Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/104/21. The contractual start date was in September 2011. The draft report began editorial review in October 2015 and was accepted for publication in June 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Christine Roffe reports personal fees from Air Liquide outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Roffe et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Stroke is the third most common cause of death worldwide. 1 With approximately 110,000 strokes per annum in England, it accounts for 11% of deaths. 2 Stroke mortality is cited as 20–30% within 1 month in the 2007 National Stroke Audit report. 2 More recent data suggest lower rates: between 14% and 17% for the UK3 and 14.5% in the USA. 4 Improvements in processes of care have significantly contributed to this reduction. 4 However, half of all stroke survivors are left dependent on others for everyday activities,2 making stroke the largest cause of complex disability. 5 Care on specialist stroke units has been shown to reduce death and disability significantly. 6 It does, however, remain unclear which aspects of stroke care are crucial for improving outcome. Prevention and treatment of hypoxia could potentially be one of the reasons for better outcome with specialist stroke care.
Scientific background
Prevalence of hypoxia and effects on outcome
Mild hypoxia is common in stroke patients and may have significant adverse effects on the ischaemic brain. 7 Hypoxia with oxygen saturations falling below 92% has been observed in 24% of continuously monitored stroke patients within the first 24 hours of symptom onset. 8 While healthy adults with normal cerebral circulation can compensate for mild hypoxia by an increase in cerebral blood flow,9 this is not possible in the already ischaemic brain after stroke. 10–13 Hypoxaemia with oxygen saturations falling below 90% in the first few hours after hospital admission is associated with a doubling of mortality14 and a trebling of the rate of institutionalisation. 15 Patients on a stroke unit are more likely to receive oxygen than patients on a non-specialised general ward16 and less likely to be hypoxic. 17 An observational study of processes of care has shown that hypoxia increases the risk of an adverse outcome fivefold if only some of the hypoxic episodes are treated with oxygen, but has no adverse effect on outcome if all episodes of hypoxia are treated with oxygen. 15 Prophylactic oxygen treatment could prevent hypoxia and secondary neurological deterioration.
Potential adverse effects of oxygen treatment
However, oxygen treatment is not without side effects. 18 It impedes early mobilisation and could pose an infection risk. There is evidence from animal models and in vitro studies that oxygen encourages the formation of toxic free radicals, leading to further damage to the ischaemic brain,19–22 especially during reperfusion. Marked changes in adenosine triphosphate and related energy metabolites develop quickly in response to acute ischaemia and tissue hypoxia. These alterations are only partially reversed on reperfusion despite improved oxygen delivery. Ischaemia-induced decreases in the mitochondrial capacity for respiration result in reduced oxygen consumption and further increase free radical generation during reperfusion. 23 Oxidative stress has also been implicated in the activation of cell signalling pathways, which leads to apoptosis and neuronal cell death. 24,25 While much research points towards adverse effects of hyperoxia in the ischaemic brain, there is also evidence to support the notion that therapy-induced eubaric hyperoxia may be neuroprotective. 19,20,26,27 Routine oxygen supplementation (ROS) for acute myocardial infarction has been abandoned after a clinical trial showed no benefit and potential harm. 28
Randomised controlled trials of oxygen treatment after acute stroke
Evidence from randomised controlled trials of oxygen supplementation after acute stroke is conflicting and insufficient to guide clinical practice. A quasi-randomised study of oxygen supplementation for acute stroke by Rønning and Guldvog29 has shown that routine oxygen treatment in unselected stroke patients does not reduce morbidity and mortality. Subgroup analyses suggested that patients with severe strokes were more likely to benefit than those with mild strokes, but the study size was too small to define patients who are likely to derive benefit with certainty. 29 A very small (n = 16) study of high-flow oxygen treatment after acute stroke showed that cerebral blood volume and blood flow within ischaemic regions improved with hyperoxia. By 24 hours, magnetic resonance imaging of the brain showed reperfusion in 50% of hyperoxia-treated patients compared with 17% of control patients (p = 0.06) but no long-term clinical benefit at 3 months. 30 In the Stroke Oxygen Pilot Study (ISRCTN 12362720), the flow rate of oxygen was lower (2 or 3 l/minute dependent on baseline oxygen saturation) and treatment was continued for longer (72 hours). Neurological recovery at 1 week was better in the oxygen group than in the control group. 31 While there was no difference in the mRS at 6 months, there was a trend for better outcome with oxygen after adjustment for differences in baseline stroke severity and prognostic factors. 32 In contrast to the earlier study by Rønning and Guldvog,29 oxygen was as effective in mild as in severe strokes. These results are promising, but need to be confirmed in a larger study.
Recommendations for oxygen treatment in national and international clinical guidelines
Clinical guidelines for oxygen supplementation after stroke are not based on evidence from randomised clinical trials, and have changed over time without obvious reason. The European Stroke Organization (2008)33 stated that ROS to all stroke patients had not been shown to be effective, but that adequate oxygenation was important, and that oxygenation can be improved by giving oxygen at a rate of > 2 l/minute (no target saturation or supporting evidence given). In 2003, the American Stroke Association Guideline34 recommended keeping the oxygen saturation at or above 95%. The 2005 update25 of the guideline made no change to the recommendations. In 2007, the advice was revised to say that oxygen saturation should be maintained at or above 92%,35 but in 2013 the Association reverted to recommending maintenance of an oxygen saturation at or above 95%. 36 In the UK, the National Clinical Guideline for the management of people with stroke recommended keeping oxygen saturation within normal limits in 2005 and in 200837,38 and in 201239 it specified that this means maintaining an oxygen saturation ≥ 95%. None of the recommendations is based on evidence from controlled clinical trials. A survey of British stroke physicians showed that there is uncertainty among physicians treating patients with stroke about which treatment approach to take, and when to give oxygen. 40
Rationale for the Stroke Oxygen Study
Hypoxia is common after acute stroke and is associated with worse outcomes. Prevention of hypoxia could avert secondary brain damage and improve recovery. Evidence from randomised controlled trials is conflicting, and insufficient to guide clinical practice. This is reflected in clinical uncertainty and conflicting guidelines based on the same evidence. An adequately powered study of ROS is needed to provide reliable information on which recommendations can be based.
Aims
The aim of the Stroke Oxygen Study (SO2S) is, first, to establish whether or not ROS will improve outcome after stroke and, second, to determine whether or not oxygen given at night only is more effective than oxygen given continuously.
Rationale for the fixed dose oxygen regimen used in the Stroke Oxygen Study
A fixed dosage scheme was chosen to keep the design of the study as simple as possible, so that any recommendations resulting from the study outcome can be carried out in day-to-day clinical practice.
Rønning and Guldvog29 have shown that giving oxygen at a rate of 3 l/minute to all stroke patients during the first 24 hours after hospital admission does not improve overall outcome. They did not report baseline oxygen saturation, or changes in saturation on treatment. It is therefore possible that some patients were undertreated, and that others achieved too high oxygen levels, leading to an increase in free radical generation in the ischaemic penumbra. There were no other data from clinical studies to inform recommendations for the dose of oxygen to give for routine supplementation at the time the study was designed. The European Stroke Organization suggested a dose of 2–4 l/minute and the American Stroke Association Guideline recommended keeping the oxygen saturation at or above 95%,34,41,42 but these recommendations were not based on evidence from controlled clinical trials. In the absence of data to the contrary, it was reasonable to assume that treatment should restore oxygen saturation to the normal range.
Normal oxygen saturation in adults is 95–98.5%,43 in healthy older individuals it is reported to be lower, at 95% [standard deviation (SD) 2.5%]. 44 Oxygen saturation in stroke patients who are normoxic at recruitment is about 1% lower than that of age-matched community control patients. 45 We have conducted a dose titration study for oxygen after acute stroke and found that 2 l/minute oxygen by nasal cannula increases oxygen saturation by 2% and 3 l/minute by 3%. 46 We also found that oxygen masks were less likely to be tolerated than nasal cannulae, leading to poorer treatment compliance with the former. For this study, we therefore decided to give oxygen by nasal cannula. A dosage regimen of 3 l/minute in individuals with a baseline oxygen saturation of ≤ 93% and 2 l/minute in individuals with a baseline saturation > 93% was therefore considered likely to prevent hypoxia without increasing oxygen saturation beyond the upper limit of the normal range.
Rationale for giving routine oxygen supplementation at night only
Patients are more likely to be hypoxic at night
The mean nocturnal oxygen saturation is about 1% lower than awake oxygen saturation in both stroke patients and control patients. 45 This study has also shown that a quarter of patients who are normoxic in the day have significant hypoxia during the night. About 60–70% of stroke patients suffer from sleep apnoea early after stroke. 47–49
The development of hypoxia is more likely to be missed at night
It is more difficult to observe patients in the darkened room, and, unless there are reasons to suspect the patient is unwell, nurses do not usually wake the patient for routine observations. The development of hypoxia is therefore more likely to be missed at night.
Nocturnal hypoxaemia is more likely to lead to brain tissue hypoxia at night
A study in healthy volunteers has shown that hypoxaemia leads to a compensatory increase in cerebral blood flow during wakefulness, but not during sleep, and is therefore more likely to result in brain tissue hypoxia at night. 50
Nocturnal oxygen supplementation does not interfere with the patient’s daytime mobility
Early mobilisation is an important aspect determining good outcome. 16 Patients who are attached to monitoring equipment, or to oxygen supplementation, are less likely to be mobilised than patients not attached to such equipment.
Giving routine oxygen at night only may prevent a significant number of otherwise undetected episodes of hypoxia without interfering with the patient’s daytime rehabilitation.
Chapter 2 Methods
Trial design
The Stroke Oxygen Study is a multicentre, prospective, randomised, open, blinded-end point (PROBE) trial. For this controlled, single-blind, parallel group trial patients were randomised in a 1 : 1 : 1 ratio to one of three study arms:
-
continuous oxygen supplementation
-
nocturnal oxygen supplementation only
-
no ROS, unless required for clinical reasons other than stroke.
Both the Stroke Oxygen Study protocol and the statistical analysis plan have been published in an open-access journal. 51,52
Hypothesis
The main hypothesis was that fixed low-dose oxygen treatment during the first 3 days after an acute stroke improves outcome compared with no oxygen.
The secondary hypothesis was that restricting oxygen supplementation to night-time only is more effective than continuous supplementation.
Participants
Recruitment
Patients were recruited by research nurses and clinicians from 136 hospitals (secondary care) across England, Northern Ireland and Wales (see Appendix 1 for a list of recruiting centres). All recruiting hospitals had acute stroke units, were able to carry out the required observations four times a day and had a stroke-trained principal investigator. Screening, baseline, randomisation and 1-week follow-up data were collected and entered online into the trial database by the research staff based in the local hospital.
Inclusion criteria
-
Adult patients (aged ≥ 18 years) with clinical diagnosis of acute stroke.
-
Within 24 hours of hospital admission.
-
Within 48 hours of stroke onset.
Exclusion criteria
-
Definite indication or contraindication to oxygen treatment at a rate of 2–3 l/minute.
-
Stroke not the main clinical problem or patient has another serious life-threatening illness likely to lead to death within the next 12 months.
Consent
Fully informed consent was sought from all research participants. Research nurses or clinicians explained the study to potential participants, who were also given a patient information sheet explaining the trial. Owing to the acute nature of stroke and the intervention being tested, there was not a 24-hour consideration period between receiving the information and taking informed consent. In patients who were unable to give fully informed consent, assent was sought from either the patient’s next of kin or from an independent physician. Fully informed consent was obtained in patients who regained capacity during the first week following randomisation.
Interventions
Patients were randomised to one of the following three groups:
-
Continuous oxygen: oxygen via nasal cannula continuously (day and night) for 72 hours after randomisation. The flow rate was set at 3 l/minute if baseline oxygen saturation was ≤ 93% or 2 l/minute if baseline saturation > 93%.
-
Nocturnal oxygen: oxygen via nasal cannula overnight (21:00–07:00) for three consecutive nights. The flow rate was set at 3 l/minute if baseline oxygen saturation was ≤ 93% or 2 l/minute if baseline saturation > 93%.
-
Control group: no ROS during the first 72 hours after randomisation unless required for other clinical reasons.
The oxygen used for the trial was supplied by the hospital through either wall-mounted sockets or portable or stationary oxygen bottles, depending on local practice.
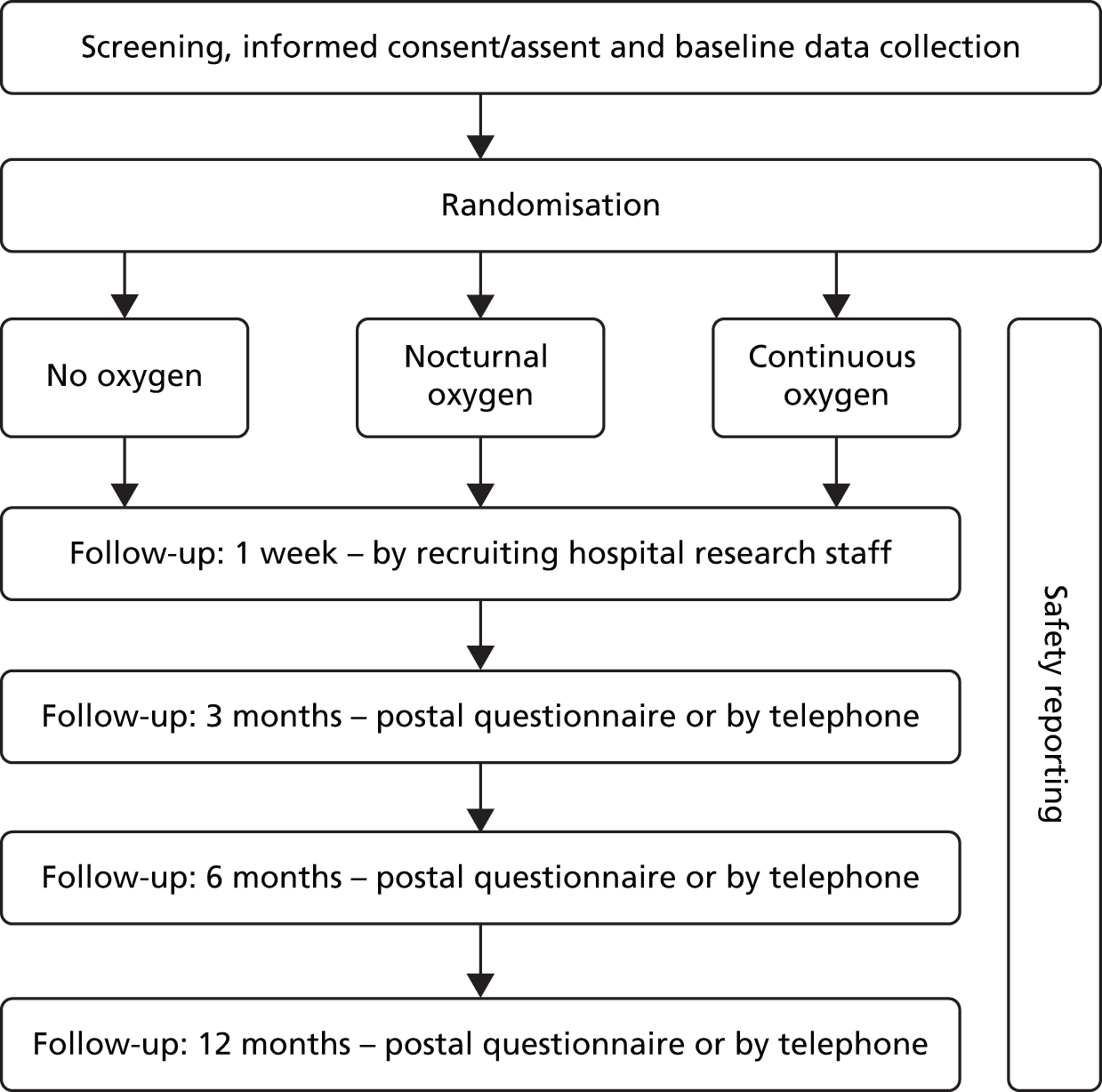
Follow-up
Patients were followed up at 1 week by research staff at the recruiting hospital, and then at 3, 6 and 12 months via a postal questionnaire from the trial co-ordinating centre (Figure 1). The case report form is presented in Appendix 2.
FIGURE 1.
Trial design.
Assessments
See Table 1 for a summary of patient assessments and timings.
| Outcome measure | Screening | Baseline | Week 1 | 3 months | 6 months | 12 months |
|---|---|---|---|---|---|---|
| Eligibility | ✓ | |||||
| Demographics | ✓ | |||||
| Glasgow Coma Scale | ✓ | |||||
| Medical history | ✓ | |||||
| Oxygen treatment prior to randomisation | ✓ | |||||
| Prognostic factors (SSV) | ✓ | |||||
| NIHSS (neurological function) | ✓ | ✓ | ||||
| Antibiotics, antipsychotics and sedatives during week 1 | ✓ | |||||
| Highest blood pressure and highest heart rate during first 72 hours | ✓ | |||||
| Oxygen saturation during first 72 hours | ✓ | |||||
| Compliance with oxygen/control treatment | ✓ | |||||
| CT/MRI diagnosis | ✓ | |||||
| Final diagnosis | ✓ | |||||
| Date of discharge (when appropriate) | ✓ | ✓ | ✓ | ✓ | ||
| Discharge locationa | ✓ | |||||
| TOAST classification | ✓ | |||||
| Modified Rankin Scale (disability) | ✓b | ✓ | ✓ | ✓ | ||
| Adverse events | ✓ | ✓ | ✓ | ✓ | ||
| Living arrangements | ✓ | ✓ | ✓ | |||
| Hospital readmissions | ✓ | ✓ | ✓ | |||
| Barthel Index (activities of daily living) | ✓ | ✓ | ✓ | |||
| EQ-5D-3L (quality of life) | ✓ | ✓ | ✓ | |||
| NEADL | ✓ | ✓ | ✓ | |||
| Patient-reported outcome measures (memory, sleep, eyesight and speech) | ✓ | ✓ | ✓ | |||
| Participants’ awareness of trial allocation | ✓ | ✓ | ✓ | |||
| Who completed the follow-up questionnaire | ✓ | ✓ | ✓ | |||
| Co-recruitment with other trials | ✓ | ✓ | ✓ | ✓ |
Baseline assessment
This was done by the research team randomising the patient, and was either entered online for patients randomised via the web or sent to the trial centre by fax for patients randomised via telephone. The initial assessment included baseline demographics, date and time of the event, whether or not the patient had been given oxygen in the ambulance or in the emergency department, and how much, comorbidities [chronic obstructive pulmonary disease, other chronic lung problems, heart failure (congestive cardiac failure), ischaemic heart disease, and atrial fibrillation], the Glasgow Coma Scale (GCS) score,53 score on the Six Simple Variables (SSV) outcome predictor tool,54 the National Institutes of Health Stroke Scale (NIHSS) score,55,56 the type of consent (by patient or legal representative) and the date and time of randomisation.
Week 1 assessment
The week 1 assessment was performed by a member of the local research team trained in the assessment tools at 7 days (± 1 day) after enrolment. In patients who were discharged before the end of 1 week, or who could not be followed at 7 days, the assessment was conducted at discharge. Data were entered online or sent to the trial centre via fax. The week 1 assessment included neurological function (NIHSS), vital status, adverse events, whether or not the patient was prescribed oxygen for clinical indications during the first 72 hours, information on compliance with the treatment, details of other treatments (antibiotics during week 1, thrombolysis, sedatives or antipsychotics), physiological variables (highest heart rate, highest systolic and diastolic blood pressure, highest and lowest oxygen saturation during the first 72 hours, and the highest temperature during week 1), the result of the computerised tomography (CT) or magnetic resonance imaging head scan (cerebral infarct/primary intracerebral haemorrhage/subdural haemorrhage/brain tumour/head scan not performed/other), the final diagnosis [ischaemic stroke/transient ischaemic attack (TIA)/primary intracerebral haemorrhage/cerebrovascular accident (CVA) without CT confirmation of aetiology/other], and the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification of stroke aetiology. 57 Compliance was assessed by asking whether or not oxygen was prescribed on the drug chart, whether or not it was signed, and whether or not it was stopped before the end of 72 hours. After an amendment of the case report form [version 1, amendment 3 (30 August 2009)], additional details were recorded for the final 4143 patients. These included a more in-depth assessment of compliance with a record of oxygen saturation at 06:00, 12:00 and 00:00, whether or not oxygen treatment was in place at 06:00 and 00:00; whether or not the patient had been enrolled into another study, and which, the pre-stroke modified Rankin Scale (mRS) and the European Quality of Life-5 Dimensions, three levels (EQ-5D-3L), whether or not this was reported by the patient or a relative, discharge destination (if discharged), and whether or not a new brain haemorrhage was identified on a second CT of the head (if conducted).
Three-, 6- and 12-month assessments
The 3-, 6- and 12-month follow-up assessments were performed centrally by the Stroke Oxygen Study team. Following a call to the participant’s general practitioner to confirm that the participant was alive and the contact details were the same as those on the trial database, postal questionnaires were sent to all participants at 3, 6 and 12 months post randomisation. The questionnaires contained the discharge date, the mRS,58 the Barthel Index (BI),59 the EQ-5D-3L and the EQ-VAS,60–62 the Nottingham Extended Activities of Daily Living scale (NEADL),63 questions regarding current abode, whether or not the patient had been readmitted to hospital since the stroke, patient-reported outcome measures (sleep, speech and memory), and a question on whether or not they remembered which treatment they were randomised to. If the questionnaire was not returned within a few weeks, a data assistant at the trial co-ordinating centre telephoned the patient and completed the questionnaire over the telephone with either the patient or a relative/carer. See Table 1 for a summary of all patient assessments and timings.
Outcomes
Primary outcome
The primary outcome measure was disability assessed by the mRS at 90 days post randomisation. 58,64 The mRS is an ordinal scale that ranges from 0 for a patient with no disability to 5 for extreme disability. Death was included in the scale as a score of 6.
Secondary outcome measures at 1 week
The number of patients with neurological improvement (≥ 4-point decrease from baseline in the NIHSS score or a value of 0 at day 7),55,56 NIHSS score, mortality, the lowest and highest oxygen saturation during the 72-hour treatment period and the number of patients whose oxygen saturation fell below 90% were all secondary outcome measures.
Secondary outcome measures at 3, 6 and 12 months
Mortality, the number of patients alive and independent (mRS score of ≤ 2), the number of patients living at home, the BI of activities of daily living, quality of life EQ-5D-3L and European Quality of Life Visual Analogue Scale (EQ-VAS) and the NEADL index were all secondary outcome measures for 3-, 6- and 12-month time points.
