Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/165/01. The contractual start date was in June 2013. The draft report began editorial review in April 2017 and was accepted for publication in August 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Leone Ridsdale secured funding from the Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. Laura H Goldstein reports that her independent research also receives support from the National Institute for Health Research (NIHR) Maudsley Biomedical Research Unit at the South London and Maudsley NHS Foundation Trust and King’s College London. She receives royalties from Goldstein LH and McNeil JE (editors) Clinical Neuropsychology. A Practical Guide to Assessment and Management for Clinicians. 2nd edn. Chichester: Wiley-Blackwell, 2013; and from Cull C and Goldstein LH (editors) The Clinical Psychologist’s Handbook of Epilepsy: Assessment and Management. Abingdon-on-Thames: Routledge; 1997. Sabine Landau reports grants from NIHR Maudsley Biomedical Research Unit at the South London and Maudsley NHS Foundation Trust and King’s College London during the conduct of the study and received a grant from NIHR Health Technology Assessment. Stephanie JC Taylor is on the Health Technology Assessment Clinical Trials Board and reports grants from the NIHR Collaboration for Leadership in Applied Health Research and Care North Thames at Barts Health NHS Trust. The authors received a contribution from Sanofi UK to enable printing of the patient workbooks.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Ridsdale et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The original funding application referred to the study intervention as SMILE. Over time, we have found that SMILE is a common acronym for other interventions (including other interventions for epilepsy). For this reason, we used SMILE (UK) in our publications. To remain consistent, we will use SMILE (UK) in our report.
Background
In 2010, the National Institute for Health Research (NIHR) issued a call for a randomised controlled trial (RCT) on a self-management education programme for people with epilepsy (PWE) (see Appendix 1). The call specified that the trial was to include people with poorly controlled epilepsy who were aged ≥ 16 years. It was specified that effectiveness should be evaluated based on quality of life (QoL) measures and that participants should be followed up 1 year post intervention. Two existing studies were cited, one based in Germany1 and one in North America. 2 Both featured 2-day self-management education programmes. The investigators of this study responded to the NIHR call by offering to adapt the intervention developed in German-speaking countries for the UK. Funding was agreed for the current RCT.
The trial was named Self-Management education for adults with poorly controlled epILEpsy [SMILE (UK)]. 3 This study was a pragmatic, parallel design, multicentre RCT in the UK. 4 The broad objective of the research was to evaluate the clinical effectiveness and cost-effectiveness of a group self-management programme for people aged ≥ 16 years who were experiencing ‘poorly controlled’ epilepsy. This was defined as epilepsy resulting in two or more seizures per year.
Epilepsy in the UK: causes, challenges and consequences
Epilepsy is a common neurological condition defined as the occurrence of two or more seizures more than a day apart. 5 Prevalence rates range from 4.2 to 9 per 1000 people6 and currently up to 1% of the UK population live with the condition. 7
The aetiology of epilepsy is variable. It can arise as a result of cerebrovascular disease (i.e. stroke), head trauma, congenital abnormalities and neurodegenerative disease (i.e. Alzheimer’s disease) or it can be idiopathic. 8 Mortality risk varies for PWE and is higher than in the general population. 9
The NHS recommends that epilepsy care should consist of a combination of regular general practitioner (GP) consultations, adherent medication use, patients’ knowledge of seizure triggers and adequate self-management. 10 Pharmacological intervention is currently the mainstay of treatment. Most PWE are able to eliminate seizure occurrence through use of antiepileptic drugs (AEDs). However, up to 40% are considered to have drug-resistant epilepsy. 11 The present research focuses on patients who experience epilepsy with two or more seizures per year, despite medical intervention, which, following the wording of NIHR guidelines, is defined as ‘poorly controlled epilepsy’.
Epilepsy is costly to UK health-care services. Recurrent seizures are a significant cost for the NHS,4 particularly when services are used on an emergency basis. 12 From 2014 to 2015, almost 130,000 people were admitted to UK hospitals for epilepsy and around 83,000 used emergency services. 13 Indirect costs of epilepsy are also likely to be high as a result of missed days of employment14 and early retirement because of illness. 15 Given the direct and indirect financial consequences of epilepsy (i.e. emergency service use, time off work), better ambulatory care management strategies offer a potential for secondary prevention and could be cost-effective for health-care service providers. 16
Epilepsy diagnosis is associated with significant psychological and social costs. Being labelled negatively as ‘epileptic’ can be accompanied by discrimination. 17,18 This further undermines well-being and QoL. 19,20 Educators and health-care service providers are mindful of the complex psychosocial nature of epilepsy,21 particularly as poor QoL and psychosocial well-being has a bidirectional relationship with seizure frequency and severity. 22 Structured education programmes that target psychosocial and biological aspects of seizure management may result in better outcomes for people with poorly controlled epilepsy and could improve QoL.
Improving quality of life of people with epilepsy
In the planning stages of an intervention designed to improve the lives of PWE, and using QoL as a primary outcome measure, the factors that could affect QoL were considered. The factors that were identified will be presented in the following sections.
Seizures
Seizure frequency and QoL are linked. PWE are likely to have similar QoL to the general population when seizures are controlled. 23 QoL is inversely associated with the amount of time since last seizure (i.e. ‘seizure recency’). 24 Given this association, even a minor improvement in seizure frequency may have a beneficial effect on health-related quality of life (HRQoL)25 and vice versa.
Seizures are associated with physical consequences that can negatively affect QoL. For instance, sustaining a serious injury during a seizure can be associated with long-lasting repercussions. Thus, minimising seizure frequency is also likely to reduce the ongoing consequences of seizure-related injury.
Antiepileptic drugs
The primary means of treating epilepsy is with AEDs; however, patients may also experience debilitating side effects from their medication. Adverse effects of medication significantly reduce QoL. 26 Apart from seizure frequency, adverse side effects from AEDs may be the biggest predictor of poor QoL in PWE. 24
Failure to comply with treatment regimens (i.e. ‘non-adherence’) also has serious implications for PWE. Non-adherence may result in increased seizure frequency, decreased QoL and missed employment. 27 Results of one AED adherence study showed that depression is associated with medication non-adherence. 28 This suggests a complex association between medication use, QoL and psychosocial well-being. These factors could be targeted by self-management intervention.
Psychological comorbidity
Psychosocial consequences may be as important as the physical consequences of epilepsy. Although treatment success is often reliant on biomedical factors, there are also important psychosocial issues to consider when managing the condition. 21 There is an elevated risk of poor mental health following epilepsy diagnosis. 29 PWE are more likely to experience higher rates of depression,30 anxiety31 and suicidal ideation. 32 Depressive symptoms can diminish QoL33,34 and influence seizure frequency. 35,36 Moreover, some forms of psychological conditions frequently co-occur,36 such as anxiety and depression, having a further impact on seizure frequency. Given the complex relationship between seizure frequency and psychological well-being, interventions that address both treatment targets seem worthwhile.
Stigma and resilience
Stigma is a major factor that influences QoL in PWE. 37,38 Although public perceptions about epilepsy have changed,38 negative beliefs and lack of knowledge continue to perpetuate stigma and discrimination. 39 In a survey of attitudes of the UK general public, participants felt epilepsy was embarrassing, frightening and meant being unable to drive or participate in employment. 40 Participants attributed seizures to stress, intoxication and mental health problems. These findings illustrate some of the prevalent, general attitudes and misconceptions about epilepsy. Despite epilepsy being a neurological condition, psychological and social factors are often attributed to its development,41 which may exacerbate feelings of ‘differentness’ and isolation in PWE.
Scambler and Hopkins42 divided stigma into felt versus enacted. Enacted stigma is discrimination against PWE based on a belief that the attributes of PWE are undesirable or unacceptable. Low employment rates among PWE may partly be a manifestation of enacted stigma. 38 Felt stigma is linked with the fear and shame tied to being ‘epileptic’. 42 Felt stigma is associated with low self-esteem, anxiety and depression. 38 Dilorio et al. 43 suggest that people with frequent seizures may internalise felt stigma and have unhelpful perceptions about their treatment. On the other hand, resilience can help to combat the diminished QoL associated with epilepsy diagnosis. 24 Those involved with epilepsy care can help PWE to combat stigma by giving them information and support strategies for overcoming stigma and discrimination. 38
Mastery and control
The term mastery is used to describe a state of confidence in which a person feels able to independently overcome the challenges with which they are faced. 44 Mastery is used in combination with self-efficacy to reduce stress and develop positive new behaviours. 45 In the literature, the term ‘self-mastery’ has been used interchangeably with self-efficacy. Evidence suggests when a person feels more confident in their ability to cope and manage their illness effectively, they are more likely to put self-management behaviours into practice. 46 Therefore, a sense of mastery and control is important for PWE as they have the potential to have an impact on seizure control and QoL. 47
Age and socioeconomic factors
The effect of epilepsy-related challenges on QoL can be related to the person’s life stage. 48 In a focus group study of UK adolescents, epilepsy diagnosis had an effect on QoL and identity formation. 49 In a study from the USA, seizure frequency was less concerning for older adults and their QoL than maintaining ‘normalcy’ in social and emotional function. 50 Other lifestyle variables such as socioeconomic status are also important for QoL in PWE. For instance, low socioeconomic status is related to an elevated risk of developing epilepsy,51,52 frequent hospital visits53 and attrition from epilepsy care,54 all of which can be associated with QoL. In interpreting the results of an intervention aiming to improve QoL and reduce seizure frequency, consideration of these factors will be important.
Summary of quality of life research
Living with epilepsy can influence QoL in PWE. In the present study, we aim to test the effect of an intervention designed to improve psychological, physical and social consequences of epilepsy. In considering the complexity of the above factors in managing this condition, the benefits of ‘self-management’ have been proposed in the literature. This addition to epilepsy treatment will be discussed in the remainder of this chapter.
Self-management education
What is self-management?
There is no widely accepted description of what self-management means, but the Department of Health has used the 2002 definition from Barlow et al. :55
Self-management refers to the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a chronic condition.
Barlow et al. 55
The notion that self-management encompasses all of the aspects of living with a chronic condition is summarised in the following:
Self-management is defined as the tasks that individuals must undertake to live with one or more chronic conditions. These tasks include having the confidence to deal with medical management, role management and emotional management of their conditions.
Adams and Corrigan. p. 5756
Corbin and Strauss identified these tasks (i.e. medical, role and emotional management) as the key aspects of disease self-management. 57 It is axiomatic that those living with a long-term condition (LTC) must be self-managing their condition. Thus, interventions that focus on self-management are really directed at supporting people to manage their LTC optimally (i.e. supported self-management interventions).
To facilitate the core tasks of self-management described above, Lorig and Holman58 proposed the acquisition of five core self-management skills including ‘problem-solving, decision-making, appropriate resource utilisation, forming a partnership with a health care provider, and taking necessary actions’. For a schematic representation of how this might be incorporated into an intervention, refer to Appendix 2.
Extensive qualitative research has identified a number of common themes relating to self-management support among individuals living with a wide range of LTCs. These common themes include:
-
the need for collaborative relationships with health-care professionals (HCPs)
-
the need for support from HCPs regarding information and education
-
medication adherence issues
-
the need for emotional and peer support
-
the need for carers to balance support and independence
-
the individuality of each person’s experience of illness
-
the importance of psychological support to help with adjustment for some people with LTCs. 59
The first two items on this list are reported in most studies looking at the experience of people living with any LTC. 57
What are self-management interventions?
Self-management interventions teach behaviours that seek to alleviate the consequences of a chronic illness. 60 The objectives are to facilitate patients taking an active role in their own health care by encouraging autonomy and providing accurate information on symptom management. 61
Recent work has attempted to identify the different activities that could be involved in supporting self-management (Box 1). 62 This taxonomy, derived from an expert advisory workshop and a systematic overview of the literature, describes only the potential components of self-management interventions as described in the literature. 59 Efficacy of these components in different LTCs has not been established. How, for example, does self-management support work?
-
Information about the LTC and/or its management.
-
Information about available resources.
-
Establishing specific clinical safety plans and/or rescue medication.
-
Regular assessment and evaluation.
-
Offering the patient feedback and monitoring.
-
Practical support regarding treatment.
-
Provision of equipment.
-
Providing opportunities to practise practical self-management.
-
Providing opportunities to practise everyday activities.
-
Providing opportunities to communicate with a HCP.
-
Providing training on psychological approaches.
-
Provision of easy access to advice or support when needed.
-
Peer support.
-
Lifestyle advice and support.
Note that not all of these components are appropriate for self-management support of all LTCs and the effectiveness of the individual components in self-management support for different LTCs is often unknown.
Self-management courses have been successfully implemented in the UK for other LTCs such as diabetes mellitus and arthritis [e.g. Dose Adjustment For Normal Eating (DAFNE),63 Diabetes Education for Self-Management for Ongoing and Newly Diagnosed (DESMOND),64 X-PERT65]. 55 DESMOND, for instance, is a group education programme based on psychosocial theories of learning and self-mastery as a basis for behaviour change. 66 The diabetes mellitus course is generally 1 or 2 days long and consists of non-didactic teaching methods. 66 Increased self-confidence and empowerment have been reported following these targeted self-management education courses. 64,65,67 Thus, previous research suggests that short-term self-management programmes can be of benefit in UK health-care settings and diabetes mellitus courses are now offered to all people with diabetes mellitus in the UK free of charge.
Why self-management for people with epilepsy?
The National Institute for Health and Care Excellence (NICE) recommends that PWE are given adequate, structured information, and empowered to be successful in managing their condition. 68 Past studies indicate that PWE want to know more about how to effectively manage their condition. 69,70
Although PWE might wish to speak with someone other than their doctors about managing their epilepsy;70 many do not know who to ask or lack the confidence to do so. 21 The level of information needed to enhance seizure control and facilitate patient empowerment may require more detail than can be delivered through traditional outpatient consultation. 71 Interventions that aim to improve knowledge, confidence and empowerment may encourage PWE to seek support from a wider range of services to help manage their condition. At present, there are no standardised group education programmes available for PWE in the UK.
A Cochrane review of epilepsy interventions found that there is a potential benefit of self-management groups, although it is essential that further empirical evidence is generated. 72 The review was later updated, indicating that evidence exists to support the benefit of self-management and epilepsy nurse specialist (ENS) involvement in interventions. 73 However, the review stressed the limitations of findings to particular settings and called for improved service models for widespread intervention delivery. In order to test the usefulness of self-management interventions in helping improve QoL for PWE across the UK, further research is needed. 74,75
What evidence exists on self-management for people with epilepsy?
Epilepsy is a condition that requires targeted behaviour and management (such as medication adherence and avoidance of seizure triggers) to alleviate epilepsy-related consequences. For PWE, self-management strategies are defined as the behaviours that minimise frequency and severity of seizures76 and improve overall QoL. 77 Good self-management strategies may also help PWE to potentially feel more confident about health-care decisions and overcome psychosocial consequences of living with epilepsy. 76
At the time of developing the research protocol for this project, there was little evidence on the efficacy of group self-management programmes for PWE. Two key studies on group-based interventions were highlighted during trial design. The interventions that informed development of the SMILE (UK) trial are described below.
Sepulveda Epilepsy Education
The Sepulveda Epilepsy Education (SEE) programme was developed with an aim of meeting the complex psychosocial and educational needs for a wide range of PWE in the USA. 2 Helgeson et al. 2 evaluated the effect of the 2-day SEE intervention on psychological and physical outcome measures (i.e. self-efficacy, AED adherence). With a sample size of n = 38 (18 treatment, 20 waiting list control), limited statistically significant differences were found between groups at follow-up (Table 1). Nevertheless, compared with controls, the treatment group showed a significant decrease in seizure-related fear, hazardous self-management behaviour and misconceptions about epilepsy. The study also showed a statistically significant decrease in self-rated depression in the treatment group (p < 0.0007) immediately after participating in the course; however, this did not persist at their 4-month follow-up. No other statistically significant differences were observed between groups at the last follow-up, 4 months after enrolment (i.e. seizure frequency, self-efficacy).
| Study | Setting | Sample | Design | Key findings |
|---|---|---|---|---|
| Helgeson et al.2 | Medical care clinic, CA, USA | PWE n = 38 | Pre-/post-test controlled outcome study; 4-month follow-up period | Statistically significant increase in understanding of epilepsy at 4-month follow-up. Statistically significant decrease in seizure-related fear, hazardous self-management behaviour and misconceptions about epilepsy. Statistically significant increase in AED compliance in treatment group at follow-up |
| Limitations: small sample size. Short follow-up period | ||||
| May and Pfäfflin1 | Epilepsy centres in Austria, Germany and Switzerland | PWE n = 242; 16–80 years | Pre-/post-test randomised study; 6-month follow-up period | Statistically significant improvement at follow-up in epilepsy-related knowledge and coping |
| In treatment group, seizure outcomes improved and participants reported feeling more satisfied with AED therapy | ||||
| Limitations: per-protocol analysis may bias the results. No long-term follow-up |
Modular Service Package for Epilepsy
The Modular Service Package for Epilepsy (MOSES) intervention was originally developed in Germany. 78 After programme development, MOSES was tested in three German-speaking countries in a RCT. 1
The programme was adapted for both in- and out-patient contexts, and the course content was covered over 2 consecutive days. Participants were recruited from 22 specialist outpatient clinics and allocated to either the treatment or the waiting list control group receiving treatment as usual (TAU). Their sample contained 242 adults with epilepsy aged > 16 years with no other major comorbidity (see Table 1).
The final follow-up was carried out at 6 months. At this time point, course attendance had a positive effect on epilepsy-related knowledge, overall coping ability, seizure frequency and medication use. Thus, the authors concluded that MOSES is effective and reduces seizure frequency. They did not find, however, any effect of the intervention on a generic measure of QoL, self-esteem and other aspects of coping with epilepsy (i.e. information seeking). 1 They used the Short Form questionnaire-36 items during the trial, which may not have adequately detected the effect of the education programme. An epilepsy-specific measure of QoL [e.g. Quality Of Life In Epilepsy 89 (QOLIE-89) or Quality Of Life In Epilepsy 31 (QOLIE-31)] may be more sensitive in future research. 1
Overall, the effect of the MOSES programme on seizure frequency was encouraging and MOSES has now been routinely offered across Germany for approximately 15 years. Its success in practice led us to hope that it may be appropriate in UK health settings. Thus, MOSES was selected to trial for use in the UK.
These two seminal studies offered important conclusions about the benefits of self-management education in group settings. However, these studies lacked:
-
a sample size adequate to detect significant differences in QoL with enough statistical power while minimising type 1 errors (false positives)
-
a long-term follow-up period (e.g. at least 1 year)
-
a process evaluation of self-management courses using qualitative methods to gain the patient’s perspective
-
a health economics evaluation of the cost-effectiveness of self-management courses
-
an assessment of implementation fidelity to determine whether or not the intervention was delivered according to protocol.
The SMILE (UK) programme was designed to address these topics.
Summary and methodological rationale
Epilepsy is a highly stigmatised condition, associated with multiple potential psychosocial consequences. Past research indicates that many PWE want to know more about their condition,69,70 but may not know where to find information. This suggests that offering guidance and information may be valuable for PWE in the UK.
Self-management education offers an opportunity to address a gap in outpatient or community service provision. Self-management programmes are different to traditional educational offerings as they are designed to educate and empower those living with chronic health-care conditions. 79 No such programme has been evaluated for UK-based health-care services for epilepsy.
Research objectives
The main aim of this research, as required by the NIHR call, was to evaluate the effect of an intervention [the SMILE (UK) course] on patient QoL compared with TAU. The primary outcome was assessed using total QOLIE-31-P (Quality Of Life In Epilepsy 31-P)80 score at 12-month follow-up. Secondary outcomes included seizure frequency and recency, impact of epilepsy, adverse effects of medication, depression, anxiety, stigma and self-efficacy (measured via mastery and control). We also collected health economics data to determine the cost-effectiveness of the intervention.
Objectives of this research were to:
-
refine the content and delivery of the SMILE (UK) course after receiving feedback from Epilepsy Action Information Reviewers, who assessed the content of research materials for PWE
-
recruit and provide training for SMILE (UK) course facilitators
-
conduct a pilot study with volunteers to determine the suitability of outcome measures in terms of ease of completion
-
obtain qualitative feedback on the SMILE (UK) course by conducting an external pilot study with Epilepsy Action volunteers
-
describe the experiences of those who attended the SMILE (UK) course, as well as perceptions of barriers to attendance and benefits of the programme
-
assess the delivery of SMILE (UK) courses by conducting a fidelity analysis
-
test the hypothesis that participants with poorly controlled epilepsy would report improved QoL 12 months after being offered the SMILE (UK) course with TAU compared with those who received TAU alone
-
evaluate changes in secondary outcome measures at 6- and 12-months after randomisation
-
assess the cost-effectiveness of the SMILE (UK) course
-
highlight training requirements for implementing SMILE (UK) in the UK
-
disseminate findings to researchers, service users and commissioners of policy development.
Taking into account Medical Research Council (MRC)81 guidelines, the SMILE (UK) trial was undertaken in a series of stages.
-
The SMILE (UK) intervention was adapted for a UK context based on existing evidence from a similar intervention developed in Germany1 and an early English translation.
-
A complex intervention protocol was developed and published. 3
-
The SMILE (UK) intervention was piloted externally. 82
-
A statistical analysis plan was published. 4
-
A process evaluation of participants’ views of the courses was undertaken. 83
-
A baseline description of SMILE (UK) participants was combined with an analysis of which outcome measures are related to QoL (measured by QOLIE-31-P). 84
-
A fidelity analysis was undertaken on complex intervention delivery. 85
-
Clinical effectiveness and cost-effectiveness follow-up data were collected for both trial arms and analysed.
-
Results would be disseminated when the study was complete.
Chapter 2 Intervention development
Introduction
The SMILE (UK) intervention was adapted from MOSES, an educational treatment programme designed in Germany for PWE. It is a group course designed with input from specialists, non-medical professionals and patient groups. The intervention was originally developed by Ried et al. 78 for German-speaking adults aged > 16 years with any severity of epilepsy. The course is also suitable for patients with both epilepsy and mild learning difficulties. This chapter will outline the process by which the intervention was developed for use by PWE in the UK. A schematic of this process can be found in Appendix 3.
Overview of the MOSES intervention
The MOSES programme was designed to promote coping with epilepsy, increase participation in everyday activities and improve general self-esteem. Content is focused on supporting patients to become experts in managing their epilepsy, which is also consistent with NICE guidelines. 68
The MOSES developers did not adopt a specific behavioural change model. 78 Instead, they designed the intervention pragmatically, incorporating three levels of information processing to achieve change:78
-
the cognitive level (providing information)
-
the emotional level (identifying and discussing emotions)
-
the behavioural level (discussing actual activities).
Cognitive, emotional and behavioural aims are included in all nine MOSES modules, which were subsequently adapted for the SMILE (UK) intervention. Ried et al. 78 specified educational aims for PWE taking part in the course, as well as for those teaching the courses. 78 Although not conceptualised as such,78 key evidence-based behavioural change elements include:86
-
education about self-monitoring (of seizures)
-
obtaining support
-
increasing confidence (through identifying previous strengths and successes)
-
exposure to role models, both within the formal teaching material and to others within the group teaching format
-
provision of encouragement from others
-
discussion within the sessions of realistic outcome expectations.
The MOSES programme is intended to improve participants’ self-mastery (i.e. their confidence in being able to perform behaviours)87 to manage their condition. It focuses on a number of lifestyle changes, including obtaining sufficient sleep, avoiding alcohol, reducing stress, obtaining social support, understanding and coping with adverse effects of medication, following their prescribed medication schedule and planning ahead for collecting medicines. 88 Self-mastery may also be increased by exposure to the experiences of other PWE and facilitators during the course. By improving the self-mastery of PWE and their expectations regarding the outcomes of treatment and seizures,89 it was anticipated that MOSES and, therefore, SMILE (UK) would lead to better outcomes for PWE.
The MOSES programme has a flexible timetable arrangement. It can be run over a short period of time (i.e. a weekend) or longer (i.e. weekly sessions for up to 8 weeks) if preferred. 78 Topics covered during the programme include the following. 78
-
Living with epilepsy: identifying and expressing how it feels having epilepsy and how it felt being first diagnosed.
-
Epidemiology: teaching how common epilepsy is and learning about famous people with the condition.
-
Basic knowledge: causes of epilepsy and different types of seizures.
-
Diagnostics: tools and techniques used for epilepsy diagnosis.
-
Therapy: treatments for epilepsy, such as AEDs.
-
Self-control: recognising what can cause seizures and using countermeasures to interrupt auras.
-
Prognosis: discussing seizure control and the possibility of seizure freedom.
-
Psychosocial aspects: discussing the impact that epilepsy has on daily life, relationships, employment and day-to-day functioning.
-
Network epilepsy: talking about help that is available from self-help groups and other institutions.
The content is delivered in a workshop-style environment in which patients are encouraged to share their experiences with group members and engage with structured teaching activities.
The programme’s aims address understanding and coping with epilepsy, how it is diagnosed and treated, how to be more involved with the treatment plan, how it can impact life socially and at work, how to become independent and lead a normal life, and especially how to become expert representatives for one’s own condition. 78
At the conclusion of the course, it was hoped that participants would gain a deeper understanding of epilepsy, have generated ideas within the group about effective coping strategies and learn how to manage their condition autonomously.
Mechanisms whereby MOSES modules may lead to improvement in QoL in PWE are outlined in Table 2. Numbers in the table refer to the specific MOSES modules 1–9, as listed above.
| Factors influencing QoL in PWE | Relevant MOSES modules | Specific content |
|---|---|---|
| Seizures (frequency and recency) |
4. Diagnostics 5. Therapy 6. Self-control 7. Prognosis |
Knowing difference between seizure types; accurate recording of seizures and their semiology to assist better medical management Understanding importance of tests (e.g. blood tests) to monitor treatment; use of aids (e.g. dosette boxes) to assist adherence; encourage use of seizure diary to monitor progress/help medical management achieve better seizure control; being able to explain treatment to others and why one may have to take drugs at certain times; planning medication for holidays etc.; what to do if a dose is omitted; understanding other non-pharmacological treatments (e.g. surgery, vagus nerve stimulation) Learning to identify, record and respond to seizure triggers; learning to identify and respond to seizure auras (using countermeasures) Learning about factors likely to improve prognosis (i.e. to increase chances of becoming seizure free) |
| Seizures (injury) |
5. Therapy 6. Self-control 8. Psychosocial aspects |
Being able to tell others about one’s seizures, what they look like; how others should respond to prevent injury Learning to identify, record and respond to seizure triggers; learning to identify and respond to seizure auras (using countermeasures) Learning how to minimise risks at home/work |
| AEDs | 5. Therapy | Reducing/improving acceptance of AED adverse effects |
| Psychological comorbidity |
1. Living with epilepsy4. Diagnostics 5. Therapy 6. Self-control 8. Psychosocial aspects |
Dealing with anger/anxiety about having epilepsy Understanding the purpose of/reducing anxiety about different investigations Reducing psychiatric comorbidities associated with adverse effects of some AEDs Improving seizure control Improving self-esteem, using problem-solving approaches and seeking psychological support; seeking neuropsychological assessment to identify cognitive difficulties and address these; identifying own positive attributes and weaknesses |
| Stigma |
2. Epidemiology 5. Therapy 7. Prognosis 8. Psychosocial aspects 9. Network epilepsy |
Learning that epilepsy is common and can affect everybody; learning about achievement potential of PWE Provision of advice on family planning; learning that taking medication can be part of everyday life Learning what can still be achieved if PWE do not achieve seizure freedom Learning to reduce unnecessary restrictions on activities; maintaining social contacts; engaging in physical exercise; understanding relevant disability legislation and entitlements Identifying/contacting/joining relevant organisations/support groups |
| Resilience | 1. Living with epilepsy | Helping with reactions to diagnosis and planning future coping strategies |
| Age |
7. Prognosis 9. Network epilepsy |
Learning about what can be achieved at different ages even if PWE do not achieve seizure freedom Identifying age-appropriate sources of support/services |
In order to teach the MOSES programme, course facilitators are required to have experience of facilitating groups and to have completed a MOSES-specific training seminar. 78 Facilitators are nurses, psychologists, clinicians or social workers. Resources for facilitators during programme delivery consist of a ‘trainer manual’ and a workbook for group participants. Various techniques were developed to teach the course material in an interactive way and these were also used for SMILE (UK).
The course modules can be delivered on separate days over a period of weeks. For the MOSES trial, researchers offered the nine course modules over 2 consecutive days. The ideal group size was thought to be between 7 and 10 participants, with a maximum of 12;78 however, the group size in the RCT was not specified. 1
Presently, MOSES is offered to all PWE aged ≥ 16 years, regardless of seizure frequency, who can follow 90-minute teaching sessions. 90 It has been offered for approximately 15 years with great success, with over 20 courses scheduled in the current year. 90
Developing the SMILE (UK) intervention
There were a number of components involved with adapting the intervention for use in the UK. We adapted the patient workbook and teaching manual, liaised with patient user groups and trained UK-based facilitators to deliver the course. The following sections will outline how this was undertaken prior to intervention delivery. A schematic representation of the timeline is presented in Appendix 3.
Adapting the participant workbook and teaching manual for the United Kingdom
The SMILE (UK) research team obtained course material from the MOSES group. Most content had been translated from German except for personal testimonies from PWE. Personal testimonies were translated into English within the research department. Each SMILE (UK) collaborator edited and revised one module of the patient workbook. Some module titles were modified for ease of understanding.
Changes were made to several sections. For example, the section on ‘Famous PWE’ was modified to include celebrities and public figures that would be recognisable to people living in the UK. The section on ‘Networks’ was also changed to reflect local support network information. Epilepsy Action contributed as subeditors during this part of the intervention adaptation. They reviewed the use of English throughout the patient workbook to reduce the required reading age and improve accessibility for a wide range of audiences. Current information on antiepileptic medication was adapted from information available from the Epilepsy Society. Local regulations (e.g. regarding insurance and driving) were changed to reflect UK legislation.
Overview of the SMILE (UK) intervention
The SMILE (UK) course is a group-based, interactive education programme based on MOSES, developed to provide strategies for individuals living with poorly controlled epilepsy in the UK. 3 SMILE (UK) was designed to be delivered in 16 hours over 2 days.
The number of people per group was intended to range from 6 to 12 participants (including carers or family). All participants assigned to the treatment group were given the option to have a carer or significant other accompany them to the course if they wished. This was encouraged to make the participant feel supported, but also to add another perspective to group conversations (i.e. family or carer perspective). The group number was selected based on the original MOSES research78 and because it might have helped allay anxiety about speaking in front of a large audience and would thus be small enough to share personal experiences comfortably.
Two trained HCPs acted as group facilitators during the course, wherein attendees progressed through set modules and participated in group discussions. A workbook was used throughout the course so participants could become familiar enough with it to be able to use it at home. The programme covered the same nine modules offered by MOSES (i.e. living with epilepsy, epidemiology, basic knowledge, diagnostics, therapy, self-control, prognosis, psychosocial aspects, and network epilepsy)78 renamed for SMILE (UK) (see Table 3).
SMILE (UK) teaching materials
The SMILE (UK) course is taught using a number of items, including two sets of workbooks (teaching manual and participant workbook), a flip chart for teaching demonstration, stickers for group exercises (used with flip chart), and Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) slides. Each of these teaching resources will be discussed in the following sections.
Teaching manual
A teaching manual was developed based on the MOSES ‘trainer workbook’. 78 For copyright reasons, specific content will not be provided here (please see Acknowledgements for more information). Content can be requested from the MOSES group. The aim of this resource is to guide facilitator teaching of the SMILE (UK) course. In the preface section of the book, it is suggested that course content may be negotiated between participants and facilitators in the future. The teaching manual is divided into two sections.
Section 1
This contains six introductory modules: (1) a preface, (2) SMILE (UK) teaching aims, (3) structure and implementation of SMILE (UK), (4) requirements for teaching SMILE (UK), (5) key terms, and (6) sources for further information. The guide stresses the need to provide participants with accurate information and the importance of adopting an interactive teaching style. Facilitators are encouraged to adapt their teaching methods and speed according to the needs of the group. Overall teaching goals include (1) promotion of active learning, (2) support sympathetic and friendly communication between course participants and (3) fostering a stimulating and varied learning environment.
Section 2
This contains a summary of each module from the participant workbook. Each module contains information on the duration of each session, teaching methods and materials. Teaching advice is given as a guideline that can be used flexibly or supplemented during the course. Throughout each module, suggestions are made to facilitators to indicate where certain teaching materials (i.e. flip chart, stickers) can be used. Throughout the course, facilitators are encouraged to allow time for social interaction and discussion rather than focusing solely on teaching.
Participant workbook
Participants were provided with a workbook at the beginning of the SMILE (UK) course and encouraged to use it as a home reference after the course had finished. Each of the nine modules (Table 3) begins with a summary page (about the topic, aims and contents), and includes note-taking space, interactive questions (e.g. what does my doctor need to know about my seizures?) and a final summary of key points (see Acknowledgements for more information about copyright).
| Module | Topic | Objective | Page |
|---|---|---|---|
| Introduction | 5 | ||
| One | Living with epilepsy | How to recognise and express different emotions that you may experience because of epilepsy. How to develop better ways to cope with epilepsy | 25 |
| Two | PWE | How common is epilepsy in the UK? When are you most likely to develop epilepsy? Famous PWE and what they have achieved | 47 |
| Three | Basic knowledge | The causes of epileptic seizures, how seizures develop and how to identify the different seizure types | 57 |
| Four | Diagnosis | How to observe and describe seizures accurately. How to document seizures, the results of investigations and understand the different diagnostic methods | 67 |
| Five | Treatment | An overview of the most common AEDs and different treatment options. How to actively participate in your treatment | 81 |
| Six | Self-control | How to avoid seizure triggers and become aware of auras/warnings. Working out what might be relevant to developing abilities of self-control | 113 |
| Seven | Prognosis | The chances of achieving seizure freedom and the chances of staying seizure-free after stopping AEDs. Options if seizure freedom is not achieved | 131 |
| Eight | Personal and social life | How to improve self-esteem and social contacts. Support for independent living, sports and professional life, driving regulations and how to explain epilepsy to others | 136 |
| Nine | Network | Addresses and other information related to treatment, psychosocial support and specific information for your epilepsy | 193 |
Not all workbook material is covered during the 2-day programme, so facilitators suggest that participants refer to the book in their own time and share content with family and friends. At the end of each module, there is a series of questions that encourage further consideration of issues raised and further teaching session. Ample time is provided for sharing problems and solutions.
Course slides
Facilitators are provided with a Microsoft PowerPoint file to show slides during the course. In the original MOSES study, course facilitators were encouraged to use overhead transparencies. We opted for PowerPoint slides based on these transparencies adapted for a UK audience. Slides correspond to participant workbook content and provide supplementary information.
Flip chart
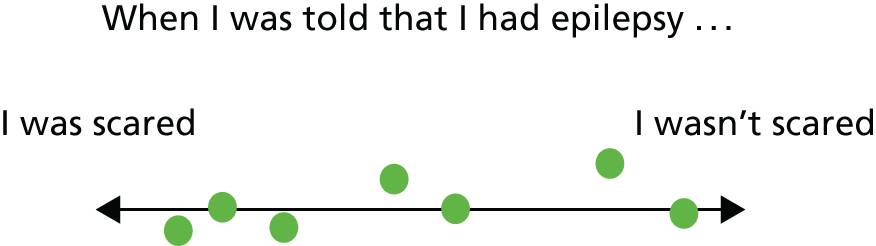
A freestanding flip chart with large paper sheets was set up in full view of course participants. Throughout each day, discussion statements were written on the flip chart and participants were asked to respond during a group discussion. For example, participants were given ‘dot’ stickers on the first morning and these were used throughout the course as a method to compare participants’ views on certain topics. Questions were asked about how participants felt regarding certain statements and they were asked to place a sticker on a scale of responses (Figure 1). The array in the placement of stickers along the scale was then used as a way to initiate discussion on the topic.
FIGURE 1.
Example of reflective exercise using stickers on a flip chart. Participants were asked to place a sticker (represented by a green circle) on the scale according to how they felt.
SMILE (UK) course delivery
To balance out emotional and teaching topics, the modules were delivered to participants in a different order than in the workbook. Each day of the course followed set schedules (see Appendix 4) corresponding to the workbook modules described in Table 3. The timing of course activities followed an organised but not prescriptive structure. The SMILE (UK) course was divided into four sessions: day 1 morning session, day 1 afternoon session, day 2 morning session and day 2 afternoon session. Each session lasted 3 hours and contained a tea/coffee break. Lunch was provided between the morning and afternoon sessions.
There were always two facilitators present at the course so that should a seizure occur, one facilitator could continue the course while the other attended to the participant. One facilitator led each module while the other assisted. Who would lead each module was discussed between the two facilitators prior to the start of the course. The course began with individual introductions of course facilitators and participants. One facilitator explained housekeeping rules and building facilities. The first module on ‘living with epilepsy’ had a heavy emotional component and was divided into two parts. The first part focused on discussing experiences and naming emotions experienced following diagnosis. Course participants were reminded that there are no ‘right or wrong feelings’ in response to living with epilepsy. The point of this module was that thoughts and feelings about epilepsy can be shared with others. The second part consisted of a discussion on how group members had coped with their condition in the past. Depending on the size of the groups, there was likely to have been a range of experiences and feelings described regarding diagnosis length, seizure severity and seizure management.
The ‘people with epilepsy’ module contained discussions about famous PWE and risk factors/causes of the condition. Facilitators compared the prevalence of epilepsy with other chronic health conditions to show how common epilepsy is. Module three on ‘basic knowledge’ covered causes of epilepsy, epileptic activity in the nerve cell and where seizures originate from in the brain. Participants were asked if they knew what type of seizures they had and were given a chance to share their experiences with the group.
Module six covered ‘self-control’. During this section, participants were asked to place a sticker on the flip chart with other course participants, to show how preventable they believed seizures were. Course facilitators then shared instances where this may or may not be possible, discussed triggers and how to keep records of seizures. Module nine addressed personal support networks for PWE. Participants were asked to share their own sources of support they used to cope with epilepsy. Local support networks were also discussed and the day closed with an overall summary of the course.
Day 2 began with a brief outline of what would be covered for the day, as well as ‘checking in’ with how participants felt about day 1 (see Appendix 4). Emotional responses to the first day were discussed together as a group. The first module of the day was a discussion on ‘treatment’. The facilitators outlined different treatment options available in the UK. Ideas and strategies were shared on how participants could actively be involved with their treatment plans. Participants were asked to write down personal drug therapy goals in their workbook.
The next module on ‘diagnosis’ listed diagnostic methods during this session and, with time permitting, described means of assessing seizure activity, such as electroencephalogram (EEG) information collected during routine assessment. Module seven focused on ‘prognosis’. Participants were asked when or if they expected to become seizure free. This topic was discussed as a group and the facilitator explained their answers from a medical viewpoint. Factors affecting prognosis were also discussed.
The final topic of day 2 was on ‘personal and social life’, which covered ways of helping people cope and overcome challenges associated with epilepsy in daily life. At the end of the course, participants were thanked for their participation. Those who wished to remain in contact with other attendees were able to give their permission for contact details to be circulated by e-mail at a later date (if not shared by individual group members during the course already).
SMILE (UK) course facilitators
The teaching model for SMILE (UK) required there to always be two course facilitators present during teaching. In international provisions of group education courses for PWE, various clinicians have acted as group facilitators. These have included psychologists,46 researchers,91 social workers and neurologists. 71
The literature is unclear on ‘who is best’ to deliver self-management education programmes. Past research suggests that lay persons might be well placed to deliver self-management groups; however, limited long-term benefits have been demonstrated. 79 Based on national literature92 and staff availability,93 we chose to recruit ENSs and EEG technicians to run the course.
In the UK, ENSs are likely to be part of outpatient epilepsy care. The role of an ENS is to provide specialist outpatient care for PWE and to support primary and secondary care teams. 94 They incorporate social support and counselling in their role, but also focus on consultation and advice for PWE. ENS services may be provided in person or over the telephone on topics ranging from AED side effects, pregnancy queries and overall support. 94
Past UK-based research found that ENSs were important support for PWE, especially those with a recent epilepsy diagnosis and with long term epilepsy. 95 ENSs are well-placed to take group courses with PWE because they possess specialist knowledge of managing epilepsy and experience of managing interpersonal dynamics and psychological issues (such as those that occur in group settings).
