Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/37/64. The contractual start date was in April 2009. The draft report began editorial review in April 2017 and was accepted for publication in November 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Matthew T Thompson reports grants and personal fees from Endologix Inc. and Medtronic Ltd (Watford, UK) and personal fees from Gore Medical (Neward, DE, USA) outside the submitted work. Richard J Grieve is a member of the Health Technology Assessment Commissioning Board. Roger M Greenhalgh serves as a salaried director of Biba Medical Ltd (London, UK) and has an equity interest in the company and serves as an expert witness on behalf of patients with vascular disease.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Ulug et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Abdominal aortic aneurysm (AAA) is a common degenerative disease, with a current prevalence of ≈2% in men aged 65 years; the prevalence increases with age. AAA is about fourfold less common in women. Small AAAs are rarely associated with symptoms but their natural history is progressive enlargement until rupture occurs. Following several randomised trials, a screening programme for 65-year-old men was rolled out, between 2009 and 2013, to cover the UK. Elective aneurysm repair is recommended when the diameter is > 5.5 cm (about three times the normal aortic diameter); the operative mortality for elective procedures for screen-detected aneurysms is low. 1–3 Without intervention, a ruptured AAA is fatal and the overall mortality is > 80%. About half of patients with ruptured aneurysms die in the community. More than one-third of patients who arrive in accident and emergency departments do not reach the operating theatre alive. Among the patients who reach the operating theatre (for open surgical repair under general anaesthesia), only half will leave hospital alive. These stark figures changed little over the 50 years between 1960 and 2010. 4 Ruptured aneurysm is a common vascular emergency, involving various symptoms that are not specific to AAA rupture, usually back or abdominal pain and collapse. It tends to occur in elderly patients who often have comorbidities that may preclude a successful repair, especially if the diagnosis is not made promptly.
Ruptured aortic aneurysms are a common cause of death in the UK and, in the last century, infrarenal AAAs caused ≈8000 deaths per annum in England and Wales. The incidence of both AAAs and ruptured AAAs continued to increase year on year until about 2000 in both men and women. 5,6 More recently, the number of deaths from ruptured AAAs has declined rapidly because of the reducing prevalence of smoking in the population and the increasing number of elective aneurysm repairs in the population aged > 75 years, using the minimally invasive endovascular repair. 7 Nevertheless, when this trial started in 2009, there were still 2581 deaths from AAA ruptures (285 of these in women) in England and Wales. Until about 2004, routine practice was to direct patients with a suspected ruptured AAA directly to the operating theatre for open repair, without preoperative computerised tomography (CT). In England, there were about 1300 open surgical repairs for ruptured aneurysms each year, with 30-day and in-hospital mortality being similar at 47–48%. 4,8,9
For open AAA repair, the aneurysm is usually approached by a midline laparotomy, carefully moving aside the colonic viscera and cross-clamping the aorta, below the renal arteries wherever possible, and inserting a prosthetic inlay graft, using a tube configuration with sutured anastomoses to the aorta both proximally and distally to exclude the aneurysm wherever possible (Figure 1). When the aneurysm extends beyond the aorta into the common iliac arteries, the use of a bifurcated graft becomes necessary. These procedures are conducted under general anaesthesia and the patients require a critical care bed postoperatively. The in-hospital care of those patients undergoing open surgical repair of a ruptured aneurysm is costly, as many days are spent in the intensive care unit (a mean of 3.5 days for uncomplicated cases and 9.5 days for complicated cases) and the average hospital stay is long. Recuperation after discharge following open surgery for a ruptured aneurysm can take up to 6 months, having a further impact on the resources of the family, social care and general practice. Although the recovery may be slow, the evidence shows that the results of elective open repair are durable and there is no need for longer-term follow-up. Interestingly, the operative mortality of open surgery appears to be independent of hospital volume, although this may be influenced by case selection. 10
FIGURE 1.
Schematics of aneurysm repair, showing (left) open repair, (centre) endograft insertion for endovascular repair and (right) endovascular repair in progress.
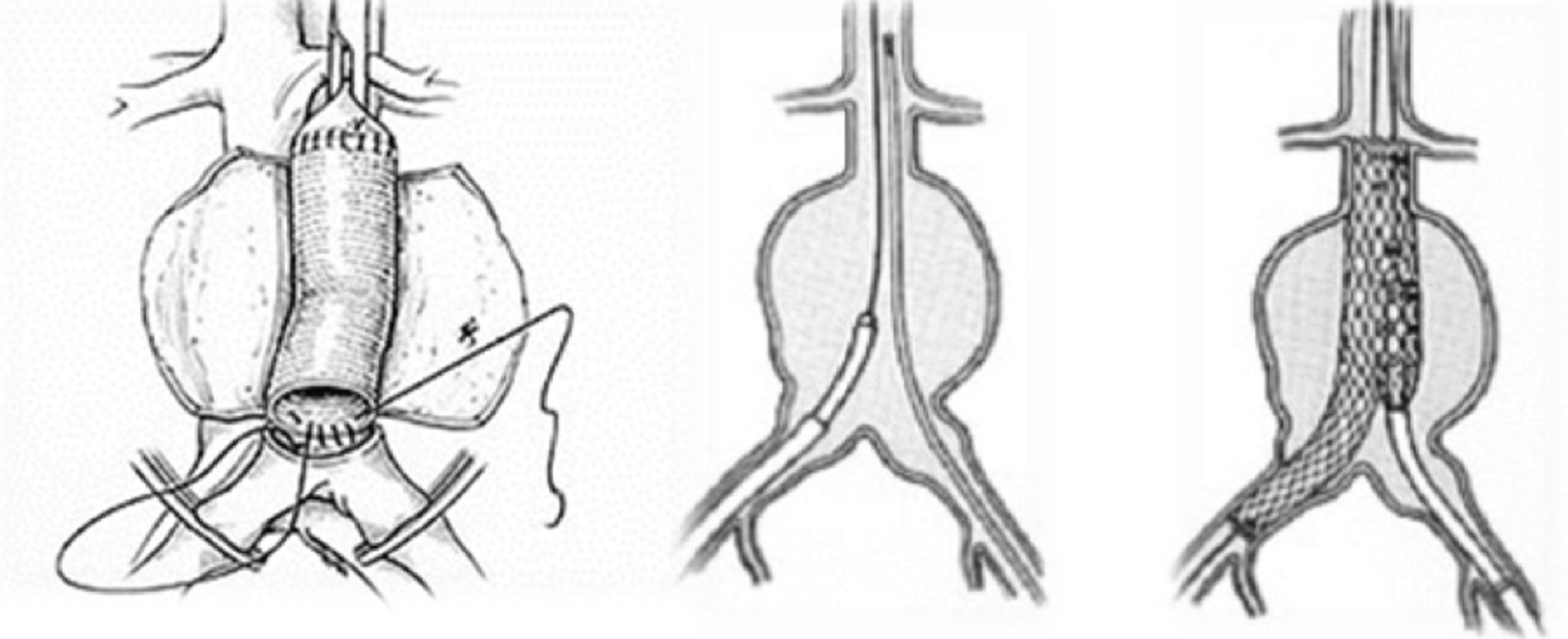
Urgent CT angiography is necessary to confirm the diagnosis of ruptured aneurysm and plan the feasibility of endovascular repair. In general, aneurysm neck diameter, length and angulation should be ≤ 3.4 cm, ≥ 10 mm and < 60°, respectively. Catheters are used to deliver the endograft through the femoral arteries, so that tortuous, very narrow or calcified femoral arteries are additional contraindications to endovascular repair. The femoral arteries are accessed via small incisions in the groin and, under imaging control, the endograft is delivered, positioned and secured in place (see Figure 1). This procedure requires specialist imaging equipment and the presence of a CT radiographer as part of the endovascular team. The configuration of the endograft is either bifurcated or aorto-uni-iliac. When the latter endografts are used, the contralateral iliac artery is occluded and a femoro-femoral crossover graft is used to supply blood to the contralateral limb. Endovascular repair can be conducted under local or regional anaesthesia, although general anaesthesia often is used and becomes necessary for most femoro-femoral crossover grafts. Some patients may not need to be transferred to a critical care bed and, in general, recovery is faster after endovascular repair than after open surgical repair. However, in contrast with open repair, continued surveillance of the endograft is necessary and reinterventions for endoleaks and other problems are not uncommon. This is likely to require at least two additional CT scans in the year following endovascular repair.
When this trial was first discussed, observational studies (synthesised in systematic reviews11–14) were reporting much lower 30-day mortality for endovascular repair of ruptured AAAs and some such studies11 have suggested that this should become the new gold standard treatment of ruptured aneurysms without the need for a randomised trial. In addition, the in-hospital and 1-year costs of treating ruptured aneurysms by endovascular repair may be up to 40% lower than for treatment by open repair. 15 However, a single centre pilot randomised trial16 with 32 participants, carried out in Nottingham, UK, showed a 30-day mortality of > 50% for participants treated with either endovascular aneurysm repair (EVAR) or open repair.
By 2004, randomised trials had shown the advantage of EVAR for elective repairs through reducing 30-day operative mortality threefold compared with open repair,3,17 and endovascular technology, no longer confined to academic centres, was widely available and preferred by patients. It remained an open question whether or not endovascular repair would have the same impact on reducing mortality from ruptured AAAs. Importantly, it was considered that only ≈55% of patients with a ruptured AAA would be anatomically suitable for endovascular repair, so that, for many patients, endovascular repair would not be an option. 14
Therefore, the design of any randomised trials was a question of considerable debate. In the Netherlands, a small randomised trial18 in the Amsterdam region started in 2007. This trial considered only patients with a ruptured AAA of relative haemodynamic stability after CT had demonstrated the anatomical feasibility of EVAR and then randomised these participants to either EVAR or open repair. 18 The primary outcome measure of this trial was combined mortality and morbidity at 30 days and, with optimistic power calculations, it was planned to randomise just 80 participants. Later, in 2010, the trial was extended to include 120 participants; the results were finally reported in late 2013. 19 A trial20 with a similar design started in France in 2008, with the aim of recruiting 160 participants, but it used the rather weak methodology of randomising participants by week of the year. The French trial recruited 107 participants and reported the early outcomes in late 2014. Both the Dutch trial and the French trial had a selective recruitment policy. In contrast, the Immediate Management of the Patient with Rupture: Open Versus Endovascular repair (IMPROVE) trial,21 the last European trial to start, larger than all of the other trials combined, had a non-selective recruitment policy for participants with the clinical diagnosis of ruptured aneurysm, so that health policy implications could be addressed.
Objectives of the IMPROVE trial
In participants with a clinical diagnosis of ruptured AAA who were randomised to either a strategy of endovascular repair (if feasible, with open repair when not feasible) or to a strategy of open repair:
-
Assess whether or not the endovascular strategy was associated with a lower 30-day and mid-term mortality (the primary outcome measure).
-
Determine the proportion of participants in each group who were discharged directly to home (disposal).
-
Assess whether or not the endovascular strategy was associated with lower 30-day costs.
-
Determine the proportion of participants who were suitable for endovascular repair and operational barriers to endovascular repair.
-
Assess whether or not an endovascular strategy was associated with improved 1-year survival and quality of life (QoL) and was cost-effective.
-
Identify subgroups of participants who derived greater benefit from endovascular repair.
The trial was initially funded by a Health Technology Assessment Emergency and Trauma Care initiative. As the trial progressed, it was extended to include three further objectives:
-
Assess whether or not an endovascular strategy was associated with improved 3-year survival and QoL and was cost-effective.
-
Conduct an individual patient meta-analysis of participants randomised in all of the European randomised trials [Amsterdam Acute Aneurysm trial (AJAX),19 Endovasculaire versus Chirurgie dans les Anévrysmes Rompus (ECAR)22 and IMPROVE23], with respect to common 30-day and 1-year outcomes.
-
Develop a risk score for 48-hour mortality for patients with a ruptured AAA.
In addition, the resources and information gathered during the trial have stimulated associated projects including the development of best practice guidelines24 for the transfer of patients and the influence of aortic morphology on mortality and reinterventions, as well as several collaborative projects.
Chapter 2 Methods
The methods for the IMPROVE trial, given in this chapter, are reproduced, in part, from published work. 23 © The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com.
Trial design
The IMPROVE trial was a multicentre randomised trial that randomised patients with a clinical diagnosis of ruptured AAA to either an endovascular strategy of immediate CT and emergency EVAR, with open repair for those who were anatomically unsuitable for EVAR (endovascular strategy group), or to the standard treatment of emergency open repair (open repair group). This trial was conducted in 30 eligible centres (29 in the UK and one in Canada). The eligibility of each centre to participate in the trial was determined by its clinical credentials, including audited volumes of elective EVAR of > 20 cases per year out of ≥ 50 cases of aortic surgery, evidence of good interdisciplinary teamworking, team availability for ≥ 66% of the week, rapid access to emergency CT (target of 20 minutes) and audited experience of emergency EVAR (five or more cases). The trial protocol and the guidelines for fluid management, anaesthesia and management of abdominal compartment syndrome as well as statistical analysis plans are available at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/ (accessed 16 November 2017).
All patients aged > 50 years with a clinical diagnosis of ruptured AAA or ruptured aortoiliac aneurysm, diagnosed by a senior trial hospital clinician (either in emergency medicine or vascular surgery), were recorded and were eligible for inclusion. The first brief consent process could be written, verbal or from a relative and, if necessary, in England only, from a non-treating physician, using the Mental Capacity Act 2005. 25 Participants were re-consented for continued participation in the trial during the recovery period.
Patients were excluded if they had had a previous aneurysm repair or a current rupture of an isolated internal iliac aneurysm, aorto-caval or aorto-enteric fistulae, a recent anatomical assessment of the aorta (e.g. awaiting elective EVAR), a connective tissue disorder or if intervention was considered futile (patient moribund).
Randomisation
An independent contractor (Sealed Envelope Ltd, London, UK) provided telephone randomisation, with computer-generated assignation of participants in a 1 : 1 ratio, using variable block size and stratified by centre. The date and time of randomisation, together with type of initial consent (written/verbal/other), were recorded automatically. The randomisation was communicated by e-mail to the trial manager, site principal investigator and trial co-ordinator. Participants were randomised either to an endovascular strategy (immediate CT followed by EVAR if locally determined as anatomically suitable and open repair when not suitable) or to immediate open repair, with CT being optional. Because this was a surgical trial, neither investigators nor participants could be masked to the treatment allocation. Adherence to the allocated treatment group, wherever possible, was reinforced by on-site training and newsletters.
Recruitment and progress
The first participant was randomised into the trial in September 2009; recruitment was initially slow because of the regulatory difficulties in opening centres. By the end of the recruitment phase, 29 centres had been opened in the UK and one had been opened in Canada, but some of the UK sites were subsequently closed by mergers and the creation of larger vascular centres. Recruitment closed in July 2013 after 613 eligible participants had been randomised, 316 to the endovascular strategy and 297 to open repair. Full details of the progress of the trial are given in Appendix 1.
Data verification, role of the computerised tomography core laboratory in the diagnosis of aneurysm rupture, symptomatic unruptured aneurysm or other cause of admission
All consent forms were audited, and source data for a minimum of 15% of participants at each centre were verified. Admission CT scans were sent for analysis in the trial core laboratory (St George’s Hospital, London) and were subject to expert review for the presence of rupture. Aneurysm rupture was defined in accordance with the protocol. Briefly, evidence on CT scans of the presence of blood or haematoma outside the aneurysm wall (abdominal aorta and/or common iliac artery) constituted a diagnosis of aneurysm rupture. If no CT scan was available, the diagnosis of rupture was made intraoperatively. In those participants who had not undergone CT or laparotomy, diagnosis was deduced from the underlying cause of death. All participants who were randomised in the UK were registered to obtain automatic reporting of the date and cause of death from the Office for National Statistics (via NHS Digital).
Participants who were admitted with symptoms attributable to an AAA but in whom there was no robust evidence of aortoiliac rupture (core laboratory diagnosis or laparotomy findings), and who underwent repair semielectively during the same admission, were categorised as having ‘symptomatic, non-ruptured aneurysm’. Other participants had primary hospital discharge diagnoses that were unrelated to AAAs.
The core laboratory also measured detailed aneurysm morphology in accordance with the method previously described. 26 The repeatability of the key variables of maximum aortic diameter, neck diameter, neck length, neck conicality, proximal and distal neck angles and maximum common iliac diameter was assessed using Bland–Altman methods. 27
Participant follow-up
Participants were followed up closely through their primary hospital admission, and the following were reported: stays in intensive therapy units and other high-dependency units and readmissions to theatre with types of complications and adverse events. Participants also were followed up at 3, 12 and 36 months with questionnaires for QoL [EuroQol-5 Dimensions (EQ-5D)] and use of health resources, in addition to clinical follow-up for aneurysm-related complications and reinterventions. Between the primary hospital discharge and 3 years, participants were followed up for aneurysm-related reinterventions both prospectively and by annual audit of hospital notes, with a final QoL questionnaire on trial exit at 36 months. Mortality reporting, including the cause, for all UK participants was from the Office for National Statistics (via NHS Digital) and was from local state sources for Canadian participants. All of the case report forms (CRFs) are provided at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/, accessed 16 November 2017. In addition, because > 90% of participants were recruited from England, Hospital Episode Statistics (HES) data were used to cross-check reinterventions and identify those taking place at non-trial centres for the 12- and 36-month analyses.
The IMPROVE trial approvals
Ethics approval for the participation of patients in England and Wales was from the South Central Berkshire Research Ethics Committee (08/H0505/173), in Scotland from the Scotland A Research Ethics Committee 08/MRE00/90 and in Canada from the University of Western Ontario Health Sciences Research Ethics Board (17698). Approval for the use of routine NHS data for participants lost to follow-up in England and Wales was obtained from the National Information Governance Board [Ethics and Confidentiality Committee (ECC) 4-03 (f) 2012].
Trial outcomes
The primary outcome measure was survival at 30 days after randomisation. The trial, comparing the groups as randomised, had 94% power to detect (as significant at 5%) a difference in 30-day mortality of 14% with 600 participants enrolled. This was based on estimated 30-day mortalities of 47% and 21% for participants receiving open repair and EVAR, respectively,4,14 an estimate of 55% of participants being anatomically suitable for EVAR after CT and an estimate that 5% of both randomised groups would not have a proven diagnosis of ruptured AAA and no 30-day mortality. 16 Hence, the estimated 30-day mortality was 44.7% in the open repair group [(0.95 × 47) + (0.05 × 0)] and 30.4% in the endovascular strategy group [(0.55 × 21) + (0.4 × 47) + (0.05 × 0)].
Early secondary outcome measures included 24-hour mortality, in-hospital mortality, costs of primary admission, number of reinterventions, time of discharge and the place to which the participant was discharged from the trial hospital after the primary admission. Details of specialist care bed-days, organ support and reinterventions were recorded primarily to inform costs. In the first 30 days, reinterventions were categorised as rebleeding, limb ischaemia, mesenteric ischaemia, abdominal compartment syndrome or other, with details of others being provided as free text. Complications such as strokes or myocardial infarction were not recorded separately from the need for specialist stroke or coronary care unit bed-days.
The unit costs of the stents and consumables used for rupture repair were taken from manufacturers’ list prices and published sources (see Appendix 2, Table 28). Salary costs for rupture repair were calculated by combining staffing levels reported from a survey of 10 IMPROVE trial centres (see Appendix 2, Table 29) with published staffing costs. The costs per critical care bed-day by Healthcare Resource Group were taken from the Payment by Results database. 28 Unit costs for outpatient visits and community service use were obtained from a recommended source for health and social care costs. 29
In addition, the impact of aortic morphology on reinterventions and mortality has been assessed. To optimise generalisability, most of these listed outcomes have also been assessed in an individual patient data meta-analysis that was conducted across all three recent European trials (see Individual patient data meta-analysis of three European ruptured aneurysm trials).
Longer-term outcomes measures (at 1 and 3 years) took a health economic perspective, in addition to measuring mortality and reinterventions. QoL, using the EQ-5D, was reported at 3, 12 and 36 months after randomisation, and the costs of aneurysm-related care were evaluated to 3 years. This enabled cost-effectiveness evaluations at 1 and 3 years. The detailed methods for the health economic and other analyses are encompassed within their statistical analysis plans (www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/; accessed 16 November 2017) and are detailed in Chapter 5.
Oversight of the trial
The Trial Steering Committee, chaired by Professor Ian Roberts (members listed in Appendix 1), was responsible for ensuring that adequate recruitment strategies were in place and that the ethics framework for conducting this emergency surgery trial was adhered to.
An independent Data Monitoring Committee, chaired by Professor Charles Warlow (members listed in Appendix 1), reviewed the data, with interim analyses carried out after the enrolment of 50, 200 and 400 participants, and agreed that it was safe to continue the trial. The statistician on this committee was the responsible statistician for the Dutch ruptured aneurysm trial (AJAX). 19
The Trial Management Committee, chaired by Professor Janet T Powell (members listed in Appendix 1), took responsibility for the day-to-day running and timely reporting of the trial. The progress of the trial and recruitment information are detailed in Appendix 1.
Patient and public involvement in the trial
Before the trial was designed, a group of seven patients was consulted. Their viewpoints were incorporated into the trial design (e.g. place of discharge from primary admission) and can be found in the published supplement to the 1-year outcomes paper. 23
The Trial Steering Committee included Anne Cheetham, the wife of a patient who survived an open repair of a ruptured AAA after a stormy course. She also is active in the Circulation Foundation (a peripheral arterial disease charity).
At the end of the trial, a small group of patients and members of the public (associated with the Circulation Foundation) were consulted about their views on the seriousness and fear of reinterventions that may become necessary after the original aneurysm repair. Their view of the severity of reinterventions (detailed at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/; accessed 16 November 2017) was different from that of clinicians. These views of the patients and members of the public have been incorporated into the 3-year analysis results, in which reinterventions are also reported according to the views about the most severe reinterventions. Further research on the development and use of metrics that are important to patients is suggested in Chapter 9.
A patient focus group (run by Professor Matt Bown in Leicester) and Anne Cheetham provided input for the plain English summary of the trial.
Timelines for reporting of IMPROVE trial outcomes
The reporting of IMPROVE trial outcomes took place in three phases: (1) 30-day outcomes, (2) 1-year outcomes and (3) 3-year outcomes. The 3-year outcomes analysis was delayed by 4/5 months because of changes in application procedures at NHS Digital (the provider of both mortality reporting and HES data).
Prespecified statistical analysis plans
For each analysis conducted, a prespecified analysis plan was prepared and approved by the Trial Management Committee and by the Data Monitoring and Ethics Committee for the main 30-day, 1- and 3-year analyses. The analysis plans were then placed on the trial websites (www.Improvetrial.org and www.imperial.ac.uk/medicine/improvetrial). These analysis plans, together with full details of the different analyses, are provided at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/ (accessed 16 November 2017).
All analyses, except the causal analyses, were intention-to-treat analyses. The primary outcome measures assessed the proportion of participants surviving for 30 days in each of the randomised groups, following an intention-to-treat policy using a Pearson’s chi-squared test without continuity correction. The primary outcome measure was then adjusted for age, sex and Hardman index using logistic regression (with the age and Hardman index considered as continuous), providing an adjusted odds ratio (OR) (Hardman index is a validated risk scoring system for ruptured aneurysms). 30,31 Missing baseline data were multiply imputed using chained equations to increase the precision of the estimates:32 the variables used for imputation are provided at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/ (accessed 16 November 2017). Sensitivity analyses were undertaken: (1) including centre as a random effect in a generalised linear mixed model and (2) restricting analysis to participants with a confirmed diagnosis of rupture only. A complier average causal effects model was also fitted to obtain an unbiased estimate of the potential policy effect if participants had adhered to trial allocation. 33 Specifically, participants who were randomised to the endovascular strategy but who were subsequently found to be not anatomically suitable and thus were treated by open repair were considered to have adhered to trial allocation. Otherwise, reasons for crossover were classified as non-adherence (see www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/, accessed 16 November 2017, for further details). Prespecified subgroup analyses were conducted for all outcomes (age, sex and Hardman index).
Secondary end-point analyses were undertaken to assess time to in-hospital mortality and time to discharge using competing risks methodology, with in-hospital mortality and discharge as the two competing risks. Gray’s non-parametric test was used to compare cumulative incidence curves. 34 Hospital costs were also evaluated, with full details of the methods provided at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/ (accessed 16 November 2017).
A causal analysis in the ruptured AAA population only was conducted to determine the effect of an endovascular strategy compared with open repair in this population if all participants had adhered to the IMPROVE trial policy design (i.e. a CT scan plus EVAR if found anatomically suitable compared with open repair). The causal analysis estimates the effect of the interventions in participants, as randomised, on the primary outcome (30-day mortality) in a complier population. 33 This provides an unbiased estimate of the true treatment effect, subject to certain modelling assumptions,35 which would not be possible with a per-protocol analysis.
For the causal analyses, participants who were randomised to the endovascular strategy who were found to be not anatomically suitable and underwent open repair were classified as having adhered to randomisation. Failure to receive the allocated treatment for any other reason was classified as non-adherence. Participants who had no operation (died before repair) were excluded from the analysis under the assumption that their outcome would be the same no matter what group they were randomised to. Participants who had a converted operation (EVAR converted to open repair) in the group randomised to the endovascular strategy, and who were anatomically suitable, were considered to have adhered to randomisation. Participants who had a converted operation after randomisation to open repair were classified as non-adherent.
Subsequent analyses of mortality and reinterventions at 1 and 3 years followed a similar pattern but included analysis of AAA-related mortality, reporting of other causes of death and full health economic evaluations. The reinterventions at these later time points were categorised as arterial, laparotomy related or other and according to their severity as perceived by (1) clinicians and (2) patients and members of the public (details of the coding of 30-day reinterventions and later reinterventions are provided at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/; accessed 16 November 2017).
The cost analysis took a UK NHS and Personal Social Services perspective36 and included costs up to 1 or 3 years after randomisation, for all randomised participants. Resource use measures were taken from the IMPROVE trial CRFs for the initial hospital admission, readmissions and outpatient visits to the study hospitals that were related to ruptured AAAs. Other outpatient visits and community service use (visits to the family doctor and home nursing) were taken from responses to a health services questionnaire administered to surviving ruptured AAA participants at 3 and 12 months after randomisation. The specific resource use categories included were (1) medical devices and consumables for each intervention (see Appendix 2, Table 28), (2) length of hospital stay during the primary admission, including critical and specialist unit bed-days and extent of organ support, (3) all reinterventions during the primary admission, whether or not directly associated with the ruptured aneurysm (including time in the operating theatre or endovascular suite), and use of devices and consumables, (4) readmissions related to the ruptured aneurysm (a sensitivity analysis was undertaken including all readmissions) and (5) outpatient and community services whether their use was related to the ruptured aneurysm or other conditions. The unit costs of the stents and consumables used for rupture repair were taken from manufacturers’ list prices and published sources (see Appendix 2, Table 28). Salary costs for rupture repair were calculated by combining staffing levels reported from a survey of 10 IMPROVE trial centres (see Appendix 2, Table 29) with published staffing costs.
Health-related QoL (HRQoL) was measured using a generic measure, the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), which requires patients to describe their health on five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension requires patients to state whether they have ‘no problems’ ‘some problems’ or ‘severe problems’. A participant’s described health at each time point was valued in accordance with health state preferences from the general population to calculate EQ-5D-3L utility scores, which are anchored on a scale from 0 (death) to 1 (perfect health). 37 Quality-adjusted life-years (QALYs) were assessed at 3 months, 1 year and 3 years. For survivors at 3 months, QALYs were calculated using the EQ-5D-3L scores at 3 months, assuming a EQ-5D score of 0 at randomisation and a linear interpolation between randomisation and 3 months. This implies that, at day 30, the EQ-5D utility score is approximately one-third of that at 3 months. For decedents between randomisation and 3 months, we assumed zero QALYs. For those surviving up to 12 months, we assumed a linear interpolation, using the EQ-5D-3L scores at 3 months and 12 months. For decedents between 3 months and 12 months for which a EQ-5D-3L score at 3 months was available, a linear interpolation was applied between EQ-5D-3L at 3 months and the date of death, at which point a EQ-5D-3L score of 0 was applied. A similar approach was used for decedents between 1 and 3 years.
The mean differences in 1- or 3-year QALYs and costs [measured in Great British pounds (GBP) and converted into US dollars (USD)]38 between the endovascular strategy and the open repair strategy were reported. The differences in mean costs and QALYs were plotted on the cost-effectiveness plane and were used to calculate incremental net benefits (INBs) by valuing the incremental (difference in mean) QALYs at recommended thresholds of willingness to pay for a QALY gain36 and subtracting from this the incremental costs.
Cost-effectiveness results were also reported for the prespecified subgroups (according to age, sex and Hardman index). Missing data on baseline covariates (Hardman index), QoL and costs were addressed with multiple imputation, using the same variables as the clinical analyses32 (full details at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/; accessed 16 November 2017). Sensitivity analyses were undertaken to assess whether or not the cost-effectiveness results were robust to alternative assumptions (rationale and details in Chapter 5). Additional work concerning methods and assumptions for missing QoL data is discussed in Chapter 8.
Total costs at 1 year and 3 years were calculated by combining the resource use with unit costs at 2012 and 2016 prices, respectively (in GBP), and then converting them to USD. 38 This conversion rate was based on purchasing power parities, which avoided the impact of short-term currency fluctuations, and recognised the relative purchasing power of the USA compared with the UK in 2012 and 2016. In the base case, incremental costs were reported as unadjusted mean differences between randomised groups, together with 95% confidence intervals (CIs).
The differences in average costs and QALYs between the randomised groups were used to calculate the incremental net monetary benefits (INMBs). We valued the incremental QALYs according to threshold of willingness to pay for a QALY gain recommended by the National Institute for Health and Care Excellence (NICE) (between £20,000 and £30,000 per QALY),36 and subtracted from this the incremental cost. INBs were reported overall, and for the same prespecified subgroups as for the clinical end points.
The uncertainty around the differences in average costs and QALYs between the randomised groups is illustrated in the cost-effectiveness plane. We estimated the incremental costs and QALYs with a seemingly unrelated regression model. 39 To express the uncertainty in the estimation of the incremental costs and QALYs, we used the estimates of the means, variances and the covariance from the regression model to generate 500 estimates of incremental costs and QALYs from the joint distribution of these end points, assuming asymptotic normality. We then plotted these incremental costs and QALYs in the cost-effectiveness plane. We also reported cost-effectiveness acceptability curves, by calculating the probability that, compared with open repair, the endovascular strategy is cost-effective at alternative levels of willingness to pay for a QALY gain. Sensitivity analyses were conducted, varying the base-case assumptions, including costs of the devices, use of additional theatre staff, for participants with a proven ruptured AAA only and including hospital readmissions for all causes taken from the Health Resources Questionnaire (available at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/; accessed 16 November 2017).
The rationale for the sensitivity analyses and details of resource use, multiple imputation and handling of missing data are described in Chapter 5.
Individual patient data meta-analysis of three European ruptured aneurysm trials
The three recent European trials20,40,41 for the management of ruptured AAAs have published their methods and results. AJAX19 included only participants in whom CT revealed both probable rupture and aortic anatomy suitable for endovascular repair using aorto-uni-iliac stent grafts and all participants had to be eligible for both open and endovascular repair. AJAX randomised 116 participants in three centres between 2004 and 2011, using sealed envelopes for randomisation to either open or endovascular repair. The ECAR trial22 had a similar selection methodology and design but used the additional criterion for haemodynamic stability of a systolic blood pressure of > 80 mmHg and used both aorto-uni-iliac and bifurcated stent grafts. The ECAR trial randomised 107 participants, with treatment allocation by weekly rotation to either open or endovascular repair, in 14 centres between 2008 and 2012. In contrast, the IMPROVE trial randomised participants with an in-hospital clinical diagnosis of ruptured aneurysm, before CT, either to an endovascular strategy (with open repair if endovascular repair was not anatomically feasible) or to open repair. The IMPROVE trial randomised 613 participants in 30 centres between 2009 and 2013.
The three data sets were merged, based on the largest trial (IMPROVE), enabling estimation of Hardman index scores for all participants.
Statistical analysis
The primary analyses considered mortality according to the groups, as randomised, within each trial, irrespective of the different trial designs. The timing of death was assessed from randomisation (for the IMPROVE trial) and from hospital admission (for the AJAX19 and ECAR22 trials). Logistic regression analysis was used to estimate the OR of both 30- and 90-day mortality for endovascular repair (or endovascular strategy) compared with open repair, adjusting for trial to obtain a one-stage fixed-effect pooled estimate. Further analysis was conducted by estimating the OR separately for each trial and then pooling using random-effects meta-analysis, estimating between-study heterogeneity using a method of moments. 42 The proportion of between-trial variability beyond that expected by chance was quantified using the I2 statistic. 43 Analyses were then adjusted for age, sex and Hardman index score. 30,31 Multiple imputation using chained equations was used to account for missing baseline covariates in adjusted analyses. 32
Secondary analyses were conducted on a restricted subgroup of more homogeneous participants from the three trials. The restrictions imposed were (1) a confirmed diagnosis of rupture and (2) anatomical suitability for EVAR. EVAR suitability was an entry criterion for inclusion in both the AJAX19 and ECAR22 trials. For the IMPROVE trial, suitability for EVAR was defined as local CT assessment of suitability; if not assessed locally, a ‘within liberal instructions for use’ definition from a core laboratory CT analysis was used.
Kaplan–Meier survival plots and cumulative incidence of time to primary hospital discharge, by randomised group, were produced within each trial separately and were accompanied by a log-rank test. A Cox proportional hazards model was used to assess whether or not the randomised groups differed in terms of the cause-specific hazard of primary hospital discharge (competing in-hospital mortality notwithstanding).
The age, sex and Hardman index scores of the subgroups were assessed for differences in the effect of the endovascular and open strategies by including an interaction term between the subgroup and randomised group in a logistic regression model.
The reporting of reinterventions was very different across the three trials; all trials reported on the use of occlusion balloons and the incidence of the abdominal hypoperfusion syndromes (abdominal compartment syndrome and mesenteric and colonic ischaemia),44 although the abdominal compartment syndrome data for AJAX were collected retrospectively. The largest trial, IMPROVE, did not report complications such as myocardial infarction, whereas complications were reported for both the AJAX and ECAR trials.
In addition, AAA diameter, aortic neck diameter, aortic neck length and proximal neck α angulation (aortic morphology) were assessed within each trial for their effect on 30-day mortality. These analyses are not a comparison between randomised groups and, therefore, were adjusted for the following potential confounding factors: age, sex (the AJAX and IMPROVE trials), Hardman index, admission systolic blood pressure, mean arterial pressure, treatment commenced and randomised group (the IMPROVE trial only). Each of the other four morphological variables was analysed, both adjusted and unadjusted for the other three variables.
Development of a new risk score
The purpose of this work was to develop a novel, point-of-care, risk score based on both physiological and imaging data that are immediately available in the emergency department to identify patients with ruptured AAAs in whom aneurysm repair or transfer to a specialist centre for repair is futile. The definition of futile is death within 48 hours of presentation, either with or without aneurysm repair.
The principal outcome measure is mortality within 48 hours of randomisation. The secondary outcome measure is death within 30 days of randomisation.
Participants from the IMPROVE trial were used to develop the score, excluding participants with a final diagnosis other than ruptured AAA (incidental or symptomatic AAA) but including participants with aortoiliac ruptures. All participants with ruptured AAA or aortoiliac rupture were considered in the risk score regardless of whether or not an operation was carried out. These criteria resulted in 536 participants, before the exclusion of those with missing risk factor data (e.g. CT results were not available).
External validation of the risk score will be conducted in participants from the AJAX19 and ECAR22 randomised controlled trials (RCTs), the wider Amsterdam cohort for AJAX, which included those unsuitable for randomisation in AJAX, and data from Stockholm. There also will be internal validation of derived risk scores using 10-fold cross-validation.
Full details of the statistical analysis are provided at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/ (accessed 16 November 2017).
Other risk scores for ruptured AAAs have been proposed. From the available information in the IMPROVE, AJAX and ECAR trials, it may be possible to calculate these other risk scores. We will compare the discrimination (c-statistic) of the published risk scores, calculated in the IMPROVE, AJAX and ECAR trials, with the derived risk score. This will initially be done using 48-hour mortality as the outcome measure. However, as the published risk scores were derived from longer-term (in-hospital or 30-day) mortality, we will also compare the discrimination of these risk scores against our derived risk score using 30-day mortality as the outcome measure.
Chapter 3 Mortality and other 30-day outcomes in the IMPROVE trial and the other ruptured aneurysm trials
The first pilot randomised trial of endovascular repair compared with open repair of ruptured AAAs was conducted in Nottingham and randomised 32 unselected patients between September 2002 and December 2004. 45 The 30-day mortality was 53% in each group. This contrasted with results from observational studies, which indicated that the 30-day mortality following emergency EVAR for ruptured AAAs was much lower, at about 25%. 46,47 Since the time of this initial trial, experience with EVAR, devices and imaging have all improved. Moreover, observational studies continued to report similarly low 30-day mortality for EVAR and reported a 30-day mortality of closer to 50% for open repair. 14,48 Therefore, there was a clear need for larger multicentre randomised trials.
Design of the recent randomised trials
The first of the later randomised trials (AJAX)19 started in the Netherlands in 2004 and had a selective recruitment design. Participants with a suspected rupture underwent CT. Next, if rupture was observed and the aortic morphology was suitable for EVAR, relatively haemodynamically stable patients were approached for consent for randomisation (using sealed envelopes) to either EVAR or open repair. Just 116 out of a target of 120 participants were randomised between 2004 and 2011 at three centres. A French trial (ECAR)22 started in 2008, with even more selective patient recruitment: only haemodynamically stable patients (systolic blood pressure of > 80 mmHg) with aortic morphology suitable for EVAR were included and the randomisation was based on a weekly rotation of treatment (EVAR one week, open repair the next). This trial recruited 107 participants at 14 centres between 2008 and 2012. The IMPROVE trial was much larger, with a total target recruitment of 600 unselected patients (all-comers), randomised at the point of in-hospital clinical diagnosis, before CT, to confirm either the diagnosis or morphological suitability for EVAR. This trial randomised 613 participants between 2009 and 2013. The different trial designs are shown in Figure 2. The first results of 30-day mortality came from the Dutch trial (AJAX) in August 2013,14,19 after recruitment to the IMPROVE trial had closed on 21 July 2013. AJAX reported a low 30-day mortality for both randomised groups (21% for the EVAR group and 25% for the open repair group; p = 0.66); however, this was a secondary outcome measure. The primary outcome measure was 30-day mortality and major complications, which also did not differ significantly between the randomised groups. 19
FIGURE 2.
Comparison of designs for three European trials comparing endovascular and open repair for a ruptured AAA.