Sample size
The original sample size calculation of 6000 patients was based on a mean mRS score of 3.51 (SD 2.03). These values came from the first 200 patients in the Stroke Oxygen Pilot Study. 32 A 5% dropout rate was assumed, along with 5% missing outcome data, giving a total of 10% lost to follow-up. The sample size of 6000 patients provided 90% power to detect small (0.2 mRS point) differences between ROS (continuous and nocturnal groups combined) and no oxygen (control group) at a p-value of ≤ 0.01 and 90% power at a p-value of ≤ 0.05 to detect small (0.2 mRS point) differences between continuous and nocturnal oxygen supplementation.
The sample size calculation was revised in October 2012 without any knowledge of interim results. Recalculation was conducted using ordinal methods to match with the Statistical Analysis Plan. The study size was subsequently consequently revised to 8000 patients. Protocol version 2 amendment 4 (18 October 2012) gave greater power to investigate any differential effectiveness of oxygen compared with control within subgroups, in particular those with more severe disease.
Randomisation and allocation
Patients were randomised via a computer-generated web-based randomisation system at the Birmingham Clinical Trials Unit. Randomisation was performed using minimisation with the following factors: the SSV prognostic index for independent survival at 6 months (cut-off points ≤ 0.1, > 0.1 to ≤ 0.35, > 0.35 to ≤ 0.70 and > 0.70), oxygen treatment before randomisation (yes, no and unknown), baseline oxygen saturation on air (< 95% and ≥ 95%), and time since stroke onset (≤ 3 hours, > 3 to ≤ 6 hours, > 6 to ≤ 12 hours, > 12 to ≤ 24 hours and > 24 hours). Study centre was not included as a minimisation variable to avoid potentially high rates of allocation prediction and selection bias. Patients were randomised via a web-based randomisation program at the level of the individual on a 1 : 1 : 1 basis to either no oxygen, nocturnal oxygen or continuous oxygen. Enrolment and intervention assignment was performed by the clinical team at the recruiting centre.
Blinding
This study was open, as placebo treatment (room air) would have similar side effects as the active treatment (e.g. infection and immobilisation), but no potential benefit, and could thus bias the data in favour of the treatment group. The main outcomes were ascertained at 3 months by central follow-up, which ensured that the assessor was blind to the intervention. Assessment was by postal questionnaire, or, when participants did not respond to the letters, by telephone interview. It is possible that the patients completing the follow-up questionnaire or responding to the interview questions may have had some recollection of being treated with oxygen or not. Patients were asked to state on the questionnaire if they remembered/could guess which treatment group they were in. This was compared with the actual allocation to quantify potential bias.
Statistical methods
The analysis is by intention to treat. The primary outcome measure is disability (mRS) at 90 days after randomisation. The mRS is an ordinal scale ranging from 0 (no disability) to 5 (extreme disability). Patients who were classified as dead at the 3 months were allocated a mRS score of 6, thus creating a 0 to 6 scale.
The mRS was analysed using an ordinal logistic regression model. Both an unadjusted (primary) and adjusted (secondary) analysis were performed. For each outcome variable, the unadjusted analysis is the primary analysis and the covariate-adjusted analysis is the secondary analysis. Adjusted analyses incorporated the following covariates: age, sex, baseline NIHSS score, baseline oxygen saturation and the SSV prognostic index for 6-month independence. For analysis of mortality data, the prognostic index for 30-day survival was used in place of that for 6-month independence.
Participants who died before the assessment point did not have data for NIHSS, BI, EQ-5D and EQ-VAS, or NEADL. To avoid bias in favour of the treatment arm with higher mortality (should this be the case), death was included in the analysis of the NIHSS, BI, EQ-VAS and the NEADL as the worst outcome on the scale. 65
For continuous outcomes means and SDs or medians and interquartile ranges (IQRs) are reported, as appropriate. Unadjusted analyses used an unrelated t-test, with the mean difference between treatments and corresponding confidence interval (CI) reported. In the event of major deviations from the assumptions of the t-test, an appropriate alternative analysis was used. The adjusted analysis used analysis of covariance methods, with the covariates specified earlier included in the analysis.
For dichotomous outcomes, percentages were compared across the treatment comparisons using a chi-squared test (unadjusted analysis). The adjusted analysis of dichotomous outcomes used binary logistic regression with the covariates listed earlier. Odds ratios (ORs) and CIs are reported. The number needed to be treated was to be calculated, if significant effects were to be seen. 66 As there were no differences, this was not done.
For ordinal secondary outcomes, the analyses described for the mRS were applied.
Data at 6 and 12 months
The longer-term follow-up data at 6 and 12 months were analysed at each time point using the same methods as those listed previously. In addition, analyses were performed across 3-, 6- and 12-month time points using a longitudinal repeated measures analysis, in this case linear mixed models. 67
The treatment effect was initially assumed to be constant over time; further analyses were carried out to investigate the effects of including time and a treatment-by-time interaction in the models.
Mortality was analysed using log-rank methods (unadjusted analysis) with Kaplan–Meier plots. The adjusted analysis used Cox regression methods, including the covariates listed above. In the covariates, the prognostic index for 30-day survival replaced that for independence at 6 months. The proportional hazards assumption associated with the Cox regression was tested via Schoenfeld residuals. Hazard ratios and 95% CIs are reported for both the unadjusted and adjusted analyses.
Planned subgroup analyses
These were performed in respect of the primary outcome measure only, based on a risk-stratification approach. 68 The subgroups comprise:
-
NIHSS score at baseline as indicator of stroke severity (0–4, 5–9, 10–14, 15–20 and > 20)
-
baseline % oxygen (O2) saturation (< 94%, 94–94.9%, 95–97% and > 97%)
-
treatment with O2 prior to randomisation (yes/no)
-
time in hours since onset of stroke (< 4 hours, 4 to < 7 hours, 7 to < 13 hours, 13 to < 24 hours and ≥ 24 hours)
-
final diagnosis (haemorrhage, infarct, TIA and other)
-
TOAST classification of infarct aetiology
-
GCS score (motor plus eye score:< 10 and 10)
-
age (< 50 years, 50–80 years and > 80 years)
-
history of chronic obstructive airway disease or asthma (yes/no)
-
history of heart failure (yes/no)
-
thrombolysed (yes/no)
-
baseline SSV risk score for independence at 6 months (≤ 0.1, > 0.1 to 0.35, > 0.35 to 0.7 and > 0.7).
These subgroup effects were analysed by means of an interaction term;69 however, pairwise hypothesis tests between the levels of the subgroup factor were not performed owing to the likely low level of statistical power. Subgroup-specific estimates are reported descriptively with 99% CIs and displayed graphically on a forest plot.
Health economics methods
Overview
A within-trial economic evaluation was conducted alongside the clinical trial in order to estimate the cost-effectiveness and cost–utility of ROS compared with no oxygen supplementation (no ROS) after stroke, over 12 months’ follow-up. Further analysis compared all three trial arms: (1) no ROS, (2) nocturnal oxygen for three nights, and (3) 72 hours’ continuous oxygen. The base-case economic evaluation adopted an NHS/Personal Social Services perspective. The cost-effectiveness analysis calculated the cost per additional day of home time gained, using information on length of stay in hospital and discharge destination. The cost–utility analysis (CUA) used quality-adjusted life-years (QALYs) as the benefit measure, in which QALYs take into account the survival and quality of life of an individual. The reporting of this analysis follows the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 70
Health outcomes
Information on length of stay in hospital due to stroke, discharge destination and any readmissions to hospital were used to calculate the number of home time days per patient over a 12-month period. Home time has been previously used as an outcome measure in other stroke trials. 71,72 When data on actual discharge location were not available, the response to a question regarding place of residence at 3 months was used as a proxy for the discharge location. Discharge to institutional care (nursing home or residential care) was not counted as home time. The actual length of stay for readmissions was not available for all patients in the trial; therefore, a sample of 100 readmissions was analysed to determine the mean length of stay for a readmission. This value was attached to all patients who had a readmission, and used in the calculation of home time gained. Although the intention was to calculate home time gained in the first 90 days, as there were poor data on the date of readmission, home time gained was calculated over the full 12 months.
The EQ-5D-3L questionnaire73 was completed at 3, 6 and 12 months, and when a patient had died during the 12 months, the date of death was noted and a value of zero (equivalent to dead) was assumed from the date of death. EQ-5D-3L data were not collected at baseline, as it was not possible for a patient admitted for a stroke to fill in a health-related quality of life questionnaire. Therefore, we used a previously published method74 and assumed that the EQ-5D-3L score for all patients at baseline was zero, and the change in quality of life between baseline and 3 months was linear. An alternative method for baseline EQ-5D-3L score was used in a sensitivity analysis by assuming that the baseline quality of life was equal to the EQ-5D-3L value at 3 months. Quality of life estimates were derived from EQ-5D-3L responses provided by patients at each time point by applying the standard UK tariff values. 75 These estimates were then used to calculate total QALYs over 12 months for every individual in the study, using the area under the curve approach.
Resource use and costs
The costs included in the analysis related to oxygen administration as prescribed by the trial, additional oxygen required for other clinical reasons, length of stay in hospital, readmission to hospital and long-term care on discharge from hospital. All costs in the analysis were in UK pounds (£), based on a price year of 2013–14. Unit costs were obtained from published standard sources of costs for NHS procedures,76 staff costs77 and previously published research (Table 2). Health and Community Health Services pay and price indices were used to inflate costs, when appropriate. 77 Unit costs are listed in Table 2.
| Health-care resource | Unit cost (£) | Source |
|---|---|---|
| Oxygen supplementation | ||
| Staff costs (per hour) | ||
| Sister/charge nurse | 51 | Curtis, 201477 |
| Registered nurse | 34 | |
| Student/research nurse | 21 | |
| Registrar | 40 | |
| Consultant | 101 | |
| Physiotherapist | 33 | |
| Allied professional | 23 | |
| Housekeeping | 26 | |
| Equipment cost (per item) | ||
| Oxygen tubing | 6.35 | University Hospitals of North Midlands NHS Trust supplies document, 201578 |
| Portable oxygen cylinder | 20.52 | |
| Nasal tubes | 4.85 | |
| Oxygen mask | 4.79 | |
| Stroke inpatient stay | ||
| Non-elective stay (10 days) | 4171 | NHS Reference Costs 2013–14 76 |
| Excess bed-day | 275 | |
| TIA/mimic inpatient stay | ||
| Non-elective stay (4 days) | 1775 | NHS Reference Costs 2013–14 76 |
| Excess bed-day | 235 | |
| Readmission | 2837 | NHS Reference Costs 2013–14 76 |
| Long-term carea | ||
| Stroke care after discharge for an independent patient (annual cost) | 1412 | Sandercock et al., 200279 |
| Stroke care after discharge for a dependent patient | 18,578 | |
For the purposes of estimating the cost of oxygen administration as prescribed in the trial, it was assumed that, once a patient was allocated to a treatment arm, costs of treatment were incurred. The cost of oxygen administration was adjusted for those patients for whom continuous oxygen was prescribed for clinical reasons outside the trial treatment, as described in Table 3. Information on resources required for oxygen administration, including any additional staff time and equipment required for each treatment group, was collected prospectively during the trial using a short questionnaire filled in by a representative in 24 participating hospitals (see Appendix 3). The cost of each resource type was calculated in order to determine the cost of oxygen supplementation per trial arm (Table 4). All institutions included in the trial were assumed to have the same expertise and to have followed similar protocols in the management of patients.
| No oxygen | Nocturnal oxygen | Continuous oxygen |
|---|---|---|
| Add 3 days of continuous oxygen | Add 1.5 days of continuous oxygen | Double the cost for the amount of oxygen given |
| Resource use and cost per patient (UK £, 2013/14) | Value (£) | Source |
|---|---|---|
| Nocturnal ROS | ||
| Nursea | 23.6 | SO2S trial data (Curtis, 201477) |
| Allied professionalsb | 23.0 | SO2S trial data (Curtis, 201477) |
| Doctorc | 1.9 | SO2S trial data (Curtis, 201477) |
| Physiotherapist | 1.5 | SO2S trial data (Curtis, 201477) |
| Housekeeping | 0.1 | SO2S trial data (Curtis, 201477) |
| Oxygen tubing | 4.4 | SO2S trial data |
| Portable oxygen cylinder | 2.7 | SO2S trial data |
| Nasal tubes | 6.5 | SO2S trial data |
| Oxygen mask | 0.6 | SO2S trial data |
| Total cost per patient | 64.0 | |
| Continuous ROS | ||
| Nursea | 32.1 | SO2S trial data (Curtis, 201477) |
| Allied professionalsb | 29.0 | SO2S trial data (Curtis, 201477) |
| Doctorc | 1.8 | SO2S trial data (Curtis, 201477) |
| Physiotherapist | 2.2 | SO2S trial data (Curtis, 201477) |
| Housekeeping | 0.1 | SO2S trial data (Curtis, 201477) |
| Oxygen tubing | 4.4 | SO2S trial data |
| Portable oxygen cylinder | 5.6 | SO2S trial data |
| Nasal tubes | 6.7 | SO2S trial data |
| Oxygen mask | 0.6 | SO2S trial data |
| Total cost per patient | 83.0 | |
Patients in the trial had a stroke, TIA or a stroke mimic. For a stroke, a NHS reference cost for a CVA was assumed, as the majority of strokes were ischaemic. As there were five different categories for CVA, taking into account the number of complications and comorbidities, the median was calculated for the cost of a non-elective long stay, cost of an excess bed-day and average length of stay. These values were then adjusted for the length of stay in hospital for each patient, either adding or subtracting a bed-day cost for length of stay under or over the median length of stay. For patients who had a TIA or stroke mimic, the NHS reference cost for a TIA was assumed, using the same methodology as for CVA to adjust for length of stay. As full data on the details and length of stay of any non-elective readmissions during the 12 months were not available, the overall average cost of a non-elective admission (for all categories) was used.
Patient-level resource use on long-term care beyond the initial hospital admission was unavailable; however, the responses to the mRS provided information on the level of dependence. Patients were categorised as independent (mRS score of 0–2) or dependent (mRS score of 3–5) using data from the 3-month questionnaire. The annual cost of independent and dependent stroke after discharge from hospital was obtained from Sandercock et al. 79 and updated to 2013/14 costs. This annual cost was then adjusted to take into account the time not in hospital over the 12-month period.
For the purpose of the analysis, the costs of acute stay in hospital and long-term care were not included in the cost-effectiveness analysis to avoid double counting, as the measure of outcome was home time gained.
Analysis
The EQ-5D-3L and mRS data were not available for all randomised patients, and therefore, multiple imputation (MI) was used. MI is a statistical technique that retains overall population variability and the relationship between observations, and is considered useful when > 10% of data are missing. As > 10% of the data were missing within the trial, these were treated as missing at random and estimated using the Markov chain Monte Carlo MI method. Imputation of missing EQ-5D-3L scores used methods proposed by Simons et al. ,80 in which the whole index score was imputed. The percentage of missing EQ-5D-3L data at 12 months was 18%; therefore, 25 simulated, complete versions of the data set were produced using Stata 12.1 software (StataCorp LP, College Station, TX, USA). The results of each of the simulated data sets were combined to produce estimates and CIs to incorporate missing data uncertainty. MI was carried out at all time points. Appendix 4 contains further detail on the imputation methods.
As the majority of cost and outcome data are usually skewed, normal parametric methods are not appropriate for calculation of the differences in means. Bootstrapping is a non-parametric approach that can be used to compare arithmetic means without making any assumptions regarding the sampling distribution. In this analysis, 3000 bootstrapping replications were undertaken in order to calculate the 95% CIs around the differences in mean costs and outcomes.
The incremental cost-effectiveness analysis was carried out at 12 months, based on the outcome of home time gained, and the incremental cost-effectiveness ratio (ICER) was expressed in terms of the cost per 1 additional day of home time gained at 12 months. A CUA was also carried out at 12 months and the ICERs were expressed as cost per additional QALY gained. The presentation of results in QALYs allows comparison of the results with other available published studies. The analysis was conducted according to the intention-to-treat principle, in line with the main trial analysis, and discounting was not applied as the duration of follow-up was only 1 year. First, an analysis of ROS compared with no ROS was conducted by including all patients in the nocturnal and continuous ROS arms in the overall ROS comparator. A subsequent analysis considered all three trial arms, using the principle of dominance: that is, if one of the trial arms was shown to be both more costly and less effective than at least one of the alternative interventions, then that option would be seen as dominated and excluded from remainder of the analysis.
A range of one-way deterministic sensitivity analyses were carried out to explore the robustness of the base-case cost–utility results for ROS compared with no ROS, and to assess the uncertainty associated with input parameters. The following sensitivity analyses were undertaken:
-
changing the costs of oxygen treatment (increasing/reducing the costs by 20%)
-
changing the acute inpatient costs of stroke (reducing/increasing the costs by 20%)
-
changing the costs of stroke care after discharge (reducing/increasing the costs by 20%)
-
assuming that quality of life changes between base-case and 3 months took place immediately, therefore allocating the 3 month EQ-5D-3L value to baseline.
In addition, a probabilistic sensitivity analysis of the base-case analysis (cost-effectiveness analysis and CUA) was carried out to enable the simultaneous exploration of uncertainty in the cost and outcome data, using 5000 simulations. The results of the probabilistic sensitivity analysis are presented using cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs). The CEAC graphically represents the probability that an intervention is cost-effective at different ICER thresholds.
All analyses were carried out using Stata version 12.1 and Microsoft Excel® 2007 (Microsoft Corporation, Redmond, WA, USA).
Patient and public involvement
We conducted focus group meetings with stroke survivors when preparing the protocol for SO2S (see Ali et al. 46 for detail). Stroke survivors and their carers considered the study important. They considered the outcomes relevant, but suggested others that were not adequately covered by the formal assessment tools. These included memory, speech problems and sleep. We designed questions to specifically address these points and included them in the assessments at 3, 6 and 12 months. We also discussed consent issues, as many stroke patients are unable to give fully informed consent soon after the stroke, because of the nature of their brain injury. Some of the stroke patients were concerned that asking relatives to provide consent on behalf of the patients would put them under too much stress at a time when they were anxious and worried. We explained that there was an option of allowing an independent physician to consent on behalf of the patient. We included patient and carer representatives as collaborators and as members of the trial management group. Over the time of the study the initial collaborators (Linda and Peter Handy) became unable to contribute further for health reasons. Towards the end of the study Norman Phillips and Brin Helliwell provided advice to the trial management group and input into the report.
Ethical approval
The protocol was approved by the North Staffordshire Ethics Committee (06/2604/109) on 24 January 2007. The protocol can be accessed at http://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-15-99 (accessed 1 June 2016).
Clinical trials registration
European Union Drug Regulating Authorities Clinical Trials (EudraCT) number 2006-003479-11 and Current Controlled Trials International Standard Randomised Controlled Trial Number (ISRCTN) 52416964.
Chapter 3 Results
Recruitment
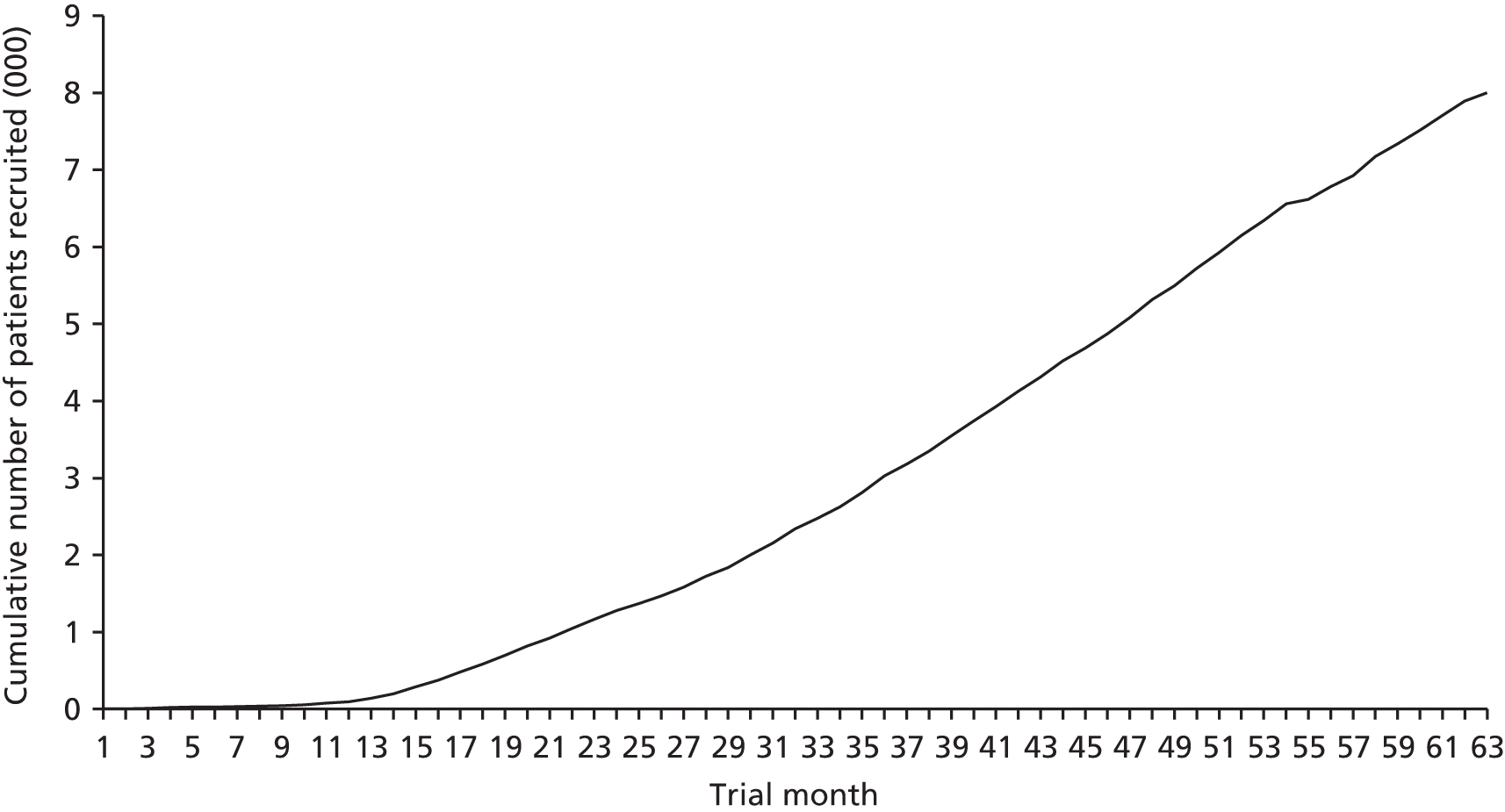
In total 8003 patients were recruited between 24 April 2008 and 17 June 2013 (Figure 2). Participants were randomised to the three groups: 2668 to the control group, 2668 to the continuous oxygen group and 2667 to the nocturnal oxygen group. All follow-up assessments were completed by December 2014.