The chief investigator who was responsible for recruiting facilitators found that ENSs in London and the South Thames were enthusiastic about the course. Nevertheless, many felt overstretched by their NHS roles and did not have the capacity to volunteer additional time delivering the course. ENSs on the whole choose Monday to Friday work hours and it seemed unlikely that they would undertake courses at the weekends.
The decision to have EEG technicians as facilitators was primarily because of their specialist experience of epilepsy diagnosis and training in medical aspects of epilepsy. EEG technicians collect diagnostic information and have specific experience in assessing PWE. These clinicians were available at King’s College Hospital (KCH) and were willing to be involved with the trial. Many have higher education training (e.g. Master of Science-level education) and an interest in teaching others about epilepsy. Furthermore, the lead neurophysiologist at KCH (Dr F Brunnhuber) had previously trained to deliver MOSES and was able to assist with recruiting of EEG staff at the hospital and also mentored the facilitators during their SMILE (UK) training and delivery of the programme. Although EEG technicians do work out of hours, the reimbursement allowed for locum substitutions is for weekdays only.
Facilitator training
The chief investigator and co-investigators of the study underwent several stages in recruiting and training of SMILE (UK) facilitators. LR was responsible for recruitment of facilitators and staff.
The first step involved the engagement of London- and Kent-based ENSs. Requests to hospitals were generally received well, although were dependent on resource availability. Several centres had staff who expressed enthusiasm to teach, but had workloads that prevented their involvement with the study.
After an initial group of prospective facilitators had applied, the trial chief (LR) and a co-investigator (AJN) held interviews to assess each candidate’s suitability for SMILE (UK) facilitator training. A final group of facilitators was selected (see Appendix 5). In total, there were eight female and three male facilitators; seven were EEG technicians and four were ENSs. Four facilitators had a Bachelor of Science-level degree and seven had a Master of Science in epilepsy or epileptology. The average length of experience in epilepsy care for the group was 20 years (range 5–40 years).
SMILE (UK) facilitator training course
Three clinicians/researchers from the MOSES group came to London to conduct a 2-day facilitator training course held on 10 and 11 June 2013 (for topics covered during training, see Appendix 6). The study chief investigator (LR) and a co-investigator (LHG) attended the training course as well.
The MOSES trainers went through the SMILE (UK) course material, showed a facilitator training digital versatile disc (DVD), and went through group-based exercises with trainee facilitators. Attendees were provided with a ‘teaching manual’, which is organised similarly to the patient’s workbooks, with indications on the techniques to use such as mind maps and writing out participant answers on a flip chart or slides. Attendees were able to ask questions on why activities were undertaken in a certain way and learn from the experiences of the MOSES facilitators who were undertaking the course with patients in Germany. Strategies were shared for enhancing participant engagement and understanding, such as focusing on topics in the workbook relevant or of interest to course participants (e.g. if participants already use a seizure diary, move on to another topic), or asking someone to clarify what they mean (e.g. ‘Can you please elaborate on that?’ or ‘Could you give an example?’). When teaching SMILE (UK), facilitators were encouraged not to follow all workbook content sentence by sentence. All sessions during the 2-day facilitator training were video-recorded so that facilitators could review them. On conclusion of the SMILE (UK) training course, all facilitators received a certificate of completion. Throughout the SMILE (UK) study period, there was attrition of facilitators owing to work commitments in their clinics. New facilitators received training by watching the video of the 2-day training session and sitting in on an active SMILE (UK) course.
Patient and public involvement during SMILE (UK)
Patient and epilepsy care groups were consulted throughout intervention development in order to assure acceptability of the treatment technology. The Epilepsy Action Research Network (EARN) is the largest national user group that supports academic research for PWE and so became user partners in the SMILE (UK) trial. Members of EARN are PWE, carers and members of the public who are familiar with health research.
To verify the content and reading levels were appropriate for PWE in the UK, documents intended for patients such as the patient information sheet and the participant workbook were sent to Epilepsy Action Information Reviewers. Comments received were on the reading difficulty and on the volume and level of information given in the participant workbook. It was decided to give workbooks out at the beginning of the course, in order to go through it selectively during the sessions. With this arrangement, participants could ask questions on content covered in the workbook and choose for themselves if they wanted to read further once they had completed the course, but not be put off by its length and complexity before the course began.
Service user feedback from EARN was sought during the design of the study protocol (see Appendix 1). Information was provided about the study, then PWE were asked, via Epilepsy Action’s website and online forum, if group-based education was something that they would find helpful.
In order to ensure that findings from SMILE (UK) would be communicated to the public on study completion, a dissemination plan was drafted. We actively sought feedback from Epilepsy Action about this plan by discussing it during collaborators’ meetings. Following the completion of the trial, a website would be created with study results and researchers or facilitators would attend meetings to present final results.
Two key individuals represented service user groups and remained in regular contact throughout the trial. We involved Mary-Jane Atkins from the patient/service user group at KCH in the early phases of the research (pre-pilot). Mary-Jane assisted by participating in the external pilot study82 and completing the battery of assessment questionnaires used during the baseline and follow-up assessments. She provided feedback on her experience of completing the questionnaires in order to ensure that they could be answered comfortably (i.e. easy to fill in, ≤ 60 minutes to complete). As the trial was being undertaken, the epilepsy services manager from Epilepsy Action (Angela Pullen) attended and participated in collaborator meetings throughout the study.
External pilot of SMILE (UK)
The external pilot was conducted prior to starting the main RCT. The aims were to identify whether or not any changes would be required to content or delivery and to explore the beliefs and understandings of those attending the SMILE (UK) course. The results from the pilot study were published82 and this section serves as a summary of the study.
Methods
Recruitment
We aimed to test the SMILE (UK) course with two groups of 10 PWE. Volunteers were sought by placing an advertisement in Epilepsy Today96 and via Epilepsy Action’s website. 97 From March to May 2013, 22 participants were recruited. Eleven did not participate as a result of illness, work obligations, being uncontactable or fatigue (and other effects of a recent seizure). A further one participant declined to take part in the research interviews after attending the SMILE (UK) course. In the end, 10 volunteers participated in the external pilot study
SMILE (UK) course delivery
Two pilot courses were given from 9:30 until 17:00, each over 2 days. Two sets of course facilitators gave the course, and this also served as a practice after their training. Each course had an ENS and an EEG technician facilitating. The courses were held at an education facility part of King’s College London (KCL), located next to KCH. The course days followed the schedule described in Appendix 4. All volunteers received the participant workbook. Courses were generally delivered by an ENS and an EEG technician working in tandem. This pairing enabled their knowledge and skills to combine optimally.
Data collection
The groups were then asked to provide feedback on their experience. Data were collected in two ways: three participants completed a focus-group interview and seven completed one-on-one semistructured interviews (two over the telephone, four in person and one by e-mail). Both took place less than 1 month after participants took part in the pilot SMILE (UK) course. The focus group lasted 60 minutes and the semistructured interviews lasted between 20 and 30 minutes.
Interview procedure
Discussion topics were established with input from Epilepsy Action. 82 The resulting topic guide was flexible and served as a prompt during discussions (see Appendix 7). Topics focused on (1) reasons for interest in the SMILE (UK) pilot study, (2) views on content covered during the course, (3) appraisal of group learning processes, (4) appraisal of teaching methods and (5) overall utility of the SMILE (UK) course. To minimise chances of bias, the study interviewer was not involved with facilitating or co-ordinating the SMILE (UK) programme. The topic guide was flexible in that prompts could be revised on the spot and returned to throughout the interview sessions.
Data analysis
Interviews and focus group discussions were audio-recorded and transcribed verbatim. The transcripts were checked by the interviewer and two co-investigators (MM and LR). Analysis began by manual marking of topics in margins. This process took on an iterative format of going back and forth between notes and emerging themes. Software (NVivo 9, QSR International, Warrington, UK) was used to formally code themes from transcripts and grouping into broader themes.
Results and discussion
Many themes emerging from the pilot study were similar to those from the process evaluation presented in Chapter 9. For these common themes, data will be presented together. In the section that follows, we will present the results relevant to the intervention that helped us to shape the SMILE (UK) course for the RCT. Full characteristics of volunteers from the pilot are also presented in Chapter 9. Briefly, six females and four males participated, aged from 21 to 60 years, having had epilepsy for 8 to 52 years.
Reasons for participating
Most volunteers said they chose to participate in the pilot study for general interest, especially as it offered a chance to meet other PWE. Two saw it as a way to be involved in developing a new intervention and one saw it as a way to help in taking control of her life. These reasons give insight into why PWE may wish to participate in the SMILE (UK) RCT.
SMILE (UK) content
Out of the nine modules, four were found to be especially useful. These were module 3, basic knowledge; module 4, diagnosis; module 6, self-control; and module 8, personal and social life. This was useful to know when we considered how to evaluate implementation fidelity of SMILE (UK) (described in Chapter 7). Importantly, the volunteers did not find any parts of the course to be redundant.
SMILE (UK) duration
The volunteers considered the duration of the course days to be too long and would have preferred to spread the course over 3 days. This was considered by the research team. As the target group was PWE with active seizures, there would be a higher risk of non-completion of the course as a result of seizures if it was spread out over more days. PWE may also not want to participate because of the longer time commitment if the course was longer. As a result of this, the investigators decided to keep the course length to 2 days.
SMILE (UK) materials
All volunteers considered the participant workbook to be a valuable source of information that could be used as a reference for the future. It was also a source of information for their family and friends. One common criticism was not incorporating the participant workbook into the course. Volunteers said they would have like to have time to take notes during sessions and to fill in some of the relevant exercises. Following this, the facilitators were instructed to include the workbook more often by indicating on which page participants could find the relevant information and invite them to complete relevant exercises (e.g. describing what happens to them during a seizure). To ensure course facilitators implemented this, workbook use throughout the course was measured in the implementation fidelity assessment (see Chapter 7).
Two participants had seizures during the course, reinforcing the value of holding SMILE (UK) near hospital emergency department (ED) services. It also confirmed the need to have two facilitators at every course so that one could assist with the seizure while the other continued with the group.
Other topics emerged, such as the positive value of a group setting, using new information to empower their discussions with HCPs and improving their personal lives. Full results from this external pilot will be presented in Chapter 9.
Summary
By incorporating local information on epilepsy care and approaching local epilepsy care networks, we adapted a German-based intervention called MOSES78 for use in the UK. Training was provided to 11 facilitators (ENSs and EEG technicians) in order to be able to deliver the SMILE (UK) intervention. A teaching manual and participant workbook were adapted from the MOSES course materials. Workbooks and course content were largely unchanged from MOSES, with the exception of topics such as local support networks, UK regulations and famous PWE. The SMILE (UK) course contained a mix of teaching modules, largely containing factual topics and others touching on emotional aspects of life with epilepsy. This balance offers a unique opportunity for PWE to gain knowledge about their condition but also to share with people like themselves. The pilot study of two SMILE (UK) courses resulted in positive feedback. The volunteers concluded that there were no major barriers perceived with running this kind of intervention in the UK.
Chapter 3 Methods
Study design
The trial was designed as a multicentre, pragmatic, parallel arm RCT of a complex intervention. Participants were randomised in a 1 : 1 intervention-to-control ratio. The intervention group received TAU and was offered the SMILE (UK) course, while the waiting list control group received TAU until the 12-month follow-up assessment. The intervention was offered to the TAU group once they had completed the final trial assessment. In addition, the study contained two qualitative elements: an external pilot and a nested process evaluation. Prior to the RCT, an external pilot was conducted of SMILE (UK) (see Chapter 2). For the nested process evaluation, 20 participants were recruited after having attended the SMILE (UK) course (see Chapter 9).
Study settings
The chief investigator (LR) visited and arranged for local principal investigators (PIs) to recruit participants from epilepsy clinics in eight hospitals in south London and south-east England: KCH (Lambeth, London); University Hospital Lewisham (Lewisham, London); the National Hospital for Neurology and Neurosurgery (NHNN) (Camden, London); St George’s Hospital (Wandsworth, London); Croydon University Hospital (Croydon, Surrey); Princess Royal University Hospital (Bromley, Kent), Darent Valley Hospital (Dartford, Kent); and Guy’s and St Thomas’ Hospital (Southwark and Lambeth, London).
The trial was run from a central location at KCL. Throughout the study, face-to-face meetings between participants and research workers were held at a location convenient to the participant – at KCH (Denmark Hill campus in Southwark, London), a public place or their own homes.
Trial approval and monitoring
The study was approved by the National Research Ethics Service Committee London – Fulham (Research Ethics Committee reference 12/LO/1962; see Appendix 8). The study was approved by the local research and development departments at each recruitment site. The study was monitored regularly by the Trial Steering Committee (TSC) and the Data Monitoring and Ethics Committee (DMEC). Meetings with both groups were held at least once a year with interim reports sent when needed.
Screening and recruitment
Participants were recruited from eight sites in London and south-east England. Local PIs identified patients from having attended neurology clinics within the previous 12 months.
Stage 1
Individuals having attended a neurology clinic were sent a letter from the local clinical PI along with information about the study in a patient information sheet (see Appendix 9). At this stage, patients had the chance to opt out from the next stage of the study. They had a 3-week period to return their opt-out slip in a pre-paid envelope. As this stage did not involve screening medical notes, patients contacted may not have necessarily met the eligibility requirements. The only requirement at this stage was that they had attended the neurology clinic within the past year.
Stage 2
At the second stage of recruitment, medical notes were screened by clinicians at the local hospital and a second invitation was sent to eligible participants along with the patient information sheet. Once again, patients had the opportunity to opt out from further contact from the research team. Again, at this stage, patients had 3 weeks to return their opt-out slip. Patients who did not opt out of either stage were contacted by the research team to invite them to enrol in the study.
Stage 3
At this point of contact, the research team telephoned eligible participants who had not opted out to invite them to join the study. Research workers confirmed eligibility criteria of interested individuals and arranged a face-to-face meeting to explain the study in more detail, obtain informed consent and conduct a baseline assessment. Participants gave written consent themselves. All meetings were arranged at participants’ homes or public locations convenient for them.
Eligibility criteria
Inclusion criteria:
-
confirmed epilepsy diagnosis (all epilepsy-related conditions and seizure types included)
-
current AED prescription
-
aged ≥ 16 years
-
ability to give informed consent, contribute during groups and complete questionnaires in English
-
have experienced two or more seizures in past 12 months (self-reported).
Exclusion criteria:
-
only having seizures that are psychogenic or non-epileptic in origin
-
only experiencing seizure related to acute neurological illness or substance misuse
-
diagnosis of a serious psychiatric disorder or terminal illness
-
participation in other epilepsy-related research.
Informed consent
Written informed consent was obtained during a face-to-face meeting between the participant and a research worker, arranged either at their home or somewhere convenient for them. The study was explained in detail and participants were given the opportunity to ask questions. Once the consent form was signed (see Appendix 10), baseline data were collected.
Randomisation
Two to three weeks following enrolment, participants were randomised in batches of 12–24 participants per recruitment site to obtain a sufficient group size for the intervention. For each batch, participants were randomised in a 1 : 1 ratio between the SMILE (UK) group and the TAU group using fixed block sizes of two, to ensure equal number of participants in each group. Thus, the randomisation was stratified by the location of recruitment sites (KCH, University Hospital Lewisham, the NHNN, St George’s Hospital, Croydon University Hospital, Princess Royal University Hospital, Darent Valley Hospital, Guy’s and St Thomas’ Hospital).
To reduce bias, randomisation was carried out by the King’s Clinical Trials Unit at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN). The trial manager had the only access to the online randomisation request system. For each batch, participant information was entered into the system by the trial manager who then sent the request for randomisation to the Clinical Trials Unit. This ensured that randomisation was performed independently of the research or statistical teams. An e-mail was generated to the trial manager reporting the assigned group for each participant, thus the trial manager remained unblinded. A letter was posted to each participant to advise them of the group to which they had been allocated.
Blinding and protection from bias
Treatment allocation could not be kept from participants. In addition, the trial manager and administrator were unblinded as they were involved in contacting participants to attend the course. Research workers collecting data, the trial statistician and health economist, along with investigators, remained blinded. Participants were asked to not disclose their allocated group during assessments. To ensure that the blinding process worked, research workers completed a ‘Research Worker Treatment Guess’ form after the 12-month follow-up assessment or at point of withdrawal of participants leaving the study early. They stated to which group they thought the participant had been randomised and then whether this was a guess or whether they already knew. If unblinding had occurred during the course of the study, they noted how this happened. Any reporting to oversight boards was also done in a blinded manner, excepting in closed DMEC reports in which data were reported in a semi-blinded manner (i.e. groups were labelled A and B without specifying the treatment allocation).
Intervention delivery
The SMILE (UK) course was held at several locations during the trial. Locations were chosen based on being easily accessible, familiar to the patient and having access to emergency care services, if required. Originally, it was hoped that all courses would be offered at the hospital nearest the patient; however, this was not possible because of challenges with off-site room bookings and staff availability. Therefore, participants were mostly invited to attend a course at KCH. The data for course attendance are given in Chapter 5.
All participants were sent a venue map and room location by post in advance. When possible, and to facilitate group interaction, chairs were arranged in a semicircle. A flip chart was set up for writing notes and discussion points. Workbooks, pens, name badges and stickers were ready for participants on arrival and a sign-in sheet was positioned beside the workbooks.
Completion of follow-ups
Every effort was made to minimise dropouts at follow-ups, including researcher phone calls to ask whether or not the questionnaire was received and whether or not any help was needed at 6-month follow-ups, and mailing questionnaires by post and e-mail for 12-month follow-ups. On completion of the final questionnaire, participants were given a £20 store voucher. We also opted for additional procedures to improve response rates when posting questionnaires,98 such as using colour printing for the participant questionnaires.
Adverse event reporting
Information about adverse events (AEs) was collected at the 6- and 12-month follow-ups by a research worker. An AE was defined as a health-related event that was experienced by a trial participant. The AEs were self-reported by participants and any change in health was recorded. Seizures and any event related to a seizure (including hospitalisations) were not recorded, as these are expected events related to the nature of poorly controlled epilepsy. Any AEs requiring hospitalisation (unrelated to seizures) or prolongation of hospital stay, that were life-threatening, or that resulted in death or in persistent disability were recorded as serious adverse events (SAEs). Status epilepticus was considered as a SAE. SAEs were reported to the site PI and reviewed by the chief investigator. A list of AEs and SAEs was included in the reports to the trial oversight committees and to the ethics committee.
Summary of changes to project protocol
During the 6-month follow-up period, some participants were slow in returning their posted questionnaire. Similarly, during the 12-month follow-up stage, it became apparent that some participants could not commit to a meeting to complete questionnaires in person. The protocol was amended to include sending questionnaires by post or e-mail or completing them over the telephone for both follow-up time points, depending on the preference of the participant.
The nested process evaluation was to be conducted after the 12-month follow-up. During the first few interviews, it became apparent that some participants could not remember details of the course so long after they attended. Thus, the protocol was amended to have interviews within 6 months of attending the course. To ensure that there was no impact on the design of the study (i.e. the only contact with researchers was a postal contact at 6 months and face-to-face contact at 12 months), it was decided to invite only participants from the TAU group to interviews, as they would have already completed their 12-month assessment. As the interviews for the process evaluation were done by two researchers, independent from the main trial assessments, no unblinding occurred.
Outcome measures
Primary outcome measure
The primary outcome measure used in the SMILE (UK) study is the QOLIE-31-P score at 12 months post randomisation. It is a short, easy-to-use measure based on the original QOLIE-31. 80 The QOLIE-31-P varies slightly from the original QOLIE-31 with an addition of one item at the end of each subscale, asking the participant to rate the degree of ‘distress’ caused by that particular topic. The measure was completed at baseline and at 6- and 12-month follow-up (Table 4). The questionnaire provides a total QoL score and contains seven individual subscales: (1) energy level (four items), (2) emotional wellbeing (five items), (3) social functioning (five items), (4) cognitive function (six items), (5) medication effects (two items), (6) seizure worry (five items) and (7) overall QoL (two items). 80 The scale asks participants to reflect on how they felt over the past 4 weeks. Scores range from 0 to 100, for which higher rank indicates better overall QoL. Internal consistency reliability (Cronbach’s alpha) has previously been reported as α = 0.79–0.85. 80
| Outcome | Measures | Items | Time point | ||
|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | |||
| Primary outcome | QOLIE-31-P80 | 39 | ✓ | ✓ | ✓ |
| Secondary outcomes | ‘Seizure control’ (seizure frequency using two scales35,99 and last seizure date) | 2 + 1 | ✓ | ✓ | ✓ |
| ‘Impact of epilepsy’100 | 9 | ✓ | ✓ | ✓ | |
| ‘Medication adherence’ (10-item subscale from ‘Epilepsy Self-management Scale’)89 | 10 | ✓ | ✓ | ||
| ‘Medication adverse effects’ (from QOLIE-31)80 | 2 | ✓ | ✓ | ||
| Psychological distress (14-item HADS)101 | 14 | ✓ | ✓ | ||
| ‘Perceived stigma’ (from ‘Stigma of Epilepsy Scale’)102 | 3 | ✓ | ✓ | ||
| ‘Self-mastery’ over epilepsy (epilepsy-specific scale)103 | 6 | ✓ | ✓ | ||
| ‘Health Services Use’ and ‘Work Status’ in the past 12 months (modified ‘Client Service Receipt Inventory’)104 | 8 | ✓ | ✓ | ||
| ‘Health status’ (EQ-5D-5L)105 | 5 | ✓ | ✓ | ||
Secondary outcome measures
A number of measures were used to assess secondary outcomes (see Table 4).
Seizure control
Seizure control was assessed by three questions in total. Two questions were about frequency35,99 with a recall period of 6 months at 6-month follow-up, and 12 months at 12-month follow-up. A third question was on seizure recency: ‘Please tell us the date of your last seizure’ (day/month/year). All three questions were asked at baseline and both 6- and 12-month follow-up assessments.
Impact of epilepsy scale
The Impact of Epilepsy scale100 contains 10 items that assess the perceived impact of epilepsy on social relationships, occupation, personal health, standard of living and future aspirations. The scale was used at baseline and at both the 6- and the 12-month follow-up. It was purposely adapted for use during the SMILE (UK) trial (see Appendix 11), using the first 10 questions of the revised Impact of Epilepsy scale106 and the Likert-style response options from the original scale. 100 Response options included ‘a lot’ scored as 1, ‘some’ scored as 2, ‘a little’ scored as 3, and ‘not at all’ scored as 4. Reverse scoring was used when appropriate for negatively worded questions. A higher overall score on this measure indicates less perceived impact of epilepsy. Research on the revised and original scale has reported α = 0.65–0.83. 100,106
Medication adherence
Ten items about medication adherence were taken from the Epilepsy Self-Management Scale89 at baseline and 12-month follow-up. This scale asks participants to evaluate how they took their medication in the previous 6 months. The scale contains a series of statements about medication use, such as ‘I take my seizure medication the way my doctor orders it’. Response options include (1) never, (2) rarely, (3) sometimes, (4) most of the time and (5) always. After reverse scoring appropriate items, a higher overall score indicates better adherence to treatment plans or, in other words, better medication-related self-management behaviours. Internal consistency scores have been acceptable in past research, with α = 0.81–0.86. 107,108
Psychological distress
Psychological distress was measured at baseline and 12-month follow-up using the 14-item Hospital Anxiety and Depression Scale (HADS). 101 This is a commonly used measure that provides a score each for anxiety and depression, and an overall total score. The questionnaire is widely used in outpatient and inpatient settings with people affected by a range of health-care conditions. 109 Anxiety and depression scores are grouped into symptom categories: 0–7 normal, 8–10 borderline and 11–21 case. 101 Internal consistency for these scales has been reported as α = 0.82 (depression) and α = 0.83 (anxiety). 109
Perceived stigma
Perceived stigma was assessed by the 3-item Stigma of Epilepsy Scale102 at baseline and 12-month follow-up. The measure assesses felt stigma, and contains questions on the extent to which PWE believe that others may treat them as inferior, avoid them, or are uncomfortable with them because of epilepsy. 102 Response options include a four-point Likert scale: (0) ‘not at all’, (1) ‘yes, maybe’, (2) ‘yes, probably’ and (3) ‘yes, definitely’. Total scores range from 0 (does not feel stigmatised) to 1–6 (mildly to moderately stigmatised) to 7–9 (highly stigmatised). In past research, internal consistency has been reported as α = 0.82. 110
Self-mastery of epilepsy
Self-mastery over epilepsy was measured using a six-item epilepsy-specific scale. 103 The scale contains six statements such as ‘there is really no way I can solve some of the problems I have with my epilepsy’, which can be answered as (1) ‘strongly agree’, (2) ‘agree’, (3) ‘disagree’ and (4) ‘strongly disagree’. Internal consistency for this scale has previously been reported as α = 0.70. 103 This measure was administered at baseline and 12-month follow-up.
Data collection for health economics
For health economics, data on employment, hospital service use, other health service use, home help and medication were collected using the Client Service Receipt Inventory (CSRI)104 modified to include only data related to epilepsy. This questionnaire documented service use over the previous 12 months. Data were collected at baseline and 12-month follow-up.
The EuroQol-5 Dimensions, five-level version (EQ-5D-5L) was used to determine quality-adjusted life-years (QALYs). 105 This questionnaire asks one question on each of five topics: mobility, self-care, usual activity, pain/discomfort and anxiety/depression. The participants were asked to answer based on how they felt that day. The questionnaire also has a visual analogue scale (VAS) component, which asks the patient to rate their QoL on a scale of 0–100. Data were collected at baseline and 12-month follow-up.
Data management
Data were collected at baseline (pre-randomisation) and at 6 and 12 months after randomisation. Full assessments were performed at baseline and at 12 months during face-to-face meetings, when possible, while an abbreviated questionnaire was sent by post at the 6-month follow-up (see Table 4). On receiving the completed questionnaire, patients were contacted by telephone to obtain information about any AEs over the past 6 months. At 12 months, data were collected about AEs over the previous year.
Demographic and clinical data collected at enrolment included the following: initials, date of birth, gender, years since epilepsy had been diagnosed, other medical conditions, attained qualifications, living arrangements, marital status and ethnicity. The first four digits of the person’s postcode were recorded to obtain the Index of Multiple Deprivation (IMD). 111 The IMD score measures the level of deprivation based on where a person lives. The scores are distributed into quintiles where a normal distribution would have 20% in each group. Data were collected on source document worksheets (i.e. manual questionnaire forms) and transferred to an electronic case report form (eCRF) system (Infermed MACRO version 4.0; London, UK). Source document worksheets were kept in the central research office with restricted access at KCL, in locked file cabinets. Research workers entered data and the trial manager performed data entry checks; 80% of the primary outcome data were checked and 50% of secondary outcomes were assessed, except for the CSRI for which 70% of questionnaires were checked. eCRFs to check were chosen at random using a random number generator.
Participant contact information was kept on a secure central network server with access only granted to study staff. Any electronic file with patient information was password protected. Only members of the research team (trial manager, research workers and trial administrator) had passwords to the files. Information about participants’ invitations and attendance to courses was kept in separate files with access given only to the trial manager and administrator. All computers used were password protected and held in an office with restricted access.
Participant interviews were recorded on digital recorders and transferred to computers on secured networks. Recordings were sent to a transcription service and subsequently deleted. Interviews were anonymised and kept on password-protected computers. A printed set of all interviews was kept in the study office.
Compliance to intervention
For those in the intervention group, SMILE (UK) course attendance was logged on ‘treatment attendance logs’ by the trial manager or administrator present during the course. Any missed sessions were recorded. The occurrence of seizures during the course was recorded, including the length of seizure, whether the participant could continue the course or had to leave, and whether this caused a significant disturbance to the course. Carers were permitted to attend the course if this was requested by the participant. This was also noted in the treatment attendance logs. Data from logs were transferred into the eCRF by the trial manager or administrator. The eCRF database was set up such that the course attendance information was kept separate from follow-up assessments to facilitate blindness of the researchers and the trial statistician.
Non-attendance by participants at the course was recorded on course completion forms that noted the date of missed course, the reason and whether or not the participant accepted a place on an alternative date.
Facilitators
Demographic data regarding course facilitators were collected and recorded in a separate electronic database on MACRO. Data included initials, gender, current occupational role, years in current role, highest level of education reached, specialist qualifications, size of current caseload, prior experience in facilitating patient groups, and pay scale grade point. Each facilitator was given a randomised therapist identification number to ensure anonymity and this number was recorded on treatment attendance logs for each participant.
Power calculations and sample size
The analysis was planned as an intention-to-treat (ITT) analysis with two equal-sized treatment arms. The primary outcome was the total QOLIE-31-P score at 12 months post randomisation. When the study began, two drug trials at the time had used this questionnaire showing an effect size of d = 0.33112 and 0.59. 113 A sample size of 320 participants, with a 1 : 1 randomisation scheme would detect an effect size of d = 0.4 (a change of 6–7 points on the total score) with 91.3 % power, using a two-sided analysis of covariance with significance set at p ≤ 0.05. This sample size also took into consideration the variation in intervention delivery by estimating that 160 participants would attend the course, with 10 present per course and an intraclass correlation coefficient of 0.025 between QOLIE-31-P scores of different course groups. A previous study on people with severe epilepsy estimated a follow-up loss of 25% after 1 year. 12 Based on these data, we targeted a sample size of 428 (214 per treatment arm) to ensure 320 participant data at the final follow-up.
Baseline data analysis
Baseline data were checked and baseline data were locked once recruitment and randomisation were completed. Baseline data analysis included descriptive statistics of participant demographics and their baseline values of outcome measures. These are presented when appropriate as means with standard deviations (SDs), medians with 25–75% interquartile ranges (IQRs) and minimum to maximum ranges. Additional analyses were performed, not initially as part of the statistical analysis plan, but as part of the baseline data assessment. 84 These were associations between different data collected (demographics and outcome measures) evaluated using simple linear regressions. Results of the associations are represented by Pearson’s correlation coefficients (r). When comparing categorical values, predicted means of QOLIE-31-P were calculated within each factor to enable comparisons. One category within each factor was used as a reference. For example, when looking at the association between gender and QOLIE-31-P, we used the predicted means of males as the reference to which females were compared.
Trial outcome analysis
All statistical analyses were prespecified in a statistical analysis plan. 4
Primary outcome analysis
Outcome data are presented, when appropriate, as means with SD, medians with 25–75% IQR and minimum to maximum ranges. All analyses of outcome measures followed the ITT principle as we aimed to evaluate the effectiveness of the SMILE (UK) intervention. In other words, participants were analysed in the groups to which they were allocated, irrespective of intervention receipt. The primary outcome was total QOLIE-31-P score at 12 months post randomisation.
Prior to the statistician becoming unblinded, a binary variable was created to represent participants who received full intervention [in the SMILE (UK) arm only] and a second variable represented whether primary outcome data were present or missing. An independent statistician tested whether or not intervention receipt was predictive of missing primary outcome data using a chi-squared test. In addition, the relationship between baseline variables and missing primary outcome data was assessed using logistic regressions, which looked at the predictive effects of each baseline variable separately. A number of baseline variables were found to be predictive of missingness (see next paragraph) and treatment receipt also predicted missingness in the intervention arm; therefore, multiple imputation was used to produce inferences that are valid under such a missing at random data-generating process. The number (proportion) of missing QOLIE-31-P data was 4 (1.0%) at baseline, 91 (22.5%) at the 6-month follow-up and 73 (18.1%) at the 12-month follow-up.
Multiple imputation consists of two steps: an imputation step and an analysis step. As a guiding principle, all variables included in the analysis model need to be included in the imputation step; further variables can be included in the imputation step only. The first step was to impute missing values using multivariate imputation by chained equations (MICE). All predictors of dropout (gender, education, comorbidities and treatment receipt within active arm) were included to allow them to drive missingness. All variables that formed part of the analysis model were included: trial arm [SMILE (UK) course or TAU], educational group within SMILE (UK), the randomisation stratified by trial centre (the site from which participants were recruited) and baseline QOLIE-31-P. The MICE model was used to impute QOLIE-31-P at baseline, 6 and 12 months simultaneously (i.e. QoL at different time points was allowed to predict QoL missingness and can provide extra precision if predictive of QoL values). A total of 100 imputations were run. Once the multiple imputation was complete, the Stata® (StataCorp LP, College Station, TX, USA) command ‘mi estimate’ was used as a prefix so that the imputed data sets were used for the analysis (i.e. Rubin’s rules were used to generate inferences based on multiple imputed data sets). The analysis model was a linear mixed-effects model: total QOLIE-31-P score at 12 months post randomisation was the dependent variable. Baseline QOLIE-31-P score, trial centre and treatment arm were included as fixed effects and educational group was included as a random effect within the intervention arm only. The random effects were added to account for potential clustering as a result of participants attending the same educational group in the SMILE (UK) arm.
Secondary outcome analyses
There were seven secondary outcomes that were measured as continuous variables: (1) HADS-anxiety (HADS-A), (2) HADS-depression (HADS-D), (3) self-mastery and control, (4) impact of epilepsy, (5) medication adherence, (6) medication AEs, and (7) stigma of epilepsy. All of these were analysed in the same way as QOLIE-31-P (i.e. using MICE followed by linear mixed model for the respective secondary outcome variable). The only terms that were amended between each analysis were the relevant outcome measures.
There were two secondary outcomes that were not continuous variables and they had to be analysed slightly differently to reflect their distributions: (1) seizure recency and (2) seizure frequency.
Seizure recency was constructed from the date of last seizure and counts the ‘number of days since last recalled seizure’. In addition to this variable allowing for positive values only, it has a maximum. As the last recalled seizure can, by definition, involve recall bias (this was assumed at 6 months), and as the eligibility criteria for the RCT stated that participants had to have at least two seizures in the last year, seizure recency maximums were defined as follows: 548 days at baseline (1.5 years); 730 days at 6-month follow-up (2 years); and 913 days at 12-month follow-up (2.5 years). Therefore, in order to analyse seizure recency, the variable was transformed onto a continuous scale using the logit transformation. This transformation converted days into a proportion and then mapped the odds onto the continuous scale via applying the logarithm. The logit-transformed recency variable was then analysed in the same way as the other continuous variables.
Seizure frequency was collected on two different scales: Baker et al. 99 and Thapar et al. 35 The seizure frequency variable as measured by the Baker scale was analysed as a binary outcome: less than one seizure per month versus one or more seizure per month. A similar analysis approach was used as above, except MICE imputed missing outcome values by assuming a logistic regression and a logistic mixed-effects model was used as the analysis model. Similarly, seizure frequency as measured by the Thapar scale was analysed as an ordered categorical outcome: 0–3 seizures, 4–6 seizures, 7–9 seizures or ≥ 10 seizures. In addition, the MICE and mixed-effects models used ordinal logistic regression.
Intervention receipt
The primary analysis was modelled as an ITT analysis. Owing to non-receipt of the intervention [attending all four SMILE (UK) sessions] being fairly high (38.5%), a complier average causal effect (CACE) analysis was performed to assess the efficacy of the intervention in the presence of non-attendance. For this purpose, binary intervention receipt of SMILE (UK) intervention was defined as attending all sessions (i.e. receiving full treatment).
An instrumental-variables approach was used to estimate CACE for the primary outcome. Specifically, a two-stage least squares estimate of an instrumental-variables regression was used (‘ivregress 2sls’ in Stata). The estimator obtained by this approach is equivalent to that obtained by, first, regressing the treatment received variable on the treatment allocated variable and baseline covariates and, second, controlling for the saved residuals, regressing QOLIE-31-P on the treatment received variable and baseline covariates.
Statistical analysis was performed using Stata version 14.
Qualitative methods
Recruitment
Participants were approached to take part in the process evaluation. They were largely recruited from the TAU arm but also included some from the intervention arm. Participants were purposefully chosen to ensure a variety in age, gender, ethnicity and severity of epilepsy was represented.
Interview procedure
Semistructured interviews were held with topics selected after completing the external pilot study, and in collaboration with research staff that held these interviews. All participants were interviewed face to face. The interviews were undertaken by two researchers. Interview times were variable, ranging from 25 to 40 minutes each.
Analysis
All interviews were recorded and then transcribed verbatim by an external third party. Transcripts were checked for accuracy by the researchers who conducted the interviews. Sample transcripts were checked by two collaborators. Researchers then undertook a line-by-line coding approach with initial codes noted in the margins of the transcripts and then grouped into broader themes. This process involved regular discussion between the researchers and study supervisors.
Full methods are presented in Chapter 9.
Summary
As requested by our NIHR funders, we used QoL measured 12 months after randomisation as our primary outcome, with secondary variables such as seizure frequency, psychological distress and stigma. Data were collected from participants at baseline and at 6 and 12 months after randomisation. Outcome analysis followed an ITT protocol.
Chapter 4 Recruitment and intervention delivery
Introduction
Participants were recruited from eight epilepsy clinics in their hospitals in London and south-east England. The intervention was subsequently delivered to a total of 150 participants with 18 course offerings. The interventions were attended partially or fully by 74% of participants randomised into the treatment group.
Participant flow through study
Recruitment
The process of generating patient lists and sending out study information occurred from September 2013 until May 2015. At the start of trial recruitment, lists were created with the names of patients seen at eight different epilepsy clinics within the past year (≥ 4000 patients in total; Figure 2). Letters were sent to these patients explaining the study and giving them the chance of a first opt-out of further contact. During this first stage of opt-outs, 262 people sent in opt-out slips or were unable to be reached by post.
FIGURE 2.
Recruitment pathway for SMILE (UK). Reproduced with permission from Ridsdale et al. 114 2018. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/.

Recruitment sites were opened progressively through the study and recruitment overlapped between sites. For this reason, medical notes were screened by instalments for those not opting out, resulting in 2854 patient notes screened. When medical notes had been screened by clinical staff, 569 patients were identified as not eligible. A second letter was then sent to the remaining patients (n = 2285) with another option to opt out of the study. During this second stage of opt-outs, 299 patients either returned their slips or could not be reached by post.
In summary, the two opt-out stages resulted in 529 opt-out slips being received (reasons provided in Table 5). Those who had not already opted out were contacted by telephone in order to assess their eligibility and interest in the study.
| Reason | Total |
|---|---|
| Opted out | 349 |
| Unable to make time commitment | 64 |
| Complaint, confidentiality issues | 3 |
| No reason given | 150 |
| Does not need or want information, meeting others | 25 |
| Does not wish to take part | 40 |
| Already taken part in the pilot/been invited | 3 |
| Does not want King’s team to look at notes | 23 |
| Does not want to talk about diagnosis | 15 |
| Lives too far away, cannot access site | 24 |
| Other | 2 |
| Ineligible: self-exclusion via opt-out method | 180 |
| Does not meet seizure requirement | 78 |
| Language | 5 |
| Does not have epilepsy | 28 |
| Unable to complete, learning disabilities or other comorbidity (self or carer reported) | 69 |
| Excluded: medical notes screening | 569 |
| Ineligible: via telephone contact | 370 |
| Uncontactable | 528 |
Recruitment progressed in sequence at each of the centres involved with the study (Table 6). Owing to overlapping 12-month follow-ups with continuous recruitment, we were able to adjust recruitment targets in light of a higher than anticipated retention rate. Recruitment ultimately finished on 6 August 2015, with an enrolment rate of 37% (n = 407; eligible patients, n = 1088).
| Site | Date of | Number recruited (randomised) | |
|---|---|---|---|
| First patient randomised | Last patient follow-up | ||
| KCH | 5 December 2013 | 25 May 2016 | 99 (97) |
| Lewisham Hospital | 17 March 2014 | 18 February 2016 | 14 (14) |
| NHNN | 1 July 2014 | 16 May 2016 | 163 (163) |
| St George’s Hospital | 3 July 2015 | 30 June 2016 | 36 (36) |
| Croydon University Hospital | 4 June 2015 | 22 June 2016 | 17 (17) |
| Princess Royal University Hospital | 18 February 2015 | 22 April 2016 | 27 (27) |
| Darent Valley Hospital | 18 March 2015 | 28 April 2016 | 10 (10) |
| Guy’s and St Thomas’ Hospital | 20 January 2015 | 7 July 2016 | 41 (40) |
| Total | 5 December 2013 | 7 July 2016 | 407 (404) |
Enrolment
Of those contacted by research workers (n = 1458), 46.7% declined to participate in the study, 27.9% consented and 25.4% were ineligible. The process of enrolment (i.e. participant consent and baseline assessment) began in December 2013 at KCH and concluded at the beginning of August 2015 at St George’s Hospital.