The IMPROVE trial
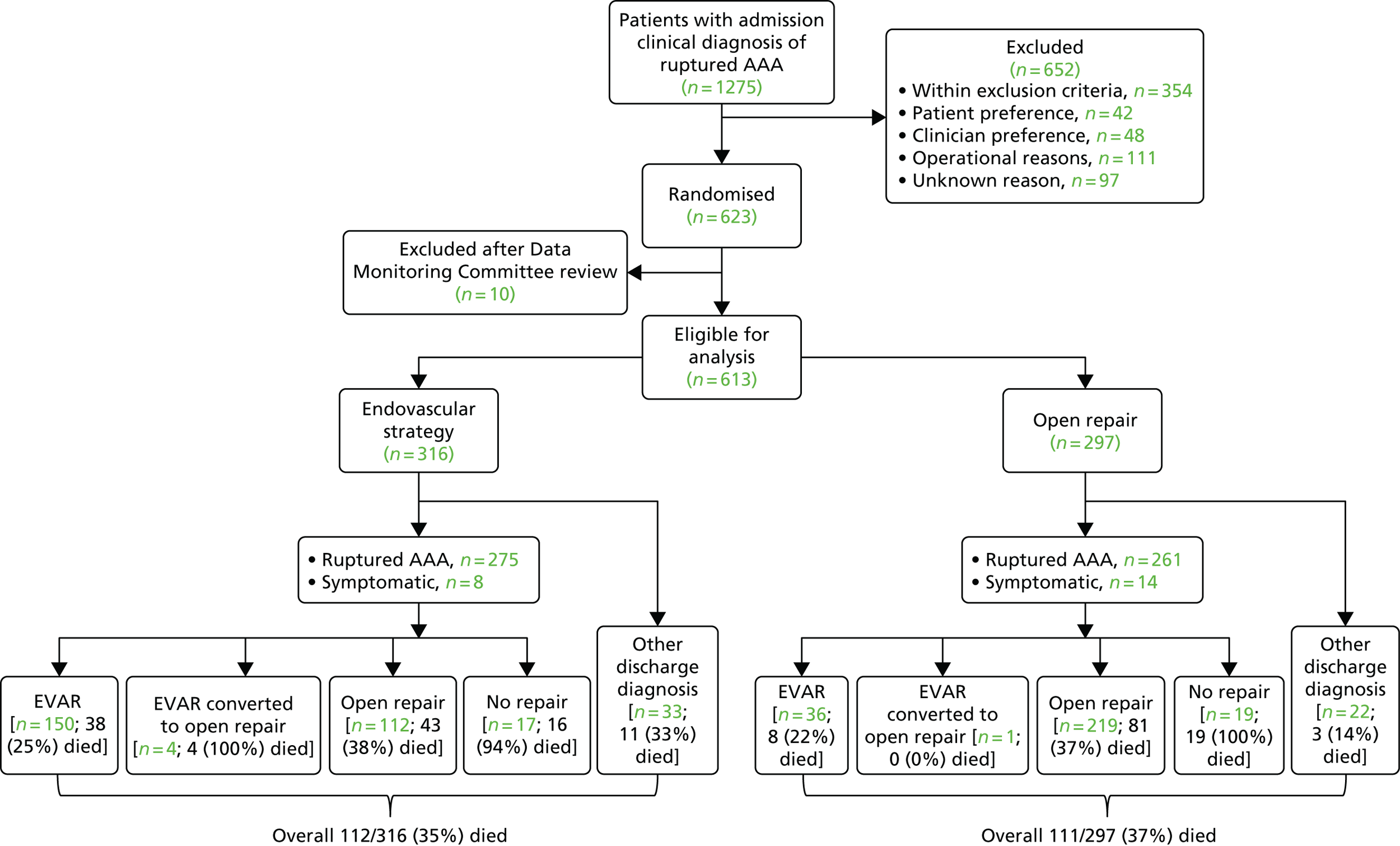
The 30-day outcomes for the IMPROVE trial were first presented at the Annual Scientific Meeting of the Vascular Society of Great Britain and Ireland at the end of November 2013, followed by publication by the IMPROVE trial investigators. 21 in January 2014. Between September 2009 and July 2013, 613 participants with an in-hospital diagnosis of ruptured AAA, made by a senior hospital clinician, were randomised either to EVAR (with the default of open repair when EVAR was not anatomically feasible; n = 316) or to open repair (n = 297). Randomisation, which was computer based, was carried out by an independent contractor with the assignation of participants in a 1 : 1 ratio using variable block sizes. These 613 participants constitute 48% of 1275 patients admitted at the trial hospitals with a diagnosis of ruptured AAA during the trial recruitment period. The recruitment rate and list of participating centres are shown in Appendix 1 and at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/, accessed 16 November 2017, respectively. A Consolidated Standards of Reporting Trials (CONSORT) diagram and the baseline characteristics of the randomised participants are given in Figure 3 and Table 1, respectively.
FIGURE 3.
A CONSORT diagram showing the flow of participants through the trial, with 30-day mortality for each group. Details of the post-randomisation exclusions (n = 10) are given in Appendix 1.
| Variable | Missing data (n) | Trial group | |
|---|---|---|---|
| Endovascular strategy (N = 316) | Open repair (N = 297) | ||
| Age (years), mean (SD) | 0 | 76.7 (7.4) | 76.7 (7.8) |
| Male sex, n/N (%) | 0 | 246/316 (78) | 234/297 (79) |
| Admission blood pressure (mmHg), mean (SD) | 12 | N = 306 | N = 295 |
| Systolic | 110.3 (32.9) | 110.4 (31.2) | |
| Diastolic | 65.3 (21.4) | 66.8 (22.5) | |
| Admission haemoglobin (g/dl), mean (SD) | 6 | 11.2 (2.5); N = 312 | 11.1 (2.3); N = 295 |
| Admission creatinine (µmol/l), median (IQR) | 13 | 117 (94–152); N = 312 | 115 (93–151); N = 288 |
| Acute myocardial ischaemia ECG, n/N (%) | 52 | 22/291 (8) | 23/270 (8) |
| Loss of consciousness, n/N (%) | 27 | 29/305 (10) | 21/281 (7) |
| Hardman index score,a n (%) | 74 | N = 282 | N = 257 |
| 0 | 93 (33) | 69 (27) | |
| 1 | 130 (46) | 126 (49) | |
| 2 | 46 (16) | 48 (19) | |
| 3 | 11 (4) | 12 (5) | |
| 4 | 2 (1) | 2 (1) | |
| 5 | 0 (0) | 0 (0) | |
| CT carried out, n/N (%) | 0 | 305/316 (97) | 266/297 (90) |
| Maximum aortic diameter (mm), mean (SD) | 86 | 84 (19); N = 263 | 81 (18); N = 264 |
| Time from randomisation to theatre admission | |||
| Ruptures (minutes), median (IQR) | 6 | 47 (28–73) | 37 (22–62) |
| Symptomatic aneurysms (hours), median (IQR) | 2 | 3.6 (3.1–15.6) | 3.0 (1.5–17.6) |
The diagnosis of ruptured aneurysm was confirmed either by CT or in surgery in 275 out of 316 participants (87%) in the endovascular strategy group and in 261 out of 297 participants (88%) in the open repair group. In the endovascular strategy group, 64% of participants (174/272) were considered suitable for EVAR after CT. A further eight participants (3%) in the endovascular strategy group and 14 participants (5%) in the open repair group had a repair of a symptomatic intact aneurysm in the same admission. The 55 participants (33 in the endovascular strategy group and 22 in the open repair group) with a final diagnosis unrelated to an AAA had a wide range of other primary diagnoses, varying from ruptured thoracic aortic aneurysm to urinary tract infection, but 45 of these participants (82%) had an asymptomatic AAA. One further participant had a thoracoabdominal aneurysm, and only 9 out of 613 participants (1.5%) did not have an aortic aneurysm.
The primary outcome measure was 30-day mortality. The overall 30-day mortality was 35.4% (112/316 participants) in the endovascular strategy group and 37.4% (111/297 participants) in the open repair group (OR 0.92, 95% CI 0.66 to 1.28; p = 0.62). However, in each group, some participants died before aneurysm repair or breached the randomisation allocation. In the endovascular repair group, 28 participants who were morphologically suitable for EVAR (26 with rupture and 2 without rupture) received emergency open repair for operational reasons (e.g. staff or endovascular suite not available or not yet available when the patient was deteriorating). In the open repair group, 33 participants with a rupture had emergency EVAR, mainly because the anaesthetist deemed them unsuitable for general anaesthesia, and a further three participants had delayed elective repair with EVAR. The detailed results are presented in Figure 3. Overall, 548 out of 613 participants (89%) adhered to the trial protocol. Among 502 participants with a ruptured aneurysm who received repair, the 30-day mortality was 32% (84/259 participants) in the endovascular strategy group and 36% (87/242 participants) in the open repair group (OR 0.86, 95% CI 0.59 to 1.24).
Limited subgroup analyses (age, sex and Hardman index) were also conducted for the primary outcome measure (30-day mortality by randomised group). There were no clear differences between the randomised groups according to age (above or below 77 years) or Hardman index (0, 1 or ≥ 2). However, there was weak evidence that the endovascular strategy was more effective in women than in men (p = 0.02). Among women, 30-day mortality was 37% (26/70 participants) in the endovascular strategy group and 57% (36/63 participants) in the open repair group, compared with 35% (86/246 participants) and 32% (75/234 participants), respectively, among men.
The secondary outcome measures at 30 days included 24-hour mortality, in-hospital mortality (Table 2), number of reinterventions, time and place of discharge, and costs. The first three of these outcomes were similar between the randomised groups and are reported in full by Powell et al. 21 In the endovascular strategy group, nearly all of the patients discharged (94%) within 30 days were discharged home, compared with only 77% in the open repair group (p < 0.001) (see Table 2). The mean resource use costs to 30 days was £13,433 [standard deviation (SD) £10,354] in the endovascular strategy group and £14,619 (SD £12,353) in the open repair group (mean difference –£1186, 95% CI –£2997 to £625) (see Appendix 2, Table 30).
| Discharge status | Trial group, n (%) | |
|---|---|---|
| Endovascular strategy (N = 316) | Open repair (N = 297) | |
| Discharged alive from trial hospital | ||
| Yes | 201 (64) | 183 (62) |
| No | 115 (36) | 114 (38) |
| Place of discharge | ||
| Home | 189 (94) | 141 (77) |
| Another hospital: routine bed | 7 (3) | 28 (15) |
| Another hospital: intensive care | 0 (0) | 1 (1) |
| Nursing home | 0 (0) | 3 (2) |
| Residential home | 1 (1) | 3 (2) |
| Sheltered accommodation | 1 (1) | 0 (0) |
| Other | 3 (1) | 7 (4) |
The mean use of critical care was 4.5 bed-days (SD 5.9 bed-days) in the endovascular strategy group, compared with 6.3 bed-days (SD 7.7 bed-days) in the open repair group. Postoperatively, renal replacement therapy was used in 78 participants: 32 in the endovascular strategy group and 46 in the open repair group, but only one participant, in the open repair group, was discharged on dialysis. Specialist coronary care was used in 11 participants (total of 38 bed-days), nine in the endovascular strategy group and two in the open repair group (total of 4 bed-days) within 30 days of randomisation; there was no additional coronary care between 30 and 90 days. A total of 28 participants in the endovascular strategy group and 47 participants in the open repair group were still in hospital 30 days after randomisation.
Individual patient meta-analysis of the three recent European trials
By 2015, four European trials had reported results, and searching the published literature and clinical trial databases did not reveal any further trials. The ECAR trial22 was presented to the European Society for Vascular Surgery in September 2014 and published results in 2015, with 30-day mortality rates (the primary outcome measure) similar to those reported in AJAX:19 19% for the EVAR trial group and 24% for the open repair group. Unfortunately, half of the data for the Nottingham trial16 had been lost, but the other three trials agreed to pool their data for an individual patient meta-analysis.
The baseline descriptions of the participants in the three trials are compared in Table 3. The IMPROVE trial had recruited older patients, less haemodynamically stable patients (lower admission blood pressure) and a higher proportion of women than the other two trials. There were other important differences too, with the AJAX and ECAR trials using predominantly aorto-uni-iliac configurations of endografts so that the procedure could not be completed under local anaesthesia, and the ECAR trial aggressively monitoring for abdominal hypoperfusion syndromes postoperatively (abdominal compartment syndrome and colonic and mesenteric ischaemia).
| Numbers, clinical details and baseline variables | Trial | ||
|---|---|---|---|
| AJAX (N = 116) | ECAR (N = 107) | IMPROVE (N = 613) | |
| Randomised group, n (%) | |||
| EVAR/endovascular strategy | 57 (49.1) | 56 (52.3) | 316 (51.5) |
| Open repair | 59 (50.9) | 51 (47.7) | 297 (48.5) |
| Ruptured AAA suitable for EVAR,a n (%) | N = 113 | N = 104 | N = 310 |
| EVAR/endovascular strategy | 57 (50.4) | 54 (51.9) | 168 (54.2) |
| Open repair | 56 (49.6) | 50 (48.1) | 142 (45.8) |
| Procedure started, n (%) | |||
| EVAR | 57 (49.1) | 56 (52.3) | 192 (31.3) |
| Aorto-uni-iliac | 49 (86) | 43 (77) | 39 (20) |
| Open repair | 59 (50.9) | 50 (46.7) | 331 (54.0) |
| Tube | 50 (88) | 26 (54) | 268 (81) |
| No aneurysm repair | 0 (0) | 1 (0.9) | 90 (14.7) |
| Age (years), mean (SD) | 74.2 (9.4) | 74.4 (10.6) | 76.7 (7.6) |
| Male sex, n (%) | 99 (85) | 97 (91) | 480 (78) |
| Maximum AAA diameter (cm), mean (SD) | 7.6 (1.6) | 7.7 (2.0) | 8.4 (1.9) |
| Aneurysm neck length (mm), median (IQR) | 25 (19–34) | 22 (16–32) | 22 (10–34) |
| Admission blood pressure (mmHg),b mean (SD) | 87 (27); N = 113 | 108 (30); N = 104 | 81 (24); N = 601 |
| Hardman index score, n (%) | N = 61 | N = 105 | N = 539 |
| 0 | 26 (43) | 41 (39.0) | 164 (30.4) |
| 1 | 19 (31) | 44 (41.9) | 254 (47.1) |
| 2 | 12 (20) | 11 (10.5) | 94 (17.4) |
| ≥ 3 | 4 (7) | 9 (8.6) | 27 (5.0) |
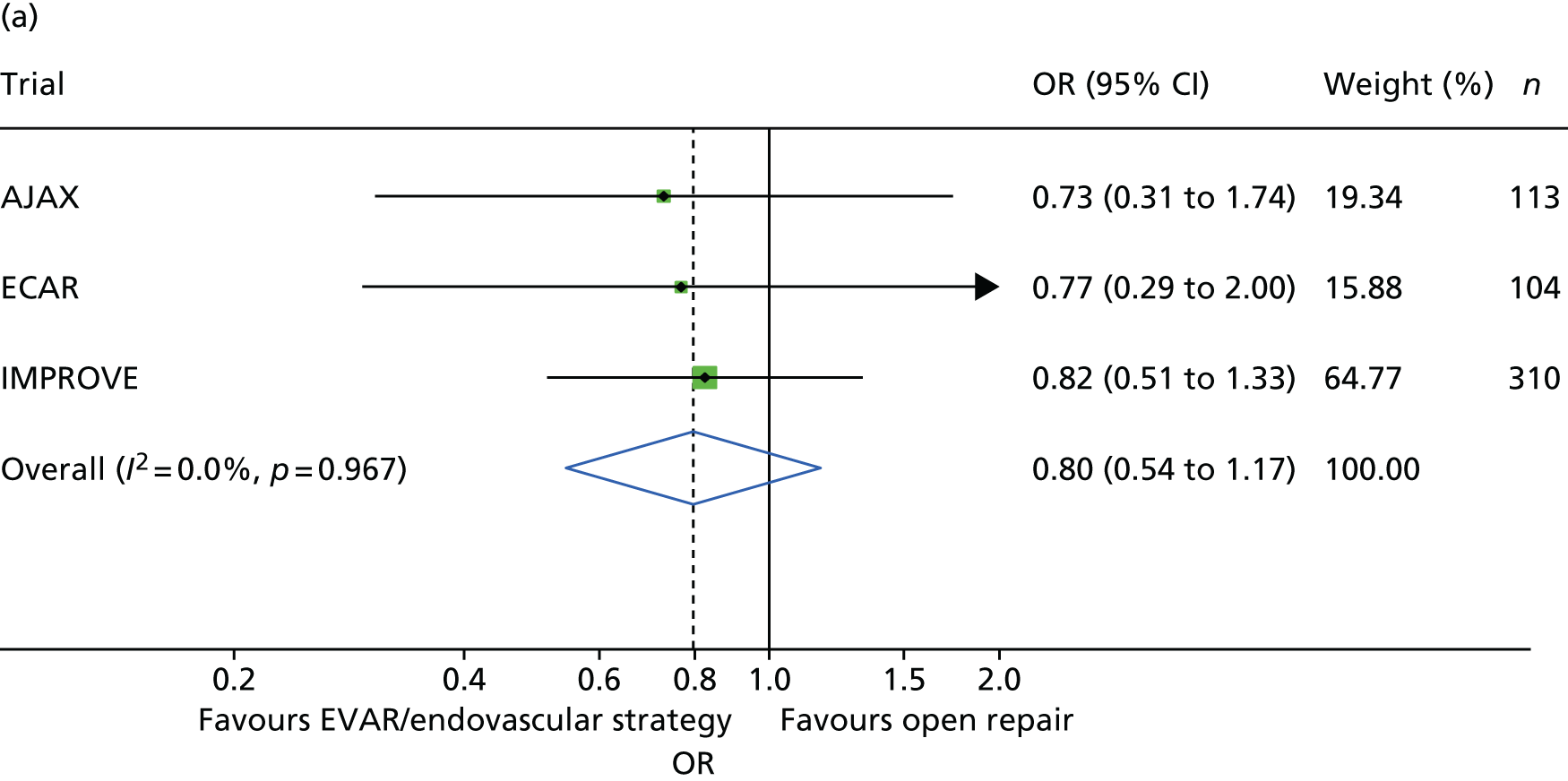
Survival to 30 days by trial and by randomised group is shown in Figure 4. Additionally, the Kaplan–Meier curves for restricted IMPROVE trial participants who were morphologically suitable for endovascular repair (and more similar to the participants in the AJAX and ECAR trials) are shown in Appendix 2, Figure 24. Mortality was higher for both randomised groups in the IMPROVE trial than in either the AJAX or ECAR trials. However, restricting the IMPROVE trial population to those patients who were morphologically suitable for EVAR reduced the mortality in both groups. When the results from the three trials were pooled in a meta-analysis, there was no evidence of heterogeneity (pooled OR 0.88, 95% CI 0.66 to 1.18) (Figure 5). When only patients with a ruptured aneurysm who were eligible for both EVAR and open repair were included (AJAX, n = 113; ECAR, n = 104; and IMPROVE, n = 310), the pooled OR reduced slightly (OR 0.80) but the CI widened (95% CI 0.54 to 1.17) (see Figure 5).
FIGURE 4.
The 30-day mortality by randomised group across the three recent European ruptured aneurysm trials.
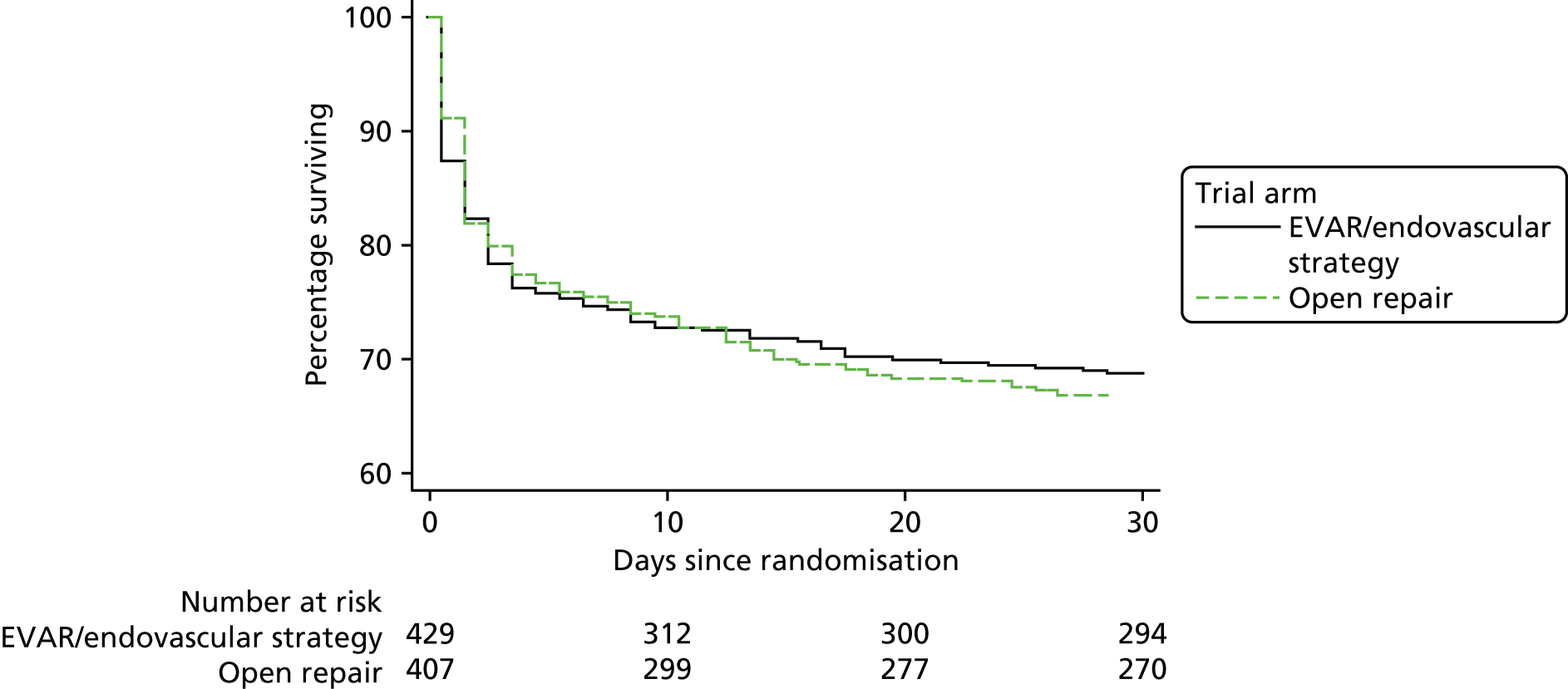
FIGURE 5.
The 30-day mortality by randomised group. (a) Restricted to 527 participants with a ruptured AAA who were eligible for both EVAR and open repair; and (b) 834 participants (two ECAR trial participants lost to follow-up before 30 days).

There was little evidence of treatment effect changing according to age or Hardman index. There were no early deaths among women who had EVAR in the ECAR trial. However, in the pooled analyses, women benefited more than men from an endovascular strategy (OR 0.47, 95% CI 0.23 to 0.97). For abdominal compartment syndrome, the highest rate was reported from the ECAR trial. Over the three trials, there was no significant difference in the incidence of abdominal compartment syndrome between the randomised groups, although the AJAX team is currently conducting per-protocol analyses. However, there was an indication that the incidence of colonic and mesenteric ischaemia was lower after EVAR or an endovascular strategy than after open repair (pooled ORs 0.57, 95% CI 0.32 to 1.01) (Table 4). A higher rate of ischaemic colitis after open repair than after endovascular repair has recently been reported in the analysis of a large US database. 49
| Trial | Trial group, n/N (%) | |
|---|---|---|
| EVAR/endovascular strategy | Open repair | |
| AJAX | ||
| ACS | 5/57 (9) | 2/59 (3) |
| CMI | 2/57 (4) | 5/59 (8) |
| ECAR | ||
| ACS | 8/56 (14) | 1/51 (2) |
| CMI | 4/56 (7) | 8/51 (16) |
| IMPROVEa | ||
| ACS | 14/259 (5.4) | 13/243 (5.3) |
| CMI | 14/259 (5.4) | 19/243 (7.8) |
The three trials were set in different health-care systems, leading to differences in discharge policies, including the use of ‘step-down’ care. For instance, many of the French ECAR trial participants were discharged to other facilities for convalescent care, whereas more participants in the IMPROVE trial were discharged directly to home. Over the three trials, for those participants discharged alive from the main trial hospital, the primary hospital stay was shorter for participants in the EVAR or endovascular strategy groups [pooled hazard ratio (HR) for discharge 1.24, 95% CI 1.04 to 1.47] (see Appendix 2, Figure 22).
The individual patient data meta-analyses have been reported with 90-day mortality50 as the primary outcome measure;51 again, there was no difference in mortality between the randomised groups, but EVAR appeared to be more effective in women than in men.
Discussion
The best evidence comes from the synthesis of evidence from randomised trials, and 30-day mortality is the standard surgical outcome measure. This best evidence, from the 836 participants from the three recent trials, shows that there is no significant difference in 30-day mortality between EVAR or an endovascular strategy and open repair (pooled OR 0.88, 95% CI 0.66 to 1.18), with no evidence of heterogeneity. The absolute differences between the trials, lowest mortality in the ECAR trial and highest mortality in the IMPROVE trial, probably relate to the selection criteria for participants in the three trials, AJAX and ECAR being highly selective and IMPROVE being unselective and ‘real world’. The influence of some of these selection criteria on 30-day mortality is explored and discussed in Chapter 4. The flexibility of individual patient meta-analysis also allowed only specific participant subsets to be included in the analysis. All trials included some participants in whom rupture was not confirmed, at laparotomy in the AJAX and ECAR trial and either at CT scan or at laparotomy in the IMPROVE trials. At 30 days, there was no evidence that EVAR offered a survival advantage in participants with a confirmed rupture who were eligible for both endovascular and open repair, although the pooled OR had reduced to 0.80.
One of the surprising findings from the IMPROVE trial was that there was weak evidence that an endovascular strategy was more effective in women than in men, partly attributable to the very high mortality after open repair in women. Despite the scant number of women in the other two trials, this finding was maintained on meta-analysis. This could suggest that EVAR should be a more common treatment option for women, although further research is needed. There was no clear effect of age.
These main findings from the trials have not been universally welcomed. The AJAX and ECAR trials have been dismissed as being too small and criticised for their use of aorto-uni-iliac endografts; certainly both trials took a long time to recruit not very many patients. The IMPROVE trial was more ‘real world’ but has been criticised for including participants who did not have a ruptured aneurysm or who died before repair could be accomplished. This serves to highlight the difference in time taken to start definitive repair with either EVAR or open repair. In each trial, the time taken to bring a participant to endovascular repair was significantly longer than for a participant to have open repair started; this difference was 0.48 hours, 1.6 hours and 0.16 hours for the AJAX, ECAR and IMPROVE trials, respectively. There is weak evidence showing that time to treatment influences survival adversely. 52,53 This might be have been a contributor to mortality in the EVAR groups of the AJAX and ECAR trials but, for the IMPROVE trial, the time difference was much shorter. Misdiagnosis might also contribute to the mortality rate in the EVAR group because diagnosis, even after CT, can be in doubt. 27 In both the AJAX and IMPROVE trials, other bleeding diagnoses were shown to be the cause of admission in a few participants at laparotomy, whereas, at endovascular repair, such diagnoses would have been missed. Since the inception of the trials and since publication of the trial results, there have been several observational studies to show how team training and protocols can enhance performance and reduce mortality. 54,55 This could include improved reading of CT scans and a reduction in the time to start EVAR. Team training could also limit misjudgement about attempting EVAR because, in the randomised trials, conversion to open repair was associated with 100% mortality. However, part of the reported reduction in mortality from team training might also have been achieved by different selection of patients for repair; few other studies report the number of untreated patients.
The appropriate time to subject new technologies to a randomised trial is controversial. The IDEAL (Idea, Development, Exploration, Assessment and Long-term follow-up) recommendations56 suggest that a trial should be considered when an intervention is sufficiently well evolved to warrant evaluation, but without the expectation that the intervention will continue to develop. Before the start of the IMPROVE trial, the uptake of EVAR for ruptured aneurysms was low and patchy and NICE recommended emergency EVAR for evaluation purposes only. 57 Therefore, the IMPROVE trial might have started when experience and team training for emergency EVAR was rather limited in some centres. However, with its widespread coverage of experienced EVAR centres that participated across the UK, the trial has acted as stimulus to evaluate the new technology. Data taken from HES for 2014/15 show that, in England, 36.4% of emergency aneurysm repairs in men and 31.7% in women were conducted using EVAR (Professor Jonathon Michaels, University of Sheffield, 2016, personal communication).
Other criticisms of the IMPROVE trial include the fact that we did not highlight the relatively low mortality in the participants who actually received EVAR58 and that, partly because of the improvements in intensive care medicine, there may be many ‘long-stayers’ and, therefore, 90-day outcome measures are more relevant than 30-day outcome measures. 59 Even after 90 days, there was no difference in mortality between the randomised groups in any of the three trials considered. 51 Surprisingly, few sources in the literature have highlighted another important outcome measure for patients: time to discharge and place of discharge. Regarding this outcome, there is a clear benefit from the EVAR/endovascular strategy, with times to discharge being much shorter than after open repair, which was observed in all three trials. These trials were set in different health-care systems and only the IMPROVE trial specifically reported discharge to home, which was much more common after the endovascular strategy (94%) than after open repair (77%).
Summary
Neither IMPROVE, the largest of the three recent trials, nor pooled data from the three trials identified that an endovascular strategy or EVAR led to lower 30-day mortality. At the time of rupture, the main clinical concern is to stop the bleeding and it is recognised that the initial repair, either endovascular or open, may need to be supported by further reinterventions beyond 30 days. For these reasons, longer-term follow-up is needed for all principal outcome measures, as well as patient QoL and cost-effectiveness, which were not assessed at 30 days, and to advise whether or not the benefits of an endovascular strategy for subgroups or secondary outcomes, observed in IMPROVE and the other trials, are maintained in the longer term.
Chapter 4 Factors other than the type of repair that influence 30-day survival of patients with a ruptured abdominal aortic aneurysm
The type of aneurysm repair, endovascular or open surgery, is not the only factor that influences the outcome for patients with a ruptured AAA. First, the patient may present at the emergency department of a hospital that does not offer emergency vascular surgery and a decision will be made as to whether or not the patient should be transferred to a specialist vascular centre. Then, the time taken for patients to be transferred to a vascular centre could be influential. 53,60 Next, there is evidence that operative mortality after rupture is lower in the larger-volume vascular centres10 and that, at least in England, operative mortality for all types of emergency surgery is lower if patients present within working hours. 61 Even larger-volume centres cannot usually offer sufficiently sized prospective case series in which to investigate other hospital, patient and clinical factors that may be associated with better or worse patient outcomes. To date, the largest patient series focused on endovascular repair reported on 473 patients who were treated between 1998 and 2011 from centres in two countries. 10 The IMPROVE trial recruited 613 eligible patients with a clinical diagnosis of abdominal aortic rupture between late 2009 and the summer of 2013. Some 536 participants had a confirmed aneurysm rupture, together with 22 urgent symptomatic aneurysms, making this the largest contemporary prospective cohort in which to investigate both the factors used for primary adjustment of 30-day mortality results (age, sex, Hardman index) and the role of factors, such as hospital presentation, time to surgery, fluid replacement therapy, aortic morphology and type of anaesthesia, on the outcomes of emergency surgery. As expected, Hardman index score was highly predictive of 30-day mortality, but mortality also increased with age; for each 5-year increase in age, the OR was 1.37 (95% CI 1.21 to 1.56) (Table 5).
| Variable | OR | 95% CI | p-value (z-test) |
|---|---|---|---|
| Age (per 5-year increase)a | 1.20 | 1.04 to 1.38 | 0.015 |
| Sex | |||
| Female | 1.00 | 0.45 to 1.14 | 0.162 |
| Male | 0.72 | ||
| Hardman index score (per 1-unit increase) | 1.62 | 1.26 to 2.08 | < 0.001 |
| Randomised group | |||
| Open repair | 1.00 | 0.62 to 1.28 | 0.535 |
| Endovascular strategy | 0.89 | ||
Transfer guidelines
At the beginning of the IMPROVE trial, we conducted a survey of the participating centres about the type of patient with a ruptured AAA who would accept for transfer from another hospital that did not offer emergency vascular surgery. It was clear that there was a wide disparity of opinion about suitable patients for transfer. This stimulated a Delphi consensus approach, working with the vascular surgeons, emergency medicine physicians and anaesthetists from the IMPROVE trial centres, as well as emergency medicine physicians and senior trainees across the NHS Wessex Clinical Strategic Network. Eventually, this generated best practice guidelines,24 endorsed by the Royal College of Emergency Medicine, the Vascular Society of Great Britain and Ireland and the Royal College of Radiologists. 62 These guidelines emphasise that there is no upper age limit for transferring patients and that all patients aged < 85 years, alert or with fluctuating consciousness, with moderate or minimal systemic disease and who need no or some help with daily living should be transferred, whereas those with cardiac arrest in current episode should not. Speed is accepted as important, and specialty trainees should be allowed to arrange transfer (target of ≤ 20 minutes of clinical diagnosis) if consultants are not on-site. CT confirmation of diagnosis is not necessary before transfer (although ultrasound assessment is desirable) and transfers should not be delayed by waiting for specific tests. A systolic blood pressure of ≥ 70 mmHg is sufficient for transfer without the need for intravenous fluids, unless deterioration occurs, in which case any fluids should be administered in small boluses only.
Use of the IMPROVE trial as a large observational series of patients with abdominal aortic aneurysm rupture
Before the IMPROVE trial had closed to randomisation, the possibility of using the patient series to investigate a number of hypotheses relating to other factors that might influence 30-day survival had been discussed and analysis plans had been formulated to address the following hypotheses:
-
Thirty-day mortality is lower in patients randomised during routine working hours than at other times.
-
Patients transferred from other hospitals to trial centres have lower Hardman index scores and better outcomes than those arriving primarily at the trial centre.
-
Lowest blood pressure is not associated with 30-day survival because overadministration of fluid and/or blood products is associated with higher mortality.
-
General anaesthesia for endovascular repair is associated with higher operative mortality than local anaesthesia.
-
Aortic morphology, particularly increasing aneurysm diameter and hostile aneurysm necks, is associated with high 30-day mortality.
All of these analyses focused on the 558 participants who had a confirmed diagnosis of either ruptured AAA or acute symptomatic AAA.
Time of presentation
Those participants who were randomised out of hours had higher 30-day mortality (144/362) than those randomised within routine working hours (65/196) (primary adjusted OR 1.47, 95% CI 1.00 to 2.17; p = 0.048). 63 However, there was little evidence that the efficacy of the endovascular strategy compared with open repair in those participants who were randomised out of hours was different from that in those randomised during routine working hours (test of interaction p = 0.100). This suggestion that mortality is higher among patients who are admitted out of hours is supported by data from the National Inpatient Sample in the USA. Among 5800 ruptured AAA participants, the in-hospital mortality was significantly higher among those participants admitted at weekends than among those admitted during the week (OR 1.32, 95% CI 1.13 to 1.55; p = 0.0004), and only 77% of those admitted at weekends underwent same-day repair, compared with 80% admitted during the week (p = 0.004). 52 Given the large sample size, Groves et al. 52 tried to identify factors contributing to the increased (32% higher) mortality at the weekend. It was not related to differential use of EVAR but, at weekends, participants received blood transfusion more often than during the week. Higher mortality among patients admitted at weekends may be a widespread phenomenon – it also has been reported in Italy64 and in Canada,65 where in-hospital mortality was 42% at weekends compared with 36% during the week – and might be attributable to the lower availability of specialised teams for treating patients or to staffing levels more generally.
Patient transfers: primary presentation compared with secondary presentation
The characteristics of participants with direct and secondary presentation to trial centres, including Hardman index scores, were similar, although a higher proportion of secondary presentation patients (221/335, 70%) were randomised out of hours. After adjustment for this, there was no difference in the 30-day mortality of those participants who were admitted directly to the trial centre and those (233 out of 335) transferred from other hospitals (adjusted OR 0.76, 95% CI 0.52 to 1.12). There are several potential explanations for this result, including the possibility that only lower-risk patients were transferred or that the highest-risk patients died during transfer. One recent study from the USA has suggested that 17% of patients die during transfer. 60
Preoperative variables: fluid administration and blood pressure
There was no evidence from IMPROVE trial participants that the volume of fluids administered before arrival in the operating theatre had a significant effect on postoperative mortality in a multivariable model. However, the volume of fluids given was recorded in only 302 participants. In contrast, there was a strong inverse association between the lowest in-hospital preoperative measured systolic blood pressure and mortality, with no significant association between this blood pressure measurement and the fluid volume administered. The association between the lowest systolic blood pressure and 30-day mortality showed no evidence of non-linearity [adjusted OR per 10-mmHg increase in blood pressure of 0.88 (95% CI 0.82 to 0.94)] (see Appendix 2, Figure 23). The 30-day mortality among participants whose blood pressure was above and below the threshold value of 70 mmHg was 34% and 51%, respectively.
General anaesthesia compared with local anaesthesia
Overall, among participants undergoing EVAR (n = 186), the 30-day mortality associated with procedures conducted under local anaesthesia only appeared to be substantially lower. After adjustment for age, sex, Hardman index, lowest systolic blood pressure and randomised group, local anaesthesia was associated with a fourfold reduction in 30-day mortality (adjusted OR 0.27, 95% CI 0.10 to 0.70; p = 0.007). Local anaesthesia was more commonly used with bifurcated graft configurations whereas general anaesthesia was more commonly used with aorto-uni-iliac configurations because of the requirement for a femoro-femoral crossover graft, which causes considerable ischaemic leg pain that cannot be tolerated under local anaesthesia. Even after adjustment for graft configuration, the benefits of local anaesthesia remained highly significant. By 2016, there was increasing recognition that endovascular repair of ruptured AAA should be conducted under local anaesthesia whenever possible. 63,66,67
Aortic morphology and the hostile aneurysm neck
Nearly all patients presenting with a clinical diagnosis of ruptured AAA undergo CT, so that rupture can be confirmed and aortic morphology can be assessed rapidly to determine whether or not the patient is a suitable candidate for EVAR. One liberal definition of suitability for endovascular repair that has been used by laboratories for centralised assessment of CT scans includes an aneurysm neck length of ≥ 10 mm, a neck diameter of < 32 mm and a neck angle of < 60°. 68 A retrospective Swiss study69 found that the 30-day mortality for open repair among patients whose aneurysm falls outside this definition was eight to nine times higher than in those whose aneurysm complied with this definition, whereas a Dutch study70 found no difference. Others have suggested that mortality following rupture in patients with very large aneurysms is worse than mortality in patients with smaller aneurysms,71 but good-quality data to support such suggestions are scant.
The IMPROVE trial core laboratory held good-quality CT scans for 458 participants with confirmed AAA rupture. First, working to a predefined analysis plan, the main morphological features (Figure 6)72 were compared in participants in whom treatment was initiated with either EVAR or open repair (Table 6). It is immediately evident that the main difference between the EVAR participants and the open repair participants is in the aneurysm neck length. Overall, if the aortic morphology was within the liberal instructions for use (IFU) defined in the previous paragraph, 30-day mortality was marginally lower than for participants with aortic morphology outside the liberal IFU (adjusted OR 0.64, 95% CI 0.41 to 1.01; p = 0.054), with the association being stronger for those in whom open repair had started. There was a stronger and linear inverse association of 30-day mortality with neck length (adjusted OR 0.72, 95% CI 0.57 to 0.92), again with the association being stronger for those in whom open repair had started; both ORs were adjusted for age, sex, Hardman index, lowest recorded systolic blood pressure and randomised group. The relationship between aneurysm neck length and mortality is shown in Figure 7, with data on neck length category and treatment started given in Table 7.
FIGURE 6.
Primary morphological variables for analysis.

| Morphological criteria | N | Trial commenced, mean (SD) | p-valuea | Total, mean (SD) (N = 458) | |
|---|---|---|---|---|---|
| EVAR commenced (N = 177) | Open repair (N = 281) | ||||
| Within liberal IFU | 389 | 108 (71.5) | 119 (50.0) | < 0.001 | 227 (58.4) |
| Maximum aneurysm diameter (mm) | 427 | 85.6 (18.2) | 86.3 (17.0) | 0.59 | 86.0 (17.4) |
| Aortic neck diameter at distal renal artery (mm) | 374 | 25.1 (3.8) | 25.9 (4.7) | 0.21 | 25.6 (4.4) |
| Neck length (mm) | 409 | 29.2 (14.8) | 19.5 (15.8) | < 0.001 | 23.3 (16.1) |
| Conicality (% change per 1-mm length) | 361 | 0.46 (0.89) | 0.93 (1.99) | 0.030 | 0.73 (1.63) |
| Proximal neck angle (degrees) | 406 | 31.0 (19.0) | 33.9 (20.9) | 0.17 | 32.7 (20.2) |
| Maximum common iliac diameter (mm) | 404 | 21.5 (8.1) | 20.6 (9.3) | 0.040 | 21.0 (8.8) |
FIGURE 7.
Association between neck length and proximal aortic neck length. Univariate effect of quintiles of neck length on 30-day mortality (complete case analysis with 409 participants), after adjustment for age, sex, Hardman index, lowest recorded systolic blood pressure, randomised group and treatment commenced.

