Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/35/32. The contractual start date was in January 2014. The draft report began editorial review in July 2016 and was accepted for publication in July 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Vinidh Paleri is a member of the National Institute for Health Research (NIHR) Health Technology Assessment programme Interventional Procedures Panel, and has received travel expenses to disseminate the trial results, as well as expenses from DP Medical Systems (Chessington, UK) and Merck & Co., Inc. (Kenilworth, NJ, USA); the expenses paid by Merck & Co., Inc., include fees to speak at a meeting. Nikki Rousseau and Tim Rapley report grants from NIHR during the conduct of the study.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Paleri et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Each year the NHS in England and Wales treats approximately 9000 new patients with head and neck squamous cell cancers (HNSCCs). Patients with oropharyngeal HNSCC formed the major group of patients who were eligible for this research project. The incidence of oropharyngeal cancer in the UK more than doubled in the 10 years between 1995 and 2006. 1 In Scotland, oropharyngeal cancer is the fastest-rising of all cancers. 2 In the USA, it is estimated that in 2020 oropharyngeal cancer will be more common than cancer of the uterine cervix. 3
Advanced (stages III and IV) HNSCCs are now treated non-surgically by radiation therapy (RT) or chemoradiation therapy (CRT). In CRT, chemotherapy is delivered concurrently with RT, potentiating tumour kill, but also toxicity, and consequently profoundly affecting eating and drinking by causing a range of side effects: loss of taste, dry mouth, pain, loss of appetite and impaired swallow mechanism. Over 90% of patients receiving this treatment option need nutritional support for severe dysphagia and weight loss, both during and after treatment. When necessary, nutritional support can be delivered through a pre-treatment gastrostomy tube or nasogastric tube.
Some clinicians advocate that, to maintain nutritional status, patients whose pre-treatment swallow function and oral intake are adequate should be fitted with a gastrostomy tube pre-treatment and continue with an oral diet during treatment, until they are no longer able to take adequate amounts of oral nutrition. Conversely, others offer patients with adequate pre-treatment swallow function the option of continued oral feeding, until they are unable to take oral nutrition adequate to maintain nutritional status and then proceed with (reactive) passage of a nasogastric tube as and when necessary. 4,5 Generic guidance suggests that gastrostomy tubes should be placed in patients who need enteral tube feeding for > 4 weeks. 6 Each year approximately 2500 gastrostomies are performed in HNSCC patients in the UK. The insertion costs alone are approximately £3M per annum.
Gastrostomy tube placement is an invasive procedure with a small, but defined, risk of acute serious complications;7 25–35% of patients retain the tube for > 1 year after CRT, and 10% retain it for > 2 years. 8 A gastrostomy tube has a major impact on patients’ and carers’ quality of life (QoL),9,10 as it can leak, leading to soiling of clothes and therefore interference with family life, intimate relationships and hobbies. 11 Although nasogastric tube placement is relatively simple, the small diameter of the tube means that it is prone to blockage, and, thus, repeated placement is necessary. If care is not taken to ensure correct placement, nasogastric tube misplacement in the lungs and subsequent feeding can lead to significant morbidity, now categorised as a ‘never event’ by the Department of Health and Social Care. 12 Systematic reviews have failed to demonstrate evidence for functional, nutritional, QoL or health economic benefits of either approach. 13,14 UK practice is correspondingly variable and no robust data are available. 15 Both nasogastric and gastrostomy tube users need community support, with greater needs for nasogastric tube users. The National Patient Safety Agency recommends that a full multidisciplinary-supported risk assessment should be carried out and documented before a patient with a nasogastric tube is discharged from acute care to the community. There is evidence that clinicians in some areas opt for gastrostomy tubes because of barriers to the delivery of nasogastric tube nutritional support in the community. However, a British Society of Gastroenterology survey in 2011 showed that only 64% of gastrostomy tube services offer an aftercare service. 16
Long-term dysphagia is now recognised as the principal functional consequence of CRT for HNSCC, and patients report this as a top concern. 14,17 Dysphagic patients and those dependent on tube feeds (gastrostomy and nasogastric tubes) need significant long-term supportive care, and suffer from an impaired QoL. 9 The effect of an enteral feeding route on the swallowing outcome is not well understood. Gastrostomy placement reduces the need for the patient undergoing chemoradiation to swallow to maintain nutritional status. Thus, it is likely that patients using gastrostomy tubes exhibit a reduction in use of the swallowing musculature. This reduction combined with the mucositis caused by radiation has been hypothesised to increase the risk of fibrosis in the muscles and pharyngo-oesophageal stricture.
The most severe CRT reaction that causes dysphagia is complete closure of the gullet, which is devastating for the individual and has substantial costs for the NHS. The risk of this may be higher with the use of a gastrostomy tube, which bypasses the gullet, unlike a nasogastric tube, which maintains a degree of oesophageal patency. Reconstruction requires complex major reconstructive surgery of the upper aerodigestive tract, with direct care costs of ≈£32,000 per patient18 and significant morbidity for the patients involved, even after intensity-modulated radiotherapy (IMRT), a new method of delivering RT that aims to limit morbidity by sparing the dose to some structures such as the salivary glands. There are national guidelines recommending that the proportion of HNSCC patients treated with IMRT should be increased. 19 However, with respect to swallowing outcomes, IMRT has been shown to increase stricture rates by 3.3 times;20 up to 46% of HNSCC patients treated with IMRT may need oesophageal dilatation,21 an intervention that requires inpatient care, is distressing to the patient and is associated with complications. Another intervention to prevent the development of chronic dysphagia is swallowing therapy, prescribed either prophylactically, to maintain function,22 or post treatment, to increase muscle range and flexibility. 23 Research into this field presents numerous challenges, including issues with recruitment, retention and adherence to rehabilitation programmes. 24
A systematic review14 suggested that the feeding route during treatment may have an impact on swallowing performance after CRT. Four retrospective studies,25–28 one prospective study29 and one randomised controlled trial (RCT) with small patient numbers30 identified that swallowing difficulties were more prevalent in patients receiving a prophylactic gastrostomy tube, even in the long term. However, existing research on the association between early gastrostomy tube feeding and long-term swallowing impairment has been inconclusive as a result of low participant numbers, caused by the use of insensitive dysphagia measurements, retrospective observational cohort study design and mixed treatment types with limited long-term follow-up. 31 Only two RCTs have been reported in the literature. 30,32 One recruited from a single Australian centre in an area of low population density. 30 The other recruited two-thirds of eligible patients, but details of gastrostomy tube placement were unclear and the sample included post-surgical patients who received an adjuvant, and, therefore, lower, dose of radiotherapy, with swallowing outcomes limited to a subsection of a QoL questionnaire. 32 A Cochrane review13 concluded that there was insufficient evidence to determine the optimal method of enteral feeding for patients with HNSCC receiving RT or CRT.
The two feeding methods (nasogastric tubes and gastrostomy tubes) have never been properly compared to establish which leads to better swallowing outcomes for patients, despite calls for better information to guide patient and clinician decisions. 13,25,30,31 This RCT was conducted to compare the two feeding methods (pre-treatment gastrostomy tube vs. oral feeding plus as-needed nasogastric tube) in patients with no, or only minimal, swallowing problems. Because a similar trial in Australia30 failed to recruit enough patients, we planned to first carry out a feasibility study to see whether or not a RCT is possible and how it should be conducted. Thus, research in this area may serve to direct resources appropriately, reduce unnecessary interventions and, thus, reduce morbidity and mortality, and improve swallowing outcomes.
Chapter 2 Methods and design
Aim and objectives
The aim of this study was to determine whether or not a definitive RCT in head and neck cancer patients with minimal swallowing problems undergoing CRT, comparing prophylactic gastrostomy tube feeding with oral feeding plus as-needed nasogastric tube feeding, was feasible. The TUBE trial feasibility phase was considered to be a necessary prelude to a full trial of these complex interventions, to assess whether or not an adequate proportion of eligible patients could be recruited into the study, in accordance with both quantitative and qualitative data.
The objectives were to:
-
explore barriers to, and facilitators of, trial implementation, and to use this information to improve recruitment and retention
-
willingness of participants to be randomised, to accept and persist with allocated treatment and to comply with assessments
-
willingness of clinicians [including clinical oncologists, surgeons, nutritionists and speech and language therapists (SALTs)] to recruit patients
-
qualitative assessment of patient and carer perspectives on trial participation, barriers to randomisation among non-participants, acceptability of assessment tools and experience of tube feeding and the conduct of and compliance with the trial protocol, and reasons for, and characteristics of, patients dropping out
-
-
carry out preliminary estimation of key parameters to inform definitive study design and study processes
-
confirm the primary outcome measure and associated power calculations for a definitive trial primary outcome with consideration of possible primary outcomes, including incidence of dysphagia, as measured by the common toxicity criteria, and dysphagia-related QoL, as measured by the MD Anderson Dysphagia Inventory (MDADI) HNSCC-specific self-report scale (variation and differences in change from baseline over time)
-
trial subsidiary QoL outcomes, measured using the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires, the EORTC Quality of Life Questionnaire – C30 (EORTC QLQ-C30) and the EORTC QLQ – Module for Head and Neck Cancer (EORTC QLQ-H&N35), and the Short Form questionnaire-36 items (SF-36), a multipurpose, short-form health survey and data collection tool for use of health and Personal Social Services (PSS) and patient costs
-
monitor nutritional parameters – body mass index, weekly weight changes (during treatment) and quantity of enteral nutrition consumed
-
derivation of an algorithm for when the nasogastric tube should be placed in the oral intake arm that is acceptable to patients and nutritionists
-
-
explore the cost-effectiveness of the two tube-feeding options and provide health economics metrics
-
assess the economic value of information based on a modelling exercise informed by the feasibility study and the existing systematic reviews
-
provide a preliminary estimate of the costs, effects and relative cost-effectiveness of the alternative methods of nutritional support based on the modelling exercise.
-
Design
A mixed-methods multicentre study to establish the feasibility of a RCT of feeding methods in patients with stage III or IV head and neck cancer receiving CRT with curative intent. The work was conducted over 24 months.
The components were a:
-
multicentre randomised controlled pilot trial comparing oral feeding plus pre-treatment gastrostomy tube with oral feeding plus as-required nasogastric tube feeding in patients with HNSCC. Patients were randomised on a 1 : 1 basis and stratified by IMRT and by the treating centre
-
qualitative process evaluation to inform the trial design by investigating patient, carer and staff experiences of trial participation
-
modelling exercise to synthesise available evidence and provide preliminary estimates of cost-effectiveness and value of information to inform future research.
Setting
The trial recruited from five tertiary NHS centres for HNSCC, two in the north and one in the south of England, and two in Wales, identified from respondents to our national survey. 15
Ethics considerations
Ethics approval was sought and granted by the Newcastle and North Tyneside 2 Committee, NHS Health Research Authority, reference number 14/NE/0045.
Target population
The target population was patients with stage III or IV HNSCC who received primary CRT with curative intent.
The inclusion criteria were:
-
grade 1 pre-treatment dysphagia, as defined by the Common Terminology Criteria for Adverse Events, version 4.0 (defined as asymptomatic/symptomatic/able to eat a regular diet)
-
consent to be randomised.
The exclusion criteria were:
-
declined to participate
-
unable to give informed consent
-
could not receive a gastrostomy for medical reasons
-
did not receive treatment with curative intent
-
malnourished and requiring immediate initiation of enteral feeding.
Primary outcomes for pilot trial
The primary outcome is feasibility, defined as the willingness of:
-
patients to be randomised, assessed by a review of patient screening logs and defined as –
-
the number of patients consenting to be randomised as a proportion of all patients approached about the trial, with reasons for non-consent
-
a qualitative assessment of the barriers to, and facilitators of, recruitment
-
-
clinicians to randomise patients, assessed by qualitative interviews
-
an assessment of retention and drop-out rates, defined as –
-
the number of patients who start randomised treatment as a proportion of the number randomised, with reasons for early drop-outs
-
the number of patients who complete randomised treatment as a proportion of the number randomised, with reasons for early drop-outs (including death)
-
a qualitative assessment of the barriers to, and facilitators of, data collection and participant retention.
-
-
Interventions
Pre-chemoradiation therapy gastrostomy arm
The endoscopic or radiologic gastrostomy tube was placed before the commencement of CRT. Given the pragmatic nature of this study, and the equivalent success rates of either technique, the choice of insertion method was left to the treating centre’s local protocols. Patients continued with oral feeding and commenced using liquid nutrition through the gastrostomy tube when they were unable to maintain an adequate oral intake to meet their nutritional requirements (< 75% of requirements based on a dietetic assessment of 24-hour recall by patients).
No pre-chemoradiation therapy gastrostomy arm
This group continued with oral feeding or had a nasogastric tube, if required, during treatment. The decision to place a nasogastric tube was based on clinical assessment, patient request and published guidelines. 6
Secondary outcome measures
Compliance with interventions and trial processes was defined as the number of patients who completed patient-reported outcomes at each time point, including baseline, with reasons given for non-compliance. Outcome measures for a definitive trial were to be rehearsed in the pilot trial, and applied before treatment and at 3 and 6 months after treatment (5 and 8 months from randomisation). The following measures were used:
-
The MDADI is a self-administered questionnaire designed specifically for evaluating the impact of dysphagia on the QoL of patients with head and neck cancer. 33 This is the proposed primary outcome measure in a definitive trial.
-
Performance Status Scale Normalcy of Diet scale, a clinician-rated scale of diet textures.
-
Two questionnaires were used to assess QoL outcomes. The EORTC QLQ-C30 (version 3.0) is a cancer-specific instrument, has a multidimensional structure, is appropriate for self-administration and is applicable across a range of cultural settings. 34 The EORTC QLQ-H&N35 is the head and neck module for EORTC QLQ-C30 (version 3.0) and is intended for use in conjunction with the QLQ-C30 in patients with head and neck cancer. 35
-
The SF-36 is a multipurpose, short-form health survey with 36 questions. It yields an eight-scale profile of functional health and well-being scores, as well as psychometrically based physical and mental health summary measures and a preference-based health–utility index that will be used to derive quality-adjusted life-years (QALYs) for a cost–utility analysis conducted as part of a subsequent full trial.
-
The use of health and PSS and costs to patients and their families/carers: as it will be necessary to elicit the costs to patients, their families/carers, and the NHS and PSS for the definitive economic evaluation, the tools required to elicit these costs were identified. These included case report forms and questionnaires to capture use of services and patient/family/carer costs. The content of these forms was developed in consultation with the study team, our existing item bank of questions, web-based resources, such as www.dirum.org, and experience of other RCTs of nutritional interventions, such as the recent SIGNET (Scottish Intensive care Glutamine or selenium Evaluative Trial). 36 The tool developed was administered at 3 and 6 months after treatment.
-
Monitoring nutritional parameters – body mass index before treatment and at 3 and 6 months following treatment, weekly weight changes during treatment and the quantity of enteral nutrition consumed.
-
Monitoring oral health – oral health assessment before commencing treatment and the end of 6 months for all dentate patients, including a full dental chart with panoramic radiographs before treatment and at 6 months, periodontal and oral hygiene assessment and plaque scores.
-
Other clinical outcomes to be recorded –
-
the number of pharyngeal/oesophageal dilatations per patient
-
tumour status at follow-up (decisions made as per local practice); clinically disease free, alive with disease, died of disease or died of other causes
-
tube dislodgements
-
migration from nasogastric group to gastrostomy tube group.
-
-
The assessment and reporting of incidence of adverse events (AEs) and serious adverse events (SAEs).
Sample size
The feasibility trial sample size and target recruitment is a total of 60 patients (30 per randomised intervention). 37 As a feasibility study, the trial will provide an assessment of recruitment and compliance rates to inform future definitive trial design. Statistical analyses will be conducted in accordance with a confidence interval (CI) approach rather than a hypothesis-testing approach. Analyses will be descriptive, reporting rates as proportions with CIs and graphical analyses of longitudinal data. Recruitment is dependent on the number of patients approached, but should be no lower than 50%. The upper limit of the 95% CI for the proportion of patients recruited should exceed 50%. If 120 patients were approached and 50% were recruited (60 patients randomised), the 95% CI for the recruitment rate would have a width of ± 9%. This would provide a good level of accuracy to assess the acceptability of the recruitment rate.
Screening, recruitment and consent
Identification and screening of participants
All potentially eligible patients were identified from the head and neck cancer multidisciplinary team (MDT) meetings, subject to satisfying the trial inclusion and exclusion criteria. This information was captured on site screening records and later transferred into an electronic screening form on the trial electronic data capture system. Whether or not a patient is eligible to participate in a trial can often be ascertained by referring to their records; in this trial, when further information was necessary, the principal investigator (PI) gathered it by taking a careful history from the participant. An eligibility screening form was completed by the investigator to document participants’ fulfilment of the entry criteria for all patients who were considered for the study and who were subsequently included or excluded.
Following recruitment discussions and randomisation, the screening records were updated to document the recruitment outcome details of everyone invited to participate in the study. The log-recorded information related to the inclusion and exclusion criteria and whether patients wanted to be part of the randomised feasibility trial and/or the qualitative interviews. The regular review and completion of the screening logs by sites ensured that potential participants were approached only once.
The screening assessments (as per routine clinical practice) usually occurred 1–2 weeks before the collection of baseline data and randomisation.
Recruitment procedures
All recruitment sites were full research sites. Eligible patients were seen at routine appointments for CRT planning. The PI or a delegated member of the clinical team (often a research nurse) invited patients to participate in the trial; they explained the trial to the patient, gave them the patient information sheet (PIS) and answered any questions.
There were two versions of the PIS to account for whether patients allocated to the pre-CRT gastrostomy arm were to have the gastrostomy tube inserted under X-ray guidance or endoscopically. Sites determined which version was appropriate for their patients depending on the sites chosen method of insertion.
Because the subject population was small, the information sheets and consent forms were available only in English. Interpreters were to be provided, if necessary, for patients who had difficulty understanding English.
Patients were encouraged to take the information leaflet home and discuss it with their family and friends. After receiving the study information, they were given a reasonable length of time (a minimum of 24 hours) to decide whether or not they wanted to participate. A research nurse followed up all invited patients with a telephone call and arranged to discuss the study further and/or take consent at the patient’s next hospital appointment (e.g. this could be a mould-making appointment, kidney function test appointment or magnetic resonance imaging-planning appointment).
If a patient refused to join the trial, their reason for refusal was sought. If the patient initially joined and subsequently withdrew, the reason for withdrawal was also sought. The rights of patients to refuse to participate or to withdraw without giving a reason were respected.
Consent procedures for the randomised trial
Informed consent discussions for the randomised feasibility trial were undertaken by appropriate site staff involved in the study (as per the delegation log), including medical staff and research nurses, and patients were given the opportunity to ask any further questions.
The delegated site staff taking consent ensured that patients had understood the information and that they would be asked to sign and date the consent form. The consent form was witnessed and dated by a member of the research team who had documented and delegated responsibility to do so.
The original signed consent form was retained in the investigator site file; a copy was placed in the clinical notes and another copy was given to the participant. Copies of consent forms were faxed to Newcastle Clinical Trials Unit to centralise the monitoring of the consent process. Patients provided specific consent for their general practitioner (GP) to be informed of their participation in the study. At the time of giving consent, participants were asked to fill in the baseline study questionnaires, which were sent to or kept in the site trial office.
Patients’ rights to refuse to participate without giving reasons were respected.
At this stage, patients were asked to indicate whether or not they were happy for the qualitative researcher to approach them later for an interview. Both those who did and those who did not consent to participate in the randomised trial were invited to take part in the qualitative interviews. The outcomes of the consent process were updated on the screening logs.
Study intervention details
Study participants were randomised to one of two treatment arms: the pre-CRT gastrostomy arm or the no pre-CRT gastrostomy arm.
Patients in both arms were given information about the treatment and the intervention involved. This was delivered by the PI at the centre and reinforced by the research nurse. Other health-care professionals (HCPs), such as dietitians, gastrostomy nurses, clinical nurse specialists and SALTs, also participated in the information-giving process.
Pre-chemoradiation therapy gastrostomy arm
The insertion of the gastrostomy tube took place before CRT commenced, approximately 2 or 3 weeks after most patients were randomised. When patients were receiving induction chemotherapy, gastrostomy tube insertion took place in either the week before cycle 2 of induction or during the week before CRT.
Gastrostomy tubes were inserted into the stomach, through an abdominal incision, by either endoscopic or radiological guidance, both being functionally equivalent. Given the pragmatic nature of this study, and the equivalent success rates of the techniques, the choice of insertion method was left to the treating clinician/centre. Patients were encouraged to continue with oral feeding throughout CRT, unless, or until, they were unable to maintain an adequate oral intake to meet their nutritional requirements (see guidance below) or were unable to swallow. At this stage, the use of liquid nutrition through the gastrostomy tube was commenced.
No pre-chemoradiation therapy gastrostomy arm
This group of patients continued oral feeding throughout CRT, unless, or until, they were unable to maintain an adequate oral intake (see guidance below) or were unable to swallow when a nasogastric tube was inserted under local anaesthesia and liquid nutrition via a nasogastric tube commenced. Confirmation of correct placement was made based on National Patient Safety Agency and local guidelines. The decision to place a nasogastric tube was based on clinical assessment, patient request and published guidelines.
Guidance on when to initiate enteral feeding in both treatment arms
National guidelines state that tube feeding should commence when a patient is at risk of malnutrition and has an inadequate oral intake. In practice, this is quite difficult to determine without collecting detailed food or oral supplements intake data to determine when oral intake is inadequate. Such an exercise was beyond the scope of this study.
As a guideline for this protocol, we set a figure of < 75% of requirements as a measure of inadequacy, and this would equate to about 1–2 lb (0.5 kg) of weight loss per week. This guideline applied to both treatment groups. The < 75% threshold was not ascertained by exact measurement, but based on a dietetic assessment of 24-hour recall by patients.
This < 75% guideline is more conservative and would lead to less weight loss in this patient population than the European Society for Clinical Nutrition and Metabolism guidelines, which stipulate a threshold of 60% and predict continued poor oral intake for > 10 days.
Tube removal in both treatment arms
Tube removal was determined by dietetic assessment. Once a patient was able to take > 75% of estimated nutritional requirements by mouth, and their weight was stable, the gastrostomy tube or nasogastric tube was removed.
Randomisation and blinding
Randomisation
Randomisation was administered centrally by the Newcastle Clinical Trials Unit internet-accessed secure web-based system with accessibility 24 hours per day, with inbuilt validation/plausibility checks at time of data entry.
A block-stratified block method (based on permuted random blocks of variable length) was used to allocate participants to the two groups in a 1 : 1 ratio. Randomisation was stratified by centre to allow for any differences in care or case mix that could alter outcomes. The use of induction chemotherapy was not a stratification factor in this feasibility trial, but it was recorded at the time of randomisation to inform possible stratification factors in a definitive trial. An individual not otherwise involved with the study produced the final randomisation schedule for use by this system.
The PI at the site, or an individual with delegated authority, was able to access the web-based randomisation system. Patient screening identification, their initials and the details of stratifying variables, was entered into the web-based system, which returned a unique patient trial number and the randomised treatment allocation. Participants were informed of their treatment group at the point of randomisation.
Blinding
Owing to the nature of the tube-feeding interventions, it was not practicable to blind the research nurses to the treatment allocated to patients for the follow-up assessments. The baseline data assessments were completed by research nurses before randomisation to reduce the chance of bias.
Trial data
Patient assessments and data collection
Research nurses in each unit co-ordinated the assessments and data collection, once written consent had been taken. Whenever possible, the first research visits were co-ordinated with patients’ pre-treatment planning appointments, and took place in the treating hospital. Some of the assessments were collected as part of routine clinical information (i.e. height/weight, oral health assessment) and permission was sought as part of the consent process to access this clinical information in order to avoid duplication. Whenever possible, patients were encouraged to complete the questionnaires at the research visit while the research nurse was available to give assistance as appropriate and to increase the rate of returns. The research nurse identified the time point for the follow-up research visits. These visits were combined with follow-up cancer surveillance appointments at the head and neck cancer unit, whenever possible.
The patient visits for the feasibility RCT and associated data were collected as follows.
Initial screening visit
At this appointment patients were provided with information about the trial and an eligibility screening form was completed.
Consent and baseline visit(s), and randomisation
The consent and baseline visit took place at least 24 hours after the patient had been provided with the trial information. The patient’s eligibility was reconfirmed and their informed consent was taken, after which the baseline assessments and baseline questionnaires were completed.
The baseline data included site of disease, patient demographic information, tumour, node and metastasis classification, a record of whether or not induction chemotherapy was planned, a record of whether or not IMRT was planned, and whether or not the patient had been given any pre-treatment swallowing exercises and, if so, if the patient had complied with these (recorded as yes or no). Questionnaires included the MDADI, EORTC QLQ-C30 (version 3.0), EORTC QLQ-H&N35 and the SF-36.
Clinical assessments included body mass index and usual weight, measurement on the Performance Status Scale, normalcy of diet and data from oral health assessment performed as standard NHS care (includes information from a panoramic radiograph, a dental chart, periodontal and oral hygiene assessment plaque scores, oral opening measurement and oral dryness).
After the baseline data and consent visit took place, randomisation was performed. Patients were informed of their randomisation allocation and given the opportunity to ask further questions.
Intervention visit
Interventions were performed as follows (timing was dependent on the treatment arm):
-
pre-CRT gastrostomy arm – gastrostomy tubes were inserted after the consent and baseline visit, and randomisation, but before any treatment (CRT/IMRT) took place
-
no pre-CRT gastrostomy arm – nasogastric tube was inserted after the consent and baseline visit, and randomisation, at a point when the patient and/or clinician assessed that this was most appropriate.
Weekly data collection
Information recorded included:
-
dates of induction chemotherapy plus number of cycles received
-
regime of concomitant chemotherapy and number of cycles received
-
details of RT technique, dose and field size, including mean dose to the pharyngeal constrictors
-
weekly weight
-
Performance Status Scale measurement, normalcy of diet
-
gastrostomy tube site infections
-
radiographs associated with tube placements
-
pH problems requiring nasogastric tube placement
-
tube changes (e.g. nasogastric tube to nasojejunal tube or nasogastric tube to another nasogastric tube)
-
degree of reliance on feeding tube use (nasogastric tube, nasojejunal tube or gastrostomy tube)
-
feed-related hospital admissions
-
number of dietetic consultations
-
quantity of enteral feed prescribed and quantity consumed
-
district nurse visits and dates
-
tumour status – clinically disease free, alive with disease or died of disease or other causes
-
AEs.
Follow-up visit at chemoradiation therapy completion
All outcome measures were recorded at the end of treatment in addition to the migration of patients from nasogastric tube to gastrostomy tube, replacement episodes and removal of nasogastric tube/gastrostomy tube, if applicable.
Follow-up visit at 3 months post chemoradiation therapy completion
All outcome measures were collected as per the end of treatment. In addition to the use of health and PSS and costs to patients and their families/friends (excluding the use of weekly/biweekly services in a cancer clinic), data on the number of pharyngeal/oesophageal dilatations were collected per patient. Information on access to rehabilitation services was recorded, including frequency of dietetic and speech and language therapy follow-up.
Follow-up visit at 6 months post chemoradiation therapy completion
The same measures as per 3 months post CRT were recorded. Oral health assessment data were included.
Criteria for progression to a Phase III trial
The decision to move to a Phase III trial is based on:
-
adequate timely recruitment with a 50% recruitment rate
-
completeness of outcome measurement (MDADI at 6 months) – excluding those individuals who died during the study period, these outcome data should be successfully collected in at least 80% of participants, as this is the proposed primary outcome of a definitive Phase III trial
-
economic criteria of the economic value-of-information analysis to suggest that further research is likely to be worthwhile.
Qualitative process evaluation (objective 1)
The integrated qualitative process evaluation explored the barriers to, and facilitators of, trial implementation. Through regular feedback of emerging findings to the trial team, this process informed the day-to-day running of the feasibility trial and optimised recruitment and retention. It also aimed to identify aspects of trial processes that could be improved for the definitive trial.
Normalisation process theory
Analyses of barriers to, and facilitators of, trial participation were informed by normalisation process theory (NPT). 38 NPT considers factors that affect implementation in relation to four key areas: (1) how people make sense of a new practice, in this case the trial (coherence); (2) the willingness of people to sign up and commit to the new practice (cognitive participation); (3) people’s ability to take on the work required of the practice (collective action); and (4) activity undertaken to monitor and review the practice (reflexive monitoring). This theory is increasingly being used in studies of the implementation of interventions in health care (www.normalizationprocess.org). In the TUBE trial, an assessment was made of how well trial processes were introduced and incorporated at each site for both patient and professional groups.
Methods and sampling
In keeping with the principles of rigorous qualitative research, the actual sample size was informed by the point of data saturation. In response to the study context, in some cases fewer interviews or observations were conducted and in others additional data were collected in the light of emerging analysis or study events. A balance was achieved between spread of data (to avoid missing key events or issues) and depth of data (a manageable data set that allowed for in-depth analysis).
Data collection focused on three inter-related phases during the trial. After appropriate consent was obtained, interviews were undertaken with health professionals and patients, and recruitment discussions were observed.
Pre-implementation of the TUBE pilot trial
To understand the context of the trial, professionals’ views and expectations and patient pathway mapping, formal interviews were conducted with key individuals involved in treatment planning for HNSCC (e.g. nurses, speech therapists and ear, nose and throat surgeons).
Patient recruitment phase
Patients’ and professionals’ experiences of recruitment were the focus of this phase. Patients were recruited from multidisciplinary clinics, with research nurses providing trial information. Qualitative interviews were conducted with patients who consented to take part in the trial. The focus of these interviews was the patients’ experiences and understandings of trial processes (e.g. the information they were given, the recruitment encounter, their ideas and/or concerns about randomisation and consent) and the intervention (their willingness to undergo either feeding option; their ideas and/or concerns about the impact on health and acceptability).
Patients were interviewed within 2 weeks of the recruitment discussions and offered a choice of location and method of interview (telephone or face to face). As people often discuss clinical decisions with others,39 those patients who wanted to involve a family member in their interview were able to do so. Further interviews were conducted with professionals involved in some aspect of patient recruitment. Research nurses were given a digital voice recorder and asked to record their recruitment discussions with patients (with the appropriate permission).
Patient follow-up phase
Patients took part in qualitative interviews approximately 8 months after recruitment to explore the acceptability of the assessment tools and investigate their experiences of the intervention.
Qualitative study consent details: interviews
During the recruitment discussion, as part of which written consent was taken for participation in the TUBE trial, patients, and any friends or family present, were asked if they were willing to be contacted about an interview for the qualitative substudy. If so, their written consent was taken and their contact details were made available to the qualitative researcher, who accessed the data on the study database. In addition, patients were given a separate information sheet. Written consent was obtained before the start of the face-to-face interviews; the qualitative researcher kept a record of verbal consent for those interviews conducted over the telephone.
Patient and family/friend interviews
The qualitative researcher telephoned patients who expressed interest in the qualitative interview element of the study (including those who consented to and those who declined randomisation) to further discuss their participation. Patients and family/friends from sites in the North of England were offered a choice of location and method of interview (telephone or face to face). Patients and family/friends elsewhere were offered telephone interviews only. Patients who wanted to involve a family member in their interview were able to do so.
Interviews with patients took place at two time points: 1 or 2 weeks after the initial recruitment discussions (to understand the barriers to, and facilitators of, recruitment) and during patient follow-up (to understand patients’ experiences of trial participation).
Summary of interview data collection
-
Took place 1–2 weeks after the recruitment discussion.
-
Trial participants’ experiences of recruitment, views on supplementary feeding, reasons for participation and feelings about randomised allocation.
-
Family and friends’ (of participants) experiences of recruitment, views on supplementary feeding, reasons for participation and feelings about randomised allocation.
-
Trial decliners’ experiences of recruitment, views on supplementary feeding and reasons for declining.
-
Family and friends’ (of decliners) experiences of recruitment and views on supplementary feeding.
-
Eight months’ follow-up.
-
Trial participants’ experiences and views of the TUBE trial (outcome measurement, etc.) and experiences of supplementary feeding.
Patient consent for observation/audio-recording of recruitment discussions
The observation and audio-recording of recruitment discussions have been key to improving recruitment processes in other randomised trials. We designed a three-stage consent process for this part of the study, balancing the need for informed consent with the need to not disrupt the process of consent for the TUBE trial. The main study information sheet, given to patients before the recruitment discussion, included a brief outline of the purpose and design of the observation or audio-recording of recruitment discussions.
-
At the start of the recruitment discussion, verbal consent to record the conversation was obtained. It was explained that more information about this would be given during the discussion and that there would be an opportunity to rescind consent at the end. Everyone present had to give verbal consent; if anyone declined, then the audio-recording did not take place.
-
Written consent for audio-recording was taken as part of the consent process for the TUBE trial. There were separate consent forms for those declining participation in the TUBE trial and for family/friends present. Everyone present had to give written consent for audio-recording; if anyone declined, then the recording had to be deleted immediately (while the person declining was present).
-
Those patients and family/friends who gave written consent for the audio-recording to be kept were given a follow-up information sheet. Prominently on the front page of this information sheet was the information that patients and family/friends had a further opportunity to change their minds about audio-recording by getting in touch with either the recruitment nurse or the qualitative study team.
Clinician interviews and observations
A qualitative researcher conducted the interviews with clinicians. Written informed consent was obtained to audio-record the face-to-face interviews and recruitment discussions. When telephone interviews were conducted, verbal consent was obtained at the start.
The aim of these interviews was to:
-
understand and map processes of care in relation to supplementary tube feeding in patients undergoing CRT for head and neck cancer
-
understand perspectives on supplementary feeding in patients undergoing treatment for head and neck cancer
-
understand experiences of the TUBE trial, including those of patient recruitment.
The selection of clinicians for interview was purposive and informed by factors such as emerging variation in recruitment rates between sites and changes in key personnel.
Qualitative data management and analysis
The interviews and recruitment discussions were audio-recorded with participants’ consent, transcribed verbatim and edited to ensure anonymity of the respondent. Data were managed using NVivo version 10 software (QSR International, Warrington, UK). The analysis was theoretically informed by the NPT and conducted in accordance with the standard procedures of rigorous qualitative analysis,39 including open and focused coding, constant comparison, memoing,40 deviant case analysis41 and mapping. 42 We undertook independent coding and cross-checking and a proportion of data were analysed collectively in ‘data clinics’, where the research team shared and exchanged interpretations of key issues emerging from the data.
Relationship between process evaluation and pilot trial
The findings were regularly fed back to the study team and appropriate changes were made to processes during the lifetime of the study. These included a ‘feed-forward’ to sites aiming to address health professionals’ concerns about patient overload, and content in the TUBE newsletters.
Economic analysis (objective 3)
The aims of the economic analysis were to (1) perform a within-trial economic evaluation for this feasibility study; (2) carry out a preliminary estimate of the relative cost-effectiveness of the alternative methods of nutritional support assessed through a modelling exercise from a NHS and PSS perspective; and (3) use the decision-analytic model to estimate the value of future research using variants of a value-of-information analysis.
Within-trial economic evaluation
Should a definitive economic evaluation be conducted in the future, costs to patients and their families/carers, the NHS and PSS would need to be elicited. In a trial-based economic evaluation, the resources used by each patient, such as hospital admissions, consultations and medication use, are normally recorded during the trial. 43 As part of this feasibility study, we planned to develop the tools necessary to elicit these costs, that is, data collection forms and questionnaires to capture the use of hospital and primary care services and patient/family/carer costs, and to summarise the data collected using these tools to inform a future trial. We also intended to use the data to inform the model-based economic evaluation and the adjustments to the data collection tools that would be required for a full economic evaluation. These data collection tools were tailored to reflect the needs of the study participants and to ensure that there was no double-counting of the use of services.
All patients randomised to one of the two trial arms – pre-treatment gastrostomy tube feeding or nasogastric tube feeding – were followed during the trial, and, when available, information was recorded about their health service utilisation related to tube feeding. The data collected were not statistically analysed but, rather, summarised descriptively for completeness.
Economic modelling
A bespoke decision-analytic economic model was developed to estimate the costs, effects and relative cost-effectiveness of the two methods of nutritional support. The clinical pathways of patients in the feasibility study were used to help inform the model structure. For the purposes of clarity, and for improved flow, the methodology for the economic model is discussed in Chapter 3.
Value-of-information analysis
The economic model was able to provide only a preliminary indication of the relative clinical effectiveness and cost-effectiveness of the different methods of nutritional support. An additional purpose was to help inform decisions about the direction of future research. This was explored through variants of a value-of-information analysis: an expected value of perfect information (EVPI) analysis and an expected value of partial perfect information (EVPPI) analysis.
A value-of-information analysis can quantify the expected gain in net benefit from obtaining further information to inform a decision. A decision based on existing information will be uncertain, so it may turn out to be incorrect if more information becomes available in the future. If the decision turns out to be incorrect, then there will be a cost in terms of lost health benefit and wasted resources. Quantifying the value of an incorrect decision, alongside the probability of making an incorrect decision, allows us to estimate the EVPI. The EVPI for a decision problem must exceed the cost of future research to make additional investigation worthwhile.
As well as determining the EVPI around the decision as a whole, value-of-information approaches can be used for particular elements of the decision with the purpose of focusing research in areas in which the elimination of uncertainty might have the most value. An EVPPI analysis can be used to estimate the expected value of removing uncertainty surrounding specific parameters or groups of parameters to identify areas in which future research should focus on identifying more precise and reliable estimates of specific pieces of information, such as relative effectiveness, costs or utilities. EVPI places an upper value on conducting further research overall, while EVPPI places an upper value on conducting further research on a specific area of information. Therefore, if the EVPI or EVPPI exceeds the expected costs of that additional research, then it is potentially cost-effective to acquire more information by undertaking this research.
Expected value of perfect information and EVPPI at an individual level were estimated non-parametrically from the model. However, to inform decisions, population EVPI estimates were also assessed based on the number of individuals who would be affected by the additional information over the anticipated lifetime of the technology. Given the fact that additional research in the present will produce net monetary benefits in the future, a discount rate was applied. Formally, a population EVPI estimate can be expressed as:
where I is the incidence in the period, t is the period, T is the total number of periods for which information from research would be useful and r is the discount rate. 44
To estimate population EVPI, we estimated that there are > 9000 patients diagnosed with HNSCC per year in the UK, based on epidemiological data. However, only half of these patients require nutritional support post CRT. Consequently, an approximate estimation of the number of patients who are going to benefit from further research is 4500 per year. It was assumed that this incidence was constant over time. Because of the uncertainty in the length of time over which the information from further research would be useful, a number of time horizons were included in the analysis (10 years, 20 years, 64 years45 and an unbounded time horizon). 46 A discount rate of 3.5%, the current UK recommendation, was applied.
The EVPI and EVPPI were assessed for both the base-case cost-effectiveness analysis and the worst-case scenario for the nasogastric tube-feeding cost-effectiveness analysis, which were explored in the alternative probabilistic sensitivity analysis (PSA).
Patient and public involvement
Patient and public involvement has played a key role in the design of this study. Jeremy Franks, our ‘expert patient’ and co-author, has been a member of our research team from the outset, regularly attending Trial Management Group (TMG) meetings to ensure that a patient perspective was consistently presented during decision-making throughout the study, and also attending Trial Steering Committee meetings.
In planning the research, we consulted two HNSCC support groups, one in Newcastle upon Tyne and one in Sunderland. Attendees included patients and carers following surgery or chemoradiotherapy treatment. All had experience of either a gastrostomy tube or a reactive nasogastric tube. They strongly agreed that research into swallowing outcomes was warranted. Members were presented with five potential research topics, and rated this study as a high priority. A further group of nine patient volunteers also reviewed this study and made suggestions, such as the time point and approach for patient interviews, which were incorporated into the study design.
An additional patient and carer group, attended by 12 patients and family members, was convened after funding was achieved during the development of the patient recruitment process and materials. The meeting was chaired by Jeremy Franks, with support from other team members. Attendees were provided with draft copies of the patient recruitment materials in advance of the meeting, and these were discussed in detail during the meeting. A particular focus of the discussion was the timing of patient contact: patients highlighted the risk of overload at the point of treatment planning, but agreed that some information about the trial did need to be provided at this stage. It was agreed that brief initial information would be provided at treatment planning, but the detailed information and discussion about the trial would be organised for a subsequent occasion. Discussion also focused on the need for verbal consent to audio-record recruitment consultations. Patient feedback – that this was very acceptable, that it was routine in many other situations (e.g. telephone calls to call centres) and that, if anything, it would give greater confidence in the quality of the information being provided – was also helpful when seeking research ethics approvals.
Jeremy Franks’ involvement in the study has been facilitated by his employment at Newcastle University, albeit in a different faculty (the Department of Agricultural Sciences). This has enabled him to attend project meetings regularly, which would have been very difficult for someone in employment in another organisation. Jeremy Franks’ employment at Newcastle University did not impede his ability to present a patient perspective; he had no prior familiarity with health research, commenting, for example, on his observation of the demanding organisation and approvals needed to conduct health research. Our study, therefore, benefited from the combination of an expert patient providing management input throughout, plus ‘responsive’ as-needed input from a broader patient panel.
Chapter 3 Results
Preliminary estimation of key parameters for definitive study
Recruitment and randomisation
Recruitment
The trial is closed to recruitment. The first patient was randomised on 10 July 2014 and the trial closed to recruitment with a total of 17 patients randomised. The last patient was randomised on 30 June 2015. The database was frozen on 5 May 2016.
Randomisation
Participants were randomised in a 1 : 1 ratio to either the pre-CRT gastrostomy arm or the no pre-CRT gastrostomy arm using a block-stratified block method (based on permuted random blocks of variable length), implemented using the Newcastle Clinical Trials Unit internet-accessed secure web-based system. Randomisation was stratified by site (Table 1).
| Site | Eligible (N = 84) | Approached (N = 75) | Declined (N = 58) | Consented (N = 17) | Treatment allocation, n | |
|---|---|---|---|---|---|---|
| Gastrostomy (N = 9) | Nasogastric (N = 8) | |||||
| 1 | 7 | 7 | 6 | 1 | 1 | 0 |
| 2 | 28 | 19 | 17 | 2 | 1 | 1 |
| 3 | 37 | 37 | 27 | 10 | 5 | 5 |
| 4 | 6 | 6 | 4 | 2 | 1 | 1 |
| 5 | 6 | 6 | 4 | 2 | 1 | 1 |
Ineligible patients
Ineligible patients were those randomised who were later found not to adhere to the trial eligibility criteria. No ineligible patients or protocol violators were recorded in this trial.
Trial population
Baseline patient characteristics
Demographic, clinical and surgical baseline characteristics and trial stratification factors at randomisation were compared across treatment groups descriptively. Descriptive statistics were tabulated by treatment group and overall. No significance testing was carried out owing to the randomised nature of the study.
Demographic characteristics included age, sex, weight and height. Clinical characteristics included the tumour, node and metastasis classification, dental characteristics and Performance Status Scale score (eating in public, understandability of speech, normalcy of diet). Trial factors included stratification factors (site), whether or not induction chemotherapy was planned and whether or not IMRT was planned (Tables 2 and 3).
Limited data are available on the characteristics of all eligible patients, including those not approached and/or recruited (n = 84). Age at screening was available for 83 out of 84 patients; the median age of these 83 individuals was 62 years [interquartile range (IQR) 58–67 years; range 38–85 years].