FIGURE 2.
Timeline of patients recruited to SO2S over a 5-year period.
Consent and patient flow through the study
Fully informed consent was given by 6991 (87%) of patients and assent was given by a relative, carer or independent legal representative for 1012 (13%) patients (Table 5). At the 7-day review, six (0.7%) assented patients refused consent and were withdrawn from the study. A further 22 assented patients were withdrawn between days 7 and 90, six between days 90 and 180, and two between day 180 and the end of the study. Of those patients who gave informed consent, 40 were withdrawn by day 7, 114 were withdrawn from the study between day 7 and day 90 post randomisation, 37 were withdrawn between day 90 and 180, and 30 withdrew between days 180 and 365 (Figure 3).
| Variable | Trial arm | ||
|---|---|---|---|
| Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | |
| Consent given by the patient, n (%) | 2329 (87) | 2340 (88) | 2322 (87) |
| Consent given by a relative, carer or independent legal representative, n (%) | 339 (13) | 327 (12) | 346 (13) |
| Withdrawal from trial by 7 days, n (%) | |||
| Total | 16 (0.6) | 20 (0.7) | 4 (0.15) |
| Patients who gave initial consent themselves | 14 (0.5) | 17 (0.6) | 3 (0.11) |
| Patients included by a legal representative | 2 (0.1) | 3 (0.1) | 1 (0.04) |
| Withdrawal from trial by 90 days, n (%) | |||
| Total | 56 (2.1) | 63 (2.4) | 57 (2.1) |
| Patients who gave initial consent themselves | 48 (1.8) | 56 (2.1) | 44 (1.6) |
| Patients included by a legal representative | 8 (0.3) | 7 (0.3) | 13 (0.5) |
| Withdrawal from trial by 180 days, n (%) | |||
| Total | 77 (2.9) | 72 (2.7) | 70 (2.6) |
| Patients who gave initial consent themselves | 67 (2.5) | 63 (2.4) | 55 (2.1) |
| Patients included by a legal representative | 10 (0.4) | 9 (0.3) | 15 (0.6) |
| Withdrawal from trial by 365 days, n (%) | |||
| Total | 89 (3.3) | 81 (3.0) | 81 (3.0) |
| Patients who gave initial consent themselves | 78 (2.9) | 71 (2.7) | 66 (2.5) |
| Patients included by a legal representative | 11 (0.4) | 10 (0.4) | 15 (0.6) |
FIGURE 3.
Consolidated Standards of Reporting Trials (CONSORT) flow chart for SO2S.

Baseline data
Patient age and sex distribution were similar in all three groups. Overall mean age was 72 years (SD 13 years) and in total 4398 (55%) patients were male. Prognostic factors, including independence in activities of daily living before the stroke (n = 7332, 92%), were also well matched across the trial arms. There was little to no difference in the participants’ medical history, with ischaemic heart disease (n = 1602, 20%), heart failure (n = 657, 8%), atrial fibrillation (n = 1995, 25%) and chronic lung conditions (n = 812, 10%) recorded in each group. Patients were enrolled relatively late at a median 20:43 (IQR 11:59–25:32) hours:minutes after symptom onset. The majority of patients had a final diagnosis of ischaemic stroke (n = 6555, 82%) recorded at the 7-day review. Primary intracerebral haemorrhage was diagnosed in 588 (7%) patients, TIA in 168 (2%) and non-stroke conditions in 292 (4%); this information was missing for 106 patients (1%). The median GCS score was 15 (IQR 15–15) and mean and median NIHSS scores were 7 and 5, respectively (range 0–34); again these were well balanced across the trial arms. Twenty per cent of patients received oxygen prior to randomisation and the mean oxygen saturation was 96.6% in the continuous (SD 1.7) and nocturnal oxygen (SD 1.6) treatment groups, and 96.7% (SD 1.7) in the control group (Table 6).
| Variable | Trial arm | ||
|---|---|---|---|
| Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | |
| Demographic characteristics | |||
| Age (years) | |||
| Mean (SD)a | 72 (13) | 72 (13) | 72 (13) |
| Median (IQR) | 74 (64–82) | 75 (65–82) | 74 (64–82) |
| Male sex; n (%)a | 1466 (55) | 1466 (55) | 1466 (55) |
| Prognostic factors | |||
| Living alone, n (%)a | 861 (32) | 857 (32) | 907 (34) |
| Independent in basic activities of daily living, n (%)a | 2451 (92) | 2431 (91) | 2450 (92) |
| Normal verbal response, n (%)a | 2190 (82) | 2207 (83) | 2196 (82) |
| Able to lift affected arm, n (%)a | 1998 (75) | 2022 (76) | 1996 (75) |
| Able to walk, n (%)a | 660 (25) | 704 (26) | 677 (25) |
| Probability of 30-day survival, median (IQR) | 0.92 (0.86–0.95) | 0.92 (0.86–0.95) | 0.92 (0.86–0.95) |
| Probability of being alive and independent at 6 months, median (IQR)a | 0.44 (0.12–0.71) | 0.42 (0.12–0.71) | 0.42 (0.12–0.71) |
| Blood glucose (mmol/l), mean (SD) | 7.1 (2.5) | 7.0 (2.4) | 7.1 (2.5) |
| Concomitant medical problems | |||
| Ischaemic heart disease, n (%) | 573 (21) | 515 (19) | 514 (19) |
| Heart failure, n (%) | 224 (8) | 217 (8) | 216 (8) |
| Atrial fibrillation, n (%) | 638 (24) | 673 (25) | 684 (26) |
| Chronic obstructive pulmonary disease/asthma, n (%) | 253 (9) | 242 (9) | 245 (9) |
| Other chronic lung problem, n (%) | 29 (1) | 24 (1) | 19 (1) |
| Details of the qualifying event | |||
| Time since symptom onset (hh:mm), mean (IQR)b | 20:44 (11:53–25:33) | 20:32 (12:05–25:31) | 20:45 (11:57–25:31) |
| Ischaemic stroke, n (%)b | 2187 (82.0) | 2165 (81.1) | 2203 (82.6) |
| Intracranial haemorrhage, n (%)b | 185 (6.9) | 207 (7.8) | 196 (7.3) |
| TIA, n (%)b | 52 (1.9) | 50 (1.9) | 66 (2.5) |
| Stroke without imaging diagnosis, n (%)b | 104 (3.9) | 106 (4.0) | 84 (3.1) |
| Not a stroke, n (%)b | 101 (3.8) | 98 (3.7) | 93 (3.5) |
| Missing, n (%)b | 39 (1.5) | 41 (1.5) | 26 (1.0) |
| GCS score (3–15), median (IQR) | 15 (15–15) | 15 (15–15) | 15 (15–15) |
| Thrombolysed, n (%)b | 447 (17) | 410 (15) | 447 (17) |
| NIHSS score (0–42), median (IQR) | 5 (3–9) | 5 (3–9) | 5 (3–9) |
| Oxygenation | |||
| Oxygen given prior to randomisation (yes), n (%)a | 531 (20) | 531 (20) | 539 (20) |
| Oxygen saturation on room air (%), mean (SD)a | 96.6 (1.7) | 96.6 (1.6) | 96.7 (1.7) |
Treatment adherence
Treatment adherence in the two intervention arms was similar, with 2158 (81%) patients completing the 72 hours of continuous oxygen therapy and 2225 (83%) patients receiving the nocturnal oxygen. In the continuous group, 433 (16%) patients did not receive the full 72 hours of oxygen therapy, and in the nocturnal group 361 (14%) did not. Discharge from hospital was the main reason for stopping before the 72 hours had passed (Table 7). In the control group trial oxygen was not prescribed for 2229 (84%) participants, but was prescribed for 23 (1%) participants; this information was not available for 406 (15%) control participants.
| Variable | Trial arm | ||
|---|---|---|---|
| Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | |
| Trial oxygen prescribed in the drug chart and signed, n (%) | 1369 (51.3) | 1426 (53.5) | 21 (0.8) |
| Trial oxygen prescribed in the drug chart but not signed, n (%) | 789 (29.6) | 799 (30) | 2 (0.1) |
| Trial oxygen stopped before 72 hours, n (%) | 433 (16.2) | 361 (13.5) | 10 (0.4) |
| No oxygen prescribed for the trial as per randomisation, n (%) | 4 (0.2) | 10 (0.4) | 2229 (83.5) |
| No data, n (%) | 73 (2.7) | 71 (2.6) | 406 (15.2) |
In a subgroup of 4144 patients spot checks of adherence to oxygen treatment were made at 00:00 and 06:00 on nights 1, 2, and 3 of the intervention (Table 8). On the first night, adherence to oxygen treatment was reasonable, with oxygen in place for 82% and 78% for the continuous and nocturnal oxygen groups, respectively, at midnight and in 82% and 72%, respectively, at 06:00. During nights 2 and 3, considerably fewer spot checks were recorded as positive, with the lowest being 56% and 51% for the continuous and nocturnal oxygen groups at 06:00 of night 3. In the control group, oxygen was recorded as being in place at 00:00 in 2%, 3%, and 3% at 00:00 on nights 1, 2, and 3, respectively, and in 3% at 06:00 for each of the 3 days. The percentages are for a total of all patients randomised after the protocol change and included patients who were no longer in hospital.
| Variable | Trial arm | ||
|---|---|---|---|
| Continuous oxygen (N = 1381) | Nocturnal oxygen (N = 1381) | Control (N = 1382) | |
| Staff checked and signed that oxygen is in place at midnight | |||
| Night 1, n (%) | 1134 (82) | 1074 (78) | 31 (2) |
| Night 2, n (%) | 970 (70) | 931 (67) | 38 (3) |
| Night 3, n (%) | 803 (58) | 772 (56) | 37 (3) |
| Staff checked and signed that oxygen is in place at 6 am | |||
| Night 1, n (%) | 1132 (82) | 994 (72) | 37 (3) |
| Night 2, n (%) | 954 (69) | 863 (62) | 45 (3) |
| Night 3, n (%) | 774 (56) | 703 (51) | 39 (3) |
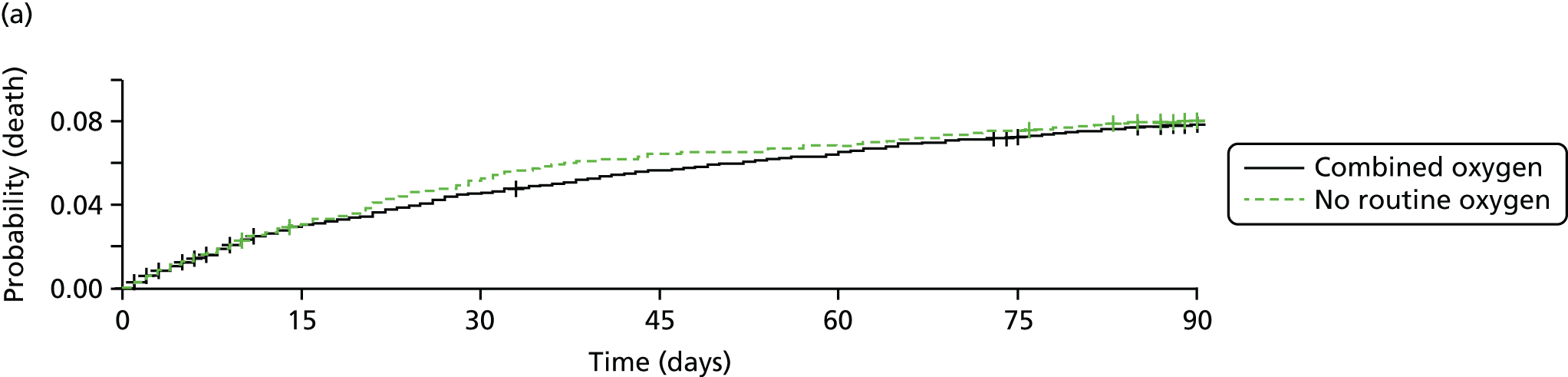
Primary outcome
The distribution of scores for mRS at 90 days is shown in Figure 4. Oxygen supplementation did not improve the level of disability either in the comparison of the combined oxygen group against control or in the comparison of continuous versus nocturnal oxygen, both in the primary unadjusted analysis and in the covariate-adjusted analysis. The unadjusted OR for a better outcome (lower mRS) was 0.97 (95% CI 0.89 to 1.05; p = 0.5) for combined oxygen versus control (see Figure 4a), and 1.03 (95% CI 0.93 to 1.13; p = 0.6) for continuous oxygen versus nocturnal oxygen (see Figure 4b). Analyses adjusted for the covariates age, sex, baseline NIHSS score, baseline oxygen saturation and the SSV prognostic index yielded very similar results [an OR of 0.97 (95% CI 0.89 to 1.06; p = 0.5) for the combined oxygen group vs. control and an OR of 1.01 (95% CI 0.92 to 1.12; p = 0.8) for continuous oxygen vs. oxygen at night only].
FIGURE 4.
The primary outcome: mRS at 3 months. (a) Comparison 1 (combined oxygen vs. control); and (b) comparison 2 (continuous oxygen vs. nocturnal oxygen). Adapted with permission from Roffe et al. 81
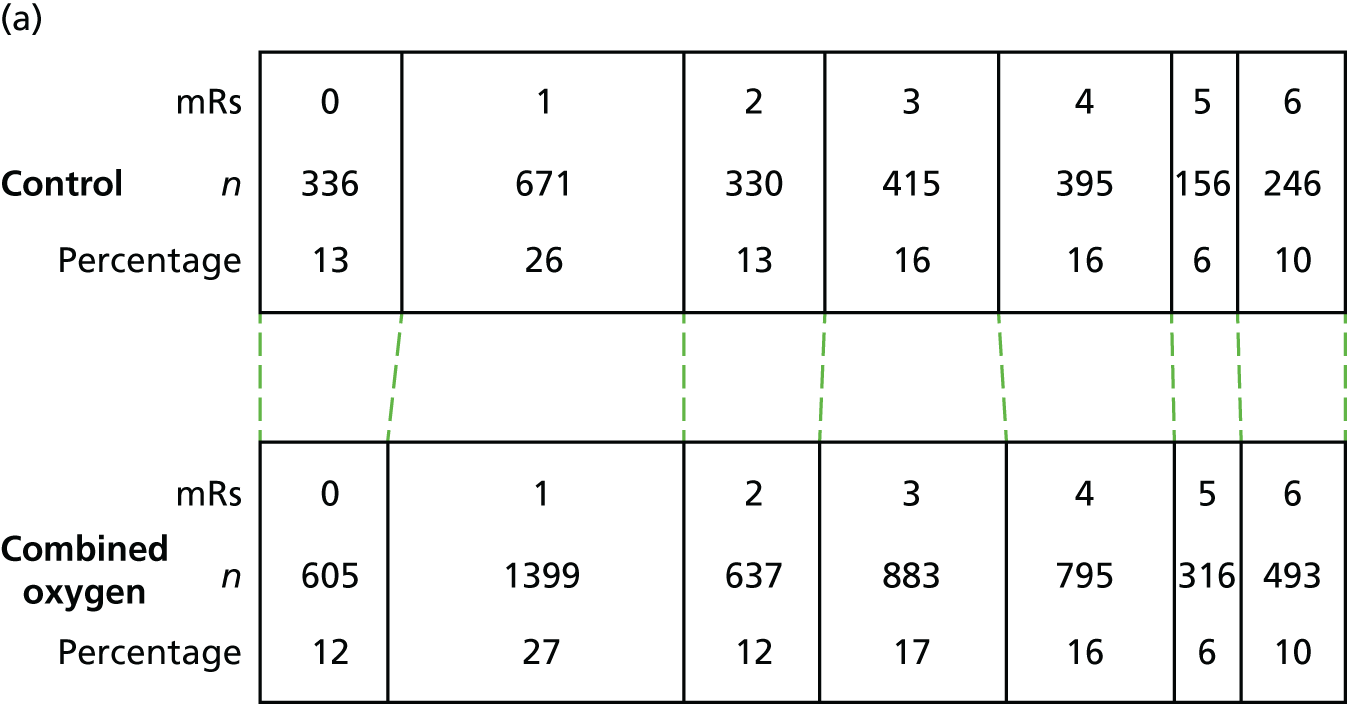

The primary outcome of the SO2S was the mRS score at 90 days post stroke as a measure of disability and dependence: (1) comparing the control group (no oxygen supplementation) with both the continuous (72 hours, day and night) and nocturnal (for three nights only) groups combined; and (2) comparing continuous oxygen with nocturnal oxygen.
Sensitivity analyses for the primary outcome
Sensitivity analyses (Table 9) show very similar results for the complete case analysis and the analysis using MI for missing values, both confirming that oxygen treatment does not improve the primary outcome. The best- and worst-case imputations indicate the plausible maximum bounds of any potential bias from missing data (under a missing not at random assumption), showing improvement in outcome for oxygen for the best-case analysis and worse outcomes with oxygen for the worst-case analysis, with similar effect sizes in both directions. The adherers-only analyses showed a minor difference between the combined oxygen groups and control, with slightly lower odds for a good outcome with oxygen for the comparison of the combined oxygen groups with control, which was not statistically significant.
| Variable | OR (95% CI); p-value | |
|---|---|---|
| Oxygen vs. no oxygena | Continuous vs. nocturnalb | |
| Complete case analysis | 0.970 (0.892 to 1.054); 0.471 | 1.025 (0.931 to 1.129); 0.611 |
| MI analysisc | 0.974 (0.895 to 1.061); 0.549 | 1.031 (0.933 to 1.135); 0.530 |
| Best-case imputationd | 1.178 (1.085 to 1.279); < 0.001 | 1.221 (1.111 to 1.342); < 0.001 |
| Worst-case imputatione | 0.803 (0.740 to 0.872); < 0.001 | 0.862 (0.784 to 0.947); 0.002 |
| Adherers onlyf | 0.925 (0.833 to 1.028); 0.148 | 0.981(0.853 to 1.127); 0.782 |
Subgroup analyses
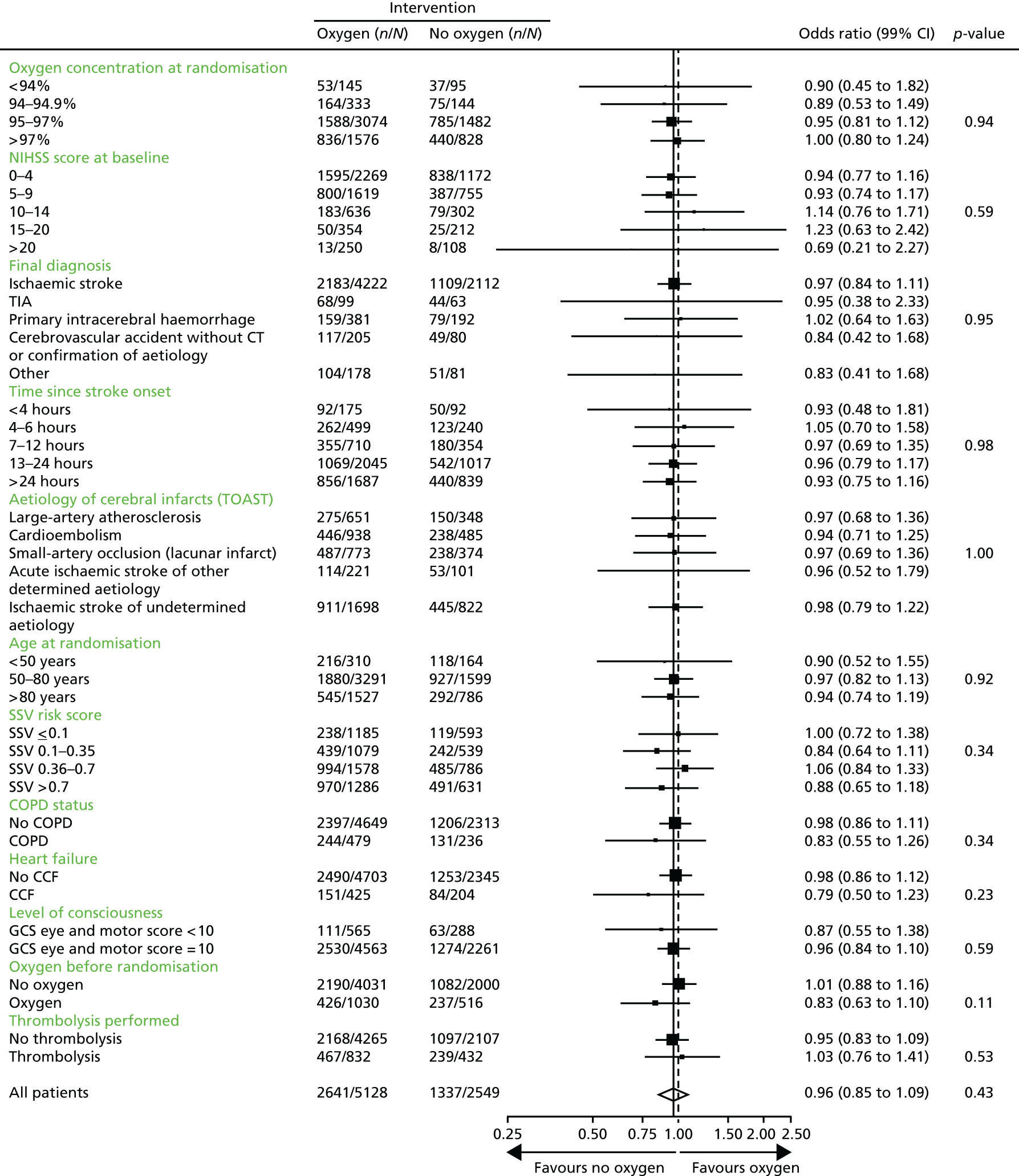
The predefined subgroup analyses are shown in Figure 5. There was no indication that treatment effectiveness differed for any of the predefined subgroups (oxygen treatment before enrolment, oxygen saturation on air at randomisation, NIHSS score, final diagnosis, time since stroke onset, aetiology, age, SSV prognostic index, level of consciousness, and history of heart failure or of chronic obstructive airways disease).
FIGURE 5.
Forest plot of subgroup analyses: alive and independent (mRS score of ≤ 2) at 3 months of oxygen (continuous and nocturnal combined) compared with control. n is the total number of events and N is the total number of events plus non-events for that group. COPD, chronic obstructive pulmonary disease; CCF, congestive cardiac failure.
