Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/16/01. The contractual start date was in January 2015. The draft report began editorial review in April 2016 and was accepted for publication in August 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Richard Wakefield has provided consulting advice and spoken for General Electric with regard to ultrasound technologies and has also received speaker fees from AbbVie Inc. for ultrasound-related projects. Cristina Estrach has been a member of advisory boards for and/or received speaker fees from AbbVie Inc., Chugai Pharma (UK) Ltd and General Electric Co. Her institution has received educational grants from Pfizer Inc.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Simpson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterised by progressive, irreversible joint damage, impaired joint function, pain and tenderness caused by swelling of the synovial lining of the joints (synovitis) and is manifested with increasing disability and reduced quality of life. 1 The primary symptoms are pain, morning stiffness, swelling, tenderness, loss of movement, fatigue and redness of the peripheral joints. 2,3 RA is associated with substantial costs both directly (associated with drug acquisition and hospitalisation) and indirectly, because of reduced productivity. 4 RA has long been reported as being associated with increased mortality,5,6 particularly as a result of cardiovascular events. 7
Diagnosis of rheumatoid arthritis
The initial classification criteria for RA were produced in 1987 by the American College of Rheumatology (ACR). 8 National Institute for Health and Care Excellence (NICE) clinical guideline 799 provides a summary of the ACR criteria, with patients needing to have at least four of the seven criteria to be given a diagnosis of RA: morning stiffness lasting at least 1 hour, swelling in three or more joints, swelling in the hand joints, symmetric joint swelling, erosions or decalcification on radiography of the hand, rheumatoid nodules and abnormal serum rheumatoid factor. For the first four criteria these must have been present for at least a period of 6 weeks. However, in the clinical guideline9 the guideline development group preferred a clinical diagnosis of RA rather than the ACR criteria to permit early treatment, as consistent with European League Against Rheumatism (EULAR) recommendations. 10
In 2010, the ACR and EULAR jointly published RA classification criteria, which focused on features present at earlier stages of the disease that are associated with persistent and/or erosive disease rather than defining the disease by its late-stage features. 11 The classification criteria allocate scores to the characteristics of joint involvement, serology, acute-phase reactants and duration of symptoms to produce a total score between 0 and 10, with those scoring ≥ 6 and with obvious clinical synovitis being defined as having ‘definite RA’ in the absence of an alternative diagnosis that better explains the synovitis. 11 The growing recognition of the accuracy of modern imaging [such as magnetic resonance imaging (MRI) and ultrasound (US)] over clinical examination (CE) in detecting synovitis was highlighted in these new classification criteria, which allow the extent of joint involvement to be determined by imaging. 11
Epidemiology
There are an estimated 400,000 people in England and Wales with RA,12 with approximately 10,000 incident cases per year. 13 The disease is more prevalent in females (1.16%) than in males (0.44%),13 with the majority of cases being diagnosed when patients are aged between 40 and 80 years14 and a peak incidence in those in their 70s. 13
Measurement of disease activity and damage progression
Synovitis can be detected by CE. Monitoring may involve taking swollen joint counts (SJCs) or tender joint counts (TJCs). Biomarkers may be used to detect evidence of systemic inflammation, for example anaemia in chronic disease or elevated levels of C-reactive protein (CRP) or an elevated erythrocyte sedimentation rate (ESR). 9,15 Common measures of disease activity, disability and response to treatment are presented in Table 1. ACR response19 and EULAR response20 measures have dominated the measurement of improvement in RA symptoms. The ACR response has been widely adopted in randomised controlled trials (RCTs) although studies have shown that the value can vary between trials because of the timing of the response. 21 The EULAR response criteria and the ACR20 improvement criteria (see Table 1) were found to have reasonable agreement in the same set of clinical trials, although van Gestel et al. 22 stated that the EULAR response criteria showed better construct and discriminant validity than the ACR20 criteria. The Disease Activity Score 28 joints (DAS28; see Table 1) can be used to classify both the disease activity of the patient and the level of improvement estimated. The EULAR response has been reported less frequently in RCTs than the ACR response, although the EULAR criteria are much more closely aligned to the treatment continuation rules stipulated by NICE,9 which require a DAS28 improvement of > 1.2 to continue treatment. 22
| Measure | Description |
|---|---|
| Health Assessment Questionnaire (HAQ)16 | A patient-completed disability assessment with established reliability and validity. Scores range from 0 to 3, with higher scores indicating greater disability. The HAQ scale is a discrete scale with step values of 0.125, resulting in 25 points on the scale |
| Visual analogue scale (VAS) | A global assessment of the extent of disease, which may be assessed by the patient or the physician. This method may also be used for patient-reported pain. The VAS usually consists of a 100-mm line that represents the total range of the disease extent/pain levels experienced. Patients/physicians mark a point on the line |
| DAS28-ESR17 | This assesses 28 joints (shoulder, knee, elbow, wrist, MCP joints one to five, PIP joints one to five, bilaterally) in terms of swelling (SW28) and of tenderness to the touch (TEN28). It also incorporates measures of the ESR and a subjective assessment (SA) on a scale of 0–100 made by the patient regarding disease activity in the previous week. The DAS28-ESR score is calculated using the following equation: 0.56 × TEN280.5 + 28 × SW280.5 + 0.70 × ln(ESR) + 0.014 × SA. A DAS28-ESR of ≤ 3.2 indicates inactive disease; a DAS28-ESR of > 3.2 and ≤ 5.1 indicates moderate disease; a DAS28-ESR of > 5.1 indicates very active disease |
| DAS28-CRP17 | Similar to the DAS28-ESR but uses the CRP value instead of the ESR. The DAS28-CRP is calculated using the following equation: [0.56 × sqrt(TEN28) + 0.28 × sqrt(SW28) + 0.36 × ln(CRP + 1)] × 1.10 + 1.15 |
| Clinical Disease Activity Index (CDAI)18 | A composite index based on SJCs (0–28), TJCs (0–28), CRP, patient global assessment of disease activity VAS (0–10 cm) and physician global assessment of disease activity VAS (0–10 cm) |
| Simplified Disease Activity Index (SDAI)18 | A simplified version of the CDAI based on SJCs (0–28), TJCs (0–28), patient global assessment of disease activity VAS (0–10 cm) and physician global assessment of disease activity VAS (0–10 cm) |
| ACR response19 | An ACR20 response requires a 20% improvement in TJCs; a 20% improvement in SJCs; and a 20% improvement in at least three of the following five ‘core set items’: physician global assessment, patient global assessment, patient pain, self-reported disability (using a validated instrument) and ESR or CRP. ACR50 and ACR70 responses require 50% and 70% improvements, respectively |
| EULAR response20 | Participants are classified as good, moderate or non-responders based on the individual change in DAS28 and the DAS28 reached.20 The relationship between change in DAS28 and DAS28 reached and EULAR response is shown in Table 2 |
The relationship between change in DAS28 and DAS28 reached and EULAR response is shown in Table 2. Depending on the initial DAS28 of the patient, this change in DAS28 would equate to either a good or moderate EULAR response, as shown in the second column of Table 2.
| DAS28 at end point | Improvement in DAS2823 | ||
|---|---|---|---|
| > 1.2 | > 0.6 and ≤ 1.2 | ≤ 0.6 | |
| ≤ 3.2 | Good | Moderate | None |
| > 3.2 and ≤ 5.1 | Moderate | Moderate | None |
| > 5.1 | Moderate | None | None |
Both the ACR criteria and the DAS28-based EULAR criteria rely on subjective measurements and can be influenced by patients’ pain threshold and comorbidities. 24 The DAS28 has been criticised as patients achieving DAS remission can still have synovitis as evidenced by imaging. 25,26 Alternatively, chronic pain experienced by patients or deformity and residual fibrous tissue leading to the detection of swelling by manual examination of joints can raise the DAS, thus overestimating disease activity. 24 If DAS measurement is based on CRP levels, it can be influenced by therapies; for example, tocilizumab (TCZ; RoActemra®, Roche Products Ltd, Welwyn Garden City, UK) has a direct effect on CRP through interleukin-6 and can therefore artificially lower the DAS28. 27 Unlike these subjective measurements, imaging could provide an objective measurement of synovitis.
The role of imaging
Imaging techniques used in the diagnosis and monitoring of RA include US, conventional radiography, MRI and computerised axial tomography (CT). Initial diagnosis of RA, or differential diagnosis of forms of arthritis, may be aided by imaging. There is a EULAR recommendation that US, MRI and conventional radiography can improve the certainty of a RA diagnosis if there is diagnostic doubt. 28
In terms of detecting inflammation, there is evidence to demonstrate that both US and MRI detect more cases of synovitis than CE. 9,28 The benefit of US as an addition to CE will be influenced by the ability of this subclinical synovitis to predict disease progression. Prognostic studies of US-detected synovitis were sought in this report (see Chapter 3). Because of their three-dimensional acquisition, both US and MRI are also more sensitive than conventional radiography at detecting erosion damage and early signs of erosion. 9 MRI can detect bone oedema, which may be a predictor of radiographic progression. 28
Although MRI may be more sensitive than US for detecting erosions,29 US and MRI have comparable abilities to detect synovitis. 28–30 Arthritis Research UK30 suggests that US has an advantage over other imaging techniques as it can be used immediately after CE to assess symptomatic areas or clinical abnormalities and this has the added advantage of improving clinical skills. 30
There is no conclusive gold standard (sometimes referred to as a reference standard) for assessing synovitis. When compiling their 2009 guidelines, NICE9 recognised that many clinicians had limited access to US and so it considered clinician examination as the gold standard for the detection of synovitis in practice. It is possible that US has become more widespread since then. To address this issue, a survey was conducted to determine the usage of US in UK clinical practice (see Appendix 1).
Conventional radiography is commonly used to measure disease progression. 9 In clinical trials this may be carried out using the total Sharp score (TSS), the van der Heijde-modified total Sharp score (vdHSS)31 or the modified Genant et al. 32 scoring method, which measure erosions and joint space narrowing, although systematic scoring of radiographs in routine clinical care is very uncommon.
Treatment strategies based on a therapeutic outcome target (known as ‘treat to target’; TTT) are becoming more widely used within rheumatology. 33–35 Typically, targets are either remission or low disease activity (when remission is not attainable). These are usually clinically defined [e.g. DAS of < 2.6, Simplified Disease Activity Index (SDAI) score of ≤ 3.3]. 34,36 However, as a previous review has reported that US has been shown to be superior to CE for detecting synovitis, providing that this is linked to later outcomes,28 US-defined remission may be a more appropriate target for TTT strategies. This previous review thoroughly examined the use of US to detect synovitis;28 however, it was published prior to the publication of the treatment studies, including RCTs, in this report. Research is starting to examine the effectiveness of US compared with clinical treatment targets within TTT strategies in RA. 36,37
Significance for the NHS
Because previous NICE technology appraisals have recommended a number of biological disease-modifying anti-rheumatic drugs (bDMARDs) (see Current service provision), with a potential sequence of three bDMARDs, there has been a considerable increase in expenditure on RA interventions as bDMARDs are markedly more expensive than conventional disease-modifying anti-rheumatic drugs (cDMARDs). Any imaging technology that could inform decisions on when to start, or to taper or discontinue, treatments has the potential to benefit the NHS. The frequency of monitoring would influence cost-effectiveness.
Further detailed information on RA can be found in NICE clinical guideline 79. 9 Additional information can also be located in the British Society for Rheumatology (BSR) guidelines. 38
Current service provision
Traditionally, patients have been treated with cDMARDs, which include methotrexate (MTX), sulfasalazine (SSZ), hydroxychloroquine (HCQ), leflunomide, ciclosporin and gold injections as well as corticosteroids, analgesics and non-steroidal anti-inflammatory drugs (NSAIDs). However, more recently, a group of drugs has been developed consisting of monoclonal antibodies and soluble receptors that specifically modify the disease process by blocking key protein messenger molecules (such as cytokines) or cells (such as B-lymphocytes). 9 Such drugs have been labelled as bDMARDs.
A simplified model of the RA clinical pathway has been provided by NICE,39 with the use of US falling into the monitoring and review box. For the purposes of this report, modelling assumes that US would be used only at points in the pathway where a change of treatment is being considered, specifically for those patients for whom a clinician is considering tapering treatment to assess the current level of synovitis and for those patients for whom a clinician is contemplating escalating treatment to rule out non-synovitis-related reasons.
Clinical guidelines
For people with newly diagnosed RA, NICE clinical guideline 799 recommends a combination of cDMARDs [including MTX and at least one other disease-modifying anti-rheumatic drug (DMARD) plus short-term glucocorticoids] as first-line treatment, ideally beginning within 3 months of the onset of persistent symptoms. When combination therapies are not appropriate (e.g. when there are comorbidities or the patient is pregnant), DMARD monotherapy is recommended. When DMARD monotherapy is used, emphasis should be on increasing the dose quickly to obtain the best disease control. For the purposes of this report, the term ‘intensive DMARDs’ has been used to denote treatment with multiple cDMARDs simultaneously.
Current National Institute for Health and Care Excellence technology appraisal guidance
National Institute for Health and Care Excellence guidance [technology appraisal (TA) 375]40 recommends the use of the tumour necrosis factor inhibitors (TNFis) etanercept (ETN; Enbrel®, Pfizer Ltd, Sandwich, UK), infliximab (IFX; Remicade®, Merck Sharp & Dohme Ltd, Hoddesdon, UK), adalimumab (ADA; Humira®, AbbVie Ltd, maidenhead, UK), certolizumab pegol (CTZ; Cimzia®, UCB Pharma, Slough, UK) and golimumab (GOL; Simponi®, Merck Sharp & Dohme Ltd), in combination with MTX, in people with RA after the failure of two cDMARDs, including MTX, and who have a DAS28 of > 5.1 and when bDMARDs have not been tried previously. TA375 also recommends the use of TCZ and abatacept (ABT; Orencia®, Bristol-Myers Squibb, Uxbridge, UK) as alternatives to TNFis in the same circumstances.
Technology appraisal 375 did not recommend the use of bDMARDs in patients with a DAS of ≤ 5.1 nor in patients in whom cDMARDs have failed. 40
The National Institute for Health and Care Excellence has also issued guidance on the treatment of RA after the failure of a TNFi (TA195,41 TA22542 and TA24743).
National Institute for Health and Care Excellence clinical guideline 799 recommends the use of intensive cDMARDs – two cDMARDs used in combination, rather than two cDMARDs used sequentially – although this latter option is acceptable.
A simplified summary of the typical pathway recommended by NICE would be the use of intensive cDMARDs followed by a bDMARD, followed by rituximab (RTX; MabTheraR, Roche Products Ltd, Welwyn Garden City, UK) plus MTX and then TCZ before returning to cDMARDs. If RTX or MTX is contraindicated then ADA, ETN, IFX or ABT in combination with MTX or ADA or ETN monotherapy can be used instead (TA19541), as can TCZ in combination with MTX (TA24743) and GOL in combination with MTX (TA22542). A NICE single technology appraisal recommended the use of CTZ after an inadequate response to a TNFi for patients with a DAS28 of > 5.1 for whom RTX is contraindicated or not tolerated. 44
National Institute for Health and Care Excellence criteria for continuing treatment
Each of the NICE TAs state that for patients to continue treatment with a bDMARD there must have been an improvement in DAS28 of at least 1.2 points at 6 months. If this criterion has not been met, treatment should be stopped and the next intervention in the sequence initiated.
Current service cost
The decision problem compares the use of US for the monitoring of synovitis in addition to CE compared with CE alone, assuming that there are US facilities within the hospital. As such, no attempt has been made to estimate the costs of providing a rheumatology service without US monitoring, as it is presumed that these costs, such as maintenance costs, consumable costs and receptionist costs, will be the same regardless of whether or not US monitoring is undertaken for RA patients. No analyses have been undertaken assuming that the machinery required to perform US would be bought primarily for the use of RA patients, although it is expected that in such circumstances the costs per US scan would be markedly higher.
Description of the technology under assessment
In US, reflected pulses of high-frequency sound are used to assess soft tissue, cartilage and bone surfaces. 30
Grey-scale ultrasound (GSUS) (also known as B-mode ultrasound) displays different intensities of echoes, denoting different densities of tissue, in black, white and shades of grey. 30 GSUS can measure synovial hypertrophy and effusion. 45 Doppler US is based on the principle that sound waves increase or decrease in frequency when objects (such as blood cells) move towards or away from the transducer, respectively. 30 Thus, power Doppler ultrasound (PDUS) can be used to detect the volume of blood flow. 30
Musculoskeletal US can be used for evaluating joint and soft tissue pathology. 30 Within the field of rheumatology, US can be used for the initial diagnosis of arthritis and for US-guided injections or aspirations (which fall outside the scope of this report). 30 It can also be used to assess the extent of inflammatory disease and determine the response to therapy. 30 A significant advantage of US over other imaging technologies such as MRI and CT is the ability to focus on the area of symptoms or clinical abnormality with the US probe immediately after CE. 30
The ACR has published guidelines on the use of US in rheumatology. 46 This included several recommendations across a range of disorders and various stages of the clinical pathway, including that it is reasonable to use US to evaluate inflammatory disease activity. 46
The EULAR recommendations on imaging for RA, published in 2013, covered several imaging technologies and pathologies across stages of the clinical pathway. 28 Recommendations included that US be used to detect and monitor inflammation and that imaging be used to predict the response to therapy.
Ultrasound is useful for distinguishing inflammatory from non-inflammatory joint disease. Effusion may indicate synovial inflammation; however, abnormally thickened hypoechoic intra-articular tissue may indicate synovitis in the absence of effusion. 30 PDUS can be used to assess synovitis as it can detect minimal increases in perfusion in the synovium. 30 US can detect subclinical synovitis. 28,30 Subclinical synovitis may predict structural deterioration in RA patients; thus, prognostic studies were sought (see Chapter 3) as progression can occur in RA patients in remission. 47,48 The benefit of US as an addition to CE will be influenced by the ability of this subclinical synovitis to predict disease progression.
Interpreting US scans is a skill. In the past, there have been concerns about inter-rater reliability; however, a review published in 201049 found that both intra-rater and inter-rater reliability for still-image interpretation was high for both GSUS and PDUS. Limited evidence is available for assessing image acquisition reliability. 50 The Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) US task force has addressed many issues involved in measuring RA and has looked specifically at the variability of US. 30,51 In the past, GSUS was used to assess the presence or absence of synovitis, but this was followed by the creation of semiquantitative scoring systems (Table 3). 45 PDUS used to detect vascularity may be quantified30 (see Table 3). Concerns had also been voiced regarding intermachine variability, but with current high-end systems this is now less of a concern. 30,45 D’Agostino et al. ,60 as reported by Arthritis Research UK,30 showed that there is good reliability between experts using different types of US machine. There may be some variability in synovitis throughout the day; response to cDMARDs may be detected within 6–8 weeks and response to bDMARDS may be seen as early as 1 week. 61,62 The optimal frequency of testing will depend on the frequency of treatment decisions. It is envisaged that the number of US tests conducted per year would be aligned to the frequency at which clinical decisions regarding changing the treatment for a patient would be undertaken.
| Reference | Scoring system |
|---|---|
| Szkudlarek et al. 200352 | Joint effusion (compressible anechoic intracapsular area):
|
| Scheel et al. 200553 | Synovitis and effusion included in a combined scoring method (the larger the anechoic structure or extent of synovial hypertrophy seen on US images, the higher the assigned score):
|
| Naredo et al. 2005,54 Naredo et al. 2007,55 Naredo et al. 200856 | Subjective grading of joint effusion and synovitis from 0 to 3:
|
| Karim et al. 200457 | Semiquantitative assessment for degree of synovitis:
|
| Schmidt et al. 200058 | Subjective grading of echogenity of effusion on a 0–3 scale:
|
| Klauser et al. 200459 | Criteria for grading intra-articular vascularisation with colour Doppler US/PDUS:
|
At the OMERACT 7 meeting in 2004 an international panel of experts agreed on the first definitions of sonographic pathology. 51 This meeting achieved consensus in US definitions of joint effusion, bursal effusion and synovitis. Studies following on from that meeting, undertaken within the EULAR/OMERACT network, were reported at the OMERACT 8 meeting in 2006. 63 The international panel of experts concluded that the standardised definitions produced by OMERACT had improved inter-rater and intra-rater reliability for detecting and grading synovitis of the hand joints. 63 The OMERACT US special interest group defined a Global Synovitis Score, which combines synovial hypertrophy and the PDUS signal in a composite score and has acceptable reliability. 64,65
Although the 28-joint count is well established for CE, the optimal joint count for US is currently unclear. Joint counts featuring smaller numbers of joints are more feasible for a clinical encounter. 45 Several studies have compared various different joint counts;45,66–70 however, there is no clear consensus on the optimal joint count at present and joint counts vary between studies, rendering comparison across studies difficult. Research is ongoing.
Patient experience of ultrasound
Ultrasound is relatively inexpensive and safe, avoiding the exposure to radiation that is necessary for conventional radiography and CT. US is a painless and harmless procedure, being non-invasive and not radioactive. As confirmed by this study’s patient advisor (see Appendix 2), US is comfortable for the patient, unlike MRI, which can be noisy, can make the patient feel claustrophobic and requires lying still for a prolonged period of time. It should be noted that only one patient advisor contributed to this report.
The advent of portable US machines means that scans can be carried out at the bedside or in the outpatient clinic without the need for a second appointment in the radiology department. Most scans for RA involve the hand or wrist joints or focus on the most affected joint and take < 20 minutes.
Current usage in the NHS
Few published data were identified on the current usage of US for RA in the NHS. Therefore, a survey was conducted to address this issue (see Appendix 1).
A published survey conducted in 200571 analysed data from 126 UK rheumatologists. It found that 93% (117/126) of the surveyed rheumatologists used US results in patient management, with 33% (41/126) conducting the US examination themselves. Of the 60% (76/126) who referred patients to other departments for US, the majority referred patients to the radiology department (56%, 71/126). Synovitis was the second most common indication (estimate from graph: 87%, 110/126) for investigation by US, after tenosynovitis. The authors acknowledge the possibility that US use was overestimated because of the sample of rheumatologists being targeted at imaging events and because of response bias (126 questionnaires returned out of 250 circulated).
Another survey of 100 rheumatologists, conducted in 2009 (Richard Wakefield, University of Leeds, 22 March 2016, personal communication), found that most used US results to detect inflammatory arthritis (84%), but fewer used US for prognostic assessment (44%) or disease monitoring (32%). In total, 28% had conducted the US examination themselves and 81% had referred patients to the radiology department. Of those surveyed, 40% reported that US frequently influenced patient management (Richard Wakefield, personal communication).
The Targeted Ultrasound Initiative,72 founded in 2012, provides training and resources regarding US. A survey of its international users suggests that > 90% use US for diagnosis and the assessment of remission and approximately 80% use US for routine monitoring (Richard Wakefield, personal communication).
Anticipated costs associated with the intervention
The cost of monitoring synovitis using US was estimated using data provided by our clinical experts (Professor Philip Conaghan, University of Leeds; Dr Cristina Estrach, Aintree University Hospitals NHS Foundation Trust; Professor Christopher Edwards, University of Southampton; and Dr Richard Wakefield, University of Leeds) and a survey (see Appendix 1) sent to members of the BSR. Our clinical advisors stated that US used for monitoring synovitis would be undertaken as an outpatient appointment and that contrast would not be required. A broad consensus was reached that two-thirds of scans would take < 20 minutes with one-third taking ≥ 20 minutes. Based on this advice, it was assumed that two-thirds of scans would be costed as NHS reference cost RD40Z (£55.03) and the remainder would be costed as NHS reference cost RD42Z (£59.90), resulting in a weighted cost per scan of £56.66. 73 Therefore, if it is assumed that, on average, four USs are performed on a patient every year, this would equate to a yearly cost of US monitoring of £226.62. No distinction has been made between different types of US for two reasons. First, detailed data on the differential costs have not been identified. Second, there was no clear evidence of a distinction between different US modes regarding the association with synovitis levels detected and in the successful tapering of treatment. Four US scans were chosen as the base case as it was assumed that clinicians may actively monitor patients in whom they had tapered treatment. This assumption was tested in sensitivity analyses.
Chapter 2 Definition of the decision problem
Decision problem
Purpose of the decision to be made
The aim of this assessment was to systematically review the evidence on the use of US examination in addition to clinical assessment, compared with CE only, in relation to how synovitis in patients with RA can be used to predict prognosis or response to treatment and to investigate the cost-effectiveness of US joint examination for monitoring synovitis in terms of guiding decisions about when treatment can be tapered or which patients should be progressed to more intensive treatment.
Intervention
The included intervention was US examination of joints in patients with RA used in addition to CE to detect synovitis. US technologies included were GSUS and PDUS.
Population/setting
The population was adult patients with a confirmed diagnosis of RA at any point in the disease pathway. The setting was secondary care.
Relevant comparators
The comparator was assessment of synovitis by CE, without the use of US technology. This included assessment of inflammatory biomarkers68 such as ESR or CRP15 and the use of disease activity scoring tools68 such as the DAS28,17 Health Assessment Questionnaire (HAQ),16 SDAI18 and Clinical Disease Activity Index (CDAI)18 (see Table 1).
Outside the scope of the decision problem
Ultrasound used in the initial diagnosis of arthritis or to determine the type of arthritis or US to guide injections.
Key factors to be addressed
The review aimed to address the clinical value of US in addition to CE for detecting synovitis at different joints and at different points in the disease pathway, compared with CE alone. The review aimed to investigate the economic costs and benefits associated with the use of US to monitor synovitis in RA.
Overall aims and objectives of the assessment
The review aimed to:
-
investigate reported data on the detection of synovitis in RA patients by US and CE and the ability of US and clinically detected synovitis to predict clinical outcome or response to RA treatment
-
investigate the economic costs and benefits associated with the use of US to monitor synovitis in RA
-
suggest key areas for primary research.
Chapter 3 Assessment of ultrasound studies
Methods for reviewing ultrasound studies
The search for evidence on US for monitoring synovitis in RA was undertaken systematically following the general principles recommended in the PRISMA statement. 74 The search strategies used are provided in Appendix 3.
Identification of studies
The following electronic databases were searched:
-
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (1946 to October 2015)
-
EMBASE (via Ovid) (1974 to October 2015)
-
Cochrane Database of Systematic Reviews (CDSR) (via Wiley Online Library) (1996–2015)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via Wiley Online Library) (1898–2015)
-
Health Technology Assessment (HTA) database (via Wiley Online Library) (1989–2015)
-
Database of Abstracts of Reviews of Effects (DARE) (via Wiley Online Library) (1946–2014)
-
NHS Economic Evaluation Database (NHS EED) (via Wiley Online Library) (1968–2014)
-
Science Citation Index Expanded (via Web of Science) (1900 to October 2015)
-
Science Citation Index and Conference Proceedings Index (via Web of Science) (1900 to October 2015)
-
ClinicalTrials.gov (US National Institutes of Health) (October 2015)
-
European League Against Rheumatism Abstract Archive (via Web of Science) (October 2015)
-
American College of Rheumatology and Association of Rheumatology Health Professionals (via Web of Science) (October 2015)
-
OMERACT conference proceedings (via Web of Science) (October 2015).
In addition to electronic database searching, bibliographies of systematic reviews and included studies were checked for studies meeting the review inclusion criteria.
Initial scoping searches identified eight relevant guidelines. 9,24,28,30,46,75–77 Bibliographies of these guidelines were also checked.
Following the submission of the report for peer review, the full results of one of the included studies [Targeting Synovitis in Early Rheumatoid Arthritis (TaSER)] were published. 78 On the request of a peer reviewer, the results from this publication have been included. The searches were not updated. However, as this was a publication of an already included study, with data relevant to the treatment strategies, it was decided that the publication would be included. Additionally, the Aiming for Remission in Rheumatoid Arthritis (ARCTIC) trial, although not published in full at the time of peer review, was published in a preliminary conference abstract in November 2015. 79 It was decided that this publication would also be discussed.
Inclusion and exclusion criteria
Population
Adult patients diagnosed with RA. 11
Intervention
The intervention was US of joints in patients with RA, as used to assess synovitis. Studies were not excluded on the basis of type of US technology used. US technologies included were GSUS and PDUS.
Comparators
The comparator was assessment of synovitis by CE without the use of US technology (in the same patients). This included assessment of inflammatory biomarkers such as ESR or CRP15 and the use of disease activity scoring tools such as the DAS2817 and HAQ. 16
Outcomes
Outcomes were diagnostic accuracy measured by sensitivity and specificity (CE with reference US, or reported US with reference CE, or sufficient data reported to calculate sensitivity or specificity); responsiveness to change in inflammation as measured by standardised response mean (SRM);80 synovitis detection rate; prognostic sensitivity or prognosis associated with baseline measures; prediction of response to treatment; or the use of US in clinical decision-making.
For diagnostic accuracy data, studies were accepted if they reported sensitivity or specificity or if they provided sufficient data to calculate sensitivity or specificity.
Study design
Diagnostic studies, prognostic studies and studies investigating prediction of response to treatment, or the influence of US on treatment decisions, whether as a one-off US test or as serial US testing, were included. Studies were not excluded based on quality alone; however, for prognostic studies, the method and setting of outcome measurement had to be the same for all study participants. 81
Additionally, systematic reviews were sought and were used to identify studies meeting the inclusion criteria for the review.
Exclusion criteria
The exclusion criteria were as follows:
-
US used for RA diagnosis only (for initial diagnosis of arthritis or for differentiating between types of arthritis)
-
US used to guide injections
-
US used for inflammatory conditions other than RA
-
studies with healthy control subjects unless outcome data were reported separately for the RA subgroup
-
studies of US compared with another imaging technology (or other diagnostic method) for which clinical comparator data were not available or for which data did not allow comparison of US with CE
-
studies with a low proportion (< 80%) of patients diagnosed with RA, unless outcome data were reported separately for the RA subgroup
-
studies of US reporting inter-rater or intra-rater reliability only
-
studies of tenosynovitis not reporting data about synovitis
-
animal models
-
preclinical and biological studies
-
narrative reviews, editorials and opinions
-
non-English-language papers
-
abstracts reporting insufficient details to allow inclusion.
This review concentrated on US as the intervention. There is no conclusive gold standard/reference standard for assessing synovitis related to disease progression. This review investigated the association of US-detected synovitis with later outcomes in prognostic studies (see Assessment of prognostic studies). This can be considered as validating the test results. 28 For detection of synovitis, it may be considered that surgery is the gold standard/reference standard, allowing direct visualisation of the synovial membrane. 57 In clinical practice, this would not be used for monitoring synovitis, and limiting studies to those including surgery would limit the amount of studies eligible for the review. In terms of other imaging techniques that have a potential role in monitoring, it has been suggested that MRI may be used as a gold standard/reference standard. However, it has been reported that US and MRI detect similar rates of inflammation. 28 Scintigraphy and positron emission tomography (PET) have been reported to detect similar rates of inflammation to CE. 28 Other imaging techniques with a potential for use in RA patients are conventional radiography and CT scans. US is more likely to be practical than other imaging techniques in assessing synovitis. US is more comfortable for the patient than MRI, is less expensive and has an advantage over other imaging techniques as it can be used immediately after CE to assess symptomatic areas. 30 Limiting studies to those including an additional imaging technique would have limited the number of studies eligible for the review. As this review concentrated on US as the intervention, the decision was taken not to compare US-detected synovitis with synovitis detected by other imaging techniques.
Study selection
Titles and abstracts were examined by one reviewer. Study selection based on full texts was carried out by two reviewers, with discrepancies resolved by discussion.
Data extraction strategy
Data relevant to the decision problem were extracted from all studies by one reviewer using a standardised data extraction form. All data extracted were checked thoroughly by a second reviewer. Data were extracted without blinding to authors or journal. Discrepancies were resolved by discussion.
Quality assessment strategy
The methodological quality of each included study was assessed by one reviewer and checked by a second reviewer. Discrepancies were resolved by discussion. Diagnostic studies were assessed using criteria based on the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool. 82 The QUADAS tool was selected as it is a validated, evidence-based tool that has been widely used in diagnostic review. Prognostic studies were assessed using criteria taken from the Generic Appraisal Tool for Epidemiology (GATE)83 and the Quality in Prognosis Studies (QUIPS)81 tool. These are both validated tools that have been used for reviewing prognostic studies. Two items of particular relevance to RA studies were adapted from recommendations on inclusion criteria and study design for RA studies by Karsh et al. 84
Methods of analysis/synthesis
For calculations of diagnostic accuracy, US was counted as the reference standard and the accuracy of the clinical comparator was assessed using sensitivity, that is, the proportion of true positives (TPs), and specificity, that is, the proportion of true negatives (TNs), calculated as follows: sensitivity is the number of TPs divided by the sum of the TPs and the false negatives (FNs); specificity is the number of TNs divided by the sum of the TNs and the false positives (FPs).
Data were tabulated and discussed. Evidence synthesis would be attempted unless precluded by heterogeneity (of population, intervention, comparator or outcomes).
Survey
In addition to the systematic review, a survey was conducted to investigate current usage of US in treatment decisions for RA patients. The survey was publicised by the BSR through newsletters. The questions used in the survey are provided in Appendix 1. The survey was available to clinicians from December 2015 to February 2016.
Results
Quantity and quality of research available
A total of 2724 records were identified from the electronic databases following deletion of duplicate records.
Eight guidelines were identified through grey literature searching. 9,24,28,30,46,75–77 Eight systematic reviews of relevance to the decision problem were also identified. 25,29,45,50,85–88 Guidelines were published on US in rheumatology by the ACR,46 Arthritis Research UK30 and EULAR,75 which also published guidelines on imaging in RA. 28 Guidelines on the management of RA were published by NICE9,77 and the Scottish Intercollegiate Guidelines Network. 76 Reviews were published on the use of US in inflammatory arthritis29,85–87 or US for use in RA including the detection of synovitis and defining remission. 24,25,45,50,88 The bibliographies of these guidelines and reviews were searched to identify studies meeting the review inclusion criteria, identifying an additional 26 records. This led to a total of 2750 records being screened.
The study selection process is provided in Figure 1. At title and abstract sift, 2596 records were excluded. The 63 records excluded at full paper sift are presented with reasons for exclusion in Appendix 4.
FIGURE 1.
Flow diagram of study inclusion.

A total of 75 articles53,55,68,69,89–158 describing 58 studies were included in the review; following the searches but prior to publication of this report, one of the studies identified as ongoing by the searches (ARCTIC) was published as an abstract. 79 Data extraction forms for these included studies are provided in Appendix 5.
Study quality assessment forms for the included studies are provided in Appendix 6.
Ten89–98 of the 58 included studies were reported as abstracts only and so limited details were available.
Of the 58 studies included, diagnosis of RA was reported as being confirmed using the ACR or EULAR classification criteria for RA, either the 2010 criteria11 or an earlier version,8 in all except nine studies. 89,90,92–96,98,99
An established US scoring system was referenced in all but nine89,91,94,95,100–104 of the 58 included studies. According to the hierarchy of evidence published by Merlin et al. ,159 diagnostic accuracy studies are of higher quality if they are blinded and recruit consecutive patients and prospective cohort studies are of higher quality than other forms of prognostic studies. Of 33 studies53,92,96,102–116,118–132 reporting diagnostic accuracy or detection rates of synovitis, 13102–104,107,109–111,113–115,118,119,124 were blinded comparisons of consecutive patients. Of 15 studies of prognosis,55,69,97,98,113,134,135,137–144 all but one,138 an ancillary study to a RCT, were prospective cohort studies.
Studies reporting the detection of synovitis are described in Appendix 7 (see Tables 138–140). Diagnostic data were extracted from 37 publications53,68,92,96,99,102–132,146 reporting on 33 studies. One of these studies113,146 also provided prognostic data.
For most of the studies providing diagnostic data (see Appendix 7, Table 138), CE had a high specificity and low sensitivity when using PDUS or GSUS as the reference standard. This indicates some agreement between CE and US, with US detecting synovitis in some joints that CE did not detect and only a few cases in which CE detected synovitis and US did not. This agrees with the higher detected rates of synovitis for US over CE (see Appendix 7, Table 139) reported in the majority of studies.
However, five studies found lower rates of detection of synovitis with US than with CE. 106,115,123,125,130 Also, there were mixed results in three studies, with higher detection rates for GSUS than for CE but lower detection rates for PDUS than for CE in two studies108,119 and lower detection rates for PDUS than for CE for metacarpophalangeal (MCP) joints but higher detection rates for PDUS than for CE for proximal interphalangeal (PIP) and wrist joints in one study. 131
The five studies106,115,123,125,130 that found lower rates of detection with US than with CE were some of the older trials; however, there were other trials from the early 2000s that did not report this finding and so this cannot explain the difference between these trials and trials finding a higher rate of detection with US than with CE. Study quality did not explain the differences between the study results, although one of the studies reporting a lower rate of detection with US than with CE had a small sample size (n = 6), and the differences did not appear to be caused by the US scoring system used (0–1 vs. 0–3 or 0–4; see Table 3).
It may be that the types of joint examined could explain the differences between the study results. Of the studies finding lower rates of detection with US than with CE, three studies106,115,130 investigated ankle and/or foot joints and one study123 included metatarsophalangeal (MTP) joints; however, this study also assessed PIP and MCP joints, which have been shown to have higher rates of detection of synovitis using US than using CE. 125 This agrees with one within-study comparison of joints, which reported that CE had a lower sensitivity for MTP joints than for MCP or PIP joints. 160 However, heterogeneity between other studies, suggesting that US is less useful for foot and ankle joints than for wrist and hand joints, means that there is uncertainty in the findings.
The detection of subclinical synovitis would be useful only if clinically relevant, as investigated using prognostic studies.
Prognostic data were extracted from 23 publications55,56,69,97,98,100,113,134–139,141–148,150,151 reporting on 15 studies. One of these studies was an ancillary study to a RCT. 138 The other studies were prospective cohort studies in which a cohort of patients was assessed at baseline with US and with CE and was then followed up to assess the association of these baseline measures with a given outcome. Prognostic data are provided in Tables 6–8.
Treatment data were extracted from 16 publications89–91,93–95,100,101,139,152–157 reporting on 13 studies. Following the searches but prior to publication of this report, one of the studies identified as ongoing (ARCTIC) was published as an abstract. 79 This abstract was included to provide treatment data, meaning that 14 studies in total provided data relating to treatment. Six of these were prospective cohort studies that measured the association of an outcome at follow-up with the US and CE variables measured at baseline93,101 or US and CE variables measured after 4 months of treatment139 or at the time of treatment discontinuation or tapering. 95,155,156 One study reported data from a prospective cohort in which baseline US and CE variables were tested for association with an outcome taken from a RCT of RA treatment. 95,132 Two studies were RCTs comparing treatment strategies with or without US. 79,154 One of these RCTs154 also provided data on treatment decisions. Treatment decisions were also investigated using observational data. 89–91,94,152 Treatment data are provided in Tables 9–14.
Meta-analyses were not performed because of heterogeneity in the type of US used, US scoring systems used (see Table 3), joints assessed, clinical comparators (type of examination; use of composite clinical scoring), outcome measures and follow-up times.
Assessment of prognostic studies
Heterogeneity of trials precluded meta-analysis. Therefore, no summary estimates of effect were available, which is a limitation of the review. Significance values refer to the association of baseline US and CE measures with later outcome measures. Radiographic progression measures are of importance to patients as they reflect structural damage, which is associated with loss of function over time. Other outcomes assessed are of importance to patients as they reflect disease activity and function. Studies investigated whether or not baseline measures could be predictive of later outcomes, rather than directly comparing different baseline measures. As well as heterogeneity of outcome measures, studies differed in the statistics reported to assess the association of baseline US and CE measures with clinical outcome at follow-up, including Spearman correlations (R) and odds ratios (ORs) (univariate analysis unless otherwise stated).
Two studies113,134 reported the sensitivity of US and CE measures to predict progression (Table 4). These were prospective cohort studies in which patients were tested with US and underwent CE at baseline and were then followed up and evaluated for the outcome measures of progression. Boyesen and Haavardsholm134 used a measure of GSUS inflammation based on synovitis and tenosynovitis and found that this US measure had a similar sensitivity for predicting erosive progression as MRI at 12 months, indicating that the majority of patients with a high US score, or high DAS, relapsed. This measure of GSUS inflammation134 had a higher specificity than DAS28 (although neither US nor DAS28 had high specificity) for predicting erosive progression as measured by MRI at 12 months, indicating that more patients with a low GSUS score than a low DAS did not progress. Ikeda et al. 113 found that GSUS, PDUS and Disease Activity Score 28 joints using C-reactive protein (DAS28-CRP; see Table 1) had similar sensitivities for predicting radiographic progression at 24 weeks (see Table 4). GSUS had a lower specificity than PDUS and DAS28 for predicting radiographic progression. 113 This study subgrouped patients by recently retrieved treatment and found that in MTX-treated patients (n = 16) and TCZ-treated patients (n = 17) PDUS had higher specificity than DAS28-CRP, but that specificities were similar in TNFi-treated patients (n = 24) (see Appendix 5). Both of these studies were prospective cohort studies, at level II in the study quality hierarchy, although the study by Ikeda et al. 113 was a better-quality and more rigorous study given that blinding was unclear in the study by Boyesen and Haavardsholm134 (see Appendix 8).
| Study | Population | Outcome | Patients with outcome, n (%) | US measure | Sensitivity (95% CI), % | Specificity (95% CI), % | Clinical measure | Sensitivity, % | Specificity, % |
|---|---|---|---|---|---|---|---|---|---|
| Boyesen 2011134 | 79 patients, dominant wrist | MRI erosive progression at 12 months (≥ 1-unit increase in RAMRIS) | 53 (67.1) | GSUS inflammation score cut-off values < 0.5 vs. ≥ 0.5a | 79 (NR) | 55 (NR) | DAS28 remission/low vs. moderate/highb | 74 (NR) | 21 (NR) |
| Ikeda 2013113 | 57 patients, 28 joints of DAS28 | Radiographic progression (mTSS) at 24 weeksc | 21 (36.8) | Total GSUS score cut-off values of < 62 | 56 (NR) | 57 (NR) | DAS28-CRP cut-off of < 9.0 | 64 (NR) | 81 (NR) |
| Total PDUS score cut-off values of < 21 | 69 (NR) | 76 (NR) |
Eleven studies (with 14 publications55,69,97,113,134–136,138–141,144,148,150) reported an association of US and CE prognostic factors with radiographic progression/erosion (Table 5). These were prospective cohort studies in which patients were tested with US and underwent CE at baseline and were then followed up and evaluated for the outcome measures of progression.
| Study | Population | Joints | Follow-up | Outcome | Type of US | Correlation of US synovitis with outcome | Type of CE | Correlation of CE with outcome |
|---|---|---|---|---|---|---|---|---|
| Backhaus 201369 | 453 patients | US7 score | 12 months | Bone erosion (US7 erosion sum score) | US7 synovitis sum score in GSUS | R ± SD: 0.140 ± 0.038 (95% CI 0.066 to 0.215; p < 0.001) | DAS28, ESR, CRP | R ± SD: DAS28 of 0.621 ± 0.141 (95% CI 0.345 to 0.898; p < 0.001); ESR 0.008 ± 0.010 (95% CI −0.011 to 0.027; p = 0.399); CRP 0.001 ± 0.009 (95% CI −0.017 to 0.018; p = 0.945) |
| US7 synovitis score in PDUS | R ± SD: 0.099 ± 0.051 (95% CI −0.001 to 0.200; p = 0.053) | |||||||
| Boyesen 2011134 | 84 patients | Dominant wrist | 1 year | MRI erosive progressiona | GSUS (univariate analysis)b | OR 2.15 (95% CI 1.23 to 3.75; p = 0.01) | DAS28 (univariate analysis) | OR 1.15 [95% CI 0.80 to 1.64; p = 0.46 (NS)] |
| GSUS (multivariate analysis)b,c | OR 2.01 (95% CI 1.14 to 3.53; p = 0.02) | ESR (univariate analysis) | OR 1.02 [95% CI 0.98 to 1.06; p = 0.29 (NS)] | |||||
| GSUS synovitis (radiocarpal joint assessed in the dorsal midline) | OR 7.2 (95% CI 0.9 to 61.0; p = NS) | CRP (univariate analysis) | OR 1.04 [95% CI 0.98 to 1.10; p = 0.16 (NS)] | |||||
| Brown 2008135 (same study as Ikeda 2007136) | 90 patients in clinical remission | Dominant hand MCP joint and wrist | 12 months | Progressive radiographic damage (both hands and feet)d | PDUS signal | OR 12.21 (95% CI 3.34 to 44.73; p < 0.001) | Painful joint count | OR 3.32 (95% CI 0.39 to 28.30; p = 0.273) |
| GSUS synovial hypertrophy | OR 1.92 (95% CI 0.49 to 7.54; p = 0.350) | TJC | OR 2.17 (95% CI 0.26 to 18.10; p = 0.473) | |||||
| SJC | OR 1.69 (95% CI 0.43 to 6.67; p = 0.457) | |||||||
| Ikeda 2007136 (same study as Brown 2008135) | 107 patients in remission (clinician assessed) on DMARDs | Dominant hand MCP and wrist | 12 months | Radiographic progression (direct assessment of individual joints) | GSUS synovial hypertrophy score | Mann–Whitney U-test p = 0.027 | Painful joints | Chi-squared test p = 0.284 |
| PDUS score | p < 0.001 (in asymptomatic joints only p = 0.002) | Tender joints | p = 0.389 | |||||
| Increased PDUS signal | Likelihood ratio 7.02, chi-squared test p = 0.037 | Swollen joints | p = 0.417 | |||||
| Cavet 2009138 | 24 patients | 10 MCP joints100 | 110 weeks | Radiographic progressione | GSUS synovial thickening | R = 0.59 (p-value NR) | Biomarkers (93 serum proteins) at 6 weeks (multivariate model) | Significant correlation R = 0.79 (p-value NR) |
| Power Doppler area) | R = 0.77 (p-value NR) | |||||||
| Dougados 2013139,149 | 59 patients | MCP (×10), PIP (×10), wrist (×2) and MTP (×10) joints | 2 years | Radiological progressionf | GSUS (0 vs. 1–3) | OR 1.64 (95% CI 1.08 to 2.47) | Clinical synovitisg | 0 vs. 1–3: OR 2.08 (95% CI 1.39 to 3.11; p < 0.001); 0–1 vs. 2–3: OR 1.64 (95% CI 1.14 to 2.36; p = 0.008) |
| GSUS (0–1 vs. 2–3) | OR 1.91 (95% CI 1.18 to 3.10) | Tender joints | OR 1.53 (95% CI 1.02 to 2.29; p = 0.04) | |||||
| PDUS 0 vs. 1–3 | OR 1.80 (95% CI 1.20 to 2.71; p = 0.005) | Tender joints with clinical synovitis | OR 1.89 (95% CI 1.25 to 2.85; p = 0.002) | |||||
| PDUS 0–1 vs. 2–3 | OR 1.36 (95% CI 0.78 to 2.38; p = 0.278) | |||||||
| Ikeda 2013113 | 57 patients (MTX, n = 16; TNFi, n = 24; TCZ, n = 17) | Finger, wrist, elbow, shoulder and knee | 24 weeks | Radiographic damagee | GSUS (cumulative total GSUS score) | R = 0.062; p = 0.649 (MTX, r = 0.210, p = 0.436; TNFi, r = 0.027, p = 0.900; TCZ, r = 0.492, p = 0.179) | Cumulative DAS28-CRP | R = 0.342, p = 0.009 (MTX, r = 0.487, p = 0.056; TNFi, r = 0.308, p = 0.144; TCZ, r = 0.369, p = 0.145) |
| PDUS (cumulative total PDUS score) | R = 0.357; p = 0.006 (MTX, r = 0.679, p = 0.004; TNFi, r = 0.153, p = 0.476; TCZ, r = 0.353, p = 0.165) | |||||||
| Naredo 200755 | 42 RA patients starting DMARDs (38 patients followed up to 1 year) | 28 joints | 1 year | Total radiographic score progression (radiographic erosion score and JSN score)e | Joint count for GSUSh | GSUS r = 0.61; p < 0.001 | SJC, TJC, DAS28, HAQ, ESR, CRP | SJC, r = 0.46, p < 0.01; TJC, r = 0.36, p < 0.05; DAS28, r = 0.40, p < 0.05; HAQ, r = 0.36, p < 0.05; ESR/CRP, NS |
| Joint count for PDUS signali | PDUS r = 0.59; p < 0.001 | |||||||
| Naredo 2008140 | 278 patients with complete data (of 367 RA patients starting TNFis) | 86 intra-articular and periarticular sites in 28 joints | 1 year | Total radiographic score progression (radiographic erosion score and JSN score);e multivariate analysisj | Time integrated values of US joint count for PDUS signal | Total radiographic score (R = 0.59) significant; erosion score (R = 0.64) significant; JSN score NS | ESR, CRP | Total radiographic score: ESR (R = 0.59) significant; ESR for erosion or JSN score NS; baseline CRP level weak predictive value for JSN score progression (R = 0.2); CRP NS for erosion and total scores |
| Osipyants 201397,150 | 35 patients | Wrists | 1 year | Radiographic progression (mTSS)e | PDUS signals of residual inflammation of the wrists at 6 months | Significantly correlated (r = 0.696; p = 0.011); mTSS at 1 year significantly higher [93 (range 56–131) vs. 32.5 (range 19.5–83) units; p < 0.04] in patients with middle- or high-active PDUS signals (n = 27) (SJC > 1, PDUS > 1) than in cases with lower imaging activity or the absence of the PD signals (n = 8) at 6 months | SJC | Non-significantly lower radiographic progression over 1 year in those with SJC ≤ 1 [55 (range 31–116) vs. 88.5 (range 55–130); p < 0.205] than in those with SJC > 1 (n = 25) |
| Reynolds 2009141 | 25 patients (providing data at the 2-year follow-up of 40 patients recruited) | MCP and PIP joints | 2 years | Erosion progressionk | GSUS and PDUS | Mann–Whitney U-test synovial score (0–3) NS; mean synovial thickness (cm) NS; pre-contrast PDUS score (0–3) NS; post-contrast PDUS score (0–3) NS | ESR | NS |
| Yoshimi 2013144 | 22 RA patients in clinical remission (of 31 recruited) | Bilateral wrists and all of the MCP and PIP joints | 2 years | Radiographic progressione | Total PDUS score | Total PDUS score significantly higher in patients with radiographic progression than in patients without (6.00 ± 6.44 vs. 0.87 ± 1.15; p = 0.0011) | SJC, DAS28, gVAS and the serum levels of ESR, CRP and MMP-3 | Significant differences in SJC (0.33 ± 0.79 vs. 1.29 ± 0.70; p = 0.0040) and DAS28-ESR (p = 0.0010) and DAS28-CRP (p = 0.041) between the radiographic progression group and the non-progression group. Borderline significance for TJC (p = 0.054). No significant difference in other clinical parameters (gVAS and the serum levels of ESR, CRP and MMP-3) between the radiographic progression group and the non-progression group |
| Total GSUS score | No significant difference in the total GSUS score, (12.6 ± 12.4 vs. 8.80 ± 5.78; p = 0.57), between the radiographic progression group and the non-progression group |
Of the 11 studies, seven55,69,113,138–140,144 found that US and at least one of the clinical measures investigated could significantly predict radiographic progression, most of which were of high quality (with the exception of two studies69,138 in which blinding was unclear). Three studies found that US could significantly predict radiographic progression whereas the clinical comparator could not (DAS28-ESR/ESR/CRP;134 joint counts;135 SJC97), two97,134 of which were less rigorous in terms of study quality, with blinding being unclear. One study141 found that neither US nor the clinical comparator (ESR) was significantly associated with later progression; this study was more rigorous in terms of study quality. All of the studies were prospective cohort studies, at level II in the study quality hierarchy, with the exception of one study,138 which was an ancillary study to a RCT (see Appendix 8).
The majority of the studies reported a significant correlation between US and radiographic progression, using GSUS55,69,134–136,138,139,148 or PDUS;55,69,97,113,134–136,138–140,144,148 most of these studies were rigorous in terms of study quality, with four less rigorous studies in which blinding was unclear. 69,97,134,138
In some cases, GSUS was not significantly correlated with radiographic progression whereas PDUS was (see Table 5). There was no significant correlation with radiological progression for GSUS in the study by Ikeda et al. ,113 which did not find improved sensitivity or specificity above those for DAS28-CRP at 24 weeks (see Table 4). This study also found that PDUS was significantly correlated with radiological progression in the MTX-treated subgroup but not in bDMARD-treated patients.
Grey-scale ultrasound was not significantly correlated with radiographic progression in a study of 22 patients in clinical remission whereas there was a significant correlation between PDUS and radiographic progression. 144 One study135,136 found that PDUS was significantly associated with later radiographic outcomes. Another study of 25 patients found that neither GSUS nor PDUS was significantly correlated with erosion progression at 2 years’ follow-up. 141
Some studies reported a significant association of CE with radiographic progression (see Table 5). DAS28 was significantly correlated with radiographic progression in four studies,55,69,113,144 although not in the study by Boyesen and Haavardsholm134 nor in the bDMARD subsets in the study by Ikeda et al. 113 HAQ score was significantly correlated with radiographic progression in the only study reporting this outcome. 55 ESR and CRP did not significantly correlate with radiographic progression,55,69,134,141,144 with the exception of the study by Naredo et al. ,140 which found that ESR and total radiographic score progression were significantly associated. Biomarkers (serum proteins) were significantly correlated with modified total Sharp score (mTSS) in the only study reporting this comparator. 138
Swollen joint count and TJC were significantly correlated with radiographic progression in three studies55,139,144 but not in two studies97,135,136 and painful joint count was not significantly correlated with radiographic progression in one study. 135,136
Power Doppler ultrasound was significantly associated with radiographic progression in 10 studies in which it was measured. Associations reported as ORs were as follows: OR 12.21 [95% confidence interval (CI) 3.34 to 44.73; p < 0.001]135 and OR 1.80 (95% CI 1.20 to 2.71; p = 0.005). 139 Associations reported at correlation coefficients were as follows: r = 0.099 (p = 0.05);69 r = 0.357 (p = 0.006);113 r = 0.59 (p < 0.001);55 r = 0.696 (p = 0.011);97 r = 0.59 (p-value not reported);140 and r = 0.77 (p-value not reported). 138 Mann–Whitney U-test results reported were p < 0.001136 and p = 0.0011. 144
One study141 reported a non-significant association of baseline PDUS with the outcome measure. This different result could not be explained by study quality or the joints assessed and, although the authors suggested that the scoring system used was not as sensitive as that used in other studies, other studies that used semiquantitative scores found significant associations. This study did differ from most in that the outcome of erosion was measured by US. The other study that used US to measure the end point was the study reporting borderline significance for association. 69
Grey-scale ultrasound was significantly associated with radiographic progression in six studies. Significant associations reported were as follows: Mann–Whitney U-test p = 0.027,136 r = 0.140 (p < 0.001),69 r = 0.61 (p < 0.001),55 OR 2.15 (95% CI 1.23 to 3.75; p = 0.01)134 and OR 2.08 (95% CI 1.39 to 3.11). 139 A moderate correlation was found between GSUS and radiographic progression in one study. 138 GSUS was not significantly associated with radiographic progression in three studies: OR 1.92 (95% CI 0.49 to 7.54; p = 0.350);135 R = 0.062 (p = 0.649);113 and Mann–Whitney U-test non-significant. 141 One of these studies113 had a shorter follow-up time (24 weeks) than the others, but the other two studies135,141 with non-significant associations had similar follow-up times to the studies finding significant associations. These two studies135,141 were two of the older studies included in the review, which may indicate a difference in the US machines used; however, because of the heterogeneity between studies, it is uncertain why these studies differed from those reporting a significant association. The difference between studies reporting significant associations and studies reporting non-significant associations could not be explained by study quality, joints assessed or how the end point was measured.
The DAS28 joints was significantly correlated with radiographic progression in one study measuring progression using the ultrasound 7 score (US7) score (r = 0.621, p < 0.001)69 and three studies measuring progression using the mTSS [r = 0.342, p = 0.009;113 r = 0.40, p < 0.05;55 and p = 0.0010 (DAS28-ESR) and p = 0.041 (DAS28-CRP) (only p-values reported)144]. DAS28 was not significantly correlated in one study measuring MRI erosive progression. 134 Given heterogeneity between trials, it is uncertain whether outcome measure was the factor explaining the difference between trials finding a significant association, or not, between DAS28 and later radiographic progression.