Table 2 shows that the majority of patients were recruited from site 3 (59%) and were male (94%), with a median age of 64 years (range 49–76 years) and a median weight of 84.8 kg (range 54.6–113 kg), balanced between the randomised groups. No patients had restrictions with eating in public and all had full normalcy of diet.
| Patient characteristic | Treatment allocation | Total (N = 17) | |
|---|---|---|---|
| Gastrostomy (N = 9) | Nasogastric (N = 8) | ||
| Site, n | |||
| 1 | 1 | 0 | 1 |
| 2 | 1 | 1 | 2 |
| 3 | 5 | 5 | 10 |
| 4 | 1 | 1 | 2 |
| 5 | 1 | 1 | 2 |
| Sex, n | |||
| Male | 9 | 7 | 16 |
| Female | 0 | 1 | 1 |
| Age (years) | |||
| Median (IQR, range) | 65 (64–67, 59–68) | 60 (58–72, 49–76) | 64 (60–68, 49–76) |
| Height (cm) | |||
| Median (IQR, range) | 171 (161–183, 152–185) | 173.5 (170–177.5, 162–185) | 172 (170–180, 152–185) |
| Usual weight (kg) | |||
| Median (IQR, range) | 81.1 (74.8–85, 54.6–104) | 89.4 (82.8–96.2, 77.3–113) | 84.8 (77.5–91.2, 54.6–113) |
| Given pre-treatment swallowing, n | |||
| No | 5 | 5 | 10 |
| Yes | 3 | 2 | 5 |
| Not known | 1 | 1 | 2 |
| Performance Status Scale, n | |||
| Eating in public | |||
| No restriction of place, food or companion | 9 | 8 | 17 |
| Understandability of speech | |||
| Always understandable | 9 | 7 | 16 |
| Understandable most of the time | 0 | 1 | 1 |
| Normalcy of diet | |||
| Full diet with no restrictions | 9 | 8 | 17 |
Table 3 shows that 65% of patients had human papillomavirus (HPV)-positive oropharyngeal tumours, and three patients in the nasogastric arm had nasopharynx tumours. Two out of nine patients (22%) randomised to the gastrostomy group and six out of eight patients (75%) randomised to the nasogastric group had a T3/T4 tumour stage. One out of nine patients randomised to the gastrostomy group and three out of eight patients randomised to the nasogastric group were node negative. All nine patients randomised to the gastrostomy group and six out of eight patients randomised to the nasogastric group were free of distant metastases.
| Tumour characteristics | Treatment allocation, n | Total (N = 17) | |
|---|---|---|---|
| Gastrostomy (N = 9) | Nasogastric (N = 8) | ||
| Site of disease, n | |||
| Nasopharynx | 0 | 3 | 3 |
| Oropharynx (HPV-positive) | 7 | 4 | 11 |
| Oropharynx (HPV-negative) | 1 | 0 | 1 |
| Larynx | 0 | 1 | 1 |
| Hypopharynx | 1 | 0 | 1 |
| Primary tumour, n | |||
| T1 | 2 | 1 | 3 |
| T2 | 5 | 1 | 6 |
| T3 | 0 | 3 | 3 |
| T4 | 2 | 3 | 5 |
| Regional lymph nodes, n | |||
| N0 | 1 | 3 | 4 |
| N1 | 1 | 0 | 1 |
| N2 | 6 | 5 | 11 |
| N3 | 1 | 0 | 1 |
| Distant metastasis, n | |||
| MX | 0 | 1 | 1 |
| MO | 9 | 6 | 15 |
| M1 | 0 | 1 | 1 |
Table 4 shows that dental information was not complete for all patients at baseline; the highest number of patients providing information for any particular component was 13 (76%). Initial decay, missing and filled teeth scores appeared balanced between the randomised groups, as did oral opening and oral dryness. Ten patients (59%) had oral hygiene aggregate scores recorded. Higher scores were reported in the gastrostomy arm, with a non-overlapping range of scores.
| Dental characteristics | Treatment allocation | Total (N = 17) | |
|---|---|---|---|
| Gastrostomy (N = 9) | Nasogastric (N = 8) | ||
| Initial presentation | |||
| Decayed (score 0–32) | |||
| Median (IQR, range), n | 2.5 (0–5, 0–9), 6 | 1 (1–1, 0–1), 5 | 1 (0–4, 0–9), 11 |
| Missing (score 0–32) | |||
| Median (IQR, range), n | 10.5 (8–14, 5–22), 6 | 12 (5–13, 4–18), 5 | 12 (5–14, 4–22), 11 |
| Filled (score 0–32) | |||
| Median (IQR, range), n | 7 (2–10, 1–14), 6 | 8 (8–8, 3–9), 5 | 8 (3–9, 1–14), 11 |
| Baseline | |||
| Decayed (score 0–32) | |||
| Median (IQR, range), n | 0 (0–0, 0–4), 6 | 0 (0–1, 0–1), 7 | 0 (0–0, 0–4), 13 |
| Missing (score 0–32) | |||
| Median (IQR, range), n | 19 (14–32, 6–32), 6 | 12 (5–14, 5–18), 7 | 14 (12–18, 5–32), 13 |
| Filled (score 0–32) | |||
| Median (IQR, range), n | 1 (0–7, 0–10), 6 | 7 (3–8, 2–9), 7 | 5 (1–7, 0–10), 13 |
| BPI | |||
| BPI_Upper L Posterior, n | |||
| 0 | 1 | 3 | 4 |
| 1 | 0 | 0 | 0 |
| 2 | 1 | 1 | 2 |
| 3 | 0 | 2 | 2 |
| 4 | 1 | 0 | 1 |
| Missing | 2 | 0 | 2 |
| Total | 5 | 6 | 11 |
| BPI_Upper Anterior, n | |||
| 0 | 0 | 1 | 1 |
| 1 | 0 | 1 | 1 |
| 2 | 2 | 3 | 5 |
| 3 | 1 | 1 | 2 |
| 4 | 1 | 0 | 1 |
| Missing | 1 | 0 | 1 |
| Total | 5 | 6 | 11 |
| BPI_Upper R Posterior, n | |||
| 0 | 1 | 0 | 1 |
| 1 | 0 | 0 | 0 |
| 2 | 1 | 2 | 3 |
| 3 | 1 | 2 | 3 |
| 4 | 1 | 1 | 2 |
| Missing | 1 | 1 | 2 |
| Total | 5 | 6 | 11 |
| BPI_Lower L Posterior, n | |||
| 0 | 1 | 2 | 3 |
| 1 | 0 | 0 | 0 |
| 2 | 2 | 1 | 3 |
| 3 | 0 | 1 | 1 |
| 4 | 2 | 1 | 3 |
| Missing | 0 | 1 | 1 |
| Total | 5 | 6 | 11 |
| BPI_Lower Arch, n | |||
| 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 |
| 2 | 2 | 4 | 6 |
| 3 | 1 | 0 | 1 |
| 4 | 2 | 2 | 4 |
| Missing | 0 | 0 | 0 |
| Total | 5 | 6 | 11 |
| BPI_Lower R Posterior, n | |||
| 0 | 0 | 2 | 2 |
| 1 | 0 | 0 | 0 |
| 2 | 3 | 1 | 4 |
| 3 | 0 | 2 | 2 |
| 4 | 2 | 0 | 2 |
| Missing | 0 | 1 | 1 |
| Total | 5 | 6 | 11 |
| OHSa | |||
| OHS_Upper Plaque, n | |||
| 0 | 1 | 3 | 4 |
| 1 | 0 | 3 | 4 |
| 2 | 0 | 2 | 0 |
| 3 | 2 | 0 | 2 |
| 4 | 0 | 0 | 0 |
| Missing | 2 | 1 | 3 |
| Total | 5 | 6 | 11 |
| OHS_Upper Calculus, n | |||
| 0 | 1 | 1 | 2 |
| 1 | 1 | 4 | 5 |
| 2 | 0 | 0 | 0 |
| 3 | 2 | 0 | 2 |
| 4 | 0 | 0 | 0 |
| Missing | 1 | 1 | 2 |
| Total | 5 | 6 | 11 |
| OHS_Upper Debris, n | |||
| 0 | 1 | 0 | 1 |
| 1 | 0 | 4 | 4 |
| 2 | 1 | 1 | 2 |
| 3 | 2 | 0 | 2 |
| 4 | 0 | 0 | 0 |
| Missing | 1 | 1 | 2 |
| Total | 5 | 6 | 11 |
| OHS_Lower Plaque, n | |||
| 0 | 1 | 2 | 3 |
| 1 | 0 | 3 | 3 |
| 2 | 2 | 0 | 2 |
| 3 | 2 | 0 | 2 |
| 4 | 0 | 0 | 0 |
| Missing | 0 | 1 | 1 |
| Total | 5 | 6 | 11 |
| OHS_Lower Calculus, n | |||
| 0 | 0 | 0 | 0 |
| 1 | 0 | 5 | 5 |
| 2 | 2 | 0 | 2 |
| 3 | 3 | 0 | 3 |
| 4 | 0 | 0 | 0 |
| Missing | 0 | 1 | 1 |
| Total | 5 | 6 | 11 |
| OHS_Lower Debris, n | |||
| 0 | 0 | 2 | 2 |
| 1 | 0 | 2 | 2 |
| 2 | 1 | 1 | 2 |
| 3 | 3 | 0 | 3 |
| 4 | 0 | 0 | 0 |
| Missing | 1 | 1 | 2 |
| Total | 5 | 6 | 11 |
| Oral opening | |||
| Upper to lower R incisors | |||
| Median (IQR, range), n | 45 (43–52, 43–53), 5 | 45 (39–48, 35–50), 5 | 45 (43–50, 35–53), 10 |
| Nearest adjacent pair | |||
| Median (IQR, range), n | No observations | ||
| Oral dryness (1–10) | |||
| Median (IQR, range), n | 0 (0–0, 0–0), 5 | 0 (0–0, 0–0), 6 | 0 (0–0, 0–0), 11 |
| Dental OHS aggregate | |||
| Median (IQR, range), n | 2.17 (2.00–2.25, 1.50–3.00), 5 | 0.83 (0.50–1.00, 0.33–1.33), 5 | 1.42 (0.83–2.17, 0.33–3.00), 10 |
Defining populations for analysis
All patients who were approached about the trial are referred to as the screening set. All patients randomised to treatment are included, retaining patients in their randomised treatment groups and including protocol violators and ineligible patients. This group of patients is referred to as the intention-to-treat (ITT) set. All patients who started treatment are referred to as the treatment set.
Treatment received
The analysis set was the ITT set. Treatment was defined as CRT plus randomised intervention. CRT treatment was delivered as per usual centre practice and was usually completed within 2 months.
Pre-chemoradiation therapy gastrostomy arm
Gastrostomy tubes were inserted after the consent and baseline visit and after randomisation, but before any treatment (CRT/IMRT) took place. Gastrostomy tubes were inserted during induction chemotherapy if necessary.
No pre-chemoradiation therapy gastrostomy arm
Nasogastric tubes were inserted after the consent and baseline visit and after randomisation, at a point when patient and/or clinician felt that this was most appropriate. Nasogastric tube placement was flexible, that is, it was carried out during or after CRT treatment, as required.
Owing to the nature of the tube-feeding interventions, it was not practicable to blind research nurses to the treatment allocated to patients for the follow-up assessments. The baseline data capture assessments were completed by research nurses before randomisation to reduce the chance of bias (see Tables 5–8).
Table 5 reports on 16 out of 17 patients receiving a randomised intervention. One patient was given the opposite intervention, as clinical staff were unable to insert a gastrostomy tube when they attempted to do so.
| Did the patient receive the intervention? | Treatment allocation, n | Total (N = 17) | |
|---|---|---|---|
| Gastrostomy (N = 9) | Nasogastric (N = 8) | ||
| Yes | 8 | 8 | 16 |
| No | 1 | 0 | 1 |
| Total | 9 | 8 | 17 |
Table 6 reports how pre-CRT gastrostomy tubes were inserted, a median of 14 days after randomisation (75% were inserted within 18 days; all were inserted within 28 days). Nasogastric tubes were inserted a median of 40 days after randomisation (75% were inserted within 53 days; all were inserted within 72 days).
| Treatment allocation | ||||||
|---|---|---|---|---|---|---|
| Gastrostomy (n = 9a) | Nasogastric (n = 8) | |||||
| Median | IQR | Range | Median | IQR | Range | |
| Number of days | 14 | 11–18 | 8–28 | 40.5 | 32–53.5 | 26–72 |
Table 7 shows that two out of nine patients randomised to receive gastrostomy tubes and and two out of eight randomised to nasogastric tubes intended to have induction chemotherapy. All patients planned to have IMRT.
| Intention at randomisation | Treatment allocation, n | Total (N = 17) | |
|---|---|---|---|
| Gastrostomy (N = 9) | Nasogastric (N = 8) | ||
| Induction chemotherapy? | |||
| Yes | 2 | 2 | 4 |
| No | 7 | 6 | 13 |
| Is the patient to have IMRT? | |||
| Yes | 9 | 8 | 17 |
Table 8 shows that two patients in each randomised arm were to receive a median of two cycles of induction chemotherapy. Eight patients in each randomised arm received a median of 65 Gy of CRT in 30 fractions. Induction chemotherapy and radiotherapy appeared to be balanced between the randomised groups.
| Received | Treatment allocation, n | |||||||
|---|---|---|---|---|---|---|---|---|
| Gastrostomy (N = 9) | Nasogastric (N = 8) | |||||||
| n | Median | IQR | Range | n | Median | IQR | Range | |
| Number of cycles of induction chemotherapy | 2 | 2 | 2–2 | 2–2 | 2 | 2 | 2–3 | 2–3 |
| Total number of fractions of radiotherapy | 8a | 30 | 30–30 | 30–30 | 8 | 30 | 30–30 | 30–65 |
| Total radiotherapy dose received (Gy) | 8a | 65 | 65–65.5 | 65–66 | 8 | 65 | 65–65 | 54–66 |
Safety analysis
Adverse events
The analysis set is the treatment set. AEs were graded on a three-point scale of intensity (mild, moderate and severe):
-
mild – discomfort is noticed, but there is no disruption of normal daily activities
-
moderate – discomfort is sufficient to reduce or affect normal daily activities
-
severe – discomfort is incapacitating, leading to an inability to work or perform normal daily activities.
A total of 230 AEs were reported in a total of 15 patients (Table 9).
| AE severity | Relatedness, n (%) | Total, N (%) | ||||
|---|---|---|---|---|---|---|
| Unrelated | Possibly | Probably | Definitely | Missing | ||
| Mild | 126 (54.78) | 2 (0.87) | 2 (0.87) | 3 (1.30) | 0 (0.00) | 133 (57.83) |
| Moderate | 74 (32.17) | 1 (0.43) | 0 (0.00) | 1 (0.43) | 0 (0.00) | 76 (33.04) |
| Severe | 20 (8.70) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 20 (8.70) |
| Missing | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.43) | 1 (0.43) |
| Total | 220 (95.65) | 3 (1.30) | 2 (0.87) | 4 (1.74) | 1 (0.43) | 230 (100.00) |
Nine (3.9%) AEs reported were possibly, probably or definitely related to the randomised intervention. These were reported in six patients. Table 10 provides a line listing of all reported AEs; shading is used in the table to show those nine events that were possibly, probably or definitely related to the randomised intervention.
| PID_ae | Rand_No | AESTDAT | AETERM | AECausality | AESER | AESeverity | RndArm |
|---|---|---|---|---|---|---|---|
| 01S006 | 101 | 26 August 2014 | Fatigue | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 26 August 2014 | Constipation | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 2 September 2014 | Vomit | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 9 September 2014 | Tinnitus | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 9 September 2014 | Fatigue | Unrelated | N | Moderate | Gastrostomy |
| 01S006 | 101 | 9 September 2014 | Low haemoglobin | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 16 September 2014 | Fatigue | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 16 September 2014 | Nausea | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 22 September 2014 | Fatigue | Unrelated | N | Severe | Gastrostomy |
| 01S006 | 101 | 22 September 2014 | Vomit | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 23 September 2014 | Oral thrush | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 23 September 2014 | Nausea | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 23 September 2014 | Throat pain | Unrelated | N | Severe | Gastrostomy |
| 01S006 | 101 | 1 October 2014 | Throat pain | Unrelated | N | Mild | Gastrostomy |
| 01S006 | 101 | 15 October 2014 | Fatigue | Unrelated | N | Moderate | Gastrostomy |
| 01S006 | 101 | 20 November 2014 | Fatigue | Unrelated | N | Mild | Gastrostomy |
| 01S009 | 102 | 22 August 2014 | Pain at gastrostomy site | Probable | N | Mild | Gastrostomy |
| 01S009 | 102 | 10 September 2014 | Constipation | Unrelated | N | Mild | Gastrostomy |
| 01S009 | 102 | 10 September 2014 | Constipated | Unrelated | N | Mild | Gastrostomy |
| 01S009 | 102 | 10 September 2014 | Xerostomia | Unrelated | N | Mild | Gastrostomy |
| 01S009 | 102 | 10 September 2014 | Loss of taste | Unrelated | N | Mild | Gastrostomy |
| 01S009 | 102 | 17 September 2014 | Thick saliva | Unrelated | N | Moderate | Gastrostomy |
| 01S009 | 102 | 17 September 2014 | Xerostomia | Unrelated | N | Moderate | Gastrostomy |
| 01S009 | 102 | 17 September 2014 | Fatigue | Unrelated | N | Moderate | Gastrostomy |
| 01S009 | 102 | 17 September 2014 | Loss of taste | Unrelated | N | Moderate | Gastrostomy |
| 01S009 | 102 | 17 September 2014 | Mucositis | Unrelated | N | Moderate | Gastrostomy |
| 01S009 | 102 | 17 September 2014 | Dysphagia | Unrelated | N | Mild | Gastrostomy |
| 01S009 | 102 | 8 October 2014 | Thick saliva | Unrelated | N | Mild | Gastrostomy |
| 01S009 | 102 | 5 December 2014 | Mucositis | Unrelated | N | Mild | Gastrostomy |
| 01S009 | 102 | 15 December 2014 | Fatigue | Unrelated | N | Mild | Gastrostomy |
| 01S009 | 102 | 5 January 2015 | Thick saliva | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 24 November 2014 | Constipation | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 24 November 2014 | Sore mouth | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 26 November 2014 | Trismus | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 26 November 2014 | Altered taste | Unrelated | N | Moderate | Gastrostomy |
| 01S014 | 106 | 26 November 2014 | Fatigue | Unrelated | N | Moderate | Gastrostomy |
| 01S014 | 106 | 26 November 2014 | Altered voice | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 26 November 2014 | Salivary change | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 26 November 2014 | Dysphagia | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 1 December 2014 | Sore mouth | Unrelated | N | Moderate | Gastrostomy |
| 01S014 | 106 | 10 December 2014 | Dysphagia | Unrelated | N | Moderate | Gastrostomy |
| 01S014 | 106 | 10 December 2014 | Salivary change | Unrelated | N | Severe | Gastrostomy |
| 01S014 | 106 | 15 December 2014 | Alopecia | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 17 December 2014 | Skin reaction | Unrelated | N | Moderate | Gastrostomy |
| 01S014 | 106 | 17 December 2014 | Fatigue | Unrelated | N | Severe | Gastrostomy |
| 01S014 | 106 | 17 December 2014 | Dysphagia | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 30 December 2014 | Thick saliva | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 18 June 2015 | Fatigue | Unrelated | N | Mild | Gastrostomy |
| 01S014 | 106 | 18 June 2015 | Altered taste | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 November 2014 | Low mood | Unrelated | N | Moderate | Gastrostomy |
| 01S016 | 107 | 26 November 2014 | Taste changes | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 26 November 2014 | Thick saliva | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 26 November 2014 | Fatigue | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 26 November 2014 | Xerostomia | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 3 December 2014 | Fatigue | Unrelated | N | Moderate | Gastrostomy |
| 01S016 | 107 | 3 December 2014 | Taste changes | Unrelated | N | Moderate | Gastrostomy |
| 01S016 | 107 | 5 December 2014 | Tinnitus | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 December 2014 | Mucositis | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 December 2014 | Infection at RIG site | Definitely | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 December 2014 | Dysphagia | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 December 2014 | Trismus | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 December 2014 | Altered voice | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 December 2014 | Constipation | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 December 2014 | Fatigue | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 December 2014 | Skin reaction | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 10 December 2014 | Trismus | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 17 December 2014 | Mucositis | Unrelated | N | Moderate | Gastrostomy |
| 01S016 | 107 | 17 December 2014 | Xerostomia | Unrelated | N | Moderate | Gastrostomy |
| 01S016 | 107 | 17 December 2014 | Thick saliva | Unrelated | N | Moderate | Gastrostomy |
| 01S016 | 107 | 17 December 2014 | Dysphagia | Unrelated | N | Severe | Gastrostomy |
| 01S016 | 107 | 23 December 2014 | Partial RIG displacement | Definitely | N | Moderate | Gastrostomy |
| 01S016 | 107 | 24 December 2014 | Nausea | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 24 December 2014 | Upper respiratory tract infection | Unrelated | N | Moderate | Gastrostomy |
| 01S016 | 107 | 24 December 2014 | Dysphagia | Unrelated | N | Moderate | Gastrostomy |
| 01S016 | 107 | 24 December 2014 | Infection at RIG site | Definitely | N | Mild | Gastrostomy |
| 01S016 | 107 | 24 December 2014 | Anaemia | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 24 December 2014 | Altered renal function | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 24 December 2014 | Fatigue | Unrelated | N | Severe | Gastrostomy |
| 01S016 | 107 | 25 December 2014 | Diarrhoea | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 30 December 2014 | Xerostomia | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 30 December 2014 | Thick saliva | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 31 March 2015 | Fatigue | Unrelated | N | Mild | Gastrostomy |
| 01S016 | 107 | 1 August 2015 | Taste changes | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 14 July 2015 | Constipation | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 14 July 2015 | Oral candidiasis | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 15 July 2015 | Altered taste | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 15 July 2015 | Xerostomia | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 21 July 2015 | Dry mouth | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 21 July 2015 | Neck pain | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 21 July 2015 | Fatigue | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 21 July 2015 | Mucositis | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 21 July 2015 | Dry nostrils | Unrelated | N | Mild | Gastrostomy |
| 01S037 | 110 | 29 July 2015 | Fatigue | Unrelated | N | Moderate | Gastrostomy |
| 01S037 | 110 | 5 August 2015 | Mucositis | Unrelated | N | Moderate | Gastrostomy |
| 01S037 | 110 | 5 August 2015 | Xerostomia | Unrelated | N | Moderate | Gastrostomy |
| 01S037 | 110 | 10 August 2015 | Neck pain | Unrelated | N | Moderate | Gastrostomy |
| 01S037 | 110 | 16 August 2015 | Skin reaction | Unrelated | N | Mild | Gastrostomy |
| 03S024 | 302 | 22 July 2015 | Fatigue | U | Gastrostomy | ||
| 04S001 | 401 | 18 March 2015 | Discharge from RIG – cream prescribed | Definitely | N | Mild | Gastrostomy |
| 04S001 | 401 | 24 April 2015 | Dry mouth | Possible | N | Mild | Gastrostomy |
| 05S005 | 501 | 17 July 2015 | Gastrostomy site infection | Possible | N | Mild | Gastrostomy |
| 05S005 | 501 | 20 July 2015 | Oral thrush | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 14 August 2015 | Mouth/throat/pain | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 21 August 2015 | Mucositis | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 21 August 2015 | Nausea | Unrelated | N | Mild | Gastrostomy |
| 05S005 | 501 | 21 August 2015 | Dysphagia | Unrelated | N | Mild | Gastrostomy |
| 05S005 | 501 | 25 August 2015 | Dizziness | Unrelated | N | Mild | Gastrostomy |
| 05S005 | 501 | 28 August 2015 | Oral thrush/sore mouth | Unrelated | N | Mild | Gastrostomy |
| 05S005 | 501 | 28 August 2015 | Dehydration | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 1 September 2015 | Dysphagia | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 7 September 2015 | Vomiting | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 7 September 2015 | Diarrhoea | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 8 September 2015 | Anaemia | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 8 September 2015 | Hypo magnesia | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 13 October 2015 | Dizziness | Unrelated | N | Mild | Gastrostomy |
| 05S005 | 501 | 23 October 2015 | Diarrhoea | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 15 November 2015 | Chest Infection? Secondary to aspiration | Unrelated | N | Moderate | Gastrostomy |
| 05S005 | 501 | 15 December 2015 | Pain in jaw | Unrelated | N | Mild | Gastrostomy |
| 05S005 | 501 | 15 February 2016 | Diarrhoea | Unrelated | N | Mild | Gastrostomy |
| 01S007 | 103 | 15 September 2014 | Fatigue | Unrelated | N | Moderate | NGT |
| 01S007 | 103 | 15 September 2014 | No appetite | Unrelated | N | Mild | NGT |
| 01S007 | 103 | 15 September 2014 | Headaches | Unrelated | N | Mild | NGT |
| 01S007 | 103 | 15 September 2014 | Altered taste | Unrelated | N | Moderate | NGT |
| 01S007 | 103 | 17 September 2014 | Thick saliva | Unrelated | N | Mild | NGT |
| 01S007 | 103 | 22 September 2014 | Rash to face | Unrelated | N | Severe | NGT |
| 01S007 | 103 | 22 September 2014 | Sore mouth/mucositis | Unrelated | N | Moderate | NGT |
| 01S007 | 103 | 24 September 2014 | Dry mouth | Unrelated | N | Moderate | NGT |
| 01S007 | 103 | 24 September 2014 | Thick saliva | Unrelated | N | Moderate | NGT |
| 01S007 | 103 | 24 September 2014 | Fatigue | Unrelated | N | Severe | NGT |
| 01S007 | 103 | 1 October 2014 | Rash to face | Unrelated | N | Mild | NGT |
| 01S007 | 103 | 1 October 2014 | Thick saliva | Unrelated | N | Mild | NGT |
| 01S007 | 103 | 4 October 2014 | Pyrexia | Unrelated | N | Mild | NGT |
| 01S007 | 103 | 7 October 2014 | Sore mouth | Unrelated | N | Severe | NGT |
| 01S007 | 103 | 7 October 2014 | Rash to face | Unrelated | N | Moderate | NGT |
| 01S007 | 103 | 14 October 2014 | Dysphagia | Unrelated | N | Moderate | NGT |
| 01S007 | 103 | 22 October 2014 | Low mood | Unrelated | N | Moderate | NGT |
| 01S007 | 103 | 15 January 2015 | Rash to face | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 23 September 2014 | Fatigue | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 24 September 2014 | Altered taste | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 30 September 2014 | Mucositis | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 1 October 2014 | Dry mouth | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 5 October 2014 | Migraine | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 6 October 2014 | Pain on swallow | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 11 October 2014 | Migraine | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 11 October 2014 | Vomiting | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 14 October 2014 | Constipation | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 15 October 2014 | Dry mouth | Unrelated | N | Moderate | NGT |
| 01S010 | 104 | 18 October 2014 | Migraine | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 20 October 2014 | Skin reaction | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 24 October 2014 | Migraine | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 29 October 2014 | Altered taste | Unrelated | N | Moderate | NGT |
| 01S010 | 104 | 29 October 2014 | Fatigue | Unrelated | N | Moderate | NGT |
| 01S010 | 104 | 29 October 2014 | Skin reaction | Unrelated | N | Moderate | NGT |
| 01S010 | 104 | 29 October 2014 | Thick saliva | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 29 October 2014 | Mucositosis | Unrelated | N | Moderate | NGT |
| 01S010 | 104 | 29 October 2014 | Pain on swallow | Unrelated | N | Moderate | NGT |
| 01S010 | 104 | 31 January 2015 | Fatigue | Unrelated | N | Mild | NGT |
| 01S010 | 104 | 2 February 2015 | Altered taste | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 5 October 2014 | Eye infections | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 8 October 2014 | Fatigue | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 8 October 2014 | Constipation | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 15 October 2014 | Headaches | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 15 October 2014 | Salivary change | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 15 October 2014 | Fatigue | Unrelated | N | Severe | NGT |
| 01S011 | 105 | 15 October 2014 | Xerostomia | Unrelated | N | Moderate | NGT |
| 01S011 | 105 | 15 October 2014 | Skin reaction | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 15 October 2014 | Altered taste | Unrelated | N | Moderate | NGT |
| 01S011 | 105 | 15 October 2014 | Dyspahgia | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 22 October 2014 | Mouth pain | Unrelated | N | Moderate | NGT |
| 01S011 | 105 | 27 October 2014 | Vomiting | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 29 October 2014 | Mucositis | Unrelated | N | Moderate | NGT |
| 01S011 | 105 | 29 October 2014 | Voice changes | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 3 November 2014 | Dysphagia | Unrelated | N | Severe | NGT |
| 01S011 | 105 | 3 November 2014 | Oedema | Unrelated | N | Moderate | NGT |
| 01S011 | 105 | 5 November 2014 | Constipation | Unrelated | N | Severe | NGT |
| 01S011 | 105 | 31 December 2014 | Mouth pain | Unrelated | N | Severe | NGT |
| 01S011 | 105 | 31 December 2014 | Altered taste | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 5 February 2015 | Fatigue | Unrelated | N | Moderate | NGT |
| 01S011 | 105 | 5 February 2015 | Dysphagia | Unrelated | N | Moderate | NGT |
| 01S011 | 105 | 4 June 2015 | Mucositis | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 4 June 2015 | Dysphagia | Unrelated | N | Mild | NGT |
| 01S011 | 105 | 4 June 2015 | Xerostomia | Unrelated | N | Mild | NGT |
| 01S017 | 108 | 18 February 2015 | Salivary change | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 18 February 2015 | Dysphagia | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 18 February 2015 | Xerostomia | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 18 February 2015 | Mucositis | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 18 February 2015 | Mouth pain | Unrelated | N | Mild | NGT |
| 01S017 | 108 | 18 February 2015 | Altered taste | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 18 February 2015 | Fatigue | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 25 February 2015 | Oedema | Unrelated | N | Mild | NGT |
| 01S017 | 108 | 25 February 2015 | Mucositis | Unrelated | N | Severe | NGT |
| 01S017 | 108 | 3 March 2015 | Xerostomia | Unrelated | N | Severe | NGT |
| 01S017 | 108 | 4 March 2015 | Voice changes | Unrelated | N | Mild | NGT |
| 01S017 | 108 | 4 March 2015 | Trismus | Unrelated | N | Mild | NGT |
| 01S017 | 108 | 4 March 2015 | Oedema | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 4 March 2015 | Fatigue | Unrelated | N | Severe | NGT |
| 01S017 | 108 | 4 March 2015 | Salivary changes | Unrelated | N | Severe | NGT |
| 01S017 | 108 | 11 March 2015 | Mouth pain | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 11 March 2015 | Oedema | Unrelated | N | Mild | NGT |
| 01S017 | 108 | 8 April 2015 | Oedema | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 8 April 2015 | Vomiting | Possible | N | Moderate | NGT |
| 01S017 | 108 | 8 April 2015 | Salivary changes | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 8 April 2015 | Mucositis | Unrelated | N | Moderate | NGT |
| 01S017 | 108 | 17 June 2015 | Fatigue | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 15 April 2015 | Vomit | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 16 April 2015 | Nausea | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 21 April 2015 | Dysphagia | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 21 April 2015 | Fatigue | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 21 April 2015 | Skin reaction | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 21 April 2015 | Altered taste | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 21 April 2015 | Xerostomia/dry mouth | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 25 April 2015 | Dysphagia | Unrelated | N | Moderate | NGT |
| 01S030 | 109 | 25 April 2015 | Mucositis | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 29 April 2015 | Thick saliva | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 1 May 2015 | Mucositis | Unrelated | N | Moderate | NGT |
| 01S030 | 109 | 1 May 2015 | Altered taste | Unrelated | N | Moderate | NGT |
| 01S030 | 109 | 1 May 2015 | Xerostomia | Unrelated | N | Moderate | NGT |
| 01S030 | 109 | 1 May 2015 | Fatigue | Unrelated | N | Severe | NGT |
| 01S030 | 109 | 6 May 2015 | Thick saliva | Unrelated | N | Moderate | NGT |
| 01S030 | 109 | 13 May 2015 | Dysphagia | Unrelated | N | Severe | NGT |
| 01S030 | 109 | 13 May 2015 | Trismus | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 13 May 2015 | Voice changes | Unrelated | N | Moderate | NGT |
| 01S030 | 109 | 13 May 2015 | Thick saliva | Unrelated | N | Severe | NGT |
| 01S030 | 109 | 13 May 2015 | Oedema | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 1 September 2015 | Thick saliva | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 1 September 2015 | Fatigue | Unrelated | N | Mild | NGT |
| 01S030 | 109 | 1 September 2015 | Dysphagia | Unrelated | N | Moderate | NGT |
| 02S001 | 201 | 18 December 2014 | Fatigue and vomiting | Probable | N | Mild | NGT |
| 03S017 | 301 | 3 June 2015 | Constipation | Unrelated | N | Mild | NGT |
| 03S017 | 301 | 13 October 2015 | Dry mouth | Unrelated | N | Mild | NGT |
The number of patients experiencing at least one ‘moderate’- or ‘severe’-grade episode of each type is reported as a percentage of those receiving treatment in each treatment group. Six out of eight patients (75%) receiving a gastrostomy tube reported a moderate or severe event, and five out of eight patients (63%) receiving a nasogastric tube reported a moderate or severe event.
The number of treatment-related AEs is reported divided by the level of association, that is, if these were ‘definitely’, ‘probably’ or ‘possibly’ related to treatment. A total of nine AEs related to the intervention (defined as possibly, probably or definitely related) were reported in six patients: seven AEs in four patients (44%) receiving a gastrostomy tube, and two AEs in two patients (22%) receiving a nasogastric tube.
With such small numbers of patients randomised, it is difficult to be conclusive, but it appears that a higher proportion of patients treated with gastrostomy experienced AEs possibly related to the gastrostomy tube (see Tables 9 and 10).
Serious adverse events
The number of patients reporting a SAE was compared descriptively across treatments. A total of 16 SAEs were reported in 11 patients. None of the SAEs were related to the randomised intervention (Table 11).
| Patient ID | SAE ID | Date of onset | Description | Related | Seriousness | Current status | Notes |
|---|---|---|---|---|---|---|---|
| 102 | SAE01 | 28 September 2014 | Gastrostomy tube pulled by patient’s dog when walking, causing pain. Admitted to hospital. Gastrostomy tube was replaced, and a subsequent tubogram showed gastrostomy placement was normal. Patient was discharged and pain was resolved | Unrelated | Involved or prolonged inpatient hospitalisation | Resolved | Completely resolved |
| 102 | SAE02 | 13 October 2014 | Patient confused and complaining of pain at gastrostomy site. A tubogram confirmed that the gastrostomy tube was in the correct position. SAE02 was split into two SAEs (SAE02 and SAE03) | Unrelated | Involved or prolonged inpatient hospitalisation | Resolved | Completely resolved |
| 102 | SAE03 | 13 October 2014 | Patient was confused and complaining of pain at gastrostomy site. Confusion probably due to secondary infection | Unrelated | Involved or prolonged inpatient hospitalisation | Resolved after i.v. antibiotics | None completely resolved |
| 107 | SAE04 | 24 December 2014 | Patient had stopped taking metformin, which resulted in unstable blood sugar levels. Patient was taking 1 g of metformin for type 2 diabetes mellitus, but misunderstood the advice about swallowing tablets and stopped taking them for 2 weeks | Unrelated | Involved or prolonged inpatient hospitalisation | Resolved | None completely resolved |
| 201 | SAE05 | 21 January 2015 | Chest sepsis secondary to neutropenia. The patient was admitted with a temperature and neutropenia and was treated with i.v. antibiotics | Unrelated | Involved or prolonged inpatient hospitalisation | Resolved | Completely resolved; deviation report and CAPA completed |
| 108 | SAE06 | 4 March 2015 | Anaemia and infected eye and mouth. Patient was seen in review clinic and admitted to hospital. Blood transfusion was given – three units. Fluconazole dose of 50 mg | Unrelated | Involved or prolonged inpatient hospitalisation | Condition still present and unchanged | Patient in hospital and halfway house |
| 18 March 2015 | Mouth remained grade 2 anaemia grade 1. Patient transferred to hospice, a half-way house between home and hospital | ||||||
| 401 | SAE07 | 13 April 2015 | Patient was admitted to hospital for emergency feeding due to dehydration and vomiting. The feeding regime prescribed was as follows: 14 May 2015, 500 ml of Jevity® Plus; 15 April 2015, 900 ml of Jevity Plus; 16 April 2015, 1400 ml of Jevity Plus; and 17 April 2015, 2000 ml of Jevity Plus | Unrelated | Involved or prolonged inpatient hospitalisation | Resolved | Ongoing |
| 109 | SAE08 | 17 May 2015 | Diarrhoea and vomiting | Unrelated | Involved inpatient hospitalisation | Resolved | Completely resolved |
| 402 | SAE09 | 13 June 2015 | Dysphagia | Unrelated | Involved inpatient hospitalisation | Condition improving | |
| 201 | SAE10 | 2 June 2015 | Metastatic SCC of lung | Unrelated | Involved inpatient hospitalisation | Ongoing (SAE15) | |
| 302 | SAE11 | 26 July 2015 | Deep-vein thrombosis in right arm | Unrelated | Involved inpatient hospitalisation | Condition improving | |
| 110 | SAE12 | 3 June 2015; date of report was 6 August 2015 | Chest infection | Unrelated | Involved inpatient hospitalisation | Recovered with sequelae | Complete |
| 302 | SAE13 | 12 August 2015 | Acute kidney injury | Unrelated | Involved inpatient hospitalisation | Condition improving | |
| 301 | SAE14 | 5 July 2015 | Constipation | Unrelated | Involved inpatient hospitalisation | Resolved | Complete |
| 202 | SAE15 | 17 August 2015 | Right ventricular mass thrombus. Patient was given anticoagulants, but thrombus increased. The patient will not receive any further treatment as they are receiving palliative care | Unrelated | Involved inpatient hospitalisation | Condition deteriorated | |
| 104 | SAE16 | 22 October 2014 | Reduced oral intake, secondary to radiotherapy, induced mucositis, leading to weight loss. Admitted to hospital on 1 November 2014 for nutritional support via a NGT. SAE asked to be submitted retrospectively after monitoring visit on 23 May 2016 | Unrelated | Involved inpatient hospitalisation | Condition improved | Complete |
Outcome data
Primary outcome measure: feasibility
Recruitment, compliance and retention are reported as a cumulative rate at the end of the follow-up phase (Figure 1).
FIGURE 1.
Consolidated Standards of Reporting Trials diagram. NGT, nasogastric tube.

The primary outcome of the trial was feasibility, defined as:
-
recruitment rate, defined as the number of patients who consented to be randomised as a proportion of all patients approached about the trial – 17 out of 75 = 0.23 (95% CI 0.13 to 0.32); the trial hypothesised that 50% of eligible patients approached would be randomised
-
compliance rate, defined as the number of patients who started randomised treatment as a proportion of the number randomised – 16 out of 17 = 0.94 (95% CI 0.83 to 1.05)
-
retention rate, defined as the number of patients who completed randomised treatment as a proportion of the number randomised – 16 out of 17 = 0.94 (95% CI 0.83 to 1.05)
-
retention at the 3-month clinician follow-up – 15 out of 17 = 0.88 (95% CI 0.73 to 1.04)
-
retention at the 6-month clinician follow-up – 15 out of 17 = 0.88 (95% CI 0.73 to 1.04).
In summary, the recruitment rate was not observed as hypothesised and, indeed, was far lower than anticipated. The reasons for this were investigated in the qualitative interviews. However, once patients were recruited to the trial, rates of compliance and retention were both high.
Secondary outcome measures
Clinician-reported measures
Table 12 shows that the retention rate at the 3-month and 6-month clinician follow-up points was high, at 0.88 (95% CI 0.73 to 1.04), and balanced between the randomised treatment groups.
| Performance Status Scale | Time point | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention visit | End of CRT | Month 3 | Month 6 | Month 12 | |||||||||||
| Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | |
| Eating in public, n | |||||||||||||||
| Always eats alone | – | – | – | 2 | 2 | 4 | – | – | – | – | – | – | – | – | – |
| Eats only at home (selected persons) | – | – | – | 2 | 1 | 3 | 1 | 0 | 1 | 1 | 2 | 3 | – | – | – |
| Eats only in presence (selected persons/places) | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | – | – | – | 1 | 0 | 1 |
| No restrictions on place, but restricted diet | – | – | – | 0 | 1 | 1 | 1 | 3 | 4 | 1 | 1 | 2 | – | – | – |
| No restrictions on place, food or companion | 7 | 3 | 10 | 2 | 2 | 4 | 4 | 3 | 7 | 6 | 4 | 10 | 3 | 2 | 5 |
| Total | 7 | 4 | 11 | 6 | 7 | 13 | 7 | 7 | 14 | 8 | 7 | 15 | 4 | 2 | 6 |
| Understandability of speech, n | |||||||||||||||
| Usually understandable | – | – | – | 0 | 2 | 2 | – | – | – | – | – | – | – | – | – |
| Understandable most of the time | – | – | – | 0 | 1 | 1 | – | – | – | 1 | 2 | 3 | – | – | – |
| Always understandable | 7 | 5 | 12 | 8 | 4 | 12 | 8 | 7 | 15 | 7 | 5 | 12 | 4 | 2 | 6 |
| Total | 7 | 5 | 12 | 8 | 7 | 15 | 8 | 7 | 15 | 8 | 7 | 15 | 4 | 2 | 6 |
| Normalcy of diet, n | |||||||||||||||
| Non-oral feeding | – | – | – | 2 | 4 | 6 | 1 | 0 | 1 | – | – | – | – | – | – |
| Cold liquids | 0 | 4 | 4 | 2 | 1 | 3 | 0 | 1 | 1 | 0 | 1 | 1 | – | – | – |
| Warm liquids | – | – | – | 2 | 0 | 2 | 0 | 1 | 1 | – | – | – | – | – | – |
| Pureed foods | – | – | – | 0 | 1 | 1 | – | – | – | 1 | 0 | 1 | – | – | – |
| Soft foods requiring no chewing | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 3 | – | – | – | – | – | – |
| Soft, chewable foods | 1 | 0 | 1 | 0 | 1 | 1 | 4 | 1 | 5 | 4 | 2 | 6 | 0 | 1 | 1 |
| Carrots, celery | – | – | – | – | – | – | 1 | 0 | 1 | – | – | – | 1 | 0 | 1 |
| Full diet with liquid | – | – | – | – | – | – | 0 | 1 | 1 | 3 | 2 | 5 | 3 | 0 | 3 |
| Full diet with no restrictions | 5 | 1 | 6 | 1 | 0 | 1 | 1 | 1 | 2 | 0 | 2 | 2 | 0 | 1 | 1 |
| Total | 7 | 5 | 12 | 8 | 7 | 15 | 8 | 7 | 15 | 8 | 7 | 15 | 4 | 2 | 6 |
| Completion rate (%)a | 70.6 | 88.2 | 88.2 | 88.2 | 35.3b | ||||||||||
At 3 months, 79% of responders reported no restrictions on eating in public, maintained at 80% at 6 months. At 3 months, 100% of responders reported that they are always understandable in speech, which reduced to 80% at 6 months. At 3 months, 20% of responders had a full diet, which increased to 47% at 6 months.
Table 13 shows that retention at the weekly clinician follow-up was highest at 1 month post intervention, and balanced across the randomised treatment groups.
| Performance Status Scale | Weekly clinical assessment | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||||||||||
| Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | |
| Eating in public, n | ||||||||||||||||||
| Always eats alone | – | – | – | – | – | – | 1 | 1 | 2 | 2 | 1 | 3 | 1 | 1 | 2 | 1 | 0 | 1 |
| Eats only at home (selected persons) | – | – | – | 1 | 0 | 1 | 2 | 0 | 2 | 3 | 1 | 4 | 1 | 2 | 3 | 0 | 1 | 1 |
| Eats only in presence (selected persons/places) | – | – | – | 1 | 1 | 2 | 2 | 0 | 2 | 0 | 1 | 1 | 2 | 0 | 2 | – | – | – |
| No restrictions on place, but restricted diet | 1 | 0 | 1 | – | – | – | – | – | – | 1 | 1 | 2 | 0 | 1 | 1 | – | – | – |
| No restrictions on place, food or companion | 8 | 5 | 13 | 6 | 4 | 10 | 3 | 4 | 7 | 2 | 2 | 4 | 2 | 2 | 4 | 0 | 1 | 1 |
| Total | 9 | 5 | 14 | 8 | 5 | 13 | 8 | 5 | 13 | 8 | 6 | 14 | 6 | 6 | 12 | 1 | 2 | 3 |
| Understandability of speech, n | ||||||||||||||||||
| No communication | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Never understandable | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 1 | 1 | – | – | – |
| Usually understandable | – | – | – | – | – | – | – | – | – | 0 | 2 | 2 | 0 | 1 | 1 | 0 | 1 | 1 |
| Understandable most of the time | 0 | 1 | 1 | – | – | – | – | – | – | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 |
| Always understandable | 9 | 4 | 13 | 7 | 5 | 12 | 9 | 5 | 14 | 9 | 4 | 13 | 7 | 3 | 10 | 0 | 1 | 1 |
| Total | 9 | 5 | 14 | 7 | 5 | 12 | 9 | 5 | 14 | 9 | 7 | 16 | 7 | 6 | 13 | 1 | 2 | 3 |
| Normalcy of diet, n | ||||||||||||||||||
| Non-oral feeding | – | – | – | – | – | – | 0 | 1 | 1 | 1 | 3 | 4 | 1 | 3 | 4 | – | – | – |
| Cold liquids | – | – | – | – | – | – | 1 | 1 | 2 | 4 | 1 | 5 | 3 | 1 | 4 | 1 | 1 | 2 |
| Warm liquids | – | – | – | 0 | 1 | 1 | 3 | 0 | 3 | 2 | 1 | 3 | 1 | 0 | 1 | – | – | – |
| Pureed foods | – | – | – | 1 | 0 | 1 | – | – | – | 0 | 1 | 1 | – | – | – | – | – | – |
| Soft foods requiring no chewing | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 2 | – | – | – | 1 | 0 | 1 | – | – | – |
| Soft, chewable foods | 3 | 0 | 3 | 4 | 1 | 5 | 1 | 2 | 3 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 1 |
| Dry bread crackers | – | – | – | 0 | 1 | 1 | – | – | – | – | – | – | – | – | – | – | – | – |
| Carrots, celery | – | – | – | – | – | – | 0 | 1 | 1 | – | – | – | – | – | – | – | – | – |
| All meats | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 1 | 1 | – | – | – |
| Full diet with liquid | 0 | 1 | 1 | – | – | – | 1 | 0 | 1 | – | – | – | – | – | – | – | – | – |
| Full diet with no restrictions | 5 | 4 | 9 | 2 | 2 | 4 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | – | – | – |
| Total | 9 | 5 | 14 | 8 | 5 | 13 | 9 | 5 | 14 | 9 | 7 | 16 | 7 | 6 | 13 | 1 | 2 | 3 |
| Completion rate (%)a | 82.4 | 76.5 | 82.4 | 94.1 | 76.5 | 17.6 | ||||||||||||
Tables 12 and 13 summarise the Performance Status Scale normalcy of diet measurements on a weekly basis, during treatment and over the course of the trial.
Patient-reported measures
Questionnaire scores were calculated and transformed as recommended by the specific research groups (MDADI, EORTC, SF-36). Scores are reported longitudinally over time, as raw scores were conditional on patient survival. Owing to small numbers, change from baseline was not calculated (see Tables 14–17). Some of the data are shown graphically in Figures 2–4.
