Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/72/01. The contractual start date was in September 2013. The draft report began editorial review in August 2017 and was accepted for publication in January 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Robert Pickard reports grants from the National Institute for Health Research (NIHR) during the conduct of the study. Thomas Chadwick reports grants from the NIHR Health Technology Assessment (HTA) programme during the conduct of the study and outside the submitted work. Katherine Walton reports grants from NIHR during the conduct of the study. Elaine McColl reports grants from the NIHR HTA programme during the conduct of the study and from the NIHR Journals Library outside the submitted work. From 2013 to 2016 she was an editor for the NIHR Programme Grants for Applied Research (PGfAR) series, with a fee paid to her employing organisation. Luke Vale reports that he is a member of the NIHR HTA Clinical Evaluation and Trials panel and is co-director of NIHR Research Design Service North East. Mohamed Abdel-Fattah reports grants from Bard Medical UK (Crawley, UK), Astellas Pharma Inc. (Tokyo, Japan), Coloplast (Humlebæk, Denmark), Pfizer Inc. (New York, NY, USA), Advanced Medical Solutions [(AMS) Winsford, UK] and Ethicon Inc. (Somerville, NJ, USA) outside the submitted work and is a member of the HTA Interventional Procedures panel. Paul Hilton reports a grant from the NIHR HTA programme during the conduct of the study and grants from the William Harker Foundation and Wellbeing of Women outside the submitted work. He was a member of the National Institute for Health and Care Excellence (NICE) Interventional Procedures Advisory Committee (2002–7), NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC)-HTA Therapeutic Procedures Panel (2007–8) and NETSCC-HTA Clinical Evaluations and Trials Prioritisation Group (2008–10). He chaired the NICE development group for clinical guideline on urinary incontinence in women (2004–7). Mandy Fader reports grants from NIHR (reference number RP-PG-0610-10078) and from the Small Business Research Initiative grant outside the submitted work. Simon Harrison reports grants from the NIHR HTA programme (reference number 11/72/01) during the conduct of the study. James Larcombe reports grants from the NIHR HTA programme during the conduct of the study and is a member of the HTA Elective and Emergency Specialist Care panel. Paul Little reports that he is director of PGfAR and editor-in-chief of the PGfAR publication in the NIHR Journals Library. James N’Dow is a member of the HTA General Board. Nikesh Thiruchelvam reports grants from Astellas, non-financial support from Astellas and personal fees from Coloplast outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Pickard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction (background and objectives)
Material from Brennand et al. 1 has been used within this chapter. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Scientific background
Clean intermittent self-catheterisation
Clean intermittent self-catheterisation (CISC) is an important management option for people who cannot empty their bladder naturally owing to bladder outlet obstruction or to failure or inco-ordination of bladder muscle contraction, most frequently associated with neurological disease. 2,3 Patients needing CISC insert a thin (typically 4.5 mm diameter) catheter up through the urethra, drain the bladder and then remove the catheter. 4 This is then repeated at set time intervals or as dictated by sensation of bladder fullness. Single-use, sterile-packed disposable catheters, typically with a hydrophilic coating, appear to be the most commonly used option in the UK, although there is no robust evidence of benefit over reusable or uncoated catheters. 5
Prevalence of use of clean intermittent self-catheterisation in the UK
There are no specific incidence or prevalence data for CISC use in the UK. The NHS England prescription database shows that approximately 66 million CISC catheters were prescribed in 2015, at a cost of £103M. 6 This estimate ties in with calculations using data from catheter manufacturers from 2013, which showed 54 million catheters dispensed at a cost of £81M (unpublished data courtesy of Doreen McClurg, Glasgow Caledonian University, February 2017). Assuming that each individual uses an average of three catheters per day,7 there are about 60,000 CISC users in England and perhaps 72,000 in the UK as a whole.
Recurrent urinary tract infection among people using intermittent catheterisation
Recurrent urinary tract infection (UTI) is the commonest adverse event (AE) experienced by CISC users, affecting between 12% and 88% of cohorts. 8 Separation of rates of asymptomatic bacteriuria, which would not normally be treated, and symptomatic UTI in these studies is often not possible. From the available literature, we estimate that 50% of users have persistent bacteriuria and about 25% suffer two or more symptomatic UTI episodes per year. 9 Neurological disease, female sex, young age and high bladder volumes have been associated with higher prevalence of UTI. 2 Rates will also vary according to the definition of symptomatic UTI used, in particular whether or not microbiological proof is required. 10 The most frequently isolated organism is Escherichia coli, accounting for 60–70% of isolates. 9 Most episodes are associated with transient symptoms such as lower abdominal pain, urethral pain and flu-like symptoms (occasionally systemic upset can occur with fever and loin pain). Rarely, bloodstream infection requiring hospital treatment ensues. Those with reduced bladder sensation may complain of cloudy urine, increased odour and worse incontinence. 11 Recurrent UTI is distressing and an additional burden for patients on top of their underlying disease and functional disability. 8 In one study,2 a cohort of 407 CISC users self-rated UTI symptoms over the previous 12 months. The findings were that 24% had no symptoms, 59% had mild symptoms, 14% had moderate symptoms and 3% had major symptoms;2 the rate of bacteriuria was 60%, with symptomatic UTI occurring in 12–18% of the 407 participants. Conservatively, using the lower rate, this suggests that 8640 CISC users in the UK suffer recurrent UTI. This is the target population for this trial.
A number of simple interventions have been trialled to reduce UTI risk for CISC users, including single-use hydrophilic catheters and antiseptics, but a Cochrane review5 and meta-analysis12 found no robust evidence for their efficacy. More recent randomised controlled trials (RCTs) reported reduced UTI risk with single-use hydrophilic catheters for patients with spinal cord injury during initial hospitalisation but increased risk of UTI in children compared with reusable, uncoated catheters. 13 A further two trials14,15 showed no benefit of cranberry extract in the prevention of UTI in CISC users. 16 The need for strategies to reduce prevalence of UTI in this population has been emphasised by recent reports from the James Lind Alliance and the National Institute for Health and Care Excellence (NICE). 17
Evidence for use of antibiotic prophylaxis
Once-daily low-dose antibiotic prophylaxis is effective for women without bladder emptying problems who suffer recurrent UTI. A systematic review and meta-analysis18 of 10 RCTs involving 403 participants showed a relative risk of UTI of 0.15 [95% confidence interval (CI) 0.08 to 0.28] in favour of antibiotic prophylaxis. AEs in trials using nitrofurantoin, trimethoprim (Kent Pharmaceuticals, Ashford, UK) or cefalexin (Sandoz Ltd, Holzkirchen, Germany) were mild and rarely associated with withdrawal, but were more frequent in the antibiotic group, with a relative risk of 1.78 (95% CI 1.06 to 3.0); gastrointestinal upset, skin rash and vaginal candidiasis predominated. Nitrofurantoin appeared to be more effective than trimethoprim but resulted in more withdrawals. These two drugs, together with cefalexin, are recommended and licensed for this indication in the UK. 19 There were no reports of serious adverse events (SAEs), such as neuropathy or pulmonary fibrosis, in the nitrofurantoin groups in randomised studies included in the Cochrane review,18 but an observation study20 of prophylactic nitrofurantoin noted one episode of possible neuropathy in 219 patients over 12 months’ use. Awareness of these conditions, together with the possibility of liver inflammation and higher risk of side effects in people with renal impairment, is advised on the Medicines and Healthcare products Regulatory Agency (MHRA) licence for nitrofurantoin. 21
Evidence for the use of antibiotic prophylaxis for CISC users suffering recurrent UTI, the focus for this trial, has been summarised in a Cochrane review updated to September 2011. 22 The review found five small, low-quality RCTs23–27 relevant to the present trial with a total of 363 participants. For the outcome of clinical UTI, two crossover trials involving children showed no difference, while one-third involving children in an unblinded, parallel-group design found an incidence density ratio of 0.69 (95% CI 0.55 to 0.87) in favour of prophylaxis using a variety of agents. For the outcome of antibiotic-treated, microbiologically proven UTI, one trial24 showed no difference, whereas a second parallel-group placebo-controlled trial23 involving 131 adults hospitalised by recent spinal injury found a risk ratio of 0.78 (95% CI 0.62 to 0.97) in favour of prophylaxis with co-trimoxazole. The review authors concluded that, although results were promising, there was inadequate evidence for the clinical effectiveness of antibiotic prophylaxis for CISC users, agreeing with a previous review. 28 None of these trials found any excess harms in the prophylaxis groups, but changes in bacterial pathogens were recorded in only one study. 23 This looked at change in bacterial sensitivity and found that 75% of participants had at least one occurrence of isolation of a resistant pathogen with no difference between prophylaxis and placebo groups. 23 Recommendations for future trials were to:
-
use incidence of symptomatic UTI as the primary outcome
-
measure antibiotic resistance
-
control for factors increasing UTI risk – sex, frequency of catheterisation, neurological cause, frequency of previous UTI and prior use of antibiotic prophylaxis.
These results and the need for further research have been highlighted in a further narrative review. 29 The most recent trial included in the Cochrane review was published in 2011;26 we updated the Cochrane search to November 2016, using the same strategy and literature database sources, and found no additional reports.
Antibiotic stewardship
The impact of prophylactic antibiotic therapy for UTI on bacterial ecology particularly of gut flora (faecal microbiome) was explored in only one of the trials23 included in the Cochrane reviews, which found no difference between prophylaxis and no-prophylaxis groups. An observational study20 of prophylaxis with nitrofurantoin in a general population with recurrent UTI found no evidence of development of faecal organisms resistant to nitrofurantoin or loss of sensitive organisms in the gut, suggesting that this drug does not have potentially harmful effects on gut commensals. In a large RCT of antibiotic prophylaxis of recurrent UTI in women with normal voiding, it was found that use of once-daily co-trimoxazole markedly increased faecal and urinary carriage of resistant E. coli but that this returned to baseline 3 months after discontinuing the antibiotic prophylactic therapy. 30 There remains a high level of public health concern regarding the empiric continuous use of antibiotics as preventative or suppressive therapy for people who suffer repeated infection given the rapid emergence of resistant strains of a number of pathogenic bacteria. 31,32
Summary with implications for trial design
This background led the UK National Institute for Health Research (NIHR) to commission a trial to determine the clinical effectiveness and cost-effectiveness of the use of once-daily antibiotic prophylaxis for UTI in users of CISC (NIHR reference number 11/72/01). The call required a trial design to determine whether or not the apparent benefit of antibiotic prophylaxis seen in a small trial23 among a specific group of CISC users could be translated to a wider population in a routine care setting and whether or not any benefits are worth the costs, both financially and in terms of harms. To fulfil this brief, we designed the Antibiotic Treatment for Intermittent Catheterisation (AnTIC) trial. We specified an experimental UTI prevention strategy using continuous once-daily low-dose prophylactic antibiotic therapy against the control strategy of no prophylaxis in adults carrying out intermittent bladder catheterisation and suffering recurrent UTI. The aim was to determine the relative clinical effectiveness in terms of reduction in rate of UTI over 12 months and cost-effectiveness in terms of incremental cost of UTI avoided. The estimates of prevalence, clinical effectiveness, cost effectiveness and harms of prophylaxis from the literature allowed us to power a trial conservatively based on what was considered to be a minimum important difference from clinician, patient and economic perspectives.
Aims and objectives
The hypothesis addressed in the trial is that a strategy of antibiotic prophylaxis reduces the rate of symptomatic antibiotic-treated UTI by ≥ 20% compared with a strategy of no prophylaxis. To investigate this hypothesis, we aimed to achieve the following objectives.
Primary objectives
-
Determine the relative impact of prophylaxis on incidence of symptomatic, antibiotic-treated UTI over 12 months.
-
Determine the incremental cost per symptomatic UTI avoided.
Secondary objectives
-
Determine the relative effect of prophylaxis on the incidence of microbiologically proven UTI.
-
Determine relative rates of fever or hospitalisation because of UTI.
-
Determine relative rates of asymptomatic bacteriuria over 12 months.
-
Record AEs related to use of prophylactic or treatment antibiotics.
-
Assess change in resistance of pathogens isolated from urine and E. coli from perianal swabs.
-
Measure overall satisfaction with prophylactic antibiotic treatment.
-
Determine the relative effect on health status among trial participants.
-
Measure difference in kidney and liver function at 12 months.
-
Measure incremental cost per quality-adjusted life-year (QALY) gained.
-
Assess participants’ willingness to pay (WTP) to avoid a UTI by contingent valuation.
-
Assess participants’ perception of benefit using qualitative methods.
Chapter 2 Methods
Material from Brennand et al. 1 has been used within this chapter. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
This chapter covers general study methods, statistical analysis and governance; details of health economic and qualitative analyses are provided in Chapters 4 and 5, respectively.
Summary of trial design
We designed an open-label, patient-randomised parallel-group superiority trial comparing an experimental strategy of once-daily low-dose antibiotic prophylaxis with a control strategy of no prophylaxis in adults undertaking CISC who suffer recurrent UTI. Because both strategies are in routine use and the choice of antibiotic agent used for prophylaxis is governed by a number of clinical and microbiological factors, we chose a pragmatic design without blinding of clinicians or participants. Central trial staff entering and managing trial data, trial staff adjudicating outcomes and laboratory staff analysing microbiological samples were blinded to participant allocation. Both groups otherwise received usual care, including on-demand, discrete treatment courses of antibiotic treatment for UTI. Inclusion criteria were broad and the trial was set in the UK community recruiting participants from both primary and secondary care UK NHS organisations to ensure that trial results could be applied to all people using CISC who suffer recurrent UTI.
Sites
From November 2013 to October 2015, we progressively established 51 research sites comprising NHS organisations affiliated to the NIHR Clinical Research Networks (CRNs) in England and equivalent organisations in Scotland that agreed to host the trial locally. The sites were grouped around seven UK secondary care centres: (1) Newcastle upon Tyne, (2) Wakefield, (3) Bristol, (4) Cambridge, (5) Southampton, (6) Glasgow and (7) Aberdeen. All seven centres had a recruitment co-ordinator funded by the trial. The central trial office was established at the Newcastle Clinical Trials Unit (NCTU), Newcastle University. The central microbiological laboratory at Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, received all urine and perianal swab specimens for culture and storage. These results were used for the microbiological outcomes of the trial but were not used for local clinical decisions. Central trial staff liaised with NIHR CRNs to identify further sites. These sites consisted of 26 hospitals (secondary care) and 19 general practices (primary care). We also established a further six participant identification centres (PICs) that identified potential participants and referred them to nearby recruitment sites. The PICs were one secondary care, three primary care and two NHS community care provider sites (Table 1).
| Site/PIC | Number of participants | |
|---|---|---|
| Randomised | Hub total | |
| Hubs | ||
| Newcastle upon Tyne | ||
| Freeman Hospital, Newcastle upon Tyne | 70 | 78 |
| Royal Victoria Infirmary, Newcastle upon Tyne | 2 | |
| Sunderland Royal Infirmary (PIC) | 2 | |
| Harbinson House, Sedgefield (primary care site) | 2 | |
| Jubilee Practice, Newton Aycliffe (primary care site) | 2 | |
| Cambridge | ||
| Addenbrooke’s Hospital | 17 | 17 |
| Bristol | ||
| Southmead Hospital | 14 | 25 |
| Bristol, Somerset and South Gloucestershire Community Care Groups (PIC) | 2 | |
| Sirona Care and Health (PIC) | 7 | |
| Weston Area Health (PIC) | 2 | |
| Glasgow | ||
| Southern General Hospital, Glasgow | 25 | 52 |
| University Hospital, Ayr | 12 | |
| Western General Hospital, Edinburgh | 15 | |
| Aberdeen | ||
| Aberdeen Royal Infirmary | 34 | 46 |
| Aberdeen primary care (PIC) | 12 | |
| Wakefield | ||
| Pinderfields Hospital, Wakefield | 25 | 32 |
| Leeds Community Healthcare (PIC) | 7 | |
| Southampton primary care sites | ||
| Portsdown Group Practice, Portsmouth | 3 | 30 |
| Adam Practice, Poole | 2 | |
| Wareham Health Centre, Wareham | 3 | |
| Bridges Medical Centre, Weymouth | 1 | |
| Ramilies Surgery, Southsea | 1 | |
| Liphook & Liss Surgery, Liphook | 1 | |
| Old Fire Station Surgery, Woolston, Southampton | 2 | |
| Forest End Surgery, Waterlooville | 5 | |
| Chawton Park Surgery, Alton | 1 | |
| Cowplain Practice, Cowplain | 2 | |
| Bermuda & Marlowe Practice, Basingstoke | 1 | |
| Swanage Medical Centre, Swanage | 1 | |
| Wellbridge Practice, Wool, Dorset | 1 | |
| Three Swans Surgery, Salisbury | 3 | |
| Swan Surgery, Petersfield | 1 | |
| Grove House, Isle of Wight | 1 | |
| Friarsgate Practice, Winchester | 1 | |
| Total hubs | 280 | |
| Other hospital sites | ||
| Coventry and Warwickshire University Hospital | 12 | |
| Queen Elizabeth Hospital, Birmingham | 5 | |
| Cheltenham General Hospital | 11 | |
| Ninewells Hospital, Dundee | 9 | |
| Southport and Formby District General Hospital | 7 | |
| Ipswich Hospital | 19 | |
| James Cook University Hospital, Middlesbrough | 11 | |
| Royal Bolton Hospital | 5 | |
| St James’ University Hospital, Leeds | 1 | |
| Kent and Canterbury Hospital | 3 | |
| New Cross Hospital, Wolverhampton | 5 | |
| Royal National Orthopaedic Hospital, Stanmore | 10 | |
| Raigmore Hospital, Inverness | 16 | |
| North Devon District Hospital | 6 | |
| Charing Cross Hospital, London | 1 | |
| Bedford Hospital | 1 | |
| Guy’s Hospital, London | 2 | |
| Total additional sites | 124 | |
Participants
Adults carrying out CISC were identified at the time of clinic reviews and from health-care records at each site. In addition, the trial was promoted to patients through the Multiple Sclerosis (MS) Society, Bladder Health UK, local patient groups and catheter delivery companies. Clinicians were encouraged, through the NIHR CRN, local and national primary and secondary care meetings and relevant professional organisations (including British Association of Urological Surgeons, British Society of Urogynaecology and the Association for Continence Advice), to introduce the trial to patients.
Patients were approached and introduced to the trial by clinical staff at the sites. If they were interested, an eligibility check was carried out in accordance with the following inclusion and exclusion criteria.
Inclusion criteria
-
Adult aged ≥ 18 years.
-
Established user of CISC who was predicted to continue using for ≥ 12 months.
-
Able to give informed consent for participation in the trial.
-
Able and willing to adhere to a 12-month follow-up period.
-
Had suffered either at least two episodes of symptomatic UTI related to CISC within the previous 12 months or at least one episode of UTI requiring hospitalisation. Or, for those currently prescribed prophylactic antibiotic for UTI, has completed a 3-month washout period without antibiotic prophylaxis. Any symptomatic UTI was treated prior to randomisation.
-
Able to take a once-daily oral dose of at least one of 50 mg of nitrofurantoin, 100 mg of trimethoprim or 250 mg of cefalexin.
Exclusion criteria
-
Aged < 18 years.
-
In a learning phase of CISC.
-
Already taking prophylactic antibiotic against UTI and declining or unable to tolerate a 3-month washout period without antibiotic prophylaxis.
-
Inability to tolerate all three of the prophylactic antimicrobial agents.
-
Women who were pregnant, breastfeeding or who intended to become pregnant during the planned period of trial participation.
-
Previous participation in this study.
-
Inability to give informed consent or complete study documents in English (the outcome measures had not been validated in other languages).
Potentially eligible patients were given or sent brief trial information. Those interested and eligible were approached by research staff and provided with full trial information. We welcomed participation of otherwise eligible patients who were already taking antibiotic prophylaxis against recurrent UTI, provided that they agreed to have a 3-month washout period without taking prophylaxis.
Consent procedures
The trial was carried out according to the principles of Good Clinical Practice33 (GCP) and the Declaration of Helsinki. 34 All participants provided written informed consent prior to randomisation and to any trial-specific procedures by signing and dating the trial consent form, which was witnessed and dated by a member of the local research team. If participants were experiencing symptomatic UTI at the time of consent, they were treated with standard antibiotic therapy and not randomised until they were symptom free. Those currently using antibiotic prophylaxis who agreed to stop for a 3-month washout period were consented to take part, but baseline data were collected, and randomisation carried out, only when the 3-month washout period was completed.
Randomisation
Participant allocation
Randomisation was administered centrally by the NCTU secure web-based system. Permuted random blocks of variable length were used to allocate participants 1 : 1 to the control and experimental groups. A statistician not otherwise involved with the trial produced the final randomisation schedule. Stratification by three variables [(1) prior frequency of UTI (fewer than four episodes per year and four or more episodes per year), (2) diagnosis of neurological lower urinary tract dysfunction (yes or no) and (3) sex (female or male)] was performed to ensure a balanced allocation within these known UTI risk factors. For those allocated to prophylaxis, an appointment was arranged with the prescribing clinician to commence an individually suitable trial drug: 50 mg of nitrofurantoin (or 100 mg according to clinician preference and local guidance), 100 mg of trimethoprim or 250 mg of cefalexin.
Progress in trial
Once in the trial, participants were encouraged through verbal, written and electronic information to contact their local site team or the central trial team if they had any concerns or questions related to the trial. Trial duration for each participant was 12 months, with an additional optional 18-month follow-up outside the trial under separate consent to provide longer-term data on treatment choice and pathogen resistance patterns. Local research staff were instructed to interview each participant, either face to face or by telephone, at 1, 3, 6, 9 and 12 months following randomisation, focusing on ensuring that there was adherence to the allocated group and that any occurrence of symptomatic antibiotic-treated UTI was documented.
Participant expenses
Expenses incurred by participants as a result of taking part, including any NHS prescription charges for trial medication, were reimbursed. Participants were also given a gift of £20 when they started the trial.
Withdrawal of participants
Participants remained in the trial unless they withdrew consent or the local principal investigator (PI), chief investigator or trial office felt that, because of serious issues, it was no longer appropriate for the participant to continue. If a participant chose to withdraw, permission was sought for the research team to continue to collect outcome data from their health-care records. Participants who withdrew later than 6 months after randomisation were included in the primary analysis. If participants withdrew completely (i.e. from intervention and follow-up), data already collected were retained up to the point of withdrawal. The reason for withdrawal was recorded if the participant agreed.
Patient and public Involvement
During protocol development, we sought the advice of a CISC user (co-investigator) on trial design, focusing on important outcomes and their definition, recruitment policies and phrasing of patient-facing documents such as the participant information sheet, consent form and further information on other measures to prevent UTI. We also consulted with patient organisations including Bladder Health UK and the MS Society. During the trial, two people who are CISC users and who responded to an advert on the MS Society website were recruited and appointed to the Trial Steering Committee (TSC). They provided continuing advice on outcome measurement and recruitment strategies, including adjustment of patient literature. In addition, they reviewed the final report and advised on dissemination of findings to a lay audience.
Outcome measurement
Primary clinical outcome
The primary clinical objective was to determine the relative clinical effectiveness of an experimental UTI prevention strategy of continuous once-daily prophylactic antibiotic therapy against the control strategy of no prophylaxis in people carrying out CISC who suffer recurrent UTI. This was assessed by comparing the incidence of patient-reported UTI over 12 months.
Participants were asked to report all episodes of symptomatic UTI for which they took a treatment course of antibiotic. This was done on a UTI record form that was returned to the central trials office [see Questionnaire (10 Jan 2018), Participant UTI Record available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. The participant recorded symptoms from a predefined list, encompassing the recommendations of the British Infection Association (BIA), the Centers for Disease Control and Prevention (CDC) and spinal cord injury UTI consensus statement. 11,35,36 The type and duration of treatment antibiotics was also recorded. In addition, at each 3-monthly review by local research staff, participants were asked about occurrence of symptomatic UTI and associated use of treatment courses of antibiotic. Details were recorded on the case report form (CRF) [see Interview Material (10 Jan 2018) available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)] and 3-monthly participant-completed questionnaire [see Questionnaire (10 Jan 2018), Participant 3 Monthly Questionnaire available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)].
Occurrence of a UTI was defined as the presence of at least one symptom together with taking a discrete treatment course of antibiotics prescribed by a clinician or as part of a patient-initiated self-start therapy.
To ensure consistent attribution, we set a hierarchy of evidence on which to base the primary outcome [see Study Documentation (10 Jan 2018), AnTIC Primary Outcome Assessment Procedure available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. First, we reviewed all UTI record forms returned by the participants. For each, we checked the information on symptoms, the identity of the antibiotic used and the duration of use. If a UTI was reported by the participant but details were missing, we then checked the 3-monthly review CRF and participant questionnaire corresponding to the time period in which the UTI occurred. Finally, if necessary, the research team at the trial site and/or the participant were contacted to confirm missing details. Only episodes in which trial documentation from UTI record forms, regular review CRFs or participant 3-monthly postal questionnaires showed symptomatic antibiotic-treated UTI were counted as fulfilling the primary outcome.
Secondary outcomes
The following secondary patient-reported and clinical outcomes were measured.
Severity of urinary tract infection
During review of each UTI record form and associated CRF, severity was classified as non-febrile or febrile, and whether or not hospitalisation was required. Febrile UTI was defined as the primary outcome plus presence of a recorded fever of > 38 °C. Hospitalisation due to UTI was defined as an unplanned visit to hospital for treatment of a UTI, which required at least one overnight hospital stay. We also asked participants to self-rate the severity of each UTI as mild, moderate or severe.
Adverse events related to both prophylaxis and treatment antibiotic use
Any AEs possibly related to the trial intervention suffered during the trial participation were recorded as free text by local research staff at 1-, 3-, 6-, 9- and 12-month trial visits on CRFs. Those occurring during treatment courses of antibiotic for UTI (adverse reactions) were recorded on the participant UTI record form [see Questionnaire (10 Jan 2018), Participant UTI Record available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. During episodes of antibiotic-treated UTI and by review of health-care records by local research staff [see Interview Material (10 Jan 2018), Participant 3-Month review interview and Health care records 3-month review available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)], AEs were categorised as mild (discomfort is noticed, but there is no disruption of normal daily activities), moderate (discomfort is sufficient to reduce or affect normal daily activities) or severe (discomfort is incapacitating, with inability to work or to perform normal daily activities). The investigator at the relevant site assessed the relationship of the AE to the trial treatment by checking against the Reference Safety Information (RSI) in the Summary of Product Characteristics (SmPCs) specific to the prescribed antibiotic, which was included in the protocol.
Overall satisfaction with prophylactic antibiotic treatment
This was measured by the participant completion of the Treatment Satisfaction Questionnaire for Medication37 (TSQM) at the end of the trial. Separate scores from the four subscales were reported: (1) effectiveness, (2) side effects, (3) convenience and (4) global satisfaction.
The relative effect on health status among trial participants
This was measured by participant completion of the Short Form questionnaire-36 items version 2 (SF-36v2; 1-week acute recall version) at baseline, after 6 and 12 months of participation and at the time of each UTI. The SF-36v2 includes 36 different questions, each of which contributes to the score that can be calculated for eight different dimensions: (1) physical functioning, (2) role limitations due to physical health, (3) bodily pain, (4) general health perceptions, (5) vitality, (6) social functioning, (7) role limitations due to emotional problems and (8) general mental health caused by either physical or emotional problems. From the scores attached to each of these dimensions, two additional summary scores were derived. These are the physical component summary (PCS) score and the mental component summary (MCS) score. The higher the value of the summary scores, the higher the level of functionality of the patient. 38
Change in kidney and liver function at 12 months
Change in kidney function was determined by comparison of the estimated glomerular filtration rate (eGFR) derived from measurement of serum creatinine and accounting for race and sex, at baseline and 12 months for each individual and each group using an online calculator [available at www.kidney.org/professionals/kdoqi/gfr_calculator (accessed 27 October 2017)]. At baseline, research staff were asked to calculate creatinine clearance using an online calculator [available at www.nuh.nhs.uk/staff-area/antibiotics/creatinine-clearance-calculator (accessed 27 October 2017)] to detect participants allocated to prophylaxis with clearance values of < 45 ml/minute who would not be able to use nitrofurantoin. Change in liver function was assessed using the measured value of the liver enzyme alanine transaminase (ALT) at baseline and 12 months, compared within individuals and according to trial group and the agent used for prophylaxis.
Microbiological outcomes
For trial purposes, the standard definition of microbiologically confirmed UTI in a symptomatic participant was the laboratory report of one or two isolates at > 104 colony-forming unit (CFU)/ml. 39 The results of culture of urine specimens sent to the central trial laboratory were preferentially used for this outcome. If a central laboratory specimen was missing and a local laboratory report was available, then this was used. For evaluation of antimicrobial resistance and assessment of asymptomatic bacteriuria, only culture and sensitivity results from specimens received by the central laboratory were used. Asymptomatic bacteriuria was defined as a positive urine culture in the absence of symptoms. Bacterial ecological change was assessed by comparing changes in resistance patterns of all pathogens isolated from urine specimens received at the central laboratory both at the time of UTI and during asymptomatic periods at baseline and at the time of 3-, 6-, 9- and 12-month reviews. Perianal swabs taken and submitted at baseline and at the 6- and 12-month visits were cultured for E. coli only.
Data collection
Summary
Outcome data from the CRFs were entered by local research staff at each site into a trial-specific database set-up using the MACRO clinical data management system (Elsevier B.V., Amsterdam, the Netherlands). Participant-completed questionnaires were collated at the central trial office and data entry outsourced to a commercial company (Ndata, North Shields, UK) for conversion to an electronic format. We made concerted efforts to obtain any missing data through contact with the sites and by direct checking with participants using their preferred means of communication (telephone, text message, e-mail or during the clinic appointment).
Trial events
Screening
General demographics and eligibility were checked. Trial Information was provided [see Patient Information Sheet (10 Jan 2018) available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)] to the participant and consent taken prior to randomisation. Participants experiencing symptomatic UTI were treated with standard antibiotic therapy and not randomised until symptom free.
Randomisation
Randomisation was performed as close as possible to the date of consent (normally immediately after). The continued eligibility of those consented participants who had completed a 3-month washout period was checked prior to their randomisation.
Follow-up
Participants were contacted by local trial staff 1 month after randomisation by telephone regarding general concerns, understanding of trial documentation and their tolerance of the prophylactic antibiotic agent (if allocated).
At 3, 6, 9 and 12 months after randomisation, telephone or face-to-face contact (according to local circumstance and participant preference) took place. Details of UTI occurrence, UTI symptoms, adverse reactions to antibiotics taken for UTI and other infections requiring treatment with antibiotics during the previous 3 months were recorded in the electronic CRF (e-CRF). Participants were asked to return a urine specimen during an asymptomatic period at 3, 6, 9 and 12 months, and a perianal swab at 6 and 12 months, to the central laboratory in postage pre-paid, safety-compliant sample packaging provided to them.
During episodes of symptomatic antibiotic-treated UTI, participants completed a UTI record form [see Questionnaire (10 Jan 2018) available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)] recording symptoms, antibiotic use, adverse reactions to treatment for UTI, participant rating of severity of UTI and details if hospitalised. They were also asked to post a urine specimen to the central laboratory prior to commencing a treatment course of antibiotics, for analysis and subsequent storage. This was in addition to urine specimens requested by the local treating clinician required for diagnosis and management of the episode locally in line with routine local diagnostic practice.
