Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/01/34. The contractual start date was in September 2008. The draft report began editorial review in January 2017 and was accepted for publication in August 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Samer Nashef received personal expenses from AtriCure, Inc. (Amsterdam, the Netherlands) for contributing to educational courses for surgeons interested in learning the maze procedure, independently of this study. AtriCure, Inc. is one of several manufacturers of atrial fibrillation ablation devices, the technology of which was used in atrial fibrillation surgery in this study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Sharples et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
The health problem
Atrial fibrillation (AF) is the most common disturbance of heart rhythm. It is characterised by an irregular heartbeat caused by low-amplitude, supraventricular oscillations. 1
The normal rhythm of the heart is sinus rhythm (SR). The stimulus to beat is triggered by the sinoatrial node. This results in atrial contraction while the stimulus is conducted to the atrioventricular node, which relays it to the ventricles, initiating ventricular contraction. AF is a condition whereby the atria beat unevenly because of abnormal electrical signals. As a result, there is no SR, the ventricles respond to the disordered atrial electrical activity in a haphazard fashion, the pulse becomes irregular and the entire heartbeat is abnormal.
Atrial fibrillation has a prevalence of 1–2% of the population in high-income countries. 2,3 In the UK, the prevalence of AF is 7.2% in patients aged ≥ 65 years and 10.3% in patients aged ≥ 75 years. 4 Prevalence is associated with age and comorbidities, such as obesity, diabetes and hypertension. With the advancing age of the population and the increasing prevalence of obesity, this proportion is likely to increase. 5
Consequences of atrial fibrillation
Atrial fibrillation can cause palpitations, chest pain, dizziness and breathlessness, and imposes a heavy burden on both patients and clinicians, as it has a considerable impact on quality of life (QoL) and NHS resources. 6
When the atria fibrillate, they lose their pumping action, and this has two very important sequelae.
There is blood stagnation in the atria, which can lead to clot formation. Blood clots can then exit the heart (thromboembolism), leading to stroke and other complications. There is an associated four- to fivefold increased risk of thromboembolic stroke in AF, and if AF is left untreated, around 1 in 25 patients will have a stroke. 7 The NHS devotes 5% of its budget to preventing and treating strokes, and 15% of strokes can be attributed to AF. 4 Drugs and other treatments can control AF, but not without complications. Routine anticoagulant drug treatment reduces the risk of stroke in AF patients by two-thirds, but this incurs an increased risk of bleeding and needs careful monitoring. The substantial burden of monitoring anticoagulant therapy usually falls on general practice, anticoagulant clinics and haematology laboratories.
When the atria do not pump, the heart is less efficient. The presence of AF exacerbates heart failure that arises from other heart conditions, and can itself cause heart failure, especially if the fibrillating atria dilate and this results in leakage of the mitral and tricuspid valves.
Treatment of AF and its consequences (antiarrhythmic and anticoagulant drugs, hospital monitoring and stroke treatment) is expensive for the NHS. Implementation of the 2006 National Institute for Health and Care Excellence (NICE) guidelines,6 on management of AF, was estimated to cost £21.86M per year. 3
Types of atrial fibrillation
Atrial fibrillation is classified into three distinct subgroups. In the Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation,8 paroxysmal AF is defined as recurrent AF (more than two episodes) that terminates spontaneously within 4 days, persistent AF is defined as AF that continues beyond 4 days and chronic or longstanding AF is persistent AF beyond 1 year. 8 These patterns of AF have slightly different electrophysiological mechanisms.
Diagnosis of atrial fibrillation
Atrial fibrillation is confirmed by electrocardiography (ECG) when the patient presents with symptoms of palpitations, as is the case with paroxysmal AF; however, both paroxysmal and more longstanding AF can be asymptomatic and, when that is the case, can be missed on the electrocardiogram, unless the AF coincides with the time at which ECG is carried out. This is especially important in assessing treatments aimed at correcting AF, as many historical series reported only the recurrence of symptomatic AF and have used only intermittent ECG for follow-up. 9 In the majority of published studies, success in the ‘resumption of SR’ was based on a single ECG recording at 12 months, which is not necessarily representative. More recently, there has been recognition of the importance of more robust AF documentation and Holter monitoring records have been reported in some AF trials. 10–12 Better evaluation of any residual AF after treatment, by continuous ECG monitoring over several days, documents the percentage of time for which a patient is in AF, and this is called the ‘AF burden’.
A number of studies (cited in Calkins et al. 8) suggest not only that AF increases the risk of a poor outcome from prospective cardiac surgery, but is also that AF an independent risk factor for early and late morbidity and mortality. This leads to the (unproven) hypothesis that efforts to eliminate pre-existing AF during cardiac surgery may improve survival and reduce adverse cardiac events after surgery.
Current treatment
Until the 1980s, AF was treated using antiarrhythmic drugs and direct current cardioversion. When that failed, AF was managed with rate control medication and anticoagulant drugs to reduce the risk of stroke. There has been a substantial development in our understanding of the pathophysiology of AF, and two very important findings have been the roles of the pulmonary veins and macro-re-entry circuits.
We now know that the majority of electrical trigger points that initiate AF lie within the pulmonary veins and not in the atria themselves. 13 Moreover, the maintenance of AF depends on the presence of macro-re-entry circuits, in which delayed conduction of the electrical signal means that the signal arrives at the originating point when that point is no longer refractory. These circuits are quite large (several centimetres). As a result of this knowledge, the maze procedure was developed in the 1980s by Cox and Boineau. 14 The procedure prescribed a number of surgical cuts aimed at achieving two objectives: electrical isolation of the pulmonary veins from the atria, thus dealing with the site of most AF trigger points, and further cuts in the atria to disrupt macro-re-entry circuits, thereby preventing the maintenance of the AF rhythm. The maze procedure therefore involves multiple cutting and sewing of the atria and pulmonary veins. Several studies have reported freedom from AF ranging from 75% to 90% after the Cox maze procedure (see Huffman et al. ’s9 systematic review and associated references), and one reported a 15-year success rate in restoring SR as high as 94%. 15 This traditional cut-and-sew technique, despite being available since 1987, has failed to achieve widespread use, as it is technically demanding and adds substantially to the operative burden of a heart operation. It is currently in very limited use by a few surgeons in a few centres and tends to be reserved for otherwise fit patients with severely symptomatic AF, who are prepared to take the risk of such a major intervention to relieve their symptoms.
Alternatives to the cut-and-sew maze procedure have been produced. A number of devices have been developed to achieve the electrical block needed, using energy sources to ablate atrial tissue. These have made a technically difficult and time-consuming operation easier, quicker and safer for cardiac surgeons to perform. Ablation devices use an energy source (heat, cold, radiofrequency or microwave) to replicate the lesion set produced by the cut-and-sew maze procedure. 16 As a rule, the procedure is safe and well tolerated and adds little to the length and burden of the operation, but these devices are a new and costly technology, which is currently being heavily marketed to treat AF. 17
There has been no direct comparison between the traditional Cox maze procedure and the ablation maze procedure,9 presumably because of the problems of incorporating such technically demanding surgery into an adequately powered randomised controlled design. However, a propensity analysis that matched patients who underwent the ablation maze procedure with those undergoing the Cox maze procedure showed no differences in freedom from AF at 3, 6 and 12 months afterwards. 18
Patients who have AF before surgery are generally older and have an increased procedural risk and other comorbidities, so that treating AF at the time of cardiac surgery may be advantageous to the patient. When the Amaze trial was planned, the only evidence supporting this came from five small randomised controlled trials (RCTs) of the ablation maze procedure as an adjunct to surgery. 11,19–22 These trials found that SR was restored in 44–94% of treated patients compared with 5–33% of control patients. The trials were small and follow-up was short. Success was mostly defined on the basis of single ECG recordings. No trial looked at patient-centred outcomes or cost-effectiveness. Despite this lack of robust evidence, the number of patients with AF undergoing open heart surgery and being offered concomitant ablation maze procedures, or ‘adjunct maze procedures’, was increasing. Although there were instances of its use as a standalone procedure, the widest use of the ablation maze procedure in the NHS was in patients already having cardiac surgery for other problems. 23 This trial was designed in response to the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme call to evaluate ablation devices that were being rapidly assimilated into NHS practice without formal assessment.
Patient benefit
It is essential to recognise that the primary justification of the ablation procedure is to treat symptomatic AF. 24 When the Amaze trial was in the planning stage, no effectiveness studies had investigated the QoL benefit and cost-effectiveness of maze procedures. Since the original grant application, four small randomised trials have reported on health-related quality of life (HRQoL) after the maze procedure. 12,25–27 No difference was reported in the overall QoL scores. However, patients in the studies by Gillinov et al. 12 and Van Breugel et al. 27 were not blinded to the treatment they received, which could have influenced the reporting of QoL outcomes. Cherniavsky et al. 25 reported improvement in the Short Form questionnaire-36 items (SF-36) score; however, the trial overall reported that the adjunct maze procedure arm did no better than coronary artery bypass graft operation (CABG) alone.
Recent evidence
A Cochrane collaboration review9 assessed the effects of adjunct AF surgery. 9 Using a comprehensive systematic review methodology, the authors identified 22 published trials (1899 participants) comparing cardiac surgery with and without adjunct AF surgery, with five additional ongoing studies and three studies not classified at the time of reporting. 10–12,19,20,22,25–40 All included studies were rated as being at a high risk of bias in at least one domain assessed. The Cochrane review9 found that AF surgery, regardless of technique, doubles the rate of freedom from AF, atrial flutter and atrial tachycardia [51.0% vs. 24.1%; relative risk (RR) 2.04, 95% confidence interval (CI) 1.63 to 2.55], with more patients not taking antiarrhythmic medication 3 months after cardiac surgery. There was little evidence of a difference between patients with AF who were treated and those who were not treated in either 30-day mortality (2.3% vs. 3.1%; RR 1.25, 95% CI 0.71 to 2.20) or all-cause mortality (7.0% vs. 6.6%; RR 1.14, 95% CI 0.81 to 1.59). However, patients who were treated for AF were more likely to be fitted with a permanent pacemaker (6.0% vs. 4.1%; RR 1.69, 95% CI 1.12 to 2.54). The review authors concluded that there remained uncertainty about the effects on cardiovascular mortality, adverse events (AEs), HRQoL and long-term outcomes. 9
In summary, there is little rigorous evidence that attempting to restore SR by treating AF with an ablation device during cardiac surgery is of benefit to the patient. Nevertheless, these devices are being incorporated into routine practice nationally and internationally. The Amaze trial provided a timely evaluation of this technology with the objective of assessing the clinical and HRQoL benefits for patients, as well as cost-effectiveness for the NHS.
Chapter 2 Amaze trial methods
Objectives
Primary objectives
The primary objectives were to compare patients undergoing the maze procedure as an adjunct to routine cardiac surgery with patients undergoing routine cardiac surgery alone, in terms of:
-
return to stable SR at 12 months
-
quality-adjusted survival over 24 months after randomisation.
Secondary objectives
The main secondary objective was to assess the cost-effectiveness of the adjunct maze procedure, relative to cardiac surgery alone, from a NHS perspective.
Other secondary objectives were to compare the following outcomes between the two arms:
-
return to stable SR at 24 months after surgery
-
overall survival
-
thromboembolic neurological complications (e.g. stroke)
-
stroke-free survival
-
anticoagulant and antiarrhythmic drug use up to 24 months post randomisation
-
HRQoL up to 24 months post randomisation, as measured by the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), the SF-36 and the New York Heart Association (NYHA)
-
resource use and costs.
Exploratory analyses
Prespecified subgroup analysis was planned to explore differences in treatment effects between:
-
patients with paroxysmal AF and non-paroxysmal AF (i.e. persistent, chronic or longstanding AF)
-
individual centres (as a random effect)
-
cardiac surgical procedures
-
surgeons.
Within the maze treatment arm, analysis was planned to explore differences between:
-
different ablation devices
-
different lesion sets treated.
Design
Overview
The Amaze trial was a Phase III, pragmatic, multicentre, double-blind, parallel-arm RCT to compare clinical, patient-based and cost outcomes for patients with pre-existing AF who undergo routine cardiac surgery either with or without an adjunct device-based ablation procedure.
Eligible patients were randomised (in a 1 : 1 ratio) to receive either:
-
planned routine cardiac surgery with no additional procedure
-
planned routine cardiac surgery with an additional device-based AF ablation procedure.
The study was reviewed and approved by the Essex 1 Research Ethics Committee (reference number 08/H0301/98) and was registered as International Standard Randomised Controlled Trial Number 82731440 (ISRCTN82731440). The trial protocol can be accessed at www.papworthhospital.nhs.uk/research/data/uploads/ptuc/protocol-v4-may-20151.pdf (accessed 2 March 2018)41 and the HESTER (Has Electrical Sinus Translated into Effective Remodelling?) substudy protocol can be accessed at www.papworthhospital.nhs.uk/research/data/uploads/ptuc/hester-study-protocol-3.pdf (accessed 2 March 2018). 42
As much as possible, the design and reporting of this trial adhered to the guidelines of the Consolidated Standards of Reporting Trials (CONSORT) statement43 and incorporated recommendations of the Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. 24
Patient and public involvement
Mr Brian Elliott was an independent member and lay representative on the Trial Steering Committee (TSC), providing input on all aspects of the trial design, conduct and recruitment strategies. Professor Paul Kinnersley has a background in primary care and public health and provided further independent advice on the conduct and progress of the trial through membership of the TSC.
Setting and investigators
Eleven acute NHS specialist cardiac surgical centres (Papworth Hospital, Cambridgeshire; Royal Brompton Hospital, London; Brighton and Sussex University Hospitals, Brighton; University Hospitals Coventry and Warwickshire NHS Trust, Coventry; Glenfield Hospital, Leicester; Wythenshawe Hospital, Manchester; Northern General Hospital, Sheffield; Guy’s and St Thomas’ Hospital, London; Derriford Hospital, Plymouth; Freeman Hospital, Newcastle upon Tyne; and Blackpool Victoria Hospital, Blackpool) participated in the study, which was co-ordinated by the Papworth Trials Unit Collaboration. For surgeons to participate, they were required to be experienced in the use of ablation devices for at least 2 years. During the trial design period, participating surgeons met to agree the permissible procedures, ablation methods and lesion sets to be treated.
Participants
Consecutive cardiac surgical patients undergoing major cardiac surgery (e.g. coronary, valve or combined operations), with a history of paroxysmal, persistent or chronic AF beginning > 3 months before the date of the operation, were screened for eligibility.
-
Paroxysmal AF was defined as recurrent AF (two or more episodes) that terminated spontaneously within 4 days. 24
-
Non-paroxysmal but persistent AF was defined as AF that continued for > 4 days.
-
Chronic or longstanding AF was defined as AF that was persistent for > 1 year.
The Amaze trial inclusion criteria included patients who:
-
were aged > 18 years
-
were scheduled to undergo elective or in-house urgent cardiac surgery (coronary surgery, valve surgery, combined coronary and valve surgery or any other cardiac surgery requiring cardiopulmonary bypass)
-
had a history of documented AF (chronic, persistent or paroxysmal) beginning > 3 months before entry into the study
-
were willing to provide written informed consent to participate.
Exclusion criteria included patients who:
-
had had previous cardiac operations
-
had had emergency or salvage cardiac operations
-
had had surgery without cardiopulmonary bypass
-
were unlikely to be available for follow-up over a 2-year period
-
were unable to provide consent.
Recruitment
The procedure for informing and obtaining consent from patients was devised to accommodate local variations in the patient pathway, but was otherwise identical for all centres. At most centres, potential participants were initially given a simple summary of the study by the local investigator at the initial surgical clinic when treatment options were discussed. Before the next attendance, the trial co-ordinator or a research nurse contacted the patients by telephone to assess interest in the trial, and posted the full patient information sheet to the homes of those who expressed an interest. Written consent was taken at the pre-admission clinic, at approximately 2 weeks prior to surgery, by the trial co-ordinator or research nurse. HRQoL questionnaires were administered by the research nurse, after consent, at this pre-admission clinic. Thereafter, patients were registered for the trial and provided with a 4-day ECG recording device and instructions on its use, to take home to monitor their heart rate for 4 days. On admission for surgery, the patient returned the 4-day ECG recorder.
Randomisation
Eligible patients who satisfied the inclusion criteria and provided written consent were randomised (in a 1 : 1 ratio) to receive either their planned cardiac surgery with no additional procedure or their routine cardiac surgery with an adjunct maze procedure.
The allocation sequence was generated by permuted block randomisation (using block sizes of 6 and 8), and randomisation was stratified by surgeon and planned cardiac procedure (CABG, aortic valve, mitral valve or combined procedure). On the day of surgery, when the patient was in the anaesthetic room, the local centre contacted the Papworth Trials Unit Collaboration by telephone. Patient details, surgeon and planned cardiac procedure were registered with the Papworth Trials Unit Collaboration, whose staff were not otherwise involved with the trial. Once registration was complete, the allocation was released to the surgical team, which was also responsible for completing the surgical clinical report form (CRF). The treatment allocation was not made available to any other staff who were directly or indirectly involved in the trial.
Blinding
Although theatre staff could not be blinded to the treatment allocation, the trial was double-blind to the extent that neither the patients themselves, nor any researchers collecting HRQoL outcomes nor the cardiologists assessing the 4-day ECG results were aware of the trial arm to which the patient had been allocated.
Patients’ medical notes were labelled to indicate that they were Amaze trial participants. Routine reports provided details of their elective surgery and the fact that they were randomised within the Amaze trial. The surgical CRF describing the research intervention (maze procedure or no maze procedure) was placed in a sealed envelope labelled ‘The Amaze Trial’ and kept in the patient’s notes. Clinicians were able to access this information in the event of a serious adverse event (SAE) considered to be related to surgery. At discharge, the data management staff retrieved the sealed envelope and uploaded the procedure details onto a secure database, before resealing the envelope and returning it to the notes. Cardiologists who analysed electrocardiograms for the primary outcome and researchers recording HRQoL outcomes did not have notes, including the procedural information, available at the time of outcome assessment.
Interventions
Control arm
The Amaze trial was planned as a pragmatic trial, following standard treatment and care as closely as possible in order to assess outcomes in a real-world context. Patients randomised to the control arm received preoperative management, elective or in-house urgent cardiac surgery and postoperative management in accordance with standardised hospital protocols.
Experimental arm
Patients randomised to the experimental arm received preoperative management and elective or in-house urgent cardiac surgery, as described in standardised hospital protocols. During the operation, the conduct of the adjunct maze procedure and the choice of lesion set was at the surgeon’s discretion. At the time of trial design, there was no evidence for the superiority of one ablation device or one energy source over another. Therefore, any AF ablation device that was routinely used within the NHS by the investigators was permitted. This allowed surgeons to use the devices with which they were most familiar and comfortable, and which were in routine use at their institution. These included bipolar and unipolar radiofrequency, ‘cut-and-sew’, cautery, cryotherapy, ultrasound, laser and microwave energy. Postoperative management, subsequent follow-up and data collection were identical to the control arm.
Standardisation between centres
In order to minimise potential confounding by other components of a patient’s care, the following aspects of the trial were standardised across participating hospitals.
Management of patients before, during and after surgery
Management was undertaken in accordance with the local site’s normal practice, irrespective of randomisation. The only exceptions were processes required to maintain the blinding of the patient, cardiologist and QoL interviewer (see Blinding).
Conducting and reporting on the adjunct maze procedure and defining the prescribed lesion set
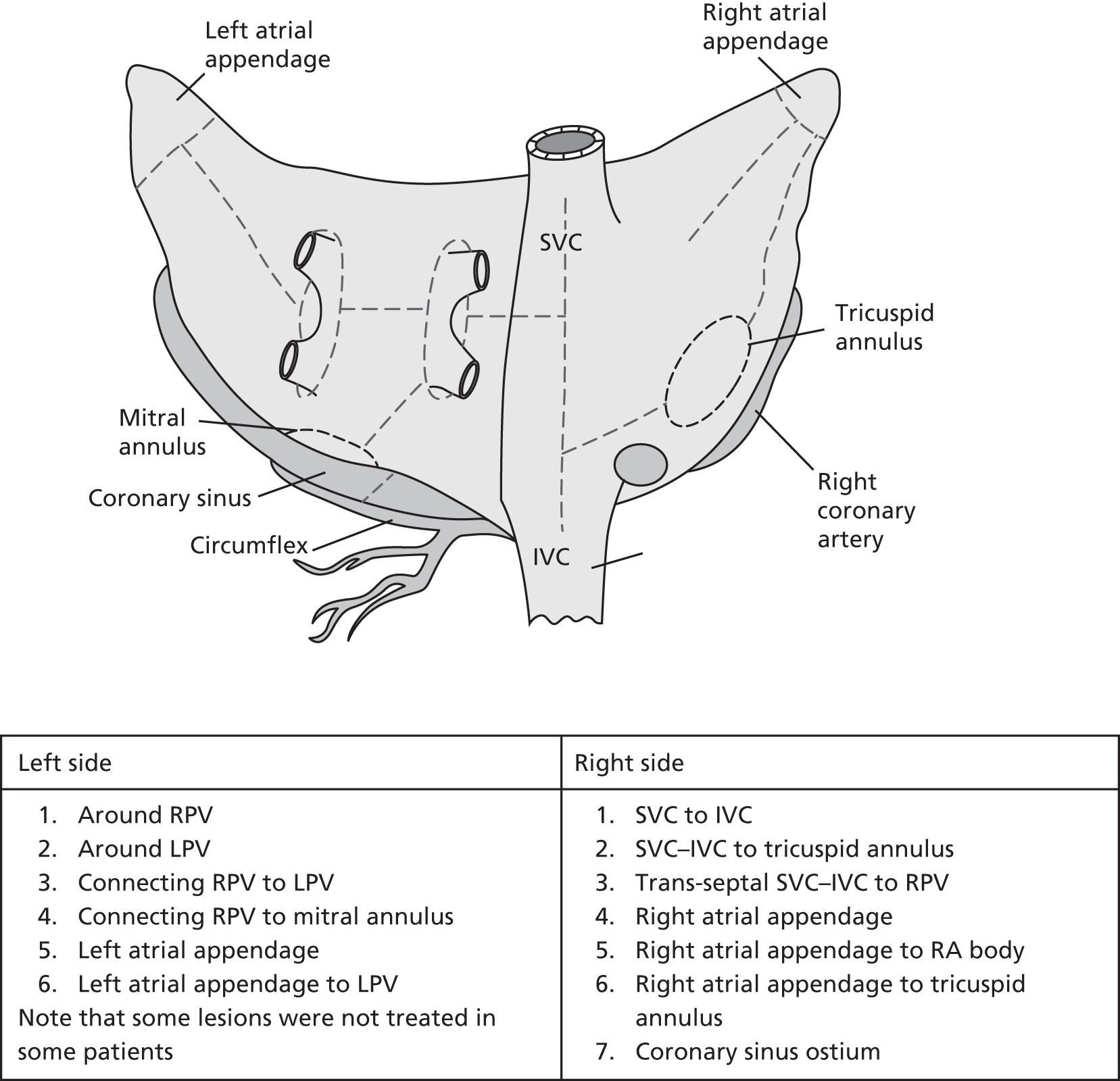
The full lesion set considered is illustrated in Figure 1, although the specific lesion set treated was left to the participating surgeon’s discretion. Details of the ablation procedure and all lesions treated were documented. Published guidelines for reporting data and outcomes for surgical treatment of AF were followed. 8,15
FIGURE 1.
Complete modified Cox maze procedure III lesion set. IVC, inferior vena cava; LPV, left pulmonary vein; RA, right atrium; RPV, right pulmonary vein; SVC, superior vena cava.
Postoperative drug use
-
Amiodarone: unless contraindicated, 200 mg three times per day was prescribed, reducing over a period of 3 weeks to 200 mg per day for 6 weeks. The drug was stopped if stable SR was established at 6 weeks. Further prescription after this period was based on individual clinical judgement.
-
Warfarin: prescribed until the patient was in stable SR. Thereafter, centres adopted normal practice.
-
Beta-blockers: prescribed at the individual clinician’s discretion.
-
Other drugs: prescribed at the individual clinician’s discretion; cardiac drugs with antiarrhythmic, antihypertensive and anticoagulant actions, including aspirin and warfarin, were documented.
Indications for cardioversion, timing and number of attempts
The protocol did not require cardioversion to be carried out at discharge, but if it was performed for clinical reasons, the details were recorded. For patients in AF at the first follow-up appointment, cardioversion was attempted within 3 months of surgery. If cardioversion was unsuccessful, then it was attempted again at 6 months after surgery.
Outcome measures
Primary outcomes
Return to sinus rhythm at 12 months
Sinus rhythm at 12 months after surgery was determined by the absence of any AF on outputs from continuous monitoring by 4-day ECG recorders. All 4-day continuous ECG recordings were analysed centrally at Papworth Hospital. Participating hospitals forwarded the anonymised secure digital cards from the ECG recorders to Papworth Hospital. Analyses using the proprietary automated software package, together with manual checking of the recording in its entirety, were completed by cardiologists who were not aware of the patient’s identity or allocated treatment arm. Total time spent in SR and in AF (AF burden) during the 4-day recording was calculated. Episodes of atrial flutter were noted and included in the AF burden.
Quality-adjusted survival over 2 years
The EQ-5D-3L was administered at randomisation, on discharge and at 6 weeks and 6, 12 and 24 months after the procedure (Table 1). 44 Although the current version of the EuroQol-5 Dimensions has five levels for each item, when the Amaze trial was designed, the three-level version was recommended for the cost-effectiveness analysis. 45 EQ-5D-3L responses were converted into utility scores reflecting values from a representative sample of the UK population. 46 Clinical effectiveness was measured by quality-adjusted life-years (QALYs) over 2 years using the area under the curve method (see Statistical analyses). 47
| Type | Questionnaire | Description |
|---|---|---|
| Generic | EQ-5D-3L |
|
| Generic | SF-36 |
|
| Specific | NYHA | Breathlessness was classified on a four-point scale:
|
Secondary outcomes
Sinus rhythm was determined by the absence of any AF on 4-day ECG recorders at 24 months after surgery. Other secondary outcomes were overall survival from the date of randomisation to date of death (all patients were registered with the Office for National Statistics’ tracking system to allow long-term follow-up of survival); stroke-free survival, defined as the time between randomisation and the date of stroke or death (whichever occurred first); incidence of hospital admission for (anticoagulant-related) haemorrhage, anticoagulant and antiarrhythmic drug usage up to 24 months after randomisation; HRQoL measured by the EQ-5D-3L, the SF-36 and the NYHA for breathlessness (completed at baseline, on discharge and at 6 weeks and 6, 12 and 24 months post surgery after randomisation); and resource use and trial-based cost-effectiveness of the adjunct maze procedure up to 24 months after randomisation.
Health-related quality of life
Health-related quality-of-life interviews were conducted by clinical research co-ordinators/research nurses in face-to-face interviews at hospital research clinics. Six-month questionnaires were administered by telephone, as patients did not have clinical appointments. The questionnaires administered are summarised in Table 1. The SF-36 consists of eight dimensions, which are the weighted sums of the questionnaire item responses. Each dimension ranges from 0 to 100, with a higher score representing better health or fewer limitations for that domain. Standardised physical and mental health scores were calculated, which, for a general UK population, are expected to be approximately normally distributed with a mean of 50 and a standard deviation (SD) of 10. 48 For missing items, we used the methods recommended in the manual. 49 Briefly, if at least half the items were available for any scale, the mean of the recorded items was imputed for the missing items. If more than half the items for a scale were missing, then the scale was recorded as missing.
Sample size
The dual primary outcomes were a clinical end point (return to SR at 12 months) and an outcome of importance to patients and service providers (quality-adjusted survival over 2 years). The maze procedure was considered effective if there was a significant effect for return to SR or if the mean difference in QALYs between the groups did not include zero. No adjustments were made to the sample size to accommodate the multiple testing of these two outcomes, which is inherent in this approach, as the focus for the QALY end point was on estimation of the treatment effect, rather than hypothesis testing. However, to guard against overinterpretation of hypothesis tests, we recommend that p-values between 0.025 and 0.05 are considered to have borderline significance.
Return to sinus rhythm at 12 months
Prior to the trial, published RCTs of ablation as an addition to cardiac surgery reported rates of return to SR at 12 months ranging from 44% to 87% in the maze procedure arms, and from 5% to 33% in the control arms. 19,22 We took a conservative estimate of the difference between the arms (45% vs. 30%) as the target effect. In order that a realistic recruitment target was achieved, 80% power was used in the calculation. Combining this with a two-sided significance of 5%, an estimated sample size of 176 in each arm (total of 352) would be sufficient to detect this effect. With planned recruitment of 400 patients, this allowed for approximately 15% death/loss to follow-up at 12 months.
Quality-adjusted survival over 2 years
The emphasis in cost-effectiveness studies is on estimation, rather than hypothesis testing, so that formal sample size calculations were considered less important. However, we provided a power calculation based on the effectiveness measure ‘QALYs at 2 years post randomisation’. We could find no studies reporting comparative QALYs in similar patients undergoing ablation and cardiac surgery. From previous studies of patients undergoing angiography for suspected ischaemic heart disease and patients with refractory angina, the SD of QALYs over 12 and 18 months was at most 0.3. 50,51 Over 2 years, the minimum clinically important improvement was considered to be 1 extra month of quality-adjusted life or 0.083 QALYs. With a sample of 200 patients per arm (total of 400), we would have exactly 79% power to detect a difference of 0.083 QALYs (at a two-sided significance of 5%).
If the accepted threshold for cost-effectiveness was in the range £20,000–30,000 per QALY and we could demonstrate a significant increase in QALYs of 0.083, then the procedure would be cost-effective for an incremental cost of, at most, £2500.
Failure to reach target recruitment
Based on audit data, our target recruitment of 400 patients was expected to be achieved in 18 months at six centres. Owing to the slower than expected accrual, recruitment terminated in September 2014, when 352 patients had been randomised, with approximately 70% power to identify the target treatment effects.
Analysis populations
Intention-to-treat population
The primary analysis used the intention-to-treat (ITT) population, defined as all randomised patients, regardless of eligibility, withdrawal, compliance with the protocol, loss to follow-up or actual treatment received. No patients withdrew consent for their data to be used, despite withdrawing from trial follow-up. Multiple imputation was used for missing primary outcomes.
Quality-of-life population
For each instrument (the SF-36 and the EQ-5D-3L), all patients who returned a completed baseline questionnaire, regardless of subsequent questionnaire return, were included in the analysis. In addition, imputation (based on planned procedure and centre) of missing baseline EQ-5D-3L scores was completed for two patients.
Safety population
All patients were included in the safety population if they underwent a surgical procedure. Patients were included in the arm corresponding to the intervention received (maze procedure completed vs. no maze procedure).
Statistical analyses
All statistical analyses and reporting complied with CONSORT guidelines where possible. 52
Formal analyses were conducted using a two-sided 5% level of significance, with no adjustment for multiple testing. All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA). The statistical analysis plan is provided in Appendix 1.
In descriptive summaries, the number of non-missing items and the mean (SD) or median (upper and lower quartiles) were summarised for continuous variables, and the number and proportion by treatment arm were summarised for each level of categorical variables.
Return to sinus rhythm
The odds of being in SR for maze procedure patients was compared with the odds for control patients, and estimated using a binary logistic regression model, including surgeon (normal random effects on the logistic scale), baseline heart rhythm and planned surgical procedure (fixed effects). The odds ratio (OR) for return to SR was reported with the 95% CI and p-value from this model. Validity of logistic regression models was assessed by examining the following statistics and graphical summaries:
-
Pearson residuals/deviance (half-normal plots)
-
leverage values
-
Cook’s distance
-
cross-validation probabilities (the probability of a particular observation, conditional on the remaining observations)
-
L-statistics (the influence of an observation on the difference in deviance as a result of fitting the treatment effect).
The percentage of time in AF across the 4 days of monitoring at baseline and at 12 months was summarised by treatment arm.
Quality-adjusted survival
For the primary outcome, QALYs over 2 years were estimated from serial measurements of the EQ-5D-3L for each patient. The UK social tariff for the EQ-5D-3L, completed at baseline and on discharge, and at 6 weeks and 6, 12 and 24 months post surgery, as estimated by Dolan et al. ,46 was applied to calculate utility values. Using actual rather than nominal times of assessment, and assuming a linear change in values between time points, patient-specific utility curves up to 24 months post randomisation were calculated. A value of zero was assigned at the date of death for patients who died. QALYs were calculated as the area under the utility curve to 24 months or date of death, whichever occurred first. In order to adjust for differences in baseline utilities, a linear regression was fitted to the utilities post treatment, with baseline utility and treatment arm as explanatory variables. For patients who did not complete all EQ-5D-3L questionnaires or those who were censored, multiple imputation was used to estimate mean QALYs (see Missing data). Model fit was assessed by examining standardised residuals and association with the predicted values, as well as identifying influential observations by referring to leverage statistics. A CI for the true difference in QALYs was estimated using a non-parametric bootstrap resampling approach. 53
Note that, for the primary outcome analysis, no discounting of the QALYs estimates was applied. However, when costs and benefits were estimated for the health economics analysis, both were discounted at 3.5% for the second year.
Missing data
Proportions of, and reasons for, missing data were investigated.
For the clinical primary end point (return to SR at 12 months), if a patient withdrew consent or was lost to follow-up within 12 months, the missing outcome (AF or SR at 12 months) was multiply imputed as a function of the baseline heart rhythm, surgeon, surgical procedure and treatment arm. 54 Rubin’s rules were used to combine imputed data sets. 55
For the patient-based primary end point (QALYs), whereby a patient died before the end of follow-up, the utility value of 0 was imputed for all subsequent assessments. If the response was missing, and the patient was alive, the missing value was imputed using the method of multiple imputation. 54 A number of sensitivity analyses related to missing data were completed; further details are provided in Appendix 1. In response to a reviewer’s request, we also provided a supportive analysis using complete cases, but with predictors of missingness included in the model.
Subgroup analysis
Prespecified subgroups are listed above (see Exploratory analysis).
For the SR end point, a logistic regression model was fitted to heart rhythm at 12 months, including baseline heart rhythm, surgeon, surgical procedure, treatment arm and subgroup variable of interest and its interaction term with the treatment arm. Within-subgroup treatment effects and the interaction effect between subgroup and treatment arm were estimated with 95% CIs and p-values.
For the QALYs end point, a linear regression model was fitted to the area under the utility curve, with baseline EQ-5D-3L score, surgeon, surgical procedure, treatment arm, subgroup variable and the subgroup-by-treatment interaction variable.
Because analysis revealed an increasing OR for the maze procedure arm relative to the control arm as the trial progressed, we explored changes in baseline characteristics, surgery and cointerventions throughout the trial in an attempt to explain this finding.