Table 14 shows that the response rates to the three patient-reported questionnaires were high at 3 months (overall > 80% of all 17 patients) and maintained at 6 months, with good response rates in both randomised groups.
| Questionnaire | Time point | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consent and baseline | End of CRT | Month 3 | Month 6 | Month 12a | |||||||||||
| Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | |
| MDADI | |||||||||||||||
| No, n | 2 | 1 | 3 | 1 | 2 | 3 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yes, n | 7 | 7 | 14 | 8 | 6 | 14 | 9 | 6 | 15 | 8 | 7 | 15 | 4 | 2 | 6 |
| Total | 9 | 8 | 17 | 9 | 8 | 17 | 9 | 8 | 17 | 8 | 7 | 15 | 4 | 2 | 6 |
| Completion rate (%) | 82.4 | 82.4 | 88.2 | 88.2 | 35.3 | ||||||||||
| EORTC QLQ-C30 | |||||||||||||||
| No, n | 0 | 1 | 1 | 1 | 2 | 3 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yes, n | 9 | 7 | 16 | 8 | 6 | 14 | 9 | 6 | 15 | 8 | 7 | 15 | 4 | 2 | 6 |
| Total | 9 | 8 | 17 | 9 | 8 | 17 | 9 | 8 | 17 | 8 | 7 | 15 | 4 | 2 | 6 |
| Completion rate (%) | 94.1 | 82.4 | 88.2 | 88.2 | 35.3 | ||||||||||
| SF-36 | |||||||||||||||
| No, n | 0 | 0 | 0 | 1 | 2 | 3 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yes, n | 9 | 8 | 17 | 8 | 6 | 14 | 9 | 6 | 15 | 8 | 7 | 15 | 4 | 2 | 6 |
| Total | 9 | 8 | 17 | 9 | 8 | 17 | 9 | 8 | 17 | 8 | 7 | 15 | 4 | 2 | 6 |
| Completion rate (%)b | 100 | 82.4 | 88.2 | 88.2 | 35.3 | ||||||||||
Given the small number of respondents, summary statistics are demonstrated as medians (ranges) between the randomised treatment groups. The data displayed in Table 15 are better represented graphically, as seen in Figure 2.
| SF-36 | Time point | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consent and baseline | End of CRT | Month 3 | Month 6 | Month 12a | |||||||||||
| Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | |
| Physical component scores | |||||||||||||||
| Median (IQR, range) | 52.03 (47.01–54.40, 32.59–56.01) | 49.72 (43.1–56.7, 28.35–60.45) | 52.03 (46.62–55.13, 28.35–60.45) | 42.35 (38.33–50.26, 29.71–53.19) | 39.05 (29.65–39.54, 24.36–42.34) | 39.37 (29.71–45.32, 24.36- 53.19) | 50.39 (41.98–51.8, 38.59–57.99) | 45.62 (45.29–48.36, 26.53–53.33) | 48.36 (41.98–51.8, 26.53–57.99) | 52.28 (49.12–55.52, 45.64–61.64) | 47.26 (37.48–52.71, 37.30–59.91) | 49.31 (45.64–53.77, 37.3–61.64) | 56.89 (55.82–58.69, 54.87–60.37) | 53.54 (47.16–59.91, 47.16–59.91) | 56.89 (54.87–59.91, 47.16–60.37) |
| Mental component scores | |||||||||||||||
| Median (IQR, range) | 55.46 (47.52–59.71, 27.68–63.83) | 49.51 (35.8–55.04, 31.14–62.99) | 49.99 (42.34–59.71, 27.68–63.83) | 38.82 (28.66–51.68, 13.89–61.62) | 27.24 (23.36–46.84, 23.28–49.63) | 34.23 (23.36–49.63, 13.89–61.62) | 57.86 (44.38–58.91, 33.38–62.34) | 45.1 (34.66–50.4, 30.25–52.08) | 50.4 (35.72–58.55, 30.25–62.34) | 57.82 (55.01–58.22, 33.86–61.98) | 50.4 (30.22–53.02, 29.18–62.19) | 54.54 (41.59–58.06, 29.18–62.19) | 49.36 (45.89–55.16, 44.62–58.74) | 46.55 (30.91–62.19, 30.91–62.19) | 49.36 (44.62–58.74, 30.91–62.19) |
| n | 9 | 8 | 17 | 6 | 5 | 11b | 9 | 6 | 15 | 8 | 7 | 15 | 4 | 2 | 6 |
FIGURE 2.
Short Form questionnaire-36 items physical (a) and mental (b) component scores over time. NGT, nasogastric tube.


Figure 2 demonstrates graphically that both the physical and mental component scores from the SF-36 questionnaire dropped from a baseline score of ≈50 following CRT, and returned to baseline level by 3 months, maintained to 6 months. This trend was observed in both randomised groups. There was a trend for patients randomised to receive gastrostomy tubes to have slightly inflated scores (a ≈5-point increase in the physical component score, and a ≈10-point increase in the mental component score) across the time points, compared with patients randomised to receive nasogastric tubes.
Given the small number of respondents, summary statistics are demonstrated as medians (ranges) across the randomised treatment groups. The data displayed in Table 16 are better represented graphically, as seen in Figure 3.
| EORTC QLQ-C30 | Time point | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consent and baseline | End of CRT | Month 3 | Month 6 | Month 12a | |||||||||||
| Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | |
| GHS (IQR, range) | 66.67 (58.33–91.67, 50.00–100.00) | 75.00 (41.67–100.00, 41.67–100.00) | 70.83 (58.33–95.83, 41.67–100) | 41.67 (29.17–58.33, 8.33–75.00) | 29.17 (0.00–66.67, 0.00–66.67) | 37.50 (16.67–66.67, 0.00–75.00) | 66.67 (50.00–83.33, 50.00–100.00) | 58.33 (25.00–83.33, 8.33–91.67) | 66.67 (50.00–83.33, 8.33–100.00) | 83.33 (66.67–83.33, 41.67–100.00) | 75.00 (66.67–83.33, 50.00–100.00) | 75.00 (66.67–83.33, 41.67–100.00) | 83.33 (75.00–83.33, 66.67–83.33) | 83.33 (66.67, 100.00, 66.67–100.00) | 83.33 (66.67–83.33, 66.67–100.00) |
| n | 9 | 7 | 16 | 8 | 6 | 14 | 9 | 6 | 15 | 8 | 7 | 15 | 4 | 2 | 6 |
FIGURE 3.
European Organisation for Research and Treatment of Cancer QLQ-C30 global health scores over time. NGT, nasogastric tube.
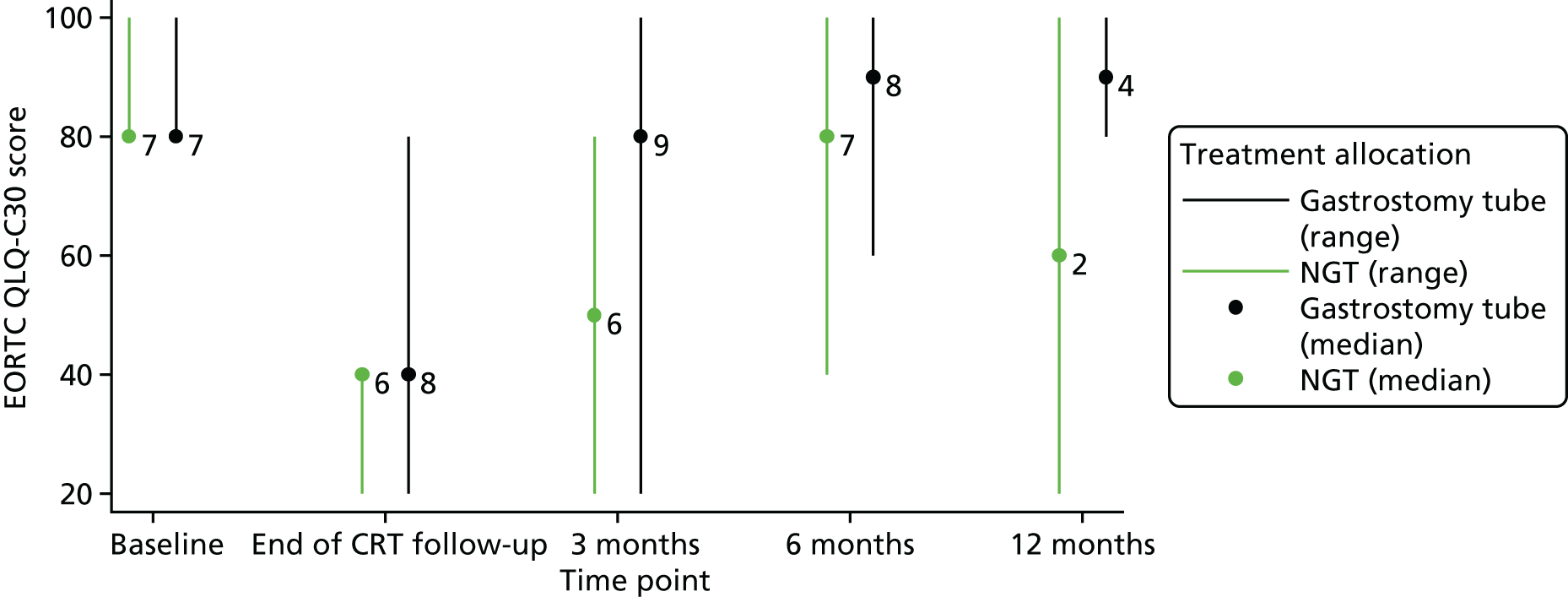
Figure 3 demonstrates graphically that the global health scores from the EORTC QLQ-C30 questionnaire dropped from a baseline score of ≈70 before CRT to a post-CRT score of ≈35. Scores improved at 3 months, but did not return to baseline level until 6 months. This trend was observed in both randomised groups. Scores appeared to be similar across the randomised groups, with a wide range of scores for both arms.
Given the small number of respondents, summary statistics are demonstrated as medians (ranges) across the randomised treatment groups. The data displayed in Table 17 are better represented graphically, as seen in Figure 4.
| MDADI | Time point | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consent and baseline | End of CRT | Month 3 | Month 6 | Month 12a | |||||||||||
| Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | Gastrostomy arm | NGT arm | Total | |
| GS (Q1), (IQR, range) | 80 (80–100, 80–100) | 80 (80–100, 80–100) | 80 (80–100, 80–100) | 40 (20–70, 20–80) | 40 (20–40, 20–40) | 40 (20–40, 20–80) | 80 (80–100, 20–100) | 50 (20–80, 20–80) | 80 (40–80, 20–100) | 90 (80–100, 60–100) | 80 (40–100, 40–100) | 80 (80–100, 40–100) | 90 (80–100, 80–100) | 60 (20–100, 20–100) | 90 (80–100, 20–100) |
| n | 7 | 7 | 14 | 8 | 6 | 14 | 9 | 6 | 15 | 8 | 7 | 15 | 4 | 2 | 6 |
FIGURE 4.
MD Anderson Dysphagia Inventory global scores over time. NGT, nasogastric tube.
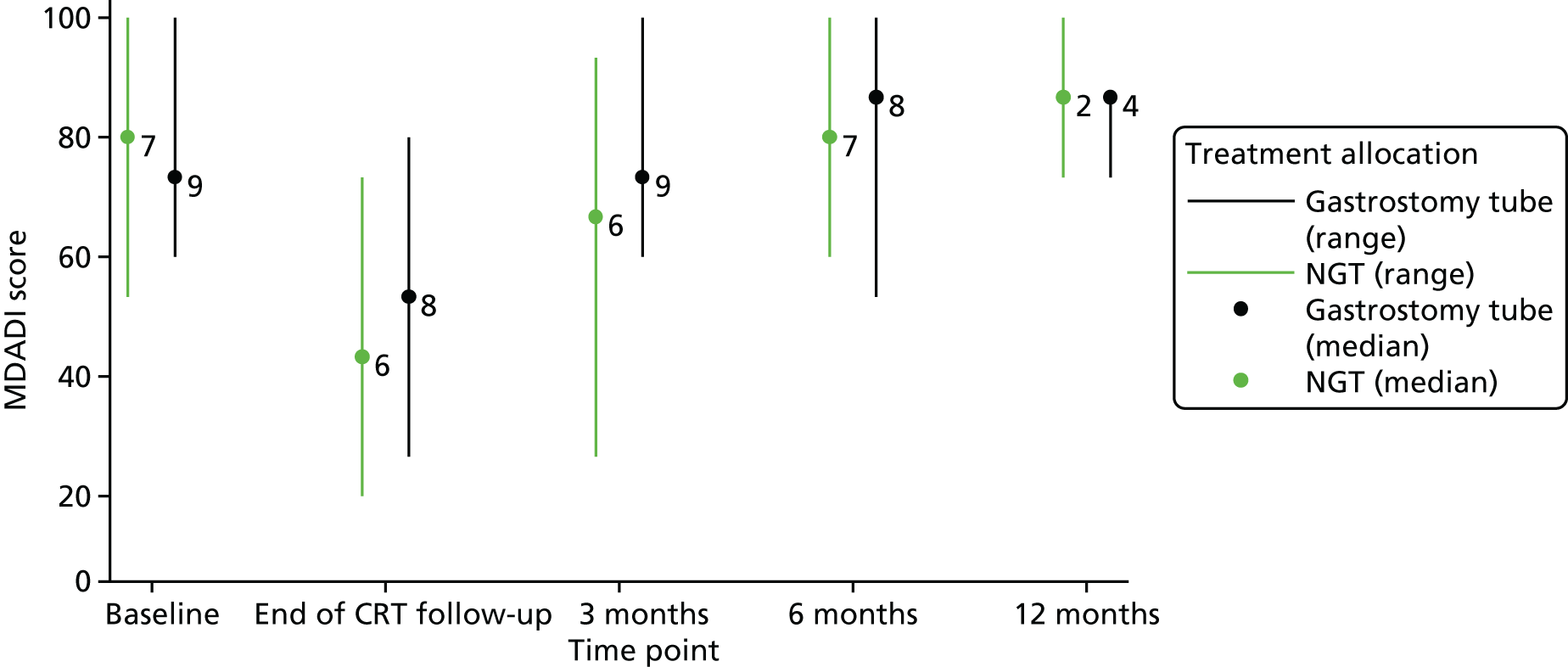
Figure 4 demonstrates graphically that the global scores from the MDADI questionnaire dropped from a baseline score of ≈80 to a score of ≈40 following CRT, and returned to baseline level by 3 months for patients randomised to receive gastrostomy tubes and by 6 months for patients randomised to receive nasogastric tubes. There was a wide range of scores in both groups.
Analyses were planned as descriptive analyses providing a comprehensive symptom profile of the patients. The mean [standard deviation (SD)] of baseline QoL scores was calculated and presented by treatment group for each domain. The mean (SD) changes in QoL from baseline to 6 months (end of the treatment period) and 12 months (end of the follow-up period) were calculated and presented by treatment group for each domain, conditional on the patients surviving.
Owing to small numbers, scores were calculated as the median (range) within a 6-month period only and are reported for global QoL scales.
Mortality
At the time of the data freeze, no patients in the trial had been reported as having died.
Planned subgroup analyses
Subgroup analyses were planned according to subgroups defined by (1) induction chemotherapy plans at randomisation (planned, not planned), (2) age at randomisation (≤ 60 years or > 60 years) and (3) patients with severely reduced levels of swallowing (yes or no). Subgroup analyses were not carried out because the trial closed early.
Decision criteria
The decision to move to a Phase III trial was based on the following:
-
Adequate timely recruitment with a 50% recruitment rate. The recruitment rate achieved was 23%, with the upper bound of the 95% CI being 33%. The justification to move forward with this design to a Phase III trial was based on an upper limit of the 95% CI exceeding 50%, which was not achieved, and, hence, the trial was not deemed feasible in terms of recruitment.
-
Completeness of outcome measurement (MDADI at 6 months). Excluding those individuals who died during the study period, the successfully collected outcome data should have been ≥ 80%, as this was to be the primary outcome for the definitive Phase III trial. The MDADI response rate at 6 months was high at an observed rate of 88%. In addition, retention to clinical assessment at 6 months was also observed at 88%, and compliance with the randomised intervention was high at 94%, and, hence, the trial was deemed feasible in terms of completeness, retention and compliance.
-
Economic criteria of the economic value-of-information analysis.
Qualitative study
Introduction
Process evaluations are central to understanding the factors that promote or inhibit the implementation of complex interventions. 47,48 A 2013 review of qualitative research within and alongside trials showed the range of issues that such work can elucidate, including issues around trial design, conduct and processes. 49 Our qualitative process evaluation draws on the NPT to elucidate factors promoting or inhibiting the TUBE trial. The NPT is an established middle-range theory of implementation that explains the normalisation of changes in practice with reference to the complex and collaborative work involved in introduction, implementation and embedding of activities. 38,50,51 The NPT focuses on collaborative work across four constructs: (1) coherence – how people make sense of an intervention; (2) cognitive participation – whether or not people are willing and able to buy into and commit to the implementation of an intervention; (3) collective action – the work, skills, trust and resources people require to implement the intervention; and (4) reflexive monitoring – how people evaluate, both formally and informally, the intervention, and how they work to adapt their practices in light of this information.
The aims of this qualitative substudy of the TUBE trial were to identify, describe and understand (1) barriers to, and facilitators of, trial recruitment; (2) issues of trial conduct; and (3) experiences of feeding tubes.
In this chapter, initially, we reported on the clinical and organisational contexts, and we focused on the range of contexts that had an impact on the implementation of the TUBE trial. We showed how sites had different histories of preferences for feeding options and that there was an ongoing debate between professional groups regarding the use of nasogastric tubes and gastrostomy tubes. In this context, the TUBE trial was consistently viewed as a worthwhile research endeavour that would provide useful evidence to guide decision-making. We showed that the TUBE trial also challenged some established patterns of work and the job specification of particular individuals or groups. Given all of these factors, we argue that the TUBE trial was not a socially ‘neutral’ exercise, but rather a collaboration that was moulded – to varying degrees of success – to a local, historically encumbered, clinical environment of pre-existing tensions.
In Operational contexts, we briefly focus on three extrinsic factors that had a direct impact on the TUBE trial. We describe delays in the process of obtaining site-level approvals for research, which meant that sites were less open to recruitment than anticipated. We then outline how, between study design and the implementation of the TUBE trial, there was a shift towards greater use of surgery in our target patient group, thus, making more patients ineligible for recruitment. Finally, we show how confusion about how to operationalise swallowing eligibility criteria may also have had an impact on recruitment.
In Integrating research, we show how sites worked to implement and embed the TUBE trial, to integrate the research pathway into the existing local clinical pathway. To successfully embed the trial, the teams at each site had to enrol a range of people and be willing to adapt their practices over time. The issue of preference, at the site, professional and individual level, had to be managed. Some teams worked collectively to present equipoise at all contacts with patients when feeding options were discussed. Some teams reported that different members of the team expressed preferences for specific feeding options at different points across the clinical and research pathway. Finally, we show how, in recruitment consultations, HCPs worked to effectively convey equipoise.
In Decision-making, we focus directly on patients’ experiences of the TUBE trial. Patients emphasised that receiving treatment for their cancer was their top priority. They understood the purpose of the research and understood what agreeing to randomisation would mean in terms of treatment and its impact on their clinical pathway. The timely and efficient progression of treatment was their central concern. The TUBE trial was generally reported in positive terms when it was perceived to fit seamlessly within a clinical pathway. We then showed the range of reasons that patients offered in relation to both accepting and declining randomisation. Finally, we outline how preferences for specific forms of feeding tubes were formed from a wide range of factors, including the perceived or stated preference of the consultant.
In the final empirical section, Living with feeding tubes, we move beyond issues of trial conduct and process to briefly focus on patients’ experiences of the different feeding treatments compared within the TUBE trial. For patients, participation was largely understood as a decision about whether or not to agree to be randomised. Post randomisation, the trial pathway was invisible and, therefore, acceptable to patients. We show how a nasogastric tube is more problematic for patients than a gastrostomy tube; insertion is more unpleasant, feeding takes longer and is more socially isolating, and dealing with problems that occur is more demanding. There was some evidence that patients who were treated with gastrostomy did retain their tubes for longer. However, they described how they consumed food and fluids orally for taste, social and other reasons, using the gastrostomy to ‘top up’ otherwise inadequate oral feeding.
Finally, in the discussion, we argue that, overall, the TUBE trial was a technically competent feasibility trial, addressing a research question that was perceived by both clinicians and patients as worthwhile. However, the clinical context that inspired the research question also played a part in undermining the practical implementation of the research across multiple sites. Although HCPs reported strong patient preferences as a barrier to recruitment, we also observed that some aspects of strong HCP preferences shaped recruitment. Centrally, although all sites experienced the same or similar barriers, such as strong patient preferences, we found that the proportion of screened to consented participants was higher when there was a strong ‘buy-in’ to the study across all HCPs at a site. When equipoise was not distributed across teams and there was a lack of interest in the study, recruitment was lower.
Data set
Nineteen interviews were conducted with HCPs: six with doctors, five with research nurses, five with dietitians and three with SALTs. Two individuals were interviewed twice, both at Woodville, the site open for longest to patient recruitment. Seven interviews were conducted at Woodville, three at Otago, four at Hamilton, three at Queenstown and two at Wellington (Table 18).
| Interview variables | Site | Total | ||||
|---|---|---|---|---|---|---|
| ‘Woodville’ | ‘Otago’ | ‘Hamilton’ | ‘Queenstown’ | ‘Wellington’ | ||
| Interviewed (patients) | 9 | 1 | 2 | 3 | 2 | 17 |
| Randomised to receive NGT | 2 | 1 | 1 | 1 | 0 | 5 |
| Randomised to receive gastrostomy tube | 4 | 0 | 1 | 1 | 1 | 7 |
| Decliners | 3 | 0 | 0 | 1 | 1 | 4 |
| Interviewed (carers) | 1 | 1 | 0 | 0 | 1 | 3 |
| Declined | 0 | 0 | 0 | 0 | 0 | 0 |
| Randomised to receive NGT | 1 | 1 | 0 | 0 | 0 | 2 |
| Randomised to receive gastrostomy tube | 0 | 0 | 0 | 0 | 1 | 1 |
| Follow-up interviews | 1 | 0 | 0 | 2 | 1 | 4 |
| Randomised to receive NGT (patients) | 0 | 0 | 0 | 1 | 0 | 1 |
| Randomised to receive gastrostomy tube (patients) | 1 | 0 | 0 | 1 | 1 | 3 |
| Randomised to receive NGT (carers) | 0 | 0 | 0 | 0 | 0 | 0 |
| Randomised to receive gastrostomy tube (carers) | 0 | 0 | 0 | 0 | 0 | 0 |
| Interviewed (patients and carers) | 11 | 2 | 2 | 5 | 4 | 24 |
| Interviewed | 5 | 3 | 4 | 3 | 2 | 17 |
| PI (oncologists, n = 3; surgeons, n = 2) | 1 | 1 | 1 | 1 | 1 | 5 |
| Research nurse | 1 | 1 | 1 | 1 | 0 | 4 |
| Dieticians | 2 | 0 | 1 | 0 | 1 | 4 |
| SALTs | 1 | 1 | 0 | 1 | 0 | 3 |
| Doctors (oncologists) | 0 | 0 | 1 | 0 | 0 | 1 |
| Doctors (other) | 0 | 0 | 0 | 0 | 0 | 0 |
| Professionals (other) | 0 | 0 | 0 | 0 | 0 | 0 |
| Follow-up interviews | 2 | 0 | 0 | 0 | 0 | 2 |
| PI (oncologists, n = 3; surgeons, n = 2) | 0 | 0 | 0 | 0 | 0 | 0 |
| Research nurse | 1 | 0 | 0 | 0 | 0 | 1 |
| Dietitians | 1 | 0 | 0 | 0 | 0 | 1 |
| SALTs | 0 | 0 | 0 | 0 | 0 | 0 |
| Doctors (oncologists) | 0 | 0 | 0 | 0 | 0 | 0 |
| Doctors (other) | 0 | 0 | 0 | 0 | 0 | 0 |
| Professionals (other) | 0 | 0 | 0 | 0 | 0 | 0 |
| Totals | 7 | 3 | 4 | 3 | 2 | 19 |
A total of 24 interviews were conducted with patients (n = 21 interviews with 17 individuals) and carers (n = 3) from across all participating sites (see Tables 18 and 22). Our recruitment strategy permitted us to follow up only patients who had consented to both the qualitative substudy and to the trial (n = 13). At follow-up, a number of patients were deemed too ill to be contacted or did not respond to contact attempts, and only four patients were successfully reinterviewed. More men than women were interviewed, reflecting the patient population for head and neck cancer.
In addition, the qualitative researcher observed the launch event and site initiation visits (SIVs) and field notes were taken. Audio-recordings were made of four recruitment consultations.
Some of the participants in the qualitative substudy were members of, or known to, the research team. Therefore, we have used pseudonyms in the current chapter of this report. Research sites have been named after New Zealand towns and cities and participants have been given a first name in place of an identification code. The ethnicity and sex implied by the pseudonym do not necessarily match those of the respondent.
Results
The results are divided into five sections. The first two focus on the clinical, organisational and operational contexts in which the TUBE trial had to be implemented. In the third section, we focus on how the TUBE trial was integrated into the day-to-day work of clinical teams. In the final two sections, we focus on patients’ engagements with the TUBE trial and their experiences of the different feeding treatments compared in the trial.
Clinical and organisational contexts
In this opening section, we focus on the range of contexts that had an impact on the implementation of the TUBE trial. We show how sites had different histories of preferences for feeding options. We show that there was an ongoing debate between professional groups regarding the use of nasogastric tubes and gastrostomy. Some sites offered only one option to the majority of the patients. Some sites were reflecting on current practice and thinking about changing options. Some, with the arrival of new team members, or the loss of old ones, were shifting emphasis. In addition to variation between sites, we show that there was considerable variation within sites in terms of preference of feeding approach. Often this related not only to perceptions of the benefits and disadvantages of each approach, but also to perceptions of the relative importance of these benefits. These tensions between the objectives and the priorities of groups of professionals underpinned some of the issues affecting the TUBE trial.
Given this context of variation in practice and preferences for supplementary feeding between and within sites, the rationale underpinning the TUBE feasibility study was clearly understood. The TUBE trial was consistently viewed as a worthwhile research endeavour, which would provide useful evidence to guide decision-making. Although HCPs identified the value of the TUBE trial in assessing the feasibility of a definitive trial, some respondents raised concerns about the perceived specificity of the research question. We also observed that the TUBE trial challenged some established patterns of work and the job specification of particular individuals or groups. There was an existing division of labour and expertise, whereby some HCP roles and responsibilities were intimately tied to specific feeding options and the organisation of care was tied to a specific modality. In this context, the TUBE trial was not a socially ‘neutral’ exercise, but rather a collaboration that was moulded – to varying degrees of success – to a local, historically encumbered, clinical environment of pre-existing tensions.
Changing patterns of feeding tube use across sites
In the past, pre-treatment gastrostomy was routinely provided to patients undergoing CRT for head and neck cancer in the UK. However, practice has shifted in many places towards the use of as-needed nasogastric tubes. At the time of TUBE trial recruitment, TUBE trial sites were broadly split between the two approaches, with Otago and Wellington favouring gastrostomy, Woodville and Hamilton favouring nasogastric tubes and Queenstown offering patients a choice between the two treatments (Table 19). Sites gave comprehensive accounts of the factors underlying the changing approach, summarised in Box 1.
| Research site | Recommendation | |
|---|---|---|
| Routine treatment | History | |
| Otago | Prophylactic gastrostomy, but moving towards reactive gastrostomy and NGT | Internal review of patient outcomes contributed to the expanded portfolio of treatment options |
| Queenstown | Patient choice with individualised guidance | Change in patient demographics cited as a reason for increased recommendation for NGT, but both options were available and treatment recommendation individualised |
| Woodville | NGT | Used to offer gastrostomy. Serious adverse patient outcomes contributed to change in routine practice |
| Wellington | Gastrostomy, but moving towards NGT | Internal review of the length of time patients rely on tube feeding contributed to increased recommendation for NGT |
| Hamilton | NGT | Used to offer gastrostomy. Staff changes and experience of serious adverse patient outcomes (at another site) contributed to change in routine practice |
-
Changing patient population (HPV cancers – younger, fitter better able to tolerate treatment).
-
Changing treatment (less toxic).
-
SAEs with gastrostomy.
-
Internal audits suggesting better tolerability and swallowing outcomes with nasogastric tube.
-
Practicalities/costs.
Although gastrostomy was the standard treatment at Otago and Wellington, HCPs at both sites indicated a state of transition away from gastrostomy at the start of the TUBE trial. Lara, an oncologist, described this process for Wellington:
There was a period of time that we were putting gastrostomy tubes, into pretty much everybody coming through for chemo/rads [chemoradiation therapy]. Then we audited our local data, and found that our patients who had prophylactic gastrostomies had them in for far, far longer than patients who had reactive NG [nasogastric] tubes, but obviously that wasn’t in the context of a randomised trial. . . . So I think just bit by bit you know probably we’re thinking harder about that decision of whether to put a gastrostomy in or not. Probably more and more we are going to reactive NG.
Lara (oncologist), Wellington
As at the Wellington site, HCPs at Otago also described having previously engaged in an internal audit of long-term outcomes in patients receiving supported tube feeding. Having reached similar conclusions to those of the team at Wellington, their approach was to offer a wider portfolio of feeding tube options, including laser-reactive gastrostomy.
In Hamilton, HCPs described an ongoing debate between professional groups regarding the use of nasogastric tubes and gastrostomy. Rebecca, a dietitian, explained that Hamilton used to offer gastrostomy predominantly. The dietetics department took a lead in organising gastrostomy placement, monitoring outcomes and providing support for patients. Over time, practice at the site moved towards counselling patients through a choice of nasogastric tube or gastrostomy. Before the TUBE trial, a change in senior oncology staff coincided with the increased use of nasogastric tubes and tension between the professional groups. Rebecca explained how these changes in practice developed:
Historically here, we were a very pro-gastrostomy site and we have always done a lot of gastrostomies. We have had a change in consultants, a retirement of our oncologists and new oncologist staff who are quite antigastrostomy, for a variety of reasons. One of the consultants had a death from one of her patients at a previous trust, which obviously had an impact.
Rebecca (dietitian), Hamilton
An important observation shared by oncologists at Hamilton and Queenstown was a change in the characteristics of patients with head and neck cancer and improvements in primary treatment. Increasingly, younger and fitter patients than those of the ‘traditional’ head and neck cancer profile were presenting with cancers linked to HPV:
The traditional head and neck cancer patients [. . .] no social support, lives alone, mostly living in a pub, smoking away and drinking 15 or 16 units a day. That’s the classic group of patients . . . [but] incidents of smoking-induced cancer is on the decline as the number of people smoking is getting less and less. [. . .]. The other group of patients . . . is increasing . . . they are non-smokers, they are young, they are working full time, have a young family, so that profile is changing. In that group of patients, the treatment modality is also slightly different [from ‘traditional’ patients] and they are motivated. They might do better [with a nasogastric tube].
Simon (oncologist), Queenstown
Simon and a second oncologist, Sally (at Hamilton), both stated that, excluding other factors, nasogastric tubes were generally more suitable than gastrostomy for the ‘new’ profile of patients. Sally explained why she recommended nasogastric tubes more often than gastrostomy:
Historically, it used to be a gastrostomy, but that was obviously pre-IMRT era, and improvement in the way that the team is structured, as well as the quality of the radiotherapy treatment [has led to increased use of nasogastric tubes]. Also, with the change in the patient population: obviously we now have a much younger, fitter, HPV-positive population who don’t necessarily have a history of drinking and smoking. We’ve had quite a few things change over the last few years.
Sally (oncologist), Hamilton
At Queenstown HCPs explained that patients were typically offered a choice between NG and gastrostomy, unless clinical and social factors (e.g. disease progression, social support, age of patient, degree of dysphagia) pointed to a specific treatment approach. HCPs at Woodville reported that NG was the ‘standard’ treatment off-study. Our practice has evolved, if you look at purely the patients who are suitable for this trial, if the trial wasn’t in existence this centre would be offering the majority of them NG tubes but that wasn’t the case even let’s say 5 years ago.
Patrick (surgeon), Woodville
The HCPs’ experience of patient mortality as a result of gastrostomy was a contributing factor for this preference. In addition to variation between sites, there was considerable variation within sites in terms of preferences for the different feeding approaches. Often this related not only to perceptions of the benefits and disadvantages of each approach, but also to perceptions of the relative importance of these advantages. This perception of the significance of the different pros and cons was also relevant in thinking about how individual clinicians felt about the outcome measures selected in the TUBE study.
Clinicians’ preferences for particular feeding approaches
In general, nasogastric tubes were seen as less pleasant for the patient; they involved irritation to an area of the body already made sensitive by the cancer treatment and were more likely to become blocked or come out, or suffer other problems that could necessitate repeated trips to and from hospital, feeding was slower and hence more disruptive to everyday life and they were a visible sign of cancer treatment:
I think possibly because these patients having so much going on in your head and neck region an NG, it can be an extra burden because it’s irritating and it’s not nice as an extra thing that they need to come back and forth to hospital for. Whereas having a gastrostomy it’s away from the head and neck.
Avani (dietitian), Woodville
Gastrostomy was seen to be less problematic, although it was acknowledged that when problems occurred these could be serious. Some HCPs pointed out that complications of nasogastric tubes could also be serious; Patrick highlighted a death he knew about that had occurred when a nasogastric tube was accidentally placed in the patient’s lungs. SALTs and dietitians speculated that nasogastric tubes ‘might’ result in better long-term swallowing, but at the cost of a more difficult experience of tube feeding than with gastrostomy:
There tends to be a lot less . . . complications for the patients [with gastrostomy], . . . I feel that if someone has [gastrostomy], it’s there, and they’re almost ready to go for treatment. If they can’t swallow, they can get their pain relief through that. If they cannot drink, we can keep them hydrated. So it keeps them out of hospital. Whereas with the NGs . . . I spend a lot of my time sorting them out. People in the community, it’s blocked, they need to come in. Or . . . it’s come out, they have vomited [up the tube, or] they do not want to be attached to the pump. They are missing out on a feed because the ambulance picked them up too early, and they had to cut the feed down because of the pump. You know, there seems to be a lot more complications through the treatment with the NG. . . . that’s just what I see . . . through the treatment, you know. At the end of it, . . . with this long-term swallow.
Charlotte (dietitian), Woodville
Gastrostomy was sometimes described as being the ‘easy’ or ‘lazy’ option:
What most people postulate is that patients get lazy [with gastrostomy] and there is a degree of that. That they have got a tube in their tummy, they can stick stuff through it, so they don’t try. So therefore the gastrostomy gets labelled as a tube that is an easy route and that we shouldn’t put them in because patients don’t try, because it is there, but we don’t really have an awful lot of evidence to support that.
Rebecca (dietitian), Hamilton
Some HCPs suggested that patients who had gastrostomy used their tube earlier, and for longer, once swallowing became difficult than those patients who had a nasogastric tube.
Health-care professionals who worked regularly with patients requiring feeding tubes explained that both methods had advantages and disadvantages, and each was appropriate to specific cases. The factors that shaped the appropriateness of each type of tube were described as ‘complex’. Jenny, a SALT at Woodville, explained:
I guess it’s just the complexity . . . thinking about all the factors that affect . . . your decision [. . .] because neither [tube] are risk free . . . swallowing outcomes is just one thing, you know, if patients all hate having an NG – even though their swallowing outcomes might be better – then that might colour you to go for a gastrostomy, and I think that’s why a lot of centres do, do a gastrostomy because it’s based on some degree on patient’s experience. Also ease of managing them and it’s not necessarily . . . based on functional outcomes. It’s about getting them through the treatment, then what happens after that?
Jenny (SALT), Woodville
Jenny identified that the different types of tube shaped the work of HCPs in managing patients: gastrostomies were easier to manage in the patient population than nasogastric tubes. The reason for this difference was that nasogastric tubes could be coughed or vomited up, pulled out or otherwise dislodged relatively easily. Gastrostomy tubes were more permanent. Moreover, nasogastric tubes required more management, essential tasks being a pH check and regular cleaning of the tube. As Charlotte highlighted, feeding could also take longer with a nasogastric tube than with a gastrostomy tube because the nasogastric tube is of smaller diameter. Jenny made a distinction between the work of managing patients’ nutrition through treatment (via tube feeding) and management of patients’ long-term functional outcomes. Her account echoes our observation that different professional groups tended to express preferences for tubes that were complementary to the priorities and esteem markers of their role specifications. Nasogastric tubes were reported across sites as having less risk in comparison to gastrostomy on outcomes including patient mortality and infection, and a suspected better long-term swallowing outcome owing to patients’ lower resistance to having a tube inserted. These advantages may have provided a better match than gastrostomy with the priorities of senior clinicians under pressure to improve measurable outcomes:
Patients who are otherwise fairly fit and they’re able to understand how to manage a tube, those are the ones that I will be recommending the nasal gastric tube because they may stand to benefit in terms of the functional outcome. [. . .] These are, again, the questions that are in TUBE. For example, functional improvement outcome versus the convenience and the improvement in the quality of life. A gastrostomy, probably the quality of life is going to be easier with a gastrostomy in the short term. However, long term, the swallowing outcome, there is some thinking that a nasal gastric tube, people who undergo that approach will have a better functional long-term outcome.
Sally (oncologist), Hamilton
Sally’s account is helpful because the TUBE trial did not assess the two tubes on convenience or QoL measures. Her description of the nasogastric tube and gastrostomy, thus, reveals her opinion of the benefits and drawbacks of both methods, with nasogastric tubes identified as potentially leading to superior ‘functional outcome’.
In comparison, arranging the placement of tubes, managing patients’ nutrition and tube care and maximising QoL during the treatment period (and beyond) was the domain of dietitians and clinical nurse specialists. Gastrostomy tube feeding represented advantages to these professional groups in terms of the execution of their daily work tasks, interactions with patients, job satisfaction, and formal and informal markers of success:
We discuss about what’s going to happen to them, . . . which methods of feeding we would use, . . . and what would happen during their journey, making sure that they’re sort of nutritionally at the place that they should be for that point. Like psychological things with eating and stuff as well. So quite a wide variety of discussions . . . it would be sort of everything to do with that social aspect [. . .] how they’re going to manage during treatment but also their lives afterwards as well.
Avani (dietitian), Woodville
These tensions between the objectives and priorities of different groups of professionals underpinned some of the issues affecting the TUBE trial.
The TUBE trial in the context of clinical variation in practice and preference for supplementary feeding
Given this context of variation in practice and preferences for supplementary feeding between, and within, sites, the rationale underpinning the TUBE feasibility study was clearly understood. The TUBE trial was consistently viewed as a worthwhile research endeavour that would provide useful evidence to guide decision-making. For example, Jenny, a SALT, explained:
I think it’s a really valuable and important study and if we can get people to randomise and agree to do it, it will be really valuable to us because that will then . . . guide us a bit better in our decision-making for feeding tube options.
Jenny (SALT), Woodville
Although HCPs identified the value of the TUBE trial in assessing the feasibility of a definitive trial, some respondents raised concerns about the perceived specificity of the research question, reflecting the differences in perceptions of the importance of different advantages and disadvantages of the different feeding approaches discussed in the previous section. A frequent remark was that long-term swallow was an important outcome, but only one consideration in a much larger matrix of factors that might guide HCPs in their advice and decision-making with patients. Although a wide range of outcome measures might be anticipated by the research team for a future definitive trial, these plans were either not evident to HCPs or too far removed from their contemporary concern of recruiting to the TUBE trial.
For some, a trial focusing on long-term swallow did not sufficiently target concerns at the forefront of patients’ minds when first considering feeding tubes. For example, Sian, a SALT at a second site, explained that:
I think people may have a preference and that might be based on lots of different things . . . I ran [a] patient engagement group . . . and the wealth of reasons . . . [for using] feeding tubes was diverse . . . this particular trial is about swallowing outcomes but . . . those other reasons will always exist.
Sian (SALT), Otago
Health-care professionals described the potential results of a future definitive RCT and its impact on clinical practice. One concern expressed by surgical and oncological professionals was that a definitive trial might be redundant, of reduced impact, or impossible to adequately recruit to given new primary treatments for head and neck cancer. Simon, an oncologist, explained that:
. . . my own personal view is that we may not find an answer, black or white. It is always going to be shades of grey I’m afraid because treatment modalities have advanced. They are going to more personalised, molecular-based treatment, and the current outcome with the modern treatment modality, the long-term tube dependency rates are much lower, less than 5% [. . .] so the percentage difference in order to find one or other [as superior] is significantly [more], means the power of the study, the number of patients is going to be much higher to find even a meaningful difference . . . We can collect the data, we may never answer the question.
Simon (oncologist), Queenstown
Health-care professionals whose work remit was closely associated with tube feeding specifically (e.g. SALTs, dietitians and specialist nurses) emphasised the potential value of a definitive trial to inform their current practice. However, these accounts also indicated an evolving clinical landscape in which current evidence needs might be eclipsed by other concerns. For example, Rebecca, a dietitian, explained:
I think as a starting point . . . TUBE is perhaps a good start. But . . . there is a big move in the head and neck fraternity that it is a very bad thing to have a feeding tube. From a dietetic perspective they the tools of our trade, a bit like saying it is very bad, a physio [physiotherapist], for a patient . . . to have a crutch to walk, you wouldn’t take the crutch away. But it seems to be, my perception nationally is that it is such a bad thing for someone to have a feeding tube, and that is the bit I don’t understand . . . I think . . . that [a definitive trial] would have some impact, but I do think some of the questions would need to be altered or you would need to be looking at perhaps slightly different outcomes.
Rebecca (dietitian), Hamilton
As indicated by Rebecca, the concept of the TUBE trial and the potential results of a definitive trial were interpreted in the context of existing job specifications and skill sets, professional identities and remits of work (e.g. ‘tools of our trade’) or, even more broadly, the potential impact on debates within the professional head and neck cancer community.
Professional roles and identities
We observed that the TUBE trial challenged some established patterns of work and the job specification of particular individuals or groups. Implementation of the trial sometimes led HCPs to adapt or change their work to fit the needs of the RCT. At Otago, some work roles were in the process of being adapted to meet the needs of new treatment modalities. TUBE trial work was integrated into the pre-trial context of change.
Avani, a dietitian at Woodville, described three domains of professional expertise mobilised in the deployment of the TUBE trial. Initially, she described the differences and similarities between the practice of SALTs and dietitians, emphasising that both groups contributed to the TUBE recruitment process. She later compared these professional groups with a third group, research nurses:
Right, so it’s not as if the speech and language therapist would give the same talk that you give?
No but . . . they might do parts of it . . . I would say possibly in the past, it’s been a bit of a bone of contention before all this, not recently, that they would give some overlapping advice about an NG or [gastrostomy]. But I would say more recently that that doesn’t happen as much, it’s more . . . joint.
So thinking about the future . . . would you perhaps think that it would be a dietitian that would be involved in the recruitment rather than a . . . language therapist?
Yeah, I would have reservations if it was just the speech and language therapist, I would prefer if it was both of us together because . . . there would be questions about ‘well what difference would that be for my swallow?’ And I can give a very brief [overview], but it’d be, you know it’s their [area of expertise], that’s them . . . But definitely not without [a dietitian] yeah, yes! Because the nutritional implications of it would be . . .
[talks over] And what if it was just the research nurse? . . .
You see I would worry about that because, [nothing] against [Jackie or Samantha the research nurses], but they’re the nurse, they’re not a dietitian they don’t know all the ins and outs of why one method would be different and how we would feed. They can give . . . the PIS, yeah but that’s different from . . . practical [experience] . . .
At the start of the quoted interview, Avani described a past tension – a ‘bone of contention’ – between SALTs and dietitians at Woodville regarding overlapping skill sets. However, she emphasised that this disagreement occurred before the trial and ‘not recently’, and that current working relationships emphasised collaboration rather than competition. She compartmentalised clinical skills and information into distinct domains that could be claimed and ‘owned’ by each professional group in turn: nutritional topics for dietitians, physical swallowing issues for SALTs and research-specific work for research nurses. Avani’s account suggested a negotiation about clinical domains that pre-dated the TUBE trial. Figuratively, she sketched a virtual map of professional boundaries as she described them to the interviewer. In this context, the TUBE trial was not a socially ‘neutral’ exercise, but rather a collaboration that was moulded – to varying degrees of success – to a local, historically encumbered clinical environment.
At Hamilton, the TUBE RCT highlighted pre-existing tensions between three professional groups of HCPs, each of whom claimed areas of expertise that converged on nutrition support tubes. HCPs at two sites described the reaction of colleagues who perceived the TUBE trial as potentially threatening their role specification and job security. A consultant surgeon, Patrick, reported that:
If you look at staff it’s their personal held beliefs, you know some don’t think it’s a good [idea]. There is a couple of people in [Woodville] who felt very strongly that patients should be given gastrostomy and not anything else. And they respond to this partly because their job is based on looking at gastrostomy, so clearly there is a bias there, so these have been barriers from a staff perspective.
Patrick (surgeon), Woodville
At Otago, HCPs reflected on how the TUBE trial had been discussed before its implementation. A central concern was how to reconcile the research question with the job specification of a specialist nurse on site. Emma explained that:
I think it’s difficult to say actually, I think that we had to have quite a stiff talk locally about equipoise, because we have a feeding tube nurse [Katie] used to be called a [gastrostomy] nurse. We had to tread quite carefully to start with and actually just sort of make [her] realise that [her] job wasn’t sort of on the line, which I think is what [she] worried about if we switched to nasogastric feeding. I think it’s just there’s been a morph of her job plan and her role . . . [which has] morphed extraordinarily over the last 5 years and [Katie is] essentially a feeding nurse now, and [her] job role actually has shrunk, but rather than that mean redundancy or something, it’s like well there’s new avenues, new things . . . She looks after the NGs and she’s going to become our new part-time swallowing nurse.
Emma (surgeon), Otago
Nasogastric feeding is a technique that facilitates the reactive (rather than prophylactic) placement of a gastric tube. Emma described several vectors of change at Otago that potentially shaped the job specification of Katie the ‘feeding nurse’. The TUBE trial assessed gastrostomy, Katie’s specialist area, in comparison with nasogastric tubes, and, therefore, may have been perceived by Katie as a ‘threat’ to her employment or status (formal assessment of gastrostomy vs. nasogastric tubes might have worked in favour of HCPs whose work role was aligned with the technology shown to be superior. However, some HCPs reported that they did not welcome what they perceived to be a gamble that might result in the erosion of their job specification and/or skill set); changes in feeding support practices at the site had already begun to shape Katie’s ‘morphed’ and atrophied role. Her future within the clinical team would be driven by adaptation (‘new avenues, new things’) as treatment modalities changed. Sian, a SALT at Otago, reflected on the implications of a definitive RCT comparing nasogastric tube with gastrostomy on swallowing outcomes. Specifically, she felt that the evidence supporting one form of feeding tube over another might undermine the infrastructure invested by some clinical sites in the less effective method, and this might in turn affect jobs. Although job specifications could be adapted to take account of changing technologies (as in Emma’s case), concern about ‘shrinking’ roles or redundancy could be intrinsically harmful. Patrick, quoted earlier, reported that some HCPs were reluctant to participate in a trial that could ultimately reduce the utility of their specialist skill sets.