Secondary and explanatory outcomes at 1 week
Oxygenation
Results for the highest oxygen saturation, the lowest oxygen saturation, the number of patients who had desaturations below 90% and the number of patients who needed additional oxygen over and above the trial prescription during the first 72 hours are shown in Table 10. The highest and lowest oxygen saturations recorded during the treatment period increased significantly (p < 0.001), by 0.8% and 0.9% respectively, in the continuous oxygen group when compared with the control group. This was also seen in the nocturnal oxygen group with highest and lowest oxygen saturations increased by 0.5% and 0.4%, respectively (p < 0.001). Severe hypoxia was recorded in a small number of patients (143, 2%), but was significantly less common in the combined oxygen group than in the control group. Significantly more patients in the combined oxygen group than in the control group required oxygen in addition to the trial intervention (OR 1.36, 99% CI 1.07 to 1.73; p = 0.0008). This was also not statistically different when comparing continuous with nocturnal oxygen (OR 1.23, 99% CI 0.96 to 1.59; p = 0.03), as significance was defined as < 0.01 for secondary outcomes.
| Variable | n (N = 8003) | Trial arm | Oxygen vs. control, OR or MD (99% CI) | Oxygen vs. control, p-value | Continuous vs. nocturnal, OR or MD (99% CI) | Continuous vs. nocturnal, p-value | ||
|---|---|---|---|---|---|---|---|---|
| Continuous oxygen (n = 2668) | Nocturnal oxygen (n = 2667) | Control (n = 2668) | ||||||
| Highest oxygen saturation (%)a | 7860 | 99.1 (99.1 to 99.2), n = 2620 | 98.8 (98.7 to 98.9), n = 2609 | 98. 3 (98.2 to 98.3), n = 2631 | 0.69 (0.61 to 0.77) | < 0.0001c | 0.32 (0.22 to 0.41) | < 0.0001c |
| Lowest oxygen saturation (%)a | 7860 | 95.0 (94.9 to 95.1), n = 2619 | 94.5 (94.4 to 94.6), n = 2610 | 94.1 (94.0 to 94.2), n = 2631 | 0.62 (0.48 to 0.76) | < 0.0001c | 0.48 (0.32 to 0.63) | < 0.0001c |
| Oxygen saturation < 90%b | 7860 | 39 (1.5%), n = 2619 | 30 (1.1%), n = 2610 | 74 (2.8%), n = 2631 | 0.46 (0.30 to 0.71) | < 0.0001d | 1.30 (0.69 to 2.44) | 0.27d |
| Need for additional oxygenb | 7809 | 254 (9.8%), n = 2599 | 209 (8.1%), n = 2589 | 176 (6.7%), n = 2621 | 1.36 (1.07 to 1.73) | 0.0008d | 1.23 (0.96 to 1.59) | 0.03d |
Neurological recovery at 1 week
Data for neurological recovery and mortality at 1 week are shown in Table 11. The median (IQR) NIHSS score at week 1 was 2 (1–6) in all three treatment groups. There was no difference in the number of patients who improved by 4 or more NIHSS points between baseline and week 1. Mortality by day 7 was very low in all three treatment groups with 50 (1.9%), 35 (1.3%) and 45 (1.7%) deaths in the continuous oxygen, nocturnal oxygen and control groups, respectively.
| Variable | n (N = 8003) | Trial arm | Oxygen vs. control, OR or MD (99% CI) | Oxygen vs. control, p-value | Continuous vs. nocturnal, OR or MD (99% CI) | Continuous vs. nocturnal, p-value | ||
|---|---|---|---|---|---|---|---|---|
| Continuous oxygen (n = 2668) | Nocturnal oxygen (n = 2667) | Control (n = 2668) | ||||||
| Neurological improvement, n (%) | 7778 | 1016 (39.2%), n = 2591 | 1029 (39.7%), n = 2591 | 1037 (39.9%), n = 2596 | 0.98 (0.86 to 1.11) | 0.68a | 0.98 (0.85 to 1.13) | 0.71a |
| NIHSS, median (IQR) | 7778 | 2 (1–6), n = 2591 | 2 (1–6), n = 2591 | 2 (1–6), n = 2596 | –0.04 (–0.43 to 0.34) | 0.78b | 0.12 (–0.32 to 0.57) | 0.47b |
| Death by 7 days, n (%) | 7959 | 50 (1.9%), n = 2651 | 35 (1.3%), n = 2645 | 45 (1.7%), n = 2663 | 0.95 (0.59 to 1.53) | 0.78a | 1.43 (0.81 to 2.54) | 0.11a |
| Highest heart rate (b.p.m.), mean (SD) | 7859 | 87.2 (16.6), n = 2618 | 88.0 (16.5), n = 2609 | 87.7 (15.7), n = 2632 | –0.07 (–1.06 to 0.92) | n/a | –0.83 (–2.01 to 0.35) | n/a |
| Highest systolic blood pressure (mmHg), mean (SD) | 7864 | 162.4 (24.6), n = 2621 | 162.8 (24.8), n = 2610 | 164.6 (24.7), n = 2633 | –1.96 (–3.48 to 0.44) | n/a | –0.35 (–2.11 to 1.41) | n/a |
| Highest diastolic blood pressure (mmHg), mean (SD) | 7861 | 89.5 (15.3), n = 2621 | 90.2 (15.5), n = 2609 | 90.9 (15.7), n = 2631 | –1.10 (–2.06 to 0.15) | n/a | –0.72 (–1.82 to 0.37) | n/a |
| Highest systolic blood pressure > 200 mmHg (n) | 7864 | 180, n = 2621 | 186, n = 2610 | 205, n = 2633 | n/a | n/a | n/a | n/a |
| Highest diastolic blood pressure > 100 mmHg (n) | 7861 | 531, n = 2621 | 552, n = 2609 | 606, n = 2631 | n/a | n/a | n/a | n/a |
| Sedative use, n (%) | 7916 | 140 (5.3%), n = 2634 | 161 (6.1%), n = 2631 | 154 (5.8%), n = 2651 | 0.98 (0.76 to 1.28) | n/a | 0.86 (0.63 to 1.17) | n/a |
| Antibiotic treatment, n (%) | 7916 | 400 (15.2%), n = 2634 | 393 (14.9%), n = 2631 | 403 (15.2%), n = 2651 | 0.99 (0.83 to 1.17) | n/a | 1.02 (0.84 to 1.24) | n/a |
| Highest temperature (°C) up to 7 days, mean (SD) | 7877 | 37.1 (0.6), n = 2623 | 37.2 (0.6), n = 2617 | 37.1 (0.6), n = 2637 | 0.01 (–0.03 to 0.04) | n/a | –0.01 (–0.05 to 0.03) | n/a |
Explanatory analysis at 1 week
Exploratory analyses (see Table 11) did not show evidence of increased stress levels (higher heart rates, higher blood pressure, or need for sedation) in oxygen-treated patients compared with control patients. There was also no evidence that oxygen treatment was associated with more infections, with no differences in the highest temperature or the need for antibiotics.
Secondary and exploratory outcomes at 3 months
There was no difference in mortality, the number of patients alive and independent at 3 months, the number of participants who lived at home, the BI, the NEADL, EQ-5D-3L, EQ-VAS, sleep, speech or memory between the oxygen-treated and control groups or between the groups receiving continuous and nocturnal oxygen treatment (Table 12).
| Variable | n (N = 8003) | Trial arm | Oxygen vs. control, OR or MD (99% CI) | Oxygen vs. control, p-value | Continuous vs. nocturnal, OR or MD (99% CI) | Continuous vs. nocturnal, p-value | ||
|---|---|---|---|---|---|---|---|---|
| Continuous oxygen (n = 2668) | Nocturnal oxygen (n = 2667) | Control (n = 2668) | ||||||
| Death by 3 months | 7677 | 257 (10.0%), n = 2567 | 236 (9.2%), n = 2561 | 246 (9.7%), n = 2549 | 1.00 (0.81 to 1.23) | 0.96b | 1.10 (0.86 to 1.40) | 0.33b |
| Death by 90 daysa (date) | 8003 | 222 (8.3%), n = 2668 | 194 (7.3%), n = 2667 | 214 (8.0%), n = 2668 | 0.97 (0.77 to 1.22) | 0.73b | 1.16 (0.89 to 1.51) | 0.15b |
| Alive and independenta | 7677 | 1325 (51.6%), n = 2567 | 1316 (51.4%), n = 2561 | 1337 (52.5%), n = 2549 | 0.96 (0.85 to 1.09) | 0.43b | 1.01 (0.87 to 1.17) | 0.87b |
| Living at homea | 6859 | 1961 (85.8%), n = 2285 | 1947 (84.8%), n = 2295 | 1947 (85.4%), n = 2279 | 0.99 (0.82 to 1.20) | 0.91b | 1.08 (0.87 to 1.34) | 0.35b |
| Barthel ADL index [0 (worst) to 100 (best)]c | 6549 | 70.2 (68.7 to 71.8), n = 2169 | 71.1 (69.6 to 72.6), n = 2194 | 70.9 (69.3 to 72.4), n = 2186 | –0.18 (–2.60 to 2.24) | 0.85d | –0.86 (–3.65 to 1.93) | 0.43d |
| Nottingham Extended ADL [0 (worst) to 21 (best)]c | 7528 | 9.66 (9.38 to 9.93), n = 2520 | 9.54 (9.26 to 9.81), n = 2501 | 9.77 (9.49 to 10.05), n = 2507 | –0.17 (–0.62 to 0.28) | 0.32d | 0.12 (–0.40 to 0.64) | 0.55d |
| Quality of life (EQ-5D-3L) [–0.59 (worst) to 1 (best)]c | 7248 | 0.50 (0.48 to 0.51), n = 2413 | 0.50 (0.48 to 0.51), n = 2428 | 0.49 (0.48 to 0.51), n = 2407 | 0.004 (0.02 to 0.03) | 0.71d | –0.003 (–0.03 to 0.03) | 0.78d |
| Quality of life (EQ-VAS) [0 (worst) to 100 (best)]c | 6675 | 55.4 (54.2 to 56.7), n = 2251 | 55.7 (54.4 to 56.9), n = 2216 | 55.5 (54.2 to 56.7), n = 2208 | 0.10 (–1.93 to 2.12) | 0.90d | –0.24 (–2.57 to 2.09) | 0.79d |
| Sleep as good as before the strokea | 6584 | 1407 (64%), n = 2194 | 1436 (65%), n = 2208 | 1419 (65%), n = 2182 | 0.98 (0.85 to 1.13) | – | 0.96 (0.82 to 1.13) | – |
| No significant speech problemsa | 6716 | 1957 (88%), n = 2229 | 1957 (87%), n = 2246 | 1939 (87%), n = 2241 | 1.09 (0.89 to 1.32) | – | 1.06 (0.84 to 1.34) | – |
| Memory as good as before the strokea | 6646 | 981 (44%), n = 2222 | 1000 (45%), n = 2224 | 971 (44%), n = 2200 | 1.02 (0.89 to 1.16) | – | 0.97 (0.83 to 1.13) | – |
Long-term outcomes (3, 6 and 12 months)
Survival
Mortality at 90 days (Figure 6) was similar in the oxygen (both groups combined) and control groups (hazard ratio 0.97, 99% CI 0.78 to 1.21; p = 0.8) and in the groups recieving continuous oxygen or oxygen at night only (hazard ratio 1.15, 99% CI 0.90–1.48; p = 0.1).
FIGURE 6.
Kaplan–Meier survival graph comparing (a) oxygen (combined) with control (no ROS) at 90 days; and (b) continuous vs. nocturnal oxygen. Oxygen compared with no oxygen: unadjusted hazard ratio for a worse outcome – 0.97 (99% CI 0.78 to 1.21; p = 0.8). Continuous compared with nocturnal oxygen: unadjusted hazard ratio for a worse outcome –1.15 (99% CI 0.90 to 1.48; p = 0.1).

Survival was the same throughout the 365 days of follow-up for patients treated with continuous oxygen and with nocturnal oxygen, and those in the control group (Figure 7).
FIGURE 7.
Kaplan–Meier survival graph comparing (a) oxygen (combined) with control (no ROS) at 365 days; and (b) continuous compared with nocturnal oxygen.


Functional outcomes
Figure 8 shows the mean mRS score at 3, 6 and 12 months. There was no change in the level of disability over the 3-, 6- and 12-month follow-up assessment points in any of the three groups. The proportion of patients in each mRS score category is shown in Figure 9 for the combined oxygen group compared with the control group and in Figure 10 for the continuous oxygen group compared with the nocturnal oxygen group. Oxygen has no effect on the level of disability at any time point, whether given continuously or at night only.
FIGURE 8.
Modified Rankin Scale score at 3, 6 and 12 months.
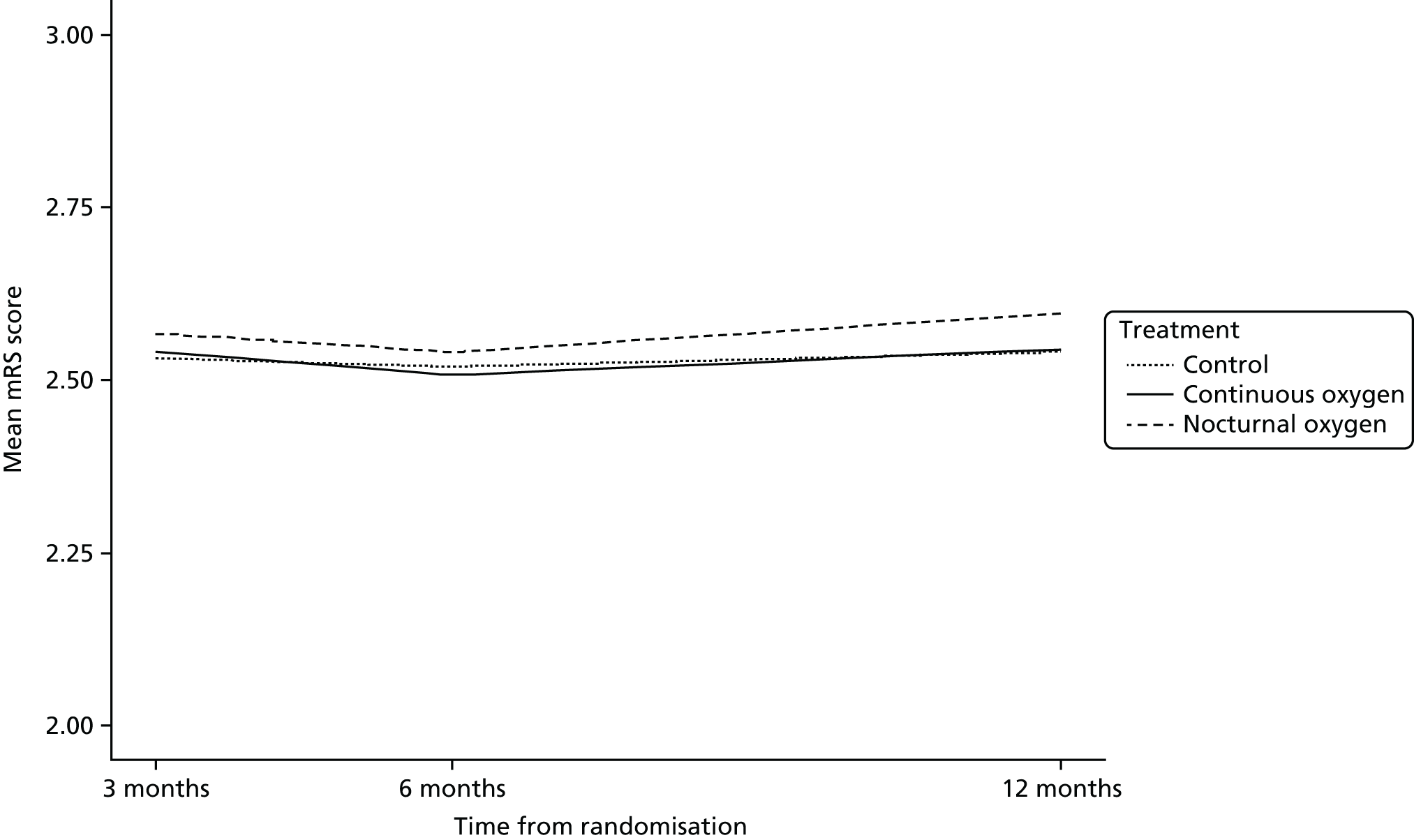
FIGURE 9.
Functional outcome of the combined (continuous and nocturnal) oxygen group compared with the control group at (a) 3; (b) 6; and (c) 12 months post randomisation.
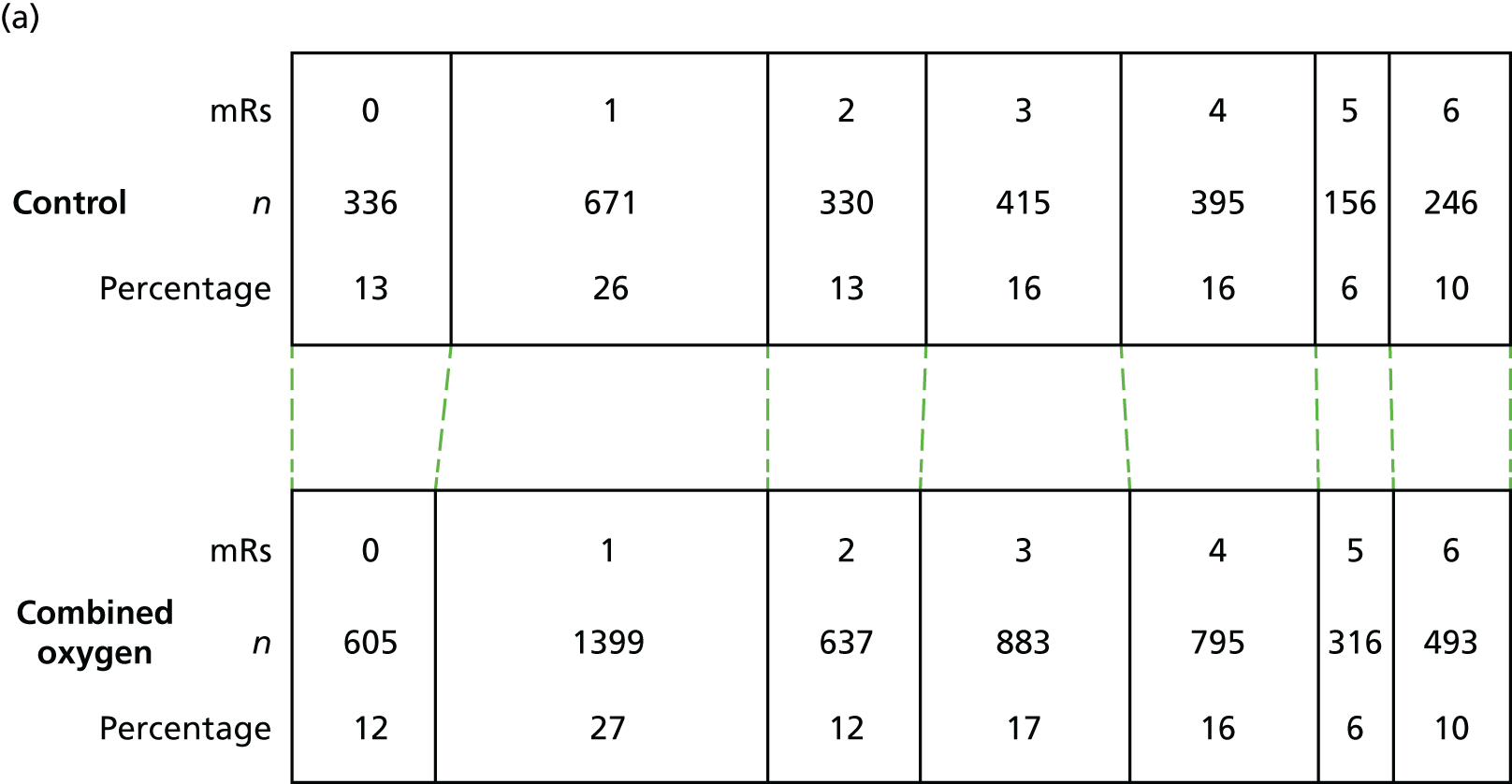
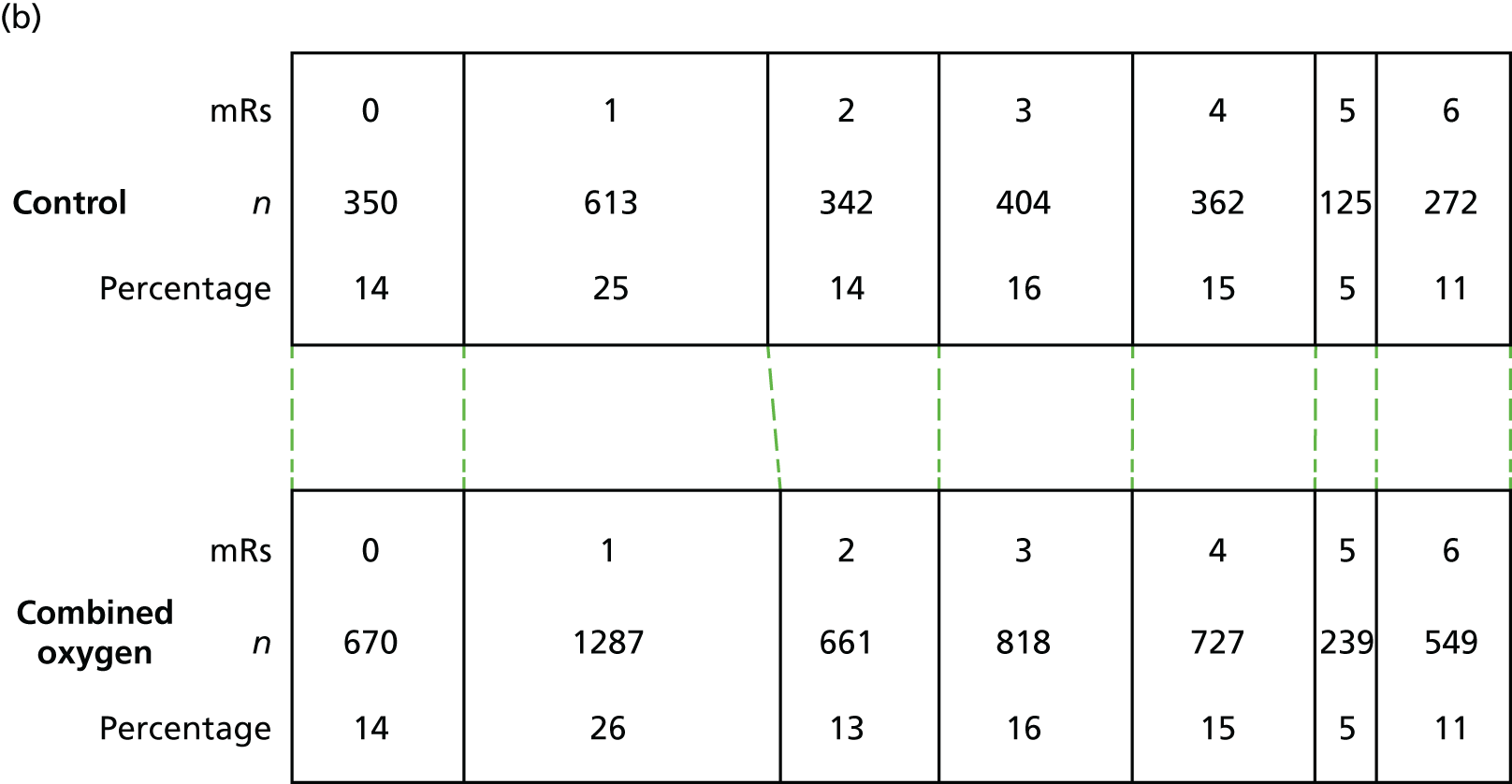

FIGURE 10.
