekar et al., 201119 | UK (London) | MDx BioRobot (Qiagen, Crawley, UK) | 12.4 (11–14) | 586 | 410 | 176 | 84 |
| Banch Clausen et al., 201420 | Denmark | QIAsymphony SP (Qiagen, Hilden, Germany); MagNA Pure LC (Roche Ltd, Rotkreuz, Switzerland); MagNA Pure Compact Instrument (Roche Ltd, Rotkreuz, Switzerland) | 25 (23–28) | 12,668 | 7830 | 4838 | 274 |
| Chitty et al., 201412 | UK (Bristol) | MDx BioRobot (Qiagen, Crawley, UK) | 19 (5–35) | 4913 | 2890 | 2023 | 393 |
| Finning et al., 200817 | UK (Bristol) | MDx BioRobot (Qiagen, Crawley, UK) | 28 (8–38) | 1869 | 1156 | 713 | 64 |
| Grande et al., 201322 | Spain | COBAS® AmpliPrep (Roche Ltd, Rotkreuz, Switzerland) | 24–26 | 282 | 186 | 96 | NR |
| Soothill et al., 201518 | UK (Bristol) | MDx BioRobot (Qiagen, Crawley, UK) | 15–17 (mostly) | 499b | 315 | 184 | 61 |
| Thurik et al., 201521 | The Netherlands | MagNa Pure 96 (Roche Ltd, Rotkreuz, Switzerland) | 26 | 18,383b | 11,283 | 7100 | NR |
| Wikman et al., 201223 | Sweden | MagNA Pure LC (Roche Ltd, Rotkreuz, Switzerland) | 8–40 | 3291c | 2073 | 1218 | 13 |
The sample size (number of patients/samples analysed) of studies ranged from 282 to 18,383. Most studies recruited pregnant women with a median gestational age of 10–28 weeks. Most participants were of white European ethnicity. All studies used maternal plasma as their sample source. A robotic DNA extraction instrument was employed in all studies. The studies used a number of robotic platforms such as MDx BioRobot (Qiagen, Crawley, UK), MagNa Pure 96 (Roche Ltd, Rotkreuz, Switzerland), MagNA Pure LC (Roche Ltd, Rotkreuz, Switzerland) and COBAS® AmpliPrep (Roche Ltd, Rotkreuz, Switzerland). For PCR, all studies targeted at least two exons (generally exons 5 and 7) and used at least two controls for RHD assay (RhD-positive DNA and RhD-negative DNA) except for the study by Wikman et al. ,23 which targeted only exon 4 and used glyceraldehyde 3-phosphate dehydrogenase (GAPDH) DNA as a control. The reference standard used in all studies was cord blood serology, except for Akolekar et al. ,19 which did not describe the reference standard. Inconclusive results were reported in all but two studies. 21,22 Appendix 4 presents further details of included studies.
Risk of bias of the included studies
Each of the eight full-text papers was assessed for risk of bias using a modified version of the QUADAS-2 tool containing 14 items. Table 3 presents a summary of the results for the risk of bias across all studies in the four main domains: patient selection, index test, reference standard, and flow and timing. Appendix 5 presents results of quality assessment for the individual studies. Despite some gaps in reporting, most studies were considered to have a low risk of bias for these four domains. NIPT as an automated procedure was deemed to have a limited risk of human error, and multiple controls were used for RHD assays in all studies except one. 23 Cord blood serology was the reference standard in all studies. The index test of NIPT was conducted independently of the reference standard and the results of one were considered unlikely to influence the results of the other, so the risk of incorporation bias was considered low.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Akolekar et al., 201119 | High | High | Unclear | Unclear | High | Low | Unclear |
| Banch Clausen et al., 201420 | Low | Low | Low | Low | Unclear | Low | Low |
| Chitty et al., 201412 | Low | Low | Low | Low | Low | Low | Low |
| Finning et al., 200817 | Low | Low | Low | Low | Low | Low | Low |
| Grande et al., 201322 | Low | Low | Low | Low | Low | Low | Low |
| Soothill et al., 201518 | Low | Unclear | Low | Low | Low | Low | Low |
| Thurik et al., 201521 | Low | High | Low | High | Low | Low | Low |
| Wikman et al., 201223 | Low | Low | Low | Low | Unclear | High | Low |
It appears that most studies prospectively recruited consecutive samples from clinical practice. Only three studies stated that multiple pregnancies were included. 17,22,23
Multiple pregnancies can pose specific challenges for NIPT (e.g. twin fetuses may have discordant RhD status). Excluding them from the analyses may have introduced patient selection bias, although it was deemed unlikely that this bias would substantially affect diagnostic accuracy estimates. Only three studies stated that their diagnostic threshold was prespecified during the conduct of the screening programme. 12,17,20
None of the studies reported whether or not there were any adverse events from the index test or reference standard.
Two studies19,21 were judged as having a high risk of bias. Akolekar et al. 19 stated that the targeted RhD-negative women were selected from a database; however, it was unclear whether or not this selection was performed on a random basis. The study recruited a large proportion of people with African ethnicity (19.3%), and so it may not be representative of the general population of pregnant women in the UK. This, combined with the fact that RHD variant analyses were not performed, may have contributed to the larger than average proportion of inconclusive results (15%). Akolekar et al. 19 excluded inconclusive results from their analyses, thereby potentially inflating their diagnostic accuracy estimates. Characteristics of the reference standard were also poorly reported.
Thurik et al. 21 excluded multiple pregnancies from their analysis and only 80% of participants received a reference standard. Reasons why cord blood serology was not performed in a significant proportion of the study population were not reported. The study also stated that their prediction algorithm was judged daily and adjusted as needed, and it was likely that this introduced bias in the diagnostic accuracy estimates (the authors reported the estimated impact of these changes on their diagnostic accuracy results).
The results of the studies were considered broadly applicable to the use of high-throughput NIPT for nationwide screening purposes in the UK, except for two studies. 19,23 The test used by Wikman et al. 23 targeted only exon 4, unlike all other included studies, which targeted at least two exons (5, 7 and/or 10). It is generally advocated that a combination of exons 5 and 7 is targeted to discriminate the pseudogene RHDφ, which is particularly present in individuals of African origin. 6,59 In addition, most participants in Wikman et al. 23 received NIPT in the first trimester of pregnancy. There is evidence to suggest that NIPT is less accurate before around 11 weeks’ gestation. These potential issues may have negatively affected the diagnostic accuracy of the test. Although it was a UK study, Akolekar et al. 19 recruited a significantly higher proportion of patients with African ethnicity (19.3%) than the population of pregnant women in the UK (3%). 60 As patients with black African ethnicity may be harder to diagnose, because of the high prevalence of RHDφ in this population, this may limit the applicability of the study’s findings to the UK population of pregnant women.
Overall, the majority of included studies were judged as having a low risk of bias, but two studies, Akolekar et al. 19 and Thurik et al. ,21 were judged as having a high risk of bias.
Meta-analyses of diagnostic accuracy
This section presents the results of the meta-analyses of the diagnostic accuracy studies. One key issue when considering the diagnostic accuracy of NIPT is how women with inconclusive test results are handled. It is expected that, in the UK, such women will be treated as having a positive test with no further testing. Although this was the policy in the three high-quality studies performed in Bristol, data on inconclusive tests were not reported in two studies. 21,22
Given these differences we considered four approaches to the diagnostic analysis:
-
women with inconclusive tests treated as test positive (including Thurik et al. 21 and Grande et al. 22 studies)
-
women with inconclusive tests treated as test positive (excluding Thurik et al. 21 and Grande et al. 22 studies)
-
excluding all women with inconclusive test results
This last analysis is likely to represent the most plausible results for UK practice, assuming that the methods used in Bristol are retained nationwide.
In all analyses, women whose NIPT was conducted at or before 11 weeks’ gestation were excluded when possible because of concerns that the diagnostic accuracy is poorer before 11 weeks and that the test should not be conducted before then (see Subgroup analyses). Some tests were performed between 8 and 11 weeks’ gestation in two studies,17,23 most women were tested between 8 and 12 weeks’ gestation in Wikman et al. 23 and < 8% of tests were performed before 11 weeks in Finning et al. ,12 but it was not possible to remove those women from the analysis.
In diagnostic analyses it is conventional to report results in terms of sensitivity (women who correctly test positive) and specificity (women who correctly test negative). NIPT is highly accurate and the focus should be on women with an incorrect test result, so in these analyses results are presented in terms of the FPRs (women incorrectly testing positive and so offered unnecessary anti-D) and FNRs (women incorrectly testing negative and so at risk of sensitisation, as they do not receive anti-D treatment).
A summary of all the results of the bivariate meta-analyses of FPRs and FNRs is presented in Table 4.
| Analysis case | Number of studies | FNR (at risk of sensitisation) | FPR (unnecessary anti-D) | ||
|---|---|---|---|---|---|
| Estimate (%) | 95% CI | Estimate (%) | 95% CI | ||
| Inconclusive tests treated as test positive (including Thurik et al.21 and Grande et al.22) | 8 | 0.34 | 0.15 to 0.76 | 3.86 | 2.54 to 5.82 |
| Inconclusive tests treated as test positive (excluding Thurik et al.21 and Grande et al.22) | 6 | 0.38 | 0.15 to 0.94 | 4.37 | 2.79 to 6.78 |
| Excluding all women with inconclusive test results | 8 | 0.35 | 0.15 to 0.82 | 1.26 | 0.87 to 1.83 |
| Studies conducted in Bristol only | 3 | 0.21 | 0.09 to 0.48 | 5.73 | 4.58 to 7.16 |
It can be seen that results are broadly consistent across the four scenarios. NIPT is very accurate among women with a RhD-positive fetus: only 2–4 in 1000 of such women will have a negative test result and so be at risk of sensitisation as a result of not being offered anti-D. NIPT is slightly less accurate among women with a RhD-negative fetus: between 1.3% and 5.7% of such women will test positive (depending on the analysis performed) and so may be offered NIPT unnecessarily. If women with inconclusive test results are excluded from analyses, the FPR was 1.3%, rising to 3.9–4.4% if women with inconclusive test results are treated as having tested positive. This suggests that the main cause of test error is treating women with an inconclusive NIPT result as if they had tested positive.
Assuming that 60% of RhD-negative women have a RhD-positive fetus, about 0.5% of women have a conclusive, but incorrect, positive test result. About 0.1–0.2% of women have a false-negative test result.
We consider the results of each analysis in more detail in the following sections.
Considering inconclusive results as test positive
Figure 2 shows forest plots of FNRs and FPRs when counting an inconclusive test result as being test positive. The results of these figures are slightly different from those in Table 4, because the figure shows separate analyses of FPR and FNR, rather than a full bivariate analysis.
FIGURE 2.
Forest plots of (a) FNR and (b) FPR when counting an inconclusive test result as being test positive.


There was some evidence of inconsistency across studies. The I2-statistic for heterogeneity was 75% for the FNR and 99% for the FPR. It should be noted that these high heterogeneities are, in part, a consequence of the high accuracy of the test and the large size of the studies (and consequent small within-study variance, because I2 increases as the average within-study variance declines). They do not necessarily indicate any clinically meaningful differences between studies. The heterogeneity in FPRs is likely to be a consequence of differing reporting and handling of inconclusive tests.
Figure 3 shows the results of each study, the results of the bivariate analysis (black circle) and the summary HSROC curve (black curve) for this analysis. As for other analyses, this is presented in terms of FPR and FNR rather than sensitivity and specificity. This plot shows the consistency of false-negative results, except for two outlying studies. 19,23 The Wikman et al. 23 study performed most NIPT in the first trimester, earlier than other studies. As discussed later (see Subgroup analyses), the timing of NIPT may have an impact on the FNR. The studies are less consistent in FPRs. This is most probably because the studies have different numbers of inconclusive test results and different methods of handling such results. As women with an inconclusive result are treated as RhD positive, women with an inconclusive result but a RhD-negative fetus will have a false-positive result. There may also be some heterogeneity because of differences in the threshold used and how different testing machines operated.
FIGURE 3.
Hierarchical summary receiver operating characteristic and bivariate analysis when counting an inconclusive test result as being RhD positive.

When excluding the two studies that did not report numbers of inconclusive tests,21,22 the results were broadly similar, as seen in Table 4. The forest plots of FPR and FNR for this analysis are given in Appendix 6.
Excluding inconclusive results
We considered the diagnostic accuracy of NIPT, excluding all inconclusive test results, to identify the ‘optimal’ diagnostic accuracy in which a test result is obtained for every woman. This analysis excluded women who were difficult to diagnose, so it may overestimate diagnostic accuracy. Forest plots for FNR and FPR are shown in Figure 4.
FIGURE 4.
Forest plots of (a) FNR and (b) FPR excluding women with inconclusive test results.

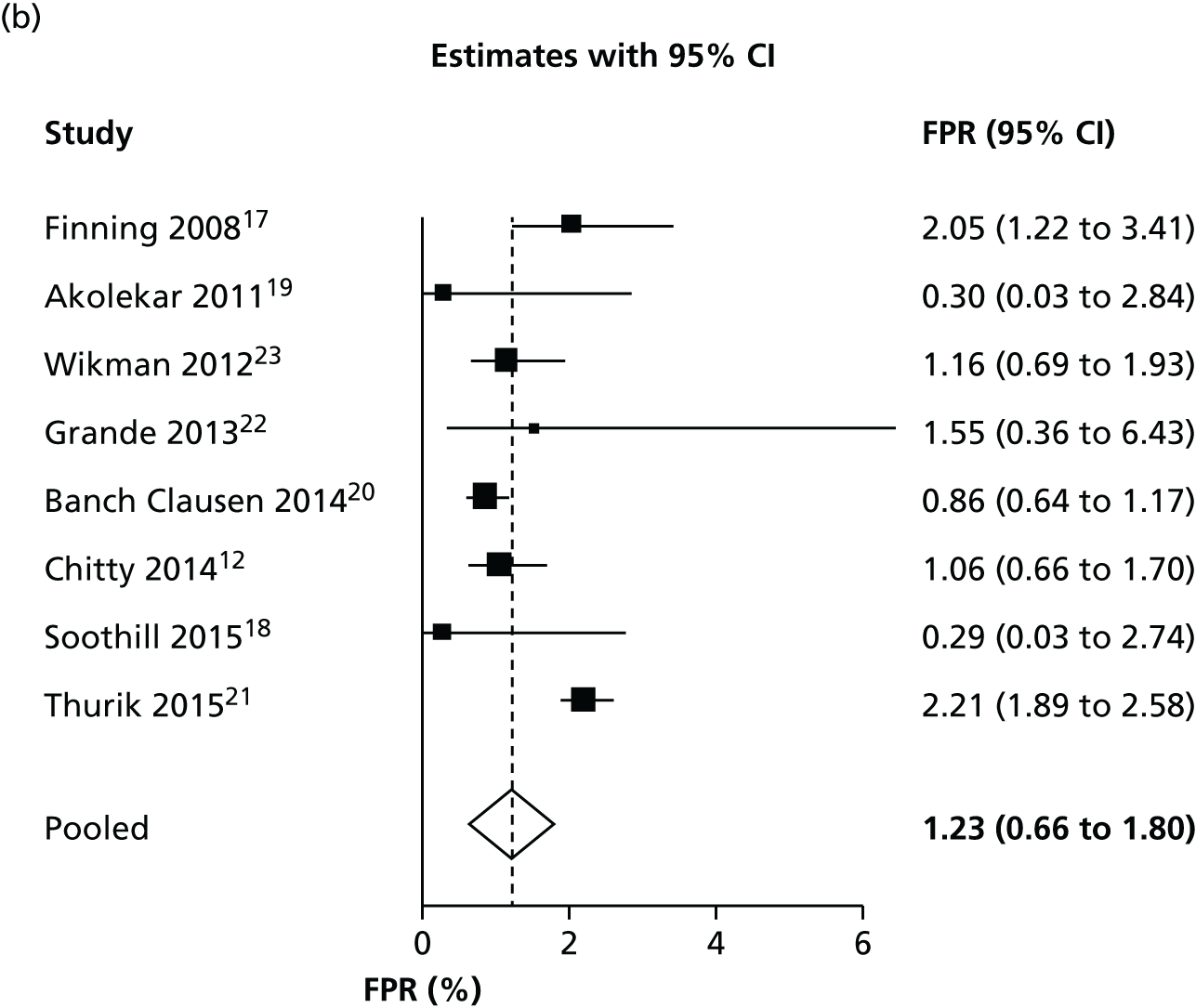
Excluding women with inconclusive test results has no meaningful impact on false-negative results (as those women are always assumed to have a positive result). It does, however, considerably reduce the FPR. The FPR, at 1.2%, is low but still considerably higher than the FNR. This suggests that NIPT is more accurate in women with a RhD-positive fetus than in those with a RhD-negative fetus. There was some evidence of heterogeneity across studies. The I2-statistic for heterogeneity was 75% for the FNR and 99% for the FPR. The ROC plot with bivariate and HSROC analyses is given in Appendix 6.
Bristol studies
We performed a subgroup meta-analysis of only the high-quality studies based in Bristol12,17,18 in order to assess the most likely performance of NIPT in the UK. We excluded the study by Akolekar et al. 19 (based in London but with NIPT run in Bristol) from this analysis on the grounds of it having a high risk of bias, as it was not primarily intended to assess NIPT screening and because of the limited applicability of recruited participants. A higher proportion of people with African ethnicity (19.3%) in this study means that it may not be representative of the general population of pregnant women in the UK.
In this analysis, women with an inconclusive test result were treated as having a positive result, in line with the practice in the studies.
As observed in Table 4 and Figure 5, the three Bristol studies have a slightly lower FNR and a higher FPR than other studies. This suggests that the Bristol high-throughput NIPT approach in which the MDx Bio Robot machine is used may be using a different test threshold from other countries, which further minimises false-negative findings, with a consequent increase in the FPR. This may explain some of the heterogeneity observed in previous analyses.
FIGURE 5.
Forest plots of (a) FNR and (b) FPR for the Bristol studies.
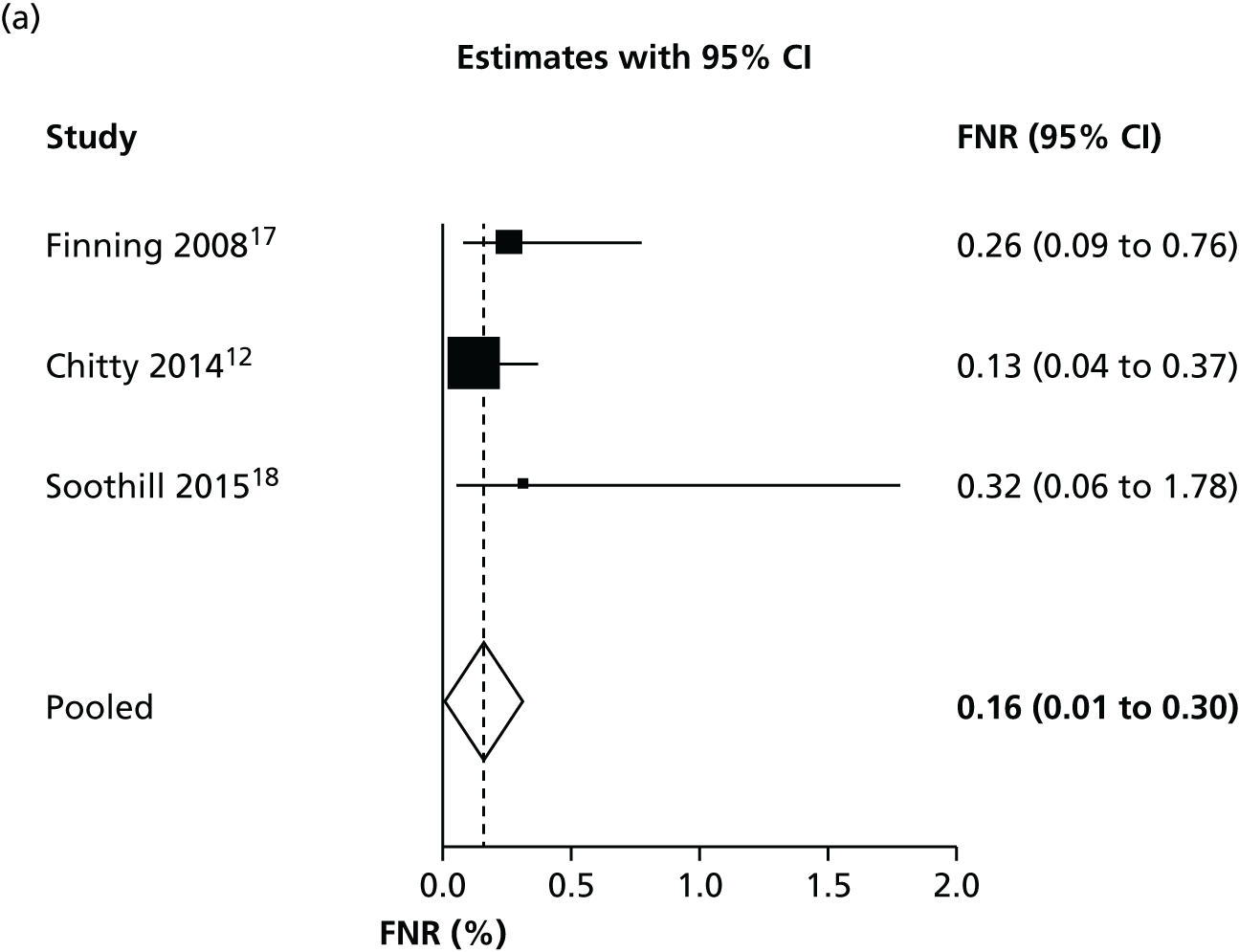
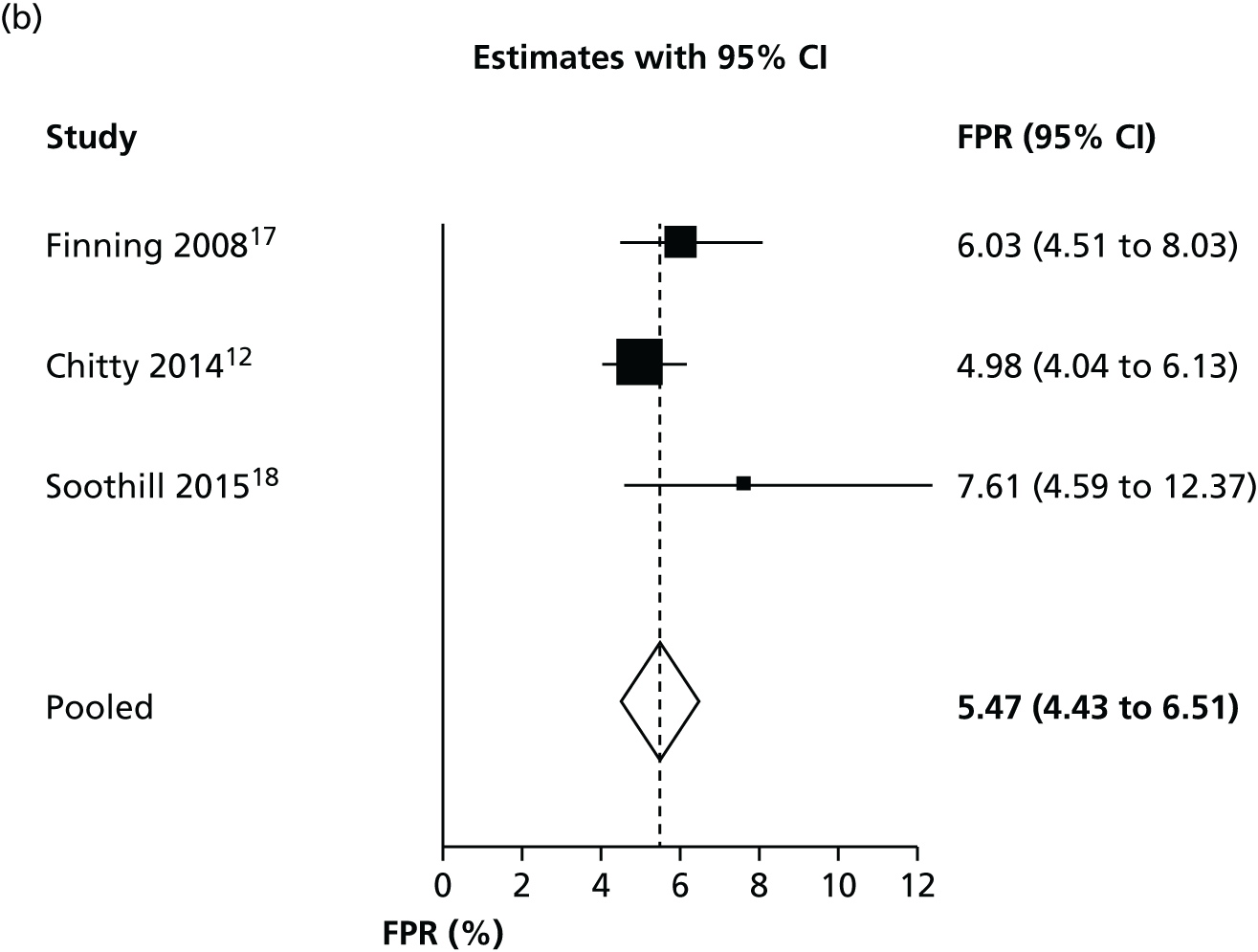
If inconclusive tests results were excluded from the Bristol studies, the summary FNR was 0.263% (95% CI 0.13% to 0.56%) and the FPR was 1.474% (95% CI 0.82% to 2.63%). This confirms that most false-positive results arise from treating women with an inconclusive test result as being test positive.
Inconclusive test results
As seen in Table 4, treating women with inconclusive test results as if they had a positive test has a substantial impact on diagnostic accuracy. Knowing the incidence of inconclusive test results is therefore important when determining diagnostic accuracy. Table 5 summarises the rates of and reasons for inconclusive test results across included studies. When reported, the most common reasons for inconclusive results were the presence of a maternal/fetal RHD variant.
| Study | Location | RhD-positive fetuses (%) | Inconclusive test results (%) | RhD-positive fetuses in women with inconclusive test results (%) | Reported reasons for inconclusive results (number of cases) |
|---|---|---|---|---|---|
| Akolekar et al., 201119 | UK (London) | 70.0 | 14.3 | 85.7 | Insufficient DNA (n = 5); RHD variant (n = 44); NR (n = 40) |
| Banch Clausen et al., 201420 | Denmark | 61.8 | 2.2 | 66.8 | Maternal weak D (n = 93); maternal silent RHD variant (n = 38); high level of maternal background DNA (n = 29); technical problems (n = 19); maternal DVI (n = 14); weak PCR signal (n = 13); suspected maternal RHD positive (n = 3); no reported cause (n = 65) |
| Chitty et al., 201412 | UK (Bristol) | 58.8 | 7.0 | 76.6 | NR |
| Finning et al., 200817 | UK (Bristol) | 61.9 | 3.4 | 54.7 | Insufficient DNA (n = 30); suspected maternal RHD gene (n = 25); failure to extract DNA from plasma (n = 1) |
| Grande et al., 201322 | Spain | 66.0 | NR | NR | NR |
| Soothill et al., 201518 | UK (Bristol) | 63.1 | 12.2 | 77.0 | NR |
| Thurik et al., 201521 | The Netherlands | 61.4 | NR | NR | Maternal RHD variant (n = 55); fetal variant (n = 45); weak PCR signals (n = 70); incorrect blood sample (n = 11) |
| Wikman et al., 201223 | Sweden | 63.0 | 0.4 | 38.5 | RHD variant (n = 14); no second sample (n = 18, of which 13 were spontaneous abortions and miscarriages) |
These results show that there is considerable variation in the rates of inconclusive tests across studies. The most likely cause for this variability is differences in how NIPT was conducted (e.g. different numbers and types of exons considered). However, even in the studies in which tests were conducted in Bristol using the same test, there is considerable unexplained variation. Differences in the characteristics of study populations (e.g. different proportions of people of black African ethnicity) may also explain some of this variation.
We performed a meta-analysis to estimate average rates of inconclusive test results. The results of this analysis are shown in Table 6. Based on these results, we would estimate that 6.7% of women in the UK would have an inconclusive test result, but this is subject to considerable uncertainty.
| Studies included | Estimated inconclusive rate (%) | 95% CI (%) |
|---|---|---|
| All reporting inconclusive tests | 4.0 | 1.5 to 10.3 |
| Bristol studies12,17,18 only | 6.7 | 3.7 to 11.7 |
Table 5 also shows that, in general, most women with an inconclusive test result have a RhD-positive fetus (and it is more common than in the general population) and so treating all women with inconclusive test results is reasonable, if no further testing is possible. However, there are still many women with a RhD-negative fetus who would receive anti-D unnecessarily.
Subgroup analyses
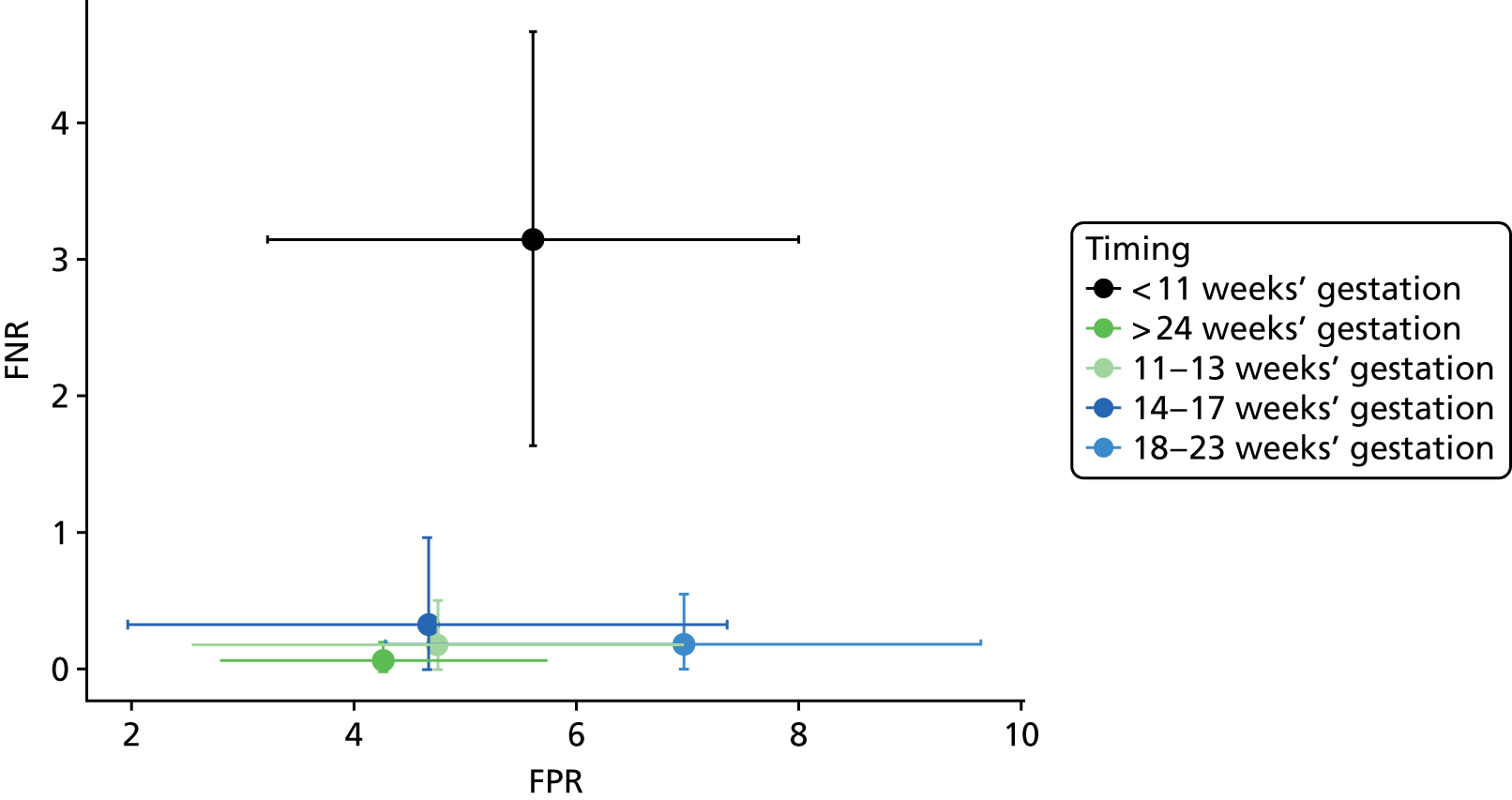
We considered the effect of the timing of NIPT on its diagnostic accuracy. Figure 6 shows the FNRs plotted by gestational age at time of high-throughput NIPT. It suggests that FNRs after 11 weeks’ gestation were consistent, irrespective of timing, but that FNRs were higher before 11 weeks’ gestation. Figure 7 shows the FPRs plotted by gestational age at time of high-throughput NIPT. There was no obvious pattern from this figure. Only one study12 examined test performance at multiple time points. Figure 8 shows the FPRs and FNRs at different times for this study. It indicates that FNRs were higher before 11 weeks’ gestation and were generally stable after 11 weeks’ gestation. We did not perform any formal statistical analyses on the timing data (such as a metaregression) because the relationship appears to be a step change in accuracy, rather than a linear trend over time. These results together suggest that NIPT is insufficiently accurate before around 11 weeks’ gestation (i.e. in first trimester) but is accurate at any time after the end of the first trimester.
FIGURE 6.
False-negative rate by gestational age at time of NIPT.

FIGURE 7.
False-positive rate by gestational age at time of NIPT.

We also considered the impact of the timing of high-throughput NIPT on the number of inconclusive test results (Figure 9). Despite the data from Wikman et al. 23 being heterogeneous, there appears to be a trend that the percentage of inconclusive results for this test reduces as the gestational age increases from 11 weeks. This is most obvious in the Chitty et al. 12 study, which reported numbers of inconclusive tests at different times.
FIGURE 9.
Inconclusive results by test timing.
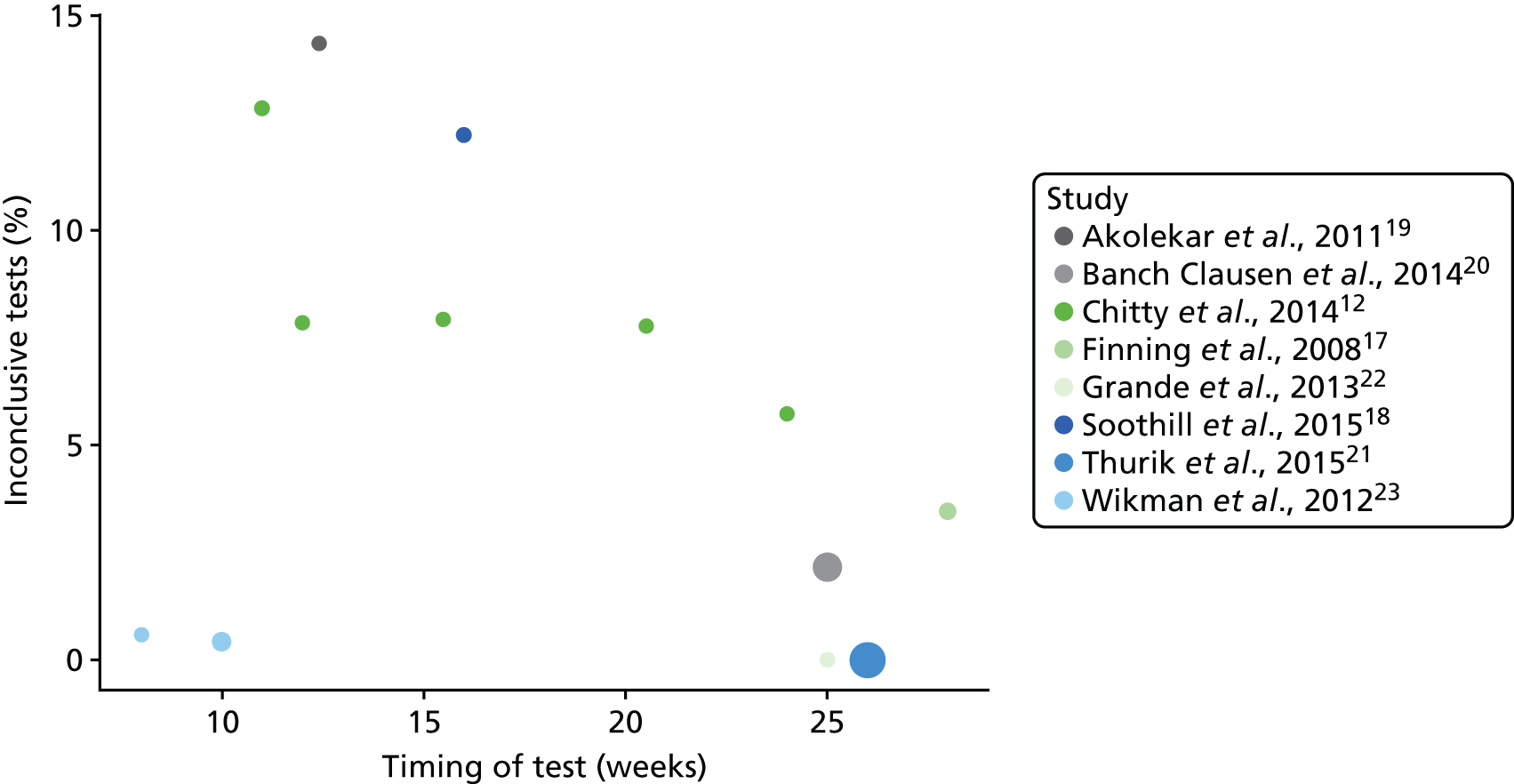
We were unable to conduct any subgroup analysis based on ethnicity, as the relevant data were not reported in any publication. As all studies were conducted in Europe, numbers of participants of non-white ethnicity were few. Any diagnostic analysis of non-white ethnicities may therefore not give reliable results.
Because each country used a different machine to perform NIPT, a subgroup analysis by type of NIPT method was not feasible, as it would be confounded by study location. We have considered a subgroup analysis including the Bristol-based studies only, as reported in Meta-analyses of diagnostic accuracy.
Sensitivity analyses
We performed two post hoc SAs. The first excluded the two studies considered to have a risk of bias19,21 and the second excluded the Wikman et al. 23 study, as this included a substantial number of women with NIPT performed before 11 weeks’ gestation. Bivariate meta-analyses as in Table 4 were performed excluding these studies. The results are presented in Appendix 6.
Excluding the two studies that were considered to have high risk of bias had limited impact on the FPRs and FNRs and does not alter any conclusions. Excluding the Wikman et al. 23 study marginally reduced the FNRs, which is consistent with the finding that the FNR is higher before 11 weeks’ gestation. It also slightly increased the FPR when counting inconclusive test results as positive. This is because there were few inconclusive tests in the Wikman et al. study (see Table 5). None of the SAs meaningfully alters any of the conclusions of these meta-analyses.
Results: assessment of clinical effectiveness
Characteristics of the included studies
Table 7 presents a summary of the characteristics of the seven studies included in the review of clinical effectiveness studies. All studies were observational and conducted in European countries, including Denmark, the Netherlands, Spain, the UK and Sweden. The sample size of studies ranged from 284 to 15,126. All participants were RhD-negative pregnant women and most participants were white European. Most studies recruited women with a gestational age median of 10–26 weeks. Three studies reported using routine antenatal anti-D prophylaxis (RAADP) at between 28 and 30 weeks.
| Study | Location | Study dates | Sample sizea | Gestational age at time of NIPT (weeks) | Routine antenatal anti-D prophylaxis | Comparator |
|---|---|---|---|---|---|---|
| Banch Clausen et al., 201420 | Denmark: one region | January–June 2010 | 591 | Median 25 | 250–300 µg at 29 weeks | Postnatal anti-D only (n = 109) |
| Banch Clausen et al., 201224 | Denmark: nationwide | January–June 2010 | 2312 | Median 25 | 250–300 µg at 29 weeks | None |
| Damkjaer et al., 201227 | Denmark: one hospital | June–September 2010 | 239 | Mean 27 | 250–300 µg at 29 weeks | None |
| de Haas et al., 201225 | The Netherlands: nationwide | July 2011–January 2012 | 15,126b | Mean 26 | 250 µg at 30 weeks and after birth | None |
| Grande et al., 201322 | Spain: Barcelona | February 2010–October 2011 | 284 | Range 24–26 | NR | None |
| Soothill et al., 201518 | England: three NHS trusts in south-west England | April–September 2013 | 529 | Range 15–26 | 500 or 1500 µg (timing NR) | None |
| Tiblad et al., 201326 | Sweden: Stockholm area | September 2009–March 2012 (reference cohort: 2004–8) | 8347c | Median 10 (range 3–40) | 250–300 µg at 28–30 weeks | Postnatal anti-D only (historical control) (n = 18,546) |
Only two studies compared women receiving NIPT to controls. 20,26 One study26 compared patients undergoing NIPT with routine management with no NIPT and routine postnatal anti-D prophylaxis only (historical control). The other comparative study20 reported data on anti-D compliance in a small subgroup of participants from one region in Denmark, comparing participants receiving NIPT with those receiving no NIPT.
Risk of bias of the included studies
The results of the quality assessment of the two comparative studies are given in Appendix 7. In summary, both studies had significant limitations. Tiblad et al. 26 was considered as having a serious risk of bias, primarily owing to concerns about patient selection, confounding and missing data. Banch Clausen et al. 20 was considered as having a critical risk of bias across all outcomes because of concerns about patient selection and lack of adjustment for potential confounders. The generalisability of these two studies to the UK context was limited given that participants in the control group did not receive RAADP.
The remaining five studies reported non-comparative effectiveness data for women receiving NIPT only. We did not perform a formal quality assessment of these studies for clinical effectiveness, as we considered the evidence from non-controlled studies to be of poor quality.
Results of studies on clinical effectiveness
Studies reported various clinical effectiveness outcomes, including sensitisation rate, NIPT uptake, rates of women receiving antenatal and postpartum anti-D prophylaxis and number of women avoiding unnecessary anti-D immunoglobulin use. We performed a narrative synthesis owing to the considerable heterogeneity in outcomes and study designs.
Sensitisations
One study reported data on the incidence of sensitisation (defined as having developed anti-D antibodies after the first trimester) and haemolytic disease of the newborn infant. Tiblad et al. 26 compared targeted routine antenatal anti-D in the first trimester with routine care (postnatal anti-D only, historical control) in the Stockholm region, Sweden. The study reported that the incidence of RhD sensitisation in the cohort that underwent high-throughput NIPT was 0.26% (95% CI 0.15% to 0.36%, n = 8347), compared with 0.46% (95% CI 0.37% to 0.56%, n = 18,546) in the historical control cohort. The absolute risk difference in the incidence of sensitisation was 0.20%. The high-throughput NIPT for targeted antenatal anti-D was associated with a significant risk reduction in sensitisation (unadjusted RR 0.55, 95% CI 0.35 to 0.87) compared with historical controls. An updated analysis by Neovius et al. 58 found an adjusted odds ratio of 0.41 (95% CI 0.22 to 0.87). In addition, this study reported one case of severe haemolytic disease diagnosed soon after birth in a nulliparous mother who did not receive routine anti-D prophylaxis.
Non-invasive prenatal testing uptake
Rates of NIPT uptake are presented in Table 8. Seven studies reported on uptake rates of NIPT screening. 18,20,22,25–27 Uptake rates ranged from 70% to > 95% across the studies. In the pilot study conducted by Soothill et al. 18 in three maternity services in the south west of England, only 70% of eligible women joined the study in the initial 6 months. The larger English study conducted by Chitty et al. 12 reported that 88% of the 3069 participants consented to receive RHD genotyping. The only country that reported nationwide NIPT screening uptake data was the Netherlands, where > 95% of eligible women underwent fetal RHD genotyping. The studies generally noted that uptake is likely to increase over time if a nationwide screening programme is implemented.
| Study | Country | Rates of NIPT uptake, % (n/N) |
|---|---|---|
| Banch Clausen et al., 201420 | Denmark | 84.2 (581/690) |
| Chitty et al., 201412 | England | 88 (372/3069) |
| Damkjaer et al., 201227 | Denmark | 90 (215/239) |
| de Haas et al., 201225 | The Netherlands | > 95 (15,126/approximately 15,750) |
| Grande et al., 201322 | Spain | 94 (284/302) |
| Soothill et al., 201518 | England | 70 (approximately) (numbers not reported) |
| Tiblad et al., 201326 | Sweden | 89 (8374/9380) |
Antenatal anti-D prophylaxis uptake
Rates of women receiving antenatal anti-D uptake according to NIPT uptake are presented in Table 9. Four studies reported uptake rates of RAADP in women who accepted NIPT and received a positive result, ranging from 86% to 96.1%. 20,26,27,49 One study reported nationwide data in women receiving RhD genotyping in the Netherlands, where 96.1% of approximately 18,383 women received antenatal prophylaxis anti-D. Tiblad et al. 6 reported a slightly lower rate, with 90% of 5104 women with a positive NIPT result receiving RAADP. Further data on uptake of RAADP in women who received a negative result (two studies),18,22 those who received an inconclusive result (one study)18 and those who refused NIPT (two studies)18,27 were limited. None of the included studies reported whether or not all women who received antenatal anti-D prophylaxis received the intended dosage at the intended time, or what proportion of women received additional anti-D owing to a potentially sensitising event.
| RAADP | % (n/N) | Source | Country |
|---|---|---|---|
| 1. Uptake of RAADP with no NIPT (current practice) | 99 (n = 5276) receiving at least one injection; 87.5% (n = 5276) receiving the correct dose at the correct time; 90%a (NR/5276) receiving all injections at correct doses | bUK anti-D audit8 | UK |
| 100 (10/10) | Soothill et al., 201518 | England | |
| 2. Uptake of RAADP in those who refuse NIPT | 0 (0/23) | Damkjaer et al., 201227 | Denmark |
| 80 (4/5) | Soothill et al., 201518 | England | |
| 3. Uptake of RAADP in those who accept NIPT and receive a positive result | 93.2 (330/354) | Banch Clausen et al., 201420 | Denmark |
| 86 (NR) | Damkjaer et al., 201227 | Denmark | |
| 90 (4590/5104) | Tiblad et al., 201326 | Sweden | |
| 96.1 (of approximately 18,383) | van der Ploeg et al., 201549 | The Netherlands | |
| 4. Uptake of RAADP in those who accept NIPT and receive an inconclusive result | 100 (5/5) | Soothill et al., 201518 | England |
| 5. Uptake of RAADP in those who accept NIPT and receive a negative result | 6 (1/18) | Soothill et al., 201518 | England |
| 5 (5/95) | Grande et al., 201322 | Spain | |
| Postnatal routine anti-D uptake | |||
| 6. Uptake of postnatal anti-D with no testing | 98.4 (91.6% had the correct dose at the correct time) (NR/3392) | bUK anti-D audit8 | UK |
| 95.7 (66/69) | Banch Clausen et al., 201420 | Denmark | |
| 7. Uptake of postnatal anti-D in those who refuse NIPT | > 99 (NR) | Damkjaer et al., 201227 | Denmark |
| 8. Uptake of postnatal anti-D in those who accept NIPT and receive a positive result | 99.7 (353/354) | Banch Clausen et al., 201420 | Denmark |
| 99.3 (151/152) | Damkjaer et al., 201227 | Denmark | |
| 92 (of approximately 18,383) | van der Ploeg et al., 201549 | The Netherlands | |
| 9. Uptake of postnatal anti-D in those who accept NIPT and receive an inconclusive result | No data | N/A | N/A |
| 10. Uptake of postnatal anti-D in those who accept NIPT and receive a negative result | 0 (0/227) | Banch Clausen et al., 201420 | Denmark |
| 0 (0/85) | Damkjaer et al., 201227 | Denmark | |
| 0.087 (2/NR) | Banch Clausen et al., 201224 | Denmark | |
| 0 (NR) | Soothill et al., 201518 | England | |
Postpartum anti-D prophylaxis uptake
Rates of women receiving postpartum anti-D uptake according to NIPT uptake are presented in Table 9. Three studies reported uptake of postnatal anti-D prophylaxis in women who accepted NIPT and received a positive result, ranging from 92% to 99.7%. 20,27,49 One study reported nationwide data in women receiving RhD genotyping in the Netherlands, where 92% of approximately 18,383 women received postnatal prophylaxis anti-D. A subgroup analysis by Banch Clausen et al. 20 (including a total of 690 pregnancies) found a slightly higher uptake of postnatal anti-D among women who received NIPT (99.7%, 353/354) than in those who did not undergo NIPT (95.7%, 66/69). Another Danish study reported a similar rate among women who received NIPT (99.3%, 151/152). 27 None of the included studies reported whether or not all women who received postpartum anti-D prophylaxis received the intended dosage at the intended time.
Reduction in anti-D use
Three non-comparative studies reported outcome measures relating to anti-D doses administered. Soothill et al. 18 reported a significant 6% reduction per month of anti-D administration (95% CI 4% to 8%, Poisson regression) within 6 months in the three maternity services in the south-west of England. The total use of anti-D doses fell by about 29%, corresponding to 35% of RhD-negative women not receiving anti-D in their pregnancy unnecessarily. Similar results were also observed in Banch Clausen et al. study,20 which reported that, of 12,668 pregnant women, 4706 (37.1%) avoided unnecessary anti-D administration within 2 years of prenatal RHD screening programme. The study by Grande et al. 22 reported that, of 95 women carrying a RhD-negative fetus, five requested anti-D administration; unnecessary anti-D administration was therefore avoided in 95% of women carrying a RhD-negative fetus.
Adverse events
None of the studies reported any data on adverse events of either NIPT or antenatal anti-D administration. In particular, there were no data on adverse reactions (such as allergic reactions) to anti-D, on transmission of blood-borne diseases, or on social consequences of NIPT (such as revealing false paternity). No studies reported data on health-related quality of life and patients’ anxiety associated with NIPT.
Simulation study of clinical effectiveness
As seen in the review of clinical effectiveness (see Results: assessment of clinical effectiveness), very limited comparative evidence on the clinical outcomes of NIPT has been reported. In order to better understand the probable consequences of implementing NIPT, and basing anti-D administration on its results, we performed a simulation study.
The parameters of this simulation study are drawn primarily from the systematic reviews of diagnostic accuracy and clinical effectiveness. Prevalence and diagnostic accuracy parameters are derived from the three high-quality Bristol-based studies12,17,18 whenever possible to best represent the UK population. Data on compliance with NIPT and anti-D are drawn from a recent audit of antenatal anti-D administration in the UK, or papers in the clinical effectiveness review, favouring UK-based results whenever available. Some important parameters, such as incidence of sensitisation with and without anti-D, were not reported in any papers included in the diagnostic accuracy or clinical effectiveness reviews. To inform other parameter estimates for this simulation, we conducted an additional literature search to identify relevant systematic reviews of antenatal anti-D prophylaxis. Four relevant reviews61–64 were identified. These reviews provided data on the probability estimates of the events used in the simulation study, including sensitisation and compliance rates. These reviews are summarised in Appendix 8.