| Treatment commenced | Neck length (mm), % (n/N) | Total, % (n/N) | ||||
|---|---|---|---|---|---|---|
| 0–4 | 5–9 | 10–14 | 15–29 | ≥ 30 | ||
| Overall | 50 (30/60) | 49 (17/35) | 43 (17/40) | 29 (40/139) | 24 (33/135) | 34 (137/409) |
| EVAR | 33 (2/6) | 63 (5/8) | 20 (2/10) | 27 (18/66) | 24 (17/71) | 27 (44/161) |
| Open repair | 52 (28/54) | 44 (12/27) | 50 (15/30) | 30 (22/73) | 25 (16/64) | 38 (93/248) |
These results suggest that a single morphological parameter, aneurysm neck length, appears to have a significant influence on operative mortality following surgery for a ruptured AAA, independent of known confounders. As the aneurysm neck shortens, conventional EVAR either becomes impossible or, if attempted, carries a very high mortality rate. This relationship has also been suggested to hold for open repair. 69 We also observed a strong relationship between short aneurysm neck length and high mortality after open repair, which explains why mortality after open repair remains high; many of these patients have juxtarenal aneurysms. Open juxtarenal aneurysm repair requires cross-clamping of the aorta above the renal arteries, with inevitable compromise of the visceral circulation, especially in shocked patients. In contrast, for longer aneurysm necks (≥ 18 mm when infrarenal aortic clamps for open repair can readily be deployed), the operative mortality rates for open repair and EVAR are closely similar (≈25%). Perhaps surprisingly, we did not identify any association between maximum aortic diameter (or any other morphological variable assessed) and mortality following repair of a ruptured AAA. Our findings may also help to explain why population-level observational studies and meta-analyses of observational studies report that, for ruptured aneurysms, operative mortality for EVAR is about half of that for open repair (the patients with long aneurysm necks have EVAR and those with short aneurysm necks have open repair), as well as why the mortality following the repair of a ruptured AAA is much higher in women than in men (in women, the AAA has a much shorter proximal neck).
These findings of the association between aneurysm neck length and mortality need to be verified in an independent cohort, but there are few large unselective cohorts available with detailed morphology of both endovascular and open repairs. However, we have shown, in an individual patient data meta-analysis, that the association for open repair was also observed for patients randomised in the AJAX19 and ECAR22 trials (in which all participants were morphologically eligible for EVAR). 51 There is support for such findings from Baderkhan et al. ,73 who reported, based on 112 participants undergoing EVAR, that both the number of reinterventions and 3-year mortality were significantly higher for those repaired within the IFU than for those repaired outside the IFU. However, others may hold different opinions: van Beek et al. 70 studied 279 consecutive participants undergoing open repair and concluded that participants with ‘hostile’ and ‘friendly’ aortic necks had similar 30-day mortality. 70
For our work on risk scoring (see Chapter 8), we are using data from 192 unselected patients in the Stockholm area,74 and the association will be tested in this cohort in the future. This future work will add depth to the current debate regarding whether or not aortic neck pathology influences survival following repair of ruptured aneurysms.
Summary
To ensure that patients across the UK who present with a clinical diagnosis of ruptured AAA have similar access to transfer to specialist vascular centres, multidisciplinary best practice clinical guidelines for the transfer of patients with a clinical diagnosis of ruptured AAA have been developed. Using the participants from the IMPROVE trial as an observational cohort of participants with ruptured AAAs, we have been able to identify several important factors, other than the type of aneurysm repair (endovascular or open), that influence the outcome of this deadly disorder. Some of these findings suggest ways to improve the outcomes of patients with ruptured AAAs, particularly through the use of local anaesthesia for emergency EVAR. Other findings, such as the higher mortality for participants admitted out of hours or at weekends, are observed throughout surgery and have been noted in other large series. The guidelines for hypotensive haemostasis75–77 have been developed in trauma patients aged < 55 years; in a much older population, we have shown the adverse effect of blood pressures of < 70 mmHg on mortality and this is just one piece of evidence that contributes to the debate about the definitions of hypotensive haemostasis in the elderly population. 75–78 The inverse association between proximal aneurysm neck length and 30-day mortality is probably crucial to understanding why observational series always report a much more favourable outcome for emergency EVAR than for emergency open repair; these observational series do not compare like with like, as patients with long aneurysm necks receive EVAR whereas those with short and challenging aortic necks receive open repair.
Chapter 5 One-year outcomes, survival, reinterventions and health economics
Some of the material in this chapter has been reproduced in part from published work. 23 © The Authors 2015. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com.
Overview
A ruptured AAA is an acute event and the success of aneurysm repair is assessed predominantly by 30-day mortality. Although there are many reports of 30-day mortality (see Chapter 3), there are far fewer reports of outcomes at ≥ 1 year. The largest series reporting on the comparative effectiveness of endovascular repair and open repair in the longer term comes from the Medicare data set in the USA. 47 In propensity-matched cohorts, Edwards et al. 47 observed a relative survival advantage at 1 year for patients of all ages who had undergone EVAR rather than open repair, and this advantage persisted for 4 years, before being dissipated. Rather similar, more recent, results have come from the Vascular Study Group of New England. 79 Such studies, from large registries, focus on mortality and reinterventions only and provide no data concerning patient QoL, costs or cost-effectiveness. All previous studies of QoL come from the period when only open surgery was used for repair and use data from repairs that were conducted before 2000. There are a few reports of the relative cost-effectiveness of endovascular repair compared with open repair for ruptured aneurysms. One report comes from AJAX,80 in which cost-effectiveness was reported as incremental cost-effectiveness per death prevented to 6 months. They found that, at 6 months, EVAR was not cost-effective. Another report, with the opposite conclusion, comes from a retrospective analysis of participants at a single UK centre but without QoL having been measured. 81 The French randomised trial, ECAR,22 did not attempt to assess cost-effectiveness. Therefore, the IMPROVE trial23 is the first study to assess the full cost-effectiveness of an endovascular strategy compared with open repair for ruptured aneurysms.
Additional methods for cost-effectiveness analyses: categories of resource use, handling of missing data and rationale for assumptions for the base-case and sensitivity analyses
The specific resource use categories included in the cost-effectiveness analysis were (1) medical devices and consumables for each intervention (see Appendix 2, Table 28); (2) length of hospital stay during the primary admission, including critical and specialist unit bed-days and extent of organ support; (3) all reinterventions during the primary admission, whether or not directly associated with the ruptured aneurysm, including time in the operating theatre or endovascular suite, devices and consumables; (4) readmissions related to the ruptured aneurysm and a sensitivity analysis including all readmissions; and (5) outpatient and community service costs whether related to the ruptured aneurysm or other conditions.
Missing data on baseline covariates, resource use and QoL variables were handled with multiple imputation using chained equations. Under this approach, each variable was imputed, conditional on fully observed baseline variables, such as age and sex, and all other imputed variables, with the same variables being used for the clinical and health economic analyses. All the variables considered for multiple imputation and, for each variable, the number of missing values and the imputation model chosen are reported in Appendix 2, Table 31.
The major incomplete resource use components, such as time in the operating theatre, length of stay in critical care or on routine wards (within either primary admission or readmission) and the use of community care, were addressed with multiple imputation. For those participants with ruptured AAA for whom aneurysm repair was commenced, missing resource use components were imputed from those participants with ruptured AAA with observed resource use data. Participants who did not have a ruptured AAA, and had no information recorded on being in critical care, were assumed to stay on a routine ward for their entire hospital stay.
In the case of participants with a proven ruptured AAA who failed to return the QoL questionnaire that was administered at 3 or 12 months, their EQ-5D scores were imputed from those of other ruptured AAA survivors. For example, of the participants with ruptured AAA for whom repair had commenced and who were eligible for the 3-month follow-up, 66 did not complete the EQ-5D questionnaire. For these 66 participants with missing EQ-5D scores at 3 months, EQ-5D scores were imputed using EQ-5D data from those 252 participants with ruptured AAA who fully completed the EQ-5D questionnaire at 3 months (see Appendix 2, Table 32). Hence, these imputations did not use information from those participants who had died prior to either time point (who were assigned a EQ-5D score of 0), participants with a ruptured AAA in whom repair had not commenced or participants who had a symptomatic AAA. In the case of participants who were alive but otherwise ineligible for follow-up, we assumed that the EQ-5D score at either time point was the average of the EQ-5D scores at baseline for participants with ruptured AAA presenting for elective repair,41 in that their EQ-5D scores were 0.75, 0.75 and 0.74 at baseline (preoperatively) and at 3 and 12 months postoperatively, respectively.
The main assumptions made in the base-case scenario, and how each was relaxed in sensitivity analyses, are shown in Appendix 2, Figure 26. The results of the sensitivity analysis were reported as the mean INB with corresponding 95% CIs. These assumptions, which were relaxed in the sensitivity analyses, included:
-
Covariate adjustment. The base case reported unadjusted mean differences of both incremental costs and QALYs, assuming randomisation had ensured that there were no imbalances in key prognostic factors such as age, sex and Hardman index. In the sensitivity analysis, we adjusted for any chance imbalances in age, sex and Hardman index using seemingly unrelated regression.
-
Distributional assumptions on costs and QALYs. The base case assumed that costs and QALYs were normally distributed when reporting the 95% CIs around incremental costs and QALYs. In sensitivity analyses, we assessed the robustness of the cost-effectiveness results to alternative distributional assumptions about both outcomes. The sensitivity analysis considered a gamma distribution for costs as they had a right-skewed distribution. For QALYs, the sensitivity analysis also considered a gamma distribution because a large proportion of decedents had 0 QALYs and the remainder of the distribution was, again, right-skewed.
-
Staffing levels in the operating theatre. In the base case, we assumed the minimum number of staff required in the operating theatre to undertake either type of repair. In sensitivity analyses, we allow for additional staff used in some IMPROVE trial centres according to the results of the staffing survey.
-
Prices of devices for the endovascular procedure (stent grafts). In the base case, unit costs for the devices and consumables of endovascular intervention were taken from manufacturing list prices, assuming all hospitals would pay the same for these items, irrespective of the number of cases. In sensitivity analysis, we considered a cost per case for the device of £4000–10,000, which may reflect, for example, differential prices according to the number of cases, because the pricing of endovascular stent grafts varied remarkably across different English hospitals because of different discounts.
-
Participants without proven rupture. For participants without a proven ruptured AAA, resource use beyond the primary admission and QoL data were not collected because postoperative participant consent was not required. In the base case, assumptions were made about EQ-5D-3L score based on participants from an elective surgery trial; participants who did not have an aneurysm repair were assumed to stay on a routine hospital ward for the primary admission and subsequently assumed to have no reinterventions or readmissions and one outpatient visit. To assess whether or not the overall cost-effectiveness results were sensitive to the inclusion of these participants and the requisite assumptions, we ran a sensitivity analysis in which we excluded them from the sample.
-
Readmissions. The base case only included costs from ruptured AAA-related readmissions to study centres that were recorded in the CRFs. Sensitivity analysis allowed for other readmissions, by using information collected in the health services questionnaire, although it was not always clear which of these admissions were aneurysm-related. For these readmissions, we assumed the average readmission cost from those readmissions recorded in the CRFs.
Results
Mortality
Between 3 months and 1 year after randomisation, two participants in the open repair group were lost to follow-up (leaving 295 participants in this group available for 1-year analysis). By 1 year, 98 participants in the endovascular strategy group and 103 participants in the open repair group had died (the flow of participants through the IMPROVE trial to 3 years is shown in Figure 8). At 1 year, mortality was 41.1% for the endovascular strategy group compared with 45.1% for the open repair group (OR 0.85, 95% CI 0.62 to 1.17). Details and causes of death are given in Table 8. As at 30 days, the endovascular strategy was more effective in women than men (interaction p = 0.034). Again, we conducted an individual patient meta-analysis of mortality across the three recent European randomised trials, which included both participants as randomised and the 308 IMPROVE trial participants with a proven diagnosis of rupture only who were suitable for endovascular repair. 82 The survival curves to 1 year for each trial and for the restricted IMPROVE trial population are shown in Appendix 2, Figure 24a. By 1 year, the ECAR trial suffered from almost 20% of participants being lost to follow-up, so that the apparent separation of the survival curves was not significant. There was no significant difference in mortality between the randomised groups for any trial, with the pooled OR being 0.84 (95% CI 0.63 to 1.11), with no significant heterogeneity (see Appendix 2, Figure 24b). Even if only the restricted population from the IMPROVE trial was included in the meta-analysis, the pooled OR only reduced to 0.80 (95% CI 0.56 to 1.16); data are not shown. However, it is interesting to note the better survival of the participants from the IMPROVE trial who were anatomically suitable for endovascular repair (compare parts a and b in Appendix 2, Figure 24a), in keeping with our earlier findings that overall 30-day mortality was lower (irrespective of type of repair) in participants with long aneurysm necks. 83
FIGURE 8.
A CONSORT diagram showing participant flow and follow-up to 3 years. a, Number of deads/(alive + died); b, with 26 participants who had open repairs in breach of protocol; c, with 33 participants who had EVARs in breach of protocol; and d, participants withdrew consent to be contacted about completing EQ-5D questionnaires, but allowed their other data to be used (five participants per randomised group). Completion rates reported indicate fully completed questionnaires.

| Variable | Missing data (n) | Trial group, n (%) | |
|---|---|---|---|
| Endovascular strategy | Open repair | ||
| Deaths | N = 316 | N = 297 | |
| Within 30 days | 0 | 112 (35.4) | 111 (37.4) |
| Before primary hospital discharge | 0 | 115 (36.4) | 114 (38.4) |
| Before overall hospital discharge | 0 | 115 (36.4) | 116 (39.1) |
| Within 1 year | 2 | 130 (41.1) | 133 (45.1) |
| Cause of death | 2 | ||
| AAA | 107 (33.9) | 116 (39.3) | |
| Myocardial disease | 4 (1.3) | 0 (0.0) | |
| Pulmonary disease | 6 (1.9) | 4 (1.4) | |
| Cancer | 4 (1.3) | 2 (0.7) | |
| Stroke and other vascular | 7 (2.2) | 8 (2.7) | |
| Other | 2 (0.6) | 3 (1.0) | |
| Participants with reinterventionsa | N = 259 | N = 243 | |
| AAA-related reintervention | 0 | 55 (21.2) | 49 (20.2) |
| Non-AAA-related reintervention | 0 | 6 (2.3) | 11 (4.5) |
| Number of reinterventions per persona | |||
| 0 | 0 | 201 (77.6) | 187 (77.0) |
| 1 | 0 | 42 (16.2) | 38 (15.6) |
| 2 | 0 | 11 (4.3) | 13 (5.4) |
| ≥ 3 | 0 | 5 (1.9) | 5 (2.1) |
If the overall benefit of an endovascular strategy confers only a relative 15% lower mortality at 1 year, we would have needed to randomise almost 5000 participants to show a significant difference in 1-year mortality.
Reinterventions
By 1 year, the number of reinterventions per person was very similar in the endovascular strategy group and the open repair group of the IMPROVE trial, with 78% of participants needing no reintervention in the endovascular strategy group compared with 77% in the open repair group and, overall, the reintervention rate of participants following hospital discharge was rather low. The very different reporting of complications and reinterventions across the IMPROVE,23 AJAX80 and ECAR22 trials meant that a full meta-analysis was not feasible. In the IMPROVE trial, complications were not captured and reinterventions were reported mainly from a health economic perspective. However, a meta-analysis has been conducted for some of the rarer events, particularly amputation. In the IMPROVE trial, there were a total of nine amputations above the knee and more proximally, eight of which occurred within 1 year of randomisation. Within 1 year, five amputations were in the endovascular strategy group and three in the open repair group. However, seven out of eight of these amputations were in participants who received open repair. In a meta-analysis with AJAX (three amputations in the open repair group) and the ECAR trial (two amputations in the open repair group), the risk of amputation was much lower after EVAR (OR 0.20, 95% CI 0.05 to 0.88), with no evidence of heterogeneity.
Resource use
The data came from CRFs and questionnaires from the 502 participants in whom repair of a rupture had started. Data completeness at 12 months in the endovascular strategy group was 89% for CRFs and 79% for questionnaires. Data completeness at 12 months in the open repair group was 91% for CRFs and 79% for questionnaires. The imputation of missing data, including for participants without a final diagnosis of ruptured AAA, has been discussed in Additional methods for cost-effectiveness analyses: categories of resource use, handling of missing data and rationale for assumptions for the base-case and sensitivity analyses.
Table 9 summarises the resource use up to 1 year after randomisation, related to primary admission, readmissions and community care. Operation time was lower for participants who were randomised to an endovascular strategy than for those randomised to the open repair procedure (see Table 9); mean time in theatre was 157 minutes (SD 100 minutes) and 180 minutes (SD 108 minutes) for endovascular strategy and open repair procedure, respectively. The endovascular strategy had, on average, a higher cost of medical devices (including the stent graft) and consumables compared with the open repair procedure (see Table 9). Participants in the endovascular group had a relatively lower mean cost in critical care compared with the open repair group (£6300 vs. £9280, respectively). Theatre and general medical costs were relatively similar between groups. Participants in the endovascular strategy stayed, on average, for fewer days in hospital compared with participants in the open repair group; mean time in hospital was 14 days (SD 21.6 days) compared with 20.1 days (SD 31.6 days), respectively. Participants in the endovascular strategy group were discharged earlier from critical care units (mean 5.1 days compared with 7.4 days in the open repair group) and fewer participants were transferred to other (secondary) hospitals than in the open repair group: 10 (3%) compared with 36 (12%), respectively. One participant in each randomised group was discharged on haemodialysis. The costs related to stay in secondary hospitals were lower for the endovascular strategy group than for the open repair group. The costs of outpatient visits and community care were comparable between randomised groups. The number of hospital readmissions up to 1 year after randomisation was higher for the endovascular group than for the open repair group: 26 readmissions (8%) and 12 readmissions (4%), respectively. Participants across the randomised groups had similar service use after hospital discharge, including outpatient, general practitioner and nurse visits (see Table 9).
| Component | Resource use | Cost (£) | ||
|---|---|---|---|---|
| Endovascular strategy (N = 316) | Open repair (N = 297) | Endovascular strategy (N = 316) | Open repair (N = 297) | |
| Primary admission | ||||
| Time in emergency room (minutes), mean (SD)a | 93 (370) | 73 (157) | 135 (138) | 118 (50) |
| Devices and consumables, mean (SD) | 4337 (2913) | 2540 (2053) | ||
| Time in theatre (minutes), mean (SD)b | 157 (100) | 180 (108) | 2057 (1299) | 2110 (1276) |
| Days in critical care, mean (SD) | 5.1 (10.6) | 7.4 (11.1) | 6300 (16,289) | 9280 (15,003) |
| Days on routine ward, mean (SD)c | 7.3 (12.2) | 7.5 (12.5) | 1973 (3213) | 2044 (3406) |
| Participants with at least one reintervention, n (%)d | 58 (18) | 56 (19) | ||
| Number of reinterventions, mean (SD) | 0.26 (0.6) | 0.32 (1.0) | 545 (1388) | 642 (1765) |
| Transfer to secondary hospital, n (%)e | 10 (3) | 36 (12) | ||
| Number of days in secondary hospital, mean (SD) | 0.7 (4.5) | 4.7 (21.0) | 174 (1158) | 1208 (5452) |
| Readmissions | ||||
| Number of readmissions, n (%) | 26 (8) | 12 (4) | ||
| Number of readmissions, mean (SD) | 0.1 (0.5) | 0.05 (0.2) | 284 (1805) | 119 (863) |
| Total days in hospital, mean (SD) | 14.0 (21.6) | 20.1 (31.6) | ||
| Total hospital cost, mean (SD) | 15,804 (19,318) | 18,062 (20,296) | ||
| Outpatient and community care, mean (SD) | ||||
| Outpatient visits | 3.2 (5.7) | 2.9 (8.5) | 397 (718) | 292 (483) |
| Days in nursing home | 0 (0) | 1.8 (22.0) | 0 (0) | 192 (2309) |
| Family doctor visits | 2.8 (3.9) | 2.5 (3.8) | 153 (216) | 139 (209) |
| Community nurse visits | 2.2 (6.7) | 2.1 (7.4) | 40 (120) | 38 (134) |
| Outpatient and community care total costs | 590 (902) | 661 (1468) | ||
| Total cost, mean (SD) | 16,394 (19,543) | 18,723 (20,599) | ||
| Incremental cost (95% CI) | –2329 (–5489 to 922) | |||
The net effect of the higher intervention cost, but lower critical care and secondary hospital care costs, was that the mean total cost per participant was lower for the endovascular strategy group (£16,394) than that for the open repair group (£18,723); the mean cost difference at 1 year was –£2329 (95% CI –£5489 to £922).
Health-related quality of life
At 3 months after randomisation, a higher proportion of participants in the endovascular strategy group than in the open repair group reported ‘no problems’ on the physical health dimensions (mobility, self-care and pain) of the EQ-5D-3L questionnaire (see Appendix 2, Table 32). For example, 74 participants (54%) in the endovascular group reported ‘no problems’ in mobility compared with 51 participants (45%) in the open repair group. At 12 months after randomisation, the distribution of the health status profiles was more similar between randomised groups, but a higher proportion of the open repair participants reported ‘severe problems’ with self-care, usual activities and pain. For example, seven participants (7%) randomised to the open repair group reported extreme pain at 12 months, compared with only one participant (1%) in the endovascular group (see Appendix 2, Table 32).
The mean EQ-5D-3L utility scores were higher in the endovascular strategy group compared with the open repair group (Table 10); the mean differences in the EQ-5D-3L score (among ruptured AAA survivors) were 0.087 (95% CI 0.017 to 0.158; p = 0.015) and 0.068 (95% CI –0.004 to 0.140; p = 0.063) at 3 and 12 months post randomisation, respectively. The QoL (using the EQ-5D-3L) reported at 1 year following emergency repair is very similar to that reported by participants undergoing elective repair. 41 This is an interesting finding given that participants enrolled in the randomised trial of elective EVAR compared with open repair were, on average, 3 years younger than those in the IMPROVE trial.
| Outcome measure | Trial group, n (%) | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| Endovascular strategy | Open repair | ||||
| n | Mean (SD) | n | Mean (SD) | ||
| EQ-5D-3La,b at 3 months for ruptured AAA survivors | 168 | 0.76 (0.24) | 150 | 0.67 (0.32) | 0.087 (0.017 to 0.158) |
| EQ-5D-3La,b at 12 months for ruptured AAA survivors | 161 | 0.77 (0.20) | 140 | 0.71 (0.35) | 0.068 (–0.004 to 0.140) |
| QALYsb,c for all randomised participants | 316 | 0.40 (0.35) | 297 | 0.35 (0.35) | 0.052 (–0.005 to 0.108) |
| Total costb (£) | 316 | 16,394 (19,543) | 297 | 18,723 (20,599) | –2329 (–5489 to 922) |
| INMBb,d (£) (95% CI) | 3877 (253 to 7408) | ||||
The relative gains in both QoL and mortality led to a higher mean QALY value at 12 months for participants in the endovascular strategy (0.36 QALYs) compared with that in the open repair group (0.30 QALYs); incremental QALY gain was 0.052 (95% CI –0.005 to 0.108; p = 0.072).
Cost-effectiveness
At 12 months post randomisation, the QALY gain coupled with a lower mean total cost led to a positive INMB (assuming a threshold of £30,000 per QALY gain) for the endovascular strategy compared with the open repair randomised group: £3877 (95% CI £253 to £7408) (see Table 10). The uncertainty in the mean cost, mean QALYs and their joint (normal) distribution is represented on the cost-effectiveness plane (see Appendix 2, Figure 25). The majority of the points are in the quadrant that shows that the endovascular strategy has a lower mean cost and higher mean QALYs, suggesting that this intervention is likely to be cost-effective. The probability that the endovascular strategy compared with open repair is cost-effective is > 0.9, irrespective of how much the decision-maker or society is willing to pay for a QALY gain.
The cost-effectiveness of endovascular repair compared with open repair does not differ by participant subgroup (Table 11). Female participants appeared to benefit relatively more from the endovascular strategy – incremental QALY gain was 0.133 (95% CI 0.02 to 0.247) – but they also had higher costs, mostly associated with longer stays in critical care. The subgroup of participants with worse preoperative Hardman index scores (of ≥ 2) had similar results. The 95% CIs of the INB included zero for all subgroups, except for men (£37 to £8012). The results of the sensitivity analysis suggest that the relative cost-effectiveness of the endovascular strategy compared with open repair is robust to departures from the different base-case assumptions (see Appendix 2, Figure 26). For example, when we considered a higher cost (£10,000) for the endovascular stent, the INB remained positive, although the 95% CI included zero. Similarly, restricting the analysis to the participants with a proven ruptured aneurysm led to a similar 95% CI (£143 to £8467) of the INB compared with the base case.
| Subgroup | Incremental | INB (95% CI)a, £ | p-value | |
|---|---|---|---|---|
| Cost (95% CI), £ | QALYs (95% CI), £ | |||
| Age (years) | ||||
| ≤ 77 | –2032 (–6579 to 2514) | 0.025 (–0.050 to 0.101) | 2797 (–2252 to 7846) | 0.719 |
| > 77 | –2560 (–7005 to 1885) | 0.064 (–0.012 to 0.140) | 4483 (–501 to 9467) | |
| Sex | ||||
| Male | –3264 (–6831 to 302) | 0.025 (–0.035 to 0.086) | 4025 (37 to 8012) | 0.661 |
| Female | 1882 (–4861 to 8626) | 0.133 (0.020 to 0.247) | 2112 (–5421 to 9646) | |
| Hardman index score | ||||
| 0 | –2513 (–8383 to 3357) | 0.034 (–0.064 to 0.131) | 3525 (–3064 to 10,114) | 0.631 |
| 1 | –2561 (–7277 to 2155) | 0.020 (–0.061 to 0.101) | 3161 (–2115 to 8437) | |
| ≥ 2 | –863 (–7350 to 5623) | 0.114 (0.004 to 0.224) | 4290 (–2898 to 11,478) | |
Main findings of the IMPROVE trial at 12 months
The IMPROVE trial showed no significant survival benefit at any time point for an endovascular strategy (using a standard endovascular device whenever anatomically and operationally possible, with open repair as a default option) compared with open repair during the first 12 months after randomisation, although, again, women benefited more than men from the endovascular strategy. There was no evidence of a difference in the number or rate of reinterventions (including those for endoleaks) at any time during the first year, with no significant interaction on the basis of age, sex or preoperative Hardman index. In contrast, there were gains for the endovascular strategy group compared with the open repair group with respect to patient-preferred outcomes of faster discharge, more home discharges and better HRQoL (using EQ-5D-3L) and, overall, the endovascular strategy was cost-effective. The cost-effectiveness results were robust to alternative departures from base-case assumptions, including prices for stent grafts, specification of the regression model and AAA type (ruptured, incidental or symptomatic).
Interpretation
There are some potential reasons for why there was no difference in survival between the randomised groups. First, shock with systemic organ damage might lead to very high early mortality after ruptured AAA repair, irrespective of the type of repair. Second, the operative mortality from open repair was lower than was anticipated. Third, we now know that aortic anatomy, particularly aneurysm neck length, has an important influence on mortality. In particular, the group of patients who are not candidates for standard endovascular repair (≈40%) has the highest operative mortality, especially for open repair, and the group of patients who are anatomically suitable for the endovascular procedure has a much lower mortality after either EVAR or open repair. 83 Fourth, the trial was designed to be inclusive and consider whether or not the availability of an endovascular service would improve the outcome of all patients with a rupture, not just those anatomically suitable for EVAR. So, inevitably, the endovascular strategy group included a significant proportion of participants who had to be treated with open repair. Moreover, if the overall benefit of an endovascular strategy confers only a relative 15% lower mortality at 1 year, we would have needed to randomise almost 5000 participants to show a significant difference in 1-year mortality.
Among survivors, however, the findings suggesting faster recovery in the endovascular group are notable. These were (1) a shorter average hospital stay, (2) a greater probability of being discharged to home and (3) better HRQoL than the open repair group. The between-group mean differences in QoL, 0.087 (3 months) and 0.068 (1 year), exceed the minimum clinically important difference of 0.03,84 although the mean difference was no longer statistically significant 1 year after randomisation. The average EQ-5D-3L utility scores of the endovascular strategy group at 3 and 12 months (0.76 and 0.78, respectively) were slightly higher than the scores for those undergoing elective EVAR in the EVAR 1 trial41 (0.71 and 0.74, respectively), whereas the EQ-5D-3L scores of the open repair group at 3 and 12 months (0.69 and 0.74, respectively) were similar to the scores for those undergoing elective open repair (0.67 and 0.75, respectively). In addition, the risk of reintervention for endoleak up to 1 year post operation in the endovascular group (8%) was not significantly different to that in the open repair group, and seems generally lower than the 10–23% range suggested in previous reports. 85,86
Participants were discharged home earlier and returned to ‘normal’ HRQoL quicker in the endovascular strategy group than in the open repair group, which translated into lower health-care costs. Together with the gains in survival and QoL, the endovascular strategy is likely to be good value for money in the UK NHS. Given the relative robustness of the results to the different analytical assumptions, this intervention is also likely to be cost-effective in other health-care settings.
Comparison with other studies
At the time of writing, the Dutch AJAX80 and French ECAR22 trials have published outcomes at 6 months and 12 months, respectively. The individual participant data meta-analysis of these trials and the IMPROVE trial indicated that there was no significant survival benefit for the endovascular strategy compared with open repair, although there was some heterogeneity between studies/countries. 82 The ECAR trial did not report HRQoL or cost-effectiveness but AJAX found (1) no between-group differences in 6-month QoL and (2) that endovascular repair was not cost-effective. 80 These findings contrast with those from the IMPROVE trial, probably because the IMPROVE trial included a wider range of patients (more representative of total ruptured AAA caseload). In addition, AJAX used aorto-uni-iliac devices (with subsequent femoro-femoral crossover grafting) for endovascular repair (resulting in a more expensive procedure) and applied unit costs from a single centre.
Limitations
First, this was a pragmatic trial and, among those participants with a ruptured AAA, the endovascular procedure was started in only 58% of those who were randomised to the endovascular strategy (with 26 endovascular-suitable participants receiving open repair for operational reasons) and 33 participants in the open repair group receiving endovascular repair (primarily because they were deemed unfit for general anaesthesia). Second, data completion was very good, including questionnaire responses, but ≈40% of randomised participants had missing QoL or resource use data (at either 3 or 12 months post randomisation). Missing data were addressed with multiple imputation, which assumes that any systematic differences in outcomes can be explained by the variables included in the imputation model. Third, there was no adjustment for testing of multiple hypotheses (except for subgroup analyses) but all reported outcomes were prespecified. Fourth, reinterventions and costs following EVAR for rupture may increase after 1 year and all participants were followed up for 3 years to address this (see Chapter 6).
Summary
We have presented clinical effectiveness and cost-effectiveness evidence from a large randomised trial, conducted in the challenging setting of emergency patients requiring an immediate operation to avoid death. It was based on the randomisation of unselected patients, including appropriate representation of women, to optimise the generalisability of the findings, and all centres were accredited for providing EVAR in routine and emergency practice. In the IMPROVE trial, and in meta-analysis with the other two European trials, there is no evidence that an endovascular strategy or endovascular repair offers a survival benefit at 1 year. Although, in the IMPROVE trial, the endovascular strategy offers no overall survival benefit for participants with ruptured AAAs compared with open repair at 1 year after randomisation, this chapter outlines the evidence that suggests that a wider provision of emergency endovascular services is likely to be a cost-effective use of health-care resources.
Chapter 6 Three-year outcomes, survival, reinterventions and health economics
Overview
There are very few studies that have followed up patients with a ruptured AAA in the medium or longer term (> 12 months after rupture) and these, including one from the Vascular Study Group of New England79 and one from the Amsterdam cohort with ruptured aneurysms,87 are non-randomised and mainly retrospective. Such studies are subject to confounding by baseline aortic morphology83 and other factors.
After full recruitment and analysis of 30-day results, longer-term projections of the mortality data from the IMPROVE trial suggested that ≈50% of the randomised participants remained alive at 3 years. Therefore, it was considered to be very important to incorporate accurate estimates of longer-term survival, QoL, reinterventions, cost and, hence, cost-effectiveness for future policy recommendations and, thus, the trial was extended to include 3 years of follow-up for all participants. Even at 1 year, the endovascular strategy was cost-effective, driven by better QoL scores than in the open repair group. Health-care costs were non-significantly lower in the endovascular strategy group than in the open repair group and there was no significant mortality difference between the groups. 83
In randomised trials of the repair of intact aneurysms, the early survival benefit of endovascular repair compared with open repair is eroded within the first 2 or 3 years after aneurysm repair and, in the longer term, the rate of reinterventions after endovascular repair is double the rate after open repair. 79,88,89 Would similar trends following the repair of ruptured aneurysms erode the benefits of the more rapid recovery and early QoL gains after endovascular repair, and hence cost-effectiveness, in the medium or longer term? Data from the Vascular Study Group of New England suggested that, after 3–5 years, survival was similar for those participants who had endovascular repair and those who had open repair (although many participants had been lost to follow-up) and that patient comorbidities and shock on admission were the main determinants of longer-term survival. 79 The Amsterdam cohort study (the total population of ruptures admitted to Amsterdam hospitals from which AJAX participants were selected) was dominated by open repair participants but showed that any early survival benefit of endovascular repair had been eroded by 2 years; thereafter, survival was similar in participants treated by open repair or endovascular repair, with ≈50% of participants remaining alive at 3 years. 90 This Amsterdam cohort study also showed that, after 2 years, reinterventions were slightly, but not significantly, more common after endovascular repair than open repair but, for those discharged alive, reinterventions were twice as common after endovascular repair.
In this chapter, we report the follow-up of all participants from the IMPROVE trial for mortality for ≥ 3 years, to the end of July 2016, and the complete follow-up of the subgroup of greatest clinical interest, the 502 participants in whom repair of a ruptured aneurysm was commenced (for mortality, aneurysm-related reinterventions and QoL), to the 3-year time point, analysed by the group to which these participants were randomised. This, in turn, enables 3-year cost-effectiveness evaluations. The analysis plan prespecified the reporting for both the entire cohort of 613 participants and the principal sensitivity analysis for the 502 participants in whom repair of a ruptured aneurysm had started. The analysis plan paralleled that of the 1-year outcomes reported in Chapter 5, except for the classification of the severity of the reinterventions, an assessment of how baseline aortic morphology influenced reinterventions, the inclusion of two additional subgroups for analyses (by aneurysm neck length and lowest systolic blood pressure on admission) and the specification of mortality and reinterventions by time: acute (0–90 days), mid-term (3 months to 3 years) and beyond 3 years. To enable comprehensive reporting of all aneurysm-related reinterventions, including those at non-trial hospitals, the participants who were randomised in Scotland were audited carefully, including the correspondence section of the notes and participant contact, as relevant. All reinterventions that took place in England were cross-validated by data from HES, which also provided details of aneurysm-related admissions and reinterventions at non-trial hospitals. However, because these data were available only for readmissions or reinterventions within 3 years of primary admission discharge, full reporting of aneurysm-related reinterventions beyond 3 years of follow-up was not possible. The cost-effectiveness analysis was therefore limited to the reporting of 3-year, rather than lifetime, outcomes, using the same methods as described in detail in Chapters 2 and 5.
Results
Study population and treatments
The CONSORT diagram (see Figure 8) shows the follow-up of participants to 3 years after randomisation. In the endovascular strategy group, 310 participants had an aortoiliac aneurysm, 27 had an asymptomatic AAA and another acute diagnosis, 14 had an acute symptomatic aneurysm, 275 had an AAA rupture and six did not have an aortic aneurysm. In the open repair group, 294 participants had an aortoiliac aneurysm, 19 had an asymptomatic AAA and another acute diagnosis, eight had an acute symptomatic aneurysm, 261 had an AAA rupture and three did not have an aortic aneurysm. In total, in 536 participants (259 in the endovascular strategy group and and 243 in the open repair groups) blood was found to breach the aneurysm sac (rupture): 34 participants died before repair and repair was started in 502 participants (the critical subgroup for this analysis). In the endovascular strategy group, 300 out of 316 participants underwent CT, of whom 186 (62%) were considered anatomically suitable for EVAR; EVAR was commenced in 154 participants, and a further 26 participants underwent open repair against protocol (mainly because a staffed endovascular suite was not immediately available). In the open repair group, 33 participants underwent EVAR (mainly because they were poor candidates for general anaesthesia). Between 30 days and 3 years, two participants in each randomised group emigrated and were lost to follow-up. At randomisation, the mean age was 77 years, 22% of the participants were women and the mean AAA diameter was 8.3 cm (baseline characteristics by randomised group are given in Table 1).
Mortality in all 613 participants
Overall follow-up for mortality was 4.9 years (median 4.7 years; range 0.1–7.1 years), with a mean of 2.5 person-years of observation (to death or censoring). There were 179 deaths (22.0 per 100 person-years) in the endovascular strategy group and 183 deaths (25.2 per 100 person-years) in the open repair group (Table 12) (HR 0.92, 95% CI 0.75 to 1.13; p = 0.41), with similar findings for aneurysm-related mortality (HR 0.89, 95% CI 0.69 to 1.16; p = 0.41) and after adjustment (see Appendix 2, Figure 27 and Table 33a and b). Kaplan–Meier survival curves (Figure 9) show a slight divergence after the acute phase (0–90 days), with lower mortality in the endovascular strategy group between 3 months and 3 years (HR 0.57, 95% CI 0.36 to 0.90), before the curves converge again by 6 years. At 3 years, mortality was 48% and 56% in the endovascular strategy group and open repair group, respectively (OR 0.73, 95% CI 0.53 to 1.00; p = 0.053). The increased number of deaths in the open repair group between 3 months and 3 years was not aneurysm-related (see Table 12; this table also gives more detailed causes of death). Subgroup analysis suggested that the endovascular strategy might be more effective in reducing mortality in women than in men, but there were no differences associated with age or Hardman index (see Appendix 2, Figure 28). By 3 years, 48% and 56% of participants had died and the mean numbers of life-years were 1.72 and 1.61 in the endovascular strategy group and open repair group, respectively (p = 0.32).
| Time period | Cause of death | Trial group, n (%) | HR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Endovascular strategy | Open repair | ||||
| All follow-up | AAA-related | 112 (63) | 120 (66) | ||
| Cardiovascular | 26 (14) | 23 (13) | |||
| Pulmonary | 13 (7) | 15 (8) | |||
| Cancer | 19 (11) | 13 (7) | |||
| Other | 9 (5) | 12 (7) | |||
| Total | 179 | 183 | 0.92 (0.75 to 1.13) | 0.41 | |
| Baseline to 3 months | AAA-related | 104 (87) | 112 (95) | ||
| Cardiovascular | 8 (7) | 3 (3) | |||
| Pulmonary | 5 (4) | 0 (0) | |||
| Cancer | 1 (1) | 0 (0) | |||
| Other | 2 (2) | 3 (3) | |||
| Total | 120 | 118 | 0.98 (0.76 to 1.26) | 0.88 | |
| 3 months to 3 years | AAA-relateda | 5 (16) | 5 (11) | ||
| Cardiovascular | 12 (38) | 16 (34) | |||
| Pulmonary | 5 (16) | 10 (21) | |||
| Cancer | 7 (23) | 10 (21) | |||
| Other | 2 (6) | 6 (13) | |||
| Total | 31 | 47 | 0.57 (0.36 to 0.90) | 0.015 | |
| > 3 years | AAA-related | 3 (11) | 3 (17) | ||
| Cardiovascular | 6 (21) | 4 (23) | |||
| Pulmonary | 3 (11) | 5 (28) | |||
| Cancer | 11 (39) | 3 (17) | |||
| Other | 5 (18) | 3 (17) | |||
| Total | 28 | 18 | 1.44 (0.80 to 2.62) | 0.23 | |
FIGURE 9.
Kaplan–Meier estimates for overall survival, by randomised group. For (a) all 613 participants who were randomised (log-rank test p = 0.40); and (b) the 502 participants with a confirmed rupture for whom repair had started (log-rank test p = 0.186).
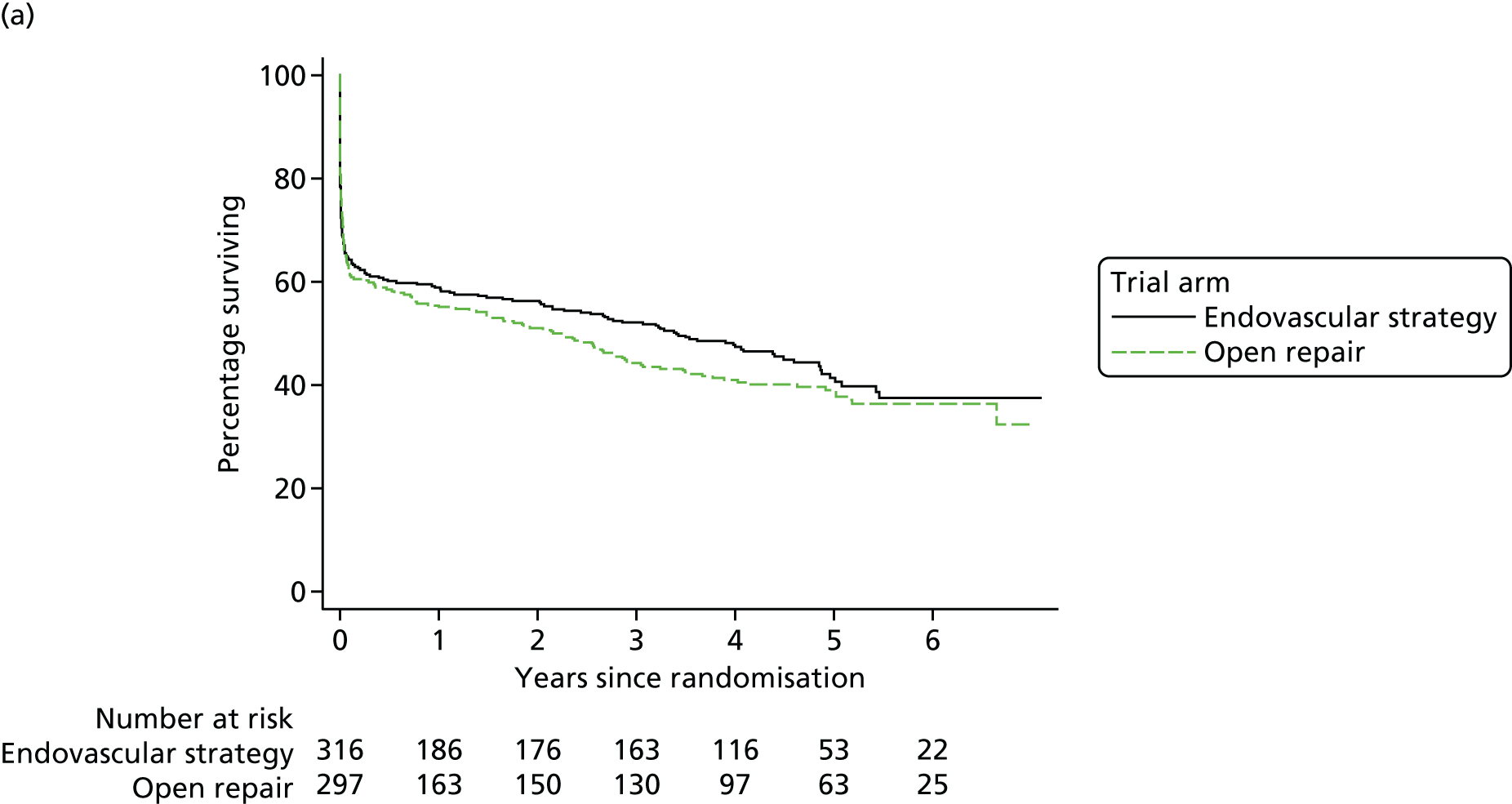
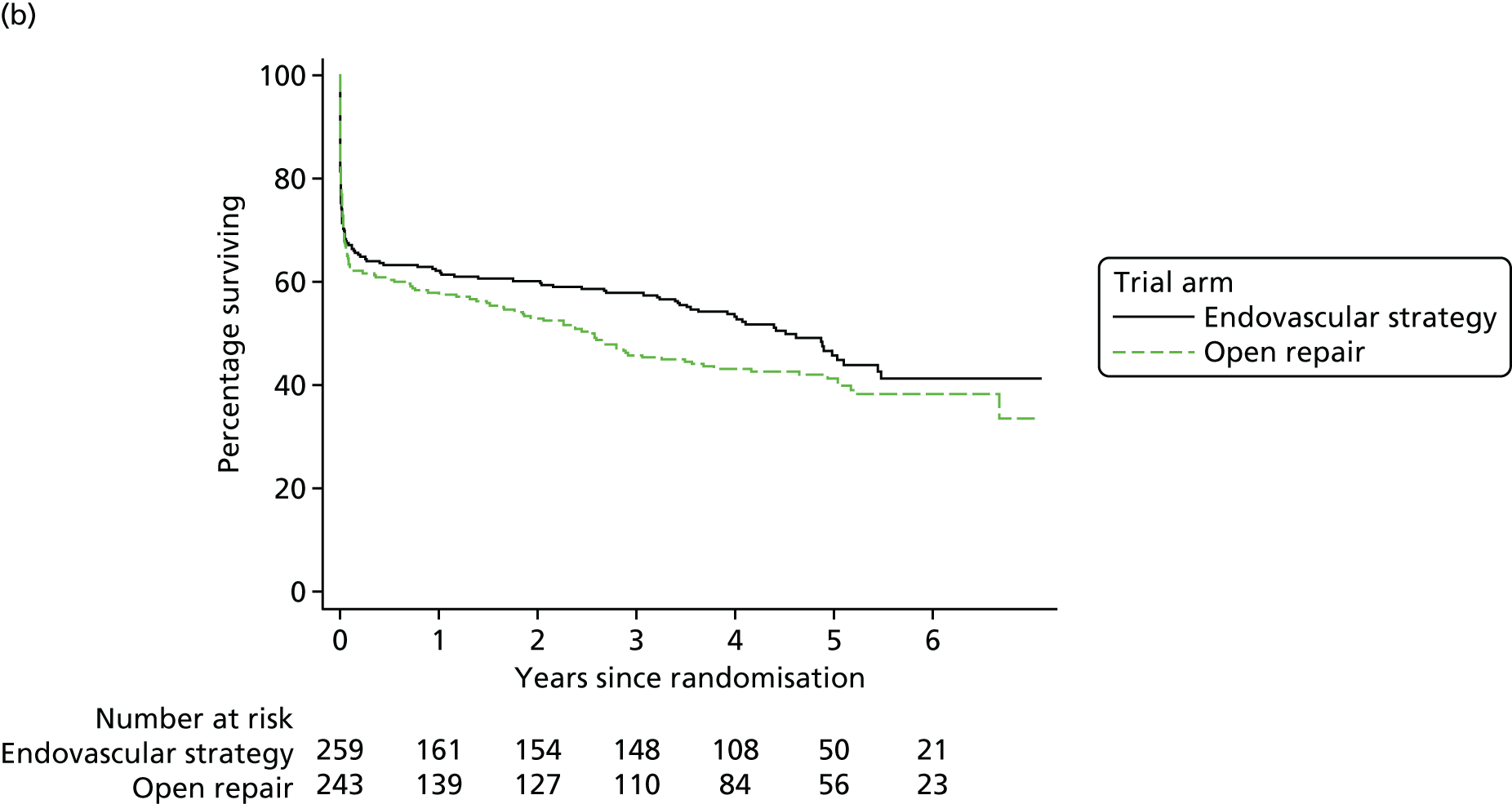
Principal subgroup analysis: mortality in 502 participants with a confirmed rupture and repair started
The baseline characteristics of these participants are shown in Table 13. Kaplan–Meier curves for the 502 participants with a confirmed rupture and in whom repair had started follow a similar pattern to those for the full trial cohort (see Figure 9b) (overall HR 0.86, 95% CI 0.68 to 1.08; p = 0.19). By 3 years, 109 out of 259 participants in the endovascular strategy group (42%) and 131 out of 243 participants in the open repair group (54%) had died. The OR for 3-year mortality was 0.62 (95% CI 0.43 to 0.89; p = 0.009). Because ≈12% of these 502 participants (26 in the endovascular strategy group and 33 in the open repair group) had not complied with their randomised treatment, a causal analysis was conducted for treatment compliers only; the OR for 3-year mortality was slightly lower (OR 0.54, 95% CI 0.34 to 0.85; p = 0.008).
| Variable | Missing data, (n) | Trial group, n (%) | |
|---|---|---|---|
| Endovascular strategy (N = 316) | Open repair (N = 297) | ||
| Age (years), mean (SD) | 0 | 76.0 (7.4) | 76.2 (7.6) |
| Sex, n (%) | 0 | ||
| Male | 209 (81) | 195 (80) | |
| Female | 50 (19) | 48 (20) | |
| Admission blood pressure (mmHg), n (%) | 9 | ||
| Systolic | 108.7 (33.1) | 109.0 (31.1) | |
| Diastolic | 65.1 (22.0) | 65.3 (22.7) | |
| Admission haemoglobin (g/dl), mean (SD) | 4 | 11.2 (2.5) | 11.0 (2.3) |
| Admission creatinine (µmol/l), median (IQR) | 11 | 122 (95–154) | 116 (95–151) |
| Hardman index (0–5), n (%) | 57 | ||
| 0 | 83 (36) | 60 (28) | |
| 1 | 103 (44) | 97 (46) | |
| 2 | 36 (15) | 43 (20) | |
| 3 | 9 (4) | 10 (5) | |
| 4 | 2 (1) | 2 (1) | |
| 5 | 0 (0) | 0 (0) | |
| CT carried out, n (%) | 0 | ||
| Yes | 251 (97) | 216 (89) | |
| No | 8 (3) | 27 (11) | |
| Core-laboratory-measured maximum aortic diameter (cm), mean (SD) | 68 | 8.7 (1.7) | 8.4 (1.8) |
| Neck length (mm), mean (SD) | 93 | 24 (17) | 23 (16) |
| Time to AAA repair, randomisation to theatre admission (minutes), median (IQR) | 5 | 47 (28–73) | 37 (22–62) |
Over the entire period of follow-up that compared the groups as randomised, the unadjusted HR for aneurysm-related mortality was 0.89 (95% CI 0.67 to 1.18; p = 0.41) and the adjusted HR was similar, at 0.92 (95% CI 0.69 to 1.23). A post hoc analysis showed that the effect of sex was stronger in this subgroup of 502 participants, particularly for aneurysm-related deaths (HR in women 0.44, 95% CI 0.24 to 0.81, and HR in men 1.09, 95% CI 0.79 to 1.52; interaction p = 0.010).
Reinterventions in 502 participants with confirmed rupture and repair started
There were 230 aneurysm-related reinterventions recorded within 3 years of randomisation: 121 in the group assigned to an endovascular strategy and 109 in the group assigned to open repair (rate of reintervention HR 1.02, 95% CI 0.79 to 1.32; p = 0.88). (In the entire cohort of 613 participants, there were two further aneurysm-related reinterventions, after repair of symptomatic, non-ruptured aneurysms, before 3 years.) The reinterventions, categorised by severity (details at www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/; accessed 16 November 2017) and whether they were arterial, laparotomy related or other, are shown in Table 14. Overall, and by time period (acute 0–90 days or 3 months to 3 years), the reintervention rates in the 502 participants in whom repair of a ruptured aneurysm had started were not significantly different between the randomised groups, with ≈28% of each group needing at least one reintervention. The cumulative incidence of participants with at least one intervention to 3 years is shown in Figure 10a and the cumulative incidence of participants with at least one arterial or laparotomy-related reintervention (4* and 5*; see www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/, accessed 16 November 2017, for categorisation) for a life-threatening condition is shown in Figure 10b. New reinterventions for life-threatening conditions continued to occur at a much slower, but steady, rate between 3 months and 3 years in both of the randomised groups (see Appendix 2, Table 34). The HRs for risk of reintervention, both overall and by time, remained similar after adjustment (see Appendix 2, Table 35).
| AAA-related reintervention | Participants with ruptured AAA in whom repair started (N = 502) | |
|---|---|---|
| Endovascular strategy | Open repair | |
| Overall | ||
| Participants with at least one reintervention, n/N (%) | 75/259 (29) | 65/243 (27) |
| Reinterventions/person-years (rate per 100 person-years), (%) | 121/463.8 (26.0) | 109/387.3 (28.1) |
| Arterial, n (%) | 93 (77) | 62 (57) |
| Laparotomy, n (%) | 16 (13) | 34 (31) |
| Other, n (%) | 12 (10) | 13 (12) |
| Severity of arterial reintervention, n (%) | ||
| 1* | 0 (0) | 1 (2) |
| 2** | 24 (26) | 10 (16) |
| 3*** | 32 (34) | 11 (17) |
| 4**** | 9 (10) | 5 (8) |
| 5***** | 28 (30) | 35 (56) |
| Severity of laparotomy reintervention, n (%) | ||
| Major | 5 (31) | 4 (12) |
| Minor | 11 (69) | 30 (88) |
| 0–90 days | ||
| Participants with at least one reintervention, n/N (%) | 57/259 (22) | 51/243 (21) |
| Reinterventions/person-years (rate per 100 person-years), (%) | 81/43.6 (185.6) | 88/38.9 (226.1) |
| Arterial, n (%) | 60 (74) | 49 (56) |
| Laparotomy, n (%) | 11 (14) | 27 (31) |
| Other, n (%) | 10 (12) | 12 (14) |
| Severity of arterial reintervention, n (%) | ||
| 1* | 0 (0) | 1 (2) |
| 2** | 13 (22) | 6 (12) |
| 3*** | 21 (35) | 9 (18) |
| 4**** | 5 (8) | 3 (6) |
| 5***** | 21 (35) | 30 (61) |
| Severity of laparotomy reintervention, n (%) | ||
| Major | 2 (18) | 3 (11) |
| Minor | 9 (82) | 24 (89) |
| 3 months to 3 years | ||
| Participants with at least one reintervention, n/N (%) | 27/167 (16) | 15/146 (10) |
| Reinterventions/person-years (rate per 100 person-years), (%) | 40/420.1 (9.5) | 21/348.3 (6.0) |
| Arterial, n (%) | 33 (83) | 13 (62) |
| Laparotomy, n (%) | 5 (13) | 7 (33) |
| Other, n (%) | 2 (5) | 1 (5) |
| Severity of arterial reintervention, n (%) | ||
| 1* | 0 (0) | 0 (0) |
| 2** | 11 (33) | 4 (31) |
| 3*** | 11 (33) | 2 (15) |
| 4**** | 4 (12) | 2 (15) |
| 5***** | 7 (21) | 5 (38) |
| Severity of laparotomy reintervention, n (%) | ||
| Major | 3 (60) | 1 (14) |
| Minor | 2 (40) | 6 (86) |
FIGURE 10.
Time to first reintervention for the 502 participants in whom repair of a ruptured aneurysm had started. (a) All reinterventions and (b) reinterventions for life-threatening conditions (arterial or laparotomy related).
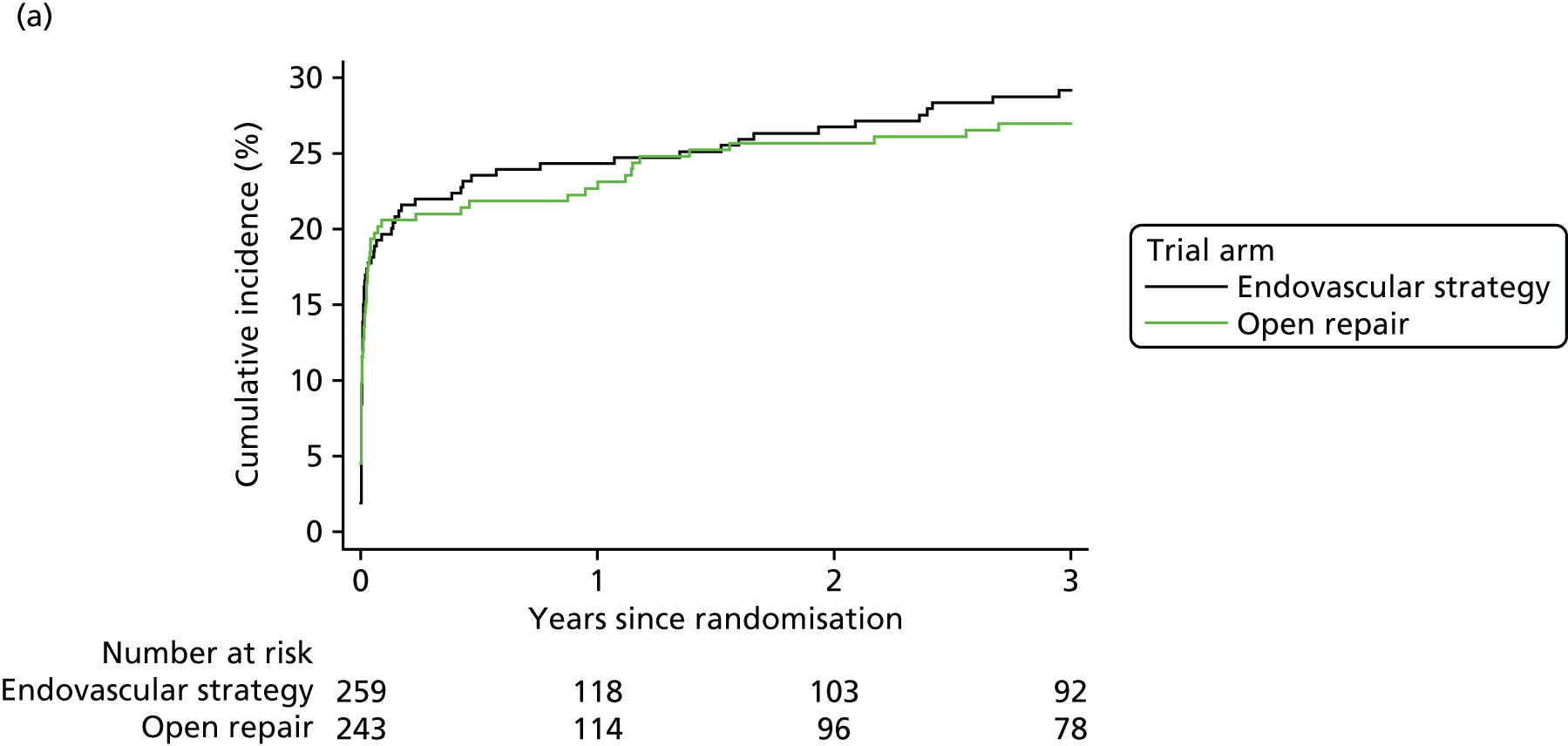
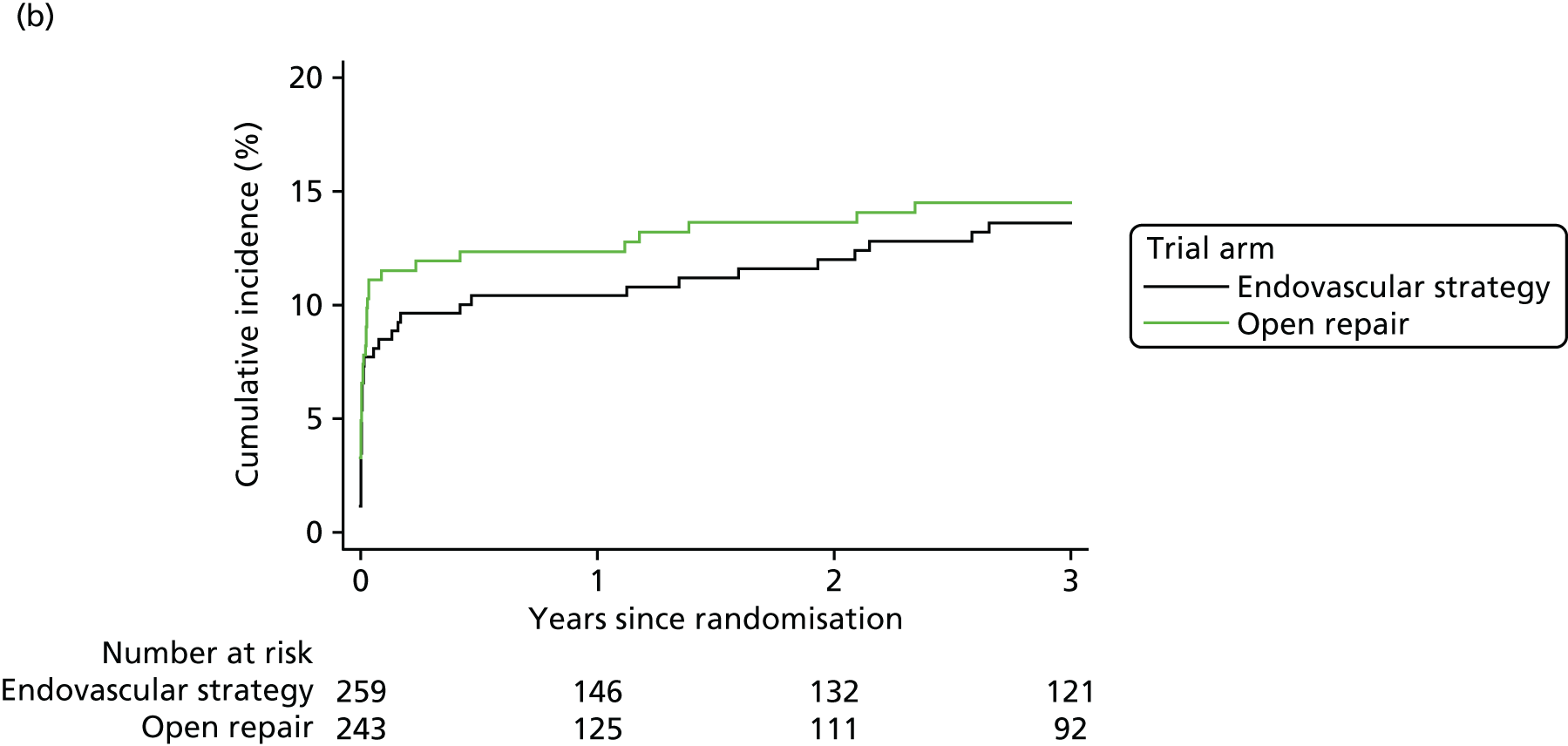
The aneurysm-related reinterventions were also assessed by indication for treatment received in the 502 ruptures in which repair was started (see Appendix 2, Table 36), both by intention to treat and by treatment received, although this latter comparison is subject to bias. It shows that the burden of reinterventions in the first 90 days is much higher after open repair (see Appendix 2, Table 36a). Nine of these reinterventions were carried out at readmission in the endovascular strategy group, compared with only one at a readmission in the open repair group. Between 3 months and 3 years, there were more reinterventions after EVAR (12.5 per 100 person-years and affecting 21% of participants) than after open repair (5.0 per 100 person-years affecting 9% of participants; p < 0.001), but the majority of reinterventions after EVAR were minor arterial reinterventions (see Appendix 2, Table 36b).
Narrative descriptions of some adverse outcomes requiring reintervention
A small group of patients and members of the public considered amputation to be the most adverse reintervention, whereas clinicians did not (see www.journalslibrary.nihr.ac.uk/programmes/hta/073764/#/; accessed 16 November 2017). There were eight amputations within the first 3 years, five in the endovascular strategy group and three in the open repair group, but seven of these occurred following open repair. Other reinterventions that were considered to be very serious by the patients and members of the public included those for graft infection or secondary rupture, complete repetition of the primary operation and a permanent stoma. Such reinterventions (n = 19) took place in eight participants in the endovascular strategy group and in 11 participants in the open repair group.
Overall, six cases of graft infection were reported. It is worth noting that, following EVAR, the only cases of graft infection were after implantation of aorto-uni-iliac endografts, with the infection being in the femoro-femoral crossover graft (two cases of graft infection in 36 aorto-uni-iliac grafts). It is also worth reporting that type I endoleak after EVAR was reported in 12 out of 186 participants (seven type Ia and five type Ib endoleaks), and 11 of these cases were detected > 6 months following repair. In due course, beyond 3 years, three cases of secondary rupture were reported; two cases following a type 1b endoleak and one case following a type 1a endoleak. Only one secondary rupture was reported after open repair.
Effect of baseline aortic morphology on reinterventions
We also investigated whether or not baseline aortic morphology was associated with the rate of reinterventions (either arterial only or all aneurysm-related). These analyses included only participants with a CT scan measured in the core laboratory and excluded those with common iliac aneurysm (CIA) ruptures. The analyses were by treatment received and not by randomised group. The results (see Appendix 2, Table 37) show that both arterial aneurysm-related and all-aneurysm-related reinterventions tended to increase with an increasing CIA diameter, particularly following EVAR. Following EVAR, for all reinterventions the HR per 9-mm increase is 1.32 (95% CI 1.01 to 1.72) and for arterial reinterventions the HR is 1.48 (95% CI 1.13 to 1.93). For open repair, the strongest association was with increasing aneurysm neck diameter. However, given the multiple comparisons that were conducted, these results should be considered as hypothesis-generating only.
Quality of life, resource use and cost-effectiveness for all 613 participants to 3 years
The response rate for the QoL questionnaires (EQ-5D-3L) at 3 years was very high (> 85%) (see Figure 8). Although the endovascular strategy group had better QoL in the first year after randomisation, by 3 years the QoL was similar between the randomised groups (Figure 11) and similar to that of an age- and sex-matched population. QoL, QALYs and resource use, all following multiple imputations, are shown in Table 15. Complete case analyses are given in Appendix 2, Table 38. The mean QALY gain at 3 years for the endovascular strategy group was 0.166 (95% CI 0.002 to 0.311 QALYs), appearing to be slightly higher for women and those with the highest baseline Hardman index scores but otherwise similar across subgroups (Table 16).
FIGURE 11.
Quality of life (EQ-5D-3L) gained up to 3 years (shaded area) for 613 participants by randomised group.
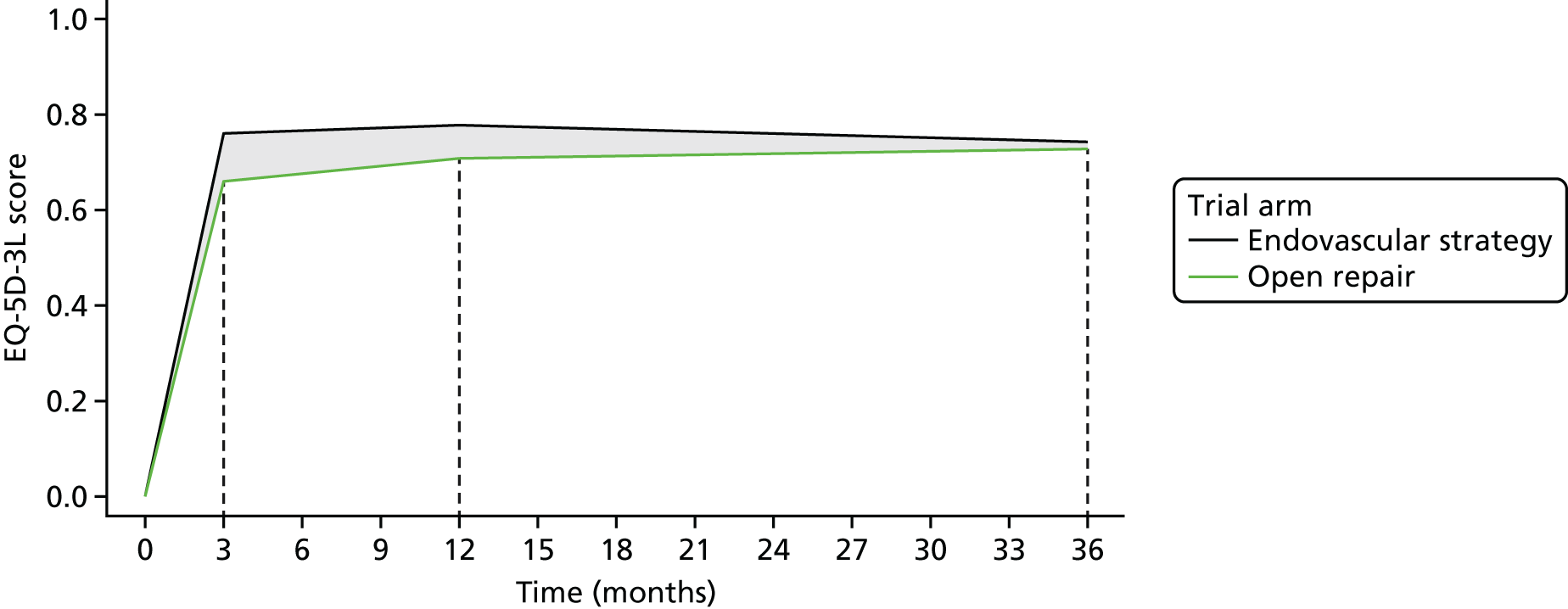
| EQ-5D-3La for ruptured AAA survivors | Trial group, n (%) | Mean difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Endovascular strategy | Open repair | |||||
| n b | Mean (SD) | n b | Mean (SD) | |||
| 3 months | 168 | 0.76 (0.23) | 150 | 0.66 (0.31) | 0.097 (0.031 to 0.163) | 0.004 |
| 12 months | 161 | 0.78 (0.19) | 140 | 0.71 (0.33) | 0.068 (0.002 to 0.134) | 0.045 |
| 36 months | 150 | 0.74 (0.25) | 112 | 0.73 (0.31) | 0.013 (–0.069 to 0.096) | 0.751 |
| Life-years for all randomised participants | 316 | 1.72 (1.43) | 297 | 1.61 (1.41) | 0.115 (–0.110 to 0.341) | 0.315 |
| QALYs (3-year) for all randomised participantsc | 316 | 1.22 (1.11) | 297 | 1.05 (1.10) | 0.174 (–0.005 to 0.353) | 0.057 |
| Subgroup | Incremental | INB (95% CI)a | p-value | |
|---|---|---|---|---|
| Cost (95% CI) | QALYs (95% CI) | |||
| Sex | ||||
| Male (n = 480) | –4066 (–7573 to –557) | 0.092 (–0.099 to 0.283) | 6824 (204 to 13,444) | 0.440 |
| Female (n = 133) | 2817 (–3806 to 9439) | 0.358 (0.038 to 0.712) | 7914 (–4401 to 20,229) | |
| Hardman index score | ||||
| 0 (n = 164) | –4017 (–9758 to 1725) | 0.016 (–0.141 to 0.465) | 8878 (–1686 to 19,442) | 0.279 |
| 1 (n = 254) | –2448 (–7022 to 2126) | 0.005 (–0.244 to 0.254) | 2594 (–5943 to 11,131) | |
| ≥ 2 (n = 121) | –923 (–7306 to 5461) | 0.423 (0.092 to 0.755) | 13,622 (2025 to 25,219) | |
| Neck length (mm) | ||||
| < 22 (n = 234) | –769 (–5595 to 4057) | 0.216 (–0.027 to 0.459) | 7243 (–1216 to 15,702) | 0.461 |
| ≥ 22 (n = 247) | –4250 (–8859 to 358) | 0.111 (–0.131 to 0.352) | 7568 (–959 to 16,095) | |
| Lowest systolic blood pressure (mmHg) | ||||
| < 90 (n = 263) | –3881 (–8604 to 842) | 0.067 (–0.185 to 0.319) | 5881 (–2949 to 14,711) | 0.275 |
| ≥ 90 (n = 305) | –1540 (–5879 to 2798) | 0.213 (–0.013 to 0.439) | 7932 (–14 to 15,878) | |
Table 17 summarises the resource use and costs up to 3 years after randomisation, related to primary admission and aneurysm-related readmissions, including those for reinterventions. This information is provided for the time periods of 0–1 years and 1–3 years. Between 1 and 3 years, participants were not asked to complete health resource usage questionnaires, in part because of problems with recall over this follow-up period. Therefore, the cost of any outpatient care and community care during this period is not included. Between 1 and 3 years, the proportion of participants being readmitted for aneurysm-related reasons was higher in the endovascular strategy group, but the proportion who received aneurysm-related reinterventions was similar in each group. Hospital stay was, on average, a few days shorter in the endovascular group than in the open repair group [mean total number of days in hospital 14.4 days (SD 23.4 days) compared with 20.5 days (SD 32.1 days), with a total incremental cost difference of –£2607 (95% CI –£5949 to £735)].
| Resource and time period | Resource use | Cost (£) | ||
|---|---|---|---|---|
| Endovascular strategy (N = 316) | Open repair (N = 297) | Endovascular strategy (N = 316) | Open repair (N = 297) | |
| Between randomisation and 1 year | ||||
| Primary admission | ||||
| Days in critical care, mean (SD) | 5.3 (12.0) | 7.4 (11.9) | 6672 (16,430) | 9674 (16,446) |
| Days on routine ward, mean (SD) | 7.0 (11.9) | 7.8 (12.0) | 1835 (3107) | 2031 (3158) |
| Other resource use,a mean (SD) | 6563 (3779) | 4757 (2896) | ||
| Transfer to secondary hospital, n (%) | 10 (3) | 36 (12) | ||
| Number of inpatient days, mean (SD) | 0.7 (4.5) | 4.8 (21.1) | 175 (1159) | 1245 (5479) |
| Total days in primary admission, mean (SD) | 13.0 (20.5) | 20.0 (31.9) | 15,245 (18,356) | 17,707 (20,842) |
| Readmissions | ||||
| One or more readmission, n (%) | 27 (9) | 11 (4) | ||
| Number of inpatient days, mean (SD) | 0.7 (4.6) | 0.2 (1.5) | 215 (1469) | 57 (433) |
| Reinterventions | ||||
| One or more reintervention, n (%) | 65 (23) | 60 (22) | 520 (1323) | 655 (1711) |
| Outpatient and community care,b mean (SD) | 525 (739) | 829 (5772) | ||
| Total days in hospital up to 1 year, mean (SD) | 13.7 (21.3) | 20.2 (31.9) | 16,505 (19,084) | 19,248 (22,365) |
| Between 1 and 3 years | ||||
| Readmissions | ||||
| One or more readmission, n (%) | 24 (8) | 12 (4) | ||
| Number of inpatient days, mean (SD) | 0.7 (4.4) | 0.3 (2.3) | 270 (1667) | 137 (981) |
| Reinterventions | ||||
| One or more reintervention, n (%) | 20 (6) | 11 (4) | 107 (626) | 98 (690) |
| Total days in hospital up to 3 years, mean (SD) | 14.4 (23.4) | 20.5 (32.1) | 16,878 (19,624) | 19,483 (22,412) |
| Incremental cost (95% CI) | –2605 (–5966 to 702) | |||
| INMBc (95% CI) | 7637 (1820 to 13,454) | |||
When the incremental costs and QALYs are represented on the cost-effectiveness plane, most of the estimates (88%) are in the quadrant that designates the endovascular strategy as ‘dominant’, with lower mean costs and higher mean QALYs (Figure 12a). The INMB of the endovascular strategy compared with open repair is positive, at £7367 (95% CI £1829 to £13,454), remains similar across subgroups (see Appendix 2, Table 39) and is robust to a range of assumptions (see Appendix 2, Figure 29). The probability that the endovascular strategy was more cost-effective was > 0.90 at all realistic thresholds of willingness to pay for a QALY gain (Figure 12b). Similarly, there was no significant evidence that the INMBs differed by participant subgroups.
FIGURE 12.
(a) Uncertainty in the mean cost (GBP) and QALY differences and their joint distribution for the endovascular strategy compared with open repair for 613 participants; and (b) cost-effectiveness acceptability curve at 3 years, reporting the probability that the endovascular strategy is cost-effective at a range of alternative thresholds of willingness to pay for a QALY gain.
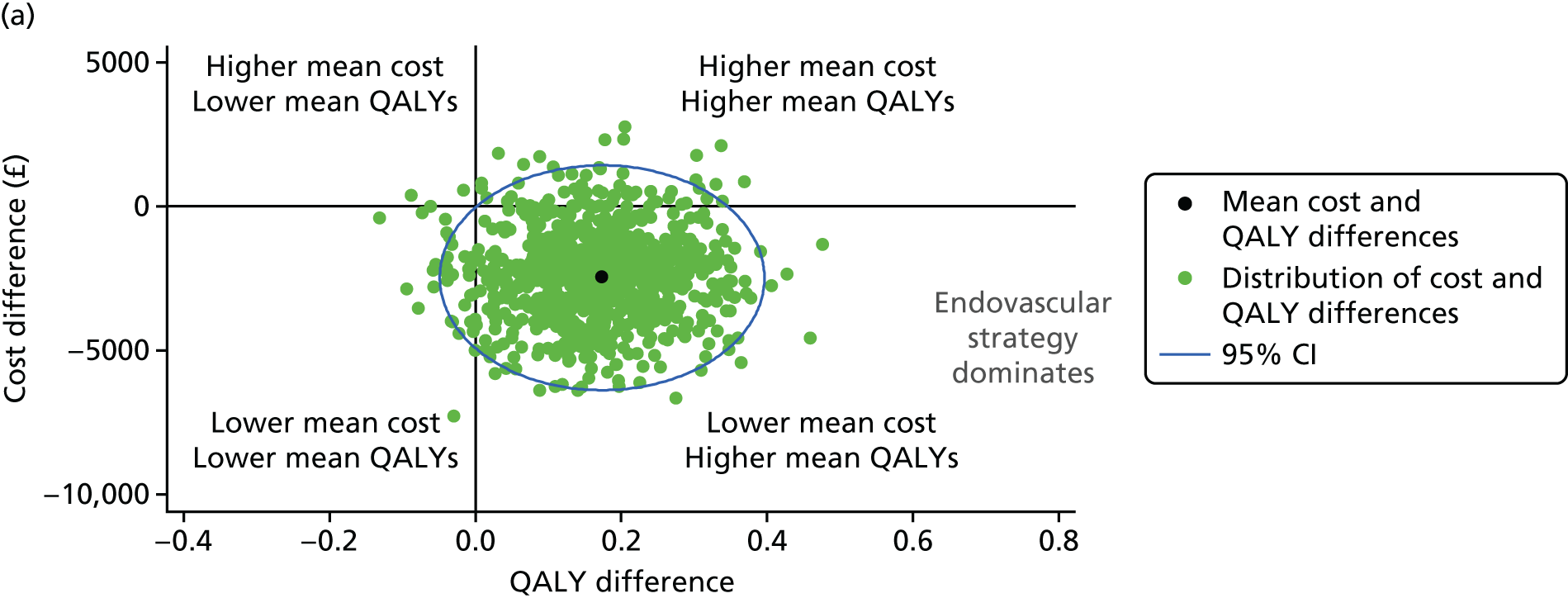