Other outcomes
Studies reporting outcomes other than radiographic progression (measures of disease activity) are shown in Table 6. These were prospective cohort studies in which patients were tested with US and underwent CE at baseline and were then followed up and evaluated for outcome measures of disease activity. All studies used measures in the baseline CE that were not the same as the outcome measure at follow-up (although some studies additionally reported assessment of the outcome measure at baseline (DAS28 and HAQ score;55 components of the composite outcome measure145).
| Study | Population | Joints | Follow-up | Outcome | Type of US | Correlation of US synovitis with outcome | Type of CE | Correlation of CE with outcome |
|---|---|---|---|---|---|---|---|---|
| Scirè 2009137 (same study as Bugatti 2012147) | 43 patients achieving clinical remission | Bilateral shoulder, elbow, wrist, MCP, PIP, sternoclavicular, acromioclavicular, knee, ankle and MTP joints | 6 months | Clinical relapse | US joint count > 2 (multivariable)a | OR 4.6 (95% CI 0.4 to 49.5; NS) | SJC > 1 | OR 0.6 (95% CI 0.1 to 5.5) |
| PDUS > 0 | OR 12.8 (95% CI 1.6, 103.5; p < 0.05) | DAS28 of > 1.1 | OR 9 (95% CI 0.7 to 110.3) | |||||
| Bugatti 2012147 (same study as Scirè 2009137) | 161 patients with early RA | Hands | 12 months | Low disease activity (i.e. a DAS of < 2.4) | PDUS synovitis scored (0–3) | OR 0.94 [0.31 to 2.83; p = 0.91 (NS)] | Serum levels of the chemokine CXCL13 | NS |
| Naredo 200755 | 42 patients with RA starting DMARDs (38 followed up for 1 year) | 28 joints | 1 year | DAS28 | Joint count for GSUSb | GSUS r = 0.63; p < 0.001 | SJC, TJC, DAS28, HAQ, ESR, CRP | DAS28, r = 0.75, p < 0.001; HAQ; r = 0.66, p < 0.001; TJC, r = 0.50, p < 0.01; SJC; r = 0.45, p < 0.01; ESR/CRP, r = 0.49, p < 0.01 |
| Joint count for PDUS signalc | PDUS r = 0.63; p < 0.001 | |||||||
| HAQ | Joint count for GSUSb | GSUS NS | SJC, TJC, DAS28, HAQ, ESR, CRP | HAQ, r = 0.82, p < 0.001; DAS28; r = 0.49, p < 0.01; TJC, r = 0.50, p < 0.01; SJC NS; ESR NS | ||||
| Joint count for PDUS signalc | PDUS NS | |||||||
| Osipyants 201397,150 | 35 | Wrists | 6 months | HAQ | PDUS | Significant (r = –0.821; p = 0.003) | DAS28-CRP, SDAI | Association with baseline DAS28 or SDAI NS. Subgroup [disease duration < 2 years (n = 9)]: HAQ score at 6 months correlated with baseline DAS28-CRP (r = 0.705, p = 0.02) and SDAI (r = 0.678, p = 0.03) |
| Ramirez García 201498 | 28 | Knees and hands (wrists, MCP, PIP flexor and extensor tendons of the hand) | 12 months | Relapse from clinical remission (i.e. a DAS28 of < 2.6) | PDUS | p = 0.034; logistic regression model OR 6.18d | ESR, TGFβ1 | ESR, p = 0.046; TGFβ1, p = 0.082 |
| Saleem 2012142 | 93 RA patients in clinical remission as determined by their treating rheumatologist | Hand and wrist | 12 months | Flare – defined as any increase in disease activity requiring a change in therapy [24/93 (26%)] | PDUS and GSUS | PDUS (unadjusted OR 4.08, 95% CI 1.26 to 13.19; p = 0.014); GSUS score was not significantly associated (p = 0.658) | HAQ-DI, TJC, SJC, RAQoL, CRP, DAS28, SDAI | HAQ-DI per 0.1 unit OR 1.27 (95% CI 1.07 to 1.52; p = 0.006). RAQol OR 1.10 (95% CI 1.01 to 1.20; p = 0.036). Other variables were NS: SJC, p = 0.690; TJC, p = 0.827; CRP, p = 0.308; DAS28 remission, p = 0.499; SDAI remission, p = 0.616 |
| Wakefield 2007143 | 10 | Bilateral glenohumeral, elbow, wrist, MCP, PIP, knee, tibiotalar, midtarsal and MTP joints | 46 weeks | Time to clinical remission | GSUS score | R = –0.221 (NS) | Baseline DAS28 | r = 0.627; p = 0.071 (NS trend) |
| PDUS score | R = –0.289 (NS) | |||||||
| Yoshimi 2014145 | 22 patient with RA in clinical remission (of 31 recruited) | Bilateral wrists and all of the MCP and PIP joints | 2 years | Remission, defined by ACR/EULAR Boolean criteria; score of ≤ 1 for all of TJC, SJC, CRP and PGA components | PDUS total score | p = 0.020 | DAS28-CRP, DAS28-ESR, SJC, TJC, CRP, ESR | NS for all: DAS28-CRP, p = 0.76; DAS28-ESR; p = 0.38; SJC; p = 0.060; TJC, p = 1.00; CRP, p = 0.17; ESR, p = 0.39 |
| GSUS total score | p = 0.020 |
In three cases, US and at least one clinical comparator measure were associated with the later outcome [ESR,98 Health Assessment Questionnaire Disability Index (HAQ-DI)142 and DAS55]. Three studies found that US was significantly associated with the later outcome but the clinical comparator was not (DAS28-CRP or SDAI;97 DAS28-CRP, DAS28-ESR, SJC, TJC, CRP and ESR;145 and SJC and DAS28137). In the study by Naredo et al. ,55 the clinical comparator DAS28 was associated with the later HAQ outcome, but the US joint count of 28 joints was not. The authors suggested that their results may differ from those of other studies because early RA patients were used in their study and, at this point of disease, functional status is related to inflammatory activity more than residual structural damage.
Low disease activity (i.e. a DAS of < 2.4) at 1 year was not significantly correlated with PDUS or the biomarker chemokine (C-X-C motif) ligand 13 (CXCL13). 147
The Disease Activity Score 28 joints at 1 year was significantly correlated with GSUS and PDUS and HAQ, SJC, TJC and ESR/CRP. 55 Relapse from clinical remission (i.e. of DAS28 of < 2.6) was significantly correlated with PDUS and ESR but not transforming growth factor beta 1 (TGFβ1). 98 Relapse (i.e. a DAS of ≥ 1.6) at 6 months was significantly correlated with PDUS, SJC and DAS but not GSUS. 137 Time to clinical remission (DAS28 of < 2.6) was not significantly correlated with GSUS, PDUS or DAS28 at baseline in a study of 10 patients. 143
Remission, defined by ACR/EULAR Boolean criteria [score of ≤ 1 for all of the TJC, SJC, CRP and patient global assessment components], at 2 years was significantly correlated with PDUS and GSUS, but not with the clinical comparators DAS28, SJC, TJC, CRP or ESR. 145
Flare, defined as any increase in disease activity requiring a change in therapy, at 12 months was significantly correlated with PDUS, HAQ and Rheumatoid Arthritis Quality of Life (RAQoL), but not with GSUS, SJC, TJC, CRP, DAS28 or SDAI. 142
Two studies investigated HAQ, one of which found that HAQ score at 1 year of follow-up was not significantly correlated with GSUS, PDUS, SJC, or ESR, but was with DAS28 and TJC. 55 One study97 found that HAQ score at 6 months was significantly correlated with PDUS, but not with DAS28 or SDAI (DAS28 and SDAI were significantly correlated in the subgroup with a disease duration of < 2 years but there were only nine patients in this subgroup).
Treatment studies
Treatment response or strategies
Nine studies79,93,95,100,101,139,154–156 reported data relating to treatment response or strategies. RA was reported as being diagnosed by 2010 ACR/EULAR criteria11 in two studies154,155 and by pre-2010 ACR/EULAR criteria8 in four studies;100,101,139,156 the criteria used were not reported in two studies. 93,95 Established semiquantitative scoring systems were used by Dougados et al. ,139 Inanc et al. ,93 Naredo et al. 156 (scoring system published by Wakefield51), Iwamoto et al. 155 (scoring system published by Naredo et al. 140) and Dale et al. 154 (scoring system published by Szkudlarek et al. 52). The heterogeneity of the trials precluded meta-analysis. Therefore, no summary estimates of effect are available, which is a limitation of the review. Significance values referred to the association of baseline US and CE measures with treatment measures (such as treatment persistence or response to treatment tapering). These measures could be of importance to patients in so far as they can be used to refine treatment.
Ultrasound was compared with CE as potential predictors of treatment persistence or response (Table 7). The study by Ellegaard et al. 101 investigated patients starting TNFi treatment to determine whether or not treatment persistence at 1 year of follow-up was associated with US and clinical measures [TJC, SJC, CRP, visual analogue scale (VAS), HAQ and DAS28]. This was a prospective cohort study in which patients were tested with US and underwent CE at baseline and were then followed up and evaluated to investigate the ability of these factors to predict treatment adherence, defined as patients remaining on TNFi therapy at the 1-year follow-up. Among US measures, the square root of the US Doppler colour fraction (USDCF), a measure of hyperaemia, was the only measure that significantly predicted TNFi continuation (p = 0.008). None of the clinical measures assessed, including SJC, TJC, DAS28 and CRP, was significantly associated with treatment persistence. 101 In the study by Inanc et al. 93, when considering EULAR response compared with no response after 3 months, baseline PDUS (p = 0.029) and GSUS (p = 0.020) differed significantly between responders and non-responders. This was a prospective cohort study in which patients were tested with US and underwent CE at baseline and were then followed up and evaluated to investigate the association with EULAR response at 3 months’ follow-up. Two of the clinical measures assessed also significantly differentiated between responders and non-responders [pain VAS (p = 0.009) and SJC (p = 0.05)], whereas other clinical measures (DAS28, TJC, ESR and CRP) did not. 93
| Study | Population | Joints | Follow-up | Outcome | US type | US association with outcome | CE association with outcome |
|---|---|---|---|---|---|---|---|
| Ellegaard 2011101 | 109 patients starting TNFis (ADA, ETN or IFX) | Wrist (most affected) | 1 year (n = 69 with US follow-up) | Treatment persistence (continued with TNFi) | USDCF square roota | Treatment persistence vs. dropouts because of lack of efficacy, p = 0.024; treatment persistence vs. all dropouts, p = 0.008 | Treatment persistence vs. dropouts because of lack of efficacy (all NS): TJC, p = 0.86; SJC, p = 0.98; CRP, p = 0.86; VAS (patient global), p = 0.08; HAQ, p = 0.416; DAS28, p = 0.943. Treatment persistence vs. all dropouts (all NS): TJC, p = 0.321; SJC, p = 0.486; CRP, p = 0.453; VAS (patient global), p = 0.240; HAQ, p = 0.098; DAS28, p = 0.375 |
| Inanc 201493 | 43 patients starting TNFis (drugs NR) | 28 joints according to EULAR guideline | 3 months | EULAR no response vs. response | PDUS | p = 0.029 | Pain VAS, p = 0.009; SJC, p = 0.05; other clinical measures NS (DAS28, p = 0.90; TJC, p = 0.12; HAQ, p = 0.31; ESR, p = 0.61; CRP, p = 0.98) |
| GSUS | p = 0.020 | ||||||
| EULAR good/moderate response (multivariate analysis)b | PDUS | OR 0.88 (95% CI 0.68 to 0.94; p = 0.04) | NR | ||||
| GSUS | NS |
Patients with persistent synovitis measured by GSUS or PDUS (Table 8), after 4 months of bDMARD treatment, were significantly more likely to have radiological progression at 2 years’ follow-up. 139 The study by Dougados et al. 139 was a prospective cohort study in which 4 months of biological therapy were prescribed, with further follow-up up to 2 years. The association of lack of response to treatment at 4 months, measured by US or CE using a semiquantitative scoring system, with radiological progression at 2 years was assessed. For persistent synovitis as measured by CE, this did not reach significance. 139 Taylor et al. 100 reported prognostic data from a study that was part of a RCT comparing MTX plus IFX with MTX plus placebo in aggressive early RA. Baseline US and CRP were tested for association with radiological progression at 54 weeks. US could significantly predict radiological progression in MTX plus placebo-treated patients (p = 0.020), but not in IFX plus MTX-treated patients (p = 0.479). 100 However, there were only 12 patients in each group in this study. Baseline CRP did not significantly predict progression in either treatment group. 100
| Study | Population | Follow-up duration | Joints | Outcome | Type of US | US association with outcome | Type of CE | CE association with outcome |
|---|---|---|---|---|---|---|---|---|
| Taylor 2004100 | 24 (IFX + MTX, n = 12; MTX + PBO, n = 12) | 54 weeks | Hands and feet | Radiological progression at 54 weeksa | Baseline synovial thickness | MTX + PBO, r = 0.69, p = 0.020; IFX + MTX, r = –0.23, p = 0.479 (NS) | Baseline CRP | MTX + PBO, r = 0.58, p = 0.077; IFX + MTX, r = 0.19, p = 0.562 |
| Baseline synovial vascularity | MTX + PBO, r = 0.78, p = 0.005; IFX + MTX, r = –0.28, p = 0.372 (NS) | |||||||
| Dougados 2013139 | 59 (ETN, n = 34; ADA, n = 23; IFX, n = 2) | 2 years | Wrist, MCP, PIP and MTP joints | Radiological progressionb | GSUS (> 0 on scale from 0 to 3), persistent synovitis after 4 months of treatment | OR 3.14 (95% CI 1.50 to 6.55; p = 0.002) | CE synovitis (> 0 on scale 0–3) persistent synovitis after 4 months of treatment | OR 1.70 (95% CI 0.93 to 3.12; p = 0.086) |
| PDUS (> 0 on scale from 0 to 3), persistent synovitis after 4 months of treatment | OR 2.79 (95% CI 1.19 to 6.56; p = 0.019) |
One prospective cohort study with 6 months’ follow-up155 reported the ability of US and CE to predict relapse following discontinuation of bDMARD (TNFi or TCZ) treatment (Table 9). GSUS- and PDUS-detected synovitis at the time of treatment discontinuation (total GSUS score of ≥ 14, total PDUS score of ≥ 3) had higher positive predictive values (PPVs) than DAS28 (≥ 1.5) for predicting relapse within 6 months. Relapse was defined as a DAS28 of > 3.2 in conjunction with escalation of anti-rheumatic treatment. Using these cut-off points patients were more likely to relapse if they had high GSUS (p = 0.007) or PDUS (p = 0.001) scores, but this was not the case for high DAS28 (p = 0.297). Few patients with high US scores did not relapse (GSUS, n = 2/10, PDUS, n = 1/9), but several patients with high DAS28 did not relapse (n = 15/28). However, negative predictive values (NPVs) were similar for GSUS, PDUS and DAS28 as most patients with scores below the cut-off point for US or DAS did not relapse.
| Study | Population | Follow-up | Joints | Outcome measure | US typea | US PPV (95% CI), % | US NPV (95% CI), % | US sensitivity(95% CI), % | US specificity(95% CI), % | CE typea | DAS28 PPV, % | DAS28 NPV, % | DAS28 sensitivity, % | DAS28 specificity, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iwamoto 2014155 | 40 patients in clinical remission who had discontinued bDMARDs | 6 months | 134 synovial sites in 40 joints | Relapse DAS28 of > 3.2 and anti-rheumatic treatment escalated | GSUS cut-off point ≥ 14 (n = 10) | 80 (NR) | 73 (NR) | 50 (NR) | 92 (NR) | DAS28 cut-off point ≥ 1.5 (n = 28) | 46 | 75 | 81 | 38 |
| PDUS cut-off point ≥ 3 (n = 9) | 89 (NR) | 74 (NR) | 50 (NR) | 96 (NR) | DAS28 cut-off point < 1.5 (n = 12) | 25 | NR | NR | NR |
This study155 also compared the median values of several variables for patients with and without relapse (Table 10); the total GSUS score differed significantly between the groups (p = 0.005), as did the total PDUS score (p = 0.002), whereas clinical variables (DAS28, SDAI, CDAI and HAQ) did not. Two other prospective cohort studies95,156 investigated the association of outcomes with US assessment or CE at the time of treatment discontinuation or tapering (see Table 10).
| Study | Population | Follow-up | Joints | Outcome | US type | US association with outcome | CE association with outcome |
|---|---|---|---|---|---|---|---|
| Iwamoto 2014155 | 42 RA patients in clinical remission, discontinued biological therapya | 6 months | 134 synovial sites in 40 joints | Relapse (i.e. a DAS28 of > 3.2) and anti-rheumatic treatment escalated | Total GSUS score | p = 0.005 | All NS: DAS28-ESR, p = 0.609; DAS28-CRP, p = 0.389; SDAI, p = 0.180; CDAI, p = 0.275; HAQ-DI, p = 0.721 |
| Total PDUS score | p = 0.002 | ||||||
| Luengroongroj 201595 | 32 RA patients in clinical remission on DMARD(s), about to stop or reduce doseb | 3 months | NR | Disease flare (arthritis symptoms and signs detected) | PDUS (median total score) | Univariate analysis: OR 2.14 (95% CI 1.13 to 4.05) (significant; p-value NR). Multivariate analysis:c NS (trend); OR 3.06, (95% CI 0.95 to 9.84; p = 0.06) | Number of DMARDs: univariate analysis – OR 5.88 (95% CI 1.12 to 30.88) (significant); in multivariate analysis NS. DAS28-CRP: NS by univariate or multivariate analysis |
| Naredo 2015156,157 | 77 RA patients in sustained clinical remissiond | 12 months (n = 35, 45.5%, tapering failure at 12 months) | 42 (including hands and feet) | bDMARD tapering failure (increase in bDMARD doses and/or the presence of clinical disease activity according to both DAS28 and SDAI criteria) | PDUS global index for Doppler synovitis (DSI) calculated | Significant associations: higher DSI (p < 0.0005); and DSI > 0 (p < 0.0005) at baseline. Multivariate analysis: DSI > 0 (OR 29.92, 95% CI 6.81 to 131.40; p < 0.0005) | Significant associations: longer duration of RA (p = 0.009), higher number of previous synthetic DMARDs (p = 0.003), higher DAS28 (p = 0.011), higher SDAI (p = 0.003) at baseline. Multivariate analysis: DAS28 of ≥ 2.2 (OR 5.81, 95% CI 1.62 to 20.93; p = 0.007). Other clinical variables (duration of remission) were not significant predictors in multiple regression analysis |
| GSUS | GSUS was not a significant predictor of tapering failure in the multiple regression analysise |
A study of patients in clinical remission with a reducing dose of bDMARDS156 found that PDUS could significantly predict tapering failure (p < 0.0005), defined as an increase in bDMARD dose and/or the presence of clinical disease activity according to both DAS28 and SDAI criteria (see Table 10), whereas GSUS was not significantly associated with tapering failure. Some clinical variables could also significantly predict tapering failure, namely DAS28 (p = 0.011) and SDAI (p = 0.003). 156 In a study of patients in clinical remission discontinuing or tapering DMARDS (bDMARDs or cDMARDs not specified),95 PDUS significantly predicted disease flare (p = 0.06 by multivariate analysis) (see Table 10), as did the number of DMARDs taken at baseline, whereas DAS28 did not.
One RCT154 investigated treatment strategies with and without US (Table 11). In this study, patients were given the same treatment for 3 months and then either step-up DMARD escalation strategies guided by DAS28 alone (n = 57) (target = DAS28 of < 3.2) or step-up DMARD escalation strategies guided by DAS28 plus PDUS assessment of a limited joint set (n = 53) (target = PDUS signal in one or fewer joints). Dale et al. 154 found that, at 18 months, significantly more patients in the group receiving PDUS had attained Disease Activity Score 44 joints (DAS44) remission (p = 0.046). Since drafting this report, the full results of this study have been published. 78 At 18 months’ follow-up, significantly more patients in the group receiving PDUS had attained DAS44 remission (p = 0.03) (see Table 11). However, for the two co-primary end points, change from baseline in DAS44 and rheumatoid arthritis magnetic resonance imaging scoring system (RAMRIS) erosion score, there was no significant between-group difference (p = 0.72 and p = 0.33, respectively). A preliminary publication of the ARCTIC study results,79 identified post search, also investigated treatment strategies with and without US in a randomised comparison (see Table 11). The primary end point (a DAS of < 1.6 and no swollen joints at 16, 20 and 24 months and no progression in vdHSS between 16 and 24 months) was reached by 26 patients in the US strategy group and 21 in the control group, with no significant difference between the groups.
| Study | Population | Follow-up duration | Outcome | US treatment strategy group | Clinical treatment strategy group | Comparison |
|---|---|---|---|---|---|---|
| Dale 2013154 (TaSER) | 110 early RA patients randomised to 3 months of the same treatment and then step-up DMARD escalation strategies guided by either DAS28 alone (n = 57) (target: DAS28 of < 3.2) or DAS28 + PDUS assessment of a limited joint set (n = 53) (target: PDUS signal in one or fewer joints) | 18 months | DAS44 remission (i.e. a DAS44 of < 1.6) | Strategy PDUS and DAS28: significant improvement in DAS44 (mean change −2.76, 95% CI −0.84 to 0.33; p = 0.39). DAS44 remission: 33% at 3 months, 65% at 18 months | Strategy DAS28: significant improvement in DAS44 (mean change in DAS44 −2.51, 95% CI NR). DAS44 remission: 43% at 3 months, 44% at 18 months | After 18 months, more patients in the PDUS group had attained DAS44 remission (p = 0.046) (at 3 months, difference was not significant: p = 0.32) |
| Dale 201678 (TaSER) | 111 RA or undifferentiated arthritis patientsa | 18 months | DAS44 remission (i.e. a DAS44 of < 1.6) |
(n = 54) DAS44 remission: 35 (66%) |
(n = 57) DAS44 remission: 25 (43%) |
DAS44 remission (p = 0.03) |
| Mean change in DAS44 | –2.69 (significant improvement) | –2.58 (significant improvement) | Non-significant difference between groups (p = 0.72) | |||
| Median (IQR) change in RAMRIS erosion score | 0.5 (0.0–1.0) | 1.0 (0.0–2.0) | Non-significant difference between groups (p = 0.33) | |||
| Haavardsholm 201579 (ARCTIC) | 130 early RA patients | 24 months | Primary end point was a DAS of < 1.6, a SJC44 of < 1 and a ΔvdHSS of < 0.5 between 16 and 24 months |
(n = 118) Strategy PDUS and GSUS and DAS: 26 (22.0%) |
(n = 112) Strategy DAS: 21 (18.8%) |
p = 0.54 |
| DAS remission (i.e. a DAS of < 1.6) | 80 (67.8%) | 75 (67.0%) | p = 0.89 | |||
| Median (IQR) change in vdHSS total score between 0 and 24 months | 1.0 (0–2.5) | 1.5 (0.5–3.0) | p = 0.09 | |||
| Median (IQR) change in vdHSS erosion score between 0 and 24 months | 0.5 (0–1.5) | 1.0 (0.5–2.0) | p = 0.04 |
For both randomised trials (TaSER and ARCTIC), there was no significant difference in primary end point between the clinical strategy group and the strategy group based on US and clinical measures. Both trials found a significant advantage of the strategy including US for one of the secondary outcomes (DAS remission in the TaSER study and erosion score in the ARCTIC study), but not for the other secondary outcomes (ACR core set variables in the TaSER study; CDAI or SDAI remission or EULAR response in the ARCTIC study). This suggests that adding US to a DAS-based strategy is not beneficial after 18–24 months. It is uncertain whether this is because of a genuine lack of improvement caused by the US strategy or because of other factors. Both trials included early RA patients only and PDUS (the ARCTIC study also used GSUS) and have some incorporation bias in terms of the clinical strategy and primary outcome being DAS related (although in the TaSER study the strategy used DAS28 and one of the co-primary outcomes was DAS44 and in the ARCTIC study the primary outcome was a composite measure of remission that included DAS).
Treatment decisions
Six studies89–91,94,152,153 reported on the use of US in addition to clinical measures and the impact on treatment decisions (Table 12). These were fairly small studies, with sample sizes ranging from 1789 to 10994 (see Appendix 8). Four studies89,90,94,153 took place in rheumatology clinics in the UK, one152 was from the USA and one91 was from France. Four studies89–91,152 did not report the source of funding, although three89–91 of these stated that the authors had no conflicts of interest. Two studies94,153 were supported by pharmaceutical companies. Dale et al. 153 stated that neither of their funders had any role in the design, performance, analysis, interpretation or reporting of the study.
| Study | Source of funding | Population, number of patients | Setting | US and treatment decisions |
|---|---|---|---|---|
| Bhamra 201489 | NR | 17 | Nuffield Orthopaedic Hospital Emergency Rheumatology Clinic, UK | In 15/17 (88%) patients, treatment escalation was directly influenced by GSUS findings |
| Ceponis 2014152 | NR | 51 | San Diego Health System, CA, USA | US use modified the bDMARD and/or cDMARD used in seven cases, but did not affect the overall treatment plan (p > 0.15) or overall cDMARD (p < 0.062) or bDMARD use (p > 1.0). Use of US increased physicians’ confidence (p < 0.0005) and patients reported that their confidence in physicians’ medical decisions had increased (88.4% of cases) |
| Ciurtin 201390 | NR | 39 | Department of Rheumatology, University College Hospital, London, UK | Study identified 9/39 (23%) RA patients with active synovitis with a positive Doppler signal that prompted a change of treatment |
| Dale 2014153,161 (TaSER) | Chief Scientist’s Office, Scottish Executive, and Pfizer UK | 53 | Three Glasgow teaching hospital sites, UK | On 120 occasions (29%), GSUS findings contradicted the DAS28 and led to modified treatment decisions |
| Gandjbakhch 200891 | NR | 52 | University rheumatology centre, Paris, France | US results caused a change in treatment in 13% of patients. Confidence of the clinician, measured using a VAS (0–100), increased by 11 points (95% CI 5.9 to 16.9 points) following US (p < 0.001) |
| Kelly 201394 | AbbVie | 109 | Four secondary care rheumatology clinics: two in London, one in Southampton and one in Antrim, UK | The US-monitored group (n = 54) had a significantly shorter time to initiation of DMARDs (1.45 vs. 2.38 months) than the non-US group (n = 55) (t-test, p = 0.02) |
When the percentage of treatment decisions modified by the additional use of US was reported, this ranged from 13% to 88% of cases89–91,153 (for studies based in the UK the percentages were 23%,90 29%153 and 88%89).
One study89 examined treatment decisions in clinician-evaluated patients by survey and found that 15 out of 17 (88%) treatment escalations were influenced by the addition of US. Of two observational studies, one91 found that US (in addition to CE and DAS28) influenced treatment decisions in only 7 out of 52 (13%) patients; however, clinician confidence was significantly improved following US (p < 0.001). The other observational study94 found a significant difference in time to initiation of DMARDs (p = 0.02) (the US-monitored group had a shorter time to initiation than the group undergoing clinical evaluation alone) (see Table 12). Two studies investigated the utility of routine US and reported a change in treatment following US in 9 out of 39 (23%) patients with clinician-evaluated synovitis90 and a modification of the bDMARD or cDMARD used in 7 out of 51 patients (compared with clinical evaluation of swollen joints and CDAI);152 clinician confidence was also significantly improved following US (p < 0.0005) in one of the studies. 152 A study of treatment strategy153 reported that, on 120 occasions (29%), GSUS findings contradicted the DAS28 and led to modified treatment decisions (see Table 12).
Survey
A survey of UK rheumatology units (see Appendix 1) with 31 respondents suggested that US is already being used in some units for modifying treatment decisions in RA. Twenty respondents (65%) said that they used US for monitoring synovitis. Additionally, one respondent said that their unit planned to use US in the future. Survey respondents were self-selecting and so the sample may have been biased. Only 31 responses were received by the end of February 2016 and the small sample size is a limitation of the survey.
Discussion
This systematic review aimed to investigate the value of US in addition to CE for monitoring synovitis and whether or not US could be used to guide treatment decisions. There were few RCTs available and thus lower-quality study designs were included.
Thirty-three53,92,96,102–116,118–132 studies provided diagnostic data (see Appendix 7). The majority of these studies reported that US detected more cases of synovitis than CE alone. The detection of subclinical synovitis would be useful only if clinically relevant, with prognostic studies suggesting that US-detected synovitis was associated with radiographic progression.
Power Doppler ultrasound was significantly associated with radiographic progression in all studies in which it was measured. GSUS was significantly associated with radiographic progression in some, but not all, studies in which it was measured. Similarly, DAS was significantly associated with radiographic progression in some, but not all, studies in which it was measured. Studies varied in terms of interventions, comparators and outcomes, making it difficult to draw firm conclusions from the available evidence. Few studies were identified that compared US with CE and their effect on treatment; however, these studies suggested that US was superior to CE alone in predicting response to treatment tapering or discontinuation. Few data were identified regarding the additional influence of US in current practice, but studies suggested that US was used in treatment decisions and could increase physician confidence in those decisions. A small survey of UK rheumatology units (see Appendix 1) suggested that US is already being used in some units for modifying treatment decisions in RA.
US could also distinguish synovitis from inflammation resulting from other pathologies. Most of these studies assessed synovitis in hand and wrist joints, reporting that US detected more cases of synovitis in these joints than CE; this was also the case for elbow and shoulder joints. Foot and ankle joints were less likely to show an advantage of US over CE. The majority of studies investigating responsiveness found similar responsiveness for US and clinical comparator measures including DAS28110,111,113,116,132 SDAI111,116,117 CDAI111,113 SJC111 TJC111 and CRP. 129
Fifteen studies55,69,97,98,113,134,135,138–144,147 provided prognostic data. Although the design of these studies was of high quality (prospective cohort), data reported were correlations and sample sizes ranged from 10 to 453 (in total, data were available from 1523 patients but study heterogeneity precluded meta-analysis). The majority of studies investigating radiographic progression reported that US was a significant predictor, either GSUS55,69,134,135,138,139 or PDUS. 55,69,97,113,135,138–140,144,160 There were mixed results regarding the association of clinical comparators with outcome measures. Regarding other outcome measures, PDUS was a significant predictor in the majority of studies for DAS28,55,98,137 ACR/EULAR remission145 and flare. 142 There were conflicting results for the association between HAQ score and US. 55,97 The data suggested that there was a stronger association with outcomes for PDUS than for GSUS.
Nine studies79,93,95,100,101,139,154–156 reported data relating to the impact of US on treatment response or strategies. Two were RCTs79,154 and the others were prospective cohort studies 93,95,100,101,139,155,156 Sample sizes ranged from 24 to 130, with data available from 627 patients in total (although study heterogeneity precluded meta-analysis). Six small (sample size 17–109 patients; 321 patients in total) studies89–91,94,152,153 reported observational data on the impact of US on treatment decisions.
One study101 found that US was the only measure that significantly predicted TNFi continuation. One study93 found that baseline PDUS and GSUS differed significantly between EULAR responders and non-responders. US-measured treatment response was associated with radiological progression100,139 and PDUS and GSUS were better predictors of relapse following treatment discontinuation than DAS28. 155 PDUS was more highly associated with tapering failure than clinical variables156 and PDUS significantly predicted disease flare on treatment whereas DAS28 did not. 95
Randomised controlled trial evidence of early RA patients reported that the addition of PDUS to a DAS28-based treat-to-target strategy led to significantly more patients attaining DAS44 remission, but there was no significant between-group difference in change from baseline in DAS44 and RAMRIS erosion score. 154 In the ARTIC study,162 there was no significant between-group difference (PDUS, GSUS and DAS vs. DAS) in the primary end point, which consisted of a composite of a DAS of < 1.6 and no swollen joints at 16, 20 and 24 months and no progression in vdHSS between 16 and 24 months. Both RCTs were subject to incorporation bias, with DAS being used in the treatment strategy groups and forming part of the primary outcome measure; however, the use of DAS reflects real-world practice. Outcome measures not subject to incorporation bias were the erosion scores, with RAMRIS not being significantly different between groups in the TaSER study154 and the vdHSS erosion score having a significant advantage for the strategy with additional US in the ARCTIC study. 162 Only two RCTs154,162 were found comparing US strategies with clinical strategies alone. In clinical practice, a diagnostic test should only be considered when there is clinical uncertainty. These two studies did not explore the value of US when added only in cases of clinical uncertainty, for example, when there was discrepancy between DAS and clinical evaluation. Several small studies have used US in combination with clinical measures for treatment decisions. 26,163,164 Most studies relied on one-off US measurements at baseline; however, the two RCTs employed US serially as part of treatment strategies. Heterogeneity of trials precluded meta-analysis. Therefore, no summary estimates of effect are available, which is a limitation of the review.
Treatment decisions were modified by the additional use of US in 13%–88% of case. 89–91,153 Clinician confidence was significantly improved following US. 91,152 A study of treatment strategy153 reported that, on 120 occasions (29%), GSUS findings contradicted the DAS28 and led to modified treatment decisions.
Guidelines and reviews were identified that were of relevance to this report,9,24,25,28–30,45,46,50,75–77,85–88 although none had a scope that was identical to the systematic review conducted here. The bibliographies of these guidelines and reviews were searched and studies meeting the systematic review inclusion criteria were included in the review.
Guidelines and reviews differed in scope from this report by not being restricted to RA29,46,85–87 or by looking at the initial diagnosis of RA,28,30,46,88 by not being restricted to synovitis9,25,28–30,46,75–77 or not including synovitis85 or by focusing on management9,76,77 or not covering management decisions. 24,25,86,88 Some imaging reviews differed from the review in this report by including US-guided injections,30,46 by not being restricted to US as an imaging technique28,29,45,87 or by focusing on inter-rater reliability. 50
The EULAR guidelines on imaging in RA28 covered all of the questions addressed by the scope of this report, apart from the percentage of treatment decisions influenced by the addition of US to CE. However, as these guidelines were published in 2013, they could not include the more recent publications that this report included. As the studies relating to treatment identified in this report were nearly all from 2013 or later, only one101 of these studies was included in the published EULAR guidelines. 28
The findings of this report were in agreement with these published guidelines and reviews in that US detects synovitis that is not apparent on CE and that US detects synovitis in patients in clinical remission. 24,25,28,30,45,88 The findings of this report were also in agreement with these published guidelines and reviews in that US can assess the course of disease,30,76,87 with similar responsiveness to that of DAS28. 28
With regard to prognosis, the findings from this report agreed with the published guidelines and reviews in that PDUS is a predictor of erosive progression and disease flare. 25,28,76,88
Other reviews have suggested that there is still a need for more evidence regarding which joints should be assessed by US for the most effective practice. 24,45
Most studies were carried out outside the UK and, as such, treatment pathways may differ from treatment pathways in UK patients. However, the results from this report are generalisable to UK practice in terms of studies using international definitions of RA. Additionally, most used standardised semiquantitative scoring systems for US measures of synovitis and clinical measures that are used internationally, for example DAS28. Treatments assessed included cDMARDs and bDMARDs, as would be relevant to UK practice.
Chapter 4 Assessment of cost-effectiveness
Literature reviews undertaken
Reviews were undertaken to identify literature on the:
-
cost-effectiveness of the use of US for monitoring synovitis in RA
-
economic impact of tapering bDMARDs in the treatment of RA.
For brevity these have been denoted the ‘monitoring’ search and the ‘tapering’ search, respectively. The search strategies for these searches are provided in Appendix 3.
Following deduplication, 226 hits were obtained in the monitoring search and 54 hits were obtained in the tapering search. Abstracts were sifted by two reviewers and, because of the relatively small numbers of hits obtained, a sensitive approach was taken in which any paper felt to be of potential relevance by either reviewer was retrieved as a full paper. From the sift, five papers were retrieved from the monitoring search165–169 and 19 papers were retrieved from the tapering search. 170–187 All papers were reviewed and, when their reference lists indicated that further papers would potentially be of benefit, these were also reviewed.
Papers potentially relating to the cost-effectiveness of ultrasound for monitoring synovitis or tapering drug doses
Three cost-effectiveness studies relevant to the decision problem were identified although all of these were related to tapering of the amount of drugs used. 176–178 The first study by Kobelt et al. 176 concluded that, in a situation in which a considerable proportion of patients achieve remission, dose adjustments would increase the cost-effectiveness of ETN. A similar conclusion was produced in a later publication by Kobelt et al. ,177 whereby dose reduction was assumed to be the most cost-effective strategy for patients with moderate disease rather than full-dose ETN or no ETN use. Krieckaert et al. 178 assessed the cost-effectiveness of personalised treatment for RA based on clinical response and drug levels at 6 months. For patients who had a EULAR response at 6 months, a decision to stop treatment, continue treatment or prolong the interval between ADA doses was made based on the drug levels detectable within patients. The authors concluded that tailoring ADA based on short-term outcome and drug levels was cost-effective compared with usual care.
No papers were identified on the cost-effectiveness of US use in monitoring levels of synovitis. Thus, any analysis undertaken within this report is, to the authors’ knowledge, the first such assessment. The consensus from the three papers regarding the cost-effectiveness of tapering RA treatment indicates that dose reductions would represent a cost saving that could be used in other areas of the health service; in addition, providing that response and/or remission was maintained, dose reductions would also be beneficial to patients because of the avoidance of potential adverse events associated with taking bDMARDs. A review of adverse events associated with bDMARDs within RCTs is reported by Stevenson et al. 23
Papers potentially relating to the efficacy of conventional or biological disease-modifying anti-rheumatic drugs when the dose has been tapered
Evidence regarding the continued efficacy of cDMARDs or bDMARDs following a reduction in dose or withdrawal of treatment was identified in papers retrieved in the literature review (both clinical and cost-effectiveness reviews) and from references within these papers. When abstracts were identified, searches for a later publication were undertaken. Only the most recent papers were included when multiple papers related to the same study unless the older papers contained data that were not included in the more recent paper. Furthermore, papers known to our clinical experts but not identified by other means were also included.
A limitation of this approach is that the sensitivity of the search strategies was not high, with a minority of the final set of papers also identified within the tapering search. As such, potentially relevant papers may have been omitted, although for the purposes of this report this was deemed acceptable as the intention was to provide a broad overview rather than to fully detail the evidence.
A brief summary of the methods and conclusions from these papers is provided in Table 13, with papers listed in reverse chronological order. The evidence suggests that drug tapering, treatment holidays or treatment withdrawal in patients induced into remission or low disease activity can occur without harm in a sizeable proportion of patients, although the evidence for withdrawal of treatment in patients receiving ADA or CTZ is arguably weaker than that for IFX. Tapering drug doses would have many benefits including reduced drug acquisition costs and potential avoidance of the adverse events associated with DMARDs.
| Study | Setting | Summarised detailsa | Summarised authors’ conclusions |
|---|---|---|---|
| Fautrel 2016188 | France | An 18-month equivalence randomised trial including (1) patients receiving ETN and ADA at stable doses for ≥ 1 year, (2) patients in remission according to the DAS28 for ≥ 6 months and (3) patients with stable joint damage. In total, 137 patients were randomised to standard maintenance (n = 73) or to a regime using an algorithm to increase the period between injections based on the DAS28, every 3 months, until completely stopping (n = 64). In the algorithm arm bDMARDs were stopped for 39%, tapered for 36% and maintained at full dose for 20%. The status of the remaining patients (5%) was not clear. Relapse was more common in the algorithm arm (76.6% vs. 46.5%; p = 0.0004). However, there was no difference in structural damage progression (p = 0.8) | The tapering algorithm was not equivalent to the maintenance strategy, resulting in more relapses without impacting structural damage progression |
| Galloway 2015189 | UK | A pragmatic 12-month RCT evaluating if tapering bDMARDs causes loss of response. In total, 103 patients receiving ETN or ADA and with a DAS28 of < 3.2 for > 3 months were randomised to one of three groups for 6 months: (1) constant bDMARD dose, (2) 33% tapered dose or (3) 66% tapered dose. Flares, defined as an increase in DAS28 to ≥ 3.2 and one or more swollen joint, occurred in 7/50 (14%) control subjects, 6/48 (13%) in the 33% tapering group and 14/38 (37%) in the 66% tapering group. Post-tapering flares resolved when TNFi was restarted. The OR for a flare in the 33% tapering group compared with the 66% tapered group was 4.2 (95% CI 1.3 to 14.5). There were no significant differences at 6 months (p-value not reported) | Good responses are maintained after bDMARD doses are tapered by one-third. Tapering by two-thirds results in more flares, but these respond to restarting bDMARDs and did not adversely affect disability progression. The 33% tapering strategy retains responses at substantially reduced drug costs |
| Ghiti Moghadam 2015190 | The Netherlands | An open-label RCT assessing whether RA patients with a DAS28 of < 3.2 in the previous 6 months can effectively and safely stop and restart bDMARDs. Patients were randomised 2 : 1 to stop or continue their current bDMARD. A flare was defined as a DAS28 of ≥ 3.2 with an increase of ≥ 0.6 compared with the previous DAS28. In total, 531 patients were randomised to stop treatment and 286 were randomised to continue treatment. At 6 months, significantly more patients in the stop group (29.3%) had experienced a DAS28 flare than in the continuation group (9.7%) (p < 0.0001). At 12 months, these values were 40.8% and 14.4%, respectively (p < 0.0001). Of the patients who restarted a TNFi within the first 26 weeks after stopping, 83% had regained a DAS28 of < 3.2 6 months later, with a median time to regained DAS28 of < 3.2 of 12 weeks (95% Cl 10.9 to 13.1 weeks) | During a 12-month follow-up period, 59% of RA-patients with a DAS28 of < 3.2 were able to stop bDMARDs without experiencing a flare. The data suggest that bDMARDs can be restarted effectively and safely |
| Luengroongroj 201595 | Thailand | A prospective study to assess whether or not US can be used to predict relapse after tapering of DMARDs. Thirty-two patients with established RA and clinical remission defined by a CDAI of < 2.8 were enrolled. Patients on a maintenance dose had their dose reduced to a half-dose, whereas those on lower doses had their treatment withdrawn. A relapse was defined as having arthritis symptoms and clinical signs. Four patients (12.5%) relapsed within 3 months | Reducing doses of DMARD for a short period of time appears to be safe; however, close monitoring for disease relapse is needed, especially in patients with subclinical synovitis |
| Marks 201526 | England | Prospective cohort study analysing the possibility of reducing the dose of bDMARDs by one-third. Seventy patients were recruited who were in disease remission (i.e. a DAS28 of ≤ 2.6), had had an absence of synovitis on PDUS for > 6 months and who were not taking corticosteroids. Combined DAS28 and PDUS remission was maintained by 96% at 3 months, 63% at 6 months, 37% at 9 months and 34% at 18 months. Those who continued on the reduced doses were more likely to have lower DAS28 scores on initiation of bDMARD therapy and to be rheumatoid factor negative | Combined clinical and US assessment identifies individuals in remission who may be suitable for bDMARD dose reduction |
| Tanaka 2015191 | Japan | Prospective cohort study analysing the possibility of discontinuing ADA for 1 year. A flare was defined as a DAS28 of ≥ 3.2. In total, 75 patients who were steroid free and with a DAS28 of < 2.6 for 6 months participated in the study; 52 patients chose to discontinue ADA whereas 23 chose to remain on treatment. The proportion of patients maintaining remission was significantly higher in the continuation group. In patients with deep remission (i.e. a DAS28 of ≤ 1.98) there was no significant difference between the groups. ADA readministration to those with a flare was effective | The possibility of remaining ADA free for a year was demonstrated, particularly in those with deep remission |
| van Herwaarden 2015187 | The Netherlands | A randomised, controlled, open-label, non-inferiority study (n = 180) evaluating disease activity-guided dose reductions of ADA or ETN (n = 121) vs. usual care (n = 59) over an 18-month period. Patients in the dose reduction arm increased the injection interval every 3 months until flare, defined as a DAS28 score increase of > 1.2 or an increase of > 0.6 and a DAS score of ≥ 3.2, or bDMARD discontinuation. Following a flare the last effective dosing regimen was reinstated. A major flare was defined as a flare with a duration of > 3 months. Dose reduction was non-inferior to usual care: 12% had a major flare in the dose reduction arm compared with 10% in the usual care arm. bDMARDs could be successfully stopped in 20% of patients, but dose reduction was reportedly not possible in 37%. Although functional status, quality of life, relevant radiographic progression and adverse events did not differ between strategies, flares (73% vs. 27%) and radiographic progression (32% vs. 15%) were more frequent in the dose reduction arm | A strategy of disease activity-guided dose reduction is non-inferior to usual care with regard to major flaring, resulting in successful dose reduction or stopping in two-thirds of patients |
| van Vollenhoven 2015192 | Europe (16 European sites) | A randomised, double-blind trial assessing the impact of dose reductions of ETN in 91 patients with stable low disease activity (i.e. a DAS28 of ≤ 3.2) receiving ETN treatment. Following an 8-week screening period, 73 patients were randomised to the usual dose of ETN (50 mg) (n = 23), a half-dose (25 mg) (n = 27) or placebo (n = 23). Sixty-six patients completed the study. The proportions of patients who did not flare at week 48 were 52% for full-dose ETN, 44% for half-dose ETN and 13% for placebo. The majority of patients who flared regained low disease activity with 50 mg of ETN | In patients who have achieved stable low disease activity on ETN, continuing treatment is superior to placebo. Reduced-dose ETN was also more effective than placebo in maintaining a favourable response, suggesting that a maintenance strategy with reduced-dose ETN may be possible |
| Emery 2014193 | Global (57 sites in Europe and Asia) | In total, 306 patients with early active disease were enrolled and treated with ETN plus MTX. A total of 193 patients with a DAS28 of ≤ 3.2 at week 39 of treatment and a DAS28 of < 2.6 at week 52 were randomised to receive one of 25 mg of ETN plus MTX, MTX alone or placebo for 39 weeks. Patients with a DAS28 of ≤ 3.2 at week 39 of the randomised period had all study treatment withdrawn. At the end of the randomised period, significantly more patients on combination therapy had sustained remission than patients receiving the remaining strategies: 40/63 (63%) of the combination therapy group, 26/65 (40%) of the MTX group and 15/65 (23%) of the placebo group. Following the treatment withdrawal period, the combination therapy group was no longer significantly better at sustaining remission than the MTX group (p = 0.10): 28/63 (44%) of the combination therapy group, 19/65 (29%) of the MTX group and 15/65 (23%) of the placebo group. No significant between-group differences were observed in radiographic progression of disease (p ≥ 0.34 for all comparisons). Serious adverse events were reported in three patients (5%) in the combination therapy group, two (3%) in the MTX alone group and two (3%) in the placebo group (p-value not reported) | Patients with early RA who achieved remission while receiving 50 mg of ETN plus MTX had better disease control with 25 mg of ETN plus MTX than MTX alone or placebo. No significant difference was observed in radiographic progression between the three groups |
| Iwamoto 2014155 | Japan | A prospective study of patients on bDMARDs to assess whether or not US assessment of synovitis predicts relapse after withdrawal of bDMARDs. 42 patients were enrolled who were in clinical remission (i.e. a DAS28 of < 2.6) and who agreed to withdraw treatment. The mean duration of remission was 23 months (range 3–73 months) | Comprehensive US assessment predicted relapse within a short term after discontinuation of bDMARDs |
| Naredo 2014156 | Spain | 77 patients treated with bDMARDs were recruited if they met the following criteria: (1) stable dose of treatment in the previous 12 months, (2) sustained clinical remission based on DAS28 or SDAI in the previous 12 months, (3) ≤ 5 mg/day of prednisone treatment in the previous 6 months and (4) not having needed NSAIDs for > 1 week nor local corticosteroid injections in the previous 6 months. bDMARDs were tapered by increasing the duration between doses or reducing the dose. Tapering failure (assessed at 6 and 13 months) was defined as an increase in the dose and/or disease activity on the DASA28 or SDAI. In total, 23 (29.9%) patients were tapering failures at ≤ 6 months and 35 (45.5%) were tapering failures at 12 months. Significant predictors of failure at 6 months (p ≤ 0.05) were longer duration of RA, a higher number of synthetic DMARDs, a higher DAS28 at baseline, a higher SDAI at baseline, a higher global index for Doppler synovitis (DSI) at baseline and a DSI > 0 at baseline | The results suggested that the presence and grade of Doppler-detected synovitis may predict biologic therapy tapering failure in RA patients in sustained clinical remission |
| Smolen 2014182 | Global (161 sites worldwide) | Patients with low disease activity [i.e. a DAS28 of < 3.2 at weeks 22 and 26 from the Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab (OPTIMA trial)194] were randomised to either ADA continuation or withdrawal for a period of 52 weeks or, if not on ADA, were maintained on MTX monotherapy. A total of 207 patients on ADA were rerandomised (105 continued to take ADA); 112 patients continued on MTX monotherapy. In total, 95/103 (92%) patients continuing ADA treatment maintained a DAS28 of < 3.2 compared with 75/90 (83%) whose treatment was withdrawn (p = 0.0569); 90/103 (87%) continuing ADA had a DAS of < 2.6 compared with 62/90 (69%) whose treatment was withdrawn (p = 0.017) | Outcomes were similar regardless of whether ADA was continued or withdrawn in patients who initially responded to ADA |
| Aguilar-Lozano 2013195 | Mexico | A prospective cohort study of patients (n = 45) in remission (i.e. a DAS28 of ≤ 2.6) with no swollen joints following cessation of TCZ. In total, 20 patients maintained remission during the 12-month follow-up period. Of the 25 who relapsed, 14 (56%) did so within 3 months of the last dose of TCZ | Long-term clinical remission is possible in a number of patients with RA after the suspension of TCZ |
| Detert 2013196 | Germany | Prospective trial comparing the use of ADA with placebo for 24 weeks after which both groups received MTX alone for 24 weeks. The trial recruited 172 people with active early (≤ 12 months) RA. At week 48, there was no statistically significant difference in DAS28 score between the groups (p = 0.41), although changes in radiographic progression significantly favoured the ADA group | A greater, and significant, reduction in radiographic progression was seen in the ADA arm, but this was not the case for DAS28 |
| Holroyd 2013163 | England | A prospective study of people in clinical and US remission (i.e. a DAS28 of < 2.6 and PDUS = 0) who had their dose of bDMARDs reduced by one-third. In total, 56 of 321 patients met the eligibility criteria. Of these, 42 (75%) remained on the tapered dose for a mean of 8.7 months. Fourteen patients (25%) flared and returned to the full dosages at a mean of 5.9 months | Using US alongside clinical assessment may increase the likelihood of selecting patients who could successfully reduce the dose of bDMARDs while maintaining clinical and US remission |
| Nishimoto 201327 | Japan | Tis study examined the possibility of drug-free remission induced by TCZ monotherapy. In total, 187 patients were enrolled who had achieved a DAS28 of < 3.2. Loss of efficacy was defined as either a DAS28 of > 3.2 at two consecutive visits or initiation of additional treatments on patient request. A DAS28 of < 3.2 was maintained in 65 patients at 24 weeks after discontinuation and 24 patients at week 52; 19 patients (10%) were drug free for 52 weeks, with 17 patients meeting the criteria for remission (i.e. a DAS28 of < 2.6). Low serum interleukin 6 (IL-6) and normalisation of matrix metalloproteinase 3 (MMP-3) levels at cessation of TCZ monotherapy were identified as independent predictive markers for longer duration of low disease activity | TCZ monotherapy may induce bDMARD-free remission or low disease activity without concomitant use of synthetic DMARDs |
| Smolen 2013183 | Global (80 sites in Europe, Latin America, Asia and Australia) | RCT (PRESERVE) of adult patients with an initial DAS28 of > 3.2 and ≤ 5.1 that had been reduced to low disease activity (mean DAS28 of ≤ 3.2 between weeks 12 and 36 and a DAS28 of ≤ 3.2 at week 36). Patients were assigned to 50 mg of ETN (n = 202), 25 mg of ETN (n = 202) or placebo (n = 200). In total, 166/201 (83%) of those receiving 50 mg of ETN had low disease activity compared with 84/197 (43%) of those receiving placebo and 159/201 (79%) of those receiving 25 mg of ETN. Significantly more patients receiving either dose of ETN had low disease activity than patients receiving placebo (p < 0.0001). The authors reported that there was no significant difference between the two ETN doses (p < 0.379) | Conventional or reduced doses of ETN maintain low disease activity more effectively than placebo |
| Smolen 2014197 | Global (31 European sites) | 52-week double-blind RCT (CERTAIN) including a 24-week treatment period and 28-week follow-up period in patients with low to moderate disease activity and stopping therapy in patients in sustained remission. Patients had a CDAI of > 6 and ≤ 16, two or more tender joints, two or more swollen joints and either a ESR of ≥ 28 mm/hour or CRP level of > 10 mg/l at screening and baseline. In total, 194 patients were randomised to placebo (n = 98) or CTZ (n = 96). A total of 20 patients receiving CTZ and 7 patients receiving placebo achieved remission, defined as a CDAI of ≤ 2.8 at both weeks 20 and 24, and had treatment withdrawn. Only 3/17 CTZ and 2/6 placebo patients maintained remission until week 52, although reinstitution of CTZ induced a renewed improvement. Retreatment with placebo did not occur | The data suggest that CTZ cannot be withdrawn in patients achieving remission |
| Chatzidionysiou 2012198 | Sweden | Randomised controlled open-label pilot study evaluating whether or not remission can be sustained after cessation of ADA in patients with a DAS28 of < 2.6 for ≥ 3 months (n = 31). Remission was rarely maintained in patients who discontinued ADA. The proportion with sustained remission was significantly lower than among those continuing on ADA | ADA discontinuation may be feasible only in a minority of patients with RA in stable clinical remission |
| Haragai 2012199 | Japan | A retrospective study to assess the cessation of ADA monotherapy in patients with low disease activity (i.e. a DAS28 of < 2.7). In total, 24 patients continued ADA treatment, with 22 patients ceasing treatment. Of these, 14/22 patients did not restart bDMARDs, with 4/22 maintaining low disease activity for 52 weeks | Some RA patients who have achieved low disease activity can discontinue ADA without increasing disease activity. This should be confirmed in a prospective, randomised study |
| Kaine 2012200 | USA | A prospective study (n = 167) to assess the temporary interruption of ABT treatment. After a 12-week introduction period, 120 patients were randomised to either placebo or continuation of ABT treatment. Following this, 79 patients on placebo were reintroduced to ABT. A non-significant increase in immunogenicity (p = 0.119) was observed following ABT withdrawal. Safety was comparable across treatment schedules | A stop–start schedule of ABT was well tolerated with little impact on safety or efficacy |
| van der Maas 2012201 | The Netherlands | An observational cohort study assessing the feasibility of down-titrating or discontinuing IFX. IFX was down-titrated by 3 mg/kg (25% of the original dose) every 8–12 weeks until discontinuation or a flare in patients with a DAS28 of < 3.2 for ≥ 6 months. Flares (i.e. a DAS28 of > 3.2) were treated with the last effective dose of IFX. IFX could be discontinued in 16% of the cohort and down-titrated in 45%. There was no statistically significant difference in patients’ quality of life (p < 0.152) after down-titrating and mean costs per patient were reduced by €3474 | In the majority of patients with a stable DAS28 of < 3.2 and stable IFX treatment, IFX can be down-titrated or discontinued without influencing patients’ quality of life, generating a considerable cost saving |
| Klarenbeek 2011202 | The Netherlands | To determine the relapse rate and predictors of relapse in patients in sustained clinical remission following the withdrawal of treatment. In total, 115 patients in the BeSt study203 achieved a DAS28 of < 1.6 for > 6 months and all treatment (including conventional DMARDs) was discontinued. A total of 59/115 patients maintained drug-free remission for a median duration of 23 months; 53/115 restarted treatment as the DAS28 reached > 1.6 (median duration to restarting treatment 23 months); and 3 patients were lost to follow-up. In total, 39/53 people who restarted treatment attained remission within 3–6 months of restarting treatment | Approximately 25% of patients with RA achieved drug-free remission; 46% restarted DMARD monotherapy because of a relapse, the majority of whom again achieved clinical remission without showing radiological progression during the relapse |
| van den Broek 2011204 | The Netherlands | Post hoc analyses (n = 104) of the BeSt study203 to identify predictive factors for maintaining low disease activity (i.e. a DAS28 of ≤ 2.4) for 6 months without IFX treatment. Low disease activity was maintained in 52% of patients, with a higher success rate in those initially treated with IFX. Of those who flared (48%), 84% regained low disease activity with IFX treatment. Predictive factors for requiring IFX treatment were smoking, long IFX treatment duration (≥ 18 months) and shared epitope | Maintaining IFX-free low disease activity was successful in the majority of patients and, of those who did flare, the large majority regained low disease activity following treatment with IFX |
| Bejarano 2010205 | England | Prospective cohort study (n = 20) of patients with poor prognosis of RA with < 1 year of symptoms. Patients were randomised to receive IFX or placebo for 1 year; these were then removed and patients were treated with MTX monotherapy alone in accordance with standard clinical care. At 8 years, disease activity data were collected. At follow-up, four patients in the IFX group were in remission compared with none in the placebo group. One patient in the IFX group achieved drug-free remission. Median DAS28 was significantly lower in the IFX group than in the placebo group (2.7 vs. 4.3; p = 0.02) | A remission induction regime with IFX for 1 year in early RA can improve long-term clinical outcomes |
| Saleem 2010206 | England | Prospective cohort study (n = 47) of patients in remission attempting to define markers that are predictive of sustained remission following cessation of bDMARD treatment. Of the 47 patients, 27 had received initial treatment and 20 delayed treatment. Two years after treatment withdrawal, 59% in the initial treatment group and 15% in the delayed treatment group had sustained remission. In the initial treatment group, secondary analyses showed that shorter symptom duration was the only clinical predictor of successful treatment withdrawal. Several immunological parameters were significantly (p < 0.05) associated with sustained remission | In patients in remission, short duration of untreated symptoms and immunological parameters are associated with successful withdrawal of bDMARDs |
| Tanaka 2010184 | Japan | Prospective cohort study (n = 114) analysing the possibility of discontinuing IFX after achieving low disease activity (i.e. a DAS28 of < 3.2). When low disease activity was maintained for > 24 weeks, IFX was withdrawn (n = 102). A total of 56 patients (55%) maintained low disease activity at 1 year post IFX treatment; 46 patients were not classed as successful, with IFX being restarted or having a DAS28 of > 3.2 at 1 year | After achieving low disease activity through IFX treatment, the majority of patients remained in this state without IFX treatment |
| Brocq 2009207 | France | A prospective cohort study of 304 patients taking a bDMARD for RA. Those who achieved remission (i.e. a DAS28 of < 2.6 for at least 6 months without NSAIDs or prednisolone) (n = 21) had their bDMARD removed; this was reinstated following a relapse (i.e. a DAS28 of > 3.2) | A relapse occurred within 12 months in 75% of patients who had ceased bDMARD treatment. Relapsing patients responded well to the resumption of the same bDMARD |
| van der Kooij 2009208 | The Netherlands | An analysis of patients from the BeSt study203 with a sustained DAS28 of < 1.6 for at least 6 consecutive months who discontinued treatment at year 3. Of the entire cohort, based on the four treatment strategies in the BeSt study, the percentage of patients in drug-free remission at the end of year 4 ranged from 8% to 18%, with a non-significant (p = 0.14) difference across strategies | In patients with recent-onset RA, drug-free remission was achieved in up to 18% given DAS-driven treatment |
| Nawata 2008209 | Japan | A prospective cohort of 172 patients with active RA were provided with IFX treatment. After induction and maintenance of clinical remission (i.e. a DAS28 of > 2.6), tapering of corticosteroids and/or NSAIDs was attempted. If clinical remission was maintained for > 24 weeks, discontinuation of IFX was considered. In total, 52 patients met the remission criteria and nine patients discontinued IFX and maintained remission for a mean of 14 months without recurrence. The duration of disease was shorter and the points from Steinbrocker’s stage classification were significantly lower in the IFX-discontinued group than in the IFX-continued group | The study indicates that DAS28 could be a good indicator of whether or not to perform a strategic reduction of IFX. The findings imply that early intervention with IFX appears to be advantageous for achieving clinical remission and for discontinuing IFX after clinical remission |
| van den Bilj 2007210 | The Netherlands | Analysis from the BeSt study203 of 120 patients with early RA receiving IFX treatment. Sixty-seven patients had persistent (> 6 months) low disease activity (i.e. a DAS28 of ≤ 2.4) and had IFX tapered and finally withdrawn at a median time of 10 months. Ten patients had IFX withdrawn but experienced a disease flare and resumed IFX treatment after a median of 4 months. This represents a bDMARD-free maintenance success rate of 67/120 (56%) during a 2-year follow-up period | 56% of patients with early RA, initially treated with IFX, could discontinue IFX after achieving a DAS28 of < 2.4 |
| Quinn 2005211 | England | A 12-month double-blind study (n = 20) attempting to induce remission with or without IFX treatment in those with symptoms for < 12 months. Of those on IFX (n = 10) who responded to treatment (n = 9), seven maintained their DAS28, whereas two had increases in DAS28 in the following 52 weeks after withdrawal of treatment. No patient had a functional deterioration as measured by the HAQ after withdrawal of IFX treatment | Functional and quality of life benefits were sustained a year following the withdrawal of IFX treatment |
| Buch 2004212 | England | Prospective 2-year extension of the Anti-TNF Therapy in RA with Concomitant Therapy (ATTRACT) study213 (n = 17). All patients had a flare, with 15 choosing retreatment with IFX, although one stopped treatment because of attempted pregnancy. After retreatment the ACR response was comparable in 12 out of 14 patients and worse in 2 out of 14 patients. No adverse reactions were observed | IFX treatment can be restarted after an interval of several months without any observable problems |
The potential advantages of using ultrasound for monitoring synovitis
The possibility of a reduced bDMARD burden was not considered by the manufacturers, the Assessment Group or the NICE Appraisal Committee in the recent evaluation of bDMARDs,40 indicating that tapering of treatment in patients who have responded well may not reflect routine clinical practice. A relevant question is, ‘Why is this the case?’. If it is because clinicians are unwilling to risk treatment reductions in patients whose disease may worsen if dosing is amended, then monitoring synovitis in patients such that treatment could be reinstated, or the dosage amended, when disease activity reoccurred could have the potential to be a cost-saving strategy and also one that could be beneficial to patients. In a survey sent to members of the BSR (see Appendix 1), 27 out of the 31 (87%) respondents replied that US is used to make decisions regarding RA therapy, with the majority stating that US was used to make decisions whether to start or stop medication or to taper or increase dosages. There was no consensus among the respondents about how often US was used in RA patients, although it appeared that this would likely be dependent on patients’ symptoms and the uncertainty around disease activity; a minority of respondents indicated that US would be routinely undertaken on patients at visits to the clinic. Although the data provided by those BSR members responding to the survey are helpful, it is not clear to what extent selection bias could have influenced the results. It cannot be ruled out that the majority of those who chose to respond were advocates of the use of US for monitoring synovitis.