Table 10 summarises the parameter estimates used in the simulation and gives their source. All these parameter estimates assume the current practice of offering antenatal anti-D at around 28 weeks and offering postpartum anti-D on the basis of a cord blood test (assumed to be 100% accurate). We assume that there are no adverse consequences of administering anti-D. We note that this simulation considers only women who would be eligible for NIPT at the time it would be received. Women who might not receive NIPT, for example because the father is confirmed as RhD negative, are excluded.
| Probability | Estimate (%) | Source |
|---|---|---|
| RhD-positive fetus | 60.7 | Bristol-based diagnostic studies12,17,18 |
| RhD-positive fetus (with inconclusive NIPT) | 70.7 | Bristol-based diagnostic studies12,17,18 |
| False-negative NIPT | 0.21 | Diagnostic meta-analysis (of the Bristol studies) |
| Inconclusive NIPT | 6.7 | Bristol-based diagnostic studies12,17,18 |
| False-positive test (if conclusive) | 1.5 | Diagnostic meta-analysis (of the Bristol studies) |
| Compliance with antenatal anti-D (without NIPT) (received at least one dose of anti-D) | 99 | UK NHS Blood and Transplant, 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 |
| Uptake of NIPT | 96 | de Haas et al., 201225 (clinical effectiveness review) |
| Compliance with postpartum anti-D | 99 | UK NHS Blood and Transplant, 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 |
| Compliance with antenatal anti-D (if NIPT refused or missed) | 80 | Soothill et al., 201518 (clinical effectiveness review) |
| Compliance with antenatal anti-D (if NIPT inconclusive) | 99 | Soothill et al., 201518 (clinical effectiveness review) |
| Uptake of antenatal anti-D in women with negative NIPT | 6 | Soothill et al., 201518 (clinical effectiveness review) |
| Compliance with postpartum anti-D after NIPT process | 99 | No data, assumed same as without NIPT |
| Sensitisation with antenatal anti-D and postpartum anti-D | 0.35 | Pilgrim et al., 200962 (HTA report) |
| Sensitisation with only postpartum anti-D | 0.95 | Pilgrim et al., 200962 (HTA report) |
| Sensitisation with no anti-D | 10.7 | Pilgrim et al., 200962 (HTA report) and Crowther and Middleton65 |
| Subsequent pregnancy in sensitised women | 62 | Used by Chitty et al., 2014,12 no source given |
| Death of RhD-negative fetus in sensitised women | 5 | Used by Chitty et al., 2014,12 no source given |
The simulation study assumes that these input probabilities are accurate and does not account for any uncertainty in their estimation. Therefore, results of the simulation study should be considered illustrative of the probable consequences of the use of NIPT and not definitive estimates of effect.
The results of the simulation study are summarised in Table 11. These results are subject to a Monte Carlo error of approximately ± 0.002%.
| Outcome | Treatment approach | Percentage of women |
|---|---|---|
| Antenatal anti-D given | Universal anti-D | 99 |
| Based on NIPT | 65.9 | |
| Unnecessary anti-D given (RhD-negative fetus) | Universal anti-D | 38.9 |
| Based on NIPT | 5.7 | |
| Anti-D not given (RhD-positive fetus) | Universal anti-D | 0.6 |
| Based on NIPT | 1.2 | |
| Sensitised during or after pregnancy | Postpartum/emergency anti-D only | 0.641 |
| Universal anti-D | 0.281 | |
| Based on NIPT with postpartum anti-D | 0.284 | |
| Based on NIPT with no postpartum anti-D for test negatives | 0.294 | |
| Deaths in subsequent pregnancies | Postpartum/emergency anti-D only | 0.0198 |
| Universal anti-D | 0.0086 | |
| Based on NIPT with postpartum anti-D | 0.0091 | |
| Based on NIPT with no postpartum anti-D for test negatives | 0.0091 |
These results show that using NIPT leads to a substantial reduction in antenatal anti-D prophylaxis use, from 99% of RhD-positive women (i.e. assuming 99% compliance) to 65.9%. This decline is similar in magnitude to that observed by Soothill et al. 18 This is a consequence of the substantial drop in unnecessary anti-D administration in women with RhD-negative fetuses, from 39% of women to 5.7%. Using the NIPT approach means that about 1.2% of women miss out on potentially beneficial prophylaxis, mainly because of non-compliance, compared with 0.6% with universal anti-D administration.
Because sensitisation is rare, very few additional women will be sensitised if NIPT is used. Assuming that all women still receive a postnatal cord blood test and anti-D if required, NIPT will result in about three extra sensitisations per 100,000 women. If cord blood testing is not performed, then there will be approximately 13 extra sensitisations per 100,000 women. These increases are small compared with the total number of sensitisations attributable to failure of anti-D treatment (around 284 per 100,000 women) and compared with not using antenatal anti-D at all (around 641 per 100,000).
The use of NIPT is unlikely to have any meaningful impact on mortality in subsequent pregnancies. Even if postpartum anti-D is never given to women with a negative NIPT result, only approximately five extra deaths will occur per 1 million RhD-negative women.
This simulation assumes that women who do not receive NIPT, for whatever reason, would still be offered, and generally receive, antenatal anti-D. As a SA we consider the impact of a strategy of requiring NIPT as a prerequisite to antenatal anti-D, or, equivalently, of assuming that women who do not comply with NIPT would not comply with the whole antenatal anti-D immunisation process. These results are shown in Table 12.
| Outcome | Treatment approach | Percentage of women |
|---|---|---|
| Antenatal anti-D given | Universal anti-D | 99 |
| Based on NIPT | 62.7 | |
| Unnecessary anti-D given (RhD-negative fetus) | Universal anti-D | 38.9 |
| Based on NIPT | 4.5 | |
| Anti-D not given (RhD-positive fetus) | Universal anti-D | 0.6 |
| Based on NIPT | 3.2 | |
| Sensitised during or after pregnancy | Postpartum/emergency anti-D only | 0.641 |
| Universal anti-D | 0.281 | |
| Based on NIPT with postpartum anti-D | 0.296 | |
| Based on NIPT with no postpartum anti-D for test negatives | 0.309 | |
| Deaths in subsequent pregnancies | Postpartum/emergency anti-D only | 0.0198 |
| Universal anti-D | 0.0086 | |
| Based on NIPT with postpartum anti-D | 0.0096 | |
| Based on NIPT with no postpartum anti-D for test negatives | 0.0096 |
These results show that anti-D administration rates will be further reduced (to 62.7%) if women who do not receive NIPT do not receive antenatal anti-D. The number of women who miss out on potentially beneficial anti-D will rise to 3.2%. This means that there will be more sensitisations: an extra 15 per 100,000 women if postpartum cord blood testing continues or 28 per 100,000 if it is withdrawn.
This simulation study suggests that the use of NIPT to determine antenatal anti-D use will substantially reduce the number of women receiving anti-D unnecessarily and so is likely to be beneficial, provided that the cost of the test does not outweigh this saving. The use of NIPT could also reduce the use of anti-D administration after potentially sensitising events during pregnancy, in women with a negative test result. The additional number of sensitisations compared with a universal offering of antenatal anti-D is very small, provided that care is taken to ensure that women who do not receive NIPT are still offered, and receive, anti-D.
The results suggest that if a woman receives a conclusive NIPT, then test cord blood testing could potentially be withdrawn and postpartum prophylaxis offered on the basis of NIPT. This conclusion depends on whether or not the increase in sensitisations (approximately 13 per 100,000 RhD-negative women) is considered ethically acceptable and cost-effective.
Results: assessment of implementation
Characteristics of included studies
Table 13 presents a summary of the characteristics of the 12 studies13,17,18,20–28 included in the review of implementation of high-throughput NIPT. Most of these were also included in the diagnostic accuracy and/or clinical effectiveness reviews. These studies were conducted in five countries: Denmark, the UK, Spain, the Netherlands and Sweden. Fetal RhD screening programmes were implemented nationally in the Netherlands and Denmark and regionally in England, Sweden and Spain. Most included studies were large cohort studies that reported implementation data as well as diagnostic accuracy data. One study was a UK-based survey (London). The number of included women ranged from 282 to 18,383.
| Study | Location | Study dates | Sample sizea | Gestational age (weeks) at time of NIPT, median (range) |
|---|---|---|---|---|
| Finning et al., 200817 | England: Birmingham and Sheffield centre of the National Blood Service | NR | 1869 | 28 (8–38) |
| Soothill et al., 201518 | England: south west, three NHS trusts | April–September 2013 | 526 | 15–17 (mostly) |
| Oxenford et al., 201328 | England: four hospitals (Birmingham, London, Newcastle, Sunderland) | NR | 289 (270 survey respondents, 19 interviews/focus groups) | > 12 |
| Banch Clausen et al., 2014;20 Banch Clausen et al., 2012;24 Banch Clausen et al., 2013;13 and Damkjaer et al., 201227 | Denmark: nationwide, five regions | 2010–11 | 14,547 | 25 (73% between 23 and 28) |
| de Haas et al., 2012;25 Thurik et al., 201521 | The Netherlands: nationwide | July 2011–January 2012 | 18,383b | 26 |
| Grande et al., 201322 | Spain: Barcelona, six maternity care units | February 2010–October 2011 | 282 | 24–26 |
| Wikman et al., 2012;23 and Tiblad et al., 201326 | Sweden: Stockholm, 83 maternity care centres, six delivery units | September 2009–March 2012 (reference cohort: 2004–8) | 8374c | 8–40 |
Results of implementation studies
Table 14 presents a summary of implementation data for high-throughput NIPT. All the large cohort studies reported high diagnostic accuracy of high-throughput NIPT (see Meta-analyses of diagnostic accuracy) and suggested that high-throughput RhD genotyping of fetuses in all RhD-negative women was feasible. These studies reported high compliance with anti-D immunoglobulin administration and moderate to high compliance with NIPT (see details in Results of studies on clinical effectiveness).
| Screening programme (country) | Study | Details of screening programme | General results | Issues to implementation | Authors’ practical advice | Authors’ research recommendation |
|---|---|---|---|---|---|---|
| Denmark | Banch Clausen et al., 201420 | National programme delivered in five regions in Denmark | Very good screening accuracy (see diagnostic review). False-negative results were mainly because of poor DNA yields or handling errors. False-positive results were a result of contamination and genetic variants. Inconclusive results were because of weak D genotypes. High compliance with anti-D/moderate compliance with NIPT (see effectiveness review) | The challenges to implement the prenatal RHD screening programme are related to programme anti-D prophylaxis compliance |
Implement external quality assurance programmes as well as regular in-house testing to optimise effectiveness of the screening programme Postnatal prophylaxis should be based exclusively on the result from the prenatal RHD screening. An increased effort to improve anti-D prophylaxis compliance is important to further reduce the number of RhD immunisations Issuing focused statements to GPs may avoid sending samples from early pregnancy, which may help reduce false-negative results Increase information given directly to pregnant women, GPs, midwives and obstetricians and systems, such as a reminder system integrated into the GPs’ software, which may help to increase women’s compliance with the programme |
None |
| Banch Clausen et al., 201224 | Earlier report on Danish screening programme | As above | There may be challenges in the logistics concerning the transportation of samples from remote sites to testing laboratories and in getting results back to the correct GP | Cord blood typing continues to ensure that postnatal anti-D is given if NIPT compliance is poor. RhD testing should be based on a single sample | Long-term follow-up is required to assess clinical effects of NIPT screening | |
| Clausen et al., 201313 | Paper focused on issues around transportation of blood samples in the Danish screening programme | Total DNA declines over time from sampling. Fetal DNA was not generally affected over time from sampling | Not applicable. The paper did not consider implementation of the screening programme as a whole | The aim should be for a transportation time of up to 4 days and no more than 7 days | None | |
| Damkjaer et al., 201227 | Earlier report on Danish screening programme, focused on compliance issues | Compliance with NIPT was around 90%, improving over time | No additional implementation issues reported | For GPs:
|
None | |
| UK (Bristol) | Finning et al., 200817 | Two regions in England (Birmingham and Sheffield), centres of the National Blood Service for routine ABO and RhD blood grouping and antibody screening | Very good diagnostic accuracy (see review). Inconclusive results were often a result of substantial maternal DNA, for example because samples were old | No issues to implementation were reported. The modest apparent increase in risk of sensitisation in false-negative women might be offset by an increased uptake of prophylaxis among mothers who have been correctly identified as carrying a RhD-positive fetus |
If the policy on routine antenatal prophylaxis were changed to a single dose of anti-RhD immunoglobulin given at 30 weeks’ gestation in RhD-negative women, then RHD genotyping testing at 28 weeks would be suitable Commencement of anti-D treatment at 30 weeks’ gestation, rather than 28 weeks’, has been considered an option in the UK. Anti-D could be avoided after sensitising event in test-negative women. Treating inconclusive results as positive seems to be the best approach Testing only samples that are < 7 days old would increase logistical issues of transport over large geographic areas but would reduce the risk of false-negative results |
Feasibility trials on testing maternal blood samples obtained during the earlier stages of pregnancy are required |
| Soothill et al., 201518 | Three maternity services in the south west of England | 29% drop in use of anti-D at a cost reduction of £60,000 per year |
It is possible to implement routine cffDNA fetal blood grouping in RhD-negative women in the NHS The requirements of patient information, patient consent, sample handling, sample transfer and implementation of the changed management were all successfully met |
This service should be extended to the whole of the UK, because it has led to a more targeted use of anti-D. The cost of the tests seems to be covered by the resulting savings in the use of anti-D immunoglobulin. Continued use of anti-D in women who can be shown to have RhD-negative fetuses may be unethical | Further research on high-throughput NIPT to improve the test accuracy and reduce the inconclusive rates is required | |
| UK (London) | Oxenford et al., 201328 | Survey conducted in one hospital in London, UK | This study investigated women’s preferences and information needs for routine implementation of NIPT. Around 290 women included: 92.1% agreed that NIPT should be offered. Only 75.9% said they would accept the test. Women preferred having the test when most accurate, even if later in pregnancy |
Women hold positive views regarding the introduction of routine fetal RhD genotyping using cffDNA but women’s current knowledge of rhesus blood groups and anti-D administration was found to be limited Although women may agree to extra appointments for the test, health professionals (n = 13) all thought that this may be impractical |
Developing information leaflets and health professional training will be critical for successful implementation | None |
| Spain | Grande et al., 201322 | Six health centres of Barcelona-West health district in Spain | High diagnostic accuracy (see diagnostic review). False-negative results were mainly related to specific DNA extraction methods, prolonged storage time before sample processing and early gestational age | No issues to implementation were reported | High-throughput NIPT of exons 5, 6, 7 and 10, before 28 weeks’ gestation in their mixed population should be considered for further clinical application | None |
| The Netherlands | Thurik et al., 201521 | One region in the Netherlands | Discordant test results were mainly caused by RhD variant genes and weak PCR signals and the ‘vanishing twin’ phenomenon | No issues to implementation were reported | Discordant positive results due to co-twin demise would have greater clinical impact in other non-invasive prenatal tests. The authors therefore advised documenting a vanishing twin at any early pregnancy scan and counselling against NIPT. False-positive findings will have little impact in NIPT, as the test causes only unnecessary anti-D use | Prospective studies in pregnancies with a vanishing twin will be required to test whether or not discrepant NIPT results may be compatible with a vanishing co-twin as a source of a third genomic cell line |
| de Hass et al., 201225 | Earlier report on the Netherlands screening programme | Compliance with NIPT screening was around 95%. The FPR was 1.1% | It is possible to guide both antenatal and postnatal anti-D immunoprophylaxis by fetal RHD screening in maternal blood obtained at 27 weeks’ gestation. No further issues relating to implementation were reported | None stated | A longer period of evaluation based on local analyses of cord blood testing is required | |
| Sweden | Wikman et al., 201223 | 83 maternity care centres in the Stockholm area, Sweden | NIPT had high diagnostic accuracy with > 99% sensitivity and specificity. Before 8 weeks’ gestation, fetal RhD genotype could not be reliably determined (see diagnostic accuracy review) |
Fetal RHD detection in early pregnancy in a routine clinical setting is feasible and accurate. No further issues relating to implementation were reported This screening programme can be included in the routine antenatal care management and will not require any extra appointment for maternal blood sampling |
NIPT should not be performed before 8 weeks’ gestation. Maternal DNA levels may be too large after 4 days’ storage for reliable testing in first trimester | The cost-effectiveness of fetal RHD screening combined with targeted antenatal Rh prophylaxis will be an important area for further research |
| Tiblad et al., 201326 | See Wikman et al., 201223 | RhD immunisation rate was 0.26% in the screening cohort and 0.46% in historical controls (see effectiveness review) | Using first-trimester screening significantly reduces the incidence of new RhD immunisation but test sensitivity is lower than for later screening | No further advice given | Cost-effectiveness of first-trimester screening should be evaluated |
One UK study18 conducted in the south west of England stated that it is feasible to implement routine cell-free fetal DNA fetal blood grouping in RhD-negative women in the NHS. This study also stated that the requirements of patient information, patient consent, sample handling, sample transfer and implementation of the changed management were all successfully met.
A number of studies reported issues related to the implementation of prenatal RhD screening programmes. For example, Banch Clausen et al. 20 stated that the challenges to the implementation of the prenatal RhD screening programme were related to programme anti-D prophylaxis compliance. Another study by Banch Clausen et al. 24 noted that there may be challenges in logistics concerning the transportation of samples from remote sites to testing laboratories and in getting results back to the correct general practitioner.
The UK-based survey28 investigated 290 women’s preferences and information needs for routine implementation of NIPT. A total of 92.1% women agreed that NIPT should be offered but only 75.9% stated that they would accept the test. Women preferred having the test when it was most accurate, even if later in pregnancy. The study revealed that women’s current knowledge of rhesus blood groups and anti-D administration was limited, which could be a barrier to implementation. Although women may agree to extra appointments for NIPT, health professionals recruited from one London hospital thought that this may be impractical. The data from this survey showed that women hold positive views regarding the introduction of routine fetal RhD genotyping using cell-free fetal DNA. Given women’s limited knowledge of rhesus blood groups and anti-D administration, the authors stated that developing information leaflets and health professional training will be critical for successful implementation. They stated that this work will be important for the development of policies and guidelines on the introduction of fetal RhD genotyping into routine care.
Several studies offered practical advice for implementing high-throughput NIPT. For example, Finning et al. 17 stated that if the policy on routine antenatal prophylaxis were changed to a single dose of anti-RhD immunoglobulin given at 30 weeks’ gestation in RhD-negative women, then RhD genotyping testing at 28 weeks would be suitable. This study also suggested that treating inconclusive results as positive seems to be the best approach to minimise the risk of not treating women with a RhD-positive fetus. Another recent UK (Bristol) study18 stated that this service should be extended to the whole of the UK, because it has allowed the use of anti-D in a more targeted way and the cost of the tests seems to be offset by the resulting savings in the use of anti-D. This study also stated that continued use of anti-D in women who can be shown to have RhD-negative fetuses may be unethical. Banch Clausen et al. 24 recommended continuing cord blood typing in practice to ensure that postnatal anti-D is given if NIPT compliance is poor. Damkjaer et al. 27 suggested improvement in relevant knowledge on prenatal RhD screening among general practitioners and midwives in Denmark.
Clausen et al. 13 focused on issues around transportation of blood samples in the Danish screening programme and suggested that the aim should be for a transportation time of up to 4 days and no more than 7 days. Wikman et al. 23 noted that testing before 8 weeks may be inappropriate because of the instability of samples and consequent difficulties of transportation.
In summary, the findings from these studies suggest that high-throughput NIPT for fetal RhD screening in all RhD-negative women is feasible. They also suggest that effective education, particularly for pregnant women but also for general practitioners and midwives, on the role of NIPT and the importance of anti-D immunisation is important. Any nationwide NIPT screening programme will require careful logistical management to ensure that blood samples are transported to laboratories and tested quickly and that results are reliably returned to general practitioners and midwives. NIPT could be carried out at any time between 25 and 28 weeks, preferably as part of an existing antenatal appointment. Anti-D, if required, should be administered as a single dose at around 30 weeks.
Clinical effectiveness summary and conclusions
Diagnostic accuracy
Eight studies12,17–23 were included in the diagnostic review of high-throughput NIPT. There were three studies based in Bristol (UK). 12,17,18 The majority of included studies were judged as having a low risk of bias.
Meta-analyses found that high-throughput NIPT had very good diagnostic accuracy. In the primary analyses, in which women with inconclusive test results were treated as if positive, the summary FNR (women at risk of sensitisation) was 0.34% (95% CI 0.15% to 0.76%) and the FPR (women needlessly receiving anti-D) was 3.86% (95% CI 2.54% to 5.82%).
The three high-quality studies performed at Bristol,12,17,18 which were most representative of UK practice, had a lower FNR of 0.21% (95% CI 0.09% to 0.48%), with a consequently higher FPR of 5.73% (95% CI 4.58% to 7.16%). This difference may be partly because the NIPT used in Bristol had a different test threshold to other countries to further reduce false-negative results.
The FPR found is mostly a consequence of treating women who have an inconclusive test result (approximately 7% of non-invasive prenatal tests in the UK) as if they had a positive test. Excluding these women from analysis gave a lower FPR of 1.26% (95% CI 0.87% to 1.83%). It may therefore be possible to reduce the FPR by further targeted testing of women with an initially inconclusive result.
The diagnostic accuracy performance of high-throughput NIPT varied by gestational age. The data suggest that high-throughput NIPT is insufficiently accurate before around 11 weeks’ gestation (i.e. in first trimester) but is accurate at any time after the end of the first trimester. One study12 also suggested that the number of inconclusive results may decline over time. Hence, NIPT cannot be recommended before the second trimester and may be best performed later in the second trimester.
Clinical effectiveness
Seven studies18,20,22,24–27 were included in the clinical effectiveness review. Only two studies had a control group. All studies were judged as having a high risk of bias. As all except one were conducted in non-UK countries, the generalisability of their findings to the UK setting is limited because of variations in national guidelines and health policies between countries (e.g. prescription of RAADP). One large prospective cohort study26 reported that use of high-throughput NIPT for targeted antenatal anti-D prophylaxis was associated with a significant risk reduction in sensitisation (adjusted odds ratio 0.41, 95% CI 0.22 to 0.87) compared with historical controls (routine management, postpartum anti-D only).
Uptake rates of NIPT were reported in seven studies, ranging from 70% in a pilot study conducted in England to > 95% in an established national programme in Denmark. Uptake rates of RAADP in women who accepted NIPT and received a positive result were moderate to high, ranging from 86% to 96.1% (four studies). Uptake rates of routine postnatal anti-D prophylaxis in women who accepted NIPT and received a positive result were reported in three studies and were generally high, ranging from 92% to 99.7%.
Three non-comparative studies evaluated changes in anti-D use following the implementation of NIPT. All found that the use of NIPT reduced the total use of anti-D immunoglobulin doses, which fell by 29% in one UK study18, because around 35% of RhD-negative women avoided receiving anti-D unnecessarily.
As the quality of the clinical effectiveness evidence was limited, we performed a simulation study, based on the findings of our reviews, to assess the probable clinical consequences of implementing NIPT. Its results were broadly consistent with the review evidence. It suggested that NIPT, when compared with offering anti-D to all RhD-negative women, would substantially reduce the use for anti-D from 99% of women to 65.9%. The number of women receiving anti-D unnecessarily would fall from 38.9% to 5.7%. The number missing out on potentially beneficial anti-D (because of a false-negative test result or non-compliance) depends on the compliance rate but could increase from 0.6% to between 1.2% and 3.1%.
The impact of NIPT on sensitisation rates (compared with universal anti-D use) also depends on compliance. Sensitisation rates may increase by 3–15 sensitisations per 100,000 women if postpartum cord blood testing is continued, or 13–28 per 100,000 women if cord blood testing is withdrawn and postpartum anti-D given on the basis of the NIPT result. Ensuring that women who do not receive NIPT are still offered, and receive, antenatal anti-D will minimise the number of additional sensitisations.
Implementation
Twelve studies were included in the review of implementation. Most of the included studies were large cohort studies reporting implementation data along with diagnostic accuracy data, although one study was a UK-based survey. As most studies were conducted in non-UK countries, the generalisability of their findings to the UK settings is limited because of variations in national guidelines and health policies between countries. All the large cohort studies suggested that high-throughput RhD genotyping of fetuses in all RhD-negative women was feasible and should be recommended. A number of studies reported issues of implementation such as those relating to programme anti-D prophylaxis compliance. Some studies emphasised the importance of short transport times of samples and the need for good management of transporting samples. Some studies also identified the need for greater knowledge of NIPT among physicians, midwives and pregnant women.
Conclusions
High-throughput NIPT for fetal RhD status is an accurate diagnostic test, if performed after 11 weeks’ gestation. It has a FNR (women remain at risk of sensitisation) of around 0.2% and a FPR (women receive unnecessary anti-D) of around 5.7%. The test gives an inconclusive result in around 7% of women in the UK. Owing to limited evidence, the accuracy of NIPT in non-white women and multiple pregnancies is unclear. Treating inconclusive tests as if they were positive is the cause of most false-positive results. Giving antenatal anti-D immunoglobulin on the basis of NIPT, rather than to all RhD-negative women, will reduce the use of anti-D and largely eliminate unnecessary use of anti-D in women who do not need it because they have a RhD-negative fetus. Some women will, however, continue to receive anti-D unnecessarily because of an inconclusive test result.
Although the evidence was limited, it appears that using NIPT will lead, at worst, to only a small increase in the number of sensitisations compared with universal use of anti-D. The simulation suggested that achieving high compliance with both NIPT and antenatal anti-D (particularly in women who do not receive NIPT) is important in order to achieve good clinical effectiveness and to reduce the sensitisation rate. It may be clinically reasonable to withdraw postpartum cord blood testing and base postpartum anti-D administration on the results of NIPT. All large implementation studies suggested that high-throughput NIPT in all RhD-negative women was feasible and should be recommended. Key issues of implementation include ensuring anti-D prophylaxis compliance, effective management of transporting samples and greater knowledge of NIPT among physicians, midwives and pregnant women.
Chapter 4 Systematic review of existing cost-effectiveness evidence
This chapter provides an overview of the existing cost-effectiveness evidence for the use of high-throughput NIPT for rhesus D status in RhD-negative women not known to be sensitised to the RhD antigen. We assessed the relevance of these data to inform UK practice and the current assessment, as set out in the NICE scoping documentation. 66 For each cost-effectiveness study we describe the manner in which NIPT is assumed to impact on the care pathway and summarise how existing cost-effectiveness studies have characterised the impact of NIPT on routine antenatal care costs, routine antenatal anti-D immunoglobulin administration, management of potentially sensitising events and postnatal administration of anti-D immunoglobulin. The findings from the review informed the development of a new decision-analytic model, reported in Chapter 5.
Methodology of the cost-effectiveness review
Searches
In addition to the searches conducted for the review of clinical evidence (see Chapter 3), the following databases were searched up to December 2015 for cost-effectiveness evidence: NHS EED, EconLit and IDEAS database via Research Papers in Economics (RePec). The bibliographies of relevant studies were also searched. Citations of identified studies were searched for any relevant publications published after the initial search.
Selection criteria
A broad range of studies was considered in the review, including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. Only full economic evaluations that compared two or more options and considered both costs and consequences (i.e. cost-minimisation, cost-effectiveness, cost–utility and cost–benefit analyses) were included in the review.
Study selection
Relevant studies were then selected in two stages. Titles and abstracts identified by the search strategy were examined independently by two researchers (PS and SG) and screened for possible inclusion. Disagreements were resolved by discussion. Full texts of the potentially relevant studies were obtained. Two researchers (PS and SG) examined these independently for inclusion or exclusion, and disagreements were resolved by discussion.
Data extraction
One reviewer (PS) independently extracted details from full-text studies on objectives, setting, population, comparators, analytical approach, data on costs and outcomes (short- and long-term) and main results/conclusions. Another reviewer (SG) checked extracted data and disagreements were resolved by discussion.
Critical appraisal
A quality appraisal was carried out using the checklist of Drummond and Jefferson. 67 This checklist evaluates the extent to which each review result provides detail on different aspects, such as study design, data collected and their use in the economic evaluation and analysis and interpretation of results. One reviewer (PS) independently assessed the quality of all included studies according to all these domains. The quality assessment was checked by another reviewer (SG). Any disagreements were resolved by consensus.
Results of the review of existing cost-effectiveness evidence
Quantity of research available
Number and type of studies included
The initial search of economic databases identified a total of 31 references. After the initial screening of titles and abstracts, 10 were considered to be potentially relevant and were ordered for full-text paper screening. Of those, seven met the selection criteria and were included in the review. 58,68–73 A flow diagram of the selection process is reported in Figure 10.
FIGURE 10.
Assessment of cost-effectiveness: summary of study selection and exclusion.
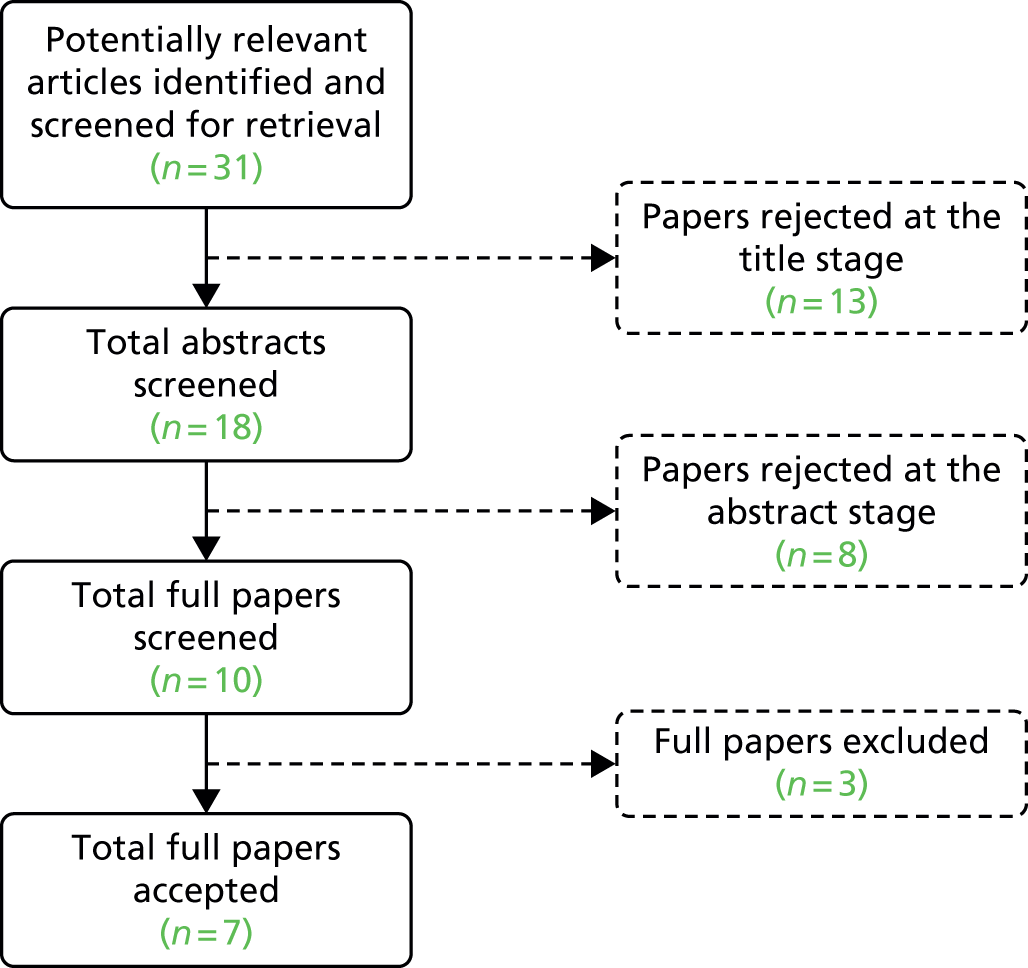
Number and type of studies excluded
A list of full-text papers that were excluded is given in Appendix 9. These papers were excluded because they failed to meet one or more of the inclusion criteria, including lack of full-text publications and ineligible study design.
Characteristics of included studies
The characteristics of the seven studies are summarised in Table 15. The large majority of studies specified the target population as being unsensitised RhD-negative pregnant women or RhD-negative pregnant women not known to be sensitised to the RhD antigen. Macher et al. 70 and Hawk et al. 72 stated that their analysis considered RhD-negative pregnant women but they were not clear about women’s sensitisation status at study entry. Only two studies68,73 explicitly stated that a high-throughput NIPT method was being used for the comparative assessment, although for the other studies this was considered implicit, as the test diagnostic performance was considered similar to the high-throughput studies. One study71 explicitly focused on providing NIPT to all RhD-negative women, as the test for sensitisation was conducted only if the NIPT result was positive.
| Study | Objectives | Setting/perspective | Population | Analytical approach | Diagnostic comparators | Outcomes | Main results |
|---|---|---|---|---|---|---|---|
| Szczepura et al., 201168 | Cost-effectiveness analysis of NIPT implementation in England and Wales | English and Welsh NHS | Unsensitised RhD-negative pregnant women | Economic analysis of NIPT implementation. For each scenario a threshold analysis was performed to identify the circumstances under which NIPT might be considered cost saving compared with RAADP | Two scenarios compared:
|
Costs (including NIPT royalty fees), additional sensitisations/year | Analysis performed did not support routine implementation of NIPT in England and Wales for unsensitised RhD-negative pregnant women. Net financial benefit of implementing mass NIPT as an add-on (while maintaining current postnatal testing) was found to be negligible in England and Wales. NIPT implementation is unlikely to produce important clinical benefits: the number of sensitisations was estimated not to fall appreciably and the sensitisations are expected to rise if NIPT sensitivity is below 99.9% |
| Benachi et al., 201269 | Cost-minimisation analysis of NIPT on the costs of managing RhD-negative pregnant women, whether or not they are sensitised | French NHS | Unsensitised RhD-negative pregnant women | A prospective follow-up of RhD-negative women during their pregnancy | Four scenarios compared:
|
Costs, except for potentially sensitising events; no clinical outcomes were considered in the analysis | NIPT performed early during pregnancy (i.e. end of first trimester and beginning of second trimester) was found to be cost saving compared with RAADP during the third trimester |
| Macher et al., 201270 | Cost-minimisation analysis of NIPT (multiplex real-time PCR assay for fetal cell-free DNA) in the plasma of pregnant women | Andalusian government, Spain | RhD-negative pregnant women | An analysis of feasibility of routine RhD status determination into the clinical setting using NIPT targeted towards two exons of the RHD gene and one exon of SRY gene |
No diagnostic comparators were presented Three ways of detecting fetal RhD using NIPT were compared:Testing was performed on RhD-negative women in weeks 10–28 of pregnancy. The consequences of test results were not explored |
Test accuracy; cost of assay per sample | The routine determination of fetal RhD status using NIPT is feasible. The use of multiplex real-time PCR allows the improvement of the response of the laboratory, saving time and reagent costs and opening the door to a complete automatisation of the process |
| Duplantie et al., 201371 | Cost-effectiveness analysis of strategies to prevent RhD alloimunisation | Public health-care system of Quebec, Canada | Unsensitised RhD-negative pregnant women | Computer-based simulation model with virtual population of 10,000 RhD-negative pregnant women
|
Four scenarios compared:
|
Clinical:
|
The four proposed strategies for prevention and treatment of sensitisation were found to be similar in terms of their effectiveness. In terms of cost-effectiveness, two options were found to be superior: RAADP and immunological Rh typing of the father. NIPT was found not to be a cost-effective option unless its cost is lowered RAADP remained the preferred option for the prevention of maternal sensitisation |
| Hawk et al., 201372 | Cost-effectiveness of NIPT for targeted prophylaxis | US health system (Medicaid and Medicare) | RhD-negative women | Decision tree model using a decision tree structure comparing three relevant scenarios | Three scenarios compared:
|
Costs per RhD woman, morbidity and mortality attributable to haemolytic disease | Non-invasive fetal RhD testing was not found to provide any economic benefit for the management of RhD-negative women. RAADP and postpartum prophylaxis guided by cord blood typing remained the most cost-beneficial option for the management of RhD-negative women |
| Neovius et al., 201658 | Cost-effectiveness of first-trimester NIPT for targeted antenatal vs. no RAADP or vs. non-targeted RAADP | Swedish health service | Unsensitised RhD-negative pregnant women | Decision-analytic model based on a population-based cohort study. Markov model with cohort simulation and three health states: ‘not sensitised’, ‘sensitised during pregnancy’ or ‘sensitised from start of pregnancy’ | Three scenarios compared:
|
Screening, pregnancy, delivery and future pregnancies related costs, additional costs per sensitisation averted | NIPT for targeted RAADP was found to be cost saving as well as more effective than no RAADP. Introduction of targeted prophylaxis was expected to save money, reduce sensitisations and avoid unnecessary exposure of pregnant women to a plasma product in short supply |
| Teitelbaum et al., 201573 | Cost-effectiveness of non-invasive fetal RhD determination | Canadian NHS | Unsensitised RhD-negative pregnant women | Decision-analytic modelling – decision trees to model costs and benefits of targeted vs. RAADP in Alberta over 1 year | Two scenarios compared:
|
Number of women sensitised in 1 year, doses of anti-D administered per pregnancy in 1 year, cost per pregnancy | Implementation of a programme of targeted anti-D prophylaxis using NIPT was found to be both feasible and cost saving with no increase in the risk of sensitisation. With higher sample throughput (i.e. in a national programme) the cost per patient was expected to decrease owing to economies of scale |
Most studies58,68,71–73 evaluated the cost-effectiveness of introducing NIPT in the management pathway of RhD-negative pregnant women compared with alternative strategies. These studies explored a range of alternative strategies to prevent sensitisation. Except for Szczepura et al. 68 and Macher et al. ,70 two strategies were common across the studies: (non-targeted) RAADP at around 28–30 weeks to every (unsensitised) RhD-negative pregnant women; and use of NIPT for fetal RhD typing with prophylaxis guided by test results (targeted RAADP) for RhD-negative pregnant women. Duplantie et al. 71 also explored the immunological determination of the father’s RhD type to target RAADP. Most studies considered the introduction of NIPT at a single time point, usually at first routine antenatal care appointment occurring between 8 and 12 weeks’ gestation. Benachi et al. 69 compared alternative timings of NIPT by considering the cost consequences of performing NIPT during the first and the third gestation trimesters. With the exception of the Duplantie et al. 71 study, for which insufficient information is provided, all cost-effectiveness studies evaluated the consequences of introducing NIPT in terms of avoiding RAADP but also in terms of the impact it had on postpartum treatment.
Three studies58,68,73 aimed to evaluate the short-term costs and consequences of sensitisation in RhD-negative women. Duplantie et al. 71 and Hawk et al. ,72 however, estimated long-term outcomes relating to morbidity and mortality attributable to haemolytic disease of the fetus and/or newborn infant. Furthermore, two studies58,71 explicitly considered in their analysis women’s first and subsequent pregnancies, presenting cost-effectiveness results for each scenario.
Benachi et al. 69 and Macher et al. 70 are cost-minimisation studies, as no health outcomes were considered, restricting their analysis to an evaluation of the impact of the test on the costs of managing the target population. A variety of cost components were considered across these two studies, such as anti-D immunoglobulin, genotyping, antibody testing.
The cost-effectiveness studies evaluated different strategies in different health systems, including England and Wales, Canada, Sweden and the USA. Except for Sweden, where only postpartum administration of anti-D (conditional on having a RhD-positive baby) is recommended, current guidance for the prevention of sensitisation in these countries is routine prophylactic administration of anti-D, with further prophylactic doses for potentially sensitising events and post partum. The two cost-minimisation studies69,70 evaluated the cost implications of introducing NIPT in the French and Spanish (namely the Andalusia region) health-care settings. Current guidance on the prevention of sensitisation in these countries was not clearly stated. Macher et al. 70 focused mainly on addressing questions relating to the accuracy and implementation of different NIPT methodologies into current clinical practice in Spain.
Quality of included studies
A summary of the results of the quality appraisal of the seven included studies is provided in Table 16.
| Criteria | Study | ||||||
|---|---|---|---|---|---|---|---|
| Szczepura et al., 201168 | Benachi et al., 201269 | Macher et al., 201270 | Duplantie et al., 201371 | Hawk et al., 201372 | Neovius et al., 201658 | Teitelbaum et al., 201573 | |
| Study design | |||||||
| The research question is stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The economic importance of the research question is stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The viewpoint(s) of the analysis are clearly stated and justified | Yes | Yes | No | Yes | Partial | Yes | No |
| The rationale for choosing alternative programmes or interventions compared is stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The alternatives being compared are clearly described | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The form of economic evaluation used is stated | Partial | Partial | No | Yes | Partial | Yes | Partial |
| The choice of form of economic evaluation is justified in relation to the question addressed | No | No | No | Partial | No | Partial | No |
| Data collection | |||||||
| The source(s) of effectiveness estimates used are stated | Yes | N/A | N/A | Yes | Yes | Yes | Yes |
| Details of the design and results of the effectiveness study are given (if based on a single study) | No | N/A | N/A | No | No | Yes | No |
| Details of the methods of synthesis or meta-analysis of estimates are given (if based on a synthesis of a number of effectiveness studies) | No | N/A | N/A | N/A | N/A | N/A | No |
| The primary outcome measure(s) for the economic evaluation are clearly stated | Yes | No | No | Partial | Partial | Yes | Yes |
| Methods to value benefits are stated | N/A | N/A | N/A | No | No | No | N/A |
| Details of the subjects from whom valuations were obtained are given | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Productivity changes (if included) are reported separately | No | No | No | No | No | No | No |
| The relevance of productivity changes to the study question is discussed | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Quantities of resource use are reported separately from their unit costs | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Methods for the estimation of quantities and unit costs are described | Yes | No | Yes | Yes | Partial | No | Partial |
| Currency and price data are recorded | Yes | No | Yes | Yes | Partial | Yes | Partial |
| Details of currency of price adjustments for inflation or currency conversion are given | No | No | No | No | No | Partial | No |
| Details of any model used are given | No | No | No | Yes | Yes | Yes | Yes |
| The choice of model used and the key parameters on which it is based are justified | N/A | N/A | N/A | Partial | Partial | No | Partial |
| Analysis and interpretation of results | |||||||
| Time horizon of costs and benefits is stated | No | No | No | Partial | No | Yes | No |
| The discount rate(s) are stated | No | No | No | N/A | No | Yes | No |
| The choice of discount rate(s) is justified | N/A | N/A | No | N/A | N/A | Yes | N/A |
Study design
All studies stated their research question and provided a rationale for it. Most studies failed to clearly mention which economic approach was being taken; the ones that did only partially justified their choice. Five of the seven studies were cost-effectiveness analyses using a decision-analytic modelling approach, typically based on a decision tree. Most of these restricted their assessment to the more short-term outcome of sensitisation, although Duplantie et al. 71 and Hawk et al. 72 explicitly dealt not only with sensitisations, but also with a broader outcome set, such as the impact on infant health and/or on subsequent pregnancies. The remaining two studies were cost-minimisation studies, with no evidence cited to support this approach. None of the studies considered any adverse effects associated with the provision of NIPT or the administration of anti-D immunoglobulin. None of the studies considered the clinical effectiveness and/or cost-effectiveness of NIPT in ethnic minority groups. Except for one study,68 most studies were not explicit in considering that most NIPT performance assessments have been undertaken in white European populations and, thus, its reliability in minorities is still to be fully demonstrated. Overall justifications and descriptions of the alternatives being compared were generally clear, with most studies comparing more than two alternative scenarios. The viewpoint of the analyses was mentioned in most studies and implicitly justified by the public health systems in which the studies were conducted.
Data
Studies utilised evidence on costs and/or effects from a variety of sources. Sources for the diagnostic accuracy of NIPT Fetal RhD genotyping were based mainly on diagnostic studies aimed at verifying test performance, including three studies58,69,70 that considered evidence collected from subjects in the underlying cohort studies. These types of observational studies are inherently prone to bias and tools exist to appraise them [e.g. Standards for Reporting Diagnostic accuracy studies,74 Quality Assessment of Diagnostic Accuracy Studies (QUADAS)75 or the more recent update QUADAS-214]. To our knowledge, these tools were not used to appraise the study findings. Sources for the effectiveness of anti-D immunoglobulin varied across the different studies and were not based on systematic reviews but mainly on jurisdiction-specific sensitisation estimates. Studies that considered broader outcomes associated with sensitisation (i.e. haemolytic disease and impact of future pregnancies) populated these parameters with relevant published evidence. 76–78
Three studies reported the methods of collecting health-care resource use data and the unit costs applied to them. The majority specified the currency and price date; however, almost all failed to provide details on whether or not any price and currency conversion adjustments were made. One study72 did not report unit costs and quantities separately. No study valued health benefits or examined changes in productivity or its associated costs.
Two key aspects in these studies were the unit cost of the diagnostic test itself and the cost of the anti-D immunoglobulin treatment. The cost of NIPT varied significantly across studies from approximately €20.0070 (2012 prices) to US$45072 (2013 prices) per sample, with some including blood type, RhD determination and antibody screen. The NIPT cost range in the studies that explicitly stated that a high-throughput method was being assessed varied from £16.2568 (2011 prices) to CA$34.4573 (2015 prices). This may indicate that studies reporting a high unit cost for NIPT71,72 were not based on a high-throughput process. The majority of studies that provided a reference for the NIPT cost figures obtained these from the government58,71,73 or from laboratory genetic test companies. 72 A relevant consideration in relation to the cost of NIPT is whether or not the test is also subject to additional royalty fees that could affect the unit cost. For the majority of studies it is not clear if this fee was already included in the diagnostic test unit cost. Only the study by Szczepura et al. 68 explicitly considered this aspect by exploring the robustness of the results by varying the fee from zero to £46.50, the latter cost being the unit cost of a commercial testing kit including the royalty fee. Significant variation was also found in the unit costs per dose of anti-D immunoglobulin, which varied from £33.5068 (2011 prices) to US$462.0072 (2013 prices). None of the studies considered the potential for further costs associated with the introduction of NIPT in terms of additional antenatal care appointments or counselling with regard to test implications.
Analysis and interpretation of results
The two cost-minimisation studies69,70 took a simple approach and evaluated direct medical costs associated with the management of the RhD-negative pregnant women. Of the five cost-effectiveness studies, only one58 explicitly stated the time horizon of costs and benefits and the discount rate used in the analysis. Uncertainty was assessed in the majority of studies58,71–73 using deterministic sensitivity and scenario analysis. Only one of these58 reflected the need to jointly consider uncertainty in all parameter inputs through probabilistic methods.
Except for Szczepura et al. ,68 all cost-effectiveness studies mentioned the timing for when NIPT was offered to pregnant women. This was generally assumed across studies to happen at around 12 weeks’ gestation (typically at first routine antenatal care appointment). This assumption was largely supported by the fact that sufficiently high test diagnostic accuracy levels were expected at that stage of the pregnancy. Benachi et al. 69 found that greater cost savings were possible when NIPT was given in the first trimester than in the third trimester owing to the avoidance of costs associated with the management of potentially sensitising events in the intervening period. Their analysis shows that NIPT early in pregnancy (first trimester) was a cost-reduction strategy in comparison with performing the test later in pregnancy (third trimester), saving, on average, €38.00 per patient (2012 prices).
Teitelbaum et al. 73 and Szczepura et al. 68 were the only two research studies that, in their analyses, factored in the issue of NIPT fetal RhD genotyping producing inconclusive results and therefore performing SA over the inconclusive rate. Their analyses assumed that inconclusive test results would be treated as positive test results and, thus, women were assumed to receive RAADP.
Generally, the cost-effectiveness studies highlighted that the main limitations of their analysis were the external validity of the results, the uncertainty over the cost of the test and the associated royalty fee, the cost of clinically managing sensitisations, the fact the ethnic background of the target population had not been fully accounted for and the impact of this on the reliability of test assays.
Results of included studies
In terms of conclusions, conflicting results were reported across the existing economic studies. Three studies68,71,72 reported NIPT fetal RhD genotyping not to be cost-effective or of no economic benefit. Hawk et al. 72 and Szczepura et al. 68 reported that the main factor driving these factors was the cost of the test itself (i.e. the clinical and economic benefits were not sufficient to offset the additional costs of the test). Szczepura et al. 68 also stated that the implementation of NIPT in the clinical pathway of the RhD-negative pregnant woman was not expected to produce important clinical benefits. Supporting this was an estimation of the potential rise in the number of sensitised women if NIPT sensitivity fell below 99.9%.
Two studies58,69 reported that NIPT is cost saving compared with no RAADP (i.e. compared with postpartum anti-D only). Only one study73 found NIPT for targeted RAADP to be cost saving compared with non-targeted RAADP, which also estimated no increase in the risk of sensitisation if NIPT were to be used. Duplantie et al. 71 found that targeting of RAADP based on the immunological RhD typing of the father is cost-effective compared with the use of NIPT.
Overall, the quality of the included studies’ findings is uncertain because of a lack of reporting of the validity of the diagnostic accuracy outcomes used. Furthermore, although SA exercises were generally done over some key parameters, the degree of uncertainty in the cost-effectiveness estimates is generally difficult to establish.
Relevance to the NHS and current decision problem
One of the key aspects of this review is to address how relevant study assumptions and findings are to the UK. None of the study approaches and findings reviewed was considered to be generalisable to the decision problem as set out in the NICE scope for the current diagnostic assessment. The scope for this decision problem includes an evaluation of the introduction of NIPT at different gestation points, the impact of the test result on the administration of anti-D immunoglobulin treatment routinely and post partum, and the impact of sensitisation on infant health and/or on subsequent pregnancies. Only one68 of the seven economic studies reviewed directly relates to the UK. This study, however, did not explicitly explore how the introduction of NIPT could impact on costs relating to potentially sensitising events. In addition, it assumed that postpartum testing and treatment would be unaffected by NIPT results. Furthermore, no assessment of the timing of NIPT or any consideration of the impact on subsequent pregnancies was undertaken. Therefore, limited UK-specific information exists that explicitly relates to the decision problem as specified in the scope for this diagnostic appraisal. Although some studies are from Canada and the USA, countries in which similar guidance to that in the UK exists on the prevention of sensitisation, relevance to the UK and generalisability of findings can be questioned, as there are crucial health-care system differences and differences in how anti-D immunoglobulin policies have been implemented over recent decades.