Functional outcome of the continuous oxygen group compared with the nocturnal only oxygen group at (a) 3; (b) 6; and (c) 12 months post randomisation.


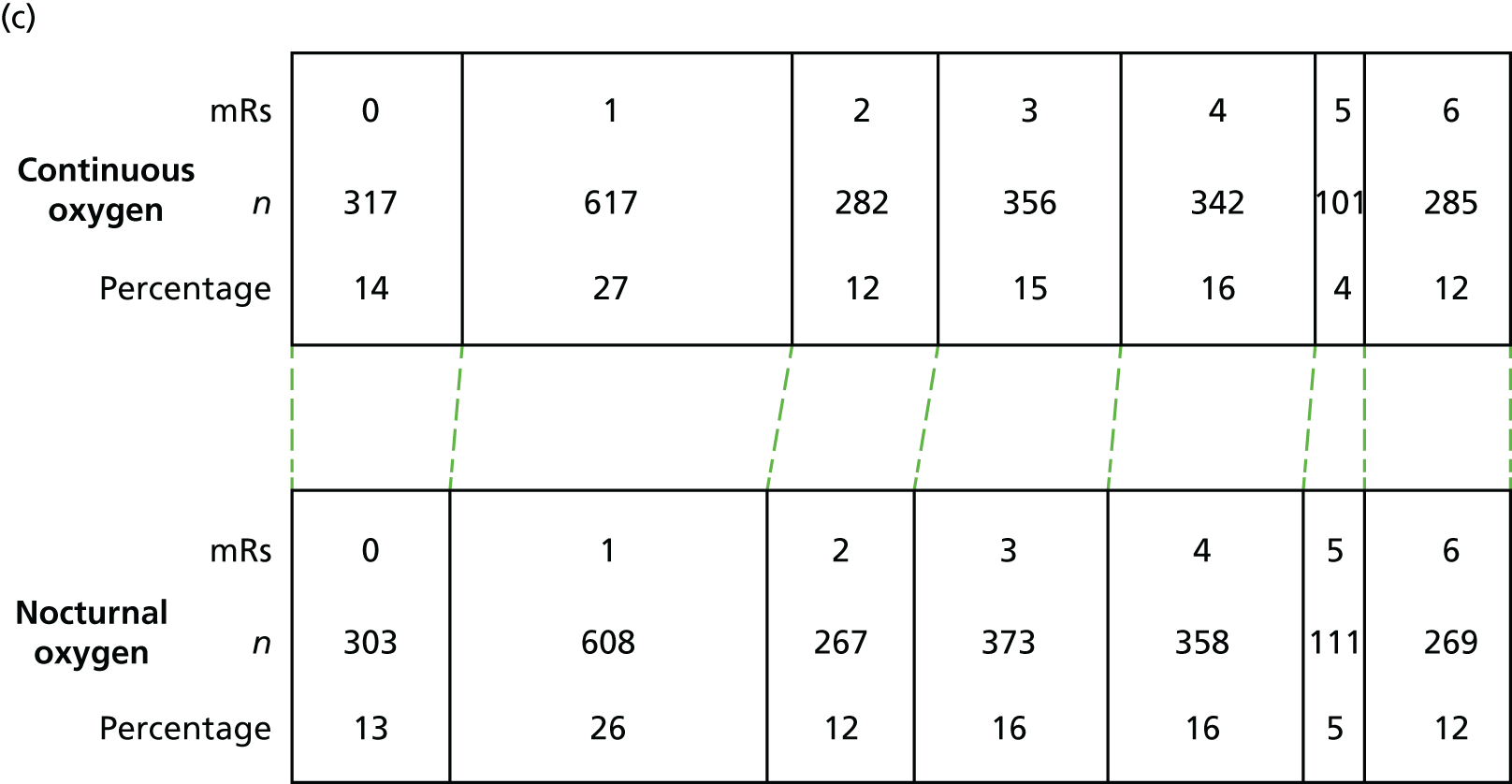
The number of patients who are alive and independent was similar in all three treatment groups and did not change with time (52%, 53% and 53%, respectively, at each of the three time points). Performance of activities of daily living (BI), ability to conduct extended activities of daily living (NEADL), and quality of life (EQ-5D-3L) were no different between the combined oxygen groups or between continuous and nocturnal oxygen at 90, 180 and at 365 days and were similar at each of the three time points (Table 13).
| Variable | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | |||||||
| Continuous oxygen (n = 2668) | Nocturnal oxygen (n = 2667) | Control (n = 2668) | Continuous oxygen (n = 2668) | Nocturnal oxygen (n = 2667) | Control (n = 2668) | Continuous oxygen (n = 2668) | Nocturnal oxygen (n = 2667) | Control (n = 2668) | |
| Death by follow-up time pointa | 257 (10.0%), n = 2567 | 236 (9.2%), n = 2561 | 246 (9.7%), n = 2549 | 280 (11.3%), n = 2475 | 269 (10.9%), n = 2476 | 272 (11.0%), n = 2468 | 285 (12.4%), n = 2300 | 269 (11.8%), n = 2289 | 279 (12.3%), n = 2268 |
| Death by 90 daysa (date) | 222 (8.7%), n = 2566 | 194 (7.6%), n = 2565 | 214 (8.4%), n = 2550 | – | – | – | – | – | – |
| Alive and independenta | 1325 (51.6%), n = 2567 | 1316 (51.4%), n = 2561 | 1337 (52.5%), n = 2549 | 1313 (53.1%), n = 2475 | 1305 (52.7%), n = 2476 | 1305 (52.9%), n = 2468 | 1216 (52.9%), n = 2300 | 1178 (51.5%), n = 2289 | 1215 (53.6%), n = 2268 |
| Living at homea | 1961 (85.8%), n = 2285 | 1947 (84.8%), n = 2295 | 1947 (85.4%), n = 2279 | 1910 (87.6%), n = 2181 | 1932 (88.0%), n = 2196 | 1888 (86.4%), n = 2184 | 1774 (88.4%), n = 2006 | 1766 (88.0%), n = 2007 | 1728 (87.1%), n = 1983 |
| Barthel ADL index [0 (worst) to 100 (best)]b | 70.2 (68.7 to 71.8), n = 2169 | 71.1 (69.6 to 72.6), n = 2194 | 70.9 (69.3 to 72.4), n = 2186 | 71.4 (69.9 to 72.9), n = 2175 | 70.9 (69.4 to 72.5), n = 2158 | 71.1 (69.6 to 72.7), n = 2158 | 70.3 (68.6 to 72.0), n = 1945 | 70.1 (68.5 to 71.7), n = 1963 | 70.7 (69.0 to 72.3), n = 1954 |
| Nottingham Extended ADL [0 (worst) to 21(best)]b | 9.66 (9.38 to 9.93), n = 2520 | 9.54 (9.26 to 9.81), n = 2501 | 9.77 (9.49 to 10.05), n = 2507 | 9.85 (9.57 to 10.14), n = 2442 | 9.74 (9.45 to 10.02), n = 2442 | 10.01 (9.72 to 10.30), n = 2426 | 10.09 (9.79 to 10.39), n = 2254 | 9.80 (9.50 to 10.10), n = 2239 | 10.15 (9.84 to 10.45), n = 2218 |
| Quality of Life (EQ-5D-3L) [–0.59 (worst) to 1 (best)]b | 0.44 (0.42 to 0.46), n = 2413 | 0.44 (0.42 to 0.46), n = 2428 | 0.43 (0.41 to 0.45), n = 2407 | 0.42 (0.40 to 0.44), n = 2338 | 0.43 (0.41 to 0.45), n = 2353 | 0.43 (0.40 to 0.45), n = 2328 | 0.41 (0.39 to 0.43), n = 2103 | 0.42 (0.40 to 0.45), n = 2110 | 0.41 (0.39 to 0.44), n = 2081 |
| Quality of life (EQ-VAS) [0 (worst) to 100 (best)]b | 55.4 (54.2 to 56.7), n = 2251 | 55.7 (54.4 to 56.9), n = 2216 | 55.5 (54.2 to 56.7), n = 2208 | 56.9 (55.6 to 58.2), n = 2164 | 57.1 (55.8 to 58.4), n = 2148 | 56.9 (55.6 to 58.2), n = 2133 | 56.1 (54.7 to 57.6), n = 1900 | 57.0 (55.6 to 58.4), n = 1903 | 56.7 (55.3 to 58.1), n = 1872 |
| Sleep as good as before the strokea | 1407 (64%), n = 2194 | 1436 (65%), n = 2208 | 1419 (65%), n = 2182 | 1339 (64%), n = 2103 | 1368 (65%), n = 2114 | 1363 (65%), n = 2100 | 1249 (66%), n = 1888 | 1243 (64%), n = 1930 | 1196 (63%), n = 1889 |
| No significant speech problemsa | 1957 (88%), n = 2229 | 1957 (87%), n = 2246 | 1939 (87%), n = 2241 | 1923 (89%), n = 2152 | 1921 (89%), n = 2163 | 1900 (89%), n = 2140 | 1739 (90%), n = 1940 | 1749 (90%), n = 1954 | 1750 (91%), n = 1925 |
| Memory as good as before the strokea | 981 (44%), n = 2222 | 1000 (45%), n = 2224 | 971 (44%), n = 2220 | 888 (42%), n = 2127 | 914 (43%), n = 2132 | 888 (42%), n = 2110 | 800 (42%), n = 1920 | 782 (41%), n = 1930 | 762 (40%), n = 1893 |
Outcomes considered important by stroke survivors
Outcomes considered important by stroke survivors during the pre-study focus group meetings (sleep, speech and memory) are shown in Table 13. There was also no difference in these outcomes between the continuous oxygen, nocturnal oxygen and control groups. At 90 days, 87% had no significant speech problems and 65% considered their sleep as good as before the stroke, but only 44% reported their memory as being as good as before the stroke. The data were similar at 180 and 365 days.
Length of hospital stay and readmissions
Data on length of stay were available for 2435 (91%), 2401 (90%) and 2446 (92%) of participants in the continuous oxygen, nocturnal oxygen and control groups. The mean (SD) length of stay in hospital was 18.6 days (50.9 days), 18.6 days (52.0 days) and 18.0 days (56.4 days), respectively (Table 14). The rate of readmissions (Table 15) increased with time, but was similar for all three treatment groups (14%, 17% and 20% at 3, 6 and 12 months, respectively).
| Length of stay in hospital | Trial arm | ||
|---|---|---|---|
| Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | |
| Randomisation to final discharge, mean (SD) | 18.6 days (50.9 days) | 18.6 days (52.0 days) | 18.0 days (56.4 days) |
| No data, n (%) | 233 (9%) | 266 (10%) | 222 (8%) |
Place of abode
Details of place of abode throughout the follow-up period are shown in Table 15. The proportion of patients living in their own homes, with relatives, in residential nursing, or continuing care homes was similar in all three groups. The proportion of participants living in their own homes or with family members was 76%, 74% and 68% at 3, 6 and 12 months. Only a few patients were residing in institutions (care home, nursing home or continuing NHS care) at each of the three time points (7%, 7% and 6%, respectively).
Blinding
Details of who completed the follow-up questionnaires, and whether or not the person completing the questionnaire remembers the treatment the participant was allocated to, are presented in Table 16. The proportion of participants who completed the follow-up questionnaires personally and unaided by another person was 33%, 29% and 28% at 3, 6 and 12 months. The proportions were similar for all three groups. As participants were not blinded to the intervention, we included a question at follow-up to assess whether or not they remembered which treatment group they were in. At 3 months, 49%, 35% and 45% in the continuous oxygen group, the nocturnal oxygen group and the control group, respectively, remembered their allocation correctly. At 6 months, 46%, 32% and 41%, respectively, remembered allocation correctly, and at 12 months correct memory of allocation was still recorded in 41%, 28% and 35% of participants.
| Variable | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | |||||||
| Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | |
| Where do you live now? | |||||||||
| In own home, n (%) | 1961 (73.5%) | 1948 (73.0%) | 1947 (73.0%) | 1911 (71.7%) | 1932 (72.4%) | 1888 (70.8%) | 1774 (66.5%) | 1766 (66.2%) | 1728 (64.8%) |
| In the home of a relative, n (%) | 86 (3.2%) | 79 (3.0%) | 62 (2.3%) | 78 (2.9%) | 69 (2.6%) | 61 (2.3%) | 67 (2.5%) | 54 (2.0%) | 56 (2.1%) |
| In a residential home, n (%) | 62 (2.3%) | 63 (2.4%) | 73 (2.7%) | 57 (2.1%) | 57 (2.1%) | 75 (2.8%) | 54 (2.0%) | 53 (2.0%) | 72 (2.7%) |
| In a nursing home, n (%) | 70 (2.6%) | 114 (4.0%) | 111 (4.2%) | 97 (3.6%) | 114 (4.3%) | 120 (4.5%) | 92 (3.45%) | 118 (4.4%) | 109 (4.09%) |
| In a continuing care home, n (%) | 37 (1.4%) | 34 (1.3%) | 25 (0.9%) | 15 (0.6%) | 8 (0.3%) | 9 (0.3%) | 6 (0.2%) | 1 (0.04%) | 5 (0.19%) |
| Not left hospital yet since stroke, n (%) | 60 (2.3%) | 50 (2.0%) | 43 (1.6%) | 7 (0.3%) | 4 (0.1%) | 12 (0.5%) | 1 (0.04%) | 2 (0.07%) | 1 (0.04%) |
| Other, n (%) | 9 (0.3%) | 9 (0.3%) | 18 (0.7%) | 17 (0.6%) | 12 (0.5%) | 19 (0.7%) | 12 (0.5%) | 13 (0.49%) | 12 (0.4%) |
| Withdrawn or no data, n (%) | 383 (14.4%) | 370 (14.0%) | 389 (14.6%) | 486 (18.2%) | 471 (17.7%) | 484 (18.1%) | 662 (24.81%) | 660 (24.8%) | 685 (25.68%) |
| Have you been admitted to hospital again for any reason after you were discharged? | |||||||||
| Yes, n (%) | 379 (14%) | 356 (13%) | 380 (14%) | 456 (17%) | 457 (17%) | 434 (16%) | 540 (20%) | 550 (21%) | 546 (21%) |
| No, n (%) | 1831 (69%) | 1883 (71%) | 1850 (69%) | 1717 (64%) | 1732 (65%) | 1743 (65%) | 1456 (55%) | 1449 (54%) | 1423 (53%) |
| No data, n (%) | 458 (17%) | 428 (16%) | 438 (17%) | 495 (19%) | 478 (18%) | 491 (19%) | 672 (25%) | 668 (25%) | 699 (26%) |
| If yes, how many times? | |||||||||
| Once, n (%) | 285 (11%) | 272 (10%) | 296 (11%) | 305 (11%) | 322 (12%) | 295 (11%) | 341 (13%) | 358 (13%) | 363 (14%) |
| More than once, n (%) | 91 (3%) | 80 (3%) | 83 (3%) | 145 (5%) | 131 (5%) | 134 (5%) | 195 (7%) | 186 (7%) | 181 (7%) |
| No data, n (%) | 3 (0.1%) | 4 (0.1%) | 1 (< 0.1%) | 6 (0.2%) | 4 (0.1%) | 5 (0.2%) | 4 (0.1%) | 6 (0.2%) | 2 (0.1%) |
Co-enrolment in other research studies
Participants in SO2S were permitted to co-enrol in other studies, if they wished, and if other studies were available. Participants were asked if they were enrolled in other studies at each of the three follow-up points. The results are shown in Table 16. The proportion of participants who reported enrolment in other studies was low, with no differences between treatment groups, and did not change over time (3%, 4% and 3% at 3, 6 and 12 months, respectively).
| Variable | Time points | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | |||||||
| Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | Continuous oxygen (N = 2668) | Nocturnal oxygen (N = 2667) | Control (N = 2668) | |
| Can you remember which of the treatments you were given as part of the trial? | |||||||||
| Yes (correct), n (%) | 1314 (49%) | 942 (35%) | 1194 (45%) | 1228 (46%) | 849 (32%) | 1082 (41%) | 1084 (41%) | 749 (28%) | 940 (35%) |
| Yes (incorrect), n (%) | 222 (8%) | 530 (20%) | 105 (4%) | 223 (8%) | 588 (22%) | 120 (4%) | 185 (7%) | 515 (19%) | 117 (5%) |
| I do not know, n (%) | 545 (21%) | 621 (23%) | 749 (28%) | 537 (20%) | 577 (22%) | 747 (28%) | 515 (19%) | 567 (21%) | 699 (26%) |
| No data, n (%) | 587 (22%) | 574 (22%) | 620 (23%) | 680 (26%) | 653 (24%) | 719 (27%) | 884 (33%) | 836 (32%) | 912 (34%) |
| Have you taken part in any other research trials since the start of this study? | |||||||||
| Yes, n (%) | 78 (3%) | 85 (3%) | 87 (3%) | 95 (4%) | 78 (3%) | 116 (4%) | 75 (3%) | 87 (3%) | 98 (4%) |
| No, n (%) | 1870 (70%) | 1856 (70%) | 1838 (69%) | 1815 (68%) | 1870 (70%) | 1775 (67%) | 1711 (64%) | 1704 (64%) | 1673 (63%) |
| No data, n (%) | 720 (27%) | 726 (27%) | 743 (28%) | 758 (28%) | 719 (27%) | 777 (29%) | 882 (33%) | 876 (33%) | 897 (33%) |
| Who completed the questionnaire? | |||||||||
| Patient, n (%) | 888 (33.3%) | 840 (31.5%) | 873 (32.7%) | 810 (30.4%) | 763 (28.6%) | 783 (29.3%) | 742 (27.8%) | 760 (28.5%) | 767 (28.8%) |
| Patient with some help, n (%) | 266 (10.0%) | 238 (8.9%) | 233 (8.7%) | 192 (7.2%) | 187 (7.0%) | 166 (6.2%) | 187 (7.0%) | 176 (6.6%) | 164 (6.1%) |
| A relative, friend or carer, n (%) | 405 (15.2%) | 442 (16.6%) | 393 (14.7%) | 312 (11.7%) | 328 (12.3%) | 325 (12.2%) | 298 (11.2%) | 303 (11.4%) | 296 (11.1%) |
| Researcher over the telephone, n (%) | 657 (24.6%) | 692 (26.0%) | 720 (27%) | 824 (30.9%) | 872 (32.7%) | 845 (31.7%) | 709 (26.6%) | 715 (26.8%) | 701 (26.3%) |
| Researcher in hospital clinic, n (%) | 18 (0.7%) | 22 (0.8%) | 13 (0.5%) | 10 (0.4%) | 7 (0.3%) | 18 (0.7%) | 8 (0.3%) | 4 (0.1%) | 10 (0.4%) |
| Other, n (%) | 15 (0.5%) | 17 (0.6%) | 16 (0.6%) | 12 (0.4%) | 12 (0.4%) | 13 (0.5%) | 12 (0.4%) | 18 (0.7%) | 6 (0.2%) |
| No data, n (%) | 419 (15.7%) | 416 (15.6%) | 420 (15.8%) | 508 (19.0%) | 498 (18.7%) | 518 (19.4%) | 712 (26.7%) | 691 (25.9%) | 724 (27.1%) |
Safety outcomes
The number of patients who experienced at least one serious adverse event at 90, 180 and 365 days is shown in Table 17. Details of serious adverse events within the first 90 days are given in Table 18.
| Variable | n (N = 8003) | Trial arm | Combined oxygen vs. control, OR (99% CI) | Combined oxygen vs. control, p-value | Continuous vs. nocturnal, OR (95% CI) | Continuous vs. nocturnal, p-value | ||
|---|---|---|---|---|---|---|---|---|
| Continuous oxygen (N = 2668), n (%) | Nocturnal oxygen (N = 2667), n (%) | Control (N = 2668), n (%) | ||||||
| Number of patients with at least one SAE by 90 days | 964 | 348 (13%), n = 2668 | 294 (11%), n = 2667 | 322 (12%), n = 2668 | 1.00 (0.83 to 1.20) | 1.0 | 1.21 (0.97 to 1.51) | 0.02 |
| Number of patients with at least one SAE by 180 days | 1385 | 485 (18%), n = 2668 | 442 (17%), n = 2667 | 458 (17%), n = 2668 | 1.02 (0.86 to 1.19) | 0.8 | 1.12 (0.93 to 1.35) | 0.1 |
| Number of patients with at least one SAE by 365 days | 2034 | 708 (27%), n = 2668 | 675 (25%), n = 2667 | 651 (24%), n = 2668 | 1.08 (0.94 to 1.25) | 0.1 | 1.07 (0.91 to 1.25) | 0.3 |
| Variable | Trial arm | Total (N = 8003) | ||
|---|---|---|---|---|
| Continuous oxygen (N = 2668), n | Nocturnal oxygen (N = 2667), n | Control (N = 2668), n | ||
| Cardiovascular | 54 | 48 | 37 | 139 |
| DVT | 4 | 3 | 3 | 10 |
| PE | 18 | 6 | 9 | 33 |
| Central nervous system | 177 | 141 | 178 | 496 |
| Agitation | 3 | 0 | 0 | 3 |
| Anxiety | 1 | 1 | 2 | 4 |
| Central nervous system other | 3 | 5 | 2 | 10 |
| Cerebral oedema | 10 | 2 | 6 | 18 |
| Complication of initial stroke | 36 | 26 | 23 | 85 |
| Confusion | 2 | 3 | 0 | 5 |
| Dementia | 0 | 0 | 2 | 2 |
| Extension of initial stroke | 34 | 32 | 45 | 111 |
| Functional symptoms | 0 | 2 | 0 | 2 |
| Haemorrhagic transformation | 8 | 8 | 16 | 32 |
| Headache | 1 | 3 | 2 | 6 |
| Intracerebral bleed | 12 | 8 | 4 | 24 |
| Intracranial/extracerebral bleed | 1 | 1 | 4 | 6 |
| Recurrent stroke | 41 | 36 | 42 | 119 |
| Seizure | 10 | 7 | 18 | 35 |
| TIA | 13 | 7 | 10 | 30 |
| Vertigo | 1 | 0 | 0 | 1 |
| Vomiting | 1 | 0 | 2 | 3 |
| Cutaneous | 1 | 0 | 0 | 1 |
| Gastrointestinal | 15 | 9 | 13 | 37 |
| Genitourinary | 11 | 10 | 21 | 42 |
| Haematological | 0 | 1 | 0 | 1 |
| Immunological | 0 | 0 | 0 | 0 |
| Miscellaneous | 40 | 33 | 37 | 110 |
| Respiratory | 76 | 84 | 97 | 257 |
| Chest infection | 2 | 4 | 3 | 9 |
| Hypoxia | 3 | 1 | 6 | 10 |
| Pneumonia | 69 | 78 | 87 | 234 |
| Respiratory other | 2 | 1 | 1 | 4 |
| Oxygen-related | 0 | 0 | 0 | 0 |
| Drying of mucous membranes | 0 | 0 | 0 | 0 |
| Respiratory depression | 0 | 0 | 0 | 0 |
| Other | 30 | 23 | 23 | 76 |
| Total | 426 | 358 | 418 | 1202 |
Health economics analysis results
A total of 7898 trial participants (no ROS, n = 2629; continuous ROS, n = 2636; and nocturnal ROS, n = 2633) formed the data set for the analysis. All base-case analyses were conducted on the imputed data set. The analyses for the comparison of ROS with no ROS are presented first, followed by all analyses for the comparison of continuous ROS and nocturnal ROS with no ROS and, finally, the deterministic sensitivity analyses for ROS compared with no ROS.