Secondary end point analysis
Return to stable SR at 24 months was analysed in a similar way to return to SR at 12 months, using a binary logistic regression model, including baseline heart rhythm, surgeon, surgical procedure and treatment arm.
Overall survival was summarised using Kaplan–Meier methods for the time between randomisation and death. Patients who were alive at the end of the study, or who withdrew before the end of follow-up, were censored at the date they were last seen. Similarly, stroke-free survival, defined as the time between randomisation and the date of stroke or death, whichever occurred first, was summarised using Kaplan–Meier methods. Patients who were alive and stroke free at the end of the study, or who withdrew without having suffered a stroke before the end of follow-up, were censored at the date they were last seen. Cox regression models were used to estimate hazard ratios (HRs) for maze procedure patients relative to control patients.
Patients who had a stroke within 12 months of surgery, and the overall proportion of stroke events, were calculated by treatment arm, using the total number of patients participating in the trial as the denominator. The relationship between stroke and treatment arm was tested by Fisher’s exact test, and the difference in stroke rates was reported along with 95% CIs for differences in proportions.
Short Form questionnaire-36 items dimension scores and summarised mental component score (MCS) and physical component score (PCS) were analysed using a linear regression model, including time point, treatment arm, time-by-treatment-arm interaction and baseline SF-36 scores (all modelled as fixed effects), and allowing random intercepts for patients.
Drug use for each arm was tabulated by time point (at baseline, discharge, 6 weeks and 6, 12 and 24 months) and drug category. Logistic regression for the outcome of each patient (1 = have one or more drugs during time period t, 0 = have no drugs during time period t) was fitted, including drug category, time period of drug usage, baseline drug usage and treatment arm as independent variables. ORs were estimated with 95% CIs and p-values.
The occurrence of atrial flutter and atrial tachycardia (organised atrial arrhythmia) and junctional rhythm were summarised by arm. The relationship between the completeness of the lesion set and the occurrences of organised atrial arrhythmia and junctional rhythm was tabulated.
Safety analysis
The number of AEs in each category and deaths from any cause were summarised by treatment arm, corresponding to the treatment received. Events were summarised according to whether or not they met the criteria of SAEs, severity and relationship to the procedure.
Economic analysis
Data collection and sources
NHS resource use was collected during primary admission and at the 6-week and 6-, 12- and 24-month follow-ups. Research nurses/clinical trial co-ordinators extracted data about inpatient stay from individual patient records and administered bespoke questionnaires about follow-up health service use either face to face or by telephone/post (for those who missed an appointment). Hospital records were checked to validate patient-reported hospital readmission.
Resources related to the primary admission (from randomisation to discharge) included theatre use (initial operation and returns to theatre), intensive care (days) and cardiac and acute care wards (days). The total length of stay was compared with the sum of recorded days in an intensive care unit (ICU) and ward, and any double-counting that was identified was subtracted. For patients who were not discharged home, subsequent admissions to rehabilitation centres or acute hospitals were added. Surgery-specific resource use, including equipment and energy sources for the maze procedure, were retrieved from patient notes.
The resource use recorded during follow-up was divided into three categories: hospital readmissions (length of stay in hospitals or rehabilitation centres), 16 types of test [e.g. cardiac related, magnetic resonance imaging (MRI) and radiographic] and 12 types of health-care visit [e.g. accident and emergency (A&E), outpatient, primary and community health services]. In all resource-use calculations, a value of zero was assigned to any unused resource item, including all resource items after death.
Medication use was limited to antiarrhythmic, anticoagulant and antiplatelet drugs and seven classes of cardiac drugs (beta-blockers, diuretics, calcium channel blockers, nitrates, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and statins). The daily dose of each drug type was based on patient record review for inpatient stay and patient reports after discharge. As specific drug names were not recorded for cardiac drugs, the chief investigator (SN) identified the most likely drugs to be prescribed, and the daily dose was taken to be the most common reported daily dose in the CRFs for that category of drugs. For example, bisoprolol and atenolol were the assumed drugs when beta-blocker was indicated, with the dose equalling that reported by 90% of respondents.
The total amount of each drug used per patient was estimated by taking a mid-value of daily dose at consecutive follow-up time points, multiplied by duration between follow-up time points, and costed using the NHS Prescription Services Electronic Drug Tariff56 and the British National Formulary (2016). 57 Missing drug use at each time point was replaced, depending on the nature of missingness, as follows: patients who died were assigned zero medication use from date of death; if a patient indicated use of a drug at only one follow-up, the duration was taken to be the mid-point between this and the next follow-up (except for drugs recorded only at baseline, which were excluded, as the trial focused on drugs post randomisation). For patients who either had completely missing drug data at a follow-up time point or were lost to follow-up, costs were multiply imputed using chained equations with predictive mean matching, stratified by treatment arm (see Missing data).
Unit costs were multiplied by the frequency of resource use to provide total resource cost for each item. National estimates of unit prices were sourced58,59 to increase generalisability. For resources for which national prices were not available, estimates were sourced either from the literature (e.g. 24-hour blood pressure monitoring and chest radiography) or from Papworth Hospital (e.g. theatre cost and cost of device). The hospital and community health services pay and price index58 was applied to adjust for inflation when necessary (see Appendix 2, Table 23). The ablation device was costed at £3000 per patient for high-intensity focused ultrasound, and £1250 per patient for all other methods. All resource costs, from the date of operation (randomisation) up to 2 years post randomisation, were summed, with year 2 costs discounted at 3.5%. 45
Health-related quality of life, assessed using the EQ-5D-3L and SF-36 questionnaires, was an important outcome, and is described in Secondary end point analysis. SF-36 health state responses were converted to the Short Form questionnaire-6 Dimensions (SF-6D) utility scale using values from the UK population. 47 QALYs, as described in Statistical analyses on page 12, were discounted at 3.5% in year 2 for the cost-effectiveness analysis. 45
Missing data
Missing baseline variables that were required for the imputation model, for example missing baseline EQ-5D-3L assessments, were replaced by the mean value for each trial arm. 60 Logistic regression identified variables that were related to missingness.
Missing resource use and utility data were imputed jointly using chained equations with predictive mean matching. The imputation models included age, sex, paroxysmal AF and baseline EQ-5D-3L score, and were stratified by trial arm. A total of 60 imputed data sets were created to attain a stable imputation. The distribution of imputed values was checked for comparability with observed data [e.g. counts of general practitioner (GP) visits, matched observations].
For 28 resource-use variables (tests and health-care visits), multiple imputation at each data collection point was not possible, as a result of the small numbers of events, and, therefore, the annual average for each resource-use variable was imputed for each arm. To assess the sensitivity of results to this assumption, an alternative imputation model was fitted, in which each test and health-care visit was multiplied by the corresponding unit cost. All tests and (separately) all health-care visits were grouped for each trial data collection point using total cost, and multiple imputation was applied to these categories of resource-use cost.
Incremental cost-effectiveness analysis and sensitivity analyses
Differences in estimated costs and QALYs between trial arms were explored using two-sample t-tests with equal variances. Linear regression analysis was used to adjust for differences in age, sex, baseline EQ-5D-3L score, AF at baseline and, for QALYs only, the primary surgery [isolated mitral valve replacement or repair (MVR), isolated CABG, isolated aortic valve replacement or repair (AVR), CABG and MVR, CABG and AVR and all others]. The incremental cost-effectiveness ratio (ICER) was calculated using adjusted mean estimates of costs and QALYs from ‘seemingly unrelated regression’, to allow for correlation between costs and effects at the patient level, and for skewness of data.
One thousand bootstraps were generated for each sample for the probabilistic sensitivity analysis. Costs for the resource-use components were sampled from gamma distributions and applied to the bootstrapped samples, and the total costs and QALYs for each sample were estimated using seemingly unrelated regression. The probability that the maze procedure was cost-effective was considered at varying willingness-to-pay (WTP) threshold values, using cost-effectiveness planes, the cost-effectiveness acceptability curve and incremental net monetary benefit (INMB).
Deterministic and probabilistic sensitivity analyses were used to explore the robustness of cost-effectiveness results that adopted different methodological approaches or assumptions. These analyses included the use of SF-6D QALYs, clinical effectiveness as measured by conversion of AF to SR, complete case analysis, examining costs and QALYs only up to discharge, examining the impact of outliers, excluding maze device cost, limiting the patient group to those randomised from April 2001 (to match the time-based post hoc statistical analysis) and an alternative imputation technique. The probability distribution of unit costs could not be resampled when the alternative imputation technique was used, as the imputation was for cost at each follow-up point, rather than resource use.
Chapter 3 Trial results: clinical effectiveness and health-related quality of life
Recruitment and compliance
Between 25 February 2009 and 6 March 2014, 1013 patients were screened for the Amaze trial in 11 UK specialist cardiac surgery centres: (1) Papworth Hospital, Cambridgeshire (n = 546); (2) Glenfield Hospital, Leicester (n = 186); (3) Derriford Hospital, Plymouth (n = 95); (4) Freeman Hospital, Newcastle (n = 72); (5) Northern General Hospital, Sheffield (n = 49); (6) Blackpool Victoria Hospital, Blackpool (n = 27); (7) Royal Brompton Hospital, London (n = 16); (8) Guy’s and St Thomas’ NHS Foundation Trust, London (n = 13); (9) Wythenshawe Hospital, Manchester (n = 10); (10) University Hospitals Coventry and Warwickshire NHS Trust, Coventry (n = 4); and (11) Brighton and Sussex University Hospitals, Brighton (n = 3). The flow of Amaze trial patients from the initial screening to final follow-up is illustrated in Figure 2.
FIGURE 2.
Patient flow through the Amaze trial. PAPVD, partial anomalous pulmonary venous drainage.
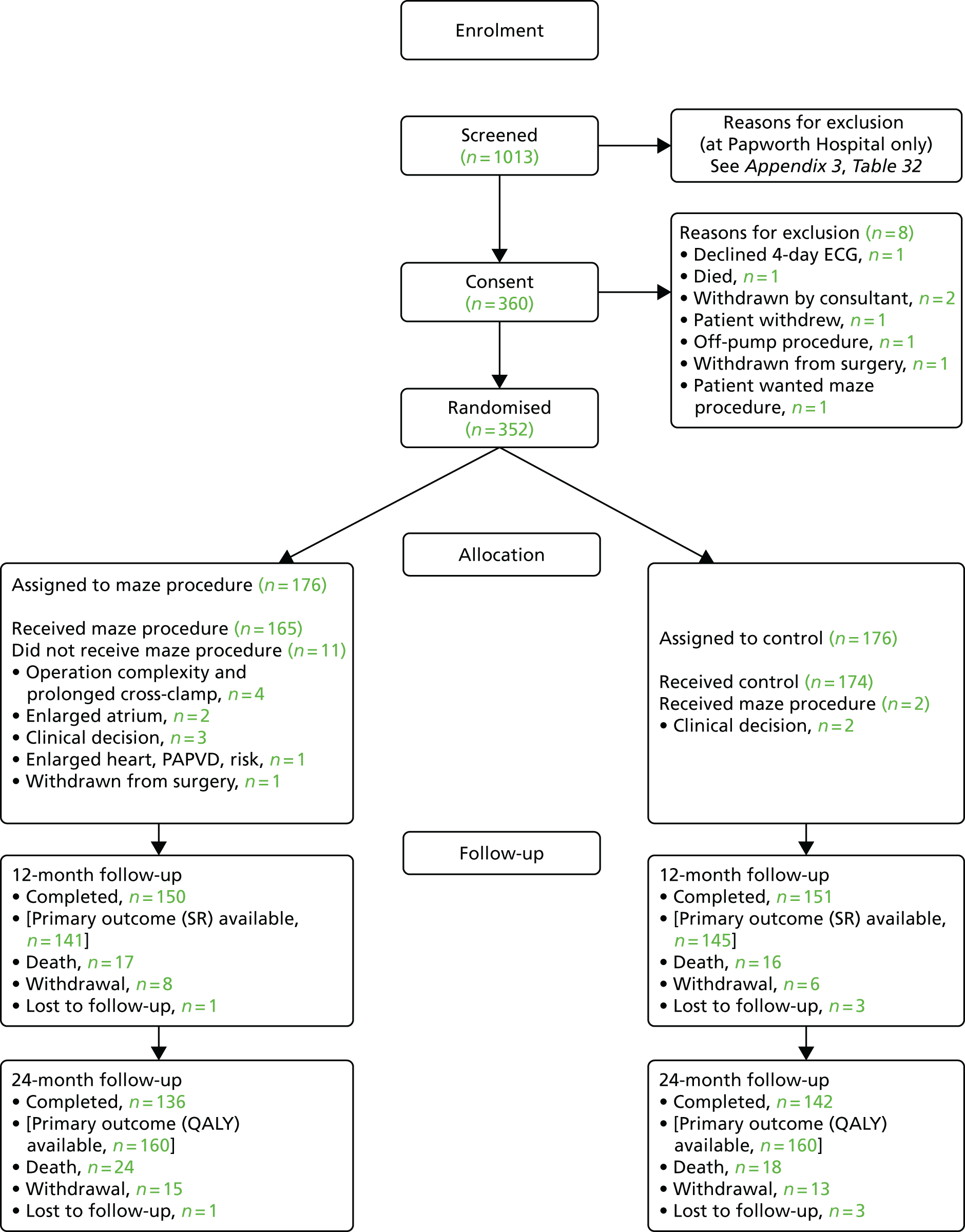
A total of 661 patients were excluded at screening, but screening logs were only completed for all patients in the co-ordinating centre (Papworth Hospital). At Papworth Hospital, 366 out of 546 patients (67%) were excluded between registration and randomisation (see Appendix 3, Table 32); 107 of these patients declined to participate, mostly because of concerns about either the trial requirements or the planned surgery they were about to undergo. Only one patient cited concerns about not knowing the treatment arm until 2 years after the procedure (as a result of patient blinding), although 38 patients declined without giving a reason. A further 115 patients were excluded by the consultant surgeon, with 49 (43%) of these patients undergoing the maze procedure outside the trial (as a result of severe or symptomatic AF or patient preference) and 11 patients opting for minimally invasive access or another procedure. The reason for exclusion by the surgeon was not recorded for 23 cases. Other reasons were related to trial exclusion criteria, such as patient participation in other clinical trials (n = 19), lack of time to recruit some in-house urgent cases (n = 6), not having a well-documented history of AF (n = 5) and having previous cardiac surgery (n = 3). Between screening and randomisation, eight patients died, and for a further four, their conditions deteriorated to such an extent that trial participation was not considered appropriate. A further 42 patients were excluded for various administrative reasons (see Appendix 3, Table 32).
After exclusions, 352 patients were randomised to either the planned cardiac procedure alone (n = 176) or maze procedure in addition to the planned procedure (n = 176). Thirteen patients (3.7%) did not receive their allocated treatment: 11 (6.3%) maze and two (1.1%) control patients. The maze procedure was not completed for a number of patients, as a result of (1) operation complexity and concern about prolonged cross-clamp time (n = 4); (2) an enlarged atrium or other technical difficulty (n = 3); (3) patient withdrawal from surgery after randomisation was revealed (n = 1); and (4) unrecorded surgeon decision (n = 3). Two control patients had the maze procedure as a result of perceived patient benefit by the consultant post randomisation.
Complete blinding was maintained for 339 (96%) patients. Treatment allocation was revealed in the notes of 13 patients (nine at Papworth Hospital, three at Derriford Hospital and one at Wythenshawe Hospital); of these, 10 underwent the maze procedure and three were control patients. The unblinding was attributable to initial protocol misunderstanding; after re-education of trial personnel, complete blinding was achieved for subsequent patients. The cardiologist reviewing the ECG recording did not have access to the patient’s medical notes. All patients and HRQoL assessors remained unaware of treatment allocation.
At 12 months, 150 maze procedure patients and 151 control patients (85% and 86%, respectively) remained in the trial. The reasons recorded for loss to follow-up at 12 months were death in 33 cases, patient withdrawal in 14 cases and loss to follow-up in four cases. Note that one maze procedure patient (out of 33) died just after 12 months, but the patient was too sick to complete follow-up. The clinical primary end point (SR at 12 months post randomisation) was completed for 141 (80%) maze procedure patients and 145 (82%) control patients, as 11 patients declined the 4-day ECG (eight maze procedure patients and three control patients) and, for four patients, the recordings were not usable (one maze procedure patient and three control patients). The frequency of missing outcomes and associated reasons was similar for the two trial arms.
The patient-based primary end point (QALYs up to 24 months post randomisation) was completed for 160 patients in each arm (91%). We note that patients who died during follow-up were included in the calculation of QALYs, contributing zero to the estimate from the date of death. Thirty-two patients were excluded from this analysis, as a result of either patient withdrawal from the study (n = 28) or loss to follow-up (n = 4).
Baseline characteristics
Patient characteristics at baseline are shown in Table 2. Almost 50% of cases were recruited in the co-ordinating centre (Pathworth Hospital) by 13 surgeons, and over one-quarter were recruited in the second highest recruiting centre (Glenfield Hospital) by four surgeons. The Amaze trial population had a mean age of 71.9 years (SD 7.67 years), almost two-thirds (65.9%) were men and the mean risk of in-hospital death as a result of the procedure [the 2003 logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE)61] was 6.79% (SD 5.18%). The characteristics of Amaze patients were broadly similar to those of UK NHS cardiac surgery patients, but Amaze patients were slightly older and more likely to be female, and had a slightly lower average EuroSCORE. 62 On average, the two treatment arms had similar characteristics.
| Characteristic | Treatment arm | Total (n = 352) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| Number of patients at each randomising centre, n (%) | |||
| Papworth Hospital | 89 (50.6) | 85 (48.3) | 174 (49.4) |
| Glenfield Hospital | 49 (27.8) | 44 (25.0) | 93 (26.4) |
| Derriford Hospital | 16 (9.1) | 16 (9.1) | 32 (9.1) |
| Northern General Hospital, Sheffield | 12 (6.8) | 14 (8.0) | 26 (7.4) |
| Freeman Hospital, Newcastle upon Tyne | 3 (1.7) | 5 (2.8) | 8 (2.3) |
| Guy’s and St Thomas’ Hospital, London | 1 (0.6) | 5 (2.8) | 6 (1.7) |
| Wythenshawe Hospital | 4 (2.3) | 1 (0.6) | 5 (1.4) |
| Brighton and Sussex University Hospitals | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| University Hospitals Coventry and Warwickshire NHS Trust | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Royal Brompton Hospital | – | 2 (1.1) | 2 (0.6) |
| Blackpool Victoria Hospital | – | 2 (1.1) | 2 (0.6) |
| Patient age (years) | |||
| Mean (SD) | 72.3 (7.53) | 71.4 (7.81) | 71.9 (7.67) |
| Range | 50.0–86.0 | 48.0–89.0 | 48.0–89.0 |
| Patient sex, n (%) | |||
| Male | 112 (63.6) | 120 (68.2) | 232 (65.9) |
| Female | 64 (36.4) | 56 (31.8) | 120 (34.1) |
| Body mass index (kg/m2) | |||
| Mean (SD) | 28.1 (5.27) | 27.6 (4.62) | 27.9 (4.96) |
| Range | 17.4–46.0 | 17.9–42.8 | 17.4–46.0 |
| Logistic EuroSCORE61 (%)a | |||
| Mean score (SD) | 6.94 (5.489) | 6.64 (4.869) | 6.79 (5.184) |
| Range | 0.88–30.41 | 1.40–23.85 | 0.88–30.41 |
Table 3 summarises symptoms at baseline. Heart failure symptoms, defined by the NYHA classification, were common, with 40.3% of patients reporting mild symptoms or slight limitations during ordinary activity, and 41.4% of patients reporting either marked or severe limitations, even during mild activity or at rest. Symptoms of angina, as defined by the Canadian Cardiovascular Society’s grading scale for angina pectoris, were less common, with 73.3% of patients being angina free at baseline and only a small proportion (5.7%) reporting moderate or severe limitations as a result of angina.
| Classification | Treatment arm, n (%) | Total number of patients (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| CCS classification | |||
| Class 0 | 125 (71.0) | 133 (75.6) | 258 (73.3) |
| Class 1 | 13 (7.4) | 17 (9.7) | 30 (8.5) |
| Class 2 | 21 (11.9) | 16 (9.1) | 37 (10.5) |
| Class 3 | 10 (5.7) | 8 (4.5) | 18 (5.1) |
| Class 4 | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Missing/not known | 6 (3.4) | 1 (0.6) | 7 (2.0) |
| NYHA classification at baseline | |||
| I | 31 (17.6) | 30 (17.0) | 61 (17.3) |
| II | 74 (42.0) | 68 (38.6) | 142 (40.3) |
| III | 59 (33.5) | 71 (40.3) | 130 (36.9) |
| IV | 10 (5.7) | 6 (3.4) | 16 (4.5) |
| Missing/not known | 2 (1.1) | 1 (0.6) | 3 (0.9) |
Other markers of cardiac function were also similar between the two arms (Table 4). For example, approximately two-thirds of patients (66.5%) had a left ventricular ejection fraction (LVEF) of > 50% at baseline, and 2.6% of patients had suffered a recent myocardial infarction (MI). The frequency of other risk factors for heart disease was similar between the two arms: 3.4% of patients were insulin-dependent diabetics, 12.5% were non-insulin-dependent diabetics, 37.8% were treated for high cholesterol and 57.4% had hypertension. The frequency of comorbidities was similar in both treatment arms, with chronic obstructive pulmonary disease (COPD) present in 9.7% of patients and pulmonary hypertension present in 15.1% of patients. In both treatment arms, 6.3% of patients had a history of cerebrovascular accidents and 8.8% had previous transient ischaemic attacks, with 2.8% of these patients having neurological dysfunction at baseline (see Appendix 3, Table 33).
| Marker | Treatment arm, n (%) | Total number of patients (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| Left ventricular function | |||
| Poor (LVEF of < 30%) | 4 (2.3) | 8 (4.5) | 12 (3.4) |
| Moderate (LVEF of 30–50%) | 50 (28.4) | 56 (31.8) | 106 (30.1) |
| Good (LVEF of > 50%) | 122 (69.3) | 112 (63.6) | 234 (66.5) |
| Recent MI | 4 (2.3) | 5 (2.8) | 9 (2.6) |
| Previous PCI | 16 (9.1) | 14 (8.0) | 30 (8.5) |
| Congestive cardiac failure | 5 (2.8) | 1 (0.6) | 6 (1.7) |
| Diabetes | |||
| Insulin dependent | 5 (2.8) | 7 (4.0) | 12 (3.4) |
| Non-insulin dependent | 27 (15.3) | 17 (9.7) | 44 (12.5) |
| Hyperlipidaemia/hypercholesterolaemia | 70 (39.8) | 63 (35.8) | 133 (37.8) |
| Systemic hypertension | 103 (58.5) | 99 (56.3) | 202 (57.4) |
Table 5 documents patients’ medical history associated with AF. For 26.1% of patients, AF was paroxysmal; the 73.9% of patients who had non-paroxysmal AF included almost 60% of patients classed as having chronic/longstanding AF and 13.9% classed as having persistent intermittent symptoms. Over two-thirds (68.5%) of patients had AF for > 12 months. Only 4.3% of patients had been fitted with a permanent pacemaker and 13.4% had previously undergone cardioversions; previous ablation had been attempted in slightly more maze procedure patients (1.7%) than control patients (0.6%). Anticoagulant and antiarrhythmic drugs were prescribed for 77.6% and 83.2% of patients, respectively.
| Marker of AF | Treatment arm, n (%) | Total (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| AF classification | |||
| Paroxysmal (intermittent) | 44 (25.0) | 48 (27.3) | 92 (26.1) |
| Persistent (intermittent) | 30 (17.0) | 19 (10.8) | 49 (13.9) |
| Chronic/longstanding (continuous) | 102 (58.0) | 109 (61.9) | 211 (59.9) |
| AF-related medical history | |||
| 0–3 months ago | 4 (2.3) | 2 (1.1) | 6 (1.7) |
| 3–6 months ago | 25 (14.2) | 25 (14.2) | 50 (14.2) |
| 6–12 months ago | 31 (17.6) | 23 (13.1) | 54 (15.3) |
| > 12 months ago | 115 (65.3) | 126 (71.6) | 241 (68.5) |
| Not known | 1 (0.6) | – | 1 (0.3) |
| Permanent pacemaker | 7 (4.0) | 8 (4.5) | 15 (4.3) |
| Time of pacemaker implant | |||
| 0–3 months ago | – | 2 (1.1) | 2 (0.6) |
| 3–6 months ago | 2 (1.1) | – | 2 (0.6) |
| 6–12 months ago | 2 (1.1) | 2 (1.1) | 4 (1.1) |
| > 12 months ago | 3 (1.7) | 4 (2.3) | 7 (2.0) |
| Previous cardioversions | |||
| 0–3 months ago | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| 3–6 months ago | 1 (0.6) | – | 1 (0.3) |
| 6–12 months ago | 5 (2.8) | 3 (1.7) | 8 (2.3) |
| > 12 months ago | 17 (9.7) | 19 (10.8) | 36 (10.2) |
| Previous ablation | 3 (1.7) | 1 (0.6) | 4 (1.1) |
| Arrhythmias other than AF/flutter | 2 (1.1) | 2 (1.1) | 4 (1.1) |
| Any anticoagulant use at baseline | 137 (77.8) | 137 (77.3) | 274 (77.6) |
| Any antiarrhythmic use at baseline | 145 (82.4) | 148 (84.1) | 293 (83.2) |
Table 6 summarises the HRQoL for the EQ-5D-3L utility score and the SF-36 dimensions at baseline. The mean EQ-5D-3L utility score was 0.75 (SD 0.22) at baseline, which compares well with the UK norms of 0.78 (SD 0.26) for people aged 65–74 years and 0.73 (SD 0.27) for people aged ≥ 75 years. 63 Thus, patients selected for cardiac surgery, who entered the Amaze trial, have comparable limitations to the general population of the same age, as measured by this generic HRQoL scale. In contrast, the mean scores for the SF-36 dimensions were very much lower than the published norms at baseline, particularly for the physical dimensions (see Table 6). 64 The mean standardised MCS at baseline was 50.19 (SD 10.32) for this population, almost exactly the same as the mean score for the UK population, whereas the mean for the standardised PCS was 30.59 (SD 13.36), which is significantly lower than the mean (SD) score for the UK population.
| HRQoL measurement | Treatment arm, mean score (SD) | Total, mean score (SD) (n = 352) | UK norm, mean score (SD) | |
|---|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | |||
| EQ-5D-3L utility score | 0.74 (0.22) | 0.75 (0.21) | 0.75 (0.22) | – |
| SF-36 dimensions | ||||
| Bodily pain | 72.00 (28.47) | 72.26 (26.18) | 72.13 (27.30) | 81.49 (21.69) |
| General health | 57.22 (19.11) | 55.61 (20.76) | 56.41 (19.94) | 73.52 (19.90) |
| Physical function | 47.18 (26.16) | 48.40 (27.33) | 47.79 (26.72) | 88.40 (17.98) |
| Role emotional | 71.10 (41.76) | 65.90 (45.61) | 68.49 (43.75) | 82.93 (31.76) |
| Role physical | 27.75 (37.20) | 30.57 (40.84) | 29.17 (39.04) | 85.82 (29.93) |
| Social functioning | 64.73 (29.19) | 64.44 (31.81) | 64.58 (30.49) | 88.01 (19.58) |
| Vitality | 43.67 (21.73) | 44.71 (23.76) | 44.19 (22.74) | 61.13 (19.67) |
| Mental health | 75.24 (15.44) | 73.51 (18.21) | 74.37 (16.88) | 73.77 (17.24) |
| PCS | 30.18 (13.17) | 31.00 (13.56) | 30.59 (13.36) | 50 (10) |
| MCS | 50.81 (9.92) | 49.58 (10.69) | 50.19 (10.32) | 50 (10) |
Surgical results
Table 7 summarises the surgical procedures completed; the most common were isolated MVR (24.7%), CABG (19.6%) and AVR (15.6%), followed by combined CABG with either AVR (10.5%) or MVR (7.7%). All other procedures were combinations of CABG and/or multiple valve procedures, with the exception of two patients for whom no procedure could be completed.
| Procedure | Treatment arm, n (%) | Total (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| Actual procedure category | |||
| MVR | 39 (22.2) | 48 (27.3) | 87 (24.7) |
| CABG | 35 (19.9) | 34 (19.3) | 69 (19.6) |
| AVR | 32 (18.2) | 23 (13.1) | 55 (15.6) |
| CABG and AVR | 16 (9.1) | 21 (11.9) | 37 (10.5) |
| CABG and MVR | 14 (8.0) | 13 (7.4) | 27 (7.7) |
| All other procedures, including none | 40 (22.7) | 37 (21.0) | 77 (21.9) |
Descriptions of surgical indices are given in Table 8. As expected, the time spent in theatre was longer for the maze procedure arm; the difference (maze procedure vs. control) in the mean length of time spent in theatre was 13.8 minutes (95% CI –4.4 to 32.0 minutes; p = 0.1375). Similarly, there was a mean difference in the time taken for cross-clamp of 5.1 minutes (95% CI –4.0 to 14.2 minutes; p = 0.2725) and in the time taken for cardiopulmonary bypass of 18.9 minutes (95% CI 9.9 to 27.8 minutes; p < 0.0001). Note that three patients’ surgical procedures were completed with a beating heart (with one patient randomised to the maze procedure arm and two patients randomised to the control arm), so that the time taken for both cross-clamp and cardiopulmonary bypass was zero minutes; on these occasions, the maze procedure was not performed. One more maze procedure patient had zero minutes recorded for cross-clamp, but 205 minutes recorded for the time taken for cardiopulmonary bypass.
| Procedure characteristic | Treatment arm | Total (n = 352) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| Total length of time (minutes) taken for cross-clamp | |||
| Mean (SD) | 82.2 (37.25) | 77.2 (48.60) | 79.7 (43.31) |
| Median (quartiles) | 74.0 (57.5–102.0) | 67.5 (51.0–92.0) | 72.0 (53.0–99.0) |
| Range | 0.0–245.0 | 0.0–530.0 | 0.0–530.0 |
| Total length of time (minutes) taken for cardiopulmonary bypass | |||
| Mean (SD) | 118.1 (43.39) | 99.3 (41.81) | 108.7 (43.59) |
| Median (quartiles) | 110.5 (84.0–145.0) | 93.0 (72.5–120.0) | 100.5 (80.0–132.0) |
| Range | 0.0–342.0 | 0.0–300.0 | 0.0–342.0 |
| Total length of time (minutes) spent in theatre | |||
| Mean (SD) | 261.2 (79.68) | 247.5 (93.27) | 254.4 (86.89) |
| Median (quartiles) | 260.0 (210.0–300.0) | 218.0 (195.0–277.5) | 240.0 (198.0–291.5) |
| Range | 75.0–582.0 | 100.0–775.0 | 75.0–775.0 |
| Excised left atrial appendage, n (%) | |||
| Yes | 97 (55.1) | 53 (30.1) | 150 (42.6) |
| No | 79 (44.9) | 123 (69.9) | 202 (57.4) |
The left atrial appendage was also significantly more likely to be excised in the maze procedure arm (55.1%) than in the control arm (30.1%).
Table 9 provides details of the lesion sets completed. Eleven patients in the maze procedure arm did not have the adjunct procedure at all. The most common ablation procedure was applied to the left and right atria and the mitral annulus (43.8%), with 22.2% applied to the left atrium and mitral annulus and 18.2% applied to the left atrium only. The mean number of lesions was 6.5 (SD 3.55) in the maze procedure arm, with 47.7% of patients having 5–9 lesions and 23.3% of patients having ≥ 10 lesions. The most common mode of delivery was bipolar radiofrequency ablation (81.8%), with unipolar radiofrequency ablation, cryotherapy and ultrasound applied to smaller numbers of maze procedures; no procedures applied laser or microwave energy.
| Lesion sets | Treatment arm | Total (n = 352) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| Number of lesions treated | |||
| Mean (SD) | 6.5 (3.55) | 0.1 (0.91) | 3.3 (4.11) |
| Median (quartiles) | 7.0 (4.0–9.0) | 0.0 (0.0–0.0) | 0.0 (0.0–7.0) |
| Range | 0.0–14.0 | 0.0–11.0 | 0.0–14.0 |
| Lesion number category, n (%) | |||
| 0 | 11 (6.3) | 174 (98.9) | 225 (63.9) |
| 1–4 | 40 (22.7) | – | 40 (22.7) |
| 5–9 | 84 (47.7) | 1 (0.6) | 85 (24.1) |
| ≥ 10 | 41 (23.3) | 1 (0.6) | 42 (11.9) |
| Lesion set treated, n (%) | |||
| I: minimal left atrial lesion set: pulmonary vein isolation either with or without left atrial appendage line | 32 (18.2) | – | 32 (9.1) |
| II: more extensive left atrial lesion set, excluding mitral annulus | 4 (2.3) | – | 4 (1.1) |
| III: more extensive left atrial only lesion set, including mitral annulus | 39 (22.2) | 1 (0.6) | 40 (11.4) |
| IV: minimal left atrial lesion set and right atrial lesion set | 2 (1.1) | – | 2 (0.6) |
| V: more extensive left atrial lesion set excluding mitral annulus and right atrial lesion set | 11 (6.3) | – | 11 (3.1) |
| VI: more extensive left atrial lesion set including mitral annulus and right atrial lesion set | 77 (43.8) | 1 (0.6) | 78 (22.2) |
| No lesions | 11 (6.3) | 174 (98.9) | 185 (52.6) |
At least one perioperative complication was recorded for 34 (19.3%) maze procedure patients and 38 (21.6%) control patients (see Appendix 3, Table 34). As expected, the most common complication was bleeding (for 10.8% of maze procedure patients and 9.7% of control patients) and pleural effusion (for 8% of maze procedure patients and 13.6% of control patients). There were no important differences in the number of patients requiring transfusion of red blood cells, platelets, fresh-frozen plasma, cryoprecipitate or human albumin (see Appendix 3, Table 35).
Intensive care unit and hospital stay did not vary by treatment arm. The median (quartiles) duration of stay in an ICU was 1.1 days (0.9–2.9 days) in the maze procedure arm and 1.0 days (0.9–2.0 days) in the control arm, whereas the median (quartiles) total length of hospital stay was 9 days (7–13 days) and 8 days (6–12 days) for the maze procedure and control arms, respectively. Eleven maze procedure patients and 12 control patients returned to an ICU on one or more occasions, with those returning having a median (quartiles) total length of stay of 4.6 days (1.3–6.5 days) and 2.5 days (1.5–10.4 days) in the maze procedure and control arms, respectively.