Chapter 5 Independent economic assessment
Overview
A de novo independent economic model was developed to assess the cost-effectiveness of high-throughput NIPT to identify fetal rhesus D status in women who are RhD negative and not known to be sensitised to the RhD antigen. The conceptualisation and development of the de novo model was informed by existing economic modelling studies described in Chapter 4, Methodology of the cost-effectiveness review and the independent economic model used to inform NICE technology appraisal (TA)156 on the clinical and cost-effectiveness of RAADP. 62 The model provides a framework for the synthesis of diagnostic accuracy reported in Chapter 3 with a range of other relevant parameters required to establish cost-effectiveness.
A decision-analytic model using a decision tree cohort approach was developed to estimate, based on best available data, the costs and health outcomes of the relevant testing and treatment strategies. The model was made up of two main elements: (1) an identification part reflecting the diagnostic performance and costs of the alternative identification strategies and (2) a treatment part that evaluated the subsequent costs and outcomes [expressed in quality-adjusted life-years (QALYs)] of alternative care pathways. The treatment part of the model was based closely on the economic model for NICE TA156 developed by researchers at the School of Health and Related Research (ScHARR), University of Sheffield. 62 This model was kindly provided on request and was subsequently modified and updated to accommodate all the required changes for the cost-effectiveness assessment of the introduction of high-throughput NIPT in pregnant RhD-negative women’s clinical pathway, as outlined in Appendix 10.
The decision model is populated using the results from the systematic clinical review on the diagnostic accuracy of high-throughput NIPT as described in Chapter 3 and other relevant parameters required to provide a link between the diagnostic accuracy of a given identification strategy, the impact on subsequent treatment decisions and the ultimate effect on health outcomes and costs. The determination of the RhD status of fetuses through high-throughput NIPT may impact the administration of anti-D immunoglobulin prophylactically following potentially sensitising events, routinely and at birth. Routine prophylactic anti-D immunoglobulin may be avoided by RhD-negative women who are indicated to be carrying a RhD-negative fetus. The use of fetal RhD status testing may also prevent further testing (i.e. FMH) as well as the administration of prophylactic anti-D immunoglobulin after a potentially sensitising event where the test result indicates a RhD-negative fetus. In addition, high-throughput NIPT for fetal RhD status determination may impact postpartum testing (i.e. cord blood typing and FMH) and postpartum anti-D immunoglobulin administration. As high-throughput NIPT is not a perfect test, women who receive inconclusive or false-positive test results will not avoid unnecessary use of anti-D immunoglobulin and the costs and consequences of suboptimal use of anti-D immunoglobulin prophylaxis in women who receive false-negative results need to be accounted for.
The following sections outline the decision problem and the structure of the model and also provide an overview of the key assumptions and data sources used to populate the model.
Overall aims and objectives of the independent economic assessment
The cost-effectiveness assessment of the use of high-throughput NIPT to identify fetal rhesus D status had the following overall main objectives:
-
To produce a de novo cost-effectiveness model assessing the cost-effectiveness of high-throughput NIPT to identify fetal RhD status in RhD-negative women not known to be sensitised to the RhD antigen.
-
To assess the impact of alternative scenarios related to the timing of the test and the impact of the test on the use of antenatal anti-D immunoglobulin prophylaxis for sensitising events and postdelivery testing and postpartum anti-D immunoglobulin administration.
Intervention and comparator pathways
Current NICE clinical guidance on antenatal care7 recommends that women be offered testing for blood group and rhesus D status in early pregnancy. All pregnant women identified as RhD-negative would be tested for the presence of RhD antibodies. Women identified as RhD-negative and found not to have RhD antibodies are not yet sensitised and form the population for this appraisal. In these women, anti-D immunoglobulin is recommended, both as prophylaxis and following potential sensitising events, to prevent sensitisation occurring. 2
Routine antenatal anti-D prophylaxis is recommended to be given as two doses at weeks 28 and 34 of pregnancy, or as a single dose between 28 and 30 weeks. Supplementary doses of anti-D immunoglobulin should also be administered prophylactically after a potentially sensitising event. 2,8 Potentially sensitising events include those that may lead to FMH, such as medical interventions (e.g. chorionic villus sampling, amniocentesis or external cephalic version), terminations, late miscarriages, antepartum haemorrhage and abdominal trauma. Following a potential sensitisation event, the recommended minimum dosage of anti-D immunoglobulin increases with gestational age (i.e. a higher dose for > 20 weeks’ gestation), and FMH testing is used to inform the actual dose after 20 weeks’ gestation.
Following birth, RhD typing should be performed on a cord blood sample to determine the RhD status of the baby. If the baby is confirmed to be RhD positive, it is recommended that previously non-sensitised RhD-negative pregnant women receive anti-D immunoglobulin within 72 hours following delivery, with the actual dose guided by FMH results. This represents the pathway and current clinical practice of the management of RhD-negative pregnant women not known to be sensitised.
The intervention technology of this assessment is high-throughput NIPT for fetal rhesus D status. By analysing cell-free fetal DNA in the plasma of RhD-negative pregnant women, high-throughput NIPT is able to predict fetal RhD genotype. High-throughput NIPT for fetal RhD status may enable prophylactic anti-D immunoglobulin to be withheld from women who are RhD-negative and carrying a RhD-negative fetus. These women could avoid unnecessary treatment with anti-D immunoglobulin, along with the potential risk associated with blood products. The results of NIPT could impact on the care pathway in the following ways:
-
For women in whom the high-throughput NIPT indicates the presence of a RhD-negative fetus:
-
avoidance of RAADP
-
avoidance of prophylactic anti-D immunoglobulin and FMH tests following potentially sensitising events
-
avoidance of cord serology testing, fetal maternal haemorrhage test and administration of anti-D immunoglobulin following delivery.
-
-
For women in whom the high-throughput NIPT indicates the presence of a RhD-positive fetus:
-
avoidance of cord serology testing in favour of routine FMH testing and postpartum anti-D immunoglobulin following delivery.
-
Model structure
Modelling methodology and scope
A decision-analytic model using a decision tree structure simulates the experience of a hypothetical cohort of RhD-negative pregnant women not known to be sensitised to the RhD antigen, with and without the introduction of high-throughput NIPT for fetal RhD status. A pregnant woman enters the model after having been identified as RhD-negative and not yet sensitised based on the results of tests from bloods drawn either at first contact with the doctor or midwife (the date at which pregnancy is reported or established) or at the booking appointment (8–12 weeks’ gestation). All further contacts between the woman and the health service are informed by the recorded test results. At the routine 16-week visit the woman is informed about her RhD status, whether or not she is sensitised and how these results impact on further management. If the woman contacts the health service following any potentially sensitising event she may be offered anti-D immunoglobulin and, if after 20 weeks’ gestation, a FMH test. Women provided with RAADP receive it at either or both of the routine visits at 28 and 34 weeks’ gestation. At delivery, a sample of cord blood may be taken and the baby’s RhD status established to guide the use of FMH tests and the administration of postpartum anti-D immunoglobulin.
All high-throughput NIPT are assumed to be performed early enough to determine the use of RAADP at 28 weeks’ gestation. Figure 11 shows the current schedule of routine antenatal care appointments and the potential placement of NIPT.
FIGURE 11.
Excerpt from NICE schedule of appointments in routine antenatal care. © NICE 2016. All rights reserved. Schedule of Appointments in Routine Care. 79 Available from: http://beta.pathways.nice.org.uk/pathways/antenatal-care/schedule-of-appointments-in-routine-antenatal-care. NICE guidance is prepared for the National Health Service in England, and is subject to regular review and may be updated or withdrawn. NICE has not checked the use of its content in this report to confirm that it accurately reflects the NICE publication from which it is taken.
In addition to the first contact/8–12 weeks’ gestation booking appointment, the points of routine contact at which blood could be drawn for NIPT are the 16-week visit and 18- to 20-week scan (at which outstanding routine screening tests are offered). Other opportunities may include attendance to receive the whooping cough vaccine and the routine 25 weeks’ gestation visit for first pregnancy only. Once the results of any high-throughput NIPT are known, they will be communicated to the woman and recorded with the potential to inform all further contacts and decisions regarding testing and treatment. We assume that RAADP and management for potentially sensitising events would be subsequently offered only to women in whom the test result indicates that their fetus is RhD positive and in whom the test result is inconclusive. For women in whom the high-throughput NIPT result is inconclusive, the existing care pathway will remain unchanged and they would receive the same management as women for whom the results of NIPT indicate a RhD-positive baby. We assume that provision of NIPT can be incorporated into routine antenatal care without requiring additional visits (to undertake the test or to communicate the results of test). Similarly, in the base case we do not model additional resources within existing antenatal care appointments to draw blood.
As previously mentioned, the model may be separated into two main elements: (1) an identification part reflecting the diagnostic performance and costs of the alternative identification strategies and (2) a treatment part evaluating the subsequent costs and outcomes (expressed in QALYs) of alternative care pathways. The main aim of the first model element is to divide the cohort according to fetal RhD status and treatment administered (i.e. routine anti-D immunoglobulin, FMH tests and anti-D immunoglobulin for potentially sensitising events, cord serology, FMH tests and postnatal anti-D immunoglobulin). This determines when receipt of anti-D immunoglobulin is appropriate (true positive in terms of NIPT result and/or postnatal cord serology and inconclusive result but pregnant with RhD-positive fetus), when avoidance of anti-D immunoglobulin is appropriate (true negative in terms of NIPT result), when anti-D immunoglobulin is unnecessary (false positive or inconclusive in terms of NIPT result and carrying a RhD-negative fetus) and when avoidance of anti-D immunoglobulin is potentially harmful (false negative in terms of NIPT result). Aspects such as the diagnostic test performance (including inconclusive results and results at different gestation timings), compliance with high-throughput NIPT and anti-D immunoglobulin treatment and the effectiveness of anti-D immunoglobulin all inform the estimation of the probability of sensitisation for each of these groups. The second model element (i.e. the treatment part) considers the short- and long-term consequences of sensitisations (i.e. fetal or neonatal death, minor and major development problems of the child) for the first, second, third and subsequent pregnancies. Costs and utilities are then evaluated for the different components and for each of the alternative pathways.
Four alternative ways in which the use of high-throughput NIPT may impact on the existing postpartum care pathway were considered.
-
NIPT postpartum scenario 1 (PP1): postpartum cord blood typing and FMH testing would continue to be performed, as per current guidelines, in all women regardless of the fetal RhD status identified through high-throughput NIPT.
-
NIPT postpartum scenario 2 (PP2): postpartum cord blood typing, FMH testing (and by implication anti-D immunoglobulin) would be withheld if high-throughput NIPT of fetal RhD status identifies a RhD-negative fetus but would continue to be performed if high-throughput NIPT was inconclusive or had identified a RhD-positive fetus.
-
NIPT postpartum scenario 3 (PP3): postpartum cord blood typing would be performed if high-throughput NIPT of fetal RhD status identifies a RhD-negative fetus. FMH testing and postdelivery anti-D immunoglobulin would be administered if high-throughput NIPT was inconclusive or identifies a RhD-positive fetus.
-
NIPT postpartum scenario 4 (PP4): postpartum cord blood typing not performed in any women. FMH testing and postdelivery anti-D immunoglobulin administered if high-throughput NIPT was inconclusive or had identified a RhD-positive fetus.
The impact that postdelivery testing has on the cost-effectiveness results is explored using separate scenarios in the model. In reality, these four separate scenarios actually represent separate and distinct testing and management strategies and, hence, could also be considered to represent relevant strategies that should be directly compared in the cost-effectiveness assessment.
The cost-effectiveness of high-throughput NIPT is determined by comparing with current practice (i.e. no use of high-throughput NIPT), which comprises (1) RAADP and supplementary anti-D immunoglobulin (as required based on potentially sensitising events) offered to all RhD-negative pregnant women and (2) further postpartum anti-D immunoglobulin offered to all RhD-negative women whose baby’s RhD status is confirmed to be positive after cord blood typing.
A schematic representation of the model is provided in Figure 12a and b. Note that this figure does not provide a comprehensive representation of all components being considered in each alternative strategy, including the postpartum scenarios. The four postpartum scenarios for how the introduction of NIPT could impact on the use of cord serology, fetal maternal haemorrhage tests and anti-D immunoglobulin use following delivery are detailed in Table 17.
FIGURE 12.
Decision-analytic model schematic representation of RhD-negative pregnant women pathways. (a) No high-throughput NIPT and RAADP (current practice, no test and RAADP); and (b) high-throughput NIPT and targeted RAADP.
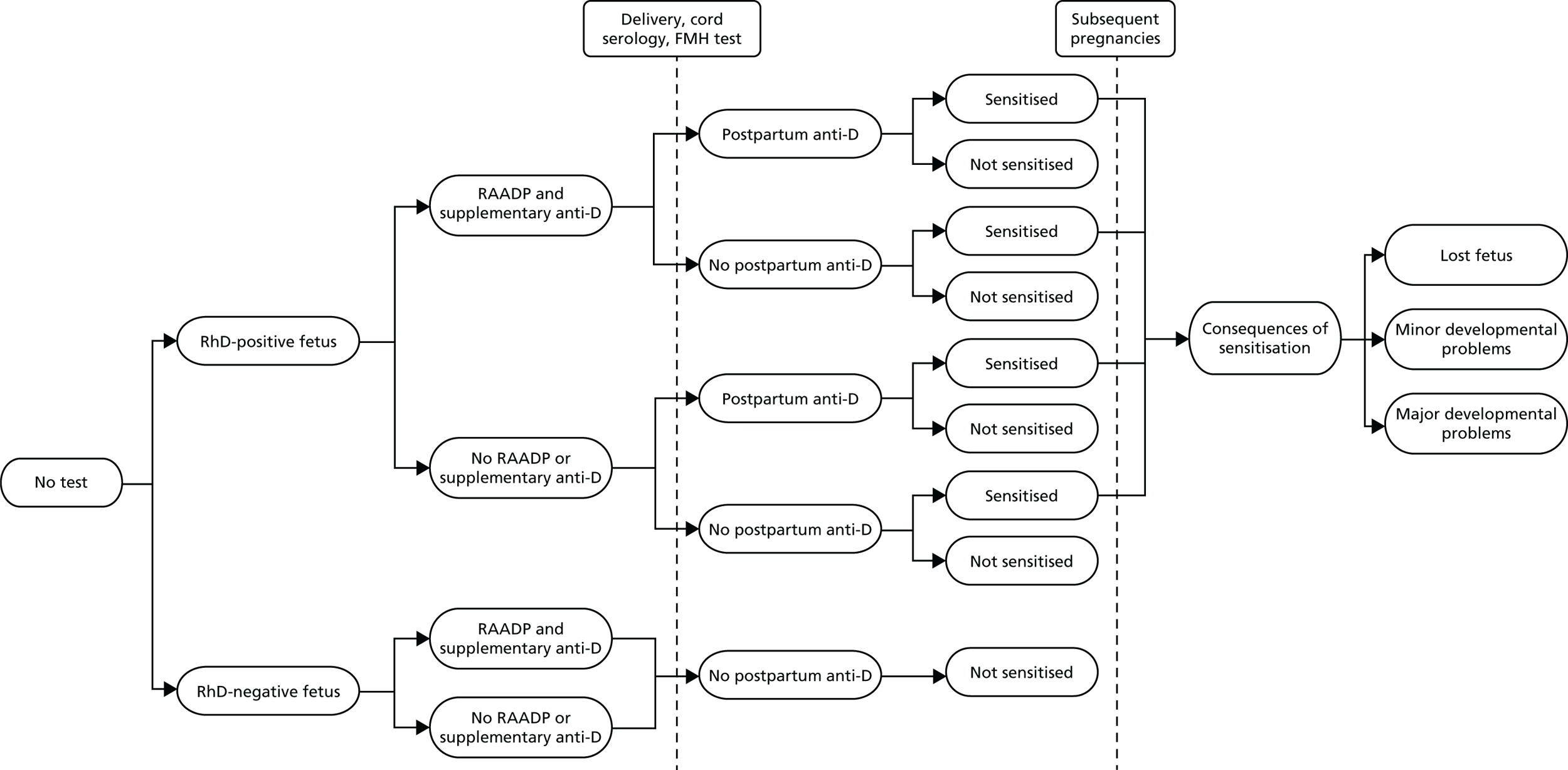

| Scenario | High-throughput NIPT result | Cord serology | FMH test | Postpartum anti-D |
|---|---|---|---|---|
| PP1 | Any | Yes | Yes if CS+ | As guided by CS and FMH test |
| PP2 | T– | No | No | No |
| T+, inc | Yes | Yes if CS+ | As guided by CS and FMH test | |
| PP3 | T– | Yes | Yes if CS+ | As guided by CS and FMH test |
| T+, inc | No | Yes | Yes with additional dose per FMH test | |
| PP4 | T– | No | No | No |
| T+, inc | No | Yes | Yes with additional dose per FMH test |
The model considers the total number of children who would be born to each RhD-negative woman in order to capture the effect of any sensitisation on all subsequent pregnancies based on national fertility rates. We assume that the consequences of sensitisation do not affect the pregnancy in which it occurs (with respect to treatments and tests administered, management and health outcomes of the resultant RhD-positive baby) but rather only subsequent pregnancies. Under current practice, a woman who is sensitised during pregnancy will be identified at the start of her next pregnancy, when she will be tested for antibodies to the RhD antigen. As a consequence of having been sensitised, the woman will be subject to more intense antenatal care in all subsequent pregnancies (see Cost of management of sensitisation) and any further RhD-positive fetuses are at risk of adverse health consequences (see Cost of high-throughput non-invasive prenatal testing and Cost of management of sensitisation). First and subsequent pregnancies together with long-term consequences of sensitisations, in terms of costs and utilities, are evaluated with a yearly cycle and a lifetime horizon. This lifetime horizon includes the full life expectancy of any fetus lost as a consequence of sensitisation. The decision model follows a NHS perspective, and all costs and effects are discounted at a rate of 3.5% each year. The main outcomes of interest within the model are the total lifetime costs and total lifetime QALYs for each of the alternative pathways. Other outcomes recorded in the model include:
-
number of sensitisations and the associated costs
-
number of affected fetuses following sensitisation
-
number of fetuses lost and associated QALY loss
-
cost per life-year gained.
What alternative scenarios have been modelled?
In addition to the five alternative pathways compared in the base-case analysis, we compare the inclusion of the high-throughput NIPT at specific gestational ages. These are determined based on available data that show how the diagnostic accuracy of the test varies with gestational age. The timing of the test is important not only in terms of diagnostic performance but also in terms of the cost of managing potentially sensitising events. Although the majority of these are thought to occur in the third trimester (weeks 29–40), any that occur prior to the use of the high-throughput NIPT will incur the cost of anti-D immunoglobulin for all women regardless of fetal RhD status. We further explore the impact of variation in compliance with anti-D immunoglobulin.
Under current guidance, more recent data on RAADP coverage indicate an uptake of approximately 99.0% in women who are still pregnant at 28 weeks and where the father is not established as RhD negative. 8 In addition, postpartum anti-D immunoglobulin current uptake is believed to be also close to 100%. 8 However, data relating to the uptake of routine and postpartum anti-D immunoglobulin in the presence of fetal RhD status identification are scarce (see Chapter 3). Finally, we consider alternative scenarios for the proportion of women in whom the NIPT result is inconclusive. The rate of inconclusive results may reach > 14% and these are typically managed as RhD-positive results (see Chapter 3, Results: assessment of diagnostic accuracy). However, women in whom the high-throughput NIPT result is inconclusive are likely to differ systematically from those in whom the test result is positive, with ethnicity being the most important factor.
Model input parameters
This section provides a description of key model input parameters and the evidence used to inform these. A full list of parameters and their characteristics is given in Table 23.
Target population
The number of pregnancies in RhD-negative women in England was estimated to be 99,225 per year. This represents a cross-section of all pregnancies and the proportions of first, second, third and subsequent pregnancies are used to characterise the total fertility rate of a typical RhD-negative woman. This estimate was based on a birth rate of 12.2 per 1000 women per year80 and assumes that 15% of the population is RhD negative. 3
Proportion of RhD-positive babies born to RhD-negative women
The RhD status of babies does not depend solely on the zygosity of the mother but also of the father. The RhD-negative gene is recessive. Following Mendel’s law on inheritance,81 if the father is homozygous (i.e. he has two RhD-positive genes) all of his children will be RhD positive, but if he is heterozygous (i.e. he has one RhD-positive gene and one RhD-negative gene) his children will have a 50% chance of being RhD-negative. Therefore, as in the NICE TA156,62 the model assumes that the proportion of RhD-positive babies born to RhD-negative women is a function of (1) the proportion of RhD-positive men (assumed to be identical to the proportion of RhD-positive women, thus, the complement of the proportion of RhD-negative women), (2) the proportion of heterozygous fathers and (3) the proportion of heterozygous fathers having RhD-positive babies. Although the probability of having a RhD-positive baby in subsequent pregnancies can be estimated conditional on knowledge of the RhD status of the first baby, we do not split the cohort in this way. The use of high-throughput NIPT among RhD-negative women not yet sensitised to the RhD antigen is not expected to be determined on the basis of the RhD status of previous offspring. It is therefore unnecessary to split the cohort according to this characteristic and so we apply the same overall rate of RhD-positive babies across all pregnancies. This equates to approximately 62%, as described in Table 18.
| Parameter | Mean value | Standard error | Distribution | Source/calculation |
|---|---|---|---|---|
| Total number of births | 659,213 | – | – | Office for National Statistics, 201382 |
| Proportion of pregnancies accounted for by Rh-negative women (a) | 15.0% | – | – | NHS Digital, Hospital Episode Statistics, 2013–143 |
| Proportion of heterozygous fathers (b) | 55.0% | 10.0% | Normal | Roman and Pernell, 200283 |
| Proportion of heterozygous fathers having RhD-positive babies (c) | 50.0% | – | – | Assumption |
| Proportion of RhD-positive babies in Rh-negative women (first baby) (d) | 61.6% | – | Uncertainty captured from above (f) | Estimate based on information above [ = (1 – a) – ((1 – a) × b × c)] |
| Probability that baby will be RhD-positive in second, third and subsequent pregnancies | 61.6% | – | Uncertainty captured from above (f) | Assumed the same as the proportion of RhD-positive babies in Rh-negative women (first baby) (d) |
Diagnostic accuracy of non-invasive prenatal testing
Data on the diagnostic accuracy of high-throughput NIPT are based on the meta-analyses summarised in Chapter 3, Results: assessment of diagnostic accuracy. The base case utilises the pooled results for the subgroup of UK (Bristol-based) studies in which inconclusive results are considered as test positive. These were considered to be the most relevant to the English setting. Sensitivity, specificity (with 95% CIs) and the correlation between these two test accuracy dimensions (on the log-odds scale) were used to inform log-normal distributions within the decision model. Note that the correlation estimate for the UK (Bristol) approach was based on only three studies (Table 19). SAs were performed based on pooled results from all studies and when inconclusive results were not considered as test positive. In general, high-throughput NIPT accuracy is consistently high across the different approaches to the diagnostic meta-analysis. The subgroup of only UK studies shows a lower FNR and a slightly higher FPR than other scenarios.
| Pooled NIPT accuracy from bivariate synthesis model | Sensitivity (mean, 95% CI) | Specificity (mean, 95% CI) | Correlation between sensitivity and 1 – specificity (log-odds scale) | Distribution |
|---|---|---|---|---|
| All studies (excluding inconclusive results) | 0.996 (0.991 to 0.999) | 0.987 (0.981 to 0.991) | 0.461 | Log-normal |
| All studies (treating inconclusive results as if testing positive) | 0.997 (0.992 to 0.999) | 0.962 (0.943 to 0.975) | –0.316 | Log-normal |
| Only studies reporting inconclusive resultsa (treating inconclusive results as if testing positive) | 0.996 (0.989 to 0.998) | 0.957 (0.932 to 0.972) | –0.074 | Log-normal |
| UK Bristol studies only (treating inconclusive results as if testing positive) | 0.998 (0.992 to 0.999) | 0.942 (0.92 to 0.959) | –1.000 | Log-normal |
Only one study12 extensively examined the test performance at multiple gestation time points. In scenario analysis these results were used to assess the cost and consequences of introducing high-throughput NIPT at different gestation ages (Table 20). We considered that high-throughput NIPT might be targeted at more specific gestational ages from 11 weeks’ gestation and not after 24 weeks’ gestation, and thus, in the model, we compared the diagnostic accuracy reported for 11–13 weeks, 14–17 weeks and 18–23 weeks (see Sensitivity analyses results).
| NIPT accuracy per gestational age (weeks)12 | Sensitivity (mean, standard error) | Specificity (mean, standard error) | Distribution |
|---|---|---|---|
| Treating inconclusive results as if testing positive | |||
| < 11 | 0.9685 (0.0079) | 0.9440 (0.0123) | Log-normal |
| 11–13 | 0.9983 (0.0023) | 0.9525 (0.0114) | Log-normal |
| 14–17 | 0.9967 (0.0045) | 0.9534 (0.0141) | Log-normal |
| 18–23 | 0.9982 (0.0003) | 0.9304 (0.0138) | Log-normal |
| > 24 | 1.0000 (0.0010) | 0.9574 (0.0076) | Log-normal |
| Excluding inconclusive results | |||
| < 11 | 0.9615 (0.0079a) | 0.9970 (0.0123a) | Log-normal |
| 11–13 | 0.9981 (0.0023a) | 0.9884 (0.0114a) | Log-normal |
| 14–17 | 0.9963 (0.0045a) | 0.9956 (0.0141a) | Log-normal |
| 18–23 | 0.9980 (0.0003a) | 0.9847 (0.0138a) | Log-normal |
| > 24 | 1.0000 (0.0010a) | 0.9900 (0.0076a) | Log-normal |
Non-invasive prenatal testing inconclusive results
In the UK studies that inform the base case for the decision model, the pooled proportion of inconclusive NIPT results was 6.7%. Across all diagnostic studies that report the number of inconclusive results this proportion is lower at 4.0%. The results of the diagnostic accuracy studies suggest that the probability of a RhD-positive baby is higher among women in whom the high-throughput NIPT is inconclusive than in all RhD-negative women (see Chapter 3, Inconclusive test results). In Proportion of RhD-positive babies born to RhD-negative women, it was estimated that the probability of RhD-negative women having RhD-positive babies in the first and subsequent pregnancies was 61.6%. In the presence of high-throughput NIPT inconclusive results it is estimated that this probability is 70.1%, irrespective of the pregnancy. This probability is slightly reduced (70.7%) if only UK studies are considered. These last two probabilities are used to estimate the positive predictive value of NIPT, and in SA around the postpartum management of women with inconclusive NIPT results (SA8).
Effectiveness of anti-D immunoglobulin
The introduction of high-throughput NIPT into the care pathway will be used to determine the level of use of anti-D immunoglobulin. Anti-D immunoglobulin affects the rate of sensitisation in women carrying RhD-positive fetuses and carries a potential risk of adverse effects as it is derived from blood products. The costs and consequences of the introduction of high-throughput NIPT are therefore determined by:
-
the efficacy of anti-D immunoglobulin in preventing sensitisation, as this determines the health and cost implications for women from whom this is incorrectly withheld because of a false-negative high-throughput NIPT result
-
the costs and adverse effects associated with administration of anti-D immunoglobulin.
The clinical effectiveness and cost-effectiveness of RAADP in RhD-negative women has been previously established in NICE TA4184 and most recently in NICE TA156. 62 No new systematic reviews of RAADP with studies not considered in TA156 were identified. We maintain consistency between the NICE TA process and the diagnostics assessment of high-throughput NIPT for fetal rhesus D status by utilising the RAADP efficacy estimated based on the same set of clinical effectiveness studies that were considered to be most representative of the UK within NICE TA156. The parameter estimates applied in our base-case analyses are based on the synthesis presented within NICE TA156. The impact of using alternative estimates reported in a related publication by Turner et al. ,63 published after NICE TA156 had been completed, is explored within a separate SA. Evidence for the clinical effectiveness of the postpartum use of anti-D immunoglobulin was sourced from a previous Cochrane review. 65 The clinical effectiveness estimates of RAADP and postpartum use of anti-D immunoglobulin reported across these separate sources are reported in Table 21.
| Source | Sensitisation, odds ratio (95% CI) | Sensitisation rate, % (95% CI) | |||
|---|---|---|---|---|---|
| RAADPa | At birth, follow-up up to 6 months, with postpartum anti-Db | (Baseline) no RAADPa | RAADP (pooled using meta-analysis) | No RAADP and no postpartum anti-D | |
| NICE TA15662 | 0.37 (0.21 to 0.65) | – | 0.95 (0.18 to 1.71) | 0.35 (0.29 to 0.40) | – |
| Turner et al.63 | 0.31 (0.17 to 0.56) | – | 0.95c (0.18 to 1.71) | 0.40 (0.16 to 0.70) | – |
| Turner et al.,63 (single dosed) | 0.42 (0.17 to 0.73) | – | 0.95c (0.18 to 1.71) | 0.30 (0.16 to 0.53) | – |
| Turner et al.,63 (two dosee) | 0.31 (0.09 to 0.65) | – | 0.95c (0.18 to 1.71) | 0.31 (0.09 to 0.62) | – |
| fCrowther et al.65 | – | 0.08 (0.06 to 0.11) | 0.95c (0.18 to 1.71) | – | 10.7 (8.0 to 13.8) |
National Institute for Health and Care Excellence technology appraisal on routine antenatal anti-D prophylaxis
The NICE TA156 found 10 relevant studies that evaluated the clinical effectiveness of RAADP. These studies varied in terms of their patient selection criteria and dosage regimens. Despite this apparent heterogeneity across studies, overall consistency of results was obtained when synthesising relevant data from different subsets of the evidence base. The result of a fixed-effect meta-analysis of two non-randomised community-based UK studies that used a dosage regimen of 500 international units (IUs) at 28 weeks and 34 weeks were considered to be most relevant to the UK. Based on these results, the introduction of RAADP, in addition to the use of anti-D immunoglobulin for potentially sensitising events and post partum, was assumed to reduce the sensitisation rate from 0.95% (95% CI 0.18% to 1.71%) to 0.35% (95% CI 0.29% to 0.40%). These sensitisation rates are conditional on anti-D immunoglobulin treatment being provided at potentially sensitising events also. This gives an odds ratio for the risk of sensitisation of 0.37 (95% CI 0.21 to 0.65) for RAADP compared with no RAADP and an absolute reduction in risk of sensitisation in RhD-negative mothers at risk (i.e. of carrying a RhD-positive child) of 0.6%. These estimates were used in the economic model, which informed the NICE TA156 and are also used to inform the base-case analysis for the de novo model presented here.
Turner et al.63
Following the publication of the NICE TA156, Turner et al. 63 revisited the effectiveness of RAADP for preventing sensitisation in pregnant RhD-negative women. This publication used alternative meta-analytic methods, which allow for the adjustment of both methodological limitations (internal biases) in the set of studies to be combined and differences in study design relative to the research question of interest (external biases). The impact of differences in dose regimen, follow-up times and study populations were evaluated by clinical experts (‘assessors’) with knowledge of anti-D immunoglobulin prophylaxis, and the impact of methodological flaws in the studies was evaluated by assessors with quantitative expertise. Elicited evidence on the bias for each study was used to adjust the study effect estimates and standard errors, while acknowledging uncertainty in the extent of bias.
After adjusting for differences in study quality and design, the pooled odds ratio for sensitisation was estimated to be 0.31 (95% CI 0.17 to 0.56), with no evidence of heterogeneity (I2 = 0%). Pooled results were similar to the ones obtained from the NICE TA156 meta-analysis, which included only two studies. Thus, this result substantiated the already existing evidence on the effectiveness of RAADP in preventing sensitisation of pregnant RhD-negative women. This odds ratio is applied in a SA for the de novo model presented here.
Postpartum use of anti-D
Current anti-D immunoglobulin postpartum prophylaxis states that following a baby’s birth, ABO and RhD typing should be performed on a cord blood sample. If the baby is confirmed to be RhD positive, all RhD-negative, previously non-sensitised, women should receive a minimum of 500 IU of anti-D within 72 hours of delivery. Maternal samples should be tested for FMH and additional dose(s) given as guided by FMH tests. 2,7
A Cochrane systematic review was identified that assessed the effectiveness of anti-D immunoglobulin in RhD-negative women who had given birth to RhD-positive babies. 65 Data on six eligible studies, comparing postpartum anti-D immunoglobulin prophylaxis with no treatment or placebo, were synthesised. The estimated odds ratio for sensitisation 6 months after birth with postpartum anti-D immunoglobulin was 0.08 (95% CI 0.06 to 0.11). The estimated odds ratio for sensitisation in subsequent pregnancies with postpartum anti-D immunoglobulin was 0.12 (95% CI 0.07 to 0.19). The former was estimated on five studies with approximately 7500 participants and the latter was based on four studies with approximately 1000 patients. Thus, on the basis of a larger sample size we assumed the former estimate to be the most representative of the effectiveness of postpartum anti-D immunoglobulin in the target population (reported in the last row of results in Table 20). Estimated benefits of postpartum anti-D immunoglobulin administration were observed irrespective of the ABO status of mother and child.
Potentially sensitising events
Following potentially sensitising events, the administration and dosage of anti-D immunoglobulin is conditional to the pregnancy stage in which the event occurs. Current guidance7 recommends that only in extraordinary sensitising events (such as ectopic pregnancy, molar pregnancy or therapeutic termination of pregnancy) should anti-D immunoglobulin be administered at < 12 weeks’ gestation. A minimum dose of 250 IU of anti-D immunoglobulin within 72 hours of the event is recommended to be administered if it occurs between 12 and 20 weeks’ gestation. For potentially sensitising events after 20 weeks’ gestation, a minimum anti-D immunoglobulin dose of 500 IU should be administered within 72 hours, with additional doses as guided by a test for FMH.
Evidence on the reported number of potentially sensitising events was found in the recent audit on anti-D immunoglobulin prophylaxis. 8 The probability of women having at least one (reported) potentially sensitising event was estimated to be 15.5%. Of these, 69.3% women were estimated to have had a FMH test and 95.8% women were estimated to have been treated with anti-D immunoglobulin following the event. It was estimated that approximately 80% of these events happened after 20 weeks’ gestation. We assume that these 80% of sensitising events are treated with the minimum required dose of 500 IU of anti-D immunoglobulin. For the remaining 20% of events (pre 20 weeks’ gestation events), we assumed that women received the minimum required dose of 250 IU of anti-D immunoglobulin.
The audit on anti-D immunoglobulin prophylaxis8 also provided information on the type of potentially sensitising event. It was estimated that the probability of women having a miscarriage (including stillbirth and intrauterine death) was 4.7%. We assumed that these fetal deaths were not a consequence of sensitisation and they are incorporated in the model only to adjust the amount of postpartum health resource consumption following delivery.
In contrast to women in whom the high-throughput NIPT result indicates that their fetus is RhD positive, women in whom the test shows that the fetus is RhD negative will not be offered prophylactic anti-D immunoglobulin treatment and will not be subject to FMH testing. This is an issue particularly for the false negatives (RhD-negative women with a RhD-positive fetus but for whom the test result was negative), as these women will, at most, receive only postpartum treatment. For women with false-negative NIPT results who receive only postpartum anti-D immunoglobulin, the model assumes a rate of sensitisation of 0.95%. This is likely to be an underestimate, as it includes receipt of anti-D immunoglobulin for potentially sensitising events. However, the only other estimate for the rate of sensitisation without RAADP is that based on no anti-D immunoglobulin at all, including no postpartum treatment (10.7%), which is likely to be a large overestimate, as the majority of events occur at birth (see Table 20). The true rate of sensitisation is likely to lie between 0.95% and 10.7%, but it appears reasonable that this rate will be closer to 0.95%.
Compliance with routine antenatal anti-D prophylaxis and postpartum anti-D immunoglobulin
The National Comparative Audit of Blood Transfusion 2013 on Anti-D Immunoglobulin Prophylaxis8 reported that, out of all eligible women, 99% received at least one RAADP injection. Full compliance (i.e. correct dose at the correct time) was found to be higher in the single-dose regime (90%) than in the two-dose regime (59%). In addition, the audit shows that a very high proportion of eligible women (98.4%) received postpartum anti-D immunoglobulin prophylaxis. Finally, for documented potentially sensitising events, it showed that approximately 96% of eligible women having these events received anti-D immunoglobulin.
Following the recent audit findings, within the de novo economic model it has been assumed that compliance with RAADP is 99.0%. This value was assumed for the base case and subject to scenario analysis, assuming a rate of 87.5% (i.e. the proportion receiving the correct dose at the correct time). Evidence from the audit points to higher compliance with the single-dose regimen than with the two-dose regimen for a number of reasons (e.g. cost, manufacturer supply, etc.) and there is a move towards the use of the single dose, over the two dose, with its market share reaching approximately 93%. 8 Thus, we did not adjust the compliance rate across RAADP regimen. In the model it has been also assumed that postpartum anti-D immunoglobulin compliance rate is 98.4%, again following evidence from the recent audit. 8 This value was subject to scenario analysis by assuming a rate of 91.6% (i.e. the proportion receiving the correct dose at the correct time).
Compliance with non-invasive prenatal testing given routine antenatal anti-D prophylaxis and postpartum anti-D immunoglobulin
The evidence for compliance with high-throughput NIPT is scarce, particularly in health systems in which the test is introduced after RAADP guidance is in place (see Chapter 3, Results: assessment of clinical effectiveness). In the absence of such evidence, and based on the already high rates of compliance assumed for current practice (99.0% for RAADP and 98.4% of women received at least one dose of anti-D immunoglobulin at RAADP and post-partum, respectively), we subsequently assume that the use of high-throughput NIPT has no additional impact on compliance. Therefore, it has been assumed that RAADP and postpartum anti-D immunoglobulin compliance is 99.0% and 98.4%, respectively, the same as in the no high-throughput NIPT scenarios.
Sensitisation outcomes
As for the independent economic model developed for NICE TA156 on RAADP, the current economic model considered a set of input parameters directly related to the consequences of sensitisation towards the fetus and the newborn infant, namely the implications of haemolytic disease. Three of these model input parameters were key to an appropriate representation of the possible health states, namely (1) the fetal loss rate per RhD-negative women at risk (i.e. carrying a RhD-positive baby), (2) the proportion of babies affected by haemolytic disease that resulted in minor developmental problems (these include, for instance, myopia, squint or delay in language and fine motor skills) and (3) the proportion of babies affected by haemolytic disease that resulted in major developmental problems (these include, for instance, severe permanent neurodevelopmental delay, such as cerebral palsy). Given the long-term consequences of these two last parameters, it was also important to consider the average duration of minor development problems and the life expectancy of an individual with major development problems.
A pragmatic literature search was performed to identify evidence sources for the outcomes associated with haemolytic disease of the fetus and newborn infant, in addition to the ones found in the NICE TA156. The literature review focused particularly on the anti-D immunoglobulin systematic reviews64,65,85 and the high-throughput NIPT diagnostic accuracy studies (see Chapter 3, Results: assessment of clinical effectiveness) as potential sources of data associated with the consequences of sensitisation. Apart from the study published by Finning et al. ,17 no other relevant evidence was found. Evidence from this study relating to the proportion of fetal or neonatal deaths (5%) and to the proportion of babies affected with mild/severe development problems (5%) was used to populate the model. In the absence of more recent data for parameters relating to the proportion of babies affected with minor development problems and the duration of these problems and relating to the life expectancy of people with major developmental problems, we used the same evidence as NICE TA156 with updated costs. It should be noted that owing to the small number of haemolytic disease-related events, the corresponding model estimates are subject to considerable uncertainty.
In the absence of more recent or relevant data, the health-related quality of life evidence used relating to the utilities of minor (0.85) and major (0.42) development problems and the associated uncertainty was assumed to be the same as those used in NICE TA156. 62
Cost of high-throughput non-invasive prenatal testing
For the base-case analysis the cost of high-throughput NIPT per sample was estimated to be (confidential information has been removed). This unit cost takes into account consumables, staffing, equipment, indirect costs and overhead costs. This is the company’s estimated cost of testing at full capacity, that is, dealing with at least 100,000 samples. An estimated royalty payment of (confidential information has been removed) of the test cost is assumed to be added to the unit cost of the test, bringing the base-case estimate of the cost of the test to (confidential information has been removed). The cost of high-throughput NIPT is discounted according to the pregnancy number in which it is being performed, accounting for an expected median time between pregnancies of around 3.2 years. The unit cost per sample may, however, fluctuate, as it is a function of capacity and predicted level of usage of each testing machine annually. The cost applied in the base-case analysis does not include transport costs for the delivery of blood samples for testing. Szczepura et al. 68 included a postage cost of £1.10 per sample in their analysis, although they recognised that the cost would be much reduced if the existing NHS transport service system was to be used.
Cost of routine antenatal anti-D prophylaxis and of anti-D immunoglobulin for potentially sensitising events and post partum
The cost of anti-D immunoglobulin was taken from the British National Formulary (BNF). 86 Currently, two brands [D-Gam® (Bio Products Laboratory Ltd, Elstree, Hertfordshire, UK) and Rhophylac®(CSL Behring LLC, Kankakee, IL, USA)] and four doses (250-, 500-, 1500- and 2500-unit vials) are available. At current prices the cost of anti-D immunoglobulin is £23.75 for D-Gam 250 IU, £33.75 for D-Gam 500 IU and £39.52 for Rhophylac. Note that current market prices of anti-D immunoglobulin may vary with supply and demand. Regional and local price negotiations exist that may make the cost anti-D immunoglobulin lower than the values indicated above.
The cost of anti-D immunoglobulin for potentially sensitising events was estimated to be £31.69, representing a weighted average of the cost of anti-D immunoglobulin 250 IU and 500 IU (minimum required) doses and their expected utilisation before and after 20 weeks’ gestation based on evidence from a recent audit. 8 The cost of RAADP was estimated to be £41.58, representing a weighted average of single-dose (1500 IU) and two-dose (2 × 500 IU) regimens and their associated market share, 92.6% versus 7.4%, respectively. 8 Similarly, the cost of anti-D immunoglobulin administered post partum was estimated to be £35.69, which reflects the expected utilisation of ‘standard’ doses: 500 IU (66.3%) and 1500 IU (33.7%). 8 Costs applied in the current economic model were discounted according to the timing of the pregnancy (the pregnancy number) in which the treatments are administered. As in NICE TA156,62 an administration cost of anti-D immunoglobulin was set to £5.
Cost of postpartum health resources used
Following birth, in current practice a cord serology test should be performed to confirm the baby’s RhD type. In addition, maternal blood samples should be tested for FMH. The costs, updated to 2015 prices, for postpartum serology (£4.18) and associated phlebotomy (£3.32) were obtained from Szczepura et al. 68 The cost of FMH testing was provided by personal communication with a NHS Blood and Transplant Manager and estimated to be £128.10 (for test by flow cytometry, NHS Blood and Transport Red Cell Immunohaemotology) (Erika Rutherford, NHS Blood and Transplant, 2016, personal communication). This cost was subject to SA, as Szczepura et al. 68 report a much lower value of £3.17 for a Kleihauer test (when updated to 2015 prices). All costs were discounted according to the timing of the pregnancy in which the resources were consumed.
Cost of management of sensitisation
The list of relevant interventions in the management of maternal and neonatal sensitisation was taken from the previous NICE TA156. 62 The proportion of individuals requiring each intervention, the estimated average number of interventions required per individual and the estimated average number of days were considered to be the same as in NICE TA15662 (Table 22). Utilisation of these resources was validated by our clinical experts, who highlighted that no significant changes in clinical practice have occurred since 2009. Similarly, the estimated annual costs for minor (£111) and major (£574) development problems was assumed to be the same as in NICE TA156 but updated to 2015 prices. Unit costs were sourced from the NHS reference costs 2014–15. 87 The total average cost per sensitisation is estimated to be £3167. Note that, owing to the multiplicity of factors affecting sensitisation and its management, the uncertainty associated with this parameter was taken from NICE TA15662 and assumed to be substantial (standard error £700).
| Intervention | Management element | Percentage of sensitised mothers/babies requiring intervention | Average number required per person | Average days per treatment | Unit cost of intervention (£) | Total cost (£) | Listed NHS Reference Costs 2014–1587 used for the unit costs |
|---|---|---|---|---|---|---|---|
| Management of maternal sensitisation | Blood tests, bilirubin, monitoring, etc. | 100 | 6 | 1 | 195 | 1172 | Code NZ19B – Ante-Natal Major Disorders with CC Score 0–1 – Regular Day or Night Admissions |
| Doppler scanning | 90 | 4 | 1 | 109 | 392 | Code NZ21Z – Ante-Natal Standard Ultrasound Scan – Outpatient Procedures | |
| In utero transfusion | 5 | 3 | 1 | 195 | 29 | Code NZ19B – Ante-Natal Major Disorders with CC Score 0–1 – Regular Day or Night Admissions | |
| Management of the sensitised baby | Phototherapy | 71 | 1 | 3 | 526 | 1121 | Code PB04D; PB05C; PB06F; PB06M (average) – Neonatal Diagnoses – Non-elective Inpatients – Short Stay |
| Exchange transfusion | 5 | 2 | 1 | 526 | 53 | Code PB04D; PB05C; PB06F; PB06M (average) – Neonatal Diagnoses – Non-elective Inpatients – Short Stay | |
| Neonatal follow-up visits | 10 | 2 | 1 | 526 | 105 | Code PB04D; PB05C; PB06F; PB06M (average) – Neonatal Diagnoses – Non-elective Inpatients – Short Stay | |
| Neonatal intensive care unit | 5 | 1 | 5 | 1176 | 294 | Code XA01Z – Neonatal Critical Care, Intensive Care – Critical Care | |
| Total | 3167 |
Model parameters and main assumptions
The parameters used within the de novo economic model, and their characteristics, as described in the preceding sections, are outlined in Table 23. Costs refer to 2015 prices.
| Parameter | Mean value | Standard error | Distribution | Source/calculation |
|---|---|---|---|---|
| Discounting | ||||
| Discount rate for utilities | 3.5% | – | – | NICE methods guidance88 |
| Discount rate for costs | 3.5% | – | – | NICE methods guidance88 |
| Target population characteristics | ||||
| Population of England (a) | 54,316,600 | – | – | Office for National Statistics, Annual Mid-year Population Estimates, 201489 |
| Crude birth rate in England: all births per 1000 population of all ages (b) | 12.18 | – | – | Office for National Statistics, Births in England and Wales, 201480 |
| Proportion of pregnancies accounted for by Rh-negative women (c) – reiterated from Table 17 | 15.0% | – | – | NHS Digital, Hospital Episode Statistics: NHS Maternity Statistics, 2013–143 |
| Number of women requiring treatment | 99,225 | – | – | Estimate based on information above [ = (a × (b/1000) × c)] |
| Proportion of first pregnancies proceeding to next pregnancy | 91.4% | – | – | Office for National Statistics, Birth Summary Tables, England and Wales90 – Characteristics of Mother 2, England and Wales – Average 2009 to 2013 |
| Proportion of second pregnancies proceeding to next pregnancy | 40.5% | – | – | Office for National Statistics, Birth Summary Tables, England and Wales90 – Characteristics of Mother 2, England and Wales – Average 2009 to 2013 |
| Proportion of third pregnancies proceeding to next pregnancy | 58.3% | – | – | Office for National Statistics, Birth Summary Tables, England and Wales90 – Characteristics of Mother 2, England and Wales – Average 2009 to 2013 |
| Median time between pregnancies (years) | 3.17 | – | – | Office for National Statistics, Birth Summary Tables, England and Wales 201490 – Characteristics of Mother 2, England and Wales, 2013 |
| Compliance | ||||
| Compliance with RAADP | 99.0% | 0.1% | Beta | NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 |
| Compliance with RAADP if high-throughput NIPT performed | 99.0% | 0.1% | Beta | Assumed the same as compliance with RAADP |
| Compliance with postpartum Anti-D immunoglobulin (dose of at least 500 IU given within 3 days of delivery) | 98.0% | 0.2% | Beta | NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 |
| High-throughput NIPT inconclusive results | ||||
| Proportion of high-throughput NIPT inconclusive results: all studies reporting inconclusives | 6.7% | 0.4% | Beta | Diagnostic accuracy review (see Chapter 3) |
| Proportion of high-throughput NIPT inconclusive results: UK Bristol studies | 4.0% | 0.1% | Beta | Diagnostic accuracy review (see Chapter 3) |
| Proportion of RhD-positive babies in high-throughput NIPT inconclusive results: all studies reporting inconclusives | 70.1% | 0.7% | Beta | Diagnostic accuracy review (see Chapter 3) |
| Proportion of RhD-positive babies in high-throughput NIPT inconclusive results: UK Bristol studies | 70.7% | 0.3% | Beta | Diagnostic accuracy review (see Chapter 3) |
| Sensitisation events | ||||
| Probability of having at least one potentially sensitising event | 15.5% | 0.5% | Beta | NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 |
| Probability of performing a FMH test given at least one potentially sensitising event | 69.3% | 1.4% | Beta | NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 |
| Probability of receiving anti-D after having at least one potentially sensitising event | 95.8% | 0.6% | Beta | NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 |
| Probability of women having a miscarriage (including stillbirth and intrauterine death) | 4.7% | 0.3% | Beta | NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 |
| Consequences of sensitisation | ||||
| Fetal loss rate per woman at risk | 5.0% | 1.0% | Beta | Finning et al. (2008)17 and the previous NICE assessment (TA156)62 |
| Proportion of babies affected by HDN with minor developmental problems | 6.0% | 2.0% | Beta | Previous NICE assessment (TA156)62 |
| Duration of minor developmental problems (years) | 16 | 5 | Beta | Previous NICE assessment (TA156)62 |
| Proportion of babies affected by HDN with major developmental problems | 5.0% | 1.0% | Beta | Finning et al. (2008)17 and the previous NICE assessment (TA156)62 |
| Life expectancy for person with major developmental problems | 59.5 | Range 40–79 | Uniform | Previous NICE assessment (TA156)62 |
| Utilities | ||||
| Utility for ‘normal’ person | 0.88 | 0.02 | Beta | Previous NICE assessment (TA156)62 |
| Utility for minor development problems | 0.85 | 0.02 | Beta | Previous NICE assessment (TA156)62 |
| Utility for major development problems | 0.42 | 0.03 | Beta | Previous NICE assessment (TA156)62 |
| Costs | ||||
| Cost of high-throughput NIPT | (Confidential information has been removed) | – | – | (Confidential information has been removed) |
| Royalty fee of high-throughput NIPT | (Confidential information has been removed) | – | – | (Confidential information has been removed) |
| Cost of RAADP | £41.58 | – | – | BNF86 and NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 – weighted average of single- and two-dose anti-D regimen costs and their market share |
| Cost of anti-D immunoglobulin for potentially sensitising events | £31.69 | – | – | BNF86 and NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 – weighted average of dose anti-D regimen cost and the likelihood of pre and post 20 weeks events |
| Cost of postpartum anti-D immunoglobulin | £35.69 | – | – | BNF86 and NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8 – weighted average of dose anti-D regimen cost and their market share |
| Cost of anti-D immunoglobulin administration per RhD-negative woman treated | £5.00 | £2.00 | Gamma | Previous NICE assessment (TA156)62 |
| Cost of postpartum blood cord serology | £4.18 | – | – | Szczepura et al.,68 updated to 2015 |
| Cost of FMH testing | £128.10 | – | – | Provided by clinical experts |
| Cost of phlebotomy | £3.32 | – | – | Szczepura et al.,68 updated to 2015 prices |
| Cost of management of a sensitised woman and sensitised neonate | £3166.72 | £700.00 | Gamma | Previous NICE assessment (TA156)62 |
| Yearly cost of minor developmental problems | £110.58 | £35.00 | Gamma | Previous NICE assessment (TA156),62 updated to 2015 prices |
| Yearly cost of major developmental problems | £573.72 | £405.73 | Gamma | Previous NICE assessment (TA156),62 updated to 2015 prices |
Within the model the following assumptions are consistent with NICE TA156:62
-
Sensitisations do not affect the pregnancy in which they occur.