Discussion
This is the only randomised comparison of treatments for ruptured AAA with comprehensive mid-term (3-year) reporting. The IMPROVE trial was designed to test the hypothesis that an endovascular strategy (the availability of endovascular repair for those deemed morphologically suitable for EVAR) would offer a 30-day survival advantage compared with open repair for participants presenting with a clinical diagnosis of ruptured AAA. The early 30-day results did not support this hypothesis,21 despite showing amore rapid patient recovery in the endovascular strategy group and later, at 1 year, evidence of better QoL and cost-effectiveness in this group. 23 However, at 3 years, the time point at which half of the participants remained alive, all of the evidence points in the same direction. There is far more convincing evidence for the benefits of being in the endovascular strategy group: interim survival is better and both QALYs gained and the INMB (cost-effectiveness) are much higher than the values at 12 months, without a significantly higher burden of aneurysm-related reinterventions, especially serious reinterventions.
Although all 613 randomised participants were followed up for mortality, more comprehensive follow-up (QoL, aneurysm-related hospital readmissions and aneurysm-related reinterventions) was limited to the subgroup of 502 participants in whom repair of a confirmed rupture was started and postoperative consent was obtained. The baseline characteristics of these 502 participants, including aortic morphology, were very similar between those assigned to the endovascular strategy group and those assigned to the open repair group. Although 34 out of 536 participants with a proven AAA rupture (blood outside the aneurysm sac) died before AAA repair was started, the number of participants who died was similar in each group (see the CONSORT diagram in Figure 8). Therefore, there should be little bias when comparing the outcomes between the groups as randomised for the principal sensitivity analysis with 502 participants. If anything, the longer time taken to start the repair in the endovascular strategy group would act as a bias against those assigned to an endovascular strategy (see Tables 1 and 13).
Over the entire period of follow-up to 7 years, there was no difference in survival between the two randomised groups, although the separation between the Kaplan–Meier curves was maximal at 3 years and of borderline significance in favour of the endovascular strategy group. At 3 years, the relative advantage of the endovascular strategy for women compared with men, first observed at 30 days (due mainly to the high perioperative mortality following open repair in women), had been attenuated, although the QALY gain at 3 years was higher in women than in men.
For the principal sensitivity analysis of 502 participants in whom repair of a confirmed rupture was started, the survival benefit of the endovascular strategy between 3 months and 3 years, and at 3 years, was more marked. This was supported by a causal analysis that suggested that mortality for treatment compliers randomised to an endovascular strategy was half of the mortality of treatment compliers in the open repair group. However, by 6 years of follow-up, the survival curves had, again, reconverged, although the number of data at this time point was limited.
In the 502 participants for whom repair of a ruptured AAA had started, overall aneurysm-related reinterventions were more severe and more often laparotomy related, rather than arterial, in the open repair group and hence were more likely to be conducted under general, rather than local, anaesthesia, and such factors might have contributed to the interim survival benefit for the endovascular strategy group at 3 years. The reconvergence of the survival curves beyond 3 years is also unexplained but, as in the situation of elective AAA repair, secondary ruptures and increased cancer deaths may have been contributory. 89 Observational series using administrative or registry data, which cannot fully account for selection bias, have also noted the reconvergence of survival curves at 4/5 years, following a strong survival benefit for EVAR during the acute period, but without discussion of underlying reasons for this. 47,79
The burden of reinterventions reflects the number of reinterventions, their severity and the associated bed-days in hospital, and probably also the type of anaesthesia used (although type of anaesthesia was not recorded, local anaesthesia would probably be used more often than general anaesthesia for arterial reinterventions, especially the less severe reinterventions). The recategorisation of reinterventions as arterial, laparotomy related or other, and by the severity of the procedure, has been useful. Particularly during the early part of follow-up, reinterventions were more severe in the open repair group (and more often laparotomy related rather than arterial). More surprisingly, new and serious reinterventions continued at a low but steady rate after the acute period (0–90 days) in both the open repair group and the endovascular group. Therefore, there was no significant excess of reinterventions in the endovascular strategy group in the later phase of follow-up, as has been indicated in other studies. 47,87
The earlier advantage of better QoL in the endovascular strategy group was no longer evident at 3 years but, importantly, the mid-term survivors had a QoL similar to, or slightly better than, that of an age–sex matched population, supporting aneurysm repair in this older population (the mean age at rupture was 77 years). The earlier gains in QoL, coupled with the survival advantage between 3 months and 3 years of the endovascular strategy group, resulted in a significant, although modest, gain in QALYs at 3 years for this group. Apart from QoL, at 3 years all other health outcomes and costs indicated that the endovascular strategy was more favourable than open repair in this population. The cost differences observed at 30 days (non-significantly in favour of the endovascular strategy group) were not eroded by an increased burden of reinterventions in later follow-up. In contrast, elective endovascular repair has been associated with higher costs and is probably not cost-effective. 91 The longer-term follow-up strengthens the positive cost-effectiveness profile of the endovascular strategy group reported at 1 year and, by 3 years, the between-group differences in both QALYs and INMB were almost double those at 1 year. This cost-effectiveness of the endovascular strategy dominates open repair, irrespective of the decision-maker’s willingness to pay for a QALY gain.
This study has several limitations. First and foremost, this was a pragmatic trial in a life-or-death emergency setting. This meant that not all randomised participants had a ruptured aneurysm, although 99% had an aortic aneurysm, and that there was significant non-compliance with allocated treatment in both randomised groups (≈10% of each group). Second, some of the participants with a ruptured aneurysm died before their aneurysm could be repaired, but the number of participants in this category was similar in each randomised group. Third, after 30-days, follow-up was focused on the subgroup of 502 participants in whom repair of a ruptured aneurysm was started. However, this subgroup of 502 participants in whom repair of a ruptured AAA had started is the most clinically relevant group and was analysed by intention to treat; thus, there should be minimal bias. Fourth, after the acute period, only aneurysm-related reinterventions were reported, but these data were complete, including procedures at non-trial hospitals collected through data linkage with routine hospital statistics, and more than half of the reinterventions were audited. Fifth, although this is by far the largest randomised trial for the management of ruptured aneurysm, with hindsight, the sample size may have been insufficient, with the CIs for mortality often being relatively wide.
There are also several strengths to this study. First, recruitment was non-selective and over half of the potentially eligible patients at the trial centres were randomised, adding to the generalisability of the results. Second, it is the first randomised study with full prospective mid-term follow-up. Third, very few participants were lost to follow-up and the response rate to QoL questionnaires was excellent.
Summary
The endovascular strategy (emergency EVAR if anatomically feasible) provides a better option for patients presenting with a ruptured AAA (especially women) with early gains in QoL, a QALY gain at 3 years and probably an interim survival advantage at 3 years without excessive aneurysm-related reinterventions, all of which combine to result in an endovascular strategy being highly likely to be cost-effective. Therefore, a strong case can be made for an endovascular strategy being more widely adopted within the NHS, and in our opinion all vascular centres should be able to offer emergency EVAR, as well as emergency open repair, at all times.
Chapter 7 Developing and validating a 48-hour mortality risk score following emergency admission for a ruptured abdominal aortic aneurysm
Overview
The purpose of this work was to develop a novel, point-of-care risk score to identify patients with a ruptured AAA in whom either immediate aneurysm repair or transfer to a specialist centre for repair would be potentially worthwhile and those in whom aneurysm repair may be futile. Important features of the risk score are that it is based on both physiological and imaging data that are immediately available in the emergency department and that it focuses on 48-hour mortality as the primary outcome measure, rather than in-hospital or 30-day mortality. The highest attrition rate following repair is in the first 48 hours (see Figure 4). The developed risk score would help to address the question of whether or not more patients in the UK should be offered the chance of aneurysm repair because there currently appears to be undertreatment in England compared with the USA. 92
The outcome considered was death within 48 hours of presentation to the emergency department where an operation, if commenced, used either open repair or EVAR. Previous risk scores have been derived but are generally based on in-hospital mortality as an outcome and are therefore sensitive to changes and improvement in critical care. 93 Furthermore, few of these risk scores have been validated externally. We therefore used the opportunity presented by the collection of individual patient data from the three largest ruptured AAA trials undertaken worldwide and two observational cohorts [the wider Amsterdam cohort87 and the STAR (STockholm Aneurysm Ruptures) cohort74] to (1) develop a 48-hour risk score in the IMPROVE trial, (2) externally validate the risk score in the AJAX, ECAR trials and the Amsterdam and STAR observational cohorts and (3) additionally assess the predictive capabilities of previously published risk scores, namely the Vascular Study Group of New England,93 the Hardman index30 and the Vancouver94 ruptured AAA risk scores, within all RCTs and observational cohorts.
Modelling
Participants for the development and validation of the risk score
Participants from the IMPROVE RCT were used to develop the score; participants with a final diagnosis other than ruptured AAA (i.e. excluding incidental or symptomatic AAA) were excluded but those with aortoiliac ruptures were included. All participants with ruptured AAA or aortoiliac ruptures are considered in the risk score regardless of whether or not aneurysm repair was commenced.
External validation of the risk score was conducted in participants from the AJAX19 and ECAR22 RCTs. The inclusion criteria for randomisation in these two RCTs, and hence the participant population, were slightly different to those in the IMPROVE trial. The IMPROVE trial randomised patients with a clinical diagnosis of ruptured AAA, before confirmation of either rupture or suitability for EVAR, and participants did not have to be haemodynamically stable. The AJAX and ECAR trials required participants to undergo CT before randomisation to confirm the presence of an aneurysm with acute haemorrhage outside the aortic wall and that patients be suitable for both EVAR and open repair and be haemodynamically stable on arrival. 19,22 Therefore, we also undertook external validation using the wider Amsterdam cohort,87 which included those participants who were unsuitable for randomisation in AJAX, as well as a large observational cohort of all patients presenting with ruptured AAAs in the Stockholm area (the STAR cohort74).
Outcome measure
The principal outcome measure that is predicted is mortality within 48 hours of randomisation. The developed 48-hour risk score was also assessed to investigate whether or not it could reliably predict 30-day mortality.
Candidate predictors
We predefined a set of variables for potential inclusion in the risk score based on the data available from the three trials of ruptured AAA (IMPROVE, AJAX and ECAR).
These variables included age, sex, admission systolic blood pressure, additional admission variables for the assessment of the Hardman index [haemoglobin, creatinine, acute myocardial ischaemia on electrocardiography and loss of consciousness] and four basic morphological features of the aneurysm that were measured using CT, which were chosen because they are required to assess the feasibility of EVAR and are relatively easy to measure in a time-critical situation (maximum aortic diameter, aortic neck diameter, aortic neck length and proximal neck angle). The AJAX and ECAR trials did not provide data regarding the volume of intravenous fluids administered or whether or not the participant suffered a preoperative cardiac arrest. Nevertheless, these variables were assessed for their predictive ability when developing the model in the IMPROVE trial data.
For the nine continuous candidate predictors (age, haemoglobin, creatinine, admission systolic blood pressure, volume of intravenous fluids administered, maximum aortic diameter, aortic neck diameter, aortic neck length and proximal aortic neck angle), non-linear relationships with the log-odds of mortality were investigated using fractional polynomials (FPs) based on complete data. 95 The powers of each predictor that were considered in the FP were –2, –1, –0.5, 0, 0.5, 1, 2 and 3, where, by convention in FP terminology, χ(0) was defined as log (χ). A FP of degree –2 was first considered with the best-fitting degree –2 FP compared with the best-fitting degree –1 FP using a chi-squared test of the difference in deviance (a p-value of > 0.1 rejected the use of the more complex degree –2 FP). Similarly, a degree –1 FP was compared with just a linear relationship. Shapes of association were plotted for each final chosen FP for a predictor to assess biological plausibility.
Missing data
To deal with missing data in the predictors, multiple imputation was carried out. The imputation model included all variables considered in the risk score plus the outcome measure (48-hour mortality), 30-day mortality, lowest systolic blood pressure, randomised group, preoperative cardiac arrest, operation received (EVAR, open repair or no operation) and hospital. Predictive mean matching was used for continuous variables and a chained equation strategy was used. Passive imputation was conducted for any non-linear relationships that were found in the prior FP exercise. Forty imputed data sets were generated. Systolic blood pressures, observed or imputed, that were < 33 mmHg were set to the lowest presumed recordable value of 33 mmHg.
Variable selection
A multivariable model was chosen using backwards selection, whereby all candidate predictors using their chosen FP transformation were initially included in the model. Estimates and standard errors (SEs) were obtained from pooling multiply imputed data sets using Rubin’s rules to give (pooled) Wald p-values. The variable with the highest Wald p-value of ≥ 0.157 was then removed from the model (note that this p-value threshold approximately corresponds to a change in deviance of 2, and is akin to using the Akaike information criterion to carry out variable selection). This process was repeated until all remaining variables had a p-value of < 0.157.
The pooled coefficients of the selected variables across the multiply imputed data sets were used to define the risk score. The predicted probability (of mortality within 48 hours) for each person was obtained using the pooled coefficients applied to data from each imputed data set separately. The resulting M = 40 linear predictors were then pooled using Rubin’s rules before being transformed back to the probability scale to obtain a single risk prediction for each person. 32
Developing a bedside predictive model
For ease of use, it was also important to develop a simplified bedside prediction model, in which continuous covariates were dichotomised and coefficients from a fitted logistic model were converted to integers, to allow a simple summation of risks associated with an individual’s risk factors. For each predictor, dichotomisation was implemented at a clinically relevant cut-off point or at a level close to the observed median.
Predictive performance
The predicted probabilities of 48-hour mortality that were obtained from the continuous model and the bedside model were assessed in terms of calibration and discrimination. Calibration was assessed by plotting observed risks compared with predicted risks within deciles of predicted risk and reporting the estimated calibration slope from a Cox proportional hazards model with the risk score as the predictor. 96 Discrimination was assessed using the area under receiver operating characteristic (AUROC) curve, which assesses the ability of the prediction model to differentiate between those who do and do not experience the outcome. The AUROC gives an estimate of the probability of correctly identifying the case in a randomly chosen case–control pair of individuals, with a value of 1 indicating perfect discrimination and a value of 0.5 indicating that discrimination is no better than chance.
Internal validation (within the IMPROVE trial data set) was conducted using 10-fold cross-validation to avoid overoptimistic estimates of predictive performance caused by overfitting. Within each cross-validation, the FP and variable selection processes were re-derived using nine-tenths of the data to obtain a new prediction model, which was then validated on the remaining one-tenth of the data. This procedure was repeated 10 times for each nine-tenths split of the data and the average AUROC over these cross-validations was calculated to give an optimism-corrected estimate.
External validation was conducted using data from the AJAX and ECAR trials to give an assessment of how well the derived risk score applies in other populations. A further assessment of the model was carried out in the wider Amsterdam cohort87 and the STAR cohort. 74
Comparison with other published ruptured-abdominal aortic aneurysm risk scores
For a comparison of the utility of the new ruptured AAA risk score for 48-hour mortality, we compared its predictive performance with the performance of other published risk scores using data from all three RCTs. Preoperative cardiac arrest was not comprehensively recorded in the three randomised trials and so was omitted from scores that used this variable. Furthermore, the use of a suprarenal clamp was not specifically recorded in these data sets and so a proxy (aortic neck length of < 10 mm) was used instead. This proxy was chosen based on a consensus arising from a questionnaire that asked ‘What is the minimal length of aortic neck required to utilise an infrarenal clamp for AAA repair?’, which was circulated at the June 2015 annual meeting of the British Society of Endovascular Therapy.
Results
Participant characteristics
A total of 536 IMPROVE trial participants with a final diagnosis of ruptured AAA met the inclusion criteria, of whom 135 (25%) died within 48 hours of randomisation. A total of 319 participants commenced open repair, 182 participants commenced EVAR and a further 35 participants did not receive an operation (were palliated or died before reaching theatre). There were 113 and 107 participants in the AJAX and ECAR trials, respectively, who met the inclusion criteria, of whom 17 (15%) and 15 (14%), respectively, died within 48 hours of randomisation. In the IMPROVE trial, participants were, on average, 2 years older and had larger aneurysms and a greater proportion of women were included (Table 18). In the Amsterdam cohort, 131 out of 514 patients (25%) died within 48 hours; the mortality rate was much higher in the STAR cohort, in which 107 out of 284 patients (38%) died, which was attributable, in part, to participants being an average of 4 years older. Patients in the STAR cohort also had, on average, lower admission systolic blood pressures and shorter aneurysm neck lengths, and a higher proportion had lost consciousness before arrival in the operating theatre.
| Candidate predictor | Trial | Cohort | |||
|---|---|---|---|---|---|
| IMPROVE (N = 536); 135 48-hour deaths (25%) | AJAX (N = 113); 17 48-hour deaths (15%) | ECAR (N = 107); 15 48-hour deaths (14%) | Amsterdam (N = 525); 131 48-hour deaths (25%) | STAR (N = 284); 107 48-hour deaths (38%) | |
| Age (years), mean (SD) [N] | 76 (8) [536] | 74 (9) [113] | 74 (11) [107] | 75 (9) [515] | 79 (9) [284] |
| Sex (male), n (%) [N] | 424 (79) [536] | 97 (86) [113] | 97 (91) [107] | 398 (77) [517] | 215 (76) [284] |
| Admission haemoglobin (g/dl), mean (SD) [N] | 11.1 (2.4) [530] | 11.5 (2.3) [113] | 10.6 (2.3) [107] | 11.1 (2.5) [499] | 11.1 (2.3) [273] |
| Admission creatinine (µmol/l), median (IQR) [N] | 118 (95–153) [524] | 106 (91–142) [107] | 114 (91–136) [105] | 110 (89–139) [478] | 117 (90–140) [268] |
| Admission systolic blood pressure (mmHg), mean (SD) [N] | 108 (32) [526] | 120 (40) [110] | 108 (30) [104] | 113 (37) [440] | 104 (39) [283] |
| Volume of i.v. fluids given before arrival in theatre (l), mean (SD) [N] | 1.06 (1.13) [391] | – | – | – | – |
| Maximum aneurysm diameter (mm), mean (SD) [N] | 86 (17) [460] | 76 (16) [92] | 77 (20) [106] | – | 81 (19) [192] |
| Aneurysm neck diameter (mm), mean (SD) [N] | 25 (4.3) [390] | 26 (4.0) [92] | 24 (4.5) [106] | – | 29 (11) [192] |
| Neck length (mm), mean (SD) [N] | 23 (17) [435] | 27 (13) [92] | 25 (14) [101] | 21 (14) [271] | 18 (18) [192] |
| Neck angle; proximal (degrees), mean (SD) [N] | 33 (20) [432] | 39 (21) [92] | 34 (26) [96] | 37 (22) [271] | 23 (16) [192] |
| Acute ischaemia detected on ECG (yes), n (%) [N] | 38 (7.7) [495] | 12 (18.5) [65] | 4 (3.7) [107] | 42 (21.7) [194] | 60 (43.2) [139] |
| Participant lost consciousness (yes), n (%) [N] | 47 (9.2) [512] | 13 (11.5) [113] | 12 (11.2) [107] | – | 68 (23.9) [284] |
| Cardiac arrest (yes), n (%) [N] | 8 (1.5) [536] | – | – | – | – |
Risk score development
Unadjusted ORs for each candidate variable are given in Table 19, after multiple imputation. Most candidate predictors were correlated with the outcome. FP modelling indicated a cubic effect of age, an inverse squared effect of admission systolic blood pressure and a log-transformed effect of neck length on the log-odds of mortality (see Appendix 2, Figure 30). Following backwards variable selection, the final variables retained in the prediction model were (1) age, (2) sex, (3) admission haemoglobin, (4) admission creatinine, (5) admission systolic blood pressure, (6) aortic neck length, (7) aortic neck angle and (8) acute cardiac ischaemia. Coefficients based on transformations of these variables are shown in Appendix 2, Table 40. A simplified ‘IMPROVE bedside score’ also was derived using an integer points system (see Appendix 2, Table 40).
| Variable | Univariate | |
|---|---|---|
| ORa (95% CI) | p-value | |
| Age (years) per 8-year increase | 1.49 (1.18 to 1.80) | < 0.001 |
| Admission haemoglobin (g/dl) per 2.4-g/dl increase | 0.63 (0.50 to 0.76) | < 0.001 |
| Admission creatinine (µmol/l) per 66-µmol/l increase | 1.37 (1.11 to 1.62) | 0.001 |
| Admission systolic blood pressure (mmHg) per 32-mmHg increase | 0.71 (0.56 to 0.86) | 0.002 |
| Volume of i.v. fluids given before arrival in theatre (l) per 1.13-l increase | 1.28 (1.03 to 1.53) | 0.014 |
| Maximum aneurysm diameter (mm) per 17-mm increase | 0.85 (0.67 to 1.03) | 0.13 |
| Aneurysm neck diameter (mm) per 4.3-mm increase | 0.95 (0.74 to 1.16) | 0.63 |
| Neck length (mm) per 17-mm increase | 0.64 (0.49 to 0.79) | < 0.001 |
| Neck angle; proximal (degrees) per 20° increase | 0.92 (0.72 to 1.12) | 0.45 |
| Sex (male) | 0.44 (0.28 to 0.69) | < 0.001 |
| Acute ischaemia detected on ECG (yes) | 2.79 (1.46 to 5.34) | 0.002 |
| Participant lost consciousness (yes) | 1.54 (0.80 to 2.94) | 0.19 |
Calibration and discrimination
Calibration plots are shown in Appendix 2, Figure 31, for both the IMPROVE score and the IMPROVE bedside score applied to the IMPROVE trial data. Both scores are generally well calibrated, particularly for individuals at a lower risk. The calibration slope was estimated as 1.07 (95% CI 0.83 to 1.32; p = 0.55) and 1.08 (95% CI 0.81 to 1.35; p = 0.54) for the IMPROVE score and IMPROVE bedside score, respectively, indicating that both low and high predictions are well calibrated.
The AUROCs of the model applied to the IMPROVE trial are provided in Table 20. The AUROC for this internal validation is derived using 10-fold cross-validation. The discriminative ability of the model is reasonable, but not exceptional, with an IMPROVE score AUROC of 0.720 (SE 0.025); this provides a slightly better predictive ability than the IMPROVE bedside score, which has an AUROC of 0.688 (SE 0.026).
| Model | Data set, AUROC (SE) | ||||
|---|---|---|---|---|---|
| Development | Validation | ||||
| IMPROVE (n = 536) | AJAX (n = 113) | ECAR (n = 107) | Amsterdam cohort (n = 514) | STAR cohort (n = 284) | |
| IMPROVE score | 0.720 (0.025)a | 0.665 (0.062) | 0.722 (0.073) | 0.737 (0.024) | 0.650 (0.034) |
| IMPROVE bedside score | 0.688 (0.026)a | 0.682 (0.055) | 0.688 (0.079) | 0.662 (0.025) | 0.594 (0.035) |
| VSGNE93 | 0.638 (0.027) | 0.628 (0.065)b | 0.672 (0.068)b | 0.655 (0.026)c | 0.655 (0.033)b |
| Hardman index30 | 0.648 (0.026) | 0.749 (0.050) | 0.730 (0.068) | 0.690 (0.026) | 0.606 (0.035) |
| Vancouver94 | 0.635 (0.028) | 0.609 (0.079)b | 0.725 (0.069)b | 0.642 (0.029)c | 0.702 (0.033)b |
External validation
The IMPROVE score and IMPROVE bedside score were validated on the four external data sets (the AJAX and ECAR trials and the Amsterdam and Stockholm area cohorts).
In AJAX, both AUROCs were < 0.70, whereas, in the ECAR trial, only the IMPROVE score gave an AUROC of > 0.70 (AUROC, 0.722; SE 0.073). In the two cohort studies, the Amsterdam cohort had a similar predictive performance to the ECAR trial, with a relatively high C-index for the IMPROVE score (0.737) but a low one for the IMPROVE bedside score (0.662), whereas the performance of both IMPROVE scores in the STAR cohort was relatively poor (AUROCs were < 0.650).
Comparison with other published risk scores
We compared the developed IMPROVE risk scores with previously published risk scores, namely the Vascular Study Group of New England risk score,93 the Hardman index30 and the Vancouver risk score. 94 The derivation of each score is given in Appendix 2, Table 41.
In terms of discrimination, the developed IMPROVE risk score outperformed other published risk scores in the IMPROVE trial data, whereas the Hardman index score outperformed the new developed (IMPROVE) scores in the AJAX and ECAR trial data for 48-hour mortality (see Table 20). There is some heterogeneity in the performance of each risk score when applied to different populations. For example, the Hardman index score performed well in the AJAX and ECAR trials but less well in the Amsterdam and STAR cohorts, perhaps attributable to the fact that the randomised trials AJAX and ECAR recruited a selected population of participants with ruptured AAA (only patients with relative haemodynamic stability and morphologically suitable for EVAR). The overall performance of each risk score in comparison with the predictive ability (discrimination) using age alone is presented in Figure 13. The average C-index increase is pooled across cohorts, weighted by the study-specific number of events. The change in C-index is highest when using the IMPROVE score, although the increase is still rather modest: ΔC = 0.08 (95% CI 0.05 to 0.11).
FIGURE 13.
Change in C-index for five ruptured AAA risk scores compared with the reference score using age alone. VSGNE, Vascular Study Group of New England. Reproduced from Sweeting et al. 97 © 2018 The Authors. BJS published by John Wiley & Sons, Inc., on behalf of BJS Society Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.