The intersection of a range of contextual clinical and organisational factors led HCPs to take additional steps to ensure the smooth integration of trial work at the site. As we outline in Integrating research, for the TUBE trial to be successfully embedded, all members of the team had to ‘buy in’ to the trial, work to enrol a range of people beyond the team and mould the timing of clinical services to fit the needs of the trial. However, before we discuss the work within clinical teams, we need to focus on the wider organisational and operational contexts, unrelated to feeding tubes, which had an impact on the implementation of the TUBE trial.
Operational contexts
In this section, we focus briefly on three extrinsic factors that had a direct impact on the TUBE trial. We describe delays in the process of obtaining site-level approvals for research, which meant that sites were open for recruitment for less time than anticipated. We then outline how, between study design and TUBE trial implementation, there was a shift towards greater use of surgery in our target patient group, thereby making many more patients ineligible for recruitment. Finally, we show how confusion about how to operationalise swallowing eligibility criteria may also have had an impact on recruitment.
Site approval process
Despite attempts to streamline processes, delays in the process of obtaining site-level approvals for research in the NHS remain common. 52–56 The TUBE trial was no exception; during the study, only one site, Woodville, was open to patient recruitment for the intended 9 months, and Queenstown and Wellington were open for just 4 and 3 months, respectively. Rather than the intended 45 months of recruitment, this meant that the study was open for 32 months across sites (Table 20).
| Site | Date opened | Months open to recruitment (approximately)a |
|---|---|---|
| Woodville | 10 July 2014 | 11 |
| Otago | 30 October 2014 | 7 |
| Hamilton | 30 October 2014 | 7 |
| Queenstown | 23 February 2015 | 4 |
| Wellington | 27 March 2015 | 3 |
Although many of the delays related to issues such as workload in trust research and development (R&D) offices that were not unique to the TUBE trial, the context of head and neck cancer care, with clinical pathways that crossed different sites and trusts, proved to be particularly challenging.
At the Wellington site, Lara, an oncologist, described some of the barriers to implementing the research. She explained that R&D approval for her site had been held up, and after some investigation she discovered that this barrier could have been averted. Lara explained some of the challenges of integrating the TUBE trial in Wellington:
One of the requirements . . . to get R&D approval was to have some formalised arrangement, in writing, between ‘Wellington’ recruiting hospital and the centre where patients are treated, is that right, a service agreement?
Yes. So this is something that’s definitely held up opening this study, locally. So we’re in a situation . . . where the cancer centre, which delivers chemotherapy and radiotherapy [and where NGs are fitted] is on a different site to the surgical centres . . . where the new patient clinic is . . . It’s also where the gastrostomy tubes are put in.
[. . .]
So . . . the TUBE study kind of crosses [sites] really for us . . . half of the interventions happen in one site and half of the interventions happen in another . . . we didn’t predict this when we initially talked about the study.
A clinical pathway divided across two clinical sites was standard practice at three research sites. However, R&D issues concerning the allocation of funds for recruited patients had not been identified as barriers in previous deployments of the trial at these other sites. Lara explained why R&D permission had been delayed:
The problem is funding . . . for that patient recruited . . . So that would go to where the study is opened [the surgical centre] but then obviously if they’re kind of contracting out some of the work to the cancer centre . . . then the cancer centre tries to kind of bill them for that . . . It’s a kind of contract between R&D departments, and what they deem something that should be charged for or not charged for. . . . it’s a changing scene . . . It’s a little bit unpredictable. [Our R&D department] suddenly request funding for something . . . seem to appear out of nowhere, to be honest. That’s a block of many, many months in a pathway. You know I feel I should have unblocked it sooner, but really . . . I wasn’t . . . aware of what the problem was.
Lara (oncologist), Wellington
Lara outlined that her site would have only ‘a couple of months’ to actively approach patients before the recruitment window closed. Moreover, she explained that the flow of eligible patients into the clinic was ‘incredibly unpredictable’. Lara reported that she felt ’incredibly frustrated’ by her experience with the R&D department and doubted that her site would meet its recruitment target.
Changes to the cancer treatment pathways
Patients were eligible for the TUBE trial if they had stage III or IV HNSCC suitable for primary CRT with curative intent. Patients who had surgery before CRT were ineligible. Between study design and implementation, there was a shift towards greater use of surgery in this patient group, reflecting changes in the types of head and neck cancer presenting and changes to the head and neck cancer teams at some sites (with new consultants favouring surgical approaches). This meant that fewer patients were eligible for consideration for the TUBE trial. At Otago, Emma described a mismatch between the form of primary treatment typically recommended at the site and the TUBE criteria for chemoradiotherapy patients:
I think the other thing is there’s quite [a] limitation on . . . who [can be recruited] . . . they have to have primary chemoradiotherapy, and a lot of our patients don’t, we’re a very surgical unit, . . . so we’ve got a much smaller . . . cohort of people to use.
Emma (surgeon), Otago
When the TMG was made aware of the impact of these changes, a request was made to the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme to widen the inclusion criteria, with the support of the Trial Steering Committee. However, this was declined:
Many patients are treated with chemoradiation therapy following surgery for head and neck cancers. We have recognised recently that with the increase in Human Papilloma Virus related cancers, the presentation is often with small, sometimes clinically undetectable primary cancers with larger neck metastases. One management option for such tumours is to perform neck surgery followed by chemoradiation therapy to the neck and the primary site. Another scenario is where small oral cavity cancers may receive surgery followed by postoperative CRT owing to the presence of adverse histological features, where swallow function is unimpaired . . . in terms of the clinical decision making regarding gastrostomy vs NG tube, this post-surgical group is the same as the group currently included in the TUBE trial. The proportion of patients needing supplementary feeding in this group is the same as for the patients receiving primary CRT, and the previous surgery does not alter the decision making in favour of either gastrostomy or NG tube. Adding these patients to the trial cohort would therefore make the trial relevant to a wider group of NHS patients, and in the definitive trial should enable us to recruit more quickly, enabling us to more rapidly provide an answer to this important clinical question.
Letter to the NIHR HTA programme requesting permission to change the inclusion criteria
Operationalising swallowing eligibility criteria
During the initial phases of recruiting a new research site into the TUBE trial, the trial manager (Karl) and sometimes other members of the research team visited the site for a SIV. This meeting offered an opportunity for HCPs at the site to speak directly to the trial manager and raise any concerns that they might have about how the trial would fit into their existing clinical pathways. An issue raised at two observed SIVs was how the eligibility criteria for the TUBE trial might be applied to the patient population. Specifically, a few HCPs expressed concern that the criteria specifying the maximum grade of dysphagia for inclusion were too stringent. Field notes, written during the course of discussion in Wellington, present a record of opinion:
The Oncologist Lara asked her team to clarify how Grade 1 dysphagia was defined. Aadhira [SALT] said that she’d tried to look up the definition before the meeting and couldn’t find one. This prompted Karl to look up the definition provided in the protocol/supporting trial documentation. A discussion ensued around the definition of Grade 1 dysphagia. The consensus was that ‘starting to avoid some foods due to discomfort’ would be ‘beyond’ stage 1. Lara and Aadhira agreed that limiting recruitment to asymptomatic patients (or at least those without any discomfort) would probably limit recruitment to the study. Lara suggested that the protocol could/should be amended to include patients with Grade 2 dysphagia. There was a brief discussion about whether this would affect the trial outcomes, with no clear resolution.
Field notes, Wellington SIV, p. 3
During a subsequent interview, Lara explained that her only concern with the study protocol was the eligibility criteria, which might limit the number of patients she would expect to recruit:
I don’t think I have any concerns with [the design of the TUBE trial] . . . There are some small things about the inclusion criteria which may limit, or which may exclude some patients, but you know . . . in general terms I don’t have a problem with the study, no.
Lara (oncologist), Wellington
The definition of grade 1 dysphagia was also discussed in the Otago SIV:
Mary [SALT] then asked about inclusion of patients with grade 2 dysphagia. Karl explained the rationale for including people at grade 1 (or below) only . . . This led to a general discussion around the definition of grade 1 dysphagia and the concept of ‘normal eating’. Whilst Karl took the position that swallowing without pain should be classed as ‘grade 1’ or below, Simon’s view [Oncologist] was that a patient who could swallow but with pain, should be classed as Grade 1 (able to eat) . . . Simon’s argument stemmed from his concern that few patients presented who did not have some discomfort/pain and that therefore the trial would struggle to recruit . . . At this point Mary joined in the conversation and Karl agreed with her point that if patients had difficulty eating then this would be classed as an ‘altered’ eating pattern and therefore ‘grade 2’ dysphagia. Simon’s response was that the dysphagia grading system was based on a general toxicity grading, not a specialist system based on symptoms of dysphagia per se, and that therefore the various categories were open to interpretation. He concluded that eating with discomfort should be considered ‘symptomatic’ grade 1, whilst not being able to eat solids would be grade 2 . . . The discussion continued with Simon emphasising the potential lack of participants due to the dysphagia grading criteria.
Field notes, Otago SIV
Karl discussed this feedback with the chief investigator and TMG. Steps were taken to clarify the swallow exclusion criteria with all sites, including via a section in the third TUBE newsletter. The extent to which patients may have been inappropriately excluded from participation in the TUBE trial because of confusion about swallow criteria is unknown, but swallowing function remained one of a variety of reasons that patients were being screened as ineligible for the TUBE trial:
[Team member] updated the team with Otago screening issues – Otago have had a number of laser patients, palliative patients and one patient that did not have good enough swallowing capabilities therefore making them ineligible for the trial.
TMG minutes, 22 April 2015
Integrating research
Health-care professionals described the TUBE trial as addressing an important research question, having a good design and being no more difficult to integrate into routine work than other trials. When some HCPs raised concerns raised about design features, these related to patient eligibility criteria, which were deemed to be overly stringent and, therefore, potentially limiting to recruitment. Research sites encountered both idiosyncratic and shared challenges – as well as successes – in integrating the TUBE trial into routine clinical activities.
In this section, we show how sites worked to implement and embed the TUBE trial, to integrate the research pathway into the existing local clinical pathway. To successfully embed the trial, the teams at each site had to enrol a range of people, and be willing to adapt their practices over time. Teams worked to allay any patient concerns that getting onto the trial pathway would disrupt the clinical pathway. They also had to manage their own concerns about overburdening patients with trial information at a time of distress, uncertainty and vulnerability. We also show how the issue of preference at the site, professional and individual level was managed. Some teams worked collectively to present equipoise at all contacts with patients in which feeding options were discussed. Some teams reported that different elements of the team expressed preferences for specific feeding options at different points across the clinical and research pathway. Finally, we show how, in recruitment consultations, HCPs work to effectively convey equipoise, the advantages and disadvantages of the two feeding tubes and the implications of trial participation, and to enable patients to explore areas of concern.
Integrating research and clinical work
At some sites, the clinical and research pathway spanned several hospitals (Figure 5). This created a challenge for research nurses at Otago and Queenstown, as they required assistance from colleagues in primary treatment sites to keep track of patients and provide trial-specific information:
. . . this study is probably causing me a lot of headaches because the patients don’t have their treatment here . . . they go to [other site]. I have access to the [other site’s] database but not all aspects of it, so I don’t see the dietitian’s annotations because I haven’t got access to that. I’m trying to get access but it takes a long time.
Kate (research nurse), Queenstown
FIGURE 5.
Clinical and research pathway. (a) Hamilton; (b) Otago; (c) Queenstown; (d) Wellington; and (e) Woodville. AHC, allied health care; AHP, allied health-care practitioner; chemo/rads, chemoradiation therapy; ENT, ear, nose and throat; G, gastronomy; GFR, glomerular filtration rate; MD, multidisciplinary; NGT, nasogastric tube; PEG, percutaneous endoscopic gastronomy; PIS, patient information sheet; RN, research nurse.
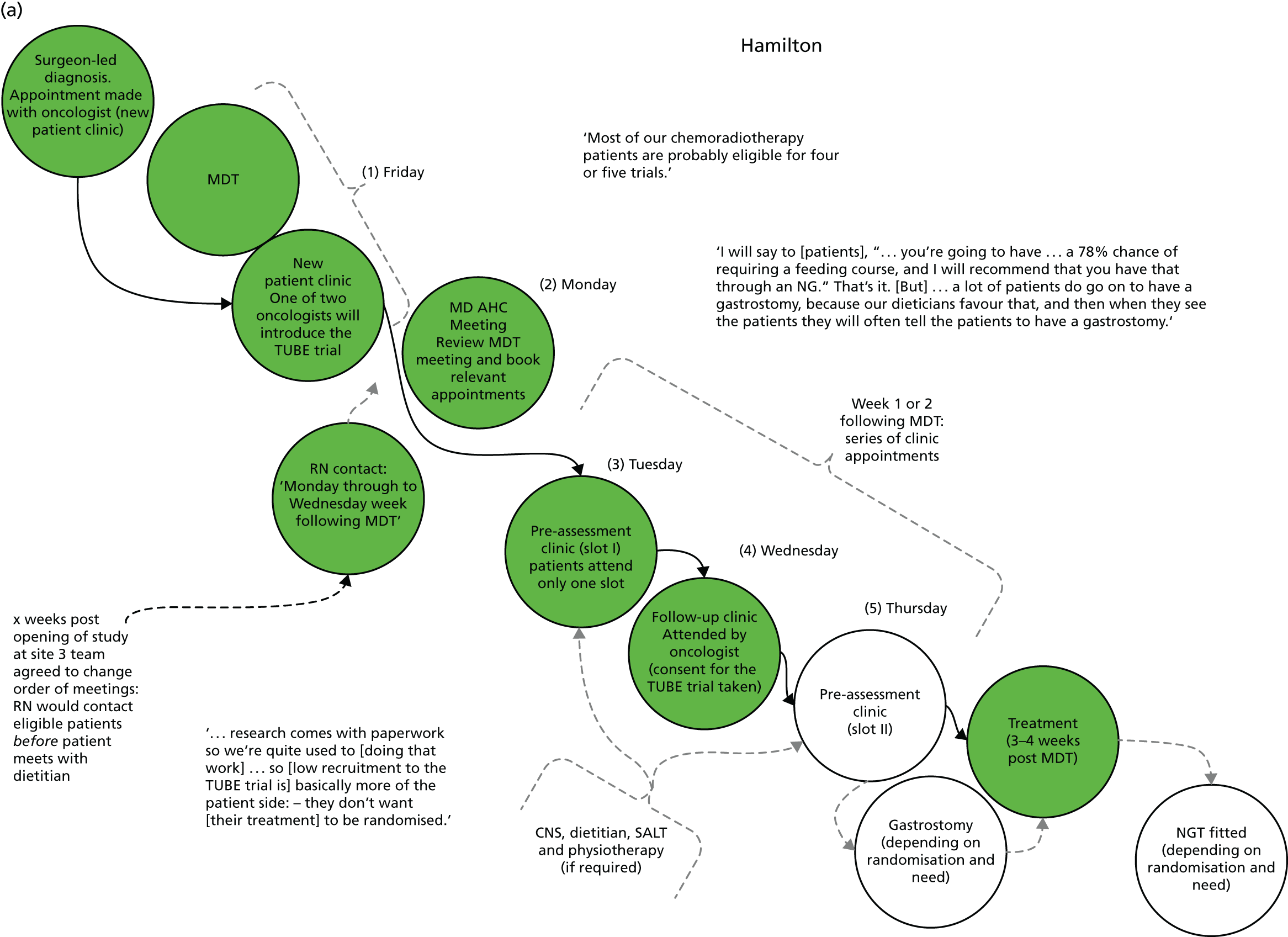
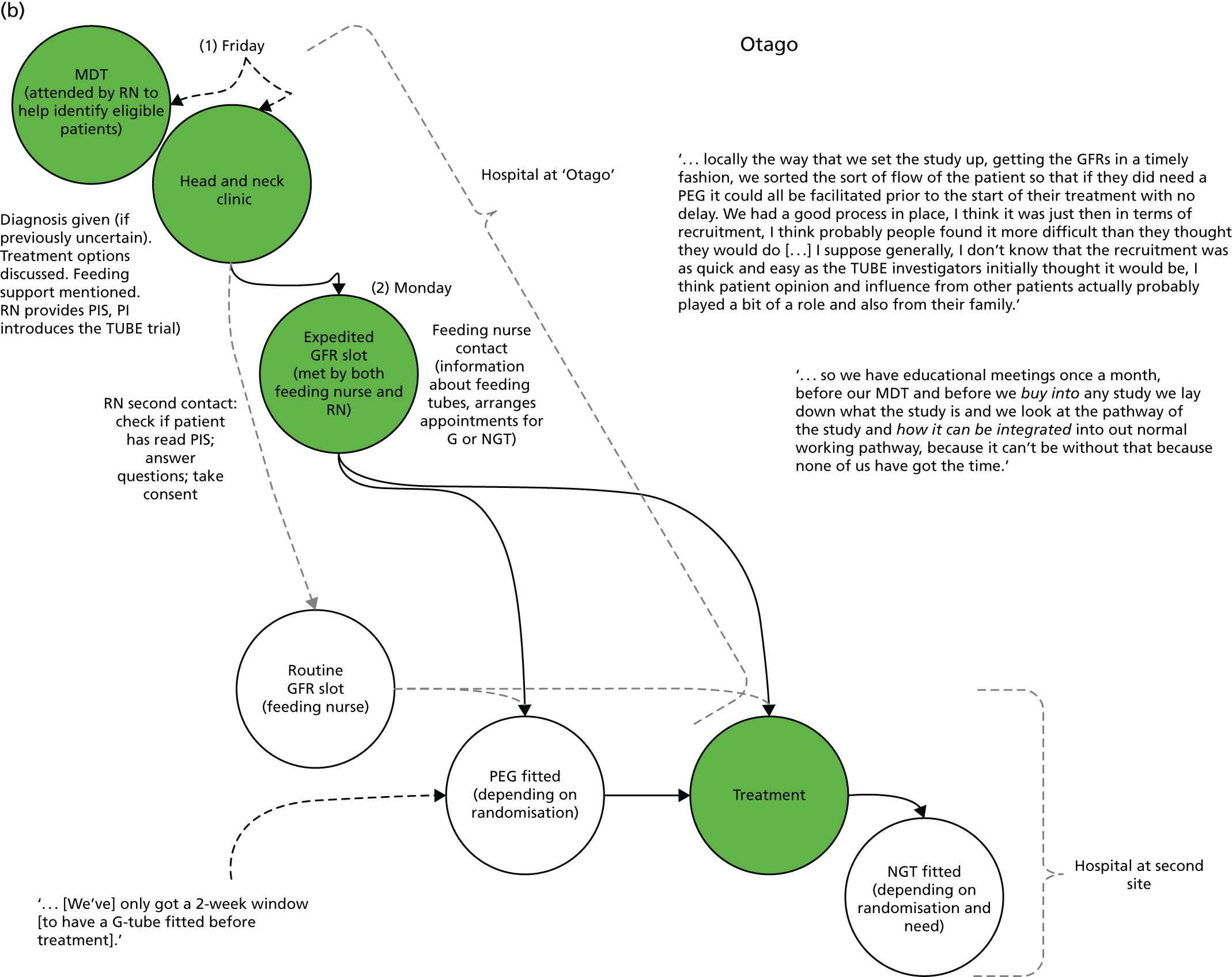



Site initiation visits organised between PIs at each site and the TUBE trial manager were designed to address practical issues of implementation. While these were observed to work well in ‘priming’ clinical teams for the research, and generating and answering initial queries, the finer, but no less important, issues of implementation were left to clinical teams to organise for themselves. Emma, a surgeon at Otago, explained how HCPs prepared to implement the TUBE trial at their site:
. . . so we have educational meetings once a month, before our MDT and before we buy into any study we lay down what the study is and we look at the pathway of the study and how it can be integrated into our normal working pathway, because [we can’t agree to do research] without that because none of us have got the time. So looking at [another study running on-site] for example, which we’re really keen to recruit to, we’ve got issues because of some of the data that needs to be collected in Woodville during the treatment. Now it’s not the data collection that’s the issue, it’s if something goes wrong and they’ve got a SAE or something like that, then who’s going to collect that data? Yeah, and that’s got to be someone with research interest. So it’s hanging on trying to get the research team in [centre] to support our patients and that is always an issue.
Emma (surgeon), Otago
Emma’s account provides a good description of the organisation of key contributors to assess if they can collectively contribute to the work involved in implementing a new set of practices. Emma identified two streams of work: the ‘pathway of the study’ and the ‘normal working pathway’. A central concern for staff in Otago was whether or not the two pathways could be combined in a way that required minimal time resources to organise. This criterion was key to whether or not they ‘buy into’ the study. Towards the end of the quotation, Emma identified that an important component of collaboration across sites was networking with colleagues who had a ‘research interest’.
Across all the sites, we observed that the success or failure of the TUBE trial hinged on whether or not potential contributors and collaborators could ‘buy in’ to the study. Notably, HCPs reported that efforts to integrate the trial with routine work were no more challenging than those required for other trials. A challenge for all sites was arranging gastrostomies. The need for a gastrostomy appointment could not be ascertained before consent and randomisation. This foreshortened the ‘window’ for gastrostomy, which, in a ‘normal working pathway’, spanned from diagnosis to just before the onset of treatment. Therefore, this issue applied regardless of whether or not sites routinely offered gastrostomy.
Health-care professionals at Otago identified a critical ‘moment’ in the clinical pathway that may have prevented them from implementing the TUBE trial. The glomerular filtration rate (GFR) assessment identified if patients were suitable for chemotherapy. If patients were found not to be suitable, then they were also ineligible for the TUBE trial. However, the appointment ‘slots’ were not at a specific time and were dictated by the medical physics department. For the TUBE trial to work at Otago, it was necessary to arrange ‘expedited’ GFR slots:
. . . so in that first MDT where we decide about the treatment options . . . the patient information sheet, because that’s the point at which we discuss feeding with them anyway. Then after that they would be contacted by the feeding nurse and also by the research nurse and if they agree to participate, then they’d have an expedited GFR slot [. . .] Glomerular filtration rate slot, so if you’re having chemo[therapy] or radiotherapy you have to have a GFR before it to see if your kidneys are good enough for your chemo[therapy], yeah, so that has to be incorporated into the pathway, and that’s done at Otago Hospital. So they come back to have that done and at that point the research nurse would meet with them and the feeding nurse would meet with them . . . at the GFR visit, and then they would discuss the options and enrol them into the trial at that point. Then when they were enrolled . . . if they were then in the [gastrostomy] arm, then the feeding nurse could facilitate getting the [gastrostomy] pre-treatment . . . cos [sic] you’ve really only got a 2-week window with everything else that’s going on . . . so they’ve got to slot it in . . . to be honest this pathway just had to work properly, so we got the pathway to work properly.
Emma (surgeon), Otago
The expedited GFR slot was crucial for the implementation of the TUBE trial at Otago. Alexandra, the research nurse at the site, explained how this was arranged:
We spoke with medical physics to see if we could get a definite time and date to get a GFR done so that . . . we could bring [patients] back [to Otago] and actually approach them in person rather than just telephoning to see if they were interested. That way, if they say, ‘Yes’, we can get consent there and then and then we can get them randomised quite quickly so we know which arm of the study they’re going into.
Alexandra (research nurse), Otago
Identifying to which arm patients had been randomised was important to HCPs at Otago, because patients receiving gastrostomy would have their tube fitted on site, whereas patients receiving a nasogastric tube could have their tube fitted at a later date at a different site. As explained above, patients receiving gastrostomy needed to have the tube fitted before treatment. Therefore, the expedited GFR was a prerequisite for the successful integration of clinical and research pathways at Otago. Centrally, integrating the trial pathway into the clinical pathway meant enrolling a large number of people and activities within and across departments and, in some cases, across organisations.
At Woodville, HCPs reported a relatively smooth integration of the TUBE trial with routine clinical practices. However, Jackie, a research nurse, identified a potential issue in the early phases of recruitment. Specifically, it was unclear if patients who decided not to take part in the TUBE trial would have the option to choose between the forms of feeding assistance. If not, patients who preferred gastrostomy might have perceived the trial as their only means of accessing that treatment:
So . . . what was the initial look of [the TUBE trial] that made you think, ‘Oh, this’ll work quite well’?
. . . the actual treatment plan wasn’t being altered it was . . . almost, something peripheral to the main treatment. But, I think the question that was in my mind, . . . – and it’s really one of the questions that you’re very likely to be asked by patients – . . . was what would happen if people didn’t go on the study. . . . and the answers to that were very vague [laughter] . . . patients weigh up how much of . . . a difference taking part in the study is compared to not taking part in the study. And . . . at this site, people would almost certainly get an NG tube. And possibly a gastrostomy but only if there was a clinical indication And . . . I think up until yesterday, that’s not exactly what I was telling the patients . . . based on the information I’d had, kind of indicating that they had a choice between a gastrostomy or an NG off the study. Which, in theory, sort of but in practice they don’t. And that’s only came to light because a patient . . . said they didn’t want to do the study . . . because they wanted a gastrostomy, and not knowing what they’d been . . . told in the first place, I left it at that. But, since then . . . they’re not going to get a gastrostomy so . . . I’ll phone that patient back.
A potential strength of the TUBE trial was the ‘low impact’ of the research design: as Jackie explained, its impact on the treatment plan was non-existent for patients randomised to receive the ‘standard’ treatment at the site (nasogastric tube) or minimal if patients received gastrostomy. However, a trial design peripheral to the standard clinical pathway also contributed to the confusion for some patients, who perceived participation as curtailing their freedom to choose between the treatment options. Later in the interview, Jackie explained how she had adapted her recruitment approach:
What I say to patients is still, kind of, evolving . . . as of today, I’ll say to patients that off the study they’ll almost certainly get a NG tube but there is a possibility – not completely rule out the gastrostomy – but make it clear that there is, there would need to be a clinical indication for that.
Jackie (research nurse), Woodville
Jackie’s account is important because it demonstrates how HCPs adapted their practice over time as they formed an understanding of the way in which trial protocols could fit within existing patterns of work.
At the start of the trial, Jackie met with Jenny, a SALT. Between them they worked out a way of integrating research information with the standard guidance that SALTs and dietitians provided to patients in routine practice. Jackie explained:
The SALT and I met and decided that two lots of information that had to be [given]. And it wasn’t going to compromise the trial information if it was given at the same time, . . . [as] the standard information people would get anyway. . . . and also, from a trial point of view, it showed the trial was not peripheral to everything, that it was very much a part of what was going on. And that other [professionals] were on board as well . . . we ran that past the dieticians, they were perfectly in agreement as well.
Jackie (research nurse), Woodville
Earlier in the interview, Jackie had used the term ‘peripheral’ to describe a positive attribute: the trial would have little impact on the clinical pathway and would, therefore, not interrupt care. However, in the excerpt above, Jackie emphasised that the trial was ‘not’ peripheral in the sense of being removed from the core business of cancer care. Jackie worked to promote confidence in the trial among both colleagues and eligible patients by emphasising that the trial was integrated into core activities and priorities: ‘a part of what was going on’. It was important that Jackie demonstrated to eligible patients that the research was regarded with esteem by the HCPs central to their care. Integrating research information with key stages in the clinical pathway facilitated this aim.
The enrolment of other HCPs was also a key task of research nurses at each research site. Jackie explained that an important step in this process was the sharing of plans and ideas concerning integration of information with the professionals key to each step in the existing clinical pathway:
. . . so [a patient] would be identified at the MDT as having the right stage of disease and . . . going for radiotherapy. Then, I would check with the SALTs or the dietitians how that person’s swallowing was, because in the MDT, they will have done a swallowing assessment and that might exclude people [. . . if] their swallowing is really compromised . . . after the patient had the treatment plan explained to them, I would join . . . the dietitian and . . . explain the trial to the patient but do that alongside . . . the dietitian explaining about the different tubes. . . . we’ve combined what I say with what the dieticians say. And that makes sense because the information is, sort of, interchangeable. It makes more sense than, for example, the dieticians going in and given a whole lot of information about the different kinds of tubes . . . and then saying, ‘Oh, but, there’s also this trial.’ . . . So they combine well and it’s much, much more fluid way of explaining the trial and the tubes at the same time to the patients.
Jackie (research nurse), Woodville
Through a series of preparatory meetings, Jackie was initially able to fit research work neatly within appointments that would typically be run by SALTs or dieticians. Centrally, when they worked as a team to introduce the trial in the least disruptive way, the recruitment process was effective:
We had, between me and then the dieticians and the speech language therapists and the surgeons and the oncologists, we had kind of formulated a plan of how patients would be approached in that they would see the surgeon first who would give them a diagnosis and decide the treatment option and if they were going for radiotherapy, plant a little seed about the study, like ‘you’ll go and see the oncologist next, they’ll mention a study by the way’, and em . . . no more than that, just planting the seed. The oncologist would do the same, but maybe in a bit more detail, once they’d described the potential side effects of the radiotherapy they would introduce the study and say ‘Well, our research nurse and our dietitians will explain that further’. So it was like a kind of seed being placed, being planted, reinforced, but with this basic minimal information, and then when the patient saw the dietitian – it was either the dietitian or the speech and language therapist, usually it would have been the dietitian – I would go in with the dietitian, we’d see the patient together so that combined getting basic nutritional support information with the study information cos it was about the same thing, and it also made the research seem like part of the whole bigger treatment plan picture.
Jackie (research nurse), Woodville
Priming patients – ‘planting the seed’ – at each stage of the clinical pathway was an effective way to introduce the trial in the least disruptive way. Providing the right amount of information at each stage slowly built an idea about the trial in patients’ minds. As Jackie notes, it enabled the trial to ‘seem like part of the whole bigger treatment plan’. However, this priming work seemed to fail in the later stages of the trial, as some HCPs earlier in the clinical pathway worked harder to actively promote the trial:
The surgeons and the oncologists would, rather than plant a seed, would go into more detail about the study, which didn’t seem like the optimal time for the patients to take that information in and consider it. . . . If surgeons mentioned too much about the study before the oncologists, before the patient could see the oncologist, patients were being asked about the study, to consider a study about something they hadn’t had the information for yet, so that was all just very kind of confusing.
Jackie (research nurse), Woodville
Rather than having a positive effect on recruitment, recruitment was reduced. Priming made patients less receptive. Some had formed an initial opinion, some were confused and some were less willing to engage in yet another discussion about the trial. The reason for this increase in information-giving at all points across the care pathway was twofold: clinician enthusiasm for the study as well as, in much later stages, attempts to manage a shortfall in recruitment at this site.
Research nurses at two research sites described how they engaged in informal, interpersonal work to smooth the path of the trial at their site. A barrier to implementing the trial in Woodville was the possibility of increased demand for gastrostomies when the site usually provided nasogastric tubes to the majority of head and neck cancer patients. The successful integration of research work within local cultures of practice is key to the successful implementation of clinical trials. ‘Global’ issues that affect all research sites within a trial can be perceived by research teams as major barriers to research. In comparison, ‘local things’ within a research site can be construed as minor barriers. However, the informal, hidden work undertaken by RCT contributors in the implementation of trial protocols 57,58 is essential. Jackie explained how the issue of increased gastrostomies at Woodville was addressed:
. . . you know with the research . . . essentially, you’re asking people to do, you’re being a pest and you’re asking people to . . . do extra things or do things differently for no real benefit to them . . . [so] the job and the role is very much building up relationships with your colleagues that you work with.
So you’re being a pest to your colleagues then?
Yes. But it’s how you go about it because you could be demanding this, demanding that or you could, . . . do it in a different way where you’re, . . . collaborating and building up a relationship with different people you work with. Which, ultimately, from a . . . very practical point of view, gets the results that you want.
For example, on TUBE, the people . . . who are randomised to gastrostomy, I know who the secretary who’ll make that appointment . . . So I take the . . . request along in person . . . Because sometimes it might be tight [for gastrostomy] but it’s more likely that they’ll accommodate the schedule if that relationship is there.
Jackie (research nurse), Woodville
To successfully implement and embed the TUBE trial, teams had to work hard to integrate the trial pathway into the existing local organisational ‘normal working pathway’. They had to be willing to enrol a range of people and activities within and across departments and organisations. They also had to be willing to adapt their practices over time as they formed an understanding of how trial protocols could fit within existing patterns of work. New practices, such as joint consultations with the research nurse and dietitian, were introduced to offer a consistent, timely and balanced account of feeding options to patients. However, too much enthusiasm across the team could also cause problems. They had to build rapport with colleagues whose work underpinned key phases in the research pathway. In this way, they proactively sought to allay any patient concerns that getting onto the trial pathway would disrupt the clinical pathway. In Accepting randomisation to the TUBE trial, we report the example of Barry in Queenstown, where, despite considerable efforts to synchronise the research and clinical pathway, mismatches did occur, with detrimental effects on patient recruitment.
Managing patients’ burden
At the time of their diagnosis, patients were given a large volume of information. This concerned their diagnosis; the details of primary treatment and likely side effects; the clinical pathway, including appointments with a range of HCPs; the possibility of feeding assistance; the known risks and side effects of feeding methods; and any research studies (including the TUBE trial) for which the patient was eligible. HCPs were aware that patients were challenged by the health and practical implications of their new situation, the volume of information that they were asked to consider and the need to make decisions during this time. Sally, an oncologist, described the content of a typical ‘new patient’ consultation:
The problem . . . is that frequently the patient has only, just that week or even that morning, been informed that they have cancer, . . . it’s already a very extended consultation. It often takes up to an hour. It’s difficult for a patient, having just had that kind of news, to really focus for a whole hour. [As the] clinician, you have to . . . explain about their cancer, explain about what sort of treatments we can offer, and then the process of chemoradiation is rather involved because often it involves induction chemotherapy by itself first . . . Then . . . chemotherapy with radiotherapy, . . . the actual procedures involved within all that and all the professionals involved in preparing them for treatment. Then when you go onto the side effects, of course they’re starting to get quite overwhelmed. At this point you’ve probably already been speaking non-stop for about 30 or 40 min[utes] to get them to this point. Unfortunately, again, there’s probably constant interruptions, people coming into the room . . . maybe even coming to see the patient as well . . . so then you talk to them about the tube. . . . Lots of patients, you do have the concern that they’re just shutting down during the consultation and they’re not really listening. As I said, to have anyone talking at you for 45 min[utes] is very difficult, and when you’ve just been told you have cancer it’s very difficult to focus.
Sally (oncologist), Hamilton
Health-care professionals were mindful of the emotional impact of diagnosis, ‘. . . that kind of news’, and the practical and decision-making work they required of patients. In addition to being asked to consider ‘factual’ information about cancer and methods of treatment, patients were required to help organise their treatment pathway. This required the patient to take on some responsibility at a time when they might have wanted to devolve control to others. Patients reported co-ordinating appointments, travelling under time constraints to unfamiliar locations (sometimes across several hospital sites) and keeping specialist HCPs abreast of delays in other parts of the clinical pathway. Sally acknowledged that there was preparatory work to orientate patients to this new role, describing ‘. . . the actual procedures involved . . . and all the professionals involved in preparing them’. Throughout interviews with both HCPs and patients, the concept that patients were often ‘overwhelmed’ during this phase of their diagnosis and treatment was common. Discussion of feeding tubes and eligibility for research studies usually occurred towards the end of the new patient consultation, at a time when some patients were struggling to absorb any more information (‘shutting down’).
As noted above, the TUBE trial research activities were designed to fit into pre-existing clinical pathways to avoid patient burden. However, at some sites, routine care required patients to attend one site for diagnosis and pre-treatment clinics and another site for their actual treatment. These pathways were complicated when patients were randomised to a treatment that was not a standard recommendation at the site. Table 19 summarises standard recommendations outside the TUBE trial by site. Figure 5 includes the clinical pathway figures for each site. A few of the HCPs using terms indicating discomfort to describe approaching patients to participate in the TUBE trial soon after diagnosis. Charlotte, a dietitian at Woodville, explained that she had not previously recruited to a trial. She reported that transitioning from the role of a therapeutic clinician to a ‘researcher-recruiter’ was ‘difficult’:
Sometimes found it a bit difficult, you know when you do feel that you’re putting upon a patient . . . cos you do have to bother them with TUBE I suppose, and it’s an extra bit of information, . . . they’ve had enough and you know that you have to kind of put it to them, I find that a bit difficult. [. . .] Some patients . . . were getting diagnosis . . . treatment plan, . . . everything, it was way too much and that was when it became, you felt a bit embarrassed and you felt, you know, that it’s not the right time.
Charlotte (dietitian), Woodville
The language used by Charlotte conveyed her sense of self-consciousness in approaching patients and asking something from them, rather than delivering a service. 59 Those HCPs who discussed discomfort in approaching patients for research often commented that the trial involved asking patients to process ‘an extra bit of information’ at a time when they were both vulnerable and in receipt of a large volume of material concerning their clinical pathway.
Not all HCPs were concerned about approaching patients about research soon after diagnosis. Jenny, a SALT, explained her perspective on recruitment:
How do you feel about that day where they’re getting . . . a lot of really unpleasant stuff that they’re hearing. How do you feel about them being offered the research at that point?
I don’t really feel strongly that it shouldn’t be done at that point because, partly because I don’t know how else it would be done, but also at that point we only said; ‘we’re doing a research study’ and they’re given information that we would be giving them anyway and then they’re given the information sheets to take away . . . and then it’s followed up so I don’t think that’s really particularly adding a lot on top of what they’re already [getting]. . . . going to be very variable because you’ll have people who . . . they’ve got all their questions. They want to know as much as they can at that point and then you’ve got people that just can’t wait to get out of the doctor’s room and they don’t want to hear anything else . . . So . . . you’re never going to get it right for everybody I don’t think. . . . I think . . . it’s important . . . to how we practice and how we treat people and that outweighs for me that . . . extra overload.
Jenny identified that, for the TUBE trial to function, it was necessary to approach patients soon after diagnosis (so that there was enough time to organise a gastrostomy if required). At the end of the excerpt she explained that the evidence produced from research ‘outweighs’ the ‘extra overload’ of the provision of research-specific information alongside clinical material. Jenny also stressed that the TUBE trial information was very similar to that which would have been provided to patients ‘anyway’ in routine clinical work. She also identified the varying clinical needs and receptiveness of patients to research.
Health-care professionals recognised the difficulty of the role of recruiters in settings such as cancer care. One HCP, Sian, identified that recruiting patients to clinical trials required a shift in the patient–clinician relationship. This switching of roles required a new set of skills and, importantly, placed HCPs in a social dynamic that was unfamiliar to them: a position whereby the ‘power’ of the interaction lay with the patient rather than the professional. 60 In the early stages of the trial, we became aware of some HCPs’ fears and concerns about overburdening patients with trial information at a time of distress, uncertainty and vulnerability. However, following qualitative work with patients, we formally reported back to them that patients felt that the timing of the approach was appropriate, given the sensitive way in which they felt the teams handled the discussions and valued the opportunity to discuss research. All except two patients either welcomed an introduction to the TUBE trial at the start of their clinical pathway or, in some cases, felt that it was relatively inconsequential or ‘no bother’ in the grander scheme of their diagnosis. The two who felt that it was an unwelcome interruption at a sensitive time nevertheless understood the rationale and the potential value of the research. Patients who accepted and declined randomisation reported positive responses to the timing of the TUBE recruitment discussion and content of the PIS:
One of the things that certainly the speech therapists, dietitians, everybody worries about is that you are overloaded with information.
Yeah, no, I didn’t feel that . . . I’d say I’d had time to process . . . and I think actually it’s, you know, it’s an appropriate time to mention it at that stage, ‘cos it does seem sensible to raise it at the earliest time really, to me anyway [laughter].
[. . .]
I mean for example I’m one of those people that always wants to know as much as possible [laughter], yeah, and that, you know, keeps me, that, that makes me feel more empowered, the more information that I’ve got, but I also recognise many people aren’t.
Patients also indicated that the information about the TUBE trial was presented and discussed in a way that avoided any pressure on them to participate, and were positive about their interactions with the research nurses:
There wasn’t any, there was definitely not any pressure. And even when you come back [research nurse] was very good, how would you put it, to step back and let you decide you don’t feel that they’re pushing you, they’re sort of letting you at your pace.
Paul, Woodville (declined randomisation)
They also reported that the presence of the study ensured that they made the right decision and so avoided decisional regret. In this way, the formal feed-forward work aimed to ameliorate the fears of HCPs who were initially concerned about the potential of overburdening patients.
Distributed equipoise
There were preferences for specific feeding options both across and within sites. However, for the TUBE trial, the key elements were whether or not and how these preferences were communicated to patients. At three of the research sites, HCPs identified a lack of individual clinical and professional equipoise as a potential barrier to recruiting patients to the TUBE trial. At Otago, HCPs described a concerted team effort to present equipoise to eligible patients in support of the study. This extra attention to equipoise was underpinned, in part, by historical practices at the site that had favoured gastrostomy:
. . . the way that we tended to manage some of our patients historically, there had to be a sort of, we had to present the evidence, i.e. that there wasn’t any, and try and make everybody within the team approach the study with equipoise, and I think that was, for us, one of the factors that we really wanted to make sure that we got right. And then from there actually, in terms of recruitment and everything I think it went reasonably well.
Emma (surgeon), Otago
To make sure that equipoise was distributed across all members of the team, this site undertook a series of ‘role play’ and practice sessions explaining the different feeding approaches. Such proactive and reflexive approaches were reported as having an impact and were reflected in the recruitment rate at Otago, which was one of the highest in the study:
I don’t really have any views with regard to the treatment arms for the outcomes for the study. And I have to admit everyone’s being pretty good who’s involved in the study, no one’s giving any opinions which has been quite interesting [. . .] they’re all, they’re very aware that they’ve got to maintain equipoise and they’re just, they are trying to do that.
Alexandra (research nurse), Otago
In this way, some sites explicitly worked to address the historical ‘preference’ of the site.
Health-care professionals at Woodville and Otago described informal and collaborative processes of evaluation regarding their activities within the TUBE RCT. Jackie, the research nurse at Woodville, reported how recruitment practices at the site had ‘evolved’ over the lifespan of the trial. Jackie described how the recruitment techniques had been adapted through communal appraisal with dietitians at the site:
Compared to when [the trial] started to now, [the recruitment approach has] kind of, evolved. So I think it will just keep evolving like that. What, certainly, has changed is the first couple of patients who said ‘no’, said no because they didn’t want a gastrostomy and the dietitians and I had a bit of a debrief about the information that we were giving. And we, kind of, concluded that what we were probably not doing was promoting gastrostomy. We were being equal but we were being so equal that it meant almost, meant that the patients would say, ‘Well, there’s no advantages, but there’s a lot of inconvenience with the gastrostomy,’ compared to the NG tube. So we did, kind of, alter and, so now explain, quite clearly, in a quite structured way, ‘This is the advantage, that’s the disadvantage . . . and this is the advantage of that, that’s the disadvantage.’
For example, I . . . think we were mentioning the gastrostomy, ‘You would be in hospital for 3 days’ and not mentioning that with the NG tube that you’d be in hospital for 3 days. So, we’ve, kind of, fine-tuned that to say that, ‘Well, you’ll be in hospital for 3 days at some point . . . it might be at the start, it might be half-way through’.
Jackie (research nurse), Woodville
Through discussion and peer feedback, HCPs at these sites actively sought to both evaluate and then change their practice in order to demonstrate a balanced and information-rich account of each feeding option. Avani, a dietitian, describes the aspects of the process she went through:
So you feel that in thinking about the experience of the recruitment, you feel that recruitment is a bit more hopeful than you first thought?
Yeah . . . we’ve had some discussions, ‘cos I’ve done two and Charlotte [another dietitian]’s done two. We’ve discussed with Jackie [the research nurse] as well how we’re discussing things and I ask Charlotte to listen to what I’d said, I haven’t listened to what she, but she’s told me what she’s said and so we’re going to try and do it so it’s exactly the same for everybody. So for example, I’d said: ‘and for the nasogastric tube you’d be hooked up to the feeding pump for a long, long time’ and she said that wasn’t quite right [laughter]! . . . So now I’ve reflected on that I’ve thought ‘right’ . . . So we’re learning from it.
Some teams worked collectively to present equipoise at all contacts with patients at which feeding options were discussed – even when, in private, they may retain a preference. They worked hard to demonstrate a consistent approach to the presentation of options.
However, some teams reported that different members of the team expressed preferences for specific feeding options at different points across the clinical pathway.
The historical preference of sites was an issue identified by HCPs when discussing how the TUBE trial was received in the workplace. This was most pronounced at Hamilton, where preparation for and care of gastrostomy patients had been overseen by one professional group. A raft of factors contributed to a long-standing difference of opinion between senior medics and allied health professionals at the site regarding the ‘optimum’ approach to tube feeding for the majority of patients receiving chemoradiotherapy for head and neck cancer. A professional whose department had built expertise in gastrostomy (and who later developed a supportive infrastructure for nasogastric tubes) reported that the expertise of her department had been overlooked by the consultant oncologists responsible for recruitment. The TUBE trial, therefore, became a focus for pre-existing debates and workplace politics. There was evidence that eligible patients were given a direct ‘recommendation’ for a nasogastric tube by their consultant:
I will say to patients, ‘. . . you’re going to have . . . a 78% chance of requiring a feeding course, and I will recommend that you have that through an NG.’ That’s it. . . . a lot of patients do go on to have [gastrostomy], because our dietitians favour that, and then when they see the patients they will often tell the patients to have a [gastrostomy].