Base-case analysis
Comparison of routine oxygen supplementation with no routine oxygen supplementation
Table 19 presents the mean outcomes per patient in terms of hospital stay, home time, EQ-5D-3L scores and QALYs. Both mean hospital stay and home time were marginally higher in the ROS group, but by less than half a day. There were no differences in mean EQ-5D-3L scores at 3, 6 and 12 months and the difference in QALYs was very small, at 0.0004 QALYs, and not significant. Table 20 presents disaggregated mean (SD) health-care costs per patient for trial oxygen and additional oxygen, acute stay costs, readmissions and long-term care, as well as total health-care costs. As expected, oxygen treatment costs were higher in the ROS arm, with all other costs slightly higher for ROS, and £206 (95% CI –£283 to £695) higher overall, compared with no ROS.
| Variable | Intervention | |
|---|---|---|
| No ROS (N = 2629) | ROS (N = 5269) | |
| Mean hospital stay (days) (95% CI) | 18.4 (17.7 to 19) | 18.7 (17.9 to 19.5) |
| Mean home time (days) (95% CI) | 313.0 (310.4 to 315.7) | 313.4 (310.2 to 136.5) |
| EQ-5D-3L scores [mean (SD)] | ||
| Baseline | 0 | 0 |
| 3 months | 0.48 (0.38) | 0.48 (0.37) |
| 6 months | 0.48 (0.37) | 0.48 (0.37) |
| 12 months | 0.46 (0.37) | 0.46 (0.37) |
| Total QALYs over 12 months | 0.4133 (0.31) | 0.4137 (0.30) |
| Difference in QALYs (95% CI) | 0.0004 (–0.0139 to 0.0148) | |
| Cost category | Intervention | |
|---|---|---|
| No ROS (N = 2629) | ROS (N = 5629) | |
| Trial prescribed oxygen | 0.65 (0.42 to 0.94) | 72.89 (72.62 to 73.15) |
| Additional oxygen | 5.52 (4.76 to 6.37) | 9.40 (8.44 to 10.32) |
| Total oxygen treatment | 6.18 (5.40 to 7.05) | 82.29 (81.30 to 83.38) |
| Acute care costs | 5130 (4856 to 5406) | 5196 (5008 to 5418) |
| Readmission | 978 (929 to 1032) | 986 (951 to 1022) |
| Stroke care after discharge | 6587 (6310 to 6865) | 6643 (6447 to 6835) |
| Total cost | 12,702 (12,309 to 13,115) | 12,908 (12,619 to 13,183) |
| Mean difference (95% CI) | 206 (–283 to 695) | |
The cost-effectiveness analysis presented in Table 21 shows that it costs an additional £71 to gain a day of home time. The CUA gave an ICER of £463,338 per QALY gained (Table 22). The cost-effectiveness plane in Figure 11 demonstrates the uncertainty around both costs and QALY differences, with points in all four quadrants. The corresponding CEAC in Figure 12 shows a 27% probability of ROS being cost-effective compared with no ROS if society was willing to pay up to £20,000 per additional QALY, also suggesting ROS is not cost-effective.
| Intervention | Mean costs (£) | Cost difference (£) | Mean days of home time | Outcome difference (days) | ICER (£/day of home time gained) |
|---|---|---|---|---|---|
| No ROS | 6.2 | – | 312.28 | – | – |
| ROS | 82.3 | 76.1 | 313.36 | 1.08 | 70.74 |
| Intervention | Mean costs (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| No ROS | 12,702 | – | 0.4133 | – | – |
| ROS | 12,908 | 206 | 0.4137 | 0.0004 | 463,338 |
FIGURE 11.
Cost-effectiveness plane for ROS compared with no ROS.

FIGURE 12.
Cost-effectiveness acceptability curve for ROS compared with no ROS.

Comparison of continuous routine oxygen supplementation and nocturnal routine oxygen supplementation with no routine oxygen supplementation
Table 23 presents the mean outcomes per patient in terms of hospital stay, home time, EQ-5D-3L scores and QALYs for all three trial arms. Mean hospital stay was the greatest in the nocturnal ROS group. Home time was lowest in the nocturnal ROS group and highest for continuous ROS, by almost 3 days compared with no ROS. There was almost no difference in mean EQ-5D-3L scores at 3, 6 and 12 months. Overall QALYs for nocturnal ROS were slightly lower (mean difference –0.0023 QALYs) than for no ROS, and slightly higher than for continuous ROS (mean difference 0.0032 QALYs), but in both cases differences were very small and non-significant. Table 24 shows the disaggregated mean (SD) health-care costs per patient for both trial and additional oxygen, acute stay costs, readmissions and long-term care, as well as total health-care costs. Oxygen treatment costs were highest in the continuous ROS arm, and there were small differences in other health-care costs between trial arms. Total health care costs were higher in both ROS arms and highest in the continuous ROS arm, £246 (95% CI –£322 to £814) higher overall compared with no ROS.
| Variable | Intervention | ||
|---|---|---|---|
| No ROS (N = 2629) | Nocturnal ROS (N = 2633) | Continuous ROS (N = 2636) | |
| Mean hospital stay (days) (95% CI) | 18.4 (17.7 to 19.0) | 19.0 (17.9 to 20.1) | 18.5 (17.4 to 19.6) |
| Mean home time (days) (95% CI) | 313.0 (310.4 to 315.7) | 310.9 (306.6 to 315.6) | 315.9 (311.4 to 320.2) |
| EQ-5D-3L scores, mean (SD) | |||
| Baseline | 0 | 0 | 0 |
| 3 months | 0.48 (0.38) | 0.48 (0.37) | 0.48 (0.37) |
| 6 months | 0.48 (0.37) | 0.47 (0.37) | 0.48 (0.36) |
| 12 months | 0.46 (0.37) | 0.46 (0.37) | 0.46 (0.36) |
| Mean (SD) total QALYs over 12 months | 0.4133 (0.31) | 0.4109 (0.31) | 0.4164 (0.30) |
| Difference in QALYs (95% CI) (vs. no ROS) | – | –0.0023 (–0.018 to 0.014)] | 0.0032 (–0.0132 to 0.0019) |
| Cost category | Intervention | ||
|---|---|---|---|
| No ROS (N = 2629) | Nocturnal ROS (N = 2633) | Continuous ROS (N = 2636) | |
| Trial prescribed oxygen | 0.65 (0.42 to 0.94) | 64.32 (64.12 to 64.50) | 81.45 (81.21 to 81.67) |
| Additional oxygen | 5.52 (4.76 to 6.37) | 3.83 (3.27 to 4.46) | 14.97 (13.32 to 16.73) |
| Total oxygen treatment | 6.18 (5.40 to 7.05) | 68.15 (67.50 to 68.79) | 96.42 (94.61 to 98.26) |
| Acute care costs | 5130 (4855 to 5404) | 5125 (4862 to 5406) | 5265 (4990 to 5583) |
| Readmission | 978 (929 to 1032) | 976 (924 to 1030) | 997 (948 to 1052) |
| Stroke care after discharge | 6587 (6310 to 5406) | 6697 (6420 to 6984) | 6588 (6305 to 6855) |
| Total cost | 12,702 (12,309 to 13,115) | 12,867 (12,471 to 13,286) | 12,948 (12,523 to 13,347) |
| Mean difference (95% CI) (vs. no ROS) | – | 165 (–393 to 725) | 246 (–322 to 814) |
The cost-effectiveness analysis presented in Table 25 shows that nocturnal ROS is dominated by no ROS. An intervention is dominated if it is more costly but less effective. Here, the outcomes for nocturnal ROS are worse, with fewer days of home time but at a higher cost than no ROS. Continuous ROS costs £25 per extra day of home time gained compared with no ROS. In the CUA, nocturnal ROS was again dominated because of lower QALYs and higher costs. Continuous ROS had an ICER of £76,997 per QALY gained, well above standard NICE willingness-to-pay thresholds of £20,000 to £30,000 per QALY (Table 26). The cost-effectiveness plane in Figure 13 displays a high level of uncertainty in both cost and QALY differences, with points in all four quadrants, and the corresponding CEAC (Figure 14) shows a 31% probability of continuous ROS being cost-effective compared with no ROS at a £20,000 per additional QALY threshold, suggesting that it is not a cost-effective intervention.
| Intervention | Mean costs (£) | Cost difference (£) | Mean days of home time | Outcome difference (days) | ICER (£/day of home time gained) |
|---|---|---|---|---|---|
| No ROS | 6.2 | – | 312.28 | – | – |
| Nocturnal ROS | 68.2 | 62.0 | 310.87 | –1.41 | Dominated |
| Continuous ROS | 96.4 | 90.2 | 315.87 | 3.59 | 25.13 |
| Intervention | Mean costs (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| No ROS | 12,702 | – | 0.4133 | – | – |
| Nocturnal ROS | 12,867 | 165 | 0.4109 | –0.0023 | Dominated |
| Continuous ROS | 12,948 | 246 | 0.4164 | 0.0032 | 76,997 |
FIGURE 13.
Cost-effectiveness plane for continuous ROS compared with no ROS.
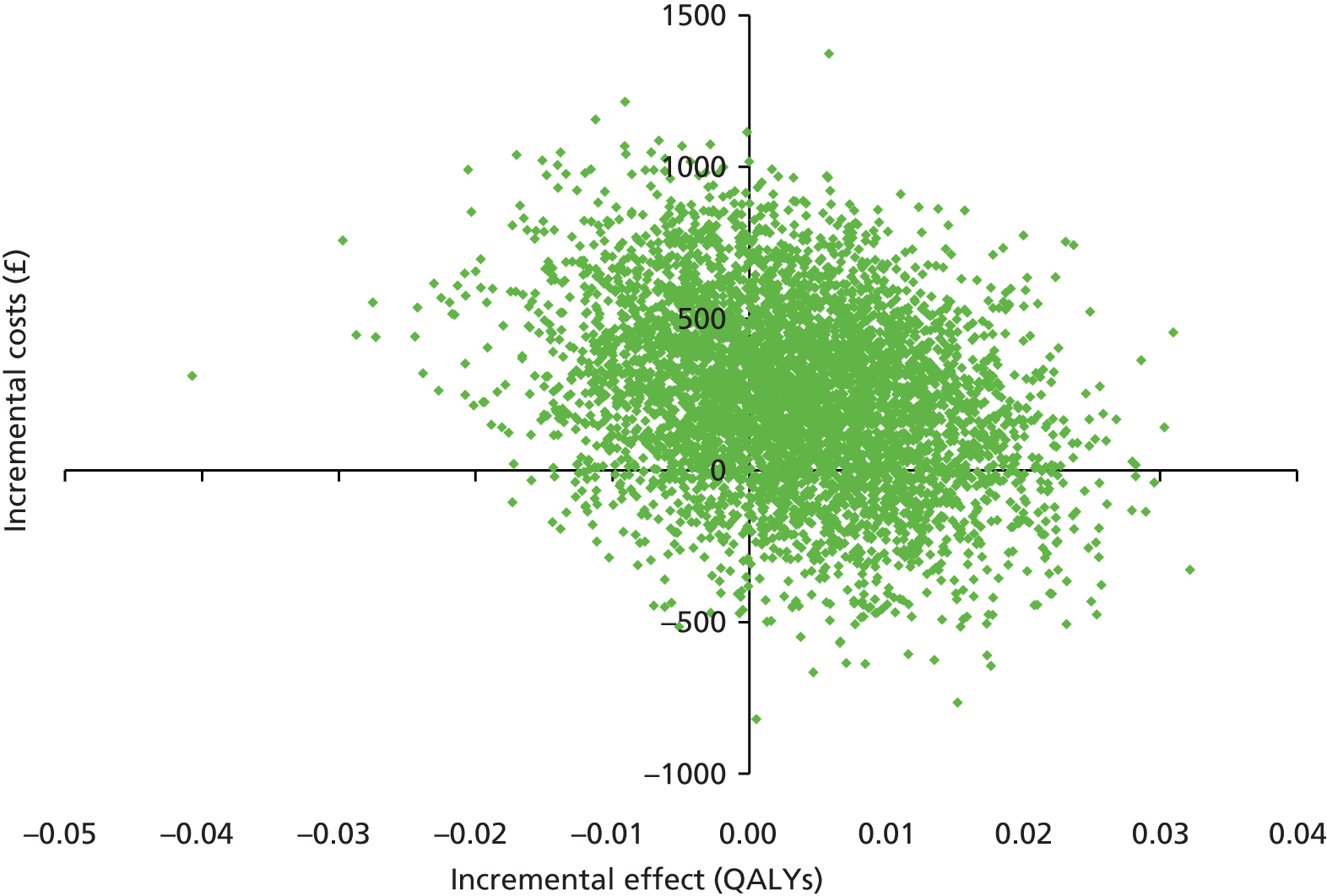
FIGURE 14.
Cost-effectiveness acceptability curve for continuous ROS compared with no ROS.

Sensitivity analysis
Deterministic sensitivity analysis was undertaken for the CUA for ROS versus no ROS. This was to assess (1) the impact on results of changing the health care cost inputs and (2) an alternative approach to valuing baseline quality of life. Three main types of cost (oxygen, acute care, long-term care) were individually changed by increasing and then decreasing the base-case value by 20% (Table 27). The difference in costs between ROS and no ROS changed very little across the board and all ICERs were over £400,000 per QALY gained. The differences in ICERs do appear to be large; however, this is a result of the sensitivity of the ICER to small changes in cost, owing to the extremely small difference in QALYs.
| Cost/outcome | Acute care costs increased by 20% | Acute care costs decreased by 20% | Total oxygen treatment cost increased by 20% | Total oxygen treatment cost decreased by 20% | Stroke care after discharge increased by 20% | Stroke care after discharge decreased by 20% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No ROS | ROS | No ROS | ROS | No ROS | ROS | No ROS | ROS | No ROS | ROS | No ROS | ROS | |
| Mean cost | 13,727 | 13,946 | 11,675 | 11,868 | 12,702 | 12,923 | 12,700 | 12,891 | 14,018 | 14,236 | 11,384 | 11,578 |
| Cost difference | – | 219 | – | 193 | – | 221 | – | 191 | – | 218 | – | 194 |
| Mean QALYs | 0.4133 | 0.4137 | 0.4133 | 0.4137 | 0.4133 | 0.4137 | 0.4133 | 0.4137 | 0.4133 | 0.4137 | 0.4133 | 0.4137 |
| QALY difference | – | 0.0004 | – | 0.0004 | – | 0.0004 | – | 0.0004 | – | 0.0004 | – | 0.0004 |
| Cost per QALY | – | 493,185 | – | 433,603 | – | 497,638 | – | 429,150 | – | 488,394 | – | 438,417 |
A further analysis was undertaken to look at the impact of applying a different method for valuing the baseline quality of life of patients (Table 28). The base-case assumed an EQ-5D-3L score of zero and a linear increase in quality of life to 3 months. The alternative analysis used the EQ-5D-3L value at 3 months for the baseline value. This increased the total mean QALYs over 12 months for both ROS and no ROS and the difference in QALYs also increased to 0.0008, but this is still a very small difference. The ICER decreased to £257,500 per QALY gained, still considerably higher than the cost per QALY threshold of £20,000 to £30,000 per QALY.
| Intervention | Mean costs (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| No ROS | 12,702 | – | 0.4729 | – | – |
| ROS | 12,908 | 206 | 0.4737 | 0.0008 | 257,500 |
Chapter 4 Discussion
The Stroke Oxygen Study is, with 8003 participants enrolled, the largest UK acute stroke trial. Over 50% of all stroke services in the UK contributed to the study, making participants representative of typical UK stroke patients. The results of SO2S show that routine low-dose oxygen supplementation after acute stroke does not improve early neurological recovery or long-term functional outcome. The observed lack of effect was consistent across all time points (1 week and 3, 6 and 12 months), all outcomes and all subgroups. There was no difference in outcome between the group given oxygen continuously and the group that was given oxygen at night only.
In the following sections potential reasons for lack of effect, limitations of the study, health economic aspects, what SO2S adds to existing evidence, what remains unknown, and implications for treatment and research will be discussed.
Potential reasons for the observed lack of effect
Mismatch of baseline clinical and demographic characteristics
The large sample size (n = 8003) makes mismatch of baseline clinical characteristics highly unlikely. Further safeguards against mismatch were built into the protocol. Randomisation by minimisation including key prognostic factors (age, sex, living alone, normal verbal component of the GCS, ability to lift both arms, the ability to walk, routine oxygen treatment during ambulance transfer and baseline oxygen saturation) ensured equal distribution of these factors across the three treatment groups. Effectiveness of minimisation was monitored continuously during the course of the study via online minimisation reports. The results in Table 6 confirm that there were no differences in demographics and baseline clinical characteristics between the three groups. The negative results of this study cannot be explained by a mismatch in baseline clinical characteristics.
The patients recruited to the study were not representative of acute stroke patients
To ensure that patients in SO2S were representative of acute stroke patients, inclusion criteria both for participating centres and for patient enrolment were wide. This is the largest and highest recruiting acute stroke study in the UK. In total 136 centres, including both acute and hyperacute stroke units, participated in the study. This means that over 50% of stroke services in the UK enrolled patients into SO2S. Stroke patients enrolled into clinical studies tend to have less severe neurological deficits than the overall stroke population unless the inclusion criteria specify that only severe patients can be included. Key reasons for this are both ethical constraints (it would not be ethical to include moribund patients who have nothing to gain from the trial intervention) and problems with obtaining informed consent in a patient group who have suffered a brain injury and are therefore often unable to make a fully informed decision. In spite of these unavoidable constraints, stroke severity in SO2S was representative of the stroke population in the UK. Participants in SO2S had a median NIHSS score of 5. While this seems low, it reflects patients admitted to acute stroke units. Data from 23,199 patients in the UK Sentinel Stroke National Audit Programme show a median NIHSS score of 4 for stroke patients in the UK. 82 The median NIHSS score of 127,950 patients with acute ischaemic stroke in the US Get with the Guidelines Register was 5,83 as in SO2S. A median NIHSS score of 5 was also recorded at baseline in a recently published Dutch study of antibiotic prophylaxis after stroke,84 which had similarly wide inclusion criteria to those of SO2S. This is a study of routine prophylactic oxygen supplementation in acute stroke. Patients with clinically significant hypoxia (e.g. oxygen saturations below 90%) who thus had indications for oxygen supplementation were not included. Our results therefore do not apply to clearly hypoxic patients. However, 717 of the participants had mild hypoxia (oxygen saturation below 95%) at the time of enrolment. The lack of benefit was the same in this subgroup as in patients who had normal or high-normal oxygen saturations at enrolment. While this study was not powered to examine this question, the results do not support the hypothesis that patients with lower baseline oxygen saturations are more likely to benefit from oxygen treatment. While we have not conducted formal screening logs for this study, discussions with participating centres about barriers to recruitment identified the requirement to recruit within 24 hours of hospital admission and the need for informed consent as the main barriers to enrolment, while clinical indications for oxygen treatment were rarely a problem. Apart from the protocol-driven exclusion of hypoxic patients, the participants in this study were as representative of the general stroke patient population as it is possible to achieve in a clinical study.
The amount of oxygen given may have been insufficient
The dose of oxygen given in SO2S was based on the results of a dose-ranging study and aimed to keep oxygen saturation within the normal range, avoiding both hyperoxia and hypoxia. The dose of oxygen given might have been too low. Studies of short-duration high-flow oxygen do not support this possibility but were too small to identify benefit or exclude harm. 30,85 The largest (n = 85) study of short-burst high-flow oxygen was terminated early because of excess mortality in the actively treated group,86 but it cannot be excluded that this difference was a result of baseline imbalance in the study groups rather than a true effect of oxygen. All three studies tested oxygen for 12 hours or less. It remains unknown whether or not higher-flow oxygen given during the whole hyperacute phase of stroke, as in SO2S, could be effective, but, given the results so far, this seems unlikely. Indeed, there is increasing concern that high-dose oxygen treatment could be harmful after stroke and in other forms of neurological injury. Hyperoxia (PaO2 > 39.99 kPa) was independently associated with mortality in a large retrospective cohort study of ventilated stroke patients. 87 Hyperoxia has also been related to delayed cerebral ischaemia in patients with subarachnoid haemorrhage (PaO2 > 23.06 kPa),88 to higher mortality and worse functional outcomes in traumatic brain injury (PaO2 > 26.66 kPa),89 and to increased in-hospital mortality following resuscitation from cardiac arrest (PaO2 > 39.99 kPa). 90 It is therefore highly unlikely that a higher dose of oxygen would have been more effective.