Cost-effectiveness analyses undertaken
The economic evaluation compared the use of US as an adjunct to CE and the use of CE only in determining the most appropriate treatment in RA. This analysis was performed using four scenarios: (1) patients who have been perceived to be clinically stable on a bDMARD and for whom the clinician may consider dose reductions; (2) patients who have been perceived to be clinically stable on a cDMARD and for whom the clinician may consider dose reductions; (3) patients who appear to have disease progression despite bDMARD treatment and for whom the clinician is contemplating amending treatment; and (4) patients who appear to have disease progression despite cDMARD treatment and for whom the clinician is contemplating amending treatment. It should be noted that the groups of patients within these four scenarios do not equate to the entire RA population. If a clinician did not believe that escalation or tapering of treatment was warranted, it was assumed that patients would not have synovitis measured with US.
All analyses were undertaken using a 1-year time horizon assuming that the decisions faced by clinicians would be recurrent, that is, that patients would continue to be monitored to ensure that the initial treatment decision remained appropriate. Given the short time horizon of the analysis, the results have not been discounted. It was assumed that if a strategy of monitoring synovitis using US was employed then this would be consistently undertaken in all patients who met the criteria of the four scenarios in all future years. In all analyses, incidental benefits and incidental costs, for example those related to detecting a condition that could have been mistaken for a worsening of RA, have been excluded from the simple model.
In the base-case analysis it was assumed that, on average, four US scans would be undertaken per year, at a cost of £226.62 per year; this was adjusted in sensitivity analyses. This frequency of monitoring in those patients for whom a clinician was considering a change of treatment or in patients following a change of treatment was considered prudent to ensure that any increase in synovitis was detected relatively early. Were four US scans per year adopted in clinical practice this could impact on sonographer capacity; if additional resources were required then the cost savings required for monitoring of synovitis with US to be cost neutral would increase.
The analyses presented detail the reductions in average drug use and the number of serious infections that would need to be avoided for US plus CE to have an incremental cost-effectiveness ratio (ICER) of £20,000 per quality-adjusted life-year (QALY) gained and £30,000 per QALY gained214 relative to CE alone. These two willingness-to-pay thresholds have been selected as they are reported in NICE’s Guide to the Methods of Technology Appraisal 2013214 and were current at the time of report writing (2016).
Serious infections were considered as potential adverse events of treatment. It was estimated that a serious infection would be associated with a cost of £1479 and a QALY loss of 0.012. 23 Although serious infections are more typically associated with bDMARDs, the possibility of this adverse event has also been included for patients on cDMARDs. The net monetary benefit of an avoided serious infection was calculated assuming both a £20,000 per QALY gained threshold and a £30,000 per QALY gained threshold. The formula for this is:
Thus, the net monetary benefit of an avoided serious infection using a £20,000 threshold is £1718 [£1479 + (0.012 × £20,000)] and using a £30,000 threshold is £1838 [£1479 + (0.012 × £30,000)].
The threshold levels for reductions in average drug use and the number of serious infections that need to be avoided are not intended to imply that monitoring synovitis with US is or is not cost-effective, rather that these are the levels at which the use of US becomes cost neutral. These threshold values may or may not be achieved in reality and it is conceivable that there would not be a saving if the use of US led to overtreatment.
It is acknowledged that the decision to amend treatment is likely to be multifactorial. However, there is not sufficient certainty in these factors, and potential heterogeneity of their implementation to allow a meaningful analysis to be conducted. We have therefore chosen to subsume all factors into threshold metrics (either in reduction of drug use or in reduction in serious infection) that would need to be achieved through the extra information supplied by the use of US as an adjunct to CE.
Analyses undertaken when dose tapering is being considered
For the modelling of strategies in which a clinician is contemplating a dose reduction, a simplistic approach has been taken using threshold analyses. This is in contrast to the protocol,215 in which it was anticipated that the model constructed for the recent NICE appraisal of bDMARDs and documented in Stevenson et al. 23 would be used. Given the uncertainty in the evidence base, it was deemed more useful to provide indicative results that focus on the key parameters related to US use, rather than to provide results of potentially spurious accuracy from a model with a much larger number of parameters, which could obscure the impact on the decision problem that we have been tasked to address.
Analyses undertaken when a change in treatment is being considered
The potential consequences associated with changing treatment are more complicated than those associated with tapering treatments as patients may have a decrease in HAQ score if they respond to the new intervention. More aggressive treatment could also potentially stop disease progression. However, as with the analyses relating to dose tapering, a simplistic approach has been undertaken to provide an indicative level of the proportion of patients not escalating treatment, because of the added information provided by a US scan, that would be result in an ICER of £20,000 or £30,000 per QALY gained. The costs of DMARDs and the impacts of a serious infection have been assumed to be the same as in the tapering analyses. Incidental benefits, for example detecting a condition that could have been mistaken for a worsening of RA, have been excluded from the model.
Costs assumed within the model
As detailed in Chapter 3 (see Anticipated costs associated with the intervention), we calculated that the use of US to monitor synovitis would be associated with a cost of £56.66. We assumed that all US appointments were outpatient appointments, that contrast was not used and that two-thirds of appointments took < 20 minutes and the remainder took ≥ 20 minutes.
For simplicity, it was assumed that clinical assessment was associated with no cost; as clinical assessment is included in both treatment strategies, and there is unlikely to be a mortality difference between strategies, this was believed not to influence the results.
The assumed cost of a bDMARD was sourced from a recent Health Technology Assessment report. 23 Several bDMARDs are subject to a commercial-in-confidence patient access scheme; however, for the interventions for which an estimated cost per year can be reported, the prices were commonly around £9200 per year. This price was assumed in our analyses. It should be noted, however, that biosimilar drugs have entered the market for IFX, ADA, RTX and ETN and thus, potentially, drug acquisition costs may decrease in future years. An additional £1608 per year was assumed for monitoring and administration for all regimens.
The assumed cost of cDMARD treatment was dependent on the actual regimen used. The most intensive course of treatment that would be used was assumed to replicate that in the study by Stevenson et al. :23 oral MTX (20 mg weekly), HCQ (6.5 mg/kg daily), SSZ (3 g daily) and oral prednisolone (7.5 mg daily). This had an estimated cost of £1826 per year when monitoring and administration costs were included and £218 per year when these costs were excluded.
In a sensitivity analysis the impact of MTX being given subcutaneously rather than orally was explored, as this is the formulation recommended for people with severe active RA, with oral MTX recommended for people with moderate to severe RA. 216 The annual cost of two 10-mg tablets of MTX per week is £39.28 whereas the annual cost of a weekly 20-mg solution for injection is £837.99. 216 The costs of intensive cDMARDs and MTX were both increased by £798.71 (£837.99 – £39.28) in the sensitivity analysis.
The costs of serious adverse advents are included; these were estimated to be £1479. 23
Utilities assumed within the model
The only utility implication considered within the model was that associated with serious adverse events. These were assumed to be associated with a QALY loss of 0.012. 23
Summarised model inputs
Table 14 provides the model parameters used in the base-case analysis.
| Parameter | Value | Reference |
|---|---|---|
| Cost of a US scan | £56.66 | Assumption based on NHS Reference Costs 2014 to 201573 |
| Number of US scans per year per patient | 4 | Assumption |
| Cost of US scans per year per patient | £226.62 | Calculated |
| Annual cost of bDMARD treatmenta | £9200 | Stevenson et al.23 |
| Annual cost of intensive cDMARD treatmenta,b | £218.17 | BNF216 |
| Annual cost of oral MTX treatmenta | £39.28 | BNF216 |
| Annual cost of subcutaneous MTX treatmenta | £837.99 | BNF216 |
| Cost of a serious infection | £1479 | Stevenson et al.23 |
| QALY loss associated with a serious infection | 0.012 | Stevenson et al.23 |
| Net monetary benefit of a serious infection avoided at a threshold of £20,000 per QALY gained | £1718 | Calculated |
| Net monetary benefit of a serious infection avoided at a threshold of £20,000 per QALY gained | £1838 | Calculated |
An illustrative example of how thresholds were calculated
An example of the calculation of a threshold is provided, assuming a cost per QALY gained threshold of £20,000 and a reduction in the costs of bDMARDs of 1%. A saving of £92 (£9200 × 1%) would be made on bDMARD acquisition costs, which would reduce the net costs of a US strategy to £134.62 (£226.62 – £92). Given a net monetary benefit of £1718 per serious infection avoided, 0.078 (£134.62/£1718) serious infections would need to be avoided to achieve a cost per QALY gained of £20,000.
Results
The cost-effectiveness of ultrasound monitoring in patients who have been stable on biological disease-modifying anti-rheumatic drugs and for whom the clinician is contemplating reducing the dose of biological disease-modifying anti-rheumatic drug
The average reduction in bDMARD use that would be required for a strategy of US monitoring to be cost neutral was calculated. This was 2.46% (£226.62/£9200). The levels of drug reduction and serious infections avoided at which US is at the threshold of cost-effectiveness are shown in Figure 2.
FIGURE 2.
The average levels of bDMARD drug reduction and serious infections avoided that result in a cost per QALY gained of £20,000 and £30,000 for the use of US in monitoring synovitis.
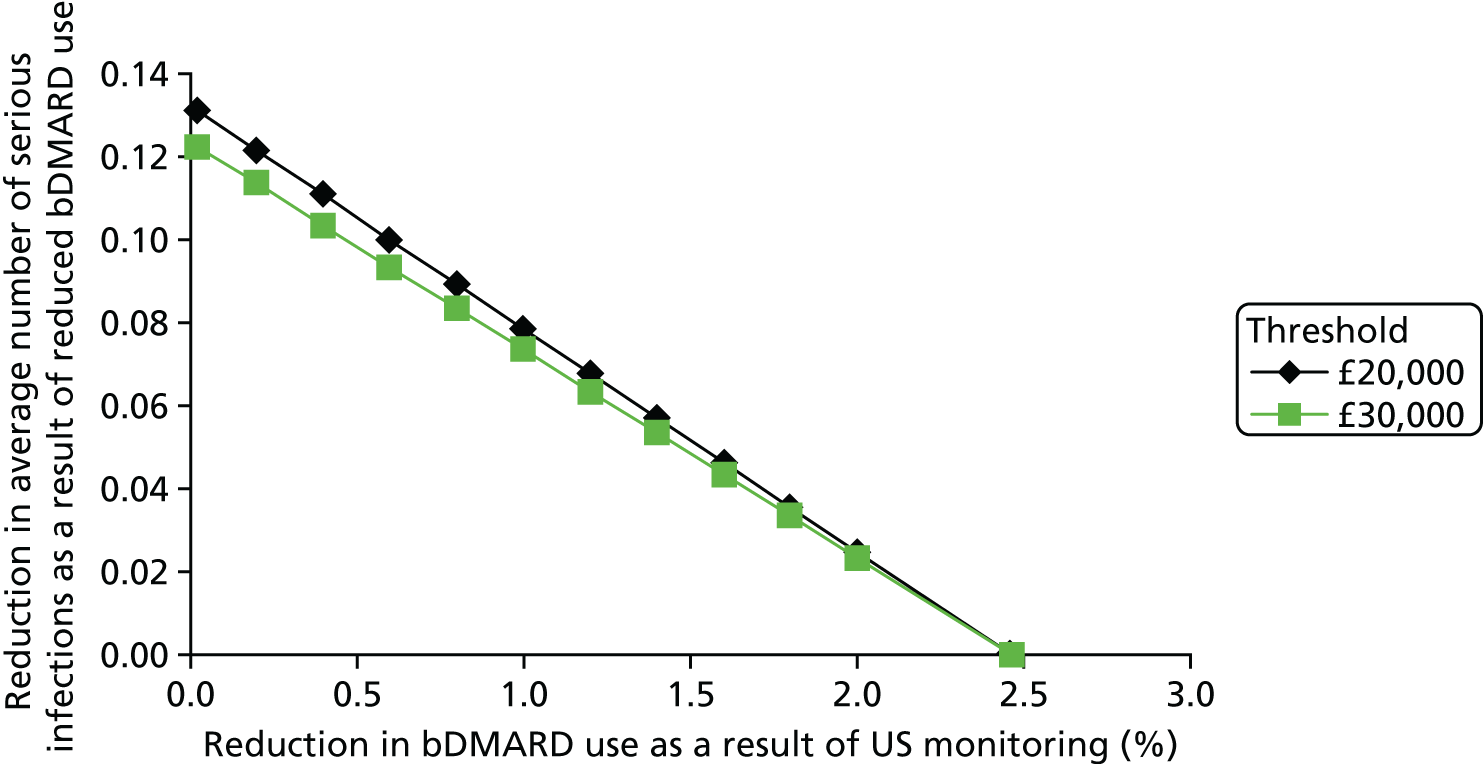
The cost-effectiveness of ultrasound monitoring in patients who have been stable on conventional disease-modifying anti-rheumatic drugs and for whom the clinician is contemplating reducing the dose of conventional disease-modifying anti-rheumatic drug
The average reduction in cDMARD use that would be required for a strategy of US monitoring to be cost neutral was calculated. It was not possible to recoup the assumed costs of US (£227) with reduced use of either intensive cDMARDs (£218 per annum) or MTX (£39 per annum). However, the levels of drug reduction and serious infections avoided at which US is at the threshold of cost-effectiveness are shown in Figure 3.
FIGURE 3.
The average levels of intensive cDMARD drug reduction and serious infections avoided that result in a cost per QALY gained of £20,000 and £30,000 for the use of US in monitoring synovitis.

The cost-effectiveness of ultrasound monitoring in patients who appear to have disease progression despite biological disease-modifying anti-rheumatic drug treatment and for whom the clinician is contemplating amending treatment
This analysis was not formally conducted as there was insufficient evidence to provide any robust assessment. The likely impact of a change in treatment for a patient on bDMARDs will vary depending on the current treatment and on whether or not the disease was progressing.
At the time of writing, NICE guidance41 recommended that patients progressing from first-line bDMARDs should receive RTX in combination with MTX as it is cheaper than other bDMARDs and has a similar efficacy. 23 As such, the immediate impact of a patient moving to RTX would be a cost saving, regardless of whether or not the disease was progressing and regardless of any US result. However, a potential treatment option has been exhausted, which may have longer-term impacts if the disease had not progressed at the time of the switch to RTX, but began progressing on subsequent treatments. It is unclear to what extent the decision to move to RTX would be influenced by the use of US to monitor synovitis.
In contrast, if a patient was on full-dose TCZ following RTX treatment, at the point that the clinician was contemplating amending treatment, current NICE guidance would allow no further bDMARDs. It is unclear to what extent the knowledge of underlying synovitis would influence a clinician’s decision to amend treatment. The threshold proportion of patients who could be moved onto RTX from their initial bDMARD, at which point the savings in bDMARD acquisition cost would offset the costs of US monitoring, has been calculated. This threshold value, assuming a cost per year of £4900 for RTX, would be £226.62/(£9200 – £4900), which is 5.3%. The components of this formula are the annual costs of US monitoring and the annual costs associated with bDMARDs and RTX.
If a patient was on a reduced-dose bDMARD because of previous tapering then the use of US to decide whether or not to increase the dose is easier to model, although the answer would be dependent on the assumed increase in dose. It has earlier been shown that the assumed costs of US per year equate to 2.46% of the annual costs of bDMARDs. The proportion of patients who would not need an increase in dose because of the information provided by US for a strategy of monitoring with US to be cost neutral can be calculated as £226.62/[assumed increase in bDMARD dose (as a percentage of the full dose) × £9200].
Thus, if a dose increase of 33% of the full dose was contemplated, the threshold proportion of patients who do not need to be treated would be £226.62/(33% × £9200), which is equal to 7.4%.
The cost-effectiveness of ultrasound monitoring in patients who appear to have disease progression despite conventional disease-modifying anti-rheumatic drug treatment and for whom the clinician is contemplating amending treatment
This analysis has again been divided into those patients on intensive cDMARDs and those on MTX alone. It has been assumed that those on MTX alone would progress to an intensive cDMARD strategy, whereas those on intensive cDMARDs would move to a bDMARD. The thresholds for patients on intensive cDMARDs are shown in Figure 4 and the thresholds for those on MTX alone are shown in Figure 5. Assuming no changes in serious infections the threshold value was 2.52% for those not progressing to bDMARDs; the threshold value could not be attained for those not progressing to intensive cDMARDs.
FIGURE 4.
The average levels of reduction in patients moving to bDMARDs and serious infections avoided that result in a cost per QALY gained of £20,000 and £30,000 for the use of US in monitoring synovitis.

FIGURE 5.
The average levels of reduction in patients moving to intensive cDMARDs and serious infections avoided that result in a cost per QALY gained of £20,000 and £30,000 for the use of US in monitoring synovitis.
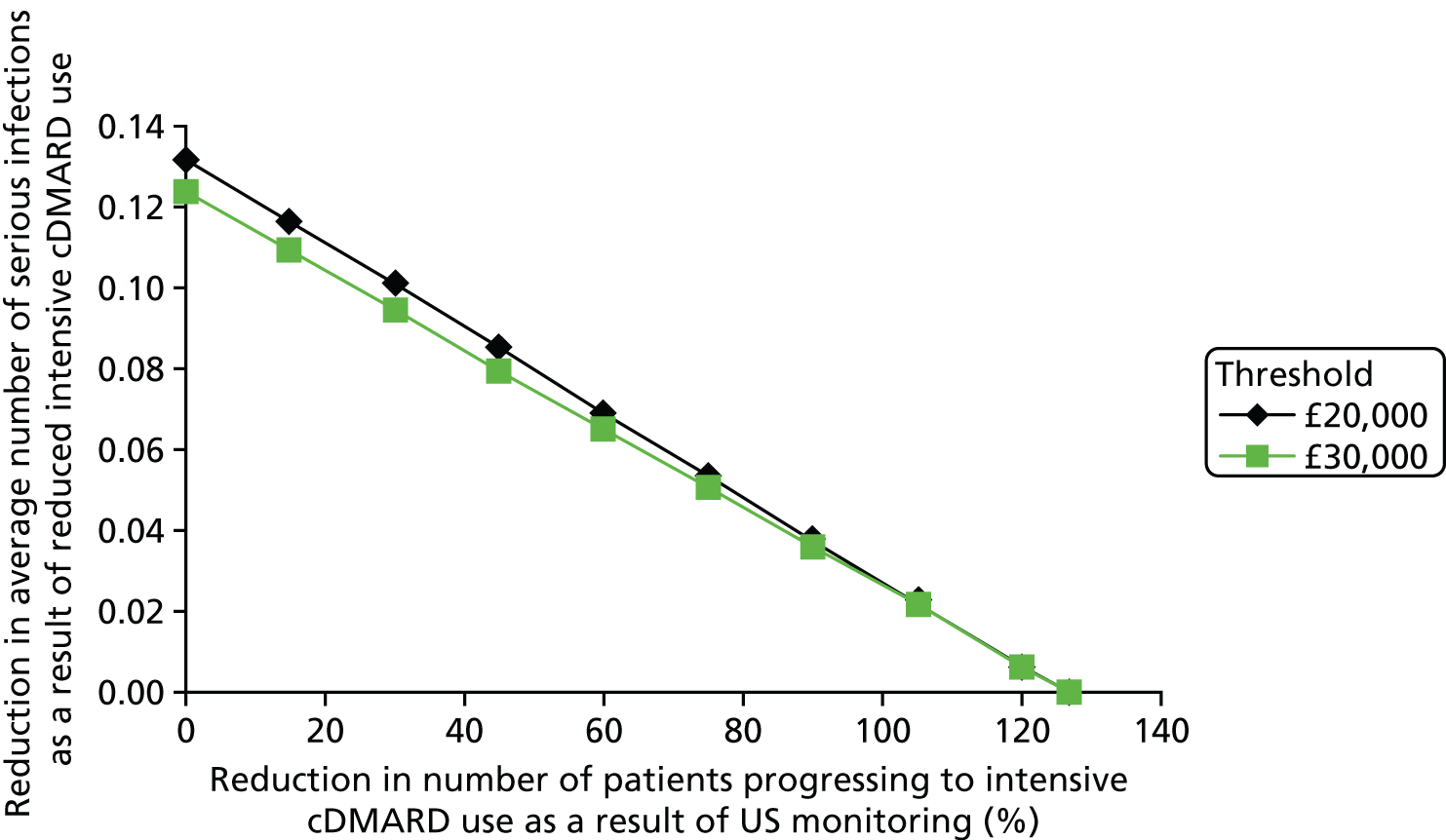
When a patient is on a reduced dose of intensive cDMARDs or MTX because of previous tapering, the threshold for the proportion of patients at which monitoring with US becomes cost neutral can be calculated in a similar manner to that illustrated for patients on bDMARDs who have received tapered medication and the clinician is contemplating increasing the dose.
Sensitivity analysis
Sensitivity analysis was undertaken assuming that the number of US scans required per patient to monitor synovitis ranged from one per year to 12 per year. The cost of a scan was assumed constant at £56.66.
Figure 6 provides data relating to the analyses in which the treatment dose was being tapered. It was calculated that, even if a scan was provided on a monthly basis, this approach would be cost neutral if the average decrease in bDMARD costs was 7.39%. This value was 15.99% for intensive cDMARDs. In contrast, for patients on intensive cDMARDs, even when only one scan was performed per year a reduction in drug costs of 26% would be needed to make US cost neutral.
FIGURE 6.
Sensitivity analysis relating reduction in drug-related costs to the assumed number of US scans undertaken per year.
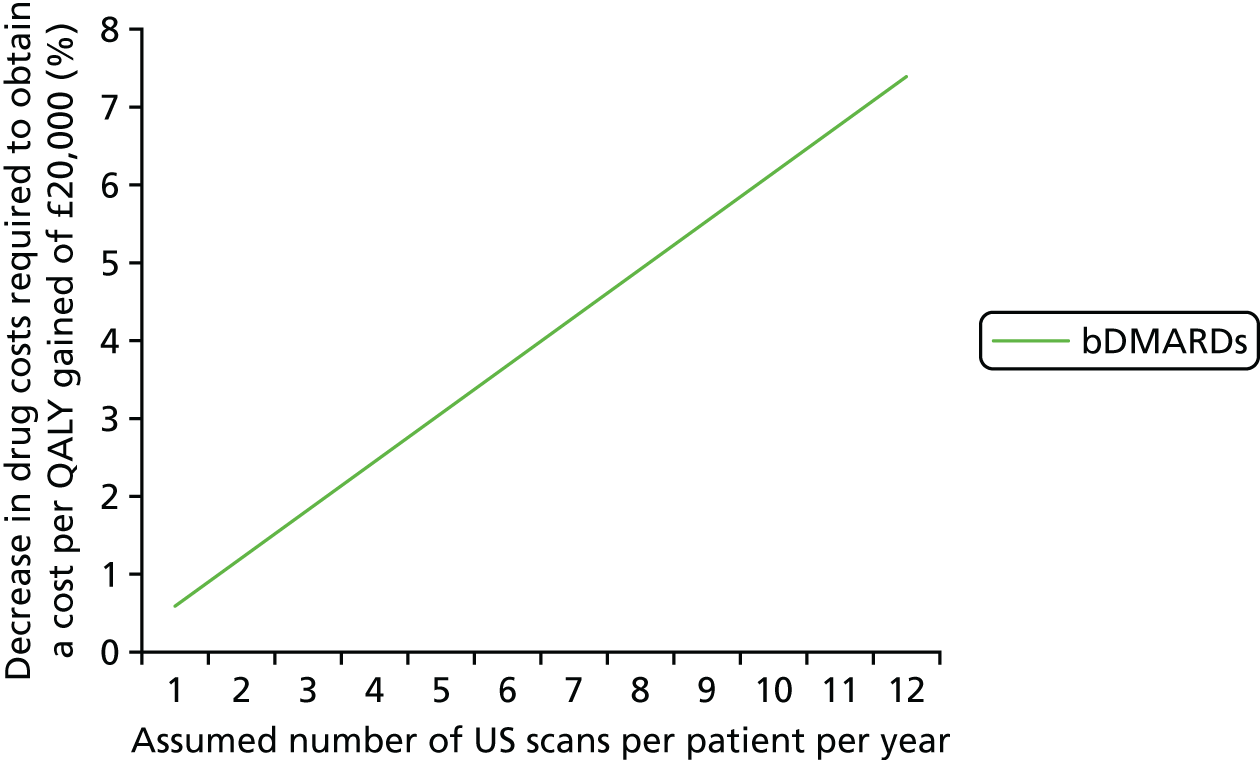
Further sensitivity analysis was undertaken looking at the percentage reduction in the cost of bDMARDs, assuming lower prices than the £9200 assumed in the base case. The motivation for this sensitivity analysis was the fact that biosimilars have entered the market. This sensitivity analysis is shown in Figure 7.
FIGURE 7.
Sensitivity analysis relating percentage reduction in bDMARD acquisition costs to the assumed price of bDMARDs.
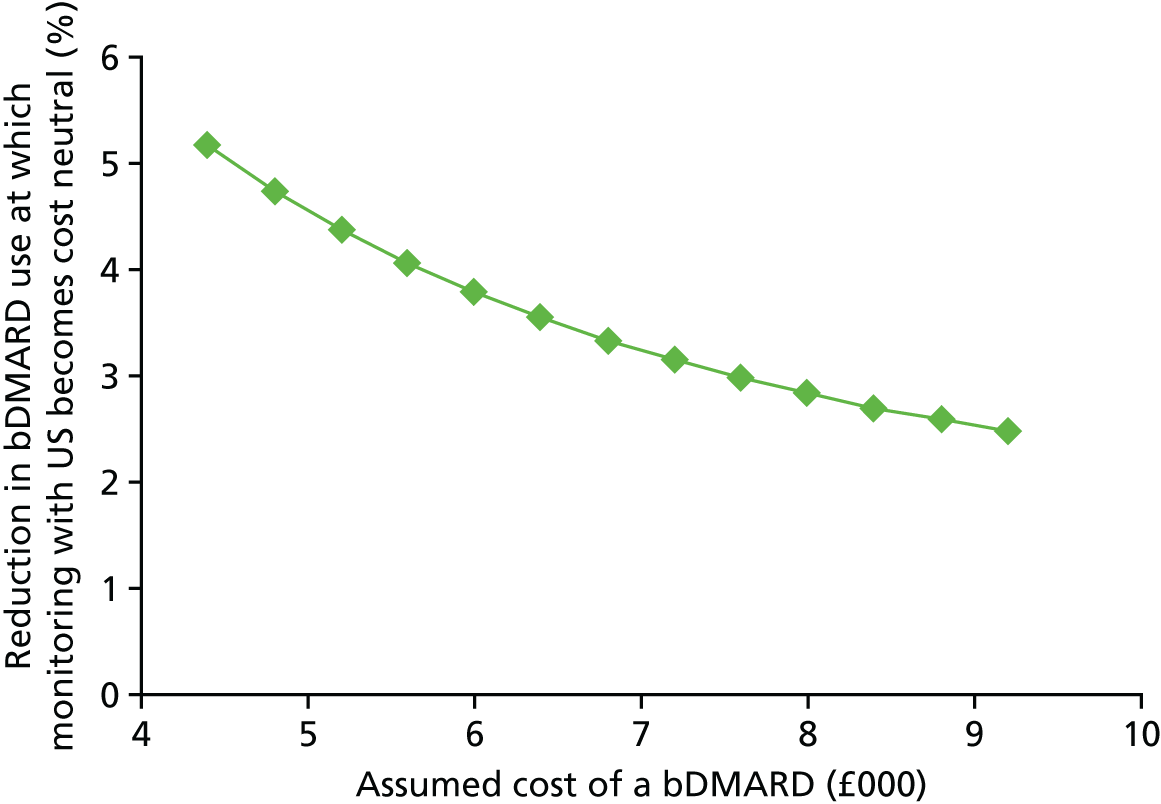
It is seen that, as the price of bDMARDs approaches 50% of the price assumed in the base case, a 5% reduction in bDMARD use would still be sufficient to make monitoring with US cost saving.
Additional sensitivity analysis was conducted to assess the impact of the increased costs associated with use of subcutaneous MTX, which was assumed to cost £798.71 per annum more than oral MTX.
The results for the tapering analyses when assuming costs associated with subcutaneous MTX are provided in Figures 8–11. The reduction in drug costs at which the use of US became cost neutral were 2.27% for patients on bDMARDs, 22.9% for those on intensive cDMARDs and 27.04% for patients on subcutaneous MTX alone.
FIGURE 8.
The average levels of bDMARD drug reduction and serious infections avoided that result in a cost per QALY gained of £20,000 and £30,000 for the use of US in monitoring synovitis assuming the use of subcutaneous MTX.

FIGURE 9.
The average levels of intensive cDMARD drug reduction and serious infections avoided that result in a cost per QALY gained of £20,000 and £30,000 for the use of US in monitoring synovitis assuming the use of subcutaneous MTX.

FIGURE 10.
The average levels of MTX drug reduction and serious infections avoided that result in a cost per QALY gained of £20,000 and £30,000 for the use of US in monitoring synovitis assuming the use of subcutaneous MTX.

FIGURE 11.
Sensitivity analyses relating reduction in drug-related costs to the assumed number of US scans undertaken per year assuming the use of subcutaneous MTX.
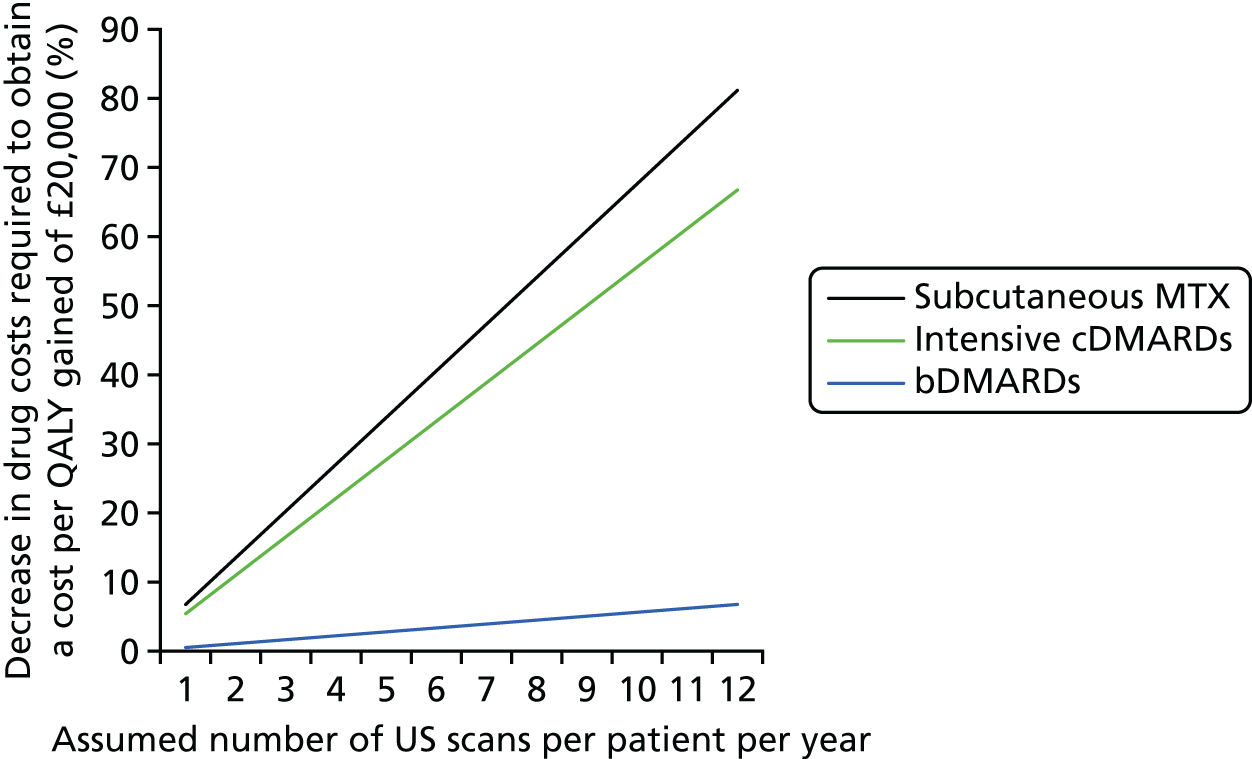
Interpretation of the results
For patients for whom a clinician is contemplating a reduction in bDMARD use because of stable disease, our analyses indicate that only a small average reduction in bDMARD use (2.46%) would be sufficient to offset the costs associated with monitoring patients with US. This value is likely to be higher than in reality should the drug burden be reduced for multiple years and the frequency of monitoring be reduced. Data collated from a review of the evidence for bDMARD tapering (see Table 13) indicate that dose reduction can occur in a sizeable proportion of patients. As such, a 2.46% reduction in bDMARD dose, which is sufficient to offset the costs associated with monitoring patients with US, could be seen to be plausible if the use of US made a clinician more confident to taper bDMARDs. An analysis was undertaken exploring how these results would change if the average price of bDMARDs falls following the introduction of biosimilars. This indicated that, if the cost was to fall by 50% of that assumed in the base case, the reduction in bDMARD costs required for the use of adjunct US to be cost neutral would remain below 5%.
Reducing intensive cDMARD drug costs or MTX costs alone would not be sufficient to offset the costs of US.
For both the bDMARD and cDMARD tapering scenarios there is the potential for fewer serious adverse events if drug intake is reduced. This has been explicitly shown in Figures 2–4. The calculations suggest that 0.13 serious infections saved per person (or approximately one per eight patients) would be sufficient to make US for monitoring synovitis cost-effective. However, this threshold may be academic only: it is not known whether or not such reductions in adverse events would be attainable only with dose reductions that would already have made monitoring with US cost saving.
For patients who appear to undergo disease progression despite bDMARD treatment and for whom the clinician is contemplating amending treatment, the decision problem is more complex. No analyses were undertaken for patients changing treatment because of the uncertain impacts of downstream treatment. However, an example calculation of the threshold value for the number of patients in whom increased treatment was not necessary was provided for patients increasing their dosage subsequent to previous tapering. The threshold value is dependent on the proposed increased dose of the bDMARD and it is uncertain whether or not US is likely to be cost-effective in this group.
The use of US in monitoring synovitis in patients on cDMARDs was modelled as there were clear next-treatment options dependent on the current regimen, namely bDMARDs for those on intensive cDMARDs or intensive cDMARDs for those on MTX alone. The results in this group were not dissimilar from those when tapering was being considered, with a relatively small (approximately 2.5%) reduction in the number of patients progressing to bDMARDs being required to make the use of US cost-effective.
Ultimately, the cost-effectiveness of US for monitoring synovitis will be dependent on how clinicians change their decisions about care given the additional knowledge provided by US. If the results do not feed into treatment strategies, then the use of US would not be cost-effective. However, evidence presented in Table 12 suggests that the use of US to monitor synovitis was fairly prevalent, with a range of 23–88% for studies based in the UK. 89,90,153 Furthermore, it was reported that clinician confidence was significantly improved (p < 0.0005) by the use of US. 152 As such, it is anticipated that the use of US in monitoring synovitis is plausibly cost-effective, particularly when activity identified on US has been shown to be predictive of flares. 142,155,156,163,164
However, the authors note that there remains considerable uncertainty around all decisions because of the lack of robust evidence and that any conclusion should be interpreted with caution.
Analysis was undertaken to assess the impact of the increased acquisition price if subcutaneous MTX were used rather than oral MTX. This reduced the thresholds required for the use of adjunct US to be cost neutral, but there remained considerable uncertainty in the result.
Discussion
The modelling undertaken was purposefully simplistic so that the key interactions between monitoring synovitis with US and decisions influencing treatment could be examined explicitly. The results presented appear to indicate that monitoring synovitis with US could be cost-effective given the evidence on the potential tapering of DMARDs presented in Table 13.
It has been assumed that monitoring with US would allow a clinician to better identify disease worsening when drugs have been tapered. If that is not the case, and irreversible damage has been caused to patients, then the thresholds presented will be favourable to US monitoring, with patients potentially costing more in direct medical costs and having a reduced health-related quality of life. Although evidence on long-term patient outcomes is limited, the majority of comparative studies have reported that there were no differences in outcomes between tapered groups and non-tapered groups and comparative and observational data have shown that patients who flared regained a favourable disease activity state when treatment was reintroduced. Evidence for successful ADA or CTZ tapering is arguably less strong than evidence for successful IFX tapering, although longer-term data are needed to form any conclusions.
If it is possible to reduce the frequency of US monitoring post DMARD tapering, or having established that treatment escalation is not required, then the threshold values presented in this report will be unfavourable to US monitoring.
Data from randomised clinical trials assessing the monitoring of synovitis with US as an adjunct to CE compared with CE alone are needed. The TaSER study78 (see Table 11) was one such RCT and the full results of this study were included in the review described in Chapter 3. Incorporating long-term radiographic data into such a study would allow an insight into whether or not irreversible damage is caused by tapered treatment. Wakefield et al. 24 state that ‘there is now a compelling argument to suggest that the addition of ultrasound assessment . . . is likely to improve the prediction on clinical outcomes’. At the time of writing, ARCTIC trial interim data had been presented in an abstract79 (November 2015) and these data were included in Chapter 3. At the time of the last search, the Targeted Ultrasound in Rheumatoid Arthritis (TURA) study217 was ongoing and had a reported 32% global recruitment rate. As an identified ongoing study, the authors checked (in February 2016) for any publications from the TURA study; however, it was still ongoing. There is no information available on when these studies will report results. The objective of the ARCTIC study was to assess whether or not the information provided by ultrasonography assessment produces better outcomes than when treatment decisions are made solely on clinical and laboratory assessments. The results from these trials are expected to reduce the decision uncertainty regarding whether or not monitoring of synovitis in RA patients with US is clinically effective and cost-effective. Furthermore, two ongoing studies (NCT02064400 and NCT01602302) were identified from a search of ClinicalTrials.gov, although neither is a RCT.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Evidence identified for this report appears to indicate that monitoring synovitis with US could potentially be cost-effective given the possibility of tapering of DMARDs or inappropriate escalation of treatment. There are a number of potential implications for NHS resources. If monitoring of synovitis becomes more widespread (and there are a number of unknowns, for example US use may vary according to the apparent clinical level of disease activity), depending on the number of US scans undertaken per year, there may be a need for more sonographers. Such increased resources would also depend on drivers outside the scope of this report. US has clinical advantages beyond the monitoring of inflammatory arthritis, both for diagnosis (e.g. for a proportion of patients with suspected inflammatory arthritis, enabling earlier intervention) and for guiding the accurate placement of needles for therapeutic injection. All of this has implications for US access in both rheumatology and radiology departments. This also raises issues regarding timely and equal access to US.
Chapter 6 Discussion
Principal findings
Studies included in the systematic review varied in interventions, comparators and outcomes, making it difficult to draw firm conclusions from the available evidence. Fifteen studies provided prognostic data. 55,69,97,98,113,134,135,137–144 Although the study designs were of high quality (prospective cohort), the data reported were correlations and sample sizes ranged from 10 to 453 (in total, data were available from 1523 patients but study heterogeneity precluded meta-analysis). PDUS was significantly associated with radiographic progression at follow-up in all studies in which it was investigated. 55,69,97,113,135,138–140,144 The majority of studies investigating radiographic progression reported that GSUS was a significant predictor; however, this was not the case in all studies of GSUS. Some studies found a significant association between DAS and later radiographic progression. PDUS was significantly associated in the majority of studies with follow-up DAS28,55,98,137 ACR/EULAR remission145 and flare. 142
Nine studies reported data regarding the use of US for monitoring treatment response or strategies. 79,93,95,100,101,139,154–156
Two were RCTs79,154 and the others were prospective cohort studies. 93,95,100,101,139,155,156 Most studies relied on one-off US measurements at baseline; however, the two RCTs employed US serially as part of the treatment strategies. Sample sizes ranged from 24 to 130, with data available from 627 patients in total (although study heterogeneity precluded meta-analysis). These studies found that US predicted treatment continuation and response to treatment. When treatment was discontinued or tapered, PDUS significantly predicted relapse or disease flare and was a better predictor than DAS28. In the RCTs, the addition of PDUS to a DAS28-based treatment strategy did not lead to significant between-group differences in change from baseline in DAS44 and RAMRIS erosion score154 or a significant between-group difference in the primary end point, which consisted of a DAS of < 1.6 and no swollen joints at 16, 20 and 24 months and no progression in vdHSS between 16 and 24 months. 162 However, in the TaSER study, the US plus DAS group had significantly more patients attaining DAS44 remission. Six small studies (sample size 17–109 patients; 321 patients in total) reported observational data on the impact of US on treatment decisions. 89–91,94,152,153 Adding US to CE led to modified treatment decisions and significantly improved clinician confidence in these decisions.
A simple model was constructed. Monitoring with US was not assumed for all patients but rather for those for whom the clinician was contemplating a change (escalation or tapering) of treatment. Screening of all patients could have wider implications for cost-effectiveness and was not considered here. The modelling undertaken indicated that only small proportions of patients tapering treatment, or not escalating treatment, were necessary for the savings associated with reduced treatment to offset the costs associated with monitoring synovitis with US. A systematic review of the literature on tapering of DMARDs indicated that some patients achieving low disease activity could have treatment tapered with no, or little, resultant harm, with the majority of those who flared regaining their previous condition on retreatment. As such, there is clearly potential for the monitoring of synovitis with US to be cost-effective.
Strengths, limitations and uncertainties
Strengths
Relevant literature on prognosis, treatment and cost-effectiveness was reviewed systematically according to a prespecified protocol. The research team was independent and experienced in the methodology and clinical advisors were experienced in the field. A patient advisor provided information on patient experience and contributed to the plain English summary.
The review findings agreed with the findings of previous reviews with regard to diagnosis and prognosis. We also compared US with CE in relation to treatment strategies/decisions, which had not previously been systematically reviewed.
Results from this study are generalisable to UK practice. The studies included in the review used international definitions of RA; most used standardised semiquantitative scoring systems for US measures of synovitis; and clinical measures were also used internationally, for example the DAS28. Treatments assessed included cDMARDs and bDMARDs, as would be relevant to UK practice.
To our knowledge, this is the first study to model the cost-effectiveness of the use of US to monitor synovitis. In addition, we also carried out a systematic review of the literature on the tapering of DMARDs.
Limitations/uncertainties
There is no conclusive gold standard/reference standard for assessing synovitis and so diagnostic accuracy comparisons may be flawed. The heterogeneity of the trials precluded meta-analysis; therefore, no summary estimates of effect were available, which is a limitation of the review. Few studies were identified that compared US with CE with regard to their effect on treatment. Few data were identified regarding the additional influence of US in current practice. The review excluded studies not published in English. The inclusion of conference proceedings may have resulted in the effectiveness of the interventions being underestimated. 218
There is a lack of long-term follow-up of studies of tapering medication. The survey conducted had few responses and was subject to selection bias.
The mathematical model was simplistic, although this was by design to enable us to focus on aspects clearly related to the impact of monitoring synovitis with US in patients with RA. There remains considerable uncertainty around the cost-effectiveness of the use of US for monitoring synovitis.
The systematic review of the literature on DMARD tapering had relatively poor sensitivity, with the majority of papers being identified from reference searching or being known to our clinical advisors. As such, relevant papers may not have been identified, although it is unlikely that these would change the broad conclusions of the review.
Chapter 7 Conclusions
Little evidence matched the decision problem. However, this systematic review found correlational evidence that US-detected synovitis was significantly associated with later radiographic progression. PDUS-detected synovitis also significantly predicted DAS28, ACR/EULAR remission and flare. Studies suggested that US was superior to CE alone in predicting response to treatment tapering or discontinuation. Studies varied with regard to interventions, comparators and outcomes, precluding meta-analysis and meaning that there is uncertainty in the available evidence. With regard to treatment strategies, studies were not all consistent with regard to the statistical significance of the benefit of US. Only two RCTs were identified and the addition of US to DAS-based strategies did not significantly influence the primary end points, although one of these RCTs significantly favoured the addition of US for the outcome of attaining DAS remission. It is uncertain whether the lack of significant influence on the primary end point is due to a genuine lack of improvement caused by the US strategy or other factors such as joint counts and types of US used within studies. Small studies suggested that US was used in treatment decisions and could increase physician confidence in those decisions. A small survey of UK rheumatology units suggested that US is already being used in some units for modifying treatment decisions in RA. Most studies relied on one-off US measurements at baseline; however, the two RCTs employed US serially as part of treatment strategies.
The purposefully simplistic modelling undertaken shows that there is potential for the monitoring of synovitis with US to be cost-effective, although there remains considerable uncertainty around this conclusion.
Implications for service provision
Limited evidence identified for this report appears to indicate that monitoring synovitis with US in those patients for whom clinicians are contemplating a change in treatment has the potential to be cost-effective, given the possibility of tapering of DMARDs or inappropriate escalation of treatment. There are a number of potential implications for NHS resources. If monitoring of synovitis becomes more widespread (and there are a number of unknowns, e.g. US use may vary depending on the apparent clinical level of disease activity), there may be a need for more sonographers, depending on the number of US scans undertaken per year. Such increased resources would also depend on drivers outside the scope of this report. US has clinical advantages beyond the monitoring of inflammatory arthritis, both in diagnosis (e.g. for a proportion of patients with suspected inflammatory arthritis, enabling earlier intervention) and in guiding the accurate placement of needles for therapeutic injection. All of this has implications for US access in both rheumatology and radiology departments. This also raises issues regarding timely and equal access to US.
Suggested research priorities
An important future research recommendation is to evaluate the role of US using methodologically robust studies that prospectively evaluate test accuracy and evaluate the role of US. For any future study thought should be given to validity and efficient design, as discussed by Bossuyt et al. ,219 who emphasise the benefits of retaining solely those patients who have discordant results (in this case, between US and CE) and randomising these patients to amended treatment or usual treatment. A feature of the decision problem in this study, however, is the temporal aspect: it could be that clinicians were inclined to taper treatment but would wait to be further convinced and would have amended treatment earlier if their view was supported by evidence from US.
The heterogeneity of trials precluded meta-analysis; therefore, no summary estimates of effect were available, which is a limitation of the review. Heterogeneity could be limited in future studies by employing protocols that use comparable US joint counts (data would have to be presented for all joints scanned so that comparisons could be made with the currently used joint sets, which vary substantially in the included joint count); comparable scoring systems for GSUS, PDUS and GSUS and PDUS combined, including similar severity scales; comparable US definitions of what constitutes ‘positive’ involvement at both the joint and the patient level (important for trials in which positive/negative findings dictate therapeutic decision-making); and the same clinical outcomes (which has been less of a problem in more recent studies that have reported a range of clinical outcomes).
Further important research questions include:
-
What are the long-term effects on function and joint damage in patients who have had treatment tapered as a result of imaging findings?
-
Which joints should be assessed with US?
-
Can a pre-selected set of joints be used for imaging or does imaging need to be guided by symptoms?
-
How often should US assessment occur and could it be restricted to situations in which a treatment decision is to be made, which should be assessed in serial testing studies?
-
Would the cost-effectiveness of US change if all patients were screened rather than only those patients for whom a change in management was being considered?
Studies including an assessment of costs and health-related quality of life would be useful to inform future health policy decisions.
Given the range of questions that still need answering, it is unlikely that one study could provide all of the answers. Different frequencies of monitoring could be compared within a randomised trial; however, feasibility or trial practice would limit how many different frequencies of monitoring could be assessed within one trial. Similarly, there are many variations of joint counts. The more joints assessed, the more time is needed for assessment. Different joint counts need to be assessed to determine the most efficient way to use US; enough joints need to be measured to warrant treatment decisions being influenced, while ensuring that the time required by sonographers and patients remains manageable. It is uncertain whether or not particular joint counts could serve all patients or whether or not the most affected joints of individual patients would need to be taken into account. Similarly, there are different systems for assessing the severity of US pathology. The OMERACT initiative has developed and validated standardised scoring systems; however, trials would need to assess, for example, whether GSUS or PDUS alone could be used to inform treatment strategies or whether or not a combination of GSUS and PDUS would be more effective.
Acknowledgements
We would like to thank Mike Holmes [School of Health and Related Research (ScHARR), University of Sheffield] for help with sifting the cost-effectiveness searches, Eva Kaltenthaler and Paul Tappenden (ScHARR) for providing comments on the draft report and Gill Rooney (Programme Manager, ScHARR) for providing administrative support and preparing and formatting the report.
We would like to thank the patient advisor who contributed to the report and the National Rheumatoid Arthritis Society (NRAS) who provided names of patients who were willing to be involved in the project. We would like to thank the BSR for providing input into the survey to investigate how US was being used in practice and for publicising it to UK rheumatology units.
This report was commissioned by the National Institute for Health Research (NIHR) HTA programme. The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA programme.
Contributions of authors
Emma Simpson (Research Fellow) led the project and conducted the systematic review.
Emma Hock (Research Fellow) conducted the systematic review.
Matt Stevenson (Professor of Health Technology Assessment) conducted the cost-effectiveness review and the economic modelling.
Ruth Wong (Information Specialist) conducted the literature searching.
Naila Dracup (Information Specialist) conducted the literature searching.
Allan Wailoo (Professor of Health Economics) commented on the modelling.
Philip Conaghan (Professor of Musculoskeletal Medicine) provided clinical advice throughout the project.
Cristina Estrach (Consultant Rheumatologist) provided clinical advice throughout the project.
Christopher Edwards (Professor of Clinical Rheumatology) provided clinical advice throughout the project.
Richard Wakefield (Senior Lecturer/ Honorary Consultant in Rheumatology) provided clinical advice throughout the project.
Data sharing statement
The data from the systematic review can be obtained from the corresponding author on request.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Scott DL, Steer S. The course of established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:943-67. https://doi.org/10.1016/j.berh.2007.05.006.
- Pincus T, Fuchs HA, Callahan LF, Nance EP, Kaye JJ. Early radiographic joint space narrowing and erosion and later malalignment in rheumatoid arthritis: a longitudinal analysis. J Rheumatol 1998;25:636-40.
- Drossaers-Bakker KW, Kroon HM, Zwinderman AH, Breedveld FC, Hazes JM. Radiographic damage of large joints in long-term rheumatoid arthritis and its relation to function. Rheumatology 2000;39:998-1003. https://doi.org/10.1093/rheumatology/39.9.998.
- Allaire S, Wolfe F, Niu J, LaValley MP, Zhang B, Reisine S. Current risk factors for work disability associated with rheumatoid arthritis: recent data from a US national cohort. Arthritis Rheum 2009;61:321-8. https://doi.org/10.1002/art.24281.
- Naz SM, Symmons DP. Mortality in established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:871-83. https://doi.org/10.1016/j.berh.2007.05.003.
- Dadoun S, Zeboulon-Ktorza N, Combescure C, Elhai M, Rozenberg S, Gossec L, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine 2013;80:29-33. https://doi.org/10.1016/j.jbspin.2012.02.005.
- Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology 2009;48:1309-13. https://doi.org/10.1093/rheumatology/kep252.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24. https://doi.org/10.1002/art.1780310302.
- Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults. London: Royal College of Physicians; 2009.
- Combe B, Landewe R, Lukas C, Bolosiu HD, Breedveld F, Dougados M, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:34-45. https://doi.org/10.1136/ard.2005.044354.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8. https://doi.org/10.1136/ard.2010.138461.
- Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800. https://doi.org/10.1093/rheumatology/41.7.793.
- Symmons DP, Barrett EM, Bankhead CR, Scott DG, Silman AJ. The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. Br J Rheumatol 1994;33:735-9. https://doi.org/10.1093/rheumatology/33.8.735.
- Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 2005;4:130-6. https://doi.org/10.1016/j.autrev.2004.09.002.
- Goëb V, Fardellone P, Sibilia J, Ponchel F. Biomarkers in rheumatoid arthritis. Mediators Inflamm 2014;2014. https://doi.org/10.1155/2014/379310.
- Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ). Clin Exp Rheumatol 2005;23:S14-18.
- Prevoo M, Van’t Hof M, Kuper H, Van Leeuwen M, Van De Putte L, Van Riel P. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44-8. https://doi.org/10.1002/art.1780380107.
- Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23:100-8.
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727-35. https://doi.org/10.1002/art.1780380602.
- van Gestel AM, Prevoo ML, van‘t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996;39:34-40. https://doi.org/10.1002/art.1780390105.
- Felson DT. Assessing the efficacy and safety of rheumatic disease treatments: obstacles and proposed solutions. Arthritis Rheum 2003;48:1781-7. https://doi.org/10.1002/art.11087.
- van Gestel AM, Anderson JJ, van Riel PL, Boers M, Haagsma CJ, Rich B, et al. ACR and EULAR improvement criteria have comparable validity in rheumatoid arthritis trials. American College of Rheumatology European League of Associations for Rheumatology. J Rheumatol 1999;26:705-11.
- Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens JW, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying anti-rheumatic drugs and after the failure of conventional disease-modifying anti-rheumatic drugs only: systematic review and economic evaluation. Health Technol Assess 2016;20.
- Wakefield RJ, D’Agostino MA, Naredo E, Buch MH, Iagnocco A, Terslev L, et al. After treat-to-target: can a targeted ultrasound initiative improve RA outcomes?. Postgrad Med J 2012;88:482-6. https://doi.org/10.1136/postgradmedj-2011-201048rep.
- Ben Abdelghani K, Miladi S, Souabni L, Kassab S, Chekili S, Laatar A, et al. Role of ultrasound in assessing remission in rheumatoid arthritis. Diagn Interv Imaging 2015;96:3-10. https://doi.org/10.1016/j.diii.2014.07.006.
- Marks JL, Holroyd CR, Dimitrov BD, Armstrong RD, Calogeras A, Cooper C, et al. Does combined clinical and ultrasound assessment allow selection of individuals with rheumatoid arthritis for sustained reduction of anti-tumor necrosis factor therapy?. Arthritis Care Res (Hoboken) 2015;67:746-53. https://doi.org/10.1002/acr.22552.
- Nishimoto N, Amano K, Hirabayashi Y, Horiuchi T, Ishii T, Iwahashi M, et al. Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) Monotherapy (DREAM) study. Mod Rheumatol 2013;24:17-25. https://doi.org/10.3109/14397595.2013.854079.
- Colebatch AN, Edwards CJ, Østergaard M, van der Heijde D, Balint PV, D’Agostino MA, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis 2013;72:804-14. https://doi.org/10.1136/annrheumdis-2012-203158.
- Aleo E, Barbieri F, Sconfienza L, Zampogna G, Garlaschi G, Cimmino MA. Ultrasound versus low-field magnetic resonance imaging in rheumatic diseases: a systematic literature review. Clin Exp Rheum 2014;32:91-8.