-
Anti-D immunoglobulin used within one pregnancy has no effect on reducing sensitisations during the next pregnancy.
-
The proportion of RhD-negative women is based on the white European population given that this group makes up > 90% of the population of England and Wales.
Furthermore, the following assumptions were made:
-
The proportion of RhD-positive babies in Rh-negative women is the same irrespective of pregnancy number.
-
The probability of having a RhD-positive baby in the general population of Rh-negative women (61.6%) is combined with the diagnostic accuracy results in terms of sensitivity and specificity (in which inconclusive results are treated as test positive) to determine the number of Rh-positive babies in the model.
-
The probability of having a RhD-positive baby in women with inconclusive test results is based on the pooled probability in the study populations used to inform the diagnostic accuracy estimates.
-
All NIPT is performed early enough to determine the use of RAADP at 28 weeks’ gestation.
-
Routine and prophylactic anti-D immunoglobulin is offered only to women whose NIPT result indicates that their fetus is RhD positive or whose results are inconclusive.
-
In women with an inconclusive NIPT result the existing care pathway is unchanged and they are treated the same as women who test positive in terms of RAADP, anti-D immunoglobulin and associated tests.
-
Women identified to receive RAADP will receive supplementary anti-D immunoglobulin at the minimum dose required for any potentially sensitising events.
-
Potentially sensitising events that involve fetal death were independent of previous sensitisation within the same pregnancy.
-
Women with false-negative test results but who are provided with cord serology and postpartum anti-D immunoglobulin have a sensitisation rate of 0.95% despite forgoing anti-D immunoglobulin treatment for potentially sensitising events.
-
Compliance with RAADP is same with and without NIPT; similarly, compliance for postpartum anti-D immunoglobulin is assumed to be the same with or without NIPT.
Analytic methods
In exploring the alternative means by which the introduction of high-throughput NIPT could impact on the postpartum care pathway, we first present results for each postpartum scenario separately compared with ‘no test and RAADP’. Thereafter, we combine them and compare them directly in a full incremental analysis.
The decision-analytic model was evaluated using 10,000 Monte Carlo simulations to reflect the joint uncertainty across all of the inputs according to the probability distributions assigned to each, as shown in Table 22. All results are presented in terms of the average over 10,000 simulations, as these provide an unbiased estimate of the expected model outcomes. The existing model non-linearity means that the deterministic results are not an accurate estimate of the mean costs and QALYs in each strategy. This non-linearity is likely to be attributable to the model being structured around the specificity and sensitivity of NIPT and the rate of sensitisation, all characterised by skewed distributions and all with baseline values close to the upper bound of 1 (sensitivity and specificity) or lower bound of 0 (rate of sensitisation). The primary results are the total expected costs and expected QALYs for each alternative strategy. Population net health benefits (NHBs) are used to summarise the cost-effectiveness results in addition to the cost-effectiveness ratio. NHBs are calculated for cost-effectiveness thresholds of £20,000 and £30,000 as shown in the equation below:
For a given cost-effectiveness threshold, the strategy with the highest net benefit is the same strategy that would be considered cost-effective when comparing incremental cost-effectiveness ratios (ICERs) against the threshold. They are useful to summarise results when there are small differences in health between strategies and when the new intervention may be less effective and less costly than current practice. In these circumstances, ICERs can be very volatile and sensitive to small changes in the denominator. Further to this, the ICER for a less costly and less effective new intervention actually represents the cost per QALY gain of introducing current practice, and this can lead to some confusion in interpretation. The introduction of the high-throughput NIPT is not expected to produce large differences in clinical outcomes and may result in lower health outcomes than RAADP if the rate of sensitisations is increased.
Results are expressed per pregnancy and for the cross-section of 100,000 pregnancies, as described in Target population. It should be noted that for the population-level results, the total number of pregnancies is distributed across time and, therefore, not all test costs or consequences are experienced in year 1. Results were initially calculated for the comparison of ‘no test and RAADP’ with ‘no test and no RAADP’ in order to illustrate the impact of the adjustments made to the model used in NICE TA15662 and to establish the baseline comparability in terms of the cost-effectiveness of the current practice, ‘no test and RAADP’. This was necessary because the benefits of a diagnostic test are reliant on there being a cost-effective treatment available. The results of this analysis are shown in Appendix 10. Throughout the main body of this diagnostic assessment report we omit the ‘no test and no RAADP’ strategy, as this is not relevant to UK current practice.
Cost-effectiveness acceptability curves are used to show the probability that each alternative strategy is cost-effective for a range of cost-effectiveness threshold. We also calculate the health consequences of the total amount of parameter uncertainty in terms of the potential health benefits that could be gained if all uncertainty were eliminated. This is the expected value of perfect information and it represents an upper bound for the value of any further research to reduce parameter uncertainty. The maximum value of further research was calculated as the difference between the expected value of basing a decision about the use of NIPT on perfect information (i.e. with no probability of error) and the expected value of that decision made on the basis of existing evidence (i.e. subject to uncertainty). This value is expressed in terms for the cross-section of 100,000 pregnancies multiplied over 10 years, as the further research may inform decisions beyond the immediate cohort of pregnancies considered in this model.
Uncertainty regarding the appropriate source of data, the appropriate assumptions or model structure and other scenarios are explored using one- and two-way SA, as described further in Sensitivity analyses.
Base-case analysis
The set of main assumptions used in the base-case analysis are shown in Table 24.
| Parameter | Assumption/evidence source |
|---|---|
| High-throughput NIPT accuracy | Bivariate meta-analysis of UK (Bristol) studies, see Chapter 3; the diagnostic test was assumed to be performed at first contact with health services |
| Effectiveness of RAADP (vs. no RAADP) | Sensitisation rate = 0.35% (NICE TA15662) |
| Uptake of RAADP (with and without high-throughput NIPT performed) | 99.0% (NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8) |
| Uptake of postpartum anti-D immunoglobulin (with and without high-throughput NIPT performed) | 98.4% (NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8) |
| High-throughput NIPT inconclusive results | Inconclusive rate of 6.2% treated as positive test results |
| Cost of high-throughput NIPT | Base-case unit cost of (confidential information has been removed) with a (confidential information has been removed) royalty fee added: (confidential information has been removed) |
| Cost of anti-D immunoglobulin | Potentially sensitising event, £31.69; RAADP, £41.58; postpartum, £35.69 |
| Cost of FMH test | £128.10 (Erika Rutherford, Business Development Manager, Red Cell Immunohaematology, NHS Blood and Transplant, 2016, personal communication) |
| Further postpartum scenario on the management of high-throughput NIPT inconclusive results | Inconclusive results are treated post delivery as positive test results |
Sensitivity analyses
A series of scenario analyses and SAs was also conducted. We focused on parameters and assumptions to which we expected that the ICER would be the most sensitive and where the available evidence was limited. The SAs are described in detail but also summarised in Table 24. We focus on the comparison of current practice with the best performing postpartum scenario in all cases unless the results of the SA affect the rank order of postpartum scenarios or suggest that multiple postpartum scenarios could potentially provide the highest NHB.
Sensitivity analysis 1
We explored alternative sources for the diagnostic performance of high-throughput NIPT. The base-case analysis utilises the results from the UK (Bristol) studies, as these are thought to be most generalisable to a UK setting. We also show the results utilising all available studies, regardless of geography. For lower estimates of sensitivity, high-throughput NIPT is expected to result in more false-negative results, which are associated with adverse health consequences in terms of additional sensitisations. For lower estimates of specificity, high-throughput NIPT is expected to result in more false-positive results, which reduce the amount of unnecessary anti-D immunoglobulin and associated management costs that is avoided.
Sensitivity analysis 2
We explored the use of high-throughput NIPT at different gestation periods. Performance results from a recent UK study12 were used to assess the cost and consequences of introducing high-throughput NIPT at 11–13 weeks, 14–17 weeks and 18–23 weeks. Note that the economic model does not incorporate the timing of a potentially sensitising event and so a threshold analysis is performed to determine the percentage of the costs that would have to occur prior to NIPT in order for the ICER to cross a threshold of £20,000 per QALY.
Sensitivity analysis 3
The base-case analysis utilised the same rate of sensitisation with ‘no test and RAADP’ as was used in the NICE TA156. 62 Subsequent to NICE TA156 a further meta-analysis was performed by Turner et al. ,63 which suggests that anti-D immunoglobulin could be marginally more effective if all studies are taken into account, reducing the rate of sensitisation with ‘no test and RAADP’ from 0.35% to 0.30%. The increased efficacy of RAADP will increase the health costs associated with false-negative results of high-throughput NIPT, as women will have incorrectly forgone a more effective treatment.
Sensitivity analysis 4
We explore the impact of an overall change in uptake of anti-D immunoglobulin. Lower uptake of RAADP will reduce the cost savings possible from avoiding unnecessary RAADP but will also affect the health consequences of additional sensitisations. However, we did not explore an effect of high-throughput NIPT on uptake. The base-case analysis assumes that the introduction of high-throughput NIPT will not alter the proportion of women who comply with the administration of anti-D immunoglobulin. Currently, few women in the UK refuse RAADP, so there is little scope for an increase in uptake. We consider that it may be possible that women who would refuse RAADP would also refuse high-throughput NIPT, but this should not impact on the cost-effectiveness of NIPT, only on throughput. Although the clinical effectiveness review identified studies that reported the rate of uptake of anti-D immunoglobulin among women provided with high-throughput NIPT, none provided a comparison with what uptake would have been in those same women without provision of high-throughput NIPT. We therefore assumed that women informed that they are carrying a RhD-positive fetus would be no more or less likely to uptake anti-D immunoglobulin than they would be if offered RAADP. Some women who are told that they are carrying a RhD-negative fetus may still demand RAADP, and this cost is not incorporated in the model. We conduct a two-way SA in which the uptake of RAADP is decreased or increased alongside the reduction of the uptake of postpartum anti-D immunoglobulin.
Sensitivity analysis 5
The base-case analysis incorporates the rate of inconclusive high-throughput NIPT results found in the UK (Bristol) studies. 12,17,18 The rate of inconclusive results will vary according to the local population demography because they are more likely in certain ethnic groups, such as in those of African ethnic origin. The rate of inconclusive results may also vary if the operation of NIPT is different in a trial setting compared with in routine use, for example if less time is spent on reprocessing initially inconclusive test results. Increasing the rate of inconclusive test results when these are treated as test positive will increase the rate of false-positive results and reduce the specificity of NIPT. This will, in turn, reduce the amount of unnecessary anti-D immunoglobulin and associated management costs that can be avoided through the use of high-throughput NIPT.
Sensitivity analysis 6
We conduct a two-way SA in which the cost per dose of anti-D immunoglobulin therapy is varied alongside the cost per high-throughput NIPT. The cost of high-throughput NIPT to the NHS is uncertain for a number of reasons: (1) the unit cost varies by throughput and so will depend on the total uptake of NIPT, (2) the unit cost of the test must be considered alongside other potential additional costs relating to the transportation of blood samples for testing, to whether or not additional antenatal visits are required to draw blood and to the delivery of test counselling and results and (3) the royalty fee charged to the NHS in addition to the unit cost of the test is uncertain. The base-case analysis includes a test cost of (confidential information has been removed) and a royalty fee of (confidential information has been removed). The base case assumes that high-throughput NIPT can be incorporated in to routine antenatal care without imposing further marginal costs to the NHS, which is likely to be favourable to any ‘test and RAADP’ strategies. We calculate the threshold NHS cost per high-throughput NIPT at which the ICER for any strategy incorporating NIPT falls below £20,000 and £30,000 per QALY. We also show how the ICER varies as the cost per test is varied between £13.20 and £24.20. The cost of anti-D immunoglobulin may be subject to discounts from the list prices utilised in the base-case analysis. We show how the cost-effectiveness results vary to –20%, –10%, +10% and +20% of list price. The cost-effectiveness of any high-throughput NIPT will be reduced as the price of anti-D immunoglobulin falls because the savings from avoiding unnecessary RAADP will be lower.
Sensitivity analysis 7
Since the introduction of RAADP there has been a move from the two-dose to the single-dose regimen for a variety of reasons, as indicated in the recent anti-D immunoglobulin prophylaxis audit. We conducted a SA that assumes a 100% use of the cheaper of the two regimens, that is, the single dose.
Sensitivity analysis 8
A further alternative way in which the use of high-throughput NIPT may impact on the existing postpartum care pathway is considered. This strategy, rather than grouping high-throughput NIPT inconclusive results with positive results, regards them as distinct from those for whom NIPT indicated a RhD-positive fetus. In this scenario postpartum cord blood typing would be performed if high-throughput NIPT of fetal RhD status identifies a RhD-negative fetus or if the test result is inconclusive. FMH testing and postdelivery anti-D immunoglobulin would be administered if a RhD-positive fetus is identified either in the positive test result group or in the inconclusive test result group.
A summary of the SA performed is listed in Table 25.
| Parameter | Assumption/evidence source | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-throughput NIPT accuracy | SA1: bivariate meta-analysis of all studies (see Chapter 3) | ||||||||||||||||
| SA2: high-throughput NIPT performance assessed at different gestation periods, using evidence from Chitty et al.12 | |||||||||||||||||
| Effectiveness of RAADP (vs. no RAADP) | SA3: sensitisation rate = 0.30% (Turner et al.63) | ||||||||||||||||
| Compliance with RAADP (with and without high-throughput NIPT performed) | SA4a: 87.5% (NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8) | ||||||||||||||||
| Compliance with postpartum anti-D immunoglobulin (with and without high-throughput NIPT performed) | SA4b: 91.6% (NHS Blood and Transplant, National Comparative Audit of Blood Transfusion – 2013 Audit of Anti-D Immunoglobulin Prophylaxis8) | ||||||||||||||||
| High-throughput NIPT inconclusive results | SA5: pooled estimates for the sensitivity and specificity replaced with the individual study results | ||||||||||||||||
| Cost of high-throughput NIPT | SA6a: varied between £13.20 and £24.20 (confidential information has been removed) | ||||||||||||||||
| Cost of anti-D immunoglobulin | SA6b: all varied ± 20% | ||||||||||||||||
| Cost of FMH test |
SA7: £3.17 (Szczepura et al. ,68 updated to 2015 prices) SA8: |
||||||||||||||||
| Further postpartum scenario on the management of high-throughput NIPT inconclusive results | NIPT resultCord serologyFMH testPostpartum anti-DT–YesYes if CS+As guided by CS and FMH testT+NoYesYes with additional dose per FMH testInconclusiveYesYes if CS+As guided by CS and FMH test | NIPT result | Cord serology | FMH test | Postpartum anti-D | T– | Yes | Yes if CS+ | As guided by CS and FMH test | T+ | No | Yes | Yes with additional dose per FMH test | Inconclusive | Yes | Yes if CS+ | As guided by CS and FMH test |
| NIPT result | Cord serology | FMH test | Postpartum anti-D | ||||||||||||||
| T– | Yes | Yes if CS+ | As guided by CS and FMH test | ||||||||||||||
| T+ | No | Yes | Yes with additional dose per FMH test | ||||||||||||||
| Inconclusive | Yes | Yes if CS+ | As guided by CS and FMH test |
Model validation
Pedro Saramago developed the model and Susan Griffin checked the model for errors. Comparisons across strategies were done to identify inconsistencies. Comparisons with the previous NICE TA156 were also done to identify the sources of any potential discrepancy.
Results of the independent economic assessment
This section reports the results of the de novo economic model developed to assess the cost-effectiveness of high-throughput NIPT to identify fetal RhD status in women who are RhD-negative and not known to be sensitised to the RhD antigen. The base-case results for the different postpartum strategies are shown first, followed by the results of performing SA on key model input parameters. All results are based on the probabilistic analysis. Detailed characteristics of each postpartum scenario are provided in Table 16.
Base-case results
Table 26 presents the results for each postpartum testing scenario separately against current practice of ‘no test and RAADP’. Total costs, total QALYs, incremental costs and incremental QALYs are presented together with incremental cost per QALY gained (ICER) and population NHBs at £20,000 and £30,000 threshold values. The results of the model suggest that for each additional sensitisation there is a loss of approximately 0.9 QALYs. Any difference in QALYs between strategies is attributable wholly to the difference in the number of sensitisations.
| Strategies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY gained) | Population NHB (λ = £20,000) | Population NHB (λ = £30,000) |
|---|---|---|---|---|---|---|---|
| Current clinical practice | |||||||
| No test and RAADP | 15,983,725 | 2,433,756 | – | – | – | 2,432,957 | 2,433,223 |
| NIPT PP1 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,400,187 | 2,433,756 | –583,538 | –0.46 | 1,269,050 | 2,432,986 | 2,433,242 |
| NIPT PP2 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,312,630 | 2,433,737 | –671,095 | –19.13 | 35,087 | 2,432,972 | 2,433,227 |
| NIPT PP3 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,498,942 | 2,433,756 | –484,783 | –0.46 | 1,054,281 | 2,432,981 | 2,433,239 |
| NIPT PP4 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,410,610 | 2,433,737 | –573,114 | –19.13 | 29,964 | 2,432,967 | 2,433,223 |
Non-invasive prenatal testing PP1 describes the use of NIPT to guide RAADP only, with all women continuing to receive cord serology with FMH testing and postpartum anti-D immunoglobulin as required, irrespective of NIPT result. This is estimated to reduce costs by £584,000 per 100,000 pregnancies and to result in lower health benefits (0.5 QALYs) than current practice.
Non-invasive prenatal testing PP2 (NIPT PP2) describes the use of NIPT to guide both RAADP and postpartum care to women who test positive or in whom the results are inconclusive, when cord serology is provided only in these women to guide FMH testing and postpartum anti-D immunoglobulin as required. This is estimated to reduce costs compared with current practice by approximately £671,000 but to result in a loss of 19.1 QALYs per 100,000 pregnancies.
Non-invasive prenatal testing PP3 (NIPT PP3) describes the use of NIPT to guide RAADP and postpartum anti-D immunoglobulin to women who test positive or inconclusive and when cord serology is used to guide FMH testing and postpartum anti-D immunoglobulin as required only to women whom NIPT indicates have a RhD-negative fetus. This is estimated to reduce costs compared with current practice by £485,000 but to result in a loss of 0.5 QALYs per 100,000 pregnancies.
Non-invasive prenatal testing PP4 (NIPT PP4) describes the use of NIPT to guide both RAADP and postpartum FMH testing and anti-D immunoglobulin to women who test positive or inconclusive and when cord serology is not provided. This is estimated to reduce costs compared with current practice by approximately £573,000 but results in a loss of 19.1 QALYs per 100,000 pregnancies.
All postpartum scenarios are cost saving but also less effective than no test and RAADP, placing them on the south-west quadrant of the cost-effectiveness plane (Figure 13). The least effective strategies are those that omit cord serology for women who test negative on NIPT. Without cord serology false negatives are not picked up at delivery and are not provided with postpartum anti-D immunoglobulin. In the model, the additional health gains are determined by the management of high-throughput NIPT false-negative test results.
FIGURE 13.
Cost-effectiveness plane of current practice (no test and RAADP) and alternative NIPT scenarios (PP1 to PP4).

Owing to these NIPT strategies being less costly and less effective than no test and RAADP, the ICERs calculated in Table 25 (and Figure 13) show the cost per QALY gained with current practice compared with high-throughput NIPT. Hence, when the ICER is above the cost-effectiveness threshold this would support the use of NIPT (no test and RAADP vs. NIPT PP1, ICER approximately £1,270,000 per QALY gained). The cost-effectiveness threshold can be used to present results in terms of NHBs, in which case the comparison is more straightforward, as the strategy with the highest NHB is preferred. All NIPT strategies have an expected NHB higher than no test and RAADP, both at threshold values of £20,000 and £30,000. Compared with no test and RAADP, NIPT PP1 has greater NHB (incremental NHB at £20,000 of approximately 14; incremental NHB at £30,000 of approximately 16, vs. no test and RAADP).
The base-case analysis assumes no adverse health impacts from use of a blood-based product, such as anti-D immunoglobulin. This is in line with the fact that widespread global use of anti-D immunoglobulin has yet to produce evidence of any adverse consequences. We illustrate how sensitive the ICER is to changes in these assumptions. Using the net benefit framework, it is possible to interpret the results of the SA around the price of anti-D immunoglobulin in terms of health impact. An increase of 20% in the cost of anti-D immunoglobulin represents a cost of £39.50 × 0.2 = £7.90. At a cost-effectiveness threshold of £20,000 per QALY, this is equivalent to assuming a health cost of 7.9/20,000 = 0.0004 QALYs per administration, or a loss of 3.5 hours of full lifetime health from every woman per dose of anti-D immunoglobulin they receive.
The incremental costs of introducing NIPT can be broken down into the cost of NIPT, the cost of managing potentially sensitising events, the cost of RAADP, the cost of postpartum tests and anti-D immunoglobulin and the cost consequences of sensitisations, and these are shown in Table 27. Although the added NIPT cost is similar across strategies (at approximately £1,585,000 per 100,000 pregnancies) it is accumulated over multiple pregnancies and so is affected by the performance of strategy in terms of the number of sensitisations. Strategies with more sensitisations (NIPT PP2 and NIPT PP4) have marginally less test cost, as sensitised women do not receive NIPT to guide RAADP in subsequent pregnancies (however, it is worth noting that it is recommended that NIPT be used in women who are sensitised in order to guide antenatal care). Similarly, all strategies save similar levels of costs from avoiding RAADP (approximately £1,544,000 per 100,000 pregnancies) and the management of potentially sensitising events (approximately £626,000 per 100,000 pregnancies). The NIPT strategies vary more markedly in their impact on postpartum testing and anti-D immunoglobulin costs. Here, NIPT PP1 is essentially the same as current practice, except for the small reduction in costs attributable to increased sensitisations, which makes women ineligible for FMH testing and anti-D immunoglobulin. NIPT PP2 decreases postpartum care costs by avoiding cord serology for women who test negative, but this comes at an increased cost of managing sensitisations, as false negatives are not picked up at delivery and women testing negative falsely are not provided with postpartum FMH tests and anti-D immunoglobulin. NIPT PP3 increases postpartum care costs because, although cord serology is avoided for those who test positive, this results in unnecessary use of FMH tests and anti-D immunoglobulin among women who test false positive (which includes those who test inconclusive but carry a RhD-negative baby). NIPT PP4 decreases postpartum care costs relative to current practice by avoiding cord serology for all women and is a combination of NIPT PP2 and NIPT PP3. As might be expected, the added cost of managing sensitisations and their associated health consequences is largest for the strategies with more sensitisations (NIPT PP2 and NIPT PP4) and is very small for strategies NIPT PP1 and NIPT PP3 (approximately £1700 per 100,000 pregnancies).
| Cost item (£) | NIPT PP1 | NIPT PP2 | NIPT PP3 | NIPT PP4 |
|---|---|---|---|---|
| NIPT cost | 1,585,117 | 1,584,861 | 1,585,117 | 1,584,861 |
| Potentially Sensitising Event management costs | –626,165 | –627,470 | –626,165 | –627,470 |
| RAADP costs | –1,544,149 | –1,544,887 | –1,544,149 | –1,544,887 |
| Postpartum test and anti-D costs | –43 | –152,771 | 98,712 | –54,790 |
| Sensitisation costs | 1703 | 69,173 | 1703 | 69,173 |
| Total incremental cost | –583,538 | –671,095 | –484,783 | –573,114 |
The assumption that the results of NIPT can be used to avoid all costs associated with the management of potentially sensitising events is favourable to NIPT and £626,000 represents the maximum cost saving in this regard. If this cost saving is reduced to £52,000, that is, if 92% of potentially sensitising events occur prior to the results of the NIPT being known, the ICER for no test and RAADP compared with NIPT PP1 would fall below £20,000 per QALY. The results of the audit indicate that 80% of potentially sensitising events occur after 20 weeks’ gestation. This suggests that incorporating NIPT into routine antenatal care when it would be provided in week 20 or earlier (see Figure 11 for schedule of appointments) could avoid upwards of 80% of the cost of managing potentially sensitising events.
We calculated the probability that each strategy would be cost-effective compared with no test and RAADP for each pair-wise comparison. NIPT PP1 and NIPT PP3 both have 99% probability of being cost-effective at a threshold of £20,000 per QALY. NIPT PP2 and NIPT PP4 have a lower probability of being cost-effective at £20,000 per QALY, at no higher than 73% compared with no test and RAADP.
Table 28 presents the fully incremental cost-effectiveness probabilistic results for high-throughput NIPT versus other strategies. Fully incremental results do not compare each NIPT strategy with current practice (i.e. no test and RAADP) but compare all NIPT scenarios simultaneously as competing alternative strategies. In this table, strategies are ranked by total costs and total QALYs, with the cheapest strategy coming first (NIPT PP2). Dominated strategies (those that have higher costs than more effective strategies) are in the bottom rows of the table. Incremental costs, incremental QALYs and, consequently, the ICER are incremental to the next cheapest, non-dominated strategy. This means that they represent the difference between the strategy in the current row compared with the strategy in the row above, as the table is ordered from least to most costly. The same applies to the incremental NHBs at £20,000 and £30,000 threshold values.
| Strategies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY gained) | Population incremental NHBs (λ = £20,000) | Population incremental NHBs (λ = £30,000) |
|---|---|---|---|---|---|---|---|
| NIPT PP2 | 15,312,630 | 2,433,737 | – | – | – | – | – |
| NIPT PP1 | 15,400,187 | 2,433,756 | 87,557 | 18.67 | 4690 | 14 | 16 |
| No test and RAADP | 15,983,725 | 2,433,756 | 583,538 | 0.46 | 1,269,050 | –29 | –19 |
| NIPT PP4 | 15,410,610 | 2,433,737 | – | – | Dominated | – | – |
| NIPT PP3 | 15,498,942 | 2,433,756 | – | – | Dominated | – | – |
In NIPT PP2 cord serology is used to identify false-positive results, thereby avoiding unnecessary FMH testing and anti-D immunoglobulin in these women, but this is withheld in women for whom NIPT indicates a RhD-negative fetus. Using the negative results of high-throughput NIPT to rule out postpartum cord serology, FMH and anti-D immunoglobulin (NIPT PP2 and NIPT PP4) has lower QALYs than no test and RAADP, NIPT PP1 and NIPT PP3. Although there are further cost savings from avoiding postpartum cord serology and anti-D immunoglobulin, the majority of sensitisations occur and can be prevented by the administration of anti-D immunoglobulin at delivery. NIPT PP2 is the cheapest strategy and provides the same QALYs as NIPT PP4. Hence, NIPT PP4 is dominated by NIPT PP2.
Providing cord serology to all women, as with NIPT PP1, will identify both the false-positive results (the small number of false positives and the proportion of women with inconclusive results who are carrying RhD-negative babies) and false-negative results. Although NIPT PP1 has higher costs than NIPT PP2 because of the additional cord serology tests, these are offset somewhat by cost savings from avoiding sensitisations in false negatives. Compared with NIPT PP2, NIPT PP1 is estimated to provide approximately 19 additional QALYs per 100,000 pregnancies, at approximately £88,000 in additional costs, corresponding to an ICER of around £5000 per QALY gained.
In NIPT PP3 cord serology is used to identify false-negative results but this is withheld in women with inconclusive results or for whom NIPT indicates a RhD-positive fetus (in favour of FMH testing and anti-D immunoglobulin). Compared with NIPT PP1, the QALY gain is not affected as the model assumes no adverse health benefits from unnecessary use of anti-D immunoglobulin. As NIPT PP3 is more costly than NIPT PP1, in the base case it is dominated by NIPT PP1.
No test and RAADP is more costly than NIPT PP1 and is the most effective strategy. The administration of RAADP and supplementary anti-D immunoglobulin for potentially sensitising events among the false negatives leads to an additional 0.5 QALYs per 100,000 pregnancies compared with NIPT PP1, at an additional cost of £584,000. This means that the ICER for no test and RAADP compared with NIPT PP1 is £1,270,000. Using high-throughput NIPT and performing cord serology irrespective of the result (NIPT PP1) has higher NHB than any other strategy.
The decision uncertainty can be shown graphically with a cost-effectiveness acceptability curve. Figure 14 shows the cost-effectiveness acceptability curves for the different scenarios being compared (i.e. no test and RAADP and alternative high-throughput NIPT scenarios – PP1 to PP4) in which we can depict the probability that each strategy is cost-effective for a range of threshold values. When all strategies are simultaneously compared, for threshold values of £20,000 and £30,000, the highest probability of being cost-effective is obtained by NIPT PP1 with 0.65 and 0.73, respectively. For the same threshold values, the probability of NIPT PP2 being cost-effective is 0.30 and 0.22, respectively. NIPT PP1 is the alternative with the highest probability of being cost-effective and also the expected cost-effective alternative for thresholds above £10,000. An estimate of the maximum value of further research, the expected value of perfect information, is estimated to be approximately £203,000 considering 10 years of cohorts of 100,000 pregnancies and using a cost-effectiveness threshold of £20,000 per QALY. If research to reduce uncertainty in the model values would cost > £203,000 this suggests that it would not represent a good investment.
FIGURE 14.
Cost-effectiveness acceptability curves of current practice (no test and RAADP) and alternative NIPT scenarios (PP1 to PP4).

Sensitivity analyses results
Several SAs were carried out to assess the sensitivity of the base-case cost per QALY findings, as detailed in Table 24. We assessed the impact of using pooled evidence from all relevant NIPT accuracy evidence rather than UK Bristol studies only and, by using recent evidence from a UK study,12 assessed the performance of high-throughput NIPT at different gestation periods. An analysis of the NIPT inconclusive results was also performed by replacing the pooled estimates for the sensitivity and specificity with the individual study results. SA was performed on the effectiveness of RAADP by using a different sensitisation rate pooled from a larger number of studies. An assessment was also carried out for the uptake rates for RAADP and postpartum anti-D immunoglobulin, with and without NIPT, decreasing these to the circumstances when the correct dose at the correct time was administered according to recent evidence. 8 In addition, we analysed the impact of altering the cost of the diagnostic test and the cost of treatment, two key components of this assessment as highlighted in the relevant literature. Finally, we have evaluated the impact of reducing the cost of the FMH test and, under an alternative postpartum scenario, assessed the management of high-throughput inconclusive results separately to the positive test results. The following sections look closely at each of these analyses and provide interpretations of obtained results relative to the base-case findings.
Sensitivity analysis 1: sensitivity analysis over the non-invasive prenatal testing accuracy using all relevant evidence
Table 29 shows the results when diagnostic accuracy for high-throughput NIPT accuracy is based on all available studies as opposed to UK (Bristol) studies only. This increases the pooled specificity by 2%, although the pooled sensitivity levels are reduced by only 0.2% (see Chapter 3, Results: assessment of diagnostic accuracy). Compared with the base case, the 2% reduction in false-positive results allows for more avoidance of anti-D immunoglobulin and associated tests, reducing total costs across all NIPT strategies by between £20,000 and £150,000 per 100,000 pregnancies. Total QALYs are marginally affected by the small 0.2% increase in false negatives, with NIPT PP2 and NIPT PP4 being the most affected, as these assume no use of cord serology post partum for women with negative results. Compared with the base case, this results in a further loss of approximately 12 QALYs per 100,000 pregnancies. Compared with no test and RAADP, NIPT PP2 and NIPT PP3 are still found to be cost saving (approximately £630,000–690,000 per 100,000 pregnancies) but NIPT PP3 is associated with a loss of approximately 1 QALY per 100,000 pregnancies compared with a loss of 31 with NIPT PP2. NIPT PP1 and NIPT PP3 are the only strategies to offer increased NHBs compared with no test and RAADP, with ICERs for no test and RAADP of approximately £830,000.
| Strategies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY gained) | Population NHB (λ = £20,000) | Population NHB (λ = £30,000) |
|---|---|---|---|---|---|---|---|
| Current clinical practice | |||||||
| No test and RAADP | 15,983,725 | 2,433,756 | – | – | – | 2,432,957 | 2,433,223 |
| NIPT PP1 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,353,678 | 2,433,756 | –630,047 | –0.76 | 829,196 | 2,432,988 | 2,433,244 |
| NIPT PP2 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,291,035 | 2,433,725 | –692,690 | –31.13 | 22,253 | 2,432,961 | 2,433,215 |
| NIPT PP3 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,351,238 | 2,433,756 | –632,487 | –0.76 | 832,406 | 2,432,988 | 2,433,244 |
| NIPT PP4 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,286,779 | 2,433,725 | –696,946 | –31.13 | 22,390 | 2,432,961 | 2,433,216 |
Sensitivity analysis 2: sensitivity analysis over the non-invasive prenatal testing accuracy at different timings using Chitty et al.12
Table 30 presents the results of providing high-throughput NIPT at different gestation periods. These are based on the analysis by Chitty et al. 12 (see Chapter 3, Results: assessment of diagnostic accuracy), with the sensitivity and specificity repeated for information. In this analysis, only the diagnostic accuracy is varied from the base-case values of 0.998 for sensitivity and 0.942 for specificity, which impacts on the probability of sensitisation. The sensitivity estimate is least favourable at 14–17 weeks’ gestation and the specificity estimate is least favourable at 18–23 weeks’ gestation, although these differences may be a result of random chance rather than systematic variation between these time points. Although this analysis does not directly take into consideration the impact of the test timing on the potential to avoid costs associated with the management of a potentially sensitising events, we estimate the threshold amount of these costs that would have to occur prior to NIPT in order for the ICER to cross the threshold of £20,000 per QALY gained. Thus, results are shown only for the best NIPT strategy within each period.
| Strategies | Sensitivity | Specificity | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY gained) | Population NHB (λ = £20,000) | Population NHB (λ = £30,000) |
|---|---|---|---|---|---|---|---|---|---|
| Current clinical practice – irrespective of NIPT timing | |||||||||
| No test and RAADP | – | – | 15,983,725 | 2,433,756 | – | – | – | 2,432,957 | 2,433,223 |
| Best postpartum scenario when NIPT performed at 11–13 weeks’ gestation | |||||||||
| NIPT PP1 (vs. no test and RAADP) | 0.9983 | 0.9525 | 15,378,009 | 2,433,756 | –605,716 | –0.39 | 1,536,731 | 2,432,987 | 2,433,243 |
| Best postpartum scenario when NIPT performed at 14–17 weeks’ gestation | |||||||||
| NIPT PP1 (vs. no test and RAADP) | 0.9967 | 0.9534 | 15,370,718 | 2,433,756 | –613,007 | –0.77 | 797,046 | 2,432,987 | 2,433,243 |
| Best postpartum scenario when NIPT performed at 18–23 weeks’ gestation | |||||||||
| NIPT PP1 (vs. no test and RAADP) | 0.9982 | 0.9304 | 15,429,067 | 2,433,756 | –554,658 | –0.36 | 1,529,418 | 2,432,984 | 2,433,242 |
As for the base case, the introduction of high-throughput NIPT results in lower health benefits than no test and RAADP. This happens irrespective of the timing at which the test is carried out. The QALY loss is slightly greater when performing NIPT at 14–17 weeks’ gestation because of the very small drop in sensitivity of 0.002, leading to more false negatives and a loss of approximately 1 QALY per 100,000 pregnancies compared with current practice, rather than a loss of approximately 0.4 QALYs if NIPT is provided at 11–13 weeks’ or 18–23 weeks’ gestation. The cost saving is greatest at 14–17 weeks because of the increase in specificity, as fewer false-positive results result in less unnecessary treatment.
The base-case results suggest that NIPT PP1 provides savings of £626,000 from avoiding the costs of managing potentially sensitising events. The audit8 indicates that 80% of potentially sensitising events occur after week 20. If NIPT PP1 is provided between 18 and 23 weeks’ gestation and £547,000 or 87% of the cost of managing potentially sensitising events occurs prior to the test, the ICER for no test and RAADP would fall below £20,000 per QALY gained. If NIPT PP3 is provided between 11 and 13 weeks’ or 14 and 17 weeks’ gestation, then approximately £598,000 or 95% of the cost of managing potentially sensitising events would have to occur prior to the test in order for the ICER for no test and RAADP to fall below £20,000 per QALY gained.
Sensitivity analysis 3: sensitivity analysis on the effectiveness of routine antenatal anti-D prophylaxis using Turner et al.
Findings from Turner et al. 63 estimated a pooled odds ratio estimate for sensitisation under RAADP (vs. no RAADP, only postpartum anti-D immunoglobulin) of 0.31 rather than 0.37 as in NICE TA15662 (Table 31). Compared with base-case results (see Table 25) the marginal reduction in the sensitisation rate (0.05% less) brings minimal changes to the total costs and QALYs estimates, as expected. The increase in effectiveness of RAADP provides reductions in total costs for all strategies and minor changes in the QALY loss associated with NIPT.
| Strategies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY gained) | Population NHB (λ = £20,000) | Population NHB (λ = £30,000) |
|---|---|---|---|---|---|---|---|
| Current clinical practice | |||||||
| No test and RAADP | 15,923,756 | 2,433,774 | – | – | – | 2,432,978 | 2,433,243 |
| NIPT PP1 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,339,945 | 2,433,773 | –583,811 | –0.50 | 1,164,285 | 2,433,006 | 2,433,262 |
| NIPT PP2 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,252,388 | 2,433,755 | –671,369 | –19.17 | 35,018 | 2,432,992 | 2,433,246 |
| NIPT PP3 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,438,716 | 2,433,773 | –485,040 | –0.50 | 967,307 | 2,433,001 | 2,433,259 |
| NIPT PP4 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 15,350,384 | 2,433,755 | –573,372 | –19.17 | 29,906 | 2,432,987 | 2,433,243 |
Sensitivity analysis 4: sensitivity analysis on the uptake of routine antenatal anti-D prophylaxis and postpartum anti-D immunoglobulin
In the base-case analysis our estimates of compliance are based on the use of anti-D immunoglobulin in women who are eligible in terms of RhD status and ignorance of the father’s status, and who remain pregnant, to receive RAADP. The National Comparative Audit of Blood Transfusion 2013 on Anti-D Immunoglobulin Prophylaxis8 reported that, out of all RhD-negative women, 87.5% received the correct dose at the correct time of RAADP. Furthermore, it reported that 91.6% received the correct dose at the correct time of postpartum anti-D immunoglobulin prophylaxis. We made use of these estimates to provide a lower bound for compliance with anti-D immunoglobulin. As for the base case, it was assumed that the use of high-throughput NIPT does not influence the uptake with anti-D immunoglobulin, that is, the uptake rate is the same irrespective of whether NIPT was previously accepted/administered.
Table 32 presents the incremental cost-effectiveness outcomes for each alternative scenario when different RAADP and postpartum anti-D immunoglobulin uptake rates are used. As the SA does not impact on the rank order of the alternative postpartum scenarios, the results are shown for NIPT PP1 only, that is, out of the five alternatives being compared, the results for the best strategy are shown together with current practice. Base-case results correspond to 99.0% and 98.4% uptake with RAADP and postpartum anti-D immunoglobulin, respectively. Overall, the results are robust to reduced compliance and there is little impact on incremental comparison between NIPT PP1 and no test and RAADP. The cost for all strategies is increased if compliance with a cost-effective treatment, such as RAADP, is reduced, although the QALY loss associated with additional sensitisations is slightly reduced.
| Strategies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY gained) | Population NHB (λ = £20,000) | Population NHB (λ = £30,000) |
|---|---|---|---|---|---|---|---|
| RAADP at 99.0% and post partum at 98.4% (base case) | |||||||
| No test and RAADP | 15,983,725 | 2,433,756 | – | – | – | 2,432,957 | 2,433,223 |
| NIPT PP1 (vs. no test and RAADP) | 15,400,187 | 2,433,756 | –583,538 | –0.46 | 1,269,050 | 2,432,986 | 2,433,242 |
| RAADP at 87.5% and post partum at 98.4% | |||||||
| No test and RAADP | 16,060,984 | 2,433,733 | – | – | – | 2,432,930 | 2,433,198 |
| NIPT PP1 (vs. no test and RAADP) | 15,477,810 | 2,433,733 | –583,174 | –0.41 | 1,430,198 | 2,432,959 | 2,433,217 |
| RAADP at 99.0% and post partum at 91.6% | |||||||
| No test and RAADP | 16,029,705 | 2,433,743 | – | – | – | 2,432,941 | 2,433,208 |
| NIPT PP1 (vs. no test and RAADP) | 15,446,384 | 2,433,742 | –583,321 | –0.43 | 1,360,214 | 2,432,970 | 2,433,227 |
| RAADP at 87.5% and post partum at 91.6% | |||||||
| No test and RAADP | 16,101,601 | 2,433,721 | – | – | – | 2,432,916 | 2,433,185 |
| NIPT PP1 (vs. no test and RAADP) | 15,518,619 | 2,433,721 | –582,982 | –0.38 | 1,532,578 | 2,432,945 | 2,433,204 |
Sensitivity analysis 5: sensitivity analysis on non-invasive prenatal testing inconclusive results
The cost saving achievable by using the high-throughput NIPT to guide anti-D immunoglobulin will depend on the rate of inconclusive test results, as for these women the current care pathway is unchanged. That is, all inconclusive results are managed as if they were test positive and, hence, unnecessary anti-D immunoglobulin continues to be provided in these women carrying a RhD-negative fetus. In order to undertake a SA around the rate of inconclusives, we replaced the pooled estimates for the sensitivity and specificity with the individual study results. Figure 15 shows how the specificity varies with the rate of inconclusives within each study. In general, a higher rate of inconclusive results will lead to a larger number of false positives and, correspondingly, a lower specificity. The cost saving achievable by using high-throughput NIPT to guide anti-D immunoglobulin will depend on the rate of inconclusive test results, as for these women the current care pathway is unchanged, that is, all inconclusive results are managed as if they were test positive, and, hence, unnecessary antenatal anti-D immunoglobulin continues to be provided in those women carrying a RhD-negative fetus.
FIGURE 15.
Specificity by rate of high-throughput NIPT inconclusive results per study.
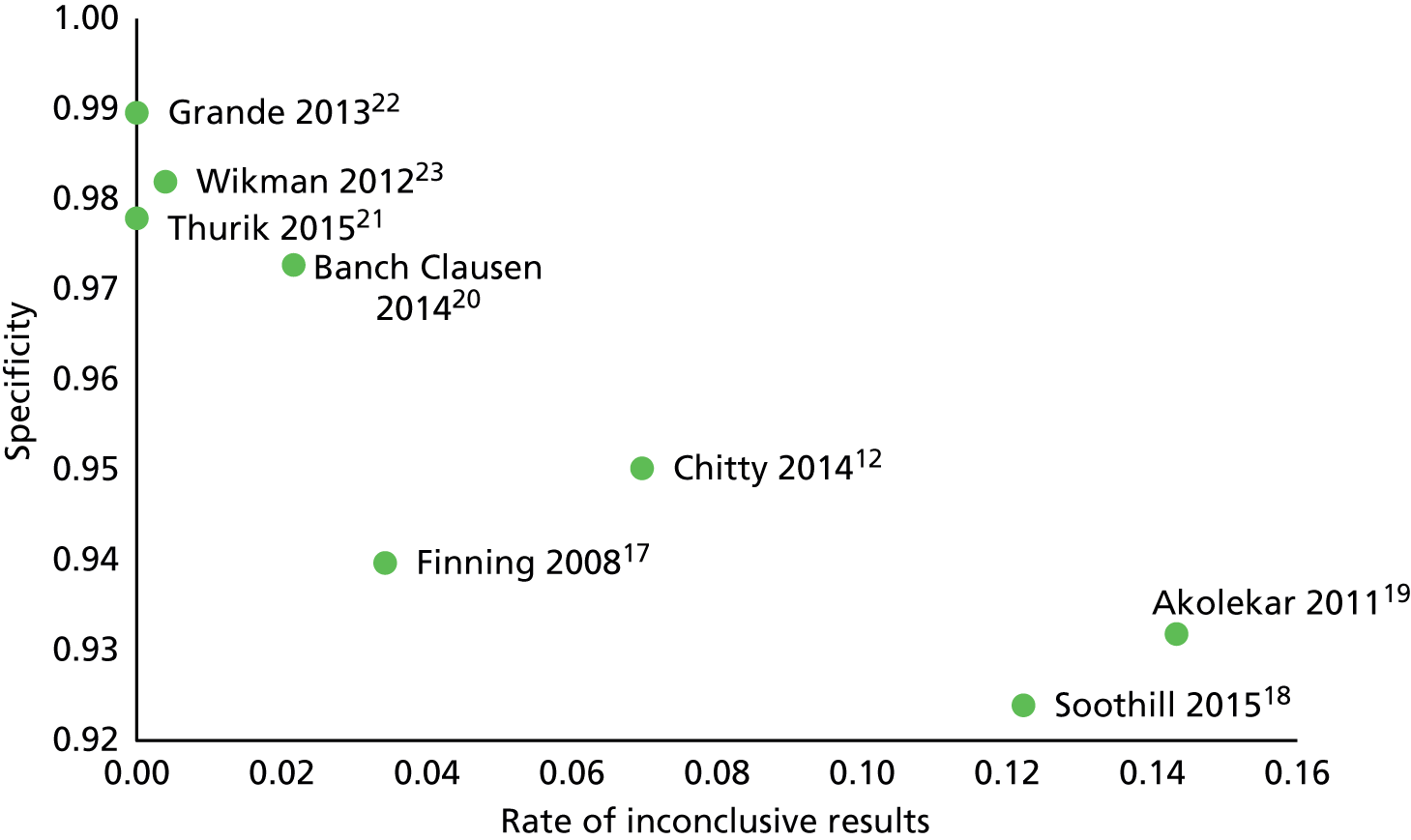
One study produced no inconclusive results and no false-negative results and so we omitted this from the SA. 22 In general, the NHBs associated with the NIPT strategies fall as the rate of inconclusive results increases, but at no point do the NHBs from NIPT PP1 or NIPT PP3 fall below those offered with no test and RAADP. Figure 16 shows the NHBs for all of the NIPT strategies. When the rate of inconclusive results is low, NIPT PP3 offers the highest NHB. This is because the amount of unnecessary postpartum FMH testing and anti-D immunoglobulin is reduced when the number of false-positive results falls. When the rate of inconclusives is high, NIPT PP1 is preferred. If the rate of inconclusives was very high, no test and RAADP would be preferred. However, the rate would have to be much higher than that observed in the set of studies underlying the evidence synthesis. Akolekar et al. 91 and Wikman et al. 23 diverge from the remaining studies in terms of the number of false-negative results and sensitivity and this impacts on the NHBs of the strategies that do not identify false negatives through cord serology (NIPT PP2 and NIPT PP4). For these two strategies, the NHB falls below that offered by current practice when the results associated with these two studies are used. Figure 17 shows how the NHBs for NIPT PP1 vary only with the rate of inconclusives.
FIGURE 16.
Population NHBs for all NIPT strategies by rate of NIPT inconclusive results per study.
FIGURE 17.
Population NHBs for NIPT PP1 by rate of NIPT inconclusive results per study.

Sensitivity analysis 6: sensitivity analysis on non-invasive prenatal testing and Anti-D costs
The unit cost of NIPT is subject to some uncertainty as it depends on throughput (the total number of samples per year) and the level of the royalty fee. The throughput determines how many machines must be bought and at what capacity they are utilised. The base-case analysis assumed sufficient machines to process all pregnancies in England in a given year. Further to this, the introduction of NIPT may impose additional costs in routine antenatal care in terms or appointments and staff time. Similarly, the cost of anti-D immunoglobulin may depart from the list price on the basis of negotiated discounts.
The results of a two-way analysis around these unit costs reported in Figure 18 show that the base case is very sensitive to both the price of NIPT and the price of anti-D. The x-axis represents the range of anti-D immunoglobulin cost from –20% to +20%. This increase/decrease in the cost of anti-D immunoglobulin is applied to all occasions in which the treatment is administered and, thus, the RAADP cost shown is indicative only, as the estimated costs of anti-D for potentially sensitising events and post partum, as described in Cost of postpartum health resources used, are omitted. The y-axis represents the range of costs per high-throughput NIPT from £17.60 to £28.60 [which may, for example (confidential information has been removed)].
FIGURE 18.
(Confidential information has been removed.)
A price increase would raise the costs associated with all strategies that provide NIPT and does not affect the ranking of the strategies. The postpartum strategy that provides the lowest NHB will be associated with the lowest threshold cost, and the postpartum strategy that provides the highest NHB will be associated with the highest threshold cost.
The threshold cost for NIPT PP1, the strategy with the highest NHB, is £24.64 (confidential information has been removed). That is, raising the cost per high-throughput NIPT to £24.64 implies that NIPT PP1 no longer offers the highest population NHB, switching to no test and RAADP. Similar results were found when the cost-effectiveness threshold was £20,000 or £30,000. NIPT PP1 strategy is always preferred over other postpartum strategies (PP2, PP3 or PP4). At no point would the price of anti-D immunoglobulin be high enough to make the omission of postpartum anti-D immunoglobulin (NIPT PP2 and NIPT PP4) look cost-effective.