Performance of the risk scores in predicting 30-day mortality
The five different risk scores also were assessed for their ability to predict 30-day mortality, the results of which are given in Appendix 2, Table 42. The performance of two IMPROVE scores in the prediction of 48-hour mortality was similar; the difference in the C-index for 30-day mortality and for 48-hour mortality varied by study and risk score. For the three previously published risk scores (Vascular Study Group of New England, Hardman index and Vancouver) the 30-day C-indices were higher than the 48-hour C-indices in all studies except for the ECAR randomised trial, underlining the fact that these scores were developed using 30-day or in-hospital mortality as the outcome measure.
Decision curve analysis
The clinical usefulness of any particular ruptured AAA risk score depends on the ability to make better decisions with a model than without it. 98 If the risk score was to be used to decide whom to operate on, or whom to transfer to a specialist centre, then a cut-off point is required to classify patients either as low risk (operate) or high risk (palliate). 96 The cut-off threshold should balance the benefit against the harms and, in an emergency setting, this threshold would naturally be very high because the harms of not treating ruptured AAA patients are dire. Nevertheless, there may be a range of very high-risk individuals in whom surgeons feel that it is futile to attempt an operation and that palliation is the best course of action. Following methodology developed by Vickers and Elkin,99 the net benefit of treating patients at different risk cut-off points compared with treating none can be quantified and the value of a ruptured AAA risk score can be assessed. The cut-off value naturally defines the benefit-to-harm ratio that a surgeon is willing to accept. For example, supposing harms and benefits can be simply expressed in terms of quality-adjusted remaining life expectancy, a surgeon treating patients with a 48-hour mortality probability of ≤ 98% quantifies the consequence of not operating when it would have been of benefit to be 98 : 2 (49 times worse than the consequence of operating unnecessarily). 96
Figure 14 shows the net benefit decision curve for the full range of possible threshold probabilities. The green horizontal line denotes the decision to treat no one (the net benefit being zero for all possible probability cut-off points). The blue line denotes the net benefit of treating everyone in relation to a chosen benefit–harm trade-off (the threshold probability) and the black line denotes the net benefit of treating only those with a mortality risk below the chosen threshold. The net benefit is higher than treating no one for higher cut-off points in which the benefit-to-harm ratio is high. At a threshold probability of 1.0 (right hand side of the figure) surgeons would value an operation as infinitely better than no operation and hence the ‘treat all’ scenario gives the highest net benefit.
FIGURE 14.
Decision curve showing the benefit of the IMPROVE ruptured AAA risk score in helping to make treatment decisions. The graph shows the expected net benefit per patient relative to no treatment in any patient. Reproduced from Sweeting et al. 97 © 2018 The Authors. BJS published by John Wiley & Sons, Inc., on behalf of BJS Society Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
In Figure 14, the black line shows the net benefit if treatment decisions are based on the IMPROVE ruptured AAA risk score. The net benefit is almost identical to that of treating everyone for thresholds of > 50%. The benefit of a ruptured AAA risk score only becomes apparent for surgeons who would treat only low-risk individuals (i.e. with 48-hour mortality risk of ≤ 40%). At a higher threshold of 90%, the ruptured AAA risk score would correctly identify all 401 survivors in the IMPROVE trial but it also correctly identifies only 2 out of 135 participants (1.5%) who died within 48 hours. This lack of sensitivity highlights why a ruptured AAA risk score is no better than a treat-all solution for most sensible thresholds.
Conclusions
The developed ruptured AAA risk score has shown that there are some important predictors of survival to 48 hours after emergency admission, including two morphological variables (neck length and angle) that complicate emergency surgical repair by influencing the feasibility of endografting. Some of these predictors were found to have a non-linear relationship with the log-odds of mortality. The derived IMPROVE score performed adequately in the IMPROVE participants and outperformed other published risk scores in validation on some, but not all, external patient populations. In general, the published ruptured AAA risk scores to date do not have sufficient predictive accuracy to enable surgeons to make life or death decisions regarding providing an operation, as highlighted in the decision curve analyses. This conclusion is in agreement with the recent study100 evaluating five different scoring systems for 30-day mortality in a cohort of Dutch patients (from outside the Amsterdam area) with a ruptured AAA. This study100 evaluated the Vancouver score and Hardman index scores, together with the Glasgow101 and Edinburgh102 scores and the newly developed Dutch Aneurysm Score,103 with areas under the curve ranging from 0.59 to 0.72. Vos et al. 100 also concluded that an almost perfect prediction is needed to withhold an intervention, and no current scoring system is capable of that.
The unconvincing performance of risk scores, when applied to data sets from which they were not derived, is disappointing. The IMPROVE risk score was novel in that it is the only risk score to focus on 48-hour mortality and to include morphological parameters. However, it does not really perform better than older scores focusing on 30-day mortality when applied to other data sets, including population-based data sets such as those from the Stockholm area of Sweden and the Amsterdam area of the Netherlands. This would suggest that further research to assess the role of risk scores in the transfer of patients, from centres without emergency vascular cover to specialist vascular centres, would not be fruitful. The inability of risk scores to predict outcomes following emergency surgical repair with sufficient accuracy indicates that any risk score should be used only for comparing different populations or adjusting population data, as the Hardman index was used in the IMPROVE trial. If the mortality risk of ruptured AAA repair cannot be predicted with sufficient accuracy, we urge that the focus should shift to offering repair to more patients and to reducing non-intervention rates for emergency ruptured AAA repair, which are currently too high in England92 and some other countries. In this context, it is of interest that, when we used a Delphi consensus approach to formulate guidelines for the transfer of patients to specialist vascular centres, cardiac arrest in the same admission was the only condition with complete agreement that the patients should not be transferred. 62 Nevertheless, there is some evidence that few patients (14%) may survive emergency repair of a ruptured AAA even after a preoperative cardiac arrest. 104 In such life or death situations, perhaps it is just not ethical to consider using risk scores to withhold treatment and we suggest that the focus should shift to ensuring that the wishes of the patient and their family are respected.
Chapter 8 Collaborative research projects emanating directly from the IMPROVE trial
The collaborative individual patient data meta-analysis, which draws data from the three recent European randomised trials for the management of ruptured AAAs, has been discussed in the chapters focusing on the 30-day and 1-year outcomes of the IMPROVE trial (Chapters 3 and 5, respectively). The IMPROVE trial achieved its target recruitment because of the enthusiasm of the many local trial investigators and has provided detailed information about the morphology of ruptured AAAs from the core laboratory at St George’s Hospital, London (under the supervision of grant applicants Matthew M Thompson and Robert J Hinchliffe). These factors have enabled the following four collaborative research projects, all of which had the approval of the IMPROVE Trial Management Committee and appropriate ethics approvals at collaborating centres as necessary:
-
The development of a practical approach to expert elicitation for RCTs with missing health outcomes, in collaboration with colleagues Alexina Mason, Manuel Gomes, Richard J Grieve and James Carpenter from the London School of Hygiene & Tropical Medicine.
-
The comparative morphology of intact and ruptured AAAs, in collaboration with colleagues at St George’s Hospital CT core laboratory (including Alan Karthikesalingam, Matthew Grima and staff) and statisticians Mohammed Hudda and Michael J Sweeting.
-
Computational fluid dynamics of ruptured AAAs, in collaboration with Barry Doyle, Karol Miller and their students at the University of Western Australia.
-
Computational flow dynamics of CIAs, again in collaboration with Barry Doyle, Karol Miller and their students at the University of Western Australia as well as Maarit Venermo (University of Helsinki) and Igor Koncar (University of Belgrade).
The development of a practical approach to expert elicitation for randomised controlled trials with missing health outcomes
The IMPROVE trial, like most RCTs, assessed participant HRQoL. In the IMPROVE trial, it was not possible to collect baseline data, but data were collected at three time points: 3 months, 12 months and 3 years after randomisation. Like most randomised trials with missing data, it was assumed that, after conditioning on the observed data, the probability of missing data does not depend on the participant’s outcome and so the data are ‘missing at random’. 16,23 This assumption is usually implausible, for example because participants in relatively poor health may be more likely to be non-responders to questionnaires or demit from routine clinical follow-up. Methodological guidelines recommend that trials undertake sensitivity analysis, which is best informed by elicited expert opinion, to assess whether or not conclusions are robust to alternative assumptions about the missing data. A major barrier to implementing these methods in practice is the lack of relevant practical tools for eliciting expert opinion.
We developed a new practical tool for eliciting expert opinion within the IMPROVE trial and demonstrate its use for the IMPROVE trial and its applicability to other randomised trials with missing QoL data. In the IMPROVE trial at 3 months post randomisation, 21% of participants did not complete HRQoL questionnaires (assessed using the EQ-5D-3L). We addressed this problem by developing a web-based tool that provides a practical approach to eliciting expert opinion about EQ-5D-3L differences between participants with missing data and those with complete data.
We developed an easy-to-use web-based elicitation tool using Shiny (RStudio Inc., Boston, MA, USA), a web application framework within the widely used statistical software, R (The R Foundation for Statistical Computing, Vienna, Austria),105 which could be administered by e-mail or in conference breaks. We adopted a graphical approach, using three cartoon characters who had undergone either open repair or endovascular repair of their ruptured AAA, minimised the administrative burden by collecting informed consent electronically and offered a £20 Amazon (www.amazon.co.uk; Amazon.com, Inc., Bellevue, WA, USA) gift card as a token of appreciation for completed surveys. ‘Good practice’ recommendations for eliciting expert opinion were followed, in particular by including a feedback question and allowing the experts to revise their answers. 106
The scale of scores for the elicitation exercise is the same as the original scale for the EQ-5D-3L utility score, multiplied by 100 for ease of completion. The expert is provided with possible scores for typical patients, with six exemplar diagnoses on the scale between –20 and 100 (based on published literature), which were chosen as they were anticipated to be familiar to our experts and because they spanned the EQ-5D-3L scale.
A total of 26 experts participated in the elicitation exercise, the majority at the Annual Meeting of the Vascular Society of Great Britain and Ireland in Bournemouth in November 2015, because the Shiny tool was not compatible with all NHS interfaces. The elicited EQ-5D-3L scores were lower on average for the participants with missing data than for those with complete data, but there was considerable uncertainty in these elicited values.
We show how this expert opinion can define informative priors within a fully Bayesian framework to carry out sensitivity analyses that allow the missing data to depend on unobserved participant characteristics. The ‘missing-at-random’ analysis found that participants randomised to the endovascular strategy, compared with those randomised to open repair, had a higher average EQ-5D score, of 0.062 (95% CI –0.005 to 0.130). Our sensitivity analysis, which used the elicited expert information as pooled priors, found that the gain in average EQ-5D-3L score for the endovascular strategy, compared with open repair groups, was 0.076 (95% CI –0.054 to 0.198).
The estimated effect of randomised group on average EQ-5D-3L score was similar across the alternative approaches to the missing data, but the sensitivity analysis resulted in greater uncertainty about this mean difference; the credible intervals from allowing the data to be non-randomly missing were wider than following the missing-at-random assumptions and the complete case analyses. These wider credible intervals recognise the variation within and across the experts in the likely differences in outcomes between those with missing data and those with observed EQ-5D-3L data.
In conclusion, we have designed and exemplified a practical tool for eliciting the expert opinion required by recommended approaches to the sensitivity analyses of RCTs. We show how this approach allows the trial analysis to fully recognise the uncertainty that arises from making alternative plausible assumptions about the reasons for missing data. This tool can be widely used in the design, analysis and interpretation of future trials. This research project has now been published in Clinical Trials,107 and to facilitate the use of this practical tool for other trials, materials have now been made available for download. 107
The comparative aortic morphology of intact and ruptured abdominal aortic aneurysms
Most AAAs are broadly fusiform in shape and, to date, the AAA diameter provides the best guide to the risk of aneurysm rupture. Some small studies have suggested that other factors, such as wall stress and the volume of luminal thrombus, might be just as important,108 but none of these factors has been subject to a prospective investigation. Because automated or semi-automated three-dimensional reconstruction of aortic CT scans is now widely available and essential to the planning of endovascular repair, we wished to test the hypothesis that morphological features (other than maximum aortic diameter) might be associated with a ruptured AAA rather than an intact AAA and hence could be useful in assessing the risk of AAA rupture.
Using a standard methodology,26 we measured the aortic morphology of 907 patients who had undergone elective repair of an intact AAA at St George’s Hospital between 2009 and 2013 and 294 cases of ruptured AAA (blood outside the AAA sac and adequate-quality CT scan) from the IMPROVE trial. All CT scans were anonymised and held only trial numbers or St George’s Hospital database identifying numbers. The following parameters were measured: proximal aneurysm neck (suprarenal aortic diameter, diameter of 1 mm below the distal renal artery, neck length, α neck angle and neck conicality), maximum AAA diameter and thrombus volume, iliac bifurcation middle angle, and common iliac artery diameter and iliac tortuosity index.
Analysis was conducted in accordance with a prespecified analysis plan using a logistic regression approach in which analyses were stratified by sex. Regression models were adjusted for two prespecified confounders: maximum aneurysm diameter and age. In order to adjust for maximum aneurysm diameter, we formed narrow categories of the diameter and adjusted for the categorised variable in the models. These narrow categories were formed after careful examinations of the overlap of the AAA diameters in both populations.
The distribution of the morphological variables by ruptured or intact AAA and by sex is shown in Table 21. The association between aneurysm morphology and rupture of an AAA, when adjusting for all eight morphology variables and the prespecified confounders, is shown in Table 22. Neck diameter was associated with rupture in women, in whom an increase of 4.1 mm led to an adjusted OR of 2.06 (95% CI 1.10 to 3.86; p = 0.02). Furthermore, aneurysm neck angle α was also associated with rupture, but only in males. The adjusted OR was 1.53 (95% CI 1.22 to 1.92; p < 0.01) per 18.3° increase.
| Measurement | Sex, mean (SD) | |||
|---|---|---|---|---|
| Males (N = 1046) | Females (N = 151) | |||
| Elective repair (n = 800) | Rupture repair (n = 246) | Elective repair (n = 103) | Rupture repair (n = 48) | |
| Aneurysm neck diameter at 1 mm (mm) | 24.2 (4) | 25.4 (3.8) | 22 (4) | 23.9 (5.2) |
| Aneurysm neck length (mm)a | 31.5 (14.1) | 30.9 (13.8) | 29 (13.2) | 29.8 (13.9) |
| Aneurysm neck angle α (degrees)a | 20.9 (16.9) | 31.5 (19) | 26.6 (20.2) | 32.9 (19.7) |
| Maximum common iliac artery diameter (mm)a | 20.2 (7.3) | 21 (8.7) | 17.8 (6.8) | 18 (5.3) |
| Neck conicality (mm) | 0.5 (0.9) | 0.6 (1.2) | 0.6 (1.3) | 0.6 (0.9) |
| Aortic aneurysm thrombus volume (mm3)a | 97.1 (85.8) | 190.8 (125) | 80 (63.5) | 109.1 (82.5) |
| Maximum iliac tortuosity indexa | 1.5 (0.2) | 1.3 (0.3) | 1.5 (0.3) | 1.2 (0.3) |
| Maximum aortic aneurysm diameter (mm) | 65.9 (11.2) | 84.3 (14.5) | 62.9 (8.6) | 70.9 (9.9) |
| Iliac bifurcation middle angle (degrees) | 60.5 (22.8) | 46.7 (15.9) | 67.0 (22.9) | 48.1 (26.5) |
| Age (years) | 80.5 (8.3) | 79.2 (7.4) | 81.8 (8.2) | 83.6 (6.8) |
| Suprarenal aortic diameter (mm) | 27.4 (3.4) | 26.1 (3.1) | 26.1 (3.9) | 24.7 (3.8) |
| Morphological variables | Sex | |||||||
|---|---|---|---|---|---|---|---|---|
| Males (n = 1044)a | Females (n = 151) | |||||||
| Univariate OR (95% CI) | p-value | Multivariable OR (95% CI) | p-value | Univariate OR (95% CI) | p-value | Multivariable OR (95% CI) | p-value | |
| Aneurysm neck diameter at 1 mm per 4.1-mm increase | 1.15 (0.96 to1.37) | 0.134 | 1.17 (0.92 to 1.49) | 0.209 | 1.55 (1.04 to 2.31) | 0.033 | 2.06 (1.10 to 3.86) | 0.024 |
| Aneurysm neck length per 14.0-mm increase | 1.13 (0.94 to 1.36) | 0.191 | 1.24 (0.99 to 1.55) | 0.060 | 0.97 (0.62 to 1.53) | 0.910 | 1.14 (0.63 to 2.08) | 0.668 |
| Aneurysm neck α angle per 18.3° increase | 1.34 (1.12 to 1.61) | 0.001 | 1.53 (1.22 to 1.92) | < 0.001 | 1.20 (0.81 to 1.78) | 0.373 | 1.10 (0.66 to 1.84) | 0.707 |
| Maximum common iliac artery diameter per 7.5-mm increase | 0.96 (0.81 to 1.15) | 0.663 | 1.22 (0.97 to 1.53) | 0.088 | 0.86 (0.53 to 1.40) | 0.549 | 0.70 (0.30 to 1.61) | 0.399 |
| Conicality per 1.0% per 1-mm increase | 0.99 (0.84 to 1.17) | 0.895 | 1.11 (0.90 to 1.37) | 0.327 | 1.04 (0.71 to 1.53) | 0.838 | 1.20 (0.70 to 2.05) | 0.502 |
| Aneurysm thrombus volume per 100.2-mm3 increase | 1.05 (0.87 to 1.28) | 0.86 | 1.08 (0.83 to 1.41) | 0.548 | 1.01 (0.56 to 1.83) | 0.974 | 0.82 (0.36 to 1.88) | 0.642 |
| Maximum iliac tortuosity index per 0.3 increase | 0.28 (0.21 to 0.37) | < 0.001 | 0.27 (0.20 to 0.37) | < 0.001 | 0.31 (0.15 to 0.63) | 0.001 | 0.28 (0.13 to 0.63) | 0.002 |
| Iliac bifurcation angle per 22.6° increase | 0.36 (0.28 to 0.47) | < 0.001 | 0.36 (0.27 to 0.49) | < 0.001 | 0.45 (0.27 to 0.76) | 0.003 | 0.49 (0.29 to 0.83) | 0.009 |
The maximum iliac tortuosity index and iliac bifurcation middle angle both showed strong evidence of an inverse association with rupture in both sexes. An increase of 0.3 in the tortuosity index led to an adjusted OR of 0.27 (95% CI 0.20 to 0.37; p < 0.01) in men and of 0.28 (95% CI 0.13 to 0.63; p < 0.01) in women. For a 22.6° increase in the iliac bifurcation middle angle, there was an adjusted OR of 0.36 (95% CI 0.27 to 0.49; p < 0.01) in men and of 0.49 (95% CI 0.29 to 0.83; p = 0.01) in women.
The results for patients, by quintiles of those morphological variables with significant associations with rupture, were also examined. Neck diameter demonstrates a positive linear trend among men across quintiles and a similar but less defined relationship in women. Neck angle, α, showed an increasing positive linear association with rupture across the quintiles, but this relationship was unclear in women. The negative linear association between quintiles of tortuosity index and rupture among men was replicated in women, except for the fourth quintile, in which the adjusted OR increased slightly. The adjusted ORs for the quintiles of iliac bifurcation middle angle showed an extremely similar relationship to that of tortuosity index, in which a linear negatively associated relationship was visible in both sexes. The discrepancies and wide 95% CIs were likely to be attributable to the small group numbers in the analysis of women.
These data suggest that iliac artery parameters, including the aortic bifurcation angle and iliac tortuosity index, in addition to aortic diameter, might be helpful in assessing the risk of rupture. The cases of ruptured aneurysms from the IMPROVE trial had (after adjustment for aortic diameter and the other morphological variables), on average, narrower aortic bifurcation angles and less iliac artery tortuosity than cases of intact aneurysms. It may also be important to note that, after adjustment for aortic diameter and other morphological characteristics, thrombus volume was not associated with ruptured aneurysms compared with intact aneurysms.
These data and analyses have several limitations, particularly the relatively poor repeatability of the measurement of proximal neck angle and iliac bifurcation angle. For these reasons, newer software has been obtained, which allows for more repeatable measurement of angles. This measurement is ongoing and, therefore, the above analyses are regarded as preliminary. They have been reported and discussed at the International Meeting for Aortic Diseases in Liège, Belgium, in September 2016. The novel findings concerning iliac bifurcation angles and tortuosity also have been considered in our ongoing collaborations with the computation flow dynamics laboratory in Western Australia (reported in the next two sections).
Computational fluid dynamics of ruptured abdominal aortic aneurysm
Knowledge of how aortic morphology influences AAA biomechanics is still developing. In a single case from the IMPROVE trial, we have considered whether or not regions of high wall stress in the aneurysm sac can predict the site of aneurysm rupture,109 with further work in progress. The effects of proximal neck angle and iliac bifurcation angle on both wall shear stress (WSS) and wall stress have been previously reported. Previously, it has been indicated that AAA wall stress increased with both iliac bifurcation angle and aortic neck angle, whereas AAA WSS remained almost constant. 110 This might suggest that AAAs with higher proximal neck angles and iliac bifurcation angles are at a higher risk of rupture. However, the recent comparisons of the morphology of ruptured AAAs and intact AAAs (see The comparative aortic morphology of intact and ruptured abdominal aortic aneurysms) have questioned these suggestions and hinted that AAAs with wider iliac bifurcations might be less prone to rupture, despite other morphological factors.
Therefore, fluid–structure interaction simulations were carried out on a range of idealised AAA geometries to conclusively determine the influence of both proximal neck angle and iliac bifurcation angle on AAA wall stress and WSS.
We found a positive linear relationship between peak AAA WSS (i.e. the peak WSS observed in the AAA sac region) and iliac bifurcation angle (see Appendix 2, Figure 32). The mean peak WSS across all geometries was 4.52 Pa with maximum and minimum values of 2.91 Pa and 6.19 Pa, respectively. Therefore, peak WSS increased more than twofold when the iliac bifurcation angle increased from 30° to 150°. Peak WSS in the AAA increased only when the proximal neck angle was > 30° and when the velocity field also changed to > 30°. In contrast, peak wall stress (but not von Mises stress) increased linearly with iliac bifurcation angle, but there was no clear association with time-averaged WSS (TAWSS) (see Appendix 2, Figure 32).
These observations from fluid–structure interaction simulations may be important to support our findings of an apparent association between bifurcation angle and likelihood of rupture, with ruptured AAAs tending to have a smaller iliac bifurcation angle than an intact AAA (see The comparative aortic morphology of intact and ruptured abdominal aortic aneurysms). The simulation data have been submitted for publication.
Morphological and computational flow dynamic characteristics of common iliac aneurysms
Common iliac aneurysms are rare (compared with AAAs); much less is known about their natural history111 and the evidence base for their management is scant, although both open repair and endovascular repair are clinically effective. 112 In the IMPROVE trial, there were seven cases of rupture of an isolated common iliac aneurysm (in the absence of a significant AAA). In scientific studies such as computational flow dynamics, CIAs have the advantage of often having a normal or nearly normal contralateral common iliac artery for comparison. Initially, our interest was focused on whether the distribution of WSS or intraluminal thrombus predicted the site of CIA rupture. Common iliac aneurysms sometimes have one or more patent distribution arteries. So, using a single case study with several branches of the internal iliac artery, we undertook a simulation study to evaluate the effect of these outflow arteries on haemodynamics in the iliac aneurysm. We concluded that accounting for small downstream arteries may not be vital to accurate computations of upstream flow. 113 Other preliminary investigations included the residence time of platelets and monocytes, velocity fields, TAWSS, oscillatory shear index and endothelial cell activation potential. We found that high cell residence times, low TAWSS, high oscillatory shear index and high endothelial cell activation potential all correlate with regions of intraluminal thrombus development. 114 Initial investigations showed that the point of CIA rupture was usually in a region of low TAWSS (Figure 15).
FIGURE 15.
Rupture (marked by an x) usually occurs in regions of low TAWSS.

After the indication that the iliac bifurcation angle and iliac artery tortuosity might be influential in discriminating the morphology of ruptured AAAs compared with intact AAAs, investigations were expanded to include a more comprehensive assessment of the morphology and computational flow dynamics of both ruptured CIAs and intact CIAs and their contralateral common iliac arteries. In particular, the underlying hypothesis was that the wider the iliac artery bifurcation was, the lower the WSS in the distal aneurysm would be, leading to an enhanced risk of rupture.
Through a network of collaborations, a total of 23 good-quality CT scans of CIAs, suitable for both morphological assessment and computational flow dynamics, were identified (10 cases of rupture and 13 cases of elective repair of an intact CIA; in 2/23 cases the aneurysms were bilateral). Visual inspection of the three-dimensional reconstructions of the CT scans suggested that the gross anatomy could be categorised as one of four types (Table 23), with most of the ruptures being of the saccular or forward-projecting type. The ruptures had a larger average CIA diameter than the intact CIAs (82.1 mm compared with 50.3 mm, respectively) and contained larger thrombus and calcium volumes than the intact CIAs. On average, the ruptured cases had wider bifurcation angles (91° compared with 67° for intact CIA cases) and more tortuous abdominal aortas than the intact CIA cases. For fusiform aneurysms, five out of eight cases had a right CIA but the other forms had predominantly left CIAs.
| CIA type | Gross anatomy | Image |
|---|---|---|
| Saccular | Large round aneurysm |

|
| Forward-projecting | The aneurysm protrudes forward with a large area in the sagittal plane, maintaining a comparatively small area in the axial and coronal views |

|
| Fusiform | A less severe dilatation that extends over a section of the artery in the direction of flow |

|
| Kinked | There is a tight bend in the CIA, with the aneurysm immediately distal to this feature; in our data, all occurred on the left side |