Other outcome data were collected by participant postal questionnaire sent by the central trial office at 3, 6, 9 and 12 months and completed by participant. This was then returned to the central trial office. Follow-up data were supplemented by regular inspection of health records by local trial site staff for documented visits to clinicians because of UTI, episodes of fever recorded as > 38 °C associated with UTI, antibiotic prescriptions for UTI, hospitalisations and results of local laboratory urine cultures. The schedule of events for the trial are summarised in Table 2.
| Intervention | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 (initial screen) | Visit 2 | Visit 3 (3 months) | Visit 4 (6 months) | Visit 5 (9 months) | Visit 6 (12 months) | At time of UTI | |||
| Consent | Baseline | Randomisation | |||||||
| Eligibility checklist | ✗ | ||||||||
| Trial discussed and patient information sheet given | ✗ | ||||||||
| Informed consent | ✗ | ||||||||
| Trial outcome UTI report form and questionnaire | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| AEs | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| SF-36v2 | ✗ | ✗ | ✗ | ✗ | |||||
| Health resource use questionnaire | ✗ | ✗ | |||||||
| Patient costs (time and travel) questionnaire | ✗ | ||||||||
| TSQM | ✗ | ||||||||
| Contingent valuation questionnaire (sent at 13 months) | ✗ | ||||||||
| Urine specimen to central laboratory | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Perianal swab to central laboratory | ✗ | ✗ | ✗ | ||||||
| Blood test for creatinine eGFR and liver enzyme (ALT) | ✗ | ✗ | |||||||
Data handling and record keeping
Data were recorded by site staff on e-CRFs in a clinical data management software package. Patient questionnaires were returned by post to the trial management office in Newcastle upon Tyne. They were checked by NCTU staff and sent to a commercial data entry organisation (Ndata), which then returned the electronic data files and paper questionnaires to the trial office for transfer to the trial database and archiving. Two reminders with a second and third copy of questionnaires were sent to participants to prompt return. Patients were allocated an individual specific trial number to allow anonymised versions of the secure database to be available to the trial team and subsequently more widely under open data access arrangements. Essential data will be retained for a period of at least 10 years following close of the trial in line with sponsor policy and the latest European Directive on GCP (2005/28/EC). 40 Data were handled, digitalised and stored in accordance with the Data Protection Act 1998. 41
Details of trial medication
Planned interventions
Both experimental and control strategies are in routine NHS use and these strategies were specified clearly in the trial information literature.
Antibiotic prophylaxis (experimental)
The agent used was selected from the alternatives of nitrofurantoin, trimethoprim and cefalexin by the responsible clinician depending on patient characteristics such as previous use, allergy, kidney function, prior urine cultures and local guidance. There is no universally agreed national guidance on appropriate agents but available evidence suggests use of 50 mg of nitrofurantoin (or 100 mg dependent on clinician preference and local guidance), 100 mg of trimethoprim or 250 mg of cefalexin, in that order of preference. 21,42,43 Kidney function was determined by creatinine clearance at baseline; if this was < 45 ml/minute, nitrofurantoin was not used. Otherwise, participants and their clinicians were asked to review the prescribing information for each drug given in the trial documentation to guide selection of the individually most appropriate initial agent. At the planned 1-month telephone review, local trial staff asked about tolerability of the prescribed medication. If there were specific and intolerable AEs, then switching to an alternative agent was advised in consultation with the relevant clinician, with the reasons for the change recorded. This process was repeated at planned 3-monthly reviews and a third agent advised, if necessary. More frequent telephone follow-up was undertaken if needed to help the participants become established on a suitable agent. The aim was to maintain participants allocated to the prophylaxis group on any one of the three prophylactic agents for as long as possible over the 12-month trial period within tolerance and safety constraints. Participants were asked to take the once-daily antibiotic prophylaxis as a single dose at bedtime. If a participant in the prophylaxis group developed symptoms and signs suggestive of breakthrough UTI, they were advised to seek treatment in their usual way, predominantly by contacting their general practitioner (GP) and starting a discrete treatment course of antibiotics. In this scenario, they were instructed to stop the prophylactic antibiotic while they were taking a treatment course and restart it again the day after the last dose of the treatment course. Clinicians and participants were advised to use a different agent for treatment to the one they were taking for prophylaxis.
No prophylaxis
The strategy applied to the control group was that of no prophylaxis. Participants self-monitored their symptoms as usual and reported to their GP or other clinician if they developed symptoms and signs suggestive of a UTI requiring treatment.
Standard care for both groups
Apart from the randomised allocation to prophylaxis and the avoidance of the prophylactic agent as treatment for symptomatic UTI, there were no differences in care between the groups. We ensured as far as possible that participants in both groups received their usual care in terms of identification and treatment of UTI, health surveillance and support related to use of CISC, and monitoring and treatment of the underlying cause of their lower urinary tract dysfunction. We recorded all health-care episodes for each participant. We considered standard care for CISC users who suffer recurrent UTI to be the use of discrete treatment courses of antibiotics as indicated by symptoms or signs of UTI. Treatment typically involved a 3- or 7-day course of an antibiotic active against urinary pathogens, depending on severity of symptoms and response to treatment. In accordance with local protocols, a urine specimen was sent for microbiological examination at the local laboratory at the time of starting antibiotic treatment. If therapy was successful, no further action would be required, whereas if symptoms did not resolve, the choice and duration of antibiotic would be reconsidered in the light of any urine culture result and, if necessary, a further urine sample submitted for analysis. 35 This suggested standard of care was emphasised in trial documentation given to participants and clinicians. Regular surveillance of kidney function using serum creatinine was also expected. Guidance was provided to participants in both groups and to their clinicians regarding use of urine testing and antibiotic options in terms of agents used and their duration of use. Participants in both groups continued their regular care with primary and secondary care clinic visits, access to continence advice and relevant patient support groups according to local practice and individual preference. All participants were given information detailing simple non-antibiotic measures that may help prevent UTI and provide symptom relief [see Study Documentation (10 Jan 2018) available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)].
Delivery of interventions
Local NHS clinicians at the site of randomisation were responsible for initiating trial medication for those participants allocated to prophylaxis, whether in secondary or primary care, with a 3-month supply of the relevant medication. The participants’ GPs were then asked to prescribe further supplies until the end of the 12-month trial treatment period. If this was not possible then the clinician at the NHS site of randomisation continued to supply the medication. If the participant wished, and if the clinician responsible for their routine care agreed, antibiotic prophylaxis was continued beyond the 12-month trial participation period but without further active monitoring from the trial research team.
Funding of trial intervention
The interventions were funded by standard NHS contracting mechanisms, having been sanctioned by local commissioning groups through local study approval mechanisms. The NHS excess treatment costs were approved by the sponsor and, for primary care, the local Clinical Commissioning Group. Any prescription charges for trial drugs incurred by participants were reimbursed from research costs. The trial intervention was low cost.
Sample size calculation
We planned to recruit 372 participants to the trial. Based on systematic reviews and expert opinion, we considered that an overall 20% reduction in symptomatic UTI rate from an average of 3 to 2.4 episodes per year represents the minimum clinically important difference. 18,22 Using the Poisson rate test, completion of the trial by 158 participants in each group, 316 in total, would give 90% power to detect this difference at the 5% level. A total of 372 would allow for a 15% attrition rate that was estimated from previous trials included in the systematic review. This gives a 92% power to detect a 25% difference in the high-frequency subgroup (from four to three episodes per year) and > 99% power for a 50% reduction in the low-frequency group (from two episodes to one episode per year) without allowance for multiple testing. At the start of the trial, we aimed to approach at least 750 eligible patients, anticipating a 50% recruitment rate.
Statistical analysis
A complete statistical analysis plan (SAP), which provides full details of all statistical analyses, variables and outcomes, was finalised and signed before the final database lock and analysis [see Statistical Analysis Plan (10 Jan 2018) available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)].
All statistical analyses were carried out on a modified intention-to-treat (ITT) basis, retaining participants in their randomised treatment groups and including those in the prophylaxis group who stopped prophylaxis but remained in the trial, those in the no-prophylaxis group who started prophylaxis but remained in the trial and those who withdrew before the end of the trial but who had ≥ 6 months of follow-up recorded.
Primary outcome measure
The primary outcome measure was the difference in incidence of symptomatic antibiotic-treated UTI during the 12-month observation period. Symptomatic UTI was defined as the presence of at least one patient-reported or clinician-recorded symptom from a predefined list encompassing the recommendations of the BIA, the CDC and spinal cord injury UTI consensus statement44 together with taking a discrete treatment course of antibiotics prescribed by a clinician or as part of a patient-initiated self-start policy. 11,35,36
The rate of UTI in each group was defined to be the incidence rate ratio (IRR), that is, the total number of UTIs suffered across all patients, allowing for differing durations of follow-up. Analyses of the primary outcome measure were performed using both the Poisson rate test (as a simple univariate approach with consideration of days in follow-up) and an IRR modelling approach to allow for the different durations of treatment for symptomatic UTIs, which reduce the number of days-at-risk for individuals. Days-at-risk was defined as the total observation period minus days spent taking treatment courses of antibiotics active against urinary tract organisms. When no information on the duration of antibiotic treatment course was available, it was assumed to last 7 days. A Poisson regression approach was used to adjust for the effects of covariates. The model selection process included the stratification factors and other baseline variables. Site was also explored as an interaction term. An analysis of the primary outcome measure was performed both for the full data set and for the separate subgroups defined by high and low baseline UTI rate (as specified during stratification for the randomisation process). The simple univariate analysis was considered to be the primary analysis for reporting purposes.
Secondary outcome measures
For the following secondary outcome measures included in the SAP, rates were defined in a similar way to the primary outcome:
-
microbiologically confirmed symptomatic UTI rate
-
febrile UTI rate
-
hospitalisation rate attributable to UTIs during the 12 months of the trial
-
asymptomatic bacteriuria rate
-
antibiotic prescription for asymptomatic UTI rate
-
AE rate (those related to prophylaxis and treatment antibiotics), including antibiotic resistance.
Statistical analyses of these outcome measures generally used the same approach as for the primary outcome.
The detection rate for resistance of all pathogens isolated from urine and strains of E. coli from perianal swabs to tested antibiotics at any point during 3-month time periods (0–3, 3–6, 6–9 and 9–12 months) was summarised by trial group. When numbers were sufficient, a chi-squared test for the 9- to 12-month samples from the prophylaxis group versus the no-prophylaxis group and tests for trend including baseline for both groups were used for analysis of the resistance patterns over the 12-month trial period. For perianal swab specimens, 6-month time periods and chi-squared tests for the 6–12 months period were used.
For TSQM scores and change in kidney and liver function at 12 months, the two-sample t-test was used as a simple univariate analysis. Furthermore, an analysis of covariance (ANCOVA) approach was employed using the covariates identified during the primary outcome modelling (for the TSQM scores, each subscale was considered separately).
For base-case analysis of the MCS and PCS of the SF-36v2 completed at baseline and 6 and 12 months, a simple Student’s t-test was used. For the adjusted analyses, linear regression was used with the component scores as the dependent variable and with treatment allocation, score at baseline and baseline characteristics as covariates. This analysis allowed assessment of the impact of the interventions after controlling for imbalances at baseline. A two-sample t-test was used as a simple univariate analysis for differences between groups in MCS and PCS. A linear regression was also performed adjusting for the baseline characteristics: age, sex, neurological bladder dysfunction or not, number of UTI episodes in the 12 months before randomisation (fewer than four vs. four or more), renal function (creatinine clearance) and MCS or PCS scores at baseline. Data from completion of the SF-36v2 at the time of UTI were excluded from this analysis but were used in the cost–utility component of the health economic evaluation.
Trial progress and monitoring
The recruitment plan set out to build progressively to a target of 372 participants over 24 months [see Study Documentation (10 Jan 2018) Gantt Chart available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. This included a 12-month feasibility study at which recruitment and patient adherence to the intervention were evaluated.
Feasibility of recruitment was analysed after 9 months of active recruitment (trial month 12) and reported in August 2014 to the TSC and the funder, with an additional safety report reviewed by the Data Monitoring Committee (DMC). Recruitment continued to be monitored by the Trial Management Group (TMG) through returns to the randomisation website. Both the funder and TSC approved continuation of the trial and a subsequent 5-month extension to the planned recruitment period including moderate over-recruitment to ensure completion to target sample size.
Sources of bias
To allow randomisation, both the eligible patient and the responsible clinician needed to be sufficiently uncertain about whether the experimental or control strategy was best for management of recurrent UTI. Given the lack of high-level evidence as to which was the more effective, trial information was provided illustrating the uncertainty and the need for a definitive trial. This aimed to ensure that any selection bias in terms of characteristics of CISC users willing to be randomised compared with those who were eligible but not willing to participate was minimised. As far as possible, we recorded reasons for declining randomisation but patients were free to decline participation and randomisation without giving a reason.
Trial literature was given to all participants and to their clinicians, detailing other measures that may reduce the risk of UTI, such as adequate fluid intake, increased frequency of catheterisation, cranberry products and, if appropriate for post-menopausal women, vaginal oestrogen supplements. To reduce the risk of participants allocated to the control of no prophylaxis being more likely to seek treatment for symptoms suggestive of UTI, and knowing that clinicians may be more likely to prescribe treatment antibiotics to this group, we gave information on use of antibiotic treatment describing indication and choice of agent in trial literature to all participants and their GPs in accordance with established guidance from the BIA and other groups. We also included advice on when to seek help regarding symptoms suggestive of UTI and use of simple measures to avoid or avert symptomatic UTI in the participant information packs.
To ensure uniformity of laboratory processing and culture techniques, we asked participants to post a specimen of urine taken at the onset of symptomatic UTI to the central laboratory at Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, for culture. The use of urine culture at a local microbiology laboratory was at the discretion of the treating clinician. Details of strategies to minimise ascertainment and attribution bias (also known as detection bias) for the primary outcome are given above in Outcome measurement.
Microbiological methods
Participants who were developing symptoms of a UTI and intended to commence a treatment course of antibiotic were instructed to send a sample of urine to the central laboratory at Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, prior to commencing antibiotic treatment. The sample was dispatched in a standard urine specimen container pre-loaded with boric acid at a concentration of 18 g/l (International Scientific Supplies Ltd, Bradford, UK), within secure packaging (Safebox™, Royal Mail Ltd, London, UK) and accompanied by a sample shipment checklist, completed by the participant, identifying the time point and type of sample. Participants were also encouraged to submit diagnostic urine samples as usual in accordance with local protocols through their GP practice or hospital clinic. These were analysed by the participant’s local microbiology laboratory, where they were processed in accordance with the laboratory’s standard operating procedures (SOPs) for the examination of urine specimens. Participants were also instructed to submit urine samples and perianal swabs to the central laboratory during asymptomatic periods as part of their baseline and 3-, 6-, 9- and 12-month assessments scheduled by the trial protocol (perianal swabs at baseline and 6 and 12 months only). Local trial staff assisted participants with this task.
Microscopy and semi-quantitative culture was carried out on all urine samples sent to the central reference laboratory. Automated microscopy was performed using the iQ200 Sprint cytometer (Beckman Coulter, High Wycombe, UK) and specimens were inoculated onto ChromidID® CPS® Elite media using a 1-µl loop and incubated for 18–24 hours in room air at 37 °C. Growth was then enumerated and the presence of up to two isolates at ≥ 1 × 104 CFU/ml was reported, in line with the UK Standards for Microbiology Investigations. 45 Bacterial counts of ≤ 103 CFU/ml and mixed cultures of three isolates or more were regarded as not significant. Presumptive identification was confirmed by matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF) (Bruker microflex, Coventry, UK). Disc diffusion susceptibility testing against a panel of 16 antimicrobial agents was carried out using Mueller–Hinton agar (Oxoid Limited, Basingstoke, UK) in accordance with the methods outlined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST). 46 For E. coli isolates, susceptibility testing was carried out in triplicate. For trial purposes, resistance patterns to the following agents were included in reported analysis: amoxicillin, cefalexin, ciprofloxacin, co-trimoxazole, co-amoxiclav, mecillinam, nitrofurantoin and trimethoprim.
Perianal swabs were inoculated onto ChromID CPS Elite media (bioMérieux, Basingstoke, UK) and examined for the presence of E. coli after overnight incubation in room air at 37 °C. As above, antimicrobial susceptibility testing was carried out in triplicate for E. coli strains using EUCAST’s disc diffusion methodology. 46
The central laboratory was accredited (ISO15189) and analyses were carried out in accordance with current standards set by the UK Health Protection Agency (now Public Health England).
Definition of end of trial
The end of the trial was defined as the last recruited participant’s final study contact at 12 months after their randomisation, which happened on 23 February 2017. Participants were consented separately for the sending of a urine specimen and perianal swab at 6 months after the end of the trial (18 months post randomisation) together with completion of an antibiotic usage questionnaire. As a proportion of these 18-month reassessments took place outside the funded trial period, the results will be communicated separately to this report as an update to the outcome of change in resistance pattern together with a descriptive result of patient choice concerning prophylaxis against UTI.
Compliance and withdrawal
Assessment of adherence
Outcome data were collected remotely, whenever feasible, by participant completion of postal questionnaires. Local research staff made use of planned routine clinical visits to check completion of trial documentation and ensure submission of urine specimen and perianal swab. Adherence to the allocated group (prophylaxis or no prophylaxis) was checked by 3-monthly contact with the participant. If crossover between the groups was reported, local trial staff explored and recorded the reasons for this with the participant and the relevant clinician by telephone or face-to-face contact. Whenever possible, participants remained in the study and continued collection of planned outcome information. The trial literature emphasised the need to adhere to the allocated strategy during the 12-month trial period if possible. Multiple switching between prophylactic agents was allowed. Previous studies47 suggested that this would affect approximately 12% of participants. The trial statistician monitored attrition rate against our anticipated maximum of 15% and reported to the TMG, TSC and DMC as appropriate.
Data monitoring, quality control and assurance
Quality control was maintained through adherence to SOPs governing the work of the sponsor, NCTU and local research teams; adherence to the study protocol and the principles of GCP; research governance; and clinical trial regulations. The MHRA agreed Type A status for the trial. An independent DMC was set up that included one methodologist, one physician not connected to the trial and one statistician (chairperson). The purpose of this committee was to monitor efficacy and safety end points; it operated in accordance with written terms of reference linked to the DMCs: Lessons, Ethics, Statistics charter. 48 Prior to completion of the trial, only the DMC and the statistician preparing reports to the DMC had access to the data separated by allocated group. The DMC met at the start of, completion of, and four times during, the study.
A TSC was established to provide overall supervision of the trial. The TSC consisted of an independent chairperson, two further independent clinicians, an independent statistician, two independent lay representatives and the chief investigator. Other members of the TMG attended as required or requested by the chairperson. The committee met approximately every 6 months during recruitment and annually thereafter for the duration of the trial.
Monitoring of study conduct and data collection was performed by a combination of central review and site monitoring visits to ensure that the study was conducted in accordance with GCP. Study site monitoring was undertaken by members of the TMG. The main areas of focus were consent, SAEs and completeness of the site file.
Trial flow chart
The trial flow for participants anticipated at the start of the trial is illustrated in Figure 1.
FIGURE 1.
Diagram showing the planned flow of participants through the trial.

Ethics and governance
The Newcastle upon Tyne Hospitals NHS Foundation Trust was the sponsor for the trial (reference 6672). Favourable ethics opinion for the trial was obtained on 1 August 2013 from the NHS Research Ethics Service Committee North East – Sunderland [Research Ethics Committee (REC) reference 13/NE/0196] and subsequent research and development and Caldicott approvals were granted by each participating site. A Type A notification submission was made to MHRA and notice of authorisation for the trial was granted with effect from 10 September 2013. Approval was sought and obtained for all substantive protocol amendments.
Trial registration and protocol availability
The trial was registered as ISRCTN67145101 on 25 October 2013. The latest version of the full protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/ (accessed 27 October 2017) and a published version is also available. 1
Table 3 summarises key changes to the original AnTIC trial protocol as approved by the REC and MHRA, when required.
| Description | Version | Date |
|---|---|---|
| Widening of inclusion criteria to include those who had had one serious UTI – in line with options that would be offered in standard care | 1.1 | 5 November 2013 |
| More frequent participant contact – to help participants as much as possible to complete UTI log and avoid ascertainment bias | ||
| Payment of any prescription charge to avoid participants being out of pocket | ||
| £20 gift to participants on study entry | ||
| Use of calculated creatinine clearance rather than eGFR – in line with recent NHS guidance for use of nitrofurantoin as UTI prophylaxis | ||
| The protocol was updated to make it clear that washout participants should be consented at the beginning of the washout period, but not randomised until the washout period was complete | 1.2 | 13 May 2014 |
| The protocol was also amended to clarify that an active, symptomatic UTI did not exclude a participant from the trial and that consent to participate could still be taken, but that the UTI should be treated before a participant could be randomised | ||
| Update to the contraindications section of the SmPC for nitrofurantoin concerning patients with kidney dysfunction with an eGFR of < 45 ml/minute. The trial protocol and all trial documentation were amended to reflect this update | 1.3 | 14 November 2014 |
| Change to the protocol to allow sites to send a second invitation letter to participants who had not responded to the initial invitation to the trial | 1.4 | 30 July 2015 |
| Update to clarify the wording around the approved RSI for the trial. The RSI contained in section 4.8 of the SmPC for the three antibiotics used in the trial was submitted to MHRA for approval. The updated SmPC for nitrofurantoin, trimethoprim and cefalexin were included in the appendices of the new protocol | 1.5 | 17 August 2016 |
Serious adverse event reporting
Guidance on AE and SAE reporting, as well as determining the degree of relatedness and assessment of causality for SAEs to study participation, was provided in the protocol. The RSI for assessment of expectedness of related events was contained in the SmPC for each of the three antibiotics and appended to the protocol. SAEs excluded UTI as this was the primary outcome collected and documented throughout the trial. All SAEs were reported for the duration of the trial and for 4 weeks after the trial intervention was stopped.
Chapter 3 Results
Recruitment
The trial was set in the UK NHS (England and Scotland). The first participant was randomised on 26 November 2013 and the last on 29 January 2016. The planned recruitment window was extended by 5 months to 26 months to accommodate the opening of more sites and to achieve the target sample size (Figure 2).
FIGURE 2.
Recruitment by month.

Participants were identified when attending NHS secondary care clinics, by search of primary and secondary care NHS health records and from commercial organisations contracted to provide NHS care (see Table 1). The recruitment strategy involved first establishing seven hubs, each with a funded recruitment co-ordinator, with two (Cambridge and Glasgow) recruiting from secondary care only, four recruiting from both secondary care and primary care/community services (Aberdeen, Bristol, Wakefield and Newcastle upon Tyne) and one from primary care only (Southampton). As the trial progressed, we opened a further 17 secondary care sites and six PICs that referred interested patients to their local hub. Overall, 340 (84%) participants were recruited from secondary care, 50 (13%) from primary care and 14 (3%) from NHS community service providers. We consented 28 patients already on prophylaxis who agreed to start a 3-month washout period, 22 of whom completed washout and were randomised.
Participant flow
The flow of participants enrolled in the trial is shown in Figure 3. A total of 1743 people were identified by study sites (73% of the estimated target of 2400) and screened for eligibility. Of these, 512 (29%) were deemed ineligible to take part by local research staff (Table 4). Of the 1231 deemed eligible and given information about the study, 232 (19%) declined the offer to participate and 76 (6%) gave other reasons for not wanting to participate. Reasons for non-participation for the remaining 519 (42%) were not recorded (an unrecorded number did not respond to postal letters of invitation). Of the 76 who gave other reasons for not wanting to participate, 20 cited age or ill health, 11 did not want to travel to the study site and 10 did not want to take prophylaxis. Following consent and collection of baseline data, 404 participants (109% of the target) were randomised, with 203 allocated to antibiotic prophylaxis and 201 to no prophylaxis. We excluded 43 participants from the primary analysis (11%; see Table 7): 30 who withdrew before completing 6 months of follow-up, one participant who died prior to the 6-month visit and 12 for whom there were insufficient data despite multiple data capture attempts by local and central trial staff (Table 5). A total of 332 participants completed the 12-month trial of allocated intervention and follow-up, surpassing the pre-set target in our sample size calculation (n = 316), with an additional 29 participants having at least 6 months of follow-up data, which allowed their inclusion in the primary analysis.
FIGURE 3.
Trial Consolidated Standards of Reporting Trials (CONSORT) diagram.
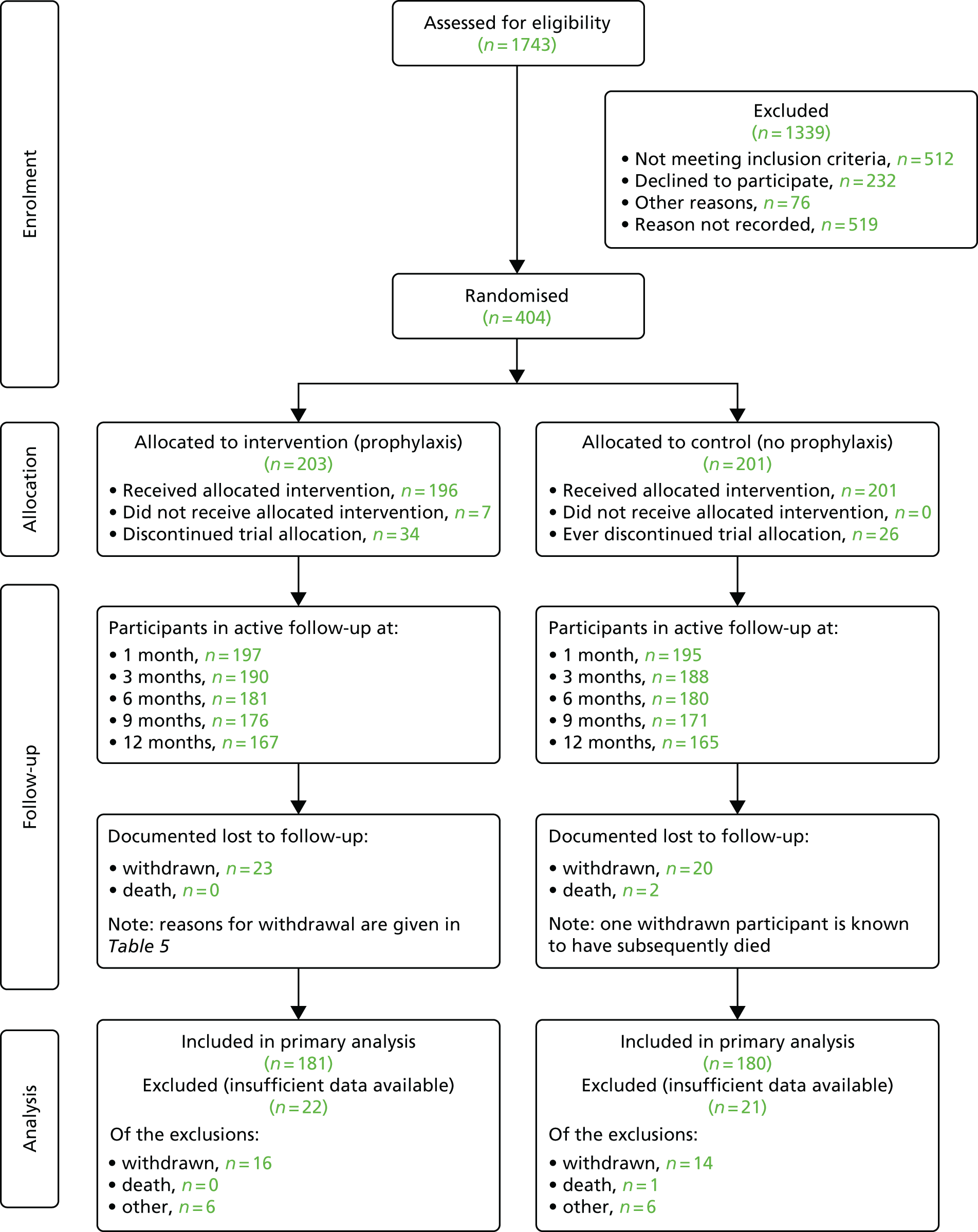
| Reasons for ineligibility | Number of ineligible participants |
|---|---|
| Aged < 18 years | 1 |
| CISC training not completed | 8 |
| Predicted CISC use was < 12 months | 101 |
| Fewer than two episodes of symptomatic UTI or one severe UTI within the previous 12 months | 176 |
| Unable to give consent for randomisation | 10 |
| Already taking prophylactic antibiotic and declining the 3-month washout period | 113 |
| Unable to take any of the three trial drugs (nitrofurantoin, trimethoprim and cephalexin) | 6 |
| Current pregnancy or breastfeeding | 1 |
| Intending to become pregnant in the next 12 months | 3 |
| Inability to adhere to the trial protocol | 4 |
| Competing research study | 2 |
| Unwilling to adhere to the 12-month follow-up period | 10 |
| Does not carryout or no longer carries out CISC | 77 |
| Total not meeting inclusion criteria | 512 |
| Reason for withdrawal | Intervention group, n | Total (N = 43), n | |
|---|---|---|---|
| Prophylaxis (N = 23) | No prophylaxis (N = 20) | ||
| Adverse reaction to prophylaxis | 3 | 0 | 3 |
| Unable to take any of the prophylactic antibiotics | 1 | 1 | 2 |
| Unwilling to continue with the study | 4 | 6 | 10 |
| Unwilling to continue as a result of comorbidities | 4 | 4 | 8 |
| Clinician decision to withdraw as a result of comorbidities | 1 | 1 | 2 |
| Study burden too great | 4 | 2 | 6 |
| Withdrawn as a result of ineligibility | 1 | 2 | 3 |
| Site unable to contact participant | 2 | 0 | 2 |
| Stopped CISC | 1 | 2 | 3 |
| Illness of family member | 2 | 0 | 2 |
| No reason recorded | 0 | 2 | 2 |
Baseline data
Key baseline measures are given in Table 6. There was no imbalance between the groups.
| Variable | Intervention group | Total (N = 404) | |
|---|---|---|---|
| Prophylaxis (N = 203) | No prophylaxis (N = 201) | ||
| Stratification factors | |||
| Sex, n (%) | |||
| Male | 115 (56.7) | 114 (56.7) | 229 (56.7) |
| Female | 88 (43.3) | 87 (43.3) | 175 (43.3) |
| Number of UTI episodes in 12 months prior to randomisation, n (%) | |||
| < 4 | 71 (35.0) | 78 (38.8) | 149 (36.9) |
| ≥ 4 | 132 (65.0) | 123 (61.2) | 255 (63.1) |
| Cause of bladder dysfunction, n (%) | |||
| Neurological | 80 (39.4) | 78 (38.8) | 158 (39.1) |
| Non-neurological | 123 (60.6) | 123 (61.2) | 246 (60.9) |
| Clinical measurements | |||
| Age (years) | |||
| Mean (SD) | 59.1 (17.0) | 60.1 (15.6) | 59.6 (16.3) |
| Weight (kg) | |||
| Mean (SD) | 78.9 (17.4) | 81.3 (16.2) | 80.1 (16.8) |
| Creatinine clearance (ml/minute) | |||
| Mean (SD) | 95.7 (40.1) | 100.4 (38.9) | 98.0 (39.5) |
| Median (IQR) | 89.8 (68.6–121.4) | 99.1 (71.9–124.2) | 93.3 (69.8–122.3) |
| Catheterisation details | |||
| Type of intermittent catheterisation, n (%) | |||
| By self | 201 (99.0) | 198 (98.5) | 399 (98.8) |
| By spouse/carer | 1 (0.5) | 2 (1.0) | 3 (0.7) |
| Missing | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| Planned future duration of need for intermittent catheterisation, n (%) | |||
| Between 1 and 2 years | 0 (0.0) | 4 (2.0) | 4 (1.0) |
| Between 2 and 5 years | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Indefinite | 182 (89.7) | 181 (90.0) | 363 (89.9) |
| Not known | 20 (9.9) | 14 (7.0) | 34 (8.4) |
| Missing | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| Route of intermittent catheterisation, n (%) | |||
| Urethra | 196 (96.6) | 195 (97.0) | 391 (96.8) |
| Mitrofanoff | 6 (3.0) | 5 (2.5) | 11 (2.7) |
| Missing | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| Type of catheter used, n (%) | |||
| Single use | 200 (98.5) | 199 (99.0) | 399 (98.8) |
| Reuseable | 2 (1.0) | 2 (1.0) | 4 (1.0) |
| Missing | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Hydrophilic-coated catheter used?, n (%) | |||
| No | 9 (4.4) | 8 (4.0) | 17 (4.2) |
| Yes | 189 (93.1) | 192 (95.5) | 381 (94.3) |
| Missing | 5 (2.5) | 1 (0.5) | 6 (1.5) |
| Frequency of CISC (per 24 hours), n (%) | |||
| Mean (SD) | 3.8 (2.2) | 4.1 (2.9) | 4.0 (2.6) |
| Median (IQR) | 4.0 (2.0–5.0) | 4.0 (2.0–5.0) | 4.0 (2.0–5.0) |
| Main functional reason for requiring intermittent catheterisation, n (%) | |||
| Bladder outlet obstruction | 49 (24.1) | 56 (27.9) | 105 (26.0) |
| Bladder failure (underactivity) | 139 (68.5) | 128 (63.7) | 267 (66.1) |
| Bladder augmentation/replacement | 13 (6.4) | 16 (8.0) | 29 (7.2) |
| Missing | 2 (1.0) | 1 (0.5) | 3 (0.7) |
| UTI | |||
| Episodes of UTI experienced by participant in previous 12 months, n (%) | |||
| Mean (SD) | 5.2 (3.3) | 5.6 (3.8) | 5.4 (3.6) |
| Median (IQR) | 4.0 (3.0–6.0) | 4.0 (3.0–7.0) | 4.0 (3.0–6.0) |
| Positive urine culture reports in previous 12 months | |||
| Mean (SD) | 2.6 (2.4) | 2.5 (2.4) | 2.5 (2.4) |
| Median (IQR) | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) |
| Approximate months of use of antibiotic prophylaxis for UTI in previous 12 months | |||
| Mean (SD) | 1.1 (2.6) | 1.0 (2.4) | 1.1 (2.5) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Central laboratory culture of urine at baseline, n (%) | |||
| No growth | 93 (45.8) | 84 (41.8) | 177 (43.8) |
| Growth of one or two isolates | 76 (37.4) | 77 (38.3) | 153 (37.9) |
| Missing | 34 (16.8) | 40 (19.9) | 74 (18.3) |
Numbers analysed
In the primary modified ITT analysis, we included 361 (89%) randomised participants who fulfilled the requirement of having documented trial follow-up of ≥ 6 months (Table 7). This total comprised 181 participants (89%) allocated to the prophylaxis group and 180 participants (90%) allocated to the no-prophylaxis group. A total of 34 participants (17%) of those in the prophylaxis group stopped taking a prophylactic agent at some point in the 12-month observation period, while 26 (13%) participants allocated to the no-prophylaxis group started prophylaxis at some point during the 12-month observation period. All such participants who ‘crossed over’ but remained in active follow-up for ≥ 6 months were analysed according to allocated group (modified ITT basis). Reasons for exclusion from main analysis are detailed in Figure 3.