Sensitivity analysis 7: sensitivity analysis over the fetal–maternal haemorrhage test cost
Reducing the cost of the FMH test to £3.17 (Szczepura et al. ,68 updated to 2015 prices) halves the estimated total costs of all strategies compared with the total costs of the base-case scenarios (Table 33). Estimated total QALYs are similar to base-case findings. NIPT PP1 is now less cost saving than current practice. This is explained by the use of the FMH test in the management of potentially sensitising events. When the cost of the FMH test is reduced, the savings from avoiding the management of potentially sensitising events are reduced. All NIPT strategies still reduce costs compared with no test and RAADP but by a lesser amount. This causes the ICER for no test and RAADP compared with NIPT PP2 and NIPT PP4 to fall below £20,000 per QALY.
| Strategies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY gained) | Population NHB (λ = £20,000) | Population NHB (λ = £30,000) |
|---|---|---|---|---|---|---|---|
| Current clinical practice | |||||||
| No test and RAADP | 8,132,447 | 2,433,756 | – | – | – | 2,433,350 | 2,433,485 |
| NIPT PP1 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 7,986,460 | 2,433,756 | –145,987 | –0.46 | 317,485 | 2,433,356 | 2,433,490 |
| NIPT PP2 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 7,915,559 | 2,433,737 | –216,888 | –19.13 | 11,339 | 2,433,341 | 2,433,473 |
| NIPT PP3 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 7,846,684 | 2,433,756 | –285,763 | –0.46 | 621,464 | 2,433,363 | 2,433,494 |
| NIPT PP4 | |||||||
| Test and RAADP (T+ only) vs. no test and RAADP | 7,775,584 | 2,433,737 | –356,862 | –19.13 | 18,658 | 2,433,348 | 2,433,478 |
Sensitivity analysis 8: sensitivity analysis on postpartum management of inconclusive results
The postpartum scenarios specified in the decision problem applied cord serology, FMH testing and postpartum anti-D immunoglobulin according to whether or not the results of NIPT were positive or negative. In this regard, we grouped inconclusive results with NIPT positive results. However, in terms of postpartum management, it may be worthwhile to regard those with inconclusive results as distinct from those on whom NIPT indicates a RhD-positive fetus. This would allow cord serology to be provided to women with negative results in order to identify false negatives and cord serology to be provided to women with inconclusive results in order to identify false positives, but for it to be withheld in women in whom NIPT indicates a RhD-positive fetus. This would result in total costs of £15,230,372 and 2,433,756 QALYs per 100,000 pregnancies. This postpartum approach would dominate all other NIPT strategies, and the ICER for no test and RAADP compared with this strategy would be £1,638,356 per QALY gained.
Table 34 summarises the results of the base-case analysis and the key SAs.
| Analysis | Total | Compared with no test and RAADP (current practice) | Compared with next best strategy | ||||
|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | ICER (£) | ICER (£) | Comparator | |||
| Base case | |||||||
| No test and RAADP | 15,983,725 | 2,433,756 | – | 1,269,050 | NIPT PP1 | ||
| NIPT PP1 | 15,400,187 | 2,433,756 | 1,269,050 | 4690 | NIPT PP2 | ||
| NIPT PP2 | 15,312,630 | 2,433,737 | 35,087 | – | – | ||
| NIPT PP3 | 15,498,942 | 2,433,756 | 1,054,281 | – | – | ||
| NIPT PP4 | 15,410,610 | 2,433,737 | 29,964 | – | – | ||
| SA1: bivariate meta-analysis of all studies | |||||||
| No test and RAADP | 15,983,725 | 2,433,756 | – | 834,396 | NIPT PP3 | ||
| NIPT PP1 | 15,353,677 | 2,433,756 | 831,178 | – | – | ||
| NIPT PP2 | 15,291,034 | 2,433,725 | 22,255 | – | – | ||
| NIPT PP3 | 15,351,238 | 2,433,756 | 834,396 | 2123 | NIPT PP4 | ||
| NIPT PP4 | 15,286,779 | 2,433,725 | 22,391 | – | – | ||
| SA2: high-throughput NIPT performance assessed at different gestation periods (Chitty et al.12) | |||||||
| 11–13 weeks’ gestation | |||||||
| No test and RAADP | 15,983,725 | 2,433,756 | – | 1,536,731 | NIPT PP1 | ||
| NIPT PP1 | 15,378,008 | 2,765,228 | 1,165,229 | 3190 | NIPT PP4 | ||
| NIPT PP2 | 15,283,278 | 2,765,206 | 31,462 | – | – | ||
| NIPT PP3 | 15,420,079 | 2,765,228 | 1,084,295 | – | – | ||
| NIPT PP4 | 15,325,344 | 2,765,206 | 29,573 | – | – | ||
| 14–17 weeks’ gestation | |||||||
| No test and RAADP | 15,983,725 | 2,433,756 | – | 797,046 | NIPT PP1 | ||
| NIPT PP1 | 15,370,717 | 2,433,756 | 604,062 | 678 | NIPT PP4 | ||
| NIPT PP2 | 15,310,563 | 2,433,724 | 15,604 | – | – | ||
| NIPT PP3 | 15,409,227 | 2,433,756 | 566,114 | – | – | ||
| NIPT PP4 | 15,349,062 | 2,433,724 | 14,712 | – | – | ||
| 18–23 weeks’ gestation | |||||||
| No test and RAADP | 15,983,725 | 2,433,756 | – | 1,529,418 | NIPT PP1 | ||
| NIPT PP1 | 15,429,066 | 2,433,756 | 1,162,227 | 6209 | NIPT PP2 | ||
| NIPT PP2 | 15,334,643 | 2,433,741 | 31,744 | – | – | ||
| NIPT PP3 | 15,593,754 | 2,433,756 | 817,141 | – | – | ||
| NIPT PP4 | 15,499,308 | 2,433,741 | 23,691 | – | – | ||
| SA3: sensitisation rate (Turner et al.63) | |||||||
| No test and RAADP | 15,923,756 | 2,433,774 | – | 1,164,285 | NIPT PP1 | ||
| NIPT PP1 | 15,339,945 | 2,433,773 | 1,164,285 | 4690 | NIPT PP2 | ||
| NIPT PP2 | 15,252,387 | 2,433,755 | 35,021 | – | – | ||
| NIPT PP3 | 15,438,716 | 2,433,773 | 970,788 | – | – | ||
| NIPT PP4 | 15,350,383 | 2,433,755 | 29,909 | – | – | ||
| SA4: uptake with RAADP (with and without high-throughput NIPT performed) | |||||||
| Uptake of RAADP at 87.5% | |||||||
| No test and RAADP | 16,060,984 | 2,433,733 | – | 1,430,198 | NIPT PP1 | ||
| NIPT PP1 | 15,477,810 | 2,433,733 | 1,430,198 | 4691 | NIPT PP2 | ||
| NIPT PP2 | 15,390,257 | 2,433,714 | 35,171 | – | – | ||
| NIPT PP3 | 15,576,545 | 2,433,733 | 1,188,057 | – | – | ||
| NIPT PP4 | 15,488,218 | 2,433,714 | 30,035 | – | – | ||
| Uptake of postpartum anti-D immunoglobulin at 91.6% | |||||||
| No test and RAADP | 16,029,705 | 2,433,743 | – | 1,360,214 | NIPT PP1 | ||
| NIPT PP1 | 15,446,384 | 2,433,742 | 1,360,214 | 4691 | NIPT PP2 | ||
| NIPT PP2 | 15,358,829 | 2,433,724 | 35,137 | – | – | ||
| NIPT PP3 | 15,545,127 | 2,433,742 | 1,129,960 | – | – | ||
| NIPT PP4 | 15,456,798 | 2,433,724 | 30,006 | – | – | ||
| Uptake of RAADP at 87.5% and postpartum anti-D immunoglobulin at 91.6% | |||||||
| No test and RAADP | 16,101,601 | 2,433,721 | – | 1,532,578 | NIPT PP1 | ||
| NIPT PP1 | 15,518,619 | 2,433,721 | 1,532,578 | 4692 | NIPT PP2 | ||
| NIPT PP2 | 15,431,068 | 2,433,702 | 35,216 | – | – | ||
| NIPT PP3 | 15,617,343 | 2,433,721 | 1,273,046 | – | – | ||
| NIPT PP4 | 15,529,017 | 2,433,702 | 30,072 | – | – | ||
| SA5: high-throughput NIPT inconclusive results rate | |||||||
| Please see Sensitivity analysis 5: sensitivity analysis on non-invasive prenatal testing inconclusive results | |||||||
| SA6: cost of high-throughput NIPT and anti-D immunoglobulin | |||||||
| Please see Sensitivity analysis 6: sensitivity analysis on non-invasive prenatal testing and Anti-D costs | |||||||
| SA7: cost of FMH test | |||||||
| No test and RAADP | 8,132,446 | 2,433,756 | – | 621,464 | NIPT PP3 | ||
| NIPT PP1 | 7,986,460 | 2,433,756 | 317,485 | – | – | ||
| NIPT PP2 | 7,915,559 | 2,433,737 | 11,340 | – | – | ||
| NIPT PP3 | 7,846,683 | 2,433,756 | 621,464 | 3809 | NIPT PP4 | ||
| NIPT PP4 | 7,775,584 | 2,433,737 | 18,658 | – | – | ||
| SA8: postpartum management of high-throughput NIPT inconclusive results | |||||||
| Please see Sensitivity analysis 8: sensitivity analysis on postpartum management of inconclusive results | |||||||
Discussion of the independent economic assessment
The evidence to support the diagnostic accuracy of NIPT is of good quality. We can combine this with established evidence for the efficacy of RAADP and postpartum anti-D immunoglobulin in order to estimate the impact of introducing NIPT on the number of sensitisations. However, there is little evidence as to the impact of sensitisations in terms of their long-term health and cost consequences. Our model suggests that each additional sensitisation costs the NHS £3167 and is associated with a loss of approximately 0.9 QALYs, but these estimates are subject to uncertainty and incorporate expert opinion.
There is uncertainty regarding the cost of introducing high-throughput NIPT. The unit cost will vary with throughput and may be subject to an additional royalty fee. Unless NIPT can be incorporated seamlessly into routine antenatal care, it may result in additional costs for blood draw, transportation of samples and antenatal care visits to administer the test and deliver counselling and results. We conducted extensive SAs to address this uncertainty and to identify the threshold cost per NIPT. The cost of high-throughput NIPT has to increase by only (confidential information has been removed) above that modelled in the base case in order for no test and RAADP to be the preferred strategy. The unit cost of high-throughput NIPT to the NHS is the most important parameter in determining cost-effectiveness. Although there is uncertainty as regards the timing of the test, our analysis suggests that this is not influential in determining the cost-effectiveness results either in terms of diagnostic accuracy or in terms of the extent of management costs for potentially sensitising events that can be avoided.
As might be expected, the potential NHBs of using NIPT to target care are reduced, as the rate of inconclusive results is increased. However, our SA indicates that, even with high-throughput NIPT inconclusive results as high as 14.3%, the introduction of NIPT compares favourably to current practice. The ability of the NIPT result to avoid unnecessary use of anti-D immunoglobulin varies systematically according to ethnicity. Although this may not be an equality issue, it should be noted that following the introduction of NIPT, any unnecessary use of anti-D immunoglobulin will be proportionately higher in ethnic groups, for example in those of African origin. We can conclude that the identification of the false-positive results is key to the estimation of the cost-effectiveness outcomes, negatively impacting the results if this rate is higher and altering the postpartum strategy that would offer the highest NHB.
There are numerous ways in which the results of high-throughput NIPT could be used to guide postpartum testing and administration of anti-D immunoglobulin. We have compared four alternative postpartum scenarios, and the results indicate that cord serology testing should be retained in women for whom NIPT indicates a RhD-negative fetus. This use of cord serology to capture false-negative results has the potential to undermine the implementation of the test if it impacts on the confidence in the NIPT results. A postpartum strategy that distinguishes between inconclusive results and positive results offers the greatest cost savings.
If the cost of the FMH test is high relative to cord serology, then it would make sense to apply cord serology to women with positive and inconclusive NIPT results. This allows for the low-cost cord serology test to avoid both the unnecessary use of a much more expensive FMH test and unnecessary postpartum anti-D immunoglobulin. It is likely that these benefits are almost entirely obtained by applying cord serology in women with inconclusive results, as 30–40% of these women would be revealed to be carrying a RhD-negative fetus. In contrast, when the results of NIPT indicate a RhD-positive fetus, the rate of false positives is very low. In the base-case analysis, women who receive inconclusive results are managed as if they test positive, but there may be potential for further cost savings if these are treated as a distinct group in terms of postpartum care. This would allow for a postpartum scenario in which cord serology was applied to women who test negative and to those who test inconclusive but for whom FMH tests and anti-D immunoglobulin are provided without cord serology in women who test positive.
Conclusions of the cost-effectiveness section
The use of high-throughput NIPT to guide the provision of anti-D immunoglobulin prophylaxis is estimated to be cost saving compared with current practice of providing RAADP to all women who are RhD-negative. The extent of the cost saving is highly sensitive to the cost of NIPT itself to the NHS, which comprises the base unit cost per test, the level of any royalty fee and any increase in antenatal care costs required to accommodate an additional test. In the base-case analysis, the extent of the cost saving is sufficient to outweigh the small increase in sensitisations and the associated small QALY loss through using NIPT. However, even a small increase in the cost imposed on the NHS of (confidential information has been removed) or more per test would cause the ICER for no test and RAADP to reduce below £20,000 per QALY.
Chapter 6 Discussion
Statement of principal findings
Diagnostic accuracy
Eight studies were included in the diagnostic review of high-throughput NIPT. There were three studies based at Bristol (UK). The majority of included studies were judged as having a low risk of bias.
Meta-analyses showed very high diagnostic accuracy of high-throughput NIPT. In the primary analyses, for which women with inconclusive test results were treated as being testing positive, the summary FNR (i.e. women at risk of sensitisation) was 0.34% (95% CI 0.15% to 0.76%) and the FPR (i.e. women needlessly receiving anti-D) was 3.86% (95% CI 2.54% to 5.82%). SAs did not materially alter the overall result.
A subgroup analysis of three high-quality studies based at Bristol (UK) showed a slightly lower FNR of 0.21% (95% CI 0.09% to 0.48%) and a higher FPR of 5.73% (95% CI 4.58% to 7.16%). This suggests that the Bristol NIPT approach may be using a different threshold for the detection algorithm that further reduces false-negative error rates, consequently increasing the false-positive error rate. The FPR found was mostly as a result of treating the roughly 7% of women (in the UK) who have an inconclusive test result as if they had a positive test. Excluding these women from analysis resulted in a lower FPR of 1.26% (95% CI 0.87% to 1.83%). We were unable to conduct the subgroup analysis based on ethnicity because of lack of relevant data from included studies.
The diagnostic accuracy performance of high-throughput NIPT varied by gestational age. The data suggest that high-throughput NIPT is insufficiently accurate before around 11 weeks’ gestation (i.e. in first trimester) but is consistently accurate at any time after 11 weeks’ gestation. This may be because of a low concentration of cell-free fetal DNA in early pregnancy92 but an increased concentration of cell-free fetal DNA after the end of the first trimester. 93
Clinical effectiveness
Seven studies18,20,22,24–27 were included in the clinical effectiveness review. Only two studies20,26 had a control group, but both studies were judged as having a high risk of bias. One large prospective cohort study26 reported that use of high-throughput NIPT for targeted antenatal anti-D prophylaxis was associated with a significant risk reduction in sensitisation (adjusted odds ratio 0.41, 95% CI 0.22 to 0.87) compared with historical controls (routine management, postpartum anti-D only).
Three non-comparative studies18,20,22 reported outcome measures relating to anti-D doses administrated. All studies found that the use of NIPT reduced the total use of anti-D immunoglobulin doses (decreasing by 29% in one UK study by Soothill et al. 18) because around 35% of RhD-negative women avoided unnecessary anti-D administration.
Four studies20,26,27,49 reported moderate to high compliance with antenatal anti-D immunoglobulin administration. The compliance with antenatal anti-D administration after a positive NIPT result ranged from 86% to 96.1% (four studies20,26,27,49). High-throughput NIPT uptake rates ranged from 70% to > 95% (seven studies. 12,18,20,22,25–27).
The results from the simulation study suggested that the use of NIPT to determine antenatal anti-D use would substantially reduce the number of women receiving anti-D unnecessarily from 38.9% to 5.7%. Results were sensitive to the rate of compliance. NIPT use could increase sensitisation rates by up to 15 sensitisations per 100,000 women if postpartum cord blood testing is continued or up to 28 per 100,000 women if cord blood testing is withdrawn and postpartum anti-D given on the basis of the NIPT result. Sensitisation rates are minimised by ensuring that women who do not receive NIPT are still offered, and receive, antenatal anti-D. The results suggest that NIPT results (if available and conclusive) could potentially be used in place of cord blood testing for administration of postpartum anti-D, if the small increase in sensitisations rates can be considered ethically acceptable.
Implementation
Twelve studies13,17,18,20–28 were included in the review of implementation. Most of the included studies were large cohort studies13,17,20,21,23–27 reporting implementation data along with diagnostic accuracy data, although one study was a survey that was based in the UK (London). 28 All the large cohort studies reported high diagnostic accuracy of high-throughput NIPT and suggested that high-throughput RhD genotyping of fetuses in all RhD-negative women was feasible and should be recommended. A number of studies reported potential issues of implementation such as those relating to programme anti-D prophylaxis compliance. 20,27 Some studies highlighted the importance of short transport times of samples and the need for effective management of transporting samples. 13,17,24 Some studies also identified the need for greater knowledge of NIPT among physicians, midwives and pregnant women. 27,28
Cost-effectiveness
Seven cost-effectiveness studies58,68–73 were included in the review. Conflicting results were identified across the existing economic studies, with three of the studies68,71,72 reporting that NIPT fetal RhD genotyping did not appear to be cost-effective. The unit cost of the test was consistently identified as a key driver of the cost-effectiveness results and the potential for the use of NIPT to result in overall cost savings. Only one of the studies68 was undertaken in a UK context, but this study did not explicitly explore how the introduction of NIPT could impact on costs relating to potentially sensitising events. For the studies undertaken outside the UK, differences in health-care systems and in implementation of anti-D immunoglobulin policies limit their relevance to UK practice. In conclusion, none of the existing studies was considered to be sufficiently generalisable to inform the specific decision problem as set out in the NICE scope for the current assessment.
A de novo independent economic model was developed to assess the cost-effectiveness of high-throughput NIPT to identify fetal rhesus D status in women who are RhD negative and not known to be sensitised to the RhD antigen. The model was made up of two main elements: (1) an identification part reflecting the diagnostic performance and costs of the alternative identification strategies and (2) a treatment part that evaluated the subsequent costs and outcomes (expressed in QALYs) of alternative care pathways. Four alternative ways in which the use of high-throughput NIPT may impact on the existing postpartum care pathway were evaluated (cord serology, FMH testing and postpartum anti-D immunoglobulin). These included scenarios in which the result of NIPT was used to guide RAADP only (with all women continuing to receive cord serology with FMH testing and postpartum anti-D immunoglobulin as required, irrespective of NIPT result) and scenarios for which the NIPT result guided both RAADP and separate aspects of postpartum care. A series of additional sensitivity and scenario analyses was also performed.
Our de novo economic model indicated that the use of high-throughput NIPT to guide the prenatal and postpartum provision of anti-D immunoglobulin prophylaxis is estimated to be cost saving compared with the current practice of providing RAADP to all women who are RhD negative. The magnitude of the cost saving appears to be highly sensitive to the cost of NIPT itself to the NHS, which comprises the base unit cost per test, the level of any royalty fee and any increase in antenatal care costs required to accommodate an additional test. In the base-case analysis, the extent of the cost saving appears sufficient to outweigh the small increase in sensitisations and the associated small QALY loss through using NIPT compared with current practice. However, even a small increase in the cost imposed on the NHS of (confidential information has been removed) or more per test would alter these conclusions.
In the base-case analysis, all four separate postpartum scenarios were estimated to be cost saving but also less effective than current practice. Based on a cross-section of 100,000 pregnancies, the magnitude of cost savings varied between approximately £485,000 and £671,000. The magnitude of the QALY loss varied between 0.5 QALYs and 19.1 QALYs (per 100,000 pregnancies). Although the magnitude of the cost savings was sufficient to outweigh the associated QALY loss when each postpartum scenario was separately compared with current practice, these four separate scenarios potentially represent separate and distinct testing and management strategies that should be directly compared. In the base-case analysis, the strategy in which the NIPT result is used to guide RAADP only (i.e. all women continuing to receive cord serology with FMH testing and postpartum anti-D immunoglobulin) was associated with the highest NHB and had the highest probability of being cost-effective for threshold values of £20,000 and £30,000 per QALY (probability of 0.65 and 0.73, respectively). However, the use of cord serology to capture false-negative results has the potential to undermine the implementation of the test if it impacts on the confidence in the NIPT results. The most efficient postpartum strategy was also shown to vary across several of the main SAs.
A postpartum strategy that distinguishes between inconclusive results and positive results offers the greatest cost savings. In the base-case analysis, women who receive inconclusive results were assumed to be managed as if they test positive, but there may be potential for further cost savings if these are treated as a distinct group in terms of postpartum care. This could allow for a postpartum scenario in which cord serology was applied to women who test negative and who test inconclusive but in which FMH tests and anti-D immunoglobulin are provided without cord serology in women who test positive.
Strengths and limitations of the assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of potentially relevant studies. These included electronic searches of a variety of bibliographic databases as well as screening of clinical trial registers and conference proceedings to identify unpublished studies. The search strategy did not restrict by study design. The review process followed recommended methods to minimise the potential for error and/or bias. The quality of the included studies was assessed and accounted for when interpreting the review results. Appropriate synthesis methods were employed by taking into account the heterogeneity of study characteristics.
There was some evidence of inconsistency in the meta-analysis of diagnostic accuracy studies. The observed heterogeneity may be explained by variations in methods used in the high-throughput NIPT approach (including diagnostic accuracy thresholds and number and types of exons targeted), gestational age at the time of testing and different methods of handling inconclusive test results. There were also variations in the reporting of included studies. Particularly, two studies19,21 did not report the number of inconclusive results of the test and some studies12,18,22 did not report detailed reasons for inconclusive results.
There was very limited evidence relating to the clinical effectiveness of high-throughput NIPT. No studies were identified reporting adverse effects of high-throughput NIPT.
Owing to limited evidence, the generalisability of the review findings to non-white women and multiple pregnancies is unclear.
Cost-effectiveness
The de novo economic model was specifically developed to address the limitations of existing studies and concerns regarding the generalisability to current UK practice. The main strength of the decision model is the linkage between the diagnostic accuracy of a given identification strategy, the impact on subsequent treatment decisions and the ultimate effect on health outcomes and costs. A key element of the model is based on the previous economic model underpinning NICE TA15662 on RAADP ensuring consistency between the separate diagnostic and TAs. A broad range of scenario analyses and SAs were undertaken to address key assumptions and uncertainties.
Uncertainties
Clinical effectiveness
In this assessment we identified very limited data on the evaluation of clinical effectiveness for using high-throughput NIPT to detect fetal RhD status in RhD-negative women. Therefore, the potential role of high-throughput NIPT in terms of its clinical impact on the care pathway and adverse effects to the mother and fetus remains unclear. In particular, we did not identify any studies reporting comparative data relating to patient-related outcomes, such as quality-of-life measures.
Owing to a lack of sufficient data from included studies, we were unable to conduct subgroup analyses based on ethnicity. Therefore, whether or not the diagnostic performance of high-throughput NIPT differs between different ethnic groups remains unclear.
In terms of implementing high-throughput NIPT in health-care settings, no studies were identified reporting compliance rates to prenatal anti-D treatment in UK settings. Although a few non-UK studies reported compliance rates to prenatal anti-D treatment, the generalisability of their findings to the UK settings remains uncertain because of variations in national guidelines and health policies between different countries.
Cost-effectiveness
There is uncertainty regarding the cost of introducing high-throughput NIPT. The unit cost will vary with throughput and may be subject to an additional royalty fee. Unless NIPT can be incorporated seamlessly into routine antenatal care, it may result in additional costs for blood draw, transportation of samples and antenatal care visits to administer the test and deliver counselling and results. We conducted extensive SAs to address this uncertainty and to identify the threshold cost per NIPT. The cost of high-throughput NIPT has to increase by only (confidential information has been removed) above that modelled in the base case in order for current practice to be the preferred strategy.
Although there remains uncertainty as regards the timing of the test, our analysis suggests that this does not appear to be influential in determining the cost-effectiveness results either in terms of diagnostic accuracy or in terms of the extent of management costs for potentially sensitising events that can be avoided.
Although the evidence to support the diagnostic accuracy of NIPT is of good quality, existing evidence informing the impact of sensitisations in terms of their long-term health and cost consequences are more limited and highly uncertain.
Other relevant factors
Owing to a lack of relevant evidence, we have not considered any adverse health impacts from the provision of a blood-based product. Although widespread global use of anti-D immunoglobulin would suggest that is it safe, there remains uncertainty as regards the potential for risk associated with prion disease or other unknown pathogens. There may also be ethical considerations concerning the unnecessary administration of a blood-based product.
We also have not considered any adverse consequences from the introduction of the high-throughput NIPT over and above the slight increase in risk of sensitisation. Women who know that they are sensitised may factor this into their family planning decisions but we have assumed no such impact within the model. It is possible that NIPT could inadvertently reveal mistaken paternity of the child in cases in which a woman’s partner knows that he is RhD negative and the baby is revealed to be RhD positive. Concerns about revealed paternity have been noted in relation to testing the father’s blood type in order to target anti-D immunoglobulin only to those women with RhD-positive partners. The inclusion of an additional prenatal test could potentially have adverse impacts on the uptake of other antenatal care if the overall quality of care is compromised by the additional test burden.
Chapter 7 Conclusions
Implications for service provision
The findings from this assessment demonstrated high diagnostic performance of high-throughput NIPT for the detection of fetal RhD status in RhD-negative women from 11 weeks’ gestation, with very low FPR and FNR. About 0.7% of women will have an incorrect test result and approximately 7% will have an inconclusive result. SAs did not materially alter the results. These findings have important implications for service provision.
The use of high-throughput NIPT as a routine screening test for fetal RhD status in RhD-negative women can largely remove unnecessary exposure to prophylactic anti-D treatment, without substantially altering the rate of sensitisations. However, there will be a very small number of women (about 0.1%) with a false-negative test result who are at increased risk of sensitisation because they do not receive antenatal anti-D prophylaxis. This risk will be increased if postnatal cord blood testing is withdrawn from clinical practice. However, the numbers of additional sensitisations is likely to be very small.
Based on a cross-section of 100,000 pregnancies, the magnitude of expected cost savings is estimated to range between £296,000 and £409,000 depending on the impact of high-throughput NIPT on postpartum management.
Suggested research priorities
For future research priorities, evidence on the diagnostic accuracy of high-throughput NIPT in women of non-white ethnicity is needed, for which large prospective cohort studies collecting diagnostic accuracy data will be required. This is of particular concern as non-white women are more likely to have less accurate test results. For example, in people with African ethnicity, because of the presence of the RHD pseudogene,5 the prenatal detection of fetal RhD type from maternal blood would lead to higher rates of false-positive results in this particular population. Future diagnostic accuracy studies should systematically record and report the number of and reasons for inconclusive results and how these were dealt with when deriving estimates of diagnostic accuracy.
Given the limited evidence on the clinical impact of NIPT, further cohort studies comparing the use of high-throughput NIPT with universal antenatal anti-D administration are required. Such studies would ideally include a consecutive and representative sample of pregnant women in the UK. These should focus on recording relevant clinical outcomes (such as sensitisation rates, test and anti-D compliance and costs and quality of life) and adjust for relevant and clearly defined confounders (such as compliance with anti-D, timing of anti-D uptake and gestational age at time of NIPT). There is also limited existing evidence on the impact of sensitisations in terms of their long-term health and cost consequences. Although well-conducted cohort studies that comprehensively assess the full impact of sensitisations over mothers and children would be ideal, the complexity and cost associated with such studies means that promoting more systematic reporting and good-quality national audit data collection may be preferred. Surveys conducted in representative samples of women that assess the impact on quality of life of NIPT appear to be warranted.
Acknowledgements
We would like to thank the following for providing advice: Dr Finning Kirstin, NHS Blood and Transplant; Professor Peter Soothill, Emeritus Professor at the University of Bristol; and Professor Lyn Chitty, Institute of Child Health, University College London.
Contributions of authors
Pedro Saramago (Research Fellow) was responsible for the cost-effectiveness section, protocol development, study selection, data extraction, development of the economic model and writing the economic sections of the report.
Huiqin Yang (Research Fellow) commented on the protocol, conducted study selection, data extraction, validity assessment, interpretation of evidence and wrote the clinical sections of the report.
Alexis Llewellyn (Research Fellow) contributed to the clinical effectiveness section, drafted the protocol, conducted study selection, data extraction and validity assessment, commented on drafts of the report and provided input to clinical sections.
Ruth Walker (Research Student) contributed to the clinical effectiveness section and conducted study selection, data extraction and validity assessment.
Melissa Harden (Information Specialist) devised the search strategy, carried out the literature searches and wrote the search section.
Stephen Palmer (Professor of Health Economics) provided project management, commented on drafts of the report and contributed to all aspects of the project.
Susan Griffin (Senior Research Fellow) contributed to the cost-effectiveness section, study selection, data extraction, development of the economic model and writing the economic sections of the report and had overall responsibility for the cost-effectiveness section of the report.
Mark Simmonds (Research Fellow) provided project management, performed the statistical analysis and wrote the simulation section, commented on drafts of the report and contributed to all aspects of the project and had overall responsibility for the clinical effectiveness section of the report.
Publication
Saramago P, Yang H, Llewellyn A, Palmer S, Simmonds M, Griffin S. High-throughout, non-invasive prenatal testing for fetal RHD genotype to guide antenatal prophylaxis with anti-D immunoglobin: a cost-effectiveness analysis [Published online ahead of print February 7 2018]. BJOG 2018.
Data sharing statement
The data used in the analyses in this report are predominantly drawn from published and publicly available sources, as cited throughout the report. Summaries of the non-confidential data and of the models used are available on request from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Kumar S, Regan F. Management of pregnancies with RhD alloimmunisation. BMJ 2005;330:1255-8. https://doi.org/10.1136/bmj.330.7502.1255.
- Qureshi H, Massey E, Kirwan D, Davies T, Robson S, White J, et al. BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfus Med 2014;24:8-20. https://doi.org/10.1111/tme.12091.
- Hospital Episode Statistics: NHS Maternity Statistics – England, 2013–14. London: NHS Digital; 2015.
- Daniels G. The molecular genetics of blood group polymorphism. Transpl Immunol 2005;14:143-53. https://doi.org/10.1016/j.trim.2005.03.003.
- Faas BH, Beckers EA, Wildoer P, Ligthart PC, Overbeeke MA, Zondervan HA, et al. Molecular background of VS and weak C expression in blacks. Transfusion 1997;37:38-44. https://doi.org/10.1046/j.1537-2995.1997.37197176949.x.
- Singleton BK, Green CA, Avent ND, Martin PG, Smart E, Daka A, et al. The presence of an RHD pseudogene containing a 37 base pair duplication and a nonsense mutation in Africans with the Rh D-negative blood group phenotype. Blood 2000;95:12-8.
- Routine Antenatal Anti-D Prophylaxis for Women Who are Rhesus D Negative. London: NICE; 2008.
- National Comparative Audit of Blood Transfusion. 2013 Audit of Anti-D Immunoglobulin Prophylaxis. Birmingham: NHS Blood and Transplant; 2013.
- The Management of Women with Red Cell Antibodies During Pregnancy. Green-Top Guideline No. 65. London: Royal College of Obstetricians and Gynaecologists; 2014.
- Geifman-Holtzman O, Grotegut CA, Gaughan JP. Diagnostic accuracy of noninvasive fetal Rh genotyping from maternal blood – a meta-analysis. Am J Obstet Gynecol 2006;195:1163-73. https://doi.org/10.1016/j.ajog.2006.07.033.
- Zhu YJ, Zheng YR, Li L, Zhou H, Liao X, Guo JX, et al. Diagnostic accuracy of non-invasive fetal RhD genotyping using cell-free fetal DNA: a meta analysis. J Matern Fetal Neonatal Med 2014;27:1839-44. http://dx.doi.org/10.3109/14767058.2014.882306.
- Chitty LS, Finning K, Wade A, Soothill P, Martin B, Oxenford K, et al. Diagnostic accuracy of routine antenatal determination of fetal RHD status across gestation: population based cohort study. BMJ 2014;349. https://doi.org/10.1136/bmj.g5243.
- Clausen FB, Jakobsen TR, Rieneck K, Krog GR, Nielsen LK, Tabor A, et al. Pre-analytical conditions in non-invasive prenatal testing of cell-free fetal RHD. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0076990.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 2001;20:2865-84. https://doi.org/10.1002/sim.942.
- Finning K, Martin P, Summers J, Massey E, Poole G, Daniels G. Effect of high throughput RHD typing of fetal DNA in maternal plasma on use of anti-RhD immunoglobulin in RhD negative pregnant women: prospective feasibility study. BMJ 2008;336:816-18. http://dx.doi.org/10.1136/bmj.39518.463206.25.
- Soothill PW, Finning K, Latham T, Wreford-Bush T, Ford J, Daniels G. Use of cffDNA to avoid administration of anti-D to pregnant women when the fetus is RhD-negative: implementation in the NHS. BJOG 2015;122:1682-6. https://doi.org/10.1111/1471-0528.13055.
- Akolekar R, Finning K, Kuppusamy R, Daniels G, Nicolaides KH. Fetal RHD genotyping in maternal plasma at 11–13 weeks of gestation. Fetal Diagn Ther 2011;29:301-6. http://dx.doi.org/10.1159/000322959.
- Banch Clausen F, Steffensen R, Christiansen M, Rudby M, Jakobsen MA, Jakobsen TR, et al. Routine noninvasive prenatal screening for fetal RHD in plasma of RhD-negative pregnant women – 2 years of screening experience from Denmark. Prenat Diagn 2014;34:1000-5. https://doi.org/10.1002/pd.4419.
- Thurik FF, Ait Soussan A, Bossers B, Woortmeijer H, Veldhuisen B, Page-Christiaens GCML, et al. Analysis of false-positive results of fetal RHD typing in a national screening program reveals vanishing twins as potential cause for discrepancy. Prenat Diagn 2015;35:754-60. https://doi.org/10.1002/pd.4600.
- Grande M, Ordoñez E, Cirigliano V, Cid J, Grau E, Pericot A, et al. Clinical application of midtrimester non-invasive fetal RHD genotyping and identification of RHD variants in a mixed-ethnic population. Prenat Diagn 2013;33:173-8. http://dx.doi.org/10.1002/pd.4035.
- Wikman AT, Tiblad E, Karlsson A, Olsson ML, Westgren M, Reilly M. Noninvasive single-exon fetal RHD determination in a routine screening program in early pregnancy. Obstet Gynecol 2012;120:227-34. https://doi.org/10.1097/AOG.0b013e31825d33d9.
- Banch Clausen F, Christiansen M, Steffensen R, Jorgensen S, Nielsen C, Jakobsen MA, et al. Report of the first nationally implemented clinical routine screening for fetal RHD in D- pregnant women to ascertain the requirement for antenatal RhD prophylaxis. Transfusion 2012;52:752-8. https://doi.org/10.1111/j.1537-2995.2011.03362.x.
- de Haas M, van der Ploeg CPB, Scheffer PG, Verlinden DA, Hirschberg H, Abbink F, et al. A nation-wide fetal RHD screening programme for targeted antenatal and postnatal anti-D. ISBT Sci Ser 2012;7:164-7. https://doi.org/10.1111/j.1751-2824.2012.01600.x.
- Tiblad E, Taune Wikman A, Ajne G, Blanck A, Jansson Y, Karlsson A, et al. Targeted routine antenatal anti-D prophylaxis in the prevention of RhD immunisation – outcome of a new antenatal screening and prevention program. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0070984.
- Damkjaer MB, Perslev A, Clausen FB, Dziegiel MH, Jørgensen FS. Study of compliance with a new, targeted antenatal D immunization prevention programme in Denmark. Vox Sang 2012;103:145-9. http://dx.doi.org/10.1111/j.1423-0410.2012.01602.x.
- Oxenford K, Silcock C, Hill M, Chitty L. Routine testing of fetal Rhesus D status in Rhesus D negative women using cell-free fetal DNA: an investigation into the preferences and information needs of women. Prenat Diagn 2013;33:688-94. http://dx.doi.org/10.1002/pd.4135.
- Chitty LS, Finning K, Massey E, Soothill P, Daniels G. Antenatal determination of fetal rhesus (RH) D status using cell free fetal DNA in the maternal circulation before 20 weeks’ gestation: is routine application practical and beneficial?. Arch Dis Child Fetal Neonatal Ed 2011;96:Fa11-Fa12. https://doi.org/10.1136/adc.2011.300160.36.
- Chitty L, Finning K, Wade A, Massey E, Soothill P, Martin W. Routine fetal RHD typing using cffDNA in RhD negative women: timing, costs and efficiency. Prenat Diagn 2012;32:58-9.
- Daniels G, Finning K, Wade A, Massey E, Soothill P, Phillips CJ, et al. Implementation of routine of fetal RHD typing in all RHD-negative pregnant women: Timing, costs, and efficiency. Vox Sang 2012;103.
- Finning K, Tovey S, Desay K, Latham T, Daniels G. UK NHS blood and transplant fetal RHD screening – giving anti-D only to those who need it!. Vox Sang 2015;109.
- Finning K, Hosken J, Latham T, Wreford-Bush T, Ford J, Daniels G, et al. NHSBT provision of a fetal RHD genotyping service pilot to reduce antenatal RhIg administration. Transfus Med 2014;24:71-2.
- Ford J, Soothill P. Cell-free DNA fetal blood group testing for RhD-negative pregnant women: implications for midwifery. Br J Midwifery 2016;24:96-9. https://doi.org/10.12968/bjom.2016.24.2.96.
- Banch Clausen F. Routine antenatal screening for fetal RHD in D negative pregnant women in Denmark to guide targeted routine antenatal anti-D prophylaxis. Transfus Med Hemother 2012;39.
- Banch Clausen F. Routine fetal genotyping for RHD in Denmark. Transfus Mede 2012;22.
- Dziegiel MH, Christiansen M, Steffensen R, Jorgensen S, Nielsen C, Jakobsen M, et al. Noninvasive prenatal screening for RHD: the 1st national antenatal directed rh prophylaxis programme – the Danish model. Vox Sang 2012;103.
- Banch Clausen F, Rieneck K, Dziegiel MH. On improving the real-time PCR-based detection of cell-free fetal DNA. Vox Sangs 2011;101:265-6.
- Steffensen R, Nielsen K, Vad J, Faergemann G, Falk L, Baech J. Routine antenatal anti-D prophylaxis and patient compliance. Vox Sangs 2012;103.
- Veldhuisen B, Thurik F, Soussan Aicha A, Woortmeijer H, van der Schoot E, de Haas M. Technical performance of the fully automated fetal RHD screening program in the Netherlands. Transfus Med 2014;24:72-3.
- Veldhuisen B, Thurik F, Jonkers R, Bossers B, Concepcion S, Woortmeijer H, et al. Molecular RhD variation of serological RhD-negative women: implications for a fetal RhD screening programme to target anti-D prophylaxis. Vox Sang 2013;105:20-1.
- Thurik FF, Soussan AA, Woortmeijer H, Page-Christiaens GCML, de Haas M, van der Schoot CE. Are false-positive results in non-invasive prenatal RHD typing caused by placental chimerism?. Prenat Diagn 2014;34:20-1.
- Thurik FF, Ait Soussan A, Woortmeijer H, Veldhuisen B, van der Schoot CE, de Haas M. Technical performance of the fully automated fetal RHD screening program in the Netherlands. Vox Sang 2014;107.
- Scheffer PG, Thurik FF, Veldhuisen B, Jonker R, Haas M, van der Schoot CE. A nation-wide fetal RHD screening program for targeted antenatal and postnatal anti-D immunoglobulin prophylaxis. Prenat Diagn 2013;33.
- van der Schoot CE, Soussan AA, Bonsel GJ, de Haas M. Non invasive screening for fetal RHD-genotype in all D-negative women is reliable and cost-effective. Blood 2005;106.
- de Haas M, van der Schoot CE, van der Ploeg CPB, Abbink F. Noninvasive prenatal screening for RHD in The Netherlands: one test for targeted antenatal and postnatal anti-d prophylaxis. Vox Sang 2012;103.
- de Haas M, van der Ploeg CPB, Veldhuisen B, Verlinden DA, Hirschberg H, Scheffer P, et al. Fetal RHD typing can be safely used to target both antenatal and postnatal anti-D prophylaxis. Vox Sang 2013;105.
- Grootkerk-Tax MG, Soussan AA, de Haas M, Maaskant-van Wijk PA, van der Schoot CE. Evaluation of prenatal RHD typing strategies on cell-free fetal DNA from maternal plasma. Transfusion 2006;46:2142-8. https://doi.org/10.1111/j.1537-2995.2006.01044.x.
- van der Ploeg CPBK, Hirschberg HJHB, de Haas M, Abbink F. Foetal Rhesus-D typing added to antenatal screening for infectious diseases and erythrocyte immunisation. Ned Tijdschr Geneeskd 2015;159.
- Wikman T, Tiblad E, Westgren M. Noninvasive prenatal screening for RHD: the Stockholm study. Vox Sang 2012;103:33-4. https://doi.org/10.1111/j.1751-2824.2012.01589.x.
- Wikman AT, Tiblad E, Karlsson A, Olsson ML, Westgren M, Reilly M. Fetal RhD detection in maternal plasma in a Swedish antenatal screening program. Transfusion 2011;51.
- Wikman AT. The Stockholm study: conclusions after 3 years fetal RHD screening in early pregnancy. Vox Sang 2013;105.
- Wikman A, Tiblad E, Karlsson A, Westgren M, Lundahl J. Detection of fetal RHD DNA in maternal plasma in early pregnancy in an antenatal screening program. Vox Sang 2010;99:25-6.
- Tiblad E, Westgren M, Karlsson A, Ates E, Wikman A. An antenatal screening program for detection of fetal RhD in the first trimester of pregnancy. Prenat Diagn 2010;30.
- Tiblad E, Wikman AT, Nordlander E, Ajne G, Karlsson A, Olerup AB, et al. First trimester non-invasive screening for fetal RHD and targeted antenatal anti-D prophylaxis. Prenat Diagn 2012;32.
- Neovius M, Tiblad E, Westgren M, Neovius K, Wikman AT. Resource utilization after first trimester noninvasive fetal RHD screening for targeted antenatal anti-D prophylaxis in RhD-negative Swedish women. Transfusion 2014;54:18A-19A.
- Tiblad E. First trimester non-invasive screening for fetal RHD and targeted antenatal anti-D prophylaxis – does it work?. Acta Obstet Gynecol Scand 2012;91.
- Neovius M, Tiblad E, Westgren M, Kublickas M, Neovius K, Wikman A. Cost-effectiveness of first trimester non-invasive fetal RHD screening for targeted antenatal anti-D prophylaxis in RhD-negative pregnant women: a model-based analysis. BJOG 2016;123:1337-46. https://doi.org/10.1111/1471-0528.13801.
- Daniels G, van der Schoot CE, Olsson ML. Report of the First International Workshop on molecular blood group genotyping. Vox Sang 2005;88:136-42. https://doi.org/10.1111/j.1423-0410.2005.00603.x.
- Pregnancy and Ethnic Factors Influencing Births and Infant Mortality: 2013. Newport: Office for National Statistics; 2015.
- Fyfe TM, Ritchey MJ, Taruc C, Crompton D, Galliford B, Perrin R. Appropriate provision of anti-D prophylaxis to RhD negative pregnant women: a scoping review. BMC Pregnancy Childbirth 2014;14. http://dx.doi.org/10.1186/s12884-014-0411-1.
- Pilgrim H, Lloyd-Jones M, Rees A. Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation. Health Technol Assess 2009;13:1-126. https://doi.org/10.3310/hta13100.
- Turner RM, Lloyd-Jones M, Anumba DO, Smith GC, Spiegelhalter DJ, Squires H, et al. Routine antenatal anti-D prophylaxis in women who are Rh(D) negative: meta-analyses adjusted for differences in study design and quality. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0030711.
- McBain RD, Crowther CA, Middleton P. Anti-D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev 2015;9.
- Crowther CA, Middleton P. Anti-D administration after childbirth for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev 1997;2. https://doi.org/10.1002/14651858.cd000021.
- High-Throughput, Non-Invasive Prenatal Testing (NIPT) for Fetal Rhesus D Status. Final Scope November 2015. London: NICE; 2015.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Szczepura A, Osipenko L, Freeman K. A new fetal RHD genotyping test: costs and benefits of mass testing to target antenatal anti-D prophylaxis in England and Wales. BMC Pregnancy Childbirth 2011;11. http://dx.doi.org/10.1186/1471-2393-11-5.
- Benachi A, Delahaye S, Leticee N, Jouannic J-M, Ville Y, Costa J-M. Impact of non-invasive fetal RhD genotyping on management costs of rhesus-D negative patients: results of a French pilot study. Eur J Obstet Gynecol Reprod Biol 2012;162:28-32. https://doi.org/10.1016/j.ejogrb.2012.02.001.
- Macher HC, Noguerol P, Medrano-Campillo P, Garrido-Marquez MR, Rubio-Calvo A, Carmona-Gonzalez M, et al. Standardization non-invasive fetal RHD and SRY determination into clinical routine using a new multiplex RT-PCR assay for fetal cell-free DNA in pregnant women plasma: results in clinical benefits and cost saving. Clin Chim Acta 2012;413:490-4. https://doi.org/10.1016/j.cca.2011.11.004.
- Duplantie J, Martinez O, Bois A, Nshimyumukiza L, Gekas J, Bujold E, et al. Cost-effectiveness of rh-negative pregnant women management. Biochim Clin 2013;37. https://doi.org/10.1016/s1701-2163(15)30864-1.
- Hawk AF, Chang EY, Shields SM, Simpson KN. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol 2013;122:579-85. http://dx.doi.org/10.1097/AOG.0b013e31829f8814.
- Teitelbaum L, Metcalfe A, Clarke G, Parboosingh JS, Wilson RD, Johnson JM. Costs and benefits of non-invasive fetal RhD determination. Ultrasound Obstet Gynecol 2015;45:84-8. http://dx.doi.org/10.1002/uog.14723.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem 2003;49:1-6. https://doi.org/10.1373/49.1.1.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. https://doi.org/10.1186/1471-2288-3-25.
- Bowman J. The management of hemolytic disease in the fetus and newborn. Semin Perinatol 1997;21:39-44. https://doi.org/10.1016/S0146-0005(97)80018-3.
- Wirthner D, Hohlfeld P, Tissot JD. Perinatal hemolytic disease. Part 1: physiopathology. J Gynecol Obstet Biol Reprod 1998;27:135-43.
- Gobalakichenane P, Lardennois C, Galene-Gromez S, Brossard V, Marpeau L, Verspyck E, et al. Perinatal management and neurological outcome of newborns hospitalized with Rhesus hemolytic disease. Gynecol Obstet Fertil 2008;36:984-90. https://doi.org/10.1016/j.gyobfe.2008.07.012.
- Schedule of Appointments in Routine Care. London: NICE; 2016.
- Births in England and Wales. Newport: Office for National Statistics; 2014.
- Ford EB. Mendelism and Evolution. London and New York, NY: Methuen & Co and John Wiley & Sons; 1960.
- Further Parental Characteristics, England and Wales. Newport: Office for National Statistics; 2013.
- Roman A, Pernell M, Decherney AH, Nathan L. Current Obstetric and Gynecologic Diagnosis and Treatment. New York, NY: McGraw-Hill Professional; 2002.
- Chilcott J, Tappenden P, Lloyd Jones M, Wight J, Forman K, Wray J, et al. The economics of routine antenatal anti-D prophylaxis for pregnant women who are rhesus negative. BJOG 2004;111:903-7. http://dx.doi.org/10.1111/j.1471-0528.2004.00226.x.
- Okwundu CI, Afolabi BB. Intramuscular versus intravenous anti-D for preventing Rhesus alloimmunization during pregnancy. Cochrane Database Syst Rev 2013;1.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- NHS Reference Costs 2014–15. London: Department of Health; 2015.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Annual Mid-year Population Estimates, 2014. Newport: Office for National Statistics; 2014.
- Birth Summary Tables, England and Wales – Characteristics of Mother 2, England and Wales – Average 2009 to 2013. Newport: Office for National Statistics; 2013.
- Akolekar R, Finning K, Kuppusamy R, Daniels G, Nicolaides KH. Fetal RHD genotype detection from circulating cell-free fetal DNA in maternal plasma in non-sensitized RhD negative women. Fetal Diagn Ther 2011;29:301-6. https://doi.org/10.1159/000322959.
- Lun FM, Chiu RW, Chan KC, Leung TY, Lau TK, Lo YM. Microfluidics digital PCR reveals a higher than expected fraction of fetal DNA in maternal plasma. Clin Chem 2008;54:1664-72. http://dx.doi.org/10.1373/clinchem.2008.111385.
- Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn 2013;33:662-6. http://dx.doi.org/10.1002/pd.4119.
- Brojer E, Zupanska B, Guz K, Orziñska A, Kaliñska A. Noninvasive determination of fetal RHD status by examination of cell-free DNA in maternal plasma. Transfusion 2005;45:1473-80. https://doi.org/10.1111/j.1537-2995.2005.00559.x.
Appendix 1 Search strategies
MEDLINE (via Ovid, http://ovidsp.ovid.com/)
Date range searched: 1946 to October Week 5 2015.
Date searched: 5 November 2015.
Records retrieved: 1815.
The search was updated on 26 February 2016, retrieving 77 records from MEDLINE and 40 records from MEDLINE In-Process & other Non-Indexed Citations.