|
Although wider bifurcation angles were associated with intact AAAs compared with ruptured AAAs, the converse would appear to hold for an isolated CIA, in which rupture is associated with wide iliac bifurcation angles. These relationships provide novel mechanistic insights into the role of haemodynamics (and cellular activation) in precipitating the catastrophic event of aneurysm rupture and are subject to ongoing computational flow dynamic investigations.
Added value from collaborations and future use of IMPROVE trial data
This chapter provides a flavour of the added value emanating from a cohort of carefully phenotyped participants in a randomised trial. Some of the projects have been completed, whereas others will continue. In addition, we hope that data from the IMPROVE trial will be used in future high-quality collaborative studies, particularly to progress the management of patients with intact and ruptured AAAs.
Chapter 9 Concluding remarks and suggestions for further research
There are few randomised trials carried out in contexts in which emergency surgery is required to save a patient’s life. As such, this trial presented important challenges in design, implementation and interpretation.
Trial design
An understanding of the trial design is pivotal to understanding and interpreting the findings of the IMPROVE trial. The pragmatic design (randomisation of patients at the point of clinical diagnosis by a senior clinician rather than after rupture had been proved on diagnostic imaging) has been criticised because it allowed the randomisation of patients without a rupture. In contrast, the design had the real advantage of reflecting the real-life situation in the emergency room, allowing for non-selective randomisation and randomisation of the most severely ill and unstable patients and stimulating rapid recruitment. Originally, another reason for the trial design was that it was feared that delays in CT and transfer to theatre in the endovascular strategy group might offset any benefits from EVAR; by the time the trial started, emergency CT scans were more accessible and CT was ubiquitous in both randomised groups. However, randomisation before CT to assess both rupture and the suitability for EVAR meant that we were comparing an endovascular strategy (EVAR with the default of open repair when either aortic morphology or operational reasons precluded EVAR) with open repair. Patients also were randomised before anaesthetic opinion about their suitability for general anaesthesia and whether or not the necessary endovascular facilities were available (such as theatre and staffing, including a CT radiographer). The IMPROVE trial was comparing different strategies for managing patients with suspected aneurysm rupture and, for this reason, was bound to have a higher rate of treatment non-compliance than most randomised trials, but it also allowed for a high external validity. The IMPROVE trial design contrasts with the ‘cleaner’ but highly selective designs of the Dutch19 and French22 randomised trials, in which patients were not randomised until after CT had indicated the presence of a rupture and when the patients were sufficiently stable to give informed consent. In the IMPROVE trial, the consent process included consent from relatives and, under the Mental Capacity Act 2005,25 if approved by a senior non-treating clinician. Overall, the IMPROVE trial was designed to inform service provisions that would result in better outcomes for patients with ruptured AAAs and to collect data on health economic outcomes as well as the primary outcome of mortality and the clinical outcome of reinterventions.
Implementation
The early phases of recruitment were slow because of regulatory difficulties and the need for sites to fulfil the credential criterion of having completed at least five cases of emergency EVAR with reasonable outcomes, a criterion approved by the Trial Steering Committee. However, in the last 18 months, recruitment was at the rate of ≈20 participants per month, with more than half of eligible (non-moribund) participants being randomised. This differs from the very selective randomisation in the Dutch19 and French22 trials, each of which took ≈7 years to recruit just over 100 participants. There has been criticism that the credential criteria did not allow for adequate experience in emergency EVAR. However, the timing of the trial was important. Recruitment took place between 2009 and 2013, before the National Abdominal Aortic Aneurysm Screening Programme was fully rolled out, which had an impact on the number of ruptures in men. The falling numbers of ruptures that have been operated on in England after this time emphasises the relevance of these considerations (Table 24).
| Year | n | Percentage of procedures | |
|---|---|---|---|
| EVAR | Open repair | ||
| 2009/10 | 1888 | 20.2 | 79.8 |
| 2010/11 | 1861 | 26.3 | 73.7 |
| 2011/12 | 1898 | 24.9 | 75.1 |
| 2012/13 | 1893 | 30.0 | 70.0 |
| 2013/14 | 1717 | 33.3 | 66.7 |
| 2014/15 | 1438 | 35.6 | 64.4 |
| 2015/16 | 1545 | 39.7 | 60.3 |
Interpretation
The early results from the IMPROVE trial showed few advantages for an endovascular strategy. By 30 days, mortality and reinterventions were similar in the two groups; only women (22% of the cohort) seemed to benefit from the endovascular strategy, in part because of their very high mortality after open repair. However, on average, participants in the endovascular strategy group (in which 64% were morphologically suitable for EVAR) spent less time in intensive care and went home sooner than those in the open repair group, and more often directly to home, leading to lower average costs. Similarly, despite their more selective designs, the Dutch and French trials also found no difference in 30-day mortality between their EVAR and open repair groups.
After 1 year, again none of the three trials showed a significantly higher mortality for open repair, although there was good evidence that the absolute mortality rate, particularly at 30 days, was influenced by aortic morphology and that those participants who were suitable for EVAR had lower mortality than those who were not suitable for EVAR, irrespective of the type of repair. This was a real advantage for the non-selective design of the IMPROVE trial. More importantly for participants in the IMPROVE trial, average QoL at both 3 and 12 months was better in the endovascular strategy than in the open repair group, with a QALY gain of borderline significance. Because the majority of costs were related to the primary admission and, at 1 year, average costs remained lower for the endovascular strategy group, the endovascular strategy was cost-effective at 1 year.
The IMPROVE trial is the only one of the three recent European trials to report comprehensive 3-year outcomes. Over time, there was a consistent trend for survival, for QoL to be slightly better and for costs to be lower, although not always significantly so, in the endovascular strategy group. These trends are amplified by 3 years.
At 3 years, the survival curves had diverged. Compared with the open repair group, the endovascular strategy had lower mortality, a finding that was particularly evident in the 502 participants for whom repair of a ruptured aneurysm had started. The reasons for the mid-term difference in survival are possibly related to the severity and invasiveness of the initial intervention and the subsequent need for intensive care. Participants in the open repair group had, on average, longer critical care stays than the endovascular strategy group (6.3 days compared with 4.2 days, respectively), and 46 participants in the open repair group required renal replacement therapy postoperatively, compared with 32 in the endovascular strategy group. Because both prolonged stay in critical care and acute kidney injury115,116 are known to be associated with higher long-term mortality, it may be the differences in critical care that result in the mid-term divergence of the survival curves.
By 3 years, there was no significant difference in QoL between the groups, but the earlier gains in QoL, together with the survival gain, led to QALYs being significantly greater for the endovascular strategy group. The reintervention rate was similar between the randomised groups, but the endovascular strategy group had less severe reinterventions, leading to the overall costs still being lower for the endovascular strategy group. These factors then combine to give a very high probability (90%) of dominance for the endovascular strategy on the cost-effectiveness plane.
Contrasting results of the randomised trials in the elective setting and emergency setting
After aneurysm rupture, an endovascular strategy offers no reduction in operative mortality at 30 or 90 days but offers an interim survival advantage at 3 years, which, together with the early gains in QoL, leads to a QALY gain after 3 years. Aneurysm-related reinterventions, particularly the more severe reinterventions, took place at a similar rate in both groups. For ruptures, the cost differences observed at 30 days (non-significantly in favour of the endovascular strategy group)21 were not eroded by an increased burden of reinterventions in later follow-up; therefore, the endovascular strategy was deemed cost-effective. All of these results are in sharp contrast to those of earlier trials of elective EVAR compared with open repair of AAAs (Table 25).
| Parameter | Repair | |
|---|---|---|
| Elective | Rupture | |
| 30-day mortality | 2.5-fold higher for open repair88 | No difference51 |
| 3-year mortality | No difference88 | Endovascular strategy better |
| Length of primary hospital stay | No difference41,117 | Shorter for endovascular strategy23 |
| Reintervention rate | Twofold to threefold higher after EVAR88 | No difference |
| Major or severe reinterventions | Twofold to threefold higher after EVAR89 | No difference |
| QoL | Better after open repair or no difference at 1 year41,118,119 | Better at 3 months and 1 year for endovascular strategy |
| Costs | EVAR higher41,120 | Endovascular strategy slightly lower |
| Cost-effectiveness | EVAR not cost-effective91,119–121 | Endovascular strategy is cost-effective |
The earlier trials of elective EVAR compared with open repair enrolled only selected participants who were known to be suitable for either treatment and mostly adhered strictly to the IFU of the likely endovascular device. In these trials in the elective setting, participants were younger, on average, by ≈3 years, and the proportion of women was smaller than in the IMPROVE trial. The trials found that 30-day mortality was 2.5 times lower for the EVAR groups than for the open repair groups but that, after 3 years, there was no difference in mortality between the groups. The reintervention rate to 5 years and beyond, was always much higher in the EVAR groups and, if anything, by 1 year, the open repair group had a higher average QoL and lower average health-care costs. Therefore, there was no evidence that EVAR was cost-effective.
The reasons for these differences in the comparative effectiveness of the endovascular strategy and open repair in the emergency and elective settings remain speculative. The shock associated with rupture, especially in those with pre-existing cardiac disease, probably kills many patients irrespective of the type of repair, but EVAR is less invasive and can be conducted under local anaesthesia, so that patients recover more rapidly than after open repair. The proportion of women in the IMPROVE trial (22%) was much higher than in the trials of elective aneurysm repair, and the advantages of the endovascular strategy were greater in women than in men. The continuing burden of major laparotomy-related reinterventions after open repair for ruptures (which are not seen after elective open repair) might contribute to both mid-term mortality and costs in the open repair group, whereas endovascular devices and the technical skills to deploy them may have improved since the trials of elective repair were conducted. The reconvergence of the survival curves beyond 3 years is unexplained too, but this phenomenon has also been observed in the analysis of Medicare47 and registry data. 79 Possible explanations include excess cancer deaths and late failure of EVAR.
Metrics for reporting reinterventions after emergency aneurysm repair and patient perspectives
The categorisation of reinterventions for the mid-term follow-up in the IMPROVE trial was based on clinician opinion of the severity of the intervention and whether or not the life of the patient was threatened if the reintervention was not successful. The scoring system used was based on the one developed for the very long-term follow-up of the EVAR 1 trial,89 with additional reinterventions that were more specific to rupture and laparotomy-related complications scored by consensus of the IMPROVE trial investigators. The acceptance of scoring reinterventions on a scale from 1 to 5 would argue that similar scoring systems, with specific reporting metrics for events such as secondary rupture and late conversions to open repair, should be incorporated into the National Vascular Registry and similar data sets. Perhaps more importantly, the low number of patients and members of the public who were consulted had very different opinions to clinicians about the severity of reinterventions. They were unanimous in ranking amputation as the most feared complication or reintervention. These observations argue for the need for further work to incorporate patients’ opinions into the metrics used for reporting the outcomes of ruptured AAA repair.
Implications for future health care
The findings from the IMPROVE trial suggest that an endovascular strategy is better than open repair for a ruptured AAA. Therefore, a strong case can be made that all providers of emergency vascular services should be able to offer both emergency EVAR and open repair at all times (24 hours a day, 7 days a week). This requires considerable logistics with respect to the availability of appropriate personnel, facilities and consumables. Not all IMPROVE trial centres could offer randomisation every day of the week because specialist endovascular teams and/or facilities were not available. As a result, 26 out of 259 participants with a confirmed rupture and morphological suitability for EVAR and who were randomised to the endovascular strategy subsequently underwent open repair because the endovascular team and/or facility was not immediately available. The evidence from other centres across the world suggests that the use of standard, well-rehearsed protocols for ruptured aneurysm repair can reduce operative mortality, particularly for EVAR. 54,122 The development of optimal protocols and training is being addressed by the relevant professional bodies in the UK. The IMPROVE trial has shown that about one-third of patients are not morphologically suitable for standard EVAR, and using EVAR in morphologically unsuitable patients is associated with a high 30-day mortality. Therefore, the real challenges in the future may be associated with:
-
providing sufficient capacity for emergency endovascular services
-
the potential further restructuring of services for emergency vascular care to allow patients to be treated in centres that also have expertise in either emergency open surgery or complex endovascular surgery (e.g. using fenestrated or branched endografts), for the treatment of patients who are not morphologically suitable for standard EVAR
-
ensuring the equitable availability and continuing cost-effectiveness of emergency vascular services.
Data from HES indicate that the proportion of emergency AAA repairs being carried out by EVAR is increasing year on year (see Table 24). The conduct and results of the IMPROVE trial may have contributed to these changes and further emphasises the need to provide sufficient capacity for emergency endovascular services. Given the findings of the IMPROVE trial, it would seem important for the health service to have sufficient capacity to ensure that the proportion of emergency AAA repairs that are undertaken by EVAR rather than open repair increases further.
Suggestions for further research
These arise both from the trial itself and the associated studies described in Chapters 4, 7 and 8. Two very crucial pieces of further research are suggested:
-
Formal assessment of whether or not avoidance of general anaesthesia improves the survival of patients undergoing EVAR for ruptured AAA repair.
-
Improving resuscitation techniques for older persons with circulatory collapse, including the identification of evidence-based blood pressure targets, which can be incorporated into guidelines. This research should include but not be limited to ruptured aneurysms.
Other significant recommendations for further research include:
-
To consider whether or not emergency paramedical staff and general practitioners could diagnose ruptured AAA with sufficient accuracy to allow the direct transfer of patients from home to a specialist vascular centre.
-
To assess metrics, other than mortality, for reporting the outcomes of emergency surgery, which include the viewpoints of patients, timelines to treatment and avoidance of secondary transfers.
-
To assess whether or not further centralisation of emergency vascular services may be required to allow provision of sufficient expertise in emergency complex endovascular repair (using fenestrated or branched endografts) and open repair of juxtarenal aneurysms and other aortoiliac aneurysms that are unsuitable for standard EVAR, as well as to increase the availability of emergency standard EVAR and open repair at all times (24 hours a day, 7 days a week).
-
With the introduction of AAA screening for men, an increasing proportion of ruptures is likely to be in women and there is a need for the acquisition and analysis of more sex-specific data that might lead to further suggestions for improving outcomes for women.
In addition, and not specifically related to ruptured AAAs:
-
to consider the practice of consent waivers and post-randomisation consent for trials of emergency medical treatment that is required to save a patient’s life.
Final words
This trial would not have been possible without the real commitment and enthusiasm of the vascular surgical community in the UK, as part of their enthusiasm to provide better-quality care for their patients.
Acknowledgements
Contributions of authors
Pinar Ulug (Trial Manager) was involved in the conduct, acquisition of data, analysis and reporting phases.
Robert J Hinchliffe (Professor of Vascular Surgery) was involved in the design, conduct and reporting phases, as well as in additional collaborative research.
Michael J Sweeting (Trial Statistician) was involved in the conduct, analysis and reporting phases.
Manuel Gomes (Senior Research Fellow in Health Economics) conducted the health economic analyses.
Matthew T Thompson (Professor of Vascular Surgery) was involved in the design, core laboratory and reporting phases, as well as additional collaborative research.
Simon G Thompson (Professor of Statistics) was involved in the design, analysis and reporting phases.
Richard J Grieve (Professor of Health Economics Methodology) was involved in the design, analysis and reporting phases, as well as additional collaborative research.
Raymond Ashleigh (Consultant Radiologist) was involved in the design, core laboratory and reporting phases, as well as in additional collaborative research.
Roger M Greenhalgh (Emeritus Professor of Surgery) was involved in the design and reporting phases.
Janet T Powell (Professor of Vascular Biology and Medicine) was involved in the design, conduct, analysis and reporting phases, as well as in additional collaborative research.
Publications
Hinchliffe RJ, Powell JT, Cheshire NJ, Thompson MM. Endovascular repair of ruptured abdominal aortic aneurysm: a strategy in need of definitive evidence. J Vasc Surg 2009;49:1077–80.
Powell JT. Time to IMPROVE the management of ruptured abdominal aortic aneurysm: IMPROVE triallists. Eur J Vasc Endovasc Surg 2009;38:237–8.
The IMPROVE Trial Management Committee. Getting research in the NHS started: can we assess new technologies in a timely manner? Lancet 2010;375:2072.
Hinchliffe RJ, Ribbons T, Ulug P, Powell JT. The transfer of patients with ruptured abdominal aortic aneurysm from general hospitals to specialist vascular centres: results of a Delphi consensus study. Emerg Med J 2013;30:483–6.
Doyle BJ, McGloughlin TM, Miller K, Powell JT, Norman PE. Regions of high wall stress can predict the future location of rupture of abdominal aortic aneurysm. CVIR 2014;37:815–18.
IMPROVE Trial Investigators. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ 2014;348:f7661.
IMPROVE Trial Investigators. Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg 2014;101:216–24.
IMPROVE Trial Investigators. An endovascular strategy for suspected ruptured abdominal aortic aneurysm brings earlier home discharge but not early survival or cost benefits. Eur J Vasc Endovasc Surg 2014;47:333–4.
IMPROVE Trial Investigators. Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomised trial. Eur Heart J 2015;36:2061–9.
IMPROVE Trial Investigators. The effect of aortic morphology on peri-operative mortality of ruptured abdominal aortic aneurysm. Eur Heart J 2015;36:1328–34.
Powell JT, Hinchcliffe RJ. Emerging strategies to treat ruptured abdominal aortic aneurysms. Expert Rev Cardiovasc Ther 2015;13:1411–8.
Powell JT, Ulug P. Flexibility in trial enrolment decisions. BMJ 2015;351:h4608.
Sweeting MJ, Balm R, Desgranges P, Ulug P, Powell JT, on behalf of Ruptured Aneurysm Triallists. Individual-patient meta-analysis of three randomised trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br J Surg 2015;102:1229–39.
Sweeting MJ, Ulug P, Powell JT, Desgranges P, Balm R, for the Ruptured Aneurysm Triallists. Ruptured aneurysm trials: the importance of longer-term outcomes and meta-analysis for 1-year mortality. Eur J Vasc Endovasc Surg 2015;50:297–302.
Mason AJ, Gomes M, Grieve R, Ulug P, Powell JT, Carpenter J. Development of a practical approach to expert elicitation for randomised controlled trials with missing health outcomes: application to the IMPROVE trial. Clin Trials 2017;14:357–67.
IMPROVE Trial Investigators. Comparative clinical effectiveness and cost-effectiveness of an endovascular strategy versus open repair for ruptured abdominal aortic aneurysm: 3-year results of the IMPROVE randomised trial. BMJ 2017;359:j4859.
Kelsey LJ, Powell JT, Norman PE, Miller K, Doyle BJ. A comparison of hemodynamic metrics and intraluminal thrombus burden in a common iliac artery aneurysm. Int J Numer Method Biomed Eng 2017;33:e2821.
Sweeting MJ, Ulug P, Roy J, Hultgren R, Indrakusuma R, Balm R, et al. Value of risk scores in the decision to palliate patients with ruptured abdominal aortic aneurysm [published online ahead of print April 6, 2018]. Br J Surg 2018.
Powell JT, Sweeting MJ, Ulug P, Thompson MM, Hinchliffe RJ, IMPROVE Trial Investigators. Re-interventions after repair of ruptured abdominal aortic aneurysm: a report from the IMPROVE randomised trial. Eur J Vasc Endovasc Surg 2018;5:625–32.
Data sharing statement
Patient-level data can be made available from the corresponding author after authorisation by the Trial Management Committee and contractual agreement with Imperial College London. Consent from participants for data sharing was not obtained but any shared data will be anonymised.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD001835.pub4.
- Davis M, Harris M, Earnshaw JJ. Implementation of the National Health Service Abdominal Aortic Aneurysm Screening Program in England. J Vasc Surg 2013;57:1440-5. https://doi.org/10.1016/j.jvs.2012.10.114.
- Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. EVAR trial participants . Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004;364:843-8. https://doi.org/10.1016/S0140-6736(04)16979-1.
- Bown MJ, Sutton AJ, Bell PR, Sayers RD. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg 2002;89:714-30. https://doi.org/10.1046/j.1365-2168.2002.02122.x.
- Best VA, Price JF, Fowkes FG. Persistent increase in the incidence of abdominal aortic aneurysm in Scotland, 1981–2000. Br J Surg 2003;90:1510-15. https://doi.org/10.1002/bjs.4342.
- Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation 2007;115:2865-9. https://doi.org/10.1161/CIRCULATIONAHA.106.671859.
- Anjum A, von Allmen R, Greenhalgh R, Powell JT. Explaining the decrease in mortality from abdominal aortic aneurysm rupture. Br J Surg 2012;99:637-45. https://doi.org/10.1002/bjs.8698.
- Aylin P, Bottle A, Majeed A. Use of administrative data or clinical databases as predictors of risk of death in hospital: comparison of models. BMJ 2007;334. https://doi.org/10.1136/bmj.39168.496366.55.
- Filipovic M, Seagroatt V, Goldacre MJ. Differences between women and men in surgical treatment and case fatality rates for ruptured aortic abdominal aneurysm in England. Br J Surg 2007;94:1096-9. https://doi.org/10.1002/bjs.5784.
- Holt PJ, Poloniecki JD, Gerrard D, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br J Surg 2007;94:395-403. https://doi.org/10.1002/bjs.5710.
- Veith FJ, Gargiulo NJ. Endovascular aortic repair should be the gold standard for ruptured AAAs, and all vascular surgeons should be prepared to perform them. Perspect Vasc Surg Endovasc Ther 2007;19:275-82. https://doi.org/10.1177/1531003507304438.
- Dillon M, Cardwell C, Blair PH, Ellis P, Kee F, Harkin DW. Endovascular treatment for ruptured abdominal aortic aneurysm. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD005261.pub2.
- Visser JJ, van Sambeek MR, Hamza TH, Hunink MG, Bosch JL. Ruptured abdominal aortic aneurysms: endovascular repair versus open surgery – systematic review. Radiology 2007;245:122-9. https://doi.org/10.1148/radiol.2451061204.
- Mastracci TM, Garrido-Olivares L, Ciná CS, Clase CM. Endovascular repair of ruptured abdominal aortic aneurysms: a systematic review and meta-analysis. J Vasc Surg 2008;47:214-21. https://doi.org/10.1016/j.jvs.2007.07.052.
- Visser JJ, van Sambeek MR, Hunink MG, Redekop WK, van Dijk LC, Hendriks JM, et al. Acute abdominal aortic aneurysms: cost analysis of endovascular repair and open surgery in hemodynamically stable patients with 1-year follow-up. Radiology 2006;240:681-9. https://doi.org/10.1148/radiol.2403051005.
- Hinchliffe RJ, Bruijstens L, MacSweeney ST, Braithwaite BD. A randomised trial of endovascular and open surgery for ruptured abdominal aortic aneurysm – results of a pilot study and lessons learned for future studies. Eur J Vasc Endovasc Surg 2006;32:506-13. https://doi.org/10.1016/j.ejvs.2006.05.016.
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004;351:1607-18. https://doi.org/10.1056/NEJMoa042002.
- Amsterdam Acute Aneurysm Trial Collaborators . Amsterdam Acute Aneurysm trial: background, design, and methods. Vascular 2006;14:130-5. https://doi.org/10.2310/6670.2006.00027.
- Reimerink JJ, Hoornweg LL, Vahl AC, Wisselink W, van den Broek TA, Legemate DA, et al. Endovascular repair versus open repair of ruptured abdominal aortic aneurysms: a multicenter randomized controlled trial. Ann Surg 2013;258:248-56. https://doi.org/10.1097/SLA.0b013e31828d4b76.
- Desgranges P, Kobeiter H, Castier Y, Sénéchal M, Majewski M, Krimi A. The Endovasculaire vs Chirurgie dans les Anévrysmes Rompus PROTOCOL trial update. J Vasc Surg 2010;51:267-70. https://doi.org/10.1016/j.jvs.2009.10.128.
- IMPROVE Trial Investigators . Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ 2014;348. https://doi.org/10.1136/bmj.f7661.
- Desgranges P, Kobeiter H, Katsahian S, Bouffi M, Gouny P, Favre JP, et al. Editor’s choice – ECAR (Endovasculaire ou Chirurgie dans les Anevrysmes aorto-iliaques Rompus): a French randomized controlled trial of endovascular versus open surgical repair of ruptured aortoiliac aneurysms. Eur J Vasc Endovasc Surg 2015;50:303-10. https://doi.org/10.1016/j.ejvs.2015.03.028.
- IMPROVE Trial Investigators . Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial. Eur Heart J 2015;36:2061-9. https://doi.org/10.1093/eurheartj/ehv125.
- Royal College of Emergency Medicine . Best Practice Guidelines for the Management and Transfer of Patients With a Diagnosis of Ruptured Abdominal Aortic Aneurysm to a Specialist Vascular Centre n.d. www.rcem.ac.uk/docs/College%20Guidelines/5z8.%20Management%20and%20Transfer%20of%20Patients%20with%20a%20diagnosis%20of%20Ruptured%20Abdominal%20Aortic%20Aneurysm.pdf (accessed 16 November 2017).
- Mental Capacity Act 2005. London: The Stationery Office; 2015.
- Ghatwary T, Karthikesalingam A, Patterson B, Hinchliffe R, Morgan R, Loftus I, et al. St George’s Vascular Institute Protocol: an accurate and reproducible methodology to enable comprehensive characterization of infrarenal abdominal aortic aneurysm morphology in clinical and research applications. J Endovasc Ther 2012;19:400-14. https://doi.org/10.1583/11-3731MR.1.
- Hoornweg LL, Wisselink W, Vahl AC, Reekers JA, van Delden OM, Legemate DA, et al. Interobserver and intraobserver variability of interpretation of CT-angiography in patients with a suspected abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg 2008;35:295-300. https://doi.org/10.1016/j.ejvs.2007.09.017.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- National Schedule of Reference Costs for NHS Trusts, 2011–2012. London: Department of Health and Social Care; 2012.
- Hardman DT, Fisher CM, Patel MI, Neale M, Chambers J, Lane R, et al. Ruptured abdominal aortic aneurysms: who should be offered surgery?. J Vasc Surg 1996;23:123-9. https://doi.org/10.1016/S0741-5214(05)80042-4.
- Acosta S, Ogren M, Bergqvist D, Lindblad B, Dencker M, Zdanowski Z. The Hardman index in patients operated on for ruptured abdominal aortic aneurysm: a systematic review. J Vasc Surg 2006;44:949-54. https://doi.org/10.1016/j.jvs.2006.07.041.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Cuzick J, Edwards R, Segnan N. Adjusting for non-compliance and contamination in randomized clinical trials. Stat Med 1997;16:1017-29. https://doi.org/10.1002/(SICI)1097-0258(19970515)16:9<1017::AID-SIM508>3.0.CO;2-V.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94. https://doi.org/10.1080/01621459.1999.10474144.
- Bellamy SL, Lin JY, Ten Have TR. An introduction to causal modeling in clinical trials. Clin Trials 2007;4:58-73. https://doi.org/10.1177/1740774506075549.
- Guide to the Methods of Technology Appraisals. London: National Institute for Health and Care Excellence; 2013.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQoL: Results from a UK General Population Survey. CHE Discussion Paper 138. York: Centre for Health Economics, University of York; 1999.
- Purchasing Power Parities and Exchange Rates for all OECD countries. Paris: OECD; n.d.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Hoornweg LL, Wisselink W, Vahl A, Balm R. Amsterdam Acute Aneurysm Trial Collaborators . The Amsterdam Acute Aneurysm Trial: suitability and application rate for endovascular repair of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2007;33:679-83. https://doi.org/10.1016/j.ejvs.2006.12.011.
- Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16090.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. https://doi.org/10.1136/bmj.327.7414.557.
- Björck M, Wanhainen A. Nonocclusive mesenteric hypoperfusion syndromes: recognition and treatment. Semin Vasc Surg 2010;23:54-6. https://doi.org/10.1053/j.semvascsurg.2009.12.009.
- Azhar B, Patel SR, Holt PJ, Hinchliffe RJ, Thompson MM, Karthikesalingam A. Misdiagnosis of ruptured abdominal aortic aneurysm: systematic review and meta-analysis. J Endovasc Ther 2014;21:568-75. https://doi.org/10.1583/13-4626MR.1.
- Egorova N, Giacovelli J, Greco G, Gelijns A, Kent CK, McKinsey JF. National outcomes for the treatment of ruptured abdominal aortic aneurysm: comparison of open versus endovascular repairs. J Vasc Surg 2008;48:1092-100. https://doi.org/10.1016/j.jvs.2008.06.036.
- Edwards ST, Schermerhorn ML, O’Malley AJ, Bensley RP, Hurks R, Cotterill P, et al. Comparative effectiveness of endovascular versus open repair of ruptured abdominal aortic aneurysm in the Medicare population. J Vasc Surg 2014;59:575-82. https://doi.org/10.1016/j.jvs.2013.08.093.
- van Beek SC, Conijn AP, Koelemay MJ, Balm R. Editor’s Choice - Endovascular aneurysm repair versus open repair for patients with a ruptured abdominal aortic aneurysm: a systematic review and meta-analysis of short-term survival. Eur J Vasc Endovasc Surg 2014;47:593-602. https://doi.org/10.1016/j.ejvs.2014.03.003.
- Moghadamyeghaneh Z, Sgroi MD, Chen SL, Kabutey NK, Stamos MJ, Fujitani RM. Risk factors and outcomes of postoperative ischemic colitis in contemporary open and endovascular abdominal aortic aneurysm repair. J Vasc Surg 2016;63:866-72. https://doi.org/10.1016/j.jvs.2015.10.064.
- Björck M. Surgery for ruptured abdominal aortic aneurysm. BMJ 2014;348. https://doi.org/10.1136/bmj.g95.
- Sweeting MJ, Balm R, Desgranges P, Ulug P, Powell JT. Ruptured Aneurysm Trialists . Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br J Surg 2015;102:1229-39. https://doi.org/10.1002/bjs.9852.
- Groves EM, Khoshchehreh M, Le C, Malik S. Effects of weekend admission on the outcomes and management of ruptured aortic aneurysms. J Vasc Surg 2014;60:318-24. https://doi.org/10.1016/j.jvs.2014.02.052.
- Salhab M, Farmer J, Osman I. Impact of delay on survival in patients with ruptured abdominal aortic aneurysm. Vascular 2006;14:38-42. https://doi.org/10.2310/6670.2006.00011.
- Starnes BW, Quiroga E, Hutter C, Tran NT, Hatsukami T, Meissner M, et al. Management of ruptured abdominal aortic aneurysm in the endovascular era. J Vasc Surg 2010;51:9-17. https://doi.org/10.1016/j.jvs.2009.08.038.
- Van Herzeele I, Sevdalis N, Lachat M, Desender L, Rudarakanchana N, Rancic Z. Team training in ruptured EVAR. J Cardiovasc Surg 2014;55:193-206.
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12. https://doi.org/10.1016/S0140-6736(09)61116-8.
- National Institute for Health and Care Excellence . Endovascular Aortic Stent–Grafts Are Not Recommended for Patients With Ruptured Aneurysms Except in the Context of Research 2009. www.nice.org.uk/guidance/ta167/chapter/1-Guidance (accessed 6 February 2018).
- Mayer D, Rancic Z, Veith FJ, Lachat M. Part two: against the motion. EVAR offers no survival benefit over open repair for the treatment of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2015;49:119-27. https://doi.org/10.1016/j.ejvs.2014.11.016.
- Björck M, Mani K. Improving outcomes for ruptured abdominal aortic aneurysm. Lancet 2014;383:933-4. https://doi.org/10.1016/S0140-6736(14)60385-8.
- Mell MW, Wang NE, Morrison DE, Hernandez-Boussard T. Interfacility transfer and mortality for patients with ruptured abdominal aortic aneurysm. J Vasc Surg 2014;60:553-7. https://doi.org/10.1016/j.jvs.2014.02.061.
- Symons NR, Moorthy K, Almoudaris AM, Bottle A, Aylin P, Vincent CA, et al. Mortality in high-risk emergency general surgical admissions. Br J Surg 2013;100:1318-25. https://doi.org/10.1002/bjs.9208.
- Hinchliffe RJ, Ribbons T, Ulug P, Powell JT. Transfer of patients with ruptured abdominal aortic aneurysm from general hospitals to specialist vascular centres: results of a Delphi consensus study. Emerg Med J 2013;30:483-6. https://doi.org/10.1136/emermed-2012-201239.
- IMPROVE Trial Investigators . Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg 2014;101:216-24. https://doi.org/10.1002/bjs.9410.
- Gallerani M, Volpato S, Boari B, Pala M, De Giorgi A, Fabbian F, et al. Outcomes of weekend versus weekday admission for acute aortic dissection or rupture: a retrospective study on the Italian National Hospital Database. Int J Cardiol 2013;168:3117-19. https://doi.org/10.1016/j.ijcard.2013.04.065.
- Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med 2001;345:663-8. https://doi.org/10.1056/NEJMsa003376.
- Mayer D, Rancic Z, Pfammatter T, Veith FJ, Lachat M. Choice of treatment for the patient with urgent AAA: practical tips. J Cardiovasc Surg 2009;50:595-8.
- Mayer D, Aeschbacher S, Pfammatter T, Veith FJ, Norgren L, Magnuson A, et al. Complete replacement of open repair for ruptured abdominal aortic aneurysms by endovascular aneurysm repair: a two-center 14-year experience. Ann Surg 2012;256:688-95. https://doi.org/10.1097/SLA.0b013e318271cebd.
- Schanzer A, Greenberg RK, Hevelone N, Robinson WP, Eslami MH, Goldberg RJ, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011;123:2848-55. https://doi.org/10.1161/CIRCULATIONAHA.110.014902.
- Dick F, Diehm N, Opfermann P, von Allmen R, Tevaearai H, Schmidli J. Endovascular suitability and outcome after open surgery for ruptured abdominal aortic aneurysm. Br J Surg 2012;99:940-7. https://doi.org/10.1002/bjs.8780.
- van Beek SC, Reimerink JJ, Vahl AC, Wisselink W, Reekers JA, Legemate DA, et al. Amsterdam Acute Aneurysm Trial Collaborators . Outcomes after open repair for ruptured abdominal aortic aneurysms in patients with friendly versus hostile aortoiliac anatomy. Eur J Vasc Endovasc Surg 2014;47:380-7. https://doi.org/10.1016/j.ejvs.2014.01.003.
- Alexander S, Bosch JL, Hendriks JM, Visser JJ, Van Sambeek MR. The 30-day mortality of ruptured abdominal aortic aneurysms: influence of gender, age, diameter and comorbidities. J Cardiovasc Surg 2008;49:633-7.
- Sternbergh WC, Carter G, York GW, Yoselevitz M, Money SR. Aortic neck angulation predicts adverse outcome with endovascular abdominal aortic aneurysm repair. J Vasc Surg 2001;35:482-6. https://doi.org/10.1067/mva.2002.119506.
- Baderkhan H, Gonçalves FM, Oliveira NG, Verhagen HJ, Wanhainen A, Björck M, et al. Challenging anatomy predicts mortality and complications after endovascular treatment of ruptured abdominal aortic aneurysm. J Endovasc Ther 2016;23:919-27. https://doi.org/10.1177/1526602816658494.
- Hultgren R, Zommorodi S, Gambe M, Roy J. A majority of admitted patients with ruptured abdominal aortic aneurysm undergo and survive corrective treatment: a population-based retrospective cohort study. World J Surg 2016;40:3080-7. https://doi.org/10.1007/s00268-016-3705-9.
- Neville AL, Nemtsev D, Manasrah R, Bricker SD, Putnam BA. Mortality risk stratification in elderly trauma patients based on initial arterial lactate and base deficit levels. Am Surg 2011;77:1337-41.
- Hasler RM, Nuesch E, Jüni P, Bouamra O, Exadaktylos AK, Lecky F. Systolic blood pressure below 110 mmHg is associated with increased mortality in blunt major trauma patients: multicentre cohort study. Resuscitation 2011;82:1202-7. https://doi.org/10.1016/j.resuscitation.2011.04.021.
- Hasler RM, Nüesch E, Jüni P, Bouamra O, Exadaktylos AK, Lecky F. Systolic blood pressure below 110 mmHg is associated with increased mortality in penetrating major trauma patients: multicentre cohort study. Resuscitation 2012;83:476-81. https://doi.org/10.1016/j.resuscitation.2011.10.018.
- Oyetunji TA, Chang DC, Crompton JG, Greene WR, Efron DT, Haut ER, et al. Redefining hypotension in the elderly: normotension is not reassuring. Arch Surg 2011;146:865-9. https://doi.org/10.1001/archsurg.2011.154.
- Robinson WP, Schanzer A, Aiello FA, Flahive J, Simons JP, Doucet DR, et al. Endovascular repair of ruptured abdominal aortic aneurysms does not reduce later mortality compared with open repair. J Vasc Surg 2016;63:617-24. https://doi.org/10.1016/j.jvs.2015.09.057.
- Kapma MR, Dijksman LM, Reimerink JJ, de Groof AJ, Zeebregts CJ, Wisselink W, et al. Cost-effectiveness and cost-utility of endovascular versus open repair of ruptured abdominal aortic aneurysm in the Amsterdam Acute Aneurysm Trial. Br J Surg 2014;101:208-15. https://doi.org/10.1002/bjs.9356.
- Rollins KE, Shak J, Ambler GK, Tang TY, Hayes PD, Boyle JR. Mid-term cost-effectiveness analysis of open and endovascular repair for ruptured abdominal aortic aneurysm. Br J Surg 2014;101:225-31. https://doi.org/10.1002/bjs.9409.
- Sweeting MJ, Ulug P, Powell JT, Desgranges P, Balm R. Ruptured Aneurysm Trialists . Ruptured aneurysm trials: the importance of longer-term outcomes and meta-analysis for 1-year mortality. Eur J Vasc Endovasc Surg 2015;50:297-302. https://doi.org/10.1016/j.ejvs.2015.04.015.
- IMPROVE Trial Investigators . The effect of aortic morphology on peri-operative mortality of ruptured abdominal aortic aneurysm. Eur Heart J 2015;36:1328-34. https://doi.org/10.1093/eurheartj/ehu521.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523-32. https://doi.org/10.1007/s11136-004-7713-0.
- Noorani A, Page A, Walsh SR, Varty K, Hayes PD, Boyle JR. Mid-term outcomes following emergency endovascular aortic aneurysm repair for ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2012;43:382-5. https://doi.org/10.1016/j.ejvs.2011.12.023.
- Hayes PD, Sadat U, Walsh SR, Noorani A, Tang TY, Bowden DJ, et al. Cost-effectiveness analysis of endovascular versus open surgical repair of acute abdominal aortic aneurysms based on worldwide experience. J Endovasc Ther 2010;17:174-82. https://doi.org/10.1583/09-2941.1.
- van Beek SC, Vahl A, Wisselink W, Reekers JA, Legemate DA, Balm R. Amsterdam Acute Aneurysm Trial Collaborators . Midterm re-interventions and survival after endovascular versus open repair for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2015;49:661-8. https://doi.org/10.1016/j.ejvs.2015.02.015.
- Powell JT, Sweeting MJ, Ulug P, Blankensteijn JD, Lederle FA, Becquemin JP, et al. EVAR-1, DREAM, OVER and ACE Trialists . Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm over 5 years. Br J Surg 2017;104:166-78. https://doi.org/10.1002/bjs.10430.
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. EVAR trial investigators . Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366-74. https://doi.org/10.1016/S0140-6736(16)31135-7.
- van Beek SC, Vahl AC, Wisselink W, Balm R. Amsterdam Acute Aneurysm Trial Collaborators . Fate of patients unwilling or unsuitable to undergo surgical intervention for a ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2015;49:163-5. https://doi.org/10.1016/j.ejvs.2014.10.007.
- Epstein D, Sculpher MJ, Powell JT, Thompson SG, Brown LC, Greenhalgh RM. Long-term cost-effectiveness analysis of endovascular versus open repair for abdominal aortic aneurysm based on four randomized clinical trials. Br J Surg 2014;101:623-31. https://doi.org/10.1002/bjs.9464.
- Karthikesalingam A, Holt PJ, Vidal-Diez A, Ozdemir BA, Poloniecki JD, Hinchliffe RJ, et al. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet 2014;383:963-9. https://doi.org/10.1016/S0140-6736(14)60109-4.
- Robinson WP, Schanzer A, Li Y, Goodney PP, Nolan BW, Eslami MH, et al. Derivation and validation of a practical risk score for prediction of mortality after open repair of ruptured abdominal aortic aneurysms in a US regional cohort and comparison to existing scoring systems. J Vasc Surg 2013;57:354-61. https://doi.org/10.1016/j.jvs.2012.08.120.
- Chen JC, Hildebrand HD, Salvian AJ, Taylor DC, Strandberg S, Myckatyn TM, et al. Predictors of death in nonruptured and ruptured abdominal aortic aneurysms. J Vasc Surg 1996;24:614-20. https://doi.org/10.1016/S0741-5214(96)70077-0.
- Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 1999;28:964-74. https://doi.org/10.1093/ije/28.5.964.
- Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J 2014;35:1925-31. https://doi.org/10.1093/eurheartj/ehu207.
- Sweeting MJ, Ulug P, Roy J, Hultgren R, Indrakusuma R, Balm R, et al. Value of risk scores in the decision to palliate patients with ruptured abdominal aortic aneurysm [published online ahead of print April 6, 2018]. Br J Surg 2018.
- Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128-38. https://doi.org/10.1097/EDE.0b013e3181c30fb2.
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565-74. https://doi.org/10.1177/0272989X06295361.
- Vos CG, de Vries JP, Werson DA, van Dongen EP, Schreve MA, Ünlü Ç. Evaluation of five different aneurysm scoring systems to predict mortality in ruptured abdominal aortic aneurysm patients. J Vasc Surg 2016;64:1609-16. https://doi.org/10.1016/j.jvs.2016.05.099.
- Samy AK, Murray G, MacBain G. Glasgow Aneurysm Score. Cardiovasc Surg 1994;2:41-4.
- Tambyraja A, Murie J, Chalmers R. Predictors of outcome after abdominal aortic aneurysm rupture: Edinburgh Ruptured Aneurysm Score. World J Surg 2007;31:2243-7. https://doi.org/10.1007/s00268-007-9181-5.
- von Meijenfeldt GC, van Beek SC, Bastos Gonçalves F, Verhagen HJ, Zeebregts CJ, Vahl AC, et al. Development and external validation of a model predicting death after surgery in patients with a ruptured abdominal aortic aneurysm: the Dutch Aneurysm Score. Eur J Vasc Endovasc Surg 2017;53:168-74. https://doi.org/10.1016/j.ejvs.2016.10.024.
- Harris DG, Garrido D, Oates CP, Kalsi R, Huffner ME, Toursavadkohi S, et al. Repair of ruptured abdominal aortic aneurysm after cardiac arrest. J Vasc Surg 2016;64:1497-502. https://doi.org/10.1016/j.jvs.2016.05.085.
- Chang W, Cheng J, Allaire J. Shiny: Web Application Framework for R 2015. https://CRAN.R-project.org/package=shiny (accessed 16 November 2017).
- Johnson SR, Tomlinson GA, Hawker GA, Granton JT, Feldman BM. Methods to elicit beliefs for Bayesian priors: a systematic review. J Clin Epidemiol 2010;63:355-69. https://doi.org/10.1016/j.jclinepi.2009.06.003.
- Mason AJ, Gomes M, Grieve R, Ulug P, Powell JT, Carpenter J. Development of a practical approach to expert elicitation for randomised controlled trials with missing health outcomes: application to the IMPROVE trial. Clin Trials 2017;14:357-67. https://doi.org/10.1177/1740774517711442.
- Bluestein D, Dumont K, De Beule M, Ricotta J, Impellizzeri P, Verhegghe B, et al. Intraluminal thrombus and risk of rupture in patient specific abdominal aortic aneurysm – FSI modelling. Comput Methods Biomech Biomed Engin 2009;12:73-81. https://doi.org/10.1080/10255840903077170.
- Doyle BJ, McGloughlin TM, Miller K, Powell JT, Norman PE. Regions of high wall stress can predict the future location of rupture of abdominal aortic aneurysm. Cardiovasc Intervent Radiol 2014;37:815-18. https://doi.org/10.1007/s00270-014-0864-7.
- Xenos M, Alemu Y, Zamfir D, Einav S, Ricotta JJ, Labropoulos N, et al. The effect of angulation in abdominal aortic aneurysms: fluid-structure interaction simulations of idealized geometries. Med Biol Eng Comput 2010;48:1175-90. https://doi.org/10.1007/s11517-010-0714-y.
- Richards T, Goode SD, Hinchliffe R, Altaf N, MacSweeney S, Braithwaite B. The importance of anatomical suitability and fitness for the outcome of endovascular repair of ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2009;38:285-90. https://doi.org/10.1016/j.ejvs.2009.05.018.
- Patel NV, Long GW, Cheema ZF, Rimar K, Brown OW, Shanley CJ. Open vs. endovascular repair of isolated iliac artery aneurysms: a 12-year experience. J Vasc Surg 2009;49:1147-53. https://doi.org/10.1016/j.jvs.2008.11.101.
- Kelsey LJ, Miller K, Norman PE, Powell JT, Doyle BJ. The influence of downstream branching arteries on upstream haemodynamics. J Biomech 2016;49:3090-6. https://doi.org/10.1016/j.jbiomech.2016.07.023.
- Kelsey LJ, Powell JT, Norman PE, Miller K, Doyle BJ. A comparison of hemodynamic metrics and intraluminal thrombus burden in a common iliac artery aneurysm. Int J Numer Method Biomed Eng 2017;33. https://doi.org/10.1002/cnm.2821.
- Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119:2444-53. https://doi.org/10.1161/CIRCULATIONAHA.108.800011.
- Williams TA, Ho KM, Dobb GJ, Finn JC, Knuiman M, Webb SA. Royal Perth Hospital ICU Data Linkage Group . Effect of length of stay in intensive care unit on hospital and long-term mortality of critically ill adult patients. Br J Anaesth 2010;104:459-64. https://doi.org/10.1093/bja/aeq025.
- Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA 2009;302:1535-42. https://doi.org/10.1001/jama.2009.1426.
- Prinssen M, Buskens E, Blankensteijn JD. Quality of life endovascular and open repair. Results of a randomised trial. Eur J Vasc Endovasc Surg 2004;27:121-7. https://doi.org/10.1016/j.ejvs.2003.11.006.
- Stroupe KT, Lederle FA, Matsumura JS, Kyriakides TC, Jonk YC, Ge L, et al. Open Versus Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group . Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm in the OVER trial. J Vasc Surg 2012;56:901-9.e2. https://doi.org/10.1016/j.jvs.2012.01.086.
- Prinssen M, Buskens E, de Jong SE, Buth J, Mackaay AJ, van Sambeek MR, et al. Cost-effectiveness of conventional and endovascular repair of abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2007;46:883-90. https://doi.org/10.1016/j.jvs.2007.07.033.
- Lederle FA, Stroupe KT, Kyriakides TC, Ge L, Freischlag JA. Open vs Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group . Long-term cost-effectiveness in the Veterans Affairs open vs endovascular repair study of aortic abdominal aneurysm: a randomized clinical trial. JAMA Surg 2016;151:1139-44. https://doi.org/10.1001/jamasurg.2016.2783.
- Mehta M, Taggert J, Darling RC, Chang BB, Kreienberg PB, Paty PS, et al. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg 2006;44:1-8. https://doi.org/10.1016/j.jvs.2006.02.057.
- Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10440.
- Dixon S, Mason S, Knowles E, Colwell B, Wardrope J, Snooks H, et al. Is it cost effective to introduce paramedic practitioners for older people to the ambulance service? Results of a cluster randomised controlled trial. Emerg Med J 2009;26:446-51. https://doi.org/10.1136/emj.2008.061424.
- Powell JT, Sweeting MJ, Ulug P, Thompson MM, Hinchliffe RJ. IMPROVE Trial Investigators . Re-interventions after repair of ruptured abdominal aortic aneurysm: a report from the IMPROVE randomised trial. Eur J Vasc Endovasc Surg 2018;5:625-32. https://doi.org/10.1016/j.ejvs.2018.01.028.
Appendix 1 Trial progress and recruitment
The IMPROVE trial organisation and committees
Grant applicants: Professor Janet T Powell (Chief Investigator), Mr Bruce Braithwaite, Professor Nicholas J Cheshire, Professor Roger M Greenhalgh, Professor Richard J Grieve, Dr Tajek B Hassan, Professor Robert J Hinchliffe, Dr Simon Howell, Dr Fionna Moore, Dr Anthony A Nicholson, Professor Chee V Soong (deceased), Professor Matthew T Thompson and Professor Simon G Thompson.
Data and trial management: Dr Pinar Ulug (Trial Manager) and Ms Francine Heatley (Trial Manager, maternity cover, 2012–13), Ms Aisha Anjum (Trial Monitor, 2012–14) and Ms Gosia Kalinowska (Trial Administrator, 2014).
Statistical analyses: Dr Michael J Sweeting and Professor Simon G Thompson.
Health economics costs analyses: Dr Manuel Gomes and Professor Richard J Grieve.
Trial Management Committee: Professor Janet T Powell (Chairperson), Dr Raymond Ashleigh, Dr Manuel Gomes, Professor Roger M Greenhalgh, Professor Richard J Grieve, Professor Robert J Hinchliffe, Dr Michael J Sweeting, Professor Matthew T Thompson, Professor Simon G Thompson and Dr Pinar Ulug.
Trial Steering Committee: Professor Ian Roberts (Chairperson), Professor Sir Peter RF Bell, Mrs Anne Cheetham, Ms Jenny Stephany (2009–2012) and Professor Alison Halliday.
Data Monitoring and Ethics Committee: Professor Charles Warlow (Chairperson), Mr Peter Lamont, Professor Jonathan Moss and Professor Jan Tijssen.
Credentialing committee: Mr Bruce Braithwaite and Dr Anthony A Nicholson.
Core laboratory: Professor Matthew T Thompson, Dr Raymond Ashleigh and Luke Thompson.
The IMPROVE trial local investigators by participating NHS trust (in order of site start date from the earliest (September 2009) to the most recent (February 2013); numbers in parentheses indicate the number of participants entered into the trial:
-
UK – Colin D Bicknell and Nicholas J Cheshire (to October 2014), Imperial College Healthcare NHS Trust, London (20); Jonathan R Boyle, Addenbrooke’s Hospital, Cambridge (40); Ferdinand Serracino-Inglott and J Vince Smyth (December 2012 to November 2013), Manchester Royal Infirmary, Manchester (69); Matthew T Thompson and Robert J Hinchliffe, St George’s Hospital, London (75); Rachel Bell, St Thomas’ Hospital, London (81); Noel Wilson, Kent and Canterbury Hospital, Canterbury (23); Matt Bown and Martin Dennis (to December 2010), Leicester Royal Infirmary, Leicester (18); Meryl Davis, Royal Free Hospital, London (1); Raymond Ashleigh, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester (21); Simon Howell, Leeds General Infirmary, Leeds (23); Michael G Wyatt, Freeman Hospital, Newcastle (23); Domenico Valenti, King’s College Hospital NHS Foundation Trust, London (2); Paul Bachoo, Aberdeen Royal Infirmary, Aberdeen (4); Paul Walker, The James Cook University Hospital, Middlesbrough (5); Shane MacSweeney, Queen’s Medical Centre, Nottingham (34); Jonathan N Davies, Royal Cornwall Hospital, Truro (5); Dynesh Rittoo and Simon D Parvin (to December 2011), Royal Bournemouth Hospital, Bournemouth (22); Syed Waquar Yusuf, Royal Sussex County Hospital, Brighton (5); Colin Nice, Queen Elizabeth Hospital, Gateshead (5); Ian Chetter, Hull Royal Infirmary, Hull (32); Adam Howard, Colchester General Hospital, Colchester (24); Patrick Chong, Frimley Park Hospital, Surrey (14); Raj Bhat, Ninewells Hospital, Dundee (8); David McLain, Royal Gwent Hospital, Newport; Andrew Gordon and Ian Lane (to June 2012), University Hospital of Wales, Cardiff (4); Simon Hobbs, New Cross Hospital, Wolverhampton (3); Woolagasen Pillay, Doncaster Royal Infirmary, Doncaster (8); Timothy Rowlands and Amin El-Tahir (to November 2012), Royal Derby Hospital, Derby (13); John Asquith, Royal Stoke University Hospital, Stoke-on-Trent (15); and Stephen P Cavanagh, The York Hospital, York (3).
-
Canada – Luc Dubois and Thomas L Forbes (to August 2014), London Health Sciences Centre and Western University, London, Ontario (13).
Trial co-ordinators: Emily Ashworth, Ayoola Awopetu, Sara Baker, Hashem Barakat, Patricia Bourke, Claire Brady, Joanne Brown, Jennie Bryce, Christine Bufton, Debbie Campbell, Tina Chance, Angela Chrisopoulou, Marie Cockell, Andrea Croucher, Gail Curran, Leela Dabee, Nikki Dewhirst, Jo Evans, Christopher Fenner, Andy Gibson, Siobhan Gorst, Moira Gough, Norma Gourlay, Lynne Graves, Michelle Griffin, Josie Hatfield, Florence Hogg, Susannah Howard, Cían Hughes, Thomas Hughes, Alex James, Elizabeth Keene, Michelle Lapworth, Ian Massey, David Metcalfe, Awad Mohalhal, Teresa Novick, Daré Oladokun, Gareth Owen, Noala Parr, David Pintar, Joanna Smee, Tom Smith, Sarah Spencer, Helen Thompson, Claire Thomson, Orla Thunder, Tom Wallace, Sue Ward, Vera Wealleans, Lesley Wilson, Janet Woods, Manu Zachariah and Ting Zheng.
Writing committee for reporting of 30-day outcomes: Janet T Powell (Chairperson), Michael J Sweeting, Matthew T Thompson, Raymond Ashleigh, Rachel Bell, Manuel Gomes, Roger M Greenhalgh, Richard J Grieve, Francine Heatley, Robert J Hinchliffe, Simon G Thompson and Pinar Ulug.
Writing committee for reporting of 1-year and 3-year outcomes: Raymond Ashleigh, Manuel Gomes, Roger M Greenhalgh, Richard J Grieve, Robert J Hinchliffe, Janet T Powell (Chairperson), Michael J Sweeting, Matthew T Thompson, Simon G Thompson and Pinar Ulug.
Referral of patients with ruptured aneurysms, trial recruitment, participant flow and completeness of participant follow-up
The trial started in April 2009, with the recruitment of 600 participants initially planned to take place between October 2009 and December 2011 in 20–24 centres across the UK. Obtaining all of the relevant permissions for the trial to start in these centres was much slower than anticipated (Figure 16). The availability of centres to start recruiting is shown in Figure 17 and recruitment to December 2011 is shown in Figure 18. The slow recruitment led us to investigate whether or not patients were being appropriately referred into the trial centres.
FIGURE 16.
Time from NHS Site-Specific Information Form submission to NHS approval by December 2010. The vertical axis shows the site number. The average time to approval for 22 centres was 7 months. Site-Specific Information Forms initiate the permission process in the Integrated Research Application System.