| Variable | Intervention group | Total (N = 404) | |
|---|---|---|---|
| Prophylaxis (N = 203) | No-prophylaxis (N = 201) | ||
| Eligible for primary analysis?, n (%) | |||
| No | 22 (10.8) | 21 (10.4) | 43 (10.6) |
| Yes | 181 (89.2) | 180 (89.6) | 361 (89.4) |
| Included in primary analysis, n | 181 | 180 | 361 |
| Baseline episodes of UTI,a n (%) | |||
| < 4 | 69 (38.1) | 68 (37.8) | 137 (38.0) |
| ≥ 4 | 112 (61.9) | 112 (62.2) | 224 (62.0) |
| Follow-up daysa | |||
| Mean (SD) | 356.0 (36.5) | 355.0 (35.5) | 355.0 (35.9) |
| Median (IQR) | 365.0 (365.0–365.0) | 365.0 (365.0–365.0) | 365.0 (365.0–365.0) |
Outcomes
Primary clinical outcome
The IRR for occurrence of symptomatic antibiotic-treated UTI over 12 months in the prophylaxis group relative to the no prophylaxis group, adjusted for days on study, was 0.52 (95% CI 0.44 to 0.61) in favour of prophylaxis, representing a 48% reduction in the rate of UTI. When number of days at risk was taken into account (discounting days taking antibiotics for UTI treatment), the IRR was 0.50 (95% CI 0.43 to 0.58). Following the modelling process, this was unchanged by additional adjustment for the following stratification factors: frequency of UTI in the 12 months prior to participation, sex and diagnosis of neurological bladder dysfunction. The modelling process additionally considered the following covariates that were non-contributory and, therefore, excluded before the final model: age, functional cause of poor bladder emptying, type of catheter, daily frequency of use of CISC, use of prophylaxis in previous 12 months, degree of renal dysfunction at baseline (creatinine clearance) and presence of asymptomatic bacteriuria at baseline (Table 8). The absolute reduction in UTI episodes over 12 months was 50% to a median [interquartile range (IQR)] of 1 (0–2) in the prophylaxis group from a median (IQR) of 2 (1–4) in the no-prophylaxis group. In the prophylaxis group, the mean [standard deviation (SD)] frequency of symptomatic antibiotic-treated UTI over 12 months was 1.3 (1.6), while in the no-prophylaxis group it was 2.5 (2.3) (see Table 8 and Figure 4a). There was no difference in effectiveness between the three agents used (nitrofurantoin, trimethoprim and cefalexin).
| Poisson regression | Coefficient | Standard error | p-value | 95% CI |
|---|---|---|---|---|
| Unadjusted | ||||
| All eligible | 0.52 | 0.04 | < 0.001 | 0.44 to 0.61 |
| Baseline episodes of UTIs < 4 | 0.46 | 0.08 | < 0.001 | 0.34 to 0.64 |
| Baseline episodes of UTIs ≥ 4 | 0.54 | 0.05 | < 0.001 | 0.45 to 0.64 |
| Adjusted for days at risk | ||||
| All eligible | 0.50 | 0.04 | < 0.001 | 0.43 to 0.58 |
| Baseline episodes of UTIs < 4 | 0.46 | 0.07 | < 0.001 | 0.33 to 0.63 |
| Baseline episodes of UTIs ≥ 4 | 0.51 | 0.05 | < 0.001 | 0.43 to 0.61 |
| Adjusted for days at risk and stratification factors | ||||
| All eligible | 0.50 | 0.04 | < 0.001 | 0.42 to 0.58 |
| Baseline episodes of UTIs < 4 | 0.46 | 0.08 | < 0.001 | 0.33 to 0.63 |
| Baseline episodes of UTIs ≥ 4 | 0.51 | 0.05 | < 0.001 | 0.42 to 0.61 |
| Frequency of UTI | Intervention group | Total (eligible N = 361) | ||
| Prophylaxis (eligible N = 181) | No prophylaxis (eligible N = 180) | |||
| Frequency of symptomatic UTI (primary outcome), n (%) | ||||
| 0 | 76 (42.0) | 40 (22.2) | 116 (32.1) | |
| 1 | 42 (23.2) | 29 (16.1) | 71 (19.7) | |
| 2 | 30 (16.6) | 39 (21.7) | 69 (19.1) | |
| 3 | 18 (9.9) | 21 (11.7) | 39 (10.8) | |
| 4 | 5 (2.8) | 20 (11.1) | 25 (6.9) | |
| 5 | 3 (1.7) | 11 (6.1) | 14 (3.9) | |
| 6 | 5 (2.8) | 7 (3.9) | 12 (3.3) | |
| 7 | 2 (1.1) | 4 (2.2) | 6 (1.7) | |
| 8 | 0 (0.0) | 6 (3.3) | 6 (1.7) | |
| 9 | 0 (0.0) | 3 (1.7) | 3 (0.8) | |
| Mean (SD) | 1.3 (1.6) | 2.5 (2.3) | 1.9 (2.0) | |
| Median (IQR) | 1.0 (0.0–2.0) | 2.0 (1.0–4.0) | 1.0 (0.0–3.0) | |
| Days at risk | ||||
| Mean (SD) | 343.2 (40.5) | 330.0 (43.6) | 336.6 (42.5) | |
| Median (IQR) | 358.0 (341.0–365.0) | 346.0 (318.5–360.0) | 353.0 (329.0–365.0) | |
FIGURE 4.
Bar charts showing frequency distribution (a–d) and mean (SEM) frequency (e–h) of primary outcome and secondary outcomes over the 12-month observation period. (a) Symptomatic (antibiotic-) treated UTI (primary); (b) microbiologically confirmed (antibiotic-) treated UTI; (c) febrile UTI; (d) asymptomatic bacteriuria; (e) symptomatic (antibiotic-) treated UTI (primary); (f) microbiologically confirmed (antibiotic-) treated UTI; (g) febrile UTI; and (h) asymptomatic bacteriuria. SEM, standard error of the mean.

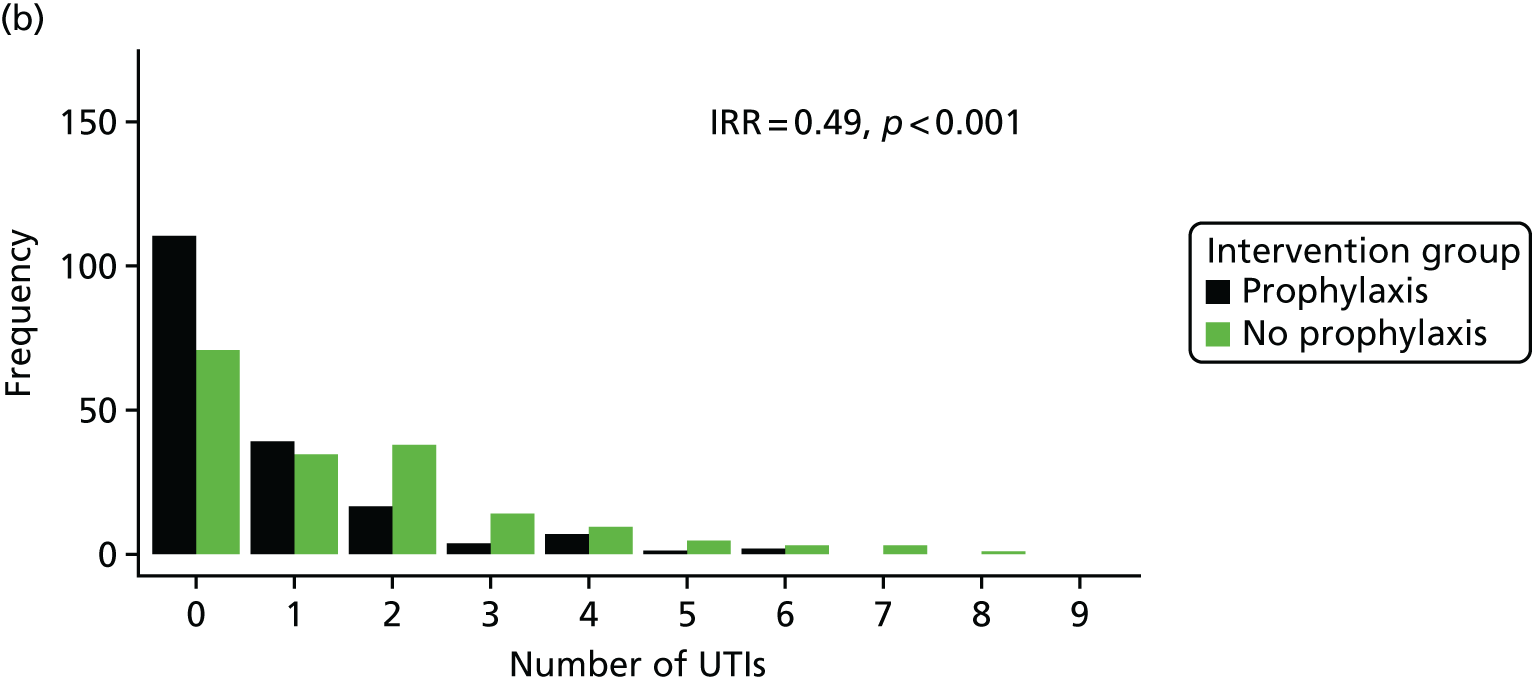


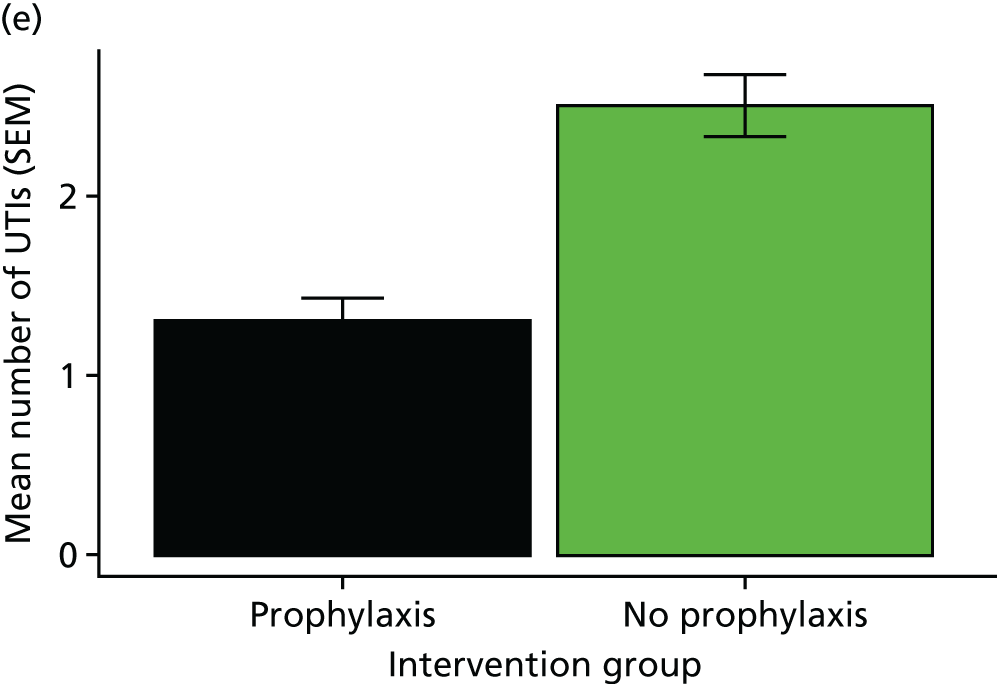
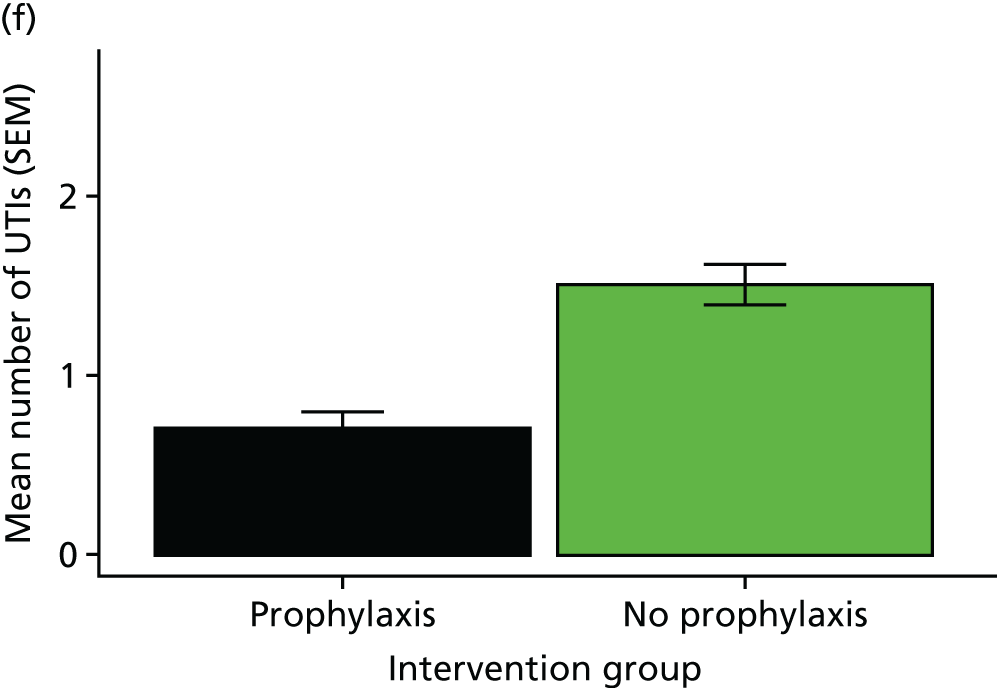
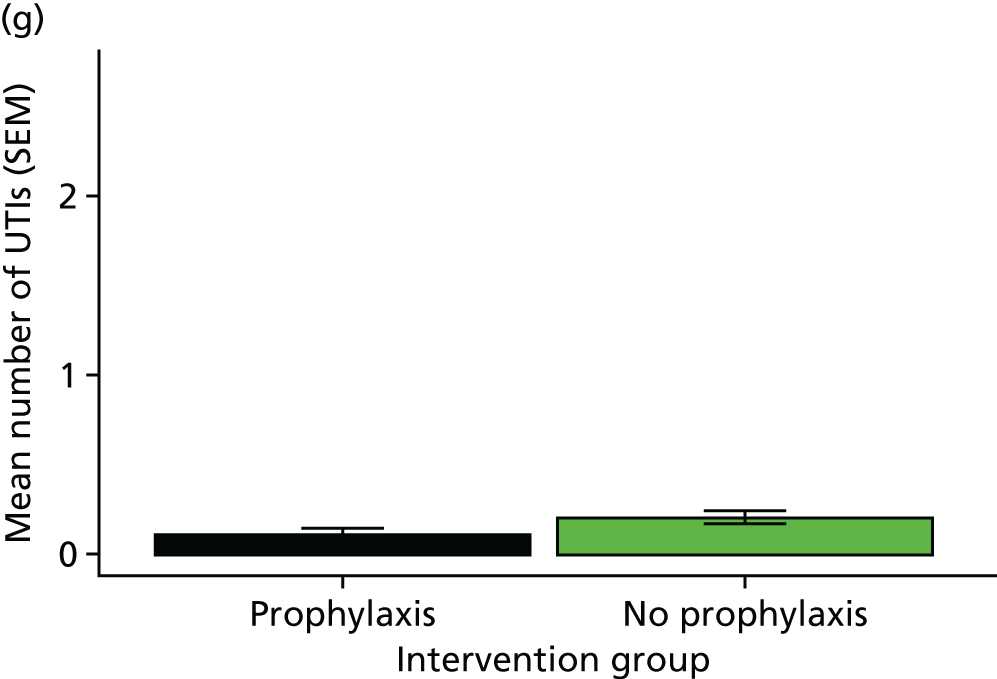
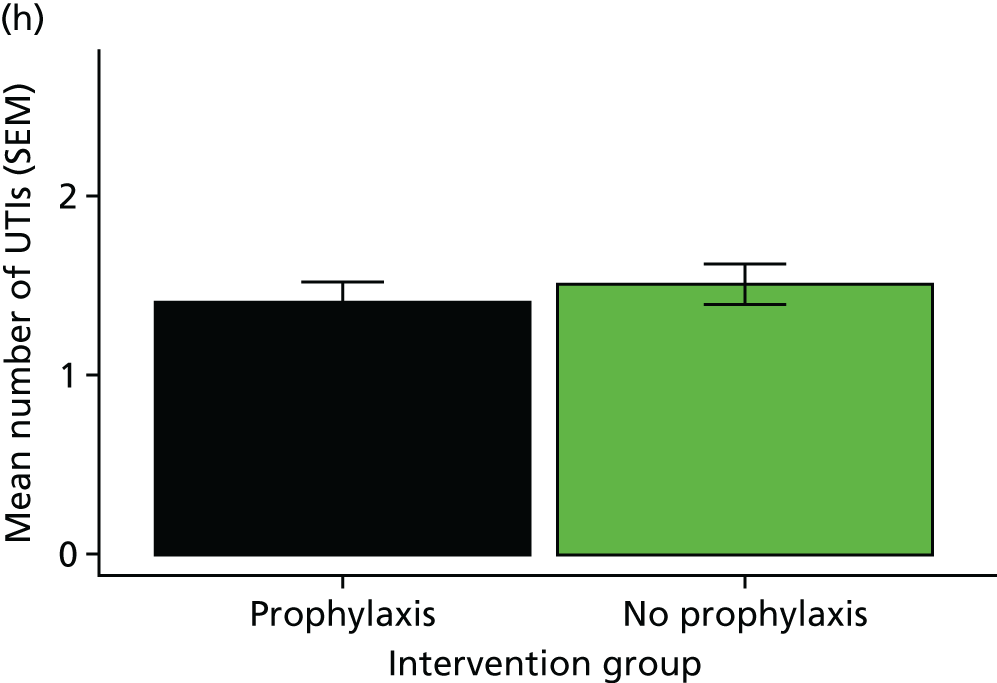
Secondary outcomes
Severity of urinary tract infection
Overall, participants rated symptoms due to UTI as mild for 31% of episodes, moderate for 43% and severe for 23% (no rating was given for 3% of episodes) (see Figure 4c). Of those participants included in the primary analysis, at least one episode of febrile UTI was suffered by 15 participants in the prophylaxis group and 22 in the no-prophylaxis group (IRR 0.71, 95% CI 0.40 to 1.26). This result was unchanged by Poisson modelling analysis with adjustment for frequency of UTI at baseline, days at risk of UTI, sex and neurological cause of bladder dysfunction. Overall, a fever of > 38 °C was recorded for participants in 49 (7%) of the 703 episodes of UTI reported. Six participants in the prophylaxis group were hospitalised as a result of UTI, compared with eight participants (including one participant with two admissions) in the no-prophylaxis group. Instances of hospitalisation were insufficient in both groups to allow for the planned formal statistical comparison.
Alternative definitions of UTI
The reduction seen with the primary trial definition of UTI (symptomatic and antibiotic treated) in the prophylaxis group was also evident using alternative definitions of UTI. For symptomatic microbiologically proven UTI, the IRR was 0.49 (95% CI 0.39 to 0.60) in favour of prophylaxis (Table 9 and see Figure 4b). This result was unchanged by adjustment for days at risk and for prior frequency of UTI, sex and neurological bladder dysfunction. There was also a considerable reduction in the number of participants suffering from four or more microbiologically proven UTIs from 22 participants in the no-prophylaxis group to 10 participants in the prophylaxis group (see Table 9). Of the 703 episodes of symptomatic antibiotic-treated UTI reported for the primary outcome, 405 (58%) were associated with a positive urine culture, with 221 being both central laboratory and local laboratory specimen positive, 114 being local laboratory urine positive with a missing or negative central specimen, and 70 being a positive central laboratory specimen with a negative or missing local laboratory specimen. A total of 298 (42%) episodes of symptomatic antibiotic-treated UTI were not associated with a positive urine sample as tested by either the central or a local laboratory. Of these, 142 had no associated urine specimen submitted for culture, 57 had no growth on culture from either the sample submitted to a central laboratory or the locally submitted sample and 99 had a single specimen submitted to either the central or local laboratory that was negative on culture. A total of 156 (22%) symptomatic, antibiotic-treated UTI episodes were associated with a negative culture.
| Poisson regression | Coefficient | Standard error | p-value | 95% CI |
|---|---|---|---|---|
| Unadjusted | ||||
| All eligible | 0.49 | 0.05 | < 0.001 | 0.39 to 0.60 |
| Baseline episodes of UTIs < 4 | 0.28 | 0.07 | < 0.001 | 0.18 to 0.45 |
| Baseline episodes of UTIs ≥ 4 | 0.57 | 0.07 | < 0.001 | 0.45 to 0.72 |
| Adjusted for days at risk | ||||
| All eligible | 0.47 | 0.05 | < 0.001 | 0.38 to 0.58 |
| Baseline episodes of UTIs < 4 | 0.28 | 0.07 | < 0.001 | 0.17 to 0.44 |
| Baseline episodes of UTIs ≥ 4 | 0.54 | 0.07 | < 0.001 | 0.43 to 0.69 |
| Adjusted for days at risk and stratification factors | ||||
| All eligible | 0.47 | 0.05 | < 0.001 | 0.38 to 0.58 |
| Baseline episodes of UTIs < 4 | 0.28 | 0.07 | < 0.001 | 0.17 to 0.44 |
| Baseline episodes of UTIs ≥ 4 | 0.55 | 0.07 | < 0.001 | 0.43 to 0.69 |
| Frequency of microbiologically confirmed UTI | Intervention group | Total (eligible N = 361) | ||
| Prophylaxis (eligible N = 181) | No prophylaxis (eligible N = 180) | |||
| Microbiological UTI frequency, n (%) | ||||
| 0 | 111 (61.3) | 71 (39.4) | 182 (50.4) | |
| 1 | 39 (21.5) | 35 (19.4) | 74 (20.5) | |
| 2 | 17 (9.4) | 38 (21.1) | 55 (15.2) | |
| 3 | 4 (2.2) | 14 (7.8) | 18 (5.0) | |
| 4 | 7 (3.9) | 10 (5.6) | 17 (4.7) | |
| 5 | 1 (0.6) | 5 (2.8) | 6 (1.7) | |
| 6 | 2 (1.1) | 3 (1.7) | 5 (1.4) | |
| 7 | 0 (0.0) | 3 (1.7) | 3 (0.8) | |
| 8 | 0 (0.0) | 1 (0.6) | 1 (0.3) | |
| Mean (SD) | 0.7 (1.2) | 1.5 (1.7) | 1.1 (1.5) | |
| Median (IQR) | 0.0 (0.0–1.0) | 1.0 (0.0–2.0) | 0.0 (0.0–2.0) | |
For asymptomatic bacteriuria detected in samples submitted to the central laboratory by participants at baseline and at 3, 6, 9 and 12 months, there was no difference between the prophylaxis and no-prophylaxis groups in the rate of positive culture in the unadjusted analysis (IRR 0.88, 95% CI 0.74 to 1.04) (see Figure 4d). When adjusted for days at risk, the subgroup of participants with low prior frequency of UTI (fewer than four per year) who were allocated to prophylaxis had a significantly lower rate of asymptomatic bacteriuria, with an IRR of 0.76 (95% CI 0.59 to 0.98). These results were unchanged by adjustment for sex and presence of neurological bladder dysfunction (Table 10). Overall, 110 (61%) participants in the prophylaxis group and 113 (63%) participants in the no-prophylaxis group had at least one positive urine culture during asymptomatic periods during their trial participation (Table 10). Antibiotic treatment for asymptomatic bacteriuria was prescribed for four participants [four in the prophylaxis group (one prescribed twice) and one in the no-prophylaxis group]. Instances of antibiotic treatment for asymptomatic bacteriuria were insufficient in both groups to allow for the planned formal statistical comparison. Isolates cultured from 3-monthly urine specimens (asymptomatic) and at the time of UTI are detailed in Table 11; E. coli dominated in both sets of specimens.
| Poisson regression | Coefficient | Standard error | p-value | 95% CI |
|---|---|---|---|---|
| Unadjusted | ||||
| All eligible | 0.88 | 0.08 | 0.14 | 0.74 to 1.04 |
| Baseline episodes of UTIs < 4 | 0.77 | 0.10 | 0.05 | 0.60 to 1.00 |
| Baseline episodes of UTIs ≥ 4 | 0.98 | 0.11 | 0.83 | 0.77 to 1.23 |
| Adjusted for days at risk | ||||
| All eligible | 0.85 | 0.07 | 0.06 | 0.71 to 1.00 |
| Baseline episodes of UTIs < 4 | 0.76 | 0.10 | 0.04 | 0.59 to 0.98 |
| Baseline episodes of UTIs ≥ 4 | 0.93 | 0.11 | 0.52 | 0.74 to 1.17 |
| Adjusted for days at risk and stratification factors | ||||
| All eligible | 0.85 | 0.07 | 0.06 | 0.72 to 1.01 |
| Baseline episodes of UTIs < 4 | 0.76 | 0.10 | 0.04 | 0.59 to 0.98 |
| Baseline episodes of UTIs ≥ 4 | 0.93 | 0.11 | 0.53 | 0.74 to 1.17 |
| Frequency of asymptomatic bacteriuria | Intervention group | Total (eligible N = 361), n (%) | ||
| Prophylaxis (eligible N = 181), n (%) | No prophylaxis (eligible N = 180), n (%) | |||
| 0 | 71 (39.2) | 67 (37.2) | 138 (38.2) | |
| 1 | 47 (26.0) | 35 (19.4) | 82 (22.7) | |
| 2 | 25 (13.8) | 32 (17.8) | 57 (15.8) | |
| 3 | 11 (6.1) | 20 (11.1) | 31 (8.6) | |
| 4 | 18 (9.9) | 11 (6.1) | 29 (8.0) | |
| 5 | 9 (5.0) | 14 (7.8) | 23 (6.4) | |
| 6 | 0 (0.0) | 1 (0.6) | 1 (0.3) | |
| Mean (SD) | 1.4 (1.5) | 1.6 (1.6) | 1.5 (1.6) | |
| Median (IQR) | 1.0 (0.0– 2.0) | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | |
| Bacterium | Isolates from, n (% of total isolates) | |
|---|---|---|
| 3-monthly surveillance urine specimens | Urine specimens submitted at time of symptomatic UTI | |
| E. coli | 499 (57.9) | 103 (54.8) |
| Klebsiella sp. | 103 (11.9) | 28 (14.9) |
| Proteus sp. | 27 (3.1) | 6 (3.2) |
| Coliforms (other) | 101 (11.7) | 27 (14.4) |
| Pseudomonas sp. | 26 (3.0) | 12 (6.4) |
| Gram negative (other) | 4 (0.5) | 0 (0.0) |
| Enterococci | 63 (7.3) | 8 (4.3) |
| Streptococci | 9 (1.0) | 1 (0.5) |
| Staphylococci | 29 (3.4) | 3 (1.6) |
| Gram positive (other) | 1 (0.1) | 0 (0.0) |
| Total isolates | 862 | 188 |
Adverse events
All SAEs reported during the trial are given in Appendix 1 (see Tables 30–33). Overall, there was no deterioration in kidney or liver function (see Appendix 1, Table 34) in either group over the 12 months of participation. One participant suffered an asymptomatic rise in the serum level of a liver enzyme (ALT) resulting from the use of nitrofurantoin as the prophylactic agent. This resolved completely on stopping nitrofurantoin. There were no reported cases of respiratory illness or peripheral neuropathy associated with prophylaxis using nitrofurantoin. One participant who had been on multiple medications, including nitrofurantoin, was admitted to hospital following a fall that was assessed as due to polypharmacy (not listed in the approved RSI as an expected AE for nitrofurantoin). The sponsor took the decision to report this to MHRA as a suspected unexpected serious adverse reaction (SUSAR). The event was submitted as a ‘drug interaction’. None of the other 51 SAEs reported was categorised as being related to study participation. Three participants died during the 12 months of trial observation: one from metastatic oesophageal cancer, one from bladder cancer and one following a fall.
Review of health records showed that 19 participants in the prophylaxis group and four in the no-prophylaxis group suffered an AE related to use of prophylactic antibiotics (Table 12). At each of the 3-monthly reviews, < 10% of the 203 participants allocated to prophylaxis reported adverse effects. Of the participant responses on UTI record forms, 28 participants in the prophylaxis group and 60 in the no-prophylaxis group reported at least one specific AE due to treatment antibiotic. The most frequent side effects were gastrointestinal disturbance (nausea and diarrhoea reported by 14% and 11% of participants respectively) and candidal infection (7% of participants) (see Table 12). The small numbers of AEs precluded formal statistical analysis.
| Number of AEs related to prophylaxis and treatment antibiotics | Intervention group, n (%) | Total (N = 404), n (%) | |
|---|---|---|---|
| Prophylaxis (N = 203) | No prophylaxis (N = 201) | ||
| Health-care record review (local trial staff) | |||
| 0 | 184 (90.6) | 197 (98.0) | 381 (94.3) |
| 1 | 17 (8.4) | 3 (1.5) | 20 (5.0) |
| 2 | 1 (0.5) | 1 (0.5) | 2 (0.5) |
| 3 | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| 3-monthly participant review (local trial staff/participant), prophylaxis antibiotics only | |||
| 1 month | 17 (8.4) | 0 (0.0) | 17 (4.2) |
| 3 months | 20 (9.9) | 0 (0.0) | 20 (5.0) |
| 6 months | 17 (8.4) | 0 (0.0) | 17 (4.2) |
| 9 months | 10 (4.9) | 0 (0.0) | 10 (2.5) |
| 12 months | 10 (4.9) | 2 (1.0) | 12 (3.0) |
| UTI record (participant) treatment antibiotics only | |||
| Skin rash | 2 (1.0) | 6 (3.0) | 8 (2.0) |
| Feeling sick (nauseated) | 20 (9.9) | 38 (18.9) | 58 (14.4) |
| Diarrhoea (loose or more frequent bowel movement) | 13 (6.4) | 31 (15.4) | 44 (10.9) |
| Thrush (candidal fungal infection) in the mouth/vagina | 10 (4.9) | 19 (9.5) | 29 (7.2) |
| Antibiotic side effects: other | 4 (2.0) | 9 (4.5) | 13 (3.2) |
Bacterial resistance to antibiotics
There were no apparent differences between the groups at baseline in resistance rates of urinary isolates to eight oral antibiotics commonly used for UTI: (1) amoxicillin, (2) cefalexin, (3) ciprofloxacin, (4) co-trimoxazole, (5) co-amoxiclav, (6) mecillinam, (7) nitrofurantoin and (8) trimethoprim.