Primary outcome results
Sinus rhythm at 12 months
Despite a history of AF, 30 patients (17.0%) in the maze arm and 32 patients (18.2%) in the control arm did not have any arrhythmias recorded by the 4-day ECG at baseline. At 12 months, 286 (81.3%) patients completed the 4-day ECG recording; of these patients, 266 (93.0%) were either in SR 100% of the time or in AF 100% of the time. Patients were classified as being in AF if any AF was observed during the 4-day ECG recording; this was decided before linking trial outcomes to either treatment arm. Among complete cases in the maze procedure arm, 87 out of 141 patients (61.7%) were in SR compared with 68 out of 145 (46.9%) control patients. In the ITT analysis, using multiple imputation of missing data, the OR for return to SR was 2.06 (95% CI 1.20 to 3.54; p = 0.0091); see Appendix 3, Table 36 for the full results. Overall, results varied substantially by surgeon, with an associated intraclass correlation coefficient (ICC) of 0.089, suggesting that 8.9% of the total variation in return to SR rates over both treatment arms resulted from surgeon effects. However, there were no differences in the treatment effect (maze procedure vs. control) among surgeons (the ICC on the treatment coefficient was zero). Table 10 shows that the difference between the treatment arms arises almost solely from an additional 19 patients in the maze procedure arm changing from having AF to SR (61 vs. 42) and 21 fewer patients remaining in AF at 12 months (50 vs. 71).
| Rhythm changes | Treatment arm, n (%) | Total (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| Change from baseline to 12 months in AF/SR | |||
| Either baseline or follow-up missing | 39 (22.2) | 33 (18.8) | 72 (20.5) |
| SR at baseline, SR at 12 months | 23 (13.1) | 25 (14.2) | 48 (13.6) |
| SR at baseline, AF at 12 months | 3 (1.7) | 5 (2.8) | 8 (2.3) |
| AF at baseline, SR at 12 months | 61 (34.7) | 42 (23.9) | 103 (29.3) |
| AF at baseline, AF at 12 months | 50 (28.4) | 71 (40.3) | 121 (34.4) |
| Change from baseline to 24 months in AF/SR | |||
| Either baseline or follow-up missing | 62 (35.2) | 50 (28.4) | 112 (31.8) |
| SR at baseline, SR at 24 months | 18 (10.2) | 23 (13.1) | 41 (11.6) |
| SR at baseline, AF at 24 months | 3 (1.7) | 4 (2.3) | 7 (2.0) |
| AF at baseline, SR at 24 months | 47 (26.7) | 23 (13.1) | 70 (19.9) |
| AF at baseline, AF at 24 months | 46 (26.1) | 76 (43.2) | 122 (34.7) |
Sensitivity analysis
An exploratory analysis of the ITT population highlighted four outlying patients in accordance with cross-validation probabilities of having undue influence; a secondary analysis excluding these patients was conducted, with little change in the results (OR 2.20, 95% CI 1.27 to 3.82). In the sensitivity analyses, the OR changed to 2.00 (95% CI 1.21 to 3.32) if only complete cases were included, 1.70 (95% CI 1.07 to 2.69) if patients who died or withdrew were assumed to have been in AF, 1.92 (95% CI 1.17 to 3.15) if patients who died were assumed to have been in AF and 1.75 (95% CI 1.10 to 2.79) using the last observation carried forward; all remained statistically significant. An additional analysis, including the variables that were most associated with a missing status for 12-month SR (baseline SR, sex, diabetes, left ventricular function, history of rheumatic fever, non-AF/atrial flutter arrhythmias and COPD), resulted in a treatment effect estimate of 2.13 (95% CI 1.27 to 3.59). As the treatment effect was significant in all sensitivity analyses, we were confident that it was robust. Finally, in order to assess the treatment effect in patients for whom the surgery was completed as planned, the complier average causal effect for the difference in 12-month SR rates between the groups (maze procedure vs. control) was calculated; this was 15.8% (95% CI 3.9% to 27.6%) compared with 14.8% (95% CI 3.2% to 26.3%) for completers.
Subgroup analysis
Figure 3 shows the results of the subgroup analysis (see also Appendix 3, Table 37); no interactions were statistically significant. The odds on returning to SR were increased by the adjunct maze procedure for both paroxysmal and non-paroxysmal AF groups. The maze patients had increased ORs on return to SR irrespective of the planned procedure, although the small numbers of patients in each subgroup meant that the ORs varied widely and were not significant in most cases.
FIGURE 3.
Forest plot showing (adjusted) odds on return to SR at 12 months after randomisation (details of lesion sets are given in Table 9). RF, radiofrequency.
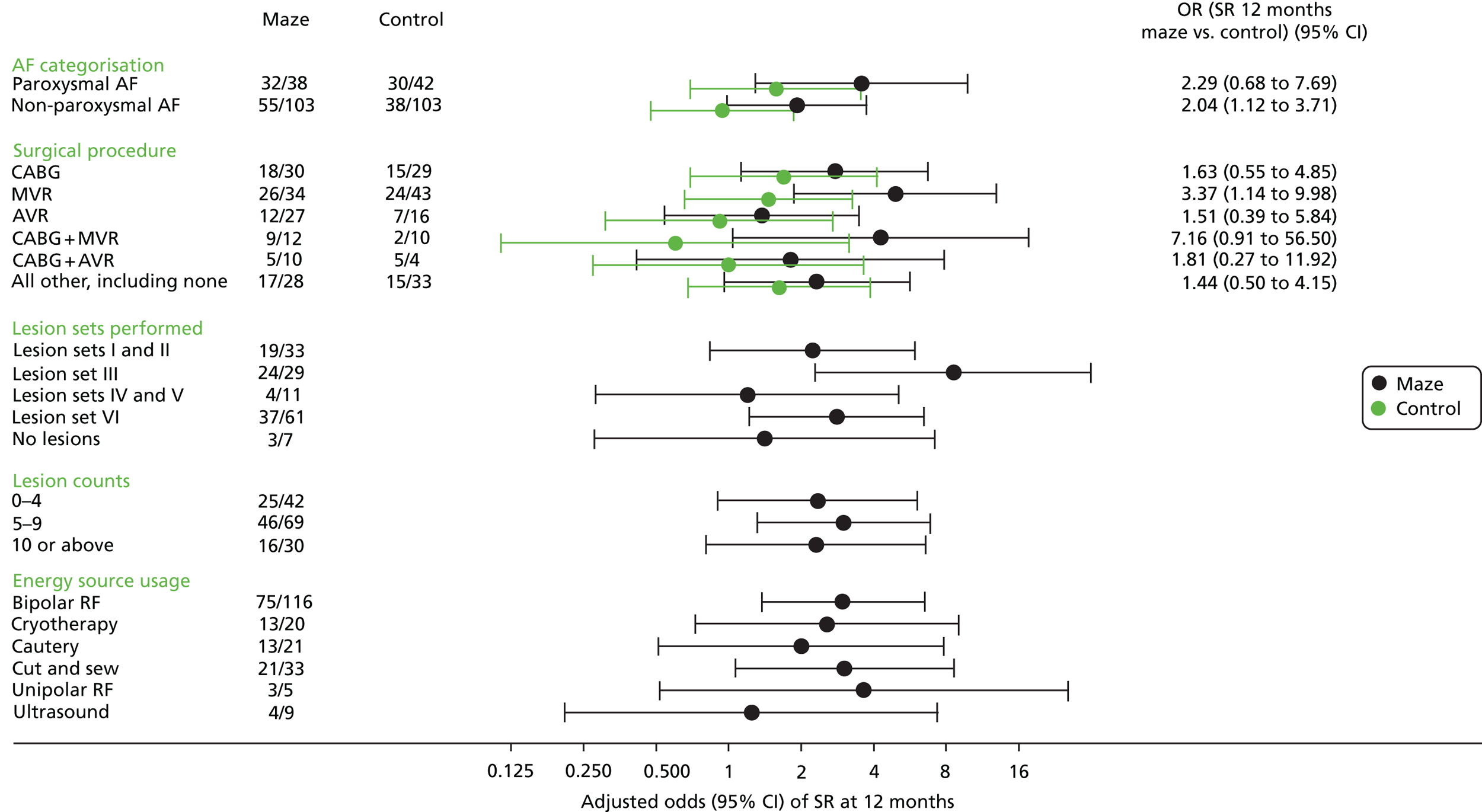
Within the maze procedure arm, there was little evidence that return to SR was associated with the number of lesions treated, although this may simply reflect the skill of the surgeon in identifying the areas to be treated (Table 11). Moreover, there was no evidence of variation in return to SR between different ablation techniques, although bipolar ablation was clearly the preferred technique for many surgeons.
| Subgroup | Number in SR/number in subgroup | Adjusted OR | 95% CI |
|---|---|---|---|
| Lesion sets performed | |||
| Lesion sets I and II | 19/33 | 2.236 | 0.838 to 5.962 |
| Lesion set III | 24/29 | 8.585 | 2.295 to 32.109 |
| Lesion sets IV and V | 4/11 | 1.198 | 0.282 to 5.095 |
| Lesion set VI | 37/61 | 2.803 | 1.214 to 6.472 |
| Lesion counts | |||
| No lesions | 3/7 | 1.412 | 0.279 to 7.142 |
| 0–4 lesions | 25/42 | 2.337 | 0.903 to 6.050 |
| 5–9 lesions | 46/69 | 3.009 | 1.320 to 6.861 |
| ≥ 10 lesions | 16/30 | 2.312 | 0.813 to 6.577 |
| Energy source usage | |||
| Bipolar RF ablation | 75/116 | 2.991 | 1.372 to 6.521 |
| Cryotherapy | 13/20 | 2.561 | 0.731 to 8.971 |
| Cautery | 13/21 | 1.998 | 0.513 to 7.789 |
| Cut and sew | 21/33 | 3.029 | 1.065 to 8.609 |
| Unipolar RF ablation | 3/5 | 3.631 | 0.517 to 25.473 |
| Ultrasound | 4/9 | 1.242 | 0.210 to 7.352 |
In October 2013, the independent Data Monitoring and Ethics Committee (DMEC) concluded that the results did not look promising and the TSC should consider stopping the trial on the basis of futility. After full and serious consideration of the recommendations made by the DMEC, the TSC decided to recommend continuation of recruitment and follow-up for the following reasons: there were no safety concerns at that stage of the trial; > 80% of patients had already been recruited; review of accruing data suggested that the results may be more promising; the trial results had the potential to have a high impact, particularly if no treatment effect was found. However, the investigators decided that the final arbiters should be the HTA programme board, which concluded that, as there were no safety concerns, it would support the continuation of recruitment to the trial until the end of May 2014. This discussion was relayed to the study teams at all sites via the steering group and circulation of the minutes, and they remained supportive of the decision to continue.
As a result of these concerns, a post hoc exploratory analysis was undertaken to assess whether or not there were changes in effects over time. The analysis found that the adjusted OR for return to SR at 12 months increased from 1.6 (95% CI 0.6 to 4.0) for the first 120 patients (considered by the DMEC) to 2.9 (95% CI 0.9 to 9.6) for the final 71 patients randomised in the final year of recruitment (2013).
Quality-adjusted life-years at 24 months
For this patient-centred primary outcome, QALYs could be estimated for 320 out of 352 patients (90.9%). The unadjusted and undiscounted mean QALYs over 2 years were 1.489 (95% CI 1.416 to 1.558) for the maze procedure arm and 1.485 (95% CI 1.403 to 1.559) for the control arm. In the primary complete-case, ITT analysis, adjusting for baseline covariates, the mean difference between the two arms (maze procedure vs. control) was –0.025 QALYs (95% CI –0.129 to 0.078 QALYs; p = 0.6319; see Appendix 3, Table 38). This difference corresponds to approximately 9 fewer days of life in perfect health for a maze procedure patient. A sensitivity analysis, which used different imputation methods for missing data and excluded any patients with outlying results, showed that these results were robust to model assumptions. Results did not vary substantially by surgeon; the average ICC across 40 multiple imputation samples was 0.001, indicating that only 0.1% of the total variation in 24-month QALYs was attributable to surgeon differences.
Sensitivity analysis
In a range of sensitivity analyses reflecting different assumptions about the missing data mechanism, the mean difference in QALYs changed only slightly, ranging from –0.029 (95% CI –0.135 to 0.078), for the last observation carried forward, to –0.010 (95% CI –0.119 to 0.100), for the analysis that adjusted for predictors of missingness (baseline EQ-5D-3L score, sex, diabetes and thoracic aorta surgery). Further details are available on request.
Subgroup analysis
The differences in QALYs at 2 years between the maze procedure and control arms are plotted in Figure 4 for a range of subgroups (see also Appendix 3, Table 39). A number of subgroups had an estimated QALY difference above our predefined minimum clinically important difference of 0.083, in favour of the maze procedure arm (isolated AVR, CABG and MVR) or the control arm (isolated MVR, CABG and AVR), but no differences were statistically significant and these values probably reflect the variation observed in small subgroup analyses. Again, there were no apparent relationships between QALYs over 2 years and any of the following: lesion sets, number of lesions treated or ablation method (Table 12).
FIGURE 4.
Forest plot showing the (adjusted) differences in QALYs at 2 years after randomisation for predefined subgroups (details of lesion sets are given in Table 9). RF, radiofrequency.
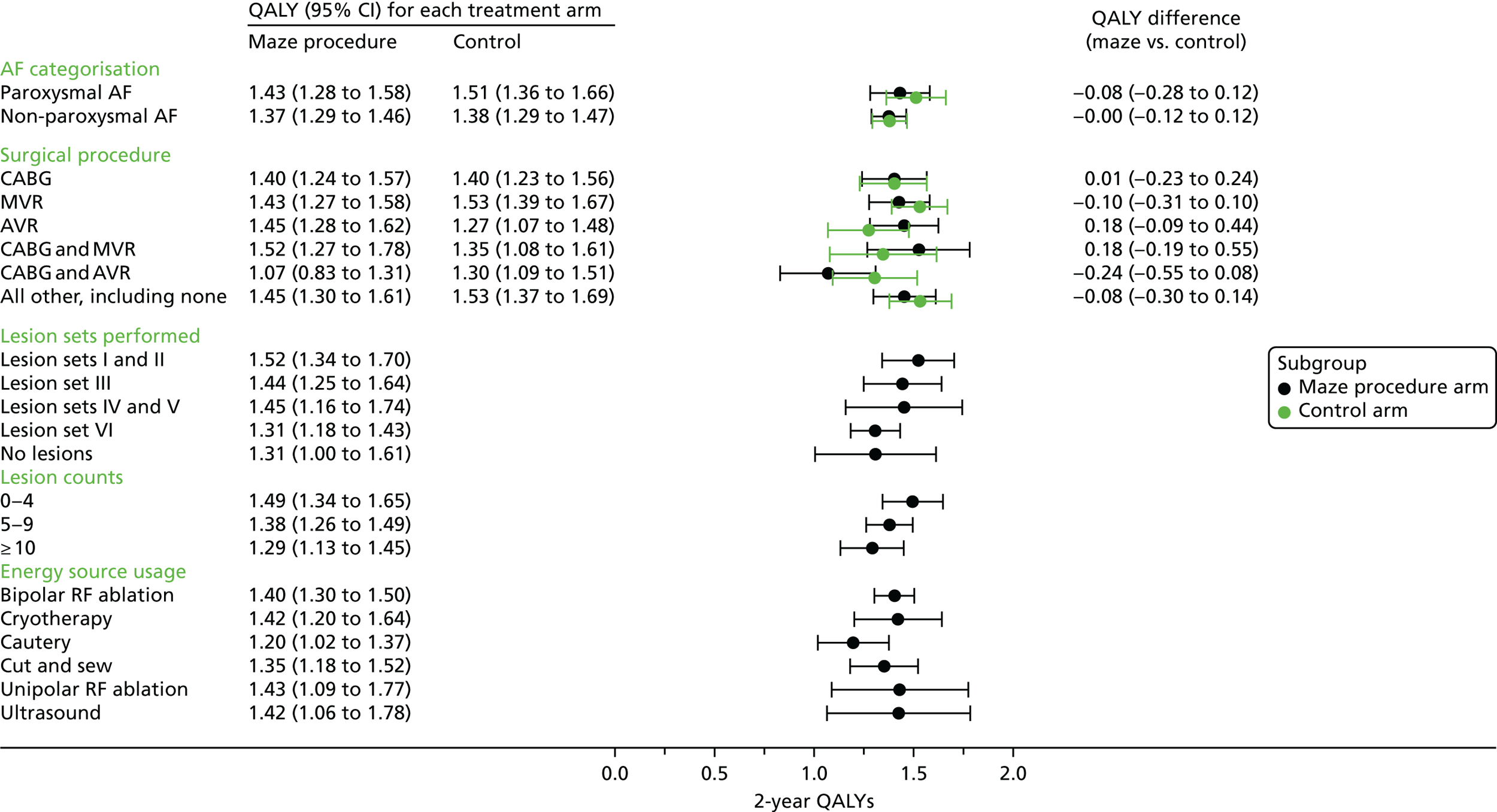
| Level | Mean | SEM | 95% CI |
|---|---|---|---|
| Lesion sets performed | |||
| Lesion sets I and II | 1.522 | 0.092 | 1.342 to 1.702 |
| Lesion set III | 1.443 | 0.100 | 1.247 to 1.639 |
| Lesion sets IV and V | 1.451 | 0.149 | 1.158 to 1.743 |
| Lesion set VI | 1.308 | 0.063 | 1.184 to 1.431 |
| Lesion counts | |||
| No lesions | 1.308 | 0.155 | 1.005 to 1.611 |
| 0–4 | 1.494 | 0.078 | 1.341 to 1.646 |
| 5–9 | 1.377 | 0.060 | 1.260 to 1.494 |
| ≥ 10 | 1.290 | 0.082 | 1.130 to 1.450 |
| Energy source usage | |||
| Bipolar RF | 1.402 | 0.052 | 1.301 to 1.503 |
| Cryotherapy | 1.420 | 0.112 | 1.200 to 1.640 |
| Cautery | 1.196 | 0.091 | 1.018 to 1.373 |
| Cut and sew | 1.351 | 0.087 | 1.181 to 1.521 |
| Unipolar RF | 1.429 | 0.175 | 1.086 to 1.772 |
| Ultrasound | 1.422 | 0.184 | 1.062 to 1.782 |
Secondary outcomes
Sinus rhythm at 24 months after surgery
At 24 months after surgery, 247 (70.2%) patients completed a 4-day ECG recording. In the maze procedure arm, 69 out of 118 (58.5%) completers were in SR compared with 47 out of 129 (36.4%) completers in the control arm. Thus, although the proportion of patients in SR decreased in both arms, the decrease was lower for the maze procedure arm. The baseline-adjusted OR for SR at 24 months was 3.24 (95% CI 1.76 to 5.96) in favour of the maze procedure arm (see Appendix 3, Table 40 for the full model results). Table 10 shows the number of people who had a change in SR between baseline and 24 months.
Survival and stroke-free survival
There were five (2.8%) postoperative deaths in the maze procedure arm and nine (5.1%) among the control patients (Fisher’s exact test, p = 0.4144). This compares with the mean predicted in-hospital death rate of 6.79% (logistic EuroSCORE,61 which is known to overestimate risk). Causes of death are listed in Appendix 3, Table 41. Between discharge and the planned 24-month follow-up date, there were 19 deaths in the maze procedure and nine in the control arm; note that three deaths in the maze procedure arm occurred just after the 24-month anniversary of their surgery.
Kaplan–Meier estimates of cumulative probability of death are plotted in Figure 5. This includes all 30 deaths in the maze procedure arm and all 25 deaths in the control arm at the end of the trial; the HR was 1.23 (95% CI 0.73 to 2.10; p = 0.437). Thus, the adjunct maze procedure did not significantly increase early or late death rates in this trial.
FIGURE 5.
Kaplan–Meier estimates of the cumulative incidence of death throughout the trial.
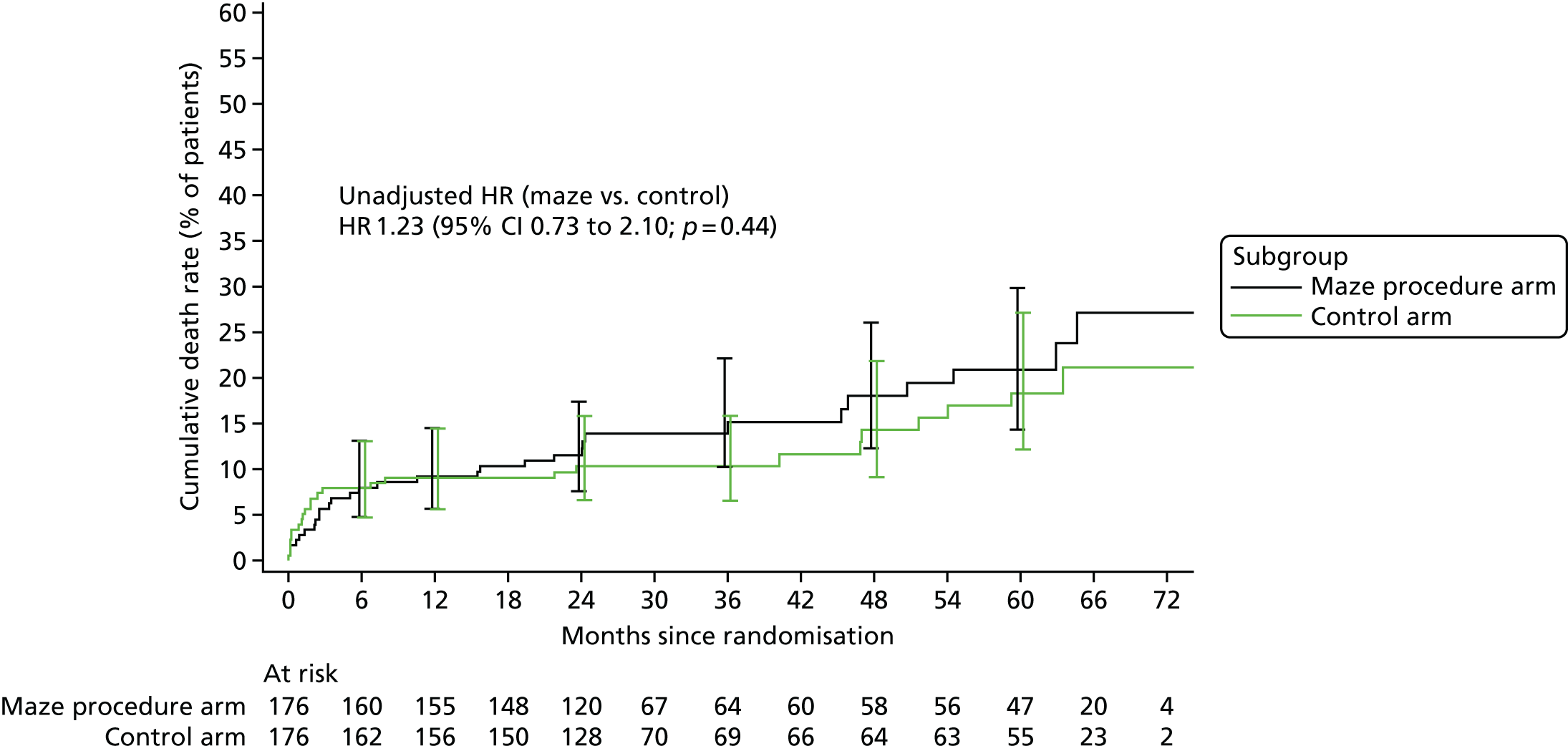
Figure 6 plots the cumulative incidence of death or stroke. During follow-up, 13 strokes were recorded in 10 (5.7%) maze procedure patients and 19 were recorded in 16 (9.1%) control patients; the difference of –3.4% (95% CI –14.1% to 7.3%) was not statistically significant (Fisher’s exact test, p = 0.3083). Moreover, there was no significant difference in stroke-free survival between the two trial arms (HR 0.99, 95% CI 0.64 to 1.53; p = 0.949).
FIGURE 6.
Kaplan–Meier estimates of the cumulative incidence of death or stroke.
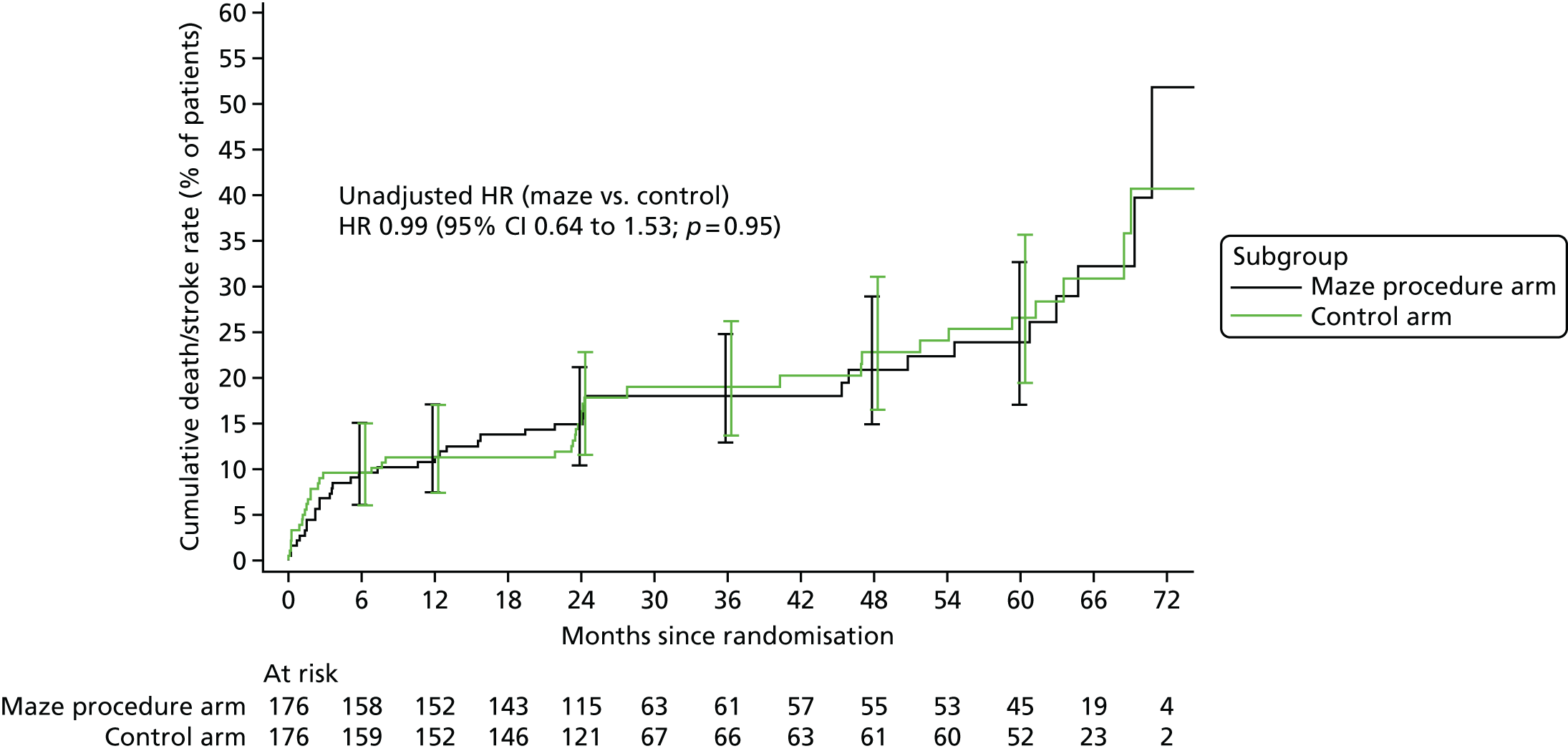
Anticoagulant and antiarrhythmic drug use
Table 13 shows that the number of patients requiring anticoagulant drug use was significantly lower in the maze procedure arm from 6 months after the procedure. Conversely, there were slightly more maze patients requiring antiarrhythmic drugs throughout follow-up, but the difference was not statistically significant at traditional levels.
| Time point for each type of drug | Treatment arm (n/N) | Adjusted OR maze procedure/control | 95% CI | p-value | |
|---|---|---|---|---|---|
| Maze procedure | Control | ||||
| Anticoagulants | |||||
| Discharge | 136/167 | 141/165 | 0.619 | 0.268 to 1.431 | 0.2616 |
| 6 weeks | 130/160 | 138/161 | 0.483 | 0.206 to 1.131 | 0.0936 |
| 6 months | 113/156 | 129/160 | 0.309 | 0.140 to 0.683 | 0.0037 |
| 12 months | 94/149 | 106/149 | 0.381 | 0.178 to 0.818 | 0.0133 |
| 24 months | 82/134 | 96/138 | 0.389 | 0.179 to 0.845 | 0.0171 |
| Antiarrhythmias | |||||
| Discharge | 138/167 | 134/165 | 1.323 | 0.623 to 2.806 | 0.4660 |
| 6 weeks | 137/160 | 134/161 | 1.547 | 0.704 to 3.399 | 0.2775 |
| 6 months | 131/156 | 127/160 | 1.603 | 0.751 to 3.423 | 0.2225 |
| 12 months | 117/149 | 109/149 | 1.541 | 0.744 to 3.192 | 0.2446 |
| 24 months | 102/134 | 97/138 | 1.613 | 0.778 to 3.344 | 0.1983 |
Further cardioversions
There was no difference between the two treatment arms in the need for further cardioversion or permanent pacemaker implants. Sixty maze procedure patients (34.1%) required 65 cardioversions and 67 control patients (38.1%) required 72 cardioversions. The cardioversion success rates for these interventions were the same [48/65 (73.8%) for the maze arm and 54/72 (75.0%) for the control arm]. Fifteen maze procedure patients (8.6%) and 17 control patients (9.7%) required pacemaker implantation.
Additional results from the electrocardiogram recordings
At baseline, 27 maze procedure patients (15.3%) and 16 control patients (9.1%) had AF or tachycardia on at least 1 day of the ECG recordings. Corresponding numbers at 12 months were 29 (16.5%) and 26 (14.8%) for the maze procedure patients and control patients, respectively, which fell to 19 (10.8%) and 18 (10.2%), respectively, at 24 months. Junctional rhythm was observed for only one (maze procedure) patient at baseline, eight maze procedure patients at 12 months and six maze procedure patients and two control patients at 24 months.
Hospital admissions for haemorrhage
There were three admissions in three patients who had the maze procedure, and two admissions in two patients who did not have the maze procedure up to 2 years after randomisation.
New York Heart Association results
Figure 7 and Appendix 3, Table 42 summarise the NYHA results for each treatment arm over the 2-year follow-up period. Among those who had complete data, there was some evidence that more patients in the maze procedure arm had symptoms of heart failure at 6 months after surgery (52.9% vs 42.7%). The difference was 10.2% (95% CI –1.4% to 21.5%; p = 0.0995), but the distribution of NYHA classes in each treatment arm was very similar thereafter.
FIGURE 7.
Comparison of NYHA classes over time.

Short Form questionnaire-36 items
Results for the eight dimensions of the SF-36 are shown in Figure 8 and Appendix 3, Table 43. For all dimensions, the two treatment arms of the trial had very similar (baseline-adjusted) SF-36 results at all follow-up points. With the exception of the pain scale, which increased (improved) only slightly, all dimensions increased substantially for both treatment arms and to a similar extent.
FIGURE 8.
Random coefficient model: mean and 95% CIs for SF-36 scores over time. Random patient effect excluded, baseline score adjusted. (a) SF-36 bodily pain score over time; (b) SF-36 general health score over time; (c) SF-36 mental health score over time; (d) SF-36 physical function score over time; (e) SF-36 role emotional score over time; (f) SF-36 role physical score over time; (g) SF-36 social functioning score over time; and (h) SF-36 vitality score over time.
