Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/189/06. The contractual start date was in April 2014. The draft report began editorial review in October 2016 and was accepted for publication in September 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Rob Anderson contributes to the work of the National Institute for Health Research (NIHR) Health Services and Delivery Research (HSDR) programme as an unpaid member of their funding advisory panel (a researcher-led funding stream). Rod S Taylor is the chairperson of the NIHR HSDR researcher-led panel, a member of the Health Technology Assessment (HTA) Efficient Study Designs Board, the NIHR Priority Research Advisory Methodology Group, the HTA General Board, the HTA Themed Call, the Core Group of Methodological Experts for the NIHR Programme Grants for Applied Research programme and the HSDR Commissioning Board (commissioned and researcher led). Willem Kuyken receives royalties from the publication of Kuyken W, Padesky CA, Dudley R, Collaborative Case Conceptualization, New York, NY: Guildford Press; 2011, outside the submitted work.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Richards et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Coronary heart disease
Globally, coronary heart disease (CHD) is the single leading cause of death. The World Health Organization estimated that ischaemic heart disease accounted for 7.4 million deaths in 2012, which is around one-third of all deaths globally. 1 In the UK, 1 in 10 women and 1 in 7 men died from CHD in 2015 (around 70,000 deaths per annum), with most deaths caused by myocardial infarctions (MIs). 2 However, the UK mortality rate from CHD is falling, largely through the introduction of evidence-based treatments and lifestyle changes leading to reductions in major cardiac risk factors (mostly smoking). The successful fall in mortality rate means that many more people are living with heart disease and may need support to manage their symptoms and prognosis. In 2015, around 2.3 million people in the UK were living with CHD, over 60% of whom were male.
As a consequence, the need to address stress, psychosocial factors (e.g. lack of social support) and other underlying mood disorders (such as depression or anxiety), has long been recognised as important within conventional cardiac care in the UK,3 Europe4 and the USA. 5
Depression and coronary heart disease
Among people who have experienced acute coronary syndromes [ACSs including ST elevation MI (STEMI), non-STEMI (NSTEMI) and unstable angina], prevalence rates for major depression are estimated to be around 20% in studies using structured clinical interview schedules. 6 The rate of depression has also been shown to be raised in individuals following coronary artery bypass grafting,7 people with unstable angina8 and those experiencing chronic heart failure. 9 Irrespective of the underlying coronary condition, the rates of depression greatly exceed the UK general population rate of 2.6%. 10
Depression is important among people with CHD, as it predicts a range of negative medical outcomes, including greater morbidity and mortality,11–15 poorer health-related quality of life (HRQoL),16–18 higher use of routine and unscheduled health care19,20 and, therefore, increased health-care costs. 21 The nature of the association between depression and CHD is complex and the mechanisms underpinning this association are unclear, but may include biological and behavioural processes (e.g. poorer adherence to cardiac risk factor management), or may be confounded by shared genetic vulnerability, environmental stresses or perseverative negative cognitive processes. 22 New-onset depression is associated with an approximate doubling of the risk of subsequent incident ACS,23,24 the worsening of associated heart failure25 and increased rates of cardiac mortality. 18 It remains unclear, however, whether new-onset depression is particularly ‘cardio-toxic’22 or whether its apparent associations with poor cardiac outcomes is confounded by, for example, the severity of the underlying cardiac disease. 18
Depression that predates an ACS, on the other hand, differs from new-onset depression in that it is predicted by risk factors that are similar to depression in the general population, including being of young age, lacking social support, experiencing ongoing life difficulties and a past history of psychiatric disorder. 22 Although such depression is associated with poorer medical outcomes, compared with people who are not depressed, the association with adverse medical outcomes appears to be less strong than that for new-onset depression. 14
In light of the high prevalence of depression among people with CHD and its association with poor medical outcomes, there is widespread national and international recognition that detection and treatment of such depression is important. 3–5
Usual health care for people with cardiac heart disease and depression
Cardiac rehabilitation service provision
In the UK, the NHS routinely offers multidisciplinary cardiac rehabilitation to people who experience an acute cardiac event. These are patients with CHD referred following admission with an ACS. The majority will have had coronary revascularisation, usually percutaneous coronary intervention (PCI) for STEMI, or coronary artery bypass graft (CABG) surgery. Many patients with heart failure may also be admitted with an ACS, and are also eligible for cardiac rehabilitation. Some patients have concomitant valve surgery with CABG, and others may have had a pacemaker or defibrillator in the context of CHD. Some patient groups, such as people with heart failure, who might benefit from cardiac rehabilitation have historically been excluded from cardiac rehabilitation. However, recent national audit data suggest that such exclusions are diminishing and that the diversity of case mix is increasing. In the UK in 2014–15, an estimated 82,127 patients commenced cardiac rehabilitation, including individuals with a primary indication of MI (19.6%), MI and PCI (28.1%), PCI (14.1%), CABG (14.8%), heart failure (4.4%), angina (3.6%), valve surgery (5.9%) and other or unknown conditions (e.g. having a pacemaker, implantable cardioverter defibrillator; 9.6%). 26
Guidance on the recommended content of usual cardiac rehabilitation care has been published by the British Association for Cardiovascular Prevention and Rehabilitation (BACPR),3 the contents of which are broadly comparable with that which is recommended across Europe4 and the USA. 5 Core components include education; exercise and physical activity; diet and weight management; medical management and psychological support. Cardiac rehabilitation follows a seven-stage care pathway (stages 0–6), as illustrated in Figure 1 and Box 1. 27
FIGURE 1.
Cardiac rehabilitation standard patient care pathway. CR, cardiac rehabilitation.

The pathway commences when a patient presents after an acute coronary event, and is identified and referred to a cardiac rehabilitation team by the hospital staff.
Stage 1After the initial referral is received, it is managed by a cardiac nurse specialist who approaches the patient, often on the hospital ward, to begin the rehabilitation process through the provision of clinical advice and educational materials. Where appropriate, the individual is also offered a clinic appointment at a community-based service after their discharge from hospital.
Stages 2 and 3Individuals who elect to attend the clinic appointment with a cardiac nurse receive a multidimensional assessment, covering both physical and mental health status (stage 2), and a care plan is developed to support their rehabilitation needs (stage 3). Individuals deemed medically suitable for a cardiac rehabilitation are then invited to attend the next available structured programme in their locality.
Stage 4Structured cardiac rehabilitation programmes offer participants the opportunity to receive further education and support in relation to cardiac risk factor modification and psychosocial health, as well as undertake a supervised exercise programme. Current guidance recommends that programmes are delivered twice weekly over a period of 8 to 12 weeks. 27
Stages 5 and 6On completion of the structured cardiac rehabilitation programme (or at the point the patient chooses to withdraw from the care pathway), when possible, the cardiac nurse specialist conducts a final assessment of the individual’s physical and mental health status (stage 5), before organising the discharge into existing community services, making any referrals that are deemed appropriate (stage 6).
The organisation and delivery of cardiac rehabilitation services are currently in a state of flux. In 2011–12, in the UK, The National Audit of Cardiac Rehabilitation: Annual Statistical Report 201328 identified 290 operationally distinct, comprehensive cardiac rehabilitation programmes (stages 2–5); by 2014–15, 308 core programmes were identified. 26 While working towards nationally agreed standards and a standardised patient care pathway, there remains considerable regional variation in the staffing and skill mix of individual services.
Patient attrition across the cardiac rehabilitation pathway also remains an area of significant challenge. It has been estimated that, for people with one of the four most prevalent indicated conditions (MI, MI and PCI, PCI or CABG) referred to cardiac rehabilitation in the UK, uptake is around 47% and varies considerably by condition (38%, 54%, 40% and 59%, respectively). 26 The number of individuals who go on to attend a structured rehabilitation programme is strongly influenced by gender. It has been estimated that around two-thirds of potentially eligible men start structured rehabilitation, compared with less than one-third of women, and the reasons underlying these differences are not well understood.
As part of the initial cardiac rehabilitation assessment with a nurse specialist (stage 2), individuals are usually asked to complete the Hospital Anxiety and Depression Scale (HADS)29 to assess mood. In 2014–15, it was estimated that 12% of patients exhibited borderline depressive symptoms (i.e. a HADS-D score of 8–10) and 7% were depressed (i.e. a HADS-D score of ≥ 11) during this initial assessment. Of the individuals who completed the final assessment of a structured cardiac rehabilitation programme (stage 5), 8% exhibited borderline depressive symptoms and 4% were depressed during this final assessment. There was considerable regional variation observed in the change scores between localities. 26 These findings indicate that there is considerable scope to improve and standardise treatment for depression among people undergoing cardiac rehabilitation.
Despite the burden of depression, in 2013, only 25 out of 260 cardiac rehabilitation services (10%) reported receiving direct psychologist input. 30 However there is recent evidence that psychologist input is increasing, with 48 out of 261 programmes (18%) reporting such input in 2014. 26 Notwithstanding this progress, the evidence still suggests that patients’ depression usually remains untreated. 31 Locally agreed referral protocols providing for access to psychological care at either the tertiary (stage 1) or community (stages 2–5) levels may be agreed. However, the precise content of such protocols, and the consistency of their implementation, is not clear from audit data.
Although it would appear that structured management of depression is not routinely provided within the majority of cardiac rehabilitation programmes, individuals may access mental health care through existing care pathways for depression provided by other mainstream NHS services. 32–34 Notwithstanding this, there is a need to deliver models of care to increase access to evidence-based depression treatments for people undergoing cardiac rehabilitation.
Effectiveness of psychological care for people with depression
Effective care for people with depressive symptoms includes the provision of psychological and/or pharmacological interventions, as well as the effective co-ordination of mental health-care services. 32–34
Effectiveness of psychological therapies in the general population
Psychological treatments, such as cognitive behavioural therapy (CBT), interpersonal therapy and antidepressant medication, are the mainstay evidence-based treatments for acute depression. Both are recommended by the National Institute for Health and Care Excellence (NICE) as first-line treatments. 33,34 However, there are problems with antidepressant medications, which include side effects, poor patient adherence and relapse risk on cessation of prescribing. Service user organisations and mental health policy advisors advocate greater availability of a range of psychological therapies, which many people prefer, in part because of the problems with antidepressant medications, but also because patients can learn skills to help manage depression. 35
Cognitive behavioural therapy has been shown to be of similar efficacy to antidepressant medication. 36 However, CBT offers two major advantages: (1) it is consistent with many service users’ preferences for non-pharmacological treatment; and, (2) it modifies the illness trajectory, as its benefits continue post treatment, by teaching skills to prevent depressive relapse in the long term. However, CBT may not suit everyone, as not all individuals engage or adhere to it because of a combination of factors (e.g. the investment of time, the burden of homework and the need to make cognitive and behavioural changes). 37 In addition, CBT is typically offered only by specialist CBT therapists, because of the significant training required; the high costs of training and employing sufficient therapists may therefore limit attempts to access CBT. The Improving Access to Psychological Therapies (IAPT)38–40 programme in England has done much to enhance access to psychological treatments (recovery rates are at approximately 50%), and recently has extended its scope to people with depression and long-term conditions, including CHD. However, the capacity of the IAPT programme to reach this patient group and its acceptability are as yet untested.
Another psychological therapy that is a potential alternative to CBT is that of behavioural activation (BA). BA alleviates depression by focusing directly on changing behaviour. 41 Behavioural theory postulates that depression is sustained by avoiding many usual activities, and as individuals withdraw and disrupt their basic routines, they become isolated from positive reinforcement opportunities in their environment. Thus, individuals may end up stuck in a cycle of depressed mood, decreased activity and avoidance of activities. 41 BA systematically disrupts this cycle through encouraging individuals to initiate action despite the presence of negative mood, counteracting their natural tendency to withdraw or avoid activities. 42 Although CBT also incorporates behavioural elements and may also increase activities, its primary techniques focus on changing maladaptive beliefs43 by initiating behavioural experiments to test specific beliefs. BA also explicitly prioritises the treatment of negatively reinforced avoidance and rumination. The rationale for BA is simpler to understand and operationalise for both patients and mental health workers. The focus of BA on context and functioning is also closely aligned to the ethos of cardiac rehabilitation services. The relative simplicity of BA makes it more straightforward to train non-mental health nurses in its application. 44–46
Evidence regarding the clinical effectiveness of BA for the treatment of depression in general populations is available from two recent systematic reviews. 47,48
Ekers et al. 47 synthesised evidence from randomised controlled trials (RCTs) of BA for depression with either control patients [waiting list, usual care (UC) or placebo] or participants taking antidepressant medication. Data from 25 trials (1088 participants) were pooled in a meta-analysis, which reported post-treatment depressive symptom levels to be significantly reduced with BA compared with controls [25 studies, n = 1088; standardised mean difference (SMD) –0.74, 95% confidence interval (CI) –0.91 to –0.56]. Although far fewer trials compared post-treatment outcomes for BA with those for antidepressant medication, the effect size estimate favoured BA (four studies, 283 participants; SMD –0.42, 95% CI –0.83 to 0.00). However, these data should be interpreted with some caution, as the upper boundary of the 95% CI bordered the value of no effect.
A second review, by Shinohara et al. ,48 compared the effectiveness of behavioural therapies for the management of depression with other psychological therapies within five major categories (cognitive–behavioural, third-wave cognitive–behavioural, psychodynamic, humanistic and integrative therapies). Synthesising data from 18 trials (690 participants), the authors concluded that the treatment response rates of behavioural therapies were comparable to other psychological therapies [risk ratio (RR) 0.97, 95% CI 0.86 to 1.09] and were broadly acceptable to participants.
It is noteworthy that both review teams noted that the methodological quality of selected studies was low and that there remains some uncertainty regarding the effectiveness of BA,47,48 particularly with regard to the longer-term outcomes. 47 However, a very recent National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme-funded non-inferiority RCT [the Cost and Outcome of Behavioural Activation versus Cognitive Behavioural Therapy for Depression (COBRA) trial]49 comparing BA with CBT has shown that BA is not inferior to CBT for the treatment of depression. 50 In this trial, which randomised 440 participants to BA or CBT, 66% of participants receiving BA or CBT were recovered from depression at 12 months post randomisation, with benefits extending to the same degree at the long-term 18-month follow-up point. The COBRA trial also reported the costs of BA to be lower than CBT (an incremental cost-effectiveness ratio of –£6865), with this difference largely attributable to BA being delivered by people with less expertise in psychological therapies. This finding is consistent with data reported from a small RCT of BA (n = 24) versus treatment as usual (n = 23), which reported an incremental cost-effectiveness ratio of –£5756 in favour of BA. 51 The evidence, therefore, is now much clearer that BA is an effective, and less costly, front-line treatment for depression in general populations.
Clinical effectiveness of collaborative care approaches in the general population
Despite the proven clinical effectiveness of depression treatments, a number of studies have shown that individuals often have difficulty accessing or maintaining contact with high-quality mental health care. Under these circumstances, effective care for people with depression may require a system-level intervention aimed at overcoming barriers to accessing services and increasing co-ordination of mental health care. Such system-level interventions can be managed in primary care as part of a stepped-care algorithm to ensure optimal access to, and co-ordination of, psychological or pharmacological interventions of proven clinical effectiveness. 32–34
Collaborative care is one example of such a system-level intervention aimed at overcoming barriers to mental health care for people with depression. Collaborative care is a complex intervention52,53 in which a number of health professionals work together to ensure the individual accesses appropriate, evidence-based care to suit their mental health-care needs. In this model, a case manager trained in mental health-care co-ordination works closely with a medical doctor [often the person’s general practitioner (GP)] and mental health specialist services. The case manager is responsible for generating a structured management plan to support the patient’s access to evidence-based treatment based on best-practice guidelines. The case manager will then implement the care plan, providing the patient with scheduled follow-ups to monitor their progress (in person or over the telephone/internet), provide specific interventions (or make the appropriate referrals), encourage treatment adherence and monitor symptoms. Another core goal of such a model is to enhance interprofessional communication across the wider care team.
A 2012 Cochrane review synthesised evidence from 79 RCTs (24,308 participants) comparing collaborative care with either UC or alternative treatments for people with depression and anxiety. 53 Although the evidence was of variable quality, the primary analysis found collaborative care to be superior to the comparator condition for reducing depressive symptoms in the short term (0–6 months, 30 studies, 5984 participants; SMD –0.34, 95% CI –0.41 to –0.27) and medium term (7–12 months, 13 studies, 4092 participants; SMD –0.28, 95% CI –0.41 to –0.15). Although one study observed long-term effects (13–24 months) in favour of collaborative care, as these data were derived from only one study (1379 participants; SMD –0.35, 95% CI –0.46 to –0.24), the generalisability of this finding should be treated with caution. There was also some evidence of benefits in other outcomes including medication use, mental HRQoL and participant satisfaction, although benefits in terms of physical quality of life remained uncertain. Although many of the published trials are not from the UK, a recent large Medical Research Council (MRC)/NIHR-funded two-arm cluster RCT (n = 581) found collaborative care to be clinically effective (and cost-effective) for people with depression in UK primary care, and of particular note is that two-thirds of this trial sample also had comorbid physical health problems. 54 A second recent cluster RCT found similar results when testing collaborative care exclusively in a UK primary care-based population with depression and a comorbid physical health problem (diabetes mellitus or CHD). 55
Clinical effectiveness of psychological care for people with coronary heart disease
In the previous section, Effectiveness of psychological therapies in the general population, we reviewed the evidence of clinical effectiveness of psychological therapies and mental health collaborative care models tested in the general population samples of people with depressive symptoms. There is also a considerable body of evidence regarding the clinical effectiveness of psychological therapies designed specifically for use for people with CHD. Similar psychological techniques (e.g. CBT, BA) may be applied in isolation or combined with other psychological interventions (e.g. stress management or problem-solving skills training) as part of multifaceted treatment packages. The target population and setting can vary. Some interventions target people with coronary disease irrespective of their baseline psychological health, whereas others adopt a more selective approach, offering interventions only to those individuals screening positive for an existing psychological condition.
Two systematic reviews56,57 have used meta-analytic techniques to synthesise evidence on the clinical effectiveness of psychological interventions for people with CHD. Direct comparisons between these reviews are problematic because of the important differences in the application of methods and definitions. Both reviews selected studies in which the direct effects of psychological interventions were compared with a comparator group (mostly usual medical care with or without cardiac rehabilitation) on measures of depressive symptoms. However, Dickens et al. 56 selected studies with any length of follow-up (ranging from 5 days to 12 months), and reported only psychological outcomes. In contrast, Richards et al. 57 selected studies that followed up participants for a minimum of 6 months (range 6 months to 10.7 years) and reported evidence for clinical events (e.g. mortality, cardiac morbidity), as well as the psychological outcomes of depressive symptoms and anxiety and stress levels. Both reviews also undertook metaregression analyses, seeking to explore whether or not intervention effectiveness was mediated by their use in unselected populations, as opposed to targeting people with existing psychological conditions. Although the studies applied different taxonomies, both reviews also sought to identify potential explanatory components of psychological interventions. Notwithstanding differences in study methods, there was some consistency in the findings from these reviews.
Analysing data from 62 studies (17,397 participants), Dickens et al. 56 observed a small but statistically significant improvement in depression for psychological interventions compared with UC (SMD 0.18, 95% CI 0.12 to 0.24). Subgroup analysis identified certain treatment components with small beneficial effects on depressive symptoms, including general education, problem-solving, skills training, exercise, CBT and relaxation. In a subgroup analysis, there was also a small effect observed in favour of psychologically based interventions (SMD 0.21; 12 trial arms), when targeted at people with coronary disease and clinical depression, as compared with untargeted populations.
In the second review, Richards et al. 57 synthesised data from 35 studies (10,703 participants), including 12 studies that recruited patients with an established psychopathology (eight studies selected people with depression), and from 19 studies reporting some levels of psychopathology within their participant samples. The interventions tested were mostly multifactorial in nature, designed to address a number of different treatment goals (e.g. reducing stress levels, improving mood states, improving coping strategies, emotional support), through combining psychological techniques (e.g. stress management, CBT or behavioural therapies).
Richards et al. 57 found no significant differences in the effects of psychological interventions versus the comparator group in terms of total mortality (23 trials, 7776 participants; RR 0.90, 95% CI 0.77 to 1.05) and the risk of subsequent revascularisation (13 trials, 6822 participants; RR 0.94, 95% CI 0.81 to 1.11) or of a non-fatal infarction (13 trials, 7845 participants; 0.82, 0.64 to 1.05). However, there was some evidence that cardiac mortality was reduced (11 trials, 4792 participants; 0.79, 95% CI 0.63 to 0.98). When considering psychological outcomes, interventions based on psychological therapies resulted in significant improvements in the participant-reported levels of depressive symptoms (19 trials, 5825 participants; SMD –0.27, 95% CI –0.39 to –0.15), anxiety levels (12 trials, 3161 participants; SMD –0.24, 95% CI –0.38 to –0.09) and stress levels (eight trials, 1251 participants; SMD –0.56, 95% CI –0.88 to –0.24). Metaregression exploring a limited number of intervention characteristics found no significant predictors of intervention effects for the outcome of cardiac mortality. Unlike Dickens et al. ,56 psychological therapies combined with adjunct pharmacology (when deemed appropriate) prescribed for an underlying psychological disorder were found to be more effective than interventions that did not combine psychological and pharmacological therapies (difference in effect size β = –0.51; p = 0.003). For anxiety, interventions recruiting participants with an underlying psychological disorder appeared to be more effective than those delivered to unselected populations (β = –0.28; p = 0.03), although this finding was not replicated for the outcome of depressive symptoms.
Although somewhat different in focus and methodology, both reviews consistently demonstrated that providing psychological interventions for people with established CHD yields small, but statistically significant, improvements in participant-reported levels of depressive symptoms. However, given the multicomponent nature of the interventions tested, combined with the diverse patient groups and settings in which such interventions were tested, it is not possible to ascertain which psychological treatment components may be most effective and for whom.
Clinical effectiveness of collaborative care approaches for people with heart disease
More recently, rather than evaluating the clinical effectiveness of psychological interventions, research is focusing on the clinical effectiveness of collaborative care for people with coronary disease and depression. Consistent with the care models developed in general populations, the focus is not to evaluate the clinical effectiveness of individual psychological therapies per se. Rather, the aim of collaborative care is to ensure that individuals with heart disease and depressive symptoms receive best-practice psychological care through appropriate assessment, symptom monitoring, referral to psychological therapists and, where appropriate, the provision of pharmacological therapy. Psychological care is delivered by a care manager and the wider clinical care team members, who work with the individual over a period of time to tailor their preferences for care with recommendations based on their symptoms and previous experiences. In some models, the care manager also provides elements of psychological therapy.
A recent systematic review by Tully and Baumiester58 synthesised data from six RCTs (1284 participants), which randomised participants to either collaborative care or a UC comparator group. Although there was no evidence of a sustained reduction in major adverse cardiac events (including mortality or morbidity) arising from collaborative care, small reductions favouring collaborative care were found in the short term (3–12 months) for depressive symptoms (six studies, 1277 participants; SMD –0.31, 95% CI –0.43 to –0.19; p < 0.00001) and anxiety symptoms (four studies; SMD –0.36, CI –0.54 to –0.17; p < 0.0001), and mental HRQoL improved (five studies; SMD 0.24, 95% CI 0.08 to 0.38; p = 0.003), although no differences were observed for physical HRQoL.
Rationale for current research
This study was undertaken in response to a NIHR commissioning brief in 2013, which invited research addressing the question: What is the clinical and cost-effectiveness of enhanced care for new onset depression post cardiac event? The brief envisaged that the intervention would involve enhanced, non-pharmacological care for depression tailored to adults with new-onset depression after an acute cardiac event. The intervention was required to be easily deliverable in the NHS, and integrated within existing NHS cardiac rehabilitation during the recovery phase.
In responding to this brief, our review of the literature regarding the clinical effectiveness of psychological treatments was inconclusive. Recent systematic reviews56–58 report modest, but statistically significant, reductions in depressive symptoms for patients with coronary disease receiving psychological treatments, although the methodological quality of studies selected is such that it precludes firm conclusions regarding which treatment components are most effective and who should be targeted for intervention.
Most intervention studies have recruited participants immediately following an acute cardiac event, and the complexity of depression in that period may have contributed to the lack of conclusive evidence regarding the treatment response. The reasons for the limited benefits of conventional depression treatments in patients with CHD are not clear, although three factors are noteworthy.
First, depression that starts after an ACS is different from depression in the general population. 22 Post-ACS depression does not have the usual risk factor profile as that observed for the general population; instead, it is associated with ongoing cardiac symptoms and increased concerns about health,59,60 which may act to reduce the response to conventional treatment. 61 Second, established psychological treatments, such as CBT, that encourage recall of past experience and challenge maladaptive thoughts may be too traumatic for people who have suffered a recent life-threatening event. Finally, the psychological therapies available through IAPT may not be widely available to people with CHD. Indeed, in 2013 a qualitative study of people undergoing cardiac rehabilitation62 concluded that management of depression should be embedded within cardiac rehabilitation teams, rather than as another source of onward referral. As UK cardiac rehabilitation services experience significant patient attrition at each stage of the rehabilitation pathway (53% attrition prior to commencing a structured programme), and 19%26 of individuals are found to have depressive symptoms upon initial assessment, another outward referral for psychological care may be a barrier to accessing timely care.
If a relatively simple psychological treatment of proven benefit in general populations of people with depression, such as BA,47,50 has the potential to be safely and effectively embedded within the cardiac rehabilitation service, this could holistically tackle the depressive symptoms at the same time as physical rehabilitation, as part of patient case management. Similarly, acknowledging that attrition from rehabilitation is high, we also believe that any attempt to enhance psychological care should include training cardiac rehabilitation nurses (who routinely screen for depressive symptoms) to apply evidence-based mental health referrals. Such a mental health collaborative care approach should also be in place for patients completing structured cardiac rehabilitation programmes, but whose depressive symptoms do not respond to psychological treatment.
Chapter 2 Aims and objectives
Feasibility study aims
The overarching aim of the feasibility study was to design an enhanced psychological care (EPC) intervention for the management of patients with new-onset depressive symptoms, composed of care co-ordination and BA, and to test its implementation within routine cardiac rehabilitation settings. We also sought to specify the psychological components within UC for patients with depressive symptoms using cardiac rehabilitation services (NHS care pathway stages 2–5; see Figure 1) following an acute cardiac event. There were five objectives:
-
(1a) To describe the content of psychological support routinely offered by cardiac rehabilitation services, including active treatments offered by rehabilitation teams, or referral practices to existing NHS mental health services.
-
(1b) To develop, and refine, the underlying intervention theory of a standardised EPC intervention (including a supporting intervention manual and training package) for implementation by cardiac rehabilitation nurses.
-
(1c) To determine the feasibility, and acceptability, of implementing/experiencing EPC from the perspectives of cardiac rehabilitation staff and patients.
-
(1d) To describe process measures, including: (1) the number of patients with new-onset depressive symptoms identified during the initial cardiac rehabilitation assessment (NHS care pathway stages 2–3); (2) participant attendance at, and adherence with, the comprehensive cardiac rehabilitation programme with an embedded EPC aimed at treating depression, and the time elapsed to the start of treatment; and (3) the psychological care co-ordination activities provided to participants when exiting from the cardiac rehabilitation care pathway.
-
(1e) To develop and undertake preliminary testing of study methods (cardiac rehabilitation team and participant recruitment, data collection procedures) required to implement a pilot trial.
To address these objectives, a multimethod feasibility study was undertaken, including a qualitative study involving cardiac rehabilitation staff and patient participants (addressing objectives 1a, 1b and 1c) and a before-and-after observational study (addressing objectives 1b, 1d and 1e).
External pilot trial aims
The main aim of the pilot trial that followed the feasibility study was to test the methods and procedures required to undertake a fully powered evaluation of the clinical effectiveness and cost-effectiveness of cardiac rehabilitation teams implementing EPC for individuals with new-onset depressive symptoms using cardiac rehabilitation services compared with cardiac rehabilitation delivering UC. There were four objectives:
-
(2a) To quantify the flow of patients from the cardiac event to the 8-month follow-up for patients entering into the cardiac rehabilitation care pathway, and to document the flow of those participants who agreed to take part in the pilot trial (i.e. eligibility, recruitment and attrition).
-
(2b) To collect participant outcome data in order to estimate the standard deviation (SD) for various continuous outcomes to inform sample size calculations (number of cardiac rehabilitation teams and participants) for a definitive trial.
-
(2c) To establish the data collection methods required to support a definitive economic evaluation.
-
(2d) To gather qualitative evidence from patient participants (including participants who did or did not adhere to the EPC, and from those patients who declined to take part in the trial) and from nurses on the acceptability of receiving/implementing EPC, on the appropriateness of study methods and procedures, and on the content of usual psychological care within cardiac rehabilitation services.
Chapter 3 Study methods
Consistent with MRC’s guidance for the development and evaluation of complex interventions,63 a two-phase study was undertaken. The first phase included a multimethod feasibility study composed of qualitative and observational methods, and the second phase entailed a pilot cluster RCT, which also tested the methods for economic data collection and included a nested qualitative study.
Feasibility study design
The feasibility study was designed to support the development and preliminary testing of the EPC intervention, and to undertake an early evaluation of study methods and procedures before undertaking the pilot RCT. A before-and-after observational study was combined with observations of UC and qualitative interviews with cardiac nurses and patient participants.
Observational study
Feasibility study intervention
Enhanced psychological care was a complex intervention, comprising nurse-led BA therapy and mental health-care co-ordination. The intervention was developed and delivered by experts within the research team who had used BA in other clinical trials conducted in general populations of patients with depression. 44,50,54 Current UK NICE guidance33 was used as the basis for the mental health-care co-ordination component of EPC. This guidance recommends the regular review of participants’ symptoms, including them in decisions about their treatment and, when necessary, referring them to their GP and/or existing community or primary care mental health services, either during or on their point of discharge from EPC.
Before taking part in the study, nurses from participating cardiac rehabilitation teams underwent a 2-day training course covering the topics of BA, mental health-care co-ordination, managing mental health risk issues (e.g. suicide ideation) and delivery of the intervention. Each nurse was given a manual (version 2, 20 August 2014; available on request from the study team) providing detailed information to support their delivery of EPC. Once trained, nurses were asked to start consecutively screening all patients for depressive symptoms during their initial cardiac rehabilitation assessment and to offer study entry to eligible participants. Nurses delivering EPC to one or more patients received weekly supervision from a clinical supervisor with mental health expertise working within the study team. Supervision was conducted by telephone, with nurses receiving individual supervision.
In the UK, the NHS routinely offers multidisciplinary cardiac rehabilitation following a seven-stage standard patient care pathway (see Figure 1). 3,27 Cardiac rehabilitation programmes usually consist of an initial assessment, with a structured 6- to 8-week programme with up to two sessions each week. However, the precise content of a cardiac rehabilitation session can vary across sites, and could include a clinic appointment during which the patient’s underlying cardiac condition would be monitored/discussed, a rehabilitation fitness session would be overseen by a nurse or there would be a group education session. It was recommended that nurses provide EPC once per week to participants, at a point in a rehabilitation session that was deemed to be appropriate based on local circumstances.
At the start of rehabilitation, the nurse provided the participant with a CADENCE (enhanced psychological CAre in carDiac rEhabilitatioN serviCEs for patients with new-onset depression compared with usual care) participant handbook (version 2, 20 August 2014), and asked that they read it before the next session. The nurse then provided an individually tailored treatment programme of BA and care co-ordination (as appropriate) across the course of the rehabilitation programme. Although there was some flexibility in how EPC was delivered, some elements of the intervention were deemed to be central. The intervention protocol advised that each EPC session should comprise:
-
The monitoring of depressive symptoms, using the Patient Health Questionnaire-9 (PHQ-9),64,65 and of anxiety, using the Generalised Anxiety Disorder-7 (GAD-7)66 instrument; these tools replaced the HADS, which cardiac nurses had previously used to monitor symptoms of anxiety and depression; this change was implemented because both GPs and community mental health services routinely use the PHQ-9 and the GAD-7 to make treatment decisions/referrals; unlike the HADS, the PHQ-9 also asks individuals to report any suicidal ideation, the identification of which is core to the safe and effective management of depression. Thus, the change from the HADS to the PHQ-9 was intended to facilitate seamless mental health-care co-ordination by ensuring that clinicians communicated the symptoms burden and risk safely and effectively.
-
Monitoring of suicide and self-harm risk at every nurse–participant contact, and procedures to manage such risks should they arise.
-
Participant self-monitoring through the completion of a mood–activity diary between rehabilitation sessions; at each session, the participant and nurse undertook a functional analysis of the diary, aiming to identify patterns of behaviour linked to high mood. Participants were encouraged to identify valued activities, and triggers to ‘depressed behaviours’; after identifying different coping strategies, over the following week, participants were encouraged to schedule other routine, pleasurable and necessary activities to replace those behaviours associated with low mood.
-
Application of mental health-care co-ordination algorithms targeted at those individuals whose low mood had not resolved during EPC (a PHQ-9 score of ≥ 10), or participants who elected not to continue with BA as part of their rehabilitation programme. On completion of cardiac rehabilitation, the participant’s GP was routinely informed of the psychological care provided, and of the last PHQ-9 and GAD-7 scores recorded during symptom monitoring.
Nurses were asked to record patient participation with EPC in their nursing notes during the course of cardiac rehabilitation attendance.
Settings and participants
The observational study aimed to recruit up to 20 eligible participants from three locality-based comprehensive cardiac rehabilitation teams in Devon, south-west England. Early discussions with cardiac rehabilitation specialists suggested that the study intervention would be delivered by cardiac nurse(s) from locality-based teams that were operationally distinct (i.e. there was no crossover of staff) from neighbouring teams. Thus, in some teams it might be necessary to train more than one nurse, particularly if two nurses work interchangeably to provide continuity of care for individual patients within their locality.
Sample size
Consistent with the feasibility design, no formal sample size calculation was undertaken; rather, a target sample (three teams, 20 participants) was deemed sufficient to provide the preliminary data required to test our feasibility study aims; that is, ensuring that nurses had sufficient experience of delivering EPC (circa eight or nine patients per team) to comment meaningfully on the intervention design.
Participant inclusion and exclusion criteria
Inclusion criteria
Adult patients (aged ≥ 18 years) referred for cardiac rehabilitation based on local clinical referral protocols were eligible to take part in the observational study. The inclusion criteria included patients admitted with an ACS (i.e. STEMI or NSTEMI and unstable angina), and/or following a coronary revascularisation procedure (i.e. CABG or PCI). At the time this study commenced, this constituted the majority of patients referred for cardiac rehabilitation services in the UK [the 2012 National Audit of Cardiac Rehabilitation (NACR)28]. Patients with a new episode of depressive symptoms were identified through nurse screening during their initial cardiac rehabilitation appointment. Those patients scoring ≥ 10 using the PHQ-9 were eligible for inclusion (note that this meant that participating teams changed their usual practice of monitoring mood using the HADS29 to monitoring mood using the PHQ-9 and the GAD-7). This cut-off point was chosen to aid identification of people with at least moderate depression,67 but also to maintain alignment with primary care psychological services (IAPT), which offer treatment for people with PHQ-9 scores of ≥ 10 upon referral from GP-based services.
Exclusion criteria
Patients who reported that they were actively treated for depression (psychological or drug therapy) in the 6 months before their acute cardiac event were excluded. The nurse also excluded patients for whom there was evidence of alcohol or drug dependency, when the participant was acutely suicidal or when there was evidence of poorly controlled bipolar disorder or psychosis/psychotic symptoms based on a clinical review (and having sought external confirmation from the GP or other clinicians as required). Potential participants also needed to have sufficiently good English-language skills to engage with both the mental health-care co-ordination and BA components of the EPC intervention, or to be willing to work with a NHS translator if required, and to provide informed consent to take part.
Participant recruitment procedure
All cardiac rehabilitation nurses were trained to apply a structured checklist to ascertain participant eligibility based on the study’s inclusion/exclusion criteria. Eligible participants were identified by cardiac rehabilitation nurses during their initial clinic appointment (NHS care pathway stage 2/3) before commencing the structured rehabilitation programme (stage 4). Eligible patients were asked by the nurse to take part in the observational study and associated qualitative interview study, and provided with brief study information to take away and review. The nurse asked for patients’ permission to pass their contact details onto a researcher, who was independent of the clinical team.
Within 2 working days of receipt of a completed ‘permission for release of personal details’ form from the cardiac team, the researcher contacted the potential participant to discuss the study. During this initial telephone call, the researcher confirmed that the patient was currently not receiving any form of active treatment for their depressive symptoms. The individual was provided with the opportunity to ask any further questions about the study. For those individuals who were happy to progress, the researcher agreed a date for the face-to-face baseline home visit within 1 week, and sent a confirmation letter, which included a detailed participant information sheet for their review. During the baseline visit, the researcher once again briefly reviewed the study eligibility criteria to ensure that there had been no major changes in their treatment for depression since referral. The researcher then went through the participant information sheet and answered any remaining questions that the individual raised. The participant was reminded that their involvement was entirely voluntary, that their usual health care would not be affected by their decision to take part, and that they could withdraw from the study at any point without giving a reason. If the individual was happy to proceed, written consent was obtained prior to completion of the baseline assessment. The baseline assessment was typically conducted before the participant commenced their comprehensive cardiac rehabilitation programme. Mental health-care co-ordination was implemented for participants who subsequently elected not to attend the rehabilitation.
Data collection and analysis procedures
The aim of this feasibility study was to collect preliminary data regarding process measures relating to the flow of participants in the study and the adequacy of data collection procedures. We therefore restricted data collection to a baseline interview and a single 5-month follow-up, and a subset of process and outcome measures was assessed.
Process measures
Process data were collected in relation to patient throughput and intervention fidelity. To ascertain study eligibility and recruitment, cardiac rehabilitation nurses completed a screening log, recording the numbers of patients attending an initial nurse assessment, the proportion identified with depressive symptoms during this assessment, the prevalence of new-onset (as opposed to existing) depression and the number of patients who were deemed eligible for study participation. The nurse screening log also captured brief, anonymised data on patient sociodemographic characteristics (e.g. age, sex, ethnicity/preferred language) and on the clinical conditions resulting in the cardiac rehabilitation referral, allowing the characteristics of our sampling frame to be described. For eligible patients, the nurse also recorded the number of participants offered study entry and the number who later agreed to be contacted by a researcher. The research team documented the number of individuals who subsequently consented to take part and who underwent a baseline interview. Descriptive statistics documenting the flow of patients through the study procedures are presented.
For this study, intervention fidelity was assessed through a review of cardiac nurse notes conducted by the researcher. We documented the numbers of people recruited to the study who subsequently did not attend the structured rehabilitation programme. For participants who commenced intensive rehabilitation with EPC, we assessed adherence by recording the number of BA sessions offered and the number of sessions attended. We reviewed the nursing notes for all participants, and recorded the number with documentary evidence of mental health-care co-ordination on exiting cardiac rehabilitation and the type of mental health referrals made (if appropriate).
Adequacy of data collection procedures
As this was a feasibility study, we restricted our focus to testing the completeness of data relating to participant-reported outcome measures at baseline and the 5-month follow-up (i.e. excluding cardiac morbidity and mortality, and health-care resource use). A full description of the participant-reported outcome measures collected at both time points is provided under the description of the pilot trial methods (see Data collection procedures). Descriptive statistics were presented on the number of participants completing assessments and the completeness of data recorded therein. Descriptive summaries of the baseline and 5-month outcomes were also calculated.
Qualitative study
Data collection procedures
Although broadly following the NHS cardiac care pathway, discussions with clinical co-applicants and participating teams indicated that there was considerable variation between the cardiac teams on how cardiac rehabilitation was delivered. Thus, before EPC was operationalised by participating teams/nurses, the qualitative researcher conducted observations of UC within each study team to assess what variations existed. These observations indicated that the study teams varied in terms of their patient waiting list times, how cardiac rehabilitation was delivered, what room facilities were available to cardiac nurses and how many cardiac rehabilitation sessions were offered to patients. These insights informed the development of the intervention and the nurse training package prior to nurses being trained and EPC being implemented. During the qualitative study, observations were also made of the nurse training. All observations were recorded by the qualitative researcher using field notes. This researcher also took notes during the early informal discussions held with participating nurse teams about the practicalities of, and barriers to, EPC delivery.
Nurses were interviewed within 4 weeks of completing EPC training, and before they started to deliver EPC, to ascertain their views of the training and to identify any problems they envisaged in delivering EPC within their rehabilitation programme. Nurses were interviewed again towards the end of delivering EPC to study participants to explore their views on the acceptability of the intervention and the study materials, and the extent to which they thought EPC could be embedded within cardiac rehabilitation services.
Nurses’ experiences of delivering the intervention were also gauged through nurse/clinical supervisor sessions being observed and field notes being taken. In addition, the clinical supervisors wrote an anonymised summary of the nurses’ experiences, detailing the issues identified during supervisory sessions relevant to EPC delivery.
Patient participants were interviewed once they had completed their EPC to explore their views and experiences of receiving it. The aim was to interview approximately 15 patients, having purposefully sampled individuals to achieve variation in relation to participant age and gender, the recruiting team and treatment adherence (including patients who refused any cardiac rehabilitation, participants who fully engaged with EPC and individuals who undertook EPC but did not complete treatment).
Nurse and participant interviews were conducted by an experienced qualitative researcher. Topic guides were used to ensure consistency across the interviews (see Appendix 1). The content of the guides was informed by the aims of the study, the research team’s knowledge of the literature and the intervention and the insights gained through observations and discussions held early on in the study with nurses and the intervention developers. The nurse and patient interview topic guides were developed in parallel to ensure that key areas were explored during both sets of interviews, allowing findings from participant and nurse interviews to then be triangulated, increasing the confidence with which conclusions could be drawn. All nurse and participant interviews were digitally recorded and transcribed verbatim. Prior to analysis, the transcripts were checked against the audio-recordings for accuracy and anonymised, with individual participants being allocated a numerical identification code.
Protocol changes when implementing the qualitative feasibility study
A number of methodological changes were made to the protocol for the qualitative elements for the feasibility study. These were made during the course of the feasibility study in response to insights gained as the work progressed.
-
The original protocol stated that a focus group would be conducted with nurses directly following their training in EPC. Once the training package had been developed, it was clear that nurses would be expected to attend a 2-day training course and that there would be little time at the end of the second day to complete a focus group with those attending. To reduce the burden on nurses, a decision was made to replace the focus group with a brief one-to-one telephone interview conducted within 4 weeks of the training.
-
We had originally planned to interview patients who were eligible for the study but who declined to take up cardiac rehabilitation to ascertain the reasons behind their decision. However, it became apparent that patients who refused cardiac rehabilitation usually did not attend the initial appointment with the nurse. As this meant that we could not determine which of these patients would be eligible for study participation (i.e. those who had low mood), these interviews were not conducted.