Urine specimens received during asymptomatic periods were split into time categories according to the date the sample was received in relation to the participant’s date of randomisation. Samples were categorised as baseline, 0–3 months, 3–6 months, 6–9 months or 9–12 months. Samples received up to 14 days after the end of these time periods were included in the preceding category to allow for delay in receipt of samples. A similar methodology was applied to perianal swabs received, but with baseline, 0–6 months and 6–12 months categories. To establish the number of participants whose specimens had isolates resistant to each antibiotic in each time category, we considered that the presence of an isolate displaying resistance in any of the surveillance urine samples received from an individual participant during each specified time period indicated that resistance was present for that participant at that time period. Figures 5–7 show resistance rates over time, plotted for eight antibiotics, by group.
FIGURE 5.
Resistance rates (%) plotted over the 12-month trial duration for pathogens isolated from urine specimens taken during symptomatic UTI, by antibiotic and group. (a) Amoxicillin; (b) cefalexin; (c) ciprofloxacin; (d) co-trimoxazole; (e) co-amoxiclav; (f) mecillinam; (g) nitrofurantoin; and (h) trimethoprim. n/N, number of participants with at least one resistant isolate/number of participants with at least one isolate; NA, not applicable.
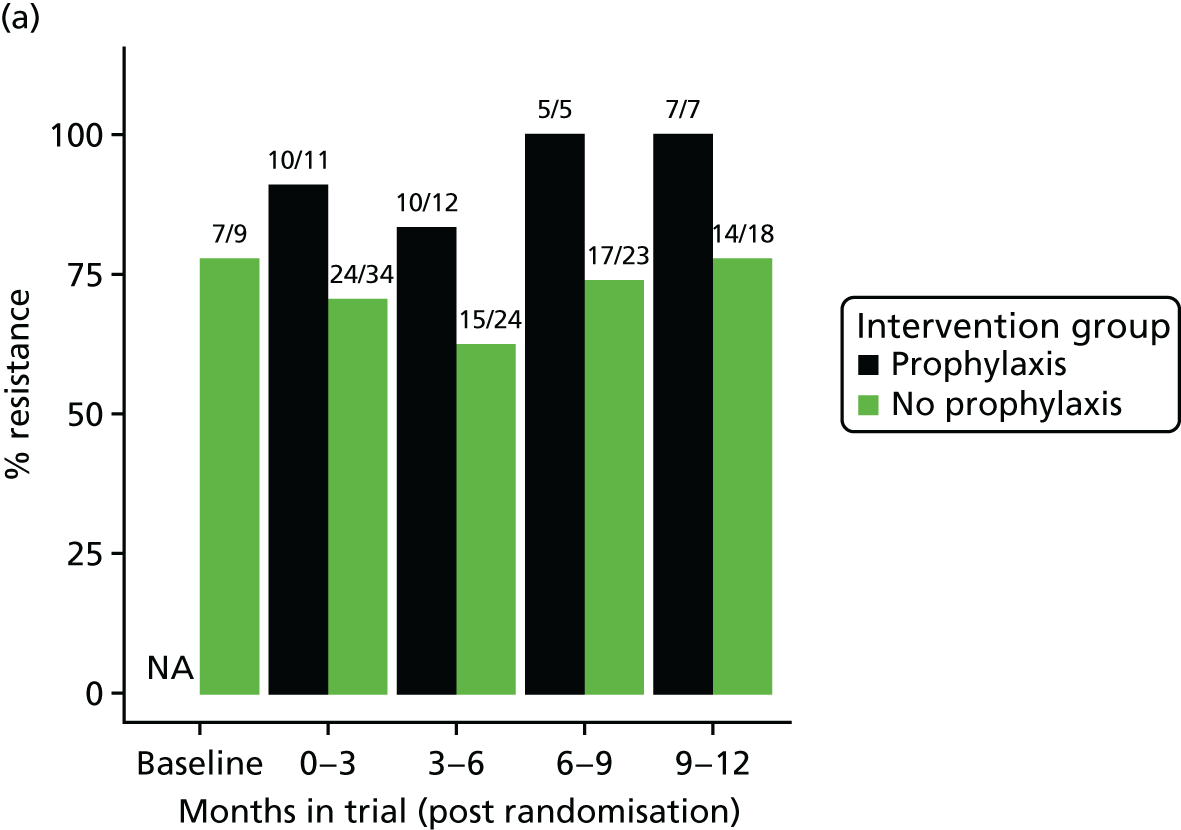




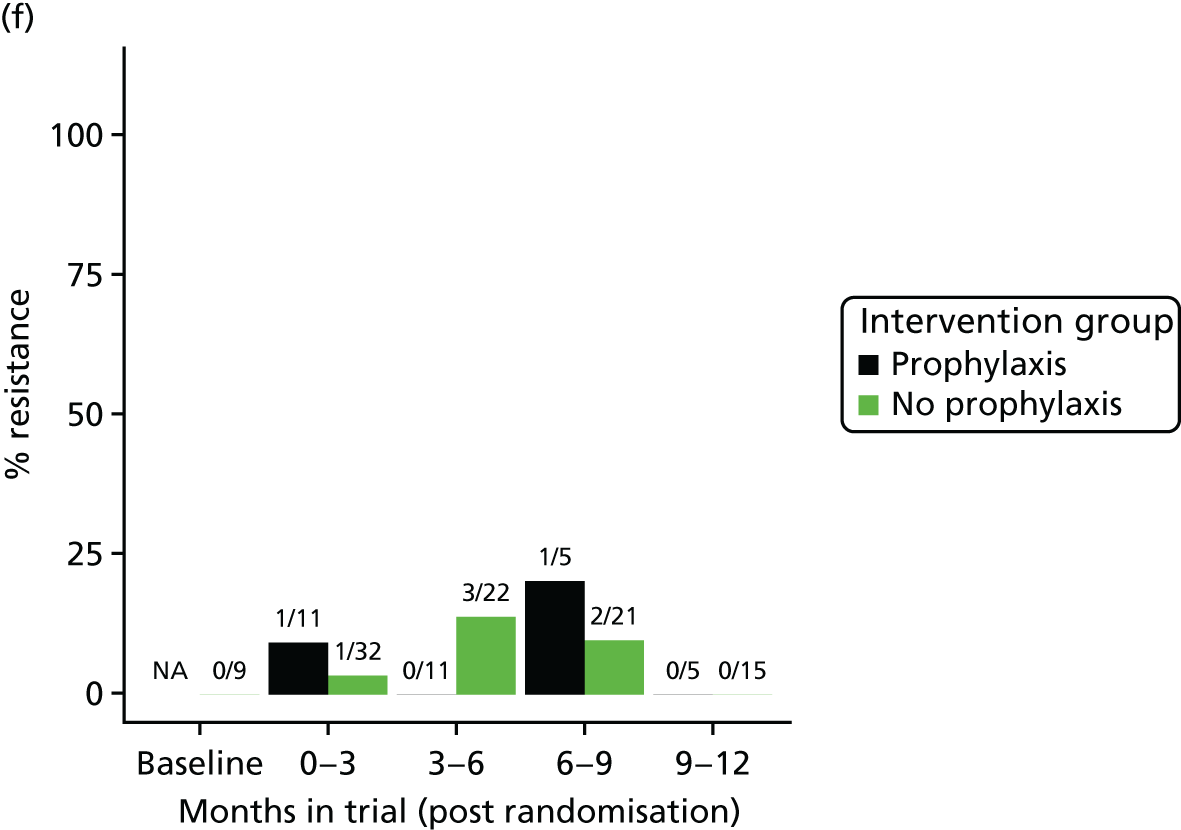


FIGURE 6.
Resistance rates (%) plotted over time for bacteria isolated from urine specimens taken during asymptomatic state, by antibiotic and group. (a) Amoxicillin; (b) cefalexin; (c) ciprofloxacin; (d) co-trimoxazole; (e) co-amoxiclav; (f) mecillinam; (g) nitrofurantoin; and (h) trimethoprim. n/N, number of participants with at least one resistant isolate/number of participants with at least one isolate.
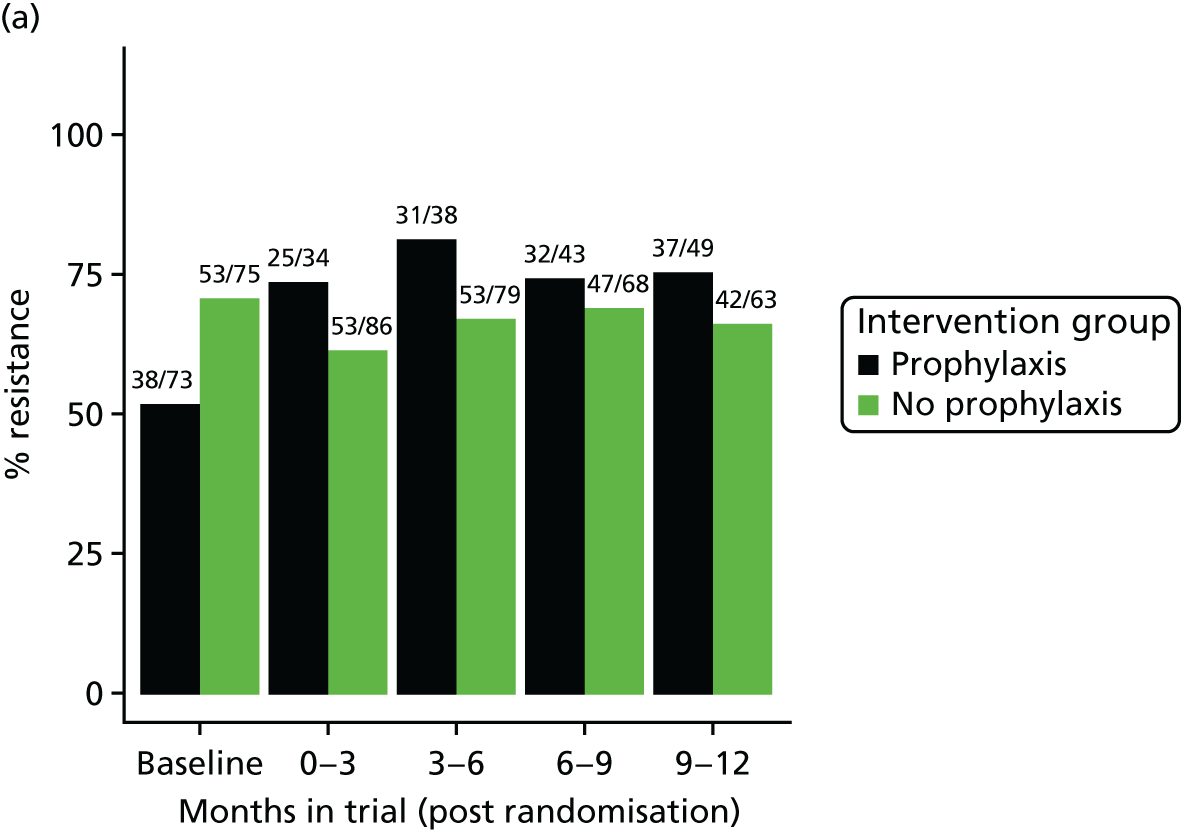

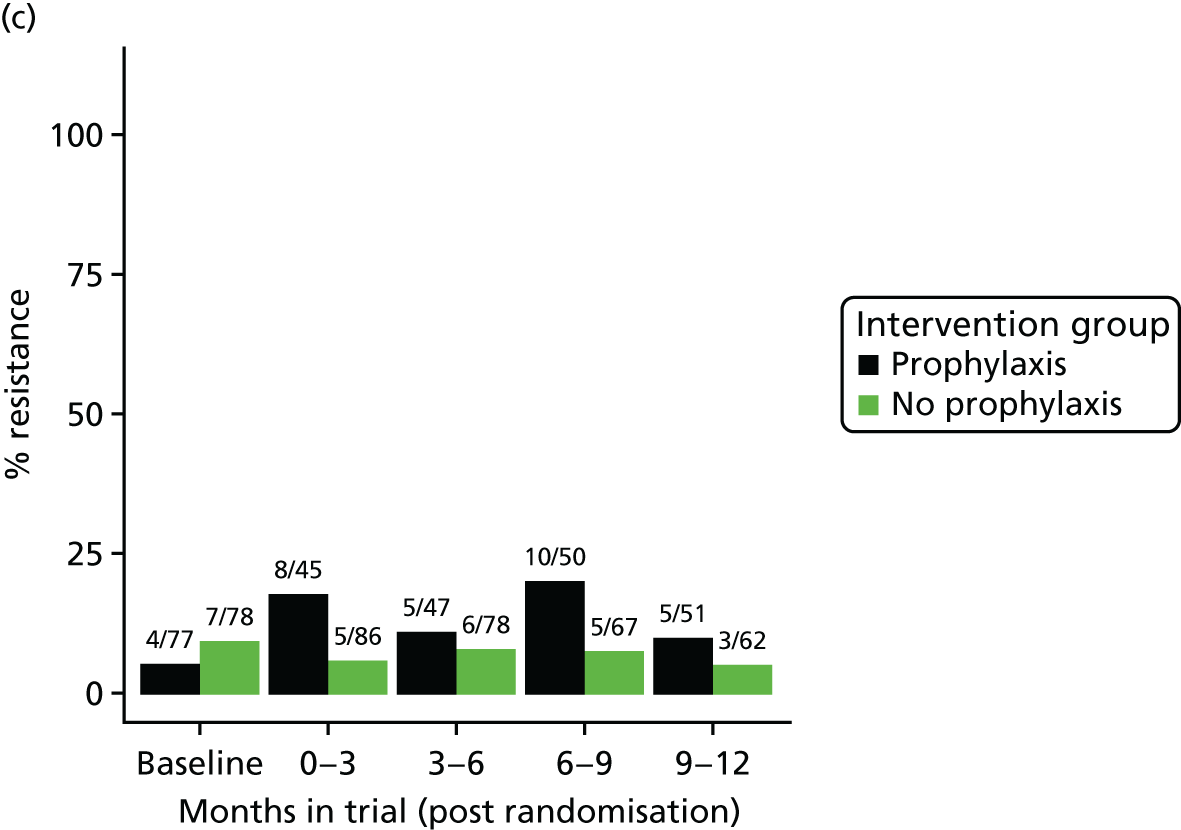


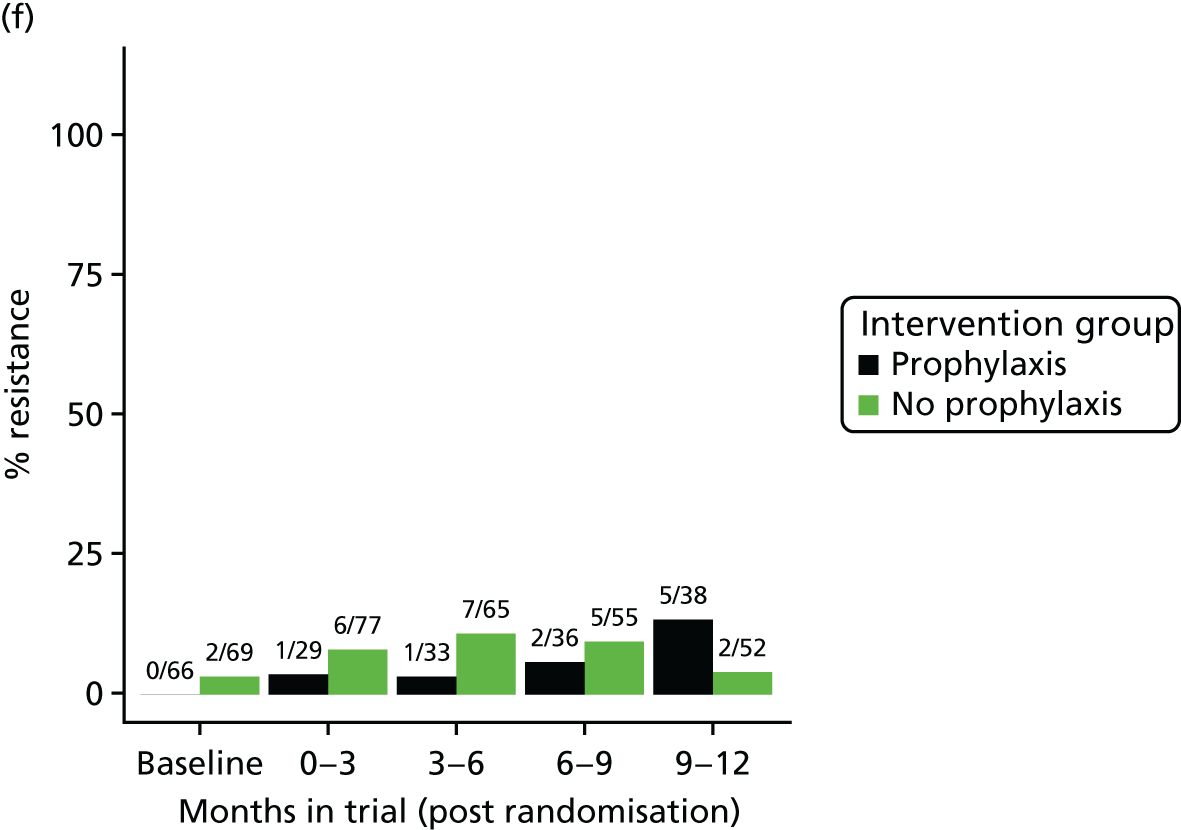
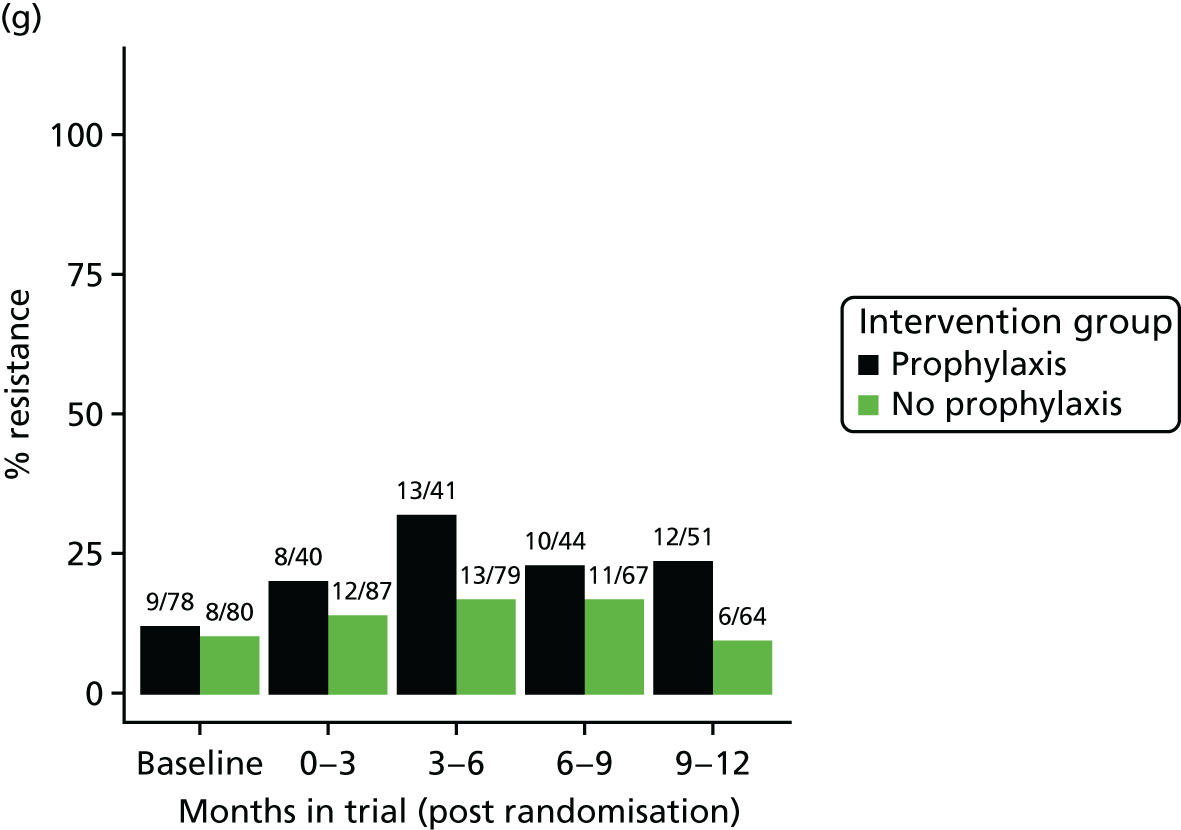

FIGURE 7.
Resistance rates plotted over time for E. coli strains isolated from perianal swabs, by antibiotic and group. (a) Amoxicillin; (b) cefalexin; (c) ciprofloxacin; (d) co-trimoxazole; (e) co-amoxiclav; (f) mecillinam; (g) nitrofurantoin; and (h) trimethoprim. n/N, number of participants with at least one resistant isolate/number of participants with at least one isolate.

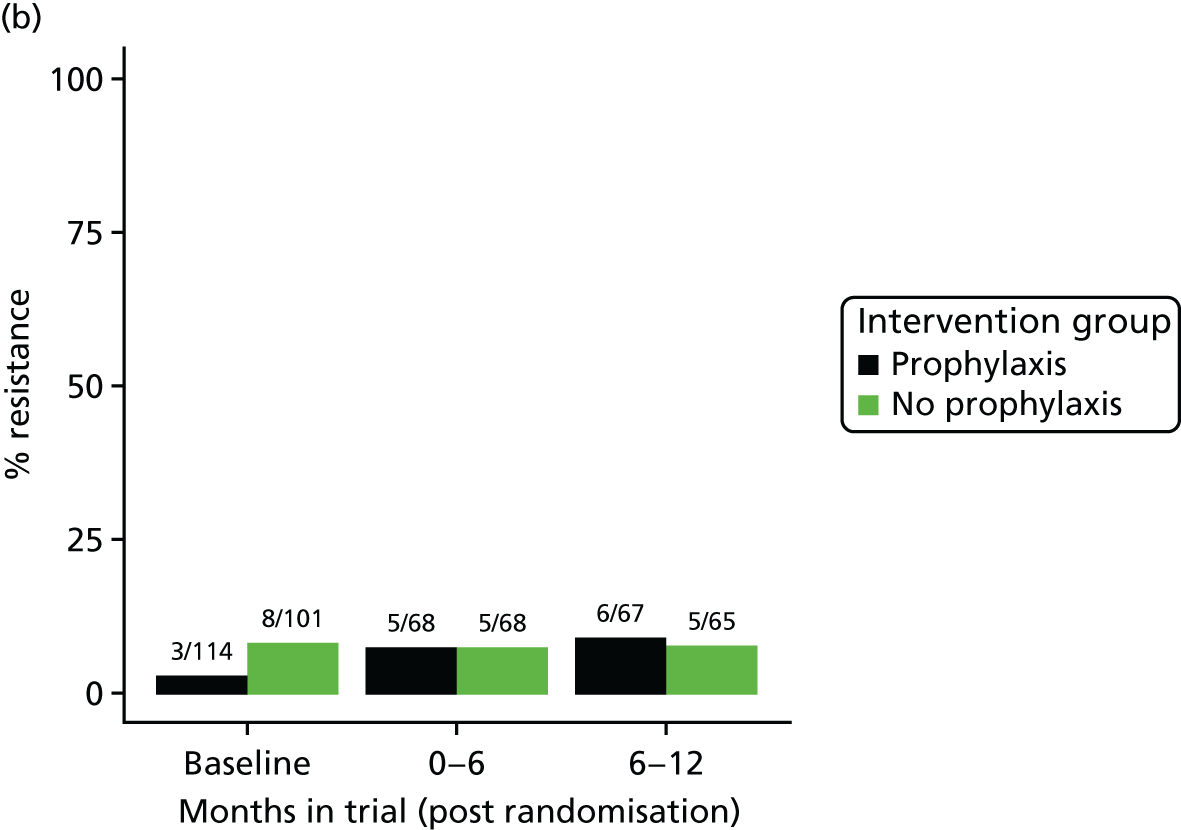

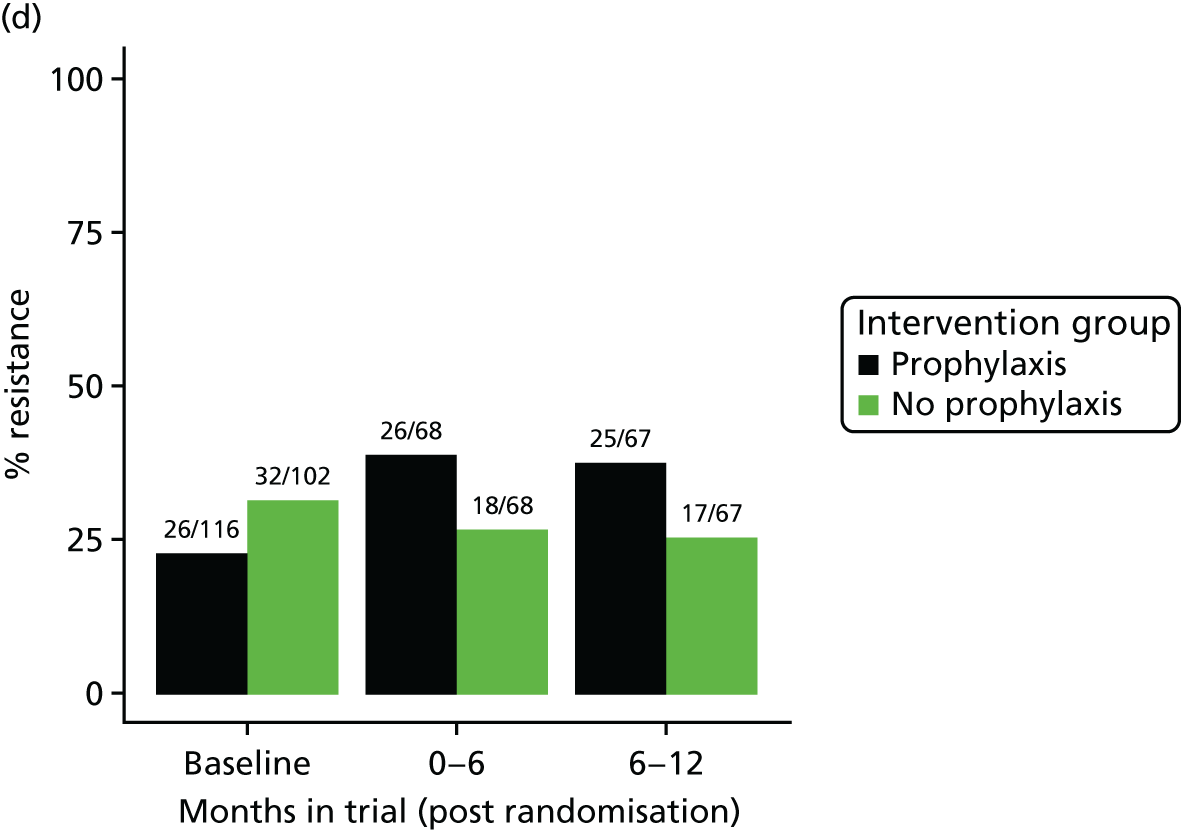
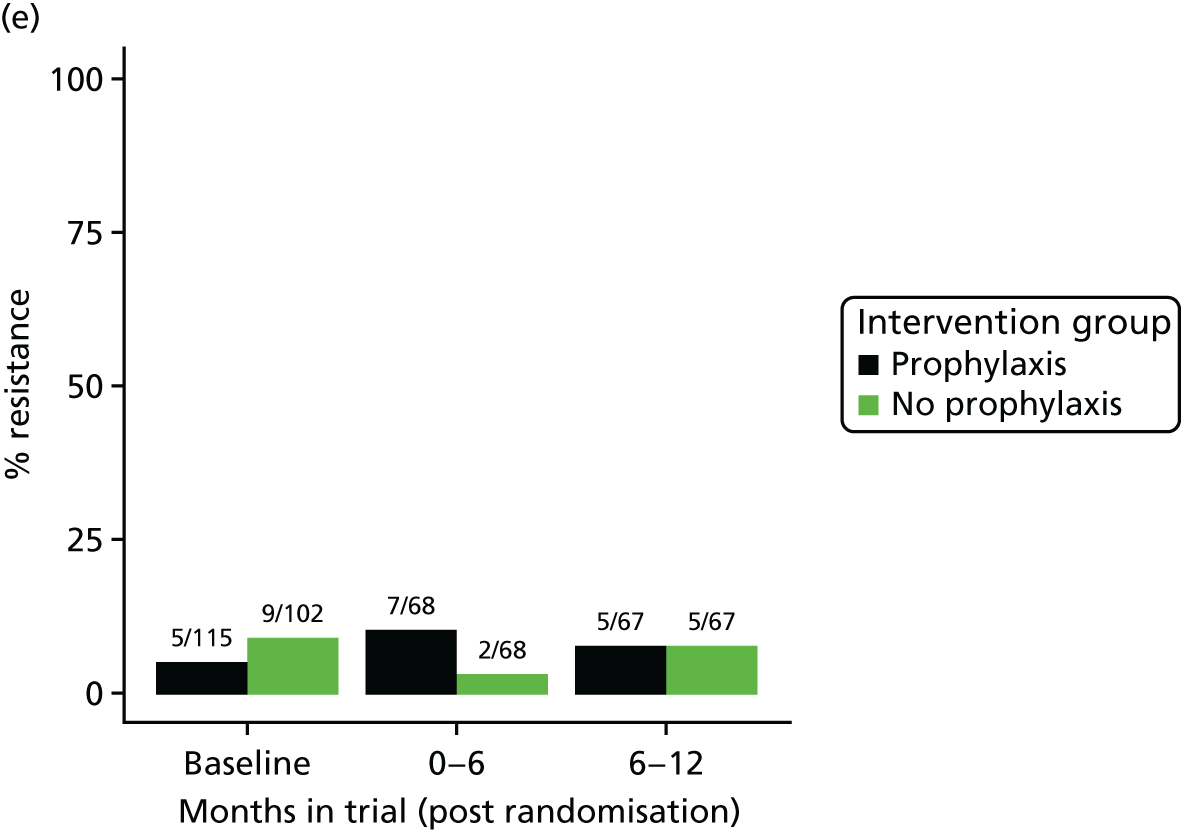



Table 13 shows the results of a test for trend during the asymptomatic state in the prophylaxis group and the no-prophylaxis group for the 3-monthly urine specimens. We observed an increase in the resistance rates with time for the majority of the antibiotics under consideration in the prophylaxis group. We did not find any evidence of increased antibiotic resistance in the no-prophylaxis group. It was considered that insufficient samples or time points were available for a trend analysis to be presented for the data from the urine samples submitted at the time of UTI or the perianal swabs.
| Antibiotic | Intervention group, test for trend | |||||
|---|---|---|---|---|---|---|
| Prophylaxis | No prophylaxis | |||||
| Slope (standard error) | Chi-squared (test for trend) | p-value | Slope (standard error) | Chi-squared (test for trend) | p-value | |
| Amoxicillin | 0.06 (0.02) | 8.44 | 0.004 | 0.00 (0.02) | 0.00 | 0.995 |
| Cefalexin | 0.05 (0.02) | 7.79 | 0.005 | 0.01 (0.02) | 0.10 | 0.752 |
| Ciprofloxacin | 0.02 (0.01) | 1.46 | 0.226 | –0.01 (0.01) | 0.426 | 0.514 |
| Co-trimoxazole | 0.06 (0.02) | 7.49 | 0.006 | –0.02 (0.02) | 0.895 | 0.344 |
| Co-amoxiclav | 0.03 (0.02) | 2.50 | 0.114 | 0.00 (0.01) | 0.02 | 0.895 |
| Mecillinam | a | a | a | a | a | a |
| Nitrofurantoin | 0.03 (0.02) | 3.46 | 0.063 | 0.00 (0.01) | 0.04 | 0.835 |
| Trimethoprim | 0.05 (0.02) | 5.81 | 0.016 | –0.02 (0.02) | 1.59 | 0.208 |
Table 14 shows the results of performing chi-squared tests for each antibiotic to compare the resistance rates in the two groups at 9–12 months (using asymptomatic state urine samples) and at 6–12 months (using perianal samples). In the asymptomatic state samples, we observe that at 9–12 months rates of resistance to nitrofurantoin (χ2 = 4.31; p = 0.038), trimethoprim (χ2 = 13.0; p < 0.001) and co-trimoxazole (χ2 = 9.79; p = 0.002) were significantly higher in the prophylaxis group than in the no-prophylaxis group. Using perianal swabs, we found no evidence that at 6–12 months rates of resistance to any antibiotics were significantly higher in the prophylaxis group than in the no-prophylaxis group.
| Antibiotic | During asymptomatic state (9–12 months) | Perianal swabs (6–12 months) | ||
|---|---|---|---|---|
| Chi-squared | p-value | Chi-squared | p-value | |
| Amoxicillin | 1.04 | 0.308 | 0.12 | 0.729 |
| Cefalexin | 0.32 | 0.571 | 0.07 | 0.793 |
| Ciprofloxacin | 1.05 | 0.306 | 0.09 | 0.770 |
| Co-trimoxazole | 9.79 | 0.002 | 2.22 | 0.136 |
| Coamoxiclav | 1.14 | 0.287 | 0.00 | 1.00 |
| Mecillinam | 2.65 | 0.103 | a | a |
| Nitrofurantoin | 4.31 | 0.038 | 0.193 | 0.661 |
| Trimethoprim | 13.0 | < 0.001 | 3.58 | 0.058 |
Satisfaction with treatment
Overall, participants allocated to prophylaxis were satisfied with their treatment. Completion of the TSQM by 144 (71%) of the 203 participants allocated to prophylaxis showed a mean (SD) score of 78 points (19 points) for effectiveness and a mean (SD) of 89 points (14 points) for convenience. Overall mean (SD) satisfaction score was 74 points (25 points) (Table 15). The TSQM is not validated for ‘no treatment’ strategies in an open-label RCT setting but, overall, this group rated mean (SD) overall satisfaction as 63 points (24 points).
| Domain | Intervention group, mean (SD), n | Total (N = 404), mean (SD), n | Two-sample t-test comparing groups, p-value | Using ANCOVA modelling to compare groups (adjusting for stratification factors), p-value | |
|---|---|---|---|---|---|
| Prophylaxis (N = 203) | No prophylaxis (N = 201) | ||||
| Effectiveness | 78.0 (19.1), 144 | 66.3 (19.5), 108 | 72.9 (20.1), 252 | < 0.001 | < 0.001 |
| Side effects | 67.4 (23.4), 22 | 67.2 (24.2), 32 | 67.3 (23.7), 54 | 0.97 | 0.94 |
| Convenience | 88.9 (13.9), 144 | 78.2 (21.4), 109 | 84.3 (18.3), 253 | < 0.001 | < 0.001 |
| Overall | 73.8 (25.4), 143 | 63.0 (24.3), 109 | 69.1 (25.5), 252 | 0.001 | < 0.001 |
At 12 months, 77 (78%) of the 99 participants allocated to prophylaxis who expressed a preference stated that they wished to continue with the treatment, whereas 21 (20%) of the 104 participants in the no-prophylaxis group who expressed a preference wanted to commence prophylaxis.