FIGURE 17.
Progress with centres that were able to recruit. The target number of centres had opened by October 2010, > 12 months behind schedule.

FIGURE 18.
Recruitment to December 2011.
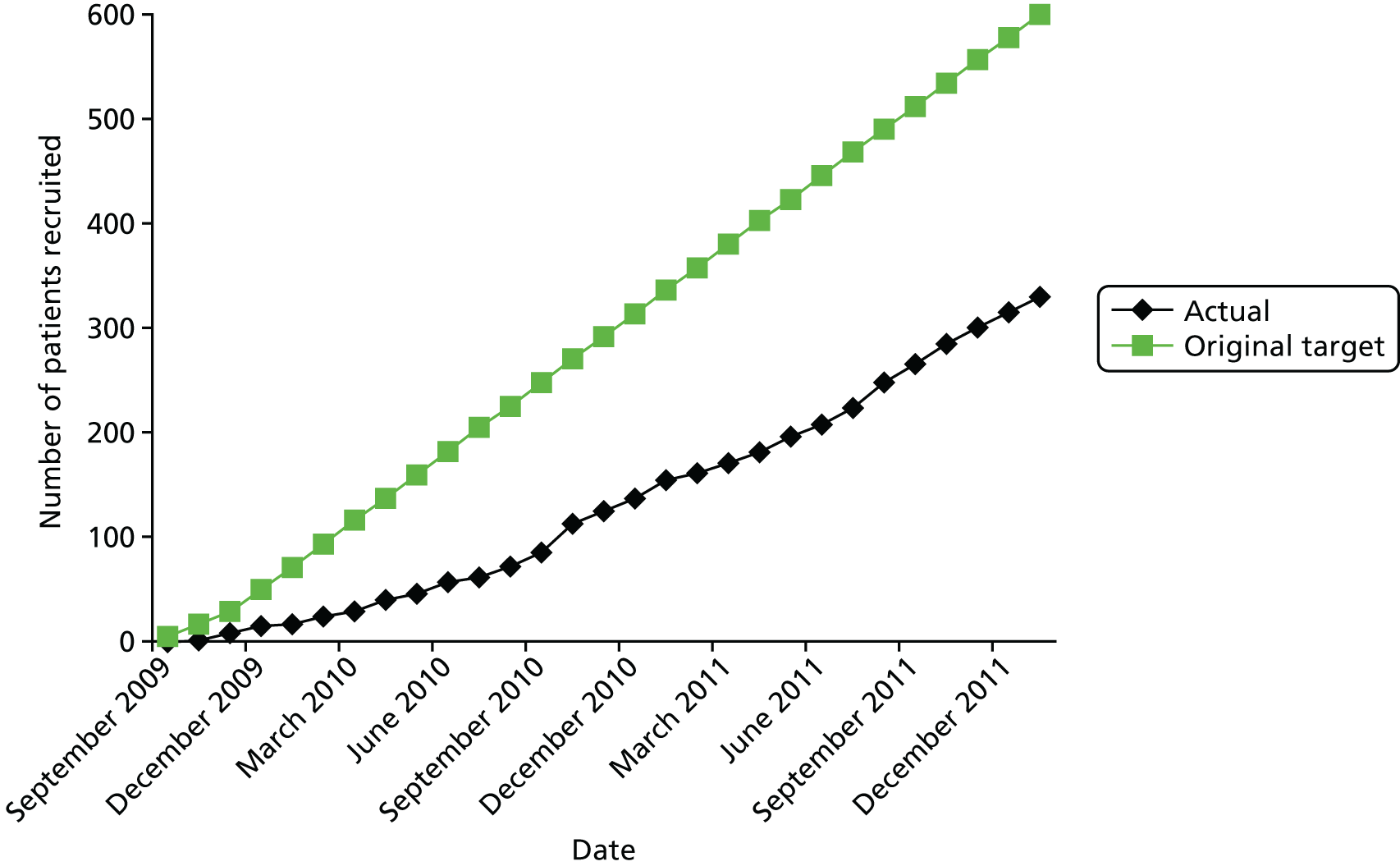
The referral of patients with ruptured abdominal aortic aneurysm and the development of guidelines for patient transfer
The aims of these studies were to explore areas of consensus and disagreement concerning the interhospital transfer of patients with a clinical diagnosis of ruptured AAA. A three-round Delphi questionnaire approach was used among vascular and endovascular surgery from the IMPROVE trial and emergency medicine specialists, from IMPROVE trial centres and the NHS Wessex Clinical Strategic Network, to explore patient characteristics and clinical management issues for emergency interhospital transfer. Agreement was reported when 70% of respondents were in agreement. Initially, there was agreement that transfer patients should be: < 85 years of age, either alert or with fluctuating consciousness, with moderate or minimal systemic disease and needing no or some help with daily living. Round 3 clarified that patients requiring inotropes and those who were institutionalised for mental infirmity should be transferred. Those with cardiac arrest in a current episode should not be transferred. There was no agreement regarding whether or not those who were institutionalised with physical infirmities, unconscious and/or intubated patients or those with severe systemic disease should be transferred. Speed was accepted as important, with agreement for specialty trainees to arrange transfer, if consultants were not on-site. Consultant–consultant discussion was recommended for patients with severe systemic disease. CT confirmation of diagnosis was considered unnecessary before transfer (but ultrasound assessment was desirable) and transfers should not be delayed by waiting for specific tests. There was no agreement about blood tests and electrocardiography before transfer or whether or not blood should accompany the patient being transferred. There was no agreement regarding whether or not specific staff and/or facilities needed to be in place at the specialist hospital. A systolic blood pressure of ≥ 70 mmHg was sufficient for transfer without the need for intravenous fluids, unless deterioration occurred. Overall, broad agreement about the type of patient who should be eligible for transfer was achieved but disagreements about patient management issues, before and during transfer, remained. These findings were reported by Hinchliffe et al. 62 and led to the development of guidelines for patient referral, which have been adopted by the Royal College of Emergency Medicine, The Vascular Society of Great Britain and Ireland and the Royal Society of Radiologists. 24
Trial extensions and final trial recruitment
Owing to the slow start to recruitment, the trial was awarded a 15-month extension, with the new target of recruiting 600 participants by the end of June 2013 and with follow-up and reporting to be completed by July 2014 (which meant that many of the participants who were recruited in 2013 would have been followed up for < 1 year). Additional centres were opened to achieve this. However, the ongoing reorganisation of vascular surgical services resulted in some centres, such as King’s College Hospital NHS Foundation Trust (London), Queen Elizabeth Hospital (Gateshead) and New Cross Hospital (Wolverhampton), being closed. The recruitment target of 600 participants was achieved during the revised recruitment period and additional participants were randomised during early July 2013, during the period when sites were being notified of the close to recruitment (Figure 19).
FIGURE 19.
Progress of recruitment.

All centres also maintained a log of non-recruited patients, providing reasons (Table 26). Both the recruited and non-recruited participants are shown in the trial CONSORT diagram (Figure 20). In summary, 1275 patients were identified with a diagnosis of ruptured AAA, 623 were randomised and 652 were not randomised.
| Participating centrea | Reason (n) | Total excluded (n) | ||||
|---|---|---|---|---|---|---|
| Within inclusion criteriab | Patient preferencec | Operationald | Clinician preference | Unknown | ||
| Imperial College London (St Mary’s Hospital and Charing Cross Hospital) | 13 | 1 | 0 | 3 | 1 | 18 |
| Addenbrooke’s Hospital | 29 | 0 | 2 | 7 | 13 | 51 |
| Manchester Royal Infirmary | 3 | 1 | 0 | 0 | 0 | 4 |
| St George’s Hospital | 11 | 1 | 0 | 1 | 5 | 18 |
| St Thomas’ Hospital | 24 | 6 | 0 | 0 | 0 | 30 |
| Kent and Canterbury Hospital | 4 | 1 | 1 | 0 | 0 | 6 |
| Leicester Royal Infirmary | 49 | 7 | 31 | 3 | 4 | 94 |
| Wythenshawe Hospital | 8 | 0 | 1 | 5 | 34 | 48 |
| Leeds General Infirmary | 22 | 4 | 4 | 3 | 6 | 39 |
| Freeman Hospital | 40 | 2 | 24 | 1 | 11 | 78 |
| King’s College Hospital | 0 | 0 | 0 | 0 | 2 | 2 |
| James Cook University Hospital | 9 | 1 | 2 | 1 | 0 | 13 |
| Queen’s Medical Centre | 44 | 2 | 1 | 13 | 0 | 60 |
| Royal Cornwall Hospital | 7 | 0 | 6 | 0 | 1 | 14 |
| Royal Bournemouth Hospital | 0 | 2 | 0 | 0 | 0 | 2 |
| Royal Sussex County Hospital | 1 | 0 | 15 | 0 | 0 | 16 |
| Queen Elizabeth Hospital | 5 | 0 | 3 | 0 | 0 | 8 |
| Hull Royal Infirmary | 5 | 1 | 0 | 8 | 0 | 14 |
| Colchester General Hospital | 19 | 3 | 0 | 0 | 15 | 37 |
| Frimley Park Hospital | 4 | 1 | 4 | 0 | 0 | 9 |
| Royal Gwent Hospital and University Hospital of Wales | 13 | 1 | 12 | 0 | 0 | 26 |
| New Cross Hospital | 3 | 0 | 2 | 1 | 0 | 6 |
| Doncaster Royal Infirmary | 4 | 0 | 1 | 0 | 1 | 6 |
| Royal Derby Hospital | 8 | 6 | 0 | 1 | 4 | 19 |
| Royal Stoke University Hospital | 6 | 0 | 0 | 0 | 0 | 6 |
| London Health Sciences Centre and Western University | 23 | 2 | 2 | 1 | 0 | 28 |
| Total | 354 | 42 | 111 | 48 | 97 | 652 |
FIGURE 20.
A CONSORT diagram for outcomes to 30 days. a, Breach of protocol; numbers include two and three symptomatics in the endovascular strategy group and the open repair group, respectively. b, 28 morphologically suitable for EVAR.
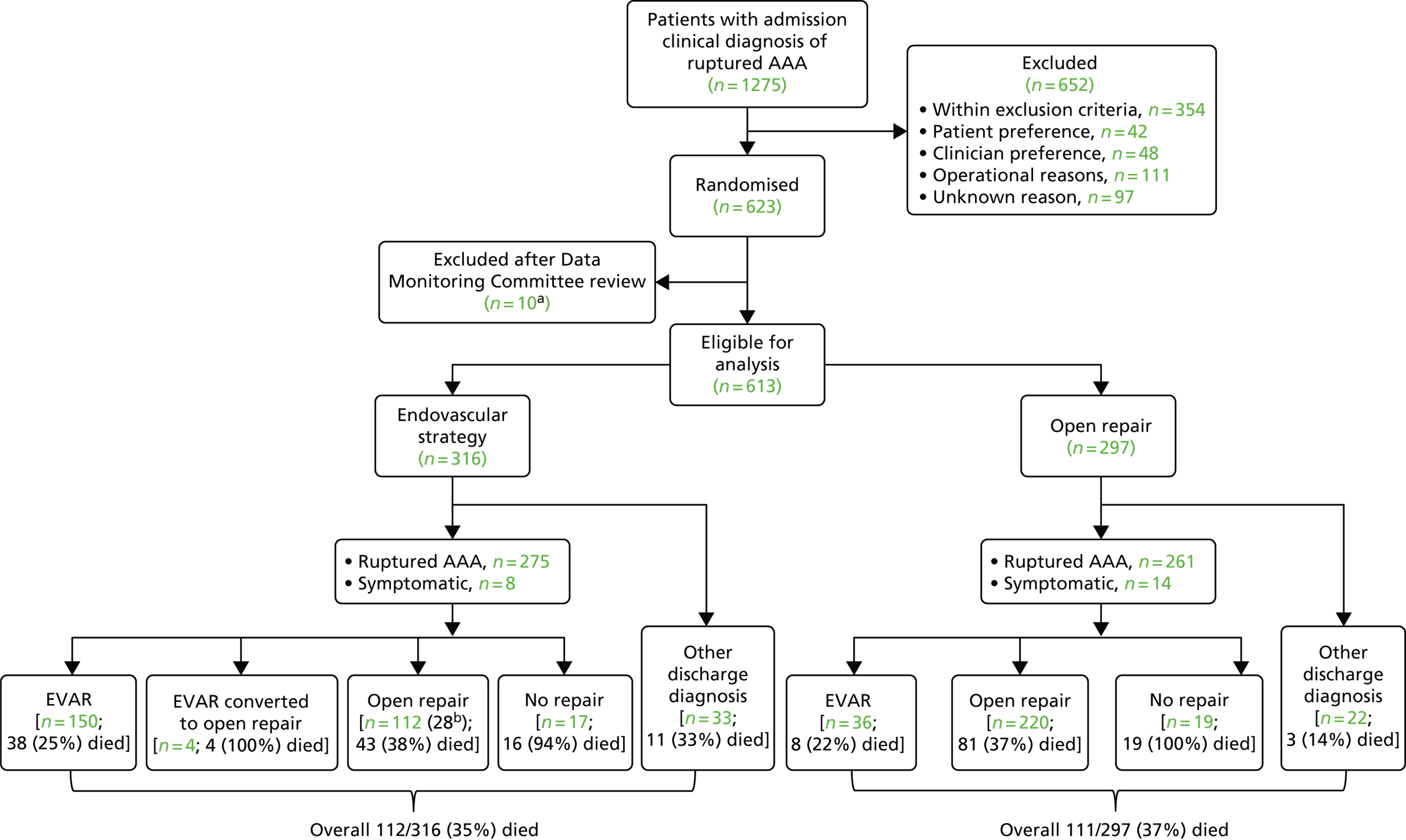
The Data Monitoring Committee approved the post-randomisation exclusion of 10 patients (three in the endovascular strategy group and seven in the open repair group) deemed ineligible because of violation of the inclusion. Two patients had a secondary rupture with previous aneurysm repair, one patient was admitted electively for aneurysm repair, three patients were randomised without consent before reaching the trial centre and the in-hospital clinical diagnosis was not ruptured aneurysm and four patients could not be identified in any hospital records. It is assumed that these four patients were randomised before reaching hospital and did not arrive alive.
The 30-day outcomes that were reported included mortality (the primary outcome measure), reinterventions, length of hospital stay, place of discharge and aneurysm morphology.
The new outcome measures that were included at the 1-year time point were QoL and use of health resources, both obtained from questionnaires, and cost-effectiveness. The Health Economist for the trial, Richard J Grieve, was spearheading methodological developments that would add to the robustness of the cost-effectiveness evaluations and we realised that half of the participants remained alive at 3 years after randomisation; a further extension request was submitted and granted. This second extension provided for cost-effectiveness reporting over a longer and more relevant time horizon and the follow-up of all participants for 3 years after randomisation, with closure and final reporting in December 2016. For the 1- to 3-year time period, the responsibility for collecting reintervention data was transferred from the local trial co-ordinators to the trial manager and audit team. Because the large majority of participants were from English sites, we planned to use HES to supplement the audit, particularly to collect information about aneurysm-related reinterventions at non-trial hospitals. Our final sources of data concerning first reinterventions are shown in Table 27.
| Time period for first reintervention (years) | Primary source according to the protocol | Participants per data source (n) | Participants identified with reinterventions (n) | |||
|---|---|---|---|---|---|---|
| Trial co-ordinator | HESa | Participant questionnaires | Audit | |||
| 0–1 | Trial co-ordinator | 162 | 5 | 2 | 22 | 191, with 0 at non-trial hospitals |
| 1–3 | Audit, supported by HES | 20 | 10 | 1 | 17 | 48, with 2 at non-trial hospitals |
Owing to delays in obtaining data from HES to report aneurysm-related admissions and reinterventions at non-trial hospitals (received 16 December 2016), a further extension of 3 months, to 31 March 2017, was obtained. CONSORT diagrams show the completeness of follow-up at 1 year and 3 years (Figures 20 and 8, respectively). At these stages, information from HES and similar sources was used to check for hospital admissions and procedures during the first 1 and 3 years after randomisation.
The trial, its extensions and its reporting deadlines are summarised in Figure 21.
FIGURE 21.
Overview of the trial. BJS, British Journal of Surgery; BMJ, British Medical Journal; HES, Hospital Episode Statistics.

Appendix 2 Additional tables and figures
| Description | Unit | Cost (£) | Source | |
|---|---|---|---|---|
| Open repair | Endovascular strategy | |||
| Medical devices and parts | ||||
| Endovascular stent and parts | Patient | 5700 | Medtronic (Minneapolis, MN, USA) and Cook Medical (Bloomington, IN, USA)a | |
| Vascular graft (straight) | Patient | 623 | Maquet (Rastatt, Germany) | |
| Vascular graft (bifurcated) | Patient | 901 | Maquet | |
| Consumables | ||||
| Endovascular package | Patient | 600 | Maquet and Cook Medical | |
| Mechanical retractor | Patient | 90 | Health-Care Equipment (Surrey, UK) | |
| Cell salvage | Patient | 74 | Davies et al. 2006123 | |
| Surgical instrument set | Patient | 51 | 51 | Health-Care Equipment |
| Anaesthetics and other drugs | Patient | 184 | 41 | British National Formulary 2012b |
| Contrast agent | ml | 0.10 | 0.10 | IMPROVE trial centresb |
| Blood | Unit | 132 | 132 | NHS Blood and Transplant 2012 |
| Platelets | Unit | 205 | 205 | NHS Blood and Transplant 2012 |
| Fresh-frozen plasma | Unit | 25 | 25 | NHS Blood and Transplant 2012 |
| CT scan | Unit | 105 | 105 | NHS Reference Costs 201229 |
| Emergency room | Minute | 0.40 | 0.40 | Dixon et al. 2009124 |
| Overheads | ||||
| Theatre | Minute | 2.65 | 2.65 | IMPROVE trial centres |
| Staffc | ||||
| Surgeon (consultant) | Minute | 2.20 | 2.20 | PSSRU 201228 |
| Surgeon (registrar) | Minute | 1.16 | 1.16 | PSSRU 201228 |
| Anaesthetist (consultant) | Minute | 2.20 | 2.20 | PSSRU 201228 |
| Anaesthetist (registrar) | Minute | 1.16 | 1.16 | PSSRU 201228 |
| ODA | Minute | 0.58 | 0.58 | PSSRU 201228 |
| Scrub nurse | Minute | 0.72 | 0.72 | PSSRU 201228 |
| Runner | Minute | 0.58 | 0.58 | PSSRU 201228 |
| Senior house officer | Minute | 0.83 | PSSRU 201228 | |
| Radiologist (consultant) | Minute | 2.20 | PSSRU 201228 | |
| Radiologist (registrar) | Minute | 1.16 | PSSRU 201228 | |
| Radiographer | Minute | 0.58 | PSSRU 201228 | |
| Radiologist nurse | Minute | 0.72 | PSSRU 201228 | |
| Critical care | ||||
| ITU/HDU: one organ supported | Bed-day | 630 | 630 | NHS Reference Costs 201229 |
| ITU/HDU: two organs supported | Bed-day | 870 | 870 | NHS Reference Costs 201229 |
| ITU/HDU: three organs supported | Bed-day | 1214 | 1214 | NHS Reference Costs 201229 |
| ITU/HDU: four organs supported | Bed-day | 1410 | 1410 | NHS Reference Costs 201229 |
| ITU/HDU: five organs supported | Bed-day | 1587 | 1587 | NHS Reference Costs 201229 |
| ITU/HDU: six organs supported | Bed-day | 1759 | 1759 | NHS Reference Costs 201229 |
| ITU/HDU: seven organs supported | Bed-day | 2000 | 2000 | NHS Reference Costs 201229 |
| Other hospital care | ||||
| Inpatient coronary care unit | Bed-day | 436 | 436 | NHS Reference Costs 201229 |
| Inpatient stroke unit | Bed-day | 309 | 309 | NHS Reference Costs 201229 |
| Inpatient routine wardd | Bed-day | 260 | 260 | NHS Reference Costs 201229 |
| Outpatient doctor visit | Visit | 139 | 139 | PSSRU 201228 |
| Outpatient nurse visit | Visit | 85 | 85 | PSSRU 201228 |
| Outpatient haemodialysis | Session | 65 | 65 | NHS Blood and Transplant, Price list 2011/12 |
| Community care | ||||
| Nursing home | Bed-day | 105 | 105 | PSSRU 201228 |
| Family doctor visite | Visit | 55 | 55 | PSSRU 201228 |
| Nurse at home visite | Visit | 18 | 18 | PSSRU 201228 |
| Staff use by type of operation | Trial centre number (n) | Total (n) | Base case | Sensitivity analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||
| Open repair | |||||||||||||
| Anaesthetist (consultant) | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 | ✓ | ✓ |
| Anaesthetist (registrar) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ✓ | ✓ |
| Vascular surgeon (consultant) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| Vascular surgeon (registrar) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| ODA (grade 5) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| Scrub nurse (grade 6) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| Runner (grade 5) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| House officer (level 2) | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | ? | ✓ |
| Second nurse (grade 5) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ? | ✓ |
| Second runner (grade 5) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | ? | ✓ |
| Second surgeon (consultant) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ? | ✓ |
| Second surgeon (registrar) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | ? | ✓ |
| EVAR | |||||||||||||
| Anaesthetist (consultant) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| Anaesthetist (registrar) | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 5 | ✓ | ✓ |
| Vascular surgeon (consultant) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | ✓ | ✓ |
| Vascular surgeon (registrar) | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| ODA (grade 5) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| Scrub nurse (grade 6) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| Runner (grade 5) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| Radiographer | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ✓ | ✓ |
| Radiologist (consultant) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | ✓ | ✓ |
| Radiology nurse (grade 6) | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 6 | ✓ | ✓ |
| Second runner (grade 5) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | ? | ✓ |
| Radiologist (registrar) | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | ? | ✓ |
| Cost component | Treatment group | |
|---|---|---|
| Endovascular strategy (N = 316) | Open repair (N = 297) | |
| Primary admissions | ||
| Time in emergency room (minutes), mean (SD)a | 93 (370) | 73 (157) |
| Cost (£) | 136 (138) | 119 (51) |
| Devices and consumables (£), mean (SD) | 4337 (2915) | 2523 (2036) |
| Time in theatre (minutes), mean (SD)b | 156 (100) | 180 (107) |
| Cost (£) | 2050 (1290) | 2101 (1264) |
| Days in critical care, mean (SD) | 4.2 (5.9) | 6.3 (7.7) |
| Cost (£) | 5249 (8779) | 8100 (11,020) |
| Days on routine ward, mean (SD)c | 5.2 (5.0) | 5.7 (6.7) |
| Cost (£) | 1425 (1591) | 1518 (1814) |
| Reinterventions, n (%) | 44 (14) | 48 (16) |
| Cost (£), mean (SD) | 172 (581) | 224 (1042) |
| Readmissions | ||
| Number of readmissions, n (%) | 6 (1.7) | 5 (1.9) |
| Cost (£), mean (SD) | 64 (554) | 34 (290) |
| Total hospital stay (days), mean (SD) | 9.8 (9.0) | 12.2 (10.2) |
| Total cost (£), mean (SD) | 13,433 (10,354) | 14,619 (12,353) |
| Incremental cost (£) (95% CI) | –1186 (–2997 to 625) | |
| Variable | Missing values, n (%)a | Imputation model |
|---|---|---|
| Baseline variables and vital status | ||
| Randomised group (endovascular strategy vs. open repair) | 0 (0) | None required |
| Age | 0 (0) | None required |
| Sex | 0 (0) | None required |
| Loss of consciousness during care episode | 27 (4) | Logistic regression |
| Admission haemoglobin | 6 (1) | Predictive mean matching |
| Admission creatinine | 13 (2) | Predictive mean matching |
| Acute myocardial ischaemia | 52 (8) | Logistic regression |
| Maximum aortic diameter | 95 (15) | Predictive mean matching |
| Aneurysm neck diameterb | 230 (38) | Predictive mean matching |
| Aneurysm proximal neck angleb | 132 (22) | Predictive mean matching |
| Aneurysm neck lengthb | 132 (22) | Predictive mean matching |
| Death within 1 year | 2 (0) | None done |
| Resource use variables | ||
| Primary admission: time in theatre | 22 (4) | Predictive mean matching |
| Primary admission: days in critical care | 13 (2) | Predictive mean matching |
| Primary admission: days in routine ward | 48 (8) | Predictive mean matching |
| Primary admission: reintervention time in theatre | 22 (4) | Predictive mean matching |
| Readmissions at 3 months: days in critical care | 20 (6) | Predictive mean matching |
| Readmissions at 12 months: days in critical care | 32 (11) | Predictive mean matching |
| Readmission at 3 months: days in routine ward | 19 (6) | Predictive mean matching |
| Readmission at 12 months: days in routine ward | 31 (10) | Predictive mean matching |
| Outpatient visits at 3 months | 37 (12) | Predictive mean matching |
| Outpatient visits at 12 months | 47 (16) | Predictive mean matching |
| Family doctor visits at 3 months | 75 (24) | Predictive mean matching |
| Family doctor visits at 12 months | 83 (28) | Predictive mean matching |
| Nurse at-home visits at 3 months | 62 (19) | Predictive mean matching |
| Nurse at-home visits at 12 months | 68 (23) | Predictive mean matching |
| QoL variables | ||
| EQ-5D at 3 months | 66 (21) | Predictive mean matching |
| EQ-5D at 12 months | 72 (24) | Predictive mean matching |
| EQ-5D component | Time point, n (%) | |||
|---|---|---|---|---|
| 3 monthsa | 12 monthsb | |||
| Endovascular strategy (N = 138) | Open repair (N = 114) | Endovascular strategy (N = 127) | Open repair (N = 102) | |
| Mobility | ||||
| 1 | 74 (54) | 51 (45) | 63 (50) | 55 (54) |
| 2 | 64 (46) | 60 (53) | 65 (51) | 48 (47) |
| 3 | 1 (1) | 3 (3) | 0 (0) | 2 (2) |
| Self-care | ||||
| 1 | 116 (84) | 76 (67) | 112 (88) | 82 (80) |
| 2 | 21 (15) | 34 (30) | 15 (12) | 20 (20) |
| 3 | 1 (1) | 5 (4) | 1 (1) | 4 (4) |
| Usual activities | ||||
| 1 | 60 (43) | 49 (43) | 59 (46) | 61 (60) |
| 2 | 71 (51) | 51 (45) | 63 (50) | 36 (35) |
| 3 | 8 (6) | 15 (13) | 5 (4) | 9 (9) |
| Pain/discomfort | ||||
| 1 | 82 (59) | 58 (51) | 76 (60) | 62 (61) |
| 2 | 53 (38) | 52 (46) | 51 (40) | 36 (35) |
| 3 | 4 (3) | 4 (4) | 1 (1) | 7 (7) |
| Anxiety/depression | ||||
| 1 | 101 (73) | 83 (73) | 93 (73) | 74 (73) |
| 2 | 37 (29) | 27 (24) | 33 (26) | 27 (26) |
| 3 | 1 (1) | 4 (4) | 2 (2) | 2 (2) |
| n | HR (95% CI) | p-value | |
|---|---|---|---|
| All-cause mortality | |||
| Unadjusted | 613 | 0.92 (0.75 to 1.13) | 0.41 |
| Adjusteda (complete cases) | 397 | 0.89 (0.69 to 1.16) | 0.39 |
| Adjusteda (multiply imputed) | 613 | 0.91 (0.74 to 1.12) | 0.38 |
| Aneurysm-related mortality | |||
| Unadjusted | 613 | 0.89 (0.69 to 1.16) | 0.41 |
| Adjusteda (complete cases) | 397 | 0.88 (0.64 to 1.22) | 0.45 |
| Adjusteda (multiply imputed) | 613 | 0.90 (0.69 to 1.16) | 0.42 |
| n (remaining under follow-up) | HR (95% CI) | p-value | |
|---|---|---|---|
| All-cause mortality | |||
| 0–3 months | 613 | 0.98 (0.76 to 1.26) | 0.88 |
| > 3 months | 373 | 0.80 (0.57 to 1.15) | 0.23 |
| Aneurysm-related mortality | |||
| 0–3 months | 613 | 0.90 (0.69 to 1.17) | 0.43 |
| > 3 months | 373 | 0.91 (0.34 to 2.43) | 0.85 |
| AAA-related reintervention | Participants with ruptured AAA in whom repair started (N = 502) | ||
|---|---|---|---|
| Endovascular strategy commenceda (N = 182) | Open repair commencedb (N = 320) | p-value | |
| Participants with at least one reintervention, n/N (%) | 52/182 (29) | 88/320 (28) | 0.80 |
| Reinterventions/person-years (rate per 100 person-years), (%) | 77/338.1 (22.8) | 153/513.0 (29.8) | 0.054 |
| Arterial, n (%) | 66 (86) | 89 (58) | < 0.001 |
| Laparotomy, n (%) | 2 (3) | 48 (31) | |
| Other, n (%) | 9 (12) | 16 (10) | |
| Severity of arterial reinterventions, n (%) | |||
| 1* | 1 (2) | 0 (0) | < 0.001 |
| 2** | 23 (35) | 11 (12) | |
| 3*** | 17 (26) | 26 (29) | |
| 4**** | 10 (15) | 4 (4) | |
| 5***** | 15 (23) | 48 (54) | |
| Severity of laparotomy reinterventions, n (%) | |||
| Major | 1 (50) | 7 (15) | 0.41 |
| Minor | 1 (50) | 40 (83) | |
| Unknown (scored 4) | 1 | ||
| Randomisation to 3 months | |||
| Participants with at least one reintervention, n/N (%) | 29/182 (16) | 79/320 (25) | 0.022 |
| Reinterventions/person-years (rate per 100 person-years), (%) | 39/33.0 (118.3) | 130/49.6 (262.0) | < 0.001 |
| Arterial, n (%) | 30 (77) | 79 (61) | 0.011 |
| Laparotomy, n (%) | 2 (5) | 36 (28) | |
| Other, n (%) | 7 (18) | 15 (12) | |
| Severity of arterial reinterventions, n (%) | |||
| 1* | 1 (3) | 0 (0) | 0.017 |
| 2** | 9 (30) | 10 (13) | |
| 3*** | 8 (27) | 22 (28) | |
| 4**** | 4 (13) | 4 (5) | |
| 5***** | 8 (27) | 43 (54) | |
| Severity of laparotomy reinterventions, n (%) | |||
| Major | 1 (50) | 3 (8) | 0.17 |
| Minor | 1 (50) | 32 (89) | |
| Unknown (scored 4) | 1 | ||
| 3 months to 3 years | |||
| Participants with at least one reintervention, n/N (%) | 26/125 (21) | 16/188 (9) | 0.002 |
| Reinterventions/person-years (rate per 100 person-years), (%) | 38/305.1 (12.5) | 23/463.4 (5.0) | < 0.001 |
| Arterial, n (%) | 36 (95) | 10 (43) | < 0.001 |
| Laparotomy, n (%) | 0 (0) | 12 (52) | |
| Other, n (%) | 2 (5) | 1 (4) | |
| Severity of arterial reinterventions, n (%) | |||
| 1* | 0 (0) | 0 (0) | 0.07 |
| 2** | 14 (39) | 1 (10) | |
| 3*** | 9 (25) | 4 (40) | |
| 4**** | 6 (17) | 0 (0) | |
| 5***** | 7 (19) | 5 (50) | |
| Severity of laparotomy reinterventions, n (%) | |||
| Major | 4 (33) | ||
| Minor | 8 (67) | ||
| Rate per 100 person-years (n/person-years) | Model | N | HR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Endovascular strategy | Open repair | ||||
| Time to first AAA-related reintervention | |||||
| 22.6 (75/332.2) | 21.0 (66/314.2) | Unadjusted | 613 | 1.10 (0.79 to 1.54) | 0.59 |
| Adjusteda (complete cases) | 397 | 1.09 (0.73 to 1.62) | 0.67 | ||
| Adjusteda (multiply imputed) | 613 | 1.10 (0.79 to 1.54) | 0.57 | ||
| Time to any AAA-related reintervention (Andersen–Gill model) | |||||
| 26.0 (121/464.5) | 28.3 (110/388.3) | Unadjusted | 613 | 1.02 (0.79 to 1.32) | 0.89 |
| Adjusteda (complete cases) | 397 | 0.93 (0.68 to 1.27) | 0.64 | ||
| Adjusteda (multiply imputed) | 613 | 1.02 (0.79 to 1.33) | 0.87 | ||
| Randomisation to 3 months | |||||
| 182.5 (81/44.4) | 222.7 (89/40.0) | Unadjusted | 613 | 0.88 (0.65 to 1.19) | 0.40 |
| Adjusteda (complete cases) | 397 | 0.78 (0.55 to 1.11) | 0.17 | ||
| Adjusteda (multiply imputed) | 613 | 0.87 (0.64 to 1.18) | 0.37 | ||
| 3 months to 3 years | |||||
| 9.5 (40/420.1) | 6.0 (21/348.3) | Unadjusted | 313 | 1.57 (0.93 to 2.67) | 0.09 |
| Adjusteda (complete cases) | 217 | 1.67 (0.82 to 3.43) | 0.16 | ||
| Adjusteda (multiply imputed) | 313 | 1.54 (0.90 to 2.62) | 0.12 | ||
| Aneurysm-related indication for 502 repairs with rupture started | Randomised to EVAR strategy (n = 259) | Randomised to open repair (n = 243) | Treated with EVAR (n = 182) | Treated with open repair (n = 320) |
|---|---|---|---|---|
| Access site | 4 | 1 | 3 | 2 |
| Abdominal compartment syndrome | 7 | 10 | 2 | 15 |
| Bowel ischaemiaa | 14 | 16 | 5 | 25 |
| Closure open abdomen | 5 | 5 | 1 | 9 |
| Distal aneurysm | 1 | 1 | 2 | 0 |
| Endograft kinkingb | 2 | 0 | 2 | 0 |
| Endoleakc | 3 | 1 | 4 | 0 |
| False aneurysm | 1 | 0 | 1 | 0 |
| Graft thrombosis/occlusion | 3 | 0 | 1 | 2 |
| Graft infection: aorta | 0 | 2 | 0 | 2 |
| Graft infection: femoro-femoral | 2 | 0 | 2 | 0 |
| Limb ischaemia | 22 | 13 | 8 | 27 |
| Ostomy (stoma) | 1 | 1 | 1 | 1 |
| Re-bleeding | 3 | 7 | 1 | 9 |
| Other indications | ||||
| Coronary or brain ischaemia | 3 | 2 | 2 | 3 |
| Miscellaneousd | 0 | 3 | 1 | 2 |
| Nutritional support | 0 | 1 | 0 | 1 |
| Pulmonary embolism | 0 | 2 | 1 | 1 |
| Renal failure | 0 | 1 | 0 | 1 |
| Tracheostomy for ventilator weaning | 5 | 7 | 2 | 10 |
| Upper GI bleed | 1 | 5 | 1 | 5 |
| Total re-interventions | 77 in 55 patients | 78 in 53 patients | 40 in 29 patients | 115 in 79 patients |
| Aneurysm-related indication for 313 repairs with rupture started who survived beyond 90 days | Randomised to EVAR strategy (n = 167) | Randomised to open repair (n = 146) | Treated with EVAR (n = 125) | Treated with open repair (n = 188) |
|---|---|---|---|---|
| Access site | 3 | 1 | 2 | 2 |
| Bowel ischaemia | 2 | 1 | 0 | 3 |
| Distal aneurysm | 3 | 4 | 3 | 4 |
| Endograft kinkinga | 2 | 2 | 4 | 0 |
| Endograft migration | 1 | 0 | 1 | 0 |
| Endoleakb | 14 | 2 | 16 | 0 |
| False aneurysm | 1 | 0 | 1 | 0 |
| Graft thrombosis/occlusion | 6 | 1 | 4 | 3 |
| Graft infection: aorta | 0 | 2 | 0 | 2 |
| Graft infection: femoro-femoral | 0 | 0 | 0 | 0 |
| Incisional hernia | 0 | 3 | 0 | 3 |
| Limb ischaemia | 2 | 2 | 3 | 1 |
| Ostomy (stoma) | 0 | 1 | 0 | 1 |
| Proximal aneurysm | 1 | 0 | 1 | 0 |
| Secondary rupturec | 2 | 0 | 2 | 0 |
| Symptomatic adhesions | 1 | 1 | 0 | 2 |
| Other indications | ||||
| Nutritional support | 0 | 1 | 0 | 1 |
| Renal failure | 1 | 0 | 1 | 0 |
| Total re-interventions | 39 in 27 patients | 21 in 15 patients | 38 in 26 patients | 22 in 16 patients |
| Morphological variable | Re-interventions | Treated with EVAR (n = 182) | Treated with open repair (n = 320) | Combined (n = 502) |
|---|---|---|---|---|
| Time to any AAA related re-intervention | ||||
| Maximum AAA diameter (per 17 mm increase) | All |
0.97 (0.72–1.32) p = .86 |
0.95 (0.77–1.18) p = .65 |
0.95 (0.80–1.12) p = .52 |
| Arterial |
1.02 (0.74–1.39) p = .94 |
0.85 (0.66–1.09) p = .21 |
0.90 (0.74–1.09) p = .28 |
|
| Aneurysm neck diameter at distal renal artery (per 4 mm increase) | All |
1.00 (0.77–1.30) p = .98 |
1.21 (0.99–1.49) p = .06 |
1.15 (0.98–1.35) p = .09 |
| Arterial |
0.98 (0.74–1.29) p = .88 |
0.94 (0.72–1.24) p = .67 |
0.95 (0.78–1.16) p = .63 |
|
| Aneurysm neck length (per 16 mm increase) | All |
0.80 (0.58–1.10) p = .16 |
0.89 (0.72–1.10) p = .28 |
0.87 (0.73–1.03) p = .12 |
| Arterial |
0.74 (0.53–1.04) p = .08 |
0.89 (0.69–1.16) p = .40 |
0.84 (0.69–1.03) p = .09 |
|
| Neck conicality (per 1.6% per mm length, change increase) | All |
0.72 (0.45–1.15) p = .17 |
0.91 (0.74–1.11) p = .36 |
0.87 (0.72–1.06) p = .16 |
| Arterial |
0.65 (0.39–1.10) p = .11 |
1.07 (0.87–1.31) p = .52 |
0.97 (0.77–1.22) p = .80 |
|
| Proximal aneurysm neck (α) angle (per 20° increase) | All |
1.01 (0.77–1.31) p = .96 |
1.05 (0.89–1.24) p = .56 |
1.04 (0.90–1.19) p = .62 |
| Arterial |
0.96 (0.72–1.29) p = .79 |
0.90 (0.70–1.16) p = .42 |
0.93 (0.77–1.12) p = .42 |
|
| Maximum common iliac diameter (per 9 mm increase)a | All |
1.32 (1.01–1.72) p = .041 |
1.06 (0.91–1.24) p = .45 |
1.11 (0.98–1.26) p = .11 |
| Arterial |
1.48 (1.13–1.93) p = .004 |
1.11 (0.92–1.35) p = .28 |
1.20 (1.04–1.39) p = .013 |
|
| Treatment group | Mean difference (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|
| Endovascular strategy | Open repair | |||||
| n | Mean (SD) | n | Mean (SD) | |||
| EQ-5D-3La for ruptured AAA survivors | ||||||
| 3 months | 138 | 0.76 (0.23) | 114 | 0.69 (0.30) | 0.073 (0.007 to 0.138) | 0.030 |
| 12 months | 127 | 0.78 (0.19) | 102 | 0.74 (0.32) | 0.043 (–0.024 to 0.110) | 0.211 |
| 36 months | 117 | 0.75 (0.24) | 91 | 0.74 (0.31) | 0.012 (–0.063 to 0.088) | 0.746 |
| Life-years (up to 3 years)b | 313 | 1.71 (1.43) | 295 | 1.60 (1.41) | 0.113 (–0.114 to 0.339) | 0.329 |
| QALYs (3-year) | ||||||
| Ruptured AAA survivors | 91 | 2.22 (0.50) | 68 | 2.16 (0.64) | 0.052 (–0.126 to 0.230) | 0.563 |
| Ruptured AAA survivors and deceased | 193 | 1.08 (1.14) | 183 | 0.88 (1.10) | 0.207 (–0.021 to 0.435) | 0.074 |
| All randomised participantsc | 250 | 1.01 (1.10) | 237 | 0.88 (1.08) | 0.131 (–0.063 to 0.325) | 0.187 |
| Subgroup | Incremental (95% CI) | INB (95% CI) | p-value | |
|---|---|---|---|---|
| Cost (£) | QALYs | |||
| Sex | ||||
| Male (n = 480) | –4066 (–7573 to –557) | 0.092 (–0.099 to 0.283) | 6824 (204 to 13,444) | 0.440 |
| Female (n = 133) | 2817 (–3806 to 9439) | 0.358 (0.038 to 0.712) | 7914 (–4401 to 20,229) | |
| Hardman index score | ||||
| 0 (n = 164) | –4017 (–9758 to 1725) | 0.016 (–0.141 to 0.465) | 8878 (–1686 to 19,442) | 0.279 |
| 1 (n = 254) | –2448 (–7022 to 2126) | 0.005 (–0.244 to 0.254) | 2594 (–5943 to 11,131) | |
| ≥ 2 (n = 121) | –923 (–7306 to 5461) | 0.423 (0.092 to 0.755) | 13,622 (2025 to 25,219) | |
| Neck length | ||||
| < 22 mm (n = 234) | –769 (–5595 to 4057) | 0.216 (–0.027 to 0.459) | 7243 (–1216 to 15,702) | 0.461 |
| ≥ 22 mm (n = 247) | –4250 (–8859 to 358) | 0.111 (–0.131 to 0.352) | 7568 (–959 to 16,095) | |
| Lowest systolic blood pressure | ||||
| < 90 mmHg (n = 263) | –3881 (–8604 to 842) | 0.067 (–0.185 to 0.319) | 5881 (–2949 to 14,711) | 0.275 |
| ≥ 90 mmHg (n = 305) | –1540 (–5879 to 2798) | 0.213 (–0.013 to 0.439) | 7932 (–14.2 to 15,878) | |
| Best-fitting model | Bedside model | ||||
|---|---|---|---|---|---|
| Variable | β (SE) | p-value | Rule | OR | Score allocateda |
| Intercept | –0.3040 (0.8024) | ||||
| (age−505)3 | 0.0028 (0.0008) | 0.001 | Aged ≥ 76 years | 1.40 | 2 |
| Haemoglobin | –0.0897 (0.0524) | 0.087 | Haemoglobin level of < 11 g/dl | 2.10 | 4 |
| (creatinine5) | 0.4522 (0.1613) | 0.005 | Creatinine concentration of ≥ 120 µmol/l | 1.63 | 3 |
| (sbp+1100)−2 | 0.4665 (0.1339) | < 0.001 | Systolic blood pressure of < 100 mmHg | 1.71 | 3 |
| log(necklength+110) | –0.7070 (0.1954) | < 0.001 | Neck length of < 15 mm | 1.92 | 4 |
| (neckangle+110) | –0.0989 (0.0614) | 0.107 | Neck angle of < 45° | 1.94 | 4 |
| Male | –0.4919 (0.2651) | 0.063 | Female | 2.11 | 4 |
| Acute ischaemia | 1.0363 (0.3659) | 0.005 | Acute ischaemia | 2.78 | 6 |
| Variable | VSGNE | Hardman index | Vancouver |
|---|---|---|---|
| Aged > 76 years | +2 | +1 | +0.062 for each 1-year increase in age |
| Cardiac arrest | +2 | +1 | +0.60 |
| Loss of consciousness | +1 | +1 | +1.14 |
| Suprarenal clamp | +1 | – | – |
| ECG ischaemia | – | +1 | – |
| Creatinine concentration of > 190 µmol/l | – | +1 | – |
| Haemoglobin level of < 9 g/dl | – | +1 | – |
| Model | Data set, c-statistic (SD) | ||||
|---|---|---|---|---|---|
| Development | Validation | ||||
| IMPROVE RCT (n = 536) | AJAX RCT (n = 113) | ECAR RCT (n = 105) | Amsterdam cohort (n = 513) | STAR cohort (n = 284) | |
| IMPROVE score | 0.715a (0.023) | 0.677 (0.052) | 0.619 (0.069) | 0.724 (0.022) | 0.693 (0.031) |
| IMPROVE bedside score | 0.691a (0.023) | 0.662 (0.054) | 0.631 (0.070) | 0.664 (0.024) | 0.632 (0.033) |
| VSGNE | 0.667 (0.023) | 0.691b (0.053) | 0.632b (0.063) | 0.682c (0.024) | 0.683b (0.031) |
| Hardman index | 0.658 (0.023) | 0.784 (0.043) | 0.694 (0.062) | 0.697 (0.023) | 0.669 (0.032) |
| Vancouver | 0.653 (0.024) | 0.700b (0.059) | 0.657b (0.064) | 0.685c (0.024) | 0.745b (0.029) |
FIGURE 22.
The HR of time to discharge from primary admission hospital (endovascular strategy/endovascular repair compared with open repair) by randomised group. Copyright © 2015 The Authors. BJS published by John Wiley & Sons, Inc., on behalf of BJS Society Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
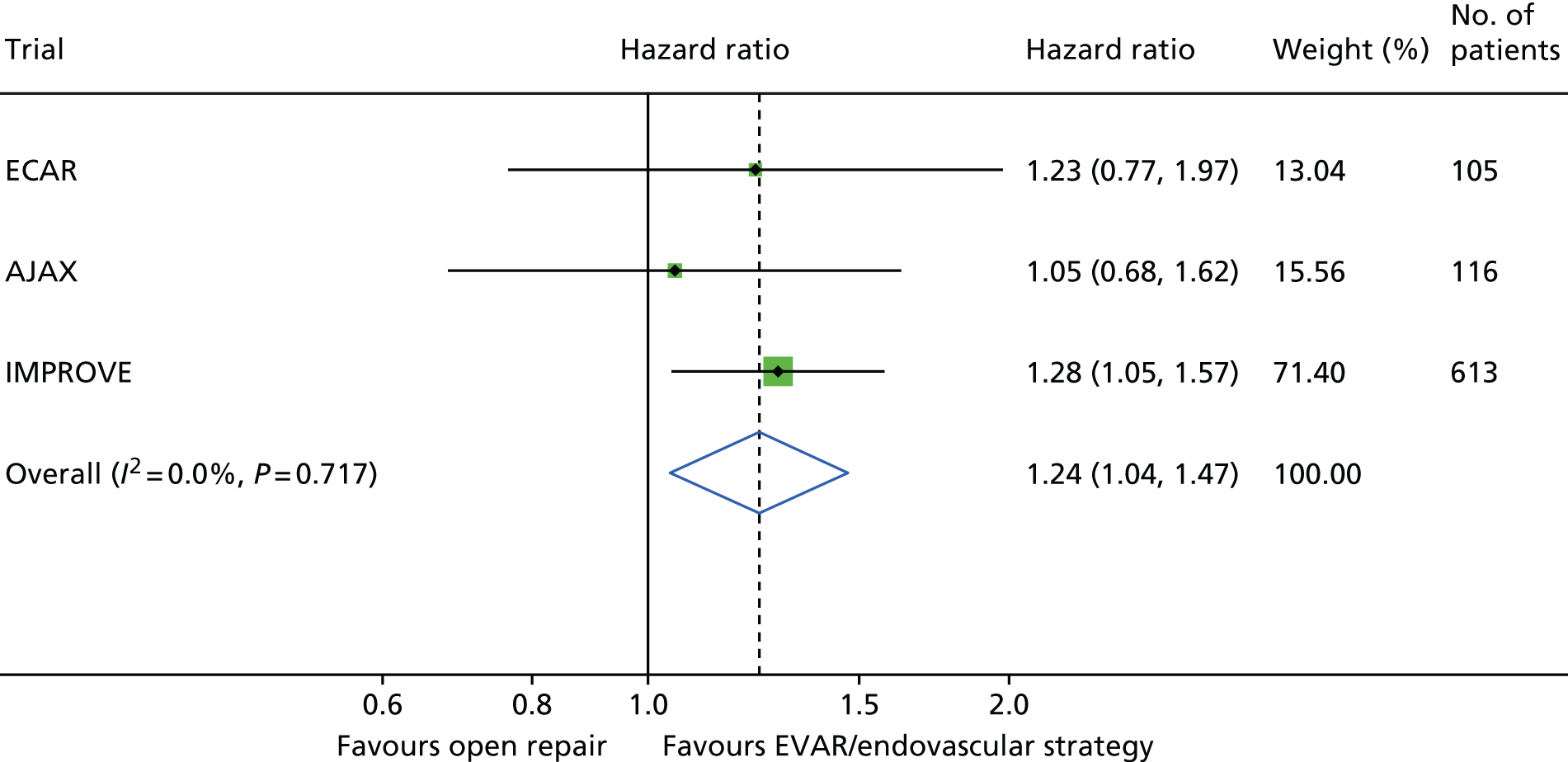
FIGURE 23.
Effect of systolic blood pressure (lowest recorded) on probability of 30-day mortality (95% CIs are presented).