-
Initially, we had planned to interview nurses on two occasions during the period in which they were delivering EPC. The first interview was scheduled to be conducted early on in the delivery of EPC to explore nurses’ initial experiences of delivering BA. The second interview was to be conducted once they had finished providing EPC. The first interview was dropped, as issues relating to the early EPC implementation formed the basis of clinical supervision sessions and, with the nurses’ consent, the supervisors were willing to let the qualitative researcher sit in on the supervision sessions.
-
The protocol stated that the qualitative researcher was to conduct observations of the nurses’ provision of the intervention to assess implementation and integration of EPC into existing care. However, as participant recruitment was much lower than anticipated (only one of the four nurses provided care to more than one patient), we elected not to conduct these observations, as we anticipated that the nurses would be uncomfortable with being observed at this early stage of delivering a new intervention.
-
Finally, we aimed to invite staff from the wider cardiac rehabilitation teams to contribute to a focus group discussion on whether or not the provision of EPC had affected them. However, it became clear that the cardiac rehabilitation nurses were working in isolation, and thus it was unlikely that the provision of EPC would impact upon other team members. Instead, a question was added to the nurse topic guide to enquire as to whether or not the nurses were aware of anyone else from the immediate clinical teams being affected by the intervention.
Our sponsors and funders were notified of all of these protocol changes.
Qualitative data analysis
Notes taken during the observations were read and re-read by the qualitative researcher, who then fed back key points that were relevant to the design and delivery of the intervention to the rest of the research team.
Interview data were analysed thematically, focusing on how study materials and nurse training could be improved in terms of making them more acceptable for nurses, and the intervention could be improved in terms of its effective implementation in practice and its acceptability to nurses and participants.
Transcripts from the nurse and participant interviews were read and re-read by two members of the research team to gain an overall understanding of the accounts given, to ascertain emerging themes and to develop an initial coding frame. The two researchers then independently coded a sample of transcripts and discussed their preliminary coding and interpretation of the data. The coding frame was then revised, with new codes being developed and existing codes being defined more clearly or deleted. Once both researchers had agreed the coding frame, transcripts were manually coded and data under each code were summarised in a table, using an approach based on framework analysis. 68 Having done this, comparisons were then made within and across the data. When coding the nurse and patient interviews, similar codes were used, when possible, to allow for triangulation of findings between data sets. Nurse and patient data sets were analysed separately to ensure that a thorough understanding of each group’s account was established before comparisons were made between the views held by patients and nurses.
The findings from the observations and interviews were fed back and discussed within the wider research team throughout the feasibility study, so that changes could be made to the intervention and study materials prior to undertaking the pilot trial.
Design of the pilot randomised controlled trial
The second phase of research included a pilot RCT, the testing of economic data collection procedures and a nested qualitative study.
An external pilot cluster RCT69,70 was undertaken. We recruited eight comprehensive cardiac rehabilitation teams (clusters) and randomised five of these to EPC plus UC embedded within cardiac rehabilitation (intervention), and the other three to usual cardiac rehabilitation care (control). The decision to allocate more clusters to the intervention arm was informed by data emerging from our feasibility study (see Chapter 4, The transition between feasibility study and pilot study), which suggested that participant recruitment would be slower than anticipated. We therefore sought to ensure that a sufficient number of nurses and patient participants had exposure to EPC to support the aims of the qualitative study interviews (i.e. recruiting enough participants with experience of EPC).
Randomisation and allocation concealment
A cluster randomised design was adopted in preference to individual randomisation to ameliorate concerns around potential contamination between the intervention and control arms (i.e. intervention participants coming into direct contact with control participants and sharing aspects of their treatment). Our EPC intervention included training for cardiac rehabilitation nurses to provide mental health-care co-ordination to participants with depressive symptoms. Once trained, we judged that it would be very difficult operationally for the nurse to apply care co-ordination to only the subset of their patients randomised to receive EPC (as would be required with individual randomisation).
Randomisation was carried out by the trial statistician (FCW), who was independent of the recruitment of rehabilitation teams or patient participants. The allocation sequence was generated using computer-generated random numbers. Cluster randomisation was balanced (to the extent possible) by team type (community, hospital or mixed community and hospital teams) and patient throughput at the initial cardiac rehabilitation assessment (NHS care pathway stage 2/3; categorised as low/high). The latter cut-off point between low/high throughputs was determined post hoc after scrutinising the mean monthly throughput across cardiac rehabilitation teams, with ‘low’ being equivalent to ≤ 22 patients per month, and ‘high’ being equivalent to > 22 patients per month (these cut-off points were based on a natural break in the distribution of team throughput assessed during a preparatory audit collecting data for a 2-month period in January and February 2015). The throughput of new patients assessed by cardiac rehabilitation teams was selected as a stratifying variable, as this appeared to be a better predictor of team workload than staffing levels, and would ensure that a sufficient number of patient participants were recruited into each trial arm. Team setting was selected as a stratifying variable, as our feasibility study suggested that teams working in hospital or community settings, or in a combination of both, may encounter different resource issues that could affect the delivery of EPC.
Randomisation took place after all eight cardiac teams had been recruited. Each team was informed of its allocation by the trial researcher.
Trial interventions
Usual care
Usual care is defined here as the standard NHS care pathway, commencing at the point of assessment undertaken by a cardiac rehabilitation specialist nurse from a locality-based cardiac rehabilitation team (see Figure 1). 3,27 Of the people invited to attend an initial cardiac rehabilitation assessment, it is estimated that less than half will attend a comprehensive rehabilitation programme offered within 2–10 weeks of assessment (stage 4). 28 Treatment as usual typically includes intensive rehabilitation for one or two sessions per week for approximately 8 weeks. Sessions generally last around 2 hours, and include structured exercise, education (e.g. managing lifestyle and cardiac risk) and some psychological input (e.g. relaxation, stress management) in order to meet the core standards of care described by the BACPR. 3 On exiting a comprehensive rehabilitation programme, patients receive a final assessment (stage 5), and discharge arrangements are made to community services (stage 6).
There is considerable debate as to what constitutes standard psychological care within cardiac rehabilitation. Although most nurses will assess the patient’s mood using the HADS during the initial assessment, and on completion of the rehabilitation programme, little is known about how this information is used as part of care planning. National audit data found that psychological expertise within locality-based cardiac rehabilitation teams is uncommon (< 10%),28 although some teams may refer patients onto specialist mental health services if they have specific concerns regarding an individual’s mental well-being.
Enhanced psychological care
Based on our feasibility findings (see Chapter 4, Refinements to the enhanced psychological care intervention prior to the pilot study), we made some important modifications to the EPC intervention before starting the pilot study. The most fundamental changes in the delivery of EPC between the feasibility and pilot phases were (1) an increased emphasis on the mental health-care co-ordination component of EPC and (2) a shift from a ‘nurse-delivered’ to a ‘participant-led’ programme of self-help BA embedded within cardiac rehabilitation, reflecting the need to reduce the impact of EPC on nurse time. The EPC training programme and supporting materials, including the participant handbook (version 5, 4 June 2015) and nurse handbook (version 5, 29 May 2015; available from the NIHR project website), were amended based on feasibility findings (see Chapter 4, The transition between feasibility study and pilot study). Nurses were trained to implement mental health-care co-ordination,33,34 including an embedded participant-led BA programme42,44–46,71,72 using the restructured materials and training package. New materials were also provided to support nurses in delivering EPC, including a structured form to insert in the participant’s clinical record for nurses to capture core data on what aspects of EPC were offered at each session (see the EPC session planner in Appendix 2).
Patient-led, nurse-supported behavioural activation therapy
As in the feasibility study, the main psychological intervention consisted of six to eight sessions of BA flexibly delivered face to face or via telephone (the latter was used for the follow-up session only). Sessions focused on the three key components of BA: activity monitoring (recording what people were doing), functional analysis (developing a shared understanding of how activity relates to mood) and activity scheduling (increasing pleasurable, routine and necessary activities that are less likely to maintain depression). Sessions focused on encouraging the participant to follow the participant handbook and review progress, and decreased the emphasis on the nurse leading the intervention. As a result, sessions were shorter, lasting 10–20 minutes, thereby reducing the burden on the nurse’s time.
The participant handbook (version 5, 4 June 2015) consisted of a structured programme aimed at enabling participants to identify and re-engage with sources of positive reinforcement from their environment and develop future strategies for managing their depressive symptoms. The handbook described the nature of the BA intervention and the structure of the treatment patients would receive, and arrangements were made to meet again to discuss the workbook and start the therapy. A functional analytical approach was adopted as part of BA; participants were helped to develop an understanding of behaviours that might interfere with meaningful, goal-oriented behaviours (e.g. negative avoidant behaviours). The handbook also explained how to self-monitor mood, and then how to identify behaviour patterns associated with low mood. Participants were encouraged to develop alternative behaviours that were goal-orientated, targeting routine, pleasurable and necessary activities, and to undertake activity scheduling of these identified behaviours. Although our pilot intervention involved a participant-led self-help BA manual, the cardiac rehabilitation nurses were trained to support participants actively.
As in the feasibility study, each session began by assessing the patient’s symptoms (using the PHQ-9 and the GAD-7) and assessing any identified risks of self-harm, suicide or risks to others. In session 1, the treatment options were briefly discussed (BA with a cardiac nurse, referral to a GP or local IAPT services, or, if appropriate, referral to psychological service provision associated with cardiac service, if available). Participants choosing BA with the rehabilitation nurse were provided with a participant handbook (version 5, 4 June 2015). The content of subsequent sessions remained flexible to accommodate different rates of progress, but also to accommodate issues with physical health that frequently came up and needed to be dealt with immediately. As serious physical problems could arise unpredictably and derail the discussions about BA, to operationalise the treatment and allow calculation of the dose of BA (the number of sessions in which BA was delivered), it was deemed to have occurred only if all of the following had occurred: an assessment of mood and risk, a discussion of progress since last time and an agreement on a plan of activity for the next week.
Early sessions (2 and 3) still focused on encouraging patients to complete a diary of their activity throughout their week, alongside a record of their mood rated on a 10-point scale (0 = lowest, 10 = normal). Mid-range sessions (3–5) focused on encouraging patients to identify the link between mood and activity (functional analysis), although the tools to achieve this were simplified. Patients were encouraged to schedule in achievable tasks (pleasurable, necessary and routine). Late sessions (6–8) continued the monitoring of mood and activity and scheduling of further activities, but with an emphasis on identifying and reinforcing the benefits of scheduled activities on mood, and considering the next steps (onwards referral if necessary, and relapse prevention).
Mental health-care co-ordination
During the feasibility study, care co-ordination was conceived as occurring after BA to ensure that care was handed on to another appropriate clinician when the cardiac rehabilitation nurses ceased to be involved. What became apparent in the feasibility study was that considerably greater flexibility in mental health-care co-ordination was required to accommodate patients with differing needs, that is, when patients refused or discontinued BA or when the nurse did not have the capacity to support BA. Furthermore, in some circumstances, a physical deterioration meant that nurses lost contact with patients (e.g. during readmission), so psychological care could be delayed. Nurses were given the flexibility to co-ordinate care with another clinician at any point during the patient’s journey, as they considered appropriate, and after discussion with their supervisor.
To deliver mental health-care co-ordination, nurses were trained to apply clinical decision-making rules based on current best practice,33,34 matching the intensity of treatment with participant preferences for mental health care. All patients referred to a cardiac rehabilitation team who agreed to undertake an initial assessment (stages 2 and 3) were routinely screened for depressive symptoms using the PHQ-9, with individuals who scored ≥ 10 being deemed eligible for inclusion. On identifying an eligible individual, the nurse explained what evidence-based treatment options were available. This could include BA self-help materials supported by the nurse, GP referral, referral to local IAPT services and/or referral to specific cardiac patient psychological support services where available. Nurses co-ordinated care by monitoring symptoms of depression and anxiety, assessing risk to self or others and agreeing a plan of care with participants that may include BA and/or referral to a GP, IAPT services or other therapeutic agencies, as described previously. As part of this care co-ordination protocol, all participants were offered patient-led, nurse-supported self-help BA, as outlined in Enhanced psychological care.
Nurses’ training in enhanced psychological care
Two cohorts of nurses underwent training. Nurses’ training comprised either 1 or 2 days of face-to-face classroom-based training, supplemented as appropriate by self-directed online teaching materials (including videos of the previous training session), which could be worked through in preparation for taught sessions. The course was delivered by experienced mental health practitioners (a liaison psychiatrist and a mental health nurse with BA accreditation) who provided training on the key techniques of mental health-care co-ordination, managing safety and risks and BA. This training allowed nurses to actively support participants during care co-ordination, and to assist participants as they worked through the BA manual alongside their cardiac rehabilitation programme. Nurses were provided with a session-by-session guide, detailing the types of care co-ordination tasks that might be relevant for participants as they moved through their 6- to 8-week comprehensive rehabilitation programme (see Appendix 3). This guide was flexible to accommodate participant preferences for care. Nurses delivering EPC to study participants also received clinical supervision by a mental health practitioner (an accredited BA therapist). Clinical supervision was obligatory for nurses who delivered EPC to participants while they had one or more EPC participants on their caseload. The sessions were usually delivered fortnightly by telephone, at a time that was convenient to the teams and which met with their clinical demands; they could be arranged either one to one or as a team. Each session lasted no more than 30 minutes.
On completion of cardiac rehabilitation (stage 5), the nurse sent structured details of a participant’s care to their GP. All participants, including those receiving BA and whose depressive symptoms did not respond (based on their PHQ-9 score), were given an opportunity to review their management options with the nurse. Here, treatment response was defined as achieving a minimum clinically important difference (MCID) for the PHQ-9 that was equivalent to a 5-point reduction in score. 73 It is important to note, however, that some people achieving this MCID may remain above the PHQ-9 diagnostic threshold (i.e. a score of ≥ 10). As part of their care co-ordination, these individuals will be referred for continuing mental health management.
Settings and study population
Cardiac rehabilitation programmes
We aimed to recruit and randomise eight locality-based comprehensive cardiac rehabilitation teams from south-west England (the geographical regions of Bristol, Cornwall, Devon, Dorset and Somerset). After excluding the four sites that took part in the feasibility study, a total of 20 teams (sites) were approached to take part between December 2014 and February 2015. In each site, the lead cardiac rehabilitation nurses from each team were invited to take part and provided detailed information about the study. In each geographical region, the recruitment letter was co-signed by the trial chief investigator and, where appropriate, the local co-applicant. In addition, the study recruitment letter was accompanied by a letter of support from a local NHS cardiac rehabilitation lead for each region.
Teams that indicated an interest were visited by the research team, whereby the trial design and methods were explained and staff were given the opportunity to ask questions. To support randomisation, teams were asked to provide some descriptive information on their staffing model and service, as well as reporting some audit data on patient throughput in the previous 2 months. In order for the team to take part, the team leader was required to provide written consent on behalf of the service. In addition, given that nurses would be required to actively contribute to the research, we also requested written consent from individual team members who would be directly involved in participant recruitment and, where appropriate, implementation of EPC. After the last team was recruited (May 2015), the trial statistician (FCW) randomised the teams into the respective allocation groups and teams were informed of their allocation.
Managing a post-randomisation withdrawal
Immediately post randomisation, one team allocated to the EPC arm withdrew from the study. In the period between providing consent to take part (April 2015) and randomisation taking place (May 2015), the team had been informed that their service was to undergo a staff consultation, with a view to a major reconfiguration of cardiac services being implemented within the next calendar year. Given this uncertainty, the team no longer felt able to commit to the study. We therefore sought to replace this team immediately. To maintain the balance between treatment arms, the site that withdrew post randomisation was replaced with a new team recruited to have a similar patient throughput (high) and population (a mixed community and hospital team delivering care to an inner-city/urban population). Although we already had two teams on a reserve list, as both were of low throughput and catering for urban/rural communities, neither were suitable replacements. Given the time constraints (i.e. the need to recruit a replacement team before the EPC training days took place in June 2015), we therefore purposively approached and recruited a cardiac rehabilitation team through our links with the BACPR (by necessity, this team was recruited within the East Midlands). Although we were unable to select a replacement site at random, the allocation was concealed from cardiac rehabilitation staff until the team had consented to take part, at which point they were informed of their allocation by the trial researcher.
Training in research procedures
All cardiac rehabilitation nurses from teams allocated to UC received training (lasting approximately 2 hours) delivered by the trial researcher on the application of study recruitment procedures. Training included the completion of study screening logs (see Appendix 4) and methods for approaching potential participants (see Appendix 5). As part of the recruitment process, teams using the HADS were required to replace this with new tools for anxiety (the GAD-7) and depression (the PHQ-9). Teams were trained in how to apply and score these new measures, and also advised on how to manage any risk or safety issues that might arise through their application.
In addition to the training in study procedures received by UC teams, teams allocated to the EPC intervention arm were asked to nominate up to three nurses per team to be trained to deliver EPC. The nurses were then invited to undergo training lasting 2 days (as described in Enhanced psychological care), before starting participant recruitment. While managing CADENCE participants, EPC-trained nurses were regularly supervised by an experienced BA practitioner recruited to support intervention delivery throughout the pilot trial.
Participant eligibility criteria
The participant inclusion and exclusion criteria applied in the pilot trial were essentially the same as those described for the observational study within the feasibility phase (see Participant inclusion and exclusion criteria). The only difference related to the underlying cardiac condition of potential participants. Over the last 3 years, UK cardiac rehabilitation services have been offering care to increasingly diverse cardiac populations. 26 Although the vast majority of patients were expected to be referred for rehabilitation as a result of an ACS, any patient referred for rehabilitation was potentially eligible, and the sample for the pilot study could now include, for example, patients with heart failure.
Participant recruitment and consent procedures
The process for identifying and recruiting trial participants followed the same procedure as outlined for the observational study conducted within the feasibility phase (see Participant recruitment procedure). Potential participants from teams allocated to UC or to the intervention group were recruited following the same procedures, with the only difference being that participant information sheets were tailored to reflect the care provided by their rehabilitation team. At the time of recruitment, all patients recruited to the trial were also asked if they would consider being approached for an interview as part of the study.
Data collection procedures
To facilitate data collection in two of our sites, which were remote from the main trial centre, and to respond to the emerging geography of the trial, it was essential that remote data collection procedures were established. We negotiated with a local clinical research network and university-based research team, both of which provided local, trained research staff to collect participant and note review data in its area. Remote staff were carefully briefed in respect of the design of the study and in data-capture procedures and processes.
Participants in both trial arms completed baseline measures before they were scheduled to commence the comprehensive cardiac rehabilitation programme. Participants were then invited to take part in two face-to-face follow-up interviews at 5 months and 8 months post baseline.
The blinding of participants, cardiac nurses or researchers extracting data on study outcomes was not possible as a result of the cluster design. However, the final analysis was carried out by the trial statistician, who was blind to treatment allocation.
Process measures
Process measures relating to the recruitment and uptake of cardiac rehabilitation nurses/teams, and participants and intervention fidelity, were collected using the methods described in the feasibility study (see Process measures).
Outcome measures
Measures were selected on the basis of evidence of their validity in this population group, and to ensure coverage of key areas of interest from a clinical and patient perspective. There was some variation in the outcome measures assessed at each time point (Table 1).
| Measure | Time point | ||
|---|---|---|---|
| Baseline | 5 months | 8 months | |
| CIS-R | ✓ | – | – |
| BDI-II | ✓ | ✓ | ✓ |
| BAI | ✓ | ✓ | ✓ |
| EQ-5D | ✓ | ✓ | ✓ |
| HeartQoL | ✓ | ✓ | ✓ |
| CSQ-8 | – | ✓ | – |
| FFT | – | ✓ | – |
| SRUQ | – | ✓ | ✓ |
| Cardiac nurse case notes review | – | – | ✓ |
| GP case notes review | – | – | ✓ |
Baseline assessment only
Participants completed a paper-based questionnaire (see Appendix 6), followed by a laptop-based administration of the Clinical Interview Schedule – Revised (CIS-R). 74,75 When possible, participants were encouraged to self-complete questionnaires, although the researcher was available to support questionnaire completion. The baseline assessment included some participant measures that were not repeated at subsequent follow-ups. The CIS-R was administered to ascertain a clinical diagnosis of depression and the severity of depression, and/or the presence of other common mental health disorders such as anxiety, according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) criteria. 76 The CIS-R score did not determine study eligibility, but allowed its comparison to the other patient-reported measures of depression symptom severity, ascertained using the Beck Depression Inventory, version 2 (BDI-II) (described in Participant questionnaires). Some short questions were included to ascertain participant sociodemographic characteristics and to document patient treatment preferences for mental health care. Participants were also asked to provide the contact details of their GP. Socioeconomic status was measured using an area-based approach, the Index of Multiple Deprivation (IMD), based on the patient’s residential postcode and divided into deciles (1 being the least deprived and 10 being the most deprived). The IMD is a composite index of neighbourhood-level socioeconomic hardship, which takes into consideration indicators in the domains of income, employment, health, education, housing and access to services (higher scores indicate more deprived neighbourhoods). 77
Baseline and follow-up assessments
We requested that NHS providers inform us immediately if a participant had died or had been admitted to hospital between baseline and the 8-month follow-up, as this constituted a serious adverse event and would need to be reported to the trial sponsor and ethics committee. A full description of the procedures for reporting/handling potential adverse events within the context of the trial is provided in Appendix 7. Cardiac rehabilitation teams were also asked to inform the study team immediately if the participant had experienced a cardiac clinical event [i.e. death and/or hospital admissions for ACS or revascularisation procedures (CABG or PCI)] or a mental health-care event (i.e. self-harm, suicidality) arising since study enrolment.
Using a structured checklist, we extracted data on process measures and intervention fidelity as described previously (see Data collection and analysis procedures), and on clinical and mental health events, cardiac risk factors (biochemical or physiological measures) and evidence of mental health-care co-ordination activities.
We also contacted the participant’s GP to request that the researcher undertook a case notes review of primary care records. During this notes review, we collected information regarding the occurrence of any new cardiac or mental health events arising since study enrolment, and of any treatments initiated with regard to the participant’s mental health needs. Data were also captured on cardiac risk factors (biochemical or physiological) and service use data for the purposes of economic evaluation (see Economic evaluation).
Participant baseline and follow-up interviews included self-reports of any cardiac- and non-cardiac-related morbidity (e.g. infarction, vascularisation) and smoking status, participants’ use of antidepressant medication78 and resource use using a structured checklist.
Depressive symptoms were measured using the BDI-II,79,80 a 21-item self-report instrument developed to measure symptom severity over the past 2 weeks, with an emphasis on affective and cognitive symptoms. Higher scores represent greater depression severity (range 0–63), and minimal (0–13), mild (14–19), moderate (20–28) and severe (29–63) symptom severity ranges were applied. There were five ways in which treatment effects were measured using the BDI-II in our trial:
-
Mean (SD) and between-group mean difference.
-
Proportion of participants demonstrating a reduction in score from baseline by > 50% (deemed a clinically important ‘response’ to treatment). 81
-
Proportion of participants demonstrating remission (going from scores of ≥ 14 to < 14).
-
Proportion of participants demonstrating a MCID in depression scores. Button et al. 82 reviewed findings from three trials that had used the BDI-II and which had also included a global measure of change; the authors found that a 17.5% reduction in BDI-II score provided the best measure of a MCID; for the purposes of this trial, we assumed that people had experienced a MCID if their depression score on the BDI-II had dropped by 17.5%.
-
A clinically significant and reliable change (CSRC), defined as whether or not a participant meets two criteria:
-
passing from above the specified threshold to below the threshold [the BDI-II remission threshold described above in (3)]
-
establishing that the magnitude of the participants’ change in score is statistically reliable.
-
To evaluate the last way, the difference between the participants’ scores at baseline and follow-up is calibrated by the standard error of the difference between two scores. 83
The Beck Anxiety Inventory (BAI)84,85 was administered, as anxiety is commonly comorbid with depression. This 21-item self-report measure asks participants to report anxiety symptoms over the last week on the following scale: 0 (none: it did not bother me at all), 1 (mildly: it did not bother me much), 2 (moderately: it was very unpleasant, but I could stand it) and 3 (severely: I could barely stand it). Item scores are summated with scores of 8–15, 16–25 and 26–63 being taken as the scoring ranges for mild, moderate or severe anxiety respectively.
Health-related quality of life was assessed using both generic and disease-specific measures. The EuroQol-5 Dimensions (EQ-5D)86 is a standardised generic measure of HRQoL that is suitable for use in people with a wide range of health conditions, and is considered to be a robust measure of health in patients with cardiovascular disease. 87 The EQ-5D is also recommended by NICE for economic evaluations in clinical trials. The index score was valued by applying the approach outlined by Devlin et al. 88 for responses to the EQ-5D, five-level version (EQ-5D-5L). This value set is based on a large and representative study sample of the general population in England, so it yields values appropriate for policy-making in the English NHS. This EQ-5D also includes a visual analogue scale (VAS), which is scored from 0 (worst imaginable health state) to 100 (best imaginable health state). The disease-specific Heart Quality Of Life (HeartQoL) scale was also administered. 89,90 The HeartQoL questionnaire comprises 14 items, with a 10-item physical subscale and 4-item emotional subscale, which are scored from 0 (poor HRQoL) to 3 (better HRQoL). Participants rate their HRQoL over the last 4 weeks. To assess when and how patients become activated over the course of their cardiac rehabilitation programme or BA treatment, the nine-item Behavioural Activation for Depression Scale – Short Form (BADS-SF) was also administered. 91 BADS-SF scores range from 0 to 54, with high scores representing higher activation.
Patient experiences of care were assessed only at 5 months (see Appendix 8), using the eight-item self-reported Client Satisfaction Questionnaire – 8 (CSQ-8)92 and an adapted version of the NHS Friends and Family Test (FFT). The CSQ-8 items are completed using four-point Likert scales (1–4), with scores ranging from a possible 8 to 32, and higher values indicating higher satisfaction. The FFT consists of a single item with a five-point rating scale (‘Extremely likely’ to ‘Extremely unlikely’) and two free-text items (‘What was good about your experience?’ and ‘What would have made your experience better?’). The free-text items were analysed using a basic content analysis.
Sample size considerations
Using the 2010–11 UK NACR audit data, 55,452 patients (for MI, PCI and CABG) attended a comprehensive cardiac rehabilitation programme offered by 1 of 280 teams, which is equivalent to an average of 200 patients per team per year. Assuming 17% of patients have depressive symptoms,28 this equates to 35 eligible patients per team each year. A central aim of the pilot study was to estimate the participant eligibility and consent rates, as no relevant UK data that are directly applicable to cluster trials are available. Two US RCTs (individual randomisation), exploring psychological/drug therapy in cardiac rehabilitation settings, observed highly variable consent rates of 85% [150/177 – the Comparison Of Depression Interventions After Acute Coronary Syndrome (CODIACS) vanguard study]93 and 37% [2481/6854 – the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study]. 94
Prior to undertaking feasibility work to inform our estimates of key parameters, we assumed a conservative patient participation rate of 50%, estimating that it would take 6 months to recruit 64 patients through the eight teams. This sample size is sufficient to estimate a follow-up percentage as low as 50% with a margin of error of ± 13%, based on the width of the 95% CI, and as high as 90% with a margin of error of ± 10%, based on the lower bound of the 95% CI. We elected to recruit eight cardiac rehabilitation teams (clusters), as this would allow us to (1) recruit sufficient patients within a set time frame deemed to be acceptable to the funder, (2) ensure that sufficient numbers of teams gained exposure to EPC delivery in diverse settings to comment on intervention delivery and (3) manage the maximum number of recruitment sites in a single-centre RCT and given the geographically dispersed nature of the data collection. Process data from the feasibility study subsequently identified that these assumptions were too optimistic (see Appendix 9).
We did not plan to use estimates from the pilot of the intracluster (i.e. the intracardiac rehabilitation team) correlation coefficients (ICCs) for key outcomes to plan the sample size for the definitive trial, as the available pilot sample was small and estimates would have been imprecise. Using 2010–11 data supplied specifically for this study by the NACR audit team (6272 patients; 119 cardiac rehabilitation teams), we estimated the ICC for depression to be 0.047 (95% CI 0.034 to 0.062). We proposed that, notwithstanding the emergence of new evidence in the interim, this estimate of the ICC would be used to inform the sample size calculation for the definitive trial.
Statistical analysis plan
Trial data estimated cardiac rehabilitation team recruitment and patient participant study completion rates. As this was a pilot study, we did not make definitive estimates of clinical effectiveness and costs; our primary aim was to document the adequacy of trial procedures, intervention acceptability and outcome measures. All pilot study data were collected and reported [in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement]95 using good practice guidelines for cluster trials. A detailed statistical analysis plan, including health economics, was developed prior to undertaking the analysis of trial data (see Appendix 10). In summary, the recruitment rate, uptake in the intervention and control arms, outcome completion rates and attrition rates were reported (with 95% CIs). It is a known feature of cluster randomised trials that when participants are recruited after clusters have been randomised this can lead to selection bias. 96 The recruitment rate and participant characteristics at baseline were compared between the trial arms to assess whether or not there was evidence of selection bias. Participant outcome data (including resource use) were analysed descriptively (e.g. means, SDs) for each arm at each follow-up. As per the commissioning brief, we reported the outcome effect sizes (i.e. between-group mean differences and 95% CIs) based on an intention-to-treat analysis. We did not use analytical methods that allow for clustering because the number of clusters and participants per cluster were too small. For this reason, and the fact that this is a pilot study, the CIs should be interpreted cautiously and no p-value is presented. We carried out a complete-case analysis, including only those participants who provided outcome data. No data were imputed as this is not a definitive trial of intervention effectiveness. Patient treatment preferences for mental health care were documented at baseline.
Economic evaluation
Adopting a societal costing perspective, we piloted the methods required to collect resource and service use data in order to estimate costs to the NHS, costs to social care and personal social services and relevant costs to participants and their carers/families (including NHS and privately funded mental health care). If feasible, we planned to distinguish the use of services for (1) depression or anxiety, (2) other mental health problems and (3) other health reasons or social needs. In addition, we also undertook a preliminary assessment of the cost of providing EPC within cardiac rehabilitation teams. Service use data were collected from both (1) routine/administrative sources (e.g. hospital records, GP records, community mental health team records and, where appropriate, social care records) and (2) via participant self-report using a Service and Resource Use Questionnaire (SRUQ). The SRUQ was adapted from the Client Services Receipt Inventory (CSRI),97 with input from the patient and public involvement (PPI) group, and some testing during the feasibility phase. A detailed plan describing the analysis of health economics data is presented in Appendix 10. In summary, we assessed the completeness, validity and reliability of patient-reported data versus routine administrative data on the types and amounts of health care/service use by participants, and over the follow-up periods planned for a definitive trial. This approach enabled better judgements to be made regarding which types of health service, care professional or other resource use were most likely to be affected by the expected trial outcomes, and to allow us to determine the optimal source of obtaining such data.
Nested qualitative study
Data collection
Data were collected during observations of staff training and during interviews with nurses and patients. The qualitative researcher observed staff training to identify what issues nurses appeared to struggle with and what practical concerns they had about EPC. Observations were recorded through extensive field notes.
All of the nurses who delivered EPC to one or more patients were invited to take part in an interview, in order to explore their experiences of recruiting patients, EPC training and delivery (including managing patients at risk of self-harm or suicide, and using the nurse and patient materials). A topic guide was used to ensure consistency across the interviews and was structured around these main areas (see Appendix 11). The researchers had planned to interview nurses once they had stopped delivering EPC, but this was not feasible, as a result of delays in patient recruitment; hence, a pragmatic decision was made to interview nurses at least 3 months after the delivery of their first EPC session, and once they had discharged at least one participant (i.e. at a time when they had had some experience of delivering EPC).
Patient participants in both arms were approached for interview shortly after completing their 5-month assessment in the trial. Prior to conducting the feasibility study, we anticipated conducting approximately 30 interviews, sampling participants who had taken part and who had either completed (n = 12) or not completed (n = 8) EPC, or who had received UC (n = 10). We had also planned to sample participants to achieve maximum variation in relation to trial arm, age, recruiting team, gender, depression score at baseline and adherence to the intervention. However, as recruitment of patients was slow within the pilot trial, our target sample was changed to include all intervention group participants (n = 15) and six UC patients, purposefully sampled where possible, on the basis of team and gender. The purpose of the interview was to discuss participants’ experiences of the psychological care received and, for those receiving EPC, the acceptability of the study intervention. The topic guides had the same core questions for both UC and intervention participants, with one section of the guide (which was specifically about EPC) being used only for the latter, and the UC participants being asked more about the usual psychological care they had received (see Appendix 1).
In addition to conducting interviews with patients recruited to the pilot trial, interviews were also held with 10 patients who were offered trial entry, but who declined to take part. Their purpose was to explore patients’ reasons for declining main trial participation. ‘Decliners’ were asked by the nurse trying to recruit them to the trial if they would be happy for their contact details to be given to the research team so that they could contact them and establish whether or not they would be willing to participate in a short telephone interview. Once the referral was received, the researcher contacted the individual to discuss the interview and arrange an interview appointment. A participant information sheet was sent to the person before they took part in the interview, and the researcher gave the person an opportunity to ask any questions before consenting and commencing the interview. A short decliners topic guide was written to guide the interview.
Written consent was gained from nurses and patients participating in the pilot trial immediately prior to being interviewed. For decliners, verbal consent was audio-recorded at the time of the interview. All interviews were conducted by an experienced qualitative interviewer. Interviews were audio-recorded, transcribed verbatim and anonymised prior to analysis.
Qualitative analysis plan
Notes taken during the nurse training were read and re-read, and key points concerning the design and delivery of the training and intervention fed back to the research team.
Analysis of the nurse and patient interview data was thematic. A sample of transcripts was read and independently coded by two qualitative researchers, who then discussed their coding and interpretation of the data. This discussion led to two coding frames being agreed: one for the nurse interviews and one for the trial participant interviews. Where possible, similar codes were used within each coding frame, as this would assist later when findings between the data sets were triangulated. Transcripts were manually coded, and data pertaining to each code were summarised in tables, using an approach based on a framework analysis. 68 Once this had been done, comparisons were made within and across the data sets to identify key themes and deviant cases, and to highlight similarities and differences between them.
As only a few decliners were interviewed, and interviews were relatively short, transcripts were read and re-read, and the main reasons for declining were summarised in a report.
Ethics and governance considerations
The Royal Devon and Exeter NHS Foundation Trust acted as the trial sponsor. The CADENCE study protocol was reviewed, and a favourable ethics opinion obtained from the National Research Ethics Service (NRES) Committee South West – Exeter (reference number 14/SW/0139). The relevant NHS research governance approvals were obtained prior to commencing fieldwork for sites participating in the feasibility and pilot phases of work. All protocol amendments were notified through existing pathways in the UK NRES and NHS research governance systems, and the trial team ensured that all participating cardiac rehabilitation teams, staff, patients and trial registries were informed of any changes to the protocol (see Appendix 12).
The CADENCE study was registered with a trials registry [as International Standard Randomised Controlled Trial Number (ISRCTN) 34701576] and has been adopted by the UK NHS Clinical Research Network (UKCRN ID: 17105). All researchers and nurses involved in participant recruitment underwent good clinical practice training.
Data management
All personal information obtained about participants or staff for the purposes of recruitment or data collection (e.g. names, addresses, contact details, personal information) was kept confidential and held in accordance with the requirements of the Data Protection Act 1998. 98 Each participant recruited to the study was assigned a research number, and all outcome data entered were encrypted and stored without the subject’s name or address. Electronic study records were held on a Structured Query Language (SQL) server database managed by the Peninsula Clinical Trial Unit (PenCTU), which was stored on a restricted-access secure server maintained by the University of Plymouth. Data were entered into the database via a bespoke web-based data entry system, encrypted using Secure Sockets Layer (SSL). Access to electronic data was permission based, with access to identifiable information limited to those processing questionnaires and directly involved in data collection from participants. Data entered onto the database were backed up according to the PenCTU standard operating procedures. Copies of the study data, retained at study sites, were securely stored for the duration of the study prior to archiving at the University of Exeter. Electronic copies of correspondence sent to study participants were stored securely on a password-protected computer at the University of Exeter, and paper-based information held in a locked filing cabinet in the research team office at the University of Exeter Medical School. Names and participant details were not passed onto any third parties, and no named individuals were to be included in the write-up of the results. The only occasion in which personal information would be passed onto a third party would be if we considered there to be a risk of serious harm to a research participant, and normally this would occur only after discussion with the person concerned and with their GP (where appropriate).
All study data will be kept for 10 years under secure conditions on the University of Plymouth and the University of Exeter secure servers. We sought consent from participants for core outcome data from the pilot study to be released in an anonymised form to an independent data repository, following publication of the key planned outcome papers. We anticipate that for the data set containing the main study outcome variables, the approximate date of release to the archive would be 1 year following completion of the grant (to allow time for the publication of key outputs). Although anonymised, as a result of the sensitive nature of the data collected, the data will be shared under special licence for educational and research purposes only. The data would be released only to users, approved by the programme management group, following completion of user identification and intended use documentation administered through the data repository. Data would also be subject to standard secure storage and usage policies of the data repository.
Safety of participants and researchers
Suicide protocols to protect staff, researchers and participants
Although we believe that the study has minimal implications for health-care staff, patient participants had depressive symptoms, and a policy was put in place regarding the management of potentially serious adverse events (e.g. self-injury or suicidality). All nurses participating in the study received training around implementing the safety protocol (see Appendix 13).
The participant information sheet informs participants that if we were very concerned about their safety or someone else’s safety, we may need to break confidentiality and inform their GP. This approach has worked effectively in our other studies on depression,99,100 and we thus adapted the tried and tested protocols.
Safety of participants and study personnel during the research interviews
Participants were asked to reflect on their health issues during study participation, and there was a risk that, in some cases, this may have caused distress. If any such difficulties occurred during a research interview, the researcher offered support to the person involved. In the unlikely event that the individual appeared significantly upset and at risk as a result of the interview, the researcher was trained to seek the participant’s permission to advise the person’s GP of this distress and to encourage them to seek further help from the support network available to them. Should there be any concern that a person was likely to cause harm either to themselves or to another person, the person’s GP would be notified immediately by a senior clinician from the study team.
As researchers were to be conducting interviews alone with participants, potentially in patients’ own homes, safeguards were put in place to protect both the participant and the researcher. Data-barring service checks were performed on any researcher taking part to ensure that they were an appropriate person to be working with vulnerable adults. To ensure the safety of the researcher, a lone-worker policy and ‘buddy system’, designed by the primary care research group, was used by the study’s researchers. This approach provides a mechanism for ensuring that the exact whereabouts of the researcher is known at any time point during fieldwork by a supervisor or a buddy.
Patient and public involvement
Patient and public involvement was sought during the protocol development. The NIHR Collaborations for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula (PenCLAHRC) hosts the Peninsula PPI group. This group has published a framework regarding PPI in research,101 and provides research training and support to facilitate the public becoming actively involved in all aspects of the research process. A co-applicant (AG, the PPI facilitator) recruited four lay advisors with relevant lived experience, each of whom was sent an overview of the emerging research protocol. Our advisors endorsed the acceptability of the intervention and the importance of embedding psychological care within cardiac care (rather than onward referral), the study design, project management arrangements and dissemination plans. Advisors fed back on participant burden (e.g. questionnaires, timing of assessments) and on whether or not patient outcomes captured areas of importance. Our advisors agreed that, whenever possible, our intervention should give patients choices over their treatment. The following practical suggestions were made: (1) patients should always be made aware of alternative treatments for depression while they are waiting for cardiac rehabilitation (with BA) to commence; (2) patients who are eligible for the study should be given the option of having their carer involved in treatment discussions and (3) as it was important, patient preferences should be recorded from the outset of rehabilitation.
Patient and public involvement continued throughout this project, with the precise roles of advisors negotiated at each stage. During the preliminary stage of the feasibility study, our PPI advisors reviewed outcome measures and informed the design of all study-related materials (e.g. participant information sheets). Members of our PPI group were also involved in the development of the intervention manual, helping to ensure its accessibility and acceptability to patients and carers. Another key area of involvement was in dissemination plans. Our lay advisors reflected on the findings emerging from both the feasibility and the pilot phases of the work, and provided important insights into recommendations arising from these findings.
Across the duration of the study, lay advisors were routinely involved in project oversight and management (see Project oversight), attending the regular project management group meetings. The lay advisors made valuable contributions to these discussions, such as advising on what would be acceptable from a patient perspective when we modified our intervention to make it more patient directed. Two (different) lay advisors also sat on the Trial Steering Committee (TSC), and on this committee, advisors had equal voting rights to academic/clinical members. Advisors were paid travel costs and for the time spent at meetings.
Project oversight
Day-to-day management
The chief investigator (JLC) and the scientific lead (SHR) supervised and monitored the strategic development and progress of the project, liaising with the wider team and methodological leads on a routine basis. The research staff members directly involved in data collection were based in Exeter. Two research fellows were appointed to manage the quantitative (observational study, RCT) and qualitative components of the study. Both research staff were responsible for managing the day-to-day aspects of the research relevant to their own role, supported by an administrator at peak periods of fieldwork activity. Katrina Turner provided qualitative expertise/supervision, Rob Anderson was the health economics lead, Obioha Ukoumunne was the statistical lead and Andy Gibson led PPI engagement.
Monthly project management meetings were organised to ensure that co-applicants and lay advisors (2–4 advisors) were informed of progress and contributed to the ongoing steering of the research. Face-to-face meetings were held every 3 months, with the remainder being held via teleconference. Co-applicants were expected to attend a minimum of one meeting every 3 months. Lay advisors were asked to attend meetings every 3 months, with their interim specific input sought as required via the PPI lead.
Trial Steering Committee
A TSC with an independent chairperson (Professor Glyn Lewis, triallist and psychiatrist) was appointed in accordance with MRC’s guidelines. Other TSC members were recruited with an appropriate range of perspectives and expertise. Members included two lay advisors (Rev. Paul Lanham and Mr Ron Grant), a cardiac rehabilitation expert (Professor Gill Furze), a qualitative methodology expert (Professor Anne Rogers) and a statistical expert (Dr Sally Kerry). Three TSC meetings were held at critical points in the project timeline (start-up, transition between feasibility and pilot study and study end). The TSC monitored the scientific quality, ethics and general progress of the project, receiving regular reports from the study team. This committee also monitored the serious adverse event and adverse event data emerging from the study. As no definitive data on clinical effectiveness/safety were collected, the TSC agreed that it would absorb the role of a Data Monitoring and Ethics Committee for the duration of the study.
Chapter 4 Feasibility study results
The feasibility study consisted of two main elements: a before-and-after observational study and a nested qualitative study, the results of which are presented below.