Janet (oncologist), Hamilton
Janet reported routinely advising patients to have a nasogastric tube, but indicated that dietitians at the site advocated gastrostomy. Janet downplayed the influence of her direct recommendation to the patient on recruitment; however, it is notable that recruitment to the trial was poor at this site. Janet suggested a strong bias by the dietitians and indicated that it was the dietitian’s recommendation, rather than hers, that influenced patients’ decision-making. Another dietitian at Hamilton offered a similar narrative regarding the influence of consultant oncologists:
Lots of the patients came to us and said that they had been told their swallow would be better if they had a nasal gastric tube, even though there is no evidence to support that, so then they wouldn’t go into the trial [. . .] Some patients unfortunately have picked up on the fact that there is a bit of a difference of opinion between the dietetic team, the speech therapy team and the medical team, but they will always go with what their consultant [oncologist] says for the vast majority.
Rebecca (dietitian), Hamilton
In this way, patients at this site are primed about which option may be appropriate for them before any detailed recruitment discussion. Some patients are caught in a dilemma. In consultations with different members of the team, they are offered different opinions. Then, in the context of a recruitment consultation, clinical uncertainty is outlined as they key issue that is driving the trial. As Rebecca highlights, their consultant’s preference strongly influences a patient’s treatment decision.
A second oncologist at Hamilton explained why, in her view, a direct recommendation to the patient was appropriate:
Since we’ve been offering them the TUBE study, what I’ve been saying to the patient is that there is a study that’s trying to evaluate which tube may have more benefit over the other and that we don’t really know the answer to the question as to which tube is the best tube. I then go on to explain to them what I recommend for them [. . .]. Ultimately, we will make clear what our recommendation is because they need guidance. Patients do not necessarily, you know, they are not clinicians. They don’t have the full knowledge as to all the different potential benefits and disadvantages.
Sally (oncologist), Hamilton
Sally is very clear that, even in the context of speaking to a potential recruit to the TUBE trial, she feels duty-bound to offer patients her recommendation. For her, patients do not have access to ‘full knowledge’ and so they need her ‘guidance’. One of her patients, James, described how he felt that his consultant, Sally, held a strong preference for nasogastric tubes:
Dr [Sally] was saying about the throat muscles, in quite simple terms, ‘use it or lose it’. That’s, I think, what she was trying to say. I think she’s an advocate of . . . ‘you’ve got to keep swallowing as long as you can’ . . . and this nose one seems to be showing that, whereas the stomach one, people give up a lot quicker. Because of the pain [they tend to use the gastrostomy tube] . . . so therefore, they’re losing the use of the muscles, so therefore, the recovery time is taking a lot longer.
James, Hamilton (randomised to receive a nasogastric tube)
James recalled an explanation given by Sally for the superiority of a nasogastric tube over gastrostomy in relation to long-term swallow outcomes. Throughout his interview, James highlighted how his consultant’s guidance was a priority for him. However, it is notable that James did decide to be randomised. In his account, he understood that the reason for the trial, in part, was to add evidence to Sally’s judgement that nasogastric tubes were superior. Participation was, therefore, a logical step for James in his support for Sally.
Enacting equipoise
Four of the sites have specific local organisational preferences for either nasogastric tubes or gastrostomy. However, in the context of the TUBE trial, HCPs needed to mark that these local preferences were, in part, uncertain, open to change and potentially not inclined towards the best option, hence the reason for asking people to think about taking part in the trial. They needed to create and sustain a position of organisational equipoise without undermining or overly selling their current practice, so as to enable a patient to have the option of being in individual equipoise themselves.
In the audio-recorded consultations, we can see how HCPs worked hard to explain two key points to patients. The first was that both types of tube were regarded as effective treatments, and were in use at different sites. However, one type of tube had been chosen over its alternative at the site the patient attended. The reasons underpinning this choice by the site were not made explicit. The second point explained to patients was that, despite the site having ‘chosen’ a standard treatment, a trial to assess which tube was ‘best’ was required. The following excerpt is taken from a follow-up recruitment consultation. This patient, Lesley, and his partner, Laura, had already taken part in an initial recruitment consultation with the research nurse. They were visiting the consultant to further discuss the options and make a final decision:
And we said, ‘Well we’d prefer to have it in the stomach because he’s already, you know, [Right] sore round that area [Right], the nose and that’ [Right. Mmm]. But we’ve, we’ve heard that it’s the, the procedure anyway to have it –
That’s right.
Down the throat, yes.
The natural process in the hospital at the minute is to have the NG tube put in as you need it when you’re going through the treatment.
Right, yes. Right.
And this is why they’re doing the research study in the first place because they don’t know whether, if, if they put a tube into your stomach, whether that improves people’s treatment and after care or whether, or whether it makes, whether it makes a difference or not.
Following Laura’s question, note here how the consultant clearly marked that routine care – ‘the natural process at the minute’ – at the hospital was the nasogastric tube. In this way, they work to confirm their understanding. Note how the consultant stated that it was current practice – ‘at the minute’ – and then went on to introduce the reason for the trial (that there is uncertainty – ‘because they don’t know’). In three of the consultations, we have a similar pattern in which the local focus on nasogastric tubes is briefly discussed, at relevant moments, in order to contextualise some aspect of the discussion about the trial processes. Notably, this works to produce routine care as a product of custom and practice at this site. As the research nurse noted to a patient and carer, ‘but that’s just local preference and the policy here. In different parts of the country, the policy’s different’.
However, with the consultation that involved both a research nurse and a dietitian, we see a slightly different approach. Centrally, the issue of nasogastric tubes as routine care at this site was repeatedly returned to as a topic throughout the whole consultation. The discussion took place approximately half-way through the consultation as the HCPs responded to the patient’s question regarding which type of tube was the more popular:
Well at this –
Well, it’s kind of at this centre –
At this centre we would use this [nasogastric tube], but everyone’s different depending on the different cases and what way you present. For you we would be using the tube in your nose to start with, but then other centres would put in the tummy tubes beforehand for everyone and that’s where we’re a bit stuck, isn’t it?
Yes, so that’s just another illustration of, there’s no agreement even between doctors or agreement between different hospitals about which is the best one to use.
Recruitment consultation, Woodville
The dietitian and the research nurse collaborated closely in positioning the local preference at Woodville for nasogastric tubes as a product of custom and practice, whereas gastrostomy tubes are given to everyone at ‘other centres’. Both HCPs worked to use clinical uncertainty as a way to reflexively demonstrate the need for the trial. Just after the sequence above, the research nurse marked nasogastric tubes as ‘the standard’ treatment at the site. The patient then raised a question about the timing of tube insertion. This was discussed in relation to the timing of gastrostomy surgery and contrasted with a nasogastric tube:
Do I get this tube irrespective, or is it in? If I go for the trial is it in early?
No, it wouldn’t be in any earlier. We would treat you exactly the same. The only difference would be that if you were allocated to the tummy tube arm, that you would have that in before you started your treatment. We’d have to get you in and it’d be there ready for you.
Right.
Whereas, if you were allocated the one in your nose, you probably wouldn’t even notice you were in the trial, because it would be exactly the same practice that we would do.
Right, yes.
Recruitment consultation, Woodville
In the excerpt above, inserting a nasogastric tube was marked as ‘exactly the same practice that we would do’. Again, inserting a nasogastric tube was formulated as the standard treatment, to the extent that to take part and receive a nasogastric tube would mean that, potentially, the patient would not ‘even notice’ that they were taking part in a trial. Taken individually, the work of marking insertion of a nasogastric tube as standard and usual practice at Woodville seems unremarkable. However, if a particular type of tube was repeatedly marked as ‘standard care’ over the course of a consultation, it is understandable that some patients may respond by being reluctant to ‘deviate’ from the established clinical pathway. We should note that this patient, later in the consultation, does outline their preference for gastrostomy, the non-standard care at this site. Patients interpreted the purpose of the trial from multiple stimuli such as information sheets and discussion with relatives, but also, importantly, their interactions with a range of HCPs at the site.
The challenge for HCPs recruiting to the TUBE trial was to present the trial as a rational exercise while also defending the local site’s standard practice of offering only one treatment to the majority of patients. Some patients responded by declining randomisation, remaining within the reassuring boundaries of standard care. Others reported that the clinical ‘uncertainty’ underpinning the TUBE trial was an opportunity to express their treatment preferences. If that preference was not the ‘standard’ treatment, then patients reported randomisation as offering them the possibility of accessing their preferred treatment. If patient’s preferred treatment was also the standard treatment, then the TUBE trial was reported by patients as having little to offer them. A third group of participants were ambivalent about the type of feeding tube they received. If the TUBE trial was perceived as having a small impact on their clinical pathway, then this group tended to report a favourable view of participation. Patients’ accounts of the decision-making process are explored in the next section.
Decision-making
In this section, we focus on how, at a time of personal turmoil, patients worked to make sense of the TUBE trial, and how they worked to decide whether or not it was right for them to take part in it. By necessity, patients who were eligible for the TUBE trial were approached soon after diagnosis, at a time when most were still processing the implications of a new, previously unanticipated, future. 61 We show how recruitment discussions were embedded in deliberations concerning diagnosis and form of primary treatment for cancer and secondary treatment (e.g. feeding tubes), and that patients emphasised that treating cancer was their first priority. Patients understood the purpose of the research and understood what agreeing to randomisation would mean in terms of treatment and its impact on their clinical pathway. The timely and efficient progression of treatment was their central concern. The TUBE trial was generally reported in positive terms when it was perceived to fit seamlessly within a clinical pathway. We then show the range of reasons that patients offered in relation to both accepting and declining randomisation. Finally, we outline how preferences for specific forms of feeding tubes were formed from a wide range of factors, including the perceived, or stated, preference of the consultant.
The local context of the TUBE trial recruitment discussions
The series of meetings concerning diagnosis, treatment, side effects and research presented patients with a range of unfamiliar and challenging possibilities. Patients described previous health and research experience to explain how they made sense of their current circumstances. Most reported that they enjoyed good physical health prior to diagnosis (n = 9), had no first-hand involvement with feeding tubes (n = 11) and that they had not previously taken part in research (n = 12). Although symptoms leading up to diagnosis varied, all emphasised how shocked they were to receive a diagnosis of cancer and how quickly treatment and associated procedures (such as crafting a protective mask for radiotherapy) were organised once a diagnosis had been made. Jenny, the spouse of a patient, explained that:
[The consultant] had told him . . . that he suspected that he may have . . . cancer of the throat and the back of his tongue . . . to say we were astounded, shocked and bowled over is a bit of an understatement . . . [After he] was diagnosed . . . it was amazing the way that the whole thing just kicked in, you know, the whole machine started to move [. . .]. He was diagnosed I think on the Tuesday . . . We went to Otago on the Friday [of the following week] and we met an awful lot of people that [. . .] It was just a whirlwind [. . .] We were introduced to everybody and their grandmother, and tests were arranged, and all of that stuff. And it was at that point that we met the lady from the hospital who told us about, obviously the trial, the study that yous [sic] were doing, and explained everything, and asked us to think about would John be prepared to be a part of it.
Jenny (relative of John, randomised to receive a nasogastric tube), Otago
Jenny’s analogy to a ‘whole machine’ which ‘started to move’ provides a good example of how patients explained the rapid implementation of a complex care pathway, including multiple interactions with HCPs across a MDT. Patients praised HCPs for the assistance they received throughout this process, and welcomed the swiftness with which care was enacted.
Patients placed their trust in the assessment of HCPs, who informed them that, despite current feelings of vitality, in the near future they would likely experience the unpleasant side effects of treatment. Bill reflected on his current health and what he had been told about the effects of chemoradiotherapy:
I’ve been told that the treatment I’m going to get with the chemo[therapy] and the radio[therapy] thing, that it is actually going to take it out of me a bit, you know, and I am going to feel tired and physically drained more than anything, . . . I’ve been told, – not knowing myself – but I’ve been told that it will actually, physically drain me [. . .] because at the moment, I’m physically fit, I’m mentally fit, and I’m alert. So, you know, it’s . . . so at the moment, everything has been as per normal.
Bill (randomised to receive gastrostomy), Queenstown
Bill made a distinction between an expert forecast of his health – ‘I’ve been told, not knowing myself’ – and his experiential state: ‘at the moment, I’m physically fit’. Patients emphasised the importance of the HCPs who helped them to understand their options, the likely effects of treatment and strategies to adapt to new dietetic needs. A perception that the clinical team were being ‘honest’ about likely outcomes was particularly valued. Jenny, the wife of the patient, John, described their experience:
They’ve been on the money with everything. So I mean [John] went in New Year’s Day to have the tube inserted, and New Year’s Eve he was eating a sausage roll . . . By the Wednesday he couldn’t take anything orally. And they told us that . . . [it] actually just makes you feel more confident in them. Because they tell you how it is, so when they’re saying to him that they’re delighted with the way things are going, with the treatment progression, you believe them. Because you’ve got no need not to believe them. Do you know what I mean?
Jenny (spouse of John, randomised to receive a nasogastric tube), Otago
Health-care professionals were mindful of the importance of honesty and the building of trust in their interactions with patients. Both HCPs and patients valued trust in their clinical relationships, and honesty was reported as nourishing trust.
A key component of participants’ accounts was how the trial fitted within the broader narrative of their care. The amount of information that patients were required to process, and the many appointments that they were asked to attend, was often overwhelming. Patients described meeting a range of HCPs in close succession, or as Jenny phrased it, ‘a whirlwind . . . everybody and their grandmother’, and reported struggling to process the emotional and practical implications of their diagnosis. In this context, information about the TUBE trial was sometimes seen as an unwelcome addition to an abundance of clinical material. Ralf, who declined to be randomised, explained that:
I don’t know who it was [that told me about the study]. I don’t remember who it was with. I don’t know whether it was with the dietitian or what. I can’t remember now.
It’s all a bit of a blur?
Yes. I’ve seen so many different people, in different hospitals, it’s unbelievable.
Right. You’re getting a bit confused by it all?
No, not confused. I’m all right at the moment, because I’ve got all my dates through for my chemo[therapy] and my radiotherapy, and everything else. At the moment, it’s levelled itself out.
So it was more that you just had so many things being given to you all at the same time?
Yes, so many different booklets on what to eat, what not to eat, this, that and the other, and everything else, you know? . . . When they start giving you a load of pamphlets . . . I think to myself, ‘I cannot go all through that.’ The whole time, you’re either at the hospital, or you’re reading about the hospital, or whatever.
Patients reported feeling a sense of control, or that their situation had ‘levelled itself out’, when the clinical pathway had been arranged: dates were set, and practical considerations such as travel arrangements were in place. Ralf made a distinction between his state of mind at the time of the interview – ‘I’m all right’ – with how he felt during diagnosis, when he was first introduced to the TUBE trial, agreeing with the interviewer’s suggestion that it had ‘all [been] a bit of a blur’. A recurring theme in patients’ interviews was a distinction between the diagnosis and treatment discussions, and a later point on the clinical pathway at which patients like Ralf reported a calmer, more determined state of mind.
Making sense of the TUBE trial
Recruitment discussions for the TUBE trial were described by patients as being embedded in deliberations concerning diagnosis and form of primary treatment for cancer and secondary treatment (e.g. feeding tubes). Patients emphasised that treating cancer was their first priority. When asked how they first heard about the TUBE trial, most patients described a conversation that flowed as a logical extension to discussions about treatment modalities, treatment effects, and the likelihood of requiring a feeding tube. Jonathan recalled his experience:
When you were approached about the study, what were your initial thoughts about it?
I didn’t know if I’d have to have a tube, but when they explained that it will get bad with the swallowing and that, I said I accept what is going to be done with the tube. They said, ‘Would you like to go in with the study?’ and I said, ‘What does that involve?’ and she told me then that there were two lots [of tubes]. She said but you can’t pick, it’s random with a computer. We’ll do a random test. I said that’s not a problem. Then she put me through the procedures and to sign and read everything about it, and I accepted that and then it’s gone from there to now [. . .]
Jonathan described a logical connection between the news that his swallowing would deteriorate, that he would probably require a feeding tube and that he was eligible to participate in a study comparing two different types of tube. Jonathan’s recollection of what the trial would involve for him as a patient was the process of randomisation: he would not be able to choose the method of feeding assistance. His account is important because one of the most significant aspects of the TUBE trial for all patients was the decision of whether or not to agree to randomise their treatment.
Comprehension of the rationale underpinning research and the design features that shape participants’ involvement is a key component of informed consent. Much has been written in the literature on participants’ recall and understanding of information about trials,62 strategies to improve informed consent procedures63 and the success of different informed consent procedures. 64 Evidence from this body of work indicates that participants’ recall of key research concepts, such as randomisation, equipoise and research aims, is usually incomplete when measured against the PIS or protocol. However, participant accounts of research design may prioritise features that are most relevant within the context of their own illness experience. Throughout the interviews with patients, we observed a range of responses concerning the rationale underpinning the TUBE trial. All patients were able to describe the trial as a comparison of two methods of tube feeding. However, responses regarding the rationale for the research varied. Bill provided a concise and coherent description of his understanding of the TUBE trial:
. . . what [the research nurse] said was that, at the moment, just say for arguments sake, France will put [nutrients] through your NG. And Germany will put [nutrients] through a [gastrostomy], through your stomach. Spain, they, you may use either/or. Nobody knows which the best is: for the patient, or for the future. So what you are actually doing, is actually studying the pros and cons of having it through the nose, and the pros and cons of having it through the gastrostomy, and then you will use this information then, to make it a standard procedure, that is my understanding of it. For the benefit of the patient in the future.
Bill (randomised to receive gastrostomy), Queenstown
Bill’s account identified that the study aimed to compare nasogastric tubes and gastrostomy and that there was a lack of agreement across clinical sites regarding which method was ‘best’ for the patient. He also described a longer-term goal: that the research might support a single method, which would become ‘standard procedure’. Noticeably lacking within Bill’s account was a description of what specific outcomes were being compared, that is, a definition of ‘best’. Rather than discuss long-term swallow, Bill referred more generally to ‘the pros and cons’ of each type of tube. Most patients explained that there was a difference of opinion across clinical sites regarding which method was superior, but few described long-term swallow specifically as a key outcome of a future trial. Craig, like Bill, also emphasised differences across clinical sites in his description of the TUBE trial:
. . . both [types of tube] have been used in different areas. I know here tend to use NG more than gastrostomy feeds, or have done in the past. But I think . . . different centres use different things. And then they all swear by what they use, [their method] is going to be the correct thing, obviously! But I think anything that can actually clarify the outcome of that is going to be good for this [condition] in general.
Craig (randomised to nasogastric tube), Woodville
Craig identified that robust preferences for the different types of feeding tube were held by different clinical sites. Differences of opinion regarding the superiority of one method over another were held at the level of a collective (e.g. a clinical research site) in both Bill and Craig’s accounts. Issues around individual clinical equipoise did not feature in patients’ accounts of the TUBE trial, likely because all but one research centre reported ‘typically’ recommending one form of tube.
The TUBE trial was reported by most patients as focusing on an important area of their care by comparing feeding tubes. However, a few patients did question whether the trial was driven from a position of clinical uncertainty or as a vehicle for proponents of one method to prove that their preferred approach was superior to colleagues. A few patients were also uncertain of what the TUBE trial aimed to achieve by comparing methods of feeding assistance. Sarah suggested that the trial might compare levels of satisfaction between patients randomised to receive a nasogastric tube and those randomised to receive gastrostomy:
In your own words, what’s your understanding of what the study is about?
I don’t know that I’m entirely clear. I think they’re looking at these two options that people are faced with, and trying to assess, relatively, levels of satisfaction with the different options. It seems to be more an experiential thing; about the patients’ experience, not necessarily the medical outcome, because I suspect that’s not really in contention.
Sarah doubted that the TUBE trial would compare nasogastric tubes and gastrostomy on nutritional or functional medical outcomes. She contrasted medical research with what she perceived to be a ‘softer’, more experiential assessment of ‘levels of satisfaction’. Sarah’s account is useful because she demonstrated two important points common to most patients’ accounts. Some patients – like Sarah – assumed that clinical questions about feeding tubes would have been established a long time ago: ‘that’s not really in contention’. Therefore, the TUBE trial was not, to use Sarah’s term, a ‘medical’ or a ‘priority’ study in the larger context of medical research and cancer treatment. Second, understanding the rationale for the TUBE trial was not as important as understanding contemporaneous concerns, such as what treatment entailed and how to proceed along a clinical pathway. This perspective was also underpinned by the perception that the TUBE trial was not a ‘medical’ study. In this regard, Sarah’s account of what she perceived to be the rationale for the TUBE trial also supported her decision not to take part.
The timely and efficient progression of treatment was a central concern for all patients. The TUBE trial was generally reported in positive terms when it was perceived to fit seamlessly within a clinical pathway. Liam offered his interpretation of the TUBE trial:
. . . I mean, it’s a minor thing, a choice between a [gastrostomy] and the nasal one, as far as I’m concerned, because it’s just a feeding tube. I mean, I could understand somebody doing clinical tests for things that are lot, lot more, [pause], I wouldn’t say ‘important’, but a lot more life-threatening. If I can put something back in a small way, I would do it . . . I mean, I consider a feeding tube, in my situation, to be a minor operation, and it’s going to help me when I finally have radiotherapy and chemo[therapy]. I could imagine that some clinical trials which probably might be drug trials or something like that, or procedures that might be more [experimental], you’d have to find out which way to go, which is more safe than other ways, you know, more or less.
Liam (randomised to receive gastrostomy), Woodville
An observation reported by most patients (and HCPs) was that the TUBE trial compared existing, routine technologies; as Liam explained, ‘. . . it’s just a feeding tube’. Neither arm of the trial involved an experimental treatment. Moreover, regardless of whether or not patients took part in the research, it was likely that they would require either type of tube to support their nutrition. In Liam’s estimation, therefore, the TUBE trial did not present a risk to his health or care, compared with clinical trials of more experimental treatments, and required almost no deviation from a standard clinical pathway. These features of the trial worked either to support or diminish its perceived attractiveness to individual patients. Patients like Liam, who perceived the trial to be unobtrusive (‘it’s a minor thing’), identified an opportunity for an altruistic act – to ‘put something back’ – via the TUBE trial, while pursuing the central activity of recovering from cancer. Some HCPs, mainly at one site, reported that when multiple research opportunities were available for patients, some opted for studies that were perceived to be more ‘important’, by virtue of their focus on novel or adapted forms of primary treatment, than a study comparing established forms of secondary treatment.
Accepting randomisation to the TUBE trial
Patients who agreed to randomisation offered three different types of reasons:
-
They had no strong preference and saw the trial as having little impact on their care pathway.
-
They had a treatment preference but viewed their contribution to this research as more important than pursuing their preference.
-
They had a preference for the treatment that was not usually available at their site, and so they agreed to randomisation as it may have given them access to that treatment.
Altruism was the most frequent motivation articulated within patients’ accounts of why they agreed to be randomised. However, the research question also contributed to a comparatively unobtrusive study design, and this was cited as attractive by patients who were all primarily concerned with the primary cancer treatment over issues of feeding. Most patients reported a desire to contribute to medicine, and to help other patients and the process of cancer treatment, for example:
What informed your decision to say, ‘Yes I’ll, I’ll be randomised’?
It is about time I put stuff back to medicine [laughter] [. . .] this is something that needs to be sorted . . . you know, both, both have been used in different areas. I know Woodville tend to use NG more than [gastrostomy] . . . But I think at different, different centres [they] use different things. And then they all swear by what they use is going to be the correct thing, obviously. But I think anything that can actually clarify the outcome of that is going to be good . . . for what you’re getting out of [medicine] you’ve got to give back sometimes as well.
[. . .]
And I don’t think I was pushed to make a decision there and then with [the consultant] . . . [there’s] nothing to lose really, . . . And it’s, it’s answering a few questions rather than actually changing particularly what goes on.
Craig explained in his interview that his treatment preference had changed since his diagnosis, from initially preferring the concept of gastrostomy to seeing benefits to receiving a nasogastric tube. Ultimately, Craig had no strong treatment preference. However, for Craig, there was ‘nothing to lose’, as the TUBE trial remained relatively ‘unobtrusive’ in his clinical pathway. He positions the study as only ‘answering a few questions’ and not ‘actually changing particularly what goes on’. In this way, the relative impact of having a choice about feeding options was seen as minor in contrast to what he saw as the primary element of his care pathway.
Another patient who accepted randomisation indicated that he was relatively uninterested in the study design or purpose, stating that ‘it’s irrelevant to me’ [Mike (randomised to receive gastrostomy), Woodville], as a research study such as the TUBE trial was largely incidental to his primary treatment. He was happy to contribute to research as a by-product of, and in recompense for (‘I owe you’), receiving treatment. Interestingly, a few patients explained that volunteering for the TUBE trial was more important to them than getting their desired treatment:
OK. So how, how did you feel about it being framed like that, that you would have no choice over [your treatment]?
. . . well it didn’t worry me, or unduly concern me, because as I say, I’m a grandfather, and I have children, and if anybody could benefit from this study, I would be more than happy for it to be either an NG, or [gastrostomy] so it didn’t make no difference to me. Personally, I would have liked to have had a choice because of social activities as well, because even though I know I won’t be as active, . . . or fit enough to go out socialising, it’s still a bit of a . . . disfiguration I suppose [to have an NG] . . . because you’ve got . . . the pipe, coming out your nose, and being stuck across your face. Whereas the other way, it would be on your stomach, nobody will actually see it as such, so it was a bit of a personal thing . . . I would prefer to have it on the stomach, rather than through the nose.
Clearly, the description of nasogastric tubes provided by Bill, in particular his concern about the associated visual impact (‘disfiguration’) in social situations, suggested that he had a reasonably strong personal preference for gastrostomy. However, Bill stated that:
I am a donor, I got a donor’s card. [. . .] So if anything did happen to me . . . they would be able to use any part of me they want to, . . . I’m quite happy to do that, . . . I’m not one of those selfish people, . . . I was brought up to help other people, . . . as I think the song says, if I can help somebody on my way, then my living won’t be in vain.
Bill (randomised to gastrostomy), Queenstown
For Bill and for others, the social good of taking part in research outweighed their personal preferences. In this way, altruism was positioned as a central motivation in such accounts.
As noted above, four of the sites had a specific local organisational preference for either nasogastric tubes or gastrostomy. If a patient’s preference was different from the standard treatment, then their only opportunity to access it was through accepting randomisation. At one of the sites where only one feeding method was offered in routine practice, recruiting HCPs indicated that patients may have participated in the TUBE trial in order to access the otherwise unavailable treatment. In a recorded recruitment consultation, Carys and her relative indicated a preference for gastrostomy, which was not normally available at the site. During the consultation, it became clear that participation in the TUBE trial would present a possibility of being given the preferred treatment:
The only concern I have is that I’ve got chronic asthma and the tube down the nose to me sounds a bit restrictive, for me. So therefore I would prefer the other one. But then there’s the worry of infection.
[explains the two different tube methods] . . . If you were not going to join the trial . . . the tube of choice would be the one that’s going down your nose. That’s the standard treatment and the standard way of supporting patients in this trust. If you did choose to go on the trial it would be randomly decided between the two. So there’s a 50% chance if you go on the trial that you’d still have the same kind of treatment you would have –
[interrupting] So you can’t elect to have the stomach tube if you weren’t going on the trial?
No. The policy here is –
The nasal tube.
The standard use, the standard tube is the nasal one.
OK, right. Well that makes it easy doesn’t it?
Yes, I’d go with that.
In this context, the local organisational preference framed the patient’s decision-making to accept randomisation. So, if someone was not in equipoise, but rather had a strong preference for the non-local option, their only choice was to enter the trial to potentially realize that preference. As one patient notes, with a preference for gastrostomy:
Yeah, we’ve got to, we’ll do the research treatment.
Right. And you understand that you might get the tube, you might get the feeding tube but you might get the –
Yeah. I’ll just get the hang of it.
Recruitment consultation, Woodville
As this was the only way to potentially access the gastrostomy treatment path, the patient felt that they had no option. Their preference (which they framed as ‘the research treatment’), which was established through the trial process itself, was not sustainable without taking part and even then, not guaranteed. Patients who had a treatment preference but could access that only through the trial explained that they would have accepted the treatment they were randomised to. Only one patient in our sample reported that they would have withdrawn from the study if they did not receive their preferred treatment.
As is common in the research on participation in trials, the majority of patients who agreed to take part situated their motives as tied to altruism. 65–67 However, as Canvin and Jacoby68 and McCann et al. 69 note, this is a ‘weak altruism’ or a ‘conditional altruism’. By this they mean that:
. . . although people may initially have a tendency to participate in a trial based on a willingness to help others or contribute to a general good, this is unlikely to lead to trial participation in practice unless people can also recognise that trial participation can benefit (or at least not harm) themselves in ways that they regard as salient.
McCann et al. ,69 2010
Thus, some patients can be altruistic and are able to demonstrate to the clinical team that they are willing to help others, as they felt that there was little or no downside for them in taking part in the research. However, in the case of some patients, taking part meant that they might also actually get access to their preferred treatment. Interestingly, the option of taking part in the qualitative substudy gave those who declined randomisation an opportunity to also be altruistic in that they could say ‘yes’ to part of the research study.
Declining randomisation to the TUBE trial
Six patients who declined randomisation agreed to take part in an interview. They offered four different types of reasons for declining:
-
They had a strong treatment preference for the usual treatment at the site.
-
They wanted to make a choice about treatment, as they needed to retain some sense of control when other elements of their life, following a diagnosis of cancer, were now out of control.
-
They felt overwhelmed by the amount of information and choices at the time of recruitment.
-
They were concerned about how taking part in the trial could impact on their clinical pathway through causing a delay in cancer treatment.
Whatever their reason for declining, all the patients worked to acknowledge the potential value of the TUBE trial:
. . . my first reaction [to the TUBE trial] was that, yes I would, in principle be very happy to support research, because, you know, I think it’s very important obviously in developing treatment in the future and you know, I’ve got a bit of a science background and recognise the importance of research.
Hmm, OK. So then you had to decide whether to take part in the trial or not.
Yes, that’s right.
And how did you make that decision? This is something that we’re interested in, how people make the decisions.
Right, yeah, because once the, you know, this particular study was, was explained to me, I mean I really made the decision I suppose based on my personal, my personal feeling how that study would impact on me.
I mean when, once it was explained what the study was about, I must admit, I felt that in this particular case, I didn’t like the idea of going into a randomised trial with two different approaches because I immediately had a particular preference . . . for one approach.
Robert prefaced his account by explaining his broader perspective, that he ‘. . . recognise[d] the importance of research’ in general terms and so was not to be seen as taking such a decision lightly. Moving through three rational stages that led to his decision, Robert first stated that the research nurse provided a more detailed explanation of the implications of the study (‘. . . once it was explained’). This discussion enabled Robert to assess the potential impact of randomisation on his clinical pathway, and the possibility of not receiving a preferred treatment cemented his decision not to be randomised. Robert emphasised that this preference for a nasogastric tube was visceral, formed from a personal ‘feeling’ that was ‘immediate’.
Sarah also had a clear preference, albeit for gastrostomy. For her, the decision was particularly salient as it was made at a time when a loss of control (over one’s body, health and time) was most acute. Sarah explained:
I’ve got no problem with the idea of research studies and us supporting and assisting in that at all; that’s how we learn. The thing that I was concerned about was, if I was going to take part in the substantive study, then the decision about whether I had a [gastrostomy] tube or a nasal tube would not be mine. One feels so out of control after a cancer diagnosis that actually, my instinct was that I need to have control over that decision; I don’t want a computer to decide it. So immediately, I expressed that. I was, at the time, very unwilling, really, to be part of the major [study], because I also had a very immediate preference of which option I wanted to take.
[. . .] To be honest, I’m sure when I get further down the road into radiotherapy, and feel like death on legs, I suspect it’ll feel quite trivial. But at the beginning of a big journey through cancer treatment, it felt like an important decision, yes.
Sarah (declined randomisation; gastrostomy), Wellington
She presented herself as a rational patient by acknowledging the value and need for medical research. However, she made a distinction between her own ‘patienthood’ and the role of ‘research subject’, prioritising the needs of the former. She described her decision not to be randomised as driven in part by ‘instinct’, and emphasised the immediacy of her response several times. Her account can be read as a description of a fundamental sense of self (self-preservation brought out in crisis). Sarah compared the intensity of the ‘moment’ of diagnosis (and subsequent treatment discussion) with what she envisaged as an extended period of treatment: ‘a big journey’. She speculated that further along her clinical pathway, during treatment, a decision between feeding tubes might seem ‘trivial’ in comparison with the side effects of treatment. Sarah declined randomisation as a result of both a strong treatment preference and a desire to retain a sense of control over her clinical pathway.
Strong patient preference for a particular method of feeding assistance was a common explanation given by HCPs for lower-than-expected recruitment:
Patients don’t want to be randomised. Patients have quite a strong voice, they have a quite strong opinion. . . . They are quite vocal in what they do and they don’t want and they do have quite strong opinions.
Rebecca (dietitian), Hamilton
Health-care professionals reported that there was not any one method – gastrostomy or insertion of a nasogastric tube – that was preferred by the majority of patients. Patients were perceived by HCPs to generally follow direct advice from their consultant. However, when uncertainty (e.g. through presentation of equipoise) or choice was presented, HCPs reported that patients tended to express their preferences. They reported that some patients, in a similar fashion to Sarah, were reluctant to give up a sense of control regarding their treatment allocation, given the lack of control they may have experienced from having been diagnosed with cancer:
A patient who came on Friday, they said to me, ‘I don’t want to take on the study because I don’t want the decision . . . on what tube to be . . .’ you know, they don’t want . . . to leave that to chance. And that is probably, an issue.
Janet (oncologist), Hamilton
For these patients, having their treatment allocated at random was a step too far.
As noted above, the diagnosis and subsequent discussion about the most appropriate care pathway were shocking and sometimes overwhelming for patients. Concerns regarding treatment procedures, side effects, practicalities of receiving treatment and possible longer-term effects of primary treatment (such as dysphagia) contributed to some patients responding that they felt that the TUBE trial contributed ‘too much’ additional complexity:
Just a step too far [. . .] I felt a bit guilty about saying no, not because of what anybody said but because I do agree with, you know, a lot of, of research and that’s the only way that you can go forward and help people. So I was just so upset at the time that I didn’t want to take part, I thought I had enough going on at that time.
Linda (declined randomisation, possibility of nasogastric if required), Woodville
For Linda, who was also recently bereaved, participation in the TUBE trial was just a ‘step too far’.
Patients were mindful of the timing of key stages in their clinical pathway, and, understandably, were keen to proceed with treatment as quickly as possible. An issue discussed by both patients and HCPs was the requirement for gastrostomy tubes to be fitted before treatment, and, subsequently, the importance of booking the procedure as soon as possible post diagnosis. One patient, Barry, expressed concern that participation in the TUBE trial would delay the onset of his treatment, particularly if he was randomised to receive gastrostomy, and so this was the issue that was a ‘step too far’ for him:
I am all for the research . . . but . . . there is no benefit for me . . . So therefore there’s no reason that I would want to jeopardise the start of my treatment or have another layer of complication or another visit to the hospital. . . . There are all these unknowns . . . when Katie [the research nurse] spoke to me, she said, ‘Well, let me tell you, [a patient] agreed on the Monday and the following Wednesday he had [a gastrostomy] done.’ I said, ‘Katie I haven’t – I don’t have that time here because I start my treatment on Sunday.’ And she was like, ‘Oh OK, we can see if we can squeeze you in.’ So I think that maybe the communication between the research [team] and [clinical team] wasn’t married up . . . I don’t think Katie knew when my treatment was starting. I didn’t want anything to compromise the start of my treatment so I didn’t want anything to be pushed back.
Barry (declined randomisation, potential to receive nasogastric), Queenstown
Patients explained that key concerns when considering participation were whether the trial would delay treatment or add complexity to the clinical pathway, and whether it would hinder or help them to receive their preferred treatment. Barry’s account touched on all of these issues. He had previously undergone surgical treatment for head and neck cancer (Table 21) and, thus, his diagnosis was both a shock and a particular disappointment for him. His chief concern was to be treated as quickly and efficiently as possible, but the TUBE trial did not facilitate this goal. Barry was worried that randomisation to gastrostomy would ‘jeopardise the start’ of his treatment and add a ‘layer of complication’ to what already seemed to be a complex clinical pathway. His account of an interaction with ‘Katie’, a research nurse, indicates that he lost faith in the research team’s capacity to co-ordinate the confluence of research and clinical pathways. Notably, he recalled Katie’s phrase ‘squeeze you in’ and repeated this throughout his account, emphasising how the research might mean that his treatment could be ‘pushed back’ if he was randomised to receive gastrostomy. Barry’s account is not primarily an example of a ‘poor’ recruitment interaction, but rather evidence of a broader issue at research sites concerning how best to integrate TUBE trial protocols within existing clinical pathways.
| ID | Sex | Prior research experience | Experience of symptoms | Prior experience of serious illness | Prior experience of tube feeding | Participation in the TUBE trial | Treatment preference | Intended treatment |
|---|---|---|---|---|---|---|---|---|
| Woodville | ||||||||
| ‘Frank’ | Male | None | I don’t feel ill either | It’s the first time I’ve been ill in my life | No personal experience. Sister-in-law worked as a nurse and was able to advise | Yes | Gastrostomy tube | Gastrostomy tube |
| Close family had cancer, however | ||||||||
| ‘Robert’ | Male | Yes (head and neck cancer-related study) | I just had a lump on my neck . . . it hasn’t been causing me any bother at all | I already knew that I had cancer . . . the neck lump had been identified as the secondary in a lymph node, but they’d had some difficulty identifying the primary | No personal experience | No | NGT | NGT |
| ‘Paul’ | Male | Unknown | . . . since it all started, I haven’t felt ill you know, and I still don’t . . . I just went to the doctor’s ‘cause I had a lump in my neck [right], and it all sort of spiralled from there | I’ve never been in a hospital before | Had a ‘camera down the throat’ | No | NGT | NGT |
| ‘Joseph’ | Male | Yes (numerous studies) | I discovered it by accident. I just was having a shave . . . and I felt this tiny little lump | Previously treated for prostate cancer | Had a NGT and hated it. Got advice from his daughter (a nurse). Gastrostomy tube fitted prior to interview | Yes | Gastrostomy tube (very strong preference) | Gastrostomy tube |
| ‘Lesley’ | Male | Unknown | He had ‘a lot of pain, constant earache for 7 or 8 months’ | Unknown | Unknown | Yes | Gastrostomy tube | NGT |
| ‘Craig’ | Male | Yes: ‘. . . there’s probably five different trials I’ve, I’ve been told about since, since I started all this’ | I wasn’t symptomatic at all | I’ve had nothing out of the NHS up until now in my life, I’ve been very lucky with my health | No personal experience: ‘. . . as part of my GP training we looked after a geriatric ward . . . that had people on PEG feeds . . . [so] it’s not . . . completely new to me’ | Yes | Gastrostomy tube → nasogastric; saw the benefits of both tubes, but was hoping to avoid having a tube inserted altogether (NGT as a ‘back up’) | NGT |
| I presented initially . . . with a large gland in my neck at my GP, by which time it had gone right down to, not quite normal, but had gone right back down | First wife passed away from serious illness | |||||||
| ‘Linda’ | Female | Unknown | Unknown | Partner passed away ‘a month or two’ prior to diagnosis | Unknown | No | Gastrostomy tube | NGT |
| ‘Mike’a | Male | Yes: ‘I’ve been in research for you people’ | Unknown | [I’ve been in hospital] quite a few times . . . Mainly for broken arms, broken legs, broken thighs a lot when I was younger | Had a ‘camera down the throat’ | Yes | Gastrostomy tube | Gastrostomy tube |
| ‘Liam’ | Male | None | [There] was just a slight lump on my neck, which started off as a cold about 12, 14 weeks ago | We’ve lived life to the full anyway. We’ve been very lucky | No personal experience: ‘I’ve never had the experience of the tube, or the PEG, for that matter’ | Yes | Gastrostomy tube | Gastrostomy tube |
| I’ve had a good life. Can’t complain | . . . my stepfather-in-law . . . 10, 15 years [back] . . . got fed through a machine . . . into his stomach. You never saw it, you weren’t involved in it. You knew it was there | |||||||
| ‘Carys’b | Female | Not interviewed (recruitment consultation only) | Not interviewed (recruitment consultation only) | Not interviewed (recruitment consultation only) | Not interviewed (recruitment consultation only) | Yes | Gastrostomy tube | NGT |
| Otago | ||||||||
| ‘John’ | Male | None | . . . some difficulty [with] . . . speaking or voice | None | No personal experience. Father fed intravenously in hospital | Yes | E → NGT | NGT |
| Hamilton | ||||||||
| ‘James’ | Male | Yes, at the time of interview the patient was taking part in two other studies (three in total) | Unknown | I’ve been a healthy person all my life; I’ve never been in hospital | No personal experience. Spoke to a neighbour with a granddaughter who had a tube. Got ‘a shock’ seeing a patient with a nasal tube | Yes | E → NGT (consultant-led) | NGT |
| Within the last 7 years prior to TUBE, father-in-law and mother both passed away from cancer | ||||||||
| ‘Ralf’ | Male | None | Unknown | Unknown | No personal experience. Wife had a NGT in hospital | No | E → gastrostomy | Unknown |
| Queenstown | ||||||||
| ‘Bill’a | Male | None | Unknown | Unknown | No personal experience. A close friend is a carer who is familiar with the use of gastrostomy | Yes | Gastrostomy tube | Gastrostomy tube |
| ‘Barry’ | Male | Yes: ‘I’ve been doing an interview, for a head and neck study when I was first diagnosed’ | Still recovering from surgery when introduced to the TUBE trial: ‘I have got a little bit of pain still in my jaw and my mouth’ | Prior to the TUBE trial had been treated via surgery for head and neck cancer: ‘The first I heard about [the TUBE trial] was when I went back in for my results of the 3-month scan, which obviously weren’t great’ | Had a ‘camera down the throat’ | No | E (leaning towards gastrostomy tube) | NGT |
| ‘Jonathan’a | Male | None | I went to the doctor’s about my throat because I had a very sore, hoarse throat | Wife passed away from lung cancer | No personal experience. Had seen other people, including his wife, with feeding tubes. Initially disliked the idea of a NGT over an extended period because of the associated restrictions on socialising | Yes | Gastrostomy tube → E | NGT |
| Wellington | ||||||||
| ‘Sarah’ | Female | None: ‘nothing involving medical research’ | Unknown | I’ve not suffered any operations in my adult life . . . I hadn’t actually been overnight in hospital for 50 years, until I went in for the PEG . . . So I haven’t suffered with ill health at all | No personal experience | No | Gastrostomy tube | Gastrostomy tube |
| ‘Gary’a | Male | None | Had some difficulty with swallowing (prior to having a gastrostomy) | Unknown | No personal experience | Yes | Gastrostomy tube | Gastrostomy tube |
| Totals | Male: n = 15; female: n = 3 | None: n = 8; yes: n = 6; unknown: n = 5 | None or minor symptoms (e.g. a small lump): n = 6; pronounced symptoms (e.g. pain, poor swallow, hoarse voice): n = 5; unknown: n = 8 | No personal experience with severe illness: n = 4; no personal (first-hand) experience, but experience of a close family member or friend who was seriously ill: n = 5; has experienced severe personal illness: n = 4; unknown: n = 6 | No personal experience: n = 7; no personal experience, observation of family or friends: n = 4; prior personal experience (camera): n = 3; prior personal experience (NGT or gastrostomy tube): n = 1; unknown: n = 4 | No: n = 6; yes: n = 12 | Gastrostomy tube: n = 11; NGT: n = 2; gastrostomy tube → E: n = 1; E → NGT: n = 2; E → NGT: n = 2; gastrostomy tube → NGT: n = 1 | Gastrostomy tube: n = 8; NGT: n = 10; unknown: n = 1; preference matched treatment: n = 12 |
Some HCPs, primarily oncologists and research nurses at one site (Hamilton), reported an additional reason why patients declined randomisation:
-
patients would not want to take part in research unless it was viewed as being relevant to the primary treatment of cancer, and was ‘high profile’.
A prominent issue at Hamilton, but one also identified at other centres, related to a number of studies being conducted at the same time, each targeting a similar patient demographic. Consequently, patients who were eligible for the TUBE trial were also eligible for a number of other studies, and would be offered participation in some or all of them. Some HCPs explained that the TUBE trial was comparatively ‘unattractive’ compared with some of the other studies available, because it involved randomising the patients (rather than simply observing their care); randomisation consisted of allocation to one of two very different treatments; the short-term result of the two methods was the same (adequate nutrition); and other studies focused on primary treatment for cancer, rather than on longer-term lifestyle and nutrition issues. This argument was used by some to explain why TUBE trial recruitment was not as successful as other studies that were recruiting a similar patient profile.
Key influences on preferences
Preferences for specific forms of feeding tubes were formed from a wide range of factors, including the experience of family and friends or the patient’s history with feeding tubes, visceral responses to a particular type of tube, seeing other patients with tubes and practical concerns about infection or social stigma. As one HCP noted, different feeding methods can be:
. . . a bit like whether they want surgery or chemo[therapy] or radiotherapy, quite often they’ve got quite a strong view on that.
Emma (surgeon), Otago
Some patients had a strong feeling underpinning their treatment preference. Common reasons for wanting to avoid a nasogastric tube included the concern that the tube would undermine relationships with children in the family (e.g. a child or grandchild), that it could be dislodged by grandchildren or activities of daily living, that it could lead to social stigma or reduce social confidence, and that it would cause physical discomfort. Some explanations for preferences were poignant. One patient, who declined randomisation because of a preference for gastrostomy, provided the following account:
The preference was for [gastrostomy] tube, mainly because I have an 8-year-old daughter, and this is a hard time for her. I think having mum going round with a tube up her nose, it’s just an incredible embarrassment for children at that age. It’s like a sort of mark; you go out and people are wondering why you’ve got it. Although a [gastrostomy] tube is a much bigger procedure, I felt it was worth it. [. . .] To be honest, I’m not likely to lose my hair with this chemo[therapy], but actually, the thing about losing hair would be easier than having a tube up my nose [. . .] from [my daughter’s] point of view. Because she would enter into buying a wig and doing that kind of stuff, but the tube up my nose marks me out as a patient on the loose.