-
Rh-Hr Blood-Group System/ (10,006)
-
(RhD or “rhesus D” or “Rh(D)” or “Rh-(D)” or Rh D).ti,ab. (3323)
-
(Rh-negative or Rh-positive).ti,ab. (898)
-
(Rhesus negative or Rhesus positive).ti,ab. (228)
-
((rh or rhesus) adj2 (factor or factors or antigen$ or system or group)).ti,ab. (3438)
-
or/1-5 (13,812)
-
Rh Isoimmunization/ (1505)
-
((isoimmuni$ or iso-immuni$ or isoimmune or iso-immune) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (1164)
-
((alloimmuni$ or allo-immuni$ or alloimmune or allo-immune) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (870)
-
((unsensiti#ed or un-sensiti#ed or non-sensiti#ed) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (25)
-
((sensiti#ation$ or sensiti#ed) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (1074)
-
((fetomaternal or feto-maternal or foetomaternal or foeto-maternal) adj2 immuni#ation).ti,ab. (80)
-
((rh or rhesus) adj2 (immuni#ation or autoimmuni#ation)).ti,ab. (695)
-
or/7-13 (4428)
-
exp Erythroblastosis, Fetal/ (11,006)
-
((hemolytic or haemolytic) adj2 (disease$ or disorder$)).ti,ab. (4465)
-
HDFN.ti,ab. (95)
-
((rhesus or rh) adj2 (disease$ or disorder$)).ti,ab. (742)
-
((rhesus or rh or RhD) adj2 (incompatib$ or antagonism)).ti,ab. (750)
-
((erythroblastoses or erythroblastosis) adj2 f?etal$).ti,ab. (760)
-
or/15-20 (13,551)
-
6 or 14 or 21 (25,723)
-
Prenatal Diagnosis/ (33,273)
-
Maternal Serum Screening Tests/ (153)
-
Hematologic Tests/ (5564)
-
((prenatal or pre-natal or antenatal or ante-natal) adj3 (test$ or screen$ or diagnos$ or determin$ or detect$)).ti,ab. (32,925)
-
((fetal or foetal or fetus$ or foetus$) adj3 (test$ or screen$ or diagnos$ or determin$ or detect$)).ti,ab. (20,036)
-
(NIPD or NIPT).ti,ab. (328)
-
or/23-28 (69,981)
-
Genotyping Techniques/ (2761)
-
((genotype$ or genotyping) adj2 (fetal or foetal or fetus$ or foetus$ or prenatal or pre-natal or antenatal or ante-natal)).ti,ab. (606)
-
((genotype$ or genotyping) adj2 (maternal or pregnan$)).ti,ab. (789)
-
((genotype$ or genotyping) adj2 (noninvasive or non-invasive)).ti,ab. (71)
-
cell-free f?etal DNA.ti,ab. (489)
-
cffDNA.ti,ab. (87)
-
or/30-35 (4483)
-
22 and 29 (1795)
-
22 and 36 (276)
-
37 or 38 (1869)
-
(editorial or comment).pt. (946,538)
-
39 not 40 (1824)
-
exp animals/ not humans/ (4,137,930)
-
41 not 42 (1815)
Key
/ = indexing term [medical subject heading (MeSH) heading]
exp = exploded indexing term (MeSH heading)
$ = truncation
# = mandated wildcard – stands for one character
? = optional wildcard – stands for zero or one character
.ti,ab. = terms in either title or abstract fields
.pt. = publication type
adj = terms next to each other (order specified)
adj2 = terms within two words of each other (any order)
Cumulative Index to Nursing & Allied Health (via EBSCOhost, www.ebscohost.com)
Date range searched: inception to 5 November 2015.
Date searched: 6 November 2015.
Records retrieved: 290.
The search was updated on 26 February 2016, retrieving 31 records.
| # | Query | Results |
|---|---|---|
| S39 | S37 OR S38 | 290 |
| S38 | S22 AND S36 | 73 |
| S37 | S22 AND S29 | 268 |
| S36 | S30 OR S31 OR S33 OR S34 OR S35 | 2737 |
| S35 | TI cffDNA OR AB cffDNA | 20 |
| S34 | TI “cell-free f#etal DNA” OR AB “cell-free f#etal DNA” | 124 |
| S33 | TI ( ((genotype* or genotyping) N2 (noninvasive or non-invasive)) ) OR AB ( (genotype* or genotyping) N2 (noninvasive or non-invasive)) ) | 21 |
| S32 | TI ( ((genotype* or genotyping) N2 (maternal or pregnan*)) ) OR AB ( ((genotype* or genotyping) N2 (maternal or pregnan*)) ) | 105 |
| S31 | TI ( ((genotype* or genotyping) N2 (fetal or foetal or fetus* or foetus* or prenatal or pre-natal or antenatal or ante-natal)) ) OR AB ( ((genotype* or genotyping) N2 (fetal or foetal or fetus* or foetus* or prenatal or pre-natal or antenatal or ante-natal)) ) | 103 |
| S30 | MM “Genetic Techniques” | 2529 |
| S29 | S23 or S24 or S25 or S26 or S27 or S28 | 22,920 |
| S28 | TI ( (NIPD or NIPT) ) OR AB ( (NIPD or NIPT) ) | 93 |
| S27 | TI ( (fetal or foetal or fetus* or foetus*) N3 (test* or screen* or diagnos* or determin* or detect*) ) OR AB ( (fetal or foetal or fetus* or foetus*) N3 (test* or screen* or diagnos* or determin* or detect*) ) | 2644 |
| S26 | TI ( (prenatal or pre-natal or antenatal or ante-natal) N3 (test* or screen* or diagnos* or determin* or detect*) ) OR AB ( (prenatal or pre-natal or antenatal or ante-natal) N3 (test* or screen* or diagnos* or determin* or detect*) ) | 5033 |
| S25 | (MH “Noninvasive Procedures”) | 1538 |
| S24 | (MH “Hematologic Tests”) | 11,530 |
| S23 | (MH “Prenatal Diagnosis”) | 5562 |
| S22 | S6 OR S14 OR S21 | 1924 |
| S21 | S15 OR S16 OR S17 OR S18 OR S19 OR S20 | 998 |
| S20 | TI ( (erythroblastoses or erythroblastosis) N2 (fetal* or foetal*) ) OR AB ( (erythroblastoses or erythroblastosis) N2 (fetal* or foetal*) ) | 16 |
| S19 | TI ( (rhesus or rh or RhD) N2 (incompatib* or antagonism) ) OR AB ( (rhesus or rh or RhD) N2 (incompatib* or antagonism) ) | 45 |
| S18 | TI ( (rhesus or rh) N2 (disease* or disorder*) ) OR AB ( (rhesus or rh) N2 (disease* or disorder*) ) | 76 |
| S17 | TI HDFN OR AB HDFN | 20 |
| S16 | TI ( (hemolytic or haemolytic) N2 (disease* or disorder*) ) OR AB ( (hemolytic or haemolytic) N2 (disease* or disorder*) ) | 298 |
| S15 | (MH “Erythroblastosis, Fetal+”) | 775 |
| S14 | S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 | 446 |
| S13 | TI ( (rh or rhesus) N2 (immuni?ation or autoimmuni?ation) ) OR AB ( (rh or rhesus) N2 (immuni?ation or autoimmuni?ation) ) | 17 |
| S12 | TI ( (fetomaternal or feto-maternal or foetomaternal or foeto-maternal) N2 immuni?ation ) OR AB ( (fetomaternal or feto-maternal or foetomaternal or foeto-maternal) N2 immuni?ation ) | 2 |
| S11 | TI ( (sensiti?ation* or sensiti?ed) N6 (rh or rhesus or maternal or pregnan*) ) OR AB ( (sensiti?ation* or sensiti?ed) N6 (rh or rhesus or maternal or pregnan*) ) | 61 |
| S10 | TI ( (unsensiti?ed or un-sensiti?ed or non-sensiti?ed) N6 (rh or rhesus or maternal or pregnan*) ) OR AB ( (unsensiti?ed or un-sensiti?ed or non-sensiti?ed) N6 (rh or rhesus or maternal or pregnan*) ) | 3 |
| S9 | TI ( (alloimmuni* or allo-immuni* or alloimmune or allo-immune) N6 (rh or rhesus or maternal or pregnan*) ) OR AB ( (alloimmuni* or allo-immuni* or alloimmune or allo-immune) N6 (rh or rhesus or maternal or pregnan*) ) | 126 |
| S8 | TI ( (isoimmuni* or iso-immuni* or isoimmune or iso-immune) N6 (rh or rhesus or maternal or pregnan*) ) OR AB ( (isoimmuni* or iso-immuni* or isoimmune or iso-immune) N6 (rh or rhesus or maternal or pregnan*) ) | 47 |
| S7 | (MH “RH Isoimmunization”) | 297 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | 870 |
| S5 | TI ( (rh or rhesus) N2 (factor or factors or antigen* or system or group) ) OR AB ( (rh or rhesus) N2 (factor or factors or antigen* or system or group) ) | 167 |
| S4 | TI ( “Rhesus negative” or “Rhesus positive” ) OR AB ( “Rhesus negative” or “Rhesus positive” ) | 24 |
| S3 | TI ( Rh-negative or Rh-positive ) OR AB ( Rh-negative or Rh-positive ) | 53 |
| S2 | TI ( RhD or “rhesus D” or “Rh(D)” or “Rh-(D)” or Rh D ) OR AB ( RhD or “rhesus D” or “Rh(D)” or “Rh-(D)” or “Rh D” or “Rh-D” ) | 492 |
| S1 | (MH “Rh-Hr Blood-Group System”) | 458 |
Key
MH = indexing term (CINAHL heading)
* = truncation
? = wildcard – stands for one character
# = optional wildcard – stands for zero or one character
TI = words in the title
AB = words in the abstract
“ “ = phrase search
N2 = terms within two words of each other (any order)
PT = publication type
Cochrane Central Register of Controlled Trials (via Wiley Online Library, http://onlinelibrary.wiley.com/)
Issue 10 of 12, October 2015.
Date searched: 6 November 2015.
Records retrieved: 16.
The search was updated on 26 February 2016, retrieving 17 records from CENTRAL.
#1 MeSH descriptor: [Rh-Hr Blood-Group System] this term only(62)
#2 (RhD or “rhesus D” or “Rh(D)” or “Rh-(D)” or “Rh D” or “Rh-D”):ti,ab,kw(94)
#3 (Rh-negative or Rh-positive):ti,ab,kw(20)
#4 (“Rhesus negative” or “Rhesus positive”):ti,ab,kw(16)
#5 (rh or rhesus) near/2 (factor or factors or antigen* or system or group):ti,ab,kw(106)
#6 #1 or #2 or #3 or #4 or #5(238)
#7 MeSH descriptor: [Rh Isoimmunization] this term only(40)
#8 (isoimmuni* or iso-immuni* or isoimmune or iso-immune) near/6 (rh or rhesus or maternal or pregnan*):ti,ab,kw(68)
#9 (alloimmuni* or allo-immuni* or alloimmune or allo-immune) near/6 (rh or rhesus or maternal or pregnan*):ti,ab,kw(22)
#10 (unsensitised or unsensitized or un-sensitised or un-sensitized or non-sensitised or non-sensitized) near/6 (rh or rhesus or maternal or pregnan*):ti,ab,kw(3)
#11 (sensitisation* or sensitization* or sensitised or sensitized) near/6 (rh or rhesus or maternal or pregnan*):ti,ab,kw(32)
#12 (fetomaternal or feto-maternal or foetomaternal or foeto-maternal) near/2 (immunisation or immunization):ti,ab,kw(1)
#13 (rh or rhesus) near/2 (immunisation or immunization or autoimmunisation or autoimmunization):ti,ab,kw(29)
#14 #7 or #8 or #9 or #10 or #11 or #12 or #13(123)
#15 MeSH descriptor: [Erythroblastosis, Fetal] explode all trees(72)
#16 (hemolytic or haemolytic) near/2 (disease* or disorder*):ti,ab,kw(99)
#17 HDFN:ti,ab,kw(3)
#18 (rhesus or rh) near/2 (disease* or disorder*):ti,ab,kw(628)
#19 (rhesus or rh or RhD) near/2 (incompatib* or antagonism):ti,ab,kw(22)
#20 (erythroblastoses or erythroblastosis) near/2 (fetal* or foetal*):ti,ab,kw(72)
#21 #15 or #16 or #17 or #18 or #19 or #20(732)
#22 #6 or #14 or #21(978)
#23 MeSH descriptor: [Prenatal Diagnosis] this term only(363)
#24 MeSH descriptor: [Maternal Serum Screening Tests] this term only(5)
#25 MeSH descriptor: [Hematologic Tests] this term only(196)
#26 (prenatal or pre-natal or antenatal or ante-natal) near/3 (test* or screen* or diagnos* or determin* or detect*):ti,ab,kw(868)
#27 (fetal or foetal or fetus* or foetus*) near/3 (test* or screen* or diagnos* or determin* or detect*):ti,ab,kw(571)
#28 (NIPD or NIPT):ti,ab,kw(10)
#29 #23 or #24 or #25 or #26 or #27 or #28(1480)
#30 MeSH descriptor: [Genotyping Techniques] this term only(18)
#31 (genotype* or genotyping) near/2 (fetal or foetal or fetus* or foetus* or prenatal or pre-natal or antenatal or ante-natal):ti,ab,kw(5)
#32 ((genotype* or genotyping) near/2 (maternal or pregnan*)):ti,ab,kw(15)
#33 ((genotype* or genotyping) near/2 (noninvasive or non-invasive)):ti,ab,kw(0)
#34 (“cell-free foetal DNA” or “cell-free fetal DNA”):ti,ab,kw(7)
#35 cffDNA:ti,ab,kw(1)
#36 #30 or #31 or #32 or #33 or #34 or #35(42)
#37 #22 and #29(33)
#38 #22 and #36(4)
#39 #37 or #38(34)
Note: The strategy above was used to search CENTRAL and CDSR. The 34 results at line #39 include Cochrane reviews, DARE, HTA and NHS EED records as well as trials from CENTRAL.
Key
MeSH descriptor = indexing term (MeSH heading)
* = truncation
:ti,ab,kw = terms in either title or abstract or keyword fields
near/2 = terms within two words of each other (any order)
next = terms are next to each other
“ “ = phrase search
Cochrane Database of Systematic Reviews (via Wiley Online Library, http://onlinelibrary.wiley.com/)
Issue 11 of 12, November 2015.
Date searched: 6 November 2015.
Records retrieved: 8.
See Cochrane Central Register of Controlled Trials for search strategy used.
The search was updated on 26 February 2016, retrieving nine records from CDSR.
Database of Abstracts of Reviews of Effects (via Centre for Reviews and Dissemination, www.crd.york.ac.uk/CRDWeb)
Date range searched: inception to 31 March 2015.
Date searched: 6 November 2015.
Records retrieved: 9.
The strategy below was used to search DARE, NHS EED and the HTA database. The hits column shows the total number of records found in all three databases.
| Line | Search | Hits |
|---|---|---|
| 1 | MeSH DESCRIPTOR Rh-Hr Blood-Group System EXPLODE ALL TREES | 16 |
| 2 | (RhD or “rhesus D” or Rh-D) | 24 |
| 3 | (Rh-negative or Rh-positive) | 7 |
| 4 | (“Rhesus negative” or “Rhesus positive”) | 9 |
| 5 | ((rh or rhesus) NEAR2 (factor or factors or antigen* or system or group)) | 18 |
| 6 | ((factor or factors or antigen* or system or group) NEAR2 (rh or rhesus)) | 1 |
| 7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 35 |
| 8 | MeSH DESCRIPTOR Rh Isoimmunization | 15 |
| 9 | ((isoimmuni* or iso-immuni* or isoimmune or iso-immune) NEAR6 (rh or rhesus or maternal or pregnan*)) | 10 |
| 10 | ((rh or rhesus or maternal or pregnan*) NEAR6 (isoimmuni* or iso-immuni* or isoimmune or iso-immune) ) | 17 |
| 11 | ((alloimmuni* or allo-immuni* or alloimmune or allo-immune) NEAR6 (rh or rhesus or maternal or pregnan*)) | 12 |
| 12 | ((rh or rhesus or maternal or pregnan*) NEAR6 (alloimmuni* or allo-immuni* or alloimmune or allo-immune)) | 8 |
| 13 | ((unsensitised or unsensitized or un-sensitised or un-sensitized or non-sensitised or non-sensitized) NEAR6 (rh or rhesus or maternal or pregnan*)) | 3 |
| 14 | ((rh or rhesus or maternal or pregnan*) NEAR6 (unsensitised or unsensitized or un-sensitised or un-sensitized or non-sensitised or non-sensitized)) | 0 |
| 15 | ((sensitisation* or sensitization* or sensitised or sensitized )NEAR6 (rh or rhesus or maternal or pregnan*)) | 6 |
| 16 | ((rh or rhesus or maternal or pregnan*) NEAR6 (sensitisation* or sensitization* or sensitised or sensitized)) | 5 |
| 17 | ((fetomaternal or feto-maternal or foetomaternal or foeto-maternal) NEAR2 (immunisation or immunization)) | 0 |
| 18 | ((immunisation or immunization) NEAR2 (fetomaternal or feto-maternal or foetomaternal or foeto-maternal)) | 0 |
| 19 | ((rh or rhesus) NEAR2 (immunisation or immunization or autoimmunisation or autoimmunization)) | 4 |
| 20 | ((immunisation or immunization or autoimmunisation or autoimmunization) NEAR2 (rh or rhesus)) | 0 |
| 21 | #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 | 29 |
| 22 | MeSH DESCRIPTOR Erythroblastosis, Fetal EXPLODE ALL TREES | 18 |
| 23 | ((hemolytic or haemolytic) NEAR2 (disease* or disorder*)) | 16 |
| 24 | ((disease* or disorder*) NEAR2 (hemolytic or haemolytic)) | 1 |
| 25 | (HDFN) | 1 |
| 26 | ((rhesus or rh) NEAR2 (disease* or disorder*)) | 3 |
| 27 | ((disease* or disorder*) NEAR2 (rhesus or rh)) | 1 |
| 28 | ((rhesus or rh or RhD) NEAR2 (incompatib* or antagonism)) | 3 |
| 29 | ((incompatib* or antagonism) NEAR2 (rhesus or rh or RhD)) | 0 |
| 30 | ((erythroblastoses or erythroblastosis) NEAR2 (fetal* or foetal*)) | 14 |
| 31 | #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 | 28 |
| 32 | #7 OR #21 OR #31 | 56 |
| 33 | MeSH DESCRIPTOR Prenatal Diagnosis | 216 |
| 34 | MeSH DESCRIPTOR Maternal Serum Screening Tests | 5 |
| 35 | MeSH DESCRIPTOR Hematologic Tests | 30 |
| 36 | ((prenatal or pre-natal or antenatal or ante-natal) NEAR3 (test* or screen* or diagnos* or determin* or detect*)) | 380 |
| 37 | ((test* or screen* or diagnos* or determin* or detect*) NEAR3 (prenatal or pre-natal or antenatal or ante-natal)) | 171 |
| 38 | ((test* or screen* or diagnos* or determin* or detect*) NEAR3 (fetal or foetal or fetus* or foetus*)) | 124 |
| 39 | ((fetal or foetal or fetus* or foetus*) NEAR3 (test* or screen* or diagnos* or determin* or detect*)) | 130 |
| 40 | (NIPD or NIPT) | 6 |
| 41 | #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 | 534 |
| 42 | MeSH DESCRIPTOR Genotyping Techniques | 6 |
| 43 | ((genotype* or genotyping) NEAR2 (fetal or foetal or fetus* or foetus* or prenatal or pre-natal or antenatal or ante-natal)) | 3 |
| 44 | ((fetal or foetal or fetus* or foetus* or prenatal or pre-natal or antenatal or ante-natal) NEAR2 (genotype* or genotyping)) | 3 |
| 45 | ((genotype* or genotyping) NEAR2 (maternal or pregnan*)) | 2 |
| 46 | ((maternal or pregnan*) NEAR2 (genotype* or genotyping)) | 2 |
| 47 | ((genotype* or genotyping) NEAR2 (noninvasive or non-invasive)) | 1 |
| 48 | ((noninvasive or non-invasive) NEAR2 (genotype* or genotyping)) | 4 |
| 49 | (“cell-free foetal DNA” or “cell-free fetal DNA”) | 7 |
| 50 | (cffDNA) | 2 |
| 51 | #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 | 18 |
| 52 | #32 AND #41 | 16 |
| 53 | #32 AND #51 | 6 |
| 54 | #52 OR #53 | 18 |
Key
MeSH DESCRIPTOR = indexing term (MeSH heading)
* = truncation
NEAR2 = terms within two words of each other (order specified)
“ ” = phrase search
EMBASE (via Ovid, http://ovidsp.ovid.com/)
Date range searched: 1974 to 2015 November 04.
Date searched: 5 November 2015.
Records retrieved: 3092.
The search was updated on 26 February 2016, retrieving 221 records.
-
blood group rhesus system/ (8133)
-
rhesus D antigen/ (785)
-
(RhD or “rhesus D” or “Rh(D)” or “Rh-(D)” or Rh D).ti,ab. (5254)
-
(Rh-negative or Rh-positive).ti,ab. (1197)
-
(Rhesus negative or Rhesus positive).ti,ab. (320)
-
((rh or rhesus) adj2 (factor or factors or antigen$ or system or group)).ti,ab. (4401)
-
or/1-6 (15,398)
-
rhesus isoimmunization/ (1536)
-
((isoimmuni$ or iso-immuni$ or isoimmune or iso-immune) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (1313)
-
((alloimmuni$ or allo-immuni$ or alloimmune or allo-immune) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (1319)
-
((unsensiti#ed or un-sensiti#ed or non-sensiti#ed) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (37)
-
((sensiti#ation$ or sensiti#ed) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (1306)
-
((fetomaternal or feto-maternal or foetomaternal or foeto-maternal) adj2 immuni#ation).ti,ab. (90)
-
((rh or rhesus) adj2 (immuni#ation or autoimmuni#ation)).ti,ab. (772)
-
or/8-14 (5218)
-
exp newborn hemolytic disease/ (11,867)
-
((hemolytic or haemolytic) adj2 (disease$ or disorder$)).ti,ab. (5302)
-
HDFN.ti,ab. (294)
-
((rhesus or rh) adj2 (disease$ or disorder$)).ti,ab. (838)
-
((rhesus or rh or RhD) adj2 (incompatib$ or antagonism)).ti,ab. (913)
-
((erythroblastoses or erythroblastosis) adj2 f?etal$).ti,ab. (739)
-
rhesus incompatibility/ (1131)
-
or/16-22 (16,217)
-
7 or 15 or 23 (30,562)
-
prenatal diagnosis/ (50,220)
-
prenatal screening/ (6356)
-
maternal serum screening test/ (145)
-
blood examination/ (10,293)
-
non invasive procedure/ (17,457)
-
diagnostic accuracy/ (195,290)
-
((prenatal or pre-natal or antenatal or ante-natal) adj3 (test$ or screen$ or diagnos$ or determin$ or detect$)).ti,ab. (40,821)
-
((fetal or foetal or fetus$ or foetus$) adj3 (test$ or screen$ or diagnos$ or determin$ or detect$)).ti,ab. (25,280)
-
(NIPD or NIPT).ti,ab. (561)
-
or/25-33 (301,546)
-
genotyping technique/ (4081)
-
((genotype$ or genotyping) adj2 (fetal or foetal or fetus$ or foetus$ or prenatal or pre-natal or antenatal or ante-natal)).ti,ab. (800)
-
((genotype$ or genotyping) adj2 (maternal or pregnan$)).ti,ab. (924)
-
((genotype$ or genotyping) adj2 (noninvasive or non-invasive)).ti,ab. (90)
-
cell-free f?etal DNA.ti,ab. (741)
-
cffDNA.ti,ab. (168)
-
or/35-40 (6300)
-
24 and 34 (3084)
-
24 and 41 (419)
-
42 or 43 (3160)
-
(editorial or note).pt. (1,117,567)
-
44 not 45 (3107)
-
animal/ (1,701,987)
-
exp animal experiment/ (1,895,782)
-
nonhuman/ (4,645,212)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,sh. (4,564,702)
-
47 or 48 or 49 or 50 (7,266,921)
-
exp human/ (16,514,549)
-
human experiment/ (344,858)
-
52 or 53 (16,515,997)
-
51 not (51 and 54) (5,693,442)
-
46 not 55 (3092)
Key
/ = indexing term (Emtree heading)
exp = exploded indexing term (Emtree heading)
$ = truncation
# = mandated wildcard – stands for one character
? = optional wildcard – stands for zero or one character
.ti,ab. = terms in either title or abstract fields
.pt. = publication type
sh. = subject heading field
adj = terms next to each other (order specified)
adj2 = terms within two words of each other (any order)
Health Technology Assessment database (via www.crd.york.ac.uk/CRDWeb)
Date range searched: inception to 31 March 2015.
Date searched: 6 November 2015.
Records retrieved: 3.
See above under Database of Abstracts of Reviews of Effects for search strategy used.
Maternity and infant care (via Ovid, http://ovidsp.ovid.com/)
Date range searched: 1971 to September 2015.
Date searched: 5 November 2015.
Records retrieved: 238.
The search was updated on 26 February 2016, retrieving 11 records.
-
Rh-Hr blood-group system.de. (26)
-
(RhD or “rhesus D” or “Rh(D)” or “Rh-(D)” or Rh D).ti,ab. (285)
-
(Rh-negative or Rh-positive).ti,ab. (81)
-
(Rhesus negative or Rhesus positive).ti,ab. (76)
-
((rh or rhesus) adj2 (factor or factors or antigen$ or system or group)).ti,ab. (57)
-
1 or 2 or 3 or 4 or 5 (439)
-
(Rh isoimmunisation or Rh isoimmunisation - therapy or “Rh isoimmunisation - prevention and control”).de. (317)
-
Alloimmunisation.de. (29)
-
((isoimmuni$ or iso-immuni$ or isoimmune or iso-immune) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (148)
-
((alloimmuni$ or allo-immuni$ or alloimmune or allo-immune) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (201)
-
((unsensiti#ed or un-sensiti#ed or non-sensiti#ed) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (9)
-
((sensiti#ation$ or sensiti#ed) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (96)
-
((fetomaternal or feto-maternal or foetomaternal or foeto-maternal) adj2 immuni#ation).ti,ab. (3)
-
((rh or rhesus) adj2 (immuni#ation or autoimmuni#ation)).ti,ab. (61)
-
7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 (616)
-
Erythroblastosis - fetal.de. (118)
-
((hemolytic or haemolytic) adj2 (disease$ or disorder$)).ti,ab. (281)
-
HDFN.ti,ab. (24)
-
((rhesus or rh) adj2 (disease$ or disorder$)).ti,ab. (96)
-
rhesus.sx. (435)
-
((rhesus or rh or RhD) adj2 (incompatib$ or antagonism)).ti,ab. (42)
-
((erythroblastoses or erythroblastosis) adj2 f?etal$).ti,ab. (27)
-
16 or 17 or 18 or 19 or 20 or 21 or 22 (669)
-
6 or 15 or 23 (1005)
-
Prenatal diagnosis.de. (4460)
-
((prenatal or pre-natal or antenatal or ante-natal) adj3 (test$ or screen$ or diagnos$ or determin$ or detect$)).ti,ab. (7133)
-
((fetal or foetal or fetus$ or foetus$) adj3 (test$ or screen$ or diagnos$ or determin$ or detect$)).ti,ab. (4763)
-
(NIPD or NIPT).ti,ab. (89)
-
25 or 26 or 27 or 28 (12,193)
-
((genotype$ or genotyping) adj2 (fetal or foetal or fetus$ or foetus$ or prenatal or pre-natal or antenatal or ante-natal)).ti,ab. (96)
-
((genotype$ or genotyping) adj2 (maternal or pregnan$)).ti,ab. (89)
-
((genotype$ or genotyping) adj2 (noninvasive or non-invasive)).ti,ab. (6)
-
cell-free f?etal DNA.ti,ab. (148)
-
cffDNA.ti,ab. (31)
-
(genotype$ or genotyping).ti,ab. (881)
-
30 or 31 or 32 or 33 or 34 (291)
-
24 and 29 (237)
-
24 and 36 (67)
-
37 or 38 (245)
-
(editorial or commentary).pt. (14,906)
-
39 not 40 (238)
Key
.de. = subject heading search
$ = truncation
# = mandated wildcard – stands for one character
? = optional wildcard – stands for zero or one character
.ti,ab. = terms in either title or abstract fields
.pt. = publication type
adj = terms next to each other (order specified)
adj2 = terms within two words of each other (any order)
NHS Economic Evaluations Database (via Centre for Reviews and Dissemination, www.crd.york.ac.uk/CRDWeb)
Date range searched: inception to 31 March 2015.
Date searched: 6 November 2015.
Records retrieved: 6.
See above under Database of Abstracts of Reviews of Effects for search strategy used.
PubMed (www.ncbi.nlm.nih.gov/pubmed)
Date searched: 26 February 2016.
Records retrieved: 112.
((((((((((((“Prenatal Diagnosis”[Mesh:NoExp]) OR “Maternal Serum Screening Tests”[Mesh:NoExp]) OR “Hematologic Tests”[Mesh:NoExp]) OR ((((test[Title/Abstract] OR tests[Title/Abstract] OR testing[Title/Abstract] OR tested[Title/Abstract] OR screen*[Title/Abstract] OR diagnos*[Title/Abstract] OR determin*[Title/Abstract] OR detect*[Title/Abstract]))) AND ((prenatal[Title/Abstract] OR pre-natal[Title/Abstract] OR antenatal[Title/Abstract] OR ante-natal[Title/Abstract] OR fetal[Title/Abstract] OR foetal[Title/Abstract] OR fetus*[Title/Abstract] OR foetus*[Title/Abstract])))) OR ((NIPD[Title/Abstract] OR NIPT[Title/Abstract])))) OR (((“Genotyping Techniques”[Mesh:NoExp]) OR ((((genotype*[Title/Abstract] OR genotyping[Title/Abstract]))) AND ((((fetal[Title/Abstract] OR foetal[Title/Abstract] OR fetus*[Title/Abstract] OR foetus*[Title/Abstract] OR prenatal[Title/Abstract] OR pre-natal[Title/Abstract] OR antenatal[Title/Abstract] OR ante-natal[Title/Abstract])) OR (maternal[Title/Abstract] OR pregnan*[Title/Abstract])) OR (noninvasive[Title/Abstract] OR non-invasive[Title/Abstract])))) OR ((“cell-free fetal DNA”[Title/Abstract] OR “cell-free foetal DNA”[Title/Abstract] OR cffDNA[Title/Abstract])))))) AND ((((((((((“Erythroblastosis, Fetal”[Mesh]) OR ((“hemolytic disease”[Title/Abstract] OR “hemolytic diseases”[Title/Abstract] OR “hemolytic disorder”[Title/Abstract] OR “hemolytic disorders”[Title/Abstract]))) OR ((“haemolytic disease” OR “haemolytic diseases” OR “haemolytic disorder” OR “haemolytic disorders”))) OR HDFN[Title/Abstract]) OR ((“rhesus disease”[Title/Abstract] OR “rhesus diseases”[Title/Abstract] OR “rhesus disorder”[Title/Abstract] OR “rhesus disorders”[Title/Abstract] OR “rh disease”[Title/Abstract] OR “rh diseases”[Title/Abstract] OR “rh disorder”[Title/Abstract] OR “rh disorders”[Title/Abstract]))) OR (((rhesus[Title/Abstract] OR rh[Title/Abstract] OR RhD[Title/Abstract])) AND (incompatib*[Title/Abstract] OR antagonism[Title/Abstract]))) OR (((erythroblastoses[Title/Abstract] OR erythroblastosis[Title/Abstract])) AND (fetal*[Title/Abstract] OR foetal*[Title/Abstract])))) OR (((((“Rh Isoimmunization”[Mesh:noexp]) OR ((((((((isoimmuni*[Title/Abstract] OR iso-immuni*[Title/Abstract] OR isoimmune[Title/Abstract] OR iso-immune[Title/Abstract]))) OR ((alloimmuni*[Title/Abstract] OR allo-immuni*[Title/Abstract] OR alloimmune[Title/Abstract] OR allo-immune[Title/Abstract]))) OR ((unsensitised[Title/Abstract] OR unsensitized[Title/Abstract] OR un-sensitised[Title/Abstract] OR un-sensitized[Title/Abstract] OR non-sensitised[Title/Abstract] OR non-sensitized[Title/Abstract]))) OR ((sensitisation*[Title/Abstract] OR sensitization*[Title/Abstract] OR sensitised[Title/Abstract] OR sensitized[Title/Abstract])))) AND ((rh[Title/Abstract] OR rhesus[Title/Abstract] OR maternal[Title/Abstract] OR pregnan*[Title/Abstract]))))) OR (((fetomaternal[Title/Abstract] OR feto-maternal[Title/Abstract] OR foetomaternal[Title/Abstract] OR foeto-maternal[Title/Abstract])) AND (immunisation[Title/Abstract] OR immunization[Title/Abstract]))) OR (((rh[Title/Abstract] OR rhesus[Title/Abstract])) AND (immunisation[Title/Abstract] OR autoimmunisation[Title/Abstract] OR immunization[Title/Abstract] OR autoimmunization[Title/Abstract])))) OR (((((“Rh-Hr Blood-Group System”[Mesh:noexp]) OR (((RhD[Title/Abstract] OR “rhesus D”[Title/Abstract] OR “Rh(D)”[Title/Abstract] OR “Rh-(D)”[Title/Abstract] OR “Rh D”[Title/Abstract]))) OR ((Rh-negative[Title/Abstract] OR Rh-positive[Title/Abstract]))) OR ((“Rhesus negative”[Title/Abstract] OR “Rhesus positive”[Title/Abstract]))) OR ((“rh factor”[Title/Abstract] OR “rh factors”[Title/Abstract] OR “rh antigen”[Title/Abstract] OR “rh antigens”[Title/Abstract] OR “rh system”[Title/Abstract] OR “rh group”[Title/Abstract]))) OR ((“rhesus factor”[Title/Abstract] OR “rhesus factors”[Title/Abstract] OR “rhesus antigen”[Title/Abstract] OR “rhesus antigens”[Title/Abstract] OR “rhesus system”[Title/Abstract] OR “rhesus group”[Title/Abstract])))))) AND ((((pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb]))) OR (((inprocess[sb] or medline[sb])) AND (“2016/02/20”[Date - Entrez] : “3000”[Date - Entrez]))))
Science Citation Index (via Web of Science, Thomson Reuters, http://thomsonreuters.com/thomson-reuters-web-of-science)
Date range searched: 1900 to 4 November 2015.
Date searched: 6 November 2015.
Records retrieved: 801.
The strategy below was used to search Science Citation Index and the Conference Proceedings Citation Index: Science. As both databases were searched together the records retrieved refer to results from both databases.
The searches for Science Citation Index and the Conference Proceedings Citation Index: Science were updated on 26 February 2016, retrieving 811 records.
| # 34 | 801 |
#32 NOT #33 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 33 | 20 |
#31 OR #30 Refined by:DOCUMENT TYPES: (EDITORIAL MATERIAL) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 32 | 821 |
#31 OR #30 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 31 | 287 |
#29 AND #19 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 30 | 744 |
#23 AND #19 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 29 | 2378 |
#28 OR #27 OR #26 OR #25 OR #24 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 28 | 79 |
TS=cffDNA Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 27 | 543 |
TS=(“cell-free foetal DNA” or “cell-free fetal DNA”) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 26 | 204 |
TS=((genotype* or genotyping) NEAR/2 (noninvasive or non-invasive)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 25 | 1222 |
TS=((genotype* or genotyping) NEAR/2 (maternal or pregnan*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 24 | 779 |
TS=((genotype* or genotyping) NEAR/2 (fetal or foetal or fetus* or foetus* or prenatal or pre-natal or antenatal or ante-natal)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 23 | 51,060 |
#22 OR #21 OR #20 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 22 | 632 |
TS=(NIPD or NIPT) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 21 | 21,197 |
TS=((fetal or foetal or fetus* or foetus*) NEAR/3 (test* or screen* or diagnos* or determin* or detect*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 20 | 36,396 |
TS = ((prenatal or pre-natal or antenatal or ante-natal) NEAR/3 (test* or screen* or diagnos* or determin* or detect*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 19 | 15,143 |
#18 OR #12 OR #5 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 18 | 5220 |
#17 OR #16 OR #15 OR #14 OR #13 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 17 | 581 |
TS = ((erythroblastoses or erythroblastosis) NEAR/2 f$etal*) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 16 | 413 |
TS = ((rhesus or rh or RhD) NEAR/2 (incompatib* or antagonism)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 15 | 1248 |
TS = ((rhesus or rh) NEAR/2 (disease* or disorder*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 14 | 102 |
TS = HDFN Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 13 | 3593 |
TS = ((hemolytic or haemolytic) NEAR/2 (disease* or disorder*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 12 | 2937 |
#11 OR #10 OR #9 OR #8 OR #7 OR #6 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 11 | 565 |
TS = ((rh or rhesus) NEAR/2 (immuni?ation or autoimmuni?ation)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 10 | 32 |
TS = ((fetomaternal or feto-maternal or foetomaternal or foeto-maternal) NEAR/2 immuni?ation) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 9 | 899 |
TS = ((sensiti?ation* or sensiti?ed) NEAR/6 (rh or rhesus or maternal or pregnan*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 8 | 15 |
TS = ((unsensiti?ed or un-sensiti?ed or non-sensiti?ed) NEAR/6 (rh or rhesus or maternal or pregnan*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 7 | 981 |
TS = ((alloimmuni* or allo-immuni* or alloimmune or allo-immune) NEAR/6 (rh or rhesus or maternal or pregnan*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 6 | 736 |
TS = ((isoimmuni* or iso-immuni* or isoimmune or iso-immune) NEAR/6 (rh or rhesus or maternal or pregnan*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 5 | 8522 |
#4 OR #3 OR #2 OR #1 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 4 | 5198 |
TS = ((rh or rhesus) NEAR/2 (factor or factors or antigen* or system or group)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 3 | 121 |
TS = (“Rhesus negative” or “Rhesus positive”) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 2 | 479 |
TS = (Rh-negative or Rh-positive) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 1 | 3491 |
TS = (RhD or “rhesus D” or “Rh(D)” or “Rh-(D)” or “Rh D” or “Rh-D”) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
Ongoing, unpublished or grey literature search strategies
ClinicalTrials.gov (https://clinicaltrials.gov/)
Date searched: 10 November 2015.
Records retrieved: 44.
RhD OR “rhesus D” OR “Rh(D)” OR “Rh-(D)” OR “Rh D” OR “Rh-negative” OR “Rh-positive” OR “Rhesus negative” OR “Rhesus positive”
The search was updated on 26 February 2016, retrieving two new records.
Conference Proceedings Citation Index: Science (via Web of Science, Thomson Reuters, http://thomsonreuters.com/thomson-reuters-web-of-science)
Date range searched: 1990 to 4 November 2015.
Date searched: 6 November 2015.
Records retrieved: 801.
See Science Citation Index for search strategy used. As both databases were searched together, the records retrieved refers to results from both databases.
The searches for Science Citation Index and the Conference Proceedings Citation Index: Science were updated on 26 February 2016, retrieving 811 records.
EU Clinical Trials Register (www.clinicaltrialsregister.eu/ctr-search/search)
Date searched: 10 November 2015.
Records retrieved: 4.
“RhD” OR “rhesus D” OR “Rh(D)” OR “Rh-(D)” OR “Rh D” OR “Rh-negative” OR “Rh-positive” OR “Rh negative” OR “Rh positive” OR “Rhesus negative” OR “Rhesus positive”
The search was updated on 26 February 2016 but no new records were retrieved.
PROSPERO (www.crd.york.ac.uk/PROSPERO)
Date searched: 10 November 2015.
Records retrieved: 4.
RhD or Rh-D or Rh-negative or Rh-positive in all fields.
The search was updated on 26 February 2016, retrieving one new record.
World Health Organization’s International Clinical Trials Registry Platform (www.who.int/ictrp/search/en)
Date searched: 10 November 2015.
Records retrieved: 29.
RhD OR rhesus OR Rh-negative OR Rh-positive.
The search was updated on 26 February 2016 but no new records were retrieved.
Guideline searches
The following websites were searched for relevant guidelines.
All guideline website searches were updated on 4 March 2016; however, no new guidelines were retrieved.
National Guidelines Clearinghouse (www.guideline.gov)
Date searched: 17 November 2015.
(rhd or rhesus or ‘rh negative” or “rh positive”)‘ and ‘(pregnan* or maternal or antenatal or ante-natal or prenatal or pre-natal or intrapartum)
A total of 23 results were retrieved and browsed for relevance; 18 relevant guidelines were found.
National Institute for Health and Care Excellence (www.nice.org.uk)
Date searched: 13 November 2015.
-
Browsed for relevant guidance in the fertility, pregnancy and childbirth section: www.nice.org.uk/guidance/conditions-and-diseases/fertility--pregnancy-and-childbirth.
-
Searched NICE website using general search box with keyword RhD.
-
Searched NICE website using general search box with keyword Rhesus.
-
Relevant guidelines found.
NHS Evidence (www.evidence.nhs.uk)
Searched on: 17 November 2015.
(rhd OR rhesus OR “rh negative” OR “rh positive”) AND (pregnan* OR maternal OR antenatal OR ante-natal OR prenatal OR pre-natal OR intrapartum) limited to guidelines.
A total of 81 results were retrieved and browsed for relevance. Seven relevant guidelines were found.
Royal College of Obstetricians and Gynaecologists (www.rcog.org.uk/en)
Date searched: 13 November 2015.
-
Browsed all guidelines.
-
Searched all guidelines by keyword – RhD or rhesus.
Four relevant guidelines were found.
Turning Research into Practice database (www.tripdatabase.com)
Date searched: 17 November 2015.
(rhd OR rhesus OR “rh negative” or “rh positive”) AND title:(pregnan* OR maternal OR antenatal OR ante-natal OR prenatal OR pre-natal OR intrapartum)
A total of 37 results were retrieved and browsed for relevance; 17 relevant guidelines were found.
UK National Screening Committee (www.gov.uk/government/groups/uk-national-screening-committee-uk-nsc)
Date searched: 13 November 2015.
Recommendations list was filtered by antenatal and the resulting list browsed.
One relevant report was found.
Search strategies: systematic reviews of antenatal anti-D prophylaxis
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid, http://ovidsp.ovid.com/)
Date range searched: 1946 to October Week 5 2015.
Date searched: 18 January 2016.
Records retrieved: 45.
The search was updated on 4 March 2016, retrieving 45 records.
-
systematic$ review$.ti,ab. (75,835)
-
meta-analysis as topic/ (14,365)
-
meta-analytic$.ti,ab. (4298)
-
meta-analysis.ti,ab,pt. (89,180)
-
metanalysis.ti,ab. (140)
-
metaanalysis.ti,ab. (1210)
-
meta analysis.ti,ab. (70,616)
-
meta-synthesis.ti,ab. (331)
-
metasynthesis.ti,ab. (166)
-
meta synthesis.ti,ab. (331)
-
meta-regression.ti,ab. (3249)
-
metaregression.ti,ab. (344)
-
meta regression.ti,ab. (3249)
-
(synthes$ adj3 literature).ti,ab. (1689)
-
(synthes$ adj3 evidence).ti,ab. (4926)
-
integrative review.ti,ab. (1177)
-
data synthesis.ti,ab. (7985)
-
(research synthesis or narrative synthesis).ti,ab. (1041)
-
(systematic study or systematic studies).ti,ab. (8551)
-
(systematic comparison$ or systematic overview$).ti,ab. (2200)
-
evidence based review.ti,ab. (1467)
-
comprehensive review.ti,ab. (8251)
-
critical review.ti,ab. (11,964)
-
quantitative review.ti,ab. (517)
-
structured review.ti,ab. (542)
-
realist review.ti,ab. (102)
-
realist synthesis.ti,ab. (73)
-
or/1-27 (187,703)
-
review.pt. (2,049,547)
-
medline.ab. (68,680)
-
pubmed.ab. (46,181)
-
cochrane.ab. (39,786)
-
embase.ab. (40,092)
-
cinahl.ab. (12,936)
-
psyc?lit.ab. (879)
-
psyc?info.ab. (10,559)
-
(literature adj3 search$).ab. (32,390)
-
(database$ adj3 search$).ab. (30,393)
-
(bibliographic adj3 search$).ab. (1461)
-
(electronic adj3 search$).ab. (11,252)
-
(electronic adj3 database$).ab. (13,910)
-
(computeri?ed adj3 search$).ab. (2857)
-
(internet adj3 search$).ab. (2045)
-
included studies.ab. (9670)
-
(inclusion adj3 studies).ab. (8188)
-
inclusion criteria.ab. (44,510)
-
selection criteria.ab. (22,215)
-
predefined criteria.ab. (1258)
-
predetermined criteria.ab. (787)
-
(assess$ adj3 (quality or validity)).ab. (48,127)
-
(select$ adj3 (study or studies)).ab. (43,640)
-
(data adj3 extract$).ab. (34,903)
-
extracted data.ab. (8161)
-
(data adj2 abstracted).ab. (3617)
-
(data adj3 abstraction).ab. (1017)
-
published intervention$.ab. (121)
-
((study or studies) adj2 evaluat$).ab. (121,595)
-
(intervention$ adj2 evaluat$).ab. (7046)
-
confidence interval$.ab. (258,288)
-
heterogeneity.ab. (106,141)
-
pooled.ab. (53,158)
-
pooling.ab. (8496)
-
odds ratio$.ab. (171,463)
-
(Jadad or coding).ab. (133,119)
-
or/30-64 (923,716)
-
29 and 65 (141,974)
-
review.ti. (299,976)
-
67 and 65 (62,549)
-
(review$ adj4 (papers or trials or studies or evidence or intervention$ or evaluation$)).ti,ab. (119,221)
-
28 or 66 or 68 or 69 (340,645)
-
letter.pt. (897,674)
-
editorial.pt. (391,059)
-
comment.pt. (647,299)
-
71 or 72 or 73 (1,445,828)
-
70 not 74 (331856)
-
exp animals/ not humans/ (4,171,020)
-
75 not 76 (321762)
-
“Rho(D) Immune Globulin”/ (1190)
-
(immune adj2 globulin adj2 rh$).ti,ab. (257)
-
anti-D.ti,ab. (2610)
-
(D-Gam or Partobulin or Rhophylac or WinRho).ti,ab. (47)
-
or/78-81 (3165)
-
77 and 82 (45)
Key
/ = indexing term (MeSH heading)
$ = truncation
? = optional wildcard – stands for zero or one character
.ti,ab. = terms in either title or abstract fields
.pt. = publication type
adj = terms next to each other (order specified)
adj2 = terms within two words of each other (any order)
Cochrane Database of Systematic Reviews (via Wiley Online Library, http://onlinelibrary.wiley.com/)
Issue 1 of 12, January 2016.
Date searched: 18 January 2016.
Records retrieved: 6.
The search was updated on 4 March 2016, retrieving six records from CDSR.
#1 MeSH descriptor: [Rho(D) Immune Globulin] this term only(51)
#2 (immune near/2 globulin near/2 rh*):ti,ab,kw(5)
#3 anti-D:ti,ab,kw(110)
#4 (D-Gam or Partobulin or Rhophylac or WinRho):ti,ab,kw(10)
#5 #1 or #2 or #3 or #4(119)
#6 #1 or #2 or #3 or #4 in Cochrane Reviews (Reviews and Protocols)(6)
Key
MeSH descriptor = indexing term (MeSH heading)
* = truncation
:ti,ab,kw = terms in either title or abstract or keyword fields
near/2 = terms within two words of each other (any order)
Database of Abstracts of Reviews of Effects (via Centre for Reviews and Dissemination, www.crd.york.ac.uk/CRDWeb)
Date range searched: inception to 31 March 2015.
Date searched on: 20 January 2016.
Records retrieved: 8.
-
(anti-D) IN DARE, HTA (15)
-
((D-Gam or Partobulin or Rhophylac or WinRho)) IN DARE, HTA (1)
-
((immune NEAR globulin NEAR rh*)) IN DARE, HTA (0)
-
((immune NEAR rh* NEAR globulin)) IN DARE, HTA (0)
-
((rh* NEAR immune NEAR globulin)) IN DARE, HTA (5)
-
((rh* NEAR globulin NEAR immune)) IN DARE, HTA (0)
-
((globulin NEAR rh* NEAR immune)) IN DARE, HTA (0)
-
((globulin NEAR immune NEAR rh*)) IN DARE, HTA (0)
-
MeSH DESCRIPTOR Rho(D) Immune Globulin IN DARE,HTA (5)
-
#1 OR #2 OR #5 OR #9 (15)
-
(#1 or #2 or #5 or #9) IN DARE (8)
-
(#1 or #2 or #5 or #9) IN HTA (7)
Health Technology Assessment database (via Centre for Reviews and Dissemination, www.crd.york.ac.uk/CRDWeb)
Date range searched: inception to 31 March 2015.
Date searched: 20 January 2016.
Records retrieved: 7.
See above under Database of Abstracts of Reviews of Effects for search strategy used.
PubMed (www.ncbi.nlm.nih.gov/pubmed)
Date searched on: 20 January 2016.
Records retrieved: 57.
The search was updated on 4 March 2016, retrieving 58 records.
(((“Rho(D) Immune Globulin”[Mesh:noexp] OR “rh* immune globulin”[Title/Abstract]) OR (“RHO(D) antibody”[Supplementary Concept] OR “RHO(D) antibody”[All Fields] OR “anti d”[All Fields])) OR (Partobulin[Title/Abstract] OR Rhophylac[Title/Abstract] OR WinRho[Title/Abstract])) AND systematic[sb]
Search strategies: cost-effectiveness
EconLit (via Ovid, http://ovidsp.ovid.com/)
Date range searched: 1886 to November 2015.
Date searched: 4 December 2015.
Records retrieved: 4.
-
(RhD or “rhesus D” or “Rh(D)” or “Rh-(D)” or Rh D).ti,ab. (3)
-
(Rh-negative or Rh-positive).ti,ab. (0)
-
(Rhesus negative or Rhesus positive).ti,ab. (0)
-
((rh or rhesus) adj2 (factor or factors or antigen$ or system or group)).ti,ab. (1)
-
((isoimmuni$ or iso-immuni$ or isoimmune or iso-immune) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (0)
-
((alloimmuni$ or allo-immuni$ or alloimmune or allo-immune) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (0)
-
((unsensiti#ed or un-sensiti#ed or non-sensiti#ed) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (0)
-
((sensiti#ation$ or sensiti#ed) adj6 (rh or rhesus or maternal or pregnan$)).ti,ab. (0)
-
((fetomaternal or feto-maternal or foetomaternal or foeto-maternal) adj2 immuni#ation).ti,ab. (0)
-
((rh or rhesus) adj2 (immuni#ation or autoimmuni#ation)).ti,ab. (0)
-
((hemolytic or haemolytic) adj2 (disease$ or disorder$)).ti,ab. (0)
-
HDFN.ti,ab. (0)
-
((rhesus or rh) adj2 (disease$ or disorder$)).ti,ab. (0)
-
((rhesus or rh or RhD) adj2 (incompatib$ or antagonism)).ti,ab. (0)
-
((erythroblastoses or erythroblastosis) adj2 f?etal$).ti,ab. (0)
-
or/1-15 (4)
Key
$ = truncation
# = mandated wildcard – stands for one character
? = optional wildcard – stands for zero or one character
.ti,ab. = terms in either title or abstract fields
adj2 = terms within two words of each other (any order)
NHS Economic Evaluations Database (via Centre for Reviews and Dissemination, www.crd.york.ac.uk/CRDWeb)
Date range searched: inception to 31 March 2015.
Date searched on: 4 December 2015.