- Taggart A, Benson C, Kane D. Ultrasound in Rheumatology. Reports on the Rheumatic Diseases 2011. www.arthritisresearchuk.org/health-professionals-and-students/reports/reports-archives/∼/media/Files/Education/Topical-Reviews/TR09-Summer-2011.ashx (accessed 25 January 2018).
- van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261-3.
- Genant HK, Jiang Y, Peterfy C, Lu Y, Redei J, Countryman PJ. Assessment of rheumatoid arthritis using a modified scoring method on digitized and original radiographs. Arthritis Rheum 1998;41:1583-90. https://doi.org/10.1002/1529-0131(199809)41:9<1583::AID-ART8>3.0.CO;2-H.
- Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631-7. https://doi.org/10.1136/ard.2009.123919.
- Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3-15. https://doi.org/10.1136/annrheumdis-2015-207524.
- Solomon DH, Bitton A, Katz JN, Radner H, Brown EM, Fraenkel L. Review: Treat to Target in Rheumatoid Arthritis: Fact, Fiction, or Hypothesis? Arthritis Rheumatol 2014;66:775-82. https://doi.org/10.1002/art.38323.
- Wakefield RJ, D’Agostino MA, Naredo E, Buch MH, Iagnocco A, Terslev L, et al. After treat-to-target: can a targeted ultrasound initiative improve RA outcomes?. Ann Rheum Dis 2012;71:799-803. https://doi.org/10.1136/annrheumdis-2011-201048.
- Østergaard M, Møller-Bisgaard S. Rheumatoid arthritis: is imaging needed to define remission in rheumatoid arthritis?. Nat Rev Rheumatol 2014;10:326-8. https://doi.org/10.1038/nrrheum.2014.63.
- Lugmani R, Hennell S, Estrach C, Birrell F, Bosworth A, Davenport G, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Guideline for the Management of Rheumatoid Arthritis (the first 2 years). Rheumatology (Oxford) 2006;45:1167-9. https://doi.org/10.1093/rheumatology/kel215a.
- National Institute for Health and Care Excellence . Rheumatoid Arthritis Overview n.d. https://pathways.nice.org.uk/pathways/rheumatoid-arthritis#path=view%3A/pathways/rheumatoid-arthritis/managing-rheumatoid-arthritis.xml&content=view-index (accessed 31 March 2017).
- Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for Rheumatoid Arthritis Not Previously Treated with DMARDs or After Conventional DMARDs Only Have Failed. London: NICE; 2016.
- Adalimumab, Etanercept, Infliximab, Rituximab and Abatacept for the Treatment of Rheumatoid Arthritis After the Failure of a TNF Inhibitor. London: NICE; 2010.
- Golimumab for the Treatment of Rheumatoid Arthritis After the Failure of Previous Disease-modifying Anti-rheumatic Drugs. London: NICE; 2011.
- Tocilizumab for the Treatment of Rheumatoid Arthritis (Rapid Review of Technology Appraisal Guidance 198). London: NICE; 2012.
- National Institute for Health and Care Excellence (NICE) . Certolizumab Pegol for Treating Rheumatoid Arthritis After Inadequate Response to a TNF-Alpha Inhibitor 2016. www.nice.org.uk/guidance/ta415 (accessed 6 February 2018).
- Mandl P, Naredo E, Wakefield RJ, Conaghan PG, D’Agostino MA. A systematic literature review analysis of ultrasound joint count and scoring systems to assess synovitis in rheumatoid arthritis according to the OMERACT filter. J Rheumatol 2011;38:2055-62. https://doi.org/10.3899/jrheum.110424.
- McAlindon T, Kissin E, Nazarian L, Ranganath V, Prakash S, Taylor M, et al. American College of Rheumatology report on reasonable use of musculoskeletal ultrasonography in rheumatology clinical practice. Arthritis Care Res 2012;64:1625-40. https://doi.org/10.1002/acr.21836.
- Mulherin D, FitzGerald O, Bresnihan B. Clinical improvement and radiological deterioration in rheumatoid arthritis: evidence that the pathogenesis of synovial inflammation and articular erosion may differ. Rheumatology (UK) 1996;35:1263-8. https://doi.org/10.1093/rheumatology/35.12.1263.
- Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 2004;50:36-42. https://doi.org/10.1002/art.11481.
- Cheung PP, Dougados M, Gossec L. Reliability of ultrasonography to detect synovitis in rheumatoid arthritis: a systematic literature review of 35 studies (1,415 patients). Arthritis Care Res 2010;62:323-34. https://doi.org/10.1002/acr.20102.
- Cheung PP, Ruyssen-Witrand A, Gossec L, Paternotte S, Le Bourlout C, Mazieres M, et al. Reliability of patient self-evaluation of swollen and tender joints in rheumatoid arthritis: a comparison study with ultrasonography, physician, and nurse assessments. Arthritis Care Res 2010;62:1112-19. https://doi.org/10.1002/acr.20178.
- Wakefield R. OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 2005;32:2485-7.
- Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen HS, Østergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum 2003;48:955-62. https://doi.org/10.1002/art.10877.
- Scheel AK, Hermann KG, Kahler E, Pasewaldt D, Fritz J, Hamm B, et al. A novel ultrasonographic synovitis scoring system suitable for analyzing finger joint inflammation in rheumatoid arthritis. Arthritis Rheum 2005;52:733-43. https://doi.org/10.1002/art.20939.
- Naredo E, Bonilla G, Gamero F, Uson J, Carmona L, Laffon A. Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis 2005;64:375-81. https://doi.org/10.1136/ard.2004.023929.
- Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum 2007;57:116-24. https://doi.org/10.1002/art.22461.
- Naredo E, Rodriguez M, Campos C, Rodriguez-Heredia JM, Medina JA, Giner E, et al. Validity, reproducibility, and responsiveness of a twelve-joint simplified power Doppler ultrasonographic assessment of joint inflammation in rheumatoid arthritis. Arthritis Rheum 2008;59:515-22. https://doi.org/10.1002/art.23529.
- Karim Z, Wakefield RJ, Quinn M, Conaghan PG, Brown AK, Veale DJ, et al. Validation and reproducibility of ultrasonography in the detection of synovitis in the knee: a comparison with arthroscopy and clinical examination. Arthritis Rheum 2004;50:387-94. https://doi.org/10.1002/art.20054.
- Schmidt WA, Völker L, Zacher J, Schläfke M, Ruhnke M, Gromnica-Ihle E. Colour Doppler ultrasonography to detect pannus in knee joint synovitis. Clin Exp Rheumatol 2000;18:439-44.
- Klauser A, Frauscher F, Schirmer M, Szkudlarek M, Court-Payen, Strandberg C, et al. Value of contrast-enhanced power Doppler ultrasonography (US) of the metacarpophalangeal joints on rheumatoid arthritis. Eur Radiol 2004;14:545-6.
- D’Agostino MA, Wakefield R, Backhaus M, Balint PV, Brwyn GA, Filippucci E, et al. Combined evaluation of influence of sonographer and machine type on the reliability of power Doppler ultrasonography (PDUS) for detecting scoring and scanning synovitis in rheumatoid arthritis (RA) patients: results of an intermachine reliability exercise. Ann Rheum Dis 2008;67.
- Lazaar H, Lhoste-Trouilloud A, Pereira B, Couderc M, Mathieu S, Soubrier M. Does rheumatoid synovitis activity vary during the day? Evaluation with color Doppler sonography. BMC Musculoskelet Disord 2017;18. https://doi.org/10.1186/s12891-017-1450-3.
- D’Agostino MA, Wakefield RJ, Berner-Hammer H, Vittecoq O, Filippou G, Balint P, et al. Value of ultrasonography as a marker of early response to abatacept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results from the APPRAISE study. Ann Rheum Dis 2016;75:1763-9. https://doi.org/10.1136/annrheumdis-2015-207709.
- Wakefield RJ, D’Agostino MA, Iagnocco A, Filippucci E, Backhaus M, Scheel AK, et al. The OMERACT Ultrasound Group: status of current activities and research directions. J Rheumatol 2007;34:848-51.
- D’Agostino MA, Conaghan PG, Naredo E, Aegerter P, Iagnocco A, Freeston JE, et al. The OMERACT Ultrasound Task Force – advances and priorities. J Rheumatol 2009;36:1829-32. https://doi.org/10.3899/jrheum.090354.
- Iagnocco A, Naredo E, Wakefield R, Bruyn GA, Collado P, Jousse-Joulin S, et al. Responsiveness in rheumatoid arthritis. a report from the OMERACT 11 ultrasound workshop. J Rheumatol 2014;41:379-82. https://doi.org/10.3899/jrheum.131084.
- Naredo E, Rodriguez M, Rodriguez-Heredia J, Campos C, Medina J, Giner E, et al. Validity, reliability and sensitivity to change of simplified power Doppler ultrasonographic assessment of the effect of biological therapy on rheumatoid arthritis joint inflammation. Ann Rheum Dis 2007;66.
- Backhaus M, Ohrndorf S, Kellner. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum 2009;61:1194-201. https://doi.org/10.1002/art.24646.
- Hammer HB, Kvien TK. Comparisons of 7- to 78-joint ultrasonography scores: all different joint combinations show equal response to adalimumab treatment in patients with rheumatoid arthritis. Arthritis Res Ther 2011;13. https://doi.org/10.1186/ar3341.
- Backhaus TM, Ohrndorf S, Kellner H, Strunk J, Hartung W, Sattler H, et al. The US7 score is sensitive to change in a large cohort of patients with rheumatoid arthritis over 12 months of therapy. Ann Rheum Dis 2013;72:1163-9. https://doi.org/10.1136/annrheumdis-2012-201397.
- Naredo E, Wakefield RJ, Iagnocco A, Terslev L, Filippucci E, Gandjbakhch F, et al. The OMERACT ultrasound task force – status and perspectives. J Rheumatol 2011;38:2063-7. https://doi.org/10.3899/jrheum.110425.
- Cunnington J, Platt P, Raftery G, Kane D. Attitudes of United Kingdom rheumatologists to musculoskeletal ultrasound practice and training. Ann Rheum Dis 2007;66:1381-3. https://doi.org/10.1136/ard.2006.065466.
- Targeted Ultrasound Initiative . About TUI n.d. http://targetedultrasound.net/about (accessed 9 January 2018).
- Department of Health and Social Care . NHS Reference Costs 2014 to 2015 2016. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 27 October 2015).
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001;60:641-9. https://doi.org/10.1136/ard.60.7.641.
- Management of Early Rheumatoid Arthritis. Edinburgh: SIGN; 2011.
- National Institute for Health and Clinical Excellence, Centre for Clinical Practice – Surveillance Programme . Surveillance Report for Guidance Executive (Update CG79) 2015. www.nice.org.uk/guidance/CG79/update/CG79/documents/cg79-rheumatoid-arthritis-surveillance-review-decision-march-20153 (accessed 27 October 2015).
- Dale J, Stirling A, Zhang R, Purves D, Foley J, Sambrook M, et al. Targeting ultrasound remission in early rheumatoid arthritis: the results of the TaSER study, a randomised clinical trial. Ann Rheum Dis 2016;75:1043-50. https://doi.org/10.1136/annrheumdis-2015-208941.
- Haavardsholm E, Aga A-B, Olsen I, Hammer HB, Uhlig T, Fremstad H, et al. Aiming for remission in rheumatoid arthritis: clinical and radiographic outcomes from a randomized controlled strategy trial investigating the added value of ultrasonography in a treat-to-target regimen. Arthritis Rheumatol 2015;67.
- Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol 2000;53:459-68. https://doi.org/10.1016/S0895-4356(99)00206-1.
- Williams K, Moons C. An Introduction to Systematic Reviews of Prognosis. Auckland, New Zealand: Cochrane Prognosis Review Methods Group; 2012.
- Whiting P, Rutjes A, Reitsma J, Bossuyt P, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. Wellington, New Zealand. BMC Med Res Methodol 2003;3. https://doi.org/10.1186/1471-2288-3-25.
- New Zealand Guidelines Group . Handbook for Preparation of Explicit Evidence-Based Clinical Practice Guidelines 2001.
- Karsh J, Keystone EC, Haraoui B, Thorne JC, Pope JE, Bykerk VP, et al. Canadian recommendations for clinical trials of pharmacologic interventions in rheumatoid arthritis: inclusion criteria and study design. J Rheumatol 2011;38:2095-104. https://doi.org/10.3899/jrheum.110188.
- Alcalde M, D’Agostino MA, Bruyn GAW, Moller I, Iagnocco A, Wakefield RJ, et al. A systematic literature review of US definitions, scoring systems and validity according to the OMERACT filter for tendon lesion in RA and other inflammatory joint diseases. Rheumatology (Oxford) 2012;51:1246-60. https://doi.org/10.1093/rheumatology/kes018.
- Joshua F, Lassere M, Bruyn GA, Szkudlarek M, Naredo E, Schmidt WA, et al. Summary findings of a systematic review of the ultrasound assessment of synovitis. J Rheumatol 2007;34:839-47.
- Keen HI, Mease PJ, Bingham CO, Giles JT, Kaeley G, Conaghan PG. Systematic review of MRI, ultrasound, and scintigraphy as outcome measures for structural pathology in interventional therapeutic studies of knee arthritis: focus on responsiveness. J Rheumatol 2011;38:142-54. https://doi.org/10.3899/jrheum.100377.
- Ten Cate DF, Luime JJ, Swen N, Gerards AH, De Jager MH, Basoski NM. Role of ultrasonography in diagnosing early rheumatoid arthritis and remission of rheumatoid arthritis – a systematic review of the literature. Arthritis Res Ther 2013;15. https://doi.org/10.1186/ar4132.
- Bhamra K, Seymour M, Swales C, Taylor P. Utility of ultrasound in the Nuffield Orthopaedic Centre Emergency Rheumatology Clinic: survey of clinical effectiveness. Ann Rheum Dis 2014;73:659-60. https://doi.org/10.1136/annrheumdis-2014-eular.4650.
- Ciurtin C, Ehrenstein M, Leandro M, Dey D, Nandagudi A, Morris V, et al. The usefulness of a musculoskeletal ultrasound (MUS) scoring system for 22 hand joints examination for the detection of early undifferentiated inflammatory arthritis and treatment decisions making in established inflammatory arthritis. Ann Rheum Dis 2013;71:295-6. https://doi.org/10.1136/annrheumdis-2012-eular.2381.
- Gandjbakhch F, Millot F, Etchepare F, Foltz V, Bourgeois P, Fautrel B. Ultrasonography examination influences therapeutic decision in RA patients. Arthritis Rheum 2008;58:S467-68.
- Hayashi N, Ogura T, Hirata A, Kujime R, Imamura M, Takenaka S, et al. The comparison between physical and ultrasound joint examination for the hand joints in patients with rheumatoid arthritis. Arthritis Rheumatol 2014;66.
- Inanc N, Ozen G, Direskeneli H. Ultrasonographic assessment of joint inflammation in rheumatoid arthritis: predictive value in response to tumor necrosis factor-alpha inhibitor treatment. Ann Rheum Dis 2014;73. https://doi.org/10.1136/annrheumdis-2014-eular.1918.
- Kelly S, Davidson B, Gorman C, Meenagh G, Reynolds P. The impact of ultrasound on the diagnosis and management of patients with rheumatoid arthritis (RA) in routine clinical care within the UK. Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-eular.351.
- Luengroongroj P, Suwannalai P, Joavisidha S. Disease flared after DMARD tapering in rheumatoid arthritis may be predicted by subclinical synovitis detected by ultrasonography. Int J Rheum Dis 2015;18.
- Mamoto K, Koike T, Okano T, Tada M, Sugioka Y, Wakitani S, et al. Comparative assessment of swollen joints in rheumatoid arthritis by patients, physicians and ultrasonography. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.1272.
- Osipyants R, Karateev D, Panasyuk E, Smirnov A, Lukina G, Glukhova S, et al. Associations between functional status and ultrasound-detected synovitis and joint damage in rheumatoid arthritis during tocilizumab treatment. Ann Rheum Dis 2013;72:A755-6. https://doi.org/10.1136/annrheumdis-2013-eular.2238.
- Ramirez García J, Ruíz-Esquide V, Celis R, Cuervo A, Cabrera S, Inciarte-Mundo J, et al. Sonographic and clinical characterization of a prospective cohort of patients with rheumatoid arthritis in clinical remission. Preliminary results. Ann Rheum Dis 2014;73. https://doi.org/10.1136/annrheumdis-2014-eular.4310.
- Zufferey P. Persistence of ultrasound synovitis in patients with rheumatoid arthritis fulfilling the DAS28 and/or the new ACR/EULAR RA remission definitions: results of an observational cohort study. Joint Bone Spine 2014;81:426-32. https://doi.org/10.1016/j.jbspin.2014.04.014.
- Taylor PC, Steuer A, Gruber J, Cosgrove DO, Blomley MJK, Marsters A, et al. Comparison of ultrasonographic assessment of synovitis and joint vascularity with radiographic evaluation in a randomized, placebo-controlled study of infliximab therapy in early rheumatoid arthritis. Arthritis Rheum 2004;50:1107-16. https://doi.org/10.1002/art.20123.
- Ellegaard K, Christensen R, Torp-Pedersen S, Terslev L, Holm CC, Konig MJ, et al. Ultrasound Doppler measurements predict success of treatment with anti-TNF-alpha drug in patients with rheumatoid arthritis: a prospective cohort study. Rheumatology (Oxford) 2011;50:506-12. https://doi.org/10.1093/rheumatology/keq336.
- Kane D, Balint PV, Sturrock RD. Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol 2003;30:966-71.
- Luukkainen R, Sanila MT. Relationship between clinically detected joint swelling and effusion diagnosed by ultrasonography in elbow joints in patients with rheumatoid arthritis. Clin Rheumatol 2005;24:228-31. https://doi.org/10.1007/s10067-004-1010-8.
- Luukkainen R, Sanila MT. Poor relationship between joint swelling detected on physical examination and effusion diagnosed by ultrasonography in glenohumeral joints in patients with rheumatoid arthritis. Clin Rheumatol 2007;26:865-7. https://doi.org/10.1007/s10067-006-0402-3.
- Balsa A, de Miguel E, Castillo C, Peiteado D, Martin-Mola E. Superiority of SDAI over DAS-28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology 2010;49:683-90. https://doi.org/10.1093/rheumatology/kep442.
- Beckers C, Ribbens C, André B, Marcelis S, Kaye O, Mathy L, et al. Assessment of disease activity in rheumatoid arthritis with (18)F-FDG PET. J Nucl Med 2004;45:956-64.
- Filippucci E, Iagnocco A, Salaffi F, Cerioni A, Valesini G, Grassi W. Power Doppler sonography monitoring of synovial perfusion at the wrist joints in patients with rheumatoid arthritis treated with adalimumab. Ann Rheum Dis 2006;65:1433-7. https://doi.org/10.1136/ard.2005.044628.
- Garrigues F, Jousse-Joulin S, Bouttier R, Nonent M, Bressollette L, Saraux A. Concordance between clinical and ultrasound findings in rheumatoid arthritis. Joint Bone Spine 2013;80:597-603. https://doi.org/10.1016/j.jbspin.2013.03.011.
- Gartner M, Mandl P, Radner H, Supp G, Machold KP, Aletaha D, et al. Sonographic joint assessment in rheumatoid arthritis: associations with clinical joint assessment during a state of remission. Arthritis Rheum 2013;65:2005-14. https://doi.org/10.1002/art.38016.
- Haavardsholm EA, Ostergaard M. Monitoring anti-TNFalpha treatment in rheumatoid arthritis: responsiveness of magnetic resonance imaging and ultrasonography of the dominant wrist joint compared with conventional measures of disease activity and structural damage. Ann Rheum Dis 2009;68:1572-9. https://doi.org/10.1136/ard.2008.091801.
- Hammer HB, Sveinsson M, Kongtorp AK, Kvien TK. A 78-joints ultrasonographic assessment is associated with clinical assessments and is highly responsive to improvement in a longitudinal study of patients with rheumatoid arthritis starting adalimumab treatment. Ann Rheum Dis 2010;69:1349-51. https://doi.org/10.1136/ard.2009.126995.
- Horikoshi M, Suzuki T, Sugihara M, Kondo Y, Tsuboi H, Uehara T, et al. Comparison of low-field dedicated extremity magnetic resonance imaging with articular ultrasonography in patients with rheumatoid arthritis. Mod Rheumatol 2010;20:556-60. https://doi.org/10.3109/s10165-010-0318-2.
- Ikeda K, Nakagomi D, Sanayama Y, Yamagata M, Okubo A, Iwamoto T, et al. Correlation of radiographic progression with the cumulative activity of synovitis estimated by power Doppler ultrasound in rheumatoid arthritis: difference between patients treated with methotrexate and those treated with biological agents. J Rheumatol 2013;40:1967-76. https://doi.org/10.3899/jrheum.130556.
- Kamishima T, Tanimura K, Shimizu M, Matsuhashi M, Fukae J, Kon Y, et al. Monitoring anti-interleukin 6 receptor antibody treatment for rheumatoid arthritis by quantitative magnetic resonance imaging of the hand and power Doppler ultrasonography of the finger. Skeletal Radiol 2011;40:745-55. https://doi.org/10.1007/s00256-010-1064-4.
- Luukkainen RK, Saltyshev M. Relationship between clinically detected joint swelling and effusion diagnosed by ultrasonography in metatarsophalangeal and talocrural joints in patients with rheumatoid arthritis. Clin Exp Rheumatol 2003;21:632-4.
- Mandl P, Balint PV. Clinical and ultrasound-based composite disease activity indices in rheumatoid arthritis: results from a multicenter, randomized study. Arthritis Care Res (Hoboken) 2013;65:879-87. https://doi.org/10.1002/acr.21913.
- Mandl P, Balint PV. Metrologic properties of ultrasound versus clinical evaluation of synovitis in rheumatoid arthritis: results of a multicenter, randomized study. Arthritis Rheum 2012;64:1272-82. https://doi.org/10.1002/art.33491.
- Naredo E, Valor L, De la Torre I, Martinez-Barrio J, Hinojosa M, Aramburu F, et al. Ultrasound joint inflammation in rheumatoid arthritis in clinical remission: how many and which joints should be assessed?. Arthritis Care Res (Hoboken) 2013;65:512-17. https://doi.org/10.1002/acr.21869.
- Pereira DF, Gutierrez M, de Buosi AL, Ferreira FB, Draghessi A, Grassi W, et al. Is articular pain in rheumatoid arthritis correlated with ultrasound power Doppler findings?. Clin Rheumatol 2015;34:1975-9. https://doi.org/10.1007/s10067-015-2964-4.
- Ribbens C, Andre B, Marcelis S, Kaye O, Mathy L, Bonnet V, et al. Rheumatoid hand joint synovitis: gray-scale and power Doppler US quantifications following anti-tumor necrosis factor-alpha treatment: pilot study. Radiology 2003;229:562-9. https://doi.org/10.1148/radiol.2292020206.
- Riente L, Delle Sedie A, Filippucci E, Scirè CA, Iagnocco A, Gutierrez M, et al. Ultrasound imaging for the rheumatologist. XXVII. Sonographic assessment of the knee in patients with rheumatoid arthritis. Clin Exp Rheumatol 2010;28:300-3.
- Riente L, Delle Sedie A, Scirè CA, Filippucci E, Meenagh G, Iagnocco A, et al. Ultrasound imaging for the rheumatologist. XXXI. Sonographic assessment of the foot in patients with rheumatoid arthritis. Clin Exp Rheumatol 2011;29:1-5.
- Salaffi F, Filippucci E, Carotti M, Naredo E, Meenagh G, Ciapetti A, et al. Interobserver agreement of standard joint counts in early rheumatoid arthritis: a comparison with grey-scale ultrasonograpky. Ann Rheum Dis 2006;65:592-3.
- Saleem B, Brown AK. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann Rheum Dis 2011;70:792-8. https://doi.org/10.1136/ard.2010.134445.
- Spiegel TM, King W III, Weiner SR, Paulus HE. Measuring disease activity: comparison of joint tenderness, swelling, and ultrasonography in rheumatoid arthritis. Arthritis Rheum 1987;30:1283-8. https://doi.org/10.1002/art.1780301111.
- Szkudlarek M, Narvestad E, Klarlund M, Court-Payen, Thomsen HS, Ostergaard M, . Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum 2004;50:2103-12. https://doi.org/10.1002/art.20333.
- Szkudlarek M, Klarlund M, Narvestad E, Court-Payen, Strandberg C, Jensen KE, et al. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther 2006;8. https://doi.org/10.1186/ar1904.
- Taniguchi D, Tokunaga D, Oda R, Fujiwara H, Ikeda T, Ikoma K, et al. Maximum intensity projection with magnetic resonance imaging for evaluating synovitis of the hand in rheumatoid arthritis: comparison with clinical and ultrasound findings. Clin Rheumatol 2014;33:911-17. https://doi.org/10.1007/s10067-014-2526-1.
- Vlad V, Berghea F, Micu M, Varzaru L, Bojinca M, Milicescu M, et al. Tenosynovitis US scoring systems follow synovitis and clinical scoring systems in RA and are responsive to change after biologic therapy. Med Ultrason 2015;17:352-60. https://doi.org/10.11152/mu.2013.2066.173.viv.
- Wakefield RJ, Freeston JE, O’Connor P, Reay N, Budgen A, Hensor EM, et al. The optimal assessment of the rheumatoid arthritis hindfoot: a comparative study of clinical examination, ultrasound and high field MRI. Ann Rheum Dis 2008;67:1678-82. https://doi.org/10.1136/ard.2007.079947.
- Xiao H, Liu M, Tan L, Liao X, Li Y, Gao J, et al. Value of ultrasonography for diagnosis of synovitis associated with rheumatoid arthritis. Int J Rheum Dis 2014;17:767-75. https://doi.org/10.1111/1756-185X.12390.
- Zufferey P. Ultrasound evaluation of synovitis in RA: correlation with clinical disease activity and sensitivity to change in an observational cohort study. Joint Bone Spine 2014;81:222-7. https://doi.org/10.1016/j.jbspin.2013.08.006.
- Gartner M, Radner H, Supp G, Mandl P, Machold K, Aletaha D, et al. Does joint sonography really add clinically important information beyond clinical joint examination?. J Miner Stoffwechs 2012;19.
- Boyesen P, Haavardsholm EA. Prediction of MRI erosive progression: a comparison of modern imaging modalities in early rheumatoid arthritis patients. Ann Rheum Dis 2011;70:176-9. https://doi.org/10.1136/ard.2009.126953.
- Brown AK, Conaghan PG, Karim, Conaghan PG. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 2008;58:2958-67. https://doi.org/10.1002/art.23945.
- Ikeda K, Brown A, Conaghan P, Karim Z, Quinn M, Peterfy C, et al. An explanation of structural deterioration in RA patients in clinical remission on DMARDS: the significance of sub-clinical inflammation detectable with imaging. Ann Rheum Dis 2007;66.
- Scirè CA, Montecucco C, Codullo V, Epis O, Todoerti M, Caporali R. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology (Oxford) 2009;48:1092-7. https://doi.org/10.1093/rheumatology/kep171.
- Cavet G, Shen Y, Abraham S, Chernoff D, Centola M, Taylor P. Predicting radiographic progression in rheumatoid arthritis with ultrasound and biomarkers. Arthritis Rheum 2009;60.
- Dougados M, Devauchelle-Pensec V, Ferlet JF, Jousse-Joulin S, D’Agostino M-A, Backhaus M, et al. The ability of synovitis to predict structural damage in rheumatoid arthritis: a comparative study between clinical examination and ultrasound. Ann Rheum Dis 2013;72:665-71. https://doi.org/10.1136/annrheumdis-2012-201469.
- Naredo E, Moller I, Cruz A, Carmona L, Garrido J. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum 2008;58:2248-56. https://doi.org/10.1002/art.23682.
- Reynolds PPM, Heron C, Pilcher J, Kiely PDW. Prediction of erosion progression using ultrasound in established rheumatoid arthritis: a 2-year follow-up study. Skeletal Radiol 2009;38:473-8. https://doi.org/10.1007/s00256-009-0670-5.
- Saleem B, Brown AK, Quinn M, Karim Z, Hensor EM, Conaghan P, et al. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis 2012;71:1316-21. https://doi.org/10.1136/annrheumdis-2011-200548.
- Wakefield RJ, Freeston JE, Hensor EMA, Bryer D, Quinn MA, Emery P. Delay in imaging versus clinical response: a rationale for prolonged treatment with anti-tumor necrosis factor medication in early rheumatoid arthritis. Arthritis Rheum 2007;57:1564-7. https://doi.org/10.1002/art.23097.
- Yoshimi R, Hama M, Takase K, Ihata A, Kishimoto D, Terauchi K, et al. Ultrasonography is a potent tool for the prediction of progressive joint destruction during clinical remission of rheumatoid arthritis. Mod Rheumatol 2013;23:456-65. https://doi.org/10.3109/s10165-012-0690-1.
- Yoshimi R, Hama M, Minegishi K, Kishimoto D, Watanabe T, Kamiyama R, et al. Ultrasonography predicts achievement of Boolean remission after DAS28-based clinical remission of rheumatoid arthritis. Mod Rheumatol 2014;24:590-8. https://doi.org/10.3109/14397595.2013.857800.
- Ikeda K, Nakagomi D, Sanayama Y, Yamagata M, Okubo A, Iwamoto T, et al. Time-integrated synovitis activity assessed by power Doppler ultrasound significantly correlates with radiographic progression in rheumatoid arthritis patients treated with methotrexate alone but not in those treated with TNF antagonists. Arthritis Rheum 2012;64.
- Bugatti S, Manzo, Manzo A, Benaglio F, Klersy C, Vitolo B, . Serum levels of CXCL13 are associated with ultrasonographic synovitis and predict power Doppler persistence in early rheumatoid arthritis treated with non-biological disease-modifying anti-rheumatic drugs. Arthritis Res Ther 2012;14. https://doi.org/10.1186/ar3742.
- Cheung P, Mari K, Devauchelle V, Bentin J, Jousse-Joulin S, D’Agostino MA, et al. Are tender joints better than synovitis to predict structural damage in rheumatoid arthritis?. Arthritis Rheumatol 2014;66.
- Dougados M, Devauchelle-Pensec V, Ferlet J-F, Jousse-Joulin S, D’Agostino M-A, Backhaus M, et al. Both clinical and ultrasonographic evaluation of synovitis are relevant to predict subsequent radiological deterioration in rheumatoid arthritis. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.3354.
- Osipyants R, Karateev D, Panasyuk E, Smirnov A, Lukina G, Glukhova S, et al. Imaging rather than clinical inflammation is associated with radiographic progression in tocilizumab-treated rheumatoid arthritis patients. Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-eular.2237.
- Rees JD, Pilcher J, Heron C, Kiely PD. A comparison of clinical vs. ultrasound determined synovitis in rheumatoid arthritis utilizing gray-scale, power Doppler and the intravenous microbubble contrast agent ‘Sono-Vue’. Rheumatology (Oxford) 2007;46:454-9. https://doi.org/10.1093/rheumatology/kel256.
- Ceponis A, Onishi M, Bluestein HG, Kalunian K, Townsend J, Kavanaugh A. Utility of the ultrasound examination of the hand and wrist joints in the management of established rheumatoid arthritis. Arthritis Care Res 2014;66:236-44. https://doi.org/10.1002/acr.22119.
- Dale J, Purves D, McConnachie A, McInnes I, Porter D. Tightening up? Impact of musculoskeletal ultrasound disease activity assessment on early rheumatoid arthritis patients treated using a treat to target strategy. Arthritis Care Res 2014;66:19-26. https://doi.org/10.1002/acr.22218.
- Dale J, Stirling A, McInnes IB, Porter D. Targeting ultrasound remission in early rheumatoid arthritis – results of the TaSER study. Arthritis Rheum 2013;65:S338-9.
- Iwamoto T, Iwamoto T, Ikeda K, Hosokawa J, Yamagata M, Tanaka S, et al. Prediction of relapse after discontinuation of biologic agents by ultrasonographic assessment in patients with rheumatoid arthritis in clinical remission: high predictive values of total gray-scale and power Doppler scores that represent residual synovial inflammation before discontinuation. Arthritis Care Res (Hoboken) 2014;66:1576-81. https://doi.org/10.1002/acr.22303.
- Naredo E, Valor L, De la Torre, I, Montoro M, Bello N, Martinez-Barrio J, et al. Predictive value of Doppler ultrasound-detected synovitis in relation to successful tapering of biology therapy in patients with rheumatoid arthritis. Ann Rheum Dis 2014;73. https://doi.org/10.1136/annrheumdis-2014-eular.3013.
- Naredo E, Valor L, De la Torre, I, Montoro M, Bello N, Martinez-Barrio J, et al. Predictive value of Doppler ultrasound-detected synovitis in relation to failed tapering of biologic therapy in patients with rheumatoid arthritis. Rheumatology (Oxford) 2015;54:1408-14. https://doi.org/10.1093/rheumatology/kev006.
- Ciurtin C, Leandro M, Dey D, Nandagudi A, Giles I, Shipley M, et al. The usefulness of a musculoskeletal ultrasound (MUS) scoring system for 22 hand joints examination for the detection of early undifferentiated inflammatory arthritis and treatment decision making in established inflammatory arthritis. Rheumatology (Oxford) 2012;51.
- Merlin T, Weston A, Tooher R. Extending an evidence hierarchy to include topics other than treatment: revising the Australian ‘levels of evidence’. BMC Med Res Methodol 2009;9. https://doi.org/10.1186/1471-2288-9-34.
- Salaffi F, Filippucci E, Carotti. Inter-observer agreement of standard joint counts in early rheumatoid arthritis: a comparison with grey scale ultrasonography – a preliminary study. Rheumatology 2008;47:54-8. https://doi.org/10.1093/rheumatology/kem286.
- Dale J, Purves D, McConnachie A, Porter D, McInnes IB. Tightening up: musculoskeletal ultrasound could further individualise treatment decisions in early rheumatoid arthritis patients treated by a step-up DMARD escalation regimen. Arthritis Rheum 2012;64.
- ClinicalTrials.gov . Aiming for Remission in Rheumatoid Arthritis (RA) – the ARCTIC Trial (ARCTIC) 2016. https://clinicaltrials.gov/ct2/show/study/NCT01205854?term=NCT+01205854&rank=1&view=record (accessed 20 October 2015).
- Holroyd CR, Davidson B, Bennett S, Waghorn D, Underhil C, Cooper C, et al. A strategy for selecting individuals with RA for reduction of anti-TNF therapy using combined clinical and ultrasound assessment. Arthritis Rheum 2013;65:S339-40.
- Lamers-Karnebeek FBG, Jansen T, van Riel P, Luime J, Jacobs J. Ultrasonography as predictor for flare in rheumatoid arthritis patients with low disease activity: nine month results from POET-US-study. Ann Rheum Dis 2015;74. https://doi.org/10.1136/annrheumdis-2015-eular.5185.
- Arshad A, Sulaiman W. Optimizing the use of traditional DMARD in RA: getting the most out of what we can afford!. J Rheumatol 2007;10:3-8. https://doi.org/10.1111/j.1479-8077.2007.00247.x.
- Garg OP, Bhakuni DS, Narayanan K, Vasdev V, Jain R. Guided interventions in rheumatology: Our experiences and innovations at a premier institute. Int J Rheum Dis 2010;13.
- Gibson N, Kissin E. The pros and cons of ultrasonography for rheumatologic conditions. J Musculoskelet Med 2011;28:289-95.
- Østergaard M, Døhn UM, Ejbjerg BJ, McQueen FM. Ultrasonography and magnetic resonance imaging in early rheumatoid arthritis: recent advances. Curr Rheumatol Rep 2006;8:378-85. https://doi.org/10.1007/s11926-006-0069-4.
- van Ingen IL, Lamers-Karnebeek F, Jansen TL. Optimizing the expediency of TNFi in rheumatoid arthritis: offering a TNFi holiday in patients having reached low-disease activity in the maintenance phase. Expert Opin Biol Ther 2014;14:1761-7. https://doi.org/10.1517/14712598.2014.955009.
- Breedveld F. The value of early intervention in RA – a window of opportunity. Clin Rheumatol 2011;30:33-9. https://doi.org/10.1007/s10067-010-1638-5.
- Brown PM, Isaacs JD. Rheumatoid arthritis: an evolutionary force in biologics. Curr Pharm Des 2015;21:2170-8. https://doi.org/10.2174/1381612821666150310141827.
- Caporali R, Scirè CA, Todoerti M, Montecucco C. The role of low-dose glucocorticoids for rheumatoid arthritis in the biologic era. Clin Exp Rheumatol 2013;31:9-13.
- Claessen SJ, Hazes JM, Huisman MA, Van ZD, Luime JJ, Weel AE. Use of risk stratification to target therapies in patients with recent onset arthritis; design of a prospective randomized multicenter controlled trial. BMC Musculoskelet Disord 2009;10. https://doi.org/10.1186/1471-2474-10-71.
- Favalli EG, Marchesoni A, Colombo GL, Sinigaglia L. Pattern of use, economic burden and vial optimization of infliximab for rheumatoid arthritis in Italy. Clin Exp Rheumatol 2008;26:45-51.
- Heather EM, Payne K, Harrison M, Symmons DP. Including adverse drug events in economic evaluations of anti-tumour necrosis factor-alpha drugs for adult rheumatoid arthritis: a systematic review of economic decision analytic models. Pharmacoeconomics 2014;32:109-34. https://doi.org/10.1007/s40273-013-0120-z.
- Kobelt G, Lekander I, Lang A, Raffeiner B, Botsios C, Geborek P. Cost-effectiveness of etanercept treatment in early active rheumatoid arthritis followed by dose adjustment. Int J Technol Assess Health Care 2011;27:193-200. https://doi.org/10.1017/S0266462311000195.
- Kobelt G. Treating to target with etanercept in rheumatoid arthritis: cost-effectiveness of dose reductions when remission is achieved. Value Health 2014;17:537-44. https://doi.org/10.1016/j.jval.2014.04.005.
- Krieckaert CL, Nair SC, Nurmohamed MT, van Dongen CJ, Lems WF, Lafeber FP, et al. Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann Rheum Dis 2015;74:361-8. https://doi.org/10.1136/annrheumdis-2013-204101.
- Radner H, Aletaha D. Anti-TNF in rheumatoid arthritis: an overview. Wien Med Wochenschr 2015;165:3-9. https://doi.org/10.1007/s10354-015-0344-y.
- Saleem B, Nizam S, Emery P. Can remission be maintained with or without further drug therapy in rheumatoid arthritis?. Clin Exp Rheumatol 2006;24:33-6.
- Scott IC, Wailoo A, Scott DL. Payers’ views on treating-to-target in rheumatoid arthritis: an English perspective. Clin Exp Rheumatol 2012;30:85-90.
- Smolen JS, Emery P, Fleischmann R, Van Vollenhoven RF, Pavelka K, Durez P, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383:321-32. https://doi.org/10.1016/S0140-6736(13)61751-1.
- Smolen JS, Nash P, Durez P, Hall S, Ilivanova E, Irazoque-Palazuelos F, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918-29. https://doi.org/10.1016/S0140-6736(12)61811-X.
- Tanaka Y, Takeuchi T, Mimori T, Saito K, Nawata M, Kameda H, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis 2010;69:1286-91. https://doi.org/10.1136/ard.2009.121491.
- Tanaka Y, Hirata S. Is it possible to withdraw biologics from therapy in rheumatoid arthritis?. Clin Ther 2013;35:2028-35. https://doi.org/10.1016/j.clinthera.2013.10.008.
- Tanaka Y. Next stage of RA treatment: is TNF inhibitor-free remission a possible treatment goal?. Ann Rheum Dis 2013;72:ii124-7. https://doi.org/10.1136/annrheumdis-2012-202350.
- van Herwaarden N, van der Maas A, Minten MJ, van den Hoogen FH, Kievit W, Van Vollenhoven RF, et al. Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial. BMJ 2015;350. https://doi.org/10.1136/bmj.h1389.
- Fautrel B, Pham T, Alfaiate T, Gandjbakhch F, Foltz V, Morel J, et al. Step-down strategy of spacing TNF-blocker injections for established rheumatoid arthritis in remission: results of the multicentre non-inferiority randomised open-label controlled trial (STRASS: Spacing of TNF-blocker injections in Rheumatoid ArthritiS Study). Ann Rheum Dis 2016;75:59-67. https://doi.org/10.1136/annrheumdis-2014-206696.
- Galloway JB, Kingsley G, Ma M, Lorente-Canovas B, Cope A, Ibrahim F, et al. SAT0150 Optimising Treatment with TNF inhibitors in rheumatoid arthritis with different dose tapering strategies: the Opttira trial. Ann Rheum Dis 2015;74. https://doi.org/10.1136/annrheumdis-2015-eular.4684.
- Ghiti Moghadam M, Vonkeman HE, Ten Klooster PM, van Riel PL, van de Laar MA, Jansen TL. SAT0157 Randomized Trial of Stopping TNF-Inhibitors in Rheumatoid Arthritis Patients with Stable Remission or Low Disease Activity in the Netherlands. Ann Rheum Dis 2015;74. https://doi.org/10.1136/annrheumdis-2015-eular.2388.
- Tanaka Y, Hirata S, Kubo S, Fukuyo S, Hanami K, Sawamukai N, et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann Rheum Dis 2015;74:389-5. https://doi.org/10.1136/annrheumdis-2013-204016.
- van Vollenhoven RF, Østergaard M, Leirisalo-Repo M, Uhlig T, Jansson M, Larsson E, et al. Full dose, reduced dose or discontinuation of etanercept in rheumatoid arthritis. Ann Rheum Dis 2016;75:52-8. https://doi.org/10.1136/annrheumdis-2014-205726.
- Emery P, Hammoudeh M, FitzGerald O, Combe B, Martin-Mola E, Buch MH, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014;371:1781-92. https://doi.org/10.1056/NEJMoa1316133.
- Kavanaugh A, Fleischmann RM, Emery P, Kupper H, Redden L, Guerette B, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis 2013;72:64-71. https://doi.org/10.1136/annrheumdis-2011-201247.
- Aguilar-Lozano L, Castillo-Ortiz JD, Vargas-Serafin C, Morales-Torres J, Sanchez-Ortiz A, Sandoval-Castro C, et al. Sustained clinical remission and rate of relapse after tocilizumab withdrawal in patients with rheumatoid arthritis. J Rheumatol 2013;40:1069-73. https://doi.org/10.3899/jrheum.121427.
- Detert J, Bastian H, Listing J, Weiss A, Wassenberg S, Liebhaber A, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis 2013;72:844-50. https://doi.org/10.1136/annrheumdis-2012-201612.
- Smolen JS, Emery P, Ferraccioli GF, Samborski W, Berenbaum F, Davies OR, et al. Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double-blind, randomised, placebo-controlled trial. Ann Rheum Dis 2014;74:843-50. https://doi.org/10.1136/annrheumdis-2013-204632.
- Chatzidionysiou K, Turesson C, Teleman A, Knight A, Lindqvist E, Larsson P, et al. A multicenter, randomized, controlled, open-label pilot study of the feasibility of discontinuation of adalimumab in rheumatoid arthritis patients in stable clinical remission. RMD Open 2016;2. https://doi.org/10.1136/rmdopen-2015-000133.
- Harigai M, Takeuchi T, Tanaka Y, Matsubara T, Yamanaka H, Miyasaka N. Discontinuation of adalimumab treatment in rheumatoid arthritis patients after achieving low disease activity. Mod Rheumatol 2012;22:814-22. https://doi.org/10.3109/s10165-011-0586-5.
- Kaine J, Gladstein G, Strusberg I, Robles M, Louw I, Gujrathi S, et al. Evaluation of abatacept administered subcutaneously in adults with active rheumatoid arthritis: impact of withdrawal and reintroduction on immunogenicity, efficacy and safety (phase Iiib ALLOW study). Ann Rheum Dis 2012;71:38-44. https://doi.org/10.1136/annrheumdis-2011-200344.
- van der Maas A, Kievit W, van den Bemt BJ, van den Hoogen FH, van Riel PL, den Broeder AA. Down-titration and discontinuation of infliximab in rheumatoid arthritis patients with stable low disease activity and stable treatment: an observational cohort study. Ann Rheum Dis 2012;71:1849-54. https://doi.org/10.1136/annrheumdis-2011-200945.
- Klarenbeek NB, van der Kooij SM, Guler-Yuksel M, van Groenendael JH, Han KH, Kerstens PJ, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis 2011;70:315-19. https://doi.org/10.1136/ard.2010.136556.
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, Van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381-90. https://doi.org/10.1002/art.21405.
- van den Broek M, Klarenbeek NB, Dirven L, Van Schaardenburg D, Hulsmans HM, Kerstens PJ, et al. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score-steered therapy: subanalysis of the BeSt study. Ann Rheum Dis 2011;70:1389-94. https://doi.org/10.1136/ard.2010.147751.
- Bejarano V, Conaghan PG, Quinn MA, Saleem B, Emery P. Benefits 8 years after a remission induction regime with an infliximab and methotrexate combination in early rheumatoid arthritis. Rheumatology (Oxford) 2010;49:1971-4. https://doi.org/10.1093/rheumatology/keq194.
- Saleem B, Keen H, Goeb V, Parmar R, Nizam S, Hensor EM, et al. Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped?. Ann Rheum Dis 2010;69:1636-42. https://doi.org/10.1136/ard.2009.117341.
- Brocq O, Millasseau E, Albert C, Grisot C, Flory P, Roux CH, et al. Effect of discontinuing TNFalpha antagonist therapy in patients with remission of rheumatoid arthritis. Joint Bone Spine 2009;76:350-5. https://doi.org/10.1016/j.jbspin.2008.11.009.
- van der Kooij SM, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Guler-Yuksel M, Zwinderman AH, Kerstens PJ, et al. Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis 2009;68:914-21. https://doi.org/10.1136/ard.2008.092254.
- Nawata M, Saito K, Nakayamada S, Tanaka Y. Discontinuation of infliximab in rheumatoid arthritis patients in clinical remission. Mod Rheumatol 2008;18:460-4. https://doi.org/10.1007/s10165-008-0089-1.
- van der Bijl AE, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Ten WS, Han KH, van Krugten MV, et al. Infliximab and methotrexate as induction therapy in patients with early rheumatoid arthritis. Arthritis Rheum 2007;56:2129-34. https://doi.org/10.1002/art.22718.
- Quinn MA, Conaghan PG, O’Connor PJ, Karin Z, Greenstein A, Brown A, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal. Arthritis Rheum 2005;52:27-35. https://doi.org/10.1002/art.20712.
- Buch MH, Marzo-Ortega H, Bingham SJ, Emery P. Long-term treatment of rheumatoid arthritis with tumour necrosis factor alpha blockade: outcome of ceasing and restarting biologicals. Rheumatology (Oxford) 2004;43:243-4. https://doi.org/10.1093/rheumatology/keg454.
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised Phase III trial. ATTRACT Study Group. Lancet 1999;354:1932-9. https://doi.org/10.1016/S0140-6736(99)05246-0.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf (accessed 20 October 2015).
- National Institute for Health Research . NETSCC, HTA. 23 April 2015. Updated Protocol n.d. https://njl-admin.nihr.ac.uk/document/download/2007323 (accessed 12 February 2018).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- UK Clinical Research Network Study Portfolio . Targeted Ultrasound in Rheumatoid Arthritis (TURA) 2016. https://www.ukctg.nihr.ac.uk/trials/trial-details/trial-details?trialId=15763 (accessed 20 October 2015).
- Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR. Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10050.
- Bossuyt PM, Lijmer JG, Mol BW. Randomised comparisons of medical tests: sometimes invalid, not always efficient. Lancet 2000;356:1844-7. https://doi.org/10.1016/S0140-6736(00)03246-3.
- Alfredo Chávez-López M, Naredo E, Carlos Acebes-Cachafeiro J, de Miguel E, Cabero F, Sanchez-Pernaute O, et al. Diagnostic accuracy of physical examination of the knee in rheumatoid arthritis: clinical and ultrasonographic study of joint effusion and BakerOs cyst. Rheumatol Clin 2007;3:98-100. https://doi.org/10.1016/S1699-258X(07)73675-6.
- Andersen M, Ellegaard K. Ultrasound colour Doppler is associated with synovial pathology in biopsies from hand joints in rheumatoid arthritis patients: a cross-sectional study. Ann Rheum Dis 2014;73:678-83. https://doi.org/10.1136/annrheumdis-2012-202669.
- Andonopoulos AP, Yarmenitis S, Sfountouris H, Siamplis D, Zervas C, Bounas A. Baker’s cyst in rheumatoid arthritis: an ultrasonographic study with a high resolution technique. Clin Exp Rheumatol 1995;13:633-6.
- Baan H, Hoekstra M, Veehof M, Van De Laar M. Ultrasound findings in rheumatoid wrist arthritis highly correlate with function. Disabil Rehabil 2011;33:729-33. https://doi.org/10.3109/09638288.2010.509459.
- Bajaj S, Lopez-Ben, Lopez-Ben R, Oster R, Alarcon G. Ultrasound detects rapid progression of erosive disease in early rheumatoid arthritis: a prospective longitudinal study. Skelet Radiol 2007;36:123-8. https://doi.org/10.1007/s00256-006-0196-z.
- Boesen M, Boesen L, Jensen KE, Cimmino MA, Torp-Pedersen S, Terslev L, et al. Clinical outcome and imaging changes after intraarticular (IA) application of etanercept or methylprednisolone in rheumatoid arthritis: magnetic resonance imaging and ultrasound-Doppler show no effect of IA injections in the wrist after 4 weeks. J Rheumatol 2008;35:584-91.
- Brown AK, Quinn MA, Karim, Quinn MA. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum 2006;54:3761-73. https://doi.org/10.1002/art.22190.
- Bruyn GA, Naredo E, Möller I, Moragues C, Garrido J, de Bock GH, et al. Reliability of ultrasonography in detecting shoulder disease in patients with rheumatoid arthritis. Ann Rheum Dis 2009;68:357-61. https://doi.org/10.1136/ard.2008.089243.
- Carotti M, Salaffi F, Manganelli P, Salera D, Simonetti B, Grassi W. Power Doppler sonography in the assessment of synovial tissue of the knee joint in rheumatoid arthritis: a preliminary experience. Ann Rheum Dis 2002;61:877-82. https://doi.org/10.1136/ard.61.10.877.
- Damjanov N, Radunovic G, Prodanovic S, Vukovic V, Milic V, Simic Pasalic K, et al. Construct validity and reliability of ultrasound disease activity score in assessing joint inflammation in RA: comparison with DAS-28. Rheumatology (Oxford) 2012;51:120-8. https://doi.org/10.1093/rheumatology/ker255.
- da Silva Chakr RM, Brenol JC, Behar M, Mendonça JA, Kohem CL, Monticielo OA, et al. Is ultrasound a better target than clinical disease activity scores in rheumatoid arthritis with fibromyalgia? A case-control study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0118620.
- Dejaco C, Duftner C, Wipfler-Freissmuth E, Weiss H, Graninger WB, Schirmer M. Ultrasound-defined remission and active disease in rheumatoid arthritis: association with clinical and serologic parameters. Semin Arthritis Rheum 2012;41:761-7. https://doi.org/10.1016/j.semarthrit.2011.09.005.
- Di Franco M. Hypovitaminosis D in recent onset rheumatoid arthritis is predictive of reduced response to treatment and increased disease activity: a 12 month follow-up study. BMC Musculoskelet Disord 2015;16. https://doi.org/10.1186/s12891-015-0505-6.
- Dohn UM, Ejbjerg B, Boonen A, Hetland ML, Hansen MS, Knudsen LS, et al. No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann Rheum Dis 2011;70:252-8. https://doi.org/10.1136/ard.2009.123729.
- Epis O, Filippucci E, Delle Sedie A, De Matthaeis A, Bruschi E. Clinical and ultrasound evaluation of the response to tocilizumab treatment in patients with rheumatoid arthritis: a case series. Rheumatol Int 2014;34:737-42. https://doi.org/10.1007/s00296-012-2638-3.
- Foltz V, Gandjbakhch F, Etchepare F, Rosenberg C, Tanguy ML, Rozenberg S, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum 2012;64:67-76. https://doi.org/10.1002/art.33312.
- Freeston JE, Brown AK, Hensor EM, Emery, Freeston JE. Extremity magnetic resonance imaging assessment of synovitis (without contrast) in rheumatoid arthritis may be less accurate than power Doppler ultrasound. Ann Rheum Dis 2008;67. https://doi.org/10.1136/ard.2007.082743.
- Fukae J, Isobe M, Kitano A, Henmi M, Sakamoto F, Narita A, et al. Positive synovial vascularity in patients with low disease activity indicates smouldering inflammation leading to joint damage in rheumatoid arthritis: time-integrated joint inflammation estimated by synovial vascularity in each finger joint. Rheumatology (Oxford) 2013;52:523-8. https://doi.org/10.1093/rheumatology/kes310.
- Fukae J. Structural deterioration of finger joints with ultrasonographic synovitis in rheumatoid arthritis patients with clinical low disease activity. Rheumatology (Oxford) 2014;53:1608-12. https://doi.org/10.1093/rheumatology/keu154.
- Funck-Brentano T, Etchepare F, Joulin SJ, Gandjbakch F, Pensec VD, Cyteval C, et al. Benefits of ultrasonography in the management of early arthritis: a cross-sectional study of baseline data from the ESPOIR cohort. Rheumatology 2009;48:1515-19. https://doi.org/10.1093/rheumatology/kep279.
- Funck-Brentano T, Gandjbakhch F, Etchepare F, Jousse-Joulin S, Miquel A, Cyteval C, et al. Prediction of radiographic damage in early arthritis by sonographic erosions and power Doppler signal: a longitudinal observational study. Arthritis Care Res 2013;65:896-902. https://doi.org/10.1002/acr.21912.
- Geng Y, Han J, Deng X, Zhang Z. Presence of power Doppler synovitis in rheumatoid arthritis patients with synthetic and/or biological disease-modifying anti-rheumatic drug-induced clinical remission: experience from a Chinese cohort. Clin Rheumatol 2014;33:1061-6. https://doi.org/10.1007/s10067-014-2634-y.
- Hameed B, Pilcher J, Heron C, Kiely PD. The relation between composite ultrasound measures and the DAS28 score, its components and acute phase markers in adult RA. Rheumatology 2008;47:476-80. https://doi.org/10.1093/rheumatology/kem383.
- Harman H, Tekeoglu I, Takçi S, Kamanli A, Nas K, Harman S. Improvement of large-joint ultrasonographic synovitis is delayed in patients with newly diagnosed rheumatoid arthritis: results of a 12-month clinical and ultrasonographic follow-up study of a local cohort. Clin Rheumatol 2015;34:1367-74. https://doi.org/10.1007/s10067-015-2926-x.
- Harman H, Tekeoglu I, Kaban N, Harman S. Factors influencing ultrasonographic remission in patients with rheumatoid arthritis. Rheumatol Int 2015;35:485-91. https://doi.org/10.1007/s00296-014-3177-x.
- Hermann KG, Backhaus M, Schneider U, Labs K, Loreck D, Zuhlsdorf S, et al. Rheumatoid arthritis of the shoulder joint: comparison of conventional radiography, ultrasound, and dynamic contrast-enhanced magnetic resonance imaging. Arthritis Rheum 2003;48:3338-49. https://doi.org/10.1002/art.11349.
- Hmamouchi I, Bahiri R, Srifi N, Aktaou S, Abouqal R, Hajjaj-Hassouni N. A comparison of ultrasound and clinical examination in the detection of flexor tenosynovitis in early arthritis. BMC Musculoskelet Disord 2011;12. https://doi.org/10.1186/1471-2474-12-91.
- Janta I, Naredo E, Martinez-Estupinan L, Nieto JC, De la Torre I, Valor L, et al. Patient self-assessment and physician’s assessment of rheumatoid arthritis activity: which is more realistic in remission status? A comparison with ultrasonography. Rheumatology (Oxford) 2013;52:2243-50. https://doi.org/10.1093/rheumatology/ket297.
- Kawashiri SY, Suzuki T, Nakashima Y, Horai Y, Okada A, Iwamoto N, et al. Ultrasonographic examination of rheumatoid arthritis patients who are free of physical synovitis: power Doppler subclinical synovitis is associated with bone erosion. Rheumatology (Oxford) 2014;53:562-9. https://doi.org/10.1093/rheumatology/ket405.
- Kawashiri SY, Suzuki T, Nishino A, Nakashima Y, Horai Y, Iwamoto N, et al. Automated Breast Volume Scanner, a new automated ultrasonic device, is useful to examine joint injuries in patients with rheumatoid arthritis. Mod Rheumatol 2015;25:837-41. https://doi.org/10.3109/14397595.2015.1040226.
- Kelly S. Angiogenic gene expression and vascular density are reflected in ultrasonographic features of synovitis in early Rheumatoid Arthritis: an observational study. Arthritis Res Ther 2015;17. https://doi.org/10.1186/s13075-015-0567-8.
- Klauser A, Demharter J, De Marchi A, Sureda D, Barile A, Masciocchi C, et al. Contrast enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: results from the IACUS study group. Eur Radiol 2005;15:2404-10. https://doi.org/10.1007/s00330-005-2884-9.
- Klauser AS, Franz M, Arora R, Feuchtner GM, Gruber J, Schirmer M, et al. Detection of vascularity in wrist tenosynovitis: power Doppler ultrasound compared with contrast-enhanced grey-scale ultrasound. Arthritis Res Ther 2010;12. https://doi.org/10.1186/ar3185.
- Krezja . Ultrasonography of the periarticular changes in patients with early active rheumatoid arthritis. Med Sci Monit 1998;4:MT366-9.
- Lillegraven S, Boyesen P, Hammer HB, Ostergaard M, Uhlig T, Sesseng S, et al. Tenosynovitis of the extensor carpi ulnaris tendon predicts erosive progression in early rheumatoid arthritis. Ann Rheum Dis 2011;70:2049-50. https://doi.org/10.1136/ard.2011.151316.