Oxygen treatment may have been ineffective because of poor compliance
Oxygen treatment can be effective only when it is given as prescribed. It is relatively easy to ensure that tablets or injections are given and to check compliance, as all drug treatments are recorded in the drug chart. Oxygen treatment is also prescribed on the drug chart, but given continuously, so that it is not possible to record compliance at all time points. Acute stroke patients are often confused and restless, and intentional or accidental removal of the nasal cannulae or oxygen mask is common. Minor benefits from oxygen treatment might therefore have been masked by poor compliance. Compliance with oxygen treatment was checked by two methods. Investigators were asked to document whether or not trial oxygen was prescribed. This was done as instructed in > 99% patients in the oxygen groups. They were also required to document whether or not the oxygen was actually given. The latter was recorded unreliably in the drug charts. Oxygen is normally prescribed only once as a continuous treatment, and nurses were not used to signing for ongoing oxygen treatment as they would for doses of medicines. To encourage more reliable recording of administration, we instructed centres to circle time points for signatures in the drug chart and put marks onto each field/time point requiring a signature (four times daily). In addition, we amended the protocol to require spot checks of compliance with oxygen treatment at different time points (00:00 and 06:00). This applied to the final 4144 patients. The results showed that oxygen was administered as prescribed in 65–83% of participants. These spot checks also showed that 3% of the control patients had oxygen in place at midnight and 6 a.m. Prescription of oxygen for clinical indications was permitted in all three treatment groups, and it was therefore appropriate that a small proportion of patients in the control group were found to have oxygen in place. While the main analysis of the study was intention to treat, a sensitivity analysis was conducted to examine the effects of non-compliance. This confirmed the main results that oxygen treatment, whether given continuously or at night-time only, did not improve outcome. The OR of the comparison of the combined oxygen groups with the control group was below 1, suggesting a possibility of harm, rather than benefit. However, this effect was very small and not significant statistically. It does nevertheless make poor compliance a less likely reason for ineffectiveness. Furthermore, oxygen treatment increased oxygen saturation in both the continuous and the night-time only treatment groups. Despite suboptimal compliance, we found highly significant increases of 0.8% and 0.9% in the highest and lowest oxygen saturations, respectively, in the oxygen groups compared with the control group during the intervention. While these changes in saturation are small, because of the S-shape of the haemoglobin dissociation curve, even small changes in oxygen saturation can reflect significant differences in the amount of oxygen dissolved in the blood. The high mean baseline oxygen saturation (96.6%) placed many participants on the flat part of the curve, where saturation changes little with increasing blood oxygen concentrations. A dose-ranging study of oxygen supplementation in stroke patients showed a 2.2% increase in oxygen saturation with 2 l/minute and a 2.9% increase with 3 l/minute. 31 In this study patients were observed continuously so that non-compliance could be excluded. However, the baseline oxygen saturation was lower at 95% and this will also have contributed to the relatively larger response. SO2S was a pragmatic study and aimed to reflect clinical practice rather than strictly supervised experimental conditions. Better compliance would require continuous observation of patients, and this is impracticable outside an intensive care unit.
Few participants in the Stroke Oxygen Study were recorded as being hypoxic
When planning the study we may have overestimated the number of patients who develop hypoxia. If hypoxia is uncommon, the effect of prophylactic treatment would be diluted. In SO2S very few (1.8%) participants in either group were recorded as having oxygen desaturations below 90%, with no differences between groups. In the Stroke Oxygen Pilot Study, 20% of patients in the oxygen group and 33% in the control group spent > 5 minutes with an oxygen saturation below 90% at night, but only 4% and 5%, respectively, spent > 60 minutes with an oxygen saturation below 90%. 31 Age, baseline oxygen saturation and NIHSS scores were similar in both studies. In the pilot study, oxygen desaturation was recorded continuously by pulse oximetry, while in SO2S pulse oximetry readings were taken from intermittent measurements recorded in the patients’ notes. It is likely that short episodes of hypoxia were missed in SO2S. This fits with evidence from a meta-analysis of continuous compared with intermittent monitoring of stroke patients, which also shows that intermittent monitoring detects fewer episodes of hypoxia than continuous monitoring. 91 A study of patients recovering from non-cardiac surgery showed that intermittent monitoring by ward nurses in a non-intensive care setting missed 90% of hypoxaemic episodes in which saturation was < 90% for at least 1 hour. 92 It is reasonable to assume that intermittent monitoring on stroke units is no more effective than that.
Neither the Stroke Oxygen Pilot Study nor SO2S found a significant difference in severe desaturations between the treatment and control groups, which may indicate that low-dose oxygen supplementation is not sufficient to prevent severe desaturations. 31 Intermittent spot checks of oxygen saturation are likely to miss a significant number of transient episodes of hypoxia. This study was not designed to monitor oxygen saturation. It is unlikely that the Stroke Oxygen Study population has had fewer episodes of hypoxia than other stroke populations monitored continuously. It is therefore very unlikely that the lack of effect can be explained by rarity of hypoxic episodes.
Oxygen may have been started too late to have an effect
In SO2S oxygen treatment was started at a median of 20 hours 43 minutes after symptom onset. This is well after the hyperacute phase of the stroke. It is therefore not possible to determine from the results of this study whether or not earlier oxygen treatment might have been more effective. However, other studies of oxygen treatment after stroke have enrolled patients earlier (Rønning and Guldvog29 within 24 hours, Singhal et al. 30 and Singhal 201086 within 8 hours, and Chiu et al. 93 within 12 hours), but also showed no beneficial effect.
Oxygen might still be beneficial, if given to the right subgroup of patients
Subgroup analyses did not identify any class of patient that was more likely to benefit from oxygen treatment. This included patients enrolled soon after onset of stroke, those with lower baseline oxygen saturation and patients with more severe strokes, the groups that were expected to be most likely to benefit from oxygen. However, patients who clearly needed oxygen because of dyspnoea or hypoxia were not enrolled into the study. The results of SO2S can therefore not be extrapolated to this subgroup.
Incomplete blinding may have introduced bias
Patients tend to expect benefit from active treatment rather than from control. For oxygen, this may apply more than for unknown new interventions, as many patients know that the brain needs oxygen and that oxygen cannot get to the brain when the blood supply is blocked. Giving more thus makes sense intuitively. Knowing whether or not they have had oxygen could thus have affected their assessment of recovery. Just under 50% of participants remembered their treatment allocation correctly at 3 months, reducing with time to 35% at 12 months. Bias towards effectiveness of oxygen treatment cannot be excluded with an open design. Bias towards assuming a worse outcome with oxygen treatment is very unlikely. SO2S did not show any effect of active treatment on any of the outcomes. While strong positive bias of the patients could have masked an adverse effect, this is unlikely. In addition, the lack of effect on functional outcomes was mirrored by a lack of effect on mortality, and the latter is not subject to bias.
Limitations to the study
Our aim was to test whether or not ROS improves outcome, not whether or not treating manifest hypoxia is beneficial. We have not included patients who were severely hypoxic (oxygen saturation below 90%) or had other definite indications for oxygen in the study. Therefore the results of the study do not apply to patients with severe hypoxia or those with definite indications for oxygen treatment not related to the stroke.
What does the Stroke Oxygen Study add to existing evidence?
There are two other published studies of low-dose oxygen supplementation after acute stroke. 30,85 In contrast to the much smaller Stroke Oxygen Pilot Study,31 we found no evidence of better early neurological recovery with oxygen. Our results confirm the findings of Rønning and Guldvog,29 who conducted a much smaller study, and found no overall benefit from oxygen given at 3 l/minute for 24 hours. While their subgroup analysis suggested a possibility of harm from oxygen in mild strokes, we found no evidence of an adverse effect on outcome in the subgroup of patients with mild strokes or with TIAs. While our results do not support routine oxygen treatment, they do provide reassurance on its safety.
A search of the literature identifying all randomised controlled studies investigating the effect of routine normobaric oxygen treatment on functional or neurological outcome in patients with acute stroke using a series of terms including ‘stroke’, ‘oxygen’ and ‘clinical trial’ up to March 2015 identified four published studies29–32,85 and one study reporting results online only. 86 A total of 979 patients were included. Oxygen was given in doses ranging from 2 to 45 l/minute and for durations of 8–72 hours. A meta-analysis was conducted including these and 8003 patients enrolled into SO2S. Statistical heterogeneity for these studies was moderate (I2 = 31%; Cochran Q; p = 0.21) and study estimates were combined using a Mantel–Haenszel method in a random-effects model. There was no reduction in mortality at 90 days (or by the end of follow-up when 90-day outcomes were not available) with oxygen treatment, with an averaged OR across studies of 1.11 (95% CI 0.84 to 1.46). Oxygen also had no effect on recovery, when reported (five out of six studies). SO2S provides clear evidence and adequate power to establish that ROS early after acute stroke does not improve outcome. As SO2S is much larger than earlier trials, the results of the meta-analysis reflect the outcome of this study. There is no indication from the other studies that giving oxygen earlier is more beneficial, or that higher doses are more effective. Unpublished results from one small study suggest that very high doses of oxygen may be associated with higher mortality (Aneesh B Singhal, Massachusetts General Hospital, 2010, personal communication). Concern about the potential of excessive doses of oxygen to cause harm has also been raised by a non-randomised observational study of 2894 ventilated stroke patients treated on intensive care units, for which hyperoxia was associated with higher mortality than both hypoxia and normoxia. 87
Rapid recruitment and study set-up
The Stroke Oxygen Study is the largest and fastest recruiting stroke study enrolling patients from the UK only. Recruitment of trial sites and set-up was facilitated by the stroke research network. This study would not have been possible without the research staff and infrastructure provided by the network.
Health economics
The results of the health economics analysis are in line with the clinical findings, demonstrating that there is no benefit from offering patients oxygen supplementation in hospital after stroke. The treatment does not result in significantly higher quality-adjusted survival and neither does it mean that patients return home more quickly, but it does increase costs of treatment. The point estimates of cost-effectiveness and the results shown in the CEACs do not suggest that the treatment is cost-effective. When the analysis considered all three trial treatments, nocturnal ROS was dominated, that is associated with slightly poorer outcomes but higher costs than no ROS. Continuous ROS resulted in about 3.5 days of home time gained, but this did not translate into significant QALY gains and the ICER was still well above the £20,000 to £30,000 NICE willingness-to-pay threshold used by UK NHS decision-makers. The CEAC also demonstrated a low probability of cost-effectiveness (31%) at £20,000/QALY. Implications for practice are that it should be recommended that patients do not need to receive oxygen supplementation unless clinically indicated.
This is the first health economics analysis of ROS compared with no ROS in stroke patients, and compared with many other economic evaluations has a very large sample size, thus increasing confidence in the robustness of the results. The main limitations of this study relate to data collection. In the majority of trial-based analyses it is possible to collect baseline quality-of-life data, which are then used in the calculation of QALYs. However, this data collection is not possible in acute admission for stroke. This creates problems in calculating QALYs; therefore, assumptions have to be made about the baseline quality of life. This issue has been explored previously and the methodology has been repeated here: the baseline quality of life score is assumed to be zero and then quality of life increases linearly to the 3-month score. 79 Obviously, patient quality of life trajectories will vary considerably, with some patients recovering very quickly, thus underestimating QALYs in the first 3 months. An alternative method was used in the analysis, with the 3-month score also being used for the baseline score. Comparison of the results of the two methods showed that there was no overall impact on the direction of the results. This remains an area in which further research is needed on the optimum way of dealing with baseline quality of life where data collection is not possible.
Detailed data were also not available on patients with regard to date of discharge and destination, or readmissions. Completion of parts of the patient questionnaires was suboptimal, and data collection on such a large number of patients also presented logistical difficulties. Therefore, assumptions were required regarding final discharge destination and an average length of readmission was assumed and an overall average cost applied, which may result in reducing the overall variability of health-care costs. Finally, the costs used for long-term care post discharge were obtained from a report from 2002. 79 More up-to-date costs of care for independent and dependent patients were sought; however, scrutiny of the literature did not yield any costs that were appropriate for this analysis and many economic evaluations in stroke since 2002 have used the same unit costs.
Future work
Post-stroke hypoxia is associated with adverse outcomes after stroke. SO2S has shown that routine low-dose oxygen supplementation does not improve this. Subgroup analysis has not identified any patient group more likely to benefit. We cannot exclude that very early enrolment could make a difference, but this was not supported by results from the small subgroup enrolled within less than 6 hours. We cannot extrapolate our results to use of high doses of oxygen, but there is currently no good support from published studies in humans. Evidence from observational studies suggests that hypoxia, especially if untreated, is associated with adverse outcomes. Hypoxia is a sign of underlying pathology (e.g. airway obstruction, pneumonia, heart failure, pulmonary embolism), which requires specific treatment. It is possible that oxygen treatment delays recognition and thereby also treatment of these complications. Future work should therefore focus on early detection and/or prevention of hypoxia.
The nature of the trial and large sample size will allow further analyses to be undertaken in the future to explore important questions regarding outcomes in the first 12 months after stroke. Two further analyses are planned. First, mean EQ-5D-3L scores will be calculated for groupings of mRS score levels to represent independence and dependence after stroke. These data will be valuable for use by future decision models for stroke, allowing the use of patient-reported utility values for model health states. The validity and the responsiveness of the EQ-5D-3L questionnaire in stroke patients will be explored by comparing responses with patient scores for the mRS, BI and the NEADL.
Conclusions
In conclusion, the results of SO2S conclusively demonstrate that low-dose oxygen supplementation in patients who are not severely hypoxic increases oxygen saturation, but is not associated with benefits after acute stroke in the whole population or in any of the subgroups. The observed lack of benefit is consistent across all outcomes and all time points up to 1 year, and the same whether oxygen is given continuously or at night-time only. Results of the health economic analysis confirm the lack of benefit of oxygen in quality of life and economic terms. Future research should address causes of hypoxia and examine methods to prevent the development of hypoxia.
Acknowledgements
This study would not have been possible without the help of many people who supported this trial in professional and lay roles. These include:
The Trial Management Group: Christine Roffe (Chairperson), Tracy Nevatte, Julius Sim, Richard Gray, Peter Crome, Natalie Ives, Jon Bishop, Sarah Pountain, Peter Handy and Linda Handy.
The Trial Steering Committee: Martin Dennis (Chairperson), Lalit Kalra, Sian Maslin-Prothero, Jane Daniels, Peta Bell and Richard Lindley.
The Data Safety and Monitoring Committee: Stephen Jackson (Chairperson), Professor Tom Robinson and Dr Martyn Lewis.
The Health Economics team at University of Birmingham: Susan Jowett and Cristina Penaloza.
The Study Team at Birmingham Clinical Trials Unit: Jon Bishop, Richard Gray, Andrew Howman, Robert Hills, Nick Hilken, Natalie Ives, Samir Mehta and Chakanaka Sidile.
The Trial Co-ordinating Centre: Alison Buttery, Clare Gething, Joy Dale, Wendy Lawton, Chris Buckley, Eddie Skelson, Nicola Mellor, Kathryn McCarron, Jean Leverett, Emily Linehan, Stephanie Edwards, Terri Oliver, Loretto Thompson, Sian Edwards, Clare Lees and Jackie Richards.
Meta-analysis: Frank Lally, Phillip Ferdinand, Girish Muddegowda and Julius Sim.
Editorial assistance: Frank Lally, David Roffe and Steve Alcock.
The National Institute for Health Research Stroke Research network and local network teams throughout the UK made rapid recruitment to this trial possible. Kathryn Jones, as the West Midlands Lead supported us locally and nationally.
Patient and public involvement: Peter and Linda Handy were involved with the trial from the start and contributed to its design. Peta Bell attended trial steering group meetings and Brin Helliwell and Norman Phillips advised on the final report.
Local investigators and research staff in the collaborating hospitals.
Contributions of authors
Christine Roffe originated the study idea, has been chief investigator for the trial and has drafted this report.
Tracy Nevatte was the trial manager and made major contributions to the draft report.
Jon Bishop did the statistical analysis.
Julius Sim contributed to the statistical analysis, conducted the sensitivity analyses and critically revised the manuscript.
Cristina Penaloza and Susan Jowett wrote the health economic section of the report.
Natalie Ives contributed to the protocol paper and the statistical analysis plan, and the interpretation of the results.
Richard Gray contributed to the development of the protocol and statistical analysis plan and the interpretation of the results.
Phillip Ferdinand, Girish Muddegowda and Julius Sim conducted the systematic review for the discussion.
Publications
Roffe C, Nevatte T, Sim J, Bishop J, Ives N, Ferdinand P, et al. Effect of routine low-dose oxygen supplementation on death and disability in adults with acute stroke: the Stroke Oxygen Study randomized clinical trial. JAMA 2017;318:1–11.
Roffe C, Crome P, Nevatte T, Gray R, Handy L, Handy P, et al. Stroke severity and mimics in patients recruited to the Stroke Oxygen Study (SO2S). Int J Stroke 2013;8:42.
Roffe C, Crome P, Nevatte T, Gray R, Handy L, Handy P, et al. Changes in the use of supplemental oxygen for stroke patients in the UK. Int J Stroke 2013;8:2.
Wu J, Nevatte T, Roffe C. The Stroke Oxygen Supplementation (SO2S) study: Comparison of postal and telephone responses of 12 months questionnaire follow up. Int J Stroke 2014;9:37.
Roffe C, Nevatte T, Crome P, Gray R, Sim J, Pountain S, et al. The Stroke Oxygen Study (SO2S) – a multi-center, prospective, randomized, open, blinded-endpoint study to assess whether routine oxygen treatment in the first 72 hours after a stroke improves long-term outcome: study protocol for a randomized controlled trial. Trials 2014;15(1):1–11.
Roffe C, Nevatte T, Crome P, Gray R, Sim J, Pountain S, et al. The Stroke Oxygen Supplementation (SO2S) study: A multi-centre, prospective, randomised, open, blinded-endpoint study of routine oxygen supplementation in the first 72 hours after stroke. Oral presentation at the European Stroke Congress, Nice, 6–9 May 2014.
Sim J, Gray R, Nevatte T, Howman A, Ives N, Roffe C. Statistical analysis plan for the Stroke Oxygen Study (SO2S): a multi-center randomized controlled trial to assess whether routine oxygen supplementation in the first 72 hours after a stroke improves long-term outcome. Trials 2014;15:229.
Roffe C, Nevatte T, Crome P, Gray R, Sim J, Pountain S, et al. The stroke oxygen study (SO2S): a multi-centre randomized controlled trial of routine oxygen supplementation within the first 72 hours after acute stroke. Oral presentation at the World Stroke Conference, Istanbul, 22–25 October 2014.
Roffe C, Nevatte T, Crome P, Gray R, Sim J, Pountain S, et al. The effect of routine oxygen supplementation on long term functional outcomes and quality of life after stroke: 6 and 12 month outcomes (The Stroke Oxygen Study). Oral presentation at the International Stroke Conference, Nashville, 11–13 February 2015.
Pountain SJ, Roffe C. Does routine oxygen supplementation in patients with acute stroke improve outcome? BMJ 2012;345:e6976.
Sim J, Teece L, Dennis MS, Roffe C and the SOS Study Team. Validation and Recalibration of Two Multivariable Prognostic Models for Survival and Independence in Acute Stroke. PLOS ONE 2016;11:e0153527.
Data sharing statement
We will make all data (with the exception of personally identifiable data) available to the scientific community once the key outputs have been published.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743-800. http://dx.doi.org/10.1016/S0140-6736(15)60692-4.
- National Audit Office, Department of Health . Reducing Brain Damage: Faster Access to Better Stroke Care 2005. www.nao.org.uk/wp-content/uploads/2005/11/0506452.pdf (accessed 1 January 2016).
- Morris S, Hunter RM, Ramsay AI, Boaden R, McKevitt C, Perry C, et al. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ 2014;349. http://dx.doi.org/10.1136/bmj.g4757.
- Fonarow GC, Saver JL, Smith EE, Broderick JP, Kleindorfer DO, Sacco RL, et al. Relationship of National Institutes of Health stroke scale to 30-day mortality in Medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc 2012;1:42-50. http://dx.doi.org/10.1161/JAHA.111.000034.
- Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability?. J Stroke Cerebrovasc Dis 2004;13:171-7. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2004.06.003.
- Stroke Unit Trialists’ Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2007;4.
- Roffe C. Hypoxaemia and stroke. Rev Clin Gerontol 2001;11:323-35. http://dx.doi.org/10.1017/S0959259801011443.
- Silva Y, Puigdemont M, Castellanos M, Serena J, Suñer RM, García MM, et al. Semi-intensive monitoring in acute stroke and long-term outcome. Cerebrovasc Dis 2005;19:23-30. http://dx.doi.org/10.1159/000081908.
- Lewis SC, Sandercock PA, Dennis MS. SCOPE (Stroke Complications and Outcomes Prediction Engine) Collaborations . Predicting outcome in hyper-acute stroke: validation of a prognostic model in the Third International Stroke Trial (IST3). J Neurol Neurosurg Psychiatr 2008;79:397-400. http://dx.doi.org/10.1136/jnnp.2007.126045.
- Nakajima S, Meyer JS, Amano T, Shaw T, Okabe T, Mortel KF. Cerebral vasomotor responsiveness during 100% oxygen inhalation in cerebral ischemia. Arch Neurol 1983;40:271-6. http://dx.doi.org/10.1001/archneur.1983.04050050039004.
- Yager JY, Thornhill JA. The effect of age on susceptibility to hypoxic-ischemic brain damage. Neurosci Biobehav Rev 1997;21:167-74. http://dx.doi.org/10.1016/S0149-7634(96)00006-1.
- Yamamoto M, Meyer JS, Sakai F, Yamaguchi F. Aging and cerebral vasodilator responses to hypercarbia: responses in normal aging and in persons with risk factors for stroke. Arch Neurol 1980;37:489-96. http://dx.doi.org/10.1001/archneur.1980.00500570037005.
- Roffe C, Corfield D. Hypoxaemia and stroke. Rev Clin Gerontol 2008;18:299-311. http://dx.doi.org/10.1017/S095925980900286X.
- Rowat AM, Dennis MS, Wardlaw JM. Hypoxaemia in acute stroke is frequent and worsens outcome. Cerebrovasc Dis 2006;21:166-72. http://dx.doi.org/10.1159/000090528.
- Bravata DM, Wells CK, Lo AC, Nadeau SE, Melillo J, Chodkowski D, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med 2010;170:804-10. http://dx.doi.org/10.1001/archinternmed.2010.92.
- Indredavik B, Bakke F, Slordahl SA, Rokseth R, Hâheim LL. Treatment in a combined acute and rehabilitation stroke unit: which aspects are most important?. Stroke 1999;30:917-23. http://dx.doi.org/10.1161/01.STR.30.5.917.
- Sulter G, Elting JW, Langedijk M, Maurits NM, De Keyser J. Admitting acute ischemic stroke patients to a stroke care monitoring unit versus a conventional stroke unit: a randomized pilot study. Stroke 2003;34:101-4. http://dx.doi.org/10.1161/01.STR.0000048148.09143.6C.
- Bateman NT, Leach RM. ABC of oxygen. Acute oxygen therapy. BMJ 1998;317:798-801. http://dx.doi.org/10.1136/bmj.317.7161.798.
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 1985;312:159-63. http://dx.doi.org/10.1056/NEJM198501173120305.
- Kontos HA. Oxygen radicals in cerebral ischemia: the 2001 Willis lecture. Stroke 2001;32:2712-16. http://dx.doi.org/10.1161/hs1101.098653.