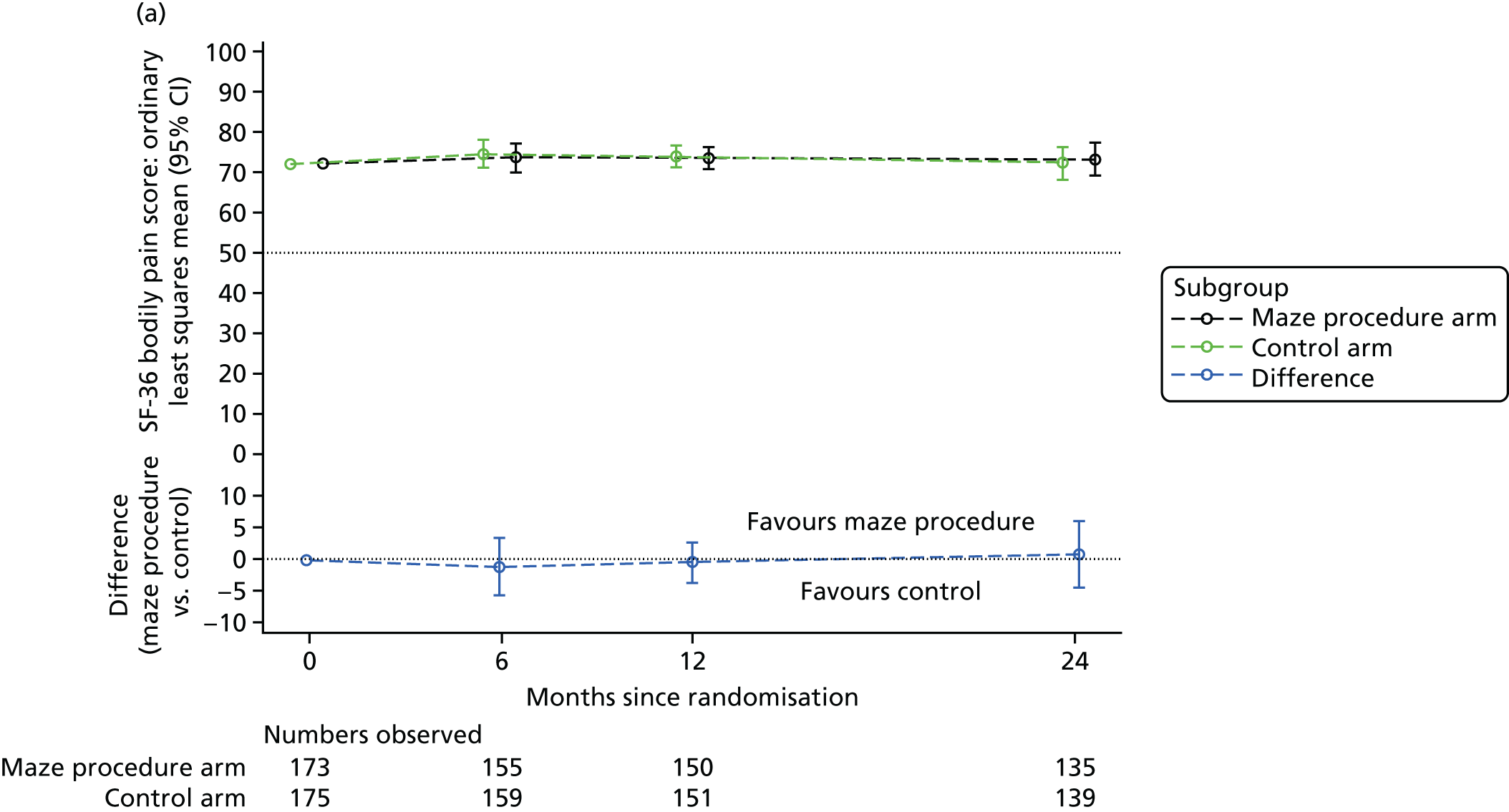
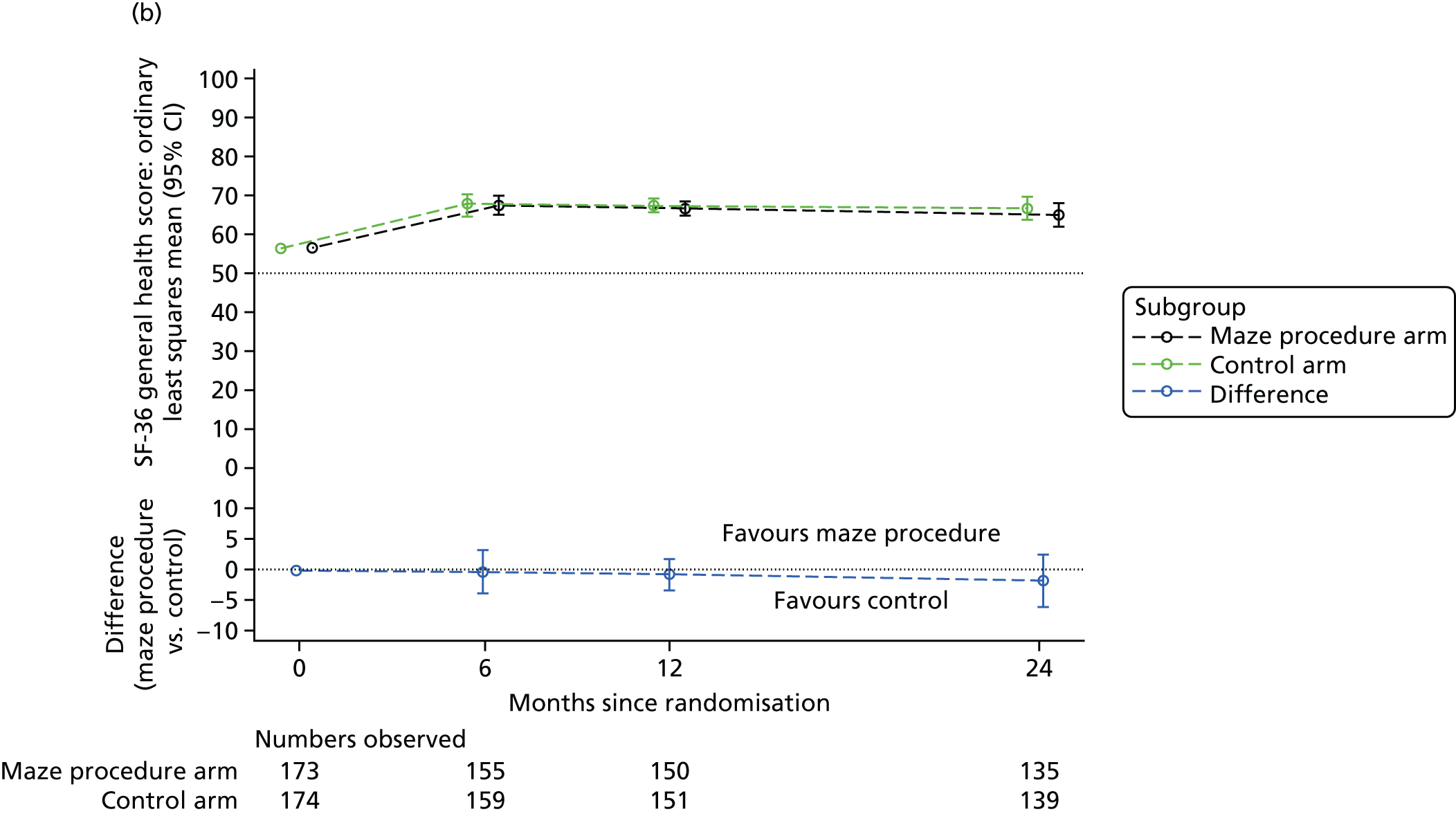

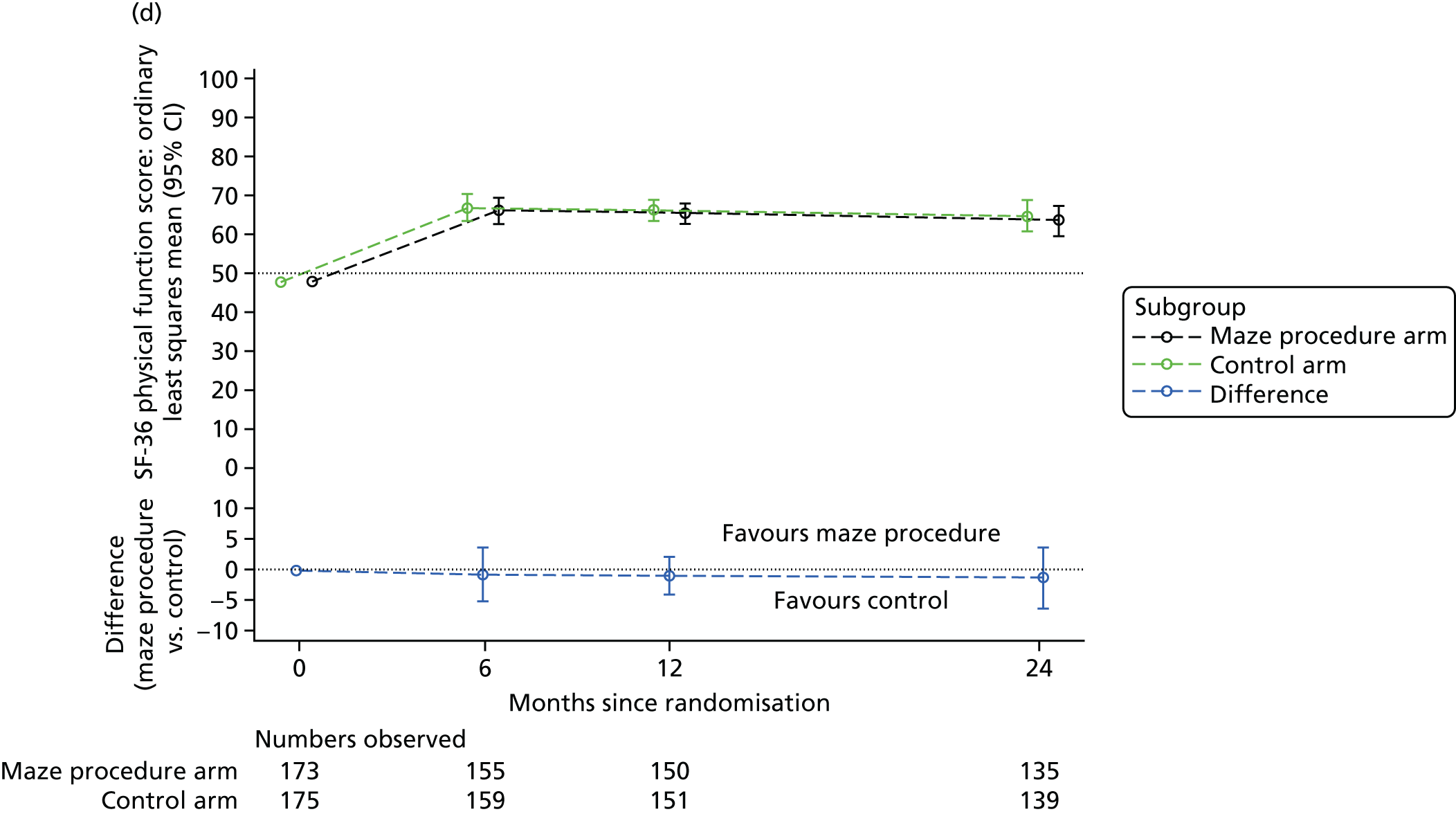

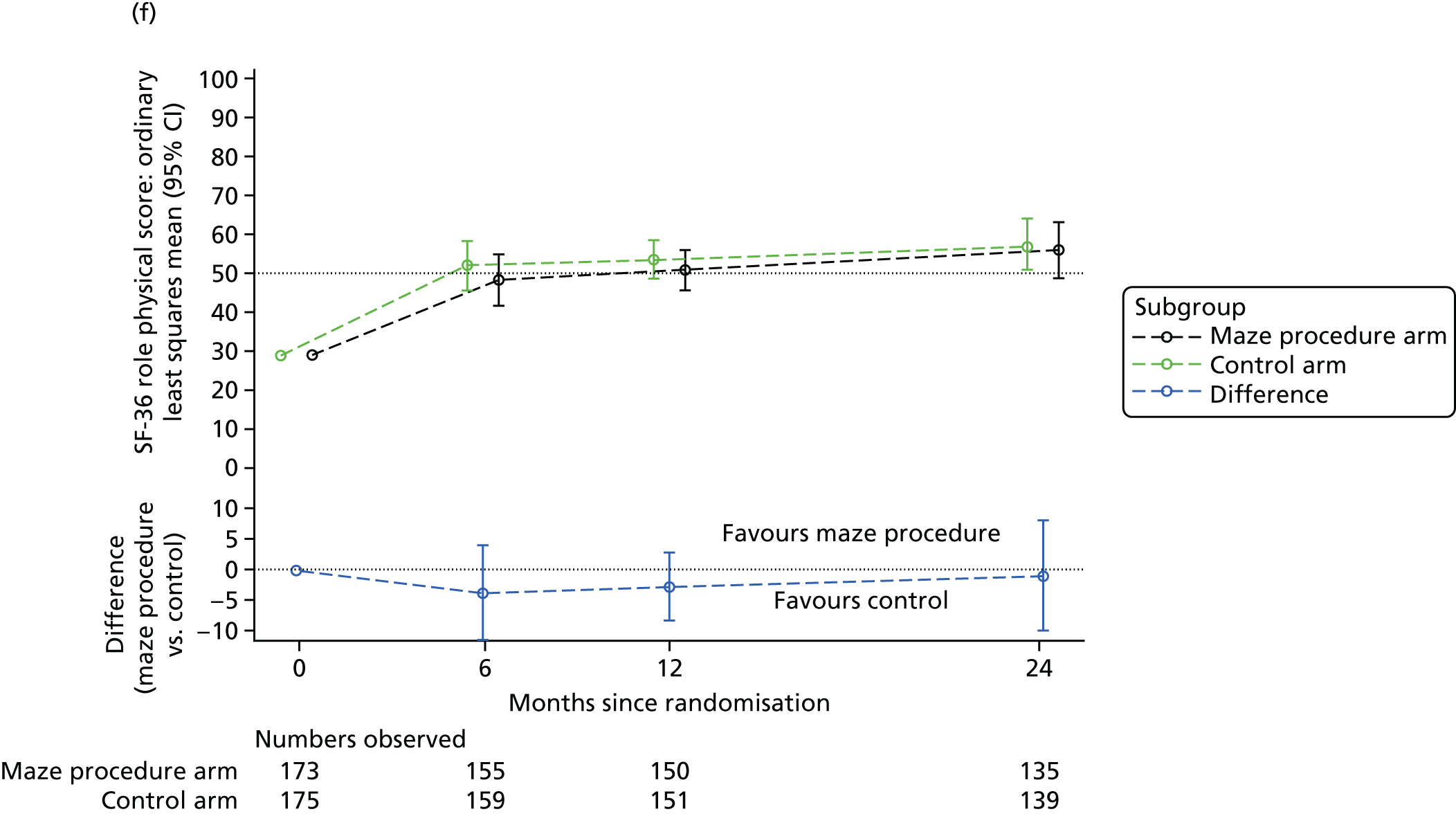


For most ‘physical’ dimensions, the mean SF-36 score increased between baseline and 6 months, but did not increase substantially thereafter. However, the ‘role limitations due to physical problems’ scale continued to improve steadily over the 2-year follow-up period, as patients recovered from the procedure and gained confidence in their ability to carry out usual activities.
The physical dimension scales all remained somewhat lower than for the general population, which may be expected given that the patients all had heart problems. The baseline-adjusted PCS was around 38 or 39 points on average at all postoperative time points for both treatment arms, which was significantly below the population average of 50 points, but was greater than baseline (Figure 9; see also Appendix 3, Table 44; p < 0.0001 for all follow-up points and both treatment arms). The improvement from baseline for the PCS was lower than that for the individual physical scales, as a result of it being a weighted average over all eight individual dimensions, some of which changed only slightly.
FIGURE 9.
Random coefficients model: mean and 95% CIs for the SF-36 PCS and MCS. Random patient intercept, baseline score adjusted. (a) SF-36 PCS over time; and (b) SF-36 MCS over time.
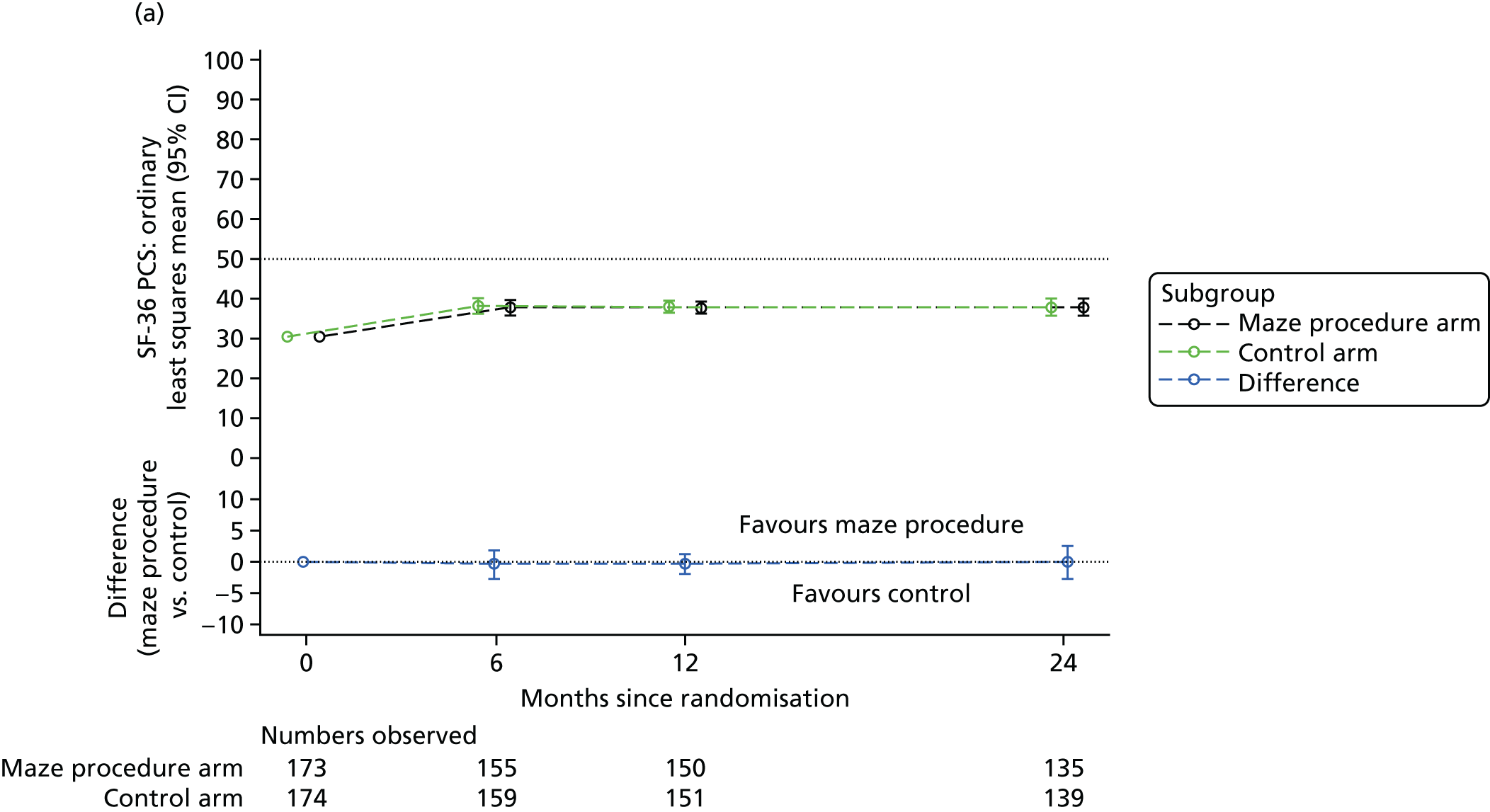
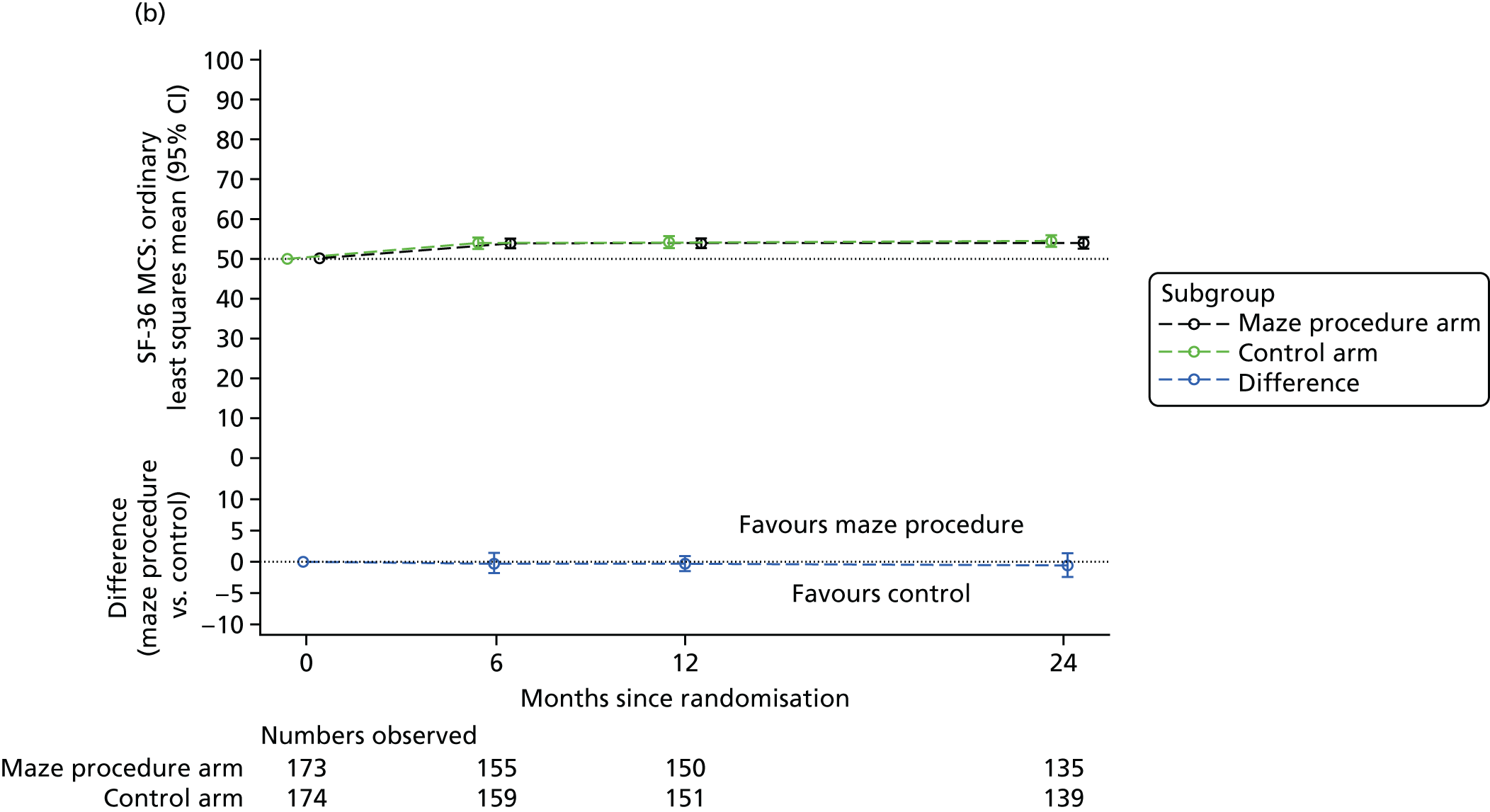
For the three scales that measured social, emotional and mental health aspects of life, a similar pattern emerged, with the arms improving between baseline and 6 months to a similar extent, but changing only slightly beyond this point. The mean MCS was very close to the general population average at baseline, and this increased by 4 points on average at 6 months post randomisation, remaining at that level for 2 years for both arms of the trial. There were no significant differences between the two treatment arms at any time point.
Finally, patients reported that their general health status, measured using the SF-36 general health scale, improved in both treatment arms to a similar extent, with no significant differences between the treatment arms at any follow-up time point.
EuroQol-5 Dimensions, three-level version
The results of the EQ-5D-3L utility score for those patients who completed the EQ-5D-3L are summarised in Appendix 3, Table 45 and Figure 10. As is common for surgical trials, there was a dip in estimated utility early after surgery, which was largely related to postoperative limitations in usual activities and self-care, and symptoms of pain/discomfort (data not shown). However, by 6 weeks after randomisation, the mean utility had increased in both treatment arms and was slightly higher than at baseline. By 6 months post randomisation, both treatment arms had a mean utility that was higher than the general population aged 65–74 years. There were no significant differences in baseline-adjusted EQ-5D-3L utility score at any follow-up time point. A similar pattern was observed for the visual analogue scale (data not presented).
FIGURE 10.
Mean and 95% CIs for the baseline-adjusted EQ-5D-3L utility score over time. B, baseline; D, discharge from hospital.
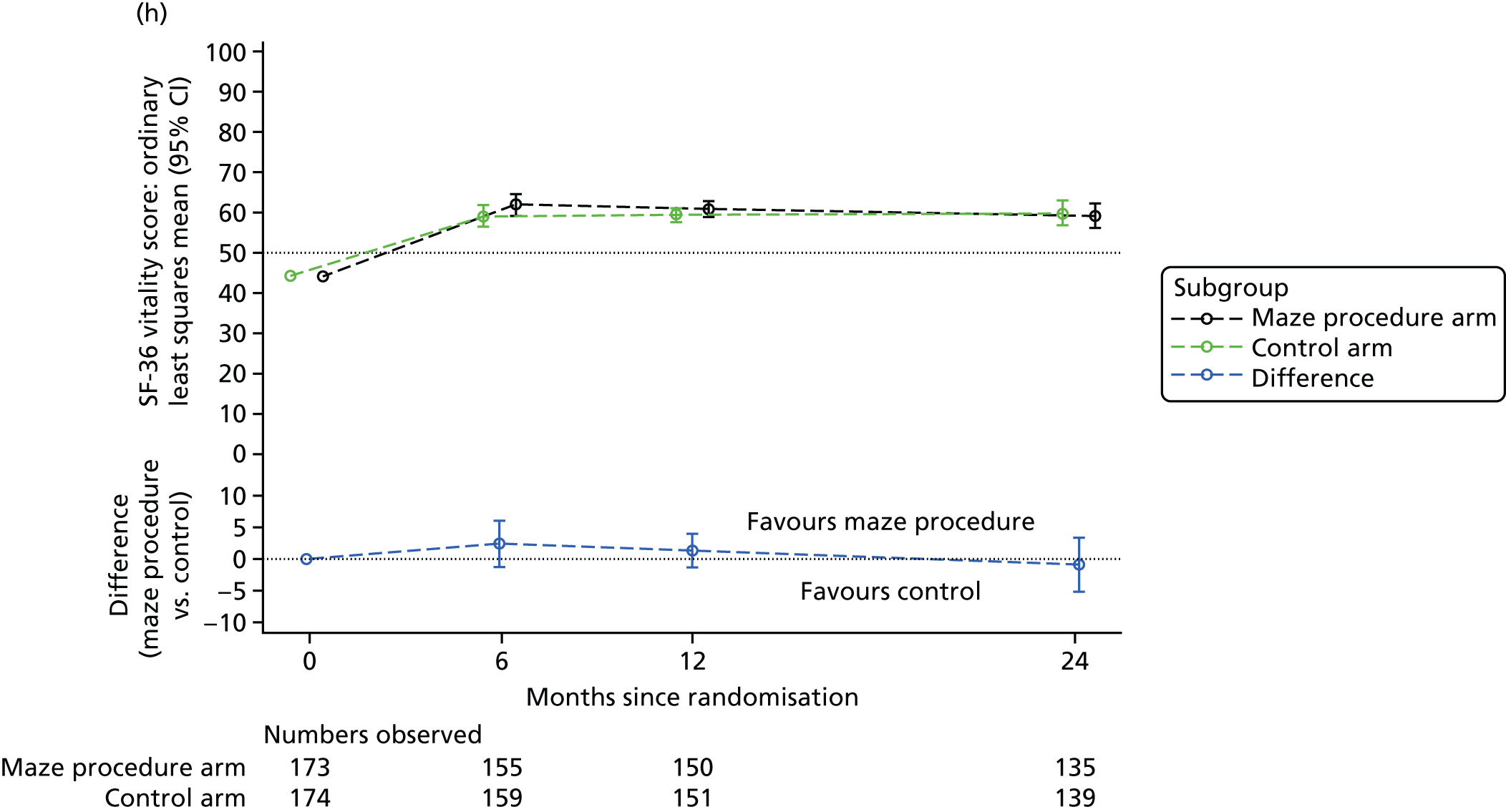
Safety
The safety results were based on 167 completed maze procedures (165 patients randomised to the maze procedure arm and two patients randomised to the control arm) and 185 non-maze cardiac procedures (11 patients randomised to the maze procedure arm and 174 patients randomised to the control arm). AEs are expected in surgical trials as a result of the high-risk nature of procedures. In the Amaze trial, a total of 560 events (136 patients) were reported after maze procedures and 589 events (157 patients) were reported after control procedures; an overview is provided in Table 14, with details in Appendix 3, Table 46. The number of these events classed as SAEs were 330 (100 patients) and 333 (111 patients) in the maze procedure and control arms, respectively. The proportion of patients having a SAE in the two treatment arms was very similar (maze procedure 100/167; control 111/185; p = 1.000). Most events were mild in severity, but 71 maze procedure patients (42.5%) and 84 control patients (45.5%) had at least one event of moderate severity, and 31 maze procedure patients (18.6%) and 38 control patients (20.5%) had a severe event. Twenty-three events in 17 patients (10.2%) were possibly related to treatment in the maze procedure arm, compared with 28 events in 19 patients (10.3%) being possibly related to treatment in the control arm; one control patient (0.5%) was admitted to hospital for investigation of an atrial flutter, which was classified as definitely related to treatment. Overall, the safety profiles of these two treatment arms were similar.
| AE category | Treatment arm, n (%) | Total (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 167) | Control (n = 185) | ||
| Any AEs/SAEs/SUSARs reported | 136 (81.4) | 157 (84.9) | 293 (83.2) |
| Any AEs reported | 103 (61.7) | 116 (62.7) | 219 (62.2) |
| Any SAEs reported | 100 (59.9) | 111 (60.0) | 211 (59.9) |
| Any SUSARs reported | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Related events reported | |||
| Definitely related | – | 1 (0.5) | 1 (0.3) |
| Possibly related | 17 (10.2) | 19 (10.3) | 36 (10.2) |
| Unrelated | 133 (79.6) | 151 (81.6) | 284 (80.7) |
| Severity | |||
| Mild | 109 (65.3) | 120 (64.9) | 229 (65.1) |
| Moderate | 71 (42.5) | 84 (45.4) | 155 (44.0) |
| Severe | 31 (18.6) | 38 (20.5) | 69 (19.6) |
Chapter 4 The ‘Has Electrical Sinus Translated into Effective Remodelling?’ substudy
Background
An important issue related to the maze procedure is that restoration of SR may not result in the return of atrial contractile function. Clinical consensus is that the increased risk of thromboembolic complications in AF is related to blood stagnation in parts of the left atrium, caused by the loss of contractile function. In addition, loss of atrial contraction also contributes to exacerbation of congestive heart failure and atrioventricular asynchrony. 65
Published literature on atrial transport following the maze procedure is limited by small sample sizes and selection bias. In addition, most studies had no matched control patients for comparison. To date, there is little rigorous evidence that attempting to restore SR by treating AF with an ablation device during cardiac surgery restores atrial transport.
Objectives
The HESTER substudy aimed to assess whether or not patients in SR at least 1 year after an adjunct maze procedure had an equivalent active left atrial ejection fraction (ALAEF) to that of control patients who had undergone cardiac surgery and were in SR both prior to and at least 1 year after surgery.
Methods
Patients and matching
The HESTER substudy was a (1 : 1) matched cohort study: the maze procedure cohort were (trial or non-trial) patients with AF who underwent the maze procedure as an adjunct to routine cardiac surgery and who were in SR at least 1 year postoperatively; the control cohort were patients who were in SR both before and at least 1 year after routine cardiac surgery.
Eligibility criteria were patients aged > 18 years, who were able to give informed consent and attend investigations, and who had a presence of ECG-confirmed SR at least 1 year after cardiac surgery. Exclusion criteria were contraindications to cardiac MRI: cardiac pacemakers, surgical clips in the head, electronic inner-ear implants, ocular metal fragments, electronic stimulators, implanted pumps and severe claustrophobia.
Consecutive eligible participants in the Amaze trial, recruited at Papworth Hospital and Glenfield Hospital, as well as consecutive non-trial AF patients who received the maze procedure as part of their routine elective surgery and were in SR at their last hospital visit, were invited to participate in the HESTER substudy. After consenting, each participant had confirmatory ECG.
A list of potential control patients was compiled from Papworth Hospital databases by an independent member of the hospital audit team. Once a maze procedure patient had consented, a list of potential matched control patients was compiled, matched for time since procedure (± 6 months), age (± 5 years), sex, type of surgery, preoperative LVEF and predicted operative mortality (logistic EuroSCORE). 61 Matching was undertaken by researchers independent of data collection and analysis. Hospital records for potential control patients were reviewed to confirm SR before surgery and the matching criteria before inviting patients to take part.
Data collection
Eligible patients underwent ECG to confirm SR, transthoracic two- and three-dimensional echocardiography and MRI to evaluate left atrial function.
All echocardiography studies were performed using a Philips IE33 ultrasound machine (Philips UK Ltd, Guildford, UK) and the British Society of Echocardiography standard minimum data set. Analysis was by Philips QLab software, version 9.0 (Philips UK Ltd, Guildford, UK), and Xcelera, version 3.2.1.712-2011 (Philips UK Ltd, Guildford, UK), reporting system.
Magnetic resonance imaging studies used a 1.5-T MRI scanner (Siemens Avanto, Erlangen, Germany) and were evaluated using Argus cardiac software, version B17 (Siemens Healthcare, Erlangen, Germany). Images were acquired in standard planes, positioned either parallel to (horizontal and vertical long-axis planes) or perpendicular to the long axis of the heart (short-axis planes), using ECG-triggering steady-state gradient echo sequences (balanced fast-field echo). Left atrial volumes were measured with Argus software.
Each investigation was performed and interpreted by a single operator blinded to the patient identity.
Outcomes
The primary outcome was ALAEF, the measurement most directly related to active left atrial contractility. Maximum left atrial volume (LAVmax), minimum left atrial volume (LAVmin) and pre A-wave left atrial volume (LAVpreA) were measured by echocardiography. ALAEF was derived as a percentage:
Each volume was measured using both the two- and four-chamber views. The four-chamber assessment of ALAEF was our primary end point, with the two-chamber version being a key secondary end point.
Volume measurements and E/A ratio (an ECG marker of left ventricular function) were secondary end points. Derived secondary end points were active stroke volume (LAVpreA – LAVmin), passive stroke volume (LAVmax – LAVpreA) and left atrial ejection fraction (LAEF):
Maximum left atrial volume and LAVmin were also measured using the MRI multiple-slice method and were, along with the LAEF derived from them, secondary end points.
Sample size
In AF, ALAEF is virtually zero, so that any measurable ALAEF after SR restoration is a marker of at least partial treatment success. In a previous study of subjects in SR, with a LAVmax of 50–70 ml, but without atrioventricular or intraventricular conduction abnormalities on a resting 12-lead ECG, the mean ALAEF (measured in four-chamber view echocardiography) was 43% with a SD of 18.2%. 66 Based on this, and the expert judgement of the investigators, the minimum clinically important difference in ALAEF was set at 1 SD, or 18.2%. Equivalence would therefore be concluded on estimating a treatment effect (maze procedure vs. control) with a two-sided 95% CI contained entirely in the interval from –18.2% to 18.2%. Based on this, 22 patients in each treatment arm would provide 80% power to demonstrate equivalence.
Statistical analyses
For the primary end point, a mixed-effects linear regression model was fitted, including fixed effects for treatment and matching variables, and random effects for the matched pairs. A variance components model was assumed for the covariance structure, with residuals at both levels assumed to be independent and normally distributed. The estimated treatment coefficient from the model was taken as the mean difference in ALAEF between maze procedure patients and control patients. Model assumptions were checked using residual plots.
The same model was fitted to each secondary end point. If the estimated random-effects variance was zero for any end point, the pairing was included as a fixed effect. No equivalence margins were specified for the secondary end points.
Analysis was undertaken in SAS version 9.4, using the PROC MIXED procedure to fit mixed-effects models by the method of restricted maximum likelihood. There were no missing values for the primary end point. For secondary end points with missing values, complete-case analyses were used. The guidelines from the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement were followed. 67
Results
Between 24 July 2013 and 8 July 2015, 22 eligible patients were recruited for each cohort and underwent echocardiography and MRI (Figure 11). All 22 control patients and 15 maze procedure patients were from Papworth Hospital, and seven maze procedure patients were from Glenfield Hospital. No patients experienced AEs during these tests.
FIGURE 11.
Recruitment and matching for the HESTER substudy.