- Lillegraven S, Prince FH, Shadick NA, Bykerk VP, Lu B, Frits ML, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis 2012;71:681-6. https://doi.org/10.1136/ard.2011.154625.
- Makinen H, Kautiainen H, Hannonen P, Mottonen T, Leirisalo-Repo M, Laasonen L, et al. Sustained remission and reduced radiographic progression with combination disease modifying antirheumatic drugs in early rheumatoid arthritis. J Rheumatol 2007;34:316-21.
- Montoro AM, Montoro Alvarez M, Chong OY, Janta I, Gonzalez C, Lopez-Longo J, et al. Relation of Doppler ultrasound synovitis versus clinical synovitis with changes in native complement component levels in rheumatoid arthritis patients treated with biologic disease-modifying anti-rheumatic drugs. Clin Exp Rheumatol 2015;33:141-5.
- Naredo E, Acebes C, Brito E, de Agustin JJ, de Miguel E, Mayordomo L, et al. Three-dimensional volumetric ultrasound: a valid method for blinded assessment of response to therapy in rheumatoid arthritis. J Rheumatol 2013;40:253-60. https://doi.org/10.3899/jrheum.121103.
- Naredo E, Hinojosa M, Valor L, Hernández-Flórez D, Mata-Martínez C, Serrano-Benavente B, et al. Does ultrasound-scored synovitis depend on the pharmacokinetics of subcutaneous anti-TNF agents in patients with rheumatoid arthritis?. Rheumatology 2014;53:2088-94. https://doi.org/10.1093/rheumatology/keu248.
- Nordal HH, Brun JG, Halse AK, Jonsson, Brun JG. The neutrophil protein S100A12 is associated with a comprehensive ultrasonographic synovitis score in a longitudinal study of patients with rheumatoid arthritis treated with adalimumab. BMC Musculoskelet Disord 2014;15. https://doi.org/10.1186/1471-2474-15-335.
- Peluso G, Michelutti A, Bosello S, Gremese E, Tolusso B, Ferraccioli G. Clinical and ultrasonographic remission determines different chances of relapse in early and long standing rheumatoid arthritis. Ann Rheum Dis 2011;70:172-5. https://doi.org/10.1136/ard.2010.129924.
- Ramirez J, Ruiz-Esquide V, Pomés I, Celis R, Cuervo A, Hernández MV, et al. Patients with rheumatoid arthritis in clinical remission and ultrasound-defined active synovitis exhibit higher disease activity and increased serum levels of angiogenic biomarkers. Arthritis Res Ther 2014;16. https://doi.org/10.1186/ar4431.
- Reiche BE, Ohrndorf S, Feist E, Messerschmidt J, Burmester GR, Backhaus M. Usefulness of power Doppler ultrasound for prediction of re-therapy with rituximab in rheumatoid arthritis: a prospective study of longstanding rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2014;66:204-16. https://doi.org/10.1002/acr.22103.
- Schmidt WA, Schicke B, Ostendorf B, Scherer A, Krause A, Walther M. Low-field MRI versus ultrasound: which is more sensitive in detecting inflammation and bone damage in MCP and MTP joints in mild or moderate rheumatoid arthritis?. Clin Exp Rheumatol 2013;31:91-6.
- Spinella A, Sandri G, Carpenito G, Belletti L, Mascia MT. The discrepancy between clinical and ultrasonographic remission in rheumatoid arthritis is not related to therapy or autoantibody status. Rheumatol Int 2012;32:3917-21. https://doi.org/10.1007/s00296-011-2259-2.
- Stramare R, Coran A, Faccinetto A, Costantini G, Bernardi L, Botsios C, et al. MR and CEUS monitoring of patients with severe rheumatoid arthritis treated with biological agents: a preliminary study. Radiol Med 2014;119:422-31. https://doi.org/10.1007/s11547-013-0369-5.
- Strunk J, Rumbaur C, Albrecht K, Neumann E, Muller-Ladner U. Linking systemic angiogenic factors (VEGF, angiogenin, TIMP-2) and Doppler ultrasound to anti-inflammatory treatment in rheumatoid arthritis. Joint Bone Spine 2013;80:270-3. https://doi.org/10.1016/j.jbspin.2012.09.001.
- Szkudlarek M, Court-Payen, Strandberg C, Klarlund M, Klausen T, Ostergaard M, . Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum 2001;44:2018-23. https://doi.org/10.1002/1529-0131(200109)44:9<2018::AID-ART350>3.0.CO;2-C.
- Taouli B, Zaim S, Peterfy CG, Lynch JA, Stork A, Guermazi A, et al. Rheumatoid arthritis of the hand and wrist: comparison of three imaging techniques. Am J Roentgenol 2004;182:937-43. https://doi.org/10.2214/ajr.182.4.1820937.
- Terslev L, Ellegaard K, Christensen R, Szkudlarek M, Schmidt WA, Jensen PS, et al. Head-to-head comparison of quantitative and semi-quantitative ultrasound scoring systems for rheumatoid arthritis: reliability, agreement and construct validity. Rheumatology (Oxford) 2012;51:2034-8. https://doi.org/10.1093/rheumatology/kes124.
- Varsamidis K, Varsamidou E, Tjetjis V, Mavropoulos G. Doppler sonography in assessing disease activity in rheumatoid arthritis. Ultrasound Med Biol 2005;31:739-43. https://doi.org/10.1016/j.ultrasmedbio.2005.02.010.
- Wakefield RJ, O’Connor PJ, Conaghan PG, McGonagle D, Hensor EMA, Gibbon WW, et al. Finger tendon disease in untreated early rheumatoid arthritis: a comparison of ultrasound and magnetic resonance imaging. Arthritis Care Res 2007;57:1158-64. https://doi.org/10.1002/art.23016.
- Watanabe T, Takemura M, Sato M, Sekine A, Fukuoka D, Seishima M, et al. Quantitative analysis of vascularization in the finger joints in patients with rheumatoid arthritis using three-dimensional volumetric ultrasonography with power Doppler. Clin Rheumatol 2012;31:299-307. https://doi.org/10.1007/s10067-011-1811-5.
- Yoshimi R, Ihata A, Kunishita Y, Kishimoto D, Kamiyama R, Minegishi K, et al. A novel 8-joint ultrasound score is useful in daily practice for rheumatoid arthritis. Mod Rheumatol 2015;25:379-85. https://doi.org/10.3109/14397595.2014.974305.
- Zheng G, Wang L, Jia X, Li F, Yan Y, Yu Z, et al. Application of high frequency color Doppler ultrasound in the monitoring of rheumatoid arthritis treatment. Exp Ther Med 2014;8:1807-12. https://doi.org/10.3892/etm.2014.2001.
- Ziswiler HR, Aeberli D. High-resolution ultrasound confirms reduced synovial hyperplasia following rituximab treatment in rheumatoid arthritis. Rheumatology (Oxford) 2009;48:939-43. https://doi.org/10.1093/rheumatology/kep139.
- Meenagh G, Filippucci E, Delle SA, Riente L, Iagnocco A, Epis O, et al. Ultrasound imaging for the rheumatologist. XVIII. Ultrasound measurements. Clin Exp Rheumatol 2008;26:982-5.
- Koski JM. Axillar ultrasound of the glenohumeral joint. J Rheumatol 1989;16:664-7.
- van Riel PLCM, van Gestel AM, Scott DL. EULAR Handbook of Clinical Assessments in Rheumatoid Arthritis. Alphen aan den Rijn: Van Zuiden; 2001.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Wakefield RJ, Balint PV, Szkudlarek, Balint PV. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 2005;32:2485-7.
- Wakefield RJ, Green MJ, Marzo-Ortega H, Conaghan PG, Gibbon WW, McGonagle D, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis 2004;63:382-5. https://doi.org/10.1136/ard.2003.007062.
- Newman JS, Laing TJ, McCarthy CJ, Adler RS. Power Doppler sonography of synovitis: assessment of therapeutic response – preliminary observations. Radiology 1996;198:582-4. https://doi.org/10.1148/radiology.198.2.8596870.
- Schmidt WA, Schmidt H, Schicke B, Gromnica-Ihle E. Standard reference values for musculoskeletal ultrasonography. Ann Rheum Dis 2004;63:988-94. https://doi.org/10.1136/ard.2003.015081.
- Nakagomi D, Ikeda K, Okubo A, Iwamoto T, Sanayama Y, Takahashi K, et al. Ultrasound can improve the accuracy of the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis to predict the requirement for methotrexate treatment. Arthritis Rheum 2013;65:890-8. https://doi.org/10.1002/art.37848.
- Lund PJ, Heikal A, Maricic MJ, Krupinski EA, Williams CS. Ultrasonographic imaging of the hand and wrist in rheumatoid arthritis. Skeletal Radiol 1995;24:591-6. https://doi.org/10.1007/BF00204858.
- Rutjes AW, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11500.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
Appendix 1 Survey
There were few publications regarding US and its use in treatment decisions (see Chapter 1). A survey was conducted of UK rheumatology units to investigate how US was being used in practice to influence therapy. The BSR publicised the survey to UK rheumatology units. Additionally, the BSR requested that respondents be questioned about the use of US in the diagnosis of RA.
The survey was available to clinicians from December 2015 to February 2016.
Only 31 responses were received by the end of February 2016. Survey respondents were self-selecting and so the sample may have been biased.
Survey form
Do you use US for diagnosis of RA, yes/no?
Do you use US for monitoring synovitis in RA, yes/no?
If you answered yes to the previous question, how frequently are patients monitored?
and what information is routinely collected (e.g. number of joints, is a particular joint count used)?
Do you use US to make decisions regarding RA therapy, yes/no?
If so, is this for decisions to start/stop medication?
Is it used for dose adjustments (e.g. tapering medication)?
Is it used for decisions regarding cDMARDs, biologics, and/or steroids?
Who conducts US, rheumatologists or radiologists or other allied health professionals (e.g. physiotherapists, nurses, podiatrists)?
Have the people conducting US received formal training in US to detect synovitis?
In which Rheumatology Unit are you based?
This survey is to provide background information for a project funded by the NIHR Health Technology Assessment programme. Survey results will be aggregated for publication and no identifying information of participants will be published. The project will be published in the Health Technology Assessment journal series. Visit the HTA programme website for more details www.nets.nihr.ac.uk/programmes/hta
Any views and opinions expressed in Health Technology Assessment journal articles are those of the authors and do not necessarily reflect those of the Department of Health.
Survey answers
Ultrasound was used in the diagnosis of RA by 27 out of 31 (87%) respondents.
Twenty respondents said they used US for monitoring synovitis (Table 15). Additionally, one respondent said that their unit planned to use US in the future. Of the 20 respondents using US for monitoring synovitis, five said that monitoring was routine, with two stating that monitoring was routine only in early arthritis. When monitoring was routine, this was every 3 or 6 months or annually. US assessment was stated explicitly to be used for cases of uncertainty regarding synovitis (12/20) or symptoms change (1/20), for distinguishing synovitis from other pathology (1/20) or for making a treatment decision or monitoring treatment (7/20). Information collected was stated by four respondents to be specific to the clinical or treatment question for which the patient was referred for US. Three respondents stated that information was collected on the presence or absence of synovitis and four stated that the number of active joints was recorded. Most respondents did not use a particular joint count, but there was one mention each of a seven-joint score, the DAS28 and 34 joints and three respondents assessed wrist and hand joints. Four respondents mentioned that grading of GSUS and PDUS was used. US was reported as being conducted by rheumatologists (23/31), radiologists (16/31) and allied professionals (6/31).
| Question | Yes, n/N (%) | No, n/N (%) | Don’t know, n/N (%) |
|---|---|---|---|
| Do you use US for monitoring synovitis in RA? | 20/31 (65) | 11/31 (35) | |
| Do you use US to make decisions regarding RA therapy? | 27/31 (87) | 4/31(13) | |
| Have the people conducting US received formal training in US to detect synovitis? | 25/31 (81) | 1/31 (3) | 5/31 (16) |
Twenty-seven respondents stated that US was used for making treatment decisions, with 20 out of 27 respondents using US for making decisions about starting or stopping medication and 4 out of 27 respondents using US for making decisions about starting medication (not stopping). US was used in making decisions around dose adjustment by 19 of the 27 respondents, with an additional two respondents stating that US was used to make decisions around escalating (but not tapering) medication. A further respondent stated that US may be used in the future for making treatment decisions.
Ultrasound was used for making treatment decisions around cDMARDs, bDMARDs and steroids by 17 respondents, for bDMARDs by three respondents and for cDMARDs and steroids by three respondents. Two respondents reported the use of US when treatment decisions were difficult for selected patients. Two respondents commented that there were plans to use US in the future for making decisions about treatment with bDMARDs.
From the responses, it can be concluded that some units do use US for making treatment decisions, but the small sample size and potential for bias mean that the results cannot be generalised across all UK rheumatology units.
Appendix 2 Patient involvement
The National Rheumatoid Arthritis Society were contacted about patient involvement in the study. It provided the names of two patients with RA who were willing to be contacted about being involved. Both patients were sent a draft of the plain English summary and were asked to provide feedback. The full report was available to provide further information. The two patients were also invited to provide comments regarding the patient experience of US and suggestions for future research priorities.
One patient did not respond. The other patient provided feedback on the plain English summary and contributed text on the patient experience of US. This feedback was helpful and was deemed to improve the accessibility of the plain English summary to a lay audience. The text of the plain English summary was amended in accordance with the patient’s suggestions. The background section of the report was amended based on information provided by the patient advisor on the patient experience of US.
Appendix 3 Literature search strategies
There were three phases of searches: (1) initial searches to identify key words, (2) the phase 1 scoping searches and (3) the phase 2 comprehensive searches. The scoping searches were conducted to determine whether or not evidence was available on US for monitoring synovitis in RA and, therefore, whether or not phase 2 of the project could be justified and whether or not any cost-effectiveness analyses could be conducted.
The phase 1 searches identified diagnostic accuracy and prognostic studies relevant to the monitoring of synovitis by US and so comprehensive searches for the diagnostic, prognostic and treatment effectiveness section of the project were conducted as planned. However, the phase 1 scoping searches revealed a lack of data to populate the planned cost-effectiveness model. Following advice from the clinical advisors and consultation with the NIHR HTA programme, it was decided that an analysis of the costs and benefits associated with US would be of value to the clinical community. However, attempting to achieve this using the model that informed the recent NICE multiple technology appraisal40 was not deemed sensible as this contained many elements that would be unnecessary when focusing on current decision problem and would add uncertainty to the results. Therefore, a simpler model was constructed, which was subject to sensitivity analyses and threshold analyses.
Initial searches to identify key words
Following the peer reviewer comments on the protocol,215 initial scoping searches for reviews and diagnostic accuracy studies were carried out on 12 March 2015 to predict the size of the evidence base. Searches for existing guidelines (national and international) were considered in the scoping searches. Existing systematic reviews were searched for in a selected number of databases by applying a specific reviews search filter. Focused diagnostic accuracy studies were searched for by applying a specific diagnostic filter. This identified 114 records, indicating that a broader search would be useful.
Phase 1 searches
Based on the initial scoping searches, design filters were not combined with the search strategies. Date and English-language limits were not applied.
The following databases were searched on 12 March 2015:
-
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
-
EMBASE (via Ovid)
-
The Cochrane Library (via Wiley Online Library)
-
Science Citation Index Expanded (via Web of Science)
-
Science Citation Index and Conference Proceedings Index (via Web of Science).
The number of records from each database (without date limits or application of a study design filter) is shown in Table 16.
| Approach | Source | Number of records |
|---|---|---|
| Electronic database | MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 459 |
| EMBASE | 1313 | |
| HTA database | 0 | |
| DARE | 3 | |
| CDSR | 0 | |
| CENTRAL | 35 | |
| NHS EED | 0 | |
| Science Citation Index Expanded and Science Citation Index and Conference Proceedings Index | 734 | |
| Electronic database and trials registry | ClinicalTrials.gov | 123 |
| Total | Retrieved | 2677 |
| Unique | 1742 | |
| Conference abstracts via Web of Science | European League Against Rheumatism Abstract Archive | 18 |
| American College of Rheumatology and Association of Rheumatology Health Professionals | 52 | |
| OMERACT conference proceedings | 6 | |
| Total | Retrieved | 76 |
| Unique | 25 | |
| Overall total | Unique records | 1767 |
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (1946–2015)
Date searched: 12 March 2015.
Search strategy
-
exp Arthritis, Rheumatoid/
-
rheumatoid arthritis.tw.
-
or/1-2
-
exp Synovitis/
-
synovitis.tw.
-
((synovial or synovium) adj5 inflam$).tw.
-
or/4-6
-
exp Ultrasonography/
-
ultrasound.tw.
-
ultrason$.tw.
-
sonography.tw.
-
echography.tw.
-
or/8-12
-
3 and 7 and 13
EMBASE (via Ovid) (1974 to 11 March 2015)
Date searched: 12 March 2015.
Search strategy
-
exp rheumatoid arthritis/
-
rheumatoid arthritis.tw.
-
or/1-2
-
exp synovitis/
-
synovitis.tw.
-
((synovial or synovium) adj5 inflam$).tw.
-
or/4-6
-
exp echography/
-
ultrasound.tw.
-
ultrason$.tw.
-
sonography.tw.
-
echography.tw.
-
or/8-12
-
3 and 7 and 13
The Cochrane Library: Cochrane Database of Systematic Reviews (1996–2015), Cochrane Central Register of Controlled Trials (1898–2015), Health Technology Assessment database (1989–2015), Database of Abstracts of Reviews of Effects (1946–2014) and NHS Economic Evaluation Database (1968–2014) (via Wiley Online Library)
Date searched: 12 March 2015.
Search strategy
#1 MeSH descriptor: [Arthritis, Rheumatoid] explode all trees
#2 rheumatoid arthritis:ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: [Synovitis] explode all trees
#5 synovitis:ti,ab,kw
#6 ((synovial or synovium) next/5 inflam*):ti,ab,kw
#7 #4 or #5 or #6
#8 ultrasound:ti,ab,kw
#9 ultrason*:ti,ab,kw
#10 sonography:ti,ab,kw
#11 or #8-#10
#12 #3 and #7 and #11
Science Citation Index Expanded and Science Citation Index and Conference Proceedings Index (1990–2015) (via Web of Science)
Date searched: 12 March 2015.
Search strategy
#10 #9 AND #4 AND #1
#9 #8 OR #7 OR #6 OR #5
#8 TOPIC: (echography)
#7 TOPIC: (sonography)
#6 TOPIC: (ultrason*)
#5 TOPIC: (ultrasound)
#4 #3 OR #2
#3 TOPIC: (((synovial or synovium) NEAR/5 inflam*))
#2 TOPIC: (synovitis)
#1 TOPIC: (rheumatoid arthritis)
ClinicalTrials.gov (US National Institutes of Health)
Date searched: 12 March 2015.
123 studies found for ultrasound | arthritis.
122 studies found for ultrasonography | arthritis.
122 studies found for sonography | arthritis.
122 studies found for echography | arthritis.
European League Against Rheumatism Abstract Archive (via Web of Science)
URL: http://scientific.sparx-ip.net/archiveeular/ (accessed 20 March 2015); published in Annals of the Rheumatic Diseases.
Date searched: 20 March 2015.
Number of results: 18.
Search strategy
#4 #3 AND #2 AND #1
#3 TOPIC: (ultrasound) OR TOPIC: (ultrason*) OR TOPIC: (sonography) OR TOPIC: (echography)
#2 TOPIC: (((synovial or synovium) NEAR/5 inflam*)) OR TOPIC: (synovitis)
#1 PUBLICATION NAME: (ann rheum dis)
American College of Rheumatology and Association of Rheumatology Health Professionals (Web of Science)
URL: http://acrabstracts.org/ (accessed 8 February 2018); published in Arthritis & Rheumatology.
Date searched: 20 March 2015.
Number of results: 52.
Search strategy
#4 #3 AND #2 AND #1
#3 TOPIC: (ultrasound) OR TOPIC: (ultrason*) OR TOPIC: (sonography) OR TOPIC: (echography)
#2 TOPIC: ((((synovial or synovium) NEAR/5 inflam*))) OR TOPIC: ((synovitis))
#1 SO=(ARTHRITIS “AND” RHEUMATISM)
An update search was carried out in May 2015, with 32 unique records retrieved (Table 17). Added to the 1767 records identified from the searches conducted in March 2015, this gave a total of 1799 records from the phase 1 electronic database searches.
| Approach | Source | Number of records |
|---|---|---|
| Electronic database | MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 11 |
| EMBASE | 20 | |
| HTA database | 0 | |
| DARE | 0 | |
| CDSR | 0 | |
| CENTRAL | 0 | |
| NHS EED | 0 | |
| Science Citation Index Expanded and Science Citation Index and Conference Proceedings Index | 21 | |
| PubMed | 24 | |
| Conference abstracts via Web of Science | European League Against Rheumatism Abstract Archive | 1 |
| American College of Rheumatology and Association of Rheumatology Health Professionals | 0 | |
| OMERACT conference abstracts proceedings | 3 | |
| Total | Retrieved | 80 |
| Final total | Records from all databases with duplicates removed | 32 unique to add to database |
The study selection criteria were refined based on the phase 1 search results. The protocol215 had stated that ‘For studies of diagnostic accuracy, study designs will be accepted into the review according to the hierarchy of evidence published by Merlin et al. 159’ As few studies were identified with diagnostic accuracy data, it was decided that diagnostic studies providing sensitivity or specificity data would be included, even if this was not the highest level of evidence according to the hierarchy,159 that is, diagnostic test accuracy studies with an independent, blinded comparator of a valid reference standard, tested on consecutive patients.
The phase 1 searches did not identify many prognostic studies or studies about response to treatment. Studies of any level according to the hierarchy of prognostic studies159 were included as well as studies with any outcome relating the use of US to treatment decisions or incorporating US to predict treatment adherence, response, failure, relapse on, or following, discontinuation of treatment and surveys looking at how US is used in treatment decisions in practice.
Phase 2 searches
The phase 1 scoping searches had identified that relevant diagnostic and prognostic studies were available and therefore the phase 2 searches were carried out. However, the phase 1 searches also revealed a lack of data to populate the planned cost-effectiveness model. As such, it was proposed that a simpler model would be constructed.
As the phase 1 searches had been successful in identifying relevant studies and had not incorporated design filters or date and English-language limits, it was decided that the same search strategies would be used, slightly broadened, for the clinical effectiveness searches in phase 2. Additionally, a cost-effectiveness filter was applied for the cost-effectiveness searches.
The following databases were searched for clinical effectiveness, cost-effectiveness and dose modification studies between 22 October and 6 November 2015:
-
BIOSIS Previews
-
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
-
EMBASE (via Ovid)
-
The Cochrane Library (via Wiley Online Library)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost)
-
PsycINFO (via Ovid)
-
Science Citation Index Expanded (via Web of Science)
-
Science Citation Index and Conference Proceedings Index (Web of Science)
-
TOXLINE (via ProQuest)
-
ProQuest Dissertations & Theses A&I (formerly Dissertation Abstracts International)
-
ProQuest Dissertations & Theses – UK & Ireland
-
EconLit.
The following trial registries, conference proceedings and websites were searched between 26 and 27 October 2015:
-
American College of Rheumatology and Association of Rheumatology Health Professionals
-
European League Against Rheumatism Abstract Archive
-
ClinicalTrials.gov
-
Current Controlled Trials
-
NICE Evidence Search
-
BSR
-
Arthritis Research UK
-
British Pain Society
-
Arthritis and Musculoskeletal Alliance
-
National Guideline Clearinghouse
-
Royal College of Physicians
-
Royal College of Radiologists
-
Royal College of Pathologists
-
Royal College of Surgeons
-
NRAS.
The phase 2 clinical effectiveness searches identified an additional 925 records. Added to the results of the phase 1 searches this gave a total of 2724 unique records from the electronic database and supplementary searches (study selection for the clinical effectiveness records is shown in Figure 1). In addition, the cost-effectiveness searches identified 226 records and a parameter search for dose modification, which was not restricted to imaging studies, identified 54 records (study selection for the cost-effectiveness review is shown in Chapter 4).
| Approach | Source | Number of records |
|---|---|---|
| Electronic database | MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 596 |
| EMBASE | 1574 | |
| CINAHL | 132 | |
| DARE | 2 | |
| CDSR | 0 | |
| CENTRAL | 1 | |
| NHS EED | 0 | |
| EconLit | 0 | |
| PsycINFO | 1 | |
| TOXLINE | 2 | |
| BIOSIS Previews | 508 | |
| Science Citation Index Expanded and Science Citation Index and Conference Proceedings Index | 975 | |
| ProQuest Dissertations & Theses A&I | 434 | |
| ProQuest Dissertations & Theses – UK & Ireland | 23 | |
| Conference abstracts via Web of Science | European League Against Rheumatism Abstract Archive | 0 |
| American College of Rheumatology and Association of Rheumatology Health Professionals | 3 | |
| OMERACT conference abstracts | 0 | |
| Grey literature | Current Controlled Trials | 1 |
| ClinicalTrials.gov | 23 | |
| BSR | 0 | |
| Royal College of Physicians | 0 | |
| Royal College of Radiologists | 3 | |
| Royal College of Surgeons | 0 | |
| NRAS | 41 | |
| Royal College of Pathologists | 0 | |
| Arthritis Research UK | 16 | |
| British Pain Society | 0 | |
| Arthritis and Musculoskeletal Alliance | 1 | |
| National Guideline Clearinghouse | 19 | |
| NICE Evidence Search | 33 | |
| Total | Retrieved total | 4388 |
| Unique total | 1205 |
Search strategies
BIOSIS Previews (via Web of Science) (1969–2015)
Date searched: 6 November 2015.
Search strategy
#1 TOPIC: (rheumat* NEAR/5 (nodule or arthritis))
#2 TOPIC: =(felty* NEAR/2 syndrome)
#3 TOPIC: =(Caplan* NEAR/2 syndrome)
#4 TOPIC: (Sjogren* NEAR/2 syndrome)
#5 TOPIC: (Sicca NEAR/2 syndrome)
#6 TOPIC: Still* disease
#7 TOPIC: Bechterew* disease
#8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
#9 TOPIC: Synovitis
#10 TOPIC: ((Synovial or synovium) NEAR/5 (inflam* or hypertrophy))
#11 #9 OR #10
#12 TOPIC: Ultrasound
#13 TOPIC: Ultrason*
#14 TOPIC: Sonography
#15 TOPIC: Echography
#16 TOPIC: Ultrasonic
#17 #12 OR #13 OR #14 OR #15 OR #16
#18 #8 AND #11 #17
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost) (1969–2015)
Date searched: 22 October 2015.
Search strategy
S1 (MH “Arthritis, Rheumatoid+”)
S2 TX (Rheumat* N5 (nodule or arthritis))
S3 (Felty* N2 syndrome)
S4 TX (Sjogren* N2 syndrome)
S5 TX (Sicca N2 syndrome)
S6 TX Still* disease
S7 TX Bechterew*
S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7
S9 (MH “Synovitis”)
S10 TS=((Synovial or synovium) NEAR/5 (inflam* or hypertrophy))
S11 #9 OR #10
S12 TX Ultrasound
S13 TX Ultrason*
S14 TX Sonography
S15 TX Echography
S16 TX Ultrasonic
S17 S12 OR S13 OR S14 OR S15 OR S16
S18 S8 AND S11 AND S17
The Cochrane Library: Cochrane Database of Systematic Reviews (1996–2015), Cochrane Central Register of Controlled Trials (1898–2015), Health Technology Assessment database (1989–2015), Database of Abstracts of Reviews of Effects (1946–2014) and NHS Economic Evaluation Database (1968–2014) (via Wiley Online Library)
Date searched: 22 October 2015.
Search strategy
#1 MeSH descriptor: [Arthritis, Rheumatoid] explode all trees
#2 (Rheumat* next/5 (nodule or arthritis)):ti,ab,kw
#3 (felty* next/2 syndrome):ti,ab,kw
#4 (caplan* next/2 syndrome):ti,ab,kw
#5 (Sjogren* next/2 syndrome):ti,ab,kw
#6 (Sicca next/2 syndrome):ti,ab,kw
#7 Still* disease:ti,ab,kw
#8 Bechterew* disease:ti,ab,kw
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 MeSH descriptor: [Synovitis] explode all trees
#11 synovitis:ti,ab,kw
#12 synovitis:ti,ab,kw
#13 #10 OR #11 OR #12
#14 MeSH descriptor: [Ultrasonography] explode all trees
#15 Ultrasound:ti,ab,kw
#16 Ultrason*:ti,ab,kw
#17 Sonography:ti,ab,kw
#18 Echography:ti,ab,kw
#19 Ultrasonic:ti,ab,kw
#20 #14 or #15 or #16 or #17 or #18 or #19
#21 #9 and #13 and #20
ClinicalTrials.gov (US National Institutes of Health)
Date searched: 26 October 2015.
91 studies found for ultrasound | rheumatoid arthritis.
Checked for duplicates: 23 imported.
Current Controlled Trials
Date searched: 27 October 2015.
Search strategy
-
text search: Synovitis condition: Rheumatoid Arthritis AND Interventions: ultrasonography 0 results found
-
Condition: Rheumatoid Arthritis AND Interventions: ultrasonography 1 result found
-
Condition: Rheumatoid Arthritis AND Interventions: ultrasonound 0 results found
-
Condition: Rheumatoid Arthritis AND Interventions: sonography 0 results found
-
Condition: Rheumatoid Arthritis AND Interventions: Echography 0 results found
EMBASE (via Ovid) (1974 to 20 October 2015)
Date searched: 20 October 2015.
Search strategy
-
exp rheumatoid arthritis/
-
(rheumat$ adj5 (nodule or arthritis)).tw.
-
felty$ adj2 syndrome).tw
-
(caplan$ adj2 syndrome).tw
-
(rheumat$ adj2 (nodule or arthritis)).tw
-
(sjogren$ adj2 syndrome).tw
-
(sicca adj2 syndrome).tw
-
still$ disease.tw
-
bechterew$ disease.tw
-
or/1-9
-
exp synovitis/
-
synovitis.tw.
-
((Synovial or synovium) adj5 (inflam$ or hypertrophy)).tw
-
or/11-13
-
exp echography/
-
ultrasound.tw.
-
ultrason$.tw.
-
sonography.tw.
-
echography.tw.
-
Ultrasonic.tw
-
or/15-20
-
11 and 14 and 21
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (1946–2015)
Date searched: 21 October 2015.
Search strategy
-
exp Arthritis, Rheumatoid/
-
(Rheumat$ adj5 (nodule or arthritis)).tw
-
(Felty$ adj2 syndrome).tw
-
(Caplan$ adj2 syndrome).tw
-
(Sjogren$ adj2 syndrome).tw
-
(Sicca adj2 syndrome).tw
-
Still$ disease.tw
-
Bechterew$ disease.tw
-
or/1-8
-
Synovitis/
-
Synovitis.tw.
-
((Synovial or synovium) adj5 (inflam$ or hypertrophy)).tw.
-
or/10-12
-
exp Ultrasonography/
-
Ultrasound.tw
-
Ultrason$.tw.
-
Sonography.tw
-
Echography.tw.
-
Ultrasonic.tw
-
or/14-19
-
9 and 13 and 20
NICE Evidence Search
URL: www.evidence.nhs.uk/ (accessed 8 February 2018).
Date searched: 26 October 2015.
52 studies found for “rheumatoid arthritis” | “Synovitis” | “ultrasonography”
Checked for duplicates: 33 imported
Science Citation Index Expanded and Science Citation Index and Conference Proceedings Index (via Web of Science) (1900–2015)
Date searched: 22 October 2015.
Search strategy
#18 #8 AND #11 AND #17
#17 #12 OR #13 OR #14 OR #15 OR #16
#16 TOPIC: Ultrasonic
#15 TOPIC: Echography
#14 TOPIC: Sonography
#13 TOPIC: Ultrason*
#12 TOPIC: Ultrasound
#11 #9 OR #10
#10 TOPIC: ((Synovial or synovium) NEAR/5 (inflam* or hypertrophy))
#9 TOPIC: Synovitis
#8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
#7 TOPIC: Bechterew* disease
#6 TOPIC: Still* disease
#5 TOPIC: (Sicca NEAR/2 syndrome)
#4 TOPIC: (Sjogren* NEAR/2 syndrome)
#3 TOPIC: (Caplan* NEAR/2 syndrome)
#2 TOPIC: (Felty* NEAR/2 syndrome)
#1 TOPIC: (rheumat* NEAR/5 (nodule or arthritis))
TOXLINE (via ProQuest) (1999–2015)
Date searched: 6 November 2015.
Search strategy
S17 S7 AND S10 AND S16
S16 S11 OR S12 OR S13 OR S14 OR S15
S15 Ultrasonic
S14 Echography
S13 Sonography
S12 Ultrason*
S11 Ultrasound
S10 S8 OR S9
S9 ((Synovial or synovium) NEAR/5 (inflam* or hypertrophy))
S8 Synovitis
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6
S6 Still* disease
S5 (Sicca NEAR/2 syndrome)
S4 (Caplan NEAR/2 syndrome)
S3 (Felty* NEAR/2 syndrome)
S2 (Rheumat* NEAR/5 (nodule or arthritis))
S1 Arthritis, Rheumatoid
PsycINFO (via Ovid) (1806 to October week 3 2015)
Date searched: 21 October 2015.
Search strategy
-
exp Rheumatoid Arthritis/
-
(Rheumat$ adj5 (nodule or arthritis)).tw.
-
(Felty$ adj2 syndrome).tw.
-
(Caplan$ adj2 syndrome).tw.
-
(Sjogren$ adj2 syndrome).tw.
-
(Sicca adj2 syndrome).tw.
-
Still$ disease.tw.
-
Bechterew$ disease.tw.
-
or/1-8
-
Synovitis.tw
-
((Synovial or synovium) adj5 (inflam$ or hypertrophy)).tw
-
or/10-11
-
exp Ultrasound/
-
Ultrasound.tw
-
Ultrason$.tw
-
Sonography.tw
-
Echography.tw
-
Ultrasonic.tw
-
or/13-18
-
9 and 12 and 19
ProQuest Dissertations & Theses A&I (via ProQuest) (1743–2015)
Date searched: 26 October 2015.
Search strategy
S1 (Rheumat* NEAR/5 (nodule or arthritis))
S2 (Felty* NEAR/2 syndrome)
S3 (Caplan* NEAR/2 syndrome)
S4 (Sjogren* NEAR/2 syndrome)
S5”Still* disease”
S6 “Bechterew* disease”
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6
S8 Synovitis
S9 ((Synovial or synovium) NEAR/5 (inflam* or hypertrophy))
S10 s8 or s9
S11 Ultrasound
S12 Ultrason*
S13 Sonography
S14 Echography
S15 Ultrasonic
S16 S11 OR S12 OR S13 OR S14 OR S15
S17 S7 AND S10 AND S16
ProQuest Dissertations & Theses – UK & Ireland (via ProQuest) (1986–2015)
Date searched: 26 October 2015.
Search strategy
S1 (Rheumat* NEAR/5 (nodule or arthritis))
S2 (Felty* NEAR/2 syndrome)
S3 (Caplan* NEAR/2 syndrome)
S4 (Sjogren* NEAR/2 syndrome)
S5 “Still* disease”
S6 “Bechterew* disease”
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6
S8 Synovitis
S9 ((Synovial or synovium) NEAR/5 (inflam* or hypertrophy))
S10 S8 or S9
S11 Ultrasound
S12 Ultrason*
S13 Sonography
S14 Echography
S15 Ultrasonic
S16 S11 OR S12 OR S13 OR S14 OR S15
S17 S7 AND S10 AND S16
European League Against Rheumatism Abstract Archive (via Web of Science)
URL: http://scientific.sparx-ip.net/archiveeular/ (accessed 9 November 2015); published in Annals of the Rheumatic Diseases.
Date searched: 9 November 2015.
Search strategy
#4 #3 AND #2 AND #1
#3 TOPIC: (ultrasound) OR TOPIC: (ultrason*) OR TOPIC: (sonography) OR TOPIC: (echography)
#2 TOPIC: (((synovial or synovium) NEAR/5 inflam*)) OR TOPIC: (synovitis)
#1 PUBLICATION NAME: (ann rheum dis)
American College of Rheumatology and Association of Rheumatology Health Professionals (via Web of Science)
URL: http://acrabstracts.org/ (accessed 8 February 2018); published in Arthritis & Rheumatology.
Date searched: 9 November 2015.
Search strategy
#4 #3 AND #2 AND #1
#3 TOPIC: (ultrasound) OR TOPIC: (ultrason*) OR TOPIC: (sonography) OR TOPIC: (echography)
#2 TOPIC: ((((synovial or synovium) NEAR/5 inflam*))) OR TOPIC: ((synovitis))
#1 SO=(ARTHRITIS “AND” RHEUMATISM)
British Society for Rheumatology
Date searched: 27 October 2015.
0 studies found for Ultrasound.
0 studies found for Ultrasonography.
0 studies found for Sonography.
0 studies found for Echography.
0 studies found for Synovitis.
Royal College of Physicians
URL: www.rcplondon.ac.uk/
Date searched: 27 October 2015.
0 studies found for Ultrasound synovitis.
0 studies found for Ultrasonography.
0 studies found for Sonography.
0 studies found for Echography.
0 studies found for Synovitis.
Royal College of Radiologists
URL: www.rcr.ac.uk/
Date searched: 27 October 2015.
0 studies found for Rheumatoid arthritis ultrasound.
0 studies found for Rheumatoid arthritis ultrasonography.
0 studies found for Rheumatoid arthritis sonography.
0 studies found for Rheumatoid arthritis echography.
0 studies found for Rheumatoid arthritis synovitis.
Royal College of Surgeons
URL: www.rcseng.ac.uk
Date searched: 27 October 2015.
0 results found for Synovitis.
Royal College of Pathologists
URL: www.rcpath.org/
Date searched: 27 October 2015.
0 studies found for Ultrasound synovitis
0 studies found for Ultrasonography synovitis
0 studies found for Sonography synovitis
0 studies found for Echography synovitis
National Rheumatoid Arthritis Society
URL: www.nras.org.uk (accessed 18 March 2015).
Date searched: 27 October 2015.
25 results found for Ultrasound synovitis.
6 studies found for Ultrasonography synovitis.
5 studies found for Sonography synovitis.
5 studies found for Echography synovitis.
Arthritis Research UK
URL: www.arthritisresearchuk.org/
Date searched: 27 October 2015.
13 studies found for Ultrasound synovitis (filter by research).
1 study found for Ultrasonography synovitis.
1 study found for Sonography synovitis.
1 study found for Echography synovitis.
British Pain Society
URL: www.britishpainsociety.org/
Date searched: 27 October 2015.
0 studies found for Rheumatoid arthritis
National Guideline Clearinghouse
URL: www.guideline.gov/
Date searched: 27 October 2015.
19 studies found for “Rheumatoid arthritis ultrasonography”.
Cost-effectiveness searches
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost) (1969–2015)
Date searched: 22 October 2015.
Search strategy
S1 (MH “Economics+”)
S2 (MH “Financial Management+”)
S3 (MH “Financial Support+”)
S4 (MH “Financing, Organized+”)
S5 (MH “Business+”)
S6 S2 OR S3 OR S4 OR S5
S7 S1 NOT S6
S8 MH Health resource allocation
S9 MH Health resource utilization
S10 S8 OR S9
S11 S7 OR S10
S12 TX (Cost or costs or economic* or pharmacoeconomic* or price* or pricing*)
S13 S11 OR S12
S14 PT Editorial
S15 PT Letter
S16 PT News
S17 S14 OR S15 OR S16
S18 S13 NOT S17
S19 (MH “Animal Studies”)
S20 S18 NOT S19
S21 (MH “Arthritis, Rheumatoid+”)
S22 TX (Rheumat* N5 (nodule or arthritis))
S23 (Felty* N2 syndrome)
S24 TX (Caplan* N2 syndrome)
S25 TX (Sjogren* N2 syndrome)
S26 TX (Sicca N2 syndrome)
S27 TX Still* disease
S28 TX Bechterew* disease
S29 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28
S30 (MH “Ultrasonography+”)
S31 TX Ultrasound
S32 TX Ultrason*
S33 TX Sonography
S34 TX Echography
S35 TX Ultrasonic
S36 S30 OR S31 OR S32 OR S33 OR S34 OR S35
S37 S18 AND S29 AND S36
EMBASE (via Ovid) (1974 to 20 October 2015)
Date searched: 21 October 2015.
Search strategy
-
Socioeconomics/
-
Cost benefit analysis/
-
Cost-effectiveness analysis/
-
Cost of illness/
-
Cost control/
-
Economic aspect/
-
Financial management/
-
Health care cost/
-
Health care financing/
-
Health economics/
-
Hospital cost/
-
(fiscal or financial or finance or funding).tw
-
Cost minimisation analysis/
-
(cost adj estimate$).mp
-
(cost adj variable$).mp
-
(unit adj cost$).mp
-
or/1–16
-
exp rheumatoid arthritis/
-
(rheumat$adj5 (nodule or arthritis)).tw synovitis.tw.
-
(felty$adj2 syndrome).tw
-
(Caplan$adj2 syndrome).tw
-
(Rheumat$adj2 (nodule or arthritis)).tw
-
(Sjogren$adj2 syndrome).tw
-
(Sicca adj2 syndrome).tw
-
Still$disease.tw
-
Bechterew$disease.tW
-
or/18–26
-
exp echography/
-
ultrasound.tw.
-
ultrason$.tw.
-
sonography.tw.
-
echography.tw.
-
Ultrasonic.tw
-
or/28–33
-
17 and 28 and 34
MEDLINE, MEDLINE Daily and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (1946–2015)
Date searched: 21 October 2015.
Search strategy
-
Economics/
-
“costs and cost analysis”/
-
Cost-effectiveness analysis/
-
Cost–benefit analysis/
-
Cost control/
-
Cost savings/
-
Cost of illness/
-
Cost sharing/
-
“deductibles and coinsurance”/
-
Medical savings accounts/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp “fees and charges”/
-
exp budgets/
-
(Low adj cost).mp.
-
(High adj cost).mp.
-
(Health?care adj cost$).mp.
-
(Fiscal or funding or financial or finance).tw.
-
(Cost adj estimate$).mp.
-
(Cost adj variable).mp.
-
(Unit adj cost$).mp.
-
(Economic$or pharmacoeconomic$or price$or pricing).tw.
-
or/1–32
-
exp Arthritis, Rheumatoid/
-
(Rheumat$adj5 (nodule or arthritis)).tw
-
(Felty$adj2 syndrome).tw.
-
(Caplan$adj2 syndrome).tw.
-
(Sjogren$adj2 syndrome).tw.
-
(Sicca adj2 syndrome).tw.
-
Still$disease.tw.
-
Bechterew$disease.tw.
-
or/34–41
-
exp Ultrasonography/
-
Ultrasound.tw.
-
Ultrason$.tw.
-
Sonography.tw.
-
Echography.tw.
-
Ultrasonic.tw.
-
or/43–48
-
33 and 42 and 49
Science Citation Index Expanded and Science Citation Index and Conference Proceedings Index (via Web of Science) (1900–2015)
Date searched: 23 October 2015.
Search strategy
#22 #21 AND #15 AND #7
#21 #16 OR #17 OR #18 OR #19 OR #20
#20 TOPIC: Ultrasonic
#19 TOPIC: Echography
#18 TOPIC: Sonography
#17 TS = Ultrason*
#16 TOPIC: Ultrasound
#15 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14
#14 TOPIC: Bechterew* disease
#13 TOPIC: Still* disease
#12 TOPIC: (Sicca NEAR/2 syndrome)
#11 TOPIC: (Sjogren* NEAR/2 syndrome)
#10 TOPIC: (Caplan* NEAR/2 syndrome)
#9 TOPIC: (Felty* NEAR/2 syndrome)
#8 TOPIC: (Rheumat* NEAR/5 (nodule or arthritis))
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6
#6 TOPIC: (Pharmacoeconomic* or price* or pricing)
#5 TOPIC: (Cost NEAR (estimate* OR variable OR unit))
#4 TOPIC: (Fiscal or funding or financial or finance)
#3 TOPIC: ((Low OR high) near cost*)
#2 TOPIC: (Cost* near/3 (analy* or benefit* or control or saving or illness* or sharing))
#1 TOPIC: Economic*
EconLit (via Ovid) (1961–2015)
Date searched: 22 October 2015.
As this database is dedicated to economic studies, no filters were used in the search.
Search strategy
-
(rheumat$adj5 (nodule or arthritis)).tw.
-
(felty$adj2 syndrome).tw.
-
(caplan$adj2 syndrome).tw.
-
(sjogren$adj2 syndrome).tw.
-
(sicca adj2 syndrome).tw.
-
still$disease.tw.
-
bechterew$disease.tw.
-
or/1–7
-
synovitis.tw.
-
((Synovial or synovium) adj5 (inflam$or hypertrophy)).tw.
-
or/9–10
-
Ultrasound.tw.
-
Ultrason$.tw.
-
Sonography.tw.
-
Echography.tw.
-
Ultrasonic.tw.
-
or/12–16
-
8 AND 11 AND 17
NHS Economic Evaluation Database (1968–2014) (via Wiley Online Library)
Searched as part of The Cochrane Library search [see The Cochrane Library: Cochrane Database of Systematic Reviews (1996–2015), Cochrane Central Register of Controlled Trials (1898–2015), Health Technology Assessment database (1989–2015), Database of Abstracts of Reviews of Effects (1946–2014) and NHS Economic Evaluation Database (1968–2014) (via Wiley Online Library) on page 101].
Parameter search
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (1946–2015)
Date searched: 20 October 2015.
Search strategy
-
Economics/
-
“costs and cost analysis”/
-
Cost-effectiveness analysis/
-
Cost–benefit analysis/
-
Cost control/
-
Cost savings/
-
Cost of illness/
-
Cost sharing/
-
“deductibles and coinsurance”/
-
Medical savings accounts/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp “fees and charges”/
-
exp budgets/
-
(Low adj cost).mp.
-
(High adj cost).mp.
-
(Health?care adj cost$).mp.
-
(Fiscal or funding or financial or finance).tw.
-
(Cost adj estimate$).mp.
-
(Cost adj variable).mp.
-
(Unit adj cost$).mp.
-
(Economic$or pharmacoeconomic$or price$or pricing).tw.
-
or/1–32
-
exp Arthritis, Rheumatoid/
-
Rheumatoid arthritis.tw.
-
(Felty$adj2 syndrome).tw.
-
(Caplan$adj2 syndrome).tw.
-
(Rheumat$adj2 (nodule or arthritis)).tw.
-
(Sjogren$adj2 syndrome).tw.
-
(Sicca adj2 syndrome).tw.
-
Still$disease.tw.
-
Bechterew$disease.tw.
-
or/34–42
-
Biologic$.tw.
-
Anti-TNF.tw.
-
TNF-antagonist.tw.
-
TNF-inhibitor.tw.
-
(Abatacept or orencia).tw
-
(Adalimumab or humira or exemptia).tw.
-
(Certolizumab or CDP870 or cimzia).tw.
-
(Etanercept or enbrel).tw.
-
(Golimumab or CNTO 148 or simponi).tw.
-
(Infliximab or remicade or remsima or inflectra).tw.
-
(Rituximab or rituxan or mabthera or zytux).tw.
-
(Tocilizumab or atlizumab or actemra or RoActemra).tw.
-
or/44–55
-
Taper$.tw.
-
Withdraw$.tw.
-
Discontinu$.tw.
-
Stop$.tw.
-
or/57–60
-
33 and 43 and 56 and 61
Ongoing studies
The search of ClinicalTrials.gov identified some relevant studies that were ongoing or for which the results were not reported at the time of writing (March 2016) (Table 19).
| NCT number | Trial title |
|---|---|
| NCT01205854 | Aiming for Remission in Rheumatoid Arthritis (RA) – the ARCTIC Trial (ARCTIC) |
| NCT02219347 | Biomarkers of Remission in Rheumatoid Arthritis (BioRRA) |
| NCT01526434 | Health-related Quality of Life and Patient-reported Outcomes in Rheumatoid Arthritis Patients Treated with Certolizumab Pegol (SONAR-12) |
| NCT02140229 | Is Ultrasound Remission a Real Remission? Does Ultrasound Permit to Achieve and Maintain the Remission in Rheumatoid Arthritis Patients More Efficiently Than Clinical Scores? (REVECHO) |
| NCT02321930 | Musculoskeletal Ultrasound Assessment of Therapeutic Response of Tofacitinib in Rheumatoid Arthritis Patients |
| NCT01717859 | Musculoskeletal Ultrasound in Predicting Early Dose Titration with Tocilizumab (RASTS) |
| NCT01443364 | Open Label Study to Assess the Predictability of Early Response to Certolizumab Pegol in Patients with Rheumatoid Arthritis (SPEED) |
| NCT02064400 | Pilot Study of Ultrasound in Rheumatoid Arthritis |
| NCT00854243 | Role of Greyscale and Power Doppler Sonography in Therapy Monitoring in Early Rheumatoid Arthritis (RA) |
| NCT02202837 | Study with Etanercept Focusing on Remission and Predictability of Remission in Real Life Clinical Practice (REACH RA) |
| NCT02056184 | Targeted Ultrasound in Rheumatoid Arthritis (TURA) |
| NCT01752309 | The Predictive Value of Ultrasound in Early Rheumatoid Arthritis (EVA) |
| NCT01282528 | Ultrasonographic Monitoring of Response to Infliximab in Patients with Rheumatoid Arthritis (ULTRA) |
| NCT00781989 | Ultrasonography as a Biomarker in Early Rheumatoid Arthritis |
| NCT01602302 | Ultrasound and Withdrawal of Biological DMARDs in Rheumatoid Arthritis (RA-BioStop) |
Appendix 4 Excluded studies
Table 20 shows the studies excluded at full-text sift, with reasons for exclusion.
| First author | Year of publication | Reason for exclusion |
|---|---|---|
| Alfredo Chávez-López220 | 2007 | Non-English language |
| Andersen221 | 2014 | No outcome data meeting review inclusion criteria |
| Andonopoulos222 | 1995 | Study not about synovitis |
| Baan223 | 2011 | No outcome data meeting review inclusion criteria |
| Bajaj224 | 2007 | No clinical comparator |
| Boesen225 | 2008 | No relevant outcomes |
| Brown226 | 2006 | No outcome data meeting review inclusion criteria |
| Bruyn227 | 2009 | No clinical comparator |
| Carotti228 | 2002 | No outcome data meeting review inclusion criteria |
| Cheung50 | 2010 | No outcome data meeting review inclusion criteria |
| D’agostino62 | 2016 | No clinical comparator |
| Damjanov229 | 2012 | No outcome data meeting review inclusion criteria |
| da Silva Chakr230 | 2015 | No outcome data meeting review inclusion criteria |
| Dejaco231 | 2012 | No outcome data meeting review inclusion criteria |
| DiFranco232 | 2015 | No outcome data meeting review inclusion criteria |
| Dohn233 | 2011 | No outcome data meeting review inclusion criteria |
| Epis234 | 2014 | No outcome data meeting review inclusion criteria |
| Foltz235 | 2012 | No clinical comparator |
| Freeston236 | 2008 | No clinical comparator |
| Fukae237 | 2013 | No clinical comparator |
| Fukae238 | 2014 | No clinical comparator |
| Funck-Brentano239 | 2009 | Not all patients have RA |
| Funck-Brentano240 | 2013 | No clinical comparator |
| Geng241 | 2014 | No outcome data meeting review inclusion criteria |
| Haavardsholm110 | 2009 | No clinical comparator |
| Hameed242 | 2008 | No outcome data meeting review inclusion criteria |
| Harman243 | 2015 | No clinical comparator |
| Harman244 | 2015 | No outcome data meeting review inclusion criteria |
| Hermann245 | 2003 | No outcome data meeting review inclusion criteria |
| Hmamouchi246 | 2011 | Study not about synovitis |
| Janta247 | 2013 | No outcome data meeting review inclusion criteria |
| Kawashiri248 | 2014 | No clinical comparator |
| Kawashiri249 | 2015 | No clinical comparator |
| Kelly250 | 2015 | No outcome data meeting review inclusion criteria |
| Klauser251 | 2005 | No clinical comparator |
| Klauser252 | 2010 | Study not about synovitis |
| Krejza253 | 1998 | Study about initial diagnosis of arthritis |
| Lillegraven254 | 2011 | Study not about synovitis |
| Lillegraven255 | 2012 | Not a US study |
| Makinen256 | 2007 | Not a US study |
| Marks26 | 2015 | No clinical comparator |
| Molenaar48 | 2004 | Not a US study |
| Montoro257 | 2015 | No outcome data meeting review inclusion criteria |
| Naredo54 | 2005 | No outcome data meeting review inclusion criteria |
| Naredo258 | 2013 | No outcome data meeting review inclusion criteria |
| Naredo259 | 2014 | No outcome data meeting review inclusion criteria |
| Nordal260 | 2014 | No outcome data meeting review inclusion criteria |
| Peluso261 | 2011 | No clinical comparator |
| Ramirez262 | 2014 | No outcome data meeting review inclusion criteria |
| Reiche263 | 2014 | No clinical comparator |
| Schmidt264 | 2013 | No relevant clinical comparator data |
| Spinella265 | 2012 | No outcome data meeting review inclusion criteria |
| Stramare266 | 2014 | No outcome data meeting review inclusion criteria |
| Strunk267 | 2013 | No outcome data meeting review inclusion criteria |
| Szkudlarek268 | 2001 | No separate data for RA patients |
| Taouli269 | 2004 | Not a US study |
| Terslev270 | 2012 | No outcome data meeting review inclusion criteria |
| Varsamidis271 | 2005 | No clinical comparator |
| Wakefield272 | 2007 | Study not about synovitis |
| Watanabe273 | 2012 | No outcome data meeting review inclusion criteria |
| Yoshimi274 | 2015 | No clinical comparator |
| Zheng275 | 2014 | No outcome data meeting review inclusion criteria |
| Ziswiler276 | 2009 | No outcome data meeting review inclusion criteria |
Appendix 5 Data extraction tables
For calculations of diagnostic accuracy, US was counted as the reference standard and the accuracy of the clinical comparator was assessed using sensitivity (i.e. the proportion of TPs) and specificity (i.e. the proportion of TNs). Sensitivity is calculated as the number of TPs divided by the sum of the TPs and FNs; specificity is calculated as the number of TNs divided by the sum of the TNs and FPs.