- Sugawara T, Fujimura M, Noshita N, Kim GW, Saito A, Hayashi T, et al. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx 2004;1:17-25. http://dx.doi.org/10.1602/neurorx.1.1.17.
- Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res 2000;301:173-87. http://dx.doi.org/10.1007/s004419900154.
- Chan PH. Role of oxidants in ischaemic brain damage. Stroke 1996;27:1124-9. http://dx.doi.org/10.1161/01.STR.27.6.1124.
- Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int 2002;40:511-26. http://dx.doi.org/10.1016/S0197-0186(01)00122-X.
- Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med 2005;38:1433-44. http://dx.doi.org/10.1016/j.freeradbiomed.2005.01.019.
- Flynn EP, Auer RN. Eubaric hyperoxemia and experimental cerebral infarction. Ann Neurol 2002;52:566-72. http://dx.doi.org/10.1002/ana.10322.
- Singhal AB, Dijkhuizen RM, Rosen BR, Lo EH. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology 2002;58:945-52. http://dx.doi.org/10.1212/WNL.58.6.945.
- Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J 1976;1:1121-3. http://dx.doi.org/10.1136/bmj.1.6018.1121.
- Rønning OM, Guldvog B. Should stroke victims routinely receive supplemental oxygen? A quasi-randomized controlled trial. Stroke 1999;30:2033-7. http://dx.doi.org/10.1161/01.STR.30.10.2033.
- Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, et al. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke 2005;36:797-802. http://dx.doi.org/10.1161/01.STR.0000158914.66827.2e.
- Roffe C, Ali K, Warusevitane A, Sills S, Pountain S, Allen M, et al. The SOS pilot study: a RCT of routine oxygen supplementation early after acute stroke – effect on recovery of neurological function at one week. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0019113.
- Ali K, Warusevitane A, Lally F, Sim J, Sills S, Pountain S, et al. The stroke oxygen pilot study: a randomized controlled trial of the effects of routine oxygen supplementation early after acute stroke – effect on key outcomes at six months. PLOS ONE 2014;8. http://dx.doi.org/10.1371/journal.pone.0059274.
- European Stroke Organization . Guidelines for the Management of Ischaemic Stroke and Transient Ischaemic Attack 2008. www.congrex-switzerland.com/fileadmin/files/2013/eso-stroke/pdf/ESO08_Guidelines_Original_english.pdf (accessed 1 January 2016).
- Adams HP, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, et al. Guidelines for the early management of patients with ischemic stroke: a scientific statement from the Stroke Council of the American Stroke Association. Stroke 2003;34:1056-83. http://dx.doi.org/10.1161/01.STR.0000064841.47697.22.
- Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007;115:e478-534. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.181486.
- Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870-947. http://dx.doi.org/10.1161/STR.0b013e318284056a.
- National Clinical Guidelines for Stroke. London: Royal College of Physicians; 2004.
- Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA). NICE: London; 2008.
- National Clinical Guideline for Stroke. London: Royal College of Physicians; 2012.
- Arora A, Gray R, Crome P, Roffe C. What do physicians with an interest in stroke consider to be best practice in the diagnosis and treatment of hypoxia after acute stroke?. Br J Cardiol 2005;12:456-8.
- Olsen TS, Langhorne P, Diener HC, Hennerici M, Ferro J, Sivenius J, et al. European Stroke Initiative Recommendations for Stroke Management-update 2003. Cerebrovasc Dis 2003;16:311-37. http://dx.doi.org/10.1159/000072554.
- Khaja AM, Grotta JC. Established treatments for acute ischaemic stroke. Lancet 2007;369:319-30. http://dx.doi.org/10.1016/S0140-6736(07)60154-8.
- Müller-Plathe O. Clinical Laboratory Diagnostics: Use and Assessment of Clinical laboratory Results. Frankfurt: TH-Books; 1998.
- Ogburn-Russell L, Johnson JE. Oxygen saturation levels in the well elderly: altitude makes a difference. J Gerontol Nurs 1990;16:26-30. http://dx.doi.org/10.3928/0098-9134-19901001-08.
- Roffe C, Sills S, Halim M, Wilde K, Allen MB, Jones PW, et al. Unexpected nocturnal hypoxia in patients with acute stroke. Stroke 2003;34:2641-5. http://dx.doi.org/10.1161/01.STR.0000095188.65567.4F.
- Ali K, Sills S, Roffe C. The effect of different doses of oxygen administration on oxygen saturation in patients with stroke. Neurocrit Care 2005;3:24-6. http://dx.doi.org/10.1385/NCC:3:1:024.
- Iranzo A, Santamaría J, Berenguer J, Sánchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology 2002;58:911-16. http://dx.doi.org/10.1212/WNL.58.6.911.
- Turkington PM, Bamford J, Wanklyn P, Elliott MW. Prevalence and predictors of upper airway obstruction in the first 24 hours after acute stroke. Stroke 2002;33:2037-42. http://dx.doi.org/10.1161/01.STR.0000023576.94311.27.
- Harbison J, Ford GA, James OFW, Gibson GJ. Sleep-disordered breathing following acute stroke. Q J Med 2002;95:741-7. http://dx.doi.org/10.1093/qjmed/95.11.741.
- Meadows GE, O’Driscoll DM, Simonds AK, Morrell MJ, Corfield DR. Cerebral blood flow response to isocapnic hypoxia during slow-wave sleep and wakefulness. J Appl Physiol 2004;97:1343-8. http://dx.doi.org/10.1152/japplphysiol.01101.2003.
- Roffe C, Nevatte T, Crome P, Gray R, Sim J, Pountain S, et al. The Stroke Oxygen Study (SO2S) – a multi-center, study to assess whether routine oxygen treatment in the first 72 hours after a stroke improves long-term outcome: study protocol for a randomized controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-99.
- Sim J, Gray R, Nevatte T, Howman A, Ives N, Roffe C. Statistical analysis plan for the Stroke Oxygen Study (SO2S): a multi-center randomized controlled trial to assess whether routine oxygen supplementation in the first 72 hours after a stroke improves long-term outcome. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-229.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81-4. http://dx.doi.org/10.1016/S0140-6736(74)91639-0.
- Weir NU, Counsell CE, McDowall M, Gunkel A, Dennis MS. Reliability of the variables in a new set of models that predict outcome after stroke. J Neurol Neurosurg Psychiatr 2003;74:447-51. http://dx.doi.org/10.1136/jnnp.74.4.447.
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-70. http://dx.doi.org/10.1161/01.STR.20.7.864.
- Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994;25:2220-6. http://dx.doi.org/10.1161/01.STR.25.11.2220.
- Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35-41. http://dx.doi.org/10.1161/01.STR.24.1.35.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. http://dx.doi.org/10.1161/01.STR.19.5.604.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61-3. http://dx.doi.org/10.3109/09638288809164103.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. http://dx.doi.org/10.1136/bmj.316.7133.736.
- Dorman P, Slattery J, Farrell B, Dennis M, Sandercock P. Qualitative comparison of the reliability of health status assessments with the EuroQol and SF-36 questionnaires after stroke. United Kingdom Collaborators in the International Stroke Trial. Stroke 1998;29:63-8. http://dx.doi.org/10.1161/01.STR.29.1.63.
- Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Is the EuroQol a valid measure of health-related quality of life after stroke?. Stroke 1997;28:1876-82. http://dx.doi.org/10.1161/01.STR.28.10.1876.
- Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301-5. http://dx.doi.org/10.1177/026921558700100409.
- Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538-41. http://dx.doi.org/10.1161/01.STR.30.8.1538.
- Ali M, Jüttler E, Lees KR, Hacke W, Diedler J. VISTA and DESTINY Investigators . Patient outcomes in historical comparators compared with randomised-controlled trials. Int J Stroke 2010;5:10-5. http://dx.doi.org/10.1111/j.1747-4949.2009.00393.x.
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 1988;318:1728-33. http://dx.doi.org/10.1056/NEJM198806303182605.
- Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-85.
- Brown H, Prescott R. Applied Mixed Models in Medicine. New York, NY: Wiley; 2006.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5. http://dx.doi.org/10.3310/hta5330.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BJOG 2013;120:765-70. http://dx.doi.org/10.1111/1471-0528.12241.
- Mishra NK, Shuaib A, Lyden P, Diener HC, Grotta J, Davis S, et al. Home time is extended in patients with ischemic stroke who receive thrombolytic therapy: a validation study of home time as an outcome measure. Stroke 2011;42:1046-50. http://dx.doi.org/10.1161/STROKEAHA.110.601302.
- Quinn TJ, Dawson J, Lees JS, Chang TP, Walters MR, Lees KR. GAIN and VISTA Investigators . Time spent at home poststroke: ‘home-time’ a meaningful and robust outcome measure for stroke trials. Stroke 2008;39:231-3. http://dx.doi.org/10.1161/STROKEAHA.107.493320.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Vainiola T, Roine RP, Pettilä V, Kantola T, Räsänen P, Sintonen H. Effect of health-related quality-of-life instrument and quality-adjusted life year calculation method on the number of life years gained in the critical care setting. Value Health 2011;14:1130-4. http://dx.doi.org/10.1016/j.jval.2011.05.047.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- NHS Reference Costs 2013–14. London: DH; 2014.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- NHS . NHS Supply Chain - North Staffordshire Hospitals NHS Trust Stroke 2015. www.supplychain.nhs.uk (accessed 1 January 2015).
- Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al. A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS. Health Technol Assess 2002;6. http://dx.doi.org/10.3310/hta6260.
- Simons CL, Rivero-Arias O, Yu LM, Simon J. Multiple imputation to deal with missing EQ-5D-3L data: Should we impute individual domains or the actual index?. Qual Life Res 2015;24:805-15. http://dx.doi.org/10.1007/s11136-014-0837-y.
- Roffe C, Nevatte T, Sim J, Bishop J, Ives N, Ferdinand P, et al. Effect of routine low-dose oxygen supplementation on death and disability in adults with acute stroke: the Stroke Oxygen Study randomized clinical trial. JAMA 2017;318:1-11. https://doi.org/10.1001/jama.2017.11463.
- Smith CJ, Bray BD, Hoffman A, Meisel A, Heuschmann PU, Wolfe CD, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc 2015;4. http://dx.doi.org/10.1161/JAHA.114.001307.
- Fonarow GC, Pan W, Saver JL, Smith EE, Reeves MJ, Broderick JP, et al. Comparison of 30-day mortality models for profiling hospital performance in acute ischemic stroke with vs without adjustment for stroke severity. JAMA 2012;308:257-64. http://dx.doi.org/10.1001/jama.2012.7870.
- Westendorp WF, Vermeij JD, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJ, et al. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015;385:1519-26. http://dx.doi.org/10.1016/S0140-6736(14)62456-9.
- Padma MV, Bhasin A, Bhatia R, Garg A, Singh MB, Tripathi M, et al. Normobaric oxygen therapy in acute ischemic stroke: a pilot study in Indian patients. Ann Indian Acad Neurol 2010;13:284-8. http://dx.doi.org/10.4103/0972-2327.74203.
- Singhal AB. Normobaric Oxygen Therapy in Acute Ischemic Stroke n.d. https://clinicaltrials.gov/ct2/show/NCT000414726 (accessed 1 January 2016).
- Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med 2014;42:387-96. http://dx.doi.org/10.1097/CCM.0b013e3182a27732.
- Jeon SB, Choi HA, Badjatia N, Schmidt JM, Lantigua H, Claassen J, et al. Hyperoxia may be related to delayed cerebral ischemia and poor outcome after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatr 2014;85:1301-7. http://dx.doi.org/10.1136/jnnp-2013-307314.
- Brenner M, Stein D, Hu P, Kufera J, Wooford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg 2012;147:1042-6. http://dx.doi.org/10.1001/archsurg.2012.1560.
- Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 2010;303:2165-71. http://dx.doi.org/10.1001/jama.2010.707.
- Ciccone A, Celani MG, Chiaramonte R, Rossi C, Righetti E. Continuous versus intermittent physiological monitoring for acute stroke. Cochrane Database Syst Rev 2013;5. http://dx.doi.org/10.1002/14651858.cd008444.pub2.
- Sun Z, Sessler DI, Dalton JE, Devereaux PJ, Shahinyan A, Naylor AJ, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg 2015;121:709-15. http://dx.doi.org/10.1213/ANE.0000000000000836.
- Chiu D, Peterson L, Elkind MS, Rosand J, Gerber LM, Silverstein MD. Glycine Antagonist in Neuroprotection Americas Trial Investigators . Comparison of outcomes after intracerebral hemorrhage and ischemic stroke. J Stroke Cerebrovasc Dis 2010;19:225-9. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2009.06.002.
- Royston P, Barthel FMS, Parmar MKB, Choodari-Oskooei B, Isham V. Designs for clinical trials with time-to-event outcomes based on stopping guidelines for lack of benefit. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-81.
Appendix 1 Participating hospitals and Stroke Oxygen Study collaborators
Patients were recruited to the SO2S from 136 hospitals across the UK. Hospitals, local principal investigators, and numbers recruited per centre are listed according to the number enrolled starting with the highest recruiting centre.
-
University Hospital of North Staffordshire (C Roffe) (478)
-
St George’s Hospital (B Moynihan) (288)
-
The Royal Liverpool University Hospital (A Manoj) (256)
-
Royal Bournemouth General Hospital (D Jenkinson) (240)
-
Kings College Hospital (L Kalra) (231)
-
Leeds General Infirmary (P Wanklyn) (204)
-
Salford Royal Hospital (C Smith) (191)
-
Southend Hospital (P Guyler) (188)
-
Countess Of Chester Hospital (K Chatterjee) (176)
-
The Royal Victoria Infirmary (A Dixit) (168)
-
Royal Sussex County Hospital (K Ali) (164)
-
Musgrove Park Hospital (M Hussain) (156)
-
Wansbeck Hospital (C Price) (155)
-
Bristol Royal Infirmary (P Murphy) (151)
-
Royal Preston Hospital (S Punekar) (149)
-
University Hospital Aintree (R Durairaj) (148)
-
Birmingham Heartlands Hospital (D Sandler) (143)
-
Pennine Acute (Rochdale) (R Namushi) (134)
-
Queen’s Hospital, Burton (B Mukherjee) (131)
-
University Hospital Coventry (Walsgrave) (P Kanti – Ray) (129)
-
Royal Devon & Exeter Hospital (Wonford) (M James) (113)
-
Royal United Hospital Bath (L Shaw) (113)
-
Royal Cornwall Hospital (Treliske) (F Harrington) (112)
-
Queen Elizabeth The Queen Mother Hospital (G Gunathilagan) (105)
-
York Hospital (J Coyle) (105)
-
University Hospital Of North Durham (B Esisi) (99)
-
Derriford Hospital (A Mohd Nor) (95)
-
Selly Oak Hospital (Acute) (D Sims) (92)
-
St Helen’s & Knowsley Hospitals Trust (V Gowda) (89)
-
Torbay District General Hospital (D Kelly) (88)
-
Charing Cross Hospital (P Sharma) (87)
-
Leighton Hospital (M Salehin) (87)
-
Kent & Canterbury Hospital (I Burger) (84)
-
New Cross Hospital (K Fotherby) (84)
-
Northwick Park Hospital (D Cohen) (83)
-
Barnsley District General Hospital (M Albazzaz) (82)
-
Blackpool Victoria Hospital (J Mcilmoyle) (82)
-
Princess Royal University Hospital (L Sztriha) (81)
-
Eastbourne District General Hospital (C Athulathmudali) (76)
-
Warrington Hospital (K Mahawish) (75)
-
City Hospitals Sunderland (N Majmudar) (69)
-
William Harvey Hospital (Ashford) (D Hargroves) (69)
-
Stepping Hill Hospital (A Krishnamoorthy) (66)
-
The James Cook University Hospital (D Broughton) (66)
-
Northampton General Hospital (Acute) (M Blake) (59)
-
Leicester General Hospital (A Mistri) (57)
-
Rotherham District General Hospital (J Okwera) (56)
-
St Peter’s Hospital (R Nari) (56)
-
Macclesfield District General Hospital (M Sein) (55)
-
Manor Hospital (K Javaid) (54)
-
Bradford Royal Infirmary (C Patterson) (53)
-
Luton & Dunstable Hospital (L Sekaran) (50)
-
Royal Blackburn Hospital (N Goorah) (50)
-
University College Hospital (R Simister) (50)
-
North Tyneside General Hospital (C Price) (48)
-
Addenbrooke’s Hospital (E Warburton) (48)
-
Queen Alexandra Hospital (D Jarrett) (47)
-
North Devon District Hospital (M Dent) (45)
-
Pilgrim Hospital (D Mangion) (44)
-
Solihull Hospital (K Elfandi) (44)
-
Norfolk & Norwich University Hospital (N Shinh) (41)
-
Gloucestershire Royal Hospital (D Dutta) (40)
-
Royal Surrey County Hospital (A Blight) (39)
-
Southport & Formby District General Hospital (P McDonald) (39)
-
Bishop Auckland General Hospital (A Mehrzad) (35)
-
Airedale General Hospital (E AdbusSammi) (34)
-
Calderdale Royal Hospital (P Rana) (34)
-
Doncaster Royal Infirmary (D Chadha) (34)
-
East Surrey Hospital (B Mearns) (34)
-
Medway Maritime Hospital (S Sanmuganathan) (34)
-
Royal Derby Hospital (T England) (33)
-
Wycombe General Hospital (M Burn) (33)
-
Princess Royal Hospital (R Campbell) (32)
-
Harrogate District Hospital (S Brotheridge) (30)
-
Peterborough City Hospital (P Owusu-Agyei) (30)
-
West Cumberland Hospital (O Orugun) (30)
-
Colchester General Hospital (R Saksena) (29)
-
Royal Hampshire County Hospital (N Smyth) (29)
-
Dorset County Hospital (H Prosche) (27)
-
Frimley Park Hospital (B Clarke) (27)
-
Royal Hallamshire Hospital (M Randall) (27)
-
Yeovil District Hospital (K Rashed) (25)
-
Poole General Hospital (S Ragab) (24)
-
Frenchay Hospital (N Baldwin) (22)
-
Princess Alexandra Hospital (S Hameed) (22)
-
West Suffolk Hospital (A Azim) (22)
-
The Ulster Hospital (M Power) (21)
-
Watford General Hospital (D Collas) (21)
-
Southampton General Hospital (N Weir) (20)
-
Craigavon Area Hospital (M McCormick) (19)
-
Royal Lancaster Infirmary (P Kumar) (18)
-
Basildon Hospital (R Rangasamy) (17)
-
City Hospital Birmingham (S Kausar) (17)
-
Nottingham University Hospitals (A Shetty) (16)
-
Antrim Area Hospital (J Vahidassr) (15)
-
Pinderfields General Hospital (P Datta) (15)
-
Royal Albert Edward Infirmary (S Herath) (15)
-
Good Hope Hospital (E Smith) (14)
-
Hereford County Hospital (C Jenkins) (13)
-
South Tyneside District General Hospital (J Scott) (13)
-
Broomfield Hospital (V Umachandran) (12)
-
Wythenshawe Hospital (E Gamble) (11)
-
Warwick Hospital (B Thanvi) (10)
-
Ipswich Hospital (J Ngeh) (9)
-
Kettering General Hospital (K Ayres) (9)
-
Nevill Hall Hospital (B Richard) (9)
-
Scarborough General Hospital (J Paterson) (9)
-
Hull Royal Infirmary (A Abdul-Hamid) (8)
-
King’s Mill Hospital (M Cooper) (8)
-
The Royal London Hospital (P Gompertz) (8)
-
Trafford General Hospital (S Musgrave) (8)
-
Altnagelvin Area Hospital (J Corrigan) (7)
-
Darent Valley Hospital (P Aghoram) (7)
-
Royal Berkshire Hospital (A Van Wyk) (6)
-
Arrowe Park Hospital (R Davies) (5)
-
Basingstoke and North Hampshire Hospital (E Giallombardo) (5)
-
Lincoln County Hospital (S Leach) (5)
-
Hexham General Hospital (C Price) (4)
-
Manchester Royal Infirmary (J Simpson) (4)
-
Salisbury District Hospital (T Black) (4)
-
Mayday University Hospital (E Lawrence) (3)
-
Russells Hall Hospital (A Banerjee) (3)
-
Worthing Hospital (S Ivatts) (3)
-
Bedford Hospital (A Elmarimi) (2)
-
James Paget Hospital (M Zaidi) (2)
-
St Richard’s Hospital (S Ivatts) (2)
-
Erne Hospital (J Kelly) (1)
-
University Hospital Lewisham (M Patel) (1)
-
Bronglais General Hospital (P Jones) (0)
-
Hillingdon Hospital (A Parry) (0)
-
Kingston Hospital (L Choy) (0)
-
Morriston Hospital (M Wani) (0)
-
North Middlesex Hospital (R Luder) (0)
-
St Helier Hospital (V Jones) (0)
-
Staffordshire General Hospital (A Oke) (0)
-
The Princess Royal Hospital (K Ali) (0)
Appendix 2 The case report form
Appendix 3 The health economic questionnaire
Appendix 4 Further details on multiple imputation of missing European Quality of Life-5 Dimensions, three levels data
We used methods suggested by Royston et al. 94 regarding the rule of thumb that the number of cycles for MI should be at least equal to the percentage of incomplete cases of the data set. In addition, when variables with missing values to be imputed are highly correlated (which was the case in our data) the index EQ-5D values at 3, 6 and 12 months were highly correlated (–0.7 and more), and more than 10 cycles of MI were needed for convergence. We used 25 cycles of imputation.
The predictive mean matching approach was used, because the predicted missing value from the imputation model is replaced with the closest (5) values from the observed complete EQ-5D-3L index. This method is used when you want to restrict the imputed values to be within the range observed for the imputed variable (–0.594 and 1 in the case of the EQ-5D index).
The following model was used:
Where pmm stands for prediction mean matching, knn refers to the number of closest values from where a missing figure would be imputed, and add(25) refers to the number of cycles of MI included.
List of abbreviations
- BI
- Barthel Index
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CT
- computerised tomography
- CUA
- cost–utility analysis
- CVA
- cerebrovascular accident
- EQ-5D-3L
- European Quality of Life-5 Dimensions, 3 levels
- EQ-VAS
- European Quality of Life Visual Analogue Scale
- GCS
- Glasgow Coma Scale
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- MI
- multiple imputation
- mRS
- modified Rankin Scale
- NEADL
- Nottingham Extended Activities of Daily Living scale
- NIHSS
- National Institutes of Health Stroke Scale
- OR
- odds ratio
- QALY
- quality-adjusted life-year
- ROS
- routine oxygen supplementation
- SD
- standard deviation
- SO2S
- the Stroke Oxygen Study
- SSV
- Six Simple Variables
- TIA
- transient ischaemic attack
- TOAST
- Trial of Org 10172 in Acute Stroke Treatment