The relative effect on health status among trial participants
Data from participant completion of the SF-36v2 available or missing at each time point for the prophylaxis group and no-prophylaxis group are shown in Table 16. This table shows the number of participants providing data at each time point falling during the 12-month study period. Overall, approximately 70% of participants completed all three assessments.
| Trial group | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | Baseline and 6 months | Baseline and 12 months | Baseline, 6 and 12 months | |
| Prophylaxis (N = 203) | ||||||
| Number of patients (%) | 197 (97.0) | 164 (80.8) | 150 (73.9) | 159 (78.3) | 147 (72.4) | 147 (72.4) |
| No prophylaxis (N = 201) | ||||||
| Number of patients (%) | 194 (96.5) | 155 (77.1) | 146 (72.6) | 155 (77.1) | 142 (70.6) | 136 (67.7) |
| Total number of patients (%) | 391 (96.8) | 319 (79.0) | 296 (73.3) | 314 (77.7) | 289 (71.5) | 283 (70.0) |
Subcategory MCS and PCS scores from participant completion of the SF-36v2 are summarised in Table 17. It presents the mean, SD, 95% CI, median, MCS and PCS scores for each group at baseline and at 6 and 12 months, by group.
| Component at time points | n (%) | Mean | SD | 95% CI | Median |
|---|---|---|---|---|---|
| Baseline | |||||
| Prophylaxis | |||||
| MCS | 194 (95.6) | 48.68 | 12.22 | 46.95 to 50.42 | 51.68 |
| PCS | 193 (95.1) | 41.02 | 12.12 | 39.30 to 42.74 | 41.61 |
| No prophylaxis | |||||
| MCS | 187 (93.0) | 49.39 | 12.05 | 47.65 to 51.13 | 52.84 |
| PCS | 186 (92.5) | 40.13 | 11.98 | 38.39 to 41.86 | 40.58 |
| 6 months | |||||
| Prophylaxis | |||||
| MCS | 148 (72.9) | 49.2 | 12.55 | 47.16 to 51.24 | 53.93 |
| PCS | 146 (71.9) | 39.85 | 12.36 | 37.47 to 41.47 | 39.66 |
| No prophylaxis | |||||
| MCS | 147 (73.1) | 46.24 | 12.85 | 44.15 to 48.34 | 49.47 |
| PCS | 144 (71.6) | 39.47 | 12.15 | 37.47 to 41.47 | 39.23 |
| 12 months | |||||
| Prophylaxis | |||||
| MCS | 139 (68.5) | 48.06 | 12.39 | 45.98 to 50.14 | 53.03 |
| PCS | 137 (67.5) | 39.43 | 13.07 | 37.23 to 45.64 | 38.42 |
| No prophylaxis | |||||
| MCS | 137 (68.2) | 46.99 | 13.08 | 44.78 to 49.21 | 51.75 |
| PCS | 134 (66.7) | 39.83 | 11.95 | 37.79 to 41.87 | 39.74 |
Details of the unadjusted and adjusted comparison between randomised groups at 6 and 12 months are reported in Table 18. For the PCS, the unadjusted analysis shows a mean increase of 0.38 (95% CI –2.45 to 3.21; p = 0.79) in the prophylaxis group compared with the no-prophylaxis group at 6 months. However, at 12 months, the prophylaxis group had a mean decrease of –0.39 (95% CI –3.39 to 2.60; p = 0.79) compared with the no-prophylaxis group. These differences in PCS scores at 6 and 12 months between groups were not statistically significant. The MCS showed a difference in mean score of 2.96 (95% CI 0.05 to 5.87; p = 0.05) at 6 months in favour of prophylaxis and 1.07 (95% CI –1.96 to 4.08; p = 0.49) at 12 months. Adjusted analysis gave broadly similar results (see Table 18).
| Unadjusted results | Mean score (SD) | Difference (95% CI) | p-value |
|---|---|---|---|
| PCS scores | |||
| 6 months | |||
| Prophylaxis | 39.85 (12.36) | 0.38 (–2.45 to 3.21) | 0.79 |
| No prophylaxis | 39.47 (12.15) | ||
| 12 months | |||
| Prophylaxis | 39.44 (13.07) | –0.39 (–3.39 to 2.60) | 0.79 |
| No prophylaxis | 39.83 (11.95) | ||
| MCS scores | |||
| 6 months | |||
| Prophylaxis | 49.20 (12.55) | 2.96 (0.05 to 5.87) | 0.05 |
| No prophylaxis | 46.24 (12.85) | ||
| 12 months | |||
| Prophylaxis | 48.06 (12.38) | 1.07 (–1.96 to 4.08) | 0.49 |
| No prophylaxis | 46.99 (13.08) | ||
| Adjusted results | Mean score (SD) | Difference (95% CI) | p-value |
| PCS scores | |||
| 6 months | |||
| Prophylaxis | 39.85 (12.36) | –0.12 (–1.82 to 1.58) | 0.89 |
| No prophylaxis | 39.47 (12.15) | ||
| 12 months | |||
| Prophylaxis | 39.44 (13.07) | –1.08 (–2.86 to 0.69) | 0.23 |
| No prophylaxis | 39.83 (11.95) | ||
| MCS scores | |||
| 6 months | |||
| Prophylaxis | 49.20 (12.55) | 3.04 (0.34 to 5.74) | 0.03 |
| No prophylaxis | 46.24 (12.85) | ||
| 12 months | |||
| Prophylaxis | 48.06 (12.38) | 1.34 (–1.45 to 4.13) | 0.34 |
| No prophylaxis | 46.99 (13.08) | ||
Chapter 4 Economic evaluation
Introduction
A within-trial health economic evaluation was planned as an integral part of the AnTIC trial in order to determine whether or not any clinical benefit found for the use of antibiotic prophylaxis was worthwhile for individual patients suffering recurrent UTIs consequent to their use of CISC and for the UK NHS. The evaluation comprised three different methodologies: (1) cost-effectiveness analysis (CEA), (2) cost–utility analysis (CUA) and (3) cost–benefit analysis (CBA). The primary aim of the economic evaluation was to determine the relative efficiency of an experimental UTI prevention strategy of continuous once-daily prophylactic antibiotic therapy against no prophylaxis in people carrying out CISC who suffer recurrent UTI. Relative efficiency in the cost-effectiveness and CUAs was estimated by taking the difference in mean cost between two trial groups and dividing it by the difference in effect. In the CBA, the monetary value of benefit minus costs provides the estimate of relative efficiency. All economic analyses were based on a modified ITT principle.
The health economic evaluation took the perspective of the health service provider (NHS) and Personal Social Services (PSS). The main costs collected relate to health service utilisation, that is, the average total cost to the NHS incurred by participants during the 12-month trial period. A wider perspective was also taken by including costs borne by the participants and their families. These include direct (e.g. travel) and indirect (e.g. time away from usual activities such as work) costs.
The results of the economic analyses are reported as the following outcomes:
-
health-care costs to the NHS over 12 months
-
direct and indirect costs to the participant and main caregiver
-
average total frequency of symptomatic antibiotic-treated UTI over 12 months
-
QALYs estimated by the Short Form questionnaire-6 Dimensions (SF-6D) derived from responses to the SF-36v2 over 12 months
-
regression models estimating the key predictors of costs and QALYs (such as age and sex) to inform calculation of incremental costs and QALYs
-
average monetary value of participants’ WTP to avoid one episode of UTI over 12 months.
When appropriate, secondary analyses using multiple imputation were applied to deal with missing data and assess the implications of this for the overall results.
Methods
This analysis has been designed and conducted to best practice conforming to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 49
NHS resource use and costs to the participant and their main caregiver
Data collection
Health service use
Data on use of secondary and primary care NHS services were collected from the health service utilisation questionnaire completed by participants at 6 and 12 months post randomisation [see Questionnaire (10 Jan 2018) available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. This instrument provided information on inpatient stays, outpatient visits, GP appointments and appointments with nursing staff. Additionally, medication costs in terms of prescribed antibiotics used for the treatment of UTI episodes and use of antibiotic prophylaxis were collected from CRFs completed by local trial staff at 3, 6, 9 and 12 months, and patient-completed UTI records [see Questionnaire (10 Jan 2018), Participant 3 Monthly Questionnaire available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. Information on all antibiotic use was collated within the primary outcome review carried out by central trial staff. In order to avoid potential double-counting of antibiotic use, the data file derived from the primary outcome review was used as the main data source for totalling antibiotic use and attributing costs.
Participant and caregiver costs
In the health service utilisation questionnaire completed at 6 and 12 months, participants gave details of any payment for private health-care costs. The time and travel questionnaire completed by participants at 12 months asked about out-of-pocket expenses relating to the attendance of their most recent appointment [see Questionnaire (10 Jan 2018), Time and Travel Questionnaire available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. These costs were then applied to one journey (their most recent appointment) made by a participant and/or their main caregiver for health care and multiplied by the number of appointments with journeys recorded over the 12-month trial period (using information collected from the health service utilisation questionnaire). In the time and travel questionnaire, participants also gave details about the main activities they, as well as their accompanying carers/relatives, would otherwise be undertaking if they had not attended appointments. This method allowed participant and caregiver costs to be separated into out-of-pocket payments for transportation and time costs of travelling and attending health-care appointments.
Derivation of costs
Patient-level data on resource use were combined with national unit costs. These unit costs were categorised into the following [see Health Economics Plan (10 Jan 2018), Table 1 available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]:
-
inpatient costs
-
outpatient costs
-
primary care costs
-
medication costs
-
direct and indirect costs to participants and their main caregiver.
Inpatient costs
In the health utilisation questionnaire completed at 6 and 12 months, participants detailed how many nights they had spent in hospital over the previous 3 months. To account for variation of NHS costs with length of stay, three different unit costs were applied, for stays of 1 night, 2–7 nights or ≥ 8 nights [see Health Economics Plan (10 Jan 2018), Table 1 available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. The cost of the first overnight stay was assumed to be highest as it involves assessment, diagnosis and frequent monitoring. The costs for nights 2–7 are based on the cost per night for a 7-night stay. The cost a stay of ≥ 8 nights is based on the cost of excess bed-days beyond 7 nights. All unit costs were obtained from freely available NHS sources. 50 The count of the number of nights participants spent in hospital over the 3-month recollected period was multiplied by the relevant cost per night. This total cost for the previous 3 months, detailed in the 6-month and 12-month questionnaires, was then doubled to provide an estimate for the 6-month total inpatient-stay cost. The cost for each participant in each study group was then averaged to give a mean (SD) inpatient cost per participant for each trial group.
Outpatient costs
Outpatient costs were categorised as emergency visits such as attendance at urgent care centres and planned visits to a clinic that did not result in an overnight stay. Data collected through the participant-completed health service utilisation questionnaire at 6 and 12 months were combined with relevant unit costs obtained from NHS Reference Costs 2015-1650 [see Health Economics Plan (10 Jan 2018), Health economics supplementary information Table 1 available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)] to calculate a total cost per participant. The average cost per participant in each group of outpatient service use was then estimated.
Primary care costs
Primary care costs were categorised as consultations with GPs or nurse clinicians. These were subcategorised on the health service utilisation questionnaire according to how the consultation was given: health centre consultation, home visit, telephone consultation or out-of-hours consultation. Each type of consultation was associated with a different unit cost. The unit costs for use of primary care services were obtained from PSSRU (Personal Social Services Research Unit) unit costs of community care [see Health Economics Plan (10 Jan 2018), Health economics supplementary information, Table 2 available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018). 51 The average cost of primary care utilised by participants in each study group was then estimated.
Medication costs
Details of antibiotics prescribed to participants for the treatment or prevention of UTI were taken from the UTI record forms completed by participants at the time of each UTI episode over the 12-month follow-up. These details were also separately recorded at the 3-monthly review. Information on antibiotic use was collated from the different sources and checked as part of the clinical primary outcome review to avoid double-counting. The unit cost of each antibiotic was obtained from the British National Formulary52 according to route of administration and dosage recorded in trial CRFs. Use of antibiotics for UTI prevention by participants allocated to the prophylaxis group was collected through the 3-monthly review CRFs. The daily dosage of the three antibiotics used for prophylaxis was 50 mg of nitrofurantoin, 100 mg of trimethoprim and 250 mg of cefalexin over 12 months. All data on antibiotic agent, dose and duration of use for treatment or prevention of UTI were combined with the associated unit cost [see Health Economics Plan (10 Jan 2018), Health economics supplementary information, Tables 3a and 3b available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. This was added to the total cost per participant and the average cost per trial group of antibiotic medication.
Costs to participants and their main caregiver
Costs to participants were defined as any direct purchase of health care such as prescription charges, over-the-counter medication and the time and travel costs of accessing health care. Responses to the question about payment for private health-care services in the health service utilisation questionnaire were used to estimate the total cost of private health care. The total costs per participant and average cost per participant of private health-care costs were estimated by adding the total private health-care costs over the 12 months for each participant and dividing by the total number of participants in each trial group.
In the time and travel questionnaire, participants indicated out-of-pocket expenses for travelling to health-care appointments and what their usual activities would have been if they had not been travelling to, or attending, medical appointments or receiving medical treatments. Data on mode of transportation were combined with routine data sources, when necessary, to derive the total cost of a return journey for travelling and accessing health-care services. 53 The total cost per participant and average travel cost per participant were then estimated.
Time costs were estimated for participants and, if they also attended appointments with the participant, their main caregiver. The cost of time of paid activities was valued at the national median wage rate per hour. 54 The cost of time of unpaid activities was valued at the national rate of non-working time. 55 Additionally, the time and travel questionnaire asked for the number of days absent from paid employment due to health problems. The national median wage rate per week divided by 37.5 (the average working week in hours) was used to provide an estimate of the participant’s hourly wage rate. The average loss in earnings due to health problems (for participants and carers) was calculated by summing the total number of days absent from paid employment over the 12-month period and dividing this by the total number of participants, by trial group. Full details on the time and travel costs to participants and their main carers are given in Health Economics Plan (10 Jan 2018); Questionnaire (10 Jan 2018), Health Service Utilisation Questionnaire; and Questionnaire (10 Jan 2018), Time and Travel Questionnaire [all available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)].
Estimation of effects
For the CEA, health outcomes were measured in natural units: UTIs avoided. For the CUA, outcomes were measured by QALYs. In the CBA, costs and benefits were valued in commensurate units [Great British pounds (GBP)]. The three subsections below describe how the ‘benefits’ side of the economic evaluation was estimated for the CEA, CUA and CBA, respectively.
Estimation of health outcomes for the cost-effectiveness analysis
The clinical effectiveness estimates used in the CEA used the same approach described in Chapter 2 for calculating the primary outcome. An average total frequency of symptomatic antibiotic-treated UTI between the trial groups over 12 months was calculated by taking the total number of symptomatic antibiotic-treated UTIs recorded by each trial participant and dividing by the total number of participants in each trial group.
Estimation of quality-adjusted life-years for the cost–utility analysis
Responses to the SF-36v2 1-week recall questionnaire were used as the basis to calculate QALYs. The SF-36v2 questionnaire was completed by participants at fixed time points (baseline and 6 and 12 months) and within the first 2 days of each episode of symptomatic antibiotic-treated UTI. The completion of the SF-36v2 was prompted by telephone, e-mail or text message every time a UTI episode was reported by either the participant or the treating clinician.
The responses to the SF-36v2 were mapped onto the SF-6D using a standard algorithm to generate utility values. 56 These utility values have a range of 0 (dead) to 1 (perfect health), with the utility value associated with suffering a UTI falling between within these boundaries. QALY values were estimated for each trial participant by using the area under the curve. 57
In order to capture a true reflection of the impact of a UTI on quality of life (QoL), we incorporated the immediate impact on QoL of having a UTI within our secondary CUA. The primary analysis incorporated QALYs calculated at the predefined intervals (baseline and then at 6 and 12 months’ follow-up). The secondary analysis incorporated an adjusted QALY calculation by including the SF-6D score when a UTI was reported. A 5-day duration was assumed for the UTI SF-6D score before symptoms start to resolve.
Estimation of willingness to pay for use in the cost–benefit analysis
Contingent valuation, a stated preference method used to attribute monetary values to health outcomes, was used to inform the CBA. 58 The contingent valuation questionnaire [see Questionnaire (10 Jan 2018) available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)] administered at 13 months’ follow-up collected information on the maximum amount (as a one-off payment) that participants would be willing to pay to avoid a UTI episode over a 12-month time period. For a given level of income, the higher the monetary value that individuals would be willing to pay, the greater the benefit that participants believe they would experience. This method enabled us to place a monetary value on the health outcome, going beyond the QALY framework, and also to conduct a CBA.
Analysis of costs and ‘benefits’
Costs
Regression techniques were applied to the data on total costs per participant (costs for the NHS and PSS, costs to the participant and caregiver, and overall costs) to identify whether or not there is a difference between trial groups, with control for potentially modifying factors such as age and sex (and also identify the key predictors of costs). An ordinary least-squares regression technique was applied (Equation 1):
In Equation 1, a dummy variable for the trial group estimates the difference in costs between the groups controlling for all other factors in the model. Estimated beta values describe the direction and magnitude of the relationship between each variable and the dependent variable. For example, if the dummy is specified as intervention = 1 and current practice = 0 and the beta estimate of the cost coefficient is + 500, this indicates that the intervention (coded as 1 in the dummy) is £500 more costly, on average, controlling for all other factors. If the coefficient was negative, then the intervention would, on average, be £500 cheaper than current practice.
Quality-adjusted life-years
As with costs, regression techniques were carried out to derive the drivers of the difference in QALYs between the groups of the trial after controlling for the key predictors of QALYs. The purpose of this regression was to determine the consequences of a UTI episode and its effect on a participant’s overall QoL (Equation 2):
The variables considered were:
-
Dependent variable:
-
QALY (total QALY score across the two groups of the trial controlling for the independent factors).
-
-
Independent variables:
-
age (A) in years and months
-
sex (S)
-
SF-6D score (SF) (baseline SF-36v2 score)
-
trial group (T) (dummy for the group of the trial to which the respondent was randomised).
-
Here, the dummy variable for the trial group estimates the difference in QALYs between the groups controlling for all other factors in the model. For example, if the dummy is specified as intervention = 1 and current practice = 0 and the beta value of the coefficient is + 0.50. this indicates that the intervention (coded as 1 in the dummy) provided 0.50 more QALYs over 12 months than current practice, on average, after controlling for all other factors. If the coefficient was negative then the intervention would, on average, provide a lower QoL than current practice.
Willingness to pay
Willingness-to-pay data are presented as mean WTP to avoid a single UTI episode over a 12-month period. The total benefits per individual are the maximum WTP to avoid one UTI episode measured in GBP, multiplied by the total count of primary outcome UTI episodes experienced by the individual over the 12-month follow-up period. As previously described, a regression model of individual covariates on WTP was specified. Data on costs and benefits (as measured via the contingent valuation survey) were combined in order to calculate the net benefit. A net benefit in terms of NHS costs and benefits, expressed in commensurate units, was calculated for each participant according to Equation 3:
where i = individual and WTP = maximum WTP threshold to avoid a UTI episode.
Therefore, the decision rule for CBA is relatively simple: if the total benefits per individual are more than the costs, this represents a gain in welfare and the strategy is deemed worthwhile. 58 Results are presented as incremental net benefits (net benefits = mean cost of intervention – mean total monetary benefits). Both stochastic and deterministic sensitivity analyses were conducted and the results presented as incremental net benefit curves.
Comparative incremental analyses of costs and outcomes between trial groups
Cost-effectiveness analysis
The CEA is the base-case economic analysis. It takes the form of a standard two-group CEA using symptomatic UTI avoided as the primary outcome measure at 12 months. Costs associated with the prophylaxis and no-prophylaxis strategies including cost of harms comprise treatment costs, health services utilisation over the 12 months’ follow-up and participant costs (as a sensitivity analysis) over the 12 months’ follow-up.
The cost and outcome regressions (detailed above) were run simultaneously using an approach referred to as a seemingly unrelated regression system. At first look, the equations seem unrelated, but the equations are related through the correlation in the errors. With this approach, the two linear regressions were estimated for the costs and count of UTI defined with a contemporaneous cross-equation error correlation (i.e. the error terms in the regression equations are correlated), given that the cost and outcome data are derived from the same individual. This analysis was conducted in Stata® (StataCorp LP, College Station, TX, USA) using the seemingly unrelated regression command sureg.
Cost–utility analysis
The CUA measured incremental cost per QALY gained through completion of SF-36v2 at baseline and 6 and 12 months. We also measured incremental cost per QALY gained incorporating the repeated completion of SF-36v2 each time a UTI was reported. This analysis also adopted a seemingly unrelated regression system.
Cost–benefit analysis
Data on costs and benefits derived from multiplying the maximum WTP to avoid one UTI over a 12-month period with the total number of UTIs per participant were combined and used to derive a net benefit value for each individual. Given that costs and outcomes were combined into a single net benefit equation, only one regression needed to be estimated. This controlled for other factors in the model, such as income, age and sex.
Missing data
Multiple imputation was used to estimate missing QALY values, controlling for randomised group allocation and age. 59 The pattern of missing SF-36v2 and health-care utilisation data was investigated to ascertain whether or not a clear pattern of missing data was observed. When no clear pattern of missing data was observed, we assumed that missing data were missing at random. 59
Sensitivity analysis
Deterministic sensitivity analysis
Deterministic sensitivity analysis was carried out to test for the effect of assumptions and variability, such as an exploration of alternative unit costs applied to the different resources used. A number of analyses were performed including one way and/or multiway, depending on the results obtained from the deterministic analysis. When appropriate, these analyses were combined with the stochastic sensitivity analysis.
In order to assess the impact on the overall results, the costs to patient and carers were added to the NHS costs in the primary CEA. In the CUA, in addition to the analyses conducted for the base case, we also assessed the impact of taking into account the disutility arising from episodes of UTI.
Stochastic sensitivity analysis
Non-parametric bootstrapping-simulations that allow a comparison of arithmetic means without making assumptions about the distribution of the costs, UTIs avoided, QALYs and net benefit (using WTP estimates) is a data-based simulation method for assessing statistical precision. Bootstrapping is based on how values of the within-trial cost per UTI avoided, QALY and net benefit would vary if the sampling process could be repeated many times. Random values were selected from the cost, UTI, QALY and net benefit data collected from within the trial, with replacement (i.e. once a random value has been used for the bootstrap resample, it is put back into the original sample). This yields a bootstrap data set derived from the complete case data on costs and outcomes from each trial group.
For the bootstrapping, 1000 reiterations were carried out. This simulation process created a sample of bootstrapped means for costs, UTIs, QALYs and net benefit, with distributions for each. The means and other parametric statistics were then calculated for the bootstrap distribution. 60 Bootstrap estimates of the difference in costs, rate of UTI, QALYs and WTP between the experimental and control groups were used to populate the cost-effectiveness plane [the horizontal axis represents the difference in effectiveness (UTIs, QALYs or WTP) between two interventions and the vertical axis represents the corresponding difference in costs].
For cost-effectiveness and CUA, combining this information with the decision-maker’s maximum WTP over a range of values (e.g. £0 to £100,000 per UTI avoided or QALY) generated a cost-effectiveness acceptability curve (CEAC) quantifying the probability that an intervention is cost-effective based on the decision-maker’s maximum WTP. 60 For the CBA, a similar approach was used. Here, the results were presented as net benefit curves and the probability that the intervention has a net benefit > 0 and is, therefore, more efficient.
Results
Response rates
Table 19 reports the response rates for the participant-completed health economics data collection tools. As the table illustrates, there was a progressive loss to follow-up over the duration of the trial follow-up. Approximately 74% of participants in the two groups of the trial completed the 12-month health service utilisation questionnaire and approximately 62% of participants completed the SF-36v2 at 12 months. The pattern of non-response was similar across both groups of the trial and at all time points. However, only 62% of participants provided resource use data at 6 and 12 months’ follow-up and < 50% of the participants completed the SF-36v2 questionnaire at all three time points of baseline and 6 and 12 months. For the WTP exercise, 198 participants provided valid responses spread equally across the two groups. Prior to carrying out this exercise we considered that around 200 responses would be sufficient.
| Data response rates | Intervention group | |
|---|---|---|
| Prophylaxis (N = 203) | No prophylaxis (N = 201) | |
| Health utilisation | ||
| 6 months | 157 | 152 |
| 12 months | 146 | 152 |
| Complete data, 6 and 12 months | 140 | 131 |
| SF-36v2 | ||
| Baseline | 178 | 178 |
| 6 months | 137 | 139 |
| 12 months | 127 | 122 |
| Complete data, baseline, 6 and 12 months | 96 | 93 |
| WTP | ||
| 13 months | 101 | 97 |
Resource use and costs
Over the 12 months of the trial, follow-up participants in both trial groups made use of a variety of different types of health care. This included use of both primary and secondary care services provided by the NHS. The most common types of health care utilised included outpatient visits and consultations with both GPs and nurses (Table 20). As would be expected, a similar pattern was observed in terms of costs (Table 21). The exception to this is the comparative importance of secondary care cost components. Although comparatively fewer participants used hospital services, the unit cost of these services was substantially higher [see Health Economics Plan (10 Jan 2018), Health economics supplementary information, Table 1 available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)]. No formal testing was conducted to identify any statistically significant differences between randomised groups. As indicated by the SDs for those people who used a service, there was substantial variation in the quantity of health care utilised, suggesting that the CIs for the difference in costs between the trial groups for any area of health-care use would be very wide and include zero (see Table 20). Tables 20 and 21 present the mean and median for comparative purposes, but IQR has been omitted for brevity.
| Resource | Intervention group | |||||||
|---|---|---|---|---|---|---|---|---|
| Prophylaxis | No prophylaxis | |||||||
| Users, n | Mean | Median | SD | Users, n | Mean | Median | SD | |
| Inpatient days in hospital | 24 | 19.42 | 10.00 | 24.22 | 36 | 12.72 | 4.00 | 17.38 |
| Day case admissions | 38 | 13.53 | 2.00 | 41.83 | 42 | 5.29 | 2.00 | 6.87 |
| Outpatient visits | 103 | 14.83 | 6.00 | 27.88 | 94 | 14.13 | 8.00 | 22.74 |
| A&E visits | 21 | 11.14 | 2.00 | 37.84 | 18 | 2.44 | 2.00 | 1.76 |
| GP surgery consultations | 113 | 13.22 | 8.00 | 25.22 | 120 | 10.05 | 8.00 | 7.65 |
| GP home consultations | 12 | 3.33 | 4.00 | 2.15 | 12 | 6.17 | 2.00 | 7.46 |
| Nurse surgery consultations | 94 | 8.66 | 4.00 | 21.46 | 83 | 10.36 | 4.00 | 30.58 |
| Nurse home consultations | 20 | 33.90 | 4.00 | 64.02 | 14 | 40.57 | 5.00 | 94.62 |
| GP telephone consultations | 43 | 5.21 | 4.00 | 4.68 | 45 | 6.36 | 4.00 | 9.34 |
| Hospital doctor telephone consultations | 7 | 3.71 | 2.00 | 2.43 | 7 | 3.71 | 2.00 | 2.14 |
| Nurse telephone consultations | 26 | 5.54 | 3.00 | 7.38 | 27 | 5.04 | 4.00 | 5.56 |
| Telephone consultations with other HCPs | 13 | 12.15 | 4.00 | 29.51 | 16 | 4.50 | 4.00 | 3.14 |
| GP out-of-hours consultations | 6 | 4.00 | 3.00 | 2.53 | 5 | 3.20 | 2.00 | 2.68 |
| Hospital doctor out-of-hours consultations | 1 | 4.00 | 4.00 | NA | 4 | 1.50 | 1.00 | 1.91 |
| Nurse out-of-hours consultations | 2 | 2.00 | 2.00 | 0.00 | 8 | 4.50 | 2.00 | 5.42 |
| Out-of-hours consultations with other clinicians | 1 | 12.00 | 12.00 | NA | 5 | 1.60 | 2.00 | 1.67 |
| Resource | Total cost (£) across the two groups time period | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to 6 months | 6–12 months | |||||||||||
| Prophylaxis | No prophylaxis | Prophylaxis | No prophylaxis | |||||||||
| Mean | Median | SD | Mean | Median | SD | Mean | Median | SD | Mean | Median | SD | |
| Inpatient days in hospital | 5195.41 | 2301.20 | 4867.04 | 3497.14 | 2301.20 | 3119.96 | 4345.30 | 2301.20 | 4998.95 | 3561.53 | 1585.60 | 4857.78 |
| Day case admissions | 1100.00 | 704.00 | 1177.14 | 1944.38 | 704.00 | 2191.52 | 6281.85 | 704.00 | 17603.86 | 1243.73 | 704.00 | 1391.93 |
| Outpatient visits | 633.27 | 420.36 | 773.37 | 791.09 | 420.36 | 869.46 | 1270.63 | 630.54 | 2967.08 | 1019.76 | 630.54 | 2339.55 |
| A&E visits | 411.21 | 293.72 | 151.68 | 326.36 | 293.72 | 97.91 | 2327.17 | 293.72 | 7071.13 | 293.72 | 293.72 | 177.12 |
| GP surgery consultations | 188.61 | 144.00 | 148.61 | 221.25 | 144.00 | 163.60 | 364.32 | 180.00 | 940.87 | 215.30 | 216.00 | 136.20 |
| GP home consultations | 148.20 | 88.92 | 102.68 | 160.06 | 177.84 | 74.40 | 133.38 | 177.84 | 86.41 | 248.98 | 88.92 | 277.42 |
| Nurse surgery consultations | 58.91 | 43.20 | 81.40 | 40.72 | 21.60 | 28.19 | 76.04 | 43.20 | 247.49 | 115.32 | 43.20 | 384.05 |
| Nurse home consultations | 280.00 | 72.00 | 551.51 | 486.00 | 72.00 | 1032.08 | 744.92 | 144.00 | 1281.96 | 609.00 | 54.00 | 1275.70 |
| GP telephone consultations | 53.84 | 28.80 | 36.12 | 53.88 | 28.80 | 39.87 | 68.52 | 57.60 | 59.83 | 90.67 | 57.60 | 132.39 |
| Hospital doctor telephone consultations | 28.80 | 28.80 | NA | 43.20 | 28.80 | 28.80 | 57.60 | 43.20 | 36.43 | 67.20 | 86.40 | 33.26 |
| Nurse telephone consultations | 18.51 | 12.96 | 11.17 | 14.10 | 8.64 | 9.64 | 24.19 | 8.64 | 31.19 | 24.59 | 17.28 | 22.54 |
| Telephone consultations with other HCPs | 44.00 | 48.00 | 18.07 | 39.27 | 48.00 | 26.88 | 204.00 | 36.00 | 451.74 | 54.00 | 60.00 | 37.95 |
| GP out-of-hours consultations | 178.88 | 134.16 | 77.46 | 134.16 | 134.16 | 0.00 | 214.66 | 134.16 | 120.00 | 268.32 | 134.16 | 232.37 |
| Hospital doctor out-of-hours consultations | 0.00 | 0.00 | NA | 210.18 | 210.18 | 210.18 | 420.36 | 420.36 | NA | 0.00 | 0.00 | NA |
| Nurse out-of-hours consultations | 21.60 | 21.60 | NA | 10.80 | 10.80 | 15.27 | 21.60 | 21.60 | NA | 61.20 | 43.20 | 63.22 |
| Out-of-hours consultations with other HCPs | 0.00 | 0.00 | NA | 210.18 | 210.18 | 210.18 | 1261.08 | 1261.08 | NA | 105.09 | 105.09 | 148.62 |
| Out-of-pocket expenses for private health care | 810.46 | 810.46 | 863.32 | 712.00 | 340.00 | 895.63 | 1387.99 | 370.16 | 2465.22 | 1185.71 | 280.00 | 1892.70 |
As would be expected, the average cost of prophylactic antibiotics was higher in the prophylaxis group (Table 22). However, the average cost of antibiotics to treat a UTI was higher in the no-prophylaxis group. Nevertheless, the SDs are large and, although we did not formally test for the possibility, it is unlikely that there would be any significant differences in cost between the two groups.