Observational study
Recruitment
Cardiac rehabilitation teams
Three cardiac rehabilitation teams (one hospital and two community) took part, recruiting study participants for a 6-month period (Table 2). A fourth team (community) was recruited at the halfway point to bolster participant recruitment, effectively screening for eligible participants for a 3-month period. In total, five nurses screened patients for study eligibility.
| Team | Number of | Location | Recruitment date | |
|---|---|---|---|---|
| Nurses | Sites | |||
| I | 2 | 2 | Hospital | August 2014 |
| II | 1 | 5 | Community | September 2014 |
| III | 2a | 6 | Community | September 2014 and January 2015 |
A cardiac rehabilitation ‘team’ consisted of one or more nurses, with access to volunteers (who helped with fitness sessions), a trainer or a physical therapist (to support patients during fitness sessions). One of the community teams also gained access to an administrator part-way through the feasibility stage of the study. A clinical psychologist was also available to the teams for referrals of patients with complex mental health issues.
Each community team was led by a single nurse. The hospital team comprised a number of nurses, two of whom were participating in the study. Two of the community team nurses visited five venues in different locations (‘sites’) within their area where elements of the cardiac rehabilitation programmes were carried out (i.e. monitoring, fitness groups, gym support, education, assessments and follow-up, administration and telephone calls). For example, one community nurse conducted fitness classes across three different sites and ran clinic appointments from two other sites. There was some overlap, in that some of the fitness classes were run together by a nurse from each community team.
Participant recruitment and retention
Participant recruitment for the feasibility study took place between September 2014 and March 2015. Participant flow from eligibility screening through to study recruitment and completion of the 5-month assessment is outlined in Figure 2.
FIGURE 2.
Feasibility study: participant flow diagram. CR, cardiac rehabilitation; DNA, did not attend.

The nurses screened 203 patients for study eligibility during their initial cardiac rehabilitation assessment, of whom 14 (7%) did not complete a PHQ-9. A total of 39 out of 203 patients (19%) were found to have depressive symptoms (i.e. score of ≥ 10 on the PHQ-9) during their initial assessment. Eight participants were already receiving active treatment for pre-existing depression and one was deemed ineligible for cardiac rehabilitation at the point of assessment, leaving 30 out of 203 participants (15%) meeting the CADENCE study definition of new-onset depression, who were therefore eligible for the trial. Of the 30 eligible patients, nine (30%) were recruited to the trial; this represents 4% of the 203 patients initially screened.
Five of the 30 participants (17%) eligible for the feasibility study were not offered study entry, the reasons for which are presented in Figure 2. Nine of those offered study entry (36%) agreed to take part and provided baseline data. All patients who were due to be followed up for the observational study completed their 5-month assessment. Based on these data, it took approximately 7.1 weeks per team to recruit each participant.
The majority of the 203 patients screened attended a community-based cardiac rehabilitation clinic (164 out of 203; 81%), with many fewer invited to attend a hospital-based service (39 out of 203; 19%). The mean age of patients attending an initial nurse screening clinic was 65.2 years (range 25–87 years), 157 out of 203 (77%) were male and the vast majority (198 out of 203; 97.5%) reported their ethnicity as ‘white’ (see Figure 2).
The sociodemographic characteristics of study participants (n = 9) recorded at baseline are presented in Table 3. The mean age of the sample recruited was 60.4 years, and most were male (7/9). All participants reported their ethnicity as ‘white’, and their preferred language was English. One-third of participants were working full time, with just under half currently in retirement. The majority of the sample were homeowners, and just under half were either married or living with a partner. The nature of the cardiac event that initiated referral to cardiac rehabilitation was recorded for eight participants. Three participants had a MI with PCI (insertion of stent, coronary angiography and left heart catheterisation), one had a MI, one had a MI with atrial flutter, two had a CABG, and one had a PCI (heart catheterisation) and left ventricular dysfunction.
| Characteristics | Baseline values (N = 9) |
|---|---|
| Gender (male), n (%) | 7 (78) |
| Mean age (years) (SD; min., max.) | 60.4 (18.2; 26, 85) |
| Ethnicity (white), n (%) | 9 (100) |
| Preferred language (English), n (%) | 9 (100) |
| Marital status, n (%) | |
| Married/living as married | 4 (44) |
| Single | 2 (22) |
| Divorced | 1 (11) |
| Widowed | 2 (22) |
| Employment status, n (%) | |
| Working full time | 3 (33) |
| Retired | 4 (44) |
| Unemployed jobseeker | 1 (11) |
| Unemployed as a result of ill health | 1 (11) |
| Housing status, n (%) | |
| Homeowner | 7 (78) |
| Tenant | 2 (22) |
Sample characteristics
Intervention fidelity
From data extracted from the notes recorded by cardiac rehabilitation nurses (Table 4), the number of cardiac rehabilitation sessions attended ranged from 1 to 18 (mean 10.1, SD 5.0 sessions attended) for the nine participants. There was evidence that the cardiac nurse discussed a mental health referral in just under half (four out of nine) of the participants. Of these four participants, only one received a GP referral for onward psychological care following completion of EPC.
| Outcome | Follow-up values (N = 8) |
|---|---|
| Cardiac rehabilitation sessions attended, n (%) | |
| 1 | 1 (13) |
| 7 | 1 (13) |
| 8 | 1 (13) |
| 11 | 2 (25) |
| 12 | 1 (13) |
| 13 | 1 (13) |
| 18 | 1 (13) |
| Mean (SD) | 10.1 (5.0) |
| Time (days) elapsed from the point of consent to the start of EPC, mean (SD), n; median (min., max.) | 9 (4), 6; 8 (4, 14) |
| Time (days) elapsed from the point of consent to nurse discharge from cardiac rehabilitation, mean (SD), n; median (min., max.) | 82 (66), 6; 69 (8, 197) |
| Time (days) elapsed from nurse discharge from cardiac rehabilitation to date of the GP discharge letter; mean (SD), n; median (min., max.) | 68 (63), 5; 103 (0, 125) |
| Psychological care co-ordination activities provided on exit from cardiac rehabilitation (N = 6), n (%) | |
| GP referral for mental health | 1 (17) |
| No evidence of care co-ordination | 5 (83) |
| Discussion with participant regarding referral to GP/IAPT services/other psychological care services, n (%) | |
| Yes | 4 (50) |
| No | 4 (50) |
| Referral made to GP/IAPT services/other psychological care services, n (%) | |
| Yes | 1 (13) |
| No | 7 (88) |
| Care accessed for depression privately (N = 5),a n (%) | |
| No | 5 (100) |
| Care accessed for depression via established NHS mental health pathways, n (%) | |
| Yes | 1 (13) |
| No | 7 (88) |
Participant-reported outcome measures
At baseline, the CIS-R was used to determine a primary and secondary psychological diagnosis. Only two patients did not receive a diagnosis, with the other participants ranging in their diagnoses from mild depressive episodes to severe depressive episodes, and one participant having agoraphobia (Table 5).
| Outcome measurement | Baseline values (N = 9) |
|---|---|
| CIS-R score, mean (SD) | 17.7 (9.9) |
| CIS-R primary diagnosis category, n (%) | |
| No diagnosis identified | 2 (22) |
| Agoraphobia | 1 (11) |
| Mild depressive episode | 2 (22) |
| Moderate depressive episode | 2 (22) |
| Severe depressive episode | 2 (22) |
| Secondary diagnosis category, n (%) | |
| No diagnosis identified | 4 (44) |
| Mild anxiety and depressive disorder | 4 (44) |
| Specific (isolated) phobia | 1 (11) |
| Do you believe you have low mood?, n (%) | |
| Yes | 8 (89) |
| No | 1 (11) |
| Do you want any professional help for your low mood? (N = 8), n (%) | |
| Strongly prefer help | 2 (25) |
| Prefer help | 4 (50) |
| Prefer not to receive help | 2 (25) |
| What type of professional help would you prefer? (N = 8), n (%) | |
| Strongly prefer non-drug help | 4 (50) |
| Prefer non-drug help | 3 (38) |
| Do not mind | 1 (13) |
Most participants (eight out of nine) reported that they felt that they had low mood, and when asked what professional help they would consider, around half indicated that a non-pharmacological approach was preferable (four out of eight), although two participants ultimately preferred not to receive any professional help.
The mean BDI-II score at baseline was 21.8 (SD 9.8) and the BAI score was 18.2 (SD 12.4). By the 5-month follow-up, participants reported lower mean levels of depression (BDI-II score of 18.0, SD 13.7) and anxiety (BAI score of 16.1, SD 14.3; Table 6). Four out of nine participants had a 50% reduction in scores from baseline at 5 months, and three out of eight were in remission (BDI-II score of < 14) at 5 months. Although all participants had reported not taking antidepressants at baseline, one participant reported taking antidepressant medication at follow-up for their low mood.
| Outcome measurement | Time point (N = 9) | |
|---|---|---|
| Baseline | 5 months | |
| BDI-II score, mean (SD); min., max. | 21.8 (9.8); 2, 36 | 18.0 (13.7); 0, 34 |
| 50% reduction in BDI-II score from baseline, n (%) | ||
| Yes | NA | 4 (44) |
| No | 5 (56) | |
| Remission (i.e. a BDI-II score of < 14), n (%)a | ||
| Yes | NA | 3 (38) |
| No | 5 (63) | |
| 17.5% reduction in BDI-II score from baseline (MCID), n (%) | ||
| Yes | NA | 4 (44) |
| No | NA | 5 (56) |
| CSRC index, n (%)a | ||
| Yes | NA | 2 (25) |
| BAI score, mean (SD); min., max. | 18.2 (12.4); 1, 44 | 16.1 (14.3); 0, 44 |
| Are you currently taking any antidepressant medication (medicine to help low mood)?, n (%) | ||
| Yes | 0 (100) | 1 (11) |
| Is your medicine for low mood prescribed by a qualified doctor?, n (%) | ||
| Yes | NA | 1 (100) |
| For how long have you been taking your medicine for low mood?, n (%) | ||
| < 6 weeks | NA | 1 (100) |
| Within the past 6 months, have you received any help for your low mood from the following people, n (%) | ||
| GP | 0 (0) | 1 (11) |
| Therapist (e.g. psychotherapist, nurse) | 0 (0) | 0 (0) |
| Another health/social care professional | 2 (22)b | 4 (44)c |
Most participants (eight out of nine) reported being ex-smokers at baseline, and at the 5-month follow-up, one participant had recommenced smoking (see Table 7). Participant-reported health outcomes, including self-reported cardiac diagnoses and other long-term health conditions, at baseline and the 5-month follow-up, are presented in Table 7. The EQ-5D-5L had a mean of 0.707 (SD 0.255) at baseline and 0.631 (SD 0.351) at 5 months; equivalent data for the mean VAS score were 55.7 (15.9) and 67.7 (21.2), respectively. The HeartQoL score had increased from a mean of 16.0 (SD 8.4) at baseline to 25.1 (SD 12.7) at 5 months.
| Characteristics and outcomes | Time point (N = 9) | |
|---|---|---|
| Baseline | 5 months | |
| Smoking status, n (%) | ||
| Never smoked | 1 (11) | 1 (11) |
| Ex-smoker | 8 (89) | 7 (78) |
| Current smoker | 0 (0) | 1 (11) |
| Time (years) since quitting smoking (ex-smokers only) | ||
| Median (IQR), n | 14.8 (0.3–30.4), 8 | |
| Smoking behaviour (current smokers only), median (IQR) | ||
| Cigarettes per day | Not recorded | 0a |
| Cigars per day | Not recorded | 10–12a |
| Pipe (g/day) | Not recorded | 0a |
| HRQoL, mean score (SD) | ||
| EQ-5D-5L | 0.707 (0.255) | 0.631 (0.351) |
| EQ-5D VAS | 55.7 (15.9) | 67.7 (21.2) |
| HeartQoL | 16.0 (8.4) | 25.1 (12.7) |
| Participant-reported heart problems, n (%) | ||
| MI | ||
| Yes | 7 (78) | 7 (78) |
| Angina | ||
| Yes | 5 (56) | 6 (67) |
| Hospital admission – non-cardiac chest pain (N = 8) | ||
| Yes | 1 (13) | 2 (22) |
| Unsure | 0 (0) | 1 (11) |
| Heart failure | ||
| Yes | 0 (0) | 1 (11) |
| Arrhythmia | ||
| Yes | 2 (22) | 5 (56) |
| Not sure | 1 (11) | 0 (0) |
| PCI procedure | ||
| Yes | 7 (78) | 5 (56) |
| Not sure | 1 (11) | 0 (0) |
| CABG procedure | ||
| Yes | 5 (56) | 5 (56) |
| Any other heart problem/procedure | ||
| Yes | 2 (22) | 1 (11) |
| Participant-reported health problems, n (%) | ||
| Asthma | ||
| Yes | 1 (11) | 1 (11) |
| Lung disease (N = 8) | ||
| Yes | 0 (0) | 0 (0) |
| Diabetes mellitus (N = 8) | ||
| Yes | 2 (22) | 2 (25) |
| Ulcer/stomach disease | ||
| Yes | 0 (0) | 1 (11) |
| Bowel disease (N = 8) | ||
| Yes | 1 (11) | 0 (0) |
| Kidney disease (N = 8) | ||
| Yes | 1 (11) | 0 (0) |
| Liver disease (N = 8) | ||
| Yes | 0 (0) | 0 (0) |
| Anaemia/blood disease | ||
| Yes | 2 (22) | 1 (11) |
| Cancer (N = 8) | ||
| Yes | 1 (11) | 1 (13) |
| Nervous system disease | ||
| Yes | 0 (0) | 0 (0) |
| Arthritis (N = 8) | ||
| Yes | 3 (33) | 4 (50) |
| Back pain | ||
| Yes | 3 (33) | 3 (33) |
| Mental health problems (N = 8) | ||
| Yes | 0 (0) | 1 (13) |
| Skin disease | ||
| Yes | 2 (22) | 1 (13) |
| Hearing/visual impairment (N = 8) | ||
| Yes | 3 (33) | 4 (50) |
Participants reported a positive experience of their cardiac rehabilitation programme, based on both the CSQ and FFT (Table 8), although one participant reported being mildly dissatisfied and ‘extremely unlikely’ to recommend the service to friends and family. Most participants reported that the quality of the service they received was excellent (six out of nine) and they were very satisfied with the amount of help they received (six out of nine). The majority of the participants reported being very satisfied overall (seven out of nine) and that they were willing to return to the programme in the future (eight out of nine). Six participants were extremely likely to recommend the service to friends and family (six out of seven).
| Patient experience item | Follow-up values (N = 9), n (%) |
|---|---|
| Quality of service | |
| Good | 3 (33) |
| Excellent | 6 (67) |
| Kind of service you wanted? | |
| Yes, generally | 4 (44) |
| Yes, definitely | 5 (56) |
| Needs met? | |
| Most of my needs have been met | 5 (56) |
| Almost all of my needs have been met | 4 (44) |
| Recommend programme? | |
| Yes, I think so | 1 (11) |
| Yes, definitely | 8 (89) |
| Satisfied with amount of help | |
| Mostly satisfied | 3 (33) |
| Very satisfied | 6 (67) |
| Services helped to deal with problems? | |
| Yes, they helped somewhat | 4 (44) |
| Yes, they helped a great deal | 5 (56) |
| Overall satisfaction | |
| Indifferent or mildly dissatisfied | 1 (11) |
| Mostly satisfied | 1 (11) |
| Very satisfied | 7 (78) |
| Come back to programme? | |
| Yes, I think so | 1 (11) |
| Yes, definitely? | 8 (89) |
| How likely are you to recommend this help or support to friends and family if they needed similar care or treatment? (N = 7)a | |
| Extremely unlikely | 1 (14) |
| Extremely likely | 6 (86) |
Acceptability of study procedures
At follow-up, eight out of nine participants had completed participant-reported outcome data for all measures. In terms of the acceptability of study procedures, most participants reported that the baseline and follow-up assessments were of ‘the right length’ and had ‘the right number of questions’, although two participants felt that there were slightly more questions in the baseline assessment than they would have liked (Table 9). Most participants did not report noticing any repetition of questions at the baseline (four out of seven) and follow-up assessments (five out of seven).
| Interview and participant assessment | Values (N = 8), n (%) |
|---|---|
| Baseline (N = 7) | |
| Length of assessment | |
| About the right length | 7 (100) |
| Number of assessments | |
| About the right number of questions | 5 (71) |
| Slightly more questions than I would have liked | 2 (29) |
| Repetitive? | |
| Not at all – I did not notice any overlap or repetition | 4 (57) |
| A little bit – I noticed some overlap or repetition | 3 (43) |
| Follow-up (N = 7) | |
| Length of assessment | |
| About the right length | 7 (100) |
| Number of assessments | |
| About the right number of questions | 7 (100) |
| Repetitive? | |
| Not at all – I did not notice any overlap or repetition | 5 (71) |
| A little bit – I noticed some overlap or repetition | 2 (29) |
Qualitative study findings
Early feedback from nurses and the patient and public involvement group
The PPI group and two cardiac rehabilitation nurses attended a preliminary meeting to discuss the intervention design facilitated by the researchers (DAR and SHR). Lay advisors included two people who had used BA and two who had received cardiac rehabilitation. The PPI group offered insights into experiencing cardiac rehabilitation and/or BA. PPI advisors and nurses also discussed the potential, and challenges, of implementing BA and mental health-care co-ordination within cardiac rehabilitation services.
The relevant points arising from this meeting were supplemented with feedback from early meetings with individual cardiac rehabilitation nurse teams. Key areas were the realisation that community teams would be conducting nearly all the initial patient assessments, which is the point at which participants enter the study, and hence the community teams would have a much bigger role in recruiting participants than the hospital team; nurses being concerned about changing to the PHQ-9 and the GAD-7 from their current mood measurement tool (the HADS) and cardiac rehabilitation services differing in how they operated, and the need to accommodate these differences during implementation of the EPC intervention.
Observations of usual care in participating sites
The qualitative researcher conducted seven observational sessions relating to three teams during the early phase of the feasibility study (Table 10). Nurses working in different regions allowed patients a choice of venue for their rehabilitation, which also enabled nurses to steer patients towards the venue that they thought would be most suitable for the individual. In each region, nurses conducted their rehabilitation programmes in different ways; for example, one community nurse tended to run group fitness class sessions, and had less private space, whereas another community nurse had more clinic time and was able to offer more one-to-one clinic appointments. Box 2 details the insights gained from these observations.
| Observation type (by team)a | Site | Setting | Number of patients presentb | Observation length (minutes) | Staff present at session |
|---|---|---|---|---|---|
| Team I: hospital | |||||
| First hospital assessment | Hospital | Clinic room | 1 | 40 | One nurse |
| Fitness class | Hospital | Physiotherapy gym | 13 | 20 | Two nurses and one volunteer |
| Post-exercise cool-down period | Hospital | Physiotherapy room | 12 | 20 | Three nurses and one volunteer |
| Health education presentation | Hospital | Physiotherapy room | 1 | 30 | Three nurses and one volunteer |
| First hospital assessment | Hospital | Clinic room | 1 | 40 | One nurse |
| Team II: community | |||||
| Individual fitness checks/training | Leisure centre | Gym | 5 | 75 | One nurse, one volunteer and one exercise trainer |
| Post-exercise cool-down period | Leisure centre | Changing room | 5 | 20 | One nurse, one volunteer and one exercise trainer |
| Pre-exercise check | Health centre A | Cafe | 7c | 20 | One nurse |
| Individual fitness checks/training | Health centre A | Gym | 6 | 60 | One nurse and one exercise trainer |
| Initial community assessment | Community hospital | Clinic room | 1 | 50 | One nurse |
| Review clinic appointment | Community hospital | Clinic room | 1 | 40 | One nurse |
| Team III: community | |||||
| Initial community assessment | Health centre B | Clinic room | 1 | 60 | One nurse |
| Initial community assessment | Health centre B | Clinic room | 1 (with wife) | 60 | One nurse |
| Initial community assessment | Health centre B | Clinic room | 1 | 60 | One nurse |
Cardiac rehabilitation is delivered in a variety of settings. In some locations, there is restricted or no space in which EPC could be delivered in privacy.
In some settings, the nurse works solo, whereas others work as a team. A solo nurse would be unable to conduct BA in privacy and without disruption, as they are responsible for the larger group of non-depressed patients. Whether solo or working as part of a team, there is very little spare time in which EPC could be embedded.
Location of careAlthough patients often have a choice of community sites, they are encouraged/referred for hospital-based rehabilitation if they have more severe health conditions (e.g. previous heart attacks) or multimorbidity.
Variation in patterns of workingSome nurses have the opportunity to provide more one-to-one appointments; others spend more time on fitness and education classes, and may see patients briefly after the class. Some patients choose not to come to the fitness classes, but could be invited to a clinic appointment if necessary.
Variation in waiting list times between sitesOnce patients are referred to the hospital team following their initial community assessment, they may wait for any length of time between 3 and 8 weeks (on top of medical recovery time) between the initial community assessment and the first hospital assessment, compared with little or no additional wait for community cardiac rehabilitation.
Concurrent psychological treatmentA clinical psychologist presents a talk to all patients in hospital rehabilitation, and takes referrals from hospital and community-based nurses.
Administrative dutiesA substantial part of the nurses’ time is taken up with administration.
Patients who decline cardiac rehabilitationNurses tend not to see any patients who decline rehabilitation. Such patients either cancel their initial assessment appointment or do not contact the nurse to make an appointment (depending on how nurses manage appointments).
Observations of nurse intervention training
The CADENCE training programme was delivered to four nurses from the three teams. Key points from the field notes taken during observations of nurse training were organised into three main categories: (1) general reflections on training (2) practical issues about the training materials and (3) intervention delivery issues. Nurses welcomed new knowledge that would help in managing patients’ mental health needs. Nurses gained new knowledge about the existence of, and how to refer patients to, existing community mental health services (e.g. IAPT) and on how to manage mental health risk issues when they arose. While undergoing the training nurses highlighted practical issues, such as the layout of the patient and nurse handbooks and how to make them more user-friendly. In terms of intervention delivery, nurses expressed concerns over how long BA would take to deliver in a typical cardiac rehabilitation session and the extra paperwork involved. Many of the points were resolved interactively during the training, whereas others (e.g. time concerns around implementation) would require monitoring and feedback once nurses implemented EPC.
Observation of clinical supervision sessions with nurses
Two telephone clinical supervision sessions with one nurse and two supervisors were observed, and six sets of supervision records were reviewed. These observations identified two key practical challenges. First, that the paperwork supporting nurse recording of EPC within the clinical notes required improvement to more accurately document the care received by the patient. For example, it was difficult to identify the number and length of BA sessions offered as part of the cardiac rehabilitation programme, and it was unclear what activities (e.g. symptom monitoring, diary review) constituted an adequate session of BA. Second, there was a need to develop procedures to ensure that nurses informed the clinical supervisors and research team when a participant was discharged from EPC. Embedded within this was ensuring that the nurse had a clear definition of when EPC ceased, as this might be different from when an individual stopped engaging with the wider cardiac rehabilitation programme.
Nurse interviews post training
All four nurses who attended the EPC training were interviewed within 4 weeks of completing their training. The interviews lasted between 25 and 36 minutes. Owing to the small number of nurses interviewed, to protect anonymity, quotations reproduced in this report from these interviews have not been tagged to indicate which interviewee is being quoted.
Nurses commented that the training was well organised, relevant and stimulating. The small group size (5–10 nurses was seen as optimal) provided a supportive and confidence-building environment. Nevertheless, the training was viewed as intense and thought-provoking (‘a good brain-ache’), with particular challenges being the initial understanding of the BA concept and how to explain BA to patients, and, later, how to explain and implement specific components of BA with patients. Role play was used throughout and, although daunting, the nurses commented on the benefits of this technique from observing and learning from each other.
Nurses were generally happy with the contents and completeness of the supporting materials provided. However, they found it difficult to differentiate between the participant handbook and nurse handbook, and requested that these were made distinctive in terms of colour/design. Nurses suggested having a few sentences and possibly some pictures in the nurse handbook that they could use with patients to help them describe/introduce BA to participants. They also requested access to electronic copies of the various materials, so that they could print copies off or e-mail them to participants. There was also some concern that the added BA materials might overload some participants, and nurses would need to make decisions about what to give out.
With regard to implementing EPC, prior to the training, nurses said that they had felt that they could provide only superficial support to patients experiencing low mood because they did not have the tools or resources to deal with it. After training, they were positive about the expected benefits of using BA with their patients:
I do a bit [working with patients with mental health issues] already but it tends not to, currently not to get to the nitty gritty of it but you just touch the surface with it and offer them some support.
Nurses expressed an eagerness to start using their new skills, but also concerns about how they would fit the BA sessions around their current workload. A few of the nurses had made plans about how they should implement the CADENCE programme within their rehabilitation sessions:
. . . we thought we’ll probably do the exercise sessions and then have the time with the client afterwards [yeah] when everybody else has gone [. . .] Rather than at the beginning where you might think, ‘oh god I’ve only got 20 minutes I’ve got to stop, no, you can’t talk anymore’, and that’s negative isn’t it?
Before participants were officially recruited to CADENCE, some of the nurses began using the PHQ-9 and the GAD-7 instead of the HADS. Unlike the HADS, the PHQ-9 included a question about suicide risk. The nurses commented that they preferred these measures and felt that the EPC training had given them the confidence to discuss mental health risk issues and a clear pathway to follow to manage these situations:
I wouldn’t have known what to do or what to say or [yeah] what actually was available for people so, and actually we never asked that question [no], we’ve never asked that question since I’ve been doing rehab[ilitation] so it is something that we should be . . .
. . . having a proper structured risk assessment and a plan of ‘what if’ just means that I wouldn’t have any worries talking about it.
Some nurses had not practised mental health-care co-ordination before, and several had not been aware that they were able to access IAPT services. Information on mental health-care co-ordination had provided nurses with options over referral, and it was evident that they now felt more able to direct patients:
I didn’t know anything about mental health-care co-ordination at all [no] before, not at all. I wouldn’t have known where to go and I wouldn’t have known what to do so it opened up a lot of avenues for us definitely.
. . . so I think in the programme, the training we had has already had an impact on enhancing the psychological services people will get through cardiac rehab[ilitation], which can only be a good thing.
Despite having been trained and being made aware that ongoing clinical supervision would be available, when imagining EPC working in practice, nurses remained concerned that they would not have enough time to deliver the intervention effectively:
. . . we’ve been given this great training and all of us we felt were very positive and we can imagine this really working, really, really well. But I think it would be really awful if we find then, well we can do this, but we can’t do it properly because we don’t have enough time.
Qualitative interviews after experiencing implementation of the intervention
Nurses and participants both commented that they felt that the EPC intervention was timely and appropriate within the context of cardiac rehabilitation. Participants explained how their cardiac event had left them feeling vulnerable and low in mood, and that they were glad to have the opportunity to discuss these feelings. Nurses remarked on how providing the intervention had provided them with new ways of supporting their patients, and the training had given them a pathway to deal more fully with individual’s mental health issues.
Nurse interviews
Nurses remained positive about moving from using the HADS to using the PHQ-9 and the GAD-7 to screen and monitor mood, describing how the training had given them the confidence to discuss arising risk issues. Their self-assurance in discussing risk also resulted from the fact that the research team had provided them with a distinct pathway to follow if a patient was identified as being at risk:
The HAD score was not that helpful and didn’t give an indication of risk . . . using GAD-7 and PHQ-9 instead of HAD, means that the screening is more efficient, I think we are much more aware of risk management and risk and what to do.
It was clear from observing supervision sessions, from the supervisor’s report and from nurse interviews, that various factors (e.g. cardiac complications, family issues) had prevented participants from regularly attending EPC sessions within their cardiac rehabilitation. Nurses found this disjointed flow a challenge for EPC delivery:
It’s been challenging, extremely challenging, I would say, because nothing is ever straightforward with patients that we’re dealing with. So you think you’re on track with dealing with maybe their mood and then suddenly they’ll have a cardiac event or something else in their life, some other illness and then of course their mood then, isn’t the priority, it’s their physical health, cos obviously with hearts, that’s going to always take priority.
Cancelled or incomplete EPC sessions (i.e. unattended or as a result of the focus of the appointment moving to mainly physical health issues) regularly needed to be rearranged. Nurses felt that delivering EPC had led to some patients being seen more often than in usual cardiac care:
Because of the BA, I was making sure I was speaking to them next week and then they’d want to come into clinic and it would create more [clinic contact], whereas before we might well have said, ‘Well, you’ve got physical symptoms, you’re seeing your GP’, or, ‘You’re seeing the cardiologist in 3 weeks’ time, give me a ring once you’ve seen them, let me know how you got on and then we’ll make a plan from there’. [. . .] whereas with BA of course, in that 3 weeks you could have seen them three times in clinic. [. . .] Taking up three half-hour slots, so I think that’s probably the reason why it took up more [time].
Nurses struggled to persuade participants to read the CADENCE participant handbook, although most participants did engage with the BA ‘homework’ (e.g. completing a daily mood and activity diary, identifying activities that promote mood). However, nurses felt less confident in knowing when to use a worksheet intended to aid functional analysis (‘triggers, response, avoidance pattern’/’triggers, response, alternative coping’).
Nurses reverted to delivering EPC by telephone, rather than face to face, when there was a lack of privacy or time. Even finding privacy to make the telephone call could be challenging:
I had to sneak up and hide in a room that luckily wasn’t booked, try and find reception to be able to make the call [for an EPC session], because I couldn’t make the call in the gym off the telephone as there was people throwing weights around.
However, nurses sensed that reviewing homework was simpler and participants engaged more fully when meeting in person rather than over the telephone.
Although EPC was seen as potentially worthwhile, nurses could not envisage incorporating the treatment into their rehabilitation sessions, in the longer term, in its present form. They found that providing the intervention was an extra burden and a source of anxiety:
Sometimes we felt slightly relieved when the PHQ come back, it’s still low, you’d [sigh], [. . .] it was relief when they scored low.
Two of the nurses had only delivered the intervention to one patient each; however, they imagined finding it too challenging to have more participants receiving the intervention on their caseloads at the same time, because of the impact on their time, clinic space and workloads. Another nurse, who had delivered EPC to four patients, three of whom had received EPC sessions concurrently, commented that the squeeze on time was the biggest challenge:
I think the [extra] time [required] effectively, and it has been really difficult, really difficult to do, and I mean I don’t feel like I’ve had a huge number of patients on it, [. . .] over the past few months, it shouldn’t have been, but it did have, bearing in mind I’m only a part-time worker, we’re lone workers, that does have an impact on it, [. . .] it’s definitely just the time.
Nurses made comments on the CADENCE materials during their training, which were revised before being implemented. In later interviews, two nurses said that they had used their nurse handbooks frequently to guide them through the EPC sessions. Nurses also made suggestions for more structured sheets for recording EPC sessions within the clinical record, as each were recording details in different ways, which meant that records were unsystematic and varied from nurse to nurse.
Participant interviews
Nine participants who took part in the observational study also agreed to be interviewed. Consistent with the nurse comments, participants admitted to not reading their CADENCE participant handbook for a range of reasons. For example, some participants said that they were dyslexic, too busy or just felt that they were provided with all they needed to know about EPC from the nurse. One participant felt that the handbook needed to be much more concise. When interviewed, participants remembered little about the contents of the handbook, except for the descriptions of patient case summaries, with which some individuals identified well. Nevertheless, most did complete the weekly mood diaries, although some said that they completed them retrospectively or changed entries they had put down earlier in the week.
By completing the mood diary regularly and discussing it with the nurse, most participants who engaged with BA commented that they had gained an awareness of how their behaviour affected their mood and vice versa. Participants had benefited from this by actively changing their behaviour in response to recognising that they felt low:
When I’m noticing that I’m on a downward spiral, I need to pick up the phone and I need to say, ‘Can we go out, can we go and have a cup of coffee somewhere, can we go and do something?’ Because actually, it breaks the mood. And I think I’ve done that relatively successfully.
Participant 4
Other benefits of BA brought up by participants were having the opportunity to talk about how they were feeling and gaining understanding into triggers for their low or improving mood. Some mentioned being more proactive in helping themselves or in finding professional help (e.g. from therapists or their GP) to avoid their mood dropping too far, and in identifying issues in their lives that they needed to address:
I think it [BA] accelerated it [mood improvement] because I had a lot on my mind and [the nurse] knew that and we were able to talk about a number of things. I was very straight with [the nurse] and very frank with [the nurse] and I found it very good, but [the nurse] didn’t offer advice. It was like a good ear, and maybe I needed that. I probably did. [. . .] yes for me it was very beneficial.
Participant 9
Several participants commented that specifically the BA component of the intervention had helped to improve their mood:
So what was great about the CADENCE pilot that I’ve done is it’s shown me that my life–work balance isn’t very good. I probably was aware of it anyway, but it certainly flagged it up. I’ve been trying to walk more and get more exercise generally, and I think by actually noting my mood during the day and during every 2 hours and jotting it down I learnt quite a bit about myself, which I wasn’t expecting.
Participant 9
She told me to keep a diary of what I did throughout the day and that’s, and like what my mood was like, and always just and stuff, basically to work out what was making me put in a bad mood and whatever. And that kind of helped.
Participant 5
As participants were often making major lifestyle changes and decisions in response to their cardiac experience (e.g. stopping smoking, moving home), it was not possible to identify if cardiac rehabilitation alone would have had the same effect.
Two participants, however, felt that receiving EPC had not helped them. One 85-year-old man, with multiple health concerns, opted to withdraw from the BA component of the intervention after a few sessions. He was having difficulty completing the paperwork and was put off by the need to constantly monitor his mood:
That’s what was required on a daily and then virtually an hourly [basis]. The bit that thoroughly put me off was the fact that I was expected to concentrate on that and know when I was having a low mood. Well, I find these moods suddenly come on you and you don’t think about them, you just try and think about something else. And you have to concentrate on noting when this mood changes. While I’m doing that, I’m not relaxing, I’m thinking about this thing that I’m supposed to be doing and it didn’t make me feel at all comfortable, I mean, it was a question of, ‘Oh, did I just have a mood change?’
Participant 2
The other participant felt that physical and mental aspects of care should be kept apart, and he would have preferred to see a male practitioner devoted to providing mental health support.
Participants recognised that the space available for EPC delivery was not always ideal. One man said that he had had EPC sessions in the changing room after the fitness session and in a café bar attached to the gym and, although he had no concerns with this, he felt that other people might feel uncomfortable explaining emotional matters in these environments. Although occasional EPC sessions provided by telephone were seen as acceptable to participants, most much preferred face-to-face contact for their sessions.
The transition between feasibility study and pilot study
Table 11 summarises the main findings from all strands of the qualitative work undertaken during the feasibility study, merged into key areas of learning. These areas were reviewed by the research team and, as a consequence, aspects of the EPC intervention and study procedures were refined prior to piloting.
| Key learning | Changes implemented for the pilot study |
|---|---|
| Variation between nursing teams in ways of working and patient pathways | |
|
Methods: questionnaire devised to send out to potential teams to assess patterns of working |
| Practical challenges and concerns over delivering effective intervention | |
| Concerns over extra time required to deliver EPC within existing services (including consultation time and extra paperwork), lack of private space to deliver EPC confidentially and without distraction and nurse availability to deliver EPC while retaining clinical oversight over patients from the wider cardiac rehabilitation groups |
EPC: intervention fundamentally redesigned from a nurse-led intervention to a patient-led, nurse-supported intervention. Greater emphasis placed on mental health-care co-ordination as the core component rather than BA. BA offered as a patient ‘self-help’ intervention, that is, participants encouraged to read the BA handbook and to complete the recommended homework (e.g. activity/mood diaries, activity scheduling). Nurses were trained to support participant engagement by introducing BA, and to encourage individuals experiencing difficulties in engaging with the self-help materials Methods: implementation to be monitored in qualitative interviews |
| Concerns over working with, and communication to, other psychological services, and the wider primary and secondary clinical care teams | EPC:
|
| Definitions of what constitutes an EPC session (e.g. length, content) were unclear, and there was ambiguity in how it should be recorded in the cardiac rehabilitation notes | Methods: structured template developed to standardise the recording of psychological treatments in nurse notes will also facilitate data extraction to support measuring intervention fidelity |
| Difficulties in engaging with clinical supervision as a result of a lack of structured clinical note-keeping (e.g. around risk issues), and nurses unsure about how to engage effectively with clinical supervision | EPC:
|
| Participants may have interrupted cardiac rehabilitation (breaks over several months) because of physical health problems, and nurses were unsure how to manage the individual’s mental health needs within this extended/interrupted timeline | EPC: intervention training and supporting materials altered to emphasise that the participant’s mental care follow best practice, that is, that mental health-care co-ordination should not be delayed because of physical health problems |
| Nurses made practical suggestions to improve the training materials, and requested scripts to introduce EPC and concepts around BA and mood | EPC: the nurse handbook and participant handbook were revised to improve user-friendliness (e.g. colour coded, scripts provided to introduce concepts) |
| The ‘TRAP/TRAC’ functional analysis tools used in the delivery of BA were not well used or understood by nurses and participants | EPC: TRAP/TRAC tools omitted from the intervention |
Refinements to the enhanced psychological care intervention prior to the pilot study
Several key refinements were made to the EPC intervention during the transition period. The most significant change was the fundamental redesign of the intervention from a nurse-led intervention, with significant emphasis on delivering the BA component within cardiac rehabilitation, to a patient-led, nurse-supported programme. The pilot intervention placed greater emphasis on the mental health-care co-ordination as the core component, rather than BA, and provided nurses with clear guidance on how to structure care. Details of the practical changes to procedures are described in Table 11. The EPC intervention tested within the pilot study is also described in detail in Enhanced psychological care.
Refining pilot study methods
Data from the qualitative study (see Table 11) were also used to refine study procedures prior to piloting. For example, to support the extraction of data relevant to the assessment of intervention fidelity, a structured form was developed (see Appendix 2) to assist nurses in recording key elements of the EPC intervention delivered to participants within each cardiac rehabilitation session. A team profile questionnaire was also developed, in order to capture information on the variability of service provision (e.g. team size, settings) across teams that elected to participate in the pilot trial. It was also apparent that even after a substantive redesign of the EPC intervention designed to reduce nurse workload, implementation issues would need to be revisited in qualitative interviews conducted with nurses and patient participants.
Data from the observational study also informed pilot study procedures in five main ways:
-
The eligibility criteria for participants recruited into the trial were broadened to reflect changing practice, whereby increasingly diverse patients were being referred for cardiac rehabilitation (i.e. patients with heart failure, not just patients with ACS).
-
The content of the case note review paperwork was revised to decrease the time burden on researchers collating detailed information around consultations with health professionals.
-
The information sought regarding participant employment and antidepressant use (via the SRUQ) was amended and simplified.
-
The assumptions underpinning the accrual of participants into the pilot RCT were deemed unrealistic based on new, previously unknown process data obtained on participant recruitment and retention (see Participant recruitment and retention). As a result, a working paper was drafted, outlining the implications regarding the design of the pilot study (see Appendix 9). In summary, we recommended that the 6-month recruitment period was not long enough to recruit sufficient participants to estimate the pilot study primary outcome (follow-up rate) with the required precision. After reviewing the sample size calculations and exploring different scenarios for our assumptions, we therefore recommended that the recruitment period be extended by 3 months (i.e. a total recruitment period of 9 months). Even with this extension, if the conditions of the feasibility study were replicated in the pilot trial, the likely accrual of participants into the pilot study might still result in the recruitment of only 43 participants (compared with the original target of 64). Despite our estimates based on feasibility data, the request to extend participant recruitment in the pilot study was rejected. As a consequence, a priori, we predicted the pilot study would fail to achieve its recruitment targets, anticipating recruitment within the region of 43 participants (half of whom would receive EPC).
-
The lower than expected participant accrual rate also had serious implications for the nested qualitative study. We had originally intended to undertake maximum variation sampling from a pool of EPC participants (see Data collection), to obtain diversity in terms of their sociodemographic characteristics, as well as their engagement (or otherwise) with EPC. In reality, the lower accrual rate meant that we had to invite most of the trial participants to take part in a qualitative interview.
Chapter 5 Pilot study results
The pilot study consisted of three main elements (i.e. a pilot RCT, an economic evaluation and a nested qualitative study), the results of which are presented below.
Pilot randomised controlled trial
Recruitment
Cardiac rehabilitation teams
The period of recruitment of the cardiac nursing teams ran from December 2014 to February 2015, with a total of 8 out of the 20 approached agreeing to participate (40%) in the pilot trial. Nine teams declined participation, two teams did not respond and one team ceased to exist as a result of service reconfiguration. Teams (clusters) were then randomised to one of the two trial arms (five to EPC and three to UC), with one team withdrawing from the EPC arm immediately post randomisation (May 2015). The mixed community and hospital-based team was replaced with another team that was similar with respect to patient throughput and population mix.