FIGURE 24.
Survival to 1 year in the AJAX, ECAR and IMPROVE trials. (a) AJAX; (b) ECAR; (c) IMPROVE (all participants); (d) IMPROVE (data for the 308 IMPROVE participants with a ruptured aneurysm who were suitable for EVAR); and (e) individual patient meta-analysis of 1-year mortality for participants as randomised in AJAX, ECAR and IMPROVE. Reprinted from Eur J Vasc Endovasc Surg, 50, Sweeting MJ, Ulug P, Powell JT, Desgranges P, Balm R, Ruptured Aneurysm Trialists, Ruptured aneurysm trials: the importance of longer-term outcomes and meta-analysis for 1-year mortality, 297–302, Copyright (2015) with permission from Elsevier.

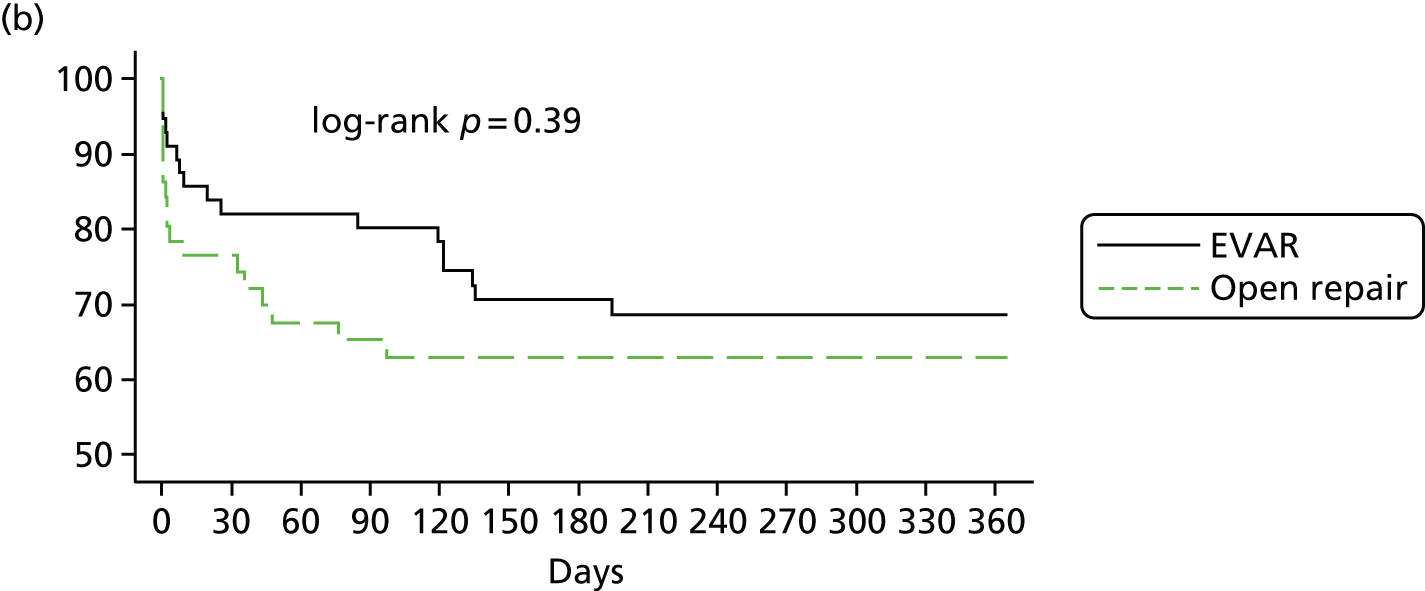

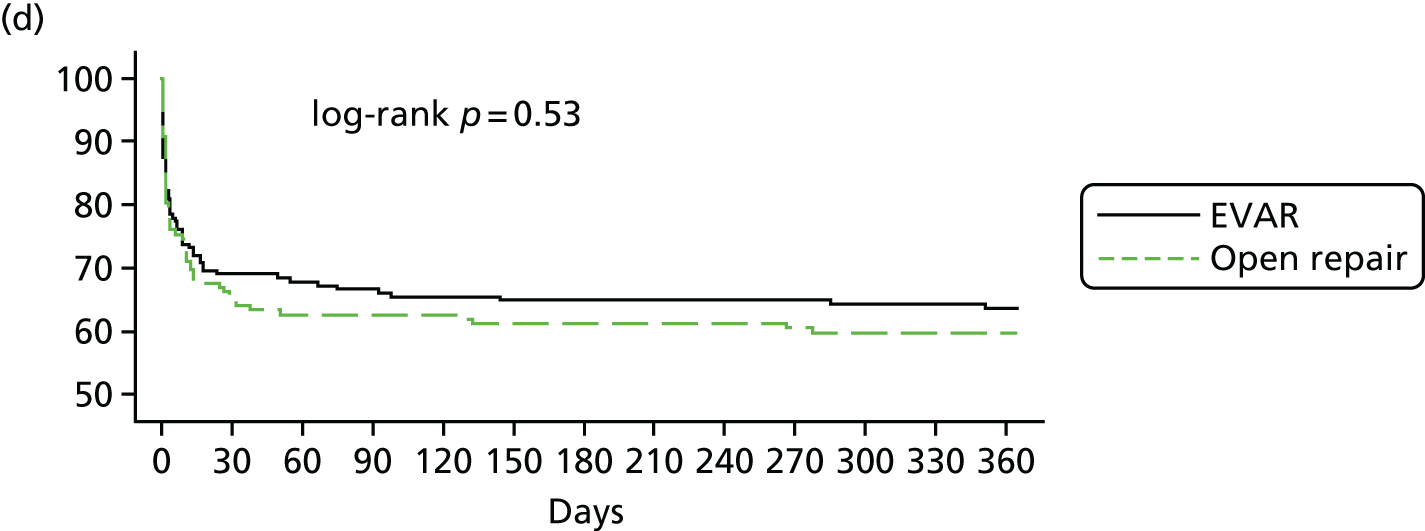

FIGURE 25.
Uncertainty in the mean cost (GBP) and QALY differences at 1 year after randomisation for 613 participants and their joint distribution for endovascular strategy compared with open repair. Reproduced with permission from the IMPROVE Trial Investigators. 23 © The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com.
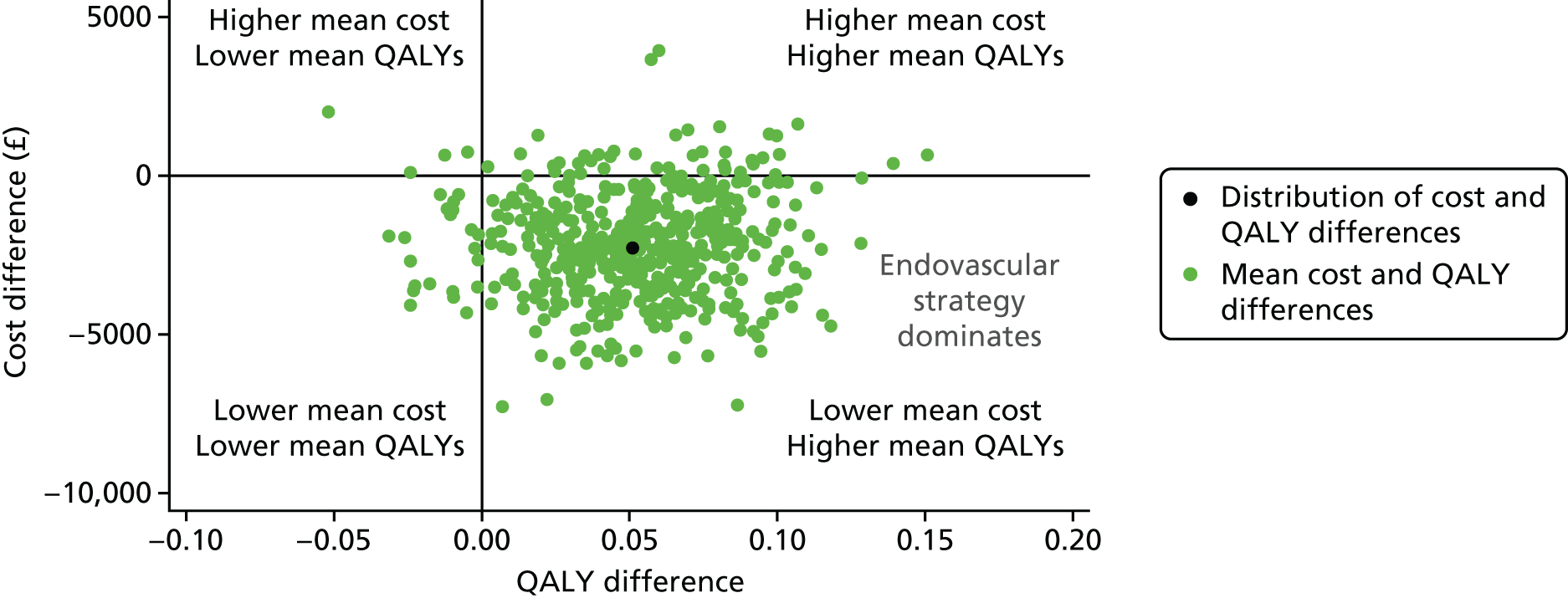
FIGURE 26.
Sensitivity analysis that considers the effect on the INMB at 1 year (at £30,000 per QALY) of alternative assumptions, compared with the base case for 613 participants.

FIGURE 27.
Kaplan–Meier survival curves for overall aneurysm-related mortality for 613 participants, by randomised group.
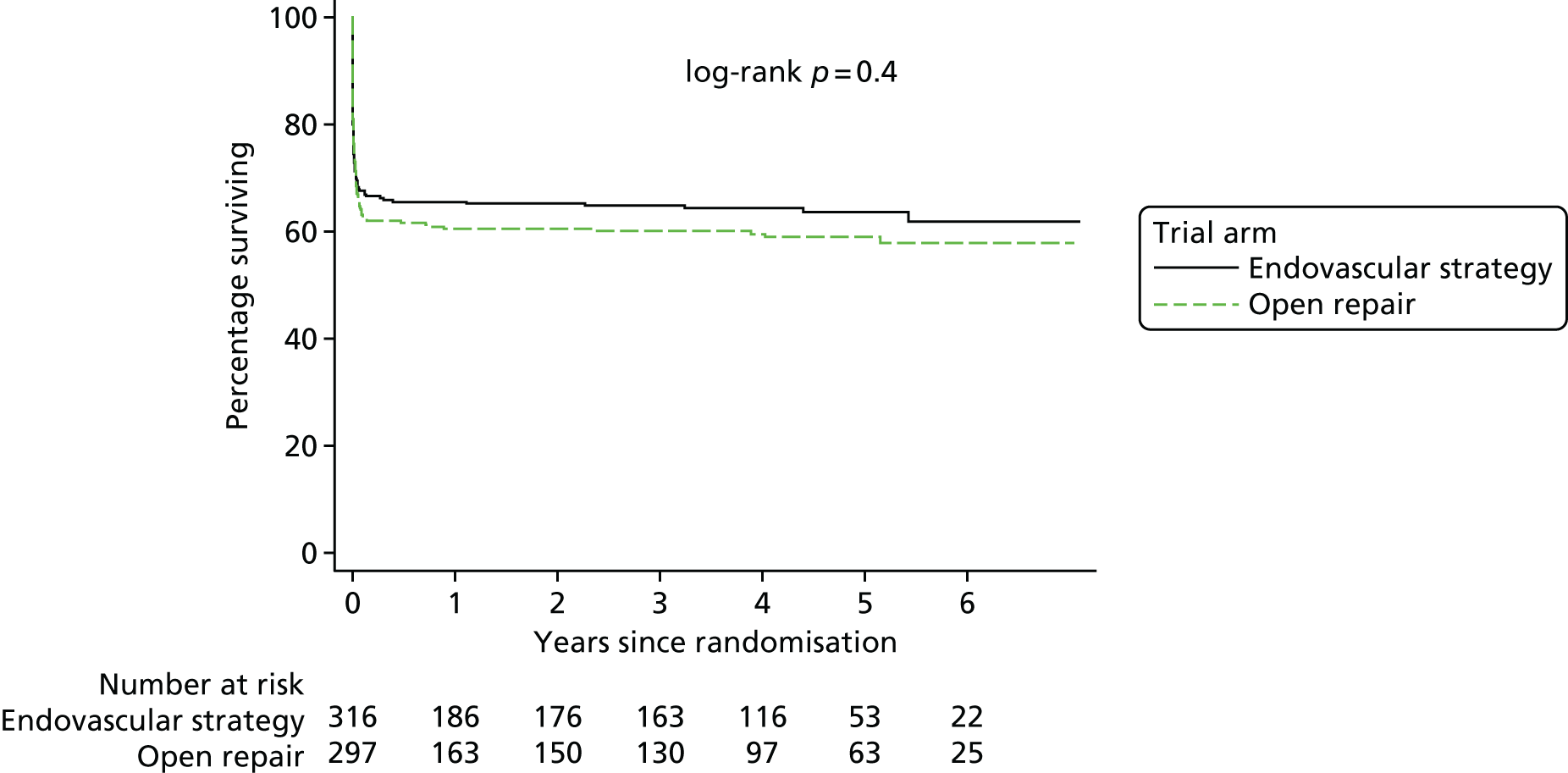
FIGURE 28.
Prespecified subgroup analyses showing HRs for the endovascular strategy group compared with the open repair group for overall mortality at 3 years in 613 participants.
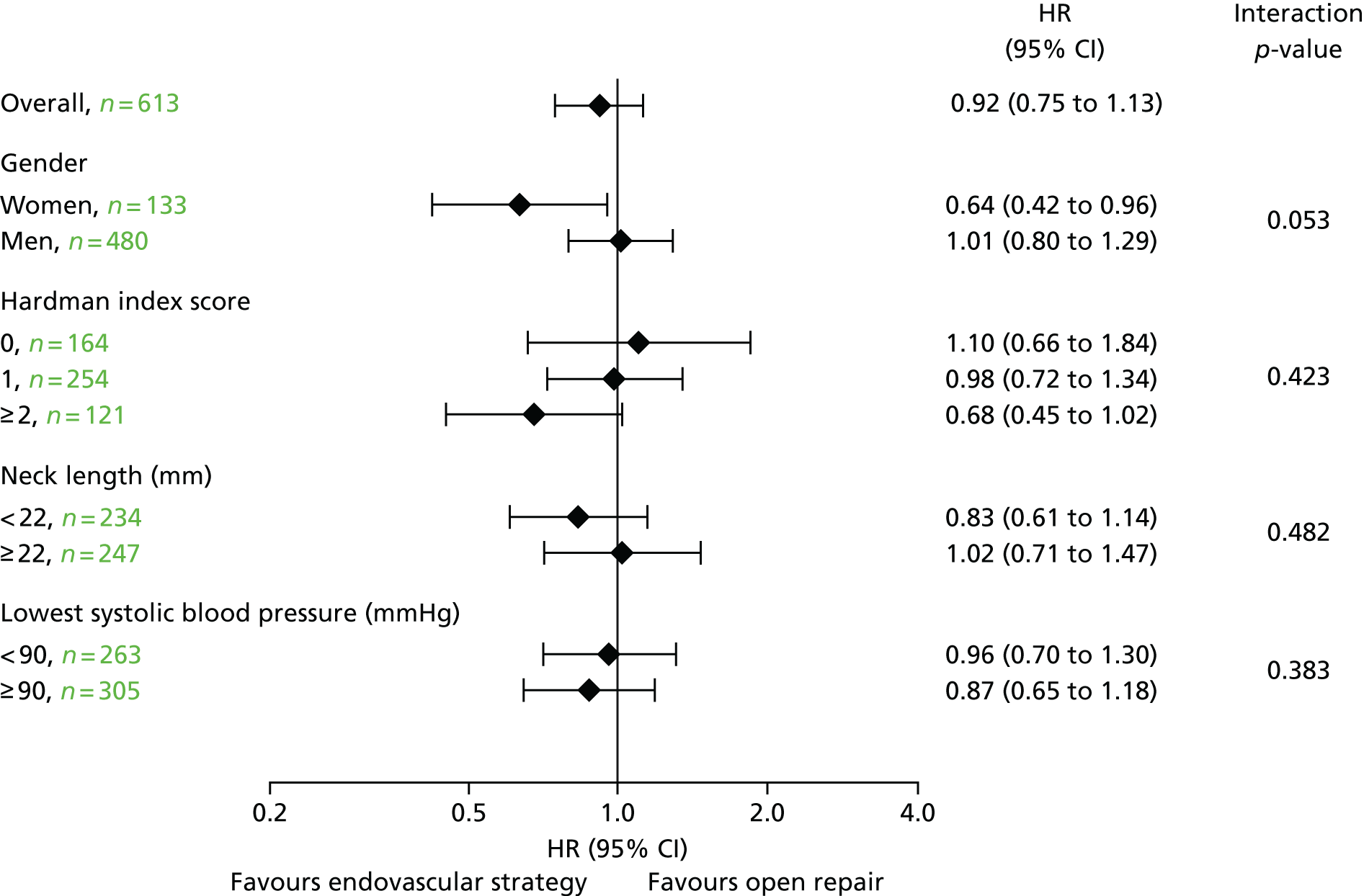
FIGURE 29.
Sensitivity analysis that considers the effect on the INMB at 3 years (at £30,000 per QALY) of alternative assumptions, compared with the base case for 613 participants.

FIGURE 30.
Non-linear effects of (a) age, (b) admission systolic blood pressure and (c) neck length on the log-odds of mortality. FP fit is shown together with estimated log-odds by deciles of candidate predictor.

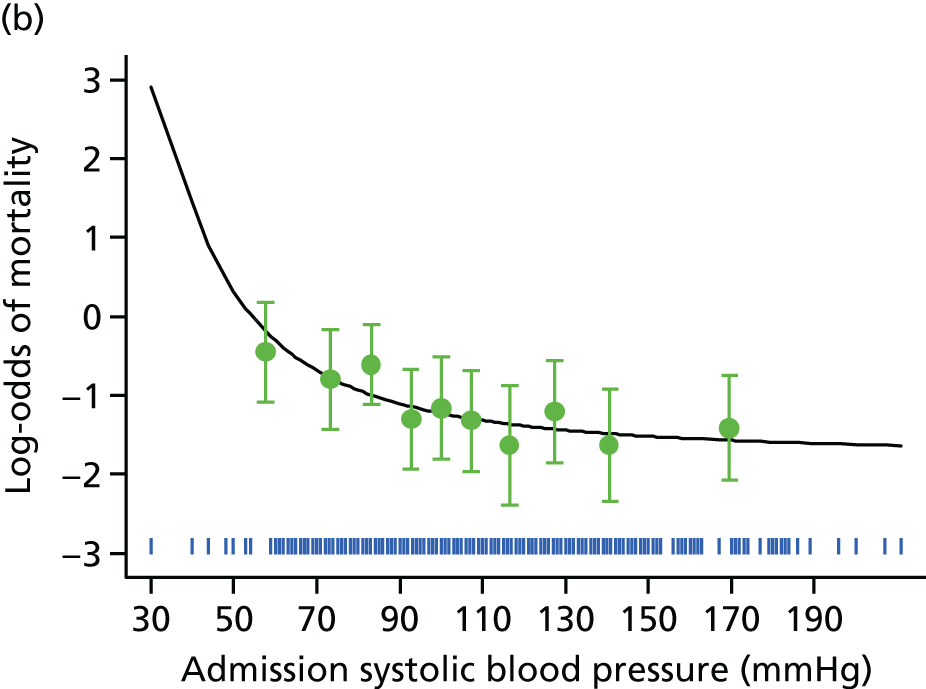
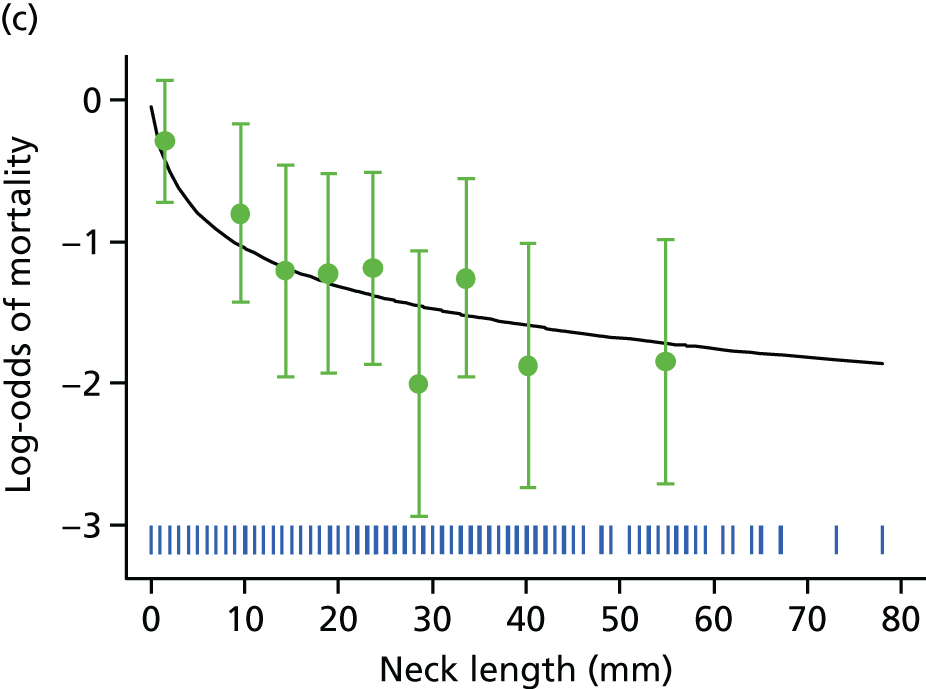
FIGURE 31.
Calibration plots for the best-fitting model and the bedside model in the IMPROVE trial data set. (a) Best-fitting model; and (b) bedside model.
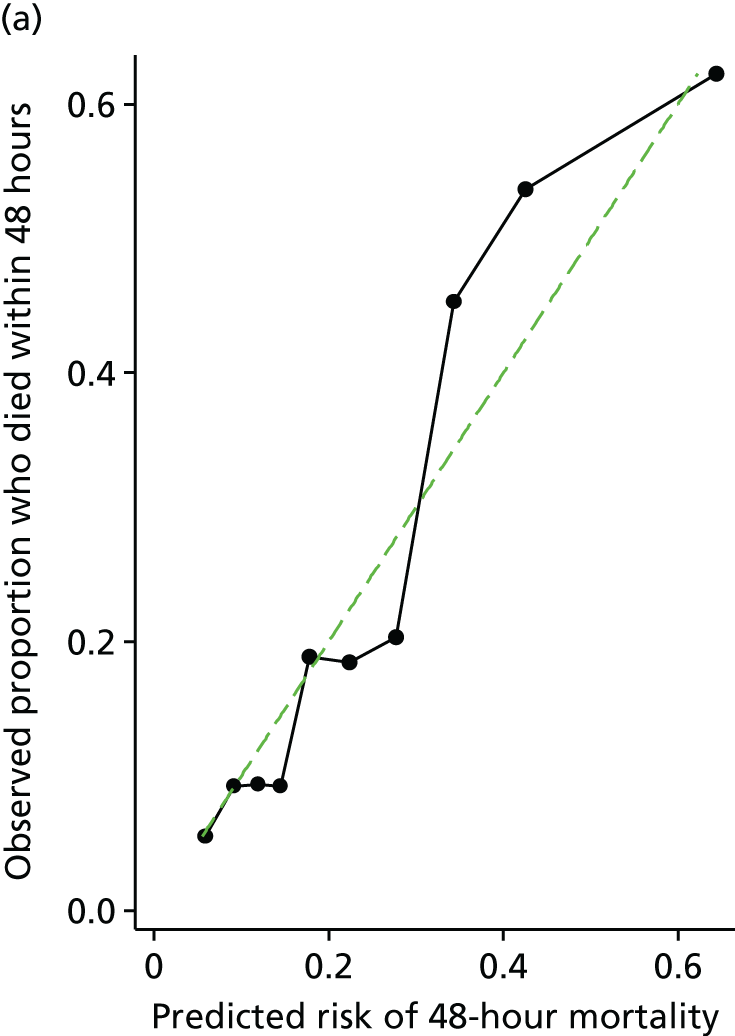
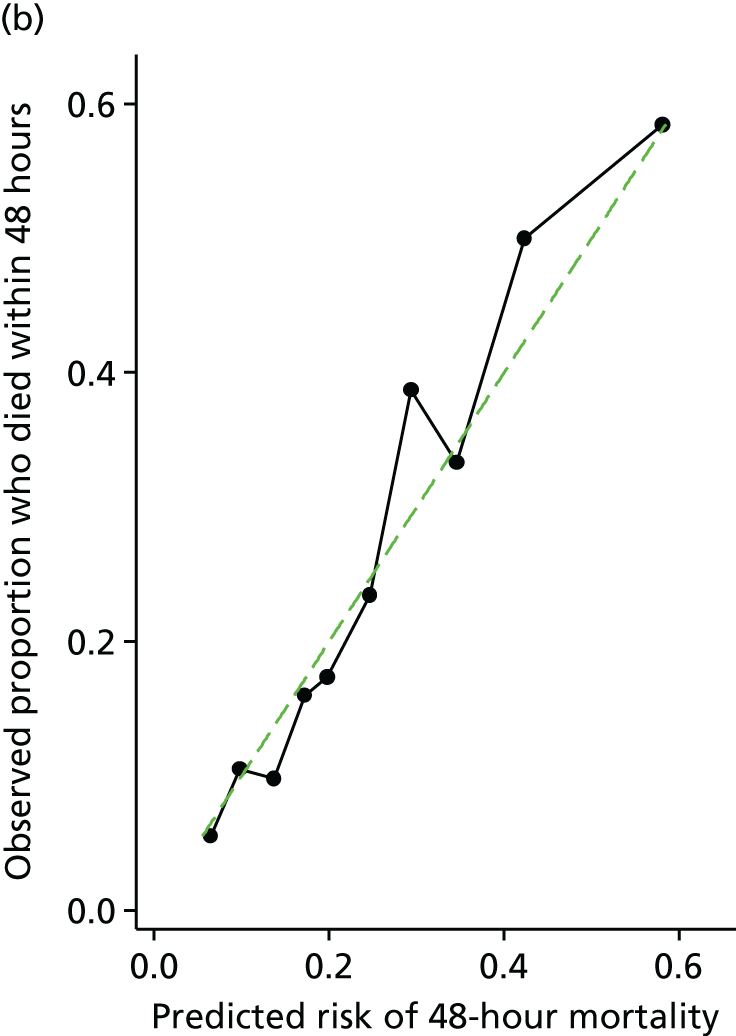
FIGURE 32.
Variation of WSSs in the aorta with iliac bifurcation angle. (a) WSS; (b) TAWSS; (c) wall stress; and (d) ECAP. ECAP, endothelial cell activation potential. MPa, megapascal.

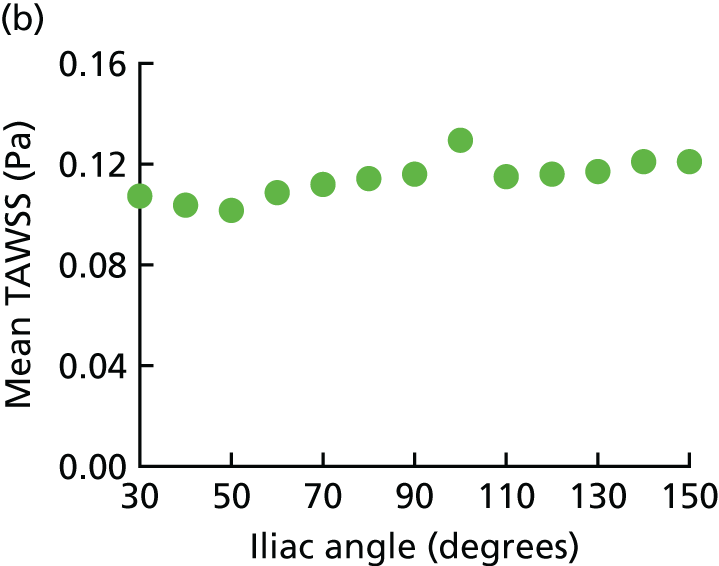

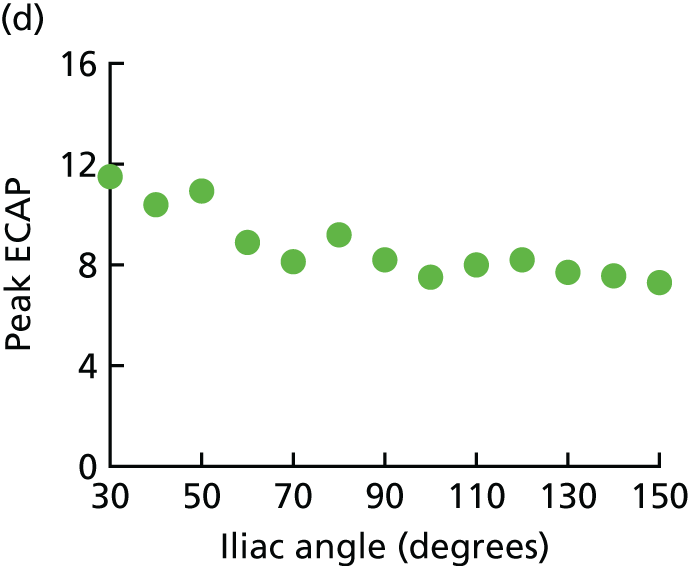
List of abbreviations
- AAA
- abdominal aortic aneurysm
- AJAX
- Amsterdam Acute Aneurysm trial
- AUROC
- area under receiver operating characteristic
- CI
- confidence interval
- CIA
- common iliac aneurysm
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CT
- computerised tomography
- ECAR
- Endovasculaire versus Chirurgie dans les Anévrysmes Rompus
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EVAR
- endovascular aneurysm repair
- FP
- fractional polynomial
- GBP
- Great British pounds
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- IFU
- instructions for use
- IMPROVE
- Immediate Management of the Patient with Rupture: Open Versus Endovascular repair
- INB
- incremental net benefit
- INMB
- incremental net monetary benefit
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- STAR
- STockholm Aneurysm Ruptures
- TAWSS
- time-averaged wall shear stress
- USD
- US dollars
- WSS
- wall shear stress