| Variable | Antibiotics for | |||
|---|---|---|---|---|
| Prophylaxis use | Treatment of UTIa | |||
| Prophylaxis | No prophylaxisb | Prophylaxis | No prophylaxis | |
| Total number of participants | 203 | 25 | 160 | 178 |
| Total number of antibiotics | 3 | 3 | 29 | 29 |
| Mean cost (£) per participant (SD) | 60.80 (72.00) | 39.70 (40.80) | 23.70 (50.10) | 40.90 (107.00) |
| Mean number of days on antibiotics per participant (SD) | 299 (110) | 135 (93) | 15 (17) | 26 (27) |
| Mean number of antibiotics types used for UTI treatment per participant (SD) | NA | NA | 2 (2.2) | 3.3 (3) |
Table 23 reports the total costs to the NHS both with and without the costs of antibiotics. It shows that the antibiotic costs constituted a small proportion of total costs. The difference in overall average total costs for each trial group is < £100. However, the SDs are large in comparison with the magnitude of total costs. The summary data also show that costs are highly skewed to the right, indicating that there are a few participants with very high costs.
| To the NHS | Intervention group, total cost (£) | |||||
|---|---|---|---|---|---|---|
| Prophylaxis (n = 140) | No prophylaxis (n = 131) | |||||
| Mean | Median | SD | Mean | Median | SD | |
| Over 12 months | 3539 | 1106 | 7809 | 3443 | 1278 | 5260 |
| Between baseline and 6 months | 1245 | 357 | 2692 | 1570 | 533 | 2905 |
| Between 6 and 12 months | 2294 | 567 | 6696 | 1873 | 631 | 3664 |
| Including UTI antibiotics between baseline and 12 months | 3555 | 1106 | 7818 | 3490 | 1390 | 5269 |
| Including UTI antibiotics and prophylaxis antibiotics between baseline and 12 months | 3615 | 1217 | 7816 | 3497 | 1427 | 5270 |
Outcomes
On average, participants in both groups of the trial rated their status as approximately 65% of full health over the 12-month follow-up period (Table 24). Adding QALY data derived from the SF-36v2 completed at the time of UTI reduced mean QALYs in both groups, but the impact was modest. The SF-6D scores at each time point and QALYs were, on average, broadly similar, but the differences in mean scores were not formally tested statistically.
| Outcome measure and time point | Intervention group | |||||
|---|---|---|---|---|---|---|
| Prophylaxis | No prophylaxis | |||||
| n | Mean | SD | n | Mean | SD | |
| SF-6D | ||||||
| At baseline | 178 | 0.668 | 0.168 | 178 | 0.663 | 0.145 |
| At 6 months | 137 | 0.659 | 0.168 | 139 | 0.644 | 0.152 |
| At 12 months | 127 | 0.649 | 0.182 | 122 | 0.643 | 0.155 |
| QALYs | ||||||
| From baseline to 6 months | 121 | 0.335 | 0.079 | 124 | 0.329 | 0.069 |
| From 6 to 12 months | 108 | 0.333 | 0.085 | 104 | 0.323 | 0.075 |
| From baseline to 12 months | 96 | 0.676 | 0.162 | 93 | 0.652 | 0.147 |
| From baseline to 6 months and utility impact of UTI and no multiple imputation | 130 | 0.302 | 0.137 | 125 | 0.301 | 0.127 |
| From 6 to 12 months and utility impact of UTI and no multiple imputation | 95 | 0.333 | 0.088 | 78 | 0.329 | 0.076 |
| From baseline to 6 months and utility impact of UTI and no multiple imputation | 93 | 0.641 | 0.205 | 74 | 0.650 | 0.170 |
On average, WTP was higher in the prophylaxis group than in the no-prophylaxis group. A similar pattern is observed for median costs, although they are lower than the mean costs, for both study groups (Table 25).
| Intervention group, WTP (£) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prophylaxis (n = 101) | No prophylaxis (n = 97) | ||||||||
| Mean | Median | SD | Maximum WTP value | Minimum WTP value | Mean | Median | SD | Maximum WTP value | Minimum WTP value |
| 158.20 | 100.00 | 214.16 | 5.00 | 1000.00 | 108.56 | 50.00 | 168.42 | 10.00 | 1000.00 |
Economic evaluation
Incremental cost-effectiveness analysis
In the unadjusted CEA, the strategy of antibiotic prophylaxis was more effective but also more costly than the strategy of no prophylaxis, with an incremental cost per UTI avoided of £99. A similar pattern was observed for the adjusted analysis although the incremental cost per UTI avoided was reduced to £33 (Table 26). How much a decision-maker believes one avoided UTI is worth in terms of patient benefit is unclear. However, some guidance can be provided by considering the incremental cost per UTI avoided in the light of the WTP data. As indicated in Table 26, the mean amount that patients are willing to pay to avoid one UTI is > £100, and this suggests that the threshold value may be greater than the incremental costs per UTI avoided.
| Investigation strategy | Cost (95% CI), £ | Incremental cost (95% CI), £a | Effect (95% CI) | Incremental effect (95% CI)a | ICER, £ | Probability that prophylaxis is cost-effective for different threshold values for society’s WTP to avoid one UTI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £200 | £500 | £1000 | £2000 | ||||||
| Primary outcome UTI: results | ||||||||||
| No prophylaxis (observations: cost n = 131/outcomes n = 180) | 3496.73 (2585.87 to 4407.59) | 118.72 | 2.50 (2.17 to 2.83) | –1.20 | 98.79 | 0.501 | 0.399 | 0.235 | 0.086 | 0.008 |
| Prophylaxis (observations: cost n = 140/outcomes n = 181) | 3615.44 (2309.43 to 4921.46) | 1.30 (1.07 to 1.53) | 0.499 | 0.601 | 0.765 | 0.914 | 0.992 | |||
| Adjusted analyses (observations n = 271) | 49.28 (–1515.45 to 1619.88) | –1.50 (1.00 to 2.01) | 32.96 | 0.507 | 0.656 | 0.821 | 0.952 | 0.998 | ||
| Primary outcome UTI patient and carer perspective: results | ||||||||||
| No prophylaxis (observations: cost n = 131/outcomes n = 201) | 4267.47 (3233.58 to 5301.36) | 114.52 | 2.50 (2.17 to 2.83) | –1.20 | 95.43 | 0.508 | 0.418 | 0.276 | 0.127 | 0.013 |
| Prophylaxis (observations: cost n = 140/outcomes n = 203) | 4381.99 (2996.62 to 5767.36) | 1.30 (1.07 to 1.53) | 0.492 | 0.582 | 0.724 | 0.873 | 0.987 | |||
| Adjusted analyses (observations n = 271) | 47.38 (–1654.76 to 1749.53) | –1.50 (1.00 to 2.01) | 31.59 | 0.476 | 0.620 | 0.782 | 0.950 | 0.998 | ||
The deterministic results alone are not sufficient to support decision-making. For this, we need to consider the imprecision surrounding estimates of costs, effects and cost-effectiveness and, for this, the probabilistic sensitivity analysis is used. Figure 8 shows the distribution of the cost and effectiveness pairs simulated by bootstrapping the result of the CEA. As this plot illustrates, for 100% of the bootstrap iterations, prophylaxis is associated with fewer UTIs. The picture for cost is more mixed, with a roughly even chance that prophylaxis would be cost saving. The results also show (Figure 9 and Table 27) that there is a 66% chance that prophylaxis would be more cost-effective than no prophylaxis should society be willing to pay £200 to avoid one UTI and that the more we are willing to pay, the more likely it is that prophylaxis would be cost-effective. However, these results are not conclusive, as there was marked variability in costs between participants..
FIGURE 8.
Cost-effectiveness plane for prophylaxis vs. no prophylaxis: adjusted bootstrapped replications for CEA.
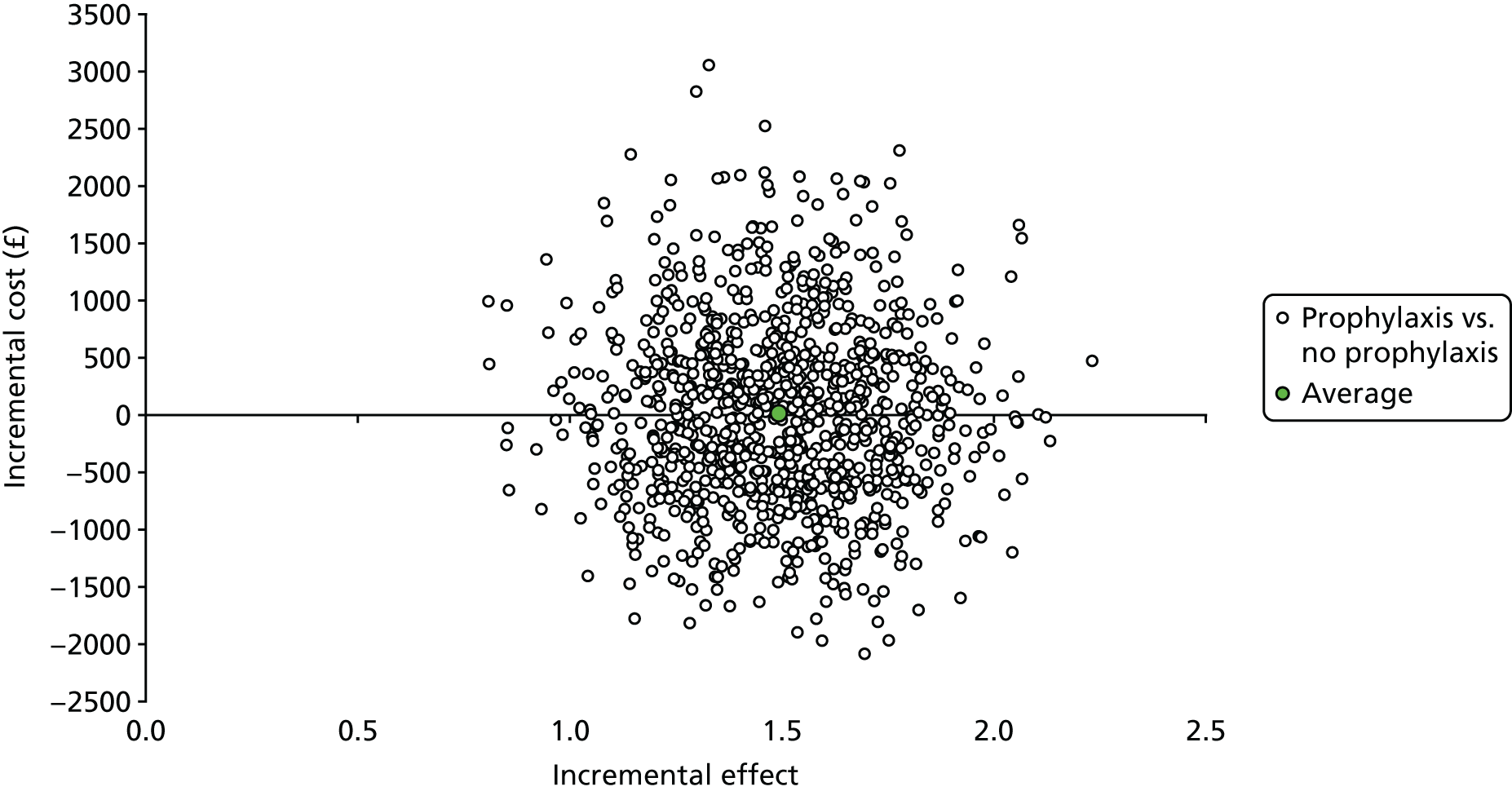
FIGURE 9.
Cost-effectiveness acceptability curves for prophylaxis vs. no prophylaxis: adjusted bootstrapped replications for CEA.
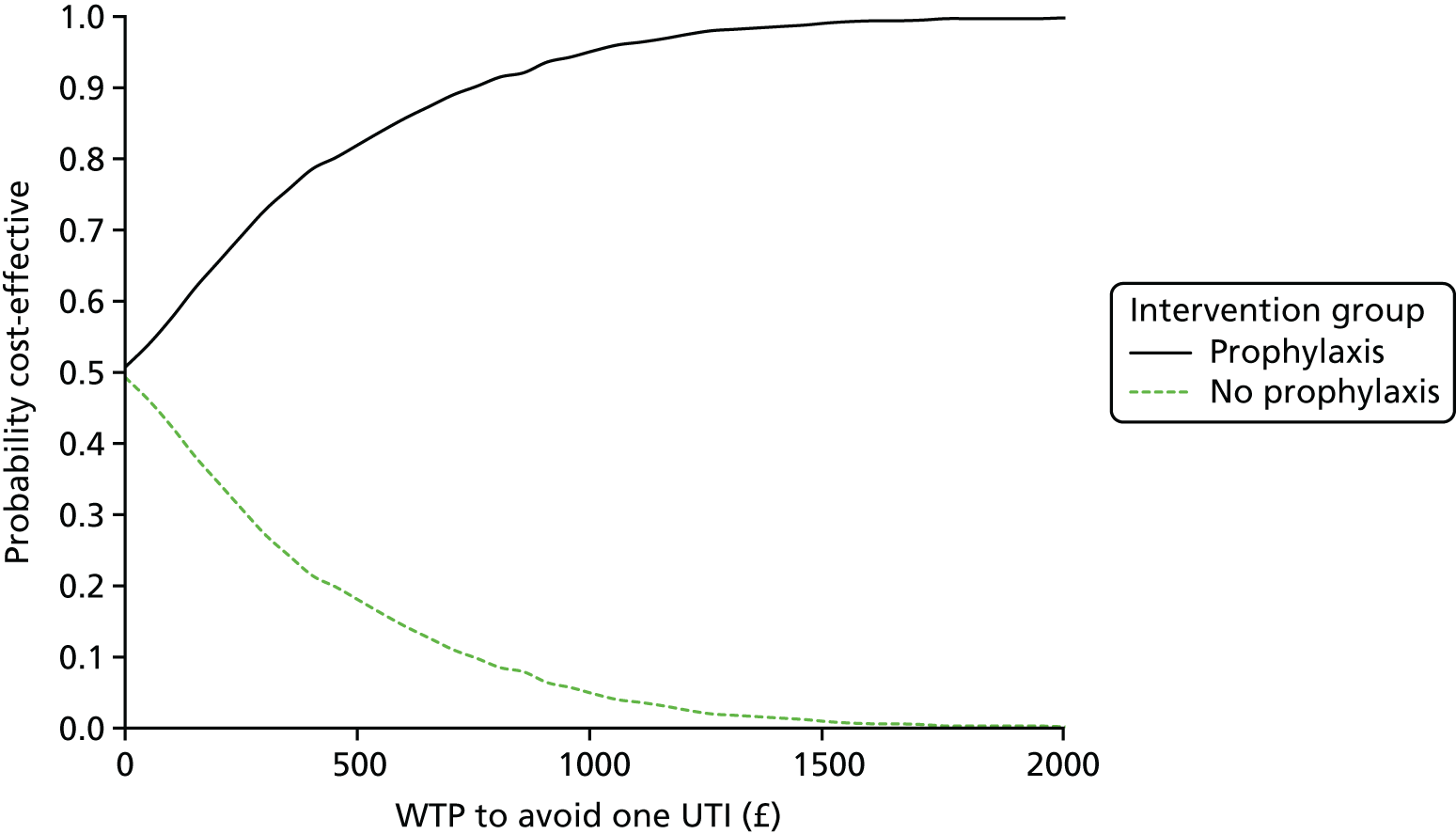
| Investigation strategy | Cost (95% CI), £ | Incremental cost (95% CI), £ | Effect (95% CI) | Incremental effect (95% CI)a | ICER (£) | Probability that prophylaxis is cost-effective for different threshold values for society’s WTP for a QALY | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| SF-36v2 results | ||||||||||
| No prophylaxis (observations: cost n = 131/outcomes n = 93) | 3496.73 (2585.87 to 4407.59) | 118.72 | 0.652 (0.622 to 0.682) | 0.023 | 5059 | 0.521 | 0.441 | 0.362 | 0.309 | 0.212 |
| Prophylaxis (observations: cost n = 140/outcomes n = 96) | 3615.44 (2309.43 to 4921.46) | 0.676 (0.643 to 0.708) | 0.479 | 0.559 | 0.638 | 0.691 | 0.788 | |||
| Adjusted analyses (observations n = 185) | –53.34 (–2136.49 to 2029.81) | 0.015 (–0.004 to 0.034) | Prophylaxis dominant | 0.540 | 0.598 | 0.646 | 0.688 | 0.743 | ||
| SF-36v2 results with UTI-specific SF-36v2 and multiple imputation | ||||||||||
| No prophylaxis (observations: cost n = 131/outcomes n = 201) | 3496.73 (2585.87 to 4407.59) | 118.72 | 0.640 (0.621 to 0.659) | 0.010 | 12,452 | 0.521 | 0.465 | 0.399 | 0.356 | 0.282 |
| Prophylaxis (observations: cost n = 140/outcomes n = 203) | 3615.44 (2309.43 to 4921.46) | 0.650 (0.628 to 0.671) | 0.479 | 0.535 | 0.601 | 0.644 | 0.718 | |||
| Adjusted analyses (observations n = 271) | 49.28 (–1515.45 to 1619.88) | 0.009 (–0.007 to 0.025) | 5481 | 0.504 | 0.545 | 0.580 | 0.616 | 0.672 | ||
Overall, the cost-effectiveness results taking the participant and carer perspective derived from the time and travel questionnaire do not change the main findings (see Table 26). Given that the average total number of contacts with NHS services is higher for prophylaxis, the average costs taking into account the costs to patients and carers are also higher in this group. There is now a reduced chance that prophylaxis would be cost saving, taking the patient and carer perspective, given that the observed outcomes (number of UTIs) do not change across the two groups, but average costs increase across both groups (but to a lesser degree for the no-prophylaxis group).
Incremental cost per quality-adjusted life-year gained
The results of the CUA detailed in Table 27 show the incremental cost per QALY gained. For both the unadjusted and the adjusted analysis, the mean QALYs from prophylaxis are greater than the mean QALYs from no prophylaxis. In the unadjusted analysis, prophylaxis is, on average, more costly, contrasting with the adjusted analysis in which the prophylaxis strategy is less costly (although the CIs around mean costs are very wide). Therefore, the incremental cost per QALY gained is less than conventional thresholds for society’s WTP for a QALY (£20,000–30,000). 61 In the unadjusted analysis and in the adjusted analysis, the prophylaxis group is dominant (less costly and more effective on average); nevertheless, these deterministic results are insufficient to guide decision-making. The results of the probabilistic analysis suggest that there is approximately a 65% chance that prophylaxis treatment would be considered cost-effective at £20,000 per QALY (see Table 27) [see Health Economics Plan (10 Jan 2018), Health economics supplementary information, Figures 1 and 3, available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)].
When the CUA was repeated taking into account the impact on QALYs of UTI together with imputation of missing data, the result in terms of incremental cost per QALY gained was similar (Figure 10 and see Table 27). In both analyses (adjusted and unadjusted), the incremental cost per QALY gained was less than typical values for WTP for a QALY. Similarly, in the probabilistic analysis there was a lower probability that use of prophylaxis would be considered cost-effective at the £20,000 threshold value (58–60%) (see Table 26) [see Health Economics Plan (10 Jan 2018), Health economics supplementary information, Figures 2 and 3, available at www.journalslibrary.nihr.ac.uk/programmes/hta/117201/#/documentation (accessed 3 April 2018)].
FIGURE 10.
Cost-effectiveness plane for prophylaxis vs. no prophylaxis: adjusted bootstrapped replications for primary CUA.

Cost–benefit analysis
In the CBA, the prophylaxis strategy was, again, costlier on average than no prophylaxis (Table 28). However, it was associated with a higher average net WTP to avoid UTIs. As before, the estimates of costs and effect were imprecise. The degree of imprecision was investigated using probabilistic sensitivity analysis (Figure 11 and see Table 28). This showed the comparatively wider spread in incremental benefits. This appears to be a consequence of combining the estimates of UTIs avoided with estimates of WTP as both variables have a degree of statistical imprecision. The results of the probabilistic sensitivity analysis suggest that the strategy of prophylaxis has a 66% chance of being considered more efficient than that of no prophylaxis (in the adjusted analysis).
| Investigation strategy | Cost (95% CI), £ | Incremental cost (95% CI), £ | Effecta (CI) | Incremental effectb (95% CI) | Incremental net benefit, £c | Probability that prophylaxis has a higher net benefit |
|---|---|---|---|---|---|---|
| Maximum WTP to avoid one UTI: results | ||||||
| No prophylaxis (observations: cost n = 131/outcomes n = 115) | 3496.73 (2585.87 to 4407.59) | 118.72 | –335.52 (–515.67 to –155.38) | 174.54 | 55.82 | |
| Prophylaxis (observations: cost n = 140/outcomes n = 123) | 3615.44 (2309.43 to 4921.46) | –160.98 (–233.76 to –88.20) | ||||
| Adjusted analyses (observations n = 202) | –85.66 (–1943.33 to 1772.01) | 208.72 (1.49 to 415.94) | Prophylaxis dominant | 0.66 | ||
FIGURE 11.
Cost–benefit plane for prophylaxis vs. no prophylaxis: adjusted bootstrapped replications for CBA.

Conclusion
The economic analyses, CEA, CUA and CBA, all suggest that, on average, a strategy of antibiotic prophylaxis is more effective and more costly than no prophylaxis. It is for decision-makers to decide if society is willing to pay the extra cost per extra unit of effect. This is true for the CEA, CUA and CBA. In the last case, the results suggest that benefits are greater than cost, but for the health-care sector when overall budgets are fixed it is unclear what benefits would be lost from elsewhere should resources be reallocated. The imprecision surrounding these results is high, but generally the balance of probabilities is in favour of prophylaxis; however, there is no evidence of a statistically significant difference at the 2.5% level (a typical level for a one-sided test, which is essentially what a CEAC portrays).
The probabilistic sensitivity analysis for the CEA shows that the prophylaxis group is promising: more effective but also more costly on average. Findings in terms of cost-effectiveness are not conclusive given that the CIs in the adjusted analysis cross zero for both the cost and effectiveness estimates.
The observed reduction in health status measured during episodes of UTI for the CUA was small, with QALYs presumably mostly determined by the generally poor underlying health of participants. Taking a decision-making approach, which focuses on which intervention has a higher probability of being cost-effective, assuming that society’s threshold value for an additional QALY is £20,000, there is a 65% probability of antibiotic prophylaxis being more efficient (as per the findings from the adjusted primary CUA).
The CBA supports the findings from the CEA in that, on average, the incremental net monetary benefits derived from a strategy of antibiotic prophylaxis are higher than no prophylaxis.
Summary
In summary, there is roughly an even chance that prophylaxis would be cost saving and a 66% chance that it would be more cost-effective than no prophylaxis should society be willing to pay ≥ £200 to avoid one UTI. Broadly, the likelihood of prophylaxis being considered cost-effective using conventional decision-making thresholds for the CUA and for the CBA were similar.
Chapter 5 Qualitative study
Introduction
Qualitative methods are increasingly being used within RCTs to explore the views and experiences of study participants. Data gathered can be an essential part of a trial’s evaluation and can highlight possible reasons for quantitative findings.
The specific aims were to explore participants’ views on:
-
the health, well-being and QoL issues related to both CISC and recurrent UTI
-
taking antibiotics for both prophylaxis and treatment
-
reasons for adherence and non-adherence to trial groups.
Methods
Design
A qualitative exploratory case-based study.
Participant recruitment and sampling
After completing the consent process for the main clinical trial, all identified eligible patients were given the further option of consenting to being approached by a qualitative researcher. Using information from the baseline questionnaire, those who consented to be approached for the qualitative study were purposively sampled to ensure that interviews were conducted with both men and women of various age groups in each group of the main trial. Participants identified in the sample were contacted and invited to undergo a telephone interview. If they were willing to be interviewed, they signed a consent form that was sent by post and a mutually convenient time to undertake the telephone interview was arranged.
Twenty-six interviews were completed between August 2015 and January 2016 with individual participants across seven study sites that were opened early in the recruitment period. The median (range) age was 56.5 years (25–81 years) and the sample comprised 15 females and 11 males. Reasons for, and duration of, CISC use are given in Table 29.
| Participant | Sex | Age (years) | Length of time (years) using CISC | Reason for use of CISC | Allocated study group |
|---|---|---|---|---|---|
| 1 | M | 54 | 5 | Spinal injury | P |
| 2 | M | 54 | 2 | MS and spinal injury | NP/Pa |
| 3 | F | 50 | 7 | MS | P |
| 4 | F | 59 | 4.5 | Overactive bladder and ulcerative colitis | NP |
| 5 | F | 54 | 15 | MS | NP/P/NPb |
| 6 | F | 72 | 3 | Overactive bladder | P |
| 7 | M | 68 | 2.5 | Urinary retention | NP |
| 8 | F | 46 | 10 | MS | NP |
| 9 | F | 29 | 26 | Spina bifida | P |
| 10 | M | 57 | 3.5 | MS | P |
| 11 | F | 53 | 5.5 | MS | P/NPc |
| 12 | F | 64 | 7 | Overactive bladder/stress incontinence | NP |
| 13 | F | 48 | 2.5 | Urinary retention | P |
| 14 | M | 67 | 2.5 | Urinary retention | P |
| 15 | M | 62 | 5 | Urinary retention | P |
| 16 | F | 51 | 3 | Prolapsed disc | P |
| 17 | M | 63 | 14 | Nerve damage from spinal column | NP |
| 18 | F | 81 | 3 | Urinary retention | NP |
| 19 | M | 51 | 3 | Transverse myelitis: paralysed from C6 down | P |
| 20 | M | 53 | 26 | Spina bifida | P |
| 21 | M | 71 | 2 | MS | NP |
| 22 | F | 69 | 4 | MS | NP |
| 23 | F | 63 | 10 | Overactive bladder post hysterectomy | P |
| 24 | F | 35 | 26 | Spina bifida (Mitrofanoff stoma) | P |
| 25 | M | 71 | 9 | Prolapsed disc | P |
| 26 | F | 25 | 20 | Mitrofanoff stoma | NP |
| Average | F: 15 | 56.5 | 8.5 | P: 15 | |
| M: 11 | NP: 11 |
Data collection
Semistructured interviews were conducted. A topic guide was used to ensure consistency while also allowing the participants to raise any other relevant issues.
Following discussions within the research team, the topic guide was designed to explore topics such as the experience and impact of using CISC on QoL, the sensory experience and impact of UTI, general illness self-management, health beliefs concerning antibiotics and the experience of taking part in the AnTIC trial (see Appendix 2, Box 1). All interviews were conducted by the researcher via telephone from a private meeting room at Glasgow Caledonian University offices following verbal confirmation of consent. Interviews were audio-recorded with a mean (range) duration of 35 minutes (17–59 minutes).
Analysis of the interviews
All interviews were audio-recorded and transcribed verbatim. Participant-identifiable information was removed from transcripts and files were saved using participants’ study identifications (IDs) (allocated at the time of randomisation) on a password-protected computer. The researcher checked all interview transcripts for accuracy and these were entered into NVivo v10 (QSR International, Warrington, UK) to be analysed through manual coding. Data were then subjected to thematic analysis to generate categories and themes appropriate to the original research questions following the six phases outlined by Braun and Clarke. 62
Team meetings were held regularly to discuss the analytical process and affirm key themes. At interview 24, it was reasoned that no new themes were apparent in the data. After discussion, it was agreed that two further interviews would be completed to satisfy the requirements for data saturation. 63 Therefore, data collection was completed after interview 26.
Results
The emotional and practical burden of CISC and UTIs was variable yet significant in participants’ lives and influenced both QoL and antibiotic use. The findings are presented under three broad categories: (1) impact on QoL, (2) views on antibiotics and (3) adherence to the AnTIC trial protocol.
‘P’ indicates that the participant was in the intervention group of the AnTIC trial (i.e. receiving low-dose prophylactic antibiotics) and ‘NP’ denotes the control or no-prophylaxis group. Participants are represented by their interview number, sex and study group.
Impact of clean intermittent self-catheterisation and urinary tract infection on quality of life
Normalisation and psychological adjustment: ‘just part of my daily life’
Participants detailed a range of experiences in relation to the impact of using CISC in their daily lives. There was a strong sense of considering the process of CISC as ‘normal’, which led to the acceptance of CISC as a bladder management tool. Many participants believed it to be a no greater burden than the way other people who do not use CISC pass urine and felt that using CISC had not changed their self-image. CISC was labelled as ‘just one of those things’ and assimilated with their own identity:
You just get on with it. To me, it’s just the same as going to the toilet. I don’t think about it really.
Participant 13, female, P
Positive appraisal of CISC intensified the perception of normalisation and eased both the physical and psychological burden of urinary problems. A number of individuals associated CISC with a new ‘lease of life’ that allowed them increased independence and personal autonomy. Two male participants were able to return to employment following CISC initiation and cited benefits such as increased financial security and increased self-esteem:
[CISC] has changed me altogether; it changed my life. In the first week I says ‘this is brilliant, best thing since coloured tellies came out, this is great, I can do what I want to do’. I don’t have a problem with it.
Participant 2, male, NP
The concept of normalisation was also closely linked to participants developing confidence and establishing routines with CISC. Incorporating the practicalities of CISC within their lifestyle enhanced psychological adjustment and often encouraged a favourable attitude towards the process. For many, this was characterised by a pragmatic and organised approach to daily living, helping protect against potential inconveniences, while being mindful of their own abilities:
I just take some [catheters] with me; it [CISC] doesn’t prevent me from getting out and about.