The timeline for obtaining local approvals is summarised in Table 12; in two areas, approvals took < 2 months from the initial contact, in five areas it took between 2.5 and 3.5 months and, in the final area, it took > 5 months. This posed a serious problem for participant recruitment, with delays experienced by teams in most areas. Of particular concern were the two areas where nearly a 2-month delay was encountered between the teams receiving all the necessary research/intervention training and then being able to implement the study protocol (i.e. effectively shortening their participant recruitment period from 6 months to 4 months).
| Team | First approach to team | Initial EOI from team | Trial allocation confirmed | EPC training delivered | First NHS R&D contact | NHS R&D approval letter issued | Participant recruitment started |
|---|---|---|---|---|---|---|---|
| A | 4 December 2014 | 19 December 2014 | 1 May 2015 | UC | 9 April 2015 | 28 May 2015 | w/c 22 June 2015 |
| B | 4 December 2014 | 19 December 2014 | 1 May 2015 | UC | 9 April 2015 | 1 June 2015 | w/c 22 June 2015 |
| C | 4 December 2014 | 5 January 2015 | 1 May 2015 | UC | 9 April 2015 | 20 July 2015 | w/c 10 August 2015 |
| D | 4 December 2014 | 8 January 2015 | 1 May 2015 | 2 June 2015 and 3 June 2015 | 9 April 2015 | 7 July 2015 and 13 July 2015 (two trusts) | w/c 3 August 2015 |
| E | 26 January 2015 | 3 February 2015 | 1 May 2015 | 2 June 2015 and 3 June 2015 | 9 April 2015 | 16 July 2015 | 18 August 2015 |
| F | 26 January 2015 | 29 January 2015 | 1 May 2015 | 13 July 2015 | 9 April 2015 | 13 July 2015 | w/c 20 July 2015 |
| G | 4 December 2014 | 10 April 2015 | 1 May 2015 | 13 July 2015 | 10 April 2015 | 10 September 2015 | 10 September 2015 |
| H | 27 May 2015 | 8 June 2015 | 9 June 2015 | 13 July 2015 | 15 June 2015 | 2 September 2015 | 2 September 2015 |
Table 13 describes the geographic location, setting, team composition, type of programme offered to the patient (including ‘usual psychological care’) and the average number of patients assessed for each of the cardiac rehabilitation nursing teams. There were two hospital-based teams and three teams based in the community, and the remaining three teams were based both in the community and at the hospital. The teams had a varying number of cardiac nurses working either part time or full time, with one team only having one part-time cardiac nurse undertaking an average of 32 assessments per month. Most of the teams offered centre-based programmes either in the community and/or at the hospital, clinic-based individual appointments and home-based programmes, with one team offering only centre-based programmes. The majority of hospital and community-based teams covered a rural setting, and two teams covered an inner-city patient population. The three UC teams had no access to psychological support integrated within their cardiac rehabilitation programme, although one team had limited access to a clinical psychologist. Three of the EPC teams had little or no access to psychological support; the other two EPC teams, located in inner-city areas, had established close links to the clinical psychology/IAPT services for mental health support.
| Team | Team location | Setting | Cardiac team composition | Type of cardiac rehabilitation programme offered (including usual psychological care)a | Average number of patients assessed per monthb |
|---|---|---|---|---|---|
| A | Hospital | Rural |
Seven nurses (two full time; five part time) One team manager (part time) |
Centre-based groups (community/hospital) Clinic-based individual appointments Home-based programmes |
40 |
| B | Community | Rural |
Seven nurses (one full time; six part time) Two administrators (part time) |
Centre-based groups (community/hospital) – some areas/teams had support from a clinical psychologist Clinic-based individual appointments Home-based programmes |
60 (for the whole service) 15–20 per area |
| C | Community | Rural |
Three nurses (part time) One team manager (part time) |
Centre-based groups (community/hospital) | 12–16 |
| D | Community | Rural/urban | Two nurses (one full time; one part time) |
Centre-based groups (community/hospital) Clinic-based individual appointments Home-based programmes |
26 |
| E | Hospital | Rural/urban |
Two nurses (part time) One team manager (part time) One administrator (part time) |
Centre-based groups (community/hospital) Clinic-based individual appointments Home-based programmes |
70 |
| F | Mixed hospital and community | Urban |
One nurse (part time) One administrator (part time) |
Centre-based groups (community/hospital) Clinic-based individual appointments Home-based programmes |
32 |
| G | Mixed hospital and community | Inner city |
Four nurses (part time) One administrator (part time) |
Centre-based groups – limited psychological input via referral to local IAPT programmes Clinic-based individual appointments Home-based programmes |
33 |
| H | Mixed hospital and community | Inner city | Six nurses split across hospital and community settings rotating between both settings |
Centre-based groups (community) Clinic-based individual appointments – part-time clinical psychologist linked to the team Home-based programmes |
50–60 |
Participant recruitment
Owing to limited numbers of patients within the individual cardiac rehabilitation teams, contrary to the statistical analysis plan (see Appendix 10), we did not attempt to report participant recruitment rates for each team on a weekly basis. We report aggregate data across teams within each trial arm for the number of participants who were eligible for, and subsequently recruited to, the trial. The flow of cardiac nursing teams and patients through the pilot stage of the CADENCE study is summarised in Figure 3. Cardiac nursing teams started patient recruitment on different dates depending on approvals (see Table 12), with individual teams recruiting for between 3 and 6 months during the period of June 2015 to 31 December 2015.
FIGURE 3.
Pilot study: CONSORT flow diagram (with cluster extension). CR, cardiac rehabilitation.
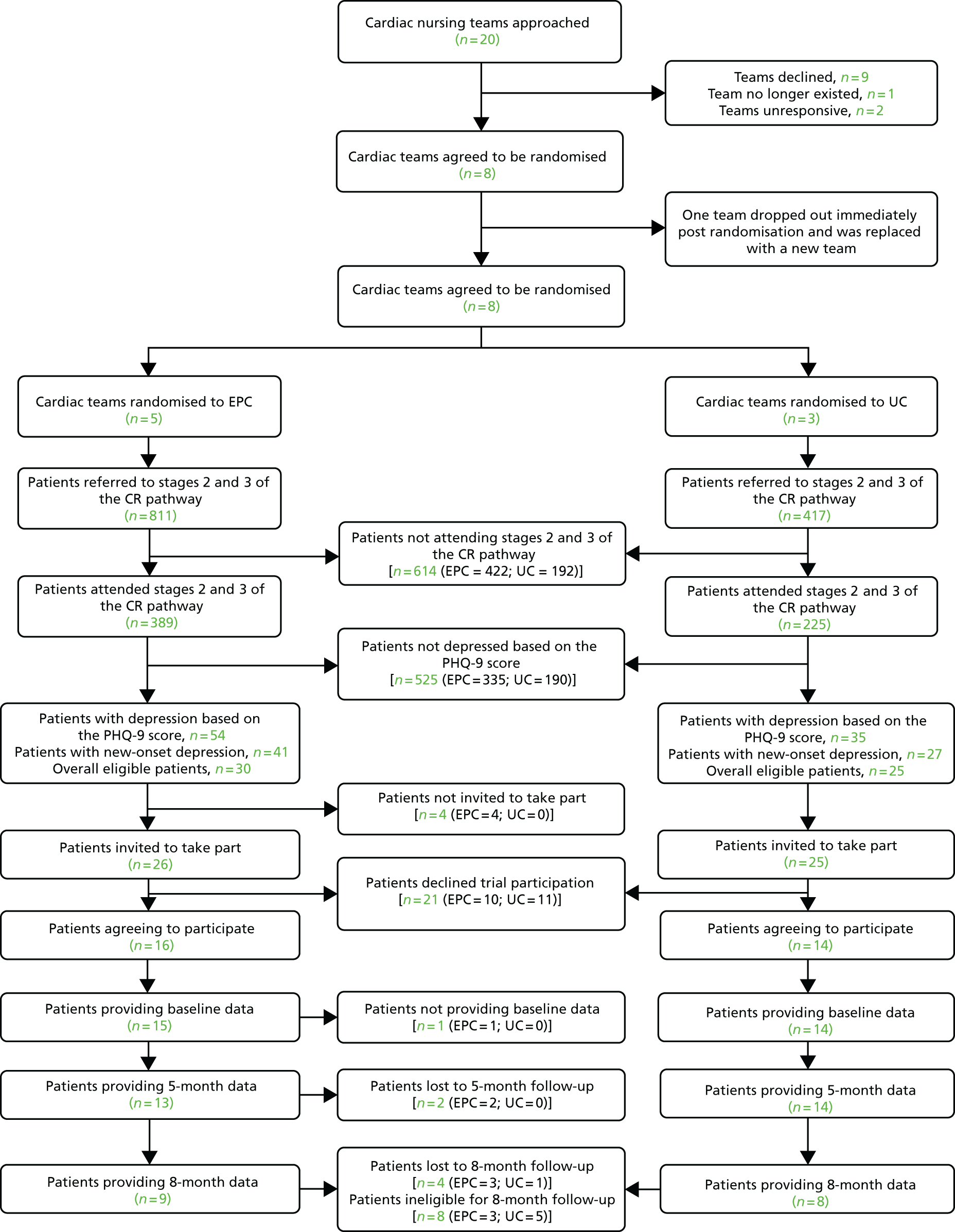
A total of 614 patients (389 in the EPC arm and 225 in the UC arm) were screened for eligibility at hospital and community-based cardiac rehabilitation clinics. The mean age of patients attending an initial nurse screening clinic was 66.2 years (range 32–95 years), with the majority being male (481 out of 614; 70.2%) and reporting their ethnicity as white (579 out of 614; 94.3%).
A total of 29 participants were randomised via their clusters (cardiac rehabilitation teams): 15 patients were randomised to the EPC arm and 14 to UC. The flow of participants at different stages of the trial is shown in Figure 3. Patients were screened at cardiac rehabilitation appointments, with 89 out of 614 (14.5%) scoring ≥ 10 on the PHQ-9 questionnaire, indicating depression. Of these 89 patients, 55 out of 614 were found to be eligible to participate in the trial (9%, 95% CI 7% to 12%); 33 were not eligible, as they were either actively being treated for depression (n = 21; 3%, 95 CI 2% to 5%) or were individuals with depression who were excluded for other reasons (e.g. alcohol and/or drug dependency problems, were currently ineligible for cardiac rehabilitation based on their cardiac condition or had language and communication problems).
Fifty-one of the 55 patients deemed to be eligible by a nurse were invited to participate. Of the four patients not invited, one patient declined any further input from the cardiac rehabilitation service, two patients were not offered EPC as the nurse was at capacity and could not take on more patients and the final patient reported a marked improvement in mood and did not feel that they required EPC. Of the 51 patients offered study entry, 21 (41%) declined participation for a variety of reasons including a preference to see their GP (n = 6); feeling better and would prefer to see how mood goes while taking part in cardiac rehabilitation (n = 3); that their mood was a long-standing problem unrelated to this current hospital stay (n = 2); or they did not want the EPC intervention (n = 2) (nine patients did not give reason). Of the 55 eligible patients, 30 out of 55 (54%, 95% CI 41% to 68%) were initially recruited (one of whom withdrew prior to providing baseline data). Four eligible patients, all in the EPC arm, were not invited to participate in the trial.
Of the denominator of 614 patients who attended an initial nurse assessment, the percentage recruited was 5% (95% CI 3% to 7%). Of the 29 participants who were recruited to the trial, 27 provided data at the 5-month follow-up (93%, 95% CI 77% to 99%). At 8 months, data were not able to be collected for 8 out of 29 participants because of late recruitment to the trial. Of the 21 participants for whom 8-month data could have been obtained, data were collected for 17 (81%, 95% CI 58% to 95%).
Sample characteristics
Sociodemographic characteristics, smoking status and baseline psychological descriptors of participants entered into the study are reported in Table 14. Both trial arms had approximately equal proportions of males and females; all participants were white and all apart from one participant reported that English was their preferred language. The mean age of the sample recruited was 62.7 years in the EPC arm and 68.1 years in the UC arm. There was, however, some imbalance between the arms, with the EPC arm having a higher proportion of participants in the three most deprived deciles (6 out of 15; 40%) than UC (2 out of 14; 14%).
| Characteristic | Treatment arm | |
|---|---|---|
| UC (N = 14) | EPC (N = 15) | |
| Gender, n (%) | ||
| Male | 7 (50) | 8 (53) |
| Age (years), mean (SD); min., max. | 68.1 (8.6); 53, 79 | 62.7 (8.9); 47, 75 |
| Ethnicity, n (%) | ||
| White | 14 (100) | 15 (100) |
| Preferred language, n (%) | ||
| English | 13 (93) | 15 (100) |
| Other | 1 (7) | 0 (0) |
| Smoking status, n (%) | ||
| Never smoked | 5 (36) | 4 (27) |
| Ex-smoker | 9 (64) | 11 (73) |
| Time since quitting smoking (ex-smokers only), n (%) | ||
| < 6 months | 3 (33) | 3 (27) |
| 6–12 months | 0 (0) | 0 (0) |
| 1–5 years | 0 (0) | 2 (18) |
| 5–10 years | 1 (11) | 0 (0) |
| > 10 years | 5 (56) | 6 (55) |
| IMD, median decile (IQR)a | 6 (4–8) | 5 (1–7) |
| Indicating cardiac event and/or proceduresb | ||
| MI (includes STEMI, NSTEMI or unclassified MI), n (%) | 1 (7) | 2 (13) |
| MI and PCI (angioplasty, angiogram or stent), n (%) | 5 (36) | 4 (27) |
| Angina, n (%) | 1 (7) | 0 (0) |
| Other heart condition,c n (%) | 3 (21) | 1 (7) |
| PCI (angioplasty, angiogram or stent), n (%) | 4 (29) | 2 (13) |
| CABG, n (%) | 0 (0) | 3 (20) |
| Other cardiac procedure (pacemaker, valve surgery), n (%) | 0 (0) | 3 (20) |
| CIS-R | (N = 13) | (N = 14) |
| Primary diagnosis category, n (%) | ||
| No psychiatric diagnosis identified | 9 (69) | 6 (43) |
| Mixed anxiety and depressive disorder – mild | 1 (8) | 1 (7) |
| Mild depressive episode | 3 (23) | 4 (29) |
| Moderate depressive episode | 0 (0) | 3 (21) |
| Secondary diagnosis category, n (%) | ||
| No psychiatric diagnosis identified | 12 (92) | 9 (64) |
| Mixed anxiety and depressive disorder – mild | 1 (8) | 1 (7) |
| Generalised anxiety disorder – mild | 0 (0) | 1 (7) |
| Mixed anxiety and depressive disorder | 0 (0) | 2 (14) |
| Specific (isolated) phobia | 0 (0) | 1 (7) |
| Emotional health | ||
| PHQ-9, mean score (SD); min., max. | 11.9 (1.8); 10, 16 | 14.2 (4.7); 10, 25 |
| BDI-II, mean score (SD); min., max. | 12.5 (4.9); 4, 20 | 18.4 (7.4); 7, 31 |
| BAI, mean score (SD) | 12.5 (4.4) | 19.3 (10.7) |
| Do you believe you have low mood?, n (%) | ||
| Yes | 11 (79) | 11 (73) |
| No | 3 (21) | 4 (27) |
| Do you want any professional help for your low mood? (N = 11), n (%) | ||
| Strongly prefer help | 1 (9) | 5 (45) |
| Prefer help | 4 (36) | 2 (18) |
| Prefer not to receive help | 3 (27) | 3 (27) |
| Strongly prefer not to receive help | 3 (27) | 1 (9) |
| What type of professional help would you prefer? (N = 11), n (%) | ||
| Strongly prefer non-drug help | 6 (55) | 9 (82) |
| Prefer non-drug help | 3 (27) | 0 (0) |
| Prefer drug-based help | 1 (9) | 0 (0) |
| Strongly prefer drug-based help | 0 (0) | 0 (0) |
| Do not mind | 1 (9) | 2 (18) |
| HRQoL | ||
| EQ-5D-5L score, mean score (SD) | 0.801 (0.097) | 0.644 (0.226) |
| EQ-5D VAS score, mean score (SD); min., max. | 63.0 (23.5); 4, 95 | 48.7 (19.3); 10, 100 |
In terms of depressive symptoms, the EPC group reported higher levels of depression at baseline than the UC group, as recorded by both the PHQ-9 (14.2 vs. 11.9) and the BDI-II (18.4 vs. 12.5) scores. At baseline, 10 out of 15 (67%) participants in the EPC arm had a BDI-II score of ≥ 14 compared with 7 out of 14 (50%) in the UC arm. The highest BDI-II score in the UC group at baseline was 20, whereas there were seven participants in the EPC group who had a score of ≥ 20 at baseline. Of note, participants from the more deprived backgrounds also had higher baseline BDI-II scores (see Table 14).
The majority of participants were former smokers (four in the EPC arm and five in the UC arm); none was current smokers. There was a wide range of time since quitting smoking, with some participants having quit several years ago and six participants having quit < 6 months prior to entering into the study.
A post hoc comparison was conducted on the sociodemographic characteristics (age, gender and ethnicity) and PHQ-9 scores of patients who were invited to participate but who declined (where sufficient data were recorded on the screening logs). Of the 51 eligible participants who were offered study entry, 15 out of 29 (52%) male patients and 15 out of 22 (68%) female patients accepted. Of 49 eligible white patients invited to participate, 19 (39%) declined, as did both of the two Asian/Asian-British patients. The mean age among 30 eligible patients who accepted participation was 63.8 years (SD 9.5 years), range 47–78 years; among 21 patients who declined, mean age was 61.1 years (SD 12.3 years; range 36–83 years). For the acceptors, mean PHQ-9 score was 13.4 (SD 4.1; range 10–25); for the decliners, mean PHQ-9 score was 14.4 (SD 4.6; range 10–24).
The indicating cardiac events, as recorded in GP or cardiac nurse records, and participant self-reported cardiac conditions at baseline are recorded in Table 15. Trial participants had experienced a wide range of cardiac events that precipitated their referral to cardiac rehabilitation according to their clinical records, the most frequent of which were MI (STEMI or NSTEMI) and PCI.
| Outcome | Time point, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | 5 months | 8 months | ||||
| UC (N = 14) | EPC (N = 15) | UC (N = 14) | EPC (N = 13) | UC (N = 8) | EPC (N = 9) | |
| Smoking status | ||||||
| Smoking status | N = 14 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Never smoked | 5 (36) | 4 (27) | 5 (36) | 4 (31) | 3 (38) | 3 (33) |
| Ex-smoker | 9 (64) | 11 (73) | 8 (57) | 9 (69) | 5 (63) | 5 (56) |
| Current smoker | 0 (0) | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 1 (11) |
| Participant-reported heart problems and procedures: have you ever had this heart problem or procedure? | ||||||
| MI | N = 14 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 8 (57) | 12 (80) | 8 (57) | 11 (85) | 4 (50) | 8 (89) |
| Not sure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Angina | N = 13 | N = 14 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 6 (46) | 6 (43) | 4 (43) | 4 (31) | 3 (38) | 3 (33) |
| Not sure | 1 (8) | 0 (0) | 1 (7) | 0 (0) | 1 (13) | 0 (0) |
| Hospital admission – non-cardiac chest pain | N = 12 | N = 14 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 0 (0) | 4 (29) | 0 (0) | 4 (31) | 0 (0) | 2 (22) |
| Not sure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Heart failure | N = 13 | N = 14 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 1 (8) | 3 (21) | 2 (14) | 2 (15) | 0 (0) | 2 (22) |
| Not sure | 0 (0) | 2 (14) | 0 (0) | 2 (15) | 0 (0) | 1 (11) |
| Arrhythmia | N = 13 | N = 14 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 2 (15) | 6 (43) | 4 (29) | 6 (46) | 1 (13) | 2 (22) |
| Not sure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PCI procedure | N = 14 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 8 (57) | 7 (47) | 9 (64) | 7 (54) | 5 (63) | 6 (67) |
| Not sure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CABG procedure | N = 13 | N = 14 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 3 (23) | 3 (21) | 3 (21) | 2 (15) | 1 (13) | 1 (11) |
| Not sure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Valve surgery | N = 13 | N = 14 | N = 13 | N = 13 | N = 8 | N = 9 |
| Yes | 1 (8) | 3 (21) | 1 (8) | 3 (23) | 0 (0) | 1 (11) |
| Not sure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other current health problems | ||||||
| Asthma | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 1 (8) | 5 (33) | 1 (7) | 4 (31) | 3 (38) | 3 (33) |
| Lung disease | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 2 (15) | 3 (20) | 3 (21) | 2 (15) | 2 (25) | 1 (11) |
| High blood pressure | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 11 (85) | 4 (27) | 8 (57) | 6 (46) | 5 (63) | 7 (78) |
| Diabetes mellitus | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 3 (23) | 1 (7) | 3 (21) | 1 (8) | 1 (13) | 2 (22) |
| Ulcer/stomach disease | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 6 (46) | 2 (13) | 2 (14) | 1 (8) | 0 (0) | 1 (11) |
| Bowel disease | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Kidney disease | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 1 (8) | 1 (7) | 1 (7) | 1 (8) | 0 (0) | 1 (11) |
| Liver disease | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 1 (8) | 1 (7) | 1 (7) | 1 (8) | 0 (0) | 1 (11) |
| Anaemia/blood disease | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 5 (38) | 0 (0) | 4 (29) | 3 (23) | 2 (25) | 1 (11) |
| Cancer | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nervous system disease | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 1 (8) | 1 (7) | 1 (7) | 1 (8) | 1 (13) | 1 (11) |
| Arthritis | N = 14 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 11 (79) | 8 (53) | 11 (79) | 8 (62) | 7 (88) | 5 (56) |
| Back pain | N = 14 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 10 (71) | 7 (47) | 10 (71) | 8 (62) | 8 (100) | 6 (67) |
| Mental health problems | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 0 (0) | 0 (0) | 1 (7) | 2 (15) | 0 (0) | 1 (11) |
| Skin disease | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 4 (31) | 1 (7) | 4 (29) | 1 (8) | 1 (13) | 0 (0) |
| Hearing/visual impairment | N = 13 | N = 15 | N = 14 | N = 13 | N = 8 | N = 9 |
| Yes | 1 (8) | 4 (27) | 2 (14) | 3 (23) | 1 (13) | 2 (22) |
Enhanced psychological care intervention delivery
Seven nurses from four teams provided EPC to one or more participants (one team recruited no participants to the study). Three teams had two nurses providing EPC and one team had a single practitioner. Most nurses had their own participants allocated to them, with some shared cases when the index nurse was unavailable.
Thirty supervision calls were made to the teams over a 7-month period (August 2015 to February 2016). Some teams opted for joint supervision (i.e. both nurses listening in to all cases), whereas others had individual calls related to their own cases, and then handed the telephone to the other team nurse.
Intervention fidelity
From data extracted from the notes recorded by cardiac rehabilitation nurses (Table 16), the number of cardiac rehabilitation sessions attended ranged from 0 to 22 (mean 8.3 sessions, SD 8.1 sessions) for the 15 EPC participants, and from 1 to 12 sessions (mean 7.4 sessions, SD 2.7 sessions) in the UC arm. The mean time between the date of consent to the start of cardiac rehabilitation was 3 days (SD 12 days) in the EPC group and 14 days (SD 2 days) in the UC group.
| Outcome | Trial arm | |
|---|---|---|
| UC (N = 14) | EPC (N = 15) | |
| Number of cardiac rehabilitation sessions, mean (SD), n; median (min., max.) | ||
| Offered | 8.8 (3.2), 14; 8 (2, 14) | 9.4 (8.1), 15; 7 (0, 24) |
| Attended | 7.4 (2.7), 14; 7.5 (1, 12) | 8.3 (8.1), 15; 7 (0, 22) |
| Number of EPC sessions attended, n (%) | ||
| 8 | NA | 8 (53) |
| 7 | 1a (7) | |
| 6 | 1b (7) | |
| 1 | 1c (7) | |
| 0 | 4 (27) | |
| Time (days) elapsed from consent date to starting cardiac rehabilitation, mean (SD), n; median (min., max.) | 14 (2), 14; 3 [–15, 27] | 3 (12), 15; 5 (–35, 22) |
| Participants who started cardiac rehabilitation prior to trial recruitment, n/N (%) | 6 (43) | 2 (13) |
| Time (days) elapsed from trial entry to start of EPC, mean (SD), n; median (min., max.) | NA | 6 (5), 11; 5 (0, 19) |
| Time (days) elapsed from trial entry to nurse discharge from cardiac rehabilitation, mean (SD), n; median (min., max.) | 64 (28), 14; 69 (13, 112) | 96 (37), 12; 89 (48, 185) |
| Time (days) elapsed from discharge to sending GP discharge letter, mean (SD), n; median (min., max.) | 5 (7), 11; 1 (0, 23) | 1 (3), 7; 0 (0, 8) |
| Psychological care co-ordination activities provided (evidence of care co-ordination during at least one attended EPC session), n (%) | NA | 5 (33) |
| Nurse referral to GP/IAPT services/other psychological care services,d n (%) | 0 (0) | 7 (47) |
| Care accessed for depression privately (participant self-report), n (%) | 0 (0) | 0 (0) |
| Care accessed for depression via established NHS mental health pathways, n (%) | 0 (0) | 0 (0) |
Consistent with the training provided, in the EPC arm there was documentary evidence that a maximum of eight sessions of EPC were offered. Just over half of the participants in the EPC arm received the maximum number of sessions (8 out of 15; 53%), whereas 4 out of 15 participants (27%) had no EPC sessions recorded. Despite the intervention being designed to place much greater emphasis on mental health-care co-ordination, documentary evidence of co-ordination activities was observed in around half of the EPC patients (7 out of 15; 47%); these individuals were referred to another NHS service (e.g. GP, IAPT, another psychological care service). This compared with 0 out of 14 UC participants (0%). No participants in either the EPC arm or the UC arm had evidence recorded in the nurse’s notes that they were accessing private mental health care.
There were no deaths recorded in either arm of the trial between baseline and the final follow-up.
Using data extracted from either the GP or cardiac nurse records, there was evidence of a small number of new cardiac events/diagnoses between baseline and the 5-month follow-up (EPC = five events; UC = four events) and a further four events (EPC = three events; UC = one event) between 5 months and 8 months. The description of cardiac events is presented in Table 17.
| Cardiac eventa | Follow-up time period in each trial arm, n (%) | |||
|---|---|---|---|---|
| Baseline to 5 months | 5–8 months | |||
| UC (N = 14) | EPC (N = 13) | UC (N = 8) | EPC (N = 9) | |
| MI (STEMI or NSTEMI) | 0 (0) | 1 (8) | 0 (0) | 0 (0) |
| ACS (not specified) | 2 (14) | 0 (0) | 0 (0) | 0 (0) |
| Angina | 0 (0) | 1 (8) | 0 (0) | 1 (11) |
| Other diagnosisb | 2 (14) | 1 (8) | 1 (13) | 2 (22) |
| PCI procedure | 0 (0) | 1 (8) | 0 (0) | 0 (0) |
Using data from the GP case notes review (Table 18), only two participants (one EPC participant and one UC participant) had evidence of a mental health event related to a newly diagnosed condition recorded within the practice records, and only one EPC participant had evidence of prescribing for antidepressant medication recorded within their GP records. Three participants had evidence of amitriptyline prescribing (one EPC participant and two UC participants) during the follow-up period. Two amitriptyline prescriptions were for 10 mg for ‘sleep problems’ and the third was for 25 mg with no recorded indication; given the low doses, these prescriptions were unlikely to be intended as antidepressant medications.
| Mental health event and use of antidepressant medication | Follow-up time period in each trial arm, n (%) | |||
|---|---|---|---|---|
| Baseline to 5 months | 5–8 months | |||
| UC (N = 14) | EPC (N = 13) | UC (N = 8) | EPC (N = 9) | |
| Mental health event(s) during follow-up | ||||
| 0 | 13 (93) | 12 (92) | 0 (0) | 0 (0) |
| 1 | 1 (7)a | 1 (8)b | 0 (0) | 0 (0) |
| 2 or more | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Using antidepressant medication at follow-up | 1 (7)c | 1 (8)d | 0 (0) | 0 (0) |
To test the feasibility of collecting physiological and biochemical measurements from routine records, we noted the availability of data from either GP or cardiac nurse records. As can be seen in Table 19, the routine recording of such measurements was highly variable; blood pressure measurements were the most frequently reported, whereas other measurements, such as glycated haemoglobin (HbA1c) and triglycerides levels, were frequently omitted from the record.
| Outcome measure availabilitya | Follow-up time point in each trial arm, n (%) | |||||
|---|---|---|---|---|---|---|
| Baselineb | 5 monthsc | 8 monthsd | ||||
| UC (N = 14) | EPC (N = 15) | UC (N = 14) | EPC (N = 15) | UC (N = 8) | EPC (N = 9) | |
| BMI (kg/m2) | 9 (64) | 6 (40) | 9 (64) | 5 (33) | 1 (13) | 4 (44) |
| Blood pressure (mmHg) | 13 (93) | 13 (87) | 13 (93) | 15 (100) | 4 (50) | 7 (78) |
| HbA1c (%) | 1 (7) | 2 (13) | 4 (29) | 1 (7) | 0 (0) | 1 (11) |
| Total cholesterol | 7 (50) | 8 (53) | 9 (64) | 6 (40) | 2 (14) | 0 (0) |
| Triglycerides | 2 (14) | 2 (13) | 5 (36) | 1 (7) | 1 (13) | 1 (11) |
Mental health risk and adverse event reporting
During the course of the pilot trial, the protocol relating to managing self-harm/suicide risk was triggered for four events in relation to three participants. Three risks were recorded by the nurse during an EPC intervention session and one managed by a study researcher during a research interview. For the three participants triggering the risk protocol, all were initially identified at ‘Level B1’ risk; that is, they reported plans/preparations to self-harm, but with protective factors identified, and the participant was advised to seek help from their GP. One individual was subsequently escalated to ‘Level B2’ risk; they had taken no action to talk to their GP regarding their mood, and their GP was contacted (with the participant’s permission) to alert them to the risk.
Although no adverse events were identified, 11 serious adverse events (regarding six participants) were recorded by the research team. All serious adverse events came to light through researcher follow-up assessments or through case note reviews of clinical records; none was reported ‘in real time’ from clinical teams. Four events occurred during the course of the treatment period, whereas seven occurred after treatment and within the trial follow-up period; all patients recovered fully. The serious adverse events all related to participant admission to hospital on account of infective conditions, asthma or cardiac complications (e.g. chest pain, additional stent, postural hypotension). None of the serious adverse events was judged by the TSC to be related to the trial intervention or research procedures.
Participant-reported outcome measures
Psychological outcomes
The participant-reported outcomes relating to their mood at each follow-up point are reported in Tables 20 (5 months) and 21 (8 months), and Figure 4. In terms of depressive symptoms, at the 5-month follow-up, the mean BDI-II score was reduced from baseline in both groups. Three participants in the EPC arm did not report a BDI-II score at the 5-month follow-up (baseline BDI-II scores were 14, 25 and 31). For 26 participants, the mean difference in BDI-II scores at the 5-month follow-up (with adjustment for baseline score), comparing EPC with UC was 1.7 (95% CI –3.8 to 7.3). For the 17 participants available for analysis, by 8 months the mean difference in BDI-II scores (EPC vs. UC) was 4.4 (95% CI –1.4 to 10.2).
| Outcome variable | Follow-up time point in each trial arm | Mean difference or ORa (95% CI) | |||
|---|---|---|---|---|---|
| Baseline | 5 months | ||||
| UC (N = 14) | EPC (N = 15) | UC (N = 14) | EPC (N = 13) | ||
| BDI-II | |||||
| Mean score (SD), n; min., max. | 12.5 (4.9), 14; 4, 20 | 18.4 (7.4), 15; 7, 31 | 7.4 (3.7), 14; 2, 14 | 10.2 (8.4), 12; 2, 33 | 1.7 (–3.8 to 7.3) |
| 50% reduction in score from baseline, n/N (%) | – | – | 7/14 (50) | 5/12 (42) | 0.71 (0.16 to 3.25) |
| Remission (score of < 14) from baseline,b n/N (%) | – | – | 6/7 (86) | 3/7 (43) | 0.13 (0 to 1.37) |
| MCID in score (17.5% reduction from baseline), n/N (%) | – | – | 11/14 (79) | 10/12 (83) | 1.36 (0.22 to 8.31) |
| CSRC in score from baseline,c n/N (%) | – | – | 3/7 (43) | 2/7 (29) | 0.53 (0.07 to 4.33) |
| BAI | |||||
| Mean score (SD), n; min., max. | 12.5 (4.4), 14; 4, 21 | 19.3 (10.7), 15; 4, 36 | 9.2 (4.0), 14; 4, 15 | 14.7 (8.3), 13; 3, 27 | 4.6 (–0.8 to 10.0) |
| EQ-5D-5L | |||||
| Mean score (SD), n; median (IQR) | 0.869 (0.085), 14; 0.893 (0.866–0.924) | 0.751 (0.182), 15; 0.751 (0.652–0.886) | 0.875 (0.115), 13; 0.901 (0.843–0.951) | 0.885 (0.065), 13; 0.866 (0.828–0.924) | 0.045 (–0.023 to 0.113) |
| EQ-5D VAS | |||||
| Mean score (SD), n; min., max. | 63 (24), 14; 4, 95 | 49 (19), 15; 10, 100 | 72 (21), 14; 30, 100 | 72 (21), 12; 30, 100 | 5 (–13 to 22) |
| HeartQoL | |||||
| Mean score (SD), n; min., max. | 21.3 (9.1), 14; 5, 37 | 15.2 (9.9), 15; 1, 35 | 31.5 (4.9), 14; 23, 38 | 22.9 (10.3), 13; 4, 41 | –8.2 (–14.9 to –1.4) |
| Outcome variable | Follow-up time point in each trial arm | Mean difference or ORa (95% CI) | |||
|---|---|---|---|---|---|
| Baseline | 8 months | ||||
| UC (N = 14) | EPC (N = 15) | UC (N = 8) | EPC (N = 9) | ||
| BDI-II | |||||
| Mean score (SD), n; min., max. | 12.5 (4.9), 14; 4, 20 | 18.4 (7.4), 15; 7, 31 | 7.0 (3.5), 8; 1, 12 | 12.6 (6.6), 9; 1, 22 | 4.4 (–1.4 to 10.2) |
| 50% reduction in score from baseline, n/N (%) | – | – | 4/8 (50) | 2/9 (22) | 0.29 (0.04 to 2.09) |
| Remission (score of < 14) from baseline,b n/N (%) | – | – | 4/4 (100) | 2/5 (40) | 0 (0 to 1.10) |
| MCID in score (17.5% reduction from baseline), n/N (%) | – | – | 5/8 (63) | 6/9 (67) | 1.20 (0.18 to 7.99) |
| CSRC in score from baseline,c n/N (%) | – | – | 2/4 (50) | 1/5 (20) | 0.25 (0 to 3.56) |
| BAI | |||||
| Mean score (SD), n; min., max. | 12.5 (4.4), 14; 4, 21 | 19.3 (10.7), 15; 4, 36 | 6.4 (4.6), 8; 0,12 | 12.7 (7.1), 9; 4, 24 | 5.0 (–1.2 to 11.1) |
| EQ-5D-5L | |||||
| Mean score (SD), n; median (IQR) | 0.869 (0.085), 14; 0.893 (0.866–0.924) | 0.751 (0.182), 15; 0.751 (0.652–0.886) | 0.876 (0.092), 8; 0.868 (0.830–0.951) | 0.827 (0.116), 9; 0.874 (0.697–0.951) | –0.041 (–0.145 to 0.064) |
| EQ-5D VAS | |||||
| Mean score (SD), n; min., max. | 63 (24), 14; 4, 95 | 49 (19), 15; 10, 100 | 64 (18), 7; 40, 90 | 64 (12), 8; 50, 80 | 1 (–17 to 19) |
| HeartQoL | |||||
| Mean score (SD), n; min., max. | 21.3 (9.1), 14; 5, 37 | 15.2 (9.9), 15; 1, 35 | 28.3 (6.7), 8; 17, 36 | 28.6 (7.6), 9; 16, 36 | 0.1 (–7.9 to 8.0) |
FIGURE 4.
Pilot study: BDI-II scores for participants with baseline and at least one follow-up observation. (a) UC; and (b) EPC.

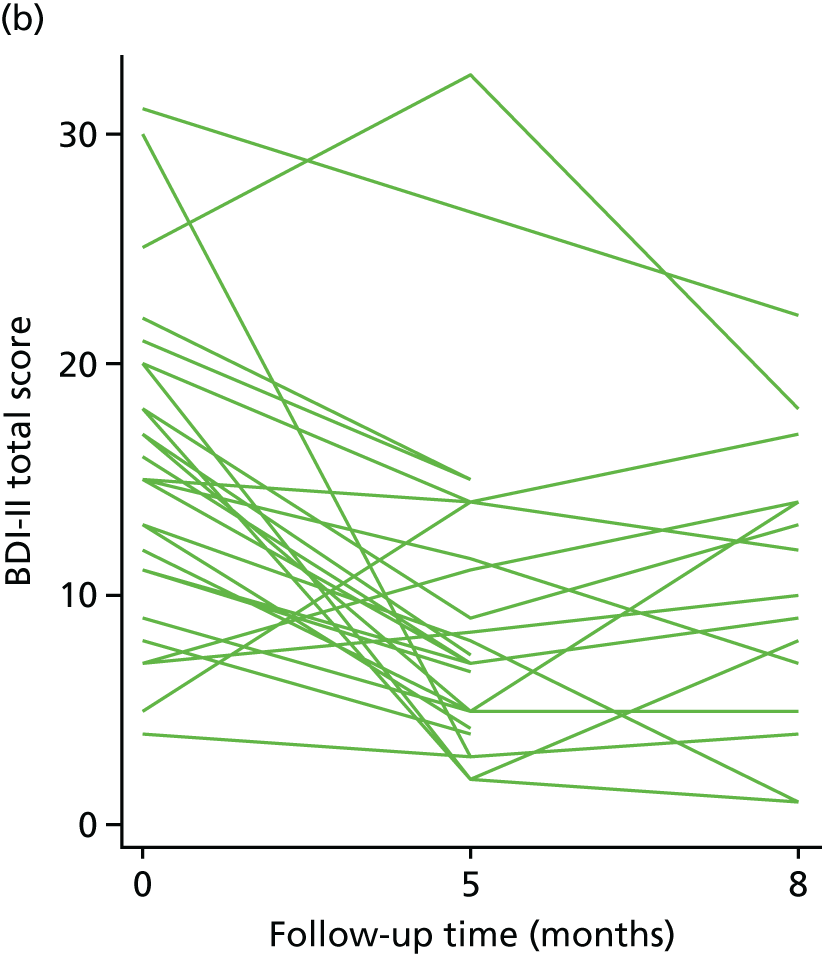
Around half the participants with data available at both baseline and 5 months (n = 26) had a 50% reduction in BDI-II scores at 5 months: 5 out of 12 (42%) in the EPC arm and 7 out of 14 (50%) in the UC arm, yielding an odds ratio of 0.71 (95% CI 0.16 to 3.25). A 17% reduction from baseline symptoms (the MCID) was observed for 10 out of 12 participants in the EPC arm (83%) and 11 out of 14 participants in the UC arm (79%); by 8 months, this was 6 out of 9 (67%) and 5 out of 8 (63%), respectively.
In the EPC arm, seven participants with a BDI-II score of ≥ 14 at baseline also had follow-up data at 5 months, of whom three (43%) were in remission (i.e. with a BDI-II score of < 14). Only seven participants in UC had a baseline BDI-II score of ≥ 14 and follow-up data at 5 months, of whom 6 (86%) were in remission at 5 months. Among these participants, in the EPC arm 2 out of 7 (29%) had a CSRC, compared with 3 out of 7 (43%) in UC.
The maximum BDI-II score at the 5-month follow-up was 14 in the UC arm. In the EPC arm, there were two participants who scored 15 and one participant who scored 33 (this participant scored 25 at baseline). Therefore, overall, in both groups, with the exception of one participant in the EPC arm, all 5-month BDI-II scores were in the moderate depression range or below.
At 8 months, the highest BDI-II score in the UC arm was 12, whereas in the EPC arm there were five participants with a score of ≥ 14. The highest score in this group was 22; this participant scored 31 at baseline and did not provide sufficient BDI-II data at 5 months. The participant who scored 33 at 5 months scored 18 at 8 months.
In terms of anxiety, the mean BAI score was also reduced at 5 months in both groups, with a between-arm mean difference between EPC and UC of 4.6 (95% CI –0.8 to 10.0). By 8 months, the between-group difference was 5.0 (95% CI –1.2 to 11.1).
Roughly equal numbers of participants in each arm believed that they had low mood, and there was a general preference in both groups to receive non-drug support for their low mood.
Other outcomes
Participant-reported HRQoL outcomes at each follow-up point are summarised in Tables 20 (5 months) and 21 (8 months). EQ-5D-5L scores within the EPC or UC groups appeared to be unchanged across time, and the between-group mean difference in EQ-5D-5L and VAS scores (adjusted for baseline score) between the EPC and UC groups at the 5-month follow-up were 0.045 (95% CI –0.023 to 0.113) and –0.014 (95% CI –0.145 to 0.064) respectively. The mean HeartQoL score had increased from baseline to 5 months in both the EPC and UC arms. The mean difference in HeartQoL score (adjusted for baseline score) between EPC and UC at the 5-month follow-up was –8.2 (95% CI –14.88 to –1.43). At the 8-month follow-up, the between-group mean difference was negligible (0.1, 95% CI –7.9 to 8.0).
One participant in the UC arm, an ex-smoker at baseline, had recommenced smoking 20 cigarettes per day by the 5-month follow-up (see Table 15). Furthermore, one participant in the EPC arm, also an ex-smoker at baseline, had recommenced smoking by 8 months.
Participant experiences of care
Participants in both arms reported a positive experience of their cardiac rehabilitation programme, based on both the CSQ-8 and the FFT reported at the 5-month follow-up, although there may be some indication of a more positive experience in EPC (Table 22).
| Patient-reported outcome | Trial arm, n (%) | |
|---|---|---|
| UC (N = 14) | EPC (N = 13) | |
| CSQ-8 | ||
| How would you rate the quality of service you have received? | N = 13 | N = 11 |
| Excellent | 9 (69) | 9 (82) |
| Good | 3 (23) | 2 (18) |
| Fair | 1 (8) | 0 (0) |
| Poor | 0 (0) | 0 (0) |
| Did you get the kind of service you wanted? | N = 13 | N = 11 |
| Yes, definitely | 8 (62) | 10 (91) |
| Yes, generally | 5 (38) | 1 (9) |
| No, not really | 0 (0) | 0 (0) |
| No, definitely | 0 (0) | 0 (0) |
| To what extent has our programme met your needs? | N = 13 | N = 11 |
| Almost all of my needs have been met | 9 (69) | 9 (82) |
| Most of my needs have been met | 3 (23) | 2 (18) |
| Only a few of my needs have been met | 1 (8) | 0 (0) |
| None of my needs have been met | 0 (0) | 0 (0) |
| If a friend were in need of similar help, would you recommend our programme to him or her? | N = 14 | N = 11 |
| Yes, definitely | 9 (64) | 11 (100) |
| Yes, I think so | 5 (36) | 0 (0) |
| No, I do not think so | 0 (0) | 0 (0) |
| No, definitely not | 0 (0) | 0 (0) |
| How satisfied are you with the amount of help you have received? | N = 13 | N = 11 |
| Very satisfied | 8 (62) | 11 (100) |
| Mostly satisfied | 5 (38) | 0 (0) |
| Indifferent or mildly dissatisfied | 0 (0) | 0 (0) |
| Quite dissatisfied | 0 (0) | 0 (0) |
| Have the services you received helped you to deal more effectively with your problems? | N = 13 | N = 11 |
| Yes, they helped a great deal | 8 (62) | 9 (82) |
| Yes, they helped somewhat | 5 (38) | 2 (18) |
| No, they really did not help | 0 (0) | 0 (0) |
| No, they seemed to make things worse | 0 (0) | 0 (0) |
| In an overall, general sense, how satisfied are you with the services you have received? | N = 13 | N = 11 |
| Very satisfied | 9 (69) | 10 (91) |
| Mostly satisfied | 4 (31) | 1 (9) |
| Indifferent or mildly dissatisfied | 0 (0) | 0 (0) |
| Quite dissatisfied | 0 (0) | 0 (0) |
| If you were to seek help again, would you come back to our programme? | N = 13 | N = 11 |
| Yes, definitely | 7 (54) | 10 (91) |
| Yes, I think so | 4 (31) | 1 (9) |
| No, I do not think so | 2 (15) | 0 (0) |
| No, definitely not | 0 (0) | 0 (0) |
| FFT | ||
| How likely are you to recommend this help or support to friends and family if they needed similar care or treatment? | N = 14 | N = 13 |
| Extremely likely | 10 (71) | 11 (85) |
| Likely | 4 (29) | 0 (0) |
| Neither likely nor unlikely | 0 (0) | 0 (0) |
| Unlikely | 0 (0) | 0 (0) |
| Extremely unlikely | 0 (0) | 0 (0) |
| Do not know | 0 (0) | 0 (0) |
| I did not receive any help or support | 0 (0) | 2 (15) |
Most participants across both treatment arms reported that the quality of the service they received was excellent (EPC = 9 out of 11 participants; UC = 9 out of 13 participants). All of the participants in the EPC arm were extremely satisfied with the amount of help they received (11 out of 11), and most of the participants in the UC arm were extremely satisfied (8 out of 13). Although most of the participants in the UC arm reported being very satisfied overall (9 out of 13), more of the EPC participants were satisfied (10 out of 11) and willing to return to the programme in the future (EPC = 10 out of 11 participants; UC = 7 out of 13 participants). The majority of participants in both arms were extremely likely to recommend the service to friends and family (EPC = 11 out of 13 participants; UC = 10 out of 14 participants).