Sample size
The recruitment target was 426 participants with a 12-month retention rate of 75% (i.e. effective sample size at 12 months of 320 participants). Owing to higher follow-up rates than expected (82% at the time), we could stop recruitment after 407 participants were consented into the study and 404 randomised. This decision was made with the approval of statisticians involved with the trial (SL, EJR) and agreed on by study collaborators and the TSC.
Randomisation into trial arms was carried out by the King’s Clinical Trials Unit as explained in Chapter 3. Early in the recruitment phase, the trial manager noticed an imbalance in the group allocation. This was attributable to a software error and was rectified by the Clinical Trials Unit. For this reason, the number of PWE was slightly unbalanced between the two groups (Figure 3).
FIGURE 3.
Retention through SMILE (UK) for intervention and control groups. Reproduced with permission from Ridsdale et al. 114 2018. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/.
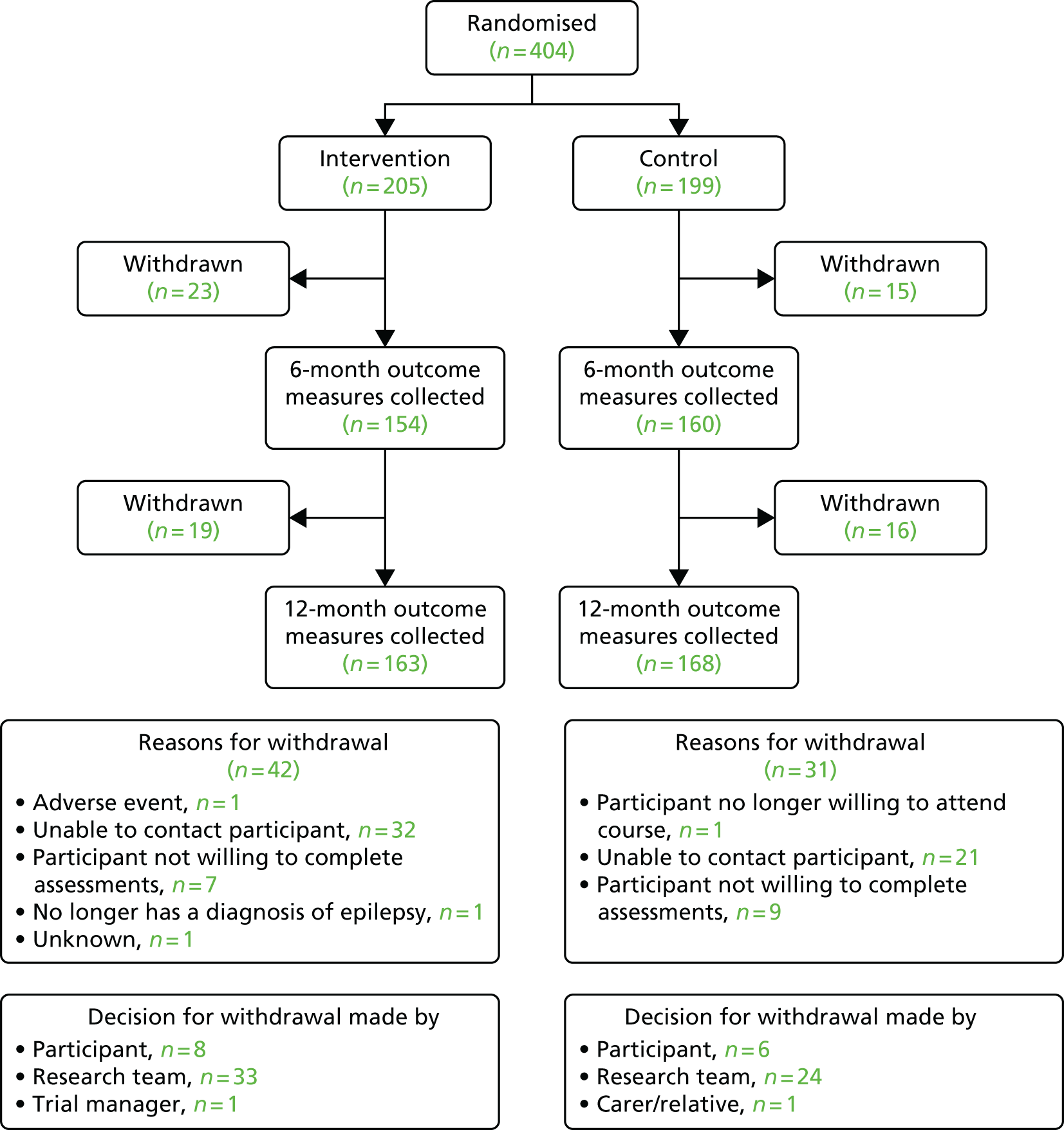
Retention and follow-up
We enrolled 404 participants into the study and 331 (82%) completed follow-up assessments at 12 months (see Figure 3). Withdrawal was defined as withdrawing from any further data collection. The most common reason for withdrawal from the trial was being unable to contact the participant. Some participants chose to withdraw as a result of time constraints, not feeling well enough to complete questionnaires or a change in personal circumstances. One participant no longer had a diagnosis of epilepsy at the 12-month follow-up and was withdrawn.
Follow-up retention rates were 77% for 6 months and 82% for 12 months. Final 6-month follow-ups were completed in February 2016. A non-response at 6 months did not lead to an automatic withdrawal from the study. Some participants who did not provide follow-up data at 6 months post randomisation subsequently provided 12-month follow-up data. Final 12-month follow-ups were completed in July 2016.
Of note, the withdrawals at 6 months were initiated by participants only. If participants could not be contacted at the 6-month follow-up, then they remained in the study and some could be reached at the 12-month follow-up. This explains why the completion rate at 12 months is higher than at 6 months.
Adverse events
The AEs were recorded at 6 months over the telephone after postal questionnaires were received and at the 12-month follow-ups. A total of 41 participants reported AEs over the course of the study. Between them, 63 AEs were reported (Table 7). Fourteen were considered mild, 21 were of moderate intensity, 26 were severe and two new psychological diagnoses were of unknown intensity. In the intervention group, 33 AEs were reported by 25 participants, while 16 participants in the control group reported 30 AEs. Twenty-two AEs were considered SAEs, but no AE or SAE was considered to be related to the intervention.
| Body system | Number of AEs (number of patients) | Related to epilepsy | Intensity | Hospital admission | Related to intervention | SAE | Outcome | Intervention group, n (%) |
|---|---|---|---|---|---|---|---|---|
| Cardiovascular | 6 (6) | None | Mild, n = 2 | 3 | None | 2 | Resolved, n = 4 | 6 (100) |
| Moderate, n = 3 | Ongoing, n = 2 | |||||||
| Severe, n = 1 | ||||||||
| Gastrointestinal | 3 (2) | None | Moderate, n = 1 | 3 | None | 1 | Resolved, n = 3 | 1 (33.3) |
| Severe, n = 2 | ||||||||
| Genitourinary | 8 (5) | None | Moderate, n = 2 | 7 | None | 5 | Resolved, n = 8 | 2 (25) |
| Severe, n = 6 | ||||||||
| Haematological | 4 (4) | None | Mild, n = 1 | 2 | None | 1 | Resolved, n = 3 | 3 (75) |
| Moderate, n = 3 | Ongoing, n = 1 | |||||||
| Musculoskeletal | 12 (10) | 2 | Mild, n = 4 | 6 | None | 2 | Resolved, n = 7 | 7 (58.3) |
| Moderate, n = 3 | Ongoing, n = 5 | |||||||
| Severe, n = 5 | ||||||||
| Neoplasia | 2 (2) | None | Moderate, n = 2 | 2 | None | 2 | Resolved, n = 1 | 1 (50) |
| Ongoing, n = 1 | ||||||||
| Neurological | 13 (11) | 7 | Mild, n = 3 | 8 | None | 4 | Resolved, n = 7 | 8 (61.5) |
| Moderate, n = 3 | With sequelae, n = 2 | |||||||
| Severe, n = 7 | Ongoing, n = 4 | |||||||
| Psychological | 6 (4) | 3 | Mild, n = 1 | 3 | None | 3 | Resolved, n = 3 | 1 (16.7) |
| Severe, n = 3 | With sequelae, n = 1 | |||||||
| Unknown, n = 2 | Ongoing, n = 2 | |||||||
| Dermatological | 1 (1) | 1 | Severe, n = 1 | 1 | None | 0 | Ongoing, n = 1 | 0 (0) |
| Ear, nose and throat | 6 (4) | None | Mild, n = 3 | 3 | None | 2 | Resolved, n = 3 | 3 (50) |
| Moderate, n = 3 | With sequelae, n = 2 | |||||||
| Ongoing, n = 1 | ||||||||
| Other | 2 (2) | None | Moderate, n = 1 | 1 | None | 0 | Resolved, n = 1 | 1 (50) |
| Severe, n = 1 | With sequelae, n = 1 |
Contamination
Three participants in the control group attended the intervention early, rather than after the 12-month follow-up. Two were invited in error and one came to the course without having been invited by the research team. These participants were analysed in their original TAU group in order to adhere to the ITT principle. They are not included in the course attendance numbers below.
Researcher unblinding
As participants knew their treatment group, there was a high risk of research workers becoming unblinded during follow-up assessments. Following every 12-month follow-up or withdrawal, researchers completed the ‘Research Worker Treatment Guess’ form. For the 331 participants completing the 12-month follow-up, research workers reported being unblinded for 56 assessments (16.9%). When the blinding remained, most guesses were that the participant was in the TAU group (73.7%) and this was mostly a random guess (random guess 73.7% vs. educated guess 9.4%).
SMILE (UK) intervention delivery
Course attendance
Eighteen SMILE (UK) intervention courses were held between December 2013 and August 2015 (Table 8). The majority were held at KCL (15 courses), which is a university-based research centre next to a teaching hospital (KCH) in south London. The remainder were held at the NHNN (two courses) and the University Hospital Lewisham (one course).
| Course number | Date | Location | Facilitators | Attendance | |||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | ||||||
| a.m. | p.m. | a.m. | p.m. | ||||
| 1 | December 2013 | KCH, WEC | Two EEG technicians | 6 | 6 | 6 | 6 |
| 2 | January 2014 | KCH, IoPPN | ENS, EEG technician | 13 | 13 | 12 | 12 |
| 3 | January 2014 | KCH, WEC | ENS, EEG technician | 6 | 7 | 8 | 8 |
| 4 | February 2014 | KCH, WEC | ENS, EEG technician | 9 | 9 | 7 | 7 |
| 5 | March 2014 | LEW | ENS, EEG technician | 5 | 5 | 5 | 5 |
| 6 | July 2014 | NHNN, QS | ENS, EEG technician | 9 | 7 | 7 | 6 |
| 7 | August 2014 | NHNN, QS | ENS, EEG technician | 12 | 11 | 12 | 11 |
| 8 | September 2014 | KCH, WEC | ENS, EEG technician | 14 | 13 | 13 | 12 |
| 9 | October 2014 | KCH, WEC | Chief investigator, EEG technician | 9 | 9 | 9 | 9 |
| 10 | November 2014 | KCH, WEC | ENS, EEG technician | 9 | 9 | 8 | 7 |
| 11 | December 2014 | KCH, WEC | ENS, EEG technician | 6 | 6 | 6 | 6 |
| 12 | January 2015 | KCH, WEC | ENS, EEG technician | 7 | 7 | 6 | 6 |
| 13 | February 2015 | KCH, WEC | ENS, EEG technician | 8 | 8 | 8 | 7 |
| 14 | March 2015 | KCH, WEC | ENS, EEG technician | 8 | 8 | 7 | 7 |
| 15 | April 2015 | KCH, WEC | ENS, EEG technician | 9 | 8 | 9 | 8 |
| 16 | June 2015 | KCH, WEC | ENS, EEG technician | 6 | 6 | 6 | 6 |
| 17 | July 2015 | KCH, WEC | ENS, EEG technician | 10 | 8 | 9 | 9 |
| 18 | August 2015 | KCH, WEC | ENS, EEG technician | 6 | 7 | 6 | 6 |
Course attendance was recorded to determine receipt of intervention. To do this, we divided the course into four sessions, and a session was defined as a half-day. Of the 205 participants who were randomised to the intervention arm, 74% (n = 151) attended at least one session and the majority [62% (n = 126)] completed all four sessions of the course (Table 9). Participants were invited up to three times to attend a course. Overall, 80 reasons for missing sessions were given by 79 participants who did not attend all four sessions. Reasons for non-attendance were typically as a result of feeling ill or prior work commitments (Table 10). We hypothesised that often lengthy travel to the venue and course length may also have been factors in course attendance rates. Seizures occurring during the course were recorded. There were six observed seizures throughout the study period lasting between 2 and 20 minutes. Of these, three were instances of non-epileptic seizures occurring in two participants, which were observed and documented by the ENS facilitating the course. One participant had been aware of having these seizures in the past. It was possible to document these instances of non-epileptic seizures as a result of the extensive experience of the ENS facilitators (> 15 years each).
| Number of sessions completed | Number of participants, n (%) |
|---|---|
| 0 | 54 (26.3) |
| 1 | 4 (2.0) |
| 2 | 7 (3.4) |
| 3 | 14 (6.8) |
| 4 | 126 (61.5) |
| Reason | Number of participants (n = 79) |
|---|---|
| Felt unwell | 18 |
| Work commitments | 10 |
| Family commitments | 3 |
| Could not travel unaccompanied | 4 |
| Other | 22 |
| Unknown | 23 |
Effect of intervention receipt on retention
We assessed whether or not attending the SMILE (UK) course was associated with retention in the intervention group (Table 11). Full receipt of the intervention was defined as having attended both days of the SMILE (UK) course/four sessions (morning and afternoon of both days). Not having received the full intervention was defined as having attended 0–3 sessions. Extent of receipt of the intervention was associated with the likelihood of remaining in the study: 120 out of the 126 participants (95.2%) who received the full intervention completed the 12-month assessment, compared with 43 of the 79 participants (54.4%) who did not receive the full intervention. This informed the need to use multiple imputation in the inferential analysis of outcome measures.
| Intervention receipt (N = 205) | Withdrawal from the trial, n (%) | Trial completed, n (%) |
|---|---|---|
| Full intervention received (4 sessions) | 6 (4.8) | 120 (95.2) |
| Received 0–3 intervention sessions | 36 (45.6) | 43 (54.4) |
| Total | 42 (20.5) | 163 (79.5) |
Summary
Participant recruitment resulted in 404 participants being enrolled into the SMILE (UK) trial. For the intervention group, 18 courses were delivered with 126 out of 205 participants attending all four sessions. Reasons for not attending included not feeling well or having work/family commitments. AEs occurring during the study period were not related to the intervention. A large proportion of participants could not be contacted at the 12-month follow-up. Despite this, 82% completed the 12-month follow-ups, which permitted adequately powered analyses.
Chapter 5 Analysis of baseline data
Introduction
We undertook an analysis of baseline data collected at enrolment for 404 PWE enrolled in SMILE (UK). Having data on such a large group of people with poorly controlled epilepsy offered a unique opportunity to describe the characteristics of this group of patients. Because our measure of QoL, QOLIE-31-P, is still not widely used, we looked at the subdomains of the scale and how they are interassociated. In addition, we assessed which of our secondary outcome measures were associated with QOLIE-31-P.
Results
Study sample characteristics
Baseline demographics of the study group are presented in Table 12. Overall, the group had an average age of 41.7 years, ranging from 16 to 85 years. There was a slightly higher proportion of females taking part (54.2%) and the majority of participants were of white ethnicity (75.2%). The IMD score, measuring levels of deprivation according to participants’ postcodes,111 indicated that the majority of our group (60.7%) lived in areas of high deprivation. The group was highly educated, with approximately 50% having post-secondary level qualifications [beyond General Certificate of Secondary Education (GCSE) level]. Despite the high level of education, only 21.8% of the group were employed full time, 13.1% were employed part time and 6.9% were self-employed. To better evaluate the proportion of participants not in work, we grouped the employment category into a binary factor looking at participants aged < 65 years. This showed that half of the group of working age were not employed. Roughly three-quarters of the group lived with others and about 40% were with a partner.
| Variable | Treatment group | Total (N = 404) | |
|---|---|---|---|
| Intervention (N = 205) | Control (N = 199) | ||
| Age (years), mean (SD) [range] | 42.5 (14.3) [16–85] | 40.8 (14.0) [17–82] | 41.7 (14.1) [16–85] |
| Age group (years), n (%) | |||
| 16–25 | 31 (15.1) | 26 (13.1) | 57 (14.1) |
| 26–35 | 40 (19.5) | 58 (29.1) | 98 (24.3) |
| 36–45 | 50 (24.4) | 45 (22.6) | 95 (23.5) |
| 46–55 | 45 (22.0) | 42 (21.1) | 87 (21.5) |
| 56–65 | 27 (13.2) | 19 (9.5) | 46 (11.4) |
| > 65 | 12 (5.9) | 9 (4.5) | 21 (5.2) |
| Gender, n (%) | |||
| Female | 115 (56.1) | 104 (52.3) | 219 (54.2) |
| Male | 90 (43.9) | 95 (47.7) | 185 (45.8) |
| Ethnicity, n (%) | |||
| White | 160 (78.0) | 144 (72.4) | 304 (75.2) |
| Mixed/multiple ethnic groups | 19 (9.3) | 21 (10.6) | 40 (9.9) |
| Black/African/Caribbean/black British | 16 (7.8) | 17 (8.5) | 33 (8.2) |
| Asian/Asian British | 7 (3.4) | 11 (5.5) | 18 (4.5) |
| Other | 3 (1.5) | 6 (3.0) | 9 (2.2) |
| IMD score, mean (SD) [range] | 25.4 (12.8) [3–60] | 26.3 (13.4) [1–59] | 25.8 (13.1) [1–60] |
| IMD quintiles, n (%) | |||
| 1 (least deprived) | 20 (9.8) | 19 (9.5) | 39 (9.7) |
| 2 | 27 (13.2) | 29 (14.6) | 56 (13.9) |
| 3 | 33 (16.1) | 31 (15.6) | 64 (15.8) |
| 4 | 70 (34.1) | 66 (33.2) | 136 (33.7) |
| 5 (most deprived) | 55 (26.8) | 54 (27.1) | 109 (27.0) |
| Highest level of education, n (%) | |||
| No formal qualifications | 31 (15.1) | 30 (15.1) | 61 (15.1) |
| Secondary level | 69 (33.7) | 62 (31.2) | 131 (32.4) |
| Further education (post secondary) | 44 (21.5) | 41 (20.6) | 85 (21.0) |
| Higher education (bachelor’s degree and higher) | 61 (29.8) | 66 (33.2) | 127 (31.4) |
| Living arrangements, n (%) | |||
| Household, living with others | 159 (77.6) | 146 (73.4) | 305 (75.5) |
| Living alone | 43 (21.0) | 52 (26.1) | 95 (23.5) |
| Other arrangements | 3 (1.5) | 1 (0.5) | 4 (1.0) |
| Marital status, n (%) | |||
| Single | 81 (39.7) | 95 (48.0) | 176 (43.8) |
| Steady relationship not cohabiting | 23 (11.3) | 21 (10.6) | 44 (10.9) |
| Married/living with partner | 83 (40.7) | 70 (35.4) | 153 (38.1) |
| Divorced/widowed | 17 (8.3) | 12 (6.1) | 29 (7.2) |
| Employment, n (%) | |||
| Employed full time | 42 (20.5) | 46 (23.1) | 88 (21.8) |
| Employed part time | 22 (10.7) | 31 (15.6) | 53 (13.1) |
| Unemployed | 94 (45.9) | 83 (41.7) | 177 (43.8) |
| Self-employed | 15 (7.3) | 13 (6.5) | 28 (6.9) |
| Retired (because of age) | 9 (4.4) | 8 (4.0) | 17 (4.2) |
| Retired (because of health) | 5 (2.4) | 3 (1.5) | 8 (2.0) |
| Student | 13 (6.3) | 15 (7.5) | 28 (6.9) |
| Housewife/husband | 5 (2.4) | 0 | 5 (1.2) |
| Employment for those aged < 65 years (binary), n (%) | |||
| Not employed | 102 (53.1) | 86 (45.3) | 188 (49.2) |
| Employed or student | 90 (46.9) | 104 (54.7) | 194 (50.8) |
| Clinical details | |||
| Years since epilepsy first diagnosed, median (IQR) [range] | 20 (8–32) [1–66] | 18 (8–32) [1–64] | 18 (8–32) [1–66] |
| Comorbidity, n (%) | |||
| None | 101 (49.3) | 118 (59.3) | 219 (54.2) |
| Another medical condition | 71 (34.6) | 61 (30.7) | 132 (32.7) |
| Psychiatric condition | 10 (4.9) | 10 (5.0) | 20 (5.0) |
| Both medical and psychiatric | 23 (11.2) | 10 (5.0) | 33 (8.2) |
Clinical details at baseline (see Table 12) showed a median 18 years since epilepsy diagnosis. Almost half of the sample reported experiencing a comorbid health condition (45.9%), including 13.2% with a current mental health diagnosis (e.g. depression, anxiety).
Associations with quality of life in people with poorly controlled epilepsy
In order to better understand the primary outcome measure, QOLIE-31-P, we undertook an in-depth analysis of the measure using values obtained at baseline. This was an additional post hoc analysis not specified in the statistical analysis plan. 84 We looked at the measure itself with its different domains, as well as what secondary outcomes are associated with QOLIE-31-P scores.
Evaluation of Quality Of Life In Epilepsy 31-P and subdomains
We found strong pairwise correlations (using Pearson’s r) between QOLIE-31-P subscales and the total score (0.63–0.71), suggesting that each subscale has a reasonable, but not overly strong, association with the total score. Higher scores on the QOLIE-31-P reflect better QoL. Correlations between subdomains were lower, indicating that each scale is measuring a sufficiently different topic in this sample (Table 13).
| Subscales | Total | Subscale | |||||
|---|---|---|---|---|---|---|---|
| Energy | Mood | Daily activity | Cognition | Medication effects | Seizure worry | ||
| Energy | 0.68 | ||||||
| Mood | 0.67 | 0.53 | |||||
| Daily activity | 0.71 | 0.42 | 0.39 | ||||
| Cognition | 0.68 | 0.44 | 0.44 | 0.43 | |||
| Medication effects | 0.68 | 0.33 | 0.27 | 0.47 | 0.38 | ||
| Seizure worry | 0.63 | 0.34 | 0.40 | 0.44 | 0.40 | 0.43 | |
| Overall QoL | 0.67 | 0.55 | 0.65 | 0.43 | 0.44 | 0.25 | 0.38 |
We then looked specifically at the relationship between QOLIE-31-P subscales and the HADS measure (Table 14). As shown in Table 14, mood and seizure anxiety were most strongly negatively associated with the anxiety subscale (HADS-A). Mood, energy and daily activity scores were most strongly negatively associated with the depression subscale (HADS-D). This finding suggests that greater psychological distress is associated with diminished QoL status.
| QOLIE-31-P and subscales | HADS | |
|---|---|---|
| HADS-A r | HADS-D r | |
| QOLIE-31-P scale (n = 400) | –0.63 | –0.66 |
| Subscale | ||
| Energy (n = 402) | –0.46 | –0.57 |
| Mood (n = 402) | –0.67 | –0.60 |
| Daily activity (n = 400) | –0.40 | –0.51 |
| Cognition (n = 402) | –0.42 | –0.46 |
| Medication effects (n = 399) | –0.35 | –0.37 |
| Seizure worry (n = 401) | –0.51 | –0.35 |
| Overall QoL (n = 400) | –0.45 | –0.56 |
Associations between variables
As shown in Table 15, total QOLIE-31-P baseline scores were associated with a number of other variables. Females scored lower on the QOLIE-31-P measure than males. Lower education level and unemployment were also associated with reduced QOLIE-31-P scores.
| Baseline characteristics (categorical) | Predicted mean (95% CI) | Coefficient (95% CI) | p-value |
|---|---|---|---|
| Gender (n = 400) | |||
| Male (reference) | 68.2 (66.2 to 70.3) | – | 0.0043 |
| Female | 64.2 (62.3 to 66.1) | –4.1 (–6.8 to –1.3) | |
| Highest level of education (n = 400) | |||
| Higher education (reference) | 68.3 (65.9 to 70.8) | – | 0.0096 |
| Further education | 67.8 (64.8 to 70.8) | –0.5 (–4.4 to 3.4) | |
| Secondary | 64.6 (62.2 to 67.1) | –3.7 (–7.1 to –0.2) | |
| No formal qualifications | 61.8 (58.2 to 65.3) | –6.6 (–10.9 to –2.2) | |
| Employment (≤ 64 years) (n = 379) | |||
| Employed or student (reference) | 69.5 (67.5 to 71.4) | – | < 0.001 |
| Not employed | 62.0 (60.0 to 64.0) | –7.5 (–10.3 to –4.7) | |
| Years with epilepsy diagnosis (n = 403) | |||
| 32 years | 67.1 (65.4 to 68.8) | 0.1 (0.01 to 0.2) | 0.037 |
| 18 years | 65.7 (64.3 to 67.2) | ||
| 8 years | 64.8 (62.9 to 66.6) | ||
| 1 year | 64.1 (61.8 to 66.4) | ||
| Comorbid condition (n = 400) | |||
| None (reference) | 68.5 (66.6 to 70.3) | – | < 0.001 |
| Another medical condition | 65.0 (62.7 to 67.4) | –3.4 (–6.4 to –0.4) | |
| Psychiatric condition | 61.5 (55.4 to 67.6) | –7.0 (–13.4 to –6.4) | |
| Both medical and psychiatric conditions | 56.8 (52.1 to 61.6) | –11.6 (–16.7 to –6.6) | |
| Seizure frequency in previous year (n = 400) | |||
| 1–3 times (reference) | 73.6 (69.7 to 77.5) | – | < 0.001 |
| 4–6 times | 68.8 (64.9 to 72.6) | –4.8 (–10.3 to 0.7) | |
| 7–9 times | 69.3 (63.7 to 74.8) | –4.3 (–11.1 to 2.5) | |
| ≥ 10 times | 64.0 (62.3 to 65.6) | –9.6 (–13.9 to –5.4) | |
| HADS-D (n = 399) | |||
| Normal (reference) | 70.8 (69.4 to 72.1) | – | < 0.001 |
| Borderline | 58.1 (55.4 to 60.8) | –12.7 (–15.7 to –9.6) | |
| Case | 47.2 (43.6 to 50.7) | –23.6 (–27.4 to –19.8) | |
| HADS-A (n = 399) | |||
| Normal (reference) | 74.4 (72.7 to 76.1) | – | < 0.001 |
| Borderline | 63.7 (61.1 to 66.3) | –10.7 (–13.8 to –7.6) | |
| Case | 56.0 (54.1 to 58.0) | –18.4 (–21.0 to –15.8) | |
| Stigma of epilepsy (n = 397) | |||
| Not stigmatised (reference) | 71.6 (69.4 to 73.8) | – | < 0.001 |
| Mild to moderate | 63.9 (62.1 to 65.8) | –7.7 (–10.6 to –4.8) | |
| Highly stigmatised | 58.9 (55.1 to 62.6) | –12.8 (–17.1 to –8.4) | |
| Self-mastery of epilepsy scale (n = 396) | |||
| 16 self-mastery score | 70.0 (68.6 to 71.4) | 2.1 (1.7 to 2.5) | < 0.001 |
| 14 self-mastery score | 65.9 (64.7 to 67.1) | ||
| 12 self-mastery score | 61.7 (60.3 to 63.1) | ||
| 6 self-mastery score | 49.2 (46.0 to 52.4) | ||
| Medication adherence scale (n = 399) | |||
| 48 medication adherence score | 66.8 (65.2 to 68.3) | 0.3 (0.05 to 0.6) | 0.023 |
| 46 medication adherence score | 66.1 (64.7 to 67.5) | ||
| 43 medication adherence score | 65.1 (63.5 to 66.6) | ||
| 16 medication adherence score | 55.9 (47.3 to 64.6) | ||
A more recent epilepsy diagnosis (i.e. within past year prior to recruitment) and high seizure frequency (≥ 10 seizures in the previous year) were strongly associated with lower QOLIE-31-P scores. Any comorbidity, especially psychiatric or both psychiatric and medical, was also associated with low QOLIE-31-P scores (see Table 15).
An association was found between reduced QoL and both HADS-A and HADS-D scores. Depression ‘caseness’ was more associated with lower QOLIE-31-P scores (predicted mean 47.2) than was anxiety ‘caseness’ (predicted mean 56.0). We found a strong association between total QOLIE-31-P and both HADS scores (anxiety –0.63, depression –0.66; Figure 4).
FIGURE 4.
Correlational analysis with quality of life (QOLIE-31-P) and secondary outcome measures. (a) Anxiety (HADS-A, r = 0.63, p < 0.001, n = 400); (b) depression (HADS-D, r = 0.66, p < 0.001, n = 400); and (c) self-management (self-mastery subscale, r = 0.49, p < 0.001, n = 399). The green line displays the ‘line of best fit’ using a simple regression analysis. 84
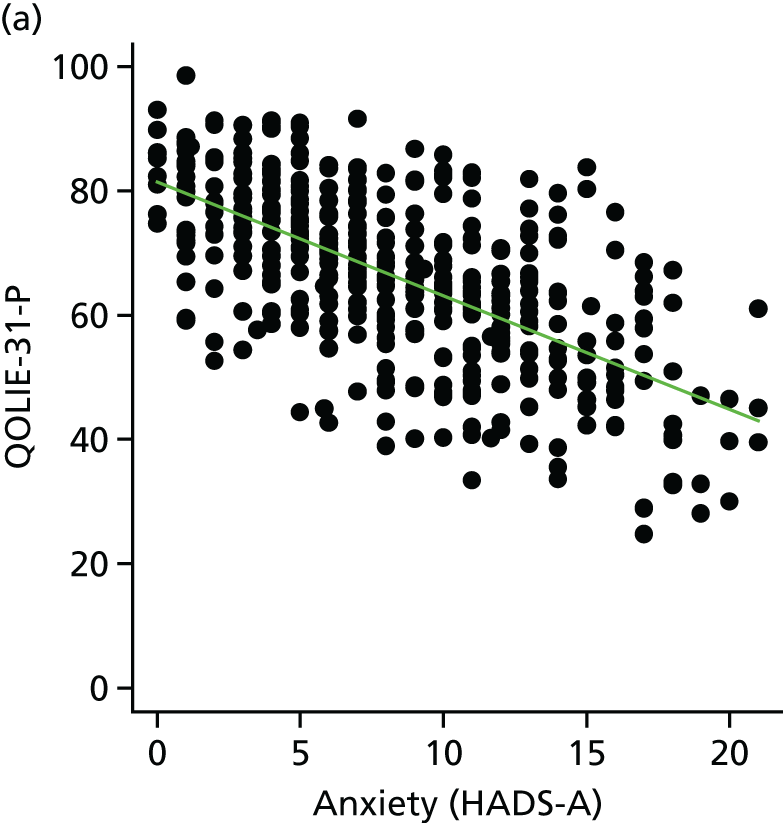
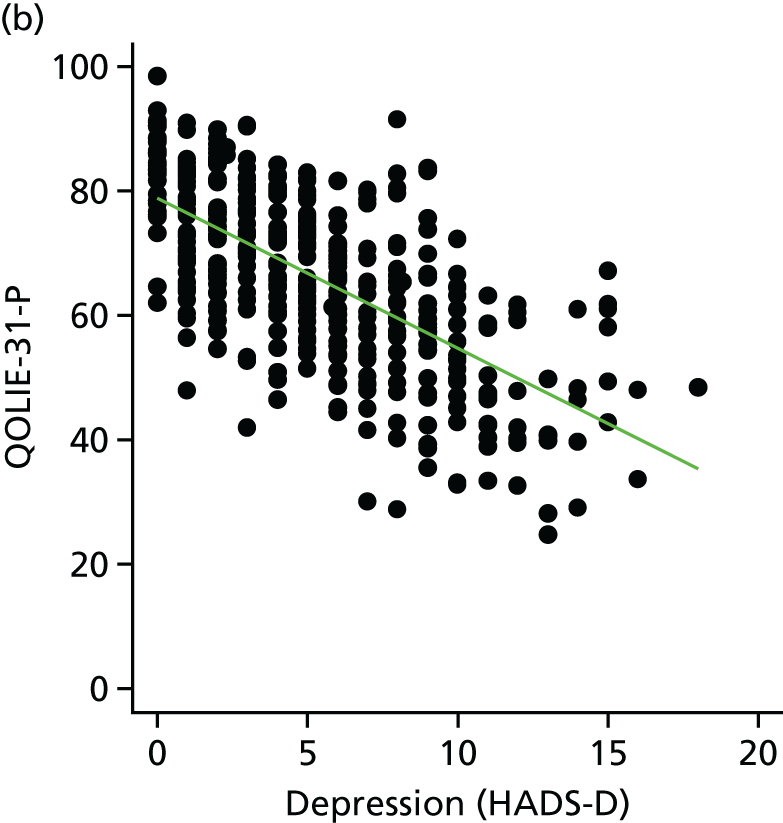
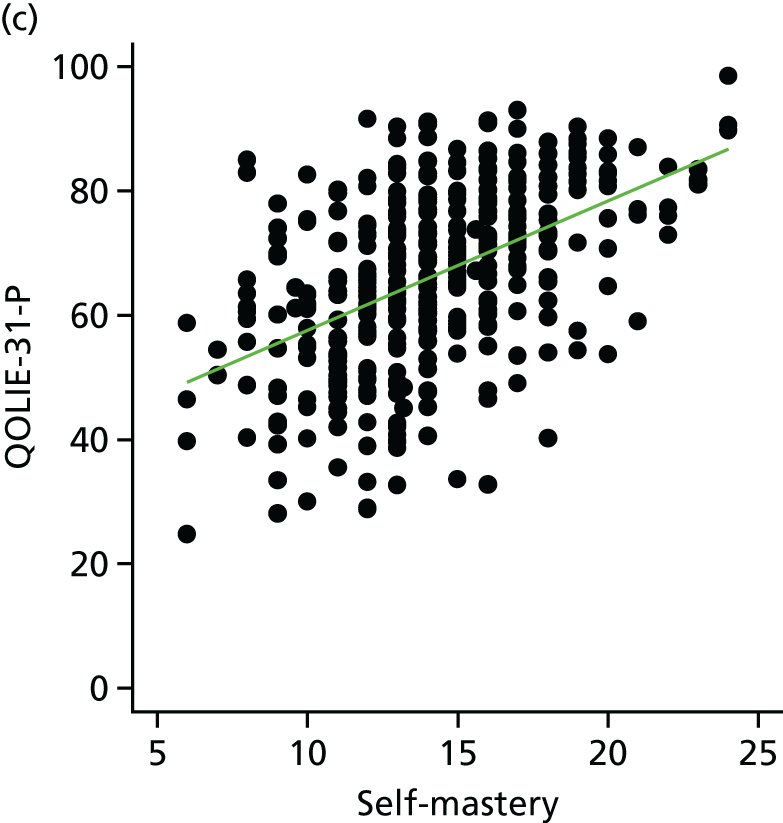
As shown in Table 15 and Figure 4, low total QOLIE-31-P was associated with low self-mastery beliefs and less medication adherence, reported using quartiles of the self-mastery and medication adherence scores. Low QoL was also associated with high levels of felt stigma.
The variables that did not show any association with QOLIE-31-P scores in the analysis were age, ethnicity, household living arrangements, marital status and IMD scores.
Summary
At baseline, our group with poorly controlled epilepsy had had active epilepsy (69% had ≥ 10 seizures in the past year) for a long time (median 18 years). There was a high level of unemployment and more than half of the participants lived in areas of high deprivation. The participants were highly educated, with about 50% having had a post-secondary education.
The various subdomains of the QOLIE-31-P scale contribute similarly to the total score. Subdomains correlated with each other but did not have high correlation coefficients, suggesting that they measure different factors of QoL.
Many of the collected participant characteristics were associated with lower QoL, for example being female, having lower qualifications, not being in employment, having a more recent diagnosis of epilepsy and having a comorbidity (especially a diagnosed psychiatric condition). Our secondary outcome measures were also associated with QOLIE-31-P. These were, in order of most associated, HADS-D, HADS-A, self-stigma, seizure frequency, self-mastery and medication adherence.
Chapter 6 Outcomes of the randomised controlled trial
Introduction
Participants completed assessments with primary and secondary outcome measures at the baseline, 6- and 12-month follow-ups. This chapter outlines results of the RCT. 114
Trial outcomes
Primary outcome: quality of life
The primary outcome measure was QOLIE-31-P measured at 12 months after randomisation to the study. This measure was also reported at 6 months post randomisation. The observed QoL outcomes are summarised in Table 16. This table shows that the two domains with consistently the lowest scores across all time points were energy and cognition. The highest scored domains were mood, daily activity and medication effects. As many published studies115–117 use QOLIE-31 without the patient-specific weighting, we calculated the total score according to the QOLIE-31 specifications for comparative purposes. These scores are presented in Table 16. Similarly to QOLIE-31-P, the observed total scores were slightly higher after 1 year in both groups. The total QOLIE-31 score improved in the intervention group by 2.7 and in the control group by 2.5 at 12 months.
| QOLIE-31 subdomain | Time point, mean (SD) [range] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | |||||||
| SMILE (UK) (n = 203) | TAU (n = 197) | Total (n = 401) | SMILE (UK) (n = 153) | TAU (n = 160) | Total (n = 313) | SMILE (UK) (n = 163) | TAU (n = 168) | Total (n = 331) | |
| Energy | 52.1 (18.7) [16.7–91.7] | 54.7 (17.4) [16.7–100.0] | 53.4 (18.1) [16.7–100.0] | 54.8 (16.6) [16.7–100.0] | 55.5 (17.3) [16.7–100.0] | 55.1 (16.9) [16.7–100.0] | 53.3 (18.4) [16.7–95.8] | 55.8 (18.0) [16.7–100.0] | 54.6 (18.2) [16.7–100.0] |
| Mood | 65.7 (18.5) [16.7–100.0] | 68.8 (16.6) [23.3–100.0] | 67.2 (17.6) [16.7–100.0] | 66.9 (17.0) [20.0–100.0] | 66.6 (16.2) [20.0–100.0] | 66.7 (16.5) [20.0–100.0] | 67.0 (17.1) [16.7–100.0] | 69.6 (17.3) [16.7–100.0] | 68.3 (17.2) [16.7–100.0] |
| Daily activity | 64.3 (23.0) [19.3–100.0] | 66.0 (24.0) [19.3–100.0] | 65.2 (23.5) [19.3–100.0] | 67.8 (20.5) [19.3–100.0] | 66.5 (22.0) [19.3–100.0] | 67.1 (21.2) [19.3–100.0] | 69.9 (20.5) [19.3–100.0] | 71.1 (23.8) [19.3–100.0] | 70.5 (22.2) [19.3–100.0] |
| Cognition | 57.8 (24.1) [18.6–100.0] | 60.6 (23.0) [18.6–100.0] | 59.2 (23.6) [18.6–100.0] | 57.9 (22.0) [18.6–100.0] | 60.3 (19.9) [18.6–100.0] | 59.1 (20.9) [18.6–100.0] | 59.4 (23.7) [18.6–100.0] | 62.9 (22.7) [18.6–100.0] | 61.2 (23.2) [18.6–100.0] |
| Medication effects | 67.9 (23.4) [21.7–100.0] | 67.5 (24.3) [21.7–100.0] | 67.7 (23.8) [21.7–100.0] | 66.2 (21.7) [21.7–100.0] | 66.5 (23.3) [21.7–100.0] | 66.3 (22.5) [21.7–100.0] | 69.2 (21.5) [21.7–100.0] | 71.8 (21.5) [21.7–100.0] | 70.5 (21.5) [21.7–100.0] |
| Seizure worry | 60.7 (21.9) [24.0–100.0] | 63.0 (21.5) [24.0–100.0] | 61.8 (21.7) [24.0–100.0] | 60.6 (20.7) [24.0–100.0] | 61.3 (21.5) [24.0–100.0] | 61.0 (21.1) [24.0–100.0] | 67.2 (21.0) [24.0–100.0] | 69.2 (22.4) [24.0–100.0] | 68.2 (21.7) [24.0–100.0] |
| Overall QoL | 62.2 (18.0) [10.0–100.0] | 63.0 (18.4) [10.0–100.0] | 62.6 (18.2) [10.0–100.0] | 63.6 (16.7) [15.0–100.0] | 62.2 (17.3) [10.0–95.0] | 62.9 (17.0) [10.0–100.0] | 64.3 (18.0) [15.0–100.0] | 63.5 (18.5) [10.0–100.0] | 63.9 (18.3) [10.0–100.0] |
| Total (non-P) | 60.9 (15.6) [24.5–93.7] | 63.1 (15.7) [24.7–97.6] | 62.0 (15.6) [24.5–97.6] | 62.3 (13.8) [33.3–95.8] | 62.8 (14.6) [26.3–94.8] | 62.6 (14.2) [26.3–95.8] | 63.6 (15.2) [28.0–95.8] | 65.6 (16.1) [26.4–95.2] | 64.6 (15.7) [26.4–95.8] |
| Total | 65.2 (14.1) [30.0–91.6] | 66.9 (14.2) [24.8–98.5] | 66.0 (14.2) [24.8–98.5] | 66.3 (13.0) [34.2–92.8] | 65.5 (14.0) [27.3–94.7] | 65.9 (13.5) [27.3–94.7] | 67.4 (13.5) [25.1–95.4] | 69.5 (14.8) [26.4–93.8] | 68.5 (14.2) [25.1–95.4] |
Importantly, the post-randomisation QoL scores in all domains were similar in the two trial arms (see Table 16). We found no statistically significant differences between the trial arms at 6 months (p = 0.195) or 12 months (p = 0.564) (Table 17).
| Outcome | Follow-up time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| Treatment effect | 95% CI | p-value | Treatment effect | 95% CI | p-value | |
| QOLIE-31-P | 1.70 | –0.87 to 4.26 | p = 0.195 | –0.72 | –3.19 to 1.74 | p = 0.564a |
| Impact of epilepsy | –0.35 | –1.83 to 1.13 | p = 0.640 | –0.96 | –2.30 to 0.38 | p = 0.159 |
| Medication adherence | – | – | – | –0.02 | –0.74 to 0.71 | p = 0.964 |
| Medication AEs | 0.03 | –0.56 to 0.62 | p = 0.923 | –0.41 | –0.97 to 0.15 | p = 0.151 |
| HADS-A | – | – | – | –0.04 | –0.83 to 0.74 | p = 0.917 |
| HADS-D | – | – | – | –0.33 | –1.14 to 0.49 | p = 0.432 |
| Stigma of epilepsy | – | – | – | 0.14 | –0.39 to 0.66 | p = 0.606 |
| Self-mastery and control | – | – | – | –0.16 | –0.94 to 0.62 | p = 0.687 |
| Seizure frequency (Baker et al.99) | – | – | – | –0.02b | –0.63 to 0.58 | p = 0.939 |
| Seizure frequency (Thapar et al.35) | – | – | – | 0.11b | –0.43 to 0.65 | p = 0.691 |
| Seizure recency (days since last seizure) | –0.19c | –0.60 to 0.22 | p = 0.371 | –0.31c | –0.70 to 0.09 | p = 0.129 |
In total, 129 (32%) participants enrolled in the study received full treatment by attending all four SMILE (UK) course sessions. This consisted of 126 randomised participants who were allocated to SMILE (UK) and three participants who were allocated to TAU alone. The CACE efficacy estimate was –1.28 (p = 0.528), which was in the same direction as the ITT effectiveness estimate of –0.72 (p = 0.564). Neither result was statistically significant.