Participant 19, male, P
Although participants generally displayed psychological adjustment to CISC, there were also some who perceived it to pose a negative impact on their QoL. Examples included impeding their ability to enjoy social activities and relax with friends, and avoiding going abroad. One participant expressed his concerns at staying away from home owing to the potential embarrassment at disclosing CISC use and associated practicalities:
The biggest thing is, it’s [CISC] fine at home, it’s just a real pain when you’re not at home. I loath to have to stay overnight ‘cause it’s more of a hassle both for you . . . you don’t want to talk to your hosts about it, but you need reasonably unencumbered access to a bathroom for yourself.
Participant 15, male, P
Time to adapt: ‘it was fine once I got used to it’
A key contributor to the theme of normalisation was the length of time taken to adapt psychologically to using CISC daily. In general, participants acknowledged a change in perspective towards CISC from initial use to how they felt following a period of time (and/or at the time of interview). This prolonged period of adaptation was often preceded by initial resistance:
At first, I didn’t like them. It took me a wee while . . . to get used to what you’re actually doing. I would say it can take as much as a year before you’re comfortable – it takes a while to find your niche I think. Now, they’re just an absolute lifesaver, it’s wonderful!
Participant 3, female, P
A number of participants attributed their immediate aversion to CISC to lack of knowledge about their bodies and the CISC process:
I wasn’t [OK with CISC] because I had no idea what was involved. But now I’m much more comfortable with it, I’m very patient with the process . . . I’m never silly about it and it’s a question of just relaxing you know, and not worrying about it.
Participant 7, male, NP
Alongside the interaction of comorbidities and aversion to CISC in general, participant 16 also expressed the impact of using CISC on her intimate relationships. She described threats to her self-esteem as a direct consequence of using CISC, factors that proved challenging to overcome:
I think the biggest issue that both of us have is that we had a really good sex life before and that has been affected massively because obviously hygiene is paramount and if my back’s not good and with catheterising, oh it’s just awful really. They [catheters] irritate my skin so I get sore skin as well. So, if you’re having intercourse and your skin’s broken you’ve got all that worry as well as all that pain, so that’s difficult. It’s just like not one little thing, it’s a few things together.
Participant 16, female, P
Perceived burden of urinary tract infection, ‘so inconvenient and uncomfortable’
Experiencing UTI was reported as intrinsic to living with bladder problems and using CISC. Symptoms were perceived on a spectrum of severity depending on the individual, the frequency of UTI and perceived disruption on one’s everyday life. One participant described the personal burden of having UTI and the challenges to treatment provision:
. . . well firstly it’s [experiencing a UTI] a bit debilitating, and secondly, it’s a bit depressing, you know, psychologically it’s not good for you. It’s also just a hindrance trying to get a doctor’s appointment, it’s quite a hassle, you can’t guarantee you’re going to get it that quickly and then you’ve got to wait to get the antibiotics, so yeah it’s just a hassle.
Participant 15, male, P
A number of participants implied that, because UTIs were so prevalent in their lives, they were highly attuned to the preliminary stages and symptoms of UTI. For some, this included normalising the feeling and experience of having a UTI to the extent that they did not perceive UTIs to have a notable impact on their lives:
. . . a bit uncomfortable, but not debilitating.
Participant 14, male, P
For these individuals, the experience of having a UTI had been internalised as ‘normal’ and a likely consequence of their bladder problems and/or comorbidities. Having a UTI or, indeed, the potential to have a UTI, did not seem to impede socialising nor going on holidays, or affect their lifestyle.
For others, the ‘hassle’ of having a UTI was more pronounced, such that UTIs were perceived to have a substantial adverse impact on overall QoL. Specifically, in relation to exacerbating comorbidities (e.g. one participant explained that having a UTI can trigger a relapse of his multiple sclerosis), juggling work commitments (e.g. to allow for GP appointments and also the impact of mood on ability to work) and interfering with social activities (e.g. avoiding social gatherings with friends):
. . . you just feel you don’t want to go out.
Participant 22, female, NP
Combined with practical implications of being in the vicinity of public conveniences/toilets:
I need to be close to a toilet all day.
Participant 17, male, NP
Two participants (one male and one female) also voiced the impact of a UTI on their relationships, including the challenges to intimacy and expressing sexuality:
It obviously makes you feel bad about yourself and it makes you feel like you want to keep away from people, go to bed a bit early. It impacts your relationship with your wife, personally, sexually, because you don’t feel good about yourself, you’ve got an infection and it’s not particularly pleasant.
Participant 20, male, P
Views on antibiotics
Nonchalant attitude: ‘I feel fine about taking them’
Participants described a range of attitudes towards antibiotics and taking antibiotics. However, many reported feeling ‘fine’ about taking them with an overarching nonchalant perspective. Antibiotics were perceived as favourable in certain circumstances, particularly if they achieved the desired results or they were deemed necessary by medical professionals:
You just do it [take antibiotics]. If the results achieve what you want it to then you’ll do it.
Participant 3, female, P
Although a relaxed attitude towards taking antibiotics was common, many participants professed that they did not wish to take antibiotics unnecessarily. Instead, they preferred to trust medical opinion and rely on health-care professionals (HCPs) to assess the need for antibiotic treatment. Alternative self-management strategies for UTIs were used, including drinking more water or cranberry juice, or waiting to see if symptoms worsened.
Ambivalence towards antibiotic resistance: ‘I’m obviously aware, but you’ve got the down side to everything’
Participants conveyed mixed awareness and understanding of the concept of antibiotic resistance. A number of participants thought that ‘overusing’ antibiotics was unwise as it could reduce their future effectiveness. The possibility of the body becoming immune or resistant to antibiotics deterred some participants from taking antibiotics, although a minority did express strong concerns specific to the hypothetical development of bacterial pathogen resistance:
If you’re on antibiotics regularly the bacteria just forms resistance. I would only take them if I felt it was absolutely necessary – I think it might be counterproductive. I’m very wary about taking them, I think it’s a big, big worry at the minute.
Participant 7, male, NP
Participant 14 elaborated to say that he would rather take low-dose prophylactic antibiotics than a treatment course of high-dose antibiotics as he prefers the thought of preventing infections with them, as opposed to treating them. A number of individuals reiterated this notion of ‘little but often’ and disclosed a positive perception of prophylaxis.
Adherence to the AnTIC trial allocation (prophylaxis and no prophylaxis)
Habitual tendencies: ‘it’s just another tablet’
For those allocated to taking prophylaxis antibiotics, there was a general sense that this behaviour had become automatic and part of an established routine. Many participants were already taking medications for comorbidities, and, so, taking another tablet became an adjunct behaviour. ‘Adding on’ an additional tablet was perceived as trivial and relatively straightforward and there was a general sense that participants did not perceive this to be a challenge or pose any difficulties:
I just took it in the morning before I went to work; it was only one a day.
Participant 13, female, P
Another mechanism for incorporating prophylaxis consumption into daily life was by exploiting already habitual behaviour. Examples include taking the tablet before going to sleep at night. Some participants relied on partners to ensure that the prophylaxis was taken each day:
I’ve got 18 tablets a day so she’s [wife] got them all morning, afternoon, evening, tea time. And she moans if I miss them.
Participant 2, male, P
A minority of individuals confessed to occasionally forgetting to take the prophylaxis. These lapses were often attributed to mitigating circumstances such as fluctuating work patterns and lifestyle choices, alongside sheer forgetfulness. Not remembering to take the prophylaxis did not seem to cause concern or endure over time, but instead was assumed as a natural event in a year-long commitment such as the AnTIC trial.
Supportive accountability: ‘it was no bother’
Adhering to the prophylactic antibiotic therapy in the AnTIC trial was aided by the perception of supportive accountability. Participants enjoyed having the support of AnTIC trial researchers and research nurses at their disposal, and often cited them as helpful, informative and friendly:
I was on it [AnTIC trial] a year and it was as if someone was listening to me. And it was nice that they kept a check on me every 3 months.
Participant 21, male, NP
Participants also cited curiosity and personal altruism for continuing with the AnTIC trial, such that adhering to the protocol was perceived as ‘giving something back’ and ‘helping others’ that may be in a similar situation. A minority also professed that they were eager to see if prophylaxis antibiotics would help them personally by reducing UTI incidence.
Discussion
Summary of findings and implications
The findings demonstrate the variation of physical and psychological impact perceived among CISC users experiencing recurrent UTI and support previous literature64 that has shown CISC to contribute, in general, to a positive impact on QoL and enhanced dignity and self-esteem. The dynamics of how participants both perceived CISC and psychologically adapted to this form of bladder management were multiple and use of CISC often had a direct impact on their perception of QoL, whether restoring, maintaining or damaging. These findings show parallels with previous research that proposed two subcategories of positive and negative impacts on QoL of using CISC. 65 In the present study, all aspects of QoL that were discussed (whether favourable, neutral or negative) contributed to a general sense of normalisation of CISC and the process of acceptance, adaptation or maladaptation to the intricacies of using CISC.
However, there were some individuals in whom this psychological distress persisted over time, or at least to the time of interview. Despite accruing 4–15 years of experience with CISC, these individuals perceived CISC as a heavy burden (both practically and psychologically) and engaged in avoidance strategies such as decreasing water intake.
Although rates may vary depending on individual circumstances, previous research66 has demonstrated UTI incidence and experiences of UTI symptoms to be common in those who use CISC. Our findings validate the theory that the variety and magnitude of UTI symptoms, alongside the recorded incidence of UTI, can present a challenge to many individuals who use CISC and experience recurrent UTI. The experience of UTI was often perceived as a burden to lifestyle, working life and social life, particularly among those who experienced more than one per month. Yet psychological distress from recurrent UTIs remained individualised, with a spectrum of connotations from ‘an inconvenience’ through to ‘untenable’ and ‘depressing’. Timeliness of access to antibiotic prescription and a need for microbiological diagnosis complicated short-term psychological distress until symptoms were eased.
Another aspect that was important to this specific population concerned the interaction of UTI incidence and comorbidities. Although some lives were transformed for the better with the introduction of CISC, some participants also discussed the negative impact of recurrent UTI on exacerbating their underlying health condition. These relapses made CISC more of a challenge and were particularly pronounced in the early stages of a UTI. This has implications for HCPs who wish to provide patient-centred care and support those with neurological diseases and bladder problems, as, although CISC can have a positive impact on some, those with comorbidities may experience additional and unforeseen difficulties.
This study enhances the understanding of views on and attitudes towards the use of antibiotics specific to UTI treatment and prevention in those who use CISC and suffer recurrent UTI. Participants seemed unconcerned at the concept of taking antibiotics for UTI treatment on an ‘as needed’ basis. Discrepancies lay in the circumstances in which this was appropriate and whether a high dose or low dose was ‘more beneficial’. For those in the intervention (prophylaxis) group who experienced a subsequent reduction in UTI incidence and symptom severity, this altered their perspective on the use of continuous low-dose antibiotics. They tended to have a positive outlook towards taking prophylaxis long term to prevent future UTI episodes. These findings highlight that a positive perception of low-dose antibiotics may be related to having positive health experiences (i.e. reductions in UTI incidence and UTI symptom severity). This study is the first to consider users’ opinions and experiences with low-dose prophylactic antibiotics. However, the attitudes and perspectives towards antibiotics presented here are specific to a population that may have normalised antibiotic use, owing to the high incidence of recurrent UTI and, thus, the gravity of antibiotic need.
Despite fears concerning bacterial resistance after repeated antibiotic use, the interviewed participants conveyed ambivalence when discussing antimicrobial resistance. This aligns with previous research67 that has shown a sample of the general public to have concerns about the AEs of taking antibiotics. However, the present study highlights that those who use CISC and experience recurrent UTI deem low-dose prophylactic antibiotics as acceptable and may even prefer this option to taking high-dose antibiotics when an infection occurs.
Fears concerning resistance influenced whether or not individuals were happy to take antibiotics as continuous prophylaxis rather than discrete treatment courses, with differences in advice given by different clinicians obscuring these perceptions further. Although we were able to interview only CISC users motivated to take part in this trial, our findings are in keeping with previous research68 that demonstrated uncertainty in primary care patients specific to the nature, cause and implications of antibiotic resistance. Moreover, participants in the present study did not regard the potential risks of antibiotic use as influencing their present behaviour in terms of using antibiotics prophylactically, implying a psychological distance from the problem. This has important implications for prescribing practice: GPs and other HCPs have a duty to individualise care and provide accessible information to help patients understand the potential risks, particularly regarding development of antimicrobial resistance, and benefits so that they can make an informed choice about whether or not to consider taking low-dose antibiotic prophylaxis.
Adhering to once-a-day low-dose antibiotics exploited mechanisms of habit formation in this population. Habits are learned dispositions to past behaviour and are triggered automatically by environmental cues. 69 We found that participants in the present study linked with pre-existing behaviours (e.g. getting ready for bed) in order to trigger taking their once-daily prophylaxis consumption. Other researchers have also demonstrated existing routines to be particularly conducive to embedding new behaviours owing to the predictability and stability of these patterns and also the reduction in cognitive effort required to perform the new behaviours. 70 By exploiting the automaticity of an already ingrained behavioural pattern, participants in the present study successfully extended their existing habits with the adjunct behaviour of taking an antibiotic.
Although there was variation in impact of the opportunity to take low-dose antibiotics, participants felt generally positive about their experience on the AnTIC trial. Research has shown that, when individuals gain personal evidence of medication effects, they are more likely to view it as favourable. 71 This, in turn, encourages their motivation to continue with this treatment. 72 Support provided by clinicians or coaches via telephone and internet platforms has also been shown to enhance adherence. 73,74 The assumption is that, although high levels of intrinsic motivation encourage adherence to treatment, extrinsic ‘help’ is also often required. 75 In the present study, the 3-monthly appointments were often perceived by participants as beneficial and may have fostered internal motivation to adhere to treatment; in respect of both once-daily prophylaxis and also the general protocol for non-prophylaxis participants. Human support in studies has been shown elsewhere to enhance adherence more than automated systems. 76
Strengths and limitations of the qualitative study
Interviewing participants in a trial of antibiotic use may have meant that we interviewed a select group of participants who held particularly positive or negative views about the use of prophylactic antibiotics. In particular, we were unable to recruit anybody to this qualitative study who had declined participation in the main trial. In addition, although this study used purposive sampling with a range of ages and from both sexes, it cannot be assumed that these experiences and opinions are universal across all individuals who use CISC and experience recurrent UTI. Furthermore, it should be noted that it included only those CISC users who suffer from repeated UTIs and, thus, their opinion of benefit from CISC use may not be the same as those who do not suffer repeated UTIs. It is also possible that only those who perceived a positive experience on the AnTIC trial were motivated to participate in the interview study. Relying on retrospective recall for descriptions of CISC experiences, UTI events and antibiotic usage may not provide a true representation of real-time perceptions. The qualitative interviews were undertaken in the first seven sites, to recruit participants who were completing their follow-up assessments and had agreed to be interviewed; participants were selected based on sex, age and group allocation. A possible limitation is that, although the age, sex and duration of CISC use across the group interviewed was representative of the general trial population, sampling based on other characteristics, such as underlying diagnosis or frequency of UTI, may have revealed further views.
Conclusion
The findings of this qualitative study should be interpreted alongside the results of the main AnTIC clinical trial. 1 The emotional and practical burden of CISC and UTI was considerable in participants’ lives and influenced the perception of QoL. The process of psychological adjustment to CISC and UTI was complex, characterised by cognitive, attitudinal and situational factors. Participant accounts detailed a nonchalant, unconcerned attitude about taking and using antibiotics for recurrent UTI. A minority of individuals felt concerned at using antibiotics prophylactically, particularly in relation to negatively affecting potential future clinical effectiveness. These attitudes had an impact on their behaviour towards taking antibiotics either prophylactically or not and were also influenced by HCP recommendations. Finally, adhering to the AnTIC trial treatment was deemed straightforward and those in the prophylaxis group exploited habitual tendencies when incorporating once-daily antibiotics into their lives.
Chapter 6 Discussion
Statement and interpretation of results
This large RCT, using a pragmatic design embedded in a standard health-care setting, has clearly demonstrated the benefit of once-daily low-dose oral antibiotic prophylaxis against symptomatic UTI for users of CISC who suffer repeated UTIs. A daily, single low dose of one of the licensed agents (nitrofurantoin, trimethoprim or cefalexin) taken over 12 months resulted in a 48% reduction in incidence of symptomatic UTI compared with no prophylaxis, with an absolute median reduction of one episode per year (from two episodes to one episode). The size of this effect was unchanged by the inclusion of possible confounders in the statistical model, including adjustment for days taking treatment courses of antibiotics for UTI, prior frequency of UTI and presence of asymptomatic bacteriuria at baseline. The reduction in numbers of participants having four or more UTIs per year in the prophylaxis group compared with the no-prophylaxis group was particularly striking [51 (28%) to 15 (8%)]. This suggests that the reduction in UTI burden was particularly worthwhile in this group of individuals. Defining UTI by the presence of symptoms and a positive urine culture (microbiologically proven UTI) showed a similar result with a relative reduction in UTI of 51% in favour of prophylaxis. Infections, when they occurred, were not clinically severe, being infrequently accompanied by a fever of > 38 °C, and few, in either group, resulted in hospitalisation. However, symptoms were rated by participants as being severe for one-third of UTI episodes.
Participants allocated to prophylaxis were satisfied by the clinical effectiveness and convenience of the therapy, and the majority of those who expressed a preference elected to continue prophylaxis after completion of trial participation. However, only 20% of participants in the no-prophylaxis group were intending to start prophylaxis. These measures of treatment satisfaction were not reflected in evidence of any clinically significant improvement in health status measured as MCS, PCS and overall scores from the SF-36v2 questionnaire, nor by QALYs derived from this questionnaire collected 6-monthly and at the time of symptomatic UTI. This may reflect confounding from underlying health problems and the continued need for CISC. The minimum clinically important difference in MCS and PCS scores in other settings and patient groups has been estimated to be between 5 and 7 points and between 5 and 8 points for MCS and PCS, respectively. 77 Only the upper confidence limit for the MCS score at 6 months is greater than these estimates of minimum important difference. This suggests that there are no clinically important lasting effects of UTI on QoL between the two groups for PCS at 6 and 12 months and for MCS at 12 months. However, it should be noted that this analysis was unlikely to reflect the acute effects of UTI on QoL. The health economic evaluation did not detect any statistically significant change in QALYs over the 12 months when adjusted for utility value during UTI. These quantitative findings are in contrast to the results of the qualitative study performed during the early part of the trial to inform trial processes and recruitment strategy. This showed that participants interviewed considered repeated UTI to be a considerable added burden and contributed to a reduced feeling of well-being. A recent systematic review78 has summarised studies reporting the effect of UTI on health status using a variety of generic measures, finding no, or minimal, change at the time of UTI. Of the papers included in this review, the most relevant to the AnTIC trial population was by Lee et al. ,79 which calculated a minimum difference in SF-6D for ‘somewhat better’ of 0.03 and for ‘somewhat worse’ of 0.10. Our unadjusted results showed a change of 0.006 in favour of prophylaxis and a worsening by 0.009 in the prophylaxis group including imputation of missing data and utility at the time of UTI. It would appear that our use of the SF-36v2 did not detect any change in well-being in the whole trial population, suggested by the qualitative study performed early in the trial.
Kidney and liver function were unchanged during 12 months of therapy. Only one participant suffered a serious adverse reaction (SAR) related to use of nitrofurantoin as the prophylaxis agent, which rapidly resolved.
A number of actual and potential harms may lessen the impact of this clear benefit. AEs related to antibiotic treatment were reported by 19 (8.4%) of those taking prophylaxis, although only 10 of them elected to stop at any point in the 12 months. Of more concern was the pattern of significantly increased resistance of potential pathogens isolated from urine specimens submitted to the central laboratory by the participants allocated to prophylaxis compared with those found in samples sent by participants in the no-prophylaxis group. This suggests that use of continuous low-dose antibiotic prophylaxis in the experimental group outweighs the increased use of treatment courses of antibiotics used by those in the control group of the trial in terms of inducing antimicrobial resistance among common UTI pathogens.
The economic analyses, CEA, CUA and CBA, all suggest that, on average, a strategy of antibiotic prophylaxis is more effective and more costly than no prophylaxis. There is roughly an even chance that prophylaxis would be cost saving and a 66% chance that it would be more cost-effective than no prophylaxis should society be willing to pay ≥ £200 to avoid one UTI. The likelihood of prophylaxis being considered cost-effective using conventional decision-making thresholds was broadly similar according to CUA and CBA.
Strengths and limitations
We carried out this trial in accordance with current best practice. We used a remote internet-based randomisation system with the assignment algorithm written by an independent statistician to ensure concealment of allocation. This included stratification for the three most important confounders. We identified other possible confounders from our literature review. All likely confounders were well-balanced across the two groups and their inclusion in the statistical model did not influence the primary result. Our chosen study design did not allow blinding of participants, clinicians or local research teams to allocation, although outcome assessors including laboratory staff and members of the central trial team involved in outcome adjudication were blinded to allocated group. We chose a primary outcome that reflected participant experience in terms of UTI symptoms and clinician action in terms of providing a treatment course of an appropriate antibiotic. At the design stage, we deliberately included different opportunities for participants to record a UTI and also included relevant fields in the 3-monthly trial visit CRF for local trial staff to record UTIs when communicating with participants directly. However, lack of participant blinding combined with the use of a patient-reported outcome may still have risked differential outcome reporting between groups. We believe that this was unlikely because UTI reports were cross-checked against other data sources (i.e. 3-monthly patient interviews collected by local trial staff and questionnaires completed every 3 months by participants). The similar findings when a definition of UTI that included microbiological confirmation was used gave further reassurance that there was limited detection bias. Two central trial staff, acting as blinded outcome assessors, checked that reports of UTI fulfilled our pre-set criteria. A third member acted as arbiter if adjudication was uncertain and rechecked 10% of the primary outcome reports. Despite these safeguards, future studies using patient-reported UTI outcome may consider computerising the adjudication process using a decision analysis algorithm to avoid observer bias. We found a mean (SD) frequency of symptomatic UTI during the 12-month trial in the control (no prophylaxis) group of 2.5 (2.3). This compares with a mean (SD) of 5.4 (3.6) reported by trial participants and supported by health-care record review by trial staff for the 12 months prior to enrolment in the AnTIC trial. There are a number of possible reasons for the lower than anticipated incidence of UTI during the 12-month trial. Most importantly, the retrospective method of detection of occurrence of UTI in the year preceding is at high risk of bias and, unlike the primary outcome, was not adjudicated to a set protocol. It may also reflect more frequent patient contact and education given during the trial regarding non-antibiotic-intervention to reduce risk of UTI. It is also possible that we did not capture all symptomatic, antibiotic-treated UTI. Although we specified counting of only symptomatic antibiotic-treated UTI prior to enrolment in written recruitment instructions, it is possible that decisions made by local trial staff during assessment of eligibility varied in rigour. Finally, in our primary outcome adjudication process we specified that antibiotic treatment courses for UTI should be ≥ 14 days apart to count as separate episodes. It is possible that baseline frequency data included episodes with courses of antibiotic < 14 days apart as separate occurrences. For this trial, the prophylactic antibiotic used was chosen from the three standard agents according to clinician and participant preference and taken according to recommended dosage. The use of higher doses or combinations of agents or different duration of use of prophylaxis was not examined.
The trial had few withdrawals, excellent completion of follow-up documents by participants and local trial staff and good completion rates for postal questionnaires. All pre-set thresholds regarding numbers of participants contributing to the primary analysis were met. The proportion and type of missing data were similar in the two groups. The trial was conducted and the results analysed in line with a published protocol1 with all pre-stated outcomes reported.
A further strength of this study was that a detailed economic evaluation was undertaken based on extensive data collected as part of the trial. The economic evaluation triangulated three different types of analyses to draw out the implications of study finding on policy, practice and research. Each of the analyses undertaken in the economic evaluation has taken a progressively wider view of what is important to study participants and the public, thereby encapsulating a wider view of benefits. Reassuringly, no major inconsistencies were observed between the economic analyses undertaken.
Another noteworthy strength was the attempt of the economic evaluation to value directly the impact of UTIs on the health and well-being of study participants. In the former case, this was by the completion of the SF-36v2 at the time of UTI. In the latter case, it was by the completion of the WTP survey.
Challenges of the economic evaluation were in measuring the impact of UTIs on health status. Specifically, challenges were faced in terms of quantifying the duration of the detrimental impact of UTIs on individuals. This was overcome by determining the duration by expert opinion. However, more information is needed on the duration of UTIs and specifically the duration in terms of the detrimental effect on QoL and on the inability to perform usual activities. The WTP study overcame the limitation of undertaking the CUA without an accurate estimate of duration of symptoms during a UTI. However, there were concerns at the outset of the study regarding the acceptability to participants of completing the WTP survey. The WTP approach is not typically used in RCTs and, although the majority of participants did not have difficulty in responding to the survey, there was a proportion of participants who conveyed uneasiness and aversion to this form of data collection (which is not unusual for this type of survey). Nevertheless, data collected in the WTP study helped put the primary clinical effect into a clearer decision-making context. CEAs reporting results in such terms as the incremental cost per UTI avoided, as was done here, have often been criticised in the economic evaluation literature as they do not provide clear guidance about how much a decision-maker should pay for a unit of effect.
Limitations of the economic evaluation are mainly concerned with the limited scope of costs. These have been presented in terms of costs of prevention and treatment of UTI to the NHS and study participants and their carers. Concern relating to the use of prophylactic antibiotics and the impact on antimicrobial resistance was not directly addressed in the economic evaluation. This impact might be assessed via a variation of approach used for budget impact assessments, although, in this case, the analysis would make use of existing work in which health and other effects have been monetarised. This analysis should take into account the risk over time of antimicrobial resistance, perhaps by means of a Markov or discrete event simulation model. In the absence of such an analysis, an extension of the CBA could be used to estimate the impact of prophylactic antibiotics on antimicrobial resistance. Here, information derived from the trial for the CBA could be combined with secondary sources of information. For example, an estimate of £1,803,341 can be made for the annual total net benefit of prophylaxis for the UK population of CISC users eligible for antibiotic prophylaxis. This was calculated by multiplication of the mean incremental net benefit value (estimated from the CBA: £208.72) and the total number of CISC users suffering repeated UTI (n = 8640). In terms of the calculation of the total cost of antimicrobial resistance for the UK population, an estimate of £19,126,662,649 could be made based on the total population of the UK and the worldwide annual cost of antimicrobial resistance. 80,81 In order to apportion the appropriate cost of antimicrobial resistance resulting from the use of antibiotics for prophylaxis, the total cost of antimicrobial resistance for the UK population needs to be adjusted to take into account that, of all the prescriptions for antibiotics in the UK, only a proportion are for prophylaxis. The mean annual number of prescriptions annually specifically for prophylaxis for CISC users, assuming monthly prescriptions, is 103,680 (8640 multiplied by 12). The estimated total number of antibiotic prescriptions for the UK annually is 3,400,000. 82 The total cost of antimicrobial resistance for the UK population, apportioned to prophylaxis for CISC users can, thus, be estimated as £583,250,701. The annual cost of antimicrobial resistance attributable to prophylaxis for CISC users outweighs the monetary benefits of a regime of antibiotic prophylaxis to the UK population (£583,250,701 and £1,803,341, respectively). This exploratory analysis of the costs associated with antimicrobial resistance resulting from prophylactic antibiotic use is based on the best available evidence: secondary sources of information combined with the results from the within-trial CBA. This imperfect calculation should be considered as one possible estimate of the costs that may be attributed to antimicrobial resistance; accordingly, it should be interpreted with caution. Primary data collection would be the optimal method to calculate the cost of antimicrobial resistance that could be attributed to this population of CISC users eligible for use of antibiotic prophylaxis. The results of our qualitative study were useful in fine-tuning recruitment strategies as the trial progressed. The analysis of views of participants concerning the detrimental effect of suffering from a UTI on their well-being provided a counterpoint to the lack of change in the measures of health status used for the trial. We were unable to recruit patients who declined participation in the trial, which may have resulted in under-representation of views more critical of antibiotic prophylaxis use.
Generalisability
Our trial included a large sample of people performing CISC who suffered from a recurrent UTI. They were drawn from geographical areas from the south coast of England to the north coast of Scotland. Inclusion criteria were broad and exclusion criteria minimised. Baseline characteristics show that the proportions of participants from various groups were representative of those found in recent Dutch83 and French84 case series. Of particular note were the similar sex and age distribution, and the proportion with neurological bladder dysfunction. The results of our trial answer the specific research question posed by the James Lind Alliance and NICE. 16,17 In addition, the results of our trial will be of great interest to the authors of the two recent Cochrane reviews12,22 that highlighted lack of robust evidence of benefit of use of antibiotic prophylaxis in users of CISC, one of which22 is currently under revision in order to improve its methods. Should this occur, this would be an ideal opportunity to incorporate the best available evidence on effectiveness into a decision-analytic model based economic evaluation. This would provide more precise estimates of cost-effectiveness.
Chapter 7 Conclusions
The benefit of antibiotic prophylaxis in reducing the incidence of clinical UTI among users of CISC who suffer from a repeated UTI is clearly demonstrated by the results of this methodologically robust trial; however, no consistent improvement in their overall health status was detected. The degree of benefit seems worthwhile from patient and NHS perspectives, but there is much uncertainty around cost estimates. The important downside of using continuous low-dose antibiotic prophylaxis in the long term is the increased development of antimicrobial resistance in pathogenic and commensal bacteria, including resistance to agents used to treat UTI. Potential future costs related to increased antimicrobial resistance were not accounted for in our within-trial health economic evaluation. An increased degree of colonisation of the urogenital tract with isolates resistant to first-line antimicrobial agents may have serious implications related to reduction in the efficacy of antimicrobial prophylaxis and UTI treatment for individual patients using CISC. Treatment of symptomatic UTI caused by multiresistant isolates may require use of broad-spectrum and parenterally administered antibiotics. There will also be increased potential for cross-infection with multiresistant isolates in the community or within health-care environments. This may result in AEs for individuals or groups of patients other than those who are taking antibiotic prophylaxis. Increased resistance of the E. coli component of the faecal microbiome is a further concern, particularly if resistance also occurs in other species not studied in our trial or affecting the balance of the constituent bacteria, and may have a serious additional impact on patient and public health. 85 Patients and clinicians will need to weigh up the potential benefit of antibiotic prophylaxis in this relatively small and specific population of CISC users in the context of appropriate antibiotic stewardship when making decisions around whether or not to start long-term prophylaxis for prevention of recurrent UTI. A cautious approach should be maintained until the risks and implications of development of antimicrobial resistance are better characterised.
Recommendations for research
-
Longer-term studies (continued beyond 12 months) of antimicrobial resistance in response to low-dose antibiotic prophylaxis against UTIs and their consequences for health-economic evaluation of therapies.
-
Identifying the optimum agent(s), duration and dose of antibiotic prophylaxis against UTI to ensure efficacy and minimisation of adverse reactions for patients, and reduced risk of antimicrobial resistance among urinary pathogens and the faecal microbiome.
-
Patient and bacterial phenotypic and genotypic studies to identify patient groups that benefit most from prophylaxis and bacterial species, and strains colonising urine and the faecal microbiome that are most likely to develop resistance with continuous exposure to low-dose antibiotics.
-
Identification of better diagnostic methods for management of UTI to enhance patient benefit and support antimicrobial stewardship.
-
From the economic perspective, further work would be valuable to update meta-analyses with the results of this trial and use these data in a model-based economic evaluation to provide more precise estimates of clinical effectiveness and cost-effectiveness.
-
Define and validate tools, including WTP exercises, to better measure the impact of UTI on QoL.
Acknowledgements
The AnTIC trial was only possible owing to the generous support and enthusiasm of a large number of individuals.
The study was funded by the NIHR Health Technology Assessment programme.
The investigators would also like to acknowledge the NIHR CRNs that provided NHS research support to the study.
The Scottish Primary Care Research Network supported the study and facilitated the identification of GP practices in Scotland.
The co-ordination of study activity at each site was supported by the research nurses. Special thanks go to our hub co-ordinators, Paul Hindmarch and Wendy Robson (Newcastle upon Tyne), Kelly Hislop-Lennie and Pauline Rachman (Southampton), Nicola Gillespie (Glasgow), Lindsay Grant and Diane Ledingham (Aberdeen), Constance Shiridzinomwa (Bristol), Steve Littler and Beverley Taylor (Wakefield), and Kelly Leonard and Lisa Geoghegan (Cambridge), for all their support in managing study activity and responding promptly to the data queries.