Analysis of the FFT free-text items identified aspects of the cardiac rehabilitation experience that were good, and those that could be improved. Of the 27 participants followed up at 5 months, 19 provided comments about their experience. Some participants (6 out of 19) mentioned that the group element of the rehabilitation was beneficial to their recovery, providing participants with additional support. Just over half the participants (10 out of 19) reported that the nurses were very supportive and that it was helpful having a named person to contact. Participants also reported the mental and physical health benefits of cardiac rehabilitation (4 out of 19 and 3 out of 19 respectively), as it improved their mood, increased their confidence and improved their physical strength. Finally, 8 out of 19 participants reported that the rehabilitation groups were informative, providing additional, reassuring information about recovery from different health professionals. As to what would make their experience better, only six participants responded, with three participants referring to the accessibility of the service provided (i.e. timings were not suitable for working people, challenges for those who are hearing impaired or the content was more relevant to patients with severe heart conditions). The remaining comments referred to the need for a balanced mix of genders in the group, as it was more heavily weighted towards male patients, the notion that cardiac rehabilitation was physically demanding for their age and the lack of information about the pain experienced in the year after the heart attack.
Methods to support economic evaluation
Cost of delivering the intervention
The estimated cost per participant receiving the EPC intervention, and the main elements of this cost, are presented in Table 23.
| Costs | Unitsa | Unit costs (£) | Total cost (£) |
|---|---|---|---|
| Initial costs | |||
| For nurse training (trainers’ and facilitators’ time, delivery and preparation) | 45 + 41 hoursb | Various | 7157 |
| Nurse training (cardiac rehabilitation nurses’ time) | 56 + 21 hoursc | 35d | 2695 |
| Leader manuals (printing) | 8 | 4 | 32 |
| Ongoing costs | |||
| Telephone clinical supervision of BA delivery | 35 hours | 50 | 1750 |
| Nurses’ time for having supervision (estimated 50 hours overall) | 50 hours | 35b | 1750 |
| Total overhead cost of providing EPC | 13,384 | ||
| Number of participants in the EPC trial arm | 15 | ||
| Mean allocated overhead cost of EPC per patient (in the feasibility trial) | 892 | ||
| Per-participant nurse time for delivering EPC within cardiac rehabilitation sessions | 1.8 hoursc | 35b | 63 |
| Patient booklet print costs | 1 | 4 | 4 |
| Estimated cost per EPC recipient | 959 | ||
The calculation presented in Table 23 makes no adjustment for the small scale of the pilot trial, and so it is inevitably an overestimate of what the actual cost would be if the intervention were to be delivered routinely to larger numbers of patients. For example, in this calculation, the initial training of nurses accounts for a significant proportion of the overall cost of providing EPC, but this would, in reality, be shared across a larger number of patients receiving EPC over a longer period.
The estimated time nurses take to deliver EPC elements within rehabilitation accounts for a relatively small amount of the overall cost (£63 of the estimated £959 per participant). This was estimated by trial researchers from the information that has been collected to describe the delivery of the intervention (see Appendix 3). However, the qualitative interviews with nurses, conducted as part of this study’s process evaluation, showed clearly that the nurses found it extremely difficult to distinguish clearly their EPC-delivering time from other aspects of the care they usually provide. Nevertheless, we believe that the cost breakdown shown may help those developing and evaluating similar nurse-delivered interventions in hospital and community settings to estimate the cost of their intervention. The estimated cost of designing and developing the intervention, including intervention manual development and the creation of related learning materials, has been separately estimated and is available from the authors.
Cost of health service use
Table 24 shows the cost of health service use by participants in the trial, based on self-reported service use in the two time periods (baseline to 5 months, and 5 months to 8 months). This analysis provides an overall indication of the types of health service use that accounted for the majority of health service costs incurred in this group. All participants reported using GP services in both periods, and the majority also had hospital outpatient appointments. Although a minority had hospital inpatient stays, these account for a large proportion of the total costs of health-care use.
| Time period and service use | Trial arm | |||
|---|---|---|---|---|
| UC (N = 14) | EPC (N = 13) | |||
| Participants with ≥ 1 contact, n (%) | Mean cost, £ (SD) | Participants with ≥ 1 contact, n (%) | Mean cost, £ (SD) | |
| From baseline to 5 months | ||||
| GP visits (including practice nurses) | 14 (100) | 335 (276) | 13 (100) | 445 (307) |
| Seeing other care professionals | 5 (38) | 31 (62) | 8 (57) | 550 (1542) |
| Hospital inpatients | 4 (29) | 456 (784) | 4 (31) | 1246 (1879) |
| Hospital outpatients | 10 (71) | 170 (213) | 11 (85) | 194 (161) |
| Emergency care (e.g. ED, 999) | 3 (21) | 28 (54) | 8 (62) | 82 (110) |
| Mean total cost (£) of health service use | 1020 (955) | 2516 (3044) | ||
| UC (N = 8) | EPC (N = 9) | |||
| From 5 months to 8 months | ||||
| GP visits (including practice nurses) | 8 (100) | 211 (123) | 9 (100) | 170 (105) |
| Seeing other care professionals | 1 (13) | 6 (16) | 3 (33) | 56 (95) |
| Hospital inpatients | 1 (13) | 221 (585) | 0 | 0 |
| Hospital outpatients | 4 (50) | 157 (207) | 5 (56) | 130 (175) |
| Emergency care (e.g. ED, 999) | 2 (25) | 70 (124) | 1 (11) | 12 (35) |
| Mean total cost (£) of health service use | 664 (822) | 368 (265) | ||
Owing to the small sample size, any differences in costs between the trial arms should be ignored, as they could easily be chance findings. For example, the > £700 difference in mean hospital inpatient costs between the EPC group and the UC-only group (baseline to 5 months) is almost wholly attributable to one participant in the EPC group who reported having two 10-day hospital admissions as a result of asthma attacks during that period (total estimated cost: £10,518). This is not likely to be due to the EPC intervention.
However, these costs could provide useful point estimates and SDs for planning future definitive randomised trials of similar interventions in similar patient groups.
Health-related quality-of-life and utility scores
The EQ-5D-5L index scores at baseline, 5 months and 8 months, calculated using the most recent and reliable UK population-derived preference weights for health states,88 are presented in Tables 20 and 21. Apart from the previously noted difference at baseline between the two trial groups, there were no notable differences or changes over time. If a definitive trial were to be conducted, these quality-of-life estimates could be used to determine a trial sample size based on the expected cost-effectiveness of the intervention.
Comparing self-report service use data with data from clinical and service records
As well as participant-reported health service use data, the study also gathered selected health service use data from clinical records held by the participant’s general practice, and those held by the participant’s cardiac rehabilitation nurse. Owing to the curtailed follow-up of some participants at 8 months, combined with study attrition, the self-reported service use data are available for small samples. Although service use data from GP records were available from all but two trial participants, complete self-reported service use data (for both baseline to 5 months and 5 months to 8 months) were available for only 17 of the 29 study participants.
For the 17 participants with data from both sources, Table 25 summarises the level of agreement in the number of services used between participant-reported data and GP records. There was poorest agreement between self-reported service use data and GP records for the number of visits to GPs or practice nurses, with no participants recalling the same number of GP visits as their GP records showed, and only 3 out of 17 participants (18%) recalling the same number of practice nurse visits. This might be expected for these frequent types of health service use. Further inaccuracy is added when asking about the type of health professional seen (whether practice nurse or GP).
| Professional seen or service visited | Mean (SD) number of visits | ICCa | Crude agreement, n (%) | Participant estimate,b n (%) | ||
|---|---|---|---|---|---|---|
| Self-reported | GP records | Underestimate | Overestimate | |||
| GP (surgery visits) | 6.1 (5.7) | 6.1 (3.8) | 0.34 | 0 (0) | 9 (53) | 8 (47) |
| Practice nurse visits | 4.7 (5.4) | 3.9 (5.1) | 0.62 | 3 (18) | 7 (41) | 7 (41) |
| A&E visits | 0.6 (1.1) | 0.7 (1.5) | 0.70 | 11 (65) | 4 (24) | 2 (12) |
| Hospital admissions | 0.5 (0.9) | 0.4 (1.0) | 0.85 | 14 (82) | 1 (6) | 2 (12) |
For the less frequent, and more significant and costly, occurrences of accident and emergency (A&E) attendances and hospital inpatient stays, the level of crude agreement between the two data sources was much higher, with 11 out of 17 participants (65%) recalling the same number of A&E attendances as their GP records show, and 14 out of 17 participants (82%) recalling the same number of hospital inpatient stays.
For all four types of health service use/episode, there seems to be no systematic tendency to either overestimate or underestimate the number of episodes of service use (with similar numbers and proportions over- and under-estimating). This partly explains the broadly similar mean number of episodes of each type. For example, although none of the participants recalled the same number of GP visits as their GP records showed, the mean number of GP visits was the same whether calculated from self-reported data or GP-recorded data. For the potential purposes of costing within an economic evaluation, this provides some evidence to support the use of self-report service use data in this patient group (and over these recall periods), so long as loss to follow-up and questionnaire non-completion could be minimised.
The cardiac rehabilitation nurse notes only consistently recorded the use of other hospital services and, thus the level of agreement could be estimated only for the number of hospital admissions (inpatient stays) reported by participants and nurse records. Overall, there was a high level of crude agreement, with 15 out of 17 (88%) agreement between participant reports and nurse records of inpatient stays for the 8-month follow-up period. The mean number of self-reported hospital admissions was 0.5 (SD 0.9), compared with 0.3 (SD 0.6) admissions, as recorded in the nurse notes. The intraclass correlation coefficient, reflecting the strength of association, was 0.55. No participants underestimated the number of hospital stays, although two participants (12%) overestimated the number of hospital admissions.
Nested qualitative interview study
The qualitative interview study results are presented for patient participants, followed by nurses (see Nurse interviews).
Patient interviews
The purpose of these interviews was to gather qualitative evidence on the content of usual psychological care and to assess the acceptability of EPC and study materials to patients. Interviews were conducted once participants had completed their 5-month assessment for the trial, and most had completed their fitness programme (including EPC where relevant).
Participant sample
Eighteen trial participants (12 in the EPC arm and six in the UC arm) were interviewed between January and June 2016. Just under half of the 18 interviewees were male (8 out of 18; 45%), and all reported their ethnicity as white British. The mean age of interviewees receiving EPC was slightly younger (64.3 years; range 52–76 years) than that of UC interviewees (72.5 years; range 65–79 years). Similarly, the mean PHQ-9 score for the EPC group was higher at baseline than that of the UC group (15.0 vs. 11.7, respectively). All but two interviews were held by telephone. The interviews with EPC participants lasted longer on average than those held with UC participants (52 minutes vs. 32 minutes).
In addition to interviewing trial participants, interviews were also held with three individuals (all male) who had declined to take part in the pilot trial (October–December 2015) to explore the reasons behind their decision (see Individuals who declined trial participation). Decliners were aged 35–60 years. The interviews were conducted by telephone and lasted for an average of 24 minutes.
Cardiac events reported by the 21 participants included heart attacks, angina, cardiomyopathy, heart failure and valve disorder treated with CABGs, stent insertion, valve replacement, medication or a combination of these. Of the 21 individuals interviewed, all said that they had attended at least an initial appointment with the rehabilitation nurse, and 18 had attended some cardiac rehabilitation that had included a fitness programme. Two participants commented that they dropped out of the fitness programme at an early stage because of a combination of health problems and logistics issues. Instead, two participants reported receiving a series of one-to-one appointments with the nurse, one overlapping with his fitness programme and the other receiving these instead of a fitness programme.
Main themes
We detail findings from the trial participant interviews before describing those from the decliner interviews. Quotations have been tagged with an individual identifier, and details of whether the individual was in the EPC arm or the UC arm or if they had declined trial participation.
There were a number of main themes identified within the patient participant interviews, including the context of cardiac care; experiences of cardiac rehabilitation; UC participants’ experiences of support for mental well-being; EPC participant’s experiences of support for mental wellbeing; integrating EPC into cardiac rehabilitation; mental and physical outcomes after receiving cardiac rehabilitation; taking part in a research study and intervention feedback.
Context of cardiac care
Enhanced psychological care and UC interviewees described the massive impact their cardiac event had had on them physically and mentally, and also on their family. As one individual explained, he felt ‘mentally and physically shell-shocked’ (EPC, participant 12) following his cardiac event. Most participants had viewed themselves as fit, strong and sociable, and having not previously experienced low mood. Many participants described still experiencing pain (chest, limbs, post operative) following hospital discharge, loss of appetite, sleeplessness, lacking energy and motivation. There was some suggestion that how they felt related not only to their body recovering from the cardiac event, but also to the new medication they now needed to take:
I felt terrible, emotionally I have never felt so low in all my life. It was a horrible, horrible experience, I just felt my life was ebbing away, and I’ve never felt that low before, and I just put it down to the medication because, and I couldn’t eat, I couldn’t sleep, I was still getting pains but nothing like I’d had, but I just felt terrible, it was just awful, I had no go in me, no energy, no focus, horrible, horrible sensation.
UC, participant 2
Most trial participants were very surprised at the overwhelming and quite sudden impact that their cardiac experience had had on their mental state:
I mean I’ve always been a busy businessman, self-employed for 30 odd years, being heavily involved in business to ending up in a situation where I couldn’t make a decision as to what day it was.
EPC, participant 12
Descriptions included a general but quite dramatic loss of self-confidence, feelings of apprehensiveness and failure, being detached or experiencing panic attacks and a loss of control over their life. Some participants (male and female) explained how they had felt suicidal at the time:
It just all came out and I was surprised at myself, to be honest, I had no idea that I was, my overwhelming feeling was disappointment that I’d actually survived and that frightened me because I’ve never felt that before [suicidal] . . . And I think that had a really big impact. And I think that, it threw up so much other stuff that it was quite frightening, to be honest. I always thought, I considered myself a very happy-go-lucky type of person.
EPC, participant 14
I sat here alright and I just watch telly and something stupid comes on the telly and it all comes back and you feel [pause] [Interviewer: Really emotional?] Yes and you don’t show it, I don’t show it but you feel it here and you feel really drained and really start thinking the worst and what’s the easiest way out which is, and then you think ‘which is the easiest way out tablets or’ [speech trailed off].
EPC, participant 9
At least four participants had given up their work following their cardiac event, and this had added to their feelings of anxiety and depression:
I was angry, frightened, upset . . . I just got worse and worse. I got very depressed, I lost my job, I loved my job. You’ve got to understand, I absolutely loved my job . . . suddenly everything had been ripped out from under my feet and I got very depressed, very anxious and felt a failure, I felt failed, that I’d let everybody down, so yes huge.
UC, participant 17
Family members were often reported to be shocked and frightened by the suddenness of the event, and it appeared to affect how they dealt with the participant:
They treated me like I was a china doll when I first came out of hospital, even though I felt like I could tackle a rugby player [laughs].
EPC, participant 14
Support from family was welcomed and described as reassuring. However, it could also be ‘stifling’ at this early stage:
I think the hardest I found with all of it is my family, it knocked the stuffing out of them, and ‘oh no, you can’t do this mum, no, you can’t do that mum’, but . . . they’re better now . . . they know that I’ll only do what I can do and if I can’t do it, I won’t do it . . . but my youngest, it took her ages to accept, ‘no, you’re not allowed to do that’. She’d get very cross with me [laughs].
UC, participant 4
Despite being close to her family, one individual who had described feeling suicidal stated that she could not share her feelings with her husband and family, and felt quite alone. In addition, she felt cut ‘adrift’ from the professional help she needed initially and lacked any information about what she could or could not do.
Several participants had very little or no family around and also felt alone and unable to cope after the ‘cosseted’ hospital environment. Thus, although some participants mentioned being contacted by a cardiac rehabilitation nurse early after discharge, for most, the weeks between being discharged from hospital and starting their rehabilitation sessions were long, and a time when they struggled emotionally and physically.
Experiences of cardiac rehabilitation
At the time of their interview, all of the EPC and UC participants interviewed had attended an initial cardiac rehabilitation assessment with the nurse. Many had also completed a fitness programme. Some had been offered attendance at further rehabilitation fitness sessions, which a few participants attended. Most of the participants described an initial one-to-one assessment, then a structured programme of exercises within a gym or leisure centre. Some commented that, after the exercises, there was usually an opportunity for light refreshment and to attend a short group talk led by the nurse or other practitioner on some aspect of rehabilitation (e.g. cholesterol, diet or medication). Occasionally, participants said that they had seen the nurse for some one-to-one sessions instead of attending a fitness programme, and some commented that a nurse had set-up exercises for them to continue at home.
Although some participants had been reluctant to attend the fitness programme, as they were apprehensive about exercising, most felt that they ought to take up the opportunity in order to aid their recovery. Many did not know what the first or subsequent appointments would entail, and some would have liked more information prior to starting.
Participants attending the rehabilitation fitness programme commented that it had benefited them, both mentally and physically. They had gained fitness, changed their diet, lost weight, talked through their cardiac concerns with a nurse, met others and gained confidence:
I can’t honestly say I remember thinking, ‘oh, I feel better doing that’, but I did think I had more confidence in myself to do it. Beforehand I was, ‘oh, I’d be frightened to do this in case, and I can’t do that in case’ and I just thought, ‘no, get on with it’.
UC, participant 5
It was also apparent that some participants enjoyed the opportunity to talk about their cardiac experiences with other participants who ‘were in the same boat’ (UC arm, participant 1). They realised that they were ‘not the only one’ (UC arm, participant 4), and listening to others’ accounts put their own in perspective. In addition, comradery and mutual experiences helped in their recovery:
I suddenly realised that here we are, all having gone through the same thing experience and at different levels and I was in the first week sort of gullible, people who were there and I’d been talking to had been there nearly 6 weeks were much more confident and I realised then that this is what it was all about, it was getting some reassurance that you weren’t on your own and it does take time and you will get better.
EPC, participant 12
By going there it was helping me a lot, it really helped me, going to the rehabilitation, it got me away from the house, and I started doing things and meeting people.
EPC, participant 18
Patients in both the EPC and UC arms built up good relationships with their nurse and perceived them as being professional and approachable.
Usual-care participants’ experiences of support for mental well-being
Usual-care participants did not feel that they had discussed their mental well-being with their nurse in any depth or, in some cases, at all:
When you went to your cardiac rehab[ilitation] sessions, did the nurse talk to you at all about your anxiety at the time or anything that she could help you with, did she give you a leaflet or did she talk about it?
No, no, because I don’t expect she knew about it. Unless she knew about it and didn’t say nothing. As far as I can remember, it was never ever talked about.
It was evident that some participants wanted to discuss how they were feeling and viewed their nurse as someone they could talk to, but felt that they had not been given the opportunity to do so by their nurse. It was also apparent that nurses had not referred, or offered to refer, them to a psychological service. One participant who had been offered a referral did not take it up, as she did not want to be labelled as depressed, but did indicate that she had wanted to talk to somebody:
I don’t really want to go and see anybody, but at the same time, I wish I’d had the opportunity to talk to somebody, but not about, I didn’t want to go down, ‘oh, she’s depressed’, I don’t want to go down that route, because I wasn’t depressed ‘depressed’, I just felt so down and low in myself.
UC, participant 2
However, occasionally, UC participants mentioned that their nurse had put forward other suggestions to help them deal with low mood. These included providing reassurance that it was normal to feel low after a cardiac event and encouragement to participate in the fitness programme as a way to improve mental health. The nurse had also given this participant the number of the department that she could ring if she felt low:
[The nurse] did say that, contrary to what I might think, but doing the gym might actually make me feel a little bit better, because then I would say I could do this or I could do that and meet different people, the same as what I was and stuff like this, and that might make me feel better. And he also said if I really felt down or anything, I had their department’s phone number and I had to feel free to use it.
UC, participant 5
Enhanced psychological care participants’ experiences of support for mental well-being
Most EPC participants welcomed the opportunity to talk about their emotions with a nurse and felt comfortable doing so:
Oh I thought it was great I could tell her anything.
EPC, participant 10
I thought it [having the opportunity to talk about low mood] was an absolutely brilliant idea. I felt very comfortable. It was almost like it’s a relief to talk to somebody as well because . . . when these sort of things happen, you don’t know who to talk to. You can talk to family members but they are not physicians or doctors or nurses, you know they don’t know about these events, they can only advise. But when you are talking to a professional you are getting information back, it is fantastic.
EPC, participant 17
Participants were pleasantly surprised that they could receive dedicated time with a nurse to discuss their emotional concerns alongside their physical needs:
It was a good surprise in that I thought the cardiac rehab would just be ‘Hello! Do a few exercises’, and that’s it. I thought it was really brilliant, that it was looked at holistically, brilliant. I came out thinking, ‘oh this is a real breath of fresh air, that people should actually look at me as a total person and not just as a patient with a dodgy heart’, sort of thing.
EPC, participant 13
Enhanced psychological care participants stated that they had a good relationship with their nurse, and were very complimentary about their abilities. However, some commented that their nurse was very busy and that they would have liked to have had even more time to discuss mental health issues. In addition, not all participants felt that nurses were best placed to deal with their mental health needs. One lady commented that she did not want to talk about mental health with a ‘physical’ health practitioner (EPC, participant 14: ‘I wouldn’t discuss my emotions with my dentist’), and had felt uncomfortable about doing so.
This lady was offered a referral to a psychological service as part of her EPC care co-ordination but eventually took up some counselling offered through her GP. The counselling sessions identified issues from her past that she had hidden or forgotten. This may have been one reason why she had felt uncomfortable about the idea of talking to her nurse.
Most EPC participants had received a CADENCE participant handbook and had read all or part of it. Participants described the section in the handbook that described a series of case scenarios as the most memorable, with many feeling that they could relate to one or another of the people, and had found this ‘absorbing’ and helpful:
Some of the people in there, they’d relate to me . . . how can I put it, they feel exactly the same as I feel. They’re old, and they think, ‘well, what, there ain’t nothing worth, what am I doing on this earth?’ And I read it a couple of times, actually, and it’s quite useful.
EPC, participant 8
No one suggested that the handbook had been too long or difficult to understand, although one participant said that he and his partner felt that the scenario outcomes had been quite negative.
Participants used the mood/activity diary and said that it had been positive in several ways: it helped get their feelings out onto paper, it highlighted patterns of behaviours that needed changing and it enabled them to compare activity levels and mood scores from earlier weeks with later weeks to see any progress they had made. As memory was described as difficult after a heart attack, being able to track progress was described as reassuring. Participants also felt that they were helping the nurse by completing it (i.e. doing what they were told to do).
Whether or not participants had completed all the BA sessions, BA was not a term most participants described themselves as being familiar with. It is not clear whether this was because participants did not recognise the term (despite it being mentioned many times in the handbook) or because nurses had not used it. Either way, not all participants were able to provide a clear description of how BA worked, with some participants feeling that the one-to-one sessions were more about having the opportunity to talk through their emotional issues; for example, when asked about the BA sessions, one participant, who was very positive about their interaction, said:
We just talked, talked about like I’m talking to you now.
EPC, participant 8
However, when prompted, participants who had completed their BA sessions were able to describe and give examples from one or more of the steps involved in BA (such as measuring mood, keeping a mood/activity diary, describing links between mood and activities or rescheduling activities and behaviour important to them), and many described discussing their completed mood/activity diary with their nurse:
I had taken part in writing down each day the sort of things that I was feeling, the sort of things that I was doing, what did, did I go for walks, if so, how far and how I generally felt in my mood and so on, so I wrote this down on a daily basis and then took it in, twice a week and then the nurse would sit down and go through it with me.
EPC, participant 12
Occasionally, a participant was able to expand and give examples of how they changed or increased activities to help enhance their mood:
We done different things so if I felt, say for instance if I wanted to go out in the garden and I felt well doing that, I tend to do it a bit more than what I did before. If I sat down and watched something and I did, as I say the telly sometimes you think ‘that rubbish is on’, and [I’d] switch it off . . . I’ve found that by going to this with [name of nurse] I found I was doing more and more and more so I still do it now, I hoovered up this morning.
EPC, participant 9
Four female participants did not complete the diaries at all, and two were not keen to continue with BA in general and were referred to other psychological services early on; one participant had concerns that the diary would be read by a family member who might be alarmed by the strength of her feelings:
I didn’t really want them to know what was going on in my head.
EPC, participant 14
The other lady was very happy to talk through her activities, but said she found the paperwork too much. However, the nurse had encouraged her to continue with EPC and the participant was happy to talk through her activities and mood instead:
I didn’t want to be filling in a load of forms and things in and they said ‘You don’t have to’, but she said ‘I’d still like to talk to you, if you didn’t mind’, and I said ‘No I don’t mind and ask your questions’, and I’m not, I didn’t like all the paperwork, it was too much . . . If I didn’t want to do it, I didn’t have to do it, they made that very clear.
EPC, participant 18
This participant was offered a referral for counselling. She did not take this up, perhaps because she felt able to talk to the nurse (‘felt as though I could speak to [the nurse] like a friend’), and because she gained a great deal out of sitting with the nurse and completing the activity diary together.
Most EPC participants felt that having dedicated one-to-one time with a nurse had been crucial to their mental recovery. Participants commented that, without this support, their recovery would have taken longer or their mood would have deteriorated more:
I honestly think had it not been for the rehab and her input, I don’t know, I mean I presumably would have progressed but I think it [would have] taken a lot longer, I think that they helped in so many ways.
EPC, participant 12
I think I’d have got really down if I hadn’t been able to talk to her.
EPC, participant 18
Male participants were particularly emphatic about the help they had received. They reported being glad to have the opportunity to express how they felt and they commented on their surprise at opening up to the nurse about their mental health, something that they would not have contemplated when they had started cardiac rehabilitation:
When she [the nurse] first met me, she would probably say ‘well actually [name of participant] was fairly very tight lipped and didn’t give anything away’, but by the end, yeah, I learned to sort of open up and I learned to sort of tell her things that I never dreamt I would. You know so that was again down to her, her being knowledgeable and applying her experience to get things out in the open, which was important to do so.
EPC, participant 12
For two participants, both of whom had had suicidal thoughts, the input from the nurse had seemed to be literally life-saving:
I think a lot of people when they commit suicide and things like that, a lot of it is to do with that because they feel by themself, they feel, well they just feel useless to everybody and nobody and the good thing with that [EPC] is they do help you. I’m not saying it’s going to help everybody it might not, but it’s certainly helped me.
EPC, participant 9
Without people like you and [name of nurse] talking to me and me talking to you, I might not be here now.
EPC, participant 8
Enhanced psychological care with dedicated nurse–patient time was perceived to help participants in a variety of ways. The sessions helped participants gain confidence to talk about their feelings and talk to others, increase their physical activity levels at home and increase their motivation to do more. They also helped some patients who were frustrated by their physical inability to complete everything to focus on several important activities and understand how their feelings and behaviours affected them:
It helped me in the sense that it was giving me answers as to why I was behaving the way I was behaving, or not doing the things I should have been doing or not having any motivation.
EPC, participant 11
However, sometimes participants found it difficult to go to their rehabilitation if they felt down, as their low mood meant that they were reluctant to go out. Although some participants had gained comfort from the occasional check-up telephone call from the nurse (and one participant had had most of their EPC sessions by telephone), participants mainly preferred the face-to-face contact, as it was more intimate and they felt that it was easier to talk in person.
Mental health-care co-ordination
Several participants had problems with panic attacks or complex emotional difficulties, or just did not get on with BA as a treatment. Four participants (including three who dropped out of BA sessions at an early stage and one who had completed all eight BA sessions) were referred on to a counsellor as a result of the nurse contacting their GP, psychological services or psychiatric services. Three participants felt that this had been beneficial. The fourth had decided not to take up the counselling offer (within psychiatric services) after the first visit, but she was now aware that she could self-refer to IAPT services, which she felt may be more confidential. Two other participants who felt better after EPC said that they would contact the nurse if they experienced a recurrence of low mood, even though they had finished the cardiac rehabilitation programme. Being referred to other services at an early stage during EPC had helped several participants to receive the care they needed without too much delay:
She would send a recommendation to my doctor that maybe I need a therapist, and she [the nurse] was amazing actually. She was brilliant and that kicked that off and now I am seeing a therapist once a week.
EPC, participant 17
Sometimes nurses had advised participants to go back to see their GP. However, six of the EPC arm participants were quite vociferous, commenting that they would not go to their GP to discuss mood issues, feeling that they would only be given antidepressants, or that GPs did not have the time to discuss mood in any depth:
I’d say, ‘what’s the point in me going to the doctors, he gives me these bloody tablets, I’m not going to live on sodding tablets, what’s the point?’
EPC, participant 9
They haven’t really got the time and you don’t like to burden them, do you, I mean, GPs are different aren’t they, well, they’ve not set something up for somebody who’s got low moods and depression in their office, have they, they’ve just got to say, ‘oh, I think you’re going through a low mood at the moment, because of what you had, oh, here’s a tablet, see you later’, type of thing, cos that’s what they do, isn’t it?
EPC, participant 11
Some EPC participants said that attending other services, such as IAPT services or a counsellor, would be preferable to seeing their GP. They were aware that they could self-refer or be referred by the nurse to IAPT services, and those who had attended IAPT services had received treatment relatively quickly (i.e. within 2 weeks).
Integrating enhanced psychological care into cardiac rehabilitation
Several participants acknowledged that a private space was not available to talk about their mental well-being, and they often received their EPC session in a corner of the gym or leisure centre. However, nurses were practised at adapting a space for their needs, and there seemed to be an understanding with other participants and helpers not to interrupt their sessions. Also, most participants seemed unbothered by the lack of privacy, even though some needed to talk about very personal and private issues:
I don’t think anybody heard for one, I think people knew not to come to the table . . . cos they obviously knew what was going on, it didn’t bother me, no, because I feel that I could have said to [name of nurse], can we go outside? I mean, she asked me if that was okay.
EPC, participant 11
Privacy was not an issue for those participants who managed to see their nurse before or after most people arrived for the fitness class.
The amount of time an EPC session took appeared to vary between participants, with one individual mentioning that his session lasted around 1 hour, and another stating that theirs was around 15–20 minutes long. Another participant would have liked more time, but recognised the need for brevity, considering the number of participants that nurses had to deal with.
Travel and parking had been concerns for at least two participants, who had consequently dropped out of the fitness and BA sessions at an early stage. One had arthritis affecting her mobility and the other had chronic obstructive pulmonary disease affecting her breathing; overall, their anxiety problems made them very reluctant to travel:
I think suffering from anxiety, going from hospital and back again has been absolutely horrific [laughs]. I don’t travel well in cars any more so I spend a lot of time with my eyes shut . . . It is pot luck to whether you get a parking spot. Literally pot luck.
EPC, participant 17
I’m exhausted when I get there and can’t breathe properly for ten minutes. But I can get through that, because that’s how my life is anyway . . . But it’s just the bicycles really, I’m terrorised that I’m going to have another [cardiac] event on the way to do the rehab because of the bicycles . . . After the [second] heart attack, I just said to them ‘don’t even put me down for it [cardiac rehabilitation]’ . . . each time there was an appointment I was cancelling them, because I was just lying awake all night getting absolutely massive panic attacks about the thought of going into town the next day. And it wasn’t because of the exercise, which I do actually feel committed to, it was because of trying to get there and get back to the bus stop again.
EPC, participant 16
Some participants recognised that nurses were ‘under tremendous pressure’ (EPC arm, participant 12), yet they remained focused and made time for participants requiring EPC. Despite wanting more time with the nurse, some participants commented that they tried hard to keep contact as brief as possible, feeling uncomfortable that they were adding to the nurse’s daily burden:
Running another one [class] tandem, trying to eat their lunches as they’re on the job there and you think, ‘gosh, yeah I can understand how overworked they are [. . .] I’m inclined not to do it’, rather than thinking, ‘hey, I’ve got demands and I need them to be met’.
EPC, participant 13
Mental and physical outcome after receiving usual cardiac rehabilitation care and cardiac rehabilitation with enhanced psychological care
Over time, several of the participants receiving UC felt that their mental well-being had gradually improved. Some had developed strategies to help themselves, such as gardening or talking to the dog, and two individuals were feeling mentally a lot better. For most of these participants, however, chronic health problems were still affecting their physical ability and overall well-being.
Many intervention participants described how the EPC and cardiac rehabilitation together had helped their physical and mental health, improved their resilience in existing activities and helped to encourage them to find other activities they enjoyed. For example, one man had learned to be less compulsive about tidiness, which could lead to a panic attack. Despite having to manage some major disabilities, he had started walking his dog again, which made him feel ‘perky’ and also meant that he saw other people. No longer feeling that life was not worth living, he felt a lot better and was grateful for the help he had received during his rehabilitation:
I’ll be honest with you, if I hadn’t gone to rehab[ilitation] I don’t think I’d be here.
EPC, participant 9
Other intervention participants had made positive life and lifestyle changes (e.g. stopped smoking, reduced alcohol intake, given up their business, talking to and supporting others) and now felt more able to manage low mood if it occurred. In general, many felt that they could cope better with their circumstances:
If your one [EPC provision] or anybody else’s service to me [had been] rubbish, I wouldn’t be any further than what I was when I had the heart attack . . . I’d come quite far in the 6, 7 months, whatever it is, I feel that I have.
EPC, participant 11
However, similar to those in the UC arm, some EPC arm participants continued to experience mental health problems, struggling with chronic mental and/or physical health issues, which had often been exacerbated by their heart condition:
I was on tablets yes for my hips and knees but I’ve never had the problems I’ve got now. Even my confidence, my balance has got, all my confidence and everything I am doing has just shattered. I walk with a stick now if I am outside. I can’t go on buses because they make me feel ill and dizzy. I’ve got a disability badge, you know so it is dreadful.
EPC, participant 17
Intervention feedback
Most EPC participants had confidence in, and felt comfortable with, a nurse providing both physical and mental support, as well as referring patients to other services. They felt that the nurse was competently able to explore emotional issues that had surfaced as a result of their cardiac experience, or, indeed, those already present, but which had not been addressed until their rehabilitation. However, both EPC and UC arm participants recognised that there was a need for more care for patients with low mood following a cardiac event, and saw the potential benefit that the CADENCE intervention could offer to patients if EPC was rolled out more widely:
It’s vitally important that hopefully this particular pilot will progress because the people who have had a heart attack need this support and if they don’t get it, I genuinely think there could be further down the line, people having problems that wouldn’t have them, you know, had they been to rehab[ilitation], had they had this particular involvement with the nurses, would have helped them progress much quicker.
EPC, participant 12
A few possible changes or improvements to the intervention were brought up by EPC participants. First, the weeks between hospital discharge and starting rehabilitation felt like a long time to some participants, and this was often when their mood was lowest and their anxiety levels were high. Several participants commented on the timing of the EPC they received – many felt that it had come at the right time, helping them to get through a very bleak period, whereas some felt that they needed the psychological care to start a lot earlier, while still in hospital or just after discharge. Participants suggested including a visit from a nurse while still in hospital to discuss options, a leaflet or a telephone call to support mental health during the period between hospital discharge and starting the cardiac rehabilitation programme:
I think they should catch you earlier, when you’re in hospital, when you’re in that hospital in bed . . . feeling down I think they should have a nurse like yourself or [name of nurse] to visit you, not every day, every other day to try to boost your morale up and I think it do . . . They [hospital nurses] don’t talk to you about the stuff like you talk.
EPC, participant 9
Ending cardiac rehabilitation and EPC was also viewed as a vulnerable time for some participants, and several voiced their concern of being suddenly cut off by cardiac rehabilitation services:
I think additional help after this [end of EPC] is a definite must for some people, it was for me, though it shouldn’t just be a cut-off thing, I don’t know how you would do that, like, ‘oh, 12 weeks and it’s cut-off’, or something like that . . . if they [your nurse] feel that you need more help [at the end of EPC], that should be offered definitely. Or if it’s a waste of time, cos you just literally will go back to how you were before, I think.
EPC, participant 11
Having to wait for their counselling to commence after ending rehabilitation was also seen by participants as an opportunity for setback. In addition, one participant felt that it would be beneficial to have regular contact with the cardiac unit to avoid consulting her GP:
If I was having say a phone call from the hospital, from the cardiac department or a cardiac unit there once every 3 months, maybe it is something I would not have to keep bothering my local doctor with.
EPC, participant 17
This sense of vulnerability could explain why participants found it very reassuring when their nurse said that they could contact them for further support if needed.
For most participants, rehabilitation of the body and the mind together was vital, and dedicated one-to-one time with a practitioner provided the medium within which most felt comfortable discussing personal emotional issues:
To have somebody that deals with the mental health side as well as the physical I think is incredibly important. As somebody who has been through this myself, I think that mental health is definitely linked to the physical part of rehabilitation and health. I think it is a serious, serious thing that it shouldn’t be just about rehabilitating the body, it should be rehabilitating the mind as well.
EPC, participant 17
I really genuinely do hope that this [study] is extended and I think from my personal point of view a bit more should be given, if it’s possible, I know that costs are involved, but you know definitely, I think there’s a future for this type of thing, without a doubt.
EPC, participant 12
Although the importance of having one-to-one sessions with the nurse was emphasised by many participants, some indicated that they could see the advantages of having some aspects of EPC in a group setting, having found it reassuring to talk about their experiences and listen to others. However, others viewed the idea of a group environment for EPC as inhibiting, feeling that they would struggle to talk about their emotions in a group context.
Individuals who declined trial participation
Individuals interviewed who declined to take part in the trial commented that they did not feel very low in mood when they started their cardiac rehabilitation programme. In addition, they thought that they could manage their mood themselves by keeping busy, doing their hobbies, talking and walking with friends, and using knowledge they had gained through their work:
Being a support worker I’ve learnt to develop you know, training, techniques and things like that where I can sort of pull myself out.
Decliner, interviewee 19
Two of the three decliners interviewed were not keen to discuss their mood with strangers or health professionals, including their GP. The third interviewee said that he would see his GP if his mood had not improved. One interviewee said that he did not like the idea of a talking therapy.
One interviewee had declined to take part because he had felt hassled by researchers when involved in a previous research study. The two others were not averse to research participation in general if they perceived that it could benefit them, but had not taken part because they felt that it was not relevant to them in their circumstances. One participant also felt that it was inconvenient:
I just find it a bit inconvenient you know, like today, no disrespect right . . . I could be getting on with a mega job I have got to do in the house because we have got a leak.
Decliner, interviewee 20
One interviewee said that he had received too much information about making changes following his cardiac event and information on study participation added to this burden:
I felt like a lot of people have [been] telling me what I’ve got to do, I’ve got to change this, got to change that and I just, kind of too much information overload.
Decliner, interviewee 19
Interviewees were not able to accurately describe the study aims or what the study would involve for them:
I was of the understanding it was just more of doctors watching and, watching my moods basically.
Decliner, interviewee 19
One interviewee perceived the EPC intervention to be a bit like going to a ‘shrink’ (decliner, interviewee 20).
Taking part in a research study
Participants from both trial arms remembered the initial screening assessment by the nurse (which involved completing the PHQ-9 and the GAD-7, going through a checklist of eligibility criteria and discussing the CADENCE intervention). Most participants in both groups were comfortable with being assessed by a nurse, although not all were used to or happy to score their mental health on a scale, and there were several participants who said that they had not completed one or more of the scores on the mood measures or activity diary to reflect how they really felt:
I know how it all works you see. You don’t put the lowest and you don’t put the highest, so even if I was feeling great I wouldn’t have put it anyway, even if I was feeling low I think, ‘well just put one up to that’.
EPC, participant 7
Consequently, although this interviewee enjoyed attending the EPC sessions and completed all the paper materials, he felt that most of the EPC sessions were irrelevant to him, as he felt that he had never been low in mood:
It’s like I told her [the nurse], ‘you have picked the wrong person in me really because I am never moody or a person who goes into moods’.
EPC, participant 7
Reasons put forward by participants for why they had decided to take part in the study were that it might do some good for themselves or others, it provided an opportunity to talk about how they were feeling or it was a way of helping out the nurse
She was looking for somebody to get it started.
EPC, participant 7
Usual-care and intervention participants’ accounts of taking part in the CADENCE study were positive; participants had not minded the fairly long baseline assessment by the researcher, had found the researchers approachable and professional and felt well informed about what was expected of them:
The way it’s been done has been extremely good and I am very impressed by it actually so yes . . . I like the fact you get a couple of home interviews and then a telephone interview which makes it easier for everybody. That’s a really good way of doing it.
EPC, participant 17
Being part of the CADENCE study had provided the continuity of care missing for some participants between the original cardiac event and the start of rehabilitation, as well as being a good opportunity to talk about the aftermath of the cardiac event:
When I came out of hospital I felt like I’d been cut adrift, this [CADENCE study] went in some kind of thread that leads back to the event.
EPC, participant 14
I’ve had the opportunity to speak to you [today at the interview] about things that I hadn’t after I had the heart attack, so to me that’s been quite a nice thing.
UC, participant 4
All participants fed back very positively and felt that they had enjoyed their participation in the study. Completing the paperwork at various stages of the study was not seen as too arduous, and participants described the researcher who visited to do baseline and follow-up assessments as ‘charming’.
Nurse interviews
Participant sample
Seven nurses (all female) from four of the five intervention teams were interviewed. These nurses had been involved in screening, recruiting, referring and delivering EPC to participants. No nurses from the fifth team were interviewed, as this team did not recruit. Interviews took place between December 2015 and March 2016. Three nurses were interviewed in person, and the remaining four nurses were interviewed by telephone. Interviews were audio-recorded and ranged in length from 38 to 64 minutes (mean length 52 minutes).
At the time of interview, each nurse had delivered EPC to between one and four patients. Six nurses reported sharing the delivery of EPC to one or more of their patients, whereas one nurse had sole responsibility for each of her patients. Nurses reported that six patients had completed EPC, six patients had withdrawn early from the BA element of EPC (five of whom received care co-ordination and one of whom did not return at all), two patients were still waiting to start EPC and two patients were receiving EPC at the time of their nurse’s interview.