Records retrieved: 6.
| 1 | MeSH DESCRIPTOR Rh-Hr Blood-Group System EXPLODE ALL TREES | 16 |
| 2 | (RhD or “rhesus D” or Rh-D) | 24 |
| 3 | (Rh-negative or Rh-positive) | 7 |
| 4 | (“Rhesus negative” or “Rhesus positive”) | 9 |
| 5 | ((rh or rhesus) NEAR2 (factor or factors or antigen* or system or group)) | 18 |
| 6 | ((factor or factors or antigen* or system or group) NEAR2 (rh or rhesus)) | 1 |
| 7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 35 |
| 8 | MeSH DESCRIPTOR Rh Isoimmunization | 15 |
| 9 | ((isoimmuni* or iso-immuni* or isoimmune or iso-immune) NEAR6 (rh or rhesus or maternal or pregnan*)) | 10 |
| 10 | ((rh or rhesus or maternal or pregnan*) NEAR6 (isoimmuni* or iso-immuni* or isoimmune or iso-immune) ) | 17 |
| 11 | ((alloimmuni* or allo-immuni* or alloimmune or allo-immune) NEAR6 (rh or rhesus or maternal or pregnan*)) | 12 |
| 12 | ((rh or rhesus or maternal or pregnan*) NEAR6 (alloimmuni* or allo-immuni* or alloimmune or allo-immune)) | 8 |
| 13 | ((unsensitised or unsensitized or un-sensitised or un-sensitized or non-sensitised or non-sensitized) NEAR6 (rh or rhesus or maternal or pregnan*)) | 3 |
| 14 | ((rh or rhesus or maternal or pregnan*) NEAR6 (unsensitised or unsensitized or un-sensitised or un-sensitized or non-sensitised or non-sensitized)) | 0 |
| 15 | ((sensitisation* or sensitization* or sensitised or sensitized )NEAR6 (rh or rhesus or maternal or pregnan*)) | 6 |
| 16 | ((rh or rhesus or maternal or pregnan*) NEAR6 (sensitisation* or sensitization* or sensitised or sensitized)) | 5 |
| 17 | ((fetomaternal or feto-maternal or foetomaternal or foeto-maternal) NEAR2 (immunisation or immunization)) | 0 |
| 18 | ((immunisation or immunization) NEAR2 (fetomaternal or feto-maternal or foetomaternal or foeto-maternal)) | 0 |
| 19 | ((rh or rhesus) NEAR2 (immunisation or immunization or autoimmunisation or autoimmunization)) | 4 |
| 20 | ((immunisation or immunization or autoimmunisation or autoimmunization) NEAR2 (rh or rhesus)) | 0 |
| 21 | #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 | 29 |
| 22 | MeSH DESCRIPTOR Erythroblastosis, Fetal EXPLODE ALL TREES | 18 |
| 23 | ((hemolytic or haemolytic) NEAR2 (disease* or disorder*)) | 16 |
| 24 | ((disease* or disorder*) NEAR2 (hemolytic or haemolytic)) | 1 |
| 25 | (HDFN) | 1 |
| 26 | ((rhesus or rh) NEAR2 (disease* or disorder*)) | 3 |
| 27 | ((disease* or disorder*) NEAR2 (rhesus or rh)) | 1 |
| 28 | ((rhesus or rh or RhD) NEAR2 (incompatib* or antagonism)) | 3 |
| 29 | ((incompatib* or antagonism) NEAR2 (rhesus or rh or RhD)) | 0 |
| 30 | ((erythroblastoses or erythroblastosis) NEAR2 (fetal* or foetal*)) | 14 |
| 31 | #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 | 28 |
| 32 | #7 OR #21 OR #31 | 56 |
| 33 | MeSH DESCRIPTOR Prenatal Diagnosis | 216 |
| 34 | MeSH DESCRIPTOR Maternal Serum Screening Tests | 5 |
| 35 | MeSH DESCRIPTOR Hematologic Tests | 30 |
| 36 | ((prenatal or pre-natal or antenatal or ante-natal) NEAR3 (test* or screen* or diagnos* or determin* or detect*)) | 380 |
| 37 | ((test* or screen* or diagnos* or determin* or detect*) NEAR3 (prenatal or pre-natal or antenatal or ante-natal)) | 171 |
| 38 | ((test* or screen* or diagnos* or determin* or detect*) NEAR3 (fetal or foetal or fetus* or foetus*)) | 124 |
| 39 | ((fetal or foetal or fetus* or foetus*) NEAR3 (test* or screen* or diagnos* or determin* or detect*)) | 130 |
| 40 | (NIPD or NIPT) | 6 |
| 41 | #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 | 534 |
| 42 | MeSH DESCRIPTOR Genotyping Techniques | 6 |
| 43 | ((genotype* or genotyping) NEAR2 (fetal or foetal or fetus* or foetus* or prenatal or pre-natal or antenatal or ante-natal)) | 3 |
| 44 | ((fetal or foetal or fetus* or foetus* or prenatal or pre-natal or antenatal or ante-natal) NEAR2 (genotype* or genotyping)) | 3 |
| 45 | ((genotype* or genotyping) NEAR2 (maternal or pregnan*)) | 2 |
| 46 | ((maternal or pregnan*) NEAR2 (genotype* or genotyping)) | 2 |
| 47 | ((genotype* or genotyping) NEAR2 (noninvasive or non-invasive)) | 1 |
| 48 | ((noninvasive or non-invasive) NEAR2 (genotype* or genotyping)) | 4 |
| 49 | (“cell-free foetal DNA” or “cell-free fetal DNA”) | 7 |
| 50 | (cffDNA) | 2 |
| 51 | #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 | 18 |
| 52 | #32 AND #41 | 16 |
| 53 | #32 AND #51 | 6 |
| 54 | #52 OR #53 | 18 |
Please note that the total number of hits at line 54 refers to the total number of results from DARE, HTA database and NHS EED.
Key
MeSH DESCRIPTOR = indexing term (MeSH heading)
* = truncation
NEAR2 = terms within two words of each other (order specified)
“ ” = phrase search
Research Papers in Economics (http://repec.org/)
Date searched: 4 December 2015.
Records retrieved: 0.
“RhD” | rhesus | “hemolytic disease” | “haemolytic disease” | HDFN | erythroblastoses | erythroblastosis | “fetomaternal immunisation” | “fetomaternal immunization” | “foetomaternal immunisation” | “foetomaternal immunization”
Key
“ ” = phrase search
| = OR
Appendix 2 Included studies
| Study (author, date) | Full title | Country | Linked publications |
|---|---|---|---|
| Included studies: diagnostic accuracy | |||
| Akolekar et al., 201119 | Fetal RHD genotyping in maternal plasma at 11–13 weeks of gestation. Fetal Diagn Ther 29:301–6 | UK (London) | None |
| Banch Clausen et al., 201420 | Routine non-invasive prenatal screening for fetal RHD in plasma of RhD-negative pregnant women – 2 years of screening experience from Denmark. Prenat Diagn 34:1000–5 | Denmark |
Full-text papers: Damkjaer et al. , 2012;27 and Banch Clausen et al. , 201224 |
| Chitty et al., 201412 | Diagnostic accuracy of routine antenatal determination of fetal RHD status across gestation: population based cohort study. BMJ 349:g5243 | UK (Bristol) |
Full-text paper: none Abstracts: Chitty et al. , 2011,29 2012;30 and Daniels et al. , 201231 |
| Finning et al., 200817 | Effect of high throughput RHD typing of fetal DNA in maternal plasma on use of anti-RhD immunoglobulin in RhD negative pregnant women: prospective feasibility study. BMJ 336:816–18 | UK (Bristol) | None |
| Grande et al., 201322 | Clinical application of midtrimester non-invasive fetal RHD genotyping and identification of RHD variants in a mixed-ethnic population | Spain | None |
| Soothill et al., 201518 | Use of cffDNA to avoid administration of anti-D to pregnant women when the fetus is RhD-negative: implementation in the NHS. BJOG 122:1682–6 | UK (Bristol) | None |
| Thurik et al., 201521 | Analysis of false-positive results of fetal RHD typing in a national screening program reveals vanishing twins as potential cause for discrepancy | The Netherlands |
Full-text paper: de Haas et al. , 201225 Abstracts: de Haas et al. , 2012;46,47 Scheffer et al. , 2013;44 Thurik et al. , 2014;42,43 van der Schoot et al. , 2005;45 and Veldhuisen et al. , 2013,41 201440 |
| Wikman et al., 201223 | Non-invasive single-exon fetal RHD determination in a routine screening program in early pregnancy. Obstet Gynecol 120:227–34 | Sweden |
Full-text papers: none Abstracts: Tiblad et al. , 2010;54 Wikman et al. , 2010,53 2011,51 2012;50 and Wikman 201352 |
| Included studies: clinical effectiveness | |||
| Banch Clausen et al., 201420 | Routine non-invasive prenatal screening for fetal RHD in plasma of RhD-negative pregnant women – 2 years of screening experience from Denmark. Prenat Diagn 34:1000–5 | Denmark |
Full-text papers: Banch Clausen et al. , 2012;24 and Damkjaer et al. , 201227 Abstracts: Banch Clausen et al. , 2011;38 Banch Clausen 2012;35,36 Dziegiel et al. , 2012;37 and Steffensen et al. , 201239 |
| Banch Clausen et al., 201224 | Report of the first nationally implemented clinical routine screening for fetal RHD in D- pregnant women to ascertain the requirement for antenatal RhD prophylaxis. Transfusion 52:752–8 | Denmark |
Full-text papers: Banch Clausen et al. , 2014;20 and Damkjaer et al. , 201227 Abstracts: Banch Clausen et al. , 2011;38 Banch Clausen 2012;35,36 Dziegiel et al. , 2012;37 and Steffensen et al. , 201239 |
| Damkjaer et al., 201227 | Study of compliance with a new, targeted antenatal D immunisation prevention programme in Denmark. Vox Sang 103:145–9 | Denmark |
Full-text papers: Banch Clausen et al. , 2012,24 201420 Abstracts: Banch Clausen et al. , 2011;38 Banch Clausen 2012;35,36 Dziegiel et al. , 2012;37 and Steffensen et al. , 201239 |
| de Haas et al., 201225 | A nation-wide fetal RHD screening programme for targeted antenatal and postnatal anti-D. ISBT Sci Ser 7:164–7 | The Netherlands |
Full-text paper: Thurik et al. , 201521 Abstracts: de Haas et al. , 2012,46 2013;47 Thurik et al. , 2014;42,43 Scheffer et al. , 2013;44 van der Schoot et al. , 2005;40–45 and Veldhuisen et al. , 2013,41 201440 |
| Grande et al., 201322 | Clinical application of midtrimester non-invasive fetal RHD genotyping and identification of RHD variants in a mixed-ethnic population | Spain | None |
| Soothill et al., 201518 | Use of cffDNA to avoid administration of anti-D to pregnant women when the fetus is RhD-negative: implementation in the NHS. BJOG 122:1682–6 | UK (Bristol) |
Full-text paper: none |
| Tiblad et al., 201326 | Targeted routine antenatal anti-D prophylaxis in the prevention of RhD immunisation--outcome of a new antenatal screening and prevention program. PLOS ONE 8(8) | Sweden |
Full-text paper: none Abstracts: Tiblad 2012;57 and Tiblad et al. , 2012,55 201456 |
| Included studies: implementation | |||
| Banch Clausen et al., 201420 | Routine non-invasive prenatal screening for fetal RHD in plasma of RhD-negative pregnant women – 2 years of screening experience from Denmark. Prenat Diagn 34:1000–5 | Denmark |
Report: Banch Clausen et al. , 201224 Full-text papers: Banch Clausen et al. , 2013;13 and Damkjaer et al. , 201227 Abstract: Banch Clausen et al. , 201138 |
| Banch Clausen et al., 201224 | Report of the first nationally implemented clinical routine screening for fetal RHD in D- pregnant women to ascertain the requirement for antenatal RhD prophylaxis. Transfusion 52:752–8 | Denmark | Linked to above |
| Clausen et al., 201313 | Pre-analytical conditions in non-invasive prenatal testing of cell-free fetal RHD. PLOS ONE 8:e76990 | Denmark | Linked to above |
| Damkjaer et al., 201227 | Study of compliance with a new, targeted antenatal D immunisation prevention programme in Denmark. Vox Sang 103:145–9 | Denmark | Linked to above |
| Brojer et al., 200594 | Non-invasive determination of fetal RHD status by examination of cell-free DNA in maternal plasma.’ Transfusion 45:1473–80 | Poland | None |
| Finning et al., 200817 | Effect of high throughput RHD typing of fetal DNA in maternal plasma on use of anti-RhD immunoglobulin in RhD negative pregnant women: prospective feasibility study. BMJ 336:816–18 | UK (Bristol) |
Full-text paper: none |
| Grande et al., 201322 | Clinical application of midtrimester non-invasive fetal RHD genotyping and identification of RHD variants in a mixed-ethnic population | Spain | None |
| Thurik et al., 201521 | Analysis of false-positive results of fetal RHD typing in a national screening program reveals vanishing twins as potential cause for discrepancy | The Netherlands |
Full-text paper: none Abstracts: Veldhuisen et al. , 201440 |
| de Hass et al., 201225 | A nation-wide fetal RHD screening programme for targeted antenatal and postnatal anti-D. ISBT Sci Ser 7:164–7 | The Netherlands | Linked to Thurik et al.21 |
| Oxenford et al., 201328 | Routine testing of fetal Rhesus D status in Rhesus D negative women using cell-free fetal DNA: an investigation into the preferences and information needs of women. Prenat Diagn 33:688–94 | UK (London) | None |
| Soothill et al., 201518 | Use of cffDNA to avoid administration of anti-D to pregnant women when the fetus is RhD-negative: implementation in the NHS. BJOG 122:1682–6 | UK (Bristol) | None |
| Wikman et al., 201223 | Non-invasive single-exon fetal RHD determination in a routine screening program in early pregnancy. Obstet Gynecol 120:227–34 | Sweden |
Full-text paper: Tiblad et al. , 201326 |
| Tiblad et al., 201326 | Targeted routine antenatal anti-D prophylaxis in the prevention of RhD immunisation–outcome of a new antenatal screening and prevention program. PLOS ONE 8:e70984 | Sweden | Linked to Wikman et al.23 |
Appendix 3 List of excluded studies
Not high-throughput non-invasive prenatal testing (123 references)
Abildinova G, Baynova M, Kamalieva B, Kostina A. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma with real-time PCR assay. Clinical Chemistry and Laboratory Medicine Conference, IFCC-WorldLab-EuroMedLab, Berlin, 2011, pp. S691.
Achargui S, Benchemsi N. Fetal rhesus D genotyping by PCR using plasma from RhD negative pregnant women. 19th Regional Congress of the International Society of Blood Transfusion, Eastern, 2009, pp. 144.
Achargui S, Tijane M, Benchemsi N. [Fetal RHD genotyping by PCR using plasma from D negative pregnant women.] Transfus Clin Biol 2011;18:13–19. http://dx.doi.org/10.1016/j.tracli.2010.10.002
Ahangari G, Zeinali S, Ebrahimi M, Mohsani F, Saremi AT. Analysis of fetal sex and RhD gene in fetal cells DNA from maternal blood by polymerase chain reaction. Middle East Fertil Soc J 2003;8:263–8.
Ahmadi MH, Amirizadeh N, Azarkeyvan A, Valikhani A, Sayyadipoor F, Navidrouyan M. Fetal RHD genotyping in plasma of Rh negative pregnant women by real time PCR. 25th Regional Congress of the International Society of Blood Transfusion, 2015, pp. 302.
Allen RW, Ward S, Harris R. Prenatal genotyping for the RhD blood group antigen: considerations in developing an accurate test. Genet Test 2000;4:377–81. http://dx.doi.org/10.1089/109065700750065126
Al-Yatama MK, Mustafa AS, Al-Kandari FM, Khaja N, Zohra K, Monem RA, Abraham S. Polymerase-chain-reaction-based detection of fetal rhesus D and Y-chromosome-specific DNA in the whole blood of pregnant women during different trimesters of pregnancy. Med Princ Pract 2007;16:327–32.
Amaral DR, Credidio DC, Ribeiro K, Cobianchi Costa D, Castilho L. Complexities on RHD genotyping in pregnant women from a multi-ethnic population. AABB Annual Meeting and TXPO, New Orleans, LA, 2009, pp. 121A.
Amaral DR, Castilho L. Fetal RHD genotyping by analysis of maternal plasma in a mixed population. Vox Sang 2010. 31st International Congress of the Society of Blood Transfusion, pp. 25.
Amaral DR, Castilho L. Evaluation of non-invasive fetal RHD genotyping in a multi-ethnic population. Transfusion 2010. AABB Annual Meeting and CTTXPO, Baltimore, MD, pp. 149A.
Amaral DRT, Credidio DC, Pellegrino J Jr, Castilho L. Fetal RHD genotyping by analysis of maternal plasma in a mixed population. J Clin Lab Anal 2011;25:100–4.
Arntfield S, Ainsworth P, Mackay J, Gagnon R. Prenatal diagnosis of fetal RhD type using free fetal DNA (ffDNA) in maternal plasma: a pilot study. Am J Obstet Gynecol 2008;199:S119.
Atamaniuk J, Stuhlmeier KM, Karimi A, Mueller MM. Comparison of PCR methods for detecting fetal RhDin maternal plasma. J Clin Lab Anal 2009;23:24–8. http://dx.doi.org/10.1002/jcla.20282
Aubin JT, Le Van KC, Mouro I, Colin Y, Bignozzi C, Brossard Y, Cartron JP. Specificity and sensitivity of RHD genotyping methods by PCR-based DNA amplification. Br J Haematol 1997;98:356–64.
Aykut A, Onay H, Sagol S, Gunduz C, Ozkinay F, Cogulu O. Determination of fetal rhesus d status by maternal plasma DNA analysis. Balkan J Med Genet 2013;16:33–8. http://dx.doi.org/10.2478/bjmg-2013-0029
Banzola I, Kaufmann I, Lapaire O, Hahn S, Holzgreve W, Rusterholz C. Isolation of serum nucleic acids for fetal DNA analysis: comparison of manual and automated extraction methods. Prenat Diagn 2008;28:1227–31. http://dx.doi.org/10.1002/pd.2154
Benachi A, Delahaye S, Leticee N, Jouannic JM, Ville Y, Costa JM. Impact of non-invasive fetal RhD genotyping on management costs of rhesus-D negative patients: results of a French pilot study. Eur J Obstet Gynecolo Reprod Biol 2012;162:28–32.
Bingulac-Popovic J, Dogic V, Babic I, Hundric-Haspl Z, Miskovic B, Mratinovic-Mikulandra J, et al. Prenatal RHD genotyping: in-house method validation. Clin Chem Lab Med 2014;52:eA13–eA14.
Bombard AT, Akolekar R, Farkas DH, VanAgtmael AL, Aquino F, Oeth P, Nicolaides KH. Fetal RHD genotype detection from circulating cell-free fetal DNA in maternal plasma in non-sensitized RhD negative women. Prenat Diagn 2011;31:802–8. http://dx.doi.org/10.1002/pd.2770
Cardo L, Garcia BP, Alvarez FV. Non-invasive fetal RHD genotyping in the first trimester of pregnancy. Clin Chem Lab Med 2010;48:1121–6.
Chan FY, Cowley NM, Wolter L, Stone M, Carmody F, Saul A, Hyland CA. Prenatal RHD gene determination and dosage analysis by PCR: clinical evaluation. Prenat Diagn 2001;21:321–6. http://dx.doi.org/10.1002/pd.60
Chinen PA, Nardozzaa LMM, Camano L, Moron AF, Pares DBS, Martinhago CD, Daher S. Non-invasive fetal RHD genotyping by real-time polymerase chain reaction using plasma from D-negative Brazilian pregnant women. J Reprod Immunol 2007;75:A8–A9.
Chinen P, Lopes C, Nardozza L, Camano L, Martinhago C, Moron A. Determination of fetal RHD genotype in maternal blood, using the real-time polymerase chain reaction technique. Int J Gynecol Obstet 2009;107:S523.
Chinen PA, Nardozza LMM, Martinhago CD, Camano L, Daher S, Pares DB, et al. Noninvasive determination of fetal Rh blood group, D antigen status by cell-free DNA analysis in maternal plasma: experience in a Brazilian population. Am J Perinatol 2010;27:759–62.
Clausen FB, Krog GR, Rieneck K, Nielsen LK, Lundquist R, Finning K, et al. Reliable test for prenatal prediction of fetal RhD type using maternal plasma from RhD negative women. Prenat Diagn 2005;25:1040–4. http://dx.doi.org/10.1002/pd.1248
Clausen FB, Krog GR, Rieneck K, Råsmark EE, Dziegiel MH. Evaluation of two real-time multiplex PCR screening assays detecting fetal RHD in plasma from RhD negative women to ascertain the requirement for antenatal RhD prophylaxis. Fetal Diagn Ther 2011;29:155–63. http://dx.doi.org/10.1159/000321347
Costa JM, Giovangrandi Y, Ernault P, Lohmann L, Nataf V, El Halali N, Gautier E. Fetal RHD genotyping in maternal serum during the first trimester of pregnancy. Br J Haematol 2002;119:255–60.
Cotorruelo C, Biondi C, Borrás SG, Galizzi S, Di Mónaco R, Racca A. Molecular determination of RhD phenotype by DNA typing: clinical applications. Ann Clin Biochem 2000;37:781–9. http://dx.doi.org/10.1258/0004563001900101
Cozac AC, Miyashiro K, Silva CG, Pinto GN, Rizzatti EG. Non-invasive fetal RHD genotyping by maternal plasma in a racially mixed population. Transfusion 2011;51:39A.
Da Silva N, Rouillac-Le Sciellour C, Menu M, Colin Y, Le Van Kim C, Cartron J, et al. Non-invasive fetal RHD genotyping on plasma DNA from RHD negative pregnant women carrying the silent RhDPsi gene. Transfusion 2009;49:132A–3A.
Doescher A, Wagner FF, Vogt C, Paul H, Ross A, Klip EJ, Petershofen EK. DNA-extraction from cell free maternal plasma with the snapcardtm method. Transfus Med Hemother 2013. Conference: 46. Jahreskongress der Deutschen Gesellschaf, pp. 34.
Doescher A, Müller TH. Noninvasive prenatal blood group genotyping. Methods Mol Biol 2015;1310:135–47. http://dx.doi.org/10.1007/978-1-4939-2690-9_12
Dovč-Drnovšek T, Klemenc P, Toplak N, Blejec T, Bricl I, Rožman P. Reliable Determination of Fetal RhD Status by RHD Genotyping from Maternal Plasma. Transfus Med Hemother 2013;40:37–43.
Sequenom Inc. Evaluation of a Noninvasive Fetal RHD Genotyping Test. Clinical trial NCT01054716. URL: https://ClinicalTrials.gov/show/NCT01054716
Faas BH, Maaskant-Van Wijk PA, von dem Borne AE, van der Schoot CE, Christiaens GC. The applicability of different PCR-based methods for fetal RHD and K1 genotyping: a prospective study. Prenat Diagn 2000;20:453–8.
Fernandez-Martinez FJ, Vicario L, Garcia-Burguillo A, Galindo A, Moreno-Garcia M, Pascual C, Moreno-Izquierdo A. Implementing RHD genotyping on cell-free fetal DNA from maternal plasma in a Spanish population. Prenat Diagn 2012. Conference: 16th International Conference on Prenatal Diagnosis and Therapy, pp. 60.
Finning KM, Martin PG, Soothill PW, Avent ND. Prediction of fetal D status from maternal plasma: introduction of a new noninvasive fetal RHD genotyping service. Transfusion 2002;42:1079–85.
Finning K, Martin P, Daniels G. A clinical service in the UK to predict fetal Rh (Rhesus) D blood group using free fetal DNA in maternal plasma. Ann N Y Acad Sci 2004;1022:119–23. http://dx.doi.org/10.1196/annals.1318.019
Gautier E, Benachi A, Giovangrandi Y, Ernault P, Olivi M, Gaillon T, Costa J. Fetal RhD genotyping by maternal serum analysis: a two-year experience. Am J Obstet Gynecol 2005;192:666–9.
Geifman-Holtzman O, Bernstein IM, Berry SM, Holtzman EJ, Vadnais TJ, DeMaria MA, Bianchi DW. Fetal RhD genotyping in fetal cells flow sorted from maternal blood. Am J Obstet Gynecol 1996;174:818–22.
Goettig S, Doescher A, Rabold U, Hundhausen T, Teixidor D, Steuernagel P, et al. Prenatal detection of Rhesus D-specific fetal DNA within exon 3, 7, 10 and intron 4 in maternal plasma from peripheral blood samples. Transfusion 2001;41:101S.
Guinchard E, Mayrand E, Rigal D. Fetal RhD genotyping from maternal plasma lyonnaise study on 196 patients. Vox Sang 2010;99:404.
Günel T, Kalelioğlu I, Ermiş H, Aydınlı K. Detection of fetal RhD gene from maternal blood. J Turk Ger Gynecol Assoc 2010;11:82–5. http://dx.doi.org/10.5152/jtgga.2010.04
Gunel T, Kalelioglu I, Gedikbasi A, Ermis H, Aydinli K. Detection of fetal RHD pseudogene (RHDΨ) and hybrid RHD-CE-Ds from RHD-negative pregnant women with a free DNA fetal kit. Genet Mol Res 2011;10:2653–7. http://dx.doi.org/10.4238/2011.October.26.1
Hahn S, Zhong XY, Bürk MR, Troeger C, Holzgreve W. Multiplex and real-time quantitative PCR on fetal DNA in maternal plasma. A comparison with fetal cells isolated from maternal blood. Ann N Y Acad Sci 2000;906:148–52.
Han S, Ryu J, Bae S, Kim Y, Yang Y, Lee K. Noninvasive fetal RhD genotyping using circulating cell-free fetal DNA from maternal plasma in RhD-negative pregnant women. J Mol Diagn 2012. Conference: 2012 Annual Meeting of the Association for Molecular Pathology, pp. 648.
Holtzman E, Geifman-Holtzman O, Jeronis S, Xiong Y, Liebermann D, Hoffman B, Prabhakaran I. Non-invasive fetal RhD genotyping and first trimester screen clinical implications for the management of RhD-negative mother. Am J Obstet Gynecol 2011. Conference: 2011 31st Annual Meeting of the Society for, pp. S290.
Hromadnikova I, Vechetova L, Vesela K, Benesova B, Doucha J, Kulovany E, Vlk R. Non-invasive fetal RHD exon 7 and exon 10 genotyping using real-time PCR testing of fetal DNA in maternal plasma. Fetal Diagn Ther 2005;20:275–80.
Hromadnikova I, Vechetova L, Vesela K, Benesova B, Doucha J, Vlk R. Non-invasive fetal RHD and RHCE genotyping using real-time PCR testing of maternal plasma in RhD-negative pregnancies. J Histochem Cytochem 2005;53:301–5.
Hudecova I, Polakova H, Rusnak I, Sisovsky V, Vlkova B, Minarik G, et al. Noninvasive prenatal RHD genotyping using cell free fetal DNA from maternal plasma. Eur J Clin Invest 2011. Conference: 45th Annual Scientific Meeting of the European Society for Clinical Investigation, pp. 18.
Hyland CA, Gardener G J, Hyett JA, Davies H, Millard G, Morris J, et al. High reliability of non-invasive prenatal assessment of fetal RHD using two independent blood samples from RhD negative pregnant women. Transfusion 2009;49:133A.
Hyland CA, Gardener GJ, Davies H, Ahvenainen M, Flower RL, Irwin D, et al. Evaluation of non-invasive prenatal RHD genotyping of the fetus. Med J Aust 2009;191:21–5.
Hyland CA, O’Brien H, Millard G, Gardener G, Hyett J, Morris J, et al. Non-invasive prenatal diagnosis of fetal RhD for an Australian obstetric population demonstrates a 2.1% rate of molecular variants in RhD negative women. Vox Sang 2010;99(Suppl. 1):399–400.
Hyland C, Millard G, O’Brien H, Tremellen A, Hyett J, Flower R, Gardener G. Non-invasive fetal rhd genotyping for D negative pregnant women. Vox Sang 2011. 22nd Regional Congress of the International Society of Blood Transfusion, Asia Tai, pp. 34.
Hyland C, Millard G, O’Brien H, Flower R, Hyett J, Gardener G. Non-invasive prenatal testing (NIPT) for fetal RHD: New strategies for management of alloimmunised RhD-negative women. Prenat Diagn 2014. 18th International Conference on Prenatal Diagnosis and Therapy, pp. 56–7.
Fernandez-Martinez FJ, Galindo-Izquierdo A, Garcia-Burguillo A, Vargas-Gallego C, Pascual C, Moreno-Izquierdo A. Evaluation of a strategy for non-invasive determination of fetal RHD status on cell-free DNA. Prenat Diagn 2010. 15th International Conference on Prenatal Diagnosis and Therapy, pp. S39.
Johnson L, McCracken SA, Morris JM, Woodland NB, Flower RL. Variation in the reliability of RHD antenatal genotyping using the polymerase chain reaction and targeting multiple exons of the RHD gene. Vox Sang 2003;85:222–3.
Keshavarz Z, Moezzi L, Ranjbaran R, Aboualizadeh F, Behzad-Behbahani A, Abdullahi M, Sharifzadeh S. Evaluation of a Modified DNA Extraction Method for Isolation of Cell-Free Fetal DNA from Maternal Serum. Avicenna J Med Biotechnol 2015;7:85–8.
Kimura M, Sato C, Hara M, Ishihara O, Ikebuchi K. Noninvasive fetal RHD genotyping by maternal plasma with capillary electrophoresis. Transfusion 2008;48:1156–63. http://dx.doi.org/10.1111/j.1537-2995.2008.01681.x
Koelewijn JM, Vrijkotte TG, de Haas M, van der Schoot CE, Bonsel GJ. Women’s attitude towards prenatal screening for red blood cell antibodies, other than RhD. BMC Pregnancy Childbirth 2008;8:49. http://dx.doi.org/10.1186/1471-2393-8-49
Kolialexi A, Tounta G, Apostolou P, Vrettou C, Papantoniou N, Destouni A, et al. Early non-invasive prenatal diagnosis of fetal RhD status and fetal gender using cell-free fetal DNA. Prenat Diagn 2012. 16th International Conference on Prenatal Diagnosis and Therapy, pp.65.
Le Sciellour C, Serazin V, De Beaumont C, Menu M. Routine fetal RHD genotyping using cell free fetal DNA: French experience at the hospital of poissy. Vox Sang 2013;105:245–6.
Legler T J. Automatable universal control reaction for fetal DNA in maternal plasma. Transfus Med Hemother 2012. Conference: 45. Jahreskongress der Deutschen Gesellschaf, pp.8.
Levi JE, Chinoca K, Liao AW, Dezan M, Dinardo CL, Jens E, et al. Determination of fetal RHD genotyping from maternal plasma in a population with a high frequency of the RHD pseudogene. Vox Sang 2015;109(Suppl. 1):315.
Li Y, Kazzaz JA, Kellner LH, Brown SA. Incorporation of fetal DNA detection assay in a noninvasive RhD diagnostic test. Prenat Diagn 2010;30:1010–12. http://dx.doi.org/10.1002/pd.2598
Lo YM, Hjelm NM, Fidler C, Sargent IL, Murphy MF, Chamberlain PF, et al. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N Engl J Med 1998;339:1734–8. http://dx.doi.org/10.1056/NEJM199812103392402
Lo YM. Non-invasive detection of fetal RHD status and other genetic characteristics by circulating nucleic acids in maternal plasma. Vox Sang 2009;97(Suppl. 1):10.
Machado IN, Castilho L, Pellegrino J Jr, Barini R. Fetal RHD genotyping from maternal plasma in a population with a highly diverse ethnic background. Rev Assoc Med Bras 2006;52:232–5.
Macher HC, Noguerol P, Medrano-Campillo P, Garrido-Marquez MR, Rubio-Calvo A, Carmona-Gonzalez M, et al. Standardization non-invasive fetal RHD and SRY determination into clinical routine using a new multiplex RT-PCR assay for fetal cell-free DNA in pregnant women plasma: results in clinical benefits and cost saving. Clin Chim Acta 2012;413:490–4.
Mackie F, Morris K, Kilby M. Diagnostic accuracy of prenatal cell-free fetal DNA testing in singleton pregnancies: a systematic review and meta-analysis. PROSPERO 2014: CRD42014007174. URL: www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014007174 (accessed 12 July 2017).
Manzanares S, Entrala C, Sanchez-Gila MM, Molina L. Noninvasive prenatal determination of fetal Rh status from cell-free fetal DNA in maternal blood. J Matern Fetal Neonatal Med 2012;25:701–71.
Manzanares S, Entrala C, Sanchez-Gila M, Fernandez-Rosado F, Cobo D, Martinez E, et al. Noninvasive fetal RhD status determination in early pregnancy. Fetal Diagn Ther 2014;35:7–12.
Mohammed N, Kakal F, Somani M, Zafar W. Non-invasive prenatal determination of fetal RhD genotyping from maternal plasma: a preliminary study in Pakistan. J Coll Physicians Surg Pak 2010;20:246–9.
Moise KJ Jr, Zhou L, Thorson J, Judd WJ. Noninvasive prenatal RhD testing. Am J Obstet Gynecol 2006;195:e20–1.
Moise K, Boring N, Shaughnessy RO, Simpson L, Wolfe H, Baxter J, et al. Circulating cell-free fetal DNA for the detection of fetal RHD status and sex: A prospective NAFTNet trial using a unique approach of reflex fetal identifiers. Am J Obstet Gynecol 2012;206:S315.
Moise KJ Jr. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol 2013;122:1306.
Moise KJ, Boring NH, O’Shaughnessy R, Simpson LL, Wolfe HM, Baxter JK, et al. Circulating cell-free fetal DNA for the detection of RHD status and sex using reflex fetal identifiers. Prenat Diagn 2013;33:95–101. http://dx.doi.org/10.1002/pd.4018
Mota MA, Dezan MR, Cruz RO, Costa TH, Conti FM, Aravechia MG, et al. Validation of a protocol for fetal rhd genotyping from maternal plasma in a multi-ethnic population. Transfusion 2013. AABB Annual Meeting and CTTXPO, Denver, 2013, pp. 170A–1A.
Mota MA, Dezan MR, Sirianni MFM, Cruz RO, Bastos EP, Silva NC, et al. An efficient protocol for fetal RHD genotyping from maternal plasma in a multi-ethnic population. Vox Sang 2014;107:188–9.
Moussa H, Tsochandaridis M, Jemni-Yacoub S, Hmida S, Khairi H, Gabert J, Levy-Mozziconacci A. Fetal RhD genotyping by real time quantitative PCR in maternal plasma of RhD-negative pregnant women from the Sahel of Tunisia. Ann Biol Clin 2012;70:683–8. http://dx.doi.org/10.1684/abc.2012.0769
Müller SP, Bartels I, Stein W, Emons G, Gutensohn K, Köhler M, Legler TJ. The determination of the fetal D status from maternal plasma for decision making on Rh prophylaxis is feasible. Transfusion 2008;48:2292–301. http://dx.doi.org/10.1111/j.1537-2995.2008.01843.x
Müller SP, Bartels I, Stein W, Emons G, Gutensohn K, Legler TJ. Cell-free fetal DNA in specimen from pregnant women is stable up to 5 days. Prenat Diagn 2011;31:1300–4. http://dx.doi.org/10.1002/pd.2889
Nardozza L, Chinen P, Lopes C, Camano L, Martinhago C, Moron A. The influence of gestational age in the determination of the fetal RHD genotype in maternal blood. Int J Gynecol Obstet 2009;107:S523.
National Collaborating Centre for Women’s and Children’s Health. Antenatal Care: Routine Care for the Healthy Pregnant Woman. London: Royal College of Obstetricians and Gynaecologists Press; 2008. URL: www.nice.org.uk/guidance/cg62/evidence (accessed 12 July 2017).
Nelson M, Eagle C, Langshaw M, Popp H, Kronenberg H. Genotyping fetal DNA by non-invasive means: extraction from maternal plasma. Vox Sang 2001;80:112–16.
Newesely-Meyer M, Singer S, Wallner S, Muhlbacher A. Comparison of Bio-Rad fetal RHD diagnosis kit and the custom-made assay from Ingenetix. Transfus Med Hemother 2012;39(Suppl. 1):64.
Onofriescu M, Nemescu D, Negura L. Noninvasive fetal RhD genotyping from maternal plasma in RhD negative women. Int J Gynecol Obstet 2009. 19th International Federation of Gynecology and Obstetrics (FIGO) World Congress of Gynecology and Obstetrics, pp. S423–4.
Pereira JC, Couceiro AB, Cunha EM, Machado AI, Tamagnini GP, Martins NP, Ribeiro ML. Prenatal determination of the fetal RhD blood group by multiplex PCR: a 7-year Portuguese experience. Prenat Diagn 2007;27:633–7. http://dx.doi.org/10.1002/pd.1760
Picchiassi E, Di Renzo GC, Tarquini F, Bini V, Centra M, Pennacchi L, et al. Non-invasive prenatal RHD genotyping using cell-free fetal DNA from maternal plasma: an Italian experience. Transfus Med Hemother 2015;42:22–8.
Polin H, Reiter A, Brisner M, Danzer M, Weinberger J, Gabriel C. Clinical application of non-invasive fetal blood group genotyping in upper Austria. Transfus Med Hemother 2013;40(Suppl. 1):36–7.
Prabhakaran I, Xiong Y, Lieberman D, Holtzman E, Montgomery O, Geifman-Holtzman O. Noninvasive fetal RhD genotyping from maternal blood-potential integration into first trimester screen. Reprod Sci 2011;18(Suppl. 1):102A.
Randen I, Hauge R, Kjeldsen-Kragh J, Fagerhol MK. Prenatal genotyping of RHD and SRY using maternal blood. Vox Sang 2003;85:300–6.
Rouillac-Le Sciellour C, Puillandre P, Gillot R, Baulard C, Métral S, Le Van Kim C, et al. Large-scale pre-diagnosis study of fetal RHD genotyping by PCR on plasma DNA from RhD-negative pregnant women. Mol Diagn 2004;8:23–31.
Rouillac-Le Sciellour C, Sérazin V, Brossard Y, Oudin O, Le Van Kim C, Colin Y, et al. Noninvasive fetal RHD genotyping from maternal plasma. Use of a new developed Free DNA Fetal Kit RhD. Transfus Clin Biol 2007;14:572–7. http://dx.doi.org/10.1016/j.tracli.2008.01.003 (unpublished).
Royal College of Physicians, NHS Blood and Transplant. National Comparative Audit of Blood Transfusion. 2013 Audit of Anti-D Immunoglobulin Prophylaxis. 2013 (unpublished).
Sapa A, Jonkisz A, Zimmer M, Kłósek A, Woźniak M. [Diagnostic utility of RHD-gene detection in maternal plasma in the prophylaxis of feto-maternal Rh-incompatibility.] Ginekol Pol 2014;85:570–6.
Sbarsi I, Isernia P, Montanari L, Badulli C, Martinetti M, Salvaneschi L. Implementing non-invasive RHD genotyping on cell-free foetal DNA from maternal plasma: the Pavia experience. Blood Transfus 2012;10:34–8. http://dx.doi.org/10.2450/2011.0021-11
Schmidt LC, Cabral ACV, Faria MA, Monken F, Tarazona-Santos E, Martins ML. Noninvasive fetal RHD genotyping from maternal plasma in an admixed Brazilian population. Genet Mol Res 2014;13:799–805.
Schwartz DW, Springer S, Schimd M, Jungbauer C, Schwartz-Jungl E, Deutinger J. Non-invasive prenatal diagnosis (NIPD) of RhD and SRY in multiple pregnancies. Transfus Med Hemother 2012. Conference: 45. Jahreskongress der Deutschen Gesellschaf, pp. 20–1.
Sedrak M, Hashad D, Adel H, Azzam A, Elbeltagy N. Use of free fetal DNA in prenatal non-invasive detection of fetal RhD status and fetal gender by molecular analysis of maternal plasma. Genet Test Mol Biomarkers 2011;15:627–31.
Sesarini C, Giménez ML, Redal MA, Izbizky G, Aiello H, Argibay P, Otaño L. [Non invasive prenatal genetic diagnosis of fetal RhD and sex through the analysis of free fetal DNA in maternal plasma.] Arch Argent Pediatr 2009;107:405–9. http://dx.doi.org/10.1590/S0325-00752009000500006
Sillence KA, Roberts LA, Hollands HJ, Thompson HP, Kiernan M, Madgett TE, et al. Fetal sex and RHD genotyping with digital PCR demonstrates greater sensitivity than real-time PCR. Clin Chem 2015;61:1399–407.
Siva SC, Johnson SI, McCracken SA, Morris JM. Evaluation of the clinical usefulness of isolation of fetal DNA from the maternal circulation. Aust N Z J Obstet Gynaecol 2003;43:10–15.
Stamna A, Zoumatzi B, Manitsa A, Vavatsi-Christaki N. Prenatal genotyping of fetal RHD in maternal plasma from RHD negative women. Haematologica 2007;92:398.
Szemes T, Minarik G, Vlkova B, Celec P, Turna J. Detection optimization and analysis of cell-free fetal nucleic acids in maternal peripheral blood for non-invasive prenatal diagnostics. FEBS J 2009. 34th FEBS Congress (2009), pp. 346–7.
Tounta G, Vrettou C, Kolialexi A, Apostolou P, Papantoniou N, Antsaklis A, et al. A multiplex PCR for non-invasive fetal RhD genotyping. Prenat Diagn 2010. 15th International Conference on Prenatal Diagnosis and Therapy, pp. S39–S40.
Tounta G, Vrettou C, Kolialexi A, Papantoniou N, Destouni A, Tsangaris GT, et al. A multiplex PCR for non-invasive fetal RHD genotyping using cell-free fetal DNA. In Vivo 2011;25:411–17.
Truglio F, Paccapelo C, Scognamiglio S, Villa M, Revelli N, Marconi M. Non-invasive prenatal RHD genotyping by analysis of circulant-free fetal DNA from maternal plasma. Transfusion 2014. AABB Annual Meeting 2014 Philadelphia, PA, pp. 151A.
Turner MJ, Martin CM, O’Leary JJ. Detection of fetal Rhesus D gene in whole blood of women booking for routine antenatal care. Eur J Obstet Gynecol Reprod Biol 2003;108:29–32.
Tynan JA, Angkachatchai V, Ehrich M, Paladino T, van den Boom D, Oeth P. Multiplexed analysis of circulating cell-free fetal nucleic acids for non-invasive prenatal diagnostic RHD testing. Am J Obstet Gynecol 2011;204:251.e1–6.
Wang XD, Wang BL, Ye SL, Liao YQ, Wang LF, He ZM. Non-invasive foetal RHD genotyping via real-time PCR of foetal DNA from Chinese RhD-negative maternal plasma. Eur J Clin Invest 2009;39:607–17. http://dx.doi.org/10.1111/j.1365-2362.2009.02,148.x
Xiong Y, Prabhakaran IM, Holtzman EJ, Jeronis S, Liebermann DA, Hoffman B, Geifman-Holtzman O. Utilization of maternal blood on Guthrie card for first trimester screen for non-invasive fetal sex determination and RhD genotyping. Am J Obstet Gynecol 2012;206(Suppl. 1):S354.
Xiong Y, Prabhakaran IM, Holtzman EJ, Jeronis S, Liebermann DA, Hoffman B, Geifman-Holtzman O. Maternal dry blood spot for non-invasive fetal RHD genotyping at first trimester. Reprod Sci 2012;19(Suppl. 1):339A.
Xiong Y, Prabhakaran I, Holtzman E, Jeronis S, Ness A, Liebermann D, et al. Using maternal dry blood spot for fetal DNA quantification, fetal RhD, and fetal gender determination in the first trimester. Am J Obstet Gynecol 2013;208(Suppl. 1):260.
Yang YF, Lee M, Liang HN, Klotzle B, Legler T, Moise KJ. A novel cell-free fetal DNA test for RHD shows 100% accuracy in non-invasive prenatal testing. Obstet Gynecol 2008;111:104S.
Yenilmez ED, Tuli A, Evruke IC, Ozgunen FT. Non-invasive fetal RHD genotyping from maternal plasma in RHD negative pregnant women. Turk J Biochem 2013. 25th National Biochemistry Congress Izmir Turkey. Conference Start, 38(s1), pp. 298.
Yenilmez ED, Tuli A, Evruke C, Ozgunen FT. Non-invasive fetal RHD genotyping in cell-free fetal DNA from maternal plasma: a Turkish pilot study. Prenat Diagn 2014. Conference: 18th International Conference on Prenatal Diagnosis and Therapy, pp. 53.
Zhang J, Fidler C, Murphy MF, Chamberlain PF, Sargent IL, Redman CW, et al. Determination of fetal RhD status by maternal plasma DNA analysis. Ann N Y Acad Sci 2000;906:153–5.
Zhong XY, Holzgreve W, Hahn S. Detection of fetal Rhesus D and sex using fetal DNA from maternal plasma by multiplex polymerase chain reaction. BJOG 2000;107:766–9.
Zhong XY, Holzgreve W, Hahn S. Risk free simultaneous prenatal identification of fetal Rhesus D status and sex by multiplex real-time PCR using cell free fetal DNA in maternal plasma. Swiss Med Wkly 2001;131:70–4. http://dx.doi.org/2001/05/smw-09660
Zhou L, Thorson JA, Nugent C, Davenport RD, Butch S H, Judd WJ. Non-invasive prenatal RHD genotyping by real-time polymerase chain reaction using plasma from D-negative pregnant women. Am J Obstet Gynecol 2005;193:1966–71.
Zhou L, Thorson J, Nugent CE, Davenport RD, Judd WJ. Non-invasive prenatal RHD genotyping by real-time PCR using plasma from RHD-negative pregnant women. Mod Pathol 2005;18:337A.
Zhou L, Wei L, Yan Q, Lazebnik N. Evaluation of a prenatal RHD genotyping strategy using fetal cell-free DNA from maternal plasma in a population with mixed ethnicity. Transfusion 2007;47:153A.
Ineligible population (10 references)
Clarke G, Hannon J, Berardi P, Barr G, Cote J, Fallis R, et al. Resolving variable maternal D typing by using serology and genotyping in selected prenatal patients. Transfusion 2015;55:149A–50A.
Daniels G, Finning K, Martin P, Summers J. Fetal blood group genotyping: present and future. Ann N Y Acad Sci 2006;1075:88–95.
de Haas M, Bossers BEM, Soussan AA, Ligthart PC, Schuitemaker LDM, Page-Christiaens GC, van der Schoot CE. Non-invasive fetal RHD genotyping and fetal sexing in maternal blood. Vox Sang 2006;9(Suppl. 3):145.
Doescher A, Vogt C, Wagner FF, Petershofen EK, Muller TH. Non-invasive prenatal blood group typing in pregnancies with known antibodies. Transfus Med Hemother 2012. Conference: 45. Jahreskongress der Deutschen Gesellschaf, pp. 9–10.
Finning K, Martin P, Summers J, Daniels G. Fetal genotyping for the K (Kell) and Rh C, c, and E blood groups on cell-free fetal DNA in maternal plasma. Transfusion 2007;47:2126–33.
Grill S, Banzola I, Li Y, Rekhviashvili T, Legler TJ, Muller SP, Zhong XY, Hahn S, Holzgreve W. High throughput non-invasive determination of foetal Rhesus D status using automated extraction of cell-free foetal DNA in maternal plasma and mass spectrometry. Arch Gynecol Obstet 2009;279:533–7.
Minon JM, Gerard C, Senterre JM, Schaaps JP, Foidart JM. Routine fetal RHD genotyping with maternal plasma: a four-year experience in Belgium. Transfusion 2008;48:373–81.
Monteiro F, Bastos P, Amorim A, Ferreira M, Tavares G, Araujo F. Non-invasive fetal RHD genotyping by real-time PCR: 3 years of experience in Portugal. Vox Sang 2012;103(Suppl. 1):215.
Ordonez E, Rueda L, Canadas MP, Fuster C, Cirigliano V. Development and validation of multiplex real-time PCR assay for non-invasive prenatal assessment of fetal RhD status and fetal sex in maternal plasma. Fetal Diagn Ther 2013;34:13–18.
Rijnders RJP, Christiaens GCML, Bossers B, van der Smagt JJ, van der Schoot CE, de Haas M. Clinical applications of cell-free fetal DNA from maternal plasma. Obstet Gynecol2004;103:157–64.
Insufficient outcome data (17 references)
Flower L, Millard GM, McGowan EC, O’Brien H, Hyett JA, Gardener GJ, Hyland CA. Genotyping to reduce anti-D immunoglobulin usage in a diverse population demographic: fetal RHD detection for mothers harbouring RHD variants. Vox Sang 2015;109(Suppl. 1):282.
Gardener G, O’Brien H, Millard G, Gibbons K, Flower R, Hyett J, Hyland C. Non-invasive prenatal testing (NIPT) for fetal RHD: evaluation of a new genotyping algorithm for mass screening. Prenat Diagn 2014. Conference: 18th International Conference on Prenatal Diagnosis and Therapy, pp. 55.
Hawk AF, Chang EY, Shields SM, Simpson KN. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol 2013;122:579–85. http://dx.doi.org/10.1097/AOG.0b013e31829f8814
Hill M, Finning K, Martin P, Hogg J, Meaney C, Norbury G, et al. Non-invasive prenatal determination of fetal sex: translating research into clinical practice. Clin Genet 2011;80:68–75. http://dx.doi.org/10.1111/j.1399-0004.2010.01533.x
Hyland C, Millard G, McGowan E, O’Brien H, Hyett J, Gardener G, Flower R. Feasibility of applying non-invasive fetal RHD gentyping to determine which D-negative pregnant women require antenatal anti-D immunoglobulin prophylaxis. HAA, 2015.
Hyland C, Millard G, McGowan E, O’Brien H, Knauth C, Tremellen A, et al. Non-invasive fetal RHD genotyping for D-negative women harbouring RHD*D-CE-D gene variants; accuracy in detection of fetal specific RHD signals. HAA, 2015.
Legler TJ, Liu Z, Mavrou A, Finning K, Hromadnikova I, Galbiati S, et al. Workshop report on the extraction of foetal DNA from maternal plasma. Prenat Diagn 2007;27:824–9.
Legler T. Fetal molecular blood group RhD determination from maternal plasma for decision making on Rh prophylaxis in D-negative pregnant women. Clin Chem Lab Med 2014. Congress of Clinical Chemistry and Laboratory Medicine, pp. eA151.
Mailloux A, Cortey AN, Da Silva N, Larsen M, Brossard Y, Carbonne B. Fetal RHD genotyping in the monitoring of RH1 negative pregnant women: The experience of the French national center for perinatal hemobiology (CNRHP). Transfusion 2009;49:132A.
Rodriguez N, Noguerol P, Garcia L, Macher H, Carmona M, Martin J, Simon JAP. Non-invasive protocol for the screening, diagnosis and treatment of hemolytic perinatal. Blood 2013;122:2405.