Summaries of the variables used in matching are presented in Table 15. Matching by sex was exact. Two pairs differed in left ventricular function (maze procedure arm: LVEF of > 50%, control arm: LVEF of 30–50%; and maze procedure arm: LVEF 30–50%, control arm: LVEF of > 50%). Only two pairs differed in operation performed: one maze procedure patient who underwent mitral and tricuspid valve surgery was matched with a control patient who underwent mitral surgery alone; another who underwent CABG and atrial septal defect repair was matched with a control patient who underwent isolated CABG. For all but three of the pairs, the length of time since surgery was longer for the maze procedure patient than the control patient. Nine pairs differed by > 6 months in the length of time since surgery. Maze procedure patients were, on average, older and 16 were older than their matched control patients, including two age differences > 5 years. Maze procedure patients tended to have a slightly higher logistic EuroSCORE. 61
| Variable | Treatment arm | |
|---|---|---|
| Maze procedure (n = 22) | Control (n = 22) | |
| Age at surgery (years), mean (SD) | 69 (9) | 66 (12) |
| Years since surgery, mean (SD) | 2.7 (1.0) | 2.9 (1.1) |
| Logistic EuroSCORE (%), mean (SD) | 4.3 (3.7) | 3.7 (2.5) |
| Left ventricular function, n (%) | ||
| Poor (LVEF of < 30%) | 1 (5) | 1 (5) |
| Moderate (LVEF of 30–50%) | 5 (23) | 5 (23) |
| Good (LVEF of > 50%) | 16 (73) | 16 (73) |
| Sex, n (%) | ||
| Male | 20 (91) | 20 (91) |
| Female | 2 (9) | 2 (9) |
| Surgery, n (%) | ||
| CABG | 5 (23) | 6 (27) |
| MVR | 8 (36) | 9 (41) |
| AVR | 2 (9) | 2 (9) |
| Combined procedures | 6 (29) | 4 (24) |
| ASD repair | 1 (5) | 1 (5) |
Summaries of the four-chamber ALAEF assessment are in Table 16. The SD in both cohorts was 8%, but maze procedure patients had a lower mean ALAEF (18%) than control patients (26%). Figure 12 shows that the ALAEF of the control patients was higher in all but three pairs. The maximum ALAEF was similar between treatment arms (41% for control patients and 39% for maze procedure patients), but the minimum for maze procedure patients (6%) was less than half that of the control patients (13%).
| End point | Treatment arm | |
|---|---|---|
| Maze procedure (n = 22) | Control (n = 22) | |
| ALAEF (%) | 18 (8) | 26 (8) |
| LAEF (%) | 31 (9) | 38 (7) |
| LAVmin (ml) | 59 (20) | 45 (14) |
| LAVpreA (ml) | 72 (22) | 60 (18) |
| LAVmax (ml) | 86 (25) | 72 (19) |
| Passive stroke volume (ml) | 13 (7) | 11 (4) |
| Active stroke volume (ml) | 13 (7) | 16 (7) |
| E/A ratio | 1.8 (0.8) | 0.9 (0.4) |
FIGURE 12.
Four-chamber ALAEF measurements for individual patients undergoing the maze procedure and their matched control patients for the HESTER substudy.

After adjusting for the matched design, the mean difference in ALAEF (four-chamber view) between maze procedure and control patients was –8.03 (95% CI –12.43 to –3.62; see Figure 13 and Appendix 3, Table 47). The 95% CI was contained entirely in the interval (–18.2 to 18.2), so our predefined criterion for equivalence was met. However, the mean ALAEF was significantly lower in maze procedure patients than in control patients (p = 0.0015).
FIGURE 13.
Forest plot showing estimated treatment effects (maze procedure vs. control) and 95% CIs for the four- and two-chamber measurements of ALAEF in the HESTER substudy. Equivalence limits (at ± 18.2%) are shown as dashed vertical lines.

For the two-chamber measurements, the mean maze–control difference was –3.48 (95% CI –8.45 to 1.49), supporting the conclusions of the primary outcome analysis.
Excluding one patient in the maze procedure cohort with a high logistic EuroSCORE61 of 16.9% did not affect the conclusion of equivalence (mean difference –7.90, 95% CI –12.55 to –3.25).
Secondary outcomes are summarised in Appendix 3, Tables 47–49. Although the summaries for the four- and two-chamber echocardiography measurements were very similar, they were not consistent with the MRI summaries.
In regression analysis, the mean E/A ratio was significantly higher and the mean LAEF (four-chamber view and MRI) was significantly lower for maze procedure patients than those for control patients (Figure 14). There were no significant differences in the other end points.
FIGURE 14.
Forest plots showing estimated treatment effects (maze procedure vs. control) and 95% CIs for secondary end points in the HESTER substudy. a, For these variables, the models were fitted with patient pairing as a fixed effect.
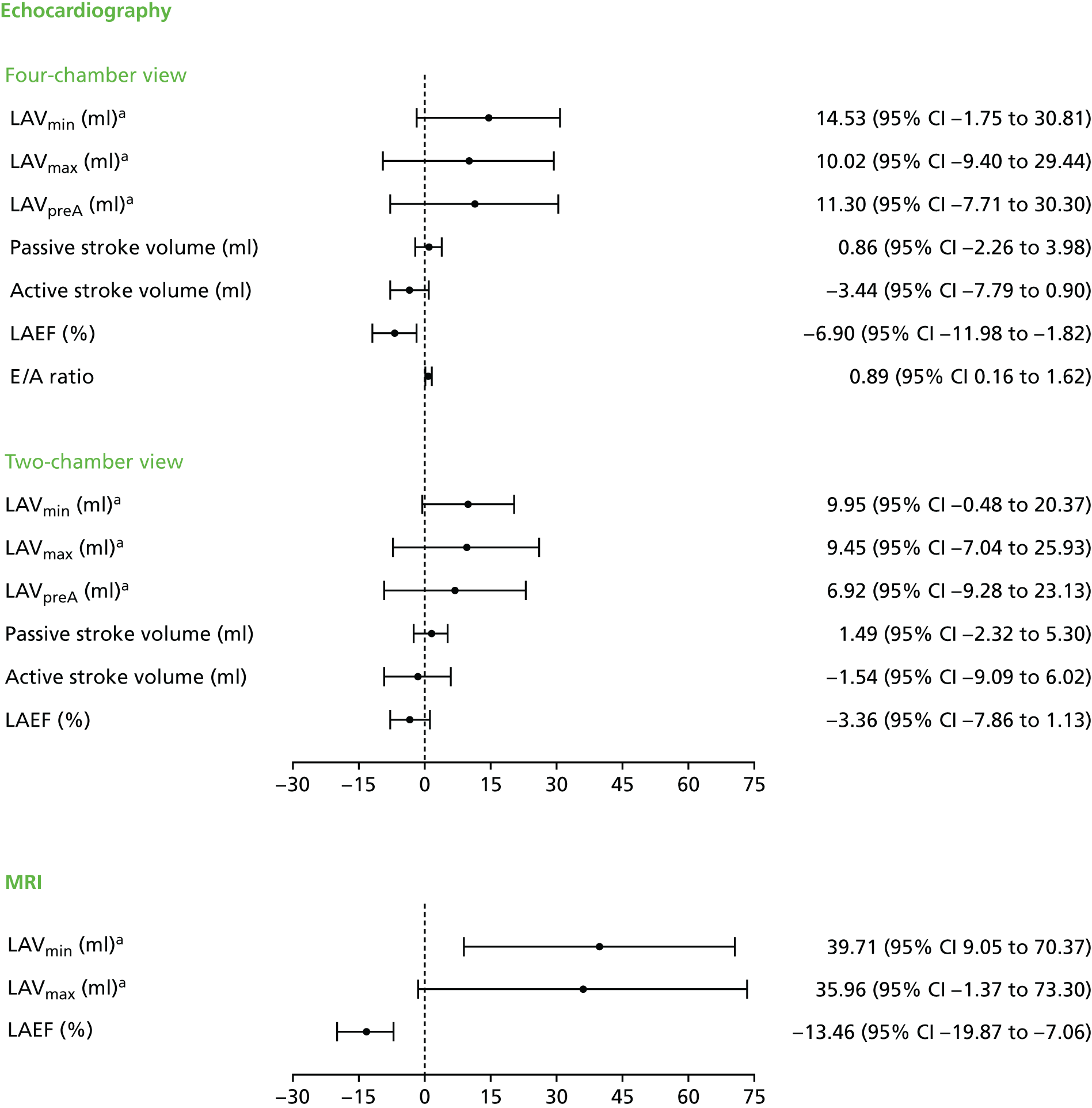
Chapter 5 Trial results: cost-effectiveness
Data completeness
There were very few item non-responses for resource use during the primary admission (one control patient and two patients from the maze procedure arm had missing length of stay data). Half the patients who were referred to a rehabilitation centre (nine maze procedure patients and six control patients) or an acute hospital (three maze procedure patients) had missing final discharge dates and, therefore, values were imputed. Excluding patients who died, the proportion of missing resource-use items at each follow-up point ranged from 6.5% to 13.4% and the proportion lost to follow-up grew from 4% at 6 weeks to 6.5% at 12 months (Table 17). The proportion of incomplete EQ-5D-3L and SF-36 scores ranged from 0.9% to 10.5% across time. Patients with poorer initial health status, measured using the EQ-5D-3L, were significantly more likely to have missing responses.
| Time point | Follow-up resource use | Treatment arm (n) | Total | |
|---|---|---|---|---|
| Maze procedure | Control | |||
| 6 weeks | Observations | 157 | 156 | 313 |
| Dead | 6 | 10 | 16 | |
| Lost to follow-up | 8 | 6 | 14 | |
| Missing | 5 | 4 | 9 | |
| 6 months | Observations | 152 | 154 | 306 |
| Deada | 14 | 14 | 28 | |
| Lost to follow-upa | 13 | 9 | 22 | |
| Missing | 5 | 5 | 10 | |
| 12 months | Observations | 147 | 146 | 293 |
| Deada | 16 | 16 | 32 | |
| Lost to follow-upa | 25 | 20 | 45 | |
| Missing | 1 | 3 | 4 | |
| 24 months | Observations | 130 | 134 | 264 |
| Deada | 23 | 18 | 41 | |
| Missing | 23 | 24 | 47 | |
Resource costs
Table 18 shows the length of stay for each stage of the primary admission, by intervention arm and control arm. Patients in the maze procedure arm had a longer stay in the ICU and the cardiac ward, and spent a longer time in an acute hospital, following referral, than those in the control arm. The distribution of resource use in the maze procedure arm included two outliers: one patient stayed in an ICU post operation for 52.9 days and, following discharge directly to their home, died a few days later; and one patient was in the cardiac ward for 144 days.
| Resource use | Treatment arm | Number of observations | Mean | Min. | Max. |
|---|---|---|---|---|---|
| Theatre time (minutes) | Maze procedure | 176 | 261.2 | 75.0 | 582.0 |
| Control | 176 | 247.4 | 100.0 | 775.0 | |
| Cardiac ward (days) | Maze procedure | 174 | 8.7 | 0.0 | 153.0 |
| Control | 176 | 7.9 | 0.0 | 33.0 | |
| ICU (days) | Maze procedure | 175 | 3.2 | 0.0 | 52.9 |
| Control | 176 | 2.4 | 0.2 | 36.2 | |
| Rehabilitation centre referral (days) | Maze procedure | 167a | 0.3 | 0.0 | 19.0 |
| Control | 170b | 1.0 | 0.0 | 84.0 | |
| Acute hospital referral (days) | Maze procedure | 173c | 2.4 | 0.0 | 144.0 |
| Control | 176d | 0.4 | 0.0 | 45.0 |
The average total cost of resources in the maze procedure arm was £3839 more than that of the control arm, and this difference was statistically significant (p = 0.0013). Table 19 shows that the primary admission had the highest cost (£14,880 in the maze procedure arm; £11,406 in the control arm) and resulted in a large proportion of the difference in cost between the trial arms (£3442; p = 0.0016). The primary admission contributed most to the variability in cost, as both arms had many high-cost outliers (Figure 15). The difference in cost between the trial arms during the primary admission stemmed largely from additional length of stay in each hospital location (surgery, ICU and cardiac ward, with the exception of rehabilitation centres), additional costs of surgical equipment to carry out the maze procedure and the influence of two outliers (see Appendix 2, Tables 25 and 26, for a detailed breakdown of resource use and total costs). Table 19 shows that, after discounting, the average cost difference was £3841 (95% CI £1514 to £6167) higher per patient for the maze procedure arm than for the control arm (p = 0.0013).
| Health service use | Treatment arm, mean cost (£) per patient (SD) | Difference in cost (£) (maze procedure vs. control) | |
|---|---|---|---|
| Maze (n = 176) | Control (n = 176) | ||
| Primary admission | |||
| Theatre use | 5225 (1594) | 4949 (1863) | 276 |
| Ablation device | 1212 (408) | 14 (133) | 1197 |
| Adult critical care | 4029 (7600) | 3065 (5586) | 964 |
| Cardiac ward | 3397 (4661) | 3064 (2014) | 333 |
| Rehabilitation | 48 (325) | 148 (1082) | –100 |
| Acute trust | 937 (6105) | 165 (1409) | 772 |
| Subtotal | 14,847 (12,474) | 11,404 (7194) | 3443 |
| Medication (whole trial period) | 618 (1584) | 681 (2765) | –63 |
| Follow-up | |||
| Readmissions | 1650 (4192) | 1220 (2994) | 430 |
| Tests | 388 (376) | 344 (283) | 44 |
| Health-care visits | 1179 (1061) | 1193 (1052) | –14 |
| Subtotal | 3217 (5629) | 2757 (4329) | 460 |
| Grand total | 18,681 (13,340) | 14,842 (8295) | 3841 |
FIGURE 15.
Comparison of the total primary admission cost with the total trial cost (with imputation).
The mean follow-up costs for the maze procedure arm were £460 more than those for the control arm, mainly because of a difference in readmissions; both treatment arms had large variations around the mean value (see Table 19). The mean cost of drugs was £63 less for the maze procedure arm than that for the control arm [the most expensive drugs used in the trial were the calcium channel blockers amlodipine and diltiazem hydrochloride (Dilzem® SR, Cephalon UK Limited, Castleford, UK), followed by acenocoumarol (Sinthrome®, Merus Labs Luxco S.a.R.L., Luxembourg City, Luxembourg)]. For more details of costs per patient, see Appendix 2, Table 23.
Comparison of Tables 24 and 25 (Appendix 2) shows that, as a result of data completeness, there was very little impact of imputation during primary admission; resource use for both the intervention and control arms increased very slightly. Further details of the total costs for the two treatment arms are given in Appendix 2, Table 26.
Quality of life
Table 20 shows that, in both years, the average QALYs in the maze procedure arm were slightly lower than in the control arm for both the EQ-5D-3L and the SF-6D, although these differences were not statistically significant. The difference in mean EQ-5D-3L QALYs per patient over 2 years was –0.044 (95% CI –0.16 to 0.07 QALYs; p = 0.44), which is also less than our a priori estimate of a minimum clinically important difference. Comparison of Tables 27 and 28 (Appendix 2) shows that imputation had no impact on these results.
| QALY type | Year of QALY | Treatment arm, mean (SD) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| EQ-5D-3L | QALY year 1 | 0.7160 (0.2583) | 0.7235 (0.2640) |
| QALY year 2 | 0.6896 (0.3066) | 0.7274 (0.2964) | |
| SF-6D | QALY year 1 | 0.6549 (0.2059) | 0.6647 (0.2152) |
| QALY year 2 | 0.6418 (0.2486) | 0.6699 (0.2374) | |
Comparing costs and effects
Table 21 shows that the mean cost per patient (with imputation, but without adjustments) was statistically significantly higher for the maze procedure arm, and that mean incremental QALYs per patient were slightly lower (although not statistically significantly different) than those in the control arm. Therefore, the maze procedure arm was dominated by the control arm and, when valuing a QALY at £20,000, the INMB of the maze procedure arm was negative.
| Cost-effectiveness parameter | Treatment arm, mean (SD) | |||
|---|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | |||
| Total cost (£) in year 1 | 17,834 | 13,225 | 13,944 | 7954 |
| Total cost (£) in year 2 (with discounting) | 818 | 2185 | 868 | 1414 |
| Total cost (£) over 2 years (present value) | 18,653 | – | 14,812 | – |
| Incremental cost (£) (maze procedure vs. control) | 3841 (95% CI 1514 to 6167) | |||
| EQ-5D-3L QALYs in year 1 | 0.7160 | 0.26 | 0.7235 | 0.26 |
| EQ-5D-3L QALYs in year 2 (with discounting) | 0.6663 | 0.30 | 0.7028 | 0.29 |
| Total QALYs over 2 years (present value) | 1.3823 | – | 1.4263 | – |
| Incremental EQ-5D-3L QALYs (maze procedure vs. control) | –0.04398 (95% CI –0.1558 to 0.0678) | |||
| ICER | Dominated | |||
| INMB (£) at a WTP of £20,000 per QALY | –4720 | |||
| INMB (£) at a WTP of £30,000 per QALY | –5160 | |||
Cost-effectiveness
Table 22 shows the primary cost-effectiveness analysis from a seemingly unrelated regression analysis of costs and QALYs, adjusted for baseline differences and correlation between costs and QALYs, with discounting for year 2. The mean difference in the cost of the maze procedure, adjusting for age, sex, baseline EQ-5D-3L score and paroxysmal AF, was £3533 (95% CI £1321 to £5746) higher than for the control intervention, and the difference was statistically significant. The mean difference in QALYs between the maze procedure arm and the control arm, after also controlling for actual procedure used, was –0.022 (95% CI –0.1231 to 0.0791 QALYs), which was not statistically significant. The maze procedure arm was therefore dominated by the control arm.
| Dependent variable | Independent variable | Coefficient | SEM | p-value |
|---|---|---|---|---|
| EQ-5D-3L QALYs | Maze procedure arm | –0.0220 | 0.0516 | 0.67 |
| Male | –0.0836 | 0.0551 | 0.13 | |
| Age (years) | –0.0045 | 0.0035 | 0.20 | |
| Baseline EQ-5D-3L score | 0.9369 | 0.1209 | < 0.01 | |
| Paroxysmal AF | –0.1053 | 0.0599 | 0.08 | |
| Actual procedure | 0.0006 | 0.0136 | 0.96 | |
| Constant | 1.3544 | 0.2855 | < 0.01 | |
| Total cost (£) per patient | Maze procedure arm | 3533 | 1129 | < 0.01 |
| Male | –2131 | 1205 | 0.08 | |
| Age (years) | 255 | 75 | < 0.01 | |
| Baseline EQ-5D-3L score | –9367 | 2645 | < 0.01 | |
| Paroxysmal AF | 2693 | 1300 | 0.04 | |
| Constant | –1691 | 6247 | 0.79 |
The plot of the estimated joint distribution of cost and QALY differences (Figure 16) shows that few estimates fall below the line representing £30,000 per QALY, which is currently considered the upper limit for cost-effective health interventions by NICE. 45 The cost-effectiveness acceptability curve (Figure 17) shows that, even at a WTP of £70,000 per QALY, the maze procedure has only a 10% probability of being cost-effective, and Figure 18 shows a continuously declining net monetary benefit of the maze procedure from around –£3500.
FIGURE 16.
Incremental cost-effectiveness plane: the maze procedure arm compared with the control arm.
FIGURE 17.
Cost-effectiveness acceptability curve for the maze procedure relative to the control arm.
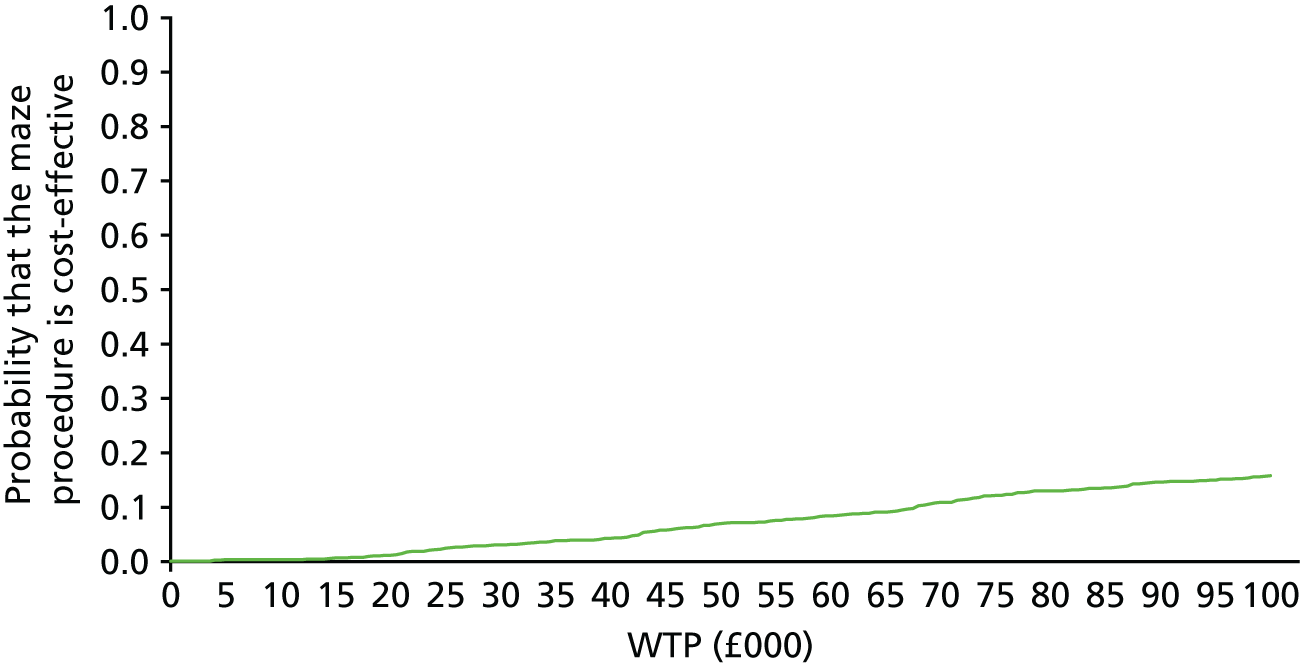
FIGURE 18.
Net monetary benefit of the maze procedure arm relative to the control arm.

Tables 29 and 30 in Appendix 2 show that none of the sensitivity analyses suggested that the maze procedure is likely to be cost-effective at £30,000 per QALY at 2 years. The closest the results came to this was limiting the analysis to patients randomised from April 2011, when the maze procedure had an ICER of £53,538 at 2 years. However, the INMB was negative and the analysis limiting patients to those randomised after April 2011 indicated that the probability of the maze procedure being cost-effective at £30,000 per QALY was < 30% (see the probabilistic sensitivity analyses in Figure 19). The final sensitivity analysis (see Appendix 2, Table 31) shows that, with imputation and controlling for baseline differences, the incremental cost per additional conversion from AF to SR was £25,220.
FIGURE 19.
Probabilistic sensitivity analyses attached to varying deterministic conditions: (a) using the SF-6D; (b) using primary admission only; (c) using a cost imputation model; (d) excluding ablation device cost; (e) excluding resource-use outliers; (f) using complete-case analysis; and (g) excluding patients randomised before 1 April 2011. Incremental cost = maze procedure cost – control cost; and incremental QALY = maze QALY – control QALY.
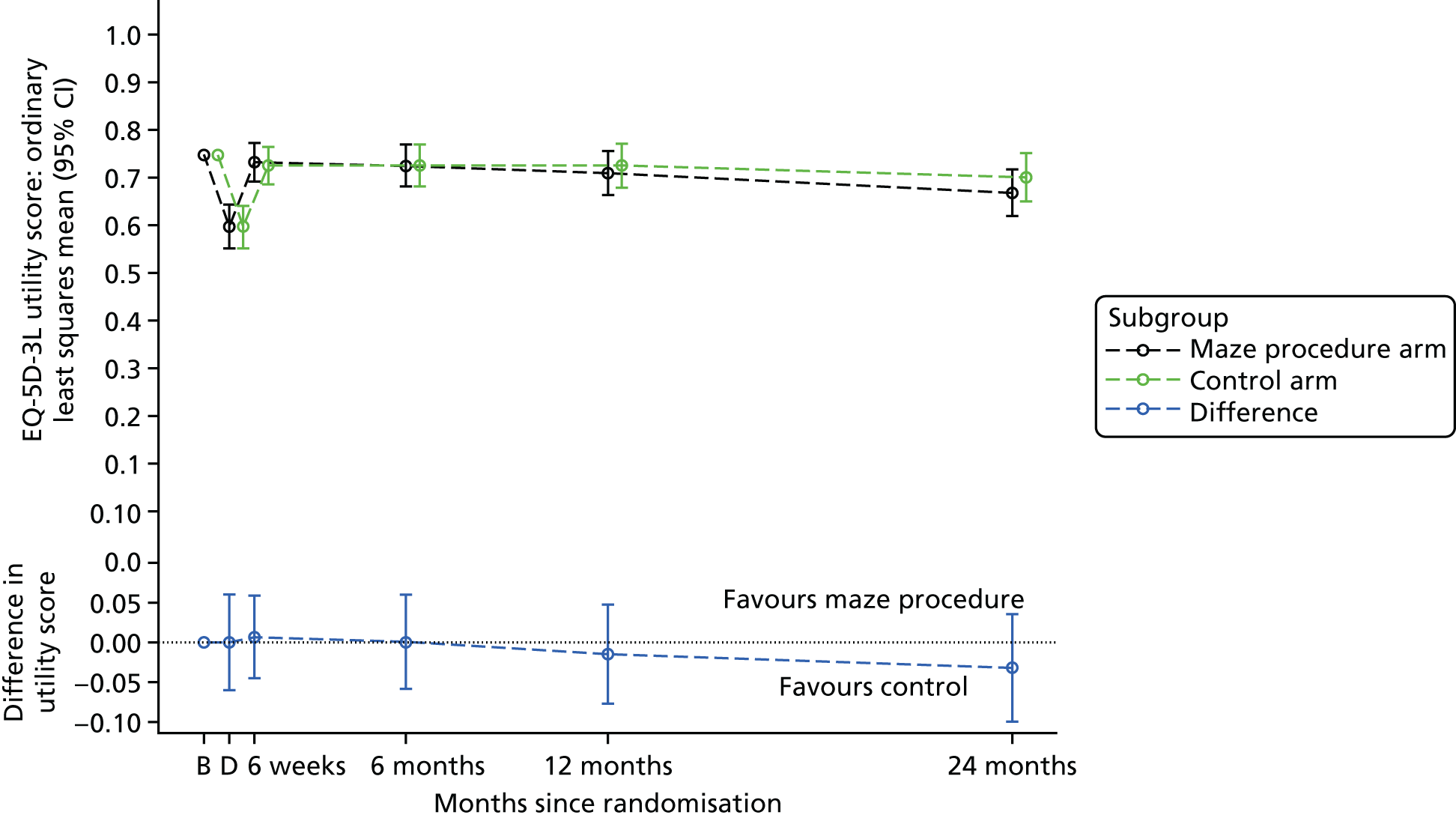



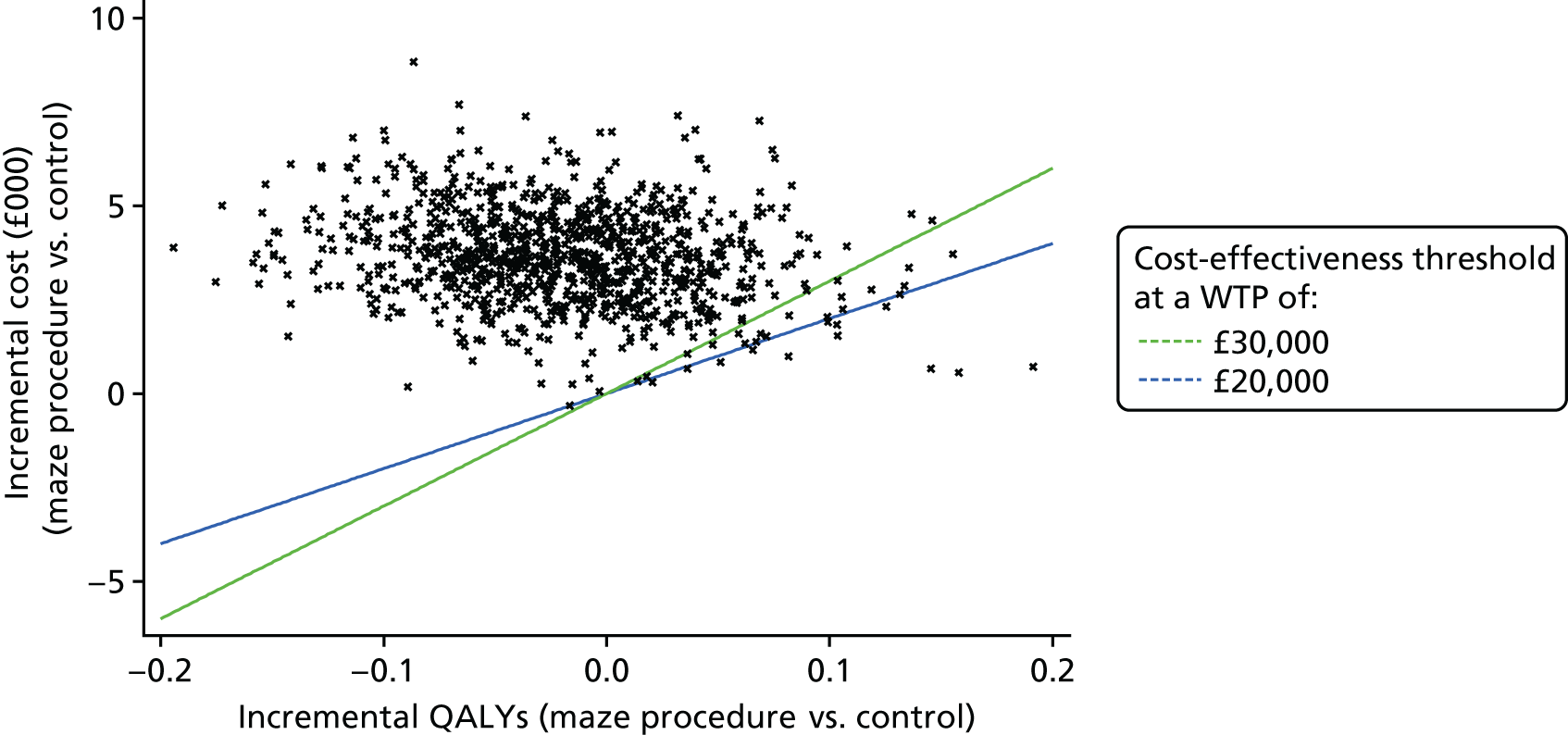
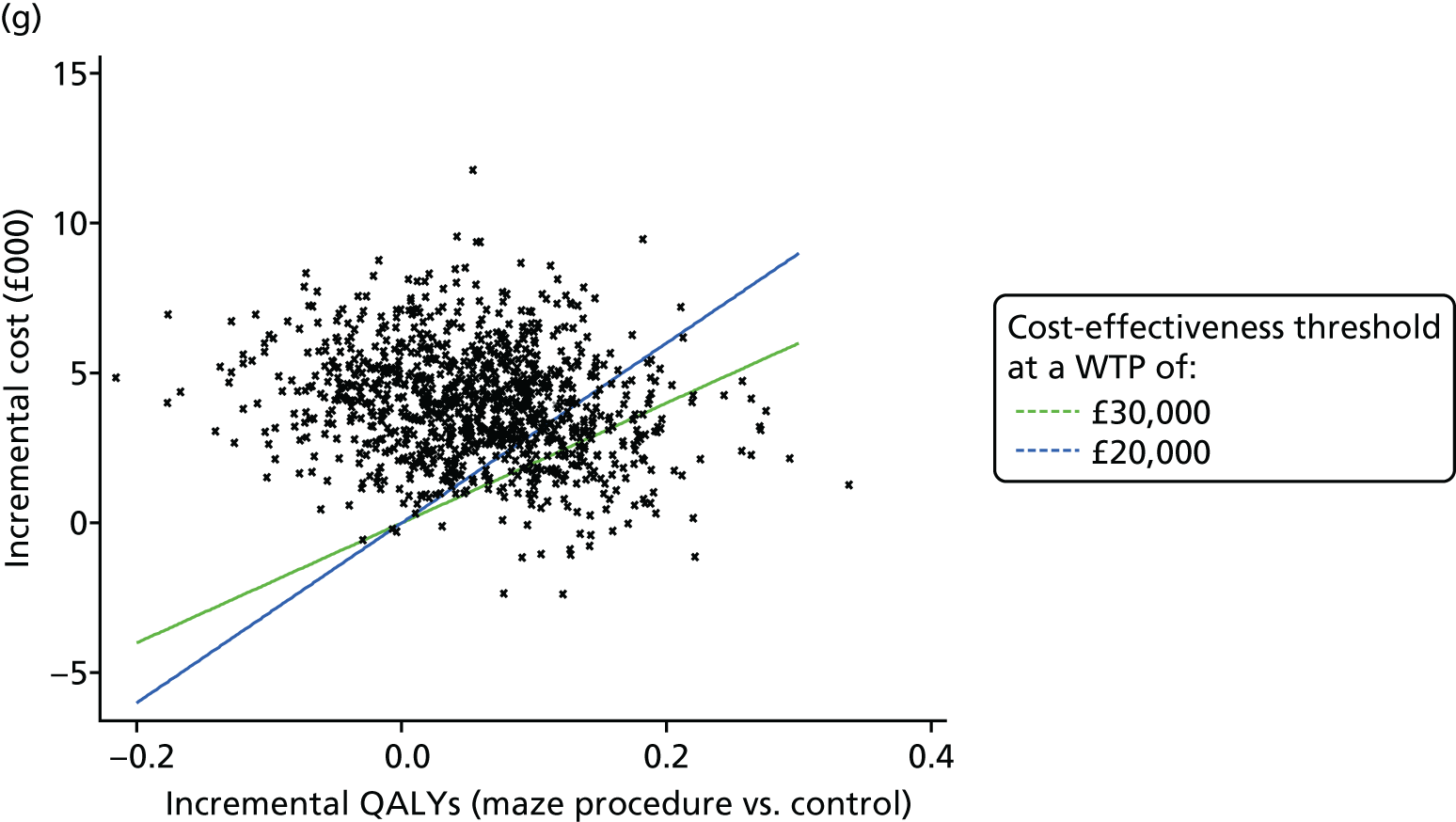
Chapter 6 Discussion
Summary of clinical trial results
The objective of the maze procedure as an adjunct to conventional cardiac surgery is to restore SR and thereby improve contractile function. If atrial function is restored, the procedure may reduce the risk of death, stroke and, via reduction in anticoagulant medication, the risk of bleeding. Improvement in patient-reported HRQoL and cost-effectiveness should follow from a reduction in these clinical events.
The Amaze trial has demonstrated a clear and important increase in the clinical primary outcome and the proportion of cardiac patients who returned to SR at 12 months (OR 2.06, 95% CI 1.20 to 3.54; p = 0.0091), and that this was maintained and increased at 24 months (OR 3.24, 95% CI 1.76 to 5.96; p = 0.0001). Moreover, the odds of returning to SR were greater in maze procedure patients, irrespective of whether patients had paroxysmal or non-paroxysmal AF, for all concomitant cardiac surgery undertaken and did not differ between surgeons (although planned surgery outcomes varied by surgeon). Although these subgroups were small and within-subgroup analyses had low statistical power, this finding adds weight to the causal link between the maze procedure and return to SR. Note that, although the OR for being in SR increased over time after randomisation, this largely resulted from a greater proportion of control patients returning to AF, rather than an increase in prevalence of SR in the maze procedure arm.
Post hoc analysis of the primary outcome showed that the OR for return to SR in early trial patients was lower than for later patients. There may be a number of reasons for this. First, it may have occurred purely by chance. Second, there was some evidence that patients recruited to the trial were more likely to have COPD as the trial progressed, which may have decreased overall success rates. Third, it may be related to greater understanding of the operative technique as the trial progressed, especially the now accepted need for multiple applications of the energy source to guarantee a transmural lesion, which has evolved during the period of the trial. 68 However, detailed examination of changes in outcomes as the trial progressed showed that control patients recruited later in the study had lower rates of return to SR than control patients recruited in the early period. Conversely, there was little difference in return to SR rates throughout the trial for the maze procedure arm. The surgical factor that also changed through the trial was the frequency of left atrial appendage excision, which decreased in control patients and increased in maze procedure patients, with a weakly significant interaction found between operation order and treatment group (the OR for left atrial appendage excision for maze procedure patients, relative to control patients, was 1.34 per 50 patients recruited; p = 0.0244). This divergence in excision rates between the arms appeared to be more common for specific surgeons who recruited more patients in the second half of the trial. The implication may be that the maze procedure is equally effective at interrupting AF in all patients, but there is substantial heterogeneity in results for control patients. Assiduous attention to cointerventions, such as left atrial appendage excision and cardioversion, is also effective, and at least some of the effect of the maze procedure may be explained by the use of these cointerventions. We stress that these post hoc exploratory analyses should be interpreted with caution, as there was little power to detect changes over time, and many patient and operative characteristics are highly correlated.
The maze procedure did not result in an increase in AEs, although 11 procedures were not completed, mainly because of technical problems or concerns regarding patient safety. There was also evidence that maze procedure patients were more likely to have their anticoagulant drugs stopped, which may result in fewer adverse bleeding events in the future.
In this pragmatic trial, there was no mandated mode of delivery of the maze procedure; radiofrequency ablation, usually bipolar, was the method of choice for a large proportion of the surgeons in the trial. The Amaze trial did not identify any clear differences in return to SR rates between methods, but the numbers of cases in these subgroups were small and these comparisons were not robust.
The lesion set treated was at the discretion of the operating surgeon, and there did not appear to be a clear relationship between the number or location of treatments and the odds of return to SR, although the power to detect these interactions was very low. The Amaze trial has confirmed that there appears to be still no practised consensus as to the optimal lesion set to be used in the adjunct maze procedure; the lesion set appeared to be more often determined by the individual surgeon and by the operative scenario than by firm guidelines and an operative plan. Nevertheless, the highest likelihood of SR restoration was observed after a left atrial ablation procedure that included the mitral isthmus lesion. Beyond that, we could discern no additional advantage of adding right atrial lesions.
In the HESTER substudy, the 95% CI for the mean difference in ALAEF, between patients in SR following the maze procedure and matched control patients who were in SR both before and after surgery, was within predefined clinically equivalent limits, although the ALAEF was statistically significantly lower in maze procedure patients. This is an important finding, as return to SR with no or minimal recovery of atrial function may not reduce the risk of thromboembolism. Absence of atrial contractility has been reported in around one-third of patients who return to SR following ablation (using a variety of surgical and catheter ablation techniques) and trials examining the return of atrial function have produced conflicting results. 65 Some reported the return of haemodynamically important function, but others suggested that the maze procedure is unlikely to achieve functional recovery, despite restoring SR. 69
It is disappointing that we found no significant differences in survival and QoL between the maze procedure and control arms at 2 years. As all patients in the Amaze trial also had conventional cardiac surgery, marked improvements were observed in the NYHA status and QoL parameters in both treatment arms from 6 months after surgery, and most of these improvements must be attributable to the conventional cardiac operation. The fact that we found no additional HRQoL benefit attributable to the maze procedure may be because of the absence of such benefit, or because such benefit exists, but is small and requires a longer follow-up period. If the rate of SR restoration falls further in the control arm as time progresses, and if there is true recovery of atrial contractility in the restored SR group, then it is reasonable to conjecture that differences in QoL may be seen in future. Cardiac surgery alone is known to produce a marked increase in QoL within months of the procedure. 70 If AF surgery and restoration of SR have a positive impact on QoL, this may only be seen in the longer term, as patients no longer in AF are less exposed to the risks of stroke, anticoagulant drug complications, antiarrhythmic complications and progressive heart failure. The finding in the HESTER substudy of functional and contractile atria is an indication that such a benefit is possible and may be seen with longer-term follow-up. A long-term health economics model was not funded by the original grant. However, continued follow-up of clinical events and HRQoL is in progress and will inform a long-term analysis.
Comparison with existing evidence
Overall, our SR restoration rate after maze procedure was consistent with other RCTs, but lower than in some published series that represent selected practitioners treating selected patients. 9 The rate of SR restoration in the control arm was much higher than expected, and remained so, despite the substantial drop at 2 years. This is a sufficient indication for considering cardioversion in AF patients after conventional cardiac surgery alone, as over one-third of patients can be in SR at 2 years.
The two adverse features of asymptomatic AF are the impact on cardiac function and the risk of thromboembolism. 71 Both are directly related to atrial function. The HESTER substudy provided evidence that atrial contractile function increases as a result of the maze procedure; therefore, restoring SR with a maze procedure might provide clinical benefits in the future.
Return to SR with no or minimal recovery of atrial function may have no benefit to the patient with respect to the risk of thromboembolism. Left atrial transport function depends on atrial contractility, atrial synchrony and left ventricular diastolic function. Absence of atrial contractility has been reported in around one-third of patients who return to SR following ablation (using a variety of surgical and catheter ablation techniques). 65 Buber et al. 65 reported that the absence of left atrial contraction was associated with a significant increase in the risk of thromboembolic stroke after the maze procedure for patients in SR. Conversely, effective left atrial transport function may be associated with reduced morbidity after successful ablation of AF. 65
Trials examining the return of atrial function have produced conflicting results. Some have reported the return of haemodynamically meaningful function, but others have suggested that the maze procedure is unlikely to achieve functional recovery, despite restoring SR. 69 In patients without atrial contraction, anticoagulant drugs significantly reduce stroke rate, but the incidence of major bleeding is increased. 72,73 Furthermore, patients with chronic AF have evidence of persistent left atrial dysfunction, even after restoration of SR by radiofrequency ablation. This suggests that global and regional atrial dysfunction may be attributable to a combination of injury from the ablation process and pre-existing disease. 66,74
Patients want to know whether or not they can safely stop taking anticoagulants after SR is restored by a maze procedure. This requires long-term follow-up and stroke surveillance to be addressed with any certainty. However, the HESTER substudy results lend some support to those advocating or already practising anticoagulant drug withdrawal. The varying rates of left atrial functional recovery after maze procedure mean that it would be prudent to measure atrial function before considering withdrawal of anticoagulant drugs.
Summary and implications of cost-effectiveness results
Given the clinical results, it is perhaps not surprising that the per-patient costs over 2 years in the maze procedure arm were statistically significantly higher than those in the control arm (£3533, 95% CI £1321 to £5746) and that there was a small non-significant reduction in discounted QALYs (–0.022, 95% CI –0.1231 to 0.0791 QALYs). With higher costs and no improvement in QoL over the 2 years of the trial, the control arm, representing current practice, dominates the new maze procedure for patients with AF within a 2-year period.
Both the deterministic and probabilistic sensitivity analyses confirmed this conclusion, showing that, within a 2-year period, the probability that the maze procedure would be considered cost-effective was < 5%. Moreover, a variety of alternative and favourable assumptions would not change the overall conclusion that, compared with current practice in the control arm, the maze procedure is not a cost-effective intervention up to 2 years. A potential caveat to this was the result from an unplanned post hoc subgroup analysis that included only the later patients (n = 200), in which the incremental effect was positive and the ICER fell to £53,500.
To date, results from the wider literature on the cost per QALY of ablation surgery in patients with AF are somewhat mixed. This first trial-based economic evaluation found that, comparing an adjunct ablation surgery with cardiac surgery over a 1-year period, an additional cost of €4426 and a mean 0.06 QALY gain translated to an ICER of €73,359. 75 The associated probabilistic sensitivity analysis indicated that, at a WTP per QALY of €30,000, the probability that the add-on ablation surgery was cost-effective was around 10%, and, therefore, was not considered cost-effective at 1 year. Our 2-year results are therefore in line with these findings. Longer-term economic models have shown that, over 5 years, either a classic ‘cut-and-sew’ maze procedure or a high-intensity ultrasound-assisted surgical ablation procedure, in addition to scheduled CABG or valve surgery, was highly cost-effective compared with drug treatment. 76 Cost-effectiveness decreased slightly if the intervention was applied with a percutaneous procedure. However, economic modelling to inform Canadian decision-makers indicated that the cost-effectiveness of AF ablation compared with antiarrhythmic medication was more favourable over longer time horizons (e.g. 10 years) and could dominate antiarrhythmic medication given time horizons of 20 years; this was not the case when considering a 5-year time horizon. 77 English decision-makers78 concluded that, having reviewed McKenna et al. 79 and Rodgers et al. ,80 duration of benefit is a key determinant of cost-effectiveness and, therefore, rejected van Breugel et al. 75 as having too short a time period for appropriate decision-making. This suggests that show be examined costs and effects over a longer time period, and preferably for longer than 5 years.
A 2013 systematic review, which included each of these studies, concluded that there was insufficient evidence to support catheter ablation as a first-line treatment, and that there was mixed evidence for second-line ablation. 81 The authors specifically lament the lack of QoL data, especially in the long term, and highlight the critical importance of this in determining cost-effectiveness. They reflect on the shortcomings of existing economic studies and, without hard evidence on such key outcomes in the medium term (1–5 years), successfully counselled the Belgian government not to extrapolate beyond a 5-year term, because of uncertainties. This highlights the importance of the planned long-term follow-up of patients from the Amaze trial and the relevance of these future data to extending economic analysis into the long term. Interestingly, an industry-sponsored, 5-year economic model published in 2014 reported that, although catheter ablation and the convergent procedure were argued to be cost-effective relative to medical management for non-paroxysmal AF, this was dependent on key assumptions about long-term maintenance of SR beyond 2 years and its relationship to future QoL. 82
Strengths and weaknesses
The Amaze trial was ground-breaking in being a relatively large, multicentre Phase III trial in a surgical setting, in which randomisation was completed during surgery, and patients, investigators and clinical staff (with the exception of the surgical team) were blinded to treatment allocation. Details of the randomised arm were sealed and released only to the clinical teams in the event of a SAE that required unblinding. Complete blinding was achieved for patients and HRQoL assessors, and only 13 patients had their treatment allocation open to the clinical teams caring for the patients, thus supporting the integrity of the trial methodology. A further strength was the longer follow-up period, which incorporated quality-adjusted survival as a co-primary outcome.
Screening logs were completed assiduously only at the co-ordinating centre, and reasons for exclusion from the trial were not always transparent, which affected the interpretation of the generalisability of the results. However, comparison of baseline characteristics with those reported in annual audit statistics and registries suggests that trial patients were slightly older and more likely to be female, and that they had a slightly lower average EuroSCORE, but otherwise were broadly representative of NHS-treated cardiac surgery patients. 62
In common with other cardiac surgical trials, prolongation of hospital stay and readmissions were common, and this is reflected in the number of SAEs recorded. There were no differences between the maze procedure and non-maze procedure patients in the number of events, their severity or relation to treatment, or in the number of patients who had events. Slow recruitment is a widespread problem in RCTs in general, and surgical trials in particular, and it is unfortunate that the Amaze trial did not achieve the target recruitment level.
In the study design, recruitment and randomisation of 400 patients were expected to be complete in 18 months. Delays in obtaining trust approvals at the recruitment sites ranged from 5 to 16 months, so that centre set-up took much longer than anticipated. In addition, a high number of staff changes across many sites, including withdrawal of Comprehensive Local Research Network support for the study at Papworth Hospital, had a significant impact on the recruitment rate.
During the trial, it became apparent that most local centres significantly overestimated their surgical activity and/or the recruitment rate for the Amaze trial. Moreover, there was heightened awareness of the maze procedure among both patients, as a result of their own personal research, and clinicians, which affected equipoise of potential recruits and decreased the number of patients approached to participate in the trial. The age distribution of the population that would be eligible for the trial also changed over time, with the proportion of elderly patients (aged ≥ 80 years) increasing. These patients were generally supportive of the research, but often declined to participate because of the need for attendance at follow-up visits and the presence of comorbidities. This was particularly true for patients living in rural areas, despite the provision of alternative methods of travel, such as taxis.
Recruitment was carefully monitored by the project management team throughout the trial. At every TSC meeting, a recruitment recovery plan was presented by the project team and reviewed. Specific measures taken to improve recruitment were as follows: (1) there was an increase in the number of sites, from 8 to 11; (2) all sites were encouraged to include all consultants who had the required experience of the maze procedure in recruitment; (3) contracts were revised to encourage ‘competitive recruitment’ across sites; and (4) extensive trial promotional material was produced to raise the profile and to encourage ‘ownership’ of the trial. Despite these measures, target recruitment was not reached within the funded time period.
Around 26% of the randomised patients (n = 92) had paroxysmal AF at baseline, and we found that being in SR during baseline ECG monitoring was strongly related to being in SR at annual follow-ups. Although all patients had a documented history of AF at baseline and satisfied the inclusion criteria for the trial, and the number of patients with paroxysmal AF at baseline were equally distributed between the two treatment arms, this could have diluted the treatment effect to some extent.
The decision by the independent DMEC to recommend that the TSC consider stopping the trial on the basis of futility meant that unblinded trial summaries were available to TSC members. We cannot rule out bias as a result of the knowledge that treatment effects were not promising at this time. Individual patients and the investigators recording primary outcomes remained unaware of group allocation, so we believe that any bias was minor. However, it is possible that clinicians drifted towards recruitment of patients with more severe AF, which in turn resulted in a larger treatment effect in the second half of the trial.
There were three follow-up data collection points (6, 12 and 24 months). Having longer gaps over which patients are asked to recall resource use has been shown to link to under-reporting of frequent events, severity of illness and those using services very intensively. 83 Despite this, neither Johnston et al. 83 nor Ridyard and Hughes84 recommended a specific interval between data collection points, but they did suggest compromising between respondent burden and collecting data on the incidence of events that drive resource use. Recent evidence published by Seidl et al. 85 reported that, in a 1-year trial evaluating management of acute MI in patients aged > 65 years, in one German hospital, three data collection points gave similar results to four data collection points. 85 In the Amaze trial, almost 95% of the difference in follow-up costs between trial arms related to admissions; as these are major and infrequent events, it is unlikely that their costs have been underestimated. There was lower resource use for primary and community care resources in the second year than in the first year, and although this could be a function of the frequency of data collection, it could also be a function of higher expected use of outpatient services following initial admission. Even so, it is not clear this would affect the incremental cost difference and, therefore, change the findings.
There was no statistically significant effect for the patient-centred primary outcome, undiscounted QALYs over 2 years, and the 95% CI for the difference between the treatment arms (maze procedure vs. control) excluded our prespecified minimum clinically important difference of 0.083 QALYs. More specifically, based on the 95% CI, we could rule out differences in QALYs of ≥ 0.083 in favour of the maze procedure arm. A long-term economic model may demonstrate a delayed effect on patient-centred outcomes, but it was outside the scope of this report.
The HESTER substudy was small and not randomised, so the results regarding restoration of contractile function were less robust. Echocardiography measurements of left atrial volumes, despite being well correlated with MRI measurements, tend to be underestimations; therefore, estimated treatment effects from MRI measurements were larger and had wider CIs than the corresponding estimates from echocardiography measurements. 86,87 Implications for the analyses of ALAEF, for which we do not have MRI measurements, are difficult to assess. Our a priori definition of clinical equivalence in the HESTER substudy was based on the SD (18.2%) of normal ALAEF reported in a published study, along with the investigators’ clinical judgement. 88 However, the SD in our sample was lower (< 8%). This may have resulted from an underestimation of the population SD in our cohort, but could be a consequence of our sample being a more homogeneous group of patients, which may limit the generalisability of our results.
There were 49 maze procedures at Papworth Hospital outside the trial; these patients either had severely symptomatic AF, so that surgeons were not in equipoise for these patients, or the patients themselves or their surgeon had a strong preference for the treatment, despite a lack of robust evidence. Thus, our results do not necessarily apply to these patients.
Implications for service
-
Ablation can be safely practised in a NHS routine cardiac surgical setting and will increase the number of patients who return to SR after surgery.
-
There is some support for anticoagulant drug withdrawal, but the varying rates of left atrial functional recovery after a maze procedure suggest that measurement of atrial function before considering withdrawal of anticoagulant drugs would be prudent.
-
The improvement in SR restoration as the study progressed suggests better patient selection and better maze procedures, including cointerventions (e.g. excision of the left atrial appendage), were performed in the later stages. This may reflect greater understanding of the lesion set and greater success in achieving truly transmural lesions. Surgeons performing the procedure may wish to audit their SR restoration rate and modify their practice if necessary.
Implications for further research
-
Continued monitoring of clinical events (deaths, strokes and haemorrhaging), resource use (especially readmissions) and HRQoL to inform a long-term health economics model is required, and for at least 5 years after surgery.
-
Further study of the relative effectiveness of different ablation techniques would clarify the choice of methodology.
Conclusions
In the Amaze trial, the maze procedure, as an adjunct to routine cardiac surgery, was safe and effective in increasing the probability of restoration of SR in patients with pre-existing paroxysmal or non-paroxysmal (persistent, longstanding or chronic) AF. The odds of being in SR approximately doubled at 12 months, and more than tripled at 24 months, after surgery. There was evidence from a small, non-randomised substudy that this was associated with improved atrial contractile function that was lower, but within the limits of clinical equivalence, when compared with patients who were in SR before surgery. The additional cost of the maze procedure per person over 2 years, relative to routine cardiac surgery, was £3500. In the 2-year follow-up period, these clinical effects had not resulted in additional improvement in HRQoL; however, given the known association between AF and subsequent neurological events and the reduced use of anticoagulants in the maze procedure arm, continued follow-up and analysis is warranted.
Acknowledgements
The trial was funded by the NIHR HTA programme, and was carried out while the first author was employed as a programme leader at the Medical Research Council Biostatistics Unit and, later, as a Division Director at the Leeds Institute of Clinical Trials Research.
We are grateful to the independent members of the TSC [Dr Dai Rowlands (chairperson), Consultant Cardiologist, Peterborough Hospital (retired); Mr Brian Elliott, independent lay member; and Professor Paul Kinnersley, School of Medicine, Cardiff University, Cardiff, UK] and the DMEC [Mr James Roxburgh (chairperson), Consultant Cardiothoracic Surgeon, Guy’s and St Thomas’ Hospital, London, UK; Dr Derick Todd, Consultant Cardiac Electrophysiologist, Liverpool Heart and Chest Hospital, Liverpool, UK; Professor Mark Sculpher, Health Economist, University of York, York, UK; and Dr Fay Cafferty, Statistician, Wolfson Institute of Preventative Medicine, London, UK] for their guidance throughout the trial.
Furthermore, we are grateful to Matt Glover for the initial health economics input, Sheridan Carter for the ECG analysis, Donna Alexander for conducting the trial, Helen Holcombe and Hannah Munday for project co-ordination and all research nurses, theatre and ward staff and patients.
The trial was conducted in collaboration with the Papworth Trials Unit Collaboration, the staff of which supported the trial design, conduct and analysis, including data management (Christine Mills, Clinical Project Manager), clinical trial operations and quality assurance (Christine Mills and Victoria Stoneman, Clinical Project Managers).
Contributions of authors
Linda Sharples (Professor of Medical Statistics) was a grant applicant, who designed the original study, led the statistical and trial methodology, oversaw the statistical analysis and drafted the manuscript. She is the guarantor for the statistical analysis.
Colin Everett (Senior Trial Statistician) analysed the Amaze trial data and contributed to the drafting of the manuscript.
Jeshika Singh (Research Fellow, Health Economics) carried out the cost-effectiveness analysis and contributed to the drafting of the manuscript.
Christine Mills (Clinical Project Manager, Project Delivery) contributed to the design, patient recruitment and project management of the trial at all centres, and also contributed to the drafting of the manuscript.
Tom Spyt (Consultant Cardiac Surgeon) contributed to the design, trial protocol, patient recruitment and management and manuscript review.
Yasir Abu-Omar (Consultant Cardiac Surgeon) contributed to patient recruitment and management and also to the drafting of the chapter on the HESTER substudy and manuscript review.
Simon Fynn (Consultant Cardiologist) contributed to the design, trial protocol, patient recruitment and management, and led the analysis of the ECG results and reviewed the manuscript.
Benjamin Thorpe (Research Methods Fellow, Statistics) analysed the HESTER substudy data and contributed to the drafting of the manuscript.
Victoria Stoneman (Clinical Project Manager, Project Delivery) contributed to the grant application and protocol for the HESTER substudy, the trial management of the HESTER substudy and the Amaze trial and the drafting of the manuscript.
Hester Goddard (Trial Manager, retired) was a grant applicant, who contributed to the protocols, managed the trial during set-up and the first 4 years and reviewed the manuscript.
Julia Fox-Rushby (Professor of Health Economics) led the health economics analysis, reviewed the methodology and contributed to the drafting of the manuscript. She is the guarantor for the cost-effectiveness analysis.
Samer Nashef (Chief Investigator, Consultant Cardiac Surgeon) was a grant applicant, who conceived and designed the original study, led the surgical methodology, oversaw surgical trial activity across all centres and drafted the manuscript. He is the overall guarantor for the study.
All authors reviewed the final version of the monograph.
Publications
Abu-Omar Y, Thorpe BS, Freeman C, Mills C, Stoneman VEA, Gopalan D, et al. Recovery of left atrial contractile function after maze surgery in persistent longstanding atrial fibrillation. J Am Coll Cardiol 2017;70:2309–11.
Nashef SAM, Fynn S, Abu-Omar Y, Spyt TJ, Mills C, Everett CC, et al. Amaze: a randomised controlled trial of adjunct surgery for atrial fibrillation [published online ahead of print 17 April 2018]. Eur J Cardiothorac Surg 2018. https://doi.org/10.1093/ejcts/ezy165
Data sharing statement
Data sets collected and/or analysed during the Amaze trial will be available from the corresponding author on reasonable request, after all trial publications have been accepted, provided that the TSC agrees and existing patient consent is consistent with the request.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: Saunders; 2014.
- Camm CF, Nagendran M, Xiu PY, Maruthappu M. How effective is cryoablation for atrial fibrillation during concomitant cardiac surgery?. Interact Cardiovasc Thorac Surg 2011;13:410-14. https://doi.org/10.1510/icvts.2011.271676.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009;104:1534-9. https://doi.org/10.1016/j.amjcard.2009.07.022.
- Hobbs FD, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9400.
- Sundt TM, Gersh BJ. Making sense of the maze: which patients with atrial fibrillation will benefit?. JAMA 2005;294:2357-9. https://doi.org/10.1001/jama.294.18.2357.
- Atrial Fibrillation: Management. London: NICE; 2006.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8. https://doi.org/10.1161/01.STR.22.8.983.
- Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2007;4:816-61. https://doi.org/10.1016/j.hrthm.2007.04.005.
- Huffman MD, Karmali KN, Berendsen MA, Andrei AC, Kruse J, McCarthy PM, et al. Concomitant atrial fibrillation surgery for people undergoing cardiac surgery. Cochrane Database Syst Rev 2016;22. http://dx.doi.org/10.1002/14651858.CD011814.pub2.
- de Lima GG, Kalil RA, Leiria TL, Hatem DM, Kruse CL, Abrahao R, et al. Randomized study of surgery for patients with permanent atrial fibrillation as a result of mitral valve disease. Ann Thorac Surg 2004;77:2089-94. https://doi.org/10.1016/j.athoracsur.2003.11.018.
- Doukas G, Samani NJ, Alexiou C, Oc M, Chin DT, Stafford PG, et al. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: a randomized controlled trial. JAMA 2005;294:2323-9. https://doi.org/10.1001/jama.294.18.2323.
- Gillinov AM, Gelijns AC, Parides MK, DeRose JJ, Moskowitz AJ, Voisine P, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399-409. https://doi.org/10.1056/NEJMoa1500528.
- Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. https://doi.org/10.1056/NEJM199809033391003.
- Cox JL, Boineau JP, Schuessler RB, Ferguson TB, Cain ME, Lindsay BD, et al. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA 1991;266:1976-80. https://doi.org/10.1001/jama.1991.03470140088029.
- Shemin RJ, Cox JL, Gillinov AM, Blackstone EH, Bridges CR. Workforce on Evidence-Based Surgery of the Society of Thoracic Surgeons . Guidelines for reporting data and outcomes for the surgical treatment of atrial fibrillation. Ann Thorac Surg 2007;83:1225-30. https://doi.org/10.1016/j.athoracsur.2006.11.094.
- Malaisrie SC, Lee R, Kruse J, Lapin B, Wang EC, Bonow RO, et al. Atrial fibrillation ablation in patients undergoing aortic valve replacement. J Heart Valve Dis 2012;21:350-7.
- Badhwar V, Rankin JS, Damiano RJ, Gillinov AM, Bakaeen FG, Edgerton JR, et al. The Society of Thoracic Surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg 2017;103:329-41. https://doi.org/10.1016/j.athoracsur.2016.10.076.
- Lall SC, Melby SJ, Voeller RK, Zierer A, Bailey MS, Guthrie TJ, et al. The effect of ablation technology on surgical outcomes after the Cox–maze procedure: a propensity analysis. J Thorac Cardiovasc Surg 2007;133:389-96. https://doi.org/10.1016/j.jtcvs.2006.10.009.
- Schuetz A, Schulze CJ, Sarvanakis KK, Mair H, Plazer H, Kilger E, et al. Surgical treatment of permanent atrial fibrillation using microwave energy ablation: a prospective randomized clinical trial. Eur J Cardiothorac Surg 2003;24:475-80. https://doi.org/10.1016/S1010-7940(03)00377-4.
- Abreu Filho CA, Lisboa LA, Dallan LA, Spina GS, Grinberg M, Scanavacca M, et al. Effectiveness of the maze procedure using cooled-tip radiofrequency ablation in patients with permanent atrial fibrillation and rheumatic mitral valve disease. Circulation 2005;112:I20-5.
- Akpinar B, Sanisoglu I, Guden M, Sagbas E, Caynak B, Bayramoglu Z. Combined off-pump coronary artery bypass grafting surgery and ablative therapy for atrial fibrillation: early and mid-term results. Ann Thorac Surg 2006;81:1332-7. https://doi.org/10.1016/j.athoracsur.2005.09.074.
- Deneke T, Khargi K, Grewe PH, Laczkovics A, von Dryander S, Lawo T, et al. Efficacy of an additional MAZE procedure using cooled-tip radiofrequency ablation in patients with chronic atrial fibrillation and mitral valve disease. A randomized, prospective trial. Eur Heart J 2002;23:558-66. https://doi.org/10.1053/euhj.2001.2841.
- McCarthy PM, Manjunath A, Kruse J, Andrei AC, Li Z, McGee EC, et al. Should paroxysmal atrial fibrillation be treated during cardiac surgery?. J Thorac Cardiovasc Surg 2013;146:810-23. https://doi.org/10.1016/j.jtcvs.2013.05.015.
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528-606. https://doi.org/10.1093/europace/eus027.
- Cherniavsky A, Kareva Y, Pak I, Rakhmonov S, Pokushalov E, Romanov A, et al. Assessment of results of surgical treatment for persistent atrial fibrillation during coronary artery bypass grafting using implantable loop recorders. Interact Cardiovasc Thorac Surg 2014;18:727-31. https://doi.org/10.1093/icvts/ivu016.
- Jessurun ER, van Hemel NM, Defauw JJ, Brutel De La Rivière A, Stofmeel MA, Kelder JC, et al. A randomized study of combining maze surgery for atrial fibrillation with mitral valve surgery. J Cardiovasc Surg 2003;44:9-18.
- Van Breugel HN, Nieman FH, Accord RE, Van Mastrigt GA, Nijs JF, Severens JL, et al. A prospective randomized multicenter comparison on health-related quality of life: the value of add-on arrhythmia surgery in patients with paroxysmal, permanent or persistent atrial fibrillation undergoing valvular and/or coronary bypass surgery. J Cardiovasc Electrophysiol 2010;21:511-20. https://doi.org/10.1111/j.1540-8167.2009.01655.x.
- Akpinar B, Guden M, Sagbas E, Sanisoglu I, Ozbek U, Caynak B, et al. Combined radiofrequency modified maze and mitral valve procedure through a port access approach: early and mid-term results. Eur J Cardiothorac Surg 2003;24:223-30. https://doi.org/10.1016/S1010-7940(03)00258-6.
- Albrecht A, Kalil RA, Schuch L, Abrahao R, Sant’Anna JR, de Lima G, et al. Randomized study of surgical isolation of the pulmonary veins for correction of permanent atrial fibrillation associated with mitral valve disease. J Thorac Cardiovasc Surg 2009;138:454-9. https://doi.org/10.1016/j.jtcvs.2009.04.023.
- Blomstrom-Lundqvist C, Johansson B, Berglin E, Nilsson L, Jensen SM, Thelin S, et al. A randomized double-blind study of epicardial left atrial cryoablation for permanent atrial fibrillation in patients undergoing mitral valve surgery: the SWEDish Multicentre Atrial Fibrillation study (SWEDMAF). Eur Heart J 2007;28:2902-8. https://doi.org/10.1093/eurheartj/ehm378.
- Budera P, Straka Z, Osmancik P, Vanek T, Jelinek S, Hlavicka J, et al. Comparison of cardiac surgery with left atrial surgical ablation vs. cardiac surgery without atrial ablation in patients with coronary and/or valvular heart disease plus atrial fibrillation: final results of the PRAGUE-12 randomized multicentre study. Eur Heart J 2012;33:2644-52. https://doi.org/10.1093/eurheartj/ehs290.
- Chevalier P, Leizorovicz A, Maureira P, Carteaux JP, Corbineau H, Caus T, et al. Left atrial radiofrequency ablation during mitral valve surgery: a prospective randomized multicentre study (SAFIR). Arch Cardiovasc Dis 2009;102:769-75. https://doi.org/10.1016/j.acvd.2009.08.010.
- Jönsson A, Lehto M, Ahn H, Hermansson U, Linde P, Ahlsson A, et al. Microwave Ablation in Mitral Valve Surgery for Atrial Fibrillation (MAMA). J Atr Fibrillation 2012;5.
- Khargi K, Deneke T, Haardt H, Lemke B, Grewe P, Müller KM, et al. Saline-irrigated, cooled-tip radiofrequency ablation is an effective technique to perform the maze procedure. Ann Thorac Surg 2001;72:S1090-5. https://doi.org/10.1016/S0003-4975(01)02940-X.
- Knaut M, Kolberg S, Brose S, Jung F. Epicardial microwave ablation of permanent atrial fibrillation during a coronary bypass and/or aortic valve operation: prospective, randomised, controlled, mono-centric study. Appl Cardiopulm Pathophysiol 2010;14:220-8.
- Pokushalov E, Romanov A, Corbucci G, Cherniavsky A, Karaskov A. Benefit of ablation of first diagnosed paroxysmal atrial fibrillation during coronary artery bypass grafting: a pilot study. Eur J Cardiothorac Surg 2012;41:556-60. https://doi.org/10.1093/ejcts/ezr101.
- Srivastava V, Kumar S, Javali S, Rajesh TR, Pai V, Khandekar J, et al. Efficacy of three different ablative procedures to treat atrial fibrillation in patients with valvular heart disease: a randomised trial. Heart Lung Circ 2008;17:232-40. https://doi.org/10.1016/j.hlc.2007.10.003.
- Vasconcelos JT, Scanavacca MI, Sampaio RO, Grinberg M, Sosa EA, Oliveira SA. Surgical treatment of atrial fibrillation through isolation of the left atrial posterior wall in patients with chronic rheumatic mitral valve disease. A randomized study with control group. Arq Bras Cardiol 2004;83:211-18.
- von Oppell UO, Masani N, O’Callaghan P, Wheeler R, Dimitrakakis G, Schiffelers S. Mitral valve surgery plus concomitant atrial fibrillation ablation is superior to mitral valve surgery alone with an intensive rhythm control strategy. Eur J Cardiothorac Surg 2009;35:641-50. https://doi.org/10.1016/j.ejcts.2008.12.042.
- Wang X, Song Y, Hu S, Wang W. Efficiency of radiofrequency ablation for surgical treatment of chronic atrial fibrillation in rheumatic valvular disease. Int J Cardiol 2014;174:497-502. https://doi.org/10.1016/j.ijcard.2014.03.153.
- Nashef S, Fynn S, Sharples L, Buxton M, Griffith G, Roberts P, et al. The Amaze Trial: A Randomised Controlled Trial to Investigate the Clinical and Cost-Effectiveness of Adding an Ablation Device-Based Maze Procedure As a Routine Adjunct to Elective Cardiac Surgery for Patients With Pre-Existing Atrial Fibrillation (Af): Study Protocol 2015. www.papworthhospital.nhs.uk/research/data/uploads/ptuc/protocol-v4-may-20151.pdf (accessed 15 December 2017).
- Nashef S, Abu-Omar Y, Gopalan D, Bushra R, Sharples L, Stoneman V, et al. The HESTER Study: Has Electrical Sinus Translated Into Effective Remodelling: Study Protocol 2014. www.papworthhospital.nhs.uk/research/data/uploads/ptuc/hester-study-protocol-3.pdf (accessed 15 December 2017).
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Dolan P, Gudex C, Kind P. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: University of York, Centre for Health Economics; 1995.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Jenkinson C, Stewart-Brown S, Petersen S, Paice C. Assessment of the SF-36 version 2 in the United Kingdom. J Epidemiol Community Health 1999;53:46-50. https://doi.org/10.1136/jech.53.1.46.
- Ware JE. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric Inc; 2000.
- Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al. Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11490.