Sarah (declined randomisation; chose gastrostomy), Wellington
Patients who preferred gastrostomy over nasogastric tube feeding reported a range of potential benefits, including larger bore of feeding tube, and, thus, faster feeding, the possibility of bolus feeding and greater independence, and having the tube ‘hidden’. They also reported being familiar with gastrostomy through friends, neighbours and family members.
Some patients, however, were concerned that gastrostomy carried a risk of infection and that it required additional surgery. As we have seen above, Ben was concerned about the potential delay in receiving treatment, as the gastrostomy was required before the onset of treatment. Notably, some felt that having a tube inserted would be symbolic of having ‘given up’ before the treatment (and attendant side effects) had even begun. Most, but not all, patients who expressed a desire to ‘do without’ feeding assistance for as long as possible accepted that they might require assistance in the future. These patients reported a preference for a nasogastric tube, as it could be inserted reactively as required. Patients also expressed a range of ‘instinctive’ responses to the concept of either a gastrostomy or a nasogastric tube. Some were unfazed by one method but found the alternative particularly distressing.
Whether declining or accepting randomisation, patients’ accounts of their participation decisions ranged from a ‘swift’ judgement through to an extended process of consideration across several days. A patient described how, over time, through reading the PIS, they shifted from an initial position of equipoise to having a strong preference for a nasogastric tube:
To be honest, I just couldn’t, when I started reading the two different things, I just couldn’t face the tube in my stomach. No, I just couldn’t face it. The more I read it, the more – I just, it was just totally alien to us. Well I didn’t have a preference for either when they asked us, mind. But there was so much going on in the room, and then it was just, ‘Well you’ve got a choice, you can be fed through your nose or your stomach’, which seemed reasonable, you know? [Laughter] It was only after I thought about it that, to be fair to them they said, ‘Take this home and read it, then we know what you think’, you know, it was when I reading the things that I suddenly said, in my mind, you know, ‘I’m not doing that one’.
Paul (declined randomisation; chose a nasogastric tube), Woodville
All patients, regardless of the temporal aspect of their decision-making, also talked about the way in which personal context shaped their decision-making. Therefore, even swift decisions were situated within a train of prior experience and across interactions with others. Some patients discussed the TUBE trial with a close circle of supporters only, such as partners or adult children. Other patients reported that conversations with acquaintances, and non-verbal observation of people with tubes in public places, shaped their participation decision. In this way, decision-making was distributed. 70 James reported a conversation with his neighbour:
[My consultant] Sally said . . . ‘We certainly didn’t want to do [gastrostomy] near the radiotherapy period, because it’s a harsh treatment . . . it’s sitting there, dormant, for 6 weeks before you have your radiotherapy, . . . you have to keep it clean, regularly, so there’s no infections . . . whereas the nose one,’. . . she said . . . ‘there’s less risk of infection’. So, you know, that made me think more of the nose one, but then when I came home, my immediate neighbour, her granddaughter has had an illness . . . she’s had this one in the stomach and the neighbour said, ‘It’s the best thing, it’s really good, you keep it clean, it’s just easy to use,’ and, ‘That’s the better . . . no one can see it’. She was pushing me along the line to have the one in the stomach, but then, as I said, I’ve got to go with the consultant.
James (randomised to receive a nasogastric tube), Hamilton
James described throughout his interview that his consultant’s guidance was a priority for him. Both HCPs and patients reported that the perceived or stated preference of consultants strongly influenced patients’ treatment decisions.
Living with feeding tubes
At the time of their first interview, only three patients reported that they had received a feeding tube (gastrostomy). Follow-up interviews were conducted with four patients. All randomised patients who took part in an initial interview were approached for a second, unless advice from HCPs suggested that contact would be unsuitable for the patient (e.g. being too ill/passing away). Some patients refused further contact or did not respond to invites. Patients who took part in a second interview were those who had progressed well through treatment. Consequently, their accounts may reflect better outcomes than most of the sample after feeding tube use.
We should note that patients largely understood participation as a decision about whether or not to agree to be randomised. Once this decision had been made, other research activities (such as questionnaires) became subsumed within the broader raft of paperwork that patients were asked to process. In this way, post randomisation, the trial pathway was both invisible and, therefore, acceptable to patients, as, in practice, it became the existing clinical pathway.
Soon after diagnosis and recruitment, patients reported that their primary concern was timely treatment and their capacity to adjust to the effects of chemoradiotherapy. The patients who were interviewed after their treatment had started, or who were interviewed twice, reported a concern with swallowing and nutrition. Therefore, although patients were informed about difficulty swallowing soon after their diagnosis, the importance of the research question – regarding long-term swallow – became increasingly evident to patients after the recruitment window to the TUBE trial had closed. In this section, we show how patients learned to adjust to life with a feeding tube, learning how to fit feeding around their day-to-day life.
Living with gastrostomy
Bill described a relatively trouble-free experience when he received a gastrostomy. Bill’s account was similar to that of the other patients who had the procedure:
But of course, I had a local anaesthetic and [the research nurse] came down and the two of us were watching it on the television, how the tube was going in. She was absolutely fascinated with it and the doctor couldn’t get over that I was so calm and watching it on the telly with her.
Bill (randomised to receive a gastrostomy), Queenstown
After insertion, patients with a gastrostomy did not immediately start to use their tube. Joseph explained how he transitioned from swallowing to using his gastrostomy tube:
. . . that’s when I went on to [the gastrostomy], when I couldn’t get any food down . . . [the gastrostomy] was in, I started me treatment and . . . I was still eating normal [. . .] But after 1 day, I just got up and I went ‘oh what’s that?’. I was having me favourite breakfast which is grated cheese, scrambled egg, chopped-up bacon and a leek chopped up . . . stow it all and just spatted it all up. And then I went to [my wife], ‘oh that’s horrible’, it just tasted vile. I says ‘oh here, give us me can of coke’ and I went ‘oh this is, what the!’ [. . .] so I says ‘oh I’ll see you when I get back, I’m going for me treatment’ when I come in and I telt [sic] the nurse she said ‘oh it’s your taste buds what’s going, you’ll have to go on the feed’. I says ‘oh great’.
Do you think you could have kept eating when it, do you think you could have kept eating and swallowing when everything tasted [bad]?
I couldn’t swallow. That was the trouble after that day, as soon as I tried it the next morning when I got up I couldn’t get it down and I thought oh I’ll get, I’ll wash it down . . . and it just went, it was just like a roll of dumpling mix in your mouth. Everything just sort of rolled up into it and I’m going, and I was having to scrape it out with me finger.
Right and it just wasn’t going to go down.
It just wouldn’t go out and I couldn’t get it washed down me throat.
Joseph explained that the time taken to complete a feeding session by using a pump and a gastrostomy tube could be limiting. However, he found that he could speed up feeding considerably via bolus feeding:
So how long does it take with the pump to get your calories in?
Probably to get one of them in, 2 hours, an hour.
And how long does it take you if you’re doing a bolus feed on your own?
On me own, 20 minutes. Oh no man, it was 2 hours to put one of them through the pump, you could do that in 6 hours for three of them and that was in the afternoon and then you could have like 2, 2 hours in the morning and 2 hours at night-time. Which is like 10 hours a day but I can do it in 20 minutes one tube which you’ve seen, it’s finished, I’ve just got to wash me stuff well that’s nowt, I’m walking about then.
Joseph also talked about fitting tube feeding around his day-to-day life. He discovered that he could use bolus feeding in public places without feeling socially uncomfortable:
I’ve [used] it in the park . . . sat down, people just sat there talking. One wife [woman] come over she says ‘is it alright if I sit here with the dog while you’re doing that?’ I says ‘nee bother’ (laughs). You know and she’s throwing to the dog and she got on rabbiting about it and she says ‘what is it like?’ I says ‘it’s for cancer of the throat’. I says ‘I’m getting fed, it’s feeding us’ . . . she says ‘cos me husband got to go in for that’, she says ‘does it hurt?’ I says ‘no’. She says ‘well he can’t make his mind up what he wants’. And I says ‘oh right’ and she asked me a couple of questions and I answered them.
Joseph (randomised to receive a gastrostomy), Woodville
Mike reflected on his preference for gastrostomy and his experience with the tube at the time of the interview. He explained that he was satisfied with having a tube that could be hidden from view. He preferred a gastrostomy:
Because it’s out the way and if I’ve got to go up the street, it’s not coming out me nose, and it’s not having [people come up and] say ‘it looks uncomfortable’ . . . [and me saying], ‘sorry to shock you’. And I feel more comfortable with it on the stomach. [. . .] I know there could be one or two problems with infection and stuff like that, . . . I’ve got somebody coming in this Friday . . . to clean the water and give the tube a rinse out, I know this is going to be a little bit uncomfortable, . . . but I’m ok, at the end of the day I feel it’s better than having it down through the nasal passages.
Mike (randomised to receive a gastrostomy), Woodville
Sarah, who at the time of the interview had not yet started using her gastrostomy tube, reported that she would have liked more information about the practicalities of using the tube and some of the potential undesirable side effects:
. . . maybe the information could have been more forthcoming about the relative efficacy in terms of feeding. So actually, it’s only recently – and I can’t remember how I’ve discovered this. Oh, I think because one of the nurses at Wellington hospital where I’m getting treatment, spoke to me about it. But when you first start using the feeding tube – because I’m not using it at the moment – sometimes, you can have reactions in terms of reflux. Your stomach says, ‘Where’s all this alien stuff coming from? It’s coming from the wrong place.’ I don’t think that was properly highlighted, probably, in the information that I had.
Sarah (declined randomisation; chose gastrostomy), Wellington
A concern identified by HCPs and patients regarding nasogastric tubes was the potential for children to be frightened by the tube or to tug on it, thereby dislodging it. Sarah explained that she decided against a nasogastric tube, as she did not want to make her young daughter uncomfortable. In the following excerpt, she described how her daughter had responded to her gastrostomy:
The thing about my daughter, on the one hand, with the NG, I think she would find that very difficult. On the other hand, with the [gastrostomy], we’ve already got an accommodation around that. She thinks it’s quite interesting that I’ve got a tube in my stomach. If it comes to feeding, she wants to be involved in that. She can.
I think with children and cancer, there’s a value in – obviously, not overloading them – but if they want to feel inside the circle, and have a role or anything like that, it can be quite useful. There was a positive thing about the [gastrostomy] . . . – having a tube down my nose, it just felt very alien and different – whereas the [gastrostomy] is something I felt we could live with and make jokes about. We call it my little alien.
Sarah (declined randomisation; chose gastrostomy), Wellington
One of the concerns with a gastrostomy is the likelihood of retaining the tube for a long period of time and delaying a return to oral feeding. At the time of the follow-up interview, one of the three patients randomised to receive a gastrostomy, Gary, still relied on his feeding tube, while the others had had their tubes removed some time previously. However, Gary described how he still ate orally when possible:
Yes. I can eat soup now and porridge and I can sip tea. Nothing too big. I still get choky like, with anything that’s too big to swallow . . . I’m still feeding myself [via the gastrostomy]. I don’t put so much water in now because I can drink water so I’d rather drink it. I’d rather have the taste of the water than not tasting the water. You don’t feel anything when it goes through your gastrostomy. You know? And it dries down – it wets your mouth this.
Gary (randomised to receive a gastrostomy), Wellington
This mix of oral and percutaneous endoscopic gastrostomy (PEG) feeding enabled Gary to start consuming fluids in social situations:
Yes. No problems. You go into town and I can even have a cup of coffee or something and I take my time. It seems like I’m getting on.
Gary (randomised to receive a gastrostomy), Wellington
Gary described how a significant barrier to a return to more substantive oral feeding was his lack of teeth, which had been removed before treatment; without functioning teeth, he was unable to chew food into a consistency that he was able to swallow:
I’m not too sure [when the gastrostomy will be removed]. I don’t know whether to see the consultant. I can’t see how; I haven’t got any teeth. They took my teeth out before the treatment so I can’t chew, well I can ‘num num’, they call it – don’t they or something – with my gums. But not too much. Nothing like crispy bread or anything like that in case it gets stuck.
Gary (randomised to receive a gastrostomy), Wellington
These problems were reiterated by Mike, who was also still waiting for new teeth, although he was managing without supplementary feeding:
Well to be quite honest I didn’t see any problems with any of the treatment, I think the only problems I’ve really got is I’ve got to wait for new teeth coming, you know, it’s been over a year since I had them taken out, and it still might be a couple of months before I can get them, get the full set. . . . cos you can’t chew anything, everything’s got to be soft and small . . . I love things like biscuits and crisps and stuff like that but they start cutting my gums and then you can’t eat them.
Mike (randomised to receive a gastrostomy), Woodville
Overall, patients who received a gastrostomy reported that they were satisfied with their treatment. Gary explained:
I was fine. I accepted it straight away . . . I didn’t think much about it. I’m still in awe in how it keeps me alive. This bottle of solution of whatever it is. I’m amazed.
Gary (randomised to receive a gastrostomy; follow-up interview), Wellington
Living with nasogastric tubes
Craig was randomised to receive a nasogastric tube and, although he did not have a tube at the time of his interview, his account, in part, vindicated proponents of the nasogastric tube. At the time of his interview, he was managing to continue eating and using nutrient supplements. At his initial interview, he reflected on his pre-treatment preference for gastrostomy. He outlined that, initially, he wanted a gastrostomy:
Because once it was in it was in . . . My feeling is possibly, looking back on it, that probably your motivation to keep eating and striving when you’ve got something that’s already there waiting to be used might not be as good . . . I’m on week 5 now, I’ve just finished my fifth chemo[therapy session] out of the six and I’m still eating.
Craig (randomised to receive a nasogastric tube; initial interview), Woodville
When the time came for insertion, the experience could be unpleasant. Jonathan’s tube was dislodged on two occasions when he was sick: he described how, while the tube was in place, he had a ‘sickly’ feeling which was relieved when the tube was removed. Each time this necessitated another unpleasant insertion experience:
Yes, when it came out it after I was sick, the last time came out . . . A nurse put [the nasogastric tube] in there and she kept on pushing and pushing and the pain was horrendous. I was shaking and there were tears in my eyes. I said, ‘Stop, I can’t take any more pain,’ and she pulled it out and she went to the other nostril and she said, ‘I’ll try in there,’ and she put it down there and that hurt as well, but she got it down in that one. I do not know if she did any damage down inside, because my throat is swollen now and I can’t manage, it’s a job to swallow . . . Oh, it was, terrible. It was only for 5 minutes at the time, I forgot about it after then. The tube was down so I was back to normal, but at the time it was terrible.
Jonathan (randomised to receive a nasogastric tube), Queenstown
Jonathan’s account of the unpleasantness of nasogastric tube insertion was supported by that of Joseph, who had a strong preference for gastrostomy after a previous experience of having a nasogastric tube, and entered the TUBE trial as it was his best chance of receiving a gastrostomy in a site that otherwise had a preference for nasogastric tubes:
I says ‘I do martial arts’, I says ‘I’ve trained to endure things like that’ and when they started putting that tube up my nose, Jesus I wish I’d have been put to sleep . . . it is the only thing I can think of is like an alien creature crawling through your body, you know when you . . . see these films where there’s somebody dying and they see the little, the spiders and that crawling up their nose and in their mouth [. . .] it’s like a scary feeling really, it feels as if your body has been taken over by something.
Joseph (randomised to receive a gastrostomy), Woodville
In his second interview, Jonathan explained that he had a fear of tube dislodgement, stemming primarily from the process of tube insertion, an event that dominated his experience of treatment:
The only thing is I [was] self-conscious because I’d be twisting and turning at night. I had to pull on the side of the window and I had to watch that I didn’t – I’d only lie on one side, you know, because otherwise I would pull the tube out. I was subconsciously concerned about that.
Jonathan (randomised to receive a nasogastric tube), Queenstown
A concern raised frequently by patients regarding the nasogastric tube was the social stigma associated with a tube visible on the face. Jonathan explained how his nasogastric tube made him feel self-conscious in public:
Yes, I wouldn’t walk around the town because all the people were looking at you . . . I don’t give a damn, myself, but [it does] make you feel a little bit [conspicuous] . . . people ask you ‘Is that oxygen’ and I said, ‘No, no,’ and explained you know. People staring when they see you, and that sticking through your nose . . . I used to do it myself anyway it’s no wonder people look at me, I used to do it myself, ‘Oh look at that poor bugger tube in his nose’ you know. Anyway it’s happened to me now so I knew what to expect. It still makes you a bit dubious going out though.
Jonathan (randomised to receive a nasogastric tube), Queenstown
Contrary to the expectation that patients with a nasogastric tube would have a tube for only short periods of time and maintain oral feeding, Jonathan described how his tube was in place for ‘months and months’, and that during that time he relied totally on the tube for nutrition:
While you had the tube in, did you manage to eat as well while the tube was in?
I couldn’t eat anything round the outside. When the tube was in, everything went down the tube.
Everything went down the tube?
Everything; injections, morphine, paracetamol, everything went down the tube.
So you weren’t swallowing anything at all?
Water. I could sip some water, but that was about my limit then.
Jonathan summarised his experience with the nasogastric tube:
If I were to have it again I would be asking for the stomach one . . . If ever I’ve got to do it again, I will be demanding the stomach one. I don’t want to go through the nose one again.
Jonathan (randomised to receive a nasogastric tube), Queenstown
Clearly, we have very limited data on this topic. However, what we have supports health professionals’ perceptions that a nasogastric tube is more problematic for patients than a gastrostomy: insertion is more unpleasant, feeding takes longer and is more socially isolating and dealing with problems that occur is more demanding. There was some evidence that patients with gastrostomy did retain their tubes for longer. However, they described how they did consume food and fluids orally for taste, social and other reasons, using the gastrostomy to ‘top up’ otherwise inadequate oral feeding. Other factors, notably issues with tooth loss, may limit a return to full oral feeding in both patients with a nasogastric tube and those with a gastrostomy.
Economic analysis
Within-trial economic evaluation
Table 22 presents data on the utilisation of health-care services at 3 and 6 months post baseline. The percentage of patients utilising health services related to tube feeding, and the frequency of service utilisation was recorded.
| Health-care services utilisation | Time period | |||
|---|---|---|---|---|
| Baseline to 3 months | 3 months to 6 months | |||
| Pre-treatment gastrostomy tube cohort, n = 5 (SD) | Nasogastric tube tube cohort, n = 8 (SD) | Pre-treatment gastrostomy tube cohort, n = 7 (SD) | Nasogastric tube tube cohort, n = 7 (SD) | |
| Percentage of patients seen by a GP | 60% | 62.5% | 43% | 71% |
| Average number of visits to a GP (for those patients who had at least one visit) | 2.33 (1.15) | 3 (1.66) | 0.66 (1.15) | 0.33 (0.57) |
| Average number of visits by a GP (for those patients who had at least one visit) | 1 (–) | 2 (0) | 0 (–) | 0.6 (1.34) |
| Average number of telephone sessions with a GP | 0.6 (1.34) | 0.625 (0.74) | 0 (–) | 0 (–) |
| Percentage of patients seen by a nurse | 0% | 12.5% | 12.5% | 25% |
| Average number of appointments with a nurse (for those patients who had at least one visit) | – (–) | 6 (–) | 1 (–) | 2.5 (2.1) |
| Percentage of patients who had seen a physiotherapist | 0% | 0% | 0% | 0% |
| Average number of appointments with a physiotherapist (for those patients who had at least one visit) | 0 | 0 | 0 | 0 |
| Percentage of patients who had an appointment with another type of HCP | 60% | 75% | 25% | 25% |
| Average number of appointments with another type of HCP (for those patients who had at least one visit) | 4 (2.6) | 3.5 (2.1) | 3.5 (3.5) | 4 (1.4) |
| Utilisation of medication | Not recorded | Not recorded | Not recorded | Not recorded |
| Percentage of patients for whom dietetic consultations were required | 43% | 62.5% | 28% | 43% |
| Average number of dietetic consultations (for those patients who had at least one consultation session) | 1.42 (1.8) | 4.75 (5.9) | 0.43 (0.78) | 1 (1.5) |
| Percentage of patients for whom speech and language therapy consultations were required | 28% | 57% | 14.3% | 43% |
| Average number of speech and language therapy consultations (for those patients who had at least one consultation session) | 0.86 (1.46) | 2.71 (3.5) | 0.43 (1.1) | 0.71 (0.95) |
The percentage of patients who had a visit with their GP was approximately 60% for both cohorts after 3 months. During months 3–6, the percentages were 71% for the nasogastric tube cohort compared with 43% for the gastrostomy tube cohort. No appointments with a physiotherapist or occupational therapist were recorded in either trial arm. The percentage of patients who had an appointment with a district nurse, an occupational workplace visit for screening or a hospital-based nurse appointment was higher during the first 3 months of the study than during months 3–6. No patients were recorded as having had a private appointment.
Finally, the percentage of patients who utilised dietetic and/or speech and language consultations was higher in the nasogastric tube group than in the gastrostomy tube group. For those patients who had at least one consultation, the average number of consultations was higher among the nasogastric tube cohort.
In addition to health service utilisation, data on the number of tube reinsertions required in each trial arm were also recorded. Table 23 shows that the percentage of patients who required a reinsertion of the originally inserted tube among the nasogastric tube cohort was more than four times higher (62.5%) than among the gastrostomy tube cohort (14.3%) during the first 3 months of the feasibility trial. In addition, the average number of tube replacements was almost three times higher in the nasogastric tube cohort (2.8) than in the gastrostomy tube cohort (1.0) during the first 3 months. No tube reinsertions were recorded in months 3–6. Finally, 6 months after baseline, 38.6% of the gastrostomy tube cohort and 87.5% of the nasogastric tube cohort no longer needed nutritional support.
| Utilisation | Time period | |||
|---|---|---|---|---|
| Baseline to 3 months | 3 months to 6 months | |||
| Pre-treatment gastrostomy cohort (SD) | Nasogastric tube cohort (SD) | Pre-treatment gastrostomy cohort (SD) | Nasogastric tube cohort (SD) | |
| Percentage of patients requiring tube reinsertion | 14.3% | 62.5% | 0% | 0% |
| Average number of reinsertions (for those patients who had a tube replaced) | 1 (–) | 2.8 (2.3) | 0 (–) | 0 (–) |
| Feeding tube still on site (%) | 71.4 | 44 | 71.4 | 12.5 |
Economic modelling
A bespoke decision-analytic economic model was developed to estimate the costs, effects and relative cost-effectiveness of the two alternative methods of nutritional support. In addition to exploring cost-effectiveness, a subsequent aim of the economic model was to estimate the EVPI and the EVPPI; these pieces of information will be used to make an economic case for funding a definitive trial and to assist in the design of that trial. The results of the EVPI and EVPPI are discussed in the final section of the economic analysis.
An overview of the key characteristics of the cost-effectiveness analysis is shown in Box 2.
Intervention: pre-treatment (prophylactic) gastrostomy tube feeding.
Comparator: as-needed nasogastric tube feeding.
Population: cohort of head and neck cancer patients undergoing CRT who require either a pre-treatment gastrostomy tube or a nasogastric tube, as and when necessary, aged 60 years.
Time frame: 6-month time horizon; 1-month time cycle.
Perspective: NHS and PSS.
Effects: major complications and AEs associated with alternative forms of nutritional support.
Costs: costs associated with alternative forms of nutritional support, switching intervention because of non-adherence to treatment, treating insertion- and tube-related AEs and fatal events.
Outcomes: mortality, QoL and QALYs.
Assessment of cost-effectiveness: cost per additional QALY gained.
Model description
An economic model developed as a Markov model was built in TreeAge Pro® (TreeAge Software, Inc., Williamstown, MA, USA) to estimate the cost-effectiveness of gastrostomy tube feeding compared with nasogastric tube feeding. The population considered was patients with head and neck cancer undergoing CRT, assuming a cohort with an average age of 60 years, to represent the average characteristics of the population with this condition and requiring this type of nutritional support. The time horizon of the model was 6 months. A time cycle of 1 month was chosen, as it was felt that a short time period would be sufficient to capture all important costs and effects, and would also be necessary because of the rapidly changing nature of the health states of head and neck cancer patients receiving nutritional support. A lifetime time horizon was not possible because of insufficient long-term data. The model structure is shown in Figure 6.
FIGURE 6.
Markov model used to assess the cost-effectiveness of ‘pre-treatment gastrostomy tube feeding’ vs. ‘nasogastric tube feeding, as necessary’. G, gastrostomy, NGT, nasogastric tube.

The entire cohort began in the ‘nutritional support’ state, which is the point at which insertion took place. From this health state, patients moved along a pathway in which they could experience an insertion-related AE (gastrostomy tube only) and subsequently remain in this health state, die from other causes, experience a fatal or non-fatal major AE or end nutritional support. Determined by the events experienced during this initial pathway, patients moved to one of three subsequent health states, comprising ‘off nutritional support’ after removal of nutritional support as the result of a sufficient increase in independent nutritional intake, ‘nasogastric tube/gastrostomy tube’ because of non-adherence at the insertion stage requiring a switch to the alternative type of nutritional support and ‘dead’ following death from other causes or from a major insertion- or tube-related event, or they remained in the original health state. Once in the ‘off nutritional support’ health state, the patients either remained in this health state or died from other causes. When patients experienced non-adherence requiring a switch to the alternative type of nutritional support, the clinical pathways described were the same as in the main alternative intervention arm. Transition probabilities (probabilities of progressing from one stage of the model to the next or probabilities of transitioning to subsequent health states) were used to link the starting point of the Markov process to the end point.
The model was designed to estimate costs from the perspective of the NHS and PSS and outcomes in terms of QALYs gained in each arm of the model,71 with costs and QALYs accumulated dependent on the transitions between health states. QALYs were generated using health utilities, which are preference weights measured on a cardinal scale of 0–1 (whereby preference can be equated with desirability) combined with quantity of life-years. 71 The difference between the costs, incidence of health outcomes and impact on QoL and mortality between treatment options was used to estimate the incremental costs and effects of gastrostomy tube feeding. The model also attempted to incorporate second-order uncertainty by assigning distributions to a selection of input parameters. All costs were for a price year of 2014–15. A 6-month time horizon was adopted; therefore, discounting of costs and effects was not required.
Decision model parameters and probabilities
The parameters and sources used in this model are summarised in Tables 24–26. Given the range of data required to populate the model, a variety of approaches were used to identify parameter estimates.
First, a comprehensive review of the literature to identify clinical effectiveness and health-related QoL evidence was conducted using a systematic search strategy (Box 3). Second, a comprehensive review of existing economic evaluations in this area was conducted. However, no published economic evaluations were identified, and data from the literature to inform parameter estimates were sparse in areas. Therefore, when available, relevant data from the feasibility trial were also utilised. As a result of the clinical uncertainty in this area, a large number of data used in the model were also estimated based on expert opinion and assumptions.
The following databases were all searched on 29 April 2016:
-
MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R): 1946 to 29 April 2016 (via Ovid)
-
PubMed: 1946 to 29 April 2016
-
Database of Abstracts of Reviews of Effects (DARE): January to March 2016 (via Ovid)
-
Cochrane Database of Systematic Reviews (CDSR): 2005 to 27 April 2016 (via Ovid)
-
HTA: January to March 2016 (via Ovid)
-
NHS Economic Evaluation Database (NHS EED): January to March 2016 (via Ovid)
-
Cochrane Central Register of Controlled Trials (CENTRAL): March 2016 (via Ovid)
-
EMBASE: 1974 to week 17 2016 (via Ovid)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL): 1946 to 29 April 2016 (via EBSCOhost).
Four search facets were developed: (1) head and neck cancers, (2) feeding tubes, (3) dysphagia and (4) chemoradiation therapies. Free-text and thesaurus terms [e.g. medical subject headings (MeSH)] were used in all facets. Truncations and adjacency syntax were used as appropriate. In most databases, facets were combined as follows:
-
1 AND 2 AND 3
-
1 AND 2 AND 4
-
A OR B.
This allowed the best coverage when head and neck cancers and feeding tubes were used alongside patients’ experiences of dysphagia or of being treated with chemoradiation therapies. This was followed by a sample search strategy for MEDLINE.
For PubMed, DARE and the CDSR, searches were combined as 1 AND 2 only, owing to small numbers of results in dysphagia and chemoradiation being included.
A total of 2736 records were retrieved from the database searches, with 730 duplicates, resulting in a total of 1858 unique records.
The model and analysis should be considered exploratory. The base-case result is aimed to present the potential cost-effectiveness of gastrostomy tube feeding over a 6-month time horizon, given the base-case assumptions and data uncertainty. The values used in the sensitivity analyses aim to test all of the assumptions and present different scenarios to show when gastrostomy tube feeding may or may not be cost-effective. The results may be indicative of the potential cost-effectiveness of gastrostomy tube feeding under differing assumptions, but should not be considered robust or exhaustive.
The main sources of evidence to inform the transition probabilities required for the model were the existing systematic reviews, the relevant literature identified during protocol development and the literature identified during a focused comprehensive review. From these sources, information on the likelihood of key events described in the economic model was sought. Additional focused searches were conducted to identify the best available evidence relevant to the UK NHS for these probabilities. However, many of the clinical parameters were not known with any certainty; therefore, some of these values were estimated after discussion with clinical experts in the study team, and wide probability distributions were applied to these estimates to reflect the extent of uncertainty. The clinical parameter values can be found in Table 21.
Because of the numerous AEs associated with nutritional support, only AEs that could be considered cost drivers or as having the potential to lead to a decrement in patient utility were included in the model for both the gastrostomy tube and nasogastric tube cohorts. For gastrostomy tube patients, there was a possibility of experiencing an insertion-related AE, or an independently defined major (tube-related) AE. These probabilities were derived as follows: for the gastrostomy tube arm, insertion-related and major AEs identified in the literature,7,72 such as infection and intra-abdominal abscess, were considered. In these studies, a meta-analysis was conducted to estimate the post-procedure complication rate for both PEG and for radiologically inserted gastrostomy (RIG). Information from both meta-analyses was used to estimate the probability of a major event occurring. First, the probability of PEG patients experiencing a major AE was estimated using the number of patients who experienced a PEG-related AE in both meta-analyses and the number of patients (who had a PEG insertion) who were at risk of experiencing an AE. The same process was followed to estimate the probability of an AE for patients who had a RIG:
To estimate a weighted average probability of AEs for percutaneous gastrostomy insertions, the total activity of PEG and RIG insertions from NHS Reference Costs 2014 to 201573 was applied. In 2014–15, the number of RIGs and PEGs was 2635 and 5497, respectively. Therefore, the probability in cycle 1 of a major AE was estimated as 67.5% (PEG activity) × 8.93% + 32.5% (RIG activity) × 7.6% = 0.08. No studies reported monthly data for subsequent cycles; therefore, based on expert advice, a constant 1.5% was used to reflect the fact that there is a low complication rate in gastrostomy tube users in the months following the month of insertion.
For nasogastric tube patients, AEs were considered to be those that led to tube reinsertion. Most of the AEs associated with nasogastric tube usage were clinically minor, and the ones that drove costs were those that led to tube reinsertion, such as tube blockage, leakage and dislodgement. Based on data from the literature, the probability of having a tube dislodged was 0.52. 74 This probability was treated as a constant, as experiencing a nasogastric tube AE does not affect the probability of experiencing the same or relevant events in the future. Patients who switched from a gastrostomy tube to a nasogastric tube were assumed to have the same probability of experiencing an AE, based on expert opinion.
For gastrostomy tube patients, a 100% probability of retaining tube feeding (i.e. not switching to the alternative type of nutritional support as a result of non-adherence at the insertion stage) was assigned, based on expert opinion. However, for nasogastric tube patients, it was advised by clinical experts that a 10% chance of patients switching from nasogastric tube to gastrostomy tube feeding as a result of non-adherence would be realistic. This event was assumed to take place only shortly after the insertion procedure, and could not occur 1 month after the procedure. Consequently, it was assumed that, after the first cycle, the probability of retaining either the nasogastric tube or the gastrostomy tube was 100%.
For all patients who retained the gastrostomy tube, there was a possibility that they may not utilise nutritional support, in either the initial cycle or subsequent cycles of the Markov model. The proportion of patients who utilised gastrostomy tube feeding within the first 2 months following insertion was estimated at 74.61%, based on data from the literature. 75 In addition, the literature indicated that 7.26% of patients never utilised their inserted gastrostomy tubes. 75 Clinical experts advised that, after 3 months, the prophylactically inserted gastrostomy tube patients would have either utilised their feeding tube or had it removed. Combining the knowledge about the cumulative percentage of patients who would have commenced their feeding support within 2 months of the insertion (74.61%) and the percentage of patients who would never commence feeding support (7.26%), it was assumed, and supported by expert opinion, that the rest of the cohort (18.13%) would have started utilising their feeding tubes in the third cycle, as, after that point, every participant would have either commenced the feeding support or had the gastrostomy tube removed.
The probability of experiencing a fatal AE was informed by clinical experts. For the gastrostomy tube patients, the likelihood of a fatal event specifically related to gastrostomy tube insertion was estimated to be 0.015, based on expert clinical input. Based on this probability, it was expected that 1.5% of the cohort would experience death after the first month. However, because this fatal event probability was intertwined with the gastrostomy tube insertion-related AEs rather than the utilisation of the tube, this figure was transformed into a monthly probability, to reflect the fact that this probability was related to gastrostomy tube insertion-related AEs. The percentage of patients who experienced AEs 1 month post insertion onwards was 8%, and the percentage of those patients experiencing death within the first month was calculated as p (probability of death from an AE) × 8% (those who experienced an AE) = 1.5% (those who experienced insertion-related mortality after month 1). Using this equation, 0.19 was estimated to be the 1-month post-insertion mortality probability for those who had an AE.
In addition to insertion-related fatal events, a low probability of experiencing death from an AE was taken into account in the analysis. The methodology used to calculate the probability of fatal events related to gastrostomy tube utilisation was the same as above. The probability of a fatal event (0.05) per cycle was assigned to the patients who were experiencing a tube-related AE. As a result, an additional 0.38% of the cohort was expected to experience death as a result of gastrostomy tube-related AEs. Therefore, a table of probabilities of dying as a result of gastrostomy tube-related AEs was created and assigned a value of 0.19 for the first cycle and 0.05 for subsequent cycles, to account for the differing probabilities of dying as a result of insertion-related AEs and tube-related AEs. When these two values were applied to the proportion of the cohort that was experiencing AEs, this led to an overall mortality of the cohort of 1.8%, which was similar to the suggestion by clinical experts that overall 6-month mortality was 1.5–2%.
For nasogastric tube patients, it was assumed that the only event that led to death was the misplacement of the nasogastric tube, and this was considered a never event. However, a constant probability of 0.0001 was assigned to this parameter, as suggested by clinical experts. For patients who switched from a gastrostomy tube to a nasogastric tube, the same probabilities were assumed.
As only summary data were available from the literature, Kaplan–Meier diagrams were used to extract information on cumulative survival at each month. In order to ensure accuracy in the extraction of data, appropriate software was used. 9 The proportion of patients who were still receiving nutritional support S(u1+1) compared with those who were at risk of ending nutritional support at the beginning S(ui) of the cycle was calculated by using the cumulative survival in order to estimate the probability of ending nutritional support per cycle:
A table was created depicting the probabilities of ending nutritional support for patients who had a prophylactically inserted gastrostomy tube and who had reactively inserted nasogastric tubes and gastrostomy tubes (the term ‘reactively inserted gastrostomy tubes’ refers to nasogastric tubes inserted after patients initially received gastrostomy tube-feeding support). In addition, exponential models were fit using Stata® (StataCorp LP, College Station, TX, USA), to provide a constant rate of transition between different health states. The exponential model fit well in the 6-month data with an R2 of over 0.80 for 6-month data from all three Kaplan–Meier diagrams used. 76 Table 24 shows the monthly probabilities and exponential values derived by this method. Table 25 shows the base-case clinical data and assumptions included in the economic model.
| Cycle | Trial arm | ||
|---|---|---|---|
| NGT (Mekhail et al.,25 2001) (n = 29) | Prophylactically inserted gastrostomy tube (Williams et al.,77 2012) (n = 71) | Reactively inserted gastrostomy tube (Mekhail et al.,25 2001) (n = 62) | |
| 1 | 0.18 | 0.022 | 0.012 |
| 2 | 0.559 | 0.095 | 0.118 |
| 3 | 0.09 | 0.177 | 0.124 |
| 4 | 0.544 | 0.097 | 0.110 |
| 5 | 0.508 | 0.14 | 0.12 |
| 6 | 0.462 | 0.117 | 0.127 |
| Exponential model probability | 0.41 | 0.11 | 0.1025 |
| Probabilities | Value | Distribution | Source |
|---|---|---|---|
| AE for NGT patients | 0.52 | Beta (α = 2.166, β = 2) | Wang et al.,74 2014 |
| AE (insertion procedure related) for gastrostomy tube patients | 0.08 (first cycle); 0.00 (subsequent cycles) | – | Assumption from expert opinion |
| AE (tube related) for gastrostomy tube patients | 0.00 (first cycle); 0.015 (subsequent cycles) | – | Assumption from expert opinion |
| AE (insertion procedure related) following switch from NGT to gastrostomy tube | 0.00 (first cycle); 0.08 (second cycle); 0.00 (subsequent cycles) | – | Assumption from expert opinion |
| AE (tube related) following switch from NGT to gastrostomy tube | 0 (first and second cycles); 0.015 (subsequent cycles) | – | Assumption from expert opinion |
| AE following switch from gastrostomy tube to NGT | 0.52 | Beta (α = 2.166, β = 2) | Wang et al.,74 2014 |
| Death from other causes | 0.000281 | – | Statistics on death78 |
| Fatal event (insertion related) for gastrostomy tube patients | 0.19 (first cycle); 0 (subsequent cycles) | – | Assumption from expert opinion |
| Fatal event (tube related) for gastrostomy tube patients | 0 (first cycle); 0.05 (subsequent cycles) | – | Assumption from expert opinion |
| Fatal event for NGT patients | 0.0001 | Beta (α = 2, β = 19,998) | Assumption from expert opinion |
| Fatal event (insertion related) following switch from NGT to gastrostomy tube | 0 (first cycle); 0.19 (second cycle); 0 (subsequent cycles) | – | Assumption from expert opinion |
| Fatal event (tube related) following switch from NGT to gastrostomy tube | 0 (first and second cycle); 0.05 (subsequent cycles) | – | Assumption from expert opinion |
| Fatal event following switch from gastrostomy tube to NGT | 0.0001 | Beta (α = 2, β = 19,998) | Assumption from expert opinion |
| Patients utilising gastrostomy tube, ending nutritional support | 0.11 | Beta (α = 2, β = 16.1818) | Williams et al.,77 2012 |
| Patients utilising NGT, ending nutritional support | 0.42 | Beta (α = 2, β = 2.761) | Mekhail et al.,25 2001 |
| Ending nutritional support following switch from NGT to gastrostomy tube | 0.1025 | Beta (α = 2, β = 17.512) | Mekhail et al.,25 2001 |
| Patients not utilising gastrostomy tube, ending nutritional support | 0.0248 (first three cycles); 0 (subsequent cycles) | – | Brown et al.,79 2014 |
| Patients not utilising gastrostomy following switch from NGT, ending nutritional support | 0 (first cycle); 0.0248 (second to fourth cycles); 0 (subsequent cycles) | – | Brown et al.,79 2014 |
| Retaining gastrostomy tube | 1 | – | Assumption from expert opinion |
| Retaining NGT | 0.9 (first cycle); 1 (subsequent cycles) | – | Assumption from expert opinion |
| Utilising gastrostomy tube | 0.49 (first cycle); 0.53 (second cycle); 0.7 (third cycle); 1 (subsequent cycles) | – | Crombie et al.,75 2015 and assumption from expert opinion |
| Utilising gastrostomy tube following switch from NGT | 1 | – | Assumption from expert opinion |
Mortality
In the age category as identified in the demographics of the study population, life tables were used to determine the probability of death for individuals in this age category. As the model time horizon was only 6 months, an assumption was made that the probability of death in each month would remain constant over the 6-month period. The monthly probability of death was estimated based on data on the annual mortality rate for patients in this age group, provided by the Office for National Statistics. 78 It was assumed that the risk of death from other causes was constant, irrespective of health state.
Resource use and costs
Information on the precise description of the resources required for each intervention was partially based on data derived from the feasibility study, augmented when necessary by members of the study group and published economic literature. From all participants recruited into the trial, resource use data were collected and costs were estimated for each method of nutritional support. Paucity of data led to other sources, as outlined above, being utilised. Unit costs were taken from appropriate routine sources, such as NHS Reference Costs 2014 to 2015,73 PSSRU80 and the British National Formulary81 for medication.
For the cost of a gastrostomy tube insertion, the non-elective short stay costs of endoscopic and radiologic insertion of a gastrostomy tube were used. The Healthcare Resource Group (HRG) code for PEG for adults is Z93A, with activity of 5497 procedures in total, whereas, for RIG, the HRG code was YF01A with a corresponding total activity of 2635. Based on the total activity of these two HRG codes reported by the NHS Reference Costs 2014 to 201573 for the specific year, 68% of the gastrostomy tube insertions were done using the percutaneous endoscopic technique. A weighted average cost was, thus, estimated. In addition to the actual insertion, patients receive information about the tube the day after the procedure, by a dietitian or a nurse specialist, for approximately 30 minutes. Insertion and education costs were combined to derive a total gastrostomy tube insertion cost of £777.
The nasogastric tube insertion procedure is less complex. The cost of intermediate upper gastrointestinal tract procedures for adults in an outpatient setting was used to inform the value of a nasogastric tube insertion cost (£167). 73 The same cost was used for nasogastric tube-related AEs (e.g. tube reinsertion). AEs associated with the gastrostomy tube are related to insertion complications and major events such as infection, and are more severe than those associated with the nasogastric tube. Clinical experts advised that AEs (both insertion-related and major events) associated with the gastrostomy tube would require an average of 4 additional days in hospital for monitoring of the patient and/or treatment of the adverse effect, and costs were estimated accordingly.
Patients who have enteral feeding tubes inserted need to be monitored to ensure that there is no tube misplacement and that the tube is functioning properly. It was assumed that monthly monitoring costs would be the same in both arms of the model, and the costs were based on a weekly 30-minute hospital dietitian-led appointment. The monthly cost of nutritional intake was also estimated based on information from the literature,82 and was assumed to be the same in both arms of the model. The cost of a tube removal was estimated based on expert clinical input. It was advised that the process takes 10 minutes, on average, for nasogastric tube removal and 20 minutes, on average, for gastrostomy tube removal, and that this procedure would be conducted by a nurse. It was assumed that patients dying as a result of an AE would receive 4 days of specialist inpatient palliative care. The cost of medical specialist palliative care for adults over 4 days (£482) was included in the model. 73 The perspective adopted for the model was that of the NHS/PSS, and a price-year of 2014–15 was applied. The unit costs included in the model can be found in Table 26.
| Unit cost | Value (£) | Distribution | Source |
|---|---|---|---|
| Gastrostomy insertion- and tube-related AE | 1183 | Gamma (α = 15.99974758, β = 73.95116667) | Assumption from expert opinion and NHS Reference Costs 2014 to 201573 |
| NGT-related AE | 167 | Gamma (α = 56.25, β = 2.968888889) | Assumption from expert opinion and NHS Reference Costs 2014 to 201573 |
| NGT-related fatal event | 482 | Gamma (α = 35.70309405, β = 13.50023052) | Assumption from expert opinion and NHS Reference Costs 2014 to 201573 |
| Gastrostomy insertion- and tube-related fatal event | 482 | Gamma (α = 35.70309405, β = 13.50023052) | Assumption from expert opinion and NHS Reference Costs 2014 to 201573 |
| Insertion of gastrostomy tube | 777 | Gamma (α = 36, β = 21.58333333) | NHS Reference Costs 2014 to 2015 73 |
| Insertion of NGT | 167 | Gamma (α = 56.25, β = 2.968888889) | NHS Reference Costs 2014 to 2015 73 |
| Monitoring for gastrostomy tube patients | 68 | Gamma (α = 36, β = 1.888888889) | Assumption from expert opinion and Unit Costs of Health and Social Care 201580 |
| Monitoring for NGT patients | 68 | Gamma (α = 36, β = 1.888888889) | Assumption from expert opinion and Unit Costs of Health and Social Care 201580 |
| Nutrition for gastrostomy tube patients | 385 | Gamma (α = 99.45488603, β = 3.874822197) | CALORIES trial82 |
| Nutrition for NGT patients | 385 | Gamma (α = 99.45488603, β = 3.874822197) | CALORIES trial82 |
| Removal of gastrostomy tube | 29 | – | Assumption from expert opinion and Unit Costs of Health and Social Care 201580 |
| Removal of NGT | 15 | – | Assumption from expert opinion and Unit Costs of Health and Social Care 201580 |
Estimation of quality-adjusted life-years
For the cost–utility analysis, estimates of clinical effectiveness and cost-effectiveness were expressed in QALYs. 71 For each health state, a health state utility was defined. A focused search to identify utility data, including a search on the Cost-Effectiveness Analysis Registry, was conducted; however, no useable data were identified, with the exception of baseline utility values. 83 Baseline utility values for patients with head and neck cancer undergoing CRT, and experiencing low-severity CRT AEs, such as dysphagia, mucositis and xerostomia, were sourced from the literature83 and utilised in the model.