| First author (study name) | Backhaus69 |
|---|---|
| Year | 2013 |
| Abstract or full paper | Full paper |
| Study design | Prognostic |
| Study objective | To determine the sensitivity to change of the US7 score among RA patients under various therapies and to analyse the effect of each therapeutic option over 1 year. To estimate predictors for the development of destructive bone changesp. 1163 |
| Population sample size | 432 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | RA |
| Population baseline characteristics |
81% female, 19% male Mean (SD, range) age 57 (12.8, 17–84) years Mean (SD, range) disease duration 8.3 (8.7, 0.08–58.3) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) |
73.2% prednisolone equivalent with a mean daily dosage of 8.8 mg per day Divided into four therapy groups at baseline: |
| Joints assessed |
CE: DAS28 US: seven-joint count – wrist, MCP 2 and 3, PIP 2 and 3, MTP 2 and 5 |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Settings for GSUS: frequency 16 MHz, length of scanner 40–42 mm Settings for PDUS: frequency 9.1 MHz, pulse repetition frequency 500–750 Hz (depending on machine setting). Machine NR Joints were evaluated for synovitis and tenosynovitis/paratenonitis and superficial bone erosions according to EULAR criteria and OMERACT definition including GSUS and PDUS GSUS was graded on the Scheel semiquantitative scale and PDUS was graded from 0 to 3 on the Szkudlarek semiquantitative scale |
| Who conducted US | NR |
| Comparator CE details | DAS28, ESR, CRP |
| Who conducted comparator CE | NR |
| Follow-up duration (if relevant) | 12 months |
| Primary outcome of study | Sensitivity to change of the US7 score among a large cohort of RA patients under various therapies (cDMARDs and/or biological therapy) and analysis of the effect of each therapeutic option over a period of 1 year |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | The US7 score was comparable to clinical and laboratory data, illustrating its potential to reflect the therapeutic response and sensitivity to change. Erosions declined significantly among patients who switched from one biologic to another, but were stable in the other groups |
| First author (study name) | Balsa105 |
|---|---|
| Year | 2010 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To investigate the accuracy of composite scores in classifying RA patients who are in remission using the absence of inflammatory activity detected by US as a gold standardp. 683 |
| Population sample size | 97 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Patients were classified as being in clinical remission by their attending rheumatologist using subjective clinical judgement |
| Population baseline characteristics |
70 female, 27 male Mean (SD, range) age 56 (12.2, 18–82) years Mean (SD, range) disease duration 5.9 (9.6, 1–18) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Taking DMARD therapy or biological agents at baseline. Excluded if taking a high dose of steroids (> 7.5 mg of prednisone daily) or had a history of intra-articular steroid joint injection during the past 6 months |
| Joints assessed | 42 joints: PIP, MCP, wrist, elbow, bilateral glenohumeral, knee, ankle and midtarsal and MTP joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Acuson Antares Siemens system with a linear probe (5–13 MHz) and a Doppler frequency of 5–8.9 MHz GSUS for synovial hypertrophy (SH) and/or joint effusion graded using a 0–3 semiquantitative scoring method (0 = no SH, 1 = mild SH, 2 = moderate SH and 3 = severe SH). PDUS graded using a 0–3 semiquantitative scoring method (0 = no PD signal, 1 = one or two vessels in small joints or up to three single vessels in large joints, 2 = less than half of the synovial area and 3 = more than half of the synovial area) |
| Who conducted US | Expert US rheumatologist |
| Comparator CE details | DAS28, SDAI |
| Who conducted comparator CE | Attending rheumatologist |
| Primary outcome of study | The relationship between clinical remission and imaging remission |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | ‘SDAI classification of remission is closer to the concept of an absence of inflammatory activity, as defined by the absence of a positive PD signal by US’ (p. 683), than DAS28 classification of remission |
| First author (study name) | Beckers106 |
|---|---|
| Year | 2004 |
| Abstract or full paper | Full papera |
| Study design | Diagnostic |
| Study objective | To assess synovitis by 18F-FDG PET in an individual joint analysis and in a global analysis of RA disease activity and to compare 18F-FDG PET parameters with clinical, biological and sonographic (US) rheumatoid parametersp. 956 |
| Population sample size | 21 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA, no DMARDs within 2 months |
| Population baseline characteristics |
17 women, 4 men Mean (range) age 48 (34–69) years Mean (range) disease duration 11 (1–24) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Previously treated with 3.4 (range 1–8) DMARDs. Seventeen patients received low-dose oral corticosteroids (mean 7 mg/day, range 4–10 mg/day of prednisolone) and all received NSAIDs. None had taken a DMARD for 2 months before study entry |
| Joints assessed | 356 joints in total: knees in all subjects and either wrists as well as MCP and PIP joints in 13 patients or ankles and the first MTP joints in the remaining eight patients |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS GSUS 13.0-MHz transducer and PDUS 5-MHz transducer (Aloka Prosound 5500). Pulse repetition frequency of 651 Hz for PDUS. Cut-off for US positivity was synovitis of ≥ 1-mm thick. PDUS scored on a 0–3 semiquantitative scale (0 = no signal, 1 = intermittent, 2 = persistent single spotting within the same location, 3 = multiple persistent spotting within the same location). Joints assessed at multiple sites (wrists and knees) were considered positive for GSUS and PDUS if at least one measurement/signal was identified |
| Who conducted US | One radiologist and one rheumatologist experienced in US |
| Comparator CE details | SJCs and TJCs |
| Who conducted comparator CE | Experienced study nurse |
| Primary outcome of study | To compare 18F-FDG PET parameters with clinical, biological and sonographic (US) rheumatoid parameters |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | 18F-FDG PET is a unique imaging technique that can assess the metabolic activity of synovitis and measure disease activity in RAp. 956 |
| First author (study name) | Bhamra89 |
|---|---|
| Year | 2014 |
| Abstract or full paper | Abstract |
| Study design | Treatment |
| Study objective | To evaluate the impact of clinic-based ultrasonography (MSUS) on the diagnosis and management of cases seen in [an] emergency rheumatology clinicp. 659 |
| Population sample size | 17 RA (of 62 in study) |
| Population diagnosis of RA | NR |
| Population eligibility details (e.g. early RA, remission) | Patients referred to rheumatology clinic (not all RA) |
| Population baseline characteristics | NR for 17 RA patients. For 62 study patients, 25 men, 38 women; mean (range) age 57.17 (range 30–88) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed | 10 MCP and PIP joints, radiocarpal joint and ulnar styloid |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS GE Logiq E9 using a linear transducer. Scoring system NR |
| Who conducted US | Consultant rheumatologist |
| Comparator CE details | NR |
| Who conducted comparator CE | Referring clinician |
| Follow-up duration (if relevant) | NA |
| Primary outcome of study | Treatment decision change following US |
| Outcome(s) reported in main body of report | Treatment decision |
| Study authors’ conclusions | There is a positive impact of US in the rheumatology clinic; specifically highlighted multiple benefits in daily practice of reduced visits, discharge at first encounter and immediate management decisionsp. 660 |
| First author (study name) | Boyesen134 |
|---|---|
| Year | 2011 |
| Abstract or full paper | Full paper |
| Study design | Prognostic |
| Study objective | To examine the associations between modern imaging modalities and joint damage measured as 1-year MRI erosive progression in early RA patientsp. 176 |
| Population sample size | 84 recruited, 79 with 1-year follow-up data |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Early RA (< 1 year), treatment NR |
| Population baseline characteristics |
65 patients (77%) female, 19 male Median (IQR) age 58 (47–67) years Median (IQR) disease duration 107 (70–188) days |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed |
CE: DAS28 US: dominant wrist |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS 8–16-MHz linear array transducer on a Diasus machine (Dynamic Imaging, Livingstone, UK). All findings were graded as 0 = none, 1 = mild, 2 = moderate or 3 = marked |
| Who conducted US | Trained user |
| Comparator CE details | DAS28-ESR |
| Who conducted comparator CE | NR |
| Follow-up duration (if relevant) | 12 months |
| Primary outcome of study | Prediction of erosions at 1 year as measured by MRI |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | GSUS inflammation and MRI bone marrow oedema were independent predictors of MRI erosive progression in early RA patients on a group level. The exact prognosis of the individual patients could not be determined by imaging alonep. 176 |
| First author (study name) | Brown135 and Ikeda136 |
|---|---|
| Year | 2008 and 2007 |
| Abstract or full paper | Full paper |
| Study design | Prognostic |
| Study objective | To evaluate the long-term significance of subclinical synovitis and its relationship to structural outcomep. 2958 |
| Setting | UK, outpatient clinics |
| Population sample size |
102 (90 with a full set of radiographs at both time points)135 107136 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | In remission (while taking cDMARDs – stable therapy for 6 months prior to baseline) |
| Population baseline characteristics |
Mean age 57 (IQR 24–81) years 67% female; 33% male Duration of RA, median (range) 7 (2–38) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | cDMARDs |
| Joints assessed | Hand and wrist – MCP, radiocarpal, ulnar carpal, distal radioulnar, intercarpal compartments |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS ATL HDI 3000 machine (ATL Ultrasound, Bothell, WA, USA) with a 10–5 MHz linear array ‘hockey-stick’ transducer, according to the EULAR guidelines. Presence and location of synovial hypertrophy (SH) and erosions were recorded according to OMERACT definitions. SH was graded using a 0–3 semiquantitative scoring method (0 = no SH, 1 = mild SH, 2 = moderate SH and 3 = severe SH). PDUS images were scored using a 0–3 semiquantitative technique (0 = normal/minimal vascularity, 1 = mild hyperaemia, 2 = moderate hyperaemia and 3 = marked hyperaemia). Erosions were scored using a similar 0–3 semiquantitative scale, according to their location and severity/size |
| Who conducted US | A single experienced sonographer |
| Comparator CE details | Duration of morning stiffness; Likert scale and VAS for fatigue, joint pain, physician’s assessment of disease activity and patient’s global impression of health and disease activity; number of painful, tender and swollen joints as assessed by an independent trained metrologist; HAQ; RAQoL; ACR; DAS28; ESR; CRP |
| Who conducted comparator CE | Consultant rheumatologist and trained metrologist |
| Follow-up duration (if relevant) | 12 months |
| Primary outcome of study |
Progression of joint damage – erosions135 Long-term radiological and clinical outcome136 |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | The present study supports the use of sensitive imaging techniques for the accurate evaluation of disease status and the prediction of outcome in patients with RA, even when the findings of standard clinical measures of inflammatory activity have returned to normalp. 2966 |
| First author (study name) | aBugatti147 and Scirè137 |
|---|---|
| Year | 2012 and 2009 |
| Abstract or full paper | Full paper |
| Study design | Prognostic |
| Study objective | To investigate whether baseline serum levels of the chemokine CXCL13 might predict clinical and ultrasonographic outcomes in patients with recent-onset RABugatti et al.,147 p. 1To evaluate the usefulness of a systematic musculoskeletal ultrasonographic assessment in the detection of residual disease activity in patients with early RA who achieved clinical remissionScirè et al.,137 p. 1092 |
| Population sample size |
161 (155 at 12-month follow-up)147 106137 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Early RA; DMARD and glucocorticoid naive |
| Population baseline characteristics |
From n = 161:147 median (IQR) age 64 (50–73) years; 112 female (69.6%), 49 male; median (IQR) disease duration 3 (2–6) months From n = 106:137 mean (SD) age 59.5 (14.4) years; 75 female, 31 male; mean (SD) disease duration 3.8 (2.8) months |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | n = 72 starting with MTX, n = 31 starting with HCQ, n = 48 starting with prednisone |
| Joints assessed | Bilateral shoulder, elbow, wrist (radiocarpal and midcarpal joint), MCP joints, PIP joints of the hands, sternoclavicular and acromioclavicular joints, knee, ankle and MTP joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS GE Logiq 9 scanner (General Electrics Medical Systems, Milwaukee, WI, USA) with a multifrequency linear array transducer (10–15 MHz), performed according to EULAR guidelines GSUS and PDUS signals were scored on 0–3 semiquantitative scales. 277 GSUS scoring: 0 = normal, 1 = mild, 2 = moderate, 3 = marked. PDUS scoring: 0 = absence or minimal flow, 1 = mild (single-vessel signal), 2 = moderate (confluent vessels), 3 = marked (vessel signals in > 50% of the joint area) |
| Who conducted US | A single experienced operator |
| Comparator CE details | SJC and TJC on the 44-joint count, Ritchie Articular Index (RAI), global health assessment on a 0–100 mm VAS, evaluator global assessment of disease activity and patient global assessment of disease activity on a 0–10 cm VAS, ESR and CRP |
| Who conducted comparator CE | NR |
| Follow-up duration (if relevant) | 12 months |
| Primary outcome of study |
Ultrasonic outcome (PDUS scores)147 Residual disease activity137 |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | CXCL13 is a promising prognostic marker in early RA, accurate in assessing the severity of synovitis and its persistence over time in response to conventional treatmentsBugatti et al.,147 p. 8The data support the specific role of US in detecting residual disease activity in early RAScirè et al.,137 p. 1096PD-positive synovial hypertrophy identifies ongoing inflammation even during remission and predicts short-term relapseScirè et al.,137 p. 1092 |
| First author (study name) | Taylor100 and Cavet138 |
|---|---|
| Year | 2004 and 2009 |
| Abstract or full paper | Full paper and abstract |
| Study design | Prognostic |
| Study objective | To investigate the ability of US and biomarkers to predict progressive joint damage |
| Population sample size | 24 |
| Population diagnosis of RA | ACR 1987 criteria100 |
| Population eligibility details (e.g. early RA, remission) |
RA patients who were followed in a 2-year blinded study comparing MTX + IFX with MTX alone in aggressive early RA RA, symptoms for 6 months to 3 years, a minimum of two swollen MCP joints despite treatment with oral MTX (minimum 8 weeks) and seropositivity for immunoglobulin M rheumatoid factor; either erosion of at least one MCP joint as demonstrated on plain radiography or GSUS or erosions of at least two MCP joints on GSUS and PDUS; stable dosage of 12.5–17.5 mg/week of folic acid at least 4 weeks prior to screening; if on corticosteroids must have been on a stable dose for 4 weeks (< 10 mg/day); screening laboratory tests100 |
| Population baseline characteristics |
18 female, 6 male100 Mean (SD) age: MTX treated 51.4 (14.0) years, IFX + MTX treated 55.2 (11.8) years100 Mean (SD) disease duration: MTX treated 1.64 (0.63) years, IFX + MTX treated 1.33 (0.64) years100 |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Treatment with MTX at a mean weekly dosage of approximately 15 mg for a mean duration of 0.91 years |
| Joints assessed | 10 MCP joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Scored for synovial thickening and for vascularity by PD area High-frequency (13-MHz) US and PD (14-MHz) imaging were performed using a 15L8 transducer (Acuson Sequoia, Siemens Medical Systems, Ultrasound Group, Issaquah, WA, USA) with constant settings in both GSUS and PDUS100 GSUS images were evaluated for synovial thickness and assigned a score of 0–5. PDUS: number of colour Doppler pixels was determined in a defined region of interest for each joint and a total vascularity score was calculated as the sum of the individual joint scores100 |
| Who conducted US | The same sonographer for all patients100 |
| Comparator CE details |
93 serum proteins associated with biological processes underlying joint damage were measured in serum samples TJC, SJC, morning stiffness duration (minutes), pain 0–10 VAS, patient and physician global assessment of disease activity 0–5 VAS, ACR 20/50/70 responses, DAS28, ESR |
| Who conducted comparator CE | An independent assessor |
| Follow-up duration (if relevant) | 110 weeks |
| Primary outcome of study | Association of US and biomarkers with modified Sharp score at 110 weeks |
| Outcome(s) reported in main body of report | Prognostic and treatment |
| Study authors’ conclusions | Both ultrasonographic imaging and quantitative serum protein biomarkers can be used to estimate rates of progression and predict joint damage in RA. Serum proteins associated with change in TSS represent multiple biological pathways. Predictive models using US and biomarkers have the potential to improve patient outcomesCavet et al.138 p. 1464 |
| First author (study name) | Ceponis152 |
|---|---|
| Year | 2014 |
| Abstract or full paper | Full paper |
| Study design | Treatment decision and diagnostic |
| Study objective | To investigate the usefulness of point-of-care hand and wrist joint US examination in patients with established RAp. 236 |
| Population sample size | 51 (also included healthy control subjects, but data reported separately for RA patients) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | RA, no history or suspicion of fibromyalgia |
| Population baseline characteristics |
Female 42 (91%), male 4 (9%) Mean (range) age 61.8 (28–82) years Mean (range) disease duration 16.6 (1–38) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Majority of patients were receiving cDMARDs, including MTX (60.9%; mean dosage 15.2 mg/week, range 5–25 mg/week) and leflunomide (15.2%; mean dosage 17.5 mg/day, range 10–20 mg/day) with or without a biologic agent [34.6%; either TNFi (n = 14), interleukin-6 inhibitor (n = 1) or RTX (n = 1)] |
| Joints assessed | MCP 1–5, PIP 2–5, wrist |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS A LOGIQ e US machine (GE Healthcare) equipped with a multifrequency 8- to 13-MHz linear transducer was used. Settings were standardised, with GSUS frequency of 12–13 MHz, gain of 58–64%, PD frequency of 6.7 MHz, gain of 9–12% and pulse repetition frequency of 0.6–0.8 Hz OMERACT definitions were used to assess joints for joint effusion and synovial hypertrophy, using a semiquantitative scale from 0 to 3 for GSUS and PDUS (0 = absence, 1 = mild, 2 = moderate and 3 = severe). 52,53 PD scoring: grade 0 = no intra-articular colour signal, grade 1 = single vessel signal(s), grade 2 = confluent colour signal in less than half of the intra-articular area and grade 3 = confluent colour signal in more than half of the intra-articular area52 |
| Who conducted US | Experienced sonographer |
| Comparator CE details | CDAI, SJC, TJC, HAQ, pain VAS, morning stiffness, fatigue VAS, patient global assessment VAS |
| Who conducted comparator CE | Four board-certified rheumatologists |
| Follow-up duration (if relevant) | NA |
| Primary outcome of study | Agreement between US and clinical findings and its impact on physicians’ confidence and clinical decision were assessed |
| Outcome(s) reported in main body of report | Treatment decision |
| Study authors’ conclusions | PD examination of the wrist and second/third MCP joints might be feasible and clinically meaningful in evaluation of disease activity in patients with established RA. US examination of the hand/wrist joints in RA increases physicians’ confidence in their clinical decisions and can help to individualise DMARD and biologic agent usep. 236 |
| First author (study name) | Ciurtin90,158 |
|---|---|
| Year | 2013 and 2012 |
| Abstract or full paper | Abstract |
| Study design | Treatment |
| Study objective | To evaluate the usefulness of a 22 hand joints scoring system, adapted from the OMERACT recommendations, in assessing and differentiating patients with established RA from those with possible or definite early undifferentiated inflammatory arthritis. To establish the usefulness of the musculoskeletal US findings in guiding treatment decisionsp. 295 |
| Population sample size | 39 RA patients (of 98 in study) |
| Population diagnosis of RA | NR (established RA) |
| Population eligibility details (e.g. early RA, remission) | Patients referred to US service with inflammatory arthritis or suspected arthritis |
| Population baseline characteristics | NR for RA subset; for overall study: 69 females [mean (SD) age 50.03 (11.2) years) and 29 males [mean (SD) age 48.8 (11.7) years) |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | 6/39 RA patients on biological therapy |
| Joints assessed | 22 wrist joints and MCP and PIP joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Machine NR. Scoring system adapted from the OMERACT recommendations; no details on scoring system reported |
| Who conducted US | Specialist US service |
| Comparator CE details | NR |
| Who conducted comparator CE | NR |
| Follow-up duration (if relevant) | NA |
| Primary outcome of study | Distinguishing RA from undifferentiated inflammatory arthritis and treatment decisions |
| Outcome(s) reported in main body of report | Treatment |
| Study authors’ conclusions | In the 22 hand joints US scoring system, the presence of joint effusion grade 1 affecting < 5 joints and minimal synovial hypertrophy affecting < 3 joints did not correlate with any laboratory evidence of inflammatory or autoimmune abnormalities |
| First author (study name) | Dale153,154 (TaSER) |
|---|---|
| Year | 2014 and 2013 |
| Abstract or full paper | Full paper |
| Study design | Treatment |
| Study objective | To determine the level of agreement and potential impact on DMARD escalation decisions and of adding musculoskeletal ultrasound (MSUS) assessment of disease activity to the DAS28Dale et al.,153 p. 19To test whether the efficacy of DAS28-driven treat-to-target DMARD strategies could be improved by the addition of a regular MSUSDale et al.,154 p. S338 |
| Population sample size | 53 in the MSUS arm (110 with RA in the whole trial) |
| Population diagnosis of RA | ACR/EULAR 2010 criteria |
| Population eligibility details (e.g. early RA, remission) | Early RA/anti-citrullinated protein antibody-positive undifferentiated arthritis, untreated |
| Population baseline characteristics |
Of the 53 patients in the MSUS arm: mean (SD) age NR; 30 females (59%); mean (SD) symptom duration 5.1 (2.8) months In the whole trial population (n = 110): median disease duration 4 months; DAS28 group contained a higher proportion of females (78%) than the MSUS group |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Step-up therapy (MTX; triple therapy; triple therapy with subcutaneous MTX; triple therapy + ETN) |
| Joints assessed | Dorsal recesses of 14 joints (second and third PIP joints, second and third MCP joints, wrist joints and second and fifth MTP joints bilaterally) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS All examinations were conducted using the same portable US machine (Voluson I, GE Healthcare) and a 10- to 16-MHz linear array probe (SP 10–16RS, GE Healthcare). PD examination was standardised using frequency high (machine preset), pulse repetition frequency 0.9 kHz, wall filter low and gain adjusted to below the level at which Doppler artefact appeared beneath bone. The presence of GS and PD synovitis positivity was graded on a 0–3 semiquantitative scale. 52 Active disease on MSUS was defined as the presence of a grade 1 or higher intra-articular PD signal in at least two joints. Therefore, the presence of a PD signal in two or more joints was used as a threshold for DMARD escalation |
| Who conducted US | Trained metrologist |
| Comparator CE details | DAS28, CRP, ESR, SJC, TJC, patient global assessment of disease activity, HAQ |
| Who conducted comparator CE | Trained metrologist |
| Follow-up duration (if relevant) | 18 months |
| Primary outcome of study | Improvements in DAS44 |
| Outcome(s) reported in main body of report | Treatment |
| Study authors’ conclusions |
Dale et al. ,153 p. 19This may allow further tailoring of DMARD therapy by supporting DMARD escalation in patients with continuing subclinical synovitis and preventing escalation in symptomatic patients with minimal clinical and/or ultrasonographic synovitis Both groups exhibited similar, very robust improvements in clinical outcomesDale et al. ,154 p. S339 |
| First author (study name) | Dougados139,149 and Cheung148 |
|---|---|
| Year | 2013 and 2014 |
| Abstract or full paper | Full paper |
| Study design | Prognostic and management |
| Study objective | To evaluate synovitis (clinical vs. ultrasound (US)) to predict structural progression in RAp. 665 |
| Population sample size | 59 with data (from 77 recruited) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | RA, minimum disease activity of at least six swollen joints by CE, eligible for TNFi (investigator opinion) |
| Population baseline characteristics |
Female 81%, male 19% Mean (SD) age 56 (12) years Mean (SD) disease duration 10 (8) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Mean number of previous cDMARDs 3.0. History of TNFis 32%. At baseline assigned to 4 months treatment with bDMARD (ETN, n = 34; ADA, n = 23, IFX, n = 2) |
| Joints assessed | MCP (×10), PIP (×10), wrist (×2) and MTP (×10) joints for US and CE |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Real-time scanners (e.g. Esaote Technos MPX, Esaote MyLab, Toshiba Aplio, Philips HD11, BK MiniFocus) using multifrequency linear transducers (7–12 MHz) Synovitis was defined according to the OMERACT definition, using a semiquantitative scale from 0 to 3 [GS: 0 = absence of synovial thickening, 1 = mild synovial thickening, 2 = moderate synovial thickening, 3 = marked synovial thickening; PD: 0 = absence of signal, no intra-articular flow, 1 = mild, one- or two-vessel signal (including one confluent vessel) for small joints and two to three signals for large joints (including two confluent vessels), 2 = moderate confluent vessels (> grade 1) and < 50% of normal area, 3 = marked vessel signals in more than half of the synovial area] |
| Who conducted US | Either a radiologist or a rheumatologist with experience |
| Comparator CE details | Clinical evaluation of synovitis and tender joints |
| Who conducted comparator CE | Either a rheumatologist or a research nurse with experience |
| Follow-up duration (if relevant) | 2 years |
| Primary outcome of study | Association between structural deterioration and the presence of baseline synovitis, or its persistence, after 4 months of therapy |
| Outcome(s) reported in main body of report | Prognostic and treatment |
| Study authors’ conclusions | This study confirms the validity of synovitis for predicting subsequent structural deterioration, irrespective of the modality of examination of joints, but also suggests that both clinical and ultrasonographic examinations may be relevant to optimally evaluate the risk of subsequent structural deteriorationp. 665 |
| First author (study name) | Ellegaard101 |
|---|---|
| Year | 2011 |
| Abstract or full paper | Full paper |
| Study design | Treatment |
| Study objective | To investigate the predictive ability of core outcomes applied in RA trials, including PDUS measurements differentiating patients who remain on TNFi therapy following 1 yearp. 506 |
| Population sample size | 109 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA, treated with TNFi (ADA, ETN or IFX) |
| Population baseline characteristics |
78 females (71.1%) Mean (SD, range) age 57.9 (13.9, 25.5–84.3) years Mean (SD, range) duration of RA 10.4 (9.0, 1.0–34.6) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | TNFi (ADA, ETN or IFX) |
| Joints assessed | Wrists (most affected) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
PDUS Scanning was performed with a US machine (Siemens, Mountainview, CA, USA) using a linear array transducer with 14-MHz centre frequency, with no adjustments of Doppler parameters performed No details provided on scoring system used |
| Who conducted US | Head of the US unit, with 20 years’ US experience; other investigators with several years of US training and 1 month of specific training on wrist scans |
| Comparator CE details | TJC, SJC, ESR, CRP, HAQ, patient general health VAS global, DAS28-CRP |
| Who conducted comparator CE | A rheumatologist unaware of the results of the US examination |
| Follow-up duration (if relevant) | 1 year |
| Primary outcome of study | Remaining on TNFi therapy at 1 year |
| Outcome(s) reported in main body of report | Treatment |
| Study authors’ conclusions | There is now evidence to support that baseline PDUS, in contrast to clinical measures, can predict which patients will remain on TNFis 1 year after initiating therapyp. 506 |
| First author (study name) | Filippucci107 | ||||
|---|---|---|---|---|---|
| Year | 2006 | ||||
| Abstract or full paper | Full paper | ||||
| Study design | Diagnostic | ||||
| Study objective | To use PDUS to evaluate changes in synovial perfusion induced by adalimumab in the wrist joints of patients with RAp. 1433 | ||||
| Setting | Two rheumatology centres in Italy | ||||
| Population sample size | 48 wrists of 24 patients | ||||
| Population diagnosis of RA | ACR 1987 criteria | ||||
| Population eligibility details (e.g. early RA, remission) | Active disease, aged ≥ 18 years, not pregnant, not in another trial of other biologic agents, no history of intra-articular steroid injections at the wrist, no changes in DMARD dose in the previous 3 months | ||||
| Population baseline characteristics |
18 female, 6 male Median (SD) age 61.5 (SD 10.5) years (IQR 48–67 years) Median (SD) disease duration 10 (SD 7.9) years (IQR 5–17 years) |
||||
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Started on ADA | ||||
| Joints assessed | Wrists | ||||
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
PDUS AU5 Harmonic (Esaote Biomedica, Genoa, Italy) with a 10- to 14-MHz linear probe, standardised with a pulse repetition frequency of 1000 Hz and a colour mode frequency of 7 MHz Representative pictures of the highest expression of intra-articular PDUS signals were obtained. A score from 0 to 3 was assigned according to the overall expression of PDUS signals at the wrist level (semiquantitative visual scale: 0 = normal or minimal degree, 1 = mild degree, 2 = moderate degree and 3 = marked degree) |
||||
| Who conducted US | An experienced operator blinded to both clinical and laboratory findings | ||||
| Comparator CE details | Physician’s global assessment of disease activity, ESR using the Westergren method, serum levels of CRP (upper reference level 4 mg/l; not measured at week 2) | ||||
| Who conducted comparator CE | An experienced rheumatologist | ||||
| Follow-up duration (if relevant) | 12 weeks | ||||
| Primary outcome of study | Change from baseline in clinical and US assessments | ||||
| Outcome(s) reported in main body of report | Diagnostic | ||||
| Study authors’ conclusions | Our study suggests that:PDUS is a feasible and sensitive imaging tool for assessing the response to treatment of synovitis at the small-joint level. In particular, we have shown that the wrist joint is a suitable anatomical site to be assessed by PDUS for detecting changes in synovial perfusion induced by systemic drug treatmentp. 1437Ongoing follow-up will add further insight into the persistence of considerable reductions in PDUS scores and their correlation with DAS28. In particular, long-term follow-up will provide information on the predictive value of rapid PDUS signal reduction for sustained remission of the disease at the small-joint level | ||||
| Joint swelling (n = 24, 192 joints) | PDUS negative | PDUS positive | Total | ||
| Clinically swollen | 13 | 61 | 74 | ||
| Clinical not swollen | 32 | 86 | 118 | ||
| Total | 45 | 147 | 192 | ||
| Joint tenderness (n = 24, 192 joints) | PDUS negative | PDUS positive | Total | ||
| Clinically tender | 10 | 58 | 68 | ||
| Clinically not tender | 35 | 89 | 124 | ||
| Total | 45 | 147 | 192 | ||
| Population | Diagnostic accuracy comparison | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
| Joint swelling (n = 24, 192 joints) | CE with reference GSUS | 41 | 71 | 82 | 27 |
| Joint swelling (n = 24, 192 joints) | CE with reference GSUS | 82 | 27 | 41 | 71 |
| Joint tenderness (n = 24, 192 joints) | GSUS with reference CE | 39 | 78 | 85 | 28 |
| Joint tenderness (n = 24, 192 joints) | GSUS with reference CE | 85 | 28 | 39 | 78 |
| First author (study name) | Gandjbakhch91 |
|---|---|
| Year | 2008 |
| Abstract or full paper | Abstract |
| Study design | Treatment decision |
| Study objective | To evaluate therapeutic decisions in daily practice and determine if US examination may influence therapeutic decisions in the management of RA patientsp. S467 |
| Population sample size | 52 |
| Population diagnosis of RA | ACR criteria |
| Population eligibility details (e.g. early RA, remission) | RA patients referred for therapy adjustment |
| Population baseline characteristics |
Sex NR Mean age 54 years Mean disease duration 10.3 years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | cDMARD, n = 43; TNFi, n = 7; no ongoing DMARDs, n = 2 |
| Joints assessed |
US: bilaterally on wrists, MCP 2–5 joints, PIP 2–5 joints, elbows, shoulders, knees and MTP 2–5 joints CE: DAS28 |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Machine and scoring system NR |
| Who conducted US | An experienced rheumatologist |
| Comparator CE details | DAS28 |
| Who conducted comparator CE | One of nine trained rheumatologists |
| Follow-up duration (if relevant) | NA |
| Primary outcome of study | Change in treatment decision (%) following US |
| Outcome(s) reported in main body of report | Treatment |
| Study authors’ conclusions | Rheumatologists’ therapeutic decision was mainly made according to disease activity assessed by the DAS28. US findings increased clinician confidence and resulted in a change in therapeutic decision for 13% of the patients. US examination seems useful to detect residual inflammatory activityp. S468 |
| First author (study name) | Garrigues108 |
|---|---|
| Year | 2013 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To evaluate concordance between CE and US of joints in a heterogeneous group of patients with rheumatoid arthritisp. 597 |
| Population sample size | 40 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Age ≥ 18 years |
| Population baseline characteristics |
29 women and 11 men Mean (SD) age 55.9 (14) years Mean (SD) disease duration 11.2 (8.7) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Most patients receiving intravenous treatment for RA |
| Joints assessed | 40 joints, namely the 28 joints included in the DAS28 (shoulders, elbows, wrists, MCP joints, PIP joints and knees) and tibiotalar and MTP joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Multiplanar GSUS (B-mode) and PDUS images were obtained using commercially available real-time scanners (Esaote MyLab60 and Philips IU22) and multifrequency linear transducers (7–12.5 MHz) Synovitis defined according to 2005 OMERACT definitions GSUS: 0–3 semiquantitative scoring: 0 = no synovial thickening; 1 = mild synovial thickening (minimal synovial thickening not bulging beyond bone surfaces); 2 = moderate synovial thickening (synovial thickening bulging beyond bone surfaces without extension along the diaphysis); 3 = marked synovial thickening (synovial thickening bulging beyond the bone surfaces with extension along at least one of the diaphysis) PDUS: 0–3 semiquantitative scoring: 0 = no signal, no intra-articular flow; 1 = mild, until three isolated points or two confluents points or one confluent point and up to two isolated points; 2 = moderate vessel confluence (> grade 1) occupying < 50% of the normal synovial surface area; 3 = marked vessel confluence occupying > 50% of the normal synovial surface area |
| Who conducted US | Two sonographers (one radiologist, one rheumatologist) |
| Comparator CE details | SJC and TJC |
| Who conducted comparator CE | Rheumatologist |
| Primary outcome of study | Concordance between CE and US |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | Ultrasound adds information to clinical examination, most notably at the shoulders, wrists and MTP joints. Concordance was moderate to strong at other joint sitesp. 597 |
| First author (study name) | Gartner109 |
|---|---|
| Year | 2013 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To compare clinically active joints with sonographically active joints in patients with RA, applying different sonographic definitions of an active jointp. 2005 |
| Setting | Rheumatology outpatient clinic in Austria |
| Population sample size | 90 patients (60 in clinical remission, 30 not in clinical remission) |
| Population diagnosis of RA | ACR 2010 criteria |
| Population eligibility details (e.g. early RA, remission) | Remission and active disease, no restriction on medication |
| Population baseline characteristics |
In clinical remission: female 78.3%; mean (SD) age 60.1 (10.8) years; mean (SD) disease duration 9.4 (8.9) years; mean (SD) DAS28 2.2 (0.5) Not in clinical remission: female 76.7%; mean (SD) age 60.1 (11.3) years; mean (SD) disease duration 10.3 (7.7) years; mean DAS28 3.8 (1.1) |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed |
CE: 28 joints US: 11 joints of each hand, including PIP, MCP and wrist joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
PDUS and GSUS Logiq E9 (General Electric) with a ML 6–15 transducer, frequency range 9–15 MHz; for PD, pulse repetition frequency was set between 500 Hz and 800 Hz and receiver gain settings were controlled to eliminate the appearance of artefacts GS and PD signals for signs of synovitis were graded using a 0–3 semiquantitative scoring system (0 = none, 1 = mild, 2 = moderate and 3 = severe)52 |
| Who conducted US | An experienced sonographer who had no access to the clinical and laboratory data and who was unaware of the results of the clinical joint examination |
| Comparator CE details | TJCs and SJCs were performed on 28 joints in all patients. Swelling and tenderness were defined in accordance with the standardised assessment recommendations from EULAR, whereby, in the presence of any doubt, a joint was considered ‘not swollen’; 100-mm VAS for pain and global assessment of disease (patient’s and evaluator’s); duration of morning stiffness (minutes); HAQ-DI; CRP, ESR; CDAI; SDAI; DAS28 |
| Who conducted comparator CE | NR |
| Primary outcome of study | Comparative analysis of sonographic and clinical joint assessment |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | . . . sonography as a tool to detect synovitis may have sufficient value only when the signals are high and that low signals may not necessarily represent inflammation and, in contrast to the clinical findings, are not related to disabilityp. 2012Thus, the present findings reveal that more detailed assessment of sonographic data is needed to fully appreciate the value of US in the follow-up of patients with RA |
| First author (study name) | Haavardsholm110 |
|---|---|
| Year | 2009 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To evaluate the responsiveness of MRI and US compared with conventional measures of disease activity and structural damage in patients with RA during the first year of treatment with TNFisp. 1572 |
| Population sample size | 36 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA (started on TNFi treatment) |
| Population baseline characteristics |
80.6% female Median (IQR) age 52.8 (24.0) years Median (IQR) disease duration 7.6 (8.0) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Started on TNFi |
| Joints assessed | One wrist for US, both hands and wrists for CE |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS All of the US measurements were performed using an 8- to 16-MHz linear array transducer (Diasus, Dynamic Imaging, Livingstone, UK) Synovitis/tenosynovitis and effusions were graded using a 0–4 semiquantitative scale (0 = none, 1 = uncertain, 2 = minimal, 3 = medium and 4 = high amount of hypoechoic material) |
| Who conducted US | An experienced ultrasonographer |
| Comparator CE details | 28 SJC, 28 TJC, patient- and investigator-perceived pain 100-mm VAS, disability via modified HAQ, DAS28-ESR, SDAI, CDAI, CRP, ESR |
| Who conducted comparator CE | A trained research nurse |
| Follow-up duration (if relevant) | 12 months |
| Primary outcome of study | SRM |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | The most responsive measure of inflammation when evaluating TNFi medication was a composite measure comprising MRI synovitis, tenosynovitis and bone marrow oedema, and this may be a promising outcome measure in clinical studiesp. 1572 |
| First author (study name) | Haarvardsholm79 (ARCTIC) |
|---|---|
| Year | 2015 |
| Abstract or full paper | Abstract |
| Study design | Treatment |
| Study objective | To investigate tight control strategies to see whether or not a treatment strategy additionally including US would lead to better clinical and radiographic outcomes compared with a conventional treatment strategy |
| Population sample size |
118 in the US arm (105 had completed the study at the time of abstract publication) 112 in the conventional strategy arm (99 had completed the study at the time of abstract publication) |
| Population diagnosis of RA | ACR/EULAR 2010 criteria |
| Population eligibility details (e.g. early RA, remission) | Early RA (symptom duration up to 2 years), DMARD naive, indication for DMARDs |
| Population baseline characteristics | NR |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | DMARD naive. Treatment strategy in trial: MTX, then triple cDMARDs, then bDMARD |
| Joints assessed | 44 joints of DAS44; PDUS and GSUS assessed 32 joints on a 0–3 scale111 |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) | PDUS and GSUS assessed on a 0–3 scale.111 Machine details NR |
| Who conducted US | Experienced sonographers |
| Comparator CE details | DAS, SJC |
| Who conducted comparator CE | NR |
| Follow-up duration (if relevant) | 2 years |
| Primary outcome of study | Patients meeting all three assessments at 16, 20 and 24 months with a of DAS of < 1.6, no swollen joints at 16, 20 and 24 months, and no progression in vdHSS (< 0.5 units) from 16 to 24 months |
| Outcome(s) reported in main body of report | Treatment |
| Study authors’ conclusions | No statistically significant difference between the two strategies for the primary outcome |
| First author (study name) | Hammer68,111 |
|---|---|
| Year | 2011 and 2010 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To examine associations between ultrasonography assessments (GS and PD) of a large number of joints and traditional assessments of diseaseHammer et al.,111 p. 1349To explore the associations between a comprehensive ultrasonographic assessment of joints, tendons and bursae and previously described reduced joint counts (7-, 12-, 28- and 44- joint score)Hammer and Kvien68 p. 1 |
| Population sample size | 20 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA, commencing ADA |
| Population baseline characteristics |
15 female (75%) Median (range) age 53 (21–78) years Median (range) disease duration 7.5 (1–26) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Starting ADA |
| Joints assessed |
US: PIP 1–5 (dorsal), MCP 1–5 (dorsal), carpometacarpal 1–5 (dorsal), wrist (radiocarpal, intercarpal and radioulnar joints) (dorsal), elbow (anterior and posterior), shoulder (glenohumeral and acromioclavicular joints) (anterior, posterior and upper), hip (anterior), knee (anterior and lateral), ankle (talocrural joint) (anterior), four major foot joints (talonavicular, subtalar, calcaneocuboidal and cuneonavicular) (anterior and lateral), tarsometatarsal 1–5 (dorsal), MTP 1–5 (dorsal) and the interphalangeal (dorsal) joint of the first toe (a total of 78 joints) CE: PIP 1–5, MCP 1–5, wrist, elbow, shoulder, knee, ankle and MTP 1–5 |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS US examinations were performed with a 5- to 13-MHz probe and fixed settings optimal for PD signals (Siemens Antares, Sonoline; Siemens Medical Solutions, CA, USA). The same US machine and the same PD setting optimised for more superficial structures (most of the joints assessed) were used throughout the study All joints were scored according to OMERACT criteria for GSUS (presence of synovitis and joint fluid) and PDUS (presence of vascularisation) (0 = none; 1 = minor; 2 = moderate; 3 = major presence) |
| Who conducted US | One experienced sonographer |
| Comparator CE details | Tenderness and swelling of 40 joints (for comparisons between clinical and US examinations, a B-mode score of ≥ 1 was used to define a joint as inflamed), patient and study nurse global disease activity VAS, ESR, CRP, DAS28-ESR, SDAI, CDAI |
| Who conducted comparator CE | One of two study nurses, both with > 5 years’ experience with joint counts in clinical studies |
| Follow-up duration (if relevant) | 12 months |
| Primary outcome of study | Association between US and CE |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | The comprehensive US assessments were associated with clinical and laboratory variables of disease activity and were highly sensitive to change during treatment with biological agentsHammer et al.,111 p. 1349The reduced joint combinations were highly associated with the 78-joint score . . . [indicating] that an approach focusing on few joints and tendons gives equivalent information about the inflammatory activity in RA patients to a comprehensive US examinationHammer and Kvien68 p. 1The optimal combination of joints and tendons for a valid, reliable and feasible US measurement should be further explored to define a US score for follow-up of RA patients on biological treatment68 |
| First author (study name) | Hayashi92 |
|---|---|
| Year | 2014 |
| Abstract or full paper | Abstract |
| Study design | Diagnostic |
| Study objective | To compare US findings with joint examination findings sorted by the presence of tenderness and/or swelling in the hand (proximal) interphalangeal (IP/PIP), MCP and wrist jointsp. S181 |
| Population sample size | 208 |
| Population diagnosis of RA | NR |
| Population eligibility details (e.g. early RA, remission) | RA patients |
| Population baseline characteristics |
158/208 female (76%) Mean age 66 years Mean disease duration NR |
| Population treatment (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed | MCP, PIP, wrists |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS US synovitis was defined as a GS imaging score of ≥ 1 (graded 0–3) or a synovial PD signal score of ≥ 2 (graded 0–3). Details of the machines and scoring systems used were not provided |
| Who conducted US | NR |
| Comparator CE details | Clinical joint assessments determined the presence of tenderness and/or swelling |
| Who conducted comparator CE | NR |
| Follow-up duration (if relevant) | NA |
| Primary outcome of study | Detection of synovitis by US or CE |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | Clinical joint examination of the IP/PIP joints overestimated, and of the wrist joints underestimated, synovitis, compared with US. The importance of US examination in daily clinical practice may differ among joint sites |
| First author (study name) | Horikoshi112 |
|---|---|
| Year | 2010 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To compare MRI and US in the detection of joint inflammation in RAp. 556 |
| Population sample size | 6 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | All patients were assessed clinically within 1 month of the study for disease activity using the DAS28-CRP |
| Population baseline characteristics |
All females Mean (SD) age 50.2 (13.4) years Mean (SD) disease duration 13.5 (8.1) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Treated with one of the following bDMARDs: IFX, ETN, ADA or TCZ |
| Joints assessed | Intercarpal joints, radioulnar joints, second to fifth PIP joints and first to fifth MCP joints, first interphalangeal and radiocarpal joints. A total of 156 joints per patient were assessed by US |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS US was performed using a Aplio SSA-700A system (Toshiba, Tokyo, Japan) with a 12-MHz linear array and a 12-MHz ‘hockey-stick’ array transducer. Joint inflammation on GSUS was defined as a hypoechoic intracapsular area and on PDUS was represented by the presence of positive findings of flow signal. These findings on both GSUS and PDUS were scored on a scale from 0 to 352 |
| Who conducted US | Two experienced rheumatologists |
| Comparator CE details | DAS28-CRP |
| Who conducted comparator CE | NR |
| Primary outcome of study | Agreement between US and MRI |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | Findings of PDUS correlated with those of MRI. Low-field MRI and PDUS are useful tools for the assessment of patients with RAp. 556 |
| First author (study name) | Ikeda113,146 | ||||
|---|---|---|---|---|---|
| Year | 2013 and 2012 | ||||
| Abstract or full paper | Full paper | ||||
| Study design | Prognostic | ||||
| Study objective | To demonstrate that structural damage progression is associated with time-integrated PDUS signals more significantly than with time-integrated DAS28 in RA patients receiving MTX or biological agentsIkeda et al.,146 p. 550 | ||||
| Population sample size | 57 (n = 57 with data at 24 weeks out of n = 69) | ||||
| Population diagnosis of RA | ACR 1987 criteria | ||||
| Population eligibility details (e.g. early RA, remission) | RA, required treatment with MTX or bDMARD | ||||
| Population baseline characteristics |
40 female (73%), male 29 Mean (SD) age 54.9 (14.0) years Median (IQR) disease duration 37 (17–111) months |
||||
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) |
MTX 60/69 (87%) Corticosteroid 41/69 (59%) TNFi: ETN, n = 10, IFX, n = 9, ADA, n = 9 |
||||
| Joints assessed | 28 joints of the DAS28 for US and CE | ||||
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Either LOGIQ 7 PRO (GE Healthcare), LOGIQ E9 (GE Healthcare), Vlamo (Toshiba Medica Systems Corporation) or HI VISION Avius (Hitachi Medical Corporation) GS and PD semiquantitative scores (0–3) (Naredo scoring). GS: 0 = absent; 1 = mild; 2 = moderate; 3 = marked. PD: 0 = absent (no synovial flow); 1 = mild (three or fewer isolated signals); 2 = moderate (more than three isolated signals or confluent signal in less than half of the synovial area); 3 = marked (signals in more than half of the synovial area) |
||||
| Who conducted US | Two rheumatologists trained in US | ||||
| Comparator CE details | 28-joint SJC and TJC, CRP, VAS for patient and physician global assessments | ||||
| Who conducted comparator CE | NR | ||||
| Follow-up duration (if relevant) | 24 weeks | ||||
| Primary outcome of study | Correlation of PD score and joint damage progression | ||||
| Outcome(s) reported in main body of report | Prognostic and diagnostic | ||||
| Study authors’ conclusions | Synovial PD activity more accurately reflects active synovial inflammation that causes joint destruction than conventional measures in RA patients treated with MTXIkeda et al.,113 p. 1967TNFis can inhibit short-term radiographic progression in the presence of active synovitis | ||||
| Sensitivity to predict non-radiographic progression at 24 weeks (CIs NR)113 | |||||
| Population | Baseline measure (optimum cut-off so different by treatment group) | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
| 57 (n = 57 with data at 24 weeks out of n = 69) | Total GS score cut-off point of < 62 | 56 | 57 | 69 | 43 |
| MTX (n = 16) | Total GS score cut-off point of < 60 | 78 | 43 | 64 | 60 |
| TNFi (n = 24) | Total GS score cut-off point of < 70 | 65 | 57 | 79 | 40 |
| TCZ (n = 17) | Total GS score cut-off point of < 62 | 60 | 71 | 75 | 56 |
| 57 (n = 57 with data at 24 weeks out of n = 69) | Total PD score cut-off point of < 21 | 69 | 76 | 83 | 59 |
| MTX (n = 16) | Total PD score cut-off point of < 20 | 89 | 86 | 86 | 89 |
| TNFi (n = 24) | Total PD score cut-off point of < 21 | 65 | 71 | 85 | 46 |
| TCZ (n = 17) | Total PD score cut-off point of < 18 | 70 | 86 | 88 | 67 |
| 57 (n = 57 with data at 24 weeks out of n = 69) | DAS28-CRP cut-off point of < 9.0 | 64 | 81 | 85 | 57 |
| MTX (n = 16) | DAS28-CRP cut-off point of < 10.8 | 89 | 71 | 80 | 83 |
| TNFi (n = 24) | DAS28-CRP cut-off point of < 11.9 | 88 | 71 | 88 | 71 |
| TCZ (n = 17) | DAS28-CRP cut-off point of < 9.0 | 80 | 71 | 80 | 71 |
| First author (study name) | Inanc93 |
|---|---|
| Year | 2014 |
| Abstract or full paper | Abstract |
| Study design | Treatment prediction, prediction of response to bDMARDs by baseline US and clinical features |
| Study objective | To investigate the ability of ultrasonographic parameters to predict which patients with RA will benefit from treatment with TNFi in terms of EULAR responsep. 468 |
| Population sample size | 43 |
| Population diagnosis of RA | Either rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positive |
| Population eligibility details (e.g. early RA, remission) | RA, bDMARD naive, starting TNFi |
| Population baseline characteristics |
34 female, 9 male Mean (SD) age: responders (n = 28) 46 (11); non-responders (n = 15) 47 (11) Mean disease duration 8.0 ± 6.7 years Mean DAS28 of 5.4 ± 1.1 |
| Population treatment (e.g. bDMARDS or cDMARDs) | Starting TNFi |
| Joints assessed | 28 joints from DAS28 |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS US synovitis GS and PD signals were semiquantitatively graded from 0 to 3 (scoring system NR) using MyLab 70 US machine (Esaote, Italy) |
| Who conducted US | Experienced sonographer |
| Comparator CE details | TJS/SJC, DAS28, HAQ scores, ESR, CRP |
| Who conducted comparator CE | NR |
| Follow-up duration (if relevant) | 12 months (with response data at 3 months) |
| Primary outcome of study | Response to TNFi related to baseline US |
| Outcome(s) reported in main body of report | Treatment |
| Study authors’ conclusions | Despite similar clinical features, baseline PD scores, despite similar clinical features, can predict which patients will respond to TNFi therapy. Patients who respond at the third month are more likely to achieve low disease activity at 1 year. Ultrasonographic response to TNFi treatment can be achieved substantially in the first 3 months, beyond which changes in US scores are mostly non-significant |
| Outcome data not added to main report | Patients who responded at the third month were significantly more likely to achieve low disease activity (p = 0.019) or remission (p = 0.008) at 1 year. There was a significant decrease in mean PD and GS sum scores from baseline to the third month (p < 0.001 for both), but not between the third and sixth months (PD, p = 0.68; GS, p = 0.77) |
| First author (study name) | Iwamoto155 |
|---|---|
| Year | 2014 |
| Abstract or full paper | Full paper |
| Study design | Prospective cohort study (treatment prediction, 6-month follow-up) |
| Study objective | To determine whether the comprehensive US assessment of synovial inflammation predicts relapse after discontinuation of treatment with a biologic agent (TNFi or TCZ) in patients with RA in clinical remissionp. 1576 |
| Population sample size | 42 (n = 40 with data at 6 months) |
| Population diagnosis of RA | ACR 1987 and ACR/EULAR 2010 criteria |
| Population eligibility details (e.g. early RA, remission) | RA, clinical remission (i.e. a DAS28 of < 2.6), on bDMARD and willing to discontinue bDMARD |
| Population baseline characteristics |
33 female, 9 male Mean (SD) age 59.6 (12.8) years Mean (SD) disease duration 8.2 (6.7) years |
| Population treatment (e.g. bDMARDS or cDMARDs) | At baseline on bDMARD, discontinued at start of study. Baseline bDMARDS: IFX, n = 17; ETN, n = 3; ADA, n = 6; GOL, n = 5; CTZ, n = 1; TCZ, n = 10. Baseline median (IQR) MTX dose 8.0 (6.0–12.0) mg/week (n = 38). Baseline median (IQR) prednisolone dose 3.0 (2.0–5.0) mg/day (n = 7) |
| Joints assessed | 134 synovial sites in 40 joints: finger, toe: interphalangeal, PIP (2–5), MCP (1–5), MTP (1–5) joints, flexor digitorum tendons; wrist: radiocarpal joint, intercarpal joints, distal radioulnar joint, compartment II/IV/VI of extensor tendons; elbow: humeroradial joint, humeroulnar joint, olecranon bursa; shoulder: glenohumeral joint, long head of biceps tendon, subacromion/subdeltoid bursae; knee: suprapatellar recess, femorotibial joint, popliteal bursa (Baker’s cyst); ankle: tibiotalar joint, extensor tendons, flexor tendons, peroneal tendons |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
Systematic multiplanar GSUS and PDUS Aplio XG, Viamo (Toshiba Medical Systems), HI VISION Avius or HI VISION Ascendus (Hitachi Medical) systems Severity of US findings was graded semiquantitatively on a scale of 0–3. Each patient’s total GS and PD scores were calculated by summing the corresponding scores of 40 joints |
| Who conducted US | Six rheumatologists trained for musculoskeletal US who were blinded to clinical information and laboratory data |
| Comparator CE details | DAS28, SJC, TJC, VAS physician and patient global assessment, HAQ-DI, ESR, CRP |
| Who conducted comparator CE | Nine rheumatologists who were blinded to the baseline US findings |
| Follow-up duration (if relevant) | 6 months |
| Primary outcome of study | Relapse rates following bDMARD discontinuation and association with baseline US values. Relapse outcome defined as a DAS28 of > 3.2 |
| Outcome(s) reported in main body of report | Treatment |
| Study authors’ conclusions | In RA patients in clinical remission receiving treatment with a biologic agent, residual synovial inflammation determined by comprehensive US assessment predicted relapse within a short time after discontinuation of the treatmentp. 1576 |
| First author (study name) | Kamishima114 |
|---|---|
| Year | 2011 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To compare quantitative MRI and PDUS with conventional measures of disease activity in RA patients treated with the anti-interleukin 6 receptor antibody TCZ in terms of responsiveness at a few months to disease activity and ability to predict structural damage at 1 yearp. 745 |
| Population sample size | 29 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA, treated with TCZ |
| Population baseline characteristics |
2 males, 27 females Mean (range) age 61 (27–74) years Median (range) duration of symptoms 8 (1–34) months |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | TCZ |
| Joints assessed | Bilateral MCP joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
PDUS A 13-MHz linear array transducer was used (Hitachi EUP-L34P, Hitachi, Tokyo, Japan). PD settings (75-dB dynamic range, medium persistence, medium frame rate, low wall filter, 1300-Hz pulse repetition frequency, medium vein flow optimisation, 1300-Hz speed velocity) were identical throughout the examinations Graded on a 0–3 semiquantitative scale (0 = absence of signal, 1 = single vessel dots, 2 = vessel dots over less than half of the synovial area, 3 = vessel dots over more than half of the synovial area)277 |
| Who conducted US | One of the three ultrasonographers specialising in musculoskeletal US |
| Comparator CE details | DAS28-ESR, TJC, SJC, VAS, CRP, ESR |
| Who conducted comparator CE | One of the five rheumatologists |
| Follow-up duration (if relevant) | 1 year (SRM at 5 months) |
| Primary outcome of study | Responsiveness to disease activity (SRM) |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | Conventional measures are responsive but less reflective of future bone destruction than image analysis . . . MRI of bone erosion and quantitative PDUS may be both responsive and predictive of structural damage progression at 1 yearp. 753 |
| First author (study name) | Kane102 |
|---|---|
| Year | 2003 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To compare US with CE:in the detection of effusion, suprapatellar bursitis and Baker’s cyst of the knee in RA to determine whether US provides additional clinical informationp. 966 |
| Population sample size | 22 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA |
| Population baseline characteristics |
20 female, 2 male Mean (SD, range) age 50.2 (15.83, 25–79) years Mean (SD, range) disease duration 10.5 (7.8, 1.5–33) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | 19 taking DMARDs (not specified if bDMARDs or cDMARDs) |
| Joints assessed | Knee |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS Real-time US was performed using an ATL (Seattle, WA, USA) HDI 3000 machine with L7- to 4-MHz and CL10- to 5-MHz probes Ultrasonographic assessment of joint effusion was recorded at each site. A knee effusion was present if hypoechoic fluid compressible by the transducer was found in either medial or lateral compartments of the knee Scoring system not reported |
| Who conducted US | An experienced rheumatologist with > 10 years’ experience in musculoskeletal ultrasonography |
| Comparator CE details | Tenderness, swelling |
| Who conducted comparator CE | An experienced rheumatologist with > 6 years’ clinical rheumatology practice |
| Primary outcome of study | Detection of effusion, suprapatellar bursitis and Baker’s cyst of the knee |
| Outcome(s) reported in main body of report | Diagnosis |
| Study authors’ conclusions | US is more sensitive than CE in the detection of suprapatellar bursitis, knee effusion and Baker’s cyst in RA. CE underestimates knee inflammation in RAp. 966 |
| First author (study name) | Kelly94 |
|---|---|
| Year | 2013 |
| Abstract or full paper | Abstract |
| Study design | Management |
| Study objective | To describe the impact of US use by rheumatologists on the diagnosis and management of RA patients in routine UK clinical practice compared with not using USp. 101 |
| Population sample size | 109 with relevant data (of 258 in the study) |
| Population diagnosis of RA | NR |
| Population eligibility details (e.g. early RA, remission) | Patients aged > 18 years, newly referred to the rheumatology clinic with suspected inflammatory arthritis |
| Population baseline characteristics |
NR for 109 patients with data relevant to this review All 258 patients: US group 31% male, non-US group 35% male; US group mean (SD) age 51.28 (15.75) years, non-US group mean (SD) age 53.12 (SD 17.34) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed | NR |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) | NR |
| Who conducted US | Consultant |
| Comparator CE details | NR |
| Who conducted comparator CE | Consultant |
| Follow-up duration (if relevant) | NA |
| Primary outcome of study | Impact of US use by rheumatologists on the diagnosis and management of RA patients in routine UK clinical practice compared with not using US |
| Outcome(s) reported in main body of report | Treatment |
| Study authors’ conclusions | Routine use of US in newly referred patients is associated with an earlier diagnosis and earlier DMARD initiation in patients with RAp. 101 |
| First author (study name) | Luengroongroj95 |
|---|---|
| Year | 2015 |
| Abstract or full paper | Abstract |
| Study design | Treatment |
| Study objective | To evaluate the role of subclinical synovitis detected by musculoskeletal US in predicting disease relapse in remission RAp. 107 |
| Population sample size | 32 |
| Population diagnosis of RA | NR |
| Population eligibility details (e.g. early RA, remission) | Remission CDAI of < 2.8 |
| Population baseline characteristics | NR |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | DMARDs, tapered during the study (not specified if bDMARDs or cDMARDs) |
| Joints assessed | NR |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
PDUS Total PD score No further details reported |
| Who conducted US | NR |
| Comparator CE details | DAS28-CRP, number of DMARDs |
| Who conducted comparator CE | NR |
| Follow-up duration (if relevant) | 3 months |
| Primary outcome of study | Disease flare |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | PDUS assessment may indicate the likelihood of remaining in a disease remission stage. It seems to be safe to reduce the dose of [DMARD] dose . . . for a short period of time, especially when DMARDs tapering is urgent. However, closed monitoring for disease relapse is needed, especially in patients with subclinical synovitisp. 107 |
| First author (study name) | Luukkainen115 |
|---|---|
| Year | 2003 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To assess the relationship between clinically detected swelling and effusion diagnosed by US in MTP and talocrural (TC) joints in patients with RAp. 632 |
| Population sample size | 30 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA |
| Population baseline characteristics |
24 females Mean (range) age 62 (36–80) years Mean (range) duration of RA 14 (0.2–27) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed | MTP, talocrural (TC) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS US measurements were carried out using a Siemens Sonoline Prima apparatus with a 7.5-MHz transducer. Diagnosis of effusion was based on the method of Koski278 The normal upper limit for MTP joints, 2.9 mm (mean + 2 SD)s, was evaluated according to the control group. If the anechogenic space was ≤ 2.9 mm it was regarded as normal and if it was ≥ 3.0 mm it was regarded as effusion. Correspondingly, the normal upper limit for TC joints, 3.0 mm (mean + 2 SDs), was taken from the control group. If it was ≤ 3.0 mm it was regarded as normal and if it was ≥ 3.1 mm it was regarded as effusion |
| Who conducted US | A doctor, no further details provided |
| Comparator CE details | Clinical assessment of MTP and TC joints by inspection and palpation according to the EULAR handbook.279 Swelling was evaluated on a scale from 0 (normal) to 1 (swelling) |
| Who conducted comparator CE | A doctor (author, from department of rheumatology), no further details provided |
| Follow-up duration (if relevant) | NA |
| Primary outcome of study | Relationship between CE and US |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | These preliminary results showed poor agreement between the clinical assessment of swelling and effusion detected by US in MTP and TC joints. Thus, US may considerably improve the diagnosis of synovitis in patients with RAp. 632 |
| First author (study name) | Luukkainen103 |
|---|---|
| Year | 2005 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To compare the relationship between clinically detected swelling and effusion diagnosed by US in elbow joints in patients with RAp. 228 |
| Population sample size | 50 (also included healthy control subjects, but data were reported separately for RA patients) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA |
| Population baseline characteristics |
44 females Mean (range) age 56 (20–77) years Mean (range) duration of RA 11.9 (0.2–32) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed | Glenohumeral (GH) joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS The diagnosis of effusion of GH joints by US was based on the method by Koski. 278 US measurements were carried out using a Siemens Sonoline Prima apparatus with a 7.5-MHz transducer US was scored using a scale of 0 (normal) or 1 (effusion) |
| Who conducted US | One doctor, no further details provided |
| Comparator CE details | Clinical assessment of GH joints was carried out using palpation according to the EULAR handbook.279 The possible synovial swelling of the joints was evaluated using a scale of 0 (normal) or 1 (swelling) |
| Who conducted comparator CE | One doctor, no further details provided |
| Primary outcome of study | Relationship between CE and US |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | The results of this study indicate that:. . . clinical assessment of swelling and evaluation of effusion by US in elbow joints in patients with RA show only fair agreement. Thus, US may improve the accuracy of the diagnosis of synovitis in many cases in these patientsp. 228 |
| First author (study name) | Luukkainen104 |
|---|---|
| Year | 2007 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To assess the relationship between swelling detected on physical examination and effusion diagnosed by ultrasonography in glenohumeral (GH) joints in patients with RAp. 865 |
| Population sample size | 50 (also included healthy control subjects, but data were reported separately for RA patients) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA |
| Population baseline characteristics |
40 females Mean (range) age 56.9 (20–77) years Mean (range) duration of RA 11.6 (0.2–30) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed | GH joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS The diagnosis of effusion of GH joints by US was based on the method by Koski. 278 US measurements were carried out using a Siemens Sonoline Prima apparatus with a 7.5-MHz transducer US was scored using a scale of 0 (normal) or 1 (effusion) |
| Who conducted US | One doctor, no further details provided |
| Comparator CE details | Clinical assessment of GH joints was carried out using palpation according to the EULAR handbook.279 The possible synovial swelling of the joints was evaluated on a scale of 0 (normal) or 1 (swelling) |
| Who conducted comparator CE | One doctor, no further details provided |
| Primary outcome of study | Relationship between CE and US |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | These results showed poor agreement between the clinical assessment of swelling and effusion detected by US in GH joints. Therefore, US may considerably improve the accuracy of diagnosis of effusion in GH jointsp. 865 |
| First author (study name) | Mamoto96 |
|---|---|
| Year | 2013 |
| Abstract or full paper | Abstract |
| Study design | Diagnostic |
| Study objective | To determine the reliability of assessments of swollen joints by patients, physicians and USp. 710 |
| Population sample size | 124 |
| Population diagnosis of RA | NR |
| Population eligibility details (e.g. early RA, remission) | Active disease (with RA), no details reported |
| Population baseline characteristics | NR |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed | Wrist, MCP, PIP bilaterally (22 joints per patient – 2728 joints in total) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Synovial hypertrophy was scored semiquantitatively using GS (score 0–3) and PD (score 0–3) signals. Swollen joints were defined as a GS score of ≥ 2. No further details reported |
| Who conducted US | A US examiner |
| Comparator CE details | DAS28, swollen joint assessment |
| Who conducted comparator CE | Attending physician and US examiner (and patient self-assessment) |
| Primary outcome of study | Sensitivity |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | US can assess swollen joints in patients with RA more sensitively than patients or attending physiciansp. 710 |
| First author (study name) | Mandl116,117 |
|---|---|
| Year | 2013 and 2012 |
| Abstract or full paper | Full paper |
| Study design | Ancillary study to RCT |
| Study objective | To evaluate the intraobserver reliability, face validity and discriminant capacity of different global US scoring systems for measuring synovitis in RAMandl and Balint,117 p. 1272To evaluate the metrological properties of composite disease activity indices in RA, utilising information derived from clinical, GSUS and PDUS examinations and to assess the classification of patients according to disease activity using such indicesMandl and Balint,116 p. 879 |
| Population sample size | 62 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Moderate RA (i.e. a DAS28 of > 3.2 and ≤ 5.1) |
| Population baseline characteristics |
50 (80.6%) female Mean (SD) age 53.8 (13.2) years Mean (SD) disease duration 8.8 (7.7) months Median (IQR) disease duration 6.5 (11) months |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) |
cDMARDs (MTX) 30 patients receiving various cDMARDs and 32 receiving ETN + MTX |
| Joints assessed | DAS28 joints (bilateral shoulder, elbow, and wrist joints, bilateral MCP joints 1–5, bilateral PIP joints 1–5, and bilateral knee joints) plus bilateral MTP joints 1–5 and bilateral ankle and talonavicular joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Systematic multiplanar GSUS and PDUS examinations were carried out with commercially available real-time scanners (e.g. Esaote MyLab70 XVG, Esaote Technos MPX, General Electric Logiq 9) using multifrequency linear transducers (6–18 MHz). US scanning techniques, GS and PD machine settings and definitions of abnormality were standardised among investigators. Sonographers were allowed to modify machine settings (e.g. gain, pulse repetition frequency) on individual machines for the best-quality images, to appropriately score each image Synovitis was defined according to OMERACT definitions. Synovitis on GSUS was evaluated using a 0–3 semiquantitative scale52 (0 = absence of synovial thickening, 1 = mild synovial thickening, 2 = moderate synovial thickening and 3 = marked synovial thickening). PD activity was evaluated using a 0–3 semiquantitative scale [0 = absence of signal, no intra-articular flow; 1 = mild, one or two vessels (including one confluent vessel) for small joints and two to three signals for large joints (including two confluent vessels); 2 = moderate confluent vessels (> grade 1) in < 50% of the synovium; 3 = marked vessel signals in > 50% of the synovium] |
| Who conducted US | Sonographers (no further details) |
| Comparator CE details | DAS28, TJC, SJC, SDAI, global disease activity patient and physician 100-mm VAS, HAQ-DI, ESR, CRP |
| Who conducted comparator CE | An investigator (no further details provided) |
| Primary outcome of study | Intraobserver reliability of composite synovitis scoring systems |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | GSUS and PDUS have better reliability than generally used clinical indices for evaluating synovitis in RAMandl and Balint,117 p. 1273Multimodal indices incorporating US and clinical data had similar metrological properties to their clinical counterparts; certain indices allowed for a significantly larger number of patients to be classified as having either high or moderate disease activityMandl and Balint,116 p. 879 |
| First author (study name) | Naredo55 |
|---|---|
| Year | 2007 |
| Abstract or full paper | Full paper |
| Study design | Prognostic |
| Study objective | To evaluate the sensitivity to change of PDUS assessment of joint inflammation and the predictive value of PDUS parameters in disease activity and radiological outcome in patients with early RAp. 116 |
| Population sample size | 42 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Early RA, starting cDMARDs |
| Population baseline characteristics |
31 female, 11 male Mean (SD, range) age 53.6 (14.1, 24–77) years Mean (SD, range) disease duration 6.8 (3.6, 1.5–12) months |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Starting cDMARDs at baseline: one cDMARD, n = 41; two cDMARDs, n = 1. Prior to study, 64.3% were taking oral corticosteroids and 85.7% were taking NSAIDs |
| Joints assessed | 28 joints for CE and US: bilaterally glenohumeral, elbow, wrist, MCPs, PIPs, knees |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Real-time scanner (Logiq 500CL; General Electric Medical Systems, Kyunngi, Korea) using multifrequency linear array transducers (7–12 MHz). Joint synovitis was defined as the presence of intraarticular effusion and/or synovial hypertrophy. PD parameters were adjusted at the lowest permissible pulse repetition frequency (PRF) to maximise sensitivity, resulting in PRF ranging from 500 Hz to 1000 Hz. PD was graded on a 0–3 semiquantitative scale (0 = absence, no intra-articular flow; 1 = mild, single-vessel signal or isolated signals; 2 = moderate, confluent vessels; 3 = marked vessel signals in more than half of the intra-articular area)54 |
| Who conducted US | Single rheumatologist, experienced, blinded |
| Comparator CE details |
DAS28, SJC, TJC, pain VAS, patient global VAS, HAQ Radiographic assessment by vdHSS measuring erosions and joint space narrowing |
| Who conducted comparator CE | Single rheumatologist, experienced, blinded (independent of US conduction) |
| Follow-up duration (if relevant) | 1 year |
| Primary outcome of study | Sensitivity to change of overall PDUS joint assessment and the predictive value of sequential PDUS parameters in clinical, functional and radiological outcomes |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | PDUS is a sensitive and reliable method for longitudinal assessment of inflammatory activity in early RA. PDUS findings may have a predictive value in disease activity and radiographic outcomesp. 116 |
| First author (study name) | Naredo140 |
|---|---|
| Year | 2008 |
| Abstract or full paper | Full paper |
| Study design | Prognostic |
| Study objective | To evaluate the validity, responsiveness and predictive value of PDUS monitoring of response to tumour necrosis factor (TNF) blocking agents in RAp. 2248 |
| Population sample size | 367 (278 with complete clinical, laboratory and PDUS data) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | All patients beginning therapy with a TNFi |
| Population baseline characteristics |
Mean (SD) age 53.7 (12.3) years Mean (SD) disease duration 9.1 (8.2) years Of the 278 patients with complete clinical, laboratory and PDUS data: 227 female, 51 male; mean (SD) age 53.3 (12.2) years; mean (SD) disease duration 9.6 (8.2) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Starting TNFi therapy |
| Joints assessed | 28 joints, including the left and right glenohumeral, elbow and wrist joints, MCP joints, PIP joints of the hands and knee joints, were assessed for tenderness and swelling |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS GSUS and PDUS examination was carried out with Logiq 5 Pro (GE Healthcare, Kyunnggi-do, Korea) scanners in 23 centres and Logiq 7 (GE Healthcare, Tokyo, Japan) scanners in two centres, using multifrequency linear array transducers (7–12 MHz). US scanning technique, GS and PD machine settings and definitions of abnormality were standardised. Synovitis, tenosynovitis and bursitis were defined according to the OMERACT definitions Synovial fluid and synovial hypertrophy were graded on a 0–3 semiquantitative scale (0 = absent; 1 = mild; 2 = moderate; 3 = marked). Synovial, tenosynovial and intrabursal blood flow at each joint was evaluated by PDUS. Pulse repetition frequencies were 500–750 Hz and colour gains were 18–30 dB The intra-articular, tenosynovial and intrabursal PD signal was graded on a 0–3 semiquantitative scale [0 = absent (no synovial flow); 1 = mild (three or fewer isolated signals); 2 = moderate (more than three isolated signals or confluent signal in less than half of the synovial area); 3 = marked (signals in more than half of the synovial area)] Modified US DAS was calculated for all patients at each visit, by replacing the SJC from the DAS28 with the count of joints with a synovial fluid, synovial hypertrophy or PD signal as determined by US (USDAS28 SF score, USDAS28 SH score and USDAS28 PD score, respectively) |
| Who conducted US | The same rheumatologist (one rheumatologist at 23 centres, two rheumatologists at two centres; all experienced in US), unaware of the clinical, laboratory and radiographic findings and not involved in treatment decisions |
| Comparator CE details | DAS28, TJC, SJC, patient-rated pain and disease activity on a 100-mm VAS, HAQ, CRP, ESR (rheumatoid factor) |
| Who conducted comparator CE | The same rheumatologist (one rheumatologist in 24 centres, two rheumatologists in one centre), who was blinded with regard to the PDUS and radiographic findings |
| Follow-up duration (if relevant) | 12 months |
| Primary outcome of study | Validity and responsiveness of comprehensive PDUS assessment of synovitis to monitor response to anti-TNF therapy |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | Study results show:. . . persistence of a synovial PD signal appears to . . . [predict] radiological progression in patients with established RA who are treated with anti-TNF agentsp. 2554 |
| First author (study name) | Naredo118 | ||||
|---|---|---|---|---|---|
| Year | 2013 | ||||
| Abstract or full paper | Full paper | ||||
| Study design | Diagnostic | ||||
| Study objective | To investigate the sensitivity for detecting subclinical synovitis of different reduced-joint US assessment models compared with a comprehensive US assessment in RA patients in clinical remissionp. 512 | ||||
| Population sample size | 67 | ||||
| Population diagnosis of RA | ACR 1987 criteria | ||||
| Population eligibility details (e.g. early RA, remission) | RA clinical remission assessed by their rheumatologist; MTX treatment for at least 2 years; neither disease flare nor changes in therapy, including corticosteroid and MTX doses, in the previous 6 months | ||||
| Population baseline characteristics |
50 female (74.6%), 17 male Mean (SD) age 60.3 (15.0) years Mean (SD) disease duration 7.5 (5.8) years |
||||
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | MTX treatment for at least 2 years | ||||
| Joints assessed | Glenohumeral, elbow, wrist, second to fifth MCP, second to fifth PIP of the hands, hip (i.e. anterior recess), knee, ankle and second to fifth MTP joints | ||||
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Real-time scanner (Mylab 70 XVG, Esaote) equipped with two multifrequency linear array transducers, a 6- to 18-MHz transducer for superficial areas and a 4- to 13-MHz transducer for deep areas GSUS synovial hypertrophy was scored on a 0–3 semiquantitative scale (0 = absent; 1 = mild; 2 = moderate; 3 = marked). The synovial PD signal was scored on a 0–3 semiquantitative scale [0 = absent (no synovial flow); 1 = mild (three or fewer PD signals); 2 = moderate (more than three PD signals in less than half of the synovial area); 3 = marked (more than three PD signals in more than half of the synovial area)] |
||||
| Who conducted US | A rheumatologist experienced in musculoskeletal US | ||||
| Comparator CE details | DAS28 and SDAI | ||||
| Who conducted comparator CE | One investigator | ||||
| Primary outcome of study | Detection of subclinical synovitis by different reduced-joint US assessment models compared with a comprehensive US assessment in RA patients in clinical remission | ||||
| Outcome(s) reported in main body of report | Diagnostic | ||||
| Study authors’ conclusions | US assessment of the wrist, MCP, ankle and MTP joints can be highly sensitive for detecting residual B-mode and Doppler joint inflammation in RA patientsp. 512 | ||||
| 67 patients | US negative, B-mode = 0 | US positive, B-mode > 0 | Total | ||
| CE positive, a DAS28 of > 2.6 | 0 | 26 | 26 | ||
| CE negative, a DAS28 of < 2.6 | 5 | 36 | 41 | ||
| Total | 5 | 62 | 67 | ||
| 67 patients | US negative, PD = 0 | US positive, PD > 0 | Total | ||
| CE positive, a DAS28 of > 2.6 | 10 | 16 | 26 | ||
| CE negative, a DAS28 of < 2.6 | 22 | 19 | 41 | ||
| Total | 32 | 35 | 67 | ||
| 67 patients | US negative, B-mode = 0 | US positive, B-mode > 0 | Total | ||
| CE positive, SDAI of > 3.3 | 1 | 44 | 45 | ||
| CE negative, SDAI of < 3.3 | 4 | 18 | 22 | ||
| Total | 5 | 62 | 67 | ||
| 67 patients | US negative, PD = 0 | US positive, PD > 0 | Total | ||
| CE positive, SDAI of > 3.3 | 18 | 27 | 45 | ||
| CE negative, SDAI of < 3.3 | 14 | 8 | 22 | ||
| Total | 32 | 35 | 67 | ||
| Diagnostic accuracy comparison, CE with reference US | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
| DAS28 of > 2.6 with B-mode US reference (> 0 on a scale of 0 to 3) | 42 | 100 | 100 | 12 | |
| DAS28 of > 2.6 with PDUS reference (> 0 on a scale of 0 to 3) | 46 | 69 | 62 | 54 | |
| SDAI of > 3.3 with B-mode US reference (> 0 on a scale of 0 to 3) | 71 | 80 | 98 | 18 | |
| SDAI of > 3.3 with PDUS reference (> 0 on a scale of 0 to 3) | 77 | 44 | 60 | 64 | |
| Diagnostic accuracy comparison, US with reference CE | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
| B-mode US (> 0 on a scale of 0 to 3), reference DAS28 of > 2.6 | 100 | 12 | 42 | 100 | |
| PDUS (> 0 on a scale of 0 to 3), reference DAS28 of > 2.6 | 62 | 54 | 46 | 69 | |
| B-mode US (> 0 on a scale of 0 to 3), reference SDAI of > 3.3 | 98 | 18 | 71 | 80 | |
| PDUS (> 0 on a scale of 0 to 3), reference SDAI of > 3.3 | 60 | 64 | 77 | 44 | |
| First author (study name) | Naredo156,157 |
|---|---|
| Year | 2014 and 2015 |
| Abstract or full paper | Full paper |
| Study design | Treatment prediction |
| Study objective | To investigate the predictive value of synovitis detected by Doppler US in relation to failed tapering of biologic therapy in RA patients in sustained clinical remissionNaredo et al.,157 p. 1408 |
| Population sample size | 77 study completers (of 80 recruited) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | RA, stable bDMARDS in the previous 12 months, clinical remission by DAS28 or SDAI, no more than 5 mg/day of prednisone, no NSAIDs for more than 1 week and no corticosteroid injections in the previous 6 months |
| Population baseline characteristics | 52 women, 25 men |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | All stable bDMARDS. Twenty-three (29.9%) patients were treated with ADA, 21 (27.3%) with ETN, 18 (23.4%) with IFX, 7 (9.1%) with TCZ, 6 (7.8%) with ABT and 2 (2.6%) with GOL |
| Joints assessed | 42 joints (including hands and feet): glenohumeral, elbow, wrist, MCP 2–5, PIP 2–5, hip, knee, ankle and MTP 2–5 joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Real-time scanner (Mylab 70 XVG; Esaote, Genoa, Italy) equipped with two multifrequency linear array transducers, a 6- to 18-MHz transducer for superficial areas and a 4- to 13-MHz transducer for deep areas, assessed by presence and grade according to OMERACT. A global index for GSUS synovitis and a global index for Doppler synovitis were calculated Synovial hypertrophy was scored on a 0–3 semiquantitative scale (0 = absent; 1 = mild; 2 = moderate; 3 = marked). The synovial PD signal was also scored on a 0–3 semiquantitative scale (0 = no synovial PD signal; 1 = mild (three or fewer PD signals within the synovial hypertrophy); 2 = moderate (more than three PD signals in less than half of the synovial hypertrophy); 3 = marked (PD signals in more than half of the synovial hypertrophy) |
| Who conducted US | Rheumatologist experienced in US |
| Comparator CE details | DAS28, SDAI, clinical and laboratory assessment every 3 months |
| Who conducted comparator CE | Two study investigators |
| Follow-up duration (if relevant) | 12 months |
| Primary outcome of study | The predictive value of synovitis detected by Doppler US in relation to failed tapering of biologic therapy at 6 and 12 months in RA patients in sustained clinical remission |
| Outcome(s) reported in main body of report | Treatment |
| Study authors’ conclusions | The presence of Doppler-detected synovitis may predict biologic therapy tapering failure in RA patients in sustained clinical remissionNaredo et al.,157 p. 1408 |
| First author (study name) | Osipyants97,150 |
|---|---|
| Year | 2013 |
| Abstract or full paper | Abstract |
| Study design | Prognostic |
| Study objective | To assess the significance of residual inflammation according to the presence of SJC and PD signals in relation to radiographic progression in patients receiving TCZOsipyants et al.,150 p. A755 |
| Population sample size | 36 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | RA patients receiving TCZ |
| Population baseline characteristics |
26 (72%) female, 10 male Median (range) age 51 (43–57) years Median (range) disease duration 54 (24–96) months |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | All patients receiving TCZ |
| Joints assessed | Bilateral, the most affected joints: wrist, MCP 2 and 3 and PIP 2 and 3 joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
PDUS ‘Voluson-i’ (GE, USA) with transducer (4–13 MHz) Each joint was scored according to the OMERACT definitions of pathology. Dichotomised into ‘non- or low-active’ (PD ≤ 1) and ‘middle- or high-active’ (PD > 1) |
| Who conducted US | Single operator |
| Comparator CE details | SJC, dichotomised into ‘non- or low-active’ (SJC ≤ 1) and ‘middle- or high-active’ (SJC > 1) |
| Who conducted comparator CE | NR |
| Follow-up duration (if relevant) | 1 year |
| Primary outcome of study | Relation of US and clinical baseline parameters with radiographic progression at 1 year. One-year radiographic progression of the hands and feet was defined when the change in the vdHSS (change in TSS) was > 0.5 units per year |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | In TCZ-treated patients the US imaging activity score appears to be more predictive of radiographic progression than SJC statusOsipyants et al.,150 p. A755 |
| First author (study name) | Pereira119 |
|---|---|
| Year | 2015 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To determine if there is a correlation between intra-articular PDUS and pain symptoms in RAp. 1975 |
| Population sample size | 72 considered in two groups: painful MCP, n = 34; painless MCP, n = 38 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Established RA, chronic swelling of at least one MCP joint for at least 3 consecutive months and stable use of DMARDs for the previous 3 months. For painful group, pain of ≥ 4 on VAS 0–10. No other concomitant inflammatory diseases, no history of hand surgery or presence of severe hand deformities and no uncontrolled comorbidities |
| Population baseline characteristics |
Female, n (%): painless group 35 (92); painful group 33 (97) Mean (range) age: painless group 60.3 (32–81) years; painful group 56.9 (29–88) years Mean (range) RA duration: painless group 17.3 (2–46) years; painful group 14.7 (2–38) years |
| Population treatment (e.g. bDMARDS or cDMARDs) | Corticosteroid use, n (%): painless group 15 (39.5), painful group 17 (50); DMARD monotherapy, n (%): painless group 17 (44.7), painful group 6 (17.6); DMARDs association, n (%): painless group 9 (23.7), painful group 11 (32.4); dose of MTX, mean (range): painless group 9.5 (0–25) mg, painful group 12.5 (0–25) mg: biologic therapy use, n (%): painless group 10 (26.3), painful group 15 (44.1); DMARD + biologic therapy use, n (%): painless group 7 (18.4), painful group 13 (38.2) |
| Joints assessed | MCP 2–5, bilaterally |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS MyLAb 60 (Esaote SpA, Genoa, Italy) and MyLab Twice (Esaote SpA, Genoa, Italy) equipped with 6- to 18-MHz broadband multifrequency linear transducer. MCP joints scanned using a multiplanar technique adopting the indications provided by EULAR guidelines. PD settings were standardised at pulse repetition frequency of 500–750 Hz and Doppler frequency between 9.1 and 11.1 MHz. OMERACT preliminary definitions were adopted Joints were examined by US according to GS, PD, erosion (present or absent) and 0–3 semiquantitative scores. PD: semiquantitative score: 0 = no intra-articular flow, 1 = single-vessel signal, 2 = confluent vessels and 3 = vessel signal in > 50% of the intra-articular area. 54 GS: semiquantitaive score: 0 = no synovial thickening, 1 = minimal synovial thickening, 2 = moderate synovial thickening with capsule distension and 3 = synovial thickening, extending to bone diaphysis52 |
| Who conducted US | Two experienced rheumatology sonographers trained for the assessment of RA |
| Comparator CE details | CE (on same day as US), SJC |
| Who conducted comparator CE | Expert rheumatologist |
| Follow-up duration (if relevant) | NA |
| Primary outcome of study | Correlation between intra-articular PD and pain symptoms |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | Intra-articular PD was not correlated with pain symptom in this studyp. 1975 |
| First author (study name) | Ramirez García98 |
|---|---|
| Year | 2014 |
| Abstract or full paper | Abstract |
| Study design | Prognostic, prospective cohort study |
| Study objective | To analyse clinical and sonographic predictors of clinical flares in patients with RA in clinical remissionp. 888 |
| Population sample size | 28 |
| Population diagnosis of RA | Criteria NR (‘diagnosed with rheumatoid arthritis’) |
| Population eligibility details | RA, clinical remission (defined as a DAS28 of < 2.6) |
| Population baseline characteristics |
23 female, 5 male Mean (SD) age 51.9 (11.9) years Mean (SD) disease duration 108.2 (SD 92.6) months |
| Population treatment | 75% on at least one cDMARD; 39.3% on biological treatment |
| Joints assessed | Knees and hands (wrists, MCP, PIP, flexor and extensor tendons) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
PDUS High sensitivity equipment (Acuson AntaresTM; Siemens AG, Erlangen, Germany) with a 8-to 12-MHz linear probe. Synovial hypertrophy (grades 0–3) and PD (grades 0–3) quantified |
| Who conducted US | Rheumatologist experienced in musculoskeletal US |
| Comparator CE details | Serum concentrations of several cytokines and angiogenic factors were analysed by enzyme-linked immunosorbent assay (ELISA) at baseline [results reported for ESR, concentration of transforming growth factor-beta1 (TGF-β1)] |
| Who conducted comparator CE | Analysis by RayBiotech Inc. |
| Follow-up duration | 12 months |
| Primary outcome of study | Clinical and sonographic predictors of clinical flares |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | Although an elevated ESR or a low concentration of TGF-β1 at baseline seems to be associated with clinical reactivation of patients with RA in remission, the PD signal is the best predictor of disease reactivation at 12 monthsp. 888 |
| First author (study name) | Reynolds141 and Rees151 |
|---|---|
| Year | 2009 and 2007 |
| Abstract or full paper | Full paper |
| Study design | Prognostic |
| Study objective | To determine whether a range of single time point US measures of synovial disease and serological characteristics are able to predict progression of US-defined erosive disease in patients with established RAReynolds et al.,141 p. 473 |
| Population sample size | 40 (25 with follow-up data) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Established RA |
| Population baseline characteristics |
29 females Median (range) age 59 (34–84) years Median (range) disease duration 6 (1–29) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Of the 25 patients followed up, 3 were on anti-inflammatory drugs only, 14 were on DMARDs and 8 were treated with a TNFi (or started during the study) |
| Joints assessed | One PIP or MCP joint from each patient was chosen as representative of one of four categories of joint based solely on the clinical signs. The four categories were (1) not swollen or tender, (2) swollen only, (3) tender only and (4) swollen and tender |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Philips HDI 5000 (Philips Medical Systems, Andover, MA, USA) with a C7- to 15-MHz ‘hockey-stick’ transducer Erosions were graded on a 0–3 semiquantitative scoring system (Lund) (0 = absence of erosions; 1 = one to two erosions; 2 = more than two erosions; 3 = areas of regional bone destruction). GS images were graded on a 0–3 semiquantitative scoring system(0 = absence of synovial hypertrophy; 1 = small degree of synovial hypertrophy; 2 = moderate synovial hypertrophy; 3 = marked synovial hypertrophy). PD images were graded on a 0–3 semiquantitative scoring system (0 = sbsence of PD signal; 1 = single-vessel dots; 2 = confluent vessel dots over less than half of the area of the synovium; 3 = confluent vessel dots over more than half of the area of the synovium) |
| Who conducted US | Two radiologists |
| Comparator CE details | Swelling, tenderness, ESR (rheumatoid factor, anti-citrullinated protein antibody) |
| Who conducted comparator CE | Baseline: experienced rheumatologist; follow-up: NR |
| Follow-up duration (if relevant) | 2 years (mean 26.8 months, range 24–32 months) |
| Primary outcome of study | Joint erosions |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | The majority of single time point US measures of synovial disease were not able to identify MCP or interphalangeal joints destined to develop progressive US-determined bone damage in patients with established RAReynolds et al.,141 p. 473 |
| First author (study name) | Ribbens120 | ||||
|---|---|---|---|---|---|
| Year | 2003 | ||||
| Abstract or full paper | Full paper | ||||
| Study design | Diagnostic and response to treatment | ||||
| Study objective | To evaluate using GSUS and PDUS and clinical assessment the response of hand joint synovitis in patients with active RA to treatment with TNFi (IFX)p. 562 | ||||
| Population sample size | 11 | ||||
| Population diagnosis of RA | ACR 1987 revised criteria | ||||
| Population eligibility details (e.g. early RA, remission) | Active RA despite MTX, six or more swollen joints, 10 or more tender joints and one of the following: morning stiffness for > 45 minutes, ESR of > 28 mm/hour or CRP of > 20 mg/l. Also, presence of erosions | ||||
| Population baseline characteristics |
7 women, 4 men Mean (range) age 54 (25–74) years Mean (range) disease duration 9 (2–31) years |
||||
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Previously treated with a mean of 3.2 (range 1–5) cDMARDs. After baseline examination, treated with IFX and MTX | ||||
| Joints assessed | Wrist, MCP and PIP joints | ||||
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS US (Aloka Prosound 5500; Aloka, Tokyo, Japan) was performed using a B-mode 13.0-MHz transducer and a PD 5-MHz pulse repetition frequency of 521 Hz. PD settings were standardised to a pulse repetition frequency of 651 Hz. Synovitis was defined as a hypoechoic or anechoic area in the joint space. Cut-off for US positivity was defined as a synovial thickness of ≥ 1 mm Doppler US measurements were simultaneously carried out and positive signals were scored according to a 0–3 semiquantitative scale (0 = no perfusion; 1 = low perfusion; 2 = moderate perfusion; 3 = intense intra-articular joint perfusion) |
||||
| Who conducted US | One radiologist and one rheumatologist, experienced in US, both examined each patient | ||||
| Comparator CE details | DAS28-ESR | ||||
| Who conducted comparator CE | An independent physician experienced in joint assessment | ||||
| Follow-up duration (if relevant) | 6 weeks | ||||
| Primary outcome of study | The response of hand (i.e. wrist, MCP and PIP) joint synovitis in patients with active RA to treatment with IFX | ||||
| Outcome(s) reported in main body of report | Diagnostic | ||||
| Study authors’ conclusions | US is a feasible imaging modality for the measurement of the response of RA small-joint synovitis to therapyp. 562 | ||||
| Additional data120 | |||||
| Wrist (20 joints) | GSUS negative | GSUS positive | Total | ||
| Clinically swollen | 2 | 13 | 15 | ||
| Clinically not swollen | 3 | 2 | 5 | ||
| Total | 5 | 15 | 20 | ||
| MCP (110 joints) | GSUS negative | GSUS positive | Total | ||
| Clinically swollen | 17 | 47 | 64 | ||
| Clinically not swollen | 19 | 27 | 46 | ||
| Total | 36 | 74 | 110 | ||
| PIP (103 joints) | GSUS negative | GSUS positive | Total | ||
| Clinically swollen | 14 | 25 | 39 | ||
| Clinically not swollen | 44 | 20 | 64 | ||
| Total | 58 | 45 | 103 | ||
| Population | Diagnostic accuracy comparison | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
| Wrist | CE with reference US | 87 | 60 | 87 | 60 |
| MCP | CE with reference US | 64 | 53 | 73 | 41 |
| PIP | CE with reference US | 56 | 76 | 64 | 69 |
| Population | Diagnostic accuracy comparison | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
| Wrist | US with reference CE | 87 | 60 | 87 | 60 |
| MCP | US with reference CE | 73 | 41 | 64 | 53 |
| PIP | US with reference CE | 64 | 69 | 56 | 76 |
| First author (study name) | Riente121 |
|---|---|
| Year | 2010 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To investigate the prevalence of US pathogenic abnormalities and to compare them with the clinical findings in the knee of RA patientsp. 300 |
| Population sample size | 100 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Patients with RA, no previous knee joint surgery and no corticosteroid injections within the previous 3 months |
| Population baseline characteristics |
79 female, 21 male Mean (SD, range) age 58 (5.74, 22–82) years Mean (SD, range) disease duration 96 (70, 12–288) months |
| Population treatment (e.g. bDMARDS or cDMARDs) | 200 knee joints |
| Joints assessed | Knee |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Logiq 9 (General Electrics Medical Systems, Milwaukee, WI, USA) with a linear probe operating at 10 MHz when studying joints and 14 MHz when studying tendons used in all centres. When synovial proliferation was detected, PD examination (pulse repetition frequency 500 Hz, Doppler frequency 7.5 MHz and Doppler gain to avoid random noise visualisation) was performed Joint effusion, synovial hypertrophy and enthesopathy and bone erosions diagnosed according to OMERACT definitions. No details of scoring system reported |
| Who conducted US | One rheumatologist experienced in musculoskeletal US in each unit |
| Comparator CE details | Pain, tenderness and swelling (presence/absence) |
| Who conducted comparator CE | A rheumatologist (not involved in US examination) |
| Primary outcome of study | Prevalence of US pathogenic abnormalities |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | US examination of the knee is more sensitive than CE in the detection of joint inflammation and allows for the identification of different patterns of pathological changes at the knee levelp. 300 |
| First author (study name) | Riente122 |
|---|---|
| Year | 2011 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To investigate the prevalence of US abnormalities in the foot of RA patients and compare these with clinical findingsp. 1 |
| Population sample size | 100 |
| Population diagnosis of RA | ACR 1987 revised classification criteria |
| Population eligibility details (e.g. early RA, remission) | RA diagnosed, attending outpatient or inpatient clinic, no prior joint surgery, no corticosteroid injection to foot within 3 months |
| Population baseline characteristics |
72 female, 28 male Mean (SD) age 56 years (4.8) Mean (SD) disease duration 65 (75) months |
| Population treatment (e.g. bDMARDS or cDMARDs) | NR (apart from no steroid injection within 3 months prior to study) |
| Joints assessed | Bilateral MTP (2–5), PIP (2–5) and midfoot joints [taloavicular, calcaneocuboid, medial, intermediate and lateral navicular-cuneiform and cuneiform-metatarsal joints and cuboid (fourth) and metatarsal (fifth) joints] |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS US examinations used a Logiq9 (General Electrics Medical Systems, Milwaukee, WI, USA) system with linear probe at 14 MHz, and a MyLab70XVG (Esaote SpA, Genoa, Italy) system with multifrequency linear probe at 16 MHz. Multiplanar scanning technique was according to EULAR guidelines (dorsal and plantar aspects). Diagnosis was according to preliminarly definitions from OMERACT, using a semiquantitative grading method (0–3) |
| Who conducted US | Rheumatologist experienced in US |
| Comparator CE details | CE for swelling, pain and tenderness |
| Who conducted comparator CE | Rheumatologist not involved in the US examination |
| Primary outcome of study | Prevalence of US abnormalities in the foot |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | US examination appears to be a useful technique to study foot joint and tendon involvement in RA patients . . . [and] is more sensitive than CE to detect joint inflammationp. 1 |
| First author (study name) | Salaffi160 |
|---|---|
| Year | 2008 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To assess the interobserver agreement of standard joint count and to compare CE with GSUS findings in patients with early RAp. 54 |
| Population sample size | 44 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Early RA (< 2 years) |
| Population baseline characteristics |
72.7% women Mean (SD) age 53 (9.8) years Mean (SD) disease duration 17 (3.8) months |
| Population treatment (e.g. bDMARDS or cDMARDs) | Prior cDMARDs in all, bDMARDs in 17% |
| Joints assessed | Acromioclavicular, glenohumeral, elbow, wrist (radiocarpal), MCP, PIP of hands, knee and ankle (tibiotalar), MTP and hip joints, bilaterally |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS US examinations were performed using an AU5 ‘Harmonic’ (Esaote Biomedica, Genoa, Italy) equipped with two broadband linear probes (7.5–10 and 10–14 MHz). Patients were evaluated using the Naredo et al. 54 scanning protocol. Joint inflammation was detected using OMERACT definitions. No details of the scoring system were reported |
| Who conducted US | A rheumatologist experienced in US |
| Comparator CE details | TJC, SJC |
| Who conducted comparator CE | Two rheumatologists |
| Primary outcome of study | Interobserver agreement of standard joint count |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | CE is far from optimal for assessing joint inflammation in patients with early RA . . . US can considerably improve the detection of signs of joint inflammation in terms of both sensitivity and reliabilityp. 54 |
| First author (study name) | Saleem124 |
|---|---|
| Year | 2011 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To assess:the accuracy of more stringent remission criteria for indicating the absence of inflammation, using US imaging assessment of synovitis as the gold standardp. 792 |
| Population sample size | 128 |
| Population diagnosis of RA | ACR criteria |
| Population eligibility details (e.g. early RA, remission) | Clinical remission |
| Population baseline characteristics |
Sex NR Mean (SD) age 54 (14) years Median (1st to 3rd quartile) disease duration 8 (5–13) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | DMARD or combination of TNFi and MTX |
| Joints assessed | Dominant-hand MCP joints 2–5 and wrist (640 joints) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS US examination was performed using the Phillips ATL HDI 3000 machine (DMARD group) and the HDI 5000 machine (combination TNFi group) using 10- to 5-MHz and 15- to 8-MHz/12- to 5-MHz ‘hockey-stick’ transducers, respectively. GSUS and PDUS were used to assess synovial hypertrophy (SH) and synovial vascularity. The presence and location of any SH and PD hyperaemia were recorded according to standardised OMERACT definitions Individual joints were scored for GS SH and PD using a validated 0–3 semiquantitative method (GS: 0 = no SH, 1 = mild SH, 2 = moderate SH, 3 = severe SH; PD: 0 = normal/minimal vascularity, 1 = mild hyperaemia, 2 = moderate hyperaemia, 3 = marked hyperaemia) |
| Who conducted US | Experienced ultrasonographers |
| Comparator CE details | Morning stiffness (minutes), 0–100 VAS for patient global assessment of health and disease activity, TJC, SJC, CRP, HAQ-DI, RAQoL |
| Who conducted comparator CE | Independent trained metrologist |
| Primary outcome of study | Relationship between clinical scores of remission, imaging-detected synovitis and quality-of-life outcomes |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | Signs and symptoms of inflammation were reduced when more stringent remission criteria were used, but the percentage of joints with PD activity was not reduced, even in those without signs or symptoms. These data suggest that PD is more sensitive than clinical criteria for accurately detecting low but clinically relevant levels of inflammation |
| First author (study name) | Saleem142 |
|---|---|
| Year | 2012 |
| Abstract or full paper | Full paper |
| Study design | Prognostic |
| Study objective | To determine the clinical, functional and imaging associations of disease flare in patients with RA in remission and any effect on long-term outcomesp. 1316 |
| Population sample size | 93 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | In clinical remission as assessed by their consulting rheumatologist; no flares in the last 6 months |
| Population baseline characteristics |
63 (67.7%) female Mean (95% CI) age 56.6 (53.9 to 59.4) years Mean (95% CI) duration of RA 7.0 (4.5 to 9.5) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | cDMARDs, stable treatment for 6 months, no indication for a change in treatment |
| Joints assessed |
Clinical: joints included in the standard 28-joint count US: dominant-hand MCP joints and wrist (intercarpal, radiocarpal, ulnar carpal and distal radioulnar compartments) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS US examination was performed using the Phillips ATL HDI 3000 machine with a 10- to 5-MHz ‘hockey-stick’ transducer (no other information presented). GSUS and PDUS were used to assess synovial hypertrophy (SH) and synovial vascularity, respectively. The presence and location of any synovial pathology were recorded according to standardised OMERACT definitions Individual joints were scored for GS SH and PD activity using a validated 0–3 semiquantitative method (GS: 0 = no SH, 1 = mild SH, 2 = moderate SH, 3 = severe SH; PD: 0 = normal/minimal vascularity, 1 = mild hyperaemia, 2 = moderate hyperaemia, 3 = marked hyperaemia) |
| Who conducted US | A single experienced ultrasonographer who was blinded to all other study findings |
| Comparator CE details | Duration of morning stiffness (minutes), 0–100 VAS for physician and patient global assessment of health and disease activity, SJC, TJC, CRP, (anti-cyclic citrullinated protein antibody, rheumatoid factor), HAQ-DI |
| Who conducted comparator CE | Independent trained metrologist |
| Follow-up duration (if relevant) | 12 months |
| Primary outcome of study | Disease flare (defined as any increase in disease activity requiring a change in therapy) |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | Patients without PD activity at baseline have a low likelihood of flaringp. 1321 |
| First author (study name) | Scheel53 |
|---|---|
| Year | 2005 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To develop an ultrasonographic synovitis scoring system suitable for evaluation of finger joint inflammation in patients with active RA and to compare semiquantitative ultrasonographic scoring with quantitative ultrasonographic measurementsp. 733 |
| Population sample size | 46 |
| Population diagnosis of RA | ACR criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA; nine patients had early RA |
| Population baseline characteristics |
37 females, 9 males Mean (range) age 53 (17–75) years Mean (SD) disease duration 8.5 (8.2) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | All treated with DMARDs, including 20 treated with bDMARDs |
| Joints assessed | Second to fifth PIP and MCP joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS US was performed with a HDI 3500 high-end US system (Advanced Technologies Laboratories, Bothell, WA, USA) using a 10- to 5-MHz ‘hockey-stick’ linear array transducer. Two criteria for active inflammation were evaluated by US: joint effusion (visualised as a black, anechoic area) and thickening of the synovial membrane (visualised as hypo- or hyperechoic structures within the region affected by effusion) Joint effusion and hypertrophy were scored on a 0–3 semiquantitative scale,52 modified to include both synovitis and effusion in a combined measure [0 = no anechoic, hypoechoic or hyperechoic structure visible (no effusion/hypertrophy); the larger the anechoic structure or extent of synovial hypertrophy seen on US images, the higher the assigned score (1 = minimal effusion/hypertrophy, 2 = moderate effusion/hypertrophy, 3 = extensive effusion/hypertrophy)] |
| Who conducted US | Investigator experienced in musculoskeletal US |
| Comparator CE details | DAS28, pain history, CRP, ESR, SJC, TJC |
| Who conducted comparator CE | No details reported |
| Primary outcome of study | Optimal US scoring method from six joint combinations [receiver operating characteristic (ROC) curve analysis] |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | US evaluation of finger joint synovitis can be considerably simplified by focusing on the palmar side and by applying semiquantitative grading instead of quantitative measurementsp. 733 |
| First author (study name) | Spiegel125 |
|---|---|
| Year | 1987 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To evaluate:the correlation of clinically graded joint tenderness and soft tissue swelling with ultrasonographic findings of the same joints and evaluate responses to treatment with fenbufen, a new NSAIDp. 1283 |
| Population sample size | 6 |
| Population diagnosis of RA | 1958 revision of diagnostic criteria for RA |
| Population eligibility details (e.g. early RA, remission) | Definite or classic RA of ≥ 1 year in duration |
| Population baseline characteristics | HR; duration of at least 1 year |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | NSAIDs; no NSAIDs for 2 weeks prior to SJC, TJC and US |
| Joints assessed | Six joints: both shoulders, wrists and knees (transversely and longitudinally) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS Performed using commercial contact B scanners, with 5.0-MHz and 7.5-MHz transducers and using a high-resolution real-time scanner, with a 7.5-MHz transducer. Degree of soft tissue proliferation and amount of effusion were evaluated by a radiologist and graded on a 0–3 semiquantitative scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). Visualisation of an effusion of < 5 mm in depth was diagnosed as a mild soft tissue change. Moderate changes were those effusions between 5 mm and 10 mm in depth, plus visualisation of echogenic soft tissue structures in the joint. Severe changes were effusions of > 10 mm in depth, plus visualisation of echogenic soft tissue abnormalities in the joint |
| Who conducted US | A radiologist |
| Comparator CE details | Degree of tenderness and swelling in each of the six joints was graded clinically on the same 4-point scale used for sonograms |
| Who conducted comparator CE | A rheumatologist |
| Primary outcome of study | Correlation of joint swelling, joint tenderness and US effusion |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | The patient’s subjective index of tenderness, the physician’s assessment of joint swelling on physical examination and the findings on ultrasonography were correlated in this study.Findings suggest that ultrasonography is useful in that it allows an objective and permanent documentation of the amount of synovial effusion and proliferation present . . .p. 1288 |
| First author (study name) | Szkudlarek126 |
|---|---|
| Year | 2004 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To compare US with MRI, conventional radiography and CE in the evaluation of bone destruction and signs of inflammation in the MTP joints of patients with RAp. 2103 |
| Population sample size | 40 patients (200 MTP joints) (also included healthy control subjects but data reported separately for RA patients) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA |
| Population baseline characteristics |
Female-to-male ratio 3 : 1 Median (range) age 56 (23–78) years Median (range) disease duration 2 (0–20) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | 35 patients being treated with DMARDs |
| Joints assessed | MCP joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS US was performed with a General Electric LOGIQ-500 unit using a 7- to 13-MHz linear array transducer. Joint effusion was defined as the compressible anechoic intracapsular area, synovitis as a hypoechoic synovial thickening (non-compressible hypoechoic intracapsular area) and bone erosions as pathological changes in the bone surface of the area adjacent to the joint GSUS findings were scored on a 0–3 semiquantitative scale for bone destruction and joint effusion,52 including a 0–3 scale for synovial thickening. Grade 3 synovial thickening was further divided into grades 3 and 4 to encompass the more advanced stages of synovial thickening. Grades 0 and 1 were considered to be physiological and grades 2 and 3 (or 4) were considered to be pathological |
| Who conducted US | The same rheumatologist, who was trained in the examination of the small joints of the hands and feet |
| Comparator CE details | Swelling and tenderness |
| Who conducted comparator CE | A consultant rheumatologist |
| Primary outcome of study | Agreement between US, MRI, CE and radiology |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | Compared with MRI, US was found to be markedly more sensitive and accurate than CE and conventional radiography for the detection and grading of destructive and inflammatory changes in the MTP joints of patients with RA. Evaluation of these joints by US may be of major clinical importance in RA, considering the early and frequent involvement of the MTP joints |
| First author (study name) | Szkudlarek127 |
|---|---|
| Year | 2006 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To investigate whether ultrasonography can provide information on signs of inflammation and destruction in RA finger joints that are not available with conventional radiography and CE and which are comparable to the information provided by MRIp. 1 |
| Population sample size | 40 patients (158 second to fifth MCP joints and 140 second to fifth PAP joints) (also included healthy control subjects but data reported separately for RA patients) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active RA [20 with established disease (> 2 years)] |
| Population baseline characteristics |
Female-to-male ratio 4 : 1 Mean (range) age 58 (23–79) years Mean (range) disease duration 5 (0–20) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | 15 being treated with DMARDs |
| Joints assessed | Second to fifth MCP joints and second to fifth PIP joints |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS Ultrasonography was performed using a General Electric LOGIQ 500 unit (General Electric, Solingen, Germany) using a 7- to 13-MHz linear array transducer. Each joint was assessed by quadrant for the presence or absence of bone erosions and the presence or absence of signs of inflammation (joint effusion and synovitis) Bone erosion defined as break in bone cortex in the area adjacent to the joint, visualised in two planes; joint effusion defined as compressible anechoic intracapsular area; and synovitis defined as uncompressible hypoechoic intracapsular area. Changes were scored according to a 0–3 semiquantitative scoring system. 52 Scoring of synovitis was widened to include grade 4 (hypoechoic area bulging out of the joint and stretching over both bone diaphyses of the joint) |
| Who conducted US | Two radiologists with expertise in musculoskeletal ultrasonography and a rheumatologist with training in the examination of the small joints of the extremities |
| Comparator CE details | Swelling and tenderness |
| Who conducted comparator CE | The consultant rheumatologist on duty |
| Primary outcome of study | Agreement between US, MRI, CE and radiology |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | This study shows that ultrasonography has the potential to improve the assessment of patients with RAp. 1 |
| First author (study name) | Taniguchi128 |
|---|---|
| Year | 2014 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To examine the usefulness of maximum intensity projection MRI for RA in the hand |
| Population sample size | 30 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | RA, not taking bDMARDs or oral steroids (18 patients in remission with a DAS28-CRP of < 2.3) |
| Population baseline characteristics |
25 women and 5 men Mean (range) age 61.5 ± 9.5 (38–81) years Mean (range) disease duration 12.5 ± 11.5 years (4 months to 45 years) |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | No bDMARDs or oral steroids, MTX in 27 patients |
| Joints assessed | US on 60 wrists and 300 MCP joints. (CE of 28 joints of DAS28) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Ultrasonography was performed using a HI VISION Avius (Hitachi Medical Corporation, Tokyo, Japan) with a linear-type (14–6 MHz) probe GS images with low echo regions within joints were considered to indicate synovitis. The PD frequency was set at 7.5 MHz and the pulse repetition frequency was set between 800 Hz and 1000 Hz. For PD images, each joint was scored on a semiquantitative scale (0–3) with a score of grade 1 or higher taken as positive (grade 0 = no flow in the synovium, grade 1 = single-vessel signals, grade 2 = confluent-vessel signals in less than half of the area of the synovium, grade 3 = vessel signals in more than half of the area of the synovium52) |
| Who conducted US | Orthopaedic surgeon trained in US examination of the small joints of rheumatoid hands. Two orthopaedic surgeons specialising in RA scored the joints independently |
| Comparator CE details | DAS28-CRP |
| Who conducted comparator CE | NR |
| Primary outcome of study | Sensitivity of CE with reference MRI or PDUS |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | CE:showed low sensitivity and high specificity compared with both MRI and PDUS images. A statistically significant correlation between the scores of MRI and PDUS images was foundp. 911 |
| First author (study name) | Vlad129 |
|---|---|
| Year | 2015 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To investigate the responsiveness of tenosynovitis of the wrist and hands compared with the responsiveness of synovitis in a 6-month follow-up period, by ultrasonography (US) in a cohort of active RA patients starting biologic therapy; to compare the responsiveness of finger flexor tenosynovitis with the responsiveness of wrist extensor tenosynovitis by US; and to describe the subclinical synovitis and tenosynovitis by US in RA patients in clinical remission |
| Population sample size | 57 (55 at follow-up) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Active disease; history of treatment with two different cDMARDs at a maximal dosage for at least 3 months each (initiating bDMARDs) |
| Population baseline characteristics |
50 female patients Mean (SD, range) age 55.28 (10.13, 26–75) years Mean (SD, range) disease duration 113.9 (105.2, 6–414) months |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Initiation of biological DMARDs |
| Joints assessed | Bilateral wrists at the level of the radius, lunate and capitate bones from the dorsal side, MCP joints 2–5 and PIP joints 2–5 from both the dorsal and the volar sides |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Ultrasonography was performed using Esaote MyLab 25, 50 and 70 equipped with a linear transducer with 12- to 18-MHz frequency, adjusted for small joint evaluation. For PD, machines were set for maximal sensitivity to detect blood flow (Doppler frequency 6.5–7.5 MHz, pulse repetition frequency 500–750 Hz, low wall filters). All settings were maintained constant throughout the study Joint synovitis was graded on separate 0–3 semiquantitative scales for GS and PD. 52 Synovitis on GS was defined as the presence of abnormal hypoechoic material within joint recesses. PD synovitis was defined as the presence of Doppler signals inside the intra-articular hypoechoic area. In addition to the semiquantitative scale, a binary grading was performed for joint synovitis [0 = no synovitis, 1 = present synovitis (including grades 1, 2 and 3 on the semiquantitative scale)], for both GS and PD US remission was defined as the absence of GS and PD synovitis in all examined joints. Low level of imaging activity was defined less strictly, allowing gradually one to a maximum of two joints or tendons with positive synovitis or tenosynovitis, binary graded (GS and PD global scores for binary evaluation of synovitis and tenosynovitis ≤ 1 or ≤ 2) |
| Who conducted US | Rheumatologist with ≥ 5 years’ experience in musculoskeletal US (at each site) |
| Comparator CE details | TJC, SJC, patient VAS for pain and general disease evaluation, physician VAS evaluation, ESR, CRP, CDAI and SDAI |
| Who conducted comparator CE | Rheumatologist. One joint assessor at each of five sites (same one for the duration of the study) |
| Follow-up duration (if relevant) | 6 months |
| Primary outcome of study | Responsiveness of synovitis (vs. responsiveness of tenosynovitis) |
| Outcome(s) reported in main body of report | Diagnostic (SRM) |
| Study authors’ conclusions | Tenosynovitis US scoring in RA may be as good as synovitis scoring for characterisation of disease activity and responsivenessp. 352 |
| First author (study name) | Wakefield130 |
|---|---|
| Year | 2008 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To compare CE and US with high-field MRI for the detection of rearfoot and midtarsal joint synovitis and tenosynovitis of the ankle tendons in patients with established RAp. 1678 |
| Population sample size | 22 |
| Population diagnosis of RA | Modified ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | RA with symptoms of midfoot and rearfoot disease |
| Population baseline characteristics |
14 female, 8 male Mean (range) age 52 (33–70) years Mean (range) disease duration 6.8 (1–20) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | All patients were on stable doses of NSAIDs. Ten were taking MTX monotherapy, one was taking SSZ, one was taking HCQ, one was taking gold and six were on a combination of MTX and a TNFi |
| Joints assessed | Right tibiotalar, subtalar, talonavicular and calcaneocuboid joints (tendons also examined) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS ATL HDI (Advanced Technologies Laboratories, High Definition Imaging, Bothell, WA, USA) 3000 machine employing a 10- to 5-MHz linear array ‘hockey-stick’ transducer Synovitis was defined as an abnormal hypoechoic area within the joint, compatible with the OMERACT US group definition |
| Who conducted US | An experienced sonographer; a second experienced sonographer examined five patients |
| Comparator CE details | CE for synovitis (and tenosynovitis) |
| Who conducted comparator CE | A podiatrist; a second podiatrist examined five patients |
| Primary outcome of study | Sensitivity of US and CE for detecting synovitis and tenosynovitis, with a reference standard of MRI |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | CE was sensitive but US was more specific in identifying hindfoot pathology in RA compared with the reference standard of MRIp. 1678 |
| The authors suggest:a need for standardisation of acquisition and interpretation of US images of the hindfootp. 1678 | |
| Outcome data additional to main report | Interobserver variability between ultrasonographers was low, although this was based on five patients only |
| First author (study name) | Wakefield143 |
|---|---|
| Year | 2007 |
| Abstract or full paper | Full paper |
| Study design | Prognostic |
| Study objective | To examine the longitudinal relationship between clinical remission and imaging remission with the hypothesis that it may be more appropriate to aim for persistent absence of imaging synovitis rather than clinical remissionp. 1564 |
| Population sample size | 10 |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | Patients with early RA (< 12 months of symptoms). None of the patients had ever received DMARDs or biological treatments and none had received corticosteroids during the preceding month |
| Population baseline characteristics |
5 male, 5 female Median (range) age 52.5 (21–78) years Median (range) disease duration 6 (3–11) months |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | None of the patients had ever received DMARDs or biological treatments and none had received corticosteroids during the preceding month. At the start of the study, patients were started on a bDMARD (IFX) and rapidly escalating MTX |
| Joints assessed | CE: 28 joints of DAS. US: 42 joints (bilateral glenohumeral, elbow, wrist, MCP, PIP, knee, tibiotalar, midtarsal and MTP joints) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
GSUS and PDUS Philips HDI 5000 (Phillips Medical Systems, Best, the Netherlands) employing either 12- to 5- or 13- to 7-MHz linear transducers Individual joints were scored for synovitis using a semiquantitative scoring method on a 0–3 scale for both GS and PD (not referenced). Scores were expressed per joint and as a total score. Absence of imaging synovitis was arbitrarily defined as both a total GS and a total PD score of 0 |
| Who conducted US | An experienced sonographer |
| Comparator CE details | DAS28 assessment (also HAQ and the RAQoL) questionnaire and CRP). Clinical remission was defined as a DAS28 of < 2.6 and clinical response was defined as a decrease in DAS28 of > 1.2 |
| Who conducted comparator CE | Treating clinician |
| Follow-up duration (if relevant) | 46 weeks |
| Primary outcome of study | Spearman’s rho correlation coefficients between baseline GS, PD scores and DAS28, time to clinical remission and time spent in clinical remission |
| Outcome(s) reported in main body of report | Prognostic |
| Study authors’ conclusions | Even when clinical remission is achieved with TNFis, the absence of imaging synovitis may not be achievedp. 1566 |
| First author (study name) | Xiao131 |
|---|---|
| Year | 2014 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To investigate the value of US for diagnosing synovitis associated with RAp. 767 |
| Population sample size | 46 (also healthy control subjects, but data reported separately for RA patients) |
| Population diagnosis of RA | ACR 1987 criteria |
| Population eligibility details (e.g. early RA, remission) | RA patients |
| Population baseline characteristics |
31 females, 15 males Age [average, no further details (range)] 51 (21–67) years Mean (SD, range) disease duration 8.7 (8.7, 0.08–30.0) years |
| Population treatment (e.g. bDMARDS or cDMARDs) | NR |
| Joints assessed | 828 joints – CP 2–5, PIP 2–5 and wrist joints bilaterally |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
PDUS Ultrasonography was performed using the GE Logiqbook XP colour ultrasonic apparatus (General Electric Company, Fairfield, CT, USA) with high-frequency linear matrix probes, the centre frequency of which was 11 MHz OMERACT diagnostic criteria were used for reference. A 0–3 semiquantitative score59 was used for PDUS (0 = no blood image; 1 = single-vessel image; 2 = several vessels that are partially confluent; 3 = confluent vessels covering more than half of the area of the synovium) |
| Who conducted US | Two skilled doctors who had undergone specialist training in US |
| Comparator CE details | Tenderness and/or swelling |
| Who conducted comparator CE | Experienced doctors from rheumatology departments |
| Primary outcome of study | US synovitis detection |
| Outcome(s) reported in main body of report | Diagnostic |
| Study authors’ conclusions | US is a valid method for diagnosing early-stage synovitis, with high-accuracy cut-off points for MCP, PIP and wrist joints set at 2.5, 2.6 and 5.2 mmp. 767 |
| First author (study name) | Yoshimi144,145 | ||||||
|---|---|---|---|---|---|---|---|
| Year | 2013 and 2014 | ||||||
| Abstract or full paper | Full paper | ||||||
| Study design | Prognostic | ||||||
| Study objective | To assess whether US can predict progressive joint destruction during clinical remission of RAYoshimi et al.,144 p. 456 | ||||||
| Population sample size | 31 | ||||||
| Population diagnosis of RA | ACR 1987 criteria | ||||||
| Population eligibility details (e.g. early RA, remission) | RA in clinical remission (clinical remission criteria using DAS28-ESR of < 2.6 or DAS28-CRP of < 2.3) for at least 2 months | ||||||
| Population baseline characteristics |
Male 4, female 27 Mean (SD) age 55.2 (13.4) years Median (range) disease duration 5 years 0 months (2 months to 6 years 5 months) |
||||||
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) | Biological therapy: n = 13 (IFX, n = 4; ETN, n = 9); DMARDs: n = 28 (MTX, n = 23; SSZ, n = 6; tacrolimus, n = 1); steroids: n = 9 (up to 5 mg/day); drug free: n = 1 | ||||||
| Joints assessed | 28 joints (as for DAS28) for clinical assessment; 22 of these joints (excluding bilateral glenohumeral, elbow and knee joints) for US | ||||||
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
PDUS and GSUS Aplio SSA-700 A apparatus (Toshiba, Tokyo, Japan) with 12-MHz linear array transducers PD signals were graded on a 0–3 semiquantitative scale [0 = absent (no synovial flow); 1 = mild (single-vessel signal or isolated signals); 2 = moderate (confluent signals in less than half of the synovial area); 3 = marked (signals in more than half of the synovial area)]. 54 For the PIP and MCP joints, GS images were scored on a 0–3 semiquantitative scale [0 = none (no synovial thickening); 1 = mild (filling the angle between the periarticular bones without bulging over the line linking the tops of the bones); 2 = moderate (synovial thickening bulging over the line linking the tops of the periarticular bones but without extension along the bone diaphysis); 3 = severe (synovial thickening bulging over the line linking the tops of the periarticular bones and with extension to at least one of the bone diaphyses)]. 52 For wrists, GS images were scored on a 0–3 semiquantitative scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) on subjective appraisal |
||||||
| Who conducted US | Experienced rheumatologists | ||||||
| Comparator CE details | TJC, SJC, CRP, ESR, matrix metalloproteinase, DAS28-ESR and DAS28-CRP | ||||||
| Who conducted comparator CE | Rheumatologists | ||||||
| Follow-up duration (if relevant) | 2 years | ||||||
| Primary outcome of study | Relationship between US at baseline and radiographic progression at 2 years | ||||||
| Outcome(s) reported in main body of report | Prognostic | ||||||
| Study authors’ conclusions | PDUS detects synovitis causing joint destruction even when the patient is in clinical remissionYoshimi et al.,144 p. 456 | ||||||
| Prognostic sensitivity (no clinical comparator sensitivity data reported) | |||||||
| Study | Population | Measure being assessed for prediction | US measure | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
| Yoshimi 2013144 | 22 in RA clinical remission | Radiographic progression at 2 years | Total PD scores of > 1 | 100 (95% CI 59.0 to 100) | 73.3 (95% CI 44.9 to 92.2) | 63.6 | 100 |
| First author (study name) | Zuffery99,132 |
|---|---|
| Year | 2014 |
| Abstract or full paper | Full paper |
| Study design | Diagnostic |
| Study objective | To evaluate whether RA patients considered to be in remission according to clinical criteria sets still have persisting US synovitis. Also, to evaluate the capacity of the US score to discriminate between the patients with clinically active disease and those in remissionZuffery,132 p. 220To evaluate the correlation between clinical measures of disease activity and a US scoring system for synovitis applied by many different ultrasonographers in a daily routine care setting within the Swiss registry for RA (SCQM) and further to determine the sensitivity to change of this US scoreZuffery,99 p. 35 |
| Population sample size |
30799 536 in a cross-sectional cohort and 183 in a longitudinal cohort132 |
| Population diagnosis of RA | NR |
| Population eligibility details (e.g. early RA, remission) | Clinical remission |
| Population baseline characteristics |
Active disease (n = 167): 82% female; mean (SD) age 57 (13) years; median (IQR) disease duration 6.8 (2.4–16.5) years DAS remission (n = 129): 70% female; mean (SD) age 55 (15) years; median (IQR) disease duration 6.1 (3.0–12.2) years ACR/EULAR remission (n = 69): 67% female; mean (SD) age 53 (15) years; median (IQR) disease duration 4.4 (2.6–10.2) years DAS remission only (n = 71): 78% female; mean (SD) age 56 (15) years; median (IQR) disease duration 7.2 (3.0–16.5) years |
| Population treatment at baseline (e.g. bDMARDS or cDMARDs) |
48–59% on biological therapy99 57% on biological therapy132 |
| Joints assessed | 22 joints (knee, elbow, wrist and fingers bilaterally) |
| Type(s) of US and US details (including the machine used, scoring system used and diagnostic cut-off if applicable) |
Multiplanar GSUS and Doppler mode (PDUS) The SONAR score is a semiquantitative score employing both multiplanar GSUS and Doppler mode (PDUS). Synovitis was graded from 0 to 3 according to the OMERACT consensus. PD scoring was graded on a 0–3 scale according to OMERACT recommendations. Some operators did not look for PD on the dorsal aspect of the joints when only grade 1 synovitis on GSUS was present on the volar side. Those missing values were assumed to be equivalent to grade 0 on PD |
| Who conducted US | 30 ultrasonographers trained on the SONAR score |
| Comparator CE details | ACR/EULAR, DAS28, ESR and CRP |
| Who conducted comparator CE | Could be performed by the ultrasonographer or by another physician |
| Follow-up duration (if relevant) | Mean (SD) follow-up duration 11.7 (5.6) months |
| Primary outcome of study | Correlation between clinical and US data |
| Outcome(s) reported in main body of report | Diagnostic (SRM) |
| Study authors’ conclusions | This observational study confirms that many patients considered to be in clinical remission according the DAS and the ACR/EULAR definitions still have residual synovitis on USZuffery,99 p. 35The SONAR score is practicable and . . . demonstrates significant correlations with the degree of as well as change in disease activity as measured by DAS. On the level of the individual, the US score shows many discrepancies and overlapping results existZuffery,132 p. 220 |
Appendix 6 Quality assessment
Relevant items to assess study bias were taken from Karsh et al. ,84 the QUADAS tool,82 GATE83 and the QUIPS tool. 81
These are validated, widely used tools. Other tools are available. The QUADAS-2 tool280 is a more recent version of the QUADAS tool, which could have been used if the QUADAS tool was not sufficient, namely if there had been concerns about applicability of studies to the review. Applicability had been addressed in the review inclusion/exclusion criteria.