Secondary outcomes
Seizure control
Overall, at baseline (Table 18) 69.3% of the group experienced ≥ 10 seizures in the previous 12 months (Thapar scale) and 72.2% had one or more seizures per month (Baker scale). As intended, the study group enrolled in the SMILE (UK) trial had highly active epilepsy despite being prescribed AEDs. The percentage of participants experiencing such high levels of seizures according to the Thapar scale remained fairly constant over the three study assessments. Thus, there were no statistically significant differences between trial arms in the Thapar scale at either 6 or 12 months (p = 0.691) (see Table 17).
| Outcome measure | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | |||||||
| SMILE (UK) (N = 205) | TAU (N = 199) | Total (N = 404) | SMILE (UK) (N = 154) | TAU (N = 160) | Total (N = 314) | SMILE (UK) (N = 163) | TAU (N = 168) | Total (N = 331) | |
| Seizure frequency in last 12 months (Thapar et al.35), n (%) | n = 205 | n = 199 | n = 404 | n = 161 | n = 168 | n = 329 | |||
| None | – | – | – | 7 (4.3) | 17 (10.1) | 24 (7.3) | |||
| 1–3 times | 19 (9.3) | 30 (15.1) | 49 (12.1) | 16 (9.9) | 23 (13.7) | 39 (11.9) | |||
| 4–6 times | 29 (14.1) | 22 (11.1) | 51 (12.6) | 23 (14.3) | 14 (8.3) | 37 (11.2) | |||
| 7–9 times | 15 (7.3) | 9 (4.5) | 24 (5.9) | 10 (6.2) | 6 (3.6) | 16 (4.9) | |||
| ≥ 10 times | 142 (69.3) | 138 (69.3) | 280 (69.3) | 105 (65.2) | 108 (64.3) | 213 (64.7) | |||
| Seizure frequency in last 6 months (Thapar et al.35), n (%) | n = 147 | n = 157 | n = 304 | ||||||
| None | 15 (10.2) | 27 (17.2) | 42 (13.8) | ||||||
| 1–3 times | 27 (18.4) | 27 (17.2) | 54 (17.8) | ||||||
| 4–6 times | 21 (14.3) | 20 (12.7) | 41 (13.5) | ||||||
| 7–9 times | 16 (10.9) | 3 (1.9) | 19 (6.3) | ||||||
| ≥ 10 times | 68 (46.3) | 80 (51.0) | 148 (48.7) | ||||||
| Seizure frequency in last 12 months (Baker et al.99), n (%) | n = 199 | n = 196 | n = 395 | n = 160 | n = 168 | n = 328 | |||
| None | – | – | – | 7 (4.4) | 17 (10.1) | 24 (7.3) | |||
| < 1 per month | 52 (26.1) | 58 (29.6) | 110 (27.8) | 59 (36.9) | 56 (33.3) | 115 (35.1) | |||
| ≥ 1 per month | 147 (73.9) | 138 (70.4) | 285 (72.2) | 94 (58.8) | 95 (56.5) | 189 (57.6) | |||
| Seizure frequency in last 6 months (Baker et al.99), n (%) | n = 145 | n = 155 | n = 300 | ||||||
| None | 15 (10.3) | 27 (17.4) | 42 (14.0) | ||||||
| < 1 per month | 40 (27.6) | 34 (21.9) | 74 (24.7) | ||||||
| ≥ 1 per month | 90 (62.1) | 94 (60.6) | 184 (61.3) | ||||||
| Seizure recency in last 12 months (days since last recalled seizure), median (IQR) [range] | n = 199; 33 (19–64) [1–351] | n = 186; 34 (17–63) [3–457] | n = 385; 34 (18–63) [1–457] | n = 161; 10 (2–66) [0–705] | n = 164; 14 (3–121) [0–815] | n = 325; 13 (2–95) [0–815] | |||
| Seizure recency in last 6 months (days since last recalled seizure), median (IQR) [range] | n = 143; 16 (6–55) [0–545] | n = 146; 19 (5–68) [0–611] | n = 280; 17 (5–62) [0–611] | ||||||
| Impact of epilepsy, mean (SD) [range] | n = 203; 24.4 (8.0) [10–40] | n = 196; 24.4 (8.1) [10–40] | n = 399; 24.4 (8.0) [10–40] | n = 153; 25.1 (7.3) [10–40] | n = 155; 24.8 (8.2) [10–40] | n = 308; 25.0 (7.8) [10–40] | n = 162; 25.4 (7.9) [10–40] | n = 166; 26.0 (8.7) [10–40] | n = 328; 25.7 (8.3) [10–40] |
| Medication adherence, median (IQR) [range] | n = 201; 46.7 (43.3–48.9) [16.7–50.0] | n = 198; 46.7 (43.3–48.9) [26.7–50.0] | n = 399; 46.7 (43.3–48.9) [16.7–50.0] | n = 162; 47.8 (45.6–48.9) [27.8–50.0] | n = 165; 47.8 (45.6–48.9) [35.6–50.0] | n = 327; 47.8 (45.6–48.9) [27.8–50.0] | |||
| Medication AEs, median (IQR) [range] | n = 201; 7 (5–10) [2–10] | n = 198; 7 (4–10) [2–10] | n = 399; 7 (5–10) [2–10] | n = 151; 7 (5–10) [2–10] | n = 159; 7 (5–10) [2–10] | n = 310; 7 (5–10) [2–10] | n = 163; 7 (5–10) [2–10] | n = 168; 8 (6–10) [2–10] | n = 331; 8 (5–10) [2–10] |
| Anxiety (HADS-A), mean (SD) [range] | n = 204; 9.0 (5.0) [0–21] | n = 199; 7.8 (4.8) [0–21] | n = 204; 8.4 (4.9) [0–21] | n = 162; 8.1 (4.6) [0–20] | n = 167; 7.6 (4.3) [0–20] | n = 329; 7.9 (4.4) [0–20] | |||
| Depression (HADS-D), mean (SD) [range] | n = 204; 5.5 (3.9) [0–18] | n = 199; 5.0 (3.9) [0–16] | n = 204; 5.3 (3.9) [0–18] | n = 162; 5.7 (4.1) [0–20] | n = 167; 5.7 (4.3) [0–18] | n = 329; 5.7 (4.2) [0–20] | |||
| Anxiety categories (HADS-A), n (%) | n = 204 | n = 199 | n = 204 | n = 162 | n = 167 | n = 329 | |||
| Normal (scores of 0–7) | 83 (40.7) | 104 (52.3) | 187 (46.4) | 78 (48.1) | 93 (55.7) | 171 (52.0) | |||
| Borderline (scores of 8–10) | 44 (21.6) | 35 (17.6) | 79 (19.6) | 28 (17.3) | 27 (16.2) | 55 (16.7) | |||
| Case (scores 11–21) | 77 (37.7) | 60 (30.2) | 137 (34.0) | 56 (34.6) | 47 (28.1) | 103 (31.3) | |||
| Depression categories (HADS-D), n (%) | n = 204 | n = 199 | n = 204 | n = 162 | n = 167 | n = 329 | |||
| Normal (scores 0–7) | 144 (70.6) | 146 (73.4) | 290 (72.0) | 114 (70.4) | 124 (74.3) | 238 (72.3) | |||
| Borderline (scores 8–10) | 37 (18.1) | 34 (17.1) | 71 (17.6) | 29 (17.9) | 15 (9.0) | 44 (13.4) | |||
| Case (scores 11–21) | 23 (11.3) | 19 (9.5) | 42 (10.4) | 19 (11.7) | 28 (16.8) | 47 (14.3) | |||
| Stigma of epilepsy, median (IQR) [range] | n = 203; 1 (0–4) [0–9] | n = 198; 2 (0–4) [0–9] | n = 401; 1 (0–4) [0–9] | n = 161; 2 (0–4) [0–9] | n = 167; 1 (0–4) [0–9] | n = 328; 1 (0–4) [0–9] | |||
| Stigma categories, n (%) | n = 203 | n = 198 | n = 401 | n = 161 | n = 167 | n = 328 | |||
| Not stigmatised (score of 0) | 76 (37.4) | 72 (36.4) | 148 (36.9) | 54 (33.5) | 57 (34.1) | 111 (33.8) | |||
| Mild to moderate (scores of 1–6) | 100 (49.3) | 103 (52.0) | 203 (50.6) | 86 (53.4) | 83 (49.7) | 169 (51.5) | |||
| Highly stigmatised (scores of 7–9) | 27 (13.3) | 23 (11.6) | 50 (12.5) | 21 (13.0) | 27 (16.2) | 48 (14.6) | |||
| Self-mastery, mean (SD) [range] | n = 201; 13.9 (3.4) [6–23] | n = 198; 14.3 (3.3) [6–24] | n = 399; 14.1 (3.3) [6–24] | n = 162; 14.5 (3.4) [7–24] | n = 167; 14.6 (3.5) [6–24] | n = 329; 14.6 (3.4) [6–24] | |||
Seizure frequency measured by the Baker scale showed fewer participants having one or more seizures per month at the follow-up times. However, overall post-randomisation Baker scores were similar between the two trial arms and not statistically significant.
Finally, the number of days since last recalled seizure was lower at 6 and 12 months than was reported at baseline. However, there was also no statistically significant difference in seizure recency at follow-ups between the two trial arms (see Table 17). Seizure control is difficult to interpret as there is recall bias in this patient population, who frequently have memory problems and can also be unaware of seizures occurring.
Impact of epilepsy
Scores from the Impact of Epilepsy scale were unchanged from baseline values at the 6- and 12-month follow-ups (see Table 18). There were no differences between the treatment groups at either of the follow-up time points (see Table 17).
Medication adherence
There was a 1-point improvement in the means of both treatment groups at the final follow-up. As the baseline scores were high, we did not anticipate that much improvement on this scale could be possible. In addition, 12 months after randomisation, there were no differences between the two treatment groups (see Table 17).
Medication adverse effects
The impact of adverse effects from epilepsy medication was determined from two questions in the QOLIE-31-P. The maximum score for the two questions is 10 and higher scores indicate less impact of adverse effects. Scores were similar at all time points (see Table 18). There were no statistically significant differences between the trial arms at the 6- or 12-month follow-ups (see Table 17).
Psychological distress
Psychological distress, specifically anxiety and depression scores, was captured using HADS. The participants were divided into categories based on scores for HADS-A and HADS-D. About one-third of the total group had case scores for anxiety at baseline in both groups. About 10% of the group had case scores for depression in both treatment groups at baseline (see Table 18).
At 12 months, there were no significant differences between the intervention and control groups (see Table 17). There was a slightly higher proportion of participants with case anxiety scores in the intervention group than in the control group, but this was not significant (see Table 18). In addition, there was a higher proportion of participants with HADS-D case scores in the control group at the 12-month follow-up (see Table 18).
Stigma of epilepsy
Felt stigma was measured using a scale with scores from 0 to 9 (see Chapter 4). The median scores were similar at baseline and at 12 months (see Table 18). These scores were categorised into ‘not stigmatised’, ‘mild–moderate stigma’ and ‘highly stigmatised’. At baseline, approximately 60% of the sample felt some level of stigma (majority in mild-to-moderate group) because of epilepsy. At the 1-year follow-up, this level of felt stigma persisted in both treatment groups with no significant difference between the two (see Tables 17 and 18).
Chapter 7 Implementation fidelity
Introduction
Many complex interventions are delivered within group settings, such as self-management education courses. When testing such interventions, it is important to monitor how well the intervention was delivered. In a RCT setting, in which an intervention is delivered on many separate occasions to different groups of participants by various facilitators, it is especially important to assess how the components of the intervention were delivered in each setting. Intervention delivery can influence trial outcomes in complex intervention RCTs. An ‘implementation fidelity’ study provides a structured method for assessing how an intervention was delivered in a RCT setting. The main objective of this study was to evaluate whether or not the SMILE (UK) intervention had been delivered as specified in the original protocol. To our knowledge, this is the first study evaluating implementation fidelity of a group self-management course for epilepsy. For the purposes of our implementation fidelity study, we evaluated facilitator adherence (to module content) and facilitator competence during the intervention delivery.
Facilitator adherence can be defined as the degree to which facilitators followed the protocol in delivering specific aspects of the course. 118,119 Competence is defined as the quality of intervention delivery. This includes aspects such as group interaction and pacing of delivery. 119 A proxy for facilitator competence is the evaluation of didactic teaching methods. This is when a facilitator spends a high proportion of time speaking rather than having an interactive discussion with course participants. Educational courses that are solely based on didactic teaching have limited impact on behaviour change. 120 During the SMILE (UK) intervention, we assessed implementation fidelity by developing an instrument that contained checklist items on facilitator competence and adherence.
Method
For full details on SMILE (UK) intervention development, see Chapter 2. As described in Chapter 5 in more detail, 18 courses were delivered by 12 SMILE (UK) course facilitators. All of these course offerings were audio-recorded and, of those, approximately 25% were chosen for the fidelity evaluation. 121 The trial manager selected five courses in a purposeful manner to ensure the maximum number of facilitators could be evaluated. As courses were given by teams of two, this resulted in 10 facilitators being evaluated. To ensure courses were not selected based on content, a second researcher listened to the quality of the recordings. The courses selected for fidelity evaluation were numbers 1, 8, 10, 13 and 15. During the intervention arm of the trial, group sizes ranged from 6 to 13 participants. At the time of enrolment into the RCT, participants gave their written consent to have the SMILE (UK) courses audio-recorded. Courses were not recorded if a participant did not agree to this when giving written informed consent. This work did not lead to any unblinding as the trial manager was unblinded throughout the study. Two raters independent from the study listened to the full content of the recordings and were not involved in data collection for the RCT.
Instrument development
The process of developing the implementation fidelity tool was based on past research. 118,122 As MOSES [and, thus, SMILE (UK)] was not developed according to a behaviour change model, we began by determining which modules of the course were likely to influence behaviour change. Eight out of nine modules were identified by study co-investigators; these were further reduced after reviewing suggestions from participants in the external pilot study. 82 Participants in the external pilot identified modules 3 (basic knowledge), 4 (diagnosis) and 6 (self-control) as those from which they had learned the most. Module 8 was also identified as helpful; however, this module is subject to participant input and, thus, is highly variable between course sessions. For this reason, module 8 was excluded from the fidelity analysis. The three modules ultimately selected for fidelity assessment were content heavy. Discussion points included knowledge about epilepsy, the science behind clinical diagnosis, treatment options and seizure auras or triggers.
Adherence
In order to assess facilitator adherence, we created a checklist based on facilitator workbook content. Each checklist item could be awarded a score from 0 to 2 (0, content undelivered; 1, partial delivery; and 2, full content delivery). In total, there were five checklist items for module 6 and six checklist items for modules 3 and 4 (maximum score of 2/2 per item).
Competence
We selected four criteria for evaluating facilitator competence: ‘group interaction’, ‘overall impression’, ‘didacticism’ and ‘trainer techniques’. Scores for ‘group interaction’ were allocated based on the number of participants who interacted with the facilitator (0, one participant was speaking; 1, two or three participants had spoken; or 2, four or more had participated in a discussion during the group session). As with past research,118 ‘overall impression’ was assessed to understand how well the modules were delivered. Scores ranged from 1 to 4 (1, poor performance – the session consisted of didactic teaching strategies with few instances of interaction; 2, average performance – there was some interaction and participant input, but not a great deal of cohesiveness 3, good performance with some didactic-style teaching and considerable group participation and cohesiveness; and 4, indicates excellent performance – minor instances of didactic teaching and major instances of group participation).
The total amount of speaking time was used as a proxy for ‘didacticism’ during recorded sessions. An annotation programme called ELAN (Max Planck Institute for Psycholinguistics, The Language Archive, Nijmegen, the Netherlands)123,124 was used to record instances of facilitator speech in number of seconds. Filler words used by facilitators such as ‘yeah’ and ‘oh’ were not scored. Total speech time was divided by the length of the module and was expressed as a percentage.
Finally, throughout each of the module recordings, raters also scored the number of the times that a facilitator used teaching resources such as a flip chart or PowerPoint slide.
Instrument testing
We conducted a test of the fidelity instrument to ensure its feasibility, with two members of the research team assessing a course delivered to the control group. For the fidelity assessment of the five courses, two independent raters were recruited to reduce the possibility of bias. The raters were trained to use the instrument by evaluating a control group offering of the course. A scoring guide was also developed in order to minimise the possibility of scoring error or inconsistency. This included a list of specific topics that the facilitator would need to cover during each module to receive a maximum score. For instance, in module 3 a score of 2 out of 2 would be awarded for the section on ‘seizure types’ if the following categories were described: generalised tonic–clonic, absence, complex partial, simple partial and myoclonic.
Statistical analysis
Several approaches were employed to measure inter-rater reliability. A weighted kappa statistic was used with ordinal scores derived from checklist items. Intraclass coefficients were used with continuous scores that were derived from didactic speech time measurements. The per cent agreement was calculated for items on the instrument checklists in order to assess the frequency of raters giving the same scores. Simple regression analysis was used to examine the associations between scores for different measures.
Results
Fidelity measure evaluation
In total, 15 recorded sessions were analysed (i.e. five courses each with three modules) and 85 items were scored. The results were charted in a table then assessed for inter-rater reliability. With a weighted kappa score of 0.67, there was substantial agreement with allocated ratings. 125 Percent agreement was also high, at 81.2%.
A similar approach was taken in assessing the competence measure. There was also substantial agreement between raters with a weighted kappa statistic of 0.65 and percentage of agreement at 60.0%. The intraclass coefficient for didacticism was high at 0.97 (p < 0.0001), which indicates a high degree of reproducibility. Most sessions received the maximum score of 2 for ‘group interaction’, and thus this item was not assessed for inter-rater agreement.
SMILE (UK) course evaluation
Adherence
We averaged the scores for each module of the five evaluated sessions in order to determine a total adherence score. The average adherence score ranged from 1.5 to 1.6 (Table 19). The median score was 2 out of 2 for modules 3 and 6, and 1.8 out of 2 for module 4.
| Module | Module score, mean; median (range) | Checklist item | Total item score, mean; median (range) |
|---|---|---|---|
| Module 3: basic knowledge | 1.5; 2 (1.3–1.9) | How do seizures develop? | 2; 2 (2–2) |
| What are the different seizure types? | 2; 2 (2–2) | ||
| What happens during a seizure? | 1.1; 1 (0–2) | ||
| What are some examples of seizure types? | 1.8; 2 (1.5–2) | ||
| Participants are facilitated to identify personal seizure type | 1.7; 2 (1–2) | ||
| Participants facilitated to note seizure type in booklet | 0.6; 0 (0–2) | ||
| Module 4: diagnosis | 1.5; 1.8 (1.2–2) | Things that are noticed before, during and after a seizure | 2; 2 (2–2) |
| The importance of detailing specifics of a seizure | 1.6; 1.5 (1–2) | ||
| What a doctor may need to know about a seizure | 1.6; 1.5 (1–2) | ||
| Participants prompted to record details of their last seizure in handbook | 0.9; 1 (0–2) | ||
| Importance of EEG | 1.8; 2 (1–2) | ||
| Other diagnostic techniques | 1.3; 1 (1–2) | ||
| Module 6: self-control | 1.6; 2 (1.3–2) | Seizure triggers and how they vary | 2; 2 (2–2) |
| Keeping a checklist of triggers | 1.5; 1.5 (0.5–2) | ||
| Avoiding and eliminating triggers | 1.6; 2 (1–2) | ||
| What is an aura and how might it be recognised? | 1.4; 2 (0–2) | ||
| Countermeasures to achieve aura control | 1.5; 1.5 (1–2) |
Out of all five courses that were evaluated, four checklist items were rated as fully delivered by facilitators: ‘how seizures develop’, ‘seizure types’, ‘events pre/during/post seizures’ and ‘seizure triggers’. Seven items were scored at 0, indicating that the content was not delivered: five out of seven were for missing content relating to the participant workbook, one out of seven was for missing content relating to seizures and the other one out of seven was for missing content relating to seizure auras.
Competence
We evaluated group interaction by recording the amount of time that participants spoke during a session. As shown in Table 20, almost every session was judged as having four or more people interacting in the session; thus, the mean scores were close to the maximum of 2.
| Module | Mean competence score (range) | Didacticism, mean % (range) | |
|---|---|---|---|
| Group interaction | Overall impression | ||
| Module 3: basic knowledge | 1.9 (1.5–2.0) | 2.6 (1.5–3.5) | 71 (55–78) |
| Module 4: diagnosis | 1.9 (1.5–2.0) | 2.6 (1.0–4.0) | 69 (48–93) |
| Module 6: self-control | 2.0 (2.0–2.0) | 3.0 (2.0–4.0) | 58 (42–76) |
We also assessed ‘overall impression’ using a scoring scale ranging from 1 to 4. Out of the 15 sessions that were evaluated, 12 were rated as ‘average to excellent’ and three sessions were given a score below two. Modules 4 and 6 received a maximum score for overall impression for at least one session evaluated (see Table 20).
There was considerable variation in the percentage of facilitator speech, especially for module 4 (i.e. 48–93%). The regression analysis showed no association between any of the competence measures. Thus, the percentage of didacticism did not have an impact on adherence, group interaction or overall impression.
Although we set out to evaluate facilitator teaching techniques, this was not possible because of the nature of audio-recordings and ambiguity over the exact strategy in use. Raters were unable to assess this component consistently across all recordings, so the measure was removed from the analysis.
Discussion
There is a fine balance between adherence and competence in delivering complex interventions. 126,127 The SMILE (UK) course was designed to involve participant interaction with a certain allowance for adapting the material to the group. 78 This requires a high level of facilitator competence, which can have an impact on adherence of the course. In the context of a RCT, it is important that the components of the course be delivered consistently in order to evaluate their effectiveness; thus, a certain level of adherence is required. It becomes important to achieve the optimum balance between adherence and competence. How to negotiate between the two can be understood only after implementation fidelity assessments.
We developed our fidelity instrument based on published literature using audio-recordings and checklists. 118,122 We had two independent raters score the course delivery. Other studies have used self-reporting by facilitators, which increases the risk of bias. 128,129 We found that audio-recordings worked well for assessing adherence and most competence measures. However, a visual approach is needed to evaluate facilitator techniques used (e.g. flip chart or slides) as this was too difficult to assess with audio only.
Our fidelity assessment showed that our instrument could be used with ease and the inter-rater reliability was high. The addition of the novel didacticism measurement offered an objective result to the instrument and was highly reproducible.
The results of our assessment demonstrated that SMILE (UK) was delivered with high adherence to important topics while allowing for interactive sessions. Four topics received the maximum adherence score across all the sessions evaluated. Every item received a maximum adherence score in at least one session. Thus, all items listed in the facilitator’s manual could be fully delivered. We found that the majority of omitted items were related to using the participant workbook during the course. It is unknown whether or not using the workbook during the course is a factor in behaviour change. However, using the workbook throughout the 2 days was an idea raised by volunteers during the pilot test of SMILE (UK). This way, the book is a tool that participants were already familiar with, which could potentially lead to them using it again at home.
Limitations of the fidelity assessment
Only three out of the nine SMILE (UK) modules were evaluated. The three selected modules involve more teaching than the others and, thus, some level of didacticism is expected. However, despite this, the facilitators still maintained a high level of group interaction. The modules not included in the fidelity assessment were participant led and would not have been suitable for a structured evaluation. Yet, they may be important in behaviour change by increasing self-confidence. 130,131 Because some modules were not assessed, it is possible that some overlapping content was touched on in other parts of the course. Thus, an item could receive a score of 0 in our evaluation but have been delivered in another session. Our group interaction score was based on a specific number of participants interacting, but this could also have been represented by a percentage of the group, which would better account for varying group sizes. Owing to constraints, we evaluated approximately 25% of the courses delivered. We were unable to assess the same facilitator across multiple courses and thus we are unable to monitor how consistent they were throughout the study.
Conclusion
The fidelity assessment demonstrated that SMILE (UK) was delivered with high levels of adherence and competence. The implementation fidelity study offers, to our knowledge, the first such assessment of a self-management course for epilepsy. The instrument that was developed offers a multicomponent evaluation of a complex intervention.
Chapter 8 Health economics
Introduction
This chapter contains the results of the economic evaluation. Few studies have looked at the cost of epilepsy in the UK. We know of a recent Dutch study in which a cost evaluation of a self-management course for epilepsy was undertaken132 and have collaborated with the trial team in assessing the appropriateness of outcome measures for cost evaluations in epilepsy. 133 Data on health service use and QoL were collected with other secondary outcomes during the trial. The objectives were to assess the cost of epilepsy health care and whether or not SMILE (UK) is cost-effective.
Analysis plan
Perspective
The primary perspective of the economic evaluation was the NHS/Personal Social Services perspective, which, for decision purposes, is generally preferred by NICE. Other resources relevant to a wider societal perspective, such as informal care and productivity loss (because of time off work) were included in the secondary analyses (societal perspective).
Data collection: service use and costs
An adapted version of the CSRI104 was administered retrospectively to collect self-reported resource utilisation for the 12 months preceding data collection at baseline and 12-month follow-up interviews. This measure is used to collect the range of services and support accessed by study participants. The CSRI has been successfully used in a variety of adult mental health populations since its design in 1985. Patients in this study have different health needs and are likely to require varying health and social services, hence the need to adapt the CSRI to ensure we capture services relevant to PWE. The CSRI was used to record epilepsy-specific hospital services and community-based health and social care services, medication, productivity losses as a result of illness, and help with usual activities provided by family and friends. Data on the number and duration of SMILE (UK) and TAU sessions were centrally recorded as part of the RCT.
Costs are reported in Great British pounds at 2014–15 prices and, given the 1-year time horizon, discounting was deemed unnecessary. Service use collected using the CSRI was combined with nationally applicable unit costs to estimate total costs for each participant. 134,135 Productivity losses as a result of epilepsy were derived based on the human capital approach, which combines days off work with average UK wage rate. Family and friends are not generally reimbursed for their support to patients but there is still an opportunity cost to this time. The cost of this unpaid care was also estimated based on the average UK wage rate for adults.
Intervention costs were estimated based on staff time (facilitator) needed to deliver SMILE (UK) including other non-staff costs, such as training and manuals. These were applied to group-session attendance data to work out the cost per participant.
Data collection: quality of life
Health-related QoL was estimated using the EQ-5D-5L,136 a widely used standardised method for assessing health-related quality. The EQ-5D-5L is a brief self-reported preference-based measure of health that considers five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each consisting of five levels of functioning (e.g. no pain, slight pain, moderate pain, severe pain and extreme pain). This measure produces a possible 3125 distinct health states ranging from 11111 (full health) to 55555 (worst). Utility scores for each participant, between 0 (worse health) and 1 (full health)136,137 were produced by combining UK population value sets with these health states. QALYs were derived from the transformed EQ-5D-5L utility scores using the area under the curve method,138 which is the preferred measure for HRQoL in UK Health Technology Assessment research. The second part of the measure [EuroQoL-5 Dimensions (EQ-5D) VAS] takes the form of a VAS, in which the participant is required to rate their health from 0 (worst imaginable health state) to 100 (best imaginable state). It shows the respondent’s self-perceived HRQoL on a scale with endpoints labelled ‘worst’ at 0 and ‘best’ at 100.
Cost-effectiveness analysis
Stata (version 14) was used to analyse data. Data were assessed two ways: complete cases (i.e. only including participants with complete service use and QoL data) and on an ITT basis (i.e. according to the group to which they were randomised regardless of intervention receipt). Missing costs and outcome data were imputed using a single imputation method based on linear extrapolation, adjusted for baseline costs, EQ-5D-5L utility scores and QOLIE-31-P score. Other epilepsy-related variables, such as seizure frequency, were obtained by non-parametric bootstrap regressions to account for the non-normal distribution commonly found in cost data. 139
Costs and outcomes were compared between the two arms at baseline and 12-month follow-up and are presented as mean values with SDs and 95% confidence intervals (CIs). Mean differences and 95% CIs were obtained by non-parametric bootstrap regressions (1000 replications) to account for non-normal distribution often found in cost data. 139
Cost-effectiveness and cost-utility
Cost-effectiveness was assessed based on the health and social care (NHS) perspective, as well as the societal perspective, by combining the costs with data on the primary outcome (QOLIE-31-P) measure at 12 months.
Cost–utility was explored by combining total costs with QALYs, derived from EQ-5D-5L data. Assuming the intervention produced better outcomes and lower costs, it would be ‘dominant’. However, if it resulted in better outcomes but higher costs, then incremental cost-effectiveness ratios (ICERs) would be estimated to show the extra cost incurred for a 1-unit improvement on the QOLIE-31-P or one extra QALY (both at 12 months). There is no need to calculate ICERs for any combination in which one group has lower costs and better outcomes as it is then considered to ‘dominate’ the other group.
To evaluate the uncertainty around point estimates, cost-effectiveness planes (CEPs) and cost-effectiveness acceptability curves (CEACs) were created. These curves are an alternative to CIs around ICERs. Non-parametric bootstrapping was used to create a joint distribution of incremental costs and outcomes to explore the probability of SMILE (UK) or TAU being cost-effective, subject to varying willingness-to-pay (WTP) values attached to a unit improvement in the QOLIE-31-P or one extra QALY gained. One thousand cost–outcome combinations were plotted on a CEP, where the y-axis depicts the additional costs of the intervention compared with TAU, and the x-axis represents a difference in the primary outcome between the intervention and control groups.
Results were interpreted using CEACs140 to show the probability of the intervention being the cost-effective option for a range of different values placed on an improvement in outcome. The range of values for QALYs was £0 to £80,000; this includes the threshold recommended by the UK’s NIHR and NICE when judging the cost-effectiveness of a health technology. The range for improvements on the QOLIE-31-P was chosen such that values at which the intervention or TAU has a 50% and 70% and 90% likelihood of being cost-effective were identified.
A series of net benefits [calculated using the formula net monetary benefit = (ΔE) × λ – (ΔC)] were calculated for each individual for a variety of values (£0–80,000 for WTP for an additional QALY). Additional gain in outcome (ΔE) was multiplied by the ceiling ratio (λ) defined by the decision-maker’s WTP for an additional unit of health outcome, and the difference in costs (ΔC) subtracted. After calculating net benefits for each participant for each value of WTP, coefficients of differences in net benefits between the trial arms were obtained through a series of bootstrapped linear regressions (1000 repetitions), which included the same covariates used for comparisons of outcomes in the primary economic analyses. The resulting coefficients were then examined to calculate the proportion of times that the intervention group had a greater net benefit than the control group for each value of WTP. These proportions were then plotted to generate CEACs for all cost–outcome combinations.
Results
Response rates
Resource use and QoL data were available for all participants at baseline and at the 12-month follow-up. At the end of the trial, 18% of participants did not complete the final assessment (Table 21).
| Assessment | Treatment group, n (%) | Total | |
|---|---|---|---|
| SMILE (UK) | TAU | ||
| Received at least one session of SMILE (UK) | 151 (73.6) | 3 (1.5) | 154 |
| QOLIE-31-P baseline | 205 (50.7) | 199 (49.3) | 404 |
| QOLIE-31-P 12 months | 163 (49.2) | 168 (50.8) | 331 |
| EQ-5D-5L baseline | 205 (50.7) | 199 (49.3) | 404 |
| EQ-5D-5L 12 months | 163 (49.2) | 168 (50.8) | 331 |
| CSRI baseline | 205 (50.7) | 199 (49.3) | 404 |
| CSRI 12 months | 163 (49.4) | 167 (50.6) | 330 |
Service use
Participants from both groups reported contact with a variety of health professionals, including use of health and social care services and the use of AEDs (Table 22). The service use between the two trial arms was similar. As patients were recruited through their neurologist, this explains the high proportion of participants in both groups who reported contact with a neurology consultant. At baseline, resource utilisation was similar between the two trial arms with GPs being the most frequently reported contact, at an average of three visits in the previous 12 months. Contact with ENS and mental-health services (counsellors and psychologists) was also reported by several participants, with a mean contact (number of visits) of 1 and 2, respectively, for both groups. Contact with community health professionals remained relatively similar at follow-up, with notable variation in the ‘other’ category. Although a small proportion of patients (in both groups) reported contact with home workers, activity centres with private nursing and other community services, the number and duration of contacts reported was quite high.
| Resource | Resource unit of measure | Time point | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-month follow-up | ||||||||
| Participants,a n (%) | Resource use of total SMILE (UK)b (N = 205), mean (SD) | Participants,a n (%) | Resource use of total TAUb (N = 199), mean (SD) | Participants,a n (%) | Resource use of total SMILE (UK)b (N = 163), mean (SD) | Participants,a n (%) | Resource use of total TAUb (N = 167), mean (SD) | ||
| Community care | |||||||||
| GP | Contacts | 96 (46.8) | 2.26 (4.5) | 116 (58.3) | 2.67 (5.0) | 80 (49.1) | 2.11 (3.8) | 84 (50.0) | 2.40 (5.5) |
| Practice nurse | Contacts | 15 (7.8) | 0.12 (0.4) | 17 (8.5) | 0.16 (0.6) | 7 (4.3) | 0.07 (0.4) | 6 (3.6) | 0.07 (0.5) |
| Epilepsy nurse | Contacts | 64 (31.2) | 0.65 (1.2) | 82 (41.2) | 0.93 (1.6) | 65 (42.3) | 0.86 (1.6) | 56 (33.3) | 0.88 (2.0) |
| Physiotherapist | Contacts | 9 (4.4) | 0.36 (2.4) | 12 (6.0) | 0.36 (2.5) | 5 (3.1) | 0.23 (1.6) | 11 (6.6) | 0.49 (4.1) |
| Social worker | Contacts | 7 (3.4) | 0.33 (2.7) | 6 (3.0) | 0.62 (7.1) | 6 (3.7) | 0.11 (0.6) | 8 (4.8) | 0.35 (2.3) |
| Counsellor Psychologist | Contacts | 24 (11.7) | 1.54 (6.6) | 26 (13.1) | 2.47 (10.9) | 19 (11.7) | 2.27 (11.0) | 29 (17.3) | 1.93 (6.8) |
| Home help: household tasks | Contacts | 5 (2.4) | 2.00 (15.0) | 5 (2.5) | 5.76 (44.4) | 12 (7.4) | 14.93 (91.1) | 6 (3.6) | 13.89 (101.9) |
| Home help: personal care | Contacts | 1 (0.5) | 3.79 (54.3) | 6 (3.0) | 9.17 (57.1) | 6 (3.7) | 9.48 (81.5) | 2 (1.2) | 7.53 (74.8) |
| Other community services | Contacts | 5 (2.4) | 0.42 (4.3) | 7 (3.5) | 2.16 (26.1) | 13 (8.0) | 3.29 (29.4) | 6 (3.6) | 8.13 (52.3) |
| AEDs | Number of AEDs | 201 (98) | 2.08 (1.0) | 196 (98) | 2.16 (1.1) | 162 (99.4) | 2.22 (1.0) | 167 (99.4) | 2.17 (1.1) |
| Hospital inpatient care | Nights | 36 (17.6) | 0.60 (1.9) | 36 (18.1) | 1.48 (4.8) | 23 (14.1) | 0.80 (3.4) | 22 (13.1) | 1.01 (4.5) |
| Hospital outpatient care | Attendances | 83 (40.5) | 0.24 (0.8) | 58 (29.1) | 0.10 (0.3) | 13 (8.0) | 0.3 (1.5) | 11 (6.6) | 0.3 (2.0) |
| ED | Attendances | 81 (39.5) | 0.99 (2.2) | 88 (44.2) | 1.55 (3.6) | 56 (34.6) | 0.79 (1.6) | 44 (26.2) | 0.56 (1.4) |
| Clinical decision unit | Attendances | 10 (4.5) | 0.12 (0.7) | 13 (6.5) | 0.22 (1.3) | 9 (5.6) | 0.74 (0.3) | 15 (8.9) | 0.11 (0.4) |
| Hospital neurology appointment | Attendances | 191 (94.0) | 2.20 (1.7) | 187 (94.0) | 2.20 (1.5) | 152 (93.3) | 2.27 (3.8) | 145 (86.3) | 1.79 (1.5) |
| Laboratory tests/investigations | Attendances | 72 (35.1) | 0.53 (0.9) | 47 (23.6) | 0.40 (1.0) | 36 (22.1) | 0.37 (0.8) | 45 (26.8) | 0.37 (0.7) |
| Informal care | |||||||||
| Personal care | Hours/week | 27 (13.2) | 1.94 (12.6) | 16 (8.0) | 0.59 (2.9) | 19 (11.7) | 0.71 (2.5) | 12 (7.4) | 8.42 (7.5) |
| Help with medical procedures | Hours/week | 31 (15.1) | 2.16 (12.9) | 23(11.6) | 0.46 (1.7) | 25 (15.3) | 0.39 (1.5) | 27 (16.1) | 0.51 (1.7) |
| Help in home | Hours/week | 61 (29.8) | 4.59 (15.0) | 46 (23.1) | 2.38 (6.47) | 49 (30.1) | 3.56 (14.0) | 39 (23.2) | 3.20 (13.8) |
| Help outside home | Hours/week | 57 (27.8) | 3.33 (13.4) | 46 (23.1) | 1.67 (5.0) | 47 (28.8) | 2.29 (13.4) | 34 (20.2) | 2.05 (13.3) |
| Time spent ‘on call’ | Hours/week | 21 (106) | 5.73 (24.9) | 33 (16.1) | 6.24 (29.3) | 37 (22.7) | 28.80 (61.8) | 33 (19.6) | 29.52 (63.2) |
| Productivity loss | |||||||||
| Days off work because of illness | Days | 49 (23.9) | 9.61 (47.9) | 48 (24.1) | 9.21 (42.8) | 37 (24.0) | 16.3 (54.2) | 29 (18.2) | 3.19 (24.2) |
| Incapacity benefits | 94 (46.9) | 89 (44.7) | 68 (38.7) | 6 (38.1) | |||||
The use of hospital services was also similar between the two groups at baseline, except for the use of outpatient care, which was reported as higher in the SMILE (UK) group (but the number of contacts is reported as almost identical between the two groups). At the 12-month follow-up, the proportion of patients reporting use of hospital services had reduced for both groups. The percentage of participants who reported informal care was low at baseline, but those who did received substantial help from family and friends, especially for the ‘on call’ category. The number of hours spent on call per week by friends and family had increased by a large amount at follow-up for both groups. Productivity loss was reported by almost one-quarter of participants in both groups. Although remaining low at 12 months, the mean number of days taken off work reported by the SMILE (UK) group was approximately five times higher than in the control group (16.3 days vs. 3.19 days).
Costs
Intervention costs
Intervention costs were estimated through microcosting using information on staff time involved in delivering the intervention as well as other non-staff costs. Intervention costs are made up of teaching manuals, training of facilitators and the actual SMILE (UK) intervention. The total cost of 20 teaching manuals, each costing £15.70, was included in the costing. Training of facilitators was conducted by two MOSES experts (one neurologist and one psychologist, both with expertise in epilepsy) for 2 days (8 hours/day). For the intervention, the SMILE (UK) course was divided into four sessions (day 1 morning and afternoon, day 2 morning and afternoon). Each session was delivered by two facilitators for a group of eight patients for 3 hours.
The cost of training was estimated by applying the unit cost of two clinical psychologists at £140.00 each per hour, to the duration (16 hours) of training. The total was added to the costs of teaching manuals and divided by the number of participants who received the SMILE (UK) intervention.
The cost per SMILE (UK) session was estimated by combining the unit cost of two nurse specialists (£44 per hour) with the duration of each session divided by the number of participants per group, and added to the cost of training, producing £56.05 as the cost per session of SMILE (UK). A complete course of SMILE (UK) with four sessions was estimated to be £224.00. Based on the number of sessions attended by the intervention group, mean cost associated with SMILE (UK) was £175.00. Three patients in the control group erroneously attended the course and mean costs were estimated to £3.00.
Service use costs
Table 23 reports costs associated with service use specifically for epilepsy. Health and social care costs (NHS perspective) were higher at baseline for the TAU group (£3275 vs. £2227). For both groups, AEDs contributed greatly to NHS health services costs. However, inpatient care contributed the highest proportion of costs for the control group. Other high cost drivers included neurology appointments, mental health services and ED attendance. At follow-up, the SMILE (UK) group still had lower costs than TAU, but the difference was not statistically significant (–£1156, bootstrapped at 95% CI –£1755 to £1507). At this time point, costs were still dominated by AEDs and inpatient care. Paid help at home and other community services also produced large costs, particularly in the control group.
| Cost component | Time point, mean cost (£) (SD) | |||
|---|---|---|---|---|
| Baseline (n = 404) | 12-month follow-up (n = 330) | |||
| SMILE (UK) (n = 205) | TAU (n = 199) | SMILE (UK) (n = 163) | TAU (n = 167) | |
| Intervention | 154 (98) | 3 (27) | ||
| Health-care services | ||||
| GP | 88 (194) | 102 (263) | 79 (133) | 87 (217) |
| Practice nurse | 1 (5) | 1 (5) | 1 (4) | 1(4) |
| Epilepsy nurse | 16 (33) | 23 (43) | 38 (165) | 18 (43) |
| Physiotherapist | 14 (115) | 15 (124) | 10 (70) | 22 (211) |
| Social worker | 11 (85) | 12 (98) | 5 (27) | 51 (390) |
| Mental health services (counsellor/psychologist) | 195 (898) | 347 (1784) | 376 (2415) | 268 (886) |
| Home help: household tasks | 93 (724) | 194 (1593) | 360 (1738) | 925 (6952) |
| Home help: personal care | 64 (596) | 194 (1593) | 140 (1065) | 311 (3407) |
| Other community health services | 70 (757) | 90 (872) | 323 (2703) | 1075 (10,665) |
| AEDs | 701 (1233) | 719 (1039) | 881 (1500) | 756 (913) |
| Hospital inpatient care (including CDU) | 381 (1188) | 932 (3012) | 499 (2092) | 631 (2731) |
| Hospital outpatient care | 20 (84) | 7 (35) | 28 (173) | 31 (225) |
| ED | 140 (313) | 218 (510) | 111 (226) | 79 (203) |
| Neurology appointment | 387 (296) | 386 (273) | 400 (666) | 316 (260) |
| Laboratory tests/investigations | 46 (109) | 35 (108) | 33 (78) | 28 (58) |
| Health and social care costs | 2227 (2768) | 3275 (6536) | 3453 (5970) | 4608 (14,664) |
| Informal care (hours per week) | ||||
| Personal care | 1392 (9039) | 364 (2048) | 515 (1813) | 434 (2084) |
| Help with medical procedures | 1546 (9236) | 328 (1207) | 280 (1102) | 348 (1186) |
| Help in-house | 3292 (10,756) | 1708 (4640) | 2477 (9973) | 2246 (9839) |
| Out-of-home help | 2386 (9615) | 1196 (3561) | 1549 (9618) | 1391 (9543) |
| Time spent ‘on call’ | 4110 (17,839) | 4475 (21,036) | 20,655 (44,296) | 21,293 (45,405) |
| Total informal care costs | 12,726 (40,979) | 8072 (24,243) | 25,476 (53,952) | 25,711 (55,759) |
| Productivity loss | 1060 (5282) | 1016 (4717) | 1804 (5985) | 354 (2679) |
| Total societal costs | 18,151 (50,012) | 13,448 (30,224) | 30,732 (55,824) | 30,675 (58,194) |
However, total costs were different from the societal perspective for complete cases, which includes costs associated with productivity loss as a result of epilepsy and informal care. The intervention group had higher costs both at baseline (£18,151 vs. £13,448) and at follow-up (£30,732 vs. £30,675). However, the difference at 12 months was not statistically significant (–£1286, bootstrapped at 95% CI –£12,197 to £11,560). Results based on the ITT analysis were different from the complete case. The SMILE (UK) group had lower NHS costs at 12-month follow-up (£30,688 vs. £31,018 for the TAU group), but the difference was not statistically significant (–£1864, bootstrapped at 95% CI–£11,949 to £7458).
Quality of life
The SMILE (UK) group had lower QoL (measured using the EQ-5D-5L utility scores) than TAU at baseline (0.7997 vs. 0.8415) (Table 24). At follow-up, both groups reported reduced QoL, with SMILE (UK) consistently having lower scores (0.7772 vs. 0.8302 in the TAU group), which subsequently translated to lower QALYs for the intervention group in the complete case analysis. However, the difference was not statistically significant (–0.0146, bootstrapped at 95% CI –0.0362 to 0.0071). Analysis using imputed data showed similar results, producing statistically insignificant differences in QALYs (–0.0142, bootstrapped at 95% CI –0.0318 to 0.0034).
| Scale | Treatment group | Difference between groups | ||||
|---|---|---|---|---|---|---|
| SMILE (UK) | TAU | |||||
| n (%) | Mean (SD) [range] | n (%) | Mean (SD) [range] | Adjusted coefficient | 95% CI | |
| Baseline EQ-5D-5L score (N = 404) | 205 (50.7) | 0.7997 (0.2426) [–0.157–1] | 199 (49.3) | 0.8415 (0.1967) [–0.041–1] | –0.0418 | –0.0016 to 0.0844 |
| Baseline EQ-VAS (N = 404) | 205 (50.7) | 65.2 (22.6) [0–100] | 199 (49.3) | 70.0 (20.1) [0–100] | –3.7687 | –7.7942 to 0.5424 |
| 12-month EQ-5D-5L score (N = 331) | 163 (49.2) | 0.7772 (0.2552) [–0.181–1] | 168 (50.8) | 0.8302 (0.2084) [–0.065–1] | –0.0291 | –0.0143 to 0.0725 |
| 12-month EQ-VAS (N = 331) | 163 (49.2) | 64.9 (20.3) [0–100] | 168 (50.8) | 68.9 (21.6) [5–100] | –1.7472 | –5.6581 to 5.658 |
| QALYs complete case (N = 331) | 163 (49.2) | 0.7903 (0.2163) [0.01–1] | 168 (50.8) | 0.8385 (0.1757) [0.0015–1] | –0.0146 | –0.0072 to 0.0369 |
| QALYs ITT (N = 404) | 205 (50.7) | 0.7872 (0.2087) [0.01–1] | 199 (49.3) | 0.8337 (0.1762) [0.0015–1] | –0.0142 | –0.0322 to 0.0037 |
Cost-effectiveness and cost–utility analysis results
The results from the cost-effectiveness analysis are presented in Table 25. Findings from the complete case analysis show that SMILE (UK) is cost-saving, but produces fewer QALYs than TAU. The associated ICER from a NHS and social care perspective is £5548 and this is how much extra it costs for TAU to produce one extra QALY. This is below the NICE threshold of £20,000. The corresponding ICER from the societal perspective is £88,082 per QALY. Imputed results produced a better ICER from the NHS perspective at £1901 per QALY, but a higher one at £131,268 per QALY from the societal perspective.
| Scenario | Treatment group, mean (SD) | Observed Difference | Adjusted differences (95% Cl) | ICER (using adjusted figures) | |
|---|---|---|---|---|---|
| SMILE (UK) | TAU | ||||
| Complete case | (n = 163) | (n = 167) | |||
| QALYs | 0.7903 (0.2163) | 0.8385 (0.1757) | –0.0482 | –0.0146 (–0.0362 to 0.0071) | |
| QOLIE-31-P | 67.4 (13) | 69.5 (15) | –2.1 | –0.62 (–2.86 to 1.79) | |
| Health and social care costs (£) | 3453 (5970) | 4609 (14,664) | –1156 | –81 (–1755 to 1507) | 5548/QALY |
| 135/QOLIE-31-P | |||||
| Societal costs (£) | 30,732 (55,824) | 30,675 (58,194) | 57 | –1286 (–12,197 to 11,560) | 88,082/QALY |
| 2143/QOLIE-31-P | |||||
| ITT | (n = 205) | (n = 199) | |||
| QALYs | 0.7872 (0.2087) | 0.8337 (0.1762) | –0.0465 | –0.0142 (–0.0318 to 0.0034) | |
| QOLIE-31-P | 67.3 (12.7) | 69.0 (14.3) | 1.7 | –0.62 (–2.54 to 1.40) | |
| Health and social care costs (£) | 3477 (5495) | 4622 (13,537) | –1145 | –27 (–1545 to 1490) | 1901/QALY |
| 1847/QOLIE-31-P | |||||
| Societal costs (£) | 30,688 (50,239) | 31,018 (53,759) | –330 | –1864 (–11,948 to 7458) | 131,268/QALY |
| 3006/QOLIE-31-P | |||||
Findings based on the QOLIE-31-P are presented based on the ITT analysis, and the ICER from the NHS perspective is £1847 for a 1-unit improvement on the QOLIE-31-P. Society, on the other hand, would have to pay £3006 for one additional improvement on the QOLIE-31-P.
Cost-effectiveness planes (Figures 5 and 6) from the NHS and societal perspectives of complete cases show the proportion of scatter points of simulated 1000 bootstrapped mean estimates of incremental cost and outcome pairs (adjusted for baseline costs and EQ-5D-5L scores). Both figures show more scatter points (51%) in the south-west quadrant, which indicates lower costs and fewer QALYs for SMILE (UK) than TAU. Scatterplots produced from imputed data also produced similar results (Figures 7 and 8); thereby providing further evidence that SMILE (UK) had lower costs and inferior QoL than TAU. The spread of scatter points in the vertical plane indicates the existence of some uncertainty regarding the cost savings associated with the intervention compared with TAU.
FIGURE 5.
Cost-effectiveness plane of complete cases (NHS perspective) using QALYs. Scatterplot of bootstrapped resampling of joint incremental costs and QALYs of SMILE (UK) vs. TAU, adjusted for baseline costs and EQ-5D-5L scores.
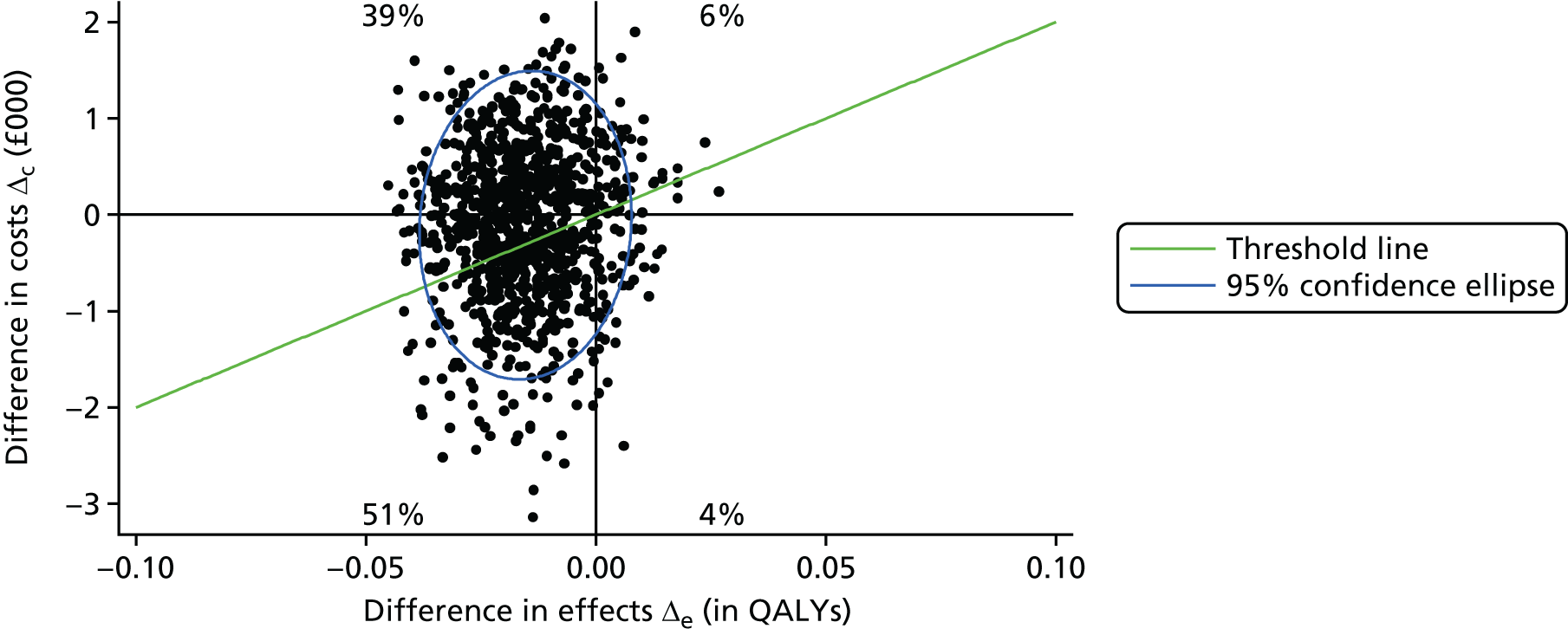
FIGURE 6.
Cost-effectiveness plane of complete cases (societal perspective) using QALYs. Scatterplot of bootstrapped resampling of joint incremental costs and QALYs of SMILE (UK) vs. TAU, adjusted for baseline costs and EQ-5D-5L scores.
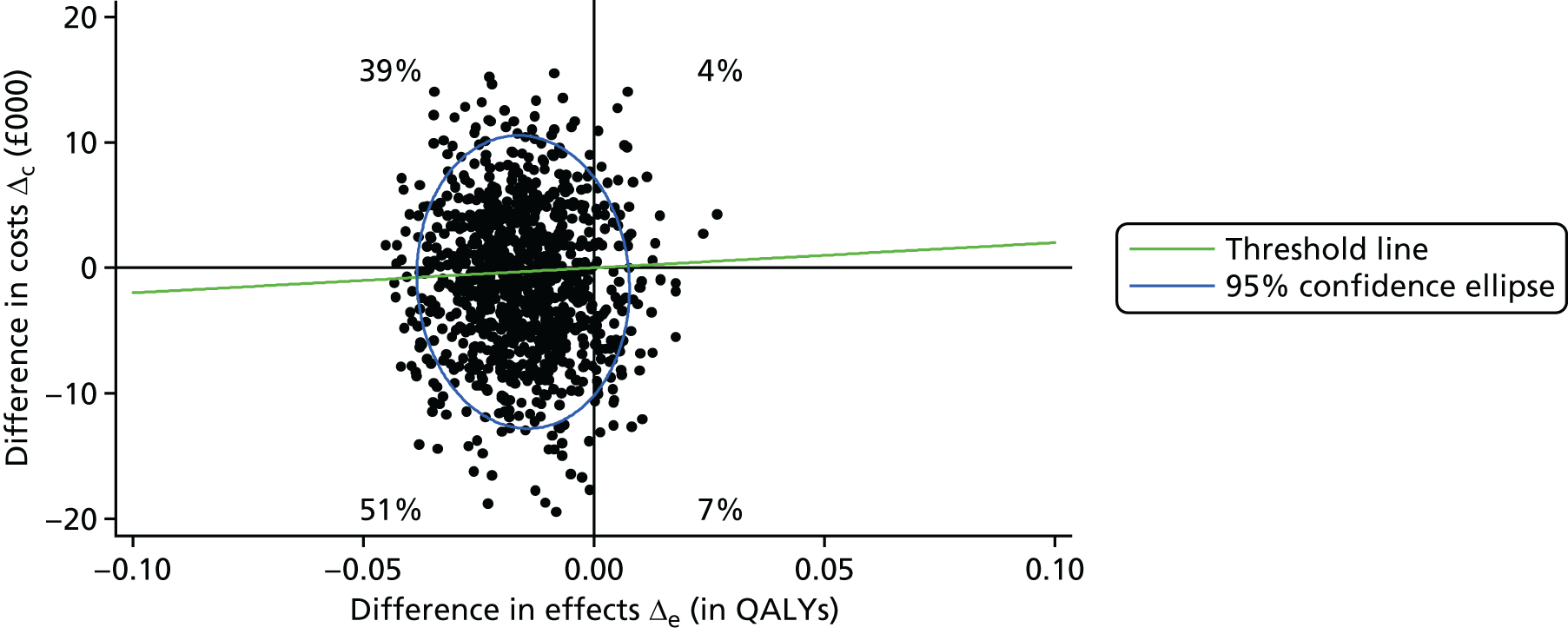
FIGURE 7.
Cost-effectiveness plane of ITT (NHS perspective) using QALYs. Scatterplot of bootstrapped resampling of joint incremental costs and QALYs of SMILE (UK) vs. TAU, adjusted for baseline costs and EQ-5D-5L.
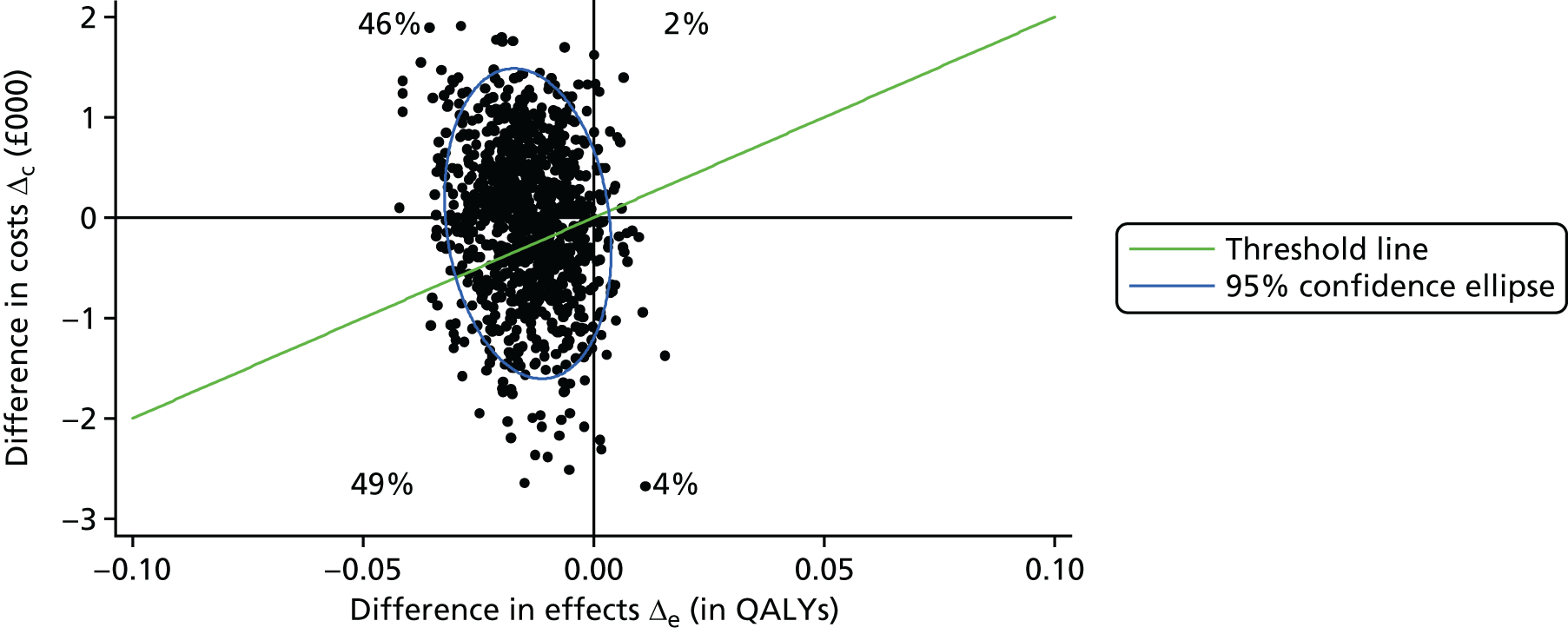
FIGURE 8.
Cost-effectiveness plane of ITT (societal perspective) using QALYs. Scatterplot of bootstrapped resampling of joint incremental costs and QALYs of SMILE (UK) vs. TAU, adjusted for baseline costs and EQ-5D-5L.
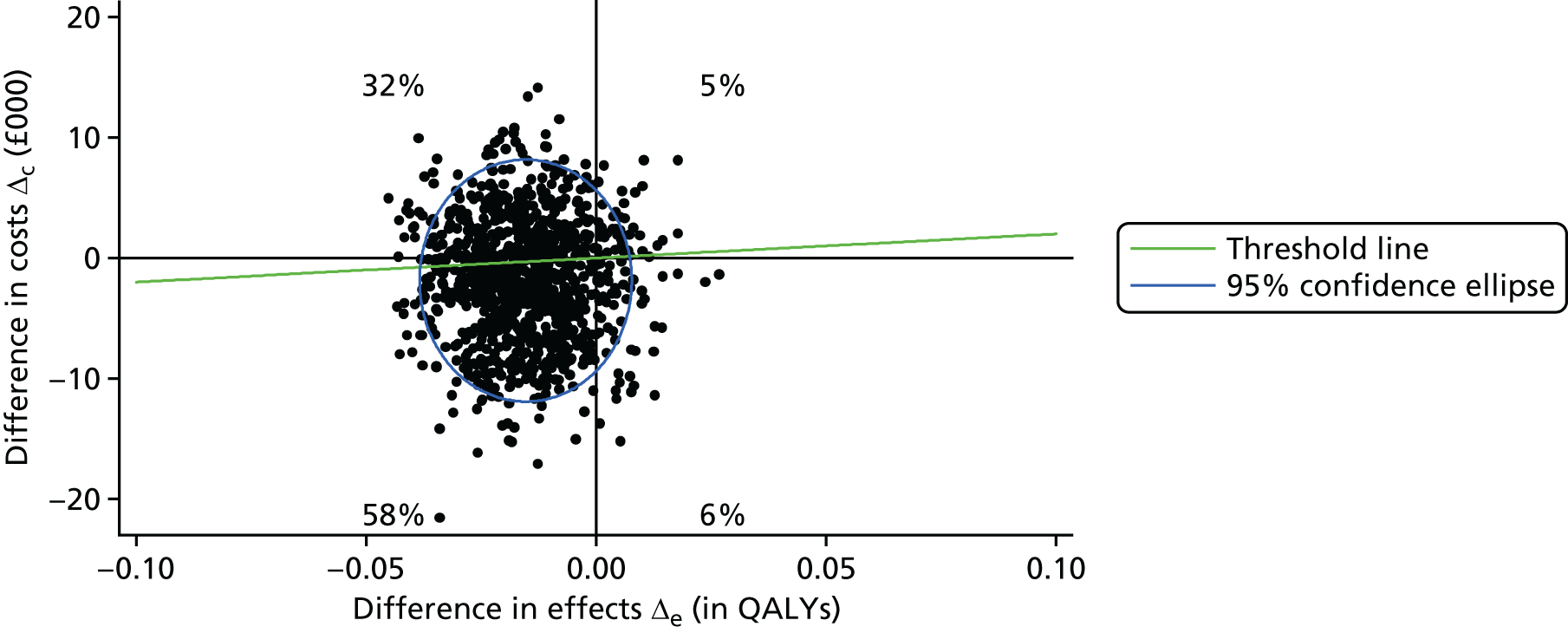
The probability of SMILE (UK) being cost-effective (compared with TAU) at the £20,000 WTP threshold from the NHS perspective (Figures 9 and 10) is slightly above 40% (for both the complete-case and the ITT analyses). However, this probability is somewhat higher (60%) from the societal perspective (complete case, Figure 11; and ITT, Figure 12) at the same threshold.
FIGURE 9.
Cost-effectiveness acceptability curve of complete cases (NHS perspective) using QALYs. CEAC showing the probability of SMILE (UK) being cost-effective compared with TAU for WTP ranges in QALYs.
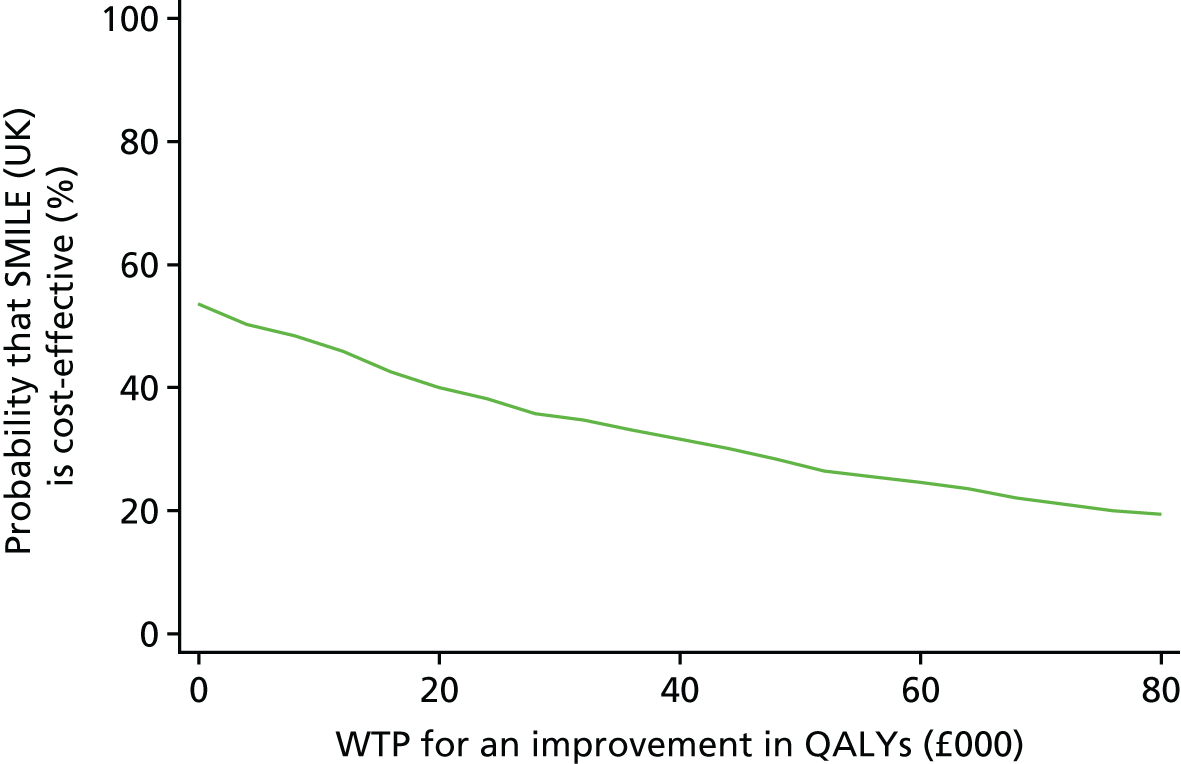
FIGURE 10.
Cost-effectiveness acceptability curve of ITT (NHS perspective) using QALYs. CEAC showing the probability of SMILE (UK) being cost-effective compared with TAU for a range of WTP.
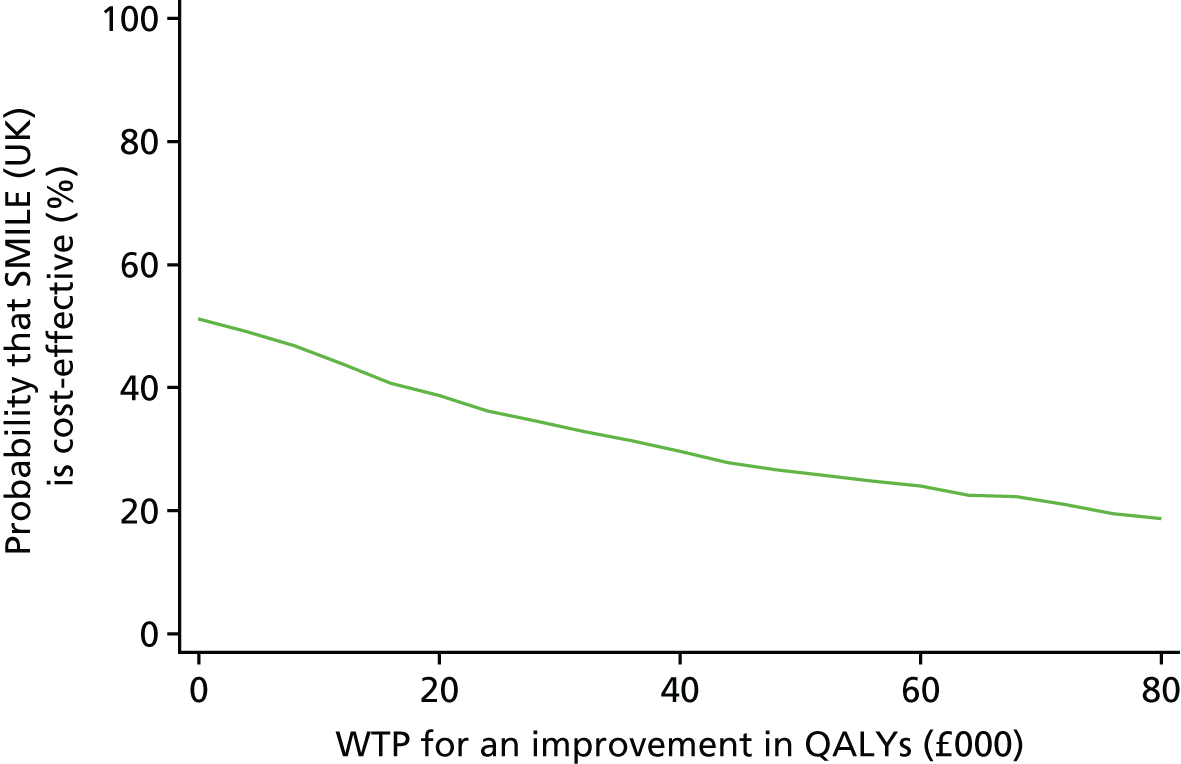
FIGURE 11.
Cost-effectiveness acceptability curve of complete cases (societal perspective) using QALYs. CEAC showing the probability of SMILE (UK) being cost-effective compared with TAU.
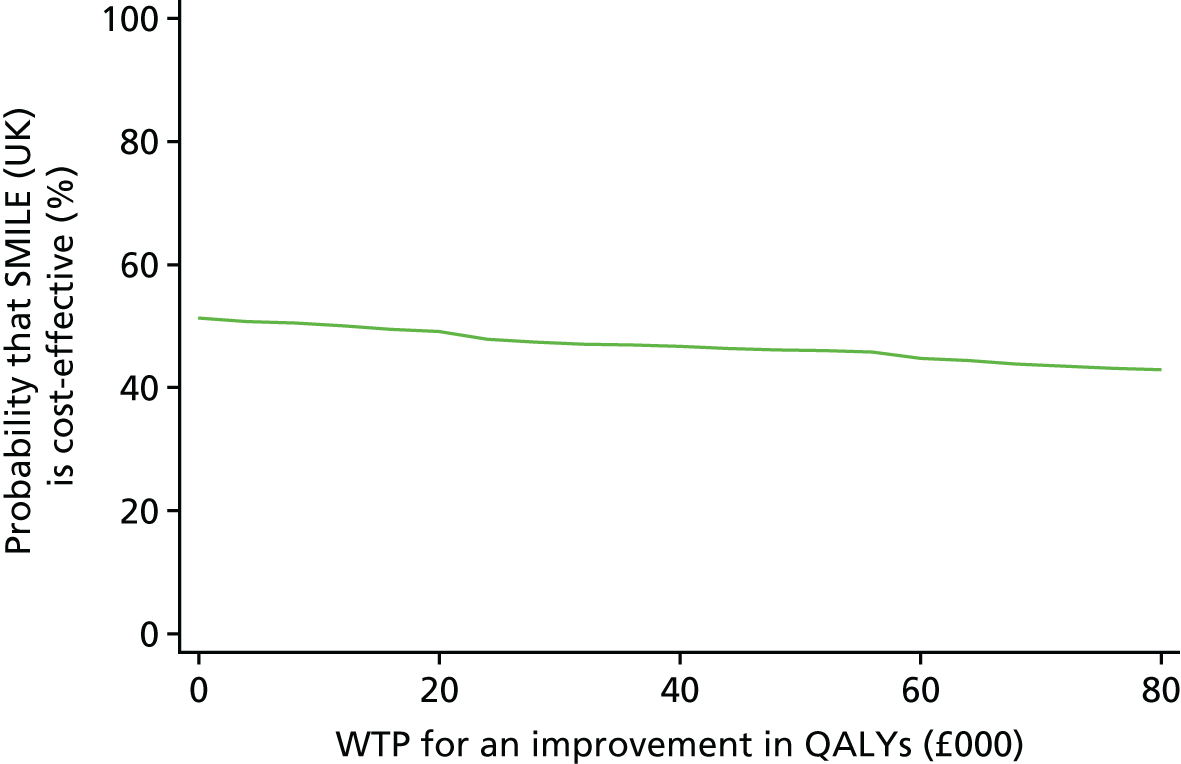
FIGURE 12.
Cost-effectiveness acceptability curve of ITT (societal perspective) using QALYs. CEAC showing the probability of SMILE (UK) being cost-effective compared with TAU for WTP ranges in QALYs.

Quality Of Life In Epilepsy 31-P
Cost-effectiveness results based on the QOLIE-31-P are similar to those emanating from QALYs. Results from the complete-case analysis show the majority (36%) of scatter points in the north-west quadrant, indicating that the SMILE (UK) group costs more and has lower QoL than the TAU group (Figure 13). For the ITT analysis, findings are slightly different from the complete-case analysis, with most scatter points located in the south-west quadrant (i.e. lower costs and lower QoL) (Figure 14).
FIGURE 13.
Cost-effectiveness plane of complete cases (societal perspective) using QOLIE-31-P. Scatterplot of bootstrapped resampling of joint incremental costs and QOLIE-31-P score comparing SMILE (UK) with TAU, adjusted for baseline costs and QOLIE-31-P.

FIGURE 14.
Cost-effectiveness plane of ITT (NHS perspective) using QOLIE-31-P. Scatterplot of bootstrapped resampling of joint incremental costs and QOLIE-31-P score comparing SMILE (UK) with TAU, adjusted for baseline costs and QOLIE-31-P.

Discussion
Offering SMILE (UK) to PWE is cost-saving but does not result in more QALYs than TAU. If we compare the intervention with TAU then we see that the ICER falls into the south-west quadrant of the cost-effectiveness plane. The positive ICERs here need careful interpretation. They indicate fewer QALYs but cost savings. The question here is whether or not the cost savings are sufficient to justify the QALY loss. From an NHS/social care perspective, the ICER is below the NICE threshold of £20,000. As such, we can conclude that the intervention SMILE (UK) does not save sufficient costs to justify the QALY loss and TAU is the preferred option. This is further indicated by the CEAC, which shows the probability that the intervention is cost-effective at £20,000 is below 50%. From a societal perspective, the intervention saves in excess of £80,000 per QALY lost.
From the NHS perspective, the main cost contributors were AEDs, inpatient care and outpatient neurological care. Costs associated with contacts with psychologists and counsellors were also high, indicating the burden of mental health in epilepsy. There was a notable reduction in the cost of hospital emergency visits at 12-month follow-up. A large proportion of epilepsy costs are associated with caregiving by family and friends and these are persistently high.
The QoL based on the EQ-5D-5L was generally high in both groups. The high mean utility scores do not seem to be uncommon in epilepsy,141 which leads us to question whether or not QoL is the appropriate outcome. Few PWE in our study reported being on the lowest levels of the five dimensions of the EQ-5D-5L, which may be consistent with the population group. Some dimensions of the EQ-5D-5L (e.g. pain) may not be relevant to PWE, whose symptoms are not necessarily chronic but recurring. This brings into question the ability of the measure to detect changes and to evaluate the impact of recurring seizures. This was also confirmed in a larger trial,142 which had large proportions reporting ‘no problems’ in the five dimensions.
Chapter 9 Process evaluation of the SMILE (UK) intervention
Introduction
Two qualitative studies were completed during the course of this research. The first study was an external evaluation of the pilot SMILE (UK) course to test the adaptation of the course with content modified for the UK population. 82 The results regarding the course content and its organisation are presented in Chapter 2. Findings regarding patients’ experiences and responses to the intervention are considered in this chapter together with the main qualitative process evaluation of the SMILE (UK) intervention. 83 Both qualitative studies have already been published82,83 and this chapter provides a summary of themes emerging from these studies.
Qualitative study: process evaluation
The process evaluation took place 2 years after the initial pilot and within 6 months of receiving the SMILE (UK) intervention. The aims of the process evaluation were to complement the quantitative evaluation of SMILE (UK) by providing more detailed descriptions of participants’ views and experiences.
Methods
Recruitment
Of the 404 participants recruited into the SMILE (UK) trial, 24 were initially approached to take part in the process evaluation. They were largely recruited from the TAU arm, but also included some from the intervention arm (before the protocol was amended; see Chapter 3). The list of participants was selected based on the register of attendance from the January, May and June 2015 courses. People were chosen to ensure that a variety in age, gender, ethnicity and severity of epilepsy was represented. Two participants were unable to remember taking part in the SMILE (UK) course and two participants were not available to complete the research interview. Overall, 20 participants agreed to take part in the qualitative interviews.
Interview procedure
Semistructured interviews were held with topics selected after completing the external pilot study, and in collaboration with research staff that held these interviews. Although the pilot study topics focused on experiences of participating in the course, the main study topics were more tailored to the patient’s experience of epilepsy, as well as their views on the SMILE (UK) course. An abbreviated topic guide can be found in Box 2 (see Appendix 7 for full guide). Topics included (1) participants’ experience of living with epilepsy, (2) negative and positive aspects of the course, (3) social contact after the course and (4) if or how they used what they had learned in the course. Interviewers probed and clarified responses as required. All participants were interviewed face to face. The interviews were undertaken by two researchers and interview times were variable, ranging from 25 to 40 minutes each.
Can you tell me a bit about your epilepsy? How did you feel when you were first diagnosed? How do you feel about it now?
Help-seeking behaviourIn the past, have you tried to find out more about your epilepsy? How?
SMILE (UK) courseWhen you first heard about SMILE (UK), how did you feel about coming on the course? How did you feel about being part of a group? How did you feel about hearing other people’s stories? Did you find out any things that were helpful?
Life after the courseDo you think the SMILE (UK) course has helped you in managing your epilepsy? Although the workbook is not an essential part of the course, have you found it useful?
Social networkHave you stayed in touch with anyone from the course?
Course improvementsWould you recommend other PWE to go on the SMILE (UK) course? Is there anything about the course that you would like to change?
Analysis
All interviews were recorded and then transcribed verbatim by an external third party. Transcripts were checked for accuracy by the researchers who conducted the interviews, and sample scripts were then checked by two collaborators (MM, LR). Researchers then undertook a line-by-line coding approach with initial codes noted in the margins of the transcripts and then grouped into broader themes. This process involved regular discussion between the researchers and study supervisors (MM, LR).
External pilot evaluation
The external pilot evaluation identified reasons for participating in the trial, views of the SMILE (UK) course and suggestions for changes (reported in Chapter 2). In addition, it provided data on their views of the intervention which complement the formal process evaluation. These findings from the two studies are briefly summarised in this chapter.
Methods
The methods of the external pilot are described in Chapter 2 and differed from the process evaluation in three main ways.
-
Data were based on 10 participants.
-
Participants were volunteers recruited through Epilepsy Action.
-
Data gathered by semistructured interviews (seven participants) and a focus group (three participants).
Despite these differences, the findings regarding patients’ experiences and responses to the SMILE (UK) intervention identified similar themes that provide support for their validity and conceptual generalisation.
Characteristics of participants
Both studies were successful in including a range of participants (i.e. age, gender, education and years since diagnosis) (Table 26). Between the pilot study and process evaluation, the main difference in participants was the higher level of education in the external pilot study with 5 out of 10 having a degree, compared with 4 out of 20 in the process evaluation. This is likely to reflect the differing sources of recruitment with the external pilot study being recruited through Epilepsy Action, while the main evaluation was based on participants recruited from usual NHS clinic settings. Similarities between the two groups were the proportion of participants with epilepsy for over 10 years (7/10 of external pilot and 15/20 of process evaluation) and the number employed (3/10 external pilot and 6/20 main evaluation).
| Participant number | Characteristic | ||||
|---|---|---|---|---|---|
| Age | Gender | Years since diagnosis | Education level | Employed | |
| External pilot | |||||
| 1 | 33 | F | 13 | PG | No |
| 2 | 21 | M | 21 | SS | No |
| 3 | 48 | M | 47 | SS | No |
| 4 | 60 | F | 44 | UG | No |
| 5 | 53 | M | 52 | SS | Yes |
| 6 | 40 | F | 34 | UG | No |
| 7 | 32 | F | 9 | SS | Yes |
| 8 | 32 | M | 8 | UG | No |
| 9 | 21 | F | 15 | SS | No |
| 10 | 29 | F | 8 | UG | Yes |
| Process evaluation | |||||
| 1 | 32 | F | 10 | SS | No |
| 2 | 81 | F | 24 | SS | No |
| 3 | 46 | M | 19 | SS | No |
| 4 | 50 | M | 20 | None | No |
| 5 | 41 | M | 29 | UG | No |
| 6 | 38 | M | 4 | UG | Yes |
| 7 | 36 | F | 35 | Other | No |
| 8 | 59 | M | 3 | None | No |
| 9 | 52 | F | 49 | None | No |
| 10 | 65 | F | 46 | UG | No |
| 11 | 55 | M | 2 | None | No |
| 12 | 54 | F | 33 | SS | No |
| 13 | 27 | F | 16 | SS | Yes |
| 14 | 51 | F | 40 | SS | No |
| 15 | 39 | M | 13 | UG | Yes |
| 16 | 22 | F | 6 | SS | No |
| 17 | 38 | M | 10 | Other | Yes |
| 18 | 54 | F | 43 | UG | No |
| 19 | 38 | M | 6 | SS | Yes |
| 20 | 58 | M | 36 | UG | Yes |
Findings
Main themes
Four themes were strongly represented in both studies (Figure 15), namely the importance of the intervention for reducing isolation, the opportunity to learn from each other, finding reassurance in the experiences of others and changing behaviours and practices after learning from others.
FIGURE 15.
Common themes reported by participants in the external pilot and process evaluation.
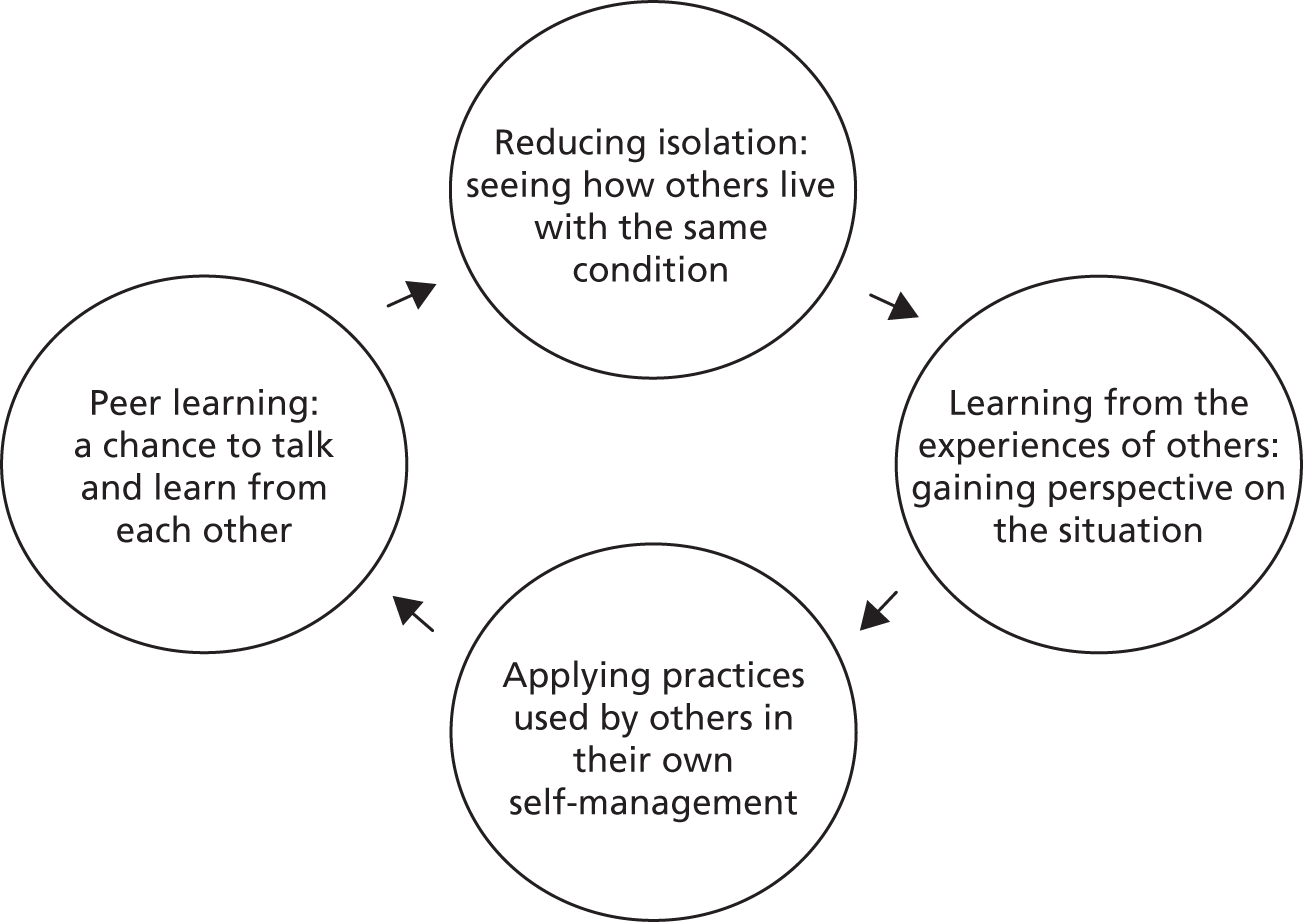
Reducing isolation
Peer support and a sense of feeling ‘less alone’ were common themes and outcomes of the intervention. Many participants had not discussed their epilepsy with anyone before other than family or health-care staff, and described how they appreciated being able to meet others with the same condition:
Nobody without epilepsy can really understand what it’s like to have a seizure . . . To see how they [other course members] deal with it makes it more easy to live with your illness because you think ‘I am not alone’.
M, 39, process evaluation
At the conclusion of the course, many attendees had shared contact information and reported that they would keep in touch with group members in the future.
Therefore, the group setting of the SMILE (UK) course provided a positive sense of being connected through the shared experience of having epilepsy:
Normally, I’d be a bit self-conscious about these groups but once I got used to it, knowing that everyone’s like, the same, as me and there’s like a big understanding amongst the group and become friends and stuff, it was actually pretty good.
M, 21, pilot study
A chance to talk and learn from each other
Epilepsy-related knowledge was imparted not only by facilitators (as with traditional teaching), but also by other group members. Participants were asked to share their experiences of coping and, by doing so, taught others about how they had overcome epilepsy-related challenges. This observation was noted by participants in both studies:
I was very keen to meet other people with epilepsy and learn new information . . . it was really interesting to see a variety of perspectives based on personal experiences . . .
F, 21, pilot study
Group members who had been living with epilepsy for a long time were able to speak with others still coming to terms with their own diagnosis. In this way, the person sharing was seen as the ‘expert’ of their own experience, with knowledge to be shared on how they had coped with past challenges.
As highlighted by the following quote, the experience of being able to share their feelings with others had the benefit of being cathartic for some:
I felt that I wanted to discuss my story . . . When I said it out loud it was a way of admitting to myself just how bad I have been over the years.
F, 54, process evaluation
Finding reassurance in the experiences of others
The benefit of social interaction during the course was multifaceted. By speaking with people who were at different stages of coping with epilepsy, those who were managing well seemed to realise how far they had come in their own process.
Many were quick to point out they were not glad that others had suffered, but grateful that their circumstances were not as bad as they could be. Therefore, the process of social comparison and interaction contributed to a change in attitude about their own epilepsy. One participant also spoke of how his perspective had shifted since participating in the interactive group sessions:
I felt like I was the only one dealing with it really and it was getting me down . . . and I met the other people who have got it, I kind of changed my mind . . . I look at it in a different way.
M, 47, process evaluation
Changes noticed after the course
Participants described a number of changes after attending the SMILE (UK) course. In the process evaluation, 12 out of 20 participants spoke of changing their self-management practices after the course. Behaviour changes after the course included reducing alcohol intake, record keeping of seizures and improved medication adherence:
The programme has helped me to understand more about epilepsy and medication. I take my treatment more seriously now.
M, 39, process evaluation
Some spoke of sharing their newfound knowledge with others. Half of the participants in the process evaluation felt they were now talking more with others about their condition.
Fourteen participants across both studies (4/10 in the pilot study and approximately 10/20 in the process evaluation) described improved self-confidence after the course. This empowered many to have an open dialogue with their neurologist, GP or ENS either to suggest or to resist changes in medication. As one participant explained:
Last time I saw my neurologist he wanted to increase the medication. After having the course, I had the confidence to say that it isn’t worthwhile increasing the medication. I know that I’d only had these seizures because I hadn’t taken the medication.
F, 22, process evaluation
Similarly, participants described feeling more informed when discussing treatment options with their clinician, empowering them to take an active role in their health care:
It’s empowering you when you got to see the doctor to be more two-way about the discussion.
F, 60, pilot study
The participant handbook was also described as a new and useful tool in improving knowledge about epilepsy. Nine out of 20 participants from the process evaluation read the workbook during or after the course. Four participants had lent it to family members or friends. The workbook was used to access what they had learned during the course and to teach others about epilepsy. As one participant remarked:
I can’t stop carrying it around. Before I used to carry around my iPad all the time and bring that out all the time. But this is what I have to read all the time.
F, 32, pilot study
Limitations of SMILE (UK)
A group course offers participants the opportunity to share their own experiences and to learn from others. In cases when language or learning abilities vary, some participants may not understand the exercises or what is being discussed. PWE who had severe learning disabilities or were not able to understand English were excluded from the RCT. However, we recruited PWE from a diverse metropolitan population with varying levels of English mastery and learning skills. For three participants in the process evaluation, this was an issue. Four participants reported memory issues, which are frequent in PWE. This later had an impact on what they retained from the course and whether or not they could remember to do things in ‘real life’. And as mentioned above, two did not remember the course at all and thus were not interviewed. Together, nine PWE had language, learning or memory problems that affected what they retained from SMILE (UK).
Summary
Similar themes arose from the pilot study and process evaluation. Participants found the course informative and particularly appreciated the opportunity to meet other PWE. Sharing their own experience with others led to some participants feeling more empowered. With the reflective exercises in SMILE (UK) (see Figure 1) using stickers on a scale, participants could compare themselves with others and, for some, this meant thinking about epilepsy in a more positive way.
Participants reported behaviour changes as well. Thirteen participants felt increased self-confidence, and this included half of those interviewed in the process evaluation. Over half of those interviewed as part of the process evaluation applied self-management practices learned during the course, such as keeping a seizure diary or reducing alcohol intake.
Memory, language and learning difficulties play a significant role in how group courses can impact PWE. A large proportion of the group interviewed as part of the process evaluation felt the impact of such issues and, thus, a 2-day course may have a limited impact at 1-year follow-ups.
To our knowledge, there is only one other study that evaluated patients’ views of a group intervention for PWE. 115 However, it was not a qualitative study but was carried out via open-response sections on a written questionnaire. PWE in both studies appreciated meeting others like them, some for the first time. This reinforces the benefit of group self-education courses for PWE.
Chapter 10 Discussion
Summary of principal findings
External pilot
An external pilot, undertaken immediately after two pre-trial SMILE (UK) courses, demonstrated the feasibility and acceptability of providing group courses among volunteers with epilepsy in the UK. 82 Having two facilitators and locating the courses near a hospital centre can be an advantage when PWE report frequent seizures.
Main outcomes
We described the characteristics of 404 participants at baseline and the relationship between clinical, psychological and social factors and an epilepsy-specific QoL measure (QOLIE-31-P). 84 Participants’ mean age was 42 years, 54% were female and 75% were white. Median time since diagnosis was long at 18 years and a large proportion (69%) had experienced ≥ 10 seizures in the prior year. Nearly half (46%) reported additional medical or psychiatric conditions, 64% reported current anxiety and 28% current depression symptoms at borderline or case level, with 63% reporting felt stigma.
Baseline QoL was measured by the mean QOLIE-31-P (range 0–100). The mean score was 66, with a wide range (25–99). Psychosocial impairment at baseline appeared to be most closely linked to impaired QoL. In order of descending magnitude of association: current depression, anxiety, a history of medical and psychiatric comorbidity, felt stigma, greater seizure frequency, low self-mastery and low self-reported medication adherence were associated with lower QoL scores.
There were no significant differences in the primary outcome measure (QOLIE-31-P at 12 months) between the treatment groups [intervention mean 67.4 (SD 13.5) vs. control mean 69.5 (SD 14.8). Nor was there a significant difference at 12 months in any of the secondary outcome measures, including seizure control, impact of epilepsy, medication adherence, medication adverse effects, psychological distress, epilepsy stigma and self-mastery. 114
A process evaluation undertaken within 6 months of the intervention found that participants valued the opportunity to meet ‘people like them’. The structured learning methods encouraged them to share and compare feelings and experiences. Specific benefits included overcoming the sense of ‘being alone’ and improving self-acceptance through meeting people with similar experience. Over half reported that this and the group-learning exercises, eliciting comparison of attitudes and experience, helped them to improve their confidence to talk openly, as well as to make changes in their health behaviours.
However, almost half of those participants approached for interviews reported they experienced language or memory problems, which limited their ability to understand and/or learn on a group course, and sometimes their ability to manage their activities or behaviour changes in practice. Memory problems are well described by many PWE, particularly those with recurrent seizures. 143 Mood disturbance (like anxiety and depression) is also associated with self-reported memory impairment in epilepsy. This makes it possible, even likely, that without psychological interventions and reinforcement of a 2-day course for people with persistent seizures, it will be difficult to demonstrate sustained improvements after a 1-year period. Even those with average memory may struggle to recall the content of a 2-day course 1 year later. For this reason, Taylor et al. 59 emphasised the importance of integrating and reinforcing self-management education during routine care.
Recruitment
Recruiting volunteers via advertisements or through user groups requires an active response from patients. Those responding are often already more engaged in their health care and can result in a group less representative of the patient group as a whole. 144 Our study recruited from routine NHS epilepsy clinics. We used an opt-out process in creating patient lists from clinic lists, reducing to an extent how engaged patients needed to be in order to remain in the recruitment process. In a patient population who frequently report memory problems, such as those with epilepsy, people may forget to respond to invitations. It is also known that men are less likely to participate in research, including self-management trials. 145 Nevertheless our recruitment process resulted in a group fairly balanced between males and females.
Owing to the UK data protection regulations, which do not allow researchers to screen notes without consent, a large number of letters were sent out to patients attending neurology clinics prior to screening for eligibility for the study. This makes it difficult to assess the rate of enrolment as the first stage of recruitment included people without a confirmed diagnosis of epilepsy, people who had moved away and some with no recent seizures. Thus, we report the rate of enrolment from the final patient list, which contained patients who had not opted out of the recruitment process and who were deemed to meet the eligibility criteria. This list contained 1986 people, of whom 1458 could be contacted and 1088 were found to be eligible. Therefore, our recruitment rate was 37%. PWE may be reluctant to commit to a 2-day course because of the unpredictable nature of epilepsy, especially if additionally experiencing anxiety, depression and/or perceived stigma. Despite this, our recruitment rate was equal to or higher than that we have achieved in a prior individual self-management intervention for PWE recruited from EDs (27%). 12
Retention
At 82%, our retention rate in the study was high. There was no difference in follow-up rates between the two intervention arms, although those in the intervention arm who attended the course were more likely to complete the study (see Table 11). From the total group, 73 participants did not complete the study. Most (53 out of 73) could not be reached for the 12-month follow-up and, therefore, were withdrawn from the study. This can be an issue in large cities and in some of our catchment areas, where there is a 17% resident turnover per year. 146 Factors associated with loss to follow-up were being female, fewer years in education and more comorbidity.
Generalisability
We have already commented on recruitment and retention, which affect generalisability.
Our complex intervention trial made considerable demands on adults with poorly controlled epilepsy in terms of completing long questionnaires, attending a 2-day course and, for some, in-depth interviews. Recruitment might have been greater if the trial had been undertaken with participants who included volunteers, who were predominantly white and who were of higher socioeconomic and educational status, if the workload required of them had been less and, in our past experience, if they were recruited by GPs in primary care. 115,145,147
The pattern of predictors of high/low recruitment to complex interventions is consistent across research and also predicts uptake of educational interventions in routine NHS practice. For example, the offer by the NHS of a course on diabetes mellitus in south London met with a 30% uptake. 146 The diabetes mellitus group found that characteristics that reduced uptake of the offer of a course included lower socioeconomic status and black and ethnic minority status. 146
We offered an epilepsy course untested in the UK, which also required the completion of a questionnaire three times over 1 year and achieved a similar response rate as the offer of a fully evaluated and commissioned course for people with diabetes mellitus in practice. 146 Our recruitment rate was similar and the response rate at 1 year compared well with a more recent study of a UK cohort of PWE in primary care. 145 That research group found that, among all LTCs they studied, PWE had the lowest response rate. We therefore believe that our findings are as representative as it is possible to be of people with long-term poorly controlled epilepsy living in cities. We believe that they are generalisable to people internationally who are in receipt of specialist care in the context of a universal health-care system.
Meaning of the study
Quantitative findings
Baseline
Among this group of people recruited from epilepsy clinics, a large proportion (69%) had experienced ≥ 10 seizures in the prior year. Moran et al. 11 found that, among a UK sample of PWE recruited through general practice, 52% had no seizures in the prior year. Among those with two or more seizures in the prior 12 months, 57% had ≥ 10 seizures in the prior year. 11 Comparing this with our sample, who were recruited from epilepsy clinics, shows that more participants in our group (69%) had frequent seizures. This may partially reflect a tendency for GPs to refer to specialists those PWE whose seizures are more difficult to control. 11
Little is known about population-level QoL among PWE in the UK. 116,145 In this context, and prior to carrying out an evaluation of the effect of a self-management course on QOLIE-31-P, we aimed to answer the questions (1) What are the clinical and psychosocial characteristics of UK adults with frequent/poorly controlled seizures? and (2) To what extent are clinical and psychosocial characteristics, which underlie constructs of QoL, associated with QOLIE-31-P?
For the purpose of comparisons to other studies, we were able to derive the more commonly reported QOLIE-31, without patient-weighting (‘P’). The mean total QOLIE-31 score of our group was similar to studies reported of PWE from other countries for people with all types of epilepsy. Our group’s mean total QOLIE-31 was 62.0 (SD 15.6), which is similar to the global mean score of 59.8 (SD 8.0). 116 Considering that PWE in our SMILE (UK) study had persistent and frequent seizures, which are generally associated with lower QoL, their QoL was better than might be expected internationally. 116,148
Comparing our results with other national studies, a recent cohort study by Peters et al. 145 of PWE in UK primary care found higher QOLIE-31 total scores than our group (baseline QOLIE-31 mean 69.9, 1-year QOLIE-31 mean 70.13). Their study included all PWE regardless of seizure frequency. Their group would be expected to have higher QoL because half to two-thirds of PWE in the population registered in primary care report no seizures in the prior year (Mark Ashworth, KCL, 2017, personal communication). The findings of Peters et al. 145 suggest that in the UK the mean QoL in all PWE is about 8 points higher than it is among PWE identified in specialist clinics with frequent seizures.
Evidence from our UK group is consistent with evidence internationally that PWE and particularly those with persistent, frequent seizures have significant psychosocial disadvantage and impaired QoL. 26,116,148–151 Luoni et al. 148 suggest that when epilepsy is accompanied by persistent seizures there is ‘a diagnostic gap’ when it comes to depression. Screening for depression has been recommended,26 but is still not routine. In PWE who have persistent seizures, anxiety symptoms are even more common than depression. This is not surprising as PWE know seizures can lead to injury, hospitalisation and even death. 7,35,152,153 Anxiety is a second diagnostic gap. 154
In the NHS, primary care groups were rewarded for annual epilepsy monitoring for 10 years but, in 2014, this scheme was withdrawn. 155 Primary care groups were also remunerated for identifying psychological comorbidity in LTCs, such as heart disease and diabetes mellitus. During this time, identification of psychological comorbidity increased. 156 Compared with other LTCs, psychological comorbidity is equally or more common in PWE. 157 This is important in PWE, particularly as depression is also a predictor of poor epilepsy control and premature death. 35,158 In the UK after diagnosis, specialists follow up/monitor less than half of PWE69 and do not generally have multidisciplinary team support to address mental health issues, even if they identify them.
Post-intervention outcomes
In our trial, a 2-day educational course for people with epilepsy and persistent seizures did not change participants’ QoL after 1 year, nor were secondary outcomes changed, including psychosocial characteristics. This is consistent with evidence from two other trials of self-management educational interventions,1,2 which were cited in the NIHR funding call (see Appendix 1). These two trials followed participants up for a shorter period, that is, at 4 and 6 months. 1,2 A more recent trial, which recruited a small, highly educated group of participants (an unspecified number of whom were volunteers) in a fee-for-service health-care context, has found aspects of QoL were improved immediately after the course, but not subsequently at the 6-month follow-up. 115 This evidence supports a hypothesis that a discrete course in isolation is not likely to affect QoL for PWE.
A Colombian trial that included an educational epilepsy course together with four cointerventions, including monthly ongoing advice from a pharmacist, demonstrated an improvement in QoL at the 6-month follow-up. 117 However, QoL at baseline was about 10 points lower than is usual in resource-rich countries, most of which have universal health care. Baseline QoL was, however, not significantly different from that pertaining in other South American countries. It is likely that improvement in QoL from a low level is more easily achievable in this context. In addition, medication adherence for LTCs is a known challenge. 159 Attendance at the course itself was poor. It may be that the regular one-to-one pharmacist intervention was key to improvement in QoL. There was no process evaluation to assess the ‘how’ and the ‘why’ of this. Nonetheless, it supports a hypothesis that when mean QoL is low at baseline, it can be sensitive to a large quantity of additional condition-specific services.
Our own, and others’, findings are that psychological factors are closely associated with QoL. 3,4,26,73,116,160–163 Therefore, it is not surprising that different sorts of interventions, which focus on treating psychological distress with cognitive behavioural therapy or with acceptance and commitment therapy, show more promise in improving in QoL for PWE. 164–166
Comparison with MOSES
The RCT methods of evaluating MOSES and SMILE (UK), and the participant groups, differed in many ways. 1
-
The participants recruited in MOSES from epilepsy centres were informed about the trial via advertisements. Participants needed to actively engage prior to enrolment.
-
There was no minimum seizure requirement to participate in the MOSES study; 42% of the group did not have a seizure in the previous 6 months.
-
A total of 35% had comorbid conditions in the MOSES evaluation compared with approximately 50% in SMILE (UK).
-
The MOSES evaluation control group had a statistically significant longer median duration of epilepsy (18.2 years) than the intervention group (13.5 years). Thus, the duration of epilepsy in the MOSES control group was similar to the SMILE (UK) group, whereas the group receiving the MOSES intervention had a more recent diagnosis.
-
Seizure frequency was measured over a period of 6 months (6 months prior to intervention and 6 months post MOSES intervention). SMILE (UK) reported seizure frequency at 6 months and 12 months post intervention. SMILE (UK) also evaluated self-reported seizure frequency over the previous 12 months with a top category of ≥ 10 seizures.
-
The analysis of MOSES outcomes was based on a per-protocol assessment, which took into account participants having completed questionnaires at both follow-ups only. From 383 recruited participants, 242 remained in the analysis (63%).
-
The MOSES study included a ‘knowledge-of-epilepsy’ questionnaire, designed specifically for the study.
-
In the MOSES study, the longest follow-up duration was 6 months after the course.
The MOSES study resulted in an improved score with regard to knowledge measured by the questionnaire designed for the study, and also with regard to coping with epilepsy scores and in seizure frequency. No improvement was found on their generic measure of QoL. Statistical analysis using a per-protocol design was used to evaluate the efficacy of an intervention, or the effect of treatment receipt. The SMILE (UK) RCT was designed to evaluate the effectiveness of SMILE (UK) or, in other words, the effect of an offer of the intervention on the group. Efficacy results tend to be greater as they only include participants who have received the intervention. Participants not completing the assessments or the course are thus excluded. This can lead to bias in interpretation of the results as randomisation is no longer in effect. In our secondary analysis, we included a CACE study for the primary outcome to account for participants not receiving the intervention. We found no difference in QOLIE-31-P total scores at the 12-month follow-up between the participants in the SMILE (UK) group who had received the full intervention and the control group.
The only measure in common with SMILE (UK) that showed an improvement with the MOSES intervention was seizure frequency (although it was assessed differently). A greater proportion of participants in the MOSES group improved in terms of seizure frequency (i.e. they improved by two seizure frequency categories). As the actual numbers of participants in each category were not published, comparisons with SMILE (UK) are not possible. As the last follow-up was short, it is unknown whether or not the changes observed at 6 months would have persisted at 1-year follow-up.
Measuring knowledge and behaviour change
Evidence from prior trials supports the hypothesis that education can affect knowledge of epilepsy scores in people with well controlled and uncontrolled epilepsy. 1,2,95 However, the follow-up periods in these trials were 4–6 months, much shorter than the 12-month follow-up used here. This may be important in light of the memory problems described particularly by people with poorly controlled epilepsy.
In an earlier trial for people whose epilepsy was long term, we found a non-significant trend towards higher knowledge of epilepsy scores following a nurse intervention. 167 In a subsequent trial of nurse advice for people with new epilepsy, we found that for those groups of participants in lower quartiles of knowledge-of-epilepsy scores at baseline, there was improved knowledge-of-epilepsy scores after the nurse intervention. 95 These trials had a short follow-up period of 6 months.
In view of the above, we did consider adding a knowledge-of-epilepsy questionnaire in this trial, but decided not to do so. The reason for this was that the number of questionnaires needed to provide the information specified by our funding agency already demanded much time and energy from participants. More questionnaires might have reduced their participation and response rate at follow-up. These were also important concerns for our funders, ourselves and our ethics committees which reviewed the research proposal.
Studies of other LTCs with large numbers of participants have examined associations between knowledge increase and adoption of taught behaviours, and between adoption of taught behaviours and health outcomes;58 however, the associations were weak. A sequential concept of the health education triggering change in health outcomes is probably too simple. Lorig et al. 168 point out that patient education can bring about changes in behaviour and in health status, but the mechanisms involved are still not clear. 168 Research in many LTCs confirms that linking education to behaviour change and other outcomes is a continuing challenge. 59,169 Given the high prevalence of LTCs and the impact that they have on the individuals and service cost, there is a need for further research on this.
We believe that such research, especially in conditions such as epilepsy, for which few complex interventions have been evaluated, should be exploratory at first. The conclusions from this study suggest researchers need to be given flexibility in determining potential outcomes, undertake cohort studies to identify predictors of change and continue to use mixed-methods research, with process evaluations to describe ‘how’ and ‘why’ complex interventions work, or fail to do so.
Process evaluation: key themes
Social isolation and stigma
In the quantitative part of the study, we found that 63% of participants felt stigmatised at baseline. In prior work we had found that stigma is more common when seizures have occurred more recently. 170 Those with newly diagnosed epilepsy have also been found to be at particular risk of perceived stigma. 171 Nevertheless, using the same stigma scale, Taylor et al. 149 measured felt-stigma among UK adults with newly diagnosed epilepsy and found a lower percentage of patients (53%) reported stigma than our group with epilepsy and persistent seizures. One inference may be that the recency and frequency of seizures is more powerful in its influence on an individual’s sense of self-stigma than the diagnosis of epilepsy itself.
The process evaluation helped us to understand this finding. It highlighted that, despite having epilepsy for a median of 18 years and being in receipt of specialist epilepsy care, PWE and people with persistent seizures still felt isolated from other people like them. In stigmatised conditions, people may tend to conceal their condition and withdraw socially. 42 We infer that either participants’ lack of sharing with peers early on after diagnosis and/or their continuing seizures may have had a continuing detrimental effect over time, reinforcing a sense of isolation and loss of self-esteem, and potentially reducing their ability to learn and apply epilepsy self-management skills.
In 1986, Scambler and Hopkins42 described felt-stigma in patients recruited from UK primary care. In the 30-year period since then, attitudes may or may not have changed to reduce this. 18 There are now also different populations living in big cities. According to the census from 2011, London’s population was just under 60% white, 19% Asian/Asian British and 13% black/African/Caribbean/black British. 172 In the context of a prior study,173 we described that felt-stigma was associated with higher usage of EDs for seizures in PWE. Non-white individuals were less likely to participate in a study of nurse-specialist education aimed at increasing self-management among those attending an ED. 174 At follow-up 1 year later, people of non-white ethnicities were more likely to use an ED for their epilepsy, generating more health-related cost. 12 In a follow-on qualitative study, we found that felt-stigma appeared greater among people from sub-Saharan Africa than it was among Caribbean people. 175 Epilepsy stigma is greater in resource-poor countries18 and these attitudes are likely to persist and may have clinical importance when epilepsy is diagnosed, and at follow-up, in Western cities with multiethnic and multiracial populations.
In this study, most participants had waited in clinics with people with similar experiences of epilepsy on each occasion they visited the hospital. But presumably they had not been aware of this or talked to others about it. In the UK, people with diabetes mellitus visit departments that are clearly labelled the Diabetes Clinic. This is not generally the case for PWE. Respect for confidentiality in a stigmatised condition may reinforce the impression that epilepsy cannot be acknowledged publicly and, in this context, PWE may be less likely to share information about their condition with others in the clinic space or elsewhere.
Learning by sharing and comparing experiences in a group
The process evaluation found that the sense of ‘feeling alone’ can be ameliorated by group learning with others with similar experiences. This may improve self-confidence and self-management. In a classical text on diffusion of innovations, Rogers176 described that for change in behaviour to be adopted, not only knowledge but also attitudes need to change. Through qualitative studies, researchers are just beginning to understand the importance of group work in stigmatised conditions. 169,177 As described earlier, the relationship between these characteristics is not clear in other conditions or in epilepsy. There is evidence from some studies of PWE, with and without seizure control, that learning more about epilepsy can enhance self-mastery/efficacy. 1,89 This relationship could not be confirmed in the quantitative aspect of our study with people with poorly controlled epilepsy at 12-month follow-up. It is possible that having recurrent seizures over time compounds people’s sense of loss of control and self-mastery. After a median of 18 years with epilepsy and persistent seizures, it is perhaps not surprising that a 2-day course has no measurable effect 1 year later. It is likely to have been too little, too late.
Memory in poorly controlled epilepsy and its implications for learning and practice
The process evaluation highlighted that language or memory problems made it more difficult for adults with recurrent seizures, many of whom also had mood disturbance, to remember new information and to remember to do things in practice. This is not rare for people with persistent seizures, as it affected about 25% of the small group of participants approached for the process evaluation. Memory impairment is well documented in epilepsy and particularly affects those with frequent seizures or mood disturbance. 143,178 Social scientists believe that beyond 2 weeks, memory for routine events declines in all people and with it the accuracy of reporting. If this is particularly so in poorly controlled epilepsy and mood disorders, it may be more appropriate to describe outcomes soon after an intervention. In addition, Taylor et al. 59 have reported that to be optimally effective in changing outcomes, interventions for all LTCs will ideally be supported and augmented by integrated hospital and community care services. For those with persistent seizures and memory issues, such liaison may be particularly important. However, in practice, it rarely occurs in a systematic way and would be more costly to provide in trials as well as in routine practice.
Cost of epilepsy and service use
Our group of 404 PWE with recurring seizures were recruited from specialist clinics and thus almost all (94%) reported seeing a neurologist twice in the previous 12 months at baseline assessment. Only about one-third had seen an ENS. Half of the group saw a GP for epilepsy in the past year and on average they had two visits. Although for the majority of PWE their epilepsy is managed in primary care, the participants in our study also have care provided in secondary or tertiary services. It may be that some may not feel the need to see their GPs for epilepsy.
About 40% of our group with persistent seizures had been to an ED once in the previous year. This is twice the proportion of PWE who attend an ED in community studies, which reflects the fact that half of PWE in the community have their seizures controlled by medications. 11,152 The total cost of service use reported at baseline for the whole group was an average of £2751 for the previous year. This increased at the 12-month follow-up to £4030. The difference is due mostly to costs for paid home help. The majority of the cost was associated with AEDs in our group. This is consistent with a study of costs in epilepsy where AEDs and frequency of seizures were reported to drive the cost of epilepsy. 20 In general, there are few studies on the cost of epilepsy. 12,173 In comparison, type I and type II diabetes mellitus cost about £9.8B a year in direct costs for the NHS for 3.8 million patients,179 which extrapolates to approximately £2500 per year per patient.
Cost-effectiveness of SMILE (UK)
The cost-effectiveness analysis of SMILE (UK) showed that, for the NHS perspective, the course is cost-saving but does not correspond to more QALYs than TAU. Further analysis concluded that the cost savings do not justify the loss of QALY. However, looking at societal costs, SMILE (UK) could be cost-effective.
The assessment of QALYs is based on the QoL of the patient group. This was measured in two ways for our study. The standard for health economic evaluations is the EQ-5D-5L. Our group displayed a high QoL using this measure compared with other studies. 142 We questioned whether or not this was the most appropriate scale to use for epilepsy as symptoms can be intermittent, unlike other LTCs. The EQ-5D-5L was found to be weakly correlated with QOLIE-31-P in a recent substudy. 142 Our group had a high total QOLIE-31-P score at enrolment and it may be hard to improve on, especially in a group having epilepsy long term. This in turn has an impact on the cost-effectiveness analysis.
Limitations
-
We used self-report data to measure outcomes. This is common and accords well with concepts such as QoL, which emphasise the experience of the individual. Reliance on patient reports of seizure frequency introduces the possibility of bias. However, there is limited consensus on how else to measure seizures over a sustained period in community studies, when many patients are not aware of, or are amnesic for, a proportion of seizures. 180
-
We also relied on patients to self-report their ED use, as PWE have been found to be reasonably accurate in recalling use of other health-care services, particularly hospital-based services, over the previous year. 181,182
-
A 2-day course may have been inconvenient for people with work and family commitments. Lengthy travel to the course during peak rush hour times may have been difficult for many PWE, especially as publicly funded, free-to-user transport passes do not cover rush hour times in England.
Strengths
-
A large sample size and good recruitment and retention in this complex intervention trial mean that we have confidence in our findings that a 2-day course does not influence QoL at 1 year. 114
-
The population from which we recruited is likely to represent people with epilepsy and persistent seizures, particularly those attending specialist clinics in cities. Our study also includes a representative proportion of males.
-
Our results may be influenced by one-third of the intervention group not attending the full course. Some trials analysed their results per protocol. 1 SMILE (UK) results were analysed according to ITT with a secondary CACE analysis on the primary outcome. 4
-
To our knowledge, our report on the fidelity of the intervention was the first time this had been reported for educational intervention trials in epilepsy. 85
-
The sample size used in the process evaluations was small, but purposefully selected, and the results helped both to explain how patients benefit from group interventions and why PWE and people with persistent seizures would require more than a 2-day course if long-term benefits are to be demonstrated. 83
-
The process evaluation highlighted that PWE felt isolated from people like themselves, despite having epilepsy for a median of 18 years and being in receipt of specialist epilepsy care.
-
The process evaluation found that patients believed group learning with others with similar experiences was helpful in reducing their sense of ‘feeling alone’. For many, the sharing of experiences improved their self-confidence and self-management.
-
The process evaluation identified that memory problems make it more difficult for some adults with recurrent seizures or mood disturbance to remember new information and to remember to do things in practice.
-
This is the second economic analysis of a complex intervention for epilepsy, and the first in the UK. 132
-
The economic analysis highlighted the health cost for poorly controlled epilepsy and the capacity burden placed by use of EDs, which is twice as much as for all PWE.
Chapter 11 Conclusions
Main conclusion
People with chronic epilepsy for a median 18 years and those with frequent seizures did not show improvement in their QoL 1 year after being offered a 2-day self-management education course. Two-thirds of the participant group reported current anxiety symptoms at borderline or case level and felt-stigma, and one-quarter reported current depression symptoms at borderline or case level. Their QoL was associated closely with these psychosocial characteristics.
We conclude that discrete educational courses are unlikely on their own to enhance QoL, particularly 1 year later. The evidence does suggest that only courses that were supported by additional cointerventions, such as ongoing monitoring by a nurse specialist or pharmacist, may have influenced QoL. 117,183 This may be because memory and/or mood disturbance is a problem for PWE, or that QoL is not much affected by an education intervention on its own. A combination of interventions may be more likely to improve outcomes in all LTCs, particularly in epilepsy with poor control. 59
As QoL is most associated with psychological and social factors, psychosocial interventions are more likely to influence QoL. 164,165 By contrast, educational interventions are more likely to show benefit by improving knowledge of epilepsy, and there is evidence that knowledge-of-epilepsy scores can be changed by educational courses. 1 However, it is more difficult to demonstrate that education changes people’s attitudes and behaviour. Ideally, future interventions will integrate education and advice given in primary and secondary care, and include a process evaluation to emphasise the ‘how’ and the ‘why’. 59 There will ideally be a system of monitoring of PWE in primary care as there is for those with diabetes mellitus, possibly with screening for anxiety and depression, and clear communication between the two sectors. 59
A process evaluation was helpful in explaining our quantitative results. Despite receiving specialist epilepsy care in addition to primary care, and having a median of 18 years’ experience of managing their epilepsy, participants reported ‘feeling alone’. Overcoming this and improving self-acceptance through meeting people with similar experience was seen to be helpful. Over half reported that this, and comparison of attitudes and experience with others, helped them improve their confidence to talk openly and make changes in health behaviours. Memory problems may be reported by at least one-quarter of those with poorly controlled epilepsy. Clinicians should encourage PWE to join groups of peers as a priority, as well as finding ways to reinforce learning about how to self-manage their epilepsy early on when QoL is more impaired.
Recommendations for research
-
A group intervention is valued for helping people overcome their sense of isolation, loss of self-esteem and self-efficacy. After a median of 18 years with seizures, some people may find a 2-day course to be too little, too late. We found that QoL was lower in people with more recent onset of epilepsy. Based on these findings, we propose that group courses be evaluated for people with newly diagnosed epilepsy earlier on. This might include testing of simple reminders to join local self-help groups.
-
We have found QoL to be closely associated with psychological distress. This finding confirms evidence from other countries. 20,148,150,151,163 If the main aim is to improve QoL, and/or mitigate anxiety and depression symptoms, then psychological interventions such as cognitive behavioural therapy or acceptance and commitment therapy are likely to hold promise for this group of PWE and require more trials in the UK. 165
-
Epilepsy is associated with stigma, as are human immunodeficiency virus (HIV) and mental ill health conditions. 42,169,177 The results from this research suggest that there needs to be a similar approach for epilepsy as in these other conditions to developing and testing interventions to reduce stigma early on after diagnosis. 169,177 This may be particularly important for groups that mat be likely to experience more self-stigma, like PWE from ethnic minority cultures and those with persistent seizures. 175
-
If the aim is particularly to help people with long-term epilepsy and frequent seizures, a range of interlinked interventions are more likely to have a positive effect, as observed in other studies. 59,117,183 They might include (1) peer-group work to reduce stigma and loss of self-esteem and self-mastery, (2) education on self-management and (3) for those with psychological distress, an intervention to reduce this.
-
When memory issues are a challenge, intermittent visits to a nurse or other professional allied to medicine should be evaluated, with additional memory prompts to assess how reinforcement of self-management learning can help recall.
-
Appropriate outcome measures need to be selected in each case, with latitude in choice provided by funders to the researchers. 184 Outcome measures should also include those deemed important by the target population. Assuming patients know what it means, QoL (‘The treatment’s effect on your quality of life’) has been ranked by users as the third most important outcome measure for trials. 184
-
The connection between knowledge, attitudes and behaviour is complex in all LTCs and requires more research for PWE. This has important implications as national and international policy is to prevent unnecessary ED use and hospital admissions. 185 A UK audit found most ED visits for seizures by PWE are clinically unnecessary; nonetheless, about half of those who come are admitted to hospital with no health gain. 16 The cost of this, and of not testing prevention strategies, is high. 12
-
Although there has been much health services research and many complex intervention trials in similar LTCs such as diabetes mellitus in the UK, there has been little in epilepsy. As so little is known, research describing PWE, developing complex interventions for them and methods of evaluation should be regarded as exploratory.
-
A cohort study, with modelling of clinical, psychological and social predictors, would help to understand determinants of QoL among UK PWE.
-
If research is to build on the shoulders of prior research for UK PWE, the development and evaluation of complex interventions should follow MRC guidelines, with process evaluation at each stage to understand ‘why’ and ‘how’ interventions work or fail to do so. 186
Implications for health care
After diagnosis has been confirmed, many people with epilepsy and persistent seizures in the UK are managed mainly in primary care. Our research focused on a large group of people with epilepsy and persistent seizures, who also attended a specialist clinic. They had chronic epilepsy, with the majority having seizures at least once a month. Our findings suggest that when clinicians encounter PWE with this seizure severity, they can expect that more often than not the PWE will feel isolated from people like themselves, feel stigmatised and suffer anxiety symptoms. Some of these social experiences and attitudes, if identified, may be ameliorated by joining groups. Clinicians might take a more active role in identifying these issues, recommending local and national user groups, and providing reminders subsequently. Clinicians, policy-makers and user groups should canvass for more research on this.
We found that anxiety and, even more so, depression symptoms are strongly associated with impaired QoL. Clinicians in primary care and NHS patients have direct access via self-referral to psychological therapists. 187 If clinicians identify PWE who have persistent seizures, they will find anxiety and depression symptoms are common and likely to be associated with a poorer QoL. Inferring from our own and others’ evidence, active identification and management of this may improve outcomes, possibly including self-management of epilepsy. 35,153 In LTCs affecting mental health, clinicians and policy-makers may grade the severity to enable a step-care approach, with the intensity of interventions varying depending on severity. 188 More research is required on this for epilepsy.
Acknowledgements
Members of the Trial Steering Committee
Anne Rogers (chairperson), Howard A Ring, Phil Smith, Henry Smithson, Ajay Thapar and Marie Edgar.
Members of the Data Monitoring Committee
Gene Feder (chairperson), Adrian Mander and Mark Manford.
SMILE (UK) facilitators
Devi Amin, Tony Hollands, Cathy Queally, Sally-Ann Remnant, Jennifer Nightingale, Peter Muthiniji, Lee Drummond, Nancy Richardson, Telma Neves, Sandra Chinyere and Marisa Pina.
SMILE (UK) research team
Gabriella Wojewodka (Trial Manager), Alison McKinlay (Research Associate), Sarah J Feehan (Research Assistant), Carly M Pearson (Clinical Trial Administrator), Siobhan Hurley (Research Assistant), Amanda Hornsby (Research Assistant), Chong Ho (Trial Administrator), Mary Lavelle (Research Associate), Laura Nichols (Trial Manager), Vikki Semik (Trial Administrator), Ines Kralj-Hans (Trial Manager), Anne Laybourne (Research Associate), Rebecca Lawton (Research Assistant) and Sarah Watkins (Research Assistant).
MOSES experts
Dieter Dennig, Margarete Pfafflin, Rupprecht Thorbecke and Franz Brunnhuber (consultant at KCH who mentored the course facilitators).
Epilepsy Action
Angie Pullen, Margaret Rawnsley (now retired) and Sue Mitchell.
Staff at collaborating hospitals
National Hospital for Neurology and Neurosurgery (Queen Square)
Matthew Walker (Principal Investigator), Fergus Rugg-Gun, Dominic Heaney and John Duncan.
Guy’s and St Thomas’ Hospital
Michalis Koutroumanidis (Principal Investigator).
Princess Royal Hospital
Jennifer Quirk (Principal Investigator).
Darent Valley Hospital
Robert S Delamont (Principal Investigator).
King’s College Hospital
Robert Elwes and Lina Nashef.
Lewisham Hospital
Ray Chaudhuri (Principal Investigator) and Asra Siddiqui.
Croydon University Hospital
Bridget MacDonald (Principal Investigator).
St George’s Hospital
Dora Lozsadi (Principal Investigator) and Hannah Cock.
SMILE (UK) materials and copyright
The SMILE (UK) teaching manual and participant workbook were translated and adapted from the materials used in the MOSES course in Germany. The use of these materials was subject to a free licence made between the MOSES group (Verein zur Förderung von Epilepsie-Schulungen, VEpS) and Professor Leone Ridsdale at KCL for the duration of the trial. The licence was granted by Dr Ulrich Specht and Dr Dieter Denning. To use the course materials, the MOSES-Geschafttsselle should be contacted via Rupprecht Thorbecke (Rupprecht.Thorbecke@mara.de) at the Epilepsy Centre Bethel, URL: www.moses-schulung.de/weitere-informationen/english-version-of-moses/.
Contributions of authors
Leone Ridsdale (Chief Investigator, Professor of Neurology and General Practice) responded to the call for research from NIHR, designed the study, helped adapt the intervention, managed the research and wrote and revised the report.
Alison McKinlay (Research Associate) contributed to the writing and preparation of the report.
Gabriella Wojewodka (Trial Manager) contributed to the conduct of the research and was involved in, writing and reviewing the report.
Emily J Robinson (Statistician) contributed to the statistical analysis of the quantitative outcome analysis, writing and reviewing the report.
Iris Mosweu (Research Associate, Health Economics) contributed to the analysis and write-up of the health economics results.
Sarah J Feehan (Research Assistant) completed follow-ups and contributed to the preparation and revisions of the report.
Adam J Noble (Lecturer of Psychology) designed the study, was involved with the conduct of the study and reviewed the report.
Myfanwy Morgan (Professor Emeritus) contributed to the design and analysis of the qualitative arms of the trial and wrote and reviewed the report.
Stephanie JC Taylor (Professor in Public Health and Primary Care) helped design the research protocol, contributed to conducting the study and reviewed the report.
Paul McCrone (Professor of Health Economics) contributed to the design and analysis of health economics data.
Sabine Landau (Professor of Biostatistics) contributed to conducting the study, conducted the statistical analysis of the quantitative outcome analysis and reviewed the report.
Mark Richardson (Professor in Epilepsy) contributed to the design of the research and reviewed the final draft of the report. He was also the PI at KCH.
Gus Baker (Professor Emeritus and Consultant Clinical Neuropsychologist) helped design the research protocol and was involved with the conduct of the study.
Laura H Goldstein (Professor of Clinical Neuropsychology) designed the research protocol, adapted the intervention for use with UK participants and wrote and reviewed the report.
Publications
Peer-reviewed publications
Kralj-Hans I, Goldstein LH, Noble AJ, Landau S, Magill N, McCrone P, et al. Self-Management education for adults with poorly controlled epILEpsy (SMILE (UK)): a randomised controlled trial protocol. BMC Neurol 2014;14:69.
Magill N, Ridsdale L, Goldstein LH, McCrone P, Morgan M, Noble AJ, et al. Self-management education for adults with poorly controlled epilepsy (SMILE (UK)): statistical, economic and qualitative analysis plan for a randomised controlled trial. Trials 2015;16:269.
Laybourne AH, Morgan M, Watkins SH, Lawton R, Ridsdale L, Goldstein LH. Self-management for people with poorly controlled epilepsy: participants’ views of the UK Self-Management in epILEpsy (SMILE) program. Epilepsy Behav 2015;52:159–64.
Wijnen BFM, Mosweu I, Majoie MHJM, Ridsdale L, de Kinderen RJA, Evers SMAA, McCrone P. Mapping of the QOLIE-31P to EQ-5D-5L utilities and comparison of the responsiveness of both instruments in epilepsy [published online ahead of print September 4 2017]. Eur J Health 2017.
Smith A, McKinlay A, Wojewodka G, Ridsdale L. A systematic review and narrative synthesis of group self-management interventions for adults with epilepsy. BMC Neurol 2017;17:114.
Wojewodka G, Hurley S, Taylor SJC, Noble AJ, Ridsdale L, Goldstein LH. Implementation fidelity of a self-management course for epilepsy: method and assessment. BMC Med Res Methodol 2017;17:100.
Ridsdale L, Wojewodka G, Robinson E, Landau S, Noble A, Taylor S, et al. Characteristics associated with quality of life among people with drug-resistant epilepsy. J Neurol 2017;264:1174–84.
Ridsdale L, Philpott SJ, Krooupa AM, Morgan M. People with epilepsy obtain added value from education in groups: results of a qualitative study. Eur J Neurol 2017;24:609–16.
Ridsdale L, Wojewodka G, Robinson EJ, Noble AJ, Morgan M, Taylor SJC, et al. The effectiveness of a group self-management education course for adults with poorly controlled epilepsy, SMILE (UK): a randomized controlled trial. Epilepsia 2018;00:1–14.
Conference presentations
Ridsdale L. Self-Management Education for Adults with Poorly Controlled Epilepsy: The SMILE Trial. Poster presentation. Joint Congress of European Neurology, Istanbul, Turkey, 31 May–3 June 2014.
Ridsdale L, Kralj-Hans I, Noble A, Landau S, McCrone P, Morgan M, et al. Self-Management Education for Adults with Poorly Controlled Epilepsy (SMILE): A Randomised Controlled Trial Protocol. Poster presentation. First Congress of the European Academy of Neurology, Berlin, Germany, 20–23 June 2015.
Ridsdale L, Philpott S, Krooupa AM, Morgan M. I Am Not Alone: Views of People with Epilepsy of Self-Management Education in Groups. Poster presentation. Second Congress of the European Academy of Neurology, Copenhagen, Denmark, 28–31 March 2016.
Ridsdale L, Wojewodka G, Noble A, Landau S, Robinson E, Mosweu I, et al. An RCT of Self-Management Education in Epilepsy: Participants at Baseline. Poster presentation. Association of British Neurologists’ Annual Meeting: ‘The Seven Ages of Man’, Brighton, UK, 17–19 May 2016.
Ridsdale L, Wojewodka G, Robinson EJ, Mosweu I, Landau S, McCrone P, et al. An RCT of Self-Management Education in Epilepsy: Characteristics of Participants at Baseline. Oral presentation. European Congress for Epileptology, Prague, Czech Republic, 11–15 September 2016.
Smith A, McKinlay A, Wojewodka G, Ridsdale L. A Review of Group Self-Management Courses for Adults with Epilepsy. Poster presentation. British Neuroscience Association and Association of British Neurologists’ joint symposium ‘Meeting of the Minds’, Cardiff, UK, 28 October 2016.
Ridsdale L, Wojewodka G, Robinson EJ, Landau S, Noble AJ, Taylor SJ, et al. Results of an RCT of Self-Management Education in Epilepsy: SMILE-UK. Poster presentation. Association of British Neurologists’ Conference, Liverpool, UK, 3–5 May 2017.
Wojewodka G, Hurley S, Taylor SJ, Noble AJ, Ridsdale L, Goldstein LH. Fidelity of a Self-Management Course for People with EpILEpsy (SMILE (UK). Oral presentation. Third Congress of the European Academy of Neurology, Amsterdam, the Netherlands, 24–27 June 2017.
Ridsdale L, Wojewodka G, Robinson E, Landau S, Noble AJ, Morgan M, et al. Self-Management education for adults with poorly controlled epILEpsy (SMILE-UK). Oral presentation. 32nd International Epilepsy Congress, Barcelona, Spain, 2–6 September 2017.
Ridsdale L, Wojewodka G, Robinson E, Landau S, Noble AJ, Morgan M, et al. Self-Management education for adults with poorly controlled epILEpsy (SMILE-UK). Poster presentation. British Chapter of the International League Against Epilepsy Annual Meeting, Leeds, UK, 4–6 October 2017.
Data sharing statement
Data can be obtained from the corresponding author (leone.ridsdale@kcl.ac.uk) on request.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- May TW, Pfäfflin M. The efficacy of an educational treatment program for patients with epilepsy (MOSES): results of a controlled, randomized study. Modular Service Package Epilepsy. Epilepsia 2002;43:539-49. https://doi.org/10.1046/j.1528-1157.2002.23801.x.
- Helgeson DC, Mittan R, Tan SY, Chayasirisobhon S. Sepulveda Epilepsy Education: the efficacy of a psychoeducational treatment program in treating medical and psychosocial aspects of epilepsy. Epilepsia 1990;31:75-82. https://doi.org/10.1111/j.1528-1157.1990.tb05363.x.
- Kralj-Hans I, Goldstein LH, Noble AJ, Landau S, Magill N, McCrone P, et al. Self-Management education for adults with poorly controlled epILEpsy (SMILE (UK)): a randomised controlled trial protocol. BMC Neurol 2014;14. https://doi.org/10.1186/1471-2377-14-69.
- Magill N, Ridsdale L, Goldstein LH, McCrone P, Morgan M, Noble AJ, et al. Self-management education for adults with poorly controlled epilepsy (SMILE (UK)): statistical, economic and qualitative analysis plan for a randomised controlled trial. Trials 2015;16.
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475-82. https://doi.org/10.1111/epi.12550.
- Ferro MA. A population-based study of the prevalence and sociodemographic risk factors of self-reported epilepsy among adults in the United Kingdom. Seizure 2011;20:784-8. https://doi.org/10.1016/j.seizure.2011.07.011.
- Ridsdale L, Charlton J, Ashworth M, Richardson MP, Gulliford MC. Epilepsy mortality and risk factors for death in epilepsy: a population-based study. Br J Gen Pract 2011;61:e271-8. https://doi.org/10.3399/bjgp11X572463.
- Forsgren L, Beghi E, Oun A, Sillanpää M. The epidemiology of epilepsy in Europe – a systematic review. Eur J Neurol 2005;12:245-53. https://doi.org/10.1111/j.1468-1331.2004.00992.x.
- Ridsdale L. Avoiding premature death in epilepsy. BMJ 2015;350. https://doi.org/10.1136/bmj.h718.
- NHS Choices . Living With Epilepsy 2014 n.d. www.nhs.uk/conditions/epilepsy/pages/living-with.aspx (accessed 24 July 2017).
- Moran NF, Poole K, Bell G, Solomon J, Kendall S, McCarthy M, et al. Epilepsy in the United Kingdom: seizure frequency and severity, anti-epileptic drug utilization and impact on life in 1652 people with epilepsy. Seizure 2004;13:425-33. https://doi.org/10.1016/j.seizure.2003.10.002.
- Noble AJ, McCrone P, Seed PT, Goldstein LH, Ridsdale L. Clinical- and cost-effectiveness of a nurse led self-management intervention to reduce emergency visits by people with epilepsy. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0090789.
- Health and Social Care Information Centre . Hospital Episode Statistics, Admitted Patient Care – England, 2014–15 (NS) 2015. http://content.digital.nhs.uk/catalogue/PUB19124 (accessed 24 July 2017).
- Begley CE, Annegers JF, Lairson DR, Reynolds TF. Methodological issues in estimating the cost of epilepsy. Epilepsy Res 1999;33:39-55. https://doi.org/10.1016/S0920-1211(98)00070-9.
- Hamer HM, Spottke A, Aletsee C, Knake S, Reis J, Strzelczyk A, et al. Direct and indirect costs of refractory epilepsy in a tertiary epilepsy center in Germany. Epilepsia 2006;47:2165-72. https://doi.org/10.1111/j.1528-1167.2006.00889.x.
- Dixon PA, Kirkham JJ, Marson AG, Pearson MG. National Audit of Seizure management in Hospitals (NASH): results of the national audit of adult epilepsy in the UK. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007325.
- Räty LK, Wilde-Larsson BM. Patients’ perceptions of living with epilepsy: a phenomenographic study. J Clin Nurs 2011;20:1993-2002. https://doi.org/10.1111/j.1365-2702.2010.03572.x.
- Jacoby A, Snape D, Baker GA. Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol 2005;4:171-8. https://doi.org/10.1016/S1474-4422(05)70020-X.
- McLaughlin DP, Pachana NA, Mcfarland K. Stigma, seizure frequency and quality of life: the impact of epilepsy in late adulthood. Seizure 2008;17:281-7. https://doi.org/10.1016/j.seizure.2007.09.001.
- Taylor RS, Sander JW, Taylor RJ, Baker GA. Predictors of health-related quality of life and costs in adults with epilepsy: a systematic review. Epilepsia 2011;52:2168-80. https://doi.org/10.1111/j.1528-1167.2011.03213.x.
- Ridsdale L. The social causes of inequality in epilepsy and developing a rehabilitation strategy: a U.K.-based analysis. Epilepsia 2009;50:2175-9. https://doi.org/10.1111/j.1528-1167.2009.02150.x.
- Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia 2004;45:544-50. https://doi.org/10.1111/j.0013-9580.2004.47003.x.
- Leidy NK, Elixhauser A, Vickrey B, Means E, Willian MK. Seizure frequency and the health-related quality of life of adults with epilepsy. Neurology 1999;53:162-6. https://doi.org/10.1212/WNL.53.1.162.
- Jacoby A, Thapar A. The contribution of seizures to psychosocial ill-health. Epilepsy Behav 2009;15:41-5. https://doi.org/10.1016/j.yebeh.2009.03.019.
- Jacoby A, Snape D, Baker GA. Determinants of quality of life in people with epilepsy. Neurol Clin 2009;27:843-63. https://doi.org/10.1016/j.ncl.2009.06.003.
- Gilliam F. Optimizing health outcomes in active epilepsy. Neurology 2002;58:9-20. https://doi.org/10.1212/WNL.58.8_suppl_5.S9.
- Hovinga CA, Asato MR, Manjunath R, Wheless JW, Phelps SJ, Sheth RD, et al. Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy Behav 2008;13:316-22. https://doi.org/10.1016/j.yebeh.2008.03.009.
- Ettinger AB, Good MB, Manjunath R, Edward Faught R, Bancroft T. The relationship of depression to antiepileptic drug adherence and quality of life in epilepsy. Epilepsy Behav 2014;36:138-43. https://doi.org/10.1016/j.yebeh.2014.05.011.
- Elliott JO, Lu B, Shneker B, Charyton C, Layne Moore J. Comorbidity, health screening, and quality of life among persons with a history of epilepsy. Epilepsy Behav 2009;14:125-9. https://doi.org/10.1016/j.yebeh.2008.10.013.
- Fuller-Thomson E, Brennenstuhl S. The association between depression and epilepsy in a nationally representative sample. Epilepsia 2009;50:1051-8. https://doi.org/10.1111/j.1528-1167.2008.01803.x.
- Mensah SA, Beavis JM, Thapar AK, Kerr MP. A community study of the presence of anxiety disorder in people with epilepsy. Epilepsy Behav 2007;11:118-24. https://doi.org/10.1016/j.yebeh.2007.04.012.
- Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia 2007;48:2336-44. https://doi.org/10.1111/j.1528-1167.2007.01222.x.
- Loring DW, Meador KJ, Lee GP. Determinants of quality of life in epilepsy. Epilepsy Behav 2004;5:976-80. https://doi.org/10.1016/j.yebeh.2004.08.019.
- Tracy JI, Dechant V, Sperling MR, Cho R, Glosser D. The association of mood with quality of life ratings in epilepsy. Neurology 2007;68:1101-7. https://doi.org/10.1212/01.wnl.0000242582.83632.73.
- Thapar A, Kerr M, Harold G. Stress, anxiety, depression, and epilepsy: investigating the relationship between psychological factors and seizures. Epilepsy Behav 2009;14:134-40. https://doi.org/10.1016/j.yebeh.2008.09.004.
- Kanner AM. Depression and epilepsy: a review of multiple facets of their close relation. Neurol Clin 2009;27:865-80. https://doi.org/10.1016/j.ncl.2009.08.002.
- Suurmeijer TP, Reuvekamp MF, Aldenkamp BP. Social functioning, psychological functioning, and quality of life in epilepsy. Epilepsia 2001;42:1160-8. https://doi.org/10.1046/j.1528-1157.2001.37000.x.
- Jacoby A, Austin JK. Social stigma for adults and children with epilepsy. Epilepsia 2007;48:6-9. https://doi.org/10.1111/j.1528-1167.2007.01391.x.
- Gzirishvili N, Kasradze S, Lomidze G, Okujava N, Toidze O, de Boer HM, et al. Knowledge, attitudes, and stigma towards epilepsy in different walks of life: a study in Georgia. Epilepsy Behav 2013;27:315-18. https://doi.org/10.1016/j.yebeh.2013.02.011.
- Jacoby A, Gorry J, Gamble C, Baker GA. Public knowledge, private grief: a study of public attitudes to epilepsy in the United Kingdom and implications for stigma. Epilepsia 2004;45:1405-15. https://doi.org/10.1111/j.0013-9580.2004.02904.x.
- Räty LK, Larsson G, Starrin B, Larsson BM. Epilepsy patients’ conceptions of epilepsy as a phenomenon. J Neurosci Nurs 2009;41:201-10. https://doi.org/10.1097/JNN.0b013e3181aaaa75.
- Scambler G, Hopkins A. Being epileptic – coming to terms with stigma. Sociol Health Illn 1986;8:26-43. https://doi.org/10.1111/1467-9566.ep11346455.
- Dilorio C, Osborne Shafer P, Letz R, Henry T, Schomer DL, Yeager K. Project EASE Study Group . The association of stigma with self-management and perceptions of health care among adults with epilepsy. Epilepsy Behav 2003;4:259-67. https://doi.org/10.1016/S1525-5050(03)00103-3.
- Morgan GA, Harmon RJ, Maslin-Cole CA. Mastery motivation: definition and measurement. Early Educat Dev 1990;1:318-39. https://doi.org/10.1207/s15566935eed0105_1.
- Dilorio C, Faherty B, Manteuffel B. The development and testing of an instrument to measure self-efficacy in individuals with epilepsy. J Neurosci Nurs 1992;24:9-13. https://doi.org/10.1097/01376517-199202000-00004.
- Au A, Chan F, Li K, Leung P, Li P, Chan J. Cognitive-behavioral group treatment program for adults with epilepsy in Hong Kong. Epilepsy Behav 2003;4:441-6. https://doi.org/10.1016/S1525-5050(03)00149-5.
- Schachter SC. Improving quality of life beyond seizure control. Epileptic Disord 2005;7:S34-8.
- Pugh MJ, Copeland LA, Zeber JE, Cramer JA, Amuan ME, Cavazos JE, et al. The impact of epilepsy on health status among younger and older adults. Epilepsia 2005;46:1820-7. https://doi.org/10.1111/j.1528-1167.2005.00291.x.
- Mcewan MJ, Espie CA, Metcalfe J, Brodie MJ, Wilson MT. Quality of life and psychosocial development in adolescents with epilepsy: a qualitative investigation using focus group methods. Seizure-Eur J Epilep 2004;13:15-31. https://doi.org/10.1016/S1059-1311(03)00080-3.
- Miller WR. Patient-centered outcomes in older adults with epilepsy. Seizure 2014;23:592-7. https://doi.org/10.1016/j.seizure.2014.04.010.
- Heaney DC, MacDonald BK, Everitt A, Stevenson S, Leonardi GS, Wilkinson P, et al. Socioeconomic variation in incidence of epilepsy: prospective community based study in south east England. BMJ 2002;325:1013-16. https://doi.org/10.1136/bmj.325.7371.1013.
- Hesdorffer DC, Tian H, Anand K, Hauser WA, Ludvigsson P, Olafsson E, et al. Socioeconomic status is a risk factor for epilepsy in Icelandic adults but not in children. Epilepsia 2005;46:1297-303. https://doi.org/10.1111/j.1528-1167.2005.10705.x.
- Begley CE, Basu R, Reynolds T, Lairson DR, Dubinsky S, Newmark M, et al. Sociodemographic disparities in epilepsy care: results from the Houston/New York City health care use and outcomes study. Epilepsia 2009;50:1040-50. https://doi.org/10.1111/j.1528-1167.2008.01898.x.
- Das K, Banerjee M, Mondal GP, Devi LG, Singh OP, Mukherjee BB. Evaluation of socio-economic factors causing discontinuation of epilepsy treatment resulting in seizure recurrence: a study in an urban epilepsy clinic in India. Seizure 2007;16:601-7. https://doi.org/10.1016/j.seizure.2007.04.008.
- Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns 2002;48:177-87. https://doi.org/10.1016/S0738-3991(02)00032-0.
- Adams KGA, Corrigan JM. The 1st Annual Crossing the Quality Chasm Summit – A Focus on Communities. Washington, DC: The National Academic Press; 2004.
- Corbin JM, Strauss A. Unending Work and Care: Managing Chronic Illness at Home. San Francisco, CA: Jossey-Bass; 1988.
- Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1-7. https://doi.org/10.1207/S15324796ABM2601_01.
- Taylor SJC, Pinnock H, Epiphaniou E, Pearce G, Parke HL, Schwappach A, et al. Health Serv Deliv Res. 2014.
- Clark NM, Becker MH, Janz NK, Lorig K, Rakowski W, Anderson L. Self-management of chronic disease by older adults: a review and questions for research. J Aging Health 1991;3:3-27. https://doi.org/10.1177/089826439100300101.
- Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 2001;4:256-62.
- Pearce G, Parke HL, Pinnock H, Epiphaniou E, Bourne CL, Sheikh A, et al. The PRISMS taxonomy of self-management support: derivation of a novel taxonomy and initial testing of its utility. J Health Serv Res Policy 2016;21:73-82. https://doi.org/10.1177/1355819615602725.
- DAFNE Study Group . Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746-9. https://doi.org/10.1136/bmj.325.7367.746.
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341. https://doi.org/10.1136/bmj.c4093.
- Deakin TA, Cade JE, Williams R, Greenwood DC. Structured patient education: the diabetes X-PERT Programme makes a difference. Diabet Med 2006;23:944-54. https://doi.org/10.1111/j.1464-5491.2006.01906.x.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. https://doi.org/10.1136/bmj.39474.922025.BE.
- Murphy K, Casey D, Dinneen S, Lawton J, Brown F. Participants’ perceptions of the factors that influence diabetes self-management following a structured education (DAFNE) programme. J Clin Nurs 2011;20:1282-92.
- The Epilepsies: The Diagnosis and Management of the Epilepsies in Adults and Children in Primary and Secondary Care: Pharmacological Update of Clinical Guideline 20. London: National Clinical Guideline Centre; 2012.
- Moran N, Poole K, Bell G, Solomon J, Kendall S, McCarthy M, et al. NHS services for epilepsy from the patient’s perspective: a survey of primary, secondary and tertiary care access throughout the UK. Seizure 2000;9:559-65. https://doi.org/10.1053/seiz.2000.0451.
- Goldstein LH, Minchin L, Stubbs P, Fenwick PB. Are what people know about their epilepsy and what they want from an epilepsy service related?. Seizure 1997;6:435-42. https://doi.org/10.1016/S1059-1311(97)80017-9.
- Helde G, Brodtkorb E, Brathen G, Bovim G. An easily performed group education programme for patients with uncontrolled epilepsy – a pilot study. Seizure 2003;12:497-501. https://doi.org/10.1016/S1059-1311(03)00050-5.
- Bradley PM, Lindsay B. Care delivery and self-management strategies for adults with epilepsy. Cochrane Database Syst Rev 2008;1. https://doi.org/10.1002/14651858.CD006244.pub2.
- Bradley PM, Lindsay B, Fleeman N. Care delivery and self management strategies for adults with epilepsy. Cochrane Database Syst Rev 2016;2. https://doi.org/10.1002/14651858.CD006244.pub3.
- Ramaratnam S, Baker GA, Goldstein LH. Psychological treatments for epilepsy. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD002029.pub3.
- Mittan RJ. Psychosocial treatment programs in epilepsy: a review. Epilepsy Behav 2009;16:371-80. https://doi.org/10.1016/j.yebeh.2009.08.031.
- Edward KL, Cook M, Giandinoto JA. An integrative review of the benefits of self-management interventions for adults with epilepsy. Epilepsy Behav 2015;45:195-204. https://doi.org/10.1016/j.yebeh.2015.01.026.
- Shaw EJ, Stokes T, Camosso-Stefinovic J, Baker R, Baker GA, Jacoby A. Self-management education for adults with epilepsy. Cochrane Database Syst Rev 2007;18. https://doi.org/10.1002/14651858.CD004723.pub2.
- Ried S, Specht U, Thorbecke R, Goecke K, Wohlfarth R. MOSES: an educational programme for patients with epilepsy and their relatives. Curr Prob E 2001;16:76-80.
- Foster G, Taylor SJ, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD005108.pub2.
- Cramer JA, Perrine K, Devinsky O, Bryant-Comstock L, Meador K, Hermann B. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia 1998;39:81-8. https://doi.org/10.1111/j.1528-1157.1998.tb01278.x.
- Medical Research Council . Developing and Evaluating Complex Interventions 2006 n.d. www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/ (accessed 24 July 2017).
- Laybourne AH, Morgan M, Watkins SH, Lawton R, Ridsdale L, Goldstein LH. Self-management for people with poorly controlled epilepsy: participants’ views of the UK Self-Management in epILEpsy (SMILE) program. Epilepsy Behav 2015;52:159-64.
- Ridsdale L, Philpott SJ, Krooupa AM, Morgan M. People with epilepsy obtain added value from education in groups: results of a qualitative study. Eur J Neurol 2017;24:609-16. https://doi.org/10.1111/ene.13253.
- Ridsdale L, Wojewodka G, Robinson E, Landau S, Noble A, Taylor S, et al. Characteristics associated with quality of life among people with drug-resistant epilepsy. J Neurol 2017;264:1174-84. https://doi.org/10.1007/s00415-017-8512-1.
- Wojewodka G, Hurley S, Taylor SJC, Noble AJ, Ridsdale L, Goldstein LH. Implementation fidelity of a self-management course for epilepsy: method and assessment. BMC Med Res Methodol 2017;17. https://doi.org/10.1186/s12874-017-0373-x.
- Michie S, Rumsey N, Fussell A, Hardeman W, Johnston M, Newman S, et al. Improving Health: Changing Behaviour. NHS Health Trainer Handbook. London: Department of Health; 2008.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215. https://doi.org/10.1037/0033-295X.84.2.191.
- Kobau R, Dilorio C. Epilepsy self-management: a comparison of self-efficacy and outcome expectancy for medication adherence and lifestyle behaviors among people with epilepsy. Epilepsy Behav 2003;4:217-25. https://doi.org/10.1016/S1525-5050(03)00057-X.
- Dilorio C, Shafer PO, Letz R, Henry TR, Schomer DL, Yeager K. Project EASE study group . Project EASE: a study to test a psychosocial model of epilepsy medication management. Epilepsy Behav 2004;5:926-36. https://doi.org/10.1016/j.yebeh.2004.08.011.
- Epilepsy Centre Bethel . MOSES Das Schulungsprogramm Fur Menschen Mit Epilepsie 2017 n.d. www.moses-schulung.de/ (accessed 15 March 2017).
- Olley BO, Osinowo HO, Brieger WR. Psycho-educational therapy among Nigerian adult patients with epilepsy: a controlled outcome study. Patient Educ Couns 2001;42:25-33. https://doi.org/10.1016/S0738-3991(00)00087-2.
- Noble AJ, Morgan M, Virdi C, Ridsdale L. A nurse-led self-management intervention for people who attend emergency departments with epilepsy: the patients’ view. J Neurol 2013;260:1022-30. https://doi.org/10.1007/s00415-012-6749-2.
- Morrish P. What is happening to English neurology?. Clin Med 2008;8:576-8. https://doi.org/10.7861/clinmedicine.8-6-576.
- Hosking PG, Duncan JS, Sander JM. The epilepsy nurse specialist at a tertiary care hospital-improving the interface between primary and tertiary care. Seizure 2002;11:494-9. https://doi.org/10.1016/S1059-1311(02)00137-1.
- Ridsdale L, Kwan I, Cryer C. Newly diagnosed epilepsy: can nurse specialists help? A randomized controlled trial. Epilepsy Care Evaluation Group. Epilepsia 2000;41:1014-19. https://doi.org/10.1111/j.1528-1157.2000.tb00287.x.
- Epilepsy Action . Epilepsy Today Magazine 2017 n.d. www.epilepsy.org.uk/involved/join/current-members/epilepsy-today (accessed 22 September 2017).
- Epilepsy Action . Epilepsy Action UK 2017 n.d. www.epilepsy.org.uk/ (accessed 22 September 2017).
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324. https://doi.org/10.1136/bmj.324.7347.1183.
- Baker GA, Jacoby A, Buck D, Stalgis C, Monnet D. Quality of life of people with epilepsy: a European study. Epilepsia 1997;38:353-62. https://doi.org/10.1111/j.1528-1157.1997.tb01128.x.
- Jacoby A, Baker G, Smith D, Dewey M, Chadwick D. Measuring the impact of epilepsy: the development of a novel scale. Epilepsy Res 1993;16:83-8. https://doi.org/10.1016/0920-1211(93)90042-6.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Jacoby A. Felt versus enacted stigma: a concept revisited. Evidence from a study of people with epilepsy in remission. Soc Sci Med 1994;38:269-74. https://doi.org/10.1016/0277-9536(94)90396-4.
- Wagner AK, Keller SD, Kosinski M, Baker GA, Jacoby A, Hsu MA, et al. Advances in methods for assessing the impact of epilepsy and antiepileptic drug therapy on patients’ health-related quality of life. Qual Life Res 1995;4:115-34. https://doi.org/10.1007/BF01833606.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: The Royal College of Psychiatrists; 2001.
- EuroQoL . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Crossley J, Jacoby A, Baker GA. The reliability and validity of the Revised Liverpool Impact of Epilepsy Scale for use in people with new-onset epilepsy. Epilepsy Behav 2013;26:175-81. https://doi.org/10.1016/j.yebeh.2012.11.046.
- Dilorio C, Faherty B, Manteuffel B. Self-efficacy and social support in self-management of epilepsy. West J Nurs Res 1992;14:292-303. https://doi.org/10.1177/019394599201400303.
- Dilorio C, Faherty B. Epilepsy self-management: partial replication and extension. Res Nurs Health 1994;17:167-74. https://doi.org/10.1002/nur.4770170304.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. https://doi.org/10.1016/S0022-3999(01)00296-3.
- Jacoby A, Baker GA, Steen N, Potts P, Chadwick DW. The clinical course of epilepsy and its psychosocial correlates: findings from a U.K. Community study. Epilepsia 1996;37:148-61. https://doi.org/10.1111/j.1528-1157.1996.tb00006.x.
- English Indices of Deprivation 2010. London: Office for National Statistics; 2011.
- Cramer JA, Arrigo C, Van Hammée G, Gauer LJ, Cereghino JJ. Effect of levetiracetam on epilepsy-related quality of life. N132 Study Group. Epilepsia 2000;41:868-74. https://doi.org/10.1111/j.1528-1157.2000.tb00255.x.
- Kaminow L, Schimschock JR, Hammer AE, Vuong A. Lamotrigine monotherapy compared with carbamazepine, phenytoin, or valproate monotherapy in patients with epilepsy. Epilepsy Behav 2003;4:659-66. https://doi.org/10.1016/j.yebeh.2003.08.033.
- Ridsdale L, Wojewodka G, Robinson EJ, Noble AJ, Morgan M, Taylor SJC, et al. The effectiveness of a group self-management education course for adults with poorly controlled epilepsy, SMILE (UK): a randomized controlled trial. Epilepsia 2018;00:1-14. https://doi.org/10.1111/epi.14073.
- Fraser RT, Johnson EK, Lashley S, Barber J, Chaytor N, Miller JW, et al. PACES in epilepsy: results of a self-management randomized controlled trial. Epilepsia 2015;56:1264-74. https://doi.org/10.1111/epi.13052.
- Saadi A, Patenaude B, Mateen FJ. Quality Of Life In Epilepsy-31 inventory (QOLIE-31) scores: a global comparison. Epilepsy Behav 2016;65:13-7. https://doi.org/10.1016/j.yebeh.2016.09.032.
- Losada-Camacho M, Guerrero-Pabon MF, Garcia-Delgado P, Martinez-Martinez F. Impact of a pharmaceutical care programme on health-related quality of life among women with epilepsy: a randomised controlled trial (IPHIWWE study). Health Qual Life Outcomes 2014;12. https://doi.org/10.1186/s12955-014-0162-8.
- Mars T, Ellard D, Carnes D, Homer K, Underwood M, Taylor SJ. Fidelity in complex behaviour change interventions: a standardised approach to evaluate intervention integrity. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003555.
- Cross WF, West JC. Examining implementer fidelity: Conceptualizing and measuring adherence and competence. J Child Serv 2011;6:18-33. https://doi.org/10.5042/jcs.2011.0123.
- Rosenbaum ME, Silverman JD, Martin LR, DiMatteo MR. The Oxford Handbook of Health Communication, Behaviour Change and Treatment Adherence. Oxford: Oxford University Press; 2013.
- Schlosser R. On the importance of being earnest about treatment integrity. Augment Altern Commun 2002;18:36-44. https://doi.org/10.1080/aac.18.1.36.44.
- Breitenstein SM, Gross D, Garvey CA, Hill C, Fogg L, Resnick B. Implementation fidelity in community-based interventions. Res Nurs Health 2010;33:164-73. https://doi.org/10.1002/nur.20373.
- Lausberg H, Sloetjes H. Coding gestural behavior with the NEUROGES – ELAN system. Behav Res Methods 2009;41:841-9. https://doi.org/10.3758/BRM.41.3.841.
- The Language Archive. ELAN n.d. https://tla.mpi.nl/tools/tla-tools/elan/ (accessed 18 October 2017).
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 337.
- Waltz J, Addis ME, Koerner K, Jacobson NS. Testing the integrity of a psychotherapy protocol: assessment of adherence and competence. J Consult Clin Psychol 1993;61:620-30. https://doi.org/10.1037/0022-006X.61.4.620.
- Rohrbach LA, Dent CW, Skara S, Sun P, Sussman S. Fidelity of implementation in Project Towards No Drug Abuse (TND): a comparison of classroom teachers and program specialists. Prev Sci 2007;8:125-32. https://doi.org/10.1007/s11121-006-0056-z.
- Hasson H, Blomberg S, Dunér A. Fidelity and moderating factors in complex interventions: a case study of a continuum of care program for frail elderly people in health and social care. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-23.
- Davison KP, Pennebaker JW, Dickerson SS. Who talks? The social psychology of illness support groups. Am Psychol 2000;55:205-17. https://doi.org/10.1037/0003-066X.55.2.205.
- Crabtree JW, Haslam SA, Postmes T, Haslam C. Mental health support groups, stigma, and self-esteem: positive and negative implications of group identification. J Social Issues 2010;66:553-69. https://doi.org/10.1111/j.1540-4560.2010.01662.x.
- Wijnen BFM, Leenen LAM, de Kinderen RJA, van Heugten CM, Majoie M, Evers S. An economic evaluation of a multicomponent self-management intervention for adults with epilepsy (ZMILE study). Epilepsia 2017;58:1398-408. https://doi.org/10.1111/epi.13806.
- Wijnen BFM, Mosweu I, Majoie MHJM, Ridsdale L, de Kinderen RJA, Evers SMAA, et al. Mapping of the QOLIE-31P to EQ-5D-5L utilities and comparison of the responsiveness of both instruments in epilepsy. [published online ahead of print September 4 2017]. Eur J Health 2017. https://doi.org/10.1007/s10198-017-0928-0.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit; 2010.
- Department of Health . NHS Reference Costs 2014 to 2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 24 July 2017).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Feng Y, Devlin N, Herdman M. Assessing the health of the general population in England: how do the three- and five-level versions of EQ-5D compare?. Health Qual Life Outcomes 2015;13. https://doi.org/10.1186/s12955-015-0356-8.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. https://doi.org/10.1002/hec.901.
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. https://doi.org/10.1192/bjp.187.2.106.
- Westerhuis W, Zijlmans M, Fischer K, van Andel J, Leijten FS. Coping style and quality of life in patients with epilepsy: a cross-sectional study. J Neurol 2011;258:37-43. https://doi.org/10.1007/s00415-010-5677-2.
- Mukuria C, Young T, Keetharuth A, Borghs S, Brazier J. Sensitivity and responsiveness of the EQ-5D-3L in patients with uncontrolled focal seizures: an analysis of Phase III trials of adjunctive brivaracetam. Qual Life Res 2017;26:749-59. https://doi.org/10.1007/s11136-016-1483-3.
- Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain 2008;131:2243-63. https://doi.org/10.1093/brain/awn127.
- Trevena L, Irwig L, Barratt A. Impact of privacy legislation on the number and characteristics of people who are recruited for research: a randomised controlled trial. J Med Ethics 2006;32:473-7. https://doi.org/10.1136/jme.2004.011320.
- Peters M, Crocker H, Dummett S, Jenkinson C, Doll H, Fitzpatrick R. Change in health status in long-term conditions over a one year period: a cohort survey using patient-reported outcome measures. Health Qual Life Outcomes 2014;12. https://doi.org/10.1186/s12955-014-0123-2.
- Harris SM, Shah P, Mulnier H, Healey A, Thomas SM, Amiel SA, et al. Factors influencing attendance at structured education for type 1 diabetes in south London. Diabet Med 2017;34:828-33. https://doi.org/10.1111/dme.13333.
- Ridsdale L, Robins D, Cryer C, Williams H. Feasibility and effects of nurse run clinics for patients with epilepsy in general practice: randomised controlled trial. Epilepsy Care Evaluation Group. BMJ 1997;314:120-2. https://doi.org/10.1136/bmj.314.7074.120.
- Luoni C, Bisulli F, Canevini MP, De Sarro G, Fattore C, Galimberti CA, et al. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia 2011;52:2181-91. https://doi.org/10.1111/j.1528-1167.2011.03325.x.
- Taylor J, Baker GA, Jacoby A. Levels of epilepsy stigma in an incident population and associated factors. Epilepsy Behav 2011;21:255-60. https://doi.org/10.1016/j.yebeh.2011.04.002.
- Whatley AD, Dilorio CK, Yeager K. Examining the relationships of depressive symptoms, stigma, social support and regimen-specific support on quality of life in adult patients with epilepsy. Health Educ Res 2010;25:575-84. https://doi.org/10.1093/her/cyq001.
- Amir M, Roziner I, Knoll A, Neufeld MY. Self-efficacy and social support as mediators in the relation between disease severity and quality of life in patients with epilepsy. Epilepsia 1999;40:216-24. https://doi.org/10.1111/j.1528-1157.1999.tb02078.x.
- Kitson AL, Shorvon SD. Clinical Standards Advisory Group Great Britain . Services for Patients With Epilepsy: Report of a CSAG Committee 1999.
- Reuber M, Hattingh L, Goulding PJ. Epileptological emergencies in accident and emergency: a survey at St James’s University Hospital, Leeds. Seizure 2000;9:216-20. https://doi.org/10.1053/seiz.2000.0386.
- Beyenburg S, Mitchell AJ, Schmidt D, Elger CE, Reuber M. Anxiety in patients with epilepsy: systematic review and suggestions for clinical management. Epilepsy Behav 2005;7:161-71. https://doi.org/10.1016/j.yebeh.2005.05.014.
- 2014/15 General Medical Services (GMS) Contract Quality and Outcomes Framework (QOF): Guidance for GMS Contract 2014/15. England: NHS England; 2014.
- McLintock K, Russell AM, Alderson SL, West R, House A, Westerman K, et al. The effects of financial incentives for case finding for depression in patients with diabetes and coronary heart disease: interrupted time series analysis. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005178.
- Walters P, Ashworth M, Tylee A. Ethnic density, physical illness, social deprivation and antidepressant prescribing in primary care: ecological study. Br J Psychiatry 2008;193:235-9. https://doi.org/10.1192/bjp.bp.107.038299.
- Fazel S, Wolf A, Långström N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet 2013;382:1646-54. https://doi.org/10.1016/S0140-6736(13)60899-5.
- Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0080633.
- Snape DA, Morgan M, Ridsdale L, Goodacre S, Marson AG, Noble AJ. Developing and assessing the acceptability of an epilepsy first aid training intervention for patients who visit UK emergency departments: a multi-method study of patients and professionals. Epilepsy Behav 2017;68:177-85. https://doi.org/10.1016/j.yebeh.2017.01.006.
- Prinjha S, Chapple A, Herxheimer A, McPherson A. Many people with epilepsy want to know more: a qualitative study. Fam Pract 2005;22:435-41. https://doi.org/10.1093/fampra/cmi024.
- Michaelis R, Tang V, Wagner JL, Modi AC, LaFrance W, Goldstein LH, et al. Psychological treatments for people with epilepsy. Cochrane Database Syst Rev 2016;2. https://doi.org/10.1002/14651858.CD012081.
- Elliott JO, Richardson VE. The biopsychosocial model and quality of life in persons with active epilepsy. Epilepsy Behav 2014;41:55-6. https://doi.org/10.1016/j.yebeh.2014.09.035.
- Lundgren T, Dahl J, Hayes SC. Evaluation of mediators of change in the treatment of epilepsy with acceptance and commitment therapy. J Behav Med 2008;31:225-35. https://doi.org/10.1007/s10865-008-9151-x.
- Dewhurst E, Novakova B, Reuber M. A prospective service evaluation of acceptance and commitment therapy for patients with refractory epilepsy. Epilepsy Behav 2015;46:234-41. https://doi.org/10.1016/j.yebeh.2015.01.010.
- Smith A, McKinlay A, Wojewodka G, Ridsdale L. A systematic review and narrative synthesis of group self-management interventions for adults with epilepsy. BMC Neurol 2017;17. https://doi.org/10.1186/s12883-017-0893-3.
- Ridsdale L, Kwan I, Cryer C. The effect of a special nurse on patients’ knowledge of epilepsy and their emotional state. Epilepsy Evaluation Care Group. Br J Gen Pract 1999;49:285-9.
- Lorig K, Seleznick M, Lubeck D, Ung E, Chastain RL, Holman HR. The beneficial outcomes of the arthritis self-management course are not adequately explained by behavior change. Arthritis Rheum 1989;32:91-5. https://doi.org/10.1002/anr.1780320116.
- Thornicroft G, Mehta N, Clement S, Evans-Lacko S, Doherty M, Rose D, et al. Evidence for effective interventions to reduce mental-health-related stigma and discrimination. Lancet 2016;387:1123-32. https://doi.org/10.1016/S0140-6736(15)00298-6.
- Ridsdale L, Robins D, Fitzgerald A, Jeffery S, McGee L. Epilepsy in general practice: patients’ psychological symptoms and their perception of stigma. Br J Gen Pract 1996;46:365-6.
- Baker GA, Jacoby A, De Boer H, Doughty J, Myon E, Taïeb C. Patients’ understanding of and adjustment to epilepsy: interim findings from a European survey. Epilepsia 1999;40:26-9. https://doi.org/10.1111/j.1528-1157.1999.tb02091.x.
- Office for National Statistics . Census Data Catalogue 2011 2011. www.ons.gov.uk/census/2011census/2011censusdata/2011censusdatacatalogue (accessed 30 March 2017).
- Ridsdale L, McCrone P, Morgan M, Goldstein L, Seed P, Noble A. Can an epilepsy nurse specialist-led self-management intervention reduce attendance at emergency departments and promote well-being for people with severe epilepsy? A non-randomised trial with a nested qualitative phase. Health Serv Deliv Res 2013;1.
- Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. Characteristics of people with epilepsy who attend emergency departments: prospective study of metropolitan hospital attendees. Epilepsia 2012;53:1820-8. https://doi.org/10.1111/j.1528-1167.2012.03586.x.
- Sonecha S, Noble AJ, Morgan M, Ridsdale L. Perceptions and experiences of epilepsy among patients from black ethnic groups in south London. Prim Health Care Res Dev 2015;16:450-60. https://doi.org/10.1017/S1463423614000437.
- Rogers EM. Diffusion of Innovations. New York, NY: The Free Press; 2003.
- Stangl AL, Lloyd JK, Brady LM, Holland CE, Baral S. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come?. J Int AIDS Soc 2013;16. https://doi.org/10.7448/IAS.16.3.18734.
- Goldstein L, Kapur N, Zeman A, Kapur N, Jones-Gotman M. Epilepsy and Memory. Croydon: Oxford University Press; 2012.
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855-62. https://doi.org/10.1111/j.1464-5491.2012.03698.x.
- Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol 2007;64:1595-9. https://doi.org/10.1001/archneur.64.11.1595.
- Hart YM, Shorvon SD. The nature of epilepsy in the general population. II. Medical care. Epilepsy Res 1995;21:51-8. https://doi.org/10.1016/0920-1211(95)00008-X.
- Jacoby A. Epilepsy and the quality of everyday life. Findings from a study of people with well-controlled epilepsy. Soc Sci Med 1992;34:657-66. https://doi.org/10.1016/0277-9536(92)90193-T.
- Helde G, Bovim G, Brathen G, Brodtkorb E. A structured, nurse-led intervention program improves quality of life in patients with epilepsy: a randomized, controlled trial. Epilepsy Behav 2005;7:451-7. https://doi.org/10.1016/j.yebeh.2005.06.008.
- Noble AJ, Marson AG. Which outcomes should we measure in adult epilepsy trials? The views of people with epilepsy and informal carers. Epilepsy Behav 2016;59:105-10. https://doi.org/10.1016/j.yebeh.2016.01.036.
- Tackling Wasteful Spending on Health. Paris: OECD Publishing; 2017.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Clark DM. Implementing NICE guidelines for the psychological treatment of depression and anxiety disorders: the IAPT experience. Int Rev Psychiatry 2011;23:318-27. https://doi.org/10.3109/09540261.2011.606803.
- Furler J, O’Neal D, Speight J, Manski-Nankervis J-A, Gorelik A, Holmes-Truscott E, et al. Supporting insulin initiation in type 2 diabetes in primary care: results of the Stepping Up pragmatic cluster randomised controlled clinical trial. BMJ 2017;356. https://doi.org/10.1136/bmj.j783.
Appendix 1 National Institute for Health Research call for research
This information can be found at www.journalslibrary.nihr.ac.uk/programmes/hta/0916501/#/.
Appendix 2 The process of adopting self-management behaviours
FIGURE 16.
The process of adopting self-management behaviours. Dotted lines represent the effects of behaviours, positive or negative, that can modify skill utilisation and a feeling of mastery. The figure is adapted from Taylor et al. 57–59 Contains information licensed under the Non-Commercial Government Licence v2.0.
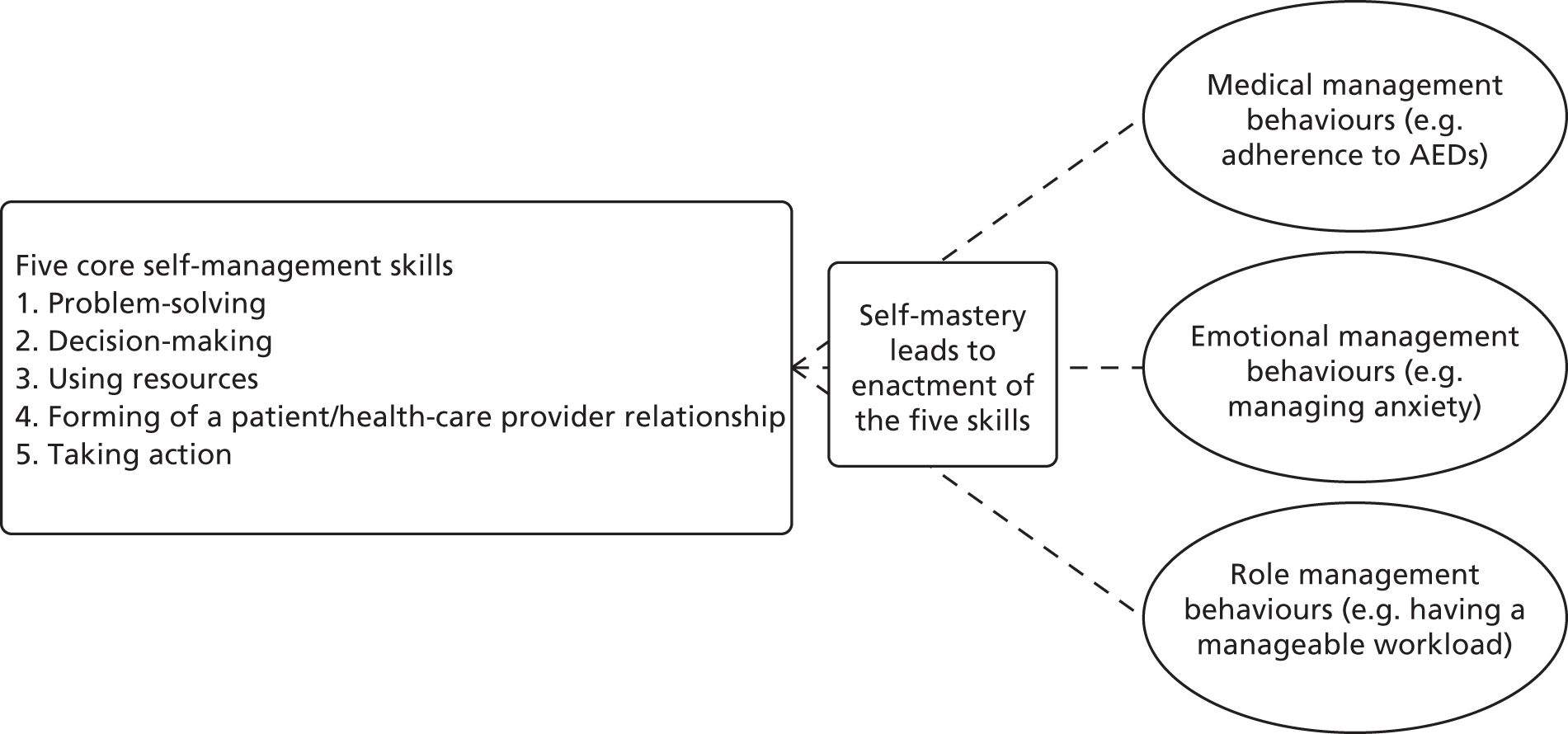
Appendix 3 SMILE (UK) intervention development timeline
FIGURE 17.
A timeline of events during the SMILE (UK) intervention adaptation.
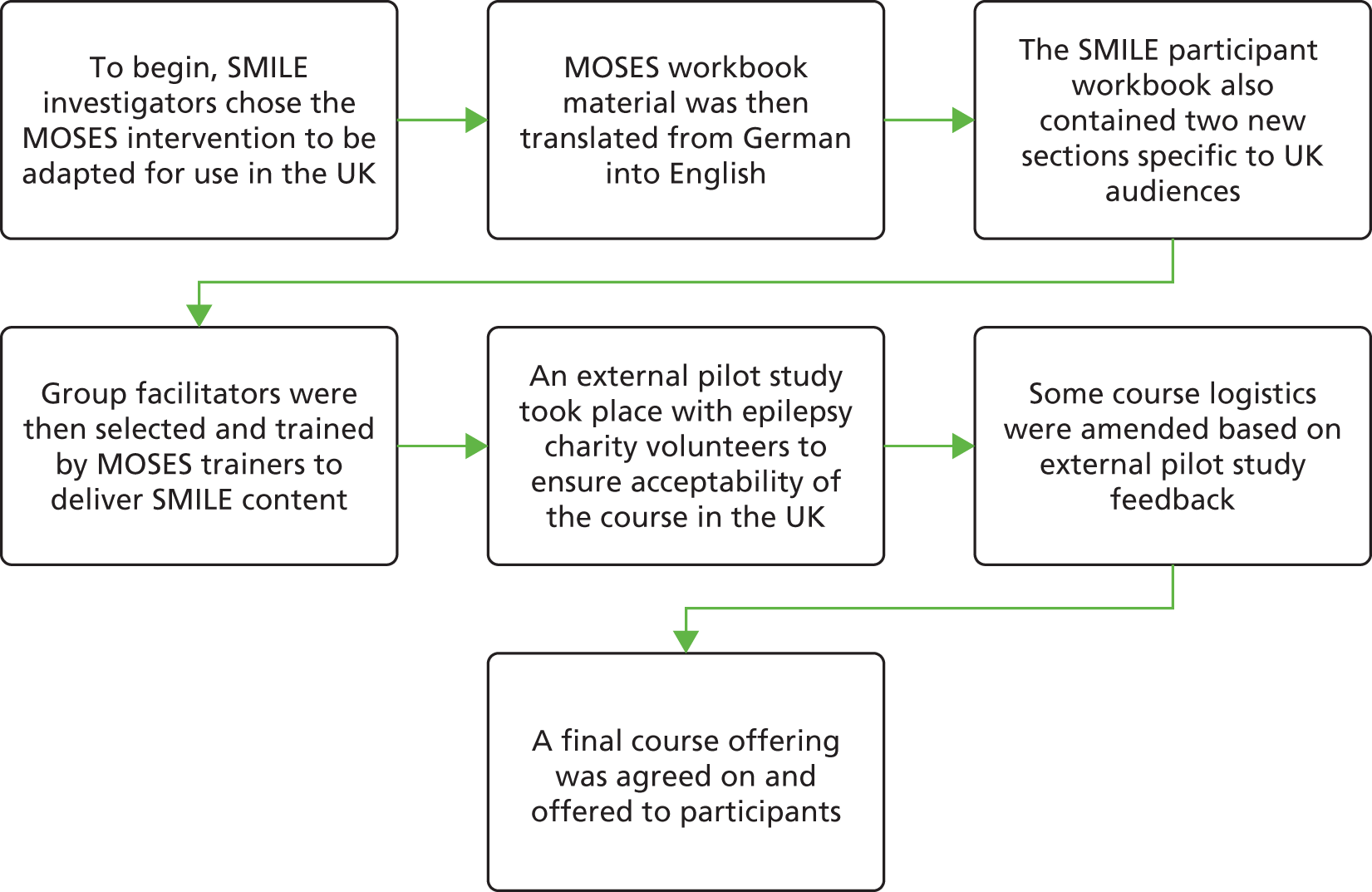
Appendix 4 SMILE (UK) course schedule
| Day 1 | ||
|---|---|---|
| Topic | Duration (minutes) | Session objective |
| Introductions | 15 | |
| Living with epilepsy part 1 (module 1) | 90 | To encourage PWE to open up about their experiences of living with their condition |
| Break (coffee/tea provided) | 15 | |
| Living with epilepsy part 2 (module 1) | 90 | As above |
| Lunch (provided) | 45 | |
| PWE (module 2) | 30 | Discuss frequencies and distribution of different epilepsy subtypes. Specific examples of PWE are discussed |
| Basic knowledge (module 3) | 60 | Describes how epileptic seizures start, develop and stop; how seizures are different; and causes of different seizures |
| Break (coffee/tea provided) | 15 | |
| Self-control (module 6) | 90 | Discuss seizure triggers and explain how seizures can be affected by behaviour |
| Network (module 9) | 15 | Provide information on support networks and means of developing a personal network that can be helpful in managing epilepsy |
| Summary of the day and close | 15 | |
| Day 2 | ||
|---|---|---|
| Topic | Duration (minutes) | Session objective |
| Welcome, ice breaker | 15 | |
| Treatment (module 5) | 90 | Provide information on various treatment options, including most commonly used AEDs. Discussion on taking active role in treatment |
| Break (coffee/tea provided) | 15 | |
| Diagnosis (module 4) | 75 | Inform participants about diagnostic investigations and methods of documenting seizures |
| Prognosis (module 7) | 45 | Describe different courses of epilepsy and instances where seizure freedom is achieved |
| Lunch (provided) | 45 | |
| Personal and social life part 1 (module 8) | 75 | Discuss impact of epilepsy on QoL and ways of cultivating self-worth and social support |
| Break (coffee/tea provided) | 15 | |
| Personal and social life part 2 (module 8) | 90 | As above |
| Summary of course and close | 10 | |
Appendix 5 List of SMILE (UK) facilitators
| Role | Facilitator characteristics | |||||
|---|---|---|---|---|---|---|
| Years in profession | Qualifications | Taught groups before | Number of treatment courses taught | Number of control courses taught | Total courses taught | |
| ENS | 32 years as RN | UG | Yes | 6 | 0 | 6 |
| 16 years as ENS | PQ | |||||
| EEG technician | 25 | UG | No | 1 | 0 | 1 |
| ENS | 25 years in profession | UG | Yes | 3 | 1 | 4 |
| 17 years as ENS | PG | |||||
| EEG technician | 26 | PG | Yes | 5 | 1 | 6 |
| EEG technician | 5 | UG | No | 3 | 1 | 4 |
| EEG technician | 23 | UG | No | 2 | 0 | 2 |
| PG | ||||||
| EEG technician | > 15 | PG | Yes | 1 | 1 | 2 |
| ENS | 19 | UG | Yes | 3 | 2 | 5 |
| PG | ||||||
| PQ | ||||||
| EEG technician | 7 | UG | No | 0 | 0 | 0 |
| ENS | 26 | UG | Yes | 1 | 1 | 2 |
| PG | ||||||
| PQ | ||||||
| EEG technician | 2 | UG | No | 4 | 1 | 5 |
Appendix 6 SMILE (UK) ‘train the trainers’ course schedule
| Time | Duration (minutes) | Content |
|---|---|---|
| 09.00–09.45 | 45 | Welcome |
| Introduction of participants – professional background, epilepsy experience, why interested in MOSES | ||
| Expectation: best and worst expectations about course | ||
| 09.45–10.10 | 25 | Presentation of work schedule |
| 10.10–10.30 | 20 | Input: Presentation of material |
| (Nine modules, trainer’s manual, exercise book, number of participants, trainer) | ||
| 10.30–10.45 | 15 | Break |
| 10.45–12.15 | 90 | Living with epilepsy (module 1) |
| Aims, going through the chapter and commenting it | ||
| Demonstrating mind map and scale/thesis | ||
| 12.15–13.00 | 45 | Lunch |
| 13.00 – 13.45 | 45 | PWE (module 2) |
| Aims, going through and commenting on it | ||
| 13.45–14.30 | 45 | Basic Knowledge (module 3) |
| Aims, going through and commenting on it | ||
| 14.30–14.45 | 15 | Break |
| 14.45–15.30 | 45 | Diagnosis (module 4) |
| Aims, going through and commenting on it | ||
| 15.30–16.15 | 45 | Input: MOSES: idea, development, aims, actual situation |
| 16.15–16.30 | 15 | Break |
| 16.30–17.45 | 75 | Therapy (module 5) |
| Aims, going through and commenting on it |
| Time | Duration (minutes) | Content |
|---|---|---|
| 09.00–09.15 | 15 | Welcome and morning round |
| 09.15–09.45 | 30 | Input: educational tools |
| 09.45–10.30 | 45 | Self-control (module 6) |
| Aims, going through and commenting on it | ||
| 10.30–10.45 | 15 | Break |
| 10.45–11.15 | 30 | Input: MODES evaluation – domains with/without effects |
| 11.15–12.15 | 60 | Prognosis (module 7) |
| Aims, going through and commenting on it | ||
| 12.15–13.00 | 45 | Lunch |
| 13.00–14.15 | 75 | Social Life (module 8) |
| Aims, going through and commenting on it | ||
| 14.15–14.30 | 15 | Network (module 9) |
| Aims, going through and commenting on it | ||
| 14.30–14.45 | 15 | Break |
| 14.45–15.00 | 10 | Input: qualification of MOSES trainers |
| 15.00–15.20 | 20 | Input: my first time with MOSES |
| 15.20–16.00 | 40 | Closing round: expectations and feedback |
| Certification of attendance |
Appendix 7 Topic guide during qualitative interviews
Pilot study
Following a brief introduction, reappraisal of consent and questions about participants’ circumstances (age, living arrangements, educational achievement), they were asked about their views and experience of taking part in the pilot SMILE (UK) programme. The main prompts (in italics) are given below:
Why did you decide to take part in the SMILE pilot?
Have you been involved in anything like this before?
Was it because it was something you had been looking for already, or was it the idea of being part of something new in epilepsy treatment, for example?
What did you think of the content of material that was delivered during the two days?
Topics covered? Were there any that were particularly useful? Any that you found you didn’t particularly like?
How did you find the way in which information was delivered? Was it easy to understand or a bit difficult?
How did you find learning with others in a group?
Were there any advantages to this for you? Were there any disadvantages for you?
Did you find it easy to participate and contribute or was this difficult?
What did you think of the different teaching methods used? (statements, mind maps, brain storming and information slides)
Did you like the different teaching methods used during the course or did you find them confusing?
How useful do you consider the course to be for the future?
Do you think you’ll be able to use anything you experienced on SMILE again? Useful to use with others in your life?
Process evaluation
‘Thanks for agreeing to take the time out and talk with me today, before we begin I just want to let you know that although I will be asking you about your experiences with SMILE, I am not actually working on SMILE and I am doing this as part of my degree at King’s. Everything you say today will be completely confidential; unless you explicitly ask for something to be passed on no one will know which responses came from you. With this in mind, I would like to encourage you to feel comfortable to express your honest opinions – which will be really useful in the further development of the course. Do you have any questions before we start?’
Patient’s epilepsy
Can you tell me a bit about your epilepsy?
When did you find out you had epilepsy?
How did you feel when you were first diagnosed? How do you feel about it now?
How do you feel you cope with your epilepsy day-to-day?
Prompts: employment, relationships, anxiety
Help-seeking behaviour
In the past, have you tried to find out more about your epilepsy?
How have you done this?
Prompts: Nurses? Clinicians? Support groups? Internet? Alternative therapies?
SMILE course
When you first heard about SMILE, how did you feel about coming on the course?
Prompt: some people may feel worried or anxious before coming on a course like this, is this something that you experienced?
Prompts: any worries about going on the course? Looking forward to it?
So thinking about the SMILE course, what was the most interesting part of the course for you?
How did you feel about being part of a group?
How did you feel about hearing other people’s stories?
How did you feel about discussing your emotions in the group?
During the course you were asked to place stickers on a line to describe how you felt about certain topics, how did you feel about doing this?
I believe there was some discussion about medication for epilepsy, was this useful?
Did you find out any things that were helpful?
Did you learn anything new about your epilepsy?
Prompts: types of seizures, triggers diagnostic techniques, other people’s experiences
Since completing the course, do you think differently about your own epilepsy?
Prompts: triggers, medication management
Life after the course
Do you think the SMILE course has helped you in managing your epilepsy?
Prompts: Medication? Triggers? Warning signs? involvement in treatment?
Have you used any of the techniques you learned on the course?
Although the workbook is not an essential part of the course, have you found it useful?
Social network
Have you stayed in touch with anyone from the course?
Prompts: How many people? Have you found this useful?
Course improvements
Would you recommend other PWE to go on the SMILE course?
Is there anything about the course that you would like to change?
Is there anything else you would like to say about the SMILE course that you have not said so far?
Appendix 8 Ethics approval letter
Appendix 9 Participant information sheet example
Appendix 10 Participant consent form
Appendix 11 Modified ‘Impact of Epilepsy’ scale
Appendix 12 Capacity development
SMILE (UK) research team training
Trial Manager: NHS – Good Clinical Practice; KCL – chairing meetings, Managing research data, unconscious bias workshop, introduction to project management, management essentials (for professional services and academic managers), psychometrics; UK trial Managers network – clinical trials project management.
Research Associate: NHS – Good Clinical Practice; NIHR – Journals Library webinar; KCL – search techniques for systematic reviews, assertiveness skills for researchers, mental health first aid, managing research projects, essentials of copyright, fundamentals of good writing, ‘publish or perish’ and journal metrics.
Research Assistant: KCL – CVs and applications for academic jobs, Microsoft Excel® intermediate, Learning to use NVivo, Desktop EndNote for PC, writing for publication in the sciences, qualitative data analysis, long document work and tutorial for video making.
Clinical Trials Administrator: NHS – Good Clinical Practice; KCL – minute-taking, assertiveness skills for researchers, CV and interview skills, search techniques for systematic reviews, pure training for administrators, and literature review workshop for health and clinical sciences.
SMILE (UK) research team activities
In November 2016, the research team arranged and attended an Advanced SMILE (UK) Facilitator Training course. The session was chaired by MOSES experts and involved SMILE (UK) facilitators’ meeting to discuss their experiences of teaching SMILE (UK). The chief investigator also presented the SMILE (UK) trial outcomes during the event.
Ridsdale L, Wojewodka G. I Am Not Alone: RCT of Group Self-management Education Baseline Findings and Views of Participants with Epilepsy. Psychological Medicine Seminar at King’s College London, London, UK, 9 May 2016.
Wojewodka G. Self-Management Education for Adults with Poorly Controlled Epilepsy: The SMILE(UK) Trial. Wohl Internal (WIN) Postdoc/Student Seminar Series at King’s College London, London, UK, 10 October 2016.
Student involvement with SMILE (UK)
Krooupa AM. Experiences and Perceived Benefits of Participation in a Self-management Intervention for Adults with Poorly Controlled Epilepsy: A Qualitative Study Nested in the SMILE (UK) Trial. Master’s thesis. London: King’s College London; 2015.
Philpott S. Experiences of Participants Completing ‘Self-management Education for Adults with Poorly Managed Epilepsy (SMILE)’: A Qualitative Study Nested in a Randomised Control Trial. Master’s thesis. London: King’s College London; 2015.
Smith A. Group Self-management Interventions for Adults with Epilepsy: A Systematic Review. Special Study Course Module for MBBS programme student project.
Smith A, McKinlay A, Wojewodka G, Ridsdale L. A systematic review and narrative synthesis of group self-management interventions for adults with epilepsy. BMC Neurol 2017;17:114.
Wijnen B. A Comparison of the Responsiveness of EQ-5D-5L and the QOLIE-31-P and Mapping of QOLIE-31-P to EQ-5D-5L in Epilepsy. PhD student project.
Wijnen BFM, Mosweu I, Majoie M, Ridsdale L, de Kinderen RJA, Evers S, et al. A comparison of the responsiveness of EQ-5D-5L and the QOLIE-31P and mapping of QOLIE-31P to EQ-5D-5L in epilepsy [published online ahead of print September 4 2017]. Eur J Health Econ 2017.
Glossary
- Absence
- A generalised seizure in which the patient loses consciousness but does not fall or have convulsions.
- Acute
- A condition that only lasts for a short amount of time or that recurs only at sporadic intervals over time.
- Adherence
- The degree to which a treatment or protocol is followed. This can be used in the context of a patient taking medication as prescribed. In addition, in this report it is used as a measure to assess whether or not facilitators delivered the SMILE (UK) components as specified by the teaching manual.
- Adverse event
- Any untoward incident experienced by a participant that does not necessarily have a causal relationship with the intervention.
- Aetiology
- The cause or set of causes attributed to a condition.
- Antiepileptic drug
- Medicine taken for the treatment of seizures.
- Arthritis
- A condition causing painful joint inflammation.
- Attrition
- Rate at which participants are lost throughout a trial.
- Aura
- A sensation that comes before the onset of a seizure. It is classified as a simple focal seizure, which can become a generalised seizure.
- Autonomous
- The ability and assertiveness to act independently.
- Blinding
- The act of ensuring that the participant group allocation is concealed from one or more individuals involved in a research study.
- Chronic
- Any condition that lasts over a long period of time.
- Clonic seizure
- The rapid contraction and relaxation of the muscles (i.e. jerking).
- Cochrane review
- A systematic review of health-care research internationally accepted as the gold standard in evidence-based health-care research.
- Cognition
- The cerebral action or process of attaining knowledge and understanding through thought, experience and the senses.
- Comorbidity
- Any condition that occurs alongside the primary diagnosis.
- Complex intervention
- An intervention that contains several interacting components.
- Complex partial seizure
- A seizure that causes a high level of impairment and loss of consciousness or memory. Other names include complex focal seizure.
- Compliance
- See Adherence.
- Computer tomography
- Scans are carried out by using 360-degree radiography and the computerised images used in assessment.
- Congenital
- Present from birth.
- Contamination
- Group contamination can occur when participants from one trial arm behave as if they are in the other arm. In the SMILE (UK) trial, this happened when participants in the treatment-as-usual group attended the course in error.
- Control(s)/group
- Participants who have not received the intervention. In the SMILE (UK) study, they received treatment as usual.
- Diabetes mellitus
- A disease in which the body’s ability to produce or respond to the hormone insulin is impaired.
- Diagnosis
- Identification of a disease or disorder.
- Didactic
- A method of teaching that provides students with the required theoretical knowledge. It mainly involves the teacher or facilitator speaking rather than having interaction or discussion between students.
- Electroencephalogram
- Form of testing used to detect abnormal electrical activity in the brain.
- Epidemiology
- The incidence and distribution of medical conditions in a population.
- Epileptic activity
- Neurological electrical activity associated with epilepsy.
- Epileptology
- The study of epilepsy.
- Facilitators
- The health-care professionals who provided the intervention to participants.
- Febrile seizure
- A type of seizure that occurs in infants or young children, which starts with a fever.
- Fidelity
- The measure of adherent and competent delivery of the intervention.
- Focal seizure
- A partial seizure that causes alterations in attention, movement or behaviour.
- Further education
- Any education beyond secondary education (e.g. Advanced levels, Bachelor of Technology).
- Generalised seizure
- A seizure that affects both hemispheres in the brain. Seizures are accompanied by a loss of consciousness and are categorised by the following categories: generalised tonic–clonic, myoclonic, absence and atonic.
- Generalised tonic–clonic seizure
- A type of seizure that combines the symptoms of both a tonic seizure and a clonic seizure. It affects both hemispheres of the brain.
- Health economics
- The study of the cost-effectiveness, value and benefits in the use of an intervention.
- Higher education
- Any education beyond further education (e.g. degree, Doctor of Philosophy).
- Idiopathic
- Of unknown origin.
- Index of Multiple Deprivation
- A measure of relative deprivation for small areas of each county in England constructed from a number of different types, or domains, of deprivation (e.g. income, education, crime).
- Intention-to-treat analysis
- All patients who were enrolled and randomly allocated to either treatment or control are analysed in the groups to which they were randomised.
- Inverse association
- A contrary relationship between two variables such that they move in opposite directions.
- Magnetic resonance imaging
- A diagnostic method that uses magnetic fields and radio waves to produce a detailed image of the body’s soft tissue and bones.
- Nerve cell
- A specialised cell transmitting nerve impulses.
- Nested process
- A research design in which levels of one factor are hierarchically included under levels of another factor.
- Neurophysiologist
- A health-care professional who specialises in the physiology of the brain.
- NHS perspective
- A health economics term used to describe cost-effectiveness for the NHS.
- Pilot study
- A study carried out on a small scale before the intended study in order to test the robustness and integrity of the intended study.
- Poorly controlled epilepsy
- An individual who experiences more than two seizures a year (National Institute for Health Research definition).
- Positron emission tomography
- An imaging test that helps to reveal how tissues and organs are functioning using a radioactive drug (tracer) to show this activity.
- Pragmatic
- A deconstructive paradigm that encourages the use of mixed methods in research eliminating a position between two opposing theoretical views.
- Prevalence
- The rate of the presence of a specific condition in the population.
- Primary care
- Health care provided in the community for people making an initial approach to a general practitioner or clinic for advice or treatment.
- Process evaluation
- A qualitative exploration of participants’ experience of the intervention.
- Prognosis
- Outlook for individuals diagnosed with a condition.
- Proxy
- A variable that is not directly relevant to the analysis, but serves in place of an unobservable or immeasurable variable.
- Psychogenic
- A symptom that presents as a medical condition but in fact has an underlying psychological cause.
- Psychosocial
- A combination of factors that originate from both psychological and social influences.
- Qualitative research
- Research that explores the underlying reasons, opinions and motivations of participants, providing rich insight into the experiences of participants in a study.
- Quality-adjusted life-year
- A generic measure of the burden of a condition, including both the quality and the quantity of life lived. It is used in economic evaluation to assess the cost-effectiveness of the intervention.
- Quantitative research
- A formal, objective process that uses numerical data to acquire information about the intervention by defining variables, investigating relationships between variables and establishing cause-and-effect interactions between variables.
- Retention
- The rate at which participants remain in the study at specified intervals throughout the process (i.e. 6 months and 12 months).
- Secondary care
- Care that is provided by a specialist on referral by a primary care physician and that requires more advanced investigation or treatment than a general practitioner can provide.
- Seizures
- Any abnormal electrical activity in the brain that can be caused by numerous factors, including those not related to epilepsy.
- Self-efficacy/mastery
- An individual’s confidence in his or her ability to accomplish behaviours essential for specific management of their condition.
- Self-management
- Any method of treatment for a condition that is initiated by the patient.
- Serious adverse event
- Any adverse event resulting in an inpatient hospital admission or prolongation of hospital stay, is life-threatening, or causes persistent disability or death.
- Societal perspective
- A health economics term for cost-effectiveness for the society.
- Status epilepticus
- An incident in which a seizure lasts > 5 minutes or when two seizures occur within 10 minutes of each other with no period of recovery in between.
- Symptomatic
- Any indication that a symptom is the result of a specific medical condition. It can also mean displaying symptoms that are characteristic of a specific medical condition.
- Taxonomy
- The process of classification according to a predetermined system, with the result providing a conceptual outline for discussion or analysis.
- Tonic seizure
- A seizure that involves the experience of muscles becoming rigid and stiff.
- Treatment as usual
- A study condition in which patients continue to receive the treatment they were receiving prior to being recruited into the study.
- Triggers
- Any factor that an individual attributes as causing an acute seizure.
- Visual analogue scale
- A psychometric response scale in the form of an image used in questionnaires to determine subjective characteristics or attitudes that cannot be directly measured.
List of abbreviations
- AE
- adverse event
- AED
- antiepileptic drug
- CACE
- complier average causal effect
- CEAC
- cost-effectiveness acceptability curve
- CEP
- cost-effectiveness plane
- CI
- confidence interval
- CSRI
- Client Service Receipt Inventory
- DAFNE
- Dose Adjustment For Normal Eating
- DESMOND
- Diabetes Education for Self-Management for Ongoing and Newly Diagnosed
- DMEC
- Data Monitoring and Ethics Committee
- EARN
- Epilepsy Action Research Network
- eCRF
- electronic case report form
- ED
- emergency department
- EEG
- electroencephalogram
- ENS
- epilepsy nurse specialist
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HADS-A
- Hospital Anxiety and Depression Scale Anxiety
- HADS-D
- Hospital Anxiety and Depression Scale Depression
- HCP
- health-care professional
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IoPPN
- Institute of Psychiatry, Psychology and Neuroscience
- IQR
- interquartile range
- ITT
- intention to treat
- KCH
- King’s College Hospital
- KCL
- King’s College London
- LTC
- long-term condition
- MICE
- multivariate imputation by chained equations
- MOSES
- Modular Service Package for Epilepsy
- MRC
- Medical Research Council
- NHNN
- National Hospital for Neurology and Neurosurgery
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PI
- principal investigator
- PWE
- people with epilepsy
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QOLIE-31
- Quality Of Life In Epilepsy 31
- QOLIE-31-P
- Quality Of Life In Epilepsy 31-P
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SEE
- Sepulveda Epilepsy Education
- SMILE (UK)
- Self-Management education for adults with poorly controlled epILEpsy
- TAU
- treatment as usual
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WTP
- willingness to pay