The other utility estimates used within the model were based on strong assumptions and best-guess estimates from clinical experts. These assumptions are discussed in a later section. All utility values included in the model were transformed to reflect the time cycle of the model and to ensure that the relevant output parameters were presented in terms of QALYs. Baseline utility values and utility decrements can be seen in Table 27. The lack of utility data, along with other data limitations, means that the economic evaluation should be considered an exploratory analysis, rather than a definitive assessment of cost-effectiveness.
| Event/health state | Value | Distribution | Source |
|---|---|---|---|
| Non-adherence to gastrostomy tube, switch to NGT | –0.01 | Uniform distribution (min.: 0.0075; max.: 0.0125) | Assumption |
| Non-adherence to NGT, switch to gastrostomy tube | –0.01 | Uniform distribution (min.: 0.0075; max.: 0.0125) | Assumption |
| Utilising gastrostomy tube | –0.05 | Uniform distribution (min.: 0.03825; max.: 0.06375) | Assumption |
| Gastrostomy insertion- and tube-related AE | –0.04 | Uniform distribution (min.: 0.0025; max.: 0.0042) | Assumption |
| NGT-related AE | –0.01 | Uniform distribution (min.: 0.0075; max.: 0.0125) | Assumption |
| ‘Nutritional support’ gastrostomy tube patients | 0.65 | Uniform distribution (min.: 0.48675; max.: 0.81125) | Assumption |
| ‘Nutritional support’ nasogastric patients | 0.60 | Uniform distribution (min.: 0.45; max.: 0.75) | Assumption |
| Switch to gastrostomy tube | 0.625 | Uniform distribution (min.: 0.46875; max.: 0.7813) | Assumption |
| Switch to NGT | 0.608 | Uniform distribution (min.: 0.456; max.: 0.76) | Assumption |
| ‘Off nutritional support’ | 0.659 | Uniform distribution (min.: 0.49425; max.: 0.82375) | NHS Direct12 |
Model assumptions
Owing to the limited research in this clinical area, and the difficulties experienced in patient recruitment for the associated feasibility trial, a number of assumptions were made regarding the model structure and input parameters.
Model structure
The two arms of the model represented the decision under evaluation for the population of interest, that is, gastrostomy tube or nasogastric tube feeding for head and neck cancer patients with < 75% of nutritional intake. The model pathways for both arms were almost identical, with the exception that the gastrostomy tube arm allowed for patients to not utilise feeding support. The gastrostomy tube was inserted prophylactically, so non-utilisation was clinically feasible, whereas the nasogastric arm (in which the nasogastric tube was inserted reactively) assumed that all patients utilised feeding support.
Furthermore, the gastrostomy tube arm also defined an insertion-related AE separately from a major AE related to an inserted tube. The nasogastric tube arm, because of the fact that major AEs were considered never events, considered only minor insertion-related AEs; all events that led to tube reinsertion were considered and grouped together to inform this parameter. Owing to these small differences, the gastrostomy tube arm involved greater structural complexity than the nasogastric tube arm, although the general pathways followed by patients were the same, and the health states that patients potentially entered were identical.
Input parameters
Cost data
The model assumed that all patients received an initial insertion (nasogastric tube or gastrostomy tube) and incurred the associated insertion costs. In addition, patients also received regular monitoring while receiving nutritional support, presented as a monthly cost. It was assumed that the feeding tube was removed immediately on completion of nutritional support, and the associated cost of removal was captured.
Clinical data
The potential to switch treatment because of non-adherence at initial insertion was captured in the model for both arms. However, based on expert opinion, this would not be a possibility for the gastrostomy tube cohort, as, clinically, issues with adherence to the pre-treatment gastrostomy tube are extremely rare. Therefore, patients in the gastrostomy tube arm could not switch to the alternative type of treatment because of non-adherence. In the nasogastric tube arm, it was assumed that 10% of patients switched treatment because of non-adherence at initial insertion, based on expert opinion. It was assumed that, if a patient experienced initial non-adherence and required a switch in treatment, these patients would utilise the alternative form of nutritional support.
For the pre-treatment gastrostomy tube arm, the model assumed that AEs were related to an event at insertion or a major tube-related event, such as an infection. It was assumed that, if patients experienced an insertion-related AE in the first cycle, another major AE would not be experienced during this same cycle. In addition, as insertion occurred at the starting point, it was assumed that an insertion-related AE could be experienced only in the first cycle. It was assumed that all gastrostomy tube patients who utilised nutritional support would have commenced feeding within 3 months of insertion, based on expert clinical input. For the nasogastric tube cohort, only AEs that led to a tube reinsertion were included in the model.
Utility data
Owing to the limited utility data for this patient population from both the trial and the literature, utility estimates for the different health states and utility decrements for AEs, presented in Table 27, were largely based on expert opinion and assumptions. The base-case model assumed that the baseline utility values were greater for patients in the gastrostomy tube arm than for those in the nasogastric tube arm owing to the fact that the gastrostomy tube patients received their nutritional support prophylactically and may not have actually required feeding assistance, and owing to clinical advice that patients may be more averse to the visual drawbacks of a nasogastric feeding tube. The baseline utility values for patients ending nutritional support were assumed to be the same in both arms of the model.
To capture the utility decrement that would probably occur because of the placement of a feeding tube in the gastrostomy tube arm, a small decrement was assigned based on the original utility values for the two cohorts. An assumption was made that the difference in the baseline utility values between gastrostomy tube and nasogastric tube patients receiving nutritional support resulted from the fact that nasogastric tube patients would immediately be utilising nutritional support, and, thus, would have a lower baseline level of utility, whereas the gastrostomy tube patients would not.
For those patients who switched from a gastrostomy tube to a nasogastric tube, the baseline utility values were assumed to be marginally higher than the baseline utilities for patients in the main nasogastric tube arm, to reflect the fact that a proportion of the patients switching treatment would not necessarily be in a position where nutritional support was required, despite the model assumption that patients switching from a gastrostomy tube to a nasogastric tube would utilise nutritional support. For those who switched from a nasogastric tube to a gastrostomy tube, the baseline utilities were marginally lower than the baseline utilities for patients in the main gastrostomy tube arm, to reflect the fact that these patients would now be receiving the gastrostomy tube reactively and were guaranteed to utilise tube feeding. It was assumed that gastrostomy tube AEs were more severe than nasogastric tube events on the basis of expert opinion.
A large number of these model assumptions were varied in the sensitivity analyses.
Deterministic sensitivity analysis
A sensitivity analysis was performed to determine the impact of changing key parameters on the model results. Therefore, many of the model parameters were subject to one- and two-way sensitivity analyses, using hypothetical increases or decreases, to determine the key drivers of the model results. In addition, threshold values were explored when data were missing by varying estimates through a range thought possible (based on the advice of the stakeholders involved in the study). Deterministic sensitivity analyses were also carried out to test for the effect of assumptions and variability. The majority of the clinical parameter values were varied extensively in the sensitivity analysis.
Assessment of cost-effectiveness
The analysis was designed to generate the cost per additional QALY15 gained from gastrostomy tube feeding. When available, data were entered into the model as distributions in order to fully incorporate the uncertainty around parameter values, so that a PSA could be undertaken. In decision modelling, many of the parameter values are often estimated with a degree of uncertainty. There is a need to propagate the joint parameter uncertainty in terms of decision uncertainty, and, thus, distributions are assigned to parameter values. Relevant distributions were informed by the systematic reviews and meta-analyses, additional literature and expert opinion. The PSA was run with 1000 simulations and cost-effectiveness planes and acceptability curves were produced in order to identify the probability of gastrostomy tube feeding being cost-effective across a range of willingness-to-pay (WTP) thresholds. Estimation of costs and QALYs were calculated as the expectation over the joint distribution of the parameters. This quantification of decision uncertainty also provided the starting point for assessing the value of additional research.
Results of the cost-effectiveness analysis
This section presents the results of the cost-effectiveness analysis. The base-case cost-effectiveness analysis results should be interpreted with caution, given the assumptions made in estimating many of the parameter values.
The base-case results shown in Table 28 indicate that the intervention of gastrostomy tube feeding is absolutely dominated, that is, it is costlier and less effective than nasogastric tube feeding. A number of assumptions were made in the estimation of the input parameters, largely around utility values, which led to this result. The results from the PSA (Figure 7) show that the largest number of points are in the north-west quadrant, indicating that the intervention of gastrostomy tube feeding is likely to be costlier, and less effective, than the comparator. A very small number of these points are below the WTP threshold value of £20,000 per additional unit of effectiveness gained, indicating that the intervention is unlikely to be cost-effective. The cost-effectiveness acceptability curve shown in Figure 8 indicates that the intervention has a low probability (maximum 37%) of being cost-effective at all of the WTP values presented, whereas Table 29 presents the output from the cost-effectiveness acceptability curve and the likelihood of the intervention being cost-effective at WTP threshold values of £10,000–40,000.
| Strategy | Cost (£) | QALY | ICER (cost per QALY) (£) | ||
|---|---|---|---|---|---|
| Mean | Difference | Mean | Difference | ||
| Nasogastric tube, as necessary | 1617 | – | 0.32 | – | – |
| Prophylactic gastrostomy tube | 2690 | 1073 | 0.3 | –0.01 | Dominated |
FIGURE 7.
Cost-effectiveness plane of ‘pre-treatment gastrostomy tube’ vs. ‘nasogastric tube feeding as necessary’: base-case analysis.
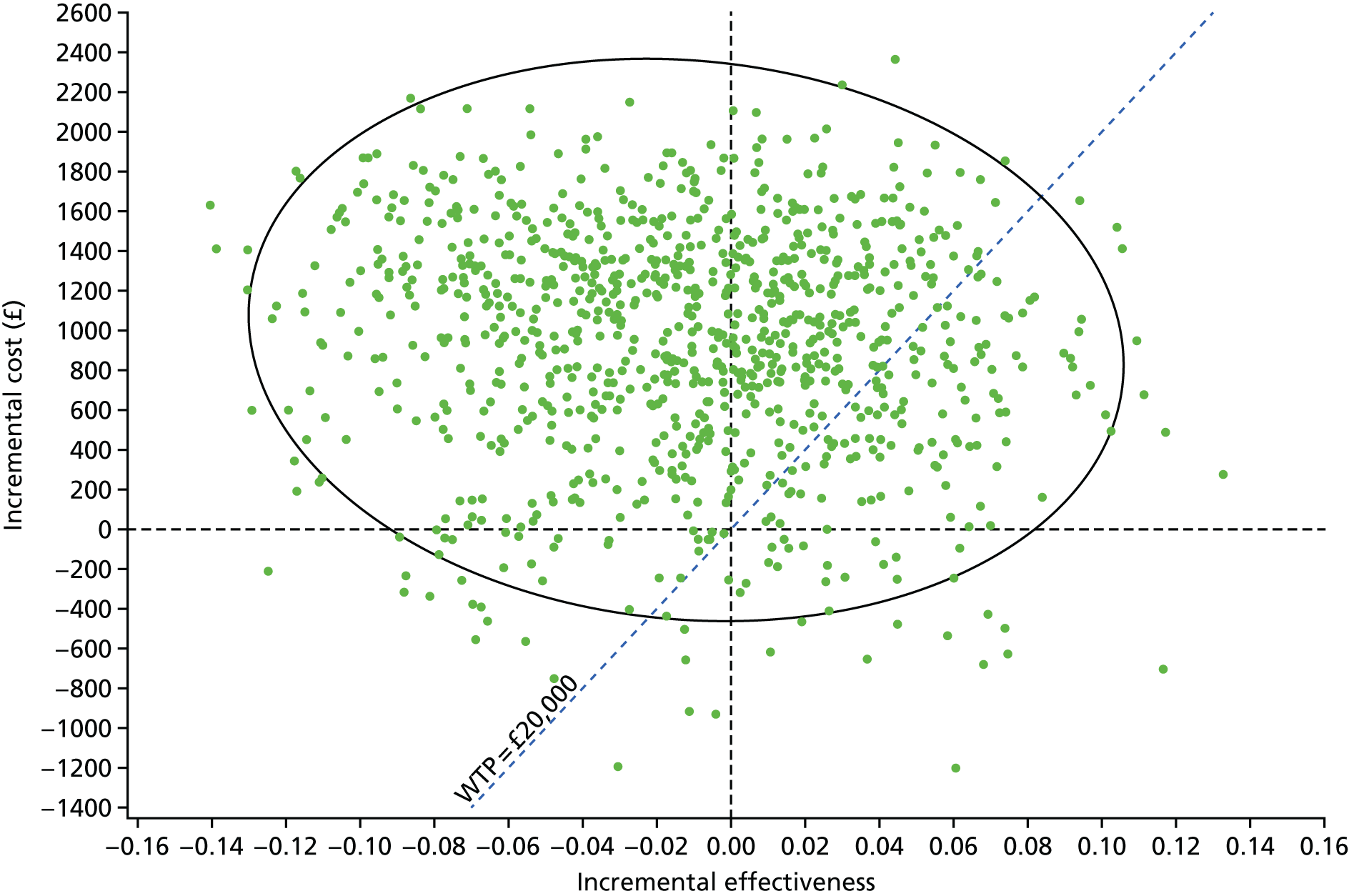
FIGURE 8.
Cost-effectiveness acceptability curve of ‘pre-treatment gastrostomy tube’ vs. ‘nasogastric tube, as necessary’: base-case analysis. NGT, nasogastric tube.
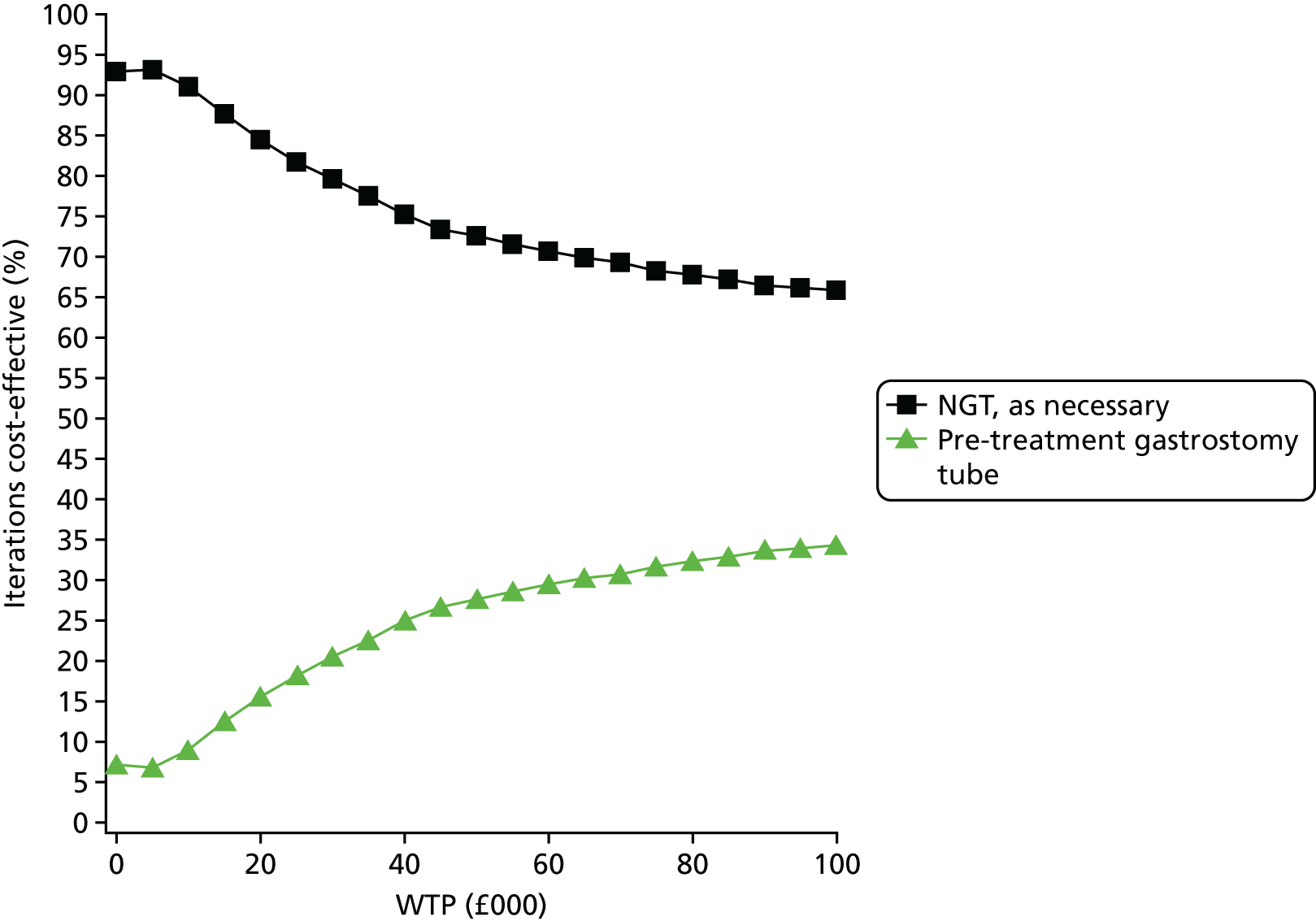
| Intervention | Mean | Probability of being cost-effective at a WTP threshold of (%) | ||||
|---|---|---|---|---|---|---|
| QALYs | Cost (£) | £10,000 | £20,000 | £30,000 | £40,000 | |
| Gastrostomy tube | 0.3 | 2708 | 8.3 | 16.6 | 21.5 | 26.6 |
| Nasogastric tube | 0.31 | 1748 | 91.7 | 83.4 | 78.5 | 73.4 |
Sensitivity analysis
Tables 29 and 30 present the results of the one-way sensitivity analysis of key model parameters, in which each variable was varied with all other parameters fixed at base-case values.
Very few of the clinical variables changed the overall results, and gastrostomy tube feeding remained a dominated strategy in the majority of cases (Table 30). Increasing the probability of an AE in the nasogastric tube arm did not change the decision, even when extreme values were used. However, when the probability of a fatal event in the nasogastric arm was increased radically (0.9), the incremental cost-effectiveness ratio (ICER) indicated that a gastrostomy tube was cost-effective at a WTP threshold of £20,000 per QALY gained. However, this was an unlikely clinical scenario. Adjusting variables associated with ending nutritional support, in both arms, could also have an impact on the decision. In the gastrostomy tube arm, when this probability was changed to an extreme value (0.7), using the gastrostomy tube became the dominant strategy. Similarly, lowering this probability dramatically (0) in the nasogastric arm resulted in use of the gastrostomy tube becoming dominant. However, again, these are unlikely clinical scenarios.
| Results | Cost difference (£) vs. NGT feeding | QALY difference vs. NGT feeding | ICER (£) for ‘prophylactic gastrostomy tube’ (cost per QALY) |
|---|---|---|---|
| Base-case result | 1073 | –0.01 | Dominated |
| Sensitivity analysis | |||
| Probability of AE for NGT patients | |||
| 0 | 1252 | –0.01 | Dominated |
| 1 | 908 | –0.01 | Dominated |
| Probability of fatal nasogastric event | |||
| 0.1 | 1089 | 0.01 | 109,391 |
| 0.3 | 1121 | 0.05 | 23,178 |
| 0.9 | 1199 | 0.14 | 8764 |
| Probability of retaining the NGT (month 1) | |||
| 1 | 1272 | –0.01 | Dominated |
| Probability of retaining the gastrostomy tube | |||
| 0.2 | 679 | –0.001 | Dominated |
| 0.7 | 842 | –0.001 | Dominated |
| Monthly probability of utilising gastrostomy tube feeding | |||
| 1 | 1379 | –0.01 | Dominated |
| Probability of ending nutritional support: gastrostomy tube arm | |||
| 0.5 | 214 | –0.01 | Dominated |
| 0.65 (extreme) | 36 | 0.001 | 111,445 |
| 0.7 (extreme) | –11 | 0.001 | Dominant |
| Probability of ending nutritional support: NGT arm | |||
| 0 | –715 | 0.01 | Dominant |
| 0.15 | 174 | –0.001 | Dominated |
Table 31 presents the result of the sensitivity analysis around the cost parameters. In the majority of cases, decreasing costs associated with the gastrostomy tube arm, or increasing costs associated with the nasogastric tube arm, did not change the overall results. The intervention remained dominated in most instances. However, when the cost of a nasogastric tube-related AE was increased dramatically (to £1200), nasogastric tube feeding became the more expensive option over the 6-month time horizon, although it was still more clinically effective. Similarly, increasing the cost of a nasogastric tube to an extreme value (£1500) meant that nasogastric tube feeding would now be a more expensive option, although it would still be more clinically effective. The large difference in cost between the two interventions in the base-case analysis meant that the decision was unlikely to change unless extreme values, which were unlikely, were used.
| Results | Cost difference (£) vs. NGT feeding | QALY difference vs. NGT feeding | ICER (£) for pre-treatment gastrostomy (cost per QALY) |
|---|---|---|---|
| Base-case result | 1073 | –0.01 | Dominated |
| Sensitivity analysis | |||
| Cost (£) of AE: gastrostomy | |||
| 5000 | 1513 | –0.01 | Dominated |
| 0 | 937 | –0.01 | Dominated |
| Cost (£) of AE: NGT | |||
| 450 | 770 | –0.01 | Dominated |
| 1100 | 74 | –0.01 | Dominated |
| 1200 | –33 | –0.01 | 2928 |
| Cost (£) of fatal event: gastrostomy | |||
| 0 (extreme value) | 1066 | –0.01 | Dominated |
| Cost (£) of fatal event: NGT | |||
| 5000 (extreme value) | 1073 | –0.01 | Dominated |
| Cost (£) of insertion: gastrostomy | |||
| 0 (extreme value) | 374 | –0.01 | Dominated |
| Cost (£) of insertion: NGT | |||
| 1200 (extreme value) | 40 | –0.01 | Dominated |
| 1500 (extreme value) | –260 | –0.01 | 22,979 |
In addition, the base-case model assumed that 100% of patients retained the gastrostomy tube, that is, no one experienced non-adherence. A variable was created to explore the impact that changing this value to identical values, as in the nasogastric tube arm (i.e. 10% experiencing non-adherence in month 1), would have on results. Gastrostomy was still dominated by using the nasogastric tube, although it became less expensive in relation to a nasogastric tube, as the probability of switching in month 1 was increased. The results of this one-way sensitivity analysis are presented in Table 32.
| Results | Cost difference (£) vs. NGT feeding | QALY difference vs. NGT feeding | ICER (£) for pre-treatment gastrostomy (cost per QALY) |
|---|---|---|---|
| Base-case result | 1073 | –0.01 | Dominated |
| Sensitivity analysis | |||
| Probability of retaining gastrostomy tube | |||
| Month 1/2/3/4/5/6: 0.9/1/1/1/1/1 | 1030 | –0.01 | Dominated |
| Month 1/2/3/4/5/6: 0.5/1/1/1/1/1 | 856 | –0.01 | Dominated |
It is evident from the base-case one-way sensitivity analyses of clinical and cost parameters that, in most instances, the decision was unlikely to change unless extreme, or unlikely, estimates were used. It should be noted that these base-case results are based largely on assumed clinical parameters in both arms of the model, assumed baseline utility values and assumed utility decrements caused by AEs. In order to explore the uncertainty in the utility estimates, a one-way sensitivity analysis of baseline utility values was conducted, and the results are presented in Table 33. Results indicate that the cost-effectiveness decision was not highly sensitive to variance in the baseline utility values and that the intervention of gastrostomy tube feeding was still not likely to be cost-effective even when the base-case utility assumptions were varied. Although many of the variations resulted in a pre-treatment gastrostomy tube becoming more effective than the comparator over the 6-month period, this difference in effectiveness is so marginal, and the cost difference so great, that the ICER is still not below the WTP threshold of £20,000 per QALY gained in any of the one-way analyses conducted.
| Results | Cost difference (£) vs. NGT feeding | QALY difference vs. NGT feeding | ICER (£) for pre-treatment gastrostomy (cost per QALY) |
|---|---|---|---|
| Base-case result | 1073 | –0.01 | Dominated |
| Sensitivity analysis | |||
| Baseline utility values of NGT arm ‘nutritional support’ | |||
| Increased to the equivalent of the gastrostomy arm | 1073 | –0.02 | Dominated |
| Baseline utility values of gastrostomy arm ‘nutritional support’ | |||
| Increased baseline utility values by 0.1 (each month) | 1073 | 0.03 | 36,890 |
| Baseline utility values of NGT arm ‘nutritional support’ | |||
| Decreased baseline utility values by 0.1 (each month) | 1073 | 0.01 | 161,751 |
| Baseline utility values of going ‘off nutritional support’ | |||
| Decreased baseline utility values by 0.1 (each month) | 1073 | 0.01 | 127,207 |
| Utility decrement of utilising the gastrostomy tube | |||
| Assuming there is no utility decrement associated with utilising the gastrostomy tube | 1073 | 0.001 | 252,139 |
| Utility decrement associated with a NGT-related AE | |||
| Ten times higher than the original NGT-related AE decrement | 1073 | –0.001 | Dominated |
Two-way sensitivity analyses were also undertaken to assess the impact of changing two key variables at the same time (Table 34). As there was uncertainty in characterising the length of time for which patients utilised tube feeding, a two-way sensitivity analysis was conducted to examine the joint effect of varying the probabilities of participants, in both cohorts, ending nutritional support. Data from Williams et al. 77 suggest that the cycle probability of ending nutritional support for nasogastric tube users could be 15–20% per cycle, rather than the 42% estimated by the Kaplan–Meier curves published by Mekhail et al. ,25 and, therefore, there was scope to explore the impact that varying these values would have on the overall results. When the probability of ending nutritional support in the gastrostomy tube arm was increased (0.15) and the probability of ending nutritional support in the nasogastric tube arm was decreased (0.12), using the gastrostomy tube was the dominant strategy. When both variables were given a value of 0.2, using the gastrostomy tube was the dominated strategy. This remained the case as both variable values were gradually increased.
| Probability of ending nutritional support for gastrostomy tube patients | Probability of ending nutritional support for NGT patients | Cost difference vs. NGT feeding (£) | QALY difference vs. NGT feeding | ICER (£) for pre-treatment gastrostomy (cost per QALY gained) |
|---|---|---|---|---|
| 0.15 | 0.12 | –100 | 0.001 | Dominant |
| 0.20 | 0.16 | –45 | 0.001 | Dominant |
| 0.20 | 0.20 | 131 | –0.001 | Dominated |
| 0.26 | 0.22 | 61 | –0.001 | Dominated |
| 0.30 | 0.30 | 250 | –0.001 | Dominated |
| 0.30 | 0.42 | 566 | –0.01 | Dominated |
Finally, a PSA was undertaken to further explore the parameters that appeared to have the potential to have an impact on the overall decision. Parameters that had the potential to have an impact on results were the cost of nasogastric tube insertion, the cost of nasogastric tube-related events and the probability of ending nutritional support in both arms of the model. A worst-case scenario for the nasogastric tube arm was explored by changing three variables in this arm to extreme values: the cost of nasogastric tube insertion was increased to £500, the cost of a nasogastric tube-related AE was increased to £500 and the probability of ending nutritional support in the nasogastric tube arm was more than halved to 0.16. The results presented in Figure 9 show that the majority of points are located in the south-east and south-west quadrants, indicating that the intervention of gastrostomy tube feeding was likely to be less costly than the comparator, following the increase in costs to the nasogastric tube arm. There appears to be a greater level of uncertainty around clinical effectiveness following the variation of these parameters. This would suggest that the probability of ending nutritional support in the nasogastric tube arm is a strong driver of results. In this PSA, the majority of points were below the WTP threshold value of £20,000 per QALY gained, indicating that gastrostomy tube feeding was likely to be cost-effective. Similarly, the results presented in Figure 10 and Table 35 indicate that gastrostomy tube feeding has an approximate 74% chance of being cost-effective at a WTP threshold of £20,000, and a high percentage chance of being cost-effective at all realistic WTP thresholds, albeit with results converging as WTP increases.
FIGURE 9.
Cost-effectiveness plane of ‘pre-treatment gastrostomy tube’ vs. ‘nasogastric tube feeding, as necessary’: worst-case scenario for ‘nasogastric tube feeding, as necessary’.

FIGURE 10.
Cost-effectiveness acceptability curve of ‘pre-treatment gastrostomy tube’ vs. ‘nasogastric tube, as necessary’: worst-case scenario for ‘nasogastric tube feeding, as necessary’. NGT, nasogastric tube.

| Intervention | Mean | Probability of being cost-effective at a WTP threshold of (%) | ||||
|---|---|---|---|---|---|---|
| QALYs | Cost (£) | £10,000 | £20,000 | £30,000 | £40,000 | |
| Gastrostomy | 0.30 | 2702 | 84.5 | 72 | 65 | 61 |
| Nasogastric tube | 0.31 | 3424 | 15.5 | 28% | 35 | 39 |
Results for the expected value of perfect information and the expected value of partial perfect information
Base-case analysis
Expected value of perfect information
Table 36 presents the individual level EVPI for all model parameters across a range of WTP thresholds for the base-case cost-effectiveness analysis. This value can be interpreted as the incremental net monetary benefit per patient, given perfect information and the removal of uncertainty around all model parameters. Another way of interpreting this is as the expected cost of the uncertainty of the decision made in the base-case cost-effectiveness analysis or the expected opportunity loss. This value ranges from £38 at a WTP threshold of £10,000 to £294 at a WTP threshold of £40,000. There is a positive relationship between the individual-level EVPI and the WTP threshold, indicating that, as the WTP threshold increases, the degree of uncertainty around the original decision also increases.
| Weight on effectiveness (WTP) (£) | EVPI (incremental net monetary benefit) (£) | Average incremental cost (£) with perfect information | Average incremental effectiveness with perfect information | Optimal strategy |
|---|---|---|---|---|
| 10,000 | 38 | –1 | 0.004 | Nasogastric tube |
| 20,000 | 98 | 75 | 0.009 | Nasogastric tube |
| 30,000 | 187 | 134 | 0.011 | Nasogastric tube |
| 40,000 | 294 | 188 | 0.013 | Nasogastric tube |
Figure 11 presents the population EVPI (4500 patients per year) across a range of WTP thresholds for four different time horizons, based on the period of time over which the information gained from further research would be useful. At a WTP threshold of £20,000, based on the estimated number of patients in this population utilising nutritional support, the cost of further research should not exceed £4.2M (10-year time horizon), £6.8M (20-year time horizon), £11.6M (64-year time horizon) or £13.1M (unbounded time horizon) in order for additional investigation to be worthwhile.
FIGURE 11.
Population EVPI vs. WTP: base-case analysis.

Expected value of partial perfect information
It is difficult to perform research to obtain perfect information for all parameters in the model. Instead, through the EVPPI, we can examine the particular parameters, or groups of parameters, for which the value of information is greatest in terms of reducing uncertainty. The EVPPI for the set of probability (clinical), cost and utility parameters was estimated at an individual level for the base-case cost-effectiveness analysis (see Tables 37–39) to identify the group that represented the most important source of uncertainty.
In Table 37 we see that the removal of uncertainty around the probability parameters led to an individual-level EVPPI of £23–87 for WTP thresholds of £10,000–40,000. For cost parameters, this value was higher than £0 for WTP thresholds above £40,000 (Table 38). Finally, the EVPPI for utility parameters for the base-case analysis ranges from £0.35 to £264 across all WTP thresholds presented (Table 39).
| Weight on effectiveness (WTP) (£) | EVPPI (incremental net monetary benefit) (£) | Average incremental cost (£) with perfect information | Average incremental effectiveness with perfect information | Optimal strategy |
|---|---|---|---|---|
| 10,000 | 1823 | –9 | 0.0014 | Nasogastric tube |
| 20,000 | 1941 | –1 | 0.0020 | Nasogastric tube |
| 30,000 | 2163 | 8 | 0.0023 | Nasogastric tube |
| 40,000 | 2287 | 10 | 0.0024 | Nasogastric tube |
| Weight on effectiveness (WTP) (£) | EVPPI (incremental net monetary benefit) (£) | Average incremental cost (£) with perfect information | Average incremental effectiveness with perfect information | Optimal strategy |
|---|---|---|---|---|
| 10,000 | 0 | 0 | 0 | Nasogastric tube |
| 20,000 | 0 | 0 | 0 | Nasogastric tube |
| 30,000 | 0 | 0 | 0 | Nasogastric tube |
| 40,000 | 0.028 | 3 | 0 | Nasogastric tube |
| Weight on effectiveness (WTP) (£) | EVPPI (incremental net monetary benefit) (£) | Average incremental cost (£) with perfect information | Average incremental effectiveness with perfect information | Optimal strategy |
|---|---|---|---|---|
| 10,000 | 0.35 | 6 | 0.000 | Nasogastric tube |
| 20,000 | 49 | 123 | 0.007 | Nasogastric tube |
| 30,000 | 147 | 184 | 0.010 | Nasogastric tube |
| 40,000 | 264 | 225 | 0.012 | Nasogastric tube |
In Figure 12, we see the individual-level EVPPI for the base-case analysis for the three different groups of parameters. The incremental net monetary benefit of reducing uncertainty around utility values is greatest for WTP thresholds of ≥ £20,000. At a WTP threshold of £10,000, the EVPPI is greatest for the probability parameters.
FIGURE 12.
Individual-level EVPPI for different groups of parameters along different WTP values (worst-case scenario): base-case analysis.
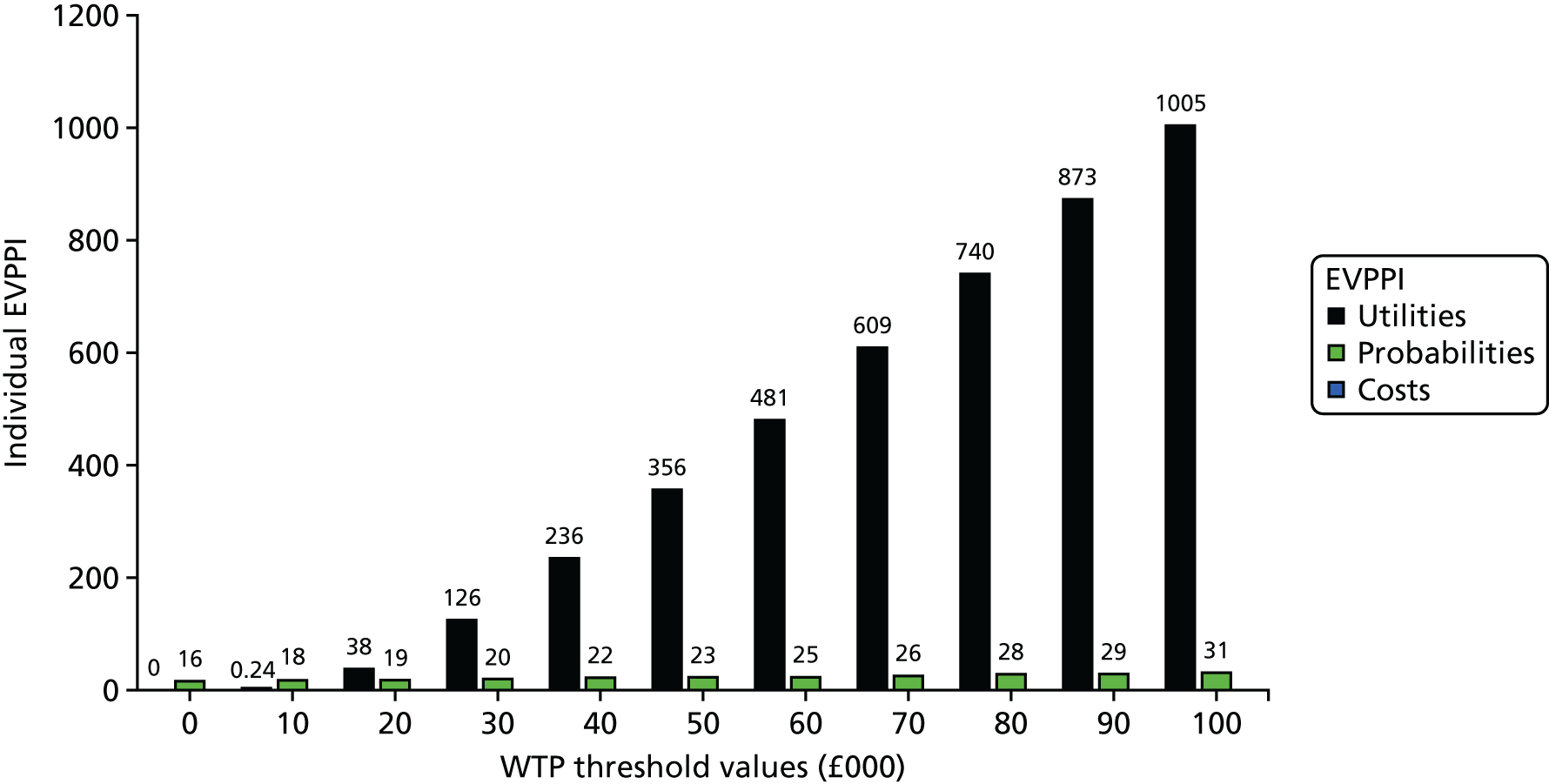
Worst-case scenario for the nasogastric tube arm
Expected value of perfect information
Table 40 presents the individual-level EVPI for all model parameters across a range of WTP thresholds for the worst-case scenario for the nasogastric tube arm cost-effectiveness analysis. This value ranges from £50 at a WTP threshold of £10,000 to £499 at a WTP threshold of £40,000. There is a positive relationship between the individual-level EVPI and the WTP threshold.
| Weight on effectiveness (WTP) (£) | EVPI (incremental net monetary benefit) (£) | Average incremental cost (£) with perfect information | Average incremental effectiveness with perfect information | Optimal strategy |
|---|---|---|---|---|
| 10,000 | 53 | 32 | 0.009 | Gastrostomy |
| 20,000 | 176 | 133 | 0.015 | Gastrostomy |
| 30,000 | 347 | 203 | 0.018 | Gastrostomy |
| 40,000 | 537 | 241 | 0.019 | Gastrostomy |
Figure 13 presents the population EVPI (4500 patients per year) for the worst-case scenario for the nasogastric tube arm cost-effectiveness analysis. At a WTP threshold of £20,000, based on the estimated number of patients in this population utilising nutritional support, the cost of further research should not exceed £7M (10-year time horizon), £11.4M (20-year time horizon), £19.7M (64-year time horizon) or £22M (unbounded time horizon) for additional investigation to be worthwhile.
FIGURE 13.
Population EVPI vs. WTP: worst-case scenario for the nasogastric tube arm.

Expected value of partial perfect information
The EVPPI for the set of probability (clinical), cost and utility parameters was also estimated at an individual level for the alternative analysis (see Tables 40–42) to identify the group that represented the most important source of uncertainty.
In Table 41, we see that the removal of uncertainty surrounding the probability parameters led to an individual-level EVPPI of £11–31 for WTP thresholds of £10,000–40,000. For cost parameters, this value is £0 across all WTP thresholds (Table 42). Finally, the EVPPI for utility parameters for the alternative analysis ranges from £17 to £577 across all WTP thresholds presented (Table 43).
| Weight on effectiveness (WTP) (£) | EVPPI (incremental net monetary benefit) (£) | Average incremental cost (£) with perfect information | Average incremental effectiveness with perfect information | Optimal strategy |
|---|---|---|---|---|
| 10,000 | 27 | 19 | 0.0046 | Gastrostomy |
| 20,000 | 110 | 125 | 0.0117 | Gastrostomy |
| 30,000 | 244 | 203 | 0.0149 | Gastrostomy |
| 40,000 | 404 | 262 | 0.0166 | Gastrostomy |
| Weight on effectiveness (WTP) (£) | EVPPI (incremental net monetary benefit) (£) | Average incremental cost (£) with perfect information | Average incremental effectiveness with perfect information | Optimal strategy |
|---|---|---|---|---|
| 10,000 | 1 | 7 | 0.0008 | Gastrostomy |
| 20,000 | 64 | 141 | 0.0103 | Gastrostomy |
| 30,000 | 184 | 199 | 0.0128 | Gastrostomy |
| 40,000 | 316 | 224 | 0.0135 | Gastrostomy |
| Weight on effectiveness (WTP) (£) | EVPPI (incremental net monetary benefit) (£) | Average incremental cost (£) with perfect information | Average incremental effectiveness with perfect information | Optimal strategy |
|---|---|---|---|---|
| 10,000 | 13 | 58 | 0.007 | Gastrostomy |
| 20,000 | 151 | 200 | 0.018 | Gastrostomy |
| 30,000 | 339 | 253 | 0.020 | Gastrostomy |
| 40,000 | 546 | 319 | 0.022 | Gastrostomy |
In Figure 14, we see the individual-level EVPPI for the three different groups of parameters for the worst-case scenario for the nasogastric tube arm cost-effectiveness analysis. The incremental net monetary benefit of reducing uncertainty around utility values was greatest across all WTP thresholds above £20,000.
FIGURE 14.
Individual-level EVPPI for different groups of parameters: worst-case scenario for the nasogastric tube arm.
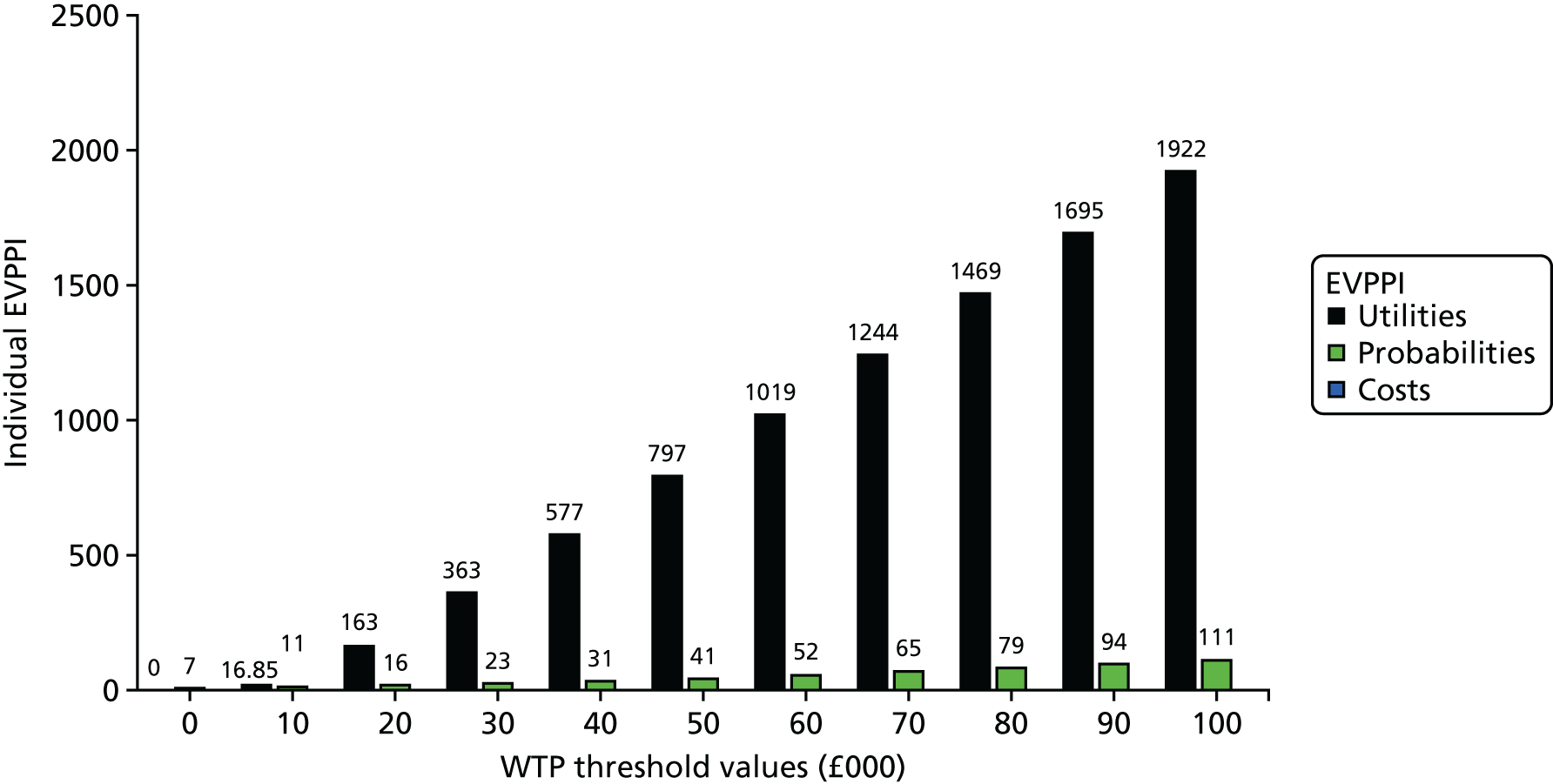
Chapter 4 Discussion
Feasibility study
Head and neck cancers include several disease processes managed within a single multidisciplinary setting, requiring complex treatment pathways and the expertise of over 10 HCP groups, often making this the most diverse type of MDT in tertiary care practice. A national survey of HCPs involved in caring for patients with head and neck cancer confirmed that issues around tube feeding during chemoradiotherapy constituted an important clinical question. As a previous study outside the UK had not succeeded in recruiting patients, the TUBE trial was planned with a robust qualitative arm to enable identification of barriers to recruitment. This is also the first study to perform a detailed assessment of qualitative issues surrounding recruitment into a surgical trial within the head and neck cancer multidisciplinary setting.
The TUBE trial aimed to explore the feasibility of conducting a RCT to compare two feeding methods for patients with HNSCC being treated with chemoradiotherapy, with a specific focus on capturing patient willingness to be randomised and clinician willingness to approach eligible patients. The success of this feasibility trial was judged on the basis of three factors: a recruitment rate of at least 50%, successful collection of outcome data in at least 80% of patients and economic criteria that suggest further research is worthwhile. The trial results indicate that the last two criteria have been met.
Although the TUBE trial did not achieve its intended recruitment rate, the data from the qualitative substudy have identified some issues that have not been previously explored as barriers to surgical studies. To our knowledge, this is the first surgical trial in which the choice of intervention (nasogastric tube vs. gastrostomy tube) could have been influenced by several HCP groups, all with different experiences and views on the subject, affecting the equipoise presented to patients. The lessons learnt from the qualitative substudy of the TUBE trial will be applicable to a wide range of surgical studies that intend to recruit from such complex settings.
From a clinical perspective, the inclusion criteria were very strictly set, and feedback from the sites indicates that relaxation of the criteria might have helped recruitment. While this would have increased recruitment to fulfil feasibility criteria, the proposed Phase III trial following on from this feasibility study was designed for patients undergoing chemoradiotherapy alone. Change in management protocols during the course of the study has also played a part in the reduction of the overall pool of patients available for recruitment into the study. HCPs continue to regard this as an important consideration, as practice is variable across the world, with no widely accepted criteria for deciding whether patients should receive a nasogastric tube or a gastrostomy tube; this is currently dictated by local practices and logistics, rather than by patient benefit or an evidence base of superiority.