Many clinicians and research staff from across the UK contributed to the study at individual sites and we express our gratitude to all staff at sites in the UK for their support of study recruitment and data collection. In particular, we would like to thank the following organisations:
-
Freeman Hospital and Royal Victoria Infirmary, the Newcastle upon Tyne Hospitals NHS Foundation Trust (PI: Anna O’Riordan)
-
Skerne Medical Group, Sedgefield (PI: James Larcombe)
-
Jubilee Practice, Newton Aycliffe (PI: Johann Brandmair)
-
Addenbrookes Hospital, Cambridge (PI: Nikesh Thiruchelvam)
-
Southmead Hospital, Bristol (PI: Anthony Timoney)
-
Southern General Hospital, Glasgow (PI: Karen Guerrero)
-
Ayr Hospital (PI: Suzanne McPhee)
-
Western General Hospital, Edinburgh (PI: Ammar Alhasso)
-
Aberdeen Royal Infirmary (PI: Mohamed Abdel-Fattah)
-
Pinderfields Hospital, Wakefield (PIs: Simon Harrison and Ian Beckley)
-
University Hospital, Coventry (PI: Rajagopalan Sriram)
-
Queen Elizabeth Hospital, Birmingham (PI: Mo Belal)
-
Cheltenham General Hospital (PIs: Kim Davenport and Faith McMeekin)
-
Ninewells Hospital, Dundee (PI: Ghulam Nabi)
-
Southport General Hospital (PI: Gurpreet Singh)
-
Ipswich Hospital (PI: Robert Brierly)
-
James Cook University Hospital, Middlesbrough (PI: Mary Garthwaite)
-
Royal Bolton Hospital (PI: Ling Lee)
-
St James’s, Leeds (PIs: Ian Eardley and Sayed Rahman)
-
East Kent Hospitals (PI: Nitin Shrotri)
-
New Cross Hospital, Wolverhampton (PIs: Nicholas Rukin and Aniruddha Chakravarti)
-
Royal National Orthopaedic Hospital, Stanmore (PI: Rizwan Hamid)
-
Raigmore Hospital, Inverness (PI: David Douglas)
-
North Devon District Hospital (PI: Eng Ong)
-
Imperial College London Hospital (PIs: Tina Rashid and Matthias Winkler)
-
Bedford Hospital (PI: Liaqat Chowoo)
-
Guys Hospital, London (PI: Jonathan Olsburgh)
-
GP practices in the Southampton area (Paul Little):
-
Portsdown Group Practice
-
Adam Practice, Poole
-
Wareham Health Centre
-
Bridges Medical Centre
-
Ramilies Surgery
-
Liphook & Liss Surgery
-
Old Fire Station
-
Forest End
-
Chawton Park Surgery
-
Cowplain Practice
-
Bermuda Practice
-
Swanage Practice
-
Wellbridge Practice
-
Three Swans Surgery
-
Swan Surgery, Petersfield
-
Grove House.
-
We would like to thank the NCTU staff, in particular the assistant trial managers Lee Munro, Sonya Carnell and Sarah Dunn, administrator Stephanie Currer and trial secretary Ruby Smith Whelan.
Institute of Health and Society staff also involved in the project were statistician Valentina Mamasoula, health economists Cristina Fernandez-Garcia (who completed the SF-36v2 analysis), Tara Homer (who contributed to the SF-36v2 analyses and overall economic evaluation), Atefeh Mashayekhi (who derived the medication costs), Julija Stoniute (who evaluated the time and travel data to derive the patient and carer time and travel costs) and Joanna Marsden (who collated literature and unit cost tables for the health economics study).
We would like to thank NHS staff of the Department of Microbiology, Freeman Hospital at Newcastle upon Tyne Hospitals NHS Foundation Trust, for careful husbandry of trial specimens and pharmacy support, in particular Michael Ford and Amritjit Singh, Department of Microbiology; and Ian Campbell, Assistant Director, Pharmacy and Medicines Management, at Newcastle upon Tyne Hospitals NHS Foundation Trust, for his advice.
We thank Sheila Wallace, Information Specialist, Cochrane Incontinence Group who performed the literature search to update the systematic review. We thank Adele Long (formerly Bristol Urological Institute) and Wendy Robson (Newcastle upon Tyne Hospitals NHS Foundation Trust) for their help in the preparation of the original application.
We would like to thank the members of the TSC, particularly the chairperson, Chris Butler, for all his support and Sarah Bittlestone (lay member) for reviewing trial material and reports. We thank the members of the DMC for all their valuable guidance: TSC members Professor Chris Butler (chairperson), Professor Emma Hall, Mr Roland Morley, Mr Dan Wood, Ms Jane J Laws and Ms Sarah Bittlestone; and DMC members Professor Graeme MacLennan (chairperson), Dr Simon Skene and Mr Julian Shah.
Finally, but most importantly, we would like to thank all our participants for agreeing to take part in the AnTIC trial.
Contributions of authors
Robert Pickard (Professor of Urology) was the study chief investigator, designed the study, was the principal applicant for funding, wrote the study protocol, supervised the overall conduct of the study, interpreted study data, and wrote and edited the final report.
Thomas Chadwick (Clinical Trials Statistician) designed the statistical plan in the study protocol, was a co-applicant for funding and led the final study statistical analysis.
Yemi Oluboyede (Health Economist and Senior Research Associate) designed the health economics analysis plan, led the health economics analysis and wrote Chapter 4.
Catherine Brennand (Trial Manager) was the trial manager for the study, reviewed study results, and co-wrote and edited the final report.
Alexander von Wilamowitz-Moellendorff (Trial Manager) was the trial manager for the study, reviewed study results, and co-wrote and edited the final report.
Doreen McClurg (Professor of Pelvic Floor Physiotherapy) contributed to the design of the protocol; was a co-applicant for funding; led the design, conduct and reporting of the qualitative interview study; and acted as PI for Glasgow.
Jennifer Wilkinson (Senior Trial Manager) contributed to the design of the protocol, was a co-applicant for funding and led the NCTU team’s involvement the study.
Laura Ternent (Health Economist and Senior Lecturer) contributed to the design of the protocol, health economics evaluation and analysis plan, and was a co-applicant for funding.
Holly Fisher (Research Associate in Medical Statistics) conducted the main study analysis and co-wrote part of the final report.
Katherine Walton (Consultant Clinical Microbiologist) contributed to the design of the protocol, was a co-applicant for funding, and led the reporting and interpretation of microbiological results.
Elaine McColl (Professor of Health Services Research) designed the protocol, was a co-applicant for funding, reviewed study results and edited the final report.
Luke Vale (Health Foundation Professor of Health Economics) led the health economic evaluation and supervised the analysis of health status (SF-36v2), contributed to the design of the protocol, reviewed study results and edited the final report.
Ruth Wood (Database Manager) designed and set up trial-specific databases, managed central data processes, reviewed study results and edited the final report.
Mohamed Abdel-Fattah (Reader) contributed to the design of the protocol, was a co-applicant for funding and acted as PI for Aberdeen.
Paul Hilton (Consultant Urogynaecologist) contributed to the design of the protocol and was a co-applicant for funding.
Mandy Fader (Professor of Continence Technology) contributed to the design of the protocol, was a co-applicant for funding and acted as PI for Southampton.
Simon Harrison (Consultant Urologist) contributed to the design of the protocol, was a co-applicant for funding and acted as PI for Wakefield.
James Larcombe (GP) contributed to the design of the protocol and was a co-applicant for funding.
Paul Little (Professor of Primary Care Research) contributed to the design of the protocol and was a co-applicant for funding.
Anthony Timoney (Consultant Urologist) contributed to the design of the protocol, was a co-applicant for funding and acted as PI for Bristol.
James N’Dow (Professor of Urology) contributed to the design of the protocol and was a co-applicant for funding.
Heather Armstrong (Patient and Service User Representative) represented the views of patients in the design of the study, and reviewed and edited all participant documentation.
Nicola Morris (Research Manager) contributed to the design of the protocol and was a co-applicant for funding.
Kerry Walker (Academic Researcher) conducted the qualitative interviews and analysed the interview data for the qualitative study.
Nikesh Thiruchelvam (Consultant Urologist) contributed to the design of the protocol, was a co-applicant for funding, acted as PI for Cambridge and was deputy chief investigator.
All authors provided critical comments on drafts of the final report.
Publications
Brennand C, von Wilamowitz-Moellendorff A, Dunn S, Wilkinson J, Chadwick T, Ternent L, et al. Antibiotic treatment for intermittent bladder catheterisation with once-daily prophylaxis (the AnTIC study): study protocol for a randomised controlled trial. Trials 2016;17:276.
McClurg D, Walker K, Pickard R, Hilton P, Ainsworth H, Leonard K, et al. Participant experiences of clean intermittent self-catheterisation, urinary tract infections and antibiotic use on the AnTIC trial – a qualitative study. Int J Nurs Stud 2018;81:1–7.
Fisher H, Oluboyede Y, Chadwick T, Abdel-Fattah M, Brennand C, Fader M, et al. Continuous low dose antibiotic prophylaxis for adults performing clean intermittent catheterisation who suffer repeated urinary tract infection (AnTIC): a randomised, open-label, superiority trial. Lancet Infect Dis 2018; in press.
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Brennand C, von Wilamowitz-Moellendorff A, Dunn S, Wilkinson J, Chadwick T, Ternent L, et al. Antibiotic treatment for intermittent bladder catheterisation with once-daily prophylaxis (the AnTIC study): study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1389-y.
- Bakke A. Clean intermittent catheterization – physical and psychological complications. Scand J Urol Nephrol Suppl 1993;150:1-69.
- Lamin E, Newman DK. Clean intermittent catheterization revisited. Int Urol Nephrol 2016;48:931-9. https://doi.org/10.1007/s11255-016-1236-9.
- Lapides J, Diokno AC, Silber SJ, Lowe BS. Clean, intermittent self-catheterization in the treatment of urinary tract disease. J Urol 1972;107:458-61. https://doi.org/10.1016/S0022-5347(17)61055-3.
- Prieto JA, Murphy C, Moore KN, Fader MJ. Intermittent catheterisation for long-term bladder management (abridged Cochrane review). Neurourol Urodyn 2015;34:648-53. https://doi.org/10.1002/nau.22792.
- Prescribing & Medicines Team, NHS Digital . Prescription Cost Analysis England – 2015 2016. http://content.digital.nhs.uk/catalogue/PUB20200/pres-cost-anal-eng-2015-rep.pdf (accessed 27 October 2017).
- Duffy LM, Cleary J, Ahern S, Kuskowski MA, West M, Wheeler L, et al. Clean intermittent catheterization: safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc 1995;43:865-70. https://doi.org/10.1111/j.1532-5415.1995.tb05528.x.
- Di Benedetto P. Clean intermittent self-catheterization in neuro-urology. Eur J Phys Rehabil Med 2011;47:651-9.
- Wyndaele JJ. Complications of intermittent catheterization: their prevention and treatment. Spinal Cord 2002;40:536-41. https://doi.org/10.1038/sj.sc.3101348.
- Cardenas DD, Moore KN, Dannels-McClure A, Scelza WM, Graves DE, Brooks M, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PM R 2011;3:408-17. https://doi.org/10.1016/j.pmrj.2011.01.001.
- National Institute on Disability and Rehabilitation Research Consensus Statement . The prevention and management of urinary tract infections among people with spinal cord injuries. J Am Paraplegia Soc 1992;15:194-207. https://doi.org/10.1080/01952307.1992.11735873.
- Prieto J, Murphy CL, Moore KN, Fader M. WITHDRAWN: Intermittent catheterisation for long-term bladder management. Cochrane Database Syst Rev 2017;8. https://doi.org/10.1002/14651858.CD006008.pub4.
- Kiddoo D, Sawatzky B, Bascu CD, Dharamsi N, Afshar K, Moore KN. Randomized crossover trial of single use hydrophilic coated vs multiple use polyvinylchloride catheters for intermittent catheterization to determine incidence of urinary infection. J Urol 2015;194:174-9. https://doi.org/10.1016/j.juro.2014.12.096.
- Scovell J, Fletcher S, Stewart J, Khavari R. A prospective randomized double-blinded placebo control trial on the effects of cranberry supplementation on bacterial colonization and symptomatic urinary tract infections in females with neurogenic bladder dysfunction dependent on self catheterization (abstract number PD8-07). J Urol 2015;193:e192-e3. https://doi.org/10.1016/j.juro.2015.02.922.
- Gallien P, Amarenco G, Benoit N, Bonniaud V, Donzé C, Kerdraon J, et al. Cranberry versus placebo in the prevention of urinary infections in multiple sclerosis: a multicenter, randomized, placebo-controlled, double-blind trial. Mult Scler 2014;20:1252-9. https://doi.org/10.1177/1352458513517592.
- Cowan K. The James Lind Alliance: tackling treatment uncertainties together. J Ambul Care Manage 2010;33:241-8. https://doi.org/10.1097/JAC.0b013e3181e62cda.
- Healthcare-Associated Infections: Prevention and Control in Primary and Community Care. Clinical Guideline CG139. London: NICE; 2012.
- Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev 2004;3. https://doi.org/10.1002/14651858.CD001209.pub2.
- NHS Evidence: Clinical Knowledge Summaries – Urinary Tract Infection (Lower) – Women. London: NICE; 2015.
- Brumfitt W, Hamilton-Miller JM. Efficacy and safety profile of long-term nitrofurantoin in urinary infections: 18 years’ experience. J Antimicrob Chemother 1998;42:363-71. https://doi.org/10.1093/jac/42.3.363.
- Nitrofurantoin: Summary of Product Characteristics. London: MHRA; 2011.
- Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD004201.pub3.
- Gribble MJ, Puterman ML. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med 1993;95:141-52. https://doi.org/10.1016/0002-9343(93)90254-M.
- Anderson RU. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J Urol 1980;123:364-6. https://doi.org/10.1016/S0022-5347(17)55938-8.
- Mohler JL, Cowen DL, Flanigan RC. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol 1987;138:336-40. https://doi.org/10.1016/S0022-5347(17)43138-7.
- Zegers B, Uiterwaal C, Kimpen J, van Gool J, de Jong T, Winkler-Seinstra P, et al. Antibiotic prophylaxis for urinary tract infections in children with spina bifida on intermittent catheterization. J Urol 2011;186:2365-71. https://doi.org/10.1016/j.juro.2011.07.108.
- Schlager TA, Anderson S, Trudell J, Hendley JO. Nitrofurantoin prophylaxis for bacteriuria and urinary tract infection in children with neurogenic bladder on intermittent catheterization. J Pediatr 1998;132:704-8. https://doi.org/10.1016/S0022-3476(98)70364-6.
- Morton SC, Shekelle PG, Adams JL, Bennett C, Dobkin BH, Montgomerie J, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil 2002;83:129-38. https://doi.org/10.1053/apmr.2002.26605.
- Wyndaele JJ, Brauner A, Geerlings SE, Bela K, Peter T, Bjerklund-Johanson TE. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int 2012;110:E910-17. https://doi.org/10.1111/j.1464-410X.2012.11549.x.
- Beerepoot MAJ, ter Riet G, Nys S, van der Wal WM, de Borgie CAJM, de Reijke TM, et al. Cranberries vs antibiotics to prevent urinary tract infections: a randomized double-blind noninferiority trial in premenopausal women. Arch Intern Med 2011;171:1270-8. https://doi.org/10.1001/archinternmed.2011.306.
- Goff DA, Kullar R, Goldstein EJC, Gilchrist M, Nathwani D, Cheng AC, et al. A global call from five countries to collaborate in antibiotic stewardship: united we succeed, divided we might fail. Lancet Infect Dis 2017;17:e56-63. https://doi.org/10.1016/S1473-3099(16)30386-3.
- Quality Standard (QS) 6. London: NICE; 2014.
- International Council for Harmonisation . The Medicines for Human Use (Clinical Trials) Amendment Regulations 2006 Schedule 1 Part 2 2006. www.ich.org/products/guidelines/efficacy/efficacy-single/article/good-clinical-practice.html (accessed 13 February 2018).
- World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Diagnosis of UTI Quick Reference Guide for Primary Care. London: Public Health England; 2007.
- Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Healthcare Infection Control Practices Advisory Committee . Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 2010;31:319-26. https://doi.org/10.1086/651091.
- Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2. https://doi.org/10.1186/1477-7525-2-12.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. https://doi.org/10.1097/00005650-199206000-00002.
- Standards and Training Centre for Infections . National Standard Method Investigation of Urine BSOP 41 2009. www.gov.uk/guidance/uk-standards-for-microbiology-investigations-smi-quality-and-consistency-in-clinical-laboratories (accessed 14 December 2016).
- Official Journal of the European Union . Commission Directive 2005 28 EC 2005. https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/dir_2005_28/dir_2005_28_en.pdf (accessed 13 February 2018).
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Trimethoprim: Summary of Product Characteristics. London: MHRA; 2011.
- Cefalexin: Summary of Product Characteristics. London: MHRA; 2011.
- Goetz LL, Cardenas DD, Kennelly M, Bonne Lee BS, Linsenmeyer T, Moser C, et al. International spinal cord injury urinary tract infection basic data set. Spinal Cord 2013;51:700-4. https://doi.org/10.1038/sc.2013.72.
- UK Standards for Microbiology Investigations (UK SMI) B 41: Investigation of Urine. London: Public Health England; 2014.
- The European Committee on Antimicrobial Susceptibility Testing . Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 7.1 2017. www.eucast.org/clinical_breakpoints/ (accessed 27 October 2017).
- Johnson HW, Anderson JD, Chambers GK, Arnold WJ, Irwin BJ, Brinton JR. A short-term study of nitrofurantoin prophylaxis in children managed with clean intermittent catheterization. Pediatrics 1994;93:752-5.
- Damocles Study Group . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)70939-9.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- NHS Reference Costs 2015-16. London: DHSC; 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Cantebury: PSSRU, University of Kent; 2016.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2017.
- Automobile Association . Motoring Costs 2014; Purchase Price of Car When New from £13,000 to £18,000 at 10,000 Miles Year 2014. www.theaa.com/resources/Documents/pdf/motoring-advice/running-costs/petrol2014.pdf (accessed 27 October 2017).
- Office for National Statistics (ONS) . Annual Survey of Hours and Earnings: 2016 Provisional Results 2016. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2016provisionalresults (accessed 27 October 2017).
- Department for Transport . Transport Analysis Guidance (TAG) Unit A1-3 on the Analysis of User and Provider Impacts in Transport Appraisals 2014. www.gov.uk/government/publications/webtag-tag-unit-a1-3-user-and-provider-impacts-november-2014 (accessed 27 October 2017).
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Morris S, Appleby J, Parkin D. Economic Analysis in Health Care. Chichester: John Wiley & Sons; 2006.
- Drummond MF. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-Effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2011.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 Process and Methods [PMG9] 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 27 October 2017).
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. London: Sage; 1990.
- Shaw C, Logan K. Psychological coping with intermittent self-catheterisation (ISC) in people with spinal injury: a qualitative study. Int J Nurs Stud 2013;50:1341-50. https://doi.org/10.1016/j.ijnurstu.2013.01.009.
- Shaw C, Logan K, Webber I, Broome L, Samuel S. Effect of clean intermittent self-catheterization on quality of life: a qualitative study. J Adv Nurs 2008;61:641-50. https://doi.org/10.1111/j.1365-2648.2007.04556.x.
- Edokpolo LU, Stavris KB, Foster HE. Intermittent catheterization and recurrent urinary tract infection in spinal cord injury. Top Spinal Cord Inj Rehabil 2012;18:187-92. https://doi.org/10.1310/sci1802-187.
- Norris P, Chamberlain K, Dew K, Gabe J, Hodgetts D, Madden H. Public beliefs about antibiotics, infection and resistance: a qualitative study. Antibiotics 2013;2:465-76. https://doi.org/10.3390/antibiotics2040465.
- Brooks L, Shaw A, Sharp D, Hay AD. Towards a better understanding of patients’ perspectives of antibiotic resistance and MRSA: a qualitative study. Fam Pract 2008;25:341-8. https://doi.org/10.1093/fampra/cmn037.
- Gardner B. A review and analysis of the use of ‘habit’ in understanding, predicting and influencing health-related behaviour. Health Psychol Rev 2015;9:277-95. https://doi.org/10.1080/17437199.2013.876238.
- Lally P, Wardle J, Gardner B. Experiences of habit formation: a qualitative study. Psychol Health Med 2011;16:484-9. https://doi.org/10.1080/13548506.2011.555774.
- Brown MT, Bussell JK. Medication adherence: WHO cares?. Mayo Clin Proc 2011;86:304-14. https://doi.org/10.4065/mcp.2010.0575.
- Michalak J, Klappheck MA, Kosfelder J. Personal goals of psychotherapy patients: the intensity and the ‘why’ of goal-motivated behavior and their implications for the therapeutic process. Psychother Res 2004;14:193-209. https://doi.org/10.1093/ptr/kph017.
- Christensen H, Griffiths KM, Farrer L. Adherence in internet interventions for anxiety and depression. J Med Internet Res 2009;11. https://doi.org/10.2196/jmir.1194.
- Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res 2011;13. https://doi.org/10.2196/jmir.1602.
- Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med 2006;166:1620-5. https://doi.org/10.1001/archinte.166.15.1620.
- Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: systematic review. J Med Internet Res 2009;11. https://doi.org/10.2196/jmir.1138.
- Carlson ML, Tveiten ØV, Yost KJ, Lohse CM, Lund-Johansen M, Link MJ. The Minimal Clinically Important Difference in Vestibular Schwannoma Quality-of-Life Assessment: an important step beyond P < .05. Otolaryngol Head Neck Surg 2015;153:202-8. https://doi.org/10.1177/0194599815585508.
- Bermingham SL, Ashe JF. Systematic review of the impact of urinary tract infections on health-related quality of life. BJU Int 2012;110:E830-6. http://dx.doi.org/10.1111/j.1464-410X.2012.11337.x.
- Lee BB, King MT, Simpson JM, Haran MJ, Stockler MR, Marial O, et al. Validity, responsiveness, and minimal important difference for the SF-6D health utility scale in a spinal cord injured population. Value Health 2008;11:680-8. https://doi.org/10.1111/j.1524-4733.2007.00311.x.
- Oppong R, Smith RD, Little P, Verheij T, Butler CC, Goossens H, et al. Cost effectiveness of amoxicillin for lower respiratory tract infections in primary care: an economic evaluation accounting for the cost of antimicrobial resistance. Br J Gen Pract 2016;66:e633-9. https://doi.org/10.3399/bjgp16X686533.
- O’Neill J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. The Review on Antimicrobial Resistance 2014. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed 16 February 2018).
- Press Association . Number of antibiotic prescriptions in England falls, figures show. Guardian 2016. www.theguardian.com/society/2016/may/25/antibiotic-prescriptions-england-falls-figures-show (accessed 16 February 2018).
- Cobussen-Boekhorst H, Beekman J, Wijlick E, Schaafstra J, Kuppevelt D, Heesakkers J. Which factors make clean intermittent (self) catheterisation successful?. J Clin Nurs 2016;25:1308-18. https://doi.org/10.1111/jocn.13187.
- Gonzalez Chiappe S, Lasserre A, Chartier Kastler E, Falchi A, Blaizeau F, Blanchon T, et al. Use of clean intermittent self-catheterization in France: a survey of patient and GP perspectives. Neurourol Urodyn 2016;35:528-34. https://doi.org/10.1002/nau.22752.
- Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut 2016;65:330-9. https://doi.org/10.1136/gutjnl-2015-309990.
Appendix 1 Results supplementary information
| SAE ID | SAE in medical terms | Intervention group |
|---|---|---|
| 047 | Polypharmacy (falls and confusion, left-sided pneumonia) | Prophylaxis |
| SAE ID | SAE in medical terms | Intervention group |
|---|---|---|
| 023 | Adverse drug reaction (asymptomatic highly raised serum liver enzyme ALT) | Prophylaxis |
| SAE ID | SAE in medical terms | Intervention group |
|---|---|---|
| 016 | Fall (resulting in fractured spine) | No prophylaxis |
| 036 | Emergency admission via GP for haematuria; died from bladder cancer | No prophylaxis |
| 054 | Oesophageal cancer, bilateral adrenal metastases, rectal cancer | No prophylaxis |
| SAE IDa | SAE in medical terms | Intervention group |
|---|---|---|
| 002 | Abdominal pain/constipation | No prophylaxis |
| 004 | Multiple sclerosis relapse | No prophylaxis |
| 005 | Haematuria (post TURP) | No prophylaxis |
| 006 | Headache and right-sided neck pain (shunt malfunction) | Prophylaxis |
| 007 | Follicular thyroid cancer | Prophylaxis |
| 008 | Epigastric pain | Prophylaxis |
| 009 | Headache and nausea – emergency admission to hospital | No prophylaxis |
| 010 | Perforated appendix | Prophylaxis |
| 011 | Road traffic accident (bilateral femoral fractures and right distal tibial fracture) | Prophylaxis |
| 012 | Pneumonia | No prophylaxis |
| 013 | Admitted for medical observation after car accident | No prophylaxis |
| 015 | Faecal impaction | Prophylaxis |
| 017 | Acute asthma attack and anxiety | Prophylaxis |
| 018 | Exacerbation of asthma and hyperventilation/gastro-oesophageal reflux/oral candidal infection | Prophylaxis |
| 019 | Chest infection | No prophylaxis |
| 020 | Post-microdiscectomy recurrent lumbar pain | Prophylaxis |
| 021 | Transverse myelitis | Prophylaxis |
| 022 | Acute exacerbation of chronic back pain | Prophylaxis |
| 024 | Abdominal pain due to faecal loading | Prophylaxis |
| 025 | Percutaneous gastric feeding tube site infection. Unable to feed for 3 weeks. Nausea and fever | Prophylaxis |
| 026 | Mechanical fall | No prophylaxis |
| 027 | Admitted for triple bypass surgery | No prophylaxis |
| 028 | Collapsed while driving (possible cerebrovascular accident or possible seizure) | Prophylaxis |
| 029 | Chest pain, dizziness and vomiting. Epigastric pain | Prophylaxis |
| 030 | Pancreatitis | Prophylaxis |
| 031 | Recurrence of neuromyelitis optica | Prophylaxis |
| 032 | Hospital admission for osteomyelitis of right foot. Diagnosed with Charcot joint | No prophylaxis |
| 033 | Hickman line infection | Prophylaxis |
| 034 | Small cell carcinoma – transurethral resection of bladder tumour | No prophylaxis |
| 035 | Chest pain | Prophylaxis |
| 037 | Right-sided weakness attributed to somatoform disorder | Prophylaxis |
| 038 | Abdominal pain | No prophylaxis |
| 039 | Admitted to hospital for observation and review (CT of head; new intracranial changes) | No prophylaxis |
| 040 | Seizures and collapse | Prophylaxis |
| 041 | Incidental finding of abdominal aortic aneurysm – rapidly expanding | Prophylaxis |
| 042 | Admitted to hospital for neuromyelitis optica | Prophylaxis |
| 043 | Rigors and on parenteral nutrition | Prophylaxis |
| 044 | Haematuria post-intravesical botulinum toxin | No prophylaxis |
| 045 | Recurrent vomiting and generalised abdominal discomfort | No prophylaxis |
| 046 | Admitted to hospital overnight, one episode of vomiting | Prophylaxis |
| 048 | Hospitalisation for right ankle stabilisation | No prophylaxis |
| 049 | Food poisoning | No prophylaxis |
| 050 | Submeatal and urethral strictures | Prophylaxis |
| 051 | Admitted to hospital following vasovagal episode | No prophylaxis |
| 053 | Carbon monoxide poisoning | No prophylaxis |
| 055 | Ruptured left quadriceps tendon | Prophylaxis |
| 058 | Unable to catheterise the Mitrofanoff adequately | No prophylaxis |
| 059 | Chest infection. Viral illness | No prophylaxis |
| 060 | Hospitalised after vasovagal episode | No prophylaxis |
| 061 | Hospital admission. Complete dysphagia | No prophylaxis |
| 063 | Bilateral pulmonary embolism | No prophylaxis |
| Variable | Intervention group, mean (SD), n | Total (N = 404), mean (SD), n | Two sample t-test comparing groups, p-value | Using ANCOVA modelling to compare groups (adjusting for stratification factors), p-value | |
|---|---|---|---|---|---|
| Prophylaxis (N = 203) | No prophylaxis (N = 201) | ||||
| eGFR | |||||
| Baseline | 86.6 (30.2), 200 | 88.0 (26.1), 197 | 87.3 (28.2), 397 | – | – |
| 12 months | 82.3 (30.0), 123 | 83.3 (27.6), 140 | 82.8 (28.7), 263 | – | – |
| Change from baseline | –2.1 (15.0), 123 | –3.4 (14.2), n = 138 | –2.8 (14.6), 261 | 0.48 | 0.48 |
| ALT | |||||
| Baseline | 24.7 (19.9), 198 | 24.1 (15.6), 188 | 24.4 (17.9), 386 | – | – |
| 12 months | 26.1 (19.1), 118 | 24.0 (14.1), n = 135 | 25.0 (16.6), 253 | – | – |
| Change from baseline | 1.2 (19.5), 117 | –0.3 (14.7), 128 | 0.4 (17.1), 245 | 0.49 | 0.50 |
Appendix 2 Qualitative supplementary information
Can you tell me about your health at the moment?
Experiences of clean intermittent self-catheterisationWhen did you start using clean intermittent self-catheterisation (CISC)?
How long have you been using CISC?
Why do you have to use CISC?
How do you feel about using CISC?
How does this impact you on a day-to-day basis?
(Probes: any examples from lifestyle/work, home life, physical activity, sleep, family/relationships.)
Experiences of urinary tract infectionsHave you experienced any UTIs since you began using CISC?
What symptoms do you experience/how do you feel at this time?
How does this impact your day-to-day life?
What treatment is recommended for you?
Attitudes towards taking antibiotics (prior to and after study)What are your views on taking antibiotics to treat an infection?
What are your views on taking antibiotics to prevent an infection?
(Probes: e.g. keen/dislike the idea; can you think of any factors that may influence your opinion?)
Have you ever taken or do you currently take any other medication for your bladder?
(Probes: cranberry juice, herbal remedies that may relieve symptomatic episodes.)
Effect of prophylaxis (intervention group only)Did you notice any difference when you were taking the low-dose antibiotic?
(Probes: UTI prevalence and symptoms)
How do you feel now compared to when you started the study?
Self-careDo you feel you took the antibiotics in the way that was recommended to you?
If not, can you explain why?
How did you incorporate them into your daily life?
What would influence you to continue or discontinue taking the antibiotics?
ClosureThank you for talking with me today.
List of abbreviations
- AE
- adverse event
- ALT
- alanine transaminase
- ANCOVA
- analysis of covariance
- AnTIC
- Antibiotic Treatment for Intermittent Catheterisation
- BIA
- British Infection Association
- CBA
- cost–benefit analysis
- CDC
- Centers for Disease Control and Prevention
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CFU
- colony-forming unit
- CI
- confidence interval
- CISC
- clean intermittent self-catheterisation
- CRF
- case report form
- CRN
- Clinical Research Network
- CUA
- cost–utility analysis
- DMC
- Data Monitoring Committee
- e-CRF
- electronic case report form
- eGFR
- estimated glomerular filtration rate
- EUCAST
- European Committee on Antimicrobial Susceptibility Testing
- GBP
- Great British pound
- GCP
- Good Clinical Practice
- GP
- general practitioner
- HCP
- health-care professional
- ID
- identification
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ITT
- intention to treat
- MCS
- mental component summary
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MS Society
- Multiple Sclerosis Society
- NCTU
- Newcastle Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PCS
- physical component summary
- PI
- principal investigator
- PIC
- participant identification centre
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RSI
- Reference Safety Information
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAR
- serious adverse reaction
- SD
- standard deviation
- SF-36v2
- Short Form questionnaire-36 items version 2
- SF-6D
- Short Form questionnaire-6 Dimensions
- SmPC
- Summary of Product Characteristics
- SOP
- standard operating procedure
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TSQM
- Treatment Satisfaction Questionnaire for Medication
- UTI
- urinary tract infection
- WTP
- willingness to pay