As there were several types of study included in the review, not all items were applicable to all study designs. Note that when studies included healthy control subjects in addition to RA patients, in line with the exclusion criteria, studies were included only if outcome data were reported separately for the RA subgroup. As stated previously, there is currently no gold standard/reference standard for detecting synovitis objectively.
Diagnostic study quality assessment items taken from the QUADAS tool
Many items from the QUADAS tool82 did not apply as the inclusion/exclusion criteria for the review meant that all studies would meet the following criteria:
-
Was the spectrum of patients representative of the patients who will receive the test in practice?
-
Is the reference standard likely to correctly classify the target condition?
-
Did patients receive the same reference standard regardless of the index test result?
-
Was the reference standard independent of the index test?
-
Were the same clinical data available when test results were interpreted as would be available when the test is used in practice?
Items considered relevant to this review were:
-
Were selection criteria clearly described?
-
Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests?
-
Was the execution of US described in sufficient detail to permit replication of the test?
-
Was the execution of the CE described in sufficient detail to permit replication of the test?
-
Were the US results interpreted without knowledge of the results of the CE?
-
Were the CE results interpreted without knowledge of the results of US?
-
Were uninterpretable test results reported?
-
Were withdrawals from the study explained?
Prognostic study quality assessment items taken from GATE
-
Was the outcome measure assessment blinded?
-
Was the follow-up time sufficiently long (to detect important prognostic factors)?
Prognostic study quality assessment items taken from GATE and the QUIPS tool
-
Were the prognostic factors clearly defined?
-
Was the outcome measure clearly defined?
Prognostic study quality assessment items taken from the QUIPS tool
-
Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders).
-
The method and setting of outcome measurement is the same for all study participants.
-
Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment).
Quality assessment items taken from Karsh et al.84
Most quality assessment items from Karsh et al. 84 did not apply as they referred to trials of therapy. RA-specific quality assessment items taken from Karsh et al. 84 were:
-
Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA?
-
Were joint assessments performed by a trained, independent blinded joint assessor? (Broken down into questions about independence of testing and training for clinical and US examinations, for which we included experienced staff as trained).
Quality assessment forms
When studies were diagnostic studies or prognostic studies, the level of study in the hierarchy according to Merlin et al. 159 was recorded. This is as follows:
-
diagnostic study hierarchy of evidence –
-
level II – diagnostic test accuracy studies with an independent, blinded comparator of a valid reference standard, tested on consecutive patients
-
level III-1 – comparative studies with an independent, blinded comparator of a valid reference standard tested on non-consecutive patients
-
level III-2 – comparative studies not meeting criteria for higher-level evidence
-
level III-3 – diagnostic case–control studies
-
-
prognostic study hierarchy of evidence
-
level II – prospective cohort study
-
level III-1 – all-or-none study
-
level III-2 – single arm of a RCT
-
level III-3 – retrospective cohort study.
-
| First author (study name) | Backhaus69 |
|---|---|
| Year | 2013 |
| Study design | Prognostic prospective cohort |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | U |
| Were clinical and US joint assessments conducted independently? | U |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52,53,281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | U |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | U |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | P |
| First author (study name) | Balsa105 |
|---|---|
| Year | 2010 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-2 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Beckers106 |
|---|---|
| Year | 2004 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-1 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | N (semiquantitative system for PD, not referenced) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Bhamra89 |
|---|---|
| Year | 2014 |
| Study design | Treatment decision |
| If diagnostic study, what level according to hierarchy?159 | NA |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | U |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | P |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | U (no details, not referenced) |
| Was the conduct of the CE clearly described? | N |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | NA |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | NA |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Boyesen134 |
|---|---|
| Year | 2011 |
| Study design | Prognostic, prospective cohort |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | U |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (Naredo et al.54) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | U |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | N |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | U |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | U |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Brown,135 Ikeda136 |
|---|---|
| Year | 2008, 2007 |
| Study design | Prognostic (cohort) |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre-2010) ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT; 0–3 semiquantitative scale57,282,283) |
| Was the conduct of the CE clearly described? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Bugatti,147 Scirè137 |
|---|---|
| Year | 2012 |
| Study design | Prognostic (prospective cohort) |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (Meenagh et al.,277 Schmidt et al.,284 Naredo et al.56) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y for US, U for low disease activity |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Cavet,138 Taylor100 |
|---|---|
| Year | 2009, 2004 |
| Study design | Prognostic (part of RCT comparing MTX + IFX with MTX alone in aggressive early RA) |
| If prognostic study, what level according to hierarchy?159 | III-2 (prognostic), II (treatment) |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | N |
| Was the conduct of the CE clearly described? | N |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | U |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | P |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Ceponis152 |
|---|---|
| Year | 2014 |
| Study design | Treatment decision and diagnostic comparison |
| If diagnostic study, what level according to hierarchy?159 | III-1 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52,53) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Ciurtin90,158 |
|---|---|
| Year | 2013, 2012 |
| Study design | Treatment decision |
| If diagnostic study, what level according to hierarchy?159 | NA |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | U |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | U |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | NA |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | P |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT86) |
| Was the conduct of the CE clearly described? | N |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | U |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | NA |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Dale153,154 (TaSER) |
|---|---|
| Year | 2013, 2014 |
| Study design | Treatment (treat-to-target strategy RCT: DAS28 target vs. DAS28 + musculoskeletal US target) |
| If intervention study, what level according to hierarchy?159 | II |
| Were eligibility criteria clearly described? | P |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | Y |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | NA |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | N |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52) |
| Was the conduct of the CE clearly described? | P |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | N |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | N |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Dougados139,148,149 |
|---|---|
| Year | 2013, 2014 |
| Study design | Prognostic, prospective cohort; 4 months of biologic therapy, then further follow-up up to 2 years |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT54,55,281,284) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Ellegaard101 |
|---|---|
| Year | 2011 |
| Study design | Treatment (cohort study) |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | N |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | NA |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| First author (study name) | Filippucci107 |
|---|---|
| Year | 2006 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre-2010) ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (0–3 semiquantitative scale; not referenced) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE? | Y |
| Were the CE results interpreted without knowledge of the results of US? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Gandjbakhch91 |
|---|---|
| Year | 2008 |
| Study design | Treatment decision |
| If diagnostic study, what level according to hierarchy?159 | NA |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | N |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | U (no details reported; not referenced) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Garrigues108 |
|---|---|
| Year | 2013 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-2 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | N |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52,281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | U |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Gartner109 |
|---|---|
| Year | 2013 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | Y |
| Was diagnosis of RA confirmed by an earlier version (pre-2010) ACR or EULAR classification criteria for RA? | NA |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52,281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE? | Y |
| Were the CE results interpreted without knowledge of the results of US? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Haavardsholm110 |
|---|---|
| Year | 2009 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (0–4 semiquantitative score; not referenced) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Haarvardsholm79 (ARCTIC) |
|---|---|
| Year | 2015 |
| Study design | Treatment (treat-to-target strategy RCT: DAS44 strategy vs. DAS44 + MSUS strategy) |
| If intervention study, what level according to hierarchy?159 | II |
| Were eligibility criteria clearly described? | P |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | Y |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | NA |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | P |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (Hammer et al.111) |
| Was the conduct of the CE clearly described? | P |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | U |
| Were uninterpretable test results reported? | U |
| Were withdrawals from the study explained? | N |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | U |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | U |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | U |
| First author (study name) | Hammer68,111 |
|---|---|
| Year | 2010, 2011 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Hayashi92 |
|---|---|
| Year | 2014 |
| Study design | Diagnostic study |
| If diagnostic study, what level according to hierarchy?159 | III-2 |
| Were selection criteria clearly described? | N |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre-2010) ACR or EULAR classification criteria for RA? | U |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | U |
| Were clinical and US joint assessments conducted independently? | U |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | P |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (semiquantitative scoring system; not referenced) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE? | U |
| Were the CE results interpreted without knowledge of the results of US? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Horikoshi112 |
|---|---|
| Year | 2010 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-2 |
| Were selection criteria clearly described? | P |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | N |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Ikeda113,146 |
|---|---|
| Year | 2013, 2012 |
| Study design | Prognostic, prospective cohort |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (Naredo et al.56,140) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | U |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Inanc93 |
|---|---|
| Year | 2014 |
| Study design | Prospective cohort study (prediction of response to bDMARDs by baseline US and clinical features) |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | P |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | U |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | U |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Iwamoto155 |
|---|---|
| Year | 2014 |
| Study design | Prospective cohort |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | Y |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | NA |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT140,285) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE? | Y |
| Were the CE results interpreted without knowledge of the results of US? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Kamishima114 |
|---|---|
| Year | 2011 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (Meenagh et al.277) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Kane102 |
|---|---|
| Year | 2003 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | N |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Kelly94 |
|---|---|
| Year | 2013 |
| Study design | Treatment decision |
| If prognostic study, what level according to hierarchy?159 | NA |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | U |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | N |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | U |
| Was the conduct of the CE clearly described? | N |
| Were uninterpretable test results reported? | U |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Luengroongroj95 |
|---|---|
| Year | 2015 |
| Study design | Treatment – cohort (treatment tapering) |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | N |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | U |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | U |
| Was the conduct of the US examination clearly described? | N |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | U |
| Was the conduct of the CE clearly described? | N |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | U |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | U |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Luukkainen115 |
|---|---|
| Year | 2003 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | P |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | U |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | P |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | N |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | NA |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | NA |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | NA |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | NA |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | NA |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | NA |
| First author (study name) | Luukkainen103 |
|---|---|
| Year | 2005 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | N |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Luukkainen104 |
|---|---|
| Year | 2007 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | N |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Mamoto96 |
|---|---|
| Year | 2013 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-2 |
| Were selection criteria clearly described? | N |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | U |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | P |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | N |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (0–3 semiquantitative scale; not referenced) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | U |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Mandl116,117 |
|---|---|
| Year | 2012, 2013 |
| Study design | Ancillary study to RCT |
| If diagnostic study, what level according to hierarchy?159 | III-1 (diagnostic), II (intervention study) |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | N (from a trial sample) |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52,281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Naredo55 |
|---|---|
| Year | 2007 |
| Study design | Prognostic, prospective cohort |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT54) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | U |
| First author (study name) | Naredo140 |
|---|---|
| Year | 2008 |
| Study design | Prognostic (prospective cohort) |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT53,281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | U |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Naredo118 |
|---|---|
| Year | 2013 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT140,281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Naredo156,157 |
|---|---|
| Year | 2015, 2014 |
| Study design | Prospective cohort study of treatment prediction, 12 month follow-up, bDMARD tapering at baseline |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Osipyants97,150 |
|---|---|
| Year | 2013 |
| Study design | Prognostic, prospective cohort |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | U |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | U |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | U |
| First author (study name) | Pereira119 |
|---|---|
| Year | 2015 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52,54,281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE? | Y |
| Were the CE results interpreted without knowledge of the results of US? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Ramirez García98 |
|---|---|
| Year | 2014 |
| Study design | Prognostic, prospective cohort study |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | U |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (0–3 semiquantitative scoring; not referenced) |
| Was the conduct of the CE clearly described? | P |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | P |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | U |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | U |
| First author (study name) | Reynolds,141 Rees151 |
|---|---|
| Year | 2009, 2007 |
| Study design | Prognostic (prospective cohort) |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (0–3 semiquantitative scoring scales286) |
| Was the conduct of the CE clearly described? | P |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | U |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | U |
| First author (study name) | Ribbens120 |
|---|---|
| Year | 2003 |
| Study design | Diagnostic and response to treatment (before-and-after study) |
| What level of study according to hierarchy?159 | III-1 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | N (0–3 semiquantitative scale58,283) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Riente121 |
|---|---|
| Year | 2010 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-1 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE? | Y |
| Were the CE results interpreted without knowledge of the results of US? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Riente122 |
|---|---|
| Year | 2011 |
| Study design | Diagnostic, blinded comparison among non-consecutive RA patients |
| If diagnostic study, what level according to hierarchy?159 | III-1 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE? | Y |
| Were the CE results interpreted without knowledge of the results of US? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Salaffi160 |
|---|---|
| Year | 2008 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-1 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE? | Y |
| Were the CE results interpreted without knowledge of the results of US? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Saleem124 |
|---|---|
| Year | 2011 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | Y |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | NA |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT57,75,281,282) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Saleem142 |
|---|---|
| Year | 2012 |
| Study design | Prognostic (prospective cohort) |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | U |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Scheel53 |
|---|---|
| Year | 2005 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | U |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Spiegel125 |
|---|---|
| Year | 1987 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-1 |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | N (1958 criteria – no mention of ACR or EULAR) |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (0–3 semiquantitative scale; not referenced) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Szkudlarek126 |
|---|---|
| Year | 2004 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-2 |
| Were selection criteria clearly described? | U |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | U |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Szkudlarek127 |
|---|---|
| Year | 2006 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-2 |
| Were selection criteria clearly described? | U |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | U |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Taniguchi128 |
|---|---|
| Year | 2014 |
| Study design | Diagnostic comparison, blinded, not consecutive patients |
| If diagnostic study, what level according to hierarchy?159 | III-1 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (0–3 semiquantitative scale52) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Vlad129 |
|---|---|
| Year | 2015 |
| Study design | Diagnostic (responsiveness) |
| If diagnostic study, what level according to hierarchy?159 | III-1 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT52) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Wakefield130 |
|---|---|
| Year | 2008 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Wakefield143 |
|---|---|
| Year | 2007 |
| Study design | Prognostic, prospective cohort |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | Y |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (0–3 semiquantitative scoring; not referenced) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | NA |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | Y |
| First author (study name) | Xiao131 |
|---|---|
| Year | 2014 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-2 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT59,281) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE? | U |
| Were the CE results interpreted without knowledge of the results of US? | U |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| First author (study name) | Yoshimi144,145 |
|---|---|
| Year | 2014, 2013 |
| Study design | Prognostic, prospective cohort |
| If prognostic study, what level according to hierarchy?159 | II |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | N |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | Y |
| Were clinical joint assessments conducted by a trained assessor? | Y |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | Y |
| Was the conduct of the US examination clearly described? | Y |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (0–3 semiquantitative scoring52,54) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | Y |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | Y |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
| Were the prognostic factors clearly defined (i.e. the variables being assessed for influence on prognosis or therapy response)? | Y |
| Was the outcome measure clearly defined (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the outcome measure assessment blinded (i.e. the clinical prognosis or therapy response measure)? | Y |
| Was the follow-up time sufficiently long (to detect important prognostic factors)? | Y |
| Loss to follow-up is not associated with key characteristics (prognostic factor and potential confounders) | Y |
| Important potential confounders are accounted for in the study design (e.g. matching for key variables, stratification or initial assembly of comparable groups) or in statistical analyses (i.e. appropriate adjustment) | P |
| First author (study name) | Zuffery99,132 |
|---|---|
| Year | 2014 |
| Study design | Diagnostic |
| If diagnostic study, what level according to hierarchy?159 | III-2 |
| Were selection criteria clearly described? | Y |
| Was diagnosis of RA confirmed by the 2010 ACR and EULAR classification criteria for RA? | U |
| Was diagnosis of RA confirmed by an earlier version (pre 2010) of the ACR or EULAR classification criteria for RA? | U |
| Were clinical joint assessments conducted by a trained assessor? | U |
| Were US joint assessments conducted by a trained assessor? | Y |
| Were clinical and US joint assessments conducted independently? | N |
| Did population recruitment consist of consecutive patients meeting the eligibility criteria? | U |
| Is the time period between US and CE short enough to be reasonably sure that the synovitis did not change between the two tests? | Y |
| Was the conduct of the US examination clearly described? | U |
| Were US results interpreted according to an established scoring system (e.g. OMERACT)? | Y (OMERACT64,85) |
| Was the conduct of the CE clearly described? | Y |
| Were the US results interpreted without knowledge of the results of the CE (i.e. blinded)? | N |
| Were the CE results interpreted without knowledge of the results of US (i.e. blinded)? | N |
| Were uninterpretable test results reported? | Y |
| Were withdrawals from the study explained? | Y |
Appendix 7 Detection of synovitis
This review concentrated on US as the intervention. There is no conclusive gold standard/reference standard for assessing synovitis. This makes interpretation of detection rates difficult. Other imaging techniques are available for detecting synovitis in RA patients. Imaging techniques used in RA include US, conventional radiography, MRI and CT scans. Other imaging techniques may be useful for detecting disease indications other than synovitis, such as bone oedema or erosion. 9,28 It is noted that MRI has a role in diagnosing RA and for detecting other indications such as bone erosion within RA.
Ultrasound is more likely to be practical in assessing synovitis than other imaging techniques. US is more comfortable for the patient than MRI, is less expensive and has an advantage over other imaging techniques, as it can be used immediately after CE to assess symptomatic areas. 30
As for any imaging technique, the value of detecting subclinical synovitis is determined by the influence of non-clinically detected synovitis on the course of the disease. This review investigated the association of US-detected synovitis with later outcomes, in prognostic studies (see Chapter 3). This can be considered as validating the test results. 287 As this review concentrated on US as the intervention, the decision was taken not to compare US-detected synovitis with synovitis detected by other imaging techniques.
In terms of synovitis, a review published in 201328 found that scintigraphy and PET detected similar rates of inflammation to CE and that US and MRI found higher rates of inflammation than CE but with similar rates to each other (compared with CE US detected 2.18-fold synovitis and MRI 2.20-fold synovitis). Studies included in the 2013 review overlapped with studies included in this review; however, two studies128,131 included in this review that were published since the 2013 review reported synovitis detection rates in both US and MRI. Taniguchi et al. 128 reported that synovitis was detected by PDUS in 31 out of 60 (51.7%) wrist joints and 23 out of 300 (7.7%) MCP joints; more joints were reported as positive for synovitis when considering maximum intensity projection MRI grades 1 and 2 [47/60 (78.3%) wrist joints and 84/300 (28.0%) MCP joints]. Xiao et al. 131 examined 180 joints with MRI and PDUS; the results were positive for synovitis in 86 joints assessed by MRI (47.7%) and 81 joints assessed by PDUS (45.0%). Imaging techniques other than US are not considered further here.
When diagnostic accuracy data were reported, these were usually presented as the diagnostic accuracy of CE, with US as the reference standard. Because of this, Table 139 shows the sensitivity and specificity of CE with reference US. When TPs, FPs, TNs and FNs were reported or calculable, these are presented in the data extraction tables in Appendix 5, as are diagnostic accuracy data for US with reference CE.
| Study | Populationa | CE assessment of joints | Diagnostic accuracy comparison (CE with reference US) | Sensitivity of CE (95% CI), % | Specificity of CE (95% CI), % |
|---|---|---|---|---|---|
| Balsa 2010105 |
97 patients 42 joints (PIP, MCP, wrist, elbow, glenohumeral, knee, ankle and midtarsal and MTP joints) (4074 joints in total) |
SDAI of > 5 | PDUS | 66 (52 to 77) | 55 (40 to 69) |
| SDAI of > 3.3 | PDUS | 57 (44 to 70) | 74 (59 to 85) | ||
| Filippucci 2006107 |
24 patients 48 wrists 192 examinations |
Swollen | PDUSb | 41 (NR) | 71 (NR) |
| Tender | PDUSb | 39 (NR) | 78 (NR) | ||
| Gartner 2013109,133 | 90 patients (60 in clinical remission, CDAI of ≤ 2.8), 1320 joints (MCP, PIP and wrist joints) | Swollen | PDUS grade 3c and GSUS grade 3c | 25 (NR) | 100 (NR) |
| Hayashi 201492 |
208 patients 416 wrist joints, 2080 MCP joints, 2080 interphalangeal/PIP joints |
Swollen and/or tender | GSUS score of ≥ 1c or PDUS score of ≥ 2c | Wrist 53 (NR), MCP 50 (NR), interphalangeal/PIP 51 (NR) | Wrist 89 (NR), MCP 95 (NR), interphalangeal/PIP 94 (NR) |
| Kane 2003102 |
22 patients 44 knees |
Effusion | GSUS effusiond | 59 (NR) | 65 (NR) |
| Luukkainen 2003115 |
30 patients 288 MTP joints |
Swollen | GSUSd | 40 (NR) | 77 (NR) |
|
30 patients 60 talocrural joints |
Swollen | GSUSd | 46 (NR) | 60 (NR) | |
| Luukkainen 2005103 |
50 patients 100 humeroradial joints |
Swollen | GSUSe | 41 (NR) | 92 (NR) |
|
50 patients 100 olecranon fossa joints |
Swollen | GSUSe | 21 (NR) | 99 (NR) | |
| Luukkainen 2007104 |
50 patients 100 glenohumeral joints |
Swollen | GSUSe | 37 (NR) | 82 (NR) |
| Mamoto 201396 |
124 patients 2728 joints (wrist, MCP and PIP joints bilaterally) |
Swollen (assessed by patient or physician) | GSUS of ≥ 2c | Physician 47 (NR), patient 34 (NR) | NR (NR) |
| GSUS of ≥ 2 and PDUS of ≥ 1c | Physician 52 (NR), patient 37 (NR) | ||||
| Naredo 2013118 |
67 patients in clinical remissionf 28 joints (of DAS28) |
DAS28 of > 2.6 | GSUSb | 42 (NR) | 100 (NR) |
| SDAI of > 3.3 | GSUSb | 71 (NR) | 80 (NR) | ||
| DAS28 of > 2.6 | PDUSb | 46 (NR) | 69 (NR) | ||
| SDAI of > 3.3 | PDUSb | 77 (NR) | 43 (NR) | ||
| Pereira 2015119 |
Painless group: 38 patients 304 MCP joints |
Swollen | GSUSb | 64 (NR) | 55 (NR) |
| PDUSb | 75 (NR) | 50 (NR) | |||
|
Painful group: 34 patients 272 MCP joints |
Swollen | GSUSb | 66 (NR) | 51 (NR) | |
| PDUSb | 79 (NR) | 47 (NR) | |||
| Ribbens 2003120 |
11 patients 20 wrist joints, 110 MCP joints, 103 PIP joints |
Swollen | PDUSb | Wrist 87 (NR), MCP 64 (NR), PIP 56 (NR) | Wrist 60 (NR), MCP 53 (NR), PIP 76 (NR) |
| Riente 2010121 |
100 patients 200 knee joints |
Swollen and/or painful | GSUS and PDUSc | 80 (NR) | 87 (NR) |
| Riente 2011122 |
100 patients 200 foot joints |
Swollen or painful | US effusion, GSUS and PDUSc | 79 (NR) | 54 (NR) |
| Salaffi 2008160 |
44 patients 440 PIP joints, 440 MCP joints, 440 MTP joints |
Swollen | GSUS | PIP 95 (NR), MCP 95 (NR), MTP 74 (NR) | PIP 80 (NR), MCP 66 (NR), MTP 69 (NR) |
| Saleem 2011124 |
128 patients in remission (i.e. a DAS28 of < 2.6) 640 MCP and wrist joints |
Swollen | PDUS > 0c | 13 (8 to 22) | 93 (90 to 95) |
| PDUS > 1c | 20 (1 to 70) | 92 (90 to 94) | |||
| Spiegel 1987125 |
6 patients 36 joints (shoulders, wrists and knees) |
Swollen (0 vs. 1–3 on a 0–3 scale) | GSUSb | 90 (NR) | 30 (NR) |
| Szkudlarek 2004126 |
40 patients 200 MTP joints |
Swollen or tender | GSUSg | 48 (NR) | 89 (NR) |
| Szkudlarek 2006127 |
40 patients 158 MCP and 140 PIP joints |
Swollen or tender | GSUSg | 53 (NR) | 94 (NR) |
| Taniguchi 2014128 |
30 patients (18 in remission; i.e. a DAS28 of < 2.3) 60 wrists and 300 MCP joints |
Swollen or tender | PDUS referenceb | Wrist 69 (NR), MCP 57 (NR) | Wrist 89 (NR), MCP 95 (NR) |
Twenty studies92,96,102–105,107,109,115,118–122,124–128,160 reported sensitivity data. Two of these studies105,118 used DAS as the clinical comparator, whereas the others used CE for synovitis.
There was seemingly a very wide range of sensitivity (13–95%) and specificity (30–100%) of CE with US as the reference standard across the 20 studies. However, for most studies, CE had a high specificity and low sensitivity when using US as the reference standard. This indicates some agreement between CE and US, with US detecting synovitis in some joints in which CE did not and only a few cases in which CE detected synovitis and US did not. This agrees with the higher detected rates of synovitis for US over CE reported in the majority of studies (Table 140).
| Author | Populationa | Joints | Type of US | Detection of synovitis on US | Type of CE | Detection of synovitis on CE |
|---|---|---|---|---|---|---|
| Balsa 2010105 | 97 patients | 4074 joints (PIP, MCP, wrist, elbow, bilateral glenohumeral, knee, ankle and midtarsal and MTP joints) | GSUS | 92/97 patients (95%) | SDAI of > 5 | 43/97 patients (44%) |
| PDUS | 41/97 patients (42%) | SDAI of > 3.3 | 54/97 patients (56%) | |||
| Beckers 2004106 | 21 patients | 356 joints (knee, wrist, MCP, PIP, ankle and MTP joints) | PDUSb | 199/356 joints (42%) | Swollen | 266/356 joints (75%) |
| Tender | 282/356 joints (79%) | |||||
| Filippucci 2006107 | 24 patients | 48 wrists (four time points × 48 wrists totalling 192 examinations) | PDUSb | 147/192 examinations (77%) | Swollen | 74/192 examinations (39%) |
| Tender | 68/192 examinations (36%) | |||||
| Garrigues 2013108 | 40 patients | 1600 joints (shoulder, elbow, wrist, MCP, PIP, tibiotalar and MTP joints) | GSUS of ≥ 1c | 477/1600 joints (30%) | Swollen | 200/1600 joints (12%) |
| PDUS of ≥ 1c | 279/1600 joints (17%) | Tender | 317/1600 joints (20%) | |||
| GSUS of or PDUS of ≥ 1c | 479/1600 joints (30%) | |||||
| Gartner 2013109,133 | 90 patients (60 in clinical remission, CDAI of ≤ 2.8) | 1980 finger and wrist joints (1320 remission, 660 active RA) | GSUS of 1–3c | 89/90 patients (99%) | CDAI of > 2.8 | 30/90 patients (33%) |
| GSUS of 2–3c | 77/90 patients (86%) | |||||
| PDUS of 1–3c | 80/90 patients (89%) | |||||
| PDUS of 2–3c | 37/90 patients (41%) | |||||
| 60 in clinical remission (CDAI of ≤ 2.8) | 1320 finger and wrist joints | GSUS of 1–3c | 887/1320 joints (67%) | Swollen | 15/1320 joints (1%) | |
| PDUS of 1–3c | 269/1320 joints (20%) | |||||
| 30 with active RA | 660 finger and wrist joints | GSUS of 1–3c | 436/660 joints (66%) | Swollen | 97/660 joints (15%) | |
| Hayashi 201492 | 208 patients | 416 wrist joints | GSUS 1–3c or PDUS of 2–3c | 197/416 joints (47%) | Swollen | 52/416 joints (13%) |
| Tender | 20/416 joints (5%) | |||||
| Swollen and tender | 58/416 joints (14%) | |||||
| 2080 MCP joints | GSUS of 1–3c or PDUS of 2–3c | 331/2080 joints (16%) | Swollen | 106/2080 joints (5%) | ||
| Tender | 65/2080 joints (3%) | |||||
| Swollen and tender | 74/2080 joints (4%) | |||||
| 2080 interphalangeal/PIP joints | GSUS 1–3c or PDUS 2–3c | 107/2080 joints (5%) | Swollen | 43/2080 joints (2%) | ||
| Tender | 93/2080 joints (4%) | |||||
| Swollen and tender | 46/2080 joints (2%) | |||||
| Horikoshi 2010112 | 6 patients | 156 joints [interphalangeal, radiocarpal, intercarpal, radioulnar, PIP (2–5) and MCP joints] | GSUSb | 74/156 (47%) | Swollen | 7/132 joints (5%) |
| PDUSb | 10/156 (6%) | |||||
| Kane 2003102 | 22 patients | 44 knees | GSUS effusion (effusion vs. no effusion)d | 27/44 (61%) | CE for swelling (fluctuant fluid observed) | 22/44 joints (50%) |
| Luukkainen 2003115 | 30 patients | 288 MTP joints | GSUSd | 73/288 joints (25%) | Swollen | 79/288 joints (27%) |
| 30 patients | 60 talocrural joints | GSUSd | 13/60 joints (22%) | Swollen | 25/60 joints (42%) | |
| Luukkainen 2005103 | 50 patients | 100 humeroradial joints | GSUSe | 29/100 joints (29%) | Swollen | 18/100 joints (18%) |
| 50 patients | 100 olecranon fossa joints | GSUSe | 29/100 joints (29%) | Swollen | 7/100 joints (7%) | |
| Luukkainen 2007104 | 50 patients | 100 glenohumeral joints | GSUSe | 27/100 joints (27%) | Swollen | 23/100 joints (23%) |
| Naredo 2013118 | 67 patients clinical remission | 28 joints per patient (1876 in total) | GSUSb | 62/67 patients (93%) | DAS28 of > 2.6 | 41/67 patients (61%) |
| PDUSb | 35/67 patients (52%) | SDAI of > 3.3 | 22/67 patients (33%) | |||
| Pereira 2015119 | Painless group: 38 patients | 304 MCP joints | GSUSb | 193/304 joints (63%) | Swollen | 174/304 (57%) |
| PDUSb | 88/304 joints (29%) | |||||
| Painful group: 34 patients | 272 MCP joints | GSUSb | 165/272 joints (61%) | Swollen | 161/272 (59%) | |
| PDUSb | 67/272 joints (25%) | |||||
| Ribbens 2003120 | 11 patients | 20 wrist joints | PDUSb | 15/20 joints (75%) | Swollen | 15/20 joints |
| 110 MCP joints | PDUSb | 74/110 joints (67%) | Swollen | 64/110 joints | ||
| 103 PIP joints | PDUSb | 45/103 joints (44%) | Swollen | 39/103 joints | ||
| Riente 2010121 | 100 patients | 200 knees | GSUS | 140/200 joints (70%) | Swollen and/or tender | 116/200 joints |
| PDUSc | 115/140 joints (82%)f | |||||
| Riente 2011122 | 100 patients | 200 foot joints | GSUS and PDUSc | 135/200 joints (68%) | Swollen or painful | 137/200 joints |
| Salaffi 2006123 | 44 patients | 440 PIP joints | GSUSb | 104/440 joints (23.6%) | Swollen | 165/440 joints (37.5%) |
| 440 MCP joints | GSUSb | 152/440 joints (34.5%) | Swollen | 241/440 joints (54.8%) | ||
| 440 MTP joints | GSUSb | 120/440 joints (27.3%) | Swollen | 189/440 joints (43%) | ||
| Saleem 2011124 | 128 patients in remission (i.e. a DAS28 of < 2.6) | 640 MCP and wrist joints | GSUS = 0 and PDUS = 0c | 22/128 patients (17%) | Swollen | 40/128 patients (31%) |
| GSUS > 1 and PDUS > 1c | 72/128 patients (56%) | Tender | 23/128 patients (18%) | |||
| PDUS = 0c | 63/128 patients (49%) | CRP (≥ 5 mg/dl) | 46/128 patients (36%) | |||
| PDUS > 1c | 101/128 patients (79%) | |||||
| Scheel 200553 | 46 patients | 184 MCP and 184 PIP joints | GSUS 1–3c | 86% across MCP and PIP | Swollen |
138/368 (37.5%) MCP and PIP joints 73/184 MCP joints (39.7%) 65/184 PIP joints (35.3%) |
| Tender |
137/368 (37.2%) MCP and PIP joints 64/184 MCP joints (34.8%) 73/184 PIP joints (39.7%) |
|||||
| Spiegel 1987125 | 6 patients | 36 joints (shoulders, wrists and knees) | GSUSb | 71/101 (70%) | Swollen | 85/101 (84%) |
| Szkudlarek 2004126 | 40 patients | 200 MTP joints | GSUSg | 129/200 joints (65%) | Swollen or tender | 70/200 joints (35%) |
| Szkudlarek 2006127 | 40 patients | 158 MCP and 140 PIP joints | GSUSg | 194/480 joints (40%) | Swollen or tender | 121/480 joints (25%) |
| Taniguchi 2014128 | 30 patients (18 in remission; i.e. a DAS28 of < 2.3) | 60 wrists | PDUSb | 31/60 joints (52%) | Swollen or tender | 25/60 joints (42%) |
| 300 MCP joints | PDUSb | 23/300 joints (8%) | Swollen or tender | 26/300 joints (9%) | ||
| Wakefield 2008130 | 22 patients | 22 TTJ | GSUSd | 10/22 (45%) | Swollen | 17/22 (77%) |
| 22 STJ | Medial aspect 2/22 (9%), lateral aspect 14/22 (64%) | 17/22 (77%) | ||||
| 22 TNJ | 12/22 (55%) | 17/22 (77%) | ||||
| 22 CCJ | 11/22 (50%) | 12/22 (55%) | ||||
| Xiao 2014131 | 46 patients | 368 bilateral MCP joints | PDUSb | 147/368 joints (40%) | Swollen and/or tender | 180/368 joints (49%) |
| 368 bilateral PIP joints | PDUSb | 173/368 joints (47%) | Swollen and/or tender | 154/368 joints (42%) | ||
| 92 wrist joints | PDUSb | 61/92 joints (66%) | Swollen and/or tender | 56/92 joints (61%) |
Sensitivity of CE of swollen and/or tender joints with a reference standard of GSUS or PDUS in 14 studies92,96,102–104,107,109,115,119,120,124,126–128,133 ranged from 13% to 69% (MCP and PIP joints for Ribbens et al. 120). Most of these studies assessed synovitis in hand and wrist joints, but they also included ankle, elbow and shoulder joints (see Table 139). Sensitivity would range from 37% to 69% if three studies with sensitivities at the lower end of the range84,103,124 were excluded. Studies with sensitivities at the lower end of the range were those in which all patients were in remission (25%;84 13% or 20% depending on PDUS definition of synovitis124) and those investigating elbow joints (21% or 41% depending on joint studied103) or shoulder joints (37%104) in which US synovitis was scored as a binary variable rather than semiquantitatively (see Table 3 for scoring systems).
Higher sensitivities (75–95%) were found in six studies119–122,125,160 (wrist joints for Ribbens et al. 120). Of these, one study, with a sensitivity of 90%, included only six patients and differed from other studies as CE was graded from 0 to 3125 and one study was primarily investigating the difference between painful and painless joints. 119 In one study, CE had higher sensitivity for wrists (n = 20) than for other joints 120 and, in another study, CE had lower sensitivity for MTP joints than for other joints. 160 One study investigated foot joints (sensitivity 79%122) and one study investigated knee joints (sensitivity 80%21). Detection rates for synovitis were higher for CE than for US (see Table 140) in one study of foot joints. 130 This suggests that US is less useful for detecting synovitis in foot joints (not including MTP) than in other joints. However, this was not a within-study comparison and so this cannot be concluded with certainty.
Four of these studies119,120,122,125 [Ribbens (MCP joints),120] also reported lower specificities (30–55%) than the other studies (see Table 139), whereas two121,160 did not (specificities of 66–87%).
The specificity of CE of swollen/tender joints, with US as a reference standard, ranged from 60% to 100% in 14 of the studies, in which joints included were mostly wrists and hand joints but also ankle, knee, elbow and shoulder joints92,102–104,107,109,115,120,121,124,126–128,133,160 (wrist and PIP joints for Ribbens et al. 120).
Study quality hierarchy did not explain the differences between the studies. Of four studies in which CE had both higher sensitivity and lower specificity than in other trials, only one119 was of level II hierarchy (blinded comparison among consecutive patients). The other three studies120,122,125 did not include (or it was unclear if they included) consecutive patients, but were blinded comparisons. However, other studies lower on the hierarchy92,96,126–128 had results consistent with level II studies. 102–104,107,109,115,118,124,133
For studies in which US synovitis was scored on a scale from 0 to 1,103,104,115 sensitivities and specificities of CE were within the range of those reported by studies using a 0–3 or 0–4 scoring system (see Table 139). This was also the case for the study using a US score of ≥ 2 as the reference standard96 (see Table 139).
Studies using DAS as the clinical comparator assessed sensitivity and specificity for diagnosis by patient, rather than for each joint (see Tables 5 and 6). Like CE for swollen/tender joints, DAS28 had low sensitivity and high specificity when US was the reference standard. 118 SDAI also had high specificity when US was the reference standard; however, SDAI had fairly high sensitivity. 105,118
When studies reported data separately by joint, sensitivities and specificities for CE were similar for wrist, MCP and PIP joints in the study by Hayashi et al. 92 and for MTP and talocrural joints in the study by Luukkainen and Saltyshev115 Mamoto et al. 96 reported that physicians and patients assessed swelling less effectively in MCP than in PIP or wrist joints. CE of olecranon fossa joints had lower sensitivity than CE of humeroradial joints103 and CE of MTP joints had lower sensitivity than that of PIP and MCP joints,160 but with a similar specificity. In the study by Ribbens et al. ,120 CE had higher sensitivity for wrists (n = 20) than MCP (n = 110) or PIP (n = 103) joints and lower specificity for MCP joints than wrists and PIP joints.
Table 140 shows the types of US and CEs and detection rates in 25 studies. 53,92,102–109,112,115,118–128,130,131 Three studies105,109,118 (see Table 6) investigated DAS. GSUS detected synovitis in more patients than had active disease indicated by SDAI,105,118 CDAI109 or DAS28118 and this was also the case for PDUS, with the exception of PDUS in one study not detecting as many patients as met a SDAI of > 3.3. 105
Twenty-three studies53,92,102–104,106–109,112,119–128,130,131 assessed swollen and/or tender joints by CE (see Table 6). Nearly all of these studies53,92,102–104,107–109,112,115,119–122,124,126–128,131 reported a higher rate of detection of synovitis by US than the rate of detection of swelling or tenderness by CE (see Table 140). There were mixed results in two studies,108,119 with higher detection rates with GSUS than CE, but lower detection rates with PDUS than CE; in addition, one study131 found a lower detection rate for PDUS than CE for MCP joints but a higher detection rate for PDUS than CE for PIP and wrist joints.
Five studies found lower rates of detection by US than by CE, of which two studies investigated ankle and MTP joints,106,115 one looked at foot joints,130 one included MTP but also PIP and MCP joints123 and one was a study in six patients of GSUS of shoulders, wrists and knees. 125 Higher rates of detection by CE than US were suggested to be the result of inflammation from other pathology, such as osteophytes or oedema,123,130 or external factors such as obesity. 130
Table 141 shows responsiveness to change of US and CE reported as the SRM. When interpreting their data, Ikeda et al. 113 cited the study by Husted et al. ,80 in which a SRM of < 0.2 is considered nil (meaning between –0.2 and + 0.2) and a SRM of > 0.6 is considered relevant, whereas Haavardsholm and Ostergaard110 cited thresholds introduced by Cohen288 for effect sizes: < 0.2, trivial; > 0.2 to ≤ 0.5, small; > 0.5 to ≤ 0.8, moderate; > 0.8, large. Using broad definitions (small/moderate/large), most studies reporting the SRM found similar responsiveness for US and CE. GSUS had similar responsiveness to DAS28,110,111,113,116,132 SDAI,111,116,117 CDAI,111,113 SJC,111 TJC111 and CRP. 129 PDUS had similar responsiveness to DAS28,111,113,116,117,132 CDAI,111,113 SDAI,111,116117 SJC111 and CRP. 129 Looking at the types of US within studies, GSUS had similar responsiveness to PDUS. 111,113,114,129,132
| Author | Populationa | Follow-up | US SRM (95% CI) (p = value) | CE SRM (95% CI) |
|---|---|---|---|---|
| Haavardsholm 2009110 |
36 patients starting TNFis Wrists |
12 months | GSUS –0.37 (–0.89 to 0.16) (NR) | DAS28 –0.36 (–1.26 to 0.35); SDAI –0.59 (–1.31 to –0.02); CDAI –0.55 (–1.17 to –0.04) |
| Hammer 2010111 |
20 patients starting biologic therapy (ADA) MCP, PIP, elbow, shoulder, hip, knee, ankle and feet joints |
12 months | GSUS (78 joints) –1.27 (NR); PDUS (78 joints) –0.89 (NR) | DAS28 –1.32 (NR); CDAI –1.25 (NR); SDAI –1.20 (NR); assessor’s global –0.78 (NR); swollen joints (of 40) –1.29 (NR); tender joints (of 40) –1.07 (NR); ESR –0.13 (NR); CRP –0.12 (NR) |
| Ikeda 2013113 |
66 patients who were taking MTX (n = 22), TNFi (n = 27) and TCZ (n = 17) 28 joints of DAS28 |
12 weeks | Total GSUS score –1.17 (NR) (MTX –1.19, TNFi –1.48, TCZ –1.05) (NR); total PDUS score –1.37 (NR) (MTX –1.48, TNFi –1.53, TCZ –1.40) (NR) | DAS28-CRP –1.37 (NR) (MTX –1.36, TNFi –1.52, TCZ –1.49) (NR); CDAI –1.20 (NR) (MTX –1.43, TNFi –1.24, TCZ –1.18) (NR) |
| Kamishima 2011114 |
29 patients, starting TCZ MCP joints |
5 months |
PDUS (score 0–3) Sum of PDUS grades at 10 MCP joints –0.2595 (NR); PDUS joint index for vascular flow at 10 MCP joints –0.3063 (NR) |
CRP –0.6024 (NR); ESR –1.100 (NR); TJC –0.9288 (NR); SJC –0.6506 (NR); DAS28-ESR –1.9692 (NR) |
| Mandl 2013116,117 |
62 patients (n = 32 randomised to ETN + MTX; n = 30 randomised to cDMARDs) 28 joints of DAS28 |
12 weeks | GSUS and PDUS combined measure based on a DAS28 of 0.79 (0.70 to 0.88); GSUS and PDUS combined measure based on SDAI 0.9 (0.52 to 1.17) | Clinical only DAS28 0.87 (NR); clinical only SDAI 1.11 (NR) |
| Vlad 2015129 |
55 patients starting bDMARDs Wrist, MCP and PIP joints |
6 months | Global GSUS synovitis score (score 0–3, global score based on dorsal and volar scores) –1.80 (NR); global PDUS synovitis score –1.30 (NR) | CRP –0.90 (NR) |
| Zufferey 2014132 |
183 patients (most already on bDMARDs) 22 joints: knees, elbows, wrists and fingers |
11.7 months (mean) | GSUS –0.31 (–0.45 to –0.16); log(PDUS + 1) score –0.23 (–0.39 to –0.08) | DAS-CRP –0.50 (–0.65 to –0.35) |
One study found that US had higher responsiveness than ESR or CRP. 111 ESR and CRP in this study were the only measures in any study with a SRM of < 0.2 (i.e. counted as a nil effect). Two studies found US to be less responsive than the clinical comparator, with US having lower responsiveness than SDAI or CDAI110 or than CRP, ESR, TJC, SJC or DAS28-ESR. 114
Studies suggested that US detects more synovitis than CE alone. Thirty-three studies provided diagnostic data. 53,92,96,102,104,105,107–116,118–122,124–132,160 The majority of these studies reported that US detected more synovitis than CE alone. US could also distinguish synovitis from other pathologies. Most of these studies assessed synovitis in hand and wrist joints, reporting that US detected more synovitis in these joints than CE, and this was also the case for elbow and shoulder joints. Foot and ankle joints were less likely to show an advantage of US over CE. The detection of subclinical synovitis would be useful only if clinically relevant and prognostic studies suggested that US-detected synovitis was associated with radiographic progression.
Appendix 8 Characteristics of included studies
| Author (study) | Year; abstract or full paper | Data provided to review | Sample size (RA patients recruited) |
|---|---|---|---|
| Haarvardsholm (ARCTIC) | 2015; abstract79 | Treatment | 238 |
| Luengroongroj | 2015; abstract95 | Treatment | 32 |
| Naredo |
2015; full paper157 2014; abstract156 |
Treatment | 77 |
| Bhamra | 2014; abstract89 | Treatment | 17 |
| Ceponis | 2014; full paper152 | Treatment | 51 |
| Dale (TaSER) |
2014; full paper (one study arm)153 2013; abstract (both study arms)154 |
Treatment | 110 |
| Inanc |
2014; abstract93 2014; abstract93 |
Treatment | 43 |
| Iwamoto | 2014; full paper155 | Treatment | 42 |
| Kelly | 2013; abstract94 | Treatment | 109 |
| Ciurtin |
2013; abstract90 2012; abstract158 |
Treatment | 39 |
| Ellegaard | 2011; full paper101 | Treatment | 109 |
| Gandjbakhch | 2008; abstract91 | Treatment | 52 |
| Dougados/Cheung |
2014; abstract148 2013; full paper139 2013; abstract149 |
Prognostic and treatment | 77 |
| Cavet/Taylor |
2009; abstract138 2004; full paper100 |
Prognostic and treatment | 24 |
| Ramirez García | 2014; abstract98 | Prognostic | 28 |
| Yoshimi |
2014; full paper145 2013; full paper144 |
Prognostic | 31 |
| Backhaus | 2013; full paper69 | Prognostic | 432 |
| Osipyants |
2013; abstract97 2013; abstract150 |
Prognostic | 36 |
| Bugatti/Scirè |
2012; full paper147 2009; full paper137 |
Prognostic | 161 |
| Saleem | 2012; full paper142 | Prognostic | 93 |
| Boyesen | 2011; full paper134 | Prognostic | 84 |
| Reynolds |
2009; full paper141 2007; full paper151 |
Prognostic | 40 |
| Brown/Ikeda |
2008; full paper135 2007; abstract136 |
Prognostic | 107 |
| Naredo | 2008; full paper140 | Prognostic | 367 |
| Wakefield | 2008; full paper130 | Diagnostic | 22 |
| Naredo | 2007; full paper55 | Prognostic | 42 |
| Wakefield | 2007; full paper143 | Prognostic | 10 |
| Ikeda |
2012; abstract146 2013; full paper113 |
Diagnostic and prognostic | 57 |
| Pereira | 2015; full paper119 | Diagnostic | 72 |
| Vlad | 2015; full paper129 | Diagnostic | 55 |
| Hayashi | 2014; abstract92 | Diagnostic | 208 |
| Taniguchi | 2014; full paper128 | Diagnostic | 30 |
| Xiao | 2014; full paper131 | Diagnostic | 46 |
| Zufferey |
2014; full paper132 2014; full paper99 |
Diagnostic | 108 |
| Garrigues | 2013; full paper108 | Diagnostic | 40 |
| Gartner |
2013; full paper109 2012; abstract133 |
Diagnostic | 90 |
| Mamoto | 2013; abstract96 | Diagnostic | 124 |
| Mandl |
2013; full paper116 2012; full paper117 |
Diagnostic | 62 |
| Naredo | 2013; full paper118 | Diagnostic | 67 |
| Hammer |
2011; full paper68 2010; full paper111 |
Diagnostic | 20 |
| Kamishima | 2011; full paper114 | Diagnostic | 29 |
| Riente | 2011; full paper122 | Diagnostic | 100 |
| Saleem | 2011; full paper124 | Diagnostic | 128 |
| Balsa | 2010; full paper105 | Diagnostic | 97 |
| Horikoshi | 2010; full paper112 | Diagnostic | 6 |
| Riente | 2010; full paper121 | Diagnostic | 100 |
| Haavardsholm | 2009; full paper110 | Diagnostic | 36 |
| Luukkainen | 2007; full paper104 | Diagnostic | 50 |
| Filippucci | 2006; full paper107 | Diagnostic | 24 |
| Salaffi | 2006; full paper123 | Diagnostic | 44 |
| Szkudlarek | 2006; full paper127 | Diagnostic | 40 |
| Luukkainen | 2005; full paper103 | Diagnostic | 50 |
| Scheel | 2005; full paper53 | Diagnostic | 46 |
| Beckers | 2004; full paper106 | Diagnostic | 21 |
| Szkudlarek | 2004; full paper126 | Diagnostic | 40 |
| Kane | 2003; full paper102 | Diagnostic | 22 |
| Luukkainen | 2003; full paper115 | Diagnostic | 30 |
| Ribbens | 2003; full paper120 | Diagnostic | 11 |
| Spiegel | 1987; full paper125 | Diagnostic | 6 |
Glossary
- Grey-scale, or B-mode, ultrasound
- Mode of ultrasound that produces a black and white image of a tissue. It demonstrates qualitative differences between tissues and is primarily used to identify synovial hypertrophy and fluid within joints.
- Power Doppler ultrasound
- Mode of ultrasound that detects blood flow. Flow is designated by a colour (often orange or red), which is overlaid onto the corresponding grey-scale image below. There are different types of Doppler but power Doppler is thought to be most sensitive to the relatively low blood flow seen in joints.
- Sensitivity
- Proportion of true-positive results, a measure of the accuracy of a diagnostic test.
- Spearman’s rho (ρ)
- Spearman’s rank correlation coefficient, a measure of the association between two variables.
- Specificity
- Proportion of true-negative results, a measure of the accuracy of a diagnostic test.
- Standardised response mean
- The ratio of the mean changes to the standard deviation of the changes, a measure of responsiveness to change.
- Synovitis
- Swelling of the synovial lining of the joints.
List of abbreviations
- ABT
- abatacept
- ACR
- American College of Rheumatology
- ADA
- adalimumab
- ARCTIC
- Aiming for Remission in Rheumatoid Arthritis
- bDMARD
- biological disease-modifying anti-rheumatic drug
- BSR
- British Society for Rheumatology
- CDAI
- Clinical Disease Activity Index
- cDMARD
- conventional disease-modifying anti-rheumatic drug
- CDSR
- Cochrane Database of Systematic Reviews
- CE
- clinical examination
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CRP
- C-reactive protein
- CT
- computerised axial tomography
- CTZ
- certolizumab pegol
- CXCL13
- chemokine (C-X-C motif) ligand 13
- DARE
- Database of Abstracts of Reviews of Effects
- DAS
- Disease Activity Score
- DAS28
- Disease Activity Score 28 joints
- DAS28-CRP
- Disease Activity Score 28 joints using C-reactive protein
- DAS28-ESR
- Disease Activity Score 28 joints using erythrocyte sedimentation rate
- DAS44
- Disease Activity Score 44 joints
- DMARD
- disease-modifying anti-rheumatic drug
- ESR
- erythrocyte sedimentation rate
- ETN
- etanercept
- EULAR
- European League Against Rheumatism
- FN
- false negative
- FP
- false positive
- GATE
- Generic Appraisal Tool for Epidemiology
- GOL
- golimumab
- GSUS
- grey-scale ultrasound
- HAQ
- Health Assessment Questionnaire
- HAQ-DI
- Health Assessment Questionnaire Disability Index
- HCQ
- hydroxychloroquine
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IFX
- infliximab
- MCP
- metacarpophalangeal
- MRI
- magnetic resonance imaging
- MTP
- metatarsophalangeal
- mTSS
- modified total Sharp score
- MTX
- methotrexate
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- NRAS
- National Rheumatoid Arthritis Society
- NSAID
- non-steroidal anti-inflammatory drug
- OMERACT
- Outcome Measures in Rheumatoid Arthritis Clinical Trials
- OR
- odds ratio
- PDUS
- power Doppler ultrasound
- PET
- positron emission tomography
- PIP
- proximal interphalangeal
- PPV
- positive predictive value
- QALY
- quality-adjusted life-year
- QUADAS
- Quality Assessment of Diagnostic Accuracy Studies
- QUIPS
- Quality in Prognosis Studies
- RA
- rheumatoid arthritis
- RAMRIS
- rheumatoid arthritis magnetic resonance imaging scoring system
- RAQoL
- Rheumatoid Arthritis Quality of Life
- RCT
- randomised controlled trial
- RTX
- rituximab
- ScHARR
- School of Health and Related Research
- SDAI
- Simplified Disease Activity Index
- SJC
- swollen joint count
- SRM
- standardised response mean
- SSZ
- sulfasalazine
- TA
- technology appraisal
- TaSER
- Targeting Synovitis in Early Rheumatoid Arthritis
- TCZ
- tocilizumab
- TJC
- tender joint count
- TN
- true negative
- TNFi
- tumour necrosis factor inhibitor
- TP
- true positive
- TSS
- total Sharp score
- TTT
- treat to target
- TURA
- Targeted Ultrasound in Rheumatoid Arthritis
- US
- ultrasound
- US7
- ultrasound 7 score
- USDCF
- Ultrasound Doppler colour fraction
- VAS
- visual analogue scale
- vdHSS
- van der Heijde-modified total Sharp score