Our data suggest that, even if clinical, organisational and operational processes are optimised, a recruitment rate of > 50% may not be achieved for this clinical question, owing to strong patient preferences. Alternative, innovative trial designs will need to explored for further research in this field.
Overall, the TUBE trial had a good safety record, with a low incidence of AEs in both intervention arms and no SAEs recorded in relation to the trial. Inferential statistical analysis of patient-reported and clinician-rated outcomes was not possible, as a result of the recruitment rate.
Qualitative study
Our qualitative process evaluation sought to identify, describe and understand the factors that promote or inhibit the introduction, implementation and embedding of the TUBE trial. It focused primarily on issues of trial conduct and trial processes in order to understand issues of recruitment, trial participation, adaptation to local contexts and the impact on HCPs and patients and their extended support network. In this way, as the TUBE trial was a feasibility study, we sought to explore the acceptability of the trial design in practice. We did not identify any single factor that was amenable to change that would be likely to substantially alter the success of recruitment to the TUBE trial. However, what we describe is a range of inter-related factors that, taken together, led to variation in levels of recruitment across the sites, and which were potentially amenable to change.
A recent synthesis of the experiences of HCPs contributing to six pragmatic RCTs identified that:
[W]hile trial recruitment (randomisation) is a simple event, it occurs at the end of a long chain of activities leading from the design of the RCT, through the operationalization of the protocol in clinical settings, to the presentation (or not) of the RCT to patients who have been investigated for eligibility. Recruitment difficulties are often seen to arise from organisational issues, patient shortages, and strong intervention preferences, but, as this synthesis has shown, many of these issues probably reflect underlying issues among recruiters in terms of knowledge and views about evidence, equipoise, RCT design, role conflicts, specialty interests, and particular personal preferences.
Donovan et al. 84
The findings of the Donovan et al. 84 synthesis read, almost, as a summary of our findings in the TUBE trial. We have identified the following:
-
Organisational issues, especially in terms of delays in the research approval process. These issues meant that, rather than the intended 45 months of recruitment, the study was open for 32 months across sites. However, in contrast, the practical integration of the TUBE trial protocols within routine clinical pathways may have been less problematic. HCPs at each site reported that they were typically engaged in a number of research studies, and, despite the organisation and paperwork typically required for a new trial, our respondents explained that they were experienced in integrating research and clinical work.
-
Patient availability, especially in terms of the changes in cancer treatment practices between study design and the TUBE trial implementation, which led to fewer eligible patients who could be approached about recruitment. HCPs reported a range of eligibility issues, including recruitment criteria that were perceived as being ‘too limiting’ and not reflective of the ‘typical’ presentation of head and neck cancer patients.
-
Strong patient intervention preferences were a recurrent issue described by HCPs. We have shown how some patients do have strong intervention preferences. In part, such strong patient preferences emerged because of the TUBE trial. As we outlined, before the TUBE trial, only one of the five sites routinely offered patient choice with individualised guidance (see Table 19). In this way, talking about the TUBE trial to patients meant that patients were given detailed information about different feeding options, as well as access to them. However, for some patients, a strong preference meant accepting randomisation in order to potentially get access to their preferred non-local treatment.
We need to be clear that organisational issues, patient shortages and strong intervention preferences did impact on the successful implementation of the TUBE trial.
However, another layer of issues, ‘in terms of knowledge and views about evidence, equipoise, RCT design, role conflicts, specialty interests, and particular personal preferences’84 was also key. We can think about these issues in terms of the core constructs of the NPT:
-
Coherence – how did HCPs make sense of the TUBE trial? Overall, the TUBE trial was consistently viewed as being a worthwhile research endeavour, which would provide useful evidence to guide decision-making. While they identified the value and benefit of the TUBE trial in assessing the feasibility of a definitive trial, some HCPs raised concerns about the perceived specificity of the research question and trial design. For example, they felt that a focus on long-term swallow function was important, but that it was only one consideration in a much larger matrix of factors; it may not align with the range of patient priorities at the time of diagnosis and treatment discussions; new advances in primary treatment would have an impact on how a future trial should be powered; and, as feeding practices shift away from tubes, evidence produced from a full trial may become redundant. However, the TUBE trial did challenge some established patterns of work and the job specification of particular individuals or groups. There was an existing division of labour and expertise; some HCP roles and responsibilities were intimately tied to specific feeding options, and the organisation of care and financial resources was tied to a specific treatment modality. In this way, in principle, the TUBE trial made sense, but, in practice, it raised practical, organisational and political questions at some sites.
-
Cognitive participation – were HCPs willing and able to buy into and commit to the implementation of the TUBE trial? Different sites had different histories of preferences for feeding options. There was an ongoing debate between professional groups regarding the use of nasogastric tubes and gastrostomy. Some sites offered only one option to the majority of patients. Some sites were reflecting on current practice and thinking about changing options. Some, with the arrival of new team members or the loss of old ones, were shifting emphasis. In addition to variation between sites, there was also considerable variation within sites in terms of preferences for the different feeding approaches. Often this related not only to perceptions of the benefits and disadvantages of each approach, but also to perceptions of the relative importance of these advantages. In general, nasogastric tubes were seen as less pleasant for the patient. Gastrostomy was seen to be less problematic, although it was acknowledged that, when problems occurred, they could be serious. These historical tensions between the objectives and priorities of different groups of professionals underpinned some of the issues affecting the TUBE trial. In this context, the TUBE trial was not a socially ‘neutral’ exercise, but, rather, a collaboration that was moulded – to varying degrees of success – to a local, historically encumbered, clinical environment of pre-existing tensions.
The existing context of variation in practice, and preferences for supplementary feeding between and within sites, positioned the TUBE trial as a coherent practice to undertake in general terms, but simultaneously led to variation in buy-in and commitment within and across sites. The key element was whether or not individual, professional or site preferences were communicated to patients. Centrally, at some sites, teams worked collectively to build a willingness and ability to present equipoise at all contacts with patients when feeding options were discussed. At other sites, some teams reported that different members of the team expressed preferences for specific feeding options at different points across the clinical and research pathway. This had an impact on whether or not patients were offered the TUBE trial, as well as shaping the preferences of patients who were offered randomisation. In addition, initially, some HCPs reported some fears and concerns about overburdening patients with trial information at a time of distress, uncertainty and vulnerability. In this way, at the start, the TUBE trial faced questions about its legitimacy in some sites:
-
Collective action – the work, skills, trust and resources HCPs require to implement the TUBE trial. To successfully implement and embed the TUBE trial, teams had to work hard to integrate the trial pathway into the existing local organisational ‘normal working pathway’. The teams had to be willing to enrol a range of people and activities within and across departments and organisations. They also had to be willing to adapt their practices over time as they formed an understanding of how trial protocols could fit within existing patterns of work. New practices, such as joint consultations with the research nurse and the dietitian, were introduced to offer a consistent, timely and balanced account of feeding options to patients. Some teams worked to prime patients – or ‘plant the seed’ – at each stage of the clinical pathway. This was an effective way to introduce the trial in the least disruptive way. Providing the right amount of information at each stage slowly built an idea about the trial in patients’ minds. The recruitment discussions seemed relatively unproblematic; when research nurses were able to engage with patients in a timely manner, and uncontaminated by other feeding discussions, they seemed to be able to effectively convey equipoise, the advantages and disadvantages of the two feeding tubes and the implications of trial participation, and to enable patients to explore areas of concern.
-
Reflexive monitoring – how HCPs evaluate, both formally and informally, the TUBE trial, and how they work to adapt their practices in light of this information. Some sites described cycles of reflection and adaptation of trial practices, including role play, practice sessions and peer feedback. Questions of the legitimacy of approaching patients about the TUBE trial at a time of vulnerability were resolved following feed-forward information from the qualitative substudy about patients’ experiences. We reported back to the teams that patients felt that the timing of the approach was appropriate, given the sensitive way in which they felt that the teams handled the discussions, and that they valued the opportunity to discuss research. At one site, priming patients at each stage of the clinical pathway seemed to fail in the later stages of the trial. Some HCPs earlier in the clinical pathway worked harder to actively promote the TUBE trial; this was, in part, driven by information on the shortfall in recruitment at this site.
Centrally, some of the barriers to the TUBE trial may be found within the cluster of issues concerning how the trial was understood vis-à-vis current and future clinical priorities. Centrally, these are questions of whether or not HCPs saw the value and worth of the TUBE trial, as well as questions around ‘buy-in’, whether or not HCPs felt that the TUBE trial should be a legitimate part of their work, whether or not they were willing to invest in the TUBE trial, especially around issues of equipoise, and motivation over time. In Figure 15, we offer a temporal trajectory of the core questions that the key stakeholders – HCPs and patients, as well as carers – asked at different points over the duration of the TUBE trial.
FIGURE 15.
Temporal trajectory of the key questions asked at different points over the duration of the TUBE trial. NGT, nasogastric tube.

Patients’ willingness to be randomised was shaped by three considerations: (1) the integration of research activities within the clinical pathway; (2) whether or not suspension of perceived control over treatment could be tolerated by the patient; and, to a lesser extent, (3) whether or not the trial offered the possibility of accessing a desired, but normally ‘unavailable’, treatment. A key factor in patients’ decisions to take part in the TUBE trial was the perceived integration of research and clinical pathways. Patients explained that their chief priority was timely, effective treatment, and that research activities were acceptable if these could be completed without hindering the clinical pathway. The NPT predicts that novel activities are more acceptable to those required to complete them – and the intended beneficiaries of those actions (e.g. patients) – if they integrate with, and facilitate the completion of, routine work. Across three of the five research sites, respondents reported research experiences indicative of adequate research integration. When research activities disrupted the expected clinical pathway, recruitment faltered.
As noted above, recurrent issues described by HCPs were patients’ treatment preferences and their reluctance to be randomised. However, we also identified a lack of (presented) clinical equipoise, which may have discouraged some participants from participating. Centrally, although all sites experienced the same or similar barriers, such as strong patient preferences, we found that, where there was a strong ‘buy-in’ to the study across all HCPs at a site, the proportion of screened to consented participants was higher. When equipoise was not distributed across teams, and when there was a lack of interest in the study, recruitment was lower.
Overall, the TUBE trial was a technically competent feasibility trial, addressing a research question that was perceived by both clinicians and patients as being worthwhile. However, the clinical context that inspired the research question also played a part in undermining the practical implementation of the research across multiple sites. While HCPs reported strong patient preferences as a barrier to recruitment, we observed that some aspects of strong HCP preferences also shaped recruitment.
Strengths and limitations
A strength of our data is their spread across all sites. In a study with a relatively small number of participating locations, this enabled us to obtain a comprehensive account of the difficulties of trial implementation. A further strength was the multiple respondents from each site; this proved particularly relevant in this instance, in which different members of the MDT had very different perspectives on supplementary feeding, contributing to a more (or less) successful enactment of collective equipoise. Patient accounts were helpful in clarifying processes at sites, as well as in providing insight into their perspectives, and were essential to the comprehensive picture of the trial and clinical pathways obtained.
We were less successful in obtaining recordings of recruitment consultations and obtaining follow-up interviews with patients. However, the small number of recordings obtained tended to corroborate the information from both patient and professional interviews, which suggested that in the TUBE trial, formal recruitment discussions with the research nurse (and other participants) were not particularly problematic and that it was other interactions about feeding, outwith these discussions, that were likely to undermine successful presentation of equipoise. The small number of follow-up interviews was disappointing, but, in part, reflects our recruitment strategy for follow-up interviews, which precluded further contact with patients who had declined the trial, and the low level of recruitment in the TUBE trial.
Data on patient experiences of gastrostomy largely echo existing literature85,86 that suggests that experiences are largely positive; Osborne et al. 86 found that 96% of patients who had experienced a PEG would recommend this treatment to other head and neck cancer patients. There are few published data on experiences of the nasogastric tube in this population. Our limited data from patients echo the perceptions of HCPs that this intervention is more problematic, both at insertion and in everyday use, than gastrostomy. There is reason to believe that experiences of nasogastric tube feeding may be more traumatic for patients who are already experiencing considerable oral and pharyngolaryngeal morbidity. Further qualitative research to understand experiences of nasogastric treatment in head and neck cancer is urgently needed, given the increasing implementation of this approach in the UK.
Economic analysis
Within-trial evaluation
Owing to the difficulties in recruiting patients to participate in this feasibility study, which led to the early closure of recruitment, the descriptive summary of information collected using these tools would be of limited value in informing the adjustments to the data collection tools that would be required for a definitive economic evaluation. As a result of the small sample size, the feasibility study has low statistical power and, so, a reduced chance of detecting a true effect. Therefore, it is uncertain whether or not the differences detected are true.
However, the feasibility trial and within-trial evaluation data were beneficial in terms of helping to map out the clinical pathways of patients and in developing the model structure for the model-based economic evaluation. Despite the limited number of available trial data, a decision was made to continue with an economic modelling evaluation based primarily on published evidence.
Cost-effectiveness and value-of-information discussion
Only minimal robust evidence existed to inform many of the parameters included in the model. Despite the uncertainty in these data, it was decided that there was still value in utilising the decision model to further explore and characterise this uncertainty; however, we advise that results should be interpreted with caution. We do recommend that the results be used not to make decisions on the relative cost-effectiveness of the alternative interventions, but rather to aid discussion and further research in this area. The model gives an insight into the potential cost-effectiveness of pre-treatment gastrostomy tube feeding, given an assumed level of effectiveness in each treatment arm. Parameter values were varied extensively in the sensitivity analysis to test the robustness of results, and a threshold analysis was conducted in order to examine the point at which the decision would change, given a variation in key parameters. The analysis presented provides a strong platform for discussion and further exploratory research.
The results of the cost-effectiveness analysis for the base-case values indicate that the intervention of pre-treatment gastrostomy tube feeding is more costly and less effective, that is, it is absolutely dominated by the alternative of reactive nasogastric tube feeding. Therefore, gastrostomy tube feeding is not cost-effective. The base-case result was robust with regard to most clinical and cost variables. However, this result changes when important input parameters, the values of which are not known with any certainty, are varied. Within the sensitivity analyses, results were sensitive to the cost of tube insertion and AEs, the probability of dying from an AE and the probability of ending nutritional support in both arms. However, the data to inform these parameters (particularly the clinical variables) were sparse. Variations that have the potential to change the decision presented in the base-case analysis are lowering the probability of ending nutritional support in the nasogastric tube arm and increasing the cost of nasogastric tube reinsertions. This is highlighted in the worst-case scenario for nasogastric tube feeding PSA, in which pre-treatment gastrostomy tube feeding is likely to be the more cost-effective option. Many of the clinical parameters and baseline utility values are not known with any certainty and, therefore, the results should be considered not a definitive assessment of cost-effectiveness but, rather, an aid to further exploratory work in this area.
A key strength of this economic analysis is that it is the first economic model to consider the relative cost-effectiveness of pre-treatment gastrostomy tube feeding compared with reactive nasogastric tube feeding. The model can also illustrate the key parameters that have the potential to have an impact on the results. The main limitation in the analysis was the significant uncertainty around parameter values, resulting from the limited and highly uncertain available evidence. In particular, there was ambiguity around the utility values and whether or not they fully capture the effects of dysphagia among this population. This has been explored where possible by the deterministic sensitivity analysis that has been undertaken, and by applying the greatest amount of uncertainty around many of the model parameter values. Although this does not mitigate the need for more robust evidence, it does help to characterise and explore the likely impact of parameter uncertainty. However, as most distributions have been assumed, there should also be caution in interpreting the results of the PSA, as there may be biases in quantifying the uncertainty, and the direction of this bias is unknown. In some instances, the choice of distribution has been driven by the need to allow for greater uncertainty, rather than utilising more routinely used/accepted distributions for parameters (i.e. uniform distributions rather than gamma distributions for utility data).
A number of assumptions were made in the model-based economic evaluation, in terms of both the structure of the model and the input parameters included. Many of these assumptions were explored in the sensitivity analyses. Although few good-quality data currently exist to populate the model, a model structure exists for reanalysis once additional data become available.
Value-of-information analysis
The value-of-information analysis was also undertaken to estimate the expected costs of decision uncertainty predicted by the model, and the maximum values that can be placed on additional research aimed at reducing that uncertainty. The estimates obtained provide an upper bound for the value of additional research. The results of the EVPI indicate that the incremental net monetary benefit of reducing uncertainty around all model parameters is high at both an individual and population level across all WTP thresholds, for both scenarios presented. The population EVPI ranges from £4.1M (10-year time horizon) to £13.1M (unbounded time horizon), and from £7.4M (10-year time horizon) to £22M (unbounded time horizon), at a WTP threshold of £20,000 for both scenarios, respectively. These ranges are based on an estimated number of patients who would benefit from this information over this time period, and are likely to be greater than the cost of future research. Therefore, conducting additional research to eliminate uncertainty around all model parameters is highly likely to be cost-effective. In addition, for both scenarios presented, the EVPPI values at an individual level were considerable for both utility and probability parameters across all WTP thresholds, indicating that there is likely to be value in reducing uncertainty around these specific groups of parameters.
The results of the cost-effectiveness analysis need to be considered with caution, given the uncertainty in the clinical parameters (utilities and probabilities) that were used to populate the economic model. Many of these uncertainties are key drivers of the model results. The results of the value-of-information analysis confirm the considerable uncertainty that exists around these parameters, and indicate the potential value in eliminating this uncertainty. Given the issues experienced with recruitment for this feasibility trial and the results of the value-of-information analysis, future research may be best placed to focus on an outcome valuation study, with the intention of reducing decision uncertainty and informing future economic analysis.
Chapter 5 Conclusions
Feasibility trial
The study has raised important questions about tube-feeding options for patients undergoing chemoradiation for head and neck cancer, and identified ways in which organisational and operational issues can be overcome in a busy clinical setting to improve recruitment for such a trial, in which multiple professionals have a stake in deciding recruitment, driven by clinical experience and personal views. Our recruitment rate per site varied from 11% (n = 1) to 33% (n = 2). Numbers of eligible patients at some sites were very low, so we have been careful not to overinterpret these data; however, our qualitative analysis suggested that sites achieving higher recruitment rates were those that worked harder to present equipoise consistently at all patient encounters. With appropriate implementation of organisational and operational measures ahead of the trial, it should be possible to recruit one-third of patients to a future study exploring this question. More importantly, many of these measures are generic and applicable to other studies in similar settings.
The health economic argument, based on the cost-effectiveness data and the economic value-of-information analysis, is reasonably compelling to warrant a further study, the design for which will need to take into consideration the results from this study. We have also identified that few data exist in the literature on experiences of nasogastric tube feeding in patients undergoing treatment for head and neck cancer; this piece of qualitative work is needed to fill the gap in the existing literature.
Qualitative process evaluation
Overall, we have a range of relatively small issues that combined to have an impact on recruitment. Some of the barriers to the TUBE trial may be found within the cluster of issues concerning how the trial was understood vis-à-vis current and future clinical priorities. In terms of the NPT, this is about issues of coherence (does the trial make sense and is it seen as worthwhile?) and cognitive participation (engagement and commitment over time). In contrast, the practical integration of trial protocols within routine clinical practice may have been less problematic. HCPs at each site reported that they were typically engaged in a number of research studies, and, despite the organisation and paperwork typically required for a new trial, our respondents explained that they were experienced in integrating research and clinical work. However, HCPs did report a range of practical issues that limited potential recruitment to the TUBE trial. Some of those issues included recruitment criteria that were perceived as being ‘too limiting’ and not reflecting ‘typical’ presentation of head and neck cancer patients and delays in being able to get sites ‘on line’. In terms of the NPT, these are issues of interactional workability (fitting the trial’s recruitment criteria into the existing patient cohort) and issues of contextual integration (issues of adequate and timely organisational support).
Patients’ willingness to be randomised was shaped by three considerations: integration of research activities within the clinical pathway; whether or not suspension of perceived control over treatment could be tolerated by the patient; and, to a lesser extent, whether or not the trial offered the possibility of accessing a desired, but normally ‘unavailable’, treatment. A key factor in patients’ decisions to take part in the TUBE trial was the perceived integration of research and clinical pathways. Patients explained that their chief priority was timely, effective treatment, and that research activities were acceptable if they could be completed without hindering the clinical pathway. The NPT predicts that novel activities are more acceptable to those required to complete them – and the intended beneficiaries of those actions (e.g. patients) – if they integrate with, and facilitate the completion of, routine work. Across three of the five research sites, respondents reported research experiences indicative of adequate research integration. When research activities disrupted the expected clinical pathway, recruitment faltered.
Recurrent issues described by HCPs were patients’ treatment preferences and their reluctance to be randomised. However, we also identified a lack of (presented) clinical equipoise, which may have discouraged some patients from participating. So, again, this concerns issues of cognitive participation (engagement and commitment) for both the HCP and the patient. How the trial was perceived undoubtedly affected how it was implemented. Issues concerning ‘buy-in’ by HCPs are, arguably, more ‘politically sensitive’ than accounts of low recruitment levels based on technical issues. Centrally, although all sites experienced the same or similar barriers, such as strong patient preferences, we found that where there was a strong ‘buy-in’ to the study across all HCPs at a site, the proportion of screened to consented participants was higher than at other sites, where a lack of equipoise and interest in the study among HCPs was particularly low.
Overall, the TUBE trial was a technically competent trial, addressing a research question that was perceived by both clinicians and patients as being worthwhile. However, the clinical context that inspired the research question also played a part in undermining the practical implementation of the research across multiple sites. Although HCPs reported strong patient preferences as a barrier to recruitment, we observed that some aspects of strong HCP preferences also shaped recruitment.
Economic analysis
Currently, there is limited economic evidence on the cost or cost-effectiveness of nutritional support interventions for patients with head and neck cancer. This is the first economic model to attempt to estimate the cost-effectiveness of pre-treatment gastrostomy tube feeding compared with nasogastric tube feeding, as necessary, for this patient population. The within-trial economic evaluation and model-based economic evaluation results should be interpreted with caution, given the assumptions made in estimating many of the clinical parameter and utility values included in the model. Over the 6-month model time horizon, and given the assumptions made in developing and populating the model, pre-treatment gastrostomy tube feeding is not a cost-effective option. This is largely because of the significant cost difference between the interventions and the impact that clinical parameters, for which we have very little information, have on the model results. The parameters that have the greatest potential to impact results are the probability of ending nutritional support in both arms of the model and major cost parameters, such as insertion costs and AE costs. The model requires more robust data in order to conduct a definitive assessment of cost-effectiveness.
This is the first value-of-information analysis to attempt to quantify the incremental net monetary benefit, among this patient population for this type of intervention, of removing uncertainty around model parameters. Results from the base-case and worst-case scenario for the nasogastric tube arm EVPI analyses indicate that conducting additional research to eliminate uncertainty around all model parameters is highly likely to be cost-effective. Results from the base-case and worst-case scenario for the nasogastric tube arm EVPPI analyses indicate that there is likely to be value in reducing uncertainty around probability and utility parameters.
Research recommendations
Further work in this area is warranted for the following reasons:
-
The qualitative substudy has identified a range of inter-related factors that led to variation in levels of recruitment across the sites, and that are potentially amenable to change. Implementation of organisational and operational measures based on the qualitative process evaluation can increase recruitment. However, the appropriate research question and design of a future study need to be identified following consultation with patient groups and other stakeholders.
-
The TUBE trial was considered to be a valuable research endeavour to provide useful evidence to guide decision-making. The economic analysis suggests that further research in this area is likely to be cost-effective and is reasonably compelling to warrant the need for a further study into the health economics of tube feeding in this patient group.
-
Few data exist in the literature on experiences of nasogastric tube feeding in patients undergoing treatment for head and neck cancer; this piece of qualitative work is needed.
Acknowledgements
We are grateful to all the patients and HCPs who generously contributed their time and effort in the conduct of this study.
Isobel Bowe (Senior Dietitian, Newcastle upon Tyne Hospitals NHS Foundation Trust) advised on tube feeding regimes and contributed to the trial launch.
Tracey Cowper (Modern Matron, South Tyneside NHS Foundation Trust) provided expertise on nutritional issues.
June Corry (Professor of Radiation Oncology, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia) advised on study design.
Charles Kelly (Consultant Clinical Oncologist, Newcastle upon Tyne Hospitals NHS Foundation Trust) advised on the patient cohort and provided RT expertise.
Valentina Mamasoula (Statistician, Institute of Health and Society, Newcastle University) contributed to statistical analysis.
Shannon Robalino (Information Specialist, Institute of Health and Society, Newcastle University) contributed to literature review and health economics modelling.
Sheila Robson (Research Associate, Newcastle University) contributed to the qualitative substudy by conducting interviews with patients and professionals, and also contributed to the study launch event and the development of study processes.
Luke Vale (Health Foundation Chair in Health Economics, Institute of Health and Society, Newcastle University) contributed to critical review of the study design and constructed the health economics analysis plans.
Janet Wilson (Professor of Otolaryngology, Newcastle University) advised on the study design and acted as mentor for the clinical investigators.
The authors acknowledge support from The Royal Marsden NHS Foundation Trust/Institute of Cancer Research NIHR Biomedical Research Centre.
Trial Steering Committee
Professor Hisham Mehanna (University of Birmingham), Mr Shane Lester (South Tees Hospitals NHS Foundation Trust) and Dr Caitrin Tudor Smith (University of Liverpool).
Data Monitoring and Ethics Committee
Professor Steve Thomas (University of Bristol), Professor Richard Shaw (University of Liverpool) and Dr Chris Foy (University of Bristol).
Local principal investigators
Ms Helen Cocks (Sunderland Royal Hospital), Dr Nachiappan Palaniappan (Royal Gwent Hospital) and Professor Mererid Evans (Velindre Cancer Centre).
Local research nurses
Deborah Jones (Royal Gwent Hospital), Sue Kearney (Royal Gwent Hospital), Alison Kelly (Velindre Cancer Centre), Jessica La (Guy’s and St. Thomas’ NHS Foundation Trust), Pauline Oates (Sunderland Royal Hospital), Julia Scott (Freeman Hospital) and Peter Wilson (Freeman Hospital).
Contributions of authors
Vinidh Paleri (Consultant Head and Neck Surgeon and Professor of Head and Neck Surgery) had the original idea for the study, led the funding application and the protocol development, supervised the running of the lead study centre and co-ordination of centres and contributed to the analysis of, and led the drafting of, the report.
Joanne Patterson (Macmillan Speech and Language Therapist and NIHR Clinical Lecturer) contributed to the funding application, protocol development, ethics approval and methodological aspects and service user input, and liaised with the patient steering group, as well as contributing to the management of the study and drafting of the report.
Nikki Rousseau (Senior Research Associate and Deputy Director, NIHR North East Research Design Service) contributed to the funding application, protocol development, ethics approval, methodological aspects and the management of the study, and led the qualitative process evaluation, liaised with the patient steering group and contributed to the drafting of the report.
Eoin Moloney (Research Associate), constructed the health economics analysis plans and performed the health economic analysis, and contributed to the management of the study and drafting of the report.
Dawn Craig (Principal Scientist) advised on the evidence synthesis and performed the health economics analysis plans.
Dimitrios Tzelis (Research Assistant) contributed to the health economics analysis plans and analysis.
Nina Wilkinson (Research Associate) contributed to the statistical analysis and the drafting of the report.
Jeremy Franks (Expert Patient/Leader of the Patient Steering Group) liaised with and chaired patient and public involvement groups, and contributed to the management of the study and drafting of the report.
Ann Marie Hynes (Trial Manager) contributed to the protocol development, co-ordinated the recruiting centres and contributed to the management of the study and the drafting of the report.
Ben Heaven (Qualitative Methods Expert) contributed to the qualitative process evaluation.
David Hamilton (Ear, Nose and Throat Higher Surgical Trainee) contributed to the qualitative process evaluation.
Teresa Guerrero-Urbano (Consultant Clinical Oncologist) contributed to the funding application and protocol development, and was the PI at a participating site, as well as the lead oncologist for the trial.
Rachael Donnelly (Principal Dietitian) contributed to the funding application and protocol development, and led the dietetics aspect of the trial.
Stewart Barclay (Consultant Restorative Dentist) contributed to the funding application and protocol development, and led on the oral health aspects of the trial.
Tim Rapley (Senior Lecturer) contributed to the funding application, advised on the qualitative process evaluation and contributed to the management of the study and the drafting of the report.
Deborah Stocken (Senior Lecturer in Biostatistics and Clinical Trials) contributed to the funding application and protocol development, provided statistical expertise and supervised the statistical analysis, as well as contributing to the drafting of the report.
All authors were involved in the interpretation of data for the work, in revising the work critically for important intellectual content and in the final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of this report.
Publication
Paleri V, Wood J, Patterson J, Stocken DD, Cole M, Vale L, et al. A feasibility study incorporating a pilot randomised controlled trial of oral feeding plus pre-treatment gastrostomy tube versus oral feeding plus as-needed nasogastric tube feeding in patients undergoing chemoradiation for head and neck cancer (TUBE trial): study protocol. Pilot Feasibility Stud 2016;2:29.
Data sharing statement
Anonymised data from this study may be available to the scientific community subject to appropriate ethics approval. Requests for data should be directed to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Profile of Head and Neck Cancers in England: Incidence, Mortality and Survival. London: National Cancer Intelligence Network; 2010.
- Junor EJ, Kerr GR, Brewster DH. Oropharyngeal cancer. Fastest increasing cancer in Scotland, especially in men. BMJ 2010;340. https://doi.org/10.1136/bmj.c2512.
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294-301. https://doi.org/10.1200/JCO.2011.36.4596.
- Clavel S, Fortin B, Despres P, Donath D, Soulieres D, Khaouam N, et al. Enteral feeding during chemoradiotherapy for advanced head-and-neck cancer: a single-institution experience using a reactive approach. Int J Radiat Oncol Biol Phys 2011;79:763-9. https://doi.org/10.1016/j.ijrobp.2009.12.032.
- McLaughlin BT, Gokhale AS, Shuai Y, Diacopoulos J, Carrau R, Heron DE, et al. Management of patients treated with chemoradiotherapy for head and neck cancer without prophylactic feeding tubes: the University of Pittsburgh experience. Laryngoscope 2010;120:71-5.
- Nutrition Support in Adults. London: National Institute for Health and Care Excellence; 2006.
- Grant DG, Bradley PT, Pothier DD, Bailey D, Caldera S, Baldwin DL, et al. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol 2009;34:103-12. https://doi.org/10.1111/j.1749-4486.2009.01889.x.
- Chapuy CI, Annino DJ, Snavely A, Li Y, Tishler RB, Norris CM, et al. Swallowing function following postchemoradiotherapy neck dissection: review of findings and analysis of contributing factors. Otolaryngol Head Neck Surg 2011;145:428-34. https://doi.org/10.1177/0194599811403075.
- Terrell JE, Ronis DL, Fowler KE, Bradford CR, Chepeha DB, Prince ME, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 2004;130:401-8. https://doi.org/10.1001/archotol.130.4.401.
- Mayre-Chilton KM, Talwar BP, Goff LM. Different experiences and perspectives between head and neck cancer patients and their care-givers on their daily impact of a gastrostomy tube. J Hum Nutr Diet 2011;24:449-59. https://doi.org/10.1111/j.1365–277X.2011.01165.x.
- Rogers SN, Thomson R, O’Toole P, Lowe D. Patients experience with long-term percutaneous endoscopic gastrostomy feeding following primary surgery for oral and oropharyngeal cancer. Oral Oncol 2007;43:499-507. https://doi.org/10.1016/j.oraloncology.2006.05.002.
- NHS Direct . Misplaced Naso or Orogastric Tube Not Detected Prior to Use 2011. www.nrls.npsa.nhs.uk/resources/collections/never-events/core-list/misplaced-naso-or-orogastric-tube-not-detected-prior-to-use (accessed 6 July 2017).
- Nugent B, Lewis S, O’Sullivan JM. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev 2010;3. https://doi.org/10.1002/14651858.CD007904.pub2.
- Paleri V, Patterson J. Use of gastrostomy in head and neck cancer: a systematic review to identify areas for future research. Clin Otolaryngol 2010;35:177-89. https://doi.org/10.1111/j.1749-4486.2010.02128.x.
- Moor JW, Patterson J, Kelly C, Paleri V. Prophylactic gastrostomy before chemoradiation in advanced head and neck cancer: a multiprofessional web-based survey to identify current practice and to analyse decision making. Clin Oncol (R Coll Radiol) 2010;22:192-8. https://doi.org/10.1016/j.clon.2010.01.008.
- Kurien M, Westaby D, Romaya C, Sanders DS. National survey evaluating service provision for percutaneous endoscopic gastrostomy within the UK. Scand J Gastroenterol 2011;46:1519-24. https://doi.org/10.3109/00365521.2011.619278.
- Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients’ perspectives. Otolaryngol Head Neck Surg 2011;145:767-71. https://doi.org/10.1177/0194599811414506.
- Jeannon JP, Abbs I, Calman F, Gleeson M, Lyons A, Hussain K, et al. Implementing the National Institute of Clinical Excellence improving outcome guidelines for head and neck cancer: developing a business plan with reorganisation of head and neck cancer services. Clin Otolaryngol 2008;33:149-51. https://doi.org/10.1111/j.1749-4486.2008.01637.x.
- Radiotherapy Services in England 2012. London: Department of Health and Social Care; 2012.
- Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck 2012;34:967-73. https://doi.org/10.1002/hed.21842.
- Sher DJ, Balboni TA, Haddad RI, Norris CM, Posner MR, Wirth LJ, et al. Efficacy and toxicity of chemoradiotherapy using intensity-modulated radiotherapy for unknown primary of head and neck. Int J Radiat Oncol Biol Phys 2011;80:1405-11. https://doi.org/10.1016/j.ijrobp.2010.04.029.
- Paleri V, Roe JW, Strojan P, Corry J, Grégoire V, Hamoir M, et al. Strategies to reduce long-term postchemoradiation dysphagia in patients with head and neck cancer: an evidence-based review. Head Neck 2014;36:431-43. https://doi.org/10.1002/hed.23251.
- Langmore SE, Pisegna JM. Efficacy of exercises to rehabilitate dysphagia: a critique of the literature. Int J Speech Lang Pathol 2015;17:222-9. https://doi.org/10.3109/17549507.2015.1024171.
- Patterson JM, Brady GC, Roe JW. Research into the prevention and rehabilitation of dysphagia in head and neck cancer: a UK perspective. Curr Opin Otolaryngol Head Neck Surg 2016;24:208-14. https://doi.org/10.1097/MOO.0000000000000260.
- Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube?. Cancer 2001;91:1785-90. https://doi.org/10.1002/1097-0142(20010501)91:9<1785::AID-CNCR1197>3.0.CO;2-1.
- Langmore S, Krisciunas GP, Miloro KV, Evans SR, Cheng DM. Does PEG use cause dysphagia in head and neck cancer patients?. Dysphagia 2012;27:251-9. https://doi.org/10.1007/s00455–011–9360–2.
- Chen AM, Li BQ, Lau DH, Farwell DG, Luu Q, Stuart K, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2010;78:1026-32. https://doi.org/10.1016/j.ijrobp.2009.09.036.
- Oozeer NB, Corsar K, Glore RJ, Penney S, Patterson J, Paleri V. The impact of enteral feeding route on patient-reported long term swallowing outcome after chemoradiation for head and neck cancer. Oral Oncol 2011;47:980-3. https://doi.org/10.1016/j.oraloncology.2011.07.011.
- Corry J, Poon W, McPhee N, Milner AD, Cruickshank D, Porceddu SV, et al. Prospective study of percutaneous endoscopic gastrostomy tubes versus nasogastric tubes for enteral feeding in patients with head and neck cancer undergoing (chemo)radiation. Head Neck 2009;31:867-76. https://doi.org/10.1002/hed.21044.
- Corry J, Poon W, McPhee N, Milner AD, Cruickshank D, Porceddu SV, et al. Randomized study of percutaneous endoscopic gastrostomy versus nasogastric tubes for enteral feeding in head and neck cancer patients treated with (chemo)radiation. J Med Imaging Radiat Oncol 2008;52:503-10. https://doi.org/10.1111/j.1440-1673.2008.02003.x.
- Shaw SM, Flowers H, O’Sullivan B, Hope A, Liu LWC, Martino R. The effect of prophylactic percutaneous endoscopic gastrostomy (PEG) tube placement on swallowing and swallow-related outcomes in patients undergoing radiotherapy for head and neck cancer: a systematic review. Dysphagia 2015;30:152-75. https://doi.org/10.1007/s00455-014-9592-z.
- Silander E, Jacobsson I, Bertéus-Forslund H, Hammerlid E. Energy intake and sources of nutritional support in patients with head and neck cancer-a randomised longitudinal study. Eur J Clin Nutr 2013;67:47-52. https://doi.org/10.1038/ejcn.2012.172.
- Chen AY, Frankowski R, Bishop-Leone J, Hebert T, Leyk S, Lewin J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg 2001;127:870-6.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
- Bjordal K, de Graeff A, Fayers PM, Hammerlid E, van Pottelsberghe C, Curran D, et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer 2000;36:1796-807. https://doi.org/10.1016/S0959-8049(00)00186-6.
- Andrews PJ, Avenell A, Noble DW, Campbell MK, Battison CG, Croal BL, et al. Randomised trial of glutamine and selenium supplemented parenteral nutrition for critically ill patients. Protocol Version 9, 19 February 2007 known as SIGNET (Scottish Intensive care Glutamine or seleNium Evaluative Trial). Trials 2007;8. https://doi.org/10.1186/1745-6215-8-25.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j.2002.384.doc.x.
- May CR, Finch T, Ballini L, MacFarlane A, Mair F, Murray E, et al. Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-245.
- Rapley T, Silverman D. Qualitative Research: Theory, Method and Practice. London: Sage; 2010.
- Glaser B. The constant comparative method of qualitative analysis. Soc Probl 1965;12:436-45. https://doi.org/10.2307/798843.
- Seale C. The Quality of Qualitative Research. London: Sage; 1999.
- Charmaz K. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. Thousand Oaks, CA: Sage; 2006.
- Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ 2011;342. https://doi.org/10.1136/bmj.d1548.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Hall JC, Platell C. Half-life of truth in surgical literature. Lancet 1997;350. https://doi.org/10.1016/S0140-6736(05)63577-5.
- Philips Z, Claxton K, Palmer S. The half-life of truth: what are appropriate time horizons for research decisions?. Med Decis Making 2008;28:287-99. https://doi.org/10.1177/0272989X07312724.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud 2013;50:587-92. https://doi.org/10.1016/j.ijnurstu.2012.09.010.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison JW. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002889.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748–5908–4-29.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Hackshaw A, Farrant H, Bulley S, Seckl MJ, Ledermann JA. Setting up non-commercial clinical trials takes too long in the UK: findings from a prospective study. J R Soc Med 2008;101:299-304. https://doi.org/10.1258/jrsm.2008.070373.
- Kearney A, McKay A, Hickey H, Balabanova S, Marson AG, Gamble C, et al. Opening research sites in multicentre clinical trials within the UK: a detailed analysis of delays. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005874.
- Salman RA-S, Brock TM, Dennis MS, Sandercock PAG, White PM, Warlow C. Research governance impediments to clinical trials: a retrospective survey. J R Soc Med 2007;100:101-4. https://doi.org/10.1177/014107680710000227.
- Snooks H, Hutchings H, Seagrove A, Stewart-Brown S, Williams J, Russell I. Bureaucracy stifles medical research in Britain: a tale of three trials. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471–2288–12–122.
- Thompson AGH, France EF. One stop or full stop? The continuing challenges for researchers despite the new streamlined NHS research governance process. BMC Health Serv Res 2010;10. https://doi.org/10.1186/1472-6963-10-124.
- Heaven B, Will C, Moreira T. Medical Proofs, Social Experiments: Clinical Trials in Shifting Contexts. Farnham: Ashgate; 2010.
- Lawton J, Jenkins N, Darbyshire J, Farmer A, Holman R, Hallowell N. Understanding the outcomes of multi-centre clinical trials: a qualitative study of health professional experiences and views. Soc Sci Med 2012;74:574-81. https://doi.org/10.1016/j.socscimed.2011.11.012.
- Hicks C. Nurse researcher: a study of a contradiction in terms?. J Adv Nurs 1996;24:357-63. https://doi.org/10.1046/j.1365-2648.1996.20218.x.
- Brody H, Miller FG. The clinician-investigator: unavoidable but manageable tension. Kennedy Inst Ethics J 2003;13:329-46. https://doi.org/10.1353/ken.2004.0003.
- Bury M. Chronic illness as biographical disruption. Sociol Health Illn 1982;4:167-82. https://doi.org/10.1111/1467-9566.ep11339939.
- Tam NT, Huy NT, Thoa le TB, Long NP, Trang NT, Hirayama K, et al. Participants’ understanding of informed consent in clinical trials over three decades: systematic review and meta-analysis. Bull World Health Organ 2015;93:186-9H. https://doi.org/10.2471/BLT.14.141390.
- Brown RF, Butow PN, Butt DG, Moore AR, Tattersall MH. Developing ethical strategies to assist oncologists in seeking informed consent to cancer clinical trials. Soc Sci Med 2004;58:379-90. https://doi.org/10.1016/S0277-9536(03)00204-1.
- Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics 2013;14. https://doi.org/10.1186/1472-6939-14-28.
- Garcea G, Lloyd T, Steward WP, Dennison AR, Berry DP. Differences in attitudes between patients with primary colorectal cancer and patients with secondary colorectal cancer: is it reflected in their willingness to participate in drug trials?. Eur J Cancer Care (Engl 2005;14:166-70. https://doi.org/10.1111/j.1365-2354.2005.00535.x.
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56. https://doi.org/10.1016/S0895-4356(99)00141-9.
- Schaefer KM, Ladd E, Gergits MA, Gyauch L. Backing and forthing: the process of decision making by women considering participation in a breast cancer prevention trial. Oncol Nurs Forum 2001;28:703-9.
- Canvin K, Jacoby A. Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-32.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. https://doi.org/10.1186/1745–6215–11–31.
- Rapley T. Distributed decision making: the anatomy of decisions-in-action. Sociol Health Illn 2008;30:429-44. https://doi.org/10.1111/j.1467–9566.2007.01064.x.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Wollman B, D’Agostino HB. Percutaneous radiologic and endoscopic gastrostomy: a 3-year institutional analysis of procedure performance. AJR Am J Roentgenol 1997;169:1551-3. https://doi.org/10.2214/ajr.169.6.9393163.
- NHS Reference Costs 2014 to 2015. London: Department of Health and Social Care; 2015.
- Wang J, Liu M, Liu C, Ye Y, Huang G. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for patients with head and neck cancer: a systematic review. J Radiat Res 2014;55:559-67. https://doi.org/10.1093/jrr/rrt144.
- Crombie JM, Ng S, Spurgin A-L, Ward EC, Brown TE, Hughes BGM. Swallowing outcomes and PEG dependence in head and neck cancer patients receiving definitive or adjuvant radiotherapy+/− chemotherapy with a proactive PEG: a prospective study with long term follow up. Oral Oncol 2015;51:622-8. https://doi.org/10.1016/j.oraloncology.2015.03.006.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81. https://doi.org/10.1080/01621459.1958.10501452.
- Williams GF, Teo MTW, Sen M, Dyker KE, Coyle C, Prestwich RJD. Enteral feeding outcomes after chemoradiotherapy for oropharynx cancer: a role for a prophylactic gastrostomy?. Oral Oncol 2012;48:434-40. https://doi.org/10.1016/j.oraloncology.2011.11.022.
- National Life Tables: 2012 to 2014. London: Office for National Statistics; 2014.
- Brown T, Ross L, Jones L, Hughes B, Banks M. Nutrition outcomes following implementation of validated swallowing and nutrition guidelines for patients with head and neck cancer. Support Care Cancer 2014;22:2381-91. https://doi.org/10.1007/s00520-014-2180-9.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Harvey SE, Parrott F, Harrison DA, Sadique MZ, Grieve RD, Canter RR, et al. A multicentre, randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of early nutritional support via the parenteral versus the enteral route in critically ill patients (CALORIES). Health Technol Assess 2016;20. https://doi.org/10.3310/hta20280.
- Head and Neck Cancer Treatment: Utility Valuation Study. London: National Institute for Health and Care Excellence; 2006.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-5.
- Kwong JPY, Stokes EJ, Posluns EC, Fitch MI, McAndrew A, Vandenbussche KA. The experiences of patients with advanced head and neck cancer with a percutaneous endoscopic gastrostomy tube: a qualitative descriptive study. Nutr Clin Pract 2014;29:526-33. https://doi.org/10.1177/0884533614532693.
- Osborne JB, Collin LA, Posluns EC, Stokes EJ, Vandenbussche KA. The experience of head and neck cancer patients with a percutaneous endoscopic gastrostomy tube at a canadian cancer center. Nutr Clin Pract 2012;27:661-8. https://doi.org/10.1177/0884533612457181.
List of abbreviations
- AE
- adverse event
- CI
- confidence interval
- CRT
- chemoradiation therapy
- EORTC
- European Organisation for Research and Treatment of Cancer
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – C30
- EORTC QLQ- H&N35
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Module for Head and Neck Cancer
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- GFR
- glomerular filtration rate
- GP
- general practitioner
- HCP
- health-care professional
- HNSCC
- head and neck squamous cell cancer
- HPV
- human papillomavirus
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IMRT
- intensity-modulated radiotherapy
- IQR
- interquartile range
- ITT
- intention to treat
- MDADI
- MD Anderson Dysphagia Inventory
- MDT
- multidisciplinary team
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- PEG
- percutaneous endoscopic gastrostomy
- PI
- principal investigator
- PIS
- patient information sheet
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- RIG
- radiologically inserted gastrostomy
- RT
- radiation therapy
- SAE
- serious adverse event
- SALT
- speech and language therapist
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SIV
- site initiation visit
- TMG
- Trial Management Group
- WTP
- willingness to pay