- Campbell HE, Tait S, Sharples LD, Caine N, Gray TJ, Schofield PM, et al. Trial-based cost-utility comparison of percutaneous myocardial laser revascularisation and continued medical therapy for treatment of refractory angina pectoris. Eur J Health Econ 2005;6:288-97. https://doi.org/10.1007/s10198-005-0310-5.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. https://doi.org/10.1016/S0140-6736(00)04337-3.
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. London: Chapman & Hall; 1993.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987.
- NHS . NHS Prescription Services Electronic Drug Tariff 2016. www.drugtariff.nhsbsa.nhs.uk/ (accessed 10 January 2018).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- NHS Reference Costs 2014 to 2015. London: Department of Health and Social Care; 2015.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J 2003;24:881-2. https://doi.org/10.1016/S0195-668X(02)00799-6.
- Cosgriff R, Hickey G, Grant S, Bridgewater B. UK Heart Surgery: What Patients Can Expect from their Surgeons. London: National Institute for Cardiovascular Outcomes Research; 2013.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: University of York, Centre for Health Economics; 1999.
- Rowen D, Brazier J, Roberts J. Mapping SF-36 onto the EQ-5D index: how reliable is the relationship?. Health Qual Life Outcomes 2009;7. https://doi.org/10.1186/1477-7525-7-27.
- Buber J, Luria D, Sternik L, Raanani E, Feinberg MS, Goldenberg I, et al. Left atrial contractile function following a successful modified Maze procedure at surgery and the risk for subsequent thromboembolic stroke. J Am Coll Cardiol 2011;58:1614-21. https://doi.org/10.1016/j.jacc.2011.05.051.
- Boyd AC, Schiller NB, Ross DL, Thomas L. Differential recovery of regional atrial contraction after restoration of sinus rhythm after intraoperative linear radiofrequency ablation for atrial fibrillation. Am J Cardiol 2009;103:528-34. https://doi.org/10.1016/j.amjcard.2008.10.021.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. https://doi.org/10.1016/S0140-6736(07)61602-X.
- Melby SJ, Lee AM, Zierer A, Kaiser SP, Livhits MJ, Boineau JP, et al. Atrial fibrillation propagates through gaps in ablation lines: implications for ablative treatment of atrial fibrillation. Heart Rhythm 2008;5:1296-301. https://doi.org/10.1016/j.hrthm.2008.06.009.
- Yoshitake M, Takakura H, Sasaki T, Hachiya T, Onoguchi K, Taguchi S, et al. Electron beam cine CT-based evaluation of left atrial function after the maze procedure for mitral valve regurgitation. Ann Thorac Cardiovasc Surg 2010;16:91-8.
- Grady KL, Lee R, Subačius H, Malaisrie SC, McGee EC, Kruse J, et al. Improvements in health-related quality of life before and after isolated cardiac operations. Ann Thorac Surg 2011;91:777-83. https://doi.org/10.1016/j.athoracsur.2010.11.015.
- Pozzoli A, Taramasso M, Coppola G, Kamami M, La Canna G, Della Bella P, et al. Maze surgery normalizes left ventricular function in patients with persistent lone atrial fibrillation. Eur J Cardiothorac Surg 2014;46:871-6. https://doi.org/10.1093/ejcts/ezu034.
- Martinez-Comendador J, Gualis J, Marcos-Vidal JM, Buber J, Martin CE, Gomez-Plana J, et al. Efficacy of oral anticoagulation in stroke prevention among sinus-rhythm patients who lack left atrial mechanical contraction after cryoablation. Tex Heart Inst J 2015;42:430-7. https://doi.org/10.14503/THIJ-14-4572.
- Ad N, Henry L, Shuman DJ, Holmes SD. A more specific anticoagulation regimen is required for patients after the cox-maze procedure. Ann Thorac Surg 2014;98:1331-8. https://doi.org/10.1016/j.athoracsur.2014.05.088.
- Thomas L, Boyd A, Thomas SP, Schiller NB, Ross DL. Atrial structural remodelling and restoration of atrial contraction after linear ablation for atrial fibrillation. Eur Heart J 2003;24:1942-51. https://doi.org/10.1016/j.ehj.2003.08.018.
- van Breugel N, Bidar E, Essers B, Neiman F, Accord R, Severens J, et al. Cost-effectiveness of ablation surgery in patients with atrial fibrillation undergoing cardiac surgery. Interact Cardiovasc Thorac Surg 2011;12:394-8. https://doi.org/10.1510/icvts.2010.249482.
- Lamotte M, Annemans L, Bridgewater B, Kendall S, Siebert M. A health economic evaluation of concomitant surgical ablation for atrial fibrillation. Eur J Cardiothor Surg 2007;32:702-10. https://doi.org/10.1016/j.ejcts.2007.07.027.
- Assasi N, Blackhouse G, Xie F, Gaebel K, Robertson D, Hopkins R, et al. Ablation Procedures for Rhythm Control in Patients with Atrial Fibrillation: Clinical and Cost-Effectiveness Analyses. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health (CADTH); 2010.
- Atrial Fibrillation: The Management of Atrial Fibrillation. London: NICE; 2014.
- McKenna C, Palmer S, Rodgers M, Chambers D, Hawkins N, Golder S, et al. Cost-effectiveness of radiofrequency catheter ablation for the treatment of atrial fibrillation in the United Kingdom. Heart 2009;95:542-9. https://doi.org/10.1136/hrt.2008.147165.
- Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al. Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation. Health Technol Assess 2008;12. https://doi.org/10.3310/hta12340.
- Neyt M, Van Brabandt H, Devos C. The cost-utility of catheter ablation of atrial fibrillation: a systematic review and critical appraisal of economic evaluations. BMC Cardiovasc Disord 2013;13. https://doi.org/10.1186/1471-2261-13-78.
- Anderson LH, Black EJ, Civello KC, Martinson MS, Kress DC. Cost-effectiveness of the convergent procedure and catheter ablation for non-paroxysmal atrial fibrillation. J Med Econ 2014;17:481-91. https://doi.org/10.3111/13696998.2014.911185.
- Johnston K, Buxton MJ, Jones DR, Fitzpatrick R. Assessing the costs of healthcare technologies in clinical trials. Health Technol Assess 1999;3.
- Ridyard CH, Hughes DA. Methods for the collection of resource use data within clinical trials: a systematic review of studies funded by the UK Health Technology Assessment program. Value Health 2010;13:867-72. https://doi.org/10.1111/j.1524-4733.2010.00788.x.
- Seidl H, Meisinger C, Wende R, Holle R. Empirical analysis shows reduced cost data collection may be an efficient method in economic clinical trials. BMC Health Serv Res 2012;12. https://doi.org/10.1186/1472-6963-12-318.
- Rodevan O, Bjornerheim R, Ljosland M, Maehle J, Smith HJ, Ihlen H. Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. Int J Card Imaging 1999;15:397-410. https://doi.org/10.1023/A:1006276513186.
- Whitlock M, Garg A, Gelow J, Jacobson T, Broberg C. Comparison of left and right atrial volume by echocardiography versus cardiac magnetic resonance imaging using the area-length method. Am J Cardiol 2010;106:1345-50. https://doi.org/10.1016/j.amjcard.2010.06.065.
- Anwar AM, Geleijnse ML, Soliman OI, Nemes A, ten Cate FJ. Left atrial Frank–Starling law assessed by real-time, three-dimensional echocardiographic left atrial volume changes. Heart 2007;93:1393-7. https://doi.org/10.1136/hrt.2006.099366.
- Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FD, et al. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet 2011;378:1219-30. https://doi.org/10.1016/S0140-6736(11)61184-7.
- Quality and Outcomes Framework (QOF) Indicator Development Programme. Cost Impact Statement: Hypertension. London: NICE; 2013.
- Auguste P, Barton P, Hyde C, Roberts TE. An economic evaluation of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15180.
Appendix 1 Statistical analysis plan
Appendix 2 Additional tables from the health economics analysis
| Primary admission cost | Source | Consultation time/code | Mean cost (£) (SD) for 2014/15 |
|---|---|---|---|
| Theatre use | Papworth Hospital estimate | 20.00 (4.00) | |
| Adult critical care | NHS Reference Costs 2014 to 2015 59 | Total/weighted average | 1274.92 (583.33) |
| Cardiac ward | NHS Reference Costs 2014 to 2015 59 | Code: SD01A | 387.96 (77.59) |
| General ward | NHS Reference Costs 2014 to 2015 59 | Code: SD03A | 103.01 (20.60) |
| Rehabilitation | PSSRU 2015,58 1.3 | 158.57 (31.71) | |
| Acute (specialised ward) | NHS Reference Costs 2014 to 2015 59 | Code: SD01A | 387.96 (77.59) |
| Follow-up admission | |||
| ICU | NHS Reference Costs 2014 to 2015 59 | Total/weighted average | 1274.92 (583.33) |
| Cardiac ward | NHS Reference Costs 2014 to 2015 59 | Code: SD01A | 387.96 (77.59) |
| General ward | NHS Reference Costs 2014 to 2015 59 | Code: SD03A | 103.01 (20.60) |
| Day case | NHS Reference Costs 2014 to 2015 59 | Code: DC | 720.78 (144.16) |
| Follow-up tests | |||
| Angiography | NHS Reference Costs 2014 to 2015 59 | Code: EY43F | 260.00 (52.00) |
| MUGA | NHS Reference Costs 2014 to 2015 59 | Code: RN22Z | 192.39 (38.48) |
| Echocardiography | NHS Reference Costs 2014 to 2015 59 | Simple echocardiogram | 83.94 (16.79) |
| PET | NHS Reference Costs 2014 to 2015 59 | Code: RN07A | 524.77 (104.95) |
| TOE | NHS Reference Costs 2014 to 2015 59 | Complex echocardiogram | 128.49 (25.70) |
| Computerised tomography scan | NHS Reference Costs 2014 to 2015 59 | Total/weighted average | 122.31 (48.86) |
| Echocardiogram stress | NHS Reference Costs 2014 to 2015 59 | Complex echocardiogram | 128.49 (25.70) |
| MRI scan | NHS Reference Costs 2014 to 2015 59 | Total/weighted average | 146.15 (56.64) |
| Exercise test | NHS Reference Costs 2014 to 2015 59 | Field exercise testing | 287.08 (57.42) |
| ECG | NHS Reference Costs 2014 to 2015 59 | ECG monitoring | 52.13 (10.43) |
| 24-hour ECG | NHS Reference Costs 2014 to 2015 59 | ECG monitoring | 169.26 (33.85) |
| > 24-hour ECG | NHS Reference Costs 2014 to 2015 59 | ECG monitoring | 169.26 (33.85) |
| 24-hour blood pressure monitoring | Lovibond et al.89 | 61.47 (12.29) | |
| < 24-hour blood pressure monitoring | NICE Cost Statement 201390 | 38.34 (7.67) | |
| Left heart catheterisation | Papworth Hospital estimate | 1267.00 (253.40) | |
| Radiography (chest) | Auguste et al.91 | 3.46 (0.69) | |
| Follow-up health-care visits | |||
| GP visits | PSSRU 2015,58 10.8b | Per-patient contact lasting 17.2 minutes | 65.00 (13.00) |
| GP home visits | PSSRU 2015,58 10.8b | Per-patient contact lasting 11.7 minutes | 45.00 (9.00) |
| Nurse visits | PSSRU 2015,58 10.6 | Per-patient contact 15.5 minutes | 14.47 (2.89) |
| Nurse home visits | PSSRU 2015,58 10.4 | Per-patient contact 17.2 minutes | 19.38 (3.88) |
| Cardiology clinic | NHS Reference Costs 2014 to 2015 59 | Code: WF01A | 123.02 (24.60) |
| AF clinic | NHS Reference Costs 2014 to 2015 59 | Code: WF01A (cardiology clinic) | 123.02 (24.60) |
| Pacemaker | NHS Reference Costs 2014 to 2015 59 | Code: EY08E | 76.32 (15.26) |
| Physiotherapy | NHS Reference Costs 2014 to 2015 59 | Code: WF01A | 14.32 (2.86) |
| Occupational therapy | NHS Reference Costs 2014 to 2015 59 | Code: WF01A | 21.41 (4.28) |
| A&E visit | NHS Reference Costs 2014 to 2015 59 | Total/weighted average | 140.59 (141.05) |
| Wound clinic | NHS Reference Costs 2014 to 2015 59 | Code: N25AF/AN | 54.93 (10.99) |
| Cardiac rehabilitation | NHS Reference Costs 2014 to 2015 59 | Code: VC38Z | 97.84 (19.57) |
| Primary admission resource | Unit of measurement | Resource use per patient in each treatment arm | |||
|---|---|---|---|---|---|
| Maze procedure | Control | ||||
| Observation | Mean (SD) | Observation | Mean (SD) | ||
| Theatre | Minutes | 176 | 261.24 (79.68) | 176 | 247.48 (93.27) |
| Critical care (ICU) | Days | 175 | 3.17 (5.98) | 176 | 2.40 (4.38) |
| Cardiac ward | Days | 174 | 8.73 (12.08) | 176 | 7.90 (5.19) |
| Convalescence | Days | 167 | 0.29 (2.07) | 169 | 0.96 (6.96) |
| Transferred to acute trusta | Days | 173 | 2.37 (15.86) | 176 | 0.43 (3.63) |
| Follow-up admission | |||||
| 6 weeks in ICU | Days | 125 | 0.07 (0.72) | 130 | 0.04 (0.29) |
| 6 weeks in ward | Days | 125 | 2.49 (9.06) | 130 | 1.25 (4.12) |
| 6 weeks in cardiac ward | Days | 125 | 1.42 (7.29) | 130 | 0.64 (2.37) |
| 6 weeks as a day case | Days | 125 | 0.01 (0.09) | 130 | 0.00 (0.00) |
| 6 months in ICU | Days | 115 | 0.01 (0.09) | 122 | 0.02 (0.20) |
| 6 months in ward | Days | 115 | 0.59 (3.02) | 122 | 0.89 (5.40) |
| 6 months in cardiac ward | Days | 115 | 0.61 (2.75) | 122 | 0.70 (3.16) |
| 6 months as a day case | Days | 115 | 0.04 (0.24) | 122 | 0.01 (0.09) |
| 12 months in ICU | Days | 122 | 0.07 (0.46) | 122 | 0.00 (0.00) |
| 12 months in ward | Days | 122 | 0.02 (0.16) | 122 | 0.11 (0.54) |
| 12 months in cardiac ward | Days | 122 | 1.38 (5.42) | 122 | 0.56 (2.18) |
| 12 months as a day case | Days | 122 | 0.02 (0.20) | 122 | 0.01 (0.09) |
| 24 months in ICU | Days | 122 | 0.02 (0.27) | 131 | 0.00 (0.00) |
| 24 months in ward | Days | 122 | 0.63 (5.31) | 131 | 0.44 (2.06) |
| 24 months in cardiac ward | Days | 122 | 1.28 (5.07) | 131 | 1.30 (5.96) |
| 24 months as a day case | Days | 122 | 0.00 (0.00) | 131 | 0.04 (0.19) |
| Follow-up tests | |||||
| Year 1 angiography | Number of tests | 145 | 0.03 (0.20) | 148 | 0.01 (0.12) |
| Year 1 MUGA | Number of tests | 145 | 0.04 (0.50) | 148 | 0.00 (0.00) |
| Year 1 echocardiogram TTE | Number of tests | 145 | 0.46 (0.77) | 148 | 0.53 (0.83) |
| Year 1 PET scan | Number of tests | 145 | 0.00 (0.00) | 148 | 0.00 (0.00) |
| Year 1 echocardiogram TOE | Number of tests | 145 | 0.02 (0.14) | 148 | 0.02 (0.14) |
| Year 1 computerised tomography | Number of tests | 145 | 0.13 (0.40) | 148 | 0.07 (0.26) |
| Year 1 echocardiogram stress | Number of tests | 145 | 0.00 (0.00) | 148 | 0.00 (0.00) |
| Year 1 MRI scan | Number of tests | 145 | 0.05 (0.25) | 148 | 0.04 (0.20) |
| Year 1 exercise test | Number of tests | 145 | 0.04 (0.23) | 148 | 0.01 (0.08) |
| Year 1 ECG | Number of tests | 145 | 2.42 (2.06) | 149 | 2.16 (2.08) |
| Year 1 24-hour ECG | Number of tests | 145 | 0.16 (0.44) | 148 | 0.13 (0.36) |
| Year 1 > 24-hour ECG | Number of tests | 145 | 0.13 (0.46) | 148 | 0.10 (0.38) |
| Year 1 24-hour blood pressure monitoring | Number of tests | 145 | 0.02 (0.14) | 148 | 0.02 (0.18) |
| Year 1 < 24-hour blood pressure monitoring | Number of tests | 145 | 0.01 (0.12) | 148 | 0.09 (0.69) |
| Year 1 left heart catheter | Number of tests | 145 | 0.00 (0.00) | 148 | 0.00 (0.00) |
| Year 1 radiography | Number of tests | 145 | 1.61 (2.47) | 149 | 1.12 (1.34) |
| Year 2 angiography | Number of tests | 146 | 0.01 (0.12) | 150 | 0.01 (0.12) |
| Year 2 MUGA | Number of tests | 146 | 0.00 (0.00) | 150 | 0.00 (0.00) |
| Year 2 echocardiogram TTE | Number of tests | 146 | 0.21 (0.53) | 150 | 0.18 (0.42) |
| Year 2 PET scan | Number of tests | 146 | 0.00 (0.00) | 150 | 0.00 (0.00) |
| Year 2 echocardiogram TOE | Number of tests | 146 | 0.02 (0.14) | 149 | 0.01 (0.08) |
| Year 2 computerised tomography | Number of tests | 146 | 0.05 (0.26) | 150 | 0.09 (0.42) |
| Year 2 echocardiogram stress | Number of tests | 146 | 0.00 (0.00) | 150 | 0.01 (0.08) |
| Year 2 MRI scan | Number of tests | 146 | 0.05 (0.30) | 150 | 0.03 (0.16) |
| Year 2 exercise test | Number of tests | 146 | 0.00 (0.00) | 150 | 0.01 (0.12) |
| Year 2 ECG | Number of tests | 146 | 0.92 (3.67) | 150 | 0.69 (1.13) |
| Year 2 24-hour ECG | Number of tests | 146 | 0.02 (0.14) | 150 | 0.07 (0.28) |
| Year 2 > 24-hour ECG | Number of tests | 146 | 0.03 (0.16) | 150 | 0.04 (0.20) |
| Year 2 24-hour blood pressure monitoring | Number of tests | 146 | 0.01 (0.12) | 150 | 0.01 (0.08) |
| Year 2 < 24-hour blood pressure monitoring | Number of tests | 146 | 0.01 (0.12) | 150 | 0.03 (0.20) |
| Year 2 left heart catheter | Number of tests | 146 | 0.01 (0.08) | 150 | 0.00 (0.00) |
| Year 2 radiography | Number of tests | 146 | 0.19 (0.67) | 149 | 0.18 (0.57) |
| Follow-up health-care visits | |||||
| Year 1 GP visits | Number of visits | 145 | 3.77 (4.59) | 148 | 3.66 (4.28) |
| Year 1 GP home visits | Number of visits | 145 | 0.17 (0.72) | 148 | 0.24 (1.23) |
| Year 1 nurse (general practice) visits | Number of visits | 145 | 4.21 (8.74) | 148 | 4.17 (8.84) |
| Year 1 nurse home visits | Number of visits | 145 | 1.46 (4.90) | 148 | 0.86 (2.39) |
| Year 1 cardiovascular clinic | Number of visits | 145 | 1.63 (1.34) | 148 | 1.32 (1.20) |
| Year 1 AF clinic | Number of visits | 145 | 0.03 (0.20) | 148 | 0.01 (0.08) |
| Year 1 pacemaker | Number of visits | 145 | 0.10 (0.36) | 148 | 0.21 (0.81) |
| Year 1 physiotherapy | Number of visits | 145 | 0.32 (1.35) | 148 | 0.47 (2.85) |
| Year 1 occupational therapy | Number of visits | 145 | 0.01 (0.08) | 148 | 0.07 (0.41) |
| Year 1 A&E visit | Number of visits | 145 | 0.26 (0.76) | 148 | 0.27 (0.75) |
| Year 1 wound clinic | Number of visits | 145 | 0.05 (0.27) | 148 | 0.02 (0.18) |
| Year 1 cardiac rehabilitation | Number of visits | 145 | 3.09 (7.76) | 148 | 3.32 (6.93) |
| Year 2 GP visits | Number of visits | 146 | 1.55 (3.37) | 150 | 1.54 (2.56) |
| Year 2 GP home visits | Number of visits | 146 | 0.01 (0.12) | 150 | 0.09 (0.99) |
| Year 2 nurse (general practice) visits | Number of visits | 146 | 2.63 (6.87) | 150 | 2.40 (4.99) |
| Year 2 nurse home visits | Number of visits | 146 | 0.21 (1.51) | 150 | 0.15 (1.35) |
| Year 2 cardiovascular clinic | Number of visits | 146 | 0.54 (0.74) | 150 | 0.59 (0.98) |
| Year 2 AF clinic | Number of visits | 146 | 0.00 (0.00) | 150 | 0.01 (0.16) |
| Year 2 pacemaker | Number of visits | 146 | 0.08 (0.30) | 150 | 0.15 (0.50) |
| Year 2 physiotherapy | Number of visits | 146 | 0.18 (0.99) | 150 | 0.10 (0.82) |
| Year 2 occupational therapy | Number of visits | 146 | 0.03 (0.33) | 150 | 0.04 (0.42) |
| Year 2 A&E visit | Number of visits | 146 | 0.10 (0.40) | 150 | 0.17 (0.61) |
| Year 2 wound clinic | Number of visits | 146 | 0.00 (0.00) | 150 | 0.00 (0.00) |
| Year 2 cardiac rehabilitation | Number of visits | 146 | 0.36 (3.45) | 150 | 0.33 (3.76) |
| Primary admission cost | Unit of measurement | Resource use per patient in each treatment arm, mean (SD) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| Theatre use | Minutes | 261.24 (79.68) | 247.43 (93.13) |
| Adult critical care | Days | 3.16 (5.96) | 2.40 (4.38) |
| Cardiac ward (specialised ward) | Days | 8.76 (12.01) | 7.90 (5.19) |
| Rehabilitation | Days | 0.30 (2.05) | 0.93 (6.83) |
| Acute (specialised ward) | Days | 2.41 (15.74) | 0.43 (3.63) |
| Follow-up admission at week 6 | |||
| ICU | Days | 0.05 (0.61) | 0.03 (0.25) |
| General ward | Days | 1.80 (7.72) | 1.03 (3.72) |
| Cardiac ward | Days | 1.47 (6.86) | 0.50 (2.08) |
| Day case | Days | 0.01 (0.08) | 0.00 (0.00) |
| Follow-up admission at 6 months | |||
| ICU | Days | 0.01 (0.08) | 0.02 (0.17) |
| General ward | Days | 0.39 (2.45) | 0.66 (4.52) |
| Cardiac ward | Days | 0.40 (2.24) | 0.52 (2.67) |
| Day case | Days | 0.04 (0.23) | 0.01 (0.08) |
| Follow-up admission at 12 months | |||
| ICU | Days | 0.05 (0.38) | 0.00 (0.00) |
| General ward | Days | 0.02 (0.13) | 0.09 (0.48) |
| Cardiac ward | Days | 1.02 (4.58) | 0.41 (1.85) |
| Day case | Days | 0.02 (0.17) | 0.01 (0.08) |
| Follow-up admission at 24 months | |||
| ICU | Days | 0.02 (0.23) | 0.00 (0.00) |
| General ward | Days | 0.48 (4.43) | 0.44 (1.91) |
| Cardiac ward | Days | 1.06 (4.38) | 1.17 (5.24) |
| Day case | Days | 0.00 (0.00) | 0.03 (0.17) |
| Follow-up tests (year 1) | |||
| Angiography | Number of tests | 0.04 (0.22) | 0.02 (0.13) |
| MUGA | Number of tests | 0.03 (0.45) | 0.00 (0.00) |
| TTE | Number of tests | 0.49 (0.77) | 0.55 (0.80) |
| PET scan | Number of tests | 0.00 (0.00) | 0.00 (0.00) |
| TOE | Number of tests | 0.03 (0.14) | 0.03 (0.14) |
| Computerised tomography | Number of tests | 0.14 (0.38) | 0.08 (0.27) |
| Echocardiogram stress | Number of tests | 0.00 (0.00) | 0.00 (0.00) |
| MRI scan | Number of tests | 0.05 (0.23) | 0.05 (0.19) |
| Exercise test | Number of tests | 0.04 (0.22) | 0.01 (0.08) |
| ECG | Number of tests | 2.43 (1.98) | 2.34 (2.23) |
| 24-hour ECG | Number of tests | 0.15 (0.41) | 0.13 (0.34) |
| > 24-hour ECG | Number of tests | 0.16 (0.48) | 0.11 (0.39) |
| Blood pressure monitoring | Number of tests | 0.02 (0.13) | 0.02 (0.17) |
| 24-hour blood pressure monitoring | Number of tests | 0.02 (0.12) | 0.09 (0.64) |
| Left heart catheter | Number of tests | 0.00 (0.00) | 0.00 (0.00) |
| Radiography | Number of tests | 1.65 (2.38) | 1.10 (1.29) |
| Follow-up tests (year 2) | |||
| Angiography | Number of tests | 0.01 (0.11) | 0.01 (0.11) |
| MUGA | Number of tests | 0.00 (0.00) | 0.00 (0.00) |
| TTE | Number of tests | 0.22 (0.51) | 0.19 (0.40) |
| PET scan | Number of tests | 0.00 (0.00) | 0.00 (0.00) |
| TOE | Number of tests | 0.02 (0.13) | 0.01 (0.08) |
| Computerised tomography | Number of tests | 0.07 (0.26) | 0.10 (0.41) |
| Echocardiogram stress | Number of tests | 0.00 (0.00) | 0.01 (0.08) |
| MRI scan | Number of tests | 0.05 (0.28) | 0.03 (0.18) |
| Exercise test | Number of tests | 0.00 (0.00) | 0.02 (0.12) |
| ECG | Number of tests | 0.87 (3.35) | 0.71 (1.10) |
| 24-hour ECG | Number of tests | 0.03 (0.15) | 0.06 (0.26) |
| > 24-hour ECG | Number of tests | 0.03 (0.16) | 0.04 (0.19) |
| Blood pressure monitoring | Number of tests | 0.02 (0.12) | 0.01 (0.08) |
| 24-hour blood pressure monitoring | Number of tests | 0.01 (0.11) | 0.03 (0.20) |
| Left heart catheter | Number of tests | 0.01 (0.08) | 0.00 (0.00) |
| Radiography | Number of tests | 0.22 (0.68) | 0.20 (0.57) |
| Follow-up health-care visits (year 1) | |||
| GP visits | Number of visits | 3.72 (4.31) | 3.81 (4.22) |
| GP home visits | Number of visits | 0.18 (0.69) | 0.22 (1.13) |
| Nurse (general practice) visits | Number of visits | 4.16 (8.33) | 4.22 (8.58) |
| Nurse home visits | Number of visits | 1.38 (4.55) | 0.86 (2.25) |
| Cardiovascular clinic | Number of visits | 1.59 (1.30) | 1.30 (1.16) |
| AF clinic | Number of visits | 0.03 (0.19) | 0.01 (0.08) |
| Pacemaker | Number of visits | 0.11 (0.34) | 0.23 (0.81) |
| Physiotherapy | Number of visits | 0.44 (1.44) | 0.64 (3.25) |
| Occupational therapy | Number of visits | 0.01 (0.08) | 0.07 (0.38) |
| A&E visit | Number of visits | 0.29 (0.80) | 0.29 (0.74) |
| Wound clinic | Number of visits | 0.07 (0.30) | 0.02 (0.17) |
| Cardiac rehabilitation | Number of visits | 3.07 (7.34) | 3.23 (6.51) |
| Follow-up health-care visits (year 2) | |||
| GP visits | Number of visits | 1.65 (3.25) | 1.70 (2.67) |
| GP home visits | Number of visits | 0.01 (0.11) | 0.09 (0.91) |
| Nurse (general practice) visits | Number of visits | 2.67 (6.65) | 2.53 (4.96) |
| Nurse home visits | Number of visits | 0.22 (1.46) | 0.14 (1.25) |
| Cardiovascular clinic | Number of visits | 0.53 (0.72) | 0.61 (0.94) |
| AF clinic | Number of visits | 0.00 (0.00) | 0.01 (0.15) |
| Pacemaker | Number of visits | 0.11 (0.33) | 0.17 (0.49) |
| Physiotherapy | Number of visits | 0.21 (1.05) | 0.13 (0.92) |
| Occupational therapy | Number of visits | 0.02 (0.30) | 0.05 (0.43) |
| A&E visit | Number of visits | 0.13 (0.43) | 0.16 (0.57) |
| Wound clinic | Number of visits | 0.00 (0.00) | 0.00 (0.00) |
| Cardiac rehabilitation | Number of visits | 0.36 (3.20) | 0.41 (3.87) |
| Primary admission cost | Resource use (£) per patient in each treatment arm, mean (SD) | |
|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | |
| Theatre use | 5224.89 (1593.66) | 4948.52 (1862.51) |
| Ablation device | 1211.65 (408.33) | 14.20 (132.87) |
| Adult critical care | 4028.75 (7600.08) | 3064.51 (5585.89) |
| Cardiac ward | 3396.88 (4661.36) | 3064.03 (2013.71) |
| Rehabilitation | 47.75 (324.98) | 147.76 (1082.35) |
| Acute (specialised ward) | 936.84 (6105.06) | 165.33 (1408.64) |
| Subtotal | 14,846.76 (12,473.61) | 11,404.35 (7193.49) |
| Readmissions at 6 weeks | ||
| ICU | 65.19 (774.24) | 36.22 (317.57) |
| General ward | 151.58 (706.84) | 51.21 (213.78) |
| Cardiac ward | 697.67 (2994.27) | 398.99 (1444.75) |
| Day case | 4.10 (54.33) | 0.00 (0.00) |
| Readmissions at 6 months | ||
| ICU | 7.24 (96.10) | 21.73 (214.40) |
| General ward | 40.97 (230.75) | 53.55 (274.82) |
| Cardiac ward | 149.89 (951.09) | 254.60 (1753.63) |
| Day case | 28.67 (165.46) | 4.10 (54.33) |
| Readmissions at 12 months | ||
| ICU | 61.57 (489.90) | 0.00 (0.00) |
| General ward | 105.35 (471.71) | 42.72 (190.13) |
| Cardiac ward | 7.72 (52.30) | 35.27 (185.59) |
| Day case | 12.29 (121.21) | 4.10 (54.33) |
| Readmissions at 24 months | ||
| ICU | 21.73 (288.30) | 0.00 (0.00) |
| General ward | 109.45 (451.38) | 120.86 (539.50) |
| Cardiac ward | 186.27 (1720.26) | 171.94 (741.60) |
| Day case | 0.00 (0.00) | 24.57 (125.38) |
| Subtotal | 1649.69 (4192.49) | 1219.85 (2994.21) |
| Follow-up tests (year 1) | ||
| Angiography | 9.60 (57.33) | 5.17 (35.05) |
| MUGA | 6.56 (87.01) | 0.00 (0.00) |
| TTE | 40.78 (64.93) | 46.26 (67.46) |
| PET scan | 0.00 (0.00) | 0.00 (0.00) |
| TOE | 3.29 (18.52) | 3.29 (18.52) |
| Computerised tomography | 17.03 (47.00) | 10.08 (32.76) |
| Echocardiogram stress | 0.00 (0.00) | 0.00 (0.00) |
| MRI scan | 7.47 (34.12) | 6.64 (28.46) |
| Exercise test | 11.42 (64.09) | 1.63 (21.64) |
| ECG | 126.63 (103.36) | 122.18 (116.13) |
| 24-hour ECG | 25.00 (69.09) | 21.64 (57.75) |
| > 24-hour ECG | 26.93 (80.46) | 18.75 (65.98) |
| Blood pressure monitoring | 1.05 (7.98) | 1.05 (10.34) |
| 24-hour blood pressure monitoring | 0.65 (4.54) | 3.38 (24.49) |
| Left heart catheter | 0.00 (0.00) | 0.00 (0.00) |
| Radiography | 5.71 (8.24) | 3.79 (4.45) |
| Follow-up tests (year 2) | ||
| Angiography | 3.69 (29.25) | 2.95 (27.64) |
| MUGA | 0.00 (0.00) | 0.00 (0.00) |
| TTE | 18.12 (42.47) | 15.74 (33.76) |
| PET scan | 0.00 (0.00) | 0.00 (0.00) |
| TOE | 2.56 (17.32) | 1.10 (10.80) |
| Computerised tomography | 7.99 (32.03) | 12.51 (50.35) |
| Echocardiogram stress | 0.00 (0.00) | 0.73 (9.69) |
| MRI scan | 7.89 (40.95) | 4.98 (26.60) |
| Exercise test | 0.00 (0.00) | 4.89 (33.96) |
| ECG | 45.32 (174.84) | 37.17 (57.48) |
| 24-hour ECG | 5.29 (25.84) | 10.58 (43.98) |
| > 24-hour ECG | 5.29 (27.38) | 7.21 (32.45) |
| Blood pressure monitoring | 1.22 (7.61) | 0.35 (4.63) |
| 24-hour blood pressure monitoring | 0.54 (4.31) | 1.20 (7.71) |
| Left heart catheter | 7.20 (95.50) | 0.00 (0.00) |
| Radiography | 0.75 (2.36) | 0.69 (1.96) |
| Subtotal | 387.97 (375.85) | 343.96 (283.09) |
| Follow-up health-care visits (year 1) | ||
| GP visits | 241.72 (280.11) | 247.63 (274.01) |
| GP home visits | 8.05 (31.01) | 9.72 (50.95) |
| Nurse (general practice) visits | 60.17 (120.49) | 61.11 (124.06) |
| Nurse home visits | 26.75 (88.09) | 16.68 (43.53) |
| Cardiology clinic | 195.36 (160.23) | 160.42 (142.71) |
| AF clinic | 3.84 (23.85) | 0.70 (9.27) |
| Pacemaker | 8.02 (26.32) | 17.78 (61.72) |
| Physiotherapy | 7.10 (23.29) | 10.41 (52.43) |
| Occupational therapy | 0.14 (1.40) | 1.23 (6.31) |
| A&E visit | 41.14 (113.06) | 41.14 (104.50) |
| Wound clinic | 3.90 (16.28) | 1.09 (9.45) |
| Cardiac rehabilitation | 300.19 (718.22) | 315.76 (637.43) |
| Follow-up health-care visits (year 2) | ||
| GP visits | 107.29 (211.47) | 110.61 (173.24) |
| GP home visits | 0.64 (5.06) | 3.96 (41.02) |
| Nurse (general practice) visits | 38.59 (96.18) | 36.58 (71.71) |
| Nurse home visits | 4.24 (28.20) | 2.64 (24.17) |
| Cardiology clinic | 65.35 (88.02) | 75.14 (115.44) |
| AF clinic | 0.00 (0.00) | 1.40 (18.55) |
| Pacemaker | 8.02 (25.02) | 12.79 (37.30) |
| Physiotherapy | 3.39 (16.89) | 2.02 (14.80) |
| Occupational therapy | 0.38 (5.03) | 0.81 (7.11) |
| A&E visit | 18.77 (60.69) | 23.17 (80.04) |
| Wound clinic | 0.00 (0.00) | 0.00 (0.00) |
| Cardiac rehabilitation | 35.58 (313.15) | 40.03 (378.61) |
| Subtotal | 1178.66 (1060.51) | 1192.79 (1052.40) |
| Medication | ||
| Year 1 | 476.94 (1203.98) | 510.06 (2092.35) |
| Year 2 | 141.20 (456.15) | 171.29 (727.75) |
| Subtotal | 618.14 (1583.61) | 681.35 (2764.66) |
| Total | 18,681.21 (13,339.82) | 14,842.30 (8295.33) |
| Time point of utility score | Utility score by treatment arm, mean score (SD) | |
|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | |
| EQ-5D-3L score | ||
| Baseline | 0.7417 (0.22) | 0.7544 (0.21) |
| Discharge | 0.5927 (0.29) | 0.5943 (0.28) |
| 6-week follow-up | 0.7187 (0.27) | 0.7246 (0.27) |
| 6-month follow-up | 0.7312 (0.29) | 0.7350 (0.31) |
| 12-month follow-up | 0.7167 (0.31) | 0.7397 (0.30) |
| 24-month follow-up | 0.6719 (0.34) | 0.7163 (0.32) |
| SF-6D score | ||
| Baseline | 0.6599 (0.11) | 0.6651 (0.11) |
| 6-month follow-up | 0.6703 (0.23) | 0.6853 (0.23) |
| 12-month follow-up | 0.6565 (0.24) | 0.6723 (0.24) |
| 24-month follow-up | 0.6350 (0.27) | 0.6685 (0.25) |
| Time point of utility score | Treatment arm | |||
|---|---|---|---|---|
| Maze procedure | Control | |||
| Observations | Mean score (SD) | Observations | Mean score (SD) | |
| EQ-5D-3L score | ||||
| Baseline | 174 | 0.7417 (0.22) | 175 | 0.7544 (0.21) |
| Discharge | 161 | 0.6091 (0.28) | 165 | 0.6034 (0.28) |
| 6-week follow-up | 164 | 0.7378 (0.25) | 169 | 0.7339 (0.26) |
| 6-month follow-up | 169 | 0.7311 (0.30) | 174 | 0.7323 (0.31) |
| 12-month follow-up | 165 | 0.7142 (0.32) | 167 | 0.7367 (0.31) |
| 24-month follow-up | 158 | 0.6680 (0.35) | 161 | 0.7098 (0.34) |
| SF-6D score | ||||
| Baseline | 172 | 0.6623 (0.11) | 174 | 0.6644 (0.11) |
| 6-month follow-up | 168 | 0.6694 (0.23) | 171 | 0.6846 (0.24) |
| 12-month follow-up | 166 | 0.6557 (0.24) | 166 | 0.6678 (0.25) |
| 24-month follow-up | 158 | 0.6291 (0.28) | 157 | 0.6597 (0.26) |
| Scenario | Observations | Difference (maze procedure vs. control), mean (SD) | ICER (£) | INMB (£) at a WTP of | ||
|---|---|---|---|---|---|---|
| Incremental cost (£) over 24 months | Incremental QALYs over 24 months | £20,000 per QALY | £30,000 per QALY | |||
| Using EQ-5D-3L QALYs (base case) | 352 | 3533 (1129) | –0.0220 (0.0516) | Dominated | –3974 | –4194 |
| Using SF-6D QALYs | 352 | 3533 (1129) | –0.0197 (0.0433) | Dominated | –3928 | –4125 |
| Including costs and QALYs data only up to discharge | 352 | 3140 (1044) | 0.0014 (0.0018) | 2,234,462 | –3112 | –3098 |
| Excluding outliers from the analysis | 350 | 2856 (1013) | –0.0108 (0.0512) | Dominated | –3073 | –3181 |
| Alternate imputation model (cost imputation) | 352 | 4103 (1180) | –0.0214 (0.0520) | Dominated | –4530 | –4744 |
| Excluding device cost | 352 | 2336 (1128) | –0.0220 (0.0516) | Dominated | –2777 | –2997 |
| Complete-case analysis | 234 | 2210 (1108) | 0.0264 (0.0571) | 83,625 | –1682 | –1417 |
| Randomised after April 2011 | 200 | 3364 (1574) | 0.0628 (0.0693) | 53,538 | –2107 | –1479 |
| Scenario | Observations | Difference (maze procedure vs. control), mean (SD) | ICER (£) | INMB (£) at a WTP of | ||
|---|---|---|---|---|---|---|
| Incremental cost (£) over 24 months | Incremental QALYs over 24 months | £20,000 per QALY | £30,000 per QALY | |||
| Using EQ-5D-3L QALYs | 1000 | 3656 (1259) | –0.0205 (0.0545) | Dominated | –4066 | –4271 |
| Using SF-6D QALYs | 1000 | 3779 (1280) | –0.0204 (0.0443) | Dominated | –4187 | –4391 |
| Including costs and QALYs data only up to discharge | 1000 | 3003 (1125) | 0.0017 (0.0020) | 1,745,221 | –2969 | –2952 |
| Excluding outliers from the analysis | 1000 | 2936 (1123) | –0.0125 (0.0511) | Dominated | –3186 | –3312 |
| Alternate imputation model | 1000 | 3794 (1103) | –0.0144 (0.0515) | Dominated | –4082 | –4226 |
| Excluding additional device cost for maze procedure | 1000 | 2580 (1278) | –0.0239 (0.0536) | Dominated | –3058 | –3297 |
| Complete case analysis | 1000 | 2256 (1281) | 0.0277 (0.0574) | 81,516 | –1702 | –1426 |
| Randomised after April 2011 | 1000 | 3942 (1868) | 0.0551 (0.0737) | 71,505 | –2840 | –2288 |
| Dependent variable | Independent variable | Coefficient | SEM | p-value |
|---|---|---|---|---|
| Conversion of AF to SR | Maze procedure | 0.14 | 0.04 | 3.37 |
| Male | –0.05 | 0.04 | –1.12 | |
| Age | 0.008 | 0.002 | 0.03 | |
| Baseline EQ-5D-3L score | 0.23 | 0.1 | 2.3 | |
| Paroxysmal AF | 0.05 | 0.04 | 1.1 | |
| Actual procedure | –0.005 | 0.01 | –0.48 | |
| Constant | –0.25 | 0.23 | –1.07 | |
| Total cost (£) per patient | Maze procedure | 3533 | 1129 | 0.00 |
| Male | –2131 | 1205 | 0.08 | |
| Age | 255 | 75 | 0.00 | |
| Baseline EQ-5D-3L score | –9367 | 2645 | 0.00 | |
| Paroxysmal AF | 2693 | 1300 | 0.04 | |
| Constant | –1691 | 6247 | 0.79 |
Appendix 3 Additional tables
| Reason for exclusion | Frequency |
|---|---|
| Patient decision | (n = 107) |
| Too much to think about | 27 |
| Could not commit to follow-up or too far to travel | 18 |
| Patient did not want/was unsure about surgery, or was treated medically | 12 |
| Age concerns | 4 |
| GP or family concerned about trial participation | 3 |
| Patient did not want a 4-day ECG | 3 |
| Patient did not want the maze procedure | 1 |
| Uncomfortable about blinding of trial allocation | 1 |
| No reason given | 38 |
| Consultant decision | (n = 115) |
| Maze procedure carried out outside the trial | 49 |
| Minimally invasive or other procedure required | 11 |
| Withdrawn from surgery | 13 |
| Too frail/sick/comorbidities | 9 |
| Procedure was too high risk/complicated | 9 |
| Maze procedure was not desirable because of small left atrium and only paroxysmal AF | 1 |
| Not suitable – reason not specified | 23 |
| Patient failed eligibility or ethics criteria | (n = 49) |
| Patient already in another trial | 19 |
| Patient was an in-house urgent patient prior to change in eligibility criteria | 6 |
| Patient in SR | 5 |
| Patient had previous cardiac surgery | 3 |
| Patient unsuitable – reason not recorded | 16 |
| Patient died | (n = 8) |
| Other | (n = 87) |
| Not enough time to consider the trial | 25 |
| Unable to contact patient | 13 |
| Patient information sheet not given to the patient | 6 |
| Language barrier | 1 |
| Other administrative reasons – not recorded | 42 |
| Comorbidity | Treatment arm, n (%) | Total (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| COPD | |||
| Yes | 14 (8.0) | 20 (11.4) | 34 (9.7) |
| No | 162 (92.0) | 155 (88.1) | 317 (90.1) |
| Missing/not known | – | 1 (0.6) | 1 (0.3) |
| Pulmonary hypertension | |||
| Yes | 25 (14.2) | 28 (15.9) | 53 (15.1) |
| No | 151 (85.8) | 147 (83.5) | 298 (84.7) |
| Missing/not known | – | 1 (0.6) | 1 (0.3) |
| Extracardiac ateriopathy | |||
| Yes | 9 (5.1) | 6 (3.4) | 15 (4.3) |
| No | 167 (94.9) | 169 (96.0) | 336 (95.5) |
| Missing/not known | – | 1 (0.6) | 1 (0.3) |
| Neurological dysfunction | |||
| Yes | 5 (2.8) | 5 (2.8) | 10 (2.8) |
| No | 171 (97.2) | 170 (96.6) | 341 (96.9) |
| Missing/not known | – | 1 (0.6) | 1 (0.3) |
| Serum creatinine level of > 200 µmol/l | |||
| Yes | 2 (1.1) | – | 2 (0.6) |
| No | 174 (98.9) | 176 (100.0) | 350 (99.4) |
| Rheumatic fever | |||
| Yes | 7 (4.0) | 6 (3.4) | 13 (3.7) |
| Cardiomyopathy | |||
| Yes | 2 (1.1) | 4 (2.3) | 6 (1.7) |
| Marfan syndrome | |||
| Yes | 1 (0.6) | – | 1 (0.3) |
| Transient ischaemic attack | |||
| Yes | 19 (10.8) | 12 (6.8) | 31 (8.8) |
| Cerebrovascular accident | |||
| Yes | 11 (6.3) | 11 (6.3) | 22 (6.3) |
| Other clinical history | |||
| Yes | 152 (86.4) | 146 (83.0) | 298 (84.7) |
| Complication | Treatment arm, n (%) | Total (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| No complicationsa | 34 (19.3) | 38 (21.6) | 72 (20.5) |
| AF/arrhythmia | 69 (39.2) | 80 (45.5) | 149 (42.3) |
| Need for a permanent pacemaker | 6 (3.4) | 5 (2.8) | 11 (3.1) |
| Bleeding/tamponade | 22 (12.5) | 17 (9.7) | 42 (11.1) |
| Aortic dissection | – | 1 (0.6) | 1 (0.3) |
| Cardiac arrest | 2 (1.1) | – | 2 (0.6) |
| Cardiogenic shock | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Pericardial effusion | 2 (1.1) | – | 2 (0.6) |
| Other cardiac event | 4 (2.3) | 6 (3.4) | 10 (2.8) |
| Wound infection | 3 (1.7) | 4 (2.3) | 7 (2.0) |
| Infection/sepsis | 1 (0.6) | 4 (2.3) | 5 (1.4) |
| Hypotension | 5 (2.8) | 1 (0.6) | 6 (1.7) |
| Respiratory/respiratory infection | 19 (10.8) | 21 (11.9) | 40 (11.4) |
| Pleural effusion | 14 (8.0) | 25 (14.2) | 39 (11.1) |
| Pulmonary embolism | – | 1 (0.6) | 1 (0.3) |
| Pneumothorax | – | 2 (1.1) | 2 (0.6) |
| Renal failure/dysfunction | 13 (7.4) | 10 (5.7) | 23 (6.5) |
| Neurological | 3 (1.7) | 6 (3.4) | 9 (2.6) |
| Delirium | 5 (2.8) | 1 (0.6) | 6 (1.7) |
| Non-cardiac bleeding complication | 1 (0.6) | – | 1 (0.3) |
| Gastrointestinal complication | 4 (2.3) | 4 (2.3) | 8 (2.3) |
| Metabolic complication | 2 (1.1) | 4 (2.3) | 6 (1.7) |
| Peripheral oedema | – | 1 (0.6) | 1 (0.3) |
| Wound complication | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Other complication | 5 (2.8) | 2 (1.1) | 7 (2.0) |
| Perioperative death | – | 1 (0.6) | 1 (0.3) |
| Returned to theatre | 20 (11.4) | 18 (10.2) | 38 (10.8) |
| Reason for return to theatre | |||
| Bleeding | 4 (2.3) | 10 (5.7) | 14 (4.0) |
| Tamponade | 4 (2.3) | 1 (0.6) | 5 (1.4) |
| Gastrointestinal complication | 2 (1.1) | 2 (1.1) | 4 (1.1) |
| Respiratory complication | 2 (1.1) | 1 (0.6) | 3 (0.9) |
| Cardiac arrest | 2 (1.1) | – | 2 (0.6) |
| Reoperation | – | 1 (0.6) | 1 (0.3) |
| Permanent pacemaker | 1 (0.6) | – | 1 (0.3) |
| Cardiac reason | – | 1 (0.6) | 1 (0.3) |
| Arrhythmia | 1 (0.6) | – | 1 (0.3) |
| Bleeding (non-cardiac) | 1 (0.6) | – | 1 (0.3) |
| Hypotension | 1 (0.6) | – | 1 (0.3) |
| Pericardial effusion | – | 1 (0.6) | 1 (0.3) |
| Respiratory complication/infection | – | 1 (0.6) | 1 (0.3) |
| Wound | 1 (0.6) | – | 1 (0.3) |
| Cardiogenic shock | – | 1 (0.6) | 1 (0.3) |
| Pleural effusion | 1 (0.6) | – | 1 (0.3) |
| Missing/not known | 2 (1.1) | – | 2 (0.6) |
| Type of transfusion | Treatment arm, n (%) | Total (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| Total red blood cell transfusion (units) | |||
| Not known | 4 (2.3) | 1 (0.6) | 5 (1.4) |
| 0 | 98 (55.7) | 106 (60.2) | 204 (58.0) |
| ≥ 1 | 74 (42.0) | 69 (39.2) | 143 (40.6) |
| Total platelets transfusion (units) | |||
| Not known | 5 (2.8) | 3 (1.7) | 8 (2.3) |
| 0 | 128 (72.7) | 141 (80.1) | 269 (76.4) |
| ≥ 1 | 43 (24.4) | 32 (18.2) | 75 (21.3) |
| Total fresh-frozen plasma transfusion (units) | |||
| Not known | 4 (2.3) | 3 (1.7) | 7 (2.0) |
| 0 | 136 (77.3) | 139 (79.0) | 275 (78.1) |
| ≥ 1 | 36 (20.5) | 34 (19.3) | 70 (19.9) |
| Total cryoprecipitate transfusion (units) | |||
| Not known | 7 (4.0) | 6 (3.4) | 13 (3.7) |
| 0 | 157 (89.2) | 162 (92.0) | 319 (90.6) |
| ≥ 1 | 12 (6.8) | 8 (4.5) | 20 (5.7) |
| Total human albumin transfusion (units) | |||
| Not known | 5 (2.8) | 4 (2.3) | 9 (2.6) |
| 0 | 165 (93.8) | 163 (92.6) | 328 (93.2) |
| ≥ 1 | 6 (3.4) | 9 (5.1) | 15 (4.3) |
| Effect | OR of SR at 12 months (95% CI) | p > |t| |
|---|---|---|
| Intercept | 0.69 (0.34 to 1.40) | 0.3078 |
| Randomised to the maze procedure arm | 2.06 (1.20 to 3.54) | 0.0091 |
| In SR at baseline | 6.52 (2.83 to 14.98) | < 0.0001 |
| Procedure: CABG vs. MVR | 0.84 (0.37 to 1.93) | 0.6836 |
| Procedure: AVR vs. MVR | 0.42 (0.18 to 0.99) | 0.0479 |
| Procedure: CABG and MVR vs. MVR | 0.64 (0.21 to 1.93) | 0.4288 |
| Procedure: CABG and AVR vs. MVR | 0.52 (0.18 to 1.54) | 0.2373 |
| Procedure: all others (including none) vs. MVR | 0.75 (0.35 to 1.61) | 0.4642 |
| Level | Treatment arm, in SR/total | Adjusted OR (maze procedure/control) (95% CI) | p > |t| | |
|---|---|---|---|---|
| Maze procedure | Control | |||
| Paroxysmal AF | 32/38 | 30/42 | 2.286 (0.679 to 7.693) | 0.1815 |
| Non-paroxysmal AF | 55/103 | 38/103 | 2.036 (1.117 to 3.710) | 0.0204 |
| CABG | 18/30 | 15/29 | 1.625 (0.545 to 4.847) | 0.3835 |
| MVR | 26/34 | 24/43 | 3.375 (1.141 to 9.980) | 0.0279 |
| AVR | 12/27 | 7/16 | 1.511 (0.391 to 5.836) | 0.5485 |
| CABG and MVR | 9/12 | 2/10 | 7.159 (0.907 to 56.496) | 0.0618 |
| CABG and AVR | 5/10 | 5/14 | 1.810 (0.275 to 11.921) | 0.5355 |
| All others, including none | 17/28 | 15/33 | 1.437 (0.497 to 4.154) | 0.5032 |
| Effect | Estimate (95% CI) | p > |t| |
|---|---|---|
| Intercept | 0.775 (0.550 to 1.000) | < 0.0001 |
| Baseline EQ-5D-3L utility score | 0.962 (0.715 to 1.208) | < 0.0001 |
| Randomised to the maze procedure arm | –0.025 (–0.129 to 0.078) | 0.6319 |
| Surgical procedure: CABG | –0.081 (–0.238 to 0.075) | 0.3087 |
| Surgical procedure: AVR | –0.103 (–0.271 to 0.065) | 0.2280 |
| Surgical procedure: CABG and MVR | –0.043 (–0.256 to 0.170) | 0.6925 |
| Surgical procedure: CABG and AVR | –0.282 (–0.471 to –0.092) | 0.0037 |
| Surgical procedure: all others, including none | 0.010 (–0.142 to 0.161) | 0.8986 |
| Level | Treatment arm, mean (SEM) | Difference (maze procedure vs. control) (95% CI) | p-value | |
|---|---|---|---|---|
| Maze procedure | Control | |||
| Paroxysmal AF | 1.430 (0.076) | 1.511 (0.075) | –0.081 (–0.283 to 0.122) | 0.4356 |
| Non-paroxysmal AF | 1.375 (0.045) | 1.379 (0.045) | –0.004 (–0.125 to 0.116) | 0.9417 |
| CABG | 1.403 (0.083) | 1.397 (0.085) | 0.006 (–0.228 to 0.239) | 0.9626 |
| MVR | 1.426 (0.078) | 1.530 (0.071) | –0.104 (–0.310 to 0.103) | 0.3265 |
| AVR | 1.451 (0.088) | 1.272 (0.104) | 0.179 (–0.086 to 0.444) | 0.1865 |
| CABG and MVR | 1.525 (0.131) | 1.345 (0.137) | 0.179 (–0.192 to 0.550) | 0.3438 |
| CABG and AVR | 1.068 (0.122) | 1.303 (0.108) | –0.235 (–0.555 to 0.084) | 0.1491 |
| All others, including none | 1.452 (0.079) | 1.533 (0.080) | –0.080 (–0.302 to 0.141) | 0.4779 |
| Effect | OR of SR at 24 months (95% CI) | p > |t| |
|---|---|---|
| Intercept (odds of SR at 24 months for baseline AF, MVR operation and standard care) | 0.38 (0.17 to 0.83) | 0.0169 |
| In SR at baseline | 11.53 (4.48 to 29.70) | < 0.0001 |
| Randomised to the maze procedure arm | 3.24 (1.76 to 5.96) | 0.0002 |
| Procedure: AVR vs. MVR | 0.48 (0.18 to 1.29) | 0.1435 |
| Procedure: all others (including none) vs. MVR | 0.53 (0.22 to 1.31) | 0.1695 |
| Procedure: CABG vs. MVR | 0.70 (0.28 to 1.76) | 0.4499 |
| Procedure: CABG and AVR vs. MVR | 0.56 (0.16 to 1.88) | 0.3426 |
| Procedure: CABG and MVR vs. MVR | 0.80 (0.24 to 2.63) | 0.7144 |
| Cause of death by treatment arm | Months from randomisation to death |
|---|---|
| Maze procedure | |
| Haemorrhage | 0.00 |
| Pancreatitis | 0.13 |
| Unknown | 0.16 |
| Multisystem organ failure | 0.59 |
| Unknown | 0.82 |
| Subdural haematoma | 1.28 |
| Infective endocarditis | 2.14 |
| Sepsis | 2.17 |
| Cardiac and renal failure | 2.46 |
| Bladder cancer | 2.50 |
| Cardiac failure and respiratory failure | 3.29 |
| Cardiac failure | 3.48 |
| Pneumonia and stroke | 5.03 |
| Pneumonia | 5.75 |
| Sepsis | 7.26 |
| Cardiac failure | 10.51 |
| Unknown | 15.44 |
| Multiorgan failure | 15.64 |
| Aspiration pneumonia | 19.35 |
| Pneumonia | 21.75 |
| Need more information on this, as it is not a cause of death | 24.01 |
| Cerebral haemorrhage | 24.05 |
| Cardiac failure | 24.24 |
| Stroke and cancer | 35.94 |
| Unknown | 45.27 |
| Unknown | 45.83 |
| Haematological cancer | 50.66 |
| Pulmonary embolus | 54.50 |
| MI | 62.88 |
| Unknown | 64.62 |
| Control | |
| MI | 0.03 |
| Bowel ischaemia | 0.07 |
| Pneumonia | 0.10 |
| Cardiac failure | 0.13 |
| Air embolus | 0.16 |
| Bowel perforation | 0.16 |
| Multiorgan failure | 0.82 |
| Multiorgan failure | 1.05 |
| Gastrointestinal haemorrhage | 1.12 |
| Bowel ischaemia | 1.28 |
| Cardiac failure | 1.77 |
| Sepsis | 1.77 |
| Cardiac failure | 2.37 |
| Sudden cardiac death | 2.79 |
| Haemorrhage | 6.73 |
| Haematological cancer | 7.88 |
| MI | 21.78 |
| Pneumonia | 23.59 |
| Unknown | 40.21 |
| Prostate cancer | 46.85 |
| Cardiac failure | 46.91 |
| Unknown | 51.64 |
| Cardiac failure | 54.04 |
| Cardiac failure | 59.23 |
| Endometrial cancer | 63.44 |
| Classification at each time point | Treatment arm, n (%) | Total (n = 352), n (%) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| 6 months | |||
| I | 66 (37.5) | 86 (48.9) | 152 (43.2) |
| II | 53 (30.1) | 49 (27.8) | 102 (29.0) |
| III | 19 (10.8) | 15 (8.5) | 34 (9.7) |
| IV | 2 (1.1) | – | 2 (0.6) |
| Missing/not known | 36 (20.4) | 26 (14.8) | 62 (17.6) |
| 12 months | |||
| I | 73 (41.5) | 71 (40.3) | 144 (40.9) |
| II | 50 (28.4) | 58 (33.0) | 108 (30.7) |
| III | 20 (11.4) | 15 (8.5) | 35 (9.9) |
| IV | 1 (0.6) | – | 1 (0.3) |
| Missing/not known | 32 (18.1) | 32 (18.2) | 64 (18.2) |
| 24 months | |||
| I | 68 (38.6) | 64 (36.4) | 132 (37.5) |
| II | 41 (23.3) | 56 (31.8) | 97 (27.6) |
| III | 19 (10.8) | 16 (9.1) | 35 (9.9) |
| IV | 3 (1.7) | 2 (1.1) | 5 (1.4) |
| Missing/not known | 45 (25.6) | 38 (21.6) | 83 (23.6) |
| SF-36 item score at each time point | Estimate (95% CI) | p > |t| |
|---|---|---|
| Bodily pain | ||
| 6 months | –1.010 (–5.539 to 3.520) | 0.6619 |
| 12 months | –0.353 (–3.585 to 2.878) | 0.8302 |
| 24 months | 0.959 (–4.352 to 6.271) | 0.7231 |
| General health | ||
| 6 months | –0.292 (–3.860 to 3.275) | 0.8723 |
| 12 months | –0.769 (–3.314 to 1.775) | 0.5531 |
| 24 months | –1.723 (–5.907 to 2.461) | 0.4191 |
| Mental health | ||
| 6 months | –0.183 (–3.022 to 2.655) | 0.8991 |
| 12 months | –0.374 (–2.399 to 1.651) | 0.7171 |
| 24 months | –0.755 (–4.082 to 2.572) | 0.6561 |
| Physical functioning | ||
| 6 months | –0.619 (–4.963 to 3.725) | 0.7798 |
| 12 months | –0.825 (–3.923 to 2.274) | 0.6015 |
| 24 months | –1.236 (–6.331 to 3.858) | 0.6340 |
| Role emotional | ||
| 6 months | –4.050 (–10.837 to 2.736) | 0.2418 |
| 12 months | –3.013 (–7.860 to 1.834) | 0.2228 |
| 24 months | –0.939 (–8.899 to 7.021) | 0.8170 |
| Role physical | ||
| 6 months | –3.761 (–11.461 to 3.939) | 0.3380 |
| 12 months | –2.776 (–8.271 to 2.720) | 0.3218 |
| 24 months | –0.806 (–9.837 to 8.224) | 0.8609 |
| Social functioning | ||
| 6 months | –0.921 (–5.504 to 3.662) | 0.6934 |
| 12 months | –0.625 (–3.894 to 2.644) | 0.7074 |
| 24 months | –0.034 (–5.409 to 5.341) | 0.9901 |
| Vitality | ||
| 6 months | 2.641 (–1.051 to 6.333) | 0.1606 |
| 12 months | 1.534 (–1.100 to 4.168) | 0.2533 |
| 24 months | –0.680 (–5.006 to 3.646) | 0.7578 |
| SF-36 item at each time point | Treatment arm | Total (n = 352) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| PCS (UK norm: 50, SD 10) | |||
| Baseline | |||
| Mean score (SD) | 30.18 (13.17) | 31.00 (13.56) | 30.59 (13.36) |
| Missing | 3 | 2 | 5 |
| 6 months | |||
| Mean score (SD) | 38.25 (13.95) | 39.23 (13.86) | 38.75 (13.89) |
| Missing | 21 | 17 | 38 |
| 12 months | |||
| Mean score (SD) | 38.55 (14.33) | 39.99 (13.18) | 39.27 (13.76) |
| Missing | 26 | 25 | 51 |
| 24 months | |||
| Mean score (SD) | 38.28 (14.66) | 39.54 (12.88) | 38.92 (13.78) |
| Missing | 41 | 37 | 78 |
| MCS (UK norm: 50, SD 10) | |||
| Baseline | |||
| Mean score (SD) | 50.81 (9.92) | 49.58 (10.69) | 50.19 (10.32) |
| Missing | 3 | 2 | 5 |
| 6 months | |||
| Mean score (SD) | 54.37 (9.47) | 54.06 (9.42) | 54.21 (9.43) |
| Missing | 21 | 17 | 38 |
| 12 months | |||
| Mean score (SD) | 54.09 (9.64) | 54.33 (8.83) | 54.21 (9.23) |
| Missing | 26 | 25 | 51 |
| 24 months | |||
| Mean score (SD) | 54.68 (9.14) | 54.44 (9.12) | 54.56 (9.11) |
| Missing | 41 | 37 | 78 |
| EQ-5D-3L utility at each time point | Treatment arm | Total (n = 352) | |
|---|---|---|---|
| Maze procedure (n = 176) | Control (n = 176) | ||
| Baseline | |||
| Mean score (SD) | 0.74 (0.22) | 0.75 (0.21) | 0.75 (0.22) |
| Missing | 2 | 1 | 3 |
| Discharge | |||
| Mean score (SD) | 0.63 (0.27) | 0.64 (0.24) | 0.63 (0.25) |
| Missing/died | 20 | 20 | 40 |
| 6 weeks | |||
| Mean score (SD) | 0.77 (0.21) | 0.78 (0.19) | 0.77 (0.20) |
| Missing/died | 18 | 17 | 35 |
| 6 months | |||
| Mean score (SD) | 0.80 (0.21) | 0.80 (0.23) | 0.80 (0.22) |
| Missing/died | 21 | 16 | 37 |
| 12 months | |||
| Mean score (SD) | 0.79 (0.22) | 0.81 (0.20) | 0.80 (0.21) |
| Missing/died | 27 | 25 | 52 |
| 24 months | |||
| Mean score (SD) | 0.78 (0.22) | 0.80 (0.24) | 0.79 (0.23) |
| Missing/died | 41 | 33 | 74 |
| AE | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Maze procedure | Control | ||
| Total reported: events (patients) | 560 (136) | 589 (157) | 1149 (293) |
| Cardiac complications | 151 (86) | 194 (108) | 345 (194) |
| Arrhythmia | 78 (58) | 108 (80) | 186 (138) |
| Bleeding | 21 (14) | 23 (16) | 44 (30) |
| Cardiac failure | 12 (6) | 26 (20) | 38 (26) |
| Cardiac arrest | 1 (1) | 6 (6) | 7 (7) |
| MI | 2 (2) | 1 (1) | 3 (3) |
| Other cardiac event | 37 (31) | 30 (19) | 67 (50) |
| Respiratory complication | 77 (51) | 70 (47) | 147 (98) |
| Infection | 72 (49) | 63 (49) | 135 (98) |
| Prolonged hospitalisation | 60 (48) | 53 (39) | 113 (87) |
| Neurological complication | 25 (20) | 22 (20) | 47 (40) |
| Death | 16 (16) | 19 (19) | 35 (35) |
| Renal failure | 8 (5) | 8 (6) | 16 (11) |
| Thromboembolic event | 3 (3) | 5 (5) | 8 (8) |
| Vascular complication | 2 (2) | 6 (5) | 8 (7) |
| Multiple organ failure | 2 (2) | 3 (3) | 5 (5) |
| Inflammation | 3 (2) | – | 3 (2) |
| Other non-cardiac event | 141 (70) | 146 (86) | 287 (156) |
| Effect | Estimated effect size (95% CI) | p-value |
|---|---|---|
| Four-chamber echocardiography (primary analysis) | ||
| ALAEF (%) | –8.03 (–12.43 to –3.62) | 0.0015 |
| LAVmin (ml)a | 14.53 (–1.75 to 30.81) | 0.0765 |
| LAVmax (ml)a | 10.02 (–9.40 to 29.44) | 0.2889 |
| LAVpreA (ml)a | 11.30 (–7.71 to 30.30) | 0.2244 |
| Passive stroke volume (ml) | 0.86 (–2.26 to 3.98) | 0.5651 |
| Active stroke volume (ml) | –3.44 (–7.79 to 0.90) | 0.1120 |
| LAEF (%) | –6.90 (–11.98 to –1.82) | 0.0111 |
| E/A ratioa | 0.89 (0.16 to 1.62) | 0.0205 |
| Two-chamber echocardiography | ||
| ALAEF (%) | –3.48 (–8.45 to 1.49) | 0.1545 |
| LAVmin (ml)a | 9.95 (–0.48 to 20.37) | 0.0600 |
| LAVmax (ml)a | 9.45 (–7.04 to 25.93) | 0.2393 |
| LAVpreA (ml)a | 6.92 (–9.28 to 23.13) | 0.3728 |
| Passive stroke volume (ml) | 1.49 (–2.32 to 5.30) | 0.4138 |
| Active stroke volume (ml)a | –1.54 (–9.09 to 6.02) | 0.6671 |
| LAEF (%) | –3.36 (–7.86 to 1.13) | 0.1310 |
| Multiple-slice MRI | ||
| LAVmin (ml)a | 39.71 (9.05 to 70.37) | 0.0146 |
| LAVmax (ml)a | 35.96 (–1.37 to 73.30) | 0.0579 |
| LAEF (%)a | –13.46 (–19.87 to –7.06) | 0.0004 |
| End point | Treatment arm, mean (SD) | |
|---|---|---|
| Maze procedure (n = 22) | Control (n = 22) | |
| ALAEF (%)a | 19 (8) | 24 (8) |
| LAEF (%)b | 30 (8) | 35 (8) |
| LAVmin (ml)b | 63 (15) | 51 (12) |
| LAVpreA (ml)a | 77 (17) | 68 (17) |
| LAVmax (ml)b | 90 (19) | 79 (17) |
| Passive stroke volume (ml)a | 13 (7) | 11 (4) |
| Active stroke volume (ml)a | 15 (6) | 17 (8) |
| End point | Treatment arm, mean (SD) | |
|---|---|---|
| Maze procedure (n = 22) | Control (n = 22) | |
| LAEF (%) | 24 (7) | 37 (6) |
| LAVmax (ml) | 120 (36) | 88 (31) |
| LAVmin (ml) | 91 (32) | 55.23 (19) |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AF
- atrial fibrillation
- ALAEF
- active left atrial ejection fraction
- AVR
- aortic valve replacement or repair
- CABG
- coronary artery bypass graft operation
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CRF
- clinical report form
- DMEC
- Data Monitoring and Ethics Committee
- ECG
- electrocardiography
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EuroSCORE
- European System for Cardiac Operative Risk Evaluation
- GP
- general practitioner
- HESTER
- Has Electrical Sinus Translated into Effective Remodelling?
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- INMB
- incremental net monetary benefit
- ITT
- intention to treat
- LAEF
- left atrial ejection fraction
- LAVmax
- maximum left atrial volume
- LAVmin
- minimum left atrial volume
- LAVpreA
- pre A-wave left atrial volume
- LVEF
- left ventricular ejection fraction
- MCS
- mental component score
- MI
- myocardial infarction
- MRI
- magnetic resonance imaging
- MVR
- mitral valve replacement or repair
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NYHA
- New York Heart Association
- OR
- odds ratio
- PCS
- physical component score
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-36
- Short Form questionnaire-36 items
- SR
- sinus rhythm
- TSC
- Trial Steering Committee
- WTP
- willingness to pay