Main themes
There were five main themes that were identified from the nurse interviews: (1) usual psychological care, (2) nurse training and materials, (3) acceptability of EPC, (4) views and experiences of delivering EPC and (5) integration of EPC into existing pathways.
Usual psychological care
Each nurse said that they were used to screening patients for mental health symptoms either prior to hospital discharge or during the patient’s initial assessment. To screen, nurses had used either the HADS29 or, in the case of one team, two mental health screening questions and then the HADS for patients for whom the two questions suggested low mood. Based on the accounts given, it was evident that the nurses viewed the provision of psychological care as a significant part of their role:
It’s never been formally identified that actually that [psychological care] is a massive part of our role.
Nurse 3B
I’ve got to be honest, I mean, sometimes I’ve left a cardiac rehabilitation clinic and all that we have addressed is the psychological side of things and particularly for people who have clearly been affected by their cardiac event.
Nurse 4C
Although nurses commented that they had no formal arrangements in place to support patients with low mood, during the interviews it was apparent that, prior to their involvement in the CADENCE study, they had provided a range of support. Some nurses commented that they offered short group sessions, talks on stress and relaxation, or a telephone number to talk to the cardiac rehabilitation team. Nurses often referred patients back to their GP or provided a leaflet on local community mental health services. Links with local IAPT services were generally reported to be fairly good, although nurses were not clear on whether or not patients who they had encouraged to self-refer went on to make a referral. Three of the teams could also refer patients to a psychologist (who was either attached to the cardiac rehabilitation services or to the psychology department within the hospital), but there were waiting lists (for 6–8 weeks) for one-to-one services. Nurses from two of the teams also mentioned referring patients to psychiatric services for more complex problems. However, nurses had received no specific training in psychological care. In addition, some nurses commented that, having been involved in the CADENCE study, they now realised that they had lacked the skills needed to effectively support patients with low mood.
Nurse training and materials
Nurses had received structured training, a nurse handbook, record sheets, tools and paperwork for their patients and some template letters (e.g. for referral to the GP). They felt that the training they had received had covered most areas that they needed to know about. Generally, the training covered assessing and discussing and managing risk; it was clear, and the EPC risk protocol was helpful in terms of providing a pathway for action. However, one nurse felt that they needed more time to practise their new skills during training.
Most nurses commented on the frustration of not being able to start using their new skills immediately, because there was a delay (sometimes of several months) in them seeing EPC patients. This delay was attributable to delays in securing research and development (R&D) approvals, identifying eligible patients through the screening and/or patients not starting their rehabilitation immediately.
Nurses described the training manual as invaluable, and commented that they checked it regularly to ensure that they were conducting EPC and risk procedures correctly. Overall, they felt that information in the manual was well laid out and simple to find. Scripted words about how to talk about EPC to patients had also been helpful. Suggested improvements in the accompanying paperwork included having a separate template of a GP letter for patients who were screened and were then found to be ineligible for the trial but who triggered the suicide protocol. In addition, it was suggested that it should be made clearer as to when any communications with patients should be documented and that there should be a list of which paperwork was needed for each situation (e.g. for screening, EPC or supervision).
Experiences of assessing mental health risk in the pilot trial
During the CADENCE study, nurses were asked to screen for low mood using the PHQ-9 and the GAD-7 instead of the HADS, and to continue to regularly monitor patients using the PHQ-9 over the course of their EPC. Most of the nurses commented that they preferred these tools, and teams often said that they would continue to use them once the study was complete. Reasons given were that the questions were more direct, the layout was better and nurses were able to liaise more easily with primary care practitioners who used the same measures. In addition, using the PHQ-9 (which, unlike the HADS, includes a question specifically about thoughts or plans of suicide) along with the risk protocol provided by the research team reassured nurses that they were identifying and managing risk more readily:
Now it’s a lot more formalised and I’m more aware of that patient, because before I wasn’t picking them up, because I wasn’t doing the PHQ-9.
Nurse 1A
The PHQ-9 and the GAD-7 were administered either by the reception staff or by a nurse handing it out prior to a clinic appointment, or by the nurse during the patient’s clinic appointment. Either way, the nurse then discussed the patient’s score during their appointment. One team found that, occasionally, patients did not want to complete the PHQ-9 in front of them, and so the team started to give these patients the form to complete after the appointment and to leave it at reception ready for discussion at their next appointment.
Despite their preference for the PHQ-9, several nurses suggested that the PHQ-9 did not always reflect the severity of the patient’s mood. Nurses mentioned a number of reasons as to why a patient might score lower than expected: some patients did not want others to know how they felt; patients manipulated the scores so that they were not stigmatised for having mental health issues and patients did not recognise their symptoms as low mood:
They’ve been describing their symptoms and their experience in life, and yet they still come out with these practically zero scores on their scoring tools, so yeah, I think I guess every tool has the ability to be maybe underplayed or if patients maybe aren’t recognising in themselves the way that they’re feeling or they don’t want you to intrude on what they perceive to be private.
Nurse 5C
On the other hand, completing the PHQ-9 and the GAD-7 could provide a welcome opportunity for patients to discuss any mental health issues. For one patient, the nurse said that it had been like opening ’a can of worms’ (Nurse 5C), delaying her assessment of other patients. However, this person had been her first (and only) participant receiving EPC, so this situation might have been worsened by her lack of experience:
It was quite a long assessment [including EPC delivery] so it did delay the [cardiac rehabilitation] assessments of all the other patients. So we were late running on the programme that morning because of that.
Nurse 4C
Exploring and dealing with the risk of suicide was new to nearly all of the nurses. Using the PHQ-9 meant that they now found it one of the biggest challenges to deal with. Some nurses were surprised at how frequently they identified patients with thoughts of suicide or self-harm, and that patients were willing to discuss these feelings, which would often ‘come a bit out of the blue’ (Nurse 4C). Most nurses commented on how ‘nervous’ or fearful they had initially been of identifying and dealing with risk issues:
The only part of it that was a bit scary was the really low mood and was their life at risk, it’s something that made you think, which we all know about but it was asking that question, and then you think, ‘oh god’ [laughs], ‘what if they say “yes”, oh my god’.
Nurse 3B
I felt stressed every time I had to have the conversation, but I would say that the training did help prepare us to ask the right questions. I still felt like I maybe needed a bit of a prompter with the paperwork and yet when somebody’s telling you those sorts of things, it doesn’t necessarily always feel right dragging out a piece of paper [to check what action was needed on the risk protocol].
Nurse 5C
One large team, in which only a subset of nurses received full CADENCE training, reported that the key lessons from managing mental health risk training had been cascaded across the wider team and had added value to their usual practice:
It’s been a really, really useful process for the whole team . . . in the past we’ve done what we’ve had to do in terms of ringing the GP and getting them help, but having a more structured process like this again has, so that’s really helped us.
Nurse 6D
Overall, nurses had gained new clinical skills in terms of assessing patients using the PHQ-9 and the GAD-7 and following structured process when risk issues were identified. They all preferred these to their previous tools and found it reassuring that the PHQ-9 was highlighting patients who were at risk of self-harm, which previously they may not have become aware of.
Acceptability of enhanced psychological care
It was clear that nurses felt that patients varied in how acceptable they had found the intervention and whether or not they benefited. Nurses related this variation to whether or not the patient felt that the BA treatment ‘was for them’ and the extent to which they were motivated to engage with EPC. One nurse felt that the highly motivated patients were the ones who benefited the most, and those who were less motivated were often those who scored highly on the PHQ-9, suggesting that reduced motivation came along with increased depressive symptoms.
Patients who were viewed as engaging well with EPC were described as having grasped concepts of BA, and as having become more physically or socially active. It was evident that patients could be viewed as benefiting even when their PHQ-9 scores had not changed:
I definitely think it is a really good, really good thing, it’s definitely a benefit and certainly you know, of the say four patients that we’ve done, . . . two of them have got a benefit [which was reflected in their PHQ-9 score] and two of them got a benefit but it doesn’t necessarily show up in their PHQ, but certainly they’ve got something out of it.
Nurse 2B
Although some patients completed the eight BA sessions that were recommended, other patients did not fully engage in the BA element. Nurses said that some patients dropped out of BA early, as they were not keen to complete the mood diaries, and during the nurse interviews, two patients were described as thinking BA was not for them. Both patients were viewed as having complex psychological problems, and were referred to psychological or psychiatric services.
Many nurses reported that they had enjoyed being part of the pilot study and developing another clinical skill. Nurses felt that the EPC training they had received had covered most areas they needed, and it was useful to have the training manuals so that they could look back over the training.
Whatever the nurses’ experiences of individual patients, all nurses commented that there was a place for EPC in their work and that BA was an extra tool that they could use. Nurses also commented that it was good to have a designated time to talk to patients, as they felt that patients really valued this, and that it was very helpful that the BA component had been integrated within cardiac rehabilitation rather than through referral to another service, as many patients declined referrals elsewhere:
Giving them something else that they can do in the background that’s not obvious to everybody else but it is actually showing how they are improving week by week by week is fantastic, so I think yes, actually having something like behavioural activation is a really good thing . . . we’ve mentioned about the psychology service at the hospital, we’ve mentioned about the anxiety and depression clinics, we’ve mentioned about speaking to their GPs about anti-depressants, so we’d done all that before and we’d all, most of the time, ninety percent of the time people would say ‘no I don’t want to do that I’m just going to work through it, I’m going to see how I get on’. So actually being able to offer an extra option that didn’t involve all of that, people were more receptive.
Nurse 2B
Those nurses who had delivered EPC to patients a few times seemed more accepting of the intervention and recognised that it got easier to deliver as they became more familiar with it.
Clinical supervision was new to nurses, and sometimes it took them a little time to understand its purpose. Supervision was held by telephone, sometimes on a one-to-one basis and sometimes with other nurses. Nurses found clinical supervision helpful. One nurse mentioned that it had helped her to think of ways to end a BA session and move on to the next stage. Another nurse felt that it had helped to keep her on the right track and think about ways to deal with situations more creatively:
He gave me good, some pointers at times, which I made note off and I tried to act on his advice.
Nurse 1A
Supervision with others enabled nurses to learn from each other. However, supervision was fortnightly, and this meant that some of the advice and support provided was out of date by the time the supervision session came along:
Supervision would be once a fortnight in the end, so you were going over things that were a couple of weeks old and you’d seen the patient again since, that was the only tricky thing.
Nurse 3B
Nurses tried to have patient paperwork ready for each supervision call. Nurses were very busy, rushing from place to place, so did not always find this easy. However, the guide to how to structure EPC (see Appendix 2) was found to be particularly useful:
I think it was useful because we had the record of the delivery, so after each session, there was a space to document what you might have done with that particular patient, anything, and your scores, your GAD or PH [PHQ-9], so you had all that information there with you, so as long as you had that with you and you’d completed it in time with your supervision call, then it was fine.
Nurse 6D
Views and experiences of delivering enhanced psychological care
As some nurses had only seen one EPC patient, they had very little experience of delivering EPC. Nurses recognised that they would improve their skills through practice and experience.
Although some nurses said that they had initially struggled to deliver EPC because it was new to them, and because sometimes it was difficult to engage and motivate patients, they soon found ways to deliver EPC more smoothly and explain concepts more readily. One nurse who had delivered EPC to three patients commented:
Within a couple of weeks I think the patients saw very quickly how their mood was related to their activities and they got it very quickly. So after that actually the time spent with them prior to the group was more as you say coordinating them to the next phase . . . and actually moving them onto actually allocating routine activities.
Nurse 2B
When nurses saw improvement in patients, it was not always easy to tease out how much the improvement was attributable to the provision of BA, because of intense nurse contact at an early stage of the patient’s cardiac rehabilitation programmes (i.e. 10 days post MI and 4 weeks for surgical patients), early contact with other patients or because of all of these factors:
We are getting them in early and I think the confidence that they get from that is huge and so it’s difficult to know. Is it the workbook, the behavioural activation that’s helping them or is it just the fact that they’re coming into a place where there’s peers, there’s support, they’re getting confidence with what they can do, so it’s a bit of a mix, isn’t it?
Nurse 7D
Nurses felt that a lot of what they were doing in terms of spending time discussing mental health issues was what they would have done pre-study training, but it was now more formalised:
I think just having this process, using the EPC just formalises it a little bit more . . . if you’re specifically talking about mood . . . in the gym environment, finding the right opportunity and they’ll plod along and smile, ‘yeah, everything’s great’, and they’re exercising and everything . . . we tend to review patients halfway through their exercise programme to see how they’re getting on, it might not be until that point that they actually decide to open up and say, ‘well, actually, you know what, I am really struggling’.
Nurse 6D
Nurses said that most of the EPC sessions incorporating the BA component took them between 10 and 40 minutes to complete. Time was also required to follow-up patients who had not turned up.
For one nurse, patients sometimes came in regularly to see her just to have an EPC session, before having started their fitness programme. Although it used up more of her time, she felt that this encouraged them to keep on coming, and for one man it meant that he was more likely to do the fitness programme:
If they don’t see me, see us, or come into contact, we’re going to lose them. So for him, I’ve probably done an extra couple so far, but I’m hoping we’re going to try and get him on a programme.
Nurse 1A
For other nurses, EPC was delivered at the same sessions as their fitness programme. Although some nurses felt that ‘patients that are struggling psychologically are always on our radar’, they were more likely now to arrange a set time to see the patient for an EPC session, and thus spent more ’quality time’ with their CADENCE study patients than with their other patients. However, nurses recognised that patients with mental health problems usually took more time anyway, in their usual practice. In practical terms, delivering EPC was time-consuming, especially because using the PHQ-9 opened up the possibility of identifying risk issues:
The components that we have delivered have definitely been more time consuming than our usual care . . . from the perspective of running through a PHQ-9 form and . . . addressing Question 9 [which asks about suicide thoughts and plans], which can often expand the conversation and, well, understandably, and so we should be talking about that sort of thing.
Nurse 5C
Although recognised as a challenge, most of the nurses commented that if there was enough time, staff and administrative resources, integrating EPC into all cardiac rehabilitation would be ‘an ideal situation’.
Although nurses were aware that timely referral to other mental health services was important, some nurses felt that the intervention had not been delivered successfully unless the patient managed to complete all their BA sessions. Some nurses perceived BA as being crucial to EPC delivery, rather than as an optional treatment component. Nevertheless, most nurses did offer a variety of referral options on completion or on opting out of BA. Several nurses commented that they had patients who had not been keen to visit their GP to discuss their psychological issues, and had declined referral to other services for their mental health. In addition, nurses were not informed about whether or not patients they had referred to other mental health services then attended these services.
Most EPC sessions were held on a face-to-face basis, and nurses preferred this to those held by telephone. Reasons for preferring face-to-face sessions included these sessions taking less time, nurses feeling that they gained a lot in terms of understanding how the patient was by seeing them and feeling that the telephone session flowed less well.
Nurses from three of the four teams had a joint workload, which meant that they sometimes shared an EPC patient. This raised the need for nurses to communicate about specific patients. One nurse commented that this would be aided if there was space for both nurses to write in the patient’s record of delivery sheet and others mentioned the need to have an overlap in working hours in order to provide nurses time to talk about the patients that they shared.
Nurses viewed the participant handbook as clear and helpful for them to guide patients through EPC, especially when used alongside the nurse handbook:
The nurse’s handbook and the [patient] workbook are very easy and if we knew we’d got a session coming up, then I’d look at where we’re at, what are we aiming to do today, we’ve already had the support previously to help with direction, but I had no problems discussing it.
Nurse 7D
Some patients engaged in the materials more than others, and many nurses commented that patients identified with case studies in the handbook. However, it was commented that some patients felt that there was a lot of paperwork and that the handbook could be improved in terms of its look and usability:
I know that some of the booklets that we give, the British Heart Foundation booklets are, I don’t know, maybe the look of the tools, it didn’t come across as maybe being an easy tool, I know it is an easy tool to use but there’s quite a lot of information for the patient to maybe read through and think about as well in the booklet and I just wonder if that could be maybe conveyed in a slightly different way.
Nurse 5C
Two nurses said that neither of their patients had completed the activity diaries or other homework materials. This was sometimes because the patients dropped out of the BA element at an early stage, were referred elsewhere or did not come back. In one case, the patient was happy to talk about the diary with the nurse at their appointment, but did not want to complete it herself.
Some nurses found explaining and using the weekly activity diary with patients intuitive, possibly because of their past experience of using food diaries. However, some patients were described as finding the mood scoring on the diary confusing or as struggling to score themselves realistically, as it scored in the opposite way to the PHQ-9, which they had also been asked to complete:
The PHQ and GAD-7, it goes from ‘not at all’ being nought, to ‘nearly every day’ being three. So then you go to the workbook and the workbook goes from lowest mood being one, and then the most positive you are being 10, so it does the opposite.
Nurse 7D
Another issue that nurses mentioned was the need to better support for patients who struggle with literacy skills (patients with dyslexia or a low level of education or ethnic minorities). One nurse felt that there were ways to help these patients to ensure that they were not excluded:
There are ways you can work round things and what you’d probably do with somebody is actually you talk to them and you fill in their activity sheets for them, . . . [using] pictures and things like that . . . But I mean, I wouldn’t want to exclude them so we’d adapt it and we’d probably . . . for those who don’t fill in the questionnaires, you can sometimes it isn’t that they don’t want to fill them in, it’s because they can’t and say, ‘actually do you want me to, it’s quite a busy form, shall we have a look at it together?’ and then they’re more than happy to answer questions and go through it.
Nurse 7D
Most of the nurses worked across multiple venues, and many of them described how they had little or no access to a private place to deliver EPC, so they needed to find a quiet corner to discuss confidential issues with patients. Although this was not ideal, nurses felt that patients found it less intimidating than trying to find a small room. In addition, as they often worked alone, it would have been difficult to leave the fitness room, and remaining in the room allowed them to keep an eye on other patients. Another option, used by nurses, was to ask the EPC patient to come half an hour early or to stay late after the class:
Where we do our exercise rehab[ilitation] groups we do it in a room in a leisure centre so there wasn’t anywhere else to do it, so we just literally met them half an hour earlier than the group and that’s just dependent on whether the patients decide to come early or not.
Nurse 2B
Integration of enhanced psychological care into existing pathways
Nurses commented that EPC had an impact on how they managed and scheduled their time, and had increased the time spent on administration:
Definitely time, you know, the admin, the letters and things, you do to them, and thinking, ‘hang on a minute, I need to take this time back from work, and do the extra’, it’s juggling all these balls and keeping clear records of it as well.
Nurse 1A
There was also a feeling among the nurses that more administrative support would have helped:
I know our admin support lady feels, again, run ragged with all of the, she’s a part-time team worker and had to juggle a whole host of different tasks and I suppose from our point of view that the whole communicating with GPs and in a timely way . . . even if it is a clinical letter, it can sometimes take quite an effort to get it to the point of actually being sent to the GP and so I think from our point of view, we’ve got that never-ending battle of we could do with some more admin support to make the whole flow of the service easier.
Nurse 5C
As well as impinging on their designated administration time, nurses sometimes shortened their lunch breaks and delayed going home in order to fit in an EPC session. Nurses also explained how they asked colleagues to help out at their rehabilitation session, so that they could focus on the EPC patient:
. . . so what we tended to do was if we knew that this lady was coming in for her CADENCE, we would double up so that one was on the gym floor and one was [seeing the CADENCE patient].
Nurse 6D
Although nurses had up to three EPC patients on their caseload at one time, they viewed it as ‘lucky’ that they had not had to see multiple EPC patients at the same rehabilitation session or on the same day:
Say my numbers doubled overnight, which is pretty, very unlikely, [pause] I’d have to work more hours to fit them in.
Nurse 1A
But I think we were lucky and I, like I said to [name of other nurse] I don’t know whether it’s fate or him upstairs that actually did it, that we never had to have that option [more than one patient at the same session] and it worked out really well and yes, I think, yes we didn’t have to have that option [as] I think it would have been difficult.
Nurse 2B
Nurses were concerned about the possibility of needing to see more than one EPC patient at one fitness session or on the same day, as they felt that they would not have the time to deliver this care. In addition, one nurse said that to do so would be ‘mind-numbingly brain taxing’ (Nurse 2B), suggesting that they would also find it mentally exhausting. However, many of the nurses also felt that although they envisaged that it would be ‘tricky’ if it became necessary to accommodate more patients, solutions would be found:
I guess if you took this on and this became routine, then you’d make time for it because it is going to impact on the day and it’ll impact probably on the admin time, because yeah, there’s a bit of paperwork involved and there’s a little bit more documentation. I think, I guess had we had more, we may have found it more difficult, but it’s just a slightly different way of working, isn’t it?
Nurse 6D
Nurses varied in their views about how much the provision of EPC had affected other staff and patients in terms of time: some nurses felt that it only affected patients and nurses receiving/delivering EPC, whereas others commented that the wider staff workload and patients were also affected:
No it didn’t impact on other staff time, from a team point of view I think everyone just got used to it so I don’t, I think it’s very doable . . . So I think we organised it well in the way that actually that time was allocated for that patient importantly, so, but we still had time for the other patients and plus we could stay behind and see them afterwards, so I don’t think it compromised anyone else’s care or time.
Nurse 2B
Especially the leisure centre one, because we’re assessing patients, ready to start the rehab programme, and obviously, I’m tied up with one patient for half an hour some weeks with this gentleman, so sometimes we do start later and yeah there is an impact on them [other staff] definitely.
Nurse 1A
Although there were clear concerns about how to fit EPC around their usual workload, nurses wanted to make the point that they felt that EPC was a worthwhile intervention and that they were keen to explore how their days could be reorganised to enable them to deliver this additional care.
Many nurses believed that there were patients who scored much lower than the threshold score of ≥ 10 on the PHQ-9 who might benefit from EPC:
We’ve seen a hundred or so people to actually screen them and yes, lots of them are under five [score on PHQ-9] but there is quite a few of them between, that’s a seven or eight who I personally think would have benefitted from coming onto CADENCE. So yes certainly from my point of view and speaking to my colleague she found the same thing that we look at people and say they would’ve been perfect but they do not fit the criteria.
Nurse 2B
Even though people hadn’t got a high score, you could just pinpoint, you just thought ‘oh, I wish I could have offered it to so and so or so and so’, you know when you just feel that they might be the sort of person that would actually embrace it a little bit and it would help them move a little bit further emotionally as well. But obviously they didn’t fall within the criteria so.
Nurse 7D
This nurse felt that everyone should be offered EPC as a tool that could aid recovery:
I think it’s a tool that can enhance people’s recovery and I mean a lot of people won’t want it because they’re fine, but you might find that you get more people take it up that may be aren’t quite on the scoring that you’d offer it to but might be just underneath it that are struggling and so that’s how I think I could see it.
Nurse 7D
However, nurses also recognised that they could be ‘quite inundated’ (Nurse 1A) if they included patients who scored < 10. Their limited capacity led to discussions about EPC being delivered in a group setting. Nurses could see that there were advantages to providing at least some elements of EPC in a group setting (social network support and managing larger patient numbers, including those with a lower PHQ-9 score who could benefit). The benefits of patients having opportunities to talk to each other about their experiences were clearly expressed by nurses:
Patients get a huge amount of benefit just in talking to each other, don’t they, and so the problem, the trouble solving, the solutions, ‘oh I do this’, and just seeing how other people are getting on, the little supportive networks that they strike up when they’re actually in the waiting room waiting for us to assess them and they’ve already got their own counselling and social network going on there, so I do recognise the power of actually getting them together as a group.
Nurse 5C
Two nurses felt that an introductory EPC session in a group setting would work well within the short talks that were delivered after a fitness session and given either by the nurse on stress, anxiety and depression, or by the psychologist on emotional impact. These talks were well attended by patients and offered to everyone. However, it was also recognised that group sessions could be off-putting for some; not all patients would want to discuss personal views and experiences in front of other patients. One nurse also acknowledged that there were patients who could dominate group sessions, and other patients could become ‘disgruntled’ by this, so the sessions would need to be skilfully managed. Running EPC in the group setting might also ’lose that element of being able to explore deeper issues when it’s not one to one’ (Nurse 6D). Thus, it was felt that there should be a one-to-one element on offer too, especially for those who had more complex issues that they needed to discuss.
Chapter 6 Discussion
Context
The CADENCE study was undertaken in response to a 2013 NIHR HTA programme commissioning brief, which invited research addressing a specific question: ‘What is the clinical and cost-effectiveness of enhanced care for new-onset depression post cardiac event?’. In the brief, an intervention was envisaged that involves enhanced non-pharmacological care for depression tailored to adults with new-onset depression occurring soon after an acute cardiac event, and who were eligible for cardiac rehabilitation in the recovery phase. The intervention was required to be easily deliverable in the NHS and integrated within existing UK cardiac rehabilitation delivery. The proposed comparator treatment was envisaged as targeting patients receiving standard cardiac rehabilitation and the psychological care routinely provided in this setting, which national audit data suggest is very limited in size and scope. 30 It was advised that referral and uptake of IAPT services should be an assessed outcome. A feasibility study was envisaged, which should clarify the comparator and define and manualise the active intervention in the light of current UK practice. Once developed, it was anticipated that the intervention should be evaluated in an external pilot RCT to test recruitment, acceptability and possible magnitude of effect. Important outcomes were seen as validated measures of depression, cardiac risk and adherence to the rehabilitation programme, and a range of other outcomes were suggested for consideration.
In responding to this brief, our original submission was considered, shortlisted and proposed for funding. Changes from our original submission were requested with a view to containing costs, and, in response, we reduced overall research staffing and proposed shortening the overall project duration from 33 months to 28 months. This involved reducing the length of the longer-term follow-up from 12 months post randomisation to 8 months post randomisation during the pilot study, and we made explicit the rationale for these changes, which were accepted by the funders.
In addressing our aim of designing an EPC intervention for the management of patients with new-onset depressive symptoms attending routine cardiac rehabilitation settings, we successfully undertook a multimethods feasibility study recruiting cardiac rehabilitation staff and patient participants into a before-and-after observational study and a qualitative study employing both interviews and observations. This feasibility study preceded an external pilot RCT, which included a nested qualitative study, evaluating an intervention that had been modified in light of the findings of the feasibility study.
Feasibility study
We successfully recruited three cardiac rehabilitation teams in the south-west of England, drawing on local expert knowledge of services and the geographical accessibility of those services. Following screening of 203 potential participants, 30 individuals with ACS (15%) met our eligibility criteria for admission to the study and, of these, nine agreed to take part. All nine participants completed a follow-up assessment at 5 months. These participants were somewhat younger than the overall group of patients attending initial screening by cardiac nurses, but all provided routine sociodemographic information, and all undertook the baseline CIS-R assessment used to determine primary and secondary psychological diagnoses.
In the feasibility study, we restricted follow-up to a single end point at 5 months, and all participants provided psychological outcome data and data related to smoking status and a range of health outcomes at baseline and follow-up. All feasibility study participants provided information at follow-up regarding their experience of care. The burden of study procedures appeared to be broadly acceptable to patient participants.
Early direct observations of the cardiac rehabilitation nurses delivering UC indicated variations across cardiac rehabilitation teams in respect of service delivery. Variation was also observed in the facilities available for cardiac rehabilitation, the number of patients participating in group sessions and the availability of private space.
Participating cardiac nurses were successfully trained in delivering the EPC intervention, and in monitoring and managing patients with depression who were attending cardiac rehabilitation. That training was supported by the development of a nurse training manual, the content of which highlighted the key elements of EPC regarding mental health-care co-ordination and BA. Observations of nurse intervention training indicated that nurses welcomed their new knowledge and skills. Issues were identified relating to the layout of the patient and nurse handbooks, which were used to guide the content and delivery of the intervention, and there were early concerns expressed in respect of how long EPC might take to deliver in a typical ‘routine’ cardiac rehabilitation session. Observations of clinical supervision sessions with nurses identified concerns relating to the quality of paperwork initially provided to document the care given to patients, and a need was also identified to develop procedures to alert clinical supervisors and the research team when a patient was discharged from EPC.
Interviews conducted with patients as part of the feasibility study indicated that participants felt comfortable with nurses and valued having dedicated one-to-one time to talk about their mental health. Some identified the rather uncomfortable sense of having been ‘singled out’ for treatment – and, in this context, some patients felt that a group approach to delivering the intervention might have been preferable.
Interviews conducted with nurses following the training sessions highlighted overall satisfaction with the organisation and delivery of the training, and with the content and completeness of the supporting materials provided. Nurses reported that they felt that the training had been effective, resulting in greater confidence in the management of patients with psychological problems. Nurses reported benefit from the newly acquired ability to undertake a structured assessment of the patient’s psychological status, including the assessment of suicide risk. Notwithstanding this, nurses identified significant challenges in integrating the delivery of EPC within routine cardiac rehabilitation because of time pressures and the availability of suitable space to allow effective delivery of the EPC intervention. Thus, nurse feedback clearly articulated that the model of EPC tested within the feasibility study was unsustainable within routine cardiac care.
Transition between feasibility and pilot studies
Findings from the feasibility study directly informed the design and delivery of the subsequent pilot study. In particular, aspects of the EPC intervention were refined and redesigned prior to the pilot study, and a range of practical refinements of the pilot study research procedures were proposed.
It was clear that issues pertaining to nurse workload were a major concern, particularly regarding the ability of nurses delivering routine cardiac care to adopt and implement additional work relating to delivery of the intervention and the research procedures. For this reason, we refocused the EPC intervention. The change in the delivery of EPC between the feasibility and pilot phases involved an increased emphasis on the mental health-care co-ordination component of EPC and a shift from a nurse-led intervention to a patient-led, nurse-supported programme of self-help BA embedded within cardiac rehabilitation, reflecting the need to reduce the impact of EPC on nurse time, albeit with nurses trained to support participant engagement. These changes were expected (and indeed shown) to reduce length of session, although it remains possible that the reduction in nurse input may reduce the effectiveness of the intervention.
In the transition period, the eligibility criteria for participants recruited into the pilot trial were broadened, reflecting changing practice within the wider NHS cardiac rehabilitation services through which individuals with other cardiac conditions are increasingly being managed. In reality, this meant that although participants were recruited if they had experienced some form of ACS, there was an increasing likelihood that they would also have other existing cardiac comorbidities (e.g. atrial fibrillation, heart failure). In addition, data collection procedures were refined, with the case note review paperwork and information on antidepressant use being simplified, and additional information being sought regarding participant employment status. As the nurse handbook and participant handbook developed for the feasibility study were well received, practical suggestions emanating from the feasibility study informed the revision and some redesign of these materials (e.g. using colour coding and improving the scripts provided to introduce concepts).
Of significant importance, the assumptions underpinning the accrual of participants into the planned pilot RCT were deemed unrealistic when considered in the light of the findings of the feasibility study. This was carefully considered and reviewed in a paper prepared for the TSC scheduled during the transition period between feasibility and pilot studies. A proposal was submitted to the funders with a view to securing an extension of funding to allow an additional 3-month period of recruitment in the pilot trial, and an additional 3-month period for consideration of the findings and writing-up of the results. This funding request was not agreed. Before commencing the pilot trial, we therefore anticipated that we would not achieve our original pilot study recruitment target, instead anticipating recruitment of around 43 participants (instead of the original 64 targeted). This revised participant accrual rate had implications for our sampling approach within the qualitative study. Thus, we revised our pilot study protocol accordingly, by altering the randomisation ratio from 1 : 1 to five EPC teams to three UC teams to ensure that a sufficient number of participants would experience the EPC intervention.
External pilot trial
The aim of the pilot trial was to test the methods and procedures required to undertake a fully powered evaluation of the clinical effectiveness and cost-effectiveness of cardiac rehabilitation teams implementing EPC for individuals with new-onset depressive symptoms using cardiac rehabilitation services, compared with a comparator of usual cardiac rehabilitation care. We successfully recruited eight cardiac rehabilitation nursing teams, randomising these to one of two trial arms: UC or EPC plus UC. Teams were drawn from a range of settings in South West England and the East Midlands. Most teams recruited offered centre-based rehabilitation programmes. The time of our first approach to the teams to the point of participant recruitment was lengthy (9 months in one area). In two regions, participant recruitment was delayed by over 2 months from the point that all necessary research training had been completed, as we waited for NHS R&D trust approvals to be issued. This effectively reduced the recruitment window from 6 months to 4 months for this team, placing increasing pressure on our ability to achieve our target sample size.
Of the 614 cardiac patients screened for eligibility by the eight teams within a 4- to 6-month recruitment period, 55 patients were eligible to take part in the trial and, of these participants, 29 provided baseline data. Of these 29 participants, 27 (93%) provided 5-month data. Of the 21 participants eligible to provide 8-month data, 17 (81%) did so. Participants in both trial arms were broadly comparable in respect of the range of cardiac diagnoses/events that had precipitated their referral for cardiac rehabilitation.
Twenty-one patients were depressed on assessment at baseline screening by nurses, but were not eligible for inclusion on account of having pre-existing treatment for depression prior to referral to cardiac rehabilitation. The requirement for inclusion of only patients with ‘new-onset’ depression (see Rationale for current research) was set out in the original commissioning brief, and this is an area that future research commissioners might want to revisit.
Cluster randomisation, balanced by team type and patient throughput at the initial cardiac rehabilitation assessment, was successfully implemented, although one randomised team withdrew immediately following randomisation on account of coincidental service changes (a regional reconfiguration of cardiac rehabilitation service provision), which made participation impractical. At short notice, the withdrawn team was replaced with another team matching closely in terms of patient throughput and population mix.
The two trial arms were not well balanced in certain patient characteristics. Participants in the UC arm were older than those in the EPC intervention group. Participants in the EPC arm were more likely to be from deprived backgrounds, to evidence higher levels of depressive symptoms at baseline, and to report lower levels of HRQoL than participants receiving UC. Given the small number of participants and the fact that randomisation was at the level of the cluster, a balance of participant characteristics between arms would not have necessarily been expected. We anticipate that this would not prove to be an issue of concern in a larger, fully powered study.
Two participants (one in the EPC arm and one in the UC arm) were prescribed antidepressants at a therapeutic dose during the study follow-up period. In addition, three participants were prescribed amitriptyline in small doses, in the range usually prescribed by GPs for pain or sleep disturbance. Amitriptyline prescribing is potentially a cause of concern in this group of patients, as older forms of tricyclic antidepressants such as these are generally considered to be relatively contraindicated on account of the presence of cardiac disease.
We relied on case note review of cardiac nurse and GP notes to obtain certain clinical data that might reasonably be assumed to be comprehensively captured in such records. The availability of physiological and biochemical outcome data varied among participants, generally with greater availability of physiological data in GP or cardiac rehabilitation notes than of biochemical data (lipid profile). The cardiac diagnoses/procedures that precipitated referral to cardiac rehabilitation were also inconsistently reported within nurse and GP notes. A future trial should consider revising procedures to ensure that these data are adequately captured by, for example, seeking further data from cardiology services.
Participants in both trial arms were offered attendance at broadly similar numbers of rehabilitation sessions, and similar proportions of participants in both treatment arms attended these sessions. However, four out of the five participants in the intervention arm who attended one or fewer cardiac rehabilitation sessions also had higher baseline depression scores (BDI-II scores of ≥ 14). This suggests that some patients who might have benefited from EPC did not take up that opportunity, although the reasons for this were not clear. In addition, some patients were not offered EPC in the pilot because of the nurse workload, with the nurse lacking capacity to offer EPC to more than three participants at any given time. Patient factors may also be important. Qualitative interviews with trial decliners found that some patients chose not to take part in the trial as they did not identify themselves as experiencing low mood or were not keen to receive more information relating to their cardiac event. We also noted that 4 out of 10 EPC participants who attended at least six rehabilitation sessions had baseline scores below the threshold BDI-II score of 14, indicative of ‘mild’ depression.
Participants’ use of resources was collected from both self-report questionnaires completed by participants and a structured review of both GP routine records and cardiac rehabilitation records. Good or very good agreement between patient and either type of record was observed regarding A&E attendance and hospital admission, but individual-level agreement was poor in respect of the number of primary care appointments attended (when compared with GP records). Differences in the mean number of GP attendances between GP record and self-report data, however, were more similar. For the potential purposes of costing within an economic evaluation, this provides some evidence to support the use of self-report service use data in this patient group (and over these recall periods) – so long as loss to follow-up and questionnaire non-completion could be minimised.
Across the 5-month follow-up period, the mean BDI-II score improved from baseline in both arms of the trial, with the mean between-group difference in BDI-II score (EPC minus UC, adjusted for baseline scores) of 1.72 units, but with a wide CI, encompassing zero. We were able to estimate the distribution of BDI-II scores in our study population, although, being a single study, future research might want to review relevant available evidence from similar or related populations. 82 We also reported additional data on different ways to calculate and compare depression status at follow-up (e.g. remission, MCIDs). Participants in both trial arms reported improved scores for anxiety and heart-related quality of life at the 5-month follow-up, although generic HRQoL, as measured by the EQ-5D-5L, remained unchanged. However, the pilot study sample size was small and likely underpowered, and this, combined with evidence of baseline imbalance between the two trial arms and the lack of adjustment for clustering in the data, does not allow us to draw any formal inferential conclusions regarding the overall direction or magnitude of outcome changes within or between arms.
Perhaps reflecting the nature of the underlying morbidity, two individuals had recommenced smoking, one in each trial arm, by 8 months. Participants in both trial arms reported improved scores for anxiety and heart-related quality of life at 5 months, although generic HRQoL, as measured by the EQ-5D-5L, remained unchanged. Although participants in both arms reported a positive experience of their cardiac rehabilitation, there was some evidence of a more positive experience among participants of EPC when compared with UC.
From semistructured interviews with trial participants, it was clear that they viewed one-to-one dedicated nurse time for psychological care as important to achieving timely recovery from their depression. Participants saw EPC within cardiac rehabilitation as reflecting a ‘holistic approach’ to care, and the delivery of EPC occurring shortly after the cardiac event as important. Despite this, it was evident that a clearer explanation of BA as a key component of EPC was needed, and participants also made it clear that a flexible approach to delivering the intervention was needed as, in the views of at least some participants, ‘one size does not fit all’. A small number of interviews with patients who declined participation identified that their reasons for declining included not feeling that low in mood (despite having questionnaire scores indicative of depression), not being keen to discuss mood with health-care professionals and having concerns regarding ‘information overload’ at the point of attending cardiac rehabilitation.
Interviews with nurses showed that they felt appropriately equipped to deliver the EPC intervention in spite of only limited experience in delivering EPC to patients over the course of the pilot trial. The training in mental health-care co-ordination (including clinical supervision during implementation) and adoption of the PHQ-9 to measure depressive symptoms (replacing the HADS) was well received by nurses, and was collectively viewed as improving their ability to effectively manage mental health and risk. BA was seen by nurses as a viable intervention, although one that was challenging to deliver to some patients and, in particular, to some individuals who were seen as not being receptive to the approach offered. Although there was a general perception of nurses that many patients seemed to benefit from EPC, this was tempered by a view that more of their dedicated time was needed to allow the effective delivery of EPC.
Methods to support economic evaluation
As for the other components of the pilot trial, a principal aim of the economic component was to establish the data collection methods required to support a definitive economic evaluation. We piloted methods required to collect resource and service use data among trial participants that would be relevant to costs to the NHS, costs to social care and personal social services and costs to participants and their carers/families. Service use data were collected from routine/administrative sources, as well as via participant self-report, using a SRUQ (the latter being adapted from the CSRI, following input from the PPI group). Our study identified the estimated cost per participant receiving the EPC intervention, and identified the main elements contributing to this overall estimate of cost. We identified a total overhead cost of providing EPC of £13,384; a substantial proportion of this cost (93%) was accounted for by nurse training. Given the relatively small number of participants, the estimated cost per participant of £904 is likely to be an overestimate, on account of small numbers. Given the nature of the intervention, it proved difficult to identify time spent by nurses in ‘delivering EPC’, as this time was distributed across all of the nurse interaction with the participant rather than in discrete, quantifiable amounts of consultation time.
We successfully gathered information from participants regarding their use of health services across their period of involvement with the study, with data available for the majority of participants. Therefore, patient self-report service use data may be sufficient for providing mean attendance rates in trial arms. The five main categories of health service use on which we captured administrative record data appeared to be intuitively correct, although data collection was intensive, requiring significant researcher time and effort to conduct manual case note review of, and data extraction from, GP and cardiac nurse records. Although we did observe differences between trial arms in resource use estimates, we interpret this with considerable caution and would make no inference regarding the magnitude or direction of any differences on account of the small participant numbers involved. In addition, it is important to separately identify high-cost care episodes (e.g. inpatient admissions) that are unlikely to be related to the intervention or the targeted conditions, but which could cause spurious cost differences between trial arms; therefore, we captured the reasons for such care episodes. The assessment of HRQoL and utility scores using the EQ-5D-5L index scores was undertaken successfully and would have the basis, in a larger trial, of providing quality-of-life estimates that are potentially useful for determining trial sample size, based on the expected cost-effectiveness of the intervention in this population of participants.
Strengths and limitations
Strengths
The CADENCE study brought together an experienced team of researchers and mental health clinicians who designed and delivered a successful mixed-methods feasibility study and pilot trial in response to a national commissioning brief issued by the NIHR. In general, the aims of the feasibility and pilot study were achieved. We successfully recruited three cardiac rehabilitation centres to the feasibility study and eight centres to the external pilot trial (and an additional replacement pilot trial site). Nurse training was provided in respect of EPC for patients attending cardiac rehabilitation with depression.
Our qualitative data found that the training was welcomed by the cardiac nurses, who saw this as an extension of their clinical skill base and knowledge. It was clear that ‘usual psychological care’ was highly variable between teams. As a consequence of intervention training, the assessment of psychological symptoms and the risk assessment of potentially suicidal patients were welcomed. The intervention was both developed and manualised by drawing on a theoretical base previously acknowledged in NICE’s guidelines33,34 for the management of depression in people with long-term conditions. The intervention was developed by experts with experience of training NHS therapists in BA working closely with cardiac nurse specialists, and with the intervention involving regular review of the patient’s psychological state, risk and regular clinical supervision available to nurses implementing the intervention. The trial was supported by the development of two manuals (targeting nurse and patient participants), both of which were subsequently refined following feedback from patients within the trial and our PPI group, as well as from participating nurses delivering the intervention. Data from qualitative observations and interviews provided a deeper insight and understanding of our quantitative findings at each stage of the study. The manuals were modified in the light of feasibility findings in preparation for the external pilot trial. Each EPC session was structured to include the monitoring of depression and risk by nurses, participant self-monitoring (including patient self-identification of activities and triggers to depressive behaviours and thinking) supporting the replacement of negative behaviours in patients with more positive behaviours and the use of mental health-care co-ordination followed an algorithm developed for the study.
Overall, 29 participants were recruited into the pilot trial, 27 out of 29 participants (93%) were successfully followed up at 5 months and 17 out of 21 participants (81%) provided data at the 8-month follow-up. Although we did not meet our original recruitment target (64 participants, subsequently revised to 43 participants), we do believe that recruiting and retaining sufficient participants is possible within the context of a future, larger, definitive, fully powered RCT. The EPC intervention generally appeared to be acceptable to participants, with some evidence of higher satisfaction among participants receiving EPC than among those receiving UC.
In line with the commissioning brief, our intention was to recruit patients attending cardiac rehabilitation who had evidence of new-onset depression occurring since the cardiac event. Although the overall prevalence of depression in this group of patients was in line with expectations, approximately one-quarter of patients (in both the feasibility and pilot studies) had symptoms of depression when initially attending for cardiac rehabilitation and had received treatment for depression in the 6 months preceding the cardiac event, and thus were deemed ineligible for the study. In addition, a small number of depressed individuals were not offered study entry, as the nurse had effectively ‘reached capacity’ in terms of the number of participants receiving EPC at a particular point in time. We would propose that, in the planning of any future research, consideration be given to extending recruitment to include all patients with depression attending cardiac rehabilitation.
Limitations
Prior to undertaking this work, and during the course of preliminary funding consideration, we agreed a change from the NIHR commissioning brief, which had originally envisaged a 1-year follow-up period. Projected study costs and anticipated attrition among this vulnerable group of patients led to this agreement, and our study design involved primary outcome data collection at 5 months and a second (final) follow-up at 8 months. However, the CADENCE study proved to be a challenging study, with tight timelines making necessary changes to the protocol difficult to manage.
Delays encountered in securing trust R&D approval meant that, on occasion, we had nurses who were trained in delivery of the intervention but who were unable to deliver the study until local trust processes had been addressed (these delays related to the negotiation of excess treatment costs). 103 These delays were, in some settings, substantial, and were a source of frustration to the research team and the nurse participants. More significantly, these delays had the potential to undermine the effective delivery of the intervention. Some nurses experienced delays between training and operationalising their skills, although the impact of this was ameliorated, by and large, through ongoing clinical supervision once recruitment commenced. It also limited the number of patients to whom each nurse was able to offer EPC; thus, they had, by and large, only limited experience of delivering the intervention. Negotiation of excess treatment costs has been identified as an impediment to the effective delivery of nationally funded research104,105 and a source of concern within the UK health research community; resolving the issue should, we believe, be a matter of urgency.
Delays were further exacerbated by challenges in managing the range of geographically dispersed recruitment centres, and in respect of research administration and bureaucracy. Overall, we recruited 29 participants – in comparison with the 64 envisaged in the external pilot trial and the revised target of 43, which we anticipated achieving after a study extension request was declined. In the event, 8 of the 29 participants in the external pilot trial were recruited ‘late’ (i.e. at a point when we could collect only 5-month follow-up data from them before data collection closed). Although we were able to estimate our primary outcome (response rate at the 5-month follow-up), our estimate of attrition at 8 months should be interpreted with caution.
We did not recruit the anticipated numbers of participants from ethnic minorities, despite the fact that some of our recruitment centres (in Bristol and Coventry) were located in areas with an ethnic mix in line with the sociodemographic profile of England. There was also an imbalance in participant characteristics and outcomes at baseline between the pilot trial arms in respect of some key features, which is likely to have arisen by chance as a result of the small numbers of clusters and participants.
Although we achieved our stated aim of testing the adequacy of methods used for collecting economic data, consistently with our pilot study aims, we did not undertake a full economic evaluation of the interventions and, hence, for example, we did not collect baseline data on previous patient resource use. We also did not capture and cost the additional time that patients spent completing their mood–activity diaries as part of the EPC intervention. As part of a definitive trial, these methodological issues would need to be resolved.
Challenges were encountered in embedding nurse-led EPC interventions in routine cardiac rehabilitation care. Although trained in study intervention and research procedures and potentially willing to participate, nurses found it very difficult to ring-fence dedicated time in which to deliver the intervention. Following the feasibility study, we refocused the intervention more towards a patient-led intervention, informed by the principles of BA. We found from our qualitative studies that participants welcomed the intervention, with some describing substantial personal benefits in respect of increased activity and improved confidence regarding their health status. Although the EPC tested during the pilot study was designed to reduce the impact on nurse workload, it was clear that this ‘lighter’ version of EPC still posed significant challenges to the nurses attempting to integrate it into routine practice. Evidence for this can be seen in the low fidelity to care co-ordination in both the feasibility and pilot studies, with fewer than 50% of participants having such activity documented in their clinical records. This was despite much greater emphasis being placed on mental health-care co-ordination in the pilot study EPC intervention, suggesting that intervention fidelity remained problematic.
Findings in context
In June 2017, at the point that this report was being finalised, the BACPR released new guidance on the standards for core components for cardiac rehabilitation services in the UK. 106 The requirement to manage psychosocial health is more explicitly described, as is the need to access appropriately qualified psychological practitioners, either embedded within cardiac rehabilitation programmes or through appropriate access arrangements with existing mental health services. As written, the BAPCR guidance is consistent with the need for cardiac nurses to be trained to deliver a mental health-care co-ordination role similar to that tested within our EPC. 106 However, embedding BA within existing teams remains aspirational, and would still face the implementation challenges reported by nurses (albeit from a small number of participating teams).
During the conduct of this research, four important papers have been published, which are of significance. In 2012, Leung et al. 18 reported the findings of a meta-analysis of 22 cohort studies investigating the timing of depression onset to determine what time frame is associated with greater mortality and cardiac morbidity. Their work identified that, for most patients, both premorbid and postmorbid depression onsets were potentially hazardous, and the authors suggested that the question of timing may be irrelevant with respect to adverse cardiac outcomes. Given this observation, excluding patients with pre-existing depression from a future trial may be inappropriate.
The recently reported and NIHR-funded COBRA trial50 identified the potential for effective psychological therapy targeting depression, such as BA, to be delivered without the need for costly and highly trained professionals. In the context of our findings, cardiac rehabilitation nurses appear to offer potential in the delivery of BA to their patients, although important operational, structural and workload issues would need to be addressed to allow them to do so in practice. An alternative approach might be to embed IAPT-trained psychological well-being practitioners (PWPs) offering BA within cardiac rehabilitation teams, thus offering the potential for delivering effective therapy, but doing so in a way that addresses the benefits identified by our trial participants (patients and nurses) of ease of access to psychological support, and the provision of holistic care as part of routine cardiac rehabilitation care.
Members of this research team have recently updated a Cochrane review seeking to assess the clinical effectiveness of psychological interventions compared with UC for patients with CHD. 57 Including 35 RCTs in 10,703 patients, this study concluded that psychological treatments are effective in treating psychological symptoms in participants with CHD, although uncertainty remains regarding the people who might benefit most from treatment.
Finally, although not focusing on the management of depression per se, a recent study has tested the direct effect of adding a single psychological therapy to an existing cardiac rehabilitation programme. In a RCT, Blumenthal et al. 107 identified that providing cardiac rehabilitation enhanced by stress management training produced significant reductions in stress and the rate of clinical events (all-cause mortality, fatal and non-fatal MI, coronary or peripheral artery revascularisation, stroke/transient ischaemic attack and unstable angina requiring hospitalisation), when compared with standard cardiac rehabilitation. These authors suggested that stress management should be incorporated routinely into cardiac rehabilitation, thus providing further support for the incorporation of targeted psychological support in this vulnerable group of patients.
Implications for future research
Based on the findings from this study, undertaking a fully powered study examining the clinical effectiveness and cost-effectiveness of EPC for patients with new-onset depression following an acute cardiac event would represent a major logistical undertaking.
Summary of recommendations for future study designs
Based on our findings, we propose the following recommendations for the design of future research studies evaluating the impact of psychological interventions within cardiac rehabilitation:
-
Appropriately locating psychological care. The qualitative work identified the importance to both patients and nurses of having a psychological component to rehabilitation that is clearly embedded within rehabilitation service delivery – not, for example, developed as a parallel service offered within psychiatric services.
-
Embedding the CADENCE EPC intervention within current routine cardiac rehabilitation practice is not realistic. Although cardiac rehabilitation nurses can be successfully trained in the delivery of EPC to patients, significant barriers to implementation remained, relating to the lack of capacity within existing cardiac rehabilitation services. As a substantive increase in cardiac rehabilitation nurse capacity is unlikely to be feasible; a new model of care may be needed. Our data suggest that consideration should be given to delivering EPC by dedicated mental health workers, possibly such as IAPT-trained PWPs, working in an integrated manner embedded within routine cardiac rehabilitation services. The issue here is not one of lack of professional willingness by cardiac rehabilitation nurses to engage in extended roles and develop/implement psychological skills for the benefit of their patients, but, rather, the evident and limiting practical constraints rendering their full engagement in EPC impractical. Should PWPs be employed to deliver EPC, it is important that they are presented to patients as a core component of the cardiac rehabilitation programme (i.e. not as another onward referral), and that the acceptability of this approach be tested in future research.
-
Refocusing of the EPC intervention from a nurse-led to a more patient-focused intervention and revision of the intervention materials. As a result of our feasibility study findings, we altered EPC to a patient-led, nurse-supported programme and tested this in the external pilot trial (in response to the issues identified in point 2 above). Some modification of the written material used to support the delivery of EPC during the feasibility study was necessary, but, having made these modifications, the revised material appeared to be acceptable to nurses and patients engaged in the CADENCE study. Notwithstanding this, some further refinement and simplification of patient materials may still be required to aid engagement with written materials. Newer modes of intervention delivery (e.g. online materials) may also prove to be worthy of exploration. Depending on the availability of dedicated therapists within the cardiac rehabilitation services as in point 2 above, the revised, patient-focused intervention appeared to offer potential as a means of delivering EPC to this population of patients, and one which may be worthy of fuller evaluation in a larger study.
-
Need for longer follow-up period. Longer periods of follow-up (of 12 months or longer), as envisaged in the original commissioning brief, are desirable from a scientific point of view (e.g. in respect of monitoring infrequent patient safety-related issues and clinical adverse events), but their adoption would add considerably to the cost, and participant attrition rates may increase if longer follow-ups are adopted in a future trial. Although the CADENCE pilot trial proved to be a logistically challenging study to deliver, we believe that the added costs and risks of attrition are worthwhile when balanced against the need for robust evidence on the longer-term effectiveness of enhancing psychological care within cardiac rehabilitation services.
-
Adoption of the BDI-II as the primary outcome measure. The BDI-II appeared to be suitable for assessing the severity of depression, with options to examine the change in symptoms from baseline as a continuous measure, and also to examine the proportion of individuals entering remission during the course of the trial (going from scores of ≥ 14 to < 14) or those experiencing clinically important changes in symptoms. Several other outcomes we worked with might also be considered as competing for ‘primary outcome’ status in a future trial, such as depression status determined by use of the PHQ-9 measure (as adopted in the recently published ‘COBRA’ trial). However, it is important that the primary outcome measure assessing depression is different from tools used clinically to monitor mood as part of a psychological intervention. The decision on exact primary outcome is not an absolute issue, and researchers undertaking a future larger study would wish to review the issue carefully in the light of other relevant published evidence available at the time.
-
Use of a cluster intervention design. If EPC (as tested in our pilot study) was evaluated in a definitive trial, a cluster randomised design is required to mitigate the risk of contamination within participating study centres (clusters), arising as clinicians delivering the intervention might not be able to fully ‘turn on’ or ‘turn off’ EPC delivery in the management of individual patients. However, should trials embed PWPs within cardiac rehabilitation teams, cluster randomisation may not be necessary, which in turn will reduce the size, logistical challenges and costs of a future trial.
-
Patient inclusion criteria. All depressed patients attending cardiac rehabilitation following an acute cardiac event, not just those with new-onset depression following the recent acute cardiac event, should be considered for study entry.
-
Study size and logistical challenges. When considered in the light of a power calculation (reported in Sample size for a future definitive study) supporting the design of a future definitive study, it seems likely that recruitment of participating teams would need to involve a substantial proportion of all available cardiac rehabilitation teams currently operating in the UK. Given this, it seems likely too that such a study would require the early and full involvement of the NIHR Clinical Research Network, drawing on its existing structures to support the geographically dispersed recruitment process.
-
Economic evaluation methods. To avoid spurious differences in costs between trial arms, for high-cost care episodes (e.g. inpatient admissions), greater efforts should be made to collect and corroborate administrative record data, but also to prespecify research processes for deciding whether or not such episodes are related to the intervention and targeted condition(s). For less costly, but more frequent, care episodes (such as general practice attendances) and where case note review is highly resource intensive, patient self-report service use data may be sufficient for providing mean attendance rates in trial arms, so long as reasonably high levels of participant follow-up can be assured. Future resource and service use data collection for similar interventions should always give scope for patients and carers to report their time and other costs involved in receiving care or rehabilitation.
Reflections on the study findings from a PPI perspective are provided in Appendix 14.
Sample size for a future definitive study
Sample size for a cluster randomised trial
On the basis of our presented findings, a future definitive trial would need to consider a number of design parameters. We suggest that ICCs in the order of 0.025 and 0.05 would be a reasonable initial approach. In the CADENCE study, we estimated the ICC to be 0.047, based on national audit data made available through the NACR – a better basis for estimation than data obtained from a single pilot trial. Future estimates of the ICC would be wise to draw on the most currently available data at the time of undertaking the study.
An adequately powered definitive cluster RCT of the intervention should include 50 cardiac teams (clusters) and 650 participants (13 participants recruited within each cardiac team), randomising 25 cardiac teams and 325 participants to each trial arm. On average, it took a cardiac team 1.38 months to recruit a participant to our pilot study; therefore, for each team to recruit 13 participants, the length of recruitment for the definitive trial should be 18 months (i.e. 13 × 1.38).
The sample size was large enough to detect an effect size of 0.35 SD units on the BDI-II, with 90% power at the two-sided 5% level of significance. The sample size allows for within-cardiac team clustering, specifying an intracluster (intracardiac team) correlation coefficient of 0.05, and that 13 participants are recruited in each cardiac team cluster. The calculation assumes 80% follow-up at the participant level at 8 months. This level of follow-up was assumed because 17 out of 21 participants (81%) eligible to be followed up at 8 months provided such data.
The clinically meaningful effect size for the BDI-II (the MCID) was defined as a 17.5% reduction in the mean score. In our pilot study, the mean BDI-II score for the control group at the 8-month follow-up was estimated to be 7.0; a clinically meaningful reduction on the BDI-II scale is therefore 1.225 units (i.e. 7.0 × 0.175). Based on the estimated SD of the BDI-II score (3.5), the corresponding clinically meaningful effect size is 0.35 (i.e. 1.225/3.5) SD units.
The mean and SD of the BDI-II score in the control arm at 8 months was based on only eight participants. The 95% CIs were 4.1 to 9.9 for the mean and 2.3 to 7.1 for the SD. We used these ranges to select values for the mean (6, 7.4, 8) and the SD (3, 3.7, 5) of the BDI-II scores, in order to assess how sensitive our sample size calculation is to uncertainty in these parameters. Sample sizes for a cluster RCT are shown in Appendix 15 for all nine combinations of the assumed mean and SD values.
Patient and public involvement contributions to this work
Reflections on the study findings from a PPI perspective are presented in Appendix 14.
Our PPI representatives actively contributed to many of the key discussions that took place during the lifetime of the project. They regularly attended project management meetings and also met separately to discuss important issues, often in preparation for a team meeting.
The PPI group were very supportive of the idea of providing EPC to cardiac patients, given the patchiness of existing psychological input in cardiac rehabilitation and the lack of availability of this in many cases. However, the PPI group raised several issues in their discussions.
Context of patients’ lives
One of the issues that was raised by the PPI group was the importance of seeing the relationship between a patient’s cardiac event and depression in the wider context of their lives, for example, the social support available to them and other stressful events that they may be experiencing. The group also pointed out that depressive symptoms might be accompanied by other symptoms, such as those associated with post-traumatic stress. This comment appears to be supported by some of the evidence in the qualitative research, when patients reported feelings of being ‘shell shocked’ and suffering from panic attacks. Given that the cardiac nurses in the trial made the initial assessment of ‘new-onset depression’, the group wondered if the limited training given to the cardiac nurses would allow them to recognise and deal with these issues. The PPI group thought that the timing of the intervention and the level of depression that would be used as a recruitment criterion were important to maximising the benefit to patients.
Behavioural activation
The group felt very strongly that the results of the work carried out and the qualitative interview results supported the supposition that cardiac patients would benefit from EPC, but it was difficult to comment on the specific effectiveness of BA. This is because of the small number of people involved in the trial.
Patient and public involvement group members noted that the results from the qualitative study indicated that having someone to talk to about their psychological problems was important to patients. The value of the ‘listening ear’, as one interviewee put it, should not be underestimated. The findings from the qualitative work highlight the importance of the nurse–patient relationship. This may have been enhanced by the nurse training in BA and the addition of extra listening time given to the patient. The group felt that the available evidence suggested that this was beneficial to patients.
An issue raised by one of our PPI members, which may need to be taken into account in future, is the fact that many medications prescribed to cardiac patients may have significant side effects, which may impair the patient’s ability to engage with BA. Such medications can contribute to a general feeling of being ‘ill’ and/or fatigued. There was a concern that this might have a negative impact on people’s ability to successfully undertake BA, given the central focus on activity and that this is an issue that needs further exploration.
Cardiac nurses
The PPI group felt that they could see a clear benefit to the initial support being provided by cardiac nurses rather than requiring a referral to another service, although this option should be available if a patient needs more specialist help.
The group noted that the BA training that the nurses received appeared to enhance their knowledge and skills, giving them greater confidence with which to explore and discuss the wider psychological impact of a cardiac event. This suggests that psychological training for nurses could improve psychological outcomes for patients.
Resources
The PPI group was concerned about the lack of resources to deliver BA, both in terms of the lack of an appropriate room in which to carry out BA and the workload of the nurses. They were concerned that these factors might significantly impair the effectiveness of the intervention. The change from a nurse-led intervention in the feasibility study to a patient-led intervention in the pilot was carried out for several reasons, but a key one was the finding from the feasibility study that nurses were struggling to deliver the intervention. The PPI group expressed initial concerns that this change may place more of a burden on patients who are already struggling to cope with a traumatic event. However, the group felt happy with the changes that were eventually made, and did not feel that they affected patients adversely.
Concluding comments
The group were mindful of the provisional nature of the findings from the study. However, the following points were emphasised as key messages in reporting this work from a patient perspective:
-
There is clearly a need for psychological support to be offered to patients as part of routine cardiac rehabilitation.
-
It was seen as beneficial that initial support can be provided by cardiac nurses rather than requiring a referral to another service. However, having trained psychological counsellors based within rehabilitation teams would also achieve this effect, and may deal with the issue of training and workload for nurses mentioned above.
-
The opportunity to share experiences between patients was clearly valuable.
-
The ability to spend time talking one to one with someone about the psychological consequences of a cardiac event was something that patients valued.
-
The need to be able to get back in touch with someone if problems arose later on is important, for example being given some contact numbers on completing the rehabilitation.
-
Behavioural activation seems to be a promising approach to meeting the needs described above.
-
The need to provide nurses with adequate training, time, resources and support in order to carry out this role was also something that the group felt was very important.
Appendix 15 is largely informed by data collected in our pilot trial design and findings. Our primary sample size calculation indicates that we would need to recruit 50 cardiac teams from the current 303 cardiac rehabilitation teams in the UK (based on the most recent 2013–14 data). 26 We believe that engagement with key stakeholders, such as the BACPR and the NIHR Clinical Research Network, will be essential to achieve this. Although the original brief suggested embedding the intervention in routine cardiac rehabilitation, this proved to be difficult on account of time and workload constraints of cardiac nurses. In contrast, embedding IAPT-trained PWPs within cardiac teams to support the delivery of the intervention may help to overcome this important barrier to recruitment, and may also allow consideration of an individually randomised trial design.
The planned definitive cluster RCT would need to recruit a total of 650 patients over a recruitment period of 18 months. The feasibility and pilot studies only included patients with new-onset depression. Widening the inclusion criteria to those with pre-existing depression is likely to increase the recruitment rate.
Sample size for an individual randomised trial
An adequately powered definitive RCT of the intervention, such as psychological care delivered by a PWP, in which individual patients are randomised, would require a total of 34 cardiac teams (sites) and 442 participants (13 participants recruited per team), with 221 participants randomised to each trial arm. The sample size is large enough to detect an effect size of 0.35 SD units on the BDI-II with 90% power at the two-sided 5% level of significance, allowing for a 20% loss to follow-up at 8 months.
Conclusion
Cardiac rehabilitation nurses can be trained to deliver EPC, as developed by the CADENCE study. Although valued by both patients and nurses, organisational and workload constraints posed significant barriers to implementation within UK cardiac rehabilitation services. We also obtained important data that inform definitive research regarding participant recruitment and retention, and optimal methods of data collection. There remains a need to develop and test new models of psychological care within cardiac rehabilitation. This research provides definitive recommendations for how this might be progressed.
Acknowledgements
We would like to thank the staff from participating cardiac rehabilitation services for their support and engagement with the study, and the study participants for their willingness to share their experiences and time with us. We would also like to thank Julie Chudley (research administrator) for keeping us all organised, and the PPI representatives (Faith Harris-Golesworthy, Julie Harvey, Victoria Jay and Lynn Tatnell) who actively contributed to the development and delivery of the CADENCE study.
This research was also hosted and sponsored by the R&D Directorate of the Royal Devon and Exeter NHS Foundation Trust, which worked in close partnership with us. Although supported by a number of other NHS research services, we are particularly grateful for the support of the South West Peninsula Comprehensive Research Network, and Gordon McGregor of the University Hospital Coventry, for his support with data collection in two areas. We also acknowledge the support of the National Audit for Cardiac Rehabilitation (hosted by the University of York), which provided us with anonymised data to aid in the design of the trial.
We also thank the members of our TSC (Glynn Lewis, Gill Furze, Ron Grant, Sally Kerry, Paul Lanham and Ann Rogers) for their critical review and insightful comments, which were both timely and important in shaping the design and delivery of this research.
Obioha Ukoumunne is supported by the NIHR CLAHRC (South West Peninsula) at the Royal Devon and Exeter NHS Foundation Trust.
Contributions of authors
Suzanne H Richards (Senior Lecturer in Primary Care) was a co-investigator who contributed to the study design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. She provided expert insight on the study design and was the overall scientific lead.
John L Campbell (Professor of General Practice and Primary Care) was the chief investigator who contributed to the study concept, design, protocol development, study management and interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. He provided strategic oversight and leadership of the CADENCE team.
Christopher Dickens (Professor of Psychological Medicine) was a co-investigator who contributed to the study concept, design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. He provided expert insight on the application of psychological methods in rehabilitation settings, and contributed to the team who designed the EPC intervention, and the training of cardiac nurses, including ongoing support through clinical supervision.
Rob Anderson (Associate Professor of Health Economics and Evaluation) was a co-investigator and contributed to the design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. He led the plans for economic data collection and conducted and reported the analyses of resource use and cost data.
Manish Gandhi (Consultant Cardiologist) was a co-investigator and contributed to the design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. He provided expert cardiology input throughout the study, and was the principal investigator for the cardiac teams that took part in the feasibility study.
Andy Gibson (Associate Professor) was a co-investigator and contributed to the design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. He was the PPI lead for the study, actively supporting lay advisors who contributed to the study.
David Kessler (Reader in Primary Care, GP) was a co-investigator and contributed to the design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. Provided general practice and mental health expertise throughout the study, and facilitated data collection in the Bristol region.
Luke Knight (Cardiac Rehabilitation Nurse Specialist) contributed to the protocol development, study management and writing of the manuscript, and approved the final version of the manuscript. He provided expert cardiac nurse input through supporting the intervention development, and led a cardiac rehabilitation team that took part in the feasibility study.
Willem Kuyken (Professor of Clinical Psychology) was a co-investigator who contributed to the study concept, design, protocol development, study management, interpretation of the data and the writing of the manuscript, and approved the final version of the manuscript. He provided expert insight on the application of psychological methods in applied health-care settings.
David A Richards (Professor of Mental Health Services Research) was a co-investigator who contributed to the study concept, design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. He provided expert insight on the application of psychological methods in applied health-care settings and led the team who designed the EPC intervention, and the training of cardiac nurses, including ongoing support through clinical supervision.
Rod S Taylor (Professor of Health Services Research) was a co-investigator and contributed to the design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. He provided expert insight on cardiac rehabilitation service organisation and delivery, and its evaluation.
Katrina Turner (Senior Lecturer and Qualitative Methodologist) was a co-investigator and contributed to the design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. She led the qualitative elements of both the feasibility study and the pilot study, and oversaw the data collection and analyses of qualitative data.
Obioha C Ukoumunne (Associate Professor in Medical Statistics) was a co-investigator and contributed to the design, protocol development, study management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript. He led the plans for statistical analysis, and oversaw the analyses of the observational study and pilot trial data.
Antoinette Davey (Research Fellow) contributed to the pilot trial data collection and validation, trial management, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript.
Fiona C Warren (Lecturer in Medical Statistics) contributed to the statistical analysis plan, interpretation of the data and writing of the manuscript, and performed the statistical analysis of the data and approved the final version of the manuscript.
Rachel E Winder (Research Fellow) contributed to the qualitative study design and data collection, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript.
Christine A Wright (Research Fellow) contributed to the study design, protocol development, trial management and feasibility study data collection, interpretation of the data and writing of the manuscript, and approved the final version of the manuscript.
Publications
Richards SH, Dickens C, Anderson R, Richards DAR, Taylor R, Ukoumunne OC, et al. Assessing the effectiveness of EPC for patients with depressive symptoms attending cardiac rehabilitation compared with treatment as usual (CADENCE): a study protocol for a pilot cluster randomised controlled trial and qualitative interview study. Trials 2016;17:59.
Winder R, Richards SH, Campbell JL, Richards DA, Dickens C, Gandhi M, et al. Using qualitative methods to support the development and implementation of a complex intervention within cardiac rehabilitation services: experiences from the CADENCE feasibility study. Pilot Feasibil Stud 2017;3:9.
Richards SH, Dickens C, Anderson R, Richards DA, Taylor RS, Ukoumunne OC, et al. Assessing the effectiveness of Enhanced Psychological Care for patients with depressive symptoms attending cardiac rehabilitation compared with treatment as usual (CADENCE): a pilot cluster randomised controlled trial. Trials 2018;19:211.
Data sharing statement
The authors confirm that all data underlying the findings are fully available without restriction. The authors have made the clinical and economic data sets available through the University of Exeter Institutional Repository – Open Research Exeter (see https://ore.exeter.ac.uk). Access to these data is permitted by controlled requests made via the repository to the chief investigator (Professor John L Campbell: john.campbell@exeter.ac.uk). Although use will be permitted, this will be on the basis that the source of the data is acknowledged (including the funder) and it includes a reference to the data set name (CADENCE) and supporting academic reference.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- The Top 10 Causes of Death. Geneva: WHO; 2014.
- Cardiovascular Disease Statistics. London: British Heart Foundation; 2015.
- The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2012. London: BACPR; 2012.
- Piepoli MF, Corra U, Adamopoulos S, Benzer W, Bjarnason-Wehrens B, Cupples M, et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention and Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol 2014;21:664-81. https://doi.org/10.1177/2047487312449597.
- Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350-69. https://doi.org/10.1161/CIR.0000000000000019.
- Thombs BD, Ziegelstein RC, Pilote L, Dozois DJ, Beck AT, Dobson KS, et al. Somatic symptom overlap in Beck Depression Inventory-II scores following myocardial infarction. Br J Psychiatry 2010;197:61-6. https://doi.org/10.1192/bjp.bp.109.076596.
- Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001;358:1766-71. https://doi.org/10.1016/S0140-6736(01)06803-9.
- Lespérance F, Frasure-Smith N, Juneau M, Théroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000;160:1354-60. https://doi.org/10.1001/archinte.160.9.1354.
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006;48:1527-37. https://doi.org/10.1016/j.jacc.2006.06.055.
- Singleton N, Bumpstead R, O’Brien M, Lee A, Meltzer H. Psychiatric morbidity among adults living in private households, 2000. Int Rev Psychiatry 2003;15:65-73. https://doi.org/10.1080/0954026021000045967.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004;66:802-13. https://doi.org/10.1097/01.psy.0000146332.53619.b2.
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006;27:2763-74. https://doi.org/10.1093/eurheartj/ehl338.
- van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814-22. https://doi.org/10.1097/01.psy.0000146294.82810.9c.
- Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry 2011;33:203-16. https://doi.org/10.1016/j.genhosppsych.2011.02.007.
- Gale CR, Batty GD, Osborn DP, Tynelius P, Rasmussen F. Mental disorders across the adult life course and future coronary heart disease: evidence for general susceptibility. Circulation 2014;129:186-93. https://doi.org/10.1161/circulationaha.113.002065.
- Dickens CM, McGowan L, Percival C, Tomenson B, Cotter L, Heagerty A, et al. Contribution of depression and anxiety to impaired health-related quality of life following first myocardial infarction. Br J Psychiatry 2006;189:367-72. https://doi.org/10.1192/bjp.bp.105.018234.
- Dickens C, Cherrington A, McGowan L. Depression and health-related quality of life in people with coronary heart disease: a systematic review. Eur J Cardiovasc Nurs 2012;11:265-75. https://doi.org/10.1177/1474515111430928.
- Leung YW, Flora DB, Gravely S, Irvine J, Carney RM, Grace SL. The impact of premorbid and postmorbid depression onset on mortality and cardiac morbidity among patients with coronary heart disease: meta-analysis. Psychosom Med 2012;74:786-801. https://doi.org/10.1097/PSY.0b013e31826ddbed.
- Dickens C, Katon W, Blakemore A, Khara A, McGowan L, Tomenson B, et al. Does depression predict the use of urgent and unscheduled care by people with long term conditions? A systematic review with meta-analysis. J Psychosom Res 2012;73:334-42. https://doi.org/10.1016/j.jpsychores.2012.08.018.
- Frasure-Smith N, Lespérance F, Gravel G, Masson A, Juneau M, Talajic M, et al. Depression and health-care costs during the first year following myocardial infarction. J Psychosom Res 2000;48:471-8. https://doi.org/10.1016/S0022-3999(99)00088-4.
- Rutledge T, Vaccarino V, Johnson BD, Bittner V, Olson MB, Linke SE, et al. Depression and cardiovascular health care costs among women with suspected myocardial ischemia: prospective results from the WISE (Women’s Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol 2009;53:17-83. https://doi.org/10.1016/j.jacc.2008.09.032.
- Dickens C. Depression in people with coronary heart disease: prognostic significance and mechanisms. Curr Cardiol Rep 2015;17. https://doi.org/10.1007/s11886-015-0640-6.
- Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med 2002;23:51-6. https://doi.org/10.1016/S0749-3797(02)00439-7.
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003;65:201-10. https://doi.org/10.1097/01.PSY.0000058371.50240.E3.
- Dickens C, McGowan L, Percival C, Douglas J, Tomenson B, Cotter L, et al. Association between depressive episode before first myocardial infarction and worse cardiac failure following infarction. Psychosomatics 2005;46:523-8. https://doi.org/10.1176/appi.psy.46.6.523.
- The National Audit of Cardiac Rehabilitation: Annual Statistical Report 2015. London: British Heart Foundation; 2015.
- Myocardial Infarction: Cardiac Rehabilitation and Prevention of Further Cardiovascular Disease. London: NICE; 2013.
- The National Audit of Cardiac Rehabilitation: Annual Statistical Report 2013. London: British Heart Foundation; 2013.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- The National Audit Of Cardiac Rehabilitation: Annual Statistical Report 2014. London: British Heart Foundation; 2014.
- Dickens C, McGowan L, Percival C, Tomenson B, Cotter L, Heagerty A, et al. Depression is a risk factor for mortality after myocardial infarction: fact or artifact?. J Am Coll Cardiol 2007;49:1834-40. https://doi.org/10.1016/j.jacc.2007.01.075.
- Common Mental Disorders: The Nice Guidance on Identification and Pathways to Care (NICE Guideline 123). London: NICE; 2009.
- Depression in Adults with a Chronic Physical Health Problem (NICE Clinical Guideline 91). London: NICE; 2009.
- Depression: The Treatment and Management of Depression in Adults (NICE Clinical Guideline 90). London: NICE; 2009.
- Bird A. We Need to Talk: The Case for Psychological Therapy on the NHS. London: Mental Health Foundation; 2006.
- Amick HR, Gartlehner G, Gaynes BN, Forneris C, Asher GN, Morgan LC, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ 2015;351. https://doi.org/10.1136/bmj.h6019.
- Barnes M, Sherlock S, Thomas L, Kessler D, Kuyken W, Owen-Smith A, et al. No pain, no gain: depressed clients’ experiences of cognitive behavioural therapy. Br J Clin Psychol 2013;52:347-64. https://doi.org/10.1111/bjc.12021.
- Layard R. The case for psychological treatment centres. BMJ 2006;332:1030-2. https://doi.org/10.1136/bmj.332.7548.1030.
- The Depression Report. A New Deal for Depression and Anxiety Disorders. London: London School of Economics; 2006.
- Gyani A, Shafran R, Layard R, Clark DM. Enhancing recovery rates: lessons from year one of IAPT. Behav Res Ther 2013;51:597-606. https://doi.org/10.1016/j.brat.2013.06.004.
- Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: returning to contextual roots. Clin Psychol Sci Pract 2001;8:255-70. https://doi.org/10.1093/clipsy/8.3.255.
- Addis M, Martell C. Overcoming Depression One Step at a Time: The New Behavioral Activation Approach to Getting Your Life Back. Oakland, CA: New Harbinger Publications; 2004.
- Beck A, Rush AJ, Shaw B, Emery G. Cognitive Therapy of Depression: A Treatment Manual. New York, NY: Guilford Press; 1979.
- Ekers D, Richards D, McMillan D, Bland JM, Gilbody S. Behavioural activation delivered by the non-specialist: phase II randomised controlled trial. Br J Psychiatry 2011;198:66-72. https://doi.org/10.1192/bjp.bp.110.079111.
- Richards D, Bennett-Levy J, Richards D, Farrand P, Christensen H, Griffiths K, et al. Oxford Guide to Low Intensity CBT. Oxford: Oxford University Press; 2010.
- Richards D, Farrand P, Chellingsworth M. National Curriculum for the Education of Psychological Wellbeing Practitioners (PWPS). Bury: British Association for Behavioural & Cognitive Psychotherapies; 2011.
- Ekers D, Webster L, Van Straten A, Cuijpers P, Richards D, Gilbody S. Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0100100.
- Shinohara K, Honyashiki M, Imai H, Hunot V, Caldwell DM, Davies P, et al. Behavioural therapies versus other psychological therapies for depression. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD008696.pub2.
- Rhodes S, Richards DA, Ekers D, McMillan D, Byford S, Farrand PA, et al. Cost and outcome of behavioural activation versus cognitive behaviour therapy for depression (COBRA): study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-29.
- Richards DA, Ekers D, McMillan D, Taylor RS, Byford S, Warren FC, et al. Cost and Outcome of Behavioural Activation versus Cognitive Behavioural Therapy for Depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet 2016;388:871-80. https://doi.org/10.1016/S0140-6736(16)31140-0.
- Ekers D, Godfrey C, Gilbody S, Parrott S, Richards DA, Hammond D, et al. Cost utility of behavioural activation delivered by the non-specialist. Br J Psychiatry 2011;199:510-11. https://doi.org/10.1192/bjp.bp.110.090266.
- Coventry PA, Hudson JL, Kontopantelis E, Archer J, Richards DA, Gilbody S, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0108114.
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD006525.pub2.
- Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f4913.
- Coventry P, Lovell K, Dickens C, Bower P, Chew-Graham C, McElvenny D, et al. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015;350. https://doi.org/10.1136/bmj.h638.
- Dickens C, Cherrington A, Adeyemi I, Roughley K, Bower P, Garrett C, et al. Characteristics of psychological interventions that improve depression in people with coronary heart disease: a systematic review and meta-regression. Psychosom Med 2013;75:211-21. https://doi.org/10.1097/PSY.0b013e31827ac009.
- Richards SH, Anderson L, Jenkinson CE, Whalley B, Rees K, Davies P, et al. Psychological interventions for coronary heart disease. Cochrane Database Syst Rev 2017;4. https://doi.org/10.1002/14651858.CD002902.pub4.
- Tully PJ, Baumeister H. Collaborative care for comorbid depression and coronary heart disease: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009128.
- Dickens CM, Percival C, McGowan L, Douglas J, Tomenson B, Cotter L, et al. The risk factors for depression in first myocardial infarction patients. Psychol Med 2004;34:1083-92. https://doi.org/10.1017/S0033291704001965.
- Dickens C, McGowan L, Percival C, Tomenson B, Cotter L, Heagerty A, et al. Negative illness perceptions are associated with new-onset depression following myocardial infarction. Gen Hosp Psychiatry 2008;30:414-20. https://doi.org/10.1016/j.genhosppsych.2008.04.003.
- Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002;288:701-9. https://doi.org/10.1001/jama.288.6.701.
- Simmonds RL, Tylee A, Walters P, Rose D. Patients’ perceptions of depression and coronary heart disease: a qualitative UPBEAT-UK study. BMC Fam Pract 2013;14. https://doi.org/10.1186/1471-2296-14-38.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. Swindon: Medical Research Council; 2008.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Meader N, Mitchell AJ, Chew-Graham C, Goldberg D, Rizzo M, Bird V, et al. Case identification of depression in patients with chronic physical health problems: a diagnostic accuracy meta-analysis of 113 studies. Br J Gen Pract 2011;61:e808-20. https://doi.org/10.3399/bjgp11X613151.
- Kroenke K, Spitzer RL, Williams JB, Monahan PO, Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146:317-25. https://doi.org/10.7326/0003-4819-146-5-200703060-00004.
- Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998;51:1055-68. https://doi.org/10.1016/S0895-4356(98)00097-3.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. London: Routledge; 1994.
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-1.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j.2002.384.doc.x.
- Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev 2007;27:318-26. https://doi.org/10.1016/j.cpr.2006.11.001.
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol Med 2008;38:611-23. https://doi.org/10.1017/S0033291707001614.
- Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care 2004;42:1194-201. https://doi.org/10.1097/00005650-200412000-00006.
- Lewis G. Assessing psychiatric disorder with a human interviewer or a computer. J Epidemiol Community Health 1994;48:207-10. https://doi.org/10.1136/jech.48.2.207.
- Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med 1992;22:465-86. https://doi.org/10.1017/S0033291700030415.
- International Statistical Classification of Diseases. Geneva: World Health Organization; 2016.
- The English Indices of Deprivation 2010. London: Department for Communities and Local Government; 2010.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67-74. https://doi.org/10.1097/00005650-198601000-00007.
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory – Second Edition: Manual. San Antonio, TX: The Psychological Corporation; 1996.
- Beck CA, Joseph L, Bélisle P, Pilote L. QOLAMI Investigators (Quality of life in acute myocardial infarction) . Predictors of quality of life 6 months and 1 year after acute myocardial infarction. Am Heart J 2001;142:271-9. https://doi.org/10.1067/mhj.2001.116758.
- Bockting CL, Hollon SD, Jarrett RB, Kuyken W, Dobson K. A lifetime approach to major depressive disorder: the contributions of psychological interventions in preventing relapse and recurrence. Clin Psychol Rev 2015;41:16-2. https://doi.org/10.1016/j.cpr.2015.02.003.
- Button KS, Kounali D, Thomas L, Wiles NJ, Peters TJ, Welton NJ, et al. Minimal clinically important difference on the Beck Depression Inventory--II according to the patient’s perspective. Psychol Med 2015;45:3269-79. https://doi.org/10.1017/S0033291715001270.
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12-9. https://doi.org/10.1037/0022-006X.59.1.12.
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio, TX: The Psychological Corporation; 1990.
- Beck AT, Steer RA, Ball R, Ciervo CA, Kabat M. Use of the Beck Anxiety and Depression Inventories for Primary Care with Medical Outpatients. Assessment 1997;4:211-19. https://doi.org/10.1177/107319119700400301.
- EQ-5D User Guide. Rotterdam: The EuroQol Group; 1996.
- Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes 2010;8. https://doi.org/10.1186/1477-7525-8-13.
- Devlin N, Shah K, Feng Y, Mulhern B, Van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. London: Office of Health Economics; 2016.
- Oldridge N, Hofer S, McGee H, Conroy R, Doyle F, Saner H. The HeartQoL: Part II. Validation of a new core health-related quality of life questionnaire for patients with ischemic heart disease. Eur J Prev Cardiol 2014;21:98-106. https://doi.org/10.1177/2047487312450545.
- Oldridge N, Hofer S, McGee H, Conroy R, Doyle F, Saner H. The HeartQoL: Part I. Development of a new core health-related quality of life questionnaire for patients with ischemic heart disease. Eur J Prev Cardiol 2014;21:90-7. https://doi.org/10.1177/2047487312450544.
- Manos RC, Kanter JW, Luo W. The behavioral activation for depression scale-short form: development and validation. Behav Ther 2011;42:726-39. https://doi.org/10.1016/j.beth.2011.04.004.
- Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann 1982;5:233-7. https://doi.org/10.1016/0149-7189(82)90074-X.
- Davidson KW, Bigger JT, Burg MM, Carney RM, Chaplin WF, Czajkowski S, et al. Centralized, stepped, patient preference-based treatment for patients with post-acute coronary syndrome depression: CODIACS vanguard randomized controlled trial. JAMA Intern Med 2013;173:997-1004. https://doi.org/10.1001/jamainternmed.2013.915.
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106-16. https://doi.org/10.1001/jama.289.23.3106.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345. https://doi.org/10.1136/bmj.e5661.
- Eldridge S, Kerry S, Torgerson DJ. Bias in identifying and recruiting participants in cluster randomised trials: what can be done?. BMJ 2009;339. https://doi.org/10.1136/bmj.b4006.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013;381:375-84. https://doi.org/10.1016/S0140-6736(12)61552-9.
- Chalder M, Wiles NJ, Campbell J, Hollinghurst SP, Haase AM, Taylor AH, et al. Facilitated physical activity as a treatment for depressed adults: randomised controlled trial. BMJ 2012;344. https://doi.org/10.1136/bmj.e2758.
- Gibson A, Britten N, Lynch J. Theoretical directions for an emancipatory concept of patient and public involvement. Health 2012;16:531-47. https://doi.org/10.1177/1363459312438563.
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Excess Treatment Costs. London: Department of Health and Social Care; 2015.
- Palmer R, Harrison M, Cross E, Enderby P. Negotiating excess treatment costs in a clinical research trial: the good, the bad and the innovative. Trials 2016;17. https://doi.org/10.1186/s13063-016-1208-5.
- Excess Treatment Costs. London: AMRC; 2014.
- The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2017. London: BACPR; 2017.
- Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, et al. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation 2016;133:1341-50. https://doi.org/10.1161/CIRCULATIONAHA.115.018926.
Appendix 1 Feasibility study: topic guides used for qualitative interviews
Patient participants
-
Background and illness history (about the cardiac event, discharge home – well-being physically and mentally after discharge).
-
Expectations and decision to come for cardiac rehabilitation (decision to attend cardiac rehabilitation, appropriateness of cardiac nurse assessing mood and providing support for low mood).
-
Experiences of receiving BA sessions and care co-ordination (number of sessions completed, areas covered in BA sessions, areas helpful/unhelpful, what effect BA had on mood, sessions by telephone rather than face to face and physical environment for EPC delivery).
-
Using the participant handbook and BA materials (how the handbook was used, what areas were helpful/could be improved).
-
Identifying links between mood and activity level (understanding BA, making changes to behaviour).
-
Relationship with the cardiac nurse (e.g. relaxed, formal, etc., perception of nurse’s knowledge/skills to assess mental health and deliver BA treatment).
-
Treatment adherence (session attendance, what helped to stay engaged).
-
How EPC has affected physical and mental well-being (current mental and physical well-being, how best to support mental health, recommending EPC to others and suggestions about more effective EPC delivery).
-
Any other treatments received or referrals made during the study and impact on mood.
Nurses
Interview 1 (post training)
-
Experiences of the EPC training (how well did it support EPC delivery, teaching style, length).
-
Views of the nurse handbook and participant handbook (layout, content, how they will use them, any improvements required).
-
Implementing training (how knowledge gained will help to integrate EPC delivery into current practice, any gaps in training, most helpful areas, any changes required in the training).
-
Views of EPC (positive aspects, concerns, anything else needed to support delivery).
Interview 2 (after delivering the intervention)
-
Experiences of delivering BA (addressing low mood, understanding and explaining BA, areas useful or struggling with; telephone vs. face-to-face delivery).
-
Using the materials (e.g. the nurse handbook and participant handbook, mood diaries, other tools).
-
Training (extent to which training was tailored to deliver EPC, any areas needing more input).
-
Managing mental health risk issues and care co-ordination.
-
Support (feedback on supervision sessions).
-
Impact on relationship with patients and perceived mental health role.
-
Practicalities (finding space, time to deliver EPC, managing nurses’ workloads).
-
Impact of integrating EPC on the existing service on wider team(s) (factors that hinder/help/improve smooth delivery).
Appendix 2 Record of enhanced psychological care delivery
Appendix 3 Pilot study: session plan for nurses delivering enhanced psychological care within a cardiac rehabilitation programme
First cardiac rehabilitation session: explain to participant the options available, that is, supported self-help BA manual, with or without onward referral to relevant mental health-care services, depending on individual preferences. Agree mental health treatment plan and take relevant action. This might include:
-
agreeing to discuss the self-help BA book next time you meet (or arranging a special follow-up telephone appointment)
-
writing to or telephoning the patient’s GP
-
giving out details of the local IAPT service or making a referral yourself
-
arranging a specialist cardiac psychological therapy referral.
All remaining sessions: content tailored depending on whether the participant has decided to follow self-help BA manual. These sessions can be brief depending on patient progress.
-
Care co-ordination only. Review mood since last appointment, using PHQ-9 and GAD-7 if preferred. Check that the patient’s mood is not deteriorating further, that they are safe and to see if the action you agreed with the patient to take has been followed up (i.e. did the participant make the GP appointment?).
-
Self-help BA manual. At each session assess symptoms and risk, review treatment choices, support BA, and future planning. BA support is aimed at helping participants to engage with the self-help manual, explaining ideas and methods as required.
At the mid-point (around 4 weeks) dedicated clinic time should be allocated to reviewing progress and carefully review treatment options.
At the final session (around weeks 6–8) dedicated time should be allocated to reviewing progress, and structured details of the care received will be sent to their GP. Participants failing to respond to self-help BA will be referred on to their preferred management option.
Appendix 4 Nurse screening logs
Appendix 5 Quick guide for nurses referring patients to the research team
Appendix 6 Baseline patient questionnaire
Appendix 7 Standard operating procedure for adverse event reporting
Appendix 8 Five-month follow-up patient questionnaire
Appendix 9 Trial Steering Committee paper reviewing participant recruitment
A review of participant recruitment and sample size in light of feasibility study findings: implications for the pilot RCT
HTA CADENCE study: enhancing psychological care in cardiac rehabilitation services [ISRCTN 34701576].
Authors: Dr Suzanne Richards, Dr Fiona Warren, Professor Obi Ukoumunne on behalf of the CADENCE investigators.
Aim
This paper reviews the data on patient participant recruitment to the feasibility phase of the CADENCE study. It aims to review our original estimates of participant recruitment in light of feasibility study findings, and to estimate the likely recruitment into the pilot RCT.
Feasibility study data collection
Reviewing the assumptions underlying patient recruitment
The patient throughput into the feasibility study teams was lower than anticipated. The latest data for the period 23 September to 10 March 2015 identified 159 patients screened by one of the teams participating in the feasibility study. National Audit (2011–12) estimated that approximately 17 patients per month per team would be assessed, i.e. a total of 272 patients during our recruitment phase.
Patient throughput into the feasibility study is lower than national data would suggest, at around 59% (159/272).
National audit data also estimated that around 17% of patients report depressive symptoms during their initial cardiac rehabilitation assessment as assessed using the Hospital Anxiety and Depression Scale. This figure includes patients with existing and new onset depression. Our feasibility data has broadly confirmed this figure.
21% (34/159) of patients assessed during the feasibility study were found to have depressive symptoms (score 10 or more on the PHQ-9) during their initial cardiac rehabilitation assessment, of whom 7 were receiving active treatment for pre-existing depression and 1 was deemed ineligible for cardiac rehabilitation at the point of assessment.
27/159 (17%) met the CADENCE study definition of ‘new onset’ depression.
We assumed that all eligible patients would be offered treatment, and that around 50% of those offered study entry would agree to take part and enter the observational study. In reality, this estimate was too high.
5/26 (19%) of the patients eligible for feasibility study were not offered study entry. Of those offered study entry 9/26 (35%) agreed to take part. When considering only those offered study entry, approximately 43% (9/21) accepted the invitation to take part; this figure is closer to that estimated a priori.
For the purposes of sample size estimation, we assumed a conservative loss to follow-up rate of 50%. In reality, most depression trials (including our own trials e.g. COBRA, COBALT, TREAD) which collect data via researcher interviews report a loss to follow-up of < 20%. The estimation of patient attrition was not a stated aim for the feasibility study, but rather a goal for the pilot study. Notwithstanding this, we will be able to report preliminary data on this parameter arising from the feasibility study:
Due to the slower than anticipate patient recruitment, we will be unable to estimate the loss to follow-up rate at five months until late August 2015. As of 01/04/15, all three patients (100%) who were due to be followed up for the observational study completed their assessments.
Estimating patient recruitment in the pilot study
Analyses of projected CADENCE participant recruitment are based on recruitment data derived from four cardiac rehabilitation (CR) teams (RD&E, Mid Devon, Exeter and Exmouth) as of 11th March 2015. This translates into a combined recruitment period (i.e. adding the recruitment periods of the four teams together) of 16 months; equivalent to 64 team–weeks, i.e. 1 month = 4 weeks. During this period, there were 159 screenings of potential participants, resulting in recruitment of 9 participants to the trial. Based on these data, it takes approximately 7.1 ‘team–weeks’ to recruit each participant.
The pilot study will recruit eight teams (i.e. an additional four teams). If the eight teams recruit at the same rate as the existing four feasibility teams:
Over a 6-month period with all 8 teams recruiting (26 weeks; 208 team–weeks) we would anticipate recruiting approximately 29 participants.
If the recruitment period were to be extended to 9 months (39 weeks; 312 team–weeks) we would anticipate recruiting approximately 44 participants.
Revisiting the pilot study sample size calculation
Estimating patient recruitment in the pilot study
The table below summarises the margin of error with which the follow-up rate can be estimated for different hypothetical samples sizes (number of participants). The margin of error (based on the width of the 95% confidence interval for the follow-up rate) is shown under scenarios where the follow-up rate is: (a) 50%, (b) 80%.
Sample size for CADENCE pilot study: margin of error for estimating follow-up rate in different scenarios
| Sample size | Margin of error (based on width of 95% CI) | |
|---|---|---|
| Follow-up is 50% | Follow-up is 80% | |
| 67 | ± 12% | ± 9.6% |
| 64 | ± 12.3%a | ± 9.8% |
| 57 | ± 13% | ± 10.4% |
| 49 | ± 14% | ± 11.25% |
| 43 | ± 15% | ± 12% |
| 38 | ± 16% | ± 12.8% |
| 34 | ± 17% | ± 13.5% |
| 30 | ± 18% | ± 14.4% |
| 27 | ± 19% | ± 14.25% |
| 25 | ± 20% | ± 15.7% |
The 80% follow-up rate is realistic. With the 64 participants we are targeting, we can estimate a follow-up rate of 80% with margin of error ± 9.8%. If recruitment was lower than expected, say 43 participants, we could still estimate this follow-up rate with margin of error ± 12%. If recruitment was 29 participants, as we would currently project from our feasibility data, we would only be able to estimate the follow-up rate with a margin of error around ± 14.4%.
Ensuring sufficient data are available for the purposes of the qualitative interview study
In addition to ensuring that the pilot study has sufficient power to quantify the primary outcome (follow-up rate), sufficient numbers of patients must also be enrolled in the trial to support sampling for the qualitative study. Here we propose interviewing 10 ‘decliners’, i.e. people who elect not to take part in the trial, and 30 patients who take part. Trial participants from the following groups will be interviewed: 10 participants receiving usual care, 20 receiving the trial intervention (12 completers and 8 non-completers).
The (cluster) randomisation ratio in the pilot trial is 5 intervention teams: 3 control teams, equating to 40 and 24 participants per arm respectively, assuming the original target of 64 participants is achieved, or 27 and 16 participants respectively assuming a lower target of 43 participants is set.
Recommendations for pilot study recruitment
Based on the feasibility study data, the following recommendations are proposed:
-
A 6-month recruitment period will not be long enough to recruit sufficient participants to estimate the pilot study primary outcome (follow-up rate) with the required precision.
-
A 9-month recruitment period (i.e. the initial 6-month period plus a 3-month extension) is the minimum required to achieve the revised target of 43 trial participants if the conditions of the feasibility study are replicated in the pilot trial. This target is, however, challenging from the perspective of sampling for the qualitative study and may pose a threat to the quality of the qualitative research.
We acknowledge that the feasibility assumptions are conservative (e.g. participant throughput, consent rates) and may alter in favour of enhanced recruitment as the trial methods are refined, and more diverse (larger) cardiac rehabilitation teams are recruited to the pilot study. However, we cannot predict potentially enhanced recruitment based on the feasibility data available.
Granting a 3-month extension to the pilot study participant recruitment period should allow us to meet our minimum sampling requirements for both the trial and qualitative research. Should our feasibility estimates prove too conservative, and recruitment be quicker than anticipated, this extension may allow us to achieve close to our original target of 64 patients.
Appendix 10 Statistical analysis plan with health economics
1. Background
Sample size: eight cardiac rehabilitation (CR) teams will be randomised in a 5 : 3 ratio to either the intervention [comprehensive CR programme (CCRP) plus enhanced psychological care (EPC)] or usual care (CCRP only; Cadence Protocol Section 4.1). EPC consists of behavioural activation (BA) plus care co-ordination by the CCRP team. Randomisation will be balanced (to the extent possible) by team type (community or hospital) and patient throughput (categorised as low/high, cut-off point was determined post hoc after scrutinising the mean monthly throughput across CR teams, and was classed as low: 22 patients/month or fewer; high: more than 22 patients/month, based on a natural break in the distribution of team throughput). Trial participants will be assessed at baseline, 5 months and 8 months. Should any teams withdraw post randomisation, such teams will be replaced by another team with characteristics that match as closely as possible those of the withdrawn team. Participants within any replacement teams will be included in descriptive analyses but not in between-group comparisons, should such analyses be performed.
2. Process data related to patient throughput
Patient throughput will be summarised from the initial point of stages 2 and 3 of the CR referral pathway (Cadence Protocol Section 1) through to follow-up of trial participants at 8 months. For each trial arm, we will record the following:
-
number of referrals for stage 2–3 of CR referral pathway
-
number of patients that attend the initial nurse assessment for CCRP (stage 2–3 of CR referral pathway)
-
number that have depression based on the PHQ-9 (score 10 or above)
-
number with specific new-onset depression (defined as not being in receipt of active therapy for depression in the 6-month period prior to occurrence of a cardiac event)
-
number that are eligible for the trial (that is, no other exclusion criteria are met)
-
number invited to participate in the trial
-
number that agree to be contacted by the study researchers
-
number that are recruited
-
number that provide baseline data
-
number followed up at 5 months
-
number followed up at 8 months.
Subsets (ii)–(ix) are natural subsets nested within the previous subset. Subsets (x) and (xi) above are subsets of (ix), but (xi) is not a subset of (x) because of intermittent non-attendance at follow-up.
In addition, we will record the number of participants randomised to CCRP only (control) who elect to not attend subsequent intensive rehabilitation as a binary variable (do/do not attend). All participants allocated to CCRP + EPC should receive EPC, but may engage to varying degrees with the BA aspect of EPC. Participants allocated to CCRP only will have no possibility of also receiving EPC/care co-ordination in error as CR teams allocated to CCRP only will be unable to deliver EPC. Hence, the receipt of EPC differentiates the two arms, irrespective of the receipt of/engagement with BA in the CCRP + EPC arm.
We will report:
-
the number of patients who experienced a cardiac event (aged ≥ 18 years with eligibility for CR according to local protocols: CADENCE Protocol 4.3.1.4) and attended an initial CR consultation
-
the number and percentage of these patients that are eligible for participation
-
the number of eligible patients that are recruited, and this number of patients as a percentage of all patients who attended a CR consultation (point 1), and as a percentage of all eligible patients (point 2)
-
the number and percentage (with 95% confidence interval) of participants followed up at 5 and 8 months.
To estimate the time that would be required to recruit in a definitive trial the following will each be reported per week (i.e. 5 working days) per cardiac team: the number of patients who experienced a cardiac event and attended an initial CR consultation, the number of these patients who are eligible for participation and the number of eligible patients recruited as trial participants. Separate reporting of these statistics by trial arm will help identify any obvious recruitment bias resulting from randomising the cardiac teams before recruiting patients to the trial.
3. Descriptive analyses of trial participants
Numbers of CR team clusters in each of the trial arms will be reported with respect to the descriptive factors used to balance the teams across the arms. These factors are patient throughput [low or high (defined in Section 1) used as a surrogate for team size] and team organisation type (hospital or community); a third team type, mixed hospital and community was subsequently added, following the realisation that some teams were a mixture of these types.
Participants will be described, separately in each trial arm, with respect to socio-demographic characteristics (including age, sex, ethnicity, preferred language), clinical condition,1 cardiac risk factors recorded at patient’s latest clinical assessment prior to recruitment (including body mass index, blood pressure, HbA1c levels, lipid concentrations, smoking status), clinical diagnosis of depression (using the revised Clinical Interview Schedule-Revised; CIS-R), Beck Depression Inventory (BDI) score, Beck Anxiety Inventory (BAI) score and participant treatment preferences (as recorded in the participant baseline questionnaire). Means and standard deviations (or medians and interquartile ranges) for continuous variables and percentages for categorical variables (including binarised depressed/non-depressed with regard to BDI and CIS-R) will be presented to summarise characteristics in each trial arm without formal tests of significance.
4. Analyses comparing outcomes at 5 months and 8 months between trial arms
All between-group comparisons will be performed on an intention-to-treat (ITT) basis, using observed data only. For quantitative outcomes, means and standard deviations will be presented for each trial arm along with the crude mean difference between arms, the adjusted (for stratification factors and baseline score) mean difference and confidence interval for the adjusted mean difference (no p-value will be reported). A 95% confidence interval will also be constructed for the standard deviation (pooled and for each arm individually) of each outcome as it is a study objective to estimate these. For dichotomous outcomes, the percentage with the outcome of interest (e.g., percentage who have a subsequent cardiac event) will be presented for each trial arm along with the crude odds ratio between arms, adjusted odds ratio and 95% confidence interval for the adjusted odds ratio (no p-value will be reported). No inferential conclusions (within arm or between arms) will be made.
The outcomes to be compared are:
-
BDI (continuous), response (50% reduction from baseline; binary), remission (< 14; binary), minimum clinically important difference (MCID; added post hoc; binary),2 clinically significant and reliable change (CSRC; binary)3
-
Beck Anxiety Inventory (continuous)
-
health-related quality of life – European Quality of Life-5 levels (EQ-5D-5–5L; continuous)
-
health-related quality of life – HeartQoL (continuous)
-
Client Satisfaction Questionnaire (at 5 months only – frequencies for each item)
-
Friends and Family Questionnaire (at 5 months only – frequencies)
-
cardiac mortality (binary)
-
all-cause mortality (binary)
-
whether the participant experienced a new cardiac event during follow-up (binary)
-
whether the participant experienced a mental health event during follow-up (Cadence Protocol Section 4.4.1.5) (binary)
-
whether the participant was using antidepressant medication at the time of follow-up (binary).
For the BDI and BAI scores, if a patient has up to two missing items, we will replace the missing items with the average of the reported items in order to generate an overall total score (if there are more than two missing items the overall score will be considered as missing). Similarly, for the HeartQoL, if there is only one item missing, this will be replaced by the average of the reported items; if more than one item is missing the overall score will considered as missing. The EQ-5D-5L score will be calculated using the algorithm developed by Devlin et al. 4 The CSRC is a measure of whether a participant meets two criteria: (i) passing from above the specified threshold to below the threshold (for the BDI, the remission threshold of 14 was used) and (ii) establishing that the magnitude of the participant’s change in score is statistically reliable. To evaluate (ii), the difference between the participant’s scores at baseline and follow-up is calibrated by the standard error of the difference between the two scores. 3 With regard to the CSRC for the BDI, we plan to use a Cronbach α value of 0.90 and SD of 10.8 as reported for the calibration sample in a previous study. 5
Cardiac risk factors (biochemical and physiological) will not be compared descriptively by group. We will report only the availability of these measures (irrespective of the data source, i.e. GP notes or CR team notes) during two sections of the follow-up period: baseline to 5-month follow-up, and 5-month to 8-month follow-up (as a post hoc decision, due to lack of data, only availability of data between baseline and 5-month follow-up).
Cardiac events include the following: myocardial infarction (MI), subdivided where possible into MI with ST segment elevation (STEMI), and non-STEMI; revascularisation procedure [coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI)]; and hospital admission due to other acute coronary syndrome (ACS) events, e.g. acute angina.
All analyses will be performed using Stata v.14, by a statistician who is unaware of the cluster (CR team) allocations.
5. Process data related to delivery of intervention
For the CCRP arm, we will summarise patient attendance at and adherence with the programme, reporting:
-
the time elapsed from point of consent to start of CCRP treatment
-
the time elapsed from point of consent to start of EPC treatment
-
the number of CR sessions (a) offered and (b) attended
-
the number of attended CR sessions with documented evidence of EPC
-
the time elapsed from point of consent to nurse discharge from psychological care
-
the time elapsed from point of consent to nurse discharge from CCRP [date of discharge letter to General Practitioner (GP)]
-
documentary evidence of psychological care co-ordination activities provided to patients on exit from CCRP (based on case notes review; presence/absence of discharge letter from CR nurse)
-
documentary evidence of discussion with participant regarding referral to GP/IAPT (Improving Access to Psychological Therapies) for mental health condition (binary by referral type)
-
documentary evidence of referrals made to (a) GP; (b) IAPT and (c) other psychological care services (binary by referral type)
-
care accessed for depression privately (derived from participant reported Health Economics Service Resource Use Questionnaire (SRUQ; binary).
For the usual-care arm, we will summarise:
-
care accessed for depression via established NHS mental health pathways, for example, GP, IAPT, other pathway (binary by type of care)
-
the time elapsed from point of consent to start of CCRP treatment
-
the time elapsed from point of consent to nurse discharge from psychological care
-
the time elapsed from point of consent to nurse discharge from CCRP (date of discharge letter to GP)
-
documentary evidence of psychological care co-ordination activities provided to patients on exit from CCRP (based on case notes review; presence/absence of discharge letter from CR nurse)
-
documentary evidence of discussion with participant regarding referral to GP/IAPT for mental health condition (binary by type of referral)
-
documentary evidence of referrals made to (a) GP; (b) IAPT; and (c) other psychological care services (binary by type of referral)
-
care accessed for depression privately [derived from participant reported Health Economics Service and Resource Use Questionnaire (SRUQ); binary].
6. Economic evaluation
Reminder of study objectives relating to economic evaluation:
Objective (2c) to establish the data collection methods required to support a definitive economic evaluation (alongside any definitive fully powered trial).
Economic data collection:
-
Service use data.
-
Cost of providing EPC within CR – preliminary assessment.
6.1. Service use data
-
Routine administrative sources (hospital records, GP records).
-
Service and Resource Use Questionnaire – [SRUQ; adapted Client Services Receipt Inventory (CSRI) with input from a Patient and Public Involvement (PPI) group]. The question items will be based on previous versions of the CSRI questionnaire which was developed for people with mental health problems living in the community, and has been adapted and used in a number of studies by health economists at University of Exeter Medical School. It has questions which comprehensively capture information about the use of:
-
hospital care (inpatient, A&E, outpatient)
-
primary care (GP, practice nurse, telephone/face-to-face/home visits)
-
use of other care professionals
-
receipt of disability or illness-related welfare allowances/payments
-
receipt of informal unpaid care (from relatives or friends)
-
receipt of medication for specific conditions.
-
To reflect the likely primary clinical outcomes, most of these questions will focus on items (i) to (vi) above in relation to mental health problems. It will also aim to link to the planned process evaluation data; for example, where referral data will be recorded about the psychological services to which patients in this group have been referred.
The SRUQ will also include a free-text survey question which asks the participant to directly report any difficulties in understanding or answering the service and resource use questions.
6.2. Cost of providing enhanced psychological care within cardiac rehabilitation
Trial records and dialogue with those directly involved in developing and delivering the intervention. This will particularly cover the amount of additional time for CR nurses to become trained in delivering the intervention, and to deliver the BA and care co-ordination components of the intervention.
6.3. Economic ‘data analysis plan’
Since the primary aim is to establish – that is, to try out and refine – the intended data collection methods required to support a definitive economic evaluation, the ‘data analysis plan’ will comprise the following elements:
-
A full estimation of service and resource use of the pilot RCT participants on the basis of available data sources and draft questionnaire items, documenting the estimation process and the difficulties encountered and any unexpected omissions or suspected inaccuracies.
-
Descriptive statistics of the completeness of data from the different sources and at different time points (i.e. item response for those who started completing a follow-up questionnaire).
-
Assessment of the validity or accuracy of self-reported service use data, where they can be compared with routine administrative sources [using kappa scores, percentage where both sources of data agree (in number of contacts of each type individually), and percentage of participants who overestimated and underestimated the number of each type of contact compared with case records]. In general, routine administrative sources will be presumed to be more accurate than self-reported service use data. However, extreme differences between participant reported and routine administrative data sources will be sampled and investigated to see if delays or errors in clinical record-keeping or data entry may explain some of the difference (for example, if a participant recalls a hospital admission which has not been captured by routine hospital records).
-
Qualitative assessment of the difficulty of completing the SRUQ questionnaire. Including assessment of responses to a free-text survey question which asks the participant to directly report any difficulties in understanding or answering the service and resource use questions.
-
Description of any difficulties encountered in processing the data.
-
The preliminary assessment of the cost of delivering the EPC intervention within CR will be developed iteratively by the study’s health economist co-investigator, as a spreadsheet with a number of the relevant stakeholders (i.e. trial manager, intervention developers, cardiac nurses) and wherever possible separately identify those costs which are effectively ‘sunk costs’ – and would be unlikely to be incurred again (or to the same extent), for example, development of the intervention manual; and are mainly associated with the running of the RCT rather than the delivery of the intervention per se.
-
The spreadsheet will have rows for those resources or staff time involved in designing and developing the intervention (meetings, materials/manuals); training cardiac nurses in EPC (nurse time, trainer time, training materials); any additional cardiac nurse time involved in screening for depression and additional nurse time to co-ordinate care or deliver BA. Wherever possible, it will separately show the number of units of resource (e.g. minutes of cardiac nurse time) and the unit price of each type of health-care resource (e.g. £ per minute of nurse time).
7. Statistical analysis plan with health economics audit trail
| Date of statistical analysis plan with health economics | Statistical analysis plan with health economics version number | Date presented to trial management group/Trial Steering Committee | Significant amendments since previous version |
|---|---|---|---|
| 30 March 2015 | 3.0 | 14 April 2015 | Addition of template tables |
| 9 May 2016 | 4.0 | 9 May 2016 |
Discussion of how to include replacement teams in analyses (Section 1) To perform descriptive analyses only, due to small numbers of patients in each group (Section 4) To report biochemical/physiological measurements in terms of availability only, and not to report descriptively the measures themselves (Section 4) Client satisfaction questionnaire to be reported as item frequencies only, i.e. not linearised and reported as a continuous outcome (Section 4) Inclusion of FFT (Section 4) |
| 27 May 2016 | 4.1 | 26 September 2016 | Definition of cardiac event added (Section 5) |
| 27 May 2016 | 4.1 | 26 September 2016 | Addition of health economics template tables |
| 26 September 2016 | 4.2 | N/A | Added MCID for BDI as outcome, added references |
8. Statistical analysis plan with health economics reference list
1. British Heart Foundation. National Audit of Cardiac Rehabilitation. 2012. URL: www.cardiacrehabilitation.org.uk/docs/2012.pdf (accessed 30 March 2015).
2. Button KS, Kounali D, Thomas L, Wiles NJ, Peters TJ, Welton NJ, et al. Minimal clinically important difference on the Beck Depression Inventory – II according to the patient’s perspective. Psychol Med 2015;45:3269–79.
3. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12–19.
4. Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality Of Life: AnEQ-5D-5L Value Set for England. Research Paper 16/01. London: Office of Health Economics; 2016.
5. Brouwer D, Meijer RR, Zevalkink J. Measuring individual significant change on the Beck Depression Inventory-II through IRT-based statistics. Psychother Res 2013;23:489–501.
Appendix 11 Pilot study: topic guides for patient participant and nurse interviews
Pilot topic guide: enhanced psychological care patient participants
-
Background and illness history (about the cardiac event, discharge home – well-being physically and mentally after discharge).
-
Expectations and decision to come for cardiac rehabilitation (decision to attend cardiac rehabilitation, number of sessions attended and cardiac rehabilitation engagement).
-
Experiences of care provided by the cardiac nurses for low mood (appropriateness of cardiac nurse assessing mood, causes of low mood and provision of support for low mood).
-
Experiences of receiving BA sessions and care co-ordination (number of sessions completed, areas covered in BA sessions, areas helpful/unhelpful, what effect BA had on mood, sessions by telephone rather than face to face and physical environment for EPC delivery).
-
Using the participant handbook and BA materials (how the handbook was used, what areas were helpful/could be improved).
-
Identifying links between mood and activity level (understanding BA, making changes to behaviour).
-
Relationship with the cardiac nurse (e.g. relaxed, formal etc., perception of nurse’s knowledge/skills to assess mental health and deliver BA treatment).
-
Treatment adherence (session attendance, what helped to stay engaged).
-
How EPC has affected physical and mental well-being (current mental and physical well-being, how best to support mental health, recommending EPC to others and suggestions about more effective EPC delivery).
-
Any other treatments received or referrals made during the study and impact on mood.
-
Experience of being part of a research study (e.g. information received from the nurse and from the researcher, experiences of completing the assessments, any aspects of the study that needed to be improved for patients).
Pilot topic guide: usual-care patient participants
-
Background and illness history (about the cardiac event, discharge home – well-being physically and mentally after discharge).
-
Expectations and decision to come for cardiac rehabilitation (decision to attend cardiac rehabilitation, what cardiac rehabilitation entailed, number of sessions attended and cardiac rehabilitation engagement).
-
Experiences of usual care provided by the cardiac nurses for low mood (appropriateness of cardiac nurse assessing mood, causes of low mood and provision of support for low mood and referral to other services).
-
Relationship with the cardiac nurse (e.g. relaxed, formal, etc., perception of nurse’s knowledge/skills to assess mental health).
-
Description of current physical and mental well-being (current mental and physical well-being, reasons for any improvements/worsening and how best to support mental health).
-
Any other treatments received or referrals made during the study and impact on mood.
-
Experience of being part of a research study (e.g. information received from the nurse and from the researcher, experiences of completing the assessments and any aspects of the study that needed to be improved for patients).
Pilot topic guide: decliner patients
-
Experiences of being approached about the CADENCE study (e.g. how approached, understanding of study aims and what was involved).
-
Reasons for declining research participation (e.g. feelings when asked about participation in study about low mood, main reasons for choosing not to participate and factors that would have encouraged participation).
-
Previous experiences of research participation and psychological therapy.
-
Thoughts on having a talking therapy (e.g. thoughts on BA or other talking therapies, experiences of treatment in the past).
-
Current mental and physical well-being (e.g. current feeling, any receipt of help for low mood and anything specific to be aware of when treating cardiac patients with low mood).
Pilot topic guide: cardiac rehabilitation nurses
-
Experiences of delivering BA (e.g. addressing low mood in usual care, understanding and explaining BA, areas useful or struggling with, acceptability of BA as a treatment by cardiac nurses, use of EPC documentation and telephone versus face-to-face delivery).
-
Using the materials (e.g. the nurse handbook and participant handbook, mood diaries and other tools, confidence using the tools with patients and how patients used the materials).
-
Training (e.g. extent to which training was tailored to deliver EPC, any areas needing more input).
-
Managing mental health risk issues and care co-ordination (e.g. experiences of dealing with patients at mental health risk, role in care co-ordination and experiences of referral of patients to psychological care services).
-
Support (e.g. feedback on supervision sessions).
-
Impact on relationship with patients and perceived mental health role (e.g. advantages and disadvantages of delivering EPC to patients by cardiac nurses).
-
Practicalities (e.g. finding space, time to deliver EPC and managing nurses’ workloads).
-
Impact of integrating EPC on the existing service on wider team(s) (e.g. factors that hinder/help/improve smooth delivery).
-
Acceptability and appropriateness of methods for the CADENCE study (e.g. screening process and referral of patients to study).
Appendix 12 Ethics committee-approved changes to the study protocol
| Amendment number | Summary of proposed amendment |
|---|---|
| 1 |
Revised inclusion/exclusion criteria The TSC met on 24 July 2014 to discuss the study protocol. The TSC recommended a change to the study inclusion and exclusion criteria in relation to patient participants – patients with early-stage dementia should not be excluded from study participation if they met all other criteria. A similar view was expressed with regard to patients with a pre-existing diagnosis of severe mental illness (e.g. schizophrenia or bipolar depression) whose symptoms of psychosis were well controlled |
| 1 |
Economic data collection Routine/administrative sources to include community mental health records to reflect revised inclusion criteria |
| 2 |
Revision of cluster randomisation ratio A 5 : 3 randomisation ratio (EPC plus usual care : usual care) was informed by data emerging from our feasibility study and seeks to ensure that sufficient nurses and patients have EPC exposure to support the aims of the qualitative study interviews |
| 2 |
Extension of patient recruitment period in pilot trial phase by 3 months Propose to recruit 64 patients from eight teams in 9 months, which affects the timeline described in Section 7 of the protocol |
| 2 |
Revision of variables used to stratify randomisation ‘team setting’ and ‘average monthly patient flow’ (throughput) are proposed as alternative stratifying variables |
| 2 |
Training provided for nurses allocated to the EPC intervention arm Based on findings emerging from the feasibility phase, it was proposed that there should be the following training and support for nurses in the trial EPC arm: |
| 2 |
Patient population for pilot trial
|
| 2 |
Addition of the NHS FFT to trial follow-up questionnaires In line with current NHS policy to use the FFT to evaluate patient experience of services, we added one item to our follow-up questionnaires to assess whether patients would recommend the services they received for their low mood within the cardiac rehabilitation programme to others. The item includes the standard NHS question stem and 5-point response scale plus two free-text questions: ‘What was good about your experience?’ and ‘What would have made your experience better?’ |
| 2 |
Changes to qualitative study It was proposed to conduct one in-depth interview with EPC-trained nurses (rather than two), taking place towards the end of EPC delivery once the nurse had gained some experience of delivering EPC. A researcher still observed the training and made detailed field notes. The number of teams in the intervention arm and the number of nurses predicted to be involved in EPC delivery were amended to reflect the proposed change in randomisation ratio. We proposed to offer flexibility to patients who declined study entry (but agreed to a one-off interview) whether their interview was conducted by telephone or face to face. A similar choice was available for patients who entered the trial and also agreed to an interview |
| 3 |
Participant recruitment period in pilot trial returned to 6 months Owing to the funder confirming that they would not fund an extended recruitment period, we returned our proposed recruitment period to the original 6 months. The funder’s decision also affects the timeline described in Section 7 of the protocol |
| 3 |
Addition of the BADS-SF to trial baseline and follow-up questionnaires To enable us to measure when and how participants become ‘activated’ over the course of their cardiac rehabilitation programme or BA treatment, we proposed to add the nine-item BADS-SF questionnaire to all trial assessments – i.e. baseline, 5-month and 8-month follow-ups. We did not anticipate that this short scale would add significantly to the length of assessments that participants would be asked to complete |
| 4 |
Addition of questions to the SRUQ Following consultations with the PPI group and nurses, additional questions to the employment history were added and medications sections for further clarification |
| 5 |
End of study date changed Following a discussion with our funding agency, the NIHR HTA programme, on 6 April 2016 we were granted a 3-month no-cost extension to allow for the timely write-up of the study. Our end of study date was therefore 31 October 2015 |
| 5 |
Follow-ups Most patients had an 8-month follow-up, but given that our primary outcome is obtained at 5 months, we have agreed with the HTA programme that, for the final nine patients recruited to the study, a final follow-up data collection will only be done at 5 months. For these nine patients, we will not have 8-month data |
Appendix 13 Standard operating procedure for cardiac rehabilitation nurses: assessing, reporting and monitoring risk of self-harm and suicide
Appendix 14 Patient and public involvement commentary
Our PPI representatives actively contributed to many of the key discussions that took place during the lifetime of the project. They regularly attended project management meetings and also met separately to discuss important issues, often in preparation for a team meeting.
The PPI group were very supportive of the idea of providing EPC to cardiac patients, given the patchiness of existing psychological input in cardiac rehabilitation and the lack of availability of this in many cases. However, the PPI group raised several issues in their discussions.
Context of patients’ lives
One of the issues that was raised by the PPI group was the importance of seeing the relationship between a patient’s cardiac event and depression in the wider context of their lives, for example, the social support available to them and other stressful events that they may be experiencing. The group also pointed out that depressive symptoms might be accompanied by other symptoms, such as those associated with post-traumatic stress. This comment appears to be supported by some of the evidence in the qualitative research, where patients report feelings of being, ‘shell shocked’ and suffering from panic attacks. Given that the cardiac nurses in the trial made the initial assessment of ‘new onset depression’ the group wondered if the limited training given to the cardiac nurses would allow them to recognise and deal with these issues. The PPI group thought that the timing of the intervention and the level of depression that would be used as a recruitment criterion were important to maximising the benefit to patients.
Behavioural activation
The group felt very strongly that the results of the work carried out and the qualitative interview results supported the supposition that cardiac patients would benefit from EPC, but it was difficult to comment on the specific effectiveness of BA. This was attributable to the small number of people involved in the trial.
Patient and public involvement group members noted that the results from the qualitative study indicated that having someone to talk to about their psychological problems was important to patients. The value of the ‘listening ear’, as one interviewee put it, should not be underestimated. The findings from the qualitative work highlight the importance of the nurse/patient relationship. This may have been enhanced by the nurse training in BA and the addition of extra ‘listening time’ given to the patient. The group felt that the available evidence suggested that this was beneficial to patients.
An issue raised by one of our PPI members, which may need to be taken into account in future, is the fact that many medications prescribed to cardiac patients may have significant side effects that may impair a patient’s ability to engage with BA. Such medications can contribute to a general feeling of being ‘ill’ and/or fatigued. There was concern that this might have a negative impact on people’s ability to successfully undertake BA, given the central focus on activity and that this is an issue which needs further exploration.
Cardiac nurses
The PPI group felt that it could see a clear benefit to the initial support being provided by cardiac nurses rather than requiring a referral to another service, although this option should be available if a patient needs more specialist help.
The group noted that the BA training that the nurses received appeared to enhance their knowledge and skills, giving them greater confidence with which to explore and discuss the wider psychological impact of a cardiac event. This suggests that psychological training of nurses could improve psychological outcomes for patients.
Resources
The PPI group were concerned about the lack of resources to deliver BA, both in terms of the lack of an appropriate room to carry out BA and the workload of the nurses. They were concerned that these factors might significantly impair the effectiveness of the intervention. The change from a nurse-led intervention in the feasibility study to a patient-led intervention in the pilot study was carried out for several reasons, but a key one was the finding from the feasibility study that nurses were struggling to deliver the intervention. The PPI group expressed initial concerns that this change may place more of a burden on patients who are already struggling to cope with a traumatic event. However, the group felt happy with the changes that were eventually made, and did not feel that they affected patients adversely.
Concluding comments
The group was mindful of the provisional nature of the findings from the study. However, the following points were emphasised as key messages in reporting this work from a patient perspective:
-
There is clearly a need for psychological support to be offered to patients as part of routine cardiac rehabilitation.
-
It was seen as beneficial that initial support can be provided by cardiac nurses, rather than requiring a referral to another service. However, having trained psychological counsellors based within rehabilitation teams would also achieve this effect, and may deal with the issues of training and workload for nurses.
-
The opportunity to share experiences between patients was clearly valuable.
-
The ability to spend time talking one to one with someone about the psychological consequences of a cardiac event was something that patients valued.
-
The need to be able to get back in touch with someone if problems arose later on is important (e.g. being given some contact numbers on completing the rehabilitation).
-
Behavioural activation seems to be a promising approach to meeting patient needs for psychological support.
-
The need to provide nurses with adequate training, time, resources and support in order to carry out this role was also something that the group felt was very important.
Appendix 15 Sample size calculation for a definitive trial
The table below summarises the sample size calculations for a definitive cluster randomised trial. Estimates are based on different assumed true values of the mean and SD of the BDI-II measure for the control group at 8 months.
| Scenario | BDI-II score | Clinically meaningful effect (MCID) | Required total sample size (n) | ||
|---|---|---|---|---|---|
| Mean (SD) | BDI-II score | Effect size | Cardiac teams | ||
| 1 | 6 (3) | 1.05 | 0.35 | 54 | 702 |
| 2 | 6 (3.5) | 1.05 | 0.3 | 72 | 936 |
| 3 | 6 (5) | 1.05 | 0.21 | 148 | 1924 |
| 4 | 7 (3) | 1.225 | 0.41 | 40 | 520 |
| 5 | 7 (3.5) | 1.225 | 0.35 | 54 | 702 |
| 6 | 7 (5) | 1.225 | 0.25 | 104 | 1352 |
| 7 | 8 (3) | 1.4 | 0.47 | 30 | 390 |
| 8 | 8 (3.5) | 1.4 | 0.4 | 42 | 546 |
| 9 | 8 (5) | 1.4 | 0.28 | 84 | 1092 |
Glossary
- Acute coronary syndrome
- A set of signs and symptoms arising from decreased blood flow to the heart, usually attributable to one of three problems: ST elevation myocardial infarction, non-ST elevation myocardial infarction or unstable angina.
- Behavioural activation
- A psychological therapy applied to the management of depression, which applies a functional-analytic approach, which asks the client to develop an understanding of the relationships between actions and emotions, and to alter behaviours that reduce low mood.
- Comprehensive cardiac rehabilitation programme
- A structured rehabilitation programme aimed at supporting people recovering from an acute cardiac event. Core components include education, exercise and physical activity, diet and weight management, medical management and psychological support.
- Mental health-care co-ordination
- The systematic monitoring of patient mood by a designated health-care professional, combined with the offering of evidence-based therapies designed to modify/manage the patient’s underlying mental health condition.
List of abbreviations
- A&E
- accident and emergency
- ACS
- acute coronary syndrome
- BA
- behavioural activation
- BACPR
- British Association for Cardiovascular Prevention and Rehabilitation
- BADS-SF
- Behavioural Activation for Depression Scale – Short Form
- BAI
- Beck Anxiety Inventory
- BDI-II
- Beck Depression Inventory, version 2
- CABG
- coronary artery bypass graft
- CADENCE
- enhanced psychological CAre in carDiac rEhabilitatioN serviCEs for patients with new-onset depression compared with usual care
- CBT
- cognitive behavioural therapy
- CHD
- coronary heart disease
- CI
- confidence interval
- CIS-R
- Clinical Interview Schedule – Revised
- CLAHRC
- Collaborations for Leadership in Applied Health Research and Care
- COBRA
- Cost and Outcome of Behavioural Activation versus Cognitive Behavioural Therapy for Depression
- CODIACS
- Comparison Of Depression Interventions After Acute Coronary Syndrome
- CONSORT
- Consolidated Standards of Reporting Trials
- CR
- cardiac rehabilitation
- CSQ-8
- Client Satisfaction Questionnaire – 8
- CSRC
- clinically significant and reliable change
- CSRI
- Client Services Receipt Inventory
- ENRICHD
- Enhancing Recovery in Coronary Heart Disease
- EPC
- enhanced psychological care
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FFT
- Friends and Family Test
- GAD-7
- Generalised Anxiety Disorder-7
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HbA1c
- glycated haemoglobin
- HeartQoL
- Heart Quality Of Life scale
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IAPT
- Improving Access to Psychological Therapies
- ICC
- intracluster correlation coefficient
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- MCID
- minimum clinically important difference
- MI
- myocardial infarction
- MRC
- Medical Research Council
- NACR
- National Audit of Cardiac Rehabilitation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRES
- National Research Ethics Service
- NSTEMI
- non-ST elevation myocardial infarction
- OR
- odds ratio
- PCI
- percutaneous coronary intervention
- PenCTU
- Peninsula Clinical Trial Unit
- PHQ-9
- Patient Health Questionnaire-9
- PPI
- patient and public involvement
- PWP
- psychological well-being practitioner
- R&D
- research and development
- RCT
- randomised controlled trial
- RR
- risk ratio
- SD
- standard deviation
- SMD
- standardised mean difference
- SQL
- Structured Query Language
- SRUQ
- Service and Resource Use Questionnaire
- SSL
- Secure Sockets Layer
- STEMI
- ST elevation myocardial infarction
- TSC
- Trial Steering Committee
- UC
- usual care
- VAS
- visual analogue scale