Rouillac-Le Sciello C, De Beaumont C, Velard C, Bourdon F, Mailloux A, Serazin V, et al. Non invasive fetal RhD genotyping from maternal plasma: validation of the free DNA fetal kit RhD using the CFX96 real-time system. Vox Sang 2010;99(Suppl. 1):404.
Rouillac-Le Sciellour C, De Beaumont C, Andry A, Velard C, Bourdon F, Serazin V, Menu M. Evaluation of a RHD blood group system genotyping test using multiplex PCR. Vox Sang 2013;105:235.
Rouillac-Le Sciellour C, De Beaumont C, Andry A, Velard C, Bourdon F, Serazin V, Menu M. Improvement of non invasive fetal RHD genotyping from maternal plasma: development of a multiplex PCR test. Vox Sang 2013;105:246.
Brossard Y. Routine Fetal RhD Genotyping for RhD- Pregnant Women. Clinical trial NCT00832962. URL: https://ClinicalTrials.gov/show/NCT00832962 (accessed 12 July 2017).
Sbarsi I, Isernia P, Montanari L, Zuffardi O, Badulli C, Bergamaschi P, et al. Set up and validation of real-time PCR technology for molecular RhD typing of cell free foetal DNA in maternal plasma: the experience of Pavia. Vox Sang 2010;99(Suppl. 1):398.
Scheffer PG, Van Der Schoot CE, Bossers BEM, Ligthart PC, Schuitemaker LDM, De Haas M. Non-invasive fetal blood group genotyping with DNA from maternal plasma: a seven-year clinical experience. Vox Sang 2010. Conference: 31st International Congress of the International Society of Blood Transfusion, pp. 24.
SensiGene fetal RHD genotyping. Lansdale, PA: HAYES, Inc.; 2013.
Ineligible reference standard (three references)
Brojer E, Zupanska B, Guz K, Orziñska A, Kaliñska A. Noninvasive determination of fetal RHD status by examination of cell-free DNA in maternal plasma. Transfusion 2005;45:1473–80.
Orzinska A, Guz K, Kopec I, Michalewska B, Nowaczek-Migas M, Brojer M. Ton-invasive fetal blood group genotyping: the decade of Polish experience. Vox Sang 2010;99(Suppl. s1):24–5.
Orzińska A, Guz K, Dębska M, Uhrynowska M, Celewicz Z, Wielgo M, Brojer E. 14 Years of Polish Experience in Non-Invasive Prenatal Blood Group Diagnosis. Transfus Med Hemother 2015;42:361–4. http://dx.doi.org/10.1159/000440821
Ineligible study design (29 references)
Moise K. A noninvasive test for Fetal RHD genotype. Clinical trial NCT00871195. URL: https://ClinicalTrials.gov/show/NCT00871195 (accessed 12 July 2017).
Avent ND. Large scale blood group genotyping. Transfus Clin Biol 2007;14:10–15.
Bills VL, Soothill PW. Fetal blood grouping using cell free DNA – an improved service for RhD negative pregnant women. Transfus Apher Sci 2014;50:148–53.
Bui TH. Non-invasive fetal RHD determination using exon sequencing for routine screening in early pregnancy. J Perinat Med 2013. Conference: 11th World Congress of Perinatal Medicine, 20130619.
Clausen FB, Damkjaer MB, Dziegiel MH. Non-invasive fetal RhD genotyping. Transfus Apher Sci 2014;50:154–62.
Daniels G, Finning K, Martin P, Summers J. Fetal RhD genotyping: a more efficient use of anti-D immunoglobulin. Transfus Clin Biol 2007;14:568–71. http://dx.doi.org/10.1016/j.tracli.2008.03.007
Finning K, Daniels G, Martin P, Soothill P. Detection of fetal Rhesus D gene in whole blood of women booking for routine antenatal care. Eur J Obstet Gynecol Reprod Biol 2003;110:117.
Finning K, Martin P, Daniels G. The use of maternal plasma for prenatal RhD blood group genotyping. Methods Mol Biol 2009;496:143–57. http://dx.doi.org/10.1007/978-1-59745-553-4_11
Flegel WA. Blood group genotyping in Germany. Transfusion 2007;47(Suppl. 1):47–53.
Freeman K, Szczepura A, Osipenko L. Quality of Rh genotyping studies and diagnostic accuracy estimation. Am J Obstet Gynecol 2007;197:116–18.
Freeman K, Szczepura A, Osipenko L. Non-invasive fetal RHD genotyping tests: a systematic review of the quality of reporting of diagnostic accuracy in published studies. Eur J Obstet Gynecol Reprod Biol 2009;142:91–8.
Fyfe TM, Ritchey MJ, Taruc C, Crompton D, Galliford B, Perrin R. Appropriate provision of anti-D prophylaxis to RhD negative pregnant women: a scoping review. BMC Pregnancy Childbirth 2014;14:411. http://dx.doi.org/10.1186/s12884-014-0411-1
Geifman-Holtzman O, Grotegut CA, Gaughan JP. Diagnostic accuracy of noninvasive fetal Rh genotyping from maternal blood – a meta-analysis. Am J Obstet Gynecol 2006;195:1163–73.
Gooch A, Parker J, Wray J, Qureshi H. Guideline For Blood Grouping And Antibody Testing In Pregnancy. London: British Society for Haematology; 2006. pp. 22.
Jayatilleke N. Antenatal Screening for Rhesus D Status and Red Cell Allo-Antibodies. London: UK National Screening Committee; 2013.
Koracin JG, Modric Z. IzvanstaniCne nukleinske kiseline ploda u krvi majke - dijagnostiCke moguCnosti, Perspektive i izazovi. Gynaecologia et Perinatologia 2013;22:150–6.
Legler TJ, Müller SP, Haverkamp A, Grill S, Hahn S. Prenatal RhD Testing: A Review of Studies Published from 2006 to 2008. Transfus Med Hemother 2009;36:189–98. http://dx.doi.org/10.1159/000216580
Legler TJ. Prenatal Rhesus Testing. In Mayr WR, editor. State of the Art Presentations. Malden, MA: Wiley-Blackwell; 2010. pp. 7–11.
Li R, Lu Y, Xu S, Guo Y, Wang Z, Chen W, Wang C. [Sensitivity and specificity of noninvasive prenatal fetal RhD genotesting: a meta-analysis.] Zhonghua Yi Xue Za Zhi 2014;94:2677–80.
Mohan A, Seth S. Foetal RhD genotyping using DNA extracted from maternal plasma. Natl Med J India 1999;12:118–19.
NSW Kids and Families. Maternity - Rh (D) Immunoglobulin (Anti D). North Sydney, NSW: Ministry of Health, NSW; 2015.
Qureshi H, Massey E, Kirwan D, Davies T, Robson S, White J, et al. BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfus Med 2014;24:8–20.
Royal College of Obstetricians and Gynaecologists. The Use of Anti-D Immunoglobulin for Rhesus D Prophylaxis (archived). London: Royal College of Obstetricians and Gynaecologists; 2011. URL: www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg22/ (accessed 12 July 2017).
The Norwegian Knowledge Centre for the Health Services. Determination of fetal rhesus D status from maternal plasma of rhesus negative women. Oslo: The Norwegian Knowledge Centre for the Health Services; 2014.
The Society of Obstetricians and Gynaecologists of Canada. (2005). Amended Canadian Guideline for Prenatal Diagnosis (2005) Change to 2005-Techniques for Prenatal Diagnosis. [online] The Society of Obstetricians and Gynaecologists of Canada. URL: http://sogc.org/guidelines/amended-canadian-guideline-for-prenatal-diagnosis-2005-change-to-2005-techniques-for-prenatal-diagnosis/.
van der Schoot CE, Soussan AA, Koelewijn J, Bonsel G, Paget-Christiaens LG, de Haas M. Non-invasive antenatal RHD typing. Transfus Clin Biol 2006;13:53–7.
Wenstrom KD. Commentary on: Effect of high throughput RHD typing of fetal DNA in maternal plasma on use of anti-RhD immunoglobulin in RhD negative pregnant women: prospective feasibility study. Obstet Gynecol Surv 2008;63:499–500.
Wright CF, Burton H. The use of cell-free fetal nucleic acids in maternal blood for non-invasive prenatal diagnosis. Hum Reprod Update 2009;15:139–51. http://dx.doi.org/10.1093/humupd/dmn047
Zhu YJ, Zheng YR, Li L, Zhou H, Liao X, Guo JX, Yi P. Diagnostic accuracy of non-invasive fetal RhD genotyping using cell-free fetal DNA: a meta analysis. J Matern Fetal Neonatal Med 2014;27:1839–44. http://dx.doi.org/10.3109/14767058.2014.882306
Appendix 4 Characteristics of diagnostic accuracy studies
| Study | Country | Study dates | Number tested | Number analysed | Gestational age (weeks), median (range) | Ethnicity (%) | Multiple pregnancies included? | DNA extraction tool | PCR technology | Multiple testing performed? |
|---|---|---|---|---|---|---|---|---|---|---|
| Akolekar et al., 201119 | England | NR | 591 | 586 | 12.4 (11–14) | White European 77.3, Asian 1.2, African 19.3, mixed 2.2 | No | MDx BioRobot (Qiagen, Crawley, UK) | ABI 7900 detection system (ABI, Applied Biosystems, Foster City, CA, USA) | Yes (for RHD variants) |
| Banch Clausen et al., 201420 | Denmark | 2010–11 | 14,547 | 12,668 | 25 (73% between 23 and 28) | NR | NR | QIAsymphony SP; MagNA Pure LC; MagNA Pure Compact Instrument (Roche Ltd, Rotkreuz, Switzerland) | ABI 7900 detection system (Applied Biosystems) LightCycler 480 (Roche) PCR ABI 7500 (Applied BioSystems) | NR |
| Chitty et al., 201412 | England | 2009–12 | 4913 | 4913 | 19 (5–35) (18% under 11 weeks) | White European 78, Asian 6, Black or mixed race 4, unknown 12 | No | MDx BioRobot (Qiagen, Crawley, UK) | ABI Prism 7900HT (Applied Biosystems) | Up to four samples per woman |
| Finning et al., 200817 | England | NR | 1997 | 1869 | 28 (8–38) (92% at 26–32) | White European 55, Asian 8, African 2, other 2, unknown 33 | Yes (n = 13 pregnancies) | MDx BioRobot (Qiagen, Crawley, UK) | ABI Prism 7900HT (Applied Biosystems) | NR |
| Grande et al., 201322 | Spain | February 2010–October 2011 | 284 | 282 | 24–26 | White European 84, Asian 1.5, African 1.8, Latin American 12, other 0.7 | Yes (n = 16 pregnancies) | COBAS AmpliPrep (Roche Ltd, Rotkreuz, Switzerland) | 7300 Real-Time PCR System (Applied Biosystems) | Yes, two independent assays performed in triplicate for all |
| Soothill et al., 201518 | England | April–September 2013 | 526 | 499 | 15–26 | NR | No | MDx BioRobot (Qiagen, Crawley, UK) | NR | NR |
| Thurik et al., 201521 | The Netherlands | July 2011–October 2012 | 24,986 | 18,383 | 26 | NR | No | MagNa Pure 96 (Roche Ltd, Rotkreuz, Switzerland) | StepOnePlus Real-Time PCR System (Applied Biosystems) | Yes, PCR in triplicate |
| Wikman et al., 201223 | Sweden | September 2009–May 2011 | 4118 | 3291 | 10 (3–40) (75.5% first trimester, 10% tested before 8 weeks) | NR | Yes (n = 61 pregnancies) | MagNA Pure LC (Roche Ltd, Rotkreuz, Switzerland) | PCR ABI 7500 (Applied BioSystems) | Yes, PCR on all samples in triplicate 211 samples reanalysed because of uninterpretable results |
Appendix 5 Risk of bias and applicability of findings of diagnostic accuracy studies
| Study | Was a consecutive sample of patients enrolled? | Did the study avoid inappropriate exclusions? | Were key study population characteristics reported? (including ethnicity, GA, multiple pregnancies) | Risk of bias | Applicability: are there concerns that the included patients do not match the target population? |
|---|---|---|---|---|---|
| Akolekar et al., 201119 | Unclear | No, excluded multiple pregnancies | Yes | High, reporting of selection process limited, much higher proportion of African than general population (19.3%) | Yes, much higher proportion of people of African ethnicity than general population (19.3%) |
| Banch Clausen et al., 201420 | Unclear, not stated but seems likely | Unclear, appears fine | No, population characteristics (including ethnicity) NR | Low | Unclear, population characteristics (including ethnicity) NR |
| Chitty et al., 201412 | Unclear, not stated but seems likely | No, excluded multiple pregnancies | Yes | Low | No |
| Finning et al., 200817 | Unclear, not stated but seems likely | Yes | Yes | Low | No |
| Grande et al., 201322 | Unclear | Yes | Yes | Low | Yes, ethnic distribution differs from general UK population (12% Latin American) |
| Soothill et al., 201518 | Unclear, not stated but seems likely | Yes | No, ethnicity and multiple pregnancy NR. Gestational range could be inferred but was not clearly reported | Low | No |
| Thurik et al., 201521 | Unclear, not stated but seems likely | No, multiple pregnancies excluded and treated as positive | No, ethnicity and number of multiple pregnancies NR | Low | Yes, exclusion of multiple pregnancies |
| Wikman et al., 201223 | Unclear, not stated but seems likely | Unclear, exclusion criteria not reported | No, ethnicity NR | Low | Unclear, ethnicity unknown |
| Study | Were the index test results interpreted without knowledge of the results of the reference standard? | If a threshold was used, was it prespecified? | Were results from replicate samples dealt with appropriately? | Were results from multiple pregnancies dealt with appropriately | Risk of bias: could the conduct or interpretation of the index test have introduced bias? | Applicability: are there concerns that the index test, its conduct or interpretation differ from the review question? | Reporting: did the study report any adverse effect of the index test? |
|---|---|---|---|---|---|---|---|
| Akolekar et al., 201119 | Unclear, likely not | Unclear, thresholds were reported, but unclear if prespecified | Yes | N/A, only singleton pregnancies | High, inconclusive results were not included in the main analysis. This may have inflated the accuracy estimates | Low | No |
| Banch Clausen et al., 201420 | Yes | Yes | Unclear, NR | Unclear, NR | Low | Low | No |
| Chitty et al., 201412 | Yes | Yes | Unclear, NR | N/A, only singleton pregnancies | Low | Low | No |
| Finning et al., 200817 | Yes | Unclear, unclear if prespecified | Unclear, NR | Yes | Low | Low | No |
| Grande et al., 201322 | Unclear | Unclear, unclear if prespecified | Yes | Yes | Low | Low | No |
| Soothill et al., 201518 | Unclear, presumably as in Chitty et al.12 | Unclear, presumably as in Chitty et al.12 | Unclear, NR | Unclear, NR | Unclear, Presumably as in Chitty et al.12 | Low | No |
| Thurik et al., 201521 | Unclear, unclear for back-up plasma analysis, yes for samples not reanalysed | No, prediction algorithm is judged daily and adjusted as needed.If we would have strictly followed the computed algorithm, the repeat rate would have been almost halved, with the expense of one false-negative and 20 more false-positive results | Yes | No, all treated as positive and prescribed anti-D | High, change of diagnostic algorithm after start of study may have introduced bias | Low | No |
| Wikman et al., 201223 | Unclear, likely not | Unclear | Yes | Yes | Low | High, only exon 4 was targeted | No |
| Study | Is the reference standard likely to correctly classify the target condition? | Were the reference standard results interpreted without knowledge of the results of the index test? | Risk of bias: could the reference standard, its conduct or its interpretation have introduced bias? | Applicability: are there concerns that the study used a non-standard reference standard? | Reporting: did the study report any adverse effect of the reference standard? |
|---|---|---|---|---|---|
| Akolekar et al., 201119 | Unclear, method NR | Unclear, NR | Unclear, method NR | Unclear, method NR | No |
| Banch Clausen et al., 201420 | Yes | Unclear, NR | Low | Low | No |
| Chitty et al., 201412 | Yes | Unclear, NR | Low | Low | No |
| Finning et al., 200817 | Yes | Yes | Low | Low | No |
| Grande et al., 201322 | Yes | Unclear, NR | Low | Low | No |
| Soothill et al., 201518 | Yes | Unclear, NR | Low | Low | No |
| Thurik et al., 201521 | Yes | Unclear, NR | Low | Low | No |
| Wikman et al., 201223 | Yes | Unclear, NR | Low | Low, author contacted: appropriate except 5% of samples processed in citrate tubes | No |
| Study | Was there an appropriate interval between index test(s) and reference standard? | Did all patients (who provided data) receive a reference standard? | Did all patients receive the same reference standard? | Were all patients included in the analysis? | Risk of bias: could the patient flow have introduced bias? |
|---|---|---|---|---|---|
| Akolekar et al., 201119 | Yes | No, only those with reference standard result and live birth were included in the study | Unclear | No, only those with reference standard result and live birth were included in the study | Low |
| Banch Clausen et al., 201420 | Yes | No | Yes | Yes | Low |
| Chitty et al., 201412 | Yes | No, 185 without cord blood result, but unlikely significant bias | Yes | No, 13% excluded for various reasons (all reported) | Low |
| Finning et al., 200817 | Yes | No, four did not because of fetal death | Yes | No, 128 fetal phenotypes were not available for paired analysis because 124 cord samples were untraceable and there were four fetal deaths | Low |
| Grande et al., 201322 | Yes | Yes, appears so | Yes | No, only two RhD-positive mothers who underwent NIPT were excluded | Low |
| Soothill et al., 201518 | Yes | No, 5% did not have cord blood serology results | Yes | Yes | Low |
| Thurik et al., 201521 | Yes | No, 80% did. No reason provided for 20% not providing cord blood serology | Yes | No, 20% samples received NIPT but not cord serology | High, 20% samples received NIPT but not cord serology. No reasons provided |
| Wikman et al., 201223 | Yes | No, 11% pregnancies with no reference standard measurement | No, 5% citrate samples (author contacted) | No, 11% pregnancies with no reference standard measurement | Low, despite limitations, risk of diagnostic accuracy results being significantly affected was not considered high |
Appendix 6 Additional figures and tables for diagnostic accuracy analyses
FIGURE 19.
Forest plots for analysis case 2.
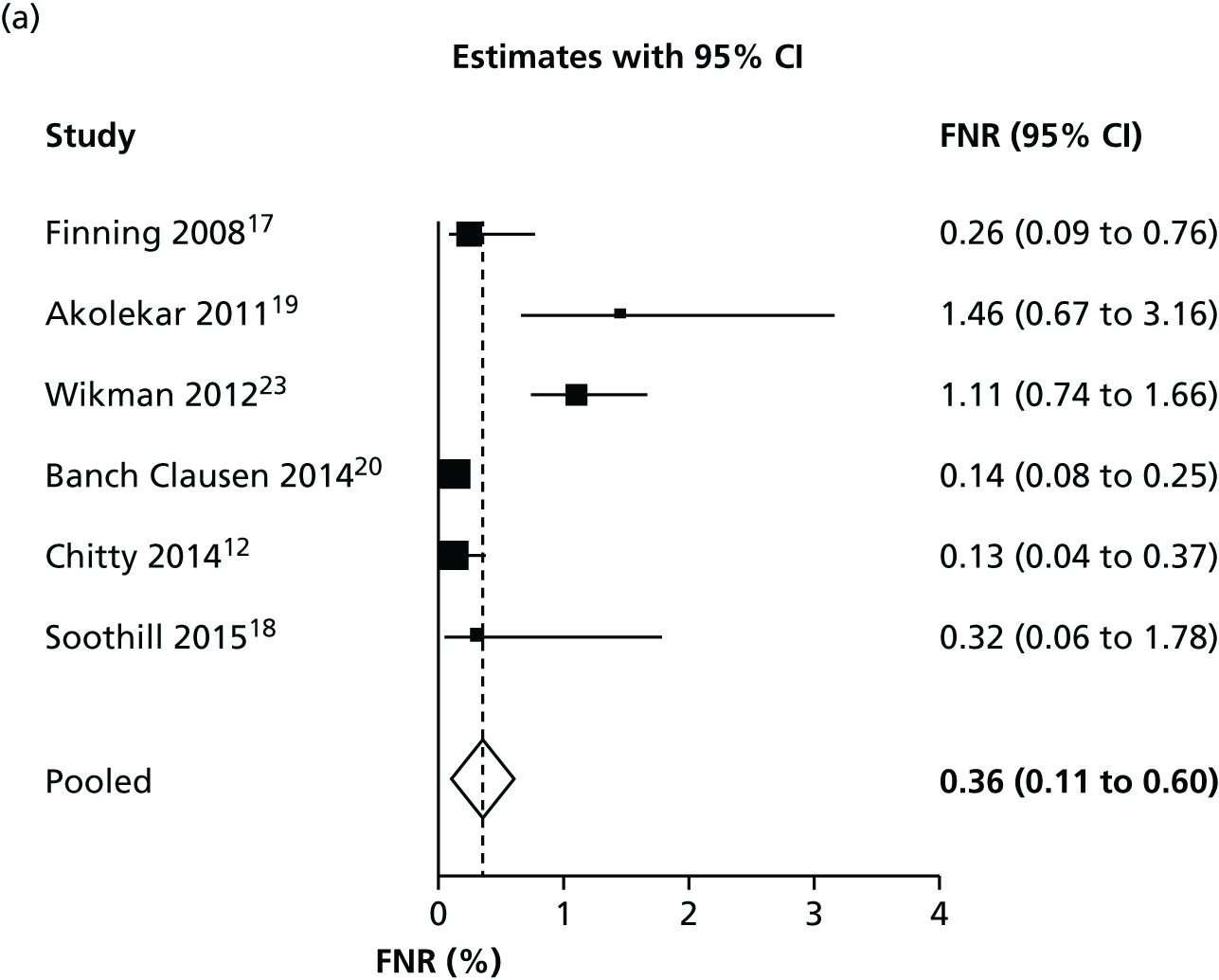
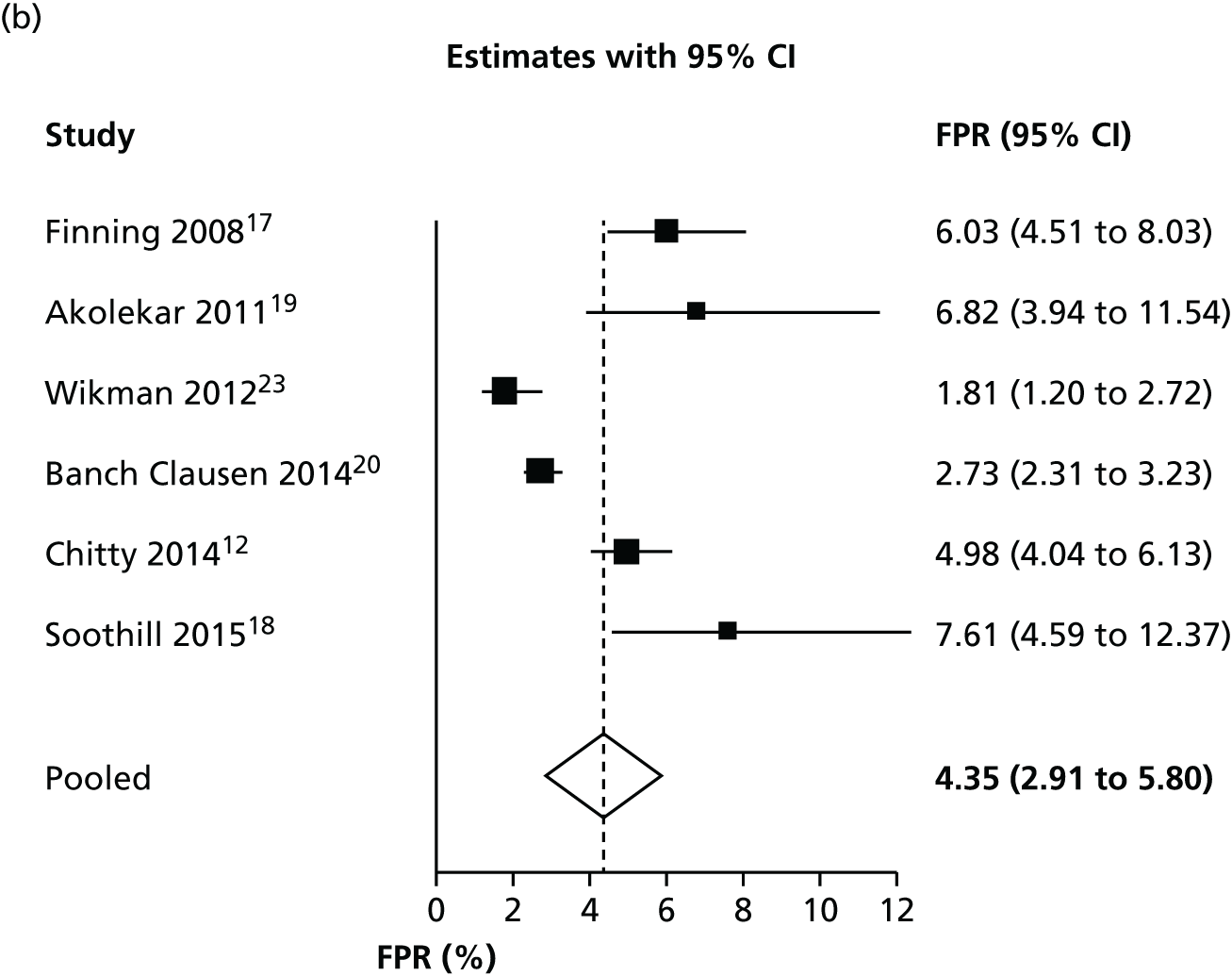
Receiver operating characteristic plot for analysis case 3
FIGURE 20.
Receiver operating characteristic plot for analysis case 3.
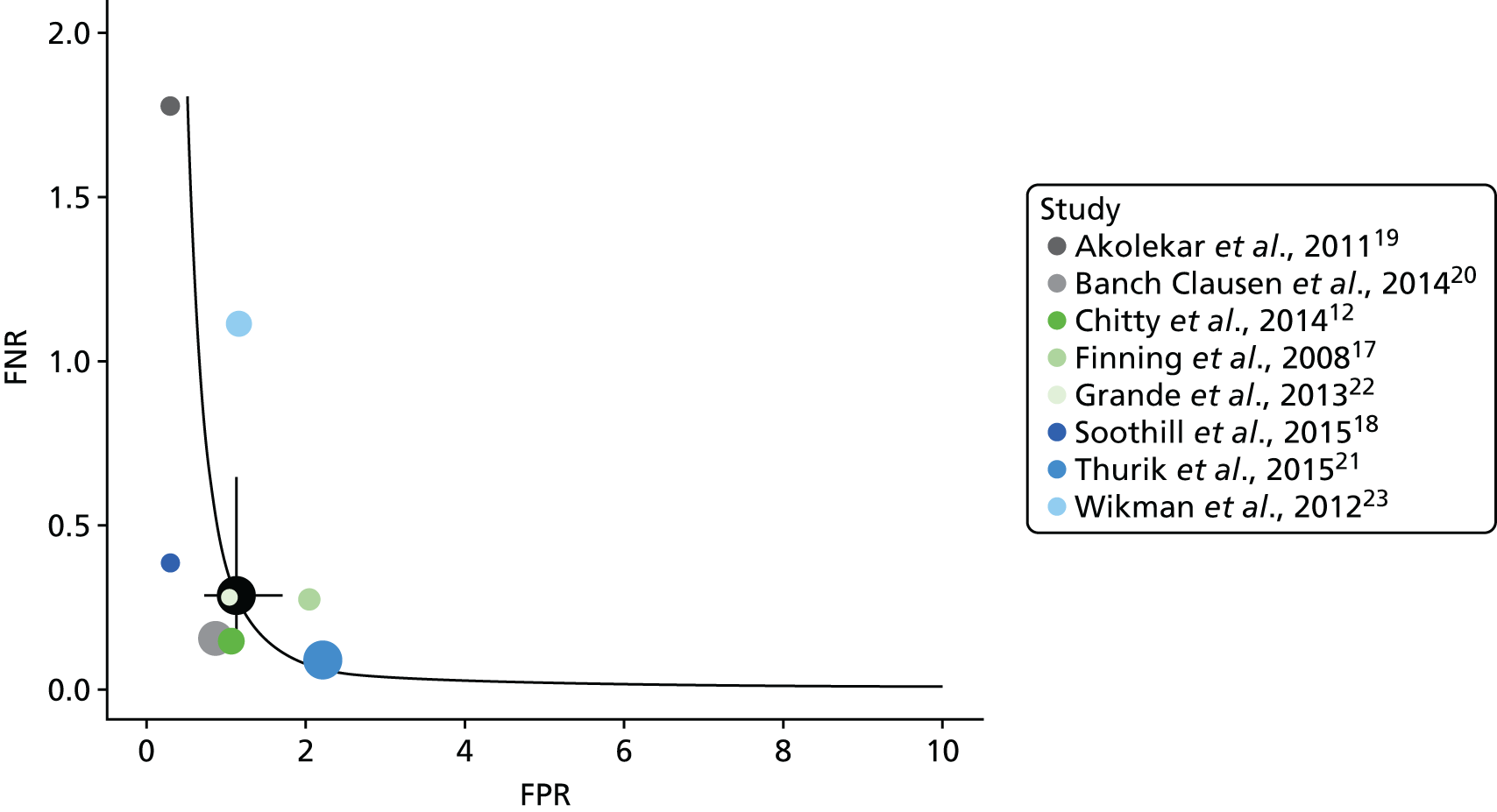
| Analysis | FNR, % (95% CI) | FPR, % (95% CI) |
|---|---|---|
| Excluding Akelokar et al., 201119 and Thurik et al., 201521 | ||
| Inconclusives treated as positive (with Grande et al., 201322) | 0.315 (0.14 to 0.70) | 3.837 (2.36 to 6.19) |
| Inconclusives treated as positive (without Grande et al., 201322) | 0.260 (0.10 to 0.65) | 4.004 (2.40 to 6.60) |
| Excluding inconclusives | 0.349 (0.16 to 0.77) | 1.205 (0.87 to 1.67) |
| Excluding Wikman et al., 201223 | ||
| Inconclusives treated as positive (with Thurik et al., 201521 and Grande et al., 201322) | 0.292 (0.13 to 0.65) | 4.478 (2.92 to 6.81) |
| Inconclusives treated as positive (without Thurik et al., 201521 and Grande et al., 201322) | 0.334 (0.13 to 0.84) | 5.245 (3.54 to 7.71) |
| Excluding inconclusives | 0.279 (1.12 to 0.67) | 1.142 (0.69 to 1.90) |
Appendix 7 Quality assessment of clinical effectiveness studies
This appendix presents quality assessment tables performed for the two comparative studies included in the review of effectiveness studies.
When multiple outcomes were assessed within the same study, risk-of-bias judgements did not differ across outcomes unless otherwise specified. Further details of the quality assessment, including prespecified target randomised trials, target comparisons and specified confounding domains are available on request.
For full guidance, see Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ACROBAT-NRSI. A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI). Version 1.0.0. 2014. URL: www.riskofbias.info (accessed 1 March 2016).
Risk-of-bias assessment: Banch Clausen et al.20
Outcomes and results assessed
| Outcomes assessed |
Compliance with prenatal anti-D Compliance with postnatal anti-D Compliance with RHD screening |
| Specific results being assessed |
Compliance with antenatal anti-D: 93.2% vs. not applicable (not recommended in patients not receiving RHD screening) Compliance with postnatal anti-D: 99.7% vs. 95.7% Compliance with RHD screening: 84.2% |
Risk-of-bias table for Banch Clausen et al.20
| Bias domain | Signalling question | Judgement | Comment |
|---|---|---|---|
| Bias attributable to confounding | Is confounding of the effect of intervention unlikely in this study? | PN | Unadjusted analyses |
| Were participants analysed according to their initial intervention group throughout follow-up? | PY | ||
| Did the authors use an appropriate analysis method that adjusted for all the critically important confounding domains? | No | Unadjusted analyses | |
| Risk-of-bias judgement | Critical | Analyses not adjusted for several potential confounders (including potential sensitising event, anti-D prophylaxis compliance, gestational age) | |
| What is the predicted direction of bias due to confounding? | Unpredictable | ||
| Bias in selection of participants into the study | Was selection into the study unrelated to intervention or unrelated to outcome? | No | |
| Do start of follow-up and start of intervention coincide for most subjects? | Yes | ||
| Were adjustment techniques used that are likely to correct for the presence of selection biases? | No | ||
| Risk-of-bias judgement | NI | Only participants from one of the five regions over 1 year (690/12,668) were included. Reasons were not provided | |
| What is the predicted direction of bias due to selection of participants into the study? | Unpredictable | ||
| Bias in measurement of interventions | Is intervention status well defined? | Yes | RHD screening |
| Was information on intervention status recorded at the time of intervention? | PY | ||
| Was information on intervention status unaffected by knowledge of the outcome or risk of the outcome? | Yes | ||
| Risk-of-bias judgement | Low | ||
| What is the predicted direction of bias due to measurement of outcomes or interventions? | Towards null | Low risk of bias | |
| Bias due to departures from intended interventions | Were the critical cointerventions balanced across intervention groups? | NI | No information on non-routine anti-D and whether or not it was measured as separate from routine administration |
| Were numbers of switches to other interventions low? | Yes | N/A | |
| Was implementation failure minor? | PY | No information but unlikely | |
| Were adjustment techniques used that are likely to correct for these issues? | No | ||
| Risk-of-bias judgement | Low | ||
| What is the predicted direction of bias due to departures from the intended interventions? | Towards null | Low risk of bias | |
| Bias due to missing data | Are outcome data reasonably complete? | NI | No information on missing data |
| Was intervention status reasonably complete for those in whom it was sought? | NI | ||
| Are data reasonably complete for other variables in the analysis? | No | Lack of reported data on confounders | |
| Are the proportion of participants and reasons for missing data similar across interventions? | NI | No information on missing data | |
| Were appropriate statistical methods used to account for missing data? | N/A | ||
| Risk-of-bias judgement | NI | ||
| What is the predicted direction of bias due to missing data? | Unpredictable | ||
| Bias in measurement of outcomes | Was the outcome measure objective? | Yes | |
| Were outcome assessors unaware of the intervention received by study participants? | NI | ||
| Were the methods of outcome assessment comparable across intervention groups? | PY | ||
| Were any systematic errors in measurement of the outcome unrelated to intervention received? | NI | ||
| Risk-of-bias judgement | Low | No information to suggest otherwise | |
| What is the predicted direction of bias due to measurement of outcomes? | Towards null | ||
| Bias in selection of the reported result | Is the reported effect estimate unlikely to be selected, on the basis of the results, from: | ||
| Multiple outcome measurements within the outcome domain? | PY | ||
| Multiple analyses of the intervention–outcome relationship? | PY | ||
| Different subgroups? | NI | Only participants from one of the five regions over 1 year (690/12,668) were included. Reasons were not provided | |
| Risk-of-bias judgement | NI | ||
| What is the predicted direction of bias due to selection of the reported result? | Unpredictable | ||
| Overall bias | Risk-of-bias judgement | Critical | Only participants from one of the five regions over 1 year (690/12,668) were included. Analyses were not adjusted for any potential confounders |
| What is the overall predicted direction of bias for this outcome? | Unpredictable | Unpredictable because of insufficient information, although may be more likely to favour the intervention |
Risk-of-bias assessment: Tiblad et al.26
Outcomes and results assessed
| Outcomes assessed | Sensitisation (measured as development of anti-D antibodies after the first trimester of pregnancy or post partum) |
| Results | Adjusted odds ratio 0.41 (95% CI 0.22 to 0.78), 0.19% vs. 0.46% (favours intervention) |
Risk-of-bias table for Tiblad et al.26
| Bias domain | Signalling question | Judgement | Comment |
|---|---|---|---|
| Bias due to confounding | Is confounding of the effect of intervention unlikely in this study? | No | Study with historical control and insufficiently adjusted analysis |
| Were participants analysed according to their initial intervention group throughout follow-up? | No | In the reference group, no routine postpartum antibody testing was performed. The outcome was measured in the first trimester of the subsequent pregnancy | |
| Were intervention discontinuations or switches unlikely to be related to factors that are prognostic for the outcome? | PN | ||
| Did the authors use an appropriate analysis method that adjusted for all the critically important confounding domains and for time-varying confounding? | PN | Analyses adjusted for NIPT sensitivity. No significant differences in gestational age and preterm births. Compliance with RAADP not adjusted for | |
| Were confounding domains that were adjusted for measured validly and reliably by the variables available in this study? | N/A | ||
| Risk-of-bias judgement | Serious | Study with historical control. No adjustment for RAADP compliance or sensitising event | |
| What is the predicted direction of bias due to confounding? | Unpredictable | ||
| Bias in selection of participants into the study | Was selection into the study unrelated to intervention or unrelated to outcome? | No | The control group was historical, pretargeted routine anti-D prophylaxis. In the reference group, immunisation after delivery was defined as presence of anti-D antibodies in the first trimester in the subsequent pregnancy. Routine antibody testing at 25 weeks in nulliparous women in routine management group was not performed |
| Do start of follow-up and start of intervention coincide for most subjects? | PN | Only clear for intervention group, probably not for routine management group | |
| Were adjustment techniques used that are likely to correct for the presence of selection biases? | No | ||
| Risk-of-bias judgement | Serious | The control group was historical, pre-targeted routine anti-D prophylaxis. In the reference group, immunisation was defined as presence of anti-D antibodies in the first trimester in a subsequent pregnancy. This means that any pregnant woman with no recorded subsequent pregnancy was excluded | |
| What is the predicted direction of bias due to selection of participants into the study? | Unpredictable | Insufficient information to assess, although it is possible events were underestimated in the reference group as sensitisation was not measured post partum in this group. On the other hand, it is plausible, as the authors stated, that not all women in the reference cohort had a subsequent pregnancy when antibodies from sensitisation late in the third trimester or at delivery in the previous pregnancy would be found, leading to rates of new RhD immunisations being somewhat underestimated | |
| Bias in measurement of interventions | Is intervention status well defined? | Yes | |
| Was information on intervention status recorded at the time of intervention? | Yes | ||
| Was information on intervention status unaffected by knowledge of the outcome or risk of the outcome? | Yes | ‘Hard’ outcome | |
| Risk-of-bias judgement | Low | ||
| What is the predicted direction of bias due to measurement of outcomes or interventions? | Towards null | ||
| Bias due to departures from intended interventions | Were the critical cointerventions balanced across intervention groups? | NI | |
| Were numbers of switches to other interventions low? | PY | ||
| Was implementation failure minor? | NI | ||
| Were adjustment techniques used that are likely to correct for these issues? | No | ||
| Risk-of-bias judgement | Low | ||
| What is the predicted direction of bias due to departures from the intended interventions? | Unpredictable | ||
| Bias due to missing data | Are outcome data reasonably complete? | NI | In the control group, it appears that any pregnant woman with no recorded subsequent pregnancy was excluded |
| Was intervention status reasonably complete for those in whom it was sought? | NI | Insufficient information | |
| Are data reasonably complete for other variables in the analysis? | No | Limited data on participants excluded from the analyses as there was no recorded subsequent pregnancy in the reference group | |
| Are the proportion of participants and reasons for missing data similar across interventions? | NI | ||
| Were appropriate statistical methods used to account for missing data? | No | ||
| Risk-of-bias judgement | NI | In the control group, it appears that any pregnant woman with no recorded subsequent pregnancy was excluded (based on Tiblad et al.26) | |
| What is the predicted direction of bias due to missing data? | Unpredictable | ||
| Bias in measurement of outcomes | Was the outcome measure objective? | Yes | |
| Were outcome assessors unaware of the intervention received by study participants? | NI | No mention of blinding | |
| Were the methods of outcome assessment comparable across intervention groups? | Yes | ||
| Were any systematic errors in measurement of the outcome unrelated to intervention received? | PN | ||
| Risk-of-bias judgement | Low | ||
| What is the predicted direction of bias due to measurement of outcomes? | Towards null | ||
| Bias in selection of the reported result | Is the reported effect estimate unlikely to be selected, on the basis of the results, from: | ||
| multiple outcome measurements within the outcome domain? | PN | ||
| multiple analyses of the intervention–outcome relationship? | PN | ||
| different subgroups? | PN | ||
| Risk-of-bias judgement | Low | ||
| What is the predicted direction of bias due to selection of the reported result? | Towards null | ||
| Overall bias | Risk-of-bias judgement | Serious | Primarily because of risk of selection bias, confounding and missing data |
| What is the overall predicted direction of bias for this outcome? | Unpredictable | Unpredictable because of insufficient information. Note: the generalisability of the study findings to the UK is limited given that RAADP is recommended as part of routine care |
Appendix 8 Summary of anti-D reviews
| Review | Review details | Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Anti-D group | Control | Outcome | Anti-D group | Control group | RR | Lower CI | Upper CI | |
| McBain et al., 201564 | 2 | Anti-D after 28 weeks | No treatment (standard care) | Alloimmunisation in pregnancy or post partum | 5 | 13 | 0.42 | 0.15 | 1.17 |
| 2 | Alloimmunisation within one year | 6 | 16 | 0.39 | 0.10 | 1.62 | |||
| 1 | Positive Kleihauer at birth | 73 | 119 | 0.60 | 0.46 | 0.79 | |||
| 1 | Jaundice | 1 | 4 | 0.26 | 0.03 | 2.30 | |||
| Turner et al., 201263 | 10 | Anti-D (500 IU) 28–34 weeks | Standard postpartum or at sensitisation | Postpartum sensitisation | 0.31 | 0.17 | 0.56 | ||
| Pilgrim et al., 200962 | 8 (total) | Anti-D (various doses) 28–34 weeks | No antenatal anti-D | Sensitisation | |||||
| 4 | 500 IU | 0.30% | 0.89% | 0.33 | 0.20 | 0.55 | |||
| 3 | 1500 IU | 0.34% | 1.60% | 0.20 | 0.13 | 0.29 | |||
| 2 | 500 IU community | 0.35% | 0.95% | 0.37 | 0.21 | 0.65 | |||
| 1 | Compliance | 90% dose 1, 79% dose 2 | |||||||
| Fyfe et al., 201461 | 8 | Not described | None | Compliance | 80–90% | ||||
Appendix 9 Existing cost-effectiveness evidence: list of excluded papers
1. Bernhofen DM. The empirics of comparative advantage: overcoming the tyranny of nonrefutability. Rev Int Econ 2005;13:1017–23.
2. Druzic G. Bankarski sustav u RH. [Banking System in the Republic of Croatia. With English summary.] Zbornik Radova Ekonomskog Fakulteta u Rijeci: Casopis za Ekonomsku Teoriju i Praksu. J Econ Bus 2002;20:67–90.
3. Du Laney T, Dibner M, Moise K. Pharmacoeconomic analysis of prenatal determination of fetal RHD genotype through non-invasive maternal serum testing. Am J Obst Gynecol 2006;195:S119.
4. Duan Q, Liao TW. Optimization of blood supply chain with shortened shelf lives and ABO compatibility. Int J Prod Econ 2014;153:113–29.
5. Leistikow EA, Collin MF, Savastano GD, de Sierra TM, Leistikow BN. Wasted health care dollars. Routine cord blood type and Coombs’ testing. Arch Pediatr Adolesc Med 1995;149:1147–51.
6. Ma KK, Rodriguez MI, Cheng YW, Norton ME, Caughey AB. Should cell-free DNA testing be used to target antenatal rhesus immune globulin administration? J Matern Fetal Neonatal Med 2015;29:1866–70.
7. Moise KJ. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol 2013;122:1306. http://dx.doi.org/10.1097/AOG.0000000000000036
8. Roque H. Fetal RhD genotyping by maternal serum analysis: a two-year experience. Am J Obstet Gynecol 2006;194:905–6.
9. Szczepura A, Bonsel G, Krauth C, Osipenko L, Haverkamp A. Fetal RHD typing: Is fetal RHD typing in all RhD negative women cost effective? BMJ 2008;336:906. http://dx.doi.org/10.1136/bmj.39556.499549.80
10. van der Schoot CE, Soussan AA, Bonsel GJ, de Haas M. Non invasive screening for fetal RHD-genotype in all D-negative women is reliable and cost-effective. Blood 2005;106:165A.
Appendix 10 Previous National Institute for Health and Care Excellence technology appraisals
Two previous TAs were carried out on RAADP. The more recent appraisal (NICE TA156) concluded that, compared with having no RAADP, RAADP reduces the incidence of sensitisation and, consequently, of haemolytic disease of the newborn infant. The economic analysis undertaken suggested that RAADP given to all RhD-negative pregnant women was likely to be cost-effective at a threshold of around £30,000 per QALY gained (Table 43). The total cost of providing RAADP to RhD-negative multigravidae in England and Wales was estimated to be around £2M–2.6M per year (2008 values). Table 43 considers only results relating to the multigravidae option as, in the current work, we assume that anti-D immunoglobulin and high-throughput NIPT would be provided in all eligible pregnancy (women RhD-negative and not previously sensitised) and not restricted based on whether or not it was the woman’s first pregnancy.
| Strategies | Incremental cost (£) | Number of sensitisations avoided | Number of affected pregnancies avoided | Number of fetal losses avoided | Life-years gained | Incremental QALYs | Cost per sensitisation avoided (£) | Cost per affected pregnancy avoided (£) | Cost per fetal loss avoided (£) | Cost per life-year gained (£) | ICER, cost per QALY gained (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No RAADPa | 1,796,546 | 630.5 | 353.4 | 14.1 | 2,878,648 | 2,533,240 | – | – | – | – | – |
| 2 × 500 IU RAADP (multi) | 2,645,120 | 232.9 | 72.1 | 2.9 | 120.4 | 100.0 | 11,358 | 36,679 | 916,982 | 21, 977 | 26,455 |
| 1 × 1500 IU RAADP (multi) | 2,010,568 | 232.9 | 72.1 | 2.9 | 120.4 | 100.0 | 8634 | 27,880 | 697,002 | 16,705 | 20,108 |
An updated assessment of RAADP was done under the current assessment. The following amendments and updating were performed:
-
We made amendments to discount the total QALYs according to the timing of subsequent pregnancies and to retain a constant probability of RhD-positive fetus per pregnancy across the whole cohort of RhD-negative pregnant women.
-
We updated the model to the current price year and more recent NHS reference costs.
-
We updated the model to more recent population values, estimates of birth rates and sensitisation.
The previous model compared RAADP plus postpartum anti-D immunoglobulin with postpartum anti-D immunoglobulin only. Many elements that were common to both arms were omitted from the model but we are required to introduce them as they may be affected by the introduction of high-throughput NIPT. The following alterations to address the current decision problem were performed:
-
We included the costs relating to potentially sensitising events (including phlebotomy, FMH test and anti-D immunoglobulin treatment).
-
We included the costs relating to postpartum treatment (including cord serology, phlebotomy, FMH test and anti-D immunoglobulin treatment).
The routine anti-D immunoglobulin characterised in our model is determined by the results of the audit. We used actual rates of single- and two-dose regimen implementation to determine a weighted cost that is based on the lowest BNF price available. As a result of the amendments, the update and, most significantly, the introduction of additional doses of anti-D immunoglobulin for potentially sensitising events and post partum, the total costs in our updated model are significantly higher for every strategy but the QALYs are not markedly different (Table 44). The total cost of RAADP is estimated to be £16.7M and the total QALYs 2.4 million. The updated results are in line with the previous HTA showing that, under a probabilistic set up, RAADP has an ICER of £14,444 compared with no RAADP. This is lower than the previous estimate of £20,108, largely a result of the reduced unit cost of anti-D immunoglobulin based on updated BNF prices and the increased birth rate.
| Strategiesa | Incremental cost (£) | Number of sensitisations avoided | Number of affected pregnancies avoided | Number of fetal losses avoided | Life-years gained | Incremental QALYs | Cost per sensitisation avoided (£) | Cost per affected pregnancy avoided (£) | Cost per fetal loss avoided (£) | Cost per life-year gained (£) | ICER, cost per QALY gained (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deterministic results | |||||||||||
| No RAADPb | 12,412,184 | 356.8 | 202.8 | 10.14 | 2,764,972 | 2,433,227 | – | – | – | – | – |
| RAADP | 3,576,953 | 218.69 | 124.38 | 6.22 | 257.46 | 195.13 | 16,356 | 28,758 | 575,167 | 13,893 | 18,331 |
| Probabilistic results | |||||||||||
| No RAADPb | 13,203,011 | 406.29 | 249.07 | 12.47 | 2,764,874 | 2,432,875 | – | – | – | – | – |
| RAADP | 3,476,596 | 249.07 | 152.84 | 7.66 | 317.40 | 240.69 | £13,959 | 22,747 | 454,043 | 10,953 | 14,444 |
Glossary
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A theoretical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- False negative
- An incorrect negative test result – the number of diseased persons with a negative test result.
- False positive
- An incorrect positive test result – the number of non-diseased persons with a positive test result.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test for which performance is being evaluated.
- Markov model
- An analytic method particularly suited to modelling repeated events or the progression of a chronic disease over time.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Metaregression
- A statistical technique used to explore the relationship between study characteristics and study results.
- Opportunity costs
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity that result from varying the diagnostic threshold.
- Reference standard
- The best currently available diagnostic test against which the index test is compared.
- Sensitivity
- The proportion of people with the target disorder who have a positive test result.
- Specificity
- The proportion of people without the target disorder who have a negative test result.
- True negative
- A correct negative test result – the number of non-diseased persons with a negative test result.
- True positive
- A correct positive test result – the number of diseased persons with a positive test result.
List of abbreviations
- ACROBAT-NRSI
- A Cochrane Risk Of Bias Assessment Tool: for Non-Randomised Studies of Interventions
- BNF
- British National Formulary
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- DARE
- Database of Abstracts of Reviews of Effects
- DNA
- deoxyribonucleic acid
- FMH
- fetal–maternal haemorrhage
- FNR
- false-negative rate
- FPR
- false-positive rate
- HSROC
- hierarchical summary receiver operating characteristic
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IU
- international unit
- MeSH
- medical subject heading
- NHB
- net health benefit
- NHS EED
- NHS Economic Evaluations Database
- NICE
- National Institute for Health and Care Excellence
- NIPT
- non-invasive prenatal testing
- PCR
- polymerase chain reaction
- PP1
- postpartum scenario 1
- PP2
- postpartum scenario 2
- PP3
- postpartum scenario 3
- PP4
- postpartum scenario 4
- QALY
- quality-adjusted life-year
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies 2
- RAADP
- routine antenatal anti-D prophylaxis
- RhD
- rhesus blood group (D antigen)
- ROC
- receiver operating characteristic
- RR
- relative risk
- SA
- sensitivity analysis
- TA
- technology appraisal
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